Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/110/02. The contractual start date was in March 2017. The draft report began editorial review in October 2020 and was accepted for publication in January 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Armstrong-Buisseret et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Bacterial vaginosis (BV) is a common condition that predisposes women to potentially serious comorbidities, such as human immunodeficiency virus (HIV) infections, other sexually transmitted infections (STIs), pelvic inflammatory disease (PID), and preterm birth, miscarriage and other adverse pregnancy outcomes. 1–5 Typical symptoms include vaginal discharge accompanied by an unpleasant fishy odour that frequently occurs in association with menstruation and can have a significant impact on the woman’s quality of life. 5
In women of reproductive age, the pH of the vagina is normally moderately acidic, in part because of the presence of lactobacilli species that produce lactic acid, which helps to prevent the overgrowth of other vaginal bacteria. An alteration in the usual vaginal flora occurs in BV, with a loss of lactobacilli and an associated increase in pH to more alkaline levels, which allows the proliferation of other primarily anaerobic bacteria, including Gardnerella. 5,6 The exact pathophysiological mechanism responsible for BV remains to be elucidated, although the sexual transmission of bacteria and the development of a biofilm containing specific bacterial species may be contributory factors to the dysbiosis observed in the vaginal flora. 5,7,8
Guidelines recommend the use of antibiotics as the first-line treatment for BV, with oral metronidazole (Flagyl, Sanofi) having been a standard choice for over 25 years and producing cure rates of up to 85% at 4 weeks post treatment. 9–11 However, antibiotic side effects can affect adherence to treatment and, although antibiotics may be initially effective, BV symptoms frequently recur within a few months. 10,12–14 This results in repeated antibiotic use and the potential for antibiotic resistance to develop.
Public Health England data from 2018 indicate that over 86,000 women had a diagnosis of BV when presenting at a sexual health clinic in England15 and symptoms are likely to recur in about one-third of women in the 3 months following initial treatment. 10,12–14 New treatment options are, therefore, required to reduce antibiotic use, provide better efficacy and lower recurrence rates.
Rationale for the VITA trial
Given that intravaginal lactic acid gel (pH 4.5) replicates the production of lactic acid by lactobacilli in the normal vagina, the use of lactic acid gel as treatment for BV could reduce antibiotic exposure in the population, as recommended in the Tackling Antimicrobial Resistance 2019–2024: The UK’s Five-year National Action Plan16 and A European One Health Action Plan Against Antimicrobial Resistance (AMR). 17 The avoidance of systemic antibiotics would help maintain the balance of the gut bacteria (microbiome) in individual participants and reduce the potential for the development of antimicrobial resistance (AMR) in the community. In addition, it would provide an alternative treatment for women who have failed to respond to current treatment for BV with systemic antibiotics.
The aim of the metronidazole Versus lactic acId for Treating bacterial vAginosis (VITA) trial was to determine whether or not using intravaginal lactic acid gel to replace vaginal acidity would be better than oral metronidazole for the symptomatic resolution of recurrent BV. Previous small studies of daily intravaginal acid gel or pessary for the treatment of BV have reported inconsistent results, with little difference between dosing regimens (23–93% efficacy with the more common once-daily dosing vs. 18–100% with twice-daily dosing). 13,18–23 For the VITA trial, a once-daily dose of 4.5% intravaginal lactic acid gel for 7 days was used because this was likely to be a more acceptable regimen than twice-daily dosing. UK management guidelines for BV10 do not currently include lactic acid as a recommended treatment because there is insufficient evidence from randomised controlled trials (RCTs) on reproducible efficacy. It was, therefore, anticipated that the VITA trial would advance our understanding by assessing whether or not lactic acid gel is effective and well tolerated for the treatment of recurrent BV, and whether or not it can reduce antibiotic usage in this large group of women. The comparator was 400-mg oral metronidazole tablets twice daily for 7 days and was chosen because it is recommended as first-line therapy in the UK national BV treatment guidelines,10 being active against a wide range of the anaerobic bacteria associated with BV and being commonly used in clinical practice supported by evidence from RCTs. 24
In addition, a qualitative assessment was performed to explore factors affecting the acceptability of, and adherence to, intravaginal treatment for BV and how these could be improved. A pragmatic trial design was used to maximise its relevance to patients and clinicians and to facilitate rapid adoption of the trial results into clinical practice.
Bacterial vaginosis is a common disease with serious physical and psychological sequelae. There is, therefore, the potential for a substantial health gain if a more effective and well-tolerated regimen, which also reduces antibiotic exposure, can be identified. The prospects for the study findings to influence clinical practice were high based on the multicentre approach including primary care, robust study design, existing widespread availability of lactic acid gel and identified need to limit antibiotic use to reduce the development of AMR.
Chapter 2 Methods
Text in the this chapter is reproduced with permission from Armstrong-Buisseret et al. 25 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
The full VITA trial protocol is available on the National Institute for Health Research project web page (www.fundingawards.nihr.ac.uk/award/15/110/02) and a summary protocol has been published. 25 Consolidated Standards of Reporting Trials (CONSORT) guidelines have been followed for data analysis and reporting. 26
Regulatory approval for the trial was given by the Medicines and Healthcare products Regulatory Agency on 12 July 2017 (reference 16719/0230/001–0001; European Union Drug Regulating Authorities Clinical Trials 2016-004483-19) and ethics approval was given by the London – Harrow Research Ethics Committee on 9 September 2017 (reference 17/LO/1245). Local research and development departments gave their own approval prior to recruitment commencing at each participating site. The trial was registered on the International Standard Randomised Controlled Trial Number (ISRCTN) register as ISRCTN14161293 on 8 September 2017 (https://doi.org/10.1186/ISRCTN14161293; accessed 27 April 2021).
There were no updates made to the protocol after the original approved version (version 1.0); however, the following changes were introduced to the trial procedures and the collection and analysis of some outcome measures:
-
As per the protocol, the Short Form questionnaire-12 items (SF-12) health survey was administered at baseline, 2 weeks and 6 months. In addition, it was administered at 3 months (which was also included as a secondary outcome), although this was inadvertently not stated in the protocol.
-
Participant-reported outcomes were collected via web-based questionnaires as detailed in the protocol. During the course of the trial, a follow-up telephone call was introduced to try to improve the collection of key outcomes for participants for whom the week 2 and 6-month web-based questionnaires had not been completed, despite several reminders being sent. The key outcome information was a subset of the information included in the web-based questionnaires. Collection of these data via a telephone call was not specifically stated in the protocol; however, consent to be contacted via telephone was included on the informed consent form.
-
To assist with interpretation of the primary outcome, additional subgroup analyses for symptom resolution at week 2 were included as follows, although these were inadvertently not stated in the protocol:
-
number of episodes of BV in the 12 months before baseline (1, 1–3 or > 3)
-
total time with BV in the 12 months before baseline (< 2 weeks, ≥ 2 weeks and < 3 months, ≥ 3 months).
-
-
An additional secondary objective was included that was to compare the time to resolution of BV symptoms, although this was inadvertently not stated in the protocol.
Trial objectives
The primary objective was to determine whether or not intravaginal lactic acid gel is better than oral metronidazole for symptomatic resolution of recurrent BV.
The secondary objectives were to:
-
compare the time to first recurrence of BV symptoms
-
compare the frequency of BV episodes over 6 months
-
compare the frequency of BV treatments required over 6 months
-
compare microbiological resolution of BV on microscopy 2 weeks after presentation
-
compare the time to resolution of BV symptoms
-
compare the tolerability profiles of lactic acid gel and metronidazole
-
compare the adherence to lactic acid gel with adherence to metronidazole tablets
-
compare the acceptability of use of lactic acid gel with that of the use of metronidazole tablets
-
determine the prevalence of concurrent STIs at baseline and week 2
-
compare quality of life (measured using the SF-12 health survey27)
-
compare the cost-effectiveness of intravaginal lactic acid gel with that of oral metronidazole tablets.
In addition, samples for further microbiological analysis, including gene sequencing, were collected for future investigation into the factors associated with successful treatment.
Outcome measures
The primary outcome was participant-reported resolution of BV symptoms at week 2. Secondary outcome measures were as follows:
-
time to first recurrence of BV as reported by participants
-
number of participant-reported BV episodes over 6 months
-
number of participant-reported BV treatment courses over 6 months
-
microbiological resolution of BV on microscopy of vaginal smears taken at week 2 and analysed at a central laboratory
-
time to participant-reported resolution of BV symptoms
-
tolerability of lactic acid gel and metronidazole assessed by participant reporting of side effects (including nausea, vomiting, taste disturbance, vaginal irritation, diarrhoea and abdominal pain) and via participant interviews
-
participant-reported adherence to treatment
-
acceptability of treatments via qualitative assessment in a subgroup of participants
-
prevalence of concurrent STIs (gonorrhoea, chlamydia and trichomoniasis) from vaginal swabs taken at baseline and week 2, and analysed at a central laboratory
-
quality of life as assessed by the SF-12 health survey27 at baseline, 2 weeks, 3 months and 6 months
-
comparative cost-effectiveness of intravaginal lactic acid gel and oral metronidazole tablets.
Participant-reported outcome measures were collected using web-based questionnaires, with several reminders sent to encourage completion. During the later stages of the trial, a follow-up telephone call was attempted to collect key outcomes from the week 2 and 6-month questionnaires when these had not been completed.
Trial design and setting
The VITA trial was an open-label, multicentre, parallel-arm RCT. Participants were randomised 1 : 1 to receive either intravaginal lactic acid gel treatment (intervention) or oral metronidazole tablets (control). The treatment was for 7 days, with follow-up taking place at 2 weeks, 3 months and 6 months after randomisation. A health economic evaluation was performed to assess the cost-effectiveness of the study treatments from a UK NHS perspective (see Chapter 4). In addition, a subgroup of participants were interviewed to further explore the adherence, tolerability and acceptability of treatment (see Chapter 5).
Women presenting with symptoms of BV and a history of one or more episodes within the previous 2 years that had been resolved with treatment were approached by a member of the site research team to determine whether or not they were interested in participating in the trial. In normal clinical practice, a diagnosis of BV would be made based on an assessment of symptoms alone or in conjunction with the microscopy appearances of a vaginal smear; therefore, microscopy confirmation of BV was not required for entry into the trial.
Recruitment was planned to take place in approximately 25 primary care general practices and 15 sexual health centres and gynaecology clinics via several routes in the UK (Figure 1).
FIGURE 1.
The VITA trial participant pathways in primary and secondary care settings. EPR, electronic patient record; SOC, standard of care. a, Acting as a participant identification centres: identification and referral of women with BV to recruiting centre; b, identification and recruitment at local clinic or referral to a recruiting sexual health centre depending on local facilities. Reproduced with permission from Armstrong-Buisseret et al. 25 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
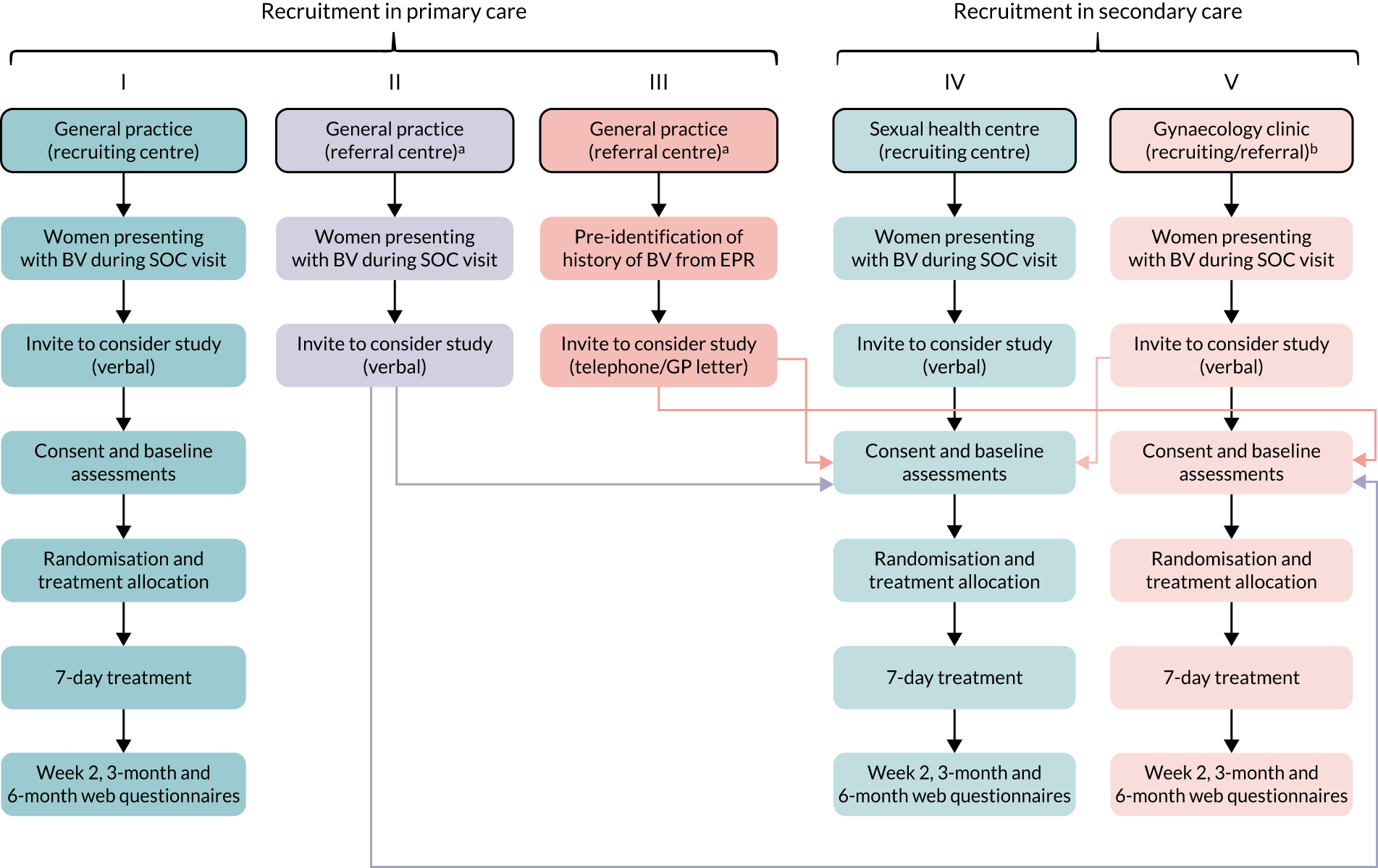
Primary care (general practices)
-
Opportunistic identification of women presenting with BV in general practices that were VITA trial recruiting centres with trained research staff on site. Participants were identified, consented, randomised and prescribed study treatment at the practice. These research-ready sites required on-site availability of trained research nurses and facilities to directly consent and randomise patients.
-
Opportunistic identification and referral of women with BV attending general practices without on-site research staff (participant identification centres) to local participating VITA trial recruiting centres for invitation to participate in the trial.
-
Pre-identification of women with a history of BV by general practitioners (GPs) from electronic patient records/primary care databases. GPs would provide potential participants with information on the trial via telephone or letter and invite them to attend a local recruiting centre for consent if they developed BV and were interested in participating.
In addition, GP practices could use computerised ‘pop-up’ alerts to support the identification and recruitment of suitable women when they presented with possible BV symptoms.
Secondary care
-
Opportunistic identification of women presenting with BV in sexual health centres that were VITA trial recruiting centres with trained research staff on site. Participants were identified, consented, randomised and dispensed study treatment at the centre. These research-ready sites required on-site availability of trained research nurses and facilities to directly consent and randomise patients.
-
Opportunistic identification of women presenting with BV in gynaecology clinics that either were VITA trial recruiting centres with trained research staff on site where participants would be identified, consented, randomised and prescribed study treatment within the clinic; or acted as VITA trial referral clinics (participant identification centres) where women presenting with BV could be referred to a nearby participating recruiting sexual health centre for invitation to participate in the trial.
Participants and eligibility
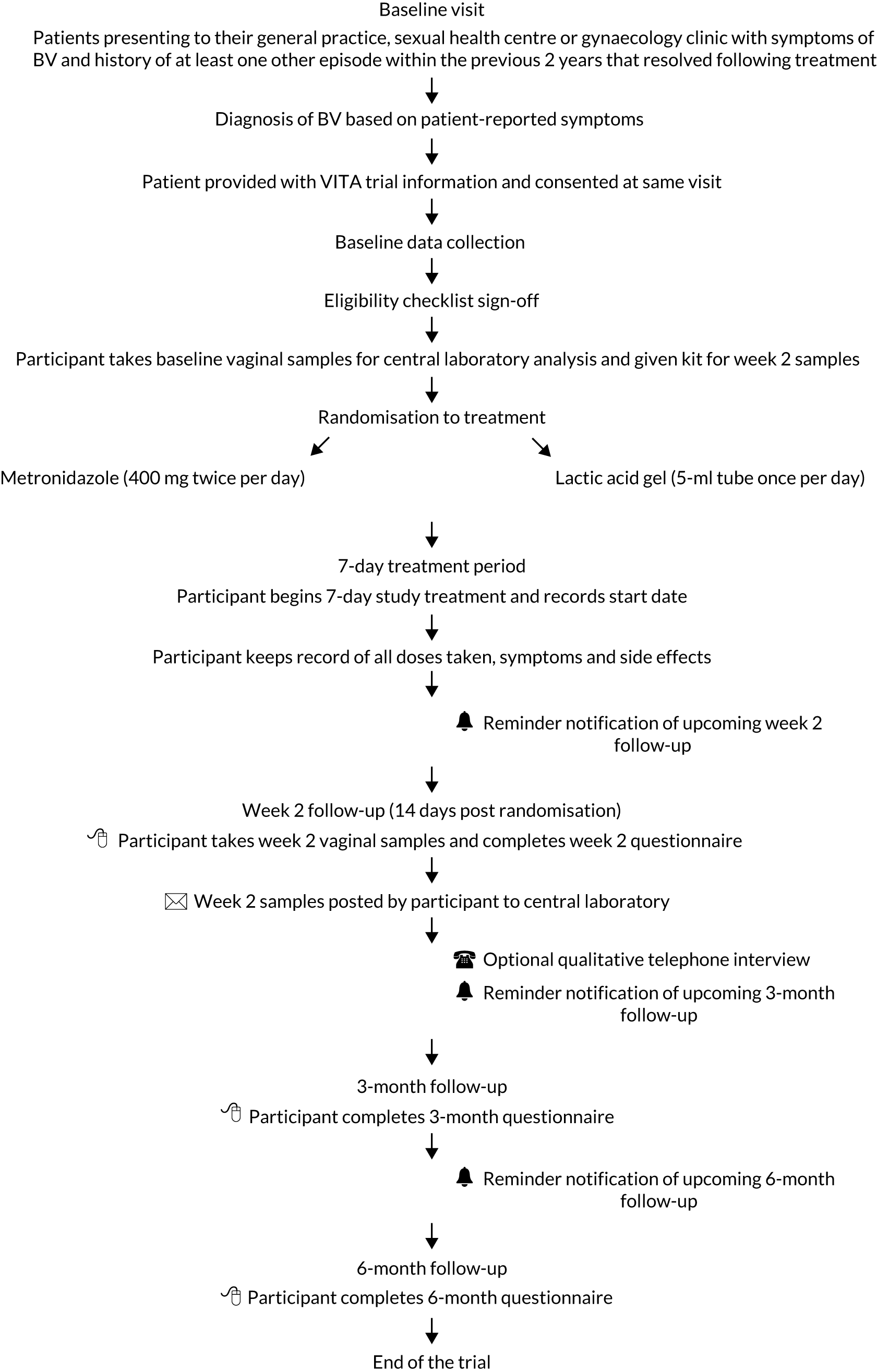
The flow of participants from presentation to follow-up is shown in Figure 2.
FIGURE 2.
Participant flow through the trial. Reproduced with permission from Armstrong-Buisseret et al. 25 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
Inclusion criteria
Individuals had to meet all of the following inclusion criteria to be included in the trial:
-
aged ≥ 16 years
-
clinical diagnosis of BV based on patient-reported symptoms of discharge with an unpleasant (typically fishy) odour, with or without positive microscopy according to local site practice
-
history of at least one previous episode of BV in the past 2 years (clinically diagnosed or patient reported) that had been resolved with treatment
-
willing to use either intravaginal lactic acid gel or oral metronidazole tablets for the management of BV
-
willing to take their own vaginal samples
-
willing to avoid vaginal douching during treatment
-
willing to provide contact details and be contacted for the purpose of collecting follow-up information
-
willing to avoid sexual intercourse or use effective contraception for the 7-day duration of study treatment (condoms were not considered to be effective contraception owing to a potential interaction with the lactic acid gel)
-
access to the internet and e-mail and willing to complete web-based follow-up questionnaires in English
-
written informed consent.
Exclusion criteria
Individuals were excluded from the trial if they met any of the following exclusion criteria:
-
contraindications or allergy to lactic acid gel or metronidazole tablets
-
pregnant or breastfeeding
-
patients currently trying to conceive and not willing to avoid sexual intercourse or use effective contraception for the 7-day duration of study treatment
-
using oral antibiotics (other than the study treatment) or antifungal agents concurrently, within the last 2 weeks or planned use within the next 2 weeks
-
using topical vaginal antibiotics, antifungals or acidifying products (other than the study treatment) concurrently, within the last 2 weeks or planned use within the next 2 weeks
-
previous participation in this study
-
current participation in another trial involving an investigational medicinal product (IMP).
Contraindications and concomitant medications
Metronidazole
As per the exclusion criteria, any known hypersensitivity to metronidazole, other nitroimidazole derivatives or any of the ingredients in metronidazole tablets would exclude patients from the trial. Sites were advised to refer to the summary of product characteristics (SmPC) for metronidazole for details, but to take particular note of the following:
-
Alcohol was to be avoided (including products containing alcohol) during the course of treatment and for 48 hours afterwards.
-
Warfarin (warfarin, Ranbaxy) – elevated international normalised ratio (INR) and bleeding events have been reported with concurrent use of warfarin and metronidazole.
Lactic acid gel
There is no SmPC for lactic acid gel, but sites were advised to take particular note of the following:
-
Shellfish allergy – some lactic acid gel brands may contain glycogen obtained from oysters.
-
Condom use – the effects of lactic acid gel on condom degradation have not been fully determined. Therefore, it was advised that condoms should not be assumed to be an effective method of contraception during the 7-day treatment period with lactic acid gel.
Concomitant medications
Concomitant medications relevant to BV, such as oral or topical antibiotics and/or antifungals, were recorded at baseline to determine participant eligibility.
Screening and consent
Screening
Women either pre-identified by or presenting to referring or recruiting general practices, sexual health centres or gynaecology clinics who had symptoms of BV (and a history of one or more episodes within the previous 2 years that resolved following treatment) were invited (by telephone or letter) or approached by a member of the site research team to determine whether or not they were interested in participating in the trial. If they were interested, they were given a participant information sheet.
Women who were identified in general practices and gynaecology clinics that were acting as referral centres were introduced to the trial and directed to a local recruiting practice, sexual health centre or gynaecology clinic for consent if they were interested in participating in the trial.
A screening log was maintained at each recruiting site detailing all patients approached about the study, the number of patients agreeing to participate, the reasons for not participating and, where relevant, the route of referral.
Consent
Women were given time to read the participant information sheet and had the opportunity to ask the site research team any questions about the trial prior to consent. Written informed consent was requested during the same clinic visit by the principal investigator (PI) or the delegated study doctor or nurse prior to performing any trial-related procedure. A copy of the completed informed consent form was given to the participant, a copy was filed in the medical notes and the original copy was placed in the investigator site file.
The consent process included optional consent to be approached by researchers from the University of Warwick for a qualitative telephone interview. When participants who had given this optional consent were contacted to arrange an interview, verbal consent that they remained willing to take part was recorded by the researcher before the interview began.
Participants recruited from sexual health centres and gynaecology clinics were also asked for their optional consent to inform their GP that they were taking part in the trial.
Randomisation and blinding
After obtaining informed consent, baseline data were collected by a member of the site research team. Participant eligibility was confirmed by the PI (or the delegated study doctor) prior to randomisation. Participants were randomised 1 : 1 to receive lactic acid gel or metronidazole using a remote internet-based randomisation system developed and maintained by Nottingham Clinical Trials Unit (NCTU). The concealed allocation system used a minimisation algorithm with the following variables and levels: site, type of site (general practice and sexual health clinic), number of episodes of BV in the previous 12 months (0, 1–3 and > 3) and whether or not they had had a female sexual partner in the previous 12 months (yes/no). The allocation system was held on a secure University of Nottingham server.
Given that this was an open-label trial, there was no blinding to treatment allocation of the participants, site research teams or trial team. However, the central laboratory staff performing BV microscopy and STI testing were blinded to participant’s treatment allocation. In addition, the trial statistician remained blinded to treatment allocation until after database lock. Analyses requiring knowledge of treatment codes were conducted by an independent statistician. Data presented to both the trial team and the Trial Steering Committee (TSC) were aggregated, that is they were not split by treatment allocation.
Trial intervention
There were two treatment arms in the trial:
-
lactic acid gel – 5 ml of gel inserted into the vagina before bedtime each day for 7 days
-
metronidazole tablets – 400 mg taken orally twice daily, approximately 12 hours apart, for 7 days.
Metronidazole tablets were an IMP in the trial and are licensed for use in the treatment of BV as per the SmPC. Lactic acid gel is a registered medical device consisting of a colourless viscous gel administered through an intravaginal tube applicator. Known side effects of lactic acid gel include vaginal irritation, for example redness, stinging and itching. In rare cases an allergic skin reaction, for example severe redness, swelling or burning, may occur.
Treatment supplies, labelling and storage
Participants received their study treatment via the routine method of dispensing used in the setting of each recruiting site. This could be via dispensing directly from standard clinic stocks or the provision of a standard prescription to be taken to a pharmacy for dispensing. Any licensed brands of metronidazole or lactic acid gel could be used and the brand of lactic acid gel was recorded by the participant in the web-based questionnaire.
Trial-specific labelling was not required given that the IMP has a marketing authorisation in the UK and was being used within the terms of its marketing authorisation. The IMP was dispensed to a trial participant in accordance with a prescription given by an authorised health-care professional and was labelled in accordance with the requirements of Schedule 5 to The Medicines for Human Use (SI 1994/31 94) (Marketing Authorisations Etc.) Regulations 199428 that apply to relevant dispensed medicinal products.
The IMP was stored in accordance with usual site policy and as per manufacturer’s instructions. Accountability records for treatment dispensing were in accordance with local site procedures and no additional trial-specific accountability was mandated.
Dosing schedule
It was requested that treatment was started on the day of receipt, and participants were advised to record their actual start date and time (morning or evening) of dosing in a paper patient diary that was given at the baseline visit. If they were menstruating at the time, those on the lactic acid gel arm were advised to delay starting treatment until menstruation had finished. Participants were also asked to use this diary with log all subsequent doses taken and/or missed doses over the treatment period to aid compliance with the treatment schedule and to record any symptoms, side effects or additional health-care use. The patient diaries were intended to be used as an aid for participants when completing their web-based questionnaires at 2 weeks, 3 months and 6 months.
No treatment or dose modifications were expected in this trial. Where a dose was accidentally missed, participants were advised to follow the manufacturer’s instructions or to seek advice from their physician. In the case of any missed dose, participants were advised to continue to complete their treatment course. Lactic acid gel was to be inserted vaginally before going to bed. Metronidazole tablets were to be taken during or after meals with a glass of water and not to be crushed or chewed, and were to be swallowed whole.
Trial assessments and procedures
All assessments and procedures performed at each time point for participants are indicated in Table 1. Assessments carried out at baseline included:
-
Demographics.
-
Symptoms.
-
Previous BV episodes.
-
Medical history.
-
Sexual history.
-
Concomitant medication.
-
Contraception and condom use.
-
Verbal confirmation that the participant was not pregnant.
-
SF-12 health survey.
-
Vaginal samples for BV/STI screening. Participants took their own vaginal samples following instruction from site personnel, and sites sent the baseline samples to a central laboratory at University Hospitals Birmingham NHS Foundation Trust, which is accredited under the UK Accreditation Service to perform the tests.
| Trial procedure | Baseline | Follow-up | |||
|---|---|---|---|---|---|
| Baseline | Post randomisation | 2 weeks | 3 months | 6 months | |
| Assessments | |||||
| Informed consent | ✗ | ||||
| Baseline data collection | ✗ | ||||
| Eligibility screen | ✗ | ||||
| Vaginal swabs for BV/STI screen | ✗ (participant) | ✗ (participant) | |||
| Randomisation | ✗ | ||||
| Posting of vaginal swabs to central laboratory | ✗ (site) | ✗ (participant) | |||
| Intervention | |||||
| Lactic acid gel (intervention arm) | ✗ (7-day treatment) | ||||
| Metronidazole (control arm) | ✗ (7-day treatment) | ||||
| Follow-up | |||||
| Participant web-based questionnaires | ✗ (site) | ✗ | ✗ | ✗ | |
| Telephone interviews for the qualitative substudy | ✗ (2–4 weeks from randomisation) | ||||
After randomisation into the trial, participants took their first dose of study treatment and continued taking study treatment for 7 days.
At 2 weeks, participants took their own vaginal samples using kits provided at the baseline visit and sent them to the central laboratory using pre-addressed and prepaid envelopes. They also completed a web-based questionnaire (see Appendices 1 and 2) with details of symptoms, treatment adherence and tolerability, any known side effects, health-care use, additional BV treatments, sexual history, contraception/condom use and another SF-12 health survey. A £15 voucher was provided to each participant on completion of the week 2 questionnaire as a thank you for their time. If requested to do so by a participant, the time frame for completing the questionnaire was extended.
Participants were also asked to complete two further web-based questionnaires at 3 months (see Appendix 3) and 6 months (see Appendix 4), with details of BV recurrence, sexual history, health-care use, additional BV treatments, contraception/condom use and the SF-12 health survey. Those not responding to requests to complete the week 2 and 6-month web-based questionnaires were contacted by telephone to collect follow-up data.
Participants could discontinue study treatment at any time but remain in the trial, taking week 2 vaginal samples and completing all follow-up questionnaires. They could also withdraw from the follow-up assessments at any time. Reason(s) for withdrawal were requested, but participants were not obliged to provide these.
Collection and analysis of vaginal samples
The central laboratory performed the following tests on vaginal samples taken at baseline and at week 2:
-
Microscopic assessment of BV based on a Gram-stained vaginal smear using the Ison–Hay scoring system. 29
-
Nucleic acid amplification tests (NAATs) for chlamydia, gonorrhoea and trichomoniasis. Positive results were returned within 1–2 months to the recruiting site to review (PI and research nurse) and to arrange further testing or treatment in accordance with local protocols.
These trial-related tests did not form the basis for patient management at the baseline visit; clinicians took additional tests that were processed locally to inform immediate patient care as indicated by the patient’s clinical presentation.
Substudies
Optional consent was sought from trial participants to store residual vaginal swabs taken at baseline and week 2 for future ethics-approved research, including microbiological analysis and gene sequencing into the factors associated with BV and its successful treatment.
Adverse events and pregnancy reporting
The safety profiles of the treatments in this trial are well characterised. To provide secondary outcome data to compare the tolerability of the two treatments, specified side effects experienced during study treatment were reported. The following were regarded as expected for the purpose of the trial and were reported on the week 2 questionnaire completed by the participant: nausea, vomiting, taste changes, vaginal irritation, abdominal pain and diarrhoea. Serious adverse events (SAEs) were not anticipated in this low-risk trial, but were recorded if reported by participants and were followed up until resolution or stabilisation.
Although lactic acid gel is considered safe for use in pregnancy and metronidazole is frequently prescribed for treatment of BV in pregnancy, caution is advised for their use in pregnant women and participants were asked to confirm that they were not pregnant as part of the screening process. Participants were also asked to confirm their pregnancy status during their follow-up period. Any pregnancies reported during the period between randomisation and week 2 were followed up for outcomes.
Data management
All baseline trial data were entered by site staff into a trial-specific database through the electronic case report form (MACRO 4.2.1 version 3800; Elsevier, London, UK), with participants identified by their unique trial number and initials only. All data collected after the baseline visit, that is at week 2, 3 months and 6 months, were entered by participants into the trial-specific database using a web-based questionnaire. The database was developed and maintained by NCTU. Access to the database was restricted and secure, and all data transactions were logged in a full audit trail.
Participant contact details were stored in a separate secure database using encryption with restricted password-protected access. Only appropriate members of the site team and the trial team had access to these data.
Statistical considerations
Analysis of outcome measures
A full statistical analysis plan was developed and agreed to prior to database lock and unblinding of the analysing statistician, and all analyses were carried out using Stata® version 15.1 (StataCorp LP, College Station, TX, USA). Continuous variables were summarised in terms of the mean, standard deviation (SD), median, lower and upper quartiles, minimum, maximum and number of observations. Categorical variables were summarised in terms of frequency counts and percentages. Descriptive statistics of demographic and clinical measures were used to assess the balance between the treatment arms at baseline, but no formal statistical comparisons were made.
The primary approach to between-arm comparative analyses was by modified intention to treat, that is it included all participants who were randomised and without imputation of missing outcome data. Sensitivity analyses were conducted to investigate the impact of missing data, additional baseline variables and adherence to allocated treatment.
Evaluation of the primary outcome was performed using a generalised estimating equation for the binary outcome that included factors used in the minimisation, with site as the panel variable. This was changed prior to database lock (and before finalising the statistical analysis plan and unblinding the trial statistician) from the originally planned mixed-effects model owing to model non-convergence because some sites had very small numbers of participants. It had been planned to also include whether or not vaginal douching had occurred in the 3 months prior to randomisation, but model convergence issues resulted in this being excluded from the model. The comparison of lactic acid gel with oral metronidazole was presented using the risk difference of the proportion of participants who reported resolution of symptoms at week 2, along with the 95% confidence interval (CI). The adjusted risk ratio and 95% CI were also presented. Owing to non-convergence of several of the models, sensitivity analyses also used a random-effects logit model including the same factors, presenting the odds ratio and 95% CI.
Secondary outcomes were analysed using appropriate regression models dependent on data type (e.g. binary, continuous, count and survival), and included factors used in the minimisation and baseline value of the outcome where measured. The analyses of secondary outcomes were considered supportive to the primary outcomes, and estimates and p-values, where presented, were interpreted in this light.
Presentations of quantitative tolerability data were descriptive. Frequency counts and percentages of the proportion of participants reporting nausea, vomiting, taste disturbance, vaginal irritation, abdominal pain and diarrhoea were presented by treatment arms.
Planned subgroup analyses
The primary analysis for symptom resolution was investigated to determine whether or not treatment effectiveness differed according to the following subgroups:
-
presence of concomitant STI (yes/no)
-
BV confirmed by positive microscopy (yes/no)
-
number of episodes of BV in the 12 months before baseline (0, 1–3 and > 3)
-
total time with BV in the 12 months before baseline (< 2 weeks, ≥ 2 weeks and < 3 months, ≥ 3 months).
A further subgroup analysis was planned to determine whether or not treatment effectiveness differed according to the type of centre at which the participant presented (sexual health vs. GP/other clinics); however, this was not performed given that no gynaecology clinics and only one GP practice took part in the trial. Between-group treatment effects were provided for each subgroup, but interpretation of any subgroup effects was based on the treatment–subgroup interaction and 95% CI, estimated by fitting an appropriate interaction term in the regression models. Given that the trial was powered to detect overall differences between the groups rather than interactions of this kind, these subgroup analyses were regarded as exploratory. An interaction term could not be fitted when investigating the subgroup for the presence of concomitant STI at baseline owing to the small number of participants with a STI.
Feasibility
There was no planned interim analysis of treatment efficacy. However, an assessment of recruitment and adherence to treatment was performed using data from the first 6 months of participant recruitment. This was to determine how feasible it was that the trial would be able to adequately address its primary and secondary objectives.
The TSC and Data Monitoring Committee (DMC) used the following criteria as a guide to determine whether or not the trial should progress:
-
Review of the number of participants completing their week 2 assessment against the following targets –
-
> 90% – continue the trial
-
65–90% – review recruitment and retention procedures to identify underlying problems and put in place strategies to address these, with review in 6 months
-
35–65% – review recruitment and retention procedures to identify underlying problems and put in place strategies to address these. Ongoing review over 6 months and terminate the trial if the recruitment trajectory does not indicate that full recruitment can occur within an acceptable recruitment period
-
< 35% – terminate the trial.
-
-
Review of adherence to lactic acid gel and metronidazole against the following predefined targets –
-
median adherence 5–7 days per week – continue the trial
-
median adherence 3–4 days per week – review data from the qualitative interviews on adherence and tolerability to identify underlying problems and put in place strategies to address these, with review in 6 months
-
median adherence < 3 days per week – terminate the trial.
-
Power calculation/sample size calculation
Assuming that 80% of participants receiving oral metronidazole would achieve resolution of symptoms,24,30–32 1710 participants (855 in each treatment arm) were required for analysis to detect a 6% increase in response rate to 86% in those receiving lactic acid gel (risk ratio 1.08) at the 5% significance level (two-sided) with 90% power. To allow for loss to follow-up of 10% (i.e. non-collection of the primary outcome), a total of 1900 participants were required to be recruited.
Patient and public involvement
Patient and public involvement (PPI) representatives provided input during the development of the grant application into the trial design, including feedback on the importance of the research question, the effect size used in the power calculation and ideas on how to make participation in the trial appealing to potential recruits. They also requested the addition of the secondary outcome of time to recurrence of BV because it was felt that this was an important measure. In addition, PPI representatives reviewed all participant-facing documents prior to submission for ethics approval. These documents included the participant information sheet; informed consent form; participant invitation letter; participant kit instruction leaflet; all participant questionnaires, reminders and diaries; all advertising materials; and the qualitative interview schedule.
The PPI representatives sat on the TSC as independent members and contributed to the oversight of the trial, including reviewing and interpretating the results and providing input to the Plain English summary. Once the trial results have been published, a summary will be disseminated to participants, and PPI representatives will be invited to review the summary prior to distribution.
Chapter 3 Clinical results
Sites
Recruitment was originally planned to take place at approximately 25 primary care general practices and 15 sexual health outpatient and gynaecology clinics in the UK. However, during the course of the trial, a total of one general practice and 21 sexual health centres were opened to recruitment; the general practice and 19 of the sexual health centres recruited at least one participant. Although the Primary Care Clinical Research Network was involved in the planning of the trial, fewer primary care centres than expected had sufficient research nurse resources to support recruitment and, with approval from the TSC, additional sexual health centres were identified to take part. One gynaecology clinic was approached but none was identified that was willing to participate in the trial.
Recruitment
Recruitment took place between October 2017 and June 2019. It was originally planned that recruitment would continue until 1900 participants had been randomised, with an initial projected date for completion of recruitment of November 2019. However, in May 2019 the DMC reviewed the unblinded trial data at a planned meeting and its recommendation from this review was that trial recruitment should be stopped, because its opinion was that the primary research question had been answered with the number of participants, recruited at that time. There were no concerns raised around any safety issues. To ensure that this was a robust decision, further analyses were conducted that were reviewed by the DMC in June 2019, and its recommendation remained the same. The TSC supported this opinion and recruitment into the trial was terminated on 28 June 2019, with follow-up of ongoing participants continuing for 6 months (completed 26 February 2020 to allow sufficient time for receipt of the final questionnaires after sending all reminders), as per the protocol. No additional information about the reason for the recommendation was provided to any member of the trial team.
During the recruitment period, a total of 3141 patients were approached (Figure 3), of whom 2618 (83%) were excluded prior to consent; the main reason given was not having a history of at least one previous episode of BV within the last 2 years (n = 695) (see Appendix 5, Table 28). A total of 523 (17%) participants consented to take part in the trial, of whom five were excluded after consent because they were not eligible (n = 3) or they changed their mind about participating (n = 2) (see Figure 3). This gave a total of 518 participants who were randomised into the trial (metronidazole arm, n = 259; lactic acid gel arm, n = 259), who represented 16% of those presenting with BV and 99% of those who consented.
FIGURE 3.
The CONSORT flow diagram. a, Prescribed lactic acid gel as refused allocated study treatment; b, ineligible as taking warfarin, prescribed lactic acid gel instead; c, no study treatment given as participant received medication to treat thrush; d, preferred metronidazole after being randomised to lactic acid gel; e, included as one of the two withdrawn before week 2 in the next box down; f, includes outcomes obtained from the week 2 questionnaire for which a date of resolution was given without an answer to the ‘Have your BV symptoms cleared’ question, and primary outcome data collected by telephone, includes outcomes obtained from the 3-month questionnaire asking about resolution by week 2; g, at least one data item entered on the questionnaire; and h, at least one data item entered on the questionnaire or obtained by telephone.
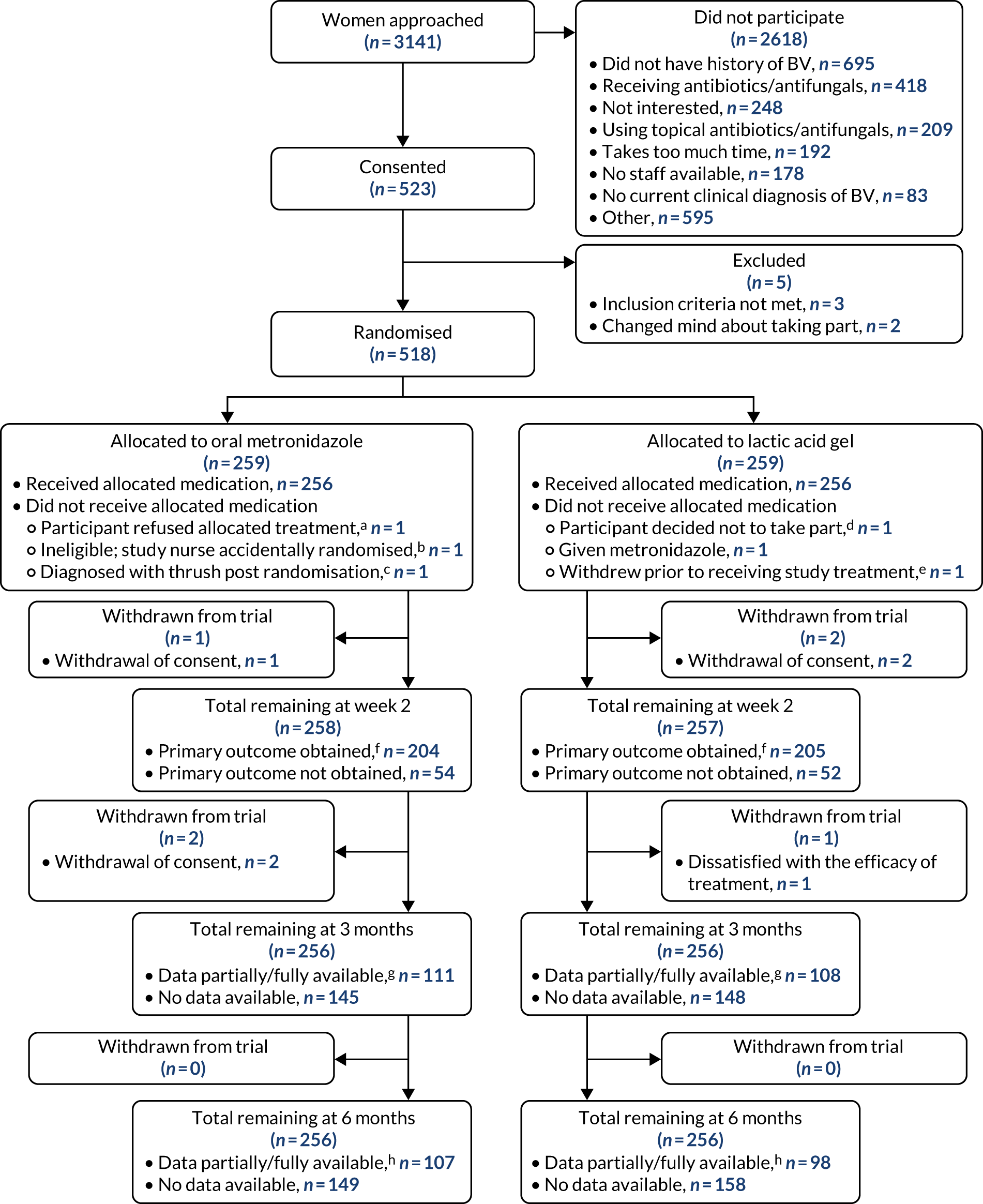
A total of three participants (metronidazole arm, n = 1; lactic acid gel arm, n = 2) withdrew their consent in the first 2 weeks, resulting in 258 participants in the metronidazole arm and 257 in the lactic acid gel arm remaining in the trial at week 2 (see Figure 3). A further three participants withdrew between week 2 and 3 months, two from the metronidazole arm (both because of withdrawal of consent) and one from the lactic acid gel arm (because of being dissatisfied with the efficacy of the treatment), giving a total of 512 participants remaining in the trial at 3 months (256 per arm). There were no known participant withdrawals between 3 months and 6 months.
Of the 22 sites that were opened, 20 screened and recruited at least one participant (see Appendix 5, Table 29).
Baseline characteristics
The baseline characteristics of the participants were similar between the two treatment arms (Table 2). The age of the participants ranged from 16 to 58 years, with a mean of 29 (SD 8.3) years. Forty-eight per cent of the participants were of white ethnicity and 23% were black Caribbean. Vaginal douching was carried out by 12% of the participants in the 3 months before baseline. A total of 198 (38%) participants had experienced more than three episodes of BV in the previous 12 months, and BV was confirmed microscopically at baseline in 436 (84%) participants by local laboratories and in 266 (51%) participants using Ison–Hay grade 329 by the central laboratory.
| Characteristic | Treatment arm | Total (N = 518) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Age at randomisation (years) | |||
| Mean (SD) | 29.0 (8.41) | 29.4 (8.12) | 29.2 (8.26) |
| Median (25th, 75th centile) | 27 (23, 34) | 27 (23, 34) | 27 (23, 34) |
| Minimum, maximum | 16, 58 | 18, 57 | 16, 58 |
| Ethnicity, n (%) | |||
| White | 125 (48) | 126 (49) | 251 (48) |
| Black Caribbean | 62 (24) | 57 (22) | 119 (23) |
| Mixed race | 24 (9) | 27 (10) | 51 (10) |
| Black African | 26 (10) | 15 (6) | 41 (8) |
| Other | 8 (3) | 8 (3) | 16 (3) |
| Other Asian (non-Chinese) | 5 (2) | 6 (2) | 11 (2) |
| Indian | 4 (2) | 5 (2) | 9 (2) |
| Black (other) | 1 (< 0.5) | 6 (2) | 7 (1) |
| Chinese | 1 (< 0.5) | 4 (2) | 5 (1) |
| Pakistani | 3 (1) | 1 (< 0.5) | 4 (1) |
| Bangladeshi | 0 | 3 (1) | 3 (1) |
| Not given | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Vaginal douching in the past 3 months, n (%) | |||
| Yes | 36 (14) | 25 (10) | 61 (12) |
| No | 223 (86) | 233 (90) | 456 (88) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Frequency of douching per month, n (%) | |||
| 0–2 | 9 (25) | 6 (24) | 15 (25) |
| 3–4 | 9 (25) | 5 (20) | 14 (23) |
| 5–6 | 1 (3) | 3 (12) | 4 (7) |
| ≥ 7 | 17 (47) | 11 (44) | 28 (46) |
| Current use of oral contraceptive pill, n (%) | |||
| Yes | 45 (17) | 44 (17) | 89 (17) |
| No | 214 (83) | 214 (83) | 428 (83) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Type of contraceptive pill, n (%) | |||
| Combined oral contraceptive pill | 27 (60) | 30 (68) | 57 (64) |
| Progesterone-only pill | 18 (40) | 14 (32) | 32 (36) |
| Past history of BV | |||
| Approximate age when BV first occurred (years) | |||
| n | 258 | 258 | 516 |
| Mean (SD) | 23.8 (7.26) | 23.4 (6.49) | 23.6 (6.88) |
| Median (25th, 75th centile) | 22 (18, 27) | 22 (19, 27) | 22 (19, 27) |
| Minimum, maximum | 14, 58 | 11, 50 | 11, 58 |
| Number of previous episodes of BV in the past 12 months, n (%) | |||
| 0 | 3 (1) | 3 (1) | 6 (1) |
| 1–3 | 157 (61) | 157 (61) | 314 (61) |
| > 3 | 99 (38) | 99 (38) | 198 (38) |
| Approximate total length of time in past year with BV symptoms, n (%) | |||
| < 2 weeks | 56 (22) | 40 (15) | 96 (19) |
| ≥ 2 weeks and < 3 months | 135 (52) | 130 (50) | 265 (51) |
| ≥ 3 months | 68 (26) | 88 (34) | 156 (30) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| BV confirmed at baseline visit (local laboratory), n (%) | |||
| Yes | 217 (84) | 219 (85) | 436 (84) |
| No | 31 (12) | 29 (11) | 60 (12) |
| Not tested | 11 (4) | 10 (4) | 21 (4) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Baseline sample Ison–Hay grade for BV (central laboratory),a n (%) | |||
| 0 (no bacteria) | 1 (< 0.5) | 1 (< 0.5) | 2 (< 0.5) |
| 1 (normal flora) | 48 (19) | 62 (24) | 110 (21) |
| 2 (intermediate BV) | 62 (24) | 61 (24) | 123 (24) |
| 3 (confirmed BV) | 138 (53) | 128 (49) | 266 (51) |
| U (Gram-positive cocci) | 3 (1) | 1 (< 0.5) | 4 (1) |
| Missing | 7 (3) | 6 (2) | 13 (3) |
The differences in BV microscopy results obtained from local and central laboratory analyses of samples are available in Appendix 5, Table 30.
Medical and sexual histories are summarised in Appendix 5, Tables 31 and 32. The vast majority of participants were HIV negative (99%) and around half (48%) reported having had thrush in the 12 months before baseline. A total of 365 (71%) participants had a sexual partner at baseline and 51 (10%) had a female sexual partner in the 12 months before baseline.
Participant-reported symptoms at baseline are summarised in Table 3, and included 470 out of 518 (91%) participants with genital discharge, 440 out of 518 (85%) with an offensive vaginal smell, 193 out of 518 (37%) with vaginal irritation and 406 out of 518 (78%) had both discharge and an offensive smell.
| Symptom | Treatment arm | Total (N = 518) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Abnormal genital discharge,a n (%) | |||
| Yes | 239 (92) | 231 (89) | 470 (91) |
| No | 20 (8) | 27 (10) | 47 (9) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| For those with discharge, length of time present during this episode (days) | |||
| n | 239 | 231 | 470 |
| Mean (SD) | 31 (58.6) | 31 (57.0) | 31 (57.7) |
| Median (25th, 75th centile) | 14 (7, 28) | 14 (7, 28) | 14 (7, 28) |
| Minimum, maximum | 1, 365 | 1, 365 | 1, 365 |
| Offensive vaginal smell,b n (%) | |||
| Yes | 227 (88) | 213 (82) | 440 (85) |
| No | 32 (12) | 45 (17) | 77 (15) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| For those with smell, length of time present during this episode (days) | |||
| n | 227 | 212 | 439 |
| Mean (SD) | 26 (45.0) | 32 (55.2) | 29 (50.2) |
| Median (25th, 75th centile) | 10 (5, 28) | 14 (7, 30) | 14 (6, 28) |
| Minimum, maximum | 1, 351 | 1, 365 | 1, 365 |
| Presence of both discharge and smell, n (%) | |||
| Yes | 211 (81) | 195 (75) | 406 (78) |
| No | 48 (19) | 63 (24) | 111 (21) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Vaginal irritation, n (%) | |||
| Yes | 100 (39) | 93 (36) | 193 (37) |
| No | 159 (61) | 165 (64) | 324 (63) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| For those with irritation, length of time present during this episode (days)c | |||
| n | 100 | 93 | 193 |
| Mean (SD) | 27 (101.8) | 22 (48.1) | 25 (80.4) |
| Median (25th, 75th centile) | 7 (3, 21) | 7 (3, 14) | 7 (3, 20) |
| Minimum, maximum | 1, 999 | 1, 364 | 1, 999 |
Data completeness
All of the 518 randomised participants were considered in the analysis of primary and secondary outcomes. Details of where data were missing, for example because of questionnaires or samples not being returned, are given below.
Questionnaires and telephone calls
The completion of data via web-based questionnaires and telephone calls was similar between the two treatment arms (see Appendix 5, Tables 33 and 34). The web-based questionnaire response rates were 318 out of 515 (62%) at week 2, 219 out of 512 (43%) at 3 months and 176 out of 512 (34%) at 6 months (see Appendix 5, Table 33). Further key data were obtained by telephone, with week 2 primary outcome information on BV resolution provided by an additional 88 out of the 202 participants for whom contact was attempted (success rate of 44%); secondary outcomes on recurrence were given by an additional 29 out of the 105 participants for whom contact was attempted at 6 months (success rate of 28%).
The median (minimum–maximum) time from randomisation to return of the week 2 questionnaires was 15 (14–55) days, with most of the questionnaires being returned within 28 days. For week 2 telephone data, the median time for obtaining these was 55.5 (29–155) days. This latter time was much longer than that for the questionnaires because the decision to collect data by telephone was made part-way through the trial to try to improve response rates. In addition, participants were given up to 28 days after randomisation to return questionnaires before a telephone call was attempted. The median time to return of the 3- and 6-month questionnaires was 93 (89–113) days and 186 (181–208) days, respectively. Six-month telephone data were collected after a median (minimum–maximum) time of 231 (203–265) days.
Week 2 vaginal samples
Week 2 vaginal samples were received by the central laboratory from 301 (58%) participants and numbers were similar between the treatment arms (see Appendix 5, Table 35). Overall, 280 out of 515 (54%) participants reported a primary outcome at week 2 and returned their week 2 samples, with similar numbers in both treatment arms (see Appendix 5, Table 35).
Nucleic acid amplification test analysis for sexually transmitted infections
Kits were provided for participants to take vaginal swab samples at baseline and week 2 for NAAT analysis of STIs. At each time point, one swab sample was placed into a tube containing a transport fluid and the tube was shipped directly to the central laboratory that was responsible for performing the analysis. On 16 January 2019, an issue was identified at three sites that some kits had been given to participants that contained time-expired NAAT sample tubes. The manufacturer of the tubes confirmed that there were no stability data past the point of expiry; therefore, it was decided that any samples received in expired tubes would be considered as void. An investigation took place into the expiry dates of existing stock at all sites and it became apparent that use of expired sample tubes was a wider issue.
Given that the expiry dates of sample tubes were not recorded by the manufacturer, site or NCTU, an audit was conducted at the central laboratory on 4 June 2019 of all NAAT samples received to determine whether or not sample tubes had expired prior to the date that each sample was taken. This audit revealed that the central laboratory had discarded some residual samples in error; therefore, for some participants it was not possible to determine whether or not the sample tubes had expired before use. For those baseline NAAT samples that were available at the central laboratory and for which expiry dates could be checked, a total of 339 samples were confirmed as being valid and within the expiry date, and a further 32 samples were confirmed as being void (i.e. the tube had expired prior to use) (see Appendix 5, Table 36). Expiry status could not be confirmed for 138 baseline samples because, although the central laboratory records indicated that these had been received, the residual samples had since been discarded. The remaining nine baseline samples either were not received by the central laboratory according to their records (n = 7) or were received but without accurate identifiable information (n = 2). For those week 2 NAAT samples that were available at the central laboratory and for which expiry dates could be checked, a total of 224 samples were confirmed as being valid and within the expiry date, and a further 19 samples were confirmed as being void (i.e. the tube had expired prior to use) (see Appendix 5, Table 36). Expiry status could not be confirmed for 58 week 2 samples because, although the central laboratory records indicated that these had been received, the residual samples had since been discarded. The remaining 214 week 2 samples either were not received by the central laboratory according to their records (n = 213) or were received but without accurate identifiable information (n = 1).
Sites followed their usual guidelines for treatment of suspected STIs during the participant’s baseline visit and clinical care of participants was not dependent on these NAAT results, which were taken purely for trial purposes.
Protocol non-compliance
Of the 518 participants who were randomised, six did not receive their allocated treatment (metronidazole arm, n = 3; lactic acid gel arm, n = 3) as a result of refusing to take the allocated study treatment (n = 1), being found to be ineligible post randomisation (n = 1), being diagnosed post randomisation with thrush, requiring antifungal treatment (n = 1), declining to take part post randomisation (n = 1), being given the other study treatment (n = 1) or withdrawing prior to receiving study treatment (n = 1) (see Figure 3). For a further two participants allocated to the lactic acid gel arm, the wrong value of the minimisation variable ‘any female sexual partners in previous 12 months’ was entered onto the system; they both received lactic acid gel and continued in the trial. No further incidents of non-compliance were recorded.
Primary outcome
Primary outcome data were available for 409 (79%) participants (see Figure 3): 321 who entered their primary outcome data into the questionnaire and 88 for whom primary outcome data were collected via a follow-up telephone call. Of the 321 participants who provided the primary outcome on the questionnaire, 316 entered this on the week 2 questionnaire (314 answered yes or no to the primary outcome question and two did not respond to the yes/no question but provided a date of resolution) and a further five with missing primary outcome data at week 2 provided a response on the 3-month questionnaire indicating either that their BV had resolved by week 2 or that their BV was ongoing.
Resolution of BV symptoms was higher in the metronidazole arm than in the lactic acid gel arm: 143 out of 204 (70%) participants in the metronidazole arm reported resolution at week 2, compared with 97 out of 205 (47%) participants in the lactic acid gel arm. The adjusted risk difference was –23.2% (95% CI –32.3% to –14.0%) and the adjusted risk ratio was 0.67 (95% CI 0.57 to 0.79) (Table 4). The analysis was adjusted for site, number of BV episodes in the 12 months before baseline (0, 1–3 or > 3) and female partner in the 12 months before baseline (yes/no), but not for vaginal douching owing to non-convergence of the model.
| Resolution of BV at week 2 | Treatment arm, n (%) | Adjusted risk difference (95% CI)a | Adjusted risk ratio (95% CI)a | Adjusted odds ratio (95% CI)a,b | |
|---|---|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||||
| Yes | 143 (70) | 97 (47) | –23.2% (–32.3% to –14.0%) | 0.67 (0.57 to 0.79) | 0.38 (0.25 to 0.57) |
| No | 61 (30) | 108 (53) | |||
| Missing | 55 | 54 | |||
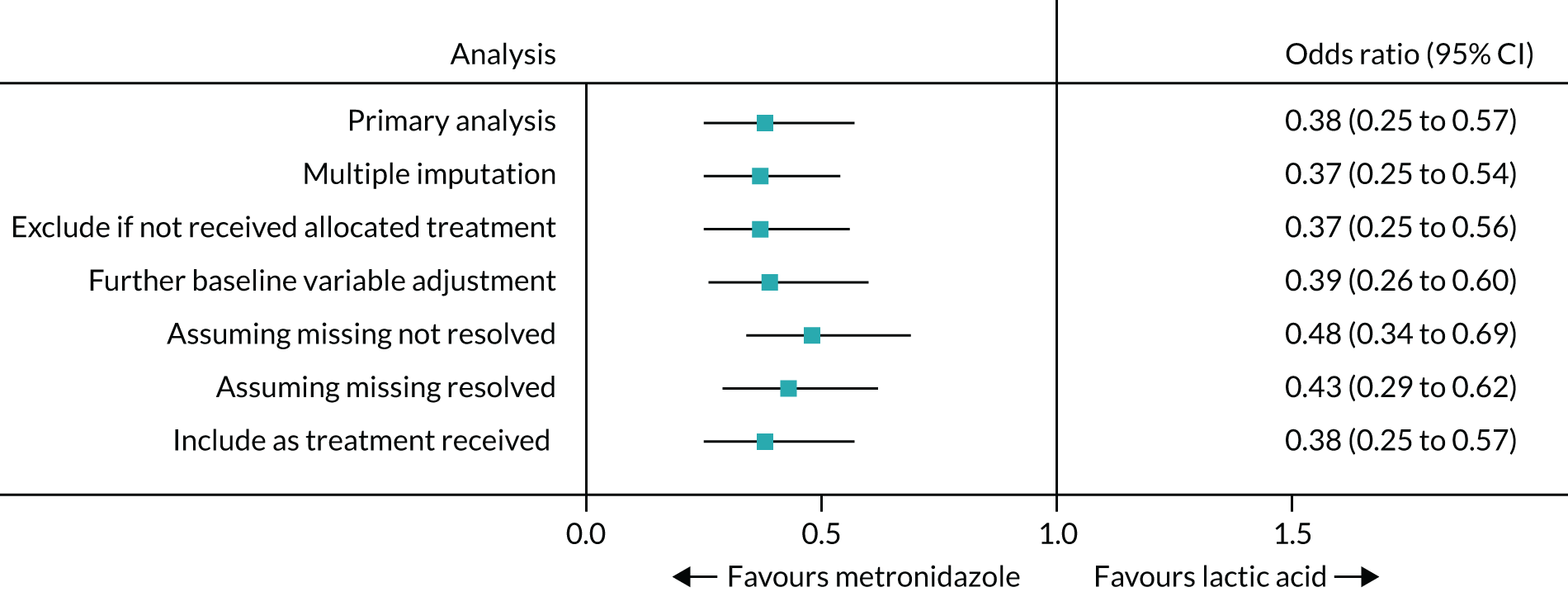
The sensitivity analyses showed similar results (Table 5 and Figure 4; see also Table 4). Odds ratios were presented for comparison of the sensitivity analyses owing to non-convergence of two of the models for treatment difference.
| Scenario | Resolution of BV symptoms, n (%) | Adjusted risk difference (95% CI)a | Adjusted odds ratio (95% CI)a |
|---|---|---|---|
| Multiple imputation of missing resolution datab | |||
| Oral metronidazole (N = 259) | (70) | Not estimablec | 0.37 (0.25 to 0.54) |
| Intravaginal lactic acid gel (N = 259) | (47) | ||
| Excluding those who did not receive allocated treatment | |||
| Oral metronidazole (N = 256) | 143 (70) | –23.5% (–32.6% to –14.3%) | 0.37 (0.25 to 0.56) |
| Intravaginal lactic acid gel (N = 256) | 96 (47) | ||
| Further adjustment for baseline variablesd | |||
| Oral metronidazole (N = 259) | 143 (70) | Not estimablec | 0.39 (0.26 to 0.60) |
| Intravaginal lactic acid gel (N = 259) | 97 (47) | ||
| Assuming missing symptom resolution as not resolved | |||
| Oral metronidazole (N = 259) | 143 (55) | –17.8% (–26.2% to –9.3%) | 0.48 (0.34 to 0.69) |
| Intravaginal lactic acid gel (N = 259) | 97 (37) | ||
| Assuming missing symptom resolution as resolved | |||
| Oral metronidazole (N = 259) | 199 (77) | –18.5% (–26.3% to –10.7%) | 0.43 (0.29 to 0.62) |
| Intravaginal lactic acid gel (N = 259) | 153 (59) | ||
| Included in arm as treatment received | |||
| Oral metronidazole (N = 258) | 144 (71) | –23.6% (–32.8% to –14.5%) | 0.38 (0.25 to 0.57) |
| Intravaginal lactic acid gel (N = 258) | 96 (48) | ||
FIGURE 4.
Forest plot for the primary outcome and sensitivity analyses.
A planned post hoc investigation showed that resolution rates were lower in both treatment arms when the data were collected via questionnaires than via telephone calls (see Appendix 5, Table 37). Of the 158 participants in the metronidazole arm who provided resolution data via a questionnaire, 105 (66%) reported that symptoms had resolved, whereas of the 46 who provided these data via a telephone call, 38 (83%) reported that symptoms had resolved. Of the 163 participants in the lactic acid gel arm who provided resolution data via a questionnaire, 75 (46%) reported that symptoms had resolved, whereas of the 42 who provided the data via a telephone call, 22 (52%) reported that symptoms had resolved.
Of those participants who reported resolution of symptoms at week 2, 154 in the metronidazole arm and 158 in the lactic acid gel arm also gave information on whether or not any additional treatment had been taken for their BV (Table 6). A total of 22 out of 154 (14%) participants in the metronidazole arm and 20 out of 158 (13%) participants in the lactic acid gel arm had taken additional treatment, of whom 12 out of 22 (55%) and 7 out of 20 (35%), respectively, had resolution of their symptoms. Although resolution rates were lower for those taking additional treatment, the difference between treatment arms was similar with an unadjusted risk difference for resolution with additional treatment of –19.5% (95% CI –49.0% to 10.0%) compared with –19.6% (95% CI –31.1% to –8.1%) for resolution without additional treatment (see Table 6).
| Resolution of BV | Treatment arm, n (%) | Unadjusted risk difference (95% CI) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| With additional treatment | |||
| N | 22 | 20 | |
| Yes | 12 (55) | 7 (35) | –19.5% (–49.0% to 10.0%) |
| No | 10 (45) | 13 (65) | |
| Without additional treatment | |||
| N | 132 | 138 | |
| Yes | 90 (68) | 67 (49) | –19.6% (–31.1% to –8.1%) |
| No | 42 (32) | 71 (51) | |
There were no statistically significant treatment-by-subgroup interactions. The number of valid STI samples available at baseline and week 2 was small owing to the expiry date issue and, consequently, the number of participants with a STI (from a valid sample) at baseline and who had resolution data at week 2 (n = 8) was too small to allow any analysis. In each of the other subgroups, resolution rates were consistently higher in the metronidazole arm than in the lactic acid gel arm (Tables 7 and 8). Treatment outcomes in the subgroup of participants who had confirmation of a BV diagnosis by positive microscopy are given in Microbiological resolution of bacterial vaginosis on microscopy of vaginal smears at week 2.
| Baseline subgroup | Treatment arm: BV resolution at 2 weeks, n (%) | |||||
|---|---|---|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |||||
| Yes | No | Missing | Yes | No | Missing | |
| Presence of concomitant STI at baselinea | ||||||
| Yes | 5 (100) | 0 | 7 | 0 | 3 (100) | 0 |
| No | 85 (67) | 41 (33) | 26 | 66 (50) | 67 (50) | 36 |
| Missing | 53 | 20 | 22 | 31 | 3 | 18 |
| BV confirmed by positive baseline microscopy (central laboratory Ison–Hay grade 3) | ||||||
| Yes | 83 (75) | 28 (25) | 27 | 49 (49) | 52 (51) | 27 |
| No | 58 (65) | 31 (35) | 25 | 46 (46) | 55 (54) | 24 |
| Missing | 2 | 2 | 3 | 2 | 1 | 3 |
| Number of episodes of BV in 12 months before baseline | ||||||
| 0 | 1 (100) | 0 | 2 | 2 (67) | 1 (33) | 0 |
| 1–3 | 91 (75) | 31 (25) | 35 | 61 (51) | 59 (49) | 37 |
| > 3 | 51 (63) | 30 (37) | 18 | 34 (41) | 48 (59) | 17 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 |
| Total time with BV in 12 months before baseline | ||||||
| < 2 weeks | 31 (72) | 12 (28) | 13 | 20 (57) | 15 (43) | 5 |
| ≥ 2 weeks and < 3 months | 78 (73) | 29 (27) | 28 | 54 (53) | 48 (47) | 28 |
| ≥ 3 months | 34 (63) | 20 (37) | 14 | 23 (34) | 45 (66) | 20 |
| Missing | 0 | 0 | 0 | 0 | 0 | 1 |
| Subgroup | Treatment difference (95% CI)a | Estimate of treatment–subgroup interaction (95% CI); p-valueb |
|---|---|---|
| Presence of concomitant STI at baseline | ||
| No (n = 259) | –17.8% (–29.6% to –6.0%) | Not enough data to analyse |
| Yes (n = 8)c | –100% (not calculable) | |
| BV confirmed by positive baseline microscopy (central laboratory Ison–Hay grade 3)d | ||
| No (n = 190) | –19.6% (–33.5% to –5.8%) | 2.1% (–11.6% to 15.7%); 0.77 |
| Yes (n = 212) | –26.3% (–38.9% to –13.6%) | |
| Number of episodes of BV in 12 months before baseline | ||
| 0 (n = 4) | –33.3% (–86.7% to 20.0%) | |
| 1–3 (n = 242) | –23.8% (–35.6% to –11.9%) | –2.0% (–21.2% to 17.0%); 0.83 |
| > 3 (n = 163) | –21.5% (–36.5% to –6.5%) | |
| Total time with BV in 12 months before baseline | ||
| < 2 weeks (n = 78) | –15.0% (–36.1% to 6.2%) | |
| ≥ 2 weeks and < 3 months (n = 209) | –20.0% (–32.8% to –7.1%) | –3.1% (–27.8% to 21.6%); 0.81 |
| ≥ 3 months (n = 122) | –29.1% (–46.2% to –12.0%) | –11.1% (–38.5% to 16.4%); 0.43 |
Secondary outcomes
Time to first recurrence of bacterial vaginosis
Among those participants who reported symptom resolution by week 2 (metronidazole arm, n = 143; lactic acid gel arm, n = 97), data on recurrence at 3 months were available for 73 (51%) and 50 (52%) participants, respectively, and at 6 months for 72 (50%) and 46 (47%) participants, respectively (Table 9). Of these participants, 37 out of 73 (51%) in the metronidazole arm and 23 out of 50 (46%) in the lactic acid gel arm reported recurrence by 3 months, whereas 51 out of 72 (71%) and 32 out of 46 (70%) reported recurrence by 6 months, respectively (see Table 9). The median [standard error (SE)] times to recurrence, allowing for censored times (when there was no reported recurrence up to 6 months, the 6-month data were used to calculate the censored time; if there was no reported recurrence up to 3 months and 6-month data were missing, the 3-month data were used; times were not available for recurrences reported by telephone at 6 months), were 92 (34.6) days in the metronidazole arm and 124 (SE not calculable) days in the lactic acid gel arm. It was possible to calculate only the lower limits of the 95% CIs (as there were not enough uncensored ‘events’) and these were 71 and 74 days, respectively (see Table 9). Including only those who had a time to recurrence gave median times of 54 (n = 43) days and 66 (n = 25) days in the metronidazole and the lactic acid gel arms, respectively. Times to first recurrence were not compared between treatment arms using statistical tests given that they comprised only those who had symptom resolution within 2 weeks and any comparison would, therefore, not be between randomised arms. A post hoc investigation showed that baseline characteristics were similar between treatment arms for the subset of participants resolving by week 2 (see Appendix 5, Table 38).
| Resolution/recurrence of BV | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Resolved by 2 weeks (n) | 143 | 97 |
| Recurred within 3 months, n/N (%) | 37/73 (51) | 23/50 (46) |
| Recurred within 6 months, n/N (%) | 51/72 (71) | 32/46 (70) |
| Number with time to recurrence (including censored times) (n) | 73 | 50 |
| Median time to recurrence (SE) (days) | 92 (34.6) | 124 (–)a |
| 95% CI (days) | (71 to –)a | (74 to –)a |
A Kaplan–Meier plot of recurrence against time is shown in Figure 5.
FIGURE 5.
Kaplan–Meier plot of recurrence of BV symptoms against time.
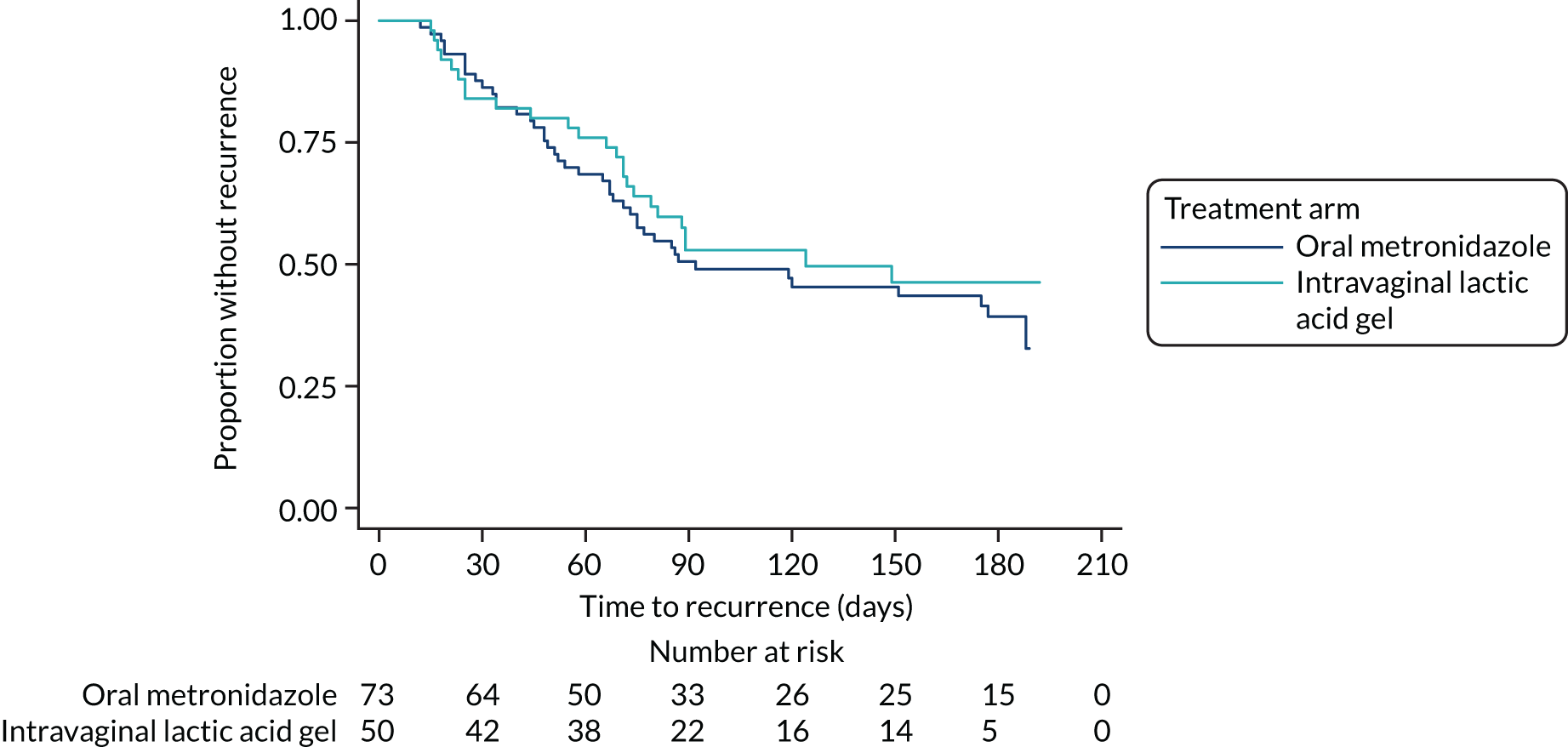
There were few participants (n = 13) who resolved, had time to recurrence data and reported that additional treatment was taken during the first 2 weeks; therefore, the difference between those with and those without additional treatment could not be assessed (see Appendix 5, Table 39).
Time to recurrence was censored at 6 months if data were available at both 3 months and 6 months, but no recurrence had been reported. Of the times used in the analysis, 30 (41%) in the metronidazole arm and 25 (50%) in the lactic acid gel arm were censored times.
Including only those with data at both 3 months and 6 months gave the percentage recurring at 6 months as 60% (32/53) in the metronidazole arm and 56% (18/32) in the lactic acid gel arm. This removes inflation of the recurrence rate resulting from needing an episode at only one time for recurrence, but data at both times to record no recurrence.
Number of participant-reported bacterial vaginosis episodes over 6 months
The number of participants whose symptoms resolved by week 2 and who had complete episode data available at both 3 months and 6 months was small (metronidazole arm, n = 48; lactic acid gel arm, n = 29). There was little difference between treatment arms in the number of subsequent episodes of BV between week 2 and 6 months for those who had resolved by week 2. Both treatment arms had a median of one episode over the 6-month period, with a maximum of six episodes in the metronidazole arm and 10 episodes in the lactic acid gel arm (Table 10). The adjusted incidence rate ratio was 0.97 (95% CI 0.56 to 1.69). The analysis was adjusted for site, number of BV episodes in the 12 months before baseline and female partner in the 12 months before baseline (yes/no). Although vaginal douching could be included in the negative binomial model, it made little difference to the estimates (incidence rate ratio 1.00, 95% CI 0.58 to 1.72) and it was more consistent with the other analyses not to include this variable. The incidence rate is defined as the number of episodes per time at risk, that is if both treatment arms were followed up for the same length of time (in this case 6 months), the ratio of episodes in the metronidazole arm compared with the lactic acid gel arm was estimated as 0.97.
| Resolution/persistence of BV | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Resolved by week 2 | n = 143 | n = 97 |
| Number of episodes within 3 months per participant whose symptoms resolved within 2 weeks | ||
| Total resolved by week 2 with 3-month episode data | 71 | 46 |
| Median number of episodes (25th, 75th centile) | 0 (0, 1) | 0 (0, 1) |
| Minimum, maximum | 0, 4 | 0, 8 |
| Number of episodes within 6 months per participant whose symptoms resolved within 2 weeks | ||
| Total resolved by week 2 with complete episode dataa | 48 | 29 |
| Median number of episodes (25th, 75th centile) | 1 (0, 3) | 1 (0, 2) |
| Minimum, maximum | 0, 6 | 0, 10 |
| Did not resolve by week 2 | n = 61 | n = 108 |
| Number of episodes within 3 months per participant whose symptoms did not resolve within 2 weeks | ||
| Total not resolved by week 2, with 3-month episode data | 26 | 40 |
| Median number of episodes (25th, 75th centile) | 1 (0, 2) | 1.5 (0, 2) |
| Minimum, maximum | 0, 6 | 0, 5 |
| Number of episodes within 6 months per participant whose symptoms did not resolve within 2 weeks | ||
| Total not resolved by week 2, with complete episode dataa | 16 | 25 |
| Median number of episodes (25th, 75th centile) | 1 (0, 2) | 3 (1, 4) |
| Minimum, maximum | 0, 13 | 0, 93b |
Number of participant-reported bacterial vaginosis treatment courses over 6 months
Data on the number of BV treatment courses over the 6 months of the trial (excluding the study treatment) for those who had resolved by week 2 were available for 59 out of 143 (41%) participants in the metronidazole arm and 35 out of 97 (36%) participants in the lactic acid gel arm (see Appendix 5, Table 40). For those resolving by week 2, the median number of BV treatment courses received between week 2 and 6 months was similar between the treatment arms (metronidazole arm, median = 1; lactic acid gel arm, median = 1), with an adjusted incidence rate of 1.03 (95% CI 0.53 to 2.01) (see Appendix 5, Tables 40 and 41), and are explored further in the health economic analysis (see Chapter 5).
Microbiological resolution of bacterial vaginosis on microscopy of vaginal smears at week 2
The number of participants with central laboratory microbiological confirmation of BV (Ison–Hay grade 3) on vaginal smears taken at baseline was 138 participants in the metronidazole arm and 128 participants in the lactic acid gel arm (Table 11). Of those participants, 77 (56%) in the metronidazole arm and 73 (57%) in the lactic acid gel arm had central laboratory BV results available at week 2. Microbiological resolution of BV at week 2 in those with confirmed BV at baseline was higher in the metronidazole arm (59/77 participants; 77%) than in the lactic acid gel arm (31/73 participants; 42%), with an adjusted risk difference of –34.3% (95% CI –49.1% to –19.5%) (see Table 11). Microbiological resolution was defined as having Ison–Hay grade 3 at baseline followed by Ison–Hay grades 0, 1, 2 or U at week 2.
| Resolution of BV | Treatment arm, n (%) | Adjusted risk difference (95% CI)a | Adjusted odds ratio (95% CI)a | |
|---|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |||
| Number with baseline-confirmed BV (Ison–Hay grade 3 by central laboratory) | 138 | 128 | ||
| Microbiological resolution of BV by week 2 | ||||
| Yes | 59 (77) | 31 (42) | –34.3% (–49.1% to –19.5%) | 0.22 (0.10 to 0.46) |
| No | 18 (23) | 42 (58) | ||
| Missing | 61 | 55 | ||
Of those participants with microbiological confirmation of BV at baseline, resolution of symptoms occurred in 83 out of 111 (75%) participants in the metronidazole arm and 49 out of 101 (49%) participants in the lactic acid gel arm.
When rates of resolution were compared between microbiological results and participant-reported symptomatic results, they were similar (60% indicating microbiological resolution and 61% indicating symptomatic resolution) (see Appendix 5, Table 42). However, these results were not consistent in about one-third of cases with data; that is, 25 out of 145 (17%) samples did not indicate microbiological resolution while the participant did report symptomatic resolution, and a further 24 out of 145 (17%) samples showed microbiological resolution without symptomatic resolution.
Time to participant-reported resolution of bacterial vaginosis symptoms
The median time to participant-reported resolution of BV symptoms (overall, not just up to week 2) was 14 days in both treatment arms (see Appendix 5, Table 43), with missing times in the first 2 weeks being substituted by 14 days [40/142 (28%) and 22/97 (23%) of times were substituted in the metronidazole and lactic acid gel arms, respectively], and times for those not recording resolution censored at the latest time without resolution (adjusted difference 0, 95% CI –1.9 to 1.9; 134 values were censored). Without censoring, the median times were 7 days in the metronidazole arm and 8.5 days in the lactic acid gel arm (Figure 6; see Appendix 5, Table 43), and without censoring or substitution median times were 5 days in both arms.
FIGURE 6.
Kaplan–Meier plot of time to resolution of BV symptoms at any time up to 6 months.
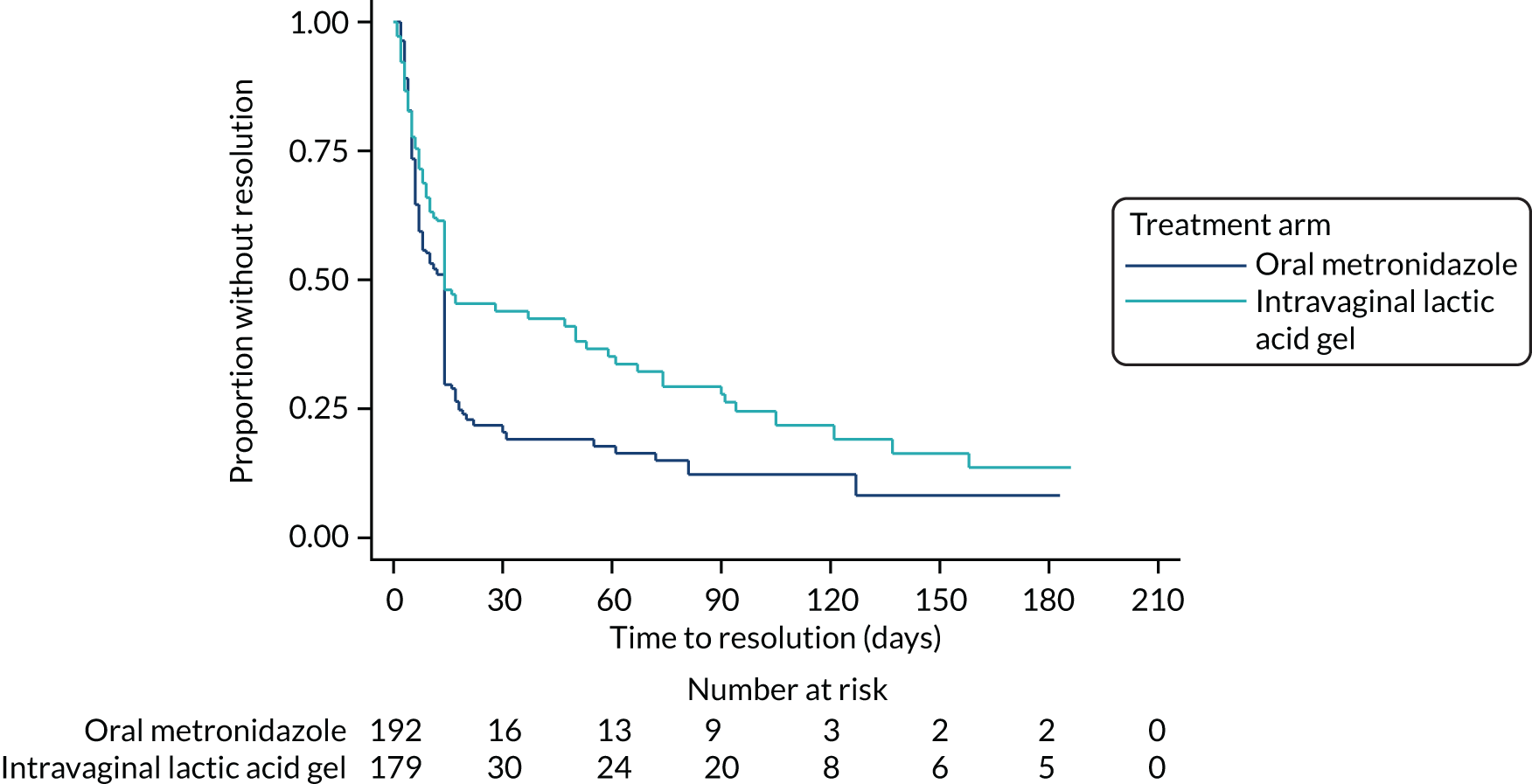
The majority of participants did not report using additional treatment, but, of those who did, median times were slightly longer than for those without (Figure 7; see Appendix 5, Table 43).
FIGURE 7.
Kaplan–Meier plot of time to resolution of BV symptoms at any time up to 6 months, split by additional treatment. Lines plotted are for oral metronidazole and intravaginal lactic acid gel either with or without additional treatment.
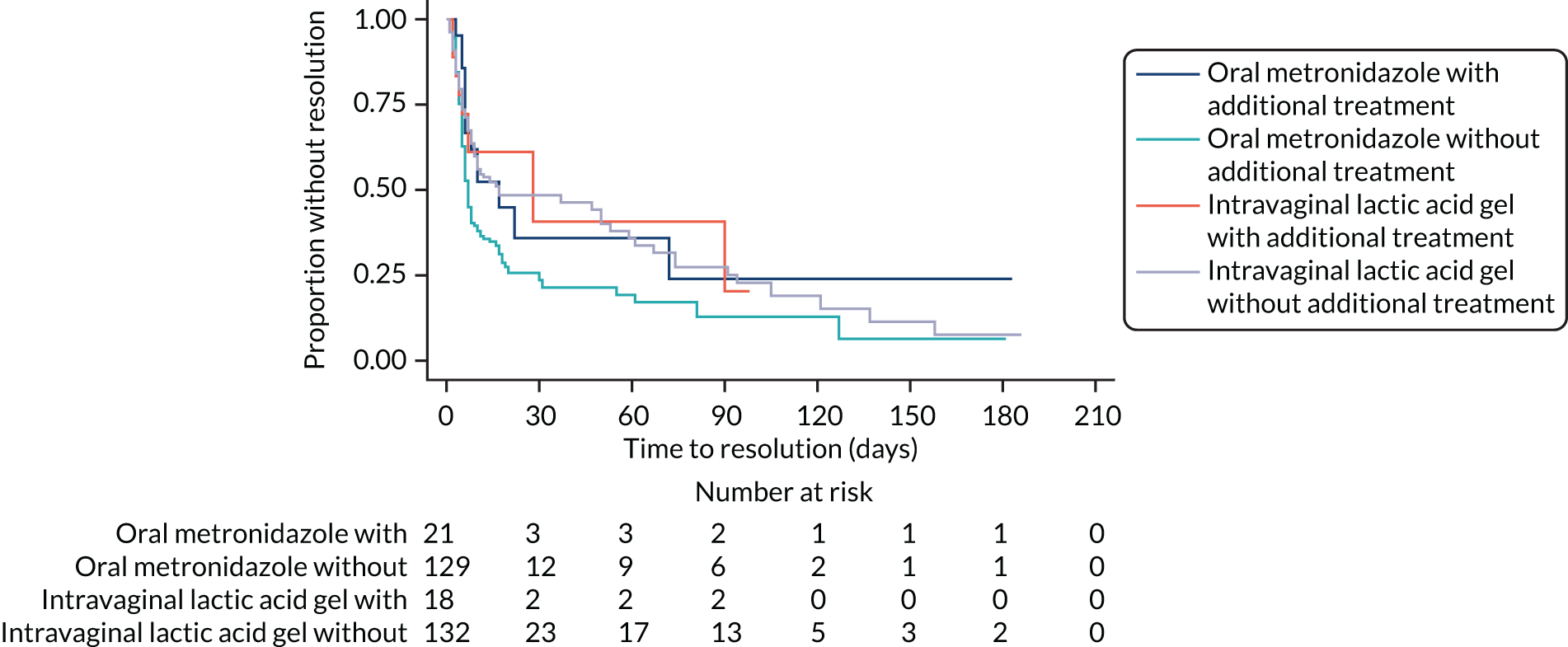
Tolerability of study treatments assessed by participant reporting of side effects
The description of the tolerability data included 516 participants summarised according to the study treatment that they received: 258 in each treatment arm. One participant in each arm did not receive any study treatment [one in the metronidazole arm because they received clotrimazole (Canesten®, Bayer) for thrush diagnosed post randomisation and did not provide any safety data; one withdrew from the lactic acid gel arm prior to receiving any study treatment] (see Figure 3). Of the 516 participants included in the tolerability analyses, two in each arm received the other study treatment from that allocated (see Figure 3). In addition, of the 258 participants in the metronidazole arm who were included in the tolerability analyses, two withdrew (one of whom was allocated lactic acid gel), whereas no participants withdrew of the 258 participants in the lactic acid gel arm who were included in the tolerability analyses.
In those participants returning week 2 questionnaires, there was a higher reported incidence of side effects in the metronidazole arm than in the lactic acid gel arm, particularly of nausea (32% vs. 8%, respectively), taste changes (18% vs. 1%, respectively) and diarrhoea (20% vs. 6%, respectively) (Table 12).
| Side effect | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Nausea | ||
| Yes | 50 (32) | 13 (8) |
| No | 103 (66) | 144 (89) |
| Missing | 3 (2) | 4 (2) |
| Vomiting | ||
| Yes | 9 (6) | 2 (1) |
| No | 141 (90) | 152 (94) |
| Missing | 6 (4) | 7 (4) |
| Taste changes | ||
| Yes | 28 (18) | 2 (1) |
| No | 127 (81) | 156 (97) |
| Missing | 1 (1) | 3 (2) |
| Vaginal irritationa | ||
| Yes | 44 (28) | 34 (21) |
| No | 110 (71) | 125 (78) |
| Missing | 2 (1) | 2 (1) |
| Abdominal pain | ||
| Yes | 31 (20) | 27 (17) |
| No | 123 (79) | 132 (82) |
| Missing | 2 (1) | 2 (1) |
| Diarrhoea | ||
| Yes | 31 (20) | 9 (6) |
| No | 123 (79) | 150 (93) |
| Missing | 2 (1) | 2 (1) |
Further details of these side effects are available (see Appendix 5, Tables 44–51). The proportion of participants with vaginal irritation at week 2 was lower in the group who did not have irritation at baseline than in the group who did (see Appendix 5, Tables 48 and 49).
Participant-reported adherence to treatment
Of the 318 participants who returned a week 2 questionnaire, 316 (99%) reported that they took at least some of their study treatment (Table 13). A total of 294 (92%) participants took at least 85% of their course: 146 out of 157 (93%) participants in the metronidazole arm compared with 148 out of 161 (92%) participants in the lactic acid gel arm (see Table 13). For 113 out of 157 (72%) participants in the metronidazole arm, the antibiotic was easy or very easy to take, whereas 142 out of 161 (88%) participants in the lactic acid gel arm reported that their treatment was easy or very easy to use. The brands of lactic acid gel that were used are reported in Appendix 5, Table 53.
| Participant-reported adherence | Treatment arm | Total (N = 518) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Number of participants returning questionnaire | 157 | 161 | 318 |
| Participant took/used any randomised treatment, n (%) | |||
| Yes | 156 (99) | 160 (99) | 316 (99) |
| No | 1 (1) | 1 (1) | 2 (1) |
| Missing | 0 | 0 | 0 |
| Course of study treatment completed, n (%)a | |||
| Yes | 144 (92) | 147 (91) | 291 (92) |
| No | 13 (8) | 14 (9) | 27 (8) |
| Missing | 0 | 0 | 0 |
| Percentage of treatment course completed | |||
| n | 157 | 161 | 318 |
| Mean (SD) | 94% (18.4%) | 95% (12.9%) | 95% (15.8%) |
| Median (25th, 75th centile) | 100% (100%, 100%) | 100% (100%, 100%) | 100% (100%, 100%) |
| Minimum, maximum | 0%, 100% | 0%, 100% | 0%, 100% |
| Received at least 85%, n (%) | 146 (93) | 148 (92) | 294 (92) |
| Reason if treatment course not completed, n (%)b | |||
| Accidentally missed | 10 (48) | 7 (58) | 17 (52) |
| Did not like using/taking it | 1 (5) | 1 (8) | 2 (6) |
| Side effects of treatment | 4 (19) | 0 | 4 (12) |
| Otherc | 6 (29) | 4 (33) | 10 (30) |
| Ease of taking study treatment, n (%) | |||
| Very easy | 63 (40) | 81 (50) | 144 (45) |
| Easy | 50 (32) | 61 (38) | 111 (35) |
| Neither easy nor difficult | 35 (22) | 16 (10) | 51 (16) |
| Difficult | 7 (4) | 2 (1) | 9 (3) |
| Very difficult | 1 (1) | 0 | 1 (< 0.5) |
| Missing | 1 (1) | 1 (1) | 2 (1) |
| Time from randomisation to treatment start (days)d | |||
| n | 156 | 156 | 312 |
| Median (25th, 75th centile) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
| Minimum, maximum | 0, 26 | 0, 17 | 0, 26 |
Prevalence of concurrent sexually transmitted infections at baseline and week 2
Prevalence of concurrent STIs at both baseline and week 2 was very low (Table 14; see Appendix 5, Tables 54–56). Out of the samples from unexpired kits (both known and uncertain expiry), there was none positive for gonorrhoea at baseline or week 2, between 1% and 5% positive for chlamydia, and 0–2% positive for trichomoniasis.
| STI present (gonorrhoea, chlamydia or trichomoniasis) | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Baseline samples received by the central laboratory | 253 | 256 |
| Week 2 samples received by the central laboratory | 148 | 153 |
| Baseline (excludes out-of-date sample kits) | ||
| N | 237 | 240 |
| Yes | 20 (8) | 8 (3) |
| No | 214 (90) | 230 (96) |
| Missing | 3 (1) | 2 (1) |
| Week 2 (excludes out-of-date sample kits) | ||
| N | 140 | 142 |
| Yes | 8 (6) | 3 (2) |
| No | 126 (90) | 136 (96) |
| Missing | 6 (4) | 3 (2) |
| Baseline (excludes out-of-date sample kits and those with unknown expiry status) | ||
| N | 166 | 173 |
| Yes | 12 (7) | 3 (2) |
| No | 152 (92) | 169 (98) |
| Missing | 2 (1) | 1 (1) |
| Week 2 (excludes out-of-date sample kits and those with unknown expiry status) | ||
| N | 110 | 114 |
| Yes | 5 (5) | 1 (1) |
| No | 103 (94) | 110 (97) |
| Missing | 2 (2) | 3 (3) |
Out of the 339 baseline samples that were confirmed as being valid, none had gonorrhoea, 10 (3%) had chlamydia and five (1%) had trichomoniasis (see Appendix 5, Tables 54–56). Out of the 224 week 2 samples confirmed as being valid, none had gonorrhoea, five (2%) had chlamydia and one (< 1%) had trichomoniasis (see Appendix 5, Tables 54–56).
Overall, there were 12 participants in the metronidazole arm and three in the lactic acid gel arm with a diagnosed STI at baseline. By week 2, some participants had acquired a STI, whereas in others the infection had resolved (Table 15).
| STI at baseline | Treatment arm: STI at week 2 (n) | |||
|---|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |||
| Yes | No | Yes | No | |
| Yes | 2 | 2 | 1 | 2 |
| No | 3 | 84 | 0 | 97 |
An analysis of the prevalence of STIs at week 2, adjusting for baseline STIs and site, showed little difference between the treatment arms, with an odds ratio of 0.15 (95% CI 0.01 to 1.60) (Table 16).
| Excludes out-of-date sample kits and those with expiry date unknown | Treatment arm, n (%) | Adjusted odds ratio (95% CI)a | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Number with a STI at baseline | 12 | 3 | |
| Number with a STI at week 2 | |||
| Yes | 5 (5) | 1 (1) | 0.15 (0.01 to 1.60) |
| No | 103 (95) | 110 (99) | |
| Missing | 151 | 148 | |
Additional follow-up data
Time with bacterial vaginosis recurrence after first resolution of symptoms
The total time with BV recurrence symptoms (in weeks) if they resolved within 2 weeks was reported by a subset of participants. Over the 6-month trial period, this was similar between the two treatment arms (see Appendix 5, Table 57). At 3 months, 37 out of 143 (26%) participants in the metronidazole arm reported recurrence compared with 23 out of 97 (24%) participants in the lactic acid gel arm. At 6 months, 51 out of 143 (36%) participants in the metronidazole arm reported recurrence compared with 32 out of 97 (33%) participants in the lactic acid gel arm.
Status of symptoms for those without resolution at week 2
The status of symptoms for those without resolution at week 2 was ‘better but not cleared/disappeared’ or ‘improved initially but worsened again’ in more than half of the participants and similar in both treatment arms (see Appendix 5, Table 58). In total, 39 out of 60 (65%) participants in the metronidazole arm reported ‘better’ or ‘improved’ compared with 64 out of 106 (60%) participants in the lactic acid gel arm.
Symptoms at week 2
Around one-third of participants reported the presence of typical BV symptoms at week 2, including genital discharge, offensive vaginal smell and vaginal irritation (see Appendix 5, Table 59). Slightly fewer participants (33%) in the metronidazole arm than in the lactic acid gel arm (41%) reported genital discharge at week 2. This was similar for those having an offensive vaginal smell (27% in the metronidazole arm vs. 40% in the lactic acid gel arm). Fewer participants reported vaginal irritation at week 2 in the lactic acid gel arm than in the metronidazole arm (23% vs. 30%, respectively).
Symptoms at recurrence compared with typical symptoms
Where reported, recurrence symptoms were mostly typical of usual BV symptoms in both treatment arms (see Appendix 5, Table 60). At 3 months, 28 out of 37 (76%) participants in the metronidazole arm reported recurrence symptoms that were ‘always’ typical of usual symptoms, compared with 15 out of 23 (65%) participants in the lactic acid gel arm. The corresponding results at 6 months were 22 out of 51 (43%) participants in the metronidazole arm and 13 out of 32 (41%) participants in the lactic acid gel arm. There was only one report of recurrence symptoms seldom being typical of usual symptoms, and this was in the lactic acid gel arm.
Additional medication for bacterial vaginosis
Nearly half of all participants in both treatment arms reported taking additional medication for BV after the first 2 weeks. Metronidazole tablets and lactic acid gel were the most frequently used additional medication, with similar proportions of each being taken in both treatment arms (see Appendix 5, Tables 61 and 62). A total of 52 out of 111 (47%) participants in the metronidazole arm and 51 out of 108 (47%) participants in the lactic acid gel arm took additional medication within 3 months, whereas 44 out of 92 (48%) and 39 out of 84 (46%) participants, respectively, took additional medication within 6 months.
Antibiotics for other conditions/illness
Around 10% of participants in both treatment arms took antibiotics for other conditions or illness in each reporting time period (see Appendix 5, Tables 63 and 64). In total, 15 out of 157 (10%) participants in the metronidazole arm and 21 out of 161 (13%) participants in the lactic acid gel arm took antibiotics for other conditions within the first 2 weeks, 8 out of 111 (7%) and 12 out of 108 (11%), respectively, took antibiotics within 3 months, and 9 out of 92 (10%) and 10 out of 84 (12%), respectively, took antibiotics within 6 months.
Vaginal thrush post randomisation
In the first 2 weeks post randomisation, of those returning a week 2 questionnaire, 42 out of 157 (27%) participants in the metronidazole arm and 27 out of 161 (17%) participants in the lactic acid gel arm reported developing thrush, for which around half of the participants took treatment (see Appendix 5, Table 65). Between week 2 and 3 months, of those returning a 3-month questionnaire, 20 out of 111 (18%) participants in the metronidazole arm and 26 out of 108 (24%) participants in the lactic acid gel arm developed thrush. Of those returning a 6-month questionnaire, 23 out of 92 (25%) and 20 out of 84 (24%) participants developed thrush between 3 and 6 months, respectively, in the two arms. The overall incidence over the 6 months was 60 out of 259 (23%) participants in the metronidazole arm and 58 out of 259 (22%) participants in the lactic acid gel arm (where no incidence over 6 months meant that ‘no’ was reported on all three questionnaires).
Sexual contact post baseline
Having sex, use of condoms and new sexual partners were similar between the two arms (see Appendix 5, Table 66). Nearly half of all participants reported having sex between the start of study treatment and the week 2 assessment in both treatment arms [71/157 (45%) in the metronidazole arm vs. 67/161 (42%) in the lactic acid gel arm]. Around three-quarters of participants did not use condoms [52/71 (73%) in the metronidazole arm vs. 48/67 (72%) in the lactic acid gel arm].
Vaginal douching post randomisation
Few participants (< 9%) reported vaginal douching during the trial, with similar percentages in each treatment arm (see Appendix 5, Table 67). At week 2, 6 out of 157 (4%) participants in the metronidazole arm reported that vaginal douching had been performed, compared with 6 out of 161 (4%) participants in the lactic acid gel arm. At 3 months, 6 out of 111 (5%) compared with 8 out of 108 (7%) participants, respectively, reported vaginal douching. At 6 months, 6 out of 92 (7%) compared with 7 out of 84 (8%), respectively, reported vaginal douching.
Participant-reported sexually transmitted infections diagnosed from 2 weeks post baseline
The incidence of participant-reported STIs diagnosed from 2 weeks post baseline was low (see Appendix 5, Table 68). In the metronidazole arm, there were five episodes of gonorrhoea, three of chlamydia, four of trichomoniasis and three of PID. In the lactic acid gel arm, there was one episode of gonorrhoea, four of chlamydia, zero of trichomoniasis and five of PID.
Other reasons reported by participants for not returning a week 2 sample
Other reasons reported by participants for not returning a week 2 sample are given in Appendix 5, Table 69. These are summarised when a sample was not received by the laboratory; often the questionnaire was completed before taking the sample. There were eight in total, five of which were because of the participant having their period (three in the metronidazole arm and two in the lactic acid gel arm).
Symptoms over the 6 months
A post hoc investigation looked at the number of participants whose symptoms resolved and remained so for 6 months compared with those whose symptoms did not resolve or who had symptoms at some point in the 6 months (see Appendix 5, Table 70). Of those with data reported at all three time points over the 6 months, 21 out of 91 (23%) participants in the metronidazole arm and 14 out of 88 (16%) participants in the lactic acid gel arm resolved and remained so for the 6 months.
Additional safety data
There were no participant-reported hospitalisations in the 2 weeks from randomisation, either for BV or for treatment-related side effects, and no SAEs or suspected unexpected serious adverse reactions were reported. There were two known pregnancies, both in the lactic acid gel arm. The estimated conception dates were 20 days pre randomisation (participant chose to have an induced abortion) and 63 days after randomisation (outcome unknown as the site was unable to make contact with the participant).
Chapter 4 Health economic evaluation
Introduction
The global economic burden of BV is substantial, with almost US$4.8B spent on treatments each year around the world. 9 The standard antibiotic treatment for BV, metronidazole, is associated with side effects and high recurrence rates,9,33 and alternative options are, therefore, required to reduce antibiotic use, provide better efficacy and reduce the recurrence of this condition. Intravaginal lactic acid gel has been proposed as an alternative treatment; however, more evidence is required on the costs of this treatment compared with oral metronidazole, in addition to assessing its clinical effectiveness. The objective of the economic evaluation in the VITA trial was to compare the cost-effectiveness of intravaginal lactic acid gel with oral metronidazole in the treatment of recurrent BV. 25 In addition, given that the trial was investigating whether or not intravaginal lactic acid gel could be used to help reduce antibiotic exposure, exploratory analyses were included to investigate the costs associated with antibiotic resistance as a penalty cost for antibiotic use. It is important to consider this type of cost because economic evaluations that include antibiotic treatments but do not include the cost of AMR may provide suboptimal information for decision-makers. 34
Methods
The health economic analysis involved both a cost-effectiveness analysis (using the primary clinical outcome) and a cost–utility analysis. The economic evaluation was conducted from a health-care (NHS) perspective. The methods used for this within-trial analysis were guided by National Institute for Health and Care Excellence recommendations35 and followed Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines for reporting. 36 Discounting was not required for costs and outcomes because participants were followed up for a period of 6 months.
Health outcomes
The cost-effectiveness analysis reflected the primary outcome of the trial, which was participant-reported resolution of BV symptoms at week 2. The cost–utility analysis assessed health-related quality of life (HRQoL) using the SF-12 health survey (version 2), which was completed by participants at baseline, week 2, 3 months and 6 months. The SF-12 was selected as a measure of HRQoL because previous research had suggested that the derived utility scores [Short Form questionnaire-6 Dimensions (SF-6D)] were more efficient at detecting differences in self-reported health status in a related area (maternal health). 37 An algorithm developed by Brazier and Roberts38 was used to predict the SF-6D preference-based score from the SF-12 health survey. The values were obtained from a survey of the UK population to derive a utility-based algorithm. The SF-6D is a preference-based measure that is composed of a six-dimensional health state classification and uses information from the SF-12 health survey. 38 The SF-6D values take into account societal preferences for health states and, therefore, can be used to obtain quality-adjusted life-years (QALYs). Following the trapezium rule, these data were used to calculate QALY gain at 6 months. 39
Resource use and costs
For the economic evaluation, the perspective that was adopted was that of the NHS (health-care perspective), meaning that only direct health-care costs were considered. Self-reported web-based questionnaires were distributed to all participants at each follow-up point to collect resource use data in primary and secondary care settings, such as treatment use, GP visits, clinic visits, additional medication for BV and thrush, and other health-care resource use to estimate the costs associated with the administration of both treatments. A separate analysis was conducted to consider any ‘out-of-pocket’ expenses incurred by participants, including the cost of non-prescribed medications as part of the sensitivity analysis.
The following assumptions were made in relation to resource use:
-
Where participants reported that a health resource was utilised without specifying the number of health-care contacts, a minimum number of contacts was assumed. For example, if a GP visit was reported without further data, it was assumed that there had been one GP visit. Although it was planned to use multiple imputation to account for missing data, this was not possible owing to the extent of missing data. 40
-
If thrush was reported and treatment obtained, it was assumed that the participant had obtained one course of the treatment specified.
Valuation of resource use
A microcosting approach was used to estimate the total costs associated with each treatment arm. This approach assigned costs to each component of resource use to capture the participant-level variation in costs.
Data on unit costs were obtained from routine and published literature, such as Unit Costs of Health and Social Care41 and NHS reference costs. 42 The prices of study treatment and additional medication were obtained from the British National Formulary. 43 Relevant items of resource use, their associated unit costs, their description and the source from which these costs were obtained are given in Appendix 5, Table 71. All costs were reported in UK currency [Great British pounds (GBP)] for 2018–19. Where necessary, costs were inflated using the Hospital and Community Health Services Pay and Prices Index. 41 Baseline characteristics were not controlled for in relation to costs because exploratory analyses suggested that baseline characteristics were not significant predictors of costs.
Analysis
Main analysis
Initially, a cost–consequence analysis was carried out in which all costs and outcomes were reported in a disaggregated manner. The costs and outcomes associated with the alternative treatment, intravaginal lactic acid gel, were compared with those for oral metronidazole. A cost-effectiveness analysis was also carried out with results reported in terms of the cost per case successfully treated at week 2. In addition, the cost–utility analysis evaluated the results in terms of cost per QALY gained at 6 months.
The cost-effectiveness analysis was presented as cost per case successfully resolved at week 2 for both treatment arms. For the cost–utility analysis, the incremental cost-effectiveness ratio (ICER) was estimated by dividing the difference in mean total cost between the study treatments by the difference in QALYs at 6 months.
The approach for the cost-effectiveness analysis was intention to treat, to reflect the approach taken in the clinical analysis. For the cost–utility analysis, a complete-case approach was adopted. Any participant randomised in error was analysed as randomised in keeping with the clinical analysis. Given that cost data are likely to be positively skewed, a bootstrapping approach was undertaken to calculate 95% CIs around mean costs. 44 This approach was used to generate repeated random samples with replacement from the original data. 44 In this approach, if the CIs of the difference in mean costs between arms cross zero, this indicates no significant difference.
All analyses were performed using Stata® version 16 (StataCorp LP, College Stations, TX, USA). Only a within-trial analysis was conducted. In view of the trial results, a longer-term decision-analytic model extrapolating findings beyond the trial period was not deemed necessary.
Sensitivity analysis
A bootstrapping approach was employed to determine the sampling uncertainty around the final outcomes by generating 5000 replications of incremental cost and benefit estimates. 45 The estimates of both cost-effectiveness and cost–utility analyses were presented on a cost-effectiveness plane as a scatterplot to aid interpretation. 46 A number of deterministic sensitivity and scenario analyses were carried out to explore the impact of variation in the estimated values and assumptions on the results. Taking a broader perspective, we considered out-of-pocket costs of non-prescribed medication at week 2 and 6 months for the primary and secondary outcomes. The cost of metronidazole was increased to take into account that the more expensive metronidazole Flagyl® (400 mg), which was administered in the trial rather than a cheaper alternative. In addition, the BV resolution rate at week 2 was varied using the data reported in Table 5 (see Chapter 3); this assumed that if all participants did not report symptoms at that time point, their BV had resolved.
Given that limited data on thrush treatment were provided at 3 and 6 months, the number of episodes of vaginal thrush was used and combined with the cost of clotrimazole to estimate the cost of thrush treatment over 6 months. The assumption was that each participant with thrush symptoms was administered one course of clotrimazole pessary for each episode.
A further analysis was conducted assuming that all those who developed symptoms post treatment would be given an additional course of oral metronidazole, irrespective of the treatment arm that they were allocated to. Data from a post hoc analysis showed that 77% of participants in the metronidazole arm developed symptoms after finishing treatment, along with 84% of participants in the lactic acid gel arm (see Appendix 5, Table 70). These findings were used and presented in the sensitivity analysis, assuming that each participant who developed symptoms was treated with one additional course of oral metronidazole.
The cost associated with antibiotic resistance has been estimated as a penalty cost for using antibiotics. 34 The impact of including such a penalty cost was analysed as part of the sensitivity analysis. Owing to a lack of evidence on the costs associated with AMR for metronidazole, a range of costs of AMR associated with different antibiotics was used to represent the potential economic cost of consuming oral metronidazole in terms of increasing resistance. 47 Economic costs from both a health-care perspective and a societal perspective were used in the sensitivity analyses, drawing on published estimates. 47 These estimates were converted from US dollars (USD) to GBP using the market exchange rate (1 USD = 0.76 GBP).
Results
Questionnaire response rate
As reported in Chapter 3, 518 participants were randomised into the trial, of whom 294 (57%) completed the resource use questionnaire at week 2: 143 in the metronidazole arm and 151 in the lactic acid gel arm. A total of 123 (24%) participants completed the resource use questionnaire at all time points (week 2, 3 months and 6 months) and were included in the base-case analysis at 6 months. The number of participants who responded to the resource use questionnaire and SF-12 health survey at different time points is provided for each treatment arm (see Appendix 5, Table 72). The baseline characteristics for those who completed resource use questionnaires at week 2 and at all time points are available (see Appendix 5, Table 73). It had been planned to use multiple imputation to generate estimates of missing values based on the distribution of observed data;48,49 however, given that resource use questionnaire data were missing for > 40% of participants at different time points, multiple imputation could not be used and a complete-case analysis was adopted. 40
Health outcomes
The mean SF-6D scores at each time point for each treatment arm are presented in Table 17. The results indicate that the differences in the mean values between the two treatment arms were not significant. The QALY gains were slightly lower for the lactic acid gel arm than for the metronidazole arm, with a mean difference of –0.003 QALYs (95% CI –0.013 to 0.009 QALYs). However, this difference was not statistically significant.
| Quality of life | Treatment arm, mean (SD) | Mean bootstrap difference (95% CI) | |
|---|---|---|---|
| Oral metronidazole (n = 61) | Intravaginal lactic acid gel (n = 48) | ||
| SF-6Da | |||
| Baseline | 0.786 (0.127) | 0.765 (0.126) | –0.021 (–0.066 to 0.026) |
| Week 2 | 0.711 (0.076) | 0.712 (0.068) | 0.001 (–0.025 to 0.031) |
| 3 months | 0.692 (0.069) | 0.688 (0.068) | –0.004 (–0.029 to 0.022) |
| 6 months | 0.696 (0.075) | 0.684 (0.085) | –0.012 (–0.044 to 0.017) |
| QALYs | |||
| QALYs gained at 6 months | 0.351 (0.030) | 0.348 (0.028) | –0.003 (–0.013 to 0.009) |
Cost and resource use
Week 2
The mean resource use and costs at week 2 are presented in Table 18 for each treatment arm. On average, participants in the lactic gel acid arm reported more health-care resource use than those in the metronidazole arm.
| Resource use | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Oral metronidazole (N = 143) | Intravaginal lactic acid gel (N = 151) | |||||
| Participants accessing the service (n) | Mean resource use per participant (SD) | Mean cost per participant (SD) (£) | Participants accessing the service (n) | Mean resource use per participant (SD) | Mean cost per participant (SD) (£) | |
| GP contact | ||||||
| Face to face | 6 | 0.04 (0.20) | 1.64 (7.85) | 10 | 0.08 (0.32) | 3.10 (12.35) |
| Telephone | 1 | 0.01 (0.17) | 0.22 (2.59) | 3 | 0.03 (0.20) | 0.41 (3.07) |
| Nurse | ||||||
| Face to face | 0 | 0 | 0 | 0 | 0 | 0 |
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| NHS walk-in centre | ||||||
| Face to face | 6 | 0.06 (0.28) | 2.18 (11.12) | 4 | 0.03 (0.21) | 1.29 (8.32) |
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| NHS 111 | ||||||
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| Pharmacy | ||||||
| Face to face | 6 | 0.05 (0.27) | 1.43 (7.23) | 6 | 0.06 (0.31) | 1.75 (9.10) |
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| Sexual health clinic | ||||||
| Face to face | 18 | 0.17 (0.49) | 20.48 (59.67) | 25 | 0.29 (0.98) | 34.74 (119.02) |
| Telephone | 6 | 0.04 (0.20) | 0.33 (1.57) | 9 | 0.06 (0.24) | 0.46 (1.85) |
| NHS outpatient | ||||||
| Face to face | 5 | 0.10 (0.62) | 14.10 (89.32) | 3 | 0.03 (0.24) | 4.77 (34.95) |
| Telephone | 3 | 0.04 (0.35) | 1.58 (13.29) | 0 | 0 | 0 |
| Out-of-hours service | ||||||
| Face to face | 1 | 0.01 (0.08) | 0.48 (5.69) | 1 | 0.01 (0.08) | 0.45 (5.53) |
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| A&E | ||||||
| Face to face | 1 | 0.01 (0.08) | 0.93 (11.12) | 2 | 0.01 (0.11) | 1.76 (15.26) |
The mean total cost was slightly higher for participants in the lactic acid gel arm (£55.38) than for those in the metronidazole arm (£48) at week 2 (Table 19). The mean difference in costs between the two arms was £7.38 (95% CI –£20.15 to £36.38), although the difference was not statistically significant. The main costs for both arms related to primary and secondary care costs (88–90% of total costs). In general, the difference in costs between the treatment arms was related to the need for additional GP and sexual clinic consultations. Hence, the initial analysis found that treatment with intravaginal lactic acid gel resulted in slightly higher health-care costs.
| Type of health-care cost | Treatment arm, mean cost (SD) (£) | Mean bootstrap difference (95% CI) (£) | |
|---|---|---|---|
| Oral metronidazole (n = 143) | Intravaginal lactic acid gel (n = 151) | ||
| Study treatment | 3.97 (NA) | 5.25 (NA) | 1.28 (NA) |
| Primary and secondary care | 43.35 (112.00) | 48.44 (113.49) | 5.09 (–22.09 to 33.67) |
| Additional medication: prescribed | 0.707 (2.49) | 1.42 (4.21) | 0.71 (–0.05 to 1.51) |
| Total cost | 48.00 (112.68) | 55.38 (134.89) | 7.38 (–20.15 to 36.38) |
Six months
A breakdown of the resource use data and the mean health-care costs by treatment arm is presented in Table 20. Those in the lactic acid gel arm generally reported more health service utilisation than those in the metronidazole arm.
| Resource use | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Oral metronidazole (N = 69) | Intravaginal lactic acid gel (N = 54) | |||||
| Participants accessing the service (n) | Mean resource use per participant (SD) | Mean cost per participant (SD) (£) | Participants accessing the service (n) | Mean resource use per participant (SD) | Mean cost per participant (SD) (£) | |
| GP contact | ||||||
| Face to face | 12 | 0.28 (0.70) | 10.74 (27.48) | 18 | 0.69 (1.38) | 26.72 (54.01) |
| Telephone | 4 | 0.13 (0.59) | 2.02 (9.17) | 5 | 0.19 (0.62) | 2.87 (9.56) |
| Nurse | ||||||
| Face to face | 2 | 0.03 (0.17) | 0.63 (3.67) | 5 | 0.11 (0.37) | 2.41 (8.08) |
| Telephone | 1 | 0.01 (0.12) | 0.11 (0.94) | 2 | 0.04 (0.19) | 0.29 (1.49) |
| NHS walk-in centre | ||||||
| Face to face | 7 | 0.13 (0.42) | 5.09 (16.26) | 2 | 0.06 (0.30) | 2.17 (11.78) |
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| NHS 111 | ||||||
| Telephone | 4 | 0.07 (0.31) | 1.03 (4.46) | 0 | 0 | 0 |
| Pharmacy | ||||||
| Face to face | 8 | 0.17 (0.54) | 5.10 (15.86) | 16 | 0.54 (1.00) | 15.74 (29.42) |
| Telephone | 0 | 0 | 0 | 1 | 0.02 (0.14) | 0.26 (1.91) |
| Sexual health clinic | ||||||
| Face to face | 31 | 1.12 (1.74) | 136.14 (211.86) | 30 | 1.39 (2.30) | 169.44 (280.83) |
| Telephone | 9 | 0.23 (0.69) | 1.81 (5.37) | 9 | 0.22 (0.88) | 1.73 (6.89) |
| NHS outpatient | ||||||
| Face to face | 7 | 0.23 (0.89) | 33.39 (128.67) | 5 | 0.20 (0.88) | 29.33 (126.28) |
| Telephone | 3 | 0.09 (0.51) | 3.27 (19.06) | 1 | 0.04 (0.27) | 1.39 (10.23) |
| Out-of-hours service | ||||||
| Face to face | 2 | 0.03 (0.17) | 1.97 (11.49) | 1 | 0.02 (0.14) | 1.26 (9.25) |
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| A&E | ||||||
| Face to face | 1 | 0.01 (0.12) | 1.93 (16.01) | 1 | 0.04 (0.27) | 4.93 (36.20) |
| Telephone | 0 | 0 | 0 | 0 | 0 | 0 |
| Other services: GP | ||||||
| Three face-to-face visits and three telephone calls | 0 | 0 | 0 | 3 | 6 | 2.98 (21.91) |
Over the 6-month follow-up period, the accumulated total mean costs were £273 in the lactic acid gel arm and £214 in the metronidazole arm (Table 21). For both arms, the highest proportion of costs related to the utilisation of primary and secondary care services. The cost difference between the two arms was £58.60; however, this difference in costs was not statistically significant (95% CI –£55.05 to £185.32).
| Type of health-care cost | Treatment arm, mean cost (SD) (£) | Mean bootstrap difference (95% CI) (£) | |
|---|---|---|---|
| Oral metronidazole (n = 69) | Intravaginal lactic acid gel (n = 54) | ||
| Study treatment | 3.97 (NA) | 5.25 (NA) | 1.28 (NA) |
| Primary and secondary care | 203.23 (295.55) | 258.54 (359.33) | 55.31 (–57.38 to 184.12) |
| Additional medication: prescribed | 8.28 (16.45) | 6.84 (9.85) | –1.16 (–3.74 to 1.32) |
| Total cost | 214.48 (302.45) | 273.08 (366.14) | 58.60 (–55.05 to 185.32) |
Base-case analysis
Cost-effectiveness analysis
The results of the base-case cost-effectiveness analysis are presented in Table 22. It is evident that lactic acid gel was less clinically effective than metronidazole in terms of participants with resolved symptoms at week 2, and that the average costs were higher. The average cost per participant with resolved BV for those in the lactic acid gel arm was £147.00, compared with £86.94 for those in the metronidazole arm.
| Economic evaluation | Treatment arm | |
|---|---|---|
| Oral metronidazole (n = 259) | Intravaginal lactic acid gel (n = 259) | |
| Mean total cost (£) for participants who returned the week 2 questionnaire | 48.00 | 55.38 |
| Number of participants with resolution of BV symptoms at week 2a | 143 | 97 |
| Cost (£) per participant with resolved BVb | 86.94 | 147.00 |
Cost–utility analysis
The base-case cost–utility analysis demonstrated that intravaginal lactic acid gel was dominated by oral metronidazole, which meant that the alternative treatment was likely to be more costly and less effective at 6 months (Table 23). The analysis suggested that oral metronidazole resulted in more QALYs and was less costly than intravaginal lactic acid gel at 6 months; however, the differences in costs and outcomes were not statistically significant.
| Economic evaluation | Treatment arm | |
|---|---|---|
| Oral metronidazole (n = 61) | Intravaginal lactic acid gel (n = 48) | |
| Mean cost per participant (£) | 214.48 | 273.08 |
| Incremental cost (£) | – | 58.60 |
| Mean QALYs | 0.351 | 0.348 |
| Incremental QALYs | – | –0.003 |
| ICER (cost per QALY)a | – | Dominated |
Sensitivity analysis
The results of the deterministic sensitivity analyses in which we explored the impact of varying the assumptions and for different scenarios are presented for both the cost-effectiveness and the cost–utility analyses in Tables 24 and 25. Using alternative cost estimates for metronidazole and including the cost of non-prescribed (out-of-pocket) medications administered at different time points did not affect the overall results. Including additional costs of the management of thrush at 3 and 6 months and a further course of treatment for those who developed symptoms post study treatment did slightly increase the total mean cost for both arms, but intravaginal lactic acid was still more costly than oral metronidazole.
| Deterministic sensitivity analysis scenario | Treatment arm, average cost per participant with resolved BV (£) | |
|---|---|---|
| Oral metronidazole | Intravaginal lactic acid gel | |
| Including the cost of non-prescribed (out-of-pocket) additional medication | 88.82 | 149.87 |
| Varying the cost of metronidazole to £6.34 | 91.61 | 147.00 |
| Assuming missing data on symptom resolution as symptoms resolved | 62.47 | 93.75 |
| Considering economic cost of AMR: health-care perspective | 87.03–88.24 | 147.00 |
| Considering economic cost of AMR: societal perspective | 88.86–119.65 | 147.00 |
| Deterministic sensitivity analysis scenario | Treatment arm | Mean difference | ICER | ||||
|---|---|---|---|---|---|---|---|
| Oral metronidazole | Intravaginal lactic acid gel | ||||||
| Mean cost (£) | Mean QALYs | Mean cost (£) | Mean QALYs | Cost (£) | QALYs | QALYs per cost | |
| Including the cost of non-prescribed (out-of-pocket) additional medication | 220.08 | 0.351 | 281.92 | 0.348 | 61.84 | –0.003 | Dominated |
| Including cost of thrush treatment at 3–6 months | 215.81 | 0.351 | 274.19 | 0.348 | 58.38 | –0.003 | Dominated |
| Including cost of treating symptoms after initial study treatment | 217.53 | 0.351 | 276.39 | 0.348 | 59.92 | –0.003 | Dominated |
Considering the cost of AMR associated with the use of metronidazole led to an increased mean cost for the metronidazole arm, but the average cost per participant with resolved BV remained higher for intravaginal lactic acid gel across all estimates for the potential cost of AMR that was used.
The results of 5000 bootstrap replications plotted on the cost-effectiveness plane for the primary analysis (Figure 8) suggest that lactic acid gel is likely to be less clinically effective than metronidazole because all replicates are to the left of the origin (worse health outcome). However, there is some uncertainty around the difference in costs between the treatment arms, as replicates are distributed almost equally across the north-west quadrant (less clinically effective and greater cost) and the south-west quadrant (less clinically effective and lower cost). Accordingly, there is uncertainty around whether intravaginal lactic acid gel was more or less costly than oral metronidazole at week 2.
FIGURE 8.
Cost-effectiveness plane of the primary outcome at week 2 (oral metronidazole vs. intravaginal lactic acid gel).
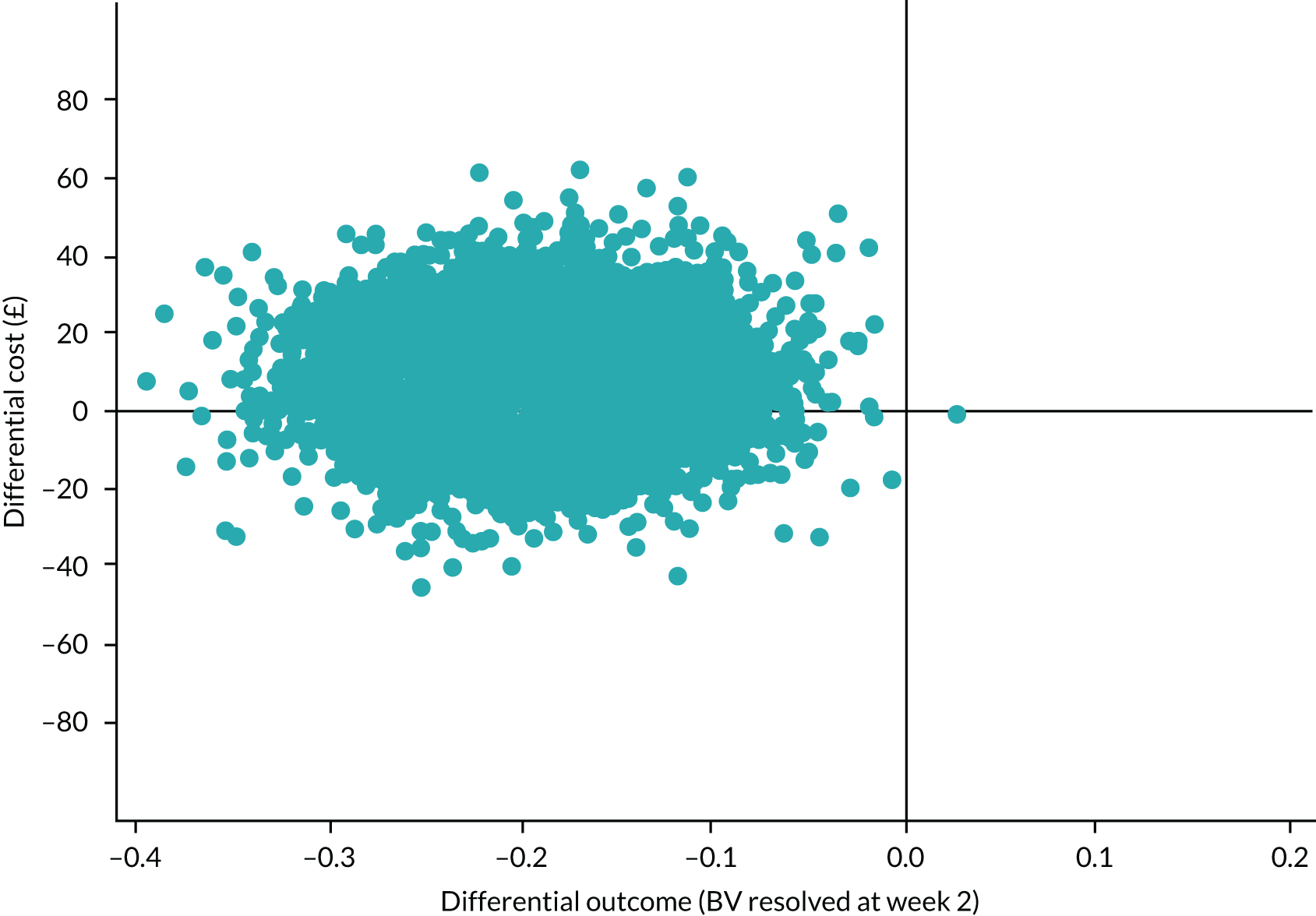
For the cost–utility analysis, the cost-effectiveness plane (Figure 9) shows that the majority of the bootstrap replicates were located in the north-west quadrant, suggesting that intravaginal lactic acid gel is more costly and less clinically effective than oral metronidazole at 6 months. However, some uncertainty was apparent, as some replicates were found in the north-east quadrant (more effective and more costly) and in the south-west quadrant (less clinically effective and less costly).
FIGURE 9.
Cost-effectiveness plane of the secondary outcome at 6 months (oral metronidazole vs. intravaginal lactic acid gel).
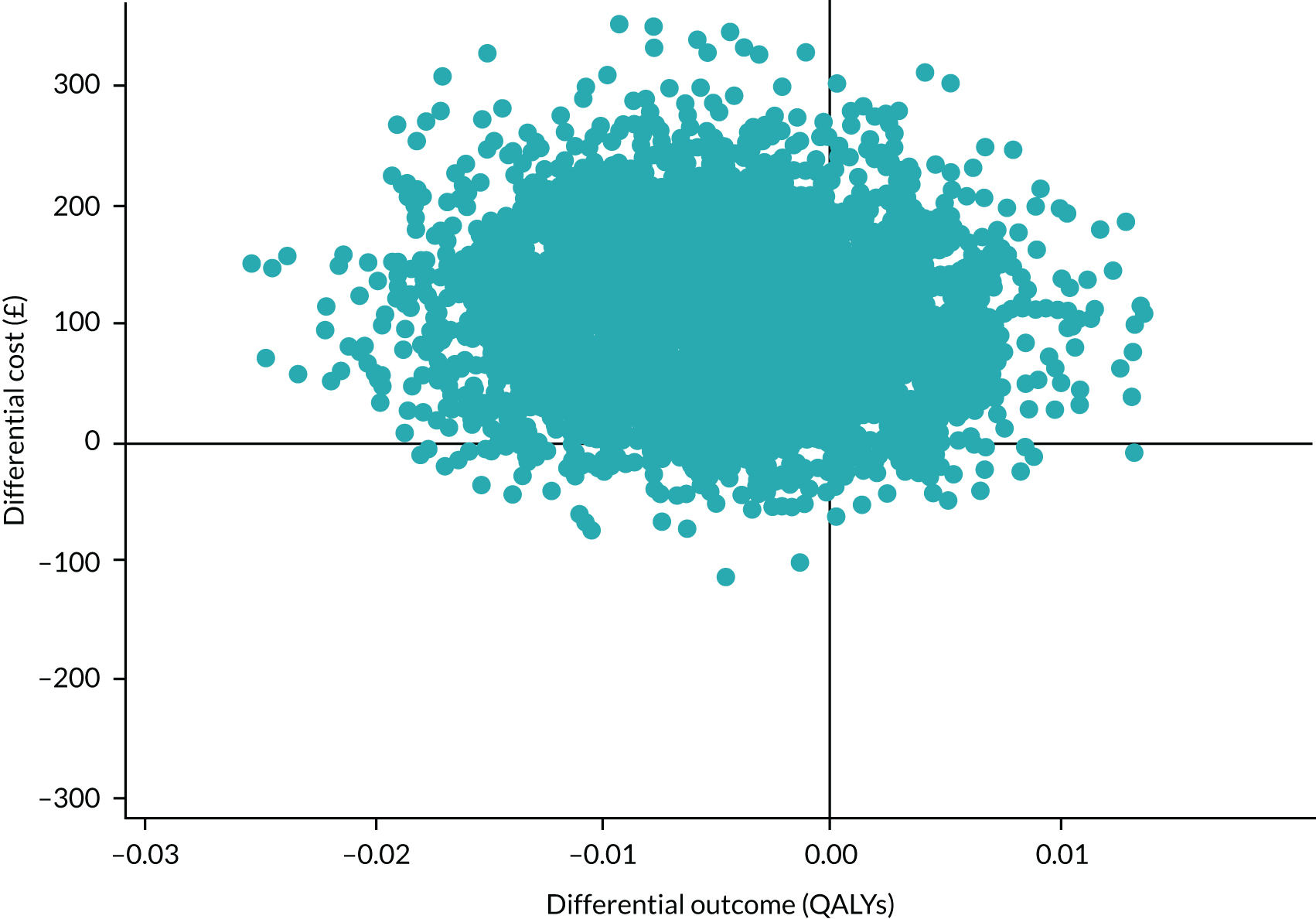
Discussion
Principal findings
The results of the primary analysis suggest that intravaginal lactic acid gel is less clinically effective and slightly more costly than oral metronidazole in resolving BV symptoms by week 2. At week 2, lactic acid gel was associated with an additional cost of £7.38 (95% CI –£20.15 to £36.38) per participant. The additional cost was mainly attributed to the utilisation of secondary care resources, mainly sexual health clinics. The total cost might have been lower if more participants from primary care (general) practices had been recruited into the trial. The cost per participant with resolved BV was £147.00 in the lactic acid gel arm, compared with £86.94 in the metronidazole arm. The cost-effectiveness plane that incorporates the uncertainty around each point estimate in the results shows that, relative to oral metronidazole, intravaginal lactic acid gel is likely to be less effective. However, there is uncertainty around the difference in costs, suggesting that intravaginal lactic acid gel could be either more or less costly than oral metronidazole.
The cost–utility analysis showed that intravaginal lactic acid gel was dominated by oral metronidazole at 6 months, although there was some uncertainty around the differences in costs and outcomes.
Strengths and limitations
The strength of the economic evaluation is that it was based on a multicentre RCT exploring the potential cost-effectiveness of intravaginal lactic acid gel compared with the current standard treatment of oral metronidazole. To our knowledge, this is the first economic evaluation conducted alongside a trial that has explored intravaginal lactic acid gel as an alternative treatment for BV. Detailed data on resource use and health outcomes were collected at different time points using web-based questionnaires that were completed by participants.
The main limitation of the analysis was the small sample size and the level of missing data on resource use and quality of life at all time points. Accordingly, multiple imputation methods could not be employed. This could have introduced bias and affected the final results. Another limitation was that the follow-up was for 6 months only, which may not capture the longer-term effects of the treatments given that the trial focused on participants with recurring BV. Some pragmatic assumptions were required to address missing data relating to resource use and thrush management, but the impact associated with these assumptions was explored as part of the sensitivity analysis.
The economic cost of AMR was explored as part of the sensitivity analysis. However, there is considerable uncertainty around the role of AMR in relation to recurrent BV50 and our exploration of the potential costs associated with AMR was, therefore, necessarily limited.
Recommendations and conclusions
The early termination of recruitment into the trial limited the ability to obtain definitive evidence on the cost-effectiveness of intravaginal lactic acid gel compared with oral metronidazole. The data collected suggest that, in both arms, participants needed to access further health care. Further research would be beneficial to reduce the uncertainty in the findings around resource use and health outcomes, particularly over the longer term. In addition, there is a need for greater understanding of the externalities associated with AMR in connection with recurrent BV. It is currently unclear what the best approaches would be for BV, as more powerful second- and third-line antibiotics are not usually given. Instead, patients are usually prescribed further treatment with metronidazole or clindamycin. 10
Chapter 5 Qualitative study of participants’ views on the acceptability of and treatment preferences for recurrent bacterial vaginosis
Introduction
Qualitative methods of data collection and analysis enable an in-depth exploration of participants’ views and perceptions of their experiences. This is particularly valuable in discussion of little-known, complex and sensitive topics51 such as BV: ‘a common condition of unknown aetiology’. 52 Furthermore, there is inconclusive evidence about which treatment regimen is most effective for women with recurrent BV, and its propensity for recurrence makes treatment of BV a challenge. 53 Greater treatment acceptance is associated with higher adherence, better compliance and improved persistence. 54 To this end, acceptability has become a key consideration in the design, evaluation and implementation of health-care interventions. 55 However, very few studies have qualitatively explored the acceptability and tolerability of different BV treatment options. 56
This qualitative substudy from the VITA trial was outlined at the start of the research design phase, as per the published protocol. 25 The value of hearing women’s voices as well as collecting their clinical BV results from the microscopy slides and web-based questionnaires at different time points would allow the research team to better understand what was deemed more acceptable and tolerable given their experiences of the condition. To do this, during interviews we explored a diverse subgroup of participants’ prospective, concurrent and retrospective perspectives in relation to the trial. We examined the acceptability and tolerability of study treatments for participants along with their preferences for biomedical treatment, what factors they perceived to contribute to the development and recurrence of BV, and how BV made them feel physically and emotionally.
Methods
Design
This qualitative substudy was completed during recruitment into the VITA trial and the results have been published. 57 The lead qualitative researcher (JAW) worked independently of the trial team and was blinded to the post-treatment results. All participants who were included in the interview subgroup had given optional consent to be contacted about the interview at the time of randomisation to the trial. Those who gave consent and had completed study treatment were consecutively sampled from both treatment arms. An invitation to take part in the interview was e-mailed to participants, which was followed by a telephone call invitation if they did not respond to the e-mail. Interviews were scheduled for a time that was convenient to the participants and were expected to take approximately 20 minutes. It was planned to interview approximately 30 participants (15 in each arm) and recruitment continued until data saturation was reached.
Data collection and analysis
Semistructured interviews took place from January 2018 to May 2018, which was part-way through recruitment into the trial, and were conducted over the telephone using a qualitative interview schedule (see Appendix 6). Only the qualitative researcher (JAW) and the participant were involved, and consent was verbally confirmed at the start of the interview. Participants were given a unique interview code to identify all data collected from them. All interviews were audio-recorded, transcribed and anonymised, and lasted between 10 and 45 minutes. Qualitative data were managed using NVivo 11 (QSR International, Warrington, UK) and all files were stored on a secure server at the University of Warwick. Data from the interviews were not analysed or triangulated with those from the web-based questionnaires.
Data were coded thematically58 and then comparatively by two researchers, with the codes being based on interview questions and emergent themes. Word-spotting of descriptive adjectives assisted in summarising all contextual data describing BV symptoms. Data were synthesised using a framework approach by comparison with the acceptability of health interventions framework55 to reflect the extent to which women receiving a health-care intervention consider treatment to be appropriate based on their anticipated or experiential cognitive and emotional responses. We considered constructs, such as perceived effectiveness and burden, according to women’s perspectives on BV treatments.
The analysis of the data required a sound narrative of the participants’ history of BV, including previous and current treatments. This helped to ensure that the analysis reflected the participants’ voices as faithfully as possible. To achieve a prolonged and intense engagement with the totality of the data, we concurrently analysed data. This helped to ensure that questions were being asked in a way that captured participants’ descriptions of their experience of BV, possible triggers of BV, perceived effectiveness of both treatments and treatment outcomes.
The quotations included fairly represent the participants’ opinions reflecting similarities, differences and nuances of opinion, experience and perception.
Results
Across the six trial sites that were open to recruitment during the interview time frame, a total of 52 participants were invited to take part in the interview, of whom 18 either were not contactable or declined to take part and one withdrew from the trial. This gave a total of 33 participants who were interviewed (lactic acid gel arm, n = 20; metronidazole arm, n = 13). The participants were from diverse ethnic backgrounds and ranged in age from 21 to 51 years; just over one-third of participants had experienced BV more than three times. Baseline characteristics of the interview subgroup are summarised in Table 26 and show that the interviewees were somewhat representative of the main trial population.
| Characteristic | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 13) | Intravaginal lactic acid gel (N = 20) | |
| Age at randomisation (years) | ||
| Mean (SD) | 34.0 (8.20) | 30.6 (5.93) |
| Ethnicity, n (%) | ||
| Black Caribbean | 5 (38.5) | 6 (30.0) |
| White | 2 (15.4) | 7 (35.0) |
| Black African | 3 (23.1) | 2 (10.0) |
| Mixed race | 0 | 3 (15.0) |
| Other Asian | 1 (7.7) | 1 (5.0) |
| Indian | 1 (7.7) | 0 |
| Pakistani | 1 (7.7) | 0 |
| Chinese | 0 | 1 (5.0) |
| Number of previous episodes of BV in the past 12 months, n (%) | ||
| 0 | 0 | 0 |
| 1–3 | 5 (38.5) | 16 (80.0) |
| > 3 | 8 (61.5) | 4 (20.0) |
We present our findings under four main themes:
-
theme 1 – context of BV
-
theme 2 – experiences and perspectives around the acceptability and use of treatment for BV
-
theme 3 – treatment preference for BV
-
theme 4 – participant’s views on which treatment they would suggest that other women take.
Theme 1: context of bacterial vaginosis
Characteristics of the physical symptoms of bacterial vaginosis
The symptoms of BV were described by participants in terms of colour, texture, smell and physical sensations using a range of adjectives (Box 1).
Colour of the discharge: dark greenish, brown, yellowy, translucent, magnolia, creamy white, dark in colour, clear or whitish, pinky, cloudy.
Texture of the discharge: solidified, crustated, mucousy, opaque, very fluid, like lotion, watery, milky, thick.
Malodour of the discharge: rancid, very smelly, sour smell, foul kind of odour, fishy, seafood odour, potent smell, vinegary, disgusting, horrendous, stinking smell.
Physical sensation associated with the discharge: itchiness, slight burning, irritation (symptoms worsened pre- and post-menstruation).
Reproduced with permission from Anstey Watkins et al. 57 This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. The box includes minor additions and formatting changes to the original.
The characteristics of BV, such as an offensive smell and abnormal vaginal discharge, that so greatly affect women’s lives are consistent with findings from other qualitative studies. 52,59 There is a complex interaction of multiple components, including the vaginal microbial ecosystem and the human host, that is modulated by a woman’s behaviour and environment and further complicates the overall ‘BV cause equation’. 60
Impact of bacterial vaginosis on participants’ lives
The symptoms of BV affected participants psychologically and physically beyond the bacterial symptomatology, leading to emotional trauma. Feelings of depression, anxiety and self-consciousness were described. The social burden of living with recurrent BV has also been described by others. 56
Participants noted that vaginal discharge malodour affected personal relationships and made them have feelings of ‘embarrassment’, ‘paranoia’ and ‘body-consciousness’, as well as being ‘self-aware’ that their partners, children, colleagues and even strangers could smell it:
I just don’t feel comfortable because I feel like I always smell.
V33, aged 33 years: lactic acid gel
I sat on the bus and I think people can smell me and I just stay away from people, you know.
V24, aged 37 years: lactic acid gel
Episodes and recurrence of bacterial vaginosis
Bacterial vaginosis was described ‘a nightmare’, always returning and persisting. Its recurrence led to participants’ frustration and confusion as to how and why they get BV. Some could start to see patterns in its recurrence:
Like me now, I’ve mastered BV; I know when it’s coming.
V45, aged 34 years: metronidazole tablets
Perceived triggers of bacterial vaginosis
Participants identified a number of different reasons for developing BV, such as:
-
Sexual practices, for example unprotected, unsafe sexual intercourse (e.g. not using condoms); multiple sexual partners (same or not same sex); and male semen post ejaculation.
-
The use of contraceptive coils, such as an intrauterine device [copper or hormonal implant, Mirena (Bayer, Reading, UK) branded], menstrual cycle, application of tampons, not changing tampons regularly or forgetting to wash hands before the application of tampon.
-
Hygiene practices, that is the type of soap product used by the sexual partner on their ‘intimate areas’; personal bathing in perfumed bubble bath water; the use of ‘cheap’ shower gels; cleaning with an antiseptic solution; or activities such as douching were described by almost all participants as detrimental to the development of BV (as described previously where lifestyle behaviours such as douching, bathing and using vaginal cleaning agents were significantly associated with BV due to the disruption of vaginal flora61).
-
Type of clothing material such as non-cotton underwear, type of laundry detergent such as fabric softener and wearing perfumed sanitary pads.
-
Lifestyle choices such as consumption of poor diets including caffeinated energy drinks and high-fat processed foods, excessive cigarette smoking or alcohol consumption. These behaviours were all attributed to working shifts, having multiple jobs, a lack of time and stress at work, all of which women felt contributed to BV.
Self-help: changes in personal hygiene habits, self-help medication and home remedies
Some participants had been advised by nurses to mainly wash with water only, dermatological soaps or emollient creams. The frequency of washing habits to remove BV discharge varied from several times per day owing to religious beliefs or to commonly twice per day or to every few days to weekly. Many were concerned about the chemicals in soaps affecting their natural pH balance, as it ‘still feel like it might be passing down there’ (V23, aged 23 years: metronidazole tablets).
Self-medication included using over-the-counter liquid lactic acid gels or waxy bullet-like pessaries bought either from a pharmacy or online, or the use of homemade remedies including steaming, apple cider vinegar or yoghurt internally in the vagina. This is congruent with women in Australia who were frustrated and dissatisfied with current treatment regimens for BV, in particular antimicrobials, leading them to take ineffective non-evidence-based therapies. 59
Theme 2: experiences and perspectives around the acceptability and use of treatment for bacterial vaginosis
Prior experience of treatment for bacterial vaginosis
Only one participant had never been prescribed oral metronidazole for BV before and one other had never used lactic acid gel previously.
Intervention treatment: acceptability and ease of use of lactic acid gel
The majority of participants who were randomised to receive lactic acid gel described the tube applicator as comfortable and easy to use with no side effects, yet it could be messy:
Yeah, it was easy to use, it was fine yeah. Simple to follow instructions and everything yeah.
V9, aged 37 years: lactic acid gel
Obviously there is some sort of drippiness that happens a bit. But as it happens mostly overnight, it hasn’t affected me during the day at all.
V8, aged 25 years: lactic acid gel
Two participants said that the gel applicator leaked even when applying it lying down correctly as instructed, which caused concern as to whether or not they had received the full dose. A similar experience was reported in another study, in which reasons given for not accepting the lactic acid gel were messiness and leakage. 62
Perceived effectiveness of the lactic acid gel
The lactic acid gel was described as fast acting and improvement was felt and seen quickly. As a result, participants would use it ‘happily’ again in the future:
Yes. I mean, you know, so it’s gone, so assuming it stays away then yes, it did definitely work.
V41, aged 33 years: lactic acid gel
Well I felt like, oh my God, at last something’s going to work you know, within 7 days I’m going to be fine . . . and I can also have a drink as well.
V24, aged 37 years: lactic acid gel
Negative aspects of the lactic acid gel
Gel application every night was presented as a ‘faff’ because participants had to change their bedtime routine. Others said that they were scared to cough as the gel may leak:
I think there’s still some in there still doing good, but there’s some that comes out. So, I don’t know if it will affect it or not.
V5, aged 38 years: lactic acid gel
A few participants felt that they could not drink water or urinate after application. In addition, abstaining from sexual intercourse while using the gel was considered an inconvenience.
Control treatment: acceptability and ease of use of oral metronidazole
Overall, antibiotics were generally not acceptable to participants because of the frequency with which they were prescribed. However, metronidazole was considered an effective first-line prescribed treatment, particularly for ‘intense’ cases of BV.
The confidence in metronidazole clearing up the BV was high because it was classified as an ‘antibiotic’ drug. Therefore, participants were willing to take these tablets to get ‘rid of that BV’ (V46, aged 42 years: metronidazole tablets).
Perceived effectiveness of metronidazole
There were differences in how quickly metronidazole cleared up the BV, but effects were short-lived before the next episode began and improvements were temporary. Concerns about antibiotic resistance were common:
I don’t really like taking antibiotic, because I know your body can become like resistant to them.
V11, aged 22 years: lactic acid gel
Yet, paradoxically in these cases, participants were still prepared to take the tablets because ‘they worked’:
The metronidazole . . . I remember when I took it, everything was cleared up and then it just came back out of nowhere.
V11, aged 22 years: lactic acid gel
Negative aspects of metronidazole
The process of taking the antibiotics twice per day was defined as a chore:
Even if I halve it, I can’t swallow them, they’re quite big. Two, the taste. Then it causes me to be ill, makes me feel nauseated more.
V44, aged 51 years: metronidazole tablets
Theme 3: treatment preference for bacterial vaginosis
Participants described their individual preferences based on the effectiveness of treatment (what they think worked) and based on personal choice (what they would rather take given individual lifestyle factors or beliefs). Treatment preference was usually dependent on the severity of symptoms, the time interval between perceived recurrences and the advantages and disadvantages of each treatment (Table 27).
| Treatment preference depending on | Treatment arm | |
|---|---|---|
| Oral metronidazole | Intravaginal lactic acid gel | |
| Severity of symptoms | Severe BV | Mild BV |
| How often the treatment can be taken | Short-term treatment | Long-term treatment |
| Perceived speed of cure | Quick results (all cleared up) | Quick to mid-term results |
| Duration between perceived recurrence | Longer durations between episodes | Shorter duration between episodes |
| Perceived effectiveness | Know antibiotic tablets work better = strong form of treatment | Prefers using the lactic acid gel = less intense form of treatment |
| Treatment option | First-line treatment on the first sign of ‘severe’ symptoms | First-line treatment on the first sign of ‘mild’ symptoms |
| Back-up drug (if lactic acid gel did not work the first time around) | Use in addition to antibiotics or straight after a course of antibiotics | |
| Description of treatment type | Last-resort treatment | Substitute treatment/precaution/preventative treatment |
Treatment preferences of lactic acid gel over metronidazole tablets
Positive factors
-
The tube applicator was comfortable.
-
The availability of self-medicated lactic acid gel without requiring health-care consultation for a prescription.
-
Insertion of the gel directly to the target area at which they have the symptoms.
-
No risk of antibiotic resistance.
-
Minimal side effects.
-
Do not have to abstain from alcohol, thus altering social behaviours.
-
Can bring instant relief (but did not cure the BV, rather it delayed the onset of the next episode).
-
Idea that the gel can be used preventatively straight after having sexual intercourse to reduce risk of BV.
-
Reduced discharge and the associated odour to some degree.
Negative factors
-
Too much gel liquid in the tube.
-
Not affordable if self-bought.
Treatment preference of metronidazole tablets over lactic acid gel
Positive factors
-
It is easier to swallow the tablets than insert the gel and can take tablets anywhere (e.g. at work).
-
Can still have sexual intercourse.
-
More effective and faster to clear symptoms.
-
Period of time between recurrence was longer when prescribed antibiotics.
-
Reduced discharge and the associated odour to some degree.
Negative factors
-
Stigma associated with being seen at a sexual health clinic to get treatment.
-
Time off work to go to the appointment or simply ‘fed up going to the clinic’ (V46, aged 42 years: metronidazole tablets).
Poor outcomes and disbelief that there was actually a cure for bacterial vaginosis led to the following choices
-
Delay in seeking treatment as they felt that any treatment was ineffective.
-
Do nothing and live with the symptoms.
‘I didn’t actually go and seek any help at that point. And then I waited a few months’ V41, aged 33 years: lactic acid gel
-
Seek treatment when at their ‘wits’ end’ owing to the ‘life sentence’ of BV and taking antibiotics frequently.
-
Self-medicate using home remedies; buying gel was deemed as unaffordable.
Theme 4: participants’ views on which treatment they would suggest that other women take
Participants were asked which treatment they would recommend, if any. Lactic acid gel was considered a milder option and a useful ‘top-up’-type treatment for minor cases of BV:
I would recommend to try it, because it’s better than nothing, definitely. But I’m very interested in trying the gel to see if that has a better impact. I think it’s socially better as well.
V23, aged 23 years: metronidazole tablets
Taking metronidazole was regarded as a safer option to clear up moderate to severe BV, with longer time periods between episodes:
Probably the antibiotics, because it seemed to get rid of it for longer.
V9, aged 37 years: lactic acid gel
One participant said that she would like to take both treatments to test the foolproof method and ensure that ‘it does not come back’ (V7, aged 26 years: lactic acid gel). Others felt that lactic acid gel worked well enough and that they would be happy to be on it for longer periods of time.
Discussion
There is much debate around the causes of BV and optimal treatments, with usually ‘more questions than answers’. 63 However, this qualitative assessment has enabled insight into what women think about their treatment options for BV and what influences their choice.
This substudy has revealed that participants hold quite complex views on treatment57 and have provided rich accounts of why they prefer either treatment. In general, participants preferred to opt for intravaginal lactic acid gel, even if they perceived the treatment to be less effective than metronidazole therapy. By using gel, they avoided taking antibiotics but felt that they were still ‘doing something’ and benefited from its soothing effect. Many participants were confused about the cause of BV, describing many different possible triggers and none.
The diverse ethnicity of the participants who took part in the interview reflects both the prevalence of BV60 and the ethnic mix of the site localities, with nearly half of the sample self-identifying as black Caribbean or black African and just over one-quarter as white.
For some participants in this substudy, BV was a cause of despair. All participants associated BV with being sexually active, but many were confused about its cause. Participants talked about unprotected sexual intercourse, use of tampons and douches, using an intrauterine contraceptive device and poor hygiene or use of cheap soap as triggers for BV.
Limitations
Many of the participants had previous experience in taking both treatment options, and this meant that these experiences merged when recalling when and what they had taken, especially when taken back to back.
Implications for health care
Although metronidazole proved to be more effective at treating BV in the short term in the VITA trial, recurrence within 6 months in a subset of participants who had initial resolution and were available for follow-up was similar in both treatment arms. In many instances, women were likely to still want to use lactic acid gel for its soothing effect and to avoid recurrent use of antibiotics. Participants were willing to purchase lactic acid gel themselves, although some found that this was not affordable. To achieve better informed and shared decision-making about treatments, health professionals need an understanding of what women think about the use of antibiotics or intravaginal gel when weighing up the pros and cons of the available options.
Recommendations for research
The overall findings of the substudy are not straightforward to communicate given the higher treatment effectiveness of metronidazole but preference, despite this, in some women for lactic acid gel. Given that women already struggle to understand BV and its cause, research is needed to understand how best to communicate treatment recommendations.
Chapter 6 Discussion
Summary of main findings
The VITA trial compared oral metronidazole with intravaginal lactic acid gel for the treatment of women with recurrent BV. Metronidazole was more clinically effective, with resolution of BV symptoms in 70% of participants, compared with 47% for those receiving lactic acid gel, 2 weeks after treatment.
The recurrence of BV within 6 months, in a smaller subset of participants who had initial resolution and were available for follow-up, was similar across arms [metronidazole: 51/72 (71%); lactic acid gel: 32/46 (70%)]. A higher incidence of some side effects was reported with metronidazole than with lactic acid gel (nausea: 32% vs. 8%; taste changes: 18% vs. 1%; diarrhoea: 20% vs. 6%, respectively).
The findings of the economic evaluation suggest that intravaginal lactic acid gel is less clinically effective and slightly more costly than oral metronidazole in resolving BV symptoms by week 2. The cost–utility analysis showed that intravaginal lactic acid gel was dominated by oral metronidazole at 6 months. Intravaginal lactic acid gel was associated with slightly fewer QALYs gained and more resource use than metronidazole. However, there is uncertainty around the difference in costs in both analyses, suggesting that intravaginal lactic acid gel could be either more or less costly than oral metronidazole and there is some uncertainty around the differences in QALY gains in the cost–utility analysis.
Qualitative interviews with a small subgroup of participants found that both treatments were acceptable. However, there was high awareness that recurrence of symptoms was likely, with an associated emotional and physical impact in some. In general, participants preferred to use intravaginal lactic acid gel, even if they perceived it to be less effective than antibiotics. By using lactic acid gel, they avoided taking antibiotics and some of their associated side effects, but felt that they were still ‘doing something’ and appreciated its immediate local soothing effect.
The trial was undertaken in symptomatic women with recurrent BV who self-referred for first-contact health care to either a sexual health clinic or a primary care clinic. The findings are, thus, likely to be generalisable to women with recurrent BV across these and wider primary care settings.
Existing evidence
Current treatment of bacterial vaginosis
Current international guidelines recommend the use of antibiotics as first-line treatment for BV. In practice, this is usually metronidazole (given either orally or as a topical intravaginal gel) and less often clindamycin. 10,33,64 Metronidazole has a broad spectrum of actions against anaerobic bacteria often associated with BV and a minimal effect on commensal vaginal lactobacilli. 65 In those with frequent BV recurrences, short courses of antibiotics to treat each symptomatic episode are recommended or regular antibiotic therapy over several weeks or months as prophylaxis.
Treatment of BV using these antibiotic regimens leads to cure within 2–4 weeks in 51–82% of patients,66 but BV symptoms recur in the majority (69–80%) of patients over the next 12 months. 12,67 The cause of initial treatment failure and subsequent frequent relapse is not known, but has been postulated to be because of AMR, failure to re-establish the normal lactobacilli-dominated vaginal flora,68 the development of a treatment-resistant bacterial biofilm on the vaginal mucosa69 and/or reinfection from a sexual partner. 70 The use of metronidazole is associated with drug side effects including vaginal candidiasis, nausea, vomiting, change in taste perception and diarrhoea,71,72 which can limit acceptability of and adherence to treatment. Patients also dislike taking multiple courses of antibiotics to treat BV and are concerned that they may develop antibiotic resistance. 57 Preventing and reducing AMR is a priority in many countries, and improving antibiotic stewardship through optimisation of and reduction in antibiotic use is a central theme of the UK’s 5-year plan to address this issue. 16
Antibiotic therapy and resistance
Following treatment, resistance to clindamycin commonly develops in the vaginal bacteria that are associated with BV,73 and resistance to metronidazole is also found in Gardnerella vaginalis74,75 and Atopobium vaginae,76 which are commonly associated with BV. Repeated antibiotic use fails to eradicate BV-associated bacteria in a substantial proportion of patients with recurrent symptoms, which also suggests that resistance might be important. 76,77 In addition to their effects on the vaginal microbiome, metronidazole and clindamycin frequently cause disruption of the gut microbial flora, with associated gastrointestinal side effects and a risk of progression to pseudomembranous enterocolitis. 78–80
Alternative bacterial vaginosis treatment approaches
The limited initial efficacy, frequent subsequent recurrences, risk of AMR and unpleasant side effects of antibiotics, in addition to patient preference in this group of trial participants, suggest that alternative approaches for the treatment of BV are needed.
A number of different approaches have been explored to try to improve the outcome of BV treatment. These include high-dose metronidazole,81 antibiotic combination therapy,82 extended release clindamycin,83 agents to disrupt the bacterial biofilm84 and probiotics. 85,86 At best, these have provided a modest improvement in efficacy compared with standard treatment87 and have not been added as recommended therapy into treatment guidelines.
Lactic acid is produced by commensal lactobacilli in the vagina and the resulting low vaginal pH helps prevent the anaerobic bacterial overgrowth that characterises BV. 88 The loss of lactobacilli that occurs in women with BV is associated with a rise in pH, and the therapeutic use of intravaginal lactic acid gel aims to restore the level of acidity to normal and, thus, inhibit the growth of BV-associated bacteria. Previous studies have been small and evaluated a variety of different intravaginal acid gels or pessaries for the treatment of BV. These have led to a variable response, with between 18% and 100% of patients achieving resolution of their BV. 13,18–23,89,90 The regimen used most often in previous trials was once per day application for 1 week, and increasing the frequency of dosing did not appear to improve the response rate (efficacy 23–93% for once per day compared with 18–100% for twice per day).
What the current trial adds
Efficacy of lactic acid gel for the treatment of bacterial vaginosis
In the VITA trial, which included a large sample of symptomatic participants with frequent recurrent BV, we found that lactic acid gel was less effective in clearing BV symptoms than oral metronidazole. However, a substantial proportion of participants in both arms did not respond to either treatment (30% of those receiving metronidazole and 53% of those receiving lactic acid gel). Moreover, in those who did respond, the limited data we had suggested that the subsequent risk of recurrent BV was very similar in both treatment arms.
In a post hoc analysis, we found no major differences in the demographic, behavioural or disease characteristics of those who responded to metronidazole compared with those who responded to lactic acid gel. Specifically, the proportion with ‘severe’ BV (based on the number of episodes or duration of symptoms in the past year) was the same in both arms. A greater proportion of participants in the lactic acid gel arm had positive microscopy for BV at week 2 (28% vs. 10% in the metronidazole arm). Overall treatment success over the 6 months of the trial was poor in both arms, with only 23% of those in the metronidazole arm and 16% of those in the lactic acid gel arm having symptom resolution and no recurrences over the 6 months following treatment, although these estimates are based on < 50% of the original trial population.
Metronidazole was associated with notably more side effects than lactic acid gel in the 2 weeks after taking study treatment, including one-quarter of participants who reported vaginal candidiasis (27% in the metronidazole arm vs. 17% in the lactic acid gel arm). However, adherence to both therapies was nevertheless high.
Our previously published qualitative study undertaken in a subset of VITA trial participants57 indicates that women dislike taking repeat courses of metronidazole to treat BV and many prefer lactic acid gel, despite perceiving it to be less effective. A preference for the lactic acid gel was based on a desire to apply treatment directly to the site of the BV, the gel having an immediate soothing effect, concerns that frequent use of antibiotics would lead to resistance and the ready availability of lactic acid gel without requiring a medical consultation or prescription. Avoiding the need to take antibiotic tablets twice per day and being able to drink alcohol were also reported as being benefits of using lactic acid gel, whereas greater efficacy and tablets being more convenient than using intravaginal lactic acid gel were advantages of metronidazole. Participants’ preferences for treatment were, thus, not based solely on short-term effectiveness but also related to ease of use, side effects, the individual’s value system and beliefs, and the possible long-term consequences of treatment.
Health economics
The health economic analysis found lactic acid gel to be more costly than oral metronidazole for the treatment of BV, which was mostly linked to reduced efficacy, leading to increased utilisation of secondary care. This difference persisted in a number of sensitivity analyses but there was uncertainty around the difference in costs, suggesting that intravaginal lactic acid gel could be either more or less costly than oral metronidazole. We attempted to include a ‘penalty’ cost for the risk of AMR associated with the use of metronidazole. This reduced the cost difference, but a robust methodology to measure the cost of resistance is not available and it is difficult to know how accurate our estimates of the cost of resistance were.
Safety, tolerability and adherence
Oral metronidazole is recognised to cause systemic side effects71 and participants who received it reported more gastrointestinal symptoms than those in the lactic acid gel arm, although these were generally of mild severity. Vaginal irritation occurred in 21% of those receiving lactic acid gel, which was similar to those receiving metronidazole (28%). Antibiotics are known to increase the risk of vaginal candidiasis,91 and thrush was reported to occur after treatment in 27% of participants in the metronidazole arm compared with 17% of those in the lactic acid gel arm in the 2 weeks after initiating treatment.
No SAEs or suspected unexpected serious adverse reactions were reported.
Rates of adherence to metronidazole for the treatment of BV have been reported to be only 50–68%. 92 However, in our clinical trial setting adherence was high, with > 90% of participants in both arms finishing their treatment course. Participants found that both treatments were easy to take, and the most common reason for missing a dose was forgetting to take it. However, these data were based on the 61% of participants who returned their week 2 questionnaire. It is not known whether or not those who did not return their questionnaires took their study treatment.
Clinical and microscopy diagnosis of bacterial vaginosis
Entry into the VITA trial and assessment of treatment response were based on the presence or absence of participant-reported BV symptoms to mirror ‘real life’ in most clinical primary care practice. Participants were symptomatic at the time of randomisation and had a history of at least one other episode of BV within the past 2 years that had resolved following treatment. Vaginal discharge can have multiple causes, although BV is typically associated with a characteristic fishy odour. A pre-planned analysis that was restricted to participants who had positive microscopic confirmation of BV29 at baseline showed a similar response rate to that seen in the primary analysis: resolution of BV symptoms in 75% of participants receiving metronidazole and 49% for those receiving lactic acid gel. Resolution at week 2, confirmed via microscopy, occurred in 77% of participants receiving metronidazole compared with 42% of those receiving lactic acid gel, suggesting that metronidazole may have a greater effect on the pattern of vaginal bacteria seen on microscopy than lactic acid gel. However, the clinical relevance of greater microbiological resolution of BV (compared with clinical symptom resolution) is uncertain because, following a clinical treatment response (resolution of symptoms) in either treatment arm, no differences were detected in the frequency or timing of BV recurrences over the next 6 months. Caution is, however, required in interpreting the limited data that were available over the 6-month follow-up period.
We chose resolution of BV symptoms as the primary end point to maximise the trial’s relevance to clinical practice where, in many settings, microscopy is not available and management is based on history and examination. In addition, microbiological evidence of BV can be present without symptoms93 and cyclical changes in the vaginal flora (including BV-type flora) can occur in the absence of treatment,94 which makes interpretation of microbiological cures difficult. Microscopy diagnosis of BV at week 2 was included as a secondary outcome in the trial and was based on the criteria described by Ison and Hay,29 as recommended in the UK National Guideline for the management of Bacterial Vaginosis. 10 Diagnosis using Ison and Hay criteria29 correlates with the two other commonly used approaches to microbiologic BV diagnosis: Amsel’s criteria29 and Nugent’s score. 95,96
From a patient perspective, the symptoms of discharge and malodour are the main causes of the physical and emotional distress associated with BV, have an impact on self-esteem and restrict sexual activity. 56,57 Our findings are, therefore, of direct relevance to patients and those providing treatment for BV.
Other factors that might have affected treatment efficacy
The VITA trial enrolled participants with severe recurrent BV. Women with frequent recurrent BV respond less well to treatment81 and this was evident in our trial as the response rate was lower in both treatment arms in those with multiple recent episodes of BV. However, the frequency of prior BV did not predict symptom resolution differently between the two treatment arms. In those successfully treated with either metronidazole or lactic acid gel, the same proportion (around one-third) reported having BV on more than three occasions in the past year.
Participants were advised to pause using lactic acid gel if they were menstruating heavily, reflecting usual practice, but overall adherence to therapy was high with few missed doses.
Strengths and limitations
The VITA trial was a large trial that successfully included an ethnically diverse patient population. Our findings help to inform evidence-based care for women with severe recurrent BV who suffer considerable physical and psychological sequelae56,57 and incur considerable costs to health-care systems. 9 There were no major protocol violations recorded and participants had good adherence to the study treatments. Our trial design was robust and the primary findings remained consistent on multiple sensitivity analyses. The trial was pragmatic and reflected common clinical practice, ensuring that our results are likely to be applicable in a range of primary care settings.
We recognise several limitations or potential limitations. It was not practical or feasible to blind participants or clinicians to the treatment allocation, which raises the possibility of reporting bias. Our previous work suggests that women with recurrent BV may consider lactic acid gel to be less effective than metronidazole. 57 However, we think that this is unlikely to be a major cause of bias because a pre-planned secondary analysis of outcomes based on microscopy findings (carried out by technicians who were blind to treatment allocation) showed a similar, if slightly larger, difference in treatment efficacy to that found in the primary analysis.
The primary outcome, participant-reported resolution of BV symptoms, was collected both through a web-based questionnaire and via direct questioning during a telephone call. The proportion reporting symptom resolution was higher when data were collected over the telephone in both treatment arms, particularly in the metronidazole arm (83% via the telephone compared with 66% via the questionnaire who reported resolution). The telephone call was used as a ‘back-up’ when the questionnaire had not been completed and, therefore, the time interval after treatment to collect data was longer than that for the web-based questionnaire, raising the possibility of response or recall bias in the telephone group. Despite this, we do not believe that this is likely to have had a major effect because telephone collection of the primary outcome was used for a minority of participants only (88/409; 22%) and a similar number of telephone calls had to be made in both treatment arms.
The loss to follow-up rate for collection of the primary end point was 21% compared with a predicted rate of 10%. 25 Sensitivity analyses were undertaken including assumptions that all participants with missing data had symptom resolution or that they all did not have symptom resolution. In both scenarios, the adjusted risk difference was –18%, compared with the primary analysis risk difference of –23%. It is, therefore, possible that the difference in efficacy between metronidazole and lactic acid gel is not as large as we found, but the short-term (2 weeks after starting treatment) efficacy of metronidazole is very likely to remain substantially greater than lactic acid gel. The recurrence rate in both arms may also have been elevated because ‘no recurrence’ required reporting an absence of symptoms at all follow-up time points, whereas ‘recurrence’ required reporting of symptoms at a only single time point, that is missing data were more likely to be recorded as a ‘recurrence’ (given that this needed to be reported as ‘yes’ at only one time point) rather than ‘no recurrence’ (given that this needed to be reported as ‘no’ at every time point).
A large number of participants did not provide data at week 2 (with the exception of the primary outcome for which telephone calls were made to obtain these data) or at 3 and 6 months post treatment. Various assumptions, therefore, had to be made owing to incomplete or inconsistent recording of the data provided, meaning that some outcomes were based on small and potentially non-random subsets of participants.
Data for the full 6 months of the trial were available for < 40% of randomised participants, and for outcomes related to recurrence and the number of additional treatment courses it was available for even fewer participants. To be classed as a recurrence, participants had to have data to show that their BV symptoms had resolved within the 2 weeks after treatment had started. Therefore, any between-arm comparisons were between non-randomised groups and any results need to be interpreted with caution. Although participants who had symptom resolution in both treatment arms appeared similar in many measured characteristics, there were some differences, and indeed they could have differed with respect to characteristics that were not measured. In addition, there may have been differences between the two arms in the participants who provided follow-up data with respect to their actual recurrence and/or characteristics associated with their recurrence(s). This may also have influenced the quality of questionnaire completion. We do not have information to assess this.
Although the sample size remained large in comparison with previous BV treatment trials (219 participants at 3 months and 176 participants at 6 months), given the low proportion of those originally randomised and the potential for bias in the between-treatment comparisons, caution is required in interpreting the longer-term trial findings.
Data on resource use were available for < 40% of randomised participants at different time points. Excluding those who did not complete the resource use questionnaire can introduce bias and some degree of inefficiency. Further research would be required to estimate the resource use and HRQoL associated with use of oral metronidazole and lactic acid gel with more precision, particularly over the longer term.
Chapter 7 Conclusions
Conclusions
In the VITA trial, participants with recurrent BV had a substantially higher response to treatment at 2 weeks with oral metronidazole than with intravaginal lactic acid gel, but treatment failure was relatively common in both arms. Subsequent recurrences of BV in the subset of participants who provided longer-term data appeared to be frequent and did not differ between those who had an initial treatment response following either therapy. Metronidazole is more likely to be cost-effective, with lower associated resource use and higher efficacy than lactic acid gel, but there is uncertainty surrounding the resource use estimates. Participants interviewed in a qualitative substudy about their experiences of treatment for BV disliked taking repeated course of antibiotics for BV and in general preferred lactic acid gel even if its short-term efficacy was lower.
Implications for health-care practice
In the absence of an effective treatment for recurrent BV, shared clinical decision-making with women about their therapeutic options assumes particular importance. The VITA trial provides robust measures of treatment response and side effects for metronidazole and lactic acid gel at 2 weeks, with additional, more limited, data over a 6-month time period. These findings can help women with BV and their clinicians make more informed decisions about therapy, taking account of women’s individual contexts and preferences.
Women should be informed of the higher initial clinical response to metronidazole but that recurrence of BV following either of these treatments is common. For some women, oral metronidazole may be favoured because of its higher short-term efficacy. However, other women may prefer intravaginal lactic acid gel if ease of use, avoiding antibiotic side effects and resistance, or access to treatment without need for medical prescription are of greater importance.
Recommendations for research
In the absence of effective curative therapy, further investigation of non-antibiotic continuous or intermittent treatment regimens to control the symptoms of recurrent BV are required to improve quality of life in this patient group.
Further analysis of vaginal samples collected in the trial would be useful to identify whether or not microbiological factors, such as specific bacterial subspecies, inflammatory co-factors or antimicrobial susceptibility, affect the short- and long-term response to metronidazole or lactic acid gel in a subgroup of women with BV.
Further development of the methods for incorporating costs and the impact of AMR specific to the antibiotic consumed in relation to recurrent BV is required.
Acknowledgements
We would like to thank the TSC for their advice and support throughout the trial: chairperson Professor Jackie Cassell (University of Brighton), Dr Gary Brook (Central Middlesex Hospital), Dr Andrew Winter (NHS Greater Glasgow and Clyde) and Ms Jo Josh (PPI representative); Mr David Roberts-Jones and Ms Georgie Bayliss also helped out as prior PPI representatives. Our thanks also go to the DMC for their input and guidance throughout the trial: chairperson Professor Chris Butler (University of Oxford), Dr Philip V Hay (St George’s University Hospitals NHS Foundation Trust) and Professor Siobhan Creanor (University of Plymouth).
We would also like to thank the following people who helped towards the successful completion of the trial: Professor Tracy Roberts (Professor of Health Economics, contributed to the development of the trial protocol and mentored Louise Jackson), Dr Sukhwinder Thandi (Clinical Trial Manager from September 2016 to June 2019, co-author on the published protocol paper and qualitative paper), John Watson (Clinical Trial Administrator), Richard Swinden (Clinical Trial Administrator), Stella Tarr (Data Manager), Lisa Evans (Data Administrator), Emily Wallbanks (Data Administrator), Chris Rumsey (Database Systems Developer), Sharon Ellender (Monitor), Kirsty Sprange (Senior Trial Manager), Wei Tan (independent statistician) and Tessa Lawrence (Research Manager).
We are grateful to the participants and staff from all of the sites who took part in the trial; the main contacts from each site are listed in alphabetical order below:
-
Axess Sexual Health Clinic, Royal Liverpool & Broadgreen University Hospital Trust, Liverpool – Damitha Edirisinghe (PI) and Graham Sweeney.
-
Bolton Centre for Sexual and Reproductive Health, Bolton NHS Foundation Trust, Bolton – Alison Loftus and Emile Morgan (PI).
-
Burrell Street Sexual Health Centre, Guys & St Thomas’ NHS Foundation Trust, London – Nori Achyuata (PI) and Hibo Mahdi.
-
University Hospital of Wales, Cardiff and Vale University Health Board, Cardiff – Sinead Cook (PI) and Jennie Williams.
-
Cripps Health Centre, Nottingham NHS Clinical Commissioning Group, Nottingham – Simon Royal (PI).
-
Derby Sexual Health, Derbyshire Community Health Services NHS Trust, Derby – Ade Apoola (PI) and Joanna Wright.
-
Elton John Centre, Brighton and Sussex University Hospitals NHS Trust, Brighton – Marion Campbell and Gillian Dean (PI).
-
Grahame Hayton Unit, Barts Health NHS Trust, London – Helena Miras and Liat Sarner (PI).
-
Hammersmith Broadway, Chelsea and Westminster Hospital NHS Foundation Trust, London – Michael Rayment (PI) and Alex Schoolmeesters.
-
Integrated Sexual Health Services, Coventry & Warwickshire Partnership NHS Foundation Trust, Coventry – Nyaradzo Nyamayaro and Huda Taha (PI).
-
John Hunter Clinic, Chelsea and Westminster Hospital NHS Foundation Trust, London – Michael Rayment (PI) and Alex Schoolmeesters.
-
Leeds Sexual Health, Leeds Teaching Hospitals NHS Foundation Trust, Leeds – Michelle Loftus-Keeling and Janet Wilson (PI).
-
Mortimer Market Centre, Central and North West London NHS Foundation Trust, London – Richard Gilson (PI) and Gaynor Lawrenson.
-
Trafalgar Clinic, Lewisham & Greenwich NHS Trust, London – Praveen Jayadeva (PI) and Tarik Moussaoui.
-
Heartlands Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham – Gerry Gilleran and Stephen Taylor (PI).
-
Royal Bournemouth Hospital, The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust, Bournemouth – Kate Schroeder (PI) and Annamaria Wilce.
-
Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield – Meena Chopra and Karen Rogstad (PI).
-
Sexual Health, West Middlesex Hospital, London – Ursula Kirwan and Michael Rayment (PI).
-
St Mary’s Hospital, Imperial College Healthcare NHS Trust, London – Wilbert Ayap and Dawn Wilkinson (PI).
-
The Florey Sexual Health & Contraceptive Services, Royal Berkshire NHS Foundation Trust, Berkshire – Fabien Chen (PI) and Sheila O’Connor.
-
The Redwoods Centre, Stafford & Shropshire Midlands Partnership Foundation NHS Trust, Shrewsbury – Paula Coventry and Andrea Ng (PI).
-
Whittall Street Clinic, University Hospitals Birmingham NHS Foundation Trust, Birmingham – Magda Nowacka and Jonathan DC Ross (PI).
Contributions of authors
Lindsay Armstrong-Buisseret (https://orcid.org/0000-0002-8045-5464) (Clinical Trial Manager) managed delivery and co-ordination of the trial from June 2019 onwards. She also drafted sections of the Health Technology Assessment (HTA) report and revised it critically for important intellectual content.
Clare Brittain (https://orcid.org/0000-0002-2950-0733) (Senior Trial Manager) was responsible for overseeing the delivery and management of the trial and revised the HTA report critically for important intellectual content.
Joe Kai (https://orcid.org/0000-0001-9040-9384) (Clinical Professor and Head of Primary Care) contributed to the development of the trial protocol and revised the HTA report critically for important intellectual content.
Miruna David (https://orcid.org/0000-0002-6756-0550) (Consultant Microbiologist) contributed to the development of the trial protocol and revised the HTA report critically for important intellectual content.
Jocelyn Anstey Watkins (https://orcid.org/0000-0003-4984-1057) (Research Fellow) undertook the qualitative study including conducting all telephone interviews, analysing the data and drafting the paper for publication along with the qualitative chapter for this report. She also revised the HTA report critically for important intellectual content.
Mara Ozolins (https://orcid.org/0000-0001-7929-9807) (Medical Statistician) carried out the statistical analysis and interpretation of clinical results. She also drafted sections of the HTA report and revised it critically for important intellectual content.
Louise Jackson (https://orcid.org/0000-0001-8492-0020) (Senior Lecturer in Health Economics) contributed to the development of the trial protocol, conducted the health economic analysis and revised the HTA report critically for important intellectual content.
Zainab Abdali (https://orcid.org/0000-0002-2736-5427) (Research Fellow in Health Economics) conducted the health economic analysis and revised the HTA report critically for important intellectual content.
Trish Hepburn (https://orcid.org/0000-0002-0936-6696) (Senior Medical Statistician) contributed to study design and conduct, statistical analysis and interpretation of data. She also drafted sections of the HTA report and revised it critically for important intellectual content.
Frances Griffiths (https://orcid.org/0000-0002-4173-1438) (Professor of Medicine in Society) led the qualitative study. She conceptualised the study design, contributed to data analysis and revised the HTA report critically for important intellectual content.
Alan Montgomery (https://orcid.org/0000-0003-0450-1606) (Professor of Medical Statistics and Clinical Trials) contributed to study design and conduct, interpretation of data and revised the HTA report critically for important intellectual content.
Jane Daniels (https://orcid.org/0000-0003-3324-6771) (Professor of Clinical Trials) contributed to study conduct, interpretation of data and revised the HTA report critically for important intellectual content.
Alice Manley (https://orcid.org/0000-0002-3804-1751) (Research Manager) contributed to study design and conduct as well as support with the delivery of the trial.
Gillian Dean (https://orcid.org/0000-0001-5599-2594) (Consultant) contributed to the development of the trial protocol and revised the HTA report critically for important intellectual content.
Jonathan DC Ross (https://orcid.org/0000-0002-4193-6919) (Professor of Sexual Health and HIV; VITA Chief Investigator) was responsible for identifying the research question, and contributed to study design, conduct and analysis. He also drafted sections of the HTA report and revised it critically for important intellectual content.
Publications
Anstey Watkins J, Ross JDC, Thandi S, Brittain C, Kai J, Griffiths F. Acceptability of and treatment preferences for recurrent bacterial vaginosis – topical lactic acid gel or oral metronidazole antibiotic: qualitative findings from the VITA trial. PLOS ONE 2019;14:e0224964.
Armstrong-Buisseret L, Brittain C, David M, Dean G, Griffiths F, Hepburn T et al. Metronidazole versus lactic acid for treating bacterial vaginosis (VITA): protocol for a randomised controlled trial to assess the clinical and cost-effectiveness of topical lactic acid gel for treating second and subsequent episodes of bacterial vaginosis. Trials 2019;20:648.
Data-sharing statement
The data sets analysed during the VITA trial will be available on request from the corresponding author. Access to the data will be subject to review of a data sharing and use request by a committee including the chief investigator and sponsor and will be granted only on receipt of a data-sharing and use agreement. Any data shared will be pseudo-anonymised, which may have an impact on the reproducibility of published analyses. Data from the qualitative substudy cannot be shared publicly beyond that contained within this report because it is human research data on a potentially sensitive subject relating to sexual health.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008;22:1493-501. https://doi.org/10.1097/QAD.0b013e3283021a37.
- Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001251.
- Ness RB, Kip KE, Hillier SL, Soper DE, Stamm CA, Sweet RL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol 2005;162:585-90. https://doi.org/10.1093/aje/kwi243.
- Leitich H, Brunbauer M, Bodner-Adler B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol 2003;188:752-8. https://doi.org/10.1067/mob.2003.167.
- Hay P. Bacterial vaginosis [version 1; peer review: 2 approved]. F1000Res 2017;6. https://doi.org/10.12688/f1000research.11417.1.
- Jones A. Bacterial vaginosis: a review of treatment, recurrence, and disparities. J Nurse Pract 2019;15:420-3. https://doi.org/10.1016/j.nurpra.2019.03.010.
- Bagnall P, Rizzolo D. Bacterial vaginosis: a practical review. JAAPA 2017;30:15-21. https://doi.org/10.1097/01.JAA.0000526770.60197.fa.
- Schwebke JR. Bacterial vaginosis: are we coming full circle?. J Infect Dis 2009;200:1633-5. https://doi.org/10.1086/648093.
- Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis 2019;46:304-11. https://doi.org/10.1097/OLQ.0000000000000972.
- British Association for Sexual Health and HIV (BASHH) . UK National Guideline for the Management of Bacterial Vaginosis 2012 2012.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. Recomm Rep 2015;64:1-137.
- Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006;193:1478-86. https://doi.org/10.1086/503780.
- Simoes JA, Bahamondes LG, Camargo RP, Alves VM, Zaneveld LJ, Waller DP, et al. A pilot clinical trial comparing an acid-buffering formulation (ACIDFORM gel) with metronidazole gel for the treatment of symptomatic bacterial vaginosis. Br J Clin Pharmacol 2006;61:211-17. https://doi.org/10.1111/j.1365-2125.2005.02550.x.
- Joesoef MR, Schmid GP. Bacterial vaginosis: review of treatment options and potential clinical indications for therapy. Clin Infect Dis 1995;20:72-9. https://doi.org/10.1093/clinids/20.supplement_1.s72.
- Public Health England . Sexually Transmitted Infections (STIs): Annual Data Tables: Table 4 2018. www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables (accessed 25 February 2020).
- HM Government . Tackling Antimicrobial Resistance 2019–24. The UK’s Five-Year National Action Plan 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf (accessed 5 December 2019).
- European Commission . A European One Health Action Plan Against Antimicrobial Resistance (AMR) 2017. https://ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf (accessed 5 December 2019).
- Fiorilli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a policarbophil-carbopol acidic vaginal gel: results from a randomised double-blind, placebo-controlled trial. Eur J Obstet Gynecol Reprod Biol 2005;120:202-5. https://doi.org/10.1016/j.ejogrb.2004.10.011.
- Boeke AJ, Dekker JH, van Eijk JT, Kostense PJ, Bezemer PD. Effect of lactic acid suppositories compared with oral metronidazole and placebo in bacterial vaginosis: a randomised clinical trial. Genitourin Med 1993;69:388-92. https://doi.org/10.1136/sti.69.5.388.
- Holley RL, Richter HE, Varner RE, Pair L, Schwebke JR. A randomized, double-blind clinical trial of vaginal acidification versus placebo for the treatment of symptomatic bacterial vaginosis. Sex Transm Dis 2004;31:236-8. https://doi.org/10.1097/01.OLQ.0000118423.20985.E7.
- Andersch B, Forssman L, Lincoln K, Torstensson P. Treatment of bacterial vaginosis with an acid cream: a comparison between the effect of lactate-gel and metronidazole. Gynecol Obstet Invest 1986;21:19-25. https://doi.org/10.1159/000298923.
- Fredricsson B, Englund K, Weintraub L, Olund A, Nord CE. Bacterial vaginosis is not a simple ecological disorder. Gynecol Obstet Invest 1989;28:156-60. https://doi.org/10.1159/000293556.
- Decena DC, Co JT, Manalastas RM, Palaypayon EP, Padolina CS, Sison JM, et al. Metronidazole with Lactacyd vaginal gel in bacterial vaginosis. J Obstet Gynaecol Res 2006;32:243-51. https://doi.org/10.1111/j.1447-0756.2006.00383.x.
- Lugo-Miro VI, Green M, Mazur L. Comparison of different metronidazole therapeutic regimens for bacterial vaginosis. A meta-analysis. JAMA 1992;268:92-5. https://doi.org/10.1001/jama.1992.03490010094036.
- Armstrong-Buisseret L, Brittain C, David M, Dean G, Griffiths F, Hepburn T, et al. Metronidazole versus lactic acid for treating bacterial vaginosis (VITA): protocol for a randomised controlled trial to assess the clinical and cost effectiveness of topical lactic acid gel for treating second and subsequent episodes of bacterial vaginosis. Trials 2019;20. https://doi.org/10.1186/s13063-019-3731-7.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-32.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Great Britain . The Medicines for Human Use (Marketing Authorisations Etc.) Regulations 1994 1994.
- Ison CA, Hay PE. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect 2002;78:413-15. https://doi.org/10.1136/sti.78.6.413.
- Hay PE. Therapy of bacterial vaginosis. J Antimicrob Chemother 1998;41:6-9. https://doi.org/10.1093/jac/41.1.6.
- Larsson PG. Treatment of bacterial vaginosis. Int J STD AIDS 1992;3:239-47. https://doi.org/10.1177/095646249200300402.
- Hillier SL, Lipinski C, Briselden AM, Eschenbach DA. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol 1993;81:963-7.
- Sherrard J, Wilson J, Donders G, Mendling W, Jensen JS. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int J STD AIDS 2018;29:1258-72. https://doi.org/10.1177/0956462418785451.
- Oppong R, Smith RD, Little P, Verheij T, Butler CC, Goossens H, et al. Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract 2016;66:e633-9. https://doi.org/10.3399/bjgp16X686533.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.ncbi.nlm.nih.gov/books/NBK395867/ (accessed 27 April 2020).
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Petrou S, Morrell J, Spiby H. Assessing the empirical validity of alternative multi-attribute utility measures in the maternity context. Health Qual Life Outcomes 2009;7. https://doi.org/10.1186/1477-7525-7-40.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Med Res Methodol 2017;17. https://doi.org/10.1186/s12874-017-0442-1.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- NHS . National Cost Collection 2019 2020. www.england.nhs.uk/national-cost-collection/ (accessed 20 May 2020).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 20 May 2020).
- Campbell MK, Torgerson DJ. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM 1999;92:177-82. https://doi.org/10.1093/qjmed/92.3.177.
- Glick HA, Briggs AH, Polsky D. Quantifying stochastic uncertainty and presenting results of cost-effectiveness analyses. Expert Rev PharmacoEcon Outcomes Res 2001;1:25-36. https://doi.org/10.1586/14737167.1.1.25.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. https://doi.org/10.1177/0272989X9001000308.
- Shrestha P, Cooper BS, Coast J, Oppong R, Do Thi Thuy N, Phodha T, et al. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control 2018;7. https://doi.org/10.1186/s13756-018-0384-3.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Gray A, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2010.
- Bradshaw CS, Sobel JD. Current treatment of bacterial vaginosis – limitations and need for innovation. J Infect Dis 2016;214:14-20. https://doi.org/10.1093/infdis/jiw159.
- Pollock K, Wilson E. Care and communication between health professionals and patients affected by severe or chronic illness in community care settings: a qualitative study of care at the end of life. Health Serv Deliv Res 2015;3. https://doi.org/10.3310/hsdr03310.
- Bradshaw CS, Brotman RM. Making inroads into improving treatment of bacterial vaginosis – striving for long-term cure. BMC Infect Dis 2015;15. https://doi.org/10.1186/s12879-015-1027-4.
- Hull CE, McLella AR. Acute and recurrent bacterial vaginosis. Clin Rev 2016;26:43-4.
- Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012;6:39-48. https://doi.org/10.2147/PPA.S24752.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Bilardi JE, Walker S, Temple-Smith M, McNair R, Mooney-Somers J, Bellhouse C, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0074378.
- Anstey Watkins J, Ross JDC, Thandi S, Brittain C, Kai J, Griffiths F. Acceptability of and treatment preferences for recurrent bacterial vaginosis – topical lactic acid gel or oral metronidazole antibiotic: qualitative findings from the VITA trial. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0224964.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Bilardi JE, Walker SM, Temple-Smith MJ, McNair RP, Mooney-Somers J, Vodstrcil LA, et al. Women view key sexual behaviours as the trigger for the onset and recurrence of bacterial vaginosis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0173637.
- Turovskiy Y, Sutyak Noll K, Chikindas ML. The aetiology of bacterial vaginosis. J Appl Microbiol 2011;110:1105-28. https://doi.org/10.1111/j.1365-2672.2011.04977.x.
- Payne SC, Cromer PR, Stanek MK, Palmer AA. Evidence of African-American women’s frustrations with chronic recurrent bacterial vaginosis. J Am Acad Nurse Pract 2010;22:101-8. https://doi.org/10.1111/j.1745-7599.2009.00474.x.
- McGowan I, Gomez K, Bruder K, Febo I, Chen BA, Richardson BA, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). AIDS 2011;25:1057-64. https://doi.org/10.1097/QAD.0b013e328346bd3e.
- Pirotta M, Fethers KA, Bradshaw CS. Bacterial vaginosis – more questions than answers. Aust Fam Physician 2009;38:394-7.
- Centers for Disease and Control Prevention . 2015 Sexually Transmitted Diseases Treatment Guidelines. Bacterial Vaginosis 2015. www.cdc.gov/std/tg2015/bv.htm (accessed 6 August 2020).
- Ling Z, Liu X, Chen W, Luo Y, Yuan L, Xia Y, et al. The restoration of the vaginal microbiota after treatment for bacterial vaginosis with metronidazole or probiotics. Microb Ecol 2013;65:773-80. https://doi.org/10.1007/s00248-012-0154-3.
- Muzny CA, Kardas P. A narrative review of current challenges in the diagnosis and management of bacterial vaginosis. Sex Transm Dis 2020;47:441-6. https://doi.org/10.1097/OLQ.0000000000001178.
- Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis 2009;22:82-6. https://doi.org/10.1097/QCO.0b013e32832180c6.
- Sobel JD, Kaur N, Woznicki NA, Boikov D, Aguin T, Gill G, et al. Prognostic indicators of recurrence of bacterial vaginosis. J Clin Microbiol 2019;57:e00227-19. https://doi.org/10.1128/JCM.00227-19.
- Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment: 2018 update. Curr Opin Infect Dis 2019;32:38-42. https://doi.org/10.1097/QCO.0000000000000516.
- Norris Turner A, Carr Reese P, Snead MC, Fields K, Ervin M, Kourtis AP, et al. Recent biomarker-confirmed unprotected vaginal sex, but not self-reported unprotected sex, is associated with recurrent bacterial vaginosis. Sex Transm Dis 2016;43:172-6. https://doi.org/10.1097/olq.0000000000000414.
- Sanofi . Metronodazole Summary of Product Characteristics 2019. www.medicines.org.uk/emc/product/9238/smpc#UNDESIRABLE_EFFECTS (accessed 4 May 2020).
- Sobel JD, Ferris D, Schwebke J, Nyirjesy P, Wiesenfeld HC, Peipert J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006;194:1283-9. https://doi.org/10.1016/j.ajog.2005.11.041.
- Beigi RH, Austin MN, Meyn LA, Krohn MA, Hillier SL. Antimicrobial resistance associated with the treatment of bacterial vaginosis. Am J Obstet Gynecol 2004;191:1124-9. https://doi.org/10.1016/j.ajog.2004.05.033.
- Schuyler JA, Mordechai E, Adelson ME, Sobel JD, Gygax SE, Hilbert DW. Identification of intrinsically metronidazole-resistant clades of Gardnerella vaginalis. Diagn Microbiol Infect Dis 2016;84:1-3. https://doi.org/10.1016/j.diagmicrobio.2015.10.006.
- Deng ZL, Gottschick C, Bhuju S, Masur C, Abels C, Wagner-Döbler I. Metatranscriptome analysis of the vaginal microbiota reveals potential mechanisms for protection against metronidazole in bacterial vaginosis. MSphere 2018;3:e00262-18. https://doi.org/10.1128/mSphereDirect.00262-18.
- Bradshaw CS, Tabrizi SN, Fairley CK, Morton AN, Rudland E, Garland SM. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J Infect Dis 2006;194:828-36. https://doi.org/10.1086/506621.
- Balkus JE, Srinivasan S, Anzala O, Kimani J, Andac C, Schwebke J, et al. Impact of periodic presumptive treatment for bacterial vaginosis on the vaginal microbiome among women participating in the preventing vaginal infections trial. J Infect Dis 2017;215:723-31. https://doi.org/10.1093/infdis/jiw622.
- Elliott TS, Stone JW. Review article: metronidazole and the anaerobic gut flora. Aliment Pharmacol Ther 1990;4:227-38. https://doi.org/10.1111/j.1365-2036.1990.tb00467.x.
- Tedesco FJ. Clindamycin and colitis: a review. J Infect Dis 1977;135:S95-8. https://doi.org/10.1093/infdis/135.supplement.s95.
- Meadowcroft AM, Diaz PR, Latham GS. Clostridium difficile toxin-induced colitis after use of clindamycin phosphate vaginal cream. Ann Pharmacother 1998;32:309-11. https://doi.org/10.1345/aph.17251.
- Sobel JD, Kaur N, Woznicki NA, Boikov D, Aguin T, Gill G, et al. Conventional oral and secondary high dose vaginal metronidazole therapy for recurrent bacterial vaginosis: clinical outcomes, impacts of sex and menses. Infect Drug Resist 2019;12:2297-307. https://doi.org/10.2147/IDR.S213853.
- Bradshaw CS, Pirotta M, De Guingand D, Hocking JS, Morton AN, Garland SM, et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0034540.
- Broumas AG, Basara LA. Potential patient preference for 3-day treatment of bacterial vaginosis: responses to new suppository form of clindamycin. Adv Ther 2000;17:159-66. https://doi.org/10.1007/BF02853158.
- Marrazzo JM, Dombrowski JC, Wierzbicki MR, Perlowski C, Pontius A, Dithmer D, et al. Safety and efficacy of a novel vaginal anti-infective, TOL-463, in the treatment of bacterial vaginosis and vulvovaginal candidiasis: a randomized, single-blind, phase 2, controlled trial. Clin Infect Dis 2019;68:803-9. https://doi.org/10.1093/cid/ciy554.
- van de Wijgert J, Verwijs MC. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG 2020;127:287-99. https://doi.org/10.1111/1471-0528.15870.
- Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med 2020;382:1906-15. https://doi.org/10.1056/NEJMoa1915254.
- Balkus JE, Carter KA, McClelland RS. Lessons from suppressive therapy and periodic presumptive treatment for bacterial vaginosis. Curr Infect Dis Rep 2019;21. https://doi.org/10.1007/s11908-019-0688-3.
- Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 2015;6. https://doi.org/10.3389/fphys.2015.00164.
- Haggerty CL, Ness RB, Amortegui A, Hendrix SL, Hillier SL, Holley RL, et al. Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am J Obstet Gynecol 2003;188:141-8. https://doi.org/10.1067/mob.2003.87.
- Powell A, Ghanem KG, Rogers L, Zinalabedini A, Brotman RM, Zenilman J, et al. Clinicians’ use of intravaginal boric acid maintenance therapy for recurrent vulvovaginal candidiasis and bacterial vaginosis. Sex Transm Dis 2019;46:810-12. https://doi.org/10.1097/OLQ.0000000000001063.
- Shukla A, Sobel JD. Vulvovaginitis caused by Candida species following antibiotic exposure. Curr Infect Dis Rep 2019;21. https://doi.org/10.1007/s11908-019-0700-y.
- Bartley JB, Ferris DG, Allmond LM, Dickman ED, Dias JK, Lambert J. Personal digital assistants used to document compliance of bacterial vaginosis treatment. Sex Transm Dis 2004;31:488-91. https://doi.org/10.1097/01.olq.0000135990.21755.51.
- Schwebke JR, Desmond R. Natural history of asymptomatic bacterial vaginosis in a high-risk group of women. Sex Transm Dis 2007;34:876-7. https://doi.org/10.1097/OLQ.0b013e318073bd82.
- Keane FE, Ison CA, Taylor-Robinson D. A longitudinal study of the vaginal flora over a menstrual cycle. Int J STD AIDS 1997;8:489-94. https://doi.org/10.1258/0956462971920631.
- Chawla R, Bhalla P, Chadha S, Grover S, Garg S. Comparison of Hay’s criteria with Nugent’s scoring system for diagnosis of bacterial vaginosis. Biomed Res Int 2013;2013. https://doi.org/10.1155/2013/365194.
- Forsum U, Jakobsson T, Larsson PG, Schmidt H, Beverly A, Bjørnerem A, et al. An international study of the interobserver variation between interpretations of vaginal smear criteria of bacterial vaginosis. APMIS 2002;110:811-18. https://doi.org/10.1034/j.1600-0463.2002.1101107.x.
- Pharmaceutical Services Negotiating Committee . Services and Commissioning 2020. https://psnc.org.uk/services-commissioning/ (accessed 20 May 2020).
- NHS . Reference Costs 2017 18 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 30 May 2020).
Appendix 1 VITA participant questionnaire: lactic acid week 2
Appendix 2 VITA participant questionnaire: metronidazole week 2
Appendix 3 VITA participant questionnaire: 3 months
Appendix 4 VITA participant questionnaire: 6 months
Appendix 5 Additional results tables
| Reason IDa | Reason | Total, n (%) |
|---|---|---|
| A | Not interested | 248 (9) |
| B | Takes too much time | 192 (7) |
| C | Current treatment will be successful | 68 (3) |
| D | Too many extra/intrusive samples | 9 (< 0.5) |
| E | Outside age range (< 16 years) | 5 (< 0.5) |
| F | Does not have access to the internet/e-mail | 1 (< 0.5) |
| G | Does not have current clinical diagnosis of BV | 83 (3) |
| H | Does not have history of at least one episode of BV within the last 2 years | 695 (27) |
| I | Did not provide written informed consent | 1 (< 0.5) |
| J | Has known contraindication or allergy to metronidazole or lactic acid | 26 (1) |
| K | Is pregnant or breastfeeding | 47 (2) |
| L | Receiving oral antibiotics or antifungal agents, concurrently (± 2 weeks) | 418 (16) |
| M | Using topical vaginal antibiotics, antifungals or acidifying products concurrently (± 2 weeks) | 209 (8) |
| N | Not willing to avoid sexual intercourse or use effective contraception for 7-day BV treatment | 38 (1) |
| O | Not willing to avoid vaginal douching for 7-day BV treatment | 3 (< 0.5) |
| P | Current participation in another clinical trial involving an IMP | 2 (< 0.5) |
| Q | Previous participation in this study | 60 (2) |
| R | Other reason | 335 (13) |
| S | No staff available | 178 (7) |
| Total | 2618 | |
| Site | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Oral metronidazole | Intravaginal lactic acid gel | ||
| Sexual health centres | |||
| Birmingham: Whittall Street Clinic | 85 (33) | 91 (36) | 176 (35) |
| London: Hammersmith Broadway | 47 (18) | 46 (18) | 93 (18) |
| Leeds: Leeds Sexual Health | 24 (9) | 23 (9) | 47 (9) |
| Brighton: Elton John Centre | 18 (7) | 15 (6) | 33 (6) |
| London: Grahame Hayton Unit | 16 (6) | 17 (7) | 33 (6) |
| London: St Mary’s Hospital | 16 (6) | 14 (6) | 30 (6) |
| London: Mortimer Market Centre | 8 (3) | 8 (3) | 16 (3) |
| Liverpool: Axess Sexual Health Centre | 8 (3) | 7 (3) | 15 (3) |
| Sheffield: Royal Hallamshire Hospital | 9 (4) | 5 (2) | 14 (3) |
| Bournemouth: Royal Bournemouth Hospital | 5 (2) | 6 (2) | 11 (2) |
| Coventry: Integrated Sexual Health Services | 5 (2) | 6 (2) | 11 (2) |
| London: Burrell Street Sexual Health Centre | 5 (2) | 3 (1) | 8 (2) |
| Derby: Derby Sexual Health | 3 (1) | 4 (2) | 7 (1) |
| Cardiff: University Hospital of Wales | 2 (1) | 3 (1) | 5 (1) |
| London: John Hunter Clinic | 1 (< 0.5) | 2 (1) | 3 (1) |
| Shrewsbury: The Redwoods Centre | 1 (< 0.5) | 2 (1) | 3 (1) |
| Birmingham: Heartlands Hospital | 1 (< 0.5) | 1 (< 0.5) | 2 (< 0.5) |
| Bolton: Bolton Centre for Sexual and Reproductive Health | 1 (< 0.5) | 0 | 1 (< 0.5) |
| London: Trafalgar Clinic | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Total | 255 | 254 | 509 |
| General practices | |||
| Nottingham: Cripps Health Centre | 4 | 5 | 9 |
| Total: all sites | 259 | 259 | 518 |
| Ison–Hay grade for BV (from central laboratory)a | Local BV results, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|
| Positive | Negative | Not tested | Missing | ||
| 0 (no bacteria) | 2 (0.5) | 0 | 0 | 0 | 2 (< 0.5) |
| 1 (normal flora) | 73 (17) | 32 (53) | 5 (24) | 0 | 110 (21) |
| 2 (intermediate BV) | 112 (26) | 8 (13) | 3 (14) | 0 | 123 (24) |
| 3 (confirmed BV) | 236 (54) | 17 (28) | 13 (62) | 0 | 266 (51) |
| U (Gram-positive cocci) | 3 (1) | 1 (2) | 0 | 0 | 4 (1) |
| Missing | 10 (2) | 2 (3) | 0 | 1 (100) | 13 (3) |
| Total | 436 | 60 | 21 | 1 | 518 |
| Participant characteristic | Treatment arm | Total (N = 518) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| HIV positive, n (%) | |||
| Yes | 2 (1) | 1 (< 0.5) | 3 (1) |
| No | 257 (99) | 257 (99) | 514 (99) |
| Missing | 0 | 1 (< 0.5%) | 1 (< 0.5) |
| Past history of immunosuppression other than HIV, n (%) | |||
| Yes | 2 (1) | 6 (2) | 8 (2) |
| No | 257 (99) | 252 (98) | 509 (98) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Reported reason for immunosuppression, n (%) | |||
| Took immunosuppressant drugs | 1 (50) | 1 (17) | 2 (25) |
| Has inherited immune deficiency | 1 (50) | 0 | 1 (13) |
| Othera | 0 | 5 (93) | 5 (83) |
| Had vaginal thrush in the past 12 months, n (%) | |||
| Yes | 123 (47) | 127 (49) | 250 (48) |
| No | 136 (53) | 131 (51) | 267 (52) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Number of episodes of vaginal thrush in past 12 months | |||
| 1, n (%) | 49 (40) | 58 (46) | 107 (43) |
| 2, n (%) | 39 (32) | 27 (21) | 66 (26) |
| > 2, n (%) | 35 (28) | 42 (33) | 77 (31) |
| Median (25th, 75th centile) | 2 (1, 3) | 2 (1, 4) | 2 (1, 3) |
| Minimum, maximum | 1, 12 | 1, 90 | 1, 90 |
| Participant characteristic | Treatment arm | Total (N = 518) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Has current sexual partner, n (%) | |||
| Yes | 192 (74) | 173 (67) | 365 (71) |
| No | 67 (26) | 85 (33) | 152 (29) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Approximate time with current/most recent sexual partner for those with partner (months) | |||
| n | 191 | 173 | 364 |
| Median (25th, 75th centile) | 12 (4, 48) | 11 (3.7, 36) | 12 (3.8, 36) |
| Minimum, maximum | 0.2, 300 | 0.1, 240 | 0.1, 300 |
| Approximate time since most recent sexual intercourse (days) | |||
| n | 259 | 257 | 516 |
| Median (25th, 75th centile) | 10 (4, 30.4) | 10 (4, 28) | 10 (4, 30.4) |
| Minimum, maximum | 1, 4018 | 0, 609 | 0, 4018 |
| Approximate total number of sexual partners over the past 12 months (including current) | |||
| N | 259 | 258 | 517 |
| 0, n (%) | 5 (2) | 2 (1) | 7 (1) |
| 1, n (%) | 102 (39) | 106 (41) | 208 (40) |
| 2, n (%) | 67 (26) | 78 (30) | 145 (28) |
| 3–5, n (%) | 62 (24) | 46 (18) | 108 (21) |
| 6–10, n (%) | 18 (7) | 21 (8) | 39 (8) |
| > 10, n (%) | 5 (2) | 5 (2) | 10 (2) |
| Missing, n (%) | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Median (25th, 75th centile) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) |
| Minimum, maximum | 0, 20 | 0, 30 | 0, 30 |
| Gender of sexual partners in lifetime, n (%) | |||
| Male | 233 (90) | 229 (88) | 462 (89) |
| Female | 6 (2) | 5 (2) | 11 (2) |
| Both male and female | 20 (8) | 24 (9) | 44 (8) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Female sexual partner in past 12 months, n (%) | |||
| Yes | 26 (10) | 25 (10) | 51 (10) |
| No | 233 (90) | 234 (90) | 467 (90) |
| Types of sexual contact in past 12 months, n (%)a | |||
| Vaginal sex | |||
| Yes | 251 (97) | 253 (98) | 504 (97) |
| No | 2 (1) | 3 (1) | 5 (1) |
| Missing | 6 (2) | 3 (1) | 9 (2) |
| Giving oral sex | |||
| Yes | 206 (80) | 217 (84) | 423 (82) |
| No | 47 (18) | 39 (15) | 86 (17) |
| Missing | 6 (2) | 3 (1) | 9 (2) |
| Receiving oral sex | |||
| Yes | 190 (73) | 204 (79) | 394 (76) |
| No | 63 (24) | 52 (20) | 115 (22) |
| Missing | 6 (2) | 3 (1) | 9 (2) |
| Receiving anal sex | |||
| Yes | 51 (20) | 45 (17) | 96 (19) |
| No | 202 (78) | 211 (81) | 413 (80) |
| Missing | 6 (2) | 3 (1) | 9 (2) |
| Use of condoms | |||
| Yes always, including oral sex | 9 (3) | 3 (1) | 12 (2) |
| Yes always, except oral sex | 28 (11) | 35 (14) | 63 (12) |
| Yes sometimes | 109 (42) | 102 (39) | 211 (41) |
| No | 113 (44) | 118 (46) | 231 (45) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Completeness of patient questionnaire data and interval to receipt of data | Treatment arm | Total (N = 518) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Week 2 questionnaire, n (%) | |||
| Total withdrawn before questionnaire expected | 1 | 2 | 3 |
| Questionnaire expected | 258 | 257 | 515 |
| Questionnaire returneda | 157 (61) | 161 (63) | 318 (62) |
| Questionnaire not returnedb | 101 (39) | 96 (37) | 197 (38) |
| Primary outcome data collected by telephone | 46 | 42 | 88 |
| Time from randomisation to questionnaire return (days) | |||
| n | 157 | 161 | 318 |
| Median (25th, 75th centile) | 15 (14, 19) | 15 (14, 19) | 15 (14, 19) |
| Minimum, maximum | 14, 35 | 14, 55 | 14, 55 |
| Time from randomisation to telephone data collection (days) | |||
| n | 46 | 42 | 88 |
| Median (25th, 75th centile) | 50.5 (33, 86) | 56 (37, 77) | 55.5 (34, 85) |
| Minimum, maximum | 29, 155 | 29, 153 | 29, 155 |
| 3-month questionnaire, n (%) | |||
| Total withdrawn before questionnaire expected | 3 | 3 | 6 |
| Questionnaire expected | 256 | 256 | 512 |
| Questionnaire returneda | 111 (43) | 108 (42) | 219 (43) |
| Questionnaire not returnedb | 145 (57) | 148 (58) | 293 (57) |
| Time from randomisation to questionnaire return (days) | |||
| n | 111 | 108 | 219 |
| Median (25th, 75th centile) | 92 (92, 97) | 93 (92, 97) | 93 (92, 97) |
| Minimum, maximum | 89, 109 | 89, 113 | 89, 113 |
| 6-month questionnaire, n (%) | |||
| Total withdrawn before questionnaire expected | 3 | 3 | 6 |
| Questionnaire expected | 256 | 256 | 512 |
| Questionnaire returneda | 92 (36) | 84 (33) | 176 (34) |
| Questionnaire not returnedb | 164 (64) | 172 (67) | 336 (66) |
| Recurrence data collected by telephonec | 15 | 14 | 29 |
| Time from randomisation to questionnaire return (days) | |||
| n | 92 | 84 | 176 |
| Median (25th, 75th centile) | 186 (183, 189) | 186 (182.5, 189) | 186 (183, 189) |
| Minimum, maximum | 181, 196 | 181, 208 | 181, 208 |
| Time from randomisation to obtaining telephone data (days) | |||
| n | 15 | 14 | 29 |
| Median (25th, 75th centile) | 233 (219, 244) | 231 (222, 239) | 231 (222, 239) |
| Minimum, maximum | 203, 265 | 213, 239 | 203, 265 |
| Frequency and completeness of telephone follow-up | Treatment arm, n (%) | Total (N = 518), n (%) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Week 2 | |||
| Primary outcomes to be obtained by telephone | 101 (39) | 96 (37) | 197 (38) |
| Participants attempted follow-up by telephonea | 103 (100) | 99 (100) | 202 (100) |
| Primary outcome obtained by telephone | 46 (45) | 42 (43) | 88 (44) |
| Primary outcome not obtained by telephone | 57 (55) | 57 (57) | 114 (56) |
| Contact made, but resolution status not known by participant | 1 (2) | 0 | 1 (1) |
| Contact made, but unwilling to provide outcome | 4 (7) | 9 (16) | 13 (11) |
| Contact not made (three attempts) | 52 (91) | 48 (84) | 100 (88) |
| 6 months | |||
| Secondary outcomes to be obtained by telephoneb | 58 | 61 | 119 |
| Participants attempted follow-up by telephoneb | 53 (91) | 52 (85) | 105 (88) |
| Presence of recurrence outcome obtained by telephone | 15 (28) | 14 (27) | 29 (28) |
| Number of episodes outcome obtained by telephone | 7 (13) | 7 (13) | 14 (13) |
| Both outcomes obtained by telephone | 13 (25) | 13 (25) | 26 (25) |
| Contact made, but recurrence outcome not known by participant | 2 | 0 | 2 |
| Contact made, but number of episodes not known by participant | 2 | 1 | 3 |
| Neither outcome obtained by telephone | |||
| Contact made, but unwilling to provide either outcome | 2 (4) | 4 (8) | 6 (6) |
| Contact not made (three attempts) | 34 (64) | 34 (65) | 68 (65) |
| Sample characteristics | Treatment arm, n (%) | Total (N = 518), n (%) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Number of samples expected | 258 | 257 | 515 |
| Number of week 2 samples received by the laboratory | 148 (57) | 153 (60) | 301 (58) |
| Number with primary outcome and sample results | 135 (52) | 145 (56) | 280 (54) |
| Primary outcome from the questionnaire | 121 | 132 | 253 |
| Primary outcome by telephone | 14 | 13 | 27 |
| Sample characteristics | Treatment arm, n (%) | Total (N = 518), n (%) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| Baseline samples and expiry dates | |||
| Sample kits in date | 166 (64) | 173 (67) | 339 (65) |
| Excluded out-of-date sample kits | 16 (6) | 16 (6) | 32 (6) |
| Sample kits with expiry status unknown | 71 (27) | 67 (26) | 138 (27) |
| No identifiable sample receiveda | 6 (2) | 3 (1) | 9 (2) |
| Week 2 samples and expiry dates | |||
| Sample kits in date | 110 (42) | 114 (44) | 224 (43) |
| Excluded out-of-date sample kits | 8 (3) | 11 (4) | 19 (4) |
| Sample kits with expiry status unknown | 30 (12) | 28 (11) | 58 (11) |
| Participant withdrawn before week 2 | 1 (< 0.5) | 2 (1) | 3 (1) |
| No identifiable sample receivedb | 110 (42) | 104 (40) | 214 (41) |
| Resolution of BV at week 2 | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Data from all sources | ||
| Yes | 143 (70) | 97 (47) |
| No | 61 (30) | 108 (53) |
| Missing | 55 | 54 |
| Questionnaire data onlya | ||
| Yes | 105 (66) | 75 (46) |
| No | 53 (34) | 88 (54) |
| Missing | 101 | 96 |
| Telephone data only | ||
| Yes | 38 (83) | 22 (52) |
| No | 8 (17) | 20 (48) |
| Missingb | 58 | 58 |
| Characteristics of those who resolved by week 2 | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 143) | Intravaginal lactic acid gel (N = 97) | |
| Age at randomisation (years) | ||
| n | 143 | 97 |
| Mean (SD) | 29.4 (8.43) | 30.5 (8.50) |
| Median (25th, 75th centile) | 27 (23, 35) | 29 (24, 35) |
| Minimum, maximum | 17, 55 | 19, 55 |
| Ethnicity, n (%) | ||
| White | 68 (48) | 43 (44) |
| Black Caribbean | 36 (25) | 28 (29) |
| Mixed race | 15 (10) | 9 (9) |
| Black African | 11 (8) | 7 (7) |
| Other | 6 (4) | 0 |
| Other Asian (non-Chinese) | 3 (2) | 2 (2) |
| Indian | 3 (2) | 2 (2) |
| Chinese | 0 | 3 (3) |
| Pakistani | 1 (1) | 1 (1) |
| Bangladeshi | 0 | 1 (1) |
| Black (other) | 0 | 1 (1) |
| Not given | 0 | 0 |
| Vaginal douching in the past 3 months, n (%) | ||
| Yes | 21 (15) | 6 (6) |
| No | 122 (85) | 91 (94) |
| Missing | 0 | 0 |
| Frequency of douching per month, n (%) | ||
| 0–2 | 6 (29) | 2 (33) |
| 3–4 | 5 (24) | 1 (17) |
| 5–6 | 0 | 0 |
| ≥ 7 | 10 (48) | 3 (50) |
| Current use of oral contraceptive pill, n (%) | ||
| Yes | 25 (17) | 18 (19) |
| No | 118 (83) | 214 (83) |
| Missing | 0 | 0 |
| Type of contraceptive pill, n (%) | ||
| Combined oral contraceptive pill | 15 (60) | 13 (72) |
| Progesterone-only pill | 10 (40) | 5 (28) |
| History of BV | ||
| Approximate age when BV first occurred (years) | ||
| n | 142 | 97 |
| Mean (SD) | 23.9 (7.18) | 24.3 (7.40) |
| Median (25th, 75th centile) | 22 (19, 28) | 23 (19, 28) |
| Minimum, maximum | 15, 53 | 11, 50 |
| Number of previous episodes of BV in the past 12 months, n (%) | ||
| 0 | 1 (1) | 2 (2) |
| 1–3 | 91 (64) | 61 (63) |
| > 3 | 51 (36) | 34 (35) |
| Approximate total length of time in past year with BV symptoms, n (%) | ||
| < 2 weeks | 31 (22) | 20 (21) |
| ≥ 2 weeks and < 3 months | 78 (55) | 54 (56) |
| ≥ 3 months | 34 (24) | 23 (24) |
| Missing | 0 | 0 |
| BV confirmed at baseline visit (local microscopy), n (%) | ||
| Yes | 125 (87) | 82 (85) |
| No | 10 (7) | 11 (11) |
| Not tested | 8 (6) | 4 (4) |
| Missing | 0 | 0 |
| Baseline sample Ison–Hay grade for BV (central laboratory), n (%) | ||
| 0 (no bacteria) | 1 (1) | 0 |
| 1 (normal flora) | 19 (13) | 25 (26) |
| 2 (intermediate BV) | 38 (27) | 21 (22) |
| 3 (confirmed BV) | 83 (58) | 49 (51) |
| U (Gram-positive cocci) | 0 | 0 |
| Missing | 2 (1) | 6 (2) |
| Thrush in the last 12 months (before baseline), n (%) | ||
| Yes | 68 (48) | 48 (49) |
| No | 75 (52) | 49 (51) |
| Has current sexual partner (baseline), n (%) | ||
| Yes | 109 (76) | 65 (67) |
| No | 34 (24) | 32 (33) |
| Female sexual partner (in 12 months before baseline), n (%) | ||
| Yes | 12 (8) | 11 (11) |
| No | 131 (92) | 86 (89) |
| Genital discharge at baseline, n (%) | ||
| Yes | 129 (90) | 84 (87) |
| No | 14 (10) | 13 (13) |
| Offensive vaginal smell at baseline, n (%) | ||
| Yes | 120 (84) | 82 (85) |
| No | 23 (16) | 15 (15) |
| Vaginal irritation at baseline, n (%) | ||
| Yes | 45 (31) | 41 (42) |
| No | 98 (69) | 56 (58) |
| Resolved by week 2 | Participants with time data (n) | Median time to recurrence (SE) (days)b | 95% CIb |
|---|---|---|---|
| No additional medication | |||
| Oral metronidazole (n = 90) | 56 | 177 (–) | 75 to – |
| Intravaginal lactic acid gel (n = 67) | 41 | 149 (–) | 71 to – |
| With additional medication | |||
| Oral metronidazole (n = 12) | 9 | 87 (20.9) | 12 to 175 |
| Intravaginal lactic acid gel (n = 7) | 4 | 124 (27.5) | 21 to – |
| All with medication data | |||
| Oral metronidazole (n = 102) | 65 | 119 (46.2) | 75 to – |
| Intravaginal lactic acid gel (n = 74) | 45 | 149 (–) | 72 to – |
| Number of treatment courses | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| For those resolving by week 2 | n = 143 | n = 97 |
| Number of BV treatment courses between week 2 and 3 months per participant | ||
| Number of 3-month questionnaires returned | 111 | 108 |
| Number of participants resolving by week 2, with 3-month additional treatment data | 76 | 52 |
| Median (25th, 75th centile) | 0 (0, 1.5) | 0 (0, 1) |
| Minimum, maximuma | 0, 9 | 0, 9 |
| Number of BV treatment courses between week 2 and 6 months per participant | ||
| Number of 6-month questionnaires returned | 92 | 84 |
| Number of participants resolving by week 2, with 3- and 6-month additional treatment data | 59 | 35 |
| Median (25th, 75th centile) | 1 (0, 3) | 1 (0, 2) |
| Minimum, maximuma | 0, 14 | 0, 14 |
| For those not resolving by week 2 | n = 61 | n = 108 |
| Number of BV treatment courses between week 2 and 3 months per participant | ||
| Number of 3-month questionnaires returned | 111 | 108 |
| Number of participants with 3-month additional treatment data | 33 | 53 |
| Median (25th, 75th centile) | 1 (0, 3) | 1 (0, 2) |
| Minimum, maximuma | 0, 7 | 0, 13 |
| Number of BV treatment courses between week 2 and 6 months per participant | ||
| Number of 6-month questionnaires returned | 92 | 84 |
| Number of participants with 3- and 6-month additional treatment data | 19 | 30 |
| Median (25th, 75th centile) | 1 (0, 5) | 2 (0, 4) |
| Minimum, maximuma | 0, 9 | 0, 19 |
| Number of treatment courses | Treatment arm | Adjusted incidence rate ratio (95% CI)a | |
|---|---|---|---|
| Oral metronidazole (n = 259) | Intravaginal lactic acid gel (n = 259) | ||
| Median number of courses between week 2 and 3 months | 0 | 0 | 0.66 (0.32 to 1.35) |
| Median number of courses between week 2 and 6 months | 1 | 1 | 1.03 (0.53 to 2.01) |
| Symptomatic resolution | Microbiological resolution, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 63 (43) | 25 (17) | 88 (61) |
| No | 24 (17) | 33 (23) | 57 (39) |
| Total | 87 (60) | 58 (40) | 145 |
| Treatment arm | Resolved by, n (%) | Median time (days) to resolution (SE); minimum, maximum | 95% CIa (days) | ||
|---|---|---|---|---|---|
| Week 2 | 3 months | 6 months | |||
| With or without additional treatment (including missing data) | |||||
| Oral metronidazole (N = 192) | 143 (70) | 156 | 158 | 14 (0.65); 2, 183 | 8 to 14 |
| Intravaginal lactic acid gel (N = 179) | 97 (47) | 117 | 124 | 14 (3.41); 1b, 186 | 14 to 47 |
| With or without additional treatment (excluding missing data) | |||||
| Oral metronidazole (N = 150) | 102 (66) | 113 | 115 | 7 (0.54); 2, 183 | 6 to 10 |
| Intravaginal lactic acid gel (N = 150) | 74 (47) | 92 | 99 | 28 (14.61); 1b, 186 | 10 to 53 |
| With additional treatment | |||||
| Oral metronidazole (N = 21) | 12 (55) | 14 | 14 | 17 (6.2); 3, 183 | 6 to – |
| Intravaginal lactic acid gel (N = 18) | 7 (35) | 10 | 11 | 28 (7.2); 2, 98 | 5 to – |
| Without additional treatmentc | |||||
| Oral metronidazole (N = 129) | 90 (68) | 99 | 101 | 7 (0.49); 2, 181 | 6 to 8 |
| Intravaginal lactic acid gel (N = 132) | 67 (49) | 82 | 90 | 17 (14.27); 1b, 186 | 10 to 53 |
| Nausea | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Nausea reported, n (%) | ||
| Yes | 50 (32) | 13 (8) |
| No | 103 (66) | 144 (89) |
| Missing | 3 (2) | 4 (2) |
| Severity (how participant was affected), n (%) | ||
| Able to eat normally | 31 (62) | 10 (77) |
| Ability to eat or drink fluids significantly decreased | 16 (32) | 3 (23) |
| Unable to eat or drink fluids | 2 (4) | 0 |
| Missing | 1 (2) | 0 |
| How long after starting study treatment did side effect start? n (%) | ||
| < 2 hours | 10 (20) | 2 (15) |
| 2 to < 6 hours | 6 (12) | 0 |
| 6 to < 24 hours | 8 (16) | 0 |
| 1–3 days | 16 (32) | 6 (46) |
| > 3 days | 8 (16) | 4 (31) |
| Missing | 2 (4) | 1 (8) |
| Approximate duration (hours) | ||
| n | 48 | 11 |
| Median (25th, 75th centile) | 48 (24, 108) | 7 (2, 72) |
| Minimum, maximum | 0, 192 | 1, 96 |
| Fully resolved at week 2, n (%) | ||
| Yes | 45 (90) | 10 (77) |
| Noa | 5 (10) | 2 (15) |
| Missing | 0 | 1 (8) |
| Vomiting | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Vomiting reported, n (%) | ||
| Yes | 9 (6) | 2 (1) |
| No | 141 (90) | 152 (94) |
| Missing | 6 (4) | 7 (4) |
| Severity, n (%) | ||
| 1 episode in 24 hours | 6 (67) | 1 (50) |
| 2–5 episodes in 24 hours | 1 (11) | 0 |
| ≥ 6 episodes in 24 hours or i.v. fluids needed | 0 | 0 |
| Missing | 2 (22) | 1 (50) |
| How long after starting study treatment did side effect start? n (%) | ||
| < 2 hours | 3 (33) | 0 |
| 2 to < 6 hours | 2 (22) | 0 |
| 6 to < 24 hours | 2 (22) | 0 |
| 1–3 days | 1 (11) | 1 (50) |
| > 3 days | 0 | 0 |
| Missing | 1 (11) | 1 (50) |
| Approximate duration (hours) | ||
| n | 8 | 1 |
| Median (25th, 75th centile) | 24.5 (4.5, 38) | 1 (1, 1) |
| Minimum, maximum | 0, 72 | 1, 1 |
| Fully resolved at week 2, n (%) | ||
| Yes | 6 (67) | 2 (100) |
| Noa | 2 (22) | 0 |
| Missing | 1 (11) | 0 |
| Taste change | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Taste change reported, n (%) | ||
| Yes | 28 (18) | 2 (1) |
| No | 127 (81) | 156 (97) |
| Missing | 1 (1) | 3 (2) |
| Severity, n (%) | ||
| Mild | 14 (50) | 2 (100) |
| Moderate | 13 (46) | 0 |
| Severe | 1 (4) | 0 |
| Missing | 0 | 0 |
| How long after starting study treatment did side effect start? n (%) | ||
| < 2 hours | 9 (32) | 0 |
| 2 to < 6 hours | 2 (7) | 0 |
| 6 to < 24 hours | 5 (18) | 0 |
| 1–3 days | 11 (39) | 0 |
| > 3 days | 0 | 1 (50) |
| Missing | 1 (4) | 1 (50) |
| Approximate duration (hours) | ||
| n | 28 | 2 |
| Median (25th, 75th centile) | 87 (36, 120) | 24 (0, 48) |
| Minimum, maximum | 0, 202 | 0, 48 |
| Fully resolved at week 2, n (%) | ||
| Yes | 26 (93) | 2 (100) |
| Noa | 1 (4) | 0 |
| Missing | 1 (4) | 0 |
| Vaginal irritation | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Vaginal irritation reported at week 2, n (%) | ||
| Yes | 44 (28) | 34 (21) |
| No | 110 (71) | 125 (78) |
| Missing | 2 (1) | 2 (1) |
| Severity, n (%) | ||
| Mild | 19 (43) | 13 (38) |
| Moderate | 19 (43) | 18 (53) |
| Severe | 4 (9) | 3 (9) |
| Missing | 2 (5) | 0 |
| How long after starting study treatment did side effect start? n (%) | ||
| < 2 hours | 7 (16) | 8 (24) |
| 2 to < 6 hours | 0 | 2 (6) |
| 6 to < 24 hours | 2 (5) | 1 (3) |
| 1–3 days | 19 (43) | 12 (35) |
| > 3 days | 13 (33) | 11 (32) |
| Missing | 3 (7) | 0 |
| Approximate duration (hours) | ||
| n | 40 | 34 |
| Median (25th, 75th centile) | 76.5 (48.5, 168) | 72 (48, 120) |
| Minimum, maximum | 24, 720 | 1, 336 |
| Fully resolved at week 2, n (%) | ||
| Yes | 22 (50) | 21 (62) |
| Noa | 21 (48) | 13 (38) |
| Missing | 1 (2) | 0 |
| Vaginal irritation for those with baseline irritation | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Total returning questionnaire who had baseline irritation | 52 | 53 |
| Vaginal irritation reported at week 2, n (%) | ||
| Yes | 25 (48) | 15 (28) |
| No | 25 (48) | 38 (72) |
| Missing | 2 (4) | 0 |
| Severity, n (%) | ||
| Mild | 11 (44) | 7 (47) |
| Moderate | 9 (36) | 7 (47) |
| Severe | 4 (16) | 1 (7) |
| Missing | 1 (4) | 0 |
| How long after starting study treatment did side effect start? n (%) | ||
| < 2 hours | 6 (24) | 4 (27) |
| 2 to < 6 hours | 0 | 2 (13) |
| 6 to < 24 hours | 1 (4) | 1 (7) |
| 1–3 days | 9 (36) | 5 (33) |
| > 3 days | 8 (32) | 3 (20) |
| Missing | 1 (4) | 0 |
| Approximate duration (hours) | ||
| n | 23 | 15 |
| Median (25th, 75th centile) | 96 (72, 168) | 72 (48, 168) |
| Minimum, maximum | 24, 720 | 1, 168 |
| Fully resolved at week 2, n (%) | ||
| Yes | 8 (32) | 9 (60) |
| Noa | 16 (64) | 6 (40) |
| Missing | 1 (4) | 0 |
| Vaginal irritation for those without baseline irritation | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| And without baseline irritation | 104 | 108 |
| Vaginal irritation reported at week 2, n (%) | ||
| Yes | 19 (18) | 19 (18) |
| No | 85 (82) | 87 (81) |
| Missing | 0 | 2 (2) |
| Severity, n (%) | ||
| Mild | 8 (42) | 6 (32) |
| Moderate | 10 (53) | 11 (58) |
| Severe | 0 | 2 (11) |
| Missing | 1 (5) | 0 |
| How long after starting study treatment did side effect start? n (%) | ||
| < 2 hours | 1 (5) | 4 (21) |
| 2 to < 6 hours | 0 | 0 |
| 6 to < 24 hours | 1 (5) | 0 |
| 1–3 days | 10 (53) | 7 (37) |
| > 3 days | 5 (26) | 8 (42) |
| Missing | 2 (11) | 0 |
| Approximate duration (hours) | ||
| n | 17 | 19 |
| Median (25th, 75th centile) | 72 (48, 72) | 72 (48, 120) |
| Minimum, maximum | 48, 240 | 1, 336 |
| Fully resolved at week 2, n (%) | ||
| Yes | 14 (74) | 12 (63) |
| Noa | 5 (26) | 7 (37) |
| Missing | 0 | 0 |
| Abdominal pain | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Abdominal pain reported, n (%) | ||
| Yes | 31 (20) | 27 (17) |
| No | 123 (79) | 132 (82) |
| Missing | 2 (1) | 2 (1) |
| Severity, n (%) | ||
| Mild | 16 (52) | 12 (44) |
| Moderate | 13 (42) | 11 (41) |
| Severe | 2 (6) | 3 (11) |
| Missing | 0 | 1 (4) |
| How long after starting study treatment did side effect start? n (%) | ||
| < 2 hours | 3 (10) | 6 (22) |
| 2 to < 6 hours | 7 (23) | 1 (4) |
| 6 to < 24 hours | 4 (13) | 2 (7) |
| 1–3 days | 13 (42) | 11 (41) |
| > 3 days | 4 (13) | 6 (22) |
| Missing | 0 | 1 (4) |
| Approximate duration (hours) | ||
| n | 31 | 24 |
| Median (25th, 75th centile) | 72 (24, 123) | 72 (15.5, 108) |
| Minimum, maximum | 1, 240 | 1, 240 |
| Fully resolved at week 2, n (%) | ||
| Yes | 24 (77) | 22 (81) |
| Noa | 7 (23) | 3 (11) |
| Missing | 0 | 2 (7) |
| Diarrhoea | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 258) | Intravaginal lactic acid gel (N = 258) | |
| Total number of questionnaires expected | 256 | 258 |
| Total returning questionnaire | 156 | 161 |
| Diarrhoea reported, n (%) | ||
| Yes | 31 (20) | 9 (6) |
| No | 123 (79) | 150 (93) |
| Missing | 2 (1) | 2 (1) |
| Severity, n (%) | ||
| Mild | 15 (48) | 4 (44) |
| Moderate | 11 (35) | 4 (44) |
| Severe | 4 (13) | 0 |
| Missing | 1 (3) | 1 (11) |
| How long after starting study treatment did the side effect start? n (%) | ||
| < 2 hours | 4 (13) | 1 (11) |
| 2 to < 6 hours | 1 (3) | 0 |
| 6 to < 24 hours | 9 (29) | 0 |
| 1–3 days | 13 (42) | 5 (56) |
| > 3 days | 3 (10) | 3 (33) |
| Missing | 1 (3) | 0 |
| Approximate duration (hours) | ||
| n | 30 | 8 |
| Median (25th, 75th centile) | 72 (48, 120) | 36 (24, 48) |
| Minimum, maximum | 1, 240 | 2, 72 |
| Fully resolved at week 2, n (%) | ||
| Yes | 28 (90) | 8 (89) |
| Noa | 3 (10) | 1 (11) |
| Missing | 0 | 0 |
| Reason | Treatment arm | |
|---|---|---|
| Oral metronidazole (n = 258) | Intravaginal lactic acid gel (n = 258) | |
| Period started during treatment | 0 | 5 |
| Misplaced treatment | 1 | 0 |
| Started treatment late owing to social engagements | 1 | 0 |
| Vaginal itching and bleeding | 0 | 1 |
| Lower abdominal pain | 0 | 1 |
| Misunderstood how to take treatment | 1 | 0 |
| Was not prescribed study treatment | 1 | 0 |
| Unknown | 1 | 0 |
| Total | 5 | 7 |
| Brand used | Intravaginal lactic acid gel (n = 259), n (%) |
|---|---|
| Number of participants returning week 2 questionnaire | 161 |
| Balance Activ® (BBI Healthcare, Crumlin, UK) | 67 (42) |
| Relactagel® (Kora Healthcare, Swords, Ireland) | 90 (56) |
| Canesbalance® (Bayer, Reading, UK) | 0 |
| Brand unknowna | 1 (1) |
| Missing | 3 (2) |
| Gonorrhoea | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Baseline samples received by the central laboratory | 253 | 256 |
| Week 2 samples received by the central laboratory | 148 | 153 |
| Baseline (excludes out-of-date sample kits) | ||
| n | 237 | 240 |
| Positive | 2 (1) | 1 (< 0.5) |
| Negative | 233 (98) | 237 (99) |
| Indeterminate | 1 (< 0.5) | 1 (< 0.5) |
| Equivocal | 0 | 0 |
| Missing | 1 (< 0.5) | 1 (< 0.5) |
| Week 2 (excludes out-of-date sample kits) | ||
| n | 140 | 142 |
| Positive | 1 (1) | 0 |
| Negative | 134 (96) | 139 (98) |
| Indeterminate | 2 (1) | 1 (1) |
| Equivocal | 0 | 0 |
| Missing | 3 (2) | 2 (1) |
| Baseline (excludes out-of-date sample kits and those with an unknown expiry date) | ||
| n | 166 | 173 |
| Positive | 0 | 0 |
| Negative | 165 (99) | 172 (99) |
| Indeterminate | 0 | 1 (1) |
| Equivocal | 0 | 0 |
| Missing | 1 (1) | 0 |
| Week 2 (excludes out-of-date sample kits and those with an unknown expiry date) | ||
| n | 110 | 114 |
| Positive | 0 | 0 |
| Negative | 109 (99) | 111 (97) |
| Indeterminate | 1 (1) | 1 (1) |
| Equivocal | 0 | 0 |
| Missing | 0 | 2 (2) |
| Chlamydia | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Baseline samples received by the central laboratory | 253 | 256 |
| Week 2 samples received by the central laboratory | 148 | 153 |
| Baseline (excludes out-of-date sample kits) | ||
| n | 237 | 240 |
| Positive | 15 (6) | 5 (2) |
| Negative | 220 (93) | 233 (97) |
| Indeterminate | 1 (< 0.5) | 1 (< 0.5) |
| Equivocal | 0 | 0 |
| Missing | 1 (< 0.5) | 1 (< 0.5) |
| Week 2 (excludes out-of-date sample kits) | ||
| n | 140 | 142 |
| Positive | 6 (4) | 2 (1) |
| Negative | 128 (91) | 137 (96) |
| Indeterminate | 2 (1) | 1 (1) |
| Equivocal | 1 (1) | 0 |
| Missing | 3 (2) | 2 (1) |
| Baseline (excludes out-of-date sample kits and those with an unknown expiry status) | ||
| n | 166 | 173 |
| Positive | 8 (5) | 2 (1) |
| Negative | 157 (95) | 170 (98) |
| Indeterminate | 0 | 1 (1) |
| Equivocal | 0 | 0 |
| Missing | 1 (1) | 0 |
| Week 2 (excludes out-of-date sample kits and those with an unknown expiry status) | ||
| n | 110 | 114 |
| Positive | 4 (4) | 1 (1) |
| Negative | 104 (95) | 110 (96) |
| Indeterminate | 1 (1) | 1 (1) |
| Equivocal | 1 (1) | 0 |
| Missing | 0 | 2 (2) |
| Trichomoniasis | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Baseline samples received by the central laboratory | 253 | 256 |
| Week 2 samples received by the central laboratory | 148 | 153 |
| Baseline (excludes out-of-date sample kits) | ||
| n | 237 | 240 |
| Positive | 4 (2) | 2 (1) |
| Negative | 230 (97) | 236 (98) |
| Indeterminate | 2 (1) | 1 (< 0.5) |
| Equivocal | 0 | 0 |
| Missing | 1 (< 0.5) | 1 (< 0.5) |
| Week 2 (excludes out-of-date sample kits) | ||
| n | 140 | 142 |
| Positive | 1 (1) | 1 (1) |
| Negative | 134 (96) | 139 (98) |
| Indeterminate | 2 (1) | 1 (1) |
| Equivocal | 0 | 0 |
| Missing | 3 (2) | 1 (1) |
| Baseline (excludes out-of-date sample kits and those with an unknown expiry status) | ||
| n | 166 | 173 |
| Positive | 4 (2) | 1 (1) |
| Negative | 160 (96) | 171 (99) |
| Indeterminate | 1 (1) | 1 (1) |
| Equivocal | 0 | 0 |
| Missing | 1 (1) | 0 |
| Week 2 (excludes out-of-date sample kits and those with an unknown expiry status) | ||
| n | 110 | 114 |
| Positive | 1 (1) | 0 |
| Negative | 108 (98) | 112 (98) |
| Indeterminate | 1 (1) | 1 (1) |
| Equivocal | 0 | 0 |
| Missing | 0 | 1 (1) |
| Time with symptoms of recurrence for those who resolved within 2 weeks | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| For those who recurred within 3 months, time with symptoms between week 2 and 3 months | ||
| Number who resolved by week 2 | 143 | 97 |
| Number who recurred by 3 months | 37 | 23 |
| Time with symptoms | ||
| < 1 week | 8 (22) | 5 (22) |
| 1–2 weeks | 11 (30) | 4 (17) |
| > 2 to 4 weeks | 9 (24) | 6 (26) |
| > 4 weeks | 6 (16) | 6 (26) |
| Missing | 3 (8) | 2 (9) |
| For those who recurred within 6 months, time with symptoms between 3 and 6 months | ||
| Number who resolved by week 2 | 143 | 97 |
| Number who recurred by 6 months | 51 | 32 |
| Time with symptoms | ||
| < 1 week | 6 (12) | 1 (3) |
| 1–2 weeks | 2 (4) | 4 (13) |
| > 2 to 4 weeks | 10 (20) | 5 (16) |
| > 4 weeks | 5 (10) | 6 (19) |
| Missing | 28 (55) | 16 (50) |
| Symptom status | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Number not resolving by week 2 | 60 | 106 |
| Status of symptoms for those not resolving | ||
| Better, but not cleared/disappeared | 29 (48) | 39 (36) |
| Improved initially, but worsened again | 10 (16) | 25 (23) |
| No change | 9 (15) | 13 (12) |
| Worse | 3 (5) | 8 (7) |
| Missing | 10 (16) | 23 (21) |
| Symptom status | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole, (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Number of participants returning the week 2 questionnaire | 157 | 161 |
| Genital discharge | ||
| Yes | 52 (33) | 66 (41) |
| No | 99 (63) | 93 (58) |
| Missing | 6 (4) | 2 (1) |
| Offensive vaginal smell | ||
| Yes | 42 (27) | 65 (40) |
| No | 111 (71) | 94 (58) |
| Missing | 4 (3) | 2 (1) |
| Vaginal irritation | ||
| Yes | 47 (30) | 37 (23) |
| No | 104 (66) | 122 (76) |
| Missing | 6 (4) | 2 (1) |
| Were recurrence symptoms typical of usual symptoms? | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| > 2 weeks to 3 months | ||
| Number with recurrence between 2 weeks and 3 months | 37 | 23 |
| Always | 28 (76) | 15 (65) |
| Sometimes | 6 (16) | 6 (26) |
| Seldom | 0 | 0 |
| Missing | 3 (8) | 2 (9) |
| > 3 to 6 months | ||
| Number with recurrence between 3 and 6 months | 51 | 32 |
| Always | 22 (43) | 13 (41) |
| Sometimes | 2 (4) | 2 (6) |
| Seldom | 0 | 1 (3) |
| Missing | 27 (53) | 16 (50) |
| Additional medication for BV | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| During the first 2 weeks | ||
| Number of participants returning the week 2 questionnaire | 157 | 161 |
| Number of participants taking at least one additional medication for BV | 22 (14) | 20 (12) |
| Number of participants taking the following medications for BV | ||
| Metronidazole tablets | 10 (6) | 15 (9) |
| Metronidazole vaginal gel | 4 (3) | 2 (1) |
| Lactic acid vaginal gel | 8 (5) | 11 (7) |
| Clindamycin cream | 5 (3) | 4 (2) |
| Other treatmentsa | 8 (5) | 3 (2) |
| From week 2 to 3 months | ||
| Number of participants returning the 3-month questionnaire | 111 | 108 |
| Number of participants taking at least one additional medication for BV | 52 (47) | 51 (47) |
| Number of participants taking the following medications for BV | ||
| Metronidazole tablets | 27 (24) | 21 (19) |
| Metronidazole vaginal gel | 5 (5) | 10 (9) |
| Lactic acid vaginal gel | 23 (21) | 25 (23) |
| Clindamycin cream | 8 (7) | 2 (2) |
| Other treatmentsa | 10 (9) | 4 (4) |
| From 3 to 6 months | ||
| Number of participants returning the 6-month questionnaire | 92 | 84 |
| Number of participants taking at least one additional medication for BV | 44 (48) | 39 (46) |
| Number of participants taking the following medications for BV | ||
| Metronidazole tablets | 18 (20) | 15 (18) |
| Metronidazole vaginal gel | 4 (4) | 6 (7) |
| Lactic acid vaginal gel | 27 (29) | 21 (25) |
| Clindamycin cream | 4 (4) | 2 (2) |
| Other treatmentsa | 8 (9) | 6 (7) |
| During the first 2 weeks | From week 2 to 3 months | From 3 to 6 months |
|---|---|---|
| Oral metronidazole treatment arm | ||
|
|
|
| Intravaginal lactic acid gel treatment arm | ||
|
|
|
| Antibiotics for other conditions/illness | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| During the first 2 weeks | ||
| Number of participants returning the week 2 questionnaire | 157 | 161 |
| Number of participants taking at least one antibiotic (other than for BV) | 15 (10) | 21 (13) |
| Number of participants taking the following antibiotics (other than for BV) | ||
| Amoxicillin (Amoxicillin, Accord Healthcare) | 5 (3) | 11 (7) |
| Flucloxacillin (Flucloxacillin, Kent Pharma) | 4 (3) | 3 (2) |
| Doxycycline (Doxycycline, Kent Pharma) | 5 (3) | 3 (2) |
| Othera | 4 (3) | 5 (3) |
| From week 2 to 3 months | ||
| Number of participants returning the 3-month questionnaire | 111 | 108 |
| Number of participants taking at least one antibiotic (other than for BV) | 8 (7) | 12 (11) |
| Number of participants taking the following antibiotics (other than for BV) | ||
| Amoxicillin | 2 (2) | 4 (4) |
| Flucloxacillin | 1 (1) | 0 |
| Doxycycline | 3 (3) | 5 (5) |
| Othera | 6 (5) | 8 (7) |
| From 3 to 6 months | ||
| Number of participants returning the 6-month questionnaire | 92 | 84 |
| Number of participants taking at least one antibiotic (other than for BV) | 9 (10) | 10 (12) |
| Number of participants taking the following antibiotics (other than for BV) | ||
| Amoxicillin | 3 (3) | 1 (1) |
| Flucloxacillin | 2 (2) | 1 (1) |
| Doxycycline | 4 (4) | 2 (2) |
| Othera | 2 (2) | 10 (12) |
| During first 2 weeks | From week 2 to 3 months | From 3 to 6 months |
|---|---|---|
| Oral metronidazole treatment arm | ||
|
|
|
| Intravaginal lactic acid gel treatment arm | ||
|
|
|
| Vaginal thrush post randomisation | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Number of participants returning the questionnaire at week 2 | 157 | 161 |
| Developed vaginal thrush in the 2 weeks since study treatment started | ||
| Yes | 42 (27) | 27 (17) |
| No | 110 (70) | 132 (82) |
| Missing | 5 (3) | 2 (1) |
| Treatment taken in 2 weeks post start of study treatment | ||
| None | 25 (60) | 12 (44) |
| Clotrimazole | 13 (31) | 13 (48) |
| Fluconazole | 5 (12) | 1 (4) |
| Itraconazole | 1 (2) | 1 (4) |
| Other | 2 (5) | 2 (7) |
| Developed vaginal thrush 2 weeks to 3 months post randomisation | ||
| Number of participants returning questionnaire at 3 months | 111 | 108 |
| Yes | 20 (18) | 26 (24) |
| No | 87 (78) | 78 (72) |
| Missing | 4 (4) | 4 (4) |
| Number of episodes | ||
| 0 | 0 | 1 (4) |
| 1 | 10 (50) | 10 (38) |
| 2 | 7 (35) | 9 (35) |
| > 2 | 3 (15) | 4 (15) |
| Missing | 0 | 2 (8) |
| Developed vaginal thrush 3 to 6 months post randomisation | ||
| Number of participants returning questionnaire at 6 months | 92 | 84 |
| Yes | 23 (25) | 20 (24) |
| No | 69 (75) | 55 (65) |
| Missing | 0 | 9 (11) |
| Number of episodes | ||
| 0 | 0 | 0 |
| 1 | 15 (65) | 14 (70) |
| 2 | 7 (30) | 2 (10) |
| > 2 | 1 (4) | 4 (20) |
| Missing | 0 | 0 |
| Overall incidence of thrusha | ||
| Yes | 60 (23) | 58 (22) |
| No | 42 (16) | 30 (12) |
| Missing | 157 (61) | 171 (66) |
| Sexual contact | Treatment arm | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Number of participants returning the week 2 questionnaire | 157 | 161 |
| Sex in 2 weeks after start of treatment? n (%) | ||
| Yes | 71 (45) | 67 (42) |
| No | 81 (52) | 92 (57) |
| Missing | 5 (3) | 2 (1) |
| Time after starting treatment until having sex | ||
| n | 71 | 67 |
| Median (25th, 75th centile) | 6 (3, 9) | 7 (3, 9) |
| Minimum, maximum | 0, 17 | 0, 15 |
| Use of condoms, n (%) | ||
| Yes always, including oral sex | 8 (11) | 6 (9) |
| Yes always, except oral sex | 9 (13) | 8 (12) |
| Yes sometimes | 2 (3) | 3 (4) |
| No | 52 (73) | 48 (72) |
| Missing | 0 | 2 (3) |
| New sexual partners, n (%) | ||
| Yes | 12 (8) | 14 (9) |
| No | 72 (46) | 64 (40) |
| Missing | 73 (47) | 83 (52) |
| Sex from week 2 to 3 months n (%) | ||
| Number of participants returning 3-month questionnaire | 111 | 108 |
| Yes | 94 (85) | 89 (82) |
| No | 14 (13) | 16 (15) |
| Missing | 3 (3) | 3 (3) |
| Use of condoms, n (%) | ||
| Yes always, including oral sex | 7 (7) | 6 (7) |
| Yes always, except oral sex | 14 (15) | 6 (7) |
| Yes sometimes | 11 (12) | 7 (8) |
| No | 62 (66) | 68 (76) |
| Missing | 0 | 2 (2) |
| New sexual partners, n (%) | ||
| Yes | 23 (21) | 21 (19) |
| No | 75 (68) | 76 (70) |
| Missing | 13 (12) | 11 (10) |
| Sex from 3 months to 6 months, n (%) | ||
| Number of participants returning the 6-month questionnaire | 92 | 84 |
| Yes | 74 (80) | 67 (80) |
| No | 18 (20) | 9 (11) |
| Missing | 0 | 8 (10) |
| Use of condoms, n (%) | ||
| Yes always, including oral sex | 10 (14) | 2 (3) |
| Yes always, except oral sex | 9 (12) | 8 (12) |
| Yes sometimes | 13 (18) | 5 (7) |
| No | 42 (57) | 52 (78) |
| Missing | 0 | 0 |
| New sexual partners, n (%) | ||
| Yes | 22 (24) | 24 (29) |
| No | 53 (58) | 43 (51) |
| Missing | 17 (18) | 17 (20) |
| Vaginal douching post randomisation | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Randomisation to week 2 | ||
| Number of participants returning the week 2 questionnaire | 157 | 161 |
| Vaginal douching | ||
| Yes | 6 (4) | 6 (4) |
| No | 141 (90) | 143 (89) |
| Missing | 10 (6) | 12 (7) |
| > 2 weeks to 3 months | ||
| Number of participants returning the 3-month questionnaire | 111 | 108 |
| Vaginal douching | ||
| Yes | 6 (5) | 8 (7) |
| No | 68 (61) | 76 (70) |
| Missing | 37 (33) | 24 (22) |
| > 3 to 6 months | ||
| Number of participants returning the 6-month questionnaire | 92 | 84 |
| Vaginal douching | ||
| Yes | 6 (7) | 7 (8) |
| No | 86 (93) | 67 (80) |
| Missing | 0 | 10 (12) |
| STIs diagnosed | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | |
| Gonorrhoea | ||
| > 2 weeks to 3 months | ||
| Number of participants returning the questionnaire | 111 | 108 |
| Number of episodes | ||
| 0 | 95 (86) | 93 (86) |
| 1 | 2 (2) | 0 |
| 2 | 0 | 0 |
| > 2 | 0 | 0 |
| Missing | 14 (13) | 15 (14) |
| > 3 to 6 months | ||
| Number of participants returning the questionnaire | 92 | 84 |
| Number of episodes | ||
| 0 | 83 (90) | 64 (76) |
| 1 | 1 (1) | 1 (1) |
| 2 | 1 (1) | 0 |
| > 2 | 0 | 0 |
| Missing | 7 (8) | 19 (23) |
| Chlamydia | ||
| > 2 weeks to 3 months | ||
| Number of participants returning the questionnaire | 111 | 108 |
| Number of episodes | ||
| 0 | 96 (86) | 90 (83) |
| 1 | 0 | 4 (4) |
| 2 | 1 (1) | 0 |
| > 2 | 0 | 0 |
| Missing | 14 (13) | 14 (13) |
| > 3 to 6 months | ||
| Number of participants returning the questionnaire | 92 | 84 |
| Number of episodes | ||
| 0 | 82 (89) | 65 (77) |
| 1 | 1 (1) | 0 |
| 2 | 0 | 0 |
| > 2 | 0 | 0 |
| Missing | 9 (10) | 19 (23) |
| Trichomoniasis | ||
| > 2 weeks to 3 months | ||
| Number responding to questionnaire | 111 | 108 |
| Number of episodes | ||
| 0 | 94 (85) | 93 (86) |
| 1 | 2 (2) | 0 |
| 2 | 1 (1) | 0 |
| > 2 | 0 | 0 |
| Missing | 14 (13) | 15 (14) |
| > 3 to 6 months | ||
| Number responding to questionnaire | 92 | 84 |
| Number of episodes | ||
| 0 | 83 (90) | 65 (77) |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| > 2 | 0 | 0 |
| Missing | 9 (10) | 19 (23) |
| Pelvic inflammatory disease | ||
| > 2 weeks to 3 months | ||
| Number responding to questionnaire | 111 | 108 |
| Number of episodes | ||
| 0 | 94 (85) | 93 (86) |
| 1 | 1 (1) | 1 (1) |
| 2 | 0 | 0 |
| > 2 | 0 | 0 |
| Missing | 16 (14) | 14 (13) |
| > 3 to 6 months | ||
| Number responding to questionnaire | 92 | 84 |
| Number of episodes | ||
| 0 | 82 (89) | 62 (74) |
| 1 | 2 (2) | 4 (5) |
| 2 | 0 | 0 |
| > 2 | 0 | 0 |
| Missing | 8 (9) | 18 (21) |
| Reason | Treatment arm (n) | |
|---|---|---|
| Oral metronidazole (n = 259) | Intravaginal lactic acid gel (n = 259) | |
| Had period | 3 | 2 |
| Travelling abroad | 0 | 1 |
| Misunderstood instructions | 0 | 1 |
| Alternative diagnosis for symptoms made | 0 | 1 |
| Total | 3 | 5 |
| Symptoms post treatment | Treatment arm, n (%) | |
|---|---|---|
| Oral metronidazole (N = 91) | Intravaginal lactic acid gel (N = 88) | |
| Yes | 70 (77) | 74 (84) |
| No | 21 (23) | 14 (16) |
| Resource use | Unit cost (£) | Description | Source |
|---|---|---|---|
| Study treatments | |||
| Oral metronidazole | 3.97 | 400 mg twice daily for 7 days. Metronidazole 400 mg (AAH Pharmaceuticals, Coventry, UK) | BNF 201943 |
| Intravaginal lactic acid gel | 5.25 | 5 ml once daily for 7 days. Balance Activ BV | BNF 201943 |
| Health services | |||
| GP: face to face | 39.00 | Per-patient contact lasting 9.22 minutes, including direct care staff costs | PSSRU 201941 |
| GP: telephone | 15.52 | Telephone triage: GP led | PSSRU 201941 |
| Nurse: face to face | 21.72 | Duration of contact: 15.5 minutes; per-hour cost: £84 | PSSRU 2015 for duration multiplied by 2019 cost41 |
| Nurse: telephone | 7.80 | Telephone triage nurse led | NHS reference costs 2018/1942 |
| Pharmacy consultation: face to face | 29.30 | Community pharmacy | Pharmaceutical Services Negotiating Committee website97 |
| Pharmacy consultation: telephone/online | 14.00 | Community pharmacy | Pharmaceutical Services Negotiating Committee website97 |
| NHS 111 | 14.26 | Assumed to be equivalent to pharmacy consultation by telephone | |
| NHS walk in: face to face | 39.00 | Same as GP face to face | PSSRU 201941 |
| NHS walk in: telephone | 15.52 | Same as GP telephone | PSSRU 201941 |
| NHS outpatient: face to face | 135.00 | General | PSSRU 201941 |
| NHS outpatient: telephone | 37.60 | Average cost of e-consultation | PSSRU 201941 |
| A&E: face to face | 174.00 | Gynaecology: consultant led | NHS reference costs 2018/1998 |
| Specialised sexual health clinic: face to face | 122.00 | Non-consultant led, non-admitted, face-to-face attendance, first | NHS reference costs 2018/1998 |
| Specialised sexual health clinic: telephone | 7.80 | Nurse triage: GP led | PSSRU 201941 |
| NHS out of hours: face to face | 68.00 | A&E emergency medicine, category 1 investigation with category 1 or 2 treatment | NHS reference costs 2018/1998 |
| NHS out of hours: telephone | 37.60 | Average cost of e-consultation | PSSRU 201941 |
| Additional medication | |||
| Metronidazole vaginal gel (Zidoval, Mylan) | 4.31 | 5 g once daily for 5 days; 0.75% Zidoval (Mylan, Hatfield, UK) | BNF 201943 |
| Clindamycin phosphate cream (Dalacin, Pfizer) | 10.86 | 5 g once daily for 7 days; 2% Dalacin Cream (Pfizer Ltd, Sandwich, UK) | BNF 201943 |
| Clotrimazole pessary | 5.54 | 500-mg single dose | BNF 201943 |
| Fluconazole | 0.87 | 150-mg single dose | BNF 201943 |
| Itraconazole | 3.14 | 200 mg twice daily for 1 day | BNF 201943 |
| Questionnaire | Treatment arm, n (%) | All participants (n = 518) | |
|---|---|---|---|
| Oral metronidazole (N = 259) | Intravaginal lactic acid gel (N = 259) | ||
| NHS resource use | |||
| Week 2 | 143 (55) | 151 (58) | 294 (57) |
| 3 months | 106 (41) | 99 (38) | 205 (40) |
| 6 months | 91 (35) | 75 (29) | 166 (32) |
| All time points | 69 (27) | 54 (21) | 123 (24) |
| SF-12 | |||
| Baseline | 257 (99) | 254 (98) | 511 (≈ 99) |
| Week 2 | 141 (54) | 156 (60) | 297 (57) |
| 3 months | 99 (38) | 97 (37) | 196 (38) |
| 6 months | 89 (34) | 71 (27) | 160 (31) |
| All time points | 61 (23) | 48 (18) | 109 (21) |
| Characteristic | Participants who completed resource use questionnaire at | |||||
|---|---|---|---|---|---|---|
| Treatment arm | Total (N = 294) | Treatment arm | Total (N = 123) | |||
| Week 2 | All time points | |||||
| Oral metronidazole (N = 143) | Intravaginal lactic acid gel (N = 151) | Oral metronidazole (N = 69) | Intravaginal lactic acid gel (N = 54) | |||
| Age at randomisation (years) | ||||||
| n | 143 | 151 | 294 | 69 | 54 | 123 |
| Mean (SD) | 29.8 (8.1) | 29.9 (8.4) | 29.8 (8.3) | 31.2 (8.3) | 31.7 (8.6) | 31.4 (8.4) |
| Minimum, maximum | 18, 55 | 18, 55 | 18, 55 | 18, 55 | 19, 51 | 18, 55 |
| Ethnicity, n (%) | ||||||
| White | 70 (49) | 73 (48) | 143 (49) | 41 (59) | 27 (50) | 68 (55) |
| Black Caribbean | 32 (22) | 33 (22) | 65 (22) | 13 (19) | 14 (26) | 27 (22) |
| Mixed race | 13 (9) | 20 (13) | 33 (11) | 2 (3) | 7 (13) | 9 (7) |
| Black African | 11 (8) | 10 (7) | 21 (7) | 4 (6) | 4 (7) | 8 (7) |
| Other | 5 (3) | 2 (1) | 7 (2) | 2 (3) | 0 | 2 (2) |
| Other Asian (non-Chinese) | 4 (3) | 1 (1) | 5 (2) | 1 (1) | 1 (2) | 2 (2) |
| Indian | 3 (2) | 3 (2) | 6 (2) | 2 (3) | 1 (2) | 3 (2) |
| Black (other) | 1 (1) | 4 (3) | 5 (2) | 1 (1) | 0 | 1 (< 1) |
| Chinese | 1 (1) | 3 (2) | 4 (1) | 1 (1) | 0 | 1 (< 1) |
| Pakistani | 3 (2) | 1 (1) | 4 (1) | 2 (3) | 0 | 2 (2) |
| Bangladeshi | 0 | 1 (1) | 1 (< 0.5) | 0 | 0 | 0 |
| Not given | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of previous episodes of BV in the past 12 months, n (%) | ||||||
| 0 | 1 (1) | 2 (1) | 3 (1) | 0 | 0 | 0 |
| 1–3 | 80 (56) | 90 (60) | 170 (58) | 38 (55) | 37 (69) | 75 (61) |
| > 3 | 62 (43) | 59 (39) | 121 (41) | 31 (45) | 17 (31) | 48 (39) |
| Female sexual partners in past 12 months, n (%) | ||||||
| Yes | 15 (10) | 13 (9) | 28 (10) | 8 (12) | 4 (7) | 12 (10) |
| No | 128 (90) | 138 (91) | 266 (90) | 61 (88) | 50 (93) | 111 (90) |
Appendix 6 VITA qualitative interview schedule outline

20-minute interview
Interview schedule for patients
Introductions
Introduction of researcher
Reminder of study (interviewee will have received participant information sheet and signed consent form)
Confirmation that participant is happy to proceed
Explanation of what happens to the data:
-
Telephone interviews will be audio recorded. The audio recordings will be stored securely. For analysis the recording will be transcribed with anonymised.
Reminder that the interviewee can stop the interview at any time.
Opening questions
Tell me about your experiences while taking part in the VITA study?
Focusing on the treatment
Thank you. Tell me about the treatment you received.
Probes used as needed:
-
What were your initial expectations of the treatment?
-
Tell me how you used it.
-
What was good about the treatment?
-
What was not so good about the treatment?
-
Any difficulties with using it?
-
Did you have to change what you do day to day when using it?
How satisfied are you with the treatment you received overall?
Focusing on the modifications to make it more acceptable.
Is there anything that you would add to or change about the treatment you received?
Is there anything else that you would like to say about the treatment?
Would you recommend treatment to other patients with similar problems to your own?
Probes:
-
Explore reasons for their response.
Focusing on use of the intervention in the other arm of the trial and its acceptability compared to what the patient received
You received treatment X and some of our patients received treatment Y which involves (description of how the treatment is used). Still thinking about the treatment you received, what are your views on treatment Y?
Probes:
-
What advantages/disadvantages do you think treatment Y may have in comparison to what you received?
Close of interview
Thanks.
Any questions from interviewee.
Reminder of study contact details.
List of abbreviations
- AMR
- antimicrobial resistance
- BV
- bacterial vaginosis
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DMC
- Data Monitoring Committee
- GBP
- Great British pound
- GP
- general practitioner
- HIV
- human immunodeficiency virus
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMP
- investigational medicinal product
- ISRCTN
- International Standard Randomised Controlled Trial Number
- NAAT
- nucleic acid amplification test
- NCTU
- Nottingham Clinical Trials Unit
- PI
- principal investigator
- PID
- pelvic inflammatory disease
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- SmPC
- summary of product characteristics
- STI
- sexually transmitted infection
- TSC
- Trial Steering Committee
- VITA
- metronidazole Versus lactic acId for Treating bacterial vAginosis
