Notes
Article history
The research reported in this monograph was funded as project number 96/17/01.
Declared competing interests of authors
Colette Hoare has been funded by the NHS Health Technology Assessment (HTA) R&D programme. Professor Hywel Williams: four of Hywel Williams' staff are currently employed on NHS HTA or Regional Research and Development grants.Two other Cochrane Skin Group staff members receive NHS R&D core support. He is a paid editor for the Drugs and Therapeutics Bulletin. He is also a life member of the National Eczema Society. He does not work as a consultant to any Pharmaceutical Company although he has received payment from Novartis for lectures on the epidemiology of atopic eczema in 1999. One of his research staff was previously funded by Crookes Healthcare International to conduct a pilot study into the role of nurses in running dermatology follow-up clinics. Professor Alain Li Wan Po has acted as occasional lecturer or consultant for Boots HealthCare Ltd, Novartis, Zyma, SmithKline Beecham, Yamanouchi and Warner Lambert.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2000. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising.
Chapter 1 Background and aims
The problem of atopic eczema
What is atopic eczema?
Atopic eczema is a chronic inflammatory skin condition characterised by an itchy red rash that favours the skin creases such as folds of elbows or behind the knees. The eczema lesions themselves vary in appearance from collections of fluid in the skin (vesicles) to gross thickening of the skin (lichenification) on a background of poorly demarcated redness. Other features such as crusting, scaling, cracking and swelling of the skin can occur. 1 Atopic eczema is associated with other atopic diseases such as hay fever and asthma. People with atopic eczema also have a dry skin tendency, which makes them vulnerable to the drying effects of soaps. Atopic eczema typically starts in early life, with about 80% of cases starting before the age of 5 years. 2
Is atopic eczema ‘atopic’?
Although the word ‘atopic’ is used when describing atopic eczema, it should be noted that around 20% of people with otherwise typical atopic eczema are not atopic as defined by the presence of positive skin prick test reactions to common environmental allergens, or through blood tests, which detect specific circulating immunoglobulin E (IgE) antibodies. 3 The word ‘atopic’ in the term atopic eczema is simply an indicator of the frequent association with atopy and the need to separate this clinical phenotype from the ten or so other forms of eczema such as irritant, allergic contact, discoid, venous, seborrhoeic and photosensitive eczema, which have other causes and distinct patterns. The terms atopic eczema and atopic dermatitis are synonymous. The term atopic eczema or just eczema is frequently used in the UK, whereas atopic dermatitis is used more in North America. Much scientific energy has been wasted in debating which term should be used.
How is atopic eczema defined in clinical studies?
Very often, no definition of atopic eczema is given in clinical studies such as clinical trials. This leaves the reader guessing as to what sort of people were studied. Atopic eczema is a difficult disease to define as the clinical features are highly variable. This variability can be in the skin rash morphology (e.g. it can be dry and thickened or weeping and eroded), in place (e.g. it commonly affects the cheeks in infants and skin creases in older children) and time (it can be bright red one day and apparently gone in a couple of days). There is no specific diagnostic test that encompasses all people with typical eczema and which can serve as a reference standard. Diagnosis is therefore essentially a clinical one.
Up until the late 1970s, at least 12 synonyms for atopic eczema were in common usage in the dermatological literature, and it is not certain if physicians were all referring to the same disease when using these terms. A major milestone in describing the main clinical features of atopic eczema was the Hanifin and Rajka diagnostic criteria of 1980. 4 These are frequently cited in clinical trial articles, and they at least provide some degree of confidence that researchers are referring to a similar disease when using these features. It should be borne in mind however that these criteria were developed on the basis of consensus, and their validity and repeatability is unknown in relation to physician's diagnosis. 3 Some of the 30 or so minor features have since been shown not to be associated with atopic eczema, and many of the terms, which are poorly defined, probably mean something only to dermatologists. Scientifically developed refinements of the Hanifin and Rajka diagnostic criteria, mainly for epidemiological studies, have been developed by a UK working party, and these criteria have been widely used throughout the world. 5 According the these criteria,6 in order to qualify as a case of atopic eczema, the person must have:
-
an itchy skin condition plus three or more of:
-
past involvement of the skin creases, such as the bends of elbows or behind the knees
-
personal or immediate family history of asthma or hay fever
-
tendency towards a generally dry skin
-
onset under the age of 2 years
-
visible flexural dermatitis as defined by a photographic protocol.
Binary or continuous disease?
It is unclear whether atopic eczema is an ‘entity’ in itself or whether it is part of a continuum when considered at a population level. Some studies have suggested that atopic dermatitis score is distributed as part of a continuum. 3 Although it may be appropriate to ask the question: “How much atopic eczema does he/she have?” as opposed to “Does he/she have atopic eczema – yes or no?”, most population and clinical studies require a categorical cut-off point and tend to include well-defined and typical cases.
Is it all one disease?
It is quite possible that there are distinct subsets of atopic eczema, for example those cases associated with atopy and those who have severe disease with recurrent infections. Until the exact genetic and causative agents are known, it is wiser to consider the clinical disease as one condition. Perhaps sensitivity analyses can be done for those who are thought to represent distinct subsets (e.g. those who are definitely atopic with raised circulating IgE to allergens, or those with severe disease and associated asthma). 3
The prevalence of atopic eczema
Atopic eczema is a very common problem. Prevalence studies in the last decade in Northern Europe suggest an overall prevalence of 15–20% in children aged 7–18 years. 7 Standardised questionnaire data from 486,623 children aged 13–14 years in the International Study of Asthma and Allergies in Childhood (ISAAC) suggest that atopic eczema is not just a problem confined to Western Europe, with high prevalence found in many developing cities undergoing rapid demographic change. 8 There is reasonable evidence to suggest that the prevalence of atopic eczema has increased two- to three-fold over the past 30 years, the reasons of which are unclear. 9 No reliable incidence estimates are available for atopic eczema.
Age
Atopic eczema is commoner in childhood, particularly in the first 5 years of life. One study of 2365 patients who were examined by a dermatologist for atopic eczema in the town of Livingston, Scotland, suggested that atopic eczema is relatively rare over the age of 40, with a 1-year period prevalence of 0.2%. 10 Nevertheless, adults over 16 years made up 38% of all atopic eczema cases in that community. Adults also tend to represent a more persistent and severe subset of cases.
Severity distribution
Most cases of childhood eczema in any given community are mild. One recent study by Emerson and colleagues found that 84% of 1760 children aged 1–5 years from four urban and semi-urban general practices in Nottingham were mild, as defined globally by the examining physician, with 14% of cases in the moderate category and 2% in the severe category. 11 Disease severity was not the only determinant of referral for secondary care, however. This severity distribution was very similar to another recent population survey in Norway. 12
How does atopic eczema affect people?
Direct morbidity has been estimated in several studies using generic dermatology quality-of-life scales. It has been found that atopic eczema usually accounts for the highest scores when compared with other dermatological disease. Specific aspects of a child's life that are affected by atopic eczema are:
-
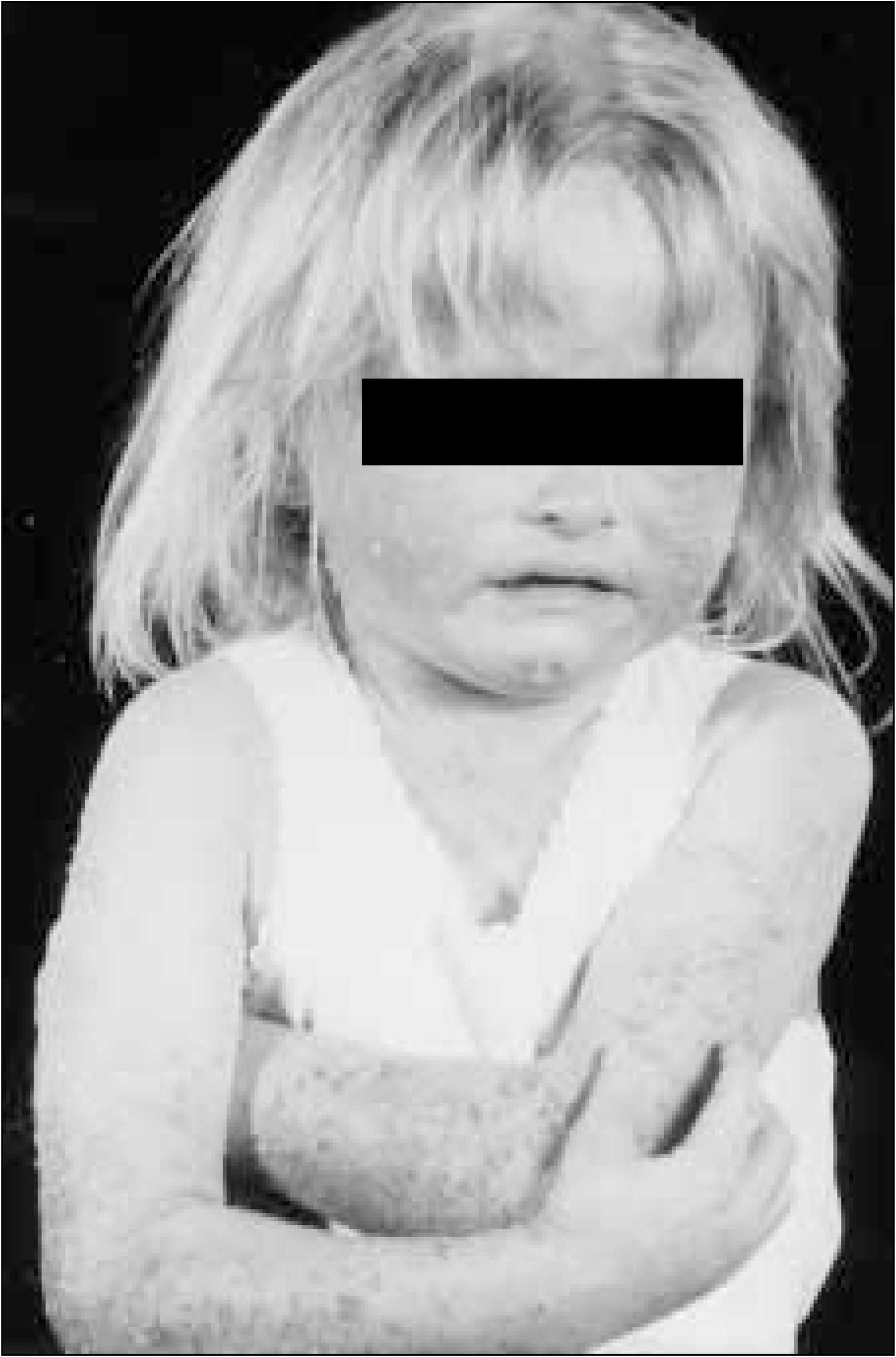
itch and associated sleep disturbance (Figure 1)
-
ostracism by other children and parents
-
the need for special clothing and bedding
-
avoidance of activities such as swimming, which other children can enjoy, and
-
the need for frequent applications of greasy ointments and visits to the doctor.
Family disturbance is also considerable with sleep loss and the need to take time off work for visits to healthcare professionals. 7
FIGURE 1.
Despite the public's tendency to trivialise skin disease, the suffering associated with the intractable itching of atopic eczema can be greater than other illnesses such as asthma and heart disease. 7
Economic costs
In financial terms, the cost of atopic eczema is potentially very large. One recent study of an entire community in Scotland estimated the mean personal cost to the patient at £25.90 over a 2-month period with the mean cost to the health service of £16.20. 13 If these results were extrapolated to the UK population, the annual personal costs to patients with atopic eczema based on lower prevalence estimates than recent studies suggest would be £297 million. The cost to the health service would be £125 million and the annual cost to society through lost working days would be £43 million making the total expenditure on atopic eczema £465 million per year (1995 prices). Another recent study from Australia found that the annual personal financial cost of managing mild, moderate and severe eczema was Aus$330,818 and $1255, respectively, which was greater than the costs associated with asthma in that study. 14
What causes atopic eczema?
Genetics
There is strong evidence to suggest that genetic factors are important in the predisposition to atopic eczema. In addition to family studies, twin studies have shown a much higher concordance for monozygotic (85%) when compared with dizygotic twins (21%). 15 Preliminary work has suggested that a marker for IgE hyper-responsiveness might be located on chromosome 11q, but this has not been consistent. It is possible that the tendency to atopic eczema might be inherited independently from atopy.
Environment
While genetic factors are probably a very important factor for disease predisposition, there are numerous general and specific clues that point strongly to the crucial role of the environment on disease expression. 16 It is difficult to explain the large increase in atopic eczema prevalence over the past 30 years, for instance, in genetic terms. 9 It has been shown that atopic eczema is commoner in wealthier families. 17 It is unclear whether this positive social class gradient is a reflection of indoor allergen exposures or whether it reflects a whole constellation of other factors associated with ‘development’. Other studies have shown an inverse association between eczema prevalence and family size. 18 This observation led to the ‘hygiene hypothesis’, that is that children in larger families were protected from expressing atopy because of frequent exposure to infections. 19 Some evidence for this protective effect of infections on atopic eczema has been shown in relation to measles infection in Guinea Bissau. 20
Migrant studies also point strongly to the role of environmental factors in atopic eczema. It has been shown that 14.9% of black Caribbean children living in London develop atopic eczema (according to the UK diagnostic criteria) compared with only 5.6% for similar children living in Kingston, Jamaica. 21 Other migrant studies reviewed elsewhere have consistently recorded large differences in ethnic groups migrating from warmer climates to more prosperous cooler countries.
Further work has suggested that the tendency to atopy may be programmed at birth and could be related to factors such as maternal age. 22 The observation that many cases of atopic eczema improve spontaneously around puberty is also difficult to explain in genetic terms alone. 2 Specific risk factors for eczema expression in the environment are still not fully elucidated. Allergic factors such as exposure to house dust mite may be important but non-allergic factors such as exposure to irritants, bacteria and hard water may also be important. 23
Pathophysiology
A number of mechanisms and cells are thought to be important in atopic eczema and these are reviewed in detail elsewhere. 1,24 Microscopically, the characteristic appearance of eczema is that of excess fluid between the cells in the epidermis (spongiosis). When severe, this fluid eventually disrupts the adjacent cells in the epidermis to form small collections of fluid, which are visible to the naked eye as vesicles. In the chronic phase, atopic eczema is characterised by gross thickening of the epidermis (acanthosis) and an infiltrate of lymphocytes in the dermis. The theory that unifies the various abnormalities of atopic eczema suggest that blood stem cells carrying abnormal genetic expression of atopy cause clinical disease as they infiltrate and remain in the mucosal surfaces and skin. There appears to be a failure to switch off the natural predominance of Th2 helper lymphocytes, which normally occurs in infancy, and this leads to an abnormal response of chemical messengers called cytokines to a variety of stimuli. The underlying mechanism of disease may be either abnormalities of cyclic nucleotide regulation of marrow-derived cells or allergenic over-stimulation that causes secondary abnormalities. Some studies have suggested a defect in lipid composition and barrier function of people with atopic eczema – a defect that is thought to underlie the dry skin tendency and possibly enhanced penetration of environmental allergens and irritants, leading to chronic inflammation.
Does atopic eczema clear with time?
Although the tendency towards a dry and irritable skin is probably lifelong, the majority of children with atopic eczema appear to ‘grow out’ of their disease, at least to the point where the condition no longer becomes a problem in need of medical care. A detailed review of studies that have determined the prognosis of atopic eczema has been reported elsewhere. 2 This review suggested that most large studies of well-defined and representative cases suggest that about 60% of childhood cases are clear or free of symptoms from disease in early adolescence. Many such apparently clear cases are likely to recur in adulthood, often as hand eczema. The strongest and most consistent factors that appear to predict more persistent atopic eczema are early onset, severe widespread disease in infancy, concomitant asthma or hay fever and a family history of atopic eczema.
How is atopic eczema treated?
The management of atopic eczema in the UK was summarised in a paper jointly produced by a British Association of Dermatology and Royal College of Physicians Working Party in 1995. 25 The article described the management of atopic eczema in three stages. The first line of treatment involved providing an adequate explanation of the nature of disease as well as advice on avoiding irritants. The role of emollients in adequate quantities was emphasised, as well as prompt treatment of secondary infections. Topical steroids were highlighted as the mainstay of treatment, though care regarding the duration of treatment, site and age of the person treated was emphasised. Antihistamines were only recommended for their sedative action. Cognitive behavioural techniques were also mentioned as being important to some families. Allergen avoidance, for example the reduction of house dust mite or dietary intervention, was described as a second-line treatment, as was treatment with ultraviolet light under specialist care. Third-line treatment (always under the care of a specialist) included such treatments as short bursts of systemic corticosteroids, cyclosporin A, evening primrose oil and Chinese herbal medicines.
These recommendations were made on the basis of consensus from a wide range of practitioners and patient advocates. Although some recommendations were based on RCTs, many were not. It is unclear, therefore, how many of these recommendations are truly beneficial to patients. New developments since the publication of these recommendations include increased use of a double layer of protective bandages (‘wet-wraps’) with or without topical steroids, ‘newer’ once-daily topical corticosteroids such as mometasone and fluticasone, and possibly some increased use of potent systemic agents such as cyclosporin A. Other new potent topical preparations such as tacrolimus and ascomycin derivatives are probably going to become available in the near future. 26
How is care organised in the UK?
Most children with atopic eczema in the UK are probably managed by the primary care team. This includes advice from pharmacists, health visitors, practice nurses and family practitioners. About 4% of children with atopic eczema are referred to a dermatologist for further advice. 11
The quality of service provided by secondary care for eczema sufferers has recently been audited by the British Association of Dermatologists. Although most departments provided a high-quality service, some aspects of care, such as the administration of simple standardised record forms could be improved. 27,28
Compliance (or more correctly, concordance) seems to be a major cause of apparent treatment failures and a recent study suggested that this was often due to a poor understanding of the chronic nature of the disease, a fear of topical corticosteroids and the belief that all atopic eczema is caused by a specific allergy. A survey in Nottingham has found that most mothers worry that topical steroids cause adverse effects, though many were not able to distinguish between weak and strong ones. 29
The National Eczema Society is the UK's self-help organisation for atopic eczema sufferers and people with other forms of eczema. It has a well-organised information service and national network of activities geared to help eczema sufferers and their families. Sources of alternative care for atopic eczema sufferers abound in the community ranging from the highly professional to elaborate expensive diagnostic and therapeutic measures of dubious value.
How are the effects of atopic eczema captured in clinical trials?
Outcome measures used in trials have recently been reviewed by Finlay. 30 Most outcome measures have incorporated some measure of itch as assessed by a doctor at periodic reviews or patient self-completed diaries. Other more sophisticated methods of objectively recording itch have been tried. Finlay drew attention to the profusion of composite scales used in evaluating atopic eczema outcomes. These usually incorporate measures of extent of atopic eczema and several physical signs such as redness, scratch marks, thickening of the skin, scaling and dryness. Such signs are typically mixed with symptoms of sleep loss and itching and variable weighting systems are used. It has been shown that measuring surface area involvement in atopic eczema is fraught with difficulties,31 which is not surprising considering that eczema is, by definition, ‘poorly-defined erythema’. Charman and colleagues recently performed a systematic review of named outcome measure scales for atopic eczema and found that of the 13 named scales in current use, only one (Severity Scoring of Atopic Dermatitis, SCORAD) had been fully tested for validity, repeatability and responsiveness. 32 Quality-of-life measures specific to dermatology include the Dermatology Quality of Life Index30 and SKINDEX. 33 The Children's Dermatology Life Quality index has been used in atopic eczema trials in children.
The authors are aware that most clinical trials of atopic eczema have been short term, that is about 6 weeks. This seems inappropriate in a chronic relapsing condition. Very few studies have considered measuring number and duration of disease-free relapse periods. It is impossible to say whether modern treatments have increased chronicity at the expense of short-term control in the absence of such long-term studies.
Why is a systematic review needed?
The authors suspect that little research has been done into primary and secondary prevention of atopic eczema. Research is also probably very limited in non-pharmacological areas of treatment such as psychological approaches to disease management. Even for traditional pharmaceutical preparations, the choice of treatments for atopic eczema by patients or their practitioners is complicated by a profusion of preparations whose comparative efficacy is unknown. 5 Thus, the current British National Formulary lists 19 classes of topical corticosteroids available for treating atopic eczema and a total of 63 preparations that combine corticosteroids with other agents such as antibiotics, antiseptics, antifungals and keratolytic agents. 34 How can a family practitioner make a rational choice between so many preparations?35
Systemic treatments for severe atopic eczema have only been partially evaluated. There are plenty of trials, for instance on expensive drugs such as cyclosporin A (which may have serious long-term adverse effects), yet to the best of our knowledge, there is not a single controlled trial on oral azathioprine – a much cheaper and possibly safer and more effective treatment that is currently widely used by British dermatologists. 36 In other areas, there is a profusion of small studies, which do not have the power to adequately answer the therapeutic questions posed.
The authors are also aware that many clinical trials have not asked patients enough of what they think about the various treatments under test. There is an opportunity in a systematic review, therefore, to redress the balance of outcome measures used in clinical trials towards the sort of measures that are clinically meaningful to patients and their carers.
Public concern over long-term adverse effects such as skin thinning and growth retardation from use of topical corticosteroid preparations has not been matched by long-term studies on atopic eczema sufferers. Individuals with atopic eczema often resort to self-prescribed diets, which can be nutritionally harmful, or they may turn to ‘alternative’ tests and treatments which may turn out to be beneficial or expensive and harmful.
Thus, there is considerable uncertainty about the effectiveness of the prevention and treatment of atopic eczema. This combination of high disease prevalence, chronic disability, high financial costs, public concerns regarding adverse effects, lack of evaluation of non-pharmacological treatments, concern regarding the clinical relevance of trial outcome measures and the profusion of treatments and care settings of unknown effectiveness is why a scoping systematic review of atopic eczema treatments is needed. It is hoped that the review will form the basis for identifying, prioritising and generating further primary, secondary and methodological research.
Summary of the problem of atopic eczema
-
The terms atopic eczema and atopic dermatitis are synonymous.
-
The definition of atopic eczema is a clinical one based on itching, redness and involvement of the skin creases.
-
About 20% of people with clinically typical atopic eczema are not ‘atopic’.
-
The word ‘atopic’ in atopic eczema serves to distinguish it from the ten or so other types of ‘eczema’.
-
Atopic eczema affects about 15–20 % of UK schoolchildren.
-
About 80% of cases in the community are mild.
-
Adults form about one-third of all cases in a given community.
-
Disease prevalence is increasing for unknown reasons.
-
The constant itch and resultant skin damage in atopic eczema can lead to a poor quality of life for sufferers and their families.
-
The economic costs of atopic eczema to both State and patient are high.
-
Genetic and environmental factors are both critical for disease expression.
-
Non-allergic factors may be just as important as allergic factors in determining disease expression and persistence.
-
Imbalances of T-lymphocytes and skin barrier abnormalities are both important in explaining the pathological processes of atopic eczema.
-
About 60% of children with atopic eczema are apparently clear or free of symptoms by adolescence.
-
Early onset, severe disease in childhood and associated asthma/hay fever are predictors of a worse prognosis.
-
Current first-line treatment in the UK includes emollients, topical corticosteroids, and sedative antihistamines.
-
Second-line treatments include allergen avoidance and ultraviolet light.
-
Third-line treatments include systemic immunomodulatory treatments such as cyclosporin A and azathioprine.
-
Most people with atopic eczema are managed by the primary care team.
-
Some people with atopic eczema seek alternative treatments.
-
A systematic review is needed to map out where high quality research has been conducted to date with the aim of resolving some areas of uncertainty and in order to identify knowledge gaps to be addressed by further primary research.
Research questions asked in this review
The remit of this project is to provide a summary of RCTs of atopic eczema with the main aim of informing the NHS R&D Office and other research commissioners of possible research gaps for further primary, secondary or methodological research. It is also hoped that the review will be of some use to healthcare providers, physicians involved in the care of people with atopic eczema and also to atopic eczema sufferers and their families by placing current treatments in context with their evidence base. The main research questions asked in this review are therefore:
-
What therapeutic interventions have the RCTs of atopic eczema covered so far? The main output of this coverage question is a summary of research gaps for further research, with research commissioners, charities and researchers as the main target audience.
-
What treatment recommendations can be made by summarising the available RCT evidence using qualitative and quantitative methods? The main output for this question are detailed summaries of available RCT evidence for different interventions for atopic eczema along with the authors' interpretation of the data based on the quality, magnitude of treatment effect, and clinical relevance of that evidence.
An impossible task?
It is unrealistic to attempt to summarise the entire ‘treatments of atopic eczema’ into a single Cochrane-style systematic review, as such a task would take years and cover several volumes. Atopic eczema is a complex disease with at least 40 different treatment approaches and specific questions that can be asked of each treatment group. What is more realistic is to produce a ‘sketch’ or ‘map’ of RCTs of atopic eczema, to quantitatively summarise a few areas of conflicting studies where possible, and to qualitatively review the others in a form that would be helpful to clinicians and patients. Such an approach could also act as ‘seed reviews’ for subsequent, more detailed Cochrane systematic reviews.
A question or data-driven review?
The very broad-ranging scoping nature of this review implies that it cannot be hypothesis-driven. Even in just one area of atopic eczema management such as dietary prevention, there are at least six separate systematic reviews that can be asked of the available data:
-
Does maternal avoidance of certain potentially allergenic foods prevent atopic eczema and if so, by how much in offspring at high risk (i.e. family history of atopy) versus those at normal risk?
-
Does dietary manipulation in pregnancy reduce the severity of atopic eczema in offspring?
-
Does exposing infants to allergens at an early stage of their immune development help by making them tolerant to substances that they will inevitably encounter in later life?
-
Does exclusive breastfeeding protect against atopic eczema?
-
Does prolonged breastfeeding with supplementation protect against atopic eczema?
-
Does the early introduction of solids bring on atopic eczema?
Trying to answer similar questions for each of the 40 or so interventions used for the treatment of atopic eczema would be impossible in one short report.
This review is therefore unashamedly a data-driven one. It is a review that aims to map out what has been done in terms of RCTs in atopic eczema to date and to reflect and comment on the coverage of already researched areas in relation to questions that are commonly asked by physicians and their patients.
The authors are aware that there is a danger that a data-driven review can serve to amplify and perpetuate current trends in evaluating minor differences between a profusion of similar pharmacological products. The authors have mitigated against this inevitable hazard by drawing attention to gaps that have not been addressed when summarising the reported studies, and also by including a comprehensive section on ‘unanswered questions’ in chapter 14 of this report, based on the views of contemporary researchers, physicians and patients.
Chapter 2 Methods
General methods structure
This review has been prepared along the guidelines developed by the University of York37 and those issued by the NHS Health Technology Assessment (HTA) programme38 and uses methods developed by the Cochrane Collaboration39 where possible.
Types of studies included in the review
Only RCTs of treatments for atopic eczema were included in the data summaries as other forms of evidence are associated with higher risks of bias. In order to be included as an RCT, a randomisation procedure was described, the study compared two or more treatments in human beings, and the study was prospective. In addition, the RCTs had to be concerned with therapeutic issues in relation to the prevention or treatment of atopic eczema. Thus, RCTs that involved evaluating cellular or biochemical responses of patients with atopic eczema after testing or injecting them with substances such as histamine were not included. Although they might inform future therapy, they were not therapeutic trials. Studies of possible increased incidence of drug adverse effects in atopic people compared with non-atopic people were also excluded. Studies also had to include at least one clinical outcome. Therefore, studies that only reported changes in blood tests or cellular mechanisms were excluded.
| Definite atopic eczema | Possible atopic eczema | Not atopic eczema |
|---|---|---|
| (include if study was an RCT) | (implies original paper must be obtained and read before a judgement is made to include or exclude by one of the authors based on additional features such as a good clinical description of atopic eczema with atopy) | (implies that the authors did not accept this term as representing atopic eczema) |
| Atopic eczema | Periorbital eczema | Seborrheic eczema |
| Atopic dermatitis | Childhood eczema | Contact eczema |
| Besnier's prurigo | Infantile eczema | Allergic contact eczema |
| Neurodermatitis atopica (German) | ‘Eczema’ unspecified | Irritant contact eczema |
| Flexural eczema/dermatitis | Constitutional eczema | Discoid/nummular eczema |
| Endogenous eczema | Asteatotic eczema | |
| Chronic eczema | Varicose/stasis eczema | |
| Neurodermatitis | Photo-/light-sensitive eczema | |
| Neurodermatis (German) | Chronic actinic dermatitis | |
| Dishydrotic eczema | ||
| Pompholyx eczema | ||
| Hand eczema | ||
| Frictional lichenoid dermatitis | ||
| Lichen simplex | ||
| Occupational dermatitis | ||
| Prurigo |
Study participants
Studies were included if participants were babies, children or adults who have atopic eczema (syn. atopic dermatitis) according to Hanifin and Rajka diagnostic criteria,4 or as diagnosed by a physician. Terms used to identify trial participants with definite, possible and definitely not atopic eczema are shown in Table 1. Those studies using terms in the ‘definitely not atopic eczema’ category such as allergic contact eczema were excluded. Those studies using terms in the ‘possible atopic eczema’ category, such as ‘childhood eczema’ were scrutinised by one of the authors and only included if the description of the participants clearly indicated atopic eczema (i.e. itching and flexural involvement).
Main outcome measures
Changes in patient-rated symptoms of atopic eczema such as itching (pruritus) or sleep loss were used where possible. Global severity as rated by patients or their physician were also sought. If these were not available, then global changes in composite rating scales using a published named scale, or where not possible, the author's modification of existing scales or new scales developed within the study were summarised. Adverse events were also included if reported. The selection of outcome measures was explored in more detail in a focus group of consumers held by one of the authors.
Secondary outcome measures
Secondary outcomes measures were changes in individual signs of atopic eczema as assessed by a physician, for example:
-
erythema (redness)
-
purulence (pus formation)
-
excoriation (scratch marks)
-
xerosis (skin dryness)
-
scaling
-
lichenification (thickening of the skin)
-
fissuring (cracks)
-
exudation (weeping serum from the skin surface)
-
pustules (pus spots)
-
papules (spots that protrude from the skin surface)
-
vesicles (clear fluid or ‘water blisters’ in the skin)
-
crusts (dried serum on skin surface)
-
infiltration/oedema (swelling of the skin), and
-
induration (a thickened feel to the skin).
Search strategy
Electronic searching
In order to retrieve all RCTs on atopic eczema treatments in accordance with inclusion criteria, a systematic and mainly electronic search strategy was carried out. The Cochrane Collaboration Handbook39 and the Centre for Reviews and Dissemination guidelines for systematic reviews37 were used as templates.
The following electronic databases have been searched:
-
MEDLINE40 (1966 to end of 1999)
-
EMBASE41 with its higher yield of non-English reports (1980 to end of 1999)
-
The Cochrane Controlled Trials Register (CCTR)42 and
-
The Cochrane Skin Group Specialised Trials Register. 43
Disease terms for atopic eczema (as a textword and MeSH term) are shown in appendix 1.
Possible trials were identified from each of the four databases by:
-
MEDLINE (Index Medicus online): the Cochrane Collaboration ‘highly sensitive electronic search string’ for RCTs was used (appendix 1). Years 1966–December 1999 were searched and yielded over 3000 references using the disease search terms in appendix 1. An iterative approach was used with retrieved papers. Once trials on specific drug types were obtained, an additional MEDLINE search was carried out employing these specific drug terms (e.g. tacrolimus) or their developmental names (e.g. FK506) combined with a general skin search (appendix 1). References were checked for possible additional RCTs of atopic eczema. Review articles were also retrieved in hard copy form and references were checked for further RCTs.
-
EMBASE (Excerpta Medica online): due to the different format of this database, an alternative search strategy was employed which was developed by the BMJ Publishing Group for its Clinical Evidence series (appendix 1). 44 Years 1980–December 1999, (the only years fully searchable on OVID), were searched. This yielded over 1000 references using the same eczema terms as for MEDLINE in Table 1. Trials that might have been on the EMBASE database from 1974 to 1979 would have been picked up by CCTR (see below), which has compiled its search of the entire EMBASE database since its inception in 1974.
-
CCTR: the Cochrane Library, Issue 4, 1999 was searched for controlled trials within the CCTR section by exploding the disease-specific search terms separated by the boolean ‘AND’ with the advanced search option. These include clinical controlled trials (quasi randomisation) and RCTs (randomisation).
-
Cochrane Skin Group Specialised Register: this was searched with the disease-specific terms and kind help of the Cochrane Skin Group Trials Search Coordinator.
Handsearching
As there are over 200 specialist dermatology journals and none specific to atopic eczema, separate handsearching was not done for this report. Some trials published in journals not listed in the main bibliographic databases or published within the body of a letter to the editor might therefore have been missed. However, results of handsearching of specialist dermatology journals by the Cochrane Skin Group are kept on the Cochrane Skin Group Specialised Register of trials, which was searched. This included results of handsearching the following dermatology journals as at July 2000:
-
Acta Dermato-Venereologica Supplementum 1970–91
-
Archives of Dermatology 1976–98
-
British Journal of Dermatology 1991–97
-
Clinical & Experimental Dermatology 1976–99
-
Cutis 1967–99
-
International Journal of Dermatology 1985–98
-
Journal of Investigative Dermatology 1991–97
-
Journal of the American Academy of Dermatology 1987–99.
In addition, conference proceedings of previous symposia such as the Atopic Dermatitis Symposium, held every 3 years (initially set up by Professor Georg Rajka), and all meeting abstracts for the annual meetings of the Society of Investigative Dermatology, European Academy of Dermatology and British Association of Dermatologists have been handsearched by one of the authors and the results made available to the Skin Group Specialised Register. Furthermore, one of the authors has been prospectively handsearching five dermatology journals (Clinical Experimental Dermatology, British Journal of Dermatology, Journal of the American Academy of Dermatology, Journal of Investigative Dermatology and Paediatric Dermatology) since January 1998, and any possible atopic eczema trials were accessed further by the team.
Other trial source
In addition to checking citations in retrieved RCTs and review articles, additional trials were sought by personal contact with atopic eczema researchers, and by writing to 37 pharmaceutical companies with a product or developing product in the area of atopic eczema.
Filtering
With the 3899 references yielded from the initial searches of MEDLINE, EMBASE and CCTR a filtering process began. This was carried out manually to assess whether the reference fitted the preliminary labels of ‘trial’ and ‘atopic eczema’.
Not all references had abstracts; therefore, ‘titles only’ had to be included as possible trials to avoid premature judgement. Where doubt existed from the abstract or title, the full paper was requested and scrutinised further by two of the authors. Papers labelled as ‘rejects’ were categorised with another label to specify why they were not suitable for inclusion. This was carried out by one reviewer and checked by a second reviewer in any cases of possible uncertainty.
All papers were catalogued on a specialised ProCite database. 45
Non-English studies
Studies published in non-English languages were screened by international colleagues (listed in the acknowledgement section) to see if they were possible RCTs with full data abstraction if this proved to be the case.
Data assessment
After assessment of retrieved papers for inclusion/exclusion criteria, the final list of included RCTs were subject to data abstraction with view to pooling or qualitative summary. Data abstraction forms were developed and used for those treatment groups where pooling appeared likely. Data for pooling were abstracted by two authors with discrepancies checked by a third if required. Data for qualitative summary were abstracted by one author and checked by a second.
Study quality
Methodological quality of each study was assessed using a previously described scheme where the three potential sources of bias were evaluated,46 namely:
-
the quality of the randomisation procedure
-
the extent to which the primary analysis included all participants initially randomised (i.e. an intention-to-treat analysis)
-
the extent to which those assessing the outcomes were aware of the treatments of those being assessed (blinding).
These three factors have been consistently shown to predict possible bias in effect estimates. 47 A descriptive component, rather than a score-based system, was used to quality rate the studies so that readers can see which aspects of the study reporting were deficient. Due to the sheer size of this scoping review, report authors were not blinded to the identity of the RCT authors when quality rating or data abstracting. Such blinding would have needed to be very thorough (to the point of having to conceal the interventions) as many of the RCTs are well known to one of the abstracting authors.
Quantitative data synthesis
Where pooling made sense clinically in terms of the interventions, study participants and common clinical outcomes, a meta-analysis was performed using both a fixed- and random-effects model depending on whether there was evidence of statistical heterogeneity. Odds ratios of improvement compared with the comparison intervention was used in the pooling exercises. The inverse of the variance of the outcome measures was used as weights for pooling the data from different trials. Sources of heterogeneity, such as differences in patients or formulation of interventions, were explored within the meta-analysis.
Methods of presenting qualitative results
Summarising the evidence of treatment and harm from 283 RCTs covering at least 47 different interventions in a way that would be helpful to healthcare commissioners, providers, physicians and users is challenging. There is always a conflict in such a situation of providing too much information resulting in loss of the general picture or of omitting important details in some specific areas. Readers are therefore encouraged to read the original studies for themselves where doubt occurs as to the reported data or author's conclusions in this paper.
For qualitative data summaries, the authors have adopted two systems.
-
Where six or more RCTs are identified, these are summarised in tabular form, noting the interventions and comparator plus any other treatments permitted concurrently during the study (co-treatments), study population and sample size, study design and duration, outcome measures used in the study, main reported results, quality of reporting and specific comments relating to the study. The table is introduced with a brief summary of the rationale for use of the drug and the way it is used, and appended by any additional general comments and a summary of key points.
-
Interventions with five or fewer RCTs have been summarised in text form in a way similar to that used in the BMJ Publishing Group's Clinical Evidence series. 44 After a brief introduction, the evidence of benefits from included RCTs is presented for each study, followed by a section on harms of treatment, followed by a section on author's interpretation of the data.
In many of the studies, over ten outcome measures have been reported, and it would have been impractical to document every one when presenting the reported results in the above two formats. In deciding which results to highlight in the ‘main reported results’ sections therefore, the authors have adopted a systematic approach of:
-
patient-rated global improvement or itch or sleep loss, then
-
global severity score based on several skin signs, or
-
individual skin sign scores,
in that order of preference. In many studies evaluating multiple clinical signs of atopic eczema, only those that were statistically significant (post-hoc) were highlighted in the paper's conclusions or abstract. The authors have mitigated against this post hoc bias by reporting results relating to excoriations, erythema, extent or lichenification (in order of preference) if global or other more clinically meaningful summary measures were not reported.
If pre-existing systematic reviews were identified for any of the interventions, these were highlighted at the beginning of the results sections and described in more detail. Help in deciding which outcomes were important to patients was obtained by running a focus group of four participants recruited through the Cochrane Skin Group Consumer Network.
Separating trial data from authors' opinions
Throughout the report, the authors of the current review have been careful to make a clear distinction between the facts abstracted from individual studies and the respective author's interpretation of what those results or lack of results mean. Thus, actual data on efficacy and possible harms have been clearly separated from the author's ‘comment’ section. In the comment sections of the tables, the authors have commented on issues such as clinical relevance, quality of reporting, possible sources of bias, generalisability of the study, clinical implications and research gaps.
Identifying treatments with no RCTs and future research priorities
Given the inescapable fact that a data-driven review can only identify treatments for which some evidence exists, the authors sought to list those other treatments that are currently used throughout the world in atopic eczema which are not necessarily supported by RCTs. This was done by mailing colleagues through professional networks, requesting them to add any interventions that were missing from a list of treatments supported by RCTs drawn up by the authors. Eighteen out of 23 physicians from six different countries responded to this request.
In order to help the authors identify future research priorities, another sample of colleagues was approached asking them to indicate the top five ‘unanswered’ questions in atopic eczema therapy today. Three purposive samples were sent this question on a personal letter: 12 colleagues internationally renowned for clinical atopic eczema research in the UK and abroad, four general practitioner colleagues with a known interest in skin disease, eight consultant dermatologists in England, Wales, Scotland and Ireland working in district general hospitals who did not have a declared special interest in atopic eczema, six consumer members of the Cochrane Skin Group and the seven Steering Group members of the European Dermato-Epidemiology Network (EDEN). Responses were collated by the authors and new themes were added as they became apparent.
Chapter 3 Results
Included studies
A total of 283 trials were finally included. In order to help summarise the interventions, groupings were constructed on the basis of: whether treatments dealt with prevention of new disease or treatment of established disease, and similar pharmacological drug type (e.g. topical steroids), similar intervention type (e.g. dietary measures) or convenience (e.g. non-pharmacological treatments) (Table 2).
| Intervention groupings | No. of studies | References |
|---|---|---|
| A. Prevention of atopic eczema | 20 | |
| Prevention by allergen avoidance during pregnancy | 8 | 48–55 |
| Prevention by allergen avoidance after birth | 12 | 56–67 |
| B. Established atopic eczema | 254 | |
| Topical corticosteroids | 83 * | 68–150 |
| Other topicals | 12 | |
| Coal tar | 1 | 151 |
| Emollients | 6 | 152–157 |
| Lithium succinate | 1 | 158 |
| Tacrolimus | 3 | 159–161 |
| Ascomycin | 1 | 162 |
| Antimicrobial/antiseptics | 10 | 163–172 |
| Antihistamines and mast cell stabilisers | 51 | |
| Antihistamines | 21 | 173–193 |
| Chromone compound/sodium cromoglycate | 20 | 84, 194–121 |
| Nedocromil sodium | 3 | 213–215 |
| Ketotifen | 2 | 216,217 |
| Doxepin | 4 | 218–221 |
| Tiacrilast | 1 | 222 |
| Dietary interventions | 37 | |
| Dietary restriction in established atopic eczema | 9 | 223–231 |
| Evening Primrose Oil | 14* | 232–245 |
| Borage oil | 5 | 246–250 |
| Fish oils | 4 | 251–254 |
| Pyridoxine | 1 | 255 |
| Vitamin E and multivitamins | 3 | 256–258 |
| Zinc supplementation | 1 | 259 |
| Non-pharmacological | 18 | |
| House dust mite reduction | 8* | 260–267 |
| Avoidance of enzyme-enriched detergents | 1 | 268 |
| Specialised clothing | 3 | 269–271 |
| Salt baths | 1 | 272 |
| Nurse education | 1 | 273 |
| Bioresonance | 1 | 274 |
| Psychological approaches | 3 | 106,275,276 |
| Ultraviolet light | 7 | 277–283 |
| Systemic immunomodulatory agents | 30 | |
| Allergen–antibody complexes | 2* | 284,285 |
| Cyclosporin A | 13* | 286–298 |
| Levamisole | 1 | 299 |
| Platelet activating factor antagonist | 1 | 300 |
| Interferon-gamma | 3 | 301–303 |
| Thymodulin | 2 | 304,305 |
| Thymostimulin | 2 | 306,307 |
| Thymopentin | 4 | 308–311 |
| Immunoglobulin | 1 | 312 |
| Transfer factor | 1 | 313 |
| Complementary therapies | 8 | |
| Chinese herbs | 4 | 314–317 |
| Homeopathy | 1 | 318 |
| Aromatherapy | 1 | 319 |
| Hypnotherapy/biofeedback | 1 | 320 |
| Massage therapy | 1 | 321 |
| Miscellaneous | 7 | |
| Nitrazepam | 1 | 322 |
| Ranitidine | 1 | 323 |
| Theophylline | 1 | 324 |
| Salbutamol | 1 | 325 |
| Papaverine | 2 | 326,327 |
| Suplatast tosilate | 1 | 328 |
| Total RCTs | 283 |
Excluded studies
Details of excluded studies are shown in Figure 2. Further details on the 146 RCTs that included atopic eczema participants and which were excluded in the final stages are shown in appendix 2. The commonest reasons for exclusion were ‘eczema’ unspecified and combining the results of atopic eczema patients with patients who had other dermatoses.
FIGURE 2.
Details of excluded studies and process involved
Prevention of atopic eczema
Given the high and rising prevalence of atopic eczema, prevention of atopic eczema has to be a desirable goal. It seems to be a far more logical one than treating sick individuals who present themselves after a long chain of pathological effects with potentially toxic and expensive medicines, which at best only ameliorate disease symptoms. Disease prevention can be considered at several levels: that of preventing allergen sensitisation at birth (which may or may not lead to atopic disease), prevention of manifest atopic eczema in childhood, the prevention of severe disease (without necessarily altering the total prevalence of disease) and the prevention of other atopic diseases such as asthma, which may follow atopic eczema. When considering disease prevention, it is important to be clear about whether a high-risk approach (i.e. intervening with parents who have atopic disease) or a low-risk population-based approach is being used in order to try and prevent atopic eczema developing in their offspring. Although it may sound an obvious strategy to simply target children known to be at high risk of developing atopic eczema, it has been previously suggested that a high-risk approach would prevent about 31% of children from developing atopic eczema compared with about 50% if the entire population was targeted. 5 It is also important that studies that purport to prevent atopic eczema follow-up children for a long time (i.e. 4 years or more), to ensure that the programme does not just simply delay the onset of disease to a time when it could have an even more damaging effect on the child's development.
Most prevention studies have focused on the role of allergen avoidance (mainly dietary) in early life, and these are summarised in the next sections. The authors were not able to find any RCT evidence of other forms of disease prevention such as avoidance of soaps, regular use of emollients or deliberate exposure to allergens at a critical time of thymic development during infancy to try to induce tolerance to allergens.
One small study62 on 40 pregnant Venezuelan women with a history of atopic disease, randomised 20 mothers to an educational and nutritional programme (which was not clearly defined) and 20 to no intervention in an open fashion, and followed the offspring to evaluate the efficacy of the programme in preventing atopic disease. No cases of atopic eczema (definition based on the Hanifin and Rajka guide) were noted in the 20 intervention children aged 4 years, whereas ten out of 20 children in the non-intervention group had developed atopic eczema by this time. Similar beneficial differences were noted for bronchial hyper-reactivity and rhinitis. Randomisation was not described, and the study was unblinded.
One ambitious RCT of a cohort of 817 infants aged 1–2 years with atopic eczema, the Early Treatment of the Atopic Child (ETAC) study,329 tested the hypothesis that long-term treatment with the non-sedating antihistamine cetirizine, at a dose of 0.25 mg/kg twice daily, could prevent the development of asthma. Although there was no overall difference in the incidence of asthma between the cetirizine and placebo intention-to-treat populations, there was a reduced risk for developing asthma in subgroups who were sensitised to grass pollen and house dust mite, who made up 20% of the study population. Data on the severity of atopic eczema in the two treatment groups have not been published to date.
Atopic eczema prevention by allergen avoidance during pregnancy
Dietary prevention
The idea of trying to prevent atopic eczema by avoiding potentially allergenic foods and other allergens such as house dust mite during pregnancy and early life is an attractive one. Parents often believe that foods are an important cause of atopic eczema and expectant mothers are often highly motivated to do what they can to prevent illness in their offspring, particularly if there is a strong family history of atopic disease.
Many questions can be asked in relation to such prevention, for example:
-
Does avoidance of certain potentially allergenic foods prevent atopic eczema and if so by how much in offspring at high risk (i.e. family history of atopy) versus those at normal risk?
-
Does exposing infants to allergens at an early stage of their immune system development help by making them tolerant to such substance, which they will inevitably encounter in later life?
-
Does such a programme simply delay the onset of disease and does it decrease disease severity?
-
Do the benefits to children outweigh the rigorous long-term measures needed to undertake such dietary exclusions?
-
Does exclusive breastfeeding protect against atopic eczema or does prolonged breastfeeding protect against atopic eczema?
-
Does the early introduction of solids bring on atopic eczema?
All of these questions require different studies. Most have been observational in nature. This is understandable for breastfeeding, as the decision to breastfeed is not something that can be easily subject to an RCT. The decision to breastfeed can also be inextricably linked to possible confounding factors such as social class and family history of atopy, rendering observational studies of such issues difficult to interpret. These have been reviewed by Kramer. 330
A Cochrane systematic review331 has evaluated three trials of maternal antigen avoidance during pregnancy for preventing atopic disease in general in infants of women at high risk of atopy. 48,52,53 Kramer's review331 of 504 women showed that the combined evidence did not suggest a strong protective effect or maternal antigen avoidance during pregnancy on the development of atopic eczema and other allergic diseases in the first year of life of their children and some evidence that such avoidance could lead to lower birth weight. The trials also suggested a non-significant increase in pre-term birth in the intervention groups. Cord blood IgE levels were similar in both groups.
Seven RCTs48–50,52–55 that have looked at dietary manipulation during and after pregnancy are summarised in Table 3. All included studies have involved children at high risk of developing atopic eczema because of atopic disease in close family members. Although some of the interventions are broadly similar, pooling is probably not justified in view of the differences in foods avoided, duration of avoidance after birth and whether the mother continued to avoid the foods during lactation. Some key points emerging from these five studies are as follows.
-
Lack of blinding seriously threatens the validity of the studies. Even if assessors were reported to be blind to the dietary allocation, it is possible that unblinded parents revealed their allocation to assessors. Independent assessment of disease status (e.g. by using coded photographic records) is one way of reducing such a possibility.
-
Disease definition is often quite vague or nonexistent in these studies. Disease definition is particularly important in the first year of life to separate atopic eczema from simple irritant eczema and seborrhoeic dermatitis of infancy.
-
Studies that have examined avoidance of potentially allergenic foods during pregnancy produce conflicting results with two suggesting benefit and four no benefit. The highest quality reported study54 found no benefit.
-
Methodological difficulties such as failure to comply with protocols, lack of blinding and complex interventions can probably be overcome by closer involvement with consumers and by use of more objective outcome measures.
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Chandra et al., 198653 | Dietary antigen avoidance of milk, dairy products, egg, fish, beef and peanuts throughout pregnancy and lactation vs no dietary restriction | 121 women with a previous history of a child with atopic eczema | Prospective RCT with infants followed-up until age 1 year | Proportion of infants who developed atopic eczema and severity/extent of atopic eczema | Of 109 mothers who completed study, 17 out of 55 (30.9%) children born to the dietary restriction group had eczema by 1 year compared with 24/54 (44.4%) children in the control group (p = 0.069) | Concealment of allocation unclear | Unclear if reported benefit was sustained after age 1 year |
| (Canada) | Severity scores lower in intervention group | Unblinded study | Lack of blinding serious threat to validity of study findings | ||||
| No ITT analysis (12/121 drop-outs some of whom withdrew for undisclosed reasons) | More mothers in the control group formula-fed, which could explain increased eczema | ||||||
| Miskelly et al., 198854 | Pregnant mothers restricting their daily milk intake to half a pint during pregnancy and during lactation and addition of soya-based milk if needed vs no such restrictions | 487 mothers with at least one family member suffering from atopic disease (238 intervention, 249 control) | Prospective RCT with infants followed-up until age 1 year | Atopic eczema, wheeze, nasal discharge and skin-prick tests | Eczema during first year in 41% of intervention group vs 34% in control group (p > 0.05) | Clear description of method of randomisation and concealment in sealed envelopes | High-quality study that tested hypothesis that cows' milk in early life increase risk of allergic disease and found no evidence to support this |
| Wales | Similar for wheeze and nasal discharge | Physician examining children reported to be unaware of allocation status | Definition of atopic eczema vague | ||||
| Clear description of flow of study participants | |||||||
| ITT analysis carried out | |||||||
| Lilja et al., 198948 | Maternal diet low in hens' egg and cows' milk (‘reduced diet’) vs diet with one hen's egg and 1 l of milk in last 3 months of pregnancy | 162 mothers with respiratory allergy to animal dander and/or pollens giving birth to 166 infants | Prospective randomised study with children followed-up to age 18 months of age | Proportion developing atopic diseases and positive skin-prick tests | Of 163 evaluable children, no difference in prevalence of asthma, rhinitis, urticaria or atopic eczema | Method and concealment of allocation unclear | Marked differences (64% vs 46%) in mothers with personal history of atopic eczema in the ‘reduced’ vs high dairy intake groups at baseline |
| Sweden | Proportion of obvious, probable and possible cases of atopic eczema in reduced group was 33% compared with 28% in other group | Study assessors reported to be unaware of diet allocation | No definition of atopic eczema given | ||||
| No ITT analysis | |||||||
| Zeiger & Heller, 1995,49 199351 | Mothers who avoided cows' milk, eggs and peanuts during last trimester of pregnancy and lactation and whose children avoided cows' milk until 1 year, egg until 2 years and fish until 3 years vs mothers and their children who followed standard feeding practices | 288 children born to parents at high risk of atopic disease | Prospective randomised study with children followed-up to age 7 years | Proportion developing atopic eczema and other atopic diseases, food allergy and skin-prick tests | Of 165 children evaluable at age 7 years, no differences in atopic eczema prevalence, allergic rhinitis, asthma, serum IgE noted | Concealment of allocation unclear | Very large drop-out from original randomisation |
| USA | Period of prevalence for atopic eczema in intervention group at age 7 years (estimated from graph) = 7%, identical to control group | Study patients were unblinded due to nature of intervention | Bias mis-classification of outcome cannot be ruled out due to unblinding | ||||
| Investigators recording atopic eczema also probably not blinded | Some changes between the 2 groups that were apparent at age 2 years (food allergy and milk sensitisation) were no longer present at age 7 years | ||||||
| No ITT analysis | Differences in eczema treatment not recorded | ||||||
| Falth-Magnusson & Kjellman,199252 | Total abstinence of cows' milk and eggs from week 28 of pregnancy until delivery vs normal food throughout pregnancy | 209 pregnant mothers from families with at least one allergic family member | Prospective randomised study with children followed-up to age 5 years | Prevalence of allergic disease including atopic eczema, skin-prick testing, and IgE | Of 209 evaluable children at age 5 years, 29% of children in the dietary group vs 24% in the control group had reported atopic eczema (p > 0.05) | Method and concealment of allocation unclear | Similar limitation of unblinding to other studies, though similar proportion with objective atopy argues against bias by observers |
| Sweden | Proportion with asthma, hay fever and objective atopy very similar in both groups | Study unblinded | Atopic eczema mainly based on history with some physical examination | ||||
| No ITT (hough very few drop-outs) | Remarkably high follow-up rate for such a long study (95%) | ||||||
| Hide et al., 1994,50 199655 | Mothers during last trimester of pregnancy and during lactation excluded dairy products, egg, fish and nuts or soy-based milk plus measures to reduce house dust mite at home vs mothers whose infants were fed conventionally with no control of house dust mite | 120 children identified before birth as being at high risk for atopy | Prospective randomised study with children followed-up to age 4 years | Atopic eczema (prevalence and severity), asthma, rhinitis and skin-prick tests | At age 2 years, 13.8% in the prophylactic and 24.2% in the control group had examined atopic eczema | Method and concealment of allocation unclear | Promising and persistent difference in atopic eczema prevalence in intervention group |
| England | At age 4 years, this difference persists (8% and 15% with atopic eczema, respectively) | Study participants unblinded but some attempt at blinding study assessor | Unclear if reported benefit was die to prenatal diet, feeding practices after birth or reduction in house dust mite | ||||
| No differences in eczema severity | No ITT analysis | ||||||
| Atopy also significantly less common in intervention group |
Prevention of atopic eczema through allergen avoidance and dietary manipulation after birth
One Cochrane systematic review331 of maternal antigen avoidance during lactation pooled three studies53,61,332 and found some benefit on the prevention of atopic disease in offspring from maternal avoidance of allergenic foods while breastfeeding. Methodological shortcomings in all three trials (mainly loss of blinding) argue for caution in interpreting the results. RCTs that have examined the usefulness of manipulating the diet of lactating mothers and their infants after birth with a view to preventing atopic eczema56–61,63–67 are summarised in Table 4. A further trial332 has been excluded because no separate data on atopic eczema have been given. These studies share similar methodological concerns regarding disease definition and unmasking of blinding.
Other summary points are as follows.
-
The Moore and colleagues63 study illustrates the difficulty of trying to randomise mothers to breastfeed.
-
There is no evidence to support the use of soya milk as opposed to cows' milk supplementation to children as a means of preventing or delaying onset of atopic eczema.
-
There is some evidence to support the use of extensively hydrolysed cows' milk formulae over regular cows' milk formulae in preventing atopic eczema in high-risk families, though the extent to which these unpalatable formulations can be taken up in practice is unclear.
-
There is some evidence that maternal avoidance of allergenic foods during lactation may reduce the incidence of subsequent atopic eczema and other allergic disease.
-
Some studies (e.g. Chandra et al. ,61 Marini et al. ,65 and Porch et al. ,57) mix up results of observational data with randomised participants in such a way as to render it difficult to make valid comparisons.
-
Most of the studies refer to children born to families with atopic disease.
House dust mite
No RCTs evaluating the sole use of anti-house dust mite measures to prevent atopic eczema were identified. The study by Hide and colleagues55 evaluated the combined effect of dietary allergen reduction and house dust mite reduction during pregnancy and after birth, but it is impossible to say from this study whether it was the diet, house dust mite reduction or both that was responsible for the observed benefit. Future studies evaluating a combination of interventions simultaneously should consider factorial designs in order to tease out which components of the intervention have been beneficial.
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Kjellman & Johansson 197956 | Supplementing breastfeeding with either soy or cows' milk from weaning to age 9 months | 48 children whose parents both had atopic disease | Parallel study with children followed-up for 4 years | Obvious and probable atopic disease (atopic dermatitis, asthma, gastrointestinal allergy, urticaria and rhinitis) | 13/25 infants in the soy group compared with 11/25 in the cows' milk group developed obvious or probable atopic dermatitis (NS) | Method of randomisation and concealment not specified | Small study, which did not provide any evidence to suggest that soy supplements had any benefit for a range of allergic diseases over cows' milk |
| Sweden | No significant differences in other allergic diseases between the two groups | Probably single-blind | |||||
| Two drop-outs with no ITT analysis | |||||||
| Moore et al., 198563 | Breastfeeding for at least 3 months and avoidance of solids for this time with soya-based supplementation if required vs standard advice plus cows' milk formula if needed | 525 mothers (250 in experimental and 275 in control group) | Prospective RCT with infants followed-up until aged 1 year | Prevalence of eczema at 3, 6 and 12 months | Because only 26% of mothers in experimental and control groups exclusively breast-fed their infants, results were not analysed as a randomised study but as an observational one | Method of randomisation and concealment of allocation unclear | An ambitious large study that illustrates the difficulty in randomising mothers to breastfeed |
| England | Person recording skin lesions reported to be unaware of feeding group | ||||||
| No ITT analysis | |||||||
| Chandra et al.,67 1989a | Four different infant feeding formulae: hydrolysate cows' milk formula vs soy-based formula vs conventional cows' milk vs exclusive breastfeeding for 4 months or more | 288 ‘high-risk’ infants with family history of atopic disease among first-degree relatives. 72 children in each of the four intervention groups | Prospective RCT with infants followed up for 6 months | Atopic eczema development, eczema severity, wheezing illness, nasal discharge | Of 263 evaluable children, 7.4% in the hydrolysate group, 29.9% in the conventional milk group, 27.9% in the soya group and 18.3% in the exclusive breast-fed group had atopic eczema (not tested for statistical significance in paper) | No details on randomisation procedure or concealment thereafter | Unclear if all four groups were randomised and at what point |
| Canada | Eczema severity score did not differ between all four groups | Two examining physicians reported to be blind to formula status of child | Description I methods suggest that the exclusive breast-fed group were not randomised at all | ||||
| Incidence of any disease of allergic atopic eczematiology was lowest in hydrolysate group (p < 0.005) | No ITT analysis | Atopic eczema notoriously difficult to separate from seborrhoeic eczema at up to age 6 months | |||||
| Chandra et al., 1989b61 | Two studies: | 97 mothers who chose to breastfeed and 124 who did not | Prospective RCT with infants followed-up for 18 months | Development of atopic eczema and eczema severity | Eczema less common and milder in severity in babies who were breastfed and whose mothers were on a restricted diet (11/49 (22%) vs 21/48 (48%)) | Method of concealment of allocation unclear | Unclear if blinding of assessors was successful |
| Canada | i) mothers who planned to breastfeed allocated to either diet restricted (milk, dairy products, eggs, fish, peanuts and soybeans) or normal diet while breastfeeding | In infants fed hydrolysate, soy or cows' milk, 9/43 (21%), 26/41 (63%) and 28/40 (70%), respectively developed eczema | No blinding of eczema in breast-fed groups | Vague definition of atopic eczema, which could easily be confused with seborrhoeic eczema of infancy | |||
| ii) mothers who did not plan to breastfeed allocated to one of three formula feeds (hydrolysate, soy milk or conventional cows' milk) | Blinding of observers reported for formula-fed babies | Several exclusion from each group after randomisation | |||||
| No ITT analysis | |||||||
| Lucas et al., 199060 | Two trials: | 777 preterm infants (birth weight < 1850 g) born to parents at no increased of atopic disease | Prospective RCT with infants followed-up for 18 months after term | Development of eczema, asthma or wheezing, and allergic food reactions | No difference in the incidence of allergic disease between dietary groups in either trial at 18 months after term | Randomisation and concealment described | Large study with high-quality reporting |
| England | i) evaluated donor milk vs preterm formula as sole diet or (separately randomised) as a supplement to mother's expressed milk | In subgroup with a family history of atopy, early exposure to cows' milk increased risk of developing eczema (odds ratio 3.6 in those with a family history vs 0.7 in those without; p < 0.05) | Observers reported to be blind to infants' initial diet | Description of atopic eczema based on ‘characteristic distribution’ unclear | |||
| ii) infants allocated term vs preterm formula | ITT analysis | Finding of increased risk in atopic families post hoc and needs testing in other studies | |||||
| Validity of results to non-preterm infants unclear | |||||||
| Mallett & Henocq, 199259 | Infants assigned to hydrolysate formula vs adapted cows' milk formula | 177 infants (92 in hydrolysate group and 85 in adapted formula group) selected from a birth cohort whose immediate family had a history of allergic disease confirmed by medical records | Prospective RCT with infants followed-up for until aged 4 years | Atopic eczema occurrence and severity, asthma, food intolerance and objective tests of atopy (total IgE) | Eczema significantly more common at 4 months, age 2 and age 4 years | No details on randomisation procedure or concealment thereafter | Unblinded study |
| France | Both interventions were either alone or with breastfeeding for 4 months | At age years, 7.1% (5/70) of children had eczema in the hydrolysate group compared with 25.9% in the cows' milk group (p < 0.001) | No mention of blinding in study | No definition of eczema given | |||
| No evidence of protective effect of hydrolysate for asthma | No ITT analysis with a 30% drop-out rate at age 4 years | Despite randomisation, 58% in hydrolysate group vs 33% in cows' milk group chose to breastfeed, which could confound results | |||||
| Vandenplas et al., 199566 | Assignment to partial whey-hydrolysate formula vs regular cows' milk during first 6 months of life | 75 infants with atopic disease in at least two first-degree relatives and whose mothers chose not to breastfeed | Prospective RCT with infants followed-up for until aged 5 years | Cows' milk protein sensitivity, atopic eczema prevalence and severity, allergic rhinitis and asthma | Of 58 evaluable children at age 5 years, 7/28 children on whey had developed eczema vs 8/30 on cows' milk (NS) | No details on randomisation procedure or concealment thereafter | No description of atopic eczema definition |
| Belgium | No difference in eczema severity | Some attempt at blinding of mothers and observers | Due to the obvious taste difference to hydrolysate, unblinding highly likely | ||||
| Cumulative atopic disease of any type was 29% vs 60% in the whey vs cows' milk group | No ITT analysis with a 23% drop-out rate at age 5 years | ||||||
| Marini et al., 199665 | Hydrolysed milk formula vs conventional cows' milk formula | Children born to mothers with high atopic risk participating in an allergen avoidance programme (n = 279) whose mothers had insufficient breast milk | Prospective RCT with infants followed-up for until aged 3 years | Atopic eczema, recurrent wheeze, rhinitis, urticaria and gut symptoms | Unclear which results refer to the randomised groups | Randomisation procedure or concealment thereafter unclear | Small underpowered randomised study occurring within a large case control study |
| Italy | These mothers randomised to breastfeeding plus hydrolysate (n = 32) and breast plus cows' milk (n = 28) | Of the 25 evaluable children on hydrolysate plus breast milk, three (12%) had eczema at 3 years vs 3/22 (13.6%) evaluable children in the breastfeeding plus cows' milk group (NS) | Mothers unblinded; physicians reported to be unaware of dietary allocation | Very difficult to relate results to original randomisation schedule as so many groups from the observational part of the study mixed together | |||
| No ITT analysis with a 22% drop-out rate at age 3 years | |||||||
| Odelram et al., 199664 | Hydrolysed cows' milk formula vs ordinary cows' milk formula | 82 infants with at least two atopic family members or one atopic parent whose mothers exclusively breast-fed for 9 months | Prospective RCT with infants followed-up for until aged 18 months | Atopic eczema occurrence, asthma, rhinitis and food allergy | Of the 82 randomised infants, 11 mothers chose to continue exclusive breastfeeding and these joined nine other children to form a nonrandomised comparison group of 20 children | Method of randomization described | Small study where there is loss of originally randomised groups due to mothers' preferences |
| Finland and Sweden | Lactating mothers and their infants also avoided milk egg and fish until 12 months | Also skin-prick tests and IgE analysis | Data for atopic eczema in the remaining 32 children randomised to hydrolysate and 39 to ordinary cows' formula, were not given, but reported as not statistically significant | Study not blinded | Authors point out the difficulties in randomising such a group and conclude that “we can spare high atopy-risk families this (hydrolysate) extra burden” | ||
| No ITT analysis | |||||||
| Oldaeus et al., 199758 | Extensively hydrolysed vs partially hydrolysed vs regular cows' milk formula in infants at weaning stage | 155 infants with a family history of allergy who avoided cows' milk for first 9 months and no eggs or fish until 12 months | Prospective RCT with infants followed-up for until aged 18 months | Cumulative prevalence of asthma, atopic eczema and rhinitis and IgE antibodies | In the 55 children randomised to extensively hydrolysed formula, 17% had developed eczema by 18 months vs 44% of the 51 children in the partially hydrolysed group and 41% of 49 children in the regular cows' milk group | Randomisation procedure or concealment thereafter unclear | Atopic eczema definition unclear |
| Sweden | Breastfeeding mothers avoided same foods | Mothers unblinded to cows' milk formula but some attempt at blinding between hydrolysate formulae | Degree of success of blinding unclear | ||||
| Physicians reported to be unaware of formula | |||||||
| No ITT analysis | |||||||
| Porch et al., 199857 | Three different infant feeding formulae: extensively hydrolysed casein formula, partially hydrolysed whey formula and soy formula | 181 infants recruited before birth if one or both parents had a history of allergy | Prospective, randomised, doubleblind parallel study of 12 months duration | Development of atopic dermatitis in infants based on examination by a paediatrician nurse and/or physician, chronic or recurrent vomiting and diarrhoea in first year of life persisting longer than 3 weeks or recurring over 2 months or more | Of the 130 infants who completed the 1-year study, two out of 40 (5%) on soy formula, five out of 42 (12%) on partially hydrolysed whey formula and three out of 31 (10%) on the extensively hydrolysed formula developed atopic dermatitis by 1 year | Clear description of method of randomisation and blinding: parents, hospital staff, physicians and investigators reported to be unaware of allocation status | Another study evaluating quite a select group of motivated parents with atopic disease who chose not to breastfeed their infants |
| USA | Only those infants whose mothers decided not to breastfeed were randomly assigned to one of three feeding groups | Observational group of infants who were breast-fed | These differences were not statistically different | No ITT analysis carried out | No statistical differences in atopic dermatitis were noted across the three randomised feeding groups, and the rate of atopic dermatitis (12%) was similar in the non-randomised breastfed group | ||
| Symptoms of food intolerance were similar in all groups |
Chapter 4 Topical corticosteroids
Topical corticosteroids have been one of the cornerstones of treatment of atopic eczema for almost 40 years. Hydrocortisone was first developed in 1952 and improved various eczematous dermatoses when applied topically. 333 Since then, another 30 or so compounds have been developed, each in different formulations such as creams, oily creams or ointments, and often in combination with other ingredients such as antibiotics. They vary in strength (as measured by ability to constrict blood vessels rather than clinical anti-inflammatory or skin thinning effect) from very mild (e.g. hydrocortisone), to very strong fluorinated products (e.g. clobetasone propionate). Systemic adverse effects are rare and include suppression of the pituitary–adrenal axis and Cushing's syndrome. Local adverse effects include spread of untreated fungal infection, irreversible striae (stretch marks) and prominent fine blood vessels, contact dermatitis, perioral dermatitis and worsening of acne and mild loss of pigmentation. The adverse effect that undoubtedly causes the most concern is that of skin thinning. 334
We located 83 RCTs on the use of topical steroids in atopic eczema. 68–150 Sixty-five other RCTs on use of topical steroids, summarised in appendix 2, had to be excluded as they did not give a sufficiently clear description of the patients, or the results of patients with atopic eczema and other inflammatory dermatoses were mixed up together. Due to the large number of studies, the 83 included RCTs are summarised in appendix 3 and separated into groups according to the sort of questions they address (though some RCTs straddle more than these categories). These groups will be commented on in turn. Some quantitative pooling of data was attempted for the question of once- versus twice-daily corticosteroid usage in view of the importance of this question to the NHS and patients.
Topical corticosteroids versus placebo
Quality of reporting of studies in the 1960s to 1980s was generally poor, and methodological details scant. Studies that only report patient preference data give us little idea of the magnitude of the benefit. Those studies that do report magnitude of benefit suggest a large treatment effect. We could not find one RCT comparing betamethasone 17-valerate and placebo, which is worrying as this is used as the ‘standard’ comparator for most new topical corticosteroids developed subsequently. Nearly all studies were less than 1 months' duration.
Topical corticosteroids versus other topical corticosteroids
This group of RCTs represents the largest in this section (n = 40). Most trials are again of poor quality, and have tended to mix atopic eczema patients with a whole range of other patients. In these studies, responses to the same topical corticosteroids for different conditions are in many cases quite different, though it is unclear how many of these observations are due to different sample sizes in the different groups. It is difficult to make any summary statement on this group of trials as there are no trials that compare all of the contenders for the most effective and safest topical corticosteroid together. Thus drug A has been compared with drug C, drug C against drug D, drug D against drug A, Drug B against drug C, but never all together. It is difficult therefore to make any ranking conclusions. Some batches of RCTs introducing a ‘new’ topical corticosteroid are oddly country-specific despite being marketed by international companies. Many of the RCTs introducing a ‘me too’ product claim equivalence against a standard preparation, erroneously making the assumption that no evidence of statistical difference is the same as evidence of equivalence.
Two newer topical corticosteroids (fluticasone propionate and mometasone furoate) have been introduced in the UK over the past 10 years and claim to have less systemic absorption and an efficacy profile that permits them to be used once as opposed to twice daily. 335 The RCTs that have compared these two substances have invariably compared the ‘new’ agents against twice-daily older agents and demonstrated reasonable equivalence. They have subsequently and rightly been marketed as ‘once-daily’ treatments for atopic eczema, possibly giving the impression that once-daily application of other topical corticosteroids is not as effective as twice daily. The RCTs evaluating these newer agents have been careful therefore to only include a once-daily comparison of their product against a twice-daily comparator. This has occasionally introduced problems with patient masking. The absence of a once-daily comparator (e.g. beta-methasone 17-valerate) is thus a pity in these RCTs as it is possible that most of the ‘older’ established topical steroids can also be used once daily. This point becomes important when one considers that the cost of 100 g of cream is £3.95, £14.05 and £13.90 for betamethasone, mometasone and fluticasone creams, respectively.
Topical corticosteroids versus other topical preparations
Only four RCTs are described in detail in this section, but probably many more described in other result sections could have been included as topical betamethasone and hydrocortisone are used as standard comparators. It is difficult to evaluate treatment efficacy in those studies without a placebo arm (e.g. the tar versus hydrocortisone study). There is one useful and well-described study, which does not provide any evidence of benefit of hamamelis above placebo, whereas some benefit for hydrocortisone was present. Blinding can be a problem for other topicals such as tar.
Topical corticosteroids plus additional active agents
Several RCTs have evaluated the possible benefit of adding in various antimicrobial/antiseptics to topical corticosteroids. Despite their widespread use, we located only one RCT comparing plain betamethasone 17-valerate against a combination of betamethasone 17-valerate plus fusidic acid,111 which provided no evidence of improved efficacy of the combination product in patients with infected atopic eczema. Similarly, we found only one RCT that compared 1% hydrocortisone with fusidic acid against 1% hydrocortisone alone. 128 That study did not find any evidence to support a benefit of the combination product above plain hydrocortisone in patients with moderately severe atopic eczema. Another study90 failed to demonstrate any additional benefit of adding in gentamicin to betamethasone.
Different formulations of the same topical corticosteroids
Two points are worthy of note in this section. The first is that there is some evidence that composition of vehicle can affect efficacy,130 though long-term studies are needed to see whether these benefits are at the expense of adverse effects. The second point is that cosmetic preference may be very important even when equivalence is suggested in a comparison of two different formulations of the same preparation,144 though this is not consistent between studies even in the same countries. 143
Once-daily versus more frequent use of the same topical corticosteroids
Several trials have investigated whether single daily topical application of corticosteroid is as effective as more frequent applications. The question being addressed is important from several perspectives:
-
the patient's perspective because this would make therapy much less burdensome and potentially safer because of the reduced risk of adverse effects of corticosteroid, and
-
the NHS perspective because of reduced cost of therapy.
Results
Unfortunately few trials met our inclusion criteria and of the three that did, the methods of assessment were disparate. The response rate, defined as the proportion of patients who obtained at least a good response with treatment, allowed us to assess comparative efficacy. However, because of the disparate study designs, the estimates from the individual trials were not pooled.
Table 5 is a contingency table of the data from the three eligible trials. The estimated differences in response rates with once-daily versus more frequent applications, are also shown in Table 5 and Figure 3. It is clear that in none of the studies were more frequent applications superior to once-daily application. While the point estimates suggest that a small difference in favour of more frequent applications cannot be excluded, it is doubtful whether this is practically meaningful. This was also the conclusion of a recent review that considered this question in relation to all inflammatory dermatoses. 336
| Study and analysis | Stratum | Contingency table | Risk difference | 95% CI (near exact) | |||
|---|---|---|---|---|---|---|---|
| a | b | c | d | ||||
| Bleehen et al., 199587 | 1 | 110 | 113 | 27 | 20 | −0.047 | −0.138 to 0.045 |
| ITT | |||||||
| Bleehen et al., 199587 | 2 | 108 | 110 | 29 | 27 | −0.015 | −0.111 to 0.082 |
| PP | |||||||
| Koopmans et al., 199569* | 3 | 71 | 74 | 4 | 1 | −0.040‡ | −0.118 to 0.025‡ |
| Koopmans et al., 199569† | 4 | 70 | 74 | 5 | 1 | −0.053§ | −0.136 to 0.013§ |
| Sudilovsky et al., 198195 o.d. vs t.d.s. | 5 | 99 | 100 | 17 | 16 | −0.009 | −0.101 to 0.084 |
FIGURE 3.
Estimated risk difference for topical steroids
Koopmans and colleagues69 concluded that the proportion of patients who were cleared of eczema in the twice-daily group was higher than that in the once-daily group. Re-analysis of their data shows that this is the case, using doctors' assessment of clearance (rate difference –0.21; 95% confidence interval (CI) –0.36 to –0.06) but not the patients' assessments (rate difference 0.13; 95% CI –0.28 to 0.02). While the results of their trial do not exclude the possibility that Koopman and colleagues' claim may be true, theirs was the only trial that reported results in sufficient detail to test their claim.
Prevention of relapse using topical corticosteroids
One RCT has broken new ground by trying to evaluate the usefulness of topical corticosteroids in preventing relapse rather than the usual trend of just trying to demonstrate short-term efficacy. The study's methodological deficiencies and missing data discussed elsewhere337 made it difficult to say whether prevention of relapse was due to application of steroids to previously healed sites or due to application to ‘new’ areas, in which case, the study has become a straightforward vehicle-controlled comparison. The study did however suggest a benefit of using intermittent topical corticosteroid to prevent relapse. Generalising from the 37.5% subsample of original study participants is also problematic. Nevertheless, this is one of the first studies to look at long-term outcomes of this chronic disease in a pragmatic way. The study also provided useful detailed data suggesting that topical corticosteroids do not produce any skin thinning when used in short bursts over a long period.
Trials that have specifically examined adverse effects of topical corticosteroids
RCTs are not the best studies to evaluate adverse effects. A more detailed search for other surveillance and case-control data is needed, which is beyond the scope of this review. Those RCTs that have specifically gathered data on skin thinning and suppression of the pituitary–adrenal axis have failed to find any evidence of harm, though these studies are very short term. The study on prevention of relapse104 also found no evidence of skin thinning after 4 months, intermittent use of a potent topical corticosteroid. Four other RCT studies of topical corticosteroid use in healthy volunteers reviewed elsewhere334 show skin thinning at 6 weeks, which reversed within 4 weeks of stopping. While there are undoubtedly occasional horror stories of individuals developing Cushing's syndrome, permanent skin thinning and striae after long-term use of potent topical corticosteroids in large areas, there is no evidence to suggest that they are a problem for typical clinical use characterised by bursts of 1–2 weeks' treatment followed by ‘holiday’ periods with emollients only. Nevertheless, steroid ‘phobia’ is now firmly established in the UK among both patients and doctors. 29
Trials that evaluated oral steroids
Large treatment effects are observed in studies that compare systemic steroids versus placebo in terms of short-term disease response. We could not identify any longer-term studies evaluating the usefulness of oral prednisolone (continuous or intermittent) against other systemic agents such as cyclosporin in the treatment of severe atopic eczema.
Additional unanswered questions
Dilution of topical corticosteroids
This is widely practised in the UK on the basis that dilution might reduce adverse effects but maintain the same degree of efficacy. 338 We could find no RCT evidence to demonstrate this in patients with atopic eczema. The largest manufacturer of topical corticosteroids does not recommend dilution as there is a wide range of preparations currently available covering a wide range of potencies (Glaxo Wellcome, personal written communication, May 1998). Nevertheless, such a practice occurs with sufficient frequency to stimulate companies such as Glaxo Wellcome to provide ready diluted preparations such as Betnovate RD™ 1:4 ointment of known stability, purity and compatibility. The possibility of compromising on stability, compatibility and microbiological purity following extemporaneous dilution of topical corticosteroids seems unnecessary with the current range of products of different strength. 339–341 It is recognised that widespread use of diluted topical corticosteroids reflects a perceived increased safety margin compared with undiluted products by prescribers and their patients, though such a belief may be a false reassurance unless there are clear data to say otherwise. Use of diluted products by prescribers may also indicate the need for a wider range of products towards the less potent end of the spectrum, particularly for the very young.
What is the most effective and safest way of using topical corticosteroids?
Atopic eczema is most commonly a condition that comes and goes, and constant use of topical corticosteroids is unnecessary and indeed undesirable in view of long-term local and systemic adverse effects. Thus, most practitioners probably use topical corticosteroids in short bursts followed by ‘holiday’ periods of emollients only. 342 Others may start with a potent preparation and then decrease to a lower potency preparation as the condition improves (the step-down approach). We could find no RCT evidence to throw light on these important issues. A pragmatic RCT set up to compare short bursts of a potent steroid versus longer-term use of a weak preparation is currently being conducted in Nottingham with support from Trent Regional R&D.
Summary of topical corticosteroids
-
RCTs of topical corticosteroids versus placebo are few, but suggest a large treatment effect.
-
It is difficult to make recommendations about the ‘best’ topical corticosteroid as most trials have only compared one against another one, but seldom against the same one and never all together.
-
The market is currently saturated with many different strengths and formulations of topical corticosteroids.
-
Many older studies confuse absence of statistical evidence of superiority of one compound over another with evidence supporting therapeutic equivalence.
-
Three RCTs have compared antibiotic/topical corticosteroid combinations versus corticosteroids alone in infected and non-infected atopic eczema.
-
None have demonstrated superior clinical efficacy of the combination antibiotic/corticosteroid above corticosteroid alone despite their frequent use in clinical practice.
-
There is some evidence to suggest that the type of vehicle used for a topical corticosteroid may enhance its efficacy.
-
Patient preference on the basis of cosmetic acceptability may be important for long-term use even when equivalent effects are demonstrated between two treatments.
-
There is no clear RCT evidence to support the use of twice-daily over once-daily topical corticosteroid administration.
-
Based on this evidence, it would be justifiable to use once-daily corticosteroids as a first step in all patients with atopic eczema.
-
Such a policy could halve the drug bill for topical corticosteroids overnight, and possibly increase compliance and reduce adverse effects, though changing well-engrained practice may be challenging.
-
There is need for a cost-effectiveness study comparing betamethasone 17-valerate once daily with once-daily mometasone furoate and fluticasone propionate ointments.
-
There is no RCT evidence to suggest that skin thinning is a problem for correct use of topical corticosteroids, though other non-RCT evidence should be considered before making firm conclusions.
-
No RCTs have compared oral steroids with other systemic agents in patients with severe atopic eczema.
-
There is no RCT evidence to support the notion that diluting topical corticosteroids reduces adverse effects while maintaining efficacy in people with atopic eczema.
-
There are some concerns that extemporaneous dilution of topical corticosteroids could affect stability, compatibility and microbiological purity.
-
Such a practice, although firmly established with some practitioners, seems unnecessary given the wide range of different preparations of different potencies currently available.
-
We could find no RCT evidence to support different approaches to using topical corticosteroids such as comparing shorter bursts of strong preparations versus longer-term treatment with weaker preparations.
-
A pragmatic RCT is currently underway by this research team which addresses this last question.
Chapter 5 Other topical agents
Topical coal tar
Although less commonly used today in Northern Europe, coal tar was used to treat chronic atopic eczema for many decades. We have located one RCT conducted by Niordson and Stahl,151 which compared one type of coal tar preparation (Clinitar™, CHS, UK) versus conventional 1% crude coal tar in the same cream, in 27 patients (mainly children) with atopic eczema in a right/left comparison study of 4 weeks' duration. As the preparations were designed to be cosmetically different, only investigator blinding was possible. The title of the RCT is ‘Treatment of Psoriasis…’, which is clearly a misprint as the entire article refers to atopic eczema.
Benefits
Of the 23 evaluable patients after 4 weeks, infiltration, redness, skin thickening, scratch marks and dryness reduced by about 50% in both treatment groups. None of the differences were statistically significant. No statistically significant differences in parents preference for treatment was found. There was a statistically significant (p < 0.001) preference by patients or their parents for Clinitar cream compared with the crude coal tar cream, however, at the end of the study.
Harms
Four patients complained of stinging and itching, one on the site treated with Clinitar cream, two with coal tar cream and one with both. All were shown with patch testing to be an allergic reaction.
Comment
Although coal tar has been used for many years in atopic eczema, it is a pity not to discover any RCTs demonstrating its efficacy compared with vehicle alone. It is difficult to say from this study comparing two different preparations of coal tar whether both were vehicle effects or whether two active treatments were being compared. Both treatment groups improved equally during the study. Although cosmetic acceptability was higher for the Clinitar group, this was an unblinded assessment in patients who might have been eager to please the investigators. Method of randomisation was not described and no intention-to-treat analysis was performed. Further RCTs should compare the most cosmetically acceptable form of coal tar treatment versus vehicle alone and versus other common topical treatments such as topical steroids. There is a theoretical risk that tar is a possible carcinogen based on observational studies of occupational groups working with tar components.
Emollients
Skin dryness is a very common feature of atopic eczema, so much so that it is a diagnostic criterion. 3 Skin dryness can lead to inflammation, and vice versa, and which event occurs first has been debated over the years. 343 There is considerable biochemical evidence to suggest that there are specific abnormalities in skin lipids (ceramides) of atopic eczema skin. A dry skin is less supple than normal skin, and this can lead to painful cracks, particularly overlying joints. Another consequence of the atopic dry skin is impaired barrier function, both in terms of keeping undesirable things out (such as bacteria) and retaining useful things, such as water. Staphylococcus aureus, a common secondary pathogen in atopic eczema, is also known to exhibit enhanced adherence to the dry surface skin cells in atopic eczema.
Few would dispute that a dry skin is an important feature of atopic eczema, but the rationale for reversing this dryness with moisturisers (emollients) is less clear. One rationale is simply to relieve the feeling and appearance of ‘dryness’, which many eczema sufferers choose to do. Another rationale is that emollients may have a soothing effect on itching and soreness. Emollients are often purported to have a topical steroid-sparing effect. Another question to pose is whether emollients have any low grade anti-inflammatory activity without the use of additional topical steroids. Some have even advocated a form of ‘total emollient therapy’ for children with atopic eczema. 344 It is possible that emollients might play a role in decreasing the incidence and severity of secondary infections. It is also possible that emollients may prevent allergen sensitisation if used from a very young age in high-risk infants, or the opposite is possible (i.e. enhanced allergen penetration because of alteration of the skin barrier fat solubility characteristics). It is also possible that regular emollient use may reduce relapses.
There are thus many questions that need to be answered by RCTs in terms of the use of emollients. Emollients are almost universally recommended as first-line therapy for atopic eczema treatment in the UK. 25 Currently there are at least 30 different types of emollients listed in the British National Formulary, and this excludes different formulations (e.g. lotion, cream or ointment). A further group of ten bath additives are also available. 34 Emollients mainly act by either occluding water loss from the outer layers of the skin (e.g. white soft paraffin), by improving water binding of the skin (e.g. urea) or by directly adding water to the dry outer layers of the skin (e.g. aqueous cream).
Some are used as bath additives, and most are applied directly to the skin. It is often recommended to apply emollients after a bath so that water is retained in the skin. Some have advocated using emollients under damp cotton bandages at night in the ‘wet-wrap’ technique.
Despite our rigorous searching efforts, only five published RCTs152,154–157 could be included in this section. Many other possible trials were excluded because they were not randomised (or randomisation was unclear), many included a range of skin conditions with no separate results for atopic eczema (e.g. Newbold345), and some (e.g. Pigatto et al. 153) did not present any actual clinical data but instead concentrated on a host of biometric measurements, the clinical significance of which was unclear. The use of emollients with added antiseptics is discussed elsewhere in chapter 9 (Non-pharmacological treatments), where it was concluded that the addition of antiseptics did not show any benefit.
Benefits
The Kantor and colleagues study154 compared the use of an oil-in-water emollient (Moisturel™, not available in the UK) versus a water-in-oil emollient (Eucerin™, Beiersdorf, UK) in 50 patients of all ages with symmetrical atopic eczema using a left/right comparison design for a period of 3 weeks. The study was split into two RCTs, which compared the two preparations in either a cream (Study 1) or lotion (Study 2). Test limbs affected by atopic eczema were treated once daily with the designated emollients and once daily with 2.5% hydrocortisone cream, and were assessed by an independent physician for redness, scaling/crusting, itching, burning/stinging and a global eczema severity on a scale of 0–3. Global severity reduced from 1.28 to 1.00 and 1.92 to 0.96 in the Eucerin and Moisturel cream groups. respectively (n = 25) and from 1.91 to 0.68 and 1.91 to 0.91 in the Eucerin and Moisturel lotion groups, respectively (n = 22). Although differences from baseline were statistically significant, there were no significant differences between the two emollients.
The Hanifin and colleagues study156 compared the effects of adding an emollient called Cetaphil™ (manufactured by the study sponsor) applied three times daily to twice-daily application of 0.05% desonide lotion (a topical steroid) versus twice-daily topical desonide alone in 80 patients with atopic eczema for a 3-week period. The study was investigator-blinded only. Efficacy variables included seven symptoms and signs measured on a scale of 0–9, with a maximum score of 63. Global assessment by investigators was also recorded. At the end of 3 weeks, there was a 70% and 80% relative reduction in total score from a baseline of 24.23 and 24.13 for the desonide alone compared with the desonide/moisturiser side, respectively (p < 0.01). The sides treated by desonide alone showed complete clearing according to physician global evaluation in about 10% of people compared with 11% in the side treated with combination (data estimated from graph).
The Wilhelm and colleagues157 and Andersson and colleagues155 studies both evaluated the benefit of emollients containing urea preparations – a substance intended to improve water-binding capacity of the outer layer of skin. In the Wilhelm study, 80 patients with subacute atopic eczema and associated dry skin were randomised to apply a topical formulation containing 10% urea (manufactured by the study sponsors) versus the vehicle base as ‘placebo’ in a right/left forearm comparison for 4 weeks. Skin redness was improved in 70% of patients at the site of the 10% urea preparation compared with 30% on the vehicle site. Similar differences were noted for induration, but not for summary score. Measurement of outer skin moisture using a capacitance meter showed statistically increased hydration in the 10% urea group when compared with vehicle alone.
The Andersson and colleagues study155 compared a ‘new’ cream containing 5% urea as the active substance with an established licensed cream containing 4% urea and 4% sodium chloride in a parallel double-blind study of 48 adults with atopic eczema in Sweden. Patients were asked to apply the creams at least once daily for 30 days to dry, eczematous areas. Clinical disease severity measured by a physician on scale with a maximum score of 1600, showed a significant benefit for both creams, but with no statistical differences between the preparations. Actual data were not given and data were difficult to read from the figure as most data points were scrunched up towards the lower range of the large scale. Patient evaluation on a visual analogue scale (maximum of 14 meaning ‘no dry skin’) changed from 7.5 at baseline to 10 at Day 31 for those on the ‘new’ cream compared with a change from 7 at baseline to 9 at the end of treat-ment for the established cream (estimated from graph). There was no statistical difference between the two groups in terms of biometric measure-ments of water content or water loss through the outer layer of the skin.
Ammonium lactate is another substance thought to improve the water-binding capacity of the outer layer of the skin. This was the subject of Larregue and colleagues' study,153 comparing 6% ammonium lactate with its cream base only, in 46 children aged 6 months to 12 years with atopic dermatitis. The study was a within-person comparison of two symmetrical sites on patients recruited from France and Italy. Outcome measure included pruritus, and clinical objective measures included redness, dryness (xerosis), desquamation, lichenification, hyperkeratosis, and presence of papules. These were graded on a scale of 0–3. Intensity items were only partially reported in the results section, and suggested a reduction in lichenification, hyperkeratosis and dryness in both groups but slightly more in the ammonium lactate group. This was reported to be statistically significant at Day 15 for lichenification and for erythema at Day 30 (the final evaluation point of the study). Tolerance, as evaluated by the patients, was very similar in both groups. Results for itching symptoms were not given.
Harms
No adverse effects were reported in the first two studies with the exception of one patient in the Kantor and colleagues study154 experiencing a burning sensation when the oil-in-water emollient was applied. In the Hanifin and colleagues study,156 14% of the patients reported stinging or burning on the side treated with desonide compared with 12% on the side treated with a combination at week 1. Most patients (96% versus 4%) preferred the combination treatment. Transient burning was noticed in four and five patients when treated with urea and vehicle creams, respectively in the Wilhelm and Scholermann study. 157 No adverse effects were described in the Andersson and colleagues study. 155 Other adverse effects of emollients include an occlusion folliculitis on hair-bearing skin and accidents from slipping while climbing into the bath due to the use of emollient bath additives.
Comment
The first two studies were of extremely short duration and quality of reporting was generally poor with little description of randomisation method, limited blinding and no intention-to-treat analysis. The Kantor study failed to show any benefit of one emollient preparation over another (in the presence of a moderate potency topical steroid), and the Hanifin study suggested that regular use of an emollient with a topical steroid may result in a small increase in treatment response compared with a topical steroid alone. Very few physicians prescribe a course of topical steroids without an emollient, but it was good to see some RCT evidence that such a policy is justified. Neither study was designed to show a steroid-sparing effect for emollients.
The two studies on urea preparations showed a possible benefit of a urea-containing preparation compared with vehicle, and the other (which compared two preparations both containing urea in different concentrations) failed to show any benefit of a new preparation containing the higher concentration of urea. Quality of reporting on randomisation, blinding and intention-to-treat analysis was poor in both studies. Similar findings were present in the Larregue lactate study.
It is extremely disappointing, particularly in relation to the questions posed at the beginning of this section, to see a virtual absence of clinically useful RCT data on the use of emollients in atopic eczema. Many studies have been performed with emollients, but these have concentrated on ‘objective’ measurements such as transepidermal water loss and surface profilimetry. Despite their scientific ring, such measurements are often highly variable in terms of measurement reliability and more importantly, their clinical significance to a child with chronic atopic eczema is often completely unclear. In addition to measuring efficacy of emollients in treating mild atopic eczema lesions or the dry skin associated with atopic eczema, it is essential that future RCTs of emollients measure long-term tolerability, patient preferences and cosmetic acceptability as these are probably key determinants for successful long-term use.
The history of emollient use in atopic eczema is a good example of the inverse research law in dermatology, whereby the quality and quantity of evidence is inversely proportional to their frequency of use. 346 Emollients have become consecrated through usage and are firmly implanted in the treatment regimens of most European healthcare practitioners. Although emollients many have many beneficial actions,347 there is an urgent need to answer several basic questions about their use, preferably through industry-independent RCTs. Although other clinical and laboratory studies may continue to shed some light on the use of emollients in atopic eczema, RCTs are needed to address the unanswered clinical questions of most relevance to eczema sufferers and their carers. The top ten questions that need answering through RCTs with regard to use of emollients are as follows.
-
Do some emollients have a useful therapeutic effect (with or without wet-wraps) for treating minor flares of atopic eczema when compared with other emollients or very mild topical steroids?
-
Are some emollients effective in reducing itch and soreness associated with dry skin in atopic eczema compared with no emollients or other emollients?
-
Do emollients have a topical steroid-sparing effect without loss of efficacy in the long-term management of atopic eczema?
-
Does the regular use of emollients in between eczema flares treated by topical steroids help to reduce relapse rates?
-
Does the use of emollients in children born to atopic parents reduce the incidence of allergen sensitisation and subsequent clinical disease?
-
Does the regular use of emollients prevent painful cracks (fissures) on the hands of atopic eczema sufferers?
-
Which emollients do children and adults prefer at different sites of their body?
-
Does the pouring of an expensive emollient into a bath provide any additional benefit to having an ordinary bath and then applying an emollient directly to the skin afterwards?
-
Does the use of regular emollients reduce the incidence and severity of secondary infection in atopic eczema?
-
How common is clinically relevant sensitisation to emollient constituents such as lanolin?
It might be argued that because clinical opinion on the use of emollients is so deeply engrained that the position of clinical equipoise348 for testing emollients passed some 20 years ago. On the other hand, patients and the NHS spend a vast amount on emollients and trials would be justified on the basis of potential economic savings alone. It is understandable that it is not in the interests of the pharmaceutical industry to invest much into RCTs of the comparative efficacy of established emollients.
Lithium succinate ointment
Based on observation for the possible benefit for lithium succinate ointment for the treatment of seborrhoeic dermatitis, Anstey and Wilkinson158 conducted a small RCT of 8% lithium succinate versus inactive placebo ointment in a right/left comparison of flexural arm eczema for 2 weeks.
Benefits
Fourteen patients (mean age 16 years) with mild-to-moderate atopic eczema and symmetrical lesions were enrolled and three dropped out. One developed redness on both arms and subsequent patch testing confirmed a contact allergy to wool alcohol present in the ointment. At the end of 2 weeks, there was slight improvement for overall impression and global score compared with baseline and changes were virtually identical in the active and placebo group. None of the changes were statistically significant between the two groups.
Harms
No adverse effects were reported within this study apart from the contact allergy to wool alcohol. Patients given topical lithium should be monitored for lithium toxicity if large quantities are applied.
Comment
Although this was a very small study published in correspondence form only, the detailed results section is particularly informative as CIs around the treatment differences are presented. Because of the small standard deviations within patient differences, the study excluded moderate and large treatment effects. Method of randomisation and concealment of allocation and blinding was unclear and no intention-to-treat analysis was performed.
Tacrolimus
Tacrolimus or FK506 is a macrolide lactone isolated from the bacterium Streptomyces tsukubaensis. It is an effective immunosuppressant drug used for the prevention of rejection after kidney and liver organ transplantation. Because of its lower molecular weight and higher potency compared with cyclosporin, it was proposed as an effective topical agent for inflammatory skin conditions such as atopic eczema. Its use in dermatology has been reviewed elsewhere. 349 Two RCTs have been published on the use of tacrolimus ointment in atopic eczema – one in adults161 and in children. 159 A further open study was published by Nakagawa and colleagues350 in 1994. We are aware of at least four other ongoing trials sponsored by the manufacturer, comparing tacrolimus with vehicle and hydrocortisone.
Benefits
In the adult study,161 215 patients with moderate-to-severe atopic eczema aged 13–60 years were randomised to a parallel group study of either 0.03%, 0.1% or 0.3% tacrolimus ointment or vehicle alone. No concurrent treatment except emollients were allowed and the study duration was 3 weeks. Compared with baseline, the percentage of patients with completely resolved or markedly improved skin lesions at the treatment site according to an overall assessment by a physician were 60%, 82%, 72% and 8% (data estimated from graphs) in the 0.03%, 0.1%, 0.3% tacrolimus ointment and vehicle groups, respectively. Results were similar for patients' overall assessments, though the data were not shown. Median percentage decrease in a summary score for redness, swelling and itch was markedly greater in the tacrolimus groups compared with vehicle (66%:83%) when compared with placebo (22.5%). All of the changes were statistically significant for comparisons of placebo versus the three tacrolimus concentrations, but there were no statistical differences between the three tacrolimus concentrations.
The tacrolimus ointment for children with atopic eczema study had the same four comparison groups as the adult study with 180 children of moderate-to-severe atopic eczema aged 7–16 years recruited from 18 study centres in North America. For the patients' (or parents') assessment of itching, the median percentage improvement from baseline to the end of the 3-week trial was 50.5% for vehicle, 88.7% for 0.03% tacrolimus, 73.6% for 0.1% tacrolimus, and 77.1% for 0.3% tacrolimus. Only the differences between vehicle and the different tacrolimus concentrations were statistically significant. Similar percentage improvements were seen for physician's global evaluation. The mean percentage improvement for a modified eczema area and severity index at the end of the treatment for each of the three tacrolimus groups (72% for the 0.03% preparation, 77% for the 0.1%, and 81% for the 0.3% preparation) was significantly better than the vehicle group (26%; p < 0.001).
Harms
There was a significantly higher sensation of burning at the site of application in the adult tacrolimus patients. Itching and redness were also reported in the adult study. Blood concentrations of tacrolimus remained very low throughout the adult study. No serious adverse effects were reported in the children's study, though again burning and increased itching at the site of application during the first 4 days of the study were reported in about a quarter of the patients. Only seven of the 254 blood samples evaluated contained more than 1 ng/ml of tacrolimus. To put this into context, increased toxicity in transplant patients has been associated with concentrations of greater than 20 ng/ml.
Another study of potential skin thinning of tacrolimus has been reported by Reitamo and colleagues,160 whereby the authors evaluated the potential of 0.1% tacrolimus ointment versus vehicle control versus betamethasone valerate (a potent topical corticosteroid) applied in a randomised order to apparently normal areas of skin on the abdomen under occlusion for 7 days. They found no evidence of skin thinning as measured by ultrasound in the tacrolimus group (compared with 8.8% median decrease in skin thickness relative to a vehicle control for betamethasone). They did not find any increase in chemical markers for collagen breakdown in the tacrolimus group whereas these were present in the steroid group.
Comment
Both of these RCTs were well reported with a good description of randomisation, blinding and both with intention-to-treat analyses. The flow diagram of the trial profile in the Boguniewicz and colleagues159 trial was particularly refreshing. Magnitude of the clinical benefit measured in a number of ways was moderately large in tacrolimus in both adults and children when compared with placebo, and little difference was observed between the different concentrations of tacrolimus. Although no more serious adverse effects have been reported to date, much larger numbers and experience with the drug is needed before topical tacrolimus can be declared safe for the widespread use of atopic eczema in the community. Both of the trials were of extremely short duration and longer-term trials are needed to evaluate the benefit of tacrolimus in managing the chronicity of atopic eczema. Both trials are placebo-controlled and therefore lack the crucial comparisons against short bursts of moderate-to-potent topical corticosteroids and also against the newly developed and very similar topical ascomycin derivatives. Such comparative trials should include cost-effectiveness, and they should also be pragmatic in order to capture the tolerability of the topical agents in the community, where transient burning may result in lower compliance than that in organised clinical trials. The lack of skin thinning is a potential advantage of tacrolimus, along with ascomycin, though the evidence that serious skin thinning occurs with correct use of topical corticosteroids in the community is lacking. Both of these RCTs were sponsored by the manufacturer, Fugisawa USA Inc.
Ascomycin derivatives
Topical ascomycin (SDZ ASM 981, Novartis Pharma AG) is a cytokine inhibitor. It inhibits activation of T-lymphocyte cells by inhibiting T-cell proliferation antigen-specific activation. Based on some success in psoriasis, it has been tried on atopic dermatitis. Two RCTs on ascomycin have been found, one of which was published in full at the time of writing this report. 162
Benefits
The van Leent and colleagues study of 1998162 randomised 34 patients with moderate atopic eczema to a double-blind placebo-controlled right/left comparison study. Topical 1% ascomycin cream was applied twice daily (n = 16) or once daily (n = 18) and compared with a corresponding placebo cream base for symmetrical lesions on the arm on one or other side. The trial duration was 21 days, and all participants were adults. At the end of 21 days, those in the twice-daily group showed a 71.9% decrease in baseline severity score compared with 10.3% in the placebo group (p < 0.001). Changes were also beneficial in the once-daily ascomycin group, but the magnitude was not so impressive with a 37.7% reduction in atopic dermatitis severity index in the active versus a 6.2% reduction of score in the placebo group (p = 0.002). Three out of 15 patients showed total clearance of their lesion in the twice-daily ascomycin group compared with none in the placebo group. None of the patients in the once-daily group (neither active nor placebo) showed any clearance of their lesions.
The other study has been published in abstract form only351 and preliminary results were shown at a recent satellite symposium sponsored by the manufacturers. The study randomised patients to one of four different concentrations of ascomycin, vehicle, or betamethasone 17-valerate cream. Results presented at the meeting suggested that the ascomycin cream was significantly more effective than the vehicle alone in a dose-dependent manner, but not as effective as the topical corticosteroid. Full publication of the study is awaited before further comments can be made.
Harms
None of the patients in the van Leent study were shown to have any skin irritation or other local adverse effects. Blood values of ascomycin remained low throughout the study. In another study published in abstract form only,352 ascomycin 1% cream did not cause any thinning of the skin in 16 healthy volunteers who applied the cream to their forearm for 4 weeks.
Comment
This was a well-reported study, though the method of randomisation and concealment of allocation in randomisation was not described. Blinding was well described and intention-to-treat analysis was performed. The study showed large treatment effects when presented in terms of relative treatment difference of a composite severity system when compared with placebo. The magnitude of the benefit was almost twice as large in the twice-daily group compared with the once-daily group, though the percentages are not directly comparable as they were not derived from the same randomised population. Although the atopic dermatitis severity index scores were unequivocally beneficial in the active group, it was disappointing not to see any patients' views on efficacy for pruritus incorporated in the main outcome measures. The small placebo effect of the cream is also unusual for atopic eczema trials, and possibly points to unblinding of the interventions. The fact that only three out of 16 patients showed total clearing of their eczema lesion on the arm after twice-daily continual application for 3 weeks is also slightly disappointing, bearing in mind the clearance potential of moderately potent topical steroids. Nevertheless, this study suggests that topical ascomycin derivatives are effective in moderately severe atopic eczema in adults, and is a welcome addition to the treatment modalities available. More comparative trials are needed, particularly against intermittent use of potent topical steroids and tacrolimus ointment in order to evaluate the place of ascomycin therapy in atopic eczema. Longer-term safety studies are also needed to ascertain rare but serious adverse effects from systemic absorption.
Summary of other topical agents
Coal tar
-
One small RCT has suggested no difference in efficacy between Clinitar cream and 1% crude coal tar, though parents preferred the Clinitar preparation. It is difficult to say whether the efficacy changes reported from baseline were due to the vehicle or active drug. Further RCTs comparing the most cosmetically acceptable coal tar preparation versus other topical treatments and vehicle alone are needed.
Emollients
-
Although the use of emollients has become established as one of the firmest rituals of atopic eczema treatment, the RCT evidence for its efficacy is very sparse.
-
There is a reasonable rationale for using emollients in atopic eczema in terms of their steroid-sparing effect providing this is not accompanied by loss of efficacy.
-
There is little RCT evidence to form a rationale for choosing between different emollients.
-
As emollients vary tremendously between themselves in terms of greasiness and water content, and because they need to be used long term, patient preference is a crucial factor.
-
The correct emollient is therefore the one that the patient will use regularly.
-
There is little RCT evidence to support the use of emollients in atopic eczema.
Lithium succinate ointment
-
One small RCT failed to show any benefit of lithium succinate ointment when compared with placebo for the treatment of flexural eczema on the upper arms.
Tacrolimus
-
Topical tacrolimus has been shown to be an effective short-term treatment for atopic eczema in children and adults.
-
Transient burning and redness at the site of application is a common adverse effect.
-
Future trials of tacrolimus should compare it against short bursts of topical corticosteroids and against ascomycin derivatives in a cost-effectiveness analysis. Such studies should be of long duration (i.e. 4 months or more), in order to capture the effect on chronicity of disease.
Ascomycins
-
Topical ascomycin derivatives have shown to be markedly effective in moderate atopic eczema when compared with placebo in the trial of 3 weeks of twice-daily applications.
Chapter 6 Antimicrobial and antiseptic agents
The relationship between secondary infection or skin colonisation with the bacterium S. aureus and atopic eczema disease activity has been debated for many years, and is still far from clear. People with atopic eczema carry S. aureus in about 90% of clinically involved areas and about 75% of clinically uninvolved areas. 353S. aureus represents about 90% of the total aerobic bacterial flora of such individuals compared with 30% in normal skin. 354 The density of S. aureus tends to increase with the clinical severity of the atopic eczema lesions. It has been suggested that the dry skin of atopic eczema is deficient in certain inhibitory fatty acids, which may encourage growth of the organism, and the organism may also show enhanced adherence properties to skin cells obtained from atopic eczema sufferers compared with normal controls. 355
Few clinicians would dispute that grossly infected atopic eczema with oozing and sore pus spots requires treatment with some form of antibiotic or antiseptic, and that the bacteria are contributing at least in part to that particular flare-up. The role of S. aureus in non-clinically infected atopic eczema skin or for borderline infection (e.g. with just redness and oozing) is far from clear however,356 and the reliability of physicians to diagnose ‘clinically infected atopic eczema’ is probably poor. Skin swabs taken for bacteriological culture are of little use due to the almost universal colonisation of atopic eczema skin with S. aureus, though such swabs may reveal additional bacteria such as Streptococci spp.
If S. aureus does play a pathogenic role in atopic eczema, then this could be due to a direct chemical irritation, a non-specific reaction of the protein A component of the bacterium with immune cells, and by the production of specific exotoxins called superantigens, which are capable of large populations of T-lymphocytes distant from the site of colonisation, giving rise to widespread activation of eczematous lesions. 357
Although in many cases of non-clinically infected atopic eczema, the presence of S. aureus could be considered as an ‘innocent bystander’, which has simply colonised a dry and broken skin surface, there is at least thus some rationale for considering a role of S. aureus in more acute forms of atopic eczema. This has led to the use of many antimicrobial compounds such as oral antibiotics active against S. aureus given in short or prolonged courses, topically applied antibiotics, and antiseptic agents applied directly or by mixing with emollients applied directly to the skin or within bath additives. Topical corticosteroid/antibiotic contributions have already been discussed in chapter 5 (Other topical agents).
A total of ten RCTs163–172 evaluating the possible benefit of antmicrobial or antiseptic agents in atopic eczema were located and these are presented in Table 6. Three of the studies evaluated the use of oral antibiotics, one evaluated topical antibiotics, and five evaluated antiseptic agents. Another study evaluated the use of an anti-yeast preparation and this has also been included in this section for completeness. In view of the differences in the types of interventions (e.g. topical or oral) and different patient populations (e.g. those with clinically infected as opposed to uninfected eczema), it did not make sense to pool the studies quantitatively.
Comment
Table 6 shows quality of reporting was generally disappointing in the studies. Missing data and small sample sizes rendered interpretation difficult. The RCT results suggest that the potential role of S. aureus in atopic eczema may have been overstated in the past, and this combined with concerns of selecting resistant strains argue against the general use of antiseptics and antimicrobials in clinically uninfected atopic eczema. Normalisation of the affected eczematous skin by treatment with a topical steroid alone decreases S. aureus colonisation dramatically,164 suggesting that topical steroids alone are an effective means of decreasing bacterial skin colonisation in atopic eczema.
| Study | Interventions (co-treatments) | Study population and sample size follow-up | Trial design, description and | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Salo et al., 1988169 | Oral erythromycin acistrate (EA) 400 mg three times daily vs oral erythromycin stearate (ES) 500 mg three times daily for 5 to 12 days (topical steroids and emollients) | 42 patients (aged 15 to 66 years) admitted with clinically infected atopic eczema | Parallel group randomised doubleblind study for 5 to 12 days | Investigator and patient assessment of treatment efficacy on a five-point Likert scale and adverse effects | Mean duration of treatment was 7.7 days and 7.5 days in the EA and ES groups, respectively | Method of randomization and concealment unclear | Difficult to evaluate as two ‘actives’ were being compared in the presence of inpatient care and potent co-treatment |
| Finland | Most had bacteriological isolates of S. aureus and four had combined Staph/Strep infection | At the end of treatment, 75% and 83% of those in the EA and ES groups, respectively, were noted to show ‘good’ or ‘very effective’ improvement | No ITT analysis | The fact that seven patients with clinically infected eczema had no bacteriological evidence of infection confirms the difficulty understanding the link between disease and bacteria | |||
| Similar results according to patients | |||||||
| Gastrointestinal adverse effects similar in both groups | |||||||
| Weinberg et al., 1992170 | Oral cefadroxil 50 mg/kg/day in two equal doses vs placebo for 2 weeks (no mention of co-treatments) | 33 patients aged 6 months to 12 years with bacteriologically confirmed superinfected atopic eczema caused by either S. aureus or mixed Staph/Strep infection | Parallel group randomised doubleblind study for 2 weeks | Clearance of superinfection (assessed clinically), eczema severity, number of patients with positive cultures and global improvement | Of 30 evaluable patients, all 13 in the cefadroxil group no longer had clinical evidence of superinfection at the end of the study compared with six out of 15 in the placebo group | Method of randomization and concealment unclear | Results clearly in favour of cefadroxil for these children with infected atopic eczema |
| South Africa | Number of patients with positive isolates fell from 13 to four and from 17 to nine in the cefadroxil and placebo groups, respectively | No description of blinding | Poor quality of reporting | ||||
| Physician-rated global improvement recorded marked or moderate improvement in 84 of cefadroxil compared with 53% of placebo-treated patients | No ITT analysis | ||||||
| Ewing et al., 1998163 | Oral flucloxacillin 250 mg daily or matched placebo four times daily for 4 weeks (topical steroids, emollients and antihistamines) | 50 children aged 1–16 years who did not have any signs suggestive of bacterial infection | Parallel group randomised doubleblind study for 1 month with an 8-week follow-up period | Change in baceteriological count of S. aureus, patient compliance, and composite eczema severity scores | Although mean S. aureus counts decreased significantly in those treated with flucloxacillin, clinical efficacy scores did not change between the two groups in any systematic way | Good description of randomisation, blinding, but no ITT analysis (five drop-outs by week 4) | An important study that did not find any evidence to support prolonged use of antistaphylococcal antibiotics in those with clinically uninfected atopic eczema |
| England | The difference in bacteriological counts at 14 days after stopping treatment were no longer significant (p = 0.32) | Flucloxacillin only temporarily changed skin colonisation by S. aureus | |||||
| Methicillin-resistant strains were commoner in those on flucloxacillin | |||||||
| Lever et al., 1988167 | Topical mupirocin ointment vs placebo for 2 weeks (topical steroids and emollients) | 49 patients aged 2–56 years with relapsing atopic eczema without overt secondary skin infection | Double-blind randomised crossover period with a 2-week run-in, two 2-week crossover periods and a further 4-week follow-up | Type and counts of bacterial isolates, composite clinical severity score and extent involved by disease | Bacterial count for 45 evaluable patients was significantly reduced in those receiving topical mupirocin but not in the placebo group, although recolonisation occurred in the 4-week follow-up period (17% of whom had developed a ‘new’ strain that had not been previously isolated) | Method of randomization and concealment unclear | Crossover design not ideally suited to a study of antibiotics with delayed actions on the skin |
| Scotland | Patients' assessment of appearance, itch and sleep | For the first treatment period, total skin severity score fell from a mean of 69.9 to 68 in the placebo group compared with a fall from 59.5 to 37.6 in the mupirocin group (p < 0.002); changes for surface area were not so marked | No ITT analysis; no analysis of period or carry over effect | Some evidence of atopic eczema improvement in the first study period in favour of mupirocin | |||
| Patient assessments were statistically in favour of the mupirocin for the first treatment period | Results suggest a significant carry-over effect between 1st and 2nd periods | Concern for selection of resistant strains | |||||
| Stalder et al., 1992164 | Proprietary brand of chlorhexidine solution compared against 1:20,000 dilution of potassium permanganate solution for 7 days in addition to topical desonide (a topical steroid) | 20 children aged 5 months to 9 years | Parallel doubleblind randomised study of 1 week duration | Bacterial counts, composite clinical severity score and patient reported tolerance | Total severity score fell from 8.8 at day 0 to 5.7 at the end of the 7 days for chlorhexidine and from 11.1 to 8.8 for the permanganate group (p = 0.63) | Poor quality of reporting with very few methodological details | Difficult to interpret with such a tiny study and the comparison of two active treatments |
| France | No details given if they were clinically infected | Intensity and number of affected sites also showed very little difference between the two groups | Scanty methodological detail | ||||
| Bacterial counts feel substantially in both groups but they were not statistically significant (p = 0.37) and baseline scores in the two groups were quite different | The clinical tolerance data were the most useful | ||||||
| Clinical tolerance was ‘good’ in both groups | |||||||
| Sasai-Takedatsu et al., 1997171 | Comparison of spraying infants with water twice a day for 1 week or an acid electrolytic water (pH < 2.7) using a spray gun (no co-treatment allowed in this period) | 22 children aged 2 to 56 months with mild-to-moderate atopic eczema | Parallel randomised double blind study of 1 week duration | Colony counts of S. aureus, composite grading score, and scores for itching and sleep disturbance | Colony counts decreased by around 50% in the active but not in the water group (though baseline scores were quite different) | Although the study was described as randomised, there is serious cause to challenge this in the methods section whereby the authors state that the 22 patients were ‘arbitrarily divided by a referee physician into two groups of 11’; blinding also seems unlikely due to the acidic taste and sensation of the acid | Difficult to interpret the clinical data as the correct statistical comparison has not been done and because of the short duration of the study |
| Japan | Global severity scores fell from 9 to 5 in the active group compared with a rise from 7 to 8 in the water group; scores for itching and sleep also decreased in the active group but not in the water group | Some serious concerns about the study quality | |||||
| Although the authors found a statistically significant change in all of these measures for the active group compared with baseline, they did not do the appropriate test of difference between the two groups | The ethics of spraying an acid onto young infants is also a cause for concern | ||||||
| Harper, 1995166 | Comparison of a standard proprietary bath emollient (Oilatum™) vs the same with the two added antiseptics 6% w/w benzalkonium chloride and 2% triclosan (Oilatum Plus™) used daily at a dose of 15 ml to the bath for 4 weeks (topical steroids) | 30 children aged 1–9 years with recurrent infections and/or frequent exacerbation | Randomised crossover study of two 4 week treatment periods with a 2-week washout period in between | Composite sign and symptom score (max. 100), patient recorded global overall impression and global change scales | Based on 26 evaluable patients, the change from baseline score (baselines scores not given) was 9.0 for those using the antiseptic emollient compared with 2.7 for those with regular emollient at 4 weeks | Method of randomisation was described, but no ITT analysis | Both this study and the Holland study are published in a ‘round table’ discussion document sponsored by the manufacturer |
| England | Patient rated scores did not show any significant differences between the two treatments (data not shown) | Only statistical tests of change in scores from baseline for each treatment separately rather than the appropriate test of the difference in score changes between the two treatments | Difficult to interpret in view of the wrong statistical tests being used and missing patient-reported data | ||||
| Re-analysis of data comparing the change in score between the two treatments at 4 weeks did not confirm any superiority of the antiseptic emollient | |||||||
| Holland et al., 1995165 | Comparison of a standard proprietary bath emollient (Oilatum) vs the same with the two added antiseptics 6% w/w benzalkonium chloride and 2% triclosan (Oilatum Plus) used daily for 4 weeks (co-treatments not mentioned) | 15 patients aged 4–34 years with moderate-tosevere atopic eczema with S. aureus on their skin | Parallel randomised double-blind study of 4 weeks' duration | Clinical scores of signs, symptoms and extent and bacterial counts | At the end of 4 weeks' treatments, clinical scores in the emollient/antiseptic group had fallen more than those in the emollient only group, but these were statistically significant | No description of randomisation process or ITT analysis | Difficult to interpret the lack of demonstration of efficacy in such a tiny study with high drop-outs |
| England | There was no statistically significant difference in S. aureus counts between the two groups at the end of the treatment period | Although described as a parallel study, patients were paired for matching pre-treatment S. aureus population densities | |||||
| Five drop-outs in the emollient only group | |||||||
| Hizawa et al., 1998172 | Daily povidone-iodine solution to one arm vs nil else on opposite side (emollients only) | 16 volunteers with atopic eczema aged 12–29 years with similar eczema lesions in each elbow fold | Right/left investigator-blinded comparison study of 1 week duration | Physician-assessed before and after photographs and colony counts of S. aureus | Of 15 evaluable patients, physicians reported an improvement in the povidone-treated sites (p < 0.01), but not on the control sites | Unclear method and concealment of randomisation | Worth pursuing in a larger double-blind study as povidone-iodine is a cheap antiseptic with good antistaphylococcal properties |
| Japan | Bacterial colonisation was significantly reduced on the treated but not untreated site | Investigator masking suspect as iodine stains the skin | This is study is inconclusive in view of threat of unblinding, short duration, and failure to perform the appropriate statistical tests | ||||
| No summary data of differences between treatments reported | |||||||
| Broberg & Faergemann, 1995168 | After a course of antibiotics, patients were allocated to a combination active against Pityrosporum yeasts (a cream containing the antifungal miconazole plus hydrocortisone applied twice daily to the head and neck and ketoconazole shampoo twice weekly) vs plain hydrocortisone cream and shampoo base (emollients) | 60 patients aged 14–53 years with atopic eczema affecting the head and neck of whom 83% were positive for P. ovale on culture at start | Parallel randomised double-blind trial of 6 weeks' duration | Modified SCORAD (a composite sign and symptom score) and reduction in P. ovale counts | Of 53 evaluable patients, severity score fell from 58.6 at baseline to 33.2 after 4 weeks in the antifungal group compared with 60.1 at baseline to 22.9 in the standard group (NS) | No description of randomisation method, allocation concealment and no ITT analysis | Despite widespread use of antifungals for atopic eczema affecting the head and neck, this RCT does not suggest that there is any additional benefit over conventional treatment and that colonisation by the yeast P. ovale may be a secondary phenomenon |
| Sweden | P. ovale colonisation rates fell significantly in the antifungal group but not in the standard treatment group |
Summary of antimicrobial and antiseptic agents
-
Some patients with atopic eczema develop overt signs of clinical infection which is usually due to the bacterium S. aureus.
-
Most atopic eczema patients' skins are colonized with S. aureus.
-
There is no RCT evidence that oral antibiotics are of any benefit in clinically uninfected atopic eczema.
-
There is some evidence that a short course of cefadroxil is of benefit in clinically infected atopic eczema.
-
There is some evidence from a short-term study that topical mupirocin may improve atopic eczema activity as well as reduce bacterial counts, though there is concern regarding the emergence of resistant strains with such an approach.
-
There is no evidence that antiseptics are of benefit in atopic eczema when applied directly to the skin or in the bath.
-
One small study of 1-week duration in Japan suggested that spraying an acidic solution on babies with atopic eczema might result in an improvement of disease activity.
-
A study of head and neck atopic eczema failed to show any benefit of antifungal creams and shampoos directed against the yeast Pityrosporum ovale.
-
Topical steroids alone are an effective way of reducing skin colonisation by S. aureus.
Chapter 7 Antihistamines and mast cell stabilisers
Antihistamines
Itching is the central and often most distressing feature of atopic eczema. Antihistamines have long been prescribed for atopic eczema in the belief that they reduce itching by blocking the action of histamine on its receptors in the skin. The role of histamine in the itch of atopic eczema is unclear, and it may only play a small part. Histamine receptors are of two types, named H1 and H2, respectively. Both types are found in the skin. Most antihistamines that have been tried in atopic eczema are of the H1 type. These H1 antihistamines can be further subdivided into those with a sedating (e.g. chlorpheniramine) and those with a less-sedating action (e.g. cetirizine). Although lack of sedation may be desirable in the daytime, it is often stated that oral antihistamines are only effective in atopic eczema if they are sedative. 25 It is suggested that sedating antihistamines are effective because of their central sedating effect rather than any action on peripheral histamine blockade. 178
Regardless of how antihistamines might work in atopic eczema, it is useful to consider the evidence of whether they help at all. We located 21 RCTs of antihistamines of various types in the treatment of atopic eczema,173–193 and these are summarised in Tables 7–9. A further study358 was excluded as data on atopic eczema patients were not separated from patients with urticaria.
Comment
Quality of studies
Generally, the quality of study reporting was very poor, with some (e.g. Hjorth186 and Foulds & MacKie184) not containing any clinical effective-ness data at all. At least eight of the studies used a crossover study design, which, as others have noted,359 is perhaps not the best design in view of potential carry-over and period effects. Such effects were only formally tested for in one study,178 and in the absence of such testing, only comparisons for the first treatment period can be evaluated with confidence. Atopic eczema is also a very unstable disease, with flares occurring within 24 hours, making it an unsuitable condition for evaluation by crossover design. The clinical usefulness of small but statistically significant changes in mean itch scores between treatment groups is also very difficult to judge from the papers.
Ongoing systematic review
No statistical pooling has been attempted in this review as there is an ongoing, more detailed Cochrane Skin Group systematic review of antihistamines in atopic eczema which includes the authors of this report. 360 This is likely to be published later in 2000. Although the outcome data in Table 8 suggests that some pooling for itch might be possible for most studies, the ongoing Cochrane Skin Group review of antihistamines in atopic eczema is already encountering difficulty in pooling due to missing vital information such as baseline data, type of scale used, and standard errors.
Sedative antihistamines
Those studies that have evaluated sedating antihistamines against placebo176,182,184,185 do not show any evidence of a clear benefit for itch or global improvements. All the studies are quite small however.
H2 antihistamines
Similarly, those studies182,184 that have evaluated the benefit of the H2 antihistamine, cimetidine, alone or in combination with H1 drugs have not shown any benefit of the H2 agents, though both studies were under-powered to detect even a modest improvement.
Less-sedative antihistamines
Those studies that have included comparative data on less-sedating antihistamines versus placebo177–179,187–190,192 show mixed results. The largest study of 817 children followed-up for 18 months as part of the ETAC study,329 has only published safety data to date, but the authors are aware that preliminary data presented at a previous meeting (Diepgen T, oral communication, 1999) on SCORAD scores361 between the placebo and cetirizine groups did not show any differences. The full publication of the ETAC atopic eczema outcome data is eagerly awaited. The second largest (n = 187) and relatively well-reported study by Hanuksela and colleagues190 compared three different doses of cetirizine with placebo. They showed a possible benefit with cetirizine, but only at four times the normal recommended dose and at the expense of some sedation. The remaining studies show a mixture of no effect and some effect, but need to be interpreted cautiously in the absence of baseline data and other missing important data in the reports.
Studies that have compared different antihistamines or doses against each other are very difficult to interpret in the absence of a clear demonstration of benefit in placebo-controlled studies.
Other systematic reviews
One systematic review on the use of antihistamines in atopic eczema has recently been published. 362 That study missed nine of the RCTs identified in this report173–175,180,185–187,192,193 and also evaluated non-randomised studies in their qualitative analysis based on study quality. The authors suggested that the best-quality evidence did not support a useful effect of antihistamines in relieving the itch of atopic eczema.
Sodium cromoglycate
Sodium cromoglycate (SCG) is used widely in the management of bronchial asthma and allergic rhinitis. Its effectiveness is thought to be at least partly due to inhibition of release of inflammatory mediators from mast cells following antigen encounter. 203 The drug has an impressive safety record and as immunological mechanisms are known to be important in atopic eczema, several investigators have evaluated its use for this condition. In addition, increased intestinal permeability to macromolecules is thought to be one of the predisposing factors to food allergy in children with atopic dermatitis, and orally administered SCG is thought to reduce intestinal permeability. 211 Opinions remain divided over the value of SCG in atopic eczema. We therefore undertook a systematic review of relevant RCTs. 84,194–212
Results and discussion
A summary of the studies retrieved, their characteristics and the outcomes used are presented in Tables 10–17. The studies using oral SCG generally reported little or no beneficial effect for the drug compared with placebo. From those results it is probably safe to conclude that orally administered SCG is of little value in atopic eczema.
The results of trials of topical disodium cromoglycate (DSCG) are conflicting. Most of the studies that have reported positive effects were from the same laboratory84,194,201 and have used solutions of the drug rather than semi-solid formulations. While differences in product formulation may have accounted for the differences in observed responses, it is possible that the study populations may also have had an effect. One of the Kimata studies201 also included oxatomide in both the control and treatment arms so that treatment effects from his two studies cannot be quantitatively compared. The other two studies provide estimates which suggest that at 2 weeks, the itch scores decreased to a comparable extent with both DSCG and beclomethasone dipropionate (Table 18). The magnitude of change in itch scores obtained with those two drugs were somewhat higher than the difference seen between DSCG and placebo.
The study by Moore and colleagues198 also suggests that SCG was effective in atopic eczema with severity scores substantially reduced within 1 month of initiation of therapy. However, the individual symptom scores are not reported so that quantitative comparison with the Kimata studies is not possible.
Nedocromil sodium
Nedocromil sodium, a mast cell stabiliser, is the disodium salt of pyranoqiunoline dicarboxylic acid and is similar to SCG in pharamcological action. Its mode of action prevents the release of inflammatory mediators from mucosal mast cells, blocking the late cutaneous reactions in mast cell-dependent allergic reactions.
Three RCTs213–215 that evaluated nedocromil sodium in atopic eczema were identified. Two studies213,214 evaluated nedocromil cream versus placebo cream and the other215 evaluated oral nedocromil versus placebo.
Benefits
The study by Kemmett and Barnetson213 (abstract only) evaluated topical 4% nedocromil sodium cream versus matching placebo, in 32 atopic eczema patients over a 4-week period. There were no significant differences between treatment and control groups as determined by clinical assessment and IgE levels (no data given). Patient-assessed relief of itch, redness and weeping was recorded but no data were given.
The paper by van Bever and Stevens214 evaluated topical 4% nedocromil sodium cream versus vehicle only, in 26 adults and children with atopic eczema over a 4-week period. Patients and clinicians could not detect any difference between the two treatments as determined by daily score card for itch, sleep and overall severity of skin lesions and clinical examination for severity of skin lesions.
The study by Benton and colleagues215 evaluated oral nedocromil sodium 100 mg three times daily versus placebo, in 22 adults with moderate-to-severe atopic eczema over a 4-week period. Patient diary cards for itch, redness and weeping and clinician's overall opinion showed no significant differences between active treatment and placebo.
Harms
Benton and colleagues performed full blood counts and tests of renal and hepatic function each month to determine any drug toxicity. The authors report no abnormalities were found in the laboratory data, and the drug was well tolerated apart from one patient who developed persistent diarrhoea, which ceased on withdrawal of the drug. Van Bever and Stevens report 17 episodes of flaring of symptoms, nine were in the nedocromil sodium group. One other patient reported dryness of skin and another furunculosis.
Comment
All three studies were randomised but method and concealment of randomisation was unclear, all described as double blind. The Kemmett and colleagues study was in abstract form only so little information was available and no data were given for results. The van Bever and Stevens study did not specify whether daily score card was patientor doctor-assessed, and no actual data were given. It was unclear how many people were enrolled in the Benton and colleagues study. The results do not show any evidence to support benefit of nedocromil sodium, though the studies were relatively small and over short periods of time.
Ketotifen
Ketotifen is a benzocycloheptathiophene with antihistaminic and anti-anaphylactic properties. Its action is said to resemble SCG.
Benefits
We located two RCTs reporting the use of ketotifen for atopic eczema216,217 one on adults and one on children. The study in children217 evaluated ketotifen 1–2 mg twice daily versus placebo on 42 atopic children with asthma and allergic rhinitis (15 had eczema) for a period of 4 months. Parent-assessed diary cards of asthma symptom scores plus night itch, day itch and redness of skin were the primary outcome measures. No statistically significant beneficial effect of ketotifen was shown in asthma, allergic rhinitis or eczema.
Falk216 evaluated ketotifen 1 mg twice daily versus placebo in 60 adults with atopic eczema over a 3-month period. The eczema was assessed for itch, sleep loss, erythema, lichenification and overall efficacy of treatment. Improvement of itch over baseline on a scale of 1–3 was 2.40 reduced to 1.20 for ketotifen (p < 0.01) versus 2.30 reduced to 1.60 for placebo (p < 0.05).
Harms
Apart from slight drowsiness no other adverse effects were reported.
Comment
The White and colleagues study217 was primarily evaluating ketotifen for asthma; however, 15 of the children also had eczema. Being a small sub-group the authors conclude the number of patients with eczema was too small for meaningful analysis. The method and concealment of randomisation were unclear, though the study was described as double-blind. Withdrawals or drop-outs were not mentioned.
The Falk study216 showed no appropriate test of differences between the two treatments and no standard errors were given, therefore the results are difficult to interpret. The method and concealment of randomisation were unclear, though the study was described as double-blind. Four dropped out, and there was no intention-to-treat analysis.
Topical doxepin cream
Doxepin is a tricyclic antidepressant drug, which also has powerful antihistamine properties by antagonising both H1 and H2 histamine receptors. On the basis of the putative role of histamine in the itch of atopic eczema and potent antihistamine antagonising effects of doxepin, topical preparations of doxepin have been tried in people with atopic eczema and other itchy skin conditions.
Benefits
Four RCTs218–221 that evaluated topical doxepin in atopic eczema patients were identified. A further study of weal response in atopic eczema patients was excluded because no atopic eczema outcomes were reported. 363 One trial364 was excluded as it was only published in abstract form with few data. Of the remaining four RCTs, two evaluated topical doxepin versus vehicle cream218,219 and the other two evaluated the possible additional benefit of topical doxepin to treatment with topical corticosteroids. 220,221 Statistical pooling of the Breneman and colleagues219 and Drake and colleagues221 studies was not possible because separate data on atopic eczema were not given in the latter study. Pooling was not attempted in the Berberian and colleagues220 and Drake and colleagues221 studies because different strengths of topical triamcinolone were used.
The study by Drake and colleagues218 evaluated topical 5% doxepin cream versus vehicle only, applied four times daily, in 270 patients with atopic eczema over a 7-day period. Relief of itch (as recorded by a physician) was reported in 85% of doxepin and 57% of vehicle-treated patients by Day 7. A statistically significant relief in itch as recorded by patients on a 100 mm visual analogue scale (where 0 = no relief and 100 = complete relief) was also noted in the doxepin versus vehicle groups (68.6 versus 54.6, respectively after 7 days with baseline of 0 for both groups). Physician-reported eczema severity was also reported to be better in the doxepin group, though no data were given.
The paper by Breneman and colleagues219 is more difficult to asses as the RCT reported within this paper included a mixture of 47 patients with atopic eczema and 49 with lichen simplex (another form of localised eczema), and results were not presented separately. There was no clinically or statistically significant difference in patient-assessed itch relief at the end of the 7-day RCT.
The study by Berberian and colleagues220 evaluated the possible additional benefit of adding 5% doxepin to commonly used topical steroids in an 8-day study of 349 patients with atopic eczema. Four groups were randomly allocated to 2.5% hydrocortisone, 0.1% triamcinolone acetonide, 2.5% hydrocortisone plus 5% doxepin or 0.1% triamcinolone acetonide plus 5% doxepin, applied four times daily. At the end of 8 days, the mean visual analogue scale value for patient-recorded itch in the doxepin/ hydrocortisone group versus hydrocortisone group was 77.8 and 68.3, respectively (where 100 = complete relief from itching). For the doxepin/triamcinolone versus triamcinolone groups, the relief scores were 94.9 versus 90.5, respectively (p < 0.05). Baseline scores were not reported. Statistically significant effects were noted in the groups containing the doxepin from Day 1 onwards, but the magnitude of these effects diminished with each consecutive day. Physicians' global evaluation of eczema severity at the end of the 8 days was not clinically or statistically significantly different.
The Drake and colleagues study221 was mainly a pharmacokinetic study comparing doxepin hydrochloride 5% cream alone with doxepin plus 0.025% triamcinolone acetonide in 24 adults for 7 days. Only limited efficacy data were given. The paper stated that there were no significant differences in severity of atopic eczema between the two treatments at any point throughout the course of treatment. Pruritus severity scores (one of six itching assessment methods used in this study) demonstrated statistically significant greater improvement in the doxepin/triamcinolone group at 8 days (p = 0.001), though actual data for this and the other pruritus outcomes were not given.
Harms
Transient stinging or burning was commoner in doxepin-treated patients (e.g. 16 versus three in the Drake study). Drowsiness was also a problem (37 for doxepin versus three in the vehicle-treated patients in the Drake study), resulting in 16 dropouts from the doxepin arm versus three in the vehicle arm. Somnolence was also noted in four out of 22 participants in the Drake study. 221
Comment
The quality of reporting in the studies was quite good – methods of randomisation (though not subsequent concealment of allocation), a description of blinding and an intention-to-treat analysis were present in all three studies above. The fact that many patients developed drowsiness and stinging resulting in differential drop-outs in study arms raises concerns regarding the success of blinding in these studies. All four RCTs were sponsored by the manufacturer and were conducted by the same group of US investigators. Two of the above studies suggest that there is some evidence that topical doxepin produces some additional relief for itching in the short term (24–48 hours) when compared with vehicle. Whether this initial relief is clinically useful is doubtful, particularly when problems of drowsiness are taken into account. These short-term effects on itch become clinically insignificant over a 1-week period. None of the studies have demonstrated a clinically useful benefit of doxepin on atopic eczema severity or control even over the very short 1-week assessment periods. The studies have also used outcome measures scales that include itch as a prominent feature, or they have used several methods to assess itch, thereby increasing the likelihood of demonstrating a significant difference between the groups for itch. Longer-term independent studies (i.e. at least 6 months) evaluating combinations of doxepin plus other commonly used topical agents in atopic eczema are needed.
Tiacrilast
Tiacrilast is an important mast cell degranulation inhibitor in in vitro and in animal studies. As mast cells and their mediators are possibly involved in atopic eczema, it has been tried in a topical preparation in atopic eczema. One RCT222 compared 3% tiacrilast in the hydrogel formulation with vehicle alone in a multicentre study of 37 adults.
Benefits
In this right/left comparison study of 28 days, a lesion of atopic eczema was rated as responding if the sum of its rating (a composite scale of signs and itching) decreased by at least 33% from baseline to the end of treatment. Of 32 evaluable patients, 78% were noted to respond on the active drug versus 75% compared with a vehicle (p = 0.614). Median changes of efficacy parameters from baseline to end of treatment were also very similar between the two groups.
Harms
Treatment was well tolerated except for one patient who experienced burning at the site of drug application (site not specified).
Comment
This is one of the few RCTs in atopic eczema to pre-specify a measure of ‘success’ of its complex efficacy ratings scale. Method of randomisation was unclear and no intention-to-treat analysis was performed. Although this study was under-powered, the complete lack of difference between active and vehicle argues against a large treatment effect.
Summary of antihistamines and mast cell stabilisers
-
There is no RCT evidence to suggest that sedating oral antihistamines have a clinically useful benefit in atopic eczema.
-
There is limited and conflicting RCT evidence that less-sedating oral antihistamines have a clinically useful benefit in atopic eczema.
-
It is possible that any benefit can only be achieved with doses much higher than is currently recommended.
-
The largest and highest quality study of antihistamines in 817 children followed for 18 months has yet to report its outcome data on atopic eczema severity.
-
The current RCT evidence does not support the routine use of antihistamines in atopic eczema.
-
There is no evidence to support the use of oral SCG in atopic eczema.
-
The results of trials of topical DSCG are conflicting.
-
Most of the studies that reported positive results are from the same study laboratory and need to be repeated elsewhere.
-
There is no RCT evidence to support the use of nedocromil sodium treatment in atopic eczema.
-
There is no RCT evidence that shows any benefit to oral ketotifen in atopic eczema.
-
Two RCTs suggest that topical doxepin might produce some additional relief of itching compared with vehicle alone in the first 48 hours.
-
None of the studies of topical doxepin have demonstrated a clinically useful benefit on eczema severity.
-
Drowsiness may occur with topical doxepin.
-
All of the studies of topical doxepin have been conducted by the same research team – sponsored by the manufacturer.
-
Longer-term independent RCTs of topical doxepin are needed.
-
There is no evidence to support the benefit of topical tiacrilast in atopic eczema.
| Study | Design | No. of patients | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawals and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Berth-Jones & Graham-Brown, 1989178 | Crossover RCT | 28 | 11–67 | 1 week | Stable | Terfenadine 120 mg b.d. | Placebo | Topical steroid | Four: failure to comply |
| Emollients | |||||||||
| Doherty et al., 1989179 | Parallel RCT | 49 | 16–58 | 2 weeks | Clinical diagnosis of atopic eczema | Acrivastine 8 mg t.d.s. vs Terfenadine 60 mg t.d.s. | Placebo | Twice daily 0.05% clobetasone butyrate and aqueous cream | Four active, one placebo |
| Foulds & MacKie, 1981184 | Multiple crossover RCT | 21 | 14–29 | 3 × 2 weeks | Life-long atopic eczema | Cimetidine + placebo vs Sedative H1 + placebo vs Cimetidine + H1 | Placebo | Ichthammol + emulsifier + 25 mg promethazine hydrochloride | One loss to follow-up |
| Placebo | |||||||||
| H1 | |||||||||
| Frosch et al., 1984182 | Multiple crossover RCT | 18 | 14–43 | 3 × 4 weeks | 3-year history of atopic eczema | Cimetidine + chlorpheniramine vs Chlorpheniramine + Placebo vs Placebo + placebo | Placebo | Bland greasy ointment | Two personal reasons |
| 0.1% betamethasone | |||||||||
| Hamada et al., 1996192 | Parallel RCT | 64 | 7–? | 6 weeks | Mild to severe | Terfenadine 60 mg b.d. + alclometasone propionate (0.1%) ointment b.d. | Betamethasone valerate 0.1% b.d. | None | Five |
| (Japanese translation) | |||||||||
| Hannuksela et al., 1993190 | Parallel RCT | 178 | 18+ | 4 weeks | Moderate to severe | Three different doses of cetirizine 10 mg, 20 mg and 40 mg daily | Placebo | Emollients | 51 total |
| 1% hydrocortisone | 20 adverse effects, 19 non-compliers | ||||||||
| Henz et al., 1998187 | Parallel RCT | 74 with atopic eczema 244 total including urticaria | 17–67 | 2 weeks | Moderate to severe pruritus | Azelastine 4 mg vs Cetirizine 10 mg | Placebo | * | 37 total but unclear how many in atopic eczema group |
| Hjorth, 1988186 | Crossover RCT | 30 | * | 2 weeks | Atopic eczema with history of contact urticaria | Terfenadine 60 mg b.d. | Placebo | * | * |
| Ishibashi et al., 1989(a)174 | Parallel RCT | 157 GIR* | 1–15 | 4 weeks | Mild to severe | E-0659 (azelastine hydrochloride) 0.017 /kg/day | 0.07mg/kg/day and 0.13mg/kg/day azelastine hydrochloride | White vaseline | 15 |
| (Japanese translation) | 168 OSR | 11 | |||||||
| 159 GUR | Nine | ||||||||
| Ishibashi et al., 1989(b)193 | Parallel RCT | 169 GIR/ | 6–? | 4 weeks | Mild to severe | E-0659 (azelastine hydrochloride) 4 mg/day and 2 mg/day | Ketotifen 2mg/day | White vaseline and topical hydrocortisone | 11 |
| (Japanese translation) | GUR* | One | |||||||
| 179 OSR | |||||||||
| Klein & Galant, 1980191 | Parallel RCT | 20 | 2–16 | 1 week | Acute exacerbations of atopic eczema | Hydroxyzine 1.25 mg/kg/day | Cyproheptadine 0.25 mg/kg/day | Lubriderm lubricating cream | * |
| Langeland et al., 1994188 | Six consecutive crossover RCTs | 16 | 19–37 | 12 weeks | Moderate-severe | Loratadine 10 mg | Placebo | Emollients | * |
| Mild topical steroid | |||||||||
| La Rosa et al., 1994189 | Parallel RCT | 23 | 6–12 | 8 weeks | Hanifin and Rajka | Cetirizine 5 mg/day for 30 kg and under | Placebo | * | One voluntary withdrawal |
| 10 mg/day for over 30 kg | |||||||||
| Monroe, 1992176 | Parallel RCT | 41 out of 59 | 18–65 | 1 week | * | 10 mg loratadine o.d. placebo b.d. | Placebo t.d.s. | Topical treatment but not specified | None |
| 25 mg hydroxyzine t.d.s. | |||||||||
| Patel et al., 1997180 | Parallel RCT | 118 | 12–65 | 2 weeks | At least moderate severity | 10 mg/day loratadine | Cetirizine 10 mg/day | * | Ten failure to meet entry criteria or report for follow-up |
| Savin et al., 1979185 | Unclear if parallel or crossover RCT | 12 | 23–38 | 3 nights over 4 weeks | Severe atopic eczema | Trimeprazine tartrate 20 mg | Placebo | Yes but not specified | * |
| Trimeprazine tartrate 50 mg | |||||||||
| Savin et al., 1986181 | Multiple Crossover RCT | 10 | * | 10 days | Long standing atopic eczema | LN2974 15 mg | Placebo | Routine topical treatment but not specified | * |
| Simons, 1984183 | Crossover RCT | 12 | 1–14 | 4 days | Severe widespread | Hydroxyzine 1.4 mg/kg | Hydroxyzine 0.7 mg/kg | * | Unclear |
| Simons, 1999175 | Parallel RCT | 817 | 12–24 | 18 months | Atopic eczema with family history | Cetirizine 0.25 mg/kg b.d. | Placebo | Yes but no details given | 99 |
| Wahlgren et al., 1990177 | Crossover RCT | 25 | 17–42 | 3 days | Persistent atopic eczema | Terfenadine 60 mg b.d. | Placebo | 1% hydrocortisone | None |
| Clemastine 2 mg b.d. | |||||||||
| Zuluaga de Cadena et al., 1989173 | Parallel RCT | 52 | 2–6 | 4 weeks | Not specified | Hydroxyzine 25 mg daily in three divided doses | Three active treatments | Emollients only | Eight total (six on hydroxyzine and two on Astemizole treatment) |
| (Colombia translated) | Terfenadine 10 mg daily in two divided doses vs Astemizole 5 mg daily in one dose |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing/weeping | Oedema | Inflammation | Crusts | Infiltration | Induration | Patient itch | Doctor itch | Patient sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berth-Jones & Graham-Brown, 1989178 | • | • | |||||||||||||||||||||||
| Doherty et al., 1989179 | • | • | |||||||||||||||||||||||
| Foulds & MacKie, 1981184 | • | • | • | • | • | • % body surface area | |||||||||||||||||||
| Frosch et al., 1984182 | • | • | • | • | |||||||||||||||||||||
| Hamada et al., 1996192 | • | • | • | • | • | • | |||||||||||||||||||
| Hannuksela et al., 1993190 | • | • | • | • | • | • | • | • | • | ||||||||||||||||
| Henz et al., 1998187 | • | • | • | • | |||||||||||||||||||||
| Hjorth, 1988186 | • | ||||||||||||||||||||||||
| Ishibashi et al., 1989(a) | • | • | • | • | • | • | |||||||||||||||||||
| Ishibashi et al., 1989(b) | • | • | • | • | • | • | |||||||||||||||||||
| Klein & Galant, 1980191 | • | • | • | • | • | ||||||||||||||||||||
| Langeland et al., 1994188 | • | ||||||||||||||||||||||||
| La Rosa et al., 1994189 | • | • | • | • | • | • | • | • | |||||||||||||||||
| Monroe, 1992176 | • | • | |||||||||||||||||||||||
| Patel et al., 1997180 | • | • | • | • | • | • | • | • | |||||||||||||||||
| Savin et al., 1979185 | • | ||||||||||||||||||||||||
| Savin et al., 1986181 | • | ||||||||||||||||||||||||
| Simons et al., 1984183 | • | ||||||||||||||||||||||||
| Simons, 1999175 | Safety study: drop-outs and serious events | ||||||||||||||||||||||||
| Wahlgren et al., 1990177 | • | ||||||||||||||||||||||||
| Zuluaga de Cadena et al., 1989173 | • | • | • | • | • | • | |||||||||||||||||||
| Study | Main reported results | Authors' conclusions | Comment and quality |
|---|---|---|---|
| Berth-Jones & Graham-Brown, 1989178 | There was no evidence of any difference between terfenadine and placebo. The mean scores for itch over the last 4 days of treatment (and standard errors of the means) were 23.95 (±4.9) for the terfenadine phase and 25.13 (±5.1) for the placebo phase. There was no evidence of carry-over or period-effect when pruritus scores were assessed | There was no benefit from terfenadine | Method and concealment of randomisation unclear, study described as double-blind. Four withdrawals for failure to comply, not clear at which point and in which initial arm. No ITT analysis. Low power to detect carry-over effect in only 20 patients. No data in graphical form. First and last period data not presented separately. Terfenadine double normal dose |
| Doherty et al., 1989179 | Acrivastine significantly reduced itching when compared with placebo according to the doctor's assessment (p = 0.021). Both acrivastine (p = 0.026) and terfenadine (p = 0.037) improved the patient's condition significantly more than placebo according to the patient's assessment of the degree of benefit obtained. No significant differences were found between the two active treatments | Acrivastine and terfenadine can partially relieve itching in atopic eczema | Method and concealment of randomisation unclear, study described as double-blind. Visual analogue data only given for Day 7. Five drop-outs (four active, one placebo), no ITT analysis. Unclear what was being assessed and what was meant by ‘careful examination of the skin’ |
| Foulds & MacKie, 1981184 | Although it was found that there was a significant difference between individual patients for patient-assessed day pruritus (p < 0.001) and night pruritus (0.01 < p < 0.025) there was no difference between the treatment periods | This study does not demonstrate any significant advantage in adding an H2 receptor antagonist to the H1 receptor antagonist commonly used in young adults with severe chronic atopic eczema | Method and concealment of randomisation unclear, study described as double-blind. Only one loss to follow-up, no ITT analysis carried out. No actual data given for clinical outcomes – only p-value for statistical comparisons |
| Frosch et al., 1984182 | Analysis of cimetidine plus chlorpheniramine results for weeks 2, 3 and 4 for both day- and night-time patient-assessed itch compared with chlorpheniramine and placebo failed to show any significant difference | The combined administration of H1 and H2 receptor antagonists is of no benefit in the treatment of atopic eczema | Randomisation was conducted according to a Latin square, study described as double-blind. Baseline itch not given, therefore unable to calculate change. No standard errors given. Missing baseline data.Two drop-outs, no ITT analysis carried out |
| Hamada et al., 1996192 | Itching score and scratch marks were improved significantly. Physician ‘improved’ and ‘markedly improved’ was 89.3% in antihistamine and steroid group compared with 50% in the topical steroid only group | Combination of terfenadine ingestion and topical alclometasone application is more effective than betamethasone application only | Used different topical steroids in each intervention and no oral placebo. No ITT |
| Hannuksela et al., 1993190 | There was a non-significant difference between groups in patient-assessed pruritus intensity at baseline. All groups improved significantly (p = 0.005).This improvement was significantly more pronounced for cetirizine 40 mg compared with placebo | The sedation observed probably was partly responsible for pruritus relief, authors suggest that cetirizine has other properties responsible for skin lesion healing | Method and concealment of randomisation unclear.A high drop-out rate of 51, 20 for side-effects (mainly sedation) and 19 non-compliers, doesn't specify drug group. No ITT analysis carried out. Possible benefit of cetirizine when used at four times normal dose, but at the expense of sedation |
| Henz et al., 1998187 | Mean overall % response rate based on physician's global score was 36.4%, 25.0% and 27.3% in the azelastine, cetirizine and placebo groups, respectively. Baseline data and exact numbers of atopic eczema patients in each group were not stated. Mean itching score dropped from 2.2 to 1.4 in the cetirizine group and from 2.2 to 1.2 in both azelastine and placebo groups (estimated from graphs) | The data underline the low efficacy of antihistamines in atopic eczema | Neither drug reduced itching significantly more than placebo. Statistics not given for atopic eczema patients, no description of what constituted a response, placebo looks very impressive, clearly no difference in atopic eczema patients. High drop-out rate of 37, no ITT analysis carried out |
| Hjorth, 1988186 | Terfenadine reduced severity of itch in approximately 52% of patients, 34% reported no change and 14% reported increased severity of itch. No data given for placebo | Terfenadine is if value in some patients with atopic eczema and a history of contact urticaria | No outcome data given and no information whatsoever on placebo response. Method and concealment of randomisation unclear, study described as double-blind. Unclear if any drop-outs or withdrawals. Author since deceased |
| Ishibashi et al., 1989(a)174 (Japanese translation) | No significant difference in general improvement rating, overall severity rating and general usefulness rating among the three dose groups.A significant difference in improvement ratio was found among three dose group in the signs if itch, papules, erythema and lichenification | No translated data available | No translated data available |
| Ishibashi et al., 1989(b)193 (Japanese translation) | No difference in final general improvement rating or general usefulness rating among the three groups. The effectiveness and usefulness in the treatment of atopic eczema were considered similar for the three groups. There was a significant difference in overall safety rating between the 4 mg/day and 2 mg/day groups. The safety rating was higher in the 2 mg/day group than in the 4 mg/day group. The overall safety rating showed no significant difference between the 4 mg/day and ketotifen groups or the 2 mg/day and ketotifen groups | No translated data available | No translated data available |
| Klein & Galant, 1980191 | The group receiving hydroxyzine had a daytime percentage improvement of 32.14 ± 4.98 (mean ± SEM) over their baseline pruritus for the entire week, which is significantly greater (p < 0.001) than the percentage improvement for the cyproheptadine group of 6.21 ± 4.90 | This study suggests that hydroxyzine is more effective than cyproheptadine for the management of pruritus associated with atopic eczema in children | Unstable data shown on a graph with inflationary % scale but no actual data given. Method and concealment of randomisation unclear, study described as double-blind. Not clear if any drop-outs or withdrawals |
| Langeland et al., 1994188 | The study detected a significant effect of loratadine, as compared with placebo, on patient-assessed pruritus during the day and night and severity of rash | Loratadine may be tried as an adjuvant therapy in the management of severe and moderate atopic eczema, in patients complaining of pruritus | Complex design, six consecutive crossovers. Changes in pruritus on VAS all small differences. No data for period or carry over effects shown. Method and concealment of randomisation unclear, study described as double-blind (block randomised) |
| La Rosa et al., 1994189 | Patient diary card scores showed a statistically significant decrease in erythema and other cutaneous symptoms such as lichenification, in the cetirizine group. Improvement over baseline total mean global score of 230 for cetirizine reduced to 155 after 8 weeks treatment, and 205 baseline for placebo reduced to 180 (p > 0.05) after 8 weeks treatment (estimated from graph) | The results of this preliminary study suggest that cetirizine can effectively control pruritus and other cutaneous symptoms in children suffering from atopic eczema without noticeable adverse effects | Method and concealment of randomisation unclear, study described as double-blind. Only one drop-out (voluntary withdrawal). Higher baseline scores in those on active treatments suggest that regression to the means could partly amount for results |
| Monroe, 1992176 | The daily pruritus score decreased 57% in the 14 patients treated with loratadine, 38% in the 14 patients treated with hydroxyzine, and 33% in the 13 placebo patients | Loratadine demonstrates a significant antipruritic effect in atopic eczema | Patients excluded if unresponsive to antihistamines. No baseline values given. Method and concealment of randomisation unclear, study described as double-blind. Very short study at 1 week |
| Patel et al., 1997180 | Loratadine reduced patients perceived severity of their overall condition by 20.8% at endpoint. Incidence of somnolence was 9% with cetirizine and 3% with loratadine | In the management of symptoms of atopic eczema, loratadine is as effective as cetirizine and is less sedating | Study excludes non-responders before study started but not told how many. The report suggests ITT but fails to carry it out. Unclear if either drug is of benefit in absence of placebo group. Method and concealment of randomisation unclear, study described as double-blind |
| Savin et al., 1979185 | Neither of the drugs altered the likelihood of scratching bout beginning in wakefulness or in any stage of sleep. However, both drugs, especially trimeprazine, made sleep less broken, and the reduced time spent in stage 1 of sleep accounted for a modest reduction in the overall amount of scratching during the night | Both trimeprazine and trimipramine sleep becomes less broken, with a lessening of the time awake and in stage 1 sleep, and that these actions, which may be helpful to some patients, were associated with a modest reduction in the number and length of scratching bouts | Unclear if parallel or crossover study. Length of study unclear. Method and concealment of randomisation unclear, study described as double-blind. Withdrawals or drop-outs not mentioned in this study. Unclear if the changes in sleep pattern helped the patient's eczema |
| Savin et al., 1986181 | No significant difference was detected between the limb movement times on placebo and on active treatment with LN2974.The difference between the mean scores of the visual analogue assessment of itching on placebo and on LN2974 did not reach statistical significance although tending to favour LN2974 | No significant suppression of scratching, as measured by limb movement meters, or of itching, recorded on VASs, could be demonstrated | No actual data given. Method and concealment of randomisation unclear, study described as double-blind. Unclear if any withdrawals or drop-outs |
| Simons et al., 1984183 | The scores for atopic eczema severity and distribution were significantly reduced at the end of treatment for both doses of hydroxyzine (p ≤0.05) | Hydroxyzine 0.7 mg/kg three times daily was as effective as hydroxyzine 1.4 mg/kg three times daily in relieving pruritus and promoting resolution of the skin lesions | This trial was buried in the middle of a case-series. Only presented mean score at end of treatment rather than mean change in score. Itch data and baseline scores not given. Sample size unclear. Point of randomisation was after the single dose study. Method and concealment of randomisation unclear, study described as double blind. Unclear of any withdrawals or drop-outs. Far too small a study to establish equivalence effects |
| Simons, 1999175 | During the 18 month long study, only 48 children treated with cetirizine and 51 treated with placebo dropped out ‘for any reason’ (p = 0.737); of these, only 11 and 15 children, respectively, dropped out because of symptoms or events (p = 0.421). Serious events were reported in 37 children (9.3%) receiving cetirizine and in 54 children (13.6%) receiving placebo. Hospitalisations were reported in 36 children receiving cetirizine and in 47 receiving placebo (p = 0.189). Fatigue and insomnia were slightly, but not statistically significantly, raised in cetirizine group. No difference in somnolence between cetirizine and placebo. No efficacy data given | The safety of cetirizine has been confirmed in this prospective study, the largest and longest randomised, double-blind, placebo-controlled, safety investigation of any H1 antagonist ever conducted in children and the longest of prospective safety study of any H1 antagonist conducted in any age group | Good description of randomisation and blinding. Well reported study. 99 drop-outs, no ITT analysis carried out. Clinical efficacy outcome data eagerly awaited. |
| Wahlgren et al., 1990177 | No significant difference in itch intensity between the three treatment periods was detected with Pain-Track, nor was there any difference in time awake without pruritus. No significant changes in itch magnitude appeared during each period (Days 0–3) | The antipruritic effect of 3 days of treatment with terfenadine (non-sedative) and clemastine (sedative) did not differ from that found with placebo | Very short trial of only 3 days. Method and concealment of randomisation unclear, study described as double blind. No withdrawals or drop-outs. Underpowered |
| Zuluaga de Cadena et al., 1989173 (Colombia) translated | At the end of the 4-week evaluation period, eight out of 15 patients on terfenadine compared with eight out of 17 patients on astemizole and six out of 8 patients on hydroxyzine had improvement in itch. Global improvement was noticed in 14 out of 15 cases on terfenadine, 15 out of 17 cases on astemizole and seven out eight cases on hydroxyzine. Improvements in other outcome measures was similar between all three groups. Other laboratory measures were also recorded | Antihistamines may be beneficial in atopic eczema with astemizole conferring a prolonged benefit | Outcome measures and their combination were quite complex. Method and concealment of randomisation unclear. Study described as single (investigative)-blind. No ITT analysis. Small numbers and no placebo group |
| Study | Design | No. of patients | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawals and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Atherton et al., 1982203 | Crossover, double-blind, randomised | 29 | 2–10 | 4 weeks | Varying severity | Oral SCG 100 mg q.d.s. | Placebo | Emollients, corticosteroids, antihistamines | One due to systemic cortico-steroids |
| Birkeland et al., 1981208 | Parallel, double-blind, randomised | 28 | 19–48 | 6 weeks | * | Oral DSCG 6 mg q.d.s. | Placebo | All stopped | * |
| Burks & Sampson, 1988207 | Crossover, double-blind, randomised | 10 | 3–15 | 1 week | * | Oral cromolyn 30–40 mg/kg/day | Placebo | * | None |
| Businco et al., 1986205 | Crossover, double-blind, randomised, ITT | 31 | 0.5–10 | 8 weeks | Severe enough to require continuous treatment | Oral aqueous solution SCG | Placebo | Other treatment kept to a minimum, no steroids allowed | Six |
| Graham et al., 1984197 | Crossover, double-blind, randomised, no ITT | 29 | 3–12 | 6 weeks | Chronic | Oral SCG 100 mg q.d.s. for 3 weeks 200 mg q.d.s. for 3 weeks | Placebo | Hydrocortisone and antihistamines | Eight |
| Antibiotics if infected | |||||||||
| Kavli & Larsen, 1981204 | Crossover, double-blind, randomised, no ITT | 35 | 15–42 | 2 weeks | * | FPL 57787 (chromone carboxylic acid) 18 mg q.d.s. | Placebo | 1% hydrocortisone | 18 |
| Larsen & Larsen, 1979206 | Parallel, double-blind, randomised | 14 | 18+ | 6 weeks | * | FPL 57787 6 mg q.d.s. | Placebo | 1% hydrocortisone | * |
| Larsen & Jacobsen, 1980195 | Crossover, double-blind, randomised | 23 | 18–41 | 6 weeks | * | FPL 57787 18 mg q.d.s. | Placebo | 1% hydrocortisone | Three (one due to adverse effects) |
| Lindskov & Knedsen, 1983210 | Crossover, double-blind, randomised | 24 | 4–37 | 6 weeks | Severe widespread | Oral DSCG 200 mg q.d.s (adults) 100 mg q.d.s. (children) | Placebo | Moisture cream, oil bath and hydrocortisone butyrate | None |
| Ventura et al., 1996211 | Parallel, double-blind, randomised | 83 | 0.1–1.5 | 4 weeks | * | Oral DSCG 100–120 mg/kg/day q.d.s. | Placebo | Corticosteroids (Locoidon, hydrocortisone butyrate) emollients | * |
| Study | Design | No. of patients | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawals and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Ariyanayagum et al., 1985202 | Parallel, double-blind, randomised | 46 | 16–65 | 12 weeks | * | 4% SCG | Placebo | Hydrocortisone 1% Terfenadine (adults) Clemastine (children) | Seven withdrew: three SCG, four placebo |
| Croner et al., 1981200 | Parallel, double-blind, randomised | 22 | 2–16 | 6 weeks | Moderate to severe | 10% SCG w/w in white soft paraffin | Vehicle | Antihistamines if required | * |
| Haider, 1977196 | Parallel, double-blind, randomised | 44 | 0.42–14 | 12 weeks | Chronic | 10% SCG in white soft paraffin | Placebo | None | 20: treatment ineffective |
| Hiratsuka et al., 199684 | Parallel, double-blind, randomized | 43 | 5.2–14.6 | 2 weeks | * | Topical SCG (concentration not given | Betnovate | None | * |
| Kimata & Igarashi, 1990194 | Parallel, double-blind, randomised | 45 | 0.8–3 | 4 weeks | Moderate to severe | Cromolyn nebulizer solution | Placebo | None | None |
| Kimata & Hiratsuka, 1994201 | Parallel, double-blind, randomised | 53 | 4.1–14.2 | 4 weeks | Moderate to severe | SCG nebulizer plus oxatomide (1.5 mg/kg/day) | Placebo (water solution plus oxatomide) | Oxatomide | Four: two from placebo group: ineffective two from active group: incorrect use of treatment |
| Kjellman & Gustafsson, 1986209 | Parallel, double-blind, randomised | 40 | 1–18 | 12 weeks | * | SCG 4% oil in water | Placebo | 1% hydrocortisone antihistamines emollients and potent topical steroids | Three: one from SCG group lack of compliance two from placebo group deterioration of eczema |
| Moore et al., 1998198 | Crossover, randomised, not blinded | 26 | 0.5–18 | 4 weeks | Moderate to severe | Cromolyn sodium inhalation solution 0.21% | Placebo | 0.1% triamcinolone, 1% hydrocortisone | Five |
| Pike & Atherton, 1988212 | Parallel, double-blind, randomised | 36 | 1–14 | 12 weeks | * | SCG oil in water cream | Placebo | * | |
| Thirumoorthy & Greaves, 1978199 | Right/left comparison, double-blind, randomised | 11 | 1–1.5 | 4 weeks | Moderate to severe | DSCG 10% in white soft paraffin | Placebo | None | Five drop-outs no data given |
| Study | Outcome measure | Scale |
|---|---|---|
| Atherton et al., 1982203 | Parent diary card for day-time itch and night-time sleep loss | 0–3 scale |
| Clinical evaluations 20 areas of skin surface for erythema, vesiculation and/or crusting, excoriation and lichenification | ||
| At end of study general well-being and severity of eczema | +2 to −2 (very much better to very much worse) | |
| Birkeland et al., 1981208 | Clinical assessments of 14 regions for colour, scaling, infiltration, itching of the three most active eczematous regions | 1–3 scale (1 = none, 2 = slight, 3 = marked) |
| Total serum IgE and reduction in disease activity | ||
| Burks & Sampson, 1988207 | Parent symptom diary cards: rash distribution, pruritus, urticaria | 0–3 scale |
| Businco et al., 1986205 | Clinician-assessed body divided into ten areas for redness, weeping, vesiculation, crusting, excoriations, lichenification | 0–3 score total body score 60, max score for all parameters 240 |
| Parent-assessed diary card for itching and sleep disturbance due to itching, weeping, redness of skin | 0–3 scale max score 12 | |
| Graham et al., 1984197 | Patient diary card for pruritus, sleeplessness, severity and area of eczema | 0–4 scale |
| Clinical assessment on a homunculus for severity and area. | 0–4 scale | |
| Dryness and excoriation also noted | ||
| Kavli & Larsen, 1981204 | Clinician-assessed disease extent and severity. | |
| Patient diary cards for itching and sleep loss and severity for lichenification, excoriation, redness | 0–3 scale (none-severe) | |
| Larsen & Larsen, 1979206 | Scaling, colour, lichenification, general assessment of the eczema and severity of itch | * |
| Larsen & Jacobsen, 1980195 | Clinician-assessed dryness, lichenification, excoriation | 0–3 scale |
| Lindskov & Knudsen, 1983210 | Clinician-assessed lichenification, eczema, and overall disease | Scales 0–2, 0–3 and 0–4, respectively |
| Patient- or parent-assessed day- and night-time itching and general severity of eczema | 0–5mm VAS | |
| Ventura et al., 1996211 | Clinician-assessed erythema, exudation, lichenification, eczema extension and itch | * |
| Study | Outcome measure | Scale |
|---|---|---|
| Ariyanayagam et al., 1985202 | Patient diary card for pruritus, sleeplessness, severity of eczema and use of concomitant therapy | 0–3 point scale patient-assessed signs and symptoms |
| Severity assessed on erythema, lichenification, vesiculation, dryness and excoriation over four main areas | 0–6 point scale physician-assessed over four main areas for signs | |
| Croner et al., 1981200 | Patient diary cards for itching (day and night), sleep disturbance and severity of eczema on face, trunk, arms and legs | 0–3 score where 0 = no symptoms and 3 = severe symptoms |
| Haider, 1977196 | Physician-assessed inflammation, lichenification and cracking of the arms and legs | 0–2 point scale physician-assessed signs over two areas |
| Patient diary card for severity of itching (day and night) and sleep disturbance | 0–3 point scale patient-assessed itch and sleep loss | |
| Hiratsuka et al., 199684 | Physician-assessed inflammation, lichenification, cracking on 15 body areas | 0–2 point scale in ascending order of severity |
| Patient diary cards for itching and sleep disturbance | 0–3 point scale patient-assessed diary cards | |
| Kimata & Igarashi, 1990194 | Signs: inflammation, lichenification and cracking assessed on four body areas max score 24, 0–8 mild, 9–16 moderate, 17–24 severe (only scores > 9 entered) | 0–2 point scale in ascending order of severity |
| Symptoms: sleep and itching patient-assessed record card | 0–3 scale | |
| Kimata & Hiratsuka, 1994201 | Physician-assessed signs lichenification, inflammation and cracking on 15 body areas, max. score = 30 | 0–2 scale |
| Patient-assessed symptoms itch and sleep loss on a diary card | 0–3 scale | |
| Kjellman & Gustafsson, 1986209 | Patient diary cards for itch, sleep disturbance and overall severity (redness, vesiculation and crusting, excoriation, lichenification) | 0–3 scale |
| Moore et al., 1998198 | Physician-assessed erythema, vesiculation, crusting and cracking, scaling, and lichenification in 12 body areas, (Rule of Nines) | 0–3 scale (none to severe) max. per area = 15 |
| Pike & Atherton, 1988212 | Diary charts recording pruritus, sleep disturbance by physician | Body score chart |
| Thirumoorthy & Greaves, 1978199 | Patient diary card for itching | * |
| Clinical responses of the two sides assessed weekly by clinician and patient | * |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing | Inflammation | Crusts | Infiltration | Induration | Itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atherton et al., 1982203 | • | • | • | • | • | • | • | • | • | ||||||||||||||
| Birkeland et al., 1981208 | • | • | • | ||||||||||||||||||||
| Burks & Sampson, 1988207* | • | RoNAA | |||||||||||||||||||||
| Businco et al., 1986205† | • | • | • | • | • | • | |||||||||||||||||
| Graham et al., 1984197 | • | • | • | • | |||||||||||||||||||
| Kavli & Larsen, 1981204 | • | • | • | • | |||||||||||||||||||
| Larsen & Larsen, 1979206‡ | • | • | |||||||||||||||||||||
| Larsen & Jacobsen, 1980195 | • | • | • | ||||||||||||||||||||
| Lindskov & Knudsen, 1983210§ | • | • | • | • | VAS | ||||||||||||||||||
| Ventura et al., 1996211¶ | • | • | • | • |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing | Inflammation | Crusts | Infiltration | Induration | Itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ariyanayagam et al., 1985202 | • | • | • | • | • | ||||||||||||||||||
| Croner et al., 1981200 | • | • | • | ||||||||||||||||||||
| Haider, 1977196 | • | • | • | ||||||||||||||||||||
| Hiratsuka et al., 199684 | • | • | • | ||||||||||||||||||||
| Kimata & Igarashi, 1990194 | • | • | • | ||||||||||||||||||||
| Kimata & Hiratsuka, 1994210 | • | • | • | ||||||||||||||||||||
| Kjellman & Gustafsson, 1986209 | • | • | • | • | • | ||||||||||||||||||
| Moore et al., 1998198 | • | • | • | • | • | • | RoNAA | ||||||||||||||||
| Thirumoorthy & Greaves, 1978199 | • |
| Study | Main reported results | Authors' conclusions | Quality |
|---|---|---|---|
| Atherton et al., 1982203 | No difference detected at 4 weeks between the effects of DSCG and placebo | The results do not confirm previous anecdotal reports of effectiveness of SCG in children with atopic eczema | Small and short-term study. Randomisation, blinding and 4-week washout period appear adequate |
| Birkeland et al., 1981208 | No significant changes were found between the severe and mild atopic eczema for number of regions involved at the first visit and reduction in disease activity during the trial. Serum IgE in relation to T and B cells shows nonsignificant differences in the figures in the severe and mild atopic dermatitis for T cells and B cells. | No benefit could be proven for the drug in the clinical investigation or any change in the immunological tests during the trial. There was no demonstrable differences in the applied immunoparameters between mild and severe atopic dermatitis | Method and concealment of randomisation unclear, study described as double-blind. Unclear whether any drop-outs |
| Burks & Sampson, 1988207 | SCG (40 mg/kg/day) did not protect against food-induced symptoms in patients with atopic eczema and egg hypersensitivity | Oral SCG is of no benefit in the treatment of children with atopic eczema and food hypersensitivity | Only ten children were studied and eight reacted to food challenge in this crossover study |
| Businco et al., 1986205 | Increase in symptom score higher when patients were given DSCG than placebo | SCG seems to have reduced exacerbations of atopic eczema caused by food allergens | Randomisation, blinding and 2-week washout period appear adequate. Small and short-term study. Patients had history of food hypersensitivity |
| Graham et al., 1984197 | Mean eczema scores for severity and area not different between groups receiving DSCG and placebo | Tailored diets were of value but SCG did not produce a significant additional effect | Randomisation, blinding and 2-week washout period appear adequate. Small and short-term study. Patients had history of food hypersensitivity |
| Kavli & Larsen, 1981204 | A significant reduction in patient-assessed itch was found for chromone carboxylic acid in the placebo, washout, chromone group (p < 0.05) after 6 weeks' treatment. Significant differences were found for lichenification, excoriation and redness in the placebo, washout, chromone group for clinically assessed signs (p = 0.05). No significant differences were found between chromone followed by placebo groups at 3 weeks treatment | Systemic chromone derivatives relieve certain symptoms in patients with atopic dermatitis. However, statistically significant results in favour of chromone carboxylic acid were obtained only in the group that started on placebo and only after the first 3 weeks of treatment. This may be due in part to the reduced sample at 6 weeks, and possibly also to an increasing awareness during the trial on the part of the patients of antigen avoidance | Method and concealment of randomisation unclear, study described as double-blind. Over half enrolled patients dropped out (n = 18) mainly due to increased severity of atopic eczema or ineffective treatment, no ITT analysis carried out |
| Larsen & Larsen, 1979206 | No statistically significant differences in the clinician's scores for any parameter | Unable to prove the new chromone drug to be effective in systemic treatment of atopic dermatitis | Method and concealment of randomisation unclear, study described as double-blind. No drop-outs. No results data given. Small sample over a short period of time |
| Larsen & Jacobsen, 1980195 | There were no statistically significant differences in the clinical assessments, in the patients' diary cards, or in the use of hydrocortisone cream. Eleven patients preferred the active period, while nine patients preferred the placebo period | This trial could not demonstrate any effect of chromone compound in systemic treatment of atopic eczema. Furthermore, the applied dose resulted in some dyspeptic adverse effects | Method and concealment of randomisation unclear, study described as double-blind. Three drop-outs, no ITT. No results data given. Small sample over a short period of time. Authors conclude: “Our first study [Larsen & Larsen206] gave some evidence that FPL 57787 might be effective in the treatment of [atopic eczema]”. However, it gave no evidence of benefit. The later study used three times (18 mg t.d.s.) the earlier dose of 6 mg t.d.s. |
| Lindskov & Knudsen, 1983210 | No significant differences between the two treatments in the patients' assessments | Unable to confirm the favourable results of DSCG in atopic eczema reported by others | Crossover trial with no washout period. Small study of 14 adults and ten children |
| Ventura et al., 1996211 | No difference in eczema score in the DSCG and placebo groups | The usefulness of dietary treatment is confirmed. Orally given DSCG does not seem capable of preventing a secondary sensitisation in patients with cows' milk protein allergy | Parallel group trial. Randomisation and blinding adequate |
| Study | Main reported results | Authors' conclusions | Quality |
|---|---|---|---|
| Ariyanayagam et al., 1985202 | Mean eczema severity score reduced significantly at 12 weeks compared with 3 weeks in patients on DSCG but not on placebo. The same effects were seen with daytime itch and night-time itch | Topical SCG as a long-term measure may be useful | Double-blind parallel group with randomisation. Short-term study of 12 weeks with an open label follow-up of 1 year |
| Croner et al., 1981200 | No significant group differences found except for less frequent use of steroids | Topical DSCG did not add to the drug's success in bronchial asthma and atopic eczema. Steroid-sparing effect could be worthwhile | Small short-term study. Parallel group randomised double-blind trial |
| Haider, 1977196 | Significantly more withdrew for lack of effect from the placebo than the DSCG arms (16/21 vs 4/21) | Safe alternative to topical steroids in the treatment of atopic eczema in children | Small short-term study. Parallel group randomised double-blind trial |
| Hiratsuka et al., 199684 | Equivalent to beclomethasone dipropionate in reducing eczema scores at 2 weeks | Both DSCG and beclomethasone diproprionate produced remarkable eczema improvement | |
| Kimata & Igarashi, 1990194 | Itch scores, eczema scores and sleep scores all improved by week 2 | Topical cromolyn solution was found to be very effective | Double-blind randomisation appeared adequate |
| Kimata & Hiratsuka, 1994201 | Itch scores, eczema scores and sleep scores all improved with DSCG but not with placebo | DSCG adds to the effect of oxatomide | Double-blind randomisation appeared adequate |
| Kjellman & Gustafsson, 1986209 | No significant change in itch scores or sleep disturbance reported | Topical DSCG did not relieve the patients' eczema | Double-blind randomisation appeared adequate |
| Moore et al., 1998198 | At 1-month crossover period, the group receiving DSCG first has a higher reduction in eczema scores than did those who received placebo first | Topical DSCG has a significant anti-inflammatory effect on moderate-to-severe atopic eczema | Crossover study with satisfactory blinding and randomisation |
| Pike & Atherton, 1988212 | No numerical data reported | No statistically significant effect between the active and placebo treatment | Letter |
| Thirumoorthy & Greaves, 1978199 | Letter | No significant difference between DSCG and placebo | Eight patients only. Few details given |
| Study | Stratum | No. of treatment patients | No. of control Patients | Mean difference | 95% CI |
|---|---|---|---|---|---|
| Hiratsuka et al., 199684 | 1 | 21 | 21 | 1.65 | 1.19 to 2.11 (DSCG in itch scores week 0 to week 2) |
| Hiratsuka et al., 199684 | 2 | 22 | 22 | 1.26 | 0.60 to 1.91 (beclomethasone week 0 to week 2) |
| Kimata & Igarashi, 1990194 | 3 | 25 | 201 | 0.47 | 0.47 to 1.53 (difference in itch scores DSCG minus placebo) |
Chapter 8 Dietary interventions
Dietary restriction in established atopic eczema
In addition to examining the role of dietary exclusion of possible allergenic foods during pregnancy or lactation with a view to preventing the development of atopic eczema, some RCTs have examined the role of dietary exclusions to improve the severity of established atopic eczema. No systematic reviews of dietary manipulation in established atopic eczema could be identified, although some of the published studies have been reviewed by Charman. 334 The eight RCTs examining the role of elimination diets in established atopic eczema are summarised in Table 19 (Atherton 1980 is the same paper as Atherton 1978). RCTs of dietary exclusion in the prevention of atopic eczema that have also estimated eczema severity in those developing eczema have already been discussed in Tables 3 and 4. In the three studies looking at dietary exclusion during pregnancy, 61,66,67 there was no difference in eczema severity between the intervention and control groups. In the studies looking at dietary exclusion during breastfeeding, one reported improvement in eczema severity in the intervention versus control group,53 while the other55 did not.
Summary of dietary restriction in established atopic eczema
-
None of the interventions and study populations were considered sufficiently similar to each other to warrant statistical pooling.
-
Elimination diets are difficult for families and patients to follow, even in the highly motivated environment of a clinical trial.
-
Drop-out rates are particularly high for elimination diets and those containing hydrolysate milk substitutes.
-
Those RCTs that employ a parallel design with an unblinded normal control diet risk biasing the motivation and ancillary care in favour of the active group.
-
Those studies that place all participants on exclusion diets and introduce the suspected offending food versus a control, risk introducing another allergen (e.g. soya) or introducing the suspected allergen (e.g. cows' milk) in an altered and controlled way that does not mimic real life.
-
Marked order effects suggest that the crossover study is not the best method of assessing the benefits of dietary exclusion.
-
There is little evidence to support an egg-and milk-free diet in unselected atopic eczema patients.
-
There is no evidence to support the use of an elemental- or few-foods diet in atopic eczema.
-
There is some evidence that the addition of a probiotic such as Lactobacillus may be beneficial for atopic eczema in those already on a cows' milk whey hydrolysate diet, though in the absence of a control group on no special diet it is hard to say if this is a real benefit.
-
There is some evidence to support the use of an egg-free diet in infants with suspected egg allergy who have positive specific IgE to eggs in their blood.
-
Methodological concerns such as poor concealment of randomisation allocation, lack of blinding and high drop-out rates without an intention-to-treat analysis suggest that the above studies should be interpreted with great caution.
-
Future studies should be longer term, more pragmatic and ensure that randomisation is concealed.
-
If participant blinding is not possible, objective outcomes such as photographic records viewed by independent blinded observers should be used.
Apart from elimination diets for the treatment of established atopic eczema, uncontrolled elimination diets followed by double-blind placebo-controlled food challenges with foods suspected to aggravate symptoms have also been tried in atopic eczema. 365–369 Although such blind challenges have sometimes been performed in random sequence, they are not the same as RCTs of food elimination. Instead they try to answer the question: ‘Does food X make a particular child's atopic eczema worse?’ The precise relationship between such food challenge studies and long-term benefits of exclusion of those suspected foods to atopic eczema sufferers is not clear. Up to 63% of selected children with atopic eczema exhibit one or more reactions (mainly in the skin) to foods in double-blind placebo-controlled challenges,370 though such reactions are lost after 1–2 years in 26–66% of patients. 371 Blood and skin-prick tests are usually only helpful in predicting clinical response if they are negative. 372,373 It should also be borne in mind that this high negative predictive value has only been shown in relation to provocation of symptoms after double-blind challenge and not clinical response following food elimination, which are not necessarily the same thing. The relationship between atopic eczema and ‘food sensitivity’, which is subdivided into food hypersensitivity, food intolerance and allergic adverse reactions to foods (immunologically mediated), is a complex one and readers are referred to a clear evidence-based work by David374 for further information.
Supplementation with essential fatty acids
Polyunsaturated fatty acids are essential components of all cell membranes. There are two families of such essential fatty acids: n-6 (e.g. linoleic and arachadonic acid) and n-3 (e.g. eicosapentanoic acid). Some of these substances are precursors of a group of substances called eicosanoids, which may play an important part in the inflammatory and immunological processes of atopic eczema. Alterations in linoleic acid metabolism have been demonstrated in some patients with atopic eczema, suggesting that a defect in the enzymatic conversion of this essential fatty acid by δ-6-desaturase might be responsible for defects in the lipid barrier of the skin, a decreased postnatal maturation of T-lymphocytes, and the decreased production of anti-inflammatory metabolites in the skin. These observations are the rationale for dietary supplementation with essential fatty acids in atopic eczema. Such supplementation includes evening primrose oil, containing 8–10% gamma-linoleic acid (GLA), and more recently borage oil (containing at least 23% GLA). Topical use of evening primrose oil has also been tried. Fish oils are particularly rich in n-3 fatty acids, and it has been suggested that these may compete with n-6 fatty acids in a way that might reduce the inflammatory components of atopic eczema.
We located five RCTs of oral borage oil supplementation, 246–250 four RCTs of fish oil supplementation, 251–254 four RCTs of topical evening primrose oil,232–234,241 two of which repeated the same study,233,234 and ten published RCTs of oral evening primrose oil235–240,242–245 (including one study published twice242,245) for the treatment of atopic eczema, and these are described in Tables 20–29, respectively.
Comment
Other and ongoing systematic reviews
A previous meta-analysis of nine placebo-controlled RCTs of evening primrose oil in 1989 conducted by the manufacturers concluded that evening primrose oil had a modest beneficial effect. 375 They included seven small unpublished studies, and these have not been made available within the public domain for others to evaluate their results and quality. They excluded the largest study by Bamford and colleagues240 in their main analysis as they implied that the investigators had allowed the active and placebo capsules to become mixed up on the basis of a subsequent blood analysis conducted within the company. These allegations have been denied by Bamford (Bamford J, personal written communication, 1996), and it is difficult to see how such a mix up could have occurred given that this was one of the best-quality reported studies with several measures in place to ensure concealment of allocation.
A further meta-analysis of 20 published and unpublished RCTs of evening primrose oil has been conducted by two of the authors of the current report376 in 1997 for the Department of Health, but permission to publish the data has not been granted.
There is currently an ongoing, more detailed systematic review of oral GLA supplementation being conducted by one of the authors within the Cochrane Skin Group, which hopes to publish its findings later this year. Pooling has therefore not been attempted in this scoping review. It is reasonable to consider pooling borage oil with evening primrose oil studies on the basis that the purported active agent is GLA.
Borage oils
Only one large well-reported RCT has evaluated borage oil in atopic eczema,249 and that study found no overall benefit or hint of benefit when the two main groups were compared. However, a subgroup analysis (proportion unspecified) of the best complying patients and those with changes in blood tests, suggested that there could be a benefit in this subgroup. The authors were rightly cautious in interpreting these post hoc findings, and in the absence of more detailed analyses, it is difficult to say how much of the positive effect was due to taking the capsules in the required dose and how much was due to the blood test changes. It is difficult to generalise from the subgroup data without knowing more about the poor compliance of some patients, as that itself may be a useful outcome measure of the pragmatic usefulness of the intervention. The subgroup analysis does however call for an RCT of people who are able to demonstrate an increase in GLA metabolites in the blood as an entry criterion. The four remaining RCTs were quite small, with two246–248 suggesting an improvement, and two247–250 suggesting none.
Fish oils
The two smaller RCTs of fish oils suggest some possible benefit in atopic eczema, and the magnitude of relative benefit was very large in the Gimenez-Arnau and colleagues study253 (though baseline data were not given). The largest and best reported independent study by Soyland and colleagues252 did not show any hint of difference in benefit between fish oils and placebo.
Topical evening primrose oil
The pilot study of 12 patients by Anstey and colleagues241 suggested a possible benefit of topical evening primrose oil for patient-assessed changes but not physician-assessed benefit. The success of blinding in that study is suspect as topical evening primrose oil is known to produce an odour on contact with the skin,377 and the placebo cream was different from the composition of the vehicle used with the active agent. The second study, which compared three increasing doses of GLA with placebo234 did not show any hint of a dose-response effect, but the sample size in each group was very small. The two studies of skin barrier function described by Gehring and colleagues232 do not provide any evidence of a useful clinical benefit for topical evening primrose oil above vehicle control at the end of the 4-week treatment period, regardless of the formulation.
Oral evening primrose oil
The two largest240,243 and best-reported studies did not show any evidence of benefit for evening primrose oil in atopic eczema. The remaining moderate-sized (between 50 and 100 patients) studies show conflicting results,235,237,238,245 ranging from no hint of improvement235 to a definite modest 10–20% benefit for some outcome measures when compared with placebo. The three small studies all suggest a benefit to evening primrose oil.
Pyridoxine
Pyridoxine (vitamin B6) is an essential water-soluble vitamin and is a core factor in many of the body's chemical pathways. Based on an earlier double-blind placebo-controlled study published in abstract form only,378 a larger and fully reported RCT was conducted by Mabin and colleagues255 of pyridoxine versus placebo in the treatment of atopic eczema in children with moderate-to-severe disease.
Benefits
Forty-one of the 48 children in this parallel-group RCT study were evaluable at 4 weeks. Median skin severity score increased from 92.3 at the beginning of the trial of the pyridoxine group to 109.0 at the end of the 4-week period. In the placebo group, the median skin severity score fell from 125.5 to 77.0 at the end of the treatment period. The difference between the median change in skin scores was 29.2 (95% CIs ranging from a benefit of pyridoxine of 19.5 to a benefit of placebo of +85.0). There was no statistical difference for skin severity score, daytime itch or nocturnal itch score. With regard to parental observation, 16% in both groups felt that overall the skin was better (p = 0.95).
Harms
No serious adverse effects were described in the study, though one child developed a non-specific erythematous rash while taking pyridoxine, and another taking placebo was reported to be much more itchy than usual.
Comment
This was a well-reported study with method of randomisation and allocation concealment and blinding clearly described. No intention-to-treat analysis was performed and no adjustment of the different baseline scores was made. Some of the significant improvement of the placebo group could partly be due to a regression to the mean phenomenon. The study did not provide any evidence to support any benefit of pyridoxine in the treatment of atopic eczema despite the earlier favourable report.
Vitamin E and multivitamins
Three very different RCTs256–258 have evaluated vitamin supplementation in atopic eczema. The first was an RCT reported by Czeizel and Dobo in 1994 of the Hungarian optimal family planning programme study looking at the effect of multivitamin supplementation around the time of conception and afterwards on postnatal development compared with the use of a tablet containing trace elements. 256 The other RCT257 was conducted to evaluate the possible benefit of supplementation with selenium and vitamin E compared with placebo in adults with atopic eczema. The rationale for this second trial was that reduced concentrations of selenium had been observed in whole blood of patients with atopic dermatitis. The final RCT was a study in Japan of vitamin E in combination with vitamin B2 compared with each vitamin separately. 258
Benefits
In the Czeizel study, data were available on 4122 pregnancies that ended in a live birth. Of these, 2090 were randomised to the ‘active’ multivitamin supplementation, compared with 2032 in the trace element supplementation group. A whole range of postnatal development factors were collected in the study, including a physical examination and study of medical records in 90% of the evaluated infants. At the end of the 17-month period, there were no significant differences in the occurrence of chronic diseases between the two groups, with the exception of atopic dermatitis and wheezy bronchitis. Fifteen out of 2090 receiving multivitamin supplementation had developed atopic eczema (four had a parent with atopic dermatitis) compared with four out of 2032 receiving trace element supplementation (none of these children's parents had atopic disease). The authors suggested that these unexpected findings may be a chance effect.
In the Fairris and colleagues study,257 60 adults with atopic eczema were randomised in a 12-week double-blind study to three groups taking either 600 μg of selenium alone, 600 μg of selenium plus 600 IU of vitamin E or a placebo. Using a severity assessment based on several skin signs at several body sites, mean severity score fell from 21.0 to 13.7 in the selenium only group, from 21.8 at baseline to 15.3 in the selenium plus vitamin E group, and from 20.4 to 14.5 in the placebo group. None of these differences were statistically significant. There was, however, a significant increase in concentration of selenium in whole blood of those taking selenium.
In the Hakakawa and Ogino study,258 59 participants with mild-to-moderate atopic eczema of the dry type were randomised to vitamin E (d-α-tocopherol) 100 mg plus vitamin B2 (riboflavin butyrate 20 mg), or vitamin E 100 mg or vitamin B2 alone for 4 weeks. Of the 49 evaluable participants, response as measured by physician-assessed overall usefulness and global rating, was greater in the combination vitamin group than in the single vitamin group.
Harms
The Hungarian study,256 in a sense, has detected a possible unwanted harm of an increase of allergic diseases in those given multivitamin supplements in early pregnancy. No adverse effects were reported in the studies of selenium and vitamin supplementation.
Comment
Although the Hungarian study is not really a therapeutic trial of an intervention in atopic eczema, it is nevertheless a large RCT that has found a statistical significant increase in atopic eczema and in wheezy bronchitis and asthma in those receiving multivitamin supplementation compared with trace element supplementation. The numbers of patients in these groups are very small and the results therefore may probably be due to chance. Nevertheless, these findings should be examined in other independent randomised birth cohort studies.
The study of selenium and vitamin E,257 while small, probably excluded moderate-to-large treatment effects. A method of randomisation was described (unusual for such an early study), though no intention-to-treat analysis was performed.
The short Japanese study258 on the use of combination of vitamin E and B2 is difficult to interpret in the absence of a placebo-controlled study of either compound. The validity of the study is also threatened by difficulties in blinding and post hoc subgroup analysis of dry skin subtypes at different time intervals.
Zinc supplementation
Oral supplements of zinc salts have become popular remedies for a range of unrelated medical disorders. They have been specifically recommended for the treatment of atopic eczema possibly because skin lesions are an important feature of zinc deficiency. One RCT conducted by Ewing and colleagues in 1991259 evaluated the possible benefit of oral zinc sulphate at a dose of 185.4 mg/day versus placebo in 15 children with atopic eczema aged 1–16 years for a total of 8 weeks.
Benefits
At the end of the 8-week period, the mean combined disease severity score increased from 36.1 at baseline to 48.7 in the zinc group compared with a change from 34.6 at baseline to 39.3 in the placebo group. Erythema score, surface area score and use of steroids, emollients and antihistamines were also almost identical in both groups and none were statistically significantly different.
Harms
No adverse effects were reported in this small RCT.
Comment
Despite the usual reservations on lack of description of the randomisation process and no intention-to-treat analysis, this small RCT illustrates the importance of an unbiased comparison of possible treatment benefits when compared with earlier enthusiastic claims of benefit. Even though the trial was quite small, the complete lack of treatment benefit along with small standard errors, argues against missing any moderate-to-large treatment effects.
Summary of dietary interventions
Essential fatty acid supplementation
-
The largest and best-reported study on the use of borage oil supplementation in atopic eczema did not suggest any overall benefit compared with placebo.
-
That study did suggest that a further RCT in those who are able to take high doses consistently and who have demonstrable changes in a blood test might be justified.
-
The largest and best-reported study on fish oil supplementation in atopic eczema did not show any benefit above placebo.
-
There is no good RCT evidence to support the use of topical evening primrose in atopic eczema, though it has never been put to the test in a large RCT.
-
The nine published RCTs that have evaluated the use of oral evening primrose oil have shown conflicting results.
-
The two largest and well-reported studies of evening primrose oil do not show any benefit over placebo.
-
The RCTs of GLA (evening primrose oil and borage oil) are the subject of an ongoing Cochrane Skin Group systematic review due to report later in 2000.
Pyridoxine
-
One well-supported RCT does not support any benefit of pyridoxine in the management of a child with atopic eczema.
Vitamin E and multivitamins
-
One large randomised trial of multivitamin supplementation in early pregnancy has suggested an unexpected increase in atopic dermatitis in children born to mothers randomised to multivitamins compared with trace elements. Although this is probably a chance finding, it needs to be looked at specifically in other similar cohort intervention studies.
-
One small trial of selenium and vitamin E supplementation in adults with atopic eczema failed to provide any evidence of a beneficial effect on clinical disease activity.
-
A Japanese study of short duration found that vitamin E in combination with vitamin B2 was more effective than either vitamin alone in the treatment of dry eczematous skin. The clinical significance of these results is difficult to interpret in the absence of a placebo.
Zinc supplementation
-
One RCT has failed to show any benefit of zinc supplementation in atopic eczema.
| Study | Interventions (co-treatments) | Study population and sample size | Trial design description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Atherton et al., 1978230 | Egg and cows' milk exclusion diet (with soya milk substitution) vs control diet with egg and cows' milk (hydrocortisone, emollients and antihistamines) | 36 children aged 2–8 years attending a dermatology clinic with clinically typical atopic eczema | Crossover design that examined three 4-week periods; during the first and third periods, patients were placed on an egg and mill elimination diet and randomly allocated to a soy-based preparation or one containing egg and cows' milk | Eczema area and activity using an unpublished composite score, degree of adherence to diet and skin-prick tests | Of 20 children who completed the trial, 13 showed an improvement during the trial diet period, six showed no change and one showed deterioration | Method of randomization and concealment unclear | Marked order effect i.e. improvements greater at end of first vs second period whatever the diet content |
| England | On control diet period, three showed improvement, 11 no change and six deteriorated | Study described as double-blind, though some unblinding of parents cannot be excluded | Soya milk (which itself can be allergenic in atopic eczema) used as ‘control’ food | ||||
| Itch was not statistically significant between the two groups | No ITT analysis and high (44%) drop-out rate | ||||||
| Munkvad et al., 1984229 | Elemental diet (amino acids, essential fatty acids, glucose, trace elements, sorbic acid and vitamins) vs a blended diluted diet of foodstuffs consumed by hospital inpatients (emollients, a topical steroid and antihistamines) | 33 adults with atopic eczema covering more than 10% of the body, 13 of whom had a history of intolerance to one or more food elements | Parallel group RCT of hospitalised patients on diets for 3 weeks | Various unpublished extent and intensity signs scored between –3 and +3, photographs before and after, patient itch and sleep, and various serum markers of inflammation | Of 25 evaluable patients, five out of 16 improved on the elemental diet compared with four out of nine on the placebo diet (NS) | Method of randomization and concealment unclear | History of food intolerance in patients not confirmed during study |
| Denmark | A ‘major activity’ score of > 100 was defined as the criterion for a positive response to treatment | Itch, sleeplessness, antihistamine use and immunological tests were no different between the two groups | Unclear if the reported ‘double-blinding’ was successful in view of the different composition of the two diets. | Small study of an intervention that is unpalatable, impractical and requires hospitalisation and dietetic input | |||
| No ITT analysis with a 24% drop-out rate | |||||||
| Cant et al., 1986228 | Exclusion of egg and cows' milk (with soya substitute) in mothers of infants with atopic eczema who were exclusively breastfeeding vs inclusion of egg and milk (topical steroids and emollients) | 19 mothers and babies with established atopic eczema | 12-week crossover study divided into three 4-week periods; during first two periods, mothers excluded cows' milk, egg and other foods from their diet and were randomised in first or second period for milk substitutes containing cows' milk and egg or soya | Combined area/intensity score (unpublished) with a max. score of 60 | Of 17 mothers completing the study, the activity scores decreased by 20% in four babies on soya and one on egg and milk | Method randomization described | Well reported, though very small study conducted alongside a before and after study |
| England | Normal diet in third period | No statistically different mean scores between the two groups | Concealment of allocation unclear | Soya used as control diet | |||
| Marked period effect in that children of mothers on normal diet in third period continued to improve | Study described as double-blind, though almost half mothers correctly identified substitutes | ||||||
| ITT analysis was attempted | |||||||
| Neild et al., 1986226 | Egg- and cows' milk-free diet (using soya as substitute) vs normal diet (topical steroids and antihistamines) | 53 unselected atopic eczema outpatients aged 1–23 years | Crossover trial with three 6-week periods; during first and third periods, patients were placed on an egg and cows' milk exclusion diet and randomised to either soya or a milk containing egg and cows' milk | Patient reported itch and sleep loss, use of co-treatments, composite score of area and intensity and skin-prick tests | Of 40 evaluable patients, there was little difference for change in score (area, itch co-treatment use) between the treatment periods and none were statistically significant | Method of randomisation and concealment of allocation unclear | High drop-out rate due to diet too difficult to adhere to |
| England | Study reported as ‘double-blind’, though test substances might have tasted differently | Unclear if there was a period or carry-over effect | |||||
| No ITT analysis and high (25%) drop-out rate | CIs suggested that if anything, patients did worse on the exclusion vs normal diet | ||||||
| Mabin et al., 1995227 | Children allocated to three groups: i) few foods diet (eliminating all but five to eight foods) plus whey hydrolysate; ii) few foods diet plus casein hydrolysate; or iii) remain on usual diet (antihistamines and topical steroids) | 85 children (median age 2.3 years) with atopic eczema which persisted despite conventional treatment and involving more than 12% of body | Parallel single-blind RCT with follow-up until 6 weeks | Skin severity score incorporating extent and severity, and parental record of itch, sleep loss and global improvement | Of 46 evaluable patients, 16 (73%) of the 22 controls and 15 (58%) of the 24 who received diet showed a greater than 20% improvement in skin severity score | Method of randomization clearly described | Good description of patient flow and interventions |
| England | Breast-fed children were excluded | Improvement in skin severity score in controls and daytime itch score in the whey hydrolysate group was statistically significantly in the 12 statistical outcome comparisons made | Concealment of allocation unclear | 35 out of 39 drop-outs were in the diet group, illustrating the difficulty of adopting the few foods diet in even a motivated hospital group | |||
| Study described as investigator-blind | No evidence to support benefit from diet and some evidence suggesting that control diet was better | ||||||
| No ITT analysis and very high drop-out rate (46%) | |||||||
| Isolauri et al., 1995231 | Children with atopic eczema allocated to whey hydrolysate vs an amino-acid derived formula containing no peptides | 45 children who were not being breast-fed, who had been fed substitute cows' milk for at least 6 months and who showed a positive reaction to a masked challenge with cows' milk | Parallel prospective randomised study drawing patients from an initial study to determine cows' milk allergy | Atopic eczema severity (extent, intensity of signs and symptoms) measured by the SCORAD system | Weight gain and infant length was statistically less in the whey hydrolysate group | Method and concealment of randomisation allocation unclear | Highly selected population |
| Finland | Children were followed-up for 8 months | Infants' growth was also measured | Eczema severity decreased from a SCORAD of 17 to 5 in 22 children on whey hydrolysate compared with a baseline of 21 to final score of 4 at 8 months in the amino acid group | Randomised part of the study probably not blinded | Study mainly concerned comparison of growth in amino-acid vs hydrolysate formulae | ||
| No drop-outs | Main statistical comparison of change in eczema severity between the two groups not reported in results, though children in amino acid group had higher baseline score | ||||||
| Majamaa & Isolauri, 1997224 | Cows' milk elimination (extensively hydrolysed whey formula) with and without a probiotic (Lactobacillus GG) | 27 infants with clinical history suggestive of cows' milk allergy who were confirmed as being sensitive to cows' milk by double-blind placebocontrolled challenge | Parallel randomised prospective study monitored for 1 month | Atopic eczema severity measured by SCORAD | Of 27 evaluable children, the median SCORAD at baseline was 21 and 26 in the whey alone vs whey plus probiotic groups, respectively; these decreased to 19 and 15, respectively, at the end of 1 month | Method and concealment of randomisation allocation unclear | Authors report statistical significance for the change in score from baseline to the end of the study separately for each intervention, but do not test the difference between the two treatments |
| Finland | 19% of the children also had gastrointestinal symptoms | No a priori statement of minimum clinically significant benefit | No blinding reported | Regression to the mean could have accounted for the greater improvement in the probiotic group | |||
| No drop-outs | |||||||
| Lever et al., 1998225 | Egg exclusion diet for young children as advised by a dietician vs general advice from a dietician only (mild-to-moderate topical steroids and emollients) | 62 children all with positive IgE blood antibodies to egg, only seven of which had a history suggestive of egg allergy | Parallel randomised prospective study of 4 weeks' duration | Eczema severity as assessed by extent in % terms and a composite severity score in 16 body sites | Of 55 evaluable children, the area involved by eczema reduced from 19.6% to 10.9% in egg-free group compared with 21.9% to 18.9% in control group (p = 0.02) | Method of randomization unclear | Study suggested that egg-free diet in those with a positive RAST test to egg may be useful |
| Scotland | Severity score reduced from 33.9 to 24.0 in the egg-free group compared with 36.7 to 33.5 in the control group (p = 0.04) | Randomisation performed by same dietician who was giving the intervention | Methodological concerns such as lack of randomisation concealment and increased motivation and ancillary care in intervention group could have resulted in bias | ||||
| Parents unblinded; assessor reported as blinded | |||||||
| No ITT analysis | |||||||
| Co-treatment use not reported |
| Study | Design | No. of patients | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawls and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Bahmer & Schafer, 1992246 | Parallel, RCT | 12 | 20–48 | 4 months | Mean ADASI score in borage and palm oil 1.63 and 1.89, respectively | Borage oil, two capsules containing 500 mg t.d.s. | Palm oil in similar dose | Unclear | Unclear |
| Borrek et al., 1997247 | Crossover, RCT | 24 | 3–17 | 14 weeks | Chronic eczema | Borage oil | Corn seed oil | Antihistamines, corticosteroids | Two |
| Buslau & Thaci, 1996248 | RCT | 50 | 12 weeks | Mild to moderate | 2 × 1 g borage oil | Placebo 2 × 1 g palm oil | Emollients | 18 | |
| Henz et al., 1999249 | Parallel, RCT | 160 | 14–65 | 24 weeks | Relatively stable and moderate | Borage oil, 500 mg three capsules daily | Miglyol lipid as placebo three capsules daily | Topical diflucortolone-21 valerate | 19 placebo drop-outs, 17 borage oil |
| Valsecchi et al., 1996250 | Parallel, RCT | 31 | 2–11 | 14 weeks | * | Borage oil, 500 mg capsule containing 80 mg GLA + linoleic acid + small amounts of palmintic acid, oleic and stearic acids | Placebo (liquid paraffin) | Unlimited emollient | One placebo, two borage oil |
| 15–38 |
| Study | Design | No. of patients | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawls and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Bjornboe et al., 1987251 | Parallel, RCT | 31 | 16–56 | 12 weeks | Unclear | Max-Epa fish oil (n-3) ten capsules daily | Olive oil | Topical steroids | Eight |
| Gimenez-Arnau et al., 1997253 | Parallel | 48 | Mean age 24.2 | 12 weeks | Chronic and severe | Eicosapentaenoic acid + docosahexaenoic acid (fish oil) vs linoleic acid (vegetable oil) | Placebo (oleic acid) | No other treatment permitted | * |
| Soyland et al., 1994252 | Parallel, RCT | 145 | 18–64 | 4 months | Moderate to severe | Six capsules daily fish oil | Corn oil as placebo | Antihistamines, hydrocortisone, emollients | * |
| Study | Design | No. of patients | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawls and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Anstey et al., 1990241 | RCT, within person right/left arm parallel | 12 | 4–46 | 14 days | Typical mild to moderate | Evening primrose oil cream in a water in oil emulsion | E45 cream™ | ‘Usual topical treatments outside test areas’ | One |
| Ferreira et al., 1998234 | Parallel, RCT | 23 | 3–15 | 4 months | In remission | Emollients containing 10% GLA vs borage oil (24% GLA) vs rose hip oil (35–40% GLA) | Atoderm™ emollient without essential fatty acids | None | Two |
| Gehring et al., 1999232 | Two within-person right/left forearm parallel studies | 20 in each study | 19–42 | 4 weeks | Atopy score of 10 or over | Study 1: Evening primrose in an amphiphilic oil in water emulsion | Study 1: Vehicle was 20% miglyol | None mentioned | One |
| Study 2: Evening primrose oil in a water-in-oil emulsion | Study 2: Vehicle was liquid paraffin |
| Study | Design | No. of patients | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawls and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Bamford et al., 1985240 | Crossover, RCT | 154 | 2–16 | 3 months | Active and using a topical steroid | < 15 years 2–4 capsules evening primrose oil b.d. | 500 mg liquid paraffin and 10 IU of vitamin E | Emollients, topical steroids, oral antihistamines | 31 drop-outs: 14 evening primrose oil, 17 placebo |
| 16–66 | > 15 years 6–8 capsules evening primrose oil b.d. | ||||||||
| Berth-Jones & Grahan-Brown, 1993243 | Parallel, RCT | 133 | 7–12 | 16 weeks | * | Epogam™ 500 mg (containing GLA) vs Efamol™ 107 mg (contains fish oil) | Placebo (olive oil) | Topical steroids, emollients, antihistamines | 21 drop-outs |
| 13–60 | |||||||||
| Biagi et al., 1994237 | Parallel, RCT | 51 | 2.2–8.5 | 8 weeks | * | High-dose evening primrose oil 0.5 g/kg/day | Placebo olive oil = 10 mg vitamin | Weak topical steroids, emollients | Three drop-outs |
| Low-dose evening primrose oil 50% mix 0.5 g/kg/day + placebo capsules | |||||||||
| Bordoni et al., 1987239 | Parallel, RCT | 24 | 2–4 | 4 weeks | * | Efamol, 0.5 g/day | Olive oil placebo | Weak steroids, emollients | Not mentioned |
| Hederos & Berg, 1996235 | Parallel | 60 | 1–16 | 16 weeks | Need regular topical steroids | Epogam, 500 mg evening primrose oil, 40 mg gammalinolenic acid, 10 mg Vitamin E | Placebo 500 mg sunflower + 10 mg vitamin E | Usual treatment allowed, steroid/antihistamines | Two from evening primrose oil group |
| Humphreys et al., 1994238 | Parallel | 58 | Adults | 16 weeks | Moderately severe | Evening primrose oil 500 mg + vitamin E 10 mg 12 x daily | Liquid paraffin 300 mg with 10 mg vitamin E | Topical steroids | Six: four placebo and two active |
| Lovell et al., 1981244 | Crossover | 32 | 1.5–13 | 3 weeks each | Atopic eczema for at least 6 months | Efamol, (500 mg evening primrose oil + 45 mg gamma-linolenic acid) | Liquid paraffin | Mild topical steroids | Not mentioned |
| 14–32 | Adults – four capsules b.d., | ||||||||
| Children – two capsules b.d. | |||||||||
| Schalin-Karrila et al., 1987236 | Parallel, RCT | 25 | 19–31 | 12 weeks | Moderate to severe | Evening primrose oil (360 mg linoleic acid, 50 mg oleic acid, 45 mg gamma-linolenic acid) four capsules b.d. | Placebo 500 g liquid paraffin | Emollient, oral antihistamines, mild topical steroids | One evening primrose oil; not mentioned in placebo group |
| Wright & Burton, 1982242 | Crossover | 99 | 0.8–11 | 12 weeks | Moderate to severe | Efamol (360 mg linoleic acid, 45 mg gamma-linolenic acid divided into three different doses for adults and two different doses for children) | Placebo 500 mg liquid paraffin | Mild topical steroids, emollients, oral antihistamines | 16 adults, three children |
| 15–58 |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing/weeping | Oedema | Inflammation | Crusts | Infiltration | Induration | Patient itch | Doctor itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bahmer & Schafer, 1992246 | |||||||||||||||||||||||||
| Borrek et al., 1997247 | • | • | • | • | • | • | • | • | • | • | Multiple area | Costa | |||||||||||||
| Buslau & Thaci, 1996248 | • | • | • | • | • | ||||||||||||||||||||
| Henz et al., 1999249 | • | • | • | • | • | • | • | • | • | • | Multiple area | Costa | |||||||||||||
| Valsecchi et al., 1996250 | • | • | • | • | • | • | • | • |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing/weeping | Oedema | Inflammation | Crusts | Infiltration | Induration | Patient itch | Doctor itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
| Bjornboe et al., 1987251 | • | • | • | • | • | • | • | ||||||||||||||||||
| Gimenez-Arnau et al., 1998253 | • | RoNAA | Ra jka | ||||||||||||||||||||||
| Soyland et al., 1994252 | • | • | • | • | • | • | • |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing/weeping | Oedema | Inflammation | Crusts | Infiltration | Induration | Patient itch | Doctor itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Topicals | |||||||||||||||||||||||||
| Anstey et al., 1990241 | • | • | • | • | • | • | • | • | |||||||||||||||||
| Ferreira et al., 1998234 | • | • | |||||||||||||||||||||||
| Gehring et al., 1999232* | |||||||||||||||||||||||||
| Orals | |||||||||||||||||||||||||
| Bamford et al., 1985240 | • | • | • | • | • | • | • | ||||||||||||||||||
| Berth-Jones & Graham-Brown, 1993243 | • | • | • | • | • | • | • | • | • | • | • | • | RoNAA | Leicester/Costa | |||||||||||
| Biagi et al., 1994237 | • | • | • | • | • | • | • | • | |||||||||||||||||
| Bordoni et al., 1987239 | • | • | • | • | • | • | • | • | • | ||||||||||||||||
| Hederos & Berg, 1996235 | • | • | • | • | • | • | • | • | • | ||||||||||||||||
| Humphreys et al., 1994 | • | • | • | • | • | • | |||||||||||||||||||
| Lovell et al., 1981244 | No data available | ||||||||||||||||||||||||
| Schalin-Karrila et al., 1987236 | • | • | • | • | • | ||||||||||||||||||||
| Wright & Burton, 1982242 | • | • | • | • | • | ||||||||||||||||||||
| Study | Main reported results | Authors' conclusions | Quality/comment |
|---|---|---|---|
| Bahmer & Schafer, 1992246 | Using within-patient change in ADASI score, five out of seven patients treated with borage oil showed a favourable effect compared with one out of five treated with palm oil | Positive effect of borage oil in atopic eczema warrants a larger study | Pilot study awaiting full translation. Novel use of time-series data to analyse within-patient changes in ADASI score |
| Borrek et al., 1997247 | After 10–14 weeks of treatment there was no improvement of the eczema under active compared with placebo. Both groups showed improvement while taking placebo. This result could be seen in the objective investigations (Costa score, three times per treatment period) as well as in the daily patients documentation. The patients whose eczema has improved under borage oil (n = 10) had no special characteristics, so that authors could not identify any responder-type | There was no improvement of the eczema under borage oil group compared with placebo | Small study, which showed no difference between active drug and placebo. Awaiting translation for methodological quality |
| Buslau & Thaci, 1996248 | Of the 32 evaluable patients, 14 out of 18 patients (78%) in the borage oil group compared with six out of 14 (43%) patients in the palm oil group showed a significant improvement in ADASI score compared with baseline | Borage oil showed good effects on the course of mild-to-moderate atopic eczema | Awaiting full translation. No ITT analysis carried out and large drop-outs. Unclear what a ‘significant improvement’ meant to patients in terms of magnitude of response |
| Henz et al., 1999249 | The reduction in Costa score points was similar in the placebo- and borage oiltreated groups, though improvement of individual symptoms over placebo was observed for erythema, vesiculation, crusting, excoriation, lichenification, and insomnia, but not for pruritus (no data given). No statistically significant differences were noted between the two treatment groups regarding the primary efficacy criterion ‘corticosteroid dosage until response’ (p = 0.8949). Significant benefit shown in a subgroup of ‘good compliers’ | This study shows no overall efficacy of gamma-linolenic acid-containing borage oil in atopic eczema, with steroid use being the primary response parameter, though it suggests that a subgroup of patients may benefit from this well-tolerated treatment | Method and concealment of randomisation not stated, study described as double-blind, success of blinding not recorded. No ITT analysis. Authors state that all previous evening primrose oil studies look at 8–10% gamma-linolenic acid, whereas borage oil looked at 23% gamma-linolenic acid concentration. Significant effect shown in subgroup (post hoc) of best compliers and whose blood changed. No overall difference in main comparison |
| Valsecchi et al., 1996250 | There was no statistically significant difference (p = 0.165) between the mean reduction from baseline clinical score of the placebo (48.4) and the GLA group (70.8). Mean baseline score was higher in the GLA group at 281.0 compared with 251.3 in placebo | Dietary supplements of GLA resulted in a significant improvement in the clinical conditions of atopic eczema compared with baseline; however, a simultaneous improvement of the clinical status, compared withy baseline, was observed with placebo, and there was no significant difference between the two treatments | Method and concealment of randomisation not stated, blinding not stated, no ITT. Published as a letter only. No difference between the two groups, but study under-powered to detect modest benefits |
| Study | Main reported results | Authors' conclusions | Quality/comment |
|---|---|---|---|
| Bjornboe et al., 1987251 | The total patient's symptom score showed significantly greater improvement in the experimental group compared with control group; mean change 11.3 and 1.3, respectively, baseline scores not given (p < 0.02). The physician-assessed scores showed no statistically significant difference between the groups | Results favoured the experimental group with regard to scale itch and overall subjective severity compared with the controls | Method and concealment of randomisation unclear, study described as double-blind. Eight withdrawals and drop-outs, no ITT analysis carried out. Discrepancy of outcomes between patients and physicians. Multiple outcomes |
| Gimenez-Arnau et al., 1998253 | Only 6-week results presented for all three groups due to high drop-out rate in vegetable oil and placebo groups. This showed a 75% reduction in median Rajka scores in the fish oil group compared with 5.3 in the placebo and 8.8 in the vegetable oil groups (p < 0.001). Baseline scores not given | Linoleic acid is useful to treat atopic eczema | Method and concealment of randomisation unclear, study described as double-blind. Very scant methods and results data. No mention of withdrawals or drop-outs |
| Soyland et al., 1994252 | The mean clinical score for the six parameters evaluated the physicians showed an improvement from 4.4 to 3.1 (30%, p < 0.001) in the fish oil group, and from 4.2 to 3.2 (24%, p < 0.001) in the corn oil group. No significant differences between the two groups for any outcome | There was a progressive significant improvement of the clinical condition in both groups, compared with baseline scores. However, there was no significant difference between the two groups, which causes the possibility of a placebo effect | Method and concealment of randomisation unclear, study described as double-blind. Twenty-four withdrawals/drop-outs, no ITT analysis carried out. Large study with no hint of any difference of response between the two groups |
| Study | Main reported results | Authors' conclusions | Quality/comment |
|---|---|---|---|
| Topicals | |||
| Anstey et al., 1990241 | Analysis of results revealed a significant difference between the two groups in the mean absolute change in patient scores over the 14-day period (p = 0.006) and also in percentage change over 14 days (p = 0.021). In both cases the change was positive, indicating improvement in eczema and that evening primrose oil was the better cream. There were no significant differences for change in doctor's assessment | Topical evening primrose oil has potential as treatment for atopic eczema and warrants further clinical studies | Method and concealment of randomisation unclear, study described as double-blind. Marked discrepancy between patient and doctor assessment may suggest unblinding. One drop-out, no ITT analysis carried out. A very small sample over a very short time of only 2 weeks, but acknowledged as a pilot study |
| Ferreira et al., 1998234 | Clinical assessment of xerosis and pruritus revealed improvement in all four groups, slightly more pronounced in the three GLA groups. None of the changes statistically significant | GLA-containing emollients can be a useful improvement in the management of atopic eczema patients | Method of concealment of randomisation unclear, no mention of blinding. Two drop-outs/ withdrawals, no ITT analysis carried out. To be included, eczema had to be in remission, those who had eczema flare became failures. No hint of a dose/benefit between the different concentrations of evening primrose oil |
| Gehring et al., 1999232 | In study 1, which compared oil-in-water evening primrose oil emulsion to vehicle, barrier function assessed in various ways improved in both groups equally. In study 2, which compared a water-in-oil evening primrose oil emulsion to a different vehicle, the authors claimed that there was evidence of a stabilising effect of the active preparation above vehicle, yet the graphs for graphs for skin hydration and transepidermal water loss and irritation potential do not suggest any clinical or statistically differences at the end of the 4-week study | That the choice of vehicle is an important factor in the efficacy of evening primrose oil | Method and concealment of randomisation not described. No ITT analysis. Described as double-blind. This study described two different studies. In study 1, an evening primrose oil-in-water emulsion was compared with vehicle in a right/left forearm comparison in 20 participants, and in study 2, an evening primrose oil water-in-oil emulsion was compared against a different vehicle in 20 different participants. The authors then make inferences about one emulsion compared against the other without any direct data to support this. The authors' conclusions are not supported by their data. The study shows the general improvement of barrier function that occurs with oil applied to the skin, but provides no evidence of efficacy of evening primrose oil above vehicle |
| Orals | |||
| Bamford et al., 1985240 | No significant effect on erythema, scale, excoriation, lichenification, or overall severity in 123 patients with atopic eczema of average severity while they took oral doses of evening primrose oil (2 or 4 g in children, 6 or 8 g in adults). Actual data shown graphically in four figures | Evening primrose oil had no significant effect on the lesions of patients with a diagnosis of atopic eczema and lesions of average severity | Method and concealment of randomisation unclear, study described as double-blind. Thirty-one drop-outs, no ITT analysis carried out. Study very clearly written up, good information on how many patients were approached and how compliance was checked. Later correspondence by company accused authors of mixing up tablets |
| Berth-Jones & Graham-Brown, 1993243 | At 16 weeks, the mean (SEM; no. patients) improvements in Leicester scores were 8.48 (2.85; 33) for patients on Epogam, 2.54 (2.89; 35) for patients on Efamol marine, and 7.15 (2.88; 34) for those on placebo. On neither active regimen was mean improvement significantly different from placebo at 16 weeks (p = 0.74 for Epogam, 0.26 for Efamol marine) | Our study, which avoided the methodological and analytical problems of previous studies, found no effect of essential fatty acid supplementation in atopic eczema | Method and concealment of randomisation unclear. Study described as double-blind. No ITT (21 drop-outs).Well-reported study otherwise. No improvement in evening primrose oil or Efamol marine singly or combined, similar in children and adults |
| Biagi et al., 1994237 | There was a trend towards improvement in the low-dose group, which approached significance (p = 0.077) and a significant improvement in the high-dose group compared with placebo (p = 0.046) for overall physician-rated severity. There were no significant changes for the symptoms of itch and for the extent of disease in the evening primrose oil group compared with placebo | The overall severity of atopic eczema improved significantly on a high dose of evening primrose oil compared with placebo, independent of whether the children had manifestations of IgE-mediated allergy | Randomisation and concealment not stated, blinding not elaborated/tested for. No ITT analysis, three drop-outs. Benefit only in higher-dose group and for one out of three main outcome measures regardless of whether children were atopic or not |
| Bordoni et al., 1987239 | After 4 weeks, the symptoms of patients treated with evening primrose oil significantly improved (p < 0.01), in placebo-treated children the clinical status remained largely unchanged. No summary data of magnitude of benefit given, but can be visualised in figure | Evening primrose oil substantially improved the clinical symptoms of atopic eczema in two-thirds of the treated children after 4 weeks of therapy | Method and concealment of randomisation unclear,‘doctor unaware of which patients receiving which treatment’ suggests single-blind study. Drop-outs not mentioned, presume ITT analysis. Evening primrose oil suggested benefit, very short-term study. High-dose capsules for children |
| Hederos & Berg, 1996235 | Both groups of patients were substantially improved with respect to baseline but no significant differences between Epogam and placebo groups were observed. The mean % improvement from baseline for patient global assessment was 10.0 and 7.1% for Epogam and placebo, respectively. The corresponding % improvement for physician-assessed global improvements were 11.0 and 13.8% for evening primrose oil and placebo, respectively | Study demonstrated significant improvements in atopic eczema during the 16 weeks' treatment, but no significant difference was found between active and placebo treatment in a group of children who need regular treatment with topical steroids | Method and concealment of randomisation unclear. Study described as double-blind. Well described study. No size differences between two groups. ITT analysis carried out, two withdrew in evening primrose oil group |
| Humphreys et al., 1994238 | Twenty-three out of 27 patients taking active treatment showed an improvement in their clinical score for erythema by the end of the treatment period compared with 11 out of 23 in the placebo group. The results for surface damage were very similar, 12 out of 23 in the placebo group showing an improvement in clinical score, compared with 23 out of 27 in the GLA group. No benefit for lichenification | Adjunctive treatment with gamolenic acid in evening primrose oil should be considered in patients with chronic atopic eczema | Method and concealment of randomisation unclear, blinding unclear. No ITT analysis carried out, (six drop-outs) good description of drop-outs though. Statistics well described. Well-described study but three groups a little confusing. Baseline severity very different in GLA group than placebo but this was adjusted in analysis |
| Lovell et al., 1981244 | Doctor assessment baseline 6.26 (±0.24) reduced to 5.27 (±0.38) after evening primrose oil and 5.64 (±0.38) after placebo. Patient assessment baseline 5.96 (±0.16) reduced to 5.02 (±0.37) after evening primrose oil and 5.54 (±0.38) after placebo | Patients receiving Efamol showed a modest but significant improvement on both the doctor's and their own assessment | Method and concealment of randomisation unclear, study described as double-blind. Clinical significance of a change in score from 5.96 to 5.02 not clear. Possibly not done correct statistical test on differences between scores |
| Schalin-Karrila et al., 1987236 | In the evening primrose oil group, a statistically significant improvement was observed in the overall severity and grade of inflammation (p < 0.001) from baseline and a significant reduction in the surface area involved as well as in dryness and itch compared with baseline (p < 0.01). Patients in the placebo group showed a significant reduction in inflammation compared with baseline (p < 0.05). Unclear if there was a comparison of change in clinical scores between the two groups | Thus in every clinical parameter the degree of improvement was significantly greater in the evening primrose oil group than in the placebo group | Random method and concealment method not mentioned, success of blinding not recorded, yet possible that placebo group could have bowel problems given they had 4 g of liquid paraffin daily. No ITT analysis (one from evening primrose oil, not mentioned in placebo group).The evening primrose oil group started off more severe. Authors concluded that evening primrose oil superior for global severity, inflammation, dryness, itch |
| Wright & Burton, 1982242 | In the low-dose groups itch was the only symptom that responded better to evening primrose oil than placebo. In the high-dose groups the patient assessments showed that the evening primrose oil was significantly superior to the placebo with regard to itch (p < 0.003), scaling (p < 0.002), and general impression of severity (p < 0.01). The doctor assessments also showed a beneficial effect of the active treatment on the overall severity of the condition (p < 0.002).The other symptom scores showed the same trend but failed to reach statistical significance | Various doses of oral evening primrose oil in 99 patients with atopic eczema showed that the preparation produced a significant clinical improvement when taken in high dosage | Random method and concealment method not mentioned, success of blinding not recorded. No ITT analysis, 16 adults and three children dropped out. Only itch improved in low-dose groups whereas most improved in high-dose groups. Multiple significance tests. Published separately twice |
Chapter 9 Non-pharmacological treatments
House dust mite reduction
There is strong circumstantial evidence that house dust mite antigens are an important precipitating factor for atopic eczema. 267 The presence of immune sensitisation and allergic reactivity to house dust mite in the majority of atopic eczema patients, the fact that cutaneous patch tests with house dust mite extract produces an eczematous reaction in which allergen-specific helper T-lymphocytes are found, the improvement of atopic eczema when sufferers are removed to low house dust mite environments, and the exacerbation of existing atopic eczema areas following direct application of house dust mite extract,379 all argue for a possible role of house dust mite allergen in atopic eczema. There are many methods for attempting to reduce house dust mite levels in the home including the use of mattress and pillow covers that are impervious to house dust mites, frequent vacuum cleaning (with or without high performance filtration), and use of acaricidal sprays. It has been argued that measures such as sprays (e.g. benzyl benzoate or permethrin) which only kill mites380 are not effective as a sole treatment because the allergenic faeces and dead mites are still present. 267
In chapter 13 (Treatments with no RCTs), it is pointed out that no RCTs on house dust reduction as the sole therapy for prevention of atopic eczema could be identified. A total of five RCTs evaluating the role of house dust mite reduction in the treatment of established atopic eczema were identified. 260–263,267 Two further studies of changing housing environments by Sanda and colleagues381 and Fukaya and colleagues382 were excluded as the intervention groups were not randomised. Another two studies described as prospective randomised studies383,384 were excluded as they did not involve any randomised therapeutic intervention comparisons.
Benefits
The first small RCT by Colloff and colleagues262 evaluated the daily use of natamycin (a spray used to kill house dust mites) versus matched placebo spray with and without vacuum cleaning in a parallel group study for 4 months in 20 young adults with atopic eczema. They demonstrated that it was the vacuum cleaning and not the natamycin spray that had a significant impact on reducing house dust mite numbers. There was no significant clinical improvement in those who had been allocated to natamycin versus placebo. The mean symptom score (maximum score 288) in the natamycin and vacuum group changed from 55.2 at baseline to 38.6 at 4 months compared with 45.2 to 35.8 in the group with no natamycin and no daily vacuuming.
A second small, but important RCT was conducted by Tan and colleagues263 in 1996 with duplicated publication in 1998 and again in 1999. Tan and colleagues randomised 60 patients (30 adults and 30 children) for a total of 6 months to an intensive dust mite eradication regimen comprising Gore-Tex® (Intervent, UK) bedding covers, benzyltannate spray to kill mites and denature their allergens, and a high filtration vacuum cleaner, or to a control group of plain cotton bedcovers, placebo spray and a standard upright vacuum cleaner with a poor filtration performance. One trained nurse applied the bedcovers and spray each week, and participants were encouraged to vacuum bedrooms daily. They showed a dramatic and very similar reduction in concentration of house dust mite major allergen (Der p1) in bedroom carpets in both the active and placebo treatment groups at the end of 6 months. Disease activity, as recorded in terms of surface area involvement and a composite severity score (maximum score 108) measured at one point at the end of the 6 months, reduced by a small amount in both groups, but more so in the active group. The mean reduction in scores for the active and placebo groups were 12.6 and 4.2 units, respectively. Those in the active treatment group were more severe to begin with, and so an analysis of covariance was conducted to allow for baseline scores and initial house dust mite antigen levels. This showed a mean difference of 4.2 in change of score (95% CI 1.7 to 6.7 units; p = 0.008) between the two treatments. Further analysis also suggested that it was changes in the mattress and carpet dust in the bedroom that mediated much of the treatment effect. Subgroup analysis suggested that only children had a clinically and statistically significant benefit, and that there was no correlation between clinical improvement and positive skin prick tests at the study outset.
Another small study in Japan by Endo and colleagues261 evaluated the potential benefit of intensive vacuum cleaning in the rooms of 30 children with atopic eczema for a total of 12 months. Both groups were visited every 3 weeks by a team of mite specialists who either cleaned room floors, mattresses and quilts very thoroughly and encouraged parents to clean in the same way in-between visits, versus a less intensive clean (vacuum suction power reduced to 50%) with similar cleaning in-between visits. Parents were thus unblinded to the intervention. A statistically significant decrease in mite numbers in favour of the intensive cleaning group was only noted for the room floors. Clinical scores, as evaluated by a physician blind to treatment allocation, were significantly improved in the active group compared with baseline but not in the control group. Clinical scores were given in graphical form only and the appropriate statistical test of mean difference between the two treatments was not reported.
Another unblinded RCT by Nishioka and colleagues260 evaluated the benefit of encasing quilts and mattresses in microfine fibres versus simple cleaning measures alone in 57 Japanese infants with atopic eczema who were not allergic to house dust mite as determined by blood tests at the study outset. After 1 year, they found that 31% of children in the encasement group compared with 63% in the control group had serological evidence of house dust mite sensitivity (p < 0.02). The authors did not report any outcomes on atopic eczema disease activity in that paper. Correspondence with the authors suggests that there were no differences between the two groups at the end of the study for the clinical outcomes, though there was a reduction in topical corticosteroid requirement in the intervention group.
Harms
None of the studies reported any adverse events of the anti-house dust mite treatments. This does not necessarily imply that none occurred. The imposition of daily vacuuming for a long period has a cost in terms of time for parents and sufferers, as does the purchase of a high filtration vacuum cleaner, impermeable mattress covers and mite sprays.
Comment
It is a pity that so few studies on house dust mite avoidance have been performed on atopic eczema. Those that have been done tend to be small and difficult to generalise in the absence of more pragmatic studies. In none of the studies was the method of randomisation and concealment reported, and no intention-to-treat analyses were performed (though drop-outs were quite low). The validity of blinding in the Tan study in unclear due to the use of very different vacuum cleaners. It is also unclear which method of reducing house dust mites is the most efficient as studies have tended to use several measures at once. Data from the Tan and colleagues263 study suggest that just vacuuming with an ordinary household cleaner achieves similar and massive reductions of house dust mite antigen levels in bedroom carpets to those achieved by the more expensive high filtration vacuum cleaners that are advertised in patient support group magazines. The Tan and colleagues study suggests a definite benefit for a range of intensive measures to reduce house dust mite levels around the home, but the clinical relevance of the small changes in scores observed is difficult to determine in the absence of patients' evaluations or outcome measures that capture the chronicity of disease over the entire 6-month period. The Endo and colleagues261 study suggested some clinical benefit with frequent intensive vacuum cleaning, but this could not be related to reduction in house dust mite numbers. On the basis of the Tan study, the Endo study could be criticised for comparing two active treatments, and that a comparison of frequent vacuuming versus ‘normal cleaning’ would have been better given the fact that any form of active intervention for intensive or high-filtration vacuuming is difficult to blind.
Both Tan and Endo are to be commended for conducting such long-term studies. Further such studies in other populations, separating the different interventions for reducing house dust mite, are needed. It is important that such trials are as pragmatic as possible to determine which groups respond best, which interventions are the most cost-effective and whether the laborious interventions are sustainable in less-motivated people.
House dust mite hyposensitisation
Hyposensitisation refers to the technique of trying to induce an immunological and clinical tolerance to allergens that might be playing a role in allergic disease by repeated and progressive exposure to increasing amounts of allergen, as is performed for example, in hay fever desensitisation. Three RCTs that evaluated the role of desensitising atopic eczema patients to potential causative allergens were located. 264–266 Another study385 was excluded as it was unclear whether participants were randomised.
Benefit
The first small study by Glover and Atherton264 evaluated the use of a tyrosine-adsorbed extract of house dust mite in 26 children with atopic eczema who were house dust mite positive on skin-prick testing. In the first part of the study, children were randomly allocated to weekly active or placebo injections for 8 months. Clinical scores improved dramatically in both groups, but there were no obvious differences between the groups. In the second part of the study, the 13 children who had been allocated active treatment in the first part of the study were offered a further 6-month period of monthly injections. The seven who accepted were then randomly allocated to receive active treatment or placebo. Redness and skin thickening scores (but not surface damage) deteriorated more in the control group and these differences were statistically significant.
Another study by Galli and colleagues265 looked at the possible benefit of a mixture of house dust mite allergens given in an oral suspension three times weekly in children with atopic eczema who were sensitised to house dust mite. Three groups were compared, one non-randomised group of children with concurrent asthma and or rhinitis (n = 26), and two groups with exclusive atopic eczema who were randomised to oral hyposensitisation (n = 16) or no specific treatment other than ‘conventional therapy’ and measures to reduce house dust mite (n = 18). Comparison of change in clinical scores between the two randomised groups did not reveal a statistically significant or clinically relevant improvement in the active group.
A further study by Wen and colleagues266 was conducted in Shanghai with allergenic extracts of house dust mite manufactured at that university. In that study, 56 patients with atopic eczema (mean age 24.8 years) were randomly allocated to weekly injections of local allergenic extract (18 patients), a partially purified extract (20 patients) and normal saline placebo (18 patients) for 1 year. Clinical scores (unspecified) were reduced in all three groups and possibly more so in the two active groups, though no statistical tests were performed. The data was presented in graphical form only.
Harms
Apart from local discomfort at injection sites (similar in each group), no adverse effects were reported in either small study. Caution is needed based on the rare but potentially life-threatening hazard of an anaphylactic reaction when desensitisation has been done with bee sting or hay fever allergy. Wen and colleagues state in their paper that allergenic extracts of house dust mite have been used for 20 years for treating and diagnosing mite allergy in China and that there have been no recorded deaths due to anaphylaxis. Weekly injections for children are also painful and require attendance at a healthcare facility.
Comment
It is possible that the lack of statistical significance in the Glover study was due to lack of power or a large placebo effect due to the injections. Improved treatment concordance and ancillary care could also explain the impressive improvements in those having placebo injections. Similarly, the lack of obvious benefit in the Galli study could be due to inadequate numbers, other concurrent treatments such as reduction in house dust mite measures, or that the oral allergen hyposensitisation therapy was inactive via the gut route. It is difficult to make any further judgement on the Wen paper due to the scant methodological details provided.
Avoidance of enzyme-enriched detergents
Detergent enzymes may cause skin irritation and occasionally hypersensitivity reactions leading some physicians to advise atopic eczema patients to avoid the use of such detergents in favour of alternative ‘non-biological’ detergents. 386 The authors located one RCT that tested the hypothesis that enzymecontaining detergents are more likely to aggravate atopic eczema than a non-biological detergent. 268
Benefits
After a 1-month washout using their normal detergent, 26 adults with mild-to-moderate atopic eczema (mean age 25 years) were randomised in a double-blind crossover study to receive either a trial detergent containing enzyme concentrations reflecting the highest quantity in commercial enzyme-enriched detergents or a visually identical detergent without enzymes as control, for a 1-month period followed by a further month with the opposite detergent. Topical steroids were permitted during the study and weighed. In the 25 patients completing the trial, there was no hint of difference between the active detergent and the control in terms of SCORAD score (29 on active, 29 on control, with 95% CIs for the mean difference extending from –4 to +5 on a scale of 108), usage of topical steroid (44 g/month in active versus 43 g/month on control), patient-reported itch (1.3 versus 1.3), or patient-reported eczema activity (1.4 versus 1.4).
Harms
None of the patients had contact dermatitis to enzymes when patch tested at the end of the study, and there was no evidence of specific blood IgE against any of the enzymes.
Comment
Although this study was small, the virtual absence of any differences between the enzyme and nonenzyme detergents and the corresponding narrow CIs provide convincing evidence of a lack of harmful effect. The study was not sponsored by industry.
Benefit from specialised clothing
Intolerance to wool is frequently reported in atopic eczema patients and has been used as a minor criterion for diagnosing this condition. We found three RCTs269–271 evaluating clothing material in atopic eczema, two of which, by Diepgen and colleagues in 1990 and 1995,269,270 evaluated the irritative capacity of poncho-like shirts made of four different materials (cotton versus other synthetics of different fibre structure). The other RCT by Seymour and colleagues271 evaluated the clinical effects of different types of nappies on the skin of normal infants and infants with atopic eczema in a 26-week trial.
Benefits
In the Diepgen 1990 study,269 55 patients with atopic eczema were compared with 31 control patients without atopic eczema and randomised to one of four poncho-like shirts of varying fibre roughness. The intensity of itching or discomfort due to repeated wearing of these shirts was evaluated by means of a points system, whereby 10 equals a maximum comfort and 1 equals maximum discomfort. At the end of the study, those wearing cotton reported a comfort score of 8.4 compared with 7.3, 3.6 and 3.3 for the other textile shirts in increasing order of weight and fibre roughness (estimated from graph). The difference between the cotton and other fibres was significant only for the latter two groups.
The 1995 study by Diepgen and colleagues270 (published in a German textile journal) evaluated seven different garments on 20 atopic eczema patients with mild-to-moderate disease (average age 25.3 years) with and without a ‘sweat test’ designed to lower the itch threshold. The garments included cotton, and polyester garments made with different fibre roughness, yarn roughness and fabric weaves. The study was a randomised crossover study (Diepgen T, personal oral communication, January 2000), with each garment worn under standardised conditions on 4 consecutive days. Comfort, as assessed on a visual analogue scale, was statistically significantly higher for warp-knits compared with jersey knits, but no different for cotton and polyester of fine fibre construction (assessed by scanning electron microscopy). Garment comfort in all groups was reduced after sweating.
In the Seymour study,271 cloth nappies were compared with cellulose core nappies versus cellulose core nappies containing absorbent gelling material in 85 babies aged less than 20 months of age who had atopic eczema and who were recruited by an advertising campaign. Average grade of eczema on the body as well as degree of nappy rash was scored by an independent dermatologist. At the end of the 26-week period, there was no clinical or statistical difference between the different nappy types for overall grade of atopic eczema. Nappy rash, however, was significantly less in the group using cellulose nappies with absorbent gelling material, compared with the others at 26 weeks and throughout the trial (p < 0.05).
Harms
No adverse effects are reported in these small studies, though specialised cotton clothing for atopic eczema sufferers is more expensive than other synthetic fibres.
No specific adverse effects were reported in the trial of different nappies, though nappy rash itself (the main efficacy outcome of this study) could be considered an adverse effect, which is desirable to prevent.
Comment
The studies by Diepgen and colleagues in 1990 and 1995 tested the hypothesis that cotton clothing is best for atopic eczema sufferers. The success of blinding in both trials is under question in view of the different roughness of the various shirt fibres. Magnitude of effects were not stated in the Diepgen 1995 paper, and it is possible that the study could have missed small differences between cotton and polyester fabrics comfort. The purported need for specialised clothing can result in considerable increased economic burden to eczema families and to the State. The two RCTs both suggest that there is nothing special about cotton for atopic eczema sufferers apart from smooth fibres. Other synthetic fibres can be constructed with similar smooth fibres using yarns and fabric construction that is just as comfortable for atopic eczema sufferers. It would be wise to repeat such studies in the UK and elsewhere before implementing policy decisions, and public knowledge of the availability and cost of cotton alternatives would be an advantage to eczema sufferers.
It was good also to locate an RCT evaluating different nappy types in atopic eczema. It was unclear in this study if the group with atopic eczema simply wearing a cloth nappy were randomised in the same way as the other two groups and whether statistical comparisons were made to the control population who were not part of the same randomised group. The study, nevertheless, suggests that nappy rash is less severe in atopic infants who wear nappies with gel absorbent material, though there appears to be no benefit for the general eczema control elsewhere. There was no evidence to support any benefit of conventional disposable nappies over cloth nappies, though the study may have lacked power to demonstrate small differences.
Salt baths
Salt has been used for centuries in the treatments of skin diseases, particularly psoriasis, popularised by holidays at the Dead Sea where the combination of high salinity and ultraviolet light may benefit patients. Some physicians recommend regular salt baths as a measure for controlling atopic eczema, presumably based on anecdotal reports of patients' eczema clearing after bathing in the sea while on holidays. Salt could help atopic eczema because of its cleansing properties (saline is a weak antiseptic agent) or by drawing fluid out of oedematous acute eczematous skin. Despite its advocates, we could find no RCTs comparing the use of salt baths versus ordinary baths. One RCT (published in German) of 40 patients with psoriasis and atopic eczema evaluating synthetic Dead Sea salt baths plus phototherapy against 3% salt baths was excluded as results for the eight atopic eczema patients were not given separately. 387 We did locate one Japanese RCT comparing deep-sea salt versus physiological saline, which will be reported further here. 272
Benefits
One-hundred patients with mild-to-severe atopic eczema aged 15 years and over were randomised to either deep-sea water or physiological saline. Both waters were sterilised and heated to 65 degrees centigrade then sprayed onto the body before home bathing and washed away after 10 minutes. Treatment was daily for 1 week. Doctor's global evaluation and several other skin signs reduced by only a small amount (∼15%) in each group after 1 week, and there were no clinical or statistical differences in the change in scores between each group.
Harms
No adverse effects were reported in this study. There were five drop-outs for reasons not related to the treatment.
Comment
Quality of reporting was good in the Adachi study, but they compared two types of salt that could have been equally active. We agree with the author's conclusions that possibly a longer contact with sea water is necessary. The study duration was also very short (1 week). The uptake of an intervention that consists of spraying each other with a concentrated salt solution is also likely to be limited in the UK and possibly quite costly to patients. It is a pity that a simple RCT comparing salt baths versus ordinary baths has not been done. Even an RCT comparing the effect of regular versus irregular bathing would be informative given the different strongly held views of frequency of bathing recommendations in children with atopic eczema. One recent non-randomised study388 from Japan has compared the benefit of sea water therapy at a beach with and without dolphins to encourage children with severe atopic eczema to enjoy the sea water. This needs to be tested further in an RCT.
Nurse education
The effective topical treatment of a child with atopic eczema is dependent upon good management by the parents. Good parental concordance can be achieved by regular follow-up visits and good patient–physician relationships, and also by active training and information given in an educational session by a nurse or other appropriate carer. One RCT273 has evaluated the possible additional benefit of a single session provided by a nurse in educating parents of children with atopic eczema versus conventional dermatological care.
Benefits
Fifty consecutive patients aged 4 months to 6 years 2 months, with atopic eczema of varying severity were randomised to either conventional treatment by a dermatologist or the same treatment plus a single session by a nurse (‘a nurse lesson’), which included general information about atopic eczema and environmental control, information and demonstration of topical treatment and also a discussion of realistic expectations. The study lasted for 3 months and was unblinded. At the end of the 3-month evaluation period, mean eczema score (maximum score 96) had fallen from 26.4 at baseline to 7.1 in the group given standard dermatological care plus education compared with 21.3 at baseline to 10.8 for conventional care alone (p < 0.05). This comparison was not adjusted for baseline scores, which were different. Each score also showed a statistically significant reduction in favour of the education group compared with the standard dermatological care, and hydrocortisone consumption was significantly greater in the intervention group.
Harms
No adverse effects were reported in this study. Those patients in the intervention group had to attend one additional session with the nurse.
Comment
This RCT suggests a modest benefit from a single nurse education session following standard dermatological care. The authors are to be applauded for randomising the treatment groups, though the lack of blinding and failure to perform an intention-to- treat analysis limits the study quality. The study nevertheless suggests a modest benefit to a single session of nurse education, though the component of the ‘package’ of the nurse education that conferred the most benefit is unclear. It is possible for instance that most of the benefit could be due to increased and appropriate use of hydrocortisone, which tends to be underused in the community because of inappropriate fear of adverse effects. Further RCTs in other countries that use a similar educational package and blinded outcome measures are needed.
Bioresonance
Bioresonance therapy, also called biophysical information therapy (BIT) has become popular as an alternative medical treatment for a variety of allergic diseases in Europe. Bioenergy is defined as the bioelectric magnetic field which is unique to materials, and that bioelectric waves produced by people can have diagnostic and therapeutic purposes. The proponents of this theory claim that the main purpose of BIT is to give a strong impulse to spontaneous healing energies of the body for self-regulation. The ultrafine electromagnetic waves of the patient's body, as well as their disturbances and presence of allergens, are purported to be transmitted for diagnostic and therapy using brass wire electrodes analysed by a ‘bioresonance apparatus’. This electronic instrument allegedly distinguishes between pathological and normal healthy waves from a patient. Pathological waves can be reversed electronically (‘corrected to healthy ones’) by the separator, and transmitted back to the patient for a therapeutic effect. The use of such BIT is frequently accompanied by claims of complete cure for allergies. One RCT conducted in Switzerland has evaluated the efficacy of bioresonance in children with atopic eczema. 274
Benefits
Thirty-six children with atopic dermatitis admitted as inpatients to a high-altitude specialist treatment centre for atopic eczema in Davo, Switzerland were randomised according to sex, age and severity of disease to receive sham (placebo) or active treatment with the bioresonance apparatus. The bioresonance was conducted exactly as described in the specific literature and by a qualified BIT therapist for at least 4 weeks. Blinding was obtained by a specially designed switchbox operated by an engineer who kept its randomisation code outside the clinic in a sealed envelope. Patients were allowed their normal treatment with creams, emollients and dietary restrictions as required throughout the study. For the short-term outcome (at least 4 weeks), total disease severity score in the active group had fallen from 39.8 to 27.3 at the end of the study period compared with a fall from 35.3 to 26.6 at the end of the sham treatment (p = 0.23). No difference was observed for the sleep score, though pruritus score was slightly improved in the actively treated group (p = 0.12). There were no clinically or statistically significant differences between the two treatment groups in a number of immunological markers in the blood and long-term outcomes measured 8 months after the treatment.
Harms
No adverse effects were described in the study, though the therapy can attract a considerable financial cost outside of health services.
Comment
This study was very carefully reported and authors were meticulous in giving the bioresonance a ‘fair test’ by following the intervention as meticulously as possibly. The study was blinded, and randomisation and concealment of allocation were well described, though an intention-to-treat analysis was not performed (4 out of 36 children dropped out). The results do not show any evidence to support benefit of bioresonance therapy. Although the study was relatively small, the study was powered to exclude a 35% benefit of relevant treatment benefit of bioresonance therapy in the sensitive COSTA scoring method. Therefore, although small benefits cannot be excluded by the study, the study failed to show evidence of any moderate or large treatment effects of this mode of treatment. It may be argued that the treatment was tried in a situation where marked treatment benefits were already occurring as a result of inpatient stay at high altitude (with very low house dust mite levels), and ideally a similar RCT could be performed in a more usual outpatient setting.
Psychological approaches
Psychological approaches to the management of atopic eczema may be quite diverse ranging from specific cognitive approaches to behavioural approaches such as habit reversal. Psychological and emotional factors have always been considered important in atopic eczema, though it is unclear to what extent such factors are a result of the eczema rather than the other way round. It has been postulated that much of the scratching (which can be pleasurable) in atopic eczema becomes a habit, and that such habit is detrimental, as scratching damages the skin and leads to further eczema forming in the so-called scratch–itch–scratch cycle. Habit-reversal is a modified behavioural technique, which teaches patients to recognise the habit, identify situations that provoke the habit, and then to progressively train them to develop a ‘competing response practice’ such as simply touching, squeezing or tapping the itching area, or to develop other ways of moving their hands away from the itching area. 389 The technique has been described in two RCTs106,276 conducted by the same team from Sweden, and compared with topical cortiscosteroids. A further RCT has evaluated the potential benefit of three psychological approaches versus dermatological education in the prevention of relapse in atopic eczema. 275 Another RCT390 of psychiatry support for patients with eczema was excluded as close inspection revealed that the patients had eczematous dermatoses of diverse types and only one case of atopic eczema was present in that study.
Benefit
In the Melin and colleagues study,276 17 patients with atopic eczema aged 19–41 years were randomised into two groups. One group was treated with hydrocortisone cream alone and the other group was treated with the cream plus two sessions of habit-reversal treatment during the first week of the treatment period of 28 days. The study was unblinded. At the end of the assessment period, there was a 67% mean reduction in global eczema score in the habit-reversal plus hydrocortisone cream group compared with 37% score reduction in the hydrocortisone only group (p < 0.05). Total score of self-assessed annoyance was also markedly reduced in the active versus comparator groups. Mean percentage reduction of scratching episodes per day was 79% in the habit-reversal and hydrocortisone group compared with 49% in the hydrocortisone only group (p < 0.01). In the later study conducted by the same team,106 45 patients (mean age 24.8 years) were randomised in a parallel fashion to four groups for a period of 5 weeks:
-
application of hydrocortisone cream for the entire 5-week period
-
application of betamethasone valerate (a strong topical steroid) for 3 weeks followed by hydrocortisone for the remaining 2 weeks
-
application of hydrocortisone plus habit-reversal for the 5-week period
-
application of betamethasone plus habit-reversal for the first 3 weeks followed by hydrocortisone plus habit-reversal for the remaining 2 weeks.
The study was unblinded. Results are reported more fully in the section on topical corticosteroids (see chapter 4 and appendix 3). The authors reported significant differences between the behaviour therapy groups and those taking steroids alone for total skin status. Scratching was reduced by 65% in the hydrocortisone only group, 74% in the betnovate followed by hydrocortisone group, 88% in the hydrocortisone plus habit-reversal group, and 90% in the betnovate and hydrocortisone and habit-reversal groups (statistics not presented).
The study by Ehlers and colleagues in 1995275 evaluated the use of an autogenic training as a form of relaxation therapy (ATP) versus a cognitive-behavioural treatment (BT), versus a standard dermatological educational programme (DE) versus combined DE and BT (DEBT). One hundred and thirteen patients attending an outpatient clinic in Germany were randomised to these four groups and were also compared with an additional standard medical treatment group who were not part of the random assignment. Investigators were blinded as to the group allocation. The intervention was for 3 months and patients were followed-up for 1 year in order to evaluate disease relapse. At the end of 1 year, mean skin severity lesion score dropped from 29.5 to 28.8 in the DE group, 33.7 to 19.8 in the ATP group, 31.0 to 20.7 in the BT group, and 35.4 to 25.8 in the DEBT group. There were no significant differences in mean severity of itching between the four randomised groups. DEBT led to significantly larger improvement in global skin severity than DE alone and this was also accompanied by significant reductions in topical steroid use.
Harms
In the Ehlers study, the behavioural approaches required 12 weekly group sessions of 1.5 to 2 hours each with a group size of between five and seven patients. No adverse events were reported in any of the trials, though some of the drop-outs could possibly be related to the fact that extra visits were needed for the behavioural technique.
Comment
The Ehlers study275 was clearly reported and included an assessment of patient expectation of treatment benefit. No intention-to-treat analysis was performed, but drop-outs were low (nine out of 113 at 3 months). Over 14 outcome measures were reported in the study, which introduces the possibility of multiple hypothesis testing. The authors also performed statistical tests in comparison to a non-randomised control group, which may not be justified. Nevertheless, the magnitude of improvement for those receiving behavioural techniques in addition to their standard dermatological care (which included topical corticosteroids) was moderately large, and carried more weight than the Melin and Noren studies because assessments were made by an investigator blinded to the treatment allocation. The combination of habit-reversal plus judicious use of topical corticosteroids seems an attractive one and evidence from two RCTs supports its use. Both RCTs were unblinded, and conducted in the hands of enthusiasts. The generalisability of these findings to other populations should be determined in further RCTs, using objective assessment methods that are clinically meaningful, and conducted by investigators blinded to group allocation. The magnitude of the benefits in these unblinded studies were considerable, particularly when these were above that which could be expected with topical corticosteroids.
Ultraviolet light
A proportion of atopic eczema sufferers have fewer flare-ups and decreased skin lesions during summer. This observation, along with the benefit of ultraviolet light in psoriasis led to the introduction of different forms of ultraviolet light for the treatment of atopic eczema. 391
Ultraviolet radiation (UVR) makes up a fraction of the electromagnetic spectrum, which can be further subdivided into:
-
UVC – the rays that do not pass through the earth's atmosphere
-
UVB – the rays responsible for nearly all biological effects following sunlight exposure including tanning, burning and skin cancer, and
-
UVA – those rays closest to the visible spectrum that pass through glass, and are the least harmful to the skin. 392
Hence, treatment is with either UVA or UVB or a combination of both. Another form of UVA exists called PUVA (psoralen plus UVA) or psoralen photochemotherapy. Psoralen is a photoactive drug taken by mouth or mixed in a bath, which is given with UVA radiation to enhance its effectiveness. PUVA has proven efficacious in psoriasis and is currently used in the treatment of eczema. 393
The mechanisms by which ultraviolet light affects atopic eczema are not completely understood. However UVB is immunosuppressive because it blocks the function of antigen-presenting Langerhans cells and alters the production of cytokines by keratinocytes. There is also evidence that UVA is able to alter both Langerhans cell and eosinophil functions in patients with atopic eczema. 394
We located six RCTs278–283 published in six papers evaluating the use of ultraviolet light in atopic eczema, and these are summarised in Table 30. The only two RCTs of PUVA that might have included atopic eczema patients had to be excluded as it was either not clear if those with ‘chronic hand eczema’ were atopic393 or because data on the subset of atopic eczema patients were not given separately. 395 Statistical pooling of summary measures was not possible due to the differences in the type of ultraviolet light in each study and the lack of common outcomes at the same endpoints.
Comment
Generally the studies were quite small and poorly reported. Blinding was likely to have become unmasked in placebo-controlled trials due to the obvious tanning on one half of the body, along with mild burning and marked treatment effects. Although the right/left body comparison design had its limitations in terms of blinding, the lack of effect on placebo-treated body halves argues against a systemic effect of ultraviolet light treatment. Treatment effects were generally large and of rapid onset (i.e. within 1–2 weeks). Future studies should consider using a simple parallel group design and they should be of longer duration in order to capture duration of remissions.
Harms
Treatment with ultraviolet light usually ties patients to twice- or thrice-weekly visits to hospitals. Mild degrees of skin redness and burning are also common short-term adverse effects. Occasionally more severe burning may occur. There is no direct information on the long-term risk of skin cancer in atopic eczema patients undergoing ultraviolet light treatment. Although data on cohorts of psoriasis patients undergoing PUVA suggest that cancer risk only increases after around 250 treatment sessions, extrapolating from these studies to another inflammatory disease with a generally younger patient population raises some concerns. This is particularly so for melanoma skin cancer where it is thought that most risk is acquired from ultraviolet light in the first 20 years of life. Specific cohort studies of different modalities of ultraviolet light treatment of atopic eczema treatment are recommended.
Summary of non-pharmacological treatments
House dust mites
-
Considering that the circumstantial evidence for implicating house dust mites in atopic eczema is so strong, it is surprising that only three RCTs have examined the usefulness of house dust mite reduction.
-
There is some evidence that reduction of house dust mite allergen around the home can result in a benefit to atopic eczema sufferers.
-
The clinical relevance of such benefit and whether it is sustainable is unknown.
-
The most clinically useful and easiest method of reducing house dust mite allergen around the home in atopic eczema is not known.
-
There is little evidence to support the use of high-filtration vacuum cleaners above ordinary ones.
-
There is no evidence to support the sole use of sprays, which only kill house dust mites.
-
More studies on house dust mite eradication are needed, which separate the different interventions.
-
Such studies should be larger and more pragmatic than those already done and should include a cost-effectiveness analysis.
-
The role of hyposensitisation therapy in atopic dermatitis has not been adequately tested.
Enzyme detergents
-
Although parents of children with atopic eczema in the UK commonly avoid enzyme-containing detergents in the belief that alternative agents are ‘kinder’ to the skin, one Danish RCT did not find any evidence to support such a notion.
Cotton clothing
-
Two RCTs suggest that specially woven smooth synthetic garments are just as comfortable as cotton to people with atopic eczema.
-
Another RCT suggests that disposable nappies containing an absorbent gelling material result in less nappy rash than conventional disposable nappies or cloth nappies, though no benefit for atopic eczema in general was demonstrated.
Salt baths
-
A Japanese RCT of 1-week duration has not found any difference between deep-sea water and physiological saline sprays before bathing.
-
RCTs comparing salt versus ordinary baths and regular versus infrequent baths in people with atopic eczema are needed.
Bioresonance
-
There is no RCT evidence to support the use of bioresonance treatment in atopic eczema.
Psychological approaches
-
The results of three RCTs suggest that psychological interventions such as behaviourtherapy habit-reversal techniques are a useful adjunct to dermatological treatment in atopic eczema. Generalising from these RCTs to other centres with less enthusiastic and appropriately trained staff requires further evaluation.
Nurse education
-
One small unblinded RCT has suggested a modest benefit from supplementing a dermatological consultation with a single session with a dermatological nurse to provide more background information and to demonstrate the use of topical treatments.
Ultraviolet light
-
There is some RCT evidence to support the use of UVB (broad and narrow band) versus placebo in atopic eczema.
-
There is some RCT evidence to support the use of high dose UVA in preference to UVB/UVA in atopic eczema.
-
There is some RCT evidence to support the use of narrow band UVB (TLO1) in preference to ordinary UVA in atopic eczema.
-
There is some RCT evidence to indicate a benefit of high-dose UVA in the treatment of acute eczema flares, with efficacy slightly superior to topical steroids.
-
Missing evidence includes a comparison of high-dose UVA versus narrow band UVB, PUVA versus placebo, PUVA versus UVB or topical steroids, and any form of ultraviolet light versus other systemic immunomodulatory treatments.
-
Future studies should be long term (i.e. 6 months or more), in order to note the duration of remissions and the effects of ultraviolet light on disease chronicity.
-
Long-term multicentre surveillance studies are needed in large cohorts of patients in order to estimate subsequent skin cancer risk.
| Study | Interventions (co-treatments) | Study population and sample size | Trial design description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Jekler & Larko, 1988280 | UVB three times per week (20–153 mJ/cm2 up to 63–816 mJ/cm2) vs placebo (visible light) three times per week (emollients and hydrocortisone) | 17 patients over the age of 15 years, most of whom had skin type III (tans easily, seldom burns | Prospective, RCT left/right parallel study of 8 weeks' duration | Patients assessed for pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema | Improvement from baseline of 1.5 (mean) to 0.7 for UVB and 1.4 for placebo for overall clinical response (p < 0.001).Thus the total score was significantly lower for the UVB treated side | Described as randomised but method unclear. Blinding unlikely due to mild burning on UVB-treated side | Unclear if randomisation referred to side of active/placebo treatment or whether to type of minimal erythemal dose |
| Study 1 | Variables assessed on a scale of 0–3, plus a global assessment | No ITT analysis | Large magnitude of treatment effect for all parameters | ||||
| Large (11/17) drop-outs due to ‘intercurrent disease’ and lack of time for treatments | |||||||
| Jeckler & Larko, 1988280 | UVB three times per week given at 80% of minimal dose required to produce redness (MED) vs UVB at 40% of MED (emollients and hydrocortisone) | 25 patients with mean age of 25.9 years, most with skin type III | Randomised right/left side parallel study for 8 weeks | Same as for study 1 above | Clearing or considerable improvement in 15/25 on high-dose UVB vs 16/25 with low-dose (NS) | Methods very scanty | Further details of study found in Jeckler 1992 thesis. This study of high- vs low-dose UVB suggested very little difference between the two, but power of study is very limited |
| Study 2 | Randomisation unclear; probably unblinded | ||||||
| No ITT analysis | |||||||
| Jekler & Larko, 1991281 | UVA (average 8.1 mW/cm2) vs UVB (0.85 mW/cm2) three times per week (emollients and hydrocortisone) | 33 patients with mean age of 23.3 years | Prospective, randomised left/right parallel study of 8 weeks' duration | Patients assessed for pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema and an overall evaluation on a score of 0–3 (none to severe) | Improvement from mean baseline of 10.3 (range 6–18) for clinical signs (total score) decreased to 5.5 for UVA and 6.4 for UVB | Described as single-blind and randomised but methods unclear | Both treatments induced large improvements compared with baseline, with some small statistically significant change in favour of UVA |
| Mean disease duration of 19.6 years | Healing evaluated on a scale of 3 to −1 (3 = healed, –1 = worse) | Pruritus scored separately with baseline of 2.2 improving to 1.1 after UVA and 1.3 after UVB | Differential tan on UVA side of the body likely to have unblinded study | Most patients preferred UVA | |||
| Most with skin type III | 12 withdrawals and dropouts, no description given, and no ITT analysis | ||||||
| Jekler, 1992282 | Mixed UVA (74%) and UVB (26%) vs UVB three times per week | 30 patients with mean age of 24.8 years and mean disease duration of 20.5 years | Prospective, randomised left/right parallel study of 8 weeks' duration | Patients assessed for pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema and an overall evaluation on a score of 0–3 (none to severe) healing evaluated on a scale of 3 to −1 (3 = healed, –1 = worse) | A decrease from baseline score of 10.8 to 5.2 for UVAB and 6.1 for UVB (p = 0.002 for difference in scores between treatments) | Described as randomized but method unclear | This thesis also presents the two studies reported in Jeckler 1988 in more detail; a further three small left/right comparison studies are also described comparing UVA vs UVB and low-dose UVB vs UVA/B, and UVA vs UVA/B, but it is unclear if these were RCTs |
| 21/24 patients reported mild burning with UVB, which was sever in six patients compared with three episodes of mild burning with UVA/B (none severe) | No blinding | ||||||
| No withdrawals or drop-outs | |||||||
| Krutmann et al., 1992283 | High-dose UVA1 (0–130 J/cm2 o.d.) vs UVA–UVB therapy (up to 30 mj/cm2 UVB and 7.5 j/cm2 UVA daily (emollients) | 25 young adults with atopic eczema and definite atopy | Prospective, randomised, parallel study of 15 days' duration | Costa scoring system: erythema, oedema, vesicles, exudation, crusts, excoriations, scales, lichenification, pruritus, loss of sleep on a 7-point scale (0 = no lesion, 6 = extremely severe) | A decrease from baseline of 53 (overall score) to 14 after UVA1 (p < 0.001 against comparator change) | Described as ‘randomly selected patients’ but method unclear | Unclear if patients randomised but confirmed by authors |
| Comparative data for UVA-UVB not given but shown in graphical form only; UVA–UVB 52 at baseline changed to 38 (estimated from graph) | Author contact ‘treatments randomly allocated’ | Large treatment effects – all in favour of high-dose UVA1 over UVA/B | |||||
| No blinding | |||||||
| No drop-outs or withdrawals | |||||||
| Krutmann et al., 1998278 | High-dose UVA1 130 J/cm2 o.d. vs o.d. fluocortolone 0.5% cream or ointment vs UVA–UVB minimal erythema dose-dependent o.d. | 53 patients acute severe exacerbation of atopic eczema | Prospective, randomised, parallel study of 10 days' duration | Costa scoring system: erythema, oedema, vesicles, exudation, crusts, excoriations, scales, lichenification, pruritus, loss of sleep on a 7-point scale (0 = no lesion, 6 = extremely severe) | Improvement over baseline for total clinical score: high-dose UVA1 baseline of 56 reduced to 26, fluocortolone baseline of 60 reduced to 35 and UVA-UVB baseline of 60 reduced to 42 (all after 10 days' treatment); p < 0.0001 | ‘A randomisation sequence generated by random numbers’ | Very short duration |
| Mean reduction in total disease activity was 9.7 for 21 evaluable patients on narrow-band UVB, 4.8 on UVA and 0.4 on placebo, the change significant at the 5% level for narrow-band UVB vs placebo only | No blinding | Results had to be estimated from graphs | |||||
| No withdrawals or drop-outs | Useful to have a comparison with topical steroids | ||||||
| Study suggests superiority of high-dose UVA over a topical steroid | |||||||
| Reynolds et al., 1999279 | Narrow-band UVB (up to max of 1.2 J/cm2) vs UVA (up to max of 15 J/cm2) or placebo (visible light) all twice weekly (mild-to-moderate topical steroids plus emollients) | 73 adult patients with moderate-to-severe atopic eczema | Prospective, randomised, double-blind parallel study of 12 weeks' duration | Five clinical features at six separate body sites plus itch and sleep loss (VAS), and extent of disease recorded by one observer | The proportion of patients reporting reduction in itch over 24 treatments was 90% (p = 0.02) for narrowband UVB, 63% for UVA and 53% for placebo (p = 0.02 compared with placebo) | Study described as randomised (in balanced blocks), controlled, and double-blind | Published in abstract form only at time of report |
| Changes for sleep loss failed to reach statistical significance | No ITT analysis | Only 47 out of 73 patients completed study | |||||
| Study possibly partly unblinded due to lack of pigmentary changes on one side and burning in others | |||||||
| Der-Petrossian et al., 2000277 | Narrow-band UVB vs bath PUVA 1 mg/l as 8-MOP three times per week | 12 patients with severe/chronic atopic dermatitis with a mean age of 27 years ± SD of 11.3 years | Prospective, randomised, single-blind half side comparison of 6 weeks' duration | Patient-rated itch and sleep loss (VAS 0–10 cm) as part of SCORAD, doctor-rated global severity, doctor-rated global changes of modified SCORAD for eight signs and symptoms | Baseline scores of 100% SCORAD for bath-PUVA and UVB reduced by 65.7% for bath-PUVA-treated side and 64.1% for UVB-treated side (p = 0.48) | Study described as randomised, and investigator blinded | A small study that took care to ensure that both treatments were given in equal doses |
| No ITT analysis carried out; two withdrawals, one due to exacerbation of atopic dermatitis, an another due to a differential response in terms of less erythema reactions to the bath-PUVA side | Big falls in SCORAD scores for both treatments with little difference between the two |
Chapter 10 Systemic immunomodulatory agents
Allergen–antibody complexes of house dust mite
Anti-house dust mite antibodies are very common in atopic eczema patients and may play a part in the disease process. Previous studies in the field of asthma have suggested that injection of complexes of house dust mite allergen (Der p 1) with antibodies may result in clinical improvement. We found one RCT285 that evaluated the role of house dust mite allergen–antibody complex injections versus placebo in the treatment of atopic eczema. The study was covertly duplicated the following year in the Journal of the American Academy of Dermatology, and both papers were used to generate the summary as each contained additional details. The later publication documented 24 patients who were randomised compared with 23 in the original report. The missing patient is documented in the secondary report as he no longer satisfied the entrance criteria at the time of the first injection. Twenty-four adults with severe atopic eczema, all of whom had evidence of sensitisation to house dust mite were entered into a placebo-controlled study of injections of house dust mite allergen–antibody complexes 4 months after an initial washout period of 6 weeks. The study was followed by a more prolonged open period.
Benefits
Of the 23 evaluable patients at 4 months, there was a statistically significant improvement in disease intensity index in those receiving active injections. Disease intensity was measured on a complex scale, which multiplied extent versus six physical signs. This reduced from 1000 to 612 in the active and from 1000 to 859 in the placebo group. Percentage mean reduction in itching was not so large, reducing from 3.3 to 2.2 in the active group and from 3.3 to 2.6 in the placebo group.
Harms
Three patients in the active group developed a delayed-type inflammatory action at the injection site and itching increased within 24 hours after injection in four patients on active and on two patients receiving placebo therapy.
Comment
Method of randomisation and concealment of allocation was unclear in this study, though blinding was probably successful. The ‘beneficial’ treatment effects are difficult to interpret given the use of an invalidated exploded scale and absence of a patient's perspective. The modest benefit demonstrated in this study needs to be replicated elsewhere before the intervention can be recommended as a treatment option.
Cyclosporin
Cyclosporin is a polypeptide of fungal origin. It is a potent inhibitor of T-lymphocyte-dependent immune responses and interleukin 2 production. 396 Primarily, cyclosporin was introduced as an immunosuppressive agent to prevent graft rejection after tissue transplantation. In dermatology, cyclosporin is used for the treatment of immune-mediated skin diseases such as cutaneous graft-versus-host reaction, immunoglobulous diseases, psoriasis and atopic dermatitis. 391
Cyclosporin is usually restricted to short-term use in severe refractory atopic dermatitis in adults and children. 397 It requires careful monitoring due to its potential adverse effects, notably kidney toxicity and hypertension. 398 Although cyclosporin has a low degree of penetration through the skin, topical applications have been suggested for reducing the potentially serious adverse effects associated with oral cyclosporin. 297
Cyclosporin can be administered orally in doses of between 2.5 mg/kg and 5 mg/kg body weight34 or topically in an ointment or gel containing cyclosporin microcrystalline 10%. 294
Statistical methods
Rate differences were estimated for categorical variables and differences in means for continuous variables. The Mantel–Haenszel type method of Greenland and Robins399 was used to estimate the pooled rate difference for all strata under the assumption of a fixed-effects model. A CI for the pooled risk difference was calculated using the Greenland–Robins variance formula. 399 The Q (‘combinability’) statistic is given with its associated probability on k (number of strata) minus one degree of freedom. This has low power as a strict test of homogeneity. There are no comprehensive rules on when to use random effects and when to use fixed effects models; debate continues in the statistical community. 400 However, when this was significant at the 0.05 level, a random effects analysis was applied. 401 The pooled mean effect estimate was calculated using weights calculated as the inverse of the variance for each study.
In the trials, estimates of error were not reported in a consistent manner. When standard errors were reported for both the treatment and control arms, both were used in the calculations. When only the pooled estimate was given, the variances for the treatment and control arms were assumed to be the same. When quantitative values were reported in graphical form only, these were read electronically from the scanned images and the responses and their associated variances were estimated by linear interpolation as described by Poolsup and colleagues. 402
Results
Of the twelve eligible reports of RCTs (Tables 31–36), two dealt with topical cyclosporin294,297 and ten with oral cyclosporin. 286–293,295,296
Topical application
The two reports294,297 on the use of topical cyclosporin gave conflicting results. While in a study of 20 patients, De Prost and colleagues297 reported positive results based on assessments of pruritus, erythema, oozing, crusts, xerosis and lichenification, De Rie and colleagues294 reported no benefit in a study of seven patients with atopic eczema. Both studies used an intrasubject design with randomised applications to comparable lesions. Neither provided any justification for sample size chosen. On the basis of the available evidence, the efficacy of cyclosporin in atopic eczema has yet to be rigorously evaluated.
Oral therapy
Of the ten reports of RCTs of oral cyclosporin therapy, those by Zonneveld and colleagues288 and Zurbriggen and colleagues289 were comparisons of dose schedule and formulations, respectively, and therefore provided little evidence on the value of cyclosporin. One of the trials291 adopted a randomised parallel group design with 23 patients receiving cyclosporin and 23 placebo. The study showed that both disease severity and area involvement were reduced by cyclosporin treatment (5 mg/kg/day) by week 6. At the end of the 6-week trial, 15 of 19 of the patients on cyclosporin compared with six of 19 patients on placebo reported at least a moderate improvement. The estimated rate difference was 39% (95% CI 10 to 62). One small study by Harper and colleagues286 of continuous versus intermittent cyclosporin A in children, suggested that more consistent control is achieved with continuous therapy when given over a 1-year period. This longer-term study did not demonstrate any clinically significant change in kidney function (serum creatinine) and blood pressure in either group.
Three of the remaining six reports were of the same trial293,295,298 with most of the outcome data reported in the Sowden and colleagues report. 295 None of the studies compared cyclosporin with an active agent, with all three remaining RCTs being comparisons of cyclosporin with placebo. 290,292,295 Those studies gave some poolable data because of similarities in study design and the use of a consistent visual metric analogue scale for scoring itch (Table 36). However, because of the crossover design used in all three trials, only the first phase of each study was used so that our estimates were not confounded by carry-over effects. Table 37 shows the estimated mean difference in itch scores for the cyclosporin group compared with the placebo group for each of the three studies as well as the pooled estimate at the end of the first period.
Irrespective of whether a fixed- or random-effects model was used, the pooled estimate showed that cyclosporin was effective in relieving eczematous itch compared with placebo as shown in Figures 4 (fixed effects) and 5 (random effects).
Generally positive results were reported for sleep loss, area involvement, reduction in steroid use and erythema.
The small study remaining by Miranda and colleagues287 compared oral cyclosporin A with oral transfer factor for 6 months and found no statistical differences in a range of outcomes between the two. No intention-to-treat analysis was performed. Three patients in the cyclosporin A group reported excess hair growth (hypertrichosis).
Comment
There is little doubt that cyclosporin is effective for the treatment of atopic eczema when compared with placebo but that continued use is necessary for the prevention of relapse, which is rapid once therapy is discontinued. Clinical scores return close to baseline values within 8 weeks. 292 Adverse effects of the drug, notably on the liver and kidneys, suggest that long-term treatment is not justifiable. Even in short-term trials, cases of hypertension and elevations of serum bilirubin and creatinine have been reported. 291 Reducing dose schedules or prolongation of treatment-free intervals have yielded unconvincing results with obvious poorer control of the disease and modest decrease in drug exposure. As recommended by Zaki and colleagues,397 cyclosporin should be reserved for the short-term treatment of refractory disease, and even then its superiority over oral steroid therapy, a substantially cheaper alternative, is as yet untested.
| Study | Design | No. of patients | Age (years) | Duration | Severity | Oral cyclosporin dosage | Comparator | Co-treatments | Withdrawals and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Orals | |||||||||
| Allen, 1991298* | Crossover, double-blind, randomised | 33 | 17–56 | 16 weeks | Severe | 5 mg/kg/day | Placebo | Topical steroids | Four crossed over prematurely (p-c) |
| Six did not complete second phase (c-p) | |||||||||
| Munro et al., 1994292 | Crossover, double-blind, randomised | 24 | 19–48 | 8 weeks | Chronic | 5 mg/kg/day | Placebo | Topical steroids | Five |
| Salek et al., 1993293* | Crossover, double-blind, randomised | 33 | 17–56 | 16 weeks | Severe | 5 mg/kg/day | Placebo | Topical steroids | Four crossed over prematurely (p-c) |
| Six did not complete second phase (c-p) | |||||||||
| Sowden et al., 1991295* | Crossover, double-blind, randomised | 33 | 17–56 | 16 weeks | Severe | 5 mg/kg/day | Placebo | Topical steroids | Four crossed over prematurely (p-c) |
| Six did not complete second phase (c-p) | |||||||||
| van Joost et al., 1994291 | Parallel, double-blind, randomised | 46 | 17–68 | 6 weeks | * | 5 mg/kg/day | Placebo | Hydroxyzine 10 or 25 mg, emollients | 18 due to lack of response from trial medication |
| Wahlgren et al., 1990296 | Crossover, double-blind, randomised | 10 | 22–42 | 10 days | Stable,moderate or severe | 5 mg/kg/day | Placebo | 1% hydrocortisone, emollients | None |
| Zonneveld et al., 1996288 | Parallel, open, randomised, dose-finding study | 78 | 18–70 | 8 weeks | Severe, long standing | 5–3 mg/kg/day | 3–5 mg/kg/day | Antihistamines, emollients, antibiotics, steroids | 48: lack of efficacy, adverse effects, non-compliance |
| Zurbriggen et al., 1999289 | Crossover, double-blind, randomised | 14 | 20–64 | 8 weeks | Severe | 4–4.5 mg/kg Sandimmun™ | 4–4.5 mg/kg Neoral™ | Monitored steroids and emollients | One: protocol violation |
| Cordero Miranda, 1999287 | Parallel study | 23 | 3–40 | 6 months | Not stated | 4 mg/kg | Oral transfer factor in escalating dose (units not specified) | 1% hydrocortisone | Three in treatment group, two in transfer factor group |
| (Spanish, translated) | |||||||||
| Harper et al., 2000286 | Parallel, unblinded, randomised | 43 | 2–16 | 12 months | Severe | Max. of 5 mg/kg/day continuously | Max. of 5 mg/kg/day given as intermittent 3-month course | Topical steroids of any potency | Three excluded after baseline due to abnormal assessments |
| A further eleven dropped out during the study | |||||||||
| Topicals | |||||||||
| de Prost et al., 1989297 | Left/right comparison, double-blind, randomised | 20 | 2–29 | 2 weeks | Stable | 10% topical gel | Placebo | * | One to adverse effects |
| De Rie et al., 1991294 | Left/right comparison, double-blind, randomised | 8 | 3–55 | 3 weeks | * | 10% topical gel | Placebo | * | * |
| Study | Outcome measure | Scale |
|---|---|---|
| Allen, 1991298* | Erythema, purulence, excoriation or crusting, dryness or scaling, cracking or fissuring, and lichenification at six body sites | 0–3 scale |
| Extent of disease | Rule of Nines | |
| Patient-assessed sleep and itch | 0–100 mm VAS | |
| Salek et al., 1993293* | Disease activity: erythema, purulence, excoriation or crusting, dryness or scaling, cracking or fissuring, and lichenification at six defined body sites | 0–3 scale |
| Disease extent: Rule of Nines | Max. score 108 | |
| Patient-assessed itch and sleep loss | 1–100 mm VAS | |
| Patient-assessed health-related quality of life | UKSIP, EDI | |
| Sowden et al., 1991295* | Clinician-assessed disease activity of erythema, purulence, excoriation or crusting, dryness or scaling, cracking or fissuring, and lichenification at six defined body sites | 0–3 scale |
| Clinician-assessed extent of disease | Rule of Nines | |
| Patient-assessed itch and sleep loss | 0–100 mm | |
| Patient and doctor global assessments | Five-point scale | |
| Munro et al., 1994292 | Composite scale for erythema, excoriation, lichenification using Rule of Nines | 0–3 |
| Itch and sleep loss | 10 cm VAS | |
| van Joost et al., 1994291 | Physician-assessed severity in six regions for erythema, infiltration, vesicles and papules, dryness and scaling, cracking and fissuring, excoriation and crusting | 0–3 scale |
| Lichenification scored separately | 0–3 scale | |
| Physician-assessed extent of disease | Rule of Nines | |
| Physician-assessed itching and sleep loss | 0–3 scale | |
| Patient-assessed global assessment | Four-point scale | |
| Wahlgren et al., 1990296 | Patient-assessed itch | Symtrack |
| Patient-assessed itch | 100 mm VAS | |
| Physician-assessed severity at 20 areas (no details) | 0–3 grading | |
| Zonneveld et al., 1996288 | Area assessment | Rule of Nines |
| Severity assessment of six body regions for erythema, lichenification, vesicles/papules, dryness/scaling, cracking/fissuring, excoriation | 0–3 | |
| Patient-assessed itch and sleep loss | 0–3 scale | |
| Patient and physician global assessment | 0–3 scale | |
| Zurbriggen et al., 1999289 | Area assessment | Rule of Nines |
| Severity assessed at six sites for (no more detail given) | 0–10 scale | |
| Itch and sleep loss. Note: reference given for details (see Sowden paper) | ||
| Cordero Miranda, 1999287 | Physician-assessed erythema,‘eczema’, lichenification, itch, oedema | 0–3 |
| Global physician's assessment every 15 days | Five categories (cure, excellent, moderate, no change, worse) | |
| Harper et al., 2000286 | Patient-rated itch, irritability, sleep loss and global severity | 100 mm VAS |
| Doctor-rated global severity | Five-point scale | |
| SASSAD score | ||
| Doctor-rated extent using Rule of Nines | ||
| Renal function and blood pressure |
Levamisole
Levamisole hydrochloride is a drug widely used in veterinary medicine for treating helminthic parasites. The drug was found to have wide immune-enhancing properties particularly on stimulating white blood cells. Because patients with atopic eczema have some evidence of decreased cell-mediated immune responses and recurrent secondary infections, it seemed reasonable to consider a possible benefit of levamisole in cases of atopic eczema. One small RCT of levamisole in atopic eczema has been published. 299 Another double-blind placebo-controlled study of 15 children in Spain403 was published in the same year, though it was unclear if randomisation had been used in that study. The Alomar study403 showed no evidence of any benefit of levamisole. The White and Hanifin study299 will be described in more detail.
Benefits
Thirty-six patients aged 13–64 years with atopic eczema were randomised to levamisole hydrochloride or placebo according to body weight with topical triamcinolone as co-treatment. Of 26 evaluable patients at the end of the 6-month trial, there were no clinical or statistically significant differences in patients' objective improvement, frequency of infections, physician prediction of active treatment, clinical scores, or immunological markers such as IgE changes. In the active groups, six of 11 patients noticed improvement compared with six of 15 in the placebo group. Mean percentage improvement in a composite sign score (not defined) was 44% in the levamisole group and 16% in the placebo group.
Harms
One patient developed urticaria and another developed nausea and vomiting while taking levamisole.
Comments
The quality of this study was surprisingly good for such an early publication with a clear description of the blinding process and testing for success of blinding. Despite the lack of intention-to-treat analysis and very small sample size, there is not a hint of any benefit of levamisole in this study or in the Alomar study. The placebo benefit in the studies was quite remarkable in that they were greater than those on active treatment. The authors conclude that the study emphasises the need for randomised double-blind comparisons for new drugs such as levamisole in view of the previous claimed excellent results from uncontrolled studies. Although the study was under-powered to miss small treatment effects, it is unlikely that levamisole has any long-term moderate-to-large treatment benefit in atopic eczema.
Platelet-activating factor antagonist
Platelet-activating factor (PAF) is a powerful mediator of certain inflammatory reactions and has been implicated in inducing itch and contact urticaria. Thus, it seemed reasonable to try a PAF antagonist in atopic dermatitis, a disease characterised by itching and various inflammatory processes. One RCT300 compared a solution of PAF antagonist with vehicle (placebo) in a study of 44 patients who applied one solution or the other on opposite sides of symmetrical lesions of atopic eczema for a period of 28 days. Patients were mainly young adults and were recruited from five centres in Europe.
Benefits
Based on an intention-to-treat population analysis, 57% of those on the experimental PAF antagonist ‘responded’ (response not defined by authors) compared with 61% on placebo at the end of the treatment period. Based on the evaluable population of 36 patients, 18 of the experimental group showed marked improvement or total clearing on the site treated with the solution compared with 17 of the 36 for the site treated with placebo. Other parameters of atopic dermatitis severity showed similar lack of difference apart from a transient statistically significant benefit for itching at Day 14 but not on Day 28.
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing | Inflammation | Crusts | Infiltration | Induration | Itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allen, 1991298* | • | • | • | • | • | • | • | • | • | • | • | RoNAA | |||||||||||
| Salek et al., 1993293* | • | • | • | • | • | • | • | • | • | • | • | RoNAA | |||||||||||
| Sowden et al., 1991295* | • | • | • | • | • | • | • | • | • | • | • | • | • | RoNAA | |||||||||
| Munro et al., 1994292 | • | • | • | • | • | RoNAA | |||||||||||||||||
| van Joost et al., 1994291 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | RoNAA | ||||||||
| Wahlgren et al., 1990296 | • | ||||||||||||||||||||||
| Zonneveld et al., 1996288 | • | • | • | • | • | • | • | • | • | • | • | RoNAA | |||||||||||
| Zurbriggen et al., 1999289 | • | • | RoNAA |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing | Inflammation | Crusts | Infiltration | Induration | Itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| de Prost et al., 1989297 | • | • | • | • | • | • | • | • | |||||||||||||||
| De Rie et al., 1991294 | • | • | • | • | • | ADSI |
| Study | Cyclosporin group | Placebo group | ||
| No. of patients | Mean difference in itch scores (SD) | No. of patients | Mean difference in itch scores (SD) | |
| Munro et al., 1994292 | 12 | 35.9 (22.9) | 12 | 16 (20.1) |
| Sowden et al., 1991295 | 17 | 27.7 (17) | 16 | 36 (17) |
| Wahlgren et al., 1990296 | 10 | 7 (10.8) | 10 | –1 (12.7) |
| Study | Stratum | No. of treatment patients | No. of control patients | Mean difference | Approximate 95% CI |
|---|---|---|---|---|---|
| Munro et al., 1994292 | 1 | 12 | 12 | 19.9 | 2.66 to 37.14 |
| Sowden et al., 1991295 | 2 | 17 | 16 | 24.1 | 12.49 to 35.70 |
| Wahlgren et al., 1990296 | 3 | 10 | 10 | 8.0 | –2.33 to 18.33 |
Harms
Fourteen out of 15 patients complained of skin dryness and burning immediately after application of the treatment. Data were not presented separately for the active versus vehicle treatment. There was one case of possible contact dermatitis related to the trial medication and another who developed severe erythema.
Comment
There appears to be no benefit from the PAF antagonist in this small study, although it lacks the power to exclude a potentially useful benefit. Randomisation, concealment of allocation, and blinding was poorly described, though an intention-to-treat analysis was performed.
Interferon-gamma
One of the immunological abnormalities characteristic of atopic eczema is reduction in a chemical messenger called interferon-gamma. Recombinant interferon-gamma inhibits IgE synthesis by human peripheral blood lymphocytes in vitro. Since atopic eczema is characterised by excessive production of IgE in response to ingested and airborne allergens, a potential therapeutic role for a substance such as interferon-gamma, which helps to switch off IgE synthesis, has been postulated and demonstrated in an open study. 404 Two RCTs of interferon-gamma in atopic eczema were located. 302,303 An earlier abstract405 quoted in Renz and colleagues (1992)301 referred to an RCT of 14 atopic eczema patients treated by gamma-interferon or saline. It was unclear if these patients were also participants in the larger study reported in 1993,302 and the lack of methodological detail precluded further discussion of this study.
Benefits
In a 12-week multicentre study of 83 patients with severe atopic eczema aged between 2 and 65 years, daily subcutaneous injections of recombinant interferon-gamma at a dose of 50 μg/m2 was compared with placebo injections with topical corticosteroids continued as co-treatment. All patients were instructed to take oral acitaminophen (an analgesic) before and after injections to protect against headaches and aching limbs associated with interferon therapy. Those randomised to active treatment were significantly older than those on placebo. At the end of the assessment period, 45% of 40 patients on active treatment compared with 21% of 43 on placebo were reported as having greater than 50% global improvement (p = 0.016) as judged by a physician who was blinded to treatment allocation. Corresponding figures for the proportion of patients with more than 50% patient/parent-reported global improvement was 53% and 21% for active versus placebo treatment, respectively (p = 0.002). Other physical signs, such as redness and scratch marks, were statistically significantly reduced by about 30% in the active group, and a similar magnitude of improvement was seen for induration, itching, dryness and lichenification, though these were not statistically significant. Response was greatest in younger patients. Serum IgE levels did not fall significantly. Another more recent study by Jang and colleagues303 has compared high- versus low-dose interferon gamma (1.5 versus 0.5 million units/m2) versus placebo given subcutaneously three times week to patients with recalcitrant atopic dermatitis aged 18–42 years for 12 weeks. At the end of the evaluation period, clinical improvement measured by means of a composite of different physical signs and surface area, was markedly better in the two interferon gamma groups compared with placebo (p < 0.05), but not between the two interferon-gamma dose groups, except perhaps with a more rapid benefit in the first 6 weeks in the higher dose group. A host of other immunological markers were assessed.
FIGURE 4.
Difference in itch scores (fixed effects)
FIGURE 5.
Difference in itch scores (random effects)
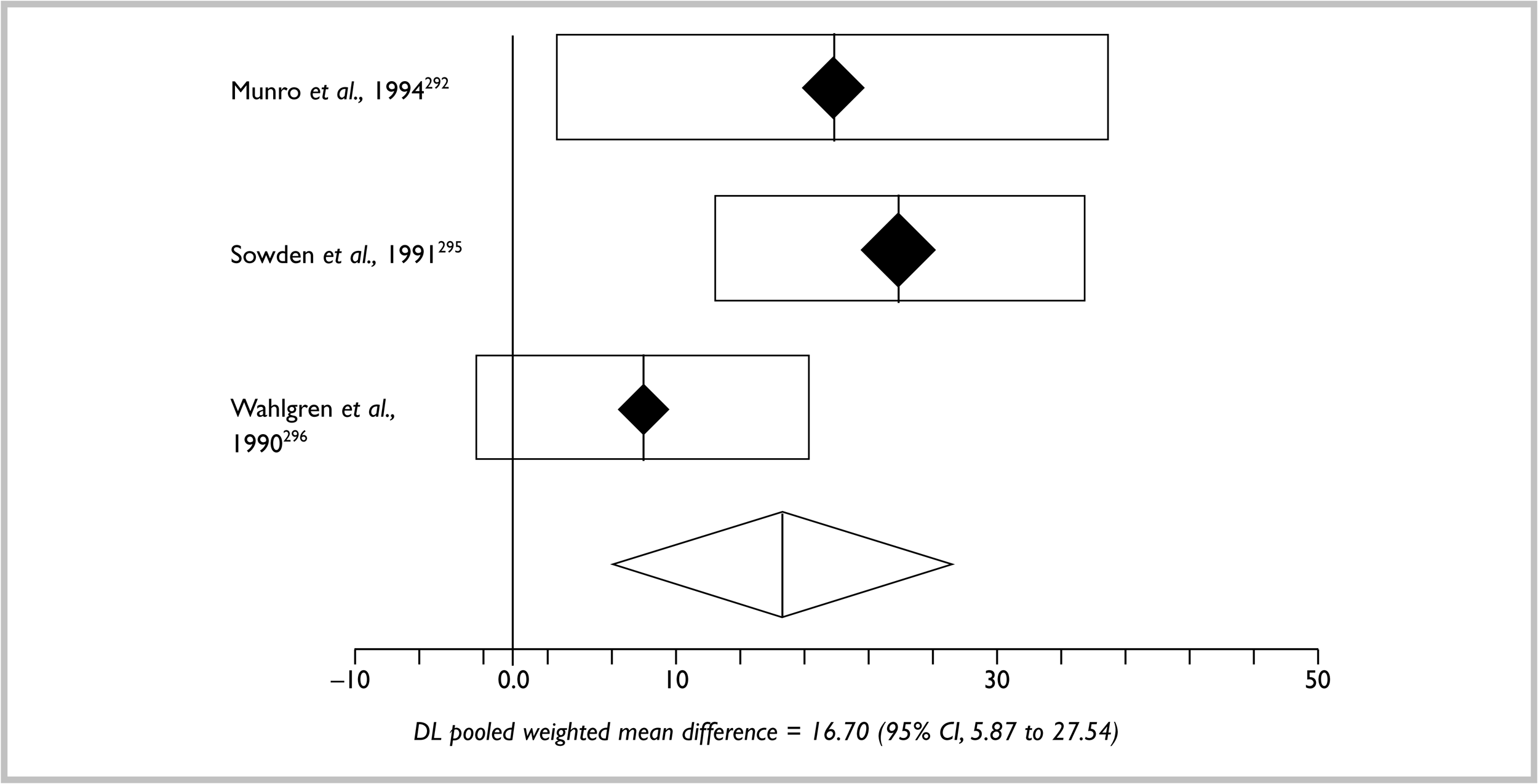
Harms
Despite taking analgesia, 60% of those on active treatment in the Hanifin and colleagues study experienced headache, 32% muscle ache and 39% chills compared with 28%, 12% and 5%, respectively, for those on placebo. A fall in white cell count occurred in five patients on interferon-gamma which normalised with continued treatment. Seven patients on active treatment had mild elevations of liver transaminase levels that did not affect therapy. Three out of the 41 patients treated with intereferon-gamma in the Jang and colleagues study discontinued therapy: two due to disease flare and one due to abnormal liver function tests. Despite taking acetaminophen as required for flu-like symptoms, 54% of those taking interferon-gamma experienced adverse effects including fever and muscle aches. Rates of adverse events in the placebo group were not described.
Comment
Quality of reporting was pleasantly high in the Hanifin study with a clear description of generation of randomisation sequence and an intention-to-treat analysis. Description of placebo and concealment of allocation was unclear, and blinding was likely to have been unmasked due to the marked therapeutic response and interferon-related adverse effects in those on active treatment. Quality of reporting was less satisfactory in the Jang and colleagues study. The methods of randomisation and concealment were not described. The fact that the three groups were of very different sizes (20 and 21 in low- and high-dose interferon versus only ten in the placebo group) suggests that some method other than simple randomisation was used. Blinding was not mentioned in the report, and an intention-to-treat analysis was not carried out.
There seems little doubt that interferon-gamma was markedly effective in these two studies of severely affected individuals, but at a cost in terms of adverse events. The inconvenience and cost of daily injections is a limiting factor in a chronic disease that can last many years.
Thymic extracts and their synthetic derivatives
Impaired T-lymphocyte cell function and sustained serum IgE levels have been described consistently in atopic eczema. This, along with observation that patients with primary T-cell immunodeficiency, such as Wiskott–Aldrich syndrome, have elevated IgE and lesions identical to atopic eczema, has prompted researchers to explore the therapeutic value of agents that promote the differentiation and function of mature lymphocytes. Initial work on calf thymic extracts given as an elixir or injection (thymomodulin and thymostimulin) was superseded by synthetic pentapeptides (thymopentin) given by injection. Thymomodulin is calf thymus acid lysate given orally in syrup form. Thymostimulin is a mixture of heat-stable polypeptides extracted from calf thymus and given by injection. Thymopentin is a synthetic pentapeptide corresponding to some of the amino acid sequences of human thymopoetin, the hormone responsible for promoting differentiation and function of mature lymphocytes.
Benefits
Thymomodulin
Two RCTs of thymomodulin in atopic eczema were identified from the same research group304,305 but could not be combined as each dealt with quite different patient groups and interventions. The Fiocchi and colleagues study304 compared thymomodulin syrup at a dose of 3 mg/kg/day with placebo in 12 children with atopic eczema followed-up for 6 months. They showed improvement in several clinical signs in the intervention group (e.g. extent decreased by 12.3 versus 24.0 in the thymomodulin and placebo groups, respectively, measured on a scale with a maximum of 60 points), and also in a number of blood immunological indices. The Cavagni and colleagues study305 compared thymomodulin syrup at a dose of 120 mg/day versus placebo in a group of 19 children with food allergy who were also placed on restriction diets. At the end of 90 days, significant improvements were noted in only one of four clinical signs (excoriations). Those on thymomodulin appeared to react less to re-challenge with foods that were considered allergic.
Thymostimulin
Two studies have evaluated thymostimulin in atopic eczema, one in adults306 and one in a mixture of adolescents and adults. 307 The Staughton and colleagues study306 has only been published in abstract form and reports that reduction in disease severity (actual values not given and results not statistically significant) was noted in an unspecified number of adults randomly allocated to thymostimulin 1.5 mg/kg twice weekly versus placebo in a crossover study. The Harper and colleagues study307 randomly allocated 29 young adults to thymopentin 1.5 mg/kg twice weekly for 10 weeks versus placebo injections. Co-treatment with topical steroids, emollients and antihistamines was allowed. Of the 26 evaluable patients, the median percentage score (measured on a multiparameter scale) in the placebo group was 99% of baseline compared with 80% in the thymostimulin group (p = 0.008), though there was no statistically significant differences for patient-assessed itch and sleep loss. Longer-term follow-up for 12 months showed a loss of the differences between the two groups within 4 weeks.
Thymopentin
Four RCTs were identified. The patient populations and interventions were not sufficiently similar to permit pooling of efficacy data. The first study by Kang and colleagues311 in 1983 was a small study of 18 participants (mean age 33 years) who were randomised to three times weekly injections of thymopentin (50 mg) or placebo for 6 weeks. Mean improvement in a compound score (maximum 18) was 2.38 and 0.82 in the active and placebo groups, respectively (p < 0.05), with five out of eight participants in the active group and two out of ten in the placebo group reporting ‘good’ improvement (p < 0.05). The second much larger study by Leung and colleagues310 in 1990 randomised 100 young adults with moderate-to-severe atopic eczema in parallel fashion to either daily subcutaneous thymopentin (50 mg) or placebo injections for 6 weeks. They found that improvement in itch was observed in 66% of thymopentin-treated versus 40% of placebo-treated patients (p = 0.02), and also statistically significant differences for global severity scores and eczema extent in favour of those on active treatment. Baseline scores and co-treatment usage was very similar in the two groups. The third study by Stiller and colleagues308 in 1994 randomised 39 adults with severe atopic eczema to three times weekly thymopentin (50 mg) or placebo for 12 weeks. They reported a statistically significant improvement in total severity score (maximum 3) from 2.19 to 1.68 in the active group versus 2.18 to 2.02 in the placebo group (p = 0.029). Overall patient-assessed improvement (on a scale of 1 to 5 where 1 = excellent, 2 = good, 3 = fair, 4 = poor and 5 = very poor) was also statistically significantly greater in the active group (from 3.11 initially to 2.78 at week 12) compared with the placebo group (from 3.00 initially to 2.92 at week 12).
The fourth study by Hsieh and colleagues309 was an unusual one, which was mainly interested in elucidating disease mechanisms. Instead of evaluating the effectiveness of thymopentin, they looked at the effect of withdrawal as surrogate evidence of efficacy. Thus they treated 16 children with three times weekly injections of thymopentin (50 mg) for 6 weeks and then randomised them to continue with either thymopentin or saline injections for a further 6 weeks. The data suggest an impressive decline in total severity score (maximum 15, with higher scores signifying worse disease) from 6.0 at 6 weeks to 12.8 at 12 weeks compared with 5.8 to 4.0 in those who continued on active injections (p < 0.001; values estimated from graph as data not given). All eight patients in the thymopentin group finished the 12-week trial, whereas three out of eight of those later randomised to placebo dropped out because of a flare-up of disease.
Harms
No information on harms was given in the Fiocchi, Cavagni, Staughton, Kang or Hsieh studies. One patient in the Harper trial was withdrawn due to possible thymopentin-induced diffuse alopecia areata. Drop-outs in the placebo and active arms of the Harper trial were very high after 12 months (about 70% in each arm). Fourteen of 48 patients (29%) and ten of 52 patients (19%) in the thymopentin and placebo groups, respectively, reported adverse effects in the Leung study, with no particular differences between the two groups apart from three patients in the active group developing local swelling at injection sites lasting up to 30 minutes. Fifteen out of eighteen patients on thymopentin compared with sixteen out of seventeen on placebo experienced adverse events in the Stiller study, which were not specified any further apart from possibly more cutaneous infections in the placebo group.
Comment
The quality of reporting of studies in this category was generally poor, with none (except the Leung study) explicitly describing randomisation procedure, concealment, success of blinding and none performing an intention-to-treat analysis. Study participants were generally well described. The Fiocchi and colleagues study failed to perform the appropriate statistical test (i.e. they only compared before and after scores for eczema severity instead of the difference between thymomodulin and placebo). The Kang and colleagues study erroneously used statistical tests for paired data when the data were unpaired, and they also put great emphasis on enhanced treatment response in patients under the age of 34 years – a post hoc finding. Success of blinding was also controversial, for example, in the Kang and colleagues study because physicians were able to guess the correct treatment in 72% of patients. Studies were generally very small, introducing the risk of discarding a potentially useful treatment because the studies were under-powered from the outset. The largest study (Leung et al. ) was well described and demonstrated a potentially clinically useful effect of thymopentin in the 6-week trial period. Although the Stiller study also showed a statistically significant benefit, the clinical relevance of the small changes in complex scores is unclear. It is also apparent that the Stiller study was part of a much larger multicentre study whereupon the authors freely admit to a policy of conducting a separate statistical analysis for each centre. However, the number of other centres is never stated, and we have been unable to locate the published data from the other studies. There are therefore strong grounds to suspect publication bias.
Despite these quality limitations, some of the studies do suggest some benefit of thymic extracts/synthetic derivatives in severe atopic eczema, and it is unclear why this mode of treatment was abandoned around 10 years ago. This could be due to cost or the fact that three times weekly injections are not a practical treatment of a chronic skin disease such as atopic eczema, particularly in children.
Immunoglobulin
One small RCT published in French was identified and translated. 312 Based on earlier observations that systemic immunoglobulin may be helpful for nasal and eye allergy, this study evaluated intramuscular injections of immunoglobulin (Allerglobulin™, not available in the UK) versus albumin in a course of ten injections over 3 months. In total, 47 adults and children over the age of 2 years were studied (mean age 15.5 years; age range 2–37 years). Eczema extent and a range of intensity items such as erythema, oedema, itching, and lichenification were recorded, as well as a global evaluation. The authors reported that 72.8% of the 22 patients receiving immunoglobulin had a global amelioration of their disease compared with only 36% of the 25 patients in the control group. Results for all of the intensity items were not fully reported, though it was commented that itching, degree of lichenification, and topography of lesions were all significantly (statistically) improved.
Harms
Adverse effects were not discussed in this paper.
Comment
Positive results are difficult to assess due to their multiplicity and unclear clinical relevance. Randomisation, concealment of allocation, and blinding was poorly described, and it was unclear whether an intention-to-treat analysis was performed. Nevertheless, the positive results of this small preliminary study deserve further work.
Transfer factor
Transfer factor is an extract from white blood cells that is thought to play a key role in cellular immunity. As cellular immunity has been claimed to be impaired in atopic dermatitis, transfer factor has been tried in this condition. One small RCT313 was identified and translated from Spanish. This study compared intramuscular injections of transfer factor with placebo injections in 24 adult outpatients with atopic dermatitis using a parallel group design for a total duration of 8 weeks. The main outcomes of interest to the authors were immunological markers in the blood such as immunolglobulin levels and T-lymphocyte subset counts. Global clinical improvement according to a physician is also recorded. At the end of 8 weeks, six out of 12 patients (50%) in the transfer factor group were reported to have experienced ‘major’ improvement compared with four out of 12 (33%) in the placebo group (not statistically significant). The 95% CI around the 17% difference between the two treatments ranged from –22% (i.e. a 22% difference in favour of placebo), to a +55% in favour of transfer factor. Various statistically significant differences in immunological parameters were noted at the end of the study.
Harms
No adverse effects were reported in this small study. No drop-outs were reported. Intramuscular injections are painful.
Comment
This was a small study lacking power to pick up even moderate clinical benefits, though it is acknowledged that clinical response was not the primary aim of the paper. The method of randomisation was clearly described, and the study was described as double-blind. The method of conceal-ment of allocation was unclear. Numbers were exactly the same in each group (n = 12), which in the absence of blocking, raises concerns as to whether simple randomisation was implemented.
Summary of systemic immunomodulatory agents
Allergen–antibody complexes
-
One small RCT has suggested benefit for allergen–antibody complex of house dust mite in the treatment of atopic eczema.
Cyclosporin A
-
There is no evidence to support the efficacy of topical cyclosporin A in atopic eczema.
-
Oral cyclosporin A is effective in atopic eczema but the long-term adverse effects on kidneys and blood pressure are a serious concern, particularly when treating a young population.
-
The value of a short holiday period of symptom relief in a chronic condition such as atopic eczema is questionable.
-
The cost-effectiveness of cyclosporin or oral steroids versus azathioprine needs to be tested.
Levamisole
-
There is little evidence to support any benefit in the use of levamisole in atopic eczema.
PAF
-
There is no evidence to support a useful treatment benefit of PAF antagonist based on the results of a small right/left comparison study in 36 patients.
Interferon-gamma
-
Daily interferon-gamma injections are an effective treatment for severe atopic eczema but at the cost of frequent flu-like symptoms despite taking analgesia.
Thymic extracts
-
There is some evidence of benefit of thymic extracts/synthetic derivatives in severe atopic eczema, and it is unclear why this mode of treatment has been abandoned.
-
Cost and the need for weekly injections are limiting factors for long-term treatment with thymopentin, particularly in children.
Systemic immunoglobulin
-
One small study of intramuscular immunoglobulin versus albumin suggests marked benefit in children and adults with atopic dermatitis. This study needs to be replicated.
Transfer factor
-
One Cuban RCT of intramuscular transfer factor did not find any evidence of any clinical benefit, though the study was too small to pick up even moderately large clinical benefits.
Systemic immunomodulatory agents
-
At present, there appear to be no effective and convenient systemic immunodulatory treatments with a good long-term safety record available for the treatment of severe atopic eczema.
Chapter 11 Complementary therapies
We define complementary therapies as a group of therapeutic and diagnostic disciplines that exist largely outside the institutions where conventional healthcare is taught and provided. 406
Chinese herbal medicine
Chinese herbal medicine forms part of a system that includes oral and/or topical Chinese herbs, acupuncture, diet and exercise for both treatment and prophylaxis of disease. Medicinal plants of various kinds can be taken orally usually in combination with others as a decoction by boiling them in water, and drinking the ‘tea’ produced, or as external applications directly to the skin. Prescriptions are individually determined based upon an overall assessment of the patient including pulse, appearance of tongue, and disease features, hence, standardised formulae are not generally prepared. Mode of action points towards anti-inflammatory and immunosuppressive properties by down-regulating local T cell-mediated reactions. 407
Benefits
We located one systematic review408 reporting two randomised trials of atopic eczema,314,315 one on adults and one on children, which the authors did not feel were appropriate to pool. Adverse effects such as slight abdominal distension and headaches were highlighted in that review. The authors conclude: “At present it is unclear whether Chinese herbal treatments of eczema do more good than harm.”
In addition to these two trials we have identified a further two,316,317 which evaluated oral Chinese herbal decoction compromising Ledebouriella seseloides, Potentilla chinensis, Clematis armandii, Rehmannia glutinosa, Paenia lactiflora, Lophatherum gracile, Dictamnus dasycarpus, Tribulus terrestris, Glycyrrhiza glabra, Schizonepeta tenuifolia, except Sheehan315 who used Anebia clematidis instead of Clematis armandii. All four RCTs are reported below.
The efficacy study of children by Sheehan and Atherton315 evaluated Chinese herbs (as above) in a decoction versus placebo comprising a mixture of ‘inert’ plant materials, once daily, in 47 children with atopic eczema over an 8-week period. Skin was assessed using a score of 0–3 for erythema, surface damage (the net effect of papulation, vesiculation, scaling, excoriation and lichenification) plus percentage area affected (maximum score 180) and patient preference. Median percentage changes of the clinical scores from baseline were 51% for Chinese herbs compared with 6.1% for placebo for erythema, and 63.1% and 6.2% change for surface damage in the herbs versus placebo groups, respectively. A 1-year follow-up study of the children concludes that Chinese herbal medicine, in the medium term, proved helpful for approximately half the children who originally took part in the RCT. 409
The adult study by Sheehan and colleagues314 evaluated Chinese herbs (as above) in a decoction versus ‘inert plants’ placebo, once daily, in 40 adult patients with atopic dermatitis. Skin was assessed using a score of 0–3 for erythema, surface damage (the net effect of papulation, vesiculation, scaling, excoriation and lichenification) plus percentage area affected. Maximum score was 180 and patients' subjective comments included itch, sleep loss and preference. Geometric mean total body score for erythema at the end of Chinese herbs treatment was 12.6 and at end of placebo phase was 113 (baseline scores not given). The geometric mean for surface damage at the end of Chinese herbs treatment was 11.3 compared with 111 at the end of placebo phase (baseline values not given).
The study by Latchman and colleagues317 evaluated the same combination of Chinese herbs as above (finely ground) versus the same Chinese herbs in a new palatable form of freeze-dried granules in 18 patients with atopic eczema over an 8-week period. Skin was assessed using a score of 0–3 for erythema, surface damage. There was a significant reduction in erythema and surface damage compared with baseline (p < 0.001). The groups showed no difference in clinical outcome between formulations.
The study by Fung and colleagues316 evaluated the same combination of Chinese herbs above versus ‘inert plants’ placebo in 40 patients with atopic eczema over an 8-week period. Scores based on the severity and extent of erythema, surface damage, lichenification and scaling were recorded. There was a general trend of clinical improvement for both Chinese herbs and placebo. There was no statistically significant treatment effect over placebo for all four clinical parameters, except for lichenification at week 4.
Harms
Unpalatability of the herbs in both active and placebo groups was a common adverse effect causing ten drop-outs in Sheehan and Atherton315 study and eight drop-outs in the Sheehan and colleagues314 study. Other adverse effects included abdominal distension, headaches, transient dizziness, gastrointestinal upsets, one lichenoid eruption and one facial herpes. There is a concern with Chinese herbs of potential hepatotoxicity; however, all the studies, except Latchman and colleagues carried out pre and post-treatment liver function tests with no abnormalities detected.
Comments
All studies were randomised but method and concealment of allocation were not described. All were described as double-blind, except Latchman and colleagues317 where no blinding was mentioned. No intention-to-treat analysis was carried out. It is questionable whether the placebo plants are truly inert in the treatment of eczema. The children study by Sheehan and Atherton315 reports large effects from Chinese herbal medicine highlighting a promising treatment of atopic eczema. This has not been replicated in the other studies, though they are all quite similar. Clearly more RCTs with larger sample sizes over a longer period of time are needed.
Massage therapy
It is possible that massage therapy might be beneficial in atopic eczema as a stress-reducing and enjoyable interaction between parent and child, by increasing peripheral circulation (which may be defective in atopic eczema) or by increasing compliance with topical treatments. One small RCT of massage therapy in young children has been identified. 321
Benefits
Twenty children with atopic eczema (mean age 3.8 years) were randomised to continue with standard therapy with topical corticosteroids, emollients and antihistamines or standard therapy plus a course of daily 20-minute massage following video demonstration for a period of 1 month. Parents in the massage group reported greater degrees of improvement in anxiety scores, tactile defensiveness, and a coping index when compared with the control group. Certain eczema activity signs (e.g. scaling and excoriation) improved statistically from baseline in the active group compared with only scaling in the control group, though the appropriate statistical comparison of differences between the two groups was not done.
Harms
No adverse effects were reported in this study. The cost of instruction by a therapist and video for one session was estimated at $30.
Comment
This small pilot study showed that parents and their children who were allocated to massage therapy in addition to their standard care were less anxious and more able to cope. Even though much of these effects could have been partly due to the unblinded nature of the study, increased coping with a chronic disease is a desirable goal. It appears that the technique of massage can be taught cheaply and quickly. It is unclear whether the technique has any specific benefit on overall atopic eczema activity, and more trials in other countries are needed.
Hypnotherapy/biofeedback
Hypnotherapy and biofeedback used to develop relaxation techniques with or without mental imagery may be beneficial in the management of atopic eczema to distract from the symptoms associated with the itch–scratch–itch cycle. 320 One RCT has been located that addresses the use of these techniques in atopic eczema. 320
Benefits
Forty-four children with atopic eczema were randomised to either hypnotherapy, biofeedback or discussion only, for a period of 20 weeks after being stabilised on topical and oral treatment in a 2-week run-in period. This study attempted to measure changes in the objective symptoms of erythema, surface damage and lichenification, which resulted from attempts to reduce children's subjective experience of itching (and subsequent scratching) using:
-
relaxation that focused specifically on reducing itching (hypnotherapy)
-
relaxation that did not involve any direct imagery per se (biofeedback)
-
an ‘attention placebo’ group who were encouraged to discuss the eczema without any mention of symptom control.
The children in the hypnotherapy and biofeedback groups showed a significant reduction from baseline in the severity of surface damage and lichenification compared with the control group. There was no difference between the two relaxation techniques. Erythema was not changed by the interventions.
Harms
No adverse effects were reported in this study.
Comments
This study shows that relaxation techniques, with or without direct imagery, may be of some benefit in the management of atopic eczema. The girls in the hypnotherapy group showed greater improvement than the girls in other groups and showed greater improvement than the boys in the hypnotherapy group. Lack of blinding threatens the validity of the study. The authors state that all the parents and children in the study were aware that the aim of the study was to help them with their symptoms further threatening the validity of the study. In particular the ‘attention placebo’ was designed to avoid mentioning symptom control. There were 13 drop-outs but no explanation was given for reasons. No intention-to-treat analysis was carried out, hence, it is not clear what effect the high number of drop-outs had on the results.
Homeopathy and aromatherapy
We located one study protocol318 in German with an English abstract assessing the efficacy of classical homeopathic treatment in 60 patients with atopic dermatitis. The patients were randomised to receive a homeopathic treatment or a placebo for a period of 8 months. The homeopathic doctor was free to change remedies, dosages or potencies if required by the reaction or a new case-picture of the patient presented according to classical homeopathy principles and guidelines.
We located one abstract319 of a preliminary study on the effect of aromatherapy on childhood atopic eczema. Sixteen children were treated with either counselling and massage with essential oils by both the therapist and the mother or the same treatment without essential oils. Parent-assessed day-time irritation score, night-time disturbance scores and general improvement scores were assessed for a period of 8 weeks. The results showed a statistically significant improvement of the eczema in the two groups of children following therapy, but there was no significant improvement shown between the experimental and control groups. Correspondence with the author confirms the study was randomised. The full report will be available shortly.
Summary of complementary therapies
Chinese herbs
-
Two studies of Chinese herbal treatment conducted in children and adults by the same research team found significant benefits compared with placebo.
-
Two further RCTs conducted by independent groups failed to demonstrate any clear clinical benefit.
-
Further larger and long-term RCTs of Chinese herbal treatment seem worthwhile.
Hypnotherapy/biofeedback
-
One unblinded study of hypnotherapy and biofeedback suggests a benefit in terms of surface damage and lichenification but not erythema.
Aromatherapy
-
One small study of massage with and without essential oils plus counselling has suggested benefits of counselling and tactile contact but no benefit from addition of essential oils.
Massage therapy
-
One small study of massage therapy in addition to standard care in children has suggested benefit in terms of reduced anxiety and better coping skills.
Chapter 12 Other interventions
Nitrazepan
Nitrazepan is a widely used benzodiazepine drug for night-time sedation. As itching at night can be a major problem for patients with atopic eczema, the benefit of nitrazepam in atopic eczema has been evaluated by an RCT conducted by Ebata and colleagues. 322
Benefits
Ten adult outpatients with atopic eczema were entered into a double-blind placebo-controlled crossover trial of three successive nights whereby they were given either 5 mg of nitrazepan or a placebo, with a washout interval of 4 days. An infrared video camera to identify bouts of scratching lasting more than 5 seconds was used to calculate the percentage of total scratch time in each group as an index of nocturnal scratching. Total scratch time was very similar between the two groups, occurring in 6.5% of the time for those taking nitrazepan compared with 5.4% of the time with placebo. Frequency of bouts of scratching was slightly less in the nitrazepan group, but the mean duration of scratching bouts was longer in the nitrazepan group compared with placebo (both comparisons statistically significant at the 5% level). Degree of itching and the condition of atopic dermatitis did not change during the 2 weeks of the study.
Harms
Although no adverse effects were mentioned in the results section, the authors comment that none of the patients experienced any rebound insomnia or residual sedative effect following the nitrazepan tablet.
Comment
This very small study lacks power to exclude moderate-to-small treatment benefits of nitrazepan, though there was no indication of any benefit in the patients studied. The most interesting thing about the study was the novel method use to assess nocturnal itch, though it remains to be seen whether this objective measure is a good predictor of general eczema improvement as measured by validated scales or patient-evaluated measures.
Ranitidine
The histamine type 2 receptor antagonist, ranitidine, modifies the immune system, possibly by its inhibition of histamine activity. Based on the observations that a few atopic patients treated with ranitidine for gastric ulcer have improved, Veien and colleagues323 conducted an RCT of ranitidine treatment for hand eczema in patients with atopic eczema versus placebo.
Benefits
Forty-seven adults with a clear description of hand eczema and atopic eczema elsewhere (allergic contact eczema excluded) were randomly allocated to oral ranitidine, 300 mg twice daily or placebo tablets of identical appearance for a total of 4 months. A potent topical steroid cream (beta-methasone valerate) and lubricating ointment to be used on the hands only was permitted throughout the trial. Thirty-eight of the 47 patients completed the 4-month trial, and intention-to-treat analysis was conducted. The total in a composite position-assessed sign score was reduced from a mean of 10.17 to 4.91 in the group receiving ranitidine and a topical steroid compared with a reduction from a mean of 10.58 to 7.46 in the group receiving placebo in the topical steroid (p = 0.07). Most of this reduction was due to significant reduction in area involvement. Seventeen out of the 23 patients treated with ranitidine reported clearing or marked alleviation compared with eight out of the 24 patients in the placebo group (p = 0.02).
Harms
No adverse effects from either ranitidine or placebo were reported in this study.
Comment
Although most trials of ‘hand eczema’ had to be excluded from this report because the nature of the eczema was unspecified, this study provides a clear description to indicate that those included probably had atopic eczema as the sole cause for their hand dermatitis. Although the method of randomisation, concealment and degree of success of blinding is unclear, the intention-to-treat analysis was helpful. The proportion of patients cleared or markedly alleviated (a combined physician/ patient score) and other composite scoring methods suggest a modest benefit of ranitidine in this subgroup of adult atopic eczema patients. It is important to replicate the results of this single RCT.
Theophylline
The β-adrenergenic theory of atopy implies a general defect of β-receptors in atopic eczema patients leading to low levels of cAMP within cells. In order to test the importance of the β-adrenergenic theory in atopic eczema, Ruzicka324 conducted a small RCT crossover study of the phosphodiesterese inhibitor theophylline (which increases cAMP levels) versus placebo in adults with atopic eczema.
Benefits
Fourteen adults were included in the study, 12 of whom were evaluable at the end of 2 weeks. They took either 300 mg of a theophylline/ethylenediamine preparation or identical placebo tablets daily in addition to antihistamines. At the end of the 2-week period, the mean number of antihistamine tablets used by the patients was 1.65 and 1.78 in the theophylline and placebo periods, respectively. Mean symptom score was 1.82 in the theophylline and 1.68 in the placebo period, and sleep disturbance was 5.0 out of 14 nights in the theophylline group compared with 4.4 out of 14 in the placebo groups. No other differences were statistically significant.
Harms
No adverse effects were mentioned in this study, but theophylline is a drug with a narrow therapeutic range which can result in cardiac toxicity.
Comment
Methodological details of this short report are scanty. Although there was no obvious difference between the two groups, the study was very small and of very short duration. It is difficult to exclude any possible benefit of theophylline on the basis of this study.
Salbutamol
Based on previous animal studies, which demonstrated that the β2-adrenoceptor agonist salbutamol can reduce inflammation, Archer and MacDonald325 conducted an RCT of salbutamol ointment (1% base in white soft paraffin, twice daily) plus a placebo oral tablet with oral salbutamol (a slow-release spandet 8 mg twice daily plus white soft paraffin placebo ointment twice daily) versus a placebo spandet and white soft paraffin only in a 2-week crossover study in 20 adults with atopic eczema.
Benefits
Itching, number of affected zones, skin thickening, vesiculation, epidermal change and redness were recorded as outcomes and none of these showed any clinically useful or statistically significant changes. Reduction in the score for redness was highlighted by the authors as being statistically significant in favour of the ointment and tablet salbutamol when compared with placebo, though baseline scores were very different. Baseline redness score for patients on salbutamol ointment was 22 at the beginning and this decreased by 9.5 at Day 14 (maximum possible score 60). Baseline median score for oral salbutamol was 29 with a median decrease of 10.5 compared with a baseline score of 14 for the placebo and median decrease of 8.5.
Harms
There were five withdrawals with three due to adverse effects. Tremor was reported by five patients taking oral salbutamol and in one patient using the salbutamol ointment. Some degree of systemic absorption of salbutamol ointment was demonstrated in two patients.
Comment
The method of randomisation, concealment of allocation, and investigator blinding in this study was not described. No intention-to-treat analysis was performed. Although the statistically significant improvement in redness in those taking salbutamol has been highlighted, this was not declared as a main outcome measure out of the six outcome measures beforehand, and could probably be explained by regression to the mean given the higher baseline scores for those taking salbutamol. Although no clinically useful benefits of salbutamol ointment were demonstrated in this study, it is probably too small to exclude even large treatment effects.
Papaverine
Papaverine is a naturally occurring compound found in opium but lacking in the narcotic activity. It is a potent inhibitor of the enzyme phosphor-diesterase and it is this property that provides a possible beneficial action for atopic eczema. Atopic eczema is characterised by elevated phosphor-diesterase levels in mononuclear cells. Papaverine has been advocated for many years for the treatment of atopic eczema, and based on a previous open study, Berth-Jones and Graham-Brown326 conducted a crossover RCT of oral papaverine versus placebo in atopic eczema. Another RCT was published a year after by Shupack and colleagues327 of a similar small placebo-controlled crossover trial of oral papaverine hydrochloride in the treatment of atopic eczema.
Benefits
In the Berth-Jones and Graham-Brown study, 50 patients with a mean age of 25.6 years were randomised to receive either papaverine hydrochloride 100 mg four times daily or 60 mg four times daily for children under 12 years, or matching placebo each for 4 weeks. All patients had moderate-to-severe atopic eczema and were allowed to continue with emollients, a bath oil and a topical steroid preparation throughout the trial. Outcome measures included itching assessed on a visual analogue scale by patients, clinical scoring of extent and severity and rate of usage of topical steroid preparation. Of the 45 evaluable patients, mean itch score in the last 7 days of each treatment period was 58.6 in the active phase compared with 55.7 in the placebo phase (maximum score 140). Clinical score (maximum of 720) in the active phase was 178 and 176 in the placebo phase. Baseline scores were not presented in the papers. Topical steroid usage was very similar between the two groups.
In the Shupack and colleagues study, 30 patients aged 18 and above were randomised into a crossover study of papaverine hydrochloride in doses of 150–300 mg three times daily compared with placebo as an adjunctive treatment to emollients and topical steroids. Of the 20 (out of 30) patients who completed both phases of the crossover, no statistically significant advantage over placebo for any of the parameters of itching, physician's and patient's global evaluation were reported. Apart from non-significant p-values, the actual data for these changes were not presented in the paper.
Harms
No serious adverse effects were reported in the Berth-Jones study and symptoms such as tiredness were similar in both groups. In the Shupack study, however, three of the study patients on active treatment developed abnormal liver function tests, which were not due to infectious hepatitis. Nausea occurred in 46% of patients on papaverine compared with 27% on placebo, though this difference was not statistically significant.
Comment
The method of randomisation in both of these studies was unclear as was concealment of allocation of randomisation. Drop-out rates were modest in both studies and no intention-to-treat analysis was performed. Although both studies were small, the Berth-Jones study in particular provides additional data to inform the reader on the possibility of missing clinically useful benefits. Based on their results, the power of their study to detect the 25% improvement in the itch score was over 85% and the power of the study to detect a 25% improvement in the clinical score was between 75% and 80%. Although the authors clearly started the trial with an enthusiasm for papaverine based on a previous open study, the study has demonstrated the need to use methods such as RCTs to reduce the possible bias associated with the reporting of such open studies. The abnormal liver function tests in the Schupack study are also a cause for concern.
Suplatast tosilate
It has been suggested that a rebound phenomenon occurs in people with atopic eczema who have been treated for prolonged periods with strong topical corticosteroids. We found one small RCT328 that evaluated the role of an anti-allergic medication called suplatast tosilate (which down-regulates production of IgE and related cytokines) versus bufexamace ointment to prevent rebound from topical steroids in atopic eczema.
Benefits
Thirty-two patients who had been treated with strong steroid ointment for several years were randomised to either bufexamace ointment (a non-steroidal antiinflammatory ointment) or bufexamace ointment and oral suplatast tosilate (400 mg/day). In the control group, 15 patients experienced the rebound phenomenon after 2 weeks compared with only two of 17 patients in the active group (rebound was undefined). Several cytokines increased in the control group but not in the active group.
Harms
No adverse effects were reported in this small study.
Comment
The issue of rebound from regular use of topical steroids is a serious and important one as it is possible that the regular use of corticosteroids increases the chronicity of disease while benefiting the short-term control of flare-ups. This small study was unblinded (the control group did not have an oral placebo) and the ‘rebound’ was completely undefined and therefore highly prone to investigator bias. The study should be followed by a randomised, controlled double-blind trial over a long period with clinical outcomes and a vehicle-only comparison group.
Summary of other interventions
Nitrazepam
-
One small RCT failed to show any benefit of nitrazepam at night for night-time scratching as detected by infrared camera.
Ranitidine
-
One RCT has suggested a modest benefit of oral ranitidine treatment above placebo for hand eczema in patients with atopic eczema. These results need to be replicated elsewhere.
Theophylline
-
One RCT of 12 patients has compared oral theophylline versus placebo for 2 weeks in atopic eczema and not found any treatment benefits.
Salbutamol
-
There is no RCT evidence to support the use of topical or oral salbutamol in atopic eczema.
Papaverine
-
Two small RCTs do not suggest that oral papaverine has a clinically important benefit in the short-term treatment of atopic eczema.
Suplatast tosilate
-
One small unblinded study suggests a possible benefit of suplatast tosilate in preventing the steroid ‘rebound phenomenon’, but is difficult to interpret in the absence of a vehicle-only group.
Chapter 13 Discussion
Treatments with no RCT evidence
As pointed out in chapter 1, a systematic review that is driven only by published RCT data can only answer questions that have been asked by such trials. Many other treatments, combinations of treatments, and management approaches are used for people with atopic eczema throughout the world. Some, such as climatotherapy are probably very rarely used if at all in the UK, whereas others such as wet-wrap bandages and oral azathioprine are used by a large number of UK dermatologists. 36 Questions need to be asked about these therapeutic options in order to identify possible research gaps. Therapies that had not been identified by our team were canvassed from colleagues and professional networks as described in chapter 2, and the results are shown in Table 38.
Division between RCTs and no RCTs is arbitrary
Table 38 probably only identifies some of the questions that can be asked of therapies that are currently used in atopic eczema. Some treatments (e.g. type IV phosphodiesterase inhibitors) are relatively new and experimental, and clinical trials will hopefully be conducted (or are currently in progress) in the near future. It should also be remembered that just because treatments for which some RCT evidence was found, mentioned in the previous chapters, this does not mean that the questions regarding each of those interventions have been answered. Thus in the section on emollients, there are at least ten important unanswered questions remaining. Similarly, just because there is one RCT evaluating deep-sea water versus physiological salt water does not mean that the evidence for salt water baths has been ‘sorted’, as no RCTs have been located that answer the more urgent question of whether salt baths have any benefit above ordinary baths. The division between treatments for which no RCTs could be found and those for which some RCTs were found is therefore somewhat arbitrary. Table 38 should be viewed as an indication of some aspects of therapies that were not discussed in the commentaries in the results chapters, rather than as a comprehensive blueprint of unanswered questions for future primary and secondary research.
| Pharmacological | Complementary treatments | Miscellaneous |
|---|---|---|
|
|
|
Difficult sites and combinations of treatments
In addition to the interventions mentioned in Table 38, consideration also has to be made of treatment of atopic eczema at specific difficult body sites such as the scalp or backs of hands, as there may be specific issues such as formulation, penetration, cosmetic acceptability and adverse effects related to such sites. It has also been pointed out that future studies should consider evaluating entire management approaches that mimic real practice. Thus, combinations of treatments such as emollients, topical steroids and education should be evaluated together rather than in isolation (Meredith B, personal oral communication, 1999).
Prevention
It is also important to always consider prevention of atopic eczema in the widest sense by means of intrauterine or early life environmental manipulation. 16 Although the links between the environment and atopic eczema are still in the early stages of research,5 energy needs to be directed at prevention as well as treatment of established cases with pharmaceutical agents that at best only control symptoms.
Diagnostic tests
Although diagnostic tests are not regarded strictly as ‘treatments’, certain tests such as patch tests also need to be evaluated as an aspect of the management of atopic eczema. This is because discovering a superimposed contact allergy (e.g. to lanolin, a steroid or a preservative in a cream) could significantly improve the disease, which had all been put down to constitutional factors. Ideally the benefit of such testing, which has considerable time and health costs if applied to all atopic eczema sufferers, needs to be put to the test by means of RCTs. This will reduce selection bias and will also permit evaluation of clinical outcomes as opposed to just positive patch test results, the clinical significance of which is not always clear in atopic eczema patients. Likewise, the popular request for ‘allergy tests’ among families of children with atopic eczema in the belief that their child's atopic eczema is caused by one specific allergy, needs to be put to the test in an RCT with long-term clinical measures rather than blood or skin tests as outcomes. This applies to the lucrative high-street industry of performing ‘allergy tests’ on vulnerable atopic eczema sufferers in addition to the more conventional tests performed in hospitals. 410
Horizon scanning
It is likely that the next 5 years will witness an increase in the number of topical pharmaceutical agents for treatment of atopic eczema. Thus treatments such as tacrolimus and ascomycin derivatives are already well down the road to development and evaluation, and others such as cytokines and phosphodiestarase inhibitors are following. Two recent review articles have considered future developments. 26,391
Validity and robustness of results
Sensitivity analyses in the traditional sense of exploring the effects of removing certain studies with particular characteristics within a meta-analysis is very limited within this report due to the little pooling that was possible. Further consideration of the validity of the results of this report is worth a brief mention in this section.
Missed studies
It is acknowledged that a predominantly electronic bibliographic database search will miss certain RCTs that have been misclassified on those databases, articles from journals not listed on those databases and unpublished studies. In terms of a reference standard for published studies, we compared the results of handsearching the entire contents of Clinical and Experimental Dermatology for atopic eczema trials and those with our electronic searching. None of the five controlled trials had been missed by our searching methods. Two were included as RCTs, two excluded as non-RCTs and a further study was excluded as it evaluated experimentally induced reactions in atopic eczema patients. Our yield of RCTs of antihistamines in atopic eczema (31 reduced to 21 after evaluating the hard copies) was very similar to the yield identified by a more intensive independent search by members of the Cochrane Skin Group (Diepgen P, personal oral communication, 2000). We found eight more RCTs for antihistamines than a recently published ‘systematic review’ of antihistamines in atopic eczema. 362
Given the fact that at least 200 specialist dermatology journals have been identified (Delamere F, personal oral communication, 2000), many of which are not registered with MEDLINE, it is likely that some RCTs of atopic eczema have been missed, particularly in non-English and less-well-read journals.
The authors also suspect that there is a large body of unpublished data held by pharmaceutical companies for various reasons. Clinicians who have lacked the time or motivation to publish their results also hold such unpublished data. It is likely that more ‘negative’ studies fall into these categories. Estimating the magnitude of this hidden part of the iceberg of evidence is difficult without additional research. In the field of evaluating evening primrose oil for instance, two of the authors of the current report were commissioned by the Department of Health in 1997 to conduct a meta-analysis of all trials. Sadly, permission to publish this report has never been granted, but it did contain the results of nine additional small unpublished studies held on file by the company. Despite writing to the company for any unpublished data for this report, no data have been forthcoming to date (Tables 39 and 40). The extent of holding unpublished data on file by pharmaceutical companies is difficult to assess, but it is likely to continue to some degree if the UK drug licensing process (which is privy to all such data) maintains its current code of keeping such data out of the public domain. 411 Some large drug companies such as Glaxo Wellcome and Schering Healthcare have recently signed-up to a policy of making all unpublished RCT data available to Cochrane reviewers. This is a welcome development. There is a case to be made for it to be compulsory that all clinical data relating to trials on patients within the NHS to be made available as part of ethical approval of any study.
| Company | Response? | Result |
|---|---|---|
| Glaxo Wellcome | Yes | All in public domain |
| Janssen-Cilag | No | |
| UCB Pharma | No | |
| Hoescht Marion | Yes | No RCTs |
| Schering-Plough | Yes | No new, all in public domain |
| Wyeth | No | |
| Stafford-Miller | Yes | No RCTs |
| Novartis | Yes | No new RCTs |
| Merck Sharp | No | |
| Pfizer | Yes | No research in this area |
| Sinclair | Yes | No RCTs |
| Fisons | No | |
| Searle | Yes | Compiling data |
| Company | Response? | Result |
|---|---|---|
| Leo | Yes | Six RCTs |
| Summary of FU9202DK unpublished trial excluded because unspecific hand eczema, Ramsay 96 already included, excluded Poyner 1996 unclear whether had atopic eczema, and Hill 1998 hand eczema, one, two included:Wilkinson 1985 and Thaci 1999 | ||
| Galderma | No | |
| Bioglan | Yes | Two RCTs |
| Berberian 1999 and Drake 1994 already included both trials on file | ||
| No unpublished papers available | ||
| Yamanouchi | No | |
| Crookes | Yes | No RCTs but a very useful file sent containing research on E45 emollient published and unpublished data |
| Seton | No | |
| Scholl | No | |
| Kestrel | No | |
| Quinoderm | No | |
| Bristol Myers | Yes | No unpublished RCTs carried out |
| Steifel | No | |
| Dermal | Yes/No | No unpublished RCTs |
| Schering Healthcare | Yes | None on file |
| Typharm | No | |
| Phyto pharmaceutics | Yes | |
| Squibb | No |
Author bias
As stated in chapter 2, blinding of authors/ institution was unrealistic as certain key words would have immediately identified the study to one of the reviewers who is very familiar with the field. Although bias in the way certain drugs or interventions are described is bound to happen in any subjective narrative report, the authors have striven to minimise such effects by adopting a standard approach and by explicitly separating the reported data from their own comments.
External validity
Only one127 out of 283 studies contained a clear indication (such as the words general practice, community or primary care in the title, abstract or methods) that the study was carried out in a primary care setting. Given that most cases of atopic eczema are treated in the UK, the generalisability of the results of the studies conducted in a hospital setting summarised in this report may be limited. The magnitude of benefit from say, a topical corticosteroid, may be reduced substantially in milder disease in the community as there is less potential to improve from a higher baseline severity score. Other issues such as patient preference, and different concordance rates in primary care may further limit the generalisability of studies conducted in well-motivated patients in hospital. It is for these reasons that future pragmatic trials should be considered in primary care.
The need for updating
Inevitably, RCTs are continually being performed in atopic eczema, and there will be a time when research stands still in order to summarise the totality of evidence. Based on our own search updates, we estimate that around one new RCT on atopic eczema is published each month. In addition, we are aware of at least ten ongoing RCTs in atopic eczema including interventions such as topical corticosteroids, Montelukast and tacrolimus through informed contacts, the Cochrane Skin Group and the National Research Register. Those making treatment guidelines or recommendations based on the results of this report are therefore advised to update their conclusions with further searches. Those topic areas that will be taken forward as Cochrane Reviews will be updated automatically as part of the Cochrane Collaboration process.
Chapter 14 Summary and conclusions
Research included in the review
Coverage and clinical relevance
To the best of our knowledge, this is the first ever comprehensive glimpse of all RCTs of atopic eczema conducted to date. The quality and quantity of 272 included RCTs covering treatments for atopic eczema is highly variable, and the clinical relevance is often difficult to understand because of complex quantitative outcome measures. It is clear that most RCTs have been about issues that are important to the Pharmaceutical Industry, often competing for a niche in a ‘me too’ market. This is understandable, but as chapter 13 pointed out, there is a major discrepancy between what answers these studies provide and what physicians and their patients often ask. As Figure 6 suggests, issues such as prevention of atopic eczema, manipulation of trigger factors and organisation of care are mere twigs in the tree of atopic eczema RCTs at present.
FIGURE 6.
The tree of RCTs of atopic eczema over the past 40 years has been a lopsided one
Much investment has gone into evaluating different topical corticosteroids, yet we still know little about the best way to use them. Little is known about other simple cheap alternatives such as topical coal tar, use of bandages and salt water baths. Many newly developed and potentially toxic drugs such as cyclosporin A have been thoroughly studied (12 RCTs), yet there is a complete absence of RCTs on some alternatives such as azathioprine and oral steroids. Such a discrepancy can give rise to the illusion that one is useful and the other is not in the current climate of evidence-based medicine, whereas the correct conclusion is that there is insufficient evidence to decide between them at present. The lack of informative RCTs for the most widely used treatments for atopic eczema (i.e. emollients and bath additives) is striking. This is particularly so when a recent detailed economic study in Nottingham suggested that the emollient and bath oils accounted for 81% of total NHS prescribing costs for children with atopic eczema in the community. 412
In addition to the ‘pull’ of the Pharmaceutical Industry's agenda, lack of public investment into researching the treatment of atopic eczema has been another factor leading to the unbalanced coverage of the tree of RCTs in atopic eczema. We did not identify one RCT of atopic eczema supported by the UK Medical Research Council in our search. Clinical trials are expensive and time consuming to run, and it is a credit to some working in the NHS that they have managed to carry out well-designed large independent RCTs in their own time. 243 It is also possible that some of the obvious gaps in researching atopic eczema treatment have not been addressed simply because researchers working in relative isolation have not asked the right questions, or because the more practical questions concerning comparison of several commonly used treatments are too difficult or are perceived as less interesting to researchers than testing a ‘new’ drug.
Despite the authors' familiarity with the subject area, some genuine surprises occurred as a result of the review, such as the finding of two RCTs that suggest cotton clothing is no better than soft synthetic fibres, identifying a well-conducted RCT on bioresonance that did not show any benefit, and finding out that there is no good RCT evidence to support the use of topical antibiotic/corticosteroid combinations. On the positive side, there were some RCTs indicating a potentially useful benefit of psychological and various non-pharmacological approaches. It was also interesting to locate five RCTs that suggested there was little, if any, advantage in using topical corticosteroids twice as opposed to once daily as this might have clear benefits to patients in terms of convenience of fewer adverse effects, as well as offering potentially very large cost savings to the NHS if adopted on a national scale.
What about non-RCT data?
While it is perfectly appropriate to use non-RCT data to answer questions on natural history and adverse effects, the authors have stuck to their policy of only considering the RCT as the study design that is able to minimise bias the most. Throughout the report there are examples of RCTs (levamisole299 in chapter 10 and papaverine327 in chapter 12) not demonstrating any benefit to an intervention despite earlier enthusiastic results from non-RCT studies. The RCT design does not guarantee freedom from bias of course, and threats to the validity of individual studies have been commented upon throughout the report.
Quality of reporting
Quality of reporting was generally very poor in most of the studies. Our primary quality criteria of a clear description of generation of the randomisation sequence, concealment of allocation of randomisation, adequate description of blinding and an intention-to-treat analysis were hardly ever fulfilled. These features have been shown to lead to biased estimates of treatment effects. 46 Some studies just reported p-values and no data. Others have performed multiple significance tests on six or more different physical signs at different time intervals and highlighted those that are positive without declaring any a priori main outcome measures. This is akin to throwing a dart and drawing a dartboard around it. 413 Other common problems were lack of CIs, failure to take different baseline scores into account, and testing changes between baseline for two drugs separately as opposed to comparing the change in scores between treatments. 414
Many studies, particularly earlier ones, were clearly under-powered. This is not necessarily a problem providing they have been interpreted correctly with CIs to present a range of plausible treatment effect, but this was seldom the case. 415 More worryingly was the fact that several authors misinterpreted lack of evidence of treatment benefit in small studies as being equivalent to evidence of no effect. Duplicate publication was also rife, as indicated by the list shown in appendix 4.
The situation has probably improved over the past 10 years, particularly with the major dermatology journals. Thus, studies such as that conducted by Boguniewicz and colleagues159 are a pleasure to read as there is a clear description and flow chart of what has happened to all those who were originally entered into the study. These changes probably reflect a change in standards of clinical trial reporting in larger journals. The recent adoption of the CONSORT statement (designed to improve clinical trial reporting) by journals such as the Archives of Dermatology, Journal of the American Academy of Dermatology and the British Journal of Dermatology is a welcome step forward. 416
Study design issues
Five points are worthy of further consideration in terms of study design for atopic eczema studies and these are discussed below.
Studies need to be longer
Perhaps the most important point is to encourage longer duration of future RCTs of atopic eczema. For most people, atopic eczema is a chronic intermittent disease, and studies that evaluate numbers of relapses and duration of symptom-free periods are required in addition to studies that measure short-term reduction in clinical signs.
Awareness of a large placebo/vehicle effect
As some studies have demonstrated,299 the placebo or vehicle effect in atopic eczema can itself account for around 30% improvement. This needs to be taken into account when conducting sample size estimates for RCTs.
Profusion of outcome measures of uncertain clinical significance
Given such a range of outcome measures and complex scales with different symptom and sign weightings in atopic eczema, there is plenty of scope for introduction of bias when assessing a new drug by selecting a scale that is likely to enhance the specific feature which the drug is designed to improve (e.g. erythema or itch). Many of the ‘named’ scales have not been tested adequately. 417 In many studies, the word ‘validated’ when referring to a scale simply meant that it had been used before. There are almost as many un-named scales as there are trials, and these may introduce a major bias towards enhancement of treatment response. 418 There has been a tendency to concentrate on the physical signs of atopic eczema in such scales to the exclusion of patient data on the basis that the former is ‘objective’ and the latter is ‘subjective’. Yet the variability of the ‘objective’ measures is often more than patient's symptoms, and their repeatability between physicians is often poor. 31 The clinical significance of a difference in a quantitative score of 13.4 between two treatments is also difficult for physicians to relate to patients. 337 Greater consideration should therefore be given to including patient-derived outcome measures alongside the physician-based scales, and use of scales should be restricted to a few well-tested ones unless there are good reasons to do otherwise. Future studies need to make it very clear whether the arm of treatment is to control symptoms, improve quality of life for patient and family, or to clear the entire rash (which may be unrealistic at present).
Crossover designs
Although the efficiency in terms of reduced numbers for a crossover design is attractive for trials of agents in atopic eczema, the fluctuating nature of the disease makes it less suitable for this type of approach. Data from the second half of a crossover study may have to be discarded in the presence of a period or carry-over effect, though this has rarely been tested for in the trials. Left/right body or limb comparisons are also popular but introduce problems with blinding and systemic absorption. Simple parallel group RCTs that are pragmatic in nature are most justifiable.
Separate atopic eczema patients from others
As can be seen from appendix 2, there is a large wastage of RCT evidence for many interventions that have included people with atopic eczema, as they have been lumped in with other inflammatory dermatoses such as psoriasis or lichen simplex, and their results not separated. Discarding such evidence might appear a little harsh. Although some might feel comfortable in generalising from studies evaluating all forms of eczema to atopic eczema, the RCT evidence discussed in the section on topical corticosteroids suggests that different inflammatory diseases respond differently to the same treatment. Atopic eczema patients therefore need to be separated from patients with other inflammatory dermatoses, or at least their results should be presented separately.
Future research priorities
Primary research
With such glaring gaps in our current knowledge about the effectiveness of treatments for atopic eczema, it is difficult to know where to start in recommending research priorities. In order to avoid bias on behalf of the authors choosing just what they think is important in future primary research, results of the survey of 25 researchers and clinicians with an interest in atopic eczema and six consumers with atopic eczema are shown in Table 41. There was remarkable similarity between the different groups in calling for research on similar themes. Research themes fall mainly into assessing the things that we already have rather than assessing the role of newer agents. Research that evaluates the delivery of whole packages of care such as involvement of nurses was also highlighted. This type of research is important when extrapolating from cost-effectiveness studies of motivated patients in secondary care to a primary care setting. It may be the case that an inexpensive single treatment delivered with a simple package of care may be more effective than an expensive therapy without any such support.
Clearly it is impossible to commission all of these proposals, and the authors prioritisation of the six most urgent research themes are shown in Table 42. Studies on disease prevention also need to be considered.
Secondary research
Perhaps one of the most useful aspects of this scoping review is that it will serve as a generator of other more detailed specific systematic reviews. Already therefore, titles for reviews on the reduction of house dust mite, antihistamines, Chinese herbs and dietary approaches have been registered with the Cochrane Skin Group, and it is hoped that others will use the data contained within this review as a backbone for additional systematic reviews.
Methodological
There is a clear need for a programme of methodological research to accompany the primary research if patients and their carers are to make sense of the study findings. Further research, such as the current research by this team to identify a simple list of patient-derived outcome measures for atopic eczema, is needed. A recent review32 of ‘named’ outcome scales used in atopic eczema identified 13 different instruments, none of which had undergone full testing for validity, repeatability and responsiveness. In addition to completing the validity testing of such ‘named’ scales, further research involving consumers and carers is required to determine a minimum list of similar outcome measures, for example a quality-of-life measure, patient-rated global disease improvement and the ‘best’ of the current named objective doctor-rated scales for use in future atopic eczema trials. This will vastly aid the comparability of studies providing such a list has been derived using the best quality external evidence with ownership from a wide range of stakeholders. Drug regulatory authorities could occupy a key role in recommending the use of such measures.
The RCTs highlighted in this study also provide an opportunity to explore more generic issues such as the relationship between magnitude of benefit and study quality or design issues. Future RCTs in dermatology can also consider using a Bayesian approach, particularly where there is pre-existing epidemiological evidence (e.g. as in the case of house dust mite and water hardness) to inform the prior probabilities. In other areas where there is a multiplicity of new interventions being introduced constantly, consideration should be given to more flexible and pragmatic approaches such as the use of tracker studies.
| Intervention | Question |
|---|---|
| Disease prevention | What is the role of vaccination in triggering atopic eczema expression? |
| Role of maternal dietary manipulation and house dust mite avoidance | |
| Emollients | Does regular use of emollients reduce disease relapse? |
| Topical corticosteroids | What is the most optimal use of topical steroids? |
| Do topical steroids suppress growth? | |
| Are the ‘newer’ once-daily topical steroids more effective than older preparations? | |
| Do topical corticosteroids cause long-term skin damage when used appropriately? | |
| Tacrolimus and ascomycin | How do the newer topical agents such as tacrolimus and ascomycin compare with topical corticosteroids? |
| Should tacrolimus or ascomycin be used after inducing a remission with topical steroids? | |
| Diets | Role of exclusion diets |
| Factors affecting treatment response | Do different patterns of atopic eczema (discoid, reverse pattern, flexural) require different treatments? |
| How important is the presence of S. aureus in managing disease? | |
| Are there any genetic markers for predicting treatment response? | |
| Trigger factors | What are the most important modifiable trigger factors? |
| Treatments for severe disease | How does ultraviolet treatment compare with oral immunomodulatory treatment in severe disease? |
| Should potentially toxic treatments be used on a rotational basis? | |
| Efficacy of agents such as azathioprine, methotrexate, anti-leukotrienes, naltrexone | |
| Disease dimensions | What are the most effective interventions at reducing the itch of atopic eczema? |
| Does any treatment alter the natural history of disease if used properly and for long enough? | |
| Which is the best treatment approach for mild-to-moderate disease? | |
| The role of tests | What is the usefulness of allergy tests in disease management |
| What is the role of allergic contact dermatitis in topic eczema? | |
| Environmental manipulation | Are water softeners effective? |
| Does removal of pets influence disease activity? | |
| How important is control of humidity and excessive heat in the home? | |
| Psychological approaches | Which are the most psychological/psychotherapeutic approaches and how well do patients respond to such approaches outside the hands of enthusiasts? |
| Bandages | How effective are wet-wraps, with and without emollients or topical steroids? |
| Organisation of care/education | How effective are educational approaches in improving the correct use of first-line treatments? |
| What is the role of specialist nurses in helping people with atopic eczema? | |
| How effective is a multiprofessional team approach compared with a dermatologist alone? | |
| How important is patient concordance in predicting disease control? | |
| Bathing | Are salt baths helpful? |
| Is there an optimal frequency of bathing? | |
| Issues of safety | Long-term adverse effects of cyclosporin in children |
| Long-term adverse effects of ultraviolet light in children |
| Question | Justification |
|---|---|
| How effective are wet-wrap bandages with topical steroids or emollients vs the same treatment and no wet-wraps? | Widespread use with no RCT backing. Potentially large and useful treatment effect but also greater potential for local and systemic adverse effects of topical steroids |
| How useful are blood allergy tests at predicting benefits from allergen avoidance? | Large demand for such tests matched by lack of evidence that they mean anything useful in terms of eczema outcomes |
| Does the installation of a water-softening device improve atopic eczema? | Sold widely to eczema sufferers. Some epidemiological evidence that hard water might be important. Modifiable environmental factor |
| What is the role of specialist nurses in managing patients with atopic eczema? | Some observational evidence of benefit in some centres. May be a cost-effective complement to current doctor-dominated approach |
| Head-to-head cost-effectiveness comparison of topical corticosteroids against topical tacrolimus or ascomycin | Newer agents likely to be taken up eagerly in view of corticosteroid ‘phobia’, but true benefit and cost–benefit unclear |
| Trials aimed at prevention of atopic eczema | Large potential health gains in high-risk populations |
Implications for healthcare
The strength of evidence supporting the various interventions have already been summarised in the key points of the results chapters. The strength of evidence in relation to those interventions which are commonly used in the UK are summarised qualitatively in Table 43. Given the virtual absence of long-term studies, the data can only refer to short-term control of disease.
Some ‘health’ warnings
Table 43 gives the authors' opinion on the value of the evidence base for the interventions considered. It is not intended as a substitute for the dose examination of the original studies in the context of local guideline and policy development. Any attempt at summarising the RCT evidence (or lack of such evidence) for such a wide range of interventions is fraught with hazard, perhaps the most important of which is that absence of RCT evidence for an intervention is not the same as providing evidence to reject that intervention. Therefore it might be entirely reasonable to continue to use a range of emollients in atopic eczema based on lower hierarchies of evidence until appropriate RCTs are done, given the fact that they have become ‘consecrated’ through usage. On the other hand, some RCTs have failed to show any benefit for some interventions and these could perhaps be looked at carefully in terms of the rationale for their continued widespread use. This is easier said than done as advice such as avoidance of synthetic fibres and enzyme-containing washing powders, and frequent bathing have all become deeply engrained in the rituals of atopic eczema advice. Similarly, use of twice-daily topical corticosteroids or dilutions of topical corticosteroids have also become deeply embedded in clinical practice. Crucial factors such as patient choice, adverse effects and cost also have to be taken into account when making recommendations.
| Interventions with reasonably established efficacy (based on at least one high-quality RCT and a clinically useful effect) | Interventions with insufficient evidence to make recommendations (only one small RCT or conflicting RCTs where the largest and best-quality RCTs do not suggest a clear and clinically useful benefit) | Interventions for which RCT evidence does not support a clinically useful benefit (at least one RCT that fails to show a convincing benefit on overall disease activity) | Interventions with no RCT evidence whatsoever |
|---|---|---|---|
|
|
|
|
Trying to split Table 43 into further first-line, second-line and third-line treatment guidelines is beyond the scope of this systematic review and is an approach that is hazardous without a much wider consultation and more detailed synthesis of adverse effect data, patient preference data and cost data, which by definition is always contextual and limited in time. Decision analysis approaches should also be used to determine which interventions are amenable to change in the future.
Summary
-
RCTs of interventions for atopic eczema have often not answered the questions of most importance to patients and their carers.
-
This mismatch is possibly due to the lack of independent investment into primary atopic eczema research.
-
There are some glaring gaps in our current knowledge regarding the use of some interventions that are commonly used such as emollients and wet-wrap bandages.
-
This review has identified some lesser known RCTs, which suggest that some interventions such as bioresonance and avoidance of synthetic clothing are ineffective.
-
The review has also failed to find any evidence to support the use of twice-daily as opposed to once-daily topical steroids or topical antibiotic/ steroid combinations as opposed to topical steroids alone in infected atopic eczema.
-
The review has helped to place non-pharmacological treatments in their context alongside conventional drug approaches.
-
Future RCTs should consider using a parallel group design and be of longer duration.
-
A profusion of outcome measures should be avoided in favour of a few clinically understandable and patient-orientated ones.
-
There is much scope for improving the standard of clinical trial reporting in atopic eczema by journals adopting CONSORT and by registering ongoing trials with the Cochrane Skin Group.
-
This review has identified several primary research gaps, which need to be addressed mainly by RCTs.
-
The review is likely to be a useful generator of future more detailed systematic reviews.
-
The RCT database contained within this report provides a good opportunity to conduct some general research into the relationship between study quality and treatment benefit.
Acknowledgements
Role of authors
Colette Hoare performed all of the searches, checked for eligible studies, obtained hard copies of studies, conducted the focus group, abstracted data from included studies, wrote the section on complementary therapies, managed the references and finalised the appendices and report layout.
Hywel Williams wrote the study proposal, supervised the day-to-day running of the project, handsearched conference proceedings, checked on excluded studies, abstracted and summarised data from most of the included studies, and wrote the final report with help from Colette Hoare.
Alain Li Wan Po provided advice on the methods, part-wrote the sections on cyclosporin, cromoglycate and once- versus twice-daily topical corticosteroids and approved the final report.
The authors wish to thank the following people for their helpful contribution to this report:
Searching
Dr Finola Delamere, Trials Search Co-ordinator, Cochrane Skin Group, Nottingham; Dr Carole Lefebvre, UK Cochrane Centre, Oxford; Mrs Christine Clarke (Manchester) and staff of the Greenfield Medical Library, University of Nottingham, colleagues in the Cochrane Complementary Medicines Field; and the BMJ Publishing Group for use of their search strategy for clinical trials on EMBASE.
Translators
Dr Urba Gonzalez (Spain) and Mrs Maxine Whitton (UK) for translation of Spanish and Portugese articles.
Dr Yukihiro Ohya (Tokyo) and Professor Toshi Aoki (Osaka) for translation of Japanese articles.
Professor Thomas Diepgen (Heidelberg), Dr Berthold Rzany (Mannheim), Dr Jan von der Werth (UK) and Ms Christine Scholtyssek (Germany) for translation of German articles.
Professor Vladimir Vlassov (Moscow) for translation of Russian articles.
Professor Alain Taieb for translation of French articles.
Dr Åke Svensson for helping to exclude a Danish study.
Ms Kirsten Lone Jensen (Copenhagen) for help in identifying Nordic RCTs.
Ms Helena Varonen (Helsinki) for helping to exclude a Finnish study.
Identifying additional treatments not on our list of RCTs
Dr D Atherton, (Hospital for Sick Children, Great Ormond Street); Professor Peter Friedmann, (Southampton); Dr J Berth-Jones, (Coventry); Dr S Lewis-Jones (Dundee); Dr J Vesty (Sunderland); Dr D Paige (Royal London Hospital); Professor A Taieb (Bordeaux); Dr C T Kennedy (Bristol); Dr M R Judge (Bolton); Dr R Chalmers (Manchester); Dr J D Wilkinson (Amersham); Dr M Glover (Newham Health Care NHS Trust London); Dr E A Bingham (Belfast); Dr A Anstey (Newport).
Authors providing additional data on published trials
Professor Thomas Diepgen (Heidelberg); Professor John Harper (London); Dr David Atherton (London); Dr Tom Poyner (Stockton-on-Tees); Dr Mary Glover (London); Professor Mark Lebwohol (New York); colleagues of the late Professor Hjorth; Professor Toshi Aoki (Osaka); Professor Frederik Bahmer (Germany); Dr Kenji Nishioka (Japan). We also wish to thank all the pharmaceutical companies mentioned in Tables 41 and 42 who kindly responded to our request for any missed or unpublished studies.
Proofreaders and commentators
Mrs Kim Thomas (Nottingham); Dr Phil Alderson (UK Cochrane Centre, Oxford); Mrs Margaret Cartman (Nottingham); Dr Carolyn Charman (Nottingham); and Miss Mara Ozolins (Nottingham). We also thank the four HTA reviewers for their helpful and thoughtful comments.
Consumers working with the Cochrane Skin Group who helped to identify unanswered questions on the treatment of atopic eczema
Mr David Potter, Mr Jack Stein, Mr John Fulton, Mrs Margaret Newton, Ms Barbara Meredith, Dr Elisabeth Curling and Mr Paul Mellows.
Individuals who helped to identify the main unanswered questions for future atopic eczema clinical trial research
Professor Andrew Finlay (Cardiff); Dr J Berth-Jones (Coventry); Dr Alex Anstey (Newport); Dr Tom Fahey (Bristol); Professor Rona MacKie (Glasgow); Dr Ian Coulson (Burnley); Dr John Adams (Cheadle); Dr Colin Munro (Glasgow); Dr Tom Poyner (Stockton-on-Tees); Professor Kevin Cooper (Cleveland, USA); Professor Jon Hanifin (Oregon, USA); Professor Tim David (Manchester); Dr Åke Svensson (Malmoe, Sweden); Dr Jan Bouwes Bavinck (Leiden, The Netherlands); Professor Luigi Naldi (Bergamo, Italy); Dr Ann Braae Olesen (Åarhus, Denmark); Professor Donald Leung (USA); Dr Pieter-van Coenraads (Groningen, The Netherlands); Professor Gimpiero Girolomoni (IRCCS, Rome); Dr Tony Avery (University of Nottingham); Dr. Dilys Harlow (Bristol); Professor Peter Friedman (University of Southampton); Dr Mary Judge (Bolton); Dr Robin Graham-Brown (Leicester); Dr Ann Bingham (Belfast); Dr Pamela McHenry (Glasgow).
Help with photocopying
Mr Steven Chambers and Mr Peter Berry.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA Programme or the Department of Health. The editors wish to emphasise that funding and publication of this research by the NHS should not be taken as implicit support for any recommendations made by the authors.
References
- Archer CB, Williams HC. Atopic dermatitis. Cambridge: Cambridge University Press; 2000.
- Williams HC, Wüthrich B, Williams HC. Atopic dermatitis. Cambridge: Cambridge University Press; 2000.
- Williams HC, Williams HC. What is atopic dermatitis and how should it be defined in epidemiological studies?. Cambridge: Cambridge University Press; 2000.
- Hanifin JM, Rajka G. Diagnostic features of atopic eczema. Acta Derm Venereol (Stockh) 1980;92:44-7.
- Williams HC, Williams HC. Atopic dermatitis. Cambridge: Cambridge University Press; 2000.
- Williams HC, Forsdyke H, Boodoo G, Hay RJ, Burney PGF. A protocol for recording the sign of visible flexural dermatitis. Br J Dermatol 1995;133:941-9.
- Herd RM, Williams HC. Atopic dermatitis. Cambridge: Cambridge University Press; 2000.
- Williams HC, Robertson CF, Stewart AW. ISAAC Steering Committee . Worldwide variations in the prevalence of atopic eczema symptoms. J Allergy Clin Immunol 1999;103:125-38.
- Williams HC. Is the prevalence of atopic dermatitis increasing?. Clin Exp Dermatol 1992;17:385-91.
- Herd RM, Tidman MJ, Prescott RJ, Hunter JAA. Prevalence of atopic eczema in the community: the Lothian atopic dermatitis study. Br J Dermatol 1996;135:18-9.
- Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol 1998;139:73-6.
- Dotterud LK, Kvammen B, Lund E, Falk ES. Prevalence and some clinical aspects of atopic dermatitis in the community of Sør-Varanger. Acta Derm Venereol 1995;75:50-3.
- Herd RM, Tidman MJ, Prescott RJ, Hunter JAA. The cost of atopic eczema. Br J Dermatol 1996;135:20-3.
- Su JC, Kemp AS, Varigos GA, Nolan TM. Atopic eczema: its impact on the family and financial cost. Arch Dis Child 1997;76:159-62.
- Schultz-Larsen F, Holm NV, Henningsen K. Atopic dermatitis. A genetic-epidemiological study in a population-based twin sample. J Am Acad Dermatol 1986;15:487-94.
- Williams HC. Atopic eczema – why we should look to the environment. BMJ 1995;311:1241-2.
- Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged?. BMJ 1994;308:1132-5.
- McNally N, Phillips D, Williams HC. Atopic dermatitis. Cambridge: Cambridge University Press; 2000.
- Strachan DP. Hayfever, hygiene, and household size. BMJ 1989;299:1259-60.
- Shaheen S. Discovering the causes of atopy. BMJ 1997;314:987-8.
- Burrell-Morris C, Williams HC, Williams HC. Atopic dermatitis. Cambridge: Cambridge University Press; 2000.
- Olesen AB, Ellingsen AR, Olesen H, Juul S, Thestrup-Pedersen K. Atopic dermatitis and birth factors: historical follow up by record linkage. BMJ 1997;314:1003-8.
- Hanifin JM, Marks RM. Eczema. London: Martin Dunitz; 1992.
- Bos JD, Wierenga EA, Smitt JHS, van der Heijden FL, Kapsenberg ML. Immune dysregulation in atopic eczema. Arch Dermatol 1992;128:1509-12.
- McHenry P, Williams HC, Bingham EA. Treatment of atopic eczema. BMJ 1995;310:843-7.
- Hanifin JM, Chan S. Biochemical and immunologic mechanisms in atopic dermatitis: new targets for emerging therapies. J Am Acad Dermatol 1999;41:72-7.
- Shum KW, Lawton S, Williams HC, Dochety G, Jones J. The British Association of Dermatologists audit of atopic eczema management in secondary care. Phase 1: audit of service structure. Br J Dermatol 1999;141:430-7.
- Shum KW, Lawton S, Williams HC, Dochety G, Jones J. The British Association of Dermatologists audit of atopic eczema management in secondary care. Phase 2: audit of service process. Br J Dermatol 2000;142:274-8.
- Charman CR, Morris A, Williams HC. Topical steroid ‘phobia’ in patients with atopic eczema. Br J Dermatol 2000;142:931-6.
- Finlay AY. Measurement of disease activity and outcome in atopic dermatitis. Br J Dermatol 1996;135:509-15.
- Charman CR, Venn AJ, Williams HC. Measurement of body surface involvement in atopic eczema: an impossible task?. Br J Dermatol 1999;140:109-11.
- Charman C, Williams HC. Outcome measures of disease severity in atopic eczema. Arch Dermatol 2000;136:763-9.
- Chren MM, Lasek RT, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of SKINDEX, a quality-of-life instrument for patients with skin diseases. Arch Dermatol 1997;133:1433-40.
- British National Formulary. London: BMJ Books; 1999.
- Williams HC. Too soon to market: problem is acute in dermatology. BMJ 1998;316.
- Lear JT, English JSC, Jones PW, Smith AG. Retrospective review of the use of azathiprine in severe atopic dermatitis. J Am Acad Dermatol 1996;35:642-3.
- Undertaking systematic reviews of research on effectiveness: CRD guidelines for those carrying out or commissioning reviews. CRD report No. 4. York, UK: NHS Centre for Reviews and Dissemination, The University of York; 1996.
- Preparation of reviews for the HTA Programme. UK: University of Southampton; 1999.
- Mulrow CD, Oxman A. How to conduct a Cochrane Systematic Review. Version 3.0.2. San Antonio: Cochrane Collaboration; 1997.
- MEDLINE Electronic Database (1966–2000) n.d. http://www.bids.ac.uk/medline/.
- EMBASE Electronic Database (1980–2000) n.d. http://www.bids.ac.uk/embase/.
- The Cochrane Library . The Cochrane Controlled Trials Register 1999.
- The Cochrane Library . The Skin Register 1999.
- Donald A, Barton S, Muthu V. Clinical evidence. London: BMJ Publishing Group; 1999.
- Procite® Software [Windows 95/NT Version 4]. USA: Research Information Systems; n.d.
- Moher D, Jadad AR, Nichol G, Penman M, Tugwell T, Walsh S. Assessing the quality of randomised controlled trials: an annotated bibliography of scales and check lists. Controlled Clin Trials 1995;16:62-73.
- Moher D, Cook DJ, Jadad AR, . Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess 1999;3.
- Lilja G, Dannaeus A, Foucard T, Graff-Lonnevig V, Johansson SGO, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of atopic diseases in infants up to 18 months of age – in vivo results. Clin Exp Allergy 1989;19:473-9.
- Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol 1995;95:1179-90.
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. J Allergy Clin Immunol 1994;93:842-6.
- Zeiger RS, Heller S. Development of nasal basophilic cells and nasal eosinophils from age 4 months through 4 years in children of atopic parents. J Allergy Clin Immunol 1993;91:723-34.
- Falth-Magnusson K, Kjellman NI. Allergy prevention by maternal elimination diet during late pregnancy – 5-year follow-up of a randomized study. J Allergy Clin Immunol 1992;89:709-13.
- Chandra RK, Puri S, Suraiya C, Cheema PS. Influence of maternal food antigen avoidance during pregnancy and lactation on incidence of atopic eczema in infants. Clin Allergy 1986;16:563-9.
- Miskelly FG, Burr ML, Vaughan-Williams E, Fehily AM, Butland BK, Merrett TG. Infant feeding and allergy. Arch Dis Child 1988;63:388-93.
- Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy 1996;51:89-93.
- Kjellman NI, Johansson SG. Soy versus cow’s milk in infants with a biparental history of atopic disease: development of atopic disease and immunoglobulins from birth to 4 years of age. Clin Allergy 1979;9:347-58.
- Porch MC, Shahane AD, Leiva LE, Elston RC, Sorensen RU. Influence of breast milk, soy or two hydrolyzed formulas on the development of allergic manifestations in infants at risk. Nutr Res 1998;18:1413-24.
- Oldaeus G, Anjou K, Bjorksten B, Moran JR, Kjellman N-IM. Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Arch Dis Child 1997;77:4-10.
- Mallet E, Henocq A. Long-term prevention of allergic diseases by using protein hydrolysate formula in at-risk infants. J Pediatr 1992;121:S95-100.
- Lucas A, Brooke OG, Morley R, Cole TJ, Bamford MF. Early diet of preterm infants and development of allergic or atopic disease: randomised prospective study. BMJ 1990;300:837-40.
- Chandra RK, Puri S, Hamed A. Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants. BMJ 1989;299:228-30.
- Perdomo-Ponce D, Benarroch L, Gonzalez-Cerrutti R, Barroso R, Carneiro F, Meijomil P. Family education, a model for allergy prevention. Invest Clin 1996;37:221-45.
- Moore WJ, Midwinter RE, Morris AF, Colley JR, Soothill JF. Infant feeding and subsequent risk of atopic eczema. Arch Dis Child 1985;60:722-6.
- Odelram H, Vanto T, Jacobsen L, Kjellman NI. Whey hydrolysate compared with cow’s milk-based formula for weaning at about 6 months of age in high allergy-risk infants: effects on atopic disease and sensitization. Allergy 1996;51:192-5.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatrica 1996:1-21.
- Vandenplas Y, Hauser B, Van den Borre C, Clybouw C, Mahler T, Hachimi-Idrissi S, et al. The long-term effect of a partial whey hydrolysate formula on the prophylaxis of atopic disease. Eur J Pediatr 1995;154:488-94.
- Chandra RK, Singh G, Shridhara B. Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Ann Allergy 1989;63:102-6.
- Camacho F, Garcia Bravo B, Diaz Perez JL, Aguirre A, Arnau C, Garcia Barbal J, et al. A comparative intraindividual double-blind assay between prednicarbate and fluocortolone in the management of atopic dermatitis. Actas Dermo Sifiliograficas 1996;87:59-63.
- Koopmans B, Lasthein Andersen B, Mork NJ, Austad J, Suhonen RE. Multicentre randomized double-blind study of locoid lipocream fatty cream twice daily versus locoid lipocream once daily and locobase once daily. J Dermatol Treat 1995;6:103-6.
- Bleeker J. Double-blind comparison between two new topical corticosteroids, halcinonide 0.1% and clobetasol propionate cream 0.05%. Curr Med Res Opin 1975;3:225-8.
- Gehring W, Gloor M. Treatment of the atopic dermatitis with a water-in-oil emulsion with or without the addition of hydrocortisone – results of a controlled double-blind randomized study using clinical evaluation and bioengineering methods. H+G Zeitschrift Fur Hautkrankheiten 1996;71:554-60.
- Reidhav I, Svensson A. Betamethasone valerate versus mometasone furoate cream once daily in atopic dermatitis. J Dermatol Treat 1996;7:87-8.
- Traulsen J. Hydrocortisone buteprate versus betamethasone valerate for once-daily treatment of atopic dermatitis. J Dermatol Treat 1997;8:109-14.
- Wolf-Jurgensen P. Efficacy of bufexamac cream versus betamethasone valerate cream in contact dermatitis: a double-blind trial. Curr Med Res Opin 1979;5:779-84.
- Haneke E. The treatment of atopic dermatitis with methylprednisolone aceponate (mpa), a new topical corticosteroid. J Dermatol Treat 1992;3:13-5.
- Lebwohl M. Efficacy and safety of fluticasone propionate ointment, 0.005%, in the treatment of eczema. Cutis 1996;57:62-8.
- Thaci D KJaKR. Fusidic acid/betamethasone 17-valerate in potentially infected atopic dermatitis. J Eur Acad Dermatol Venereol 1999;12.
- Amerio PL, Biggio P, Bossi G, Cainelli P, Cappugi P, Cerimele D, et al. Mometasone furoate 0.1% once a day in allergic contact dermatitis and in atopic dermatitis: controlled study versus betamethasone valerate. Dermatol Clin 1998;18:255-60.
- Bagatell FK, Barkoff JR, Cohen HJ, Lasser AE, McCormick GE, Rex IH, et al. A multi-center comparison of alclometasone dipropionate cream 0.05% and hydrocortisone cream 1.0% in the treatment of atopic dermatitis. Curr Ther Res Clin Exp 1983;33:46-52.
- Gelmetti C, Grimalt R, Del Campo G, Caputo R. Tolerability and efficacy of topical budesonide in the treatment of atopic dermatitis in pediatric age. G Ital Dermatol Venereol 1994;129:XIII-XVII.
- Sears HW, Bailer JW, Yeadon A. Efficacy and safety of hydrocortisone buteprate 0.1% cream in patients with atopic dermatitis. Clin Ther 1997;19:710-19.
- Wolkerstorfer A, Strobos MA, Glazenburg EJ, Mulder PGH, Oranje AP. Fluticasone propionate 0.05% cream once daily versus clobetasone butyrate 0.05% cream twice daily in children with atopic dermatitis. J Am Acad Dermatol 1998;39:226-31.
- Munkvad M. A comparative trial of clinitar versus hydrocortisone cream in the treatment of atopic eczema. Br J Dermatol 1989;121:763-6.
- Hiratsuka S, Yoshida A, Ishioka C, Kimata H. Enhancement of in vitro spontaneous IgE production by topical steroids in patients with atopic dermatitis. J Allergy Clin Immunol 1996;98:107-13.
- Majerus JP, Reiffers-Mettelock J. Sicorten: a synthetic corticosteroid for topical treatment of common dermatoses. J Int Med Res 1986;14:46-9.
- Korting HC, Schafer-Korting M, Klovekorn W, Klovekorn G, Martin C, Laux P. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur J Clin Pharmacol 1995;48:461-5.
- Bleehen SS, Chu AC, Hamann I, Holden C, Hunter JA, Marks R. Fluticasone propionate 0.05% cream in the treatment of atopic eczema: a multicentre study comparing once-daily treatment and once-daily vehicle cream application versus twice-daily treatment. Br J Dermatol 1995;133:592-7.
- Jorizzo J, Levy M, Lucky A Shavin J, Goldberg G, Dunlap F, . Multicenter trial for long-term safety and efficacy comparison of 0.05% desonide and 1% hydrocortisone ointments in the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol 1995;33:74-7.
- Kaplan RJ, Daman L, Rosenberg EW, Feigenbaum S. Topical use of caffeine with hydrocortisone in the treatment of atopic dermatitis. Arch Dermatol 1978;114:60-2.
- Wachs GN, Maibach HI. Co-operative double-blind trial of an antibiotic/corticoid combination in impetiginized atopic dermatitis. Br J Dermatol 1976;95:323-8.
- Stalder JF, Fleury M, Sourisse M, Rostin M, Pheline F, Litoux P. Local steroid therapy and bacterial skin flora in atopic dermatitis. Br J Dermatol 1994;131:536-40.
- Almeyda J, Burt BW. Double blind controlled study of treatment of atopic eczema with a preparation of hydrocortisone in a new drug delivery system versus betamethasone 17-valerate. Br J Dermatol 1974;91:579-83.
- Leibsohn E, Bagatell FK. Halcinonide in the treatment of corticosteroid responsive dermatoses. Br J Dermatol 1974;90:435-40.
- Lupton ES, Abbrecht MM, Brandon ML. Shortterm topical corticosteroid therapy (halcinonide ointment) in the management of atopic dermatitis. Cutis 1982;30:671-5.
- Sudilovsky A, Muir JG, Bocobo FC. A comparison of single and multiple applications of halcinonide cream. Int J Dermatol 1981;20:609-13.
- Fisher M, Kelly AP. Multicenter trial of fluocinonide in an emollient cream base. Int J Dermatol 1979;18:660-4.
- Roth HL, Brown EP. Hydrocortisone valerate. Double-blind comparison with two other topical steroids. Cutis 1978;21:695-8.
- Dickey RF. Parenteral short-term corticosteroid therapy in moderate to severe dermatoses. A comparative multiclinic study. Cutis 1976;17:179-83.
- Yasuda T. Clinical experiences with hydrocortisone 17-butyrate. Dermatologica 1976;152:221-9.
- Wahlgren CF, Hagermark O, Bergstrom R, Hedin B. Evaluation of a new method of assessing pruritus and antipruritic drugs. Skin Pharmacol 1988;1:3-13.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol 1991;24:603-7.
- Binder R, McCleary J. Comparison of fluocinonide in a double-blind study with betamethasone valerate. Curr Ther Res Clin Exp 1972;14:35-8.
- Korting HC, Zienicke H, Schafer-Korting M, Braun-Falco O. Liposome encapsulation improves efficacy of betamethasone dipropionate in atopic eczema but not in psoriasis vulgaris. Eur J Clin Pharmacol 1990;39:349-51.
- Van Der Meer JB, Glazenburg EJ, Mulder PGH, Eggink HF, Coenraads PJ. The management of moderate to severe atopic dermatitis in adults with topical fluticasone propionate. Br J Dermatol 1999;140:1114-21.
- Vanderploeg DE. Betamethasone dipropionate ointment in the treatment of psoriasis and atopic dermatitis: a double-blind study. South Med J 1976;69:862-3.
- Noren P, Melin L. The effect of combined topical steroids and habit-reversal treatment in patients with atopic dermatitis. Br J Dermatol 1989;121:359-66.
- Meenan FO. A double-blind comparative study to compare the efficacy of locoid c with tri-adcortyl in children with infected eczema. Br J Clin Pract 1988;42:200-2.
- Duke EE, Maddin S, Aggerwal A. Alclometasone dipropionate in atopic dermatitis: a clinical study. Curr Ther Res Clin Exp 1983;33:769-74.
- Kuokkanen K, Sillantaka I. Alclometasone dipropionate 0.05% vs hydrocortisone 1.0%: potential to induce cutaneous atrophy in children. Clin Ther 1987;9:223-31.
- Rajka G, Verjans HL. Hydrocortisone 17-butyrate (locoid) 0.1% fatty cream versus desonide (apolar) 0.1% ointment in the treatment of patients suffering from atopic dermatitis. J Int Med Res 1986;14:85-90.
- Hjorth N, Schmidt H, Thomsen K. Fusidic acid plus betamethasone in infected or potentially infected eczema. Pharmatherapeutica 1985;4:126-31.
- Heddle RJ, Soothill JF, Bulpitt CJ, Atherton DJ. Combined oral and nasal beclomethasone diproprionate in children with atopic eczema: a randomised controlled trial. BMJ Clin Res Ed 1984;289:651-4.
- Veien NK, Hattel T, Justesen O, Norholm A, Verjans HL. Hydrocortisone 17-butyrate (Locoid) 0.1% cream versus hydrocortisone (Uniderm) 1% cream in the treatment of children suffering from atopic dermatitis. J Int Med Res 1984;12:310-13.
- Sefton J, Loder JS, Kyriakopoulos AA. Clinical evaluation of hydrocortisone valerate 0.2% ointment. Clin Ther 1984;6:282-93.
- Lassus A. Clinical comparison of alclometasone dipropionate cream 0.05% with hydrocortisone butyrate cream 0.1% in the treatment of atopic dermatitis in children. J Int Med Res 1983;11:315-19.
- Lebwohl M, Lane A, Savin R, Drake L, Berman B, Lucky A, et al. A comparison of once-daily application of mometasone furoate 0.1% cream compared with twice-daily hydrocortisone valerate 0.2% cream in pediatric atopic dermatitis patients who failed to respond to hydrocortisone. Int J Dermatol 1999;38:604-6.
- Wilkinson RD, Leigh DA. Comparative efficacy of betamethasone and either fusidic acid or neomycin in infected or potentially infected eczema. Curr Ther Res 1985;38:177-82.
- el Hefnawi H, el Shiemy S, Paris R, Tadros SS. Double-blind paired comparison clinical trial of halcinonide and hydrocortisone. Cutis 1978;22:97-9.
- Bluefarb SM, Howard FM, Leibsohn E, Schlagel CA, Wexler L. Diflorasone diacetate: vasoconstrictor activity and clinical efficacy of a new topical corticosteroid. J Int Med Res 1976;4:454-61.
- Morley N, Fry L, Walker S. Clinical evaluation of clobetasone butyrate in the treatment of children with atopic eczema, and its effect on plasma corticosteroid levels. Curr Med Res Opin 1976;4:223-8.
- Bjornberg A, Hellgren L. Comparison between 2 steroid dosage forms in psoriasis and eczema. Zeitschrift Fur Hautkrankheiten 1975:13-5.
- Almeyda J, Fry L. Controlled trial of the treatment of atopic eczema with a urea-hydrocortisone preparation versus betamethasone 17-valerat. Br J Dermatol 1973;88:493-5.
- Rampini E. Methylprenisolone aceponate (mpa) – use and clinical experience in children. J Dermatol Treat 1992;3:27-9.
- Hoybye S, Balk Moller S, De Cunha Bang F, Ottevanger V, Veien NK. Continuous and intermittent treatment of atopic dermatitis in adults with momethasone furoate vs. hydrocortisone 17-butyrate. Curr Ther Res Clin Exp 1991;50:67-72.
- Chapman RS. Treatment of atopic dermatitis. Practitioner 1979;223:713-16.
- Brock W, Cullen SI. Triamcinolone acetonide in flexible collodion for dermatologic therapy. Arch Dermatol 1967;96:193-4.
- Treatment of eczemas and infected eczemas. Br J Clin Pract 1967;21:505-7.
- Ramsay CA, Savoie JM, Gilbert M, Gidon M, Kidson P. The treatment of atopic dermatitis with topical fusidic acid and hydrocortisone acetate. J Eur Acad Dermatol Venereol 1996;7:S15-S22.
- Rafanelli A, Rafanelli S, Stanganelli I, Marchesi E. Mometasone furoate in the treatment of atopic dermatitis in children. J Eur Acad Dermatol Venereol 1993;2:225-30.
- Malzfeldt E, Lehmann P, Goerz G, Lippold BC. Influence of drug solubility in the vehicle on clinical efficacy of ointments. Arch Dermatol Res 1989;281:193-7.
- Marchesi E, Rozzoni M, Pini P, Cainelli T. Comparative study of mometasone furoate and betamethasone dipropionate in the treatment of atopic dermatitis. G Ital Dermatol Venereol 1994;129:IX-XII.
- Maloney JM, Morman MR, Stewart DM, Tharp MD, Brown JJ, Rajagopalan R. Clobetasol propionate emollient 0.05% in the treatment of atopic dermatitis. Int J Dermatol 1998;37:142-4.
- Lucky AW, Grote GD, Williams JL Tuley MR, Czernielewski JM, Dolak TM, . Effect of desonide ointment, 0.05%, on the hypothalamicpituitary-adrenal axis of children with atopic dermatitis. Cutis 1997;59:151-3.
- Korting HC, Zienicke H, Braun-Falco O, . Modern topical glucocorticoids and anti-infectives for superinfected atopic eczema: do prednicarbate and didecyldimethylammoniumchloride form a rational combination?. Infection 1994;22:390-4.
- Lassus A. Alclometasone dipropionate cream 0.05% versus clobetasone butyrate cream 0.05%. A controlled clinical comparison in the treatment of atopic dermatitis in children. Int J Dermatol 1984;23:565-6.
- Sefton J, Kyriakopoulos AA. Comparative efficacy of hydrocortisone valerate 0.2 percent ointment in the treatment of atopic dermatitis. Cutis 1983;32:89-91.
- Mali JW. An evaluation of betamethasone dipropionate (diprosone) versus locacorten 0.02% cream. Dermatologica 1976;153:177-8.
- Nolting S. Treatment with topical corticosteroids in severe or resistant dermatoses. Dermatosen in Beruf Und Umwelt 1985;33:140-4.
- Van DelRey ML, Geller M, Azulay RD. Estudo duplo-cego sobre a eficacia e a seguranca do creme de alcometasoma no tratamento de dermatite atopica. An Bras Dermatol 1983;58:177-80.
- La Rosa M, Musarra I, Ranno C, Maiello N, Negri L, Miraglia Del Giudice J, et al. A randomized, doubleblind, placebo-controlled crossover trial of systemic flunisolide in the treatment of children with severe atopic dermatitis. Curr Ther Res Clin Exp 1995;56:720-6.
- Ulrich R, Andresen I. Double-blind comparative trial involving 0.5% halomethasone (Sicorten™) cream versus 0.25% prednicarbate cream in patients with acute episodes of atopic dermatiti. Fortschr Med 1991;109:49-50.
- Hoybye S, Balk Moller S, De Cunha Bang F, Ottevanger V, Veien NK. Continuous and intermittent treatment of atopic dermatitis in adults with mometasone furoate versus hydrocortisone 17-butyrate. Curr Ther Res Clin Exp 1991;50:67-72.
- Olholm Larsen P, Brandrup F, Roders GA. Report on a double-blind, left-right study comparing the clinical efficacy of mildison (hydrocortisone 1%) Lipocream™ with Uniderm™ (hydrocortisone 1%) cream in the treatment of children with atopic dermatitis. Curr Ther Res Clin Exp 1988;44:421-5.
- Andersen BL, Andersen KE, Nielsen R, Stahl D, Niordson A, Roders GA. Treatment of dry atopic dermatitis in children. A double-blind comparison between mildison Lipocream™ (1% hydrocortisone) and Uniderm™ (1% hydrocortisone) ointment. Clin Trials J 1988;25:278-84.
- Richelli C, Piacentini GL, Sette L, Bonizzato MC, Andreoli A, Boner AL. Clinical efficacy and tolerability of clobetasone 17-butyrate 0.5% lotion in children with atopic dermatitis. Curr Ther Res Clin Exp 1990;47:413-17.
- Zienicke H. Topical glucocorticoids and antiinfectives: a rational combination?. Curr Probl Dermatol 1993;21:186-91.
- Konzelmann M, Harms M. Diflorasone diacetate cream compared to betamethasone dipropionate cream in the treatment of eczemas. Schweiz Rundsch Med Prax 1983;72:709-11.
- Sanabria-Silva E, Laterza AM, Tamayo L, Ruiz-Maldonado R. Evaluation of rebound phenomenon in children with atopic dermatitis treated with topical corticosteroids. Dermatologia Revista Mexicana 1991;35:84-9.
- Harder F, Rufli T. Therapy of eczema. Once daily use of diflorasone diacetate in comparison to thrice daily use of betamethasone-17-valerate. Schweiz Rundsch Med Prax 1983;72:1240-2.
- Savin RC. Betamethasone dipropionate in psoriasis and atopic dermatitis. Conn Med 1976;40:5-7.
- Niordson AM, Stahl D. Treatment of psoriasis with clinitar cream. A controlled clinical trial. Br J Clin Pract 1985;39:67-8.
- Larregue M, Devaux J, Audebert C, Gelmetti DR. A double-blind controlled study on the efficacy and tolerability of 6% ammonium lactate cream in children with atopic dermatitis. Nouv Dermatol 1996;15:720-1.
- Pigatto PD, Bigardi AS, Cannistraci C, Picardo M. 10% urea cream (Laceran) for atopic dermatitis: a clinical and laboratory evaluation. J Dermatol Treat 1996;7:171-5.
- Kantor I, Milbauer J, Posner M, Weinstock IM, Simon A, Thormahlen S. Efficacy and safety of emollients as adjunctive agents in topical corticosteroid therapy for atopic dermatitis. Today Ther Trends 1993;11:157-66.
- Andersson AC, Lindberg M, Loden M. The effect of two urea-containing creams on dry, eczematous skin in atopic patients. I. Expert, patient and instrumental evaluation. J Dermatol Treat 1999;10:165-9.
- Hanifin JM, Hebert AA, Mays SR, Paller AS, Sherertz EF, Wagner AM, et al. Effects of a lowpotency corticosteroid lotion plus a moisturizing regimen in the treatment of atopic dermatitis. Curr Ther Res Clin Exp 1998;59:227-33.
- Wilhelm KP, Scholermann A. Efficacy and tolerability of a topical preparation containing 10% urea in patients with atopic dermatitis. Aktuel Dermatol 1998;24:26-30.
- Anstey A, Wilkinson JD. Lithium succinate ointment in the treatment of atopic eczema [1]. J Dermatol Treat 1991;2:37-8.
- Boguniewicz M, Fiedler VC, Raimer S, Lawrence ID, Leung DY, Hanifin JM. A randomized, vehiclecontrolled trial of tacrolimus ointment for treatment of atopic dermatitis in children. Pediatric Tacrolimus Study Group. J Allergy Clin Immunol 1998;102:637-44.
- Reitamo S, Rissanen J, Remitz A, Granlund H, Erkko P, Elg P, et al. Tacrolimus ointment does not affect collagen synthesis: results of a single-center randomized trial. J Invest Dermatol 1998;111:396-8.
- Ruzicka T, Bieber T, Schopf E, Rubins A, Dobozy A, Bos JD, et al. A short-term trial of tacrolimus ointment for atopic dermatitis. European tacrolimus multicenter atopic dermatitis study group. N Engl J Med 1997;337:816-21.
- Van Leent EJ, Graber M, Thurston M, Wagenaar A, Spuls PI, Bos JD. Effectiveness of the ascomycin macrolactam SDZ ASM 981 in the topical treatment of atopic dermatitis. Arch Dermatol 1998;134:805-9.
- Ewing CI, Ashcroft C, Gibbs AC, Jones GA, Connor PJ, David TJ. Flucloxacillin in the treatment of atopic dermatitis. Br J Dermatol 1998;138:1022-9.
- Stalder JF, Fleury M, Sourisse M, Allavoine Th, Chalamet C, Brosset P, . Comparative effects of two topical antiseptics (chlorhexidine vs kmn04) on bacterial skin flora in atopic dermatitis. Acta Dermato Venereol (Stockh) 1992:132-4.
- Holland KT, Bojar RA, Cunliffe WJ, Lever R, Levy J. The bacteriology of eczema. UK: The Royal Society of Medicine Press Limited; 1995.
- Harper J, Lever R, Levy J. The bacteriology of eczema. UK: The Royal Society of Medicine Press Limited; 1995.
- Lever R, Hadley K, Downey D, Mackie R. Staphylococcal colonization in atopic dermatitis and the effect of topical mupirocin therapy. Br J Dermatol 1988;119:189-98.
- Broberg A, Faergemann J. Topical antimycotic treatment of atopic dermatitis in the head/neck area. A double-blind randomised study. Acta Dermato Venereol 1995;75:46-9.
- Salo OP, Gordin A, Brandt H, Antikainen R. Efficacy and tolerability of erythromycin acistrate and erythromycin stearate in acute skin infections of patients with atopic eczema. J Antimicrob Chemother 1988;21:101-6.
- Weinberg E, Fourie B, Allmann B, Toerien A. The use of cefadroxil in superinfected atopic dermatitis. Curr Ther Res Clin Exp 1992;52:671-6.
- Sasai-Takedatsu M, Kojima T, Yamamoto A, Hattori K, Yoshijima S, Taniuchi S, et al. Reduction of Staphylococcus aureus in atopic skin lesions with acid electrolytic water – a new therapeutic strategy for atopic dermatitis. Allergy 1997;52:1012-6.
- Hizawa T, Sano H, Endo K, Fukuzumi T, Kataoka Y, Aoki T. Is povidone-iodine effective to the lesions of atopic dermatitis?. Skin Res 1998;40:134-9.
- Zuluaga de Cadena A, Ochoa de VA, Donado JH, Mejia JI, Chamah HM, Montoya de Restrepo F. Estudio comparativo del efecto de la hidroxicina la terfenadina y el astemizol en ninos con dermatitis atopica: Hospital General de Medellin-Centro de Especialistas C.E.S. 1986–1988. CES Med 1989;3:7-13.
- Ishibashi Y, Tamaki K, Yoshida H, Niimura M, Harada S, Ueda H, et al. Clinical evaluation of E-0659 on atopic dermatitis. Multicenter double-blind study in comparison with ketotifen. Rinsho Hyoka 1989;17:77-115.
- Estelle F, Simons R. Prospective long term safety evaluation of the H1-receptor antagonist cetirizine in very young children with atopic deramtitis. J Allergy Clin Immunol 1999;104:433-40.
- Monroe EW. Relative efficacy and safety of loratadine, hydroxyzine, and placebo in chronic idiopathic urticaria and atopic dermatitis. Clin Ther 1992;14:17-21.
- Wahlgren CF, Hagermark O, Bergstrom R. The antipruritic effect of a sedative and a nonsedative antihistamine in atopic dermatitis. Br J Dermatol 1990;122:545-51.
- Berth-Jones J, Graham-Brown RA. Failure of terfenadine in relieving the pruritus of atopic dermatitis. Br J Dermatol 1989;121:635-7.
- Doherty V, Sylvester DG, Kennedy CT, Harvey SG, Calthrop JG, Gibson JR. Treatment of itching in atopic eczema with antihistamines with a low sedative profile. BMJ 1989;298.
- Patel P, Gratton D, Eckstein G, Aberer W, Pryzbilla B, Chelly M, et al. A double-blind study of loratadine and cetirizine in atopic dermatitis. J Dermatol Treat 1997;8:249-53.
- Savin JA, Dow R, Harlow BJ, Massey H, Yee KF. The effect of a new non-sedative h1-receptor antagonist (ln2974) on the itching and scratching of patients with atopic eczema. Clin Exp Dermatol 1986;11:600-2.
- Frosch PJ, Schwanitz HJ, Macher E. A double blind trial of h1 and h2 receptor antagonists in the treatment of atopic dermatitis. Arch Dermatol Res 1984;276:36-40.
- Simons R, Estelle F, Simons KJ, Becker AB, Haydey RP. Pharmacokinetics and antipruritic effects of hydroxyzine in children with atopic dermatitis. J Pediatr 1984;104:123-7.
- Foulds IS, MacKie RM. A double-blind trial of the h2 receptor antagonist cimetidine, and the h1 receptor antagonist promethazine hydrochloride in the treatment of atopic dermatitis. Clin Allergy 1981;11:319-23.
- Savin JA, Paterson WD, Adam K, Oswald I. Effects of trimeprazine and trimipramine on nocturnal scratching in patients with atopic eczema. Arch Dermatol 1979;115:313-15.
- Hjorth N. Terfenadine in the treatment of chronic idiopathic urticaria and atopic dermatitis. Cutis 1988;42:29-30.
- Henz BM, Metzenauer P, O’Keefe E, Zuberbier T. Differential effects of new-generation h1-receptor antagonists in pruritic dermatoses. Allergy 1998;53:180-3.
- Langeland T, Fagertun HE, Larsen S. Therapeutic effect of loratadine on pruritus in patients with atopic dermatitis. A multi-crossover-designed study. Allergy 1994;49:22-6.
- La Rosa M, Ranno C, Musarra I, Guglielmo F, Corrias A, Bellanti JA. Double-blind study of cetirizine in atopic eczema in children. Ann Allergy 1994;73:117-22.
- Hannuksela M, Kalimo K, Lammintausta K, Mattila T, Turjanmaa K, Varjonen E, et al. Dose ranging study: cetirizine in the treatment of atopic dermatitis in adults. Ann Allergy 1993;70:127-33.
- Klein GL, Galant SP. A comparison of the antipruritic efficacy of hydroxyzine and cyproheptadine in children with atopic dermatitis. Ann Allergy 1980;44:142-5.
- Hamada T, Ishii M, Nakagawa K, Kobayashi H, Kitajima J, Chanoki M, et al. Evaluation of the clinical effect of terfenadine in patients with atopic dermatitis. A comparison of strong cortico-steroid therapy to mild topical corticosteroid combined with terfenadine administration therapy. Skin Res 1996;38:97-103.
- Ishibashi Y, Ueda H, Niimura M, Harada S, Tamaki K, Imamura S, et al. Clinical evaluation of E-0659 in atopic dermatitis in infants and children. Dose-finding multicenter study by the double-blind method. Skin Res 1989;31:458-71.
- Kimata H, Igarashi M. Topical cromolyn (disodium cromoglycate) solution in the treatment of young children with atopic dermatitis. Clin Exp Allergy 1990;20:281-3.
- Larsen FS, Jacobsen KU. Atopic dermatitis and systemic treatment with a new chromone compound (FPL 57787): a double blind clinical trial. Acta Derm Venereol Suppl (Stockh) 1980:128-9.
- Haider SA. Treatment of atopic eczema in children: clinical trial of 10% sodium cromoglycate ointment. BMJ 1977;1:1570-2.
- Graham P, Hall-Smith SP, Harris JM, Price ML. A study of hypoallergenic diets and oral sodium cromoglycate in the management of atopic eczema. Br J Dermatol 1984;110:457-67.
- Moore C, Ehlayel MS, Junprasert J, Sorensen RU. Topical sodium cromoglycate in the treatment of moderate-to-severe atopic dermatitis. Ann Allergy Asthma Immunol 1998;81:452-8.
- Thirumoorthy T, Greaves MW. Disodium cromoglycate ointment in atopic eczema [letter]. BMJ 1978;2:500-1.
- Croner S, Fagerlund E, Kjellmann NIM, Leijon I. Sodium cromoglycate ointment in atopic eczema during childhood. Opuscula Medica 1081;26:49-50.
- Kimata H, Hiratsuka S. Effect of topical cromoglycate solution on atopic dermatitis: combined treatment of sodium cromoglycate solution with the oral anti-allergic medication, oxatomide. Eur J Pediatr 1994;153:66-71.
- Ariyanayagam M, Barlow TJ, Graham P, Hall-Smith SP, Harris JM. Topical sodium cromoglycate in the management of atopic eczema – a controlled trial. Br J Dermatol 1985;112:343-8.
- Atherton DJ, Soothill JF, Elvidge J. A controlled trial of oral sodium cromoglycate in atopic eczema. Br J Dermatol 1982;106:681-5.
- Kavli G, Larsen PO. Double-blind crossover trial comparing systemic chromosome-carboxylic acid with placebo in patients with atopic dermatitis. Allergy 1981;36:597-600.
- Businco L, Benincori N, Nini G, Businco E, Cantani A, De Angelis M. Double-blind crossover trial with oral sodium cromoglycate in children with atopic dermatitis due to food allergy. Ann Allergy 1986;57:433-8.
- Larsen PO, Larsen FS. Clinical trial of a new chromone compound for systemic treatment of atopic dermatitis. Acta Derm Venereol 1979;59:270-1.
- Burks AW, Sampson HA. Double-blind placebocontrolled trial of oral cromolyn in children with atopic dermatitis and documented food hyper-sensitivity. J Allergy Clin Immunol 1988;81:417-23.
- Birkeland SA, Larsen PO, Larsen FS. Subpopulations of lymphocytes and lymphocyte transformation tests in atopic dermatitis: evaluation of a systemic treatment with a new chromone compound and comparison with a normal group. J Invest Dermatol 1981;76:367-70.
- Kjellman NI, Gustafsson IM. Topical sodium cromoglycate in atopic dermatitis. A disappointing but informative trial. Allergy 1986;41:423-8.
- Lindskov R, Knudsen L. Oral disodium cromoglycate treatment of atopic dermatitis. Allergy 1983;38:161-5.
- Ventura A, De Seta L, Martelossi S, Florean P, Maggiore G, Salvatore CM, et al. Soy allergy and DSCG in atopic eczema: ‘much ado about nothing’?. Pediatr Med Chir 1996;18:283-8.
- Pike MG, Atherton DJ. Failure of a new topical sodium cromoglycate formulation to improve atopic dermatitis [letter]. Eur J Pediatr 1988;148.
- Kemmett D, Barneston RST. Topical nedocromil sodium: a double blind placebo controlled study in atopic dermatitis. Br J Dermatol 1987;123.
- Van Bever HP, Stevens WJ. Nedocromil sodium cream in the treatment of atopic dermatitis [letter]. Eur J Pediatr 1989;149.
- Benton EC, McFarlane HA, Barnetson RS. Trial of nedocromil sodium in atopic eczema. Br J Dermatol 1990;122:817-20.
- Falk ES. Ketotifen in the treatment of atopic dermatitis. Results of a double blind study. Rev Eur Sci Med Farmacol 1993;15:63-6.
- White MP, MacDonald TH, Garg RA. Ketotifen in the young asthmatic – a double-blind placebocontrolled trial. J Int Med Res 1988;16:107-13.
- Drake LA, Fallon JD, Sober A. Relief of pruritus in patients with atopic dermatitis after treatment with topical doxepin cream. The doxepin study group. J Am Acad Dermatol 1994;31:613-16.
- Breneman DL, Dunlap FE, Monroe EW, Schupbach CW, Shmunes E, Phillips SB. Doxepin cream relieves eczema-associated pruritus within 15 minutes and is not accompanied by a risk of rebound upon discontinuation. J Dermatol Treat 1997;8:161-8.
- Berberian BJ, Breneman DL, Drake LA, Gratton D, Raimir SS, Phillips S, et al. The addition of topical doxepin to corticosteroid therapy: an improved treatment regimen for atopic dermatitis. Int J Dermatol 1999;38:145-8.
- Drake LA, Cohen L, Gillies R, Flood JG, Riordan AT, Phillips SB, et al. Pharmacokinetics of doxepin in subjects with pruritic atopic dermatitis. J Am Acad Dermatol 1999;41:209-14.
- Czarnetzki BM, Brechtel B, Braun-Falco O, Christophers E, Schopf E, Reckers-Czaschka R, et al. Topical tiacrilast, a potent mast cell degranulation inhibitor, does not improve adult atopic eczema. Dermatology 1993;187:112-4.
- Atherton DJ. Dietary antigen avoidance in the treatment of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980:99-102.
- Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol 1997;99:179-85.
- Lever R, MacDonald C, Waugh P, Aitchison T. Randomised controlled trial of advice on an egg exclusion diet in young children with atopic eczema and sensitivity to eggs. Pediatr Allergy Immunol 1998;9:13-9.
- Neild VS, Marsden RA, Bailes JA, Bland JM. Egg and milk exclusion diets in atopic eczema. Br J Dermatol 1986;114:117-23.
- Mabin DC, Sykes AE, David TJ. Controlled trial of a few foods diet in severe atopic dermatitis. Arch Dis Child 1995;73:202-7.
- Cant AJ, Bailes JA, Marsden RA, Hewitt D. Effect of maternal dietary exclusion on breast fed infants with eczema: two controlled studies. BMJ Clin Res Ed 1986;293:231-3.
- Munkvad M, Danielsen L, Hoj L, Povlsen CO, Secher L, Svejgaard E, et al. Antigen-free diet in adult patients with atopic dermatitis. A double-blind controlled study. Acta Derm Venereol 1984;64:524-8.
- Atherton DJ, Sewell M, Soothill JF, Wells RS, Chilvers CE. A double-blind controlled crossover trial of an antigen-avoidance diet in atopic eczema. Lancet 1978;1:401-3.
- Isolauri EMD, Sutas YMD, Makinen-Kiljunen SMSc, Oja Simo S MD, Isosomppi RMSc, Turjanmaa KMD. Efficacy and safety of hydrolyzed cow milk and amino acid-derived formulas in infants with cow milk allergy. J Pediatr 1995;127:550-7.
- Gehring W, Bopp R, Rippke F, Gloor M. Effect of topically applied evening primrose oil on epidermal barrier function in atopic dermatitis as a function of vehicle. Arzneimittelforschung 1999;49:635-42.
- Ferreira MJ, Fiadeiro T, Silva M, Soares AP. Topical gamma-linolenic acid therapy in atopic dermatitis. A clinical and biometric evaluation. Allergo J 1998;7:213-16.
- Ferreira MJ, Fiadeiro T, Silva M, Soares AP. Electrical conductance: a controversial parameter in the evaluation of emollients in atopic dermatitis. Skin Res Technol 1998;4:138-41.
- Hederos CA, Berg A. Epogam evening primrose oil treatment in atopic dermatitis and asthma. Arch Dis Child 1996;75:494-7.
- Schalin-Karrila M, Mattila L, Jansen CT, Uotila P. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol 1987;117:11-9.
- Biagi PL, Bordoni A, Hrelia S, Celadon M, Ricci GP, Cannella V, et al. The effect of gamma-linolenic acid on clinical status, red cell fatty acid composition and membrane micro-viscosity in infants with atopic dermatitis. Drugs Exp Clin Res 1994;20:77-84.
- Humphreys F, Symons JA, Brown HK, Duff GW, Hunter JAA. The effects of gamolenic acid on adult atopic eczema and premenstrual exacerbation of eczema. Eur J Dermatol 1994;4:598-603.
- Bordoni A, Biagi PL, Masi M, Ricci G, Fanelli C, Patrizi A, et al. Evening primrose oil (efamol) in the treatment of children with atopic eczema. Drugs Exp Clin Res 1987;14:291-7.
- Bamford JT, Gibson RW, Renier CM. Atopic eczema unresponsive to evening primrose oil (linoleic and gamma-linolenic acids). J Am Acad Dermatol 1985;13:959-65.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatol Treat 1990;1:199-201.
- Wright S, Burton JL. Oral evening-primroseseed oil improves atopic eczema. Lancet 1982;2:1120-2.
- Berth-Jones J, Graham-Brown RC. Placebo-controlled trial of essential fatty acid supplementation in atopic dermatitis. Lancet 1993;341:1557-60.
- Lovell CR, Burton JL, Horrobin DF. Treatment of atopic eczema with evening primrose oil [letter]. Lancet 1981;1.
- Wright S. Atopic dermatitis and essential fatty acids: a biochemical basis for atopy?. Acta Derm Venereol (Stockh) 1985:143-5.
- Bahmer FA, Schafer J. Treatment of atopic dermatitis with borage seed oil (glandol) – a time series analytic study. Kinderarztl Prax 1992;60:199-202.
- Borrek S, Hildebrandt A, Forster J. Gammalinolenicacid-rich borage seed oil capsules in children with atopic dermatitis. A placebo-controlled doubleblind study. Klin Padiatr 1997;209:100-4.
- Buslau M, Thaci D. Atopic dermatitis: Borage oil for systemic therapy. Z Dermatol 1996;182:131-2.
- Henz BM, Jablonska S, van de Kerkhof PCM, Stingl G, Blaszczyk M, Vandervalk PGM, et al. Doubleblind, multicentre analysis of the efficacy of borage oil in patients with atopic eczema. Br J Dermatol 1999;140:685-8.
- Valsecchi R, Di Landro A, Pansera B, Reseghetti A. Gammalinolenic acid in the treatment of atopic dermatitis [1]. J Eur Acad Dermatol Venereol 1996;7:77-9.
- Bjorneboe A, Soyland E, Bjorneboe G-EA, Rajka G, Drevon CA. Effect of dietary supplementation with eicosapentaenoic acid in the treatment of atopic dermatitis. Br J Dermatol 1987;117:463-9.
- Soyland E, Funk J, Rajka G, Sandberg M, Thune P, Rustad L, et al. Dietary supple-mentation with very long-chain n-3 fatty acids in patients with atopic dermatitis. A double-blind, multicentre study. Br J Dermatol 1994;130:757-64.
- Gimenez-Arnau A, Barranco C, Alberola M, Wale C, Serrano S, Buchanan MR, et al. Effects of linoleic acid supplements on atopic dermatitis. Adv Exp Med Biol 1997;433:285-9.
- Bjorneboe A, Soyland E, Bjorneboe GE, Rajka G, Drevon CA. Effect of n-3 fatty acid supplement to patients with atopic dermatitis. J Intern Med Suppl 1989;225:233-6.
- Mabin DC, Hollis S, Lockwood J, David TJ. Pyridoxine in atopic dermatitis. Br J Dermatol 1995;133:764-7.
- Czeizel AE, Dobo M. Postnatal somatic and mental development after periconceptional multivitamin supplementation. Arch Dis Child 1994;70:229-33.
- Fairris GM, Perkins PJ, Lloyd B, Hinks L, Clayton BE. The effect on atopic dermatitis of supplementation with selenium and vitamin E. Acta Derm Venereol 1989;69:359-62.
- Hakakawa R, Ogino Y. Effects of combination therapy with vitamins E and B2 on skin diseases. Doudle blind controlled clinical trial. Skin Res 1989;31:856-81.
- Ewing CI, Gibbs AC, Ashcroft C, David TJ. Failure of oral zinc supplementation in atopic eczema. Eur J Clin Nutr 1991;45:507-10.
- Nishioka K, Yasueda H, Saito H. Preventive effect of bedding encasement with microfine fibers on mite sensitization. J Allergy Clin Immunol 1998;101:28-32.
- Endo K, Fukuzumi T, Adachi J, Kojima M, Aoki T, Yoshida M, et al. Effect of vacuum cleaning of room floors and bed clothes of patients on house dust mites counts and clinical scores of atopic dermatitis. A double blind control trial. Arerugi 1997;46:1013-24.
- Colloff MJ, Lever RS, McSharry C. A controlled trial of house dust mite eradication using natamycin in homes of patients with atopic dermatitis: effect on clinical status and mite populations. Br J Dermatol 1989;121:199-208.
- Tan BB, Weald Dawn, Strickland Ian, Friedmann PS. Double-blind controlled trial of effect of housedust-mite allergen avoidance on atopic dermatitis. Lancet 1996;347:15-8.
- Glover MT, Atherton DJ. A double-blind controlled trial of hyposensitization to dermatophagoides pteronyssinus in children with atopic eczema. Clin Exp Allergy 1992;22:440-6.
- Galli E, Chini L, Nardi S, Benincori N, Panei P, Fraioli G, et al. Use of a specific oral hyposensitization therapy to dermatophagoides pteronyssinus in children with atopic dermatitis. Allergol Immunopath (Madr) 1994;22:18-22.
- Wen T, Wang E, Shen S, Jiang C, Tian R, Kang K, et al. Allergenic potency of smu-df extract in comparison with vus-df extract; and diagnosis and immunotherapy for atopic dermatitis and rhinitis with smu-df extract in china. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M 1992;85:217-27.
- Friedmann PS, Tan BB. Mite elimination – clinical effect on eczema. Allergy 1998;53:97-100.
- Andersen PH, Bindslev-Jensen C, Mosbech H, Zachariae H, Andersen KE. Skin symptoms in patients with atopic dermatitis using enzymecontaining detergents. A placebo-controlled study. Acta Derm Venereol 1998;78:60-2.
- Diepgen TL, Stabler A, Hornstein OP. Irritation from textiles in atopic eczema and controls. Textile intolerance in atopic eczema: a controlled clinical study. Z Hautkrankheiten 1990;65:907-10.
- Diepgen TL, Salzer B, Tepe A, Hornstein OP. A study of skin irritations by textiles under standardized sweating conditions in patients with atopic eczema. Melliand English 1995;12.
- Seymour JL, Keswick BH, Hanifin JM, Jordan WP, Milligan MC. Clinical effects of diaper types on the skin of normal infants and infants with atopic dermatitis. J Am Acad Dermatol 1987;17:988-97.
- Adachi J, Sumitsuzi H, Endo K, Fukuzumi T, Aoki T. Evaluation of the effect of short-term application of deep sea water on atopic dermatitis. Arerugi 1998;47:57-60.
- Broberg A, Kalimo K, Lindblad B, Swanbeck G. Parental education in the treatment of childhood atopic eczema. Acta Derm Venereol 1990;70:495-9.
- Schoni MH, Nikolaizik WH, Schoni-Affolter F. Efficacy trial of bioresonance in children with atopic dermatitis. Int Arch Allergy Immunol 1997;112:238-46.
- Ehlers A, Stangier U, Gieler U. Treatment of atopic dermatitis: a comparison of psychological and dermatological approaches to relapse prevention. J Consult Clin Psychol 1995;63:624-35.
- Melin L, Frederiksen T, Noren P, Swebilius BG. Behavioural treatment of scratching in patients with atopic dermatitis. Br J Dermatol 1986;115:467-74.
- Der-Petrossian M, Seeber A, Honigsmann H, Tanew A. Half-side comparison study on the efficacy of 8-methoxypsoralen bath-PUVA versus narrow-band ultraviolet B phototherapy in patients with severe chronic atopic dermatitis. Br J Dermatol 2000;142:39-43.
- Krutmann J, Diepgen TL, Luger TA, Grabbe S, Meffert H, Sonnichsen N, et al. High-dose UVA1 therapy for atopic dermatitis: results of a multicenter trial. J Am Acad Dermatol 1998;38:589-93.
- Reynolds NJ, Franklin V, Gray JC, Diffey BL, Farr PM. Effectiveness of narrow-band UVB (TL01) compared to UVA in adult atopic eczema: a randomised controlled trial. Br J Dermatol 1999;141.
- Jekler J, Larko O. UVb phototherapy of atopic dermatitis. Br J Dermatol 1988;119:697-705.
- Jekler J, Larko O. UVa solarium versus UVb phototherapy of atopic dermatitis: a paired-comparison study. Br J Dermatol 1991;125:569-72.
- Jekler J. Phototherapy of atopic dermatitis with ultraviolet radiation. Acta Derm Venereol Suppl 1992;171:1-37.
- Krutmann J, Czech W, Diepgen T, Niedner R, Kapp A, Schopf E. High-dose uva1 therapy in the treatment of patients with atopic dermatitis. J Am Acad Dermatol 1992;26:225-30.
- Leroy BP, Boden G, Lachapelle JM, Jacquemin MG, Saint-Remy JM. A novel therapy for atopic dermatitis with allergen-antibody complexes: a double-blind, placebo-controlled study. J Am Acad Dermatol 1993;28:232-9.
- Leroy BP, Boden G, Jacquemin MG, Lachapelle JM, Saint-Remy JMR. Allergen-antibody complexes in the treatment of atopic dermatitis: preliminary results of a double-blind placebo-controlled study. Acta Derm Venereol 1992:129-31.
- Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, et al. Cyclosporin for severe childhood atopic dermatitis: Short course versus continuous therapy. Br J Dermatol 2000;142:52-8.
- Cordero Miranda MA, Flores Sandoval G, Orea Solano M, Estrada Parra S, Serrano Miranda E. Safety and efficacy of treatment for severe atopic dermatitis with cyclosporin A and transfer factor. Revista Alergia Mexico 1999;46:49-57.
- Zonneveld IM, De Rie MA, Beljaards RC, Van-Der-Rhee HJ, Wuite J, Zeegelaar J, et al. The long-term safety and efficacy of cyclosporin in severe refractory atopic dermatitis: a comparison of two dosage regimens. Br J Dermatol 1996;135:15-20.
- Zurbriggen B, Wuthrich B, Cachelin AB, Wili PB, Kagi MK. Comparison of two formulations of cyclosporin A in the treatment of severe atopic dermatitis. A double-blind, single-centre, cross-over pilot study. Dermatology 1999;198:56-60.
- Wahlgren CF. Itch and atopic dermatitis: clinical and experimental studies. Acta Derm Venereol 1991:4-53.
- van Joost T, Heule F, Korstanje M, van den Broek MJ, Stenveld HJ, van Vloten WA. Cyclosporin in atopic dermatitis: a multicentre placebo-controlled study. Br J Dermatol 1994;130:634-40.
- Munro CS, Levell NJ, Shuster S, Friedmann PS. Maintenance treatment with cyclosporin in atopic eczema. Br J Dermatol 1994;130:376-80.
- Salek MS, Finlay AY, Luscombe DK, Allen BR, Berth-Jones J, Camp RD, et al. Cyclo-sporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trial. Br J Dermatol 1993;129:422-30.
- De Rie MA, Meinardi MM, Bos JD. Lack of efficacy of topical cyclosporin a in atopic dermatitis and allergic contact dermatitis. Acta Derm Venereol 1991;71:452-4.
- Sowden JM, Berth-Jones J, Ross JS, Motley RJ, Marks R, Finlay AY, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet 1991;338:137-40.
- Wahlgren CF, Scheynius A, Hagermark O. Antipruritic effect of oral cyclosporin a in atopic dermatitis. Acta Derm Venereol 1990;70:323-9.
- de Prost Y, Bodemer C, Teillac D. Double-blind randomized placebo-controlled trial of local cyclosporine in atopic dermatitis [letter]. Arch Dermatol 1989;125.
- Allen BR, Wolff K. Cyclosporin A and the skin: Proceedings of a satellite symposium to the 2nd congress of the European Academy of Dermatology and Venereology, 12 Oct. 1991. Athens, Greece. London: Royal Society of Medicine Services Ltd; 1991.
- White CR, Hanifin JM. Levamisole therapy in atopic dermatitis: randomized double-blind evaluation. Arch Dermatol 1978;114:1314-15.
- Abeck D, Andersson T, Grosshans E, Jablonska S, Kragballe K, Vahlquist A, et al. Topical application of a platelet-activating factor (paf) antagonist in atopic dermatitis. Acta Derm Venereol 1997;77:449-51.
- Renz H, Jujo K, Bradley KL, Domenico J, Gelfand EW, Leung DY. Enhanced il-4 production and il-4 receptor expression in atopic dermatitis and their modulation by interferon-gamma. J Invest Dermatol 1992;99:403-8.
- Hanifin JM, Schneider LC, Leung DY, Ellis CN, Jaffe HS, Izu AE, et al. Recombinant interferon gamma therapy for atopic dermatitis. J Am Acad Dermatol 1993;28:189-97.
- Jang I, Yang J, Lee H, Yi J, Kim H, Kim C, et al. Clinical improvement and immunohistochemical findings in severe atopic dermatitis treated with interferon gamma. J Am Acad Dermatol 2000;42:1033-40.
- Fiocchi A, Grasso U, Rottoli A, Travaglini P, Mazzanti P, Cazzola P, et al. A double-blind clinical trial on the effectiveness of a thymic derivative (thymomodulin) in the treatment of children with atopic dermatitis. Int J Immunother 1987;3:279-84.
- Cavagni G, Piscopo E, Rigoli E, Iuliano P, Bertolini P, Cazzola P. Food allergy in children: an attempt to improve the effects of the elimination diet with an immunomodulating agent (thymomodulin). A double-blind clinical trial. Immunopharmacol Immunotoxicol 1989;11:131-42.
- Staughton RCD, Byrom NA, Nagvekar NM, Harper JI, Mobayen M, Perry DE, et al. A doubleblind cross-over trial of thymostimulin in atopic eczema. Br J Dermatol 1983;109:39-4.
- Harper JI, Mason UA, White TR, Staughton RC, Hobbs JR. A double-blind placebo-controlled study of thymostimulin (tp-1) for the treatment of atopic eczema. Br J Dermatol 1991;125:368-72.
- Stiller MJ, Shupack JL, Kenny C, Jondreau L, Cohen DE, Soter NA. A double-blind, placebocontrolled clinical trial to evaluate the safety and efficacy of thymopentin as an adjunctive treatment in atopic dermatitis. J Am Acad Dermatol 1994;30:597-602.
- Hsieh KH, Shaio MF, Liao TN. Thymopentin treatment in severe atopic dermatitis – clinical and immunological evaluations. Arch Dis Child 1992;67:1095-102.
- Leung DY, Hirsch RL, Schneider L, Moody C, Takaoka R, Li SH, et al. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol 1990;85:927-33.
- Kang K, Cooper KD, Hanifin JM. Thymopoietin pentapeptide (tp-5) improves clinical parameters and lymphocyte subpopulations in atopic dermatitis. J Am Acad Dermatol 1983;8:372-7.
- Pons-Guiraud A. Value of Allerglobulin in the treatment of atopic dermatitis in children and young adults. A double-blind randomized study. Rev Med Intern 1986;7:537-42.
- Valdes Sanchez AF, Fernandez Ortega C, Gomez Echeverria AH, Gillama Niebla E, Lastra Alfonso G, Lopez Saura P. Atopic dermatitis. Treatment with transfer factor. A controlled clinical trial. Revista Alergia 1991;38:158-62.
- Sheehan MP, Rustin MH, Atherton DJ, Buckley C, Harris DW, Brostoff J, et al. Efficacy of traditional chinese herbal therapy in adult atopic dermatitis. Lancet 1992;340:13-7.
- Sheehan MP, Atherton DJ. A controlled trial of traditional chinese medicinal plants in widespread non-exudative atopic eczema. Br J Dermatol 1992;126:179-84.
- Fung AY, Look PC, Chong LY, But PP, Wong E. A controlled trial of traditional Chinese herbal medicine in Chinese patients with recalcitrant atopic dermatitis. Int J Dermatol 1999;38:387-92.
- Latchman Y, Banerjee P, Poulter LW, Rustin M, Brostoff J. Association of immunological changes with clinical efficacy in atopic eczema patients treated with traditional chinese herbal therapy (zemaphyte). Int Arch Allergy Immunol 1996;109:243-9.
- Remy W, Rakoski J, Siebenwirth J, Ulm K, Wiesenauer M. Classical homeopathic treatment in atopic dermatitis. Study protocol. Allergologie 1995;18:246-52.
- Anderson C, Lis Balchin M. The effect of aromatherapy on childhood atopic eczema (presented at the 5th Annual Symposium on Complementary Healthcare, 10–12 December 1998, Exeter). FACT: Focus on Alternative &Amp; 1998;3.
- Sokel B, Kent CA, Lansdown R, Atherton D, Glover M, Knibbs J. A comparison of hypnotherapy and biofeedback in the treatment of childhood atopic eczema. Contemp Hypnosis 1993;10.
- Schachner L, Field T, Hernandez-Reif M, Duarte AM, Krasnegor J. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol 1998;15:390-5.
- Ebata T, Izumi H, Aizawa H, Kamide R, Niimura M. Effects of nitrazepam on nocturnal scratching in adults with atopic dermatitis: a double-blind placebo-controlled crossover study. Br J Dermatol 1998;138:631-4.
- Veien NK, Kaaber K, Larsen PO, Nielsen AO, Thestrup-Pedersen K. Ranitidine treatment of hand eczema in patients with atopic dermatitis: a doubleblind, placebo-controlled trial. J Am Acad Dermatol 1995;32:1056-7.
- Ruzicka T. Effect of theophylline in atopic dermatitis: a double-blind cross-over study. Arch Dermatol Res 1980;269:109-10.
- Archer CB, MacDonald DM. Treatment of atopic dermatitis with salbutamol. Clin Exp Dermatol 1987;12:323-5.
- Berth-Jones J, Graham-Brown RA. Failure of papaverine to reduce pruritus in atopic dermatitis: a double-blind, placebo-controlled cross-over study. Br J Dermatol 1990;122:553-7.
- Shupack J, Stiller M, Meola T Jr, Orbuch P. Papaverine hydrochloride in the treatment of atopic dermatitis: a double-blind, placebocontrolled crossover clinical trial to reassess safety and efficacy. Dermatologica 1991;183:21-4.
- Kimata H. Selective enhancement of production of IgE, IgG4, and Th2-cell cytokine during the rebound phenomenon in atopic dermatitis and prevention by suplatast tosilate. Ann Allergy 1999;82:293-5.
- Allergic factors associated with the development of asthma and the influence of cetirizine in a double-blind, randomised, placebo-controlled trial: first results of ETAC. Early Treatment of the Atopic Child. Pediatr Allergy Immunol 1998;9:116-24.
- Kramer MS. Does breast feeding help protect against atopic disease? Biology, methodology, and a golden jubilee of controversy. J Pediatr 1988;112:181-90.
- Kramer MS. Maternal antigen avoidance during lactation for preventing atopic disease in infants of women at high risk (Cochrane Review). Oxford: Update Software; 1999.
- Lovegrove JA, Hampton SM, Morgan JB. The immunological and long-term atopic outcome of infants born to women following a milk-free diet during late pregnancy and lactation: a pilot study. Br J Nutr 1994;71:223-38.
- Hepburn DJ, Aeling JL, Weston WL. A reappraisal of topical steroid potency. Pediatr Dermatol 1996;31:239-45.
- Charman C, Godlee F. Clinical evidence. London: BMJ Publishing Group; 1999.
- Once-a-day topical corticosteroids. Drug Ther Bull 1995;33:21-2.
- Lagos BR, Maibach HI. Frequency of application of topical corticosteroids: an overview. Br J Dermatol 1998;139:763-6.
- Williams HC. Do topical steroids reduce relapses in adults with atopic dermatitis?. Evidence Based Dermatology Section of Archives of Dermatology 1999;135:1530-1.
- Gibson JR, Kirsch JM, Darley CR, Harvey SG, Burke CA, Hanson ME. An assessment of the relationship between vasoconstrictor assay findings, clinical efficacy and skin thinning effects of a variety of undiluted and diluted corticosteroid preparations. Br J Dermatol 1984;111:204-12.
- Clement M, du Vivier A. Concern about the current clinical practice of diluting topical glucocorticoid preparations. Clin Exp Derm 1984;9:286-9.
- Clement M, DuVivier A. Concern about the current clinical practice of diluting topical glucocorticoid preparations. Clin Exp Dermatol 1984;9:286-9.
- Deeks T. Compounding and dilution of topical steroids. Br J Pharmaceut Pract 1985:134-40.
- Hornstein O, Ruzicka T, Ring J, Przybilla B. Guidelines for topical treatment of atopic eczema. Handbook of atopic eczema. Berlin: Springer-Verlag; 1991.
- Marks R. How to measure the effects of emollients. J Dermatol Treat 1997;8:15-8.
- Cork MJ. Complete emollient therapy. The National Association of Fundholding Practices Official Yearbook. Dunstable: BPC Waterlow; 1998.
- Newbold PC. Comparison of four emollients in the treatment of various skin conditions. Practitioner 1980;224:205-6.
- Williams HC, Stevens ARJ. Dermatology. Oxford: Radcliffe Medical Press; 1997.
- Boardman L. The use of emollients in dry skin conditions. MeReC Bulletin 1998;9:45-8.
- Chalmers TC. When should randomisation begin?. Lancet 1968;i.
- Ruzicka T, Asmann T, Homey B. Tacrolimus – the drug for the turn of the millenium?. Arch Dermatol 1999;135:574-80.
- Nakagawa H, Etoh T, Ishibashi Y, Higaki Y, Kawashima M, Torii H, et al. Tacrolimus ointment for atopic eczema. Lancet 1994;344.
- Bos JD, Bartin P, de Vries HJC, Ebelin ME, Graeber M, van Leent EJM. 1999.
- Ortonne JP. SDZ ASM 981 does not induce skin atrophy: a randomised, double-blind controlled study. J Eur Acad Dermatol Venereol 1999;12.
- Leyden JE, Marples RR, Kligman AM. Staphylococcal aureus in the lesions of atopic dermatitis. Br J Dermatol 1974;90:525-30.
- Antiseptic/emollient combinations. Drug Ther Bull 1998;11:84-6.
- Noble WC, Lever RLJ. The Bacteriology of atopic eczema. London: Royal Society of Medicine Press Ltd; 1995.
- Hauser C. The role of staphylococcus aureus on atopic eczema. Int J Dermatol 1986;25:573-4.
- McFadden JP, Noble WC, Camp RDR. Superantigenic exotoxin-secreting potential of stapjylococci isolated from atopic eczematous skin. Br J Dermatol 1993;128:631-2.
- Baraf CS. Treatment of pruritis in allergic dermatoses: an evaluation of the relative efficacy of cyproheptadine and hydroxyzine. Curr Ther Res 1976;19:32-8.
- Corbett MF. Cross-over designs for clinical trials. Br J Dermatol 1991;124.
- Eysenbach G, Williams H, Diepgen TL. Antihistamines for atopic eczema (Protocol for a Cochrane Review). Oxford: Update-Software; 1999.
- Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993;186:23-31.
- Klein PA, Clark RAF. An evidence-based review of the efficacy of antihistamines in relieving pruritis in atopic dermatitis. Arch Dermatol 1999;315:1522-5.
- Sabroe RA, Kennedy CTC, Archer CB. The effects of topical doxepin on responses to histamine, substance P and prostaglandin E2 in human skin. Br J Dermatol 1997;137:386-90.
- Bernstein JE. Relief of eczema-associated pruritus by doxepin hydrochloride. J Invest Dermatol 1986;86.
- Sloper KS, Wadsworth J, Brostoff J. Children with atopic eczema I: clinical response to food elimination and subsequent double-blind food challenge. Q J Med 1991;80:677-93.
- Devlin J, David TJ. Tartrazine in atopic eczema. Arch Dis Child 1992;67:709-11.
- James JM, Bernhisel-Broadbent J, Sampson HA. Respiratory reactions provoked by double-blind food challenges in children. Am J Respir Crit Care Med 1994;149:59-64.
- Fuglsang G, Madsen G, Halken S, Jorgensen S, Ostergaard PA, Osterballe O. Adverse reactions to food additives in children with atopic symptoms. Allergy 1994;49:31-7.
- Kanny G, Hatahet R, Moneret-Vautrin DA, Kohler C, Bellut A. Allergy and intolerance to flavouring agents in atopic dermatitis in young children. Allergie Immunol 1994;26:204-6.
- Sampson HA. The immunopathogenic role of food hypersensitivity in atopic dermatitis. Acta Derm Venereol Suppl 1992;176:34-7.
- Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med 1989;321:228-32.
- Sampson HA. Comparative study of commercial food antigen extracts for the diagnosis of food hypersensitivity. J Allergy Clin Immunol 1988;82:718-26.
- Sampson HA, Ho DG. Relationship between foodspecific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol 1997;100:444-51.
- David TJ. Food and food additive intolerance in childhood. Oxford: Blackwell Scientific Publications; 1993.
- Morse PF, Horrobin DF, Manku MS, Stewart JCM, Allen R, Littlewood S, et al. Meta-analysis of placebo-controlled studies on the efficacy of epogam in the treatment of atopic eczema. Br J Dermatol 1989;121:75-90.
- Li Wan Po A, Williams HC. A systematic review of Epogam in the treatment of atopic eczema. London: Department of Health; 1997.
- MacDonald KJS, Green C, Raffle EJ. Topical evening primrose seed oil and atopic eczema. Scott Med J 1985;30.
- Koller DY, Pirker C, Jarisch R. Pyridoxine HCI improves atopic eczema dermatitis: changes of IL-1 beta, IL-2, ACTH and cortisol in plasma. Clin Exp Allergy 1992;22.
- Norris PG, Schofield O, Camp RDR. A study of the role of house dust mite in atopic dermatitis. Br J Dermatol 1988;118:435-40.
- Cameron MM. Can house dust mite-triggered atopic dermatitis be alleviated using acaridides?. Br J Dermatol 1997;137:1-8.
- Sanda T, Yasue T, Oohashi M, Yasue A. Effectiveness of house dust-mite allergen avoidance through clean room therapy in patients with atopic dermatitis. J Allergy Clin Immunol 1989;3:653-7.
- Fukaya M. Change of housing environment and withdrawal of corticosteroid as treatments of atopic dermatitis. Arerugi 1999;48:520-5.
- Darsow U, Vieluf D, Ring J. Evaluating the relevance of aeroallergen sensitization in atopic eczema with the atopy patch test: a randomized, double-blind multicenter study. Atopy patch test study group. J Am Acad Dermatol 1999;40:187-93.
- Varela P, Selores M, Gomes E, Silva E, Matos E, dos Santos L, et al. Immediate and delayed hypersensitivity to mite anitgens in atopic dermatitis. Pediatr Dermatol 1999;16:1-15.
- Pacor ML, Biasi D, Maleknia T. The efficacy of longterm specific immunotherapy for Dermatophagoides pteronyssinus in patients with atopic dermatitis. Recenti Progressi in Medicina 1994;85:273-7.
- Rothe MJ, Grant-Kels JM. Diagnostic criteria for atopic dermatitis. Lancet 1996;348:1391-2.
- Zimmermann J, Utermann S. Photo-brine therapy in patients with psoriasis and nueroderm. Hautarzt 1994;45:849-53.
- Iikura Y, Sakamoto Y, Imai T, Akai T, Matsuoka T, Sugihara K, et al. n.d.
- Noren P. Habit reversal: a turning point in the treatment of atopic dermatitis. Clin Exp Derm 1995;20:2-5.
- Brown DG, Bettley FR. Psychiatric treatment of eczema: A controlled trial. BMJ 1971;2:729-34.
- Brehler R, Hilderbrand A, Luger T. Recent development in the treatment of atopic eczema. Am Acad Dermatol 1997;36:983-4.
- Diffey BL. Human exposure to ultraviolet light. Semin Dermatol 1990;9:2-10.
- Sheehan-Dare RA, Goodfield MJ, Rowell NR. Topical psoralen photochemotherapy (PUVA) and superficial radiotherapy in the treatment of chronic hand eczema. Br J Dermatol 1989;121:65-9.
- Krutmann J, Elmets C. Photoimmunology. Oxford: Blackwell; 1995.
- Grundmann-Kollmann M, Behrens S, Peter RU, Kerscher M. Treatment of severe recalcitrant dermatoses of the palms and soles with PUVA-bath versus PUVA-cream therapy. Photodermatol Photoimmunol Photomed 1999;15:87-9.
- Thomas MD, Cook LJ. Drug points: fever associated with cyclosporin for treating atopic dermatitis. BMJ 1998;317.
- Zaki I, Emerson R, Allen BR. Treatment of severe atopic dermatitis in childhood with cyclosporin. Br J Dermatol 1996;135:21-4.
- Holden CA, Parish WE, Rook AJ WDEF. Textbook of dermatology. Oxford: Blackwell Scientific Publications; 1998.
- Greenland S, Robins JM. Estimation of common effect parameter from sparse follow-up data. Biometrics 1985;41:55-68.
- Fleiss J, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol 1991;44:127-39.
- DerSimonian R, Laird N. Meta-analysis in Clinical Trials. Controlled Clin Trials 1986;7:177-88.
- Poolsup N, de Oliveira, Li Wan Po A. Estimating clinical trial data from graphical reports. J Clin Pharm Ther 2000.
- Alomar A, Gimemez-Camarasa JM, de Moragas JM. The use of levamisole in atopic dermatitis: a prospective study. Arch Dermatol 1978;114:1316-19.
- Boguniewicz M, Jaffe HS, Izu A, Sullivan MJ, York D, Geha RS, et al. Recombinant gamma interferon in treatment of patients with atopic dermatitis and elevated IgE levels. Am J Med 1990;88:365-70.
- Schneider LC, Hanifin J, Cooper K, Boguniewicz M, Milgrom H, Jaffe HS, et al. Recombinant interferon gamma therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol 1991;87.
- Zollman C, Vickers A. ABC of complementary medicine: what is complementary medicine?. BMJ 1999;319:693-6.
- Xu X-J, Banerjee P, Rustin MHA, Poulter LW. Modulation by Chinese herbal therapy of immune mechanisms in the skin of patients with atopic eczema. Br J Dermatol 1997;136:54-9.
- Armstrong NC, Ernst E. The treatment of eczema with Chinese herbs: a systematic review of randomised clinical trials. Br J Clin Pharm 1999;48:262-4.
- Sheehan MP, Atherton DJ. One year follow-up of children treated with Chinese medicinal herbs for atopic eczema. Br J Dermatol 1994;130:488-93.
- David TJ. Conventional allergy tests. Arch Dis Child 1991;66:281-2.
- Dent THS, Hawke S. Too soon to market. Br Med J 1997;313:1157-8.
- Emerson RM, Williams HC, Allen BR. What are the prescribing costs for atopic dermatitis in young children?. Br J Dermatol 1998;139:21-2.
- Williams HC. Hywel Williams’ top 10 deadly sins of clinical trial reporting. Ned Tijd Derm Venereol 1999;9:372-3.
- Sharpe GR, Farr PM. Evening primrose oil and eczema. Lancet 1990;335.
- Williams HC, Seed P. Inadequate size of ‘negative’ clinical trials in dermatology. Br J Dermatol 1993;128:317-26.
- Cox N, Williams HC. Can you COPE with CONSORT?. Br J Dermatol 2000;142:1-7.
- Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol 2000;136:763-9.
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales – a major source of bias in randomised controlled trials of treatments of schizophrenia. Br J Psychiatry 2000;176:249-52.
- Lundell ER, Koch E. Über ein im Doppelblind-Versuch geprüftes neues Corticosteroid. Zeitschrift Fur Allgemeinmedizin 1974;50:463-6.
- Ramelet AA, Mauracher E. Treatment of resistant steroid-responsive dermatoses: a comparison of Diprolene™ and Neriforte™. Clin Trials J 1982;19:298-307.
Appendix 1 Search strategies
The Cochrane Collaboration highly sensitive electronic search string for MEDLINE (OVID)
| #1 | RANDOMIZED CONTROLLED TRIAL.pt. |
| #2 | CONTROLLED CLINICAL TRIAL.pt. |
| #3 | RANDOMIZED CONTROLLED TRIALS.sh. |
| #4 | RANDOM ALLOCATION.sh. |
| #5 | DOUBLE BLIND METHOD.sh. |
| #6 | SINGLE BLIND METHOD.sh. |
| #7 | or/1-6 |
| #8 | (ANIMAL not HUMAN).sh. |
| #9 | 7 not 8 |
| #10 | CLINICAL TRIAL.pt. |
| #11 | exp CLINICAL TRTIALS/ |
| #12 | (clin$ adj25 trial$).ti,ab. |
| #13 | ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. |
| #14 | PLACEBOS.sh. |
| #15 | placebo$.ti,ab. |
| #16 | random$.ti,ab. |
| #17 | RESEARCH DESIGN.sh. |
| #18 | or/10-17 |
| #19 | 18 not 8 |
| #20 | 19 not 9 |
| #21 | COMPARATIVE STUDY.sh. |
| #22 | exp evaluation studies/ |
| #23 | follow up studies.sh. |
| #24 | prospective studies.sh. |
| #25 | (control$ or prospectiv$ or volunteer$).ti,ab. |
| #26 | or/21-25 |
| #27 | 26 not 8 |
| #28 | 26 not (9 or 20) |
| #29 | 9 or 20 or 28 |
| #30 | explode dermatitis, atopic/ |
| #31 | dermatitis, atopic.ti,ab,rw,sh. |
| #32 | eczema, atopic.ti,ab,rw,sh. |
| #33 | eczema.ti,ab,rw,sh. |
| #34 | atopic eczema.ti,ab,rw,sh. |
| #35 | atopic dermatitis.ti,ab,rw,sh. |
| #36 | infantile eczema.ti,ab,rw,sh. |
| #37 | childhood eczema.ti,ab,rw,sh. |
| #38 | neurodermatitis.ti,ab,rw,sh. |
| #39 | besniers prurigo.ti,ab,rw,sh. |
| #40 | or/30-39 |
| #42 | 29 and 40 |
Date of last search using this strategy for this report was end of June 2000
General skin search (EMBASE and MEDLINE)
| #1 | drug |
| #2 | dermatological agent/ |
| #3 | skin/ |
| #4 | dermatology/ |
| #5 | dermatolog$.mp. |
| #6 | skin disease$.mp. |
| #7 | or/2-6 |
| #8 | 1 and 7 |
Date of last search using this strategy for this report was end of November 1999
Search string for EMBASE (OVID) developed by the BMJ Publishing Group for its Clinical Evidence series
| #1 | exp clinical trial/or clinical trial.ti,ab,hw,tn,mf. |
| #2 | exp controlled study/ |
| #3 | (clinical trial$ or controlled clinical trial$).ti,ab,hw,tn,mf. |
| #4 | (random$ or placebo$).ti,ab,hw,tn,mf. |
| #5 | double blind.ti,ab,hw,tn,mf. |
| #6 | exp Randomized Controlled Trial/ |
| #7 | or/1-6 |
| #8 | limit 7 to human |
| #9 | explode dermatitis, atopic/ |
| #10 | dermatitis, atopic |
| #11 | eczema, atopic |
| #12 | eczema |
| #13 | atopic eczema |
| #14 | atopic dermatitis |
| #15 | infantile eczema |
| #16 | childhood eczema |
| #17 | neurodermatitis |
| #18 | besniers prurigo |
| #19 | 8 and 18 |
Date of last search using this strategy for this report was end of June 2000
Appendix 2 Excluded studies
| Author | Date | Interventions | Reason for exclusion |
|---|---|---|---|
| Topical steroid vs ‘placebo’ vehicle | |||
| Cullen | 1973 | Betamethasone benzoate gel 0.025% vs placebo gel | Atopic dermatitis not separated from other dermatoses in results |
| Rosenthal | 1980 | 1980 Clocortolone pivalate 0.1% cream vs placebo cream base | Atopic dermatitis not separated from other dermatoses in results |
| Gartner | 1984 | Diproderm cream 0.05% vs placebo vehicle | Atopic dermatitis not separated from other dermatoses in results |
| Guzzo | 1991 | Halobetasol propionate 0.05% ointment vs vehicle | Atopic dermatitis not separated from other dermatoses in results |
| Lebwohl | 1996 | Fluticasone propionate 0.005% ointment vs vehicle | Unclear if ‘eczema’ is atopic dermatitis in this study, especially as most of the subjects were adult – author has been contacted for clarification |
| Schachner | Schachner 1996 | Hydrocortisone 17-butyrate ointment vs vehicle | No randomisation mentioned |
| Heuck | 1997 | Topical bedesonide vs base | The atopic dermatitis patients (study one) were part of an open case series. The two remaining RCTs in this study were all on asthma patients |
| Topical steroid vs another topical steroid | |||
| Zimmerman | 1967 | Betamethasone 17-valerate 0.05% ointment vs fluocinolone acetonide 0.025% | First study was a case series, and it is unclear if randomisation occurred in second study |
| Grater | 1967 | Flumethasone vs 0.1% triamcinolone vs 1% hydrocortisone | Atopic dermatitis not separated from other dermatoses in results |
| Rosenberg | 1971` | 0.05% fluocinonide vs 0.1% betamethasone valerate | Atopic dermatitis not separated from other dermatoses in results |
| Bluefarb | 1972 | Desonide cream 0.05% vs betamethasone valerate cream 0.1% | Atopic dermatitis not separated from other dermatoses in results |
| Meenan | 1972 | Flucinonide 0.05% vs betamethasone 17 valerate cream 0.1% | Atopic dermatitis not separated from other dermatoses in results |
| Borelli | 1973 | Clocortolone (C168) vs fluocinolone | ‘Eczema’ group not specified sufficiently |
| McCuiston | 1973 | Fluocinonide 0.01% and 0.05% vs betamethasone valerate | Not clear if randomised, outcome measures not described at all |
| Polano | 1973 | Hydrocortisone butyrate 0.1% vs triamcinolone acetonide 0.1% vs hydrocortisone acetate 1% | Atopic dermatitis not separated from other dermatoses in results |
| Stewart | 1973 | Desonide vs triamcinolone acetonide vs betamethasone 17-valerate | Atopic dermatitis not separated from other dermatoses in results |
| Nordwell | 1974 | Betamethasone 17, 21-dipropionate 0.05% cream vs fluocortolone caproate 0.25% plus fluocortolone pivalate 0.25% cream | Atopic dermatitis not separated from other dermatoses in results |
| Sparkes | 1974 | Clobetasol propionate 0.05% vs betamethasone 17-valerate ointment and cream vs fluclorolone acetonide ointment and cream and fluocinonide | Atopic dermatitis not separated from other dermatoses in results |
| Laurberg | 1975 | 1% hydrocortisone in a stabilized 10% urea cream vs betamethasone 17-valerate 0.1% cream | Atopic dermatitis results mixed up with patients with ‘atopic winter feet’ |
| Lundell | 1975 | Desoximetasone 0.25% vs fluocinolone acetonide 0.025% cream | Nature of ‘endogenous eczema’ unclear. Inadequate description to classify as atopic dermatitis |
| Ludvigsen | 1975 | Calmuril-hydrocortisone 1% cream vs triamcinolone acetonide 0.1% cream | Unclear if randomised. No study results given! |
| Meyer-Rohn | 1975 | Desoximetason 0.25% vs betamethasone-valerate 0.1% | Atopic dermatitis not separated from other dermatoses in results |
| Sudilovsky | 1975 | Halcinonide cream 0.1% vs fluocinonide 0.05% cream | Disease definition, i.e.‘eczematous dermatoses which would normally be treated with topical steroids’ not acceptable as a term synonymous with atopic eczema |
| Parish | 1976 | Betamethasone benzoate 0.025% gel vs betamethasone valerate 0.1% cream | Cannot be sure that study subjects with ‘eczematous dermatoses’ had atopic eczema |
| Thormann | 1976 | Hydrocortisone 17-butyrate vs betamethazone 17-valerate | Results of five different skin disorders mixed up and only one patient with atopic eczema |
| Roessel | 1977 | Triamcinolone acetonide benzoyl-β-amino-isobutyrate vs betamethasone dipropionate | Atopic dermatitis not separated from other dermatoses in results |
| Khan | 1978 | 1% hydrocortisone plus 10% urea vs 0.05% fluocinonide | Dry eczematous dermatoses in adults mixed up with atopic dermatitis |
| Lassus | 1979 | Clobetasone butyrate 0.05% cream vs hydrocortisone butyrate 0.1% cream | Atopic dermatitis not separated from other dermatoses in results |
| Helander | 1982 | Hydrocortisone 17-butyrate 0.1% cream vs betamethasone 17-valerate 0.1% cream | Atopic dermatitis not separated from other dermatoses in results |
| Hersle | 1982 | Diflorasone diacetate 0.05% vs betamethasone valerate | Atopic dermatitis not separated from other dermatoses in results |
| Turnbull | 1982 | Locoid vs Betnovate lotion | Study of seborrhoeic and atopic dermatitis of the scalp with results not separated |
| Gip | 1983 | 1983 Hydrocortisone 17-butyrate 0.1% cream vs betamethasone 17-valerate 0.1% cream | Atopic dermatitis not separated from other dermatoses in results |
| Schmidt | 1984 | D-homosteroids domoprednate 0.1% ointment vs 0.1% betamethasone valerate ointment | Atopic dermatitis not separated from other dermatoses in results |
| Gip | 1987 | Hydrocortisone 17-butyrate 0.1% cream vs betamethasone 17-valerate 0.1% cream | Atopic dermatitis not separated from other dermatoses in results |
| Schmidt | 1987 | Domoprednate 0.1% ointment vs hydrocortisone butyrate ointment | Atopic dermatitis not separated from other dermatoses in results |
| Handa | 1988 | Alcometasone dipropionate 0.05% ointment vs 1% hydrocortisone ointment | Atopic dermatitis not separated from other dermatoses in results |
| Panja | 1988 | Alclometasone dipropionate 0.05% cream vs 1% hydrocortisone cream | Atopic dermatitis not separated from other dermatoses in results |
| Celleno | 1990 | Alclometasone dipropionate 0.1% vs 0.1% hydrocortisone 17-butyrate | Atopic dermatitis not separated from other dermatoses in results |
| Viglioglia | 1990 | Mometasone furoate 0.1% cream once daily vs betamethasone valerate 0.1% cream twice daily | Atopic dermatitis not separated from other dermatoses in results |
| Brunner | 1991 | Halobetasol propionate 0.05% ointment vs 0.1% diflucortolone valerate ointment | Atopic dermatosis results mixed up with patients with lichen simplex |
| Datz | 1991 | Halobetasol propionate ointment 0.05% vs clobetasol 17-propionate ointment 0.05% | Atopic dermatosis results mixed up with patients with lichen simplex |
| Rajka | 1993 | Mometasone furoate 0.1% fatty cream vs betamethasone valerate 0.1% cream | Atopic dermatitis not separated from other dermatoses in results |
| Schäfer-Korting | 1993 | Prednicarbate 0.025% -0.25% vs hydrocortisone aceponate vs hydrocortisone buteprate 0.1% vs betamethasone 17-valerate 0.1% vs hydrocortisone 1% vs 2 drug-free vehicles | Conducted in healthy volunteers not atopic eczema subjects |
| Blum | 1994 | Betamethasone dipropionate 0.05% in propylene glycol vs clobetasol propionate 0.05% ointment | Atopic dermatitis not separated from other dermatoses in results |
| Delescluse | 1996 | Fluticasone propionate ointment 0.005% vs betamethasone 17, 21-dipropionate ointment 0.05% | Atopic dermatitis not separated from other dermatoses in results |
| Juhlin | 1996 | Fluticasone propionate 0.05% cream vs hydrocortisone 17-butyrate 0.1% cream | Atopic dermatitis results not separated from patients with other eczemas of a known cause |
| Meffert | 1999 | Topical methylprednisolone aceponate vs amcinonide, betamethasone valerate, hydrocortisone butyrate and vehicle | Whole range of ‘acute eczemas’ not separated in results |
| Topical steroid vs another topical | |||
| Bjornberg | 1967 | Crotamiton vs Crotamiton/hydrocortisone combo | Atopic eczema not specified/separated |
| Christiansen | 1977 | Bufexamac vs 0.1% triamcinolone acetonide, 1% hydrocortisone cream and placebo | Atopic dermatitis results not separated from other dermatoses |
| Topical steroid plus additional active agents | |||
| Bjornberg | 1966 | Topical flumethasone plus vioform vs hydrocortisone with 5, 7-Dichlor-8-hydroxy-2-methylquinolin 3% | Besnie's prurigo included, results not separated |
| Sasagawa | 1970 | Betamethasone valerate plus gentamicin sulphate vs betamethasone | Atopic dermatitis not separated from other dermatoses in results |
| Weitgasser | 1972 | Topical dexamethasone vs topical nandrolone plus chlorhexadine | Rag bag of dermatoses (atopic dermatitis not among them) and results not separated |
| Aertgeerts | 1973 | Topical dexamethasone vs topical nandrolone plus chlorhexdine | Various dermatoses lumped together |
| Carpenter | 1973 | Vioform-hydrocortisone cream vs components alone and base cream vehicle | Atopic dermatitis not separated from other dermatoses in results |
| Aertgeerts | 1976 | Dexamethasone plus chlorhexidine vs flumethasone – pivalate 0.02% plus iodochlorohydroxy-quinolone | Atopic dermatitis not separated from other dermatoses in results |
| Cunliffe | 1976 | Fluclorolone acetonite 0.025% in FAPG vs betamethasone 17-valerate plus 0.5% neomycin | Atopic dermatitis not separated from other dermatoses in results |
| Strategos | 1986 | Fusidic acid/betamethasone combination vs gentamicin – betamethasone combination | Only five patients with atopic eczema all present in only one treatment arm |
| Weitgasser | 1993 | Halometasone/triclosan cream vs betamethasone dipropionate/getamicin sulphate cream | Atopic dermatitis not separated from other dermatoses in results |
| Poyner | 1996 | Fusidic acid/hydrocortisone cream vs miconazole/hydrocortisone cream | Unclear if patients with ‘clinically infected eczema’ had atopic eczema. Author contacted for clarification |
| Comparison of different formulations of the same topical steroids | |||
| Pilgaard | 1980 | Hydroderm™ vs hydrokortison DAK™ | Atopic dermatitis not separated from other dermatoses in results |
| Once-daily vs more frequent application of topical steroids | |||
| Tharp | 1996 | Fluticasone propionate 0.05% once vs twice daily | Eczema unspecified |
| Fredricksson | 1980 | Halcinonide cream 0.1% once daily vs same cream three times daily | Psoriasis and atopic dermatitis results mixed up |
| Schmid | 1981 | Topical fluocinoloneacetonid 0.025% once daily, twice daily or interval therapy | Not clearly atopic dermatitis patients |
| English | 1989 | Betamethasone dipropionate once vs twice daily | Atopic dermatitis not separated from other dermatoses in results |
| Topical steroids in the prevention of relapse | |||
| Vickers | 1976 | Maintenance on low-potency topical steroids switching to high-potency for short periods vs use of high-potency steroid throughout treatment vs high-potency steroid regularly once daily using a low-potency steroid for the second application | Not an RCT, though a clear intention to conduct one. Subsequent RCT never published |
| Moller | 1983 | Clobetasol proprionate vs flupredniden acetate | Atopic dermatitis not separated from other dermatoses in results |
| Author | Date | Interventions | Reason for exclusion |
|---|---|---|---|
| Smith | 1961 | Trimeprazine vs methdilazine | Atopic eczema data not separated in results |
| Brown | 1971 | Psychiatric treatment | Only one case of atopic eczema |
| Chan-Yeung | 1971 | DSCG | Asthma study |
| Anonymous | 1973 | Carbamide in hyperkeratosis | Atopic eczema results not separated |
| D'Souza | 1973 | House dust mites | People had asthma or hay fever |
| Baraf | 1976 | Antihistamines: cyproheptadine vs hydroxyzine | Atopic eczema results not separated from other dermatoses |
| Baertschi | 1976 | Antibiotic prophylaxis | ‘Eczema’ only mentioned as adverse effect |
| Friedman | 1978 | Monoamione oxidase inhibitors | Unclear if any of the neurodermatits patients had atopic eczema |
| Buch-Rasmussen | 1979 | Hydrocortisone alcoholic solutions | Study of external otitis |
| Newbold | 1980 | Emollients | Atopic eczema results not given separately |
| Anonymous | 1981 | 5% butyl flufenamate vs bufexamac | Atopic dermatitis not separated from other dermatoses in results |
| Bazex | 1982 | Terfenadine vs clemastine | Atopic eczema results not separated from other dermatoses in results |
| Cooper | 1983 | Thymopoietin pentapeptide | No clinical outcomes measured or reported |
| Archer | 1984 | Adrenoreceptor agonists | Not a therapeutic trial |
| Fairris | 1984 | Superficial X-Ray therapy (of the feet) | Unclear if patients had atopic eczema |
| Fairris | 1985 | Superficial X-Ray therapy (of the hands) | Unclear if patients had atopic eczema |
| Meyrick-Thomas | 1985 | Ranitidine | Healthy atopic volunteers |
| Svensson | 1985 | Diagnostic tool based on clinical criteria | Diagnostic study ‘subjects randomly collected’ |
| Bernstein | 1986 | Doxepin hydrochloride | Abstract only |
| Niimura | 1988 | Oral acyclovir | Study of eczema herpeticum |
| Roberts | 1988 | PAF antagonist vs placebo | Not atopic eczema patients |
| Warren | 1988 | The importance of bradykinin and histamine in the skin response to antigen | Not atopic eczema patients, not a therapeutic trial |
| Burr | 1989 | Risk factors for atopic eczema | Not an RCT of an intervention for atopic eczema, instead, an observational study of risk factors for atopic eczema within another breastfeeding RCT |
| Ebden | 1989 | Evening primrose oil | Asthma not atopic eczema |
| Monroe | 1989 | Nalmefene opiate antagonist vs placebo | Atopic eczema results not presented separately |
| Sheehan-Dare | 1989 | PUVA vs superficial radiotherapy | Not clear atopic dermatitis |
| Brandrup | 1990 | Occlusive dressing | ‘Eczema’ only mentioned as adverse effect |
| Markey | 1990 | PAF | Atopic subjects without evidence of atopic eczema |
| Michel | 1990 | Cetirizine | Pollen sensitive patients unspecified |
| Heyer | 1991 | Substance P and topical mustard oil | Not a therapeutic trial |
| Peter | 1991 | Ketaconazole | Study of seborrhoeic dermatitis |
| Schafer | 1991 (a) | Evening primrose oil | No clinical outcomes |
| Schafer | 1991 (b) | Phospholipid fatty acid composition and LTB4 release of neutrophils | No clinical outcomes |
| Kerscher | 1992 | Topical steroids | Healthy volunteers |
| Korting | 1992 | Prednicarbate cream | Healthy volunteers |
| Nierop | 1992 | Auranofin | Study of asthma only |
| Olsen | 1992 | Systemic steroids with 2% minoxidil | Study of alopecia areata with eczema mentioned as adverse effect |
| Couser | 1993 | Surfactant | Unspecified eczema as outcome measure |
| Lutsky | 1993 | Loratadine syrup vs terfenadine suspension | Atopic eczema results not given separately |
| Rombo | 1993 | Malaria prophylaxis | ‘Eczema’ mentioned as adverse effect |
| Zepelin | 1993 | Omega-3 fatty acid | Psoriasis patients |
| Lee | 1994 | Surfactant mixturesHealthy volunteers | |
| Lovegrove | 1994 | Milk-free diet vs normal diet | No separate data on atopic eczema |
| Nakagawa | 1994 | Tacrolimus ointment 0.03, 0.1 and 0.3% | Randomisation not described, three actives compared in hand and neck area, unblinded |
| Soyland | 1994 | n-3 omega fatty acid supplementation | Atopic eczema severity outcome data not given |
| Syed | 1994 | Podophyllotoxin cream | Study of molluscum |
| Tegner | 1994 | Skin blanching by hydrogen peroxide | Adverse effect study of skin blanching of hydrogen peroxide |
| Zimmermann | 1994 | Balneophototherapy with daily 15% synthetic Dead Sea Salt bath and selective ultraviolet phototherapy vs balneophototherapy with daily 3% NaCl salt bath and selective ultraviolet phototherapy | Atopic dermatitis not separated from other dermatoses in results |
| Roquet | 1995 | Loratidine | Atopic subjects not necessarily having eczema |
| Simon | 1995 | Ioxaglate vs Iopamidol | Not a study of atopic eczema outcomes. A study to see if allergic reactions are commoner in one type of contrast medium in patients with atopic disease |
| Simon | 1995 | Gamma-interferon | No clinical outcomes |
| Snyman | 1995 | Betahistine | Simply ‘atopic volunteers’ not necessarily atopic eczema |
| Verwimp | 1995 | Whey-protein hydrolysate based formulas | Unclear if atopic eczema patients |
| Wahlgren | 1995 | Interleukin-2 | Laboratory experiment with no clinical outcomes, not a therapeutic trial |
| Anonymous | 1997 | Cetirizine vs placebo | No atopic eczema outcomes |
| Kalpakliogu | 1997 | Heparin | Asthma study |
| Heyer | 1997 | Opiate and H1 antagonist effects | Healthy volunteers |
| Kekki | 1997 | Skin-prick and patch-test reactivity | Diagnostics |
| Lippert | 1997 | Antigen-induced cytokine release | Not a clinical trial of a therapeutic agent. Only cytokines measured |
| Pigatto | 1997 | Colloidal grain suspensions | Not a therapeutic trial |
| Rukwied | 1997 | Cetirizine vs placebo | Experimentally-induced flare responses |
| Sabroe | 1997 | Doxepin vs terfenadine | No atopic eczema outcomes |
| Frossard | 1998 | Cetirizine | Healthy volunteers |
| Hill | 1998 | Betamethasone plus clioquinol cream vs betamethasone plus fusidic acid cream | Hand eczema |
| Lippert | 1998 | Certirizine | Laboratory experiment with no clinical outcomes |
| Sorensen | 1998 | Intravenous immunoglobulin | Study of multiple sclerosis with eczema mentioned as side effect |
| Syed | 1998 | Imiquimod 1% | Study of molluscum |
| Warnecke | 1998 | Ichthyol oil | Healthy volunteers |
| Weisshaar | 1998 | Topical capsaicin vs placebo | Effect of capsaicin on experimentally induced whealing from histamine icthyosis |
| Darsow | 1999 | Aeroallergen sensitization | Diagnostics |
| Goh | 1999 | Mometasone furoate cream vs clobetasol propionate cream | Unspecified chromic limb eczema |
| Grundmann-Kollmann | 1999 | PUVA bath vs PUVA cream | Atopic eczema results not separated |
| Ortonne | 1999 | SDZ ASM 981 vs topical steroids and vehicle | Healthy volunteers |
| Rudofsky | 1999 | Intravenous prostaglandin | Study of venous ulcers with eczema mentioned as adverse effect |
| Author | Date | Interventions | Reason for exclusion |
|---|---|---|---|
| Topical steroids | |||
| Leo Pharmaceuticals unpublished data on file | – | Fucicort® vs Betnovate | Unspecified hand eczema |
| Stahle | 1965 | Fluocinolone vs tumenol prednisolone | Description of ‘patches of eczema’ unclear |
| Stahle | 1965 | Full vs half strength betamethasone 17-valerate | Description of ‘patches of eczema’ unclear |
| Munro | 1967 | Betamethasone 17-valerate vs fluocortolone caproate ointment | ‘Eczema’ unspecified |
| Anonymous | 1969 | Flurandrenolone with clioquinol in 2 different strengths | Unclear if ‘eczema’ included atopic eczema |
| Lloyd | 1969 | Fluocinolone acetonide 0.025% vs flucinolone containing neomycin | Nature of inflammatory dermatitis unclear |
| Portnoy | 1969 | 1% hydrocortisone vs 0.2% fluocortolone | ‘Eczema’ unspecified |
| Ashurst | 1970 | Beclomethasone dipropionate vs betamethasone 17-valerate | ‘Conditions responsive to topical applications of steroids’ – unclear if this included atopic eczema |
| Ashurst | 1972 | Hydrocortisone 17-butyrate vs fluocinolone acetonide vs hydrocortisone butyrate with chlorquinaldol | Inadequate description of ‘eczema’ |
| Hall-Smith | 1972 | Betamethasone valerate vs betamethasone benzoate | Description of ‘steroid-responsive dermatoses’ insufficient |
| Harman | 1972 | Fluclorolone acetonide vs betamethasone 17-valerate | Various types of ‘dermatitis’ unclear |
| Neering | 1972 | Betamethasone 17-valerate vs triamcinolone acetanide under occlusive dressing | ‘Eczema’ unspecified |
| Sarkany | 1972 | Fluocinonide vs betamethasone valerate | Type of ‘eczema’ unclear |
| Alexander | 1973 | Hydrocortisone 17-butyrate vs betamethasone valerate 0.1% | Nature of ‘eczema’ unclear |
| Craps | 1973 | Clocortolone pivalate vs controls in 17 double-blind trials | Non-specific ‘eczema’ |
| Cullen | 1973 | Betamethasone benzoate vs placebo gel | ‘Eczematous dermatoses’ not separated |
| Marks | 1973 | Betamethasone 17-valerate 0.1% vs formocortal 0.025% | ‘Eczema of the hands’ unclear |
| Wilson | 1973 | Betamethasone 17-valerate ointment lanolin-free vs original formulation vs fluclorolone acetonide ointment | Type of eczema unclear |
| Garretts | 1975 | Fluprednylidene acetate cream vs base | Inflammatory skin disease unspecified |
| Ronn | 1976 | Betamethasone vs fluocinonide | ‘Eczema’ unspecified |
| Munro | 1977 | Betamethasone valerate ointment vs fluocinonide FAPG | ‘Eczema’ unspecified |
| Palmerio | 1977 | Halopredone acetate vs betamethasone valerate | Nature of ‘eczema’ unclear |
| Dotti | 1978 | Dexamethasone 17-valerate vs 0.1% betamethasone vs 1% hydrocortisone acetate | Nature of ‘eczematous lesions’ unclear |
| Afzelius | 1979 | Betamethasone diapropionate 0.05% vs fluocinolone acetonide 0.025% | Unclear if atopic eczema included |
| Doherty | 1979 | Diflucortolone valerate 0.3% oily cream vs clobetasone propionate 0.05% cream | ‘Chronic severe eczema’ too non-specific |
| Rosenberg | 1979 | Amcinonide vs betamethasone valerate | ‘Eczematous dermatitis’ unclear |
| Vollum | 1979 | Betamethasone valerate vs halcinonide | Nature of eczema lesions unclear |
| Allenby | 1981 | Clobetasone butyrate 0.05% vs hydrocortisone butyrate 0.1% | Unclear if atopic eczema |
| Anonymous | 1981 | Hydrocortisone 17-butyrate vs betamethasone 17-valerate creams | Unspecified uninfected eczema |
| Guenther | 1981 | Amcinonide cream 0.1% vs halcinonide cream 0.1% | Nature of ‘eczematous dermatitis’ unclear |
| Bickers | 1984 | Amcinonide vs halcinonide | Nature of ‘subacute eczematous dermatitis’ unclear |
| Johansson | 1984 | Diflorasone diacetate vs betamethasone valerate | Nature of ‘eczematous dermatitis’ unclear |
| August | 1985 | Diflucortolone vs betamethasone cream | Unspecified symmetrical eczema |
| Jegasothy | 1985 | Clobetasol propionate vs fluocinonide cream | Nature of ‘chronic eczema’ unclear |
| Jaffé | 1986 | Hydrocortisone plus potassium hydroxyquinoline vs 1% hydrocortisone plus 2% miconazole cream | Nature of ‘infected eczema’ unclear |
| Barry | 1987 | Desonide 0.05% and 0.1% cream | ‘Non-infected hand eczemas’ unclear |
| Williamson | 1987 | Hydrocortisone/urea cream vs betamethasone valerate cream | Nature of ‘dry eczema’ unclear |
| Lutsky | 1993 | Loratadine syrup vs Terfenadine suspension | Atopic dermatitis not separated from other dermatoses in results |
| Gip | 1994 | Betamethasone 17-valerate 0.1% lipocream vs betamethasone 17-valerate 0.1% cream | Nature of ‘dry chronic dermatitis’ unclear |
| Kejda | 1994 | 1% hydrocortisone cream vs Locoid 0.1% | Nature of ‘chronic eczema’ unclear |
| Nakagawa | 1994 | Tacrolimus ointment 0.03, 0.1 and 0.3% | Randomisation not described, unblinded open study |
| Tharp | 1996 | Fluticasone once daily vs twice daily | Unspecified eczema |
| Jorizzo | 1997 | Clobetasol propionate 0.05% vs emollient vehicle | ‘Eczema’ unspecified |
| Radiation | |||
| King | 1984 | Superficial radiotherapy vs simulated therapy | Nature of ‘palmar’ eczema unclear |
| Cartwright | 1987 | Grenz vs placebo | Nature of ‘bilateral hand ezcema’ unclear if atopic eczema |
| Cromoglycate | |||
| Dannaeus | 1977 | SCG vs placebo | Unspecified eczema |
| Pacor | 1992 | DSCG vs oxatomide | Nature of eczema unspecified |
| Antihistamines | |||
| Hellier | 1963 | Trimeprazine vs amylobarbitone | Unspecified eczema |
| Laugier | 1978 | Mequitazine vs placebo | Unspecified ‘dermatological conditions’ |
| Miscellaneous | |||
| de Gregorio | 1970 | Topical bendazac vs placebo vs 3% hydrocortisone acetate | Nature of ‘eczematous eruptions’ unclear |
| Fredrikksson | 1975 | Urea creams | Nature of eczematous dermatitis of hands unclear |
| Zimmermann | 1981 | Intravenous demetindenmaleat vs clemastine | Nature of ‘allergic dermatoses’ unclear |
| Fairris | 1984 | Superficial X-ray therapy | Nature of unspecified constitutional eczema of the hands unclear |
| Veien | 1985 | Oral challenge with balsam of Peru vs placebo | Various types of ‘dermatitis’ unclear |
| Lauharanta | 1991 | Emuslion cleansing vs washing with soap | Nature of ‘hand eczema’ unclear |
| Drake | 1995 | 5% doxepin cream vs vehicle cream | Description of study subjects suggests that ‘eczematous dermatitis’ did not include atopic dermatitis |
Appendix 3 Studies of steroid therapy
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Brock & Cullen, 1967126 | 0.5% triamcinolone acetonide o.d. in flexible collodion vs ‘flexible collodion’ placebo o.d. | 40 patients in total two atopic eczema patients | Prospective, randomised, double-blind parallel study | Lesion improvement | Out of two atopic eczema patients one found active site better and one found neither site better | Method and concealment of randomisation unclear, study described as double-blind, no withdrawals or drop-outs | Scant details |
| USA | Patient preference study gives little indication of magnitude of effect | ||||||
| Gehring & Glooer, 199671 | Water-in-oil emulsion b.d. for 2 weeks vs water-in-oil emulsion plus1% hydrocortisone for 1 week followed by the emulsion only in the second week | 69 patients with atopic dermatitis | Prospective, randomised, double-blind parallel-group study for 2 weeks | Doctor-assessed erythema, patient-assessed roughness and itching | Both groups improved substantially for all parameters | 69 patients enrolled, 12 did not meet all study critieria yet 63 were used in final assessment | The study demonstrates the large vehicle/placebo response in atopic eczema trial |
| Germany | Other biological measures | Trend toward greater improvement in hydrocortisone groups but not statistically significant | |||||
| Translated | |||||||
| Vanderploeg, 1976105 | 0.05% betamethasone dipropionate ointment b.d. vs vehicle placebo | 36 patients with moderate-to-severe atopic dermatitis | Prospective, randomised, double-blind study of 3 weeks' duration | Amount of scale, erythema, pruritus, thicknes of lesions and crusting on a 0–4 scale (0 = none, 4 = very severe) | Improvement over baseline for mean total symptom score was 11.4 for dipropionate and 11.2 for placebo decreasing to 1.6 for dipropionate and 8.4 for placebo at week 3; p < 0.0001 | Method and concealment of randomisation unclear, study described as double blind ‘code’, three drop-outs, no ITT | Large treatment effect |
| Global evaluation <25% = worse to 100% = excellent | |||||||
| Roth & Brown, 197887 | Hydrocortisone valerate cream 0.2% vs placebo t.d.s. | 20 atopic eczema patients | Prospective, randomised, left/right, parallel study of 4 weeks' duration | Pruritus, erythema, scaling, excoriation, lichenification | No actual data given for hydrocortisone valerate vs placebo. 75% of the patients showed excellent improvement or were better with the hydrocortisone valerate cream compared with 20% with the placebo | Method and concealment of randomisation unclear, study described as double-blind | Difficult to estimate magnitude of benefit |
| USA | Overall condition and severity of disease | Overall ratings at the end of the therapy showed hydrocortisone valerate to be significantly more effective than the placebo (p < 0.001) | Withdrawals and drop-outs not mentioned | ||||
| Sudilovsky et al., 198195 | 0.1% halcinonide cream o.d. plus cream base placebo b.d. vs cream base placebo t.d.s. | 58 atopic eczema patients | Prospective, randomised, right/left, parallel study of 3 weeks' duration | Comparative and absolute therapeutic responses: erythema, oedema, changes in size of thickness of lesions | Of 54 evaluable patients at week 3, 13 (24%) were markedly improved for halcinonide comparative clinical response vs 1 (2%) for placebo patients (p < 0.001) | Method and concealment of randomisation unclear, study described as double-blind, four drop-outs | Patients with a previous history of poor response to topical corticosteroids were excluded |
| Physician global response | Withdrawals, no ITT | ||||||
| Lupton et al., 198294 | Halcinonide ointment 0.1% vs t.d.s. ointment base placebo t.d.s. | 233 patients with mild, moderate and severe atopic dermatitis | Prospective, randomized, double-blind paired comparison study of 2 weeks' duration | Therapeutic response of lesions on each side evaluated as excellent, good, fair, poor | In Halcinonide group 64% excellent, 21% good, 10% fair and 5% poor response | Method and concealment of randomisation unclear, study described as double-blind | Big treatment effect |
| Lesion resolution evaluated for lesion size, erythema, oedema, transudation and lichenification | In placebo group 23% excellent, 21% good, 36% fair and 20% poor response | 19 lost to follow-up, no ITT | Only 4 weeks' duration | ||||
| Therapeutic response four-point scale (4 = excellent, 1 = poor) | |||||||
| Sefton et al., 1984114 | Hydrocortisone valerate 0.2% ointment b.d. vs vehicle placebo | 64 patients with mild-to-moderate atopic dermatitis | Prospective, randomised, double-blind left/right parallel study of 2 weeks' duration | Pruritus, erythema, scaling, papulation, lichenification and vesiculation | Mean global evaluation severity scores on 0–100 VAS: hydrocortisone valerate baseline score of 34.6 decreasing to 10.3 and placebo baseline score of 34.1 decreasing to 28.9 after 14 days treatment (p < 0.01) | Method and concealment of randomisation unclear, study described as double-blind (identical coded tubes), three drop-outs, no ITT | Six trials described in this paper (three RCTs) only one of which had not been published elsewhere |
| Global evaluation using an VAS 0–100, 100 most severe | |||||||
| Wahlgren et al., 1988100 | Betamethasone dipropionate 0.05% cream b.d. vs base cream placebo b.d. | 30 adult patients with persistent atopic dermatitis and chronic pruritis | Prospective, randomised, double-blind crossover study of 4 days' duration | Intensity of pruritus using Pain-Track and distribution and activity of eczema determined and excoriations counted | ‘No pruritus’ on Days 3–4 was 35.8% during betamethasone and 21.5% during placebo therapy (p = 0.0062) | Method and concealment of randomisation unclear, study described as double-blind, four drop-outs, no ITT | Very short duration (4 days) using a novel approach to measure itch |
| Stalder et al., 199491 | Desonide o.d. vs excipient o.d. | 40 children with atopic dermatitis | Prospective, randomised, double-blind parallel study of 7 days' duration | Global physician score based on extent and severity of lesion | 66.7% desonide group showed improvement or resolution compared with 15.8% in the placebo group (p < 0.001) | Method and concealment of randomisation unclear, study described as double-blind | Paper suggests that use of topical steroids alone has a big impact on bacterial colonisation |
| Local lesion score based on target area | S. aureus density decreased by log 2.2 compared with log 0.6 in the placebo group (p < 0.05) | No mention of withdrawals and drop-outs | |||||
| Various bacteriological assessments | |||||||
| Lebwohl et al., 199676 | Fluticasone propionate ointment 0.005% vs placebo vehicle | 203 patients with atopic eczema | Prospective, randomised, parallel study of 29 days' duration | Patient's self assessment of treatment efficacy | Patient's self assessment at Day 29, 81% (n = 74) found fluticasone excellent or good vs 37% (n = 28) found vehicle excellent or good | Method and concealment of randomisation unclear, study described as double-blind | Unclear why two identical large multicentre trials conducted and repeated concurrently |
| USA (Study 1) | Physician's gross assessment, severity scores of five signs and one symptom | Drug-related adverse effects were rare | Large number of withdrawals and dropouts (n = 101); no ITT analysis | ||||
| Lebwohl et al., 1996116 | Fluticasone propionate ointment 0.005% vs placebo vehicle | 169 patients with atopic eczema | Prospective, randomised, parallel study of 29 days' duration | Patient's self assessment of treatment efficacy | Patient's self assessment at day 29, 84% (n = 63) found fluticasone excellent or good vs 48% (n = 26) found vehicle excellent or good | Method and concealment of randomisation unclear, study described as double-blind | Unclear why two identical large multicentre trials conducted and repeated concurrently |
| USA (Study 2) | Physician's gross assessment, severity scores of five signs and one symptom | Drug-related adverse effects were rare | Large number of withdrawals and dropouts (n = 80); no ITT analysis | ||||
| Sears et al., 199781 | Hydrocortisone buteprate 0.1% cream vs cream base placebo o.d. | 194 patients with atopic dermatitis | Prospective, randomised, double-blind parallel study of 14 days' duration | Seven disease signs (infiltration, scaling, erythema, lichenification, vesicles, papules, and excoriation) were evaluated on a four-point scale (0 = absent, 3 = severe) | Seven sign lesion scores improvement over baseline of 9.13 for hydrocortisone reduced to 2.67 at Day 14, and placebo baseline of 9.95 reduced to 6.69 at Day 14 | Method and concealment of randomisation unclear, study described as double-blind | Large study with big treatment effects |
| Pruritus evaluated separately on same four-point scale | 26 drop-outs; no ITT | ||||||
| Overall improvement on a seven-point scale (1 = cleared, 7 = worse) and global treatment efficacy on a four-point scale (1 = good and 4 = poor) | |||||||
| Maloney et al., 1998132 | Clobetasol propionate 0.05% b.d. cream vs vehicle placebo b.d. | 81 patients with moderate-to-severe atopic dermatitis | Randomised, double-blind parallel study of 43 days' duration | Physician gross assessment based on % improvement of target lesion plus changes from baseline in mean severity scores for erythema, pruritus, induration/papulation, lichenification, erosion/oozing/crusting, and scaling/dryness and for total signs and symptoms | Gross assessment at Day 43 showed 78% of clobetasol-treated patients were good, excellent or cleared compared with 33% of placebo-treated patients | Method and concealment of randomisation unclear, study described as double-blind | Baseline scores not given |
| 20 drop-outs; no ITT |
| Study | Design | No. of subjects | Age (years) | Duration | Severity | Treatment | Comparator | Co-treatments | Withdrawals and drop-outs |
|---|---|---|---|---|---|---|---|---|---|
| Duke et al., 1983108 | Parallel, RCT | 68 | > 14 | 3 weeks | Not specified | Alclometasone dipropionate ointment 0.05% b.d | Clobetasone butyrate ointment 0.05% b.d. | Unspecified | Four |
| However, 65 included in efficacy study | |||||||||
| Fisher & Kelly, 197996 | Parallel, left/right, RCT | (240) 107 atopic eczema | 2–73 | 3 weeks | Not specified | Fluocininide 0.05% emollient cream t.d.s. | Betamethasone valerate 0.1% cream t.d.s. | Unspecified | 11 atopic eczema patients |
| Lassus, 1983115 | Parallel, RCT | 40 | 5–11 | 2 weeks | Stable or worsening > 1 week | Alclometasone dipropionate cream 0.05% b.d. | Hydrocortisone butyrate cream 0.1% b.d. | Unspecified | None |
| Lassus, 1984135 | Parallel, RCT | 43 | No data | 2 weeks | Stable or worsening > 1 week | Alclometasone dipropionate cream 0.05% b.d. | Clobetasone butyrate cream 0.05% b.d. | Unspecified | None |
| Roth & Brown, 197897 | Parallel, L/R, RCT | 19 | No data | 4 weeks | Not specified | Hydrocortisone valerate cream 0.2% t.d.s. | Betamethasone valerate cream 0.1% t.d.s. | Antihistamines (for allergic rhinitis); insulin, antibiotics and tranquilisers | Unspecified |
| Hydrocortisone valerate cream 0.2% t.d.s. | Hydrocortisone cream 1.0% t.d.s. | ||||||||
| Sefton & Kyriakopoulos, 1983136 | Parallel, L/R, RCT | 145 | No data | 2 weeks | Mild to moderate | Hydrocortisone valerate ointment 0.2% | Betamethasone valerate 0.1% ointment | Unspecified | 14: loss to follow-up or protocol violations |
| Fluocinolone acetonide 0.025% ointment | |||||||||
| Triamcinolone acetonide 0.1% ointment | |||||||||
| VanDelRey et al., 1983139 | Not translated (Spanish) | ||||||||
| Veien et al., 1984113 | Parallel, L/R, RCT | 40 | < 10 | 4 weeks | Not specified | Hydrocortisone 17-butyrate (Locoid) cream 0.1% | Hydrocortisone (Uniderm™) 1% cream | None specified | None |
| Yasuda, 197699 | Parallel, L/R, RCT | 144 | No data | 7 days | Not specified | Hydrocortisone 17-butyrate 0.1% Locoid ointment | Triamcinolone acetonide 0.1% ointment | ‘No local or systemic medications were permitted that could conceivably affect the dermatoses’ | 39 (seven drop-outs, 32 ‘not yet evaluated’) |
| Hydrocortisone acetate 1% ointment |
| Study | Outcome measure | Scale |
|---|---|---|
| Duke et al., 1983108 | Clinical signs and disease sign score: erythema, induration, pruritus | 0–3 scale (0 = absent, 3 = severe) |
| Physician's global assessment: cleared, marked improvement, moderate improvement, slight improvement, no change, exacerbation | Clear = 100%, <50% = slight improvement | |
| Fisher & Kelly, 197996 | Clinical response relative to status of lesion (moderate improvement, slight improvement, no significant change, worse) | Five-point scale (5 = clear, 1 = worse) |
| Adverse effects recorded | ||
| Patient and physician preference regarding efficacy of one agent over the other excluding cosmetic preferences | ||
| Lassus, 1983115 | Erythema, induration, pruritus | 0–3 scale (0 = absent, 3 = severe) |
| Physician global evaluation of improvement: cleared, marked improvement, moderate improvement, slight improvement, no change, exacerbation | Clear = 100%, <50% = slight improvement | |
| Lassus, 1984135 | Erythema, induration, pruritus | 0–3 scale (0 = absent, 3 = severe) |
| Investigator globally evaluated improvement in overall disease condition | ||
| Patients examined for adverse reactions | ||
| Roth & Brown, 197897 | Overall condition evaluation | Eight-point scale: severely worse (1) to cleared (8) |
| Severity scored | Four-point scale: 1 = clear, 2 = slight, 3 = moderate, 4 = severe | |
| Five symptoms: pruritus, erythema, scaling, excoriation, lichenification | Ten-point scale (0–9) 0 = clear | |
| Sefton & Kyriakopoulos, 1983136 | Investigator-assessed pruritus, erythema, scaling, papulation, lichenification, vesiculation (the distance of the mark made by an investigator from the end of the scale labelled none or clear indicated the degree of severity of the particular sign or symptom) | VAS digitalised into 0–100, 100 most severe |
| VanDelRey et al., 1983139 | ||
| Veien et al., 1984113 | Global severity of all lesions | Five-point scale (0 = none, 1 = slight, 2 = moderate, 3 = severe, 4 = very severe) |
| Therapeutic results assessed using three different degrees of improvement | Moderate, good, excellent associated with score reductions of at least 1, 2 and 3 points on the rating scale, respectively | |
| Yasuda, 197699 | Investigator-assessed decrease in erythema, scaling, oedema, subjective symptoms, such as pruritus and burning sensation | |
| Rapidity of onset of any therapeutic response, maximum degree of improvement of lesions and maintenance of response during the remainder of the treatment period were also rated as criteria for the overall evaluations of the treatment |
| Study | Erythema | Purulence | Excoriation | Dryness | Xerosis | Scaling | Lichenification | Cracking | Fissuring | Exudation | Vesiculation | Pustules/papules | Oozing/weeping | Oedema | Inflammation | Crusts | Infiltration | Induration | Itch | Sleep loss | Physician global severity assessment | Patient global severity assessment | Area assessment (method used) | Scale named (if modified specify) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duke et al., 1983108 | • | • | • | • | ||||||||||||||||||||
| Fisher & Kelly, 197996 | ||||||||||||||||||||||||
| Lassus, 1983115 | • | • | • | • | ||||||||||||||||||||
| Lassus, 1984135 | • | • | • | • | ||||||||||||||||||||
| Roth & Brown, 197897 | • | • | • | • | • | • | ||||||||||||||||||
| Sefton & Kyriakopoulos, 1983136 | • | • | • | • | • | • | • | |||||||||||||||||
| VanDelRey et al., 1983139 | ||||||||||||||||||||||||
| Veien et al., 1984113 | ||||||||||||||||||||||||
| Yasuda, 197699 | • | • | • | • |
| Study | Main reported results | Authors' conclusions | Quality |
|---|---|---|---|
| Duke et al., 1983108 | 75% improvement in alclometasone dipropionate vs 68% improvement in clobetasone butyrate for mean clinical scores erythema, induration, pruritus (p > 0.10) | Clinical sign scores improved continuously during the study period and, at the end of therapy, favoured alclometasone conclusively over clobetasone | Method and concealment of randomisation unclear, ‘blind evaluator technique’ suggests single-blind study |
| No ITT | |||
| Fisher & Kelly, 197996 | Mean clinical response 4.5 for fluocinonide and 4.38 for betamethasone on a scale of 1–5, where 5 = excellent or clear | Clinical responses favoured fluocinonide for the atopic dermatitis group (p = 0.021) | “Randomisation correlated with sequential numbers”; study described as double-blind |
| No ITT | |||
| Lassus, 1983115 | Eight out of 20 patients in alclometasone dipropionate group and seven out of 20 patients in hydrocortisone butyrate group showed 76–100% (marked or cleared) improvement in signs and symptoms (erythema, induration, pruritus) | Both creams were effective treatments for atopic dermatitis, however, alclometasone dipropionate was judged slightly more efficacious | Method and concealment of randomisation unclear, study described as double-blind |
| No drop-outs or withdrawals | |||
| Lassus, 1984135 | 85% improvement for alclometasone dipropionate group and 86% improvement for clobetasone butyrate group of signs and symptoms (erythema, induration and pruritus) | Both treatments were effective | Method and concealment of randomisation unclear, study described as double-blind |
| No drop-outs or withdrawals | |||
| Roth & Brown, 197897 | 14 out of 19 patients showed clear or excellent improvement for both hydrocortisone valerate 0.2% cream and betamethasone valerate 0.1% cream (t.d.s.) | Hydrocortisone valerate 0.2% cream was found to be as effective as betamethasone valerate cream 0.1% cream | Method and concealment of randomisation unclear, study described as double-blind |
| Withdrawals and drop-outs not mentioned | |||
| Sefton & Kyriakopoulos, 1983136 | Hydrocortisone valerate 0.2% scored 12.6 compared with 10.7 for betamethasone valerate 0.1% VAS (mean) | Hydrocortisone valerate 0.2% ointment is a safe and effective perparation for use in mild-tomoderate atopic dermatitis | “The allocation treatments in each study was accomplished by a restricted randomisation process to ensure equal frequencies of the treatments to each side in small sequences of consecutively numbered patients”. “The test preparations were supplied to investigators in coded identical tubes” |
| Hydrocortisone valerate 0.2% scored 15.6 compared with 14.5 for triamcinolone acetonide 0.1% VAS (mean) | No ITT carried out | ||
| Hydrocortisone valerate 0.2% scored 4.7 compared with 4.6 for fluocinolone acetonide 0.1% VAS (mean) | |||
| VanDelRey et al., 1983139 | Needs translating | ||
| Veien et al., 1984113 | Complete clearance of skin symptoms was found in 60% Locoid 0.1% treated patients and in 30% of Uniderm 1% treated patients | Locoid cream is significantly superior to Uniderm cream in the treatment of atopic dermatitis in children | Treatment assignment followed ‘randomised double-blind code’ |
| No withdrawals or drop-outs | |||
| Yasuda, 197699 | Improvement over baseline as reported by investigator: 11 patients found hydrocortisone 17-butyrate superior to triamcinolone, 17 found drugs comparable | Hydrocortisone 17-butyrate 0.1% ointment proved more effective than hydrocortisone acetate 1% ointment and triamcinolone acetonide 0.1% ointment | Table of numbers assured randomization |
| 17 patients found hydrocortisone 17-butyrate superior to hydrocortisone acetate, nine found them comparable (for clinical effects) | Study described as double-blind | ||
| No ITT |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Binder & McCleary, 1972102 | Fluocinonide cream 0.05% q.d.s. vs betamethasone valerate cream 0.10% q.d.s. | Ten atopic eczema patients | Prospective, randomised, left right parallel study of 2 weeks' duration | Lesion improvement | Fluocinolone was superior to betamethasone in 70% of patients | Table of randomized numbers used. Study described as double-blind | Difficult to interpret magnitude of effect. |
| No withdrawals or drop-outs | |||||||
| Almeyda & Fry, 1973122 | 10% urea and 1% hydrocortisone vs cream 0.1% betamethasone 17-valerate cream | 50 atopic eczema patients | Prospective, randomised, left, right, parallel study of 3 weeks' duration | Lesion response: excellent, good, none, deterioration | Mean response of good or excellent outcome 76% urea hydrocortisone and 78% betamethasone 17-valerate | Method and concealment of randomisation unclear. Study described as double-blind. No withdrawals or drop-outs | Study which claimed equivalence of a very mild corticosteroid preparation against a potent one. Study grossly under-empowered to establish equivalence |
| Leibsohn & Bagatell, 197493 | Halcinonide cream 0.1% t.d.s. betamethasone 17-valerate cream 0.1% t.d.s. | Nine patients with atopic dermatitis | Prospective, randomised. Left right parallel study of 3 weeks' duration | Decrease of lesion size, reduction in erythema, oedema, transudation, lichenification and scaling, relief of pruritus and pain | An excellent or good response was recorded in 63% halcinonide patients and 38% betamethasone patients for overall evaluation of therapeutic response | “Randomised according to patient's study number” Study described as double-blind. One lost to follow-up, no ITT | Study of 88 patients with mixed dermatoses, some responding differently to the treatment |
| Almeyda & Burt, 197492 | Hydrocortisone 1% UHc powder-cream vs 0.1% betamethasone 17-valerate | 36 adults and children with mild, moderate and severe atopic eczema | Prospective, randomised, left, right, parallel study of 4 weeks' duration | Clinical condition assessed as excellent if completely cleared and good if partially cleared, no improvement and deterioration | 97% ‘excellent’ or ‘good’ improvement for hydrocortisone 1% and 94% ‘excellent’ or ‘good’ improvement for betamethasone | Method and concealment of randomisation unclear. Study described as double-blind. No withdrawals or drop-outs | Another study which assumes that no evidence of a statistical difference is the same as therapeutic equivalence |
| Lundell, 1974419 (German) | 0.1% fluprednylidenaceate in vs 0.25% fluocortolone | 42 patients with severe atopic dermatitis | Prospective, randomised, left/right, parallel study of 4 weeks' duration | Erythema, scaling, weeping, itching (composite score therapeutic index) | Good effect for both preparations; fluprednylidenacetate significantly better than fluocortolone after 2nd week; therapeutic index 0.96 vs 0.86 after 4 weeks | Method and concealment of randomisation unclear, study described as double-blind | Difficult to interpret treatment effect without placebo control |
| Three withdrawals/drop-outs, no ITT | |||||||
| Bjornberg & Hellgren, 1975121 | 0.25% Desoximetasone cream b.d. vs 0.1% betametasone valerate cream b.d. | 22 patients with atopic dermatitis and 24 patients with psoriasis | Prospective, randomised, doubleblind controlled side-toside comparison for 1-2 weeks | 0–5 scale assessment of skin morphology | For atopic dermatitis patients: Desoximetason treated side was rated superior 11 times; betamethasone treated side rated superior eight times | Randomisation unclear | Very short duration |
| Germany (translated) | Scoring of superior treatment (a>b, or b>a or a=b) | No difference three times | |||||
| Bleeker, 197570 | Halcinonide 0.1% cream b.d. vs clobetasol propionate 0.05% cream b.d. | 27 moderate-to-severe atopic eczema patients | Prospective, randomised, left/right, parallel study of 2 weeks' duration | Lesions assessed for decrease in erythema, oedema, transudation, lichenification, scaling, pruritus and pain | 92% ‘excellent’ or ‘good’ overall clinical response for both halcinonide and clobetasol | Table of random assignment | No placebo arm |
| Study described as double-blind | |||||||
| No drop-outs or withdrawals | |||||||
| Morley et al., 1976120 | 0.05% clobetasone butyrate cream or ointment b.d. vs 0.0125% flurandrenolone cream or ointment b.d. | 71 atopic eczema patients children only | Prospective, randomised, left/right, parallel study of 1 week duration | Clinician-assessed lesions as healed, improved, static or worse plus clinician/patient preference for right/left side | No data on clinician rated healing of lesions given | Method and concealment of randomisation unclear, implies double blinding (neither clinician nor patient aware of identification) | No data to indicate magnitude of treatment effect |
| Patient preference data only reported, which indicated a non-statistically significant preference in favour of clobetasone butyrate | No withdrawals or drop-outs | ||||||
| Savin, 1976150 | Betamethasone dipropionate ointment 0.05% vs hydrocortisone ointment 1% b.d. | 27 patients with atopic dermatitis | Prospective, randomised, parallel study of 3 weeks' duration | Clinical effectiveness: excellent(>75%), good (50–75%), fair (25–50%), poor (<25%) | 50% betamethasone ‘excellent’ or ‘good’ response compared with 22% hydrocortisone | Method and concealment of randomisation unclear, study described as double-blind | Clear categorical data and seperation of atopic eczema and psoriasis |
| 26 moderate, one very severe | Five drop-outs; no ITT | ||||||
| Yasuda, 197699 | Hydrocortisone 17-butyrate 0.1% locoid ointment vs triamcinolone acetonide 0.1% ointment or hydrocortisone acetate 1% ointment | 144 atopic dermatitis patients | Prospective, randomised, left/right, parallel study of 7 days' duration | Decrease in erythema, scaling, oedema, subjective symptoms such as pruritus and burning sensation and improvement of lesions | Hydrocortisone 17-B superior to triamcinolone 10%, comparable 16%, inferior 3% | Table of numbers assured randomisation | Well reported with useful data on placebo and psoriasis groups |
| Hydrocortisone 17-B superior to hydrocortisone acetate 16%, comparable 9% and inferior 3% | Study described as double-blind | ||||||
| Seven drop-outs; 32 not yet evaluated hence no ITT | |||||||
| Mali, 1976137 | Betamethasone dipropionate cream vs locacorten 0.02% b.d. | 16 atopic dermatitis patients from a total of 66 steroid-responsive dermatoses | Prospective, randomised, parallel study of 3 weeks' duration | Much better, slightly better, no change, slightly worse, much worse | 19% betamethasone group much better compared with 13% Locacorten group (p > 0.10) | Method and concealment of randomisation unclear, study described as double-blind | Useful to have data separated by diseases but only 16 atopic eczema patients |
| 16 withdrawals, unclear from which group | |||||||
| Bluefarb et al., 1976119 | Diflorasone diacetate 0.05% cream vs flucinonide 0.05% cream b.d. | 210 atopic/neurodermatitis patients | Prospective, randomised, parallel study of 3 weeks' duration | Degree of therapeutic response 1–25%, 26–50%, 51–75%, 76–100% clinical resolution, no change in severity or deterioration of lesions | Improvement over baseline >50% improvement: 71% for both diflorasone and fluocinonide | Method and concealment of randomisation unclear, study described as double-blind | Some reservation on whether atopic/neurodermatitis is the same as atopic eczema |
| Nine+ withdrawals, no ITT | |||||||
| Roth & Brown, 197897 | Hydrocortisone valerate cream 0.2% vs betamethasone valerate cream 0.1% t.d.s. | 19 atopic eczema patients | Prospective, randomised, left/right parallel study of 4 weeks' duration | Symptoms pruritus, erythema, scaling, excoriation, lichenification | 74% showed clear or excellent improvement for both hydrocortisone valerate and beta-methasone valerate | Method and concealment of randomisation unclear, study described as double-blind | Underpowered study |
| Study 1 | Overall condition and severity | Withdrawals and drop-outs not mentioned | |||||
| Roth & Brown, 197897 | Hydrocortisone valerate cream 0.2% vs hydrocortisone cream 1% t.d.s. | 29 atopic eczema patients | Prospective, randomised, left/right parallel study of 4 weeks' duration | Symptoms pruritus, erythema, scaling, excoriation, lichenification | No actual data given for this study | Method and concealment of randomisation unclear, study described as double-blind | Difficult to evaluate without any data |
| Study 2 | Overall condition and severity | Overall judgement of the response to the two medications (defined as cleared, excellent, good, no effect, or worse) showed hydrocortisone valerate to be statistically superior to hydrocortisone (p < 0.05) | Withdrawals and drop-outs not mentioned | ||||
| El-Hefnawi et al., 1978118 | Halcinonide-neomycin-amphotericin ointment 0.1% vs hydrocortisone 1% ointment | Five atopic dermatitis patients | Prospective, randomised, left/right parallel study of 3 weeks' duration | Subjective and objective evaluations of responses | 100% improved or cleared for both halcinonide and hydrocortisone (cleared: 80% and 60%, respectively) | Pre-designed randomization chart | Very few atopic eczemna patients mixed up with other inflammatory skin diseases |
| Global evaluation | Study described as double-blind | ||||||
| Withdrawals and drop-outs not mentioned | |||||||
| Fisher & Kelly, 197996 | Fluocinonide 0.05% emollient cream t.d.s. vs betamethasone valerate 0.1% cream t.d.s. | 107 atopic eczema patients | Prospective, randomised, left/right parallel study of 3 weeks' duration | Clinical response relative to status of lesion | Mean clinical response 4.5 for fluocinonide and 4.38 for betamethasone on a scale of 1–5, where 5 = excellent or clear | Randomisation correlated with sequential numbers | Suspect randomization method |
| Study described as double-blind | |||||||
| 11 withdrawals/drop-outs; no ITT | |||||||
| Ramelet, 1982420 | Betamethasone dipropionate 0.05% vs diflucortolone valerate 0.3% b.d. | 12 adults with resistant atopic dermatitis | Prospective, randomised, parallel study of 14 days' duration | Physician-assessed erythema, induration, scaling, crusting, pruritus, excoriation, and pain | For overall therapeutic efficacy 83% had cleared or marked improvement in both betamethasone and diflucortolone groups | Method and concealment of randomisation unclear, study described as double-blind | Very small number of patients over very short period of time |
| Physician global assessment of therapeutic response % improved | No drop-outs or withdrawals | ||||||
| Sefton & Kyriakopoulos, 1983136 | Hydrocortisone valerate ointment 0.2% vs betamethasone valerate 0.1% ointment t.d.s. | 68 mild-to-moderate atopic eczema patients | Prospective, randomised, left/right parallel study of weeks duration | Investigator-assessed pruritus, erythema, scaling, papulation, lichenification, vesiculation on an VAS of 1–100 (100 being most severe) | Improvement over baseline for hydrocortisone 44.1 reduced to 12.6 betamethasone 43.4 reduced to 10.7 | Allocation by a restricted randomisation process in coded identical tubes | Magnitude of efficacy of 0.2% hydrocortisone valerate similar to that of a potent preparation |
| Study 1 | 14 initially lost to follow-up, plus three from this part of study | ||||||
| No ITT carried out | |||||||
| Sefton & Kyriakopoulos, 1983136 | Hydrocortisone valerate ointment 0.2% vs triamcinolone acetonide 0.1% ointment t.d.s. | 37 mild-to-moderate atopic eczema patients | Prospective, randomised, left/right parallel study of weeks duration | Investigator-assessed pruritus, erythema, scaling, papulation, lichenification, vesiculation on VAS of 1–100 (100 being most severe) | Improvement over baseline for hydrocortisone 46.4 reduced to 15.6 triamcinolone 47.9 reduced to 14.5 | Allocation by a restricted randomisation process in coded identical tubes | Three studies described in same paper |
| Study 2 | 14 initially lost to follow-up, plus one from this part of study | ||||||
| No ITT carried out | |||||||
| Sefton & Kyriakopoulos, 1983136 | Hydrocortisone valerate ointment 0.2% vs flucinolone 0.025% ointment t.d.s. | 26 mild-to-moderate atopic eczema patients | Prospective, randomised, left/right parallel study of weeks duration | Investigator-assessed pruritus, erythema, scaling, papulation, lichenification, vesiculation on VAS of 1–100 (100 being most severe) | Improvement over baseline for hydrocortisone 27.1 reduced to 4.7, fluocinolone 26.9 reduced to 4.6 | Allocation by a restricted randomisation process in coded identical tubes | Three studies described in same paper |
| Study 3 | 14 initially lost to follow-up, plus one from this part of study | ||||||
| No ITT carried out | |||||||
| Lassus, 1983115 | Alclometasone dipropionate cream 0.05% b.d. vs hydrocortisone butyrate cream 0.1% b.d. | 40 children with atopic eczema | Prospective, randomised, parallel study of 2 weeks' duration | Erythema, induration, pruritus | 76–100% improvement or ‘marked-cleared’ was seen in 40% of alclometasone patients and 35% of hydrocortisone patients | Method and concealment of randomisation unclear, study described as double-blind | Useful to have outcome data presented as categories |
| Bagatell et al., 198379 | Alclometasone dipropionate cream 0.05% vs hydrocortisone cream 1.0% t.d.s. | 249 atopic eczema patients | Prospective, randomized parallel study of 3 weeks' duration | Erythema, induration, pruritus | 71% alclometasone patients showed cleared or marked improvement compared to 69% for hydrocortisone patients | Method and concealment of randomisation unclear, study described as double-blind | Although written up as a study supporting superiority of the newer alclometasone, there is not much difference when % who are markedly improved or clear is evaluated |
| 20 withdrawals/dropouts; no ITT carried out | |||||||
| Van DelRey et al., 1983139 | Alclometasone cream 0.05% vs hydrocortisone butyrate | 30 patients over 12 years old, more than 1 year disease duration and resistant to treatment | Parallel double-blind prospective randomised trial lasting 3 weeks | Doctor-assessed erythema, hardening of the skin and scaling | Both treatments gave similar results of efficacy | Described as double-blind and randomised but method not clear | Difficult to establish equivalence in such a small study |
| Spain (translated) | After treatment improvement evaluated on a scale 1–6, where 1 = 100% improvement | Total sign score fell from 7.20 to 1.00 in the alclometasone group and from 7.14 to 0.93 in the hydrocortisone group | One patient excluded from hydrocortisone group as he had seborrheic dermatitis | ||||
| Harder & Rufli, 1983149 | Diflorasonediacetate 0.05% ointment o.d. vs betamethasone 17 valerate 0.1% ointment t.d.s. | 98 patients with eczema’ (probably atopic eczema but this is not specified in the paper) | Prospective, randomised, single-blind parallel-group study for 3 weeks | Improvement as assessed by: erythema, oedema, lichenification, induration, scaling, excoriation, itching, exulceration; each assessed with four-point scale | (Only summary data reported) | 26 drop-outs (detailed description given), no ITT analysis | One of the first studies to evaluate o.d. application vs more frequent application of a standard treatment |
| Switzerland (Basel) (translated) | Both groups achieved good results | ||||||
| No significant difference between groups | |||||||
| Konzelmann & Harms, 1983147 | Diflorasone diacetate 0.05% cream o.d. vs betamethasone dipropionate 0.1% cream | 120 patients with acute or subacute eczema | Prospective, randomised, open parallel-group study for 3 weeks | Improvement assessed by doctor on five-point scale 0–100% improvement | 85% of all patients showed grade 4 improvement (75–100%), no significant difference between groups | 18 drop-outs, which were not assessed | Similar to above study |
| Switzerland (Zurich) (translated) | |||||||
| Duke et al., 1983108 | Alclometasone dipropionate ointment 0.05% b.d. vs clobetasone butyrate ointment 0.05% b.d. | 68 atopic eczema patients | Prospective, randomized parallel study of 3 weeks' duration | Clinical score erythema, induration, pruritus, and physician global assessment | 75% improvement in alclometasone group compared with 68% improvement in clobetasone group for mean clinical score | Method and concealment of randomisation unclear, blind evaluator technique suggests single-blind study | A small equivalence study |
| Lassus, 1984135 | Alclometasone dipropionate cream 0.05% b.d. vs clobetasone butyrate cream 0.05% b.d. | 43 atopic eczema patients | Prospective, randomized parallel study of 2 weeks' duration | Erythema, induration, pruritus and physician global evaluation of improvement | 85% improvement for alclometasone group compared with 86% improvement for clobetasone group for three signs | Method and concealment of randomisation unclear, study described as double-blind | Little difference in treatment effect |
| No withdrawals or drop-outs | |||||||
| Veien et al., 1984113 | Hydrocortisone 17-butyrate (Locoid) cream 0.1% vs hydrocortisone (Uniderm) 1% cream | 40 atopic eczema patients | Prospective, randomised, left/right parallel study of 4 weeks' duration | Global severity of all lesions | Complete clearance of skin symptoms was found in 60% hydrocortisone 17-butyrate-treated patients compared with 30% hydrocortisone 1%-treated patients | Method and concealment of randomisation unclear, study described as double-blind | Treatment benefit of hydrocortisone butyrate increased as study progressed |
| No withdrawals or drop-outs | |||||||
| Nolting, 1985138 | Betamethasone dipropionate 0.05% vs desoximetasone 0.25% ointment | 33 atopic eczema patients with resistant or severe disease in a trial, which also included psoriasis patients | Prospective, randomised, parallel RCT of 2 weeks' duration | Physician global rating | 41% and 53% had clearance in the betamethasone vs desoximetasone groups, respectively (p > 0.05) | Method and concealment of randomisation unclear, study described as double-blind | Numbers of atopic eczema patients too small to make any specific comments |
| Germany (translated) | No ITT | ||||||
| Rajka & Verjans, 1986110 | Hydrocortisone 17-butyrate (Locoid) 0.1% fatty cream vs desonide (Apolar) 0.1% ointment b.d. | 30 moderate-to-severe atopic dermatitis patients | Prospective, randomised, left/right, parallel study of 4 weeks' duration | Investigator-assessed global severity and severity grades of erythema, induration and scaling | Mean global severity score over baseline of 2.8 reduced to 1.3 for hydrocortisone and 1.7 for desonide (p < 0.05) | Method and concealment of randomisation unclear, study described as double-blind | Scaling scores not given on nine out of 30 patients because they did not experience scaling throughout the trial |
| No drop-outs | |||||||
| Majerus & Reiffers-Mettelock, 198685 | Halometasone 0.05% cream or ointment vs betamethasone valerate 0.1% cream or ointment b.d. | 75 atopic dermatitis patients | Prospective, randomised, parallel study of 3 weeks' duration | Inflammation, crusting, scaling, lichenification, excoriation, induration, exudate, pruritus, pain (healing, improvement, failure) | Healing was reported in 70% of patients with halometasone cream, 60% with halometasone ointment compared with 90% on betamethasone cream, and 80% on betamethasone ointment | Method and concealment of randomisation unclear, study described as double-blind | RCT mixed inflammatory dermatoses |
| 33 drop-outs/withdrawals; no ITT | |||||||
| Ulrich & Andresen, 199141 | 0.05% Halomethasone cream b.d. vs 0.25% Prednicarbate cream b.d. (both topical steroids) | 165 patients with active episode of atopic dermatitis suitable for exclusively topical treatment | Prospective, randomised, double-blind parallel group study for 2 weeks | 1. clinical effectiveness (doctor-assessed, five-point scale) | 1. clinical effectiveness: no significant difference between groups | Randomisation criteria unclear | One of authors was an employee of the company that produces Halomethasone cream |
| Germany (translated) | 2. onset of clinical effectiveness (doctor-assessed) | 2. onset: no difference at Day 1 or 4 between groups | Authors tried to create subgroup of severely affected patients, probably retrospectively | ||||
| 3. adverse effects | 3. adverse effects: none reported | They then claim significant advantage for Halomethasone in severely affected patients | |||||
| 4. cosmetic acceptability (patient-assessed five-point scale) | 4 cosmetic acceptability: 51% vs 46% rated it ‘excellent’ (NS) | ||||||
| Haneke, 199275 | 0.1% methylprednisolone aceponate ointment o.d vs 0.1% betamethasone valerate b.d. | 94 adults with atopic dermatitis | Prospective, randomised, left/right, parallel study of 4 weeks' duration | Patient and doctor global assessments | No actual data for o.d. methylprednisolone vs b.d. betamethasone given | Method and concealment of randomisation unclear | Results of all three studies impossible to disentangle |
| Germany | Doctor assessed 11 signs and symptoms | Study described as double-blind, no ITT | |||||
| Study 1 | |||||||
| Haneke, 199275 | 0.1% methylprednisolone aceponate ointment b.d. vs 0.1% betamethasone valerate b.d. | 94 adults with atopic dermatitis | Prospective, randomised, left/right, parallel study of 4 weeks' duration | Patient and doctor global assessments | No actual data for b.d. methylprednisolone vs b.d. betamethasone given | Method and concealment of randomisation unclear | Results of all three studies impossible to disentangle |
| Germany | Doctor assessed 11 signs and symptoms | Study described as double-blind, no ITT | |||||
| Study 2 | |||||||
| Rampini, 1992123 | Methylprenisolone aceponate 0.1% cream b.d. vs prednicarbate 0.25% cream b.d. | 80 children with atopic dermatitis | Prospective, randomised, parallel study of 3 weeks' duration | Objective and subjective symptoms of erythema, exudation, scaling, hyperkeratosis, itching, burning | 97.3% methylprenisolone patients achieved complete healing or distinct improvement compared with 100% prednicarbate patients | Method and concealment of randomisation unclear, study described as double-blind | Three studies of three different comparisons in different age groups |
| Study 1 | Global therapeutic response | Two drop-outs/withdrawals; no ITT | |||||
| Rampini, 1992123 | Methylprenisolone aceponate 0.1% o.d. ointment vs prednicarbate 0.25% cream b.d. | 120 children with atopic dermatitis | Prospective, randomised, parallel study of 3 weeks' duration | Objective and subjective symptoms of erythema, exudation, scaling, hyperkeratosis itching, burning | 96.3% methylprenisolone patients achieved complete healing or distinct improvement compared with 98.1% prednicarbate patients | Method and concealment of randomisation unclear, study described as double-blind | Three studies of three different comparisons in different age groups |
| Study 2 | Global therapeutic response | 12 drop-outs/withdrawals; no ITT | |||||
| Hoybye et al., 1991124 | Momethasone furoate o.d. vs hydrocortisone 17-butyrate b.d. | 96 atopic dermatitis patients | Prospective, randomised, parallel study of 6 weeks' duration | No information given | 85% momethasone patients significantly greater improvement vs 71% hydrocortisone group (p = 0.0025) | Method and concealment of randomisation unclear, study described as investigator blind | Published in abstract form only |
| Drop-outs/withdrawals no data given | |||||||
| Gelmetti et al., 199480 | 0.025% budesonide cream vs 0.1% alclometasone dipropionate b.d. | 40 children with atopic dermatitis | Prospective, randomised, parallel study of 2 weeks' duration | % of patients who were good or excellent | 83% good or excellent for budesonide vs 94% good or excellent for alclometasone | Method and concealment of randomisation unclear; blinding unclear; no ITT | No final analysis |
| Italy (translated) | Composite scale of signs and symptoms and tolerability | (No formal statistical comparison done) | Very similar effects, small numbers over very short term | ||||
| Jorizzo et al., 199588 | Desonide 0.05% ointment vs hydrocortisone 1% ointment b.d. | 113 children with atopic dermatitis | Prospective, randomised, parallel study of 5 weeks' duration | Physician global improvement, erythema, lichenification excoriations, oozing and crusting, induration and papules | 68% desonide patients and 40% hydrocortisone had clearing or marked improvement at 5 weeks | Method and concealment of randomisation unclear, study described as investigator blind | Study followed-up by a longer 6-month follow-up study, which did not show any signs of skin thinning in either group |
| Pruritus assessed subjectively | Two drop-outs/withdrawals; no ITT | ||||||
| Camacho et al., 199668 | 0.25% prenicarbate cream vs 0.2% flucortolone monhydrate cream b.d. | 49 out-patients with atopic dermatitis aged 19–65 years | Prospective, randomised, double-blind parallel right/left comparison of 3 weeks' duration | Itch, erythema, eczema, vesicles/papules lichenification, on a scale of 0–3 | Physicians rated the prednicarbate side better in 12 patients, the flucortolone side better in seven patients and no difference in 16 patients (p = 0.30) at the end of 3 weeks | Randomisation method and concealment not described | Sponsored study of very short duration |
| Spain (translated) | Also physician and patient global evaluation of whether one side better than the other | 80% of patients recorded good to excellent improvement on the prednicarbate side compared with 63% for the flucortolone side (p = 0.10) | Stated to be double-blind | Drop-outs were not included in analysis, which is worrying given the high drop-out rate (29%) and the fact that at least two dropped out because they worsened | |||
| No statistical difference between signs and symptoms were noted | No ITT analysis (14/49 drop-outs) | ||||||
| Stinging similar in both groups | |||||||
| Lebwohl et al., 1999116 | 0.1% mometasone furoate cream o.d. vs 0.2% hydrocortisone valerate cream b.d. | 219 children with moderate-to-severe atopic dermatitis | Prospective, randomised, parallel study of 21 days' duration | Investigator assessed seven signs and symptoms on a 0–3 scale (0 = none, 3 = severe) and global assessment % improved | Mean improvement in severity score (no baselines given) at day 21 (% of patients with 100% clearance), 87.4% for mometasone and 79.7% for hydrocortisone valerate at Day 21 | Method and concealment of randomisation not clear, study described as evaluator-blind | Unclear if the o.d. vs b.d. cream was blinded (probably not) |
| USA | End-points given but unclear what they are |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Hiratsuka et al., 199684 | Beclomethasone dipropionate t.d.s. vs topical SCG t.d.s. | 43 children with moderate-to-severe atopic dermatitis | Prospective, randomised, parallel study of 2 weeks' duration | Severity of inflammation, lichenification and cracking over 15 body areas | Itch and sleep disturbance estimated from graph | Method and concealment of randomisation unclear, study described as double-blind | Study mainly concerned with cellular and immunological changes |
| Japan | Patient diary cards for itch and sleep loss | SCG baseline score 2.3 and 2.4 reduced to 0.7 and 0.5 for itch and sleep disturbance, respectively, at 2 weeks and beclomethasone baseline 2.2 and 2.3 reduced to 0.9 and 0.6 for itch and sleep loss, respectively, at 2 weeks | No information of withdrawals or drop-outs | ||||
| Laboratory tests | |||||||
| Korting et al., 199586 | Hamamelis distallate 5.35 g plus 0.64 mg ketone/100 g vs vehicle or 0.5% hydrocortisone | 72 patients with moderate-to-severe atopic dermatitis | Prospective, randomised, left/right, parallel study of 2 weeks' duration | Physician and patient global assessments 0–5 scale, where 0 = healed and 5 = worse | There was no clinical or statistical difference between hamamelis and vehicle for reduction of itching at 2 weeks | Method and concealment of randomisation unclear, study described as double-blind | Useful study with a placebo arm, which provided no evidence to support efficacy of hamamelis |
| Germany | Itch, erythema, scaling, oedema, papules, pustules, exudation, lichenification, excoriations, fissuring | Mean itch score changed from 2.1 to 0.8 for hydrocortisone and from 2.1 to 1.2 for hamamelis (p < 0.01) | Seven withdrawals/drop-outs; no ITT | ||||
| Patient recorded efficacy was also significantly improved in hydrocortisone group when compared with hamamelis | |||||||
| There were no differences between hamamelis and vehicle | |||||||
| Munkvad, 198983 | Clinitar coal tar vs 1% hydrocortisone | 30 patients with mild-to-moderate atopic eczema | Prospective, randomised, left/right, parallel study of 4 weeks' duration | Infiltration, erythema, lichenification, excoriations, dryness, doctor and patient global assessments | All five parameters reduced significantly over the 4-week period but no significant differences between the two treatments | Method and concealment of randomisation unclear | Difficult to blind due to smell of coal tar |
| Denmark | No mention of blinding | Difficult to evaluate significance of change in scores due to small sample size and lack of data | |||||
| No withdrawals/drop-outs | No placebo arm | ||||||
| Wolf-Jürgensen, 197974 | 5% bufexamac b.d. vs 0.1% hydrocortisone or placebo b.d. | 10 atopic eczema patients within a study of 72 patients with various forms of dermatoses | Prospective, randomised, parallel study of 2 weeks' duration | Patient and investigator global assessment | Change in global score was very similar for the three patients allocated to placebo, betamethasone and bufexamac | Method and concealment of randomisation unclear, study described as double-blind | Impossible to interpret differences in such a small subsample of atopic eczema patients |
| Norway | Severity of inflammation, induration, lichenification, crusts, scaling, pruritus | One withdrawal/drop-out; no ITT |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Addition of antimicrobials | |||||||
| Wachs & Maibach, 197690 | Betamethasone valerate cream vs gentamicin/betamethasone valerate vs gentamicin cream t.d.s. | 83 infected moderate-to-severe atopic dermatitis patients | Prospective, randomised parallel study of 22 days | Global assessment and overall severity, degree of inflammation, degree of infection, erythema, pruritus, pustules, crusting, exudation, vesiculation, lichenification | Improvement over baseline on a scale of 0–10 | Method and concealment of randomisation unclear, study described as double-blind | Treatment responses were very slightly larger for steroid/antibiotic combination but none statistically significant |
| USA | Betamethasone/gemtamicin group baseline score of 6.1 reduced to 1.0, betamethasone group 6.1 reduced to 1.8 and gentamicin group 6.6 baseline reduced to 4.2 | Four drop-outs; no ITT | Bacterial growth similar in all three groups | ||||
| Hjorth et al., 1985111 | Betamethasone 17-valerate 0.1% vs betamethasone 17-valerate plus 2% fusidic acid | 60 atopic dermatitis patients with potentially infected atopic eczema | Prospective, randomised, left/right, parallel study of 7 days' duration | Bacteriological swabs | Data for mean atopic dermatitis not given, only result is investigator preference: 29 no preference, 22 preferred betamethasone plus fusidic acid and nine preferred betamethasone alone | Method and concealment of randomisation unclear, study described as double-blind | Author contacted for more data but has sadly deceased |
| Clinical symptoms: vesicles, oedema, erythema, excoriation, crusting, lichenification, itching | No drop-outs or withdrawals | Study provides no evidence of improved efficacy of betamethasone/fusidic acid combination above betamethasone alone in infected atopic eczema | |||||
| Wilkinson & Leigh 1985117 | 0.1% betamethasone plus 2% fusidic acid cream vs 0.1% betamethasone plus 0.5% neomycin cream b.d. or t.d.s. | 43 infected or potentially infected atopic eczema patients | Prospective, randomised, parallel study of 2 weeks' duration | Severity of lesions assessed by patient and doctor as either very severe, severe, moderate, mild, minimal, or absent | 95% patients (91% doctors) felt lesions improved after beta-methasone plus fusidic acid after 2 weeks vs 100% patients (100% doctors) felt beta-methasone plus neomycin | Method and concealment of randomisation unclear, study described as double-blind | Difficult to interpret in the absence of a betamethasone only arm |
| UK | Swabs taken for infection | No separate data for bacteriological efficacy for atopic dermatitis | Nine withdrawals and drop-outs but unclear which type of eczema these patients had (seven types of dermatoses reported) | ||||
| Meenan, 1988107 | Hydrocortisone 17-butyrate 0.1% plus 3% chlorquinaldol vs 0.1% triamcinolone acetonide plus 0.25% neomycin plus 0.025% gramicidin nystatin | 40 children with eczema for 3 months to 14 years with secondary infection | Prospective, randomized parallel study of 14 days' duration | Pruritus, erythema, lichenification, oozing/crusting, scaling | Both treatments produced a highly significant (p < 0.001) linear reduction in the scores for all parameters, no significant difference between treatments | Method and concealment of randomisation unclear, study described as double-blind | Both agents contain an antimicrobial/antiseptic, and no steroid-only comparator |
| Skin swabs for infection | Highly significant reduction in infection for both treatments (p < 0.001) | No data given on withdrawals or drop-outs | |||||
| Zienicke, 1993146 | Prednicarbate 0.25% cream vs prednicarbate 0.25% cream plus didecyldimethyl-ammoniumchloride 0.25% | 180 superinfected atopic eczema patients | Prospective, randomised, parallel study of 34 days' duration | Redness, swelling, papulovesicles, vesicles, pustules, bullae, papules, status madidans, crusting and scaling on a score of 1–5 | Clinical score over baseline of 25 for both drugs reduced to 13.5 for prednicarbate and 13 for prednicarbate plus didecyl dimethylammoniumchloride | Method and concealment of randomisation unclear, study described as double-blind | Duplicate publication of Korting 1994 |
| 30% patietns still had S. aureus at day 34 compared with 100% at start | 44 withdrawals/drop-outs; no ITT carried out | No clinical or statistical difference between groups | |||||
| Ramsay et al., 1996128 | Fusidic acid and 1% hydrocortisone vs 1% hydrocortisone | 186 mild-to-moderately severe atopic dermatitis | Prospective, randomised, parallel study of 2 weeks' duration | Primary: percentage patients not failing treatment (included signs, withdrawal and various bacteriological criteria) | 63.7% fusidic acid plus hydrocortisone did not fail treatment compared with 50.6% in the hydrocortisone group (p = 0.11) | Method and concealment of randomisation unclear, study described as double-blind | No evidence to support a clear benefit of combination over plain hydrocortisone |
| Study 1 | Secondary: erythema, scaling, oedema, itching, serous discharge, crusting, extent of lesions and overall clinical response | Mean change in clinical scores not statistically significant (p = 0.21) | 32 drop-outs/withdrawals; no ITT | ||||
| Ramsay et al., 1996128 | Fusidic acid and 1% hydrocortisone vs 2% fusidic acid | 68 mild to moderately severe atopic dermatitis | Prospective, randomised, parallel study of 2 weeks' duration | Erythema, scaling, oedema, itching, serous discharge, crusting, extent of lesions and overall clinical response | 36.4% fusidic acid plus hydrocortisone failed treatment and 65.6% fusidic acid failed treatment (p = 0.04) | Method and concealment of randomisation unclear, study described as double-blind | Some evidence of benefit of fucidin/hydrocortisone over fucidin alone |
| Study 2 | Swabs taken | Three drop-outs/withdrawals; no ITT | |||||
| Thaci, 199977 | Fusidic acid 2% plus 0.1% betamethasone cream vs fusidic acid 2% plus 0.1% betamethasone ointment vs ointment vehicle b.d. | 59 patients with potentially infected atopic dermatitis | Prospective, randomised, parallel study of 10 days' duration | Bacteriological tests, signs and symptoms on a four-point scale, investigator-assessed overall clinical response | Overall clinical response assessed by invrestigator as ‘clearance’ or ‘marked improvement’ in 92% fusidic acid/betamethasone cream patients, in 84% fusidic acid/betamethasone ointment patients, and 25% ointment vehicle patients | Method and concealment of randomisation unclear, study described as double-blind | Abstract only |
| Germany | No statistically significant difference between the two formulations | No withdrawals or drop-outs mentioned | Only results reported in text given | ||||
| Addition of antifungal | |||||||
| Anon, 1967127 | Triamcinolone acetonide 0.1% and neomycin sulphate 0.35% vs triamcinolone acetonide 0.1% and neomycin sulphate 0.35% plus undecylenic acid 2.5% | Ten infantile eczema patients within a study of 100 patients with various skin disorders | Prospective, randomised, parallel study | No change, some improvement, marked improvement, cured | Cured or marked improvement 17% for triamcinolone acetonide and neomycin sulphate compared with 100% for triamcinolone acetonide 0.1% and neomycin sulphate plus undecylenic acid | Method and concealment of randomisation unclear, study described as double-blind | Difficult to make any conclusion in such a small subset |
| Drop-outs/withdrawals: no data given no ITT | |||||||
| Length of study not given | |||||||
| Topical steroids plus something else vs topical steroids alone | |||||||
| Kaplan et al., 197889 | Hydrocortisone 0.5% plus 30% caffeine vs hydrocortisone 0.5% vs betamethasone valerate 0.1% | 90 atopic dermatitis patients | Prospective, randomised, parallel study of 3 weeks' duration | Pruritus, erythema, scaling, lichenification, oozing, excoriation, overall global impression | Mean improvement over baseline global impression on a scale 0–5: | Method and concealment of randomisation unclear, study described as double-blind | Some evidence to suggest the addition of caffeine might have a small additional benefit |
| 2.6 to 1.6 for hydrocortisone, 2.1 to 0.8 for caffeine and hydrocortisone, 2.7 to 0.6 for betamethasone | Seven drop-outs/withdrawals; no ITT | ||||||
| Chapman, 1979125 | 0.1% hydrocortisone butyrate ointment vs 1% hydrocortisone alcohol with 10% urea b.d. | 40 atopic eczema patients split into two studies | Prospective, randomised, left/right parallel study of 3 weeks' duration | Erythema, scaling, oedema | Mean clinical improvement dry skin 73% hydrocortisone alcohol vs 80% hydrocortisone 17-butyrate (p > 0.05) wet skin 67% hydrocortisone alcohol vs 68% hydrocortisone 17-butyrate (p > 0.05) | Method and concealment of randomisation unclear, study described as double-blind | No evidence to support efficacy of the combination treatment |
| One group applied creams to dry skin, the other after wetting the skin first | Drop-outs/withdrawals; no data given | ||||||
| Norén & Melin, 1989106 | Hydrocortisone vs betamethasone valerate plus hydrocortisone vs hydrocortisone plus habit reversal vs betamethasone valerate plus hydrocortisone plus habit reversal | 45 moderate-to-severe atopic dermatitis patients | Prospective, randomised, parallel study of 5 weeks' duration | Primary: reduction in scratching, secondary: dryness, scaling, erythema, infiltration, frequency of scratching | At end of 5-week evaluation period total skin status scores (not defined in paper) fell in all four groups but more so in groups that included habit reversal (data only presented in graphical form) | Method and concealment of randomisation unclear, no blinding | Useful RCT which evaluates combinations of different treatment approaches which suggests an additional benefit of habit reversal |
| Similar changes for other skin signs presented in graphical form | Two drop-outs/withdrawals; no ITT | ||||||
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Andersen et al., 1988144 | Mildison® 1% hydrocortisone vs Uniderm 1% hydrocortisone | 96 children with atopic dermatitis | Prospective, randomised, left/right parallel study of 4 weeks' duration | Global severity of symptoms, global improvement of skin lesions, investigator and patient preference | Mean reduction in severity score over baseline of 1.7 for Mildison and Uniderm reduced to 0.7 and 0.8, respectively | Method and concealment of randomisation unclear, study described as double-blind | Little efficacy difference between treatments, yet patients preferred the Mildison preparation |
| Denmark | No withdrawals or drop-outs | ||||||
| Korting et al., 1090103 | 0.039% liposomal betamethasone dipropionate vs 0.054% commercial propylene glycol gel | 12 patients with atopic dermatitis | Prospective, randomised, left/right, parallel study of 2 weeks' duration | Investigator assessed ten signs and symptoms of eczema and proportion of patients with major improvement or healed and global effect on a 0–5 scale. where 0 = healed and 5 = worse | Although data not reported in text, estimates from the figure showed that 80% evaluable patients noted healed or major improvement in liposome group compared with 60% patients in reference group at Day 14 | Method and concealment of randomisation unclear, study described as double-blind | Small study where ten parameters measured and data only given for some to support enhanced benefit for test substance |
| Germany | Two withdrawals/dropouts; no ITT | ||||||
| Malzfeldt et al., 1989130 | Betamethasone 17-valerate 0.0056% in liquid paraffin vs betamethasone 17-benzoate 0.00056% in neutral oil | 16 patients with atopic eczema | Prospective, randomised, left/right parallel study of 5–7 days' duration | Investigator assessed five signs on a 0–3 scale (max. score 15) | In low solution capacity group mean global score fell from 11.9 at baseline to 3.8 at Day 7 compared with 11.9 to 8.2 at baseline and Day 7, respectively, for high solution capacity (p ≤ 0.01) | Method and concealment of randomisation unclear, study described as double-blind | Study suggests that vehicle can markedly affect efficacy |
| Germany | Withdrawals or drop-outs not mentioned | ||||||
| Olholm et al., 1988143 | Mildison 1% hydrocortisone vs Uniderm 1% hydrocortisone | 60 atopic dermatitis patients | Prospective, randomised, left/right parallel study of 4 weeks' duration | Lesions: global severity of atopic dermatitis, investigator and patient preference of therapeutic efficacy | Physician global assessment for those aged <10 years: the proportion of those with moderate, severe, or very severe dermatitis was 94% at baseline and 14% at 4 weeks for Mildison compared with 94% at baseline and 16% at 4 weeks for Uniderm | Method and concealment of randomisation unclear, study described as double-blind | Very similar to Andersen et al.,144 study, but this time no patient preference with regards to cosmetic acceptibility |
| For >10 years 89% baseline to 12% at 4 weeks for Mildison and Uniderm | Five drop-outs and withdrawals; no ITT |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Sudilovsky et al., 198195 | 0.1% halcinonide cream o.d. vs 0.1% 0.1% halcinonide cream t.d.s. | 149 atopic eczema patients | Prospective, randomised, right/left, parallel study of 3 weeks' duration | Comparative and absolute therapeutic responses: erythema, oedema, changes in size of thickness of lesions | Based on 116 evaluable patients at week 3 86.2% noticed good or excellent clearance in t.d.s. vs 85.3% in o.d. group | Method and concealment of randomisation unclear, study described as double-blind | Table of random numbers used |
| USA | Physician global response | No statistical differences | 33 drop-outs/withdrawals; no ITT | Implies double blinding by use of placebo | |||
| Richelli et al., 1990145 | Clobetasone 17-butyrate lotion b.d. (8 am and 3 pm) vs b.d. (3 pm and 8 pm) or o.d. (9 pm) | 30 children with atopic eczema | Prospective, randomised, right/left, parallel study of 3 weeks' duration | Itching, burning, pain, erythema, oedema, exudation, blisters, bullae, scabs, scaling, lichenification, pooled into a mean score | Data on severity scores only presented in graphical form | Method and concealment of randomisation unclear | Method and concealment of randomisation unclear |
| Italy | Serum cortisol and ACTH tests | No obvious differences between three groups | Blinding not described | Blinding not described | |||
| No supporting statistics given | Unclear of ITT | Unclear of ITT | |||||
| Haneke, 199275 | Methylprednisolone aceponate ointment o.d. vs b.d. | 88 adults with atopic dermatitis | Propsective, randomised, left/right, parallel study of 4 weeks' duration | Patient and doctor global assessments | No actual data for o.d. vs b.d. methylprednisolone aceponate given | Method and concealment of randomisation unclear | Results of all three studies impossible to disentangle |
| Germany | Doctor assessed 11 signs and symptoms | Study described as double-blind | |||||
| No ITT | |||||||
| Koopmans et al., 199569 | 0.1% hydrocortisone 17-butyrate cream b.d. vs o.d. plus vehicle o.d. | 150 adults and children over the age of 12 years suffering from atopic dermatitis | Prospective, randomised, parallel study of 4 weeks' duration | Patient and doctor assessed overall severity | 78% o.d. vs 93% b.d. (p = 0.006) noticed considerable improvement or clearance according to patient | Method and concealment of randomisation unclear | Method and concealment of randomisation unclear |
| Finland | Clinical features assessed were erythema, induration, pruritus and excoriation | Study described as double-blind | Study described as double-blind | ||||
| No ITT but only one drop-out | No ITT but only one drop-out | ||||||
| Bleehen et al., 199587 | Fluticasone propionate 0.05% cream o.d. vs b.d. | 270 moderate-to-severe atopic dermatitis patients | Prospective, randomised, parallel study of 4 weeks' duration | Patient diary cards for itch, rash and sleep disturbance | Patient diary cards revealed improvement in rash, itch and sleep loss for both treatment groups within first week. 80% in o.d. and 85% in b.d. groups defined as clinical success on ITT analysis (p = 0.35) | Method and concealment of randomisation unclear | Method and concealment of randomisation unclear |
| UK | Physician assessed six signs and global assessment | Probably investigator blinded but unclear | Probably investigator blinded but unclear | ||||
| ITT carried out | ITT carried out |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Hoybye et al., 1991142 | Mometasone furoate cream o.d. vs hydrocortisone 17-butyrate cream b.d. | 96 adult atopic eczema patients | Prospective, randomised, parallel study of 6 weeks' duration | Patient VAS for severity of eczema, 0–3 score for doctor-assessed erythema, infiltration and pruritus, global evaluation scores of 1–6 | A comparison of the evaluations made by patients on a VAS after 6 weeks showed no difference in efficacy between the two treatments (p = 0.30) | Method and concealment of randomisation unclear, study described as single-blind | Difficult to blind a o.d. treatment with a b.d. treatment |
| Denmark | Ten drop-outs/withdrawals, no ITT | Posology of treatments not given | |||||
| Vernon et al., 1991101 | Mometasone furoate 0.1% cream vs hydrocortisone 1.0% cream o.d. | 48 children with moderate-to-severe atopic dermatitis | Prospective, randomised, parallel study of 6 weeks' duration | Doctor-assessed erythema, lichenification, skin surface disruption (crusting, scaling), excoriation, and pruritus on a 0–3 scale, % body surface area and global evaluation | For the 12 evaluable patients mean percentage improvement in total sign/symptom score was 95% for mometasone vs 75% for hydrocortisone (p = 0.01) | Method of randomisation unclear, study described as single blind with an ‘unblinded investigator’ evaluations were carried out by a ‘blinded’ investigator | Efficacy advantage of mometasone (classified as potent in UK) not surprising when compared against a very mild product |
| USA | The group with more than 25% body surface area involvement showed a wider difference in favour of mometasone (92% vs 62%; p = 0.01) | 36 patients experienced clearing of eczema prior to end of study so were withdrawn; no ITT carried out | |||||
| Rafanelli et al., 1993129 | Mometasone furoate 0.1% cream o.d. vs clobetasone 0.05% cream b.d. | 60 children with atopic dermatitis | Prospective, randomised, parallel study of 3 weeks' duration | Parent-assessed efficacy of treatment on a four-point scale (excellent to poor) | Total sign/symptom score improvement over baseline, 7.8 to 1.1 (p < 0.01) for mometa-sone vs 7.2 to 2.4 for clobetasone (NS) | Method of randomisation unclear, study described as third party blind | Uncertain what type of clobetasone was tested |
| Italy | Investigator-assessed erythema, induration and pruritus, global percentage improvement | No withdrawals or drop-outs | This is important as the propionate is super potent whereas butyrate is moderately so | ||||
| Marchesi et al., 1994131 | Mometasone furoate ointment 0.1% o.d. vs betamethasone dipropionate ointment 0.05% b.d. | 60 adult patients with atopic eczema of at least moderate severity | Prospective, randomised, parallel study of 3 weeks' duration | Investigator assessed erythema, induration and pruritus on a 0–3 scale, global evaluation % improvement | 100% of mometasone and betamethasone patients had cleared or experienced good improvement by week 3 | Method of randomisation unclear, study described as third-party blind evaluator | Pity there was no comparison between o.d. betamethasone |
| Italy | No baseline values given | No withdrawals or drop-outs | |||||
| Reidhav & Svensson, 199672 | Betamethasone valerate 0.1% cream o.d. vs mometasone furoate 0.1% cream o.d. | 30 patients with atopic dermatitis aged 15–66 years | Prospective, randomised, left/right, parallel study of 4 weeks' duration | Patient assessed pruritus and smarting pain on 0–3 scale, evaluator assessed erythema, scaling, lichenification, excoriation, papules, and vesicles on a 0–3 scale (max. score 18) | No significant differences were found for any of the symptoms scored following 4 weeks' treatment with betamethasone valerate or mometasone furoate | Method and concealment of randomisation unclear, study described as double-blind | No actual efficacy data reported, only patient preference data given |
| Sweden | Ten drop-outs/withdrawals; no ITT carried out | ||||||
| Traulsen, 199773 | Hydrocortisone buteprate cream 0.1% o.d. vs betamethasone valerate 0.1% cream o.d. | 86 atopic dermatitis patients 12+ years | Prospective, randomised, left/right parallel study of 2 weeks' duration | Erythema, infiltration, lichenification, scaling, vesiculation, papules, excoriations and pruritus on a 0–4 scale | The sum of scores of 8 symptoms showed a mean reduction from 13.1 to 2.3 after 2 weeks' treatment | Method and concealment of randomisation unclear, study described as double-blind | No differences between treatments but effect sizes similar to studies of b.d. usage |
| Denmark | Patient-assessed efficacy and investigator global assessment | There were no significant differences between the two treatments | Three withdrawals/drop-outs; no ITT carried out | ||||
| Study 1 | |||||||
| Traulsen, 199773 | Hydrocortisone buteprate ointment 0.1% o.d. vs betamethasone valerate 0.1% ointment o.d. | 82 atopic dermatitis patients 12+ years | Prospective, randomised, left/right parallel study of 2 weeks' duration | Erythema, infiltration, lichenification, scaling, vesiculation, papules, excoriations and pruritus on a 0–4 scale | The mean sum of scores of five symptoms (erythema, scaling, vesicles, papules, pruritus) decreased from baseline 8.3 to 1.6 after 2 weeks for hydrocortisone buteprate vs 8.3 to 1.4 for betamethasone valerate | Method and concealment of randomisation unclear, study described as double-blind | |
| Denmark | Patient-assessed efficacy and investigator global assessment | A statistically significant difference was found in favour of betamethasone | Four withdrawals/drop-outs; no ITT carried out | ||||
| Study 2 | |||||||
| Amerio et al., 199878 | Mometasone furoate 0.1% o.d. vs betamethasone valerate b.d. | 97 atopic dermatitis patients | Prospective, randomised, parallel study of 15 days' duration | Erythema, oedema, essudate, scaling, excoriation, lichenification (objective symptoms) and pruritus and burning (subjective symptoms) | 83.1% mometasone furoate patients and 89.2% betamethasone valerate patients experienced a reduction in signs and symptoms (NS) | Method and concealment of randomisation unclear from abstract, study described as double-blind | A study reported in Italian; all information abstracted from the English abstract only |
| Italy | Unclear from abstract if any withdrawals or drop-outs | Pity there was no o.d. betamethasone | |||||
| Wolkerstorfer et al., 199882 | Fluticasone propionate 0.05% cream o.d. vs clobetasone butyrate 0.05% cream b.d. | 22 children with atopic dermatitis | Prospective, randomised, parallel study of 4 weeks' duration | SCORAD composite scale of extent and intensity of eight signs | At week 4, three fluticasone patients and one clobetasone patient were clinically healed (SCORAD <9) | Method and concealment of randomisation unclear, study described as double-blind | Small sample over a short period of time |
| The Netherlands | Only one drop-out; no ITT analysis carried out |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| VanDerMeer, 1999104 | Fluticasone propionate 0.005%(g/g) vs placebo | 54 patients with moderate-to-severe atopic dermatitis patients identified from a larger set of 112 on the basis of enhanced steroid responses | Prospective, randomized, parallel study of 16 weeks' duration | Risk of relapse and time to relapse | 68% of patients in the placebo group and 39% in the fluticasone group withdrew because of recurrence and relapse | Method and concealment of randomisation unclear, study described as double-blind | Good to see a longer-term study evaluating relapse as well as short-term efficacy |
| The Netherlands | Clinical assessment SCORAD: erythema, oedema/papulation, oozing/crusts, excoriations, lichenification, dryness, pruritus and sleep loss | Risk of relapse was 2.6 times greater in active group (95% CI, 1.2 to 5.7) | 17 withdrawals/drop-outs; no ITT | Difficult to say how much of the benefit in preventing realpse was due to treating old healed sites as opposed to treatment of new sites | |||
| Skin thickness on biopsy specimens | No significant changes were detected in either treatment group in serum cortisol levels or in skin thickness measurements | Only data up to first relapse analysed |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Lucky et al., 1997133 | Desonide 0.05% ointment vs hydrocortisone 2.5% ointment b.d. | 20 children | Prospective, randomised, parallel study of 4 weeks' duration | Hypothalamic pituitary–adrenal (HPA) axis (cortisol levels) | –1.6 and –1.3% change in cortisol levels over baseline at 28 days for desonide and hydrocortisone groups, respectively | Method and concealment of randomisation unclear, study described as open label | No evidence of HPA suppression in either group |
| USA | Five drop-outs; no ITT | Short-term study | |||||
| Sanabria-Silva et al., 1991148 | Hydrocortisone 1% vs betamethasone dipropionate 0.05% vs cold cream placebo | 45 children with atopic dermatitis | Prospective, randomised, open study of 4 weeks' duration with 10 days suspended treatment | ‘Rebound phenomenon’ reactivation of lesions with greater intensity than their pre-treatment state a few (<10) days after suspending the treatment with topical steroids, which had controlled them | Sudden suspension of topical steroids was followed by relapse in every case but in no case was there rebound | Method and concealment of randomisation unclear, open study, no blinding | Although rebound is often referred to, there was no evidence of such a phenomenon in this study |
| Spain (translated) | The extensiveness of lesions according to three signs | There was no statistical difference between the frequency of relapse in the three groups (p < 0.055) | No mention of withdrawals and drop-outs | ||||
| Photographs taken before and after treatment | |||||||
| Kuokkanen & Sillantaka, 1987109 | Alclometasone dipropionate 0.05% vs hydrocortisone 1.0% b.d. | 37 children with eczema | Prospective, randomised, left/right, parallel study of 3 weeks' duration | Cutaneous atrophy: skin thinning, shininess, striae and fine blood vessels (telangiectasia) as assessed under magnification | Signs of cutaneous atrophy were not observed at any test site either at beginning or at 3-week evaluation period | Method and concealment of randomisation unclear, study described as double blind | No evidence of skin thinning, though study duration very short |
| Finland | Efficacy similar in both groups with 88% improvement signs and symptoms in alclometasone-treated sites vs 86% hydrocortisone-treated sites | Three withdrawals/drop-outs; no ITT |
| Study | Interventions | Study population and sample size | Trial design, description and follow-up | Outcome measures | Main reported results | Quality of reporting | Comment |
|---|---|---|---|---|---|---|---|
| Dickey, 197698 | Betamethasone sodium phosphate 4.0 mg/ml injection vs dexamethasone sodium phosphate 4.0 mg/ml injection o.d. | 22 patients with moderate-to-severe atopic dermatitis | Prospective, randomised, parallel study of 24 hours' duration | Inflammation, vesiculation, pruritus, exudation, excoriations, overall evaluation | Overall evaluation on a four-point rating scale: baseline 3.44 for betamethasone reduced to 2.89 and 3.62 for dexamethasone reduced to 2.69 | Method and concealment of randomisation unclear, study described as double-blind | Although a placebo group might have been ethically difficult, patient-based views on treatment response would have been useful |
| USA | Withdrawals and drop-outs not mentioned | ||||||
| Heddle et al., 1984112 | Oral plus nasal beclomethasone dipropionate q.d.s. vs placebo | 27 children with moderate-to-severe atopic eczema | Prospective, randomised, crossover study of 4 weeks' duration | Patient assessed itch and sleep loss (VAS) | Parental scores for itch and antihistamine use were significantly lower on beclomethasone than placebo, but use of topical steroids and sleep loss did not show any significant change | Method and concealment of randomisation unclear, study described as double-blind | Crossover study with significant treatment order interactions |
| UK | Doctor assessed redness, vesiculation, crusting, excoriation, lichenification | Other significant changes especially surface damage | One withdrawal; no ITT | Large treatment effects | |||
| La Rosa et al., 1995140 | Systemic flunisolide 640–1200 μg b.d. vs placebo | 20 children with severe atopic dermatitis | Prospective, randomised, crossover study of 2 weeks' duration | Pruritus, erythema/oedema, excoriation, papulation/erosion/scaling, lichenification | Improvement over baseline for total clinical severity score: group A 75 reduced to 34 and group B 74 reduced to 29 | Method and concealment of randomisation unclear, study described as double-blind | Big treatment effects |
| Italy | Withdrawals/drop-outs not mentioned |
Appendix 4 Duplicate and triplicate publications
| Interventions | Duplicate or triplicate publications |
|---|---|
| Fish oils | Bjorneboe 1989254 = Bjorneboe 1987251 |
| Evening primrose oil | Wright 1985245 = Wright 1982242 |
| Evening primrose oil | Ferreira 1998a233 = Ferreira 1998b234 |
| Cyclosporin A | Sowden 1991295 = Salek 1993293 = Allen 1991298 |
| Corticosteroids | Sefton 1984114 = Sefton 1983136 |
| Corticosteroids | Korting 199586 = Zienicke 1993146 |
| House dust mites | Friedmann 1998267 = Tan 1996263 |
| Allergen–antibody complexes | Leroy 1993284 = Leroy 1992285 |
| Topical corticosteroids | Ottevanger 1992 = Hoybye 1991124 |
Glossary
- Atopic dermatitis
- Synonymous with atopic eczema.
- Bayesian approach
- An approach to data analysis first developed by Thomas Bayes, which tests the likelihood of something occuring in the light of prior knowledge and belief about what is likely to happen. This is different from traditional ‘frequentist’ statistics, which seeks to disprove the null hypothesis of no difference between the things being compared.
- Demarcated
- Lacks boundary.
- Erythema
- Redness.
- Inverse proportion
- A relation between two quantities such that one increases in proportion as the other decreases.
- ITT analysis
- A method of analysis for RCTs whereby all patients randomly allocated to one of the treatments in a trial are analysed together irrespective of whether or not they completed or received that treatment.
- Lichenification
- Thickening of the skin as a result of chronic scratching.
- Morphology
- Form.
- Pruritus
- Itching.
- Rebound phenomenon
- The tendency for atopic eczema to flare up immediately on stopping a treatment that suppresses the disease process.
- Recombinant
- Reformed by recombination.
- Rule of Nines
- A method for estimating extent of eczema based on dividing the body into multiples of 9%.
- Serological (serology)
- The scientific study of blood sera and their effects.
- Spongiosis
- Excess fluid between the cells in the epidermis.
- Tracker studies
- A type of experimental design suggested by Lillford for testing technologies that are rapidly changing, for example prosthetic devices. In the duration of a 3-year trial, the types of devices that were originally tested might have already been improved upon, thereby limiting the extent to which one could generalise from the findings. Tracker studies are more flexible by permitting the use of the technology as it evolves within the trial.
- Wiskott–Aldrich Syndrome
- A genetic defect affecting a gene on the x chromosome. Generally passed from mother to son. Main symptoms are proneness to infections, thrombocytopenia (low platelet counts) and eczema of differing severity.
List of abbreviations
- ADASI
- Atopic Dermatitis Area Severity Index
- b.d.
- twice daily*
- BIT
- biophysical information therapy
- CCT
- controlled clinical trial*
- CCTR
- Cochrane Controlled Trials Register
- CI
- confidence interval
- DSCG
- disodium cromoglycate
- EDEN
- European Dermato-Epidemiology Network
- ETAC
- Early Treatment of the Atopic Child (study)
- GLA
- gamma-linoleic acid
- IgE
- immunoglobulin E
- ISAAC
- International Study of Asthma and Allergies in Childhood
- ITT
- intention-to-treat*
- NS
- not significant
- o.d.
- once daily*
- PAF
- platelet-activating factor
- PP
- per protocol*
- PUVA
- psoralen plus UVA
- q.d.s.
- four times daily*
- RCT
- randomised controlled trial
- RoNAA
- Rule of Nines area assessment*
- SCG
- sodium cromoglycate
- SCORAD
- Severity Scoring of Atopic Dermatitis
- SEM
- standard error of the mean*
- t.d.s.
- three times daily*
- UVR
- ultraviolet radiation
- VAS
- visual analogue scale*
* Used only in tables