Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/12/02. The contractual start date was in September 2018. The draft report began editorial review in October 2020 and was accepted for publication in April 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Claxton et al. This work was produced by Claxton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Claxton et al.
Chapter 1 Background
Chronic heart failure
Chronic heart failure (CHF) is a significant and growing health-care challenge, as increasing numbers of people live longer and survive ischaemic heart disease. In high-income countries, 10–15% of individuals over the age of 75 years suffer from the disorder1,2 and, despite substantial improvement over the last two decades,3,4 overall prognosis remains poor. Disease morbidity and mortality are high, with a 5-year survival rate of 25% after hospitalisation for heart failure with a reduced ejection fraction (HFrEF). 5 Those living with CHF may experience persistent shortness of breath, ankle swelling, tiredness, frequent stays in hospital and reduced quality of life (QoL), as well as a shorter life expectancy.
Chronic heart failure accounts for a large proportion of UK hospital admissions (2% of bed-days and 5% of emergency admissions)6 and an NHS annual spend of around £2.3B. 7 The King’s Fund has identified heart failure as an ambulatory care-sensitive condition, where effective primary care interventions could avoid hospitalisation, have significant benefit on patients’ QoL and reduce service costs. 8 There is, therefore, an unmet and increasing need for effective therapies both to improve health and well-being and to help keep patients out of hospital and reduce the economic burden on health-care systems. To achieve comprehensive coverage of the at-risk population and to maximise both clinical effectiveness and cost-effectiveness, new treatments should be easy to deliver in primary care settings and be acceptable and safe in a broad spectrum of patients, including the elderly and those with multiple comorbidities.
Heart failure and coenzyme Q10
Heart failure is characterised by cardiomyocyte energy depletion9 due to mitochondrial dysfunction10 and adenosine triphosphate depletion,11 leading to abnormal calcium handling and impaired contractile function. 12 Coenzyme Q10 (co-Q10) is an endogenous, vitamin-like, fat-soluble quinone found in high concentrations in myocardium, liver and kidney mitochondria. It is an electron carrier crucial to mitochondrial adenosine triphosphate production13 and has antioxidant14,15 and anti-atherogenic properties. 16 Natural production of co-Q10 peaks in a person’s twenties, thereafter declining with increasing age. Cardiomyocytes in patients with heart failure are deficient in co-Q10,17,18 and low myocardial and/or circulating levels are associated with worse symptoms19–21 and poorer heart function,22 although there is inconsistency of effect of low circulating levels on prognosis. 22–24 A common but infrequently recognised feature of heart failure is micronutrient deficiency25 and the use of co-Q10 in practice may be as a single supplement or as part of a multi-micronutrient supplement.
It has been shown that oral co-Q10 supplementation (up to 300 mg/day) leads to increased serum and myocardial levels,21 but it is uncertain whether or not this increase in levels translates to clinical benefit. Co-Q10 is not available on prescription in the UK, but it can be bought over the counter.
Statins and coenzyme Q10
Statins block the production of both cholesterol and co-Q10, and there is some evidence that statin use reduces serum levels of co-Q10. 26,27 Although younger and healthier statin users appear to tolerate this depletion, it has been suggested that when this happens in CHF patients, it worsens myocardial function. Should this be the case, patients using statins may face competing risks and benefits and have a greater capacity to benefit from co-Q10.
There is divided opinion on the clinical effectiveness and potential role of co-Q10 in treating CHF. At one extreme, it has been suggested that adjunctive co-Q10 is essential for those receiving statins and that this should be noted in US black-box labelling. 28 By contrast, current National Institute for Health and Care Excellence (NICE) guidance actively lists this as a ‘do not do’ (i.e. do not offer co-Q10 or vitamin D to increase adherence to statin treatment). 6 Existing research evidence is inconclusive.
Existing clinical trial and systematic review evidence
Early uncontrolled studies suggested beneficial effects on left ventricular ejection fraction (LVEF), exercise tolerance and symptoms, at a variety of doses. 25,29,30 Most randomised trials of co-Q10 have been small, reported surrogate outcomes and had mixed results. Recent systematic reviews of single co-Q10 supplementation have been limited by the nature and incompatibility of the reported data.
A systematic review reported by Fotino et al. ,31 which included 13 randomised controlled trials (RCTs) and 395 participants, reported a 3.7% mean net increase in LVEF [95% confidence interval (CI) 1.60% to 5.74%] and a –0.3 mean change in New York Heart Association (NYHA) class (95% CI –0.66 to 0.06). A Cochrane review published in 2014,32 which included seven RCTs and 914 participants, was able to analyse only LVEF and exercise capacity owing to incomplete reporting in trial publications. It found no clear effects, concluding that ‘there is no convincing evidence to support or refute the use of co-Q10 for heart failure’. 32 Neither of these reviews included the more recently published Q-SYMBIO trial33 (420 participants), which reported a halving of all-cause risk of death [hazard ratio (HR) 0.51, 95% CI 0.30 to 0.89]. A more recent systematic review, published in July 2017,34 of 14 trials and 2149 participants, did include the Q-SYMBIO trial. This systematic review34 reported a significant reduction in mortality [relative risk (RR) 0.69, 95% CI 0.50 to 0.95] and an improvement in exercise capacity, but also stated that, owing to limitations, further trials were needed. 34 Another systematic review,35 of 16 trials and 1662 participants, also reported a significant reduction in mortality (pooled HR 0.62, 95% CI 0.40 to 0.95), as well as reduced hospitalisation (HR 0.39, 95% CI 0.29 to 0.53). 35 A more recent review36 of nutraceuticals (which did not include a meta-analysis) concluded that there was insufficient evidence to demonstrate the efficacy of co-Q10. 36 None of these systematic reviews was able to explore potential effect modifiers, such as the use of statins.
Rationale
Despite a long history, and therapeutic promise, there is considerable uncertainty about the effectiveness of co-Q10 in CHF. Most trials have been small, and systematic reviews have been limited by incomplete reporting and data limitations. As co-Q10 is classed as a nutritional supplement and is not subject to the same regulatory processes as pharmaceuticals, some trials have not undergone the same independent scrutiny as for licensed medicines. Publication bias may be substantial.
The planned individual participant data (IPD) meta-analysis would have provided an opportunity to collect unreported outcomes and data from participants excluded from published analyses,37 support time-to-event analyses38 and model covariate treatment interactions, as well as enable robust independent scrutiny of the existing trial evidence. However, despite considerable effort, insufficient IPD were available from trial investigators to support an IPD meta-analysis.
Therefore, we undertook the meta-analysis using aggregate data. Although previous systematic reviews exist, we improved on these by bringing them up to date, incorporating additional aggregate data derived from the IPD that were obtained and completing additional analyses, including comparing estimates of effectiveness in people taking statins with those in people not taking statins.
To the best of our knowledge, there was no existing economic evaluation of co-Q10 in CHF and so there was a need to explore whether or not prescription of co-Q10 could be cost-effective.
Chapter 2 Aims and objectives
The aims of this review were to:
-
undertake a high-quality systematic review and meta-analysis to assess the clinical effectiveness of co-Q10 in the management of CHF
-
develop an economic model evaluating cost-effectiveness, based on best current evidence
-
undertake a value-of-information (VOI) analysis to quantify the value of undertaking a new trial to address key uncertainties.
Coenzyme Q10 (on its own or in combination with other micronutrients) was compared with placebo or no supplementation. Short- and long-term benefits and harms were considered. A main consideration was to undertake detailed exploration of clinical heterogeneity, investigating whether or not particular types of individuals experience greater benefit (or harm) from the intervention. This was to help resolve existing uncertainty and to support the development of a linked economic model and VOI analysis.
The economic model addressed the value of co-Q10 in treating CHF, based on existing evidence, considering both health outcomes and cost. The VOI analysis assessed whether or not additional research would be valuable in supporting decisions about the use of co-Q10 in CHF.
Chapter 3 Systematic review and meta-analysis of effectiveness: methods
This systematic review was prospectively registered in PROSPERO (CRD42018106189) and is reported in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidance.
Literature searches
Bibliographic search strategies for MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and Science Citation Index were developed by an experienced information specialist and carried out during the protocol development phase of the project in October 2018. Update searches were run towards the end of the project in March 2020 to identify any new trials. An example MEDLINE search strategy is provided in Appendix 1.
Trial registers [ClinicalTrials.gov, ISCTRN, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) portal and OpenTrials.net] were also searched to identify any unpublished and/or ongoing trials. We contacted the manufacturer that provided some support for the largest co-Q10 trial for details of any further clinical trials that they had undertaken or sponsored.
Inclusion criteria
We aimed to include all relevant trials irrespective of whether they were published or unpublished, where they were carried out or which language they were managed and reported in.
Population
Trials of adult patients (aged > 18 years) with a diagnosis of HFrEF were included. Paediatric trials were excluded. Mixed population trials were eligible if data from relevant individuals (adults with CHF) were available separately. As patients with a diagnosis of heart failure with a preserved ejection fraction (HFpEF) are a clinically distinct population, trials focusing on this population were excluded. One included trial33 included a small proportion of HFpEF individuals (7% of participants had LVEF ≥ 45%) and it is possible that other trials that did not report LVEF had included some patients with HFpEF.
Intervention
Trials of co-Q10 (taken singly or as part of a multi-micronutrient supplement) given as an adjunct to co-treatment (e.g. statins) or other routine care.
Comparators
Placebo given as an adjunct to co-treatment (e.g. statins) or other routine care.
Outcomes
Intended outcomes included the following:
-
All-cause mortality (ACM) and cardiovascular mortality (time to event) [i.e. death from myocardial infarction (MI), stroke, heart failure or sudden cardiac death].
-
Major cardiovascular events (time to first event) (i.e. non-fatal MI, non-fatal stroke and revascularisation procedures).
-
Hospitalisation related to heart conditions (i.e. any, number of and duration of stays).
-
Any cardiovascular event, as above, death or any hospitalisation (composite outcome).
-
QoL measures using a validated instrument [e.g. the EuroQol-5 Dimensions (EQ-5D)].
-
NYHA functional class (or equivalent).
-
Adverse effects/side effects.
-
LVEF, which is the volumetric fraction of fluid blood ejected from the left ventricle of the heart with each contraction.
-
Exercise testing [e.g. change in 6-minute walk test (6MWT)] over a defined period.
-
B-type natriuretic peptide (BNP)/N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) level.
-
Peak oxygen consumption.
-
The NYHA classification is a simplified scale that classifies heart failure severity. It classifies patients into one of four categories based on their limitations during physical activity, including limitations to normal breathing, varying degrees of shortness of breath and/or angina pain. A higher score is associated with worse symptoms. The classification is as follows: class I – no symptoms and no limitation in ordinary physical activity (e.g. shortness of breath when walking); class II – mild symptoms (e.g. mild shortness of breath and/or angina) and slight limitation during ordinary activity; class III – marked limitation in activity due to symptoms, even during less than ordinary activity [e.g. walking short distances (20–100 m)], comfortable only at rest; and class IV – severe limitations, experiences symptoms even while at rest, mostly bedbound patients.
-
LVEF is calculated as a ratio of the volume of blood pumped from the left ventricle per beat (stroke volume) to the volume of blood collected in the left ventricle at the end of diastolic filling (end-diastolic volume). The American College of Cardiology recommends the following classification of systolic function: normal – LVEF 50–70%; mild dysfunction – LVEF 40–49%; moderate dysfunction – LVEF 30–39%; and severe dysfunction – LVEF < 30%.
-
In CHF, BNP and its amino-terminal NT pro-hormone BNP are released into circulation directly from the myocardium following end-diastolic stress as a result of increases in volume or pressure. Measurements of BNP or NT-pro-BNP are used in establishing diagnosis, prognosis and disease severity in CHF in current guidelines, with higher levels indicating poorer prognosis. 39,40 However, it has been suggested that BNP and NT-pro-BNP measurements may not be reliable end points because within-patient changes are not a consistent surrogate of changed prognosis either in studies or in clinical practice. 41
-
Study design
Only RCTs were eligible. Both parallel-group and crossover trial designs were included.
Study selection
At least two researchers (AL, LB and SS) independently screened all titles and abstracts identified in the bibliographic searches. Full publications were then obtained for potentially relevant studies and independently assessed for relevance by two members of the research team. Any discrepancies in screening decisions were resolved by discussion. Authors were contacted for further information, where necessary. For studies reported in a non-English language, native Italian- and Japanese-speaking researchers and a non-native Russian speaker assisted with study selection.
Studies that did not meet all of the inclusion criteria and were excluded at full-text screening stage were tabulated along with the reasons for exclusion (see Appendix 2).
Data collection
At least two researchers (AL, LB and SS) independently extracted data from published and unpublished study reports. Data were extracted on study design, intervention and comparator characteristics, baseline characteristics and results. Any discrepancies were resolved by discussion and, where necessary, cross-referencing IPD or contacting study authors. Where results from a single trial were reported in more than one manuscript or conference abstract, the most complete publication or, where available, the publication with IPD was preferred. Researchers who were native speakers of Italian and Japanese assisted with data collection for studies reported in those languages.
We sought to extract all possible results data from all publications of each trial. This included, where feasible, extracting data from figures, such as Kaplan–Meier plots, for re-analysis. Where possible, analyses were based on the intention-to-treat principle.
Individual participant data
Trial investigators were invited to supply data either in a standardised format, using standardised coding developed for the project, or in any reasonable format and re-coded, as necessary, by the research team. Data were requested for all randomised participants, including any who had been excluded from the original trial analyses.
After recoding, all IPD received were checked for validity, including coding errors, and for balance in randomisation, outliers and impossible or implausible data. As insufficient data were available for full IPD meta-analysis, the received IPD were summarised by calculating the numbers of events in each study arm or the means and standard deviations in each study arm. These summary data were compared with study publications to ensure the validity of both the IPD and the publications.
Critical appraisal, data checking, quality assurance and risk-of-bias assessment
Critical appraisal of included studies was based on an assessment of trial publications and protocols (if available) and by checking received data sets. Risk-of-bias assessment was carried out using the Cochrane Risk of Bias tool (version 5.2). 42 Risk-of-bias judgments (low, high or unclear) were made for the following domains in each study: participant selection, performance (blinding of participants and study personnel), outcome assessment (blinding of outcome assessor), attrition and selective outcome reporting. Decisions on participant selection bias were informed by methods reported for random sequence generation and allocation concealment, taking into account any imbalances in key baseline characteristics reported in aggregate data and IPD, where available, using additional guidance. 43 Where it was considered that lack of blinding may have affected only those outcomes that required subjective judgement (e.g. NYHA or QoL outcomes), separate risk-of-bias judgments were carried out by outcome. For crossover trials, the following additional design aspects were accounted for: presence and duration of washout period, randomisation by order of treatment, reporting of outcomes by trial phase and attrition before crossover. Trial authors whose whereabouts we had established were contacted if available information was considered insufficient to formulate a risk-of-bias judgement (i.e. unclear risk of bias), although this had limited success. Risk-of-bias assessment was performed independently by at least two researchers, and any disagreements were resolved through discussion. For studies reported in a non-English language, researchers who were native speakers of Italian or Japanese assisted with risk-of-bias assessment.
Statistical analysis methods
A statistical analysis plan, which set out the analytic methods in detail, was developed for the originally intended IPD meta-analysis. This plan was followed, as far as was feasible, for meta-analyses using published aggregate data. Analyses were performed in accordance with the intention-to-treat principle.
Outcomes analysed
The full set of protocol intended outcomes and their definitions are given in Table 1. Those outcomes that were reported in trial publications and able to be analysed are given in Table 4.
| Cardiovascular outcome | Definition |
|---|---|
| ACM | |
| Cardiovascular mortality | Any of:
|
| Major cardiovascular event | Any of:
|
| MI | Fatal or non-fatal |
| Stroke or TIA |
Fatal or non-fatal Any of: |
| Other cardiac event | Any type |
| Revascularisation | Any type, including PCI and bypass surgery |
| Hospitalisation |
Any cause Number of hospitalisations and number of days |
| CHF-related hospitalisation |
Any CHF-related cause Number of hospitalisations and number of days |
| Death, cardiovascular event or hospitalisation | Composite outcome: incidence of any of the above |
| Functional and other outcomes | |
| NYHA functional class | Grades I–IV or improvement/worsening |
| LVEF | As a percentage |
| 6MWT | Distance walked or change in distance |
| Peak oxygen consumption (VO2) | |
| NT-pro-BNP | |
| BNP | |
| QoL | Any measurement scale |
| Adverse events | Any type |
Effect measures
Dichotomous outcomes included in the meta-analyses were analysed by calculating the relative risk (RR) for the effect of co-Q10 compared with placebo. For continuous outcomes, mean differences (MDs) between treatment arms were analysed. Alternatively, standardised mean differences (SMDs) were used for trials that had different measurement scales for the same outcome. Analyses were performed in terms of change from baseline values and, as a sensitivity analysis, using only final outcomes, without adjusting for baseline values.
Hazard ratios were calculated for time-to-event outcomes, either as reported or using data extracted from survival curves.
Some outcomes were analysed in multiple ways. Death and cardiovascular outcomes were analysed as both dichotomous outcomes and survival outcomes (where survival data were available). NYHA data were dichotomised (classes III and IV vs. classes I and II) and also analysed in terms of improvement by one or more categories. RRs and 95% CIs were calculated and reported for all effect estimates included in the meta-analyses.
Trials supplying individual participant data
As the majority of included trial investigators either could not or declined to supply IPD, analyses were based on data reported in their trial publications. Investigators for two trials supplied IPD. For these two trials, summary results (numbers of patients and events, and means and standard deviations by treatment arm) were calculated using the IPD and these summary results were then pooled in meta-analyses alongside data extracted from the publications for which IPD were unavailable.
Crossover and parallel-group trials
The protocol-intended approach was to analyse crossover and parallel-group trials separately, as the two trial designs may not give comparable results. However, because of limited outcome reporting in the crossover trials, this was not feasible for all outcomes. For LVEF, crossover and parallel-group trials were analysed separately and subsequently in combination. For all other outcomes, crossover and parallel-group trials were combined and meta-analysed together, provided that the two trial designs produced broadly consistent results.
Standard meta-analysis
Initial analyses estimated the effect (RR, MD or HR) for each outcome reported in each trial (or provided as IPD). A map of the data was produced to identify the number of trials and participants for each outcome to identify where meta-analysis was feasible. Meta-analyses were performed where two or more trials reporting the outcome under consideration were available. When only one trial was available, its results were reported narratively.
Effect estimates were combined in inverse-variance random-effects meta-analyses using the standard DerSimonian–Laird two-stage approach. This generated forest plots, enabling results across trials to be compared visually, heterogeneity to be investigated and differences across subgroups to be visualised. Heterogeneity was quantified using the I2-statistic. Where there were sufficient trials, the potential for publication bias was assessed using contour-enhanced forest plots.
One-stage regression analyses
Although more commonly associated with IPD meta-analyses, ‘one-stage’ meta-analyses that combine data from all trials in a single regression model to estimate the overall effect, rather than estimating an effect in each trial and then pooling across trials, can be carried out using either IPD or aggregate data. 44,45 For example, for aggregate dichotomous data, this is carried out using the numbers of events and number of patients in each arm of each trial. This approach was preferred to conventional meta-analysis, as it uses an exact likelihood and so may be more robust where data are sparse. In this review, one-stage analysis of the available aggregate data was possible for the outcomes of ACM and NYHA class only. These used restricted maximum likelihood methods and regressed outcome against treatment, with correlated random intercept and random treatment effects (to account for heterogeneity). Model parameters for treatment effect were then converted into RR estimates, with associated 95% CIs.
As for two-stage analyses, meta-analyses were performed if at least two trials reported data for the specified outcome.
Potential effect modifiers (subgroups and meta-regression)
The impact of trial- and patient-level characteristics on treatment effect (i.e. treatment–covariate interactions) was examined.
For categorical covariates, the trials were divided into groups according to the characteristic and meta-analyses were performed within each subgroup. Meta-regression was used for continuous covariates (e.g. co-Q10 dose). The covariates considered were:
-
trials specifically comparing co-Q10 plus statin with statin alone/other trials
-
single- or multi-micronutrient supplement
-
parallel or crossover design
-
co-Q10 dose
-
duration of treatment/trial
-
percentage of patients receiving statins
-
mean baseline value of outcome
-
year of publication.
For dichotomous outcomes with sufficient data, one-stage meta-regression models were also fitted. To do this, the one-stage regression models (see One-stage regression analyses) were extended to include one parameter for the covariate of interest and one for the treatment–covariate interaction. To ensure model convergence, these parameters were assumed to be common to all trials (i.e. no random effects).
Network meta–analysis
A network meta-analysis (NMA) compared single- and multi-micronutrient supplements containing co-Q10 with co-Q10 alone and with co-Q10 in combination with statins or other concomitant treatments. Analyses were carried out for the main outcomes listed earlier (see Outcomes analysed) where sufficient trials reported that outcome. Two statistical models were used. The first was the Bayesian models of Lu and Ades,46 which are the most commonly used methods for NMA. The second used a frequentist ‘one-stage’ logistic or linear regression, including multiple treatment arms.
Software
All analyses were conducted in the R software package (version 4.0; The R Foundation for Statistical Computing, Vienna, Austria). The meta package was used for meta-analyses, forest plots and funnel plots, and the lme4 package was used for one-stage models. For the NMA, OpenBUGS 3.2.3 and the GeMTC and BRugs packages in R were used (MRC Biostatistics Unit, University of Cambridge, Cambridge, UK; URL: www.mrc-bsu.cam.ac.uk/software/bugs/openbugs/).
Patient and public involvement and engagement
Two patient and public involvement partners were involved throughout the project participating in an advisory group meeting and commenting on project materials. The patient and public involvement partners helped to conceptualise the decision model through their experiences as patients, and they commented on the protocol, Plain English summary and final report.
Chapter 4 Systematic review and meta-analysis of effectiveness: results
Study selection
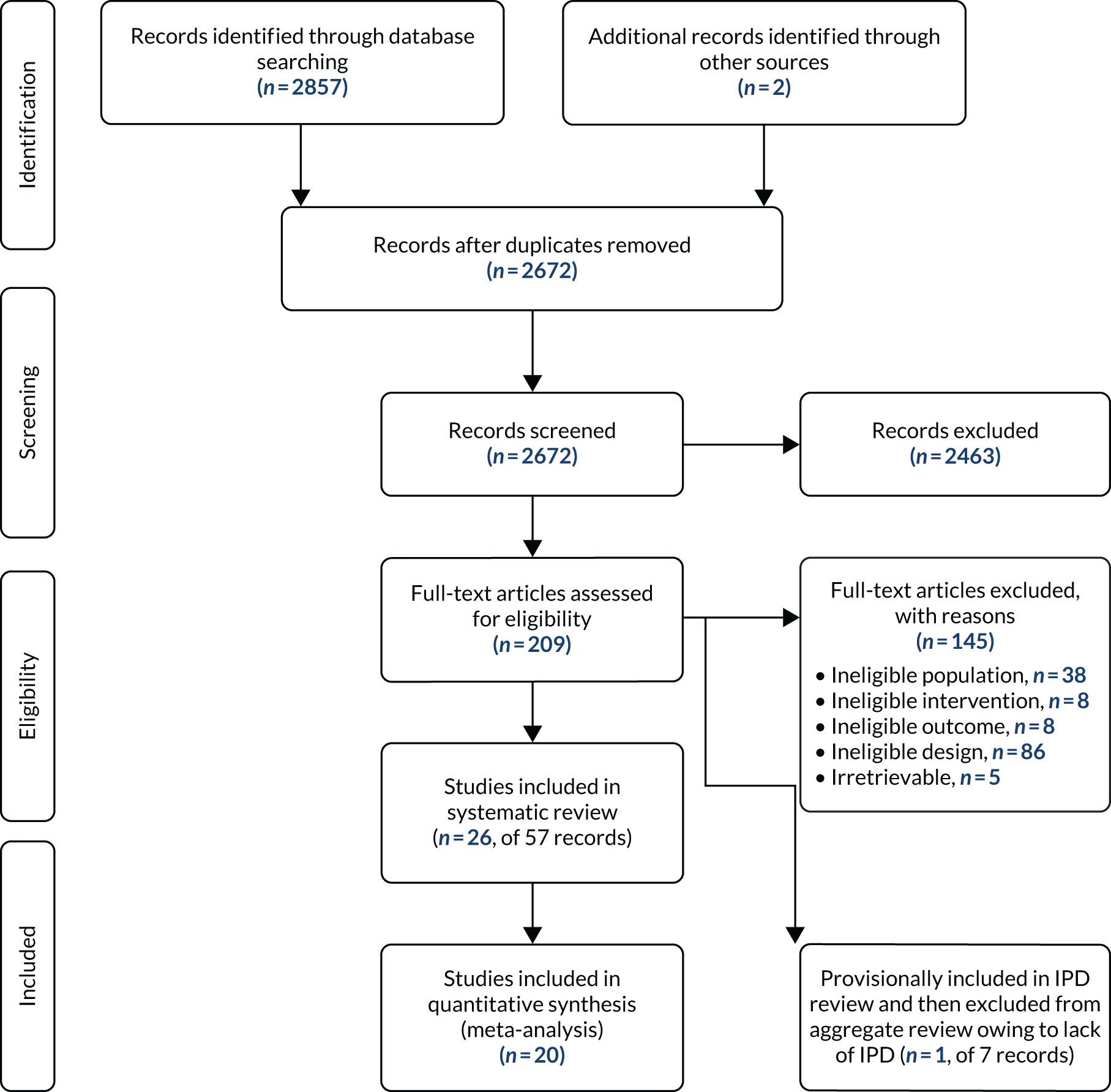
The bibliographic searches identified a total of 2675 unique references. Of these, 209 titles and abstracts potentially met the prespecified review selection criteria, and the full papers were obtained and assessed. A total of 26 unique trials29,33,47–70 met the inclusion criteria. One further community-based trial71 of elderly patients, which included a small proportion of patients who had a LVEF of < 40% (7.5%), was provisionally included in the IPD review because just those trial participants with a LVEF of < 40% could have been analysed. However, after failing to obtain IPD for this trial, it was excluded, as most participants did not have a diagnosis of HFrEF and those who did could not be analysed separately. One trial72 of patients with HFpEF was excluded in accordance with the review prespecified inclusion criteria. The study selection process is summarised in Figure 1. A list of studies excluded after the examination of full publications is provided in Appendix 2, with reasons given for exclusion. No relevant ongoing trials of co-Q10 in patients with a reduced ejection fraction were identified.
FIGURE 1.
Study selection process (PRISMA flow diagram).
Included studies
Of the 26 included trials,29,33,47–70 1733,48–51,54–57,59,60,62–64,67,69,70 had a parallel design and nine29,47,52,53,58,61,65,66,68 were crossover trials. The characteristics of the parallel and crossover trials are reported in Tables 2 and 3, respectively. All studies used a placebo control, except three,49,56,64 in which patients in the control group received only standard therapy [including diuretics, digoxin, angiotensin-converting enzyme (ACE) inhibitors and vasodilators]. Two studies56,60 were reported only as conference abstracts and two studies49,64 were reported in a language other than English.
| Trial [authors (year)] | Country/region; single/multicentre | Intervention | Control | Main inclusion criteria | n | Outcomes | Trial registration; stated funding |
|---|---|---|---|---|---|---|---|
| Berman et al. (2004)48 | Israel; single centre |
60 mg of co-Q10 twice daily for 3 months Follow-up:a 3 months |
Placebo | End-stage heart failure, awaiting heart transplantation | 32 |
NYHA class 6MWT Mortality Adverse events |
Not registered; NR |
| Davini et al. (1992)49 | Italy |
100 mg of exogenous co-Q10 daily for 4 months Follow-up:a 4 months |
Standard therapy only | NYHA classes > II CHF with dilative, valvular or ischaemic cardiopathy | 63 | NYHA class | Not registered; NR |
| Fumagalli et al. (2011)50 | Italy; single centre |
32 mg of co-Q10 plus 170 mg of creatine for 8 weeks Follow-up:a 8 weeks |
Placebo |
CHF due to LVSD LVEF of ≤ 35% |
67 |
Peak O2 consumption QoL |
Not registered; Scharper Therapeutics (Milan, Italy) |
| Garakyaraghi et al. (2015)51 | Iran; multicentre |
90 mg of co-Q10 plus 200 µg of selenium daily for 3 months Follow-up:a 3 months |
Placebo |
NYHA classes II and III LVEF of ≥ 35% |
64 |
NYHA class LVEF |
Not registered; Isfahan University (Isfahan, Iran) |
| Keogh et al. (2003)54 | Australia; multicentre |
150 mg of co-Q10 daily for 3 months Follow-up:a 3 months |
Placebo | NYHA classes II and III heart failure | 39 |
NYHA class 6MWT Adverse events |
Not registered; Blackmores Ltd (Warriewood, NSW, Australia), Pharma Nord (Vejle, Denmark) |
| Khatta et al. (2000)55 | USA; multicentre |
200 mg of co-Q10 daily for 6 months Follow-up:a 6 months |
Placebo | NYHA classes III and IV heart failure | 55 |
LVEF, Peak O2 consumption Mortality Adverse events |
Not registered; Pharma Nord, National Institute of Aging (Bethesda, MD, USA) |
| Kukharchik et al. (2016) (conference abstract)56 | Russia |
120 mg of co-Q10 daily for 3 months Follow-up:a 3 and 12 months |
Standard therapy only |
NYHA classes II and III CHF MI history |
120 | LVEF | NR |
| Kumar et al. (2007)57 | India; single centre |
270 mg of ubiquinol plus 2250 mg of L-carnitine daily for 12 weeks Follow-up:a 12 weeks |
Placebo | NYHA classes II–IV CHF | 62 |
NYHA class 6MWT Mortality Adverse events |
Not registered; Tishcon Corp. (New York, NY, USA) |
| Ma et al. (1996)59 | China; single centre |
20 mg of co-Q10 or 12.5 mg of captopril three times per day Follow-up:a 2 years |
Placebo |
NYHA classes II and III Dilated cardiomyopathy |
65 | Mortality | Not registered; Chinese Nature Scientific Fund |
| Mareev et al. (2017) (conference abstract)60 | Russia; multicentre |
co-Q10 nasal drops 90 mg/day (equivalent to 225 mg/day) for 24 weeks Follow-up:a 24 weeks |
Placebo |
NYHA classes I–IV heart failure LVEF of < 45% |
148 |
NYHA class LVEF 6MWT |
Not registered; NR |
| Morisco et al. (1993)62 | Italy; multicentre |
50 mg of co-Q10 two or three times daily for 1 year Follow-up:a 1 year |
Placebo | NYHA classes III and IV CHF | 641 | Mortality | Not registered; Italian Association of Internal Medicine (Viale Università, Rome, Italy) |
| Mortensen et al. (2014)33 | Europe, Asia and Australia; multicentre |
100 mg of co-Q10 three times daily for 2 years Follow-up:a 2 years |
Placebo | NYHA classes III and IV CHF | 420 |
NYHA class LVEF 6MWT Pro-BNP Hospitalisation Mortality Cardiovascular events Adverse events |
Retrospectively registered (ISRCTN94506234); International Coenzyme Q10 Association (Ancona, Italy); Pharma Nord, Kaneka Corporation (Tokyo, Japan) |
| Munkholm et al. (1999)63 | Denmark; single centre |
100 mg of co-Q10 twice daily for 12 weeks Follow-up:a 12 weeks |
Placebo | NYHA classes II and III heart failure | 22 | LVEF | Not registered; NR |
| Nakanishi et al. (1988)64 | Japan |
45 mg of co-Q10 per day for 5 months Follow-up:a 5 months |
Standard therapy only |
NYHA classes II and III Dilated cardiomyopathy |
16 | NYHA class | Not registered; NR |
| Pourmoghaddas et al. (2014)67 | Iran; single centre |
100 mg of co-Q10 twice daily for 4 months (plus 10 mg of atorvastatin daily) Follow-up:a 4 months |
Placebo | NYHA classes II–IV heart failure | 62 |
NYHA class LVEF Pro-BNP Mortality |
Not registered; NR |
| Witte et al. (2005)69 | UK; single centre |
Calcium, magnesium, zinc, copper, selenium, vitamin A, thiamine, riboflavin, vitamins B6, B12, C, D and E, folate and co-Q10 (150 mg) daily for 9 months Average follow-up:a 295 days |
Placebo |
CHF LVEF of ≤ 35% |
32 |
LVEF Pro-BNP Mortality HRQoL |
Not registered; none |
| Zhao et al. (2015)70 | China; single centre |
30 mg of co-Q10 daily for 12 months Follow-up:a 6 and 12 months |
Placebo |
NYHA classes II–IV heart failure LVEF of < 40% |
102 |
LVEF 6MWT Mortality |
Not registered; NR |
| Trial [authors (year)] | Country/region; single/multicentre | Intervention | Blinding | Inclusion criteria | n | Outcome | Trial registration; stated funding |
|---|---|---|---|---|---|---|---|
| Belardinelli et al. (2005)47 | Italy |
100 mg of co-Q10 daily for 4 weeks Follow-up:a 8 weeks |
Placebo | NYHA classes II and III CHF | 21 |
LVEF Peak O2 consumption Adverse events |
Not registered; NR |
| Hofman-Bang et al. (1995)52 | Sweden; multicentre |
100 mg of co-Q10 daily for 3 months Follow-up:a 6 months |
Placebo | NYHA classes II and III CHF | 79 |
NYHA class LVEF Pro-BNP Mortality Adverse events HRQoL |
Not registered; NR |
| Kawashima et al. (2020)53 | Japan; single centre |
400 mg of co-Q10 daily for 3 months Follow-up:a 7 months, including a 1-month washout period between treatments |
Placebo | EF of ≤ 40% | 20 |
Pro-BNP Adverse events |
Not registered; none |
| Langsjoen et al. (1985)58 | USA; single centre |
33.33 mg of co-Q10 three times daily for 12 weeks Follow-up:a 28 weeks |
Placebo | NYHA classes III and IV with myocardial disease | 19 |
LVEF Adverse events |
Not registered; The Welch Foundation (Houston, TX, USA) |
| Mazzola et al. (1987)61 | Italy; single centre |
60 mg of co-Q10 daily for 4 weeks Follow-up:a 8 weeks |
Placebo | Mild–moderate heart failure and chronic stable effort angina | 20 |
NYHA class 6MWT |
Not registered; NR |
| Morisco et al. (1994)29 | Italy; single centre |
50 mg of co-Q10 three times daily for 4 weeks Follow-up:a 12 weeks |
Placebo | NYHA classes II–IV CHF | 6 | LVEF | Not registered; NR |
| Permanetter et al. (1992)65 | Germany; single centre |
33.3 mg of ubiquinone three times per day for 4 months Follow-up:a 8 months |
Placebo |
NYHA classes I–III Dilated cardiomyopathy |
25 |
NYHA class Adverse events |
Not registered; NR |
| Pogessi et al. (1991)66 | Italy; single centre |
50 mg of co-Q10 daily Follow-up:a 150 days, including a 30-day washout period between treatments |
Placebo |
NYHA classes II and III LVEF of 30–50% Dilated cardiomyopathy |
20 |
LVEF Adverse events |
Not registered; NR |
| Watson et al. (1999)68 | Australia; single centre |
33 mg of co-Q10 three times daily for 12 weeks Follow-up:a 25 weeks, including a 1-week washout period between treatments |
Placebo |
CHF LVEF of < 35% |
30 |
LVEF Adverse events HRQoL |
Not registered; Health World Limited (Brisbane, QLD, Australia) |
Most trials were conducted in high-income countries. Parallel-group trials were published between 1992 and 2017, and ranged in size from 16 to 641 participants. In general, the crossover trials were older and smaller than the parallel-group trials;52 the most recent was published in 200547 and the largest comprised only 79 participants. 52 Trials varied in their inclusion criteria, although most patients included had CHF of class NYHA II or III.
Most trials used co-Q10 alone as the intervention. One trial combined co-Q10 with selenium,51 one trial used co-Q10 as part of a broader multinutrient supplement,69 one trial combined co-Q10 with creatine50 and one trial combined co-Q10 with L-carnitine. 57 Dosage regimens of co-Q10 and treatment duration varied significantly across the studies. co-Q10 doses ranged from 32 mg to 400 mg daily, and treatment lasted from 4 weeks to 2 years. Further details of included studies are reported in Table 2.
Not all of the intended outcomes listed in the protocol were reported in trial publications. Tables 4 and 5 summarise which outcomes each trial reported and whether or not these could be included in meta-analyses. Furthermore, as data were reported in a variety of ways, not every trial that reported data on an outcome could be included in the corresponding meta-analysis. Some trials reported outcomes only within discussions from which analysable data could not be readily extracted, and some data were presented only in figures or just as p-values. Overall, 20 trials33,47,48,50–52,54–57,59,60,62–64,66–70 were included in at least one meta-analysis.
| Outcome data reported | Berman et al. (2004)48 | Davini et al. (1992)49 | Fumagalli et al. (2011)50 | Garakyaraghi et al. (2015)51 | Keogh et al. (2003)54 | Khatta et al. (2000)55 | Kukharchik et al. (2016)56 | Kumar et al. (2007)57 | Ma et al. (1996)59 | Mareev et al. (2017)60 | Morisco et al. (1993)62 | Mortensen et al. (2014)33 | Munkholm et al. (1999)63 | Nakanishi et al. (1988)64 | Pourmoghaddas et al. (2014)67 | Witte et al. (2005)69 | Zhao et al. (2015)70 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NYHA | |||||||||||||||||
| LVEF | |||||||||||||||||
| (Pro) BNP | |||||||||||||||||
| 6MWT | |||||||||||||||||
| Peak VO2 | |||||||||||||||||
| QoL | |||||||||||||||||
| Hospital admission | |||||||||||||||||
| ACM | |||||||||||||||||
| CHF mortality | |||||||||||||||||
| CVD mortality | |||||||||||||||||
| CVD morbidities | |||||||||||||||||
| Non-fatal CVD events | |||||||||||||||||
| Adverse events |
| Outcome data reported | Belardinelli et al. (2005)47 | Hofman-Bang et al. (1995)52 | Kawashima et al. (2020)53 | Langsjoen et al. (1985)58 | Mazzola et al. (1987)61 | Morisco et al. (1994)29 | Permanetter et al. (1992)65 | Pogessi et al. (1991)66 | Watson et al. (1999)68 |
|---|---|---|---|---|---|---|---|---|---|
| NYHA | |||||||||
| LVEF | |||||||||
| (Pro) BNP | |||||||||
| 6MWT | |||||||||
| Peak vol | |||||||||
| QoL | |||||||||
| Hospital admission | |||||||||
| ACM | |||||||||
| CHF mortality | |||||||||
| CVD mortality | |||||||||
| CVD morbidities | |||||||||
| Non-fatal CVD events | |||||||||
| Adverse events |
In general, parallel-group trials mostly reported ACM and key short-term functional outcomes (e.g. LVEF, NYHA and 6MWT). Morbidity outcomes (including hospitalisation) and data on specific causes of death were not widely reported.
Outcome data from crossover trials were very limited, with most reporting only LVEF as an outcome, and without sufficient detail for this to be included in meta-analyses. Two trials52,68 reported some QoL data in each crossover period. Consequently, few crossover trials contributed to meta-analyses.
Study quality and risk of bias
Tables 6 and 7 summarise the risk-of-bias assessment for parallel and crossover trials, respectively, with further details reported in Appendix 3, Tables 30–33.
| Study | Selection | Performance (blinding of participants and personnel) | Outcome assessment (blinding of outcome assessor) | Attrition | Outcomes reporting | |
|---|---|---|---|---|---|---|
| Berman et al. (2004)48 | ? | + | +a | ?a | ? | ? |
| Davini et al. (1992)49 | ? | – | – | ? | ? | |
| Fumagalli et al. (2011)50 | + | + | + | + | ? | |
| Garakyaraghi et al. (2015)51 | + | + | + | + | ? | |
| Keogh et al. (2003)54 | – | + | +b | ?b | + | ? |
| Khatta et al. (2000)55 | + | + | + | + | ? | |
| cKukharchik et al. (2016)56 | ? | + | +d | –d | ? | ? |
| Kumar et al. (2007)57 | + | + | + | + | ? | |
| Ma et al. (1996)59 | ? | + | + | + | ? | |
| cMareev et al. (2017)60 | ? | + | +e | ?e | ? | ? |
| Morisco et al. (1993)62 | + | + | + | + | ? | |
| Mortensen et al. (2014)33 | + | + | + | + | ? | |
| Munkholm et al. (1999)63 | – | + | ? | + | ? | |
| Nakanishi et al. (1988)64 | – | – | – | ? | ? | |
| Pourmoghaddas et al. (2014)67 | – | + | + | + | ? | |
| Witte et al. (2005)69 | + | + | + | + | + | |
| Zhao et al. (2015)70 | + | + | + | ? | ? | |
| Study | Selection | Performance (blinding participants and personnel) | Outcome assessment (blinding of outcome assessor) | Attrition | Outcomes reporting | Contamination (washout period before crossover) | |
|---|---|---|---|---|---|---|---|
| Belardinelli et al. (2005)47 | ? | + | ? | + | ? | – | |
| Hofman-Bang et al. (1995)52 | ? | + | +a | ?a | + | ? | – |
| Kawashima et al. (2020)53 | ? | + | ? | – | ? | – | |
| Langsjoen et al. (1985)58 | – | + | ? | ? | ? | – | |
| Mazzola et al. (1987)61 | ? | + | ? | ? | ? | – | |
| Morisco et al. (1994)29 | ? | + | ? | ? | ? | – | |
| Permanetter et al. (1992)65 | – | + | ? | + | ? | – | |
| Pogessi et al. (1991)66 | ? | + | ? | + | ? | + | |
| Watson et al. (1999)68 | ? | + | + | + | ? | + | |
Most studies were not sufficiently well reported to allow a full assessment of risk of bias. Only seven studies33,51,55,57,62,67,69 reported appropriate information on randomisation methods, and only four33,48,50,69 reported appropriate information on allocation concealment. Eight trials33,50,51,55,57,62,69,70 (all of which had a parallel design) were judged to be at low risk of participant selection bias. Six trials54,58,63–65,67 were considered to be at high risk of selection bias because of differences between trial arms in the numbers of participants with important prognostic characteristics at baseline, such as age (co-Q10 patients were on average 2 and 6 years younger than participants in the control arm in two parallel trials,58,63 and were nearly 4 years younger in a crossover trial),64 sex (there were 30% more male participants in the control group of one parallel trial,58 and differences of approximately 30% in the proportion of male patients between arms in two crossover trials). 63,65 Mean LVEF was approximately 8% lower in the co-Q10 arm at baseline in one parallel trial. 67 However, many of these trials were very small and so, by chance alone, baseline imbalances that may affect outcome estimates may have occurred, even with appropriate randomisation. 43 Although difficult to ascertain, it appears that the direction of bias in the two parallel trials64,65 that had significant baseline imbalances may have favoured co-Q10. Twelve trials29,47–49,52,53,56,59–61,66,68 were rated as having an unclear risk of selection bias because of insufficient information. Attempts to gain further information from study authors whose contact details we had been able to trace were unsuccessful. It was notable that many trial publications did not present information on baseline characteristics by treatment arm, which is a basic requirement of trial reporting.
Owing to the lack of a placebo control and the use of subjective outcomes, two trials49,56 were considered to be at high risk of performance bias and outcome assessor bias. All other studies were considered to be at low risk of bias for this domain. Lack of details regarding blinding of outcome assessors meant that 13 studies29,47,48,52–55,58,60,61,63,65,66 were at unclear risk of outcome assessment bias for at least one outcome.
Owing to a significant rate of attrition across study arms (30%), one study53 was considered at high risk of attrition bias. There was no clear evidence of bias associated with outcome reporting for any of the studies; however, because there were few registration records and no published protocols setting out planned outcomes, the risk of outcome reporting bias was unclear for almost all trials. Crossover trials were generally poorly reported, with six studies29,52,53,61,66,68 failing to clearly report outcomes for each randomised sequence at all specified follow-up points. Only two of the crossover trials66,68 reported a washout period (of 30 days) following the first treatment phase and, therefore, most crossover trial evidence was rated as being at a high risk of bias because of contamination from treatment before the treatment arm was switched.
Meta-analysis results
This section presents results across all studies for each outcome listed in Table 4. Crossover trials reported few data suitable for meta-analysis and are, therefore, excluded from the meta-analyses, except for analyses of LVEF and QoL. Additional meta-analysis results are presented in Appendix 5.
For the two trials55,69 that provided full IPD, summary data created from the provided IPD were included alongside data extracted from publications of other trials. As these two trials55,69 included a total of only 87 participants between them, no separate analysis of the IPD was conducted. The publications of these two trials55,69 included data on three patients who were excluded from the main analyses. Data for these excluded patients were not included in the supplied IPD and so to ensure robustness of the data only the suppled IPD were analysed, and excluded patients were not considered in the meta-analyses.
One trial71 of co-Q10 plus selenium in a general elderly population, which included a small proportion of patients with a LVEF of < 40% (7.5%), was excluded, as results for HFrEF patients were not reported separately (had we been able to obtain IPD from this trial, we would have been able to use the HFrEF subpopulation in IPD meta-analysis). The impact of adding this study, as a whole, to the review was explored in sensitivity analyses for those main outcomes for which suitable published data were available.
All-cause mortality
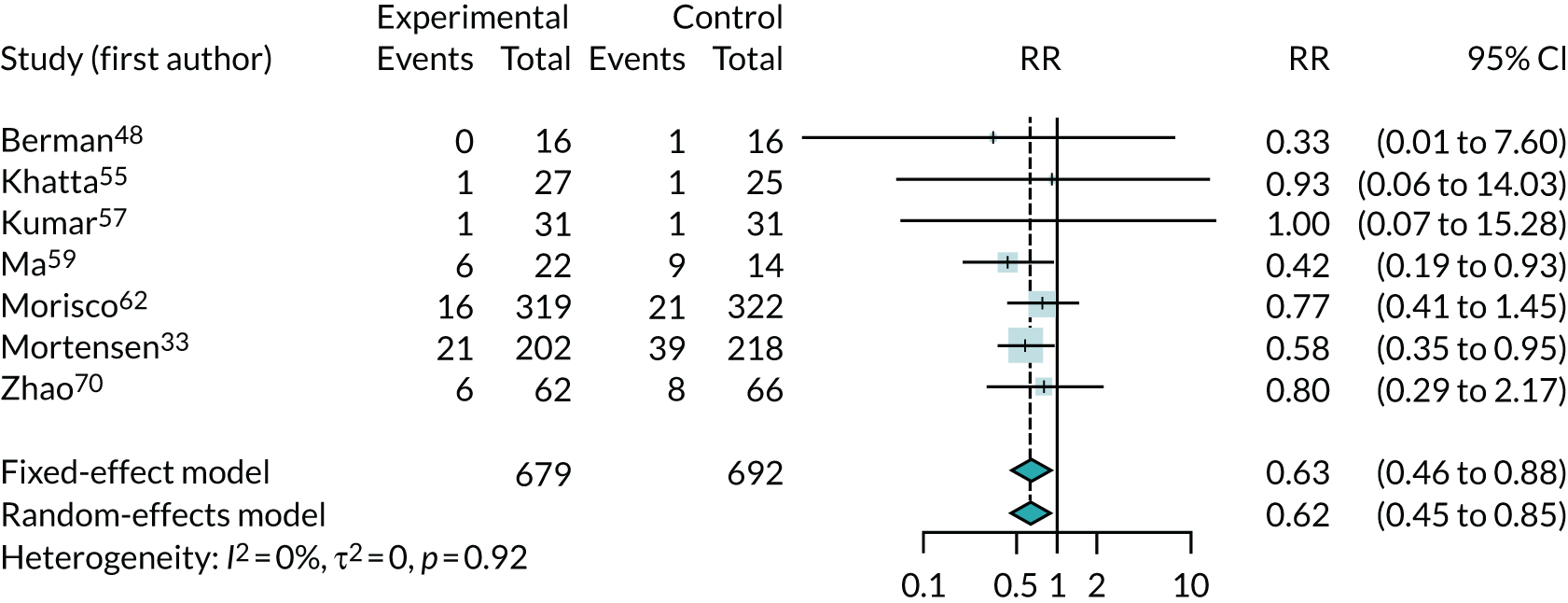
Eleven trials33,48,54,55,57,59,62,67,69,70 (involving 1589 randomised patients) reported data on ACM. Three of these trials54,67,69 reported/included no deaths and one crossover trial52 reported insufficient data for inclusion in the meta-analysis. Therefore, seven trials were included. 33,48,55,57,59,62,70
The one-stage meta-analysis of ACM, comparing co-Q10 with control, had a RR of 0.68 (95% CI 0.45 to 1.03), suggesting that co-Q10 reduced the risk of mortality, but this was not quite statistically significant. There was evidence of modest heterogeneity (τ2 = 0.23) across trials.
For comparison, Figure 2 shows the results of the standard DerSimonian–Laird random-effects meta-analysis for ACM. This found a substantial benefit of co-Q10, with a larger reduction in the risk of death and narrower CIs (RR 0.62, 95% CI 0.45 to 0.85). There was no evidence of any heterogeneity across trials (I2 = 0). This is broadly similar to the one-stage analysis. However, the one-stage analysis identified modest heterogeneity (τ2 = 0.23), which meant that the CI for the one-stage analysis results did, just, include the null value and also led to the slightly different RR estimate. The one-stage analysis was preferred because it is likely to be more robust, given the small number of deaths in several trials, and so its larger estimate of heterogeneity is more plausible.
FIGURE 2.
Meta-analysis of ACM.
A sensitivity analysis that added the Alehagen et al. trial71 to the meta-analysis slightly reduced the estimated treatment benefit (RR 0.67, 95% CI 0.51 to 0.87).
One crossover trial52 found no significant difference in deaths between treatment and placebo: four deaths occurred during the placebo and three deaths occurred during the co-Q10 treatment periods (total incidence = 8.9%). The trial52 did not report whether these events occurred before or after crossover and so was not included in the meta-analysis.
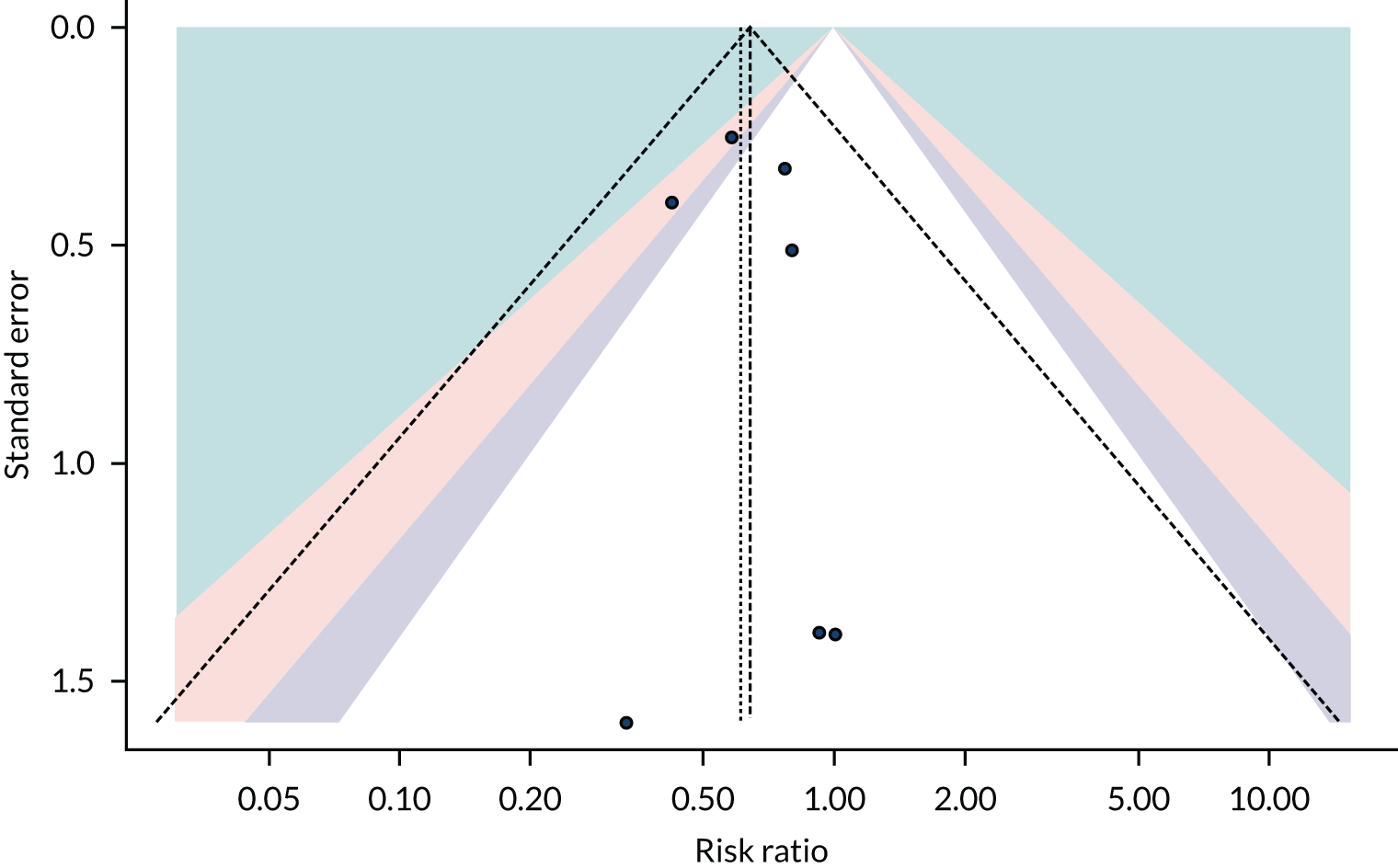
Figure 3 is a contour-enhanced funnel plot for ACM. The figure shows no indication of reporting bias or small-study effects. This was, however, based on limited numbers of trials.
FIGURE 3.
Funnel plot for ACM.
Cardiovascular mortality
Two trials33,55 (involving 472 participants) reported data on cardiovascular mortality, but the data were insufficient for meta-analysis.
The Q-SYMBIO trial33 found a substantial benefit of co-Q10, with a large reduction in cardiovascular mortality (RR 0.57, 95% CI 0.33 to 0.98). The Q-SYMBIO trial33 also reported a higher incidence of death from heart failure in the placebo arm (4.6%) than in the co-Q10 arm (0.5%) (RR 0.11, 95% CI 0.01 to 0.84). This appears to be consistent with the findings for ACM (see Figure 2).
Khatta et al. 55 recorded one death from heart failure in the placebo arm and no deaths in the co-Q10 arm.
The excluded Alehagen et al. trial71 also found a substantial benefit of co-Q10 for reducing cardiovascular mortality (RR 0.47, 95% CI 0.25 to 0.88).
Non-fatal cardiovascular events
No studies reported non-fatal cardiovascular events separately from cardiovascular disease-related deaths.
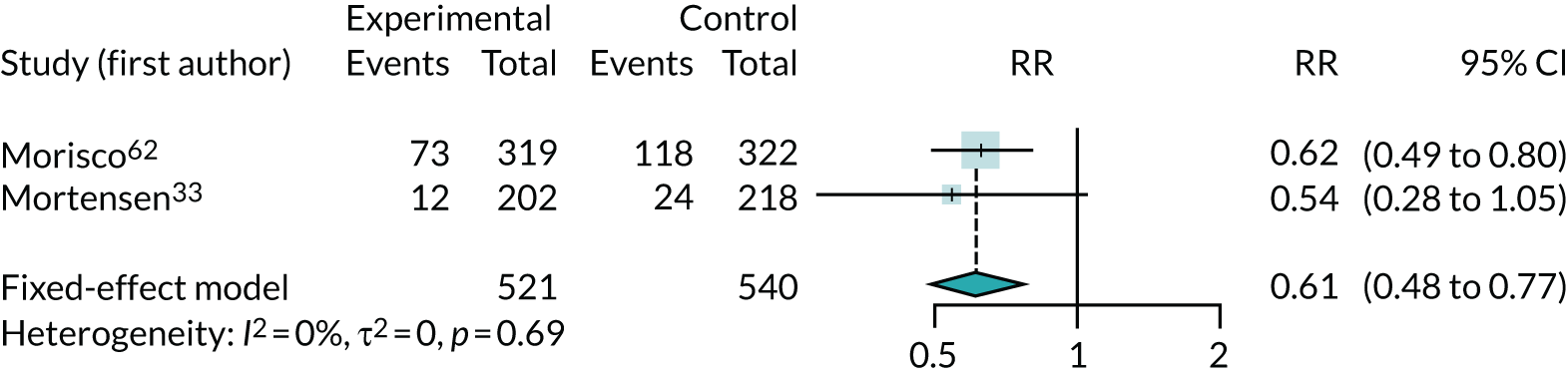
Hospitalisation (due to chronic heart failure)
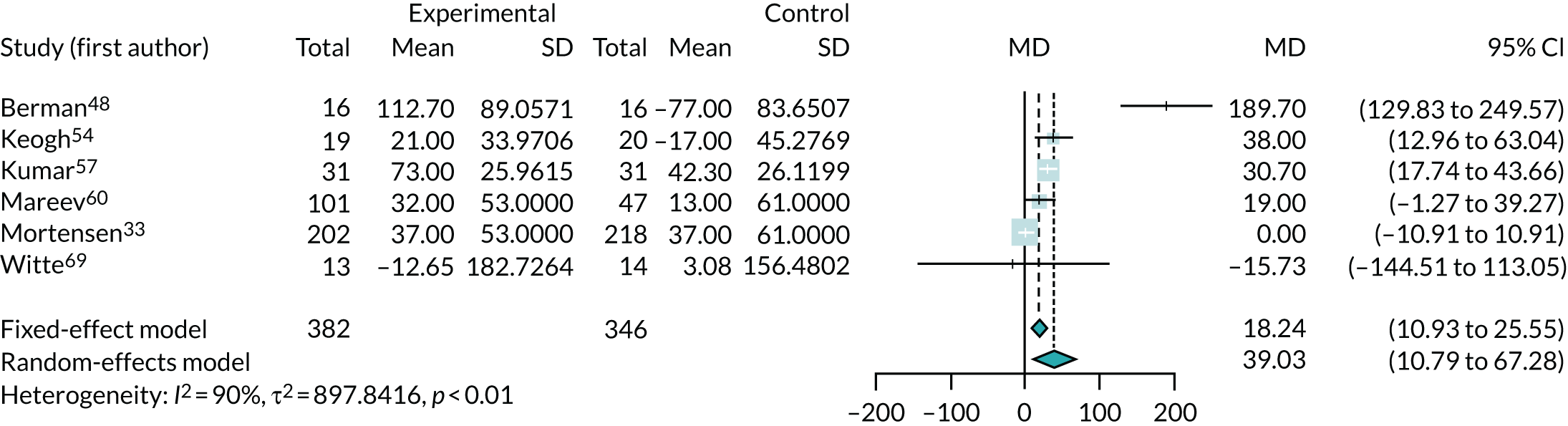
Three studies33,54,62 (involving 1100 participants) reported data on hospitalisation due to CHF, but one54 recorded no events. The meta-analysis of risk of hospitalisation for the remaining two studies33,62 showed a substantial benefit of co-Q10, with a large reduction in hospitalisation (RR 0.61, 95% CI 0.48 to 0.77) (Figure 4).
FIGURE 4.
Meta-analysis of hospitalisation due to CHF.
New York Heart Association functional class
Thirteen trials33,48,49,51,54,55,57,60,61,64,65,67,69 (involving 1038 participants) reported NYHA class as an outcome, of which seven33,48,51,54,64,67,69 provided sufficient data for meta-analysis. Two trials64,69 provided full IPD.
The NYHA is a symptoms scale that classifies patients depending on their limitations and symptoms (i.e. breathing, shortness of breath or angina pain) during physical activity. Classification ranges from I (no symptoms and no limitation in ordinary physical activity) to IV (severe limitations, symptomatic even at rest).
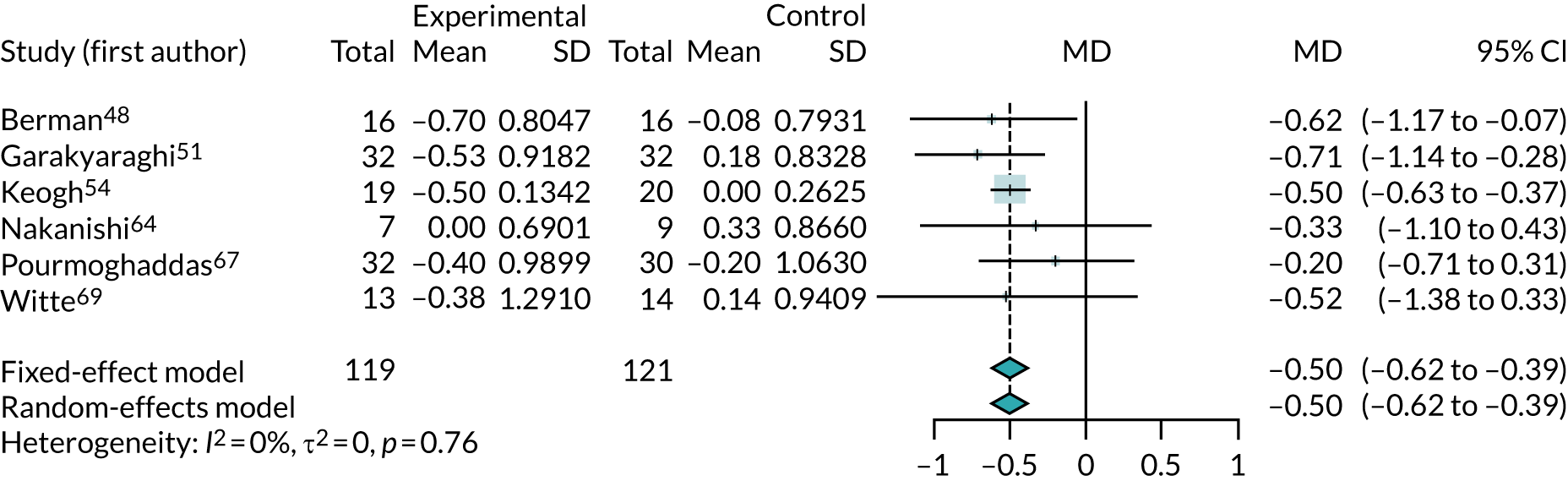
As NYHA is a categorical outcome, it would ideally be analysed as such. However, no trial reported data to permit this (i.e. trials did not provide the numbers of patients in each NYHA class). Trials mostly reported NYHA as a continuous variable (mean value in each trial arm) or in terms of numbers in NYHA class III or IV. Meta-analyses of these outcomes are presented here, but we note that these are not ideal representations of NYHA data.
Figure 5 shows the MD between arms in the change in NYHA class from baseline. This analysis treats the four-category NYHA as if it were a continuous variable and may not, therefore, be a reliable indicator of the effect of co-Q10. The results favour co-Q10, suggesting that it lowers NYHA class by approximately half of a class on average (MD 0.50, 95% CI 0.39 to 0.62). The results were homogeneous (I2 = 0).
FIGURE 5.
Meta-analysis of change from baseline in NYHA class.
Adding the excluded Alehagen et al. trial71 to this analysis gave a smaller treatment benefit (MD 0.37, 95% CI 0.07 to 0.67) and its addition resulted in substantial heterogeneity (I2 = 79%).
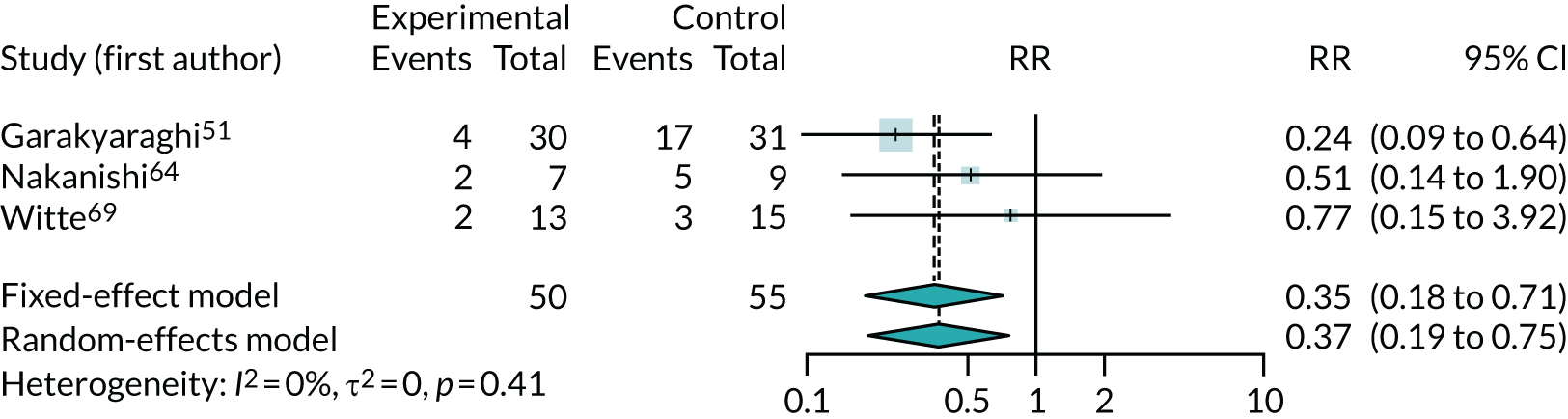
Figure 6 shows the RR of being in NYHA class III or IV after intervention. This meta-analysis could be biased if NYHA classes were not balanced across arms at the start of each trial; however, we did not find any evidence of such bias. The results suggest a large treatment benefit (RR 0.37, 95% CI 0.19 to 0.73; I2 = 0). This outcome was also analysed using a one-stage model. The RR of being in NYHA class III or IV was 0.37 (95% CI 0.17 to 0.81), which is almost identical to the two-stage results (see Figure 6), but with wider CIs.
FIGURE 6.
Meta-analysis of being in NYHA class III or IV after intervention.
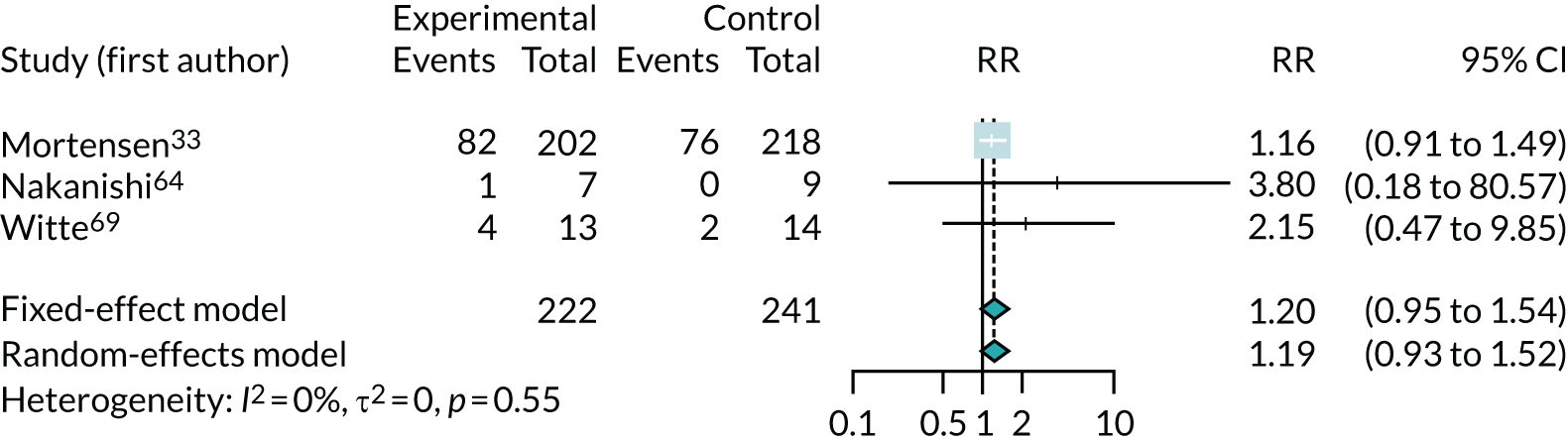
The results for improvement by one or more class in NYHA, which was reported in three trials,33,64,69 are shown in Figure 7. Note that, because this is measuring improvement, a RR of > 1 suggests a benefit from co-Q10 (greater improvement). This suggests that co-Q10 confers a modest but uncertain benefit, a finding dominated by the results of the Q-SYMBIO trial33 (RR 1.19, 95% CI 0.93 to 1.52). The results for deterioration of NYHA class (see Appendix 5, Figure 22) also found a possible modest, but highly uncertain, benefit from co-Q10 (RR 0.76, 95% CI 0.15 to 4.0). These results are based on very small numbers (just 14 patients out of 463 had a deterioration in NYHA class).
FIGURE 7.
Meta-analysis of improvement in NYHA class.
Four parallel trials49,55,57,60 could not be included in the meta-analyses because of insufficient data. Davini et al. 49 found that the percentage of patients with an improved NYHA class at 4 months was greater with co-Q10 than with placebo for patients with dilated cardiomyopathy (87% vs. 43%) and valvular disease (87% vs. 29%), but smaller for patients with ischaemic heart disease (5% vs. 27%). Khatta et al. 55 reported that only 1 of 23 patients in each of the co-Q10 and placebo arms had improved NYHA class (data from publication only; no further details reported). Kumar et al. 57 reported a greater reduction in the percentage of participants with NYHA II–IV in the co-Q10 arm (from 100% at baseline to 62% after 12 weeks) than in the control arm (from 100% to 86.2%). Mareev et al. 60 reported that the change in NYHA from baseline to 24 weeks was greater in the co-Q10 group (–0.16) than in the placebo group (–0.08).
Two crossover trials61,65 reported data on NYHA class. One trial61 reported an overall reduction in the percentage of NYHA class III patients (from 25% to 5%) at 8 weeks’ follow-up from baseline, but another trial65 found no statistically significant changes in mean NYHA functional class with co-Q10 compared with placebo at 4 months’ follow-up. Further details are reported in Appendix 4, Tables 34 and 35.
Left ventricular ejection fraction
Sixteen studies29,33,47,51,52,55,56,58,60,63,65–70 (involving 1318 participants) reported/recorded data on LVEF, but four studies29,52,58,65 did not report sufficient data to be included in any meta-analysis.
Figure 8 presents the results of the meta-analysis of the change in LVEF from baseline and shows the mean difference between the co-Q10 and control arms for each study. Substantial increases in ejection fraction percentage may indicate improved condition. These results suggest a modest benefit from co-Q10 (MD 1.76%, 95% CI 0.21% to 3.31%). The trials appear to be homogeneous (I2 = 0), although two trials have mean effects in the direction of harm. 55,63
FIGURE 8.
Meta-analysis of change from baseline in LVEF.
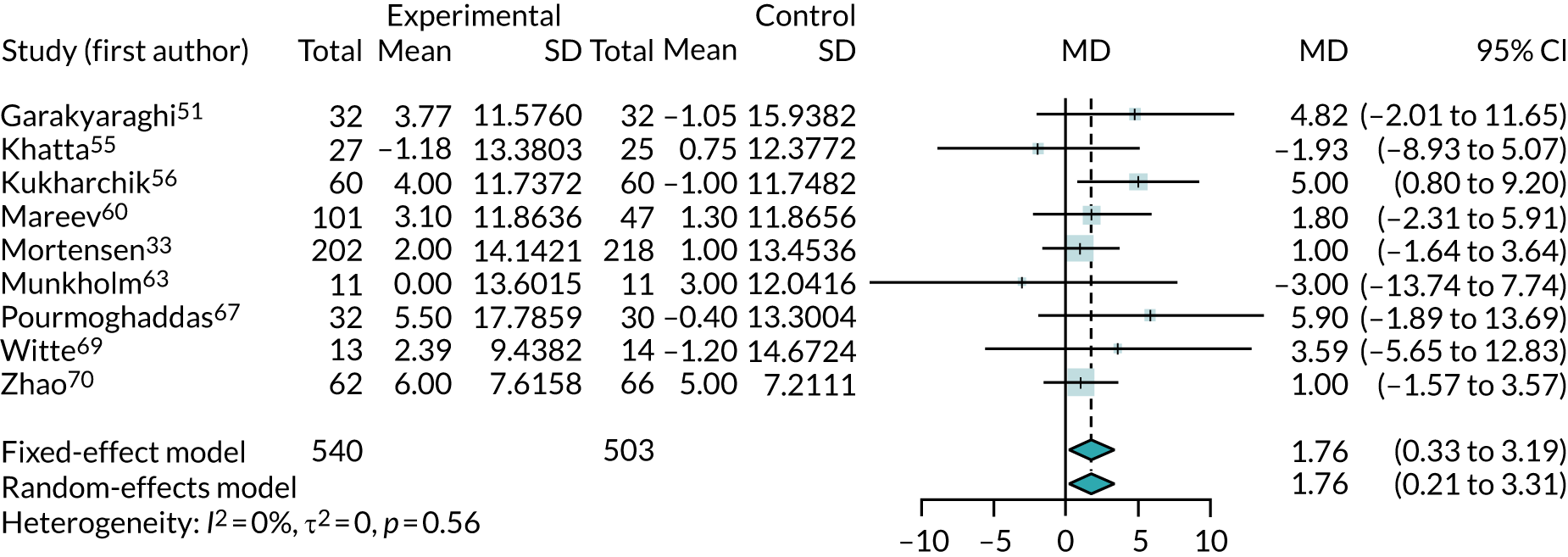
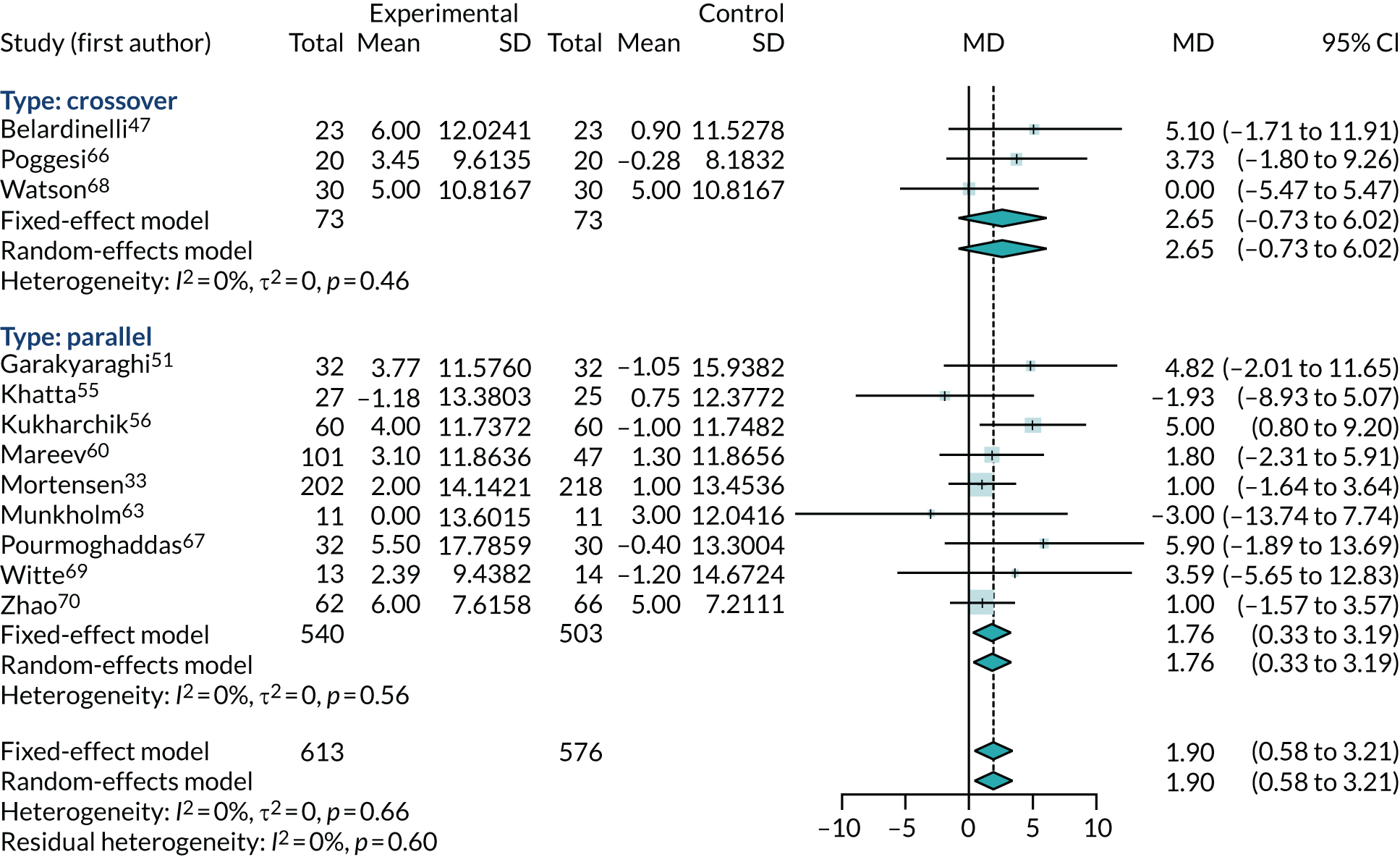
Three47,66,68 of the five crossover trials that reported LVEF supplied enough data to be incorporated into the meta-analysis. The results are shown in Figure 9. Although the crossover trials gave a slightly larger benefit from co-Q10 (MD 2.65%, 95% CI –0.73% to 6.02%), they were consistent with the parallel-group trials, with no evidence of heterogeneity. The meta-analysis combining all trials showed a modest benefit from co-Q10 (MD 1.90%, 95% CI 0.58% to 3.21%).
FIGURE 9.
Meta-analysis of change from baseline in ejection fraction percentage, including all trials.
Four studies29,52,58,65 could not be included in the meta-analysis because of insufficient data (i.e. a crossover trial with results grouped across randomised sequences)29,52,58,65 and unextractable figures. 58,65 Only one of these trials58 reported a significant improvement in LVEF from baseline that favoured co-Q10.
Of the remaining crossover trials that could not be included in the meta-analysis reported in Figure 9, two studies52,65 found no significant change from baseline to follow-up in percentage LVEF across all patients, one study52 reported a small statistically non-significant and clinically irrelevant difference (MD 0.5%, 95% CI –1% to 2%) and the other study65 did not provide any further details. One crossover trial58 found a significantly greater increase in LVEF with co-Q10 than with placebo (p < 0.0001; no further extractable details), whereas another trial29 reported improved mean percentage LVEF from baseline at 8 weeks’ follow-up across randomised sequences [from 27% (SD 11%) to 33% (SD 13%)], but did not report whether or not any differences had been observed between co-Q10 and placebo.
Figure 10 provides a contour-enhanced funnel plot for LVEF. The figure shows no indication of reporting bias or small-study effects. This was, however, based on limited numbers of trials.
FIGURE 10.
Funnel plots for LVEF change from baseline.

6-minute walk test
Six trials33,48,54,57,60,69 (involving 728 participants) reported sufficient data on 6MWT results to be included in a meta-analysis. Figure 11 presents the 6MWT results expressed as mean change from baseline in number of metres walked in 6 minutes, and meta-analyses the MD in this between co-Q10 and control arms. The results show a benefit from co-Q10 in increasing walking distance from baseline, but this is strongly influenced by the Berman et al. 48 trial, which is a substantial outlier. It is unclear why the Berman et al. 48 trial is so inconsistent with other trials. Meta-regression analyses [see Meta-regression (trial- and participant-level factors)] did not find any evidence that trial properties or baseline 6MWT values might be the cause of this inconsistency.
FIGURE 11.
Meta-analysis of 6MWT.
Removing the Berman et al. 48 trial produced a more modest benefit from co-Q10 (MD 19.89 metres, 95% CI 1.87 to 37.90 metres). However, the trials were still very heterogeneous (I2 = 76%), with the largest trial (the Q-SYMBIO trial73) showing no benefit from co-Q10.
Peak oxygen consumption
Three trials47,50,57 (including one crossover trial47) reported enough data for a meta-analysis of peak oxygen consumption (Figure 12). The results are expressed as the difference between mean change from baseline (ml/kg/minute) between co-Q10 and control arms. The meta-analysis showed no significant benefit from co-Q10 in improving peak oxygen consumption (MD 0.72 ml/kg/minute, 95% CI –0.60 to 2.05 ml/kg/minute).
FIGURE 12.
Meta-analysis of peak oxygen consumption.
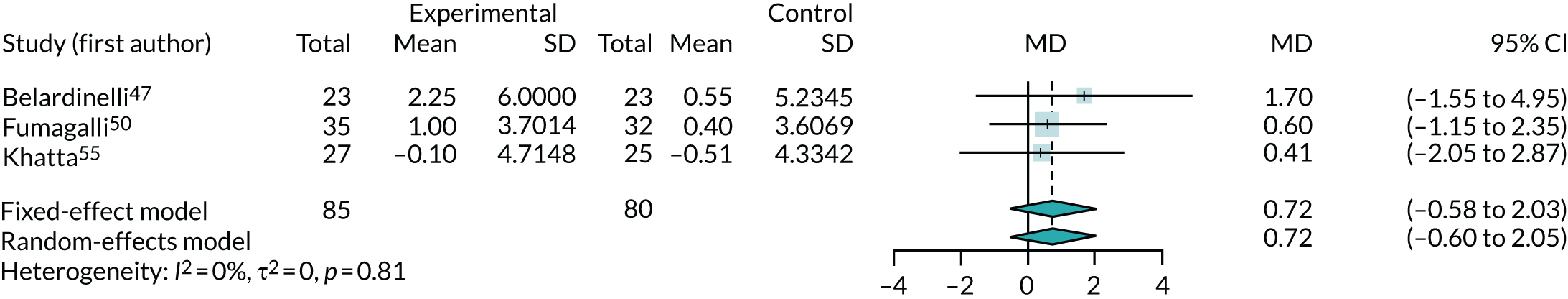
Removing the one crossover trial47 slightly reduced the observed effect (MD 0.54 ml/kg/minute, 95% CI –0.89 to 1.96 ml/kg/minute).
B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide
Three trials33,56,67 (involving 602 participants) reported data for NT-pro-BNP and two trials53,60 (involving 168 participants) reported data for BNP. Two of these trials53,56 could not be included in the meta-analyses because of insufficient data; one56 was a conference abstract and did not report sufficient statistical information, and the other53 reported data only as median values. The three other trials33,60,67 (involving 630 participants) were combined in a meta-analysis (Figure 13). As the analyses combined BNP and NT-pro-BNP, studies were pooled using SMDs. Earlier follow-up (4 months rather than 1 year) was used from one study33 to align it with the follow-up of the other trials included in the meta-analysis. Higher levels of BNP and NT-pro-BNP may indicate more severe CHF. Overall, there was no evidence that co-Q10 reduced BNP or NT-pro-BNP levels (SMD 0.56, 95% CI –0.5 to 1.62). The results were not statistically significant, were highly heterogeneous (I2 = 97%) and were unlikely to be of clinical relevance.
FIGURE 13.
Meta-analysis of BNP and pro-BNP.
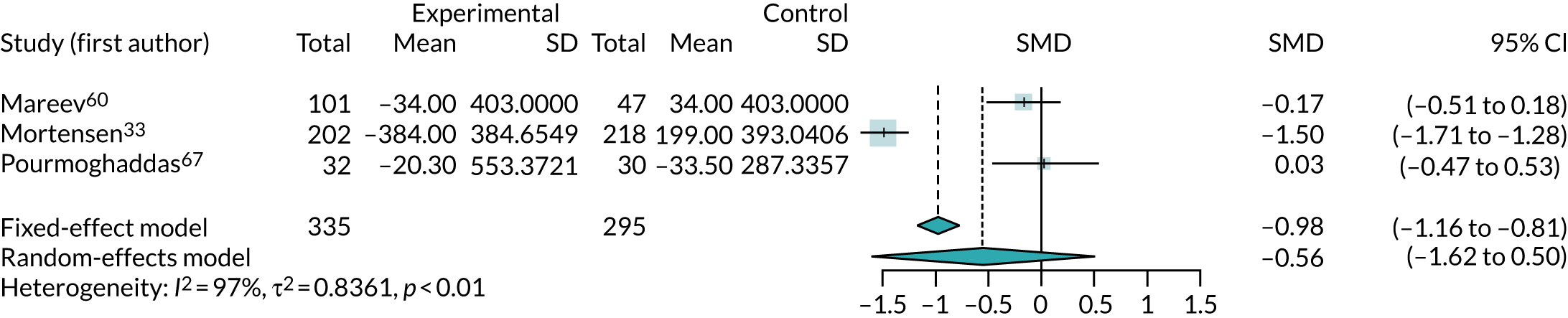
One trial,56 reported as a conference abstract, noted statistically significant changes in NT-pro-BNP levels from baseline in both co-Q10 and placebo groups. NT-pro-BNP in the co-Q10 arm was 490.7 pg/ml at baseline and 134.6 pg/ml (p < 0.05) at 3 months, although it is unclear whether these numbers were medians or means. In the placebo group, reported levels decreased significantly, from 701.3 (95% CI 271.4 to 1385.5) pg/ml at baseline to 230.8 (95% CI 178.9 to 443.4) pg/ml at follow-up (p < 0.05).
One crossover trial53 reported no significant change in median BNP levels with co-Q10 [117 pg/ml, interquartile range (IQR) 69–168 pg/ml pre co-Q10; 152 pg/ml, IQR 83–266 pg/ml post co-Q10 (p = 0.37)] or with placebo [91 pg/ml, IQR 55–165 pg/ml pre placebo; 137 pg/ml, IQR 48–291 pg/ml post placebo (p = 0.48)], and no significant difference in median BNP levels between co-Q10 and placebo.
Quality of life
Quality-of-life measures were reported in five studies50,52,60,68,69 (involving 460 participants), including three parallel-group50,60,69 and two crossover trials. 52,68 Of these trials, one parallel trial60 was reported only as conference abstract, with insufficient data for inclusion in the meta-analysis.
Different scales were used to assess QoL. Fumagalli et al. 50 used the Sickness Impact Profile, which ranges from 0% to 100%, where 0% represents a completely healthy patient and 100% represents a patient completely dependent on another person in all aspects of life. 74 Poggesi et al. 66 used the Minnesota Living With Heart Failure Questionnaire (MLWHFQ), which ranges from 0 to 105, with higher scores indicating more significant impairment in health-related quality of life (HRQoL). Witte et al. 69 applied the EuroQol Heart Failure Scale, which ranges from < 0 to 1, with higher scores indicating higher health utility. 75 Hofman-Bang et al. 52 used a tool developed by the trial centre,76 with higher scores indicating improved QoL. Finally, Mareev et al. 60 used the Scale for Heart failure to Optimise Clinical Status (SHOCS), which ranges from 0 to 20, with higher scores indicating worsening clinical condition.
As only two trials reported QoL at baseline, the meta-analysis of all four trials used QoL at the end of trial. SMDs were used to account for scales of different quality being used in each trial (Figure 14). Positive SMDs indicate improved QoL for participants receiving co-Q10, compared with control. The results suggest that there is no clear evidence of any effect of co-Q10 (SMD 0.10, 95% CI –0.12 to 0.33), with the overall effect size unlikely to be of clinical significance.
FIGURE 14.
Meta-analysis of QoL at the end of trial.
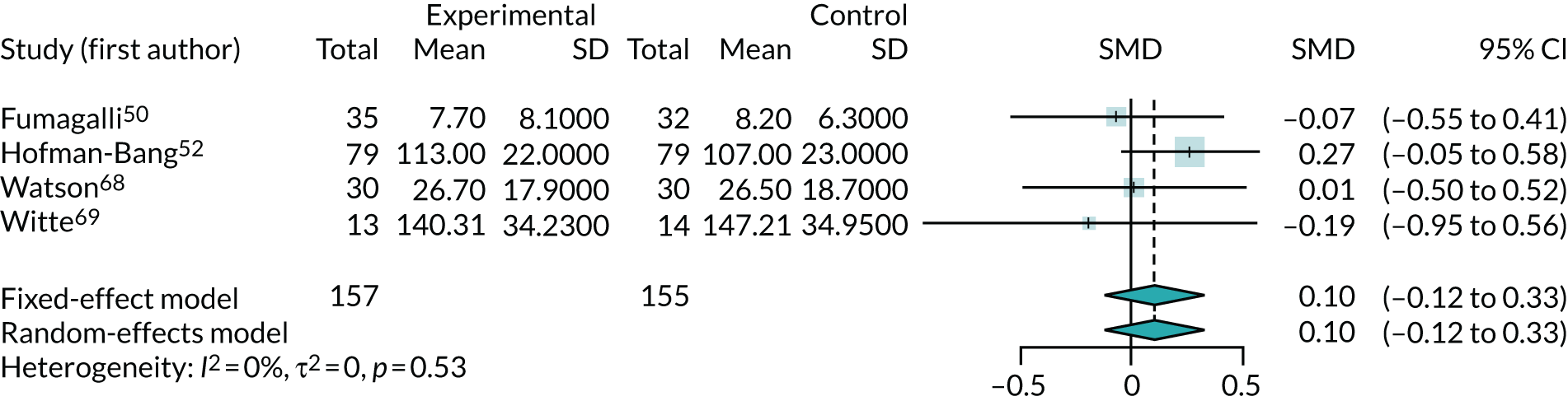
The trial by Mareev et al.,60 which was reported as a conference abstract, found that SHOCS score reduction from baseline to 24 weeks was greater in the co-Q10 group (–1.06) than in the placebo group (–0.53) (p = 0.036).
Adverse events
Twelve trials33,47,48,52–55,57,58,65,66,68 reported numbers of adverse events (of any type), of which six47,53,55,58,65,66 reported no events and two52,68 reported insufficient data for meta-analysis. Figure 15 shows the meta-analysis of the RR of any adverse event for the four remaining trials. 33,48,54,57 This figure provides no evidence of increased adverse events with co-Q10, that is the direction of effect is for reduced adverse events (RR 0.73, 95% CI 0.47 to 1.12), but with results driven largely by the Q-SYMBIO trial. 33 The most common adverse events reported in the Q-SYMBIO trial33 were gastrointestinal disturbances (2.4%), stroke (1.7%) and arrhythmia (1.7%). Cardiovascular procedures (i.e. percutaneous coronary intervention and coronary artery bypass grafting) were reported in 2.1% of participants, with no significant differences between the study arms.
FIGURE 15.
Meta-analysis of adverse events.
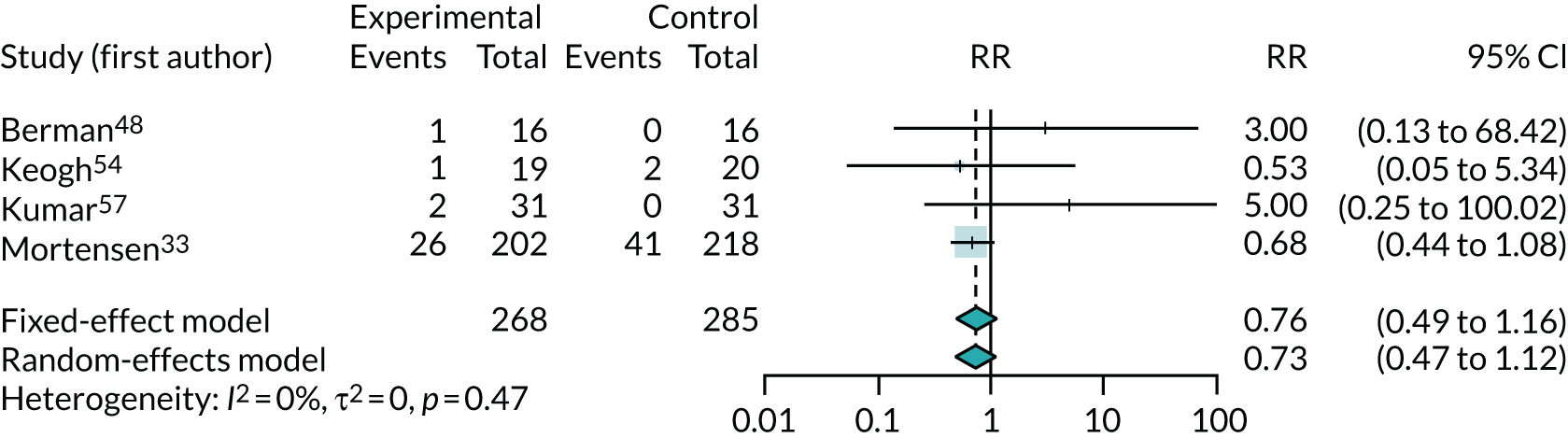
Three trials reported numbers of discontinuations due to adverse events and found no clear evidence of higher discontinuation with co-Q10 exposure (RR 1.54, 95% CI 0.32 to 7.47). However, this was based on just six events (see Appendix 5, Figure 23).
None of the trials that we were unable to include in the meta-analysis found a significant difference in adverse events between co-Q10 and control. One crossover trial52 reported 46 adverse events throughout the 6-month study period, with no significant differences between co-Q10 and placebo. Adverse events included gastrointestinal disturbances (12.7% of patients in each period), vertigo (7.6% in co-Q10 periods and 12.7% in placebo periods) and dry skin (7.6% in the co-Q10 periods and 5.1% in the placebo periods).
Two adverse events (in 20 patients) were reported in another crossover trial. 66 Epigastric burning and slight epigastralgia were recorded; however, the study did not detail whether these occurred during the co-Q10 period or the placebo period of the study.
The Alehagen et al. 71 trial, which was excluded because it included only a small proportion of co-Q10 patients, reported no significant difference in the percentage of patients with gastrointestinal symptoms or diarrhoea between the co-Q10 (4.1%) and placebo (3.2%) arms that led to study discontinuation (p = 0.60). There is no reason to expect that adverse effects would differ between CHF patients and the general elderly patients included in this trial.
Potential effect modifiers (subgroups and meta-regression)
Subgroups of trials (trial-level factors)
Where there were sufficient trials, subgroup analyses by intervention type [i.e. co-Q10 only, co-Q10 plus selenium, co-Q10 plus other micronutrient(s)] were performed. Given the limited data from crossover trials, all analyses were restricted to only parallel-group trials.
As there was only one trial of co-Q10 with selenium51 and only three multinutrient trials,50,57,69 subgroup analysis was possible only for ACM (see Appendix 5, Figure 24), LVEF (see Appendix 5, Figure 25) and change from baseline in NYHA class (see Appendix 5, Figure 26). In each analysis, there was only one or no selenium trials51 and only one multinutrient trial57,69 and so it is difficult to make formal comparisons. For LVEF, effect estimates were larger for the trials of co-Q10 with selenium and multinutrients, but both trials51,69 were small and the results were not visibly different from those of the co-Q10 only trials (I2 = 0). For ACM and NYHA class, the results varied across intervention types, but the data were very limited and heterogeneous.
No trials explicitly combined co-Q10 with statins and so this intended subgroup analysis could not be performed.
Meta-regression (trial- and participant-level factors)
Meta-regression was performed whenever at least five parallel-group trials reported both the required outcome and the regression factor. Factors considered were:
-
intended co-Q10 dose
-
intended duration of treatment
-
percentage of patients receiving statins
-
mean baseline value of outcome
-
year of publication.
Only ACM, LVEF, NYHA change from baseline and 6MWT had sufficient data for meta-regression. Table 8 summarises the meta-regression results, presenting the estimated regression parameter and the associated p-value for the regression.
| Outcome | Parameter | co-Q10 dose | Duration | Publication year | Percentage taking statins | Mean at baseline |
|---|---|---|---|---|---|---|
| ACM | Interaction (log-RR) | 0.000 | –0.011 | –0.001 | ||
| 95% CI | –0.002 to 0.002 | –0.038 to 0.015 | –0.022 to 0.02 | |||
| p-value | 0.982 | 0.439 | 0.932 | |||
| NYHA | Interaction (MD) | 0.003 | 0.020 | –0.001 | 0.010 | –0.203 |
| 95% CI | 0.001 to 0.005 | –0.103 to 0.144 | –0.028 to 0.026 | 0.003 to 0.017 | –0.939 to 0.534 | |
| p-value | 0.056 | 0.770 | 0.957 | 0.224 | 0.643 | |
| LVEF | Interaction (MD) | –0.002 | –0.175 | 0.231 | 0.015 | 0.102 |
| 95% CI | –0.015 to 0.01 | –0.532 to 0.183 | –0.076 to 0.538 | –0.055 to 0.084 | –0.032 to 0.235 | |
| p-value | 0.720 | 0.370 | 0.184 | 0.710 | 0.185 | |
| 6MWT | Interaction (MD) | –0.381 | –8.563 | –3.508 | –0.153 | |
| 95% CI | –0.718 to –0.044 | –32.6 to 15.5 | –8.022 to 1.007 | –0.855 to 0.549 | ||
| p-value | 0.091 | 0.524 | 0.202 | 0.698 |
There was no clear evidence that any parameter altered the effectiveness of co-Q10 for either ACM or LVEF. Most regression parameter estimates were near zero and p-values were large. In particular, there was no evidence that the effectiveness of co-Q10 varied with the proportion of patients taking statins.
There was some inconclusive evidence (p of < 0.1 but > 0.05) that the dose of co-Q10 affected 6MWT results; however, this was clinically counterintuitive (i.e. higher doses reduced effect) and was driven largely by one outlying trial. 48 Similarly, there was a suggestion that dose affected NYHA class results, but this was also clinically counterintuitive (i.e. reduced benefit with higher dose).
For ACM, the meta-regressions in Table 8 were supplemented with ‘one-stage’ regression models, regressing outcome against treatment and dose, treatment duration and publication year. In all of these, there was no evidence that any factor influenced the effectiveness of co-Q10, with all interaction estimates being very close to the null value of 1. There were insufficient data to perform one-stage meta-regressions for any other outcome.
Network meta-analysis
Network meta-analyses were performed for ACM, NYHA class and LVEF, as these were the outcomes with sufficient data on all interventions (i.e. co-Q10 alone, co-Q10 with selenium and multinutrients) to make analysis feasible.
All-cause mortality
The results for the Bayesian NMA of ACM are shown in Tables 9 and 10. Table 9 shows the odds ratios (ORs) for comparisons between interventions and Table 10 summarises the predicted rankings of interventions.
| Intervention | Comparator | OR | 95% CI |
|---|---|---|---|
| Co-Q10 only | Placebo | 0.54 | 0.23 to 1.10 |
| Multinutrient | Placebo | 1.04 | 0.02 to 41.67 |
| Multinutrient | Co-Q10 only | 1.91 | 0.03 to 84.01 |
| Outcome | Intervention | Mean | 95% CI |
|---|---|---|---|
| Probability of being best (%) | Placebo | 2.1 | |
| Co-Q10 only | 62.1 | ||
| Multinutrient | 35.8 | ||
| Ranking | Placebo | 2.451 | 2 to 3 |
| Co-Q10 only | 1.401 | 1 to 2 | |
| Multinutrient | 2.148 | 1 to 3 |
As for the main meta-analysis, the results suggest that co-Q10 on its own reduces ACM, compared with placebo (although the CI only just includes 1). The limited data for multinutrients mean that CIs are too wide to draw any conclusions on their effectiveness. There is no evidence of any difference between interventions, although CIs are wide. The results suggest that co-Q10 alone is the highest ranked and most effective intervention, and there is almost no chance that placebo is most effective.
New York Heart Association class
The results for the Bayesian NMA of change from baseline in NYHA class are shown in Tables 11 and 12. The results suggest that co-Q10 on its own and co-Q10 with selenium or multinutrient all give a modest improvement in NYHA class compared with placebo (although the CI always includes the null). There is no conclusive evidence of any difference between interventions.
| Intervention | Comparator | MD | 95% CI |
|---|---|---|---|
| Co-Q10 only | Placebo | 0.46 | –0.91 to 1.75 |
| Co-Q10 with selenium | Placebo | 0.70 | –2.53 to 3.82 |
| Multinutrient | Placebo | 0.53 | –3.22 to 4.25 |
| Co-Q10 with selenium | Co-Q10 only | 0.23 | –3.19 to 3.67 |
| Multinutrient | Co-Q10 only | 0.07 | –3.85 to 4.05 |
| Multinutrient | Co-Q10 with selenium | –0.16 | –5.05 to 4.66 |
| Outcome | Intervention | Mean | 95% CI |
|---|---|---|---|
| Probability of being best (%) | Placebo | 2.8 | |
| Co-Q10 only | 21.3 | ||
| Co-Q10 with selenium | 39.8 | ||
| Multinutrient | 36.0 | ||
| Ranking | Placebo | 3.1 | 1 to 4 |
| Co-Q10 only | 2.3 | 1 to 4 | |
| Co-Q10 with selenium | 2.2 | 1 to 4 | |
| Multinutrient | 2.4 | 1 to 4 |
Left ventricular ejection fraction
The results of the Bayesian NMA of LVEF are shown in Tables 13 and 14. As with the main meta-analysis, the results suggest that co-Q10 on its own improves LVEF compared with placebo (although the CI just includes the null). The limited data for the other interventions mean that CIs are too wide for any conclusions on their effectiveness to be drawn. However, both have larger estimates of benefit than co-Q10 alone. There is no conclusive evidence of any difference between interventions. The results favour co-Q10, with selenium being more effective than co-Q10 alone, but CIs are too wide for any conclusions to be drawn. The results, consequently, suggest that co-Q10 with selenium is the highest ranked and most effective intervention, followed by multinutrients, but both of these results are based on a single trial. There is almost no chance that placebo is most effective.
| Intervention | Comparator | MD | 95% CI |
|---|---|---|---|
| Co-Q10 only | Placebo | –1.87 | –4.17 to 0.38 |
| Co-Q10 with selenium | Placebo | –5.09 | –12.43 to 2.63 |
| Multinutrient | Placebo | –3.25 | –13.38 to 6.71 |
| Co-Q10 with selenium | Co-Q10 only | –3.22 | –11.01 to 4.90 |
| Multinutrient | Co-Q10 only | –1.36 | –11.68 to 8.75 |
| Multinutrient | Co-Q10 with selenium | 1.75 | –11.08 to 14.43 |
| Outcome | Intervention | Mean | 95% CI |
|---|---|---|---|
| Probability of being best (%) | Placebo | 0.1 | |
| Co-Q10 only | 8.1 | ||
| Co-Q10 with selenium | 56.1 | ||
| Multinutrient | 35.8 | ||
| Ranking | Placebo | 3.62 | 2 to 4 |
| Co-Q10 only | 2.45 | 1 to 4 | |
| Co-Q10 with selenium | 1.69 | 1 to 4 | |
| Multinutrient | 2.24 | 1 to 4 |
Meta-analysis of time-to-event data
Two trials59,73 reported data on time-to-event analyses, presenting HRs and Kaplan–Meier survival curves. One of these trials, the Q-SYMBIO trial, also reported a subanalysis of only patients recruited in Europe, in an additional publication. 73 Table 15 summarises the time-to-event data that were available in Kaplan–Meier curves. As the table shows, data were limited.
| Trial | Publication | Outcome | ||
|---|---|---|---|---|
| ACM | Cardiovascular mortality | Major adverse cardiovascular events | ||
| Ma et al. | Original59 | Yesa | No | No |
| Mortensen et al. (Q-SYMBIO) | Original33 | Yes | Yes | Yes |
| Europe subanalysis73 | Yes | No | Yes | |
Data were extracted from Kaplan–Meier curves using the method of Guyot. 77 In one case (see Table 15), no ‘numbers at risk’ table was reported and so we assumed that there were no censored patients. This might lead to bias if there was imbalance in censoring between arms and will also reduce the width of all CIs.
We used the extracted data to fit Cox proportional hazards models to each outcome in each publication. The results are shown in Figure 16. These show substantial benefits of co-Q10 in both trials for all outcomes, although all CIs are wide.
FIGURE 16.
Hazard ratios estimated from Kaplan–Meier data, with 95% CIs.
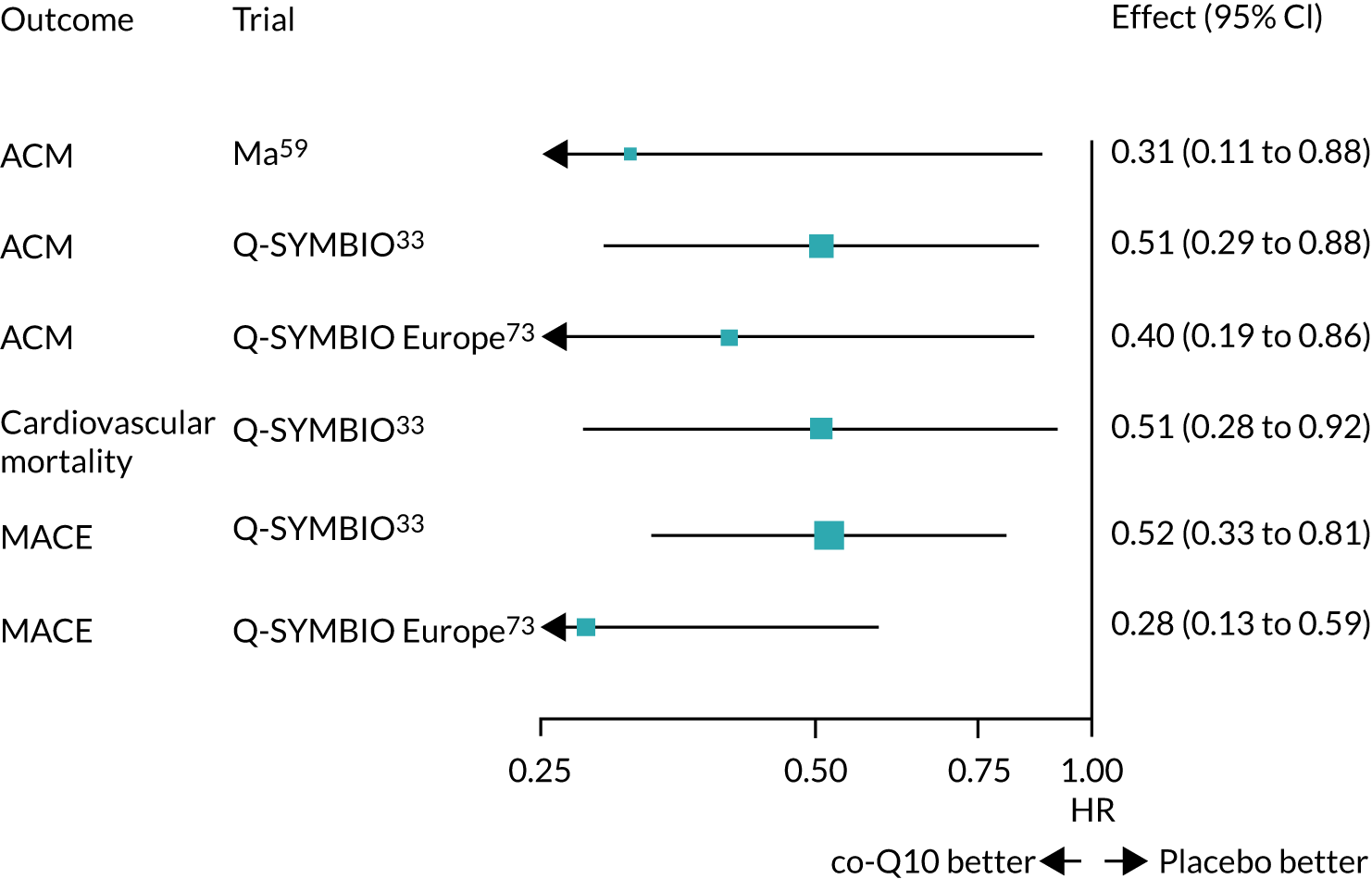
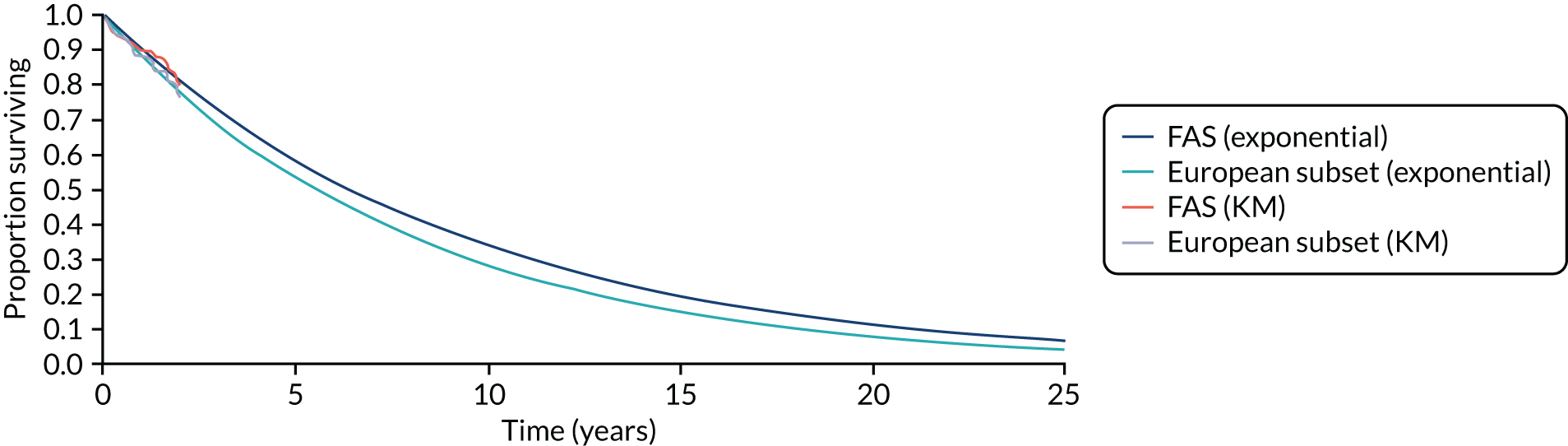
All analyses showed clear benefits from co-Q10. We note that the analyses using the Europe-only data from the Q-SYMBIO trial produced more extreme HRs than using all Q-SYMBIO trial data. 73
Summary
Table 16 summarises the results of the meta-analyses. In general, most outcomes suggested that co-Q10 was beneficial, with most being statistically significant and having little or no observable heterogeneity. Most analyses, however, were based on limited evidence and few trials. Only three outcomes were reported in five or more trials.
| Outcome | Number of trials | Number of participants | Effect estimate (RR/MD/SMD) | 95% CI | I 2 | τ2 |
|---|---|---|---|---|---|---|
| ACM (RR) | 7 | 1371 | 0.68a | 0.45 to 1.03 | 0 | 0.23 |
| Hospitalisation (RR) | 2 | 1061 | 0.61 | 0.49 to 0.77 | 0 | 0 |
| NYHA (MD in change from baseline) | 6 | 240 | 0.5 | 0.39 to 0.62 | 0 | 0 |
| LVEF (MD in change from baseline) | 9 | 1043 | 1.76 | 0.21 to 3.31 | 0 | 0 |
| 6MWT (MD in change from baseline) | 6 | 728 | 39.03 | 10.79 to 67.28 | 90 | 898 |
| Peak oxygen consumption (MD in change from baseline) | 3 | 165 | 0.72 | –0.60 to 2.05 | 0 | 0 |
| Pro-BNP or BNP (SMD in change from baseline) | 3 | 630 | 0.56 | –0.50 to 1.62 | 97 | 0.84 |
| QoL (SMD at end of follow-up) | 4 | 312 | 0.10 | –0.12 to 0.33 | 0 | 0 |
| Adverse events (RR) | 4 | 553 | 0.73 | 0.47 to 1.12 | 0 | 0 |
There was no evidence that administering co-Q10 with selenium or as part of a multinutrient modified the effectiveness of co-Q10, but there were few trials to inform this analysis.
There was no consistent evidence to suggest that co-Q10 dosage, duration of treatment, statin use or baseline values of parameters modified the effectiveness of co-Q10, but these analyses were restricted to meta-regressions using summary trial data, which lack power to detect effects.
Chapter 5 Systematic review of existing cost-effectiveness evidence
Two systematic searches were undertaken to identify existing cost-effectiveness analyses of co-Q10 and evidence for interventions used to treat CHF.
The specific aims of the review were to identify the key issues and areas of uncertainty in any existing decision-analytic models of co-Q10 and to identify any potentially relevant data sources in CHF that could be used in the development of a new decision-analytic model to assessing the cost-effectiveness of co-Q10.
Cost-effectiveness of coenzyme Q10 based on existing evidence
Methods
A broad range of types of economic evaluations were eligible for inclusion in the assessment of cost-effectiveness of co-Q10, including those that considered both costs and consequences (including cost-effectiveness, cost–utility or cost–benefit analyses) and those that considered only costs. MEDLINE, EMBASE and EconLit were searched on 13 May 2020, with no restriction on dates or language.
Full details of the search strategy used are given in Appendix 6.
Two reviewers independently assessed all obtained titles and abstracts for inclusion. Any discrepancies were resolved by discussion and consultation with a third reviewer. It was expected that all studies meeting the inclusion criteria would be summarised and used as the basis for identifying major structural issues, and the assumptions and key drivers of the cost-effectiveness of co-Q10.
Results
The systematic literature search identified 81 references (69 after deduplication), none of which met the inclusion criteria for the cost-effectiveness review of co-Q10 [i.e. none of the studies undertook an assessment of the cost-effectiveness of co-Q10 against any comparator(s)].
Cost-effectiveness of interventions for chronic heart failure
The objective of the second search was to identify studies of the cost-effectiveness of CHF interventions so that an overview of previous approaches to modelling the clinical pathway for CHF could be provided.
An existing review of cost-effectiveness studies of CHF was used as a starting point and supplemented with a new updated search. The existing review was conducted up to December 2017 to inform NICE guideline NG10678 (September 2018) for the diagnosis and management of CHF in adults. Our updated search was conducted to retrieve references published between 2010 and 2020 so that we could identify any studies that had been omitted from the existing search that would not have met the inclusion criteria in NG106 and to identify studies that had been published since the guideline was developed. 78
The following sections provide an overview of the review used in NG106, followed by the methods and results of the updated review and an assessment of identified studies that are potentially relevant for informing modelling approaches to CHF. The studies in the reviews that were considered relevant to our decision problem were reviewed in full, and the findings were used in conjunction with guidance from clinical experts to inform the development of a new decision-analytic model to assessing the cost-effectiveness of co-Q10 in CHF.
Overview of NICE guideline NG106
A total of 26 review questions were identified in the development of the NICE guideline. 78 Six questions were related to the diagnosis of CHF (three of which included elements of cost-effectiveness), 16 were related to interventions for the treatment of CHF (13 of which included elements of cost-effectiveness) and four were prognostic and qualitative questions relating to CHF.
The evidence used to inform the review questions in NG106 was identified by conducting a broad search relating to heart failure in the NHS Economic Evaluation Database (NHS EED) and the Health Technology Assessment database with no date restrictions, and of MEDLINE and EMBASE using a health economic filter, from September 2009. The general heart failure economic search was updated by the Guideline Group on 6 December 2017.
The review excluded conference abstracts, literature reviews, posters, letters, editorials, comment articles, unpublished studies and studies not in English. Studies published before 2001, or those published after 2001 but using unit costs and resource data from before 2001, were excluded. Studies from non-Organisation for Economic Co-operation and Development countries or from the USA were excluded, as were non-comparative cost analyses, including cost-of-illness studies.
Thirteen studies relating to the 13 review questions pertaining to interventions for the treatment of CHF that included elements of cost-effectiveness were identified and included in the final review. A summary of these is presented in Appendix 7, Table 36. Of those identified, three of the cost-effectiveness studies had taken a UK perspective and modelled a lifetime time horizon for CHF, and were considered relevant to standard practice for CHF. 79–81 NICE guideline NG106 also presented new health economic analyses in selected areas. 82
Methods for updating the review
A broad range of studies were considered for inclusion in the updated review of relevant cost-effectiveness evidence for interventions in CHF, including those conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that compared two or more options and considered both costs and consequences (including cost-effectiveness, cost–utility or cost–benefit analyses) were included.
MEDLINE was searched on 13 May 2020, with no restrictions on the type of intervention. The search was restricted to journal papers, and conference abstracts were excluded. Full details of the search strategy are given in Appendix 6. Only English-language papers from 2010 onwards were considered for inclusion.
Two reviewers independently assessed all obtained titles and abstracts for inclusion. Any discrepancies were resolved by discussion and consultation with a third reviewer. All studies that met the inclusion criteria were summarised and used as the basis for identifying major structural issues, assumptions and key drivers of cost-effectiveness when modelling CHF.
Results
The literature search identified 3330 potentially relevant publications. Six studies7,81,83–86 from an NHS perspective, published since 2015, met the inclusion criteria for the cost-effectiveness review. Because more applicable and recent evidence was available to inform the decision problem, studies published before 2015 were excluded.
Appendix 8 provides a description of the cost-effectiveness evidence from these six main studies7,81,83–86 and an assessment of the relevance of the data from the perspective of the NHS. A summary of the cost-effectiveness results for standard care, against which a new decision-analytic model could be validated, is given below.
Summary
The systematic review of existing cost-effectiveness evidence for co-Q10 did not identify any previous economic studies. The review of existing cost-effectiveness evidence for interventions in CHF identified six potentially useful studies7,81,83–86 to help inform the development of a new decision-analytic model to assess the cost-effectiveness of co-Q10 in CHF. Based on these six studies,7,81,83–86 the key characteristics of previous models in CHF can be summarised as follows:
-
All of the identified decision-analytic models of CHF used two health states, ‘alive’ and ‘dead’, to represent the long-term prognosis of patients. Parametric survival analysis was used to estimate mortality, whereas hospitalisations were modelled as an event within the alive health state.
-
The assumptions about the standard of care arm in the identified studies provide a useful basis for informing the baseline risk of mortality and hospitalisation in the absence of treatment.
-
The approach to modelling mortality rates varied across the studies. Some studies focused on estimating ACM rates, whereas others focused on cardiovascular mortality and used UK life tables to estimate other causes of mortality.
-
The survival models were assumed to follow an exponential or a Gompertz distribution.
-
The sources of data used to inform mortality rates were predominantly RCTs or UK-specific observational data sets.
-
The approach to modelling hospitalisation events varied across the studies, but the majority focused on all-cause hospitalisation rates, with some focused on cardiovascular hospitalisation rates only.
-
Some of the studies chose to stratify patients by NYHA class and modelled the change in NYHA class over time.
Chapter 6 Economic analysis: methods
Overview
The review of cost-effectiveness studies identified no previous economic studies examining the cost-effectiveness of co-Q10 compared with that any comparator(s) in CHF. Therefore, a new decision-analytic model was developed to assess the cost-effectiveness of adding co-Q10 to the current standard care, compared with standard care alone, in patients with HFrEF, from the perspective of the NHS.
In developing and populating the decision model, three main issues were considered central to the approach and methods employed:
-
the appropriate estimation of the baseline risk of mortality and hospitalisation rates in the absence of the intervention
-
the requirement to extrapolate outcomes beyond the time horizon of the RCTs included in the meta-analysis, and to ensure that all differences in costs, life-years gained and quality-adjusted life-years (QALYs) were appropriately quantified over a lifetime horizon
-
the need to ensure that the data inputs and modelling assumptions were relevant to informing current NHS practice.
The decision-analytic model, which was developed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), provides a framework for combining data on the clinical effectiveness of co-Q10, assumptions concerning the duration of treatment effect and the long-term impact of the intervention on mortality, hospitalisation events, QoL and other inputs that reflect current NHS practice. The model took an NHS and Personal Social Services perspective. Costs are presented in GBP at a 2019/20 price base and outcomes are presented as QALYs. Both costs and outcomes were discounted at a 3.5% annual rate in accordance with current NICE recommendations for technology appraisals. 87 Clinical advisors provided feedback on the model structure, data inputs and assumptions.
To capture uncertainty in the cost-effectiveness results, Monte Carlo simulation was used to propagate uncertainty in input parameters through the model, which were entered as probability distributions. The probabilistic analysis allows for the quantification of the expected consequences associated with the uncertainty surrounding the model results, and can be used to identify priorities for future research. In addition, scenario analyses were undertaken to explore the robustness of the cost-effectiveness results to changes in the parameter inputs and model assumptions.
The following sections outline the decision problem and the structure of the model, and an overview of the key modelling assumptions and data used to populate it. The economic analysis is reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 88
Population
The primary economic analysis considered all patients with symptomatic CHF due to left ventricular systolic dysfunction (LVSD) (i.e. HFrEF), with no further restrictions on disease severity (e.g. by NYHA class or LVEF). co-Q10 is not classed as a drug and, unlike most pharmaceutical agents that are typically considered in cost-effectiveness analysis, does not have a marketing authorisation. It is currently available as a supplement to purchase ‘over the counter’ for anyone to take. Co-Q10 has no known contraindications and, in practice, it is likely to be taken by those with ongoing symptoms of CHF, despite optimally tolerated routine therapy.
The baseline characteristics of the modelled population were informed by the characteristics of patients recruited to the ‘West Yorkshire’ data set (Table 17), a prospective observational cohort study of 1802 patients with CHF who attended one of four specialist physician-led cardiology outpatient clinics in NHS tertiary or district hospitals in West Yorkshire, UK, between 2006 and 2014. 89–91 The patients in this data set were assumed to be representative of a UK heart failure population suitable for co-Q10 and were used to inform the baseline risk of ACM and cardiovascular disease-related hospitalisation events (see Baseline event rates for mortality and Baseline event rates for hospitalisation), as well as background medical resource use (see Standard care). An entry criterion for the ‘West Yorkshire’ study was HFrEF with a LVEF of < 45. A small proportion of patients were subsequently reassessed and were found to have LVEF of > 45, although none of these patients was classified as being HFpEF. The majority of patients were in NYHA class II (50.67%), the patients’ mean age was 69.62 years and 73.20% of the cohort was male. Based on the knowledge of the clinical experts, patients had not used co-Q10 at any point during the study period.
| Characteristic | All patients (n = 1802) | LVEF of < 45 (n = 1573) | LVEF of < 45 and NYHA II–IV (n = 1287) | LVEF of < 45 and NYHA III and IV (n = 497) |
|---|---|---|---|---|
| Ejection fraction of < 45 (%) | 89.53 | 100.00 | 100.00 | 100.00 |
| Mean ejection fraction (%) | 31.96 | 30.39 | 30.18 | 29.22 |
| NYHA class (%) | ||||
| I | 18.50 | 18.08 | 0.00 | 0.00 |
| II | 50.67 | 50.29 | 61.38 | 0.00 |
| III | 29.67 | 30.30 | 36.99 | 95.77 |
| IV | 1.17 | 1.34 | 1.63 | 4.23 |
| Age (years), mean | 69.62 | 69.37 | 70.57 | 72.23 |
| Gender (male) (%) | 73.20 | 72.98 | 71.48 | 70.02 |
| Ischaemic aetiology (%) | 59.21 | 58.80 | 60.76 | 66.20 |
| History of diabetes (%) | 27.97 | 27.65 | 30.07 | 33.20 |
| History of COPD (%) | 15.76 | 15.70 | 18.18 | 20.32 |
| eGFR (ml/kg/1.73 m2), mean | 57.75 | 57.93 | 56.38 | 52.65 |
| Haemoglobin (g/dl), mean | 13.46 | 13.47 | 13.36 | 13.21 |
| Lymphocyte count (×106/ml), mean | 22.60 | 22.54 | 21.99 | 21.03 |
| IMD quintile (%) | ||||
| Rank 1 (most deprived) | 30.02 | 29.94 | 30.15 | 33.00 |
| Rank 5 (least deprived) | 13.82 | 14.37 | 13.83 | 10.46 |
| Atrial fibrillation (%) | 35.67 | 34.79 | 35.67 | 39.69 |
| ICD implanted (%) | 11.65 | 12.33 | 12.67 | 12.68 |
| CRT implanted (%) | 25.25 | 26.64 | 28.90 | 35.01 |
| Systolic blood pressure (mmHg), mean | 122.42 | 121.56 | 120.88 | 120.51 |
| Diastolic blood pressure (mmHg), mean | 71.46 | 71.25 | 70.70 | 70.14 |
| Heart rate (b.p.m.), mean | 75.32 | 75.69 | 76.09 | 76.70 |
| Prior hospitalisation (in year prior to recruitment) (%) | 45.92 | 47.30 | 47.14 | 43.44 |
Subgroup analysis
Heterogeneity in the cost-effectiveness of co-Q10 was investigated in three subgroup populations with different levels of disease severity:
-
heart failure patients with a LVEF of < 45
-
heart failure patients with a LVEF of < 45 and NYHA classes II–IV
-
heart failure patients with a LVEF of < 45 and NYHA classes III and IV.
Intervention strategies
The decision model explored the addition of co-Q10 to current standards of care for patients with CHF, compared with standard care alone. Therefore, the model evaluated two treatment strategies:
-
treatment with co-Q10 as an adjunct to standard therapy
-
treatment with standard therapy alone.
The dosing regimen for co-Q10 varied considerably across the clinical trials included in our systematic review and meta-analysis, ranging from 30 mg to 400 mg daily. 53,70 The Q-SYMBIO trial,33 the largest trial included in the meta-analysis, used a dose of 100 mg three times per day to maximise the serum level of co-Q10. Our clinical experts agreed that this dosing was suitable for the model.
In projecting the treatment pathway over the lifetime of patients, assumptions needed to be made about the duration of treatment and the duration of the effect of treatment. In the base-case analysis, treatment with co-Q10 was assumed to be lifelong, given that it has no known associated contraindications or side effects, and it may lower the risk of mortality. However, a range of additional scenarios were also explored to examine the robustness of alternative assumptions, including the impact of different treatment durations.
Background medication for patients receiving current standard care was estimated from resource use in the ‘West Yorkshire’ data set (Table 18). 89–91
| Medication | Proportion (%) | Details |
|---|---|---|
| ACE inhibitors | 90.49 | Equivalent daily dose to ramipril (licensed maximum dose of 10 mg) |
| Beta-blockers | 84.71 | Equivalent daily dose of bisoprolol (licensed maximum dose of 10 mg) |
| Mineralocorticoid receptor antagonists | 38.32 | Assumed to be 25 mg of spironolactone |
| Loop diuretic | 74.57 | Equivalent daily dose of furosemide |
Model structure
A Markov model was developed to estimate the clinical outcomes, costs and QALYs for two cohorts of patients with CHF with a reduced ejection fraction treated with (1) co-Q10 in addition to standard care and (2) standard care alone (Figure 17). In a Markov model, patients move between discrete health states over time based on the treatment pathway and their clinical progression. The systematic review of economic evaluations of CHF treatments found that the health states most frequently employed in previous models were ‘alive’ and ‘dead’, with hospitalisation events considered for those in the ‘alive’ state. Therefore, in line with previous models, we used two health states (i.e. ‘alive’ and ‘dead’) to estimate the long-term costs and benefits over the population’s lifetime. During each model cycle, the probability of death was calculated based on the cohort’s baseline characteristics and the time since they had the model. Patients accrued QALYs and health-care costs according to their hospitalisation and treatment status.
FIGURE 17.
Structure of the decision model.
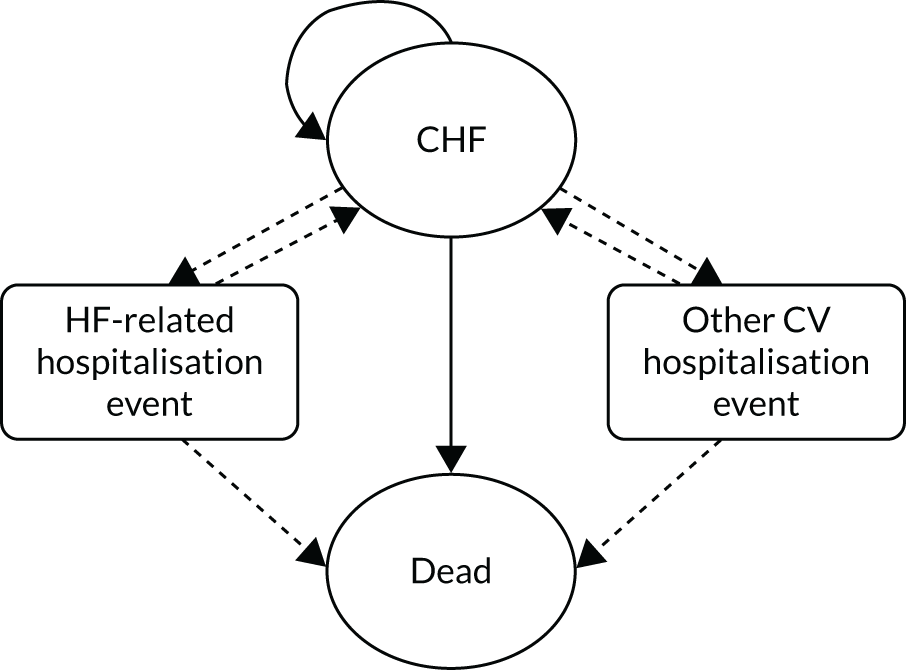
The primary events of interest were hospitalisations for major cardiovascular events and ACM. Owing to an absence of data, the baseline risk and the treatment effect on ACM were assumed to capture the interaction between the number of hospitalisations and the subsequent risk of death, and the elevated risk of death associated with hospitalisation.
The decision to model cardiovascular hospitalisation only, and not all-cause hospitalisations, was primarily data driven because the two studies in the meta-analysis that provided data on the impact of co-Q10 on hospitalisation reported outcomes for CHF-related hospitalisation only. 33,62 As the impact of co-Q10 on CHF-related hospitalisation cannot be assumed to apply to non-cardiovascular hospitalisation, the model considers hospitalisation relating to cardiovascular events only.
A 1-month cycle length with half-cycle correction was employed, which is consistent with the majority of the other economic models of CHF identified in our review of economic evaluations of interventions for CHF (see Chapter 5 Systematic review of existing cost-effectiveness evidence and Cost-effectiveness of interventions for chronic heart failure), allowing for the comparison and validation of model outcomes over the time horizon.
Heart failure is a chronic, progressive disease that requires lifelong therapy and, consequently, the cost-effectiveness analysis considered a lifetime time horizon to ensure that all costs and benefits of co-Q10 supplementation were captured.
Clinical effectiveness
Chronic heart failure-related hospitalisation and other cardiovascular hospitalisations were captured in the model as separate, distinct events. The Q-SYMBIO trial33 evaluated the impact on hospitalisation for CHF events, described as ‘unplanned hospitalisation stay for worsening of CHF’, while non-CHF cardiovascular events, such as stroke and MI, were described as ‘adverse events’. 33 In the base-case analysis, it was assumed that co-Q10 has an impact on CHF-related hospitalisation only, and the impact on other cardiovascular hospitalisation was explored in a scenario analysis.
Baseline event rates for mortality
Most of the RCTs in the systematic review of clinical effectiveness were conducted outside the UK, and patient characteristics, treatment patterns and resource use in the UK can be expected to differ from those in the centres involved in those trials. One implication is that the baseline event rates observed in the control groups of the trials are unlikely to provide reliable estimates for UK practice.
The Q-SYMBIO trial provided Kaplan–Meier survival curves for ACM for the full analysis set (FAS) and for a European subpopulation up to a period of 106 weeks. 33,73 Long-term survival was estimated by fitting a parametric exponential distribution to the control arm in the FAS and in the European subset to predict survival over a patient’s lifetime, as shown in Figure 18.
In the FAS, the exponential model predicted that 34.2% of patients would be alive after 10 years and 11.7% would be alive after 20 years. For a trial that reported that 88% of its patients were in NYHA class III, these projections appeared implausibly high, based on clinical opinion. Furthermore, this group of trial patients, who had a mean age of 62 years, was also considerably younger than those in the ‘West Yorkshire’ data set,89–91 whose mean age was 69.6 years and 50% had CHF of NYHA class II. Therefore, the cohort in the Q-SYMBIO trial33,73 was not considered representative of the patients typically seen in UK practice who would potentially receive co-Q10.
Further consideration was given to calibrating the projected survival estimates from the Q-SYMBIO trial33,73 to the long-term survival estimates from UK observational data sets of patients with CHF to make an adjustment to the hazard of mortality predicted by the Q-SYMBIO trial. 33,73 Three potential sources were identified for this purpose: (1) The Health Improvement Network (THIN) data set (1998–2012),85 (2) the Clinical Practice Research Datalink (CPRD) linked to inpatient Hospital Episode Statistics (HES) and Office for National Statistics (ONS) mortality data (i.e. CPRD–HES–ONS) (2000–17)92 and (3) the EchoCardiographic Heart Of England Screening (ECHOES) study. 93 THIN is one of the largest databases of general practitioner (GP) records, and includes data from 587 practices in the UK for patients with a code of CHF. The survival rates of those with CHF (i.e. patients with a first diagnostic label) in THIN varied considerably by age. 85 For those aged 55–64 years, the survival estimates were 91.5% at 1 year, 75.0% at 5 years and 58.4% at 10 years, whereas the corresponding survival estimates for those aged 65–74 years were 87.6%, 64.5% and 40.4%. CPRD–HES–ONS92 provides more recent trends in survival after a diagnosis of CHF in the UK. For those aged 55–64 years, the 1-, 5- and 10-year survival rates were 87.9%, 70.6% and 52.8%, respectively, whereas for those aged 65–74 years they were 83.5%, 59.1% and 35.4%, respectively. The ECHOES study93 screened a total of 6162 patients from 16 randomly selected primary care practices in England into four prespecified cohorts (i.e. the general population, diuretic users, those with a prior clinical label of CHF and a population with risk factors for CHF) to identify the prevalence and prognosis of CHF and LVSD. The 5-year survival rate was 53% in patients with CHF and LVSD (mean age of 70.5 years), and survival improved significantly with increasing ejection fraction. However, these estimates were based on a much smaller subset of the full population (only 219 patients with CHF and LVSD).
The THIN, CPRD–HES–ONS and ECHOES data sets85,92,93 are helpful for providing estimates of long-term mortality in patients with a diagnosis of CHF, but their generalisability to a HFrEF population is limited. The CPRD–HES–ONS data set,92 which provides the most recent contemporary evidence, does not specify the type of heart failure (reduced or preserved ejection fraction). There are also additional concerns regarding the reliability of GP coding and misclassification of CHF diagnosis in the THIN and CPRD–HES–ONS data sets,85,92 and the very small sample size of the population with CHF and LVSD in the ECHOES study. 93
Given these concerns, and the fact that the baseline data from the RCTs included in the meta-analysis were not considered representative of patients typically seen in UK practice, extrapolation of the Q-SYMBIO control arm (or calibration to THIN, CPRD–HES–ONS or ECHOES study survival estimates85,92,93) was not considered appropriate for informing long-term baseline survival. However, the impact of estimating ACM from the Q-SYMBIO trial was explored in a scenario analysis. 33,73
Baseline event rates specific to UK practice were therefore obtained from the ‘West Yorkshire’ data set,89–91 which prospectively recorded ACM, hospitalisations and subsequent deaths. Among the sample of patients who were followed until death, for 38.9% the cause of death was cardiovascular (of which 79.4% were related to CHF) and for 57.8% the cause was non-cardiovascular or their death was sudden. Access to this data set permitted the analysis of IPD, allowing the development of risk equations for different patient subgroups. It also permitted the estimation of survival over a longer time period than was available from the RCTs. It may also provide a better estimation of the ‘real-world’ effectiveness of standard care, as used outside a RCT setting. However, this assumes that the treatment effect of co-Q10, derived from the RCTs included in our meta-analysis on which the relative effect used in the model is based, can be generalised to the patients with CHF in the ‘West Yorkshire’ data set. 89–91
Multivariable parametric survival analysis using the ‘West Yorkshire’ data set was used to model ACM over time using baseline characteristics. Backwards, stepwise elimination was undertaken to select covariates for the final model. The model was applied both during and beyond the duration of the follow-up of the data set. The baseline risk for ACM was assumed to follow an exponential distribution, selected from potential distributions, on the basis of statistical fit and clinical plausibility, and from consultation with clinical experts, following review of projected life expectancies and discussion of the nature of the mortality hazard over time. Survival predictions for patients in each subgroup are presented in Figure 19. In scenario analyses, the Weibull distribution was used to evaluate the robustness of the results.
FIGURE 19.
Predicted baseline rate of survival.
Baseline event rates of hospitalisation
The baseline event rates of hospitalisation were obtained from a subset of the ‘West Yorkshire’ data set. 89–91 Hospitalisations in the first 1091 of 1802 patients recruited to the data set were recorded up to 1 year. In the remaining 711 patients, hospitalisations were recorded over a longer time period, with a median follow-up of 2.86 years. 90 Heart failure hospitalisation was defined as a new onset or worsening of signs and symptoms of heart failure, with evidence of fluid overload requiring at least 24-hour hospitalisation and the use of intravenous diuretics.
In the subset of patients with long-term hospitalisation data, the 1-year rate of CHF-related hospitalisation was 6.47% and non-CHF cardiovascular-related hospitalisation was 11.11%. The mean time to first CHF-related hospitalisation was 603 days and the mean time to non-CHF cardiovascular-related hospitalisation was 527 days. In a previous analysis of 1-year hospitalisation in the cohort, predictors of CHF-related hospitalisation included furosemide-equivalent dose, the presence of type 2 diabetes, hospitalisation relating to acute heart failure syndrome within the previous year and pulmonary congestion on chest radiograph. A summary of hospitalisation events in the study is given in Table 19.
| Hospitalisation variable | All patients | LVEF of < 45 | LVEF of < 45 and NYHA classes II–IV | LVEF of < 45 and NYHA classes III and IV |
|---|---|---|---|---|
| n | 711 | 630 | 536 | 177 |
| Median follow-up (years) | 2.86 | 2.78 | 2.75 | 2.72 |
| Patients with one or more heart failure hospitalisations, n (%) | 125 (17.6) | 113 (17.9) | 101 (18.8) | 38 (21.5) |
| Patients with one or more non-heart failure cardiovascular hospitalisations, n (%) | 162 (22.8) | 139 (19.5) | 118 (22.0) | 48 (27.1) |
| Mean heart failure hospitalisations (range) | 1.57 (1 to 7) | 1.57 (1 to 7) | 1.63 (1 to 7) | 1.89 (1 to 7) |
| Mean non-heart failure cardiovascular hospitalisations (range) | 1.60 (1 to 13) | 1.62 (1 to 13) | 1.67 (1 to 13) | 1.73 (1 to 13) |
To predict the rate of hospitalisation beyond the follow-up period of the study, a multivariate regression model was fitted to the hospitalisation data set. A large proportion of patients were found not to experience any CHF-related hospitalisations or other cardiovascular hospitalisation events. Among the 711 patients in the cohort for whom there were long-term hospitalisation data, 125 (17.6%) experienced a CHF-related hospitalisation and 162 (22.8%) experienced a non-CHF cardiovascular-related hospitalisation (see Table 19).
Previous economic analyses have applied a negative binomial model for all-cause hospitalisation; however, this model fits less well here, as it does not account for excess zeros. To reflect the high proportion of patients with zero events, a hurdle model was selected to predict hospitalisation events. This has two components: (1) a right-censored hurdle process, which models zero versus larger counts (i.e. whether or not a patient has any hospitalisation episodes at all) and (2) a left-truncated count process for positive counts, which predicts the number of hospitalisation episodes conditional on the patient experiencing at least one event. Modelling the two processes separately has the advantage that the fit of the count and the hurdle component can be optimised separately:94
The hurdle process was modelled using a binomial distribution. Based on discussions with clinical experts, and from plotting histograms of follow-up times and hospitalisation events, the proportion of patients who were never hospitalised for CHF or for other cardiovascular reasons was assumed not to vary over time, as it is expected that there would be a certain group of patients who would never require cardiovascular hospitalisations throughout their lifetime, regardless of their survival time. Predictions of the hurdle process (see Table 24) corresponded well to the observed data.
The count process was modelled using a Poisson distribution, which assumes a constant rate of events per unit of time. Although an increase in the number of hospitalisation events is often observed among CHF patients in the last year of life, the evidence suggests that this is attributable to non-cardiovascular causes and that the rate of hospitalisation for CHF remains constant up until the end of life. 95 Examination of the rate of hospitalisation by time did not reveal any consistent trends, with peaks in both those with a low censoring time and those with a high censoring time. This suggests that those censored earlier experienced a larger number of hospitalisations (the reasons for this are unclear, but it could reasonably be considered to be because they die earlier and, therefore, have more severe disease). Likewise, those who had been followed up for a longer amount of time experienced more episodes, most likely because they simply had a longer at-risk period. The monthly CHF-related and other cardiovascular hospitalisation rate (in those patients who experienced at least one event) for the base-case population was 2.15% and 2.38%, respectively. The monthly rate increased for subgroups who had more severe symptoms (Table 20).
| Hospitalisation variable | All patients (%) | LVEF of < 45 (%) | LVEF of < 45 and NYHA classes II–IV (%) | LVEF of < 45 and NYHA classes III and IV (%) |
|---|---|---|---|---|
| Proportion with at least one heart failure event | 16.87 | 17.48 | 18.35 | 21.66 |
| Mean monthly heart failure hospitalisation rate among those with at least one event | 2.15 | 2.19 | 2.48 | 3.92 |
| Proportion with at least one other cardiovascular event | 22.68 | 22.45 | 23.22 | 25.65 |
| Mean monthly other cardiovascular hospitalisation rate among those with at least one event | 2.38 | 2.46 | 2.59 | 3.07 |
Impact of coenzyme Q10
All-cause mortality
In the base-case analysis, the impact of co-Q10 on ACM was estimated using the one-stage meta-analysis estimate of effect (see Chapter 4 and Meta-analysis results), which included data from seven trials. 33,48,55,57,59,62,70 The RR was estimated as 0.68 (95% CI 0.45 to 1.03), suggesting that co-Q10 reduced the risk of death, but this finding was associated with a degree of uncertainty.
The seven trials33,48,55,57,59,62,70 included in the meta-analysis recruited populations with differing disease severity, with inclusion criteria ranging from patients in NYHA class I60,65 to patients with end-stage CHF awaiting heart transplant. 48 None of the trials reported that co-Q10 had a negative effect on ACM. There was no apparent relationship between the disease severity of the populations recruited to the trial and the estimated treatment benefit (shown in the two-stage analysis presented in Figure 20). Therefore, the same treatment effect estimate (i.e. RR 0.68) was assumed in the base case and in the subgroup analyses.
FIGURE 20.
Relative impact of co-Q10 on ACM. SoC, standard of care.
Alternative estimates of the treatment effect were considered in scenario analyses: (1) from the two-stage meta-analysis (RR 0.62, 95% CI 0.45 to 0.85), (2) from the Bayesian NMA (OR 0.54, 95% CI 0.23 to 1.10) and (3) from the one-stage meta-analysis, excluding the Q-SYMBIO trial33,73 (RR 0.72, 95% CI 0.43 to 1.21). The RR for ACM was varied in the probabilistic analysis using a log-normal distribution.
Long-term follow-up was not available in most RCTs. However, our clinical experts judged it plausible that the treatment effect would endure while patients remained on treatment, which is in line with other interventions for CHF, and the base-case analysis applied the duration of treatment effect for the patient lifetime. 96 In a scenario analysis, the treatment effect was limited to 4 and 10 years. Figure 20 presents the relative impact of co-Q10 on ACM when the treatment effect is applied (1) over the patient’s remaining lifetime and (2) for 4 years.
Hospitalisation
The impact of co-Q10 on the rate of hospitalisation was estimated using the results of the meta-analysis of the two trials33,62 that reported data (RR 0.61, 95% CI 0.49 to 0.77). The effect size from the Q-SYMBIO trial33,73 was used in a scenario analysis (RR 0.54, 95% CI 0.28 to 1.05).
Both trials in the meta-analysis of hospitalisation reported only the number of patients with CHF-related hospitalisation events rather than total events experienced, and, therefore, they did not provide information on the risk of rehospitalisation or treatment effect of co-Q10 on risk of subsequent CHF-related hospitalisations. The model conservatively assumed that co-Q10 has an impact on only the first CHF-related hospitalisation event in the base-case analysis, with an increased likelihood of remaining free from CHF-related hospitalisation events. Although it may be plausible that co-Q10 could have an impact on subsequent hospitalisations as well as the first recorded one, the magnitude of this effect is unknown. The impact of applying a treatment effect to subsequent hospitalisation events was explored in a scenario analysis. Further scenario analyses applied the impact of co-Q10 on other cardiovascular hospitalisation events.
Quality of life
Estimating QALYs necessitates adjusting the period of a patient’s average lifetime to account for QoL by applying an appropriate utility score to the model. Utility data are used to differentiate the health status of patients in the different health states of decision models and, ideally, are a generic measure of health directly elicited from patients. The EQ-5D is a generic instrument designed to capture patient-reported outcomes across five health domains (self-care, mobility, usual activities, pain/discomfort and anxiety/depression). Utility values typically measure patients’ QoL on a scale where zero represents death and 1 represents full health, although negative values are possible. QoL weights may be derived from the EQ-5D using country-specific values of different health statuses.
In the decision model, the baseline utility value reflected an individual who had stable CHF and was not currently experiencing a hospitalisation event. Utility decrements were applied to patients experiencing CHF-related and non-CHF cardiovascular-related hospitalisation events.
Identification of quality-of-life evidence
None of the trials identified in our meta-analysis of HRQoL was considered appropriate for the economic model because they did not present baseline HRQoL values, they did not report on a generic preference measure or they provided only mean values over time, which could not be used to inform health state utility values.
A targeted review of utility scores was undertaken to identify health state utility values and appropriate utility values for major cardiovascular events.. From the review of cost-effectiveness studies, HRQoL values from two trials, the Systolic Heart failure treatment with the IF inhibitor ivabradine Trial (SHIFT)97,98 and the Eplerenone Post-AMI Heart Failure Efficacy and Survival Study (EPHESUS),99 were considered relevant to our economic model. Utility values from SHIFT were used in the base-case analysis, whereas the utility values from EPHESUS, consisting of post-MI patients whose CHF may be more severe than the modelled population, were used in a scenario analysis.
Impact of coenzyme Q10 on quality of life
The results of our meta-analysis of the data available from four trials,50,52,68,69 suggested no clear evidence of any effect of co-Q10 on QoL (see Figure 14). Therefore, the same health state utility values were applied to co-Q10 and to standard therapy alone.
Health state utility values
The Systolic Heart failure treatment with the IF inhibitor ivabradine Trial97,98 evaluated the effect of ivabradine (Procoralan, Servier Laboratories, Suresnes, France) compared with placebo added to guidelines-driven background therapy in 6558 adult patients with CHF of NYHA classes II–IV, a LVEF of ≤ 35% and a resting heart rate of ≥ 70 beats per minute. The study collected HRQoL data using the EQ-5D questionnaire, which was administered to patients (n = 5313) in countries in which the questionnaire has been validated. UK tariff values were used in the study, regardless of the country of origin of the HRQoL data. HRQoL values from SHIFT97,98 were analysed and applied in two separate economic evaluations of ivabradine. 97,100
In one cost-effectiveness study,100 EQ-5D data were analysed using multilevel modelling, which takes into account correlation between measurements from the same individual to increase precision and avoid bias. The regression equation included coefficients for treatment allocation, baseline characteristics, NYHA class and hospitalisation episode. The baseline utility values by NYHA class for the SHIFT97,98 population were 0.82 for NYHA class I, 0.74 for NYHA class II, 0.64 for NYHA class III and 0.46 for NYHA class IV. Table 21 presents the predicted baseline utility values for the four patient subgroups of interest in the cost-effectiveness analysis, estimated using the mean utility value for each NYHA class, and weighted by the proportion of patients in each NYHA class in the ‘West Yorkshire’ data set (see Table 21). 89–91 This study also estimated the impact on HRQoL of all-cause hospitalisation; however, we did not apply this value to our economic analysis.
| Health state/event | Utility value/decrement |
|---|---|
| Baseline utility | |
| All patients | 0.721 |
| Patients with a LVEF of < 45 | 0.720 |
| Patients with a LVEF of < 45 and CHF of NYHA classes II–IV | 0.698 |
| Patients with a LVEF of < 45 and CHF of NYHA classes III and IV | 0.635 |
| Event-related utility decrements | |
| CHF-related hospitalisation | −0.084 |
| Non-CHF cardiovascular-related hospitalisation | −0.032 |
In the second cost-effectiveness study,97 the authors did not distinguish utility values by NYHA class. However, the authors considered the impact of cardiovascular hospitalisations rather than all-cause hospitalisations, and further split the impact into CHF-related and non-CHF cardiovascular-related hospitalisation, which were considered more appropriate for use in the decision model. Two regression equations were developed to estimate change in EQ-5D score from baseline, using treatment, beta-blocker use and number of hospitalisations as independent variables. The same utility decrements were applied to each hospitalisation event type regardless of patient subgroup.
A study by Göhler et al. 99 used primary data from EPHESUS99 to estimate the utility values for patients with CHF according to their NYHA classification and number of cardiovascular rehospitalisations. EPHESUS99 recruited CHF patients from a post-MI population who were considered to have greater ill-health and worse HRQoL than patients who would generally be eligible for co-Q10. The baseline utility values by NYHA class for the EPHESUS99 population were slightly lower than those estimated from the SHIFT97,98 population, reflecting the greater debilitation of the population: 0.79 for NYHA class I, 0.71 for NYHA class II, 0.62 for NYHA class III and 0.48 for NYHA class IV. The decrement was –0.024 for one rehospitalisation, –0.031 for two rehospitalisations and –0.055 for three or more rehospitalisations. These utility values were used in a scenario analysis in our model, with the mean rehospitalisation utility decrement applied for hospitalisation events.
Quality of life over time
Health-related quality of life was adjusted to reflect its decreases associated with ageing. Age- and sex-adjusted norms for the UK were adjusted downwards by approximately 13% to reflect the presence of CHF. The adjustment factor was estimated by comparing the baseline utility in SHIFT97,98 with the average utility of a person of the equivalent mean age (60 years) in the UK, derived from a nationally representative UK sample using the EQ-5D. 101
Resource use and unit costs
Costs associated with non-fatal cardiovascular events and the routine management of CHF over time were included in the model. Resource use data were identified from published sources, including national surveys and published economic analyses, and through consultation with clinical experts and service providers. Unit costs were obtained from published sources and UK-based mainstream retailers of micronutrient supplements, and applied in GBP for the financial year 2019–20.
The model includes several cost parameters: (1) the cost of co-Q10, (2) the cost of background medical management, including GP visits and other outpatient contacts, and (3) the costs of treating patients with CHF in hospital.
Coenzyme Q10
Although many patients purchase co-Q10 themselves, this analysis explores the impact of the cost of co-Q10 being borne by the NHS. The unit costs of co-Q10 were obtained from the British National Formulary (BNF) [provided as ubidecarenone (Bio-Quinone®, Pharma Nord)]. 102 The mean daily cost was estimated assuming a dosing regimen of 100 mg three times a day, as per the Q-SYMBIO trial. 33 It was assumed that compliance would be 100% if co-Q10 were to be adopted. The mean daily cost was £0.94, equating to £28.61 per month (Table 22).
| Supplier | Pack size | Pack cost (£) | Cost (£) per tablet |
|---|---|---|---|
| Ubidecarenone 100 mg (Bio-Quinone®, Pharma Nord) | 20 | 8.12 | 0.41 |
| 60 | 21.03 | 0.35 | |
| 150 | 42.09 | 0.28 | |
| Ubidecarenone 100 mg (Uniquinol®, Pharma Nord) | 60 | 29.23 | 0.49 |
| 150 | 53.14 | 0.35 | |
| Ubidecarenone 100 mg (Vega Nutritionals Ltd, Talbot, UK) | 60 | 6.00 | 0.10 |
| Ubidecarenone 100 mg (Lamberts Healthcare, Tunbridge Wells, UK) | 60 | 16.50 | 0.28 |
| Ubidecarenone 100 mg (Natures Aid, Preston, UK) | 30 | 9.42 | 0.31 |
| Mean cost (per tablet) | 0.31 | ||
Standard care
Standard care was assumed to comprise background medication and resource use for CHF, including accident and emergency referrals, outpatient contacts and GP visits. Standard care for CHF comprised a number of pharmacological agents, including ACE inhibitors, beta-blockers, mineralocorticoid receptor antagonists and diuretics. The proportion of patients receiving each type of medication and the mean dose of each medication were informed by the ‘West Yorkshire’ data set (see Table 18). 89–91 The costs of background therapies were based on average doses and utilisation reported in the ‘West Yorkshire’ data set at baseline (Table 23). Unit costs were obtained using the electronic market information tool (eMIT). 103 This provides information on the average price that the NHS pays for pharmaceuticals, which can differ from the prices listed in the BNF, and it is a more accurate and up-to-date indicator of the costs incurred by the NHS. The total monthly cost for standard therapy was estimated to be £1.94.
| Therapy | Proportion (%) of patients on medication | Mean daily dose (mg) | Mean daily cost (£) |
|---|---|---|---|
| Ramipril | 90.29 | 5.44 | 0.02 |
| Bisoprolol | 84.52 | 4.58 | 0.01 |
| Spinolactone | 38.24 | 25 | 0.01 |
| Furosemide | 74.36 | 68.72 | 0.02 |
Background resource use (Table 24) was informed by an analysis of the CPRD reported by McMurray et al. 83 The CPRD data set covers the English NHS and provides bespoke data tables that consider resource use, excluding hospitalisation and pharmacological therapies. Unit costs were from published national sources. 104,105
| Resource use | Mean annual use | Unit cost (£) | Source of unit cost |
|---|---|---|---|
| Emergency visits | |||
| GP emergency visits | 0.14 | 39 | PSSRU 2019104 |
| A&E referrals | 0.01 | 183 | NHS Reference Costs (HRG codes VB01 to VB09)105 |
| Outpatient visits | |||
| GP visits | 13.54 | 39 | PSSRU 2019104 |
| Cardiologist visits | 0.05 | 139 | NHS Reference Costs (service code 320)105 |
| Other clinician visits | 0.36 | 39 | PSSRU 2019104 |
| Other visits | |||
| GP home visits | 1.23 | 39 | PSSRU 2019104 |
| GP nursing home visits | 0.19 | 39 | PSSRU 2019104 |
| GP residential home visits | 0.04 | 39 | PSSRU 2019104 |
| GP telephone calls to patient | 0.73 | 39 | PSSRU 2019104 |
| GP visits with third parties | 7.27 | 39 | PSSRU 2019104 |
The annual cost of background resource use was estimated to be £925.27 (equivalent to a monthly cost of £77.11).
Hospitalisation
The analysis includes the costs of hospitalisations by admission type (i.e. CHF-related or non-CHF cardiovascular-related) estimated from NHS Reference Costs. 105
The reference costs are the average costs to the NHS of providing a defined service or resource in a given financial year. The costs are categorised into groups [Healthcare Resource Groups (HRGs)] according to episodes that are clinically coherent and consume similar resources. NHS reference costs provide the unit costs of a hospitalisation event and not a cost per day. This method is considered to be aligned with the process through which care is reimbursed in England and Wales. The costs of each type of hospitalisation event are based on a weighted average of short- and long-stay visits, as well as the numbers of patients who incur complications. The unit costs of hospitalisations were assumed to be the same in both arms of the model.
The unit cost of a CHF-related hospitalisation event was estimated as £1935 from HRG codes EB03A-E (heart failure or shock). A non-CHF cardiovascular-related event was estimated as £1272 from HRG codes AA22C-G (cerebrovascular accident, nervous system infections or encephalopathy), AA35A-F (stroke), EB07A-E (arrhythmia or conduction disorders) and EB10A-E (actual or suspected myocardial infarction).
Analytical approach and scenario and sensitivity analysis
Overview
The cost-effectiveness of co-Q10 as an adjunct to standard therapy in patients with CHF and reduced ejection fraction was evaluated by comparing the total expected lifetime costs and QALYs with those of standard therapy alone. The mean costs and QALYs for co-Q10 as an adjunct therapy and for standard care alone are presented, and their cost-effectiveness is compared using conventional cost-effectiveness decision rules, estimating the incremental cost-effectiveness ratio (ICER) as appropriate. The ICER uses the additional costs that one strategy incurs over another and compares this with the additional benefits.
The cost-effectiveness results are presented for the base-case population and separately for each of the scenario and subgroup populations. Scenario analyses were used to test the robustness of the cost-effectiveness results to changes in the structural assumptions of the model.
Probabilistic sensitivity analysis
Uncertainty in the data used to populate the economic model was characterised using a probabilistic analytic approach, with each input entered as an uncertain parameter with an assigned probability distribution representing its uncertainty.
The uncertainty in the probability and utility parameters was represented using beta distributions, as these values are typically bounded at 0 and 1. Log-normal distributions were used to estimate uncertainty in HRs, RRs and ORs. Gamma distributions were used to represent the uncertainty in the cost parameters, as these values are constrained to be non-negative, but often are right-skewed distributions. To account for uncertainty around the parametric models fitted to ACM and hospitalisation, outcomes were sampled using their associated variance–covariance matrices of covariates. When the variation around the mean input was not available, the standard error was assumed to be equivalent to 20% of the width of the 95% CI (normally distributed) around the point estimate.
Probabilistic sensitivity analysis was used to estimate 95% CIs around the cost-effectiveness results. The mean probabilistic estimate of costs and QALYs was estimated from 10,000 iterations of the model to estimate the uncertainty surrounding the total costs and QALYs calculated from the model. Cost-effectiveness acceptability curves (CEACs) are presented. The CEACs show the probability that co-Q10 is cost-effective at a range of threshold values that NHS decision-makers attach to an additional QALY (e.g. £20,000–30,000 per additional QALY, as used by NICE).
The base-case parameters, associated assumptions and sources are given in Appendix 9, Table 37.
Value of information
Value-of-information analysis was used to quantify the expected benefits of further research by estimating the value of reducing decision uncertainty related to the cost-effectiveness of adjunct co-Q10 compared with standard care alone in HFrEF. The maximum amount that the NHS should be willing to invest to reduce decision uncertainty can be informed by the expected value of perfect information (EVPI). 106 The EVPI evaluates the expected consequences of decision uncertainty, in terms of costs incurred and health benefits lost, should it later transpire (with further research) that the decision based on the evidence currently available is not correct. The EVPI takes into account both the probability that the decision based on existing evidence is wrong, and the magnitude of the consequences of making an incorrect decision. The consequences of making an incorrect decision because of uncertainty can be compared with the costs of conducting new research (e.g. a clinical trial) to establish the potential value of new research.
The EVPI provides an estimated value of resolving all uncertainty parameterised within the model through the provision of perfect information and provides a measure of the expected maximum return from further research. Therefore, EVPI represents an expected upper bound to the amount that a decision-maker should be willing to pay for additional evidence to support the current decision. If the EVPI exceeds the expected costs of additional research, then it is potentially cost-effective to acquire more information by undertaking this research.
Information generated in research is used to inform decisions for the population of patients who could potentially benefit from the information. This depends on the size of the benefiting population, whose decision choice will be informed by the additional research and the time horizon over which the information generated by research is useful. This means that the population-level EVPI is estimated by scaling up the individual (per-patient) EVPI by the number of people who would be affected by the information over the anticipated lifetime of the technology. This can be expressed as:
where I is the incidence in the period, t is the period, T is the total number of periods for which information from research would be useful and r is the discount rate.
The British Heart Foundation estimates that the prevalence of CHF in the UK is 658,944 (the number of people in the UK on their GP’s heart failure register),107,108 although as 920,000 people may be living with heart failure. 109 The annual incidence of CHF is estimated to be 89,986 cases, based on an age-standardised rate of 164.1 per 100,000 of the population (209.6 for men and 127.6 for women per 100,000 population). 110 Assuming a 10-year time horizon for the lifetime of the technology gives an effective CHF population of 1,433,512 (prevalent population plus incident population per annum discounted at 3.5% over the lifetime of the technology). It is assumed that 50% of this population has a reduced ejection fraction, giving an estimate of 716,756 for the population EVPI calculations. 5
The EVPI analysis quantifies the decision uncertainty predicted by the model and provides the maximum value that can be placed on additional research. However, it does not address the structural or methodological uncertainty inherent in the set of model assumptions. To explore the value of reducing decision uncertainty related to the assumptions on the key effectiveness parameter for co-Q10 on ACM, the EVPI calculations were conducted for two scenarios using different assumptions about the treatment effect on ACM and its associated uncertainty. The first scenario used the estimate of treatment effect as applied in the base-case analysis from the one-stage meta-analysis (RR 0.68, 95% CI 0.45 to 1.03). With this estimate of treatment effect, the CI around the RR indicates that there is very little uncertainty about whether or not co-Q10 has a positive impact on ACM. However, there are concerns that this estimate, which is largely influenced by the effect observed in the Q-SYMBIO trial33 (the largest trial with the greatest weight in the meta-analysis), underestimates the true uncertainty about the effect of co-Q10 on ACM. In the second scenario, the treatment effect of co-Q10 on ACM was estimated from the one-stage meta-analysis by excluding the Q-SYMBIO trial. 33 Excluding this study leads to greater uncertainty in the treatment effect of co-Q10 on ACM, with the CI estimating a higher likelihood that co-Q10 is not associated with a positive impact (RR 0.72, 95% CI 0.43 to 1.21).
Scenario analyses
Several alternative scenarios were considered, in which the assumptions used as part of the base-case results were varied. These analyses were undertaken to assess the robustness of the base-case results to variation in the sources of data used to populate the model and alternative assumptions. The alternative scenarios are summarised in Table 25.
| Scenario | Element | Position in the base-case analysis | Variation in the scenario analysis |
|---|---|---|---|
| 1 | Duration of treatment effect | Lifetime | 4 years |
| 2 | Duration of treatment effect | Lifetime | 10 years |
| 3 | Baseline ACM risk | Exponential survival model from the ‘West Yorkshire’ data set89–91 | Weibull survival model from the ‘West Yorkshire’ data set89–91 |
| 4 | ACM risk | Exponential survival model from the ‘West Yorkshire’ data set89–91 | Exponential survival model from the Q-SYMBIO trial (FAS)33,73 |
| 5 | ACM risk | Exponential survival model from the ‘West Yorkshire’ data set89–91 | Weibull survival model from the Q-SYMBIO trial (FAS)33,73 |
| 6 | ACM risk | Exponential survival model from the ‘West Yorkshire’ data set89–91 | Exponential survival model from the Q-SYMBIO trial (European subset)33,73 |
| 7 | ACM risk | Exponential survival model from the ‘West Yorkshire’ data set89–91 | Weibull survival model from the Q-SYMBIO trial (European subset)33,73 |
| 8 | Treatment effect on ACM | One-stage meta-analysis | One-step meta-analysis excluding the Q-SYMBIO trial33,73 |
| 9 | Treatment effect on ACM | One-stage meta-analysis | Two-stage meta-analysis |
| 10 | Treatment effect on ACM | One-stage meta-analysis | Bayesian NMA |
| 11 | Hospitalisation risk | Treatment effect on first heart failure event only | Treatment effect on rate of all heart failure-related hospitalisation events |
| 12 | Hospitalisation risk | No treatment effect on other cardiovascular hospitalisation event rate | Treatment effect on rate of first other cardiovascular hospitalisation events |
| 13 | Hospitalisation risk | No treatment effect on other cardiovascular hospitalisation event rate | Treatment effect on rate of all other cardiovascular hospitalisation events |
| 14 | Utility values | SHIFT97,98 data set to measure health state utility values | EPHESUS99 data set to measure health state utility values |
Validation
The model was developed by one analyst (LC) and the programming was checked by a second (CR). To ensure that the model outputs were valid, coding and formulae were checked by two modellers. Black-box testing examined the predictive validity of parameter inputs (e.g. that increasing the effectiveness of treatment reduces cost-effectiveness). All model inputs were checked against the original sources. Clinical experts were consulted on key assumptions.
Chapter 7 Economic analysis: results
Base-case results
Table 26 shows the cost-effectiveness results for the base-case scenario for all patients with HFrEF, incorporating a lifetime treatment effect for co-Q10 and assuming that the cost of co-Q10 is borne by the NHS. If the relative reduction in mortality is sustained over the patient lifetime, the number of predicted life-years gained for co-Q10 in addition to standard care was 3.80 (14.54 life-years vs. 10.74 life-years). Co-Q10 in addition to standard care is estimated to be both more effective and more costly than standard care. Co-Q10 increases the mean total costs by approximately £4878 per patient, but this results in an additional gain of 1.34 QALYs. The ICER for co-Q10 compared with standard care alone is £3650 per additional QALY gained, indicating that co-Q10 is cost-effective in this patient subgroup at the threshold of £20,000 per QALY used by NICE.
| Strategy | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Co-Q10 | 16,767 | 6.44 | 4878 | 1.34 | 3650 |
| Standard care | 11,889 | 5.11 |
Not all of the additional costs in the co-Q10 arm were attributable to the supplementation itself, although this accounted for the largest increase in costs (£3486 over the patient’s remaining lifetime). Increases in life expectancy due to co-Q10 also resulted in increased costs associated with background medication and the management of CHF. This was offset, to some degree, by the reduction in cost from averted CHF-related hospitalisations.
Probabilistic analysis and value of information
The relatively low ICER for co-Q10 compared with standard care is reflected in the estimated high likelihood that co-Q10 is cost-effective. At thresholds of £20,000 and £30,000 per QALY, the probability that co-Q10 is cost-effective is 95.2% and 95.8%, respectively. Figure 21 presents the CEAC of co-Q10 compared with standard care. Co-Q10 is associated with a high probability of cost-effectiveness at all values of the threshold of cost-effectiveness.
FIGURE 21.
Cost-effectiveness acceptability curve of co-Q10 compared with standard care.

Expected value of perfect information results
The value of the uncertainty surrounding a decision based on the expected ICER estimates is expressed using both individual (per patient) estimates of EVPI and total population EVPI, based on a 10-year time horizon. Table 27 presents the EVPI estimates for two scenarios that vary the assumptions about the treatment effect of co-Q10 on ACM.
| Cost-effectiveness threshold | Individual EVPI (£) | Population EVPI (£) | ||
|---|---|---|---|---|
| Base-case assumption | Scenario (less certain treatment effect) | Base-case assumption | Scenario (less certain treatment effect) | |
| £10,000 | 162 | 635 | 115,804,126 | 454,934,574 |
| £20,000 | 218 | 1046 | 156,406,878 | 749,798,997 |
| £30,000 | 292 | 1487 | 208,944,261 | 1,065,522,238 |
Under the base-case assumptions, the individual EVPI ranges from £162 to £292 across the range of cost-effectiveness thresholds of £10,000 to £30,000 per additional QALY. The decision uncertainty is relatively low because co-Q10 is increasingly cost-effective as the threshold is increased (i.e. the probability of decision error is low at thresholds above £10,000, as seen in Figure 21, with a chance of only approximately 5% that co-Q10 is not a cost-effective option), but the consequences of error are valued more highly as the threshold is increased. The implication is that the EVPI increases with higher values of the threshold because the opportunity losses associated with making an incorrect decision are increasing. The estimates of population EVPI ranged from £116M to £209M across the different thresholds for the base-case assumptions. These estimates demonstrate that there appears to be value in undertaking additional research to reduce the existing decision uncertainty.
When the EVPI calculations were based on more uncertain estimates of the treatment effect of co-Q10 on ACM, the individual EVPI ranged from £635 to £1487 across the range of cost-effectiveness thresholds of £10,000 to £30,000 per additional QALY. The corresponding estimates of population EVPI ranged from £455M to £1066M. The EVPI results are higher under this scenario than with the base-case assumptions, because the decision uncertainty is increased (i.e. the probability of error increases from 5% in the base case to 12% in this scenario, while the consequences of error are valued highly as the threshold is increased).
Subgroup analysis
Table 28 presents the results of the three subgroup analyses using the assumptions of the base-case analysis. The outcomes of the subgroup of patients with LVEF < 45 were broadly similar to those of the base-case population. As only a small proportion of patients from the cohort were excluded, the impact on the results was minimal in this subgroup.
| Population | Co-Q10 | Standard care | ICER (£) | ||
|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | ||
| Base case | 16,767 | 6.44 | 11,889 | 5.11 | 3650 |
| LVEF < 45 | 16,773 | 6.38 | 11,970 | 5.05 | 3595 |
| LVEF < 45 and CHF of NYHA classes II–IV | 16,034 | 5.58 | 11,735 | 4.33 | 3418 |
| LVEF < 45 and CHF of NYHA classes III and IV | 16,210 | 4.21 | 13,290 | 3.14 | 2741 |
co-Q10 was found to be more cost-effective in subgroups with greater symptomatic severity. The number of total QALYs gained and the total costs associated with supplementation with co-Q10 and with the management of CHF are smaller in the subgroups with greater symptomatic severity, reflecting the reduced life expectancy of these patients. The QALY gains associated with co-Q10 also decrease with more severe disease. The total costs and cost savings associated with CHF-related hospitalisation were also reduced in the subgroups. Although patients receiving standard care had a shorter predicted life expectancy, those in the most severe subgroup had higher costs compared to the base-case subgroup, because of a greater number of hospitalisations in the severe subgroup.
However, these results should be interpreted with some caution. The lack of IPD meant that it was not possible to estimate a treatment effect for any patient subgroups, and so the impact of co-Q10 on CHF-related hospitalisation and ACM estimated for the base-case population was applied to each subgroup. The limited evidence from the Q-SYMBIO trial33 on the composite end point (i.e. major adverse cardiovascular events) suggests that the treatment effect may be greater in NYHA class III than in NYHA class IV. Therefore, the ICER associated with co-Q10 may be somewhat higher in subgroups restricted to the more severe NYHA categories.
Scenario analysis results
Scenario analyses were undertaken to assess the robustness of the base-case results to variation in the sources of data used to populate the model and alternative, plausible, assumptions (Table 29).
| Scenario | Element | Co-Q10 | Standard care | ICER (£) | ||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | |||
| 0 | Base case | 16,767 | 6.44 | 11,889 | 5.11 | 3650 |
| 1 | RR duration 4 years | 15,486 | 5.60 | 11,889 | 5.11 | 7363 |
| 2 | RR duration 10 years | 16,236 | 6.03 | 11,889 | 5.11 | 4695 |
| 3 | Weibull survival model from the ‘West Yorkshire’ data set89–91 | 15,219 | 5.75 | 11,097 | 4.62 | 3643 |
| 4 | Exponential survival model from the Q-SYMBIO trial (FAS)33,73 | 17,600 | 6.83 | 10,995 | 4.52 | 2858 |
| 5 | Weibull survival model from the Q-SYMBIO trial (FAS)33,73 | 17,921 | 6.96 | 12,535 | 5.48 | 3634 |
| 6 | Exponential survival model from the Q-SYMBIO trial (European subset)33,73 | 18,260 | 7.14 | 10,284 | 4.05 | 2583 |
| 7 | Weibull survival model from the Q-SYMBIO trial (European subset)33,73 | 19,566 | 7.72 | 10,906 | 4.42 | 2630 |
| 8 | Effect on ACM from one-stage meta-analysis (excluding the Q-SYMBIO trial33,73) | 16,358 | 6.25 | 11,889 | 5.11 | 3905 |
| 9 | Effect on ACM from two-stage meta-analysis | 17,443 | 6.76 | 11,889 | 5.11 | 3359 |
| 10 | Effect on ACM from NMA | 18,417 | 7.21 | 11,889 | 5.11 | 3097 |
| 11 | Treatment effect on rate of all heart failure-related hospitalisation | 16,367 | 6.45 | 11,889 | 5.11 | 3326 |
| 12 | Treatment effect on rate of first other cardiovascular hospitalisation | 15,796 | 6.47 | 11,889 | 5.11 | 2873 |
| 13 | Treatment effect on rate of all other cardiovascular hospitalisation | 14,804 | 6.49 | 11,889 | 5.11 | 2107 |
| 14 | Utility values from EPHESUS99 | 16,767 | 6.36 | 11,889 | 5.04 | 3699 |
Under all scenarios, co-Q10 was associated with greater benefits and higher costs than standard care and, therefore, co-Q10 remained a cost-effective option for the treatment of CHF. Even under the most pessimistic scenarios, when the duration of treatment effect was limited to shorter time horizons after starting supplementation with co-Q10, the ICER remained well under the cost-effectiveness threshold of £20,000 (£7363 and £4695 per QALY for a duration of benefit of 4 and 10 years, respectively). When the duration of treatment effect was limited to 1 year, the ICER increased to £21,101 per QALY.
Alternative survival models, and source of data for the baseline risk of ACM, had a modest impact on the model results. The use of the Weibull model provided lower survival estimates than the exponential model used in the base-case analysis, resulting in fewer QALYs in both arms. However, the relative impact was minimal and the ICER in this scenario was very similar to that of the base-case analysis. Including survival data from the Q-SYMBIO trial33,73 was associated with poorer survival in the standard care arm and improved survival in the co-Q10 arm, leading to more favourable ICERs in these scenarios. The population of the Q-SYMBIO trial consisted of patients in NYHA classes III and IV, and so these results could be compared with the subgroup analysis presented in the preceding section (see Subgroup analysis) where the ICER was broadly similar at £2741 per QALY when a similar survival model was used. However, total QALYs predicted by the scenario using the Q-SYMBIO trial33,73 were much higher than in the associated subgroup, which may suggest that patients in the Q-SYMBIO trial were not truly in NYHA classes III and IV. Indeed, the authors of the paper acknowledged that there was a possibility that NYHA classes may have been lower than initially recorded, in the study.
When the Q-SYMBIO trial33,73 was excluded from the meta-analysis of ACM, the treatment effect was reduced, resulting in fewer QALYs gained and a slightly higher ICER. However, the treatment effect was associated with a wider CI. The impact of this greater degree of uncertainty was explored in a VOI analysis described above (see Probabilistic analysis and value of information).
Extending the treatment effect from heart failure hospitalisation to other cardiovascular hospitalisation naturally had a favourable impact on the ICER, with more hospitalisation events being averted as a result of co-Q10, leading to reduced costs and QALY losses. Under the most optimistic scenario, where co-Q10 had an impact on the rate of both first and subsequent other cardiovascular hospitalisations, the ICER was £2107 per QALY.
The use of the alternative source of utility values had a very small impact on the ICER. The use of these utility values predicts slightly fewer total QALYs in each arm, as they were estimated from a cohort of post-MI patients who were known to have lower HRQoL.
Summary of cost-effectiveness analysis
The decision model suggests that co-Q10 is a cost-effective strategy for the management of HFrEF when the cost of co-Q10 is borne by the NHS. Assuming that treatment with co-Q10 is continued over a patient’s lifetime, the ICER of co-Q10 compared with standard care alone is £3650 per QALY gained. Co-Q10 remains cost-effective when more pessimistic assumptions regarding the duration of treatment benefit are modelled (i.e. the ICER increased to £7363 and £4695 per QALY when assuming that the treatment benefit with co-Q10 is limited to 4 and to 10 years, respectively). The results are robust to a range of alternative assumptions, including applying alternative baseline event rates and incorporating an alternative source of utility data for a more severe population. In each of the scenarios, the ICER of co-Q10 is consistently under the threshold of cost-effectiveness of £20,000–30,000 per QALY that is conventionally used to establish value for money in the NHS.
The low ICER for co-Q10 compared with standard care is reflected in the estimated high likelihood that co-Q10 is cost-effective. At a threshold of £20,000 per QALY, the probability that co-Q10 is cost-effective is 95.2%.
Under the base-case assumption, the individual EVPI ranges from £162 to £292 across the range of cost-effectiveness thresholds of £10,000 to £30,000 per additional QALY. The estimates of population EVPI range from £116M to £209M across the different thresholds, demonstrating that there appears to be value in undertaking additional research to reduce the existing decision uncertainty. When the EVPI calculations were based on more uncertain estimates of the treatment effect of co-Q10 on ACM, the individual EVPI ranges from £635 to £1487 across the range of cost-effectiveness thresholds of £10,000 to £30,000 per additional QALY. The corresponding estimates of population EVPI range from £455M to £1066M.
The decision model suggests that co-Q10 may be more cost-effective in subgroups of patients with greater symptomatic severity. However, the lack of IPD means that it was not possible to estimate a treatment effect for any patient subgroups and so the impact of co-Q10 on CHF-related hospitalisation and ACM estimated for the base-case population was applied in each subgroup and, therefore, results should be interpreted cautiously.
Chapter 8 Discussion
This report presents the first economic model of the clinical effectiveness and cost-effectiveness of co-Q10 for treating HFrEF, and a comprehensive and up-to-date systematic review of the clinical trial evidence. The systematic review was conceived as an IPD meta-analysis. However, insufficient data were received, or pledged, for a full IPD meta-analysis to be completed and, instead, data synthesis used mostly the aggregate data available in trial publications. Economic modelling used IPD from a UK observational data set of patients with CHF90 to inform a number of model parameters, along with treatment effects derived from the meta-analyses.
Main findings
Overall, we identified 26 randomised trials (comprising 2250 participants) that compared adjunct co-Q10 supplementation with standard treatment alone in patients with CHF and a reduced ejection fraction. Trials varied in their inclusion criteria, although most patients had CHF of NYHA class II or III.
Meta-analysis of data from seven trials33,48,55,57,59,62,70 suggested that co-Q10 had a potentially large benefit on mortality (although the CI just crosses 1.0), reducing ACM by over 30% (RR 0.68, 95% CI 0.45 to 1.03). This benefit appeared surprisingly large for a micronutrient supplement given in addition to standard care (compared with standard care alone). The effect size was similar to or larger than that of disease-modifying pharmacological therapies96 for the secondary prevention of ACM, using commonly prescribed drugs such as ACE inhibitors,111 angiotensin receptor blockers,112 beta-antagonists113 and angiotensin neprolysin inhibitors. 83 Benefit was also shown in time-to-event analyses of survival.
By contrast, the results for short-term functional outcomes were more modest or unclear. Co-Q10 appeared to produce improvements in LVEF of around 1–2% (MD 1.76%, 95% CI 0.21% to 3.31%), which is within the measurement error of imaging modalities. 114 Results for improvement by one or more class in NYHA, which provides a measure of symptom burden, suggested a modest but uncertain improvement (RR 1.19, 95% CI 0.93 to 1.52).
Different trials contributed to the analyses of short-term functional and longer-term outcomes, making it difficult to make comparisons, but the inconsistency between short- and longer-term outcomes was notable. However, an observed disconnect between functional outcomes and mortality is not uncommon. Many treatments for CHF improve longevity while having a marginal benefit on symptoms, whereas others improve symptoms but do not extend longevity. 115 Nonetheless, the lack of obvious remodelling benefit of co-Q10 is concerning, as improved heart function is a key mechanism of improved outcome that is not seen in the present data.
Admission to hospital for CHF, which is a driver of both costs to health systems and individual well-being, was reduced by 40%, although this was based on data from only two trials. 33,62 Results for other outcomes were generally limited by a lack of data. Although most data favoured co-Q10, they were not conclusive. There was no evidence of improved QoL at the end of the trials, nor any indication that adverse events increased with co-Q10.
There was no evidence that co-Q10 dose, trial duration, values of outcome at baseline or age of the trial had any impact on the relative effectiveness of co-Q10. In particular, there was no evidence that taking statins alongside co-Q10 altered the effectiveness of co-Q10, but this was based on meta-regression, which lacks power to detect such differences. The intention to explore the potential interaction between statins and co-Q10 was a major motivation for seeking IPD and this important question remains largely unaddressed.
As few trials combined co-Q10 with selenium or other nutrients, analyses were limited, but there was no evidence that additional supplements modified the effectiveness of co-Q10 either in subgroup analyses or in NMAs. There therefore appear to be no evidential grounds for the preference of a single- or a multi-micronutrient supplement.
In economic modelling, base-case cost-effectiveness results showed incremental costs of £4878, incremental QALYs of 1.34 and an ICER of £3650 per QALY, based on a lifetime treatment duration. Although the impact of co-Q10 on ACM was associated with some uncertainty, probabilistic sensitivity analyses at cost-effectiveness thresholds of £20,000 and £30,000 per QALY showed high probability (95.2% and 95.8%, respectively) that adjunct co-Q10 was a cost-effective option relative to standard therapy alone. The cost-effectiveness results remained robust to a range of scenario analyses and demonstrated that the ICER was consistently under the threshold of cost-effectiveness of £20,00–30,000 per QALY conventionally used by NICE to establish value for money in the NHS. To exceed the £20,000-per-QALY threshold would require the duration of treatment effect to fall to ≤ 1 year, with all other assumptions held the same as in the base case. This would require a large difference in parameter values that would no longer be evidence based.
The results also suggested that co-Q10 may be more cost-effective in subgroups with more serious symptoms. However, the lack of IPD meant that it was not possible to estimate a treatment effect specific to any patient subgroup.
Analysis of EVPI was undertaken to determine the expected costs of decision uncertainty predicted by the model and the maximum value that can be placed on additional research aimed at reducing this uncertainty. This provides a benchmark value against which to compare the likely cost of new research and to decide whether or not the new research is potentially worthwhile. The population EVPI ranged between £116M and £209M under the base-case model assumptions. The probability of decision error was low at cost-effectiveness thresholds above £10,000 per QALY, but the consequences of decision error were valued highly. This, together with a large patient population who could ultimately benefit from additional research, indicates that a future trial of adjunct co-Q10 in patients with HFrEF could be valuable.
Strengths and limitations
Comprehensive literature searches were undertaken and the systematic review methods followed best practice. The decision model was developed to address a number of important evidence gaps concerning the use of co-Q10 for the management of HFrEF, notably the absence of cost-effectiveness studies undertaken from the perspective of the NHS.
Most trials were poorly reported, and many were at unclear risk of bias in several domains. Several key outcomes that we believe should have been routinely collected, including mortality, NYHA class and LVEF, were missing in several trials. This may suggest a possibility of selective outcome reporting (with less favourable outcomes going unreported), but, with few trial registration records or published protocols, our ability to explore this was limited.
Follow-up ranged from 12 weeks to 2 years after randomisation, with only five trials following patients for ≥ 1 year. 33,56,59,62,70 Most of the short-term trials examined only functional outcomes. This limited the scope of the survival analyses and the appropriateness of evidence against which to consider ‘rest-of-life’ treatment.
Obtaining trial data sets and harmonising and reanalysing participant-level data would have supported more detailed analyses, particularly those examining potential effect modifiers. In the absence of IPD, the extent to which we were able to explore these was limited and the meta-regressions performed lacked power to detect important modifying effects.
Many of the meta-analysis results were driven by the largest and most comprehensively reported trial (the Q-SYMBIO trial33), which, therefore, also had an important impact on the economic modelling. The Q-SYMBIO publication notes that survival was better than anticipated and that approximately one-fifth of patients in both the co-Q10 and the control arms were stabilised on standard therapy without diuretics. This suggests that more trial participants may have had CHF of NYHA class II than were recorded. As the Q-SYMBIO trial33 was a major driver of the meta-analysis results, this raises some concerns about the applicability of the findings to the UK population because of differences in care pathways and disease severity.
For the economic analyses, access to a UK-representative observational data set permitted analysis of IPD, allowing development of risk equations for different patient subgroups and survival over a longer time period than was available from the RCT evidence. Modelling strengths include the range of scenarios that were explored to examine the robustness of the cost-effectiveness and EVPI results that were robust to a series of alternative assumptions – and the consistency of these findings.
The cost-effectiveness and EVPI results are subject to several important limitations. The strength of the conclusions that can be drawn, based on the current set of cost-effectiveness results, clearly depends on the validity of the evidence entering the model. As noted above, a number of concerns remain about the existing evidence from co-Q10 trials relating to the treatment effect for both mortality and hospitalisations, which are key parameters in the model. Furthermore, the development of the model relied on judgements or assumptions that reflect the underlying structure of the decision problem and patient pathway. As the model structure, or functional form, approximates to real-world (clinical) practice only, this results in structural uncertainty116 (e.g. in how the model is used to extrapolate the effects over time). Methodological uncertainty may also arise from differences in model outcomes resulting from analytical choices, for example the choice of the evaluation framework (cost–benefit, cost-effectiveness or cost–utility analysis), time horizon, discount rate and comparators. 117
It should be borne in mind that both the cost-effectiveness results and the EVPI analysis quantify only the decision uncertainty predicted by the model. They do not address structural or methodological uncertainty that is inherent in the set of model assumptions and approach taken. Several methods for handling structural uncertainty have been described in the literature, but these would require additional parameterisation,118 model averaging or weighting of the scenario analyses,45 or a model discrepancy approach. 119 It was not feasible to elicit plausible weights for the scenarios analyses or to formally quantify the impact of separate modelling choices on the results. Therefore, the EVPI results depend, critically, on the model assumptions and the additional assumptions about the size of the patient population that could ultimately benefit from additional research over an appropriate time horizon. Although the EVPI results demonstrated significant value in undertaking further research to resolve uncertainty, the results present an expected upper bound to this value. A new trial would be expected to resolve only a proportion of this uncertainty and, therefore, the amount of uncertainty likely to be resolved would need to be assessed against the cost of the trial (based on its design features) to ensure that any further research is an efficient use of NHS resources.
Comparison with previous studies
Our systematic review and meta-analysis was more extensive and included a larger number of trials and participants than other recently published systematic reviews of co-Q10 for CHF. The most recent systematic reviews that included the Q-SYMBIO trial, those by Lei and Liu34 and Trongtorsak et al. ,35 both found a large improvement in ACM among co-Q10-treated patients compared with control patients (RR 0.69, 95% CI 0.50 to 0.95, I2 = 0%, and HR 0.62, 95% CI 0.40 to 0.95, respectively). The fact that these reviews had narrower CIs and reached conventional levels of statistical significance most likely reflects their two-stage approach (our two-stage analysis of ACM was also statistically significant). The results of the one-stage analysis of ACM, presented here, are preferred because this approach performs better when event rates are low, and we believe that the larger estimate of heterogeneity and the greater uncertainty is appropriately conservative. Trongtorsak et al. 35 also reported a similarly large reduction in hospitalisation with co-Q10 (HR 0.39, 95% CI 0.29 to 0.53; p < 0.001).
No previous economic models or VOI analyses have addressed the use of adjunctive co-Q10 in CHF.
Issues in obtaining individual participant data
It was disappointing that it proved so difficult to obtain IPD from co-Q10 trials, much more so than for other IPD meta-analyses that we have completed previously. For trials conducted in the 1980s and 1990s, it was difficult to trace investigators. A number of authors were deceased or had retired, and in other cases data were no longer stored or available (possibly reflecting the times during which they were collected and changing priorities for the long-term management of clinical trial data).
Although difficulty in tracing and obtaining data from older trials was understandable, it was also difficult to secure data from more recent trials. We were able to trace contacts for most trials conducted after 2000. Several trial investigators responded that the data were no longer stored and available, possibly reflecting the fact that many had been carried out in a single centre and did not appear to have involved an established clinical trials unit (trials units often take responsibility for the long-term storage of trial data), as well as changing attitudes to long-term data storage. Many trial investigators did not reply to invitations to participate in the project; some who had initially agreed to collaborate subsequently did not respond to formal requests for IPD. Overall, we secured agreement to collaborate from only four trials and ultimately received two data sets55,59 by our cut-off date of April 2020. It is not clear why it was quite so difficult to obtain data from these relatively recent trials. We know that the contacts of one trial did not support our approach and that the same trial team had concerns about other investigators that they erroneously believed that our team was working with. However, the reasons for other trial investigators’ reluctance to participate were less obvious. We offered participation in an active collaboration, and group authorship of outputs, acknowledging contribution and providing academic credit for participation and sharing data. It may be that this particular clinical community is less familiar with the concept of IPD meta-analysis, although there are long-standing examples of successful IPD meta-analysis collaborations on cardiovascular topics. 120,121 As co-Q10 is not a licensed drug, potentially those involved in its evaluation might be less familiar or comfortable with the idea of releasing data for independent analysis, as would be routine for trials involved in obtaining market authorisation for pharmaceuticals, and, therefore, perhaps less inclined to share data.
It was particularly disappointing that after initially promising interactions we were unable to secure data from the Q-SYMBIO trial33 (i.e. the largest trial), which, being multicentre, registered (retrospectively), run to good clinical practice and with clearly reported results, for a comprehensive set of outcomes, carries the hallmarks of a well-designed and well-conducted trial. Obtaining the IPD would have enabled us to explore and better understand the trial data and results, such as the effect of potential effect modifiers and the impact of censoring patients at the point of device implantation. These could have been informative for our economic modelling.
Given that several trials reported that some support had been received from co-Q10 manufacturers, independent scrutiny could generally have helped dispel possible concerns about potential conflicts of interest and influence on the evidence base. Inability to obtain and scrutinise trial data was generally of concern, given that bias assessment was rated as unclear for many trials and high risk for several trials, particularly as the meta-analysis-estimated reductions for ACM appeared unexpectedly large. Our inability to explore such issues in detail must leave some question marks on the robustness of the meta-analysis results overall.
Chapter 9 Conclusions
Co-Q10 has potential to be a clinically effective and cost-effective intervention for HFrEF, if prescribed. At a prescribed cost of < £30 per month, it is relatively inexpensive and appears to have few side effects (although it is more costly than the standard prescribed background medication, which costs around £2 per month). However, given concerns about risk of bias and the applicability of the existing evidence, before considering NHS prescription there is a need to confirm the meta-analysis findings to establish whether or not co-Q10 is genuinely effective in a typical UK population.
Additional primary research could strengthen the evidence base considerably. Given that co-Q10 has not been subject to the rigour and scrutiny of drug-licensing processes, if it were to be offered as an NHS treatment it would seem important that robust independent trial data were available to underpin that decision. Furthermore, although inexpensive and potentially cost saving to the NHS, making co-Q10 routinely available to all HFrEF patients would incur purchase costs of around £113M per year. It would, therefore, be important to understand to what extent these costs might be defrayed by reduced hospitalisation and justified by extending or improving quality of life. Moreover, adding to the pill burden of patients commonly already coping with taking five or so disease-modifying agents per day comes with a responsibility that each has been tested thoroughly and shown to be beneficial.
Should further research confirm the benefits of co-Q10, suggested in the meta-analysis, and its cost-effectiveness, as suggested by our economic model, this would support consideration of NHS prescribing, making co-Q10 available to all relevant CHF patients with a reduced ejection fraction and providing equity of access. Conversely, if further research found benefit to be limited, then this would provide important information for those who currently purchase co-Q10 at their own expense.
Implications for service provision
Currently co-Q10 is not available on NHS prescription. CHF patients who wish to use co-Q10 purchase it at their own expense. Co-Q10 is relatively inexpensive when prescribed and appears to have few side effects. Our analyses have shown that co-Q10 has potential to be a clinically effective and cost-effective intervention for HFrEF patients, from an NHS perspective. However, given concerns about possible bias, applicability and plausibility of meta-analysis effect sizes, stronger evidence may be needed before considering NHS prescription, particularly as co-Q10 has never been through the rigour and scrutiny of drug-licensing processes.
If the variation in the size of benefits on the symptoms observed between the trials included in the meta-analysis reflects differences in patient phenotypes, a targeted approach might be more appropriate, limiting use to those with the most to gain. To the best of our knowledge, there is currently no robust evidence to inform targeting, but this could be generated by a new trial.
Suggested research priorities
Given concerns about possible bias, applicability and plausibility of effect size, and as co-Q10 has never been through the rigour and scrutiny of drug-licensing processes, an adequately powered placebo-controlled RCT of co-Q10 in a typical UK HFrEF population may be warranted. Such a trial would seem to be a necessary step before NHS prescription could be considered. Our analyses suggested that the value of reducing decision uncertainty is highly likely to outweigh the costs of a new trial. The amount of uncertainty that is likely to be resolved by a new trial would need to be assessed against the cost of the trial (based on its design features) to ensure that any further research is an efficient use of NHS resources. However, the cost of a new trial is likely to be much less than the EVPI estimates.
A new trial might be designed to be relevant to primary care, to recruit typical HFrEF patients, based on clinical risk factors, and to resolve questions about whether or not co-Q10 could be particularly beneficial for patients taking statins, stratified by statin use. Outcomes should be patient centred and include mortality, CHF hospitalisation and QoL assessment. Trial duration would need to be 3–5 years to adequately capture long-term outcomes and, ideally, the trial would be linked to routine data sets to allow hospital utilisation to be captured.
A trial of patients with HFpEF could also be considered. HFpEF is a heterogeneous condition with different underlying causes and its response to treatments is likely to vary phenotypically. In the absence of existing evidence, or plausible biological hypotheses about which types of patient might benefit from any particular treatment, trials would need to be powered to detect effectiveness within phenotype subgroups and it would need to be anticipated that an intervention may be ineffective for a proportion of the trial population. Although not eligible for our systematic review, searches identified one trial72 of 30 HFpEF patients, which found no short-term improvement in diastolic function. Results from two current trials122,123 (aiming to recruit 276 and 60 participants, respectively) may provide signals of whether or not co-Q10 is potentially effective in HFpEF and whether or not a trial of co-Q10 in HFpEF would be warranted.
Any new trial should have a clear data-sharing policy in place to enable future independent scrutiny and inclusion in additional research, thereby maximising the utility of the data generated by those patients participating in the trial.
Acknowledgements
We thank Alessandro Grosso (NIHR Methods Research Fellow, Centre for Health Economics, University of York, UK) and Samriti Sharma for translation advice during the study selection and quality assessment stages of the systematic review. We also thank Claire Khouja for proofreading the report.
Contributions of authors
Lindsay Claxton (https://orcid.org/0000-0002-1795-7568) (Research Fellow) contributed to the development of the protocol, developed and built the economic analysis, wrote sections of the report pertaining to the economic analysis and contributed to the writing of all sections of the report.
Mark Simmonds (https://orcid.org/0000-0002-1999-8515) (Senior Research Fellow) contributed to the development of the protocol, developed the statistical analysis plan, performed the statistical analyses, provided oversight of data extraction and quality assessment, wrote sections of the report pertaining to meta-analyses and contributed to the writing of all sections of the report.
Lucy Beresford (https://orcid.org/0000-0001-6803-5566) (Research Fellow) performed study selection, data extraction and quality assessment, and contributed to the writing of all sections of the report.
Richard Cubbon (https://orcid.org/0000-0001-7844-3600) (Associate Professor and Honorary Consultant Cardiologist) provided access to, and answered questions regarding, the ‘West Yorkshire’ data set, was a member of the Project Advisory Group, provided feedback and advice during the project, and commented on the final report.
Mark Dayer (https://orcid.org/0000-0002-0976-9743) (Consultant Cardiologist, Honorary Senior Lecturer, University of Exeter) was a member of the Project Advisory Group, provided feedback and advice during the project and commented on the final report.
Stephen S Gottlieb (Professor of Medicine) provided and answered queries about IPD from his trial and commented on the final report.
Nick Hartshorne-Evans (https://orcid.org/0000-0003-0356-1471) (CEO, The Pumping Marvellous Foundation) was a patient and public involvement and engagement member of the Project Advisory Group, contributed to protocol development, provided feedback and advice during the project, and commented on the final report.
Bruce Kilroy was a patient and public involvement and engagement member of the Project Advisory Group, provided feedback and advice during the project, and commented on the final report.
Alexis Llewellyn (https://orcid.org/0000-0003-4569-5136) (Research Fellow) contributed to the development of the protocol, performed study selection, data extraction and quality assessment, and contributed to the writing of all sections of the report.
Claire Rothery (https://orcid.org/0000-0002-7759-4084) (Senior Research Fellow) contributed to the development of the protocol, provided advice and input to the development of the economic analysis, wrote sections of the report pertaining to the economic analysis and contributed to the writing of all sections of the report.
Sahar Sharif (https://orcid.org/0000-0001-6885-0456) (Research Fellow) performed study selection, data extraction and quality assessment, undertook communications with trial authors and contributed to the writing of all sections of the report.
Jayne F Tierney (https://orcid.org/0000-0002-4734-3014) (Professor and co-lead, Meta-analysis Programme) was a member of the Project Advisory Group, provided feedback and advice during the project, and commented on the final report.
Klaus K Witte (https://orcid.org/0000-0002-7146-7105) (Academic Fellow and Consultant Cardiologist) contributed to the development of the protocol, provided clinical advice and oversight, and contributed to the writing of all sections of the report. He also provided and answered questions about IPD from his RCT.
Kath Wright (https://orcid.org/0000-0002-9020-1572) (Information Service Manager) prepared and performed all database searches for the systematic reviews.
Lesley A Stewart (https://orcid.org/0000-0003-0287-4724) (Director of CRD and Professor of Evidence Synthesis) contributed to the development of the protocol, undertook communications with trial authors, wrote sections of the report, contributed to the writing of all sections of the report and provided oversight of the project as a whole.
Data-sharing statement
Requests for access to the aggregate data used in the systematic review should be addressed to the corresponding author. Requests for IPD from the two trials that shared data should be addressed to the trial authors directly. Queries regarding the ‘West Yorkshire’ database should be directed to r.cubbon@leeds.ac.uk.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194-202. https://doi.org/10.1001/jama.289.2.194.
- Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137-46. https://doi.org/10.1136/hrt.2003.025270.
- Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation 2006;113:799-805. https://doi.org/10.1161/CIRCULATIONAHA.104.492033.
- Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail 2011;4:396-403. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959882.
- Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA 2020;324:488-504. https://doi.org/10.1001/jama.2020.10262.
- National Institute for Health and Care Excellence (NICE) . New NICE Guidance Will Improve Diagnosis and Treatment of Chronic Heart Failure 2010.
- Cowie MR, Simon M, Klein L, Thokala P. The cost-effectiveness of real-time pulmonary artery pressure monitoring in heart failure patients: a European perspective. Eur J Heart Fail 2017;19:661-9. https://doi.org/10.1002/ejhf.747.
- Tian Y, Dixon A, Gao H. Emergency Hospital Admissions for Ambulatory Care Sensitive Conditions: Identifying the Potential for Reductions. London: The King’s Fund; 2012.
- Cadenas E, Ahmad S. Oxidative Stress and Antioxidant Defenses in Biology. London: Chapman & Hall; 1995.
- Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion 2007;7:S41-50. https://doi.org/10.1016/j.mito.2007.02.006.
- Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol 2007;37:31-7. https://doi.org/10.1007/s12033-007-0052-y.
- Witting PK, Pettersson K, Letters J, Stocker R. Anti-atherogenic effect of coenzyme Q10 in apolipoprotein E gene knockout mice. Free Radic Biol Med 2000;29:295-30. https://doi.org/10.1016/S0891-5849(00)00311-7.
- Vogt A, Kübler W. Heart failure: is there an energy deficit contributing to contractile dysfunction?. Basic Res Cardiol 1998;93:1-10. https://doi.org/10.1007/s003950050055.
- Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 2001;88:529-35. https://doi.org/10.1161/01.res.88.5.529.
- Starling RC, Hammer DF, Altschuld RA. Human myocardial ATP content and in vivo contractile function. Mol Cell Biochem 1998;180:171-7. https://doi.org/10.1023/A:1006876031121.
- Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure – a rational approach for disease treatment. Cell Metab 2015;21:183-94. https://doi.org/10.1016/j.cmet.2015.01.005.
- Folkers K, Littarru GP, Ho L, Runge TM, Havanonda S, Cooley D. Evidence for a deficiency of coenzyme Q10 in human heart disease. Int Z Vitaminforsch 1970;40:380-90.
- Kitamura N, Yamaguchi A, Otaki M, Folkers K, Yamamura Y. Biomedical and Physical Aspects of Coenzyme Q. Amsterdam: Elsevier; 1984.
- Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci U S A 1985;82:901-4. https://doi.org/10.1073/pnas.82.3.901.
- McMurray JJ, Dunselman P, Wedel H, Cleland JG, Lindberg M, Hjalmarson A, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J Am Coll Cardiol 2010;56:1196-204. https://doi.org/10.1016/j.jacc.2010.02.075.
- Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol 2008;52:1435-41. https://doi.org/10.1016/j.jacc.2008.07.044.
- Shimizu M, Miyazaki T, Takagi A, Sugita Y, Yatsu S, Murata A, et al. Low circulating coenzyme Q10 during acute phase is associated with inflammation, malnutrition, and in-hospital mortality in patients admitted to the coronary care unit. Heart Vessels 2017;32:668-73. https://doi.org/10.1007/s00380-016-0923-x.
- Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 Drug Surveillance Investigators. Mol Aspects Med 1994;15:s287-94. https://doi.org/10.1016/0098-2997(94)90040-X.
- Jameson S. Statistical data support prediction of death within 6 months on low levels of coenzyme Q10 and other entities. Clin Investig 1993;71:137-9. https://doi.org/10.1007/BF00226855.
- Witte KK, Clark AL, Cleland JG. Chronic heart failure and micronutrients. J Am Coll Cardiol 2001;37:1765-74. https://doi.org/10.1016/S0735-1097(01)01227-X.
- Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A 1990;87:8931-4. https://doi.org/10.1073/pnas.87.22.8931.
- Davidson M, McKenney J, Stein E, Schrott H, Bakker-Arkema R, Fayyad R, et al. Comparison of one-year efficacy and safety of atorvastatin versus lovastatin in primary hypercholesterolemia. Atorvastatin Study Group I. Am J Cardiol 1997;79:1475-81. https://doi.org/10.1016/S0002-9149(97)00174-4.
- Langsjoen PH. The Clinical Use of HMG CoA-Reductase Inhibitors (Statins) and the Associated Depletion of the Essential Co-Factor Coenzyme Q10; A Review of Pertinent Human and Animal Data. Silver Spring, MD: US Food and Drug Administration; 2003.
- Morisco C, Nappi A, Argenziano L, Sarno D, Fonatana D, Imbriaco M, et al. Noninvasive evaluation of cardiac hemodynamics during exercise in patients with chronic heart failure: effects of short-term coenzyme Q10 treatment. Mol Aspects Med 1994;15:s155-63. https://doi.org/10.1016/0098-2997(94)90025-6.
- Mortensen SA, Vadhanavikit S, Muratsu K, Folkers K. Coenzyme Q10: clinical benefits with biochemical correlates suggesting a scientific breakthrough in the management of chronic heart failure. Int J Tissue React 1990;12:155-62.
- Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q10 supplementation on heart failure: a meta-analysis. Am J Clin Nutr 2013;97:268-75. https://doi.org/10.3945/ajcn.112.040741.
- Madmani ME, Yusuf Solaiman A, Tamr Agha K, Madmani Y, Shahrour Y, Essali A, et al. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev 2014;6. https://doi.org/10.1002/14651858.CD008684.pub2.
- Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail 2014;2:641-9. https://doi.org/10.1016/j.jchf.2014.06.008.
- Lei L, Liu Y. Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials. BMC Cardiovasc Disord 2017;17. https://doi.org/10.1186/s12872-017-0628-9.
- Trongtorsak A, Kongnatthasate K, Susantitaphong P, Kittipibul V, Ariyachaipanich A. Effect of coenzyme Q10 on left ventricular remodeling and mortality in patients with heart failure: a meta-analysis. J Am Coll Cardiol 2017;69. https://doi.org/10.1016/S0735-1097(17)34096-2.
- Hopper I, Connell C, Briffa T, De Pasquale CG, Driscoll A, Kistler PM, et al. Nutraceuticals in patients with heart failure: a systematic review. J Card Fail 2020;26:166-79. https://doi.org/10.1016/j.cardfail.2019.10.014.
- Advanced Ovarian Cancer Trialists Group . Chemotherapy in advanced ovarian cancer: an overview of randomised clinical trials. BMJ 1991;303:884-93. https://doi.org/10.1136/bmj.303.6807.884.
- Stewart L, Clarke M. Practical methodology of meta-analyses (overviews) using updated individual participant data. Stat Med 1995;14:2057-79. https://doi.org/10.1002/sim.4780141902.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. https://doi.org/10.1016/j.jacc.2013.05.019.
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. https://doi.org/10.1093/eurheartj/ehs104.
- McLellan J, Heneghan CJ, Perera R, Clements AM, Glasziou PP, Kearley KE, et al. B-type natriuretic peptide-guided treatment for heart failure. Cochrane Database Syst Rev 2016;12. https://doi.org/10.1002/14651858.CD008966.pub2.
- Higgins G. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (Updated June 2017). London: The Cochrane Collaboration; 2017.
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods 2014;5:79-85. https://doi.org/10.1002/jrsm.1090.
- Simmonds MC, Higgins JP. A general framework for the use of logistic regression models in meta-analysis. Stat Methods Med Res 2016;25:2858-77. https://doi.org/10.1177/0962280214534409.
- Jackson D, Law M, Stijnen T, Viechtbauer W, White IR. A comparison of seven random-effects models for meta-analyses that estimate the summary odds ratio. Stat Med 2018;37:1059-85. https://doi.org/10.1002/sim.7588.
- Lu G, Ades A. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006;101:447-59. https://doi.org/10.1198/016214505000001302.
- Belardinelli R, Muçaj A, Lacalaprice F, Solenghi M, Principi F, Tiano L, et al. Coenzyme Q10 improves contractility of dysfunctional myocardium in chronic heart failure. Biofactors 2005;25:137-45. https://doi.org/10.1002/biof.5520250115.
- Berman M, Erman A, Ben-Gal T, Dvir D, Georghiou GP, Stamler A, et al. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clin Cardiol 2004;27:295-9. https://doi.org/10.1002/clc.4960270512.
- Davini A, Cellerini F, Topi PL. Coenzyme Q10: contractile dysfunction of the myocardial cell and metabolic therapy. Minerva Cardioangiol 1992;40:449-53.
- Fumagalli S, Fattirolli F, Guarducci L, Cellai T, Baldasseroni S, Tarantini F, et al. Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study. Clin Cardiol 2011;34:211-17. https://doi.org/10.1002/clc.20846.
- Garakyaraghi M, Bahrami P, Sadeghi M, Rabiei K, Taheri M, Pourmoghaddas A. Combination effects of seleniumand coenzyme Q10 on left ventricular systolic function in patients with heart failure. Iranian Heart J 2015;15:6-12.
- Hofman-Bang C, Rehnqvist N, Swedberg K, Wiklund I, Aström H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. The Q10 Study Group. J Card Fail 1995;1:101-7. https://doi.org/10.1016/1071-9164(95)90011-x.
- Kawashima C, Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Sato R, et al. Ubiquinol improves endothelial function in patients with heart failure with reduced ejection fraction: a single-center, randomized double-blind placebo-controlled crossover pilot study. Am J Cardiovasc Drugs 2020;20:363-72. https://doi.org/10.1007/s40256-019-00384-y.
- Keogh A, Fenton S, Leslie C, Aboyoun C, Macdonald P, Zhao YC, et al. Randomised double-blind, placebo-controlled trial of coenzyme Q, therapy in class II and III systolic heart failure. Heart Lung Circ 2003;12:135-41. https://doi.org/10.1046/j.1443-9506.2003.00189.x.
- Khatta M, Alexander BS, Krichten CM, Fisher ML, Freudenberger R, Robinson SW, et al. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med 2000;132:636-40. https://doi.org/10.7326/0003-4819-132-8-200004180-00006.
- Kukharchik G, Sichinava L, Burbello A, Yubrina I, Gaykovaya L, Sorokin L, et al. Analysis of changes of ECHO-cardiogram indices in patients with chronic heart failure during therapy with coenzyme Q10. Eur J Heart Fail 2016;1. https://doi.org/10.1002/ejhf.539.
- Kumar A, Singh RB, Saxena M, Niaz MA, Josh SR, Chattopadhyay P, et al. Effect of carni Q-gel (ubiquinol and carnitine) on cytokines in patients with heart failure in the Tishcon study. Acta Cardiol 2007;62:349-54. https://doi.org/10.2143/AC.62.4.2022278.
- Langsjoen PH, Vadhanavikit S, Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proc Natl Acad Sci U S A 1985;82:4240-4. https://doi.org/10.1073/pnas.82.12.4240.
- Ma A, Zhang W, Liu Z. Effect of protection and repair of injury of mitochondrial membrane-phospholipid on prognosis in patients with dilated cardiomyopathy. Blood Press Suppl 1996;3:53-5.
- Mareev VY, Minina YV, Mareev YV. Coenzyme Q-10 in treatment of patients with heart failure: Results Russian multicenter double blind placebo controlled study. Eur J Heart Fail 2017;19. https://doi.org/10.1002/ejhf.833.
- Mazzola C, Guffanti EE, Vaccarella A. Noninvasive assessment of coenzyme Q 10 in patients with chronic stable effort angina and moderate heart failure. Curr Ther Res Clin Exp 1987;41:923-32.
- Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. Clin Investig 1993;71:134-6. https://doi.org/10.1007/BF00226854.
- Munkholm H, Hansen HH, Rasmussen K. Coenzyme Q10 treatment in serious heart failure. Biofactors 1999;9:285-9. https://doi.org/10.1002/biof.5520090225.
- Nakanishi M, Yokota Y, Fukuzaki H. The chronic effect of coenzyme Q10 in dilated cardiomyopathy: echocardiographic assessment. Kokyu To Junkan 1988;36:655-60.
- Permanetter B, Rössy W, Klein G, Weingartner F, Seidl KF, Blömer H. Ubiquinone (coenzyme Q10) in the long-term treatment of idiopathic dilated cardiomyopathy. Eur Heart J 1992;13:1528-33. https://doi.org/10.1093/oxfordjournals.eurheartj.a060096.
- Poggesi L, Galanti G, Comeglio M, Toncelli L, Vinci M. Effect of coenzyme Q10 on left ventricular function in patients with dilative cardiomyopathy. A medium-term randomized double-blind study versus placebo. Curr Ther Res Clin Exp 1991;49:878-86.
- Pourmoghaddas M, Rabbani M, Shahabi J, Garakyaraghi M, Khanjani R, Hedayat P. Combination of atorvastatin/coenzyme Q10 as adjunctive treatment in congestive heart failure: a double-blind randomized placebo-controlled clinical trial. ARYA Atheroscler 2014;10:1-5.
- Watson PS, Scalia GM, Galbraith A, Burstow DJ, Bett N, Aroney CN. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol 1999;33:1549-52. https://doi.org/10.1016/S0735-1097(99)00064-9.
- Witte KK, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J 2005;26:2238-44. https://doi.org/10.1093/eurheartj/ehi442.
- Zhao Q, Kebbati AH, Zhang Y, Tang Y, Okello E, Huang C. Effect of coenzyme Q10 on the incidence of atrial fibrillation in patients with heart failure. J Investig Med 2015;63:735-9. https://doi.org/10.1097/JIM.0000000000000202.
- Alehagen U, Johansson P, Björnstedt M, Rosén A, Dahlström U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol 2013;167:1860-6. https://doi.org/10.1016/j.ijcard.2012.04.156.
- Sobirin MA, Herry Y, Sofia SN, Uddin I, Rifqi S, Tsutsui H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discov Ther 2019;13:38-46. https://doi.org/10.5582/ddt.2019.01004.
- Mortensen AL, Rosenfeldt F, Filipiak KJ. Effect of coenzyme Q10 in Europeans with chronic heart failure: a sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol J 2019;26:147-56. https://doi.org/10.5603/CJ.a2019.0022.
- Marchionni N, Ferrucci L, Baldasseroni S, Fumagalli S, Guralnik JM, Bonazinga M, et al. Item re-scaling of an Italian version of the sickness impact profile: effect of age and profession of the observers. J Clin Epidemiol 1997;50:195-201. https://doi.org/10.1016/S0895-4356(96)00318-6.
- Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J 2003;24:442-63. https://doi.org/10.1016/S0195-668X(02)00823-0.
- Wiklund I, Lindvall K, Swedberg K, Zupkis RV. Self-assessment of quality of life in severe heart failure. An instrument for clinical use. Scand J Psychol 1987;28:220-5. https://doi.org/10.1111/j.1467-9450.1987.tb00758.x.
- Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12.
- National Institute for Health and Care Excellence (NICE) . Chronic Heart Failure in Adults: Diagnosis and Management 2018. www.nice.org.uk/guidance/ng106 (accessed 24 September 2020).
- Lee D, Wilson K, Akehurst R, Cowie MR, Zannad F, Krum H, et al. Cost-effectiveness of eplerenone in patients with systolic heart failure and mild symptoms. Heart 2014;100:1681-7. https://doi.org/10.1136/heartjnl-2014-305673.
- Pandor A, Thokala P, Gomersall T, Baalbaki H, Stevens JW, Wang J, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17320.
- Pufulete M, Maishman R, Dabner L, Mohiuddin S, Hollingworth W, Rogers CA, et al. Effectiveness and cost-effectiveness of serum B-type natriuretic peptide testing and monitoring in patients with heart failure in primary and secondary care: an evidence synthesis, cohort study and cost-effectiveness model. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21400.
- Laramée P, Wonderling D, Swain S, Al-Mohammad A, Mant J. Cost-effectiveness analysis of serial measurement of circulating natriuretic peptide concentration in chronic heart failure. Heart 2013;99:267-71. https://doi.org/10.1136/heartjnl-2012-302692.
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004. https://doi.org/10.1056/NEJMoa1409077.
- Mealing S, Woods B, Hawkins N, Cowie MR, Plummer CJ, Abraham WT, et al. Cost-effectiveness of implantable cardiac devices in patients with systolic heart failure. Heart 2016;102:1742-9. https://doi.org/10.1136/heartjnl-2015-308883.
- Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, Marshall T. Survival following a diagnosis of heart failure in primary care. Fam Pract 2017;34:161-8. https://doi.org/10.1093/fampra/cmw145.
- Witte K, Hasenfuss G, Kloppe A, Burkhoff D, Green M, Moss J, et al. Cost-effectiveness of a cardiac contractility modulation device in heart failure with normal QRS duration. ESC Heart Fail 2019;6:1178-87. https://doi.org/10.1002/ehf2.12526.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9] 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 24 September 2020).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Cubbon RM, Lowry JE, Drozd M, Hall M, Gierula J, Paton MF, et al. Vitamin D deficiency is an independent predictor of mortality in patients with chronic heart failure. Eur J Nutr 2019;58:2535-43. https://doi.org/10.1007/s00394-018-1806-y.
- Drozd M, Garland E, Walker AMN, Slater TA, Koshy A, Straw S, et al. Infection-related hospitalization in heart failure with reduced ejection fraction: a prospective observational cohort study. Circ Heart Fail 2020;13. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006746.
- Witte KK, Patel PA, Walker AMN, Schechter CB, Drozd M, Sengupta A, et al. Socioeconomic deprivation and mode-specific outcomes in patients with chronic heart failure. Heart 2018;104:993-8. https://doi.org/10.1136/heartjnl-2017-312539.
- Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ 2019;364. https://doi.org/10.1136/bmj.l223.
- Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. Midlands Research Practices Consortium (MidReC) . Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J 2007;28:1128-34. https://doi.org/10.1093/eurheartj/ehm102.
- Zeileis A, Kleiber C, Jackman S. Regression models for count data in R. J Stat Softw 2008;27:1-25. https://doi.org/10.18637/jss.v027.i08.
- Madelaire C, Gustafsson F, Kristensen SL, D’Souza M, Stevenson LW, Kober L, et al. Burden and causes of hospital admissions in heart failure during the last year of life. JACC Heart Fail 2019;7:561-70. https://doi.org/10.1016/j.jchf.2019.03.018.
- Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396:121-8. https://doi.org/10.1016/S0140-6736(20)30748-0.
- Kansal AR, Cowie MR, Kielhorn A, Krotneva S, Tafazzoli A, Zheng Y, et al. Cost-effectiveness of ivabradine for heart failure in the United States. J Am Heart Assoc 2016;5. https://doi.org/10.1161/JAHA.116.003221.
- Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875-85. https://doi.org/10.1016/S0140-6736(10)61198-1.
- Göhler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS, et al. Utility estimates for decision-analytic modeling in chronic heart failure – health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009;12:185-7. https://doi.org/10.1111/j.1524-4733.2008.00425.x.
- Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the U.K. National Health Service perspective. Heart 2014;100:1031-6. https://doi.org/10.1136/heartjnl-2013-304598.
- Ara R, Brazier J. Health Related Quality of Life by Age, Gender and History of Cardiovascular Disease: Results From the Health Survey for England. Sheffield: University of Sheffield; 2009.
- British National Formulary for Children . Ubidecarenone n.d. https://bnfc.nice.org.uk/medicinal-forms/ubidecarenone.html (accessed 22 September 2020).
- NHS . Drugs and Pharmaceutical Electronic Market Information Tool (eMIT) 2011. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 22 September 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- NHS . National Cost Collection for the NHS n.d. www.england.nhs.uk/national-cost-collection/#ncc1819 (accessed 22 September 2020).
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8310.
- British Heart Foundation . Heart Statistics n.d. www.bhf.org.uk/what-we-do/our-research/heart-statistics (accessed 22 September 2020).
- British Heart Foundation . Heart &Amp; Circulatory Disease Statistics 2020 n.d. www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2020 (accessed 22 September 2020).
- Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572-80. https://doi.org/10.1016/S0140-6736(17)32520-5.
- Office for National Statistics . United Kingdom Population Mid-Year Estimate 2020. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/timeseries/ukpop/pop (accessed 22 September 2020).
- CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429-35. https://doi.org/10.1056/nejm198706043162301.
- Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772-6. https://doi.org/10.1016/S0140-6736(03)14284-5.
- CIBIS-II Investigators and Committees . The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13. https://doi.org/10.1016/S0140-6736(98)11181-9.
- O’Dell WG. Accuracy of left ventricular cavity volume and ejection fraction for conventional estimation methods and 3D surface fitting. J Am Heart Assoc 2019;8. https://doi.org/10.1161/JAHA.118.009124.
- Koshy AO, Gallivan ER, McGinlay M, Straw S, Drozd M, Toms AG, et al. Prioritizing symptom management in the treatment of chronic heart failure. ESC Heart Fail 2020;7:2193-207. https://doi.org/10.1002/ehf2.12875.
- Strong M, Oakley JE. When is a model good enough? Deriving the expected value of model improvement via specifying internal model discrepancies. SIAMASA J Uncertain Quantif 2014;2:106-25. https://doi.org/10.1137/120889563.
- Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. ISPOR-SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 6. Value Health 2012;15:835-42. https://doi.org/10.1016/j.jval.2012.04.014.
- Bojke L, Claxton K, Sculpher M, Palmer S. Characterizing structural uncertainty in decision analytic models: a review and application of methods. Value Health 2009;12:739-49. https://doi.org/10.1111/j.1524-4733.2008.00502.x.
- Strong M, Oakley JE, Chilcott J. Managing structural uncertainty in health economic decision models: a discrepancy approach. J R Stat Soc Ser C Appl Stat 2012;61:25-4. https://doi.org/10.1111/j.1467-9876.2011.01014.x.
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta-analysis. Cochrane Database Syst Rev 2010;5. https://doi.org/10.1002/14651858.CD005066.pub2.
- Antithrombotic Trialists’ Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. https://doi.org/10.1136/bmj.324.7329.71.
- Pierce JD, Mahoney DE, Hiebert JB, Thimmesch AR, Diaz FJ, Smith C, et al. Study protocol, randomized controlled trial: reducing symptom burden in patients with heart failure with preserved ejection fraction using ubiquinol and/or D-ribose. BMC Cardiovasc Disord 2018;18. https://doi.org/10.1186/s12872-018-0796-2.
- Ubiquinol Treatment in Patients With Heart Failure and Preserved Ejection Fraction n.d. https://clinicaltrials.gov/ct2/show/NCT02779634.
- Belardinelli R, Muçaj A, Lacalaprice F, Solenghi M, Seddaiu G, Principi F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J 2006;27:2675-81.
- Tilson L, McGowan B, Ryan M, Barry M. Cost-effectiveness of spironolactone in patients with severe heart failure. Ir J Med Sci 2003;172:70-2. https://doi.org/10.1007/BF02915250.
- Gutzwiller FS, Schwenkglenks M, Blank PR, Braunhofer PG, Mori C, Szucs TD, et al. Health economic assessment of ferric carboxymaltose in patients with iron deficiency and chronic heart failure based on the FAIR-HF trial: an analysis for the UK. Eur J Heart Fail 2012;14:782-90. https://doi.org/10.1093/eurjhf/hfs083.
- Cowie MR, Fox KF, Wood DA, Metcalfe C, Thompson SG, Coats AJ, et al. Hospitalization of patients with heart failure: a population-based study. Eur Heart J 2002;23:877-85. https://doi.org/10.1053/euhj.2001.2973.
- Moertl D, Steiner S, Coyle D, Berger R. Cost-utility analysis of nt-probnp-guided multidisciplinary care in chronic heart failure. Int J Technol Assess Health Care 2013;29:3-11. https://doi.org/10.1017/S0266462312000712.
- Mohiuddin S, Reeves B, Pufulete M, Maishman R, Dayer M, Macleod J, et al. Model-based cost-effectiveness analysis of B-type natriuretic peptide-guided care in patients with heart failure. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-014010.
- Thokala P, Baalbaki H, Brennan A, Pandor A, Stevens JW, Gomersall T, et al. Telemonitoring after discharge from hospital with heart failure: cost-effectiveness modelling of alternative service designs. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003250.
- Sahlen KG, Boman K, Brännström M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliat Med 2016;30:296-302. https://doi.org/10.1177/0269216315618544.
- Atienza F, Anguita M, Martinez-Alzamora N, Osca J, Ojeda S, Almenar L, et al. Multicenter randomized trial of a comprehensive hospital discharge and outpatient heart failure management program. Eur J Heart Fail 2004;6:643-52. https://doi.org/10.1016/j.ejheart.2003.11.023.
- Pulignano G, Del Sindaco D, Di Lenarda A, Tarantini L, Cioffi G, Gregori D, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med 2010;11:739-47. https://doi.org/10.2459/JCM.0b013e328339d981.
- Postmus D, Pari AA, Jaarsma T, Luttik ML, van Veldhuisen DJ, Hillege HL, et al. A trial-based economic evaluation of 2 nurse-led disease management programs in heart failure. Am Heart J 2011;162:1096-104. https://doi.org/10.1016/j.ahj.2011.09.019.
- McMurray JJV, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, et al. Cost-effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart 2018;104:1006-13. https://doi.org/10.1136/heartjnl-2016-310661.
- Donkor A, McDonagh T, Hardman S. National Heart Failure Audit April 2015 – March 2014. London: British Society for Heart Failure; 2017.
- Abraham WT, Kuck KH, Goldsmith RL, Lindenfeld J, Reddy VY, Carson PE, et al. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail 2018;6:874-83. https://doi.org/10.1016/j.jchf.2018.04.010.
- Abraham WT, Nademanee K, Volosin K, Krueger S, Neelagaru S, Raval N, et al. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J Card Fail 2011;17:710-17. https://doi.org/10.1016/j.cardfail.2011.05.006.
- Kadish A, Nademanee K, Volosin K, Krueger S, Neelagaru S, Raval N, et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J 2011;161:329-37. https://doi.org/10.1016/j.ahj.2010.10.025.
- Klersy C, De Silvestri A, Gabutti G, Raisaro A, Curti M, Regoli F, et al. Economic impact of remote patient monitoring: an integrated economic model derived from a meta-analysis of randomized controlled trials in heart failure. Eur J Heart Fail 2011;13:450-9. https://doi.org/10.1093/eurjhf/hfq232.
- Berg J, Lindgren P, Mejhert M, Edner M, Dahlström U, Kahan T. Determinants of utility based on the EuroQol Five-Dimensional Questionnaire in patients with chronic heart failure and their change over time: results from the Swedish Heart Failure Registry. Value Health 2015;18:439-48. https://doi.org/10.1016/j.jval.2015.02.003.
- Matza LS, Stewart KD, Gandra SR, Delio PR, Fenster BE, Davies EW, et al. Acute and chronic impact of cardiovascular events on health state utilities. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-0772-9.
- Hollingworth W, Biswas M, Maishman RL, Dayer MJ, McDonagh T, Purdy S, et al. The healthcare costs of heart failure during the last five years of life: a retrospective cohort study. Int J Cardiol 2016;224:132-8. https://doi.org/10.1016/j.ijcard.2016.09.021.
Appendix 1 Literature search strategies for clinical evidence
Literature searches were carried out of the following databases: Allied and Complementary Medicine Database (AMED), CENTRAL, EMBASE, Food Science and Technology Abstracts (FSTA), MEDLINE (including MEDLINE In-Process & Other Non-Indexed Citations) and Science Citation Index (SCI).
Searches of various trial registers were undertaken, including ClinicalTrials.gov, ISRCTN, OpenTrials.net and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) portal.
A total of 3433 search results were loaded into EndNote bibliographic software (Clarivate Analytics, Philadelphia, PA, USA) and deduplicated to leave a total of 2408 records.
The search strategies used are listed below.
Allied and Complementary Medicine Database
Database: Allied and Complementary Medicine Database (AMED) via Ovid.
Dates searched: 1985 to October 2018.
Search date: 11 October 2018.
Records identified: 15.
Search strategy
-
heart failure.mp. (784)
-
((heart or cardiac or myocardial) adj4 failure$).ti,ab,sh. (789)
-
cardiomyopath$.ti,ab,sh. (86)
-
1 or 2 or 3 (879)
-
exp Trace elements/(555)
-
micronutrient$.ti,ab,sh. (116)
-
Ubiquinon$.ti,ab,sh. (26)
-
ubiquinol.ti,ab,sh. (7)
-
ubidecarenone.ti,ab,sh. (0)
-
quinone.ti,ab,sh. (83)
-
neuquinon$.ti,ab,sh. (0)
-
(“bio-quinone Q10” or “bioquinone Q10”).ti,ab,sh. (0)
-
(“co-enzyme Q$” or “coenzyme Q$”).ti,ab,sh. (100)
-
(COQ10 or C0Q10 or “COQ 10” or “C0 Q10”).ti,ab,sh. (27)
-
(Q10 or “Q 10” or Q-10).ti,ab,sh. (99)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 (852)
-
4 and 16 (15).
Cochrane Central Register of Controlled Trials
Database: Cochrane Central Register of Controlled Trials.
Dates searched: issue 3 of 12, March 2020.
Search date: 11 October 2018.
Records identified: 135.
Search strategy
-
#1 MeSH descriptor: [Heart Failure] explode all trees
-
#2 ((heart or cardiac or myocardial) NEAR/4 failure*):ti,ab,kw (Word variations have been searched)
-
#3 MeSH descriptor: [Cardiomyopathies] explode all trees
-
#4 MeSH descriptor: [Cardiomyopathy, Dilated] explode all trees
-
#5 (cardiomyopath*):ti,ab,kw (Word variations have been searched)
-
#6 #1 OR #2 OR #3 OR #4 OR #5
-
#7 MeSH descriptor: [Micronutrients] explode all trees
-
#8 MeSH descriptor: [Ubiquinone] explode all trees
-
#9 (ubiquinon*):ti,ab,kw OR (ubiquinol):ti,ab,kw OR (ubidecarenone):ti,ab,kw OR (quinone):ti,ab,kw OR (neuquinon*):ti,ab,kw (Word variations have been searched)
-
#10 (“bio-quinone Q10”):ti,ab,kw (Word variations have been searched)
-
#11 (“co-enzyme Q*”):ti,ab,kw (Word variations have been searched)
-
#12 (“coenzyme Q*”):ti,ab,kw (Word variations have been searched)
-
#13 (COQ10):ti,ab,kw OR (C0Q10):ti,ab,kw (Word variations have been searched)
-
#14 (Q10):ti,ab,kw OR (“Q 10”):ti,ab,kw OR (Q-10):ti,ab,kw (Word variations have been searched)
-
#15 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14
-
#16 #6 AND #15.
EMBASE
Database: EMBASE via Ovid.
Dates searched: 1974 to 9 October 2018.
Search date: 11 October 2018.
Records identified: 1945.
Search strategy
-
exp Heart Failure/ (433,070)
-
(heart adj4 failure$).mp. (365,011)
-
(cardiac adj4 failure$).mp. (31,005)
-
(myocardial adj4 failure$).mp. (12,338)
-
exp cardiomyopathy/ (121,733)
-
cardiomyopath$.mp. (136,317)
-
1 or 2 or 3 or 4 or 5 or 6 (568,017)
-
exp Trace Elements/ (35,322)
-
micronutrient$.mp. (17,727)
-
ubiquinone/ (7221)
-
ubiquinon$.mp. (19,834)
-
ubiquinol.mp. (6323)
-
ubidecarenone.mp. (7836)
-
quinone.mp. (26,940)
-
neuquinon$.mp. (12)
-
bioquinone Q10.mp. (0)
-
bio-quinone Q10.mp. (4)
-
co-enzyme Q$.mp. (217)
-
coenzyme Q$.mp. (6347)
-
COQ10.mp. (2444)
-
COQ 10.mp. (224)
-
Q10.mp. (8216)
-
(“Q 10” or Q-10).mp. (1572)
-
C0Q10.mp. (9)
-
C0Q 10.mp. (0)
-
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 (101,092)
-
7 and 26 (2341)
-
exp animal/or exp nonhuman/or exp animal experiment/or exp animal model/ (24,850,091)
-
exp human/ (18,840,975)
-
28 not 29 (6,009,116)
-
27 not 30 (1945).
Food Science and Technology Abstracts
Database: Food Science and Technology Abstracts (FSTA) via ProQuest.
Dates searched: inception to 10 March 2018.
Search date: 11 October 2018.
Records identified: 14.
Search strategy
| S1 | (heart near/2 failure*) OR (cardiac near/2 failure*) OR (myocardial near/2 failure*) OR cardiomyopath* | 423° |
| S2 | SU.EXACT.EXPLODE(“MICRONUTRIENTS”) OR micronutrient* OR (SU.EXACT.EXPLODE(“COENZYME Q”)) OR Ubiquinone OR ubiquinon* OR ubiquinol* OR ubidecarenone OR quinone OR neuquinon* OR (“bioquinone Q10” or “bio-quinone Q10”) | 7693* |
| S3 | (“co-enzyme Q*”) OR (“coenzyme Q*”) OR ((COQ10 or C0Q10)) OR ((“COQ 10” or “C0 Q10”)) OR (Q10 or Q10 or Q-10) | 828° |
| S4 | S3 OR S2 | 8075* |
| S5 | S4 AND S1 | 14° |
MEDLINE
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily via Ovid.
Dates searched: 1946 to 9 October 2018.
Search date: 11 October 2018.
Records identified: 545.
Search strategy
-
exp Heart Failure/ (109,307)
-
(heart adj4 failure$).mp. (185,558)
-
(cardiac adj4 failure$).mp. (20,579)
-
(myocardial adj4 failure$).mp. (8237)
-
Cardiomyopathies/ (25,814)
-
Cardiomyopathy, Dilated/ (14,800)
-
cardiomyopath$.mp. (88,142)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (262,456)
-
Micronutrients/ (5085)
-
micronutrient$.mp. (14,686)
-
Ubiquinone/ (8516)
-
ubiquinon$.mp. (12,111)
-
ubiquinol.mp. (1796)
-
ubidecarenone.mp. (65)
-
quinone.mp. (20,439)
-
neuquinon$.mp. (0)
-
bio-quinone Q10.mp. (2)
-
co-enzyme Q$.mp. (127)
-
coenzyme Q$.mp. (5528)
-
(COQ10 or C0Q10).mp. (1538)
-
(COQ 10 or C0 Q10).mp. (332)
-
Q10.mp. (6430)
-
Q 10.mp. (2374)
-
Q-10.mp. (2374)
-
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (49,941)
-
8 and 25 (620)
-
exp animals/not humans/ (4,502,274)
-
26 not 27 (545).
Science Citation Index
Database: Science Citation Index via Web of Science.
Dates searched: 1900 to 10 October 2018.
Search date: 11 October 2018.
Records identified: 682.
Search strategy
| Set | ||
|---|---|---|
| # 11 | 682 |
#9 NOT #10 Indexes = SCI-EXPANDED Timespan = All years |
| # 10 | 1,842,535 |
TI = (rat or rats or mouse or mice or pig or pigs or chicken* or broiler*) Indexes = SCI-EXPANDED Timespan = All years |
| # 9 | 727 |
#8 AND #4 Indexes = SCI-EXPANDED Timespan = All years |
| # 8 | 76,498 |
#7 OR #6 OR #5 Indexes = SCI-EXPANDED Timespan = All years |
| # 7 | 12,603 |
TOPIC: (“co-enzyme Q*”) OR TOPIC: (“coenzyme Q*”) OR TOPIC: ((COQ10 or C0Q10)) OR TOPIC: ((“COQ 10” or “C0 Q10”)) OR TOPIC: (Q10) OR TOPIC:(“Q 10”) OR TOPIC: (Q-10) Indexes = SCI-EXPANDED Timespan = All years |
| # 6 | 46,872 |
TOPIC: (Ubiquinon*) OR TOPIC: (ubiquinol) OR TOPIC: (ubidecarenone) OR TOPIC: (quinone) OR TOPIC: (neuquinon*) OR TOPIC: (“bio-quinone Q10”) OR TOPIC: (“bioquinone Q10”) Indexes = SCI-EXPANDED Timespan = All years |
| # 5 | 20,044 |
TOPIC: (Micronutrient*) Indexes = SCI-EXPANDED Timespan = All years |
| # 4 | 291,670 |
#3 OR #2 OR #1 Indexes = SCI-EXPANDED Timespan = All years |
| # 3 | 98,309 |
TOPIC: (Cardiomyopath*) Indexes = SCI-EXPANDED Timespan = All years |
| # 2 | 226,278 |
TOPIC: ((heart or cardiac or myocardial) near/4 failure*) Indexes = SCI-EXPANDED Timespan = All years |
| # 1 | 214,540 |
TOPIC: (“Heart Failure”) Indexes = SCI-EXPANDED Timespan = All years |
Searches of trials registers
ClinicalTrials.gov via internet
Search date: 12 October 2018.
Records identified: 93.
Series of searches carried out.
Search strategy
-
Heart failure AND micronutrients = 74 records
-
Heart failure AND ubiquinone = 3 records
-
Heart failure AND ubiquinol = 2 records
-
Heart failure AND ubidecarone = 4 records
-
Heart failure AND quinone = 0 records
-
Heart failure AND neuquinone = 0 records
-
Heart failure AND bioquinone = 0 records
-
Heart failure AND co-enzyme Q = 3 records
-
Heart failure AND coenzyme Q = 3 records
-
Heart failure AND COQ = 2 records
-
Heart failure AND Q10 = 4 records
-
Heart failure AND Q_10 = 9 records
-
cardiomyopathies AND micronutrients = 14 records
-
cardiomyopathies AND ubiquinone = 3 records
-
cardiomyopathies AND ubiquinol = 1 records
-
cardiomyopathies AND ubidecarone = 2 records
-
cardiomyopathies AND quinone = 0 records
-
cardiomyopathies AND neuquinone = 0 records
-
cardiomyopathies AND bioquinone = 0 records
-
cardiomyopathies AND co-enzyme Q = 3 records
-
cardiomyopathies AND coenzyme Q = 3 records
-
cardiomyopathies AND COQ = 0 records
-
cardiomyopathies AND Q10 = 2 records
-
cardiomyopathies AND Q_10 = 3 records.
ISRCTN registry
URL: www.isrctn.com/
Search date: 12 October 2018.
A series of individual searches using the following terms were carried out and three relevant records were identified.
Search strategy
Ubiquinone, ubiquinol, ubidecarone, quinone, neuquinone, bioquinone, coenzyme, and Q10.
OpenTrials.net
A series of individual searches using the following terms were carried out and six relevant records were identified.
Search strategy
Ubiquinone, ubiquinol, ubidecarone, quinone, neuquinone, bioquinone, coenzyme, and Q10.
World Health Organization International Clinical Trials Registry Platform
URL: http://apps.who.int/trialsearch/
Search date: 12 October 2018.
Five unique records identified.
Search strategy
-
Heart failure AND micronutrients = 0 records
-
Heart failure AND ubiquinone = 2 records
-
Heart failure AND ubiquinol = 1 records
-
Heart failure AND ubidecarone = 0 records
-
Heart failure AND quinone = 0 records
-
Heart failure AND neuquinone = 0 records
-
Heart failure AND bioquinone = 0 records
-
Heart failure AND co-enzyme Q = 0 records
-
Heart failure AND coenzyme Q = 2 records
-
Heart failure AND (COQ10 OR C0Q10) = 3 records
-
Heart failure AND (COQ 10 OR C0 Q10) = 3 records
-
Heart failure AND (Q10 OR Q 10 OR Q-10) = 3 records
-
cardiac failure AND micronutrients = 0 records
-
cardiac failure AND ubiquinone = 3 records
-
cardiac failure AND ubiquinol = 1 records
-
cardiac failure AND ubidecarone = 0 records
-
cardiac failure AND quinone = 1 records
-
cardiac failure AND neuquinone = 0 records
-
cardiac failure AND bioquinone = 0 records
-
cardiac failure AND co-enzyme Q = 1 records
-
cardiac failure AND coenzyme Q = 3 records
-
cardiac failure AND (COQ10 OR C0Q10) = 4 records
-
cardiac failure AND (COQ 10 OR C0 Q10) = 4 records
-
cardiac failure AND (Q10 OR Q 10 OR Q-10) = 4 records
-
cardiomyopathies AND micronutrients = 0 records
-
cardiomyopathies AND ubiquinone = 0 records
-
cardiomyopathies AND ubiquinol = 0 records
-
cardiomyopathies AND ubidecarone = 0 records
-
cardiomyopathies AND quinone = 0 records
-
cardiomyopathies AND neuquinone = 0 records
-
cardiomyopathies AND bioquinone = 0 records
-
cardiomyopathies AND co-enzyme Q = 0 records
-
cardiomyopathies AND coenzyme Q = 0 records
-
cardiomyopathies AND (COQ10 OR C0Q10) = 0 records
-
cardiomyopathies AND (COQ 10 OR C0 Q10) = 0 records
-
cardiomyopathies AND (Q10 OR Q 10 OR Q-10) = 0 records.
Update MEDLINE search strategy
Database: Ovid MEDLINE(R) ALL.
Dates searched: 1946 to 16 March 2020.
Search date: 17 March 2020.
Search strategy
-
exp Heart Failure/ (118,987)
-
(heart adj4 failure$).mp. (204,092)
-
(cardiac adj4 failure$).mp. (21,965)
-
(myocardial adj4 failure$).mp. (8932)
-
Cardiomyopathies/ (27,624)
-
Cardiomyopathy, Dilated/ (15,496)
-
cardiomyopath$.mp. (95,608)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (286,729)
-
Micronutrients/ (5692)
-
micronutrient$.mp. (16,959)
-
Ubiquinone/ (9153)
-
ubiquinon$.mp. (12,971)
-
ubiquinol.mp. (1881)
-
ubidecarenone.mp. (66)
-
quinone.mp. (22,404)
-
neuquinon$.mp. (0)
-
bio-quinone Q10.mp. (2)
-
co-enzyme Q$.mp. (150)
-
coenzyme Q$.mp. (6117)
-
(COQ10 or C0Q10).mp. (1773)
-
(COQ 10 or C0 Q10).mp. (338)
-
Q10.mp. (7120)
-
Q 10.mp. (2580)
-
Q-10.mp. (2580)
-
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (55,185)
-
8 and 25 (683)
-
exp animals/not humans/ (4,679,006)
-
26 not 27 (602).
Appendix 2 Table of excluded studies at full-text screening stage
| Study | Reason for exclusion |
|---|---|
| Excluded because of ineligible study design | |
| Aalbers J. Coenzyme Q10, an antioxidant of value to reduce oxidative stress; also useful to reduce statin-induced myalgia. Cardiovasc J Afr 2012;23:170 | Letter |
| Anonymous. Coenzyme Q-10 disappoints in rigorous study. Harv Heart Lett 1999;9:6 | Letter |
| Anonymous. [Drug therapy for heart failure in hospitalized elderly patients.] G Gerontol 1995;43:95–9 | Non-randomised study |
| Aronow WS, Shamliyan TA. Dietary Micronutrient interventions to prevent hospitalization and readmission in adults with congestive heart failure. Am J Med 2018;131:492–9 | Systematic review |
| Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ J 1992;56:95–9. https://doi.org/10.1253/jcj.56.95 | Non-randomised study |
| Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 Drug Surveillance Investigators. Mol Aspects Med 1994;15:s287–94 | Non-randomised study |
| Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure (interim analysis). The CoQ10 Drug Surveillance Investigators. Clin Investig 1993;71:145–9. https://doi.org/10.1007/BF00226857 | Non-randomised study |
| Balta S, Demırkol S, Celik T. Coenzyme Q10 supplementation may improve diastolic heart functions especially coronary artery disease patients. Hemodial Int 2013;17:467–8. https://doi.org/10.1111/hdi.12037 | Letter |
| Belcaro G, Cesarone MR, Dugall M, Hosoi M, Ippolito E, Bavera P, Grossi MG. Investigation of Pycnogenol® in combination with coenzymeQ10 in heart failure patients (NYHA II/III). Panminerva Med 2010;52(Suppl. 2):21–5 | Non-randomised study |
| Belcaro G, Cesarone MR, Ledda A, Cornelli U, Dugall M, Stuard S, et al. Supportive treatment with CoenzymeQ10 in heart failure: the Irvine3 labs study on heart failure in vascular patients. Angeiologie 2010;62:9–16 | Non-randomised study |
| Belcaro G, Dugall M, Hu S, Corsi M, Hosoi M, Luzzi R, et al. Miraqule-C and mild heart failure: an 8-week registry [published online ahead of print October 22 2015]. Minerva Gastroenterol Dietol 2015 | Non-randomised study |
| Bramwell BL. Coenzyme q(10) supplementation in the treatment of heart disease. Int J Pharm Compd 2010;14:108–11 | Review |
| Carlsen SM, Fougner KJ. [Statin therapy, Q10 and heart failure. Is there any difference between statins?] Tidsskr Nor Laegeforen 1994;114:1345 | Letter |
| Cascone A, Alaimo A, Ferrari F, Cascone G. [Coenzyme Q10 in myocardial insufficiency.] Boll Chim Farm 1984;123:55S–60S | Non-comparative study |
| Cascone A, Cascone G, Alaimo A. [Treatment of patients with congestive heart failure with coenzyme Q10 in an open trial.] Boll Chim Farm 1985;124:43S–52S | Non-comparative study |
| Coenzyme Q10. J Complement Med 2008;7:36 | Non-comparative study |
| Costantini G, Bertaccini B, Favilli L. [Evaluation of efficacy and acceptability of ubidecarenone (coenzyme Q10) in treatment of congestive heart failure.] Arch Med Internal 1984;36:139–50 | Non-comparative study |
| D’Agnolo B. [Therapeutic effect of coenzyme Q10 in patients with congestive heart failure.] Boll Chim Farm 1985;124:7S–12S | Non-comparative study |
| D’Ambrosio P, Palladino A, Scutifero M, Petillo R, Orsini C, Passamano L, et al. Efficacy of Coenzime Q10 in patients with dystrophinopathic cardiomyopathy. Acta Myol 2018;37:67 | Non-randomised study |
| Davini A, Topi PL. [Metabolic therapy in cardiology: experience with Q10 coenzyme (ubidecarenone) in the treatment of heart failure.] G Ital Ric Clin Ter 1991;12:11–16 | Non-comparative study |
| Del Mar CB, Glasziou PP, Spinks AB, Sanders SL. Is coenzyme Q10 helpful for patients with idiopathic cardiomyopathy? Med J Aust 2001;174:421 | Letter |
| Del Mar CB, Glasziou PP. Is coenzyme Q(10) helpful for patients with idiopathic cardiomyopathy? Med J Aust 2001;175:447–8 | Letter |
| DiNicolantonio JJ, Bhutani J, McCarty MF, O’Keefe JH. Coenzyme Q10 for the treatment of heart failure: a review of the literature. Open Heart 2015;2:e000326. https://doi.org/10.1136/openhrt-2015-000326 | Non-comparative study |
| Dragan S, Buleu F, Christodorescu R, Cobzariu F, Iurciuc S, Velimirovici D, et al. Benefits of multiple micronutrient supplementation in heart failure: a comprehensive review. Crit Rev Food Sci Nutr 2019;59:965–81. https://doi.org/10.1080/10408398.2018.1540398 | Review |
| Flowers N, Hartley L, Todkill D, Stranges S, Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2014;12:CD010405. https://doi.org/10.1002/14651858.CD010405.pub2 | Systematic review |
| Folkers K, Langsjoen P, Langsjoen PH. Therapy with coenzyme Q10 of patients in heart failure who are eligible or ineligible for a transplant. Biochem Biophys Res Commun 1992;182:247–53 | Review |
| Folkers K, Langsjoen P. Nutrition and cardiac health. A deficiency of coenzyme Q10 is a dominant molecular cause of heart failure. J Opt Nutr 1993;2:264–74 | Review |
| Folkers K, Wolaniuk A. Progress in biomedical and clinical research on coenzyme Q10. Drugs Exp Clin Res 1984;10:513–17 | Review |
| Folkers K. Heart failure is a dominant deficiency of coenzyme Q10 and challenges for future clinical research on CoQ10. Clin Investig 1993;71:51–4. https://doi.org/10.1007/BF00226840 | Non-comparative study |
| Frustaci A, Schiavoni G, Pennestrì F, Mazzari M, Rossi E, Ferri T, et al. [Coenzyme Q10 in dilated cardiomyopathy: a biochemical approach to the treatment. Preliminary data.] Cardiologia 1985;30:533–6 | Non-randomised study |
| Ghiringhelli G. [Ubidecarenone therapy of some cardiopathies with and without decompensation. Comparison with a standard therapy.] Boll Chim Farm 1986;125:28S–33S | Non-randomised study |
| Gianrossi R, Nizzo MC, Montemanni M, Azzolini A. [Short-term trial on Ubidecarenone in combination with digitalis in the treatment of congestive heart failure.] Clin Ter Cardiovasc 1985;4:225–30 | Non-comparative study |
| Gottlieb SS, Khatta M, Fisher ML. Coenzyme Q10 and congestive heart failure. Ann Intern Med 2000;133:745–6 | Letter |
| Gutierrez-Mariscal FM, Yubero-Serrano EM, Villalba JM, Lopez-Miranda J. Coenzyme Q(10): from bench to clinic in aging diseases, a translational review. Crit Rev Food Sci Nutr 2019;59:2240–57 | Systematic review |
| Harinstein ME, Berliner JI, Shah SJ, Taegtmeyer H, Gheorghiade M. Normalization of ejection fraction and resolution of symptoms in chronic severe heart failure is possible with modern medical therapy: clinical observations in 11 patients. Am J Ther 2008;15:206–13. https://doi.org/10.1097/MJT.0b013e3181728a1d | Non-randomised study |
| Ishiyama T, Morita Y, Toyama S, Yamagami T, Tsukamoto N. A clinical study of the effect of coenzyme Q on congestive heart failure. Jpn Heart J 1976;17:32–42. https://doi.org/10.1536/ihj.17.32 | Non-randomised study |
| Iwabuchi T. Clinical efficacy of coenzyme Q10 for cardiac failure: a double-blind controlled comparison. Rinsho Kenkyu 1972;49:2604–8 | Non-randomised study |
| Jalali SF, Baradaran M, Mahdinejad Shani M, Rooshan T. [Effect of Coenzyme-Q10 in congestive heart failure.] J Babol Univ Med l Sci 2010;12:24–9 | Non-randomised study |
| Jones, Hughes K, Mischley K, McKenna D.J. Coenzyme Q-10: efficacy, safety, and use. Altern Ther Health Med 2002;8:42–55 | Non-comparative study |
| Jorat MV, Tabrizi R, Kolahdooz F, Akbari M, Salami M, Heydari ST, Asemi Z. The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2019;27:233–48. https://doi.org/10.1007/s10787-019-00572-x | Systematic review |
| JPRN-UMIN000020203. Study of a Heart Failure Treatment Using a Combination Drug Consisting of Reduced Coenzyme Q10, Astaxanthin, Citrulline, and Zinc. 2015. URL: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000022720 | Non-randomised study |
| Judy WV, Hall JH, Toth PD, Folkers K. Influence of coenzyme Q10 on cardiac function in congestive heart failure patients. Fed Proc 1984;43:432 | Non-randomised study |
| Lampertico M, Comis S. Italian multicenter study on the efficacy and safety of coenzyme Q10 as adjuvant therapy in heart failure. Clin Investig 1993;71:129–33. https://doi.org/10.1007/BF00226853 | Non-randomised study |
| Langsjoen H, Langsjoen P, Langsjoen P, Willis R, Folkers K. Usefulness of coenzyme Q10 in clinical cardiology: a long-term study. Mol Aspects Med 1994;15:s165–75 | Non-randomised study |
| Langsjoen PH, Folkers K, Lyson K, Muratsu K, Lyson T, Langsjoen P. Pronounced increase of survival of patients with cardiomyopathy when treated with coenzyme Q10 and conventional therapy. Int J Tissue React 1990;12:163–8 | Non-randomised study |
| Langsjoen PH, Folkers K, Lyson K, Muratsu K, Lyson T, Langsjoen P. Effective and safe therapy with coenzyme Q10 for cardiomyopathy. Klin Wochenschr 1988;66:583–90 https://doi.org/10.1007/BF01720833 | Non-randomised study |
| Langsjoen PH, Langsjoen PH, Folkers K. Long-term efficacy and safety of coenzyme Q10 therapy for idiopathic dilated cardiomyopathy. Am J Cardiol 1990;65:521–3 | Non-randomised study |
| Langsjoen PH, Langsjoen A, Willis R, Folkers K. Treatment of hypertrophic cardiomyopathy with coenzyme Q10. Mol Aspects Med 1997;18:S145–51 | Non-randomised study |
| Langsjoen PH, Langsjoen AM. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors 2008;32:119–28. https://doi.org/10.1002/biof.5520320114 | Non-randomised study |
| Langsjoen PH, Langsjoen JO, Langsjoen AM, Lucas LA. Treatment of statin adverse effects with supplemental coenzyme Q10 and statin drug discontinuation. Biofactors 2005;25:147–52. https://doi.org/10.1002/biof.5520250116 | Non-randomised study |
| Langsjoen PH, Langsjoen PH, Folkers K. A six-year clinical study of therapy of cardiomyopathy with coenzyme Q10. Int J Tissue React 1990;12:169–71 | Non-randomised study |
| Langsjoen PH, Langsjoen PH, Folkers K. Isolated diastolic dysfunction of the myocardium and its response to CoQ10 treatment. Clin Investig 1993;71:140–4. https://doi.org/10.1007/BF00226856 | Non-randomised study |
| Langsjoen PH, Langsjoen PH, Folkers K. Long-term efficacy and safety of coenzyme Q10 therapy for idiopathic dilated cardiomyopathy. Am J Cardiol 1990;65:521–3 | Non-randomised study |
| Langsjoen PH. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J Am Coll Cardiol 2000;35:816–17 | Letter |
| Littarru GP. [Coenzyme Q10: which dosage?] Cuore 1993;10:609–16 | Non-randomised study |
| Manzoli U, Rossi E, Littarru GP, Frustaci A, Lippa S, Oradei A, et al. Coenzyme Q10 in dilated cardiomyopathy. Int J Tissue React 1990;12:173–8 | Non-randomised study |
| Molyneux SL, Florkowski CM, George PM, Lever M, Richards AM. Coenzyme Q10 in heart failure and healthy subjects. FEBS J 2006;273:216–17 | Non-randomised study |
| Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, Richards AM. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol 2008;52:1435–41. https://doi.org/10.1016/j.jacc.2008.07.044 | Non-randomised study |
| Mortensen SA, Mortensen AL. The mitochondria in heart failure: a target for coenzyme Q10 therapy? Clin Pharmacol Ther 2014;96:645–7 | Letter |
| Mortensen SA, Vadhanavikit S, Baandrup U, Folkers K. Long-term coenzyme Q10 therapy: a major advance in the management of resistant myocardial failure. Drugs Exp Clin Res 1985;11:581–93 | Non-randomised study |
| Mortensen SA, Vadhanavikit S, Folkers K. Deficiency of coenzyme Q10 in myocardial failure. Drugs Exp Clin Res 1984;10:497–502 | Non-randomised study |
| Mortensen SA, Vadhanavikit S, Muratsu K, Folkers K. Coenzyme Q10: clinical benefits with biochemical correlates suggesting a scientific breakthrough in the management of chronic heart failure. Int J Tissue React 1990;12:155–62 | Review |
| Mortensen SA. Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure. Rationale, design and end-points of ‘Q-SYMBIO’ – a multinational trial. Biofactors 2003;18:79–89. https://doi.org/10.1002/biof.5520180210 | Review |
| NCT01474486. Feasibility and Effectiveness of Micronutrients as Palliative Care Therapy in Patients with Congestive Heart Failure. URL: https://clinicaltrials.gov/ct2/show/NCT01474486 | Non-randomised study |
| Nguyen T, Valentine R, Brand J, Welborn TL. In adults with reduced left ventricular ejection fractions (systolic heart failure), does coenzyme Q10 improve cardiac function or patient outcomes? J Okla State Med Assoc 2013;106:9–10 | Review |
| Nishimura T, Hori M. [Therapeutic effects of coenzyme Q10 on dilated cardiomyopathy: assessment by 123I-BMIPP myocardial single photon emission computed tomography (SPECT): a multicenter trial in Osaka University Medical School Group.] Kaku Igaku 1996;33:27–32 | Non-randomised study |
| Okello E, Jiang X, Mohamed S, Zhao Q, Wang T. Combined statin/coenzyme Q10 as adjunctive treatment of chronic heart failure. Med Hypotheses 2009;73:306–8. https://doi.org/10.1016/j.mehy.2009.03.027 | Review |
| Pepping J. Coenzyme Q10. Am J Health Syst Pharm 1999;56:519–21 | Review |
| Peverill R, Bowden D, Chen M, Chia M, Rosenfeldt F. Reduced selenium levels in betathalassaemia major are associated with impairment of left ventricular systolic function. Heart Lung Circ 2015;3:S182 | Non-randomised study |
| Qu H, Guo M, Chai H, Wang WT, Gao ZY, Shi DZ. Effects of coenzyme Q10 on statin-induced myopathy: an updated meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e009835. https://doi.org/10.1161/JAHA.118.009835 | Systematic review |
| Qu H, Meng YY, Chai H, Liang F, Zhang JY, Gao ZY, Shi DZ. The effect of statin treatment on circulating coenzyme Q10 concentrations: an updated meta-analysis of randomized controlled trials. Eur J Med Res 2018;23:57. https://doi.org/10.1186/s40001-018-0353-6 | Systematic review |
| Raizner AE. Coenzyme Q10. Methodist DeBakey Cardiovasc J 2019;15:185–91 | Review |
| Rengo F, Abete P, Landino P, Leosco D, Covelluzzi F, Vitale D, et al. Role of metabolic therapy in cardiovascular disease. Clin Investig 1993;71(Suppl. 8):124–8. https://doi.org/10.1007/BF00226852 | Non-randomised study |
| Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors 2003;18:91–100. https://doi.org/10.1002/biof.5520180211 | Review |
| Sacher HL, Sacher ML, Landau SW, Kersten R, Dooley F, Sacher A, et al. The clinical and hemodynamic effects of coenzyme Q10 in congestive cardiomyopathy. Am J Ther 1997;4:66–72. https://doi.org/10.1097/00045391-199702000-00003 | Non-randomised study |
| Saurabh S, Yadav A, Tiwari RK, Sharma A, Goyal YK, Jain A. Comparative study of ubiquinone (COQ10) and krill oil in dilated cardiomyopathy. Indian J Pharmacol 2014;1:S30 | Non-randomised study |
| Schneeberger W, Zilliken F, Moritz J. Clinical studies with coenzyme Q10 in patients with congestive heart failure. Drugs Exp Clin Res 1984;10:503–12 | Non-randomised study |
| Shcherbakova AG, Sigitova ON. [Assessment of quality of life after inclusion of coenzyme Q10 in the scheme of treatment of women with arterial hypertension and elevated risk of cardiovascular complications.] Kardiologiia 2010;50:19–21 | Non-randomised study |
| Shilov AM, Mel’nik MV, Voevodina ES, Osiia AO, Griaznov DA. [Prophylaxis of ischaemic heart lesions during chronic heart failure with complex therapy using Q10 coenzyme.] Anesteziol Reanimatol 2011:34–8 | Non-randomised study |
| Sinatra ST, Berman M, Ben-Gal T. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clin Cardiol 2004;27:A26–A30 | Letter |
| Sinatra ST. Coenzyme Q10: a vital therapeutic nutrient for the heart with special application in congestive heart failure. Conn Med 1997;61:707–11 | Non-randomised study |
| Sizova ZM, Zakharova VL, Alibeyli KA, Medvedev OS, Shikh EV, Bolevich SB, et al. [Ubiquinone plasma levels are correlated with brain natriuretic peptide plasma levels in patients with chronic heart failure: the potential of coenzyme Q10 combined therapy.] Serbian J Exp Clin Res 2018;19:141–9 | Review |
| Study suggests coenzyme Q10 could improve heart failure mortality. Clin Lipidol 2013;8:399–400 | Letter |
| Excluded because of ineligible population | |
| Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors 2008;32:145–9. https://doi.org/10.1002/biof.5520320117 | Participants do not have CHF |
| Chen YF, Lin YT, Wu SC. Effectiveness of coenzyme Q10 on myocardial preservation during hypothermic cardioplegic arrest. J Thorac Cardiovasc Surg 1994;107:242–7 | Surgical study |
| Chew GT, Watts GF, Davis TM, Stuckey BG, Beilin LJ, Thompson PL, et al. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. Diabetes Care 2008;31:1502–9. https://doi.org/10.2337/dc08-0118 | Participants do not have CHF |
| Damian MS, Ellenberg D, Gildemeister R, Lauermann J, Simonis G, Sauter W, Georgi C. Coenzyme Q10 combined with mild hypothermia after cardiac arrest: a preliminary study. Circulation 2004;110:3011–16 | Participants do not have CHF |
| Fedacko J, Pella D, Rybar R, Lopuchovsky T, Tuomainen P, Merkovska L, et al. the role of selenium supplementation on top of coenzyme q10 in statins treated patients with possible diastolic dysfunction of left ventricular. Eur Heart J 2012;1:613 | Participants with diastolic dysfunction |
| Fedacko J, Pella D, Rybar R. Influence of coenzyme Q10 supplementation in statin treated patients on left ventricular diastolic dysfunction. Results of randomised double-blind clinical study. Eur Heart J 2009;1:369–70 | Participants taking statins |
| Gini M, Schiavi M, Mazzola C. [Effectiveness of coenzyme Q10 therapy in patients with pulmonary cardiopathy.] Boll Chim Farm 1985;124:21S–8S | Participants do not have CHF |
| Grasso S, Pesciatini F, Cefis M, Lazzaroni A, Cerri B. Influence of ubidecarenone (CoQ10) on ECG during exercise in heart failure. Basi Razionali Terapia 1988;18:331–5 | Participants do not have CHF |
| Hicks JJ, Montes-Cortes DH, Cruz-Dominguez MP, Medina-Santillan R, Olivares-Corichi IM. Antioxidants decrease reperfusion induced arrhythmias in myocardial infarction with ST-elevation. Front Biosci 2007;12:2029–37 | Participants do not have CHF |
| IRCT201311278307N3. Evaluation of Co-enzyme Q10 in Cardiac Pre-procedural Injury of Ischaemic Heart Diseases Patients Undergoing Angioplasty. 2014. URL: https://en.irct.ir/trial/8729 | Participants do not have CHF |
| Jeejeebhoy F, Keith M, Freeman M, Barr A, McCall M, Kurian R, et al. Nutritional supplementation with MyoVive repletes essential cardiac myocyte nutrients and reduces left ventricular size in patients with left ventricular dysfunction. Am Heart J 2002;143:1092–100 | Participants do not have CHF |
| Judy WV, Stogsdill WW, Folkers K. Myocardial preservation by therapy with coenzyme Q10 during heart surgery. Clin Investig 1993;71:155–61. https://doi.org/10.1007/BF00226859 | Surgical study |
| Malm C, Svensson M, Ekblom B, Sjödin B. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol Scand 1997;161:379–84. https://doi.org/10.1046/j.1365-201X.1997.00198.x | Participants do not have CHF |
| Efficacy and Safety of Coenzyme Q10 in the Treatment of Statin-Associated Myalgia. 2007. URL: https://clinicaltrials.gov/ct2/show/NCT00590408 (accessed 7 January 2019) | Participants taking statins |
| Firefighter Aged Garlic Extract Investigation With CoQ10 as a Treatment for Heart Disease (FAITH). 2009. URL: https://clinicaltrials.gov/ct2/show/NCT00860847 (accessed 7 January 2019) | Participants do not have CHF |
| Ubiquinol Treatment in Patients With Heart Failure and Preserved Ejection Fraction. 2016. URL: https://clinicaltrials.gov/ct2/show/NCT02779634 (accessed 7 January 2019) | Heart failure with preserved ejection fraction |
| Potential Role of Water-soluble Ubiquinol in Complementary Therapy for Pediatric Dilated Cardiomyopathy. 2016. URL: https://clinicaltrials.gov/ct2/show/NCT02847585 (accessed 7 January 2019) | Paediatric participants |
| CoQ10 and D-ribose in Patients With Diastolic Heart Failure. 2018. URL: https://clinicaltrials.gov/ct2/show/NCT03133793 (accessed 7 January 2019) | Heart failure with preserved ejection fraction |
| The Effects of the Dietary Supplement CardioFlex Q10 on Reducing Cardiovascular Disease Risk Factors in Adults. 2018. URL: https://clinicaltrials.gov/ct2/show/NCT03826914 (accessed 7 January 2019) | Participants do not have CHF |
| Pagliano F. [Therapeutic activity of Q10 coenzyme (ubidecarenone) in ischaemic and sclerotic cardiopathies.] Boll Chim Farm 1986;125:40S–5S | Participants do not have CHF |
| Pepe S, Leong JY, Van der Merwe J, Marasco SF, Hadj A, Lymbury R, et al. Targeting oxidative stress in surgery: effects of ageing and therapy. Exp Gerontol 2008;43:653–7. https://doi.org/10.1016/j.exger.2008.03.011 | Surgical study |
| Pierce JD, Mahoney DE, Hiebert JB, Thimmesch AR, Diaz FJ, Smith C, et al. Study protocol, randomized controlled trial: reducing symptom burden in patients with heart failure with preserved ejection fraction using ubiquinol and/or D-ribose. BMC Cardiovasc Disord 2018;18:57. https://doi.org/10.1186/s12872-018-0796-2 | Heart failure with preserved ejection fraction |
| Rinaldi C, Tucci T, Maione S, Giunta A, De Michele G, Filla A. Low-dose idebenone treatment in Friedreich’s ataxia with and without cardiac hypertrophy. J Neurol 2009;256:1434–7. https://doi.org/10.1007/s00415-009-5130-6 | Participants do not have CHF |
| Rivera MB, Yeung CK, Robinson-Cohen C, Phillips BR, Ruzinski J, Rock D, et al. Effect of coenzyme Q(10) on biomarkers of oxidative stress and cardiac function in hemodialysis patients: the CoQ(10) Biomarker Trial. Am J Kidney Dis 2017;69:389–99 | Participants do not have CHF |
| Sharifi MH, Eftekhari MH, Ostovan MA, Rezaianazadeh A. Effects of therapeutic lifestyle change diet and Q10 plus L-carnitine supplementation on inflammatory biomarkers of in-stent restenosis, lipid profile, and left ventricular ejection fraction in myocardial infarction: a randomized clinical trial. Iranian Red Crescent Med J 2017;19 | Participants do not have CHF |
| Shidfar F, Mohseni M, Vafa M, Hajimiresmail SJ, Rahimi A. The effect of COQ10 supplementation on serum lipoproteins, IL-6, ICAM-1 and plasma fibrinogen in hyperlipidemic patients with myocard infarction. Cardiovasc Ther 2012;1:73 | Participants do not have CHF |
| Silver MA, Langsjoen PH, Szabo S, Patil H, Zelinger A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. Am J Cardiol 2004;94:1306–10 | Participants taking statins |
| Silver MA, Langsjoen PH, Szabo S, Patil H, Zelinger A. Statin cardiomyopathy? A potential role for co-enzyme Q10 therapy for statin-induced changes in diastolic LV performance: description of a clinical protocol. Biofactors 2003;18:125–7. https://doi.org/10.1002/biof.5520180214 | Participants taking statins |
| Singh AK, Aoki M, Miyata M, Mine Y, Suzuki J, Urakami T. Mitochondrial diabetes a rare case. J Nepal Paediatr Soc 2018;37:290–2 | Participants do not have CHF |
| Singh RB, Kartikey K, Charu AS, Niaz MA, Schaffer S. Effect of taurine and coenzyme Q10 in patients with acute myocardial infarction. Adv Exp Med Biol 2003;526:41–8. https://doi.org/10.1007/978-1-4615-0077-3_6 | Participants do not have CHF |
| Singh RB, Neki NS, Kartikey K, Pella D, Kumar A, Niaz MA, Thakur AS. Effect of coenzyme Q10 on risk of atherosclerosis in patients with recent myocardial infarction. Mol Cell Biochem 2003;246:75–82 | Participants do not have CHF |
| Sobirin MA. Coenzyme Q10 Supplementation in Heart Failure with Preserved Ejection Fraction Patients. URL: www.isrctn.com/ISRCTN96610559 (accessed 7 January 2019) | Heart failure with preserved ejection fraction |
| Sobirin MA, Herry Y, Sofia SN, Uddin I, Rifqi S, Tsutsui H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discov Ther 2019;13:38–46. https://doi.org/10.5582/ddt.2019.01004 | Heart failure with preserved ejection fraction |
| Turk S, Baki A, Solak Y, Kayrak M, Atalay H, Gaipov A, et al. Coenzyme Q10 supplementation and diastolic heart functions in hemodialysis patients: a randomized double-blind placebo-controlled trial. Hemodial Int 2013;17:374–81. https://doi.org/10.1111/hdi.12022 | Participants do not have CHF |
| Yang YZ, Chen RZ, Zhang JN. [Observation on collaborative treatment of dilated cardiomyopathy.] Zhongguo Zhong Xi Yi Jie He Za Zhi 2001;21:254–6 | Participants do not have CHF |
| Yeung CK, Billings FT, Claessens AJ, Roshanravan B, Linke L, Sundell MB, et al. Coenzyme Q10 dose-escalation study in hemodialysis patients: safety, tolerability, and effect on oxidative stress. BMC Nephrol 2015;16:183. https://doi.org/10.1186/s12882-015-0178-2 | Participants do not have CHF |
| Yuan Z, Liu Z, Zheng X, Ma A, Zhu J, Wang S. Protective effects of captopril and coenzyme Q10 on the mitochondrial membrane-phospholipid injury of lymphocytes in patients with dilated cardiomyopathy. J Xian Med Univ 1995;7:107–11 | Participants do not have CHF |
| Excluded because of ineligible intervention | |
| Hjalmarson A. CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). Clin Cardiol 2008;31:90 | Intervention is not co-Q10 |
| McKeag N, McKinley MC, Woodside JV, Harbinson MT, McKeown PP. A trial of micronutrient supplementation in patients with heart failure. Eur J Heart Fail 2013;1:S75 | Intervention is not co-Q10 |
| McKeag NA, McKinley MC, Harbinson MT, Noad RL, Dixon LH, McGinty A, et al. The effect of multiple micronutrient supplementation on left ventricular ejection fraction in patients with chronic stable heart failure: a randomized, placebo-controlled trial. JACC Heart Fail 2014;2:308–17. https://doi.org/10.1016/j.jchf.2013.12.008 | Intervention is not co-Q10 |
| McKeag NA, McKinley MC, Harbinson MT, Noad RL, Dixon LH, McGinty A, et al. Correction to the effect of multiple micronutrient supplementation on left ventricular ejection fraction in patients with chronic stable heart failure: a randomized, placebo-controlled trial. JACC Heart Fail 2014;2:549 | Intervention is not co-Q10 |
| McKeag NA, McKinley MC, Woodside JV, Harbinson MT, McKeown PP. The effect of multiple micronutrient supplementation in patients with chronic stable heart failure: a randomized, placebo-controlled trial. Ir J Med Sci 2013;8:S383 | Intervention is not co-Q10 |
| McMurray JJ, Dunselman P, Wedel H, Cleland JG, Lindberg M, Hjalmarson A, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J Am Coll Cardiol 2010;56:1196–204. https://doi.org/10.1016/j.jacc.2010.02.075 | Intervention is not co-Q10 |
| Strey CH, Young JM, Molyneux SL, George PM, Florkowski CM, Scott RS, Frampton CM. Endothelium-ameliorating effects of statin therapy and coenzyme Q10 reductions in chronic heart failure. Atherosclerosis 2005;179:201–6 | Intervention is not co-Q10 |
| Excluded becuase of ineligible outcome | |
| Anfossi F, Guaraggi A, Varosio GP. [Efficacy of ubidecarenone (ubiquinone 10; coenzyme Q10) in patients with ischaemic heart disease.] Arch Med Interna 1983;35:271--84 | No relevant outcomes reported |
| Cogo R, Furcolo F. [Q10 coenzyme (ubidecarenone) in the treatment of senile cardiopathy and of the toxic one from antiblastic therapy.] Boll Chim Farm 1986;125:5S--11S | No separate outcomesreported for CHF participants |
| Dai YL, Luk TH, Yiu KH, Wang M, Yip PM, Lee SW, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis 2011;216:395–401. https://doi.org/10.1016/j.atherosclerosis.2011.02.013 | No relevant outcomes reported |
| Dai YLE, Luk TH, Siu CW, Yiu KH, Chan HT, Lee S, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic heart failure. Eur Heart J 2009;1:236 | No relevant outcomes reported |
| Kawashima C, Matsuzawa Y, Akiyama E, Sato R, Konishi M, Suzuki H, et al. Ubiquinol improves endothelial function in patients with heart failure with reduced ejection fraction: a single center, randomized double-blind placebo-controlled cross-over study. Circulation 2016;134 | No relevant outcomes reported |
| Medvedev OS, Kozaeva LP, Gorodetskaya EA, Kalenikova EI. Intravenous administration of CoQ10 evokes increase in NO-mediated blood vessels relaxation. Eur J Heart Fail 2016;1:426 | No relevant outcomes reported |
| Qingyan Z, Okello E, Yanhong T, Bing W, Congxin H. Effect of coenzyme Q10 administration on the incidence of atrial fibrillation in patients with heart failure. Circulation 2010;122:e209 | No relevant outcomes reported |
| Yuan Z, Liu Z, Yang D. [The protective effects of captopril and coenzyme Q10 on the mitochondrial membrane-phospholipid injury of lymphocytes in patients with dilated cardiomyopathy.] Chin J Cardiol 1994;22:49–51, 79–80 | No relevant outcomes reported |
| Irretrievable studies | |
| Anonymous. [Coenzyme Q10 is ineffective in heart failure.] Geneesmiddelenbulletin 2000;35:10–11 | Unable to retrieve |
| Belardinelli R, Mucaj A, Lacalaprice F, Solenghi M, Principi F, Mosca F, et al. Coenzyme Q10 potentiates the effect of exercise training on the endothelium-dependent relaxation of the brachial artery in chronic heart failure. Circulation 2003;108:739 | Conference abstract: unable to retrieve |
| Hall JH, Judy WV, Folkers K. Long-term survival in coenzyme-q10 treated congestive-heart-failure patients. Circulation 1990;82:675 | Unable to retrieve |
| Li X, Xie Y, Jiang X. Combined treatment of atrovastatin and coenzyme Q10 for chronic heart failure. Clin Chem 2009;55:A6-A | Conference abstract: unable to retrieve |
| Mortensen SA, Adamanti S. [The 7th world symposium of Copenhagen: Coenzyme Q10 in the treatment of heart failure.] Cuore 1992;9:685–8 | Unable to retrieve |
Appendix 3 Risk-of-bias assessment
| Study [authors (year)] | Selection bias: overall risk | Randomisation | Allocation concealment | Baseline imbalance | |||
|---|---|---|---|---|---|---|---|
| Berman et al. (2004)48 | ? | ? | Group allocation by third external party. ‘Randomly divided into two groups according to age and gender.’ No further details | + | Centrally allocated. Participants received a personally addressed sealed envelope containing ‘code A’ or ‘code B’ and were instructed to give the envelope to the pharmacist in return for a 3-month supply of capsules of either co-Q10 60 mg/day or maize flour-based placebo. Capsules were externally identical in the two groups | ? | No baseline characteristics reported per arm for a number of key variables, including age, sex, LVEF, heart failure medication, statins, NYHA |
| Davini et al. (1992)49 | ? | ? | No information | ? | No information on allocation concealment | ? | No baseline characteristics reported per arm |
| Fumagalli et al. (2011)50 | + | ? | Randomisation list mentioned, but no details reported on how it was generated | + | The randomization list remained concealed to the investigators; both patients and physicians, who were in charge of the enrolment and assessment, were blinded toward assignment | + | No statistically significant differences in key baseline variables. Some differences in co-existing therapies (notably digoxin 71% in co-Q10 arm vs. 91% control arm), but considered acceptable and not statistically significant |
| Garakyaraghi et al. (2015)51 | + | + | Simple randomisation method (cards, with equal numbers between groups). Used equal numbers of cards labelled as A or B. Neither the patients nor the researchers were aware of the group assignments. Placebos had identical appearance to the treatment | ? | No information | + | No statistically significant differences in key baseline variables |
| Keogh et al. (2003)54 | – | ? | No information | ? | No information | – | Clinically significant differences in medication (notably digoxin 83% in co-Q10 arm vs. 59% in placebo), warfarin and diurectics. Overall, co-Q10 arm participants appeared more medicated |
| Khatta et al. (2000)55 | + | + | Randomisation was performed by using a random number generator | ? | No information on allocation concealment. Following clarification request, authors stated that assignment to treatment arm was not made until after consent. No other details were provided | + | IPD showed no significant baseline imbalances |
| Kukharchik et al. (2016)56 | ? | ? | No information (conference abstract) | ? | No information (conference abstract) | ? | No baseline characteristics reported (conference abstract) |
| Kumar et al. (2007)57 | + | + | Randomised by computer-generated numbers | ? | No information | + | Baseline characteristics similar between groups for key variables |
| Ma et al. (1996)59 | ? | ? | No information | ? | No information | ? | Study reports that ‘each group had no significant difference observed through statistical analysis’, but no baseline characteristics reported |
| Mareev et al. (2017)60 | ? | ? | No information (conference abstract) | ? | No information (conference abstract) | ? | No baseline characteristics reported (conference abstract). The abstract states ‘there was no statistically significant difference in clinical characteristics and therapy of patients’ between the two arms but no further details were reported |
| Morisco et al. (1993)62 | + | + | Randomisation by computer generation | ? | No information | + | In control conditions there was no statistically significant difference between the clinical characteristics of the two patient groups |
| Mortensen et al. (2014)33 | + | + | Randomisation code was prepared by means of a random number generator software in blocks of six | + | Sealed envelopes used and sequentially numbered coded drug packs were distributed, supervised by a central pharmacist to the local centre with the instruction to assign new patients to the next available number | + | Baseline characteristics similar between groups for key variables |
| Munkholm et al. (1999)63 | – | ? | No information | ? | No information | – | Clinically significant differences in age and sex [older in co-Q10 arm, mean 60 vs. 54 years; higher proportion of males in control arm, 54% (6/11) vs. 9/11 (81%)], although not statistically significant. NYHA class at baseline was IIIA and IIB in treatment/control |
| Nakanishi et al. (1988)64 | – | ? | No information | ? | No information | – | Co-Q10 group were, on average, 2 years younger than the control group [mean 54 (SD 7) years vs. 56 (SD 8) years] |
| Pourmoghaddas et al. (2014)67 | – | + | Patients were randomly divided into two groups, using random allocation software. The sequence generation was performed by one of the study investigators who did not play a role in the clinical assessments and delivery of drugs to patients | ? | No information | – | Lower mean percentage ejection fraction in co-Q10 arm [18.7 (SD 10.3) vs. control 26.2 (SD 9.1)] at baseline, considered clinically significant although not statistically significant |
| Witte et al. (2005)69 | + | + | Block two-by-two randomisation performed centrally by remote pharmacy | + | Randomisation was co-ordinated by a remote pharmacy with which the investigators had no contact during the study | + | Baseline characteristics were similar between groups for key variables |
| Zhao et al. (2015)70 | + | ? | Patients were randomised and divided into two groups. No information on type of randomisation method | ? | No information | + | Baseline characteristics were similar between groups for key variables |
| Study | Blinding | Attrition | Outcomes reporting | |||||
|---|---|---|---|---|---|---|---|---|
| Participants and personnel | Outcome assessor | |||||||
| Berman et al. (2004)48 | + | ‘Double blind’ and treatments looked identical. No further statement about blinding of personnel, but considered low risk | +/? | States ‘double blind’, although not clear if outcome assessor were blinded. Unclear for 6MWT, NYHA and adverse events, low risk for death | ? | Five patients of 32 randomised (16%) lost to follow-up. Unclear how many were lost in each arm | ? | No protocol |
| Davini et al. (1992)49 | – | Open label. Control group received optimal therapy only. NYHA was the only outcome | – | Open label. Control group received optimal therapy only. NYHA was the only outcome | ? | No information on loss to follow-up/exclusions | ? | No protocol |
| Fumagalli et al. (2011)50 | + | Double blind, placebo controlled The randomization list remained concealed to the investigators; both patients and physicians, who were in charge of the enrollment and assessment, were blinded toward assignment | + | The randomization list remained concealed to the investigators; both patients and physicians, who were in charge of the enrollment and assessment, were blinded toward assignment | + | It appears that none was lost to follow-up | ? | No protocol |
| Garakyaraghi et al. (2015)51 | + | Neither the patients nor the researchers were aware of the group assignments | + | Researchers were not aware of the group assignments | + | Few exclusions (three participants in the co-Q10 arm and one in the placebo arm) | ? | No protocol |
| Keogh et al. (2003)54 | + | Study is ‘double blind’ and mentions 3 months’ blinded therapy | +/? | Double blind, but not clear if outcome assessors were blinded. Unclear risk of NYHA, LVEF and adverse events. Low risk for hospitalisation and ACM | + | Two participants withdrew from the placebo group (because of adverse effects) and two withdrew from trt group (one becuase of adverse effects and one to start another medication) | ? | No protocol |
| Khatta et al. (2000)55 | + | All patients and study personnel were blinded to study group assignment until all data were final | + | All patients and study personnel were blinded to study group assignment until all data were final | + | Nine patients did not finish the study (five in the co-Q10 group and four in the placebo group). Reasons are given and these were similar between groups | ? | Protocol not available |
| Kukharchik et al. (2016)56 | + | Open label. Control group received optimal therapy only. Unlikely to affect performance bias for outcomes reported | +/– | Open label. Control group received optimal therapy only. High risk for LVEF and low risk for pro-BNP | ? | No information on loss to follow-up/exclusions | ? | No protocol |
| Kumar et al. (2007)57 | + |
Matching placebos were used |
+ |
Matching placebos were used |
+ | Five patients were excluded before randomisation (no reasons given). Four patients were excluded after randomisation (two in the co-Q10 group and two in the placebo group due to loss to follow-up or an adverse effect) | ? | No protocol |
| Ma et al. (1996)59 | + | No information on blinding but placebo was used and outcomes were objective | + | No information on blinding but all objective outcomes | + | Four of 65 patients were lost to follow-up (no reasons given) | ? | No protocol |
| Mareev et al. (2017)60 | + | Placebo was used (conference abstract) | +/? | Reports study was double blind. No further details. Unclear risk for NYHA, LVEF, 6MWT and QoL. Low risk for pro-BNP | ? | No information on loss to follow-up/exclusions | ? | No protocol |
| Morisco et al. (1993)62 | + | Reports ‘double blind’. No other information on blinding, but all outcomes were objective and placebo was used | + | Reports ‘double blind’. No information on blinding, but all objective outcomes | + | Relatively low rate of attrition and no significant difference between study arms: 23 (of 319) patients dropped out in the co-Q10 group and 18 (of 322) patients in the placebo group | ? | No protocol |
| Mortensen et al. (2014)33 | + | The randomisation code was unavailable to investigators, participants or statisticians at any time during the study until all data material had been collected, all blood samples had been analysed and statistical analysis had been performed. Placebo used | + | The randomisation code was unavailable to investigators, participants or statisticians at any time during the study until all data material had been collected, all blood samples had been analysed and statistical analysis had been performed | + | There were 36 withdrawals (four lost to follow-up and 18 discontinued in the co-Q10 group and four lost to follow-up and 10 discontinued in the placebo group), which is < 10% of the total number of participants. Reasons given were not differences between groups | ? | Retrospectively registered in 2007 |
| Munkholm et al. (1999)63 | + | Only reports that ‘Subjects were randomized into two groups in a double-blinded, placebo controlled investigation’. Placebo capsules containing only soya oil were used. All objective outcomes | ? | Only reports that ‘Subjects were randomized into two groups in a double-blinded, placebo controlled investigation’. Considered unclear for LVEF (only outcome) | + | All patients completed the study | ? | No protocol |
| Nakanishi et al. (1988)64 | – | Open label. Control group received no treatment. NYHA was the only outcome extracted | – | Open label. Control group received no treatment. NYHA was the only outcome extracted | ? | No information on loss to follow-up/exclusions | ? | No protocol |
| Pourmoghaddas et al. (2014)67 | + |
‘Double blind’ |
+ | For the blindness of physicians the drugs were delivered to patients by one of the study investigators who did not perform the echocardiography and determination of NYHA symptom class grade | + | All patients completed the study | ? | No protocol |
| Witte et al. (2005)69 | + | Patients received either micronutrient supplementation or placebo capsules in a double-blind fashion. The patients were asked to take four visually identical, opaque capsules per day of either placebo or micronutrients. Low risk for objective outcomes and for 6-minute exercise test | + | ‘Double blind’. Randomisation was co-ordinated by a remote pharmacy with which the investigators had no contact during the study | + | Only two patients dropped out because of not being able to tolerate the CMR scan (2/32) | ? | No protocol |
| Zhao et al. (2015)70 | + |
No placebo mentioned. The control group was given ‘administration of common drugs’. However, all outcomes were objective |
+ |
No placebo mentioned. The control group was given ‘administration of common drugs’ |
? | ? | No protocol | |
| Study [authors (year)] | Selection bias: overall risk | Randomisation | Allocation concealment | Baseline imbalance | Blinding | Attrition | Outcome reporting | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants and study personnel | Outcome assessor | ||||||||||||||
| Belardinelli et al. (2005)47 | ? | ? |
No further information |
? | NI | ? | Reported for all patients together only | ? | Only reports ‘double-blind study’. Unclear risk for all outcomes (LVEF, peak volume and adverse events) | ? | As per previous comment | + | Two of 23 patients dropped out (one becuase of an orthopaedic injury and one because of work-related reasons) | ? | No protocol |
| Hofman-Bang et al. (1995)52 | ? | ? | NI | ? | NI | ? | Reported for all patients together only | +/? | Only reports ‘double-blind study’ Unclear risk for LVEF and adverse events. Low risk for death | +/? | As per previous comment | + | Seven of 69 patients died, four during the placebo and three during the co-Q10 periods. Three of 69 patients were withdrawn because of acute referral to heart transplantation, unassociated illness and personal reasons, respectively | ? | No protocol |
| Kawashima et al. (2020)53 | ? | ? | NI | ? | NI | ? | No baseline characteristics reported per randomised sequence. NYHA reported for co-Q10 and placebo patients, but only grouped (sequences 1 and 2 combined for pre placebo and pre co-Q10 values) | ? | Reports ‘double blind’ and use of placebo. NYHA was the only eligible outcome reported | ? | As per previous comment | – | Six of 20 patients dropped out, of whom five received co-Q10 followed by placebo | ? | No protocol |
| Langsjoen et al. (1985)58 | – | ? |
No further information |
? | NI | – | Significant differences in age (mean 60.6 years in group A vs. mean 64.2 years in group B) and sex (75% male in group A vs. 45% male in group B) | + | ‘Double-blind and double-crossover protocol’ and states that matching placebo was used | ? | States ‘double blind’ with no further details. Unclear risk for LVEF and adverse events | ? | No information on loss to follow-up/exclusions | ? | No protocol |
| Mazzola et al. (1987)61 | ? | ? | NI | ? | NI | ? | Reports only key baseline characteristics for all patients together | ? | Reports study was double blind but no more information. Unclear risk of NYHA (only outcome reported) | ? | As per previous comment | ? | No information on loss to follow-up/exclusions | ? | No protocol |
| Morisco et al. (1994)29 | ? | ? | NI | ? | NI | ? | Reported for all patients together only | ? | Reports study was double blind but no more information. Unclear risk of LVEF (only outcome reported) | ? | As per previous comment | + | It appears that none of the six patients was lost to follow-up | ? | No protocol |
| Permanetter et al. (1992)65 | – | ? | NI | ? | NI | – | Sex was different between groups (0% female in group 1 and 31% female in group 2) | ? | Reports study was double blind but no more information. Unclear risk for all outcomes (NYHA, LVEF and adverse events) | ? | As per previous comment | + | One patient excluded from group 2 as they needed a heart transplant | ? | No protocol |
| Pogessi et al. (1991)66 | ? | ? | NI | ? | NI | ? | No baseline characteristics reported | ? | Reports study was double blind. No further details. Unclear risk for all outcomes (LVEF and adverse events) | ? | As per previous comment | + | Two patients excluded | ? | No protocol |
| Watson et al. (1999)68 | ? | ? | NI | ? | NI | ? | Reported for all patients together only | + | Low risk for all outcomes (LVEF, QoL and adverse events), as patients and outcome assessors were blinded | + | As per previous comment | + | Three of 30 patients did not complete the trial (one because of heart failure death, and two needed a heart transplant) | ? | No protocol |
| Study [authors (year)] | Order of treatment randomised? | Sufficient washout? | First- and second-period data both available? | Attrition: any differences between arms before crossover? |
|---|---|---|---|---|
| Belardinelli et al. (2005)47 | Unclear.58 Discrepancy between the 200547 and 2006124 publications. The 2005 publication reports that two sequences were randomised and the 2006 publication reports that three were randomised. Nearly identical number of patients (21 and 23) and baseline characteristics | Unclear, no information | Yes | No |
| Hofman-Bang et al. (1995)52 | Yes. Randomly assigned to treatment or placebo and switched after 3 months | Unclear, no information | No | No |
| Kawashima et al. (2020)53 | Yes | 1 month | No | Yes. Six of 20 patients dropped out, of whom five received co-Q10 followed by placebo |
| Langsjoen et al. (1985)58 | Yes. Patients were randomly assigned by pharmacy personnel of the clinic in a double-blind and double-crossover protocol so that patients of group A received co-Q10 (33.3 mg) orally three times daily for 12 weeks and then a matching placebo three times daily for 12 weeks. The patients of group B received the placebo and then co-Q10 | Unclear, no information | Yes | Unclear |
| Mazzola et al. (1987)61 | Yes. Group A received co-Q10 followed by placebo, and group B received placebo then co-Q10 | Unclear, no information | No | Unclear |
| Morisco et al. (1994)29 | Yes. Patients were randomised to one of two sequences | Unclear, no information | No | Unclear |
| Permanetter et al. (1992)65 | Yes. Groups 1 and 2 were randomised to opposite sequences. It is not clear why the numbers differed between the arms (10 vs. 15) | Unclear, no information | Yes | No |
| Pogessi et al. (1991)66 | Yes. Groups were randomised to one of two arms in period A, with crossover after washout in period B | 30 days | No | No |
| Watson et al. (1999)68 |
Yes. Patients were randomised to blinded treatment with co-Q10 or placebo |
30 days | No | No |
Appendix 4 Additional New York Heart Association results from crossover trials
Two crossover trials61,65 reported data on NYHA class. Mazzola et al. 61 measured the NYHA functional class at baseline and at 8 weeks from baseline (but not at the 4 weeks crossover). Table 34 presents the changes in NYHA functional class between baseline and 8-week follow-up. The study reported an overall reduction in the percentage of NYHA class III patients (from 25% to 5%) and an increase in percentage of NYHA class I patients (from 0% to 20%) from baseline.
| NYHA functional class | Number of participants (total n = 20) | |
|---|---|---|
| Baseline | End of study | |
| I | 0 | 4 |
| II | 15 | 15 |
| III | 5 | 1 |
| IV | 0 | 0 |
Permanetter et al. 65 recorded average NYHA functional class in 25 patients at baseline, crossover at 4 months and at 8 months from baseline. There were no statistically significant changes in mean NYHA functional class with co-Q10 compared with placebo (Table 35).
| Group | Mean NYHA functional class (SD) | ||
|---|---|---|---|
| Baseline | Under placebo | Under co-Q10 | |
| Group I (placebo/co-Q10) (n = 10) | 2.30 (0.82) | 2.00 (0.82) | 1.70 (0.48) |
| Group II (co-Q10/placebo) (n = 15)a | 2.33 (0.72) | 2.13 (0.64) | 2.38 (0.81) |
Appendix 5 Additional meta-analysis results
FIGURE 22.
Forest plot of NYHA class deterioration.
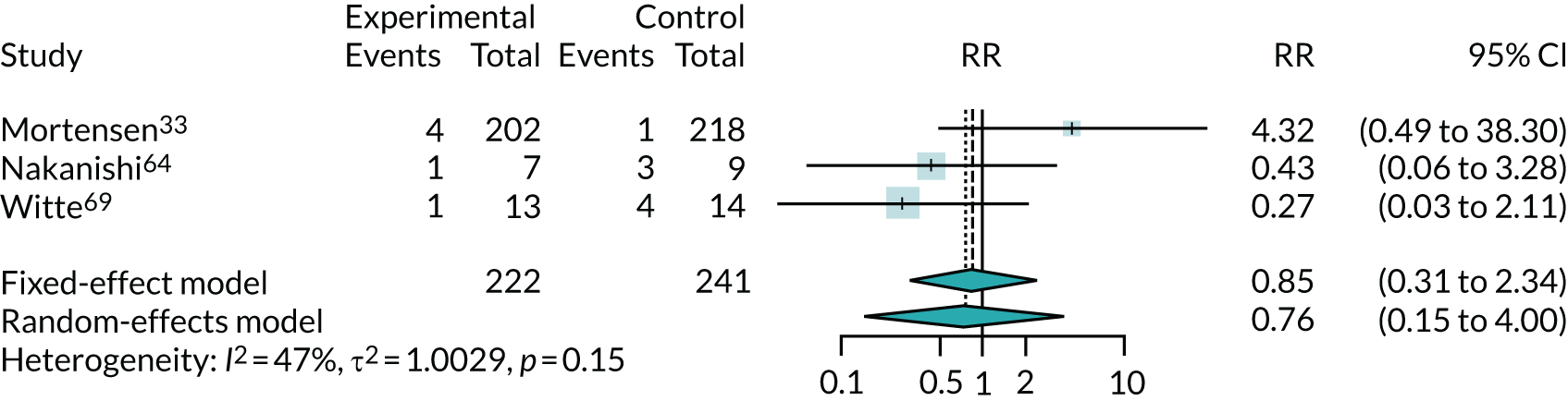
FIGURE 23.
Forest plot of adverse events leading to discontinuation.
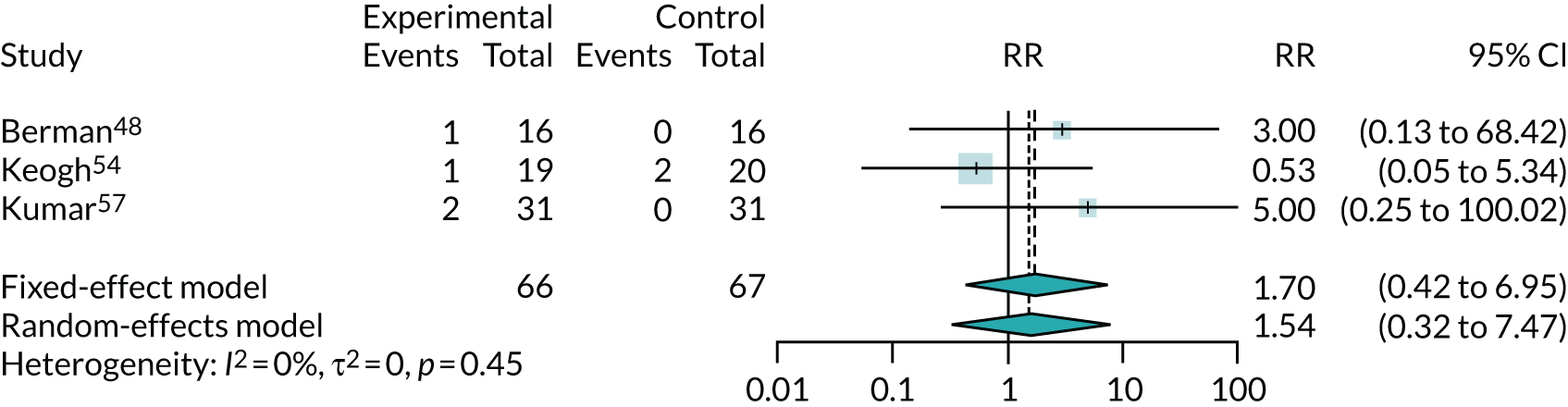
FIGURE 24.
Forest plot of ACM by intervention type.
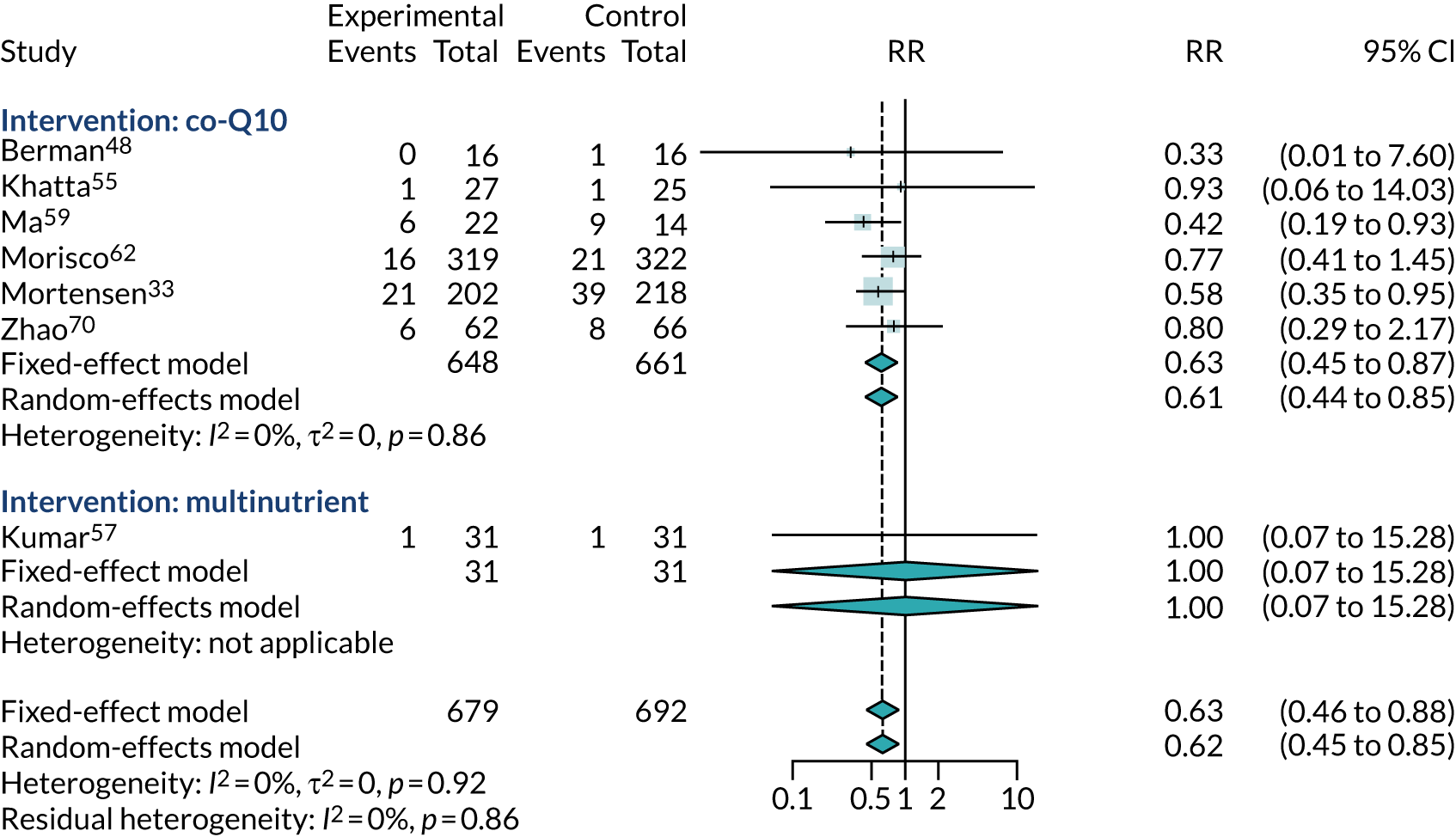
FIGURE 25.
Forest plot of LVEF change from baseline by intervention type.
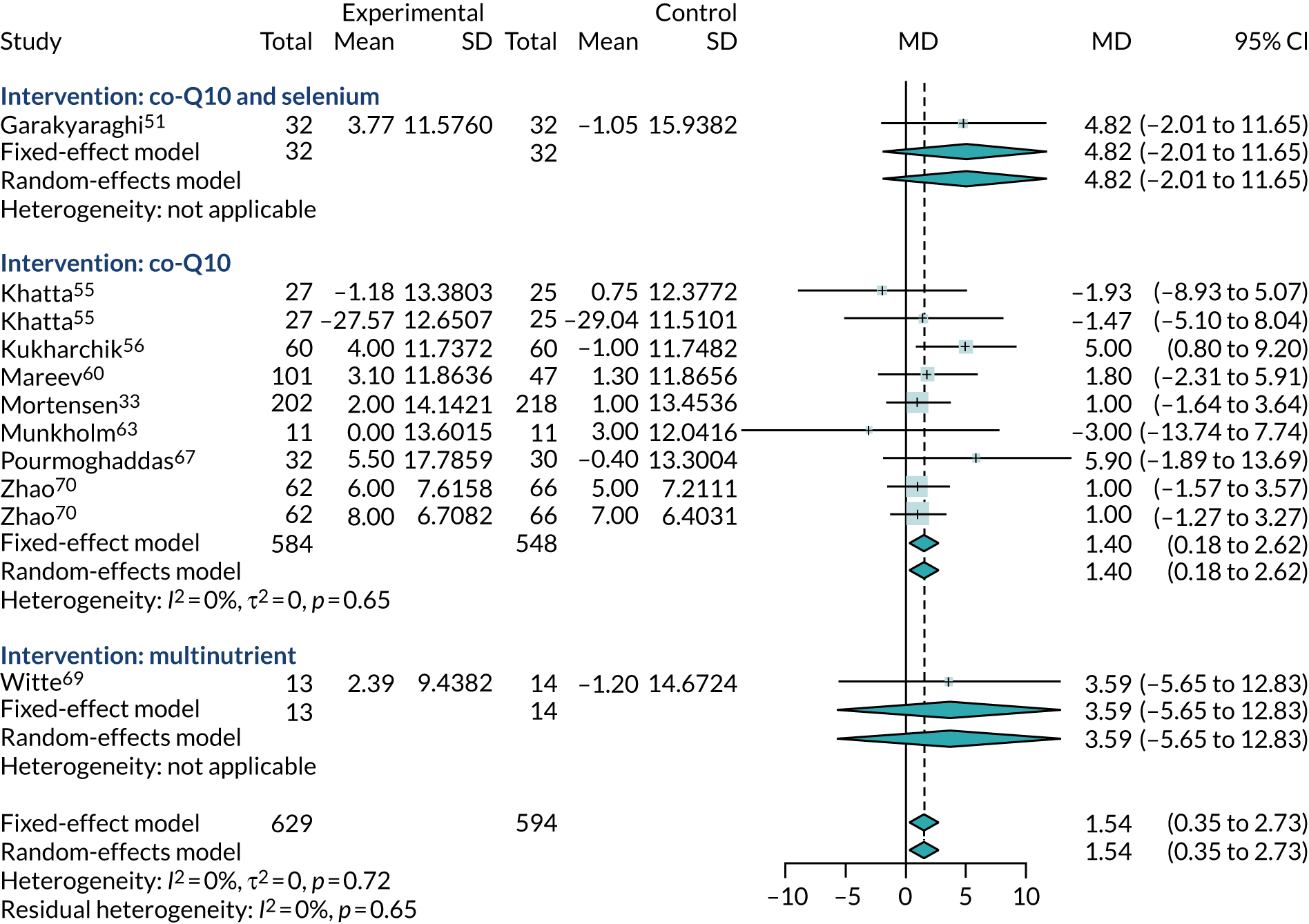
FIGURE 26.
Forest plot of NYHA subgroup by intervention type.
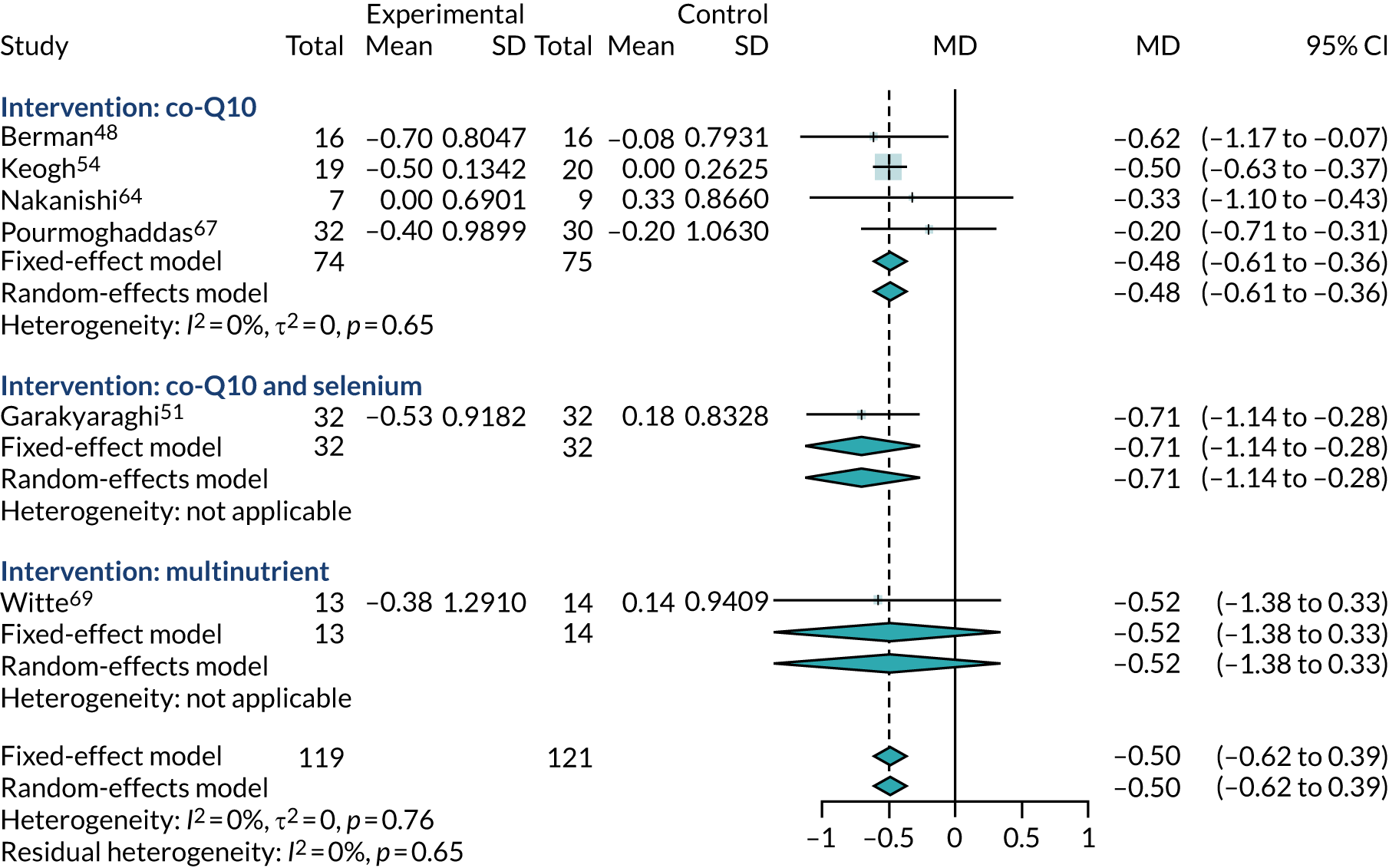
Appendix 6 Literature search strategies for cost-effectiveness evidence
Cost-effectiveness of co-Q10
Ovid MEDLINE(R) ALL via Ovid
Database: Ovid MEDLINE(R) ALL via Ovid.
Dates searched: 1946 to 12 May 2020.
Search date: 14 May 2020.
Records identified: 13.
Search strategy
-
exp Heart Failure/ (120,001)
-
(heart adj4 failure$).mp. (206,447)
-
(cardiac adj4 failure$).mp. (22,142)
-
(myocardial adj4 failure$).mp. (9025)
-
Cardiomyopathies/ (27,808)
-
Cardiomyopathy, Dilated/ (15,565)
-
cardiomyopath$.mp. (96,434)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (289,723)
-
Ubiquinone/ (9213)
-
ubiquinon$.mp. (13,068)
-
ubiquinol.mp. (1888)
-
ubidecarenone.mp. (65)
-
quinone.mp. (22,585)
-
neuquinon$.mp. (0)
-
bio-quinone Q10.mp. (2)
-
co-enzyme Q$.mp. (150)
-
coenzyme Q$.mp. (6175)
-
COQ10.mp. (1807)
-
COQ 10.mp. (336)
-
Q10.mp. (7185)
-
Q 10.mp. (2588)
-
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (38,620)
-
8 and 22 (557)
-
economics/ (27,180)
-
exp “costs and cost analysis”/or Cost Allocation/or Cost-Benefit Analysis/or Cost Control/or Cost of Illness/or Cost Sharing/or Health Care Costs/or Health Expenditures/ (234,988)
-
economics, dental/ (1911)
-
exp “economics, hospital”/or Hospital Charges/or Hospital Costs/ (24,416)
-
economics, medical/ (9069)
-
economics, nursing/ (3998)
-
economics, pharmaceutical/ (2929)
-
(economic$ or cost$ or price or prices or pricing or pharmacoeconomic$).tw. (819,404)
-
(expenditure$ not energy).tw. (29,497)
-
(value adj1 money).tw. (34)
-
budget$.tw. (28,991)
-
24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 (970,146)
-
((energy or oxygen) adj cost).ti,ab. (4079)
-
(metabolic adj cost).ti,ab. (1398)
-
((energy or oxygen) adj expenditure).ti,ab. (24,834)
-
or/36-38 (29,335)
-
35 not 39 (963,391)
-
letter.pt. (1,076,957)
-
editorial.pt. (528,227)
-
historical article.pt. (358,050)
-
41 or 42 or 43 (1,943,791)
-
40 not 44 (926,770)
-
exp animals/not humans/ (4,698,080)
-
45 not 46 (859,972)
-
23 and 47 (13)
EMBASE via Ovid
Database: EMBASE via Ovid.
Dates searched: 1974 to 2020 week 19.
Search date: 14 May 2020.
Records identified: 68.
Search strategy
-
exp Heart Failure/ (494,072)
-
(heart adj4 failure$).mp. (414,078)
-
(cardiac adj4 failure$).mp. (34,190)
-
(myocardial adj4 failure$).mp. (13,829)
-
exp cardiomyopathy/ (138,364)
-
cardiomyopath$.mp. (153,852)
-
or/1-6 (644,517)
-
exp Trace Elements/ (39,422)
-
micronutrient$.mp. (20,716)
-
ubiquinone/ (7692)
-
ubiquinon$.mp. (22,086)
-
ubiquinol.mp. (7025)
-
ubidecarenone.mp. (8793)
-
quinone.mp. (30,241)
-
neuquinon$.mp. (12)
-
bioquinone Q10.mp. (0)
-
bio-quinone Q10.mp. (4)
-
co-enzyme Q$.mp. (258)
-
coenzyme Q$.mp. (6996)
-
COQ10.mp. (2803)
-
COQ 10.mp. (232)
-
Q10.mp. (9081)
-
(“Q 10” or Q-10).mp. (1812)
-
C0Q10.mp. (9)
-
C0Q 10.mp. (0)
-
or/8-25 (113,365)
-
7 and 26 (2586)
-
health economics/ (32,637)
-
exp economic evaluation/ (303,574)
-
exp health care cost/ (288,729)
-
exp pharmacoeconomics/ (200,986)
-
or/28-31 (641,670)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (1,027,470)
-
(expenditure$ not energy).ti,ab. (39,798)
-
(value adj2 money).ti,ab. (2402)
-
budget$.ti,ab. (38,107)
-
or/33-36 (1,063,843)
-
32 or 37 (1,389,064)
-
letter.pt. (1,111,528)
-
editorial.pt. (651,226)
-
note.pt. (794,772)
-
39 or 40 or 41 (2,557,526)
-
38 not 42 (1,271,972)
-
(metabolic adj cost).ti,ab. (1507)
-
((energy or oxygen) adj cost).ti,ab. (4317)
-
((energy or oxygen) adj expenditure).ti,ab. (31,514)
-
44 or 45 or 46 (36,239)
-
43 not 47 (1,264,660)
-
exp animal/ (25,462,303)
-
exp animal experiment/ (2,538,229)
-
nonhuman/ (6,178,194)
-
(rat or rats or mouse oor mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (4,320,821)
-
49 or 50 or 51 or 52 (27,422,261)
-
exp human/ (20,837,709)
-
exp human-experiment/ (494,244)
-
54 or 55 (20,839,284)
-
53 not (53 and 56) (6,583,918)
-
48 not 57 (1,152,384)
-
27 and 58 (68)
EconLit
Database: EconLit via Ovid.
Dates searched: 1886 to 30 April 2020.
Search date: 14 May 2020.
Records identified: 0.
Search strategy
-
ubiquinon$.mp. (0)
-
ubiquinol.mp. (0)
-
ubidecarenone.mp. (0)
-
quinone.mp. (0)
-
neuquinon$.mp. (0)
-
bio-quinone Q10.mp. (0)
-
co-enzyme Q$.mp. (0)
-
coenzyme Q$.mp. (0)
-
COQ10.mp. (0)
-
COQ 10.mp. (0)
-
Q10.mp. (0)
-
Q 10.mp. (1)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (1)
-
Securities and Exchange Commission.ti. (30)
-
13 NOT 14 (0)
Cost-effectiveness of interventions for chronic heart failure
Ovid MEDLINE(R) ALL
Database: Ovid MEDLINE(R) ALL.
Dates searched: 1946 to 12 May 2020.
Search date: 13 May 2020.
Search strategy
-
exp heart failure/ (120,001)
-
cardiomyopathy, dilated/ (15,565)
-
shock, cardiogenic/ (8399)
-
exp ventricular dysfunction/ (36,990)
-
cardiac output, low/ (5496)
-
((heart or cardiac or myocardial) adj2 (failure or decompensation)).ti. (72,395)
-
((congestive or acute or decompensat* or chronic) adj2 “heart failure”).ti,ab. (62,748)
-
((dilated or congestive) adj2 cardiomyopath*).ti. (8022)
-
“cardiogenic shock”.ti. (3827)
-
((ventricular or ventricle*) adj2 (failure or insufficien* or dysfunction*)).ti. (7948)
-
((“left ventricular” or “left ventricle”) adj2 (failure or insufficien* or dysfunction*)).ti,ab. (21,412)
-
lvsd.ti,ab. (517)
-
or/1-12 (213,184)
-
letter/or editorial/or news/or exp historical article/or anecdotes as topic/or comment/or case report/ (4,278,703)
-
(letter or comment*).ti. (150,752)
-
14 or 15 (4,342,409)
-
13 not 16 (168,126)
-
economics/ (27,180)
-
exp “costs and cost analysis”/or Cost Allocation/or Cost-Benefit Analysis/or Cost Control/or Cost of Illness/or Cost Sharing/or Health Care Costs/or Health Expenditures/ (234,988)
-
economics, dental/ (1911)
-
exp “economics, hospital”/or Hospital Charges/or Hospital Costs/ (24,416)
-
economics, medical/ (9069)
-
economics, nursing/ (3998)
-
economics, pharmaceutical/ (2929)
-
(economic$ or cost$ or price or prices or pricing or pharmacoeconomic$).tw. (819,404)
-
(expenditure$ not energy).tw. (29,497)
-
(value adj1 money).tw. (34)
-
budget$.tw. (28,991)
-
18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 (970,146)
-
((energy or oxygen) adj cost).ti,ab. (4079)
-
(metabolic adj cost).ti,ab. (1398)
-
((energy or oxygen) adj expenditure).ti,ab. (24,834)
-
or/30-32 (29,335)
-
29 not 33 (963,391)
-
letter.pt. (1,076,957)
-
editorial.pt. (528,227)
-
historical article.pt. (358,050)
-
35 or 36 or 37 (1,943,791)
-
34 not 38 (926,770)
-
exp animals/ not humans/ (4,698,080)
-
39 not 40 (859,972)
-
17 and 41 (6102)
-
limit 42 to yr="2010 -Current" (3481)
Appendix 7 Relevant cost-effectiveness studies of interventions for chronic heart failure informing the development of the NICE guideline NG106
| Study | Comparators | Model structure | Limitations |
|---|---|---|---|
|
Lee et al. (2014)79 UK Mineralocorticoid receptor antagonists |
|
Discrete event simulation model using EMPHASIS-HF RCT trial data Trial follow-up: 4 years. Lifetime time horizon modelled |
Potentially serious limitations The analysis is based on estimates of the relative treatment effect and resource use from a single study, and does not reflect all available evidence. Utility values are not reported directly from patients of the EMPHASIS-HF trial. Potential bias due to commercial sponsorship of the study |
|
Tilson et al. (2003)125 Ireland Mineralocorticoid receptor antagonists |
|
Cost-effectiveness analysis reporting cost per life-year gained Time horizon: 10 years Based on RALES trial for patients with severe heart failure (NYHA classes III and IV) and LVSD |
Potentially serious limitations Analysis developed from an Irish perspective, which is reasonably comparable to the UK NHS. There is uncertainty regarding the applicability of resource use and costs from the Irish NHS in 2003 to current NHS setting |
|
Gutzwiller et al. (2012)126 UK NHS Iron supplementation for iron deficiency in heart failure |
|
Within-trial analysis of FAIRHF RCT 24-week follow-up |
Potentially serious limitations The short time horizon may not capture full costs and effects of the intervention. Lack of detailed medical resource use data. Within-trial analysis and so does not reflect full body of available evidence for all comparators |
|
Cowie et al. (2002)127 UK Home- vs. centre-based rehabilitation |
|
People with heart failure on optimised medication dosages, clinically stable for 1 month. Comparative costing from NHS perspective Follow-up: 5 years Does not include any health outcomes No discounting was undertaken |
Very serious limitations Small sample size, which has significant impact on cost per patient for the home training group. The baseline patient characteristics are not typical and also suggest that there might be selection bias |
|
Laramée et al. (2013)82 UK Biomarker-based monitoring |
|
Cost–utility analysis and Monte Carlo simulation model Lifetime horizon modelled Population split into multiple subgroups (CHF with LVSD, CHF, mean age > 75 and < 75 years) Disease progression not captured in the model |
Minor limitations Preference weights of EQ-5D scores were based on subject’s region of origin, not necessarily UK tariff (31% USA, 52% Western Europe and 14% Latin America) |
|
Moertl et al. (2013)128 Austria Biomarker-based monitoring |
|
Cost–utility analysis and Markov model 20-year time horizon modelled Population: patients with heart failure discharged after a heart failure hospitalisation |
Potentially serious limitations Austrian payer perspective. Utility scores converted from non-generic instrument using a previously published algorithm Costs and effects discounted at 5%. Cost of GP visits and drug costs were not collected for the clinical trial phase |
|
Pufulete et al. (2017);81 Mohiuddin et al. (2016)129 UK Monitoring |
|
Cost–utility analysis and Markov model Lifetime horizon modelled Population split into multiple subgroups (heart failure, HFrEF, HFpEF, mean age < 75, > 75 years) |
Minor limitations Uses a simple two-state Markov model that does not capture disease progression |
|
Pandor et al. (2013);80 Thokala et al. (2013)130 UK NHS Telemonitoring and self-monitoring |
|
Cost–utility analysis Lifetime horizon Clinical data determined from a NMA of RCT data |
Potentially serious limitations May not reflect the full body of available evidence. Utility decrement of heart failure hospitalisation considered to be overestimated |
|
Sahlen et al. (2016)131 Sweden Multidisciplinary teams |
Usual care vs. multidisciplinary team (including a heart failure nurse, palliative care nurse, cardiologist, palliative care physician, physiotherapist and occupational therapist) |
Cost–utility analysis Within-trial analysis of a RCT study 6-month follow-up Population: adults with CHF with NYHA class III or IV symptoms and a marker of severity |
Potentially serious limitations Single-centre study from a county council hospital in Sweden and therefore resource use and 2012 costs may not reflect current UK NHS context Short time horizon may not capture full costs and effects of the intervention. EQ-5D reported differently to the clinical trial evidence |
|
Atienza et al. (2004)132 Spain Multidisciplinary teams |
Usual care vs. multidisciplinary team (including a specialist cardiac nurse, primary care physician and cardiologist) |
Cost–consequence analysis (health outcomes: 1-year mortality rate, all-cause readmissions, QoL as measured by the MLWHFQ) Within-trial analysis of same paper in clinical review 16-month follow-up Population: people discharged from cardiology wards with a primary diagnosis of heart failure |
Potentially serious limitations Spanish resource use data and unit costs may not reflect current NHS context. QALYs were not used as the health outcome measure Within-trial analysis and so does not reflect the full body of available evidence available for this intervention |
|
Pulignano et al. (2010)133 Italy Multidisciplinary teams |
Usual care (primary/secondary care) vs. multidisciplinary team [including a cardiologist, experienced in geriatrics (case managers), two to four specialised nurses and the patient’s primary care physician] |
Cost-effectiveness analysis (health outcomes: death or readmission for heart failure and all-cause admission rate) Within-trial analysis of a RCT study 2-year follow-up Population: people aged > 70 years with HFrEF, discharged home after a hospitalisation |
Potentially serious limitations Italian national health service resource use and unit costs may not reflect current NHS context. QALY data were not reported clearly enough. Discounting was not applied Within-trial analysis and therefore does not reflect the full body of evidence available |
|
Postmus et al. (2011)134 Netherlands Multidisciplinary teams |
Usual care (cardiology clinic) vs. basic multidisciplinary team vs. intensive multidisciplinary team |
Cost–utility analysis Within-trial analysis of a RCT study 18-month follow-up Population: patients aged > 18 years with evidence of structural cardiac dysfunction. Also reports results for subgroups according to NYHA class No discounting was undertaken; however, the time horizon was only 18 months and so is unlikely to have a significant effect |
Potentially serious limitations Analysis undertaken from a Dutch perspective using 2009 unit costs and may not reflect current NHS context. Does not include important cost aspects, such as procedures during hospital admission. EQ-5D was not used |
Appendix 8 Description of included cost-effectiveness studies
This section summarises the six studies7,81,84,86,92,135 included in the updated review of studies informing the decision model and provides an assessment of the relevance of the data from the perspective of the NHS.
Taylor et al. (2019)92
The study by Taylor et al. 92 was designed to establish the long-term cost-effectiveness of the addition of home-based cardiac rehabilitation to usual care, compared with usual medical care alone, in patients with HFrEF. A Markov cohort model was used to capture the impact of the interventions on hospital admissions and associated increase in the mortality rate. The model used a lifetime horizon, following patients from a starting age of 78–100 years.
All-cause mortality was based on UK mortality rates from the THIN data set, 1998–2012, a retrospective cohort of 54,313 patients with a first diagnostic label of heart failure, with mean age 78 years. 85 The model assumed that survival rates followed an exponential distribution, on the basis that this distribution had previously been shown to provide a good fit in HFrEF populations. 93,129 To reflect the increased mortality rates during and after hospitalisation, the model applied a HR for survival based on alternative published data sources. 85,136
Heart failure-specific and other-cause hospital admissions were obtained from a UK cohort study of 332 patients in 1996–7. 127 A constant rate was applied throughout the time horizon of the model, irrespective of time, age and previous hospital admissions.
Costs associated with hospital admissions were obtained from NHS Reference Costs. 127 Primary and secondary usual health-care costs associated with heart failure (£815/patient/year) were informed by UK national data for heart failure and the THIN data set. 85
Health state utility values were estimated from EQ-5D data collected in SHIFT. 97
The model predicted that usual care alone was associated with total costs of £15,051 and total QALYs of 4.24.
Witte et al. (2019)86
The study by Witte et al. 86 was designed to assess whether or not cardiac contractility modulation (via the OPTIMIZER® System; Impulse Dynamics, Marlton, NJ, USA) plus standard of care was a cost-effective treatment for people with heart failure, compared with standard of care alone, from the perspective of the NHS. The baseline characteristics of patients were NYHA class III, LVEF of 25–45% and a mean starting age of 60.7 years.
The cost-effectiveness model estimated costs and QALYs over a lifetime time horizon. During each model cycle, patients with heart failure could remain stable, improve (move to NYHA class I or II), deteriorate (move to NYHA class IV) or die.
Outcomes for mortality, hospitalisation and QoL were predicted from regression equations, estimated from individual patient data from three RCTs. 137–139 A parametric exponential model was selected to model ACM. A multinomial logit model determined the proportion of patients in each NYHA class at any given time and a generalised linear mixed model (GLMM) predicted monthly all-cause hospitalisation rates by NYHA class. It was assumed that any change in NYHA class would occur within the first year and that the proportion in each class would remain constant thereafter. To account for differences in patient baseline characteristics, the model considered the following variables: time, treatment, sex, baseline age, baseline LVEF, ischaemic status and diabetic status.
The RCTs captured patients’ HRQoL via the MLWHFQ. A GLMM predicted patients’ MLWHFQ score by NYHA class. Predicted MLWHFQ scores derived from the GLMM were transformed into EQ-5D utility scores using two published algorithms.
Health-care costs associated with hospitalisations and standard of care, including outpatient visits, prescribed drugs and laboratory expenses were estimated from published sources.
The model predicted 8.91 life-years, 5.02 total QALYs and a total lifetime cost of £43,897 for patients on standard of care. Life expectancy was slightly higher in the subgroup of patients with a LVEF of 35–45% (9.03 years) than in the subgroup of patients with a LVEF of 25–34% (8.87 years).
McMurray et al. (2018)135
The study was designed to assess the cost-effectiveness of sacubitril/valsartan (Entresto; Novartis, London, UK) in the treatment of HFrEF from the perspective of three separate health-care providers in the UK, Denmark and Colombia. A Markov model was developed over a lifetime time horizon, with two health states defined as alive and dead. Hospitalisation rates, HRQoL and adverse event rates were estimated within the alive health state, whereas survival analysis was used to model cardiovascular mortality over time and non-cardiovascular mortality was estimated using national life tables adjusted to remove the risk of cardiovascular mortality. Enalapril was modelled as the base-case comparator. The mean age was 63.8 years and 69.3% of patients were in NYHA class II.
The risks of mortality and hospitalisation events were estimated through multivariable regression models, dependent on patients’ baseline characteristics and treatment. The models used data from the PARADIGM-HF (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) RCT, which enrolled patients between 2009 and 2012, with 4212 patients allocated to the enalapril (active control) arm. 83 The baseline risk of cardiovascular mortality was assumed to follow a Gompertz distribution, selected from potential distributions by clinical experts following review of projected life expectancies. The monthly risk of all-cause hospitalisation was estimated using a multivariable negative binomial regression model derived from PARADIGM-HF data.
Utility values were estimated from PARADIGM-HF data, using a multilevel model of EQ-5D to predict utility values dependent on baseline characteristics, hospitalisation, adverse events and time since randomisation.
Costs of background therapies were based on recommended doses and the utilisation reported in PARADIGM-HF at baseline. The proportion of each type of hospitalisation was taken from PARADIGM-HF and the cost was adjusted to reflect whether or not the event involved a surgical procedure. Mean annual use of background resources, such as GP and outpatient visits, was taken from an analysis of CPRD data.
The model predicted a mean life expectancy of 8.36 years for patients on enalapril. Patients had 0.89 heart failure-related hospitalisations and 2.18 cardiovascular hospitalisations over their lifetime. The mean per-patient QALY was estimated as 5.06 and the total lifetime costs were £14,814.
Cowie et al. (2017)7
This study was designed to assess the cost-effectiveness of real-time pulmonary artery pressure monitoring compared with usual care in patients with heart failure.
A Markov model was developed consisting of two health states (‘stable heart failure’ and ‘dead’). The model used a 10-year time horizon and patients were assumed to enter the model at age 70 years.
The baseline monthly risk of death was based on previous work by Griffiths et al. 98,100 A Gompertz model was fitted to the cardiovascular mortality from SHIFT,97 a RCT of ivabradine compared with standard of care, which enrolled 6558 patients between 2006 and 2009. Patients had HFrEF with a prior hospitalisation for heart failure within the last 12 months, NYHA classes II–IV, and a LFEF of ≤ 35%. The risk of non-cardiovascular mortality was estimated using age- and sex-adjusted UK national life table data, with cardiovascular mortality removed. The baseline monthly risk of heart failure-related hospitalisation was based on a meta-analysis of 21 studies, with patients with a median age of 70.7 years and 54% of patients in NYHA class III. 140
The cost of standard heart failure care was estimated to be £36.31 per month, based on previous estimates,100 and was applied to stable heart failure patients. The cost of heart failure hospitalisation was estimated from NHS Reference Costs.
Utility values for the first 12 months of usual care and treatment were based on data from the CHAMPION trial. After 12 months, utility values were assumed to decrease at a rate of 0.008 per year. 141 At 5 years, a utility value for CHF was applied. 142 Hospitalisations were associated with a temporary QoL loss, which was applied after 5 years on the assumption that the disutility associated with hospitalisations in the first 5 years was reflected in the trial utility values. 140
For patients on standard care, the model predicted a mean survival of 4.79 years, total costs of £6189 and total QALYs of 2.57 per patient.
Pufulete et al. (2017)81
The study by Pufulete et al. 81 was designed to examine the clinical effectiveness and cost-effectiveness of serum BNP testing and monitoring in patients with heart failure in primary and secondary care from a UK NHS perspective. A Markov model with a lifetime time horizon was developed and consisted of two health states (‘alive’ and ‘dead’). The analysis considered subgroups of patients based on age and LVEF status.
The baseline probability of ACM was estimated from CPRD–HES–ONS-linked data from April 2005 up to the censoring date of April 2014, which included 52,122 patients. 143 The monthly hazard rate was estimated by fitting an exponential distribution to the data, which was applied for the first 8 years of the model. Beyond 8 years, survival was estimated from age- and sex-specific ONS population life tables for the UK, inflated with a RR derived from an observational study to reflect the heart failure patient population. The monthly hazard rate of all-cause hospitalisation was also estimated from CPRD–HES–ONS-linked data and was applied throughout the lifetime of patients in the model.
Utility values used in the model were based on data from the ASCEND-HF trial, a multinational trial of > 6000 patients hospitalised with acute decompensated heart failure and randomised to nesiritide (Nesiritide Natricor; Janssen Pharmaceuticals, Beerse, Belgium) or placebo. The costs of managing patients hospitalised with HF and with stable HF in the community, stratified by age group, were estimated from on the CPRD–HES–ONS linked data.
The predicted life expectancy for patients on standard of care was 6.46 years for those aged < 75 years. The total lifetime QALYs was predicted to be 5.02 for those aged < 75 years and 2.02 for those aged ≥ 75 years. Total lifetime costs were £58,139 for those aged < 75 years and £26,093 for those aged ≥ 75 years.
Mealing et al. (2016)84
This study was designed to examine the cost-effectiveness of implantable cardiac devices in patients with systolic heart failure from the perspective of the NHS. The population comprised HFrEF patients with a starting age of 66 years, all NYHA classes and a LVEF ≤ 35%.
A meta-analysis of individual patient data from 13 RCTs was used to predict baseline rates of ACM, all-cause hospitalisation and HRQoL. 84 A series of regression equations for each outcome included covariates representing patients’ baseline prognostic characteristics.
Parametric survival analysis was used to extrapolate mortality risks beyond the follow-up period. Hospitalisation rates were assumed constant over a patient’s lifetime. Hospitalisation costs were based on information on hospitalisation type from a UK-based population study. 7 The heart failure medications for each NYHA class were estimated based on a review of literature and expert opinion.
Median survival for patients on standard care ranged from 8.96 years in NYHA class I to 1.82 years in NYHA class IV. Predicted number of hospitalisations over a lifetime ranged from 1.19 to 2.14, depending on NYHA class and QRS duration.
Appendix 9 Summary of model parameters
| Parameter | Mean | Distribution | Source |
|---|---|---|---|
| Baseline patient characteristics | |||
| Age (years) | 69.62 | Fixed | West Yorkshire data set89–91 |
| Gender (male) | 73.20% | Fixed | West Yorkshire data set89–91 |
| Ejection fraction | 31.96% | Fixed | West Yorkshire data set89–91 |
| History of diabetes | 27.97% | Fixed | West Yorkshire data set89–91 |
| History of COPD | 15.76% | Fixed | West Yorkshire data set89–91 |
| Ischaemic aetiology | 59.21% | Fixed | West Yorkshire data set89–91 |
| eGFR (ml/kg/1.73 m2) | 57.75 | Fixed | West Yorkshire data set89–91 |
| Haemoglobin (g/dl) | 13.46 | Fixed | West Yorkshire data set89–91 |
| NYHA class II | 50.67% | Fixed | West Yorkshire data set89–91 |
| NYHA class III | 29.67% | Fixed | West Yorkshire data set89–91 |
| NYHA class IV | 1.17% | Fixed | West Yorkshire data set89–91 |
| Atrial fibrillation | 35.67% | Fixed | West Yorkshire data set89–91 |
| Diastolic blood pressure (mmHg) | 71.46 | Fixed | West Yorkshire data set89–91 |
| Heart rate (b.p.m.) | 75.32 | Fixed | West Yorkshire data set89–91 |
| Lymphocyte count (×106/ml) | 22.60 | Fixed | West Yorkshire data set89–91 |
| Baseline event rates | |||
| ACM | Multivariate exponential survival (λ = 0.0002) | Multivariate normal | West Yorkshire data set89–91 |
| Heart failure-related hospitalisation: monthly probability | 0.0215 | Multivariate normal | West Yorkshire data set89–91 |
| Other cardiovascular hospitalisation: monthly probability | 0.0238 | Multivariate normal | West Yorkshire data set89–91 |
| Relative treatment effect (co-Q10 vs. standard care) | |||
| ACM | 0.68 (95% CrI 0.45 to 1.03) | Log-normal | One-stage meta-analysis |
| Heart failure-related hospitalisation (first occurrence of event) | 0.61 (95% CrI 0.49 to 0.77) | Log-normal | Two-stage meta-analysis |
| Other cardiovascular hospitalisation | 1 | Fixed | Assumption |
| Unit costs | |||
| Daily cost of co-Q10 | £0.94 (SE 0.01) | Gamma | BNF102 |
| Heart failure hospitalisation unit cost | £1948 (SE 198.80) | Gamma | NHS Reference Costs105 |
| Cardiovascular hospitalisation unit cost | £1935 (SE 197.45) | Gamma | NHS Reference Costs105 |
| GP emergency visits | £39 | Gamma | PSSRU 2019104 |
| A&E referrals | £183 | Gamma | NHS Reference Costs105 |
| GP visits | £39 | Gamma | PSSRU 2019104 |
| Cardiologist visits | £139 | Gamma | NHS Reference Costs105 |
| Other physician visits | £39 | Gamma | PSSRU 2019104 |
| GP home visits | £39 | Gamma | PSSRU 2019104 |
| GP nursing home visits | £39 | Gamma | PSSRU 2019104 |
| GP residential home visits | £39 | Gamma | PSSRU 2019104 |
| GP telephone calls to patient | £39 | Gamma | PSSRU 2019104 |
| GP visits with third parties | £39 | Gamma | PSSRU 2019104 |
| Resource use | |||
| GP emergency visitsa | 0.14 | Gamma | McMurray et al.135 |
| A&E referralsa | 0.01 | Gamma | McMurray et al.135 |
| GP visitsa | 13.54 | Gamma | McMurray et al.135 |
| Cardiologist visitsa | 0.05 | Gamma | McMurray et al.135 |
| Other physician visitsa | 0.36 | Gamma | McMurray et al.135 |
| GP home visitsa | 1.23 | Gamma | McMurray et al.135 |
| GP nursing home visitsa | 0.19 | Gamma | McMurray et al.135 |
| GP residential home visitsa | 0.04 | Gamma | McMurray et al.135 |
| GP telephone calls to patienta | 0.73 | Gamma | McMurray et al.135 |
| GP visits with third partiesa | 7.27 | Gamma | McMurray et al.135 |
| Proportion on ACE inhibitor | 90.49% | Beta | West Yorkshire data set89–91 |
| Proportion on beta-blockers | 84.71% | Beta | West Yorkshire data set89–91 |
| Proportion on MRA | 38.32% | Beta | West Yorkshire data set89–91 |
| Proportion on loop diuretic | 74.57% | Beta | West Yorkshire data set89–91 |
| Mean daily dose of ramipril (mg) | 5.44 (SE 0.0018) | Gamma | West Yorkshire data set89–91 |
| Mean daily dose of bisoprolol (mg) | 4.58 (SE 0.0018) | Gamma | West Yorkshire data set89–91 |
| Daily dose of spironolactone (mg) | 25.00 (SE 0.25) | Gamma | West Yorkshire data set89–91 |
| Mean daily dose of furosemide (mg) | 68.72 (SE 0.0254) | Gamma | West Yorkshire data set89–91 |
| Utilities | |||
| Baseline utility | 0.722 (SE 0.05) | Beta | SHIFT97,98 |
| Utility decrement for heart failure-related hospitalisation | –0.084 (SE 0.006) | Beta | Kansal et al.97 |
| Utility decrement for other cardiovascular hospitalisation | –0.032 (SE 0.005) | Beta | Kansal et al.97 |
| Length of stay: heart failure hospitalisation | 9.80 (SE 0.06) | Gamma | West Yorkshire data set89–91 |
| Length of stay: cardiovascular hospitalisation | 7.88 (SE 0.07) | Gamma | West Yorkshire data set89–91 |
| Annual discount rate | |||
| On costs | 3.5% | Fixed | NICE87 |
| On QALYs | 3.5% | Fixed | NICE87 |
Glossary
- Angina
- A chest pain or discomfort caused by a restricted blood supply to the heart. It is a common symptom of ischaemic heart disease, which is a subtype of chronic heart failure [British Heart Foundation. Angina – Causes, Symptoms & Treatments. URL: www.bhf.org.uk/informationsupport/conditions/angina (accessed 24 September 2020)].
- B-type natriuretic peptide/N-terminal pro-B-type natriuretic peptide
- B-type natriuretic peptide is a cardiac hormone used in maintaining heart function. N-terminal pro-B-type natriuretic peptide is an inactive molecule that is produced at the same time as B-type natriuretic peptide. B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide can be used to indicate heart failure, as increased levels of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide can indicate myocardial stress [Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-pro BNP in clinical routine. Heart 2006;92:843–9].
- Cardiomyopathy
- A disease that affects the function of the heart. Most types of cardiomyopathy are inherited. Dilated cardiomyopathy (i.e. when heart muscle stretches and becomes too thin) and hypertrophic cardiomyopathy (i.e. when heart muscle becomes too thick) are two common types [British Heart Foundation. Dilated Cardiomyopathy. URL: www.bhf.org.uk/informationsupport/conditions/cardiomyopathy/dilated-cardiomyopathy (accessed 24 September 2020)].
- Chronic heart failure
- A long-term condition caused by an individual’s heart functioning ineffectively, meaning that blood cannot be pumped around the body efficiently [NHS. Heart Failure. URL: www.nhs.uk/conditions/heart-failure/ (accessed 24 September 2020)].
- Cost-effectiveness acceptability curve
- A graph that plots a range of cost-effectiveness thresholds against the probability that the intervention will be cost-effective at each threshold.
- Cost-effectiveness threshold
- The maximum amount a health-care system is willing to pay to provide a new technology or intervention. The National Institute for Health and Care Excellence typically considers interventions with an incremental cost-effectiveness ratio of between £20,000 and £30,000 per quality-adjusted life-year to be cost-effective.
- Cost–utility analysis
- An analysis that aims to estimate the costs and consequences arising from making a particular policy decision (i.e. whether or not the NHS should fund a new procedure or drug). The effects or consequences of interventions are expressed in generic units of health gain, usually quality-adjusted life-years.
- Crossover trial
- A trial in which each participant receives both the active treatment and the control in sequence. In a randomised crossover trial, the sequence of treatment is randomised (Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials 2009;10:27. https://doi.org/10.1186/1745-6215-10-27).
- Ejection fraction
- The amount of blood that is pumped out of the left ventricle of the heart in each contraction. It is expressed as a percentage, with an ejection fraction of > 50% considered to be normal [British Heart Foundation. Heart Failure. BHF. URL: www.bhf.org.uk/informationsupport/conditions/heart-failure (accessed 24 September 2020)].
- Incremental cost-effectiveness ratio
- A measure that represents the economic value of an intervention compared with an alternative. The incremental cost-effectiveness ratio is the cost of generating an additional quality-adjusted life-year using the intervention of interest compared with an alternative, usually current clinical practice.
- Ischaemic heart disease
- A type of heart failure caused by the narrowing of the arteries that lead to the heart. It is caused by a build-up of fatty materials around the artery walls. Ischaemic heart disease is also known as coronary heart disease [British Heart Foundation. Coronary Heart Disease. URL: www.bhf.org.uk/informationsupport/conditions/coronary-heart-disease (accessed 24 September 2020)].
- Myocardial infarction
- More commonly known as a heart attack, myocardial infarction is caused by a blood clot that blocks the artery to the heart, leading to the heart being starved of blood and oxygen. Myocardial infarction is commonly caused by ischaemic heart disease [British Heart Foundation. Heart Attack. URL: www.bhf.org.uk/informationsupport/conditions/heart-attack (accessed 24 September 2020)].
- Parallel-group trial
- A trial in which participants are allocated to receive one of two or more interventions (e.g. either an active treatment or a control). In a randomised trial, allocation to groups is randomised (Evans SR. Clinical trial structures. J Exp Stroke Transl Med 2010;3:8–18).
- Peak oxygen consumption
- The volume of oxygen that an individual can take up at their maximum exercise capacity. Poor peak oxygen consumption can indicate heart failure (Opasich C, Pinna GD, Bobbio M, Sisti M, Demichelis B, Febo O, et al. Peak exercise oxygen consumption in chronic heart failure: toward efficient use in the individual patient. J Am Coll Cardiol 1998;31:766–75).
- Quality-adjusted life-year
- An index of health gain where survival duration is adjusted according to the patient’s quality of life over the time that they are alive. Quality of life is based on utilities, which are valuations measured on a scale between full health (utility = 1) and death (utility = 0).
List of abbreviations
- 6MWT
- 6-minute walk test
- ACE
- angiotensin-converting enzyme
- ACM
- all-cause mortality
- BNF
- British National Formulary
- BNP
- B-type natriuretic peptide
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHF
- chronic heart failure
- CI
- confidence interval
- co-Q10
- coenzyme Q10
- CPRD
- Clinical Practice Research Datalink
- ECHOES
- EchoCardiographic Heart Of England Screening
- EPHESUS
- Eplerenone Post-AMI Heart Failure Efficacy and Survival Study
- EQ-5D
- EuroQol-5 Dimensions
- EVPI
- expected value of perfect information
- FAS
- full analysis set
- GLMM
- generalised linear mixed model
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HFpEF
- heart failure with a preserved ejection fraction
- HFrEF
- heart failure with a reduced ejection fraction
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual participant data
- IQR
- interquartile range
- LVEF
- left ventricular ejection fraction
- LVSD
- left ventricular systolic dysfunction
- MD
- mean difference
- MI
- myocardial infarction
- MLWHFQ
- Minnesota Living with Heart Failure Questionnaire
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NT-pro-BNP
- N-terminal pro-B-type natriuretic peptide
- NYHA
- New York Heart Association
- ONS
- Office for National Statistics
- OR
- odds ratio
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SHIFT
- Systolic Heart failure treatment with the IF inhibitor ivabradine Trial
- SHOCS
- Scale for Heart failure to Optimise Clinical Status
- SMD
- standardised mean difference
- THIN
- The Health Improvement Network
- VOI
- value of information