Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/142/07. The contractual start date was in January 2015. The draft report began editorial review in September 2020 and was accepted for publication in April 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Banister et al. This work was produced by Banister et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Banister et al.
Chapter 1 Introduction
Background
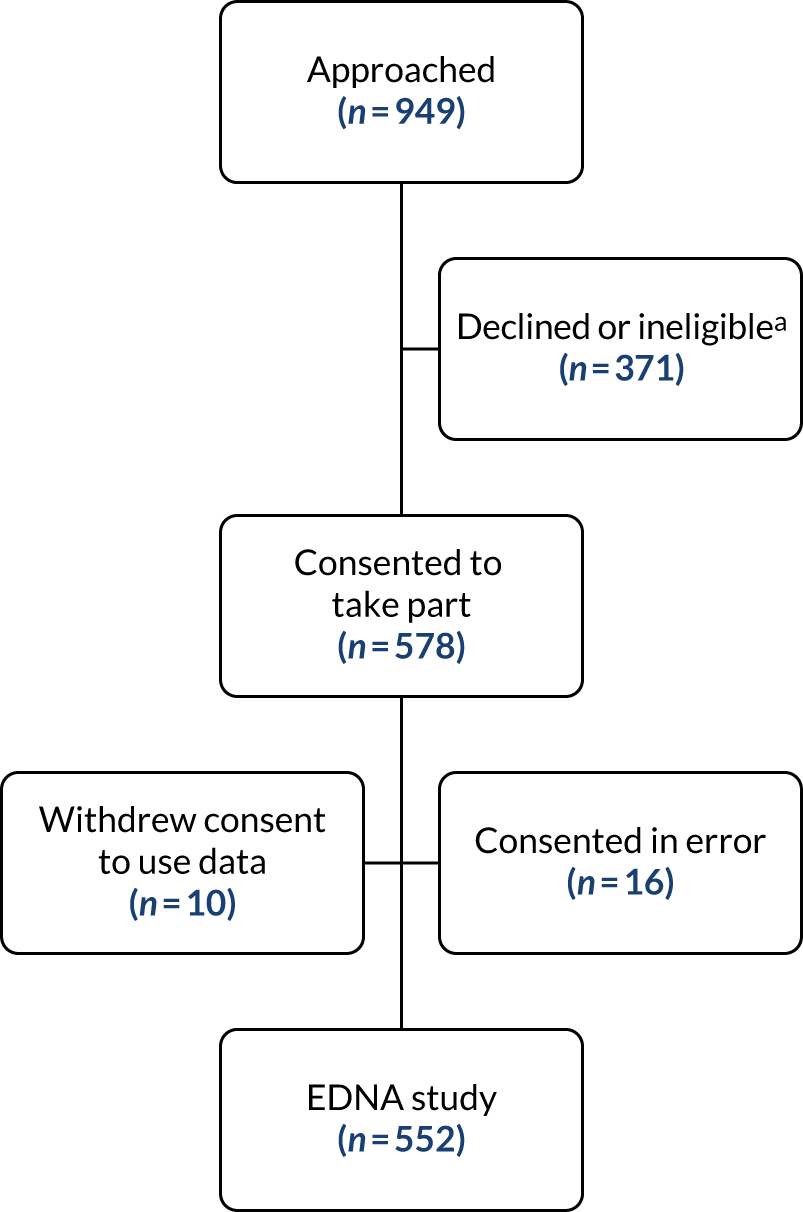
Neovascular age-related macular degeneration (nAMD), also known as wet age-related macular degeneration (AMD), causes severe visual loss and is the most common cause of blindness in people aged > 50 years in the western world. 1 nAMD is defined as the presence of neovascularisation that occurs in a setting of age-related degenerative changes in the centre of the retina, called the macula. The foci of abnormal blood vessels that either arise in the choroid and invade the subretinal pigment epithelial and subretinal spaces or arise de novo within the neurosensory retina leak fluid and blood into the macular tissues and distort its normal architecture (Figure 1). This results in a sudden onset of central visual disturbances that may range from distortion to loss of central vision. Left untreated, the exudation from the nAMD lesion along with the unchecked proliferation of the neovascular complexes destroys the macular tissues, resulting in both scarring and atrophy of the retina and its pigment epithelium (Figure 2); this rapidly progresses to severe and permanent loss of vision. 2
FIGURE 1.
Active nAMD. Each panel consists of one representative frame captured using a colour image, fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA) and optical coherence tomography (OCT).
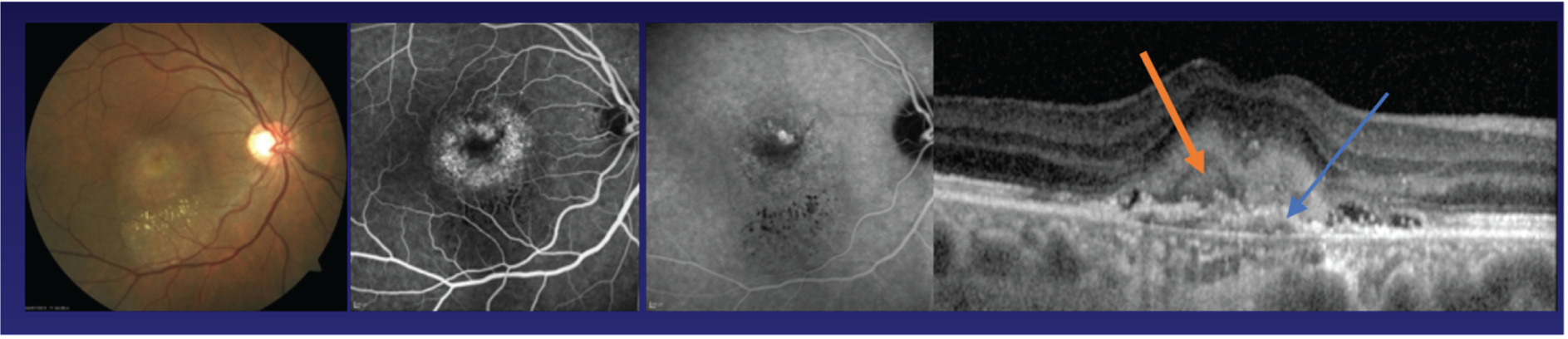
FIGURE 2.
Sequelae of untreated nAMD showing development of scarring. Panel 1, area of macular haemorrhage; panel 2, neovascular complex seen as a greenish-grey lesion; panel 3, area of neovascularisation with overlying subretinal fluid; panel 4, moderately fibrosed nAMD lesion; panel 5, fully fibrosed nAMD lesion.
Standard imaging methods used to detect nAMD are colour photography, optical coherence tomography (OCT), fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA). The panel of multimodally acquired images in Figure 1 show a typical example of nAMD. The first panel is a colour image that shows a central area of discolouration and the collection of yellow exudate. The next panel is a frame from FFA in which retinal blood vessels are outlined in sharp relief to the retinal tissue because fluorescein is retained within the vessels. There is a central bright area of leakage that represents the area of neovascularisation. The third panel is a frame from ICGA that also shows a more focal leak, as indocyanine green binds more tightly to the plasma proteins and remains within the neovascular complex within the choroid. The final panel shows a single B-scan from OCT taken through the region of the neovascular complex. The mid-layers and inner layers of the neurosensory retina are draped over the neovascular lesion, which appears as a mound of hyper-reflective material (orange arrow) located over a shallow irregular elevation of the retinal pigment epithelium (RPE) (blue arrow). This region of hyper-reflectivity is termed subretinal hyper-reflective material, and may consist of serous fluid, fibrin and/or whole blood components that have leaked into the subretinal space.
In recent years, there have been major advances in the clinical management of patients with nAMD, notably the introduction of biological therapies targeting vascular endothelial growth factor (VEGF), a protein implicated in the pathogenesis of this disease.
Anti-VEGF treatments have improved visual outcomes compared with laser therapies, which were the mainstay in past decades. 3 A number of available novel biologics suppress the exudative manifestations in a highly effective manner, resulting in a rapid improvement in visual acuity in treated eyes, averaging around two lines on the early treatment of diabetic retinopathy (ETDRS) acuity charts within 3 months of initiation. 4 All six of the currently available anti-VEGF drugs require repeated invasive administration into the vitreous cavity of the eye because they have limited durability of action. 5 Regarding visual outcomes, clinical trials and real-world studies have shown that acuity improvements seen within 3 months of treatment initiation are maintained in the medium term (about 2 years) in one-third of patients, and a further 40% of those treated will retain visual acuity at their immediate pre-treatment level. 5,6 However, there is a considerable residual burden of visual morbidity. This residual burden of visual disability is clearly evident in the outcomes reported in the pivotal clinical trials, as well as in subsequent trials and post-licensing studies. 7–9
For example, 40% of patients will have acuities of 20/50 (Snellen 6/15) or worse after 2 years of intensive treatment, and the proportion of those with 20/20 (Snellen 6/6) or better acuity (normal vision) is low (< 5%). 7
The reality is that normal vision is still a long way from being achieved despite the use of anti-VEGF therapies, which are the current standard of care. There are a multitude of reasons why these treatments do not restore normal macular function. These reasons include (1) the presence of a neovascular network with a large component of mature vessels that do not regress or permanently close with anti-VEGF treatment; (2) glial and fibrous tissue that distort the delicate cellular architecture of the retina; and (3) neural and RPE cell loss.
Figure 2 shows images of the macula of the eyes of patients with nAMD at initial presentation but at different stages of evolution of the scar. If treatment is provided at a point in time, as seen in panels 1 and 2, the likelihood of avoiding scarring increases dramatically. 10
Permanent morphological damage of the macular tissues at the time of presentation and a degree of irreversible visual loss constitute important barriers to visual gain. 11 Furthermore, in a proportion of eyes, fibrosis and atrophy progressively increase even during anti-VEGF maintenance therapy, and the worsening is more rapid in eyes with long-standing disease when treatment is initiated. 12,13 Therefore, there is a sound rationale to identify methods that detect the onset of nAMD at a stage when the cellular constituents of the retina have the potential to recover, prior to the onset of atrophy or fibrosis, and when the neovascular complexes have not matured to the point at which they are less likely to regress.
There is a body of evidence in the literature to indicate that nAMD in the first eye often remains undetected for long periods, as patients are unaware of any visual deficit because the fellow eye usually has good function and masks the deficit. 1,8 Patients are often more alert to alterations in visual function in the second eye. However, evidence indicates that the second eye has often suffered loss of acuity by the time the patient has sought help or nAMD has been detected. 14 In one study,15 which followed up patients enrolled in a laser prevention trial, the average acuity at presentation when nAMD was detected in the better-seeing eye was 20/100, which represents more than a quadrupling of the visual angle. Reasons for the delay in presentation included (1) the development of the lesion at an extrafoveal location with no early impact on acuity; (2) a lack of sudden onset of a bleed or an acute increase in exudation with involvement of the fovea by these manifestations; and (3) an adjustment to minor changes in visual function.
Approximately 8–10% of patients with nAMD in one eye will develop the same condition in the fellow eye per year. Interrogation of large electronic medical record repositories that have been constructed from patient data collected during anti-VEGF therapy treatment for nAMD from many clinical sites in the UK shows the benefits of initiating treatment when visual acuity is still good. 16 Detection of nAMD at a stage when damage to the retina is not permanent with prompt initiation of treatment could result in much better preservation of sight.
Therefore, there is a clear need for an easily and rapidly performed cost-effective monitoring test that will detect the onset of nAMD with high diagnostic accuracy.
The scale of the problem in the UK and the use of NHS resources
Neovascular age-related macular degeneration remains the most common cause of blindness and partial sight in the UK, despite improvements in treatments. 1,17 The incidence of AMD increases with age and, therefore, the burden is projected to rise steeply in future years as the population ages. Vision loss is associated with a profound impairment of quality of life, increased risk of falling, emotional distress, depression and an inability to care for self and for others. 18 Patients with bilateral vision loss may suffer from visual hallucinations (Charles Bonnet syndrome), poor sleep patterns and loss of confidence. Managing nAMD presents an enormous burden to the NHS. Ophthalmology accounts for 10% (5 million per year) of all outpatient attendances to the NHS and AMD accounts for 15% of all ophthalmology outpatient attendances. 1 This is because patients are typically seen every 2 months for up to 2 years after initiation of anti-VEGF therapy, and long-term studies from the UK show that some 50% of those who are commenced on treatment are still on active treatment or being followed up after 5 years. 19
Evidence for monitoring intervals/diagnostic performance
When active nAMD is confirmed by FFA, treatment with anti-VEGF therapy is initiated. 7,20,21 FFA is the gold-standard diagnostic test for nAMD. In the early phases of treatment (i.e. up to about 1 year), at each subsequent visit, which is usually on an 8-week cycle after the loading phase of 3-monthly dosing, patients are re-assessed to evaluate disease activity. Visual acuity, clinical biomicroscopic examination and OCT are the most commonly employed tests in the follow-up setting (see Figure 3). OCT-guided re-treatment decisions are the standard of care in almost all NHS units (see Figure 4); however, the combination of visual acuity, clinical examination and fluorescein angiography in selected cases are also used in the monitoring phase. 7,20 In the absence of disease activity on the OCT, treatment is withheld and a future review arranged, or the patient is treated and the next follow-up visit is extended.
Patients are also given an Amsler chart to self-monitor their disease and are advised to report immediately any signs of distortion or missing areas. The accuracy of this test compared with the above tests has not been systematically evaluated. Interrogation of the national ophthalmology data set, which is an amalgamation of the electronic records of some 14,500 patients who have received anti-VEGF treatments since 2009 (Usha Chakravarthy is a contributing member), shows that the average number of visits is 10 in year 1 and around eight in year 2. 22 The average interval between visits in year 1 is 35 days (± 10 days). The interval between visits increases in years 2 to 5. However, even in year 5, more than half of all people are on regular review and treatment. Thus, there is an opportunity to obtain information about unaffected fellow eyes of patients with nAMD in one eye to determine the optimum method of early detection of incipient nAMD.
New-onset nAMD responds extremely well to anti-VEGF therapy, with rapid disappearance of subretinal fluid and a more gradual resolution of fibrinous exudate and haemorrhage (see Figure 1). However, any delays can result in worsening of vision, which is mainly because of the development of fibrosis that involves the outer retina. The fibrous tissue replaces the normal cellular layers consisting of the choriocapillaris, the RPE and the photoreceptors. Furthermore, contraction of the fibrous tissue distorts both the adjacent outer retina and the overlying inner retina, changing the architecture and orientation of the cellular elements and resulting in severe visual loss. The initiation of treatment after the onset of fibrosis cannot reverse the vision loss but may stabilise visual acuity. Therefore, early detection of nAMD and treatment before the onset of fibrosis is clearly important.
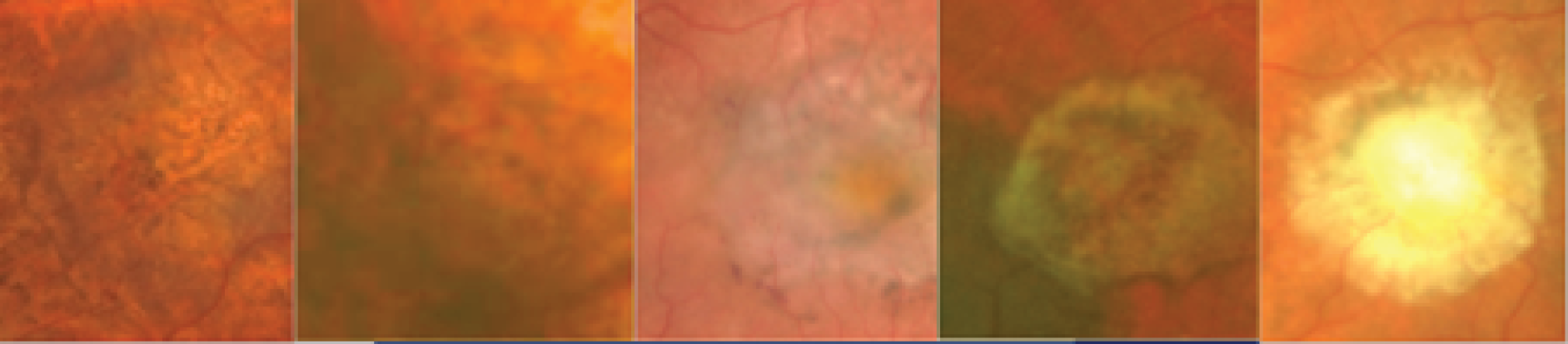
In the example shown in Figure 3, the upper panels show a colour photograph of the fundus (Figure 3a), in which the site of the neovascular complex is indicated by an asterisk; blue light autofluorescence (Figure 3b); an early frame of the fluorescein angiogram (Figure 3c), which shows leakage and pooling of fluorescein dye; and ICGA (Figure 3d), in which the crescentic-shaped outline of the neovascular complex is visible. The OCT scan in Figure 3e) shows fibrinous exudation (shown by the arrow). Anti-VEGF treatment has resulted in the resolution of subretinal fluid (white arrow) and the hyper-reflective material has shrunk after 6 months, with the eye receiving monthly anti-VEGF (yellow arrow) (Figure 3f).
FIGURE 3.
The upper panels show typical features of nAMD using a variety of imaging techniques that are commonly used in clinical practice. (a) Colour fundus photography; the asterisk shows the site of the neovascular complex. (b) Blue light autofluorescence. (c) FFA image showing a distinct and well-delineated region of bright hyper-fluorescence at the site of the nAMD lesion. (d) ICGA image showing the nAMD lesion, but without leakage obscuring the vessel complex. (e) A highly actively leaking exudative lesion. (f) OCT image of the same eye taken 6 months later and showing resolution of the exudation. The bright RPE layer is draped over the shrinking neovascular complex.
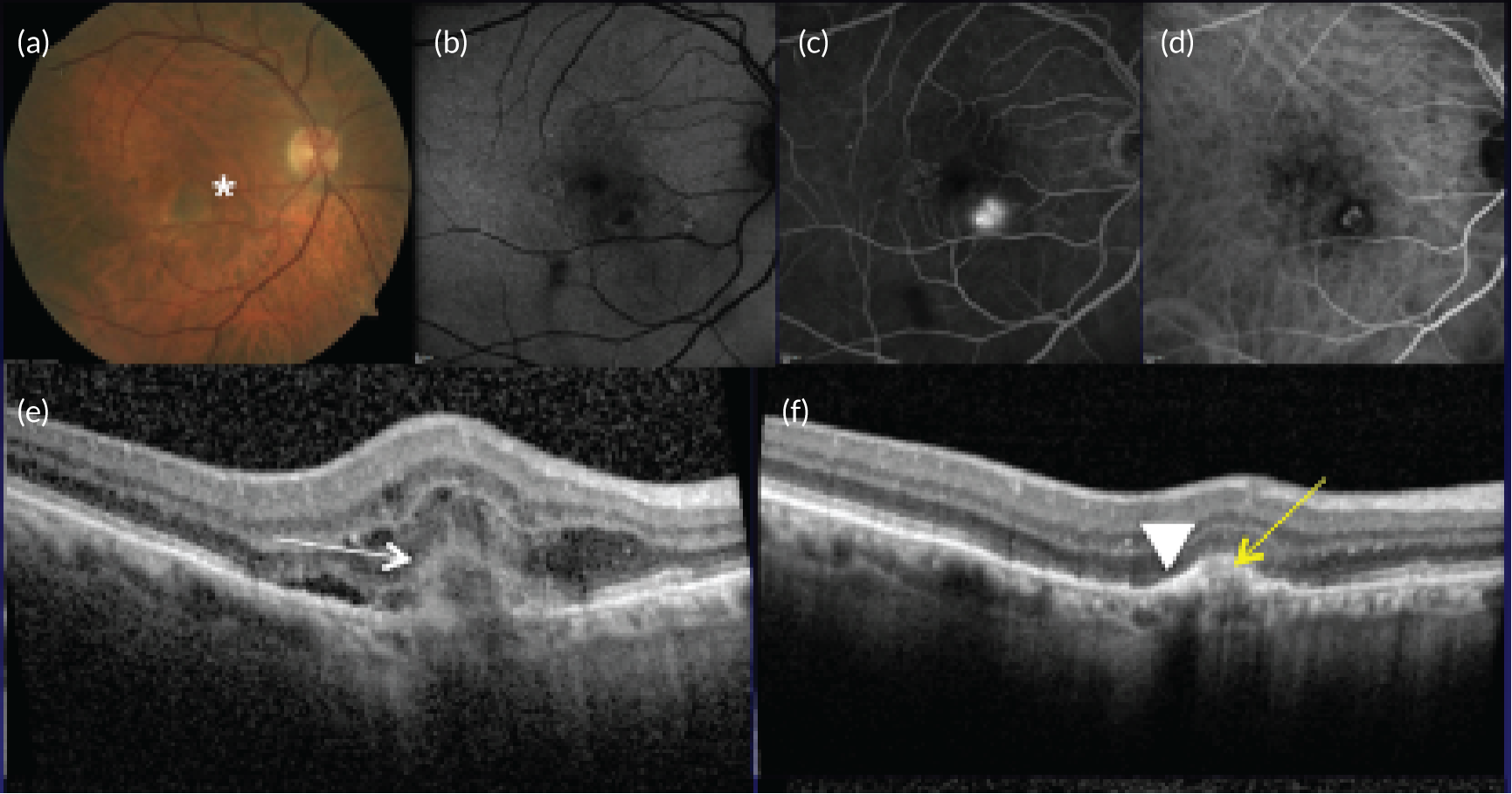
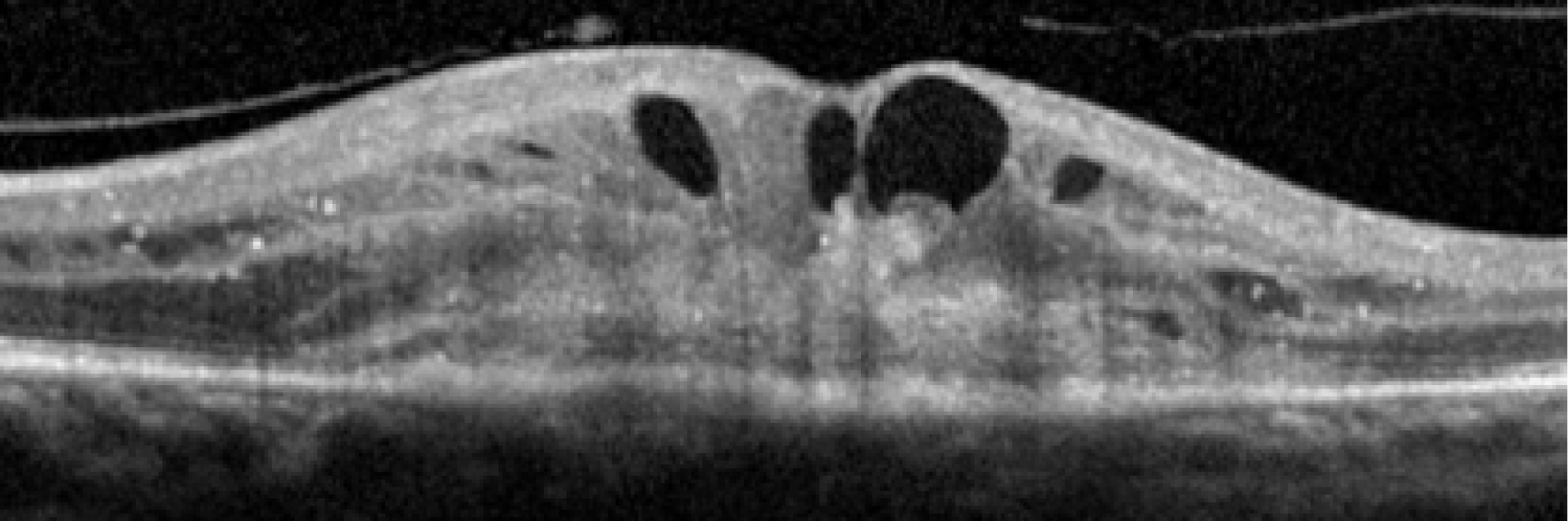
Figure 4 shows an image of an eye in which treatment was initiated at a much later stage of evolution of the nAMD lesion. The normal organisation of the outer retina into distinct hyper-reflective bands consisting of Bruch’s membrane, the RPE and the photoreceptor layers has been lost. Instead, a band of horizontally oriented disorganised layers of hypo- and hyper-reflective material can be seen, which has been shown in previous studies to correspond to fibrous tissue which represents scarring. 23 Because layers of the outer retina have become incorporated into the scar, function is grossly diminished. The overlying inner retina has multiple cystic spaces and is distorted and thickened despite treatment with anti-VEGF agents. The visual acuity did not improve despite multiple treatments.
FIGURE 4.
OCT B-scan showing a band of hyper-reflective material that has replaced the outer retina and is indicative of a fibrotic scar. The inner retina contains many hyporeflective regions, which are cystic spaces.
As stated previously, there are many reasons why the reduction of visual acuity in one eye may not be detected by the sufferer. To unmask a change in vision owing to nAMD, which is usually seen as distortion in the central visual field, a test known as the Amsler chart has been used. This is a simple test that consists of a square grid with horizonal and vertical lines and a central dot as a fixation target printed onto a sheet of paper. Patients are asked to look at the central dot with only one eye (covering the fellow eye). In the absence of nAMD, there is no separation of the retinal layers and the lines on the chart are unaltered. When nAMD is present, the RPE separates from the outer retina, with fluid accumulation in the subretinal or intraretinal locations. This results in distortion of the grid lines and/or the appearance of scotomas (blank spots) in the Amsler chart. However, the Amsler test has previously been shown to have high false positive and false negative rates, which suggests that it may be a poor screening tool. Visual acuity checks have also been used to help detect nAMD, but there may be other causes for a reduction in vision in older age groups. OCT, on the other hand, provides non-invasive images of the retina and, with the reduction in price of such devices and their widespread adoption within the optometric community, this technology offers an alternative method of early detection. To date, to the best of our knowledge, the use of OCT to detect nAMD has not been tested against the gold standard of fluorescein angiography.
Rationale for the study
Scrutiny of the outcomes from large clinical trials and real-world evidence16 shows that if treatment is commenced when acuity is better than 73 letters (Snellen equivalent 6/12), over 90% of people maintain this level of vision or better. 7,21
Better acuity is associated with smaller nAMD lesions and, thus, early detection of nAMD and prompt initiation of treatment will result in final visual outcomes that are consistent with good visual function. The aim of the Early Detection of Neovascular Age-related macular degeneration (EDNA) study was to evaluate the best test or combinations of tests that can be carried out to identify the onset of nAMD at the earliest point so that treatment can be commenced at a stage when therapeutic reversal of exudation using anti-VEGF drugs is accompanied by visual improvement and/or stabilisation of acuity without further loss. This study was possible because:
-
There is a large group of patients whose care pathway requires regular visits and monitoring (every 8 weeks), which offers the ideal situation for a study of early detection of nAMD in fellow eyes of patients with nAMD in one eye.
-
These patients are subjected to tests of function (acuity) and OCT, and it is current clinical practice to acquire information on both eyes at every visit.
-
The OCT examination is quick (performed without the need for pupillary dilatation) and the quality of the tomograms is high because all of the NHS units offering anti-VEGF therapies have invested in high-resolution spectral-domain OCT technology.
-
The patients are used to monitoring any change in their vision using the Amsler chart.
-
The patients are motivated.
-
The national ophthalmology data set has shown that attendance is high, with dropout < 10% per annum. 24
In addition, the study design allowed further valuable data to be collected regarding genetic risk factors and emerging imaging techniques. AMD features are detected even when minimal, and the time taken for AMD to progress is hugely variable between individuals and between the two eyes of an individual. The importance of genetic risk in AMD is well established. Polymorphisms in genes that encode proteins that modulate and control the alternative pathway of complement activation suggest that chronic oxidative stress and low-grade inflammation at the level of the RPE/Bruch’s complex play an important role in determining susceptibility to AMD when defined as any manifestation of this condition at any stage early or late. The genetic risk score influences the rate of progression in the second eye in persons with advanced AMD in one eye. 25 Such information could prove useful in patient counselling and for selection of potential participants for interventions that may prevent progression. With the foregoing in mind we sought to establish a DNA repository in the EDNA study as participants were enrolled at first presentation of their first eye, which would have the virtue of avoiding time-varying confounders.
In addition to providing standard OCT images (also known as structural or B-scan images), some OCT acquisition systems have the capability to acquire optical coherence tomography angiography (OCTA). This is a recent introduction to retinal imaging, which provides images of flowing blood in the microvasculature of the retina and choroid. These imaging systems are now commercially available and being increasingly used in the NHS to aid the detection and diagnosis of nAMD. In some EDNA sites, existing OCTs have been upgraded to permit acquisition of OCTA images. There are emerging data in the literature that suggest that these instruments may reveal hotspots that represent blood flow within developing neovascular membranes, which can be localised to specific layers, such as the outer retina/choroid slab, the outer nuclear layer slab or the inner retinal layers. However, data on the clinical relevance of these instruments in the early detection of nAMD are sparse and there is almost no good-quality data of the sensitivity and specificity of these instruments compared with FFA. In summary, OCTA is a developing technique that yields a variety of parameters, some of which are for the diagnosis of nAMD, but its diagnostic accuracy is not yet proven. Not all centres taking part in the EDNA study had OCTA capability; therefore, OCTA could be opportunistically collected, when available at the clinical site, alongside OCT when a FFA was conducted (triggered FFA, 18 months and 36 months) for future evaluation.
Aims and objectives of the study
The aim of the EDNA study was to identify the optimum non-invasive diagnostic test strategy that will robustly detect nAMD in fellow eyes during follow-up in secondary care of persons with nAMD in the first affected eye.
The primary objective was to determine the diagnostic monitoring performance (sensitivity and specificity) of five index tests for diagnosing nAMD against a primary reference standard of FFA determination of conversion to nAMD at the clinical site. The index tests considered were:
-
Amsler – participants completing an Amsler chart
-
fundus clinical examination – clinical evaluation of the fundus for signs of nAMD
-
OCT – clinical assessment of images captured on OCT
-
self-reported vision – participant’s subjective assessment of their vision
-
visual acuity – ETDRS visual acuity.
The secondary objectives were to:
-
develop an economic model to identify an optimal monitoring regime
-
develop a risk prediction model using baseline characteristics to predict the development of nAMD in the study eye
-
create a cohort (including a biobank) for future studies
-
create a repository of opportunistically collected OCTA imaging data to explore their potential value in the detection of new-onset nAMD.
Chapter 2 Methods
This chapter describes the EDNA study design, the methods for the diagnostic performance and other key statistical analyses. We follow the standards for reporting of diagnostic accuracy studies. 26 The methods for the health economic evaluation are described separately (see Chapter 7).
The full study protocol, including a summary of the amendments, is available on the NIHR report web page (www.journalslibrary.nihr.ac.uk/programmes/hta/1214207). The Office for Research Ethics Committees in Northern Ireland reviewed and approved this study (14/NI/1120) and the study was registered on the ISRCTN (International Standard Randomised Controlled Trial Number) registry (ISRCTN48855678).
Overview of the study design
The EDNA study was a multicentre, prospective, cohort, comparative diagnostic accuracy study that tested five index tests in a monitoring setting. At recruitment (baseline), participants had a diagnosis of nAMD in one eye and a contralateral eye (the EDNA study eye) with no evidence of active nAMD. Participants were monitored during the delivery of care in hospital eye services for up to 3 years. The study was designed to be pragmatic and have minimum impact on the current patient standard-of-care pathway. A schematic of the study design is shown in Figure 5.
FIGURE 5.
Study overview flow diagram.
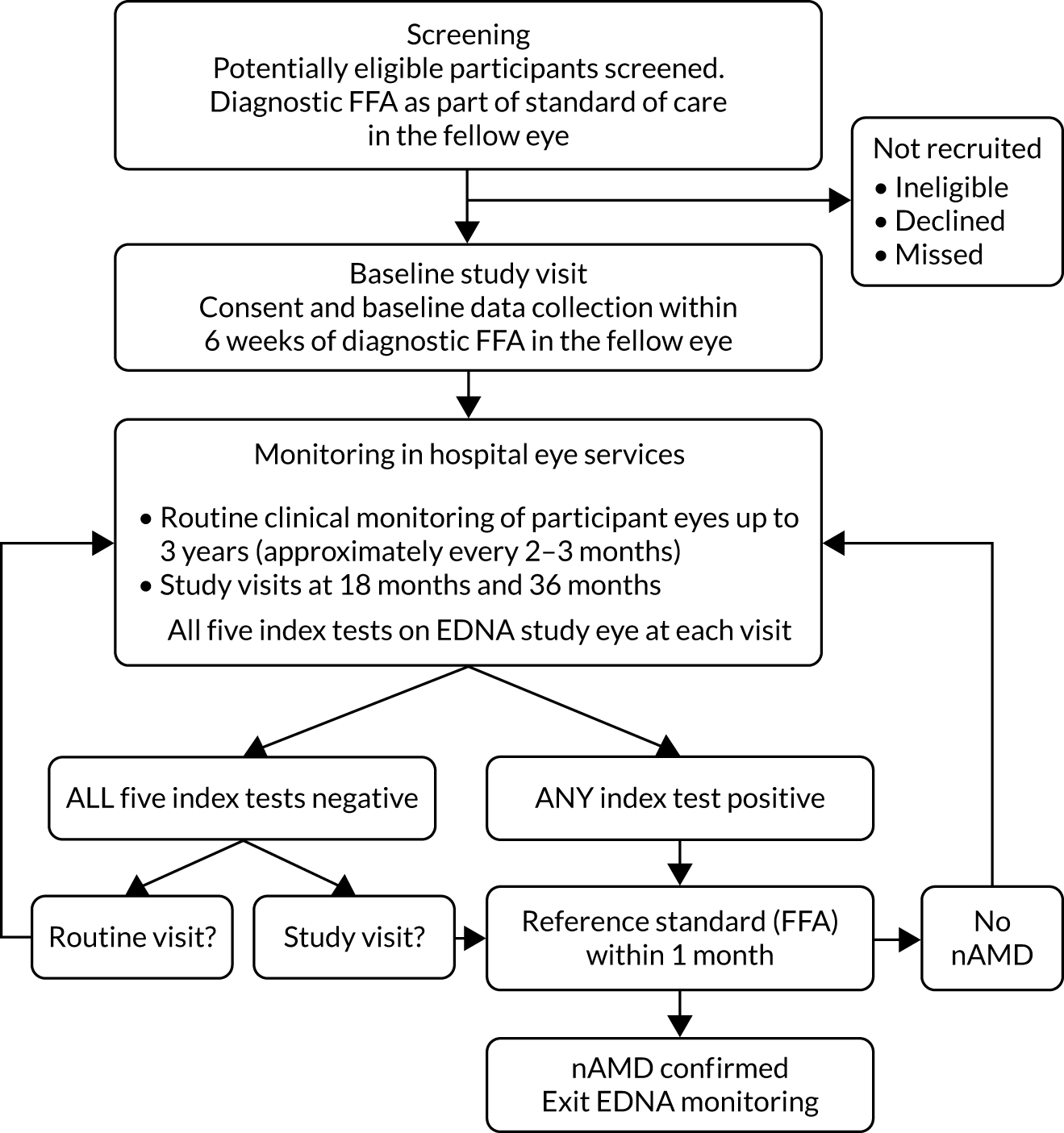
Once enrolled into the study, the diseased eye and the contralateral eye of participants were monitored at routine clinic visits at each clinical site until onset of nAMD was detected in the EDNA study eye or until 3 years after enrolment, whichever occurred first. Clinical sites tended to follow the guidelines of the Royal College of Ophthalmologists27 for monitoring of patients, but there were local variations in the manner of clinical reviews and the intervals between visits. The most common pathway in terms of standard of care in the NHS for patients newly diagnosed with nAMD was treatment with an anti-VEGF agent monthly for the first 3 months, followed by regular (approximately every 4–8 weeks depending on the anti-VEGF drug administered) assessment and re-treatment if required. At each routine monitoring visit, participants were examined and the results from the index tests that were performed at these visits in the EDNA study eye were collected.
A reference standard measurement (FFA) was requested if any of the index tests were positive (Table 1). At 18 months and at study exit (36 months), any participants who had not developed nAMD by these time points had a study visit undertaken and a detailed clinical assessment along with retinal imaging that included FFA.
| Index test | Definition of test positive |
|---|---|
| Amsler test28 | As assessed by the clinician, the appearance of a new area of distortion in the Amsler chart or regions in which the grid pattern disappears (scotoma) when previously no distortion or scotoma was present (this test was included for a participant only if test result was negative at baseline) |
| Fundus clinical examination29 | Slit-lamp biomicroscopy or fundus photography showing clinical signs of nAMD on the fundus, as determined by an expert |
| OCT30 | Abnormal findings indicative of nAMD, such as the presence in the mid-retina and outer retina of hyporeflective spaces or hyper-reflective material |
| Self-reported vision31 | The patient’s subjective assessment of vision is ‘much worse’ than the previous visit on the patient questionnaire (see Report Supplementary Material 1) |
| Visual acuity32 | Drop of ≥ 10 letters in best corrected visual acuity (BCVA) from baseline |
Further details of the index tests and reference standards are found in Description of the index tests and Description of the reference standard: fundus fluorescein angiography.
Study setting
Twenty-four secondary care, NHS ophthalmology outpatient departments (also known as hospital eye services) participated in this study.
Identification of participants and the recruitment process
Consecutive eligible patients were identified by the clinician or research nurse in each centre at the time of diagnosis of unilateral nAMD at first clinical presentation. Potentially eligible patients were given a patient information sheet and those interested in participating in the study were invited to attend a baseline study visit at which eligibility was assessed in full. Once a patient was confirmed as eligible for the study, written informed consent was obtained by an appropriately trained individual and baseline data were collected. The diagnostic FFA, collected prior to recruitment as part of routine care, was used as the baseline FFA. Other EDNA diagnostic tests (except self-reported vision) were collected at baseline if not already obtained as part of routine care within the 6 weeks prior to consent. Participants who consented to blood collection and storage had their blood collected at baseline and at study exit.
Blood collection and storage
Approximately 20 ml of blood was collected at the baseline and exit visit in participants who had consented to donating blood for future research studies. Blood was pseudonymised with the EDNA study number. The samples collected in this study were sent to Queen’s University Belfast for storage and will be stored indefinitely. Research using the samples will be conducted only after approval by a Research Ethics Committee.
Follow-up procedures: delivery of interventions
Once enrolled into the study, the participants were clinically monitored in each site in accordance with local clinical care pathways for a maximum of 3 years. At the time of the study, the standard of care in the NHS for patients newly diagnosed with nAMD was treatment with an anti-VEGF agent, which is injected into the eye with active nAMD. The treatment effect can vary by drug and by individual patient needs. Therefore, clinical care pathways include a 3-month period with monthly intravitreal injections (loading phase) followed by a maintenance phase. In the treatment maintenance phase, some patients continue to require treatment every month but in others the intervals can be extended. Clinics use varying strategies to review patients and determine their treatment needs. However, all clinics perform a clinical examination and measure visual acuity and OCT on both eyes at a large proportion of visits, except when the patient is scheduled for only an injection to the eye without assessment. On average, patients were expected to be seen approximately every 8 weeks, generating a considerable number of data in the maintenance phase. For EDNA participants, study personnel collected the two additional index tests (Amsler and self-reported change in vision in the EDNA study eye). If the baseline Amsler test of the EDNA study eye was deemed to be abnormal, this test was excluded during the follow-up monitoring phase.
If the results of any of the five index tests were positive (see Table 1), a FFA (reference standard assessment) was performed within 1 month, as detailed in the flow diagram (see Figure 5). If nAMD was diagnosed in the EDNA study eye, the participant was no longer required to undertake any index tests and exited the EDNA study. If nAMD was not detected, the participant continued in the EDNA monitoring phase.
To minimise bias in collecting index tests and reference standard results, a standard reporting order for the index tests was created, as shown in the case report forms (see Report Supplementary Material 1). Clinical teams were trained to request participants to collect self-reported vision data before the Amsler test and then the additional index tests whenever possible. FFA results were always collected after the index test results.
Study visits
Participants were examined at 18 and 36 months if they had not been diagnosed with nAMD in the EDNA study eye by these time points. At the study visit, all five index tests were undertaken along with a FFA. These study visits could take place within a 2-month time window either side of the exact due date. Therefore, participants could be monitored for up to 38 months. Participants remained in the study unless they withdrew consent or they were unable to continue for a clinical reason. All data collected up to the point of complete withdrawal were included in the analysis, unless the participant requested this to be destroyed and excluded.
Safety: serious adverse events
Within the EDNA study, only serious adverse events (SAEs) relating to the collection of blood or FFA requested during involvement in the study were recorded. SAEs relating to FFAs conducted prior to recruitment to the study or resulting from treatment to the nAMD eye were not recorded.
Reading centre assessment
An ophthalmic reading centre (Central Angiographic Resource Facility), located at Queen’s University Belfast, independently graded anonymised images (FFA, OCT, fundus photography, autofluorescence – optional) that were acquired at baseline, when any index test was positive, and at study visits (18 months and 36 months/study exit). The data from the graded images were used to provide the baseline and conversion ocular variables for the description of the cohort (see Chapters 3 and 5), the secondary reference standard 2 data set (see Chapter 4) and ocular variables for evaluation in the prognostic model (see Chapter 6). The grading protocol is provided in Report Supplementary Material 1.
Description of the index tests
Amsler chart
The Amsler chart is a grid of horizontal and vertical lines that is used to monitor a person’s central visual field. It is a simple, inexpensive diagnostic tool that aids the detection of visual disturbances caused by changes in the retina, particularly the macula (e.g. macular degeneration). After covering one eye, the person looks with the eye to be tested, fixating at the small dot in the centre of the grid printed on the Amsler test sheet. Patients with macular fluid may see distortion of the straight lines or areas of the pattern may be missing. Patients should have a normal Amsler test in the study eye at baseline. In participants who had distortion on the Amsler chart at baseline, Amsler tests were not collected in the subsequent assessments.
Fundus clinical evaluation for signs of neovascular age-related macular degeneration
Examination of the macula can reveal fluid and/or lipid exudates (yellow deposition) and/or blood. Other features of AMD, such as drusen and pigmentary irregularities, may be observed. Sometimes these latter features are obscured by the exudative manifestations or may be absent in specific AMD phenotypes, such as polypoidal choroidal vasculopathy (PCV). The fundus could be assessed using slit-lamp biomicroscopy or fundus photography.
Signs suggestive of nAMD include the following:
-
Subretinal or subretinal pigment epithelium (RPE) neovascularisation, which may be visible as a dark grey lesion. Occasionally the lesion will have a dark pigmented edge that is thought to be a result of proliferation of the RPE at the edge of the membrane.
-
Serous detachment of the neurosensory retina.
-
RPE detachment.
-
Haemorrhages – subretinal pigment epithelial, subretinal, intraretinal or preretinal. Breakthrough bleeding into the vitreous may also occur, most often indicating the presence of PCV.
-
Hard exudates (lipids) within the macular area related to any of the above and not related to other retinal vascular disease.
-
Epiretinal, intraretinal, subretinal or sub-pigment epithelial scar/glial tissue or fibrin-like deposits.
-
Retinal angiomatous proliferations – a form of intraretinal new vessel complexes arising de novo in the macular retina, which are a subtype of nAMD.
-
Choroidal polyps – spherical lesions associated with choroidal vessels that cause the RPE to be focally elevated (PCV).
Optical coherence tomography clinical assessment of images
Optical coherence tomography is a light-wave-based technology that produces cross-sectional images of the retina with scan rates and resolution parameters that have greatly improved over the last 10 years. It is a non-invasive, non-contact visual test, which is rapidly and easily performed and takes < 5 minutes to assess both eyes. 33 Tomograms are acquired by trained medical photographers. The tomogram is a sequential collection of some 25,000 A scans (reflectivity profile in depth), which are sequentially incorporated into a cross-sectional image of the retina generating a B-scan. A series of B-scans are constructed across the macular region of the eye and, depending on the orientation of the scan, can be a rectangular raster or a star pattern. The density of the scan lines can be modified from widely to tightly spaced, with the latter providing more detailed information. The scans can be displayed in three-dimensional mode to provide information on the various retinal layers. Automated segmentation algorithms provided by the manufacturer generate averaged retinal thickness and volume measurements for regions (sectors) of the retina. These algorithms have been shown to provide consistent and reliable estimates in normal eyes. However, in the presence of disease with alterations in the retinal layer anatomy, the algorithms frequently fail, leading to considerable error and variability in the segmentations; therefore, the thickness and volume measurements are generally unreliable. At present, many groups, including one of the applicants, are exploring the use of automated segmentation algorithms on the imaging outputs. Promising results indicate that automated segmentation is a reality and that subjectivity of interpretation may be replaced in the future by objective computerised assessments.
The separation of the various retinal layers can be seen on a scan and there may be deviation in the interfaces between the layers and/or alterations in reflectivity. In nAMD, abnormal dilations and the growth of blood vessels in the retina and choroid can result in fluid and/or blood seeping into the various tissue spaces, changing the normal retinal architecture and/or altering the normal reflectivity. 30 These characteristics are noted and reported by clinicians experienced in the interpretation of OCT.
When abnormalities as a consequence of nAMD develop in the retinal and choroidal circulations (such as dilation of existing vessels or growth of new vessels), there is an accumulation of fluid within the macular tissue compartments with separation of the normal tissue interfaces. In addition, seepage of haemoglobin and other cellular and proteinaceous or lipid constituents of blood into the retina can cause alterations in the internal reflectivity and homogeneity of the retinal layers. These can take the form of areas of dense hyper-reflective material or foci. The appearance of abnormalities when previously there was none, as well as their spatial localisation and distribution, can alert the clinician to the onset of nAMD even when the signs are subtle and only just discernible.
For the purpose of this study, any OCT machine could be used for data collection. No validation was required for OCT imaging data collection. There were four manufacturers of OCT used in the study: Heidelberg (Heidelberg Engineering, Heidelberg, Germany), Zeiss (Carl Zeiss Meditec, Jena, Germany), Topcon [Topcon (Great Britain) Medical Limited, Newbury, UK] and Nidek (Nidek Co. Ltd, Aichi, Japan). The most commonly used OCT was Heidelberg (13 sites); four sites had Heidelberg and Topcon, two sites had Zeiss only, two sites had Topcon only, two sites had Heidelberg and Zeiss, and one site had Nidek.
Self-reported vision: participant’s subjective assessment of their vision
The onset of exudative AMD may be heralded by the appearance of central visual blurring and distortion. Patients may complain that straight lines appear crooked or wavy when the lesion involves the central macula.
At each follow-up visit, participants were asked the following question: ‘How is your vision in the (unaffected) eye compared with the last visit?’. There were four possible answers to this question: ‘about the same or better’, ‘a bit worse’, ‘worse’ or ‘much worse’.
Visual acuity
Patients with new-onset nAMD will usually have a decrease in best corrected visual acuity (BCVA). Visual acuity is a measure of the spatial resolution of the visual processing system. It is a psychophysical test requiring a response from the person to be tested. Usually high-contrast letters of diminishing size are displayed on a chart at a set distance. The most commonly used chart is the ETDRS chart, which is based on a geometric progression of letter sizes with five letters in each row. A three-line difference in either direction from any given line represents a halving or a doubling of the visual angle. BCVA provides a measure of resolution at the fovea. A change of five or more letters (one full line) on the ETDRS chart is considered to be within the limits of the reliability and reproducibility of the measurement. Therefore, a drop of ≥ 10 letters will be considered to be a true reduction in BCVA.
Description of the reference standard: fundus fluorescein angiography
Fundus fluorescein angiography is currently the reference standard for diagnosing choroidal neovascularization (CNV) in AMD (i.e. nAMD). A fluorescein angiogram is a sequence of images of the fundus that is usually captured over a 10-minute period after injection of the non-toxic dye fluorescein isothiocyanate into a suitable peripheral vein. nAMD is diagnosed by FFA. A technician or photographer performs the test, which is interpreted by an ophthalmologist. Pupils need to be dilated prior to the test. nAMD can be classified on the basis of the temporal and spatial features of the patterns of fluorescence as observed on the FFA.
Classic choroidal neovascularization
Classic choroidal neovascularization is said to be present when an area of well-delineated hyperfluorescence appears in the early phases of the FFA. Most commonly, classic CNV represents new vessels that have breached the RPE and lie in the subretinal space. Sometimes a typical lacy pattern of hyperfluorescence is observed in the very early phase of the angiogram, which corresponds to the vascular profiles before the fluorescein has leaked out of these vessels and obscured the margins. Classic CNV also leaks aggressively and, hence, there is considerable pooling of fluorescein dye in the subretinal space in late frames of the angiogram. Multimodal imaging shows that these neovascular complexes lie between the RPE and the neurosensory retina and have a feeder vessel arising from the choroidal circulation.
Occult choroidal neovascularization
As the name suggests, occult choroidal neovascularization refers to the presence of leakage without clear evidence of neovascular profiles in the early angiographic images. Two types of occult leakage are recognised. The first is a characteristic stippled hyperfluorescence that occurs early and is located at the level of the RPE. The RPE layer is elevated and in the later phases of the angiogram there is increasing hyperfluorescence and pooling of dye in the subretinal pigment epithelial space. The pattern of leakage suggests new vessels between Bruch’s membrane and the RPE and it is, therefore, considered to be a fibrovascular pigment epithelial detachment (FPED). The second pattern of occult leakage is a more diffuse hyperfluorescence with poorly demarcated boundaries that occurs late in the angiographic phase, generally after 2 minutes have elapsed since the injection of dye. There is no corresponding hyperfluorescence in the early frames and there is shallow elevation of the RPE. This type of leakage is referred to as late leakage of indeterminate origin (LLIO). OCT has shed further light on these patterns of leakage and has revealed that the neovascular complexes of FPED and LLIO patterns are present in the subretinal pigment epithelial space causing irregular elevation of the RPE.
Retinal angiomatous proliferation
This type of neovascularisation consists of intraretinal telangiectatic blood vessels that are strongly associated with serous pigment epithelial detachments and a form of drusen known as reticular pseudodrusen.
A better scientific term for these types of drusen is subretinal drusenoid deposits because the material accumulates in the outer retina (in the zone containing the photoreceptor matrix). By contrast, classic drusen, which are the hallmark of AMD, accumulate as nodules or diffuse amounts of material between the RPE cells or between the cells and their basement membrane.
Polypoidal choroidal vasculopathy
Polyps are said to be present when individual choroidal vessels exhibit focal dilations. 34 On colour imaging, these appear as orange or red nodules. On dynamic contrast imaging (fluorescein and indocyanine green), polyps become visible, well-defined focal, round areas of hyperfluorescence or hypercyanescence, which can be pulsatile. Polyps are best visualised on ICGA because this dye is tightly bound to plasma proteins and, therefore, does not leak out into the choroidal vascular bed. Polyps are often associated with haemorrhage and lipid exudates. The presence of a haemorrhagic pigment epithelial detachment (PED) is highly suggestive of the presence of this phenotype. ICGA is recommended if the combination of FFA and OCT features suggest presence of PCV. 1
A more recent classification based on FFA and OCT combined has been proposed by an international panel of experts (Consensus on Neovascular AMD Nomenclature criteria), which included the chief investigator for the EDNA study. 35 The new definitions classify CNV into type 1 and 2 with type 1 having vessels that have not breached the subretinal space and type 2 with vessels that ramify in the subretinal space. Retinal angiomatous proliferation (RAP) lesions form category 3 and PCV has been included in type 1 sub-category aneurysmal. These criteria were applied to classify nAMD subtype by the reading centre.
Primary reference standard: fundus fluorescein angiography determination of conversion to neovascular age-related macular degeneration at the clinical site
The primary reference standard was the clinical interpretation of the FFA by the participant’s clinician. A positive reference standard (FFA) test was one that showed typical changes of nAMD, as described above, and as determined by an experienced ophthalmologist.
Secondary reference standard 1: clinical determination of conversion to neovascular age-related macular degeneration at the clinical site
In the event that the FFA was negative or inconclusive, the clinician diagnosis of nAMD was based on all of the available clinical information. Should the clinician give a positive diagnosis of nAMD during the 3-year follow-up, this was the end of EDNA study monitoring for the participant. At the EDNA study exit visit, if a FFA was not performed, the clinical diagnosis was assessed in the absence of the FFA.
Secondary reference standard 2: reading centre determination of conversion to neovascular age-related macular degeneration
All FFA results that were collected at the site were uploaded to the reading centre to independently determine the nAMD diagnosis. The methods for the grading and interpretation of the images are shown in Report Supplementary Material 1.
Outcome measures
Primary outcome measure
The primary diagnostic performance outcome was the sensitivity and specificity of the index tests in the detection of nAMD in the study eye in a monitoring setting. The primary economic outcome was the incremental costs (to the health service) per quality-adjusted life-years (QALYs) gained.
Secondary outcome measures
The secondary diagnostic performance outcomes included the diagnostic odds ratio (DOR), likelihood ratio and proportion of indeterminate tests. The performance of combinations of tests was also evaluated. Other outcomes included the time gain of early detection; the visual acuity at diagnosis; the performance of a risk predictor algorithm according to baseline characteristics; the establishment of a well-characterised cohort of clinical and biological data for future research; and the creation of a repository of optical coherence tomography angiography (OCTA) data to explore its potential value in the detection of new onset nAMD.
Sample size
The sample size calculation was based on comparative diagnostic accuracy to ensure the ability to detect differences in sensitivity and specificity between candidate tests based on McNemar’s test. 36 Under the primary analysis, a positive candidate test result was defined as any positive result during the monitoring period on the respective test. At a two-sided, 5% significance level and 90% power, a paired difference of 15% (80% to 65%) in sensitivity required 491 participants (560 participants allowing for indeterminate/missing data results, including participants lost to follow-up cumulatively of up to 12%), given a cumulative incidence of 28% at 3 years. 37 This calculation assumed a disagreement between tests of 0.30 based on data from a diagnostic study involving OCT for diagnosis glaucoma. 38 A smaller difference in specificity would be identifiable (7%; 94% to 87% with power and significance levels as before), even if the maximum level of disagreement occurs given that most participants were not expected to convert during the 3-year follow-up period. The reference sensitivity and specificity values used in this calculation are the values observed for OCT in a pilot study with a similar study design. 39
Differences in sensitivity and specificity of at least 20% would also be detected at the same power and significance levels, even if the sensitivities/specificity were substantially lower (e.g. 60% to 40%) or the number of missing data was larger (e.g. 20%). These calculations conservatively assumed maximum possible disagreement between tests. A sample of this size would be sufficient for other measures of diagnostic performance (e.g. the sensitivity and specificity of individual technologies will be estimated to 95% confidence interval (CI) of width 16% and 10%, respectively, given a sensitivity/specificity of ≥ 65%). Such a sample would also provide a sufficient sample for the generalised estimating equations (GEE) analysis, given the anticipated gain in precision owing to the use of multiple repeated measures over time. 40 Similarly, this sample will be sufficient for the development of a risk prediction model with over 130 events (conversions to nAMD) anticipated and given that 10 events per predictor variable/contract are typically recommended. 41
Statistical analyses
Analyses were conducted using Stata® version 15 (StataCorp LP, College Station, TX, USA). All analyses were conducted following a predefined detailed statistical analysis plan, which can be found in Report Supplementary Material 2. The baseline characteristics of participants are summarised descriptively in Chapter 3 from the information collected in clinic and from the reading centre assessment of the baseline images. Chapter 4 describes the results of the time to conversion and diagnostic monitoring performance analyses. Chapter 5 summarises descriptively the key imaging characteristics at the time of conversion from the reading centre analysis. Chapter 6 describes the results of the prediction model analysis.
Time to conversion to neovascular age-related macular degeneration survival analysis
The survival distribution of conversion to nAMD (defined using the primary reference standard in a first analysis and as secondary reference standard 1 in a second analysis) over the follow-up period was estimated. Participants who did not convert to nAMD were censored at the time of their last available observation. A Kaplan–Meier curve was fitted to estimate the underlying nAMD conversion distribution and the time of conversion was estimated based on the date of conversion confirmation. The distribution was estimated assuming parametric distributions and addressing the interval nature of the data using the stintreg command in Stata®. Exponential, Weibull and log-normal proportional hazard survival models were fitted. Using an accelerated time failure approach, a generalised gamma-distribution was used and related assessments of fit were summarised. The date of participants developing nAMD was assumed to be the date of the FFA confirming nAMD. In a sensitivity analysis, this date was altered to a time halfway between the positive FFA and the previous negative FFA.
Diagnostic monitoring performance of the index tests
Main analysis
Under the main analysis approach (analysis A1, see Table 3), repeated monitoring test results were collapsed over the whole monitoring period to give a single candidate test result (positive or negative) as shown in Table 2. This was compared with a single final FFA determination of conversion to nAMD at the clinical site from the participant. Several alternative approaches, in which the definitions for reference standard and index tests were varied, were predefined to test the robustness of the findings, and are described in Table 3.
| Reference standard status | Index test positive | Index test negative |
|---|---|---|
| Reference standard positive: nAMD | True positives: any reference standard result indicates nAMD in study eye AND any positive index test result during valid follow-up period | False negatives: any reference standard result indicates nAMD in study eye AND index test is negative throughout valid follow-up period |
| Reference standard negative: no nAMD | False positives: all reference standard results indicate no nAMD in study eye AND any positive index test result during valid follow-up period | True negatives: all reference standard results indicate no nAMD in study eye AND index test is negative throughout valid follow-up period |
| Analysis number | Analysis description | Reference standard definition of disease | Index test definitions |
|---|---|---|---|
| A1 (main analysis) | Primary diagnostic analysis: diagnostic performance of the individual tests. Paired comparison (McNemar’s test) of sensitivity and specificity between the tests | Primary | A |
| A2 | Secondary diagnostic analysis: diagnostic performance of the individual tests. Paired comparison (McNemar’s test) of sensitivity and specificity between the tests | Primary | B |
| A3 | Secondary diagnostic analysis: diagnostic performance of the individual tests. Paired comparison (McNemar’s test) of sensitivity and specificity between the tests | Primary | C |
| A4 | Secondary diagnostic analysis: diagnostic performance of the individual tests. Paired comparison (McNemar’s test) of sensitivity and specificity between the tests | Secondary reference standard 2 | A |
| A5 | Secondary diagnostic analysis: diagnostic performance of the individual tests. Paired comparison (McNemar’s test) of sensitivity and specificity between the tests | Secondary reference standard 2 | B |
| A6 | Secondary diagnostic analysis: diagnostic performance of the individual tests. Paired comparison (McNemar’s test) of sensitivity and specificity between the tests | Secondary reference standard 2 | C |
| A7 | Secondary diagnostic analysis: diagnostic performance of the individual tests. Paired comparison (McNemar’s test) of sensitivity and specificity between the tests | Secondary reference standard 1 | A |
| Sensitivity analyses | |||
| SA1 | Uses OCT from the reading centre. Diagnostic performance of OCT and a paired comparison of the OCT and the other tests | Primary | A: reading centre OCT |
| SA2 | A positive subjective vision is defined as ‘worse’ or ‘much worse’. Diagnostic performance of subjective vision and a paired comparison of subjective vision and the other tests | Primary | A: subjective vision ‘worse’ or ‘much worse’ |
| SA3 | A positive visual acuity is a drop of ≥ 20 letters. Diagnostic performance of visual acuity and a paired comparison of visual acuity and the other tests | Primary | A: visual acuity drop of ≥ 20 letters |
| SA4 | A positive visual acuity is a drop of 10 letters from the last FFA-confirmed false positive. Diagnostic performance of visual acuity and a paired comparison of visual acuity and the other tests | Primary | A: visual acuity drop of ≥ 10 letters from the last FFA-confirmed false positive |
| SA5 | Indeterminate FFA and indeterminate index tests taken as positive. Diagnostic performance of the individual tests and a paired comparison of sensitivity and specificity between the tests | Primary: indeterminate FFA results defined as positive | A |
| SA6 | Indeterminate FFA and indeterminate index taken as negative. Diagnostic performance of the individual tests and a paired comparison of sensitivity and specificity between the tests | Primary: indeterminate FFA results defined as negative | A |
To be included in the main analysis, the following criteria were applied for each of the five index tests separately. If the last FFA result for a participant showed no nAMD, the participant was included if there was at least one prior index test result during the follow-up period. If the last FFA result for a participant showed nAMD, and the index test result was within the previous 3 months, the participant was included. If a participant did not have an index test result within the previous 3 months of their last FFA, the last FFA was not included. In such cases, the previous FFA result from the participant obtained during EDNA monitoring was used if satisfying the two rules above. If no follow-up FFA was available that satisfied either criteria, the participant was excluded from the assessment of that index test. The valid follow-up period for the index tests was then defined as between baseline and the date of the last valid FFA (as defined above).
Index test result definition for the main analysis
For each index test, multiple test results were collapsed into a single test result. Any positive test result over the valid follow-up period was classed as an overall positive result. To be a negative index test result, all index test results must be negative. The classic 2 × 2 table for assessing diagnostic accuracy with our definitions is provided in Table 2.
Sensitivity and specificity were calculated with 95% CIs calculated using the Agresti-Coull method. 42 Positive and negative likelihood ratios were calculated with 95% CIs calculated using the method in Zhou 2002. 42 The positive and negative likelihood ratios quantify how much the estimated probability of an individual having the disease should increase or decrease given a positive or negative test result, respectively. Diagnostic odds ratios and the proportion of indeterminate tests were calculated with 95% CIs. A receiver operating characteristic curve was plotted and the area under the curve (AUC) was calculated using the trapezoidal rule for visual acuity tests. The standard error for the AUC was calculated using the method of DeLong et al. 43 and was used to form an asymptotic normal 95% CI.
Monitoring sensitivity and specificity of the tests were compared using McNemar’s statistical test (with 95% CIs produced using Newcombe’s method). 44
For comparing sensitivities under the primary analysis, McNemar’s 2 × 2 table was constructed using only participants who had a positive FFA result. They were classified as having a positive index test if the index test had any positive results during the valid follow-up period. They were classified as having a negative index test if all prior available tests were negative during their valid follow-up period.
For comparing specificities under the primary analysis, McNemar’s 2 × 2 table was constructed using only participants who had negative FFA results throughout using the same index test classification.
Alternative time periods for inclusion of the index test result
Two alternative, more restricted approaches to including test results in the analysis were predefined. Analysis A2 collapses the index tests as shown in Table 2, but used index tests from only the individual’s last 6 study months (not the entire study period). A positive index test result occurring outside the 6-month window, therefore, did not lead to a positive test result at the collapsed individual level. The individual diagnostic performance of the tests were calculated using this index test definition and the McNemar comparisons of sensitivity and specificity, as detailed above.
Analysis A3 used the index test at the individual’s last study visit only. If a participant developed nAMD according to the reference standard during the follow-up period, only the index test from the visit at which nAMD was diagnosed were used. If a participant did not develop nAMD during the follow-up period, index test data from the last visit for which their reference standard was available were used.
Secondary reference standards
Two secondary reference standards were evaluated:
-
Secondary reference standard 1 – clinical determination of conversion to nAMD at the clinical site.
Reference standard is positive if the clinical interpretation of FFA indicates nAMD or a clinician diagnosis of nAMD at any time point. The reference standard is negative if the clinical interpretation of FFA is negative/inconclusive/not carried out and the clinician diagnosis is negative.
-
Secondary reference standard 2 – reading centre determination of conversion to nAMD.
The reference standard is positive if the reading centre FFA indicates nAMD at any time point and negative if it indicates no nAMD throughout.
Combination of tests
Using the primary reference standard and all available index test results over the monitoring period, the impact of combining OCT with each of the other index tests was considered using two approaches: (1) both positive and (2) either positive. For approach (1), a visit at which both of the index tests were positive was defined as a positive combined result and a negative combined result was produced if there were no visits at which both of the index tests were positive. For approach (2), at least one positive index test result from either of the tests resulted in a positive combined result, and a negative combined result was produced if there were no positive index test results from both of the tests. Two post hoc test combinations were evaluated. One test combined Amsler, fundus clinical examination, visual acuity and self-reported vision. The other test combined Amsler, self-reported vision and visual acuity. Both combinations assumed that any positive test leads to a positive combination test result. These further combination tests were motivated by considerations of the preliminary findings for the health economic model.
Sensitivity analyses
Several sensitivity analyses were predefined using the primary reference standard, and are detailed in Table 3. These explored possible threshold effects for two of the index tests (visual acuity and self-reported vision) and inclusion of indeterminate reference standard results. A planned sensitivity analysis to use OCT test results as assessed from the reading centre as an alternative diagnostic test (rather than OCT assessed at the clinical site) was not possible because the OCT result was assessed in conjunction with an assessment of the respective FFA at the reading centre.
Subgroup analyses
We undertook a pre-planned subgroup evaluation according to the type of nAMD in the study eye (CNV and RAP) for the main analysis. These subgroup analyses were classified as exploratory and were evaluated at the two-sided 5% significance level, and the individual group sensitivity (with 95% CIs) was calculated. It was not possible to quantify the proportion of classic disease (for the CNV subtype only) as planned because the data could not be retrieved.
Secondary complex analysis utilising repeated test results
A GEE modelling approach was used to allow the simultaneous modelling of sensitivity and specificity in a regression framework and the use of multiple test results per participants over time. This had the advantage of allowing a flexible regression framework (with easy comparison between tests), allowing for clustering of observations by participants and incomplete data without requiring extensive distributional assumptions. The GEE modelling was applied using the primary reference standard.
It was assumed that at time points when index tests were available but the reference standard was not available, the reference standard result was negative until a positive reference standard finding occurred. An independent correlation structure with robust standard errors was adopted (xtgee Stata command).
Handling of missing data
The absence of a positive reference standard during a valid follow-up period was presumed to indicate a negative result. Indeterminate tests were treated as missing, but sensitivity analyses 6 and 7 looked at the impact of this assumption. For the primary analysis, if the last available reference standard was missing/indeterminate, the previous reference standard was used with index data curtailed accordingly. If no reference standards were carried out after the baseline reference standard, then that participant was excluded from the analysis. If the FFA result was indeterminate or missing, it was excluded unless a curtailed follow-up period with a valid FFA and index test result existed.
Prognostic model development
A risk prediction model using Cox regression was developed to predict development of nAMD (defined using secondary reference standard 1) in the EDNA study eye using baseline candidate predictors.
Sample size
The prognostic model was a substudy within the EDNA study and, therefore, its sample size was limited to the number of participants available for the diagnostic accuracy study.
Candidate predictors
The baseline variables that were considered for inclusion in the model were selected through discussion between the clinicians and the statisticians of the study. They included person-specific risk factors collected via a baseline case report form completed at the site (age, raised blood pressure, smoking history, cardiovascular disease, diabetes, sex, nutritional supplements, family history of AMD and body mass index); ocular variables in the EDNA study eye [previous cataract surgery, baseline visual acuity, drusen type, maximum size of drusen, most frequent size of drusen, presence of pigmentary abnormalities in the fundus, retinal thinning, choroid thinning, external limiting membrane (ELM) disruption and ellipsoid zone (EZ) disruption]; and ocular variables in the first-presenting eye (type of wet AMD, lesion size and total lesion area). Ocular variables were collected from reading centre baseline image grading, with the exception of previous cataract surgery and baseline visual acuity that were collected via a baseline case report form completed at the site. Fractional polynomials were used to explore the presence of non-linear relationships of the continuous predictors (age, baseline visual acuity and total lesion area). To assess the association between each candidate predictor and the outcome, we conducted univariate analysis using Cox regression and calculated the hazard ratio and respective 95% CIs.
Analysis and selection of candidate predictors
We used a multiple Cox regression to estimate hazard ratios and 95% CIs for the candidate predictors. We initially ran the multiple Cox regression including all candidate predictors (‘full model’). Owing to the large list of candidate predictors and given the limited sample size, a backwards elimination process was implemented using a p-value of 0.10 to select predictors for the model. The ‘final model’ was estimated by including the selected predictors from this process. As part of a sensitivity analysis, we varied the threshold (from p = 0.05 to p = 0.20) to assess its effect on the final model.
Model performance
The final model’s predictive performance was assessed in terms of discriminative ability using Harrell’s c-index and accompanying 95% CIs. As a post hoc sensitivity analysis, we assessed the impact of two variables on the c-statistic: type of drusen and presence of cataract. Calibration was assessed visually by plotting the Kaplan–Meier survival estimates of risk groups against the monitoring time. Four risk groups were calculated (divided by the 16th, 50th and 84th centiles of linear prediction risk). 45
A planned exploratory analysis of the effect on conversion of extending the model by including as a covariate CNV subtype (‘percentage classic’) was not conducted because the data could not be retrieved.
Handling of missing data in the prognostic model
Two approaches were used to handle missing data in the development of the prognostic model: (1) complete case analysis, in which participants with all candidate predictors available were included in the selection process; and (2) multiple imputation, in which missing values were replaced in candidate predictors prior to implementing the selection process. Through this process, we assessed the impact of multiple imputation in the selection of predictors in the final model. In both instances, we excluded candidate predictors with a large number of missing data (> 20%).
Data collection and processing
A case report form was used to collect participant data at baseline, and at diagnostic test results at each routine follow-up appointment and at 18 months and 36 months. Data were entered remotely by clinical research teams into a bespoke password-protected study website. A copy of the case report form is found in Report Supplementary Material 1.
Baseline measurements
At baseline, data collection included participant demographics, risk factors, whole blood (separated into white cells, serum and plasma) and baseline index test results. Where baseline measures were already documented in clinical case notes within 6 weeks prior to consent, these measures were used for entry into the baseline data collection form and they did not need to be repeated. FFA at diagnosis that confirmed nAMD in the first eye and a second eye free of nAMD were sent to the reading centre for grading along with baseline OCT and colour fundus photographs. Ethnicity data were collected from the medical records using ethnic categories defined on the Medisoft (Medisoft Ltd, Leeds, UK) EMR software.
Follow-up measurements
Data collected at all routine clinic visits after enrolment into the EDNA study for up to 3 years were extracted from the medical records. These included the index test results and, if a FFA was triggered based on positive test results, FFA results and clinical diagnosis. At 18 months and 36 months, all index tests were collected along with a FFA, unless the participant refused or there was a clinical reason to avoid FFA.
Post conversion case note review
Following conversion to nAMD, additional data about the study eye were extracted, and included number of visits, visual acuity and number of anti-VEGF treatments. These data were used to inform the health economic analysis.
Optical coherence tomography angiography
In addition to providing standard OCT images (also known as structural or B-scan images), some OCT acquisition systems have the capability to acquire OCTA. This is a recent introduction into retinal imaging, which shows images of flowing blood in the microvasculature of the retina and choroid. 46 These imaging systems are now commercially available and being increasingly used in the NHS to aid nAMD detection and diagnosis. In some EDNA sites, existing OCTs have been upgraded to permit acquisition of OCTA images.
There are emerging data in the literature that suggest that these instruments may reveal hotspots representing blood flow within developing neovascular membranes, which can be localised to specific layers, such as the outer retina/choroid slab, the outer nuclear layer slab or the inner retinal layers. 47,48 However, data on their clinical relevance to early detection of nAMD are sparse, and there are almost no good-quality data on the sensitivity and specificity of these instruments compared with FFA.
For the purposes of the EDNA study, OCTA was opportunistically collected when appropriate instrumentation was available at the clinical site at visits when a FFA was conducted owing to a test positive (triggered FFA) to provide a data set for future analysis.
Management of the study
The study management team, based within the Centre for Healthcare Randomised Trials (CHaRT), University of Aberdeen, provided day-to-day support for the recruiting centres led by a local principal investigator (PI). The PIs, supported by dedicated research nurses, were responsible for all aspects of local organisation, including recruitment of participants, delivery of index tests and reference standards, and notification of any problems or unexpected developments during the study period. The study was supervised by the project management group, which consisted of representatives from the study office and grant holders. Independent oversight of the study was provided by a Study Steering Committee comprising eight independent members, including three patient partners. There was no data monitoring committee as there was no blinding or interventional aspect to the study.
Patient and public involvement
With the support of the Macular Society (Andover, UK), we ensured active patient and public involvement (PPI) throughout the EDNA study from design to dissemination.
Oversight of the study
A panel of Macular Society patient members (initially one, later three) and the chief executive of the Macular Society served as members of the EDNA Study Steering Committee, contributing both their individual perspectives of macular disease and the perspectives of macular disease of the wider community. As integral members of the Study Steering Committee, they attended regular investigator meetings in addition to the regular steering committee meetings, and received updates on study progress between meetings. The Study Steering Committee reviewed and commented on the study design, protocol and all study documentation, including patient-facing documents that were sent to potential and recruited participants in the EDNA study. In addition, the PPI partners co-produced a patient newsletter for EDNA participants and a patient diary to help to monitor eye health, designed with visually impaired people in mind, and used their own experiences of having their eye health monitored. Having three patient representatives with different stages of disease and visual impairment enabled a wide cross-section of input and perspectives.
Dissemination
The PPI partners have been actively involved in discussions of the study results with the Study Steering Committee and investigators, and have contributed towards the preparation of the Plain English summary. They will continue to be involved in dissemination to participants and academic papers. We anticipate that the PPI partners on the Study Steering Committee will comment on the participant results newsletter/audio. It is also anticipated that the publication of the study results will be co-ordinated with press releases from the participating academic/NHS institutions and the Macular Society.
At the end of the study, the PPI partners reflected on their input and made suggestions for future research, which is included in the discussion.
Chapter 3 Baseline characteristics of participants
Recruitment of participants
Between June 2015 and March 2017, 578 participants from 24 NHS hospital eye services consented to take part in the EDNA study. Figure 6 and Table 4 show the flow of patients approached and reasons for not taking part. Following consent, 16 participants were subsequently excluded because they had been consented in error (ineligible) and 10 participants withdrew consent to the use of their data during EDNA follow-up. The remaining 552 participants formed the EDNA monitoring cohort and their baseline data are presented here.
| Reasons for not taking part in the study | Participants (N = 371), n (%) |
|---|---|
| Aged < 50 years | 3 (0.8) |
| History of nAMD in both eyes | 14 (3.8) |
| nAMD detected at baseline in the study eye | 11 (3.0) |
| Visual acuity worse than 68 letters | 93 (25.1) |
| Retinal pathology in study eye that can confound subsequent assessments | 40 (10.8) |
| Not undergoing regular monitoring in standard of care | 36 (9.7) |
| Patient declines further treatment | 3 (0.8) |
| Cannot give informed consent | 6 (1.6) |
| Unable to undergo FFA | 24 (6.5) |
| Does not wish to take part in the study | 56 (15.1) |
| Past study entry date | 54 (14.6) |
| Reason not available | 31 (8.4) |
Demographics
The baseline characteristics of the EDNA study participants are shown in Table 5. Participants were, on average, aged 77 years old and a higher proportion were women (57%). Participants were predominantly of white British ethnicity (72.6%); ethnic background was not recorded in 16.5% of clinical case notes. The average body mass index (BMI) was 28 kg/m2, more than half of the participants had hypertension (53%) and just under one-quarter of the participants had cardiovascular disease (22%). Approximately one-sixth of the participants had diabetes (16%) and around one-third were taking nutritional supplements (30%). A family history of AMD was recorded in the majority of participants (85%). Never smokers accounted for around 40% of the cohort, half were former smokers (48%) and only a small proportion were current smokers (12%).
| Characteristic | Participants (N = 552) |
|---|---|
| Age (years), mean (SD), n | 77.4 (7.7), 552 |
| Body mass index (BMI) (kg/m2), mean (SD), n | 27.6 (5.3), 423 |
| Sex, n (%) | |
| Male | 236 (42.8) |
| Female | 316 (57.2) |
| Hypertension, n (%) | |
| Yes | 292 (52.9) |
| No | 259 (46.9) |
| Missing | 1 (0.2) |
| Cardiovascular disease, n (%) | |
| Yes | 118 (21.4) |
| No | 433 (78.4) |
| Missing | 1 (0.2) |
| Family history of AMD, n (%) | |
| Yes | 82 (14.9) |
| No | 469 (85.0) |
| Missing | 1 (0.2) |
| Diabetes, n (%) | |
| Yes | 88 (15.9) |
| No | 463 (83.9) |
| Missing | 1 (0.2) |
| Co-morbidities, n (%) | |
| Yes | 48 (8.7) |
| No | 502 (90.9) |
| Missing | 2 (0.4) |
| Nutritional supplements, n (%) | |
| Yes | 165 (29.9) |
| No | 386 (69.9) |
| Missing | 1 (0.2) |
| Smoking history, n (%) | |
| Current smoker | 70 (12.7) |
| Ex-smoker | 264 (47.8) |
| Never smoked | 217 (39.3) |
| Missing | 1 (0.2) |
| Drug used to treat nAMD in fellow eye, n (%) | |
| Ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA) | 159 (28.8) |
| Aflibercept (Eylea; Bayer AG, Leverkusen, Germany) | 379 (68.7) |
| Bevacizumab (Avastin; F. Hoffmann-La Roche AG, Basel, Switzerland) | 13 (2.4) |
| Missing | 1 (0.2) |
| Ethnicitya | |
| British | 401 (72.6) |
| Irish | 2 (0.4) |
| Any other white background | 47 (8.5) |
| Indian | 5 (0.9) |
| Any other Asian background | 3 (0.5) |
| Any other black background | 1 (0.2) |
| Any other ethnic group | 2 (0.4) |
| Missing | 91 (16.5) |
Ocular characteristics
With respect to ocular characteristics in the EDNA study eye (Table 6), around 20% of eyes were pseudophakic. Around one-quarter of the cohort had an EDNA study eye that was phakic with no cataract. Of those with cataract, nuclear sclerosis was the most common form, with 89% of eyes having some degree of nuclear opacity, around one-third with cortical cataract and just under 10% with posterior subcapsular opacities (Table 7).
| Ocular characteristic | Participants (N = 552), n (%) |
|---|---|
| Study eye | |
| Right | 279 (50.5) |
| Left | 273 (49.5) |
| Baseline examination at clinic | |
| Fundus examination findings | |
| No early AMD | 136 (24.6) |
| Early AMD | 407 (73.7) |
| Geographic atrophy | 8 (1.4) |
| Missing | 1 (0.2) |
| OCT examination findings | |
| No early AMD | 151 (27.4) |
| Early AMD | 398 (72.1) |
| Co-existent atrophy | 2 (0.4) |
| Missing | 1 (0.2) |
| Amsler scotoma present | |
| Yes | 92 (16.7) |
| No | 460 (83.3) |
| Lens status and opacity | |
| Phakic: cataract present | 285 (51.6) |
| Phakic: no cataract present | 148 (26.8) |
| Pseudophakic | 117 (21.2) |
| Missing | 2 (0.4) |
| Cataract classification | Nuclear sclerosis (n) | Total (n) | |||||
|---|---|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Missing | |||
| Cortical | Grade 0 | 3 | 164 | 25 | 2 | 0 | 194 |
| Grade 1 | 20 | 37 | 13 | 0 | 2 | 72 | |
| Grade 2 | 4 | 7 | 3 | 0 | 0 | 14 | |
| Missing | 0 | 3 | 0 | 0 | 2 | 5 | |
| Total | 27 | 211 | 41 | 2 | 4 | – | |
| Posterior subcapsular cataracts | Grade 0 | 20 | 198 | 36 | 1 | 0 | 255 |
| Grade 1 | 4 | 7 | 3 | 1 | 0 | 15 | |
| Grade 2 | 3 | 1 | 2 | 0 | 0 | 6 | |
| Missing | 0 | 5 | 0 | 0 | 4 | 9 | |
| Total | 27 | 211 | 41 | 2 | 4 | – | |
Baseline features of diagnostic tests assessed in clinic in the EDNA study eye
Amsler test:28 distortion on the chart was determined to be present in 92 participants (16.7%) at baseline, with the rest having no changes on Amsler. Those with distortion at baseline were excluded from the Amsler diagnostic test analysis.
Clinical fundus examination:29 features of drusen and/or retinal pigment epithelial changes that constitute early macular degeneration were seen on slit-lamp examination in the EDNA study eye in 74% of the cohort.
OCT: features of early macular degeneration, such as drusen30 and subretinal drusenoid deposits (otherwise termed reticular pseudodrusen),50,51 were seen on OCT scans in the EDNA study eye in 72% of the cohort.
Self-reported vision:31 this was not collected at baseline because it was the first participant visit and self-reported vision was measured as a change from the previous visit.
Visual acuity:32 the mean visual acuity in the study eye was 79 letters [logarithm of the minimum angle of resolution (logMAR) 0.1, Snellen equivalent 6/6]. Figure 7 shows the visual acuity distribution at baseline.
FIGURE 7.
Visual acuity (ETDRS letters) distribution at baseline by eye. (a) The EDNA study eye; (b) the fellow eye.
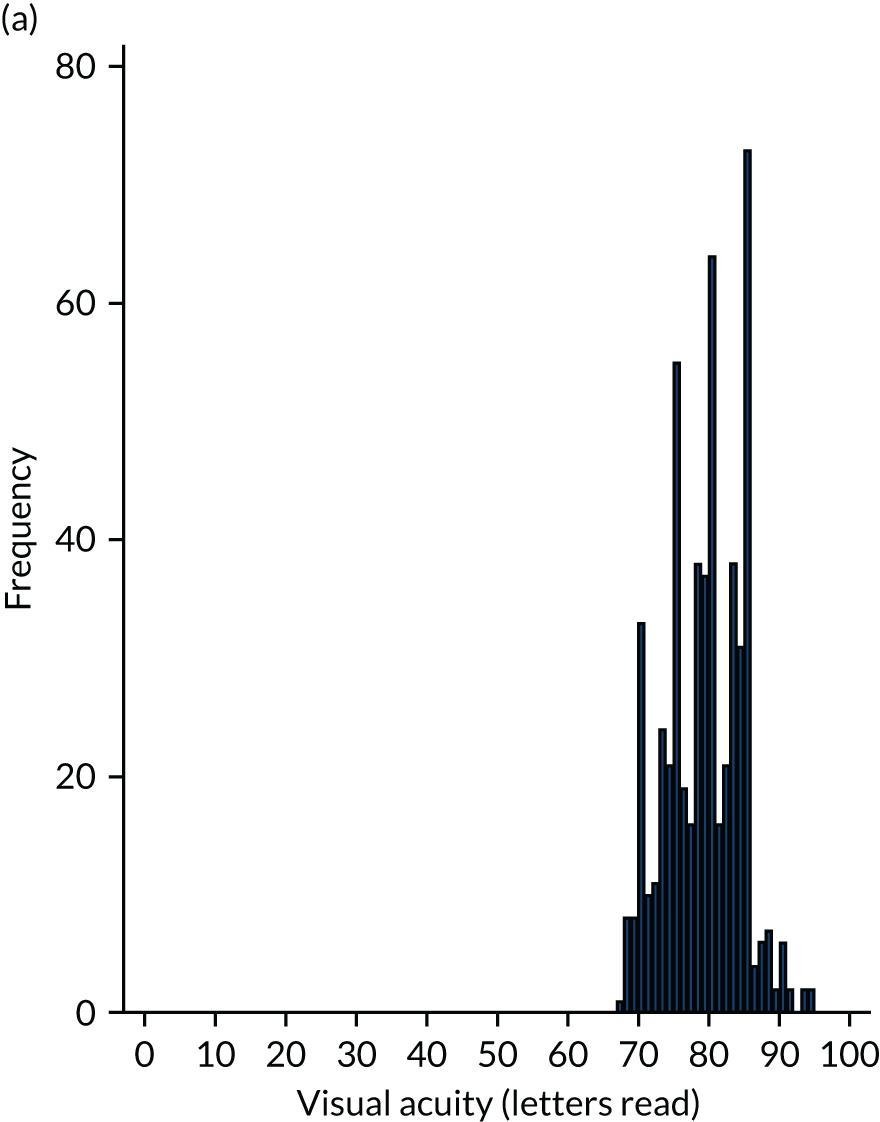
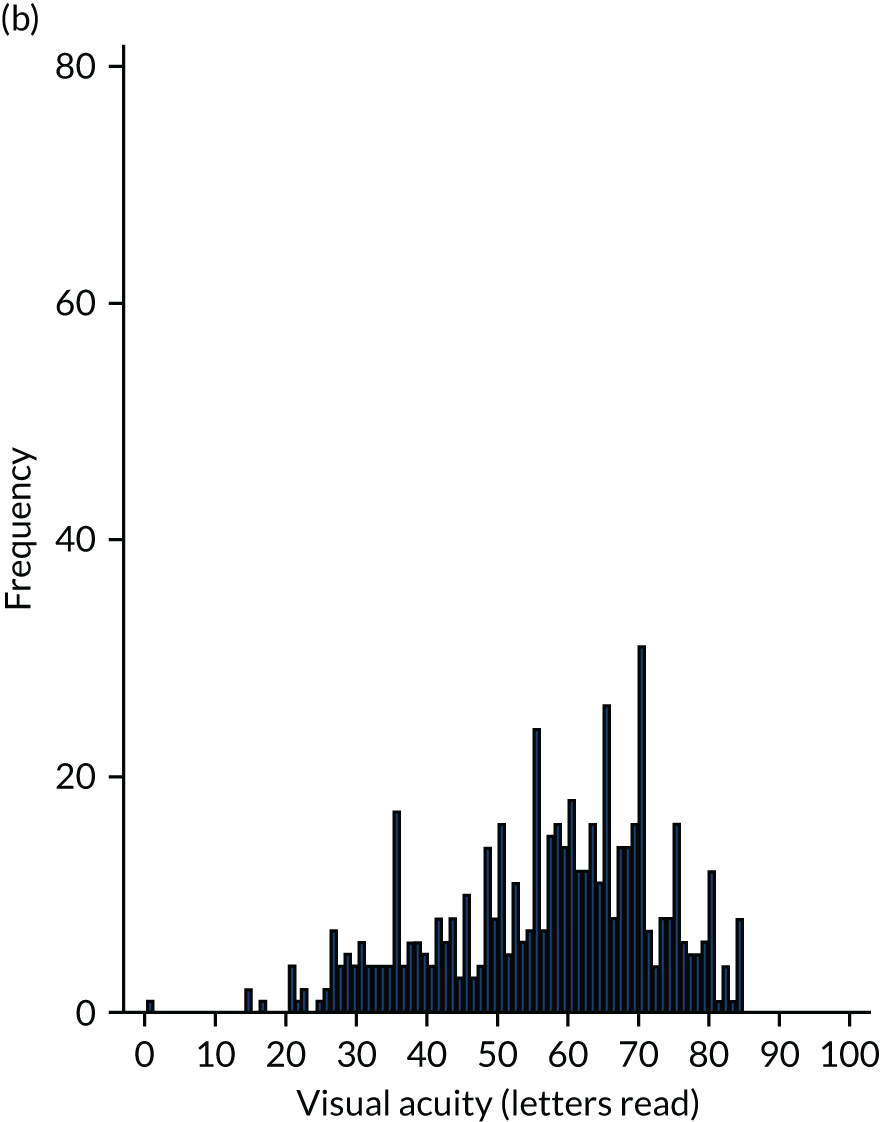
Clinical features of the eye presenting with neovascular age-related macular degeneration (fellow eye)
The mean visual acuity in fellow eyes was 57 ± 5.4 ETDRS letters. The most common anti-VEGF agent initiated was aflibercept (Eylea; Bayer AG, Leverkusen, Germany) (69%), with around 30% of participants treated with ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA). Less than 1% of eyes were treated with bevacizumab (Avastin; F. Hoffmann-La Roche AG, Basel, Switzerland). Table 8 shows the lesion characteristics at initial presentation/study enrolment in the fellow eye with active nAMD. The classification of nAMD was performed by the reading centre. 35 The reading centre graded lesion activity in the fellow eye and reported on the presence of subretinal fluid, fibrin, haemorrhage, atrophy and fibrosis based on colour and fluorescein angiography. The metric provided by the reading centre was the total lesion size measured on the enface images of the FFA and this was defined as all neovascular complex, contiguous elevated blocked fluorescence, pigment epithelial detachment and whole blood, exudate or fibrous tissue. The area of the active lesion was defined as all neovascular complex, but excluded serous pigment epithelial detachments and non-perfusing fibrous tissue even if contiguous to the active area of the lesion. Of the 552 EDNA study participants, baseline imaging data of the fellow eye were graded from 548 participants. The reading centre graded the majority of CNV as type1 CNV, type 2 CNV or mixed (89%), and 11% of CNV was classified as RAP (type 3). The size of the lesion and location are shown by composition in Table 8.
| Characteristic | n (%) or mean (SD), n | Median (P25–P75) | Minimum–maximum |
|---|---|---|---|
| FFA findings in the fellow eyea | |||
| Exudative AMD presentb | 535 (97.6) | ||
| Active CNV or RAP present | 517 (94.3) | ||
| Total GLD (mm) | 3.3 (1.8), 535 | 3.1 (2.0–4.3) | 0.1–11.0 |
| Total lesion area (mm2) | 7.7 (7.8), 535 | 5.6 (2.3–10.6) | 0.1–63.1 |
| Active lesion GLD (mm2) | 3.2 (1.8), 518 | 3.0 (1.9–4.2) | 0.1–17.2 |
| Total active lesion area (mm2) | 6.9 (7.4), 518 | 4.7 (2.1–9.8) | 0.1–63.1 |
| Type 1 MNV present | 352 (64.2) | ||
| Type 1 MNV area (mm2) | 6.1 (4.9), 352 | 4.6 (2.5–8.3) | 0.2–26.6 |
| Type 1 MNV location | |||
| Extrafoveal | 68 (19.3) | ||
| Juxtafoveal | 28 (8.0) | ||
| Subfoveal | 256 (72.7) | ||
| Type 2 MNV present | 176 (32.1) | ||
| Type 2 MNV area (mm2) | 2.6 (4.2), 176 | 1.4 (0.5–3.1) | 0.0–41.9 |
| Type 2 MNV location | |||
| Juxtafoveal | 53 (30.1) | ||
| Extrafoveal | 20 (11.4) | ||
| Subfoveal | 103 (58.5) | ||
| Type 3 MNV present | 59 (10.8) | ||
| Type 3 MNV area (mm2) | 0.2 (0.4), 59 | 0.1 (0.1–0.2) | 0.0–3.3 |
| Type 3 MNV location | |||
| Juxtafoveal | 28 (47.5) | ||
| Subfoveal | 8 (13.6) | ||
| Extrafoveal | 23 (39.0) | ||
| Fibrosis present | 50 (9.1) | ||
| Fibrosis area (mm2) | 4.3 (5.4), 50 | 2.9 (1.0–4.7) | 0.1–30.0 |
| Atrophy within lesion | 55 (10.0) | ||
| Area of atrophy (mm2) | 1.7 (2.0), 55 | 1.0 (0.3–2.3) | 0.1–9.6 |
| Location of atrophy | |||
| Extrafoveal | 18 (32.7) | ||
| Juxtafoveal | 26 (47.3) | ||
| Subfoveal | 11 (20.0) | ||
| OCT findings fellow eyea | |||
| Central foveal thickness (µm) | 396.5 (145.6), 518 | 370.5 (292.0–479.0) | 144.0–1096.0 |
Discussion
Although we are unable to compare baseline characteristics in a contemporary control population drawn from the same ethnic and geographical distributions within the EDNA study, our participants had many similarities to those observed in previous population-based and clinic enrolled studies. The baseline EDNA participant characteristics are indeed similar to other nAMD studies in terms of age and female preponderance. Increased BMI is a risk factor for nAMD and the mean BMI was above the normal range in our population. 53 Around two-thirds of our population were either past or current smokers. Smoking is known to be a strong risk factor for AMD. 54 Around one-third of our participants were taking nutritional supplements. This finding suggests that vitamin supplements have not gained much ground in the UK population despite the Age Related Eye Disease Study (AREDS),55 which found that the fellow eye of patients with nAMD benefited from vitamin supplements with a relative risk reduction of around 20%.
Around three-quarters of the EDNA study eyes exhibited features of early AMD, consisting of drusen and/or pigmentary irregularities. 56 These features are key ocular risk factors in fellow eyes of patients with nAMD in one eye. 57,58
We phenotyped the nAMD lesion in fellow eyes because we intended to use the data to develop a model to predict the onset of nAMD in the EDNA study eye. We graded key baseline characteristics, such as lesion size, lesion type and markers of lesion activity. It is notable that the lesion composition and type is often symmetrical between eyes, with the highest concordance seen in RAP lesions. 59 In the EDNA cohort, fellow eyes with nAMD exhibited neovascular lesions that arose mainly from the choroid constituting type 1, type 2 or mixed pattern. 35 Around 10% of the cohort had RAP lesions. RAP is best appreciated on ICGA video. In addition, RAP lesions eventually connect with the choroidal vasculature. Thus, it is possible that some lesions that were classified as type 1 or type 2 in the EDNA study fellow eyes may have arisen as RAP lesions, which would account for the lower prevalence than that observed in studies that have phenotyped lesions in great detail with FFA and ICGA. 23 Variations in the proportions of the different types of lesions is not uncommon in different populations. 35
A key reason for reading centre assessment of the EDNA images was to confirm the diagnosis of nAMD in the included participants and, subsequently, to confirm the onset of nAMD when the index tests and/or FFA were reported as positive by the clinical sites. In terms of the former, it was very reassuring to note that the reading centre reported only eight of the fellow eyes to have been incorrectly diagnosed as exhibiting nAMD. These cases had exudative maculopathies that were deemed to have arisen from other aetiology, such as central serous retinopathy which can mimic the features of nAMD.
We collected information about ineligible participants during the enrolment period. It was notable that around one-quarter of ineligible participants had a baseline visual acuity of < 68 letters in their EDNA study eye. This was surprising given that 68 letters on the ETDRS chart equates to a Snellen acuity of 6/12, which represents the cut-off level for the driving standard applicable to the better seeing eye. Our data, therefore, suggest that a significant minority of older adults presenting with nAMD in one eye also have visual impairment of sufficient severity in the eye free of nAMD. This impairment is at a level that can produce a considerable diminution of quality of life as driving, reading and other activities dependent on high levels of visual acuity become difficult to perform. 18,60 Possible reasons for the impaired function in the second eye of patients with nAMD in one eye are media opacities and other retinal co-morbidities. This finding has implications for social services and providers of care.
Chapter 4 Diagnostic accuracy results
The set of index tests included in this study, namely visual acuity, fundus clinical examination, macular OCT, change in vision reported by the patient and the Amsler chart, were performed at routine visits. A positive index test was a trigger for the primary reference standard, which was the clinician diagnosis of active nAMD based on the FFA results. Clinicians reviewed the angiographic sequence to confirm the diagnosis. When regions of the posterior fundus were observed to develop areas of hyperfluorescence, owing to leakage of fluorescein from the new vessels during the transit phase of the dye in the sequential images of the angiographic run, this constituted a feature of nAMD.
Overview of the analyses presented
The analysis has been carried out (see Report Supplementary Material 2) based on the following diagnostic reference standards.
Primary reference standard: fundus fluorescein angiography determination of conversion to neovascular age-related macular degeneration at the clinical site
This was undertaken by the clinician at the clinical site. We chose this as the primary reference standard because this is the recognised method of determining the onset of nAMD at the clinic. This represents the most appropriate and pragmatic standard of care.
Secondary reference standard 1: clinical determination of conversion to neovascular age-related macular degeneration at the clinical site
We included this alternative reference standard to allow for clinical interpretation of disease status based on negative or indeterminate FFA results, or when a FFA was refused by participants or deemed unsafe owing to patient characteristics. Where a FFA was unavailable, a clinical diagnosis of nAMD was made based on all available clinical information. We included this secondary reference standard because we recognised that a proportion of participants would not be put through a FFA. This arose because of two reasons: (1) although enrolled participants had to have had a FFA, some had experienced mild allergic response to fluorescein and, therefore, subsequent FFA was deemed to be unsafe; and (2) participants sometimes declined a FFA because the procedure was found to be uncomfortable, inducing nausea in some susceptible cases.
Secondary reference standard 2: reading centre determination of conversion to neovascular age-related macular degeneration
This was carried out by the reading centre after submission of the FFA images to netwORC UK (Belfast, UK). It is known that there is disagreement between clinician determination and reading centre determination of conversion to nAMD. 61 Reading centres are known to produce data with higher reliability and consistency than clinicians and, hence, have been used as the gold standard in clinical trials and studies. Therefore, we included reading centre determination as a further reference standard to confirm whether or not FFA shows conversion from the non-neovascular stage to neovascular AMD.
We further presented the results of each of the three reference standards by three timeline-specific definitions for index tests. Under each specific timeline definition of the index test, additional analyses were conducted as follows:
-
Primary reference standard – FFA determination of conversion to nAMD at the clinical site –
-
index test results cover the entire period of follow-up (main analysis):
-
– main analysis and paired comparison
-
– combining index test results
-
– subgroup analysis based on nAMD subtype
-
– sensitivity analyses varying the index test definitions for self-reported vision and severity of visual acuity loss
-
– sensitivity analyses varying the definition of an inconclusive FFA result
-
– modelling diagnostic accuracy by GEE
-
-
index test results cover the last 6 months of participant follow-up
-
index test results are based on the last visit available
-
-
Secondary reference standard 1 – clinical determination of conversion to nAMD at the clinical site –
-
index test results cover the entire period of follow-up
-
-
Secondary reference standard 2 – reading centre determination of conversion to nAMD –
-
index test results cover the entire period of follow-up
-
index test results cover the last 6 months of follow-up
-
index test results are from the last visit available.
-
Figure 8 shows a flow chart describing the cohort, the number of participants who were included within the analyses pertaining to each reference standard and the number of participants deemed to have converted from the non-exudative state to exudative AMD in the EDNA study eye under each reference standard. Of the 552 eligible participants in the EDNA cohort with a diagnosis of nAMD in the first-presenting eye (fellow eye) based on a FFA at baseline, nine participants had no follow-up data and were, therefore, excluded from all subsequent analyses. Of the 543 participants with at least one follow-up visit, 464 participants had at least one additional FFA and constituted the cohort included in the analysis using the primary reference standard for all index tests. Given that it was prespecified that participants with a positive Amsler test at baseline would be excluded, we had a smaller sample who could be included in the assessment of the performance of the Amsler test.
FIGURE 8.
Participants’ status at the end of the study per reference standard. a, Participants have at least one index test available during follow-up.
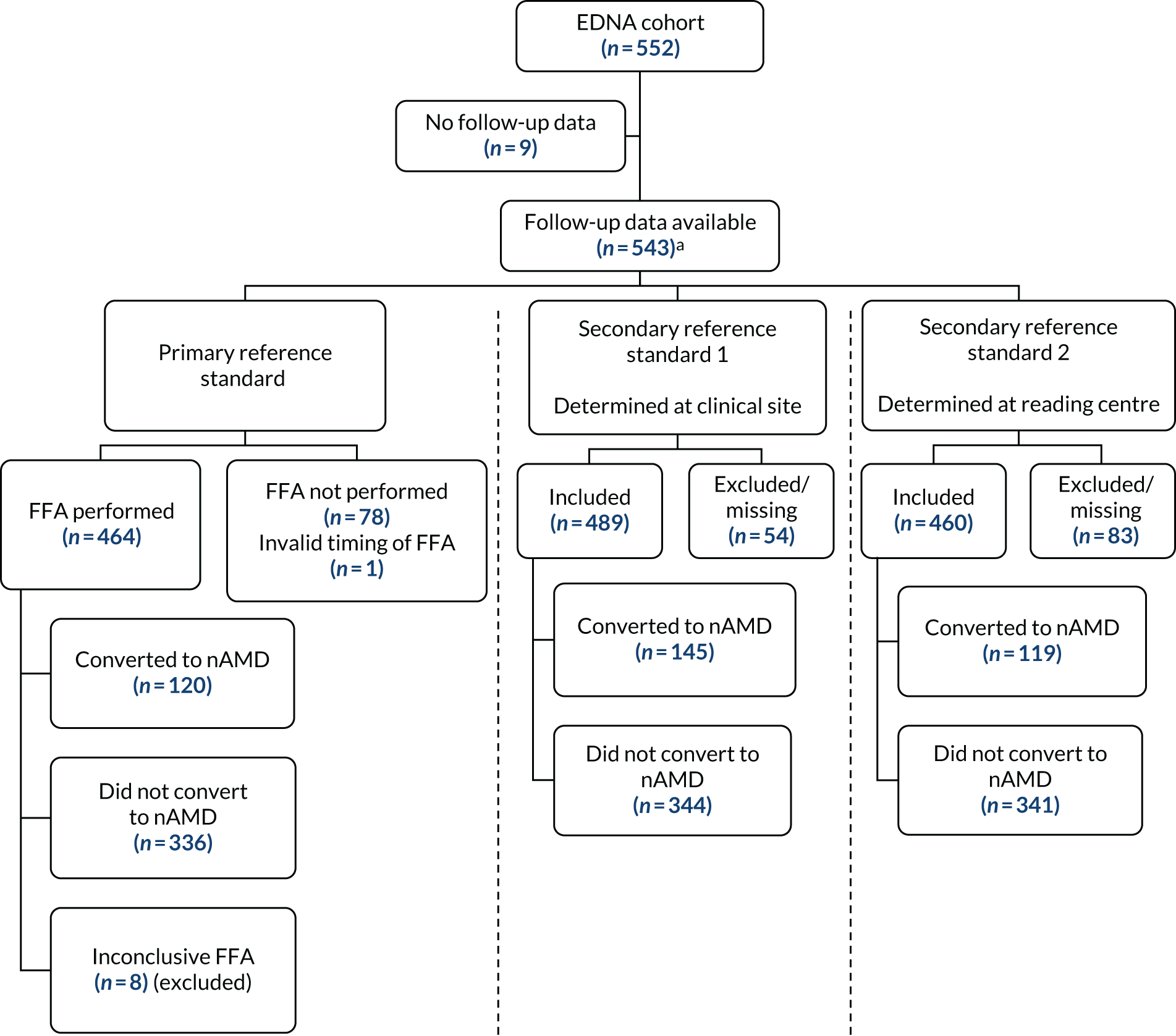
Secondary reference standard 1 represents the broadest definition of conversion to nAMD and included 489 out of the 552 eligible participants. In this cohort we had an additional 25 participants who did not have a FFA, but had a clinical determination of conversion to nAMD at the study site. In the remaining 54 participants, there was insufficient follow-up precluding use of their data in the analysis. The sample included in secondary reference standard 2 consisted of data from 460 participants whose FFA was graded at the reading centre. The differences in the reference standards defined above result in differences in the number of EDNA study eyes that were deemed to have converted to nAMD under each analysis. For a summary of the comparison between the primary and the two secondary reference standards, see Tables 25 and 26.
Monitoring and testing frequency
Table 9 shows the mean number of clinic visits and index tests undertaken during the 36-month follow-up period from the 543 participants with follow-up data (for the Amsler test this is 453 participants because 90 participants were excluded). The average number of clinic visits was 15.6 visits during follow-up (range 1–35 visits). A large proportion of all five index tests was carried out at clinical visits. The mean number of Amsler tests performed was smaller than that of other index tests. Of the participants who had a follow-up, a positive Amsler at baseline was recorded in 90 (17%) participants and, as per protocol, subsequent Amsler test results were not conducted and, therefore, these individuals were not included in any of the diagnostic accuracy analyses. Appendix 1 shows these data for the subset excluded from the main analysis. No serious adverse events were reported during the study.
| Visit/test | Eligible participants (N = 543) | ||
|---|---|---|---|
| Mean (SD), n | Minimum–maximum | P25–P75 | |
| Clinic visits | 15.6 (7.7), 543 | 1–35 | 10–21 |
| Index tests | |||
| Amsler | 13.8 (7.5), 453a | 0–35 | 8–18 |
| Fundus clinical examination | 14.2 (7.4), 543 | 0–35 | 9–19 |
| OCT | 14.5 (7.5), 543 | 1–35 | 9–19 |
| Self-reported vision | 14.0 (7.4), 543 | 0–35 | 9–19 |
| Visual acuity | 15.5 (7.6), 543 | 1–35 | 10–21 |
Primary reference standard
The following sections relate to analyses conducted using the primary reference standard.
Time to conversion to neovascular age-related macular degeneration
Of the 456 participants who were included in the primary reference standard analysis, eight participants were excluded because the FFA was reported as inconclusive by the site clinician (see Figure 8). The average number of FFAs carried out during follow-up was 1.7 FFAs (standard deviation 1.1 FFAs). In participants who developed nAMD during follow-up, the average number of FFAs carried out was 1.5 FFAs (standard deviation 0.82 FFAs) compared with 1.7 FFAs (standard deviation 0.82 FFAs) in those whose study eye did not convert to nAMD. The maximum number of follow-up FFAs that was recorded in any one participant was six.
During follow-up, 145 conversions to nAMD were detected by clinicians using clinical information and any available imaging modality. Of these conversions, 120 had a FFA performed at the time of conversion and read by the clinician, who confirmed conversion to nAMD based on this imaging modality. This yielded a crude conversion rate of 26% (95% CI 22.3% to 30.6%), with a median follow-up time of 33 months (range 0.8 to 38.5 months).
The Kaplan–Meier curve for the conversion to nAMD in the EDNA study eye, using the time of FFA confirmation of conversion (primary reference standard) as the time of conversion to nAMD, is shown in Figure 9.
FIGURE 9.
Kaplan–Meier survival estimate: months from consent to conversion to nAMD or exit from the study (primary reference standard). Raw data: the time of conversion to nAMD is the date that the FFA is conducted. Reproduced with permission from Sivaprasad et al. 52 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
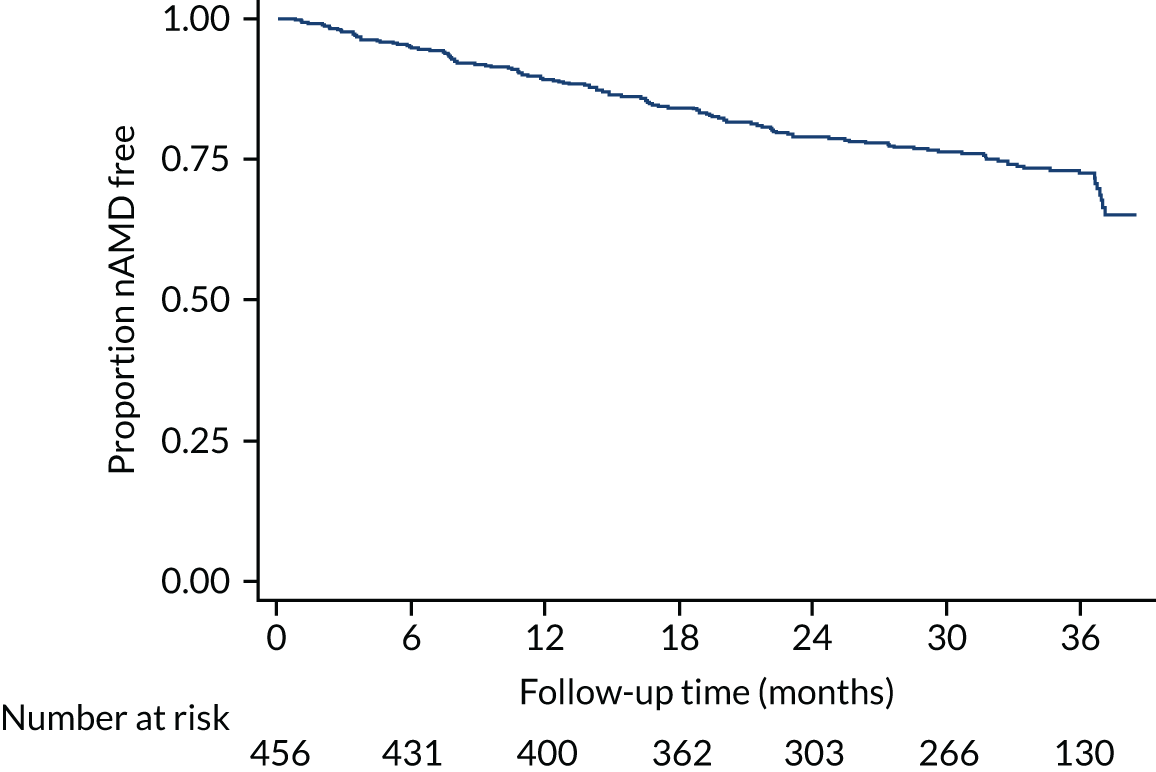
The rate of conversion to nAMD in the EDNA study eye over the follow-up period was uniform. The sharp decline seen in the Kaplan–Meier plot at 36 months reflects the small cluster of conversion events from the reduced number of participants still at risk at this time point. The revised Kaplan–Meier curve (Figure 10), in which the time of conversion was recalculated as the mid-point between the date of FFA confirmation of nAMD conversion and the last (negative) FFA, does not show this dip. In Figure 10, the curve flattens after month 12, whereas the curve in Figure 9 appears to show a more uniform risk over the first 24 months.
FIGURE 10.
Kaplan–Meier survival estimate: months from consent to conversion to nAMD or exit from the study (primary reference standard). Interpolated results: assumes that time of conversion to nAMD is midway between the last FFA without disease and the positive FFA.
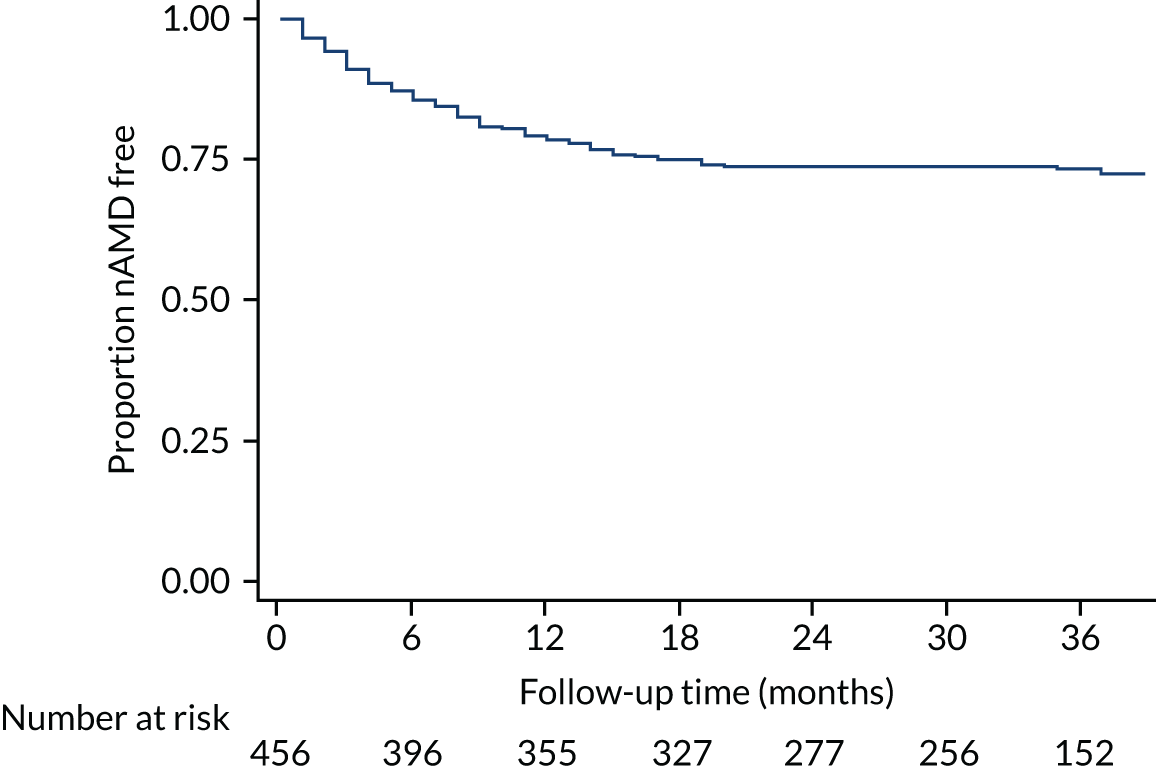
Given that the study design required an exit visit with an assessment, which would differ from usual clinical practice, we specifically examined this time point. Of the 265 participants who were at risk and who did not have nAMD detected in the EDNA study eye by month 30, 18 participants were found to have converted to nAMD after 30 months, of whom 11 were detected at the planned study exit visit and seven prior to the planned exit visit. Among these 18 study eyes, none reported all index tests as negative in the period between 30 months in the study and the planned exit visit. One participant had a negative OCT and a positive FFA result.
Diagnostic performance of the index tests: index test acquired during the entire follow-up period (main analysis)
The following results relate to the primary reference standard and analyses for which the index test results cover the entire period of follow-up.
Table 10 shows the time in days between the last index tests and the reference standard, grouped by conversion to nAMD status. In participants who converted to nAMD, the mean interval between the index test and the reference standard ranged between 30.3 days for Amsler and 6.9 days for OCT. The median interval between a positive index test and a positive reference standard is zero, which shows that, in the majority of participants who converted to nAMD, the positive index test triggered the reference standard measurement on the same day. In participants who did not develop nAMD in their EDNA study eye during follow-up, the interval between the last index test and the final FFA ranged from an average of 8.4 days for self-reported vision to 117 days for visual acuity. Similar tables for the secondary reference standards are presented in Appendix 1.
| Index test | Participants who converted to nAMD | Participants who did not convert to nAMD | ||
|---|---|---|---|---|
| Mean number of days (SD), na | Median (P25–P75) | Mean number of days (SD), n | Median (P25–P75) | |
| Amsler | 30.3 (114.4), 98 | 0 (0–13) | 82.2 (223.0), 279 | 0 (0–0) |
| Fundus clinical examination | 9.4 (28.5), 119 | 0 (0–13) | 9.7 (64.6), 335 | 0 (0–0) |
| OCT | 6.9 (17.2), 120 | 0 (0–12) | 39.0 (142.3), 335 | 0 (0–0) |
| Self-reported vision | 7.3 (13.4), 118 | 0 (0–13) | 8.4 (51.0), 337 | 0 (0–0) |
| Visual acuity | 16.3 (78.2), 120 | 0 (0–13) | 117.0 (264.1), 335 | 0 (0–21) |
The sensitivity and specificity of the index tests compared with the primary reference standard (FFA determination of conversion to nAMD at the clinical site) are shown in Table 11. The maximum available sample size, as shown in Figure 8, is 464 participants. However, data were missing for a few participants in each of the index tests. More information on missing data is available in Appendix 2. OCT had the highest sensitivity at 91.7%, followed by fundus clinical examination (53.8%). All remaining index tests, including Amsler, visual acuity and self-reported vision, had sensitivities that fell below 50%. The fundus clinical examination had the highest specificity (97.9%), followed by self-reported vision (97%) and OCT (87.8%).
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 33.7 (25.1 to 43.5) | 33/98 | 81.4 (76.4 to 85.5) | 227/279 |
| Fundus clinical examination | 53.8 (44.8 to 62.5) | 64/119 | 97.6 (95.3 to 98.9) | 327/335 |
| OCT | 91.7 (85.2 to 95.6) | 110/120 | 87.8 (83.8 to 90.9) | 294/335 |
| Self-reported vision | 4.2 (1.6 to 9.8) | 5/118 | 97.0 (94.6 to 98.5) | 327/337 |
| Visual acuity | 30.0 (22.5 to 38.7) | 36/120 | 66.3 (61.0 to 71.1) | 222/335 |
Changing the reference standard to include FFA results that were inconclusive, by classifying them as all positive or all negative, had little impact on the sensitivity and specificity of the index tests (see Appendix 1).
Table 12 shows the likelihood ratios (with 95% CIs) that indicate the probability of conversion to nAMD, with values that are furthest from 1 being the most informative; a likelihood ratio of 1 means that the test result is equally likely to be positive or negative in participants with and without nAMD. OCT, fundus clinical examination and Amsler CIs for the positive likelihood ratio did not include 1. OCT and fundus clinical examination CIs for the negative likelihood ratio did not include 1. OCT had a high positive likelihood ratio and a very low negative likelihood ratio.
| Index text | Likelihood positive ratio (95% CI) | Likelihood negative ratio (95% CI) | Diagnostic odds ratio (95% CI) |
|---|---|---|---|
| Amsler | 1.8 (1.3 to 2.6) | 0.8 (0.7 to 1.0) | 2.2 (< 0.01 to > 100) |
| Fundus clinical examination | 22.4 (16.7 to 30.1) | 0.5 (0.2 to 0.9) | 47.4 (< 0.01 to > 100) |
| OCT | 7.5 (4.1 to 13.9) | 0.1 (0.1 to 0.1)a | 79.5 (< 0.01 to > 100) |
| Self-reported vision | 1.4 (0.6 to 3.3) | 1.0 (0.5 to 1.8) | 1.4 (< 0.01 to > 100) |
| Visual acuity | 0.9 (0.6 to 1.2) | 1.1 (1.0 to 1.2) | 0.8 (< 0.01 to > 100) |
By contrast, the fundus clinical examination has a very high positive likelihood ratio, but the negative likelihood ratio is five-times worse than for OCT. Diagnostic odds ratios varied from 0.8 (visual acuity) to 79.5 (OCT).
Paired comparisons between index tests (main analysis)
Table 13 shows the paired differences (with 95% CIs) in sensitivity and specificity for detecting nAMD between two index tests along with the corresponding McNemar’s test p-values for analysis A1. Corresponding McNemar’s tests for the paired comparisons are also given. The sensitivity of the test pairs differed significantly from each other (see Table 13) except for the pair constituted by Amsler and visual acuity for which the difference in sensitivity was 9.2% and did not reach statistical significance. Differences in sensitivity between index tests varied from –16.3% (Amsler vs. fundus clinical examination) to 87.4% (OCT vs. self-reported vision). The differences in sensitivity between OCT and all of the other tests were statistically significant, with these differences ranging from 38% to 87%. The magnitude of differences in specificity between OCT and other tests was smaller; fundus clinical examination and self-reported vision specificity were marginally but significantly higher than that for OCT.
| Comparison | Difference (%) in sensitivity (95% CI); p-valuea | Difference (%) in specificity (95% CI); p-valuea |
|---|---|---|
| OCT vs. Amsler | 59.6 (48.1 to 68.6); < 0.001 | 6.9 (1.1 to 12.6); 0.02 |
| OCT vs. fundus clinical examination | 37.8 (27.6 to 47.1); < 0.001 | –9.9 (–13.9 to –6.1); < 0.001 |
| OCT vs. self-reported vision | 87.4 (79.1 to 91.9); < 0.001 | –9.3 (–13.4 to –5.3); < 0.001 |
| OCT vs. visual acuity | 61.7 (49.9 to 70.7); < 0.001 | 21.5 (15.6 to 27.3); < 0.001 |
| Amsler vs. fundus clinical examination | –16.3 (–29.0 to –2.8); 0.03 | –16.2 (–21.3 to –11.5); < 0.001 |
| Amsler vs. self-reported vision | 29.6 (19.2 to 39.7); < 0.001 | –17.3 (–22.3 to –12.7); < 0.001 |
| Amsler vs. visual acuity | 9.2 (–3.5 to 21.5); 0.21 | 12.3 (5.3 to 19.2); < 0.001 |
| Fundus clinical examination vs. self-reported vision | 50.0 (40.3 to 58.7); < 0.001 | 0.6 (–2.0 to 3.3); 0.80 |
| Fundus clinical examination vs. visual acuity | 24.4 (12.5 to 35.2); < 0.001 | 31.3 (25.9 to 36.7); < 0.001 |
| Self-reported vision vs. visual acuity | –25.2 (–34.0 to –16.5); < 0.001 | 30.7 (25.7 to 35.9); < 0.001 |
Combining test results (main analysis)
Table 14 shows the sensitivity and specificity of combinations of index test to detect nAMD, where a positive combination is defined as a positive OCT or a positive index test selected from any one of the other index tests. This definition specifies that both tests have to be negative for the combination to be deemed negative. The combination of index tests shows a marginal improvement in sensitivity compared with conducting OCT alone (sensitivity 91.7%, 95% CI 85.2% to 95.6%). Specificities are lower for a combination of index tests than for using OCT alone (specificity 87.8%, 95% CI 83.8% to 90.9%).
| OCT result combined witha | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 93.9 (87.0 to 97.4) | 92/98 | 73.3 (67.8 to 78.2) | 203/277 |
| Fundus clinical examination | 94.1 (88.1 to 97.3) | 112/119 | 86.0 (81.8 to 89.3) | 288/335 |
| Visual acuity | 96.7 (91.5 to 99.0) | 116/120 | 60.0 (54.7 to 65.1) | 201/335 |
| Self-reported vision | 91.5 (84.9 to 95.5) | 108/118 | 85.1 (80.8 to 88.5) | 285/335 |
In Table 15, the definition of the combination has been changed, and the combination test is positive only if both the OCT and the index test results are positive. With the combination defined in this manner, the sensitivity drops but the specificity improves for all of the combinations tested. See Appendix 1 for the results of two further post hoc combination tests of Amsler, fundus clinical examination, visual acuity and self-reported vision, and Amsler, visual acuity and self-reported vision; the results similarly indicated that OCT alone was a better monitoring strategy.
| OCT results combined witha | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 32.7 (24.2 to 42.5) | 32/98 | 96.8 (93.9 to 98.4) | 268/277 |
| Fundus clinical examination | 51.3 (42.4 to 60.1) | 61/119 | 99.4 (97.7 to 100.0) | 333/335 |
| Visual acuity | 25.0 (18.1 to 33.5) | 30/120 | 94.0 (90.9 to 96.1) | 315/335 |
| Self-reported vision | 4.2 (1.6 to 9.8) | 5/118 | 99.7 (98.2 to 100.0) | 334/335 |
Subgroup analysis based on neovascular age-related macular degeneration subtype
A planned subgroup analysis was conducted based on the nAMD subtype [CNV (type 1 and 2) vs. RAP (type 3)] in the study eye at conversion to nAMD. Figure 11 shows the participants included in this analysis. To be eligible for inclusion, the reading centre nAMD phenotype in the EDNA study eye at conversion to nAMD had to be available.
FIGURE 11.
Flow of participants included in the nAMD subtype subgroup analysis.
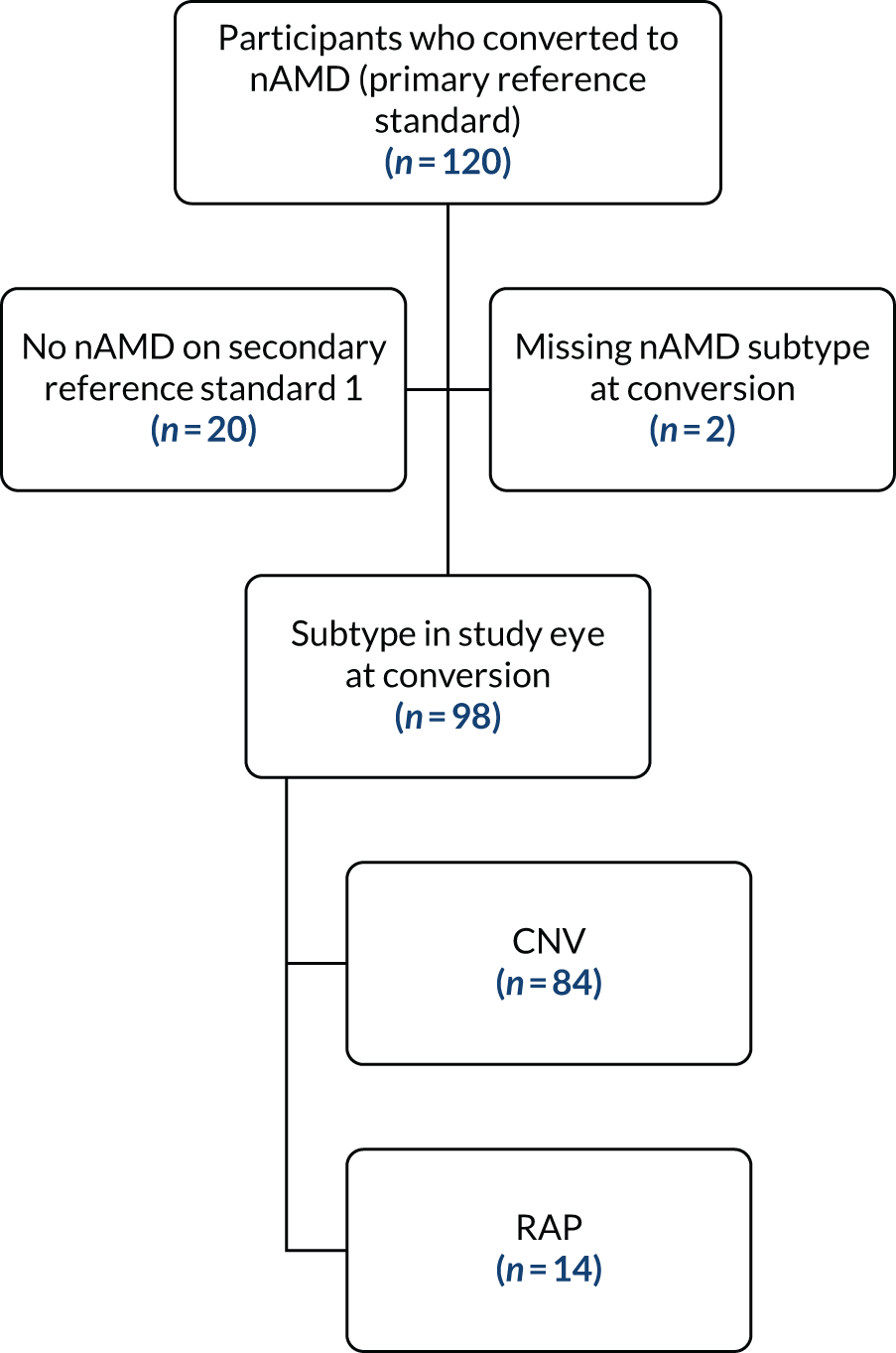
Table 16 presents the sensitivity of each index test to detect nAMD conversion in the study eye by nAMD-type subgroup. Differences in the sensitivity of detection of conversion to nAMD were seen by nAMD subtype. The Amsler test was numerically better in the RAP subgroup than in the CNV subgroup. In addition, the sensitivity of the detection of conversion to nAMD by OCT was better in the RAP subgroup at 100% than in the CNV subgroup at 91.7%.
| Index test | nAMD subtype | |||
|---|---|---|---|---|
| CNV (maximum N = 84) | RAP (maximum N = 14) | |||
| Sensitivity (%) (95% CI) | TP/nAMD | Sensitivity (%) (95% CI) | TP/nAMD | |
| Amsler | 31.9 (22.1 to 43.6) | 22/69 | 38.5 (17.6 to 64.6) | 5/13 |
| Fundus clinical examination | 57.8 (47.1 to 67.9) | 48/83 | 50.0 (26.8 to 73.2) | 7/14 |
| OCT | 91.7 (83.5 to 96.2) | 77/84 | 100.0 (74.9 to 100.0) | 14/14 |
| Self-reported vision | 4.9 (1.5 to 12.3) | 4/82 | 0.0 | 0/14 |
| Visual acuity | 32.1 (23.1 to 42.8) | 27/84 | 21.4 (6.8 to 48.3) | 3/14 |
Sensitivity analyses I: varying the definition of index tests
The definitions of two of the index tests (self-reported vision and visual acuity) were changed to estimate their impact on the sensitivity and specificity of detection of conversion to nAMD in the study eye.
On changing the test positive self-reported vision to either ‘worse’ or ‘much worse’ since the last appointment, Table 17 shows a higher sensitivity and lower specificity than the definitions used in the primary analysis (see Table 11).
| Test positive definition | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Self-reported vision: worse or much worse | 15.3 (9.8 to 22.9) | 18/118 | 92.3 (88.9 to 94.7) | 311/337 |
| Visual acuity: ≥ 10 letters since last FFA | 11.7 (7.0 to 18.8) | 14/120 | 93.1 (89.9 to 95.4) | 312/335 |
| Visual acuity: ≥ 20 letters since baseline | 10.8 (6.3 to 17.8) | 13/120 | 98.2 (96.1 to 99.3) | 329/335 |
For visual acuity, the definition of a positive test in the main analysis was a drop of ≥ 10 letters from baseline. Two changes in this test definition were made: (1) a drop of ≥ 10 letters since the last FFA and (2) a drop of ≥ 20 letters since baseline.
Both sensitivity analyses of visual acuity resulted in a lower sensitivity but a substantially higher specificity than that of the definitions used in the primary analysis (see Table 11).
Sensitivity analyses II: index test results cover the last 6 months of participants’ follow-up
Table 18 shows the sensitivity and specificity (with 95% CI) of the index tests in the last 6 months of the participants’ follow-up for detecting conversion to nAMD. The sensitivity and specificity are similar to that of the main analysis (see Table 11). The likelihood ratios (with 95% CIs) are shown in Table 19.
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 28.3 (20.3 to 37.9) | 28/99 | 92.6 (88.8 to 95.2) | 251/271 |
| Fundus clinical examination | 52.9 (44.0 to 61.7) | 63/119 | 99.4 (97.7 to 100.0) | 333/335 |
| OCT | 91.7 (85.2 to 95.6) | 110/120 | 94.6 (91.6 to 96.6) | 317/335 |
| Self-reported vision | 4.2 (1.6 to 9.8) | 5/118 | 97.9 (95.6 to 99.1) | 326/333 |
| Visual acuity | 27.5 (20.3 to 36.1) | 33/120 | 84.2 (79.9 to 87.7) | 282/335 |
| Index test | Likelihood positive ratio (95% CI) | Likelihood negative ratio (95% CI) | DOR (95% CI) |
|---|---|---|---|
| Amsler | 3.8 (2.6 to 5.5) | 0.8 (0.5 to 1.1) | 4.9 (0.1 to > 100) |
| Fundus clinical examination | 88.2 (65.8 to 118.2) | 0.5 (0.1 to 1.9) | 186.1 (5.1 to > 100) |
| OCT | 17.0 (9.2 to 31.3) | 0.1 (0.1 to 0.1)a | 193.6 (10.0 to > 100) |
| Self-reported vision | 2.0 (0.8 to 4.8) | 1.0 (0.5 to 2.0) | 2.0 (< 0.01 to > 100) |
| Visual acuity | 1.7 (1.2 to 2.5) | 0.9 (0.7 to 1.1) | 2.0 (< 0.01 to > 100) |
Table 20 shows the paired differences (with 95% CIs) in sensitivity and specificity for detecting nAMD between pairs of index tests along with the corresponding McNemar’s test for significance. Index test sensitivities were statistically significantly different in these comparisons, except for the comparison between Amsler and visual acuity. Statistically significant differences in sensitivity varied from –22.0% (self-reported vision vs. visual acuity) to 87.3% (OCT vs. self-reported vision) in magnitude.
| Comparison | Difference (%) in sensitivity (95% CI); p-valuea | Difference (%) in specificity (95% CI); p-valuea |
|---|---|---|
| OCT vs. Amsler | 64.6 (53.1 to 73.2); < 0.001 | 2.2 (–1.8 to 6.3); 0.34 |
| OCT vs. fundus clinical examination | 38.7 (28.4 to 48.0); < 0.001 | –4.8 (–7.8 to –2.3); < 0.001 |
| OCT vs. self-reported vision | 87.3 (79.0 to 91.8); < 0.001 | –3.3 (–6.5 to –0.3); 0.04 |
| OCT vs. visual acuity | 64.2 (52.4 to 73.0); < 0.001 | 10.4 (5.9 to 15.1); < 0.001 |
| Amsler vs. fundus clinical examination | –20.4 (–32.8 to –7.0); < 0.001 | –6.7 (–10.4 to –3.6); < 0.001 |
| Amsler vs. self-reported vision | 24.7 (14.6 to 34.8); < 0.001 | –6.3 (–10.1 to –3.0); < 0.001 |
| Amsler vs. visual acuity | 7.1 (–4.8 to 18.9); 0.31 | 5.2 (0.4 to 10.1); 0.04 |
| Fundus clinical examination vs. self-reported vision | 49.6 (39.8 to 58.3); < 0.001 | 1.5 (–0.3 to 3.7); 0.13 |
| Fundus clinical examination vs. visual acuity | 26.1 (14.5 to 36.6); < 0.001 | 15.2 (11.3 to 19.6); < 0.001 |
| Self-reported vision vs. visual acuity | –22.0 (–30.7 to –13.7); < 0.001 | 13.9 (10.0 to 18.2); < 0.001 |
For specificity, the differences were significant for all comparisons, except for OCT versus Amsler and fundus clinical examination versus self-reported vision. Statistically significant differences in specificity between index tests varied from –6.7% (Amsler vs. fundus clinical examination) to 15.2% (fundus clinical examination vs. visual acuity) in magnitude. The specificity of the detection of conversion to nAMD for the fundus clinical examination and self-reported vision was generally higher than that for self-reported vision and fall in visual acuity.
Sensitivity analyses III: index test results are based on the last visit available
Table 21 shows the sensitivity and specificity of index tests (with 95% CIs) for detecting conversion to nAMD using data from the last visit only. Results are similar to that of the main analysis (see Table 11). OCT has the best sensitivity, with 90.0%; all of the other index tests have a sensitivity below 50%. Fundus clinical evaluation has the highest specificity (99.7%), followed by self-reported vision (98.5%), OCT (96.4%), Amsler (93.7) and visual acuity (88.4%). Likelihood ratios (with 95% CIs) and diagnostic odds ratios are shown in Table 22.
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 26.5 (18.8 to 36.1) | 26/98 | 93.7 (90.1 to 96.1) | 253/270 |
| Fundus clinical examination | 49.6 (40.8 to 58.4) | 59/119 | 99.7 (98.2 to 100.0) | 334/335 |
| OCT | 90.0 (83.2 to 94.3) | 108/120 | 96.4 (93.8 to 98.0) | 323/335 |
| Self-reported vision | 4.2 (1.6 to 9.8) | 5/118 | 98.5 (96.4 to 99.5) | 328/333 |
| Visual acuity | 25.8 (18.8 to 34.4) | 31/120 | 88.4 (84.5 to 91.4) | 296/335 |
| Index test | Likelihood positive ratio (95% CI) | Likelihood negative ratio (95% CI) | DOR (95% CI) |
|---|---|---|---|
| Amsler | 4.2 (2.9 to 6.2) | 0.8 (0.5 to 1.2) | 5.4 (0.1 to > 100) |
| Fundus clinical examination | 165.3 (123.4 to 221.4) | 0.5 (0.1 to 3.6) | 327.1 (8.2 to > 100) |
| OCT | 25.0 (14.3 to 43.7) | 0.1 (0.1 to 0.2) | 241.0 (12.5 to > 100) |
| Self-reported vision | 2.8 (1.2 to 6.7) | 1.0 (0.4 to 2.3) | 2.9 (< 0.01 to > 100) |
| Visual acuity | 2.2 (1.6 to 3.2) | 0.8 (0.6 to 1.1) | 2.7 (< 0.01 to > 100) |
Table 23 shows the paired differences (with 95% CIs) in sensitivity and specificity for detecting nAMD between pairs of index tests along with the corresponding McNemar’s test p-values for analysis A3. The index test pairs differed significantly from each other, except for Amsler versus visual acuity. The differences in sensitivity between index tests varied from –20.3 (self-reported vision vs. visual acuity) to 85.6 (OCT vs. self-reported vision) in magnitude.
| Comparison | Difference (%) in sensitivity (95% CI); p-valuea | Difference (%) in specificity (95% CI); p-valuea |
|---|---|---|
| OCT vs. Amsler | 64.3 (52.3 to 73.1); < 0.001 | 2.6 (–1.1 to 6.4); 0.21 |
| OCT vs. fundus clinical examination | 40.3 (30.0 to 49.6); < 0.001 | –3.3 (–5.9 to –1.2); < 0.001 |
| OCT vs. self-reported vision | 85.6 (77.0 to 90.5); < 0.001 | –2.1 (–4.9 to 0.4); 0.14 |
| OCT vs. visual acuity | 64.2 (52.4 to 73.0); < 0.001 | 8.1 (4.1 to 12.3); < 0.001 |
| Amsler vs. fundus clinical examination | –18.6 (–31.0 to –5.3); 0.01 | –5.6 (–9.1 to –2.7); < 0.001 |
| Amsler vs. self-reported vision | 22.7 (12.8 to 32.6); < 0.001 | –5.6 (–9.2 to –2.5); < 0.001 |
| Amsler vs. visual acuity | 8.2 (–3.0 to 19.3); 0.20 | 2.6 (–1.7 to 7.0); 0.30 |
| Fundus clinical examination vs. self-reported vision | 46.2 (36.5 to 55.0); < 0.001 | 1.2 (–0.4 to 3.2); 0.22 |
| Fundus clinical examination vs. visual acuity | 24.4 (12.5 to 35.3); < 0.001 | 11.3 (8.0 to 15.2); < 0.001 |
| Self-reported vision vs. visual acuity | –20.3 (–28.9 to –12.2); < 0.001 | 10.2 (6.7 to 14.2); < 0.001 |
The specificity of the index test pairs also differed significantly from each other, except for the following pairs: OCT versus Amsler, OCT versus self-reported vision and fundus clinical examination versus self-reported vision.
Statistically significant differences in specificity between index tests varied from –5.6% (Amsler vs. fundus clinical examination and Amsler vs. self-reported vision) to 11.3% (fundus clinical examination vs. visual acuity) in magnitude.
Modelling diagnostic accuracy by generalised estimating equations
The sensitivity and specificity of the five index tests against FFA as the reference standard using the GEE model also confirmed that OCT had high sensitivity (90%) and specificity (97.4%) (Table 24), which was consistent with all other analyses.
| Index test | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) |
|---|---|---|
| Amsler | 27.7 (18.6 to 36.7) | 97.9 (97.4 to 98.5) |
| Fundus clinical examination | 50.4 (41.4 to 59.4) | 99.5 (99.3 to 99.7) |
| OCT | 90.0 (84.8 to 95.4) | 97.4 (96.6 to 98.2) |
| Self-reported vision | 1.8 (0.2 to 3.3) | 98.0 (95.4 to 100) |
| Visual acuity | 25.8 (18.0 to 33.7) | 93.7 (92.4 to 95.0) |
Secondary reference standard 1
The cross-tabulation between the primary reference standard and secondary reference standard 1 for the diagnosis of nAMD is shown in Table 25.
| Variable | Secondary reference standard 1a | |||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis of nAMD | Diagnosis of no nAMD | |||||||
| Primary reference standarda | FFA positive | FFA negative | FFA inconclusive | FFA not carried out | FFA positive | FFA negative | FFA inconclusive | FFA not carried out |
| Participants (n) | 120 | 12 | 5 | 8 | 0 | 324 | 3 | 17 |
| Total | 145 | 344 | ||||||
Summarising the index tests over the whole period and the paired comparisons did not alter the sensitivity or specificity compared with the primary reference standard results (see Appendix 1).
Secondary reference standard 2
The analyses conducted using reading centre FFA determination of conversion to nAMD were available for 127 participants, of whom eight were ungradable (secondary reference standard 2). The cross-tabulation between the primary reference standard and secondary reference standard 2 for diagnosis of nAMD is shown in Table 26. The agreement between the reading centre and the site result was 91%.
| FFA at the reading centre | FFA on site (n) | |||
|---|---|---|---|---|
| Positive | Negative | Inconclusive | Total | |
| Positive | 99 | 15 | 5 | 119 |
| Negative | 20 | 318 | 3 | 341 |
| Ungradable | 1 | 3 | 0 | 4 |
| Total | 119 | 333 | 8 | 464 |
The analyses conducted using secondary reference standard 2 included summarising the index tests over the whole period, the last 6 months and the last visit and the associated paired comparisons, none of which altered sensitivity or specificity compared with the primary reference standard results (see Appendix 1).
Discussion
The sensitivity and the specificity of the five index tests for the main analyses are summarised in Figures 12 and 13, respectively. Across the different reference standards and timelines analysed, OCT consistently exhibited the highest sensitivity, which ranged from 82% to 92%, and was significantly superior to all other index tests. The specificity of OCT was high, ranging from 88% to 96%. Although fundus clinical examination and self-reported vision exhibited specificities that were higher than OCT, their sensitivity was significantly lower at 60% and 10%, respectively.
FIGURE 12.
Summary of the sensitivity of the index tests across all diagnosis analyses. A1, main analysis: primary reference standard, all index test results; A2, primary reference standard, last 6 months of index tests; A3, primary reference standard, last visit index tests; A4, secondary reference standard 2, all index test results; A5, secondary reference standard 2, last 6 months of index tests; A6, secondary reference standard 2, last visit index tests; A7, secondary reference standard 1, all index test results.
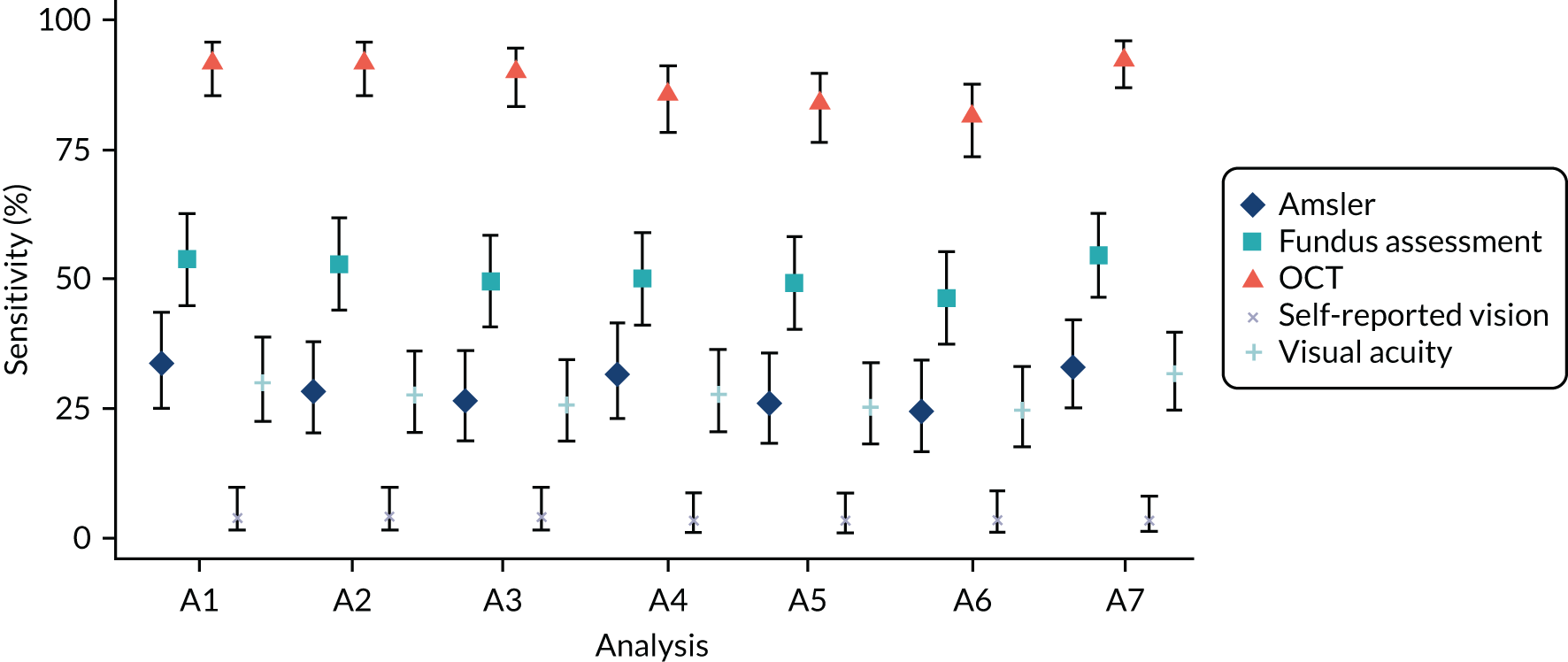
FIGURE 13.
Summary of the specificity of the index tests across all diagnosis analyses. A1, main analysis: primary reference standard, all index test results; A2, primary reference standard, last 6 months of index tests; A3, primary reference standard, last visit index tests; A4, secondary reference standard 2, all index test results; A5, secondary reference standard 2, last 6 months of index tests; A6, secondary reference standard 2, last visit index tests; A7, secondary reference standard 1, all index test results.
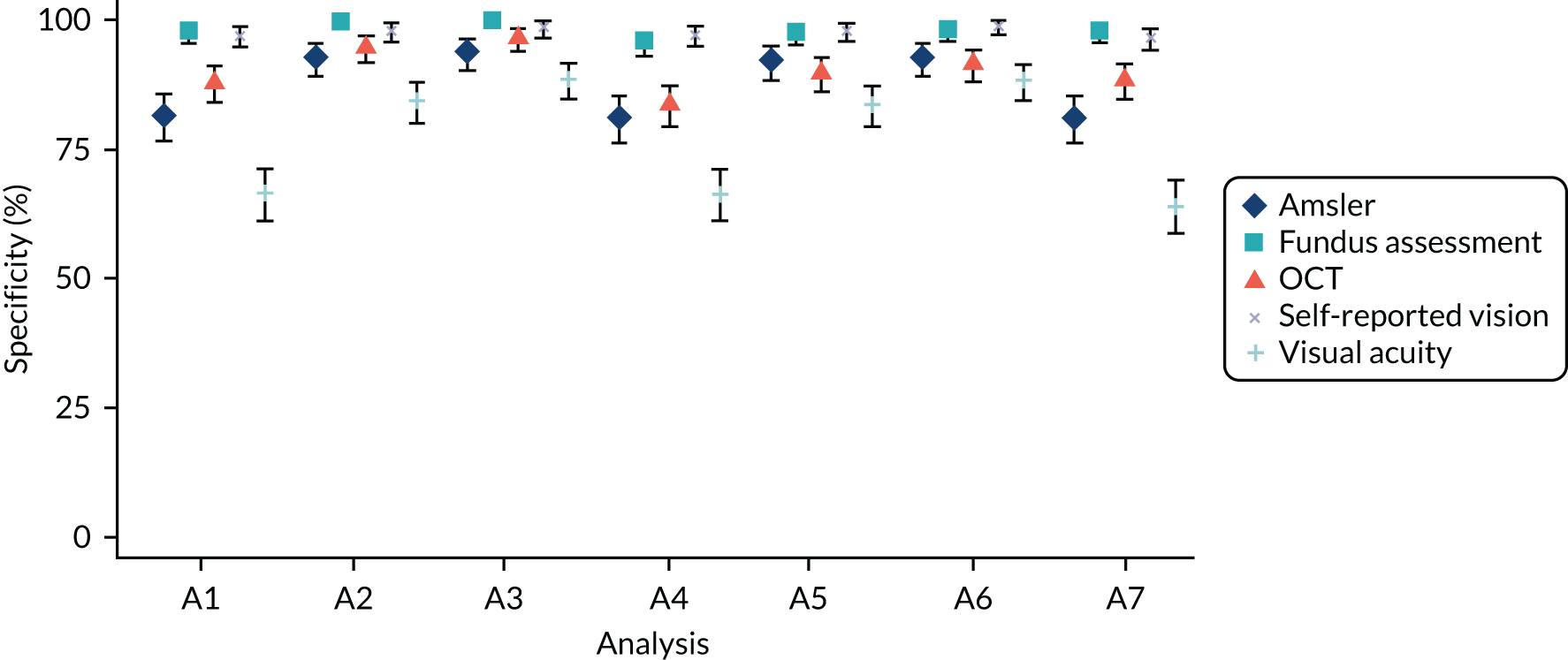
The paired comparisons assess whether or not there was statistical evidence of a difference between the tests’ diagnostic accuracy performance. Notably, we found that OCT had substantially superior sensitivity compared with all of the other tests. Although OCTs specificity was marginally lower than some of the tests, when considering monitoring strategies, there is an implicit preference for sensitivity over specificity in the context of the occurrence of repeated visits. Overall, these findings support OCT as the most superior of the non-invasive index tests selected for evaluation in the present study in terms of diagnostic accuracy in a monitoring setting.
A FFA determination of conversion to nAMD at the clinical site was the reference standard used in the main analysis. Given that clinician’s interpretation of FFA has been reported to have only moderate levels of agreement, we obtained a reading centre evaluation of all FFAs performed during the study. 62 We were, therefore, able to vary the reference standard using the reading centre determination of conversion to nAMD. In contrast to reports that have in the past shown lesser degrees of agreement between clinicians in FFA interpretation,62 we observed very high levels of agreement between the clinicians and the reading centre (90%). However, in the present study, clinicians were not asked to determine the nAMD phenotype but to merely report whether or not nAMD was present. We also varied the reference standard to include data from 25 additional participants for whom a FFA was not performed but the clinician had determined nAMD to have developed in the EDNA study eye based on clinical judgement. We found that all analyses pointed clearly to OCT as the most accurate index test.
It is of note that most of the index tests and the primary reference standard that were carried out were performed on the same day or within a few days of each other. To avoid being influenced by the FFA, site staff carried out the index tests and if any test was positive this was considered to be a trigger and a FFA was performed.
We carried out a number of sensitivity analyses. None of these altered the overall view of the value of the respective index tests. We varied the definition of two of the index tests. For the primary analysis, we chose a visual acuity drop of 10 letters from baseline, representing a fall of two lines on the ETDRS visual acuity chart, to be a positive index test. We redefined the index test as positive with a drop of 15 letters (three lines on the ETDRS chart), which represents a doubling of the visual angle, which is a considerable fall in acuity. However, the sensitivity of visual acuity as an index test did not improve. We also changed the definition of a positive test for self-reported vision from worse to much worse. Interestingly, even for participants who had experienced considerable worsening of vision in the EDNA study eye, self-reported change in function performed poorly in terms of sensitivity of the index test. We were not able to rigorously assess the reading centre OCT assessment owing to the concurrent use of the FFA assessment in the reading centre. This would have helped inform about inherent differences between OCT and FFA versus differences between one-off individual assessment (i.e. clinical site vs. reading centre).
In addition to the diagnostic accuracy of an index test, other factors need to be considered to determine the value of a test for use for monitoring in clinical practice. The results show that in the current NHS settings, the selected index tests were readily conducted. Visual acuity had the largest number of tests performed, which reflects how well the collection of visual acuity data is embedded within the system given its near universal use in ophthalmic care. Of particular note was the finding that a substantial proportion (17%) of the Amsler tests were false positives at baseline, thus precluding the use of this test in monitoring such patients. This proportion of false positives even at a point in time when nAMD was clearly absent in the EDNA study eye substantially limits the value of the Amsler, aside from its poor diagnostic accuracy. Data on the time taken to perform tests and the associated costs are addressed in Chapter 7.
The constraints of routine clinical practice that caused variations in monitoring procedures between sites, the variable gaps in visit intervals (in a small number of cases these gaps were substantial) and study-specific methodology (utilisation of non-triggered 18 months and ‘exit’ FFAs) all affected the ability to generate a precise survival curve. In particular, the estimated proportion of nAMD-free patients beyond 30 months should be interpreted cautiously (particularly beyond 38 months) owing to the gaps between clinic visits and FFAs. The impact of these constraints can be seen in the shape of the two Kaplan–Meier curves that we produced. The first curve shows an acute dip in the proportion of nAMD-free patients around month 30, which is an artefact of the study methodology. Arguably, the survival curve that assumes that conversion to nAMD occurred at the mid-point of the interval between the last visit with negative index tests and the final FFA represents a better reflection of the conversion rate and is more aligned with that observed over the first 2 years of the study (21%). Of note, the models that dealt with interval data were used to inform the health economic modelling because we considered that they better represent the survival distribution, despite that some of the assumptions that we made about the shape of survival distribution might be disputed. There was no clear sign of a variable rate of conversion over the 3-year period because the distribution of data collection points affected this assessment, particularly in the third year of follow-up.
Owing to the longitudinal nature of the data, we used different options to deal with multiple time points. In both the main and the secondary analyses, we utilised a single index result for comparison with the reference standard. In the main analysis, for which we summarised index test over the duration of follow-up, conversions occurred between 7 and 30 days earlier (on average) than the reference standard. We did not consider this interval to be of sufficient length for disease to have progressed. We varied the index tests that could be included in the secondary analyses: in one approach tests that were selected were acquired over the 6 months prior to the participants’ diagnosis; in a second approach we used the last visit test. Positive index tests, which were defined as triggers to collect the reference standard, were usually followed up within a short period of time. Therefore, the main approach to summarising index tests and the two secondary approaches yielded results that were highly similar.
To account for multiple time points, we used an approach that collapsed the data for an individual down to a single summary test result for the main analysis and associated sensitivity analyses. A secondary analysis used a GEE model to calculate sensitivity and specificity using results from each index test over time. Findings were broadly similar for sensitivity; however, specificity was marginally higher per test conducted (producing similar results to the last visit analysis, analysis A3). The model provided improved precision, resulting in tighter confidence intervals for specificity than those for single point analyses. This finding was expected given the way that the EDNA study was set up: multiple negative index tests were possible during the study follow-up and participants were assumed to be disease free until evidence of the contrary was found. Furthermore, when an index test was positive, a primary reference standard was triggered resulting in either a false positive index test or a true positive index test with an exit from the study. An independent correlation structure, with calculation of robust standard errors, was assumed to avoid the potentially dubious assumption relating to test performance for diseased and non-diseased individuals implied by using an exchangeable correlation structure. 63,64
Chapter 5 Function and morphology of the lesion in the study eye at onset of neovascular age-related macular degeneration and in the fellow eye at initial presentation, and agreement between clinician diagnosis of onset of neovascular age-related macular degeneration and reading centre determination of presence of neovascular age-related macular degeneration in the study eye
Introduction
In this chapter, we illustrate in detail the morphological features of the exudative nAMD lesions at first occurrence in the EDNA study eye. To aid comparison with late presentation, which happens in routine eye care, we compare and contrast the morphological features of the nAMD lesions at enrolment in the first-presenting eye, here termed ‘fellow eye’, with those features of the nAMD lesions detected at conversion to exudative nAMD in the second eye, the EDNA study eye. This was made possible by the detailed independent grading that was performed at netwORC UK by the trained staff, who recorded the findings using prespecified data fields.
The image-grading objectives at the reading centre in the EDNA study were to:
-
confirm the diagnosis of nAMD in the first-presenting eye (fellow eye) at enrolment (an eligibility criterion)
-
confirm the absence of exudative nAMD in the EDNA study eye at baseline
-
confirm conversion to exudative nAMD in the EDNA study eye.
The main objectives of this chapter are to describe the:
-
function and lesion morphology of the fellow eye at enrolment
-
function and lesion morphology of the EDNA study eye at conversion (phenotype of nAMD)
-
lesion metrics (size of lesion, proportion of lesion composed by neovascular complex, haemorrhage, atrophy and fibrosis) in the fellow eye at presentation and the EDNA study eye at conversion to exudative nAMD
-
lesion characteristics in cases where there was lack of agreement between clinician and reading centre on conversion to exudative nAMD in the EDNA study eye
-
lesion characteristics in cases where there was disagreement in reading centre determination between FFA and OCT – (1) nAMD was determined as present on the FFA but not on OCT and (2) nAMD was determined as absent on the FFA but present on OCT – and the effect of these disagreements on clinician determination of conversion to exudative nAMD in the EDNA study eye.
Methods
Clinicians examined the patient (fundus examination) and reviewed the OCT scan (see Figure 1). If any of the index tests were positive at any visit, this was considered to be a trigger and a FFA was performed. The triggers were: signs of nAMD in clinical examination, change in the Amsler test, patient describes a worsening in their vision (self-reported vision), drop in visual acuity of ≥ 10 letters or an abnormal OCT scan. After reviewing the FFA, the clinician determined if conversion to nAMD had occurred (primary reference standard).
In the EDNA study cohort of 552 participants, there were eight missing baseline FFA images. Although there were no missing OCT images at baseline, images from 15 OCT participants were not submitted to the reading centre. During EDNA study follow-up, there were nine participants with no follow-up data available. Of the remaining 543 participants, 460 participants had a reading centre grading (78 participants had no FFA and five participants had a FFA, but this was not sent to the reading centre). See Figure 8 and Table 26 for full details of participant flow and reading centre determination of conversion to nAMD. In the analysis described in this chapter, study eyes without a baseline FFA are excluded. For the tables comparing the morphology at conversion in the EDNA study eye with the morphology of the fellow eye at baseline, only eyes with an appropriate follow-up or trigger FFA read by the reading centre are included (n = 460).
Figure 14 shows a case in which the OCT indicated retinal thickening and acted as a trigger. The corresponding FFA was read by the clinician to show conversion to nAMD, and the reading centre-confirmed conversion to nAMD. If no trigger occurred at any routine visit, a research visit was scheduled at 18 and 36 months. At this visit, the clinician reviewed the imaging set and confirmed either the presence or the absence of nAMD. Figure 15 shows an example that did not experience any triggers, and at study exit the patient’s OCT and FFA were deemed to be free of nAMD. The reading centre determination agreed with this assessment. Reading centre data were obtained for the sole purpose of verification of conversion to nAMD and, thus, support the analysis.
FIGURE 14.
A case in which the OCT indicated retinal thickening and acted as a trigger. (a) OCT scan of the EDNA study eye at study enrolment (baseline) showing multiple large confluent drusen. There is no evidence of neovascularisation at baseline in the EDNA study eye. (b) The EDNA study eye 6 months later, showing retinal thickening that triggered a FFA. (c) Corresponding fluorescein angiogram read by the clinician as having converted to nAMD in the EDNA study eye and confirmed by reading centre grading.
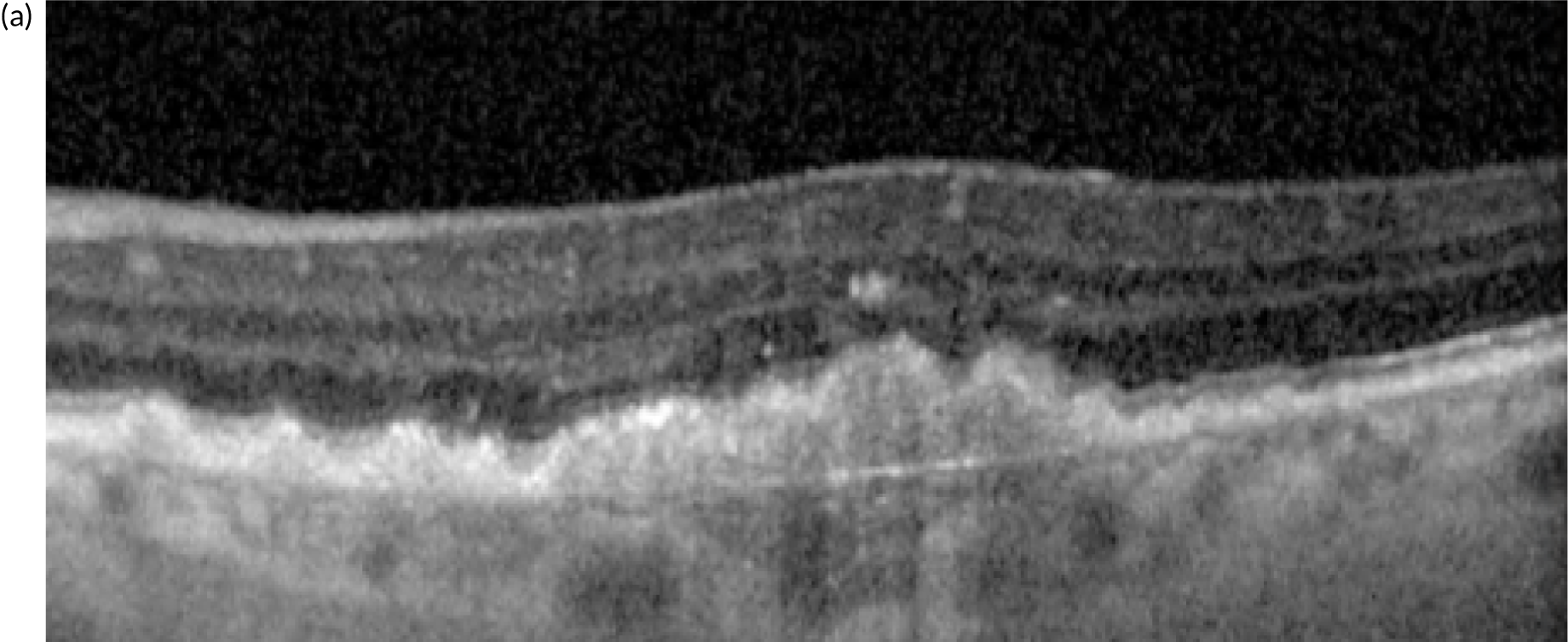
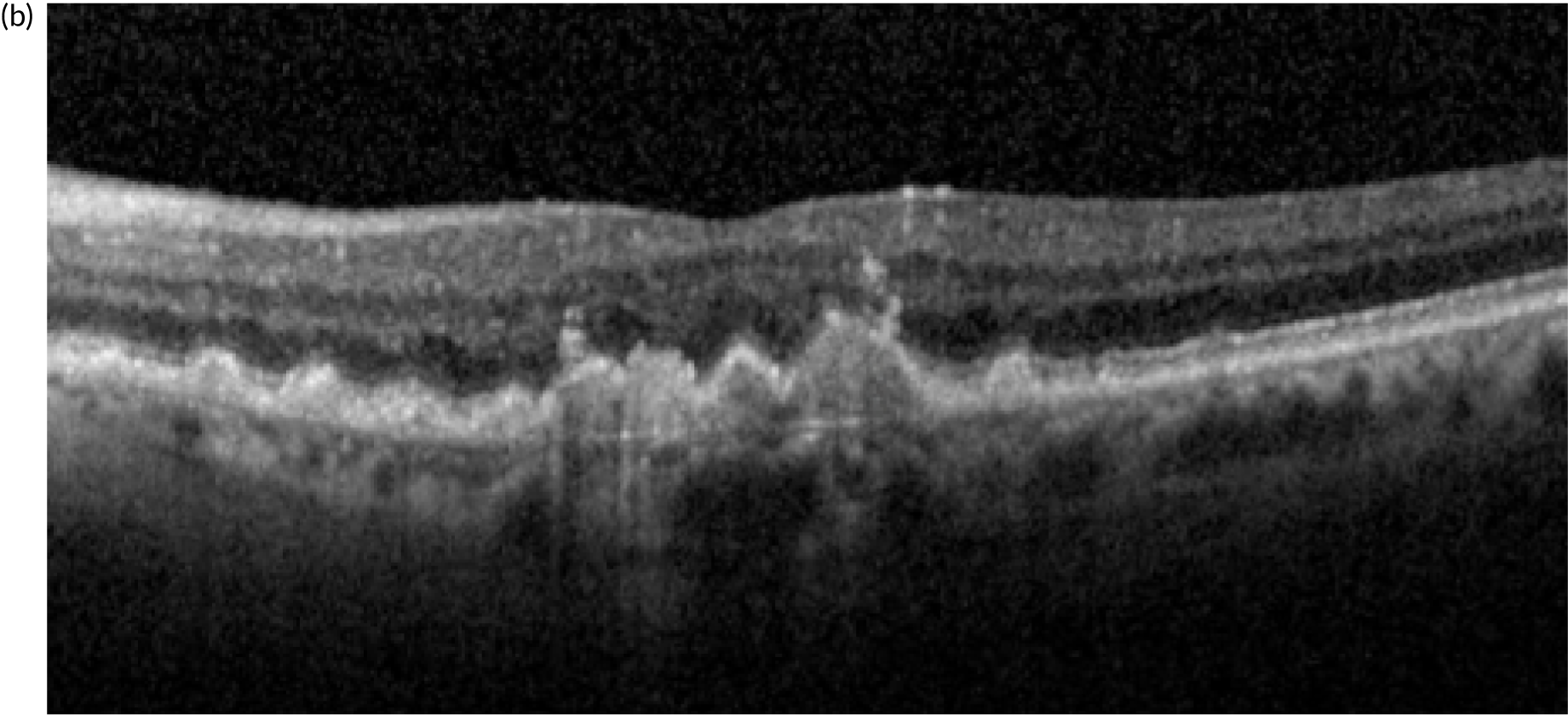
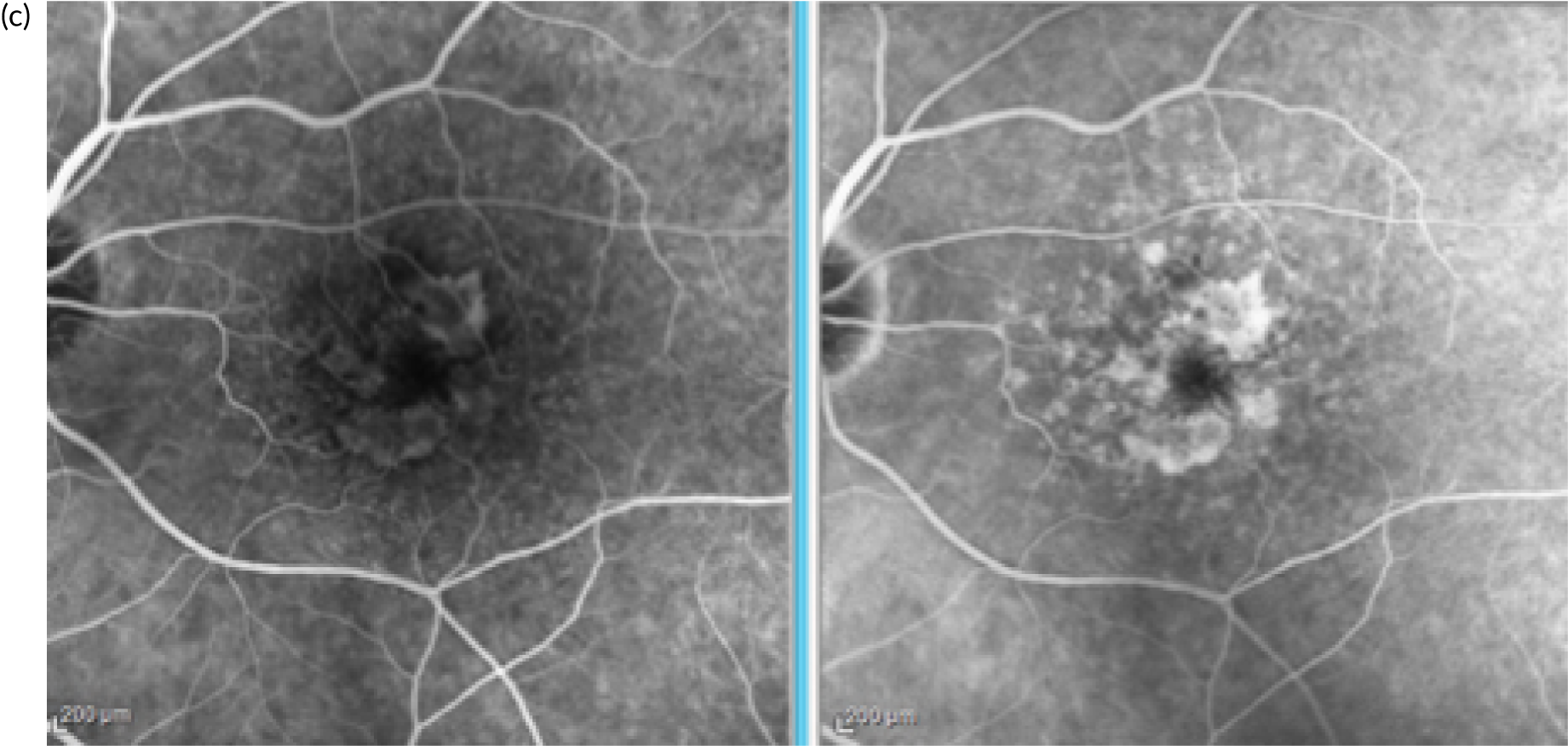
FIGURE 15.
A case in which there were no triggers and OCT and FFA were deemed free of nAMD at study exit. (a) EDNA study eye (right eye) at enrolment showing few drusen on the OCT scan. (b) At the study exit visit approximately 30 months after enrolment, there is no evidence of conversion to nAMD on OCT. (c) FFA carried out at the exit visit also confirms absence of neovascularisation.
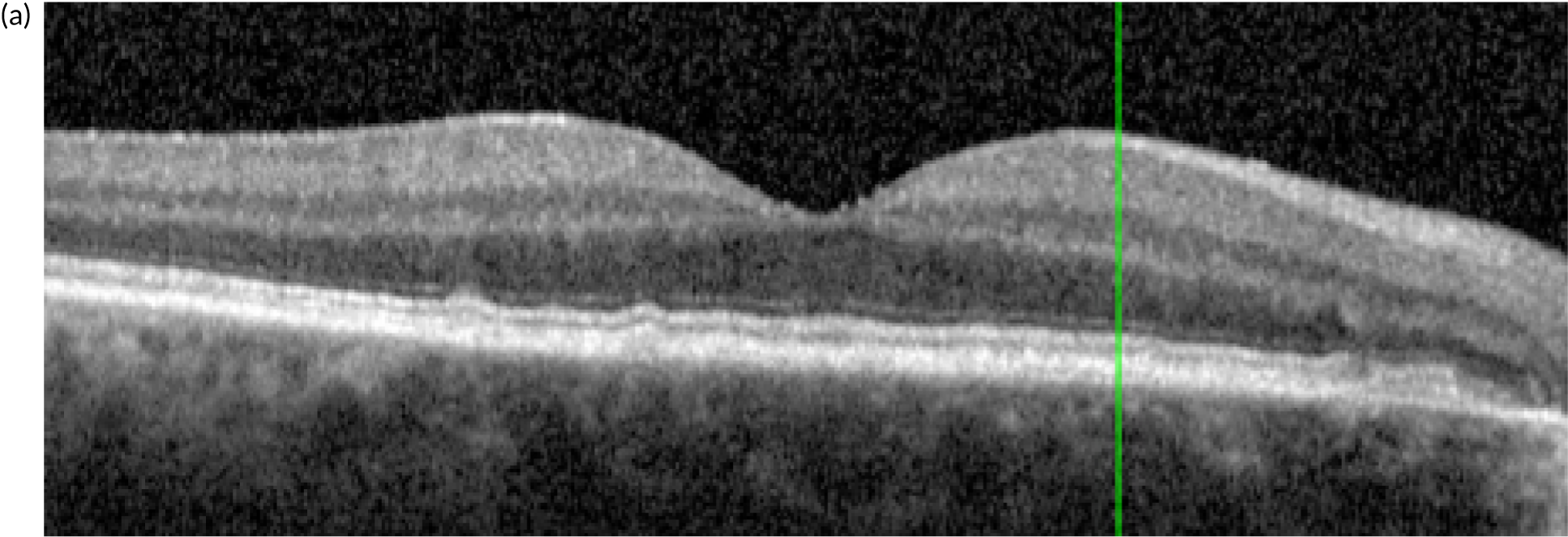
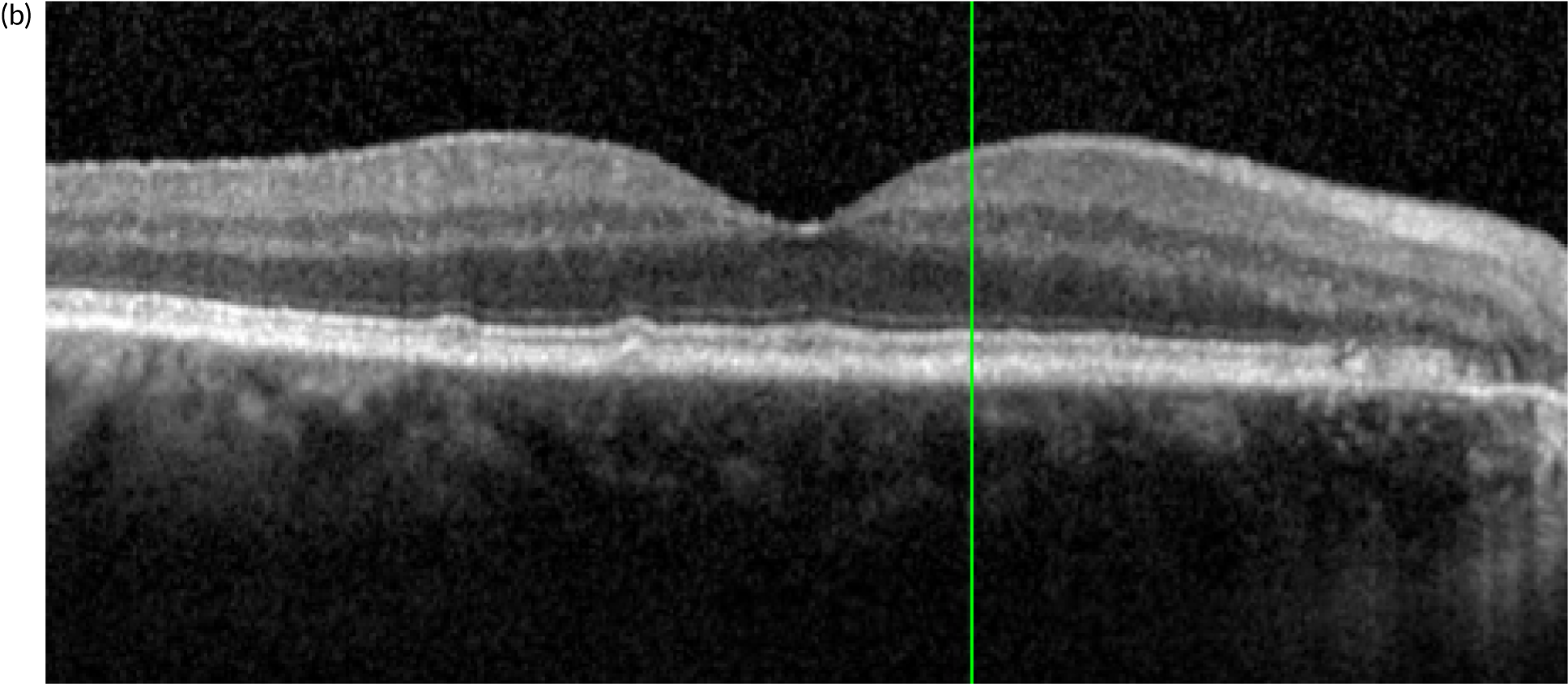
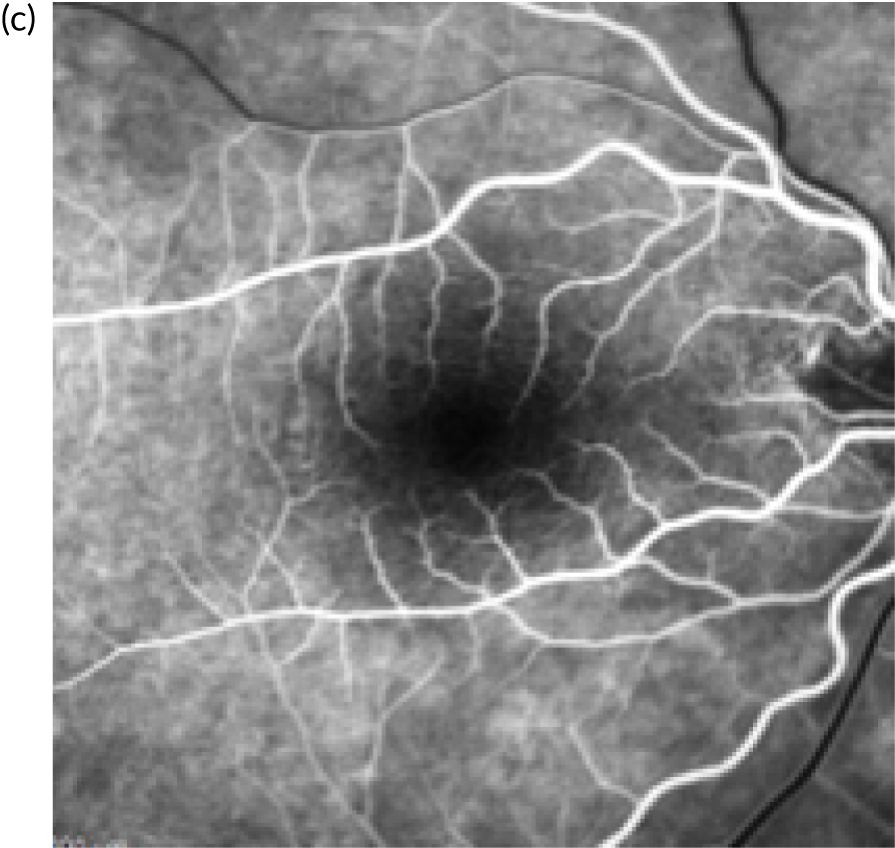
For a full description of the reading centre definitions of nAMD and conversion to nAMD, see Report Supplementary Material 1. In brief, images from the different imaging modalities (colour, OCT, FFA and, if available, ICGA) were uploaded into netWORC UK’s platform, checked by the administrative staff and subjected to a protocol-based examination by trained graders using standardised definitions.
The full EDNA image set for each patient was uploaded to the netWORC UK server from which it was accessed by graders. Graders, after logging in, are presented with an EDNA study ID for a given visit. Images are displayable on screen alongside the grading form. Each image modality is graded separately. If nAMD was not detected on one of the imaging modalities but was recorded as present on another imaging modality (e.g. present on colour but not on OCT), this was flagged as a grading discrepancy and the senior grader or site clinician was allowed to correct the mistake with arbitration. If the arbitrator agreed that there were no signs of nAMD in any given image modality, discrepancies could not be changed (i.e. genuine discordance was kept).
Reading centre data were uploaded to the CHaRT data management platform and integrated into the clinical data set. Cross-tabulation of clinician determination of conversion to nAMD versus reading centre determination was carried out.
Results
Fundus fluorescein angiography lesion characteristics in fellow eyes at study entry
At study enrolment (baseline), the reading centre classified 535 out of 548 available FFA images of fellow eyes as exhibiting nAMD (see Table 8). Of the remaining 13 eyes, eight eyes were classified by the reading centre as not exhibiting nAMD. The remaining five eyes were ungradable. The lesion characteristics of fellow eyes at initial presentation are shown in Table 8, and the reading centre grading confirmed neovascular complexes in 517 (94.3%) out of the 535 fellow eyes. The size of the lesion, as measured by the greatest linear dimension, was 5.9 mm and the area was, on average, 7.7 mm2. Two-thirds of the lesions had type 1 neovascularisation, one-fifth had type 2 and the remainder had RAP. RAP lesions were more likely to be extrafoveal, whereas type 1 were most likely to be subfoveal. Fibrosis and atrophy were present in around 10% of contralateral eyes at presentation.
Fundus fluorescein angiography lesion characteristics in EDNA study eyes at conversion
A total of 119 EDNA study eyes with a FFA were transmitted to the reading centre for grading at the visit, at which conversion to nAMD was detected by the clinician examination or because of a trigger arising from one of the test technologies. The reading centre confirmed 99% of EDNA study eyes to have nAMD based on FFA at the conversion visit. Table 27 shows the reading centre-graded lesion characteristics at baseline of fellow eyes for the 119 participants constituting the subset who developed nAMD in the EDNA study eye. The characteristics of the fellow eyes at first presentation in this subset were highly similar to those of the full sample for frequency of nAMD subtype. In fellow eyes, the diameter and area of the total lesion and that of the active neovascular complex were similar between the subset that converted to nAMD and the full sample. On comparing fellow eyes with EDNA study eyes, the proportion with type 1, 2 and 3 lesions was similar between fellow eyes at enrolment and EDNA study eyes at detection of conversion to exudative nAMD. On comparing corresponding study eyes with fellow eyes in the 119 eyes that converted to nAMD, the area dimensions of the total lesion and active neovascular complex were markedly lower in the former than in the latter (see Table 27). Lesions were also more likely to be extrafoveal or juxtafoveal for all three lesion types in the EDNA study eye than in corresponding fellow eyes. In type 1 lesions, 50% were subfoveal at detection of nAMD in the EDNA study eye compared with > 80% that were subfoveal in the fellow eye.
| FFA findings | Baseline (fellow eye) | At conversion to nAMD (EDNA study eye) | ||||
|---|---|---|---|---|---|---|
| n (%) or mean (SD); n | Median (P25–P75) | Minimum–maximum | n (%) or mean (SD), n | Median (P25–P75) | Minimum–maximum | |
| Exudative AMD present | 118 (99.2) | 118 (99.2) | ||||
| Active CNV or RAP present | 113 (95.0) | 116 (97.5) | ||||
| Total GLD (mm2) | 3.6 (2.7); 118 | 3.3 (2.3–4.5) | 0.3–25.4 | 1.9 (1.5); 118 | 1.5 (0.8–2.6) | 0.1–9.7 |
| Total lesion area (mm2) | 8.1 (7.8); 118 | 6.3 (2.5–10.6) | 0.1–48.8 | 3.4 (5.8); 118 | 1.3 (0.6–4.5) | 0.0–44.7 |
| Active lesion GLD (mm2) | 3.5 (2.7); 113 | 3.2 (2.2–4.3) | 0.3–25.4 | 1.9 (1.5); 116 | 1.5 (0.7–2.6) | 0.1–9.7 |
| Total active lesion area (mm2) | 7.6 (7.6); 113 | 5.7 (2.5–10.2) | 0.1–44.9 | 3.2 (5.7); 117 | 1.3 (0.4–4.1) | 0.0–44.7 |
| Type 1 present | 80 (67.2) | 68 (57.1) | ||||
| Type 1 area (mm2) | 6.7 (5.1); 80 | 5.7 (2.9–10.0) | 0.2–21.1 | 3.7 (3.7); 68 | 2.3 (1.1–4.9) | 0.1–17.2 |
| Type 1 location | ||||||
| Extrafoveal | 11 (13.8) | 20 (29.4) | ||||
| Juxtafoveal | 2 (2.5) | 14 (20.6) | ||||
| Subfoveal | 67 (83.8) | 34 (50.0) | ||||
| Type 2 present | 37 (31.1) | 29 (24.4) | ||||
| Type 2 area (mm2) | 2.6 (2.9); 37 | 1.6 (0.5–4.6) | 0.0–13.7 | 0.8 (0.8); 29 | 0.4 (0.2–1.0) | 0.0–3.6 |
| Type 2 location | ||||||
| Extrafoveal | 5 (13.5) | 16 (55.2) | ||||
| Juxtafoveal | 13 (35.1) | 10 (34.5) | ||||
| Subfoveal | 19 (51.4) | 3 (10.3) | ||||
| Type 3 present | 13 (10.9) | 20 (16.8) | ||||
| Type 3 area (mm2) | 0.2 (0.3); 13 | 0.1 (0.0–0.2) | 0.0–0.8 | 0.1 (0.2); 20 | 0.1 (0.0–0.1) | 0.0–1.1 |
| Type 3 location | ||||||
| Extrafoveal | 5 (38.5) | 10 (50.0) | ||||
| Juxtafoveal | 4 (30.8) | 10 (50.0) | ||||
| Subfoveal | 4 (30.8) | |||||
| Fibrosis present | 14 (11.8) | 2 (1.7) | ||||
| Fibrosis area (mm2) | 4.1 (4.5); 14 | 3.1 (0.5–4.7) | 0.2–15.2 | 2.4 (3.2); 2 | 2.4 (0.1–4.7) | 0.1–4.7 |
| Atrophy within lesion | 11 (9.2) | 10 (8.4) | ||||
| Area of atrophy (mm2) | 2.1 (2.4); 11 | 0.8 (0.2–4.2) | 0.1–7.0 | 1.4 (2.1); 10 | 0.8 (0.4–1.3) | 0.1–7.2 |
| Location of atrophy | ||||||
| Extrafoveal | 1 (9.1) | 5 (50.0) | ||||
| Juxtafoveal | 7 (63.6) | 4 (40.0) | ||||
| Subfoveal | 3 (27.3) | 1 (10.0) | ||||
In type 2 lesions, 10% were subfoveal in the EDNA study eye compared with 50% in corresponding fellow eyes. Of lesions classified as RAP, none was subfoveal in the EDNA study eye at conversion to exudative nAMD, with these lesions equally distributed between extra and juxtafoveal locations.
Fibrosis was rare in nAMD lesions at conversion in the EDNA study eye compared with fellow eyes at initial presentation (2% vs. 12%). Atrophy was present in similar proportions in the EDNA study eye at nAMD conversion compared with fellow eyes at initial presentation.
Optical coherence tomography findings in the entire cohort and in the subset of participants who converted to neovascular age-related macular degeneration in the EDNA study eye
In the fellow eyes, the average central foveal thickness was just under 400 µm at initial presentation in the 548 participants with gradable OCT images available to the reading centre (Table 28). In the 119 participants who converted to nAMD, the mean central foveal thickness in the fellow eye at presentation was 437 µm.
| Central foveal thickness | Baseline | Conversion | ||
|---|---|---|---|---|
| Mean (SD); n | Median (P25–P75) | Mean (SD); n | Median (P25–P75) | |
| Central foveal thickness (µm): fellow eye | 437.8 (156.8); 114 | 421.0 (322.0–520.0) | N/A | N/A |
| Central foveal thickness (µm): study eye | 274.6 (37.4); 113 | 276 (255–293) | 334.4 (102.3); 111 | 318.0 (275.0–372.0) |
External limiting membrane and EZ disruption was graded as present in around one-quarter and one-third of contralateral eyes, respectively, and focal atrophy in one-fifth of eyes (Table 29).
| OCT features | All participants (N = 548), n (%) | Participants who converted to nAMD (N = 119), n (%) | |
|---|---|---|---|
| Baseline | Baseline | At conversion to nAMD | |
| ELM disruption present | 146 (26.6) | 38 (31.9) | 75 (63.0) |
| EZ disruption present | 186 (33.9) | 48 (40.3) | 95 (79.8) |
| Hyporeflective spaces present within the neurosensory retina | 15 (2.7) | 2 (1.7) | 44 (37.0) |
| Focal atrophy present | 72 (13.1) | 19 (16.0) | 31 (26.1) |
| Evidence of inner nuclear subsidence | 60 (10.9) | 16 (13.4) | 22 (18.5) |
| Transmission defect present | 60 (10.9) | 16 (13.4) | 22 (18.5) |
| Normal choroid | 307 (56.0) | 60 (50.4) | 44 (37.0) |
| Thick choroid | 5 (0.9) | 1 (0.8) | 4 (3.4) |
| Thin choroid | 225 (41.1) | 56 (47.1) | 64 (53.8) |
In study eyes, at conversion to exudative nAMD the central foveal thickness was just over half of the average thickness of corresponding fellow eyes at 274 µm (see Table 28). The proportion of eyes with ELM and EZ disruption, which was similar between fellow eyes and study eyes at enrolment, had doubled in study eyes at nAMD conversion (see Table 29).
Discrepancies between fundus fluorescein angiography determination of conversion to neovascular age-related macular degeneration at clinical site and reading centre determination of conversion to neovascular age-related macular degeneration
A proportion of EDNA study eyes was determined to have nAMD at the clinical site, but did not have this confirmed by the reading centre (20 out of the 119 eyes with nAMD according to the primary reference standard). Conversely, 15 out of 333 cases that were classed as not having converted to nAMD by the clinician were determined by the reading centre to have converted to nAMD (see Table 26). This represents a kappa statistic of 0.91. In the case illustrated in Figure 16, the OCT was determined to be positive but the FFA was considered negative by the clinic, while the reading centre determined the case to have converted to nAMD both on OCT and on FFA. In this case, leakage was seen in the late frames of the angiogram in an area remote to the fovea (shown by red arrow). A further case (not shown) had a type 1 CNV that was indolent and minimal fluorescein leakage visible only on stereoscopic examination of the late angiographic frames. In this case, the clinic determined absence of conversion to nAMD whereas the reading centre determined that exudation was present.
FIGURE 16.
The OCT scan shows a shallow area of subretinal fluid. The fluorescein was deemed by the clinician to be negative. The FFA late frames show the presence of leakage (red arrow) indicating presence of nAMD.
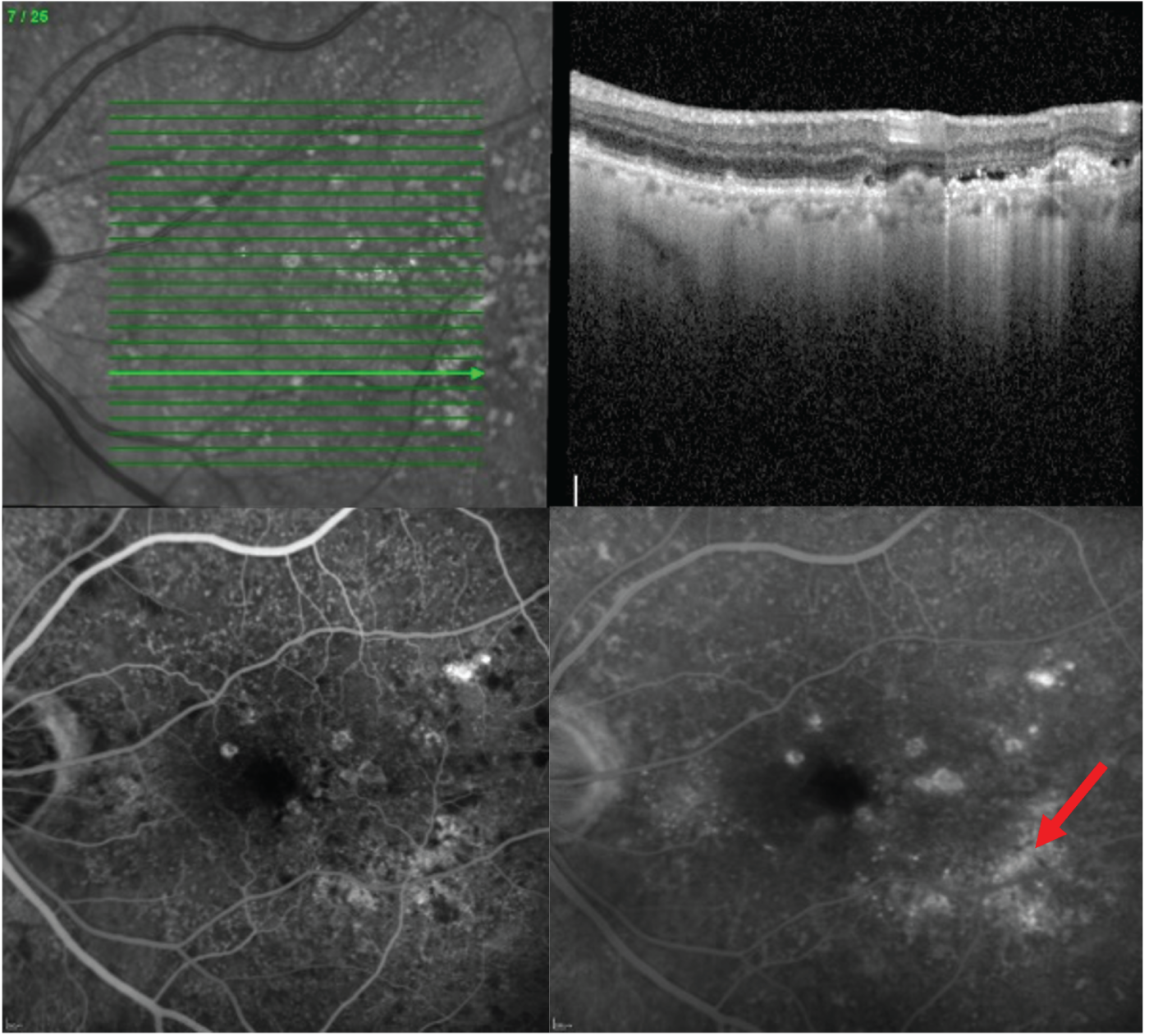
Discrepancies between fundus fluorescein angiography diagnosis and optical coherence tomography diagnosis of neovascular age-related macular degeneration at the reading centre
In total, there were 12 instances for which the FFA was positive and the OCT was negative. There were also 12 instances for which the OCT was positive and the FFA was negative. Most discrepancies had equivocal findings in either OCT or FFA, or both. In the case shown in Figure 17, the OCT was determined to be negative with no leak, but the FFA was determined to be positive. In the case shown in Figure 18, the OCT was determined to be positive, with a small focus of hyporeflectivity seen in the subretinal space in the foveal B-scan. The early frames of the FFA show some hyperfluorescence at the site corresponding to the region of presumed fluid on the OCT scan. This hyperfluorescence fades over the angiographic run, which is not thought to be a result of a true leak but staining of the RPE.
FIGURE 17.
Disagreement between OCT- and FFA-detected nAMD: example 1. (a) The OCT shows no leakage, i.e. no intra or subretinal fluid. There is a drusenoid elevation of the RPE that is non-exudative. (b) Corresponding FFA shows increasing leakage over the course of the angiogram.
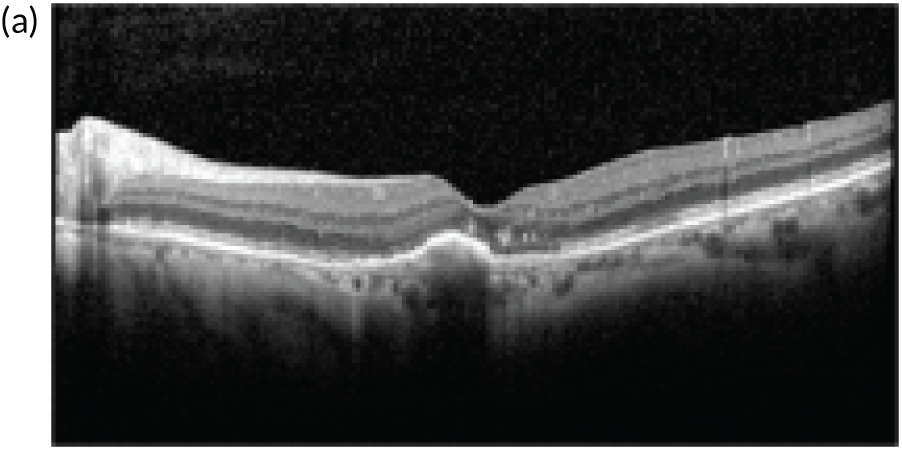
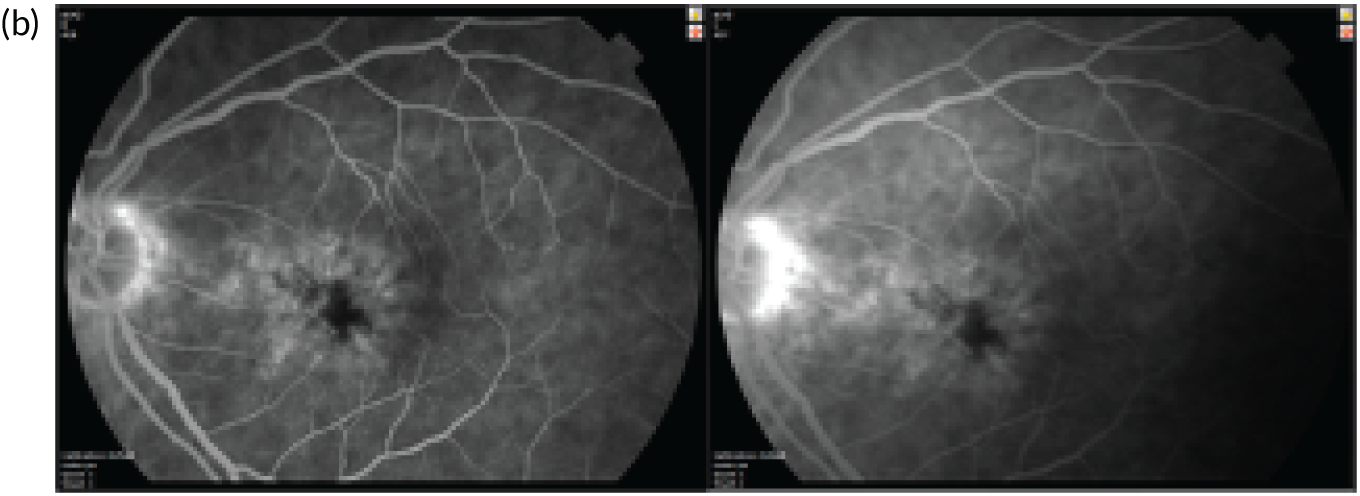
FIGURE 18.
Disagreement between OCT- and FFA-detected nAMD: example 2. (a) OCT: there is a small pocket of subretinal fluid that is located extrafoveally. (b) FFA: the early frames of the FFA show no leakage. The late frames show speckled hyperfluorescence. Additional late frames show no intensification of the speckled hyperfluorescence nor blurring of the margins of the speckles suggesting that these are just RPE abnormalities.
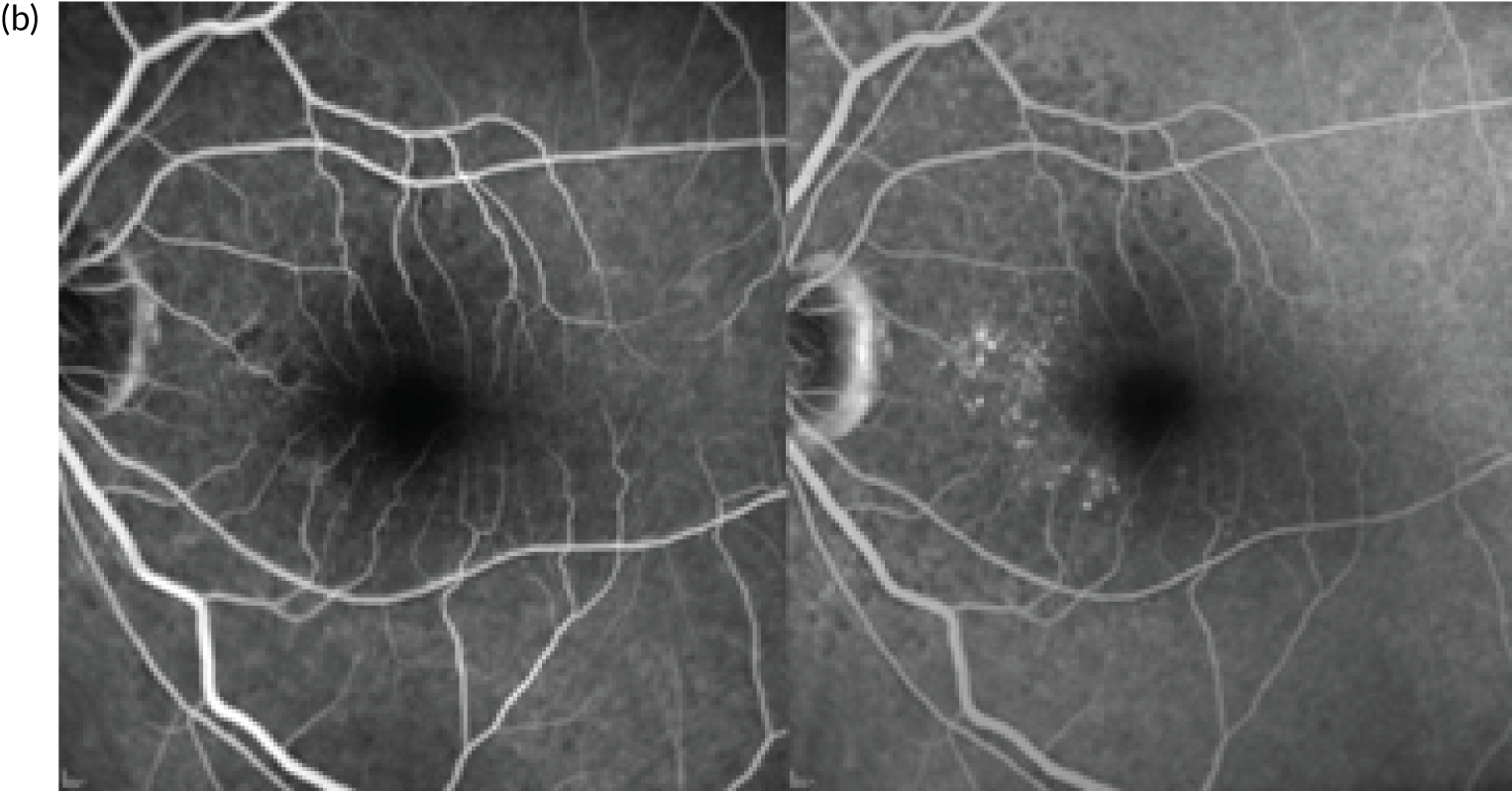
Comparison of visual acuity outcomes in study eyes and fellow eyes
In the overall group of participants, the mean visual acuity in fellow eyes at enrolment was 56.6 [standard deviation (SD) 15.5] letters. In the subset of 119 participants, the mean visual acuity in fellow eyes at enrolment was 54 letters and improved to 58 letters when conversion to nAMD was detected in the corresponding EDNA study eyes (Table 30). In the 119 eyes, the EDNA study eye mean visual acuity was 78 letters at enrolment and fell by four letters at nAMD detection.
| Visual acuity | Time point | |||
|---|---|---|---|---|
| Baseline | Conversion to nAMD | |||
| Mean (SD), n | Median (P25–P75) | Mean (SD), n | Median (P25–P75) | |
| Visual acuity (letters): fellow eye | 54.4 (15.5), 119 | 56.0 (45.0–65.0) | 58.4 (20.5), 64 | 64.0 (45.5–75.0) |
| Visual acuity (letters): study eye | 78.5 (5.2), 119 | 79.0 (75.0–83.0) | 74.3 (8.3), 64 | 75.5 (69.0–80.0) |
Table 31 shows the visual acuity distribution by nAMD status at study exit. Participants who converted to nAMD had lower mean visual acuity than those who did not convert to nAMD (73.1 vs. 77.5 letters, respectively); however, there was a lot of variability in both groups.
| Visual acuity in categories (number of letters) | Converted to nAMD (N = 120), n (%) | Did not convert to nAMD (N = 335), n (%) |
|---|---|---|
| > 93 | 0 | 3 (0.9) |
| 89–93 | 3 (2.5) | 11 (3.3) |
| 84–88 | 10 (8.3) | 76 (22.7) |
| 79–83 | 25 (20.8) | 90 (26.9) |
| 74–78 | 30 (25.0) | 74 (22.1) |
| 69–73 | 18 (15.0) | 38 (11.3) |
| 64–68 | 17 (14.2) | 24 (7.2) |
| 59–63 | 8 (6.7) | 7 (2.1) |
| 54–58 | 3 (2.5) | 4 (1.2) |
| 49–53 | 5 (4.2) | 3 (0.9) |
| 44–48 | 0 | 2 (0.6) |
| ≤ 43 | 1 (0.8) | 3 (0.9) |
Figure 19 shows the mean difference in visual acuity distribution by nAMD status at study exit. More participants who did not convert to nAMD had no change in visual acuity than those who did convert to nAMD. However, there was a lot of variability in both groups.
FIGURE 19.
Distribution of change in visual acuity (number of letters) from baseline at study exit by conversion to nAMD (primary reference standard). (a) Converted to nAMD; (b) did not convert to nAMD.
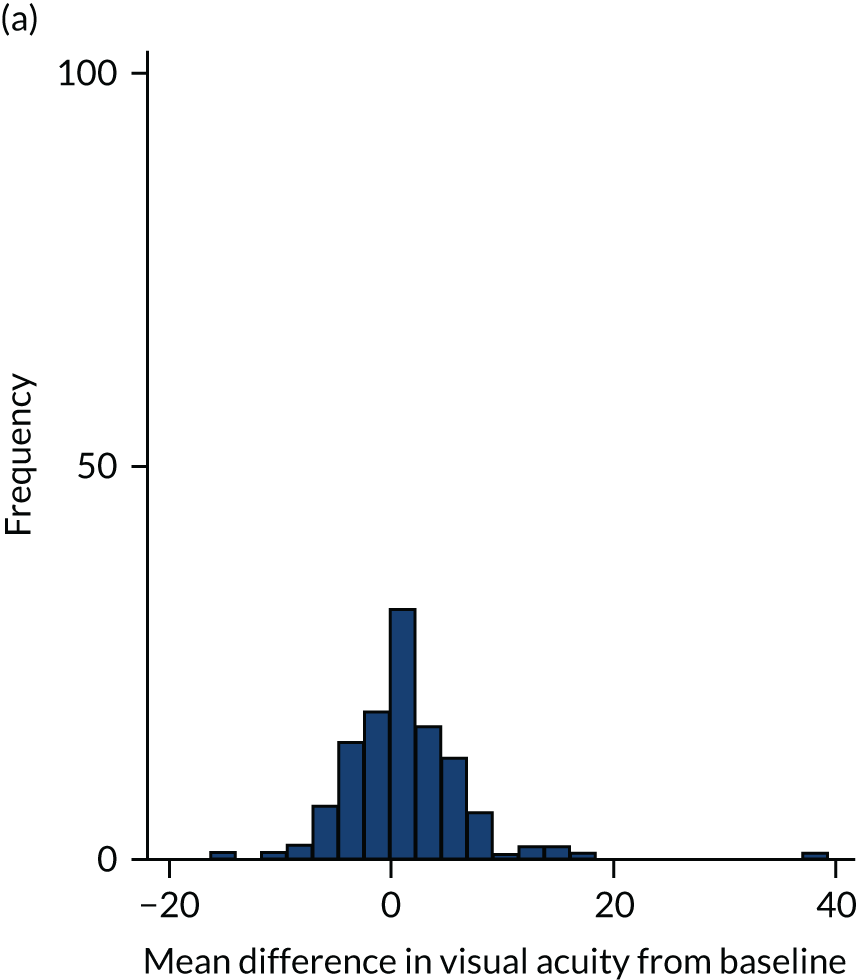
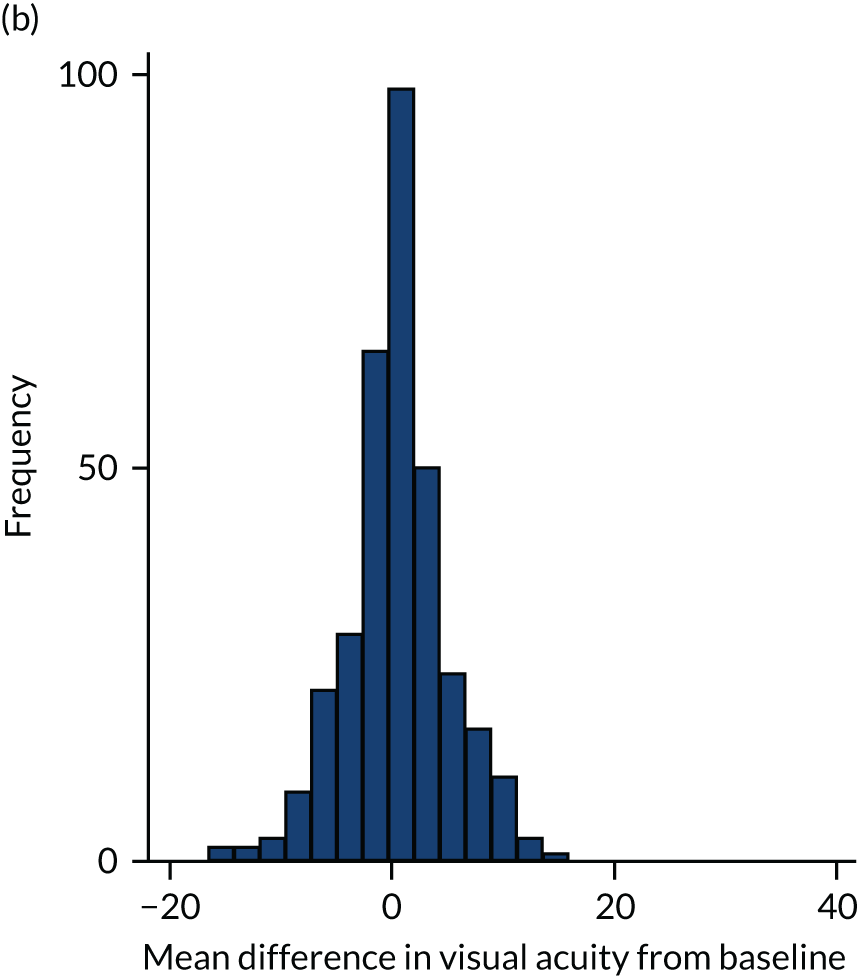
Discussion
The comparison of findings in the fellow eye at enrolment with those seen in the EDNA study eye at conversion to nAMD highlights important differences in visual acuity, lesion size, composition and location that occur with early detection compared with late presentation of nAMD.
At conversion to nAMD in EDNA study eyes, lesions were more likely to be extrafoveal, half the size or smaller than lesions that were observed at initial presentation in the corresponding fellow eyes, and more likely to be larger if type 1. Fibrosis was rare when the nAMD lesion was detected, but atrophy of the outer retina was common. These characteristics are in accordance with the better function in study eyes at detection of conversion to nAMD than that of fellow eyes at enrolment. Notably, the mean visual acuity in the study eye of 119 participants at detection of conversion to nAMD was 78 letters, compared with 54 letters in corresponding fellow eyes: a difference of approximately four ETDRS lines.
Given that 15 letters (three ETDRS lines) equates to a halving or doubling of the visual angle on the ETDRS chart, the fall represents a considerable deficit of visual acuity caused by the delayed detection of nAMD in first-presenting eyes.
The concordance between clinical decisions and reading centre determination was high, with over 91% agreement. This finding is very reassuring because previous studies have shown lower agreement between clinicians and reading centres. 65 Where discrepancies were present, it was usually because of subtle findings on the FFA, such as late frames showing slow leakage, which were more likely to be detected by graders who have more time than clinicians to review the images. Rarely, the discrepancy arose because only a limited number of B-scans were scrutinised in the clinical setting.
In a few cases, clinicians made a diagnosis of conversion to nAMD but the reading centre disagreed. Vitelliform lesions and drusenoid material, which can take up fluorescein stain and present an appearance of leakage, were a feature in the few disagreements. In such cases, clinicians may have been biased by patient’s symptoms of distortion and/or the change in visual acuity. Graders are completely impartial and receive no information, and have to base their decisions purely on the images.
The probability of missing nAMD by not routinely carrying out FFAs or indeed misdiagnosing the onset of nAMD, therefore, appears to be extremely low. It is worth noting, however, that all participants had nAMD in their first-presenting eye and, therefore, the likelihood of neovascularisation arising from nAMD was extremely high. In the routine clinical setting, in which patients may present for the first time with their first affected eye, the chance of misdiagnosis is likely to be higher.
Using only OCT to diagnose the onset of nAMD may increase the risk of treating an eye where a small volume of fluid may spontaneously resolve. It is also known that in nAMD shallow leakage through the RPE can be present and fluctuate over time. The case study example presented in Figure 16 is likely to be such an example because the nAMD lesion in the EDNA study eye was detected at the 18-month visit; however, because the clinician elected not to treat, the patient was retained in the study and presented a similar appearance to the 18-month visit at exit at 36 months.
A final point to make is that in cases of discrepancy we have assumed that FFA is perfect and, if discrepant, we are penalising OCT. Beyond the EDNA study this has huge importance. We recommend that efforts are needed to follow-up patients with discrepancies between OCT and FFA when visual acuity is normal and when patients report no symptoms without treatment.
Chapter 6 Prognostic modelling of conversion to neovascular age-related macular degeneration in the study eye
This chapter reports the findings from the risk prediction model on the development of nAMD in the EDNA study eye using predefined baseline risk factors. The methods are described separately (see Chapter 2).
Candidate predictors
Our candidate predictors for conversion to nAMD in the EDNA study eye were selected through clinical consensus in line with methodological guidelines. 66 Candidate predictors were collected at the enrolment of participants when nAMD had first been identified in the fellow eye. Summary statistics for the candidate predictors are shown in Table 32. There were 145 conversions to nAMD (defined by secondary reference standard 1) among the 489 participants in the EDNA study for whom data on outcome were available (note that the participant and conversion numbers will change for each model depending on the number of missing data in the model). The median duration of follow-up was 34 months. Participants included in the model were, on average, aged 77 years; the majority were women (57%) with hypertension (53%) and most had no family history of AMD (85%). A history of cardiovascular disease was reported in 23% of participants and diabetes in 16% of participants. Twelve per cent of participants were current smokers. Twenty per cent of participants were pseudophakic and 53% had cataracts in the EDNA study eye.
| Characteristic | Total (N = 489) |
|---|---|
| Baseline participant characteristics | |
| Age (years), mean (SD); n | 77.0 (7.5); 489 |
| BMI (kg/m2), mean (SD); n | 27.7 (5.4); 379 |
| Sex, n (%) | |
| Male | 209 (42.7) |
| Female | 280 (57.3) |
| Hypertension, n (%) | |
| Yes | 260 (53.2) |
| No | 228 (46.6) |
| Missing | 1 (0.2) |
| Cardiovascular disease, n (%) | |
| Yes | 110 (22.5) |
| No | 378 (77.3) |
| Missing | 1 (0.2) |
| Family history of AMD, n (%) | |
| Yes | 75 (15.3) |
| No | 413 (84.5) |
| Missing | 1 (0.2) |
| Diabetes, n (%) | |
| Yes | 79 (16.2) |
| No | 409 (83.6) |
| Missing | 1 (0.2) |
| Nutritional supplements, n (%) | |
| Yes | 147 (30.1) |
| No | 341 (69.7) |
| Missing | 1 (0.2) |
| Smoking history, n (%) | |
| Current | 60 (12.3) |
| Ex-smoker | 236 (48.3) |
| Never smoked | 192 (39.3) |
| Missing | 1 (0.2) |
| Baseline fellow eye characteristics | |
| Total lesion area of nAMD at detection (mm2), mean (SD); n | 7.7 (7.8); 477 |
| CNV subtype, n (%) | |
| RAP | 53 (10.8) |
| CNV | 406 (83.0) |
| Missing | 30 (6.1) |
| Baseline study eye characteristics | |
| Cataract present? n (%) | |
| Yes | 260 (53.2) |
| No | 129 (26.4) |
| Pseudophakic | 98 (20.0) |
| Missing | 2 (0.4) |
| Visual acuity, mean (SD); n | 79.1 (5.3); 489 |
| Drusen type, n (%) | |
| None | 45 (9.2) |
| Nodular | 288 (58.9) |
| Reticular | 14 (2.9) |
| Both | 130 (26.6) |
| Missing | 12 (2.5) |
| Maximum size of drusen (µm), n (%) | |
| < 63 | 99 (20.2) |
| 63–125 µm | 157 (32.1) |
| > 125 µm | 227 (46.4) |
| Missing | 6 (1.2) |
| Most frequent size of drusen (µm), n (%) | |
| < 63 | 218 (44.6) |
| 63–125 | 200 (40.9) |
| > 125 | 65 (13.3) |
| Missing | 6 (1.2) |
| Presence of pigmentary abnormalities in the fundus, n (%) | |
| Yes | 199 (40.7) |
| No | 289 (59.1) |
| Missing | 1 (0.2) |
| ELM disruption, n (%) | |
| Yes | 126 (25.8) |
| No | 337 (68.9) |
| Missing | 26 (5.3) |
| EZ disruption, n (%) | |
| Yes | 161 (32.9) |
| No | 304 (62.2) |
| Missing | 24 (4.9) |
| Retinal thinning, n (%) | |
| Yes | 64 (13.1) |
| No | 413 (84.5) |
| Missing | 12 (2.5) |
| Choroid thinning, n (%) | |
| Normal | 268 (54.8) |
| Thick | 5 (1.0) |
| Thin | 204 (41.7) |
| Missing | 12 (2.5) |
| Follow-up characteristics | |
| Time until event or study exit (months), mean (SD); n | 28.1 (10.9); 489 |
| Follow-up duration (months), median (P25–P75); n | 33.6 (20.2–36.4); 489 |
| Developed nAMD in study eye during studya | |
| Yes | 145 (29.7) |
| No | 344 (0.3) |
The ocular characteristics of the study eye were based on multimodal image grading using colour and OCT to characterise the macular drusen, pigmentary irregularities and outer retinal layer intactness. The most common type of drusen was nodular (59%). The most frequent drusen (45%) were < 63 µm in size; however, the majority of eyes (54%) had drusen > 63 μm in size. In a few cases (< 1%), data on drusen size were missing. Around 40% of participants had retinal pigmentary irregularities in their study eye. The ELM and EZ were not intact in 26% and 33% of eyes at baseline, respectively. Retinal thinning was present in one-fifth of study eyes. Choroidal thinning was graded as present in 40% of study eyes. The nAMD subtype in the first-presenting eye (fellow eye) was graded on fluorescein angiography. The majority had CNV: type 1, type 2 or mixed. Just under one-fifth were classified as RAP.
A large percentage of BMI data were missing (23%). For all other variables, < 10% of data were missing, and for most predictors ≤ 2% of the observations were missing. A table with correlations between candidate predictors is available in Appendix 3.
Univariate analysis
Table 33 presents hazard ratios with 95% CIs from a univariate analysis using a Cox proportional hazards model, that is the estimates are not adjusted for all other candidate predictors. In the univariate models, the following characteristics were significantly associated (p < 0.10) with a higher risk of developing nAMD without adjusting for other candidate predictors: smokers (vs. non-smoker); family history of AMD; lower visual acuity (study eye); thin choroid in study eye (vs. normal); retinal thinning (study eye); presence of pigmentary abnormalities (study eye); most frequent or maximum size of drusen (study eye) of 63–125 μm or > 125 μm (compared with < 63 μm); and nodular, or both nodular and reticular, drusen (compared with no drusen) in the study eye.
| Candidate predictor | Hazard ratio (95% CI); p-value |
|---|---|
| Age (years) | 1.01 (0.99 to 1.03); 0.30 |
| BMI (kg/m2) | 1.01 (0.98 to 1.04); 0.54 |
| Male vs. female | 1.00 (0.72 to 1.39); 0.99 |
| Hypertensive vs. not hypertensive | 0.97 (0.70 to 1.34); 0.85 |
| With cardiovascular disease vs. without | 0.73 (0.48 to 1.11); 0.14 |
| Family history of AMD vs. no family history | 1.43 (0.95 to 2.15); 0.09 |
| Diabetic vs. non-diabetic | 1.02 (0.66 to 1.60); 0.92 |
| Taking nutritional supplements vs. not taking nutritional supplements | 0.85 (0.59 to 1.23); 0.40 |
| Smoking status | |
| Smoker vs. non-smoker | 1.58 (0.97 to 2.57); 0.07 |
| Former smoker vs. non-smoker | 1.03 (0.72 to 1.47); 0.87 |
| Total lesion area of nAMD (mm2) at detection (fellow eye) | 1.01 (0.99 to 1.03); 0.43 |
| RAP vs. CNV (fellow eye) | 1.17 (0.71 to 1.95); 0.53 |
| Cataract present (study eye) | |
| Cataract vs. pseudophakic | 1.35 (0.84 to 2.17); 0.21 |
| No cataract vs. pseudophakic | 1.61 (0.96 to 2.68); 0.07 |
| Visual acuity (study eye) | 0.96 (0.93 to 0.99); < 0.001 |
| Drusen type (study eye) | |
| Nodular vs. none | 7.66 (1.88 to 31.14); < 0.001 |
| Reticular vs. none | 1.71 (0.15 to 18.81); 0.66 |
| Both nodular and reticular vs. none | 13.37 (3.26 to 54.81); < 0.001 |
| Most frequent drusen size (µm) (study eye) | |
| 63–125 vs. < 63 | 2.20 (1.25 to 3.85); 0.01 |
| > 125 vs. < 63 | 2.34 (1.37 to 4.01); < 0.001 |
| Maximum drusen size (µm) (study eye) | |
| 63–125 vs. < 63 | 1.84 (1.28 to 2.66); < 0.001 |
| > 125 vs. < 63 | 1.92 (1.18 to 3.12); 0.01 |
| Presence of pigmentary abnormalities vs. absence (study eye) | 1.70 (1.23 to 2.36); < 0.001 |
| ELM disruption vs. no disruption (study eye) | 1.64 (1.16 to 2.33); 0.01 |
| EZ disruption vs. no disruption (study eye) | 1.63 (1.16 to 2.28); < 0.001 |
| Retinal thinning vs. not (study eye) | 1.65 (1.08 to 2.52); 0.02 |
| Choroid thinning (study eye) | |
| Thin vs. normal | 1.44 (1.04 to 2.01); 0.03 |
| Thick vs. normal | 1.60 (0.39 to 6.53); 0.51 |
Multiple Cox regression analysis
Table 34 shows the hazard ratios for each candidate predictor included in a full model. All candidate predictors except those with a high proportion (> 20%) of missing data were included in the full model. The full model presented below includes 428 participants and 134 events. Being a smoker (vs. a non-smoker), having family history of nAMD and having nodular drusen or both nodular and reticular drusen (vs. none) in the study eye were significantly associated with having higher risk of developing nAMD.
| Candidate predictor | Hazard ratio (95% CI); p-value |
|---|---|
| Age (years) | 1.01 (0.98 to 1.04); 0.39 |
| Male vs. female | 1.06 (0.74 to 1.53); 0.75 |
| Hypertensive vs. not hypertensive | 1.00 (0.70 to 1.45); 0.98 |
| With cardiovascular disease vs. without cardiovascular disease | 0.75 (0.46 to 1.21); 0.23 |
| Family history of AMD vs. no family history | 1.43 (0.91 to 2.25); 0.12 |
| Diabetic vs. non-diabetic | 0.97 (0.59 to 1.60); 0.91 |
| Taking nutritional supplements vs. not taking nutritional supplements | 0.74 (0.49 to 1.11); 0.15 |
| Smoking status | |
| Smoker vs. non-smoker | 1.83 (1.05 to 3.20); 0.03 |
| Former smoker vs. non-smoker | 0.95 (0.65 to 1.41); 0.82 |
| Total lesion area of nAMD (mm2) at detection (fellow eye) | 1.00 (0.98 to 1.02); 0.98 |
| RAP vs. CNV | 1.11 (0.62 to 2.00); 0.73 |
| Cataract present (study eye) | |
| Cataract vs. pseudophakic | 1.42 (0.83 to 2.41); 0.20 |
| No cataract vs. pseudophakic | 1.81 (1.01 to 3.25); 0.05 |
| Visual acuity (study eye) | 0.97 (0.94 to 1.01); 0.14 |
| Type of drusen (study eye) | |
| Nodular vs. none | 5.17 (1.14 to 23.38); 0.03 |
| Reticular vs. none | 1.51 (0.13 to 18.30); 0.74 |
| Both reticular and nodular vs. none | 9.48 (2.02 to 44.46); < 0.001 |
| Most frequent drusen size (µm) (study eye) | |
| 63–125 vs. < 63 | 0.90 (0.45 to 1.82); 0.77 |
| > 125 vs. < 63 | 0.75 (0.35 to 1.59); 0.45 |
| Maximum drusen size (µm) (study eye) | |
| 63–125 vs. < 63 | 1.55 (0.97 to 2.49); 0.07 |
| > 125 vs. < 63 | 1.71 (0.90 to 3.26); 0.10 |
| Presence of pigmentary abnormalities vs. absence (study eye) | 1.28 (0.87 to 1.87); 0.21 |
| ELM disruption vs. no disruption (study eye) | 0.99 (0.52 to 1.89); 0.99 |
| EZ disruption vs. no disruption (study eye) | 1.02 (0.57 to 1.83); 0.94 |
| Retinal thinning vs. not (study eye) | 1.41 (0.79 to 2.53); 0.24 |
| Choroid thinning (study eye) | |
| Thin vs. normal | 1.18 (0.81 to 1.72); 0.40 |
| Thick vs. normal | 2.77 (0.62 to 12.28); 0.18 |
Selection of predictors and model estimation
Owing to the large number of candidate predictors, we used a backwards elimination process to obtain a final model. Fractional polynomials were used to explore the presence of non-linear relationships of the continuous predictors (age, baseline visual acuity in the study eye and total lesion area of nAMD at detection in the fellow eye) and we found no evidence of a non-linear relationship; therefore, we include these candidate predictors as measured.
A backward elimination algorithm was applied to the multiple Cox regression model, including all candidate predictors with the threshold set at 0.10. Hazard ratios for the predictor cataract present (study eye) were observed to be counterintuitive from a clinical perspective, and we decided to exclude this candidate predictor from the selection process.
Following the elimination process, the four risk factors for progression to nAMD in the final multiple prediction model were smoking, family history of nAMD, presence of pigmentary irregularities in the study eye and presence of nodular drusen in the study eye (Table 35). The presence of both nodular and reticular drusen carried the highest risk. The final model included 476 participants and 143 events (conversion to nAMD in the EDNA study eye).
| Candidate predictor | Hazard ratio (95% CI); p-value |
|---|---|
| Smoking status | |
| Smoker vs. non-smoker | 1.70 (1.04 to 2.79); 0.03 |
| Former smoker vs. non-smoker | 0.99 (0.69 to 1.41); 0.94 |
| Type of drusen (study eye) | |
| Nodular vs. none | 6.45 (1.57 to 26.45); 0.01 |
| Reticular vs. none | 1.62 (0.15 to 17.96); 0.70 |
| Both nodular and reticular vs. none | 11.81 (2.86 to 48.87); < 0.001 |
| Presence of pigmentary abnormalities vs. absence (study eye) | 1.47 (1.05 to 2.05); 0.02 |
| Family history of AMD vs. no family history | 1.24 (0.82 to 1.89); 0.31 |
Discrimination
Discrimination measures how well the model distinguishes between individuals who developed nAMD and individuals who did not develop nAMD. For the final model shown in Table 35, the c-statistic was 0.66 (95% CI 0.62 to 0.71), that is the model would predict a high risk for subjects that converted 66% of the time. This represents a modest level of discrimination.
Calibration
The calibration coefficient is 1 (95% CI 0.68 to 1.3), which suggests that the prediction model is well calibrated as anticipated. Calibration is shown visually (Figure 20) against monitoring time. Four risk groups were calculated (divided by the 16th, 50th and 84th centile of linear prediction risk). 45 Group 1 had the lowest risk and group 4 had the highest risk. Plotting Kaplan–Meier-observed survival by group, it is possible to see if the model can distinguish between observed and expected probability of remaining nAMD free. A perfectly calibrated model would result in separate Kaplan–Meier curves for each risk group. As can be seen in Figure 20, there is a separation of group 1 (lower risk) from group 2 and from the remaining groups.
FIGURE 20.
Observed nAMD probabilities over time (in months) by risk group (group 1: lowest risk; group 4: highest risk) according to model’s prediction (expected probability).
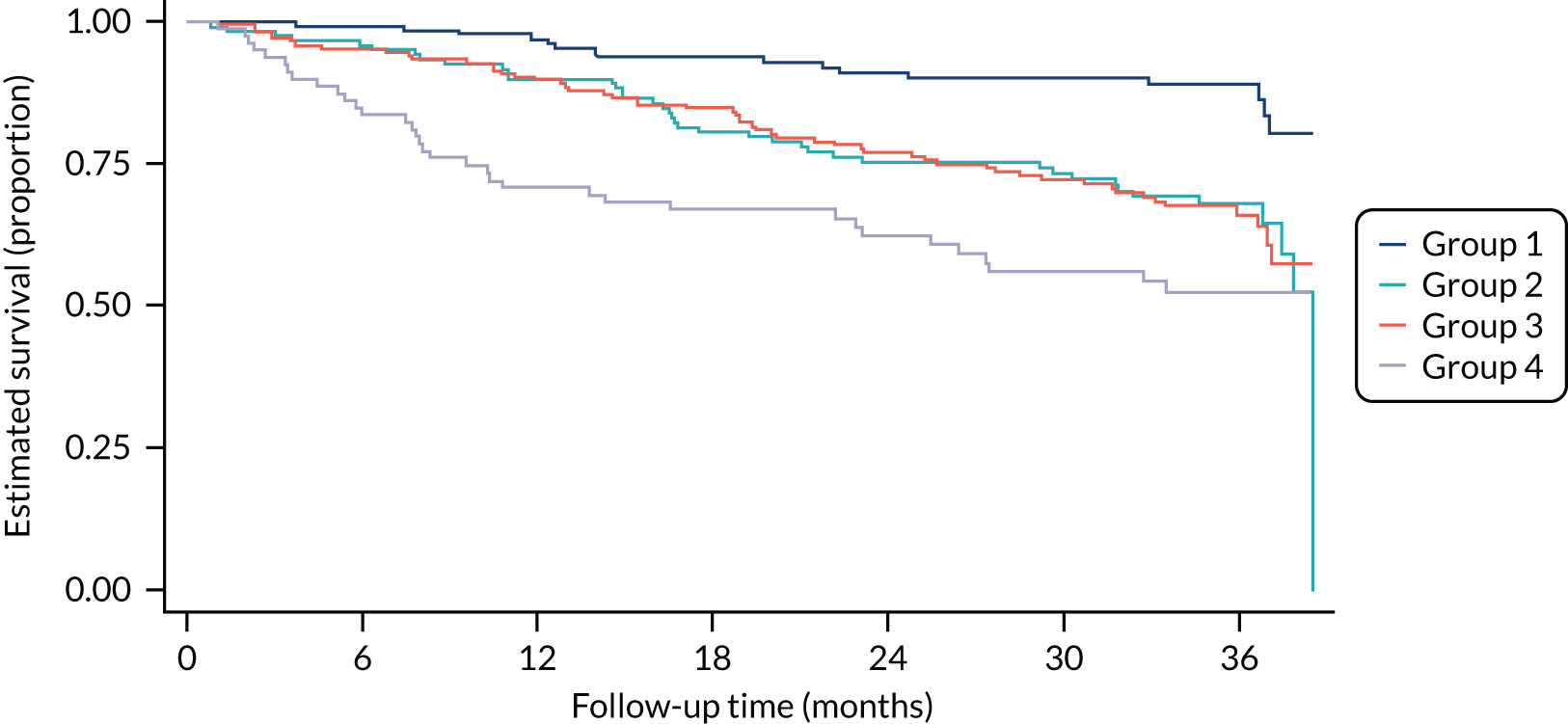
Sensitivity analysis
In developing the model, we performed the following sensitivity analyses.
Impact of including or excluding specific candidate predictors in the c-statistic
Type of drusen
Owing to the strong association that was found between the type of drusen and the outcome, we assessed its discriminative ability by itself. When the type of drusen is included in the model as a single predictor, the resulting c-statistic is 0.63.
Presence of cataract
The presence of cataract was initially included as a candidate predictor; however, owing to hazard ratios that were clinically counter-intuitive and had a negligible effect on the c-statistic (from 0.66 to 0.68), we agreed to exclude the variable from the selection process for the final model and the additional sensitivity analyses.
Varying the selection threshold for variable inclusion
We explored the impact of varying the selection threshold that was used to decide the variables to include in the model from p = 0.05 to p = 0.20 (Table 36). This resulted in similar included variables when compared with the main analysis using a selection threshold of p = 0.10 (see Table 35). The most stringent threshold (p = 0.05) led to the inclusion of only two variables as predictors: type of drusen (study eye) and smoker. The least stringent threshold (p = 0.20) selected the main model variables with two additional predictors: study eye visual acuity and retinal thinning.
| Candidate predictor | Selection threshold, hazard ratio (95% CI); p-value | ||
|---|---|---|---|
| p = 0.05 | p = 0.15 | p = 0.20 | |
| Family history of AMD vs. no family history | – | 1.31 (0.86 to 2.00); 0.21 | 1.32 (0.86 to 2.01); 0.20 |
| Smoking status | |||
| Smoker vs. non-smoker | 1.68 (1.03 to 2.74); 0.04 | 1.69 (1.03 to 2.76); 0.04 | 1.72 (1.05 to 2.81); 0.03 |
| Former smoker vs. non-smoker | 0.98 (0.68 to 1.41); 0.92 | 0.99 (0.69 to 1.42); 0.96 | 0.99 (0.69 to 1.41); 0.94 |
| Visual acuity (study eye) | – | 0.97 (0.94 to 1.00); 0.05 | 0.97 (0.94 to 1.00); 0.06 |
| Type of drusen (study eye) | |||
| Nodular vs. none | 7.62 (1.87 to 30.99); < 0.001 | 6.08 (1.48 to 24.94); 0.01 | 6.02 (1.47 to 24.69); 0.01 |
| Reticular vs. none | 1.87 (0.17 to 20.72); 0.61 | 1.59 (0.14 to 17.64); 0.71 | 1.63 (0.15 to 18.15); 0.69 |
| Both vs. none | 14.03 (3.42 to 57.59); < 0.001 | 10.80 (2.60 to 44.76); < 0.001 | 10.64 (2.57 to 44.12); < 0.001 |
| Presence of pigmentary abnormalities vs. absence (study eye) | – | 1.44 (1.03 to 2.01); 0.03 | 1.40 (1.00 to 1.96); 0.05 |
| Retinal thinning vs. absence (study eye) | – | – | 1.29 (0.83 to 2.01); 0.26 |
Multiple imputation
Variables with multiple categories were combined into binary variables to overcome the software conversion problems in running the multiple imputation model. We then applied a backwards selection process with a threshold of 0.10 to select the predictors following the same process as in the main analysis. The resulting model variables after imputation are shown in Table 37. The three predictors that were selected for the model after imputation differ from the four predictors that were selected for the model without multiple imputation. Specifically, most frequent size of drusen and study eye visual acuity are newly included in the model after multiple imputation, and type of drusen in the study eye, smoking status and family history of nAMD are all excluded from the model after multiple imputation.
| Candidate predictor | Hazard ratio (95% CI); p-value |
|---|---|
| Most frequent drusen size (µm) (study eye) | |
| 63–125 vs. < 63 | 1.44 (0.87 to 2.38); 0.16 |
| > 125 vs. < 63 | 1.54 (1.06 to 2.25); 0.02 |
| Both types of drusen vs. none or single type of drusen | 1.84 (1.30 to 2.60); < 0.001 |
| Presence of pigmentary abnormalities vs. absence (study eye) | 1.52 (1.08 to 2.12); 0.02 |
| Visual acuity (study eye) | 0.96 (0.93 to 1.00); 0.02 |
Discussion
The strongest ocular risk factor for conversion to nAMD in the second eye is the presence of nAMD in the contralateral eye. 67 However, the risk of conversion to nAMD in the second eye in individuals with unilateral nAMD varies between individuals, with about 10% of individuals developing nAMD in the first year and 50% by 5 years from developing nAMD in the first eye. 68 The EDNA study enabled us to develop a predictive model for developing nAMD in the unaffected eye (the EDNA study eye). We had access to a rich data set with a large number of potential predictors through the baseline data collection and reading centre grading. A total of 145 study eyes developed nAMD over 3 years out of 489 participants who had unilateral nAMD at baseline. This was a reasonable number of events to develop a prediction model. This was not a large data set in terms of prognostic modelling, but it was large for the clinical area. Other strengths included the use of candidate predictors that are easily obtainable in routine ophthalmic practice; that misclassification of ocular predictors was unlikely because the retinal images were graded by investigators at the study site, as well as by graders in the reading centre; and that the candidate predictors were selected through clinical consensus in line with methodological guidelines. 66 Smoking status, family history of nAMD, presence of nodular drusen with or without RPD in the study eye and presence of pigmentary abnormalities in the study eye were the four predictor variables in the final complete case model. Although these predictors are in accordance with previous studies, age was not included. Age has been an inconsistent factor in the multiple prediction models of nAMD in other cohorts. For example, both of the predictive models that were developed for conversion of second eyes to nAMD of participants in the Harbor study did not include age in the final model. 69,70 By contrast, a recent secondary analysis of second eye conversion in the VIEW1 and 2 studies showed that increasing age, female sex and lesion size in the nAMD eye were among the predictors of conversion to nAMD. 71
When we consider non-ocular risk factors, only smoking status and family history of nAMD were included in the model. Seddon et al. 72 described a combination of demographic, genetic and environmental factors, along with macular phenotype based on colour photographs, to develop and validate a model to predict progressors versus non-progressors in the AREDS cohort. In the study by Seddon et al. ,72 the definition of progression included persons with bilateral early or intermediate AMD who progressed to advanced AMD in any eye along with those who already had advanced AMD in one eye who subsequently progressed to advanced AMD in the unaffected eye. Our study design could be based only on progression to advanced AMD in the EDNA study eye because of the existence of nAMD in the first eye, which by itself is a strong risk factor. A different prediction algorithm that has been used in the past was based solely on ocular characteristics. This algorithm is the simplified AREDS scale, which used only the macular phenotype to predict progression of AMD. A graded categorical scale was used with both eyes, contributing 1 or 2 points depending on whether intermediate AMD (1 point for drusen and 1 point for pigmentary abnormalities) or nAMD was present (2 points). The scale could, therefore, extend from 0 (no features of AMD) to 4. However, a score of 4 could mean that both eyes exhibited intermediate AMD or one eye exhibited late AMD with the fellow eye having both drusen and pigmentary changes. 73 This scale, therefore, exhibited some anomalous relationships because individuals with bilateral drusen only without pigmentary abnormalities had a 5-year risk as low as 2% or with pigmentary abnormalities as high as 20%. By contrast, the presence of nAMD in one eye with intermediate AMD and pigmentary abnormalities increased the risk to 53%. Therefore, it may be that the presence of nAMD in one eye carries a much higher risk than that of bilateral intermediate AMD for conversion to nAMD. 73
The only study to find a reduction in progression from earlier stages of AMD to nAMD was the US-conducted AREDS. 68 This study enrolled over 3640 participants, and in the subset of participants with large drusen in both eyes or nAMD in one eye (approximately n = 2556) a statistically significant reduction in the rate of progression was detected at 5 years (28% progressed in the treated group vs. 20% in the supplemented group, representing a benefit of 8% in the group who received a cocktail of antioxidant vitamins and zinc). The EDNA study, although similar insofar as its prospective follow-up and ocular characteristics of AMD (our sample too had nAMD in one eye with the study eye observed until nAMD developed), was in all other respects not comparable to AREDS. In the EDNA study, we observed our participants for an average of just under 3 years, with a sample that was less than one-quarter that of the high-risk AREDS population. Although supplement use was recorded, the identity of the supplement and consistency of its use during follow-up was not tracked. Therefore, it is not surprising that nutritional supplements did not contribute to the predictive model.
Because family history of AMD was found to be a significant predictor in our data set, we suggest that this is an easier option to use in situations where DNA analysis is not possible. In the present study, although a biobank with whole blood has been established from participants who were willing to donate a blood sample, we have not yet obtained the resources to undertake appropriate genetic analysis. Even if genetic profiling is carried out, the multifactorial aetiology and gene–environment interactions that characterise AMD makes it a challenge to model accurately. 74
Ocular predictors in our model included nodular drusen with or without RPD. This was found to be the most important predictor in our model and, by itself, yielded a c-statistic of 0.63 (compared with 0.66 when including all selected predictors). The presence of nodular drusen in fellow eyes increased the risk of conversion to nAMD by six-fold compared with the absence of drusen, while the combination of nodular drusen and RPD almost doubled this risk, highlighting the strongest predictor for conversion.
Various types of drusen parameters have been assessed as risk factors for conversion and these include the number of drusen, area, volume and load. 75–77 Using the simplified scale based only on ocular risks, the fellow eye of people with unilateral advanced AMD in the AREDS cohort had a 35.4% 5-year risk of progression to advanced AMD if the fellow eye had additional risk factors of presence of large drusen and/or pigment abnormalities. This figure increased to 76.8% at 10 years. 78 Therefore, both large drusen and RPE abnormalities are considered strong risk factors for conversion to nAMD. 75–79 On OCT, pigmentary abnormalities are seen as hyper-reflective foci. In our data, in the univariate analysis, hyper-reflective foci were highly significant predictors for conversion and this finding is concordant with that of many preceding studies. 70,80 However, hyper-reflective foci were not included in the final multivariable model because these do not occur in the absence of nodular drusen, and it would appear that the prediction ability of the model is unimpaired by exclusion of the presence of pigmentary abnormalities. Decreasing visual acuity was also a predictor in our model, in keeping with previous studies. 78,81
We had a small percentage of missing data for all candidate predictors, except BMI which was excluded from the complete case model. This resulted in a final model that included 477 out of 489 participants and 143 events out of 145 events after the variable selection process. A weakness of our study was that the final model was based on a subset of EDNA participants given that we could use data only from those with information available on the state of the EDNA study eye at exit from the study. We utilised data from as many participants as possible by using secondary reference standard 1 (clinical determination of conversion to nAMD at clinical site), which was available in a larger number of participants. We were justified in using the larger sample because it was based on information that came from clinical practice and is, therefore, more applicable in the routine setting. We recognised that this strategy could have led to selection bias; however, candidate predictors considered in the model showed a similar distribution to the baseline characteristics of the whole sample (see Chapter 3). BMI was not included as a variable in the multiple imputation owing to the large number of missing data. Our sensitivity analyses highlighted some variability in the included variables for the model. When applying a less-stringent threshold for inclusion (p = 0.20), study eye visual acuity, family history of nAMD and retinal thinning in the study eye were additional significant predictors. With the most stringent threshold (p = 0.05), only smoking status and type of drusen in the study eye performed well in the model. The most frequent size of drusen and visual acuity were included only as predictors in the model after conducting multiple imputation, which suggests potential bias on the variable selection of the main model owing to missing data.
Our model had a modest level of discrimination, which was measured through the c-statistic, attributing higher-risk estimates to participants who developed nAMD than those who did not. This discriminative ability might be considered adequate depending on the research or clinical field. 82 However, the level does not seem high enough to inform changes in clinical practice regarding monitoring. In terms of the model performance, discrimination should be assessed in combination with calibration, the agreement between observed outcomes and prediction. Calibration in our model was good, with a calibration slope of one and visual examination showing the model’s ability to distinguish between higher- and lower-risk groups. Calibration is more likely to produce good results in the internal validation of a model given that the predictions are produced based on the sample available. However, calibration and discrimination need to be reassessed by doing an external validation of the model using a new set of patients, preferably in a different setting. 83
There were limitations to this work. The model applies only to second eyes of participants with nAMD in their first eye; therefore, it is a selected population and the findings should be interpreted in that light. In particular, the lack of prognostic value of age is probably because of the effect of focusing on people who already have nAMD in one eye. There may be other unmeasured or uncontrolled parameters that may confound the relationship of the current predictors, so they should not be taken as causal relationships. Other variables not readily collected might have resulted in a better prognostic model. A higher discriminatory performance had been anticipated because we believed that important characteristics in the ocular environment were included. However, we did not have information on the genetic profile of participants because carriage of well-characterised genetic polymorphisms are known to be important in increasing the risk and rapidity of progression. This and additional OCT parameters of the fellow nAMD eyes may merit further investigation as candidate predictors; intraretinal fluid at baseline has been shown to be a predictor of conversion of unaffected eyes to nAMD. The c-statistic (discriminative ability) was only 0.66, so the model cannot be implemented in routine monitoring clinics. We did not measure the model’s inherent optimism or correct its c-statistic for that. Further development of the model should focus on validating the model performance in an external population. The performance does not suggest that this model would have clinical application at this point without further improvement. However, this model adds to the growing literature on risk predictions for conversion to nAMD in the second eye, and the predictors used in this model have been included in most models, but not together.
Chapter 7 Health economics
Introduction
This chapter reports the methods and results of the economic evaluation that was conducted as part of the EDNA study. The EDNA study has provided important data on the rate of conversion to nAMD in the second eye of people being treated for nAMD in the first eye. It has also provided evidence on the diagnostic accuracy of several candidate monitoring tests for the early detection of nAMD in the second eye. However, it was not designed to assess the impact of nAMD detection on subsequent treatment and health outcomes. These longer-term impacts need to be considered when assessing the cost-effectiveness of the different monitoring tests. Therefore, a health economic model was constructed to simulate the natural history of nAMD in the second eye (EDNA study eye) and the subsequent impact of timely detection and treatment on visual acuity outcomes over time.
Objectives of the economic evaluation
The objective of the economic evaluation was to identify an optimal monitoring regime in terms of cost-effectiveness. The analysis assessed the cost-effectiveness of the five alternative index tests (i.e. Amsler, fundus clinical examination, OCT, self-reported vision and visual acuity) when used in routine practice in hospital outpatient eye services to monitor the second eye of people who are attending monitoring visits for treatment of their first eye. The perspective was that of the UK NHS.
Methods
The analysis was conducted in accordance with a predefined health economics analysis plan (see Report Supplementary Material 3). The economic decision model was developed in TreeAge Pro 2020 version R1.1 (TreeAge Software, Inc., Williamstown, MA, USA). The model utilised a Markov structure with a fixed cycle length of 1 month (i.e. the status of simulated patients was updated monthly). Microsimulation was used to analyse the model, whereby individuals were simulated to pass through the model one at a time.
Simulated individuals were defined by a set of baseline characteristics that were drawn from the EDNA study cohort: age, sex, visual acuity in the second eye (EDNA study eye) and type of nAMD (in the first eye). Parametric survival curves were fitted to the time to conversion to nAMD data from the EDNA study and were used to derive monthly probabilities of conversion to nAMD in the second eye. These probabilities were then applied in the economic model.
Following conversion to nAMD and prior to detection and treatment individuals were modelled to lose vision at the rate observed for untreated eyes with nAMD. Following correct identification by a monitoring test, confirmation by FFA and appropriate treatment, individuals were modelled to gain, maintain or lose vision in line with treated cohorts. Based on visual acuity in their second eye, simulated individuals were assigned a health-related quality-of-life weight on a zero (death) to one (full health) scale, allowing cumulative quality-adjusted life-years (QALYs) to be modelled for the alternative monitoring strategies. Health-care and social care costs associated with monitoring, treatment and severe vision loss were incorporated in the model, allowing total cumulative costs to be tracked. The modelled output was used to determine the expected differences in costs and QALYs between the monitoring strategies in an incremental cost-effectiveness analysis.
Model structure
The economic model was structured around disease status (no nAMD or nAMD), diagnosis status (undetected or detected) and treatment status (untreated or treated). A simplified schematic of the structure is provided in Figure 21. The change in visual acuity was modelled at the individual level in each model cycle and there was a simplifying assumption that patients maintain their baseline visual acuity in the EDNA study eye (second eye) until it converts to nAMD. This assumption was considered reasonable by clinical experts within the study team. Visual acuity was updated as a continuous variable in the model, taking a value between 0 and 100 letters (ETDRS). The individual’s current acuity was tracked and linked to a health-related quality-of-life weight using a published equation (see Valuation of health effects). It was assumed that visual acuity in the second eye was representative of visual acuity in the best seeing eye throughout the modelled time horizon. This was justified by the baseline visual acuity in the EDNA study eye being better or within 10 letters of visual acuity in the treated eye in 93% of EDNA study participants. Given that eyes being treated for nAMD still tend to lose vision over the long-term period, it was deemed reasonable to assume that visual acuity in the second eye will remain, on average, higher than visual acuity in the first eye over time. The modelling adopted a 25-year time horizon, with a mean starting age of 77 years (mean age in the EDNA study cohort).
FIGURE 21.
Simplified schematic of the model structure. FN, false negative; FP, false positive; Ref, reference standard; TN, true negative; TP, true positive.
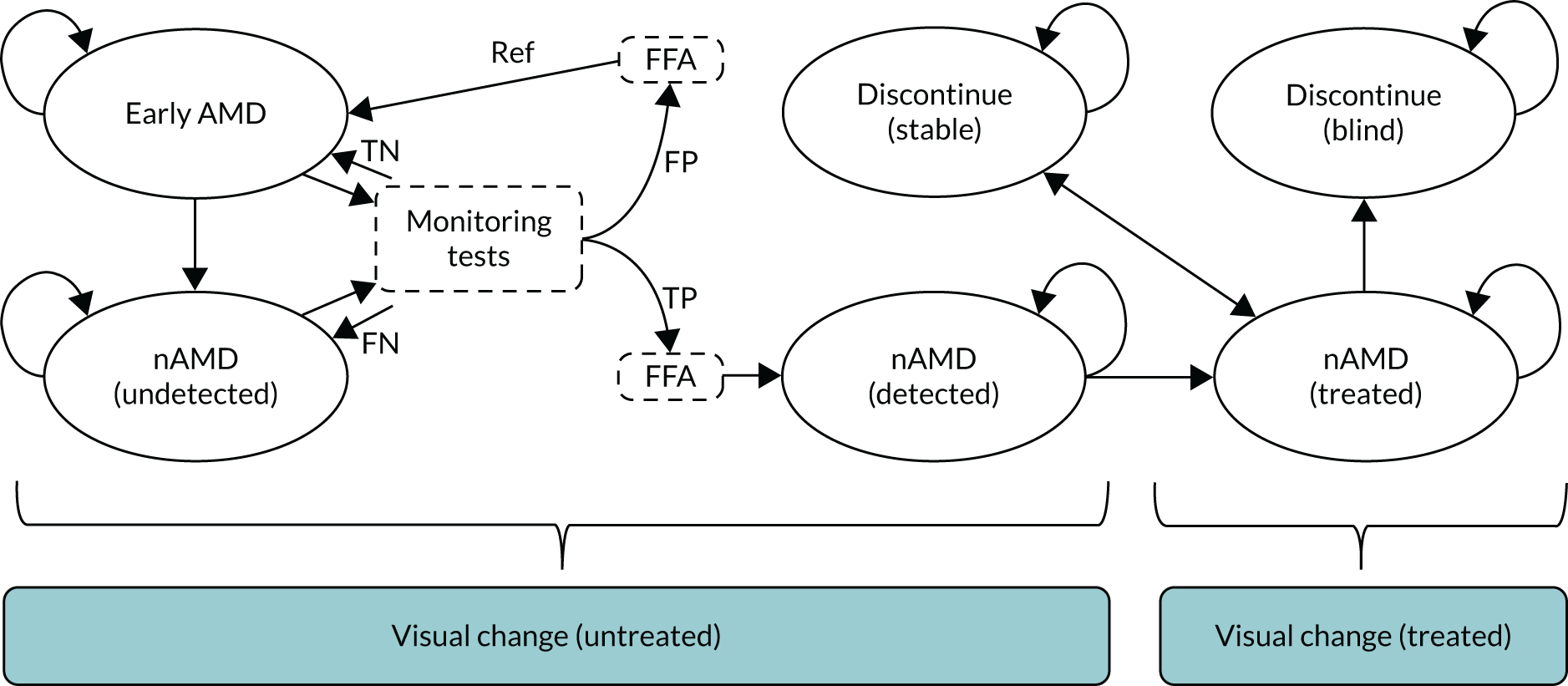
Population
The analysis was conducted for a simulated cohort of individuals with characteristics matching those of the EDNA study cohort. The baseline characteristics of simulated individuals were drawn at random from a table containing the baseline characteristics of each EDNA study participant. This ensured that any correlations between baseline characteristics were maintained in simulated individuals. Given that the EDNA study was embedded in routine NHS practice across 18 study sites, the simulated cohort can be considered representative of the NHS patient population. The average age was 77.4 years, the average baseline visual acuity in the second eye was 79 letters compared with 56.6 letters in the first eye and 57.2% of patients were female.
Comparators
The economic analysis considered each of the EDNA study monitoring tests individually. The base-case analysis followed the EDNA study protocol in assuming that positive tests would be confirmed or refuted by fluorescence angiography.
Clinical effectiveness parameters
Time to conversion to neovascular age-related macular degeneration
Conversion to nAMD in the second eye was based on the observed time to conversion to nAMD data from the EDNA study, using the primary reference standard (FFA determination of conversion to nAMD at the clinical site) to define conversion to nAMD (events). Individuals were censored at the time of their last observed FFA if no conversion to nAMD was observed. Standard parametric curves were fitted to the observed Kaplan–Meier data. The parametric distributions considered were exponential, Weibull and log-normal (Figure 22). Based on Akaike information criterion (AIC) and Bayesian information criterion (BIC), the exponential curve was identified as having the best statistical fit to the observed data (Table 38). This assumed a constant hazard of conversion to nAMD over time. Clinical opinion within the team was split between whether or not the hazard of conversion to nAMD would be expected to increase over time and whether or not the hazard of conversion to nAMD would be expected to decrease over time; therefore, it was agreed that the exponential was appropriate for application in the base case. The Weibull distribution (predicting an increasing hazard of conversion to nAMD over time) and the log-normal distribution (predicting a decreasing hazard) were tested in sensitivity analysis. The estimated rate parameter (λ) of the exponential function was used to derive monthly probabilities of conversion to nAMD in the model (transition probability) using the following equation, where t is time in months and u is the Markov cycle of one month:
The curve was extrapolated beyond the observation period of the EDNA study over the lifetime of simulated individuals.
FIGURE 22.
Kaplan–Meier curve of time to conversion to nAMD overlaid with fitted parametric survival curves.
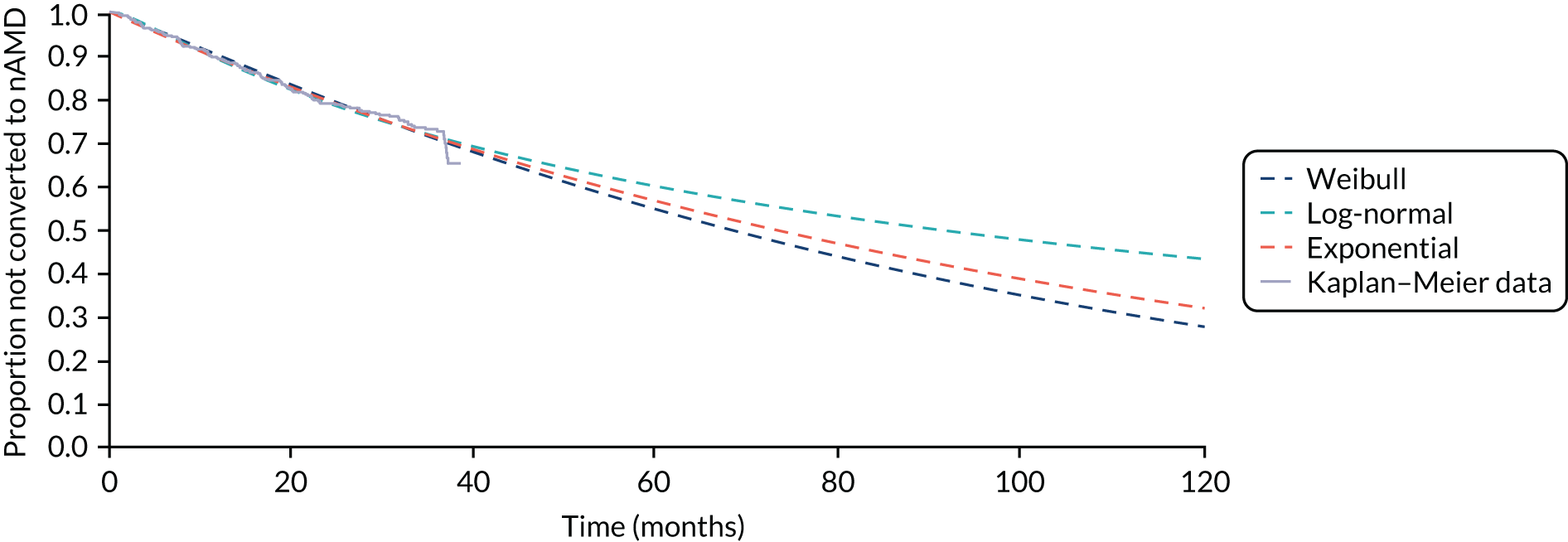
| Distribution | AIC | BIC |
|---|---|---|
| Exponential | 758.21 | 762.33 |
| Weibull | 759.31 | 767.55 |
| Log-normal | 757.80 | 766.05 |
Diagnostic accuracy
The alternative diagnostic monitoring strategies for the second eye were embedded in the economic model using the sensitivity/specificity estimates obtained for the five alternative diagnostic tests. Given that the model seeks to inform the impact of using the alternative tests repeatedly over consecutive monitoring visits, the primary diagnostic accuracy analysis was considered unsuitable for this purpose. Therefore, we used diagnostic accuracy estimates based on the analysis of index test results at the last visit (see Chapter 4, Primary reference standard), reproduced in Table 39. These provide the best available estimates of diagnostic performance at a single point in time, which was necessary for modelling the expected differences in time from conversion to nAMD to diagnosis when using the different tests. However, the repeated application in the model of the estimated specificity for each test (assuming independence within individuals over time) was found to overestimate the observed cumulative proportion of patients experiencing a false positive in the EDNA study. Therefore, we adopted adjusted specificities in the model to yield the observed cumulative proportion over the mean number of observed tests (Table 40). Sensitivity was applied independently in the model. This was considered reasonable given that the potential for correlation in test sensitivity over repeated assessments is countered by progression of the underlying disease, which may result in sensitivity increasing over time.
| Index test | Sensitivity (95% CI) (%) | True positives (n)/participants with nAMD (n) | Specificity (95% CI) (%) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 26.5 (18.8 to 36.1) | 26/98 | 93.7 (90.1 to 96.1) | 253/270 |
| Fundus | 49.6 (40.8 to 58.4) | 59/119 | 99.7 (98.2 to 100.0) | 334/335 |
| OCT | 90.0 (83.2 to 94.3) | 108/120 | 96.4 (93.8 to 98.0) | 323/335 |
| Self-reported vision | 4.2 (1.6 to 9.8) | 5/118 | 98.5 (96.4 to 99.5) | 328/333 |
| Visual acuity | 25.8 (18.8 to 34.4) | 31/120 | 88.4 (84.5 to 91.4) | 296/335 |
| Index test | Unadjusted specificity | Cumulative proportion of people experiencing a false positive during the EDNA studya | Mean number of tests in the EDNA study | Adjusted specificity per testb |
|---|---|---|---|---|
| Amsler | 0.937 | 0.1398 | 13.8 | 0.9891 |
| Fundus clinical evaluation | 0.997 | 0.02 | 14.2 | 0.9986 |
| OCT | 0.964 | 0.0965 | 14.5 | 0.9930 |
| Self-reported vision | 0.985 | 0.0177 | 14 | 0.9987 |
| Visual acuity | 0.884 | 0.182 | 15.5 | 0.9871 |
Given that the cost-effectiveness modelling was based on the expected changes in visual acuity following conversion to nAMD and that visual acuity loss of ≥ 10 letters was one of the index tests, this created a challenge to applying test sensitivity for change in visual acuity in the model; that is, the visual acuity test cannot be positive in true cases for whom no visual loss has yet occurred, and by definition it must be positive in true cases for whom visual loss of ≥ 10 letters has occurred. Therefore, we modelled visual acuity change to have sensitivity of 0% for nAMD cases prior to any vision loss and 100% following vision loss of ≥ 10 letters.
We attempted to assess the correlation between the sensitivity of the other index tests and whether or not participants with observed conversion to nAMD had lost ≥ 10 letters from baseline at the time of detection. This did not indicate a strong relationship, but the number of true positive cases with vision loss of ≥ 10 letters from baseline was small (n = 31). Given that few nAMD cases in the EDNA study eye reached more severe levels of vision loss prior to detection, we were unable to accurately determine how the sensitivity of each of the index tests changes as vision loss increases. We, therefore, made a number of assumptions about what would happen to those losing a significant amount of vision owing to undetected nAMD: (1) all individuals losing > 30 letters were assumed to present immediately to the hospital eye service and be detected; (2) 50% of individuals losing 15–30 letters were assumed to present immediately to the hospital eye service and be detected; (3) the remaining 50% of patients losing between 15 and 30 letters were detected at subsequent monitoring visits based on the test sensitivity of each test (100% when using visual acuity as the index test).
Pre-treatment change in visual acuity
There is a paucity of published data on the rate of vision loss in the early stages of nAMD immediately following conversion to nAMD. It is known that the overall trajectory of vision loss is not linear, with a rapid decline expected when nAMD first supervenes before the slope flattens somewhat. The available natural history data come from an era in which patients presented with nAMD late; therefore, reported rates of visual acuity loss will relate to the flatter part of the curve following the steeper initial decline after conversion to nAMD.
Nevertheless, a meta-analysis of older natural history data still found that > 50% of patients can expect to lose ≥ 3 lines (≥ 15 letters) over a period of 12 months from this later starting point, with 27% expected to lose ≥ 6 lines (≥ 30 letters). 2
To better inform the expected rate of visual acuity decline from the point of conversion to nAMD, we used survival analysis methods to estimate the time to losing ≥ 10 letters from visual acuity before conversion to nAMD, using post conversion to nAMD visual acuity data for the 145 patients who were judged to have developed nAMD in their EDNA study eye. This analysis relied on the fact that some patients had already experienced visual acuity loss by the time of detection, while other patients were not treated immediately. For those not treated immediately, visual acuity data at time of treatment initiation and at 1 year after conversion to nAMD were obtained from the Observing Fibrosis, Macular Atrophy and Sub retinal Highly Reflective Material – Before and After Intervention with Anti-VEGF Treatment (FASBAT) study (ISRCTN 76889861)84 for those EDNA participants who were enrolled or were otherwise extracted from the EDNA participant’s case notes. The time of conversion to nAMD was taken as the mid-point between the visit at which nAMD was detected and the preceding visit (when no nAMD was detected). The time at risk was taken as the time from conversion to nAMD to losing ≥ 10 letters or treatment initiation. If no treatment was initiated, time at risk was censored at the last available follow-up point at which visual acuity data were available. The data were used to generate a Kaplan–Meier curve, to which various parametric survival curves were fitted (Figure 23).
FIGURE 23.
Time to losing ≥ 10 letters from the point of conversion to nAMD, overlaid with the three best fitting parametric distributions.
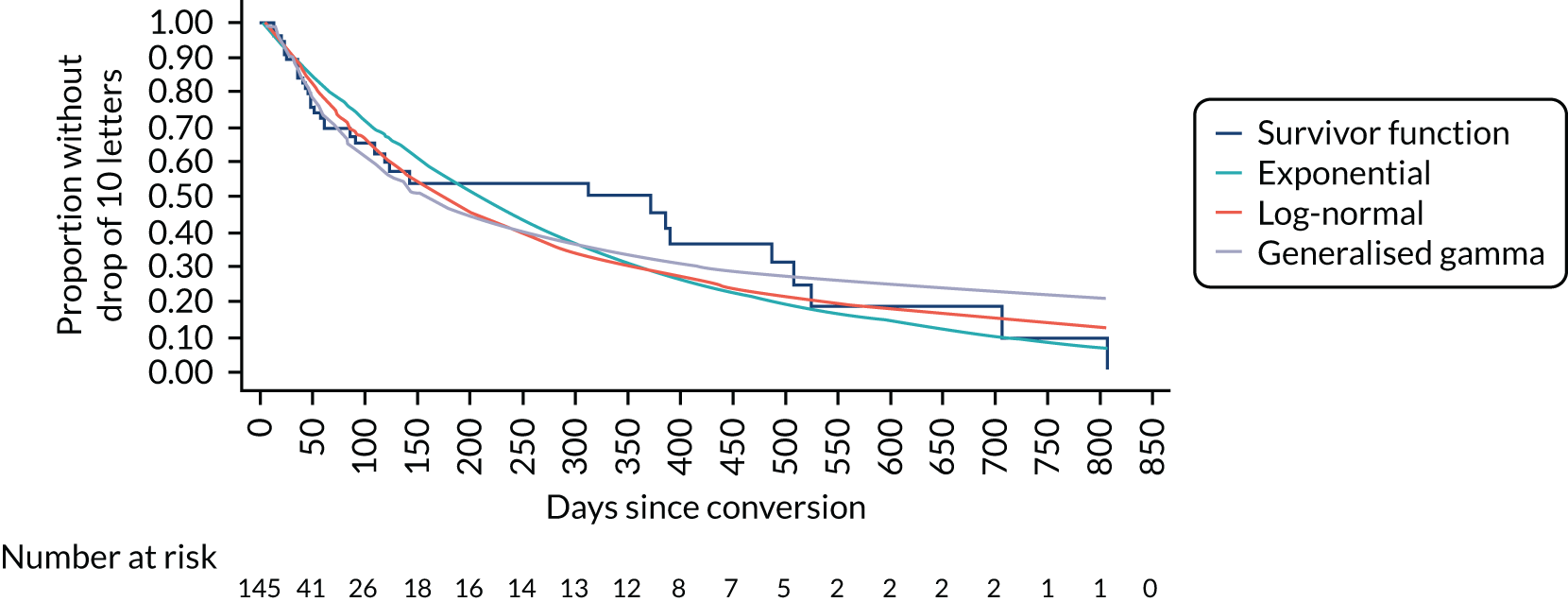
It can be noted that the data are heavily affected by censoring from an early stage owing to the tendency for early-detected patients to be treated prior to losing ≥ 10 letters, which may bias the curves upwards. Furthermore, a lack of observation time points beyond the point of conversion to nAMD made the exact timing of later visual loss events and the shape of the Kaplan–Meier curve uncertain. Nevertheless, extrapolated curves suggest that 50% of patients can expect to lose ≥ 10 letters by 150–200 days (4.9 to 6.6 months) after conversion to nAMD without treatment. Given the limitations in estimating the exact timing of events and the shape of the distribution, we selected the exponential distribution for the base-case extrapolation. This function was also most consistent with the long-term rate of post-treatment visual acuity decline applied in the model (see below) because it avoids the chance of visual acuity loss in untreated patients dropping below the rate of visual acuity decline in treated patients.
To inform the expected rate of transition to higher levels of visual acuity loss (≥ 30 letters) among those losing ≥ 10 letters, we assumed that the fellow eye prior to the development of nAMD would have had a similar visual acuity to the unaffected EDNA study eye at study baseline. We, therefore, used the difference in visual acuity between the fellow eye and the EDNA study eye at baseline to estimate the proportion of nAMD eyes that could be expected to have lost ≥ 10 or ≥ 30 letters between conversion to nAMD and the time of treatment initiation: 78.4% and 29.2%, respectively. We then assumed that 29.2% of eyes would be expected to lose ≥ 30 letters by the time that the chosen exponential extrapolation (see Figure 23) predicted that 78.4% would have lost ≥ 10 letters from conversion to nAMD (15.15 months). This was used to calculate monthly probabilities of losing ≥ 30 letters from the time of conversion to nAMD (Figure 24).
FIGURE 24.
Extrapolated time to losing ≥ 10 and ≥ 30 letters from the point of conversion to nAMD.
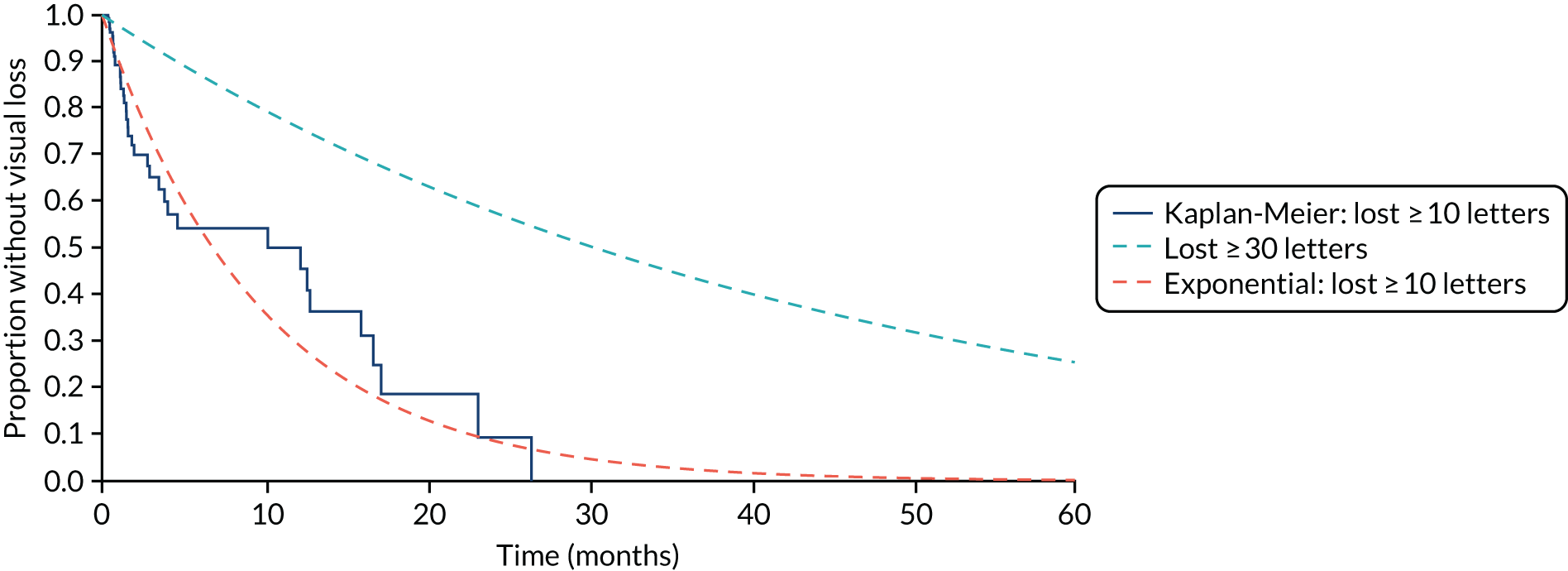
For those modelled to fall into each category of vision loss, the exact number of letters lost was drawn from conditional distributions informed by the observed difference in visual acuity between the fellow eye and the EDNA study eye at baseline. For those losing 10–29 letters, this suggested a uniform distribution (minimum 10 letters, maximum 29 letters). For those losing ≥ 30 letters, a gamma-distribution was applied to account for the skewed nature of the data (mean 42 letters, standard deviation 9.27 letters, minimum 30 letters).
Post-treatment change in visual acuity
Post-treatment visual acuity changes were informed using evidence from several sources: (1) short-term visual acuity outcomes for patients who converted to nAMD in the EDNA study and then commenced treatment; (2) randomised controlled trials of anti-VEGF treatments conducted in the UK NHS; and (3) real-world cohort studies assessing the visual acuity changes in second treated eyes with good visual acuity (> 70 letters) at baseline.
Short-term visual acuity outcomes for participants who converted to neovascular age-related macular degeneration in the EDNA study
Visual acuity outcomes at conversion to nAMD, treatment initiation, post load and 12 months after conversion to nAMD were collected, where possible, for EDNA study participants who converted to nAMD within the study follow-up period. For EDNA study participants who were recruited to the FASBAT study,84 these data were obtained with appropriate permissions from the FASBAT database. For those not recruited to FASBAT, the visual acuity data were extracted from routine case notes.
Based on the above, we were able to obtain usable post-treatment visual acuity data on 93 out of 145 study eyes judged to have converted to nAMD during the EDNA study. The post-treatment changes in visual acuity were categorised by whether or not the individual had lost ≥ 10 letters from baseline at the time of treatment initiation (Figure 25). Among those who lost ≥ 10 letters prior to treatment initiation, mean visual acuity improved by 6.47 (95% CI 2.66 to 10.28) letters following the loading phase. For those treated prior to losing ≥ 10 letters, visual acuity was 1.47 (95% CI –0.22 to 3.17) letters lower after loading. These data were used to inform the loading phase visual acuity changes applied in the model. Following the loading phase, data were available for 23 patients who had lost ≥ 10 letters prior to treatment initiation and 39 who had not. The mean rate of deterioration following the loading phase was very similar between the groups (0.34 and 0.37 letters per month, respectively).
FIGURE 25.
Short-term mean visual acuity outcomes in the EDNA study eye of participants who converted to nAMD during the study.
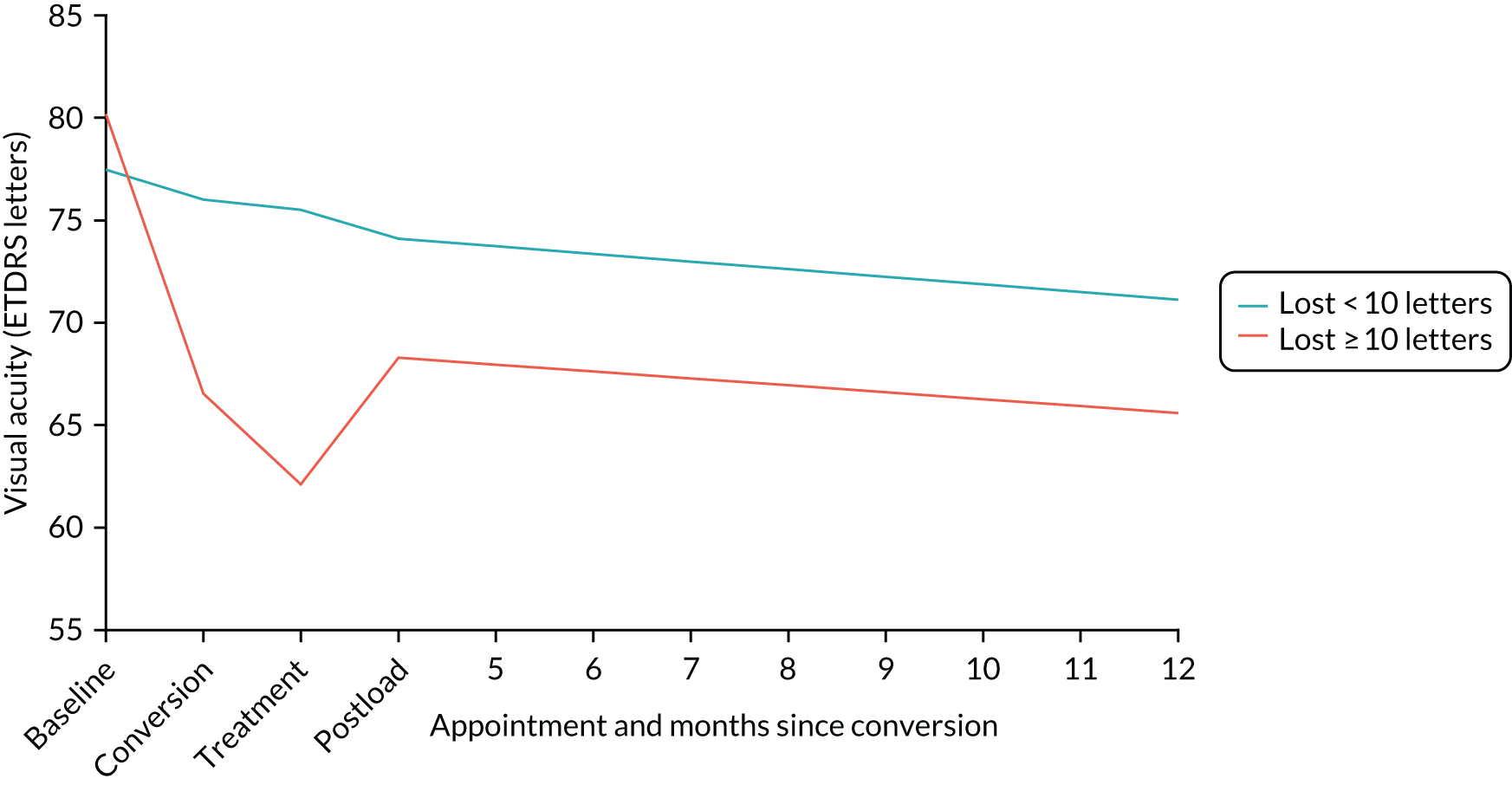
Visual acuity outcomes in randomised controlled trials
Relevant randomised controlled trials were identified from existing reviews and considered with respect to their applicability to the NHS setting. 1,85 Although several treatment protocols exist for anti-VEGF injections, systematic reviews have reported broadly comparable efficacy. 86 There is some evidence to suggest that treat-and-extend regimens (treatment interval gradually extended as long as the macula remains dry) are comparable to continuous monthly injections, and slightly superior to pro re nata (when needed) regimens. 86 With respect to medicinal products, there are two licenced anti-VEGF treatments available in Europe: ranibizumab and aflibercept. In addition, there is some limited use of off-license treatment with bevacizumab, which is a larger molecule but otherwise structurally similar to ranibizumab. Several studies have found comparable efficacy between these. 87,88
Given the above, visual acuity changes observed for patients treated with ranibizumab in the NHS-based Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN) trial87,89 were used to help inform the modelling. The ranibizumab arm of the IVAN trial included individuals randomised to continuous monthly injections and discontinuous (as needed) treatment following a pro re nata protocol, with three monthly injections given on re-initiation of treatment. 87,89 For the purpose of the current model, the visual acuity results are considered broadly generalisable to patients treated with any anti-VEGF treatment in routine NHS practice. A further benefit of the IVAN trial was the availability of long-term follow-up data for 537 participants who completed the original 24 months of trial follow-up. 9
With respect to model implementation of post-treatment visual acuity change, we followed the approach described by Claxton et al. ,90 whereby IVAN trial data were used to derive time-dependent normal distributions for visual acuity change per month. The monthly visual acuity change for each simulated individual was determined by taking a random draw from the assigned change distributions. The approach of Claxton et al. 90 was simplified by applying average monthly letter changes across months 3 to 12 and 13 to 24 (Table 41). In addition, given that the IVAN trial included a mixture of eyes with baseline visual acuity above and below 70 letters, we adjusted the loading phase change distributions to yield the loading phase changes in mean visual acuity observed for patients who converted to nAMD during the EDNA study: + 6.47 letters for those who had lost ≥ 10 letters prior to treatment initiation and –1.47 letters for those who had lost < 10 letters prior to treatment initiation.
| Variable | Point estimate (SD) | Source |
|---|---|---|
| Monthly visual acuity change (months 1–3) for eyes with visual acuity loss of ≥ 10 letters | 2.1567 (2.89) | Chakravarthy et al.87 and Claxton et al.90 |
| Monthly visual acuity change (months 3–12) for eyes with visual acuity loss of ≥ 10 letters | 0.00 (2.25) | Chakravarthy et al.87 and Claxton et al.90 |
| Monthly visual acuity change (months 13–24) for eyes with visual acuity loss of ≥ 10 letters | –0.0917 (2.17) | Chakravarthy et al.87 and Claxton et al.90 |
Longer-term visual acuity changes by visual acuity at treatment initiation
Given that most treated eyes in IVAN were symptomatic at baseline (mean visual acuity 61.4 letters), further data were required to inform the modelling for patients detected early and treated prior to significant vision loss. To do this, we reviewed studies reporting treatment outcomes for second and early treated eyes with good visual acuity at baseline. 14,16 In both studies,14,16 second-treated eyes with good visual acuity at baseline did not show the gain in vision that symptomatic first eyes show following treatment loading, confirming the observation in EDNA study participants. By contrast, these eyes remained stable over the loading phase before declining gradually at a similar rate to the first treated eyes after the loading phase. Vision in the second treated eyes remained better than vision in first treated eyes at all time points up to the end of the observed follow-up periods of 216 and 314 years. Thus, for second eyes treated prior to significant vision loss, this trajectory was assigned in the model by capping visual acuity at the baseline value and calibrating the visual acuity change distributions to yield output in line with the above observations.
Beyond 24 months, we specified a monthly visual acuity change distribution based on the average annual rate of decline observed during the long-term follow-up of IVAN participants: 4.3 letters per year (95% CI 3.7 to 4.9 letters). This was applied to eyes that initiated treatment prior to and after losing ≥ 10 letters. The modelled mean trajectories for eyes treated before and after significant vision loss are provided in Model validation below.
Treatment discontinuation
The monthly probabilities of discontinuation from monitoring and treatment were applied from 24 months post treatment initiation. These were based on discontinuation rates observed for IVAN trial participants during the period of extended follow-up after release from the trial protocol. The rates were reported by visual acuity in the study eye at the beginning of long-term follow-up. 9 Discontinuation of treatment was defined as discharge from the clinic or > 1 year of follow-up observed since the last injection to the study eye. The median times to discontinuation or the last observed event if not reached were extracted from the published Kaplan–Meier plots using digitising software. The extracted probabilities of discontinuation by corresponding time points were then converted into monthly probabilities, assuming an exponential distribution (Table 42). The data suggest a higher rate of treatment discontinuation in eyes with poorer visual acuity at 24 months post treatment initiation. Furthermore, we applied a rule in the model that treatment and monitoring would cease for futility if vision dropped below 18 letters. For those who discontinued with visual acuity above 18 letters, it was assumed that stability had been achieved and, therefore, no further changes in visual acuity were modelled unless reactivation occurred and re-initiation of treatment was required.
| Variable | Estimated probability (SE)a | Source |
|---|---|---|
| Monthly probability of treatment discontinuation by visual acuity at 24 months post-treatment initiation | ||
| ≥ 68 | 0.0091 (0.001) | Evans et al.9 |
| 53–67 | 0.0143 (0.002) | Evans et al.9 |
| 38–52b | 0.0095 (0.003) | Evans et al.9 |
| ≤ 37 | 0.0257 (0.007) | Evans et al.9 |
| Overall | 0.0174 (0.001) | Evans et al.9 |
| Average of 37 to ≥ 68 | 0.0110 (0.002) | Evans et al.9 |
| Annual probability of re-initiating treatment following 12 months of stability | ||
| Year 1 | 0.246 (0.038) | Chandra et al.;91 Sobha Sivaprasad, Moorfields Eye Hospital, 2020, personal communication |
| Year 2 onwards | 0.106 (0.038) | Chandra et al.;91 Sobha Sivaprasad, personal communication |
Given that data on the rate of treatment re-initiation were not available from the IVAN trial, we used data from another UK-based cohort to inform this. 91 Data from this cohort study showed that of 512 patients initiating anti-VEGF treatment in an NHS treatment centre, 158 (30.9%) were discharged stable, of whom 38 reinitiated treatment within a 5-year follow-up period (Sobha Sivaprasad, Moorfields Eye Hospital, 2020, personal communication). The authors provided the numbers of patients discontinuing annually, numbers at risk and times to re-initiation of treatment among those who discontinued. These data were used to approximate annual probabilities of discontinuing treatment and returning to treatment following discontinuation. The estimated monthly probability of discontinuing with stable vision (0.0092) was similar to the discontinuation probability derived for IVAN trial participants with better visual acuity outcomes at 24 months post treatment initiation. The estimated probability of treatment re-initiation was higher in the first year following a 12-month pause in treatment and lower in subsequent years (see Table 42).
An alternative source of treatment re-initiation following a 12-month pause was identified in the literature, which suggested a higher rate of return (approximately 2.9% per month) based on data from the UK Neovascular AMD database. 92 However, it did not report the treatment discontinuation rate preceding treatment re-initiation and, therefore, preference was given to the former source. Furthermore, the data provided by Sivaprasad (Sobha Sivaprasad, personal communication) were more contemporary, reflecting the re-initiation rate following discharge from a treat-and-extend maintenance regimen. The rate provided by Madhusudhana et al. 92 was based on eyes that would have discontinued on a pro re nata maintenance regimen. Clinical opinion in the team suggested that lower re-treatment rates could be expected for patients discharged from treatment on the current treat-and-extend protocols.
Valuation of health effects
The available health state utility data for quality-adjusting survival by visual acuity status were identified from searches of the published literature. Searches identified numerous potential sources. 93–101 An emphasis was placed on identifying values for visual acuity states based on the preferences of the UK general population. Given that there was some evidence to suggest that the EuroQol-5 Dimensions lack sensitivity to change in visual acuity,102 directly elicited values for specific visual acuity states were considered where these had been elicited from a sample of the UK general population using an appropriate valuation technique.
Based on consideration of the available evidence, we chose to base the visual acuity health state utilities on time trade-off values reported by Czoski-Murray et al. 101 In this study, contact lenses were used to simulate visual impairment in participants from the UK general population. Participants valued the simulated states using the time trade-off methodology, providing utility weights on a zero (death) to one (full health) scale. Czoski-Murray et al. 101 also reported a regression analysis of the health state utility values by visual acuity in the better seeing eye, with participant age included as a covariate. In line with the National Institute for Health and Care Excellence (NICE) appraisals of ranibizumab103 and aflibercept104 for the treatment of nAMD, we used this published equation to assign health state utility weights by visual acuity in the model, where SE is standard error and MAR is minimum angle of resolution:
Mortality
In line with the modelling used to support NICE guidance on the use of ranibizumab and aflibercept in the NHS, mortality was based on UK general population life tables, with risk ratios of 1.1 (95% CI 1.01 to 1.19) and 1.17 (95% CI 1.07 to 1.27) applied for visual acuity of < 75 letters and visual acuity of < 60 letters, respectively. 105 Thus, strategies that maintain higher visual acuity were modelled through evidence of association to provide a small survival benefit. The impact of removing these risks ratios was assessed in a sensitivity analysis.
Resource use and costs
The model included costs of testing and monitoring conversion to nAMD, costs of monitoring and treatment post conversion to nAMD, and health-care and personal social care costs associated with severe visual loss. All costs were expressed in 2018/19 Great British pounds.
Pre-conversion to neovascular age-related macular degeneration testing and monitoring costs
It was assumed that prior to conversion to nAMD in the second eye (EDNA study eye), treatment and monitoring of the first eye drives the frequency of outpatient visits. Therefore, marginal costs of testing the second eye at these monitoring/treatment visits were estimated and applied in the model (Table 43). In addition, the outpatient visit cost was applied for all monitoring strategies.
| Statistics | Index test modality (£) | |||||
|---|---|---|---|---|---|---|
| OCT | Fundus clinical evaluation | Amsler | Self-reported vision | Visual acuity | ||
| Slit lamp | Photography | |||||
| EDNA study eye (second eye) | ||||||
| Mean | 10.68 | 10.64 | 11.79 | 6.13 | 7.08 | 8.15 |
| Standard deviation | 5.19 | 6.32 | 7.11 | 3.05 | 6.68 | 6.32 |
| Median | 9.71 | 9.91 | 10.04 | 4.83 | 5.05 | 6.26 |
| IQR | 6.38 | 6.56 | 10.60 | 3.21 | 3.57 | 5.36 |
| First eye (nAMD at baseline) | ||||||
| Mean | 19.45 | 18.44 | 13.70 | 6.88 | 8.07 | 10.23 |
| Standard deviation | 10.14 | 8.85 | 7.33 | 2.92 | 7.35 | 6.50 |
| Median | 17.83 | 20.24 | 12.11 | 7.10 | 5.33 | 7.34 |
| IQR | 11.76 | 13.23 | 9.72 | 3.40 | 4.80 | 6.21 |
| Total | ||||||
| Mean | 30.13 | 30.79 | 25.48 | 13.01 | 15.14 | 18.38 |
| Standard deviation | 14.50 | 10.72 | 13.18 | 5.85 | 13.73 | 12.16 |
| Median | 29.12 | 31.58 | 22.15 | 12.89 | 10.82 | 13.68 |
| IQR | 16.47 | 11.58 | 16.97 | 6.14 | 8.82 | 5.36 |
A survey of participating centres was undertaken in January 2020 to gather information on the staff time required to test both eyes and only one eye with each index test. Unit costs of staff time, inclusive of overheads, were used to estimate the cost for each test. 106 Prices of test-specific equipment were obtained from centres or manufacturers and annuitised over their expected useful life span. The annual equivalent cost of each piece of equipment, inclusive of maintenance, was divided by the annual throughput reported by centres to estimate an equipment cost per patient tested. The estimated equipment costs per test were allocated between the first and the second eye based on the estimated time required to test the first and the second eye. IT storage costs were also estimated for tests that involve the acquisition of digital images (OCT and fundus photography). The total file size of the image set per eye was obtained from centres and multiplied by an estimated cost of file storage for a 10-year period.
Table 43 provides a summary of the estimated testing costs for the first eye, the second eye and overall. Further details are provided in Appendix 4.
The sensitivity analysis explored the impact of alternative costing assumptions. Given that the staff cost multipliers used in the calculation of testing costs do not account for all of the capital overheads associated with use of clinic consulting and treatment space, we assessed the impact of uplifting the test cost estimates to account for these further overheads. This was carried out by applying the ratio of allocated overhead costs to direct staffing costs (1 : 3.333) reported for ophthalmology outpatient visits across Scottish hospitals. 107 This approach may double count some of the overheads already included in the staff cost multipliers and, therefore, should provide an upper limit of the estimated marginal cost of each test.
To inform testing frequency prior to conversion to nAMD, we used the observed interval between visits in the EDNA study by year of follow-up. This indicated that the average interval between visits was ≈ 1.5 months during the first 6 months, ≈ 2 months between month 6 and month 24, ≈ 2.3 months between month 24 and month 30, and ≈ 3.3 months between month 30 and month 36. For consistency with the monthly cycle used in the model, we applied a monthly interval for the first 3 months, a 2-month interval up to month 30, followed by a 3-month monitoring interval beyond this time point. There was uncertainty beyond 36 months with respect to how long and how frequently second eyes would continue to be monitored for conversion to nAMD, and to what extent these monitoring costs would continue to be driven by treatment/monitoring visits for the first eye. For the base case it was assumed that beyond 5 years the monitoring frequency would be driven by the EDNA study eye (second eye) and that it would occur on a 6-monthly basis.
Post-conversion to neovascular age-related macular degeneration monitoring and treatment costs
From the point of conversion to nAMD in the second eye it was assumed that treatment and monitoring of the second eye would drive the frequency of visits. From this point onwards, full treatment and monitoring costs were applied to the second eye according to time since treatment initiation. The nAMD treatment and monitoring costs after conversion to nAMD were informed by mean numbers of treatment and monitoring visits per year. These estimates of resource use were derived from the data reported in available clinical trials and clinical advice (Table 44), and the associated unit costs are provided in Table 45. The base case assumed a drug treatment distribution in line with that observed in patients who commenced treatment for the EDNA study eye: aflibercept (68.7%), ranibizumab (22.3%) and bevacizumab (9%). Current list prices were applied to each drug.
| Time point | Ranibizumab | Aflibercept | ||
|---|---|---|---|---|
| Treatment visits per year, mean (SE)a | Monitoring visits per year, mean (SE)a | Treatment visits per year, mean (SE)b | Monitoring visits per year, mean (SE)b | |
| Year 1 | 10 (0.233) | 12c | 7 (0.35) | 7 (0.35) |
| Year 2 | 8 (0.233) | 12c | 6 (0.3) | 6 (0.3) |
| Year 3+ | 4 (0.277) | 9 (0.231) | 4 (0.2) | 6 (0.3) |
| Resource | Unit costs (£) | Source | Notes |
|---|---|---|---|
| Ophthalmology outpatient visit | 95.00 | 2018–19 National Cost Collection Data 108 | Code 130: outpatient consultant-led appointment in ophthalmology |
| FFA | 145.00 | 2018–19 National Cost Collection Data 108 | BZ86B: outpatient intermediate vitreous retinal procedures |
| Administration of anti-VEGF injection | 145.00 | 2018–19 National Cost Collection Data 108 | BZ86B: outpatient intermediate vitreous retinal procedures |
| Ranibizumab (Lucentis injection) | 551.00 | British National Formulary 2019109 | 1.65 mg/0.165 ml solution for injection pre-filled syringes (Novartis Pharmaceuticals UK Ltd, London, UK) |
| Aflibercept (Eylea injection) | 816.00 | British National Formulary 2019109 | 2 mg/50 µl solution for injection vials (Bayer Plc, Leverkusen, Germany) |
| Bevacizumab (Avastin injection) | 49.00 | Dakin et al. 2014110 | 1.25 mg per injection |
| Cost of blindness (health service perspective) | 562.41 per month in year 1, 541.73 per month from year 2 onwards | Mowatt et al. 2014111 | Cost per month for visual acuity of < 35 ETDRS letters |
Where vision dropped below 35 letters, health-care and social care costs associated with legal blindness were applied. These were based on the costs applied in the Health Technology Assessment model by Mowatt et al. ,111 which were inflated to 2018/19 prices using the NHS cost inflation index. 106
Model validation
Prior to running the analysis, the modelled visual acuity outcomes were checked against the input sources. Figure 26 shows the modelled mean visual acuity trajectories for typical patients commencing treatment before (early treatment) and after (late treatment) losing ≥ 10 letters. It indicates the benefit of treating eyes prior to vision loss, with better visual acuity maintained over time than that for eyes treated after a significant amount of vision loss. 14,16 The trajectories are for patients who continue treatment and, therefore, do not account for those who are discharged with stable vision. Therefore, the modelled reduction in visual acuity for the treated cohort was slightly lower than that shown in Figure 26.
FIGURE 26.
Model output: visual acuity of continually monitored/treated patients by time since treatment initiation (EDNA model). Note that with application of a visual acuity cap in the model, there is a downwards pressure on visual acuity change between month 3 and month 24, which slightly overestimates the expected visual acuity during this time-period compared with the IVAN trial.
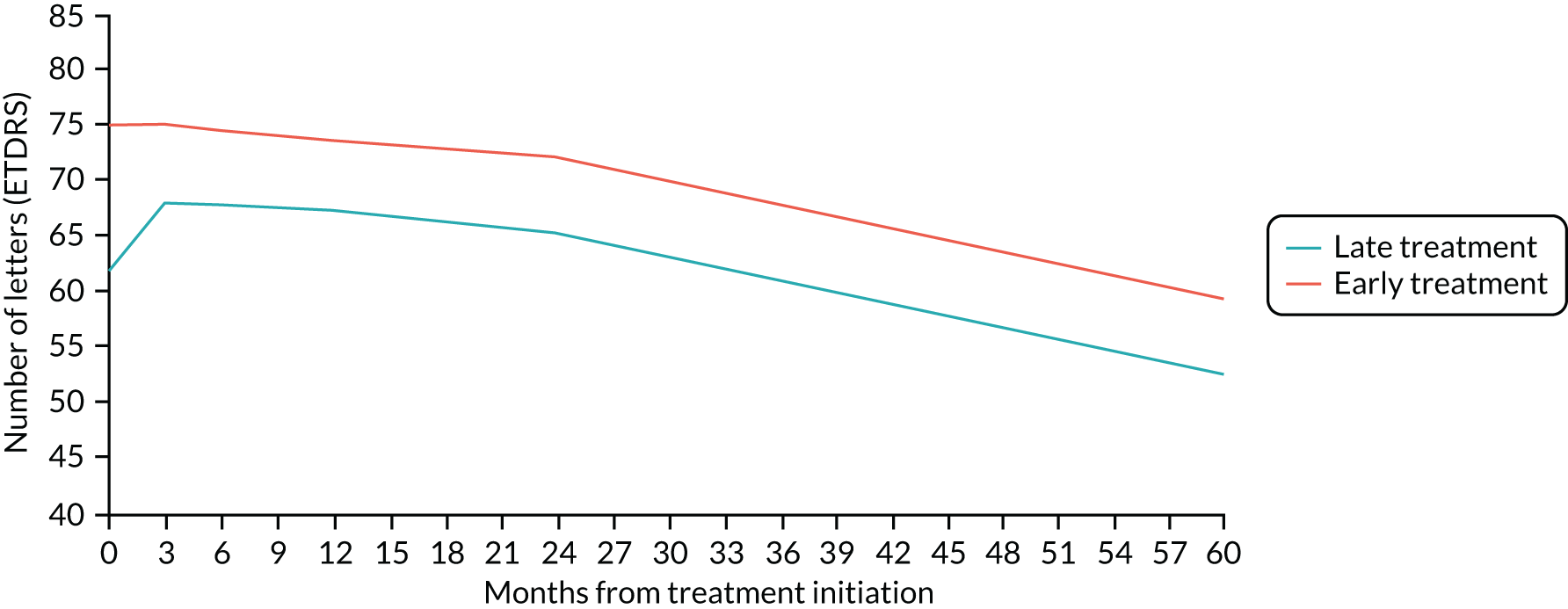
Model analysis
The analysis used first-order Monte Carlo simulation to propagate the passage of individuals through the model one at a time. A total of 200,000 individual trials were found to produce stable results. In line with the NICE reference case, future costs and QALYs were discounted at a rate of 3.5% beyond year 1. 112
Incremental cost-effectiveness ratios (ICERs) that expressed the additional cost per QALY gained were estimated by comparing each monitoring strategy with the next less costly strategy (excluding those strategies that are more costly and less effective than an alternative option). In the UK NHS, a threshold value of £20,000 to £30,000 is normally used to make judgements on cost-effectiveness of health technologies.
Probabilistic sensitivity analysis was conducted to characterise the joint uncertainty around the model outputs (incremental costs and QALYs) arising from the uncertainty around the model input parameters. Probability distributions were assigned based on the mean and the measure of variance reported for each parameter in the preceding sections (see Clinical effectiveness parameters, Valuation of health effects, Mortality and Resource use and costs). Beta-distributions were assigned to sensitivity and specificity parameters, gamma-distributions were used for costs of testing and the cost of blindness (assuming a standard error of 10% of the mean), and normal distributions were applied for regression coefficients (including the utility increment associated with change in visual acuity and age, and the exponential rate parameters for time to conversion to nAMD and time to visual acuity loss of ≥ 10 letters). Normal distributions were also used for the mean change in visual acuity during the loading phase, the long-term change in visual acuity per year for treated individuals and the probabilities of treatment discontinuation and treatment re-initiation. Second-order Monte Carlo simulation with an inner loop of first-order simulation (n = 5000) was then used to analyse the model for 1000 random draws from the assigned input probability distributions. The output from this analysis provided the probability of each monitoring test being the preferred strategy by increased cost-effectiveness thresholds. 113 Further deterministic scenario analyses were also undertaken to assess the impact on findings of uncertainty surrounding key model input parameters and structural assumptions (see Results).
Base-case assumptions
Several key assumptions of the base-case analysis are as follows:
-
Time to conversion to nAMD follows an exponential distribution, that is a constant probability of conversion over time.
-
Time to losing ≥ 10 letters from time of conversion to nAMD to treatment initiation is informed by exponential extrapolation of the EDNA study Kaplan–Meier data.
-
All positive diagnostic monitoring test results are confirmed or refuted by FFA.
-
New visual acuity loss of > 30 letters in undetected nAMD triggers self-referral and detection regardless of the testing strategy.
-
New visual acuity loss of 10–29 letters in undetected nAMD triggers self-referral and detection in 50% of individuals.
-
Undetected nAMD with a new visual acuity drop of ≥ 10 letters is detected by the visual acuity testing strategy at the next monitoring visit.
-
Long-term monitoring of second eyes that do not develop nAMD continues indefinitely beyond 36 months (twice per year from 5 years onward).
-
Long-term monitoring in patients who do not develop nAMD in the second eye is driven by the second eye beyond 5 years in the model.
-
Following conversion to nAMD and detection, patients are treated immediately with anti-VEGF injections.
-
Individuals treated prior to experiencing a significant visual acuity loss (≥ 10 letters) experience no gain in vision during the loading phase.
-
Individuals treated after experiencing a significant visual acuity loss (≥ 10 letters) experience a loading phase gain in line with the gain observed in EDNA study participants who converted to nAMD during study follow-up.
-
A percentage of patients discontinue treatment with stable disease over time but can reinitiate treatment.
Results
Base-case results
The results of the base-case analysis are provided in Figure 27 and Table 46. They indicate that more sensitive monitoring strategies generate increased health benefits at a lower cost, with the visual acuity change test being least effective and most costly and OCT being most effective and least costly. It can be noted from Figure 27 that OCT lies at the bottom right of the plane (lowest cost and highest QALYs), dominating all other test strategies.
FIGURE 27.
Cost-effectiveness results: base case.
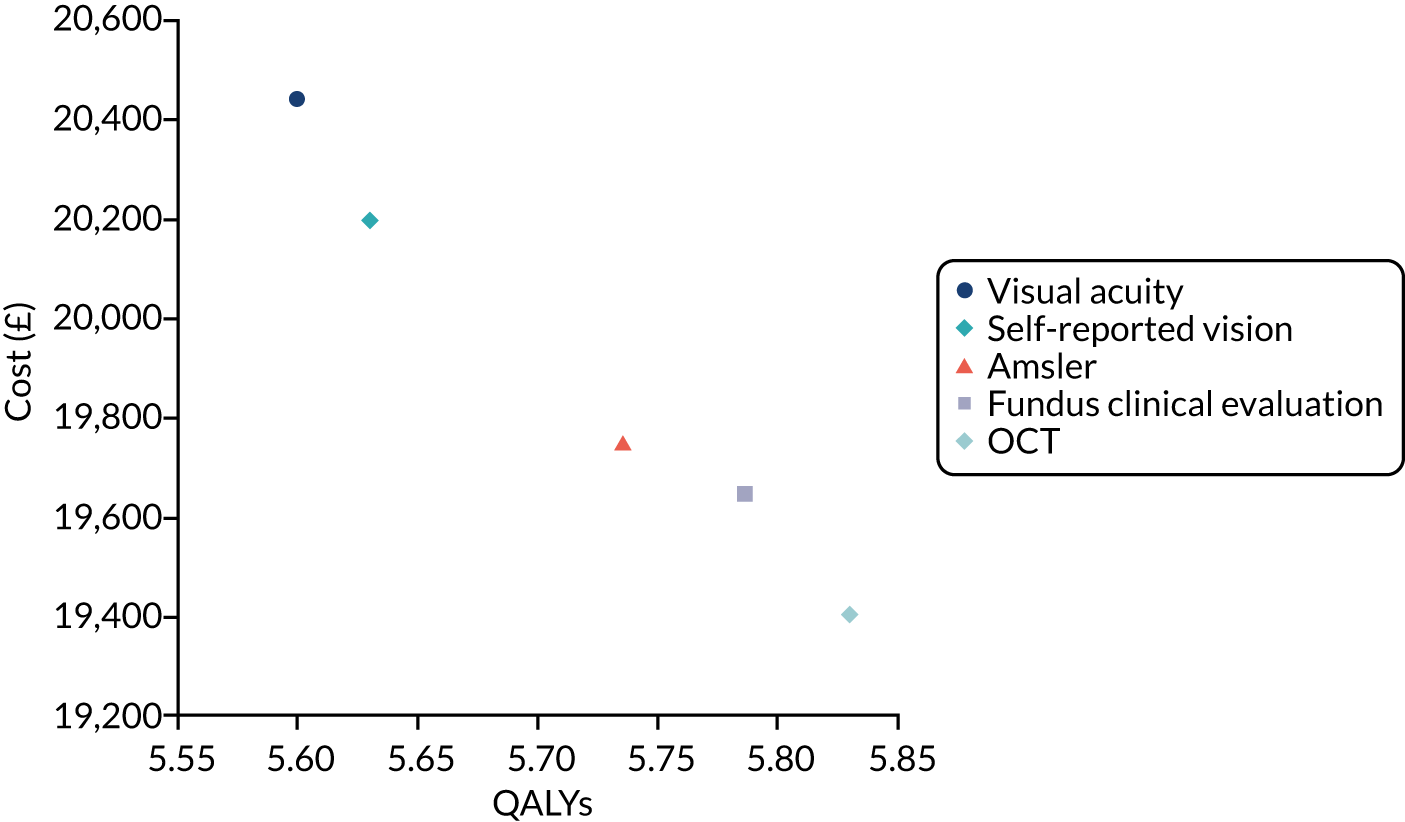
| Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| OCT | 19,406 | 5.830 | |||
| Fundus clinical evaluation | 19,649 | 243 | 5.787 | –0.044 | –5562a |
| Amsler | 19,751 | 346 | 5.736 | –0.095 | –3656a |
| Self-reported vision | 20,198 | 792 | 5.630 | –0.200 | –3961a |
| Visual acuity | 20,444 | 1039 | 5.600 | –0.230 | –4510a |
Table 47 disaggregates the total costs accrued in the model under each testing strategy. The index tests with lower sensitivity and specificity accrue moderately higher prediagnosis costs than more sensitive/specific strategies as a result of more visits prior to detection and increased chances of a false positive result. More sensitive index tests accumulate higher post-diagnosis monitoring and treatment costs owing to earlier detection. However, the increased costs of earlier treatment are countered by reduced costs associated with visual impairment and blindness. It is the lower costs of blindness resulting from earlier treatment with OCT that makes it the dominant strategy in the base case.
| Index test | Cost (£) | ||||
|---|---|---|---|---|---|
| Total | Monitoring pre diagnosis | Monitoring post diagnosis | Treatment | Blindness | |
| OCT | 19,406 | 2573 | 2576 | 10,966 | 3291 |
| Fundus clinical evaluation | 19,649 | 2588 | 2506 | 10,685 | 3870 |
| Amsler | 19,751 | 2502 | 2426 | 10,362 | 4461 |
| Self-reported vision | 20,198 | 2565 | 2261 | 9700 | 5672 |
| Visual acuity | 20,444 | 2644 | 2226 | 9562 | 6013 |
Table 48 further illustrates the benefits of OCT compared with the other strategies in terms of the expected time from conversion to nAMD to detection and treatment, and the mean visual acuity at time of treatment initiation. The modelling suggests that, compared with using visual acuity alone, OCT monitoring will bring detection forward by approximately 7.5 months, for a gain in visual acuity at time of treatment initiation of approximately 16 letters. It is this earlier initiation of treatment and maintenance of better visual acuity that drives the QALY gains compared with the other strategies.
| Index test | Time difference (months), mean (SD) | Visual acuity at first treatment, mean (SD) | ||
|---|---|---|---|---|
| Conversion to nAMD to detection | Conversion to nAMD to first treatment | Detection to first treatment | ||
| OCT | 2.5 (1.8) | 3.3 (1.8) | 0.8 (0.4) | 71.3 (13.4) |
| Fundus clinical evaluation | 4.1 (3.7) | 4.8 (3.7) | 0.7 (0.5) | 68 (14.6) |
| Amsler | 5.9 (5.4) | 6.4 (5.4) | 0.5 (0.5) | 64.4 (15.1) |
| Self-reported vision | 9.4 (8.4) | 9.5 (8.4) | 0.1 (0.3) | 57.2 (13.3) |
| Visual acuity | 10 (9.2) | 10.2 (9.2) | 0.2 (0.4) | 55.2 (11.8) |
Sensitivity analysis
To assess the sensitivity of the model findings to key assumptions and input parameters, a series of scenario analyses were undertaken. These are outlined as follows:
-
The Weibull distribution applied to model time to conversion to nAMD, giving an increasing hazard of conversion over time.
-
The log-normal distribution applied to model time to conversion to nAMD, giving a decreasing hazard of conversion over time.
-
The log-normal distribution applied to model time from conversion to nAMD to significant vision loss (≥ 10 letters).
-
The generalised gamma-distribution applied to model time from conversion to nAMD to significant vision loss (≥ 10 letters).
-
Pre-treatment visual acuity loss conditional on dropping 10–29 and ≥ 30 letters assumed to be skewed towards the lower end of the visual acuity loss ranges: mean of 12 and mean of 32 letters, respectively.
-
A reduced longer-term rate of post-treatment visual acuity decline applied: 3.1 letters per year in line with the average rate of decline estimated for younger patients (aged 70 years) in the IVAN long-term follow-up study.
-
A reduced longer-term rate of post-treatment visual acuity decline applied: 2 letters per year in line with the average rate of decline estimated for younger patients (aged 60 years) in the IVAN long-term follow-up study.
-
Distribution for long-term post-treatment rate of visual acuity loss per year assumed to be right-skewed and constrained by 0; gamma distribution with mean of 4.3 and standard deviation of 7.
-
A reduced rate of treatment discontinuation for stable vision applied (0.0092), based on data from Chandra et al. 91 (applied independent of visual acuity outcome at 24 months post treatment).
-
Removal of treatment discontinuation for stable vision (an extreme scenario to assess the impact of this uncertain parameter).
-
An increased rate of treatment re-initiation (approximately 2.9% per month) following discontinuation for stable disease, in line with data reported by Madhusudhana et al. 92
-
100% test sensitivity applied for all tests at the subsequent monitoring visit for those who lose 15–29 letters as a result of nAMD.
-
Removal of excess mortality associated with visual impairment.
-
Removal of costs of blindness.
-
Wait-to-treat policy, which assumes that visual acuity must drop below the threshold specified for ranibizumab and aflibercept in NICE guidance (≤ 70 letters).
-
Treatment instigated following OCT positive findings, without confirmation with FFA – this assumes that any patient receiving a false positive OCT result incurs 12 months’ worth of anti-VEGF treatment inappropriately, before being identified as morphologically unchanged and treatment withdrawn.
-
Increased test monitoring costs as per the increased overhead scenario outlined in Appendix 4.
The results of these scenarios are provided in Table 49. They indicate that OCT remains dominant in all but five scenarios (4, 5, 14, 15 and 16). Scenario 4 results in a significant proportion of patients with nAMD (≈ 10%) maintaining stable vision without treatment to 10 years post conversion to nAMD. Under this scenario, OCT results in the early treatment of these patients at increased cost but with no benefit over the visual acuity strategy. This undermines the cost savings and QALY gains. Nevertheless, the ICER for OCT (£9040 per QALY gained) remains well below the accepted thresholds for cost-effectiveness. Scenario 5 minimises the penalty of missing cases with less-sensitive strategies. Again, the ICER for OCT becomes positive in this case but well below the accepted thresholds. When costs of blindness are removed (scenario 14), OCT becomes the costliest strategy but, again, its ICER remains favourable against the accepted thresholds even in this extreme scenario. In scenario 15, assuming that clinicians would wait for vision to drop below 70 letters before treating, the QALY gain associated with OCT is greatly diminished and its ICER increases to £19,488. It can be noted from this scenario that the costs of all testing strategies are higher and the QALYs lower than those for the base case, which assumes that treatment is initiated on detection. This indicates that early treatment dominates delayed treatment. Scenario 16, assessing the impact of initiating treatment following an OCT positive finding and forgoing a FFA, results in the ICER increasing to £17,256. Again, the base-case OCT strategy, with confirmatory FFA, dominates this alternative approach.
| Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICERa (£) |
|---|---|---|---|---|---|
| 1. Weibull distribution applied to model time to conversion to nAMD, giving an increasing hazard of conversion over time | |||||
| OCT | 19,909 | 5.806 | |||
| Fundus clinical evaluation | 20,178 | 269 | 5.761 | –0.046 | –5898b |
| Amsler | 20,290 | 381 | 5.709 | –0.098 | –3892b |
| Self-reported vision | 20,754 | 845 | 5.601 | –0.205 | –4123b |
| Visual acuity | 20,993 | 1084 | 5.572 | –0.235 | –4617b |
| 2. Log-normal distribution applied to model time to conversion to nAMD, giving a decreasing hazard of conversion over time | |||||
| OCT | 17,288 | 5.926 | |||
| Fundus clinical evaluation | 17,501 | 212 | 5.888 | –0.037 | –5687b |
| Amsler | 17,550 | 261 | 5.843 | –0.083 | –3163b |
| Self-reported vision | 17,969 | 681 | 5.748 | –0.178 | –3826b |
| Visual acuity | 18,219 | 931 | 5.721 | –0.205 | –4545b |
| 3. Log-normal distribution applied to model time from conversion to nAMD to losing ≥ 10 letters without treatment | |||||
| OCT | 19,616 | 5.806 | |||
| Fundus clinical evaluation | 19,854 | 238 | 5.765 | –0.041 | –5773b |
| Amsler | 19,899 | 283 | 5.723 | –0.083 | –3406b |
| Self-reported vision | 20,049 | 433 | 5.644 | –0.161 | –2679b |
| Visual acuity | 20,112 | 496 | 5.620 | –0.186 | –2672b |
| 4. Generalised gamma-distribution applied to model time from conversion to nAMD to losing ≥ 10 letters without treatment | |||||
| Visual acuity | 18,853 | 5.688 | |||
| Self-reported vision | 19,311 | 458 | 5.690 | 0.002 | 293,816c |
| OCT | 19,760 | 907 | 5.788 | 0.100 | 9040 |
| Amsler | 19,833 | 73 | 5.728 | –0.060 | –1229b |
| Fundus clinical evaluation | 19,912 | 153 | 5.756 | –0.032 | –4745b |
| 5. Letter losses conditional on dropping 10–29 and ≥ 30 letters assumed to be skewed towards the highest end of the range; mean of 12 and mean of 32, respectively | |||||
| Self-reported vision | 18,728 | 5.780 | |||
| Amsler | 18,755 | 27 | 5.836 | 0.057 | 470 |
| Visual acuity | 18,831 | 76 | 5.763 | –0.073 | –1037b |
| Fundus clinical evaluation | 18,867 | 112 | 5.861 | 0.025 | 4478c |
| OCT | 18,878 | 123 | 5.883 | 0.046 | 2655 |
| 6. Reduced long-term rate of post treatment visual acuity decline from 4.3 letters per year to 3.1 letters per year | |||||
| OCT | 18,592 | 5.925 | |||
| Fundus clinical evaluation | 18,839 | 247 | 5.878 | –0.047 | –5237b |
| Amsler | 18,975 | 382 | 5.822 | –0.102 | –3733b |
| Self-reported vision | 19,455 | 863 | 5.703 | –0.221 | –3897b |
| Visual acuity | 19,690 | 1097 | 5.671 | –0.253 | –4331b |
| 7. Reduced long-term rate of post treatment visual acuity decline from 4.3 letters per year to 2 letters per year | |||||
| OCT | 17,987 | 6.016 | |||
| Fundus clinical evaluation | 18,193 | 206 | 5.965 | –0.051 | –4067b |
| Amsler | 18,289 | 302 | 5.906 | –0.110 | –2748b |
| Self-reported vision | 18,712 | 725 | 5.778 | –0.238 | –3045b |
| Visual acuity | 18,915 | 929 | 5.744 | –0.272 | –3415b |
| 8. Distribution for long-term post-treatment rate of letter loss per year assumed to be right skewed and constrained by 0; gamma distribution with mean of 4.3, standard deviation of 7 | |||||
| OCT | 19,132 | 5.954 | |||
| Amsler | 19,182 | 51 | 5.839 | –0.115 | –441b |
| Fundus clinical evaluation | 19,184 | 52 | 5.899 | –0.055 | –944b |
| Self-reported vision | 19,464 | 333 | 5.729 | –0.226 | –1475b |
| Visual acuity | 19,614 | 482 | 5.696 | –0.258 | –1870b |
| 9. Reduced rate of treatment discontinuation for stable vision to 0.0092 based on data from Chandra et al. | |||||
| OCT | 19,957 | 5.816 | |||
| Fundus clinical evaluation | 20,175 | 218 | 5.772 | –0.043 | –5028b |
| Amsler | 20,265 | 307 | 5.722 | –0.094 | –3284b |
| Self-reported vision | 20,699 | 741 | 5.619 | –0.197 | –3765b |
| Visual acuity | 20,934 | 976 | 5.588 | –0.227 | –4292b |
| 10. No treatment discontinuation for stable vision | |||||
| OCT | 23,854 | 5.723 | |||
| Amsler | 23,857 | 3 | 5.640 | –0.082 | –36b |
| Fundus clinical evaluation | 23,941 | 87 | 5.686 | –0.037 | –2373b |
| Self-reported vision | 23,978 | 124 | 5.549 | –0.173 | –715b |
| Visual acuity | 24,124 | 269 | 5.524 | –0.199 | –1356b |
| 11. Increased rate of re-initiation of therapy following discontinuation with stable vision | |||||
| OCT | 20,457 | 5.792 | |||
| Fundus clinical evaluation | 20,664 | 207 | 5.751 | –0.042 | –4973b |
| Amsler | 20,725 | 268 | 5.703 | –0.090 | –2989b |
| Self-reported vision | 21,104 | 647 | 5.602 | –0.191 | –3390b |
| Visual acuity | 21,329 | 872 | 5.574 | –0.219 | –3990b |
| 12. 100% test sensitivity applied for all tests at the next monitoring visit for those who lose 15–30 letters owing to nAMD | |||||
| OCT | 19,405 | 5.830 | |||
| Fundus clinical evaluation | 19,640 | 236 | 5.786 | –0.044 | –5359b |
| Amsler | 19,740 | 336 | 5.736 | –0.095 | –3545b |
| Self-reported vision | 20,189 | 784 | 5.630 | –0.200 | –3913b |
| Visual acuity | 20,442 | 1037 | 5.600 | –0.231 | –4497b |
| 13. No excess mortality owing to visual acuity loss | |||||
| OCT | 20,234 | 5.931 | |||
| Fundus clinical evaluation | 20,531 | 297 | 5.887 | –0.044 | –6716b |
| Amsler | 20,692 | 458 | 5.837 | –0.094 | –4876b |
| Self-reported vision | 21,259 | 1025 | 5.734 | –0.197 | –5195b |
| Visual acuity | 21,542 | 1308 | 5.705 | –0.227 | –5774b |
| 14. Removal of costs of blindness | |||||
| Visual acuity | 14,432 | 5.600 | |||
| Self-reported vision | 14,527 | 95 | 5.630 | 0.030 | 3144 |
| Amsler | 15,290 | 763 | 5.736 | 0.106 | 7231 |
| Fundus clinical evaluation | 15,779 | 489 | 5.787 | 0.051 | 9621c |
| OCT | 16,115 | 825 | 5.830 | 0.095 | 8729 |
| 15. Wait for visual acuity to drop to 70 letters or lower before initiating treatment, regardless of visual acuity at time of detection | |||||
| Self-reported vision | 20,423 | 5.580 | |||
| Amsler | 20,471 | 48 | 5.591 | 0.012 | 4159 |
| Visual acuity | 20,484 | 14 | 5.578 | –0.014 | –1001b |
| Fundus clinical evaluation | 20,630 | 159 | 5.597 | 0.005 | 31,014c |
| OCT | 20,651 | 181 | 5.601 | 0.009 | 19,488 |
| 16. Initiating anti-VEGF treatment on the back of OCT positive findings, without confirmation with FFA | |||||
| Fundus clinical evaluation | 19,649 | 5.787 | |||
| Amsler | 19,751 | 103 | 5.736 | –0.051 | –2018b |
| Self-reported vision | 20,198 | 549 | 5.630 | –0.156 | –3514b |
| OCT | 20,403 | 754 | 5.830 | 0.044 | 17,256 |
| Visual acuity | 20,444 | 42 | 5.600 | –0.230 | –180b |
| 17. Higher testing cost scenario | |||||
| OCT | 19,634 | 5.830 | |||
| Fundus clinical evaluation | 19,872 | 238 | 5.787 | –0.044 | –5452b |
| Amsler | 19,987 | 353 | 5.736 | –0.095 | –3732b |
| Self-reported vision | 20,372 | 738 | 5.630 | –0.200 | –3688b |
| Visual acuity | 20,642 | 1008 | 5.600 | –0.230 | –4376b |
It should be noted that for all of these scenarios list prices for ranibizumab and aflibercept were applied. It is known that price discounts are available to the NHS via agreed patient access schemes for both of these drugs. The discounted price is confidential and, therefore, could not be applied in the model. However, a lower unit cost for ranibizumab and/or aflibercept would further improve the cost-effectiveness of OCT.
Probabilistic sensitivity analysis
Results of the probabilistic sensitivity analysis are provided in Table 50. They show consistency with the base-case deterministic results, with OCT dominating all other monitoring tests. The cost-effectiveness acceptability curve (Figure 28) indicates that OCT has a very high chance of being the preferred strategy across the range of cost-effectiveness thresholds applied by NHS decision-making bodies.
| Index test | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| OCT | 19,660 | 5.842 | |||
| Fundus clinical evaluation | 19,900 | 240 | 5.796 | –0.046 | Dominated |
| Amsler | 20,069 | 169 | 5.744 | –0.098 | Dominated |
| Self-reported vision | 20,659 | 590 | 5.644 | –0.198 | Dominated |
| Visual acuity | 20,900 | 242 | 5.615 | –0.227 | Dominated |
FIGURE 28.
Cost-effectiveness acceptability curve.
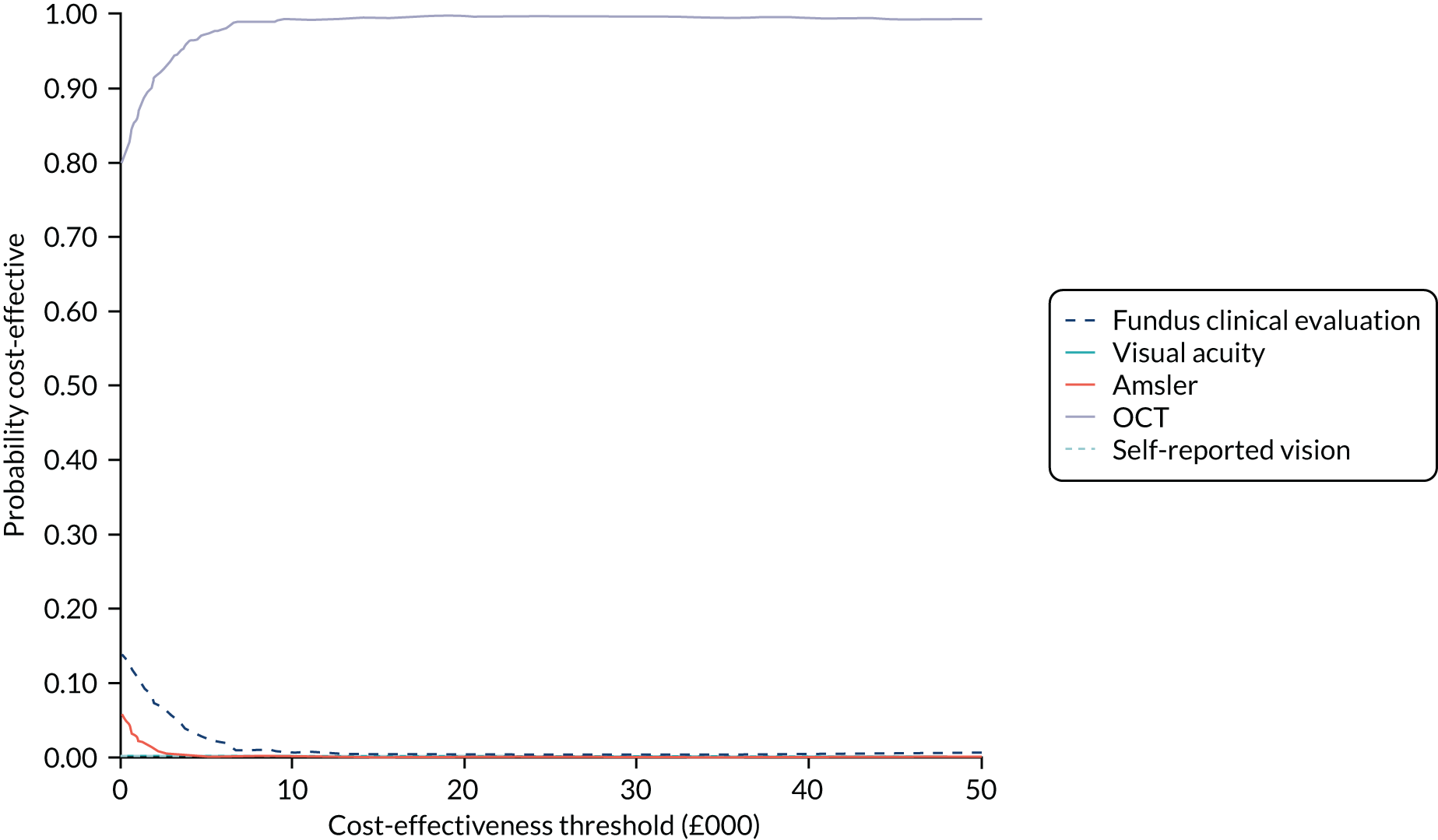
Secondary analysis using test combinations
In a secondary analysis, we assessed the cost-effectiveness when including two combination strategies: OCT or fundus clinical evaluation (test accuracy reported in Table 14), and a combination of all other tests apart from OCT (fundus clinical evaluation or Amsler or self-reported vision or visual acuity: test accuracy reported in Appendix 1). As for the single index tests, the specificity of each of the combination tests was also adjusted to account for the lack of independence across multiple observations within individuals to 0.9915 and 0.9673, respectively. The results are provided in Table 51. Under the base-case model specification, the OCT or fundus clinical evaluation combination resulted in a very small gain in QALYs compared with OCT alone. However, the increased costs resulted in the ICER being above the accepted thresholds for cost-effectiveness compared with OCT alone. The combination of all other tests, excluding OCT, resulted in higher costs and lower QALYs than OCT alone and the combination of OCT or fundus clinical evaluation.
| Strategy | Cost (£) | Incremental costs (£) | QALYs | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| Base-case model specification, with all positive tests confirmed by FFA | |||||
| OCT | 19,406 | 5.830 | |||
| Fundus clinical evaluation | 19,649 | 243 | 5.787 | –0.044 | –5560a |
| Test combination (OCT or fundus clinical evaluation) | 19,729 | 323 | 5.833 | 0.002 | 137,711 |
| Amsler | 19,752 | 23 | 5.736 | –0.097 | –233a |
| Self-reported vision | 20,199 | 470 | 5.630 | –0.202 | –2320a |
| Test combination (all tests except OCT) | 20,203 | 473 | 5.806 | –0.027 | –17,557a |
| Visual acuity | 20,445 | 716 | 5.600 | –0.233 | –3076a |
Discussion
The cost-effectiveness modelling reported in this chapter indicates that, of the five index monitoring tests, OCT results in the greatest QALYs for patients at the lowest cost to health-care and social care services over the lifetime of the simulated cohort. The increased costs that are associated with earlier detection and treatment initiation are more than compensated for by the reduction in the costs associated with severe visual impairment over the lifetime of the modelled cohort. This finding holds unless the costs associated with severe visual loss are removed; a significant proportion of patients who convert to nAMD are modelled to remain stable and not require treatment beyond 10 years post conversion to nAMD; post-conversion/pre-treatment acuity losses are substantially reduced; treating ophthalmologists wait for visual acuity to drop to ≤ 70 letters before initiating treatment; or no confirmatory FFA is assumed after OCT positive results to confirm the diagnosis prior to treatment initiation. Although OCT is no longer cost-saving in these scenarios, the ICER remains broadly favourable against cost-effectiveness thresholds typically applied in the UK NHS.
Strengths and limitations
The modelling reported in this chapter has several strengths. By adopting an individual simulation approach, individual visual acuity could be modelled as a continuous variable, which increased the sensitivity of the model to capture the impact of changes in visual acuity on health-related quality of life. Key inputs around time to conversion to nAMD and diagnostic accuracy were informed by the prospective EDNA study data, which supports the internal and external validity of the model findings. Furthermore, pre-detection testing costs and post-detection treatment costs were also based on the resource use that was reported by EDNA study participating centres and pragmatic NHS-based trials, ensuring generalisability of costs across the NHS. Finally, post-treatment visual acuity changes were carefully informed by a range of sources applicable to NHS routine practice and capture the expected differences in visual acuity trajectories by the degree of visual loss in the second eye prior to treatment initiation, ensuring that expected visual acuity gains from early identification and treatment were accurately captured.
All of the modelled strategies assumed a confirmatory FFA after a positive index test and before treatment. For this reason, the OCT strategy reflects the current NICE diagnosis guidelines to offer OCT to ‘people with suspected late AMD (wet active)’ and FFA to confirm diagnosis if OCT does not exclude neovascular disease. 11 Discussions within the EDNA project team revealed that clinical practice might vary towards initiating treatment based on OCT results without a confirmatory FFA. A scenario reflecting this was modelled. Although a strategy with OCT and without confirmatory FFA would be cost-effective at the usual UK cost-effectiveness thresholds,112 this strategy is no longer cost-saving. These results suggest that it may be more cost-effective to first confirm the diagnosis of nAMD in an OCT positive case using FFA, rather than initiating treatment for all OCT positive cases immediately. This is because of the chance of inappropriately treating small numbers of false positive patients with expensive anti-VEGF drugs for some considerable time. However, it may be that clinical judgement can be applied in practice to efficiently circumvent the need for FFA in some OCT positive cases without risking inappropriate overtreatment of false positive patients.
With respect to limitations, there is a paucity of available data on the rate of visual acuity loss in the immediate period following conversion to nAMD. Although the EDNA study was able to provide a reasonable estimate of the proportion expected to lose ≥ 10 letters within 3–6 months of conversion to nAMD, the increasing tendency to treat patients early (prior to significant vision loss) and limited observations on the few patients who experienced delayed treatment results in a degree of uncertainty around this important parameter. However, more conservative extrapolations were explored and the ICER for OCT remained favourable under these. It would take a relatively large proportion of nAMD cases to remain stable without treatment to undermine the cost-effectiveness of an OCT monitoring and immediate treatment strategy. Nevertheless, the rate of visual acuity deterioration in the immediate period following conversion to asymptomatic nAMD, prior to initiation of treatment, is one of the most important parameters in the economic model. This could benefit from further analysis of routine observational cohort data, where a significant proportion of early identified patients have undergone a period of monitoring prior to treatment initiation.
To ensure that the impact of visual loss in the EDNA study eye (second eye) was not underestimated, the second eye was assumed to represent the better seeing eye over the model time horizon. Although this will probably hold true for the majority of patients, there will be some patients whose visual acuity in the second eye drops below that of the first eye. Thus, the model may slightly overestimate the health-related quality-of-life benefits and cost savings of early detection and treatment. Given that a relatively small proportion of patients lost ≥ 10 letters prior to detection of nAMD in the EDNA study, there is remaining uncertainty relating to the sensitivity of the different diagnostic tests following substantial visual acuity loss owing to nAMD. Therefore, several assumptions were required to model the expected time to diagnosis for such patients. This included an assumption that the visual acuity test would detect all patients at the next monitoring visit if visual acuity dropped ≥ 10 letters from baseline (the trigger for the test as used in EDNA). This is a favourable assumption for the visual acuity strategy because it assumes that no such cases would be missed as a result of measurement error. Despite this, the model suggests that the visual acuity strategy results in the longest delay from conversion to detection and treatment and that it generates the lowest QALYs of all the strategies. A further assumption related to the rates of self-presentation between scheduled monitoring visits in the event of significant visual loss occurring. For the base case, it was assumed that anyone with a drop in visual acuity of > 30 letters would present immediately, while 50% of those with a drop in visual acuity of 10–30 letters would also present immediately. However, the model findings were not found to be particularly sensitive to assuming that the remaining 50% of the latter group also present more rapidly. Nevertheless, the degree of visual loss that has occurred when nAMD in the second eye is identified by changes in visual acuity, or subjective tests of visual function, remains an area of uncertainty that could benefit from further data collection. Again, the analysis of existing observational data sets could help to inform this parameter. Finally, there were limited data available to inform the rates of treatment discontinuation for stable disease and long-term rates of treatment re-initiation with contemporary anti-VEGF maintenance regimens.
Comparison with other studies
We are not aware of any other studies that have explicitly assessed the cost-effectiveness of alternative diagnostic monitoring strategies for nAMD in the second eye of patients being treated for nAMD in the first eye. A previous health technology assessment assessing the cost-effectiveness of OCT for the diagnosis and/or treatment monitoring of individuals with nAMD reported a high degree of uncertainty related to the optimal use of OCT in the nAMD monitoring pathway. 111 This was because of identified uncertainty around the diagnostic accuracy of OCT. The current study has provided robust evidence for the high diagnostic accuracy of OCT as a monitoring test for the early detection of nAMD in the second eye, and consequently provides evidence that when coupled with early treatment it is likely to offer a highly cost-effective strategy in this context.
With respect to treatment, many studies have evaluated the cost-effectiveness of anti-VEGF injections, which are widely accepted as a cost-effective use of NHS resources under agreed confidential patient access schemes. However, the majority of studies have assessed the cost-effectiveness of treating symptomatic individuals with visual acuity of 70 letters or below. We are aware of only one study that has assessed the cost-effectiveness of immediate ranibizumab treatment in patients with visual acuity better than 70 letters at detection compared with waiting for visual acuity to drop below 70 letters. 114 Using data from the nAMD UK database on patients with visual acuity above 70 letters at detection, Butt et al. 114 were able to estimate time to dropping below 70 letters without treatment and transition probabilities between visual acuity states for treated patients. Owing to the rapid decline in visual acuity in the early period after conversion to nAMD, their modelling predicted that immediate treatment would maintain better visual acuity and offer a cost-effective use of NHS resources in the short to medium term (2–10 years). Our results are consistent with respect to the finding that early detection with OCT followed by immediate treatment offers a highly cost-effective approach compared with relying on a drop in visual acuity to detect and treat nAMD in the second eye. With a 25-year horizon and the inclusion of costs associated with severe visual impairment (not included in the modelling reported by Butt et al. 114) we found that this strategy dominated the other alternatives assessed.
Policy implications
The data and modelling reported in this chapter strongly suggest that the use of OCT, compared with other available diagnostic tests, can lead to substantial reductions in the time to diagnosis and treatment of nAMD in the second eye of patients being treated for nAMD in their first eye. The early initiation of treatment in the second eye, based on FFA-confirmed OCT-positive findings, can be expected to maintain better visual acuity and health-related quality of life over time than the less sensitive monitoring strategies. Moreover, our modelling suggests that this strategy may be cost-saving in the long term compared with less-sensitive monitoring strategies that result in greater visual acuity loss occurring before treatment is initiated.
Chapter 8 Discussion
The EDNA study was a prospective longitudinal study of participants with a study eye free of nAMD at baseline and a confirmed diagnosis of nAMD in the fellow eye. Five easily performed, commonly used, non-invasive tests (self-reported vision and Amsler) and clinic-based tests (visual acuity, fundus clinical examination by slit-lamp biomicroscopy and spectral-domain OCT) consisting of measures of function and morphology were nominated as the index tests and were tested against the reference standard of FFA. Based on the detection on FFA of conversion to nAMD in the EDNA study eye, we have shown conclusively that spectral-domain OCT was found to exhibit high sensitivity and specificity compared with the reference standard of FFA. All other tests did not perform at the same level of sensitivity and specificity and were, therefore, unsuitable as replacements for FFA. Importantly, in the EDNA study data were collected during routine care with minimal study-specific requirements and, thus, the findings from this pragmatic study design are clearly applicable findings for routine clinical practice.
Principal findings
Diagnostic accuracy
The primary reference standard was the diagnosis of the onset of nAMD in the study eye based on interpretation of the FFA by the clinician at the clinical site. Against this reference standard, the sensitivity of OCT in the detection of new-onset nAMD was 91.7% (95% CI 85.2% to 95.6%) and the specificity was 87.8% (95% CI 83.8% to 90.9%). Two alternative reference standards that we prespecified were (1) the inclusion of an additional set of patients who did not have a FFA but for whom the clinician made the diagnosis based on any other available modality, and (2) a parallel determination of nAMD confirmed at a specialised reading centre. In this latter reference standard, no other information apart from images were available to the graders and, therefore, no associated cues, such as clinical history, could be used. We also tested the sensitivity and specificity of the index tests in three specified periods of follow-up: (1) the entire length of the study, (2) index tests limited to the last 6 months of follow-up and (3) limited to the last visit.
Regardless of the reference standards and the time periods over which index tests results were used, OCT had the highest sensitivity (82–92%) and was consistently and significantly superior to all other index tests. Although OCT performed extremely well for specificity with a range of 88–96%, fundus clinical examination and self-reported vision reached comparable levels. However, neither of these two tests performed well for sensitivity, which was significantly lower and not at an acceptable level (lower than 60% for fundus clinical examination and 10% for self-reported vision).
We also examined the sensitivity and specificity of pair-wise combinations of OCT with each of the other index tests. When either test was positive, sensitivity increased for most, with a best performance of 96% for the combination of OCT and visual acuity. However, specificity decreased for all combinations except for OCT combined with fundus clinical examination. This was not surprising given that the index tests apart from OCT were affected by a large number of false positives. When both of the pair-wise combinations of tests were positive, sensitivity was markedly reduced for all combinations compared with OCT by itself. With this approach, specificity was seen to increase for all combinations. We conclude from the analysis of combinations of index tests that OCT alone is sufficiently sensitive and specific for the detection of new-onset nAMD. The combination of all alternative tests against OCT further emphasised the value of OCT in a monitoring setting over the other tests.
Because the nAMD phenotype results in different morphological changes in the retinal tissue compartments, there can be differential impact on function. Notably, the RAP phenotype results in fluid pockets within the retina and, therefore, is more likely to result in distortion and worsening function as soon as nAMD develops. Therefore, we prespecified a subgroup analysis comparing CNV with the RAP subtype. OCT achieved 100% sensitivity in the detection of participants who developed RAP nAMD. However, none of the other index tests showed the same sensitivity or better specificity for RAP. Additional planned sensitivity analyses using two different reference standards for conversion to nAMD – (1) based on clinical examination without a FFA and (2) reading centre determination – also yielded results that were highly similar to that observed in the main analysis.
Patient-reported tests (Amsler/self-reported vision)
Although OCT allows for a non-invasive appreciation of macular morphology, it requires appropriate instrumentation and technical expertise for acquisition and expert clinical opinion for interpretation. Therefore, we also included two index tests that are easily self-performed, that reflect retinal function and are widely used in practice. Participants undertook the Amsler test to determine the onset of distortion and, using a questionnaire, reported any reduction in vision in the EDNA study eye. 28,31 It was notable that neither of these tests achieved even moderate levels of sensitivity or specificity. To some extent, the finding that the Amsler test was insensitive to new-onset neovascularisation is not surprising. Despite ongoing use by the optometric community and ophthalmologists, the inadequacy of the Amsler test for detecting onset of nAMD is extensively recorded in the literature, with sensitivities as low as 34%. 115 A systematic review and meta-analysis evaluating the diagnostic accuracy of the Amsler chart produced a pooled sensitivity of 78% (96% CI 64% to 87%). 116 The Amsler tests’ failure is likely because of perceptual completion, a well-recognised visual phenomenon in which surrounding features provide sufficient contextual information that the scotoma or blind spot is ‘filled in’ in the absence of neural input. 117
We were surprised at the poor sensitivity of self-reported symptoms of visual change as a marker for the onset of nAMD in the EDNA study eye. The definition of a positive test was a self-reported recognition that vision in the study eye was ‘much worse’ since the previous visit. We anticipated that the better functional status of the study eye than the fellow eye would have resulted in a greater awareness of the onset of distortion owing to nAMD. Changing the definition to include a status of either ‘worse’ or ‘much worse’ increased sensitivity only from 4.2% to 15.3%. Our finding of a poor sensitivity for self-reported vision change even in the better seeing eye is, therefore, of interest because studies investigating patient-reported outcomes during AMD treatments have consistently observed greater concordance with disease severity in the better seeing eye than that of the worse seeing eye. 118 Our data are at variance with the results from the Age-related Eye Disease Study (AREDS),119 which evaluated the responsiveness of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) to progression to advanced AMD. 25 The overall score was responsive (t = 14.0; p < 0.001) and showed moderate discrimination (AUC 0.74), and the subscales most sensitive to progression to either nAMD or geographic atrophy included general vision (AUC 0.65), near activities (AUC 0.73) and driving (AUC 0.72). One possible explanation is that, similar to the insensitivity of the Amsler in the detection of nAMD onset, fluid accumulation and leakage, which is more likely to be associated with metamorphopsia, is counteracted by perceptual filling-in rather than photoreceptor loss. However, it is also possible that the wording of our question was suboptimal for ascertaining vision changes. Our findings suggest that, although patient-reported outcomes are important in intervention trials to assess improvements in vision-related quality of life, they should not be used as tools for the detection of progression to nAMD. In this context, our findings also highlight the importance of regular eye examinations in the most at-risk age groups because relying on detection based on self-reported symptoms will almost certainly result in a lower detection rate and presentation at a more advanced stage of the disease.
Visual acuity
Visual acuity is another easily performed test and reflects central macular function. A visual acuity drop of 10 letters, representing a fall of two lines on the ETDRS chart, has been recognised as a clinically important change in function; in the EDNA study this was selected as the trigger for a FFA. However, the change in visual acuity in the EDNA study eye proved to be a poor marker for conversion to nAMD. visual acuity measurements were available at frequent time intervals in EDNA participants because fellow eyes were on active treatment for nAMD, which necessitated regular visits to clinics for assessment and intravitreal injections. Changes in visual acuity can occur because of medial opacities (cataract) and/or from the development of focal areas of outer retinal atrophy in the macular tissues. Degeneration of drusen leaving behind focal patches of atrophy is a recognised component of the pathogenetic pathways leading to nAMD. In fact, we observed that around half of all EDNA study eyes were graded as exhibiting foci of macular atrophy at the time of conversion to nAMD. This type of atrophy may be accompanied by imperceptible slow reduction in visual acuity, which is not recognised by the patient. It is also of note that the area of the neural sub-retina serving high-contrast distance visual acuity is small (< 1 °) and in some cases the location of the neovascularisation falls outside and in such cases visual acuity may be unaffected. 120 Changing the definition of a positive visual acuity test to a drop of 15 and 30 letters did not alter the sensitivity or specificity of the test.
Morphology
We performed detailed imaging in the EDNA study participants at initial presentation. Thus, we were able to compare visual acuity and morphological features of the nAMD lesion in the first eye with those at detection of nAMD in the EDNA study eye. We expected that first-presenting eyes would have only moderate impairment of vision and that the nAMD lesions would not be advanced because (1) we selected participants only at their very first presentation with nAMD and (2) of the increasing awareness of AMD in the UK population with the introduction of anti-VEGF treatments, which, in turn, resulted in a recognition at the primary-care level of urgent referral pathways into specialist retinal clinics. 1,121,122 In EDNA study eyes at nAMD detection, the average visual acuity was 74 letters compared with 54 letters in corresponding fellow eyes at initial presentation. Furthermore, first-eye lesions even at initial presentation often had severe exudative features. Comparing the features of nAMD lesions in study eyes at conversion with the features of fellow eyes at initial presentation, central subfield thickness was on average 150 µm less, lesions were half to one-third smaller and more likely to be juxta foveal or extra foveal, explaining the large differences in visual acuity between these groups. In addition, and of note, was the presence of atrophy in the macula in some 10% of eyes indicating that neural cell loss had already occurred. However, there was a low frequency of fibrosis in the nAMD lesion confirming that our selection criteria eliminated patients with long-standing disease. A high proportion of advanced nAMD lesions develop fibrosis and macular atrophy despite anti-VEGF therapy and, therefore, treating them while smaller, and prior to involvement of the fovea, may indeed prevent permanent foveal damage. 10,23 Vision lost to nAMD cannot be restored to normal levels even with intensive anti-VEGF therapy, as shown by studies that have examined large repositories of data collected in the real world. 9
The foregoing observations highlight key novel points justifying the need for early diagnosis and treatment of nAMD. Data from electronic medical records show that presenting visual acuity is a predictor of final visual acuity. Early diagnosis results in an average acuity that can be as much as 20 letters better than that of even a marginally later diagnosis. The institution of treatment at a stage when neither fibrosis nor atrophy has supervened and when lesions are small and less exudative can allow the preservation of visual acuity at good levels. 14
Prediction model
There were 145 conversions to nAMD (using secondary reference standard 1) out of 489 participants in the EDNA study, with the data available on their outcome used to develop the prediction model. Over the median follow-up duration of 34 months, we observed an approximate conversion rate of 30%. This rate was in keeping with the expected conversion rates observed in previous reports. For example, the risk of conversion to nAMD in the unaffected eye in individuals with unilateral nAMD is around 10% in the first year and some 50% by 5 years. 68
The predictors that were significant and used in the final model included lower visual acuity, cigarette smoking, family history of AMD, thin choroid, retinal thinning, presence of retinal pigmentary abnormalities, higher category of the most frequent or maximum size of drusen, and presence of nodular drusen or both nodular and reticular pseudodrusen. Age was not observed as a significant predictor. The mean age of this cohort was 77 years, which is no different from most other studies on nAMD. It is notable that age has not been shown to be a consistent risk factor. The availability of longitudinal data sets from clinical trials that have enrolled patients with nAMD has been exploited and used in secondary analyses as these repositories are generally robust with high quality information from both eyes. An analysis of the Harbor study, which analysed risk factors for the development of nAMD in fellow eyes of patients with nAMD in the first eye only, did not include age in the final model. 69,70 By contrast, a recent analysis of the VIEW (VEGF trap-eye: investigation of efficacy and safety in wet AMD) 1 and 2 studies showed that increasing age, female sex and lesion size in the nAMD eye were among the predictors of conversion to nAMD in unaffected fellow eyes. 71
It has been recognised for many years that the presence of nAMD in the first eye is by itself a strong risk factor. The simplified AREDS scale used only macular phenotype to predict progression from the non-neovascular stage to nAMD. In this scale, 2 points were assigned if one eye had nAMD and additional points could be given if the fellow eye had large drusen (1 point) and retinal pigmentary irregularities (1 point), underscoring the strength of nAMD in one eye as a key risk factor. 73 It was shown that having nAMD in one eye carries a two- to three-fold greater risk for conversion to nAMD than that of a state of bilateral intermediate AMD. 73
The progression from unilateral to bilateral nAMD in the AREDS cohort was seen in 35.4% of patients over 5 years if the fellow eye had additional risk factors of the presence of large drusen and/or pigment abnormalities. This figure increased to 76.8% at 10 years. Therefore, large drusen and RPE abnormalities are considered strong risk factors for conversion to nAMD. 75–79 Where information from OCT was available, hyper-reflective foci and drusen were the strongest contributors to predictive models. 70,80 Ferrara et al. 117 compared 40 patients who did not progress from unilateral to bilateral nAMD with another 40 patients who did progress. These patients were matched on an AMD baseline grade and follow-up interval and the comparison showed that OCT features, including total retinal thickness, pigmentary hyper-reflective material, nascent geographic atrophy and choroidal vessel abnormalities, were independently associated with progression to either form of advanced AMD (geographic atrophy or nAMD). 117 Given that both forms of advanced AMD were included in this small study, it is difficult to decipher the specific risks of nAMD. For Submacular Surgery Trials participants who had a FFA-confirmed absence of nAMD in one eye at baseline, drusen size, focal hyperpigmentation and non-foveal geographic atrophy were significantly and independently related to progression to nAMD within the 2-year follow-up. 123 No non-ocular characteristic (age, sex, history of hypertension or smoking) or ocular feature of the nAMD eye at baseline were predictive of progression to nAMD. An artificial intelligence approach to OCT feature analysis highlighted drusen volume and presence of hyper-reflective foci as significant predictors of progression to nAMD using spectral-domain OCT images from the HARBOR study. 124 Although reticular pseudodrusen are known risk factors for nAMD, they ranked only 11th out of 15 OCT risk features for conversion to nAMD in the fellow eye of the HARBOR participants when the model was developed using machine learning. 70 This may be because providing the ground truth for reticular pseudodrusen is challenging and they can disappear over time but the eye will retain the high risk for conversion to nAMD.
The ocular predictors in our model included nodular drusen with or without reticular pseudodrusen. The presence of nodular drusen in fellow eyes increased the risk of conversion to nAMD by six-fold compared with the absence of drusen, whereas the combination of nodular drusen and reticular pseudodrusen almost doubled this risk, highlighting that the presence of both drusen types was the strongest predictor for conversion. In a small study that observed 83 patients over a mean follow-up time of 2.8 years, Nathoo et al. 115 showed that drusen load (area and volume) was associated with progression to nAMD.
Decreasing visual acuity was also a predictor in our model, in keeping with previous studies. 78,81 When considering non-ocular risk factors, our models contained smoking status and family history of AMD. We did not have data on genetic risk. Seddon et al. 72 included a combination of demographic, genetic and environmental factors, and macular phenotype on colour photographs to develop and validate a model to predict progressors compared with non-progressors in the AREDS cohort. However, progressors were defined as both bilateral early or intermediate AMD and those with advanced AMD in one eye at baseline who progressed to advanced AMD.
Health economic analysis
The results of the base-case economic analysis show that OCT is expected to generate the greatest number of QALYs per patient (OCT, 5.830; fundus, 5.787; Amsler chart, 5.736, self-reported vision, 5.630; and visual acuity, 5.600) for the lowest health-care and social care costs (OCT, £19,406; fundus, £19,649; Amsler chart, £19,751; self-reported vision, £20,198; and visual acuity, £20,444) over the lifetime of the simulated cohort. The increased treatment costs associated with earlier detection with OCT were more than offset by the reductions in costs associated with severe visual impairment, compared with less-sensitive tests that resulted in greater visual acuity loss prior to detection and treatment. OCT was found to dominate the other tests or to have an ICER below accepted cost-effectiveness thresholds across the range of scenarios analyses explored. The probabilistic sensitivity analysis indicated a high probability of OCT being cost-effective across a range of cost-effectiveness thresholds typically applied by NHS decision-making bodies.
Patient perspectives of monitoring and diagnosis of neovascular age-related macular degeneration
Three patient representatives within our steering committee who had nAMD and experience of being monitored for disease in the second eye fed back their opinions and perspectives on the diagnosis of and monitoring for nAMD. Throughout the study, they highlighted a number of concerns and suggestions from a patient perspective.
Of particular concern was the poor performance of the patient-reported index tests (Amsler, visual acuity and patient-reported vision), especially considering that all three patient partners felt that self-monitoring had been very important to detect changes in their own eyesight. A highlighted challenge for patients (with and without nAMD) is how to monitor their vision consistently and the need for patient tools to support consistent self-monitoring so that it can be effective. Our PPI partners regularly highlighted a need for better education for the public on self-awareness of their eye health and understanding the importance of self-monitoring within the health-care setting. Considering the average age of the participants (aged > 70 years), the difficulties that some older people face in doing routine tasks and/or in following processes, and the possibility that vision change may be perceived to be just because of old age, may in some way explain the poor performance of the patient-reported index test results. A further challenge for patients is to understand which changes in vision are significant, especially because vision may be variable. Any well-meaning feedback from clinical teams reassuring the patient that their perceived deterioration is not clinically significant may reduce the incentive for the patient to monitor and report further deterioration. Further research to identify patient tools to help with self-monitoring and awareness in those patients not being routinely monitored was felt to be of benefit.
Strengths and limitations
Diagnostic study
A number of strengths can be highlighted for the diagnostic study. The EDNA study was a large, multicentre, prospective, comparative, diagnostic accuracy study that evaluated routinely used diagnostic tests in a monitoring setting under standard of care. The benefit of the large sample size is reflected in the precision with which sensitivity and specificity were estimated, and the large number of centres gives confidence in the generalisability of findings to the NHS.
It was a strength that the interpretation of the index tests was undertaken by investigators who were unaware of the results of the reference standard. The compliance with conducting index and reference standard tests in accordance with the study protocol was extremely high.
We used a reference standard that represents standard care, that is FFA interpreted by an expert clinician. We also explored the performance of the index tests using a reading centre to interpret the FFA results as an enhanced reference standard. It was notable that the discordance between the clinician diagnosis of nAMD and the reading centre diagnosis of nAMD was extremely low.
Among the limitations, we recognise that the population enrolled in the EDNA study consisted of subjects at high risk of developing nAMD in the second eye because they presented with nAMD in one eye, and precluded patients with moderate or poor vision from inclusion in the cohort. Furthermore, we did not have a cohort of older patients with bilateral large drusen in whom the diagnosis might have been confounded with other common exudative maculopathies, such as diabetic macular oedema and vein occlusions. Therefore, the diagnostic performance reported here is pertinent to the characteristics of the included cohort and may be different in an unselected population without any history of nAMD.
The constraints of clinical practice, including variations in monitoring practice between sites, variable gaps for individuals between visits and study-specific methodology (utilisation of non-triggered 18 month and ‘exit’ FFAs), all affected the ability to estimate the survival curve (time to conversion) accurately. In particular, estimated survival beyond 30 months should be interpreted cautiously. Although we do not know what the true conversion point of patients is, only that they converted at some point between FFA results, the sensitivity analyses did not change test conclusions.
We were not able to rigorously assess the reading centre OCT assessment owing to the concurrent use of the FFA assessment in the reading centre. This would have helped inform about the inherent differences between OCT and FFA compared with differences between one-off individual assessment (i.e. clinical site vs. reading centre).
Health economic modelling
The modelling has several strengths. By adopting an individual simulation approach, this allowed for individual visual acuity to be modelled as a continuous variable, increasing the sensitivity of the model to capture the impact of changes in visual acuity on health-related quality of life. Key inputs around time to conversion and diagnostic accuracy were informed by the prospective EDNA study cohort, which supports the internal and external validity of the model findings. Furthermore, pre-detection testing costs and post-detection treatment costs were also based on resource use reported by EDNA participating centres and pragmatic NHS-based trials, ensuring generalisability of costs across the NHS. Finally, post-treatment visual acuity changes were also carefully informed by a range of sources applicable to NHS routine practice and capture expected differences in visual acuity trajectories by the degree of visual loss in the second eye prior to treatment initiation, ensuring that expected visual acuity gains from early identification and treatment were accurately captured.
With respect to limitations, there is a paucity of available data on the rate of visual acuity loss in the immediate period following conversion to nAMD. Although the EDNA study was able to provide a reasonable estimate of the proportion expected to lose ≥ 10 letters within 3–6 months of conversion, the increasing tendency to treat early (prior to significant vision loss) and limited observations on the few patients who experienced delayed treatment results in a degree of uncertainty around this important parameter. However, more conservative extrapolations were explored and the ICER for OCT remained favourable under these. It would take a relatively large proportion of nAMD cases to remain stable without treatment to undermine the cost-effectiveness of an OCT/immediate treatment strategy.
Prognostic model
The EDNA study enabled us to develop a predictive model of developing nAMD in the study eye because the EDNA study provided a well-standardised longitudinal cohort with a large number of potential risk factors ascertained using the standardised EDNA data capture forms. There were enough conversions to nAMD in the EDNA study to develop a prediction model. We used candidate predictors that are easily obtainable in routine ophthalmic practice and we had few missing data on predictors. The misclassification of ocular predictors is unlikely because the retinal images were graded by investigators at the study site, as well as by graders in the reading centre.
There were a number of limitations to this modelling work. The model applies only to second eyes of participants with nAMD in their first eye and, therefore, it is a selected population and the findings should be interpreted in that light. In particular, the lack of prognostic value of age is probably because of the effect of focusing on people who already have nAMD in one eye. Other variables not readily collected might have resulted in a better prognostic model. However, the known strong markers are all included in the model. A higher discriminatory performance had been anticipated because what we believed were important characteristics in the ocular environment were included. However, we did not have information on the genetic profile of participants, and the carriage of well-characterised genetic polymorphisms is known to be important in increasing risk and rapidity of progression of nAMD. This and additional OCT parameters of the fellow nAMD eyes may merit further investigation as candidate predictors; intraretinal fluid at baseline has been shown to be a predictor of conversion of unaffected eyes to nAMD. The c-statistic (discriminative ability) was only 0.66, so the performance does not suggest that this model would have clinical application at this point without further improvement. However, this model adds to the growing literature on risk predictions for conversion in the second eye and the predictors used in this model have been included in most models but not together.
We did not include genetic markers in our model; however, family history of AMD is a predictor in our model and may be an easier predictor to apply in clinical practice than genetic make-up. Including genetic risk in prediction models in nAMD remains a challenge owing to its multifactorial aetiology and gene–environment interactions. 74 Despite this, family history, our closest surrogate to genetic risk, remained significant in our final model. Blood samples collected during this study will enable the exploration of the predictive ability of genetic markers in the future. For example, a recent study involving a collaboration between DeepMind (DeepMind, London, UK) and Moorfields Eye Hospital has used deep learning to predict the conversion to nAMD from OCT within a 6-month window, with sensitivity of 80% at 55% specificity and sensitivity of 34% at 90% sensitivity. Higher conversion rates were seen in patients with larger drusen volume, FPED and hyper-reflective foci. 125
Implications for health care
The EDNA study results show that OCT is the most accurate diagnostic tool to assess early nAMD in the fellow eye of people with unilateral AMD. It also shows that, compared with OCT, visual function measures have low accuracy in detecting early nAMD and these included a drop of visual acuity by 10 letters, patients’ own perception of visual deterioration and a positive Amsler test. The EDNA study has shown that there is no evidence to support the use of the Amsler test as a monitoring test in the second eye of patients with nAMD in the first eye who are managed in hospital eye services. The Amsler test may still have some value in detecting the onset of nAMD in older populations who are at risk of developing this condition and who are not being managed in hospital eye care services. It was also notable that self-reported visual function and clinic-measured visual acuity showed poor sensitivity in detecting the onset of nAMD. Our findings, therefore, call into question the current reliance of community-based monitoring systems, such as those commonly used in optometric practices.
In addition, our findings also have implications for the way that we monitor nAMD patients while they are in follow-up for treatment of the first eye. Many clinics do not regularly monitor the second eye with OCT at every visit for a number of reasons, but not because of lack of capacity. The time taken to capture a typical macular OCT scan once the patient has had an explanation of the procedure and is positioned for image capture is approximately 2–3 minutes per eye. Clinicians who were consulted during the EDNA investigator meeting stated that the marginal cost of conducting an OCT in the second eye might be negligible because to do otherwise requires the technician to take time to ensure that they are scanning the correct eye. In addition, all 18 out of the 24 NHS participating centres that responded to our costing survey reported that they routinely used OCT to monitor the treated eye, as well as the at-risk second eye. Although it is routine practice to use OCT to guide treatment decisions in the treated eye, the monitoring frequency in the second eye is governed by the monitoring frequency in the treated eye. In the economic modelling, we included a monitoring frequency in line with the average observed frequency of monitoring in the EDNA study, which was in accordance with local clinical practice and reduced over the monitoring period.
However, a variety of different clinical care pathways and strategies have been instituted in different clinical sites (personal communications from study PIs to study team) to streamline and improve patient throughput, which then could result in reduced OCT monitoring of both eyes. For example, for patients who present with nAMD in one eye and who are commenced on treatment, the drug label in some instances recommends a review with OCT at completion of the loading phase and then at 1 year. This care pathway could result in patients not receiving an OCT in either their first eye or their at-risk eye for up to 9 months. The alternative strategy of the treat-and-extend protocol for those with nAMD in one eye could also result in the patient not having to attend for intervals that might exceed 3–4 months. In addition, if patients are discharged into the community after failed treatment in the first eye, the second eye may be monitored only at very long intervals. Finally, clinical sites have reduced direct patient contact to a minimum owing to concerns about COVID-19, which results in reduced monitoring of the at-risk eye.
The EDNA study shows that if second eyes are also monitored with OCT at every clinic visit that the patients attends for treatment, earlier nAMD will be identified. The study also suggests that if regular monitoring is carried out by OCT in the fellow eyes and early nAMD is identified, treatment can be initiated before visual acuity deteriorates, which enables the second eye to be maintained at good levels of visual acuity compared with the first eye, which usually present with poorer visual acuity when the patient is already symptomatic.
The findings of the EDNA study are of relevance globally and especially in the UK given that the monitoring strategy outlined in the NICE guidelines places high reliance on self-reporting of change in visual function, which has been shown in the EDNA study to have low sensitivity and only moderate specificity. 11 Because the risk of progression to nAMD in the unaffected fellow eye of a patient with nAMD in one eye may be as high as 50% within 5 years of diagnosis, the use of OCT as a monitoring tool has considerable potential for cost savings. 72 Treatment for nAMD imposes a significant burden for patients, their carers and hospital facilities, and a diagnostic test of such high sensitivity and specificity has important implications in terms of acceptability to patients and can help streamline care pathways and reduce the impact on hospital intravitreal injection services. In terms of diagnostic accuracy, based on our findings presented here, a revised strategy to monitor such patients with OCT should be considered. Our study did not enrol participants with bilateral early AMD who would be expected to have good vision in both eyes and a low risk of between 1% and 5% over 5 years of conversion to nAMD. 72 In people with bilateral early AMD, screening for conversion to nAMD would be inappropriate and probably not cost-effective. Furthermore, to consider recommendations based on EDNA study findings in this category of bilateral early AMD patients who are normally routinely seen annually in the community by optometrists would be inappropriate.
The health economic data and modelling strongly suggest that the use of OCT, compared with other available diagnostic tests, can lead to substantial reductions in the time to diagnosis and treatment of nAMD in the second eye of patients being treated for nAMD in their first eye. The early initiation of treatment in the second eye, based on FFA-confirmed OCT positive findings, can be expected to maintain better visual acuity and health-related quality of life over time than that of less-sensitive monitoring strategies. Moreover, our modelling suggests that this strategy may be cost-saving in the longer term compared with less sensitive monitoring strategies that result in greater visual acuity loss occurring before treatment is initiated.
Recommendations for research
-
The feasibility of using OCT for the diagnosis and management of nAMD in a primary care or home monitoring setting should be investigated. Given the clear findings in this study of OCT successfully identifying nAMD in the second eye, different models of providing care should be developed and tested. The use of OCT as a monitoring device in a community setting will pose a number of challenges. This is because to detect the onset of nAMD promptly it is likely that the susceptible population will have to be screened at a rate similar to that achieved in the EDNA study, that is ranging from every 1.5 months during the first 6 months to every 3.3 months by month 36. Therefore, any study that is specifically designed to address early detection will have to use technology available in the patient’s own home. Devices for OCT home monitoring are currently undergoing testing and have shown promise. However, there are multiple potential obstacles, including transmission of the OCT images either to the retina clinician or to a monitoring hub.
-
Further development of the risk prediction model, including exploration of morphological biomarkers and additional imaging characteristics, should be undertaken to assess if model performance can be improved. Spectral-domain OCT images are of high resolution and allow an assessment of the integrity of the outer retinal layers, and the data can also be subjected to artificial intelligence analysis.
-
Longer-term visual outcomes and subsequent treatment strategies for patients with nAMD need to be assessed. The current lack of longer-term data leads to uncertainty in health economic modelling comparisons. This could be achieved using existing patient cohorts.
-
The role of artificial intelligence algorithms for improving the diagnosis and monitoring of nAMD should be explored. New imaging technologies are already being developed using artificial intelligence methods for identifying nAMD. The new technologies need to be assessed using rigorously collected patient cohorts.
-
The performance of diagnostic tests for AMD should be assessed in people aged ≥ 70 years who do not have nAMD in either eye and, therefore, who have a risk of developing nAMD in both eyes.
Conclusion
The EDNA study has clearly and rigorously demonstrated that OCT is the most accurate diagnostic tool to assess early nAMD in the second eye of people with unilateral AMD. Health economic modelling also strongly suggests that the use of OCT can lead to substantial reductions in the time to diagnosis and treatment of nAMD in the second eye and lead to cost savings. In addition, the EDNA study has identified clear risk factors for the development of nAMD in the second eye, which should be tested further.
Acknowledgements
Independent members of the steering committee
We thank the members of the EDNA steering committee, including our three patient representatives, for their valued contribution and oversight of the study and for their attendance at regular EDNA investigator days and steering committee meetings: Rupert Bourne (chairperson), Anat Loewenstein, Michael Bowen, Yemisi Takwoingi, Felicia Hart, Andrea Rodgers, Michael Warren and Cathy Yelf.
Project management group
The grant holders were Katie Banister, Augusto Azuara-Blanco, Usha Chakravarthy (chief investigator), Jonathan Cook, Heinrich Heimann, Ruth Hogg, Craig Ramsay (co-chief investigator), Graham Scotland and Sobha Sivaprasad.
The study team and CHaRT support were Lorna Aucott, Claire Cochran, Fernanda Dias da Silva, Maria Dimitrova, Gillian Ferry, Mark Forrest, Pauline Garden, Beatriz Goulao, Lorna Henderson, Rodolfo Hernandez, Pamela Jamison, Charlotte Kennedy, Neil Meharg, Alison McDonald and Samantha Wileman.
Other acknowledgements
Our thanks go to the following groups for their assistance in conducting the EDNA study.
We extend our thanks to all of the participants who took part in the study and without whom the study would not be possible.
We are grateful to all of the staff at recruitment sites who facilitated recruitment and data collection (listed below) and contributing to regular EDNA investigator meetings.
We thank the team at the reading centre (Central Angiographic Resource Facility) who conducted the grading of images for the EDNA study. Particular thanks to Clare Newell, Karleigh Kelso, Alyson Muldrew, Michelle McGauchey, Vittorio Silvestri, Tunde Peto, Savita Madhusudhan and Konstantinos Balaskas. Thanks to Philip Earle and Cieran Ennis from the BioBank at Queen’s University Belfast.
We are grateful for the support from the FASBAT study team, which was led by Richard Gale and trial manager Mia Porteous.
We are thankful for the administrative assistance from Zoe Batham and Dianne Dejean; the construction and maintenance of the EDNA study website and data entry portal by the CHaRT support team; the assistance with managing the study budget from Louise Cotterell, Anne Buckle, Sara Shields and Paula Plant; and the proofreading from CHaRT trial managers and data co-ordinators.
The Health Services Research Unit (HSRU) and the Health Economics Research Unit (HERU) are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Staff who facilitated recruitment and data collection
Belfast
Deirdre Burns, Guiseppe Casalino, Usha Chakravarthy (PI), Rebecca Denham, Lesley Doyle, Karen Gillvray, Katie Graham, Jonathan Keenan, Lisa Kelly, Nuala Jane Lavery, Georgios Mangioris, Orla McNally, Tunde Peto (PI), Louise Scullion, Vittorio Silvestri, Georgina Sterrett, Paul Wright and Graham Young.
Bradford
Helen Devonport, Faruque Ghanchi (PI), Nicci Hawes, Charlotte Hazel, Hayley Higgins, Zeid Madanat, Sarah Moss and Roopa Setty.
Bristol
Monalisa Bora, Claire Buckland, Colin Chu, Julie Cloake, Georgios Farantzos, Eleanor Hiscott, Sameer Hussein, Alice Johns, Danielle Lee, Rebecca Lunn, Stephanie Petrausluas, Adam Ross (PI), Serena Salvatore and Helen Talbot.
Cardiff
Ayad Al-Bermani, Sanjiv Banerjee (PI), Emma Dimond, Helen Hill, Kate Humphreys, Rhianon Reynolds, Pravin Sandanshiv, Chris Tetley, Hayley Westwood, Visith Sidath Wijetilleka, Caroline Young and Tafadzwa Young-Zvandasara.
Colchester
Giles Baggiony-Taylor, Elaine Chinery, Christopher Ellis, Judith Field, Alison Ghosh, Nicola Hopkins (PI), Tasneem Khatib and Jignesh Patel.
Edinburgh
Ali Al-Ani, Ana Maria Armbrecht, Shyamanga Borooah, Marion Brannan, Peter Cackett, Meg Das, Baljean Dhillon (PI), Margaret Frost, Mark Hope, Ash Khan, Margaret McDonald, Douglas Mitchell and Louise Ogilvie.
Frimley Park
Tayaba Akhter, Nadir Ali, Harbhajan Arora, Nadia Azad, Elizabeth Baker, Reynette Baroman, Leena Bhat, Manju Chandran, Maheswari Chekuri, Gloria Crawford, John Deligiannis, Mohamed El-Hefrim, Fernando Esposito, Sarah Gee, Petya Gencheva, Talat Gondal, Harinder Grewal, Jessica Haney, Ali Hassan, Wendy Hockney, Victoria Hodgson, Charlene Humphries, Psiyanta Kakai, Vijay Kakkar, Jaskiran Kaur Sandhu, Radhika Kotecha, Teena Kunnath, Stella Lee, Sally Mathew, Lesley McAllister, Brendan McIlhargey, Jessica McKean, Gail McKenzie, Lorraine McLoughlin, Geeta Menon (PI), Prashant Mistry, Narendran Nair, Karima Nesnas, Lorraine North, Rohina Patel, Ganga Pathinayake, Abigail Raguro, Darryl Ratiram, Rani Retnamma, Deana Robson, Anitha Sethumadhavan, Priyanka Srikanth, Kathryn Walters, Debbie Young, Fani Zacharaki and Louisa Zouita.
Gloucester
Fadi Alkherdahji, Jade Ayland, Liz Bristow, Victoria Edgeworth, Helen Fisher, Emily Fletcher, Rachel Healy, Katerina Ivanova, Seb James, Robert Johnston (PI), Vanessa Jones, Antonis Kaintatzis, Laura Lodge, Jennifer Mackenzie, Quresh Mohamed, Dawn Phillips, Jeffrey Pick, Claire Porter, Bridget Rosser, Peter Scanlon (PI) and Karen Townsend.
Harrogate
Bushra Al-Deiri, Tony Burton, Oana-Gabriela Graves, James Featherstone, Theodora Georgouli, Mamta Gupta, Natalie Hollingsworth, Sarah Mackenzie (PI), Karen Martin, Tracy Nixon, Kim Oses-Fred, Eve Panesar, Clare Stemp, Jo Varley and Gavin Walters.
Hillingdon
Muhammed Aliahmed, Balisee Beruessa, Doris Bilayon, Teresita Bolanos, Richard Cheong-Leen, Sheena George (PI), Charlene Hamid, Darren Hanumunthasu, Jason Ho, Neo Johnson, Kash Khuttan, Dhakshi Kumar, Ashley Long, Sonal Mehta, Rahila Naureen, Angelos Raytis, Anju Shrestha, Georgios Sotiropoulos, Denise Wheeler and Elizabeth Woodstock.
Hull
Seem Arora, Angela Atkinson, Atiq Babar, Helen Cook, Sally Cooper, Mark Costen, Louise Downey (PI), Kala Gopalakrishnan, Helen Hobman, Krishnappa Madhusudhana, Anish Mani, Christine Mayman, Muhammad Shaikh, Naomi Teal, Yvonne Waudby and Naeem Zaman.
James Paget University Hospital
Ben Burton (PI), Debbie Busby, Stephanie Cotton, Kelly Evers, Katherine MacKintosh, Helen Nutt, Lucy Palmer, Lesley Parsons, Loretta Poundall, Umair Qidwai, Mya So, Emma Stimpson, Thomas Webber and Celia Whitehouse.
Leeds
Samantha Bird, Frances Cassidy, James Cook, Swetha Devabhakthini, Mohammed Jama, Martin McKibbin (PI), Raj Mukherjee, Mike Stockton, Charmain Tidswell and Alice van Lare.
Leicester
Mandy Babbington, Kayleigh Brown, Sreekala Burgula, Patrina Christian, Melanie Chrystal, Sue Cockburn, Lee Daines, Theo Empeslidis (PI), Howard Fairey, Andrew Farmer, Konstantina Gorgoli, Vasileios Konidaris, Bhavesh Mistry, Eshmael Palmer, Michelle Rickerby, Lauren Rybicki and Konstantinos Tsaousis.
Liverpool
Julia Baxter, Alina Cordos, Karen Hawkins, Heinrich Heimann (PI), Vivian Ho, Oana Hotu, Samantha Jackson, Robert Jardine, Darina Koneva, Jae Ku, Pauline Lenfestey, Andrea Madden, Savitha Madhusudhan, Ira Mahac, Ian Pearce, Jayne Schumacher and Sandra Taylor.
Manchester
Mohammed Abid, Amy Aldridge, Tariq Aslam (PI), Aaron Bain, Konstantinos Balaskas, Rachel Bambrick, Goncalo Bento, Jay Brown, Ryan Carney, Rosalind Creer, Natalie Fox, Thomas Hamper, Mania Horani, Narcisa Ianopol, Binu John, Emma Linton, Katie McGuiness, Harriet Ndu-Melekwe, Jeremy Parkes, Laura Perry, Raisa Platt, Afeefa Rasheeth, Leanne Richards, Amy Stone (PI) and Daniel Todd.
Moorfields
Joynal Abadin, Nadine Abdelgalil, Muna Ali, Minara, Begim, Rawona Berinde, Kanom Bibi, Asma Burale, Dominic Carrington, Cristina Citu, Monica Clemo, Eleanor Connolly, Roxanne Crosby-Nwaobi, Diana Dabrickaite, Supeetha David, Jenny Elliot, Sherene Ettienne, Charlene Formento, Sash Jeetun, Layla Juma, Alexa King, Shamaine King, Tabassum Master, Aldrich Meneces, Deepthy Menon, April Neville, Luke Nicholson, Andrea Ogunyemi, Sweta Panchagnula, Namritha Patrao, Panayiotis Panayiotou, Samiul Rahman, Jayashree Ramu, Nafeesa Reece, Joshua Robinson, Vincent Rocco, Priyansha Sheel, Roy Schwartz, Anar Shaikh, Sobha Sivaprasad (PI), Hayley Thomas, Emerson Tingco and Marta Zola.
Rugby
Jeanette Allison, Susanne Armitage, Jenny Bhatti, Amritpal Chaggar, Krystal Gale, Jeanette Houston, Peter James, Charlotte Johnson, Obaid Kousha, Caitlin Lumsden, Marie McCauley, Parimala Nagarajan, Sergio Pagliarini (PI), Linzi Randle and Helen Shawl.
Sheffield
Nachiketa Acharya, Naadir Ansari, Christopher Brand (PI), Stephen Connell, Maria Edwards, Mary Freeman, Helen Pokora, Fahd Quhill, Lydia Reaney, Martin Rhodes and Katherine Wheeler.
Southampton
Hussein Almuhtaseb, Magdalena Ansari, Joanna Ballingall, Mercy Jeyeraj, Joanna Karkorz, Samir Khandhadia, Theodora Koutresi, Andrew Lotery (PI), Georgiana Matei, Marie Nelson, Thea Sass, Amanda Smith, Suresh Thulasidharan, George Tsokolas, Sirli Veetousme, Catrin Watkins and Isabela Wica.
Stoke Mandeville
Judith Abrams, Kavita Aggarwal, Ahmad Ahmado, Tayaba Akhtar, Mandeep Singh Bindra (PI), Nicola Cronbach, Amelia Davidson, Markus Groppe, Shreya Haldar, Christopher King, Katarina Manso, Libby McKerrow, Ruth Penn, Jamil Razzaque, Pavandeep Singh Sandhu and Shafak Toufeeq.
Sunderland
Steve Dodds, Louise Fairlie, Lauren Gardner, Casper Geenan, Maged Habib, Hugh Harris, Ajay Kotagiri (PI), Haifa Madi, Teresa Sandinha (PI), Gemma Sloanes, Jonathan Smith, David Steel, Nicolle Teasdale, Deepali Varma, Jade Ward, Michelle Young and Jill O’Brien.
Wolverhampton
Bhogal Singh Bhogal, Vina Bhundia, Nick Denyer, Gemma Edwards, Ibtesam Elaraoud, Imogen Hawthorne, Jennifer Howell, Sharon Hughes, Donna Jones, Susan Massey, José Maya, Nirodhini Narendran (PI), Jas Purewal, Habiba Saedon, Sara Simmons, Richard Webb, Diane Whistance-Smith and Yit Chiun Yang.
York
Archana Airody, Fiona Bailey, Robert Cann, Richard Gale (PI), Srilakshmi Gollapothu, Alison Grice-Holt, Reema Gupta, Richard Hanson, Greg Heath, Gary Lamont, Mike Pringle, Carol Sarginson, Helen Shaw, Sara Stringer, Nicola Topping, Gavin Walters and Joanne Wincup.
Contributions of authors
Katie Banister (https://orcid.org/0000-0002-2189-3755) (Research Fellow, Trial Management) contributed to the conception and design of the study, was responsible for the day-to-day conduct and management of the study, contributed to the interpretation of results, led the writing of the methods section, contributed to the writing/editing of the report, and produced the report.
Jonathan A Cook (https://orcid.org/0000-0002-4156-6989) (Associate Professor, Statistics) contributed to the conception and design of the study, was responsible for the statistical analyses, contributed to the interpretation of results and contributed to the writing/editing of the report.
Graham Scotland (https://orcid.org/0000-0001-5539-8819) (Reader, Health Economics) contributed to the conception and design of the study, was responsible for the health economics analysis, contributed to the interpretation of results, led the writing of the health economics section and contributed to the writing/editing of the report.
Augusto Azuara-Blanco (https://orcid.org/0000-0002-4805-9322) (Consultant, Ophthalmology) contributed to the conception and design of the study, the interpretation of results and the writing/editing of the report.
Beatriz Goulão (https://orcid.org/0000-0003-1490-7183) (Research Fellow, Statistics) conducted the statistical analyses, contributed to the interpretation of results, led the writing of the diagnostic accuracy and prediction modelling sections, and contributed to the writing/editing of the report.
Heinrich Heimann (https://orcid.org/0000-0002-3298-4644) (Consultant, Ophthalmology) contributed to the conception and design of the study, the recruitment and follow-up of participants, the interpretation of results and the writing/editing of the report.
Rodolfo Hernández (https://orcid.org/0000-0003-2619-8230) (Research Fellow, Health Economics) conducted the economic analysis, contributed to the interpretation of results and contributed to the writing/editing of the report.
Ruth Hogg (https://orcid.org/0000-0001-9413-2669) (Senior Lecturer, Vision Science) contributed to the conception and design of the study, the interpretation of results and the writing/editing of the report.
Charlotte Kennedy (https://orcid.org/0000-0002-1974-6318) (Research Assistant, Health Economics) contributed to the health economic analysis, the interpretation of results and the writing/editing of the report.
Sobha Sivaprasad (https://orcid.org/0000-0001-8952-0659) (Consultant, Ophthalmology) contributed to the conception and design of the study, the recruitment and follow-up of participants, the interpretation of results and the writing/editing of the report.
Craig Ramsay (https://orcid.org/0000-0003-4043-7349) (Professor, Health Care Evaluation) was co-chief investigator, contributed to the conception and design of the study, contributed to the interpretation of results, led the writing of the scientific summary and contributed to the writing/editing of the report.
Usha Chakravarthy (https://orcid.org/0000-0002-2606-3734) (Consultant, Ophthalmology) was chief investigator, led the conception and design of the study and the recruitment and follow-up of participants, contributed to the interpretation of results, led the writing of the introduction, morphology section and discussion, and contributed to the writing/editing of the report.
Publications
Perrott-Reynolds R, Cann R, Cronbach N, Neo YN, Ho V, McNally O, et al. The diagnostic accuracy of OCT angiography in naive and treated neovascular age-related macular degeneration: a review. Eye 2019;33:274–282.
Hernández R, Kennedy C, Banister K, Goulão B, Cook J, Sivaprasad S, et al. Early detection of neovascular age-related macular degeneration: an economic evaluation based on data from the EDNA study. Br J Ophthalmol 2021; in press.
Sivaprasad S, Banister K, Azuara-Blanco A, Goulão B, Cook JA, Hogg R, et al. Diagnostic accuracy of monitoring tests of fellow eyes in patients with unilateral neovascular AMD (EDNA study). Ophthalmology 2021;128:1736–47.
Data-sharing statement
All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Royal College of Ophthalmologists . Age-Related Macular Degeneration: Guidelines for Management 2013. www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-318-RCOphth-AMD-Guidelines-Sept-2013-FINAL-2.pdf (accessed June 2020).
- Wong TY, Wong T, Chakravarthy U, Klein R, Mitchell P, Zlateva G, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008;115:116-26. https://doi.org/10.1016/j.ophtha.2007.03.008.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31. https://doi.org/10.1056/NEJMoa054481.
- Khanna S, Komati R, Eichenbaum DA, Hariprasad I, Ciulla TA, Hariprasad SM. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol 2019;4. https://doi.org/10.1136/bmjophth-2019-000398.
- Bakri SJ, Thorne JE, Ho AC, Ehlers JP, Schoenberger SD, Yeh S, et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology 2019;126:55-63. https://doi.org/10.1016/j.ophtha.2018.07.028.
- Mehta H, Tufail A, Daien V, Lee AY, Nguyen V, Ozturk M, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res 2018;65:127-46. https://doi.org/10.1016/j.preteyeres.2017.12.002.
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119:1388-98. https://doi.org/10.1016/j.ophtha.2012.03.053.
- Ho AC, Albini TA, Brown DM, Boyer DS, Regillo CD, Heier JS. The potential importance of detection of neovascular age-related macular degeneration when visual acuity is relatively good. JAMA Ophthalmol 2017;135:268-73. https://doi.org/10.1001/jamaophthalmol.2016.5314.
- Evans RN, Reeves BC, Phillips D, Muldrew KA, Rogers C, Harding SP, et al. IVAN Study Group . Long-term visual outcomes after release from protocol in patients who participated in the Inhibition of VEGF in Age-related Choroidal Neovascularisation (IVAN) trial. Ophthalmology 2020;127:1191-200. https://doi.org/10.1016/j.ophtha.2020.03.020.
- Casalino G, Stevenson MR, Bandello F, Chakravarthy U. Tomographic biomarkers predicting progression to fibrosis in treated neovascular age-related macular degeneration: a multimodal imaging study. Ophthalmol Retina 2018;2:451-61. https://doi.org/10.1016/j.oret.2017.08.019.
- National Institute for Health and Care Excellence (NICE) . Age-Related Macular Degeneration [NG82]. 2018. www.nice.org.uk/guidance/ng82 (accessed July 2020).
- Bailey C, Scott LJ, Rogers CA, Reeves BC, Hamill B, Peto T, et al. Intralesional macular atrophy in anti-vascular endothelial growth factor therapy for age-related macular degeneration in the IVAN trial. Ophthalmology 2019;126:75-86. https://doi.org/10.1016/j.ophtha.2018.07.013.
- Jaffe GJ, Ying GS, Toth CA, Daniel E, Grunwald JE, Martin DF, et al. Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Macular morphology and visual acuity in year five of the comparison of age-related macular degeneration treatments trials. Ophthalmology 2019;126:252-60. https://doi.org/10.1016/j.ophtha.2018.08.035.
- Zarranz-Ventura J, Liew G, Johnston RL, Xing W, Akerele T, McKibbin M, et al. The neovascular age-related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology 2014;121:1966-75. https://doi.org/10.1016/j.ophtha.2014.04.026.
- Maguire MG, Alexander J, Fine SL. Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group . Characteristics of choroidal neovascularization in the complications of age-related macular degeneration prevention trial. Ophthalmology 2008;115. https://doi.org/10.1016/j.ophtha.2008.02.028.
- Lee AY, Lee CS, Butt T, Xing W, Johnston RL, Chakravarthy U, et al. UK AMD EMR USERS GROUP REPORT V: benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br J Ophthalmol 2015;99:1045-50. https://doi.org/10.1136/bjophthalmol-2014-306229.
- Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012;96:752-6. https://doi.org/10.1136/bjophthalmol-2011-301109.
- Stevenson MR, Hart PM, Montgomery AM, McCulloch DW, Chakravarthy U. Reduced vision in older adults with age related macular degeneration interferes with ability to care for self and impairs role as carer. Br J Ophthalmol 2004;88:1125-30. https://doi.org/10.1136/bjo.2003.032383.
- Johnston RL, Lee AY, Buckle M, Antcliff R, Bailey C, McKibbin M, et al. UK Age-Related Macular Degeneration Electronic Medical Record System (AMD EMR) users group report IV: incidence of blindness and sight impairment in ranibizumab-treated patients. Ophthalmology 2016;123:2386-92. https://doi.org/10.1016/j.ophtha.2016.07.037.
- Chakravarthy U, Evans J, Rosenfeld PJ. Age related macular degeneration. BMJ 2010;340. https://doi.org/10.1136/bmj.c981.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. IVAN Study Investigators . Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119:1399-411. https://doi.org/10.1016/j.ophtha.2012.04.015.
- Chakravarthy U, Bailey CC, Scanlon PH, McKibbin M, Khan RS, Mahmood S, et al. Direct ophthalmic healthcare resource use among patients with geographic atrophy in a large cohort from the United Kingdom. Ophthalmol Retina 2019;3:920-6. https://doi.org/10.1016/j.oret.2019.06.012.
- Casalino G, Bandello F, Chakravarthy U. Changes in neovascular lesion hyperreflectivity after anti-VEGF treatment in age-related macular degeneration: an integrated multimodal imaging analysis. Invest Ophthalmol Vis Sci 2016;57:OCT288-98. https://doi.org/10.1167/iovs.15-18753.
- Healthcare Quality Improvement Partnership . Opthalmology National Electronic Age-Related Macular Degeneration (AMD) Audit: Feasibility Report 2018. www.hqip.org.uk/resource/age-related-macular-degeneration-amd-feasibility-report/#.X1pLHGhKiUm (accessed 10 September 2020).
- Seddon JM, Reynolds R, Yu Y, Rosner B. Validation of a prediction algorithm for progression to advanced macular degeneration subtypes. JAMA Ophthalmol 2013;131:448-55. https://doi.org/10.1001/jamaophthalmol.2013.2578.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351. https://doi.org/10.1136/bmj.h5527.
- The College of Optometrists, The Royal College of Ophthalmologists . Commissioning Better Eye Care 2013. www.rcophth.ac.uk/wp-content/uploads/2014/12/AMD-guidance-25-11-13-2013_PROF_262.pdf (accessed July 2020).
- AMSLER M. Earliest symptoms of diseases of the macula. Br J Ophthalmol 1953;37:521-37. https://doi.org/10.1136/bjo.37.9.521.
- Ferris FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Beckman Initiative for Macular Research Classification Committee . Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844-51. https://doi.org/10.1016/j.ophtha.2012.10.036.
- Regatieri CV, Branchini L, Duker JS. The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2011;42:S56-66. https://doi.org/10.3928/15428877-20110627-05.
- McClure ME, Hart PM, Jackson AJ, Stevenson MR, Chakravarthy U. Macular degeneration: do conventional measurements of impaired visual function equate with visual disability?. Br J Ophthalmol 2000;84:244-50. https://doi.org/10.1136/bjo.84.3.244.
- Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt 1976;53:740-5. https://doi.org/10.1097/00006324-197611000-00006.
- Medical Advisory Secretariat . Optical coherence tomography for age-related macular degeneration and diabetic macular edema: an evidence-based analysis. Ontario Health Technology Assessment Series 2009;9:1-22.
- Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen SJ, Chen Y, Freund KB, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology 2018;125:708-24. https://doi.org/10.1016/j.ophtha.2017.11.019.
- Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology 2020;127:616-36. https://doi.org/10.1016/j.ophtha.2019.11.004.
- Obuchowski NA. On the comparison of correlated proportions for clustered data. Stat Med 1998;17:1495-507. https://doi.org/10.1002/(SICI)1097-0258(19980715)17:13<1495::AID-SIM863>3.0.CO;2-I.
- Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al. A preliminary model-based assessment of the cost-utility of a screening programme for early age-related macular degeneration. Health Technol Assess 2008;12. https://doi.org/10.3310/hta12270.
- Azuara-Blanco A, Banister K, Boachie C, McMeekin P, Gray J, Burr J, et al. Automated imaging technologies for the diagnosis of glaucoma: a comparative diagnostic study for the evaluation of the diagnostic accuracy, performance as triage tests and cost-effectiveness (GATE study). Health Technol Assess 2016;20. https://doi.org/10.3310/hta20080.
- Padnick-Silver L, Weinberg AB, Lafranco FP, Macsai MS. Pilot study for the detection of early exudative age-related macular degeneration with optical coherence tomography. Retina 2012;32:1045-56. https://doi.org/10.1097/IAE.0b03e31823fb82b.
- Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med 1998;17:1643-58. https://doi.org/10.1002/(SICI)1097-0258(19980730)17:14<1643::AID-SIM869>3.0.CO;2-3.
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10.
- Zhou X, Obuchowski NA, McClish DK. Statistical Methods in Diagnostic Medicine. New York, NY: John Wiley & Sons Inc.; 2002.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. https://doi.org/10.2307/2531595.
- Newcombe RG. Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 1998;17:2635-50. https://doi.org/10.1002/(SICI)1097-0258(19981130)17:22<2635::AID-SIM954>3.0.CO;2-C.
- Cox DR. Note on grouping. J Am Stat Assoc 1957;52:543-7. https://doi.org/10.1080/01621459.1957.10501411.
- de Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology 2015;122:1228-38. https://doi.org/10.1016/j.ophtha.2015.01.029.
- Perrott-Reynolds R, Cann R, Cronbach N, Neo YN, Ho V, McNally O, et al. The diagnostic accuracy of OCT angiography in naive and treated neovascular age-related macular degeneration: a review. Eye 2019;33:274-82. https://doi.org/10.1038/s41433-018-0229-6.
- Cole ED, Ferrara D, Novais EA, Louzada RN, Waheed NK. Clinical trial endpoints for optical coherence tomography angiography in neovascular age-related macular degeneration. Retina 2016;36:83-92. https://doi.org/10.1097/IAE.0000000000001338.
- Age-Related Eye Disease Study Research Group . The age-related eye disease study (AREDS) system for classifying cataracts from photographs: AREDS Report No. 4. Am J Ophthalmol 2001;131:167-75. https://doi.org/10.1016/S0002-9394(00)00732-7.
- Mimoun G, Soubrane G, Coscas G. Les drusen maculaires [Macular drusen]. J Fr Ophtalmol 1990;13:511-30.
- Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina 2010;30:1441-54. https://doi.org/10.1097/IAE.0b013e3181ee5ce8.
- Sivaprasad S, Banister K, Azuara-Blanco A, Goulão B, Cook JA, Hogg R, et al. Diagnostic accuracy of monitoring tests of fellow eyes in patients with unilateral neovascular AMD (EDNA study). Ophthalmology 2021;128:1736-47.
- Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr 1998;68:899-917. https://doi.org/10.1093/ajcn/68.4.899.
- Chakravarthy U, Augood C, Bentham GC, de Jong PT, Rahu M, Seland J, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology 2007;114:1157-63. https://doi.org/10.1016/j.ophtha.2006.09.022.
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD000254.pub4.
- Joachim N, Colijn JM, Kifley A, Lee KE, Buitendijk GHS, Klein BEK, et al. Five-year progression of unilateral age-related macular degeneration to bilateral involvement: the Three Continent AMD Consortium report. Br J Ophthalmol 2017;101:1185-92. https://doi.org/10.1136/bjophthalmol-2016-309729.
- Burgess D, Hawkins B, Jefferys J, Bressler N, Bressler S, Hiner C, et al. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Macular Photocoagulation Study Group. Arch Ophthalmol 1993;111:1189-99. https://doi.org/10.1001/archopht.1993.01090090041018.
- Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123:1484-98. https://doi.org/10.1001/archopht.123.11.1484.
- Lamin A, El Nokrashy A, Chandra S, Sivaprasad S. Association of longitudinal changes in drusen characteristics and retinal layer volumes with subsequent subtype of choroidal neovascularisation. Ophthalmic Res 2020;63:375-82. https://doi.org/10.1159/000505628.
- Hart PM, Chakravarthy U, Stevenson MR, Jamison JQ. A vision specific functional index for use in patients with age related macular degeneration. Br J Ophthalmol 1999;83:1115-20. https://doi.org/10.1136/bjo.83.10.1115.
- Zayit-Soudry S, Alfasi M, Goldstein M, Moisseiev J, Axer-Siegel R, Pollack A, et al. Variability among retina specialists in evaluating fluorescein angiograms of patients with neovascular age-related macular degeneration. Retina 2007;27:798-803. https://doi.org/10.1097/IAE.0b013e31802c50a3.
- Holz FG, Jorzik J, Schutt F, Flach U, Unnebrink K. Agreement among ophthalmologists in evaluating fluorescein angiograms in patients with neovascular age-related macular degeneration for photodynamic therapy eligibility (FLAP-study). Ophthalmology 2003;110:400-5. https://doi.org/10.1016/S0161-6420(02)01770-0.
- Sullivan Pepe M, Anderson GL. A cautionary note on inference for marginal regression models with longitudinal data and general correlated response data. Comm Stat Simul Comput 1994;23:939-51. https://doi.org/10.1080/03610919408813210.
- Sternberg MR, Hadgu A. A GEE approach to estimating sensitivity and specificity and coverage properties of the confidence intervals. Stat Med 2001;20:1529-39. https://doi.org/10.1002/sim.688.
- Toth CA, Decroos FC, Ying GS, Stinnett SS, Heydary CS, Burns R, et al. Identification of fluid on optical coherence tomography by treating ophthalmologists versus a reading center in the comparison of age-related macular degeneration treatments trials. Retina 2015;35:1303-14. https://doi.org/10.1097/IAE.0000000000000483.
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. https://doi.org/10.7326/M14-0698.
- Friberg TR, Bilonick RA, Brennen P. Is drusen area really so important? An assessment of risk of conversion to neovascular AMD based on computerized measurements of drusen. Invest Ophthalmol Vis Sci 2012;53:1742-51. https://doi.org/10.1167/iovs.11-9338.
- Age-Related Eye Disease Study Research Group . A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch Ophthalmol 2001;119:1417-36. https://doi.org/10.1001/archopht.119.10.1417.
- Hallak JA, de Sisternes L, Osborne A, Yaspan B, Rubin DL, Leng T. Imaging, genetic, and demographic factors associated with conversion to neovascular age-related macular degeneration: secondary analysis of a randomized clinical trial. JAMA Ophthalmol 2019;137:738-44. https://doi.org/10.1001/jamaophthalmol.2019.0868.
- Schmidt-Erfurth U, Waldstein SM, Klimscha S, Sadeghipour A, Hu X, Gerendas BS, et al. Prediction of individual disease conversion in early AMD using artificial intelligence. Invest Ophthalmol Vis Sci 2018;59:3199-208. https://doi.org/10.1167/iovs.18-24106.
- Parikh R, Avery RL, Saroj N, Thompson D, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients with age-related macular degeneration treated with intravitreal aflibercept or ranibizumab [published online ahead of print July 11 2019]. JAMA Ophthalmol 2019. https://doi.org/10.1001/jamaophthalmol.2019.1947.
- Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci 2009;50:2044-53. https://doi.org/10.1167/iovs.08-3064.
- Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol 2005;123:1570-4. https://doi.org/10.1001/archopht.123.11.1570.
- Feigl B, Morris CP. The challenge of predicting macular degeneration. Curr Med Res Opin 2011;27:1745-8. https://doi.org/10.1185/03007995.2011.603301.
- Smith RT, Sohrab MA, Pumariega NM, Mathur K, Haans R, Blonska A, et al. Drusen analysis in a human-machine synergistic framework. Arch Ophthalmol 2011;129:40-7. https://doi.org/10.1001/archophthalmol.2010.328.
- Folgar FA, Yuan EL, Sevilla MB, Chiu SJ, Farsiu S, Chew EY, et al. Drusen volume and retinal pigment epithelium abnormal thinning volume predict 2-year progression of age-related macular degeneration. Ophthalmology 2016;123:39-50.e1. https://doi.org/10.1016/j.ophtha.2015.09.016.
- Lamin A, Dubis AM, Sivaprasad S. Changes in macular drusen parameters preceding the development of neovascular age-related macular degeneration. Eye 2019;33:910-16. https://doi.org/10.1038/s41433-019-0338-x.
- Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Davis MD, et al. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS Report No. 36. JAMA Ophthalmol 2014;132:272-7. https://doi.org/10.1001/jamaophthalmol.2013.6636.
- Roberts PK, Baumann B, Schlanitz FG, Sacu S, Bolz M, Pircher M, et al. Retinal pigment epithelial features indicative of neovascular progression in age-related macular degeneration. Br J Ophthalmol 2017;101:1361-6. https://doi.org/10.1136/bjophthalmol-2016-310004.
- Fragiotta S, Rossi T, Cutini A, Grenga PL, Vingolo EM. Predictive factors for development of neovascular age-related macular degeneration: a spectral-domain optical coherence tomography study. Retina 2018;38:245-52. https://doi.org/10.1097/IAE.0000000000001540.
- Friberg TR, Bilonick RA, Brennen PM. Risk factors for conversion to neovascular age-related macular degeneration based on longitudinal morphologic and visual acuity data. Ophthalmology 2012;119:1432-7. https://doi.org/10.1016/j.ophtha.2012.02.048.
- Kappen TH, van Klei WA, van Wolfswinkel L, Kalkman CJ, Vergouwe Y, Moons KGM. Evaluating the impact of prediction models: lessons learned, challenges, and recommendations. Diagn Progn Res 2018;2. https://doi.org/10.1186/s41512-018-0033-6.
- Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW. Topic Group ‘Evaluating diagnostic tests and prediction models’ of the STRATOS initiative . Calibration: the Achilles heel of predictive analytics. BMC Med 2019;17. https://doi.org/10.1186/s12916-019-1466-7.
- ISRCTN Registry . An Observational Study Following Patients Newly Diagnosed With Neovascular or ‘Wet’ Age-Related Macular Degeneration (AMD) Looking at the Changes Within the Eye Both Before and After Treatment With Anti-VEGF Injection Therapy 2019. https://doi.org/10.1186/ISRCTN76889861 (accessed February 2020).
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2019;3. https://doi.org/10.1002/14651858.CD005139.pub4.
- Okada M, Kandasamy R, Chong EW, McGuiness M, Guymer RH. The treat-and-extend injection regimen versus alternate dosing strategies in age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol 2018;192:184-97. https://doi.org/10.1016/j.ajo.2018.05.026.
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258-67. https://doi.org/10.1016/S0140-6736(13)61501-9.
- Sangroongruangsri S, Ratanapakorn T, Wu O, Anothaisintawee T, Chaikledkaew U. Comparative efficacy of bevacizumab, ranibizumab, and aflibercept for treatment of macular edema secondary to retinal vein occlusion: a systematic review and network meta-analysis. Expert Rev Clin Pharmacol 2018;11:903-16. https://doi.org/10.1080/17512433.2018.1507735.
- Chakravarthy U, Harding SP, Rogers CA, Downes S, Lotery AJ, Dakin HA, et al. A randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN). Health Technol Assess 2015;19. https://doi.org/10.3310/hta19780.
- Claxton L, Hodgson R, Taylor M, Malcolm B, Pulikottil Jacob R. Simulation modelling in ophthalmology: application to cost effectiveness of ranibizumab and aflibercept for the treatment of wet age-related macular degeneration in the United Kingdom. PharmacoEconomics 2017;35:237-48. https://doi.org/10.1007/s40273-016-0459-z.
- Chandra S, Arpa C, Menon D, Khalid H, Hamilton R, Nicholson L, et al. Ten-year outcomes of antivascular endothelial growth factor therapy in neovascular age-related macular degeneration. Eye 2020;34:1888-96. https://doi.org/10.1038/s41433-020-0764-9.
- Madhusudhana KC, Lee AY, Keane PA, Chakravarthy U, Johnston RL, Egan CA, et al. UK Neovascular Age-Related Macular Degeneration Database. Report 6: time to retreatment after a pause in therapy. Outcomes from 92 976 intravitreal ranibizumab injections. Br J Ophthalmol 2016;100:1617-22. https://doi.org/10.1136/bjophthalmol-2015-308077.
- Tosh J, Brazier J, Evans P, Longworth L. A review of generic preference-based measures of health-related quality of life in visual disorders. Value Health 2012;15:118-27. https://doi.org/10.1016/j.jval.2011.08.002.
- Espallargues M, Czoski-Murray CJ, Bansback NJ, Carlton J, Lewis GM, Hughes LA, et al. The impact of age-related macular degeneration on health status utility values. Invest Ophthalmol Vis Sci 2005;46:4016-23. https://doi.org/10.1167/iovs.05-0072.
- Butt T, Crossland MD, West P, Orr SW, Rubin GS. Simulation contact lenses for AMD health state utility values in NICE appraisals: a different reality. Br J Ophthalmol 2015;99:540-4. https://doi.org/10.1136/bjophthalmol-2014-305802.
- Butt T, Dunbar HM, Morris S, Orr S, Rubin GS. Patient and public preferences for health states associated with AMD. Optom Vis Sci 2013;90:855-60. https://doi.org/10.1097/OPX.0b013e3182962318.
- Butt T, Tufail A, Rubin G. Health state utility values for age-related macular degeneration: review and advice. Appl Health Econ Health Policy 2017;15:23-32. https://doi.org/10.1007/s40258-016-0275-9.
- Dixon P, Dakin H, Wordsworth S. Generic and disease-specific estimates of quality of life in macular degeneration: mapping the MacDQoL onto the EQ-5D-3L. Qual Life Res 2016;25:935-45. https://doi.org/10.1007/s11136-015-1145-x.
- Hodgson R, Reason T, Trueman D, Wickstead R, Kusel J, Jasilek A, et al. Challenges associated with estimating utility in wet age-related macular degeneration: a novel regression analysis to capture the bilateral nature of the disease. Adv Ther 2017;34:2360-70. https://doi.org/10.1007/s12325-017-0620-x.
- Brown GC, Sharma S, Brown MM, Kistler J. Utility values and age-related macular degeneration. Arch Ophthalmol 2000;118:47-51. https://doi.org/10.1001/archopht.118.1.47.
- Czoski-Murray C, Carlton J, Brazier J, Young T, Papo NL, Kang HK. Valuing condition-specific health states using simulation contact lenses. Value Health 2009;12:793-9. https://doi.org/10.1111/j.1524-4733.2009.00527.x.
- Longworth L, Yang Y, Young T, Mulhern B, Hernández Alava M, Mukuria C, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18090.
- National Institute for Health and Care Excellence (NICE) . Ranibizumab and Pegaptanib for the Treatment of Age-Related Macular Degeneration 2012.
- National Institute for Health and Care Excellence (NICE) . Aflibercept Solution for Injection for Treating Wet Age–related Macular Degeneration 2014.
- Thiagarajan M, Evans JR, Smeeth L, Wormald RP, Fletcher AE. Cause-specific visual impairment and mortality: results from a population-based study of older people in the United Kingdom. Arch Ophthalmol 2005;123:1397-403. https://doi.org/10.1001/archopht.123.10.1397.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- ISD Scotland . Scottish Health Service Costs 2019. www.isdscotland.org/Health-Topics/Finance/Costs/ (accessed March 2020).
- Department of Health and Social Care (DHSC) . 2018/19/National/Cost/Collection/Data/Publication 2019.
- Joint Formulary Committee . British National Formulary 2020. www.medicinescomplete.com/about/ (accessed February 2020).
- Dakin HA, Wordsworth S, Rogers CA, Abangma G, Raftery J, Harding SP, et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005094.
- Mowatt G, Hernández R, Castillo M, Lois N, Elders A, Fraser C, et al. Optical coherence tomography for the diagnosis, monitoring and guiding of treatment for neovascular age-related macular degeneration: a systematic review and economic evaluation. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18690.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Briggs AD, Wolstenholme J, Blakely T, Scarborough P. Choosing an epidemiological model structure for the economic evaluation of non-communicable disease public health interventions. Popul Health Metr 2016;14. https://doi.org/10.1186/s12963-016-0085-1.
- Butt T, Lee A, Lee C, Tufail A. UK AMD EMR . Study Group. The cost-effectiveness of initiating ranibizumab therapy in eyes with neovascular AMD with good vision: an economic model using real-world outcomes. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-006535.
- Nathoo NA, Or C, Young M, Chui L, Fallah N, Kirker AW, et al. Optical coherence tomography-based measurement of drusen load predicts development of advanced age-related macular degeneration. Am J Ophthalmol 2014;158:757-61. https://doi.org/10.1016/j.ajo.2014.06.021.
- Do DV, Gower EW, Cassard SD, Boyer D, Bressler NM, Bressler SB, et al. Detection of new-onset choroidal neovascularization using optical coherence tomography: the AMD DOC Study. Ophthalmology 2012;119:771-8. https://doi.org/10.1016/j.ophtha.2011.10.019.
- Ferrara D, Silver RE, Louzada RN, Novais EA, Collins GK, Seddon JM. Optical coherence tomography features preceding the onset of advanced age-related macular degeneration. Invest Ophthalmol Vis Sci 2017;58:3519-29. https://doi.org/10.1167/iovs.17-21696.
- Seddon JM, Silver RE, Kwong M, Rosner B. Risk prediction for progression of macular degeneration: 10 common and rare genetic variants, demographic, environmental, and macular covariates. Invest Ophthalmol Vis Sci 2015;56:2192-202. https://doi.org/10.1167/iovs.14-15841.
- Clemons T, Chew EY, Bressler SB, McBee W. Age-Related Eye Disease Study Research Group . National Eye Institute Visual Function Questionnaire. Age-related eye diseases study (AREDS) Report No. 10. Arch Ophthalmol 2003;121:211-17. https://doi.org/10.1001/archopht.121.2.211.
- Seddon JM, Rosner B. Validated prediction models for macular degeneration progression and predictors of visual acuity loss identify high-risk individuals. Am J Ophthalmol 2019;198:223-61. https://doi.org/10.1016/j.ajo.2018.10.022.
- Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group . The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014;121:1092-101. https://doi.org/10.1016/j.ophtha.2013.11.031.
- Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015;29:721-31. https://doi.org/10.1038/eye.2015.48.
- Solomon SD, Jefferys JL, Hawkins BS, Bressler NM, Bressler SB. Submacular Surgery Trials Research Group . Risk factors for second eye progression to advanced age-related macular degeneration: SST report No. 21 Submacular Surgery Trials Research Group. Retina 2009;29:1080-90. https://doi.org/10.1097/IAE.0b013e3181b1baeb.
- Waldstein SM, Vogl WD, Bogunovic H, Sadeghipour A, Riedl S, Schmidt-Erfurth U. Characterization of drusen and hyperreflective foci as biomarkers for disease progression in age-related macular degeneration using artificial intelligence in optical coherence tomography. JAMA Ophthalmol 2020;138:740-7. https://doi.org/10.1001/jamaophthalmol.2020.1376.
- Yim J, Chopra R, Spitz T, Winkens J, Obika A, Kelly C, et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat Med 2020;26:892-9. https://doi.org/10.1038/s41591-020-0867-7.
- Department of Health and Social Care (DHSC) . Achieving a World Class Productivity in the NHS, 2009 10–2013 14: The Mckinsey Report 2010.
- Royal College of Ophthalmologists . Consultant Ophthalmologist Guidance Job Plan. 2014. www.rcophth.ac.uk/wp-content/uploads/2014/12/Consultant-Ophthalmologist-Guidance-Job-Plan-April-2014_FINAL.pdf (accessed September 2020).
- Information Services Division Scotland . Outpatients Speciality Costs 2018–19 n.d. www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Speciality-Costs/Outpatients.asp (accessed September 2020).
Appendix 1 Supplementary statistical analysis tables
This appendix contains supplementary tables to the analyses provided in Chapter 4. The statistical analysis plan contains the details of each analysis (see Report Supplementary Material 2).
Supplementary results for the main analysis
Table 52 shows the mean number of clinic visits and index tests undertaken during the 36-month follow-up period for the 87 participants excluded from the main analysis.
| Visit/test | Mean (SD); n | Minimum–maximum | P25–P75 |
|---|---|---|---|
| Clinic visits | 10.4 (7.6); 87 | 1–28 | 4–17 |
| Index tests | |||
| Amsler | 7.4 (6.9); 87 | 0–25 | 2–11 |
| Fundus clinical examination | 8.7 (6.9); 87 | 0–27 | 3–15 |
| OCT | 8.9 (7.0); 87 | 1–27 | 3–13 |
| Self-reported vision | 8.9 (6.7); 87 | 0–26 | 3–12 |
| Visual acuity | 10.2 (7.5); 87 | 1–27 | 3–17 |
Post hoc combination of index tests in the primary analysis
The following tables present the results of post hoc analyses combining test results. Two combinations of tests were considered:
-
combination A – Amsler, fundus clinical examination, visual acuity and self-reported vision test results
-
combination B – Amsler, self-reported vision and visual acuity.
In both combinations, a positive result from any of the included index tests leads to a positive combined test result and only if all index tests were negative would the combined test result be negative. These combinations were conducted both for the main analysis, utilising all test results in the monitoring period (Table 53), and separately, utilising the last visit test results only (Table 54).
| Combination | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Fundus or self-reported vision or Amsler or visual acuity | 72.2 (62.5 to 80.1) | 70/97 | 55.6 (49.7 to 61.3) | 154/277 |
| Self-reported vision or Amsler or visual acuity | 50.0 (40.3 to 59.7) | 49/98 | 57.0 (51.2 to 62.7) | 158/277 |
| Combination | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Fundus or self-reported vision or Amsler or visual acuity | 63.9 (54.0 to 72.8) | 62/97 | 85.9 (81.2 to 89.6) | 231/269 |
| Self-reported vision or Amsler or visual acuity | 39.8 (30.7 to 49.7) | 39/98 | 86.2 (81.6 to 89.9) | 232/269 |
Varying the primary reference standard test result when the fundus fluorescein angiography was inconclusive
The impact of including the inconclusive FFA results is shown in Tables 55 (inconclusive FFAs were classified as positive) and 56 (inconclusive FFAs were classified as negative). Compared with the main analysis, which excluded inconclusive FFAs, the sensitivity and specificity for the index tests remained similar.
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 31.7 (23.6 to 41.2) | 33/104 | 81.4 (76.4 to 85.5) | 227/279 |
| Fundus clinical examination | 52.8 (44.1 to 61.2) | 67/127 | 97.6 (95.3 to 98.9) | 327/335 |
| OCT | 92.2 (86.1 to 95.9) | 118/128 | 87.8 (83.8 to 90.9) | 294/335 |
| Self-reported vision | 4.0 (1.5 to 9.2) | 5/126 | 97.0 (94.6 to 98.5) | 327/337 |
| Visual acuity | 29.7 (22.4 to 38.1) | 38/128 | 66.6 (61.3 to 71.4) | 223/335 |
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 33.7 (25.1 to 43.5) | 33/98 | 81.8 (76.8 to 85.8) | 233/285 |
| Fundus clinical examination | 53.8 (44.8 to 62.5) | 64/119 | 96.8 (94.3 to 98.3) | 332/343 |
| OCT | 91.7 (85.2 to 95.6) | 110/120 | 85.7 (81.6 to 89.0) | 294/343 |
| Self-reported vision | 4.2 (1.6 to 9.8) | 5/118 | 97.1 (94.7 to 98.5) | 335/345 |
| Visual acuity | 30.0 (22.5 to 38.7) | 36/120 | 66.8 (61.6 to 71.5) | 229/343 |
Results using secondary reference standard 1
Table 57 presents index test sensitivity and specificity with 95% CIs for analysis A7 (see Report Supplementary Material 2). The results are similar to the ones found in the main analysis. OCT has the best sensitivity with 92.5%, followed by fundus clinical examination (54.9%); all the other index tests have a sensitivity of < 50%. Fundus clinical examination has the highest specificity (97.7%), followed by self-reported vision (96.5%) and OCT (88.3%).
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 33.0 (25.1 to 42.1) | 38/115 | 81.0 (76.0 to 85.1) | 230/284 |
| Fundus clinical examination | 54.5 (46.4 to 62.5) | 78/143 | 97.7 (95.4 to 98.9) | 335/343 |
| OCT | 92.4 (86.8 to 95.8) | 134/145 | 88.3 (84.5 to 91.3) | 303/343 |
| Self-reported vision | 3.5 (1.3 to 8.1) | 5/143 | 96.5 (94.0 to 98.1) | 334/346 |
| Visual acuity | 31.7 (24.7 to 39.7) | 46/145 | 63.8 (58.6 to 68.8) | 219/343 |
Likelihood ratios (with 95% CIs) showed evidence to be able to rule in the presence of nAMD for all tests, except for self-reported vision and visual acuity, which include 1.0 in their confidence intervals for positive likelihood ratio, and rule out the presence of nAMD for OCT (confidence intervals for negative likelihood ratio did not include 1.0). Diagnostic odds ratios (DORs) varied from 1.0 (visual acuity) to 93.1 (OCT) (Table 58).
| Index test | Likelihood positive ratio (95% CI) | Likelihood negative ratio (95% CI) | DOR (95% CI) |
|---|---|---|---|
| Amsler | 1.7 (1.3 to 2.4) | 0.8 (0.7 to 1.0) | 2.1 (< 0.01 to > 100) |
| Fundus clinical examination | 23.7 (18.2 to 30.9) | 0.5 (0.2 to 0.9) | 50.9 (1.4 to > 100) |
| OCT | 7.9 (4.4 to 14.2) | 0.1 (0.1 to 0.1) | 91.8 (4.4 to > 100) |
| Self-reported vision | 1.0 (0.4 to 2.4) | 1.0 (0.6 to 1.7) | 1.0 (< 0.01 to > 100) |
| Visual acuity | 0.9 (0.7 to 1.2) | 1.1 (1.0 to 1.2) | 0.8 (< 0.01 to > 100) |
Paired comparisons of sensitivity and specificity between index tests: analysis A7
Table 59 shows the paired differences (with 95% CIs) in sensitivity and specificity for detecting nAMD between pairs of index tests along with the corresponding McNemar’s test p-values for analysis A7. There was evidence that the sensitivity of all of the tests differed from each other, except for Amsler and fundus clinical examination, Amsler and visual acuity, and fundus clinical examination and visual acuity. Statistically significant differences in sensitivity between index tests varied from –34.5% (visual acuity vs. self-reported vision) to 96.6% (OCT vs. self-reported vision). There was evidence that the specificity of all of the tests differed from each other, except for fundus clinical examination compared with self-reported vision. Statistically significant differences in specificity between index tests varied from –17.6% (Amsler vs. self-reported vision) to 34.4% (fundus clinical examination vs. visual acuity).
| Comparison | Difference (%) in sensitivity (95% CI); p-valuea | Difference (%) in specificity (95% CI); p-valuea |
|---|---|---|
| OCT vs. Amsler | 66.7 (38.8 to 81.9); < 0.001 | 8.1 (2.5 to 13.8); 0.01 |
| OCT vs. fundus clinical examination | 39.3 (18.0 to 57.5); < 0.001 | –9.3 (–13.3 to –5.7); < 0.001 |
| OCT vs. self-reported vision | 96.6 (78.5 to 99.4); < 0.001 | –8.2 (–12.3 to –4.2); < 0.001 |
| OCT vs. visual acuity | 62.1 (36.6 to 77.6); < 0.001 | 25.1 (19.2 to 30.7); < 0.001 |
| Amsler vs. fundus clinical examination | –25.0 (–42.9 to –3.3); 0.06 | –17.0 (–22.0 to –12.3); < 0.001 |
| Amsler vs. self-reported vision | 30.0 (7.7 to 51.9); 0.03 | –17.6 (–22.7 to –12.9); < 0.001 |
| Amsler vs. visual acuity | 0.0 (–29.1 to 29.1); 1.00 | 14.8 (7.8 to 21.7); < 0.001 |
| Fundus clinical examination vs. self-reported vision | 57.1 (35.4 to 73.5); < 0.001 | 1.2 (–1.5 to 3.9); 0.48 |
| Fundus clinical examination vs. visual acuity | 25.0 (–1.2 to 46.9); 0.12 | 34.4 (28.9 to 39.8); < 0.001 |
| Self-reported vision vs. visual acuity | –34.5 (–52.7 to –15.8); < 0.001 | 33.2 (28.1 to 38.4); < 0.001 |
Results using secondary reference standard 2
Table 60 presents the sensitivity and specificity (with 95% CIs for analysis) for the index tests’ detection of nAMD conversion (according to the reading centre’s assessment of FFA) for analysis A4. Results are similar to the main analysis. OCT has the best sensitivity with 85.7%, followed by fundus clinical examination with 50.0%; the other three index tests have a sensitivity < 50%. Self-reported vision has the highest specificity (97.1%), followed by fundus clinical examination (95.6%), OCT (83.5%), Amsler (81.0%) and visual acuity (66.2%).
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 31.6 (23.1 to 41.5) | 30/95 | 81.0 (76.0 to 85.1) | 230/284 |
| Fundus clinical examination | 50.0 (41.1 to 58.9) | 59/118 | 95.6 (92.8 to 97.4) | 325/340 |
| OCT | 85.7 (78.2 to 91.0) | 102/119 | 83.5 (79.2 to 87.1) | 284/340 |
| Self-reported vision | 3.4 (1.1 to 8.7) | 4/117 | 97.1 (94.6 to 98.5) | 331/341 |
| Visual acuity | 27.7 (20.5 to 36.4) | 33/119 | 66.2 (61.0 to 71.0) | 225/340 |
Likelihood ratios (with 95% CIs) showed evidence to be able to rule in the presence of nAMD for all tests, except self-reported vision and visual acuity (CIs for positive likelihood ratio include 1.0), and rule out the presence of nAMD for OCT and fundus clinical examination (confidence intervals for negative likelihood ratio did not include 1.0). DORs varied from 0.8 (visual acuity) to 30 (OCT) (Table 61).
| Index test | Likelihood positive ratio (95% CI) | Likelihood negative ratio (95% CI) | DOR (95% CI) |
|---|---|---|---|
| Amsler | 1.7 (1.2 to 2.4) | 0.8 (0.7 to 1.0) | 2.0 (< 0.01 to > 100) |
| Fundus clinical examination | 11.4 (8.5 to 15.2) | 0.5 (0.3 to 0.8) | 21.7 (0.5 to > 100) |
| OCT | 5.2 (3.2 to 8.3) | 0.2 (0.1 to 0.2) | 30.3 (1.2 to > 100) |
| Self-reported vision | 1.2 (0.4 to 3.1) | 1.0 (0.5 to 1.8) | 1.2 (< 0.01 to > 100) |
| Visual acuity | 0.8 (0.6 to 1.2) | 1.1 (1.0 to 1.2) | 0.8 (< 0.01 to > 100) |
Paired comparisons of sensitivity and specificity between index tests: analysis A4
Table 62 shows the paired differences (with 95% CIs) in sensitivity and specificity between index test pairs along with the corresponding McNemar’s test p-values for analysis A4. There was evidence that the sensitivity of all index tests differed from each other, except for Amsler compared with visual acuity. Statistically significant differences in sensitivity between index tests varied from –16.8% (Amsler vs. fundus clinical examination) to 82.2% (OCT vs. self-reported vision) in magnitude. OCT had the highest sensitivity of the tests, followed by fundus clinical examination, with self-reported vision having the lowest sensitivity. There was evidence that the specificity of all tests differed from each other, except for OCT compared with Amsler and fundus clinical examination compared with self-reported vision. Statistically significant differences in specificity between index tests varied from –11.3% (Amsler vs. self-reported vision) to 29.4% (fundus clinical examination vs. visual acuity) in magnitude. Fundus clinical examination and self-reported vision tests had the highest specificities, with visual acuity having the lowest specificity.
| Comparison | Difference (%) in sensitivity (95% CI); p-valuea | Difference (%) in specificity (95% CI); p-valuea |
|---|---|---|
| OCT vs. Amsler | 56.3 (43.6 to 66.1); < 0.001 | 2.5 (–3.4 to 8.4); 0.48 |
| OCT vs. fundus clinical examination | 35.6 (25.8 to 44.5); < 0.001 | –12.1 (–16.4 to –8.0); < 0.001 |
| OCT vs. self-reported vision | 82.2 (73.3 to 87.8); < 0.001 | –13.5 (–18.1 to –9.2); < 0.001 |
| OCT vs. visual acuity | 58.0 (46.5 to 67.0); < 0.001 | 17.4 (11.1 to 23.5); < 0.001 |
| Amsler vs. fundus clinical examination | –16.8 (–29.3 to –3.6); 0.02 | –14.8 (–20.0 to –9.8); < 0.001 |
| Amsler vs. self-reported vision | 27.4 (17.0 to 37.5); < 0.001 | –17.6 (–22.6 to –13.0); < 0.001 |
| Amsler vs. visual acuity | 7.4 (–5.5 to 19.9); 0.34 | 11.3 (4.4 to 18.1); < 0.001 |
| Fundus clinical examination vs. self-reported vision | 47.0 (37.4 to 55.9); < 0.001 | –1.5 (–4.5 to 1.5); 0.40 |
| Fundus clinical examination vs. visual acuity | 22.9 (11.1 to 33.8); < 0.001 | 29.4 (23.9 to 34.9); < 0.001 |
| Self-reported vision vs. visual acuity | –23.7 (–32.5 to –15.1); < 0.001 | 30.9 (25.8 to 36.0); < 0.001 |
Summarised index tests over the last 6-month follow-up period: analysis A5
Table 63 presents the sensitivity and specificity (with 95% CIs) for the index tests' detection of nAMD conversion for analysis A5. Results are similar to the ones found in the main analysis. OCT has the best sensitivity of 84.0%; all of the other index tests have a sensitivity of < 50%. Self-reported vision has the highest specificity (97.9%), followed by fundus clinical examination (97.4%) and OCT (89.7%).
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 26.0 (18.3 to 35.7) | 25/96 | 92.1 (88.2 to 94.7) | 255/277 |
| Fundus clinical examination | 49.2 (40.3 to 58.1) | 58/118 | 97.4 (95.0 to 98.7) | 331/340 |
| OCT | 84.0 (76.3 to 89.6) | 100/119 | 89.7 (86.0 to 92.5) | 305/340 |
| Self-reported vision | 3.4 (1.0 to 8.7) | 4/118 | 97.9 (95.7 to 99.1) | 332/339 |
| Visual acuity | 25.2 (18.2 to 33.7) | 30/119 | 83.5 (79.2 to 87.1) | 284/340 |
Likelihood ratios (with 95% CIs) showed evidence to be able to rule in the presence of nAMD for all tests, except self-reported vision and visual acuity (CIs for positive likelihood ratio include 1.0), and rule out the presence of nAMD for OCT (confidence intervals for negative likelihood ratio did not include 1.0). DORs varied from 1.6 (self-reported vision) to 45.7 (OCT) (Table 64).
| Index test | Likelihood positive ratio (95% CI) | Likelihood negative ratio (95% CI) | DOR (95% CI) |
|---|---|---|---|
| Amsler | 3.3 (2.2 to 4.9) | 0.8 (0.6 to 1.2) | 4.1 (< 0.01 to > 100) |
| Fundus clinical examination | 18.9 (14.1 to 25.4) | 0.5 (0.3 to 1.0) | 36.3 (0.9 to > 100) |
| OCT | 8.2 (5.2 to 12.7) | 0.2 (0.1 to 0.2) | 45.7 (2.0 to > 100) |
| Self-reported vision | 1.6 (0.6 to 4.3) | 1.0 (0.5 to 2.0) | 1.6 (< 0.01 to > 100) |
| Visual acuity | 1.5 (1.1 to 2.2) | 0.9 (0.7 to 1.1) | 1.7 (< 0.01 to > 100) |
Paired comparisons of sensitivity and specificity between index tests: analysis A5
Table 65 shows the paired difference (with 95% CIs) in sensitivity and specificity between pairs of index tests along with the corresponding McNemar’s test p-values for analysis A5. There was evidence that the sensitivity of all of the tests differed from each other, except for Amsler compared with visual acuity. The statistically significant differences in sensitivity between index tests varied from –21.1% (Amsler vs. fundus clinical examination) to 80.5% (OCT vs. self-reported vision) in magnitude. There was evidence that the specificity of all tests differed from each other, except for OCT compared with Amsler and fundus clinical examination compared with self-reported vision. The statistically significant differences in specificity between index tests varied from –8.3% (OCT vs. Amsler) to 14.5% (self-reported vision vs. visual acuity) in magnitude.
| Comparison | Difference in sensitivity (%) (95% CI); p-valuea | Difference in specificity (%) (95% CI); p-valuea |
|---|---|---|
| OCT vs. Amsler | 59.4 (47.6 to 68.3); < 0.001 | –2.9 (–7.6 to 1.7); 0.27 |
| OCT vs. fundus clinical examination | 34.7 (25.0 to 43.6); < 0.001 | –7.6 (–11.2 to –4.5); < 0.001 |
| OCT vs. self-reported vision | 80.5 (71.4 to 86.4); < 0.001 | –8.3 (–12.2 to –4.7); < 0.001 |
| OCT vs. visual acuity | 58.8 (47.1 to 68.0); < 0.001 | 6.2 (1.2 to 11.2); 0.02 |
| Amsler vs. fundus clinical examination | –21.1 (–33.2 to –7.9); < 0.001 | –5.4 (–9.4 to –1.9); < 0.001 |
| Amsler vs. self-reported vision | 22.1 (12.2 to 32.1); < 0.001 | –6.9 (–10.7 to –3.6); < 0.001 |
| Amsler vs. visual acuity | 5.3 (–6.8 to 17.1); 0.49 | 4.7 (–0.0 to 9.5); 0.07 |
| Fundus clinical examination vs. self-reported vision | 46.2 (36.6 to 55.0); < 0.001 | –0.6 (–3.1 to 1.8); 0.79 |
| Fundus clinical examination vs. visual acuity | 24.6 (13.1 to 35.1); < 0.001 | 13.8 (9.7 to 18.3); < 0.001 |
| Self-reported vision vs. visual acuity | –21.2 (–29.8 to –12.9); < 0.001 | 14.5 (10.6 to 18.8); < 0.001 |
Index test results based on the last visit available
Table 66 presents the sensitivity and specificity of the index tests (with 95% CIs) for detecting nAMD conversion for analysis A6. Results are similar to those from the main analysis. OCT has the highest observed sensitivity of 81.5%; all of the other index tests have a sensitivity of < 50%. Self-reported vision has the highest specificity (98.8%), followed by fundus clinical examination (97.9%), Amsler (92.7%), OCT (91.4%) and visual acuity (88.2%).
| Index test | Sensitivity (%) (95% CI) | True positives (n)/participants with nAMD (n) | Specificity (%) (95% CI) | True negatives (n)/participants without nAMD (n) |
|---|---|---|---|---|
| Amsler | 24.4 (16.7 to 34.3) | 22/90 | 92.7 (89.0 to 95.3) | 255/275 |
| Fundus clinical examination | 46.2 (37.4 to 55.2) | 54/117 | 97.9 (95.7 to 99.1) | 332/339 |
| OCT | 81.5 (73.5 to 87.5) | 97/119 | 91.4 (87.9 to 94.0) | 308/337 |
| Self-reported vision | 3.6 (1.1 to 9.1) | 4/112 | 98.8 (96.9 to 99.6) | 332/336 |
| Visual acuity | 24.6 (17.7 to 33.1) | 29/118 | 88.2 (84.3 to 91.2) | 299/339 |
Likelihood ratios (with 95% CIs) showed evidence to be able to rule in the presence of nAMD for all tests (CIs for positive likelihood ratio exclude 1.0), and rule out the presence of nAMD for OCT (CIs for negative likelihood ratio did not include 1.0). DORs varied from 2.4 (visual acuity) to 46.8 (OCT) (Table 67).
| Index test | Likelihood positive ratio (95% CI) | Likelihood negative ratio (95% CI) | DOR (95% CI) |
|---|---|---|---|
| Amsler | 3.3 (2.2 to 5.1) | 0.8 (0.6 to 1.2) | 4.1 (< 0.01 to > 100) |
| Fundus clinical examination | 22.0 (16.4 to 29.6) | 0.6 (0.3 to 1.1) | 40.0 (0.9 to > 100) |
| OCT | 9.5 (6.3 to 14.3) | 0.2 (0.1 to 0.3) | 46.8 (2.0 to > 100) |
| Self-reported vision | 3.0 (1.1 to 8.0) | 1.0 (0.4 to 2.6) | 3.1 (< 0.01 to > 100) |
| Visual acuity | 2.1 (1.5 to 3.0) | 0.9 (0.7 to 1.1) | 2.4 (< 0.01 to > 100) |
Paired comparisons of sensitivity and specificity between index tests: analysis A6
Table 68 shows the paired differences (with 95% CIs) in sensitivity and specificity for detecting nAMD between pairs of index tests along with the corresponding McNemar’s test p-values for analysis A6. There was evidence that the sensitivity of all tests differed from each other, except for Amsler compared with visual acuity. The statistically significant differences in sensitivity between index tests varied from –17.9% (self-reported vision vs. visual acuity) to 77.7% (OCT vs. self-reported vision) in magnitude. There was evidence that the specificity of all of the tests differed from each other, except for OCT compared with Amsler and fundus clinical examination compared with self-reported vision. Statistically significant differences in specificity between index tests varied from –5.1% (Amsler vs. fundus clinical examination) to 10.8% (self-reported vision vs. visual acuity) in magnitude.
| Comparison | Difference in sensitivity (95% CI); p-valuea | Difference in specificity (95% CI); p-valuea |
|---|---|---|
| OCT vs. Amsler | 57.8 (45.0 to 67.4); < 0.001 | –2.2 (–6.7 to 2.1); 0.39 |
| OCT vs. fundus clinical examination | 35.0 (25.2 to 43.9); < 0.001 | –6.5 (–9.9 to –3.7); < 0.001 |
| OCT vs. self-reported vision | 77.7 (68.1 to 84.1); < 0.001 | –7.5 (–11.2 to –4.3); < 0.001 |
| OCT vs. visual acuity | 56.8 (45.0 to 66.0); < 0.001 | 3.3 (–1.4 to 8.0); 0.21 |
| Amsler vs. fundus clinical examination | –19.1 (–31.4 to –5.9); 0.01 | –5.1 (–8.9 to –1.7); < 0.001 |
| Amsler vs. self-reported vision | 22.5 (12.9 to 32.5); < 0.001 | –6.6 (–10.3 to –3.4); < 0.001 |
| Amsler vs. visual acuity | 6.7 (–4.7 to 18.0); 0.33 | 1.1 (–3.2 to 5.4); 0.73 |
| Fundus clinical examination vs. self-reported vision | 43.2 (33.5 to 52.4); < 0.001 | –0.9 (–3.2 to 1.2); 0.55 |
| Fundus clinical examination vs. visual acuity | 22.2 (10.2 to 33.4); < 0.001 | 9.8 (6.1 to 13.7); < 0.001 |
| Self-reported vision vs. visual acuity | –17.9 (–26.5 to –9.7); < 0.001 | 10.8 (7.3 to 14.7); < 0.001 |
Appendix 2 Standards for the reporting of diagnostic accuracy studies flow diagrams for the main diagnostic accuracy analysis
The following flow diagrams (Figures 29–33) detail how the participants were included/excluded for the sensitivity and specificity calculations for each index test in the main analysis in Chapter 4.
FIGURE 29.
Flow of participants for the Amsler index test (main analysis A1; primary reference standard: FFA determination of conversion to nAMD at the clinical site, index test results cover entire period of follow-up).
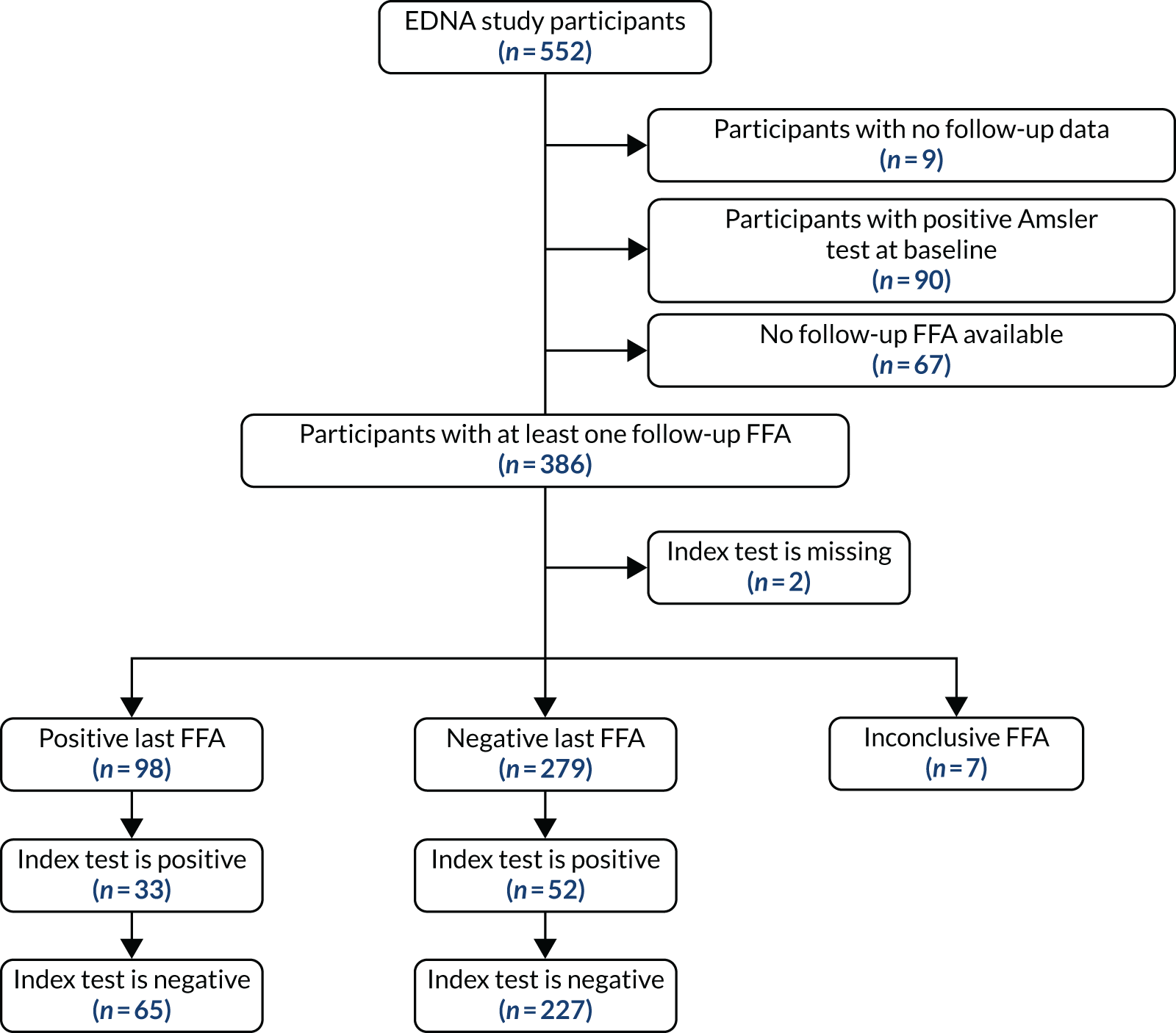
FIGURE 30.
Flow of participants for the FFA index test (main analysis A1; primary reference standard: fundus fluorescein angiography determination of conversion to nAMD at the clinical site, index test results cover entire period of follow-up).
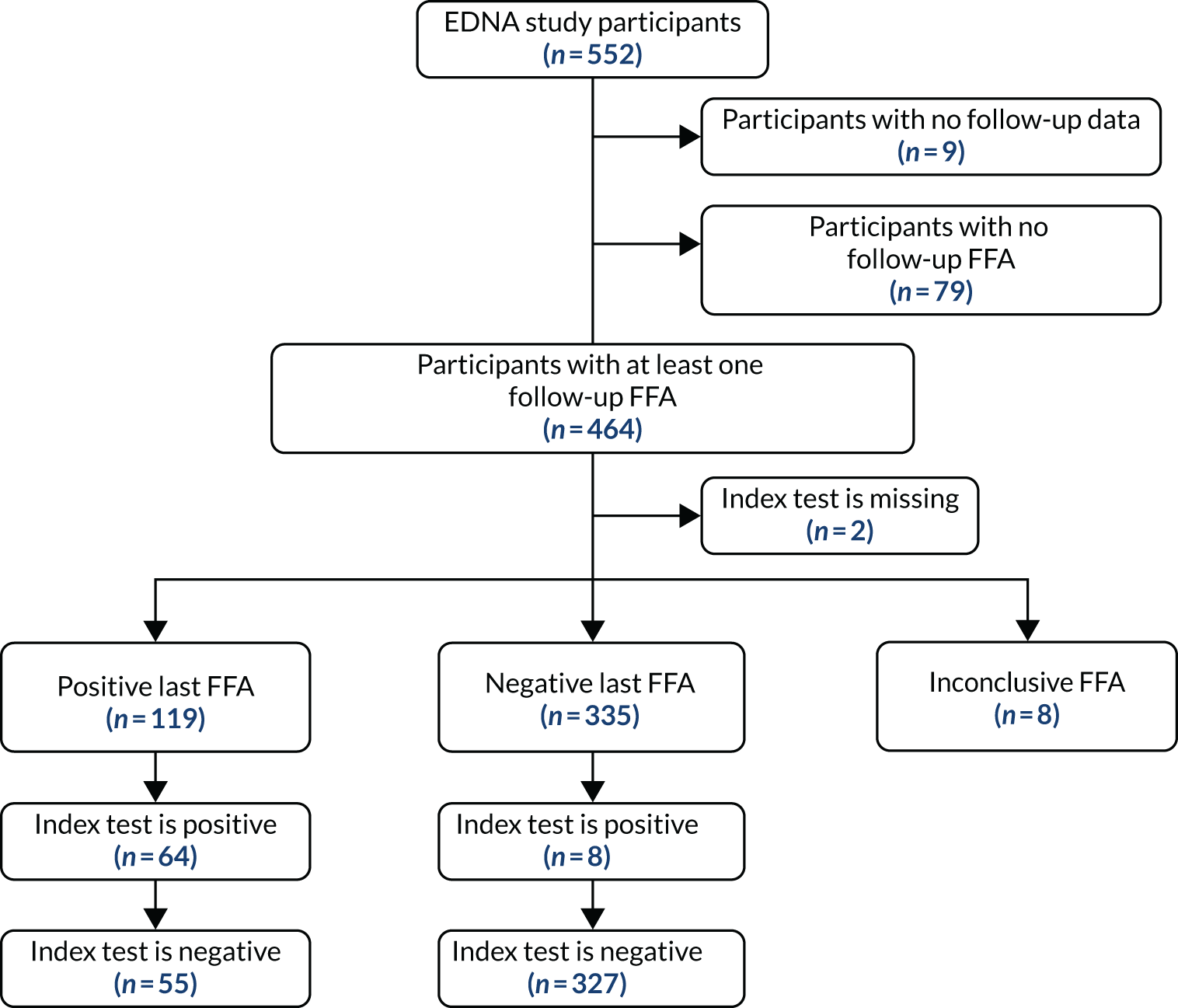
FIGURE 31.
Flow of participants for the OCT index test (main analysis A1; primary reference standard: FFA determination of conversion to nAMD at the clinical site, index test results cover entire period of follow-up).
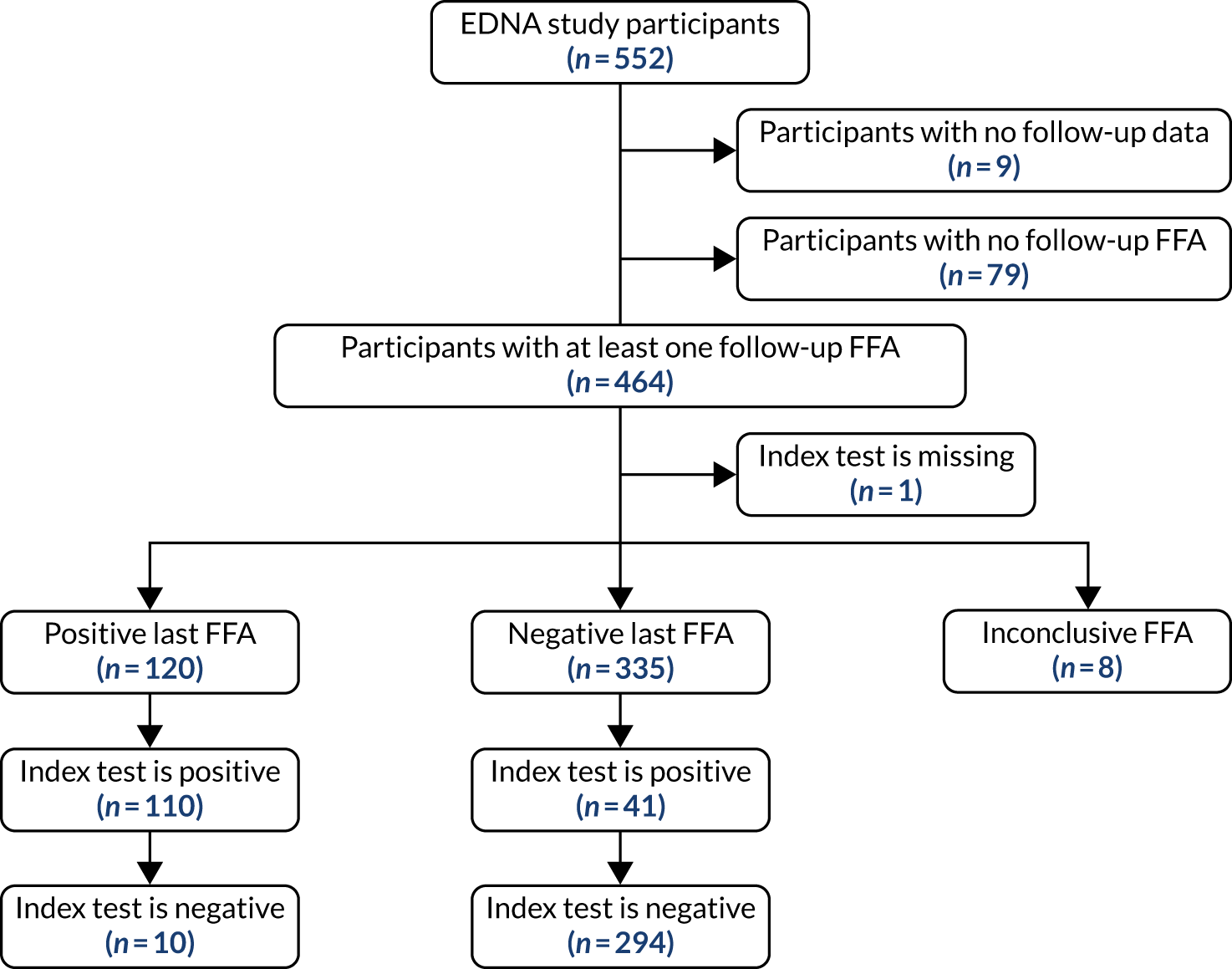
FIGURE 32.
Flow of participants for the self-reported vision index test (main analysis A1; primary reference standard: FFA determination of conversion to nAMD at the clinical site, index test results cover entire period of follow-up).
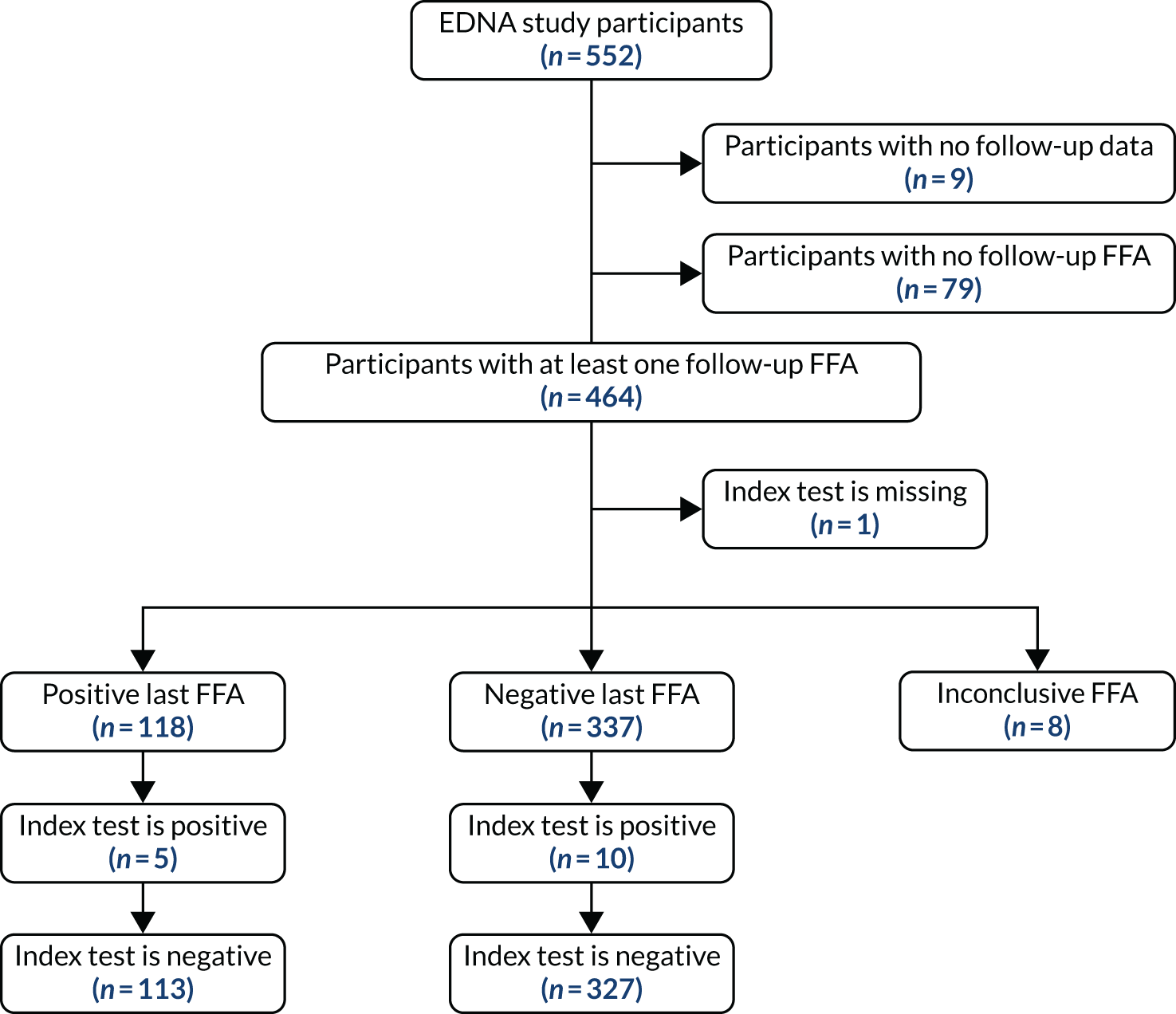
FIGURE 33.
Flow of participants for the visual acuity index test (main analysis A1; primary reference standard: FFA determination of conversion to nAMD at the clinical site, index test results cover entire period of follow-up).
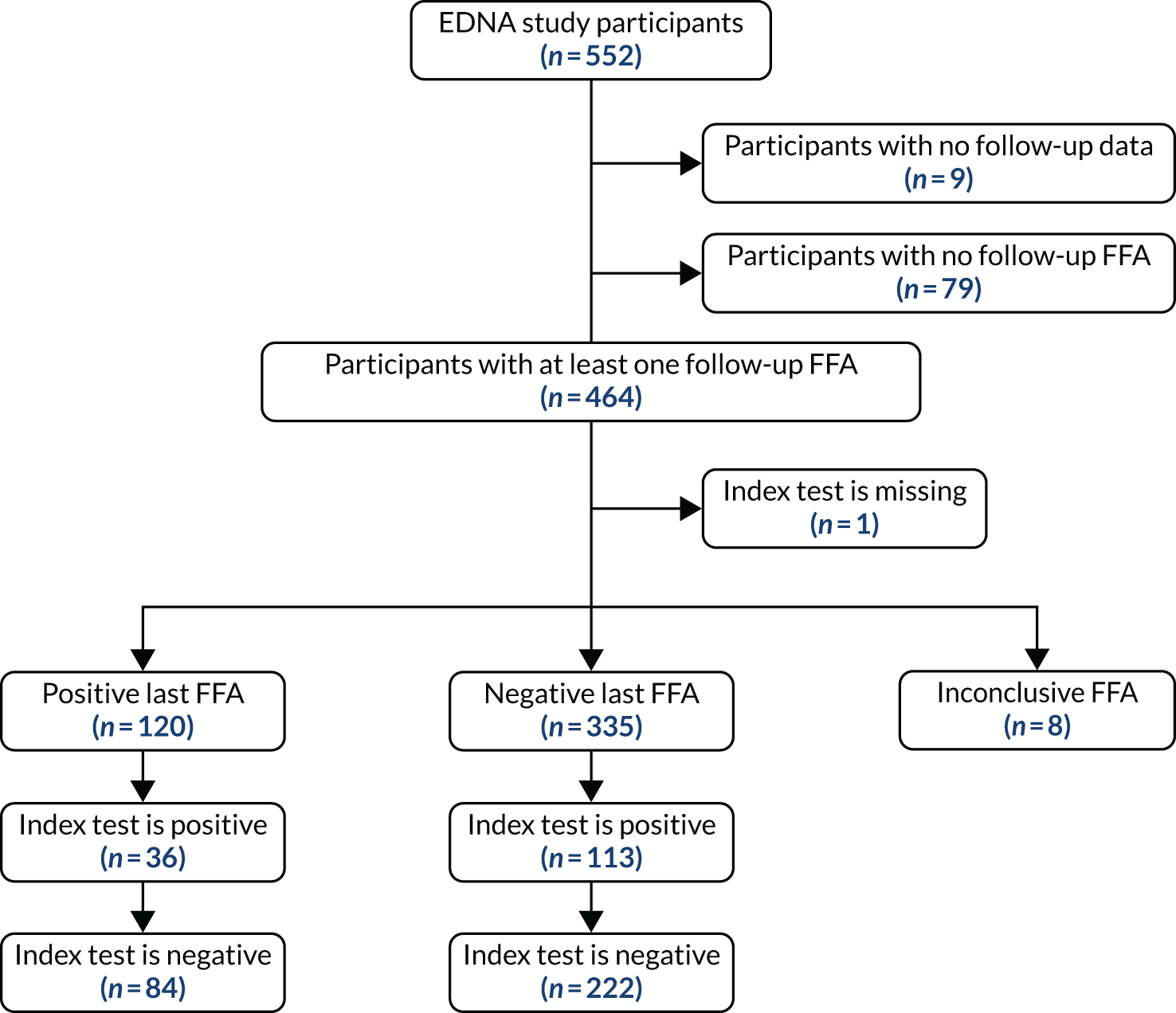
Appendix 3 Supplementary tables for prognostic modelling
Categorical variables were transformed into binary variables to calculate their correlation. Table 69 presents a clear legend and details on how the transformation was carried out. Tables 70 and 71 show the correlation between candidate predictors included in the prognostic modelling (see Table 32).
| Candidate predictor (see Table 32) | Label in correlation matrix (see Tables 70 and 71) |
|---|---|
| Smoking history (current smoker vs. not) | Smoker |
| Hypertension | Hypertension |
| Cardiovascular disease | Cardio |
| Diabetes | Diabetes |
| Nutritional supplements | Supplements |
| Family history of AMD | AMD history |
| Body mass index | BMI |
| Sex | Sex |
| Age | Age |
| Cataract present (present vs. not) | Cataract |
| Visual acuity (study eye) | Visual acuity |
| CNV subtype (fellow eye) | CNV type |
| EZ disruption (study eye) | EZ |
| ELM disruption (study eye) | ELM |
| Choroid thinning (study eye) (normal vs. not normal) | Choroid |
| Retinal thinning (study eye) | Retinal |
| Presence of pigmentary abnormalities in the fundus (study eye) | Pigmentary |
| Most frequent size of drusen (µm) (study eye) (< 63 vs. > 125) | Freq Size |
| Maximum size of drusen (µm) (study eye) (< 63 vs. > 125) | Max Size |
| Drusen type (study eye) (none vs. any type) | Drusen |
| Total lesion area of nAMD at detection (mm2) (fellow eye) | Lesion area |
| Predictors | Smoker | Hypertension | Cardio | Diabetes | Supplements | AMD History | BMI | Sex | Age | Cataract |
|---|---|---|---|---|---|---|---|---|---|---|
| Smoker | 1.00 | –0.12 | –0.05 | 0.05 | –0.10 | –0.06 | 0.02 | 0.03 | –0.25 | 0.06 |
| Hypertension | –0.12 | 1.00 | 0.18 | 0.15 | 0.07 | –0.07 | 0.21 | –0.02 | 0.17 | 0.00 |
| Cardio | –0.05 | 0.18 | 1.00 | 0.08 | –0.12 | –0.07 | 0.16 | –0.07 | 0.13 | 0.04 |
| Diabetes | 0.05 | 0.15 | 0.08 | 1.00 | –0.10 | 0.03 | 0.18 | –0.04 | –0.11 | 0.07 |
| Supplements | –0.10 | 0.07 | –0.12 | –0.10 | 1.00 | 0.16 | –0.10 | 0.15 | 0.02 | –0.02 |
| AMD history | –0.06 | –0.07 | –0.07 | 0.03 | 0.16 | 1.00 | –0.03 | 0.06 | –0.07 | –0.00 |
| BMI | 0.02 | 0.21 | 0.16 | 0.18 | –0.10 | –0.03 | 1.00 | –0.06 | –0.17 | 0.04 |
| Sex | 0.03 | –0.02 | –0.07 | –0.04 | 0.15 | 0.06 | –0.06 | 1.00 | 0.02 | –0.04 |
| Age | –0.25 | 0.17 | 0.13 | –0.11 | 0.02 | –0.07 | –0.17 | 0.02 | 1.00 | –0.10 |
| Cataract | 0.06 | 0.00 | 0.04 | 0.07 | –0.02 | –0.00 | 0.04 | –0.04 | –0.10 | 1.00 |
| Visual acuity | 0.05 | –0.03 | –0.08 | –0.04 | 0.02 | 0.01 | 0.06 | –0.05 | –0.30 | –0.05 |
| CNV type | 0.12 | –0.07 | 0.01 | 0.03 | –0.05 | 0.01 | –0.00 | –0.04 | –0.06 | –0.07 |
| EZ | –0.03 | 0.01 | –0.03 | –0.01 | 0.03 | 0.03 | 0.02 | 0.05 | 0.17 | 0.04 |
| ELM | –0.04 | 0.05 | –0.01 | –0.06 | 0.04 | 0.03 | 0.06 | 0.08 | 0.17 | 0.05 |
| Choroid | 0.06 | 0.04 | –0.03 | –0.06 | –0.07 | 0.02 | –0.01 | –0.01 | –0.18 | –0.01 |
| Retinal | –0.07 | 0.03 | –0.00 | 0.08 | –0.01 | –0.00 | 0.04 | 0.01 | 0.10 | 0.10 |
| Pigmentary | –0.01 | 0.07 | 0.08 | –0.08 | 0.04 | –0.02 | 0.04 | 0.01 | 0.10 | –0.06 |
| Freq size | 0.03 | –0.03 | 0.06 | 0.05 | –0.15 | –0.10 | 0.02 | –0.07 | –0.09 | –0.05 |
| Maximum size | 0.00 | –0.05 | 0.02 | 0.05 | –0.04 | –0.12 | 0.04 | –0.07 | –0.12 | –0.00 |
| Drusen | 0.06 | –0.03 | –0.04 | –0.05 | 0.05 | –0.03 | –0.06 | –0.02 | 0.01 | 0.01 |
| Lesion area | –0.02 | 0.05 | 0.13 | 0.04 | 0.04 | 0.02 | 0.09 | 0.03 | 0.00 | 0.01 |
| Predictors | Visual acuity | CNV type | EZ | ELM | Choroid | Retinal | Pigmentary | Freq size | Maximum size | Drusen | Lesion area |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual acuity | 1.00 | 0.07 | –0.20 | –0.24 | 0.15 | –0.12 | –0.08 | 0.12 | 0.15 | –0.02 | –0.01 |
| CNV type | 0.07 | 1.00 | –0.11 | –0.20 | –0.04 | –0.03 | –0.15 | 0.14 | 0.11 | 0.00 | 0.17 |
| EZ | –0.20 | –0.11 | 1.00 | 0.77 | –0.15 | 0.44 | 0.34 | –0.27 | –0.31 | –0.01 | 0.09 |
| ELM | –0.24 | –0.20 | 0.77 | 1.00 | –0.14 | 0.52 | 0.29 | –0.21 | –0.29 | –0.11 | 0.12 |
| Choroid | 0.15 | –0.04 | –0.15 | –0.14 | 1.00 | –0.08 | –0.05 | 0.05 | 0.08 | 0.04 | –0.07 |
| Retinal | –0.12 | –0.03 | 0.44 | 0.52 | –0.08 | 1.00 | 0.19 | –0.10 | –0.15 | –0.05 | 0.06 |
| Pigmentary | –0.08 | –0.15 | 0.34 | 0.29 | –0.05 | 0.19 | 1.00 | –0.29 | –0.28 | 0.07 | 0.06 |
| Freq Size | 0.12 | 0.14 | –0.27 | –0.21 | 0.05 | –0.10 | –0.29 | 1.00 | 0.58 | –0.16 | –0.17 |
| Max Size | 0.15 | 0.11 | –0.31 | –0.29 | 0.08 | –0.15 | –0.28 | 0.58 | 1.00 | –0.19 | –0.12 |
| Drusen | –0.02 | 0.00 | –0.01 | –0.11 | 0.04 | –0.05 | 0.07 | –0.16 | –0.19 | 1.00 | –0.08 |
| Lesion area | –0.01 | 0.17 | 0.09 | 0.12 | –0.07 | 0.06 | 0.06 | –0.17 | –0.12 | –0.08 | 1.00 |
Appendix 4 Health economics costing tables
Health economics: monitoring costs prior to conversion to neovascular age-related macular degeneration
The frequency of monitoring the EDNA study eye is dictated by the treatment and monitoring strategy of the first eye already diagnosed with nAMD. Therefore, marginal costs of assessing the EDNA study eye were calculated as the additional resources and staff time required to assess two eyes rather than just one during a monitoring appointment (assuming each test would already be performed as part of the monitoring process for the first eye). To quantify this, participating centres were asked to return a survey that requested information regarding their standard practice and how they conduct the index tests.
Survey
The survey asked centres questions regarding their determination of a positive pathology of nAMD, the bands of staff who conduct and interpret the five index tests used in EDNA, the time required to conduct and interpret these tests, and whether or not any of the tests require a second member of staff. Fundus clinical evaluation was split into slit-lamp biomicroscopy and photography. Further questions regarding the equipment used and the respective file size of digital images produced were also sent to the imaging departments of the centres.
The framing of the questions used to determine the marginal timings of the second eye asked the centres how much time they would save if they assessed only the first eye that had already been diagnosed with nAMD. This was to ensure that centres responded with only the extra time required to test the second eye and not, for example, the time to seat the patient.
Timings
Tables 72 and 73 detail the average timing in minutes for each index test calculated from the returned surveys. The timings of each test were used to determine the cost of staff time for conducting and interpreting the test. If the centre reported that more than one staff member was responsible for conducting or interpreting that test, we assumed that they conducted that test 1/n of the time, where n is the number of staff that the centre reports.
| Statistics | OCT | Fundus clinical evaluation: slit lamp | Fundus clinical evaluation: photography | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conduct | Interpret | Conduct | Interpret | Conduct | Interpret | |||||||
| nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | |
| Mean (minutes) | 8.94 | 2.67 | 5.44 | 2.03 | 7.31 | 2.71 | 5.22 | 1.81 | 7.27 | 3.65 | 4.65 | 1.97 |
| Standard deviation (minutes) | 5.01 | 1.46 | 2.62 | 1.09 | 3.03 | 1.76 | 2.56 | 1.16 | 5.24 | 2.96 | 2.52 | 1.34 |
| Median (minutes) | 7.50 | 2.50 | 5.00 | 2.00 | 7.50 | 2.25 | 5.00 | 2.00 | 6.00 | 2.29 | 4.50 | 1.75 |
| IQR (minutes) | 4.75 | 1.75 | 2.00 | 1.00 | 5.25 | 2.50 | 2.63 | 1.00 | 4.75 | 3.50 | 2.00 | 1.24 |
| N | 18 | 15 | 17 | 15 | 16 | 12 | 16 | 13 | 11 | 10 | 10 | 9 |
| Statistics | Amsler | Self-reported vision | Visual acuity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conduct | Interpret | Conduct | Interpret | Conduct | Interpret | |||||||
| nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | |
| Mean (minutes) | 4.29 | 2.00 | 2.33 | 1.00 | 3.53 | 2.14 | 2.88 | 1.45 | 7.64 | 3.34 | 3.00 | 1.82 |
| Standard deviation (minutes) | 2.93 | 1.64 | 0.82 | 0.45 | 3.36 | 1.44 | 2.48 | 1.29 | 2.71 | 1.74 | 3.00 | 1.71 |
| Median (minutes) | 4.00 | 1.82 | 2.00 | 1.00 | 2.00 | 1.50 | 2.00 | 1.00 | 7.50 | 3.00 | 2.00 | 1.00 |
| IQR (minutes) | 2.50 | 1.25 | 0.17 | 0.00 | 2.38 | 1.25 | 1.00 | 0.13 | 5.00 | 3.00 | 2.00 | 1.00 |
| N | 7 | 6 | 6 | 5 | 16 | 7 | 16 | 10 | 18 | 16 | 17 | 11 |
If a centre responded with a range of minutes, the central value was taken; for example, 5–7 minutes was recorded as 6 minutes. If a centre did not respond, the mean time of staff used across other centres was applied. This is why row ‘N’ from Tables 71 and 72 varies between tests. Some centres did not respond when asked the time saved if they assessed only one eye, often their reason was because it was standard care to assess both in a monitoring appointment.
Staff cost
The estimated staffing costs for each test are summarised in Tables 74 and 75. The hourly cost applied to staff time was taken from the PSSRU Unit costs of Health and Social Care 2019. 106 The cost per hour includes salary, employer pension contributions, national insurance and staff overheads. The length in minutes of the test does not acknowledge the additional time required per hour of patient contact for staff; therefore, a multiplier of 2.44 was applied to the cost per hour of hospital-based nurses. 126 Optometrists were considered the same as band 7 hospital-based nurses and ophthalmologists were considered as ‘consultant medical’ doctors from the PSSRU 2019. 106 However, the same multiplier to account for additional staff time required per hour of patient contact was not applied to ophthalmologists. An alternative multiplier of 1.43 was used; this was calculated using the consultant ophthalmologist guidance job plan, whereby an ophthalmologist on a standard 10 programmed activity (PA) contract would have a maximum of seven patient-facing direct clinical care (DCC) sessions. 127 Therefore, for every seven DCC sessions, three sessions were non-patient facing, leading to each hour of patient contact equalling 1.43 hours of consultant ophthalmologist’s time.
| Statistics | OCT | Fundus clinical evaluation: slit lamp | Fundus clinical evaluation: photography | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conduct | Interpret | Conduct | Interpret | Conduct | Interpret | |||||||
| nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | |
| Mean (£) | 9.27 | 4.55 | 8.29 | 4.91 | 11.26 | 6.34 | 8.00 | 4.78 | 7.40 | 6.88 | 6.45 | 4.90 |
| Standard deviation (£) | 6.93 | 2.99 | 4.85 | 2.63 | 4.93 | 3.73 | 5.09 | 2.90 | 5.59 | 5.93 | 4.44 | 3.18 |
| Median (£) | 7.02 | 3.65 | 7.51 | 4.69 | 12.08 | 6.31 | 7.25 | 4.83 | 5.51 | 4.93 | 6.29 | 4.49 |
| IQR (£) | 6.79 | 3.79 | 3.84 | 1.33 | 8.55 | 5.00 | 5.77 | 2.77 | 6.63 | 6.38 | 2.78 | 2.81 |
| N | 18 | 18 | 18 | 18 | 17 | 17 | 17 | 17 | 11 | 11 | 11 | 11 |
| Statistics | Amsler | Self-reported vision | Visual acuity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conduct | Interpret | Conduct | Interpret | Conduct | Interpret | |||||||
| nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | |
| Mean (£) | 4.22 | 3.59 | 2.66 | 2.54 | 4.74 | 4.08 | 3.33 | 3.00 | 6.78 | 5.36 | 3.43 | 2.78 |
| Standard deviation (£) | 1.99 | 2.10 | 1.10 | 1.07 | 5.48 | 4.55 | 3.16 | 2.73 | 4.06 | 4.15 | 3.23 | 2.82 |
| Median (£) | 4.51 | 2.59 | 2.42 | 2.42 | 2.42 | 2.24 | 2.24 | 2.16 | 5.45 | 3.46 | 2.42 | 2.31 |
| IQR (£) | 2.31 | 2.52 | 0.92 | 0.51 | 2.92 | 1.77 | 1.12 | 0.98 | 6.79 | 5.09 | 2.50 | 1.51 |
| N | 7 | 7 | 7 | 7 | 17 | 17 | 17 | 17 | 18 | 18 | 18 | 18 |
Assistant cost
Assistant costs were applied dependent on whether or not a centre detailed them as being required for either conducting or interpreting the test. As the question specified whether or not an additional staff member was required to carry out the test, all assistant costs were applied to the time taken to conduct the test. However, if the centre responded that the second member of staff was an ophthalmologist, then we assumed that this would be for test interpretation because all centres who did so also specified that it was for ‘equivocal cases’ or ‘at times’. For these cases, we applied the cost at 20% of the time taken to interpret the results. For all other bands of staff where the centre described the use of the staff member intermittently, the cost was applied at 50% to the time taken to conduct the test. Only four centres reported the need for an assistant during an OCT examination, of which just two used assistants every time. For the only centre that reported the need for an assistant during a fundus clinical evaluation, the use was for an ophthalmologist in the case of an equivocal diagnosis. No centres reported the need for an assistant during an Amsler chart test. Three centres reported the need for an assistant to assess self-reported vision and visual acuity; of these, one centre used an ophthalmologist for unexplained visual decline.
Equipment cost
All equipment costs per patient were calculated using equivalent annual costs divided by the centre’s reported throughput of patients per year for that index test (including other patients not participating in the EDNA study). Where this was not disclosed, a throughput of 10,000 patients per year was assumed. The total cost was applied to each eye as a function of the proportion of the total time to conduct the test. The whole equipment cost was not simply placed on the eye already diagnosed with nAMD, as the patient required extra time with the equipment to assess the EDNA study eye. Table 76 provides the estimated average equipment costs for each eye. Further detail of how these costs are calculated for each index test are provided below. We assumed zero equipment cost for the Amsler chart assessment and the patient’s subjective assessment of vision. The costs exclude VAT.
| Statistics | OCT | Fundus clinical evaluation | Visual acuity | |||||
|---|---|---|---|---|---|---|---|---|
| Slit lamp | Photography | |||||||
| EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | |
| Mean (£) | 0.88 | 1.90 | 0.14 | 0.26 | 0.58 | 0.68 | 0.02 | 0.03 |
| Standard deviation (£) | 0.39 | 0.92 | 0.07 | 0.11 | 0.27 | 0.24 | 0.01 | 0.01 |
| Median (£) | 0.82 | 1.85 | 0.14 | 0.25 | 0.53 | 0.63 | 0.02 | 0.02 |
| IQR (£) | 0.53 | 0.73 | 0.11 | 0.13 | 0.15 | 0.19 | 0.00 | 0.00 |
| N | 11 | 11 | 6 | 6 | 6 | 6 | 2 | 2 |
Optical coherence tomography
The technician surveys returned detailed the equipment used for each test: whether they were purchased or hired, the patient throughput and the expected useful lifespan of the machine. These were used to calculate the centres respective equivalent annual cost (EAC), factoring in depreciation at 3.5%. Missing information was rectified using data provided by the companies that manufacture the machines. For those centres known to use Heidelberg technology, information from the company was used to inform the EAC. This information included the cost of additional modules, useful lifespan and cost of a maintenance contract. Where discrepancies were found between company and centre responses, the centre’s response was favoured. If a centre did not use a Heidelberg machine and no other information on costings was provided by the centre, these centres did not have a cost for their machinery and the mean cost from all other centres was used.
The EAC was divided by the patient throughput per year and the cost was shared between the two eyes by the proportion of total conducting time dedicated to each eye.
Maintenance costs were reported as a cost per year by the centres, so were applied by dividing by the patient throughput per year. If the centre did not specify their maintenance contract and had a Heidelberg machine, information from Heidelberg was directly used to apply a suitable estimate for the make and model used.
Fundus clinical evaluation
In total, 17 out of the 18 responding centres reported using some form of fundus examination in a standard monitoring appointment for nAMD. Over half of the centres (n = 11) used slit-lamp biomicroscopy exclusively for fundus clinical evaluation, whereas just three centres used only photography and a further three centres used both. Some models of OCT machines are capable of capturing images of the fundus; therefore, it may be of little significance to a centre to also take an image of the fundus given that all centres report the use of an OCT. This frequency of use of the different methods of fundus clinical evaluation is detailed in Table 77.
| Fundus clinical evaluation used? | Frequency (n) |
|---|---|
| Slit lamp only | 11 |
| Photography only | 3 |
| Both | 3 |
| None | 1 |
Costs relating to the specific make and models of equipment were imputed from other centres responses where a centre did not provide this information. These values did not inform the mean costs used where a centre did not respond at all. The EAC was shared between eyes by their proportion of total time to conduct the test.
Where the centre did not specify their maintenance contract and reported a ‘Haag–Streit’ slit lamp (Clement Clarke International Ltd, London, UK), maintenance costs were incorporated into the EAC because a maintenance contract can be purchased with the machine direct from the company, which covers 10 years. Therefore, we assumed that the useful lifespan of the machine is 10 years if the centre did not specify the expected lifespan of their machine.
The cost per year of a maintenance contract for fundus clinical evaluation by photography was imputed using other centre’s responses where they reported using the same equipment
Amsler
The cost of an Amsler chart was assumed to be negligible. Furthermore, of the 18 centres that responded to the survey, just one of the five centres that use the Amsler chart to monitor patients with nAMD did so in the outpatient clinic.
Visual acuity
Information on the visual acuity equipment used by the centres was limited; only six responses to this section were received. Of the six centres that responded, there were missing values for the make and model, maintenance cost and purchase price. Therefore, the equipment cost of ETDRS letter charts for non-responsive centres was imputed from the two centres that did provide full information. No maintenance contract cost was applied to the visual acuity equipment.
Information technology cost
The OCT and fundus photography both produce files that are kept on the centre’s servers for at least 6 years. Therefore, the server capacity allocated to patients with nAMD in one eye should be at least doubled to allow for the additional images taken to monitor the EDNA study eye. Advice from the University of Aberdeen IT department recommends that, over the long term, the server capacity should be at least doubled to allow for this because servers should always have ‘free space’, aside from what is required. We assumed a total file size per visit of 74.1MB for a comprehensive OCT examination and 20MB for a fundus clinical evaluation by photography for a patient diagnosed with nAMD. The estimate of the file size of an OCT examination was provided by Heidelberg, while the size of a fundus photography image was inferred through centre responses to the technician surveys. Therefore, these file costs are not representative of an ophthalmic centre’s cost but should be acknowledged as a guide, as other models of OCT machine may produce different file sizes, take more or less images and IT costs are unknown for all centres in the EDNA study.
Centre responses cited a variety of storage lengths for patient data: between 6 years and indefinitely. For our purposes, we assumed a storage length of 10 years to be appropriate where the data are accessible. It is possible to then archive the data, which is significantly cheaper but significantly more difficult to access. The recommendation by the University of Aberdeen IT department was to increase the capacity at the beginning of the time period. Therefore, the additional storage space required for the additional OCT images of 37.05MB for an assumed 10,000 patients kept for 10 years is 5TB, where the most data in this new server at any one time is 3.705TB, leaving a reserve capacity of 1.295TB. This information was used to calculate an EAC, which was divided by the centre’s throughput and applied to the EDNA study eye only. Table 78 gives a summary of the IT costs applied to the cost per index test. Caution is encouraged because these costs are not representative of an ophthalmic centre’s cost of file storage but that of the University of Aberdeen, which is not a medical establishment. These costs were not applied to the eye diagnosed with nAMD because the EDNA study eye is the driver of this file expansion. It is assumed that the centre already has measures in place for file storage of the nAMD eye included in the overheads, which are discussed in the next section.
| Statistics | OCT | Fundus clinical evaluation: photography |
|---|---|---|
| Mean (£) | 0.33 | 0.25 |
| Standard deviation (£) | 0.11 | 0.17 |
| Median (£) | 0.30 | 0.18 |
| IQR (£) | 0.05 | 0.02 |
Total testing costs
Table 79 provides a summary of the estimate testing costs for the first (nAMD) eye and the second (EDNA) eye per patient, inclusive of staff costs (incorporating overheads), equipment and IT storage.
| Statistics | OCT | Fundus clinical evaluation | Amsler | Self-reported vision | Visual acuity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slit lamp | Photography | |||||||||||
| EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | |
| Mean (£) | 10.68 | 19.45 | 10.64 | 18.44 | 11.79 | 13.70 | 6.13 | 6.88 | 7.08 | 8.07 | 8.15 | 10.23 |
| Standard deviation (£) | 5.19 | 10.14 | 6.32 | 8.85 | 7.11 | 7.33 | 3.05 | 2.92 | 6.68 | 7.35 | 6.32 | 6.50 |
| Median (£) | 9.71 | 17.83 | 9.91 | 20.24 | 10.04 | 12.11 | 4.83 | 7.10 | 5.05 | 5.33 | 6.26 | 7.34 |
| IQR (£) | 6.38 | 11.76 | 6.56 | 13.23 | 10.60 | 9.72 | 3.21 | 3.40 | 3.57 | 4.80 | 5.36 | 6.21 |
| N | 18 | 18 | 14 | 14 | 5 | 5 | 3 | 3 | 15 | 15 | 18 | 18 |
Additional overheads scenario
Overheads were applied as part of the staff cost multipliers (see Tables 73 and 78). However, given that the staff cost multipliers do not account for all of the capital overheads associated with use of clinic consulting and treatment space, we assessed the impact of uplifting the test cost estimates to account for these further overheads. The uplift factor was calculated using the ISD Scotland Health Service Specialty Costs (November 2019) for ophthalmology consultant outpatient appointments. 128 The average total allocated cost per attendance was £41 and the total direct cost per attendance was £135. Allocated costs per attendance included administration, uniforms, property maintenance, utilities, rent and rates, furniture and depreciation. Direct costs per attendance included all staff and laboratory costs. A multiplier of 1.30 [= (135 + 41)/135] was calculated to acknowledge the allocated costs for every £1 spent on direct costs during an average consultant-led ophthalmology appointment in Scotland. This was then multiplied by the whole centre staff and assistant costs for only those index tests that centres reported using for a standard monitoring appointment for nAMD. This gave additional overhead costs for each monitoring appointment (Table 80).
| Statistics | Whole centre overheads |
|---|---|
| Mean (£) | 28.11 |
| Standard deviation (£) | 13.14 |
| Median (£) | 25.56 |
| IQR (£) | 9.32 |
| n | 18 |
The cost of overheads was then shared between the index tests by their proportion of the total test time that the patient spends at each centre for their appointment. Tables 81 and 82 give summary statistics of overheads by each index test. The n fluctuates between tests because a centre must have reported the use of that test to monitor nAMD for the multiplier of overheads to be applied to that test.
| Statistics | OCT | Fundus clinical evaluation: slit lamp | Fundus clinical evaluation: photography | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conduct | Interpret | Conduct | Interpret | Conduct | Interpret | |||||||
| EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | |
| Mean (£) | 1.87 | 3.79 | 1.32 | 2.19 | 1.59 | 2.97 | 1.15 | 2.19 | 2.02 | 1.90 | 1.52 | 1.61 |
| Standard deviation (£) | 1.09 | 2.49 | 0.79 | 1.23 | 1.01 | 1.44 | 0.71 | 1.71 | 1.14 | 0.94 | 1.01 | 0.73 |
| Median (£) | 1.59 | 3.02 | 1.19 | 2.06 | 1.48 | 3.04 | 1.11 | 1.78 | 1.84 | 1.81 | 1.47 | 1.55 |
| IQR (£) | 1.64 | 2.34 | 0.53 | 1.22 | 1.30 | 1.94 | 0.75 | 2.19 | 1.89 | 0.63 | 1.09 | 1.11 |
| n | 18 | 18 | 18 | 18 | 14 | 14 | 14 | 14 | 5 | 5 | 5 | 5 |
| Statistics | Amsler | Self-reported vision | Visual acuity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conduct | Interpret | Conduct | Interpret | Conduct | Interpret | |||||||
| EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | |
| Mean (£) | 0.95 | 0.95 | 0.67 | 0.67 | 1.04 | 1.22 | 0.93 | 0.90 | 2.15 | 2.76 | 0.91 | 1.07 |
| Standard deviation (£) | 0.89 | 0.89 | 0.12 | 0.12 | 1.07 | 1.43 | 0.93 | 0.93 | 1.18 | 1.09 | 1.07 | 1.10 |
| Median (£) | 0.54 | 0.54 | 0.69 | 0.69 | 0.58 | 0.62 | 0.60 | 0.64 | 1.71 | 2.66 | 0.58 | 0.64 |
| IQR (£) | 0.81 | 0.81 | 0.12 | 0.12 | 0.57 | 0.58 | 0.45 | 0.31 | 2.14 | 1.58 | 0.36 | 0.84 |
| n | 3 | 3 | 3 | 3 | 15 | 15 | 15 | 15 | 18 | 18 | 18 | 18 |
Given that this approach may ‘double count’ the equipment, IT costs and staffing overheads, which were calculated separately and included in the cost estimates in Table 79, the uplifted test costs in Table 83 were applied in a scenario analysis as an upper limit of the estimated marginal cost of each test.
| Statistics | OCT | Fundus clinical evaluation | Amsler | Self-reported vision | Visual acuity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slit lamp | Photography | |||||||||||
| EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | EDNA | nAMD | |
| Mean (£) | 13.27 | 24.29 | 13.22 | 24.65 | 18.34 | 18.12 | 9.64 | 9.64 | 8.54 | 9.21 | 10.42 | 13.24 |
| Standard deviation (£) | 6.18 | 11.87 | 6.45 | 10.52 | 9.77 | 7.13 | 5.84 | 5.84 | 6.98 | 7.90 | 7.06 | 7.44 |
| Median (£) | 12.39 | 21.96 | 12.49 | 26.13 | 16.92 | 20.38 | 6.82 | 6.82 | 5.62 | 5.53 | 9.10 | 10.56 |
| IQR (£) | 7.72 | 14.62 | 7.53 | 16.83 | 14.73 | 8.78 | 5.30 | 5.30 | 5.08 | 5.24 | 7.28 | 6.30 |
| n | 18 | 18 | 14 | 14 | 5 | 5 | 3 | 3 | 15 | 15 | 18 | 18 |
List of abbreviations
- AIC
- Akaike information criterion
- AMD
- age-related macular degeneration
- AREDS
- Age Related Eye Disease Study
- AUC
- area under the curve
- BCVA
- best corrected visual acuity
- BIC
- Bayesian information criterion
- BMI
- body mass index
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CNV
- choroidal neo-vascularisation
- DCC
- direct clinical care
- DOR
- diagnostic odds ratio
- EAC
- equivalent annual cost
- EDNA
- Early Detection of Neovascular Age-related macular degeneration
- ELM
- external limiting membrane
- ETDRS
- Early Treatment Diabetic Retinopathy Study
- EZ
- ellipsoid zone
- FASBAT
- Observing Fibrosis, Macular Atrophy and Sub retinal Highly Reflective Material – Before and After Intervention with Anti-VEGF Treatment
- FFA
- fundus fluorescein angiography
- FPED
- fibrovascular pigment epithelial detachment
- GEE
- generalised estimating equation
- ICER
- incremental cost-effectiveness ratio
- ICGA
- indocyanine green angiography
- ISRCTN
- International Standard Randomised Controlled Trial Number
- IVAN
- Inhibit VEGF in Age-related choroidal Neovascularisation
- LLIO
- late leakage of indeterminate origin
- nAMD
- neovascular age-related macular degeneration
- NICE
- National Institute for Health and Care Excellence
- OCT
- optical coherence tomography
- OCTA
- optical coherence tomography angiography
- PCV
- polypoidal choroidal vasculopathy
- PI
- principal investigator
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RAP
- retinal angiomatous proliferation
- RPE
- retinal pigment epithelium
- SAE
- serious adverse event
- SD
- standard deviation
- VEGF
- vascular endothelial growth factor
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/VLFL1739).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.