Notes
Article history
The contractual start date for this research was in August 2021. This article began editorial review in November 2023 and was accepted for publication in August 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Simmonds et al. This work was produced by Simmonds et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Simmonds et al.
Background
Diabetes is a major cause of poor health that affects over 4 million people in the UK. Older people, men, people of South Asian ethnicity and more deprived populations are at higher risk. 1 Diabetic retinopathy is a ‘chronic progressive, potentially sight-threatening disease of the retinal microvasculature’2,3 that is a major complication of diabetes and a common cause of sight loss. Diabetic retinopathy impairs the sight of more than 1700 people in the UK each year. 4 The most severe form, proliferative diabetic retinopathy (PDR), places the patients at a high risk of vitreous haemorrhage, retinal detachment, neovascular glaucoma and vision loss. 5,6
Panretinal (laser) photocoagulation (PRP) is the primary treatment for PDR, where a laser is applied to vascular abnormalities to prevent proliferation of new blood vessels or encourage regression in those with established new vessels. PRP is delivered over the entire periphery of the retina, by placing 1200–1600 laser burns per session, usually over two or three treatment sessions. It is known to be effective and long-lasting7 but can have side effects including peripheral visual field loss, impaired night time and colour vision, and blurred vision. There is a small risk of central scotomata if laser burns are inadvertently placed at or near the foveal centre or if the laser scar extends centrally. 8
Anti-vascular endothelial growth factor (anti-VEGF) drugs have been proposed as alternative to PRP. In the UK, the National Institute for Health and Care Excellence (NICE) has approved ranibizumab and aflibercept for the treatment of diabetic macular oedema (DMO),9,10 and they are the standard treatment for wet age-related macular degeneration. However, whether they are beneficial for the treatment of diabetic retinopathy remains to be established. There are concerns that effects may not be long-lasting, and patients may have worse outcomes than those who had laser photocoagulation without repeated re-treatment and long-term follow-up. 11,12 They have rare but potentially serious adverse effects including: ocular hypertension, retinal detachment, endophthalmitis and other intraocular inflammation, and cataracts. 13
International Council of Ophthalmology guidelines on diabetic eye care14 support laser photocoagulation and ‘appropriate use of anti-VEGF drugs’ for the management of diabetic retinopathy. When this project commenced, there was no current NICE guidance for the use of anti-VEGF drugs in people with diabetic retinopathy but without macular oedema. NICE guidance is under development,15 and this review and meta-analysis was conducted to help inform it.
Given the uncertainty around whether anti-VEGF should be used to treat diabetic retinopathy, and the need for clear guidance on this topic, a systematic assessment of the relevant evidence and appropriate synthesis were needed. In order to synthesise data from mixed comparator studies, a network meta-analysis (NMA) approach was required to assess the value, effectiveness and rank of all relevant anti-VEGF interventions.
This paper presents a systematic review and NMA of all published randomised controlled trials (RCTs) of the three main anti-VEGFs used to treat diabetic retinopathy: aflibercept, bevacizumab and ranibizumab. While all three drugs act similarly to inhibit VEGF and slow the growth of blood vessels in the eye, they are different at molecular and receptor level, and so may differ in both efficacy and safety. This is why it is important to compare the three anti-VEGFs in a NMA.
The project was funded by the National Institute for Health and Care Research (Project number NIHR132948). The main project included a systematic review and meta-analysis incorporating individual patient data (IPD) from high-quality trials. Other components of the project included a wider assessment of anti-VEGF studies, including non-randomised studies, and an economic analysis of the cost-effectiveness of using anti-VEGF to treat diabetic retinopathy. The review was registered on PROSPERO (CRD42021272642) and the full protocol is available online from the NIHR (https://fundingawards.nihr.ac.uk/award/NIHR132948).
Methods
The aim of this project was to systematically review all RCTs where anti-VEGFs were used to treat diabetic retinopathy. The review was conducted following the Centre for Reviews and Dissemination guidance on undertaking systematic reviews16 and reported according to the principles of the overarching Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 17
Inclusion criteria
All RCTs that recruited people with diabetic retinopathy (proliferative and non-proliferative); patients with a principal indication for treatment of DMO or vitreous haemorrhage were excluded. The technologies of interest were any anti-VEGF therapy, anti-VEGF combined with PRP, PRP alone and sham injection.
A full list of outcomes of interest were reported in the review protocol. This paper focuses particularly on best corrected visual acuity (BCVA), as this was the only outcome reported in all trials. The appendices to this paper report evidence on all protocol-specified outcomes reported in the trials.
Review methods
An Information Specialist (HF) designed a preliminary search strategy in Ovid MEDLINE which consisted of terms for the condition (diabetic retinopathy), that were combined with terms for the intervention (anti-VEGF, angiogenesis inhibitors, or specific drugs used for the treatment of diabetic retinopathy). A RCT study filter was applied. No date or language limits were applied. The final MEDLINE strategy was adapted for use in all resources searched. All search strategies are presented in full in Appendix 1.
The searches were performed on 27 August 2021 and were updated on 13 July 2022 and again on 26 May 2023. The following databases were searched: Ovid MEDLINE(R) ALL, EMBASE (Ovid), Science Citation Index Expanded (Web of Science), Conference Proceedings Citation Index Science (Web of Science), Cochrane Central Register of Controlled Trials [CENTRAL (Wiley)], Cochrane Database of Systematic Reviews (Wiley), Database of Abstracts of Reviews of Effects {DARE [Centre for Reviews and Dissemination (CRD)]}, PROSPERO (CRD) and Epistemonikos. The following trial registries were searched: World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov and the EU Clinical Trials Registry. Search results were imported into EndNote 20 (Clarivate Analytics, Philadelphia, PA, USA) and deduplicated.
Two researchers (RW, AL) independently screened all titles and abstracts retrieved for consideration of the full text. The reviewers then screened full texts of potentially eligible studies to determine inclusion. Disagreements were resolved through discussion or with a third reviewer (MS).
A data extraction form was developed and piloted. Data on interventions used, patient characteristics, outcomes reported, and all outcome data were extracted for all included publications. Data extraction was completed by one reviewer and checked by a second (RW, AL). Risk of bias in all included trials was assessed using the Cochrane Risk of Bias 2 tool, focusing on the BCVA outcome, given limited reporting of other outcomes. 18
Statistical analysis
Effect estimates were pooled across trials using standard DerSimonian–Laird random-effect pairwise meta-analyses, according to the duration of follow-up. Heterogeneity was assessed in terms of I219 and by inspecting the between-study heterogeneity standard deviations (SDs; τ), relative to the treatment effect size.
Network meta-analyses were performed using standard Bayesian methods of NMA in R (version 4.3.1, The R Foundation for Statistical Computing, Vienna, Austria) using the R package multinma (version 0.5.1). 11,20 This extends the standard NMA modelling approach to investigate the potential impact of patient factors (e.g. type of retinopathy) and timing of assessments on the effectiveness of anti-VEGF therapy. 20 Network consistency was checked by comparing the model fit and between-study heterogeneity from the NMA models to an unrelated mean effects model (similar to a model performing direct meta-analysis for each treatment comparison, but with a shared heterogeneity parameter). 21
Visual acuity (BCVA) in diabetic retinopathy is commonly measured using the logarithm of the minimum angle of resolution (log-MAR) and Early Treatment Diabetic Retinopathy Study (ETDRS) scales. As both are widely used, NMAs were performed for both scales. Published data were transformed from one scale to the other, as required. This paper presents results on the log-MAR scale, with ETDRS results reported in the appendices.
The potential impact of unpublished or ongoing trials on the NMAs was investigated using threshold analysis. Threshold analysis investigates where in a NMA results might not be robust to changes in the observed evidence. 22
All R code and data used for this paper are available on GitHub (github.com/marksimmondsyork/AVID).
Patient and public involvement
Patient and clinical representatives were involved in all stages of this project as part of our advisory group including: the funding application, protocol development, discussing the review and its findings, and writing this paper. Further patient and stakeholder involvement was engaged through the NICE committee currently developing guidance on diabetic retinopathy management.
Equality, diversity and inclusion
As this was a review project of existing trial data, we could not account for equality issues in this field beyond what was reported in included publications or data. We note that reporting on potential equality areas such as ethnicity or socioeconomics was limited.
Results
General results
Key findings for BCVA, DMO, vitrectomy, vitreous haemorrhage and adverse events are presented here. A full presentation of all analyses performed for all outcomes is provided in the appendices.
Figure 1 shows the PRISMA flow chart for this review. Studies excluded from the review are listed in Appendix 1. Overall, 14 RCTs were included in the meta-analyses. The searches also identified 21 other RCTs, which were unsuitable for meta-analyses. These included trials reported only as conference abstracts, not in English, published before 2010 (and therefore judged to be out-of-date), that used types of anti-VEGF not in widespread use, or did not include a PRP arm. Those trials therefore could not be reasonably included in the NMAs. These are summarised in Appendix 1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. DMO, diabetic macular edema; VH; vitreous haemorrhage; MA, meta-analysis.

The included RCTs are summarised in Table 1. Trials varied substantially in sample size from only 40 eyes up to just over 400 persons. There were six trials of ranibizumab, five of bevacizumab and three trials of aflibercept. Five trials used anti-VEGF as the intervention, while nine used anti-VEGF combined with PRP. Twelve trials were of patients with proliferative retinopathy. Two trials recruited patients with non-proliferative retinopathy; both evaluated aflibercept. 23,24 Trials of aflibercept and ranibizumab were conducted in Europe, North America or Brazil. All trials of bevacizumab were conducted in the Middle East or South Asia. BCVA was the only outcome reported consistently in all trials.
| Trial | Year | Anti-VEGF | Comparator | Location | Sample size | Follow-up | Population | Main outcome(s) |
|---|---|---|---|---|---|---|---|---|
| CLARITY23 | 2017 | Aflibercept | PRP | UK | 232 persons | 1 year | PDR | BCVA, diabetic retinopathy severity, subsequent treatment, complications |
| DRCRN Protocol W24 | 2021 | Aflibercept | Sham injection | USA/Canada | 328 persons | 2 years | Severe non-proliferative diabetic retinopathy (some DMO) | Time to proliferative diabetic retinopathy or DMO |
| PANORAMA25 | 2018 | Aflibercept (every 16 weeks vs. 8 weeks) | Sham injection | International | 402 persons | 1 and 2 years | non-proliferative diabetic retinopathy | DR severity, subsequent treatment, complications |
| Marashi26 | 2017 | Bevacizumab | PRP | Jordan/Syria | 30 persons | 1 year | PDR | BCVA, DR severity |
| Ahmad27 | 2012 | Bevacizumab + PRP | PRP | Pakistan | 54 eyes | 3 months | PDR | BCVA |
| Ali28 | 2018 | Bevacizumab + PRP | PRP | Pakistan | 60 eyes | 1 month | PDR | BCVA |
| Rebecca29 | 2021 | Bevacizumab + PRP | PRP | Pakistan | 76 eyes | 6 months | PDR | BCVA |
| Roohipoor30 | 2016 | Bevacizumab + PRP | PRP | Iran | 64 eyes | 10 months | PDR | BCVA |
| DRCRN Protocol S31 | 2018 | Ranibizumab | PRP | USA | 305 persons | 2 and 5 years | PDR | DR severity, functional impact on vision, subsequent treatment, complications |
| Ferraz32 | 2015 | Ranibizumab + PRP | PRP | Brazil | 60 eyes | 6 months | PDR | BCVA |
| PRIDE33 | 2019 | Ranibizumab + PRP | PRP | Germany | 106 persons | 1 year | PDR | BCVA, DR severity, subsequent treatment |
| PROTEUS34 | 2018 | Ranibizumab + PRP | PRP | Europe | 87 persons | 1 year | PDR | BCVA, subsequent treatment, complications |
| Sao Paulo B35 | 2011 | Ranibizumab + PRP | PRP | Brazil | 40 persons | 1 year | PDR | BCVA, pain |
| Sao Paulo A36 | 2018 | Ranibizumab + PRP (ETRDS) | Ranibizumab + PRP (PASCAL) | Brazil | 40 eyes | 1 year | PDR | BCVA |
Risk of bias
For the risk-of-bias assessment of the included trials, see Table 2 and Appendix 1. Overall, four trials were classed at low risk of bias, three moderate and seven at high risk of bias. Risk of bias across individual domains was predominately of ‘some concerns’, primarily due to poor reporting, although larger trials tended to be better reported. Concerns were most common for the outcome measurement domain, due to the lack of masking of participants and outcome assessors. Other concerns included limited description of randomisation and allocation concealment processes, and missing patients and outcome data. The direction of bias was generally unpredictable. Overall, all the trials of bevacizumab were judged to be at high risk of bias. Only the larger trials of ranibizumab and aflibercept were at low risk of bias.
| Trial | Risk-of-bias domain | Overall | ||||
|---|---|---|---|---|---|---|
| Randomisation | Deviation from intended intervention | Missing outcome data | Outcome measurement | Selective reporting | ||
| Ahmad | ! | ! | + | – | ! | High |
| Ali28 | ! | ! | ! | – | ! | High |
| CLARITY23 | + | + | + | ! | + | Low |
| Ferraz32 | ! | ! | + | + | ! | Moderate |
| Marashi26 | – | ! | ! | – | + | High |
| PANORAMA25 | + | + | ! | + | + | Low |
| PRIDE33 | ! | + | ! | – | + | Moderate |
| PROTEUS34 | ! | + | ! | – | + | Moderate |
| Protocol S31 | + | + | + | ! | + | Low |
| Protocol W24 | + | + | + | ! | + | Low |
| Rebecca29 | + | ! | ! | – | ! | High |
| RECOVERY | ! | + | + | – | + | Moderate |
| Roohipoor30 | + | ! | – | – | ! | High |
| Sao Paulo A36 | ! | ! | ! | – | ! | High |
| Sao Paulo B35 | ! | ! | ! | – | ! | High |
| + | Low risk | |||||
| ! | Some concerns | |||||
| – | High risk | |||||
Impact on vision (best corrected visual acuity)
Figure 2 summarises all the data on BCVA for anti-VEGF compared to PRP, as reported across all trials. Results are shown as difference in ETDRS letters between anti-VEGF and control arms. This plot highlights significant variation in the design of the included studies, which precludes combining them all in a standard meta-analysis and demonstrates the need for NMA and meta-regression. First, some trials compare anti-VEGF to PRP directly, while others combine anti-VEGF with PRP, therefore motivating the need for NMA. Second, the time at which BCVA is measured varied enormously across trials, from 1 month to five years. Shorter trials were generally smaller in size, more likely to use bevacizumab and possibly showed larger effect sizes.
FIGURE 2.
All BCVA data (ETDRS letters) from all trials of anti-VEGF.

Network meta-analyses of best corrected visual acuity in proliferative retinopathy
Given the variations in timing at which BCVA results were reported, two NMAs were performed:
-
Analysis up to and including 1 year of follow-up, using the longest follow-up in each trial
-
Analysis only of trials with 1 or 2 years’ follow-up
Note that trials reporting at exactly 1 year were included in both analyses. Given the clinical differences between proliferative and non-proliferative disease, the two trials of non-proliferative disease were not included in the NMA. The network diagrams for both analyses are shown in Figure 3. The size of the circles indicates the number of participants, and the width of the lines and the number of trials. Note that all the trials of bevacizumab combined with PRP had follow-up durations of < 1 year, so are not included in the analyses at 1–2 years. In both networks, there is only one trial of aflibercept and one of bevacizumab (without PRP).
FIGURE 3.
Network diagrams at (a) up to 1 year and (b) 1–2 years.
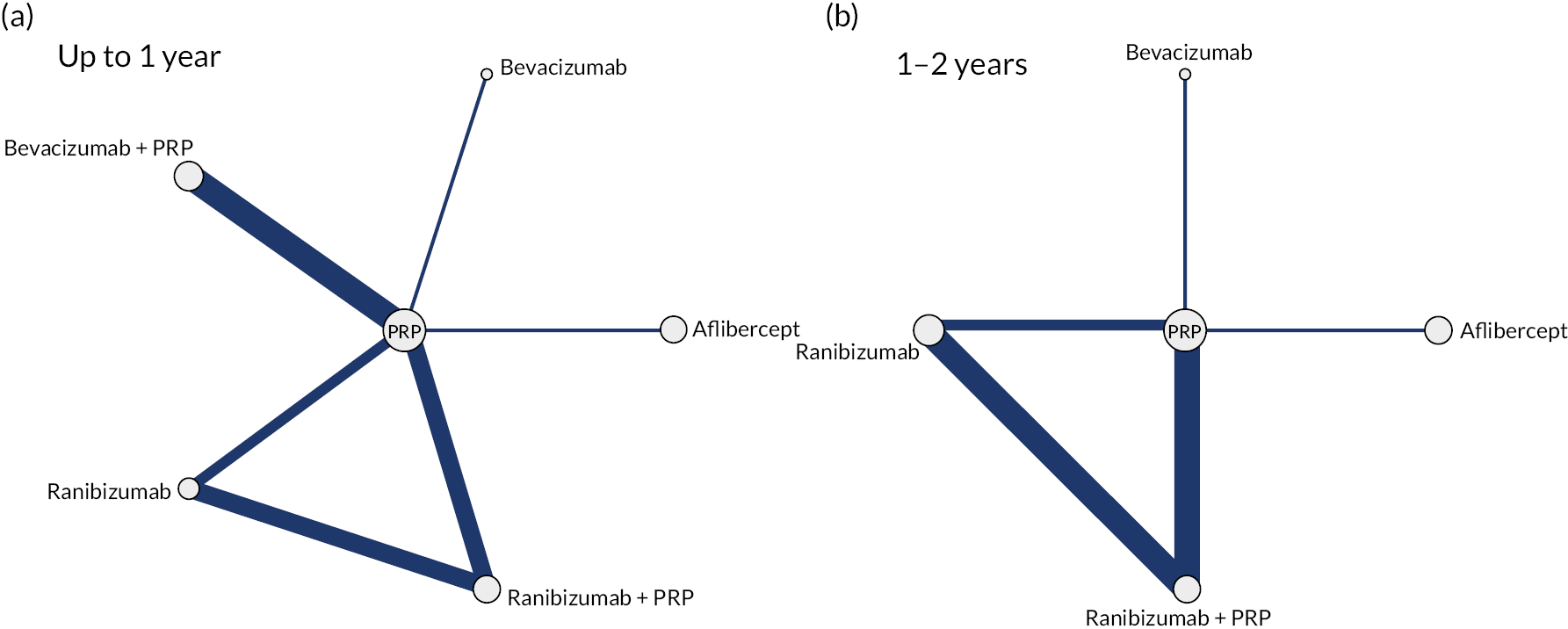
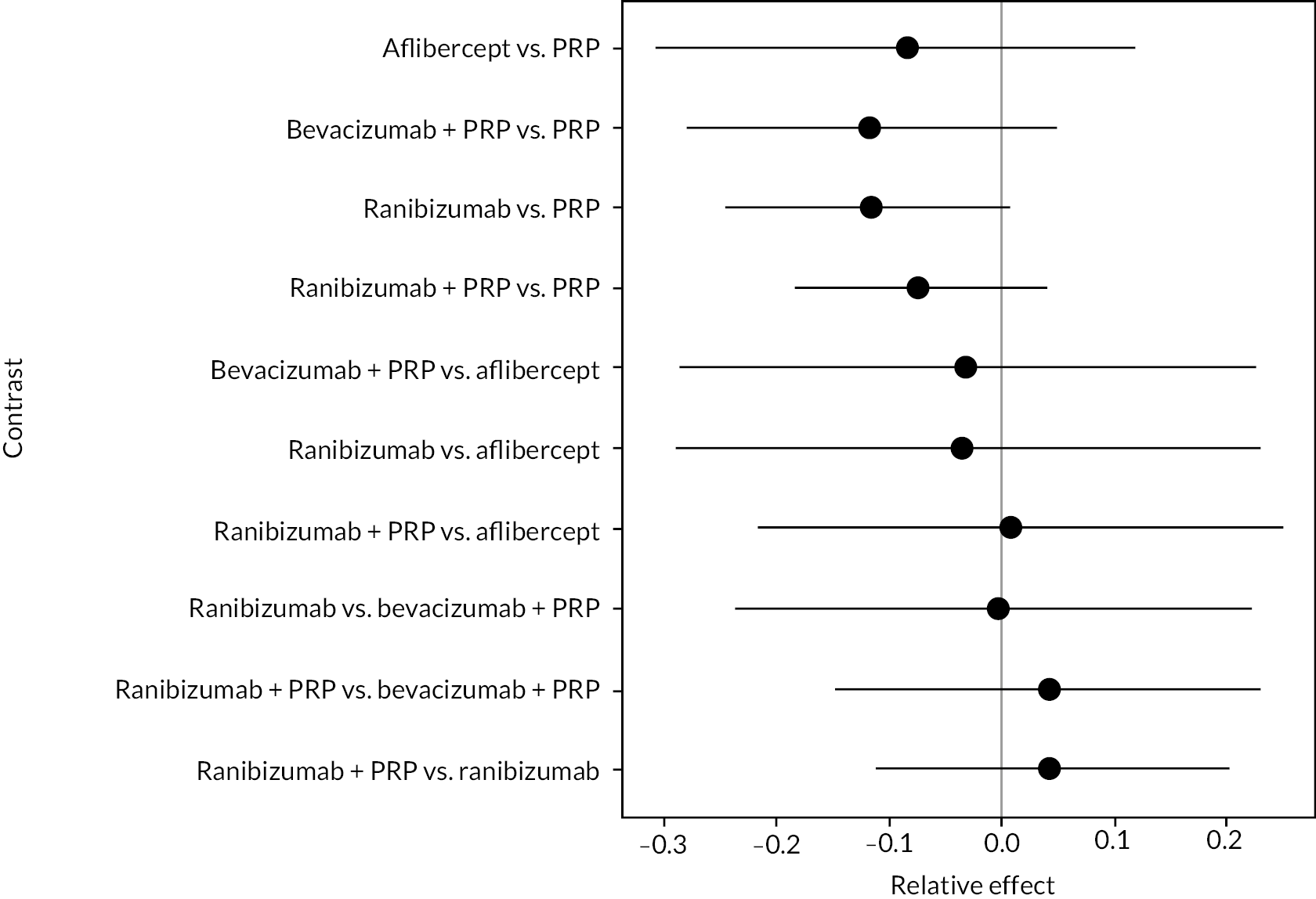
Figure 4 shows the results of all treatment comparisons from the NMA for data up to 1 year, and Figure 5 for data from 1 to 2 years. Full results of these NMAs are given in Appendix 2. In both figures, the point estimates are shown by the dots, with the horizontal lines being 95% credible intervals (CrIs). Negative relative effects (to the left of the vertical line) indicate favouring the first-named intervention.
FIGURE 4.
Comparison of interventions from NMA of BCVA up to 1 year.
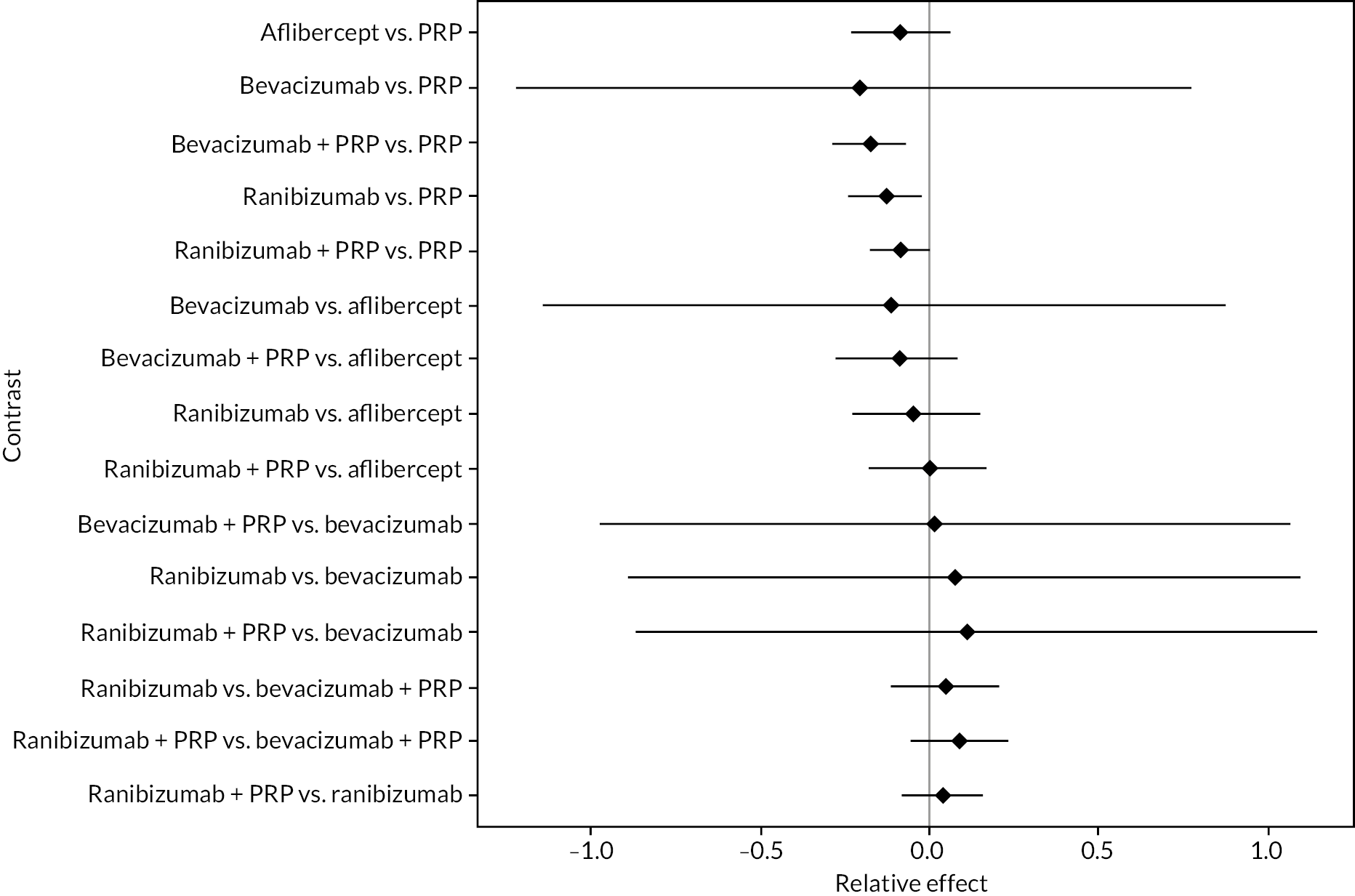
FIGURE 5.
Comparison of interventions from NMA of BCVA from 1 to 2 years.
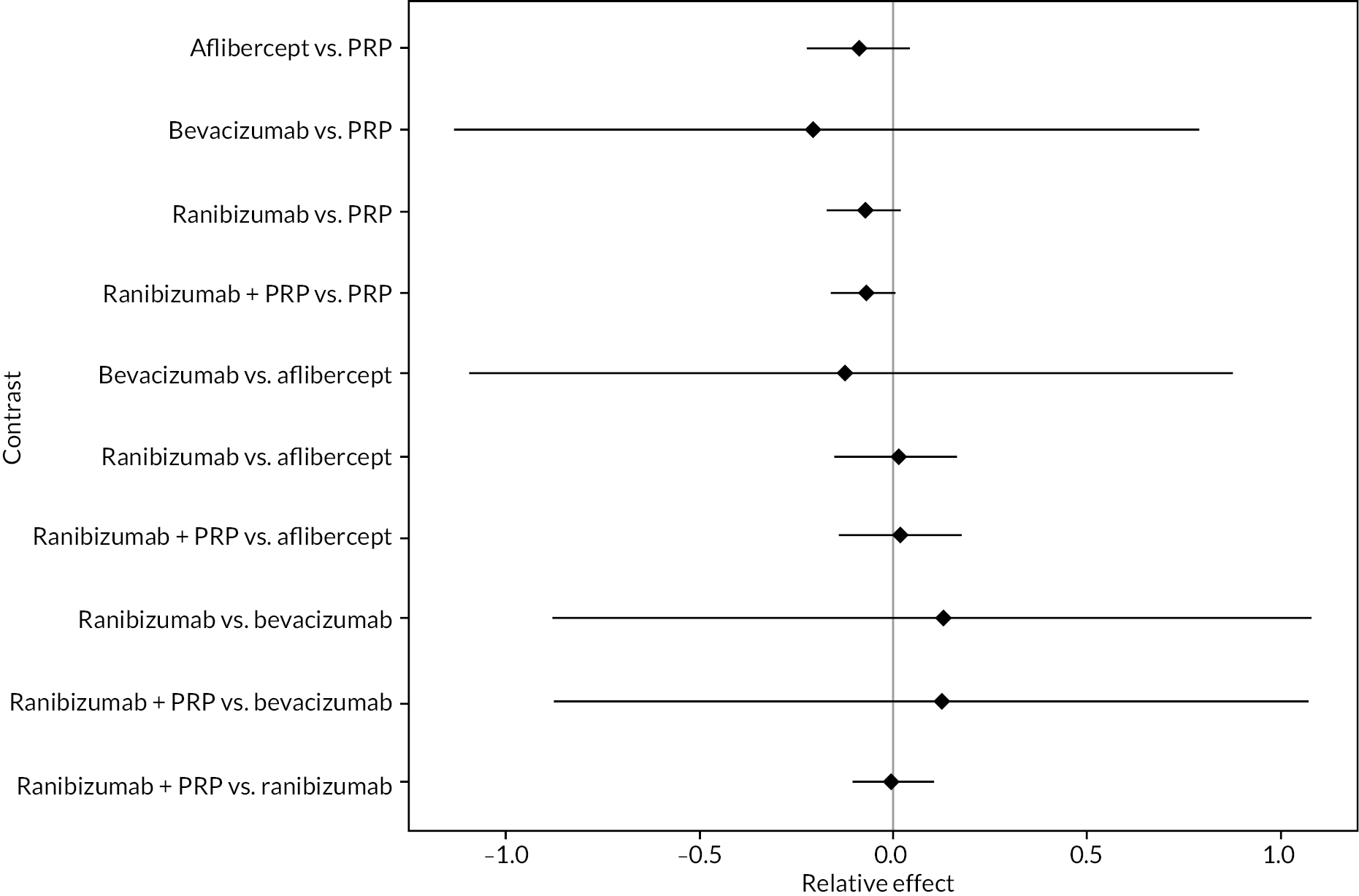
For the primary comparisons with PRP at up to 1 year, all trials favoured anti-VEGF over PRP and improved vision (reduced log-MAR scores). Changes in log-MAR scores when compared to PRP ranged from −0.078 (or 3.8 ETDRS letters) for ranibizumab with PRP to −0.198 (or 6.8 ETDRS letters) for bevacizumab. Results for aflibercept and bevacizumab (without PRP) were inconclusive because there was only one trial of each. Indirect comparisons between anti-VEGFs found no conclusive evidence that any one anti-VEGF was superior to the others. Heterogeneity across the network appeared to be modest, with an estimated heterogeneity standard error (τ) of 0.04 (95% CrI 0 to 0.14). Results for trials with a follow-up duration of 1–2 years (see Figure 5) were similar to those at up to 1 year, suggesting no obvious trend in treatment effects at up to 2 years.
Given the similarity in magnitude of effect for the various anti-VEGF agents compared to PRP, it is not surprising that the indirect comparisons between agents show no conclusive evidence of difference between any of them. There appears to be no difference between using ranibizumab alone versus ranibizumab combined with PRP, particularly at 2 years.
Treatment rankings are shown in Appendix 2 (Figures 23 and 26). Given the similarity in effect sizes across the different types of anti-VEGF, it is difficult to draw conclusions from the ranking diagrams beyond the fact that PRP alone is likely to be the least effective treatment. The limited data on bevacizumab mean its ranking is very uncertain.
Impact of follow-up time and vision at randomisation
To further examine the impact of follow-up time on the effectiveness of anti-VEGFs, we fitted a range of NMA models including time as a covariate. This meant that all trials could be combined in a single NMA, and whether the effectiveness of anti-VEGFs varied with time could be assessed. Models were also fitted including BCVA at randomisation, to account for possible variation in the effectiveness of the anti-VEGFs with initial vision (see Appendix 2).
Overall, results were very similar to the NMAs at up to 1 year and 1–2 years. Figure 6 shows the effect estimates for anti-VEGFs compared to PRP alone from a model with a linear association between anti-VEGF effect and both follow-up time and BCVA at randomisation. Estimates are presented for 1 year of follow-up and the mean BCVA at baseline across all trials (log-MAR 0.3). The pattern of effect sizes is very similar to that seen in Figures 4 and 5, but CrIs are wider, suggesting that adjusting for follow-up time and baseline BCVA leads to greater uncertainty.
FIGURE 6.
Network meta-analysis of log-MAR with adjustment for follow-up time and BCVA at baseline.
There was no clear evidence that the relative effectiveness of anti-VEGFs varied with time or with vision at randomisation. However, it should be noted that almost all the data were for follow-up times of 2 years or less. Only one trial followed up patients for 5 years, and that found no evidence of difference between anti-VEGF (ranibizumab) and PRP after 5 years. 31
Further network meta-analyses
To further compare the anti-VEGFs to each other, simplified NMAs were performed by combining treatment arms. Two NMAs were performed:
-
Comparing anti-VEGF (of any type), anti-VEGF (any type) combined with PRP and PRP alone
-
Comparing aflibercept, ranibizumab (with or without PRP), bevacizumab (with or without PRP) and PRP alone
In both cases, NMAs included adjustment for follow-up time and BCVA at randomisation. Full results for these NMAs are presented in Appendix 2. In summary, there was good evidence that, when all types of anti-VEGF were combined, anti-VEGF in general improved BCVA when compared to PRP (mean difference −0.089, 95% CrI −0.180 to −0.019), as did anti-VEGF combined with PRP compared to PRP alone (mean difference −0.108, 95% CrI −0.192 to −0.048).
When comparing the three anti-VEGFs (with or without concomitant PRP), there was no clear evidence of any difference in effectiveness between the three types of anti-VEGF; for example, there was no difference between aflibercept and ranibizumab (mean difference −0.003, 95% CI −0.166 to 0.163).
Threshold analysis
Threshold analyses of the NMAs of BCVA are reported in Appendix 2. These found that the evidence for anti-VEGF being superior to PRP was robust, but there was some uncertainty in the overall ranking of the various anti-VEGF treatments. This was probably because the evidence across the different anti-VEGFs showed very similar effectiveness.
Other outcomes
Results on outcomes other than BCVA were inconsistently reported, with most being reported in no more than three trials. Complete results for these outcomes are presented in Appendix 3. The limited data meant that NMAs were not feasible for these outcomes. A meta-analysis was performed for outcomes reported in two or more trials by assuming that the impact of anti-VEGFs is the same for all types of anti-VEGF, for anti-VEGF alone or in combination with PRP, and at all times up to 2 years. While these are strong assumptions, they may be reasonable given the results observed for BCVA, and the apparent lack of heterogeneity in the data.
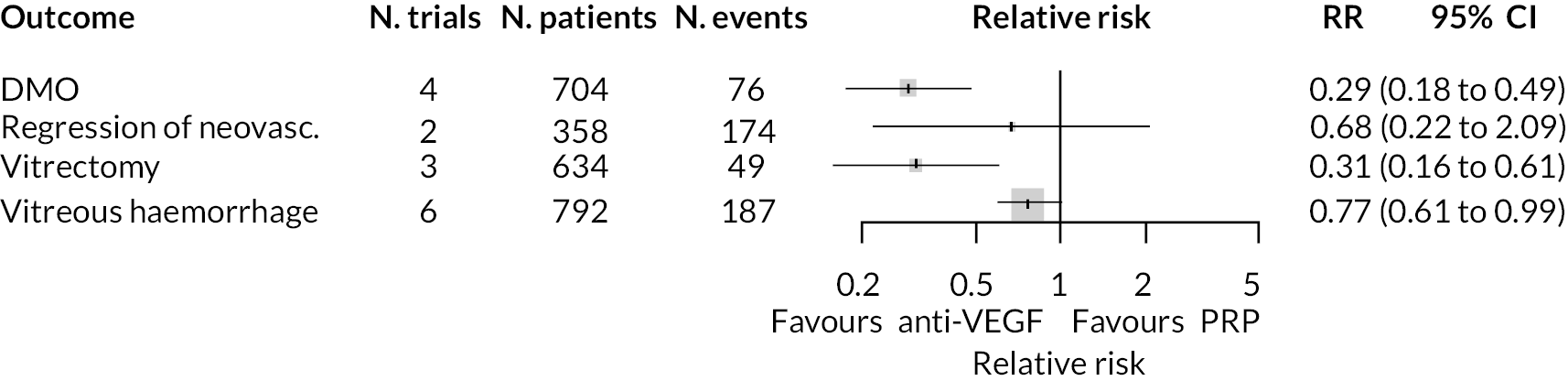
Forest plots of neovascularisation of the disc (NVD) and neovascularisation elsewhere (NVE) are shown in Appendix 3. These suggest that neovascularisation was reduced while using anti-VEGF. The results of meta-analyses for other non-vision outcomes are shown in Figure 7. Although data were limited, the results suggest that anti-VEGF treatment substantially reduces the rate of macular oedema (DMO), the need for vitrectomy and reduces the rate of vitreous haemorrhage. No data on progression of diabetic retinopathy were reported.
FIGURE 7.
Meta-analysis of non-vision outcomes. RR, relative risk.
Adverse events
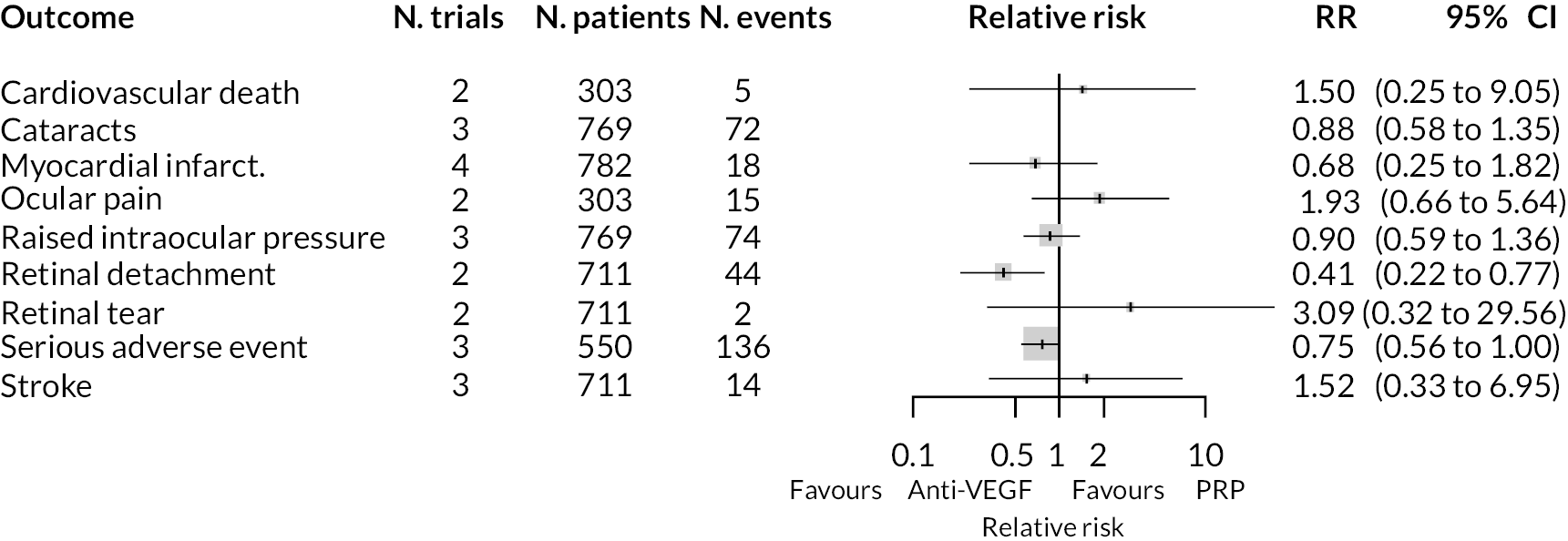
As with non-BCVA outcomes, adverse events were not widely reported, with little consistency across trials as to which adverse events were reported. A meta-analysis was performed for adverse event types reported in two or more trials by assuming that the impact of anti-VEGFs is the same for all types of anti-VEGF, for anti-VEGF alone or in combination with PRP, and at all times up to 2 years.
The meta-analysis results are shown in Figure 8. Due to the small numbers of events, and limited numbers of trials reported each adverse event, most results are inconclusive. Anti-VEGF appeared to reduce the incidence of retinal detachment. It appeared to increase the rate of ocular pain, but it was unclear whether this was procedure-related or post-intervention pain. Full results are presented in Appendix 3.
FIGURE 8.
Meta-analyses of adverse event outcomes.
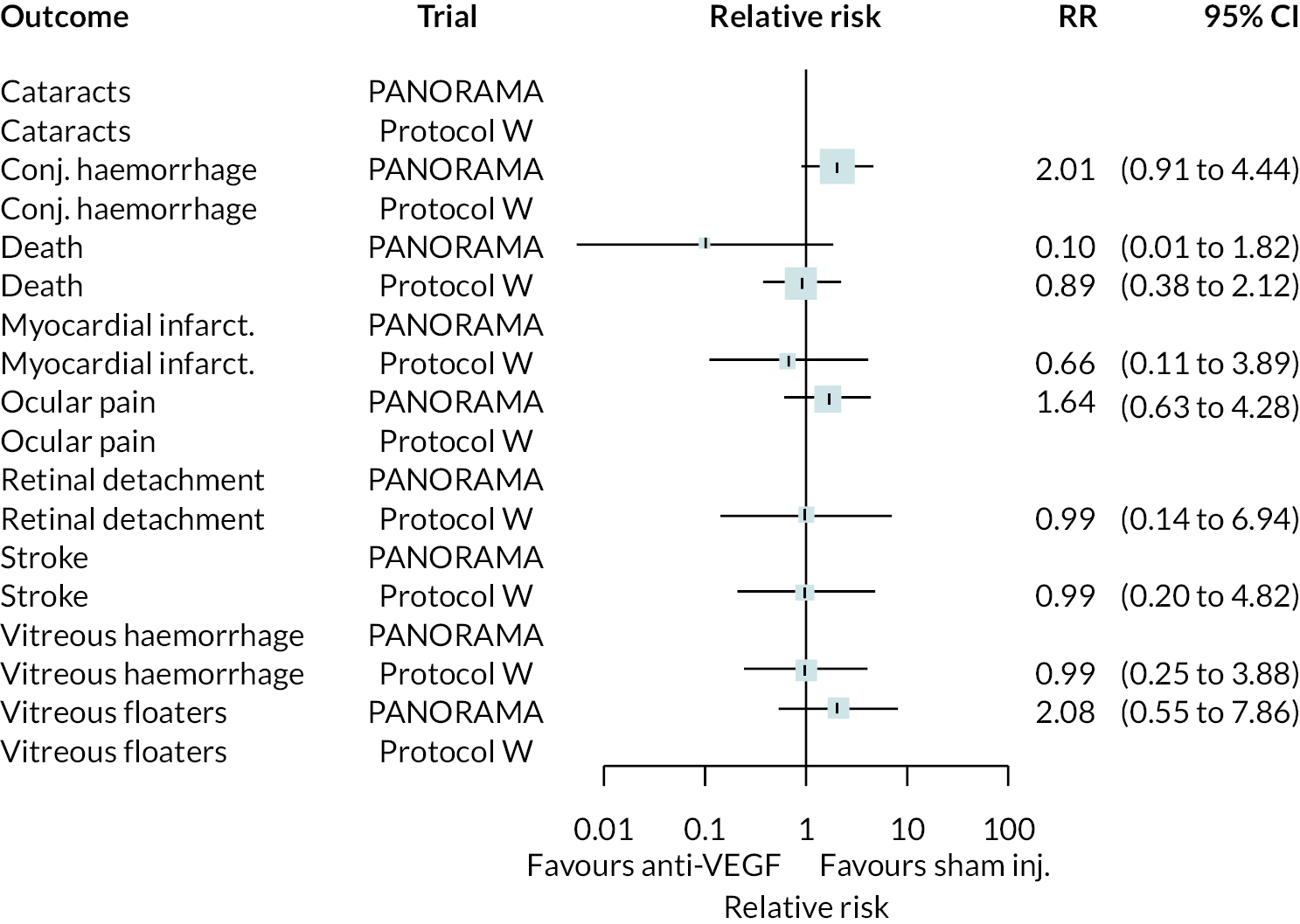
Non-proliferative retinopathy
Two trials compared aflibercept to sham injection in patients with non-proliferative retinopathy with a follow-up of 2 years (PANORAMA and Protocol W). Meta-analysis of their BCVA results found no clear evidence of any benefit of aflibercept over sham injection [mean difference (log-MAR) −0.02, 95% CI −0.05 to 0.01]. Progression to macular oedema was the only other outcome reported by both trials, with strong evidence to suggest that aflibercept reduces the risk of macular oedema [relative risk (RR) 0.283, 95% CI 0.18 to 0.44]. Protocol W reported that aflibercept reduced the rate of vitrectomy compared to sham injection (RR 0.38, 95% CI 0.24 to 0.60). Full results are presented in Appendix 4.
Protocol W found that aflibercept slowed the rate of progression to proliferative retinopathy when compared to sham injection (hazard ratio 0.40, 97.5% CI 0.28 to 0.57). PANORAMA found that more patients on aflibercept experienced a 2 point or more improvement in Diabetic Retinopathy Severity Scale (DRSS) (RR 4.41, 95% CI 2.81 to 6.94).
Discussion
This systematic review included 14 trials of anti-VEGFs used to treat diabetic retinopathy. For patients with PDR, the NMA found good, but not conclusive, evidence that anti-VEGF therapy is better at maintaining vision than PRP therapy, with a benefit of around −0.089 log-MAR (95% CI −0.179 to −0.019), or 3.6 ETDRS letters. This is within the region of variation that might be expected between eye tests without any intervention and is therefore unlikely to be clinically meaningful. 37 There was no compelling evidence to suggest that the three anti-VEGFs (aflibercept, ranibizumab and bevacizumab) differ in effectiveness; observed differences might be due to different trial populations or potential for bias. There was no conclusive evidence that combining anti-VEGF injection with PRP therapy is more effective at improving vision than anti-VEGF alone. Anti-VEGF appears to have no impact on BCVA in people with non-proliferative disease.
A further issue is the impact of time on the effectiveness of anti-VEGF therapy. Our meta-analysis found no evidence that the effectiveness waned over the first 2 years after initialising therapy. However, the one trial with a longer follow-up (Protocol S) found no benefit of ranibizumab over PRP after 5 years. 31 The longer-term value of anti-VEGF therapy therefore needs further investigation, particularly regarding how anti-VEGF treatment should be repeated over long time periods. There was some evidence that the benefit of anti-VEGF over PRP may be greater in people with poorer vision at time of injection. However, it was not possible to draw any firm conclusions on this from data presented in trial publications alone.
Data on outcomes other than visual acuity were limited, and not reported consistently across trials. Given the variations in follow-up and interventions used, NMAs were not feasible, and meta-analyses had to make the strong assumption of no difference in effect between the three anti-VEGFs, and no variation over time. Given these limitations, there was some evidence that anti-VEGFs are more effective than PRP at preventing the most serious consequences of diabetic retinopathy. They reduced the incidence of macular oedema (in both PDR and NPDR patients) and vitreous haemorrhage. In patients with NPDR, there was some evidence that aflibercept slows the rate of progression to PDR and improves retinopathy severity. This suggests that anti-VEGF may be valuable in preventing progression of diabetic retinopathy, even if its impact on vision directly is modest. Evidence on adverse events was limited due to inconsistent reporting, and small numbers of events. There was some evidence that anti-VEGF reduces the risk of retinal detachment.
Most trials were of short duration, with only one trial in PDR extending beyond 1 year. That trial found no vision benefit of anti-VEGF over PRP after 5 years, raising concerns as to the long-term efficacy of anti-VEGF therapy.
Patient and public perspectives
Patient representatives noted several key areas of continued concern. Most critically was that most trials of anti-VEGF used BCVA as their primary outcome, without any consideration of how that impacted on quality of life, ability to work, drive or care for family. The lack of long-term evidence also raised concerns because there is substantial uncertainty about how PDR will be managed and treated long term.
Conclusion
Anti-VEGF injection is only marginally better than PRP at maintaining vision and the benefit is unlikely to be clinically meaningful. There was no evidence of a difference in effectiveness between aflibercept, ranibizumab and bevacizumab, although data to compare these therapies were limited. There was no evidence to suggest that combining anti-VEGF with PRP improves effectiveness. Anti-VEGF may prevent, or delay, progression of macular oedema and vitreous haemorrhage. Some concern over bias in the trials remains.
The benefits of anti-VEGFs appear consistent for at least 2 years after initiation of treatment, but longer-term benefits are uncertain. There is some evidence that anti-VEGFs are less effective at maintaining visual acuity in people with less severe retinopathy, but this requires further investigation. Access to original individual-level trial data might aid in resolving this. Trials or observational studies of duration substantially longer than 1 year are needed to examine whether anti-VEGF may be beneficial in the long term, particularly with the requirement for long-term repeated anti-VEGF injections.
Additional information
CRediT contribution statement
Mark Simmonds (https://orcid.org/0000-0002-1999-8515): Conceptualisation (lead), Data curation (lead), Formal analysis (lead), Funding acquisition (lead), Investigation (lead), Methodology (lead), Project administration, Writing (lead).
Alexis Llewellyn (https://orcid.org/0000-0003-4569-5136): Conceptualisation, Data curation, Formal analysis, Funding acquisition, Investigation (co-lead), Methodology, Writing.
Ruth Walker (https://orcid.org/0000-0003-2765-7363): Conceptualisation, Data curation, Formal analysis, Funding acquisition, Investigation, Writing.
Helen Fulbright (https://orcid.org/0000-0002-1073-1099): Investigation, Methodology.
Matthew Walton (https://orcid.org/0000-0003-1932-3689): Conceptualisation, Funding acquisition, Writing.
Rob Hodgson (https://orcid.org/0000-0001-6962-2893): Conceptualisation, Funding acquisition, Writing.
Laura Bojke (https://orcid.org/0000-0001-7921-9109): Conceptualisation, Funding acquisition, Writing.
Lesley Stewart (https://orcid.org/0000-0003-0287-4724): Conceptualisation, Funding acquisition, Writing.
Sofia Dias (https://orcid.org/0000-0002-2172-0221): Conceptualisation, Funding acquisition, Methodology, Writing.
Thomas Rush: Conceptualisation, Funding acquisition, Writing (patient and public involvement advisor).
John G Lawrenson: Conceptualisation, Funding acquisition, Writing.
Tunde Peto (https://orcid.org/0000-0001-6265-0381): Conceptualisation, Funding acquisition, Writing.
David Steel (https://orcid.org/0000-0001-8734-3089): Conceptualisation, Funding acquisition, Writing.
Acknowledgements
We acknowledge the help and advice given by all persons involved in the NICE diabetic retinopathy guidance development process.
Data-sharing statement
Data and code to reproduce the meta-analyses are available on GitHub (https://github.com/marksimmondsyork/AVID). For all other data requests please contact the corresponding author.
Ethics statement
As this was a systematic review of existing published data, no ethics approval was required.
Information governance statement
All data used in this paper were taken from published sources: no personal data were included.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/PCGV5709.
Primary conflicts of interest: Laura Bojke declares that she was on the HS&DR Researcher-Led awards panel (December 2019–December 2022). All other authors have no conflicts of interest to declare.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the Health Technology Assessment programme or the Department of Health and Social Care.
This article was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Study registration
This study is registered as PROSPERO (CRD42021272642).
Funding
This article presents independent research funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme as award number NIHR132948.
This article reports on one component of the research award Anti-VEGF drugs compared with laser photocoagulation for the treatment of diabetic retinopathy: a systematic review and economic analysis. Other articles published as part of this thread are: [LINKS to other articles]. For more information about this research please view the award page [https://fundingawards.nihr.ac.uk/award/NIHR132948]
About this article
The contractual start date for this research was in August 2021. This article began editorial review in November 2023 and was accepted for publication in August 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Copyright
Copyright © 2024 Simmonds et al. This work was produced by Simmonds et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
List of abbreviations
- anti-VEGF
- anti-vascular endothelial growth factor
- BCVA
- best corrected visual acuity
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- DMO
- diabetic macular oedema
- DRSS
- Diabetic Retinopathy Severity Scale
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- ICTRP
- International Clinical Trials Registry Platform
- IPD
- individual patient data
- log-MAR
- logarithm of the minimum angle of resolution
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NVD
- neovascularisation of the disc
- NVE
- neovascularisation elsewhere
- PDR
- proliferative diabetic retinopathy
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRP
- panretinal photocoagulation
- RCT
- randomised controlled trial
- WHO
- World Health Organization
References
- Mathur R, Bhaskaran K, Edwards E, Lee H, Chaturvedi N, Smeeth L, et al. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: a cohort study in the Clinical Practice Research Datalink 2004–2014. BMJ Open 2017;7.
- Ghanchi F. Diabetic Retinopathy Guidelines Working Group . The Royal College of Ophthalmologists’ clinical guidelines for diabetic retinopathy: a summary. Eye (Lond) 2013;27:285-7.
- Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008;115:1859-68.
- Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Vision Loss Expert Group of the Global Burden of Disease Study . Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health 2017;5:e1221-34. https://doi.org/10.1016/s2214-109x(17)30393-5.
- The Diabetic Retinopathy Study Research Group . Four risk factors for severe visual loss in diabetic retinopathy. The third report from the Diabetic Retinopathy Study. Arch Ophthalmol 1979;97:654-5.
- Parikh R, Shah RJ, VanHouten JP, Cherney EF. Ocular findings at initial pan retinal photocoagulation for proliferative diabetic retinopathy predict the need for future pars plana vitrectomy. Retina 2014;34:1997-2002.
- Early Treatment Diabetic Retinopathy Study Research Group . Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98:823-33.
- Royle P, Mistry H, Auguste P, Shyangdan D, Freeman K, Lois N, et al. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: systematic review and economic evaluation. Health Technol Assess 2015;19:v-xxviii.
- National Institute for Health and Care Excellence . Aflibercept for Treating Diabetic Macular Oedema 2015. www.nice.org.uk/guidance/ta346/resources/aflibercept-for-treating-diabetic-macular-oedema-pdf-82602611201221 (accessed 5 July 2023).
- National Institute for Health and Care Excellence . Ranibizumab for Treating Diabetic Macular Oedema 2013. www.nice.org.uk/guidance/ta274/resources/ranibizumab-for-treating-diabetic-macular-oedema-pdf-82600612458181 (accessed 5 July 2023).
- Wubben TJ, Johnson MW, Sohn EH, Peairs JJ, Kay CN, Kim SJ, et al. Anti-vascular endothelial growth factor therapy for diabetic retinopathy: consequences of inadvertent treatment interruptions. Am J Ophthalmol 2019;204:13-8.
- Obeid A, Su D, Patel SN, Uhr JH, Borkar D, Gao X, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology 2019;126:407-13.
- Royal National Institute of Blind People (RNIB) . Anti-VEGF Treatment n.d. www.rnib.org.uk/eye-health/eye-conditions/anti-vegf-treatment (accessed 5 July 2023).
- Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018;125:1608-22.
- National Institute for Health and Care Excellence . Diabetic Retinopathy (Guidance in Development) 2023. www.nice.org.uk/guidance/indevelopment/gid-ng10256 (accessed 3 October 2023).
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care n.d. www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed 17 September 2020).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64.
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- Phillippo DM, Dias S, Ades AE, Belger M, Brnabic A, Schacht A, et al. Multilevel network meta-regression for population-adjusted treatment comparisons. J R Stat Soc Ser A Stat Soc 2020;183:1189-210.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641-56.
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses. Ann Intern Med 2019;170:538-46.
- Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. CLARITY Study Group . Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017;389:2193-203.
- Maturi RK, Glassman AR, Josic K, Antoszyk AN, Blodi BA, Jampol LM, et al. DRCR Retina Network . Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol 2021;139:701-12.
- Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol 2021;139.
- Marashi A, Abukhalaf I, Alfaraji R, Shuman Y, Salahia A. Panretinal photocoagulation versus intravitreal bevacizumab for proliferative diabetic retinopathy treatment. Adv Ophthalmol Vis Syst 2017;7. https://doi.org/10.15406/aovs.2017.07.00211.
- Ahmad M, Jan S. Comparison between panretinal photocoagulation and panretinal photocoagulation plus intravitreal bevacizumab in proliferative diabetic retinopathy. J Ayub Med Coll Abbottabad 2012;24:10-3.
- Ali W, Abbasi KZ, Raza A. Panretinal photocoagulation plus intravitreal bevacizumab versus panretinal photocoagulation alone for proliferative diabetic retinopathy. J Coll Physicians Surg Pak 2018;28:923-7.
- Rebecca MR, Shaikh FF, Jatoi SM. Comparison of efficacy of combination therapy of an intravitreal injection of bevacizumab and photocoagulation versus pan retinal photocoagulation alone in high risk proliferative diabetic retinopathy. Pak J Med Sci 2021;37:157-61.
- Roohipoor R, Sharifian E, Ghassemi F, Riazi-Esfahani M, Karkhaneh R, Fard MA, et al. Choroidal thickness changes in proliferative diabetic retinopathy treated with panretinal photocoagulation versus panretinal photocoagulation with intravitreal bevacizumab. Retina 2016;36:1997-2005.
- Gross JG, Glassman AR, Jampol LM. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial (vol,314 pg 2137, 2015). JAMA 2019;321.
- Ferraz DA, Vasquez LM, Preti RC, Motta A, Sophie R, Bittencourt MG, et al. A randomized controlled trial of panretinal photocoagulation with and without intravitreal ranibizumab in treatment-naive eyes with non-high-risk proliferative diabetic retinopathy. Retina 2015;35:280-7.
- Lang GE, Stahl A, Voegeler J, Quiering C, Lorenz K, Spital G, et al. Efficacy and safety of ranibizumab with or without panretinal laser photocoagulation versus laser photocoagulation alone in proliferative diabetic retinopathy – the PRIDE study. Acta Ophthalmol 2020;98:e530-9.
- Figueira J, Fletcher E, Massin P, Silva R, Bandello F, Midena E, et al. EVICR.net Study Group . Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS study). Ophthalmology 2018;125:691-700.
- Filho JA, Messias A, Almeida FP, Ribeiro JA, Costa RA, Scott IU, et al. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol 2011;89:e567-72.
- Messias K, Barroso RD, Jorge R, Messias A. Retinal function in eyes with proliferative diabetic retinopathy treated with intravitreal ranibizumab and multispot laser panretinal photocoagulation. Doc Ophthalmol 2018;137:121-9.
- Rosser DA, Cousens SN, Murdoch IE, Fitzke FW, Laidlaw DA. How sensitive to clinical change are ETDRS logMAR visual acuity measurements?. Invest Ophthalmol Vis Sci 2003;44:3278-81. https://doi.org/10.1167/iovs.02-1100.
Appendix 1 Systematic review processes
Database search strategies
The aim of the literature search was to identify RCTs on anti-VEGFs, angiogenesis inhibitors and other specific drugs used for the treatment of diabetic retinopathy.
An Information Specialist (HF) designed a preliminary search strategy in Ovid MEDLINE in consultation with the research team. The strategy consisted of terms for the condition (diabetic retinopathy), which were combined with terms for the intervention (anti-VEGF, angiogenesis inhibitors, or specific drugs used for the treatment of diabetic retinopathy) using the Boolean operator AND. Text word searches for terms appearing in the title and abstracts of database records were included in the strategy alongside searches of relevant subject headings. A RCT study filter was applied using the Boolean operator AND. No date or language limits were applied. The final MEDLINE strategy was adapted for use in all resources searched.
The searches were performed on 27 August 2021. The following databases were searched: Ovid MEDLINE(R) ALL, EMBASE (Ovid), Science Citation Index Expanded (Web of Science), Conference Proceedings Citation Index Science (Web of Science), Cochrane CENTRAL (Wiley), Cochrane Database of Systematic Reviews (Wiley), DARE (CRD), PROSPERO (CRD) and Epistemonikos. The following trial registries were searched: WHO ICTRP, ClinicalTrials.gov, and the EU Clinical Trials Registry.
Search results were imported into EndNote 20 and deduplicated. All search strategies are presented in full below.
The searches were updated on 13 July 2022 and again on 26 May 2023 using all the databases and strategies as used previously, except for DARE as this database is no longer updated. For each update search, the results of the databases were deduplicated against each other in a separate EndNote 20 Library before being merged with the results of the original EndNote Library and deduplicated for a second time.
Ovid MEDLINE(R) ALL
(Includes Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE)
via Ovid http://ovidsp.ovid.com/
Date range searched: <1946–25 May 2023>
Date searched: 26 May 2023
Records retrieved: 3172
The MEDLINE strategy below includes a search filter to limit retrieval to RCTs using the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity-maximising version (2008 revision); Ovid format.
Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Technical Supplement to Chapter 4: Searching for and Selecting Studies. In Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook.
-
(*Diabetes Mellitus/ or *Diabetes Complications/) and exp *Retinal Diseases/ (3199)
-
Diabetic Retinopathy/ (29304)
-
((diabet* or DM) adj3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath* or maculopath*)).ti,ab,kw. (30685)
-
(((proliferat* or PDR or pre-proliferat* or preproliferat* or non-proliferat* or nonproliferat* or NPDR or background) adj3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath*)) and (diabet* or DM)).ti,ab,kw. (7895)
-
(new blood vessel* and diabet*).ti,ab,kw. (273)
-
(((retin* or subretina* or sub-retina* or interretina* or inter-retina* or vitreoretin* or vitreo-retin* or chorioretin* or chorio-retin* or choroid* or macula* or intraocular or intra-ocular or intravitreal or intra-vitreal) adj4 (damage* or deteriorat* or degnerat* or disease* or edema or oedema or neovasculari?ation*)) and diabet*).ti,ab,kw. (13654)
-
((retinal vein* adj3 (occlu* or obstruct* or clos* or stricture* or steno* or block* or emboli*)) and diabet*).ti,ab,kw. (1473)
-
or/1‐7 (44519)
-
exp Vascular Endothelial Growth Factors/ai (9366)
-
exp Receptors, Vascular Endothelial Growth Factor/ai (3393)
-
(anti adj2 VEGF*).ti,ab,kw. (9210)
-
(anti-VEGF* or antiVEGF*).ti,ab,kw. (9455)
-
((anti vascular or anti-vascular or antivascular) adj2 endothelial growth factor*).ti,ab,kw. (5745)
-
(((vascular endothelial adj2 growth factor*) or vasculotropin or VEGF* or vascular permeability factor* or VPF) adj2 (trap* or inhibit* or antagonist*)).ti,ab,kw. (11005)
-
(vascular proliferation adj4 inhibit*).ti,ab,kw. (38)
-
or/9‐15 (28125)
-
Angiogenesis Inhibitors/ (28876)
-
exp Angiogenesis Inducing Agents/ai (118)
-
(angiogen* adj2 (antagonist* or inhibit*)).ti,ab,kw. (14831)
-
((antiangiogen* or anti angiogen* or anti-angiogen*) adj2 (agent* or drug* or effect*)).ti,ab,kw. (10949)
-
(angiostatic adj2 (agent* or drug*)).ti,ab,kw. (103)
-
((neovasculari?ation or vasculari?ation) adj2 inhibit*).ti,ab,kw. (1243)
-
or/17‐22 (45139)
-
Aflibercept*.ti,ab,kw,rn. (3315)
-
(Eylea or Zaltrap or Ziv-Aflibercept or “AVE 0005” or AVE0005 or “AVE 005” or AVE005).ti,ab,kw. (316)
-
Bevacizumab/ (14139)
-
Bevacizumab*.ti,ab,kw,rn. (22533)
-
(Avastin or Mvasi or Alymsys or Aybintio or Equidacent or Onbevzi or Oyavas or Zirabev or rhuMAbVEGF or rhuMAb-VEGF or rhuMAb VEGF or “NSC 704865” or NSC704865).ti,ab,kw. (1675)
-
(IVB adj2 inject*).ti,ab,kw. (316)
-
Ranibizumab/ (4684)
-
Ranibizumab*.ti,ab,kw,rn. (6307)
-
(Lucentis or “rhuFab V2”).ti,ab,kw. (456)
-
(IVR adj2 inject*).ti,ab,kw. (139)
-
Pegaptanib*.ti,ab,kw,rn. (671)
-
(“EYE 001” or EYE001 or Macugen or “NX 1838” or NX1838).ti,ab,kw. (140)
-
or/24‐35 (28353)
-
8 and (16 or 23 or 36) (4979)
-
randomized controlled trial.pt. (593242)
-
controlled clinical trial.pt. (95314)
-
randomized.ab. (604126)
-
placebo.ab. (238387)
-
drug therapy.fs. (2592996)
-
randomly.ab. (408822)
-
trial.ab. (649200)
-
groups.ab. (2520111)
-
or/38‐45 (5663345)
-
37 and 46 (3308)
-
exp animals/ not humans.sh. (5123796)
-
47 not 48 (3190)
-
limit 49 to yr=“2000-Current” (3182)
-
remove duplicates from 50 (3172)
Key:
/ or.sh. = indexing term (Medical Subject Heading: MeSH)
/ai = indexing term with subheading for antagonists & inhibitors
exp = exploded indexing term (MeSH)
* or $ = truncation
? = adds up to 1 additional character
ti,ab,kw = terms in either title, abstract or keyword fields
rn = registry number/name of substance
adj3 = terms within three words of each other (any order).
pt = publication type
fs = floating sub-heading
EMBASE
via Ovid http://ovidsp.ovid.com/
Date range searched: <1974–25 May 2023>
Date searched: 26 May 2023
Records retrieved: 2558
The EMBASE strategy below includes the Cochrane EMBASE RCT filter (Ovid format).
Glanville J, Foxlee R, Wisniewski S, Noel-Storr A, Edwards M, Dooley G. Translating the Cochrane EMBASE RCT filter from the Ovid interface to EMBASE.com: a case study. Health Info Libr J. 2019. doi:10.1111/hir.12269
-
*diabetes mellitus/ and exp *retina disease/ (4826)
-
exp diabetic retinopathy/ (53891)
-
((diabet* or DM) adj3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath* or maculopath*)).ti,ab,kw. (43573)
-
(((proliferat* or PDR or pre-proliferat* or preproliferat* or non-proliferat* or nonproliferat* or NPDR or background) adj3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath*)) and (diabet* or DM)).ti,ab,kw. (11148)
-
(new blood vessel* and diabet*).ti,ab,kw. (391)
-
(((retin* or subretina* or sub-retina* or interretina* or inter-retina* or vitreoretin* or vitreo-retin* or chorioretin* or chorio-retin* or choroid* or macula* or intraocular or intra-ocular or intravitreal or intra-vitreal) adj4 (damage* or deteriorat* or degnerat* or disease* or edema or oedema or neovasculari?ation*)) and diabet*).ti,ab,kw. (20734)
-
((retinal vein* adj3 (occlu* or obstruct* or clos* or stricture* or steno* or block* or emboli*)) and diabet*).ti,ab,kw. (2199)
-
or/1‐7 (70501)
-
vasculotropin inhibitor/ (7663)
-
(anti adj2 VEGF*).ti,ab,kw. (15751)
-
(anti-VEGF* or antiVEGF*).ti,ab,kw. (16291)
-
((anti vascular or anti-vascular or antivascular) adj2 endothelial growth factor*).ti,ab,kw. (7400)
-
(((vascular endothelial adj2 growth factor*) or vasculotropin or VEGF* or vascular permeability factor* or VPF) adj2 (trap* or inhibit* or antagonist*)).ti,ab,kw. (17346)
-
(vascular proliferation adj4 inhibit*).ti,ab,kw. (50)
-
or/9‐14 (38838)
-
angiogenesis inhibitor/ (20415)
-
(angiogen* adj2 (antagonist* or inhibit*)).ti,ab,kw. (20444)
-
((antiangiogen* or anti angiogen* or anti-angiogen*) adj2 (agent* or drug* or effect*)).ti,ab,kw. (15734)
-
(angiostatic adj2 (agent* or drug*)).ti,ab,kw. (125)
-
((neovasculari?ation or vasculari?ation) adj2 inhibit*).ti,ab,kw. (1718)
-
or/16‐20 (45260)
-
aflibercept/ (8877)
-
Aflibercept*.ti,ab,kw,dy,tn. (9141)
-
(Eylea or Zaltrap or Ziv-Aflibercept or “AVE 0005” or AVE0005 or “AVE 005” or AVE005).ti,ab,dy,tn. (1741)
-
bevacizumab/ (72890)
-
Bevacizumab*.ti,ab,kw,dy,tn. (75152)
-
(Avastin or Mvasi or Alymsys or Aybintio or Equidacent or Onbevzi or Oyavas or Zirabev or rhuMAbVEGF or rhuMAb-VEGF or rhuMAb VEGF or “NSC 704865” or NSC704865).ti,ab,kw,dy,tn. (11007)
-
(IVB adj2 inject*).ti,ab,kw. (395)
-
ranibizumab/ (12442)
-
Ranibizumab*.ti,ab,kw,dy,tn. (12826)
-
(Lucentis or “rhuFab V2”).ti,ab,kw,dy,tn. (3216)
-
(IVR adj2 inject*).ti,ab,kw. (197)
-
pegaptanib.dy,tn. (2470)
-
Pegaptanib*.ti,ab,kw,dy,tn. (2544)
-
(“EYE 001” or EYE001 or Macugen or “NX 1838” or NX1838).ti,ab,kw,dy,tn. (1266)
-
or/22‐35 (85594)
-
8 and (15 or 21 or 36) (8778)
-
randomized controlled trial/ (785964)
-
controlled clinical trial/ (469252)
-
Random$.ti,ab,ot. (1968994)
-
randomization/ (99178)
-
intermethod comparison/ (297283)
-
placebo.ti,ab,ot. (366311)
-
(compare or compared or comparison).ti,ot. (604093)
-
((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2766233)
-
(open adj label).ti,ab,ot. (109016)
-
((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,ot. (274477)
-
double blind procedure/ (210575)
-
parallel group$1.ti,ab,ot. (32223)
-
(crossover or cross over).ti,ab,ot. (124540)
-
((assign$ or match or matched or allocation) adj5 (alternate or group or groups or intervention or interventions or patient or patients or subject or subjects or participant or participants)).ti,ab,ot. (415063)
-
(assigned or allocated).ti,ab,ot. (489023)
-
(controlled adj7 (study or design or trial)).ti,ab,ot. (450984)
-
(volunteer or volunteers).ti,ab,ot. (282270)
-
human experiment/ (650911)
-
trial.ti,ot. (403295)
-
or/38‐56 (6311902)
-
37 and 57 (2810)
-
(rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$).ti,ot. and animal experiment/ (1227092)
-
animal experiment/ not (human experiment/ or human/) (2577203)
-
59 or 60 (2645661)
-
58 not 61 (2689)
-
limit 62 to yr=“2000-Current” (2686)
-
remove duplicates from 63 (2558)
Key:
/ or.sh. = indexing term (Emtree Subject Heading)
exp = exploded indexing term (Emtree)
* or $ = truncation
? = adds up to 1 additional character
ti,ab,kw = terms in either title or abstract fields
dy,tn = drug index terms word or drug trade name fields
adj3 = terms within three words of each other (any order).
pt = publication type
ot = original title
Cochrane Central Register of Controlled Trials
via Wiley http://onlinelibrary.wiley.com/
Date range searched: Issue 5 of 12, May 2023
Date searched: 26 May 2023
Records retrieved: 1825
-
([mh ^“Diabetes Mellitus”] or [mh ^“Diabetes Complications”]) and [mh “Retinal Diseases”] 250
-
[mh ^“Diabetic Retinopathy”] 1934
-
((diabet* or DM) NEAR/3 (retinopath* or vitreoretinopath* or chorioretinopath* or maculopath*)):ti,ab,kw 4547
-
(((proliferat* or PDR or preproliferat* or nonproliferat* or NPDR or background) NEAR/3 (retinopath* or vitreoretinopath* or chorioretinopath*)) and (diabet* or DM)):ti,ab,kw 1326
-
(“new blood” NEXT vessel* and diabet*):ti,ab,kw 32
-
(((retin* or subretina* or interretina* or vitreoretin* or chorioretin* or choroid* or macula* or intraocular or intravitreal) NEAR/4 (damage* or deteriorat* or degnerat* or disease* or edema or oedema or neovasculari?ation*)) and diabet*):ti,ab,kw 3457
-
((retinal NEXT vein* NEAR/3 (occlu* or obstruct* or clos* or stricture* or steno* or block* or emboli*)) and diabet*):ti,ab,kw 254
-
{OR #1-#7} 5751
-
[mh “Vascular Endothelial Growth Factors”/ai] 758
-
[mh “Receptors, Vascular Endothelial Growth Factor”/ai] 154
-
(anti NEAR/2 VEGF*):ti,ab,kw 1610
-
(antiVEGF*):ti,ab,kw 1523
-
((anti NEXT vascular or antivascular) NEAR/2 “endothelial growth” NEXT factor*):ti,ab,kw 699
-
(((“vascular endothelial” NEAR/2 growth NEXT factor*) or vasculotropin or VEGF* or “vascular permeability” NEXT factor* or VPF) NEAR/2 (trap* or inhibit* or antagonist*)):ti,ab,kw 2048
-
(“vascular proliferation” NEAR/4 inhibit*):ti,ab,kw 1
-
{OR #9-#15} 3671
-
[mh ^“Angiogenesis Inhibitors”] 1681
-
[mh “Angiogenesis Inducing Agents”/ai] 0
-
(angiogen* NEAR/2 (antagonist* or inhibit*)):ti,ab,kw 2126
-
((antiangiogen* or anti NEXT angiogen*) NEAR/2 (agent* or drug* or effect*)):ti,ab,kw 717
-
(angiostatic NEAR/2 (agent* or drug*)):ti,ab,kw 10
-
((neovasculari?ation or vasculari?ation) NEAR/2 inhibit*):ti,ab,kw 37
-
{OR #17-#22}2691
-
Aflibercept*:ti,ab,kw 1081
-
(Eylea or Zaltrap or Ziv NEXT Aflibercept or “AVE 0005” or AVE0005 or “AVE 005” or AVE005):ti,ab,kw 252
-
[mh ^Bevacizumab] 2633
-
Bevacizumab*:ti,ab,kw 7386
-
(Avastin or Mvasi or Alymsys or Aybintio or Equidacent or Onbevzi or Oyavas or Zirabev or rhuMAbVEGF or rhuMAb NEXT VEGF or “NSC 704865” or NSC704865):ti,ab,kw 941
-
(IVB NEAR/2 inject*):ti,ab,kw 89
-
[mh ^Ranibizumab] 1049
-
Ranibizumab*:ti,ab,kw 2266
-
(Lucentis or “rhuFab V2”):ti,ab,kw 451
-
(IVR NEAR/2 inject*):ti,ab,kw 32
-
Pegaptanib*:ti,ab,kw 166
-
(“EYE 001” or EYE001 or Macugen or “NX 1838” or NX1838):ti,ab,kw 82
-
{OR #24-#35}10087
-
#8 and (#16 or #23 or #36) 1847
-
(rat or rats or rodent* or mouse or mice or “mus musculus” or “mus domesticus” or murine or murinae or bovine or sheep or ovine or “ovis aries” or porcine):ti,ab,kw 17188
-
#37 not #38 with Publication Year from 2000 to 2023, in Trials 1825
Science Citation Index Expanded
via Web of Science, Clarivate Analytics https://clarivate.com/
Date range searched: 1900–26 May 2023
Date searched: 26 May 2023
Records retrieved: 2394
-
32 #29 NOT #30 2,394 Limited by 2000-01-01 to 2023-05-26
-
31 #29 NOT #30 2,410
-
30 TI=(animal or animals or rat or rats or rodent* or mouse or mice or “mus musculus” or “mus domesticus” or murine or murinae or porcine or pig or pigs or piglet or piglets or sow or sows or minipig or minipigs or sheep or ovine or “ovis aries” or lamb or lambs or ewe or ewes or rabbit or rabbits or leporide or leporidae or kitten or kittens or dog or dogs or puppy or puppies or monkey or monkeys or horse or horses or foal or foals or equine or bovine or calf or calves or cattle or heifer or heifers or hamster or hamsters or chicken or chickens or livestock or alpaca* or llama*) 3,259,653
-
29 #27 AND #28 2,524
-
28 TS=(random* or control* or trial* or “single blind” or “double blind” or “triple blind” or placebo)8,083,064
-
27 #6 AND #26 6,121
-
26 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 83,065
-
25 TS=(“EYE 001” or EYE001 or Macugen or “NX 1838” or NX1838) 142
-
24 TS=(Pegaptanib*) 716
-
23 TS=(IVR NEAR/2 inject*) 177
-
22 TS=(Lucentis or “rhuFab V2”) 564
-
21 TS=(Ranibizumab*) 9,347
-
20 TS=(IVB NEAR/2 inject*) 307
-
19 TS=(Avastin or Mvasi or Alymsys or Aybintio or Equidacent or Onbevzi or Oyavas or Zirabev or rhuMAbVEGF or rhuMAb-VEGF or “rhuMAb VEGF” or “NSC 704865” or NSC704865) 3,355
-
18 TS=(Bevacizumab*) 36,279
-
17 TS=(Eylea or Zaltrap or Ziv-Aflibercept or “AVE 0005” or AVE0005 or “AVE 005” or AVE005) 320
-
16 TS=(Aflibercept*) 4,076
-
15 TS=((neovascularisation or neovascularization or vascularisation or vascularization) NEAR/2 inhibit*) 1,858
-
14 TS=(angiostatic NEAR/2 (agent* or drug*)) 105
-
13 TS=((antiangiogen* or “anti angiogen*” or anti-angiogen*) NEAR/2 (agent* or drug* or effect*)) 11,802
-
12 TS=(angiogen* NEAR/2 (antagonist* or inhibit*)) 19,846
-
11 TS=(“vascular proliferation” NEAR/4 inhibit*) 44
-
10 TS=(((“vascular endothelial” NEAR/2 “growth factor*”) or vasculotropin or VEGF* or “vascular permeability factor*” or VPF) NEAR/2 (trap* or inhibit* or antagonist*)) 14,540
-
9 TS=((“anti vascular” or anti-vascular or antivascular) NEAR/2 “endothelial growth factor*”) 5,018
-
8 TS=(anti-VEGF* or antiVEGF*) 10,111
-
7 TS=(anti NEAR/2 VEGF*) 10,549
-
6 #1 OR #2 OR #3 OR #4 OR #5 43,073
-
5 TS=((“retinal vein*” NEAR/3 (occlu* or obstruct* or clos* or stricture* or steno* or block* or emboli*)) and diabet*) 1,546
-
4 TS=(((retin* or subretina* or sub-retina* or interretina* or inter-retina* or vitreoretin* or vitreo-retin* or chorioretin* or chorio-retin* or choroid* or macula* or intraocular or intra-ocular or intravitreal or intra-vitreal) NEAR/4 (damage* or deteriorat* or degnerat* or disease* or edema or oedema or neovasculari?ation*)) and diabet*) 16,980
-
3 TS=(“new blood vessel*” and diabet*) 288
-
2 TS=(((proliferat* or PDR or pre-proliferat* or preproliferat* or non-proliferat* or nonproliferat* or NPDR or background) NEAR/3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath*)) and (diabet* or DM)) 7,763
-
1 TS=((diabet* or DM) NEAR/3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath* or maculopath*)) 36,053
Key:
TS= terms in either title, abstract, author keywords, and keywords plus fields
TI= search in title field
NEAR/3 = terms within three words of each other (any order).
* = truncation
Conference Proceedings Citation Index – Science
via Web of Science, Clarivate Analytics https://clarivate.com/
Date range searched: 1990–26 May 2023
Date searched: 26 May 2023
Records retrieved: 86
-
32 #29 NOT #30 86 Limited by 2000-01-01 to 2023-05-26
-
31 #29 NOT #30 89
-
30 TI=(animal or animals or rat or rats or rodent* or mouse or mice or “mus musculus” or “mus domesticus” or murine or murinae or porcine or pig or pigs or piglet or piglets or sow or sows or minipig or minipigs or sheep or ovine or “ovis aries” or lamb or lambs or ewe or ewes or rabbit or rabbits or leporide or leporidae or kitten or kittens or dog or dogs or puppy or puppies or monkey or monkeys or horse or horses or foal or foals or equine or bovine or calf or calves or cattle or heifer or heifers or hamster or hamsters or chicken or chickens or livestock or alpaca* or llama*) 295,290
-
29 #27 AND #28 92
-
28 TS=(random* or control* or trial* or “single blind” or “double blind” or “triple blind” or placebo) 1,616,551
-
27 #6 AND #26 458
-
26 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 8,998
-
25 TS=(“EYE 001” or EYE001 or Macugen or “NX 1838” or NX1838) 14
-
24 TS=(Pegaptanib*) 39
-
23 TS=(IVR NEAR/2 inject*) 1
-
22 TS=(Lucentis or “rhuFab V2”) 29
-
21 TS=(Ranibizumab*) 564
-
20 TS=(IVB NEAR/2 inject*) 7
-
19 TS=(Avastin or Mvasi or Alymsys or Aybintio or Equidacent or Onbevzi or Oyavas or Zirabev or rhuMAbVEGF or rhuMAb-VEGF or “rhuMAb VEGF” or “NSC 704865” or NSC704865) 196
-
18 TS=(Bevacizumab*) 4,659
-
17 TS=(Eylea or Zaltrap or Ziv-Aflibercept or “AVE 0005” or AVE0005 or “AVE 005” or AVE005) 60
-
16 TS=(Aflibercept*) 577
-
15 TS=((neovascularisation or neovascularization or vascularisation or vascularization) NEAR/2 inhibit*) 177
-
14 TS=(angiostatic NEAR/2 (agent* or drug*)) 6
-
13 TS=((antiangiogen* or “anti angiogen*” or anti-angiogen*) NEAR/2 (agent* or drug* or effect*)) 634
-
12 TS=(angiogen* NEAR/2 (antagonist* or inhibit*)) 1,209
-
11 TS=(“vascular proliferation” NEAR/4 inhibit*) 6
-
10 TS=(((“vascular endothelial” NEAR/2 “growth factor*”) or vasculotropin or VEGF* or “vascular permeability factor*” or VPF) NEAR/2 (trap* or inhibit* or antagonist*)) 1,025
-
9 TS=((“anti vascular” or anti-vascular or antivascular) NEAR/2 “endothelial growth factor*”) 224
-
8 TS=(anti-VEGF* or antiVEGF*) 836
-
7 TS=(anti NEAR/2 VEGF*) 869
-
6 #1 OR #2 OR #3 OR #4 OR #5 5,826
-
5 TS=((“retinal vein*” NEAR/3 (occlu* or obstruct* or clos* or stricture* or steno* or block* or emboli*)) and diabet*) 74
-
4 TS=(((retin* or subretina* or sub-retina* or interretina* or inter-retina* or vitreoretin* or vitreo-retin* or chorioretin* or chorio-retin* or choroid* or macula* or intraocular or intra-ocular or intravitreal or intra-vitreal) NEAR/4 (damage* or deteriorat* or degnerat* or disease* or edema or oedema or neovasculari?ation*)) and diabet*) 2,140
-
3 TS=(“new blood vessel*” and diabet*) 29
-
2 TS=(((proliferat* or PDR or pre-proliferat* or preproliferat* or non-proliferat* or nonproliferat* or NPDR or background) NEAR/3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath*)) and (diabet* or DM)) 642
-
1 TS=((diabet* or DM) NEAR/3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath* or maculopath*)) 4,723
Key:
TS= terms in either title, abstract, author keywords, and keywords plus fields
TI= search in title field
NEAR/3 = terms within three words of each other (any order).
* = truncation
Cochrane Database of Systematic Reviews
via Wiley http://onlinelibrary.wiley.com/
Date range searched: Issue 5 of 12, May 2023
Date searched: 26 May 2023
Records retrieved: 14
-
([mh ^“Diabetes Mellitus”] or [mh ^“Diabetes Complications”]) and [mh “Retinal Diseases”] 250
-
[mh ^“Diabetic Retinopathy”] 1934
-
((diabet* or DM) NEAR/3 (retinopath* or vitreoretinopath* or chorioretinopath* or maculopath*)):ti,ab,kw 4547
-
(((proliferat* or PDR or preproliferat* or nonproliferat* or NPDR or background) NEAR/3 (retinopath* or vitreoretinopath* or chorioretinopath*)) and (diabet* or DM)):ti,ab,kw 1326
-
(“new blood” NEXT vessel* and diabet*):ti,ab,kw 32
-
(((retin* or subretina* or interretina* or vitreoretin* or chorioretin* or choroid* or macula* or intraocular or intravitreal) NEAR/4 (damage* or deteriorat* or degnerat* or disease* or edema or oedema or neovasculari?ation*)) and diabet*):ti,ab,kw 3457
-
((retinal NEXT vein* NEAR/3 (occlu* or obstruct* or clos* or stricture* or steno* or block* or emboli*)) and diabet*):ti,ab,kw 254
-
{OR #1-#7} 5751
-
[mh “Vascular Endothelial Growth Factors”/ai] 758
-
[mh “Receptors, Vascular Endothelial Growth Factor”/ai] 154
-
(anti NEAR/2 VEGF*):ti,ab,kw 1610
-
(antiVEGF*):ti,ab,kw 1523
-
((anti NEXT vascular or antivascular) NEAR/2 “endothelial growth” NEXT factor*):ti,ab,kw 699
-
(((“vascular endothelial” NEAR/2 growth NEXT factor*) or vasculotropin or VEGF* or “vascular permeability” NEXT factor* or VPF) NEAR/2 (trap* or inhibit* or antagonist*)):ti,ab,kw 2048
-
(“vascular proliferation” NEAR/4 inhibit*):ti,ab,kw 1
-
{OR #9-#15} 3671
-
[mh ^“Angiogenesis Inhibitors”] 1681
-
[mh “Angiogenesis Inducing Agents”/ai] 0
-
(angiogen* NEAR/2 (antagonist* or inhibit*)):ti,ab,kw 2126
-
((antiangiogen* or anti NEXT angiogen*) NEAR/2 (agent* or drug* or effect*)):ti,ab,kw 717
-
(angiostatic NEAR/2 (agent* or drug*)):ti,ab,kw 10
-
((neovasculari?ation or vasculari?ation) NEAR/2 inhibit*):ti,ab,kw 37
-
{OR #17-#22} 2691
-
Aflibercept*:ti,ab,kw 1081
-
(Eylea or Zaltrap or Ziv NEXT Aflibercept or “AVE 0005” or AVE0005 or “AVE 005” or AVE005):ti,ab,kw 252
-
[mh ^Bevacizumab] 2633
-
Bevacizumab*:ti,ab,kw 7386
-
(Avastin or Mvasi or Alymsys or Aybintio or Equidacent or Onbevzi or Oyavas or Zirabev or rhuMAbVEGF or rhuMAb NEXT VEGF or “NSC 704865” or NSC704865):ti,ab,kw 941
-
(IVB NEAR/2 inject*):ti,ab,kw 89
-
[mh ^Ranibizumab] 1049
-
Ranibizumab*:ti,ab,kw 2266
-
(Lucentis or “rhuFab V2”):ti,ab,kw 451
-
(IVR NEAR/2 inject*):ti,ab,kw 32
-
Pegaptanib*:ti,ab,kw 166
-
(“EYE 001” or EYE001 or Macugen or “NX 1838” or NX1838):ti,ab,kw 82
-
{OR #24-#35} 10087
-
#8 and (#16 or #23 or #36) 1847
-
(rat or rats or rodent* or mouse or mice or “mus musculus” or “mus domesticus” or murine or murinae or bovine or sheep or ovine or “ovis aries” or porcine):ti,ab,kw 17188
#39 #37 not #38 with Cochrane Library publication date between January 2000 and May 2023, in Cochrane Reviews 14
Key:
mh = exploded indexing term (MeSH)
mh ^ = unexploded indexing term (MeSH)
/ai = indexing term with subheading for antagonists & inhibitors
* = truncation or additional characters within a word
? = adds up to 1 additional character
ti,ab,kw = terms in either title or abstract or keyword fields
near/3 = terms within three words of each other (any order)
next = terms are next to each other
Epistemonikos
via https://www.epistemonikos.org/
Date range searched: Inception – 26 May 2023
Date searched: 26 May 2023
Records retrieved: 1026
((title:((title:(((diabet* OR proliferat* OR PDR OR pre-proliferat* OR preproliferat* OR non-proliferat* OR nonproliferat* OR NPDR OR background) AND retinopath*)) OR abstract:(((diabet* OR proliferat* OR PDR OR pre-proliferat* OR preproliferat* OR non-proliferat* OR nonproliferat* OR NPDR OR background) AND retinopath*))) OR (title:((new blood vessel* AND diabet*)) OR abstract:((new blood vessel* AND diabet*)))) OR abstract:((title:(((diabet* OR proliferat* OR PDR OR pre-proliferat* OR preproliferat* OR non-proliferat* OR nonproliferat* OR NPDR OR background) AND retinopath*)) OR abstract:(((diabet* OR proliferat* OR PDR OR pre-proliferat* OR preproliferat* OR non-proliferat* OR nonproliferat* OR NPDR OR background) AND retinopath*))) OR (title:((new blood vessel* AND diabet*)) OR abstract:((new blood vessel* AND diabet*))))) AND (title:((anti AND VEGF*)) OR abstract:((anti AND VEGF*))) OR (title:((anti-VEGF* OR antiVEGF*)) OR abstract:((anti-VEGF* OR antiVEGF*))) OR (title:(((“anti vascular” OR anti-vascular OR antivascular) AND “endothelial growth factor”)) OR abstract:(((“anti vascular” OR anti-vascular OR antivascular) AND “endothelial growth factor”))) OR (title:(((“vascular endothelial growth factor” OR vasculotropin OR VEGF* OR “vascular permeability factor” OR VPF) AND (trap* OR inhibit* OR antagonist*))) OR abstract:(((“vascular endothelial growth factor” OR vasculotropin OR VEGF* OR “vascular permeability factor” OR VPF) AND (trap* OR inhibit* OR antagonist*)))) OR (title:((angiogen* AND (antagonist* OR inhibit*))) OR abstract:((angiogen* AND (antagonist* OR inhibit*)))) OR (title:(((antiangiogen* OR “anti angiogen” OR anti-angiogen* OR angiostatic) AND (agent* OR drug* OR effect*))) OR abstract:(((antiangiogen* OR “anti angiogen” OR anti-angiogen* OR angiostatic) AND (agent* OR drug* OR effect*)))) OR (title:((Aflibercept* OR Eylea OR Zaltrap OR Ziv-Aflibercept OR “AVE 0005” OR AVE0005 OR “AVE 005” OR AVE005 OR Bevacizumab* OR Avastin OR Mvasi OR Alymsys OR Aybintio OR Equidacent OR Onbevzi OR Oyavas OR Zirabev OR rhuMAbVEGF OR rhuMAb-VEGF OR “rhuMAb VEGF” OR “NSC 704865” OR NSC704865 OR Ranibizumab* OR Lucentis OR “rhuFab V2” OR Pegaptanib* OR “EYE 001” OR EYE001 OR Macugen OR “NX 1838” OR NX1838)) OR abstract:((Aflibercept* OR Eylea OR Zaltrap OR Ziv-Aflibercept OR “AVE 0005” OR AVE0005 OR “AVE 005” OR AVE005 OR Bevacizumab* OR Avastin OR Mvasi OR Alymsys OR Aybintio OR Equidacent OR Onbevzi OR Oyavas OR Zirabev OR rhuMAbVEGF OR rhuMAb-VEGF OR “rhuMAb VEGF” OR “NSC 704865” OR NSC704865 OR Ranibizumab* OR Lucentis OR “rhuFab V2” OR Pegaptanib* OR “EYE 001” OR EYE001 OR Macugen OR “NX 1838” OR NX1838))) OR (title:(((IVB OR IVR) AND inject*)) OR abstract:(((IVB OR IVR) AND inject*))))
Filter: Publication year 2000–2023
Publication type: Systematic Reviews
= 1026
Key:
* = truncation
title: = searches in title field
abstract: = searches in abstract field
PROSPERO
via https://www.crd.york.ac.uk/prospero/
Date range: Inception – 26 May 2023
Date searched: 26 May 2023
Records retrieved: 159
-
MeSH DESCRIPTOR Diabetic Retinopathy 107
-
((diabet* or DM) adj3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath* or maculopath*)) 609
-
(((proliferat* or PDR or pre-proliferat* or preproliferat* or non-proliferat* or nonproliferat* or NPDR or background) adj3 (retinopath* or vitreoretinopath* or vitreo-retinopath* or chorioretinopath* or chorio-retinopath*)) and (diabet* or DM)) 110
-
(new blood vessel* and diabet*) 9
-
(((retin* or subretina* or sub-retina* or interretina* or inter-retina* or vitreoretin* or vitreo-retin* or chorioretin* or chorio-retin* or choroid* or macula* or intraocular or intra-ocular or intravitreal or intra-vitreal) adj4 (damage* or deteriorat* or degnerat* or disease* or edema or oedema or neovascularisation* or neovascularization*)) AND diabet*) 373
-
((retinal vein* adj3 (occlu* or obstruct* or clos* or stricture* or steno* or block* or emboli*)) and diabet*) 64
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 740
-
MeSH DESCRIPTOR Vascular Endothelial Growth Factors EXPLODE ALL TREES WITH QUALIFIER AI 0
-
MeSH DESCRIPTOR Receptors, Vascular Endothelial Growth Factor EXPLODE ALL TREES WITH QUALIFIER AI 0
-
(anti adj2 VEGF*) 327
-
(anti-VEGF* or antiVEGF*) 327
-
((anti vascular or anti-vascular or antivascular) adj2 endothelial growth factor*) 153
-
(((vascular endothelial adj2 growth factor*) or vasculotropin or VEGF* or vascular permeability factor* or VPF) adj2 (trap* or inhibit* or antagonist*)) 96
-
(vascular proliferation adj4 inhibit*) 0
-
#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 412
-
MeSH DESCRIPTOR Angiogenesis Inhibitors 40
-
MeSH DESCRIPTOR Angiogenesis Inducing Agents EXPLODE ALL TREES WITH QUALIFIER A I0
-
(angiogen* adj2 (antagonist* or inhibit*)) 74
-
((antiangiogen* or anti angiogen* or anti-angiogen*) adj2 (agent* or drug* or effect*)) 145
-
(angiostatic adj2 (agent* or drug*)) 0
-
((neovascularisation* or neovascularization* or vascularisation* or vascularization*) adj2 inhibit*) 0
-
#16 OR #17 OR #18 OR #19 OR #20 OR #21 224
-
(Aflibercept*) 141
-
(Eylea or Zaltrap or Ziv-Aflibercept or AVE 0005 or AVE0005 or AVE 005 or AVE005) 22
-
MeSH DESCRIPTOR Bevacizumab 46
-
(Bevacizumab*) 445
-
(Avastin or Mvasi or Alymsys or Aybintio or Equidacent or Onbevzi or Oyavas or Zirabev or rhuMAbVEGF or rhuMAb-VEGF or rhuMAb VEGF or NSC 704865 or NSC704865) 59
-
(IVB adj2 inject*) 0
-
MeSH DESCRIPTOR Ranibizumab 7
-
(Ranibizumab*) 142
-
(Lucentis or rhuFab V2) 23
-
(IVR adj2 inject*) 0
-
(Pegaptanib*) 30
-
(EYE 001 or EYE001 or Macugen or NX 1838 or NX1838) 5
-
#23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 500
-
#15 OR #22 OR #35 839
-
#7 AND #36 159
Key:
MeSH DESCRIPTOR = indexing term: Medical Subject Heading (MeSH)
QUALIFIER AI = indexing term subheading for antagonists & inhibitors
EXPLODE ALL TREES = exploded indexing term (MeSH)
* = truncation
adj3 = terms within three words of each other (order specified).
:TI,KW = terms in either title or keyword fields
ClinicalTrials.gov
via https://clinicaltrials.gov/
Date searched: 26 May 2023
Records retrieved: 286
Two separate searches were used in Advanced Search, retrieving 286 records in total, which were imported into EndNote 20 and deduplicated.
1. Condition or Disease: (diabetic retinopathy)
Other Terms: (Aflibercept OR Eylea OR Zaltrap OR Bevacizumab OR Avastin OR Mvasi OR Alymsys OR Aybintio OR Equidacent OR Onbevzi OR Oyavas OR Zirabev OR rhuMAb VEGF OR Ranibizumab OR Lucentis OR rhuFab OR Pegaptanib OR Macugen) = 190 hits
2. Condition or Disease: (diabetic retinopathy)
Other Terms: ((VEGF OR vascular endothelial growth factor OR vasculotropin OR vascular permeability factor or VPF) AND (anti OR trap or inhibitor or antagonist)) = 96 hits
European Union Clinical Trials Register
via www.clinicaltrialsregister.eu/ctr-search/search
Date searched: 26 May 2023
Records retrieved: 163
Two separate searches were used, retrieving 163 records in total, which were imported into EndNote 20 and deduplicated.
-
((“diabetic retinopathy”) AND (Aflibercept OR Eylea OR Zaltrap OR Bevacizumab OR Avastin OR Mvasi OR Alymsys OR Aybintio OR Equidacent OR Onbevzi OR Oyavas OR Zirabev OR “rhuMAb VEGF” OR Ranibizumab OR Lucentis OR rhuFab OR Pegaptanib OR Macugen)) = 113 hits
-
((“diabetic retinopathy”) AND ((anti OR trap or inhibitor OR antagonist) AND (VEGF OR “vascular endothelial growth factor” OR vasculotropin OR “vascular permeability factor” OR VPF))) = 50 hits
WHO International Clinical Trials Registry Platform
via https://trialsearch.who.int/
Date searched: 26 May 2023
Records retrieved: 198
Two separate searches were used in Advanced Search, retrieving 198 records in total, which were imported into EndNote 20 and deduplicated.
1. Advanced Search
Condition: (diabetic retinopathy)
Intervention: (Aflibercept OR Eylea OR Zaltrap OR Bevacizumab OR Avastin OR Mvasi OR Alymsys OR Aybintio OR Equidacent OR Onbevzi OR Oyavas OR Zirabev OR rhuMAb VEGF OR Ranibizumab OR Lucentis OR rhuFab OR Pegaptanib OR Macugen)
Recruitment Status: ALL = 194 records for 180 trials
2. Advanced Search
Condition: (diabetic retinopathy)
Intervention: ((VEGF OR vascular endothelial growth factor OR vasculotropin OR vascular permeability factor or VPF) AND (anti OR trap or inhibitor or antagonist))
Recruitment Status: ALL = 23 records for 18 trials
List of excluded studies
Randomised controlled trial of DME (35)
Bayer AG. An open-label, randomized, active-controlled, parallel-group, Phase-3b study of the efficacy, safety, and tolerability of three different treatment regimens of 2 mg aflibercept administered by intr.
Braimah IZ, Kenu E, Amissah-Arthur KN, Akafo S, Kwarteng KO, Amoaku WM. Safety of intravitreal ziv-aflibercept in choroido-retinal vascular diseases: a randomised double-blind intervention study. PLOS ONE 2019;14:e0223944.
Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, Wells JA 3rd; Diabetic Retinopathy Clinical Research and Network. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 2012;130:1153–61.
Bressler SB, Qin H, Melia M, Bressler NM, Beck RW, Chan CK, et al.; Diabetic Retinopathy Clinical Research and Network. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol 2013;131:1033–40.
Bressler SB, Liu D, Glassman AR, Blodi BA, Castellarin AA, Jampol LM, et al.; Diabetic Retinopathy Clinical Research and Network. Change in diabetic retinopathy through 2 years: secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmol 2017;135:558–68.
Department of Ophthalmology and Medical University of Vienna. A Randomized, Double-masked Study with Intraocular Bevacizumab (Avastin®) Compared with Intravitreal Ranibizumab (Lucentis®) in Patients with Persistent Diabetic Macular Edema or Persistent Active. URL: www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2008-001469-28
Dhoot D, Hill L, Tarnowski K, Stoilov I. Baseline factors associated with >= 2-step diabetic retinopathy (DR) severity improvement with ranibizumab (RBZ). Investigative Ophthalmol Visual Sci Conference 2018;59.
Dhoot DS, Hill LF, Ghanekar A, Tarnowski KW, Ali FS. Baseline factors associated with diabetic retinopathy improvement in RIDE/RISE. Ophthalmol Retina 2021;5:101–3.
Dhoot DS, Moini H, Reed K, Du W, Vitti R, Berliner AJ, Singh RP. Functional outcomes of sustained improvement on Diabetic Retinopathy Severity Scale with intravitreal aflibercept in the VISTA and VIVID trials. Eye 2022;19:19.
Dimitriou E, Theodossiadis P, Chatzirallis A, Kazantzis D, Theodossiadis G, Chatziralli E. Intravitreal ranibizumab alone or in combination with panretinal photocoagulation for the treatment of proliferative diabetic retinopathy with coexistent macular edema: long-term outcomes in real-life data. Investigative Ophthalmol Visual Sci Conference 2020;61.
Ekinci M, Ceylan E, Cakici O, Tanyildiz B, Olcaysu O, Cagatay HH. Treatment of macular edema in diabetic retinopathy: comparison of the efficacy of intravitreal bevacizumab and ranibizumab injections. Expert Rev Ophthalmol 2014;9:139–43.
Euctr-009909-25-De. Evaluation of the Efficacy and Safety of a Macugen Monotherapy versus Combined Therapies in the Treatment of Diabetic Retinopathy – A Single Centre, Randomized, Prospective Phase II Trial. 2009.
Glassman AR, Stockdale CR, Beck RW, Baker C, Bressler NM; Diabetic Retinopathy Clinical Research and Network. Evaluation of masking study participants to intravitreal injections in a randomized clinical trial. Arch Ophthalmol 2012;130:190–4.
Gonzalez VH. Pegaptanib in diabetic retinopathy: improvements in diabetic macular edema, retinal neovascularization, and diabetic retinopathy severit. Am Academy Ophthalmol 2006:192.
Gonzalez VH, Wang PW, Ruiz CQ. Panretinal photocoagulation for diabetic retinopathy in the RIDE and RISE trials: not ‘1 and done’. Ophthalmology 2019;21:21.
Gonzalez VH, Wang PW, Ruiz CQ. Panretinal photocoagulation for diabetic retinopathy in the RIDE and RISE trials: not ‘1 and done’. Ophthalmology 2021;128:1448–57.
Hassan M, Sadiq MA, Halim MS, Afridi R, Nguyen NV, Sepah YJ. Short-term effects of ranibizumab on diabetic retinopathy severity and progression. Ophthalmol Retina 2018;2:749–51.
Hassan M, Sadiq MA, Halim MS, Afridi R, Nguyen NV, Sepah YJ. Short-term effects of ranibizumab on diabetic retinopathy severity and progression in the ranibizumab for edema of the macula in diabetes – Protocol 3 with high dose (READ-3) study. Investigative Ophthalmol Visual Sci Conference 2018;59.
Irct201205029617N. Efficacy of Macular laser Photocoagulation with or without Intravitreal Injection of Bevacizumab (Avastin) or Triamcinolone Acetonide for Diffuse Diabetic Macular Edema. 2012.
Mehta H, Lim LL, Nguyen V, Qatarneh D, Wickremasinghe SS, Hodgson LAB, et al. Development of new proliferative diabetic retinopathy in the BEVORDEX trial. Ophthalmol Retina 2019;3:286–7.
Mitchell P, McAllister I, Larsen M, Staurenghi G, Korobelnik JF, Boyer DS, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmol Retina 2018;2:988–96.
NCT. Laser-Ranibizumab-Triamcinolone for Proliferative Diabetic Retinopathy. 2007. URL: https://clinicaltrials.gov/show/NCT00445003
NCT. Anterior and Posterior Segment Vascular Changes Following Laser and Anti-Vascular Endothelial Growth Factor (VEGF) Treatment of Diabetic Retinopathy. 2009.
NCT. Laser Therapy Combined with Intravitreal Aflibercept vs Intravitreal Aflibercept Monotherapy (LADAMO). 2015. https://clinicaltrials.gov/show/NCT02432547
Novartis Pharma and AG. A 12-Month, 2-Arm, Randomized, Double-Masked, Multicenter Phase III Study Assessing the Efficacy and Safety of Brolucizumab Every 4 Weeks versus Aflibercept Every 4 Weeks in Adult Patients with Vis.
Novartis Pharma Gmb and H. A Randomized, Single-blinded, Multicenter, Phase IV Study to Compare Systemic VEGF Protein Dynamics Following Monthly Intravitreal Injections of 0.5 mg Ranibizumab versus 2 mg Aflibercept until Stu.
Novartis Pharma and AG. A Two-year, Three-arm, Randomized, Double Masked, Multicenter, Phase III Study Assessing the Efficacy and Safety of Brolucizumab versus Aflibercept in Adult Patients with Visual Impairment due to D.
Novartis Pharma and AG. A Two-year, Two-arm, Randomized, Double Masked, Multicenter, Phase III Study Assessing the Efficacy and Safety of Brolucizumab versus Aflibercept in Adult Patients with Visual Impairment due to Dia.
Oxurion NV. A Phase 2, Randomised, Single-masked, Active-controlled, Multicentre Study to Evaluate the Efficacy and Safety of Intravitreal THR-317 Administered in Combination with Ranibizumab, for the Treatment.
Quark Pharmaceuticals and Inc. An Open-Label Dose Escalation Study of PF-04523655 (Stratum I) Combined With A Prospective, Randomized, Double-Masked, Multi-Center, Controlled Study (Stratum II) Evaluating The Efficacy and Safety.
Sadiq MA, Hassan M, Soliman MK, Afridi R, Do DV, Nguyen QD, Sepah YJ. Effects of two different doses of ranibizumab on diabetic retinopathy severity. Ophthalmol Retina 2017;1:566–7.
Sameen M, Khan MS, Mukhtar A, Yaqub MA, Ishaq M. Efficacy of intravitreal bevacizumab combined with pan retinal photocoagulation versus panretinal photocoagulation alone in treatment of proliferative diabetic retinopathy. Pakistan J Med Sci 2017;33:142–5.
Sasongko MB, Rogers S, Constantinou M, Sandhu SS, Wickremasinghe SS, Al-Qureshi S, Lim LL. Diabetic retinopathy progression 6 months post-cataract surgery with intravitreous bevacizumab vs triamcinolone: a secondary analysis of the DiMECAT trial. Clin Exp Ophthalmol 2020;48:793–801.
Shahraki T, Arabi A, Nourinia R, Beheshtizadeh NF, Entezari M, Nikkhah H, et al. Panretinal photocoagulation versus intravitreal bevacizumab versus a proposed modified combination therapy for treatment of proliferative diabetic retinopathy: a Randomized Three-Arm Clinical Trial (CTPDR Study). Retina 2022;42:1065–76.
Yan P, Qian C, Wang W, Dong Y, Wan G, Chen Y. Clinical effects and safety of treating diabetic macular edema with intravitreal injection of ranibizumab combined with retinal photocoagulation. Therapeutics Clin Risk Manage 2016;12:527–33.
RCT of vitreous haemorrhage or vitrectomy (86)
Ahmadieh H, Shoeibi N, Entezari SM. Intravitreal bevacizumab for early post-vitrectomy hemorrhage in diabetics: a randomized, doubleMasked clinical trial. Am Academy Ophthalmol 2008:181.
Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology 2009;116:1943–8.
Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology 2011;118:2218–26.
Albuquerque TL, Pierozzi GS, Araujo AC. C, Neto NH, Carregal TB, Martins MC, et al. Comparative, randomized, double blinded study of the use of anti-VEGF in patients with vitreous hemorrhage or tractional retinal detachment secondary to diabetic retinopathy. Investigative Ophthalmol Visual Sci 2014;55:4391.
Aleman I, Velazquez JC, Rush SW, Rush RB. Ziv-aflibercept versus bevacizumab administration prior to diabetic vitrectomy: a randomised and controlled trial. Br J Ophthalmol 2019;103:1740–6.
Arevalo JF, Lasave AF, Kozak I, Al Rashaed S, Al Kahtani E, Maia M, et al.; Pan-American Collaborative Retina Study Group. Preoperative bevacizumab for tractional retinal detachment in proliferative diabetic retinopathy: a prospective randomized clinical trial. Am J Ophthalmol 2019;207:279–87.
Bhavsar A. A randomized trial evaluating intravitreal ranibizumab or intravitreal saline for vitreous hemorrhage from proliferative diabetic retinopathy. Investigative Ophthalmol Visual Sci Conference 2013;54.
Bhavsar AR, Torres K, Beck RW, Friedman SM, Glassman AR, Maturi RK, et al.; Diabetic Retinopathy Clinical Research Network. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from Proliferative Diabetic Retinopathy Diabetic Retinopathy Clinical Research Network. JAMA Ophthalmol 2013;131:283–93.
Castillo J, Aleman I, Rush SW, Rush RB. Preoperative bevacizumab administration in proliferative diabetic retinopathy patients undergoing vitrectomy: a randomized and controlled trial comparing interval variation. Am J Ophthalmol 2017;183:1–10.
Castillo Velazquez J, Aleman I, Rush SW, Rush RB. Bevacizumab before diabetic vitrectomy: a clinical trial assessing 3 dosing amounts. Ophthalmol Retina 2018;2:1010–20.
Chelala E, Nehme J, El Rami H, Aoun R, Dirani A, Fadlallah A, Jalkh A. Efficacy of intravitreal ranibizumab injections in the treatment of vitreous hemorrhage related to proliferative diabetic retinopathy. Retina 2018;38:1127–33.
ChiCtr. Feasibility Study of Anti-VEGF Instead of Intraoperative PRP in Proliferative Diabetic Retinopathy. 2018.
ChiCtr. A Prospective and Randomized Controlled Clinical Study for Pre- and After-operative Intravitreal Injection of Anti-VEGF Combined with Pars Plana Vitrectomy. 2020.
ChiCtr. A Prospective Randomized Controlled Study of Long-acting Dexamethasone Implant to Improve the Prognosis of PDR Patients after Vitrectomy. 2021.
ChiCTR1800019455. Effects of Intraocular Injection of Different Anti-VEGF Drugs on Inflammatory Factors in Aqueous Humor of Patients with Diabetic Retinopathy. 2018.
ChiCTR2000035032. Efficacy of Different Doses of Anti-VEGF with Vitrectomy in the Treatment of Proliferative Diabetic Retinopathy. 2020.
Comyn O, Bainbridge JWB. A pilot randomized controlled trial of ranibizumab pre-treatment for diabetic vitrectomy (The RaDiVit study). Investigative Ophthalmol Visual Sci 2014;55:2302.
Comyn O, Wickham L, Charteris DG, Sullivan PM, Ezra E, Gregor Z, et al. Ranibizumab pretreatment in diabetic vitrectomy: a pilot randomised controlled trial (the RaDiVit study). Eye 2017;31:1253–8.
Comyn O, Lange C, Bainbridge JWB. Vitreous and plasma cytokine levels in subjects with advanced proliferative diabetic retinopathy in the Ranibizumab in Diabetic Vitrectomy (RaDiVit) Study. Investigative Ophthalmol Visual Sci Conference 2019;60.
Cui J, Chen H, Lu H, Dong F, Wei D, Jiao Y, et al. Efficacy and safety of intravitreal conbercept, ranibizumab, and triamcinolone on 23-gauge vitrectomy for patients with proliferative diabetic retinopathy. J Ophthalmol 2018;2018:4927259.
da R Lucena D, Ribeiro JA, Costa RA, Barbosa JC, Scott IU, de Figueiredo-Pontes LL, Jorge R. Intraoperative bleeding during vitrectomy for diabetic tractional retinal detachment with versus without preoperative intravitreal bevacizumab (IBeTra study). Br J Ophthalmol 2009;93:688–91.
di Lauro R, De Ruggiero P, di Lauro R, di Lauro MT; Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2010;248:785–91.
Diabetic Retinopathy Clinical Research and Network. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol 2013;131:283–93.
Dong F, Yu C, Ding H, Shen L, Lou D. Evaluation of intravitreal ranibizumab on the surgical outcome for diabetic retinopathy with tractional retinal detachment. Medicine 2016;95:e2731.
Dong X. Effect of ranibizumab on the efficacy of vitrectomy in patients with PDR[J]. Int Eye Sci 2019;19:809812.
Luo W. Effect of ranibizumab combined with vitrectomy on the serum VEGF-A and SDF-1 expression in patients with proliferative diabetic retinopathy. Int Eye Sci 2019;19:438–41.
Euctr-000780-21-Gb. A Randomised, Single-masked, Phase IV Pilot Study of the Efficacy and Safety of Adjunctive Intravitreal Avastin® (Bevacizumab) in the Prevention of Early Postoperative Vitreous Haemorrhage Following Diabetic Vitrectomy – Intravitreal Avastin® in Diabetic Vitrectomy. 2007.
Euctr-015559-25-Gb. Preoperative Intravitreal Ranibizumab for Persistent Diabetic Vitreous Haemorrhage: A Randomized, Double-masked, Controlled Study – Vitreous Haemorrhage Study. 2010. URL: www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009-015559-25-GB
Euctr-024062-22-Gb. A Prospective, Randomised Controlled Trial of Ranibizumab Pre-treatment in Diabetic Vitrectomy – A Pilot Study – A Pilot RCT of Ranibizumab in Diabetic Vitrectomy – The RaDiVit Study. 2011.
Farahvash MS, Majidi AR, Roohipoor R, Ghassemi F. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina 2011;31:1254–60.
Ferraz DA, Morita C, Preti RC, Nascimento VP, Maia OO, de Barros AC, et al. Use of intravitreal bevacizumab or triamcinolone acetonide as a preoperative adjunct to vitrectomy for vitreous haemorrhage in diabetics. Revista Brasileira De Oftalmologia 2013;72:12–16.
Gao S, Lin Z, Chen Y, Xu J, Zhang Q, Chen J, Shen X. Intravitreal conbercept injection as an adjuvant in vitrectomy with silicone oil infusion for severe proliferative diabetic retinopathy. J Ocul Pharmacol Ther 2020;36:304–10.
Genovesi-Ebert F, Rizzo S, Di Bartolo E, Miniaci S, Vento A, Palla M, Cresti F. Injection of intravitreal Avastin before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy. Invest Ophthamol Vis Sci 2007;48:ARVO E‐Abstract 5044.
Glassman AR, Beaulieu WT, Maguire MG, Antoszyk AN, Chow CC, Elman MJ, et al. Visual acuity, vitreous hemorrhage, and other ocular outcomes after vitrectomy vs aflibercept for vitreous hemorrhage due to diabetic retinopathy: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol 2021;139:725–33.
Han XX, Guo CM, Li Y, Hui YN. Effects of bevacizumab on the neovascular membrane of proliferative diabetic retinopathy: reduction of endothelial cells and expressions of VEGF and HIF-1alpha. Mol Vision 2012;18:1–9.
Hernandez-Da Mota SE, Nunez-Solorio SM. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur J Ophthalmol 2010;20:1047–52.
Hu BJ, Zeng Q, Liu XL, Li XR, Song WJ. Influence of intravitreal avastin on the expression of cell factors in retinal proliferative membrane in proliferative diabetic retinopathy eye. [Chinese]. Zhonghua Shiyan Yanke Zazhi/Chinese Journal of Experimental Ophthalmology 2013;31:55–59.
Hu Z, Cao X, Chen L, Su Y, Ji J, Yuan S, et al. Monitoring intraocular proangiogenic and profibrotic cytokines within 7 days after adjunctive anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy. Acta Opthalmol2021;14:14.
Jeon S, Lee WK. Intravitreal bevacizumab increases intraocular interleukin-6 levels at 1 day after injection in patients with proliferative diabetic retinopathy. Cytokine 2012;60:535–9.
Jiang TT, Gu JX, Zhang PJ, Chen WW, Chang Q. The effect of adjunctive intravitreal conbercept at the end of diabetic vitrectomy for the prevention of post-vitrectomy hemorrhage in patients with severe proliferative diabetic retinopathy: a prospective, randomized pilot study. BMC Ophthalmol 2020;20:9.
Jiao C, Spee C, He S, Mullins R, Eliott D, Hinton DR, Sohn EH. Angiofibrotic response to bevacizumab on fibrovascular membranes in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 2014;55:5821.
Jorge DM, Tavares Neto JEDS, Poli-Neto OB, Scott IU, Jorge R. Intravitreal bevacizumab (IVB) versus IVB in combination with pars plana vitrectomy for vitreous hemorrhage secondary to proliferative diabetic retinopathy: a randomized clinical trial. Int J Retina Vitreous 2021;7:35.
JPRN-UMIN. Low Dose of Intravitreal Bevacizumab (Avastin) Used as Preoperative Adjunct Therapy for Proliferative Diabetic Retinopathy. 2012.
Kanclerz P, Raczynska K. Preoperative bevacizumab as an adjunct for vitrectomy in proliferative diabetic retinopathy patients. Ophthalmologica J Int d’ophtalmologie 2016;236:58.
Li Q, Wang JH, Zhang MM, Wang Y. Effect of ranibizumab intravitreal injection before 23G-vitrectomy surgery in the treatment of patients with proliferative diabetic retinopathy [Chinese]. Int Eye Sci 2016;16:1959–61.
Li B, Li MD, Ye JJ, Chen Z, Guo ZJ, Di Y. Vascular endothelial growth factor concentration in vitreous humor of patients with severe proliferative diabetic retinopathy after intravitreal injection of conbercept as an adjunctive therapy for vitrectomy. Chine Med J 2020;133:664–9.
Manabe A, Shimada H, Hattori T, Nakashizuka H, Yuzawa M. Randomized controlled study of intravitreal bevacizumab 0.16 mg injected one day before surgery for proliferative diabetic retinopathy. Retina 2015;35:1800–7.
Meng N, Ren BC. Effect of intravitreal injection of bevacizumab for vitreous hemorrhage in patients with proliferative diabetic retinopathy [Chinese]. Int Eye Sci 2016;16:972–4.
Modarres M, Nazari H, Falavarjani KG, Naseripour M, Hashemi M, Parvaresh MM. Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol 2009;19:848–52.
NCT. Intravitreal Bevacizumab for Proliferative Diabetic Retinopathy. 2007. URL: https://clinicaltrials.gov/show/NCT00423059
NCT. Evaluation of Ranibizumab in Proliferative Diabetic Retinopathy (PDR) Requiring Vitrectomy. 2007. URL: https://clinicaltrials.gov/show/NCT00516464
NCT. Preoperative Bevacizumab for Vitreous Hemorrhage. 2008. URL: https://clinicaltrials.gov/show/NCT00596297
NCT. Safety and Efficacy of Intravitreal Ranibizumab as a Preoperative Adjunct Treatment before Vitrectomy Surgery in Proliferative Diabetic Retinopathy (PDR) Compared to Vitrectomy Alone. 2009. URL: https://clinicaltrials.gov/show/NCT00931125
NCT. Acute Changes in Intraocular Cytokines after Intravitreal Bevacizumab. 2011. URL: https://clinicaltrials.gov/show/NCT01439178
NCT. Ranibizumab in Diabetic Vitrectomy. A Prospective, Randomised Controlled Trial of Ranibizumab Pre-treatment in Diabetic Vitrectomy – A Pilot Study. 2011. URL: https://ClinicalTrials.gov/show/NCT01306981
NCT. Prospective Randomized Controlled Study of Intravitreal Injection of Bevacizumab for Proliferative Diabetic Retinopathy. 2013. URL: https://clinicaltrials.gov/show/NCT01854593
NCT. Aflibercept Injection for Proliferative Diabetic Retinopathy. 2013. URL: https://clinicaltrials.gov/show/NCT01805297
NCT. Pre-operative Intravitreal Bevacizumab for Tractional Retinal Detachment Secondary to Proliferative Diabetic Retinopathy. 2013. URL: https://clinicaltrials.gov/show/NCT01976923
NCT. Comparison of Interval Variation and Dosage in Preoperative Bevacizumab and Ziv-Aflibercept Administration in Proliferative Diabetic Retinopathy Undergoing Vitrectomy. 2015. URL: https://clinicaltrials.gov/show/NCT02590094
NCT. 25-G Vitrectomy with Ranibizumab or Triamcinolone Acetonide on PDR in China-Randomized Clinical Trial. 2015. URL: https://clinicaltrials.gov/show/NCT02447185
NCT. Intravitreal Injection of Ranibizumab versus Sham before Vitrectomy in Patients with Proliferative Diabetic Retinopathy. 2016. URL: https://clinicaltrials.gov/show/NCT02857491
NCT. Pre-vitrectomy Intravitreal Ranibizumab for Patients with Proliferative Diabetic Retinopathy Combined With Diabetic Macular Edema. 2020. URL: https://clinicaltrials.gov/show/NCT04464694
Pakzad-Vaezi K, Albiani DA, Kirker AW, Merkur AB, Kertes PJ, Eng KT, et al. A randomized study comparing the efficacy of bevacizumab and ranibizumab as pre-treatment for pars plana vitrectomy in proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina 2014;45:521–4.
Petrarca R, Soare C, Wong R, Desai R, Neffendorf J, Simpson A, Jackson TL. Intravitreal ranibizumab for persistent diabetic vitreous haemorrhage: a randomised, double-masked, placebo-controlled feasibility study. Acta Ophthalmol 2020;98:E960–7.
Qi QF, Shi YW, Guo T. Clinical observation on preoperative application of bevacizumab in proliferative diabetic retinopathy [Chinese]. Int Eye Sci 2014;14:1646–8.
Ren XJ, Bu SC, Zhang XM, Jiang YF, Tan LZ, Zhang H, Li XR. Safety and efficacy of intravitreal conbercept injection after vitrectomy for the treatment of proliferative diabetic retinopathy. Eye 2019;33:1177–83.
Reza NM, Hosein AM, Hesamsadat H, Amir EM, Narges H, Amin N. Intravitreal tissue plasminogen activator in diabetic vitreous hemorrhage. Int J Pharm Res 2019;11:823–7.
Sohn EH, He S, Kim LA, Salehi-Had H, Javaheri M, Spee C, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Arch Ophthalmol 2012;130:1127–34.
Starnes DC, Lalane R, Walia H, Farooq A, Frazier H, Marcus W, et al. Endolaserless vitrectomy with intravitreal aflibercept injection (IAI) for proliferative diabetic retinopathy (PDR)-related vitreous hemorrhage: LASER LESS TRIAL 1-year results. Invest Ophthalmol Vis Sci Conference 2019;60.
Su L, Ren X, Wei H, Zhao L, Zhang X, Liu J, et al. Intravitreal conbercept (Kh902) for surgical treatment of severe proliferative diabetic retinopathy. Retina 2016;36:938–43.
Sun M, Li MX. Study of anti-vascular endothelial growth factor medicine for proliferative diabetic retinopathy at perioperative period [Chinese]. Int Eye Sci 2015;15:1772–4.
Sun L, Tao Y. Effects of bevacizumab on CTGF and PEDF in proliferative membrane in patients with PDR [Chinese]. Int Eye Sci 2017;17:1051–4.
Tegins E, Javaheri M, Eliott D, Kim L, Salehi-Had H, Hinton D, Sohn E. 1 year clinical outcomes of a randomized clinical trial investigating pre-operative adjunctive bevacizumab for tractional retinal detachment (TRD) due to proliferative diabetic retinopathy (PDR). Invest Ophthalmol Vis Sci Conference 2013;54.
Victor AA, Gondhowiardjo TD, Waspadji S, Wanandi SI, Bachtiar A, Suyatna FD, Muhiddin H. Effect of laser photocoagulation and bevacizumab intravitreal in proliferative diabetic retinopathy: review on biomarkers of oxidative stress. Med J Indones 2014;23:79–86.
Wang YP, Chen MZ, Chen GC, Chen YJ. Clinical effect of vitrectomy with intravitreal ranibizumab for diabetic retinopathy [Chinese]. Int Eye Sci 2014;14:1257–9.
Wildan A, Winarto, Kristina TN. Aflibercept and bevacizumab injection effects on visual acuity of post vitrectomy diabetic retinopathy. Pakistan J Med Health Sci 2019;13:1214–8.
Yamaji H, Shiraga F, Shiragami C, Nomoto H, Fujita T, Fukuda K. Reduction in dose of intravitreous bevacizumab before vitrectomy for proliferative diabetic retinopathy. Arch Ophthalmol 2011;129:106–7.
Yang XC, Xu JB, Wang RL, Mei Y, Lei H, Liu J, et al. A randomized controlled trial of conbercept pretreatment before vitrectomy in proliferative diabetic retinopathy. J Ophthalmol 2016;2016:8.
Yao TT, Yang Y, Jin XL, Wang YX, Zhou YL, Xu AJ, et al. Intraocular pharmacokinetics of anti-vascular endothelial growth factor agents by intraoperative subretinal versus intravitreal injection in silicone oil-filled eyes of proliferative diabetic retinopathy: a randomized controlled pilot study. Acta Opthalmol 2020;98:e795–e800.
Yin N, Zhao S, Zhu HN. Efficacy comparison of conbercept and ranibizumab as pre-treatment for pars plana vitrectomy in proliferative diabetic retinopathy [Chinese]. Int Eye Sci 2017;17:1300–02.
Yu XQ, Cao GP, Tang MX. Effect of vitrectomy combined medication hyperplastic on patients with diabetic retinopathy [Chinese]. Int Eye Sci 2015;15:1402–04.
Zahaf A, Zghal I, Fekih O, Zayani M, Mahjoub A, Bouguila H. Preoperative intravitreal bevacizumab effects on the course of Pars Plana vitrectomy in diabetic vitreous hemorrhage. Acta Ophthalmol Conference 2015;93.
Zaman Y, Rehman AU, Memon AF. Intravitreal Avastin as an adjunct in patients with proliferative diabetic retinopathy undergoing pars plana vitrectomy. Pakistan J Med Sci 2013;29:590–2.
Zhao XL, Yang G, Yang J, Zhang JJ. Effect of intravitreal conbercept vs triamcinolone acetonide at the end of surgery on macular structure and function in patients with severe proliferative diabetic retinopathy. Int J Clin Exp Med 2017;10:14511–18.
Zhou AY, Zhou CJ, Yao J, Quan YL, Ren BC, Wang JM. Panretinal photocoagulation versus panretinal photocoagulation plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy. Int J Ophthalmol 2016;9:1772–8.
Zhou J, Liu Z, Chen M, Luo ZH, Li YQ, Qi GY, Liu T. Concentrations of VEGF and PlGF decrease in eyes after intravitreal conbercept injection. Diabetes Ther Res Treatment Educ Diabetes Related Disorders 2018;9:2393–8.
Exclude population (others) (17)
Altaweel MM. Changes in severity of diabetic retinopathy following Pegaptanib (Macugen®) Therapy. Invest Ophthamol Vis Sci 2006;47:ARVO E‐abstract 5441.
Chae JB, Joe SG, Yang SJ, Lee JY, Sung KR, Kim JY, et al. Effect of combined cataract surgery and ranibizumab injection in postoperative macular edema in nonproliferative diabetic retinopathy. Retina 2014;34:149–56.
Cheema RA, Al-Mubarak MM, Amin YM, Cheema MA. Role of combined cataract surgery and intravitreal bevacizumab injection in preventing progression of diabetic retinopathy: prospective randomized study. J Cataract Refract Surg 2009;35:18–25.
Euctr-004648-12-Es. This Is a Phase 3, Multicenter, Randomized, Masked, Controlled, Parallel Group Study of 12 Months Duration in Treatment Naïve Subjects with RVO. 2017.
Department of Ophthalmology and Medical University of Vienna. European Intravitreal Avastin® Trial 1. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2005-003132-21
JPRN-jRCTs031180307. The Effect of an Anti-VEGF Drug on Proliferative Retinopathy. 2019. URL: https://jrct.niph.go.jp/latest-detail/jRCTs031180307
Kodiak Sciences and Inc. A Prospective, Randomized, Double-masked, Active Comparator-controlled, Multi-center, Two-arm, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 Compared with Intravitreal A.
NCT. Analysis of Aqueous and Vitreous Humor. 2017. URL: https://ClinicalTrials.gov/show/NCT02067013
Novartis Farmacéutica and SA. A 12-Month, Phase IIIb, Randomized, Visual Acuity, Assessor-masked, Multicenter Study Assessing the Efficacy and Safety of Ranibizumab 0.5mg in Treat and Extend Regimen Compared to Monthly Regimen.
Novartis Pharma Services and AG. A 24-Month Randomized, Double-masked, Multicenter, Phase II Study Assessing Safety and Efficacy of Verteporfin (Visudyne®) Photodynamic Therapy Administered in Conjunction with Lucentis™ versus Luc.
Novartis Pharma Services and AG A 24-Month, Phase IIIb, Open-label, Randomized, Active-controlled, 3-Arm, Multicenter Study Assessing the Efficacy and Safety of an Individualized, Stabilization-criteria-driven PRN Dosing Regimen W.
Novartis Pharma Services and AG. A 24-Month, Phase IIIb, Open-label, Single Arm, Multicenter Study Assessing the Efficacy and Safety of an Individualized, Stabilization Criteria-driven PRN Dosing Regimen with 0.5-mg Ranibizumab IN.
Novartis Pharma Services and AG. A 24-Month, Phase IIIb, Randomized, Double-masked, Multicenter Study Assessing the Efficacy and Safety of Two Treatment Regimens of 0.5 mg Ranibizumab Intravitreal Injections Guided by Functional A.
Novartis Pharma and AG. A 64-Week, Two-arm, Randomized, Double-masked, Multi-center, Phase IIIb Study Assessing the Efficacy and Safety of Brolucizumab 6 mg Compared to Aflibercept 2 mg in a Treat-to-Control Regimen in PA.
NCT. Effects of Intravitreal Ranibizumab for Macular Edema with Nonproliferative Diabetic Retinopathy. 2016. URL: https://ClinicalTrials.gov/show/NCT02834663
Opthea Limited A Phase 3, Multicentre, Double-masked, Randomised Study to Evaluate the Efficacy and Safety of Intravitreal OPT-302 in Combination with Ranibizumab, Compared with Ranibizumab Alone, in Participants.
Yu B, Liu Z. The clinical efficacy of vitreous injection of ranibizumab in patients with ocular fundus disease and its effect on hemorheology. Int J Clin Exp Med 2019;12:11249–56.
Irrelevant intervention (3)
Abadia B, CalvoP, Ferreras A, Bartol F, Verdes G, Pablo L. Clinical applications of dexamethasone for aged eyes. Drugs Aging 2016;33:639–46.
Altun A, Kanar HS, Aki SF, Arsan A, Hacisalihoglu A. Effectiveness and safety of coadministration of intravitreal dexamethasone implant and silicone oil endotamponade for proliferative diabetic retinopathy with tractional diabetic macular edema. J Ocular Pharmacol Ther 2021;37:131–7.
CTRI. A Clinical Study to Assess and Compare the Efficacy and Safety of Hydroxychloroquine and Teneligliptin in Type 2 Diabetes Patients with Non-proliferative Diabetic Retinopathy. 2020.
Irrelevant comparator (4)
Antoszyk AN, Glassman AR, Beaulieu WT, Jampol LM, Jhaveri CD, Punjabi OS, et al. Network DRCR Retina. Effect of intravitreous aflibercept vs vitrectomy with panretinal photocoagulation on visual acuity in patients with vitreous hemorrhage from proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2020;324:2383–95.
Khodabandeh A, Fadaifard S, Abdollahi A, Karkhaneh R, Roohipoor R, Abdi F, et al. Role of combined phacoemulsification and intravitreal injection of bevacizumab in prevention of postoperative macular edema in non-proliferative diabetic retinopathy. J Curr Ophthalmol 2018;30:245–9.
Shi R, Ma Y, Wang F, Wang JP. Effects of intravitreous injection on the expression of vascular endothelial growth inhibitor in vitreous of proliferative diabetic retinopathy [Chinese]. Int Eye Sci 2015;15:985–8.
Yan P, Zhang XH, Zhang L, Li J. Effect of intravitreal injection of ranibizumab combined with voritine on hemorrhagic proliferative diabetic retinopathy and its effect on visual acuity and endothelial growth factor [Chinese]. Chine J Pharm Biotechnol 2019;26:127–30.
No relevant outcomes (1)
Khalaf H, Rostamizadeh M, Gonzalez VH. Foveal Avascular Zone in high risk proliferative diabetic retinopathy treated with intravitreal aflibercept injection (ELYSIAN). Invest Ophthalmol Vis Sci Conference 2018;59.
Inappropriate trial design (27)
Ababneh OH, Yousef YA, Gharaibeh AM, Abu Ameerh MA, Abu-Yaghi NE, Al Bdour MD. Intravitreal bevacizumab in the treatment of diabetic ocular neovascularization. Retina 2013;33:748–55.
Abdallah W, Fawzi AA. Anti-VEGF therapy in proliferative diabetic retinopathy. Int Ophthalmol Clin 2009;49:95–107.
Al-Khersan H, Hariprasad SM, Salehi-Had H. Dexamethasone and anti-VEGF combination therapy for the treatment of diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 2019;50:4–7.
Bakri SJ, Donaldson MJ, Link TP. Rapid regression of disc neovascularization in a patient with proliferative diabetic retinopathy following adjunctive intravitreal bevacizumab. Eye 2006;20:1474–5.
Beaulieu WT, Bressler NM, Gross JG; Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: patient-centered outcomes from a randomized clinical trial reply. Am J Ophthalmol 2017;177:233–233.
Bi SS, Jiang T, Chen Y, Ma XF. Effects of laser photocoagulation combined with anti-VEGF drugs at different time in the treatment of diabetic retinopathy. Int Eye Sci 2020;20:613–8.
Brown DM, Wykoff CC. Intravitreal aflibercept for proliferative diabetic retinopathy. Lancet 2017;390:2141–2141.
Browning DJ, Lee C, Stewart MW, Landers MB 3rd. Vitrectomy for center-involved diabetic macular edema. Clin Ophthalmol 2016;10:735–42.
Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006;26:699–700.
Chen PY, Wang TW, Wang WC, Liao JC, Yang SA, Hsu YT. Clinical Outcome of Diabetic Retinopathy with the Treatment of Photocoagulation versus Anti-VEGF. 2020.
Desapriya E, Khoshpouri P, Al-Isa A. Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: patient-centered outcomes from a randomized clinical trial. Am J Ophthalmol 2017;177:232–3.
Ergur O, Bayhan HA, Kurkcuoglu P, Takmaz T, Gurdal C, Can I. Comparison of panretinal photocoagulation (PRP) with PRP plus intravitreal bevacizumab in the treatment of proliferative diabetic retinopathy. [Turkish] Proliferatif diyabetik retinopati tedavisinde tek basina panretinal fotokoagulasyon (PRF) ile PRF ve intravitreal bevacizumab kombinasyonunun karsilastirilmasi. Retina-Vitreus 2009;17:273–7.
Gibson JM, McGinnigle S. Diabetes: intravitreous ranibizumab for proliferative diabetic retinopathy. Nature Rev Endocrinol 2016;12:130–1.
Glassman AR. Results of a randomized clinical trial of aflibercept vs panretinal photocoagulation for proliferative diabetic retinopathy: is it time to retire your laser?. JAMA Ophthalmol 2017;135:685–6.
Gross JG, Glassman AR. A novel treatment for proliferative diabetic retinopathy: anti-vascular endothelial growth factor therapy. JAMA Ophthalmol 2016;134:13–4.
Gupta MP, Kiss S, Chan RV. Reversal of retinal vascular leakage and arrest of progressive retinal nonperfusion with monthly anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy. Retina 2018;38:e74–e75.
Hershberger V, Hill LF, Tuomi LL, Ghanekar A. Ranibizumab-induced diabetic retinopathy improvement-results from patients at high risk for vision loss in ride/rise and Protocol S. Diabetes 2018;67:A158.
Krishnan R, Goverdhan S, Lochhead J. Intravitreal pegaptanib in severe proliferative diabetic retinopathy leading to the progression of tractional retinal detachment. Eye 2009;23:1238–9.
Krzystolik MG, Filippopoulos T, Ducharme JF, Loewenstein JI. Pegaptanib as an adjunctive treatment for complicated neovascular diabetic retinopathy. Arch Ophthalmol 2006;124:920–1.
Li J, Liu F. Clinical evidence on the treatment of non-proliferative diabetic retinopathy. Chine J Evidence-Based Med 2007;7:894–8.
Melia M, Edwards A, Kollman C. Interim analysis with sample size re-estimation for binary outcome in a trial of intravitreal ranibizumab versus saline injection for prevention of vitrectomy in eyes with proliferative diabetic retinopathy and vitreous hemorrhage. Clin Trials 2012;9:523–4.
Olsen TW. Anti-VEGF pharmacotherapy as an alternative to panretinal laser photocoagulation for proliferative diabetic retinopathy. JAMA 2015;314:2135–6.
Ospedale Sacro Cuore-Don and Calabria. Evaluation of Safety and Efficacy on Visual Acuity Outcome of Intravitreal Somministration of Bevacizumab in Patients with Diabetic Retinopathy. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2006-005315-10
Parikakis E. Laser or anti-VEGF for proliferative diabetic retinopathy. Acta Ophthalmol 2018;96:94.
Tan TE, Sivaprasad S, Wong TY. Anti-vascular endothelial growth factor therapy for complications of diabetic retinopathy-from treatment to prevention?. JAMA Ophthalmol 2023;141:223–5.
Wise J. Lucentis offers treatment alternative for diabetic retinopathy, trial finds. BMJ 2015;351:h6145.
Zucchiatti I, Bandello F. Intravitreal ranibizumab in diabetic macular edema: long-term outcomes. Dev Ophthalmol 2017;60:63–70.
Not a randomised controlled trial (17)
CHICTR-OON. Effect of Anti VEGF on the Expression of Vitreous Ang2 in Patients with PDR 2017. URL: www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-OON-17012170
Chung EJ, Kang SJ, Koo JS, Choi YJ, Grossniklaus HE, Koh HJ. Effect of intravitreal bevacizumab on vascular endothelial growth factor expression in patients with proliferative diabetic retinopathy. Yonsei Med J 2011;52:151–7.
Department of Ophthalmology and M U W. Disease-modification under treatment with aflibercept in advanced diabetic retinopathy – a pilot study.
EUCTR2006-005315-10-IT. Evaluation of Safety and Efficacy on Visual Acuity Outcome of Intravitreal Somministration of Bevacizumab in Patients with Diabetic Retinopathy – ND. 2006.
He F, Yu W. Longitudinal neovascular changes on optical coherence tomography angiography in proliferative diabetic retinopathy treated with panretinal photocoagulation alone versus with intravitreal conbercept plus panretinal photocoagulation: a pilot study. Eye 2020;34:1413–18.
IRCT138903314232N1. Intravitreal Bevacizumab (Avastin) Therapy for Proliferative Diabetic Retinopathy. 2010.
JPRN-UMIN. Evaluate the Effect of Intravitreal Bevacizumab Injection for Ocular Proliferative Diseases. 2016.
Kernt M, Cserhati S, Seidensticker F, Liegl R, Kampik A, Neubauer A, et al. Improvement of diabetic retinopathy with intravitreal Ranibizumab. Diabetes Res Clin Pract 2013;100:e11–3.
Lopez-Lopez F, Gomez-Ulla F, Rodriguez-Cid MJ, Arias L. Triamcinolone and bevacizumab as adjunctive therapies to panretinal photocoagulation for proliferative diabetic retinopathy. ISRN Ophthalmol Print 2012;2012:267643.
NCT. Intravitreal Bevacizumab for Management of Active Progressive Proliferative Diabetic Retinopathy (PDR). 2006. URL: https://ClinicalTrials.gov/show/NCT00370721
NCT. Analysis of Angiogenic Factor Levels in Eyes with Diabetic Retinopathy. 2012. URL: https://ClinicalTrials.gov/show/NCT02026843
NCT. Combined Triple Therapy in Diabetic Retinopathy (DRP). 2012. URL: https://clinicaltrials.gov/study/NCT00806169
NCT. Effect of Macugen (Pegaptanib) on Surgical Outcomes and VEGF Levels in Diabetic Patients with PDR (Diabetic Retinopathy or CSDME (Macular Edema)). 2012. URL: https://ClinicalTrials.gov/show/NCT00446381
NCT. Ziv-aflibercept in Ocular Disease Requiring Anti-VEGF Injection. 2015. URL: https://ClinicalTrials.gov/show/NCT02486484
Park YJ, Ahn J, Kim TW, Park SJ, Joo K, Park KH, Shin JY. Efficacy of bevacizumab for vitreous haemorrhage in proliferative diabetic retinopathy with prior complete panretinal photocoagulation. Eye:8.
Park JM, Lee SJ. The effect of panretinal photocoagulation and additive intravitreal bevacizumab injections on central retinal vessel diameters in diabetic retinopathy. Acta Ophthalmol Conference 2015;93.
Vidinova CN, Gouguchkova PT, Dimitrov T, Vidinov KN, Nocheva H. [Comparative clinical and ultrastructural analysis of the results from ranibizumab and aflibercept in patients with PDR]. Klinische Monatsblatter fur Augenheilkunde 2020;237:79–84.
Protocols of excluded and ongoing studies (20)
Frimley Park Hospital and NHS. Foundation Trust. A randomised controlled trial of efficacy of Pegaptanib sodium in the prevention of proliferative diabetic retinopathy.
Fakultní nemocnice Královské and Vinohrady. A randomized, 12 months, active controlled study of the efficacy of repeated doses of intravitreal aflibercept in subjects with proliferative diabetic retinopathy.
Euctr-000658-30-Ie. Randomised Controlled Trial of Intravitreal Bevacizumab vs. Conventional Treatment for Proliferative Diabetic Retinopathy – Randomised Controlled Trial of Intravitreal Bevacizumab vs. Conventional Treatment for Proliferative. 2007.
Euctr-001856-36-Fr. Efficacy and Safety of Aflibercept (Eylea®) in Proliferative Diabetic Retinopathy. 2016.
Euctr-004203-39-Cz. Study of Effect of Intravitreal Aflibercept in Subjects with Proliferative Diabetic Retinopathy. 2014.
Euctr-006795-10-Gb. A Randomised Controlled Trial of Efficacy of Pegaptanib Sodium in the Prevention of Proliferative Diabetic Retinopathy – EPPPDR. 2008.
ISRCTN. A Prospective Randomised Controlled Trial Assessing the Efficacy of Pegaptanib Sodium (Macugen®) in the Prevention of Proliferative Diabetic Retinopathy. 2010.
NCT. Ranibizumab for Treatment of Persistent Diabetic Neovascularization Assessed by Wide-Field Imaging. 2008. URL: https://clinicaltrials.gov/show/NCT00606138
NCT. Prospective, Randomized, Open Label, Phase II Study to Assess Efficacy and Safety of Macugen® (Pegaptanib 0.3 mg Intravitreal Injections) Plus Panretinal Photocoagulation and PRP (Monotherapy) in the Treatment with High Risk PDR. 2011. URL: https://clinicaltrials.gov/show/NCT01281098
NCT. Prevention of Macular Edema in Patients with Diabetic Retinopathy Undergoing Cataract Surgery. 2013. URL: https://clinicaltrials.gov/show/NCT01988246
NCT. Treatment with Intravitreal Aflibercept Injection for Proliferative Diabetic Retinopathy, The A.C.T Study. 2013. URL: https://clinicaltrials.gov/show/NCT01813773
NCT. Safety and Efficacy of Aflibercept in Proliferative Diabetic Retinopathy. 2015. URL: https://ClinicalTrials.gov/show/NCT02151695
NCT. Conbercept vs Panretinal Photocoagulation for the Management of Proliferative Diabetic Retinopathy. 2016. URL: https://clinicaltrials.gov/show/NCT02911311
NCT. Intravitreal Aflibercept as Indicated by Real-Time Objective Imaging to Achieve Diabetic Retinopathy Improvement. 2018. URL: https://clinicaltrials.gov/show/NCT03531294
NCT. Multicenter Clinical Study of Anti-VEGF Treatment on High Risk Diabetic Retinopathy (DR). 2018. URL: https://clinicaltrials.gov/show/NCT03452657
NCT. A Multicenter, Randomized Study in Participants with Diabetic Retinopathy without Center-involved Diabetic Macular Edema to Evaluate the Efficacy, Safety, and Pharmacokinetics of Ranibizumab Delivered via the Port Delivery System Relative to the Comparator Arm. 2020. URL: https://clinicaltrials.gov/show/NCT04503551
NCT. Intravitreal Bevacizumab for Nonproliferative Diabetic Retinopathy. 2020. URL: https://clinicaltrials.gov/show/NCT04511715
NCT. Study of Efficacy and Safety of Brolucizumab versus Panretinal Photocoagulation Laser in Patients with Proliferative Diabetic Retinopathy. 2020. URL: https://ClinicalTrials.gov/show/NCT04278417
NCT. Intravitreal Bevacizumab vs Laser vs Combination of Bevacizumab and Modified Laser in PDR. 2021. URL: https://clinicaltrials.gov/show/NCT04800679
TCTR. Change of OCT Findings after Intravitreal Anti-VEGF Injection in Patients with Diabetic Tractional Retinal Detachment: A Randomized Controlled Trial. 2021.
Irretrievable (1)
Neri Alvarez-Villalobos Humberto de León-Gutiérrez Fernando Ruiz-Hernandez. Safety and clinical effectiveness behavior of bevacizumab biosimilars in the intravitreal application.
Trials not included in meta-analyses
| Trial | Key paper(s) | Anti-VEGF | Comparator | Location | Sample size | Population |
|---|---|---|---|---|---|---|
| No PRP arm | ||||||
| RECOVERY | Alagorie 2021 | Aflibercept (monthly) | Aflibercept (quarterly) | 40 eyes | PDR | |
| Conference abstracts | ||||||
| Garcia | Garcia-Aguirre 2008 | Bevacizumab | PRP | Mexico | 10 persons | NPDR, PDR |
| Ernst | Ernst 2012 | Bevacizumab | PRP | USA | 10 persons | NPDR, PDR |
| MEDICARE | Dufour 2017 | Aflibercept | PRP | France | 20 persons | PDR |
| Oh | Oh 2014 CA | Bevacizumab (+ PRP) | PRP | South Korea | 125 persons | NPDR, PDR |
| Ramezani | Ramezani 2021 | Bevacizumab (+ PRP) | PRP | Unknown | 153 eyes | PDR |
| Tardieu | Tardieu 2022 | Not stated | PRP | Unknown | 40 persons | PDR |
| Papers in Chinese | ||||||
| Bi | Bi 2020 | Ranibizumab (+ PRP) | PRP | China | 120 persons | Unclear |
| Meng | Meng 2019 | Ranibizumab (+ PRP) | PRP | China | 80 persons | PDR |
| Zhou | Zhou 2017 | Bevacizumab (+ PRP) | PRP | China | 30 persons | Unclear |
| Trials from before 2010 | ||||||
| Cho | Cho 2009–2010 | Bevacizumab (+ PRP) | PRP + Triamcinolone | China | 91 eyes | NPDR, PDR |
| Mirshahi | Mirshahi 2008 | Bevacizumab (+ PRP) | PRP, Sham injection | Iran | 80 eyes | PDR |
| Tonello | Tonelo 2008 | Bevacizumab (+ PRP) | PRP | Brazil | 30 eyes | PDR |
| Unused or unspecified anti-VEGFs | ||||||
| Chen/Zhou | Chen 2017 | Unclear | PRP | China | 120 persons | PDR |
| Gonzalez | Gonzalez 2007/2009/2014 | Pegaptanib sodium | PRP | USA | 20 persons | PDR |
| He | He 2020 | Conbercept (+ PRP) | PRP | China | 44 eyes | PDR |
| Leal | Leal 2013 | Pegaptanib sodium (+ PRP) | PRP | Portugal | 22 persons | PDR |
| Wang | Wang 2019 | Conbercept (+ PRP) | PRP | China | 64 persons | NPDR, PDR |
| No protocol-specified outcomes | ||||||
| Helmy | Helmy 2023 | Ranibizumab | PRP | Egypt | 50 persons | PDR |
| Preti | Preti 2013 | Bevacizumab (+ PRP) | PRP | S. America | 42 persons | PDR |
| Rentiya | Rentiya 2022 | Ranibizumab (+ PRP) | PRP | Brazil | 30 persons | PDR |
Risk-of-bias assessment
| Randomisation process | Deviations from intended interventions | Missing outcome data | ||||
|---|---|---|---|---|---|---|
| Trial | Judgement | Comments | Judgement | Comments | Judgement | Comments |
| Ahmad 201227 | Some concerns | Randomised by ‘simple lottery’. No further details No allocation concealment method reported No evidence of significant differences in key prognostic factors |
Some concerns | No placebo States ‘the physician did not know which eye has been injected’, but the control group did not receive a placebo injection No CONSORT diagram, and no reporting of deviation from the intervention due to the trial context ITT/mITT not reported. The risk that the analysis was not based on ITT principles cannot be excluded |
Low | All participants completed the 90 days follow-up |
| Ali 201828 | Some concerns | States the study is randomised, with allocation by ‘simple lottery method’. No further details No information on whether allocation was concealed |
Some concerns | No placebo. Contralateral design No CONSORT diagram, and no reporting of deviation from the intervention due to the trial context ITT/mITT not reported The risk that the analysis was not based on ITT principles cannot be excluded |
Some concerns | No information on loss to follow-up. No evidence that the result was not biased by any possible missing outcome data Likelihood of significant missingness may be limited by relatively short follow-up duration |
| CLARITY23 | Low | Computer generated with minimisation. Central allocation by trials unit No significant baseline imbalances |
Low | No placebo. ‘The treating ophthalmologists and participants were not masked’ CONSORT diagram reported. No evidence of deviation from intended intervention due to the trial context Analyses conducted according to ITT principles |
Low | Available for 91% (211/232) at 52 weeks Appropriate sensitivity analyses for missing BCVA data with prespecified alternative scenarios were conducted and showed no evidence of bias |
| Ferraz et al. 201532 | Some concerns | Described as randomised. No other details No details on allocation concealment Contralateral design No evidence of significant differences in key prognostic factors |
Some concerns | Placebo controlled. Contralateral design Trial registry entry described as single masked (participants) Masking only reported for outcome assessors (‘examiners’ and participants), not for carers No CONSORT diagram, and no reporting of deviation from the intervention due to the trial context ITT/mITT not reported The risk that the analysis was not based on ITT principles cannot be excluded |
Low | 3% (2/60) eyes excluded due to VH in the control arm. It appears that all other randomised eyes were analysed |
| Marashi 201726 | High | Described as randomised. No other details No details on allocation concealment Eighty per cent had DME at baseline in the IVB arm vs. 20% in the control arm Although the trial is small, the difference is large and considered unlikely to be due to chance alone. No adjustments for baseline imbalance were performed |
Some concerns | No placebo No CONSORT diagram, and no reporting of deviation from the intervention due to the trial context ITT/mITT not reported The risk that the analysis was not based on ITT principles cannot be excluded |
Some concerns | No information on loss to follow-up. Follow-up duration means that the risk of at least some loss to follow-up is high No evidence that the result was not biased by any possible missing outcome data |
| PANORAMA25 | Low | Patients were randomised according to a central randomisation scheme with treatment assignments provided by an interactive voice response system/interactive web response system to the designated study pharmacist (or qualified designee) Some differences in sex at baseline: higher rate of males in 2q16 (56%) and 2q8 (60%) compared with control (52%), but no other imbalances in reported baseline characteristics |
Low | Placebo controlled. Participants, outcome assessors and study personnel were masked throughout the study period, except for study drug administration which was done by an unmasked physician Rates of participants not assessed were higher in the control group (73%) at 100 weeks (and 52 weeks) compared with aflibercept arms (84% and 83%), although participants were masked throughout the study period, and there was no evidence of changes from the intended intervention that occurred because of the trial context All participants analysed. LOCF imputation method used |
Low | Rates of participants not assessed were higher in the control group (73%) at 100 weeks compared with aflibercept arms (84% and 83%) Sensitivity analysis: primary efficacy analysis was also performed using all observed measurements (regardless of whether rescue treatment was given). Protocol also stated that for sensitivity analyses, only true missing values would be imputed using the LOCF procedure, and that baseline values would be carried forward if all post-baseline observations were missing or non-gradable Sensitivity analysis results showed similar results to main analyses for DRSS, although all are based on the LOCF principle, and sensitivity analyses were not performed for BCVA The risk that the higher rate of missingness in the control arm is partly due to its true value cannot be excluded However, due to the size of the difference in missingness, any possible bias arising is likely to be small |
| PRIDE33 | Some concerns | A number of differences in baseline characteristics, including key variables, although differences do not clearly favour one arm and may have occurred by chance. Differences in mean age (ranibizumab: 52.5, PRP: 53, ranibizumab + PRP: 55), age distribution (< 65 years: 86%, 86%, 72%); smoker (14%, 26%, 35%); duration of diabetes (25 years, 23 years, 21 years), Mean mm2 NVD + NVE: 9.4, 5.4, 4.1; ETDRS: 83.3, 80.5, 80.0 | Low | No masking Analyses conducted based on ITT principle, using LOCF |
Some concerns | 23% (25/108) of randomised participants not measured at 12 months No significant differences in rates of missingness across groups |
| PROTEUS34 | Low | Computer-generated block randomisation. Central allocation implemented through electronic platform Large and statistically significant difference in mean age [ranibizumab + PRP: 58.8 years (13.3), PRP: 52.0 (11.9)]. Non-statistically significant difference in sex (31.7% vs. 41.3% female) Difference in time since diagnosis not reported In a multivariate analysis, ‘age, HbA1c, and number of PRP treatments did not show a significant association with BCVA difference from baseline to month 12’ Re-analysis with IPD provided by trialist suggested low concerns |
Low | CONSORT diagram reported. No evidence of deviation from intended intervention due to trial context ITT-principle-based primary analysis |
Some concerns | |
| Protocol S | Low | Permuted block randomisation. Stratification by site and presence of centrally involved DME Central allocation concealment with web-based tool from trials unit No evidence of baseline imbalances |
Low | No placebo. Masking only for outcome assessors All eyes randomised received the treatment allocated Analyses conducted according to ITT principles |
Low | 83% (382/394) completed 2-year follow-up. Of those, 5% (18/394) died, 12% (48/394) withdrew or missed their visit For missing data at 2 years, statistical analysis plan reports ‘Markov chain Monte Carlo multiple imputation-based on treatment group, the randomisation stratification factors, and all available visual acuity data from assessment visits prior to 2 years’ |
| Protocol W24 | Low | Central, web-based (DRCR network) randomisation, stratified by DR severity level No evidence of baseline imbalances |
Low | Placebo controlled. Participants masked. Investigators and study co-ordinators unmasked CONSORT diagram reported. No evidence of deviation from intended intervention due to trial context Analyses conducted according to ITT principles |
Low | 68.5% (137/200, or 74.9% excluding 17 deaths) completed their 4-year visit in intervention arm, vs. 67.3% (134/199, 73.2% excluding 16 deaths) Multiple imputation (Markov model) used for missing data (assumes data are missing at random). Model included treatment group, study eye laterality, baseline DRSS, baseline visual acuity and change in visual acuity from baseline to each protocol assessment visit up to and including 4 years. Missingness documented, balanced between arms and unlikely to depend on its true value |
| Rebecca 202129 | Some concerns | Described as randomised. No other details No details on allocation concealment No evidence of significant differences in key prognostic factors |
Some concerns | No placebo No CONSORT diagram, and no reporting of deviation from the intervention due to the trial context ITT/mITT not reported The risk that the analysis was not based on ITT principles cannot be excluded |
Some concerns | No information on loss to follow-up. Follow-up duration means that the risk of at least some loss to follow-up is high No evidence that the result was not biased by any possible missing outcome data |
| Roohipoor 201930 | Some concerns | Random block method, but no further details on how allocation sequence was generated. No information on allocation concealment No evidence of significant differences in key prognostic factors |
Some concerns | No placebo. Contralateral design No CONSORT diagram, and no reporting of deviation from the intervention due to the trial context ITT/mITT not reported The risk that the analysis was not based on ITT principles cannot be excluded |
Some concerns | Significant loss to follow-up. Only 59% (19 out of 32) completed 10 months follow-up No evidence that the result was not biased by missing outcome data Reasons for loss to follow-up were not reported. The risk that at least some missingness could be due to visual acuity outcomes cannot be excluded |
| Sao Paulo A36 | Some concerns | Randomised based on a computer-generated sequence. No further details reported There were differences in age (mean PASCAL arm age was 7.5 years older than ranibizumab and 2.2 years older than ETDRS), although they were not statistically significant |
Some concerns | No placebo No evidence of deviation from the intervention due to the trial context ITT/mITT not explicitly reported |
Some concerns | 13/48 (27%) withdrew. No significant difference in withdrawal between arms No evidence that the result was not biased by missing outcome data Reasons for loss to follow-up were not reported. The risk that at least some missingness could be due to visual acuity outcomes cannot be excluded |
| Sao Paulo B35 | Some concerns | Block randomisation (blocks of 2), allocation drawn randomly by technician from one of two identical opaque envelopes. No further information on randomisation and allocation concealment No evidence of significant differences in key prognostic factors |
Some concerns | No placebo No CONSORT diagram, and no reporting of deviation from the intervention due to the trial context ITT/mITT not reported The risk that the analysis was not based on ITT principles cannot be excluded |
Some concerns | Only 72.5% (29/40) participants analysed at 48 weeks No evidence that the result was not biased by missing outcome data Significant loss to follow-up. Reported reasons for loss to follow-up were generally appropriate (incl. four deaths and two ocular events, four did not return for assessment, one not specified). No clear imbalances between arms |
| Trial | Measurement of the outcome | Selection of the reported result | Overall bias | ||
|---|---|---|---|---|---|
| Judgement | Comments | Judgement | Comments | Judgement | |
| Ahmad 201227 | High | Snellen chart, converted to log-MAR Participants unmasked (no placebo). No mention of blinding of outcome assessors Participants and study personnel may have been influenced by knowledge of the intervention |
Some concerns | Insufficient information about analysis plans | High |
| Ali 201828 | High | Appears to be ETDRS, standard scale No placebo |
Some concerns | No protocol | High |
| CLARITY23 | Some concerns | ETRDS, standard scale The lack of blinding of participants means raises some concerns, although appropriate steps were taken to mask the optometrists assessing BCVA Optometrists ‘masked to treatment allocation throughout the study. The optometrists received the participants into the visual acuity lanes with a visual acuity-specific source data worksheet that included the PIN and details of the study eye and non-study eye to be refracted, but with no previous records or case report forms by which the patient’s treatment arm could be identified’ |
Low | A SAP ‘was finalised before data lock and agreed with oversight committees’ | Low |
| Ferraz 201532 | Low | ETDRS Outcome assessors masked throughout the study period |
Some concerns | Insufficient information about analysis plans. Outcome retrospectively reported in trial registry | Some concerns |
| Marashi 201726 | High | Snellen scale, converted to log-MAR No placebo Participants and study personnel may have been influenced by knowledge of the intervention |
Low | Protocol registered around time of study start, and prespecified outcome and time point were reported | High |
| PANORAMA25 | Low | ETRDS method Outcome assessors were masked throughout the study period |
Low | Low | |
| PRIDE33 | High | ETDRS, standard. No masking of outcome assessors | Low | SAP not mentioned Protocol registered before time of study start, and prespecified outcome and time point were reported | Some concerns |
| PROTEUS34 | High | Standard ETDRS No placebo. Participants and outcome assessors were aware of the intervention Participants and study personnel may have been influenced by knowledge of the intervention |
Low | No SAP Outcome and follow-up specified in prospectively registered protocol | Some concerns |
| Protocol S31 | Some concerns | E-ETDRS Participants unmasked (no placebo), but protocol states that ‘visual acuity testers [.] will be masked to treatment group at annual visits’ |
Low | SAP v1.0 is dated March 2015 Protocol first published December 2011, primary completion dated January 2015 Outcome specified in prospectively registered protocol |
Low |
| Protocol W24 | Low | DRSS Outcome assessors masked |
Low | SAP reported and finalised before unblinded outcome data were available for analysis | Low |
| Rebecca 202129 | High | BCVA. Scale not reported, but standard outcome No placebo. Participants and outcome assessors were aware of the intervention Participants and study personnel may have been influenced by knowledge of the intervention |
Some concerns | Insufficient information about analysis plans | High |
| Roohipoor 2019 | High | BCVA measured using standard Snellen chart No placebo Participants and study personnel may have been influenced by knowledge of the intervention |
Some concerns | SAP not mentioned in protocol or publication. 10 months follow-up assessment was not pre-specified (unlike 6 months) | High |
| Sao Paulo A | High | Standard ETDRS No placebo. Participants were aware of the intervention. No masking of outcome assessor reported |
Some concerns | No SAP Outcome and follow-up specified in protocol, but unclear if prospectively registered | High |
| Sao Paulo B | High | ETDRS, converted to log-MAR No blinding of outcome assessor, who performed the interventions Participants and study personnel may have been influenced by knowledge of the intervention |
Some concerns | Insufficient information about analysis plans | High |
Appendix 2 Proliferative diabetic retinopathy: all best corrected visual acuity analyses
All figures and tables relate to the trials of PDR, excluding the two trials (PANORAMA, Protocol W) of non-proliferative retinopathy. For their results, see Appendix 4.
FIGURE 9.
All ETDRS data (as mean change from baseline) by drug and type of intervention.
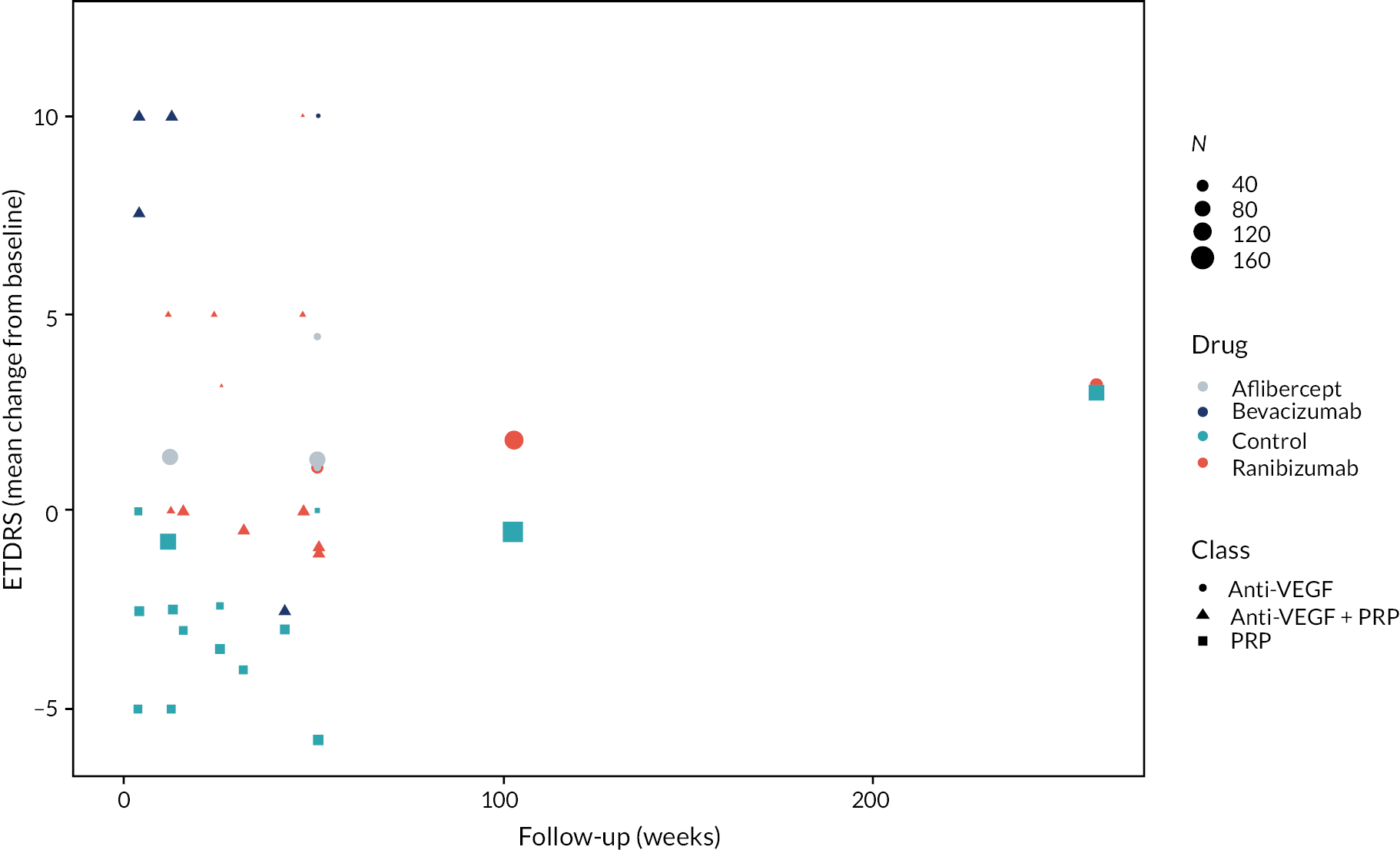
FIGURE 10.
All ETDRS data (as mean change from baseline) by trial and drug type.
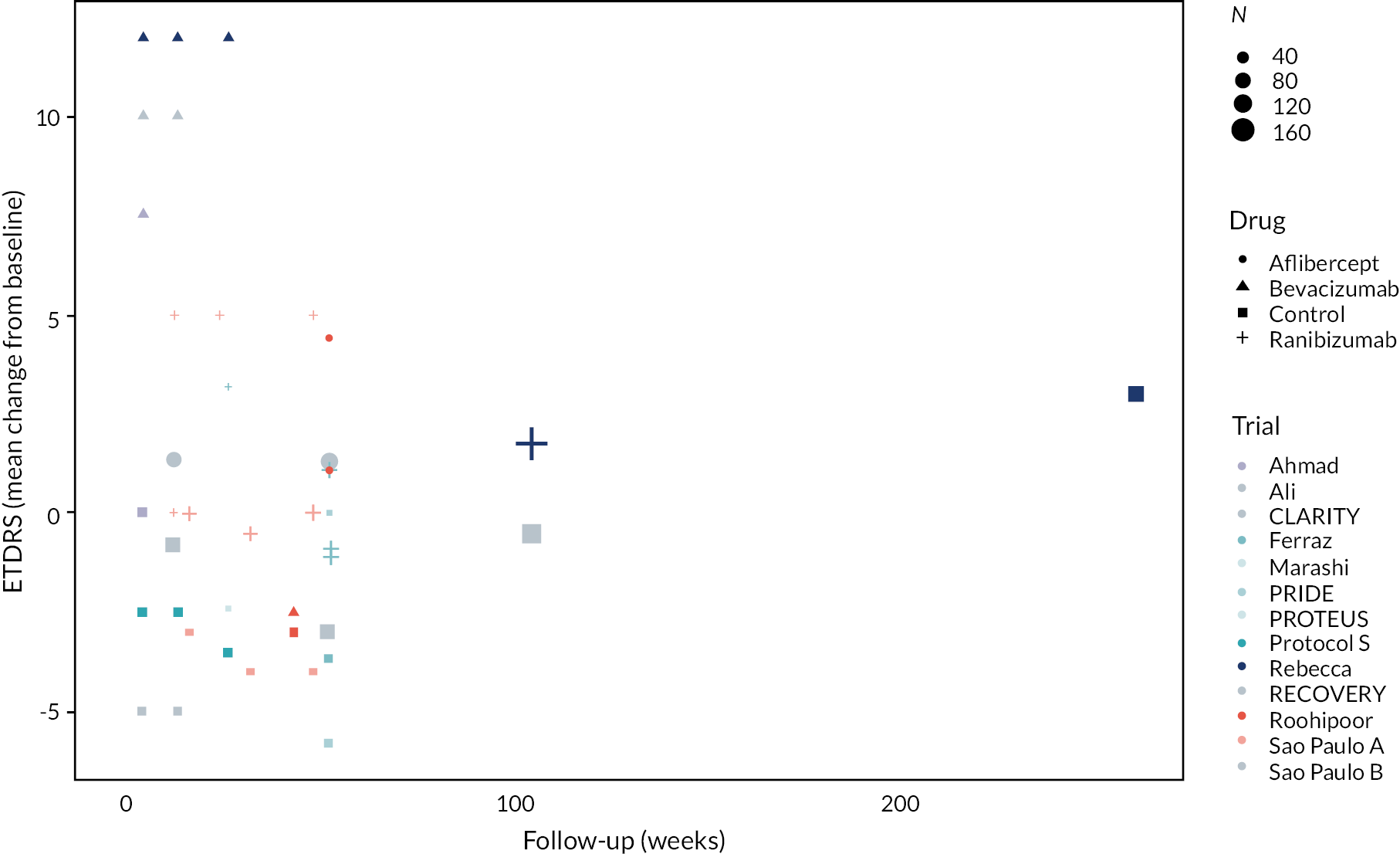
FIGURE 11.
Mean difference in ETDRS between anti-VEGF and control arms over time.
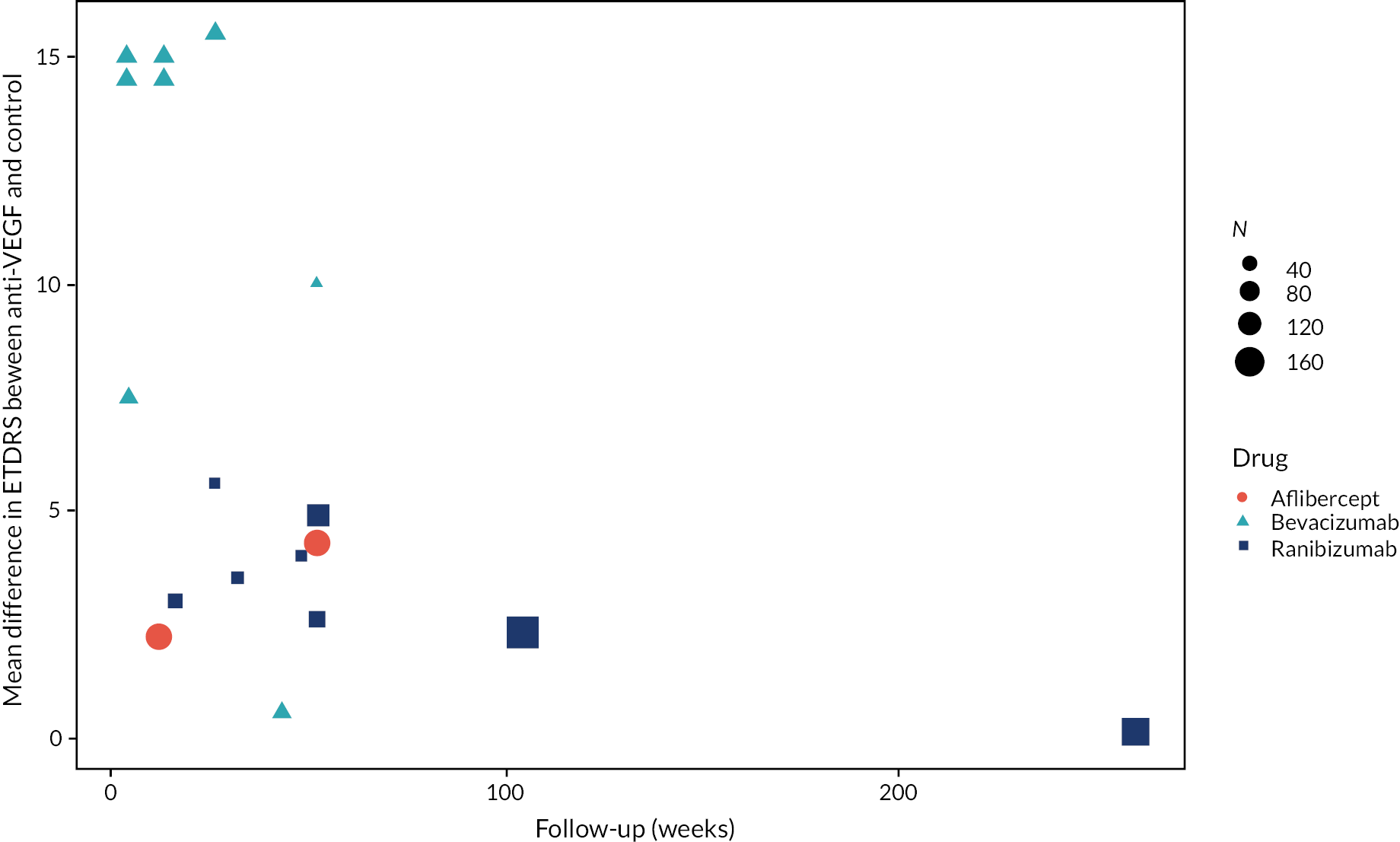
FIGURE 12.
Mean difference between anti-VEGF and control arms by ETDRS at randomisation.
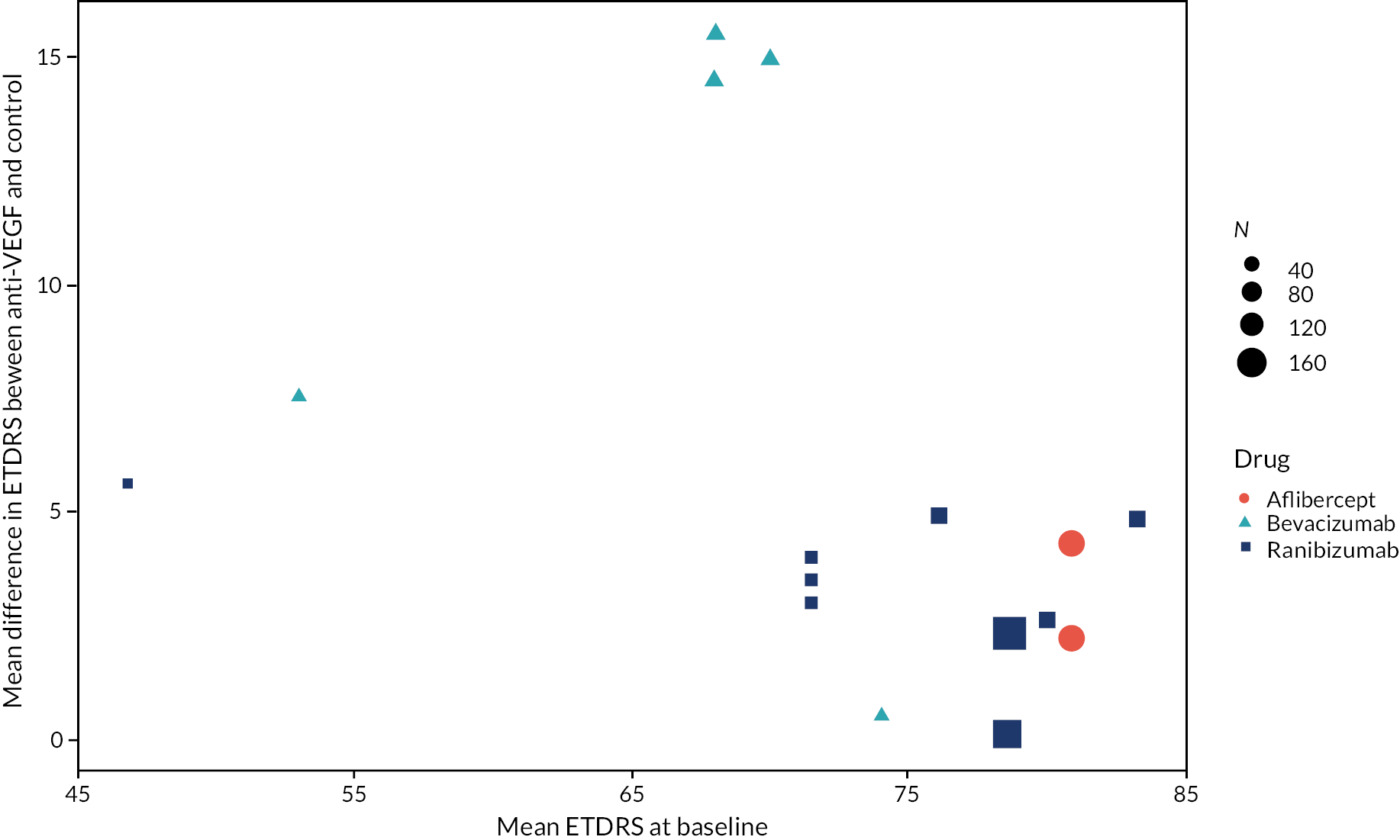
Figures and forest plots summarising best corrected visual acuity data
Note from these figures that there appears to be a possible decline in benefit to vison over time, and that the benefit of ant-VEGF may be greater in people with poorer initial vision, but these differences may be confounded by differences between types of anti-VEGF.
FIGURE 13.
Forest plot of all mean differences in ETDRS between anti-VEGF and control (right side favours anti-VEGF).
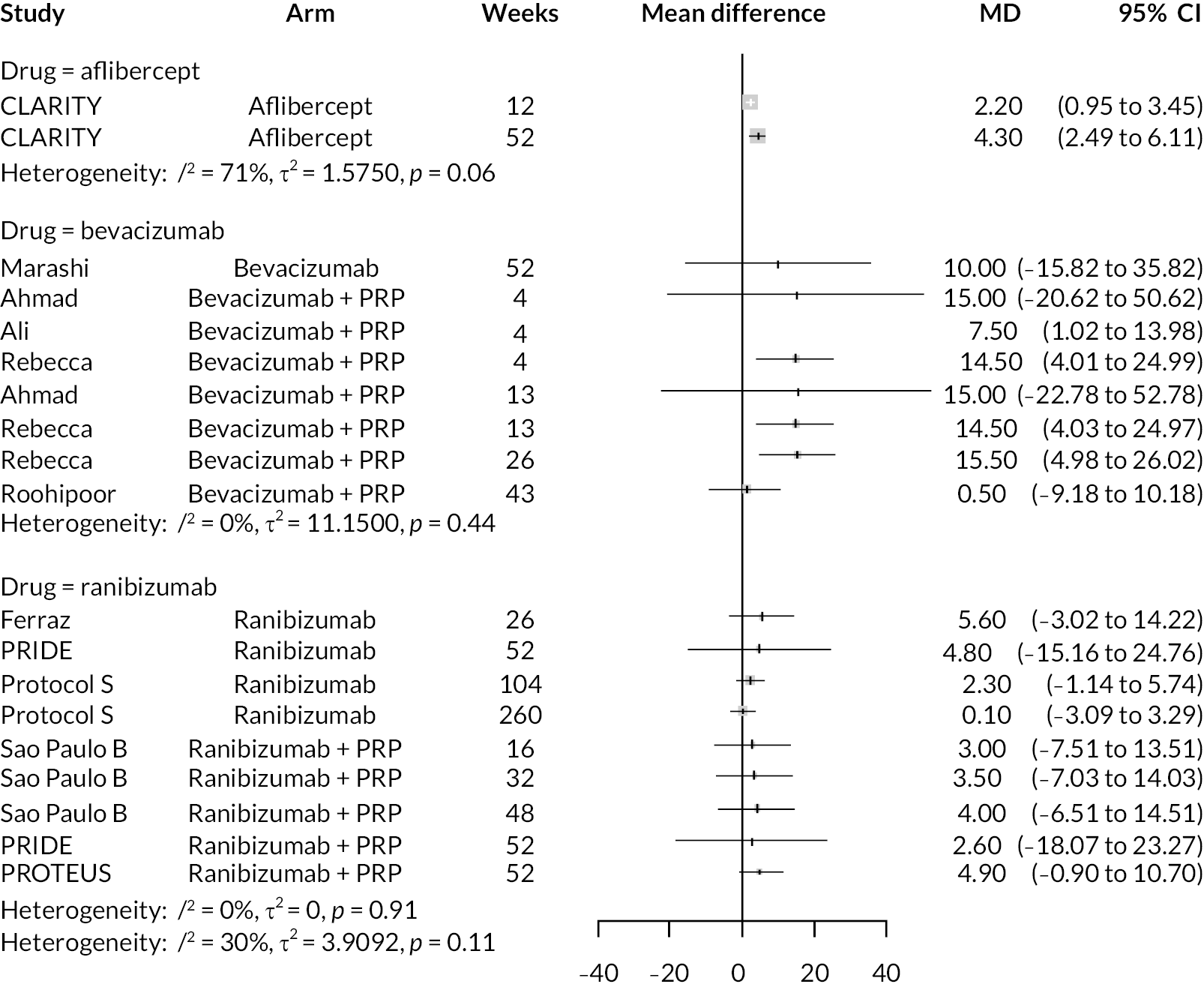
FIGURE 14.
Forest plot of all mean differences in log-MAR between anti-VEGF and control (left side favours anti-VEGF).
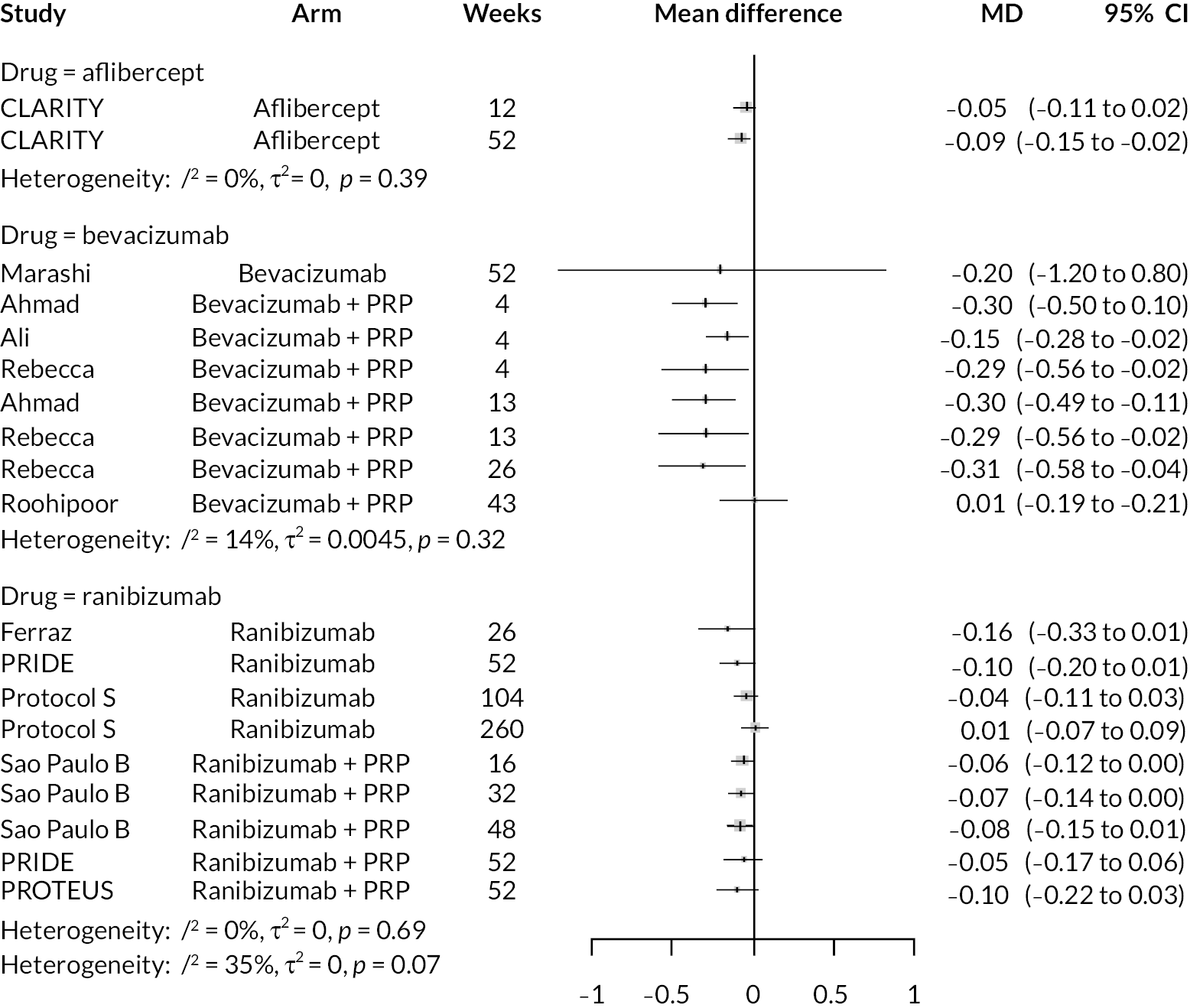
Forest plots of meta-analyses of best corrected visual acuity
Up to 1 year
FIGURE 15.
Meta-analysis of mean differences in ETDRS between anti-VEGF and control up to 1 year of follow-up (right side favours anti-VEGF).
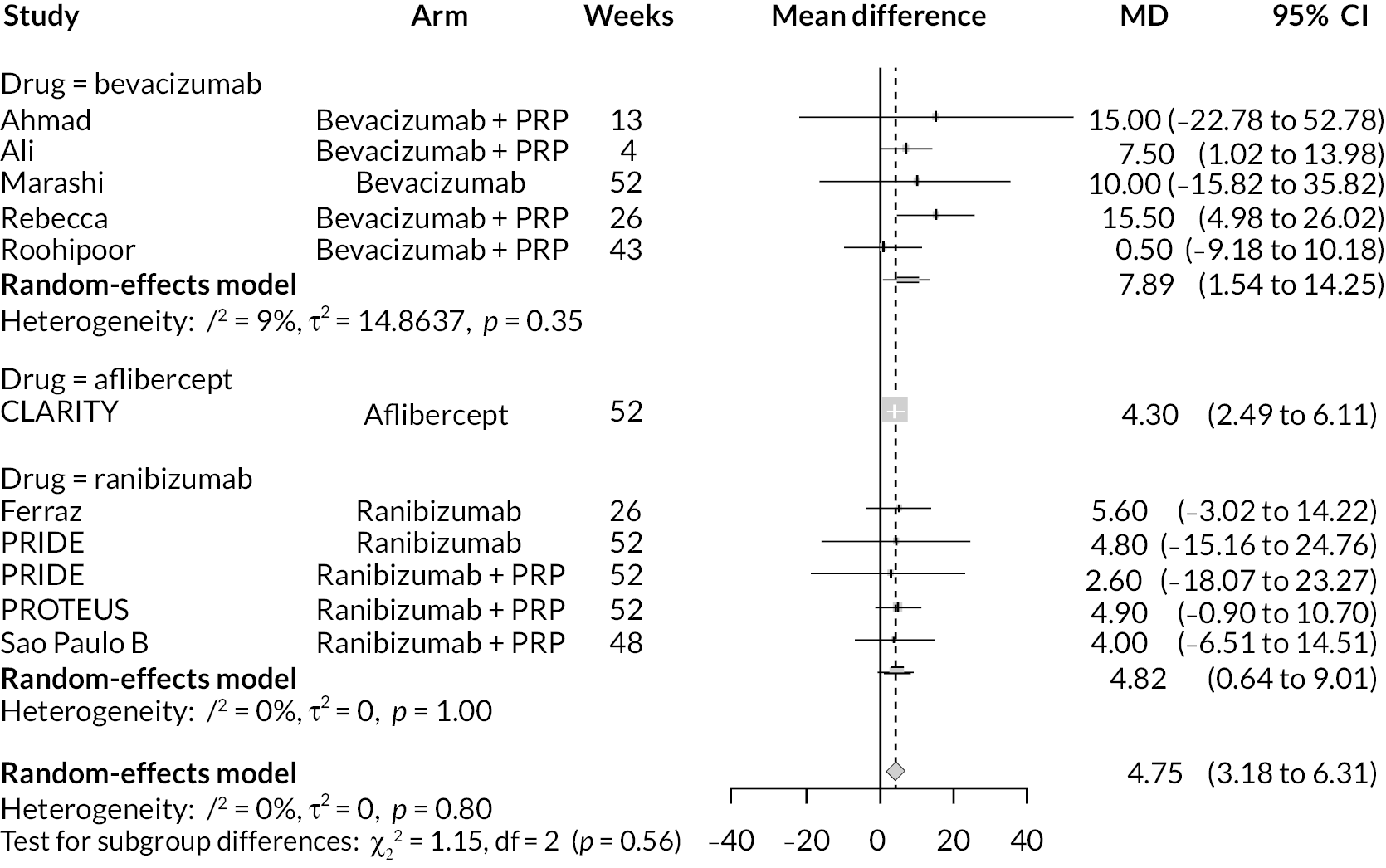
FIGURE 16.
Meta-analysis of mean differences in log-MAR between anti-VEGF and control up to 1 year of follow-up (left side favours anti-VEGF).
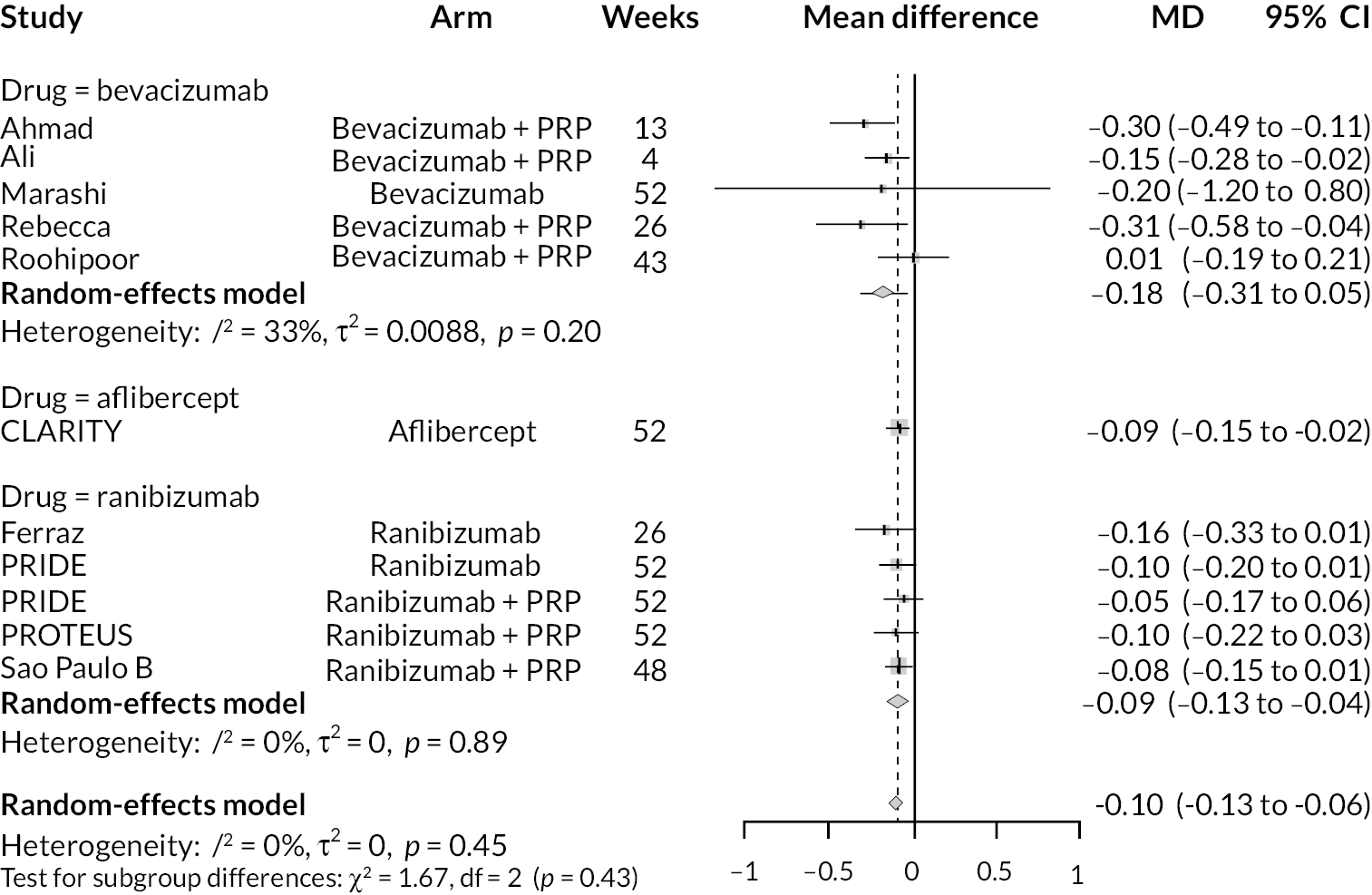
One to 2 years’ follow-up
FIGURE 17.
Meta-analysis of mean differences in ETDRS between anti-VEGF and control with 1–2 years’ of follow-up (right side favours anti-VEGF).
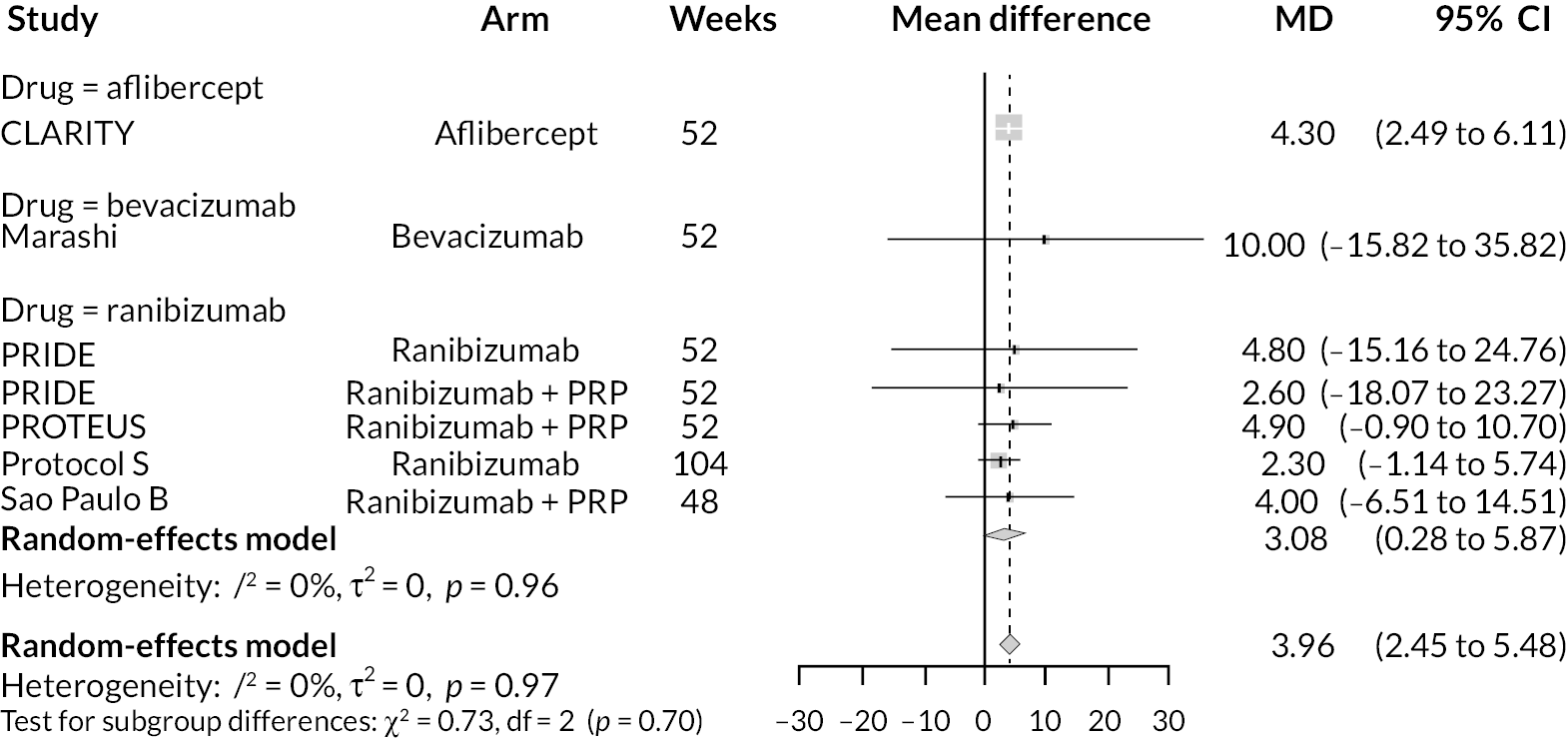
FIGURE 18.
Meta-analysis of mean differences in log-MAR between anti-VEGF and control with 1–2 years’ of follow-up (left side favours anti-VEGF).
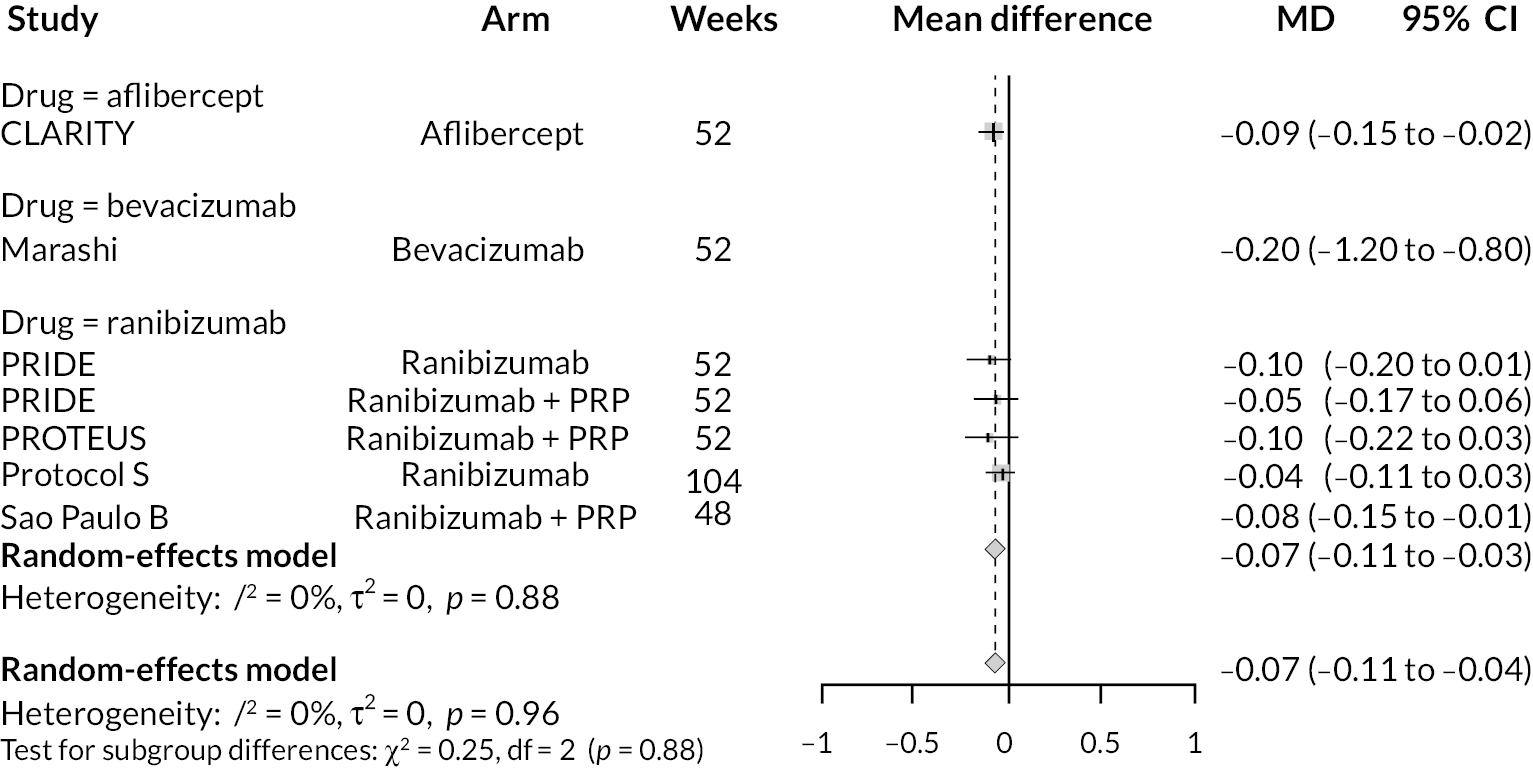
Maximum follow-up in trial (up to 2 years)
FIGURE 19.
Meta-analysis of mean differences in ETDRS between anti-VEGF and control at end of trial (right side favours anti-VEGF).
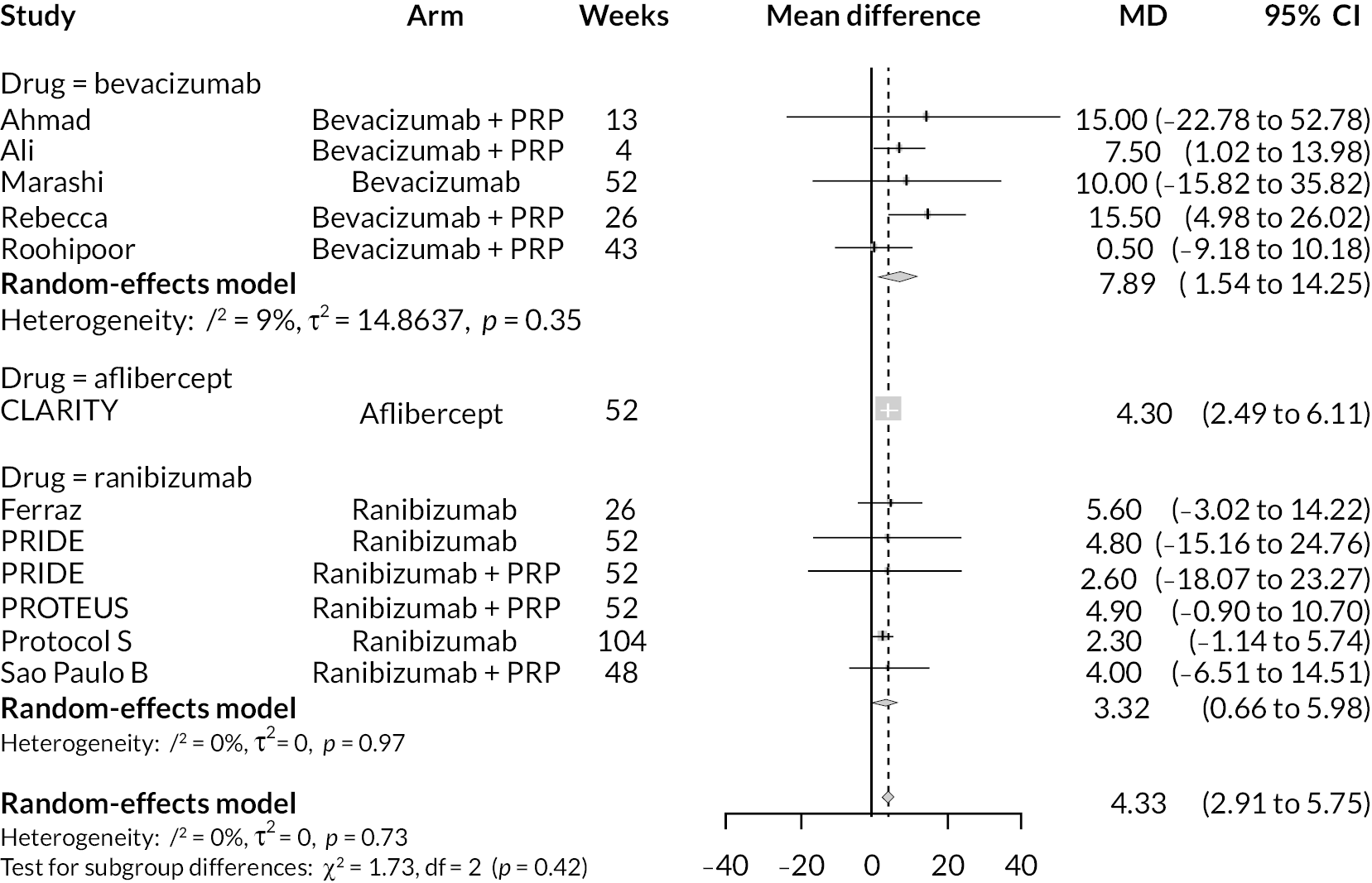
FIGURE 20.
Meta-analysis of mean differences in log-MAR between anti-VEGF and control at end of trial (left side favours anti-VEGF).
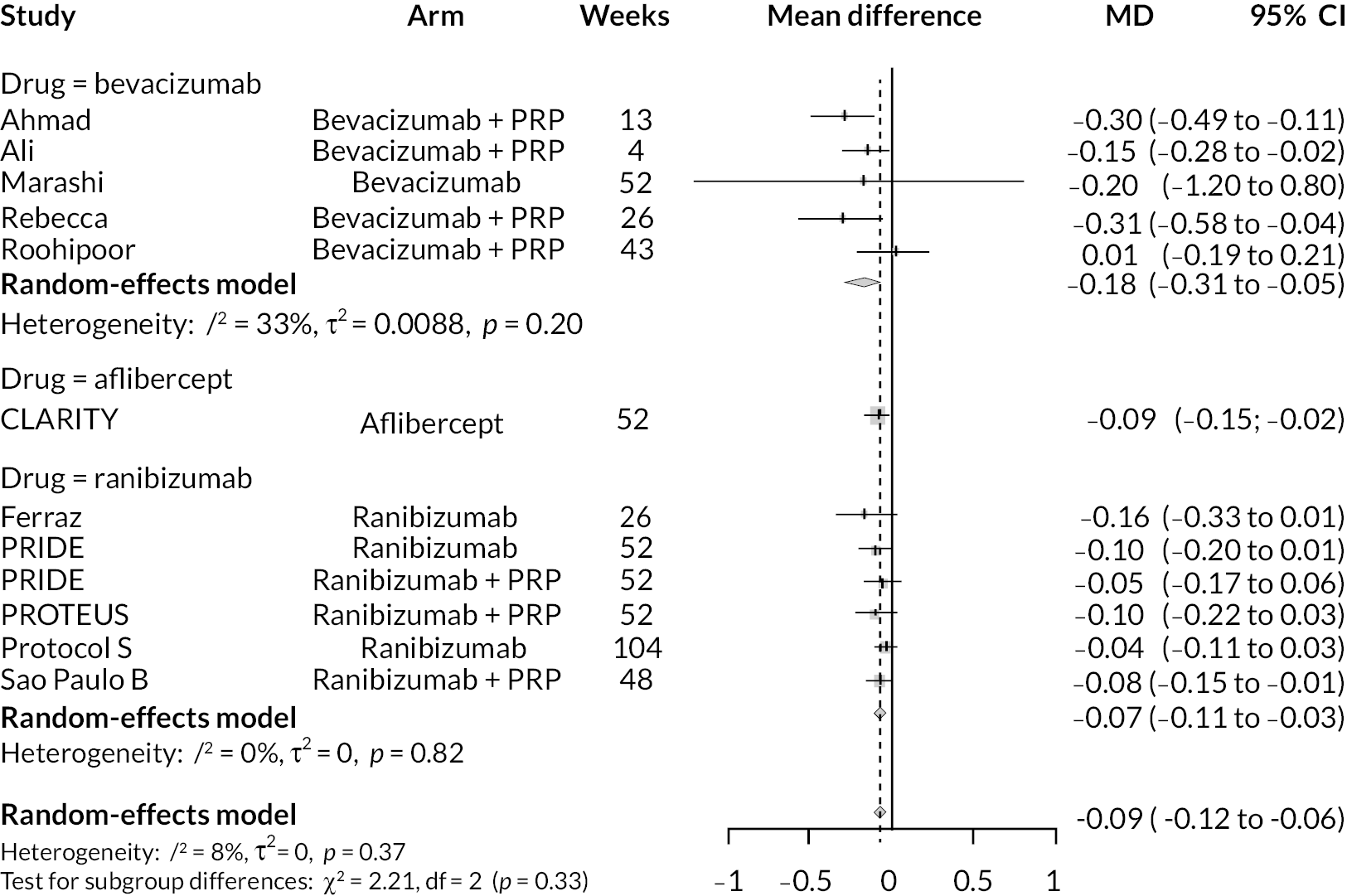
Network meta-analyses of best corrected visual acuity (using logarithm of the minimum angle of resolution)
Note: From this point forward on, meta-analyses of BCVA measured using log-MAR are presented. Some analyses using ETDRS were performed but are not included here. Similarly, only random-effects analyses are presented for simplicity, as differences between random- and fixed-effect analyses were minimal.
Analyses at up to 1 year of follow-up
FIGURE 21.
Network diagram of BCVA at up to 1 year of follow-up.
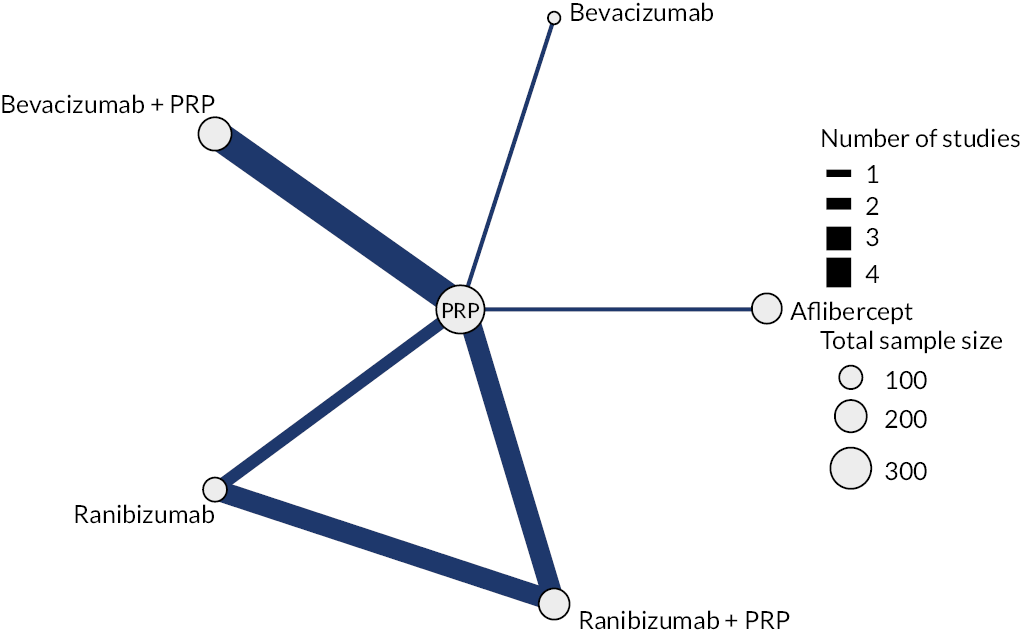
FIGURE 22.
All treatment comparisons for 1-year random-effects NMA of log-MAR.
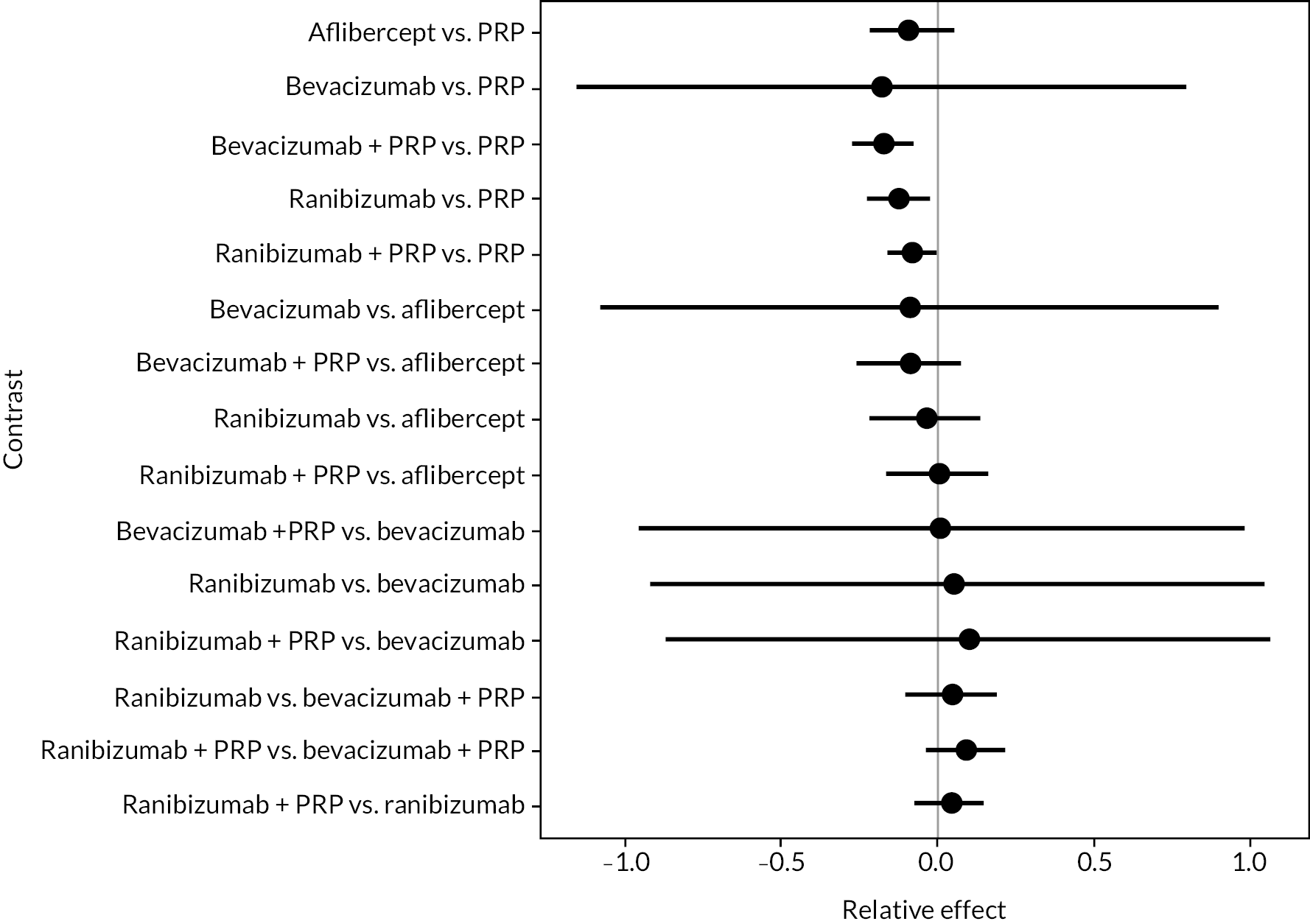
FIGURE 23.
Probability of treatments for 1-year random-effects NMA of log-MAR.
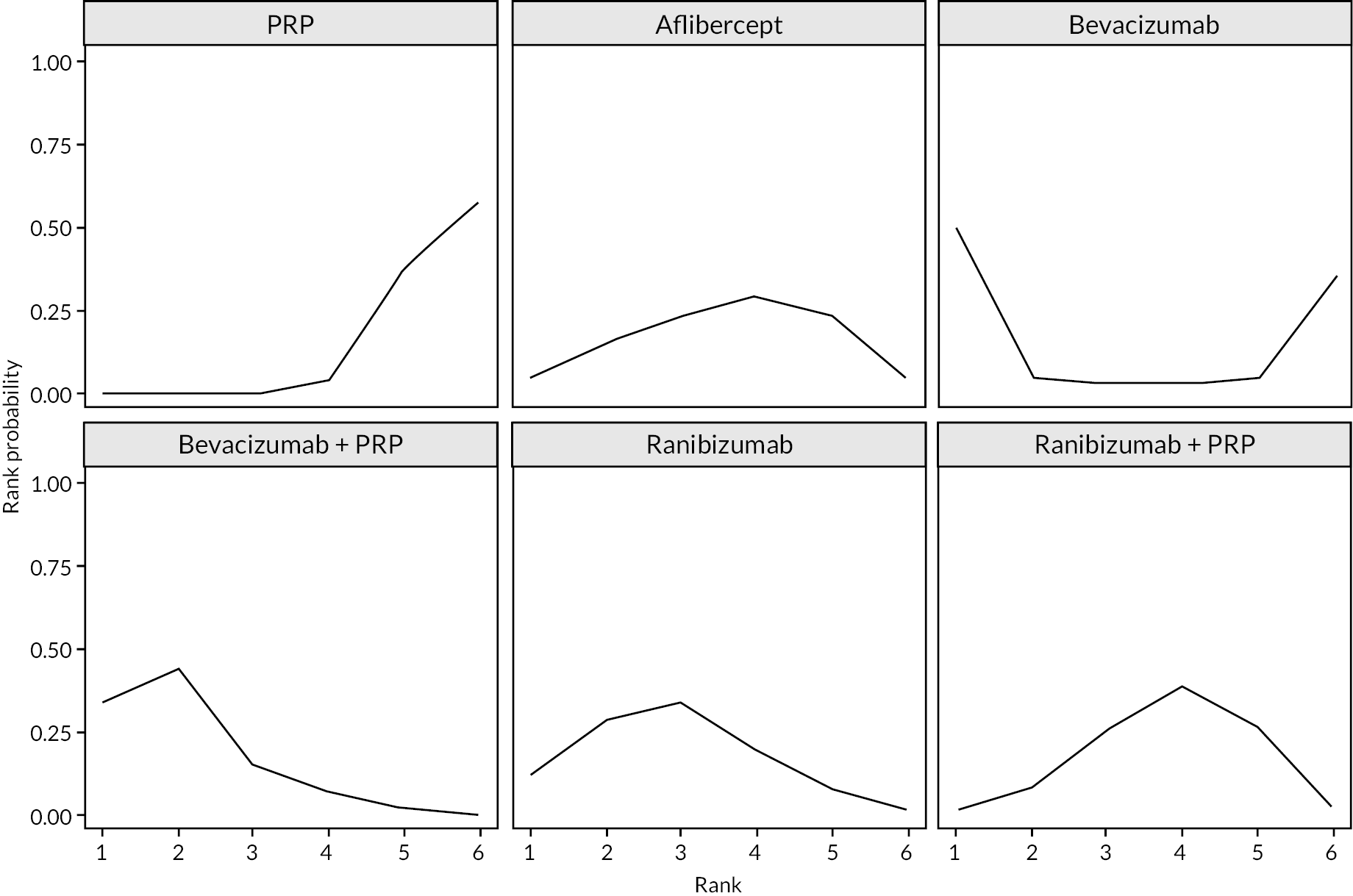
| Intervention | Comparator | Mean difference | 95% CI | |
|---|---|---|---|---|
| Aflibercept | PRP | −0.084 | −0.222 | 0.056 |
| Bevacizumab | PRP | −0.198 | −1.213 | 0.785 |
| Bevacizumab + PRP | PRP | −0.172 | −0.279 | −0.069 |
| Ranibizumab | PRP | −0.121 | −0.233 | −0.006 |
| Ranibizumab + PRP | PRP | −0.078 | −0.165 | 0.013 |
| Bevacizumab | Aflibercept | −0.115 | −1.142 | 0.853 |
| Bevacizumab + PRP | Aflibercept | −0.088 | −0.273 | 0.082 |
| Ranibizumab | Aflibercept | −0.037 | −0.213 | 0.130 |
| Ranibizumab + PRP | Aflibercept | 0.006 | −0.151 | 0.173 |
| Bevacizumab + PRP | Bevacizumab | 0.026 | −0.947 | 1.027 |
| Ranibizumab | Bevacizumab | 0.077 | -0.913 | 1.098 |
| Ranibizumab + PRP | Bevacizumab | 0.121 | −0.867 | 1.151 |
| Ranibizumab | Bevacizumab + PRP | 0.051 | −0.095 | 0.208 |
| Ranibizumab + PRP | Bevacizumab + PRP | 0.094 | −0.040 | 0.236 |
| Ranibizumab + PRP | Ranibizumab | 0.043 | −0.067 | 0.160 |
| Treatment arm | Probability of ranking | |||||
|---|---|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | 5th (%) | 6th (%) | |
| PRP | 0.00 | 0.13 | 0.65 | 4.93 | 37.65 | 56.65 |
| Aflibercept | 4.33 | 12.65 | 24.65 | 30.48 | 23.10 | 4.80 |
| Bevacizumab | 50.73 | 5.05 | 2.38 | 2.63 | 3.83 | 35.40 |
| Bevacizumab + PRP | 33.60 | 44.23 | 14.73 | 5.68 | 1.73 | 0.05 |
| Ranibizumab | 10.25 | 30.30 | 33.60 | 17.40 | 7.25 | 1.20 |
| Ranibizumab + PRP | 1.10 | 7.65 | 24.00 | 38.90 | 26.45 | 1.90 |
Analyses at 1–2 years’ follow-up
FIGURE 24.
Network diagram of BCVA at up to 1–2 years of follow-up.
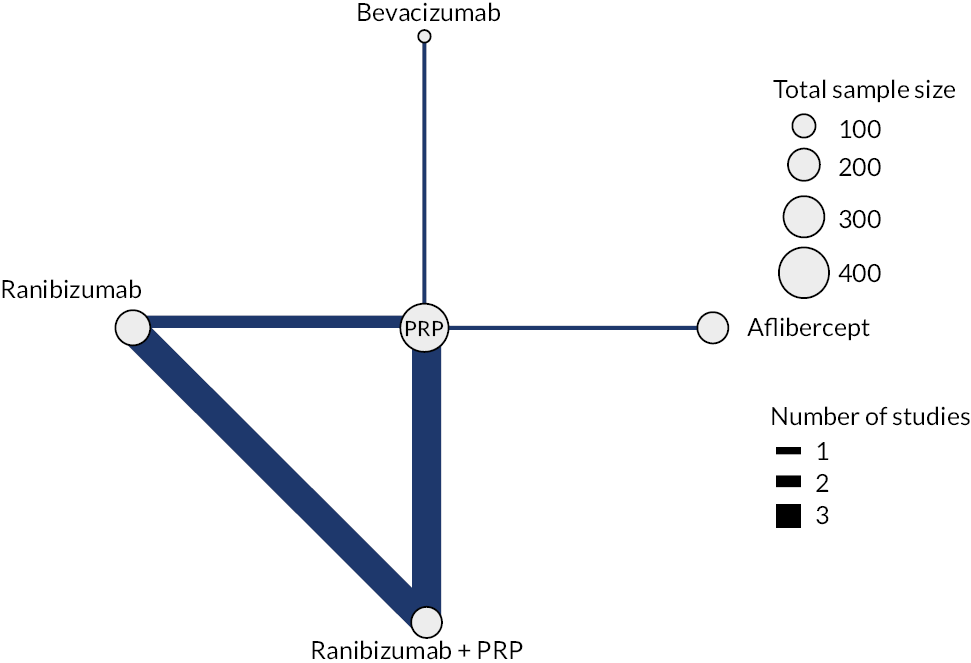
FIGURE 25.
All treatment comparisons for 1–2 year random-effects NMA of log-MAR.
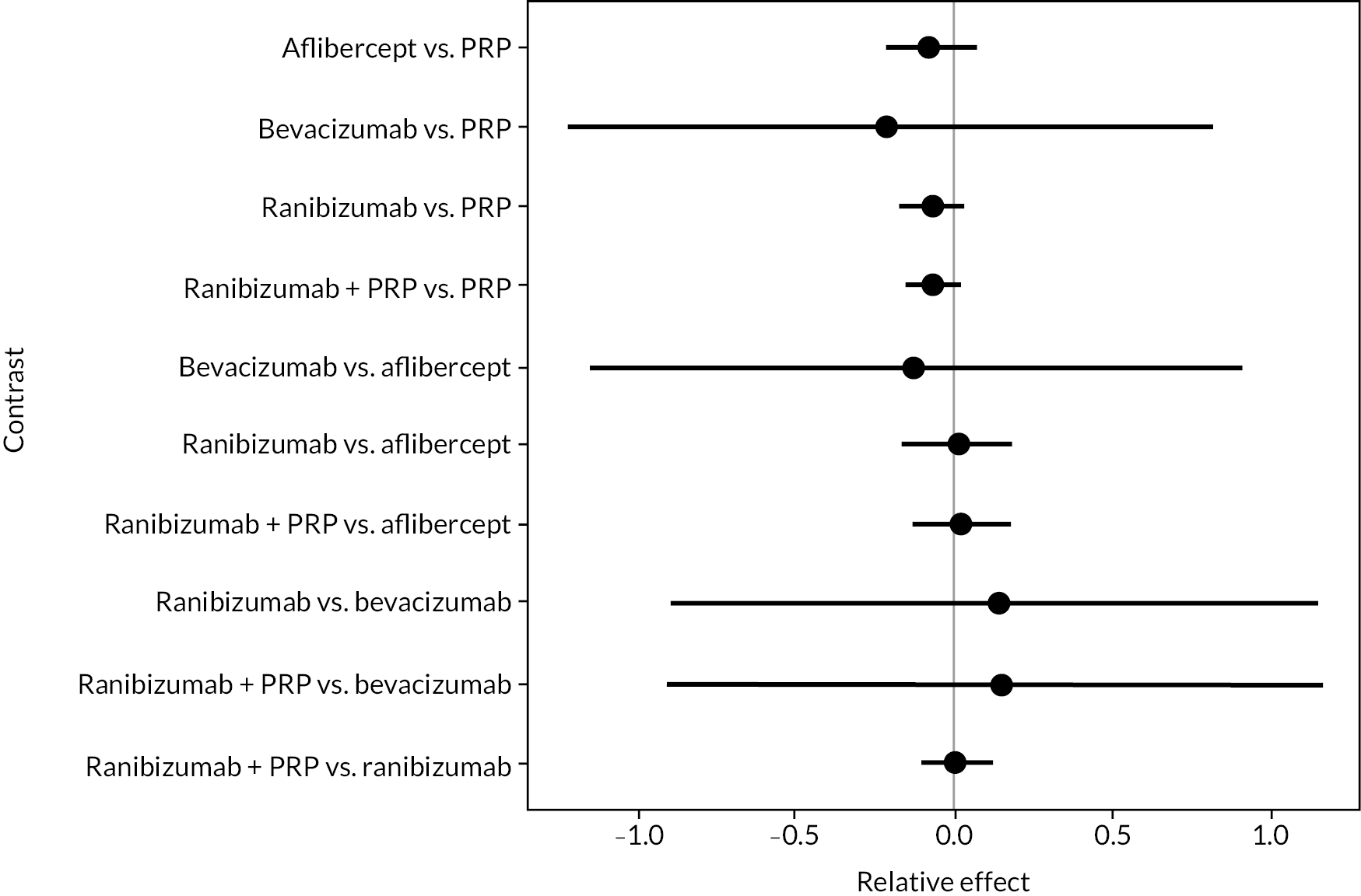
FIGURE 26.
Probability of treatments for 1–2-year random-effects NMA of log-MAR.
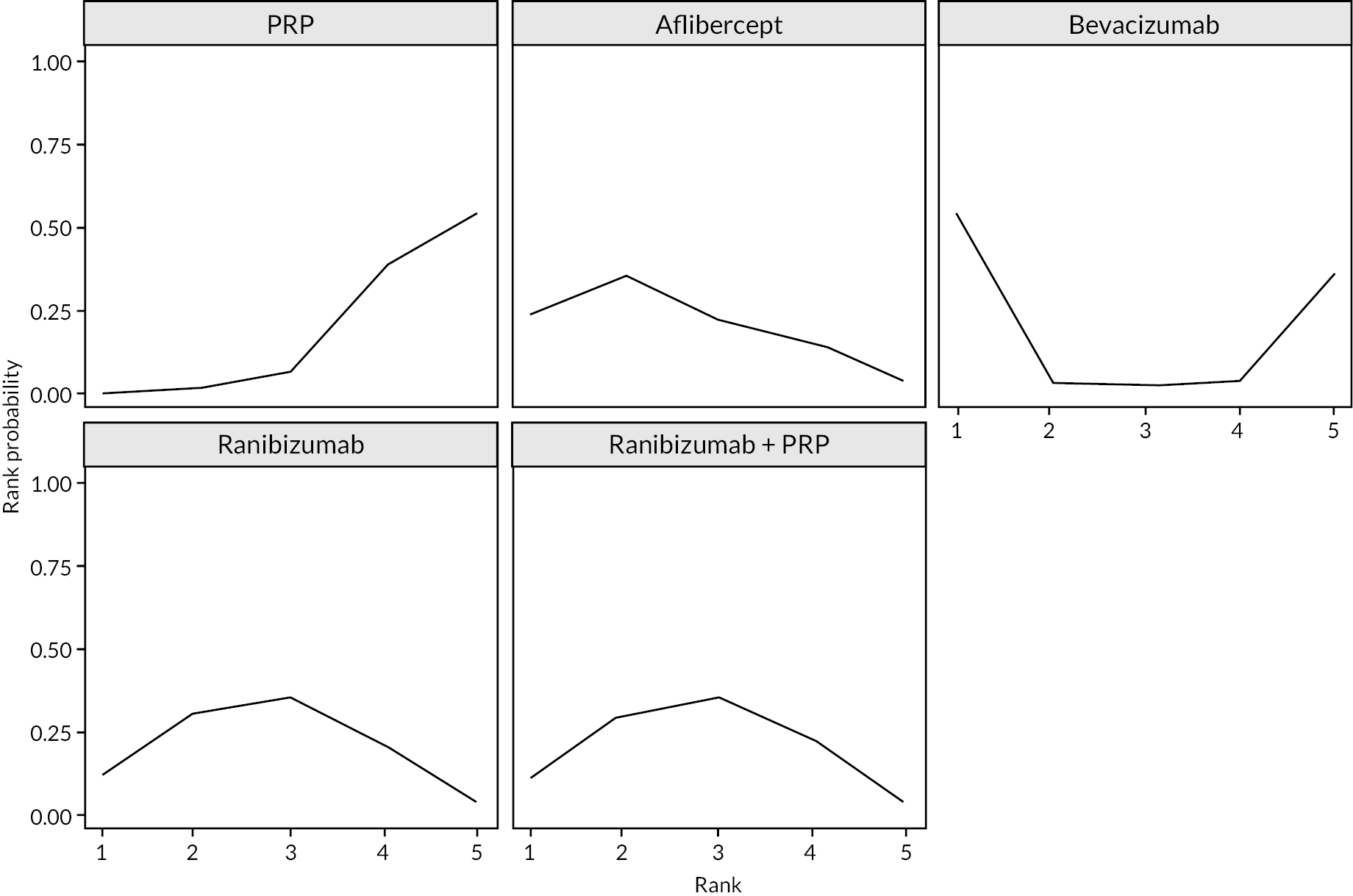
| Intervention | Comparator | Mean difference | 95% CI | |
|---|---|---|---|---|
| Aflibercept | PRP | −0.080 | −0.225 | 0.100 |
| Bevacizumab | PRP | −0.182 | −1.181 | 0.816 |
| Ranibizumab | PRP | −0.072 | −0.171 | 0.017 |
| Ranibizumab + PRP | PRP | −0.068 | −0.152 | 0.020 |
| Bevacizumab | Aflibercept | −0.102 | −1.095 | 0.899 |
| Ranibizumab | Aflibercept | 0.008 | −0.200 | 0.187 |
| Ranibizumab + PRP | Aflibercept | 0.012 | −0.174 | 0.189 |
| Ranibizumab | Bevacizumab | 0.110 | −0.887 | 1.104 |
| Ranibizumab + PRP | Bevacizumab | 0.114 | −0.885 | 1.112 |
| Ranibizumab + PRP | Ranibizumab | 0.004 | −0.100 | 0.114 |
| Treatment arm | Probability of ranking | ||||
|---|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | 5th (%) | |
| PRP | 0.05 | 1.20 | 6.25 | 37.93 | 54.58 |
| Aflibercept | 21.38 | 34.58 | 20.98 | 17.00 | 6.08 |
| Bevacizumab | 56.20 | 3.15 | 2.88 | 3.35 | 34.43 |
| Ranibizumab | 13.13 | 30.28 | 34.13 | 20.15 | 2.33 |
| Ranibizumab + PRP | 9.25 | 30.80 | 35.78 | 21.58 | 2.60 |
Analysis at maximum follow-up time (up to 2 years)
FIGURE 27.
All treatment comparisons for end-of-trial random-effects NMA of log-MAR.
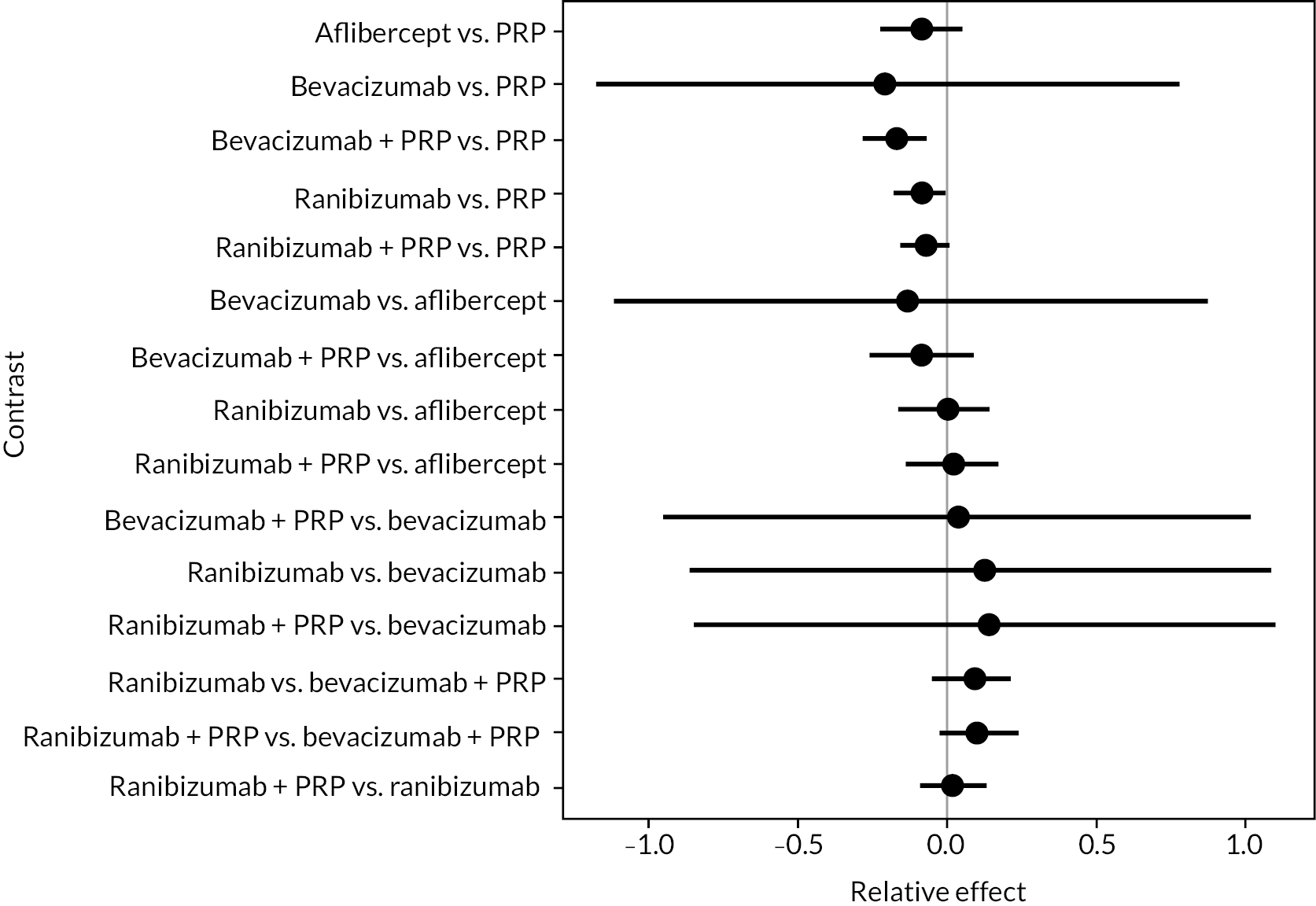
FIGURE 28.
Probability of treatments for end-of-trial random-effects NMA of log-MAR.
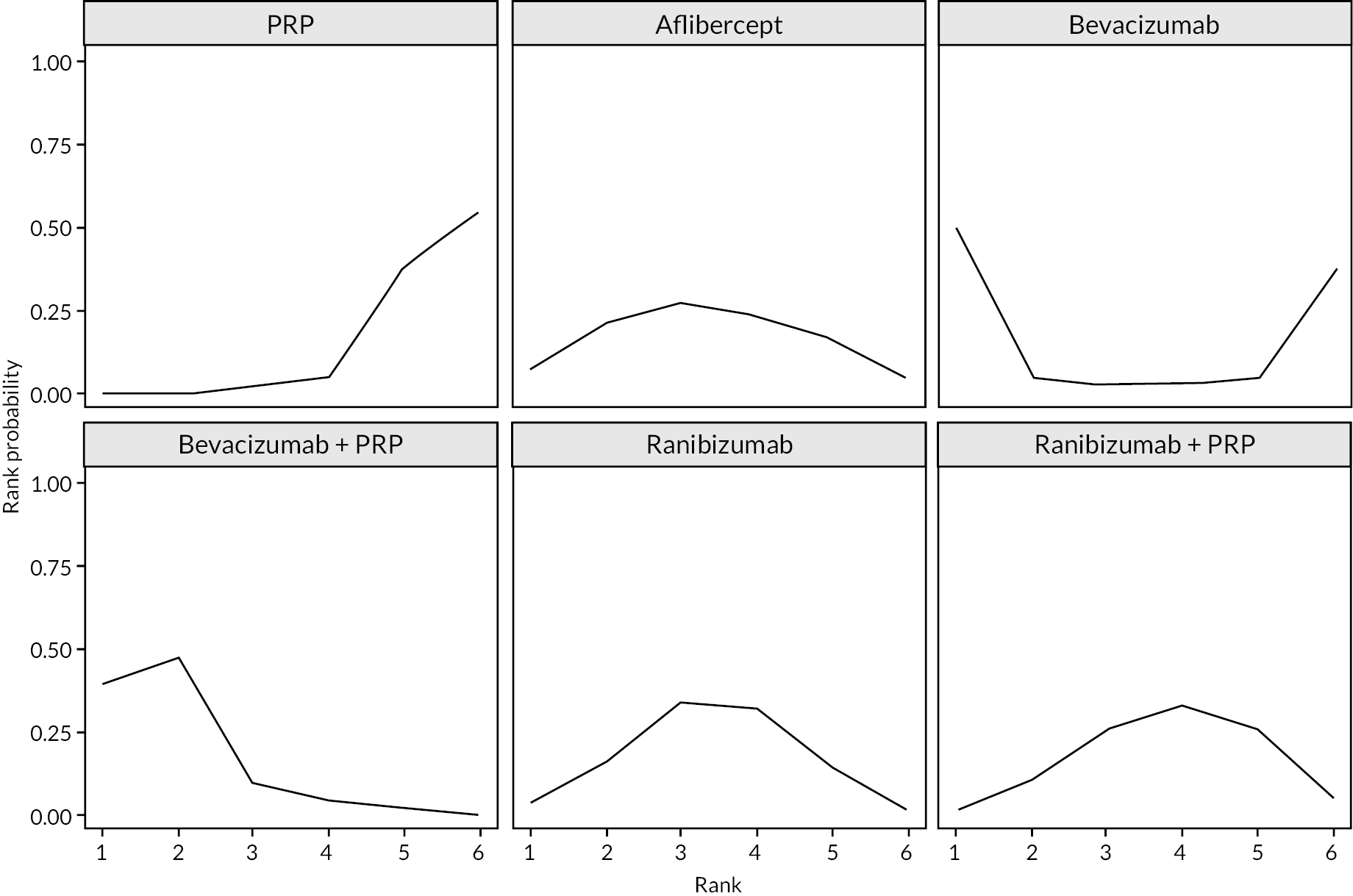
| Intervention | Comparator | Mean difference | 95% CI | |
|---|---|---|---|---|
| Aflibercept | PRP | −0.087 | −0.228 | 0.049 |
| Bevacizumab | PRP | −0.209 | −1.176 | 0.782 |
| Bevacizumab + PRP | PRP | −0.171 | −0.284 | −0.064 |
| Ranibizumab | PRP | −0.085 | −0.177 | −0.004 |
| Ranibizumab + PRP | PRP | −0.069 | −0.151 | 0.016 |
| Bevacizumab | Aflibercept | −0.122 | −1.117 | 0.881 |
| Bevacizumab + PRP | Aflibercept | −0.085 | −0.265 | 0.093 |
| Ranibizumab | Aflibercept | 0.002 | −0.167 | 0.151 |
| Ranibizumab + PRP | Aflibercept | 0.017 | −0.139 | 0.180 |
| Bevacizumab + PRP | Bevacizumab | 0.038 | −0.956 | 1.030 |
| Ranibizumab | Bevacizumab | 0.124 | −0.868 | 1.100 |
| Ranibizumab + PRP | Bevacizumab | 0.140 | −0.830 | 1.116 |
| Ranibizumab | Bevacizumab + PRP | 0.087 | −0.054 | 0.225 |
| Ranibizumab + PRP | Bevacizumab + PRP | 0.102 | −0.032 | 0.243 |
| Ranibizumab + PRP | Ranibizumab | 0.015 | −0.086 | 0.128 |
| Treatment arm | Probability of ranking | |||||
|---|---|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | 5th (%) | 6th (%) | |
| PRP | 0.00 | 0.63 | 4.80 | 35.88 | 58.70 | 0.00 |
| Aflibercept | 20.58 | 29.20 | 23.05 | 17.80 | 4.35 | 20.58 |
| Bevacizumab | 5.90 | 2.70 | 1.85 | 3.65 | 33.43 | 5.90 |
| Bevacizumab + PRP | 46.80 | 9.78 | 3.88 | 1.23 | 0.10 | 46.80 |
| Ranibizumab | 17.03 | 31.83 | 31.10 | 16.20 | 0.93 | 17.03 |
| Ranibizumab + PRP | 9.70 | 25.88 | 35.33 | 25.25 | 2.50 | 9.70 |
Network meta-analyses allowing for interaction with follow-up time and best corrected visual acuity at randomisation
Allowing for variation with follow-up time
Network meta-analyses incorporating all follow-up times (longest in each trial), allowing varying effect of anti-VEGF with follow-up time. Time variation is assumed to be the same for all types of anti-VEGF. A selection of output plots is presented. Results are presented for the predicted effects after 1 year of follow-up.
FIGURE 29.
All treatment comparisons for time-adjusted random-effects NMA of log-MAR.
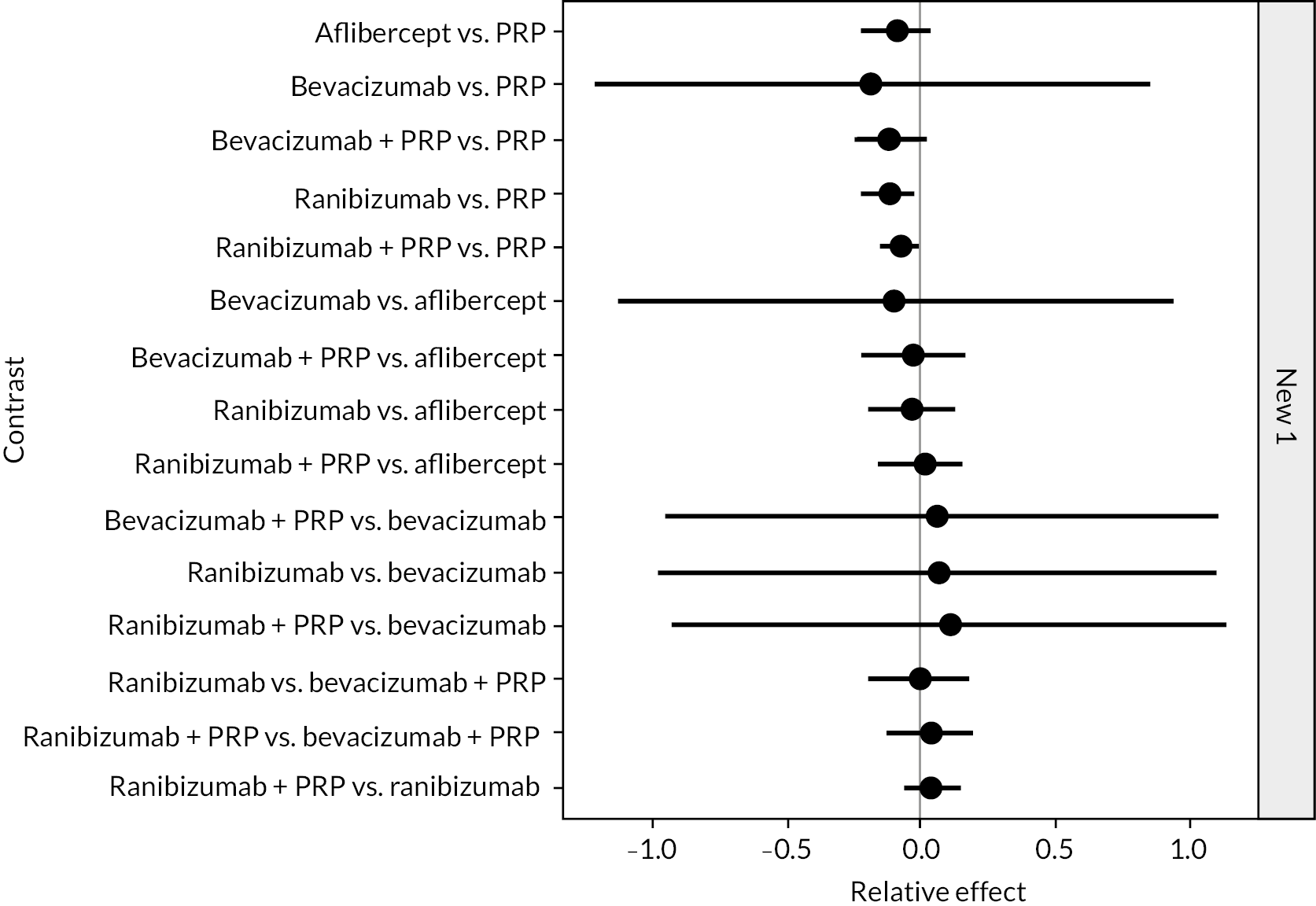
| Intervention | Comparator | Mean difference | 95% CI | |
|---|---|---|---|---|
| Aflibercept | PRP | −0.086 | −0.221 | 0.045 |
| Bevacizumab | PRP | −0.199 | −1.218 | 0.858 |
| Bevacizumab + PRP | PRP | −0.112 | −0.245 | 0.027 |
| Ranibizumab | PRP | −0.119 | −0.214 | −0.023 |
| Ranibizumab + PRP | PRP | −0.075 | −0.153 | 0.001 |
| Bevacizumab | Aflibercept | −0.112 | −1.135 | 0.945 |
| Bevacizumab + PRP | Aflibercept | −0.026 | −0.216 | 0.163 |
| Ranibizumab | Aflibercept | −0.033 | −0.200 | 0.130 |
| Ranibizumab + PRP | Aflibercept | 0.011 | −0.138 | 0.163 |
| Bevacizumab + PRP | Bevacizumab | 0.086 | −0.957 | 1.127 |
| Ranibizumab | Bevacizumab | 0.080 | −0.973 | 1.105 |
| Ranibizumab + PRP | Bevacizumab | 0.123 | −0.919 | 1.138 |
| Ranibizumab | Bevacizumab + PRP | −0.007 | −0.197 | 0.184 |
| Ranibizumab + PRP | Bevacizumab + PRP | 0.037 | −0.123 | 0.195 |
| Ranibizumab + PRP | Ranibizumab | 0.044 | −0.064 | 0.155 |
| Treatment arm | Probability of ranking | |||||
|---|---|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | 5th (%) | 6th (%) | |
| PRP | 0.00 | 0.05 | 0.65 | 5.13 | 38.10 | 56.08 |
| Aflibercept | 7.60 | 19.98 | 25.30 | 25.10 | 17.48 | 4.55 |
| Bevacizumab | 51.98 | 3.88 | 2.58 | 2.78 | 4.35 | 34.45 |
| Bevacizumab + PRP | 18.73 | 30.70 | 17.90 | 17.35 | 12.35 | 2.98 |
| Ranibizumab | 19.40 | 34.13 | 25.88 | 14.88 | 5.25 | 0.48 |
| Ranibizumab + PRP | 2.30 | 11.28 | 27.70 | 34.78 | 22.48 | 1.48 |
Allowing for variation over time and by logarithm of the minimum angle of resolution at randomisation
Network meta-analyses incorporating all follow-up times (longest in each trial), allowing for varying effect of anti-VEGF by follow-up duration and varying effect by trial mean log-MAR at randomisation. Time and log-MAR variation are assumed to be the same for all types of anti-VEGF. A selection of output plots is presented. Results are presented for the predicted effects after 1 year of follow-up and at mean baseline BCVA across trials.
FIGURE 30.
All treatment comparisons for time-adjusted and baseline BCVA adjusted random-effects NMA of log-MAR.
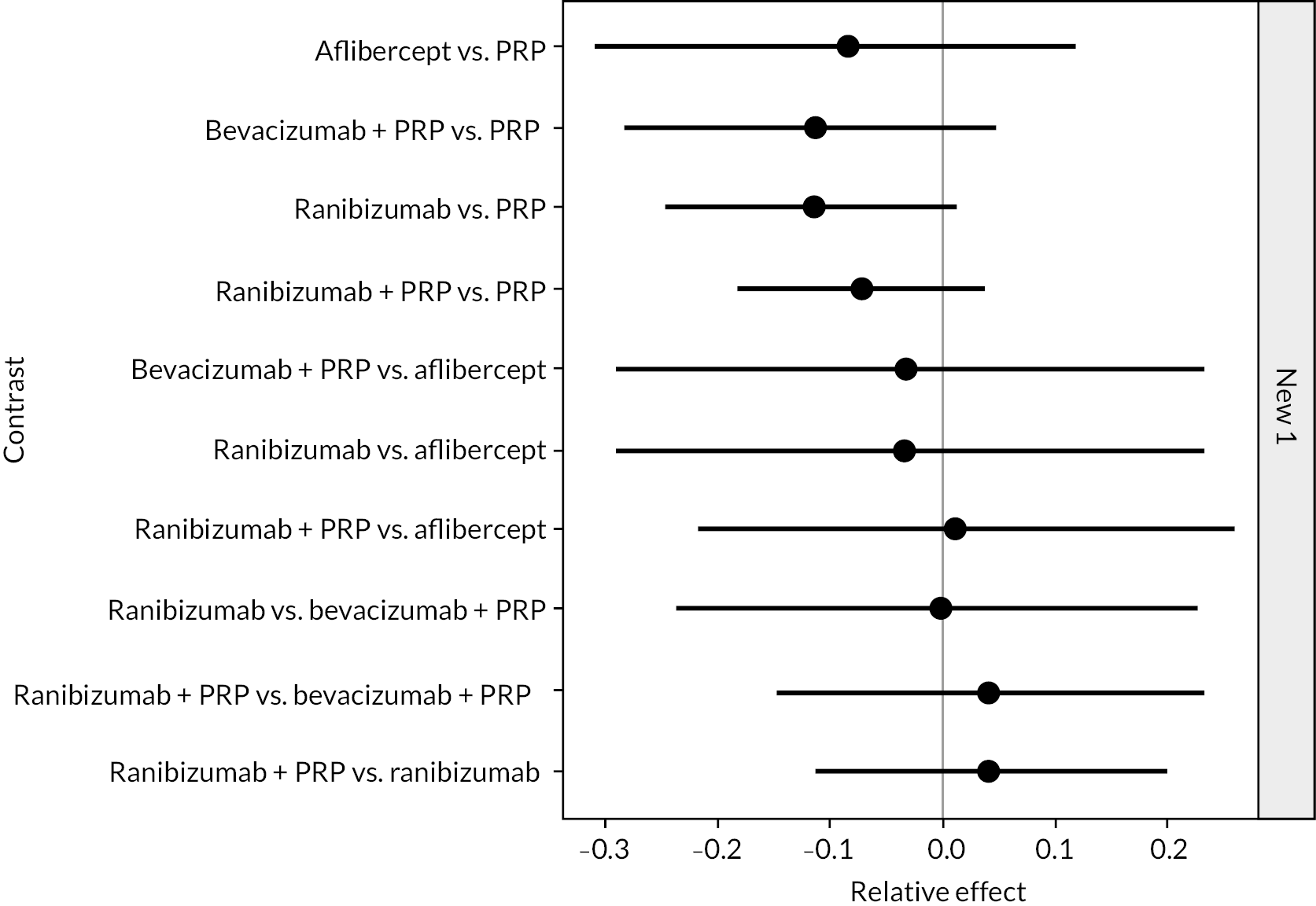
| Intervention | Comparator | Mean difference | 95% CI | |
|---|---|---|---|---|
| Aflibercept | PRP | −0.085 | −0.310 | 0.119 |
| Bevacizumab + PRP | PRP | −0.116 | −0.281 | 0.050 |
| Ranibizumab | PRP | −0.117 | −0.247 | 0.011 |
| Ranibizumab + PRP | PRP | −0.073 | −0.187 | 0.041 |
| Bevacizumab + PRP | Aflibercept | −0.031 | −0.287 | 0.228 |
| Ranibizumab | Aflibercept | −0.032 | −0.288 | 0.233 |
| Ranibizumab + PRP | Aflibercept | 0.012 | −0.218 | 0.251 |
| Ranibizumab | Bevacizumab + PRP | −0.001 | −0.239 | 0.225 |
| Ranibizumab + PRP | Bevacizumab + PRP | 0.043 | −0.151 | 0.234 |
| Ranibizumab + PRP | Ranibizumab | 0.044 | −0.112 | 0.203 |
| Treatment arm | Probability of ranking | ||||
|---|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | 5th (%) | |
| PRP | 0.08 | 0.70 | 4.23 | 19.50 | 75.50 |
| Aflibercept | 19.25 | 23.45 | 21.43 | 22.70 | 13.18 |
| Bevacizumab + PRP | 38.40 | 23.30 | 18.10 | 15.68 | 4.53 |
| Ranibizumab | 36.93 | 30.48 | 19.63 | 10.75 | 2.23 |
| Ranibizumab + PRP | 5.35 | 22.08 | 36.63 | 31.38 | 4.58 |
Network meta-analyses of reduced networks
Assuming anti-vascular endothelial growth factor and anti-vascular endothelial growth factor + panretinal photocoagulation are equivalent
This analysis assumes that anti-VEGF only arms and anti-VEGF + PRP arms have equal effect. To be used to assess differences between anti-VEGF types. A model allowing effect to vary with time and baseline log-MAR was used. Results are presented for the predicted effects after 1 year of follow-up and at mean baseline BCVA across trials.
FIGURE 31.
Results from a reduced network to compare anti-VEGFs.
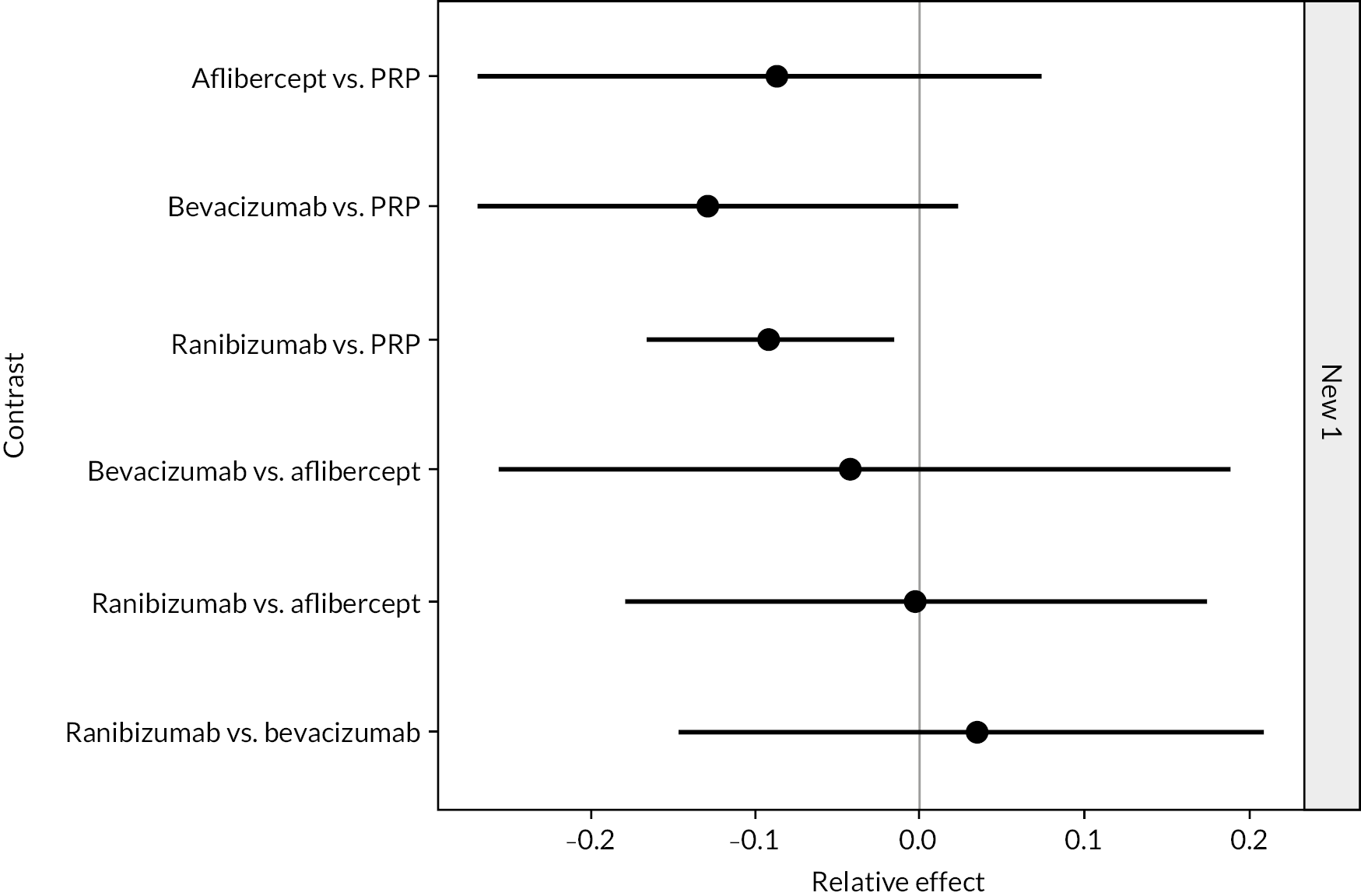
| Intervention | Comparator | Mean difference | 95% CI | |
|---|---|---|---|---|
| Aflibercept | PRP | −0.091 | −0.245 | 0.063 |
| Bevacizumab | PRP | −0.126 | −0.261 | 0.007 |
| Ranibizumab | PRP | −0.094 | −0.173 | −0.023 |
| Bevacizumab | Aflibercept | −0.035 | −0.238 | 0.174 |
| Ranibizumab | Aflibercept | −0.003 | −0.166 | 0.163 |
| Ranibizumab | Bevacizumab | 0.032 | −0.142 | 0.200 |
| Treatment | Probability of ranking | |||
|---|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | |
| PRP | 0.00 | 0.80 | 11.48 | 87.73 |
| Aflibercept | 25.08 | 32.93 | 33.35 | 8.65 |
| Bevacizumab | 53.60 | 23.33 | 20.18 | 2.90 |
| Ranibizumab | 21.33 | 42.95 | 35.00 | 0.73 |
Assuming all types of anti-vascular endothelial growth factor are equivalent
This analysis assumes that all three anti-VEGF drugs have equal effect. To be used to assess the overall effect of anti-VEGF. A model allowing effect to vary with time and baseline log-MAR was used.
FIGURE 32.
Results from a reduced network to compare treatment classes.
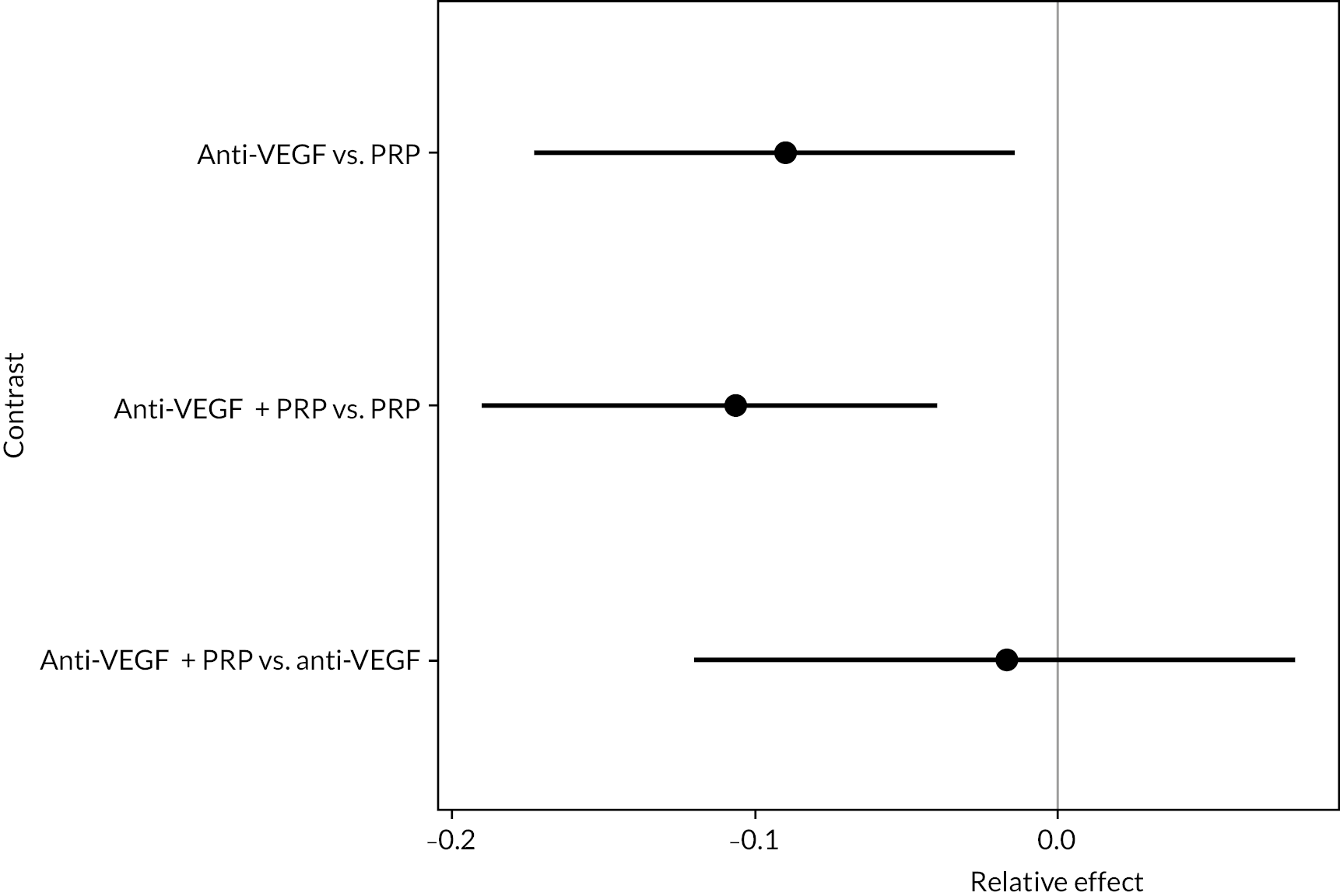
| Intervention | Comparator | Mean difference | 95% CI | |
|---|---|---|---|---|
| Anti-VEGF | PRP | −0.089 | −0.180 | −0.019 |
| Anti-VEGF + PRP | PRP | −0.108 | −0.192 | −0.039 |
| Anti-VEGF + PRP | Anti-VEGF | −0.019 | −0.126 | 0.083 |
| Treatment | Probability of ranking | ||
|---|---|---|---|
| 1st (%) | 2nd (%) | 3rd (%) | |
| PRP | 0.03 | 1.20 | 98.78 |
| Anti-VEGF | 33.05 | 65.88 | 1.08 |
| Anti-VEGF + PRP | 66.93 | 32.93 | 0.15 |
Threshold analyses
Up to 1 year
FIGURE 33.
Threshold analyses of data up to 1 year of follow-up.
One to 2 years
FIGURE 34.
Threshold analyses of data with 1–2 years of follow-up.
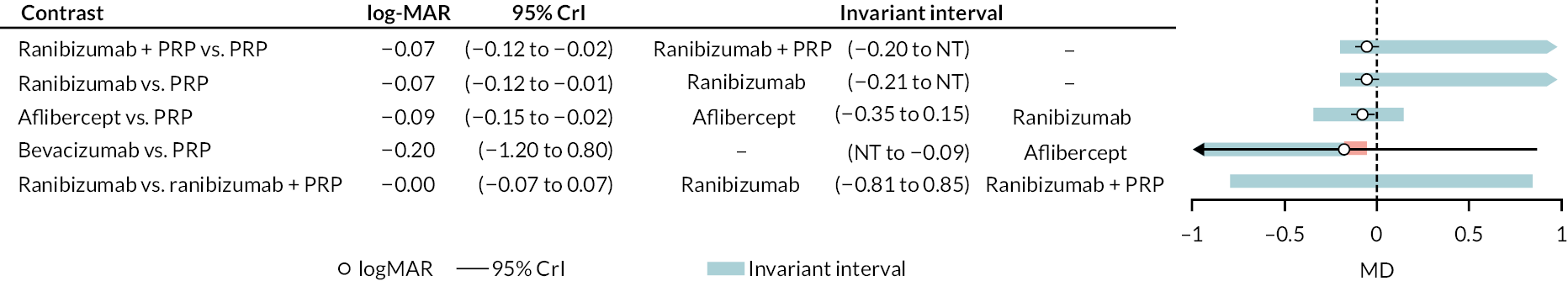
Maximum follow-up (up to 2 years)
FIGURE 35.
Threshold analyses of data at end of trial (up to 2 years).
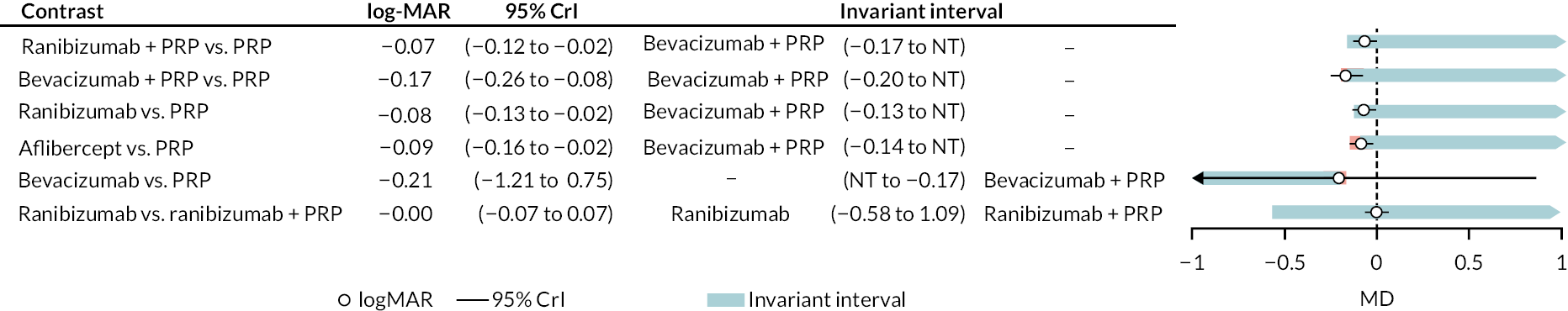
Allowing for effect variation with time and baseline logarithm of the minimum angle of resolution
FIGURE 36.
Threshold analyses of model adjusting for effect of time and baseline log-MAR.
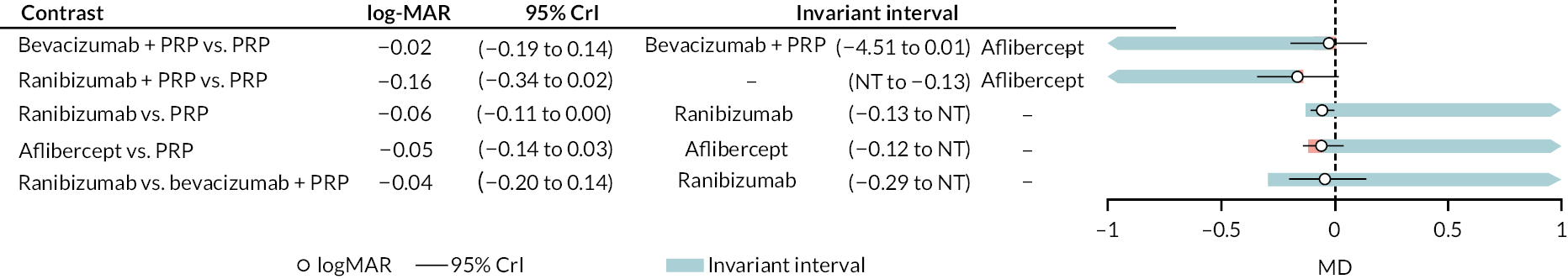
Reduced network (for comparing anti-vascular endothelial growth factors)
Adjusted for follow-up time and BCVA at baseline.
FIGURE 37.
Threshold analysis of simplified network to compare anti-VEGF types, with time and baseline BCVA adjustment.

Reduced network (comparing anti-vascular endothelial growth factor to panretinal photocoagulation)
Adjusted for follow-up time and BCVA at baseline.
FIGURE 38.
Threshold analyses of simplified network to compare anti-VEGF to PRP, adjusted for follow-up time and baseline BCVA.
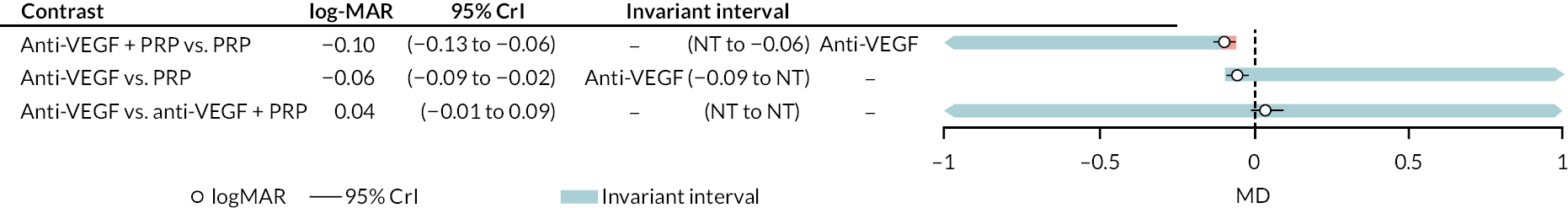
Appendix 3 Other outcomes
This appendix presents tables and figures for all analyses, using data from publications of included RCTs for outcomes other than BCVA. These mostly consist of forest plots without meta-analysis, because the evidence was generally too limited in extent, and too diverse in intervention and follow-up times, to justify a full meta-analysis.
As meta-analysis was not possible for most outcomes, the forest plots without meta-analysis include trials of proliferative and non-proliferative retinopathy, to aid comparison.
Forest plots of outcomes without meta-analysis
These forest plots show results for all anti-VEGF types, and at all follow-up times. Note that this means some trials appear more than once in a forest plot.
Neovascularisation of the disc
FIGURE 39.
Forest plot of all NVD data (left side favours anti-VEGF).
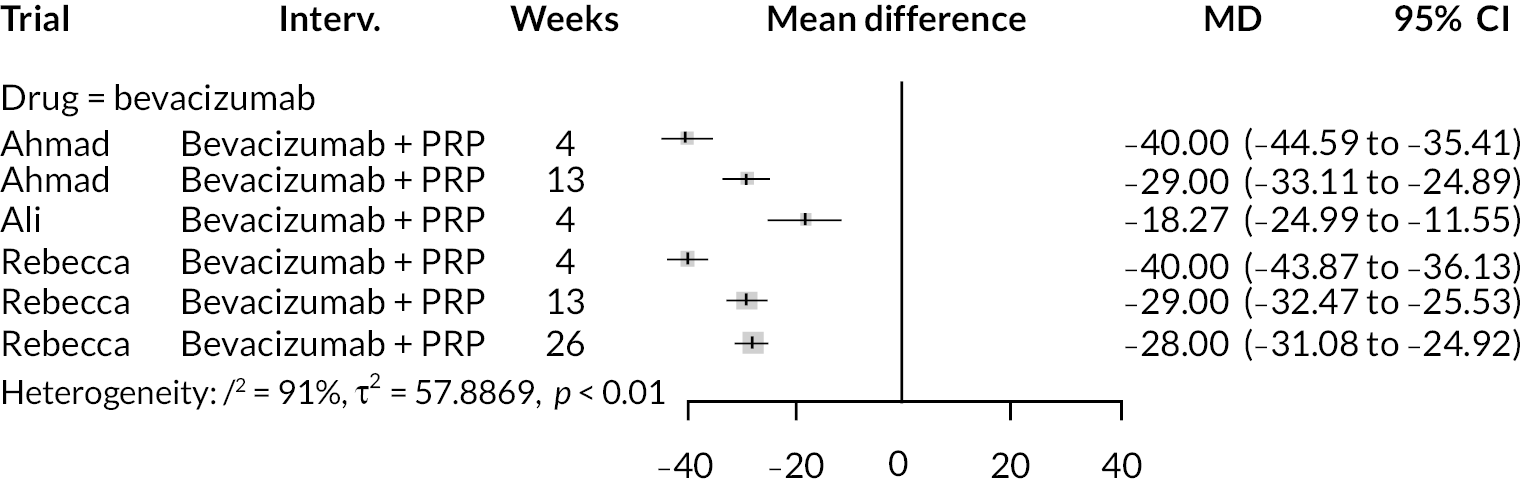
Neovascularisation elsewhere
FIGURE 40.
Forest plot of all NVE data (left side favours anti-VEGF).
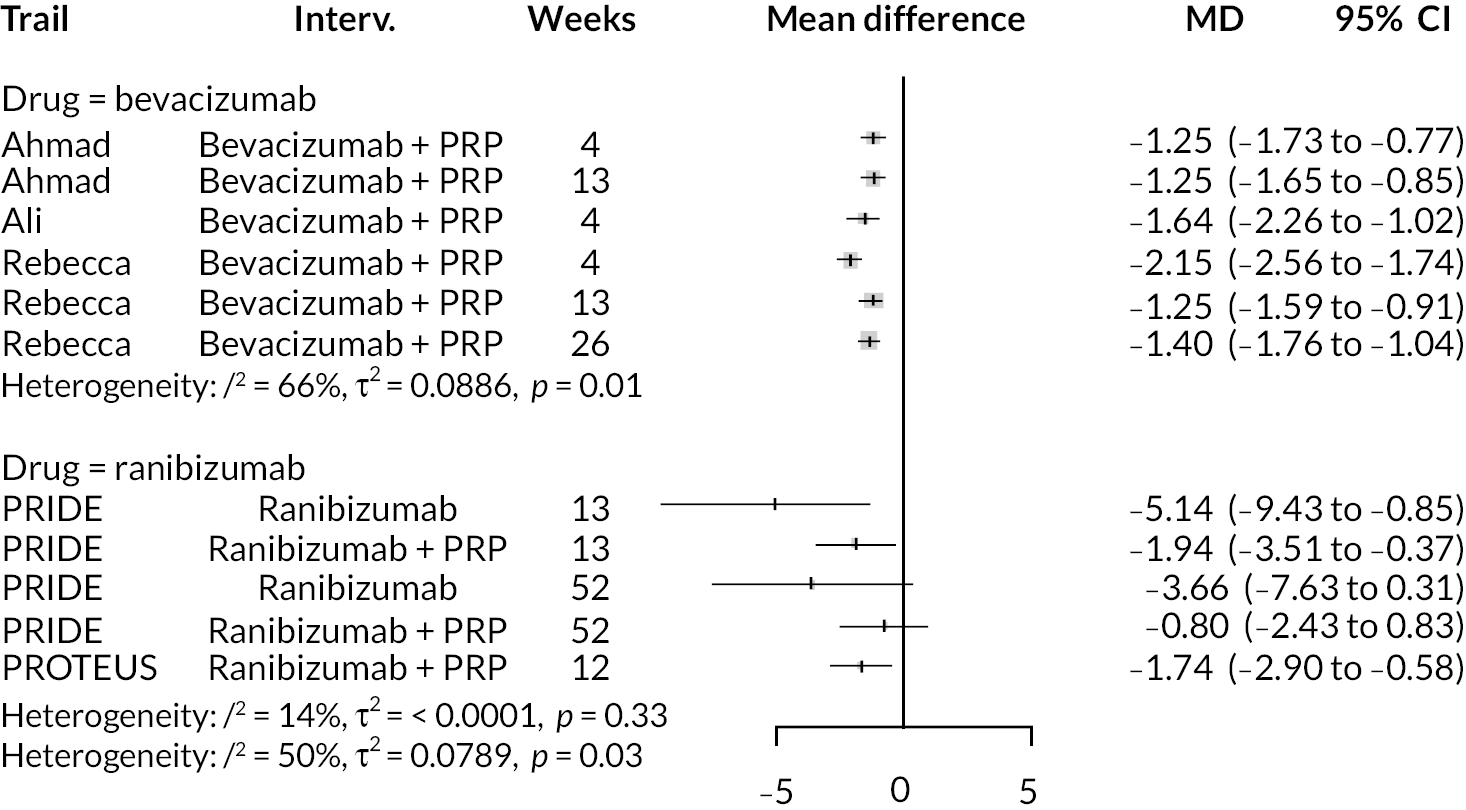
Diabetic macular oedema
FIGURE 41.
Forest plot of DME incidence (left side favours anti-VEGF).
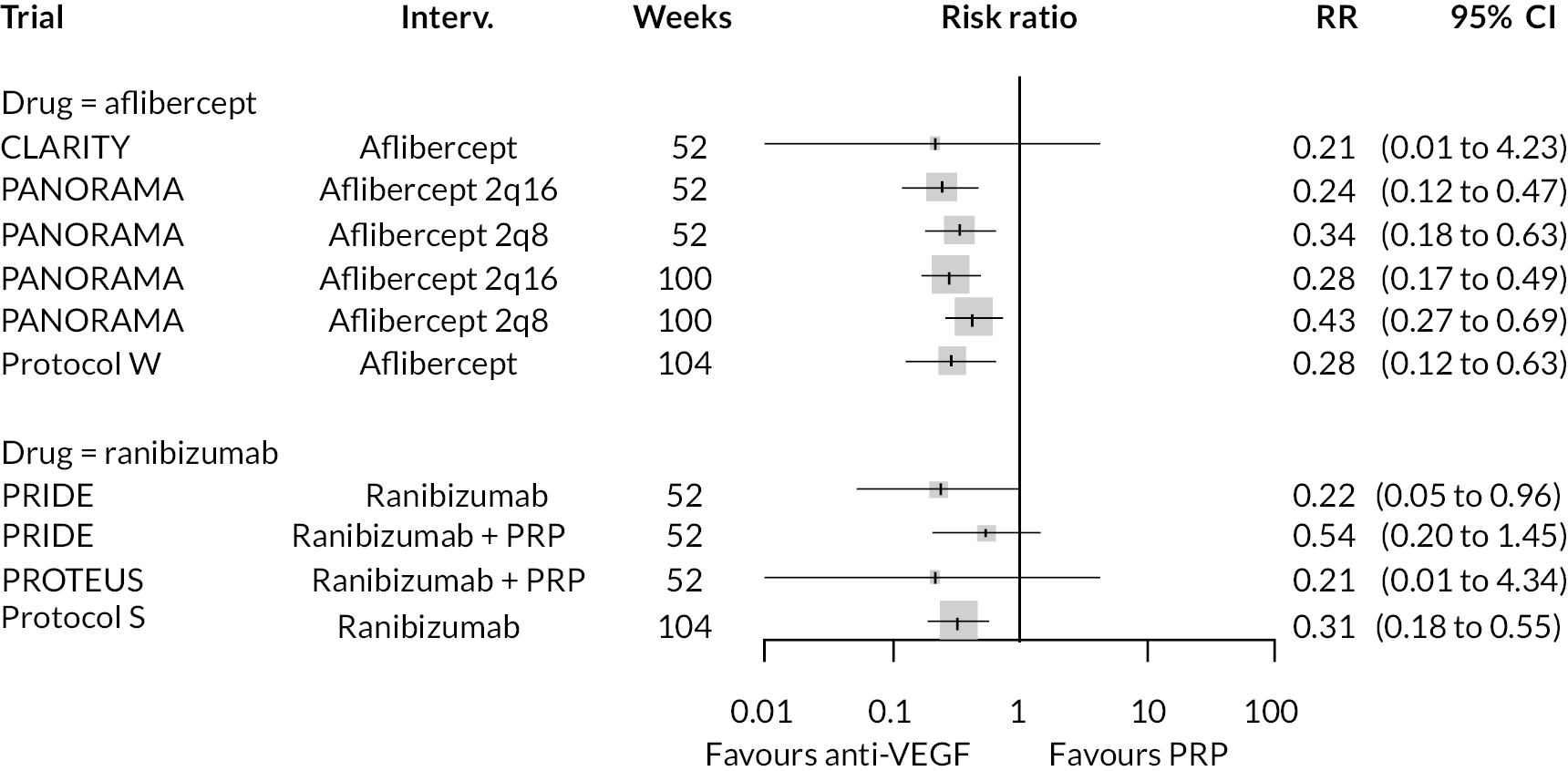
Improvement in diabetic retinopathy severity score (Diabetic Retinopathy Severity Scale)
FIGURE 42.
Forest plot of improvement in DRSS severity (right side favours anti-VEGF).
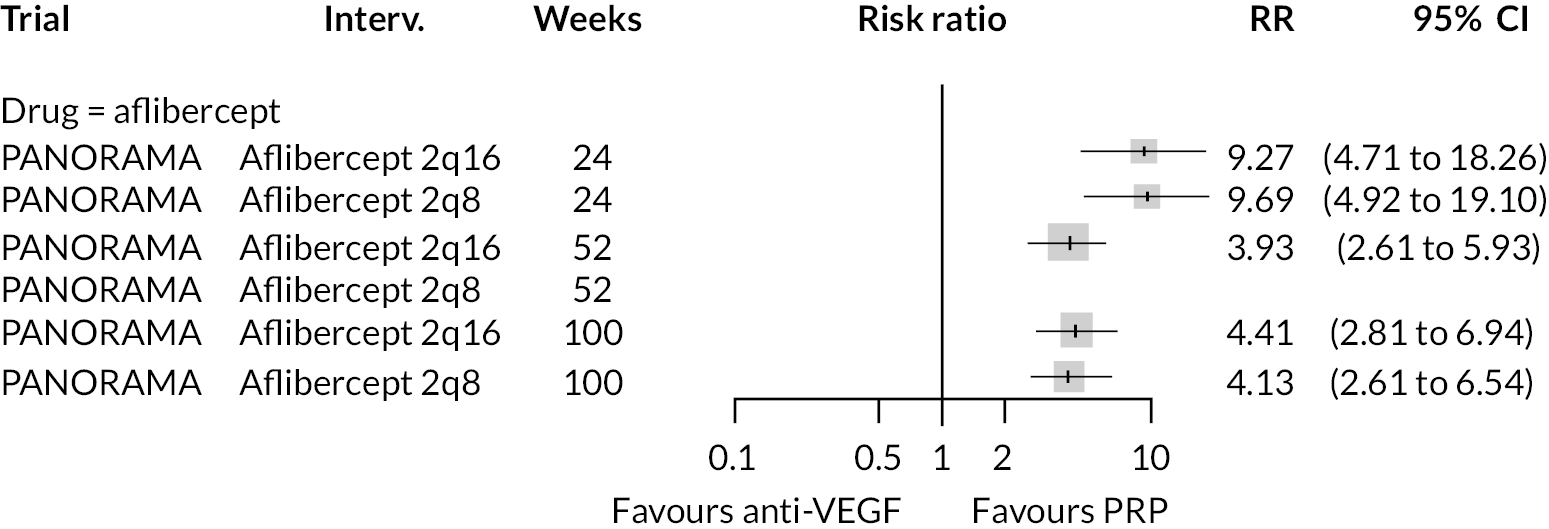
Proliferative retinopathy incidence
FIGURE 43.
Forest plot of proliferative DR (left side favours anti-VEGF).
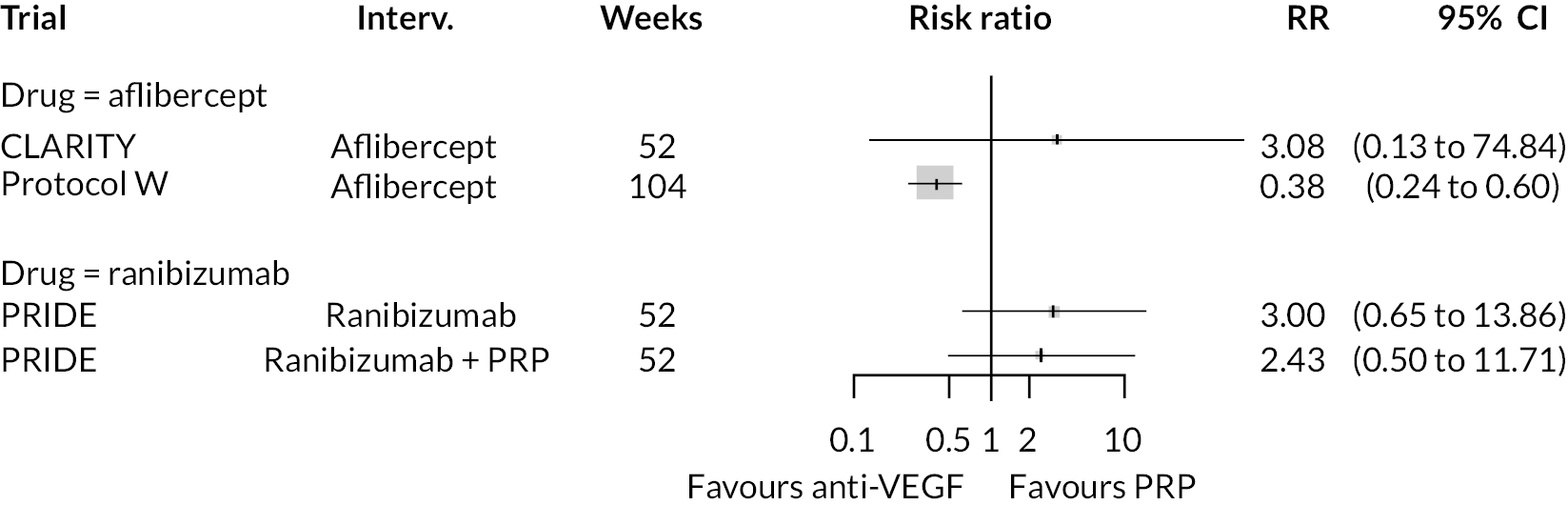
Regression of neovascularisation
FIGURE 44.
Forest plot of regressive neovascularisation (left side favours anti-VEGF).
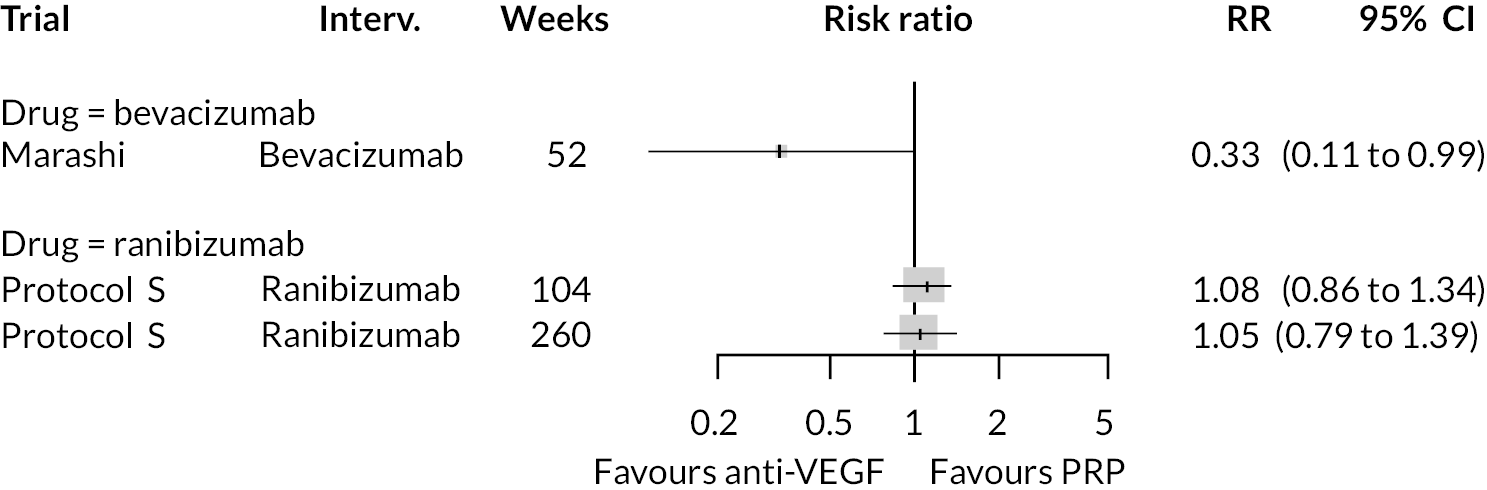
Use of other treatments
FIGURE 45.
Forest plot of use of other treatments (left side favours anti-VEGF).
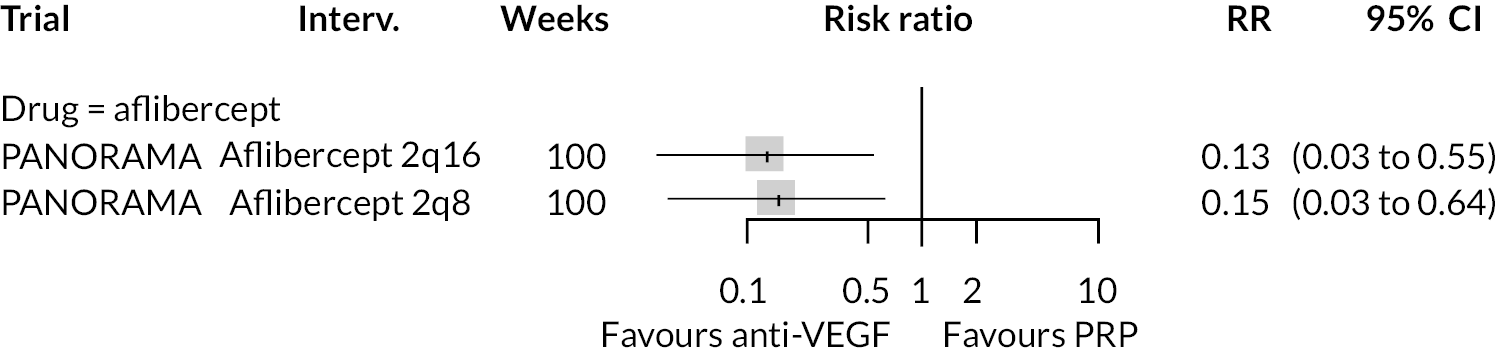
Vitrectomy
FIGURE 46.
Forest plot of vitrectomy incidence (left side favours anti-VEGF).
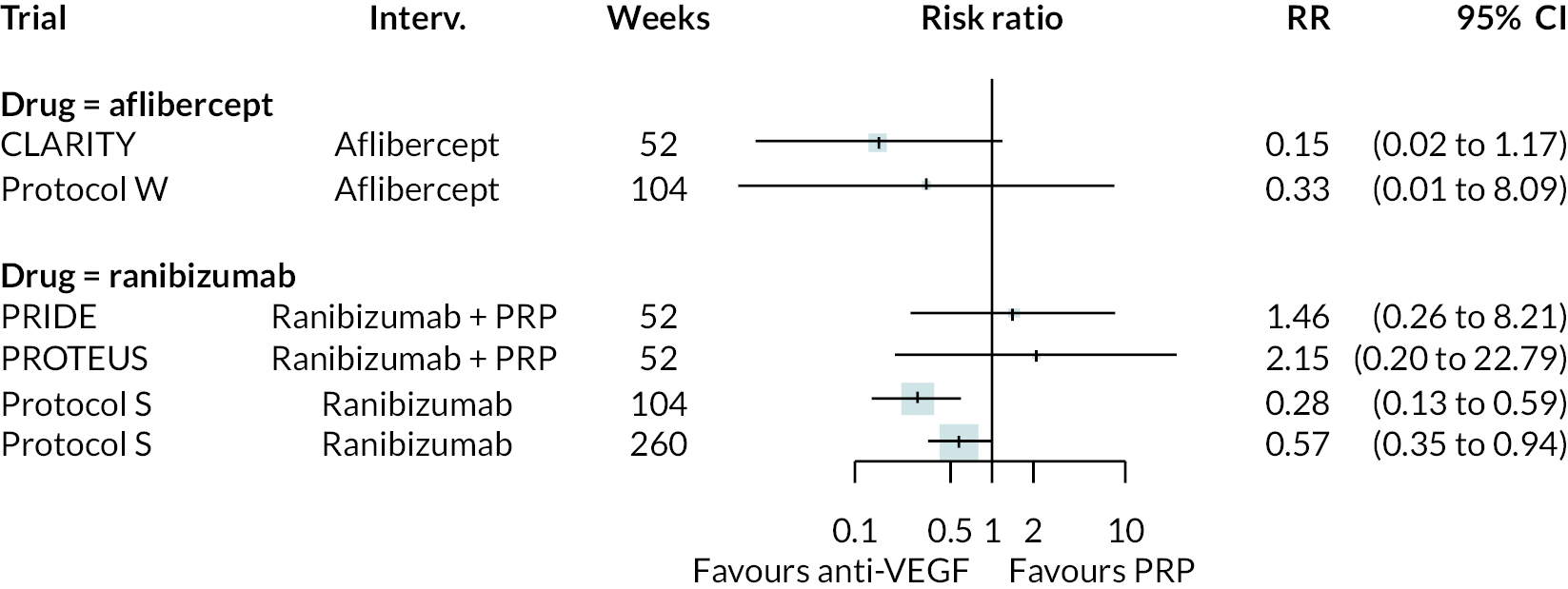
Vitreous haemorrhage
FIGURE 47.
Forest plot of vitreous haemorrhage incidence (left side favours anti-VEGF).
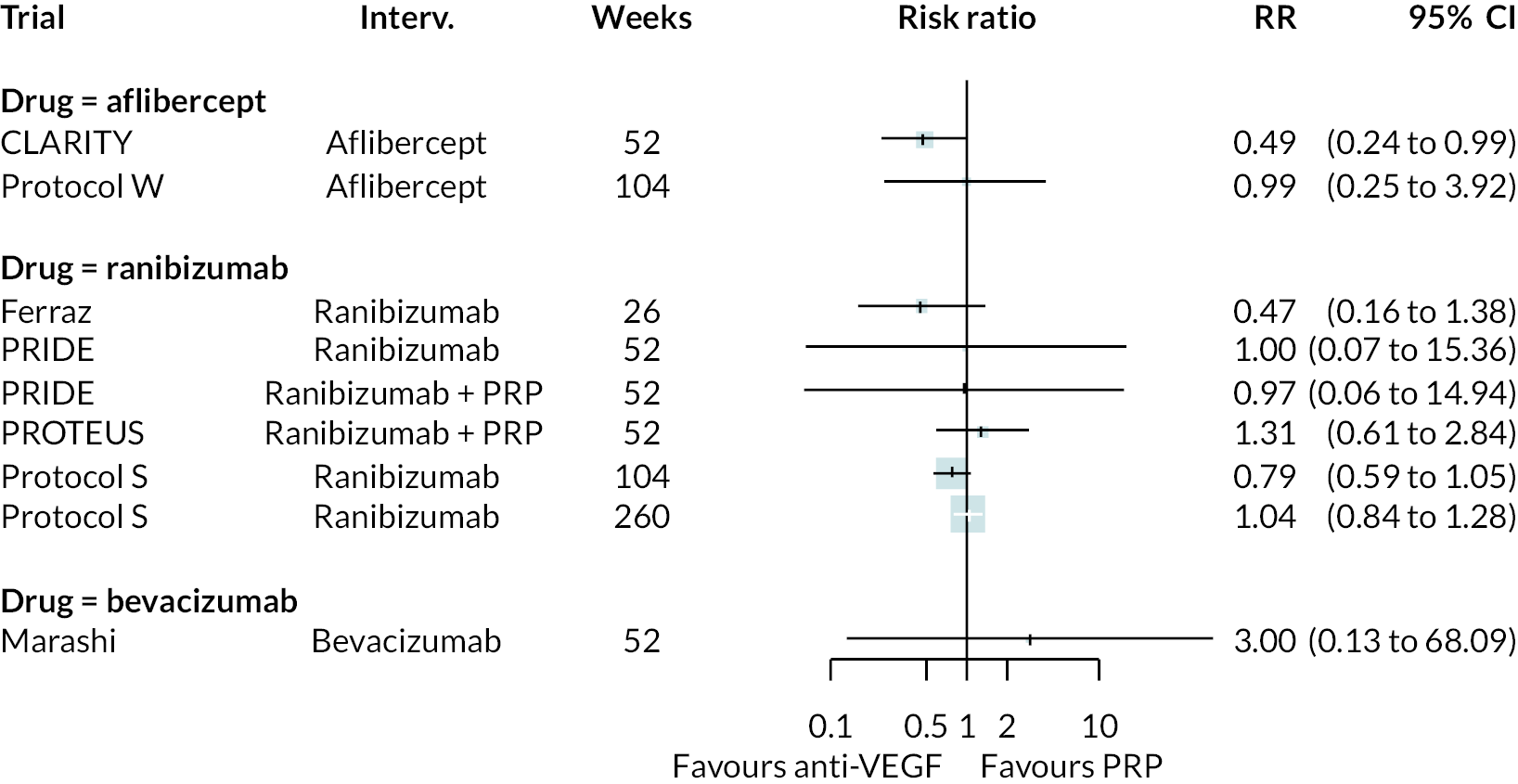
Adverse event outcomes
These forest plots show results for all anti-VEGF types, and at all follow-up times. Note that this means some trials appear more than once in a forest plot. For simplicity, only adverse event outcomes reported in two or more studies are presented.
Cataracts
FIGURE 48.
Forest plot of cataracts data (left side favours anti-VEGF).
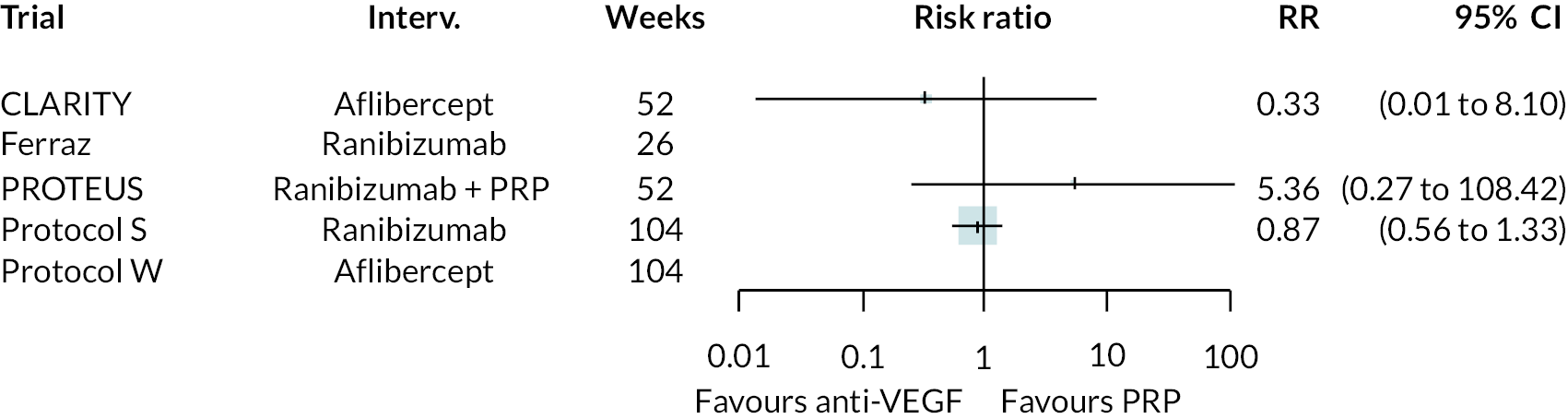
Conjunctival haemorrhage
FIGURE 49.
Forest plot of conjunctival haemorrhage data (left side favours anti-VEGF).
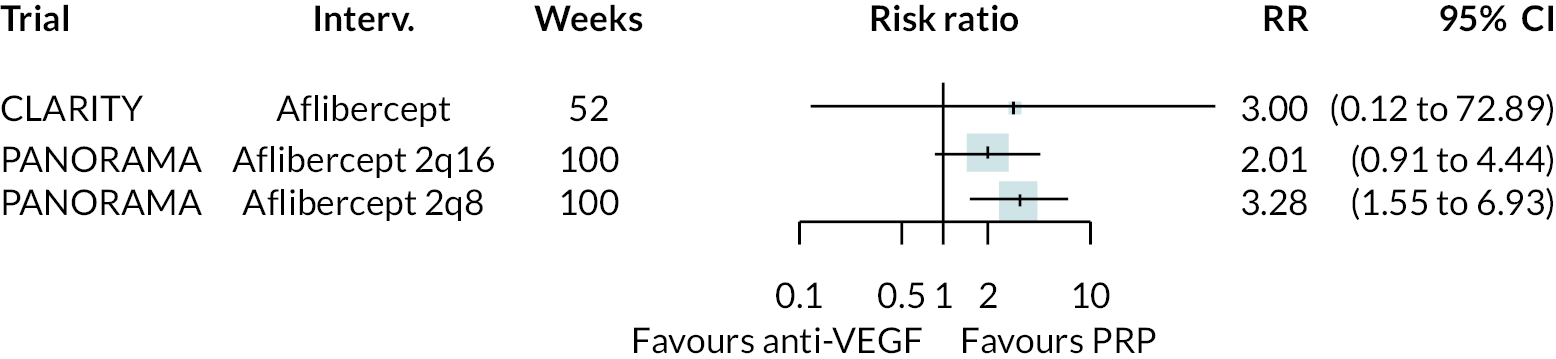
Cardiovascular mortality
FIGURE 50.
Forest plot of cardiovascular mortality data (left side favours anti-VEGF).
Death (all-cause mortality)
FIGURE 51.
Forest plot of death data (left side favours anti-VEGF).
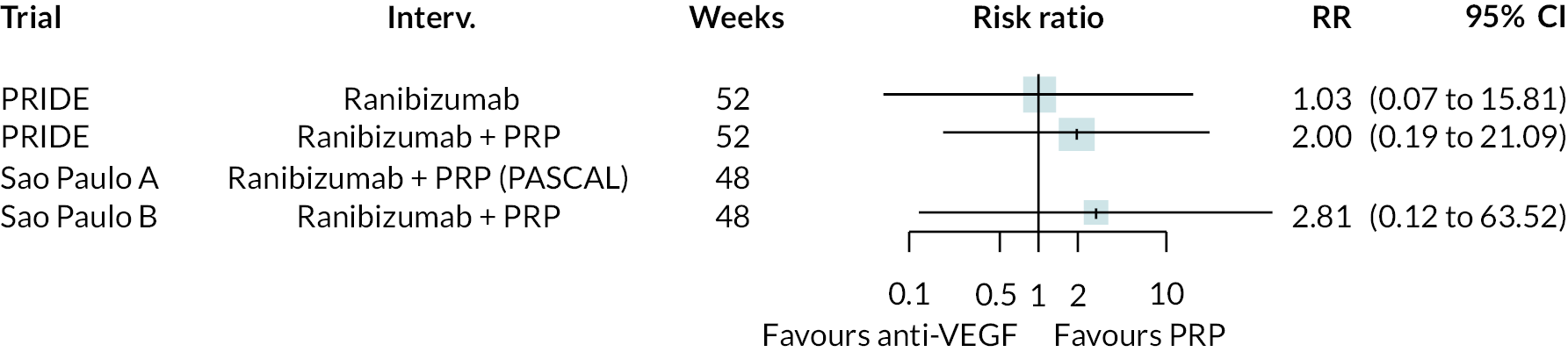
Myocardial infarction
FIGURE 52.
Forest plot of myocardial infarction data (left side favours anti-VEGF).
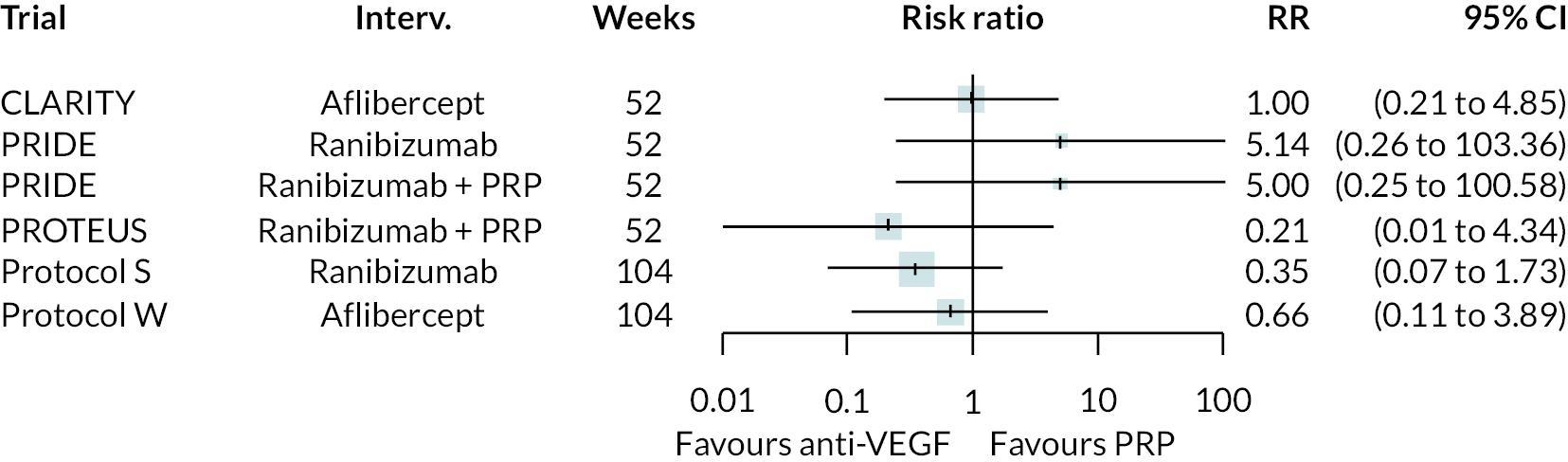
Ocular pain
FIGURE 53.
Forest plot of ocular pain data (left side favours anti-VEGF).
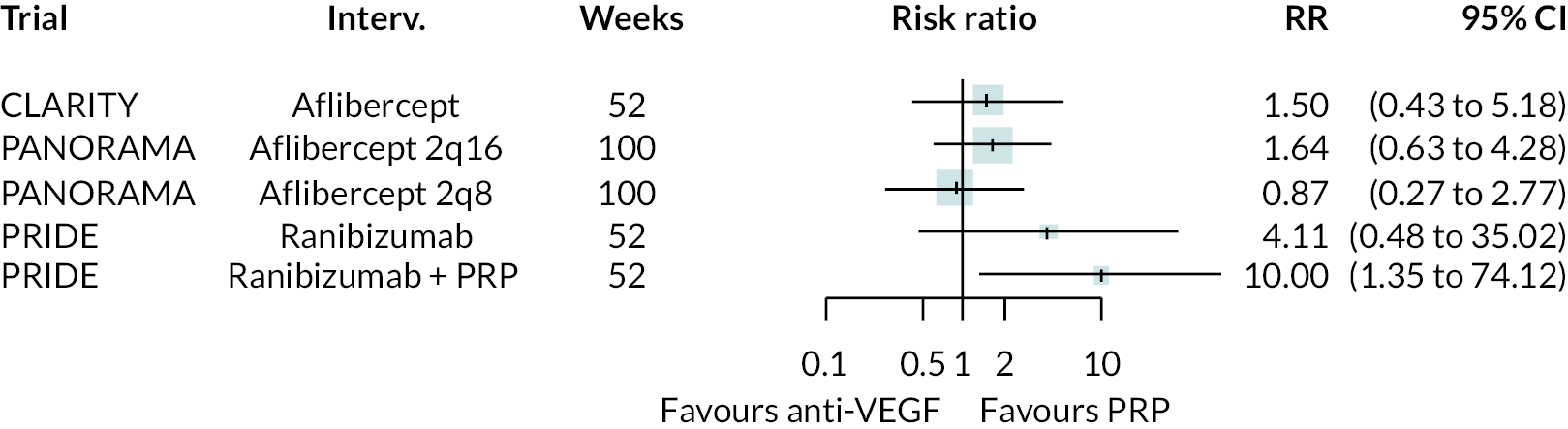
Raised intraocular pressure
FIGURE 54.
Forest plot of raised intraocular pressure data (left side favours anti-VEGF).
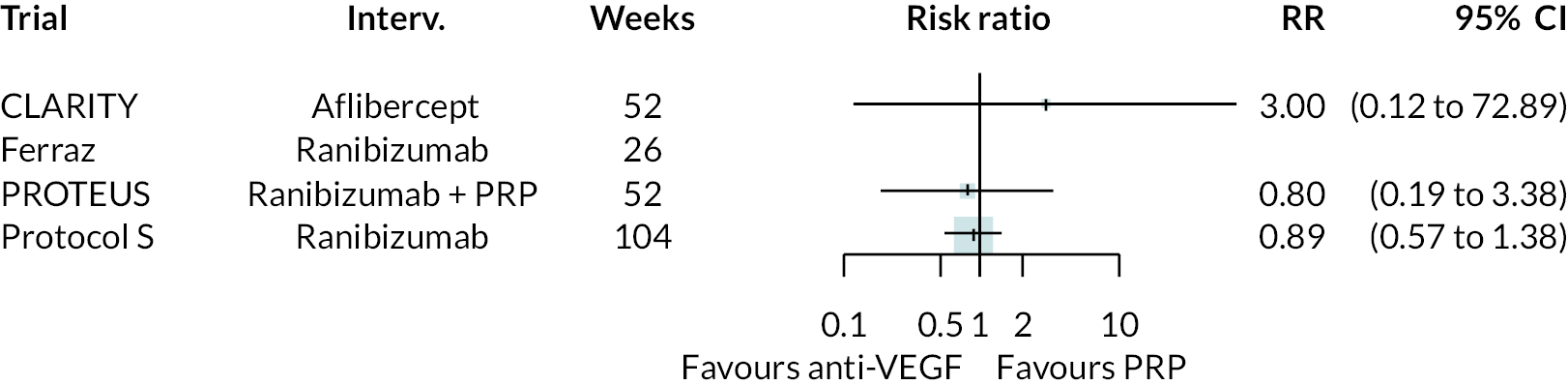
Retinal detachment
FIGURE 55.
Forest plot of retinal detachment data (left side favours anti-VEGF).
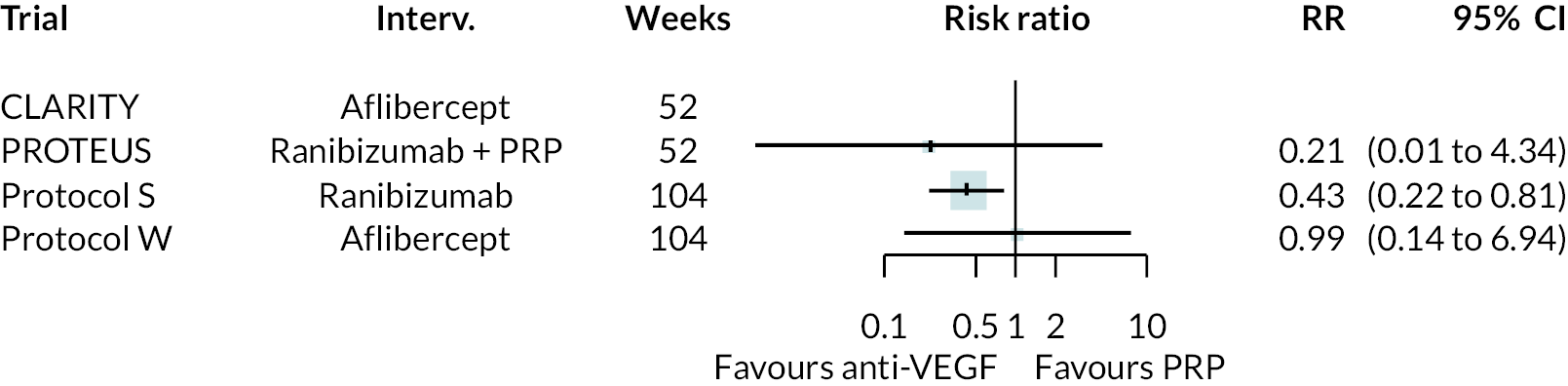
Retinal tear
FIGURE 56.
Forest plot of retinal data (left side favours anti-VEGF).
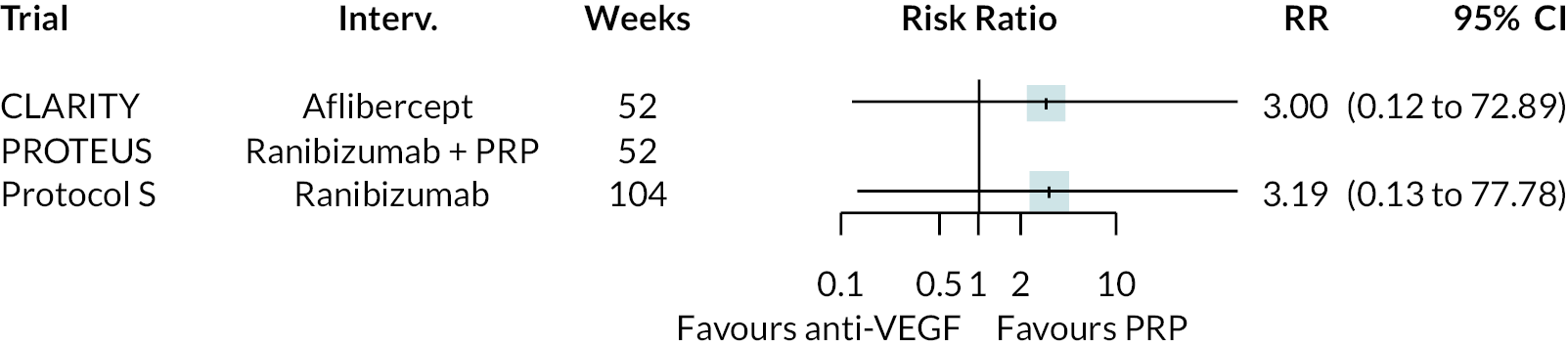
Serious adverse event (however defined)
FIGURE 57.
Forest plot of serious adverse event data (left side favours anti-VEGF).
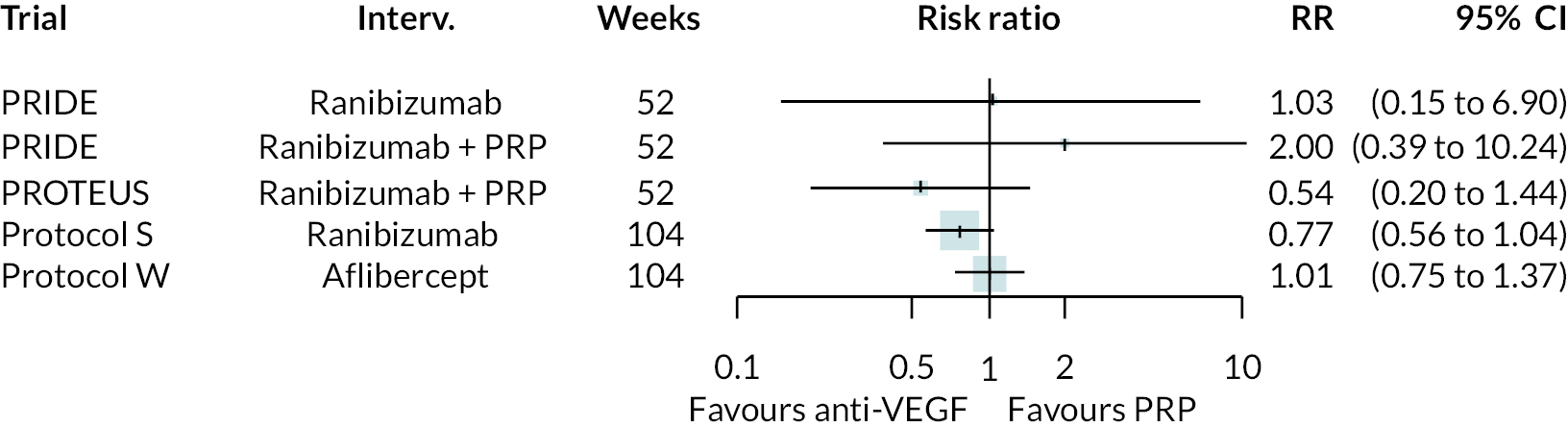
Stroke
FIGURE 58.
Forest plot of stroke data (left side favours anti-VEGF).
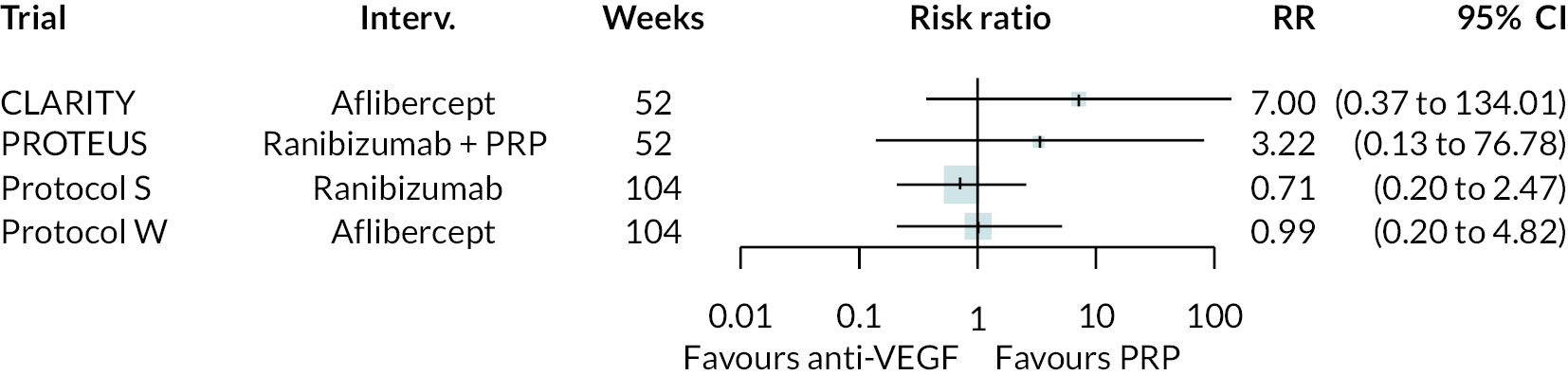
Meta-analyses of other outcomes and adverse events
All meta-analyses presented assumed that the impact of anti-VEGF on outcome (or adverse event) is the same for all types of anti-VEGF (in isolation or combined with PRP), and at all follow-up times. For trials with multiple time points, the longest follow-up was used. For trial with multiple arms, only one anti-VEGF arm was used; arms using anti-VEGF alone were preferred.
Neovascularisation elsewhere
FIGURE 59.
Meta-analysis of NVE (left side favours anti-VEGF).
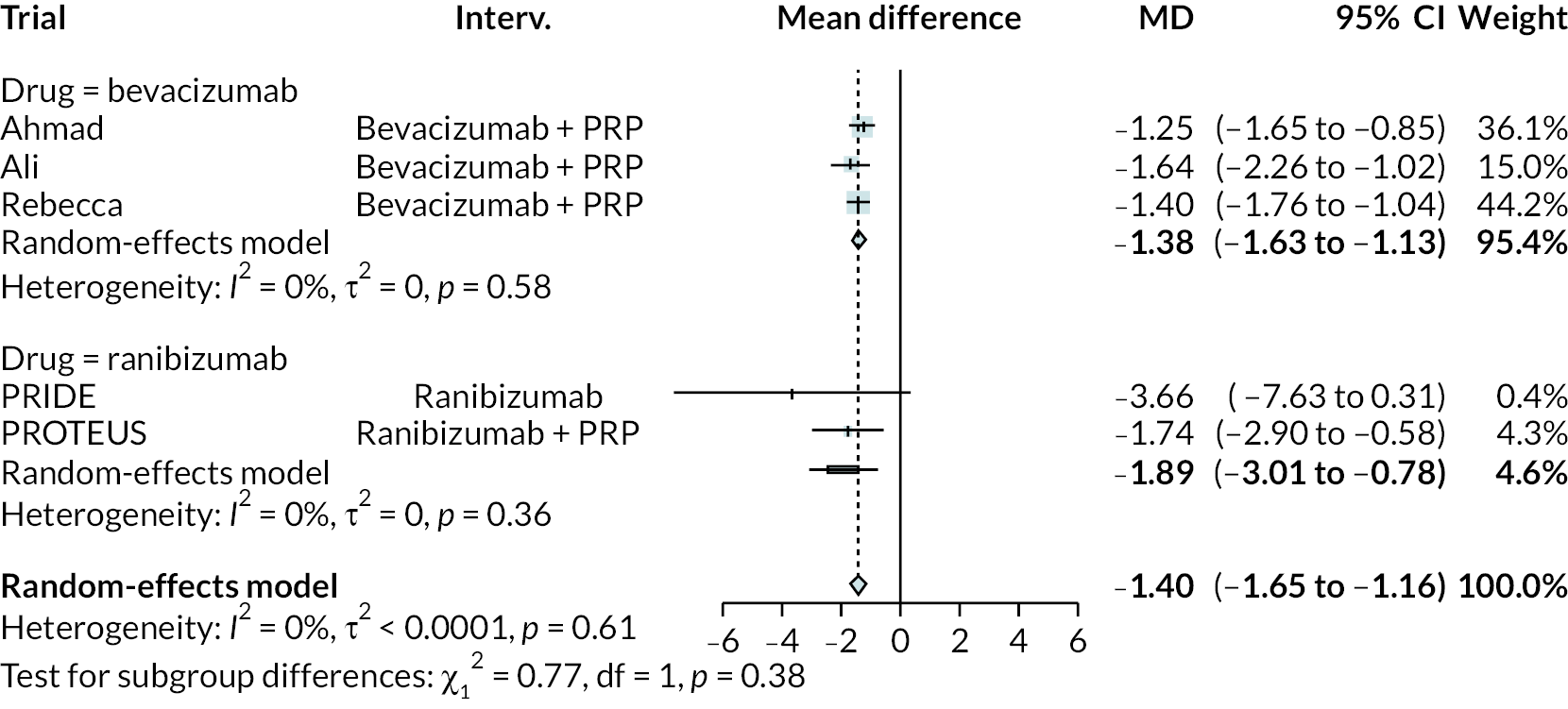
Neovascularisation of the disc
FIGURE 60.
Meta-analysis of NVD (left side favours anti-VEGF).
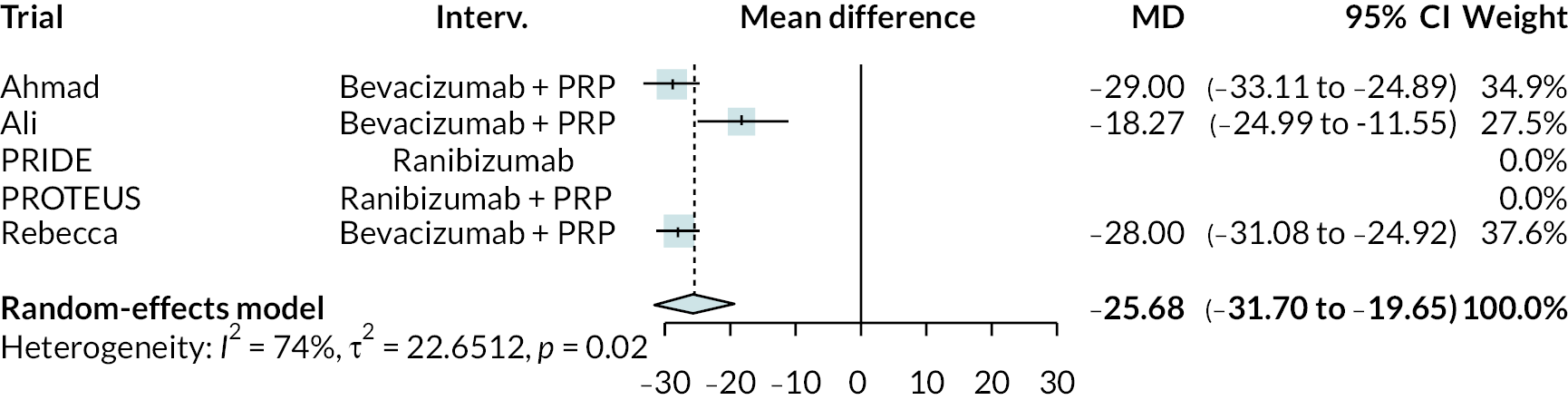
Other non-vison outcomes
This forest plot shows the summary results of each meta-analysis (each bar is a meta-analysis result). Meta-analyses are restricted to trials of proliferative retinopathy. Full forest plots for each outcome are not presented.
FIGURE 61.
Meta-analysis summary for non-vision outcomes in PDR trials (left side favours anti-VEGF).
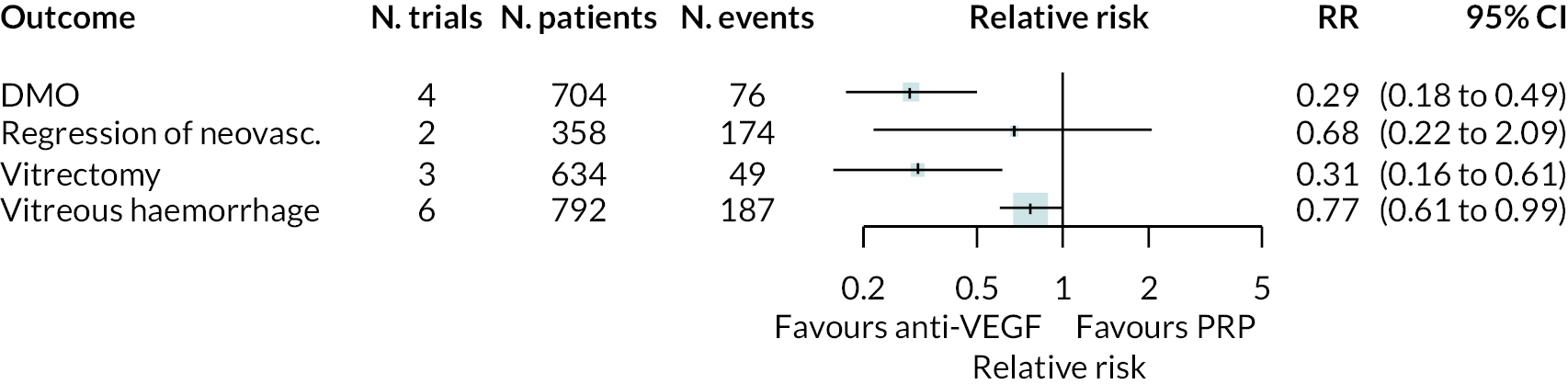
Adverse events
This forest plot shows the summary results of each meta-analysis (each bar is a meta-analysis result). Meta-analyses are restricted to trials of proliferative retinopathy. Full forest plots for each outcome are not presented.
FIGURE 62.
Meta-analysis summary for adverse events (left side favours anti-VEGF).
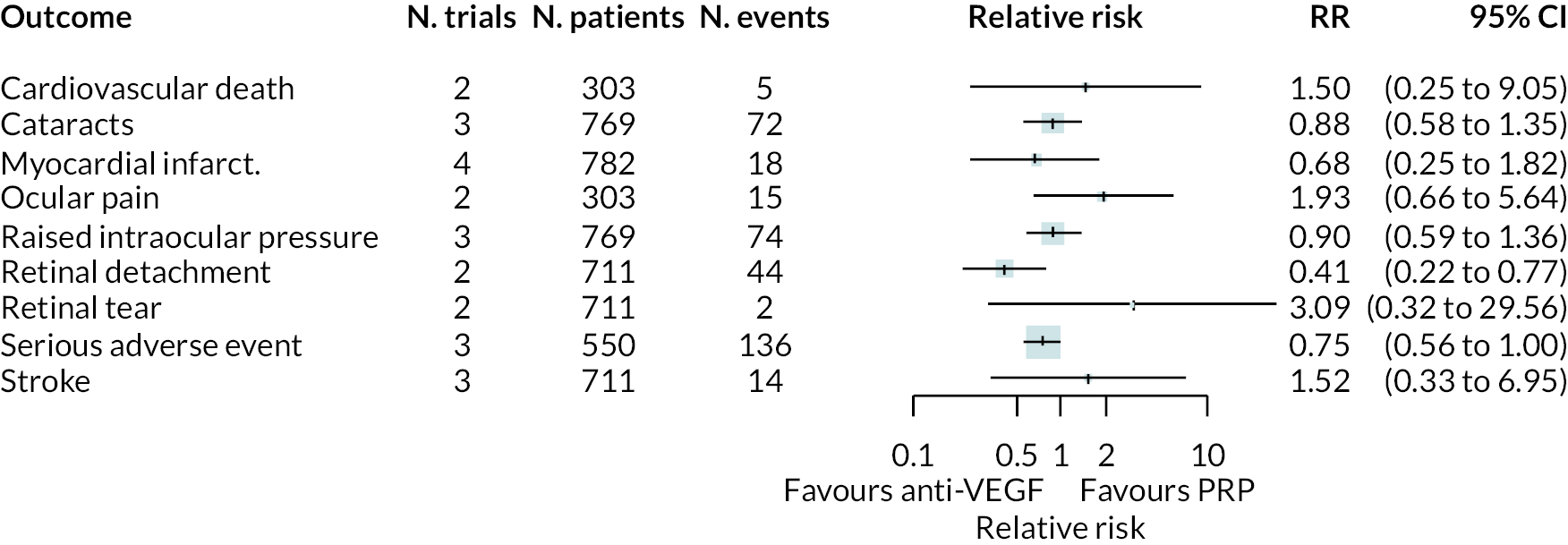
Appendix 4 Non-proliferative diabetic retinopathy
This section reports the findings of the two trials in non-proliferative retinopathy. As both trials compared aflibercept to sham injection, no NMAs were performed. PANORAMA had two aflibercept arms: injections every 8 weeks or every 16 weeks. Only the 16-week arm is analysed here, as that was the schedule used in Protocol W.
Best corrected visual acuity
FIGURE 63.
Mean difference in ETDRS after 2 years in NPDR trials.
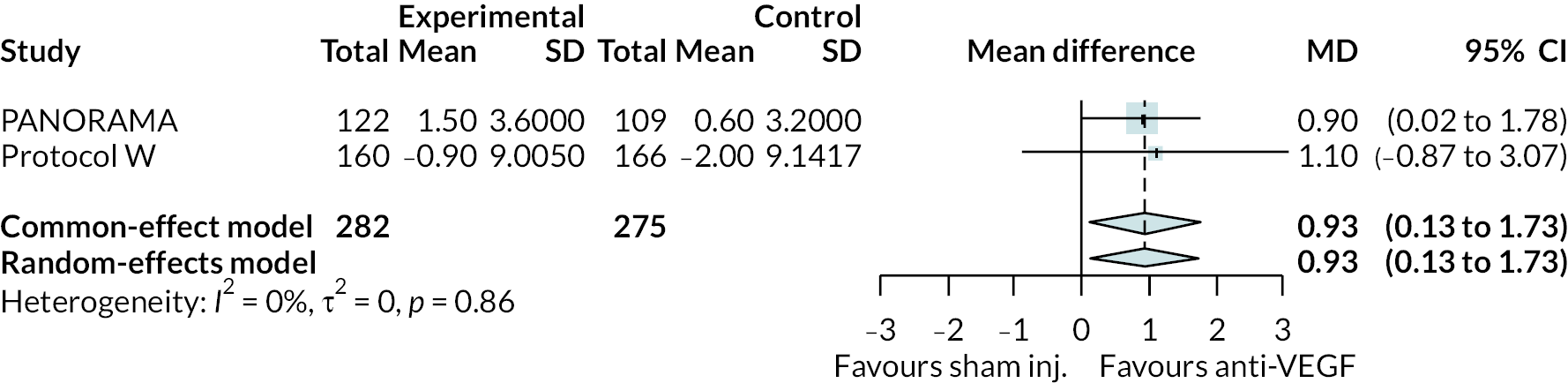
FIGURE 64.
Mean difference in log-MAR after 2 years in NPDR trials.
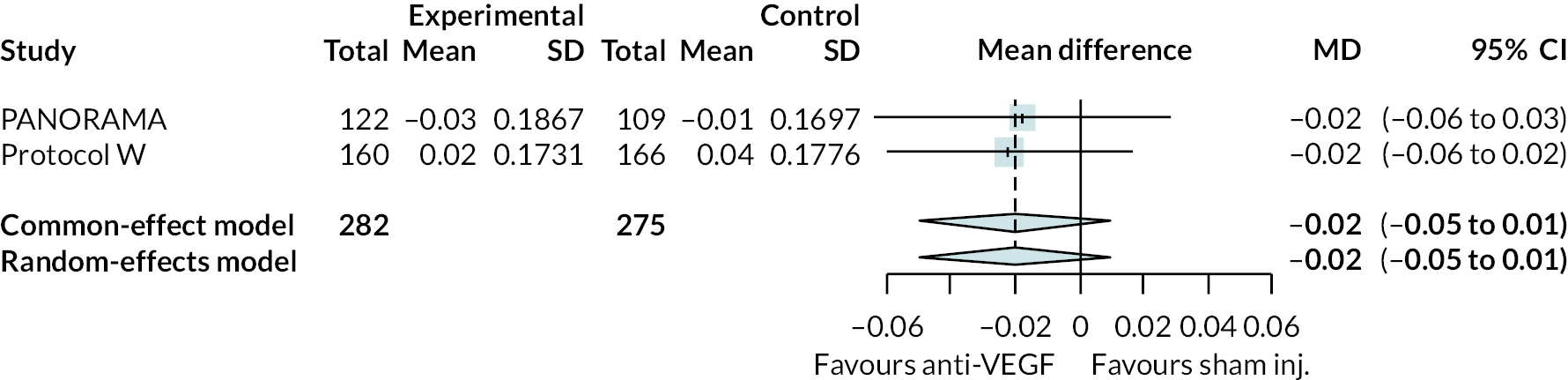
Diabetic macular oedema in non-proliferative retinopathy
Diabetic macular oedema was the only outcome other than BCVA reported in both trials of NPDR.
FIGURE 65.
Diabetic macular oedema incidence in NPDR trials.
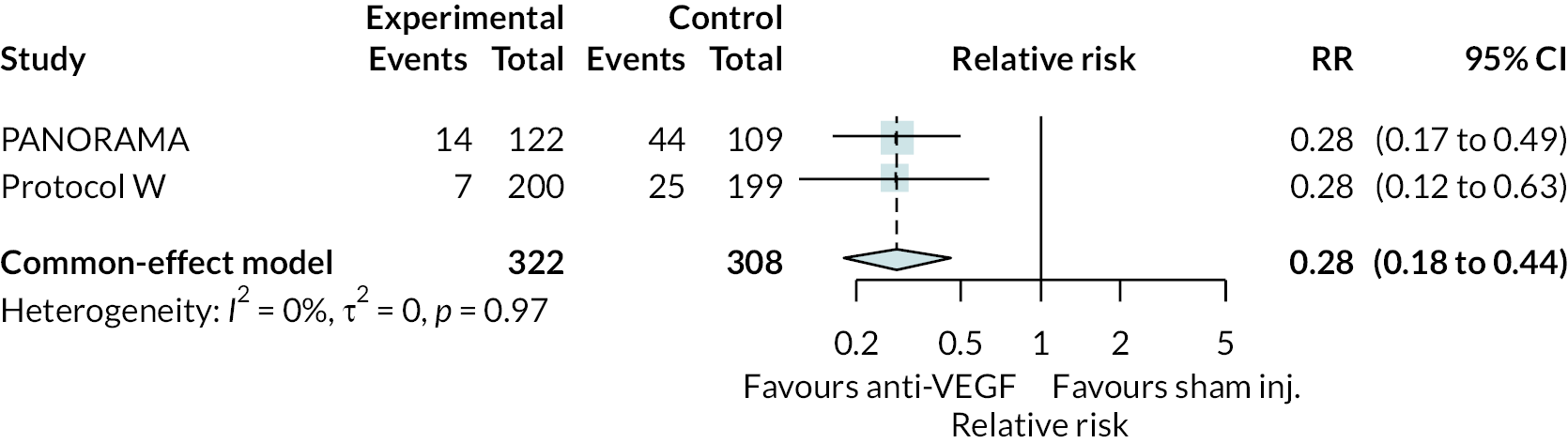
Other outcomes
FIGURE 66.
Non-BCVA outcomes in NPDR trials.
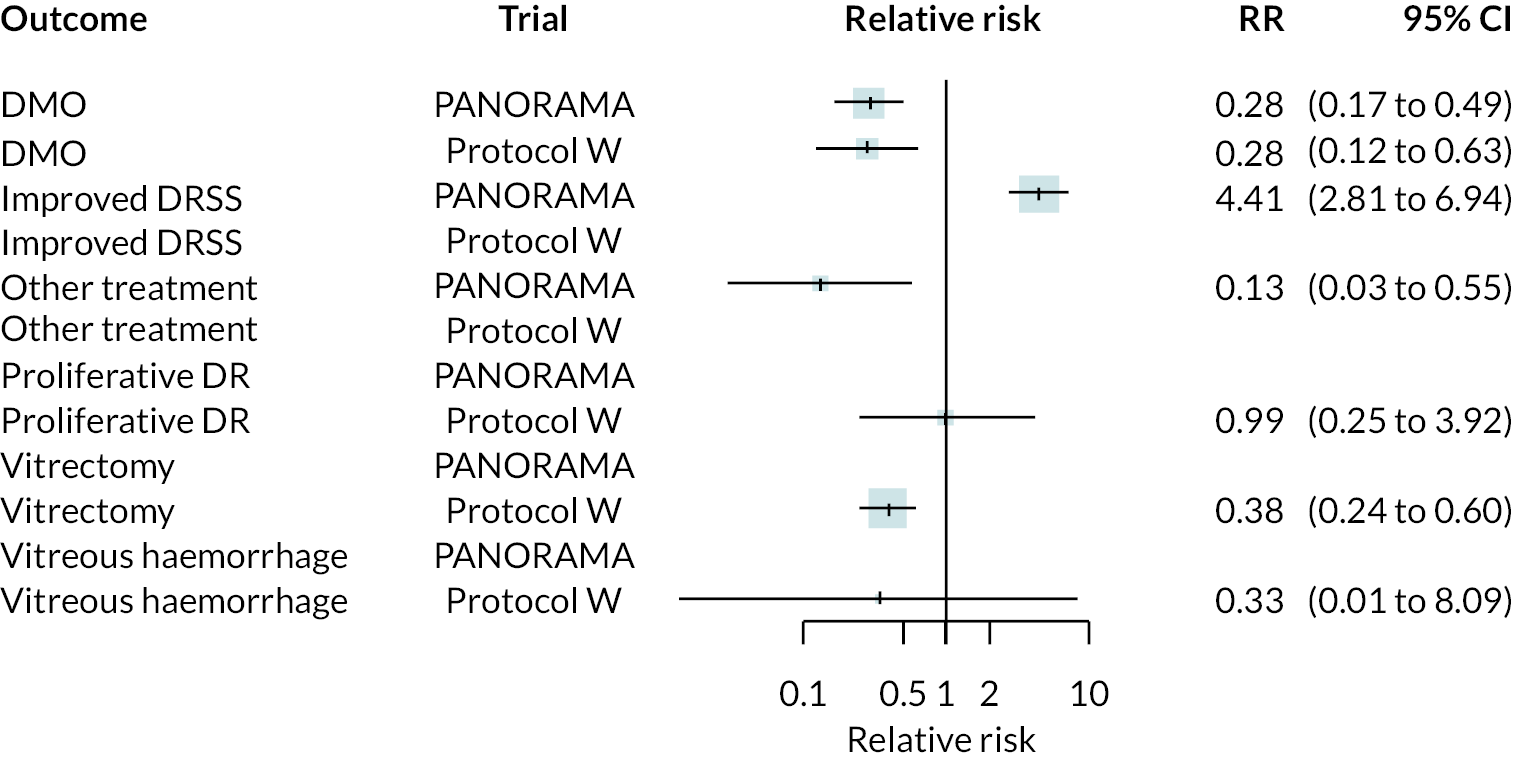
Adverse events
FIGURE 67.
Adverse event outcomes in NPDR trials.