Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1211-20001. The contractual start date was in January 2014. The final report began editorial review in August 2019 and was accepted for publication in March 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Yardley et al. This work was produced by Yardley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Yardley et al.
Chapter 1 Aims, overview and context of the research programme
Summary of aims and rationale
The overall purpose of the DIPSS (Integrating Digital Interventions into Patient Self-Management Support) programme was to address the question of how digital interventions (DIs) can be used to provide cost-effective support for patient self-management of long-term conditions in primary care. To address this question, we chose to focus specifically on improving management and, consequently, outcomes for two common, contrasting long-term conditions (i.e. hypertension and asthma). We chose contrasting clinical conditions to allow comparison of patients from different age groups, with very different patterns of symptoms and different self-management regimes, as this would enable us to consider which findings were specific to one condition and which might be more common across different conditions or management regimes. The proposed project team brought together (1) researchers with leading international expertise in e-health, hypertension, asthma, behaviour change and health economics, and in developing, trialling and implementing complex health-care interventions; and (2) patient and public involvement (PPI) representatives, including people with experience of hypertension and asthma, and representatives of two relevant patient organisations [Blood Pressure UK (London, UK) and Asthma UK (London, UK)].
Our programme of research was intended to undertake the rigorous development and evaluation necessary to maximise the likelihood of effective integration of DIs within NHS primary care, while identifying and using best practice methods of designing and delivering DIs to ensure that they were considered accessible and useful by patients and clinicians. Our specific objectives were as follows:
-
To identify key features associated with maximising feasibility, acceptability (to patients and health professionals), clinical effectiveness and cost-effectiveness of DIs.
-
To examine the range of delivery and support modes that can be used for DIs and assess their relative feasibility, acceptability [to health-care professionals (HCPs) and patients], clinical effectiveness and cost-effectiveness.
-
To optimise interventions for hypertension and asthma and to carry out feasibility studies in preparation for full randomised controlled trials (RCTs).
-
To undertake a RCT of a DI for hypertension to determine the clinical effectiveness and cost-effectiveness of integrating it into routine care.
Summary of research
We proposed three closely linked parallel workstreams (Figure 1). A behavioural and economic workstream [workstream 1 (WS1)] focused on identifying condition-specific and common factors influencing cost-effective integration of DIs into primary care. This research was embedded in two clinical workstreams that developed and trialled DIs for self-management of hypertension [workstream 2 (WS2)] and asthma [workstream 3 (WS3)].
FIGURE 1.
Research pathway diagram for DIPSS.
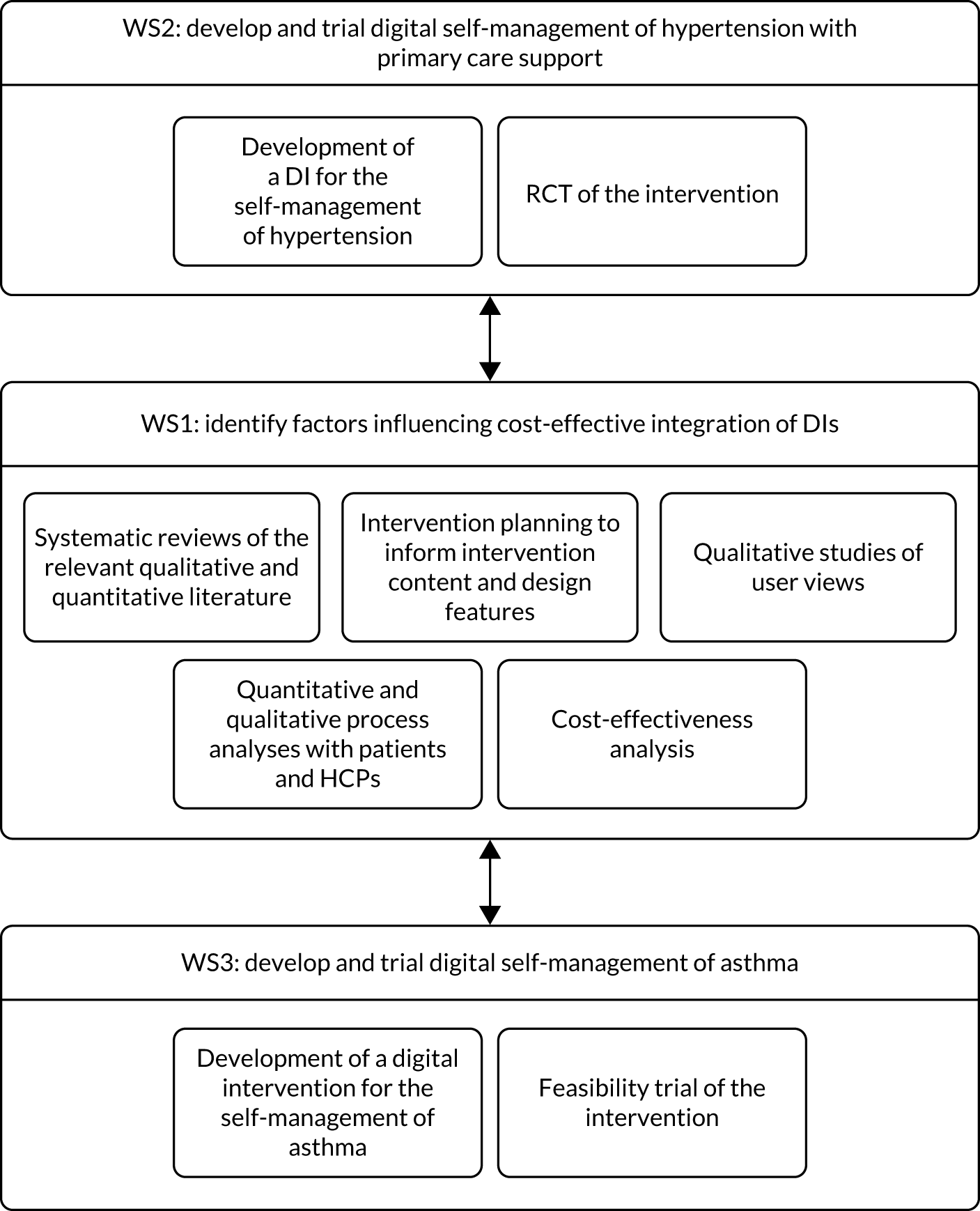
Workstream 1 undertook detailed intervention planning to identify factors influencing acceptable and cost-effective integration of DIs into primary care and, hence, the required elements and characteristics of the interventions and support to be offered for hypertension and asthma self-management in WS2 and WS3. To inform our planning, we completed systematic reviews of the relevant quantitative and qualitative literature and also drew on our primary qualitative studies of patient and HCP views and experiences.
Workstream 2 and WS3 developed DIs for self-management of hypertension and asthma (respectively), using iterative qualitative research to ensure that the DIs were viewed as acceptable and useful by patients and primary care staff. We proposed that both WS2 and WS3 would complete feasibility trials of the DIs, using quantitative and qualitative methods to evaluate patient and primary care experiences of different delivery formats. WS2 also carried out a full RCT of the cost-effectiveness for reducing blood pressure (BP) over 1 year of the optimum method(s) of delivering the DI compared with usual care, with an embedded qualitative process analysis.
There were two changes from the original proposal that affected the direction of the research programme. First, the feasibility trial for the hypertension intervention in WS2 was integrated into the main trial as an internal pilot study, as no changes to the intervention or trial procedures were required. Second, we originally proposed to test the following two intervention arms alongside a usual care arm: (1) the most intensive support (i.e. at least three face-to-face consultations and telephone and/or e-mail support, with the option of further support if required) for the DI considered likely to be feasible and cost-effective in routine care; and (2) the least intensive support (i.e. one face-to-face consultation at baseline, with further support up to the level of condition, provided only as required) for the DI considered likely to be effective in routine care. Instead, we trialled only one intervention arm (vs. a usual-care arm), which included a minimal level of nurse support (i.e. a compulsory face-to-face or telephone conversation), with the option of more face-to-face, telephone and e-mail nurse support if required. This was deemed the acceptable and efficient compromise between the two originally proposed.
Changes to the digital, clinical and research context since the research programme commenced
Changes to the digital context
When the DIPSS research proposal was written in 2013, online self-management of health was sufficiently novel that its functionality and its potential value to the NHS had to be explained in the proposal. Likewise, in our research proposal for this study, the feasibility of online self-management of health as a method of delivering interventions had to be justified to the funding panel as follows:
The problem of internet access is rapidly diminishing, even for older people and socially disadvantaged sectors of the population; in early 2011 77 per cent of households had internet access (with the proportion still growing fast), including 55 per cent of those aged 65–75.
During the lifetime of this project, the use and, therefore, potential of the internet has continued to increase in all age groups. In 2018, in the UK, 95% of adults aged 16–74 years and 47% of adults aged ≥ 75 years were recent internet users. 1 There has also been a huge proliferation in DIs, mainly provided by the private sector, with little or no evaluation of their effectiveness. Consequently, the question of whether or not DIs have a role to play supporting patient self-management has become less relevant. However, the question of how best to implement DIs and integrate them into primary care has become even more pressing. It is also important to think about how to provide this support most effectively and most cost-effectively, particularly in view of the ageing population, the inexorable rise in the prevalence of chronic and multimorbid conditions, higher expectations for medical care and the limited health-care staff resource available to manage those conditions.
During this period, there has also been a major shift from delivering and accessing DIs via computers to delivering and accessing them via mobile phones (although this shift has been more rapid among younger people than older people). When designing DIs delivered by computer, it was customary to assume that the user would devote some dedicated periods of time to accessing interactive ‘sessions’ of advice that were similar to what might be delivered by a health professional in a face-to-face meeting. As users became accustomed to accessing digital content on-the-go via smartphones, the assumptions about usage and design changed, as it became necessary to deliver advice in smaller chunks that could be accessed on a phone screen during shorter periods of time. This change in technology usage was reflected in the design of the DIPSS interventions. The HOME BP intervention was aimed at older people mainly using computers at home, whereas the My Breathing Matters intervention was developed later and had to be designed to be accessible by younger people using their phones.
Another major change in the digital context is that the digital environment is becoming better regulated. Increased data regulation relevant to all digital technology has now been introduced, such as the 2018 EU General Data Protection Regulation. 2 In addition, there is increased regulation internationally of applications considered to be medical devices, and criteria for evaluating digital health interventions are being developed by the Department of Health and Social Care, working with the National Institute for Health and Care Excellence (NICE) and other partners. 3
Changes to the clinical context
Hypertension
Blood pressure is a key risk factor for cardiovascular disease, which is the largest cause of morbidity and mortality worldwide. 4 Over 13% of NHS patients are currently recorded on hypertension registers (almost 7 million patients in England alone); however, the Health Survey for England5 found that around 40% of patients were inadequately controlled. A 10-mmHg reduction in BP is estimated to lead to a 41% reduction in stroke and a 22% reduction in coronary heart disease. 6 Every day, 670 people go to hospital because of a suspected stroke, that is, more than 100,000 strokes per year, and it is estimated that around 38,000 people will die from stroke in the UK each year. Overall, there are 1.2 million stroke survivors. This leads to NHS and social care costs of around £1.7B per year. Hypertension is the most important risk factor.
Factors responsible for suboptimal BP control include patient, physician and the health system factors. 7 The key patient factors are adherence to medication and other health behaviours. Clinical inertia is another key issue, whereby clinicians fail to intensify treatment, despite evidence of inadequate control. A Scottish study8 found that treatment was not intensified in nearly half (45%) of consultations in which patients had a single BP reading above target, and around one-third (36%) of consultations in which patients had two successive readings above target. There is evidence that self-monitoring BP is useful in improving medication adherence, reducing therapeutic inertia and controlling BP. 9–12 Finally, a recent Cochrane review concluded that ‘an organised system of registration, recall and regular review allied to a vigorous stepped care approach to antihypertensive drug treatment appears the most likely way to improve the control of high BP’. 13 Research by our team and others has shown that sustained reductions in BP can, indeed, be achieved by linking self-monitoring to pre-planned medication titration when hypertension is uncontrolled. 10,14–16 The latest NICE guidance17 recommends self-monitoring as a possible intervention for the management of hypertension, but stops short of an outright clear recommendation, perhaps because of concerns regarding the evidence base.
Asthma
The UK has one of the highest prevalence of asthma in the world. Nearly 6% of the UK population have asthma, comprising 5.4 million people, most of whom are managed in primary care. 18 Hospital admission and mortality rates for asthma showed improvements in the last decades of the last century, but these improvements have stalled since the millennium. Retrospective audits of asthma deaths have consistently suggested that poor self-management and other potentially preventable factors occur commonly in association with asthma fatalities. The largest such audit, which was carried out in the UK and was funded by the Department of Health and Social Care, found that potentially avoidable factors played a significant role in over 60% of the 195 asthma deaths audited, and that 77% lacked an agreed self-management plan and 50% lacked awareness of asthma triggers. 19
Although the UK leads the world in providing guidelines for asthma management, these guidelines have been poorly implemented and people with asthma do not receive evidence-based interventions, particularly individual action plans, which are known to impact positively on outcomes. 20 Patient education and proactive self-management have been convincingly shown to improve clinical outcomes in asthma and have been advocated in guidelines for 20 years. 19,21 People with asthma without a management plan are four times more likely to have an asthma attack that requires emergency care in hospital,22 and a national review of UK asthma deaths suggested that only one-quarter of people who died had been given a self-management plan. 23 Self-management in asthma can also encompass non-pharmacological interventions to improve control and empower the patient, such as breathing exercises or healthy behaviour changes, such as smoking cessation and weight reduction (as smoking and obesity are associated with worse prognosis in asthma). 24
Changes to the research context
In addition to the changes in the digital and clinical context of the research, during the period that this project was carried out there has been considerable development in thinking and guidance relating to intervention development and evaluation, culminating in new recommendations and guidance, for example in relation to intervention development,25 process evaluation26 and mixed-methods implementation research. 27 This project has responded to these changes as far as possible (given that it commenced prior to them) and has also actively contributed to them, as described in this report.
Chapter 2 Development of HOME BP
Parts of this section are reported in more detail in McLean et al. ,28 Morton et al. ,29 Band et al. 30 and Bradbury et al. 31,32
Introduction
Some key elements of the HOME BP intervention were informed by a previous programme of work that developed and trialled non-DIs for managing high BP. 15,33 These elements included the frequency of self-monitoring, the algorithm for interpreting BP readings and recommending appropriate action, and the procedure for the HCP to plan three potential medication changes in advance for each patient. This workstream (i.e. WS2) sought to explore how to adapt these procedures to be implemented successfully via an online intervention. This adaptation process is not simply a matter of transferring written materials into a digital delivery format. It is well established that it is vital to ensure that patients and clinicians find the intervention easy to use and are motivated and confident to implement the procedures correctly with only digital support. 34–36 In addition, a secondary aim of WS2 was to examine whether or not digital support for healthy behaviour change could contribute to better self-management of hypertension. Therefore, WS1 also involved developing and adapting our existing digital healthy behaviour change resources for use by people with hypertension in WS2.
Intervention Development Team and patient and public involvement
The Intervention Development Team included clinicians with expertise in hypertension management, e-health and lifestyle change, health psychologists, web developers, representatives of the charity Blood Pressure UK and PPI contributors. Our PPI contributors, including three patients (Shelley Mason, Keith Manship, Cathy Rice) with hypertension and/or stroke and one public contributor (Samantha Richards Hall) with a general interest in DIs, joined the project team and provided essential advice on the grant proposal. All PPI contributors were subsequently invited to each Management Committee meeting to discuss important issues arising in the planning and development of the HOME BP intervention, including decisions about support for healthy behaviour change, insights into patient burden of self-monitoring, and discussions around approaches to participant recruitment and how to promote accessibility of the patient materials.
Early prototypes of the intervention were shared with the PPI contributors for feedback from a patient perspective and this led to important changes to optimise the intervention, such as the introduction of additional optional information for patients who might like to know more about clinical risks of hypertension. PPI contributors also provided an important patient perspective during debates among the research team, for example some researchers were concerned that the motivational quiz might be irritating or hard to relate to, but PPI contributors felt that the quiz was useful and engaging. PPI contributors promoted a focus on patient priorities throughout this phase of intervention planning and development, and provided the opportunity for rapid feedback on the early development of the intervention to maximise potential to meet patients’ needs.
Objectives
This section will describe the substudies used to inform the planning and development of the HOME BP DI, based on evidence, theory and qualitative research. For each substudy or discrete research activity, we report aims, methods, results and practical implications for how it informed the intervention development. The substudies are described in chronological order as follows:
-
Phase 1: collate and synthesise evidence, including primary mixed-methods research, evidence from quantitative review of the literature and evidence from qualitative review of the literature.
-
Phase 2: behavioural analysis, identifying facilitators and barriers, and how to address them.
-
Phase 3: intervention development and optimisation, alongside developing guiding principles.
-
Phase 4: mapping facilitators and barriers on to theory.
The section ends by considering how the INDEX (IdentifyiNg and assessing different approaches to DEveloping compleX interventions) actions for developing complex interventions were met in this planning and development process. 25 The INDEX actions were published in 2019 and comprised 18 recommended actions for intervention developers to consider, collated through a systematic synthesis of intervention development approaches.
Phase 1: collating evidence from primary mixed-methods research, and evidence from quantitative and qualitative reviews of the literature
Collating evidence from a previous primary mixed-methods research study (described in Band et al.30)
Aim
The aim was to collate feedback from a small feasibility study that explored patients’ and HCPs’ experiences of managing high BP using an online intervention prototype.
Methods
The feasibility study was completed before the start of this programme grant. The online prototype of the intervention was based closely on written materials used for BP self-management in the Telemonitoring and Self-Management in the Control of Hypertension (TASMINH2) trial. 15 Eight general practices participated, recruiting 50 patients with hypertension. Semistructured qualitative interviews were conducted with a subsample of 16 patients and 3 HCPs, and a debriefing focus group was held with 8 HCPs. To inform the development of the HOME BP intervention for the current programme grant, a rapid analysis was adopted, in which the transcripts from the feasibility study were read and barriers to implementation were extracted from the data and tabulated to help consider how best to overcome them.
Results and practical implications for intervention development
Table 1 provides a list of barriers to implementation at the practice level, HCP level or patient level, and optimisation solutions actioned in the HOME BP intervention to overcome these.
| Barrier to implementation | Optimisation solutions actioned in the HOME BP intervention |
|---|---|
| Practice or HCP level | |
| GPs forgetting the procedures from their training for initiating planned medication changes |
|
| GPs not checking the prompts to change patients’ medication |
|
| Reception staff booking appointments for patients when they contacted the practice with raised readings due to a lack of awareness about the automated procedures for medication change |
|
| Patient level | |
| Low motivation for healthy behaviour changes, as patients felt they were already living healthily |
|
| Some patients did not consider hypertension to be a serious health issue that needed active management |
|
A systematic review and meta-analysis of the quantitative evidence for digital interventions for hypertension (described in McLean et al.28)
Aim
The aim was to conduct a systematic review of quantitative evidence relating to interactive DIs for hypertension.
Methods
An exhaustive search was conducted using MEDLINE, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, ERIC (Education Resources Information Center), The Cochrane Library, DoPHER (Database of Promoting Health Effectiveness Reviews), TRoPHI (Trials Register of Promoting Health Interventions), Social Science Citation Index and Science Citation Index, identifying 5606 papers for abstract screening, after which 164 papers were reviewed in full. Two independent researchers screened the search results and extracted data relating to the eligibility criteria into a standard template for comparison. Eight papers were eligible for inclusion, and for each of these eight papers a detailed data extraction was performed using prespecified fields, including study details, intervention components, participant details and outcomes. A meta-analysis was conducted using a random-effects model to explore the difference in mean change in systolic and diastolic BP.
Results
Patients using interactive DIs for BP were found to have significantly lower systolic BP than those receiving usual care in four of the seven studies. Overall, there was a weighted mean difference of –3.74 mmHg systolic BP after using interactive DIs compared with usual care. No differences were found in systolic BP reductions between interventions with or without a theoretical basis, with or without additional HCP support (e.g. sending patients personalised recommendations based on their readings and monthly counselling calls) or with more or less intensive self-monitoring regimens.
Practical implications for intervention development
The meta-analysis provided evidence that DIs can reduce BP across a range of participants. The reduction in systolic BP found in this review would be of clinical significance at a population level, with a drop of 3 mmHg reducing the chance of stroke mortality by 8%. 37
However, it was noted that only a small number of studies were included and only one study lasted longer than 12 months, meaning that sustainability of the effect was uncertain. There was also insufficient evidence to aid understanding of how different components of the interventions might work to reduce BP.
In terms of HOME BP, the meta-analysis provided support for the concept of a DI and some reassurance that this could be effective with minimal HCP support; however, the meta-analysis could not offer more specific suggestions for how to promote effectiveness.
Identification of barriers and facilitators from the qualitative literature (described in Morton et al.29)
Aims
The aim was to undertake a systematic review of qualitative evidence to explore how patients and HCPs perceived self-management DIs across a range of long-term physical health conditions, including hypertension and asthma.
Methods
A combination of search terms were developed relating to e-health, qualitative research, intervention and chronic illness. Searches were conducted using CINAHL, EMBASE, PsycINFO, MEDLINE, Web of Science and The Cochrane Library. Inclusion criteria specified that the population were adults with a chronic health condition or HCPs, the main component of the intervention was delivered digitally and promoted self-management, and that the research adopted qualitative research methods.
Data were extracted for each paper on the study, intervention details, participants, target self-management behaviours, HCP involvement, methods and main findings. A meta-ethnography approach was used to synthesise the primary studies and to generate a higher conceptual level understanding. 38 The meta-ethnography approach involved comparing key concepts between each paper and every other paper to develop a line of argument, identifying similarities and differences between the studies. The meta-ethnography synthesised research from across a range of interventions (from complex behaviour change programmes to more simplistic tele-monitoring interventions) and conditions (including hypertension, chronic heart failure, diabetes, asthma, chronic obstructive pulmonary disease and back pain).
Results
The search identified 1256 papers for abstract screening, of which 120 went to full-text screening and 30 were eligible for inclusion. Three third-order constructs were developed to explain how patients and HCPs perceive DIs: (1) ‘perceived purpose of the DI: Who is responsible’, (2) ‘perceiving meaning in self-monitored data’ and (3) ‘patients carefully consider recommended medication changes’.
Perceived purpose of the digital intervention
It appeared that patients and HCPs focus on different purposes of the intervention, with patients valuing increased self-management skills and understanding of their condition, whereas HCPs value improved clinical control. A risk in some intervention studies was that patients relied on their HCP to continually check on their health data, creating an unfeasible level of HCP burden and, therefore, the feedback messages for patients needed to clearly define who was responsible for taking action in the case of out-of-range readings. Clear feedback also helps avoid uncertainty for the patient and HCP, which can otherwise be a negative outcome of self-monitoring.
Perceiving meaning in self-monitored data
The action of self-monitoring data appeared to be powerful for patients, and simple tele-monitoring interventions alone could change how the patient perceived their condition and their role as self-managers. However, it appeared that where self-monitored data were stable over time or appeared meaningless in relation to patients’ efforts to control their condition, then this could result in frustration.
Patients carefully consider recommended medication changes
Some interventions prompted medication changes in response to self-monitored data, and it appeared that concerns and belief in the necessity of the change may influence to what extent patients adhere to these changes, and that there are possible differences in these perceptions between health conditions.
Practical implications for intervention development
The meta-ethnography highlighted several practical implications for the development of the HOME BP intervention, including the importance of providing clear actions for the patient and HCP in response to home readings, ensuring patients are responsible for responding to out-of-range readings (rather than expecting the practitioner to constantly monitor their readings), building patients’ confidence to engage with planned medication changes and increasing positive outcome expectancies for the patient and HCP for the effects of changing medication at the target threshold.
Phase 2: behavioural analysis – identifying facilitators and barriers, and how to address them (described in Band et al.30)
Aim
The aim was to map the evidence identified from the primary mixed-methods research and quantitative and qualitative literature reviews regarding influences on patient and HCP target behaviours on to intervention elements that could address these influences.
Methods
Likely facilitators of and barriers to each target behaviour for patients and HCPs were extracted from the evidence and recorded in a behavioural analysis table. Expert and stakeholder input regarding facilitators and barriers were also recorded in the table. Intervention components were then identified to optimise the facilitators and to minimise the barriers, based on stakeholder expertise and knowledge of behaviour change theory and frameworks [particularly social cognitive theory,39 normalisation process theory (NPT)35 and the behaviour change wheel (BCW)34].
Results
Four target behaviours were identified for patients: (1) engaging with the online intervention, (2) self-monitoring BP, (3) adhering to medication changes and (4) healthy behaviour change. Three behaviours were identified for HCPs: (1) engaging with the online intervention, (2) changing medication when recommended and (3) providing behavioural support to patients. A range of facilitators and barriers were collated from the evidence for each behaviour, along with suggestions for how this could inform the intervention. For example, the evidence showed that challenges for patients in engaging with regular self-monitoring included forgetting and limited time/competing priorities, and possible solutions identified included sending automated e-mail prompts via the intervention as reminders and enabling a flexible monitoring routine that patients could choose to delay by 1 week when necessary.
Practical implications for intervention development
The behavioural analysis process helped to ensure that the intervention being developed was addressing the key concerns of patients and HCPs, as informed by the literature and expert knowledge of stakeholders in the research team. The collation of facilitators and barriers also helped inform complex decisions, such as the extent and format of HCP support during the intervention, by interpreting the available evidence through an applied lens with a focus on how to promote the behaviour.
Phase 3: intervention development and optimisation alongside developing guiding principles
The HOME BP DI included both patient and HCP components. The patients completed two online training sessions, which were designed to raise motivation and teach patients how to self-monitor their BP. After the first online session, patients attended a baseline medication review with their prescriber, in which a three-step medication plan was created. The patient then completed 1 week of practise BP readings to increase confidence, after which they were reminded by e-mail to self-monitor their BP for 7 days every month. Patients entered their readings online, and if the average reading was above target for 2 consecutive months then the prescriber received an e-mail alert recommending that the next medication change in the plan was made. A third optional online session became available after 9 weeks to increase motivation and self-efficacy to engage in healthy lifestyle changes for managing high BP. From this optional session, patients could choose to complete one-off online educational modules on reducing salt, eating a healthy diet or reducing alcohol, or could sign-up to a multisession DI to support physical activity or weight loss40 [with the latter only available to those with a body mass index (BMI) over 25 kg/m2]. Supporters (i.e. nurses or health-care assistants who had completed online training for this role) were asked to send monthly support e-mails to patients throughout the trial, and to provide optional face-to-face support as needed. See Appendix 1 for full details of the intervention.
Qualitative research: think-aloud interviews and retrospective interviews with patients (described in Bradbury et al.31)
Aim
The aim was to gain an in-depth understanding of hypertensive patients’ beliefs about target behaviours and their psychosocial contexts to identify possible barriers to engagement and how best to optimise the intervention.
Methods
Twelve participants each completed three separate think-aloud interviews to explore perceptions of the three online sessions of the HOME BP intervention. Refinements were made to the intervention iteratively, such that concerns raised by the first batch of participants were addressed before conducting further think-aloud interviews with a new batch of participants. Recruitment ceased when data saturation was reached and no further issues were arising with the intervention.
At this point, 11 participants were recruited to use the intervention in a real-world setting. After using the intervention independently, including completing all three online sessions and submitting 7 days of home readings to receive online feedback, participants took part in a retrospective semistructured telephone interview to identify further ways to optimise the intervention. In addition, seven participants who did not want to use a DI to manage their BP were purposively recruited to explore their concerns and to gain insight into potential barriers to uptake.
To use the qualitative data systematically and efficiently to inform intervention modification, we developed a rapid analysis approach. The rapid analysis involved tabulating all data from the transcripts relating to the intervention and systematically deciding which changes to make to optimise behaviour change, using a set of criteria for modifications. The criteria included how important each modification was for promoting behaviour change, how easy it was to implement and whether or not it was in line with theory and evidence.
Results
The think-aloud interviews showed that many patients liked the idea of self-monitoring their BP at home and felt motivated by the training sessions to become more involved in their care. However, some barriers were also discovered and the intervention was iteratively modified to address these, as described below.
To help patients understand the rationale for the intervention, the first online training session explained that HCPs often do not change patients’ medication despite clinic readings being raised, but the intervention would address this by encouraging HCPs to plan medication changes in advance and prompt change based on accurate home readings. However, some patients did not accept this rationale, as they had high trust in their general practitioner (GP) and believed they were already receiving the best care. These beliefs undermined the rationale for the intervention and, therefore, the training session was changed to be more compatible with patients’ high regard for GP care, emphasising, instead, how home readings would help the GP and make it easier for them to provide the best care. Another barrier was that some patients felt very anxious about the risk of negative health outcomes from raised BP, which were highlighted by a quiz in the first training session. This barrier was addressed by reassuring patients at the end of the quiz that these risks could be managed effectively by taking the right medication to control BP.
The retrospective interviews conducted with patients after using the intervention independently suggested that the intervention was feasible to implement in a real-life setting, and many patients described positive responses, such as reassurance when seeing readings were well controlled. Low confidence in the accuracy of readings could arise when patients felt uncertain about how to use the monitor and, therefore, a week of practise home readings was introduced, with the option to discuss monitoring technique with the nurse to increase self-efficacy. Another barrier was reluctance to fully fasten the cuff because of discomfort, which was addressed by adding the rationale for securely fastening the cuff to the training, explaining that this was necessary to obtain accurate readings. There was also evidence of possible reluctance to receive medication changes remotely, with some patients explaining that they would want to see their GP at this point. The intervention aimed to avoid increasing face-to-face consultations to maximise cost-effectiveness. Consequently, rather than prompting patients to have an appointment at this point, further reassurance was added to patients’ feedback by reminding them that they had agreed on this medication change at the start with their GP, and patients were given the option to send any concerns they had at the time of a medication change via an e-mail for their GP to consider.
Participants who did not want to use a DI to manage their BP discussed their concerns about the behavioural changes involved, including misconceptions that the intervention would change their medication without their GP’s involvement, and concerns about internet security for health data. These perceptions informed modifications to the patient recruitment materials, which ensured that these possible barriers were addressed using accessible, clear explanations to maximise uptake to the trial.
Practical implications for intervention development
This qualitative research was essential for ensuring that the HOME BP intervention was motivating, persuasive, feasible and enjoyable for people to use, and that concerns that could interfere with engagement with the target behaviours were addressed. Additional specific barriers were discovered through this research that had not been predicted by other elements of the planning process (including stakeholder involvement), demonstrating the value of conducting this development work. The intervention was optimised to ensure that the rationale was consistent with patients’ perceptions of their care, that fears about future health were mitigated by increased self-efficacy to control BP via medication, and that patients’ confidence to use the BP monitor and change medication without an appointment with their GP was maximised.
Qualitative research: focus groups with health-care professionals (described in Bradbury et al.32)
Aim
The aim was to explore HCPs’ beliefs and concerns about implementing the HOME BP intervention in practice to optimise the intervention.
Methods
Seven focus groups were conducted with 55 HCPs after they had completed the mandatory online training session relating to the intervention (i.e. GPs, nurses and health-care assistants) or after they had read summary information about implementing the trial (i.e. reception staff and practice managers). The rapid analysis approach that our team had developed was used to identify important changes to the intervention to promote feasibility and to optimise engagement, after which further data were collected from new participants. Recruitment ceased once no concerns were emerging during the focus groups. After the intervention had been optimised, thematic analysis was conducted to gain an in-depth understanding of HCPs’ perceptions of this DI. 41
Results
In the rapid analysis, important changes were made to the online intervention training to address HCPs’ concerns about implementing intervention procedures in practice. The changes included adding evidence that the intervention was unlikely to result in more consultations (owing to fears about increased workload) and reassuring HCPs in the training session about the accuracy of home readings by explaining that patients would complete a practice week of readings. In addition, there was some concern regarding how to plan three medication changes in advance for more complex patients, and this concern was addressed by adding scenarios to the training to demonstrate how to successfully implement this behaviour. From the nurses’ perspective, some nurses were anxious about not being able to give advice when using the Congratulate, Ask, Reassure, Encourage (CARE) approach to support patients. 42 Therefore, the training was adapted to incorporate further rationale for using this approach, including the addition of quotes from our previous research42 that showed that the approach had been well received by nurses and patients, to increase confidence in the value of this approach.
Three themes were developed in the thematic analysis: (1) managing BP at home, (2) agreeing medication changes in advance and (3) supporting patients with the HOME BP intervention. It appeared that some HCPs felt that self-monitoring BP and planning medication changes could help patients become more involved in their care and improve their own management of BP, although there were some concerns about patients becoming anxious about their readings and needing more support. Some HCPs were also unsure about the benefits of planning medication changes in advance in case the changes were no longer appropriate at the time.
Practical implications for intervention development
The focus groups suggested that the HOME BP intervention was acceptable and persuasive to HCPs. However, the focus groups highlighted some important modifications needed to optimise the intervention, including adding elements designed to increase confidence in planning medication changes in advance, demonstrating the accuracy of home readings and persuading HCPs that the CARE approach is effective for supporting patients.
Guiding principles (described in Band et al.30)
Aim
The aim was to develop guiding principles that identify how the intervention design will address specific challenges to engaging with the target behaviours in this particular context and population.
Methods
Guiding principles consist of two elements. First, intervention design objectives were based on the key context-specific behavioural needs, issues or challenges identified by the review of qualitative evidence, the mixed-methods primary research and the qualitative development interviews. In addition, we consulted the Intervention Development Team who had extensive stakeholder expertise in hypertension and developing DIs, as well as knowledge of the relevant evidence base. Second, the key features of the intervention consist of intervention characteristics that address these objectives. The guiding principles were progressively refined as intervention planning proceeded, in line with ongoing accumulation of relevant quantitative and qualitative evidence.
Results
Changing medication (‘titration’) was identified as a challenging behaviour for both patients and HCPs due to concerns about side effects and doubts about necessity to increase medication when readings are borderline. Therefore, motivating users to engage in medication change was a key objective for the intervention, and several features were included in the intervention to achieve this, such as educating patients and HCPs about the benefits of medication change and providing reassurance about safety and side effects. Furthermore, the process for medication change needed to be easy for HCPs and patients to implement in practice, and this became a design objective, which could be achieved by ensuring that the procedures were as automated and compatible as possible. Cost-effectiveness and feasibility were identified as a third design objective, as the intervention needed to be appropriate to implement in primary care, with features such as online training included to help achieve this objective. The full guiding principles have been published. 30
Practical implications for intervention development
The guiding principles provided a coherent and succinct summary of the key aims of the intervention and how these would be achieved to promote its acceptability and, ultimately, its effectiveness. The guiding principles were useful to refer back to during any decisions about the intervention and they helped ensure that the central priorities were kept in mind by the research team during the day-to-day running of the project.
Phase 4: mapping facilitators and barriers on to theory (described in Band et al.30)
Aim
The aim was to comprehensively describe the intervention in terms of existing theory and programme-level theory.
Methods
Once the intervention was complete, the intervention components identified in the behavioural analysis were mapped on to theory, represented as a large table. 30 The BCW and behaviour change techniques (BCTs) taxonomy provide a standardised system of well-defined theoretical concepts for describing complex interventions and identifying the techniques they use to change behaviour. 34,43 Therefore, each intervention component was mapped on to an intervention function from the BCW, and the relevant BCT was also identified to demonstrate how the intervention was theorised to be working. In addition, the intervention components were mapped on to NPT,35 which helped to describe the mechanisms likely to be involved in implementing the target behaviours for the patient and HCP. After mapping the intervention to theory, the BCW and NPT were checked for any additional theoretical constructs that had not emerged from the evidence, but that may be important for promoting behaviour change in this intervention.
Subsequently, a logic model was developed in line with the Medical Research Council guidance for process evaluation. 26 The target behaviours were theorised to influence the primary outcome of reducing BP, and the intervention components identified in the behavioural analysis were represented as intervention processes that would change the target behaviours. In addition to the evidence from the qualitative and quantitative reviews, further non-systematic scoping literature searches were conducted to enhance understanding of the causal mechanisms shown to influence the target behaviours. 44 Potential determinants of behaviour were extracted from papers and mapped on to existing theories of behaviour change.
Results
The behavioural analysis helped to clearly characterise the intervention. When mapped on to the BCW, the HOME BP intervention components were shown to target physical and social opportunity, reflective motivation and psychological capability, using the intervention functions of environmental restructuring, education, persuasion, training and enablement. The HOME BP intervention components also mapped on to 10 different BCTs, including prompts/cues, biofeedback and behavioural practice/rehearsal. Mapping to NPT showed that the intervention was targeting several mechanisms to promote successful implementation, such as training patients to use BP monitors to increase skillset workability, and providing patients with written confirmation of medication change from their HCP to promote initiation of a medication change (from the cognitive participation construct of NPT). In addition, each construct from the BCW and NPT was evaluated in terms of how it might contribute to the HOME BP intervention, but this did not identify any additional intervention content required to change behaviour.
In terms of the logic model, outcome expectancies appeared to be important in patients’ and HCPs’ willingness to change medication, as described by social cognitive theory. 39 More specifically, beliefs about hypertension and antihypertensive treatments seemed to inform these outcome expectancies, as described by the extended common sense model. 45 Both social cognitive theory and the extended common sense model were incorporated into the logic model. In addition, in line with social cognitive theory, self-efficacy was theorised to influence engagement with self-monitoring BP. Each intervention process in the logic model was defined using NPT mechanisms to show how it sought to promote implementation. See Band et al. 30 for the full logic model.
Practical implications for intervention development
The behavioural mapping was useful for ensuring that the intervention content could be described using standard terminology, and for checking that no theoretical concepts had been missed when planning the intervention from the evidence. The logic model also explicitly described the underlying mechanisms theorised to change behaviour.
Mapping the HOME BP planning and development process to the INDEX actions
New guidance for complex intervention development has recently emerged,25 based on a taxonomy of approaches to intervention development, interviews, Delphi consultation and workshops with developers and stakeholders. O’Cathain et al. 25 completed a comprehensive review of approaches and produced 18 actions that are recommended for consideration during intervention planning and development. For completeness, Table 2 shows a retrospective mapping of the HOME BP intervention planning and development process to the 18 actions from this guidance25 [see Appendix 1 for a full description of the HOME BP intervention using the Template for Intervention Description and Replication (TIDieR) checklist46].
| Action from INDEX guidance25 | How this action was addressed in the HOME BP intervention |
|---|---|
| Identify that there is a problem in need of a new intervention | The rationale for the HOME BP intervention was identified in the funding application, based on the following existing evidence (see Chapter 1):
|
| Establish a group or set of groups to guide the development process, thinking about engagement of relevant stakeholders, such as the public, patients, practitioners and policy-makers |
The Programme Management Group (which met 3-monthly to oversee all important decisions) was set up at the proposal stage and included hypertensive patients, behaviour change specialists, health economists, policy-makers, statisticians, trial managers and clinicians All members of the Programme Management Group were invited (if interested) to join the Intervention Development Team, which met monthly (or as necessary) to oversee and guide intervention development. This Intervention Development Team included patients, clinicians and health psychologists A core Intervention Development Team, comprising the health psychologists who were developing the intervention, met weekly and worked in close consultation with key clinical academics when necessary |
| Understand the problems or issues to be addressed |
Facilitators of and barriers to key behaviours were identified from (1) reviews of the existing quantitative and qualitative evidence, and (2) in-depth primary qualitative and mixed-methods research These evidence sources enabled us to understand the specific beliefs and contextual factors that appeared to influence target behaviours |
| Make a decision about the specific problem or problems that an intervention will address, and the aims or goals for the intervention. This may involve defining the behaviours to target |
A logic model was created to map the hypothesised mechanisms (including target behaviours) through which the intervention was theorised to change behaviour and outcomes Our behavioural analysis table30 documented the target behaviours for patients and health professionals, the barriers to and facilitators of implementing them, and intervention ingredients intended to support target behaviours Guiding principles were developed to specify how the intervention would meet design objectives to promote engagement with the target behaviours in this specific population and context |
| Identify possible ways of making changes to address the problem(s). This involves identifying what needs to change, how to bring about this change and what might need to change at individual, interpersonal, organisational, community or societal levels |
The primary and secondary research and analyses helped to identify what needed to change at the individual patient and HCP levels, as well as at a organisational level in the health-care systems, and provided insights into how this might best be achieved The development and management teams reviewed and agreed the design of the intervention, informed by the evidence reviews, the behavioural analysis table and the guiding principles, together with stakeholder expertise (clinical and experiential) and knowledge of existing relevant theory and theoretical frameworks (in particular social cognitive theory,39 NPT35 and the BCW)34 |
| Specify who will change, how and when. Selections may depend on consideration of the likely impact of the change, how easy it is to change, how influential it is for the problem being addressed and how easy it is to measure |
Decisions about the appropriate target group for behaviour change, core behaviours to target and intervention outcome measurement (e.g. required sample size, trial design and duration and the primary and secondary outcomes) were informed by the funding application, previous evidence relating to BP management (especially McManus et al. 15) and the wider review of evidence undertaken as part of the intervention planning There was good evidence15 that a face-to-face version of the intervention procedures for self-management of BP was acceptable and effective, and so steps were taken to ensure that the key procedures were preserved for the online delivery (e.g. the GP creating a three-step medication plan, patient self-monitoring at home, prompting the GP when medication change was required) The evidence was less strong that healthy behaviour change would be acceptable to patients and would have clinically useful effects on BP, and so this aspect of the intervention was encouraged, but was not made a core part of the intervention |
| Consider real-world issues about cost and delivery of any intervention at this early stage to reduce the risk of implementation failure at a later stage |
As the rationale for the intervention was to provide a more feasible and cost-effective method of controlling BP, a key focus was to design the intervention to be as pragmatic, efficient and easy to implement as possible. This included creating standardised, easily disseminated online training for patients and HCPs, minimising requirements for HCP input, using automated prompts to action and providing online templates for HCP communications with patients Regular management meetings were held among stakeholders, including patient contributors and clinicians, during which optimising the feasibility of the intervention in primary care was thoroughly discussed |
| Consider whether or not it is worthwhile continuing with the process of developing an intervention |
Early review of the evidence suggested that DIs were effective for controlling BP, suggesting that it was worthwhile continuing with the development process PPI, stakeholder and qualitative feedback on prototype versions of the intervention also provided encouraging evidence that the intervention was accessible and well liked by patients, as well as acceptable and feasible for HCPs |
| Generate ideas and solutions with regard to components and features of an intervention |
Qualitative research was undertaken with a range of patients and HCPs from the target population. The research included:All interviews and focus groups were transcribed verbatim Decisions about how to optimise the intervention based on feedback were made at weekly core development meetings, and straightforward changes to overcome users’ concerns about the intervention were made directly. Any decisions that were more complex or needed clinical input were raised with the wider Intervention Development Team at monthly meetings, with PPI contributors or with the full Programme Management Group Further user feedback was sought on the revised intervention from new participants |
| Re-visit decisions about where to intervene. This can involve consideration of the different levels at which to intervene and the wider system in which the intervention will operate | The in-depth qualitative development research enabled the Intervention Development Team to review decisions about how the intervention would work, as well as the key points for support. For example, feedback from some patients after using the intervention independently indicated that they did not feel confident using the BP monitor, which led to the addition of an optional support appointment with the nurse after a week of practise readings |
| Make decisions about the content, format and delivery of the intervention | As described above, decisions about the content, format and delivery of the intervention were informed by in-depth qualitative and mixed-methods research with the target user population, reviews of the evidence, behavioural analysis and input from the Intervention Development Team and wider Management Team |
| Design an implementation plan, thinking about who will adopt the intervention and maintain it |
The grant proposal for the intervention included an implementation plan should the intervention prove effective The implementation plan involved disseminating the findings through multiple pathways, including open-access peer-reviewed publications; presentations at conferences; workshops for patients, HCPs, and policy-makers to discuss the next steps; and speaking to NHS Clinical Commissioning Groups, NHS Choices and NHS Digital. The implementation plan also specified that the intervention software would facilitate adaptation of the DI materials for future roll-out in different contexts (e.g. adapting for certain patient subgroups or adding new components). It was planned that the intervention could be used by the NHS, as well as in the private sector, third sector and by other health researchers Blood Pressure UK were involved in the project from the outset, with their chief executive officer Katharine Jenner being invited to all management meetings. OMRON (Milton Keynes, UK) were also informed of this research project, and provided the patient BP monitors for the trial |
| Make prototypes or mock-ups of the intervention, where relevant | The intervention was developed using our in-house LifeGuide software, which enabled creation of a prototype intervention that could be easily modified throughout the development process, based on user feedback (especially from think-aloud interviews). This was an essential, iterative phase of intervention development, which helped to ensure that the intervention was accessible, appropriate, feasible, motivating, convincing and persuasive for users |
| Test on small samples for feasibility and acceptability and make changes to the intervention if possible | At early stages of development, feedback on the intervention was sought from the Intervention Development Team and Programme Management Group. Subsequently, detailed think-aloud interviews (n = 36), retrospective interviews with patients who had used the intervention independently (n = 11) and focus groups with HCPs (n = 7) informed decisions about changes to the intervention |
| Test on a more diverse population, moving away from the single setting where early development of the intervention took place and seeking a more diverse sample. This can involve asking questions, such as ‘is it working as intended?’, ‘is it achieving short term goals?’, ‘is it having serious adverse effects?’ | Owing to the extensive prior development work (including a previous feasibility study that informed intervention planning) and time constraints, this project included an internal pilot study, rather than a feasibility study, to enable any final minor but essential modifications to the intervention to be made. The pilot study was carried out in 15 practices that had not been involved in the intervention development work. Although outcomes could not be assessed, the feasibility of the intervention procedures was confirmed via usage data and process interviews with patients and HCPs |
| Optimise the intervention for efficiency prior to a full RCT | The intervention was optimised to promote feasibility based on the findings during the internal pilot trial. Decisions were made by the core intervention developers when changes were very minor, but more significant changes were discussed with the Intervention Development Team. Examples of optimisations included additional reminder e-mails about healthy behaviour changes and revising the content of GPs’ e-mails about medication change to further encourage the use of remote rather than face-to-face procedures for changing patients’ medication |
| Document the intervention, describing the intervention so others can use it and offer instructions on how to train practitioners delivering the intervention and on how to implement the intervention |
The intervention was described in detail using the TIDieR checklist (see Appendix 1)46 (Note that intervention content was made available in full as a demo, and the intervention has also been described in papers30–32 and shared via workshop dissemination) |
| Develop the objectives of the outcome and process evaluations. This includes determining how outcomes and mediators of change can be measured, developing measures, specifying evaluation design, planning recruitment and considering feasibility of a full RCT |
The process evaluation was planned in consultation with the Programme Management Group, and appropriate measures were selected to capture beliefs theorised to influence adherence to the target behaviours, informed by the logic model This involved:The data were planned to be analysed independently, and a mixed-methods approach adopted for triangulating the individual findings. This would facilitate an enhanced understanding of patients’ and HCPs’ experiences and perceptions of engaging with an online intervention for managing hypertension |
Completing Table 2 provided a useful prompt and a template for describing aspects of the intervention development process that are important, but are seldom currently described, such as details of the decision-making process and planning for efficient future implementation.
Chapter 3 Evaluation of the HOME BP intervention
Parts of this section are reported in more detail in McManus et al. 47 and Morton et al. 48,49 Additional findings have been written up as a paper reporting on the HCP process analysis. 49
Objectives
This section will describe the evaluation of the HOME BP intervention during a 12-month RCT. Aims, methods, results and implications are described for each discrete piece of research as follows:
-
A RCT to assess the clinical effectiveness and cost-effectiveness.
-
A process evaluation exploring how patients and HCPs experienced and implemented the intervention in practice, including:
-
a patient qualitative process study, examining the perceived benefits and burdens of using the intervention for patients
-
a patient quantitative process study, examining engagement and usage of the HOME BP intervention by patients
-
a HCP mixed-methods process study, exploring HCPs’ experiences of and adherence to using the intervention.
-
The section finishes with a conclusions section, which draws the findings together.
Randomised controlled trial to assess clinical effectiveness and cost-effectiveness
Aim
Our aim was to establish if a DI for guided self-management of uncontrolled BP in primary care is effective compared with usual care.
Methods
Patients (n = 622) from 76 general practices across Wessex and Thames Valley regions in southern England were randomised to the trial. To be eligible, patients had to be prescribed one, two or three antihypertensive medications and have a BP reading exceeding 140/90 mmHg at baseline. An online system [URL: www.lifeguideonline.org (accessed 28 July 2022)] randomised participants to the intervention (n = 305) or usual care (n = 317) in a 1 : 1 ratio. Minimisation took account of patients’ baseline systolic BP reading, age, whether or not they had diabetes and general practice. Randomisation was concealed from participants until after completion of the baseline questionnaires. HCPs were notified of participants’ randomisation group by e-mail. The intervention group completed online training to self-monitor BP, and had planned changes to medication initiated by the GP in response to raised home readings. The intervention group were prompted to self-monitor at home for 7 days, every 4 weeks. The intervention group also had the option to make a healthy behaviour change, with online support.
Patients in both groups had a baseline medication review with their GP, as their BP was above-target at baseline. The target thresholds for home readings in the intervention group were in line with UK national guidelines17 (i.e. 135/85 mmHg) and were adjusted for patients with diabetes and for patients aged > 80 years. The difference in systolic BP at 12 months was the primary outcome, adjusting for BP at baseline, BP target, patient age and general practice. Multiple imputation was used for missing values. Cost-effectiveness analysis took an NHS perspective, in which the costs comprised that of the intervention and use of NHS BP-related services. Two economic analyses are reported: (1) cost per unit of BP reduction in a within-trial analysis and (2) a long-term cost per quality-adjusted life-year (QALY) gained.
During the trial, the target sample size was increased from 574 to 610 patients, as initial withdrawal rates suggested that it would be prudent to allow for a 20% drop out rather than 10%, although this later proved not to be necessary.
Results
Figure 2 shows the flow of participants through the trial.
FIGURE 2.
Flow of participants through HOME BP trial. a, Partial withdrawals withdrew from the intervention but consented to be followed up; and b, partial withdrawals in usual care consented to follow-up. Reproduced with permission from McManus et al. 47 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
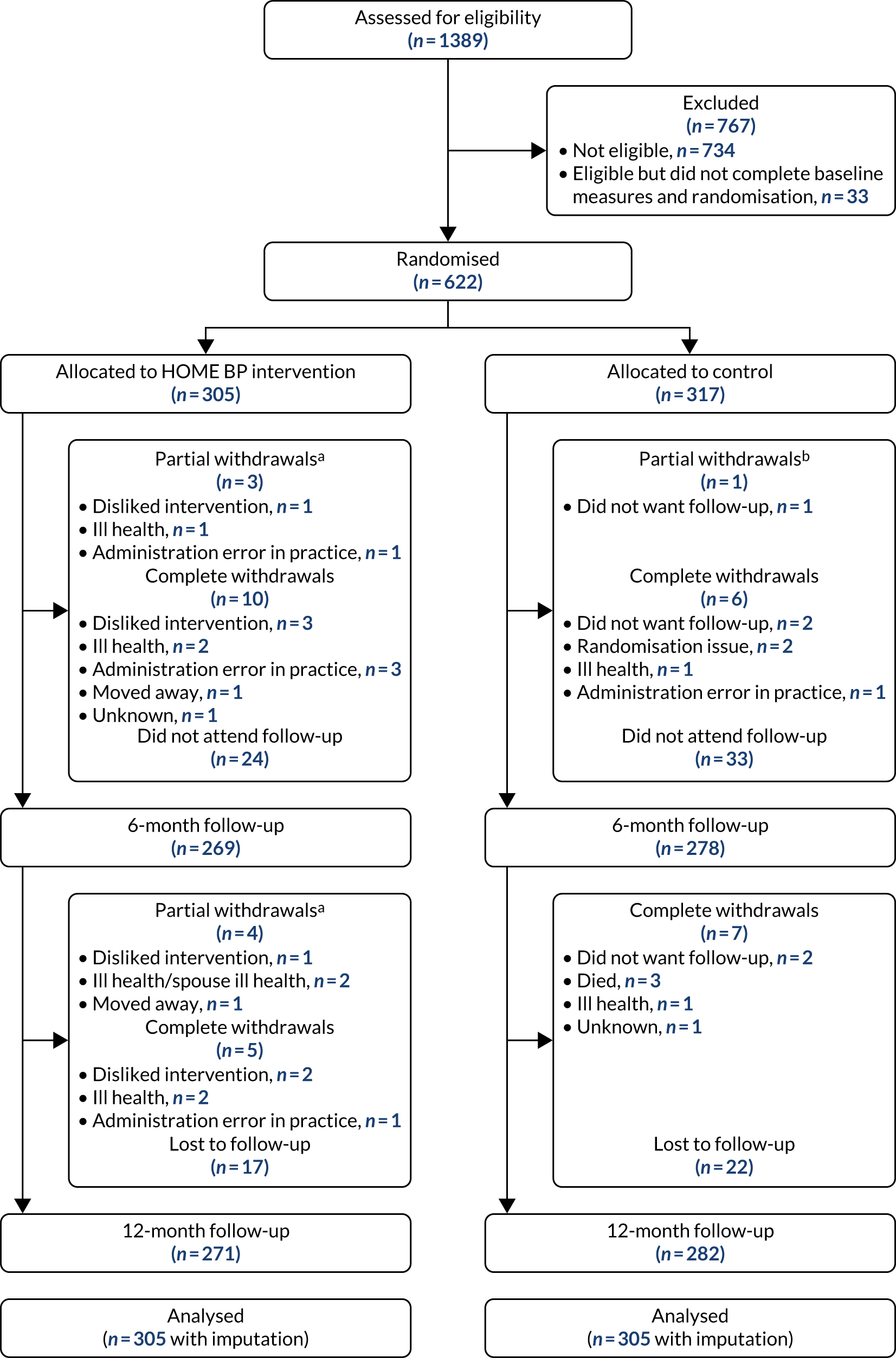
Table 3 provides baseline characteristics of the sample.
| Baseline characteristic | Randomised group | |
|---|---|---|
| Intervention (N = 305) | Usual care (N = 317) | |
| Age (years), mean (SD) | 65.2 (10.3) | 66.7 (10.2) |
| Female, n/N (%) | 145/305 (47.5) | 143/317 (45.0%) |
| Ethnicity, n/N (%) | ||
| White | 285/304 (93.8) | 299/317 (94.3) |
| Black African | 5/304 (1.6) | 3/317 (1.0) |
| Black Caribbean | 0/304 (0.0) | 1/317 (0.3) |
| Indian | 3/304 (1.0) | 0/317 (0.0) |
| Pakistani | 1/304 (0.3) | 3/317 (1.0) |
| Other | 10/304 (3.3) | 11/317 (3.5) |
| Index of Multiple Deprivation quintile, n/N (%) | ||
| 1–3 (most deprived) | 36/304 (11.8) | 27/317 (8.5) |
| 4–7 | 108/304 (35.5) | 125/317 (39.3) |
| 8–10 (least deprived) | 160/304 (52.6) | 166/317 (52.2) |
| Diabetes, n/N (%) | 24/278 (8.6) | 32/291 (11.0) |
| Of which: type 1 | 1/278 (0.4) | 1/291 (0.3) |
| Systolic BP (mmHg), mean (SD) | 151.7 (11.8) | 151.6 (11.1) |
| Diastolic BP (mmHg), mean (SD) | 86.4 (9.6) | 85.3 (9.9) |
The 12-month follow-up rate was 89% in both groups. Systolic BP at 12 months was significantly lower in the intervention group (138.4/80.2 mmHg) than in the control group (141.8/79.8 mmHg), with a difference between groups of –3.4 mmHg [95% confidence interval (CI) –6.1 to –0.8 mmHg]. For diastolic BP, the between-group difference at 12 months was –0.5 mmHg (95% CI –1.9 to 0.9 mmHg) (Table 4). Exploratory subgroup analyses suggested that the intervention had a larger effect in younger participants. Self-reported adverse effects showed no differences between the two groups. According to a self-reported symptoms scale, which was used as an indication of side effects, a significantly higher proportion of the intervention group reported weight loss at 12 months, but this was not born out on objective measurement of weight. Although engagement with self-monitoring was relatively high across the sample (with 80% of the sample completing both training sessions and at least three complete sets of BP entries), less than one-third of the sample chose to register on an optional programme for healthy behaviour change.
| BP measurement | n | Mean (SD) BP (mmHg) at time point | ||
|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||
| Systolic BP | ||||
| Usual care | 282 | 151.65 (11.10) | 140.87 (15.98) | 141.83 (16.76) |
| Intervention | 271 | 151.74 (11.82) | 138.69 (17.04) | 138.43 (15.99) |
| Diastolic BP | ||||
| Usual care | 282 | 85.27 (9.88) | 80.18 (10.32) | 79.77 (10.10) |
| Intervention | 271 | 86.44 (9.65) | 79.88 (9.68) | 80.22 (10.07) |
A within-trial cost-effectiveness analysis was conducted from an NHS perspective using data collected on use of services and on the intervention. The reduction in BP of 3.45 mmHg combined with an increased cost in the intervention arm of £38 led to incremental cost per unit of BP reduction in the base case of £11 (95% CI £5 to £29). The increased cost per patient of £38 in the intervention arm was, almost entirely, due to the cost of the intervention (£39.73) (Table 5).
| Results | Base case (imputed) | Alternative (complete cases) |
|---|---|---|
| NHS cost (£) | ||
| Usual care | 100 | 100 |
| Intervention | 138 | 138 |
| Difference | 38 | 38 |
| Difference in primary outcome at 12 months (mmHg) | 3.45 | 3.54 |
| Cost/BP (£) | 11 | 11 |
| QALY difference | –0.01 | –0.01 |
| Cost/QALY (£) | –3800 | –3800 |
The base case relies on the imputed values; however, the complete-case results were the same for cost and only slightly different for the clinical outcome (see Table 5).
Table 5 also shows a small QALY loss of 0.01 in the intervention arm. Given the combination of higher cost and very slightly reduced QALYs, this means that the intervention arm was dominated by the usual-care arm. However, as QALY differences with regard to improved BP control at 12 months are of less interest than QALY differences with regard to BP control in the longer term, the results of the life-long modelling reported below are of more interest.
The base case included use of NHS services relating to BP, and this comprised the full range of NHS services, including hospital admissions. Although few such admissions were recorded, some were elective procedures that had to have been planned before entry to the trial. Consequently, only hospital admissions that occurred after a change of medication were included in the base-case costing. To test the sensitivity of results to this assumption, a scenario was costed that included all hospital BP-related service use, regardless of timing. Although this scenario made little difference overall, the scenario reduced both the cost difference and the incremental cost-effectiveness.
The mean cost per patient in primary care was similar to the base-case costs, indicating that primary care accounted for almost all costs (Table 6). Within primary care, costs were split roughly 60 : 40 between costs attributable to consultations and costs for prescriptions. Patients in the intervention arm had slightly higher prescription costs, associated with changes in medication and/or dose. However, these costs did not increase the cost of primary care consultations because of the role of the DI. These trends were as might be expected. Further analysis of these changes is planned for a separate publication, which will include changes in the time spent by patients in managing their hypertension.
| Randomised group | Mean cost (£) per patient | ||
|---|---|---|---|
| Consultations | Prescriptions | Total primary care | |
| Usual care | 62.5 | 34.8 | 97.3 |
| Intervention | 55.8 | 40.7 | 96.5 |
As the benefits of reduced BP take the form of lowered risk of cardiovascular disease, long-term modelling was required to capture these effects. The most comprehensive approach involves estimation of lifetime benefits measured in terms of QALYs. Life-years reflect reduced mortality and quality adjustment allows for the effects of non-fatal cardiovascular events.
Rather than develop a new long-term model, we fed the results of the randomised trial into a pre-existing model, which was developed by one of the lead clinicians in the present study for previous trials of BP interventions. 50 The model TASMINH450 (Telemonitoring and Self-Monitoring of Blood Pressure for Antihypertensive Titration in Primary Care) is a Markov patient-level simulation undertaken in TreeAge 2018 (TreeAge Software, Inc., Williamstown, MA, USA). The simulation tracks the costs and consequences of individual patients passing through the model, with characteristics (taken from the trial) free to vary between patients. The model was run over the maximum lifetime of the patients (maximum of 65 years; minimum trial inclusion criteria was age 35 years), a time horizon sufficient to capture all relevant long-term costs and consequences.
All patients started in the well/no event health state. Within a 6-month time cycle, a patient had a risk of suffering a fatal or non-fatal cardiovascular event or of dying from other causes. The possible cardiovascular events in the model were stable angina, unstable angina, stroke, myocardial infarction and transient ischaemic attack. A 10-year cardiovascular risk was calculated for each individual patient, with the distribution of coronary heart disease and stroke events dependent on age and sex. Patients who suffered a non-fatal cardiovascular event transitioned to a post-event cardiovascular health state and additional clinical events were not modelled. Once a cardiovascular event had occurred, mortality risk was adjusted accordingly. The impact of each intervention in terms of event reduction was applied as a relative risk, taking into account the mean differences in systolic BP observed in the HOME BP trial.
Owing to a cost difference of £402 and a QALY difference of 0.044, the results from inputting the HOME BP trial results into the long-term TASMIN4 cost-effectiveness model put the incremental cost-effectiveness ratio (ICER) at just over £9000 (Table 7). The probability of being cost-effective at different levels of willingness to pay was explored using a cost effectivness acceptability curve. The probability of being cost-effective was 66% at willingness to pay £20,000 per QALY, rising to 80% as willingness to pay increased to £50,000. Such results compare well with those assessed for use in the NHS by NICE, albeit with a sizeable degree of uncertainty.
| Strategy | Total cost (£) | Incremental cost (£) | QALY | Incremental QALYs | Incremental cost/QALY |
|---|---|---|---|---|---|
| Usual care | 2685 | 11.562 | |||
| HOME BP intervention | 3087 | 402 | 11.606 | 0.044 | 9107 |
See Appendix 2 for the full cost-effectiveness analyses and see Report Supplementary Material 1 for a Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
The intervention group had significantly more changes to their antihypertensive medication than the usual-care group during the trial, both in terms of dose increases (relative risk 2.03, 95% CI 1.54 to 2.69) and new drugs added (relative risk 1.46, 95% CI 1.12 to 1.91); however, this had minimal impact on costs.
Further details of the secondary outcomes are reported in the main trial publication. 47
Implications
The HOME BP intervention led to significantly lower BP at 12 months among a sample of participants with raised BP at baseline. The reduction in BP in the HOME BP trial was similar to that in other comparable trials (see Appendix 2). The cost of the intervention was modest at just under £40 per patient. Although this is probably an overestimate, given that it was based on providing a novel service for relatively few people, it, nonetheless, delivered benefits that would be considered cost-effective by NICE and the NHS. Long-term modelling puts the incremental cost per QALY at just over £9000. If the intevrention was included in a suite of DIs, which seems increasingly possible, then the cost per patient would probably reduce. More generally, post-COVID, and in line with demographic trends, self-management seems likely to become more widely used in modernised health services. The work reported here provides evidence of both its clinical effectiveness and cost-effectiveness.
It was encouraging that this DI had a similar, albeit slightly smaller, effect size to previous paper-based interventions50,51 for BP management, as the HOME BP intervention offers a more feasible system for wider implementation. Furthermore, the cost of the HOME BP intervention was less than the cost of previous interventions. 50,51 It would be interesting to further explore the interaction whereby the intervention appeared to be more effective for younger participants to better understand how to optimise effectiveness for all participants in wider implementation.
Process evaluation exploring how patients and health-care professionals experienced and implemented the intervention in practice
Patient qualitative process study: perceived benefits and burdens of using the intervention for patients
Aim
The aim was to explore the benefits and burdens perceived by patients of using the HOME BP self-management intervention.
Methods
Semistructured telephone interviews were conducted with 28 patients in the intervention group and seven patients in the usual-care group. The uptake rates of those invited to interviews was 52% in the intervention group and 29% in the usual-care group. Interviews were transcribed verbatim and the data were analysed using thematic analysis, with some techniques from grounded theory. 41,52
Results
Table 8 provides the characteristics of participants in the qualitative process study.
| Characteristic | Randomised group | |
|---|---|---|
| Intervention | Usual care | |
| n | 28 | 7 |
| Age (years), median (range) | 70 (41–87) | 67 (52–77) |
| Female (%) | 71 | 43 |
| Ethnicity, n | ||
| White | 24 | 6 |
| Black African | 1 | |
| Pakistani | 1 | |
| Other | 2 | 1 |
The thematic analysis generated three perceived benefits resulting from use of the HOME BP intervention: (1) reassurance, (2) improved health and (3) motivation to engage in healthy behaviour change. Four perceived burdens were also developed from the data: (1) worrying about health, (2) uncertainty about self-monitoring, (3) guilt about not engaging with healthy behaviour change and (4) fitting self-monitoring into the day. It appeared that the beliefs patients held about their illness and treatment could influence the extent to which they experienced these benefits or burdens. For example, patients with high confidence that their BP could be controlled by medication and with low concerns around side effects or comorbidities tended to be more focused on the benefits of improved health and reassurance that the intervention could bring.
Implications
This study suggested that the benefit of reassurance from seeing well-controlled readings could encourage ongoing engagement with the intervention, even when readings were stable. However, patients with poorly controlled readings might need more support to maintain engagement over time. Intervention optimisation to minimise burdens for these users might include a guided conversation at baseline with the GP to address expectancies for medication change and to manage concerns about side effects.
More generally, these findings suggested that it is important to capture the benefits of using a self-management DI, as well as the burdens. Benefits, such as reassurance, appeared to be strong sources of motivation to keep people engaging with the intervention over time. Currently, however, measures, such as the Patient Experience with Treatment and Self-management questionnaire, focus only on the structural burden for patients, such as attending appointments or engaging in self-monitoring. 53 In addition, intervention evaluations could seek to explore the more subjective psychosocial outcomes of using an intervention, as well as objective factors, such as time and number of appointments, as these psychosocial perceptions are critical for understanding people’s experiences and, therefore, building knowledge about how best to optimise digital self-management interventions.
The participant demographics show that a higher proportion of the process study participants were female (71%) compared with the sample in the overall RCT (48%), and the average age was a little higher (70 years vs. 65 years, respectively).
Patient quantitative process study: engagement and usage of the HOME BP intervention by patients
Aims
The aim was to describe patient uptake and engagement with the HOME BP intervention and to determine which factors were associated with adherence to target behaviours.
Methods
General practices were asked to report the gender, age and postcode of all patients invited to the study to establish whether or not there was evidence of a response bias within the RCT. The online intervention automatically recorded usage data, including number of logins to the intervention and BP readings entered by participants. A subsample of 20 BP monitors was audited to compare the readings saved on the monitor with those entered by patients on the online intervention.
Participants in the RCT completed the following self-report questionnaires at baseline and at 12 months:
-
Medication Adherence Scale (MARS)54
-
Beliefs about Medication Questionnaire (BMQ)55
-
self-efficacy to engage in self-monitoring and manage BP (developed using social cognitive theory56)
-
Patient Enablement Questionnaire57
-
change in healthy lifestyle behaviours (at 12 months only).
Data analysis to explore differences between participants and non-participants in the trial included Mann–Whitney U-tests for continuous data and chi-squared tests for categorical data. Multiple regression analyses were used to explore predictors of the main outcome (i.e. systolic BP at 12 months, controlling for systolic BP and age at baseline).
Results
Data were available from 54 of the 76 general practices in the trial. The data showed no evidence of a response bias in terms of the age (U = 1539847.5, n1 = 6616, n2 = 469; p = 0.786) or gender [χ2(1, n = 8429) = 1.16; p = 0.333] of participants randomised to the trial compared with those who were not. However, there was a very small but significant difference in Index of Multiple Deprivation quintile, as indicated by home postcode, with participants who took part being from less deprived areas than those who did not take part in the study (U = 1539193.0, n1 = 7106, n2 = 468; p = 0.007).
Engagement with the DI was high, with most patients completing both core training sessions (92%) and entering a week of practise readings (88%). Seventy per cent of the intervention group continued to enter at least one BP entry into the final quarter of the study (i.e. at months 10–12). The number of BP entries patients made during the study was predicted by baseline self-reported medication adherence (MARS) and perceived concerns and necessity of BP medication (BMQ), controlling for age and baseline BP (see Appendix 3). Systolic BP at 12 months was predicted by the number of BP entries a patient made, the number of medication changes recommended and their medication necessity beliefs at baseline, controlling for baseline BP and age (see Appendix 3). An audit of BP monitors showed that readings were entered on the HOME BP intervention with 95% accuracy (557/589 readings entered accurately). Where discrepancies occurred, some appeared to be genuine errors, whereas others indicated a potentially deliberate attempt to lower the average.
In terms of engagement with the healthy behaviour change session, 95 (31%) participants completed the optional session, which described the health benefits of making healthy behaviour changes. The difference in systolic BP at 12 months between participants who did and did not complete the optional session on healthy behaviours was not significant; however, there was a trend towards participants who completed the optional session having a higher systolic BP at 12 months (with an increase of 2.78 mmHg, 95% CI –1.16 to 6.73 mmHg), after controlling for baseline systolic BP, age, sex, target category and a random effect for practice.
Of the 243 participants in the intervention group with a BMI > 25 kg/m2, 46 (19%) signed up to the weight loss intervention. Of the remaining healthy lifestyle sessions, 25 participants registered for the physical activity intervention, 24 for healthy eating, 16 for reducing salt and 6 for reducing alcohol. A significantly higher proportion of participants in the intervention group, than in the usual-care group, reported increasing the amount of fruit and vegetables in their diet during the last 12 months (37% vs. 25%, respectively) [χ2(2, n = 486) = 10.70; p = 0.005].
Practical implications
The findings suggested that engagement with self-monitoring BP throughout the intervention was high, and it was encouraging that the audit indicated that patients entered their readings on the intervention with high levels of accuracy. Entering more BP readings was predictive of lower systolic BP at 12 months, demonstrating the importance of maintaining engagement, especially when readings are poorly controlled. Although uptake to optional healthy behaviour change sessions was relatively low, the findings suggested that the intervention group may have engaged in more offline healthy behaviour change than the usual-care group, with a significantly higher proportion of patients in the intervention group reporting an increase in healthy diet at 12 months.
Health-care professionals mixed-methods process study: exploration of health-care professionals’ experiences of, and adherence to, using the intervention
Aim
The aim was to develop a detailed understanding of adherence levels, factors influencing adherence and barriers to and facilitators of implementing the intervention in primary care.
Methods
A mixed-methods approach was adopted for the HCP process evaluation. The HOME BP intervention was used by 125 HCPs across 70 general practices, and adherence data were collected either automatically by the online program or from the patients’ medical notes. The following measures of adherence were used:
-
Percentage of automated recommendations to increase patients’ medication in response to home readings that were actioned.
-
Percentage of patients for whom a three-step medication plan was created.
-
Percentage of medication changes that were issued remotely (by letter or e-mail).
Health-care professionals also completed self-report questionnaires before and after completing compulsory online training at the start of the trial, capturing perceived self-efficacy, outcome expectancies and perceived intervention acceptability for patients. The data were analysed using a combination of correlations, Mann–Whitney U-tests and chi-squared tests.
Qualitative semistructured process interviews were conducted with 27 HCPs during the trial, including GPs, nurse prescribers, nurses and health-care assistants. To begin with, all prescribers and supporters were invited to an interview (17/25 accepted our invitation) and, subsequently, purposive sampling was used to target HCPs with more patients in the study and HCPs who were acting as both a prescriber and a supporter. The interviews were transcribed and analysed using thematic analysis. 41
The quantitative and qualitative data were analysed separately to maximise the strengths of each research method and were, subsequently, integrated using triangulation in which key findings from each analysis were compared to establish whether they were in agreement, partial agreement (i.e. complemented one another), disagreement or silence. 58
Findings
The sample for the quantitative analyses included 62 prescribers (i.e. GPs or nurse prescribers; 35% female), 58 supporters (i.e. nurses or health-care assistants; 95% female) and 5 prescriber–supporters who performed both roles (60% female). The subsample of HCPs who took part in qualitative interviews included 13 prescribers (38% female), 11 supporters (91% female) and 3 prescriber–supporters (100% female).
In terms of adherence to the target behaviour of escalating patients’ antihypertensive medication in response to average home readings being above target for 2 consecutive months, 405 e-mail recommendations were sent to HCPs, of which 215 (53%) were actioned. Comparisons of recommendations actioned against recommendations that were not showed that cases where systolic BP was closer to the threshold of 135/85 mmHg, and cases in which the patient had already been recommended a medication change previously, were less likely to be actioned. Meanwhile, patient age did not appear to make a difference.
Figure 3 shows the distribution of average systolic BP readings in cases where the recommendation for a medication change was not adhered to. Figure 3 shows that in 181 of 190 cases not adhered to, the patient had a mean BP reading below 150 mmHg (note that 150 mmHg is the target for the national Quality and Outcomes Framework in UK general practice59), although there were a few higher means that did not result in a change.
FIGURE 3.
Distribution of average systolic BP readings in cases where the recommendation for a medication change was not adhered to. Note that the cases in which systolic BP was below 135 mmHg triggered a medication change recommendation due to the diastolic mean exceeding the target, rather than the systolic. SD, standard deviation.
The qualitative data also indicated that borderline readings were a reason for not changing patients’ medication when recommended, and other reasons included concerns about the lack of context in which the readings had occurred (e.g. whether or not the patient had recently experienced a stressful event or illness) and preferring to recommend healthy behaviour change instead.
Adherence to planning three medication changes in advance for each patient was 82%, but the qualitative interviews highlighted several issues with implementing this procedure in practice. Some HCPs found it challenging to plan three medication changes for more complex patients, and there were also concerns about planning in advance when side effects or changes in health might mean that the medication change is no longer appropriate. A few HCPs described negative experiences of having to update the three-step plan, which could create additional work for them and cause anxiety for the patient.
Notifying patients of medication change remotely (i.e. by e-mail or letter) occurred in 38% of cases, whereas in all other cases the HCP spoke to the patient by telephone or face to face. Adherence to sending a monthly support e-mail to patients in the trial was 56%. Qualitative data indicated that HCPs had some concerns about contacting patients remotely, for example patients might not receive or value the information.
Practice implications
This mixed-methods evaluation suggested that there were practical issues with creating a three-step medication plan for some hypertensive patients and that this process might need more flexibility to improve implementation in practice. The processes of changing patients’ medication when their BP was above-target and supporting patients’ BP management via e-mail appeared straightforward to enact, but some HCPs were doubtful about the benefit of changing their working processes in this way. It may be that changes at the organisational level to BP targets and normalising e-mail support for patients might facilitate implementation of these processes.
Conclusions
Overall, the HOME BP intervention appeared to be both clinically effective and cost-effective, with significant reductions in BP compared with usual care, for a low cost per unit of BP. The reduction in BP found in this trial is important in terms of long-term health outcomes, with an anticipated reduction of 10–15% of patients suffering a stroke and of 5–10% of patients experiencing coronary-related events.
The detailed process evaluation of patients’ and HCPs’ experiences of implementing the intervention suggested some ideas for optimising the intervention, including:
-
a guided discussion at baseline to increase patients’ and HCPs’ motivation to change medication when average BP exceeds the threshold, and to address some of the common concerns for patients about taking more medication
-
additional support for patients with continuously raised BP readings to encourage patients to maintain engagement with self-monitoring
-
acknowledgements for HCPs when patients have received information sent remotely to reassure HCPs that the information has been received and to increase the feasibility of remote support.
Chapter 4 Development of the My Breathing Matters intervention
Parts of this section are reported in more detail in McLean et al. 60 and Morton et al. 29
Objectives
In this section, we describe the intervention planning (WS1), development and testing (WS3) of the My Breathing Matters intervention, a digital self-management intervention for adults with asthma. The intervention objective was to improve functional quality of life of primary care patients with asthma, by supporting illness self-management by pharmacological means (e.g., medication adherence, appropriate health-care service use) and non-pharmacological means (e.g. breathing retraining, cognitive–behavioural therapy/stress reduction, healthy behaviour change). Development was carried out in four phases. The phases have not been reported elsewhere (apart from the development of the ‘guiding principles’61) and are, therefore, fully described in this section. The phases are described in chronological order and for each phase we report on the aims, methods, results and practical implications for how the findings informed intervention development. The four phases were as follows:
-
Collate and synthesise evidence to inform intervention planning, including a systematic review of quantitative evidence, a qualitative meta-ethnography and primary mixed-methods research.
-
Create an intervention plan, which involved developing guiding principles and carrying out behavioural analysis, to identify barriers to key behaviours and specify how these will be addressed.
-
Create an intervention prototype and use iterative qualitative interviews (i.e. think-aloud and retrospective interviews) to optimise the intervention.
-
Map the evidence onto behavioural barriers and intervention components onto theory.
At the end of this section, we demonstrate how the INDEX actions for developing complex interventions were met in this development process. 25
Intervention Development Team and patient and public involvement
The intervention development process was guided throughout by an Intervention Development Team that involved asthma-focused clinicians, psychologists, web developers, PPI contributors and representatives of Asthma UK, a key stakeholder organisation. Members of Asthma UK were invited on our Steering Committee and Intervention Development Team to provide their extensive knowledge of asthma and available self-management resources, and to help recruit PPI contributors. Asthma UK would also provide a suitable channel for national intervention dissemination once the acceptability and effectiveness of the intervention is established.
Our PPI contributors included two people with asthma (David Russell and Mark Stafford-Watson) and one public contributor with a general interest in DIs (Samantha Richards Hall). The PPI contributors provided feedback on study materials (e.g. interview topic guides, participant information sheets) and detailed feedback on the intervention prototype, and changes were made following their feedback. Two PPI contributors (David Russell and Samantha Richards Hall) provided their feedback on the key findings and final interpretations of the mixed-methods process evaluation and one PPI contributor (David Russell) was a co-author on the associated manuscript for this work, published in npj Primary Care Respiratory Medicine. 62
Phase 1: collate and synthesise evidence
Systematic review and meta-analysis of interactive digital interventions to promote self-management in adults with asthma (described in McLean et al.60)
Aim
The aim was to carry out a systematic review with meta-analysis of quantitative evidence, assessing the effects of interactive DIs to support patient self-management of asthma.
Methods
Ten electronic databases were searched to identify RCTs of interactive DIs for adults (aged ≥ 16 years) with asthma, which used usual care as a comparator. 60 Outcomes were change in clinical outcomes, patient-reported outcomes of well-being or quality of life and cost-effectiveness. Studies were eligible if they were published in peer-reviewed journals and were written in English. Two independent researchers screened potential studies, extracted data from the eligible studies and assessed risk of bias using the Cochrane collaboration tool. Where possible, meta-analysis using a random-effects model was performed.
Results
Eight publications of five trials with 593 participants were included. All five interventions provided health education and facilitated self-monitoring (e.g. monitoring symptoms, medication usage or quality of life). Three of the trials were eligible for inclusion in a meta-analysis, which showed no significant changes in asthma quality of life and asthma control (when compared with usual care) and extremely high heterogeneity. To reduce heterogeneity, one study was removed, as its’ aim was to reduce the total dose of oral prednisolone. The other two studies aimed to improve asthma control. The remaining two studies demonstrated significant improvement for asthma quality of life (standardised mean difference = 0.45) and asthma control (standardised mean difference = 0.54). No evidence of harm was identified. Most studies were likely to be underpowered for most outcomes, as they were small, of moderate quality and were short in duration.
Practical implications
Although the findings show potential for benefit, with evidence of improvements in some outcomes, the evidence base is weak due to a lack of large, robust trials. In terms of the My Breathing Matters intervention, the meta-analysis provided some, albeit weak, support for the potential efficacy of a DI for asthma. However, it could not offer more specific implications for how to promote effectiveness.
Meta-ethnography review of published qualitative studies on digital interventions for self-management of chronic physical conditions (described in Morton et al.29)
Aim
The aim was to synthesise the qualitative evidence on DIs for self-management of chronic physical health conditions to identify key barriers to and facilitators of the target behaviours in the My Breathing Matters intervention.
Methods and results
The methods and key findings of this study were reported previously [see Chapter 2, Identification of barriers and facilitators from the qualitative literature (described in Morton et al. 29)].
Practical implications
The meta-ethnography suggested that tailoring of self-monitoring feedback could be important to promote perceived necessity of medication change for patients. It was also theorised that meaningful feedback could help patients understand how their self-monitored data are influenced by lifestyle activities. To facilitate meaningful self-monitoring while minimising burden, the My Breathing Matters intervention included simple self-monitoring of domains of asthma-related quality of life. Users were asked to rate how much their asthma has affected their quality of life over the last week by reporting how often (from ‘almost all the time’ to ‘not at all’) single-item statements applied to them. For example, one statement read ‘My breathing has made some activities a bit more difficult (e.g. exercising, sleeping, working, housework or seeing friends)’. Tailored feedback on how users rated these items was then used to try to motivate users to make appropriate changes in their management of symptoms. For example, users could be recommended to do the 4-week challenge, which encouraged patients to engage in habitual optimal preventer inhaler use and to report the results.
Within the meta-ethnography, several asthma studies noted that health professionals were concerned about the additional time required to process or review DI data. 63 To ensure that the My Breathing Matters intervention minimised the demands on health professionals’ time and was easily scalable, the intervention advertised existing telephone support offered by trained nurses from Asthma UK. The telephone helpline could provide people with additional information and support to follow the behavioural advice provided in the intervention, but did not provide patients with medical advice. Patients were recommended to contact their health professional if they had any concerns about their symptoms or medications. This type of support would also be sustainable if Asthma UK were to disseminate the final intervention.
Primary mixed-methods research
Aim
The aim was to use published and directly relevant primary mixed-methods research to inform the design of the My Breathing Matters intervention.
Methods
Two primary research projects were previously conducted by members of the Project Research Team that directly informed the design and development of My Breathing Matters:
-
The Randomized trial of an Asthma Internet Self-management InterventioN (RAISIN)64,65 involved a non-blinded pilot RCT to evaluate the feasibility of a theory-informed, evidence-based online resource (i.e. ‘Living Well with Asthma’) to support self-management in people with asthma. Patients in the intervention group (n = 25) completed the Problematic Experience of Therapies Scale to identify barriers to using the website and following its advice. Quantitative usage data were also explored.
-
Breathing Retraining for Asthma – a Trial of Home Exercises (BREATHE) was a large RCT that investigated the use of breathing retraining, a non-pharmacological treatment involving exercises to retrain dysfunctional breathing. The intervention was delivered by digital versatile disc (DVD) and booklet, or by DVD and booklet plus three face-to-face sessions with a physiotherapist. 66
Members of the RAISIN and BREATHE study teams provided stakeholder input when developing the My Breathing Matters intervention, sharing their expertise and the lessons they learned from their research.
Results
Recruitment and retention in RAISIN confirmed feasibility and trends towards improved asthma-related quality of life and asthma control, which suggested that use of the Living Well with Asthma resource may improve self-management in adults with asthma, compared with usual care. To be included in the trial, participants needed to have poorly controlled asthma [as defined by an Asthma Control Questionnaire (ACQ) score of ≥ 1], and introduction questions at the start of the website revealed that 95% of users reported that asthma was negatively affecting their lives. Despite this, 42% of users reported doubting the personal relevance of the website (as measured by the Problematic Experience of Therapies Scale), stating that the intervention would be more useful to people with more severe asthma. Exploration of usage patterns revealed that, although engagement was comparable to other behaviour change websites (76% of individuals logging in), some users missed core intervention sections that they may have benefited from (e.g. sections promoting use of personal asthma action plans and attendance at annual asthma reviews). At stakeholder meetings, the RAISIN research team explained how some users found the Living Well with Asthma website large and difficult to navigate, and that it was not always clear what content they had already accessed.
In BREATHE, both breathing retraining groups demonstrated improved asthma-related quality of life compared with usual care. There was no inferiority of the DVD-only group compared with patients supported by a physiotherapist, indicating the effectiveness of self-guided breathing retraining.
Practical implications
Table 9 provides a summary of the intervention features that were included in the My Breathing Matters intervention to address each key issue identified in the primary mixed-methods research.
| Key issue identified | Intervention feature included in the My Breathing Matters intervention |
|---|---|
| RAISIN | |
| RAISIN confirmed feasibility and trends towards improved asthma-related quality of life and asthma control |
|
| Many users of the Living Well with Asthma resource reported doubting its personal relevance |
|
| Some users missed core intervention sections that they may have benefited from |
|
| The Living Well with Asthma website was large and difficult to navigate |
|
| BREATHE | |
| Both breathing retraining groups demonstrated improved asthma-related quality of life compared with usual care |
|
| No inferiority of the DVD-only group vs. patients supported by a physiotherapist |
|
Phase 2: creation of an intervention plan
Guiding principles
Aim
The aim was to develop brief guiding principles to inform intervention development.
Methods
Guiding principles are a key part of the ‘person-based approach’ (PBA), which was developed and refined in the process of completing the DIPSS research programme. PBA is discussed fully later in this report (see Chapter 6) and in Yardley et al. 36 The methods for developing the guiding principles were the same as those used in the HOME BP intervention (see Chapter 2). The development of the My Breathing Matters guiding principles is published in more detail in Yardley et al. 61
Results
The evidence collated in phase 1 suggested that most people with non-optimal asthma control, nevertheless, do not consider themselves as patients with active asthma. Therefore, one intervention design objective was to engage these people and we aimed to do this using three key features: (1) maintaining positive illness context throughout (i.e. promote health rather than illness), (2) simple unobtrusive interface to provide optional (and flexible) support only when needed and (3) demonstrating that impaired quality of life is not ‘just my breathing’, but can be improved.
The phase 1 evidence also highlighted that users are not likely to adhere to medication, nor to use an asthma management plan, and may be sceptical of necessity and efficacy of both. Therefore, a second intervention design objective was to persuade and educate users to implement appropriate pharmacological management. Key features of this objective included persuading and educating users regarding the necessity, efficacy and safety of preventative asthma medication, and tailoring appropriate information regarding medical management according to users’ current medication behaviour.
Phase 1 findings also highlighted that there were other factors contributing to increased asthma symptoms and reduced quality of life, but these are often not known or acknowledged, particularly anxiety, stress and lifestyle (e.g. smoking, obesity, physical activity). Therefore, a third intervention objective was to encourage users to employ non-pharmacological methods of improving quality of life. The intervention aimed to address this by educating users on the benefits of these methods and offer psychological methods to improve quality of life (e.g. cognitive–behavioural techniques for symptom management). The intervention also provided tailored access to, and addressed patient concerns about, relevant positive healthy behaviour changes, and this was achieved by giving users access to several previously evaluated interventions promoting healthy behaviours (i.e. smoking cessation,67 physical activity,68 weight management40 and handwashing to prevent infections69).
Practical implications
Similar to the HOME BP intervention, the guiding principles succinctly summarised the distinctive design objectives and features of the My Breathing Matters intervention to ensure that the psychosocial context and perspectives of target users was considered and accommodated throughout development.
Behavioural analysis
Aim
The aim was to systematically identify the influences on patient target behaviours and the intervention components that could address these.
Methods
The methods for the behaviour analysis were the same as those used in the HOME BP intervention (see Chapter 2). Key target behaviours were identified from the primary mixed-methods research and key barriers for each behaviour were identified across all of the evidence collated and synthesised in phase 1.
Results
Appendix 4 provides the My Breathing Matters behavioural analysis table (see Table 31). Five target behaviours were identified: (1) preventer medication adherence, (2) engagement with a personal asthma action plan, (3) attendance at annual asthma reviews, (4) engagement with breathing retraining and (5) engagement with cognitive–behavioural stress management practice. We also identified one subsidiary behaviour (i.e. effective engagement with DI) that is necessary to enact these target behaviours. The healthy behaviour changes were not included in the behavioural analysis, as the interventions targeting these behaviours were not developed as part of this research. A range of barriers were identified for each behaviour, along with suggestions for how barriers could be addressed in the intervention. For example, to address patients’ belief that breathing retraining is not as effective as medicine, we provided information regarding the rationale behind breathing retraining and stories from other asthma patients emphasising its potential benefits.
Practical implications
The behavioural analysis helped ensure that the intervention addressed key barriers identified in the literature and expert knowledge of stakeholders in the Intervention Development Team. The behavioural analysis aimed to maximise user engagement with the intervention’s key target behaviours. Specifying the key target behaviours ensured that intervention development focused on the self-management components most likely to have an impact on the intervention outcomes.
Phase 3: creating and optimising the intervention
Aims
The aims were to create an intervention prototype and to use in-depth iterative qualitative research to optimise the intervention.
Methods
An intervention prototype was developed with input from all members of the Intervention Development Team.
To explore target users’ perception of the My Breathing Matters intervention, 34 think-aloud interviews were carried out with 14 adults with asthma. Refinements were iterative in that changes were made in between each interview based on the feedback from the previous interview. Semistructured telephone interviews were then carried out with 12 additional adults with asthma who were asked to use the intervention for 2 weeks. These interviews allowed us to further explore intervention aspects that were not appropriate for single-session think-aloud testing. For example, the optimal timing for sending e-mails and releasing the different intervention content. Each negative comment from participants in both studies was recorded in a table and possible changes were discussed by the research team. Recruitment ceased for each study when no further issues were arising with the intervention that seemed important and that could be addressed.
Results
Overall, both sets of qualitative interviews showed that participants found the website acceptable and easy to navigate, and the content was easy to understand. Participants particularly liked that the website included both pharmacological and non-pharmacological content. However, several issues affecting the acceptability of the intervention were identified and the findings were used to optimise the intervention. When taking part in the think-aloud interviews, participants found the intervention-tailoring process to be too demanding and onerous. On each unique log-in, users completed a brief assessment of quality of life in five areas: (1) activities, (2) sleep, (3) stress, (4) illness and (5) reliever medication use (this tool was named ‘My Breath Check’). Participants were then signposted to relevant content based on their scores. After discussion within the Intervention Development Team, it was decided to modify the tailoring to focus on three areas only: (1) activities, (2) stress and (3) reliever inhaler use.
Several participants in the retrospective interview study appreciated the value of the My Breathing Matters tool for people with asthma generally, but did not consider the intervention relevant to them because they believed their asthma was not particularly severe or problematic for them (despite reporting impaired quality of life in ‘My Breath Check’). Indeed, participants noted that they would be unlikely to use certain intervention components that they did not consider relevant. For example, participants would not engage with the 4-week medication challenge if they believed that they were already adhering to their medication, and the support for making a personal asthma action plan was not relevant to participants if they already had created one with their GP. Findings from both studies indicated that many users did not consider the possible benefits of improved symptom control to be personally relevant. This was considered a major issue by the Intervention Development Team, as users who did not consider the intervention relevant were less likely to engage with the intervention and be motivated to change behaviours. However, to address this by introducing additional content was considered by the Intervention Development Team to be in conflict with guiding principles (i.e. simple, clear and unobtrusive). Consequently, the issue was addressed by increasing the prominence of the content that highlights the personal relevance of impaired quality of life and challenges participants’ perceptions about what it means to have active asthma by making this the first section users viewed after the sign-up process. The updated intervention was then tested with new users who felt that the intervention was personally relevant.
Practical implications
This iterative qualitative research suggested that the My Breathing Matters intervention was acceptable and persuasive to adults with asthma. The research also highlighted some important modifications to optimise the intervention, including increasing its ease of use and perceived relevance.
Phase 4: mapping the evidence onto behavioural barriers and the intervention components onto theory
Aims
-
To comprehensively describe the intervention in terms of existing theory and programme level theory.
-
To create a logic model to illustrate the hypothesised mechanisms of action that explain how the My Breathing Matters intervention is expected to lead to improvements in asthma-related quality of life.
Methods
The methods for the behaviour analysis and logic modelling were the same as those used in the HOME BP intervention (see Chapter 2). For the logic model, relevant hypothesised mechanisms were identified from a literature review of existing evidence and the considerable behavioural science expertise in the study team.
Results
Appendix 4 shows the mapping in a behavioural analysis table (see Table 31, columns 3–6). When mapped onto the BCW, the My Breathing Matters intervention components were shown to target all six target constructs: (1) psychological capability, (2) physical capability, (3) reflective motivation, (4) automatic motivation, (5) physical opportunity and (6) social opportunity. The intervention components mapped onto six intervention functions (i.e. education, persuasion, training, modelling, enablement, and environmental restructuring) and 22 different BCTs. The intervention mapped onto all four core constructs of NPT (i.e. coherence, cognitive participation, collective action and reflexive monitoring).
For the logic model (Figure 4), three types of variables proposed to mediate the impact of the My Breathing Matters intervention on asthma-related quality of life were identified: (1) behavioural adherence, including effective engagement with DIs and improved pharmacological and non-pharmacological management (e.g. preventer medication adherence, engagement with a personal asthma action plan, attendance at annual asthma reviews, engagement with breathing retraining, engagement with cognitive–behavioural stress management practice and engagement with healthy behaviour change); (2) physiological mediators (e.g. improved asthma control, improved lung function and fewer exacerbations); and (3) psychological mediators (e.g. reduced stress, improved mood and improved enablement).
FIGURE 4.
Logic model of the My Breathing Matters intervention to improve quality of life in patients with asthma. A, Uptake and engagement facilitation; b, pharmacological support; and c, non-pharmacological support. BR, breathing retraining; PAAP, personal asthma action plan.
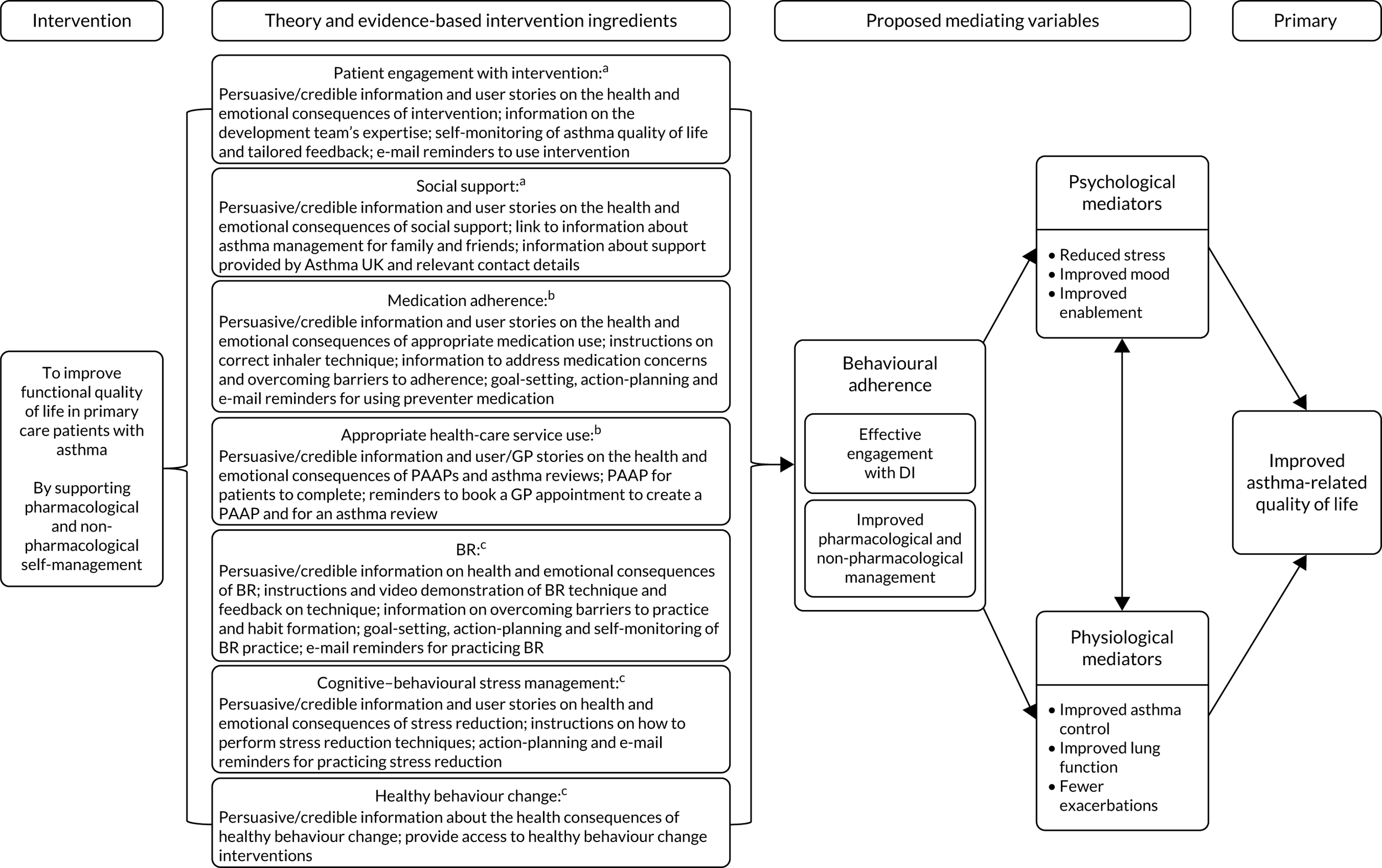
Practical implications
Similar to the development of the HOME BP intervention, the behavioural analysis was useful for ensuring that the intervention content could be described using standard terminology. The logic model explicitly illustrated the mechanisms theorised to change asthma-related quality of life.
Mapping the My Breathing Matters intervention development process to the INDEX actions
As in the HOME BP intervention development (see Chapter 2), we retrospectively mapped the My Breathing Matters intervention development process to the 18 recommended actions for consideration during intervention development provided by O’Cathain et al. 25 (Table 10).
| Action from INDEX guidance | How this action was addressed in the My Breathing Matters intervention |
|---|---|
| Identify that there is a problem in need of a new intervention | The rationale for the My Breathing Matters intervention was identified in the funding application, based on existing evidence (see Chapter 1):
|
| Establish a group or set of groups to guide the development process, thinking about engagement of relevant stakeholders, such as the public, patients, practitioners and policy-makers |
The Programme Management Group (which met 3-monthly to oversee all important decisions) was set-up at the proposal stage and included people with asthma, behaviour change specialists, Asthma UK, clinicians, health economists, policy-makers, statisticians and trial managers All members of the Programme Management Group were invited (if interested) to join the Intervention Development Team, which met monthly (or as necessary) to oversee and guide intervention development. The team included patients, clinicians and health psychologists A core Intervention Development Team, comprising the health psychologists who were developing the intervention, met weekly and worked in close consultation with key clinical academics when necessary |
| Understand the problems or issues to be addressed |
Barriers to key behaviours were identified from (1) reviews of the existing quantitative and qualitative evidence, and (2) in-depth primary mixed-methods research These evidence sources enabled us to understand the specific beliefs and contextual factors that appeared to influence target behaviours |
| Make a decision about the specific problem or problems that an intervention will address, and the aims or goals for the intervention. This may involve defining the behaviours to target |
A logic model was created to map the hypothesised mechanisms (including target behaviours) through which the intervention was theorised to change behaviour and outcomes Our behavioural analysis table documented the target behaviours for patients and the barriers for implementing them, and the intervention ingredients intended to support target behaviours Guiding principles were developed to specify how the intervention would meet design objectives to promote engagement with the target behaviours in this specific population and context |
| Identify possible ways of making changes to address the problems. This involves identifying what needs to change, how to bring about this change and what might need to change at individual, interpersonal, organisational, community or societal levels |
The primary and secondary research and analyses described above helped identify what needed to change at the individual patient level, and at a more organisational level in the health-care systems, and provided insights into how this might best be achieved The development and management teams reviewed and agreed the design of the intervention, informed by the evidence reviews, behavioural analysis table (see Table 30) and the guiding principles, together with stakeholder expertise (clinical and experiential) and knowledge of existing relevant theory and theoretical frameworks (in particular NPT and the BCW)34 |
| Specify who will change, how and when. Selections may depend on consideration of the likely impact of the change, how easy it is to change, how influential it is for the problem being addressed and how easy it is to measure |
Decisions about the appropriate target group for behaviour change, core behaviours to target and intervention outcome measurement (e.g. required sample size, trial design and duration, and the primary and secondary outcomes) were informed by the funding application, previous evidence relating to asthma management (especially Morrison et al. 64,65 and Bruton et al. 66) and the wider review of evidence undertaken as part of the intervention planning There was good evidence that a digital self-management intervention and breathing retraining delivered using digital technology (i.e. DVDs) was acceptable and effective for asthma. Therefore, steps were taken to ensure that the key behaviours were incorporated into the My Breathing Matters intervention [e.g. breathing retraining delivered using videos, a 4-week challenge to facilitate medication adherence, promoting better utilisation of health-care resources (action plans, annual asthma reviews) and providing stress management strategies] |
| Consider real-world issues about cost and delivery of any intervention at this early stage to reduce the risk of implementation failure at a later stage |
As the rationale for the intervention was to provide a more feasible and cost-effective method of managing asthma, a key focus was to design the intervention to be as pragmatic, efficient and easy to implement as possible. For example, it was self-guided and advertised existing telephone support offered by trained nurses from Asthma UK Regular management meetings were held among stakeholders, including patient contributors and clinicians, during which optimising the feasibility of the intervention in primary care was thoroughly discussed |
| Consider whether or not it is worthwhile continuing with the process of developing an intervention |
Early review of the evidence provided some, albeit weak, support for the potential efficacy of DIs for asthma, suggesting that it was worthwhile continuing with the development process PPI, stakeholder and qualitative feedback on prototype versions of the intervention also provided encouraging evidence that the intervention was accessible and well liked by patients |
| Generate ideas and solutions with regard to components and features of an intervention | Qualitative research was undertaken with a range of patients from the target population, including:
|
| Re-visit decisions about where to intervene. This can involve consideration of the different levels at which to intervene, and the wider system in which the intervention will operate | The in-depth qualitative development research enabled the Intervention Development Team to review decisions about how the intervention would work, and the appropriateness of providing additional support using the Asthma UK helpline. For example, patients during retrospective and think-aloud interviews liked the links with Asthma UK, as it added credibility to the intervention |
| Make decisions about the content, format and delivery of the intervention | Decisions about the content, format and delivery of the intervention were informed by in-depth qualitative and mixed-methods research with the target user population, reviews of the evidence, behavioural analysis, and input from the Intervention Development Team and wider Management Team |
| Design an implementation plan, thinking about who will adopt the intervention and maintain it |
The grant proposal for the intervention included an implementation plan should the intervention prove effective This involved disseminating the findings through multiple pathways, including open-access, peer-reviewed publications, presentation at conferences, and speaking to NHS Clinical Commissioning Groups, NHS Choices and NHS Digital. The plan also specified that the intervention software would facilitate adaptation of the DI materials for future roll-out in different contexts (e.g. adapting for certain patient subgroups or adding new components). It was planned that the intervention could be used by the NHS, as well as in the private sector, third sector and by other health researchers Asthma UK were involved in the project from the outset, with their Head of Health Advice Colette Harris attending management and development meetings and helping to recruit PPI team members |
| Make prototypes or mock-ups of the intervention, where relevant | The intervention was developed using LifeGuide software, which enabled creation of a prototype intervention that could be easily modified throughout the development process, based on user feedback (especially from think-aloud interviews). This was an essential, iterative phase of intervention development, which helped to ensure that the intervention was accessible, appropriate, feasible, motivating, convincing and persuasive for users |
| Test on small samples for feasibility and acceptability, and make changes to the intervention if possible | At early stages of development, feedback on the intervention was sought from the Intervention Development Team and Programme Management Group. Subsequently, detailed think-aloud interviews (n = 34) and retrospective interviews with patients who had used the intervention independently (n = 12) informed decisions about changes to the intervention |
| Test on a more diverse population, moving away from the single setting where early development of the intervention took place and seeking a more diverse sample. This can involve asking questions, such as ‘is it working as intended?’, ‘is it achieving short term goals?’, ‘is it having serious adverse effects?’ | This project included a feasibility RCT, which recruited 88 adults with asthma from a diverse mix of general practices (i.e. rural/urban, different SES and practice sizes). We built in a mixed-methods process evaluation (usage data and process interviews with patients) so that we could identify any acceptability, feasibility or engagement issues with the final intervention |
| Optimise the intervention for efficiency prior to a full RCT | The iterative qualitative findings were used to optimise the intervention to maximise user acceptability and engagement. The acceptability issues identified by the process evaluation will be addressed before a full RCT |
| Document the intervention, describing the intervention so that others can use it, and offer instructions on how to train practitioners delivering the intervention and on how to implement the intervention | The intervention was described in detail using the TIDieR checklist (see Appendix 5) |
| Develop the objectives of the outcome and process evaluations. This includes determining how outcomes and mediators of change can be measured, developing measures, specifying evaluation design, planning recruitment and considering feasibility of a full RCT |
The feasibility process evaluation was planned in consultation with the Programme Management Group This involved:The data were planned to be analysed independently, and a mixed-methods approach was adopted for triangulating the individual findings. This would facilitate an enhanced understanding of patients’ experiences of, and interactions with, a DI for asthma self-management The feasibility of trial procedures for a future definitive RCT and full quantitative process analysis was assessed. This included questionnaires measuring purported mediators (informed by the logic model) and medication and health resource use captured via review of patients’ medical notes. This identified potential improvements to the trial procedures (see Chapter 5) |
Conclusion
Similar to the HOME BP intervention, the combination of these approaches to intervention development helped ensure that the intervention was optimally persuasive, motivating and feasible to implement in practice for adults with asthma. A full description of the My Breathing Matters programme using the TIDieR checklist is provided in Appendix 5. 46 A demonstration of the My Breathing Matters intervention can be accessed via URL: www.mybreathingmatters.co.uk (accessed 29 July 2022).
Chapter 5 Evaluation of the My Breathing Matters intervention
Parts of this section are reported in more detail in Ainsworth et al. 70 and Greenwell et al. 62
Aims and objectives
In this section, we describe the evaluation of the My Breathing Matters intervention during a 12-month feasibility RCT. The aims, methods, results and implications are described for each discrete piece of research as follows:
-
A feasibility RCT to assess feasibility of trial procedures and data analysis.
-
A mixed-methods process evaluation to explore the acceptability of the My Breathing Matters intervention, including how patients experienced and used the intervention.
The section finishes with a conclusions section, which draws together all the findings.
Feasibility randomised controlled trial to assess feasibility of trial procedures and data analysis
Aims
-
To assess feasibility of trial procedures, including recruitment strategy, eligibility criteria, consent/withdrawal, randomisation and blinding.
-
To assess feasibility of data analysis, including data collection, data quality and management of trial data across trial end-point measures to inform sample size calculations for a fully powered RCT.
Methods
A total of 88 primary care patients with asthma from seven general practices in Wessex, aged ≥ 18 years, with impaired asthma-specific quality of life, were randomised to usual care (n = 44) or the intervention group (n = 44) in which they accessed the My Breathing Matters intervention. Block randomisation stratified by an average score of 4.3 on the Mini Asthma Quality of Life Questionnaire (Mini AQLQ;71 taken from a previous trial using the same inclusion criteria) was used. 66 Practices were purposively sampled to be both rural (n = 4) and urban (n = 3), with a spread across socioeconomic deprivation {mean practice deprivation index 20.60% [standard deviation (SD) 10.5%]; practice socioeconomic deprivation deciles = 2, 4, 4, 5, 8, 10, 10, in which lower deciles indicate more deprivation}. 72 Participants completed postal screening questionnaires (Mini AQLQ) to identify impaired asthma-specific quality of life, and attended a baseline appointment at their local general practice with a trained research nurse. Randomisation was carried out by a computer program and allocation was concealed from both the participant and research nurse. Participants completed postal follow-up measures after 3 months and attended a follow-up appointment after 12 months. The primary outcome was the feasibility of the trial design, including recruitment, adherence, intervention engagement and retention at follow-up. Secondary outcomes were the feasibility and effect sizes of specific trial measures, including asthma-specific quality of life (measured with the Mini AQLQ) and asthma control (measured with the ACQ). 73 Health-care utilisation data [e.g. medication use, frequency of GP consultations, accident and emergency (A&E) admissions and hospitalisations] were collected via a retrospective notes review conducted by practice staff. Exploratory analysis compared group differences in continuous primary end-point measures (i.e. Mini AQLQ and ACQ) using linear regression models, adjusted for baseline scores of each measure. The trial is registered as ISRCTN15698435.
Results
Table 11 provides the baseline demographic characteristics of the study population by group and Figure 5 shows participants’ flow through the trial. Follow-up data at 3 months was gathered from 91% of patients (intervention group, 36/44; control group, 44/44; total, 80/88) and at 12 months was gathered from 90% of participants (intervention group, 36/44; control group, 43/44; total, 79/88). At 12 months, four patients formally withdrew from the study, one patient was withdrawn as they were no longer eligible (i.e. they were referred to secondary care) and four patients did not complete their 12-month follow-up questionnaires. Nine adverse events and three serious adverse events were reported, which were unrelated to the study.
| Characteristic | Overall sample (N = 88) | Randomised group | |
|---|---|---|---|
| Intervention group (N = 44) | Control group (N = 44) | ||
| Age (years), mean (SD) | 56.6 (15.2) | 57.0 (14.2) | 56.3 (16.2) |
| Female, n (%) | 53.0 (60.2) | 27.0 (61.4) | 26.0 (59.1) |
| BMI (kg/m2), mean (SD) | 29.5 (6.1) | 28.9 (5.9) | 30.1 (6.3) |
| Length of diagnosis (years), mean (SD) | 24.0 (17.5) | 25.2 (17.2) | 22.8 (17.8) |
| Ethnicity, n (%) | |||
| White | 84 (95.5) | 42 (95.5) | 42 (95.5) |
| Other | 4 (4.5) | 2 (4.5) | 2 (4.5) |
| Smoking status, n (%) | |||
| Current | 9 (10.2) | 7 (15.9) | 2 (4.5) |
| Former | 29 (33.0) | 13 (29.5) | 16 (36.3) |
| Never | 50 (56.8) | 24 (54.5) | 26 (59.1) |
| Age left education (years), n (%) | 18.5 (5.3) | 19.4 (7.0)a | 17.7 (2.7) |
| ≤ 16 | 40 (46.5) | 18 (42.9) | 22 (50.0) |
| 17–18 | 22 (25.6) | 9 (21.4) | 13 (29.5) |
| > 18 | 24 (27.9) | 15 (35.7) | 9 (20.5) |
| Index of Multiple Deprivation, mean rank (median decile) | 17,192 (5.5) | 17,231 (6.5) | 17,212 (5) |
FIGURE 5.
A CONSORT (Consolidated Standards of Reporting Trials) flow diagram for the My Breathing Matters feasibility trial. This figure has been reproduced with permission from Ainsworth et al. 70 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
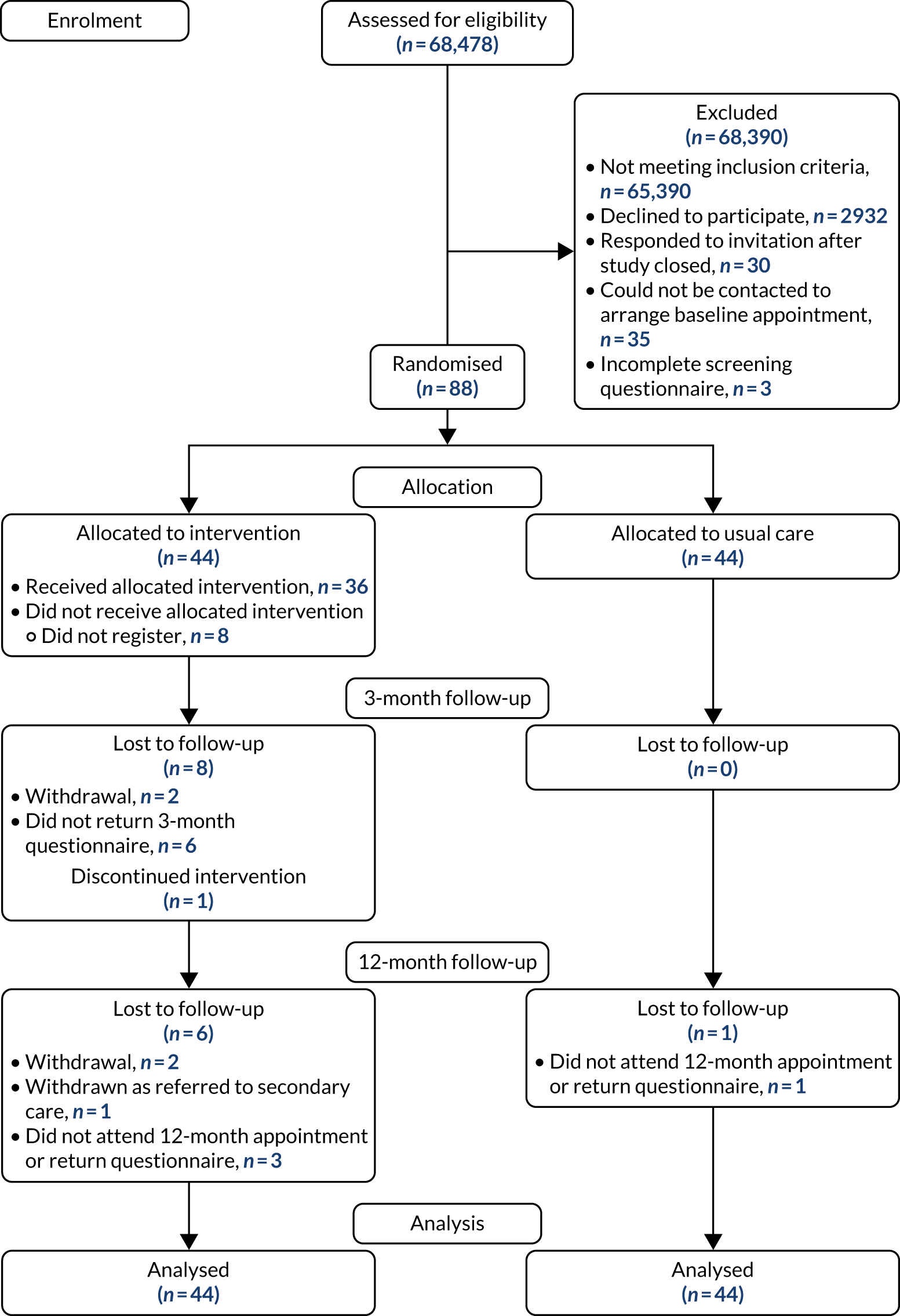
The mean Mini AQLQ and ACQ scores (and SDs) and the percentage of patients achieving a minimal clinically important difference improvement in Mini AQLQ scores of ≥ 0.5 at each time point are presented in Table 12. Patients in the intervention group who completed the 12-month follow-up measures (n = 36) had mean improvement in asthma-related quality of life (i.e. Mini AQLQ score) of 0.35 (95% CI 0.10 to 0.60), compared with an improvement of 0.21 (95% CI –0.09 to 0.51) in the control group. The between-group difference (controlling for baseline differences) was 0.18 higher (95% CI –0.21 to 0.56) in the intervention group, indicating better quality of life. In the ACQ 12-month analysis, the between-group ACQ score was 0.14 lower (95% CI –0.40 to 0.11) in the intervention group, indicating better control. These findings are not significant, but indicate consistent trends to improvement in both asthma quality of life and asthma control in the intervention group when compared with the control group. There was no statistically significant difference in the number of patients who showed minimal clinically important difference improvement at 3 or 12 months across groups. There was no suggestion of an effect on physiological measures of lung function. The data quality check, and subsequent examination by research team clinicians, found that reviews completed by the practice nurses lacked the detail to quantify the amount of medication prescribed. Specifically, the reviews did not provide information on the number of inhalers issued on each prescription or specify the device (e.g. metered dose inhaler or dry powder inhaler) prescribed in enough detail. In any future trial, data collection plans would need to ensure that these data were collected.
| Measure | Intervention group | Control group | ||||
|---|---|---|---|---|---|---|
| Baseline (n = 44) | 3 months (n = 36) | 12 months (n = 37) | Baseline (n = 44) | 3 months (n = 44) | 12 months (n = 43) | |
| Mini AQLQ, mean (SD) | 4.85 (0.94) | 5.51 (0.85) | 5.29 (0.98) | 4.78 (1.09) | 5.30 (1.07) | 5.00 (1.25) |
| Mini AQLQ minimal clinically important difference improvement (%) | 47.2 | 38.9 | 47.7 | 39.5 | ||
| ACQ, mean (SD) | 1.35 (0.66) | 0.98 (0.65) | 1.00 (0.59) | 1.56 (0.91) | 1.28 (0.87) | 1.26 (0.69) |
Implications
Our findings are that a fully powered confirmatory RCT to demonstrate the effectiveness of the My Breathing Matters intervention is feasible, requiring only minor modifications to trial procedures. In addition to this, the observed trends towards improved asthma control and quality of life in patients who were randomised to the My Breathing Matters intervention support the need for a confirmatory RCT.
Mixed-methods process evaluation to explore the acceptability of the My Breathing Matters intervention
Aim
-
To assess feasibility and acceptability of the My Breathing Matters intervention and to highlight any future modifications to optimise for a Phase III RCT.
Methods
Intervention usage data were collected to describe patters of intervention usage for all intervention participants (n = 44) over the 12-month study period. A My Breathing Matters satisfaction questionnaire was administered to participants who used the intervention (n = 36) at 12-month follow-up to assess their satisfaction with the intervention. The questionnaire included two items to assess benefits/disadvantages of using the intervention, as well as open questions to report any further benefits and disadvantages. The one-item NHS Friends and Family Test assessed how likely participants are to recommend the intervention to friends and family if they needed similar care and treatment. The Friends and Family Test used a five-point Likert scale, ranging from ‘extremely likely’ to ‘extremely unlikely’, with a ‘do not know’ option. 74
At 3-month follow-up, retrospective semistructured telephone interviews were carried out with 18 intervention participants to explore intervention participants’ views on the intervention content and design, reasons for any non-usage and any changes users experienced. Qualitative interview data were analysed using inductive thematic analysis. 41
Results
Of the participants in the intervention group, 81.8% (n = 36) logged in at least once and between 1 and 25 times (median 4; interquartile range 8). When given the choice, most users (71%) chose to look at the non-pharmacological content first (instead of the pharmacological content) and the breathing retraining module was the most viewed component, with over half of participants signing up to the breathing retraining challenge. Eighty-six per cent (n = 31) of participants indicated that they gained benefit from using the My Breathing Matters intervention and 78% (n = 28) reported that there were no, or very few, disadvantages to using the intervention. Seventy-eight per cent (n = 28) of participants said that they would recommend the My Breathing Matters intervention to friends and family if they needed similar care and treatment.
Overall, interview participants expressed positive views of the intervention and found the content easy to understand and the website easy to use. Users reported gaining several benefits from taking part in the intervention, including improvements in their asthma symptoms (e.g. reduced coughing, chest tightness and breathlessness); medication use (e.g. improved medication adherence, correct use of their inhalers and reduction in reliever inhaler use); breathing awareness, technique and posture; and identification, and ability to deal with, asthma triggers (e.g. air pollution). Participants particularly liked that the My Breathing Matters intervention provided alternative non-pharmacological strategies for managing their asthma and, consistent with the findings of the usage analysis, the breathing retraining was particularly popular.
In the My Breathing Matters intervention, content was not available all at once, rather different content was ‘unlocked’ at various time points after the user’s first visit to the website to encourage long-term engagement with the intervention. Participants’ views on this design feature were mixed. On the one hand, some participants liked this feature as it meant that the intervention content was more digestible when made available in stages and it encouraged them to practice each individual exercise fully before moving onto the next. On the other hand, some participants found the feature frustrating and annoying when they were unable to access content they wanted to view or were unclear why this feature was important.
The qualitative interviews also highlighted several factors that influenced users’ engagement with the intervention. Participants’ engagement with the My Breathing Matters intervention was influenced by their perceptions of their asthma control, their current self-management practices, the season, the time since diagnosis, their confidence with, and dislike of, computers, and their other health priorities.
Implications
The findings demonstrated that the My Breathing Matters intervention was feasible and acceptable to adults with asthma. The findings also highlighted one important future modification. Future versions of the intervention will keep the current information structure, but will provide users with an explanation for why the unlocking feature is important (e.g. it is helpful to practise easier breathing exercises before progressing onto harder ones). Participants who are still keen to progress will have the option to unlock additional content themselves so that no content is restricted. The findings also identified the type of people who would benefit more from the intervention, such as those who perceive their asthma control to be problematic and those who are motivated to improve their self-management practices.
Conclusion
Overall, the findings demonstrated that a fully powered confirmatory RCT to assess the effectiveness of the My Breathing Matters intervention is feasible. The outcomes evaluation supported the need for a confirmatory RCT, with some optimisation of specific trial procedures for recording health utilisation data to improve the quality of the data collected for a health economics analysis. The My Breathing Matters intervention appeared to be acceptable to adults with asthma and there was good user engagement with the intervention. Future iterations of the intervention should include modifications to its information structure to facilitate easy, but structured, access to content important to users.
Chapter 6 Conclusions
Objectives
Our objectives (see Chapter 1) were to develop and trial DIs to support patient self-management of two common contrasting health conditions, that is, hypertension and asthma (objectives 3 and 4, WS2 and WS3). Through the process of planning, developing and evaluating these interventions, we also aimed to generate a better understanding of what features and methods for implementing DIs could make them acceptable, feasible, effective and cost-effective to integrate into primary care (objectives 1 and 2, WS1).
Workstream 2 and WS3 fully achieved the aims of developing and trialling interventions that proved acceptable, feasible and (in the fully trialled intervention) also cost-effective. In-depth theory-, evidence- and person-based planning and development processes were undertaken for both interventions, drawing on extensive qualitative research, PPI input, review of the evidence and behavioural analysis to ensure that key behavioural barriers to engaging with the target behaviours were addressed. Feasibility studies were successfully carried out for both DI, as an internal pilot trial for the hypertension DI and as a standalone feasibility study for the asthma DI. Both studies suggested that fully powered RCTs were feasible, with only minor modifications to the intervention and the asthma health utilisation data collection methods.
A RCT recruited 622 patients in primary care to explore the clinical effectiveness and cost-effectiveness of the DI for hypertension. Eighty-nine per cent of patients completed the 12-month follow-up and the DI was found to be effective, with the intervention group having significantly lower BP at 12 months than the usual-care group. The intervention cost was £11 per unit reduction in systolic BP, which is less than what is reported previous similar studies. 50,51
Chapter structure
Implications of our findings for future digital health intervention research reflects on the generalisable insights that we obtained through this programme of research into how to develop engaging and useful DIs using the PBA, theory and evidence. Implications of our findings for integrating digital interventions for hypertension and asthma into primary care then considers the clinical implications of our research in terms of hypertension and asthma, and The contribution of patient and public involvement provides a reflection on the important contributions PPI made to this programme. Finally, Strengths and limitations describes some strengths and limitations of the research programme, and Summary and recommendations for future research summarises the recommendations for future research.
Implications of our findings for future digital health intervention research
Reflections on what was learned from the intervention development process
A specific aim of the DIPSS research programme was to identify key features associated with maximising the feasibility, acceptability (to patients and health professionals), clinical effectiveness and cost-effectiveness of DIs. In this respect, the research programme was extremely fruitful, as it provided valuable opportunities for our research team to explicitly articulate and refine the PBA that we were using to develop the interventions.
Prior to the DIPSS programme of research, we had employed core elements of the PBA to develop several interventions, but had not systematically described these methods. These methods included using a combination of primary qualitative research and behavioural theory to inform initial intervention development,75 developing ‘guiding principles’ that captured the key design aims and strategies for enhancing engagement with the intervention,76 and using ‘think-aloud’ interviews to enhance the accessibility, acceptability and persuasiveness of the intervention. 77–79 Once the DIs developed using these methods had completed trialling and process analysis, we had evidence that our interventions had a remarkably good rate of success in terms of both effectiveness40,69,80 and accessibility and acceptability. 81,82 This gave us confidence that our methods might be useful to other intervention developers. Therefore, we published an initial paper36 setting out the rationale and core methods of the PBA. We then used the opportunities provided by the DIPSS programme to refine and illustrate our PBA methods whenever possible.
The most substantial novel work undertaken to illustrate and document the application of the PBA was the intervention planning for the HOME BP intervention for management of hypertension (see Chapter 2). This intervention-planning process integrated the PBA with theory- and evidence-based approaches to intervention planning,30 and clearly delineated how these approaches made important complementary contributions to intervention planning. To identify probable barriers to and facilitators of uptake, engagement and implementation evidence was collated from a mixed-methods feasibility study, a systematic review of quantitative evidence and a synthesis of qualitative research. The evidence was then used to inform the guiding principles for intervention design, and provided input to our behavioural analysis and logic model. As the DIPSS team included leading theory-based researchers in the fields of health psychology and sociology, this paper was able to convincingly demonstrate how the PBA could be combined with mapping intervention elements onto the theoretical constructs from both disciplines.
As well as refining these intervention-planning techniques, the HOME BP intervention also provided the opportunity to enhance early techniques for developing interventions. Conducting detailed qualitative research during intervention development had already been identified as essential by the research team to inform how best to optimise interventions to overcome specific barriers arising for a population within a certain context. 83 However, a clear system for making decisions about which changes to make and recording these decisions had not yet been developed. In the HOME BP intervention, our method for recording and documenting decisions about how to optimise the intervention was systematised using an early version of the Table of Changes, which is now a core component of the PBA. The Table of Changes is a tool that offers researchers a method for categorising the reason for a change as important, easy, responding to repeated feedback or in line with stakeholder experience or the literature. 31 The Table of Changes also has the option to record if a change was not made, and why. Criteria are used for prioritising changes, identifying which are essential to promote behaviour change and which are just desirable but unlikely to impact on intervention outcomes. 84
During the period of this programme grant, we started to actively disseminate the PBA to the wider research and intervention development community by a variety of methods. As planned in the DIPSS proposal, we held three workshops funded by the DIPSS grant and used these workshops to illustrate the methods and the value of the PBA for developing the DIPSS interventions. We also presented the use of the PBA at conferences through symposia, workshops and individual papers, and we now have a dedicated website [URL: www.personbasedapproach.org (accessed 8 August 2022)] and newsletter to update the research community on the latest developments in the approach (see Report Supplementary Material 2 for a full list of dissemination events). We have found the research community very receptive to, and appreciative of, the PBA methods, and our discussions of our methods at these workshops and presentations have stimulated and helped us to develop our methods further. As the PBA has become more widely known, the PBA has, in turn, directly informed development of more generic national guidance, such as the Medical Research Council-funded INDEX guidance (see Chapters 2 and 4) and the Public Health England guidance. 25,85
The PBA evolved considerably during the course of the DIPSS research programme and continues to evolve. In particular, we have recently been focusing our attention on how best to combine stakeholder and PPI input with our PBA qualitative research methods. A useful comment from one of our PPI contributors when writing this report was that they had not felt aware of the PBA process or how they did and could contribute to it. In future, we need to introduce the aims and methods of the PBA process explicitly to PPI contributors and explain to PPI contributors how their comments on the design and qualitative findings are integrated into the PBA development process. Integrating PPI more explicitly with the PBA will be facilitated, structured and documented in future by expanding the behavioural analysis table, which informs intervention development into a template similar to the ‘Table of Changes’ and can be used at the design stage to integrate all sources of evidence informing design, including stakeholder views.
In addition to our methods for developing DIs, some of the intervention elements have proven useful across both interventions in the DIPSS programme, and for developing other interventions. In this respect, it has proved useful to not only deploy common BCTs across different interventions, but to also preserve, as far as possible, the insights we gained from our PBA work into how these techniques can be made most accessible and engaging. For example, we have learned how to simplify the format for goal-setting, self-monitoring and tailored feedback so that it is quick and easy for users to set realistic but useful goals and to receive feedback that matches their self-perceived progress. We have also developed a brief quiz format that provides an engaging method of communicating positive messages about consequences of health-related behaviour. Both these behavioural modules needed only minor modification to form a well-received part of both our DIPSS interventions, and are now also being used in numerous other DIs developed by our team and collaborators. Larger modules developed by our team are also being adapted and used in a large number of further interventions, including the modules to support physical activity and healthy eating for a range of health conditions (e.g. to promote quality of life in cancer survivors and to reduce cognitive decline in older people). We are currently building collaborations to disseminate these modules widely for clinical use, for example through new applied research collaborations and through partnerships with the private sector.
Preserving the positive holistic qualities of our interventions and their components is consistent with realist approaches to health interventions, which predict that effects of interventions may result from emergent properties of the whole intervention package and could, therefore, be altered if behaviour change elements are isolated and delivered in isolation or in a different format. However, changes in delivery format for DIs are inevitable as the technology for delivering them changes. A challenge for the future is to determine how to identify and preserve the important characteristics of DIs across digital delivery formats. 86
Implications of our findings for integrating digital interventions for hypertension and asthma into primary care
Implications of our findings for future research and practice in hypertension
The main results suggest that the HOME BP intervention, by using very efficient digital support and minimal staff resource, is clinically effective and cost-effective, as well as both feasible and acceptable for clinicians and patients. The reduction in BP found in this trial is important in terms of long-term health outcomes, with an anticipated reduction of 10–15% of patients suffering a stroke and 5–10% of patients experiencing coronary-related events. The effect size was similar to a paper-based BP management intervention,15 and the 12-month follow-up showed a greater difference between groups than the 6-month follow-up, suggesting that the intervention may have a longer-term impact. Therefore, it is potentially scalable for use in the NHS, although further economic evaluations of the long-term cost-effectiveness will better inform the potential for widespread adoption.
Current plans for wider implementation of the HOME BP algorithm for managing BP include a collaboration via the Oxford/Thames Valley Applied Research Centre for an implementation trial, sharing the results from the HOME BP intervention with those from the previous TASMINH2 and TASMIN-SR (Targets and Self-Management for the Control of Blood Pressure in Stroke and at Risk Groups) trials of self-management and the recently published trial of titration using self or telemonitored monitoring of BP. 15,33,87 This collaboration will, in turn, link to the planned national strategy for cardiovascular prevention.
At the end of the HOME BP project, we held a public engagement event to share our findings with a range of stakeholders in BP management and held discussions about possible next steps. A half-day dissemination workshop was organised, which was advertised via local general practice patient groups, Clinical Research Networks and Blood Pressure UK, and was open to anyone to register via Eventbrite (San Francisco, CA, USA). Targeted invites were also sent to all GPs and nurses who took part in the trial, as well as people working in digital health or BP management within the NHS, Public Health England, Blood Pressure UK, the British and Irish Hypertension Society and NICE. Eleven attendees were present, including a PPI contributor, three nurse practitioners with a special interest in hypertension, two GPs, a digital health tools designer and a policy-maker from Public Health England.
There was excellent participation in interactive activities throughout the event, with rich discussions between the various stakeholders. It was perceived that the HOME BP intervention was highly relevant for primary care, given the current focus on self-management and improving cardiovascular outcomes, and that it could contribute to a cultural shift where regular BP checks and ‘knowing your numbers’ is perceived to be as routine as regularly attending the dentist for check-ups.
There was enthusiasm for implementing the HOME BP intervention more widely, including suggestions for potential application in care homes, secondary care and for patients with carers, as well as in a primary care population. It was suggested that self-monitoring could be prompted monthly until a patient is well controlled, and then less frequently or when triggered by a change in circumstances, such as developing another health condition. Suggestions for ways to increase intervention feasibility included involving pharmacies in the medication change process, and managing patients’ expectations about side effects and BP variability at the outset. Practical barriers to wider implementation identified by stakeholders included the restriction on prescribing BP monitors for patients, the nurse time involved in sending monthly support e-mails to patients (which it was suggested could be overcome by automating this process) and the issue of the HOME BP intervention being unable to interact with existing medical records systems. Feedback at the end of the event suggested that people had enjoyed the workshop and found it interesting to hear the perspectives of other stakeholders.
The findings from the HOME BP process evaluation suggest that the intervention was both acceptable and feasible. The findings also highlighted simple ways to make the intervention even more effective in wider implementation. The process evaluation has already been used to inform the development of a DI for stroke patients and their carers to self-manage BP in primary care. 88 The intervention was based on the same procedures and algorithms that informed the HOME BP intervention,15,33 but changes were made to optimise engagement and adherence following insights gained from the HOME BP process evaluation. For example, the baseline medication review has been adapted to include a guided discussion between the GP and patient to manage expectations about the likelihood of medication change and to increase confidence in medication change for both parties. GP alerts regarding medication change have also been tailored to include additional evidence and rationale designed to overcome common barriers to changing medication, such as the BP readings being borderline. In addition, patients have the choice of sending their readings via SMS, an application or a website, instead of only via a website, which is intended to make the intervention available to a wider group of people and up-to-date with changing technology. The process evaluation of this intervention for stroke patients will enable us to continue learning about how best to optimise DIs for managing high BP.
Implications of our findings for future research and practice in asthma
Since the inception of the DIPSS study, asthma outcomes have remained suboptimal and effective self-management is frequently poorly achieved. Although there are a number of commercial and pharmaceutical industry-sponsored digital asthma programmes available, the programmes have failed to have widespread use or impact. The need for an effective digital self-management support intervention for people with asthma remains pressing, and is supported by our findings from the My Breathing Matters evaluation, which suggest that a fully powered RCT study of the intervention is feasible and justified. In such a trial, the health utilisation data collection methods should be improved to ensure that data quality is sufficient for a health economic analysis. This improvement can be achieved by a trained member of the study team (e.g. a dedicated research nurse) collecting data from patients’ medical records, as has been successfully used in previous studies. 66
In terms of the magnitude of benefits seen in asthma outcomes, as a pilot feasibility study we were not powered to show a significant between-groups difference, and the CIs on our likely primary outcomes (i.e. Mini AQLQ and ACQ) are accordingly wide. However, there are non-significant trends to improvement in both these outcomes, and the mean between-groups improvement we observed in Mini AQLQ score (i.e. 0.18, 95% CI –0.21 to 0.56) can be compared to the between-groups difference in Mini AQLQ scores reported in a meta-analysis by Bateman et al. 89 In a meta analysis of placebo-controlled pharmacological interventions, Bateman et al. 89 reported a between-group difference of 0.06 for short-acting B-agonists, 0.20 for leukotriene receptor antagonists, 0.30 for anti-IgE monoclonal antibody treatment and 0.35 for long-acting beta-agonists treatment for add-on pharmacological treatments in asthma patients receiving inhaled corticosteroids. We would therefore conclude that there is potential for a benefit in patient reported outcomes of an order of magnitude within the range of that seen from commonly used pharmacological treatments, thus warranting a definitive fully powered trial.
Our findings highlighted potential future improvements to the intervention design and trial methodology. On the basis of these findings, we are currently developing a proposal for a fully powered RCT of the My Breathing Matters intervention to be submitted to the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme.
The detailed development and process analysis also has implications for other digital behaviour change interventions in primary care populations with asthma. For example, we were able to develop and disseminate a digital version of the ‘Breathe Freely’ intervention, which is a NIHR HTA programme-funded breathing retraining intervention that drew directly on the breathing retraining resources provided in the My Breathing Matters intervention [available via URL: www.breathestudy.co.uk/ (accessed 8 August 2022)]. Findings from the feasibility study are also directly informing related NIHR-funded work, such as the Breathing REtraining for Asthma Trial of Home Exercises for Teenagers (BREATHE-4T), which is a NIHR Research for Patient Benefit-funded optimisation and feasibility trial of the Breathe Freely intervention for adolescents.
The contribution of patient and public involvement
The aims of PPI involvement in the programme were to ensure that (1) patients’ needs were taken into account throughout the research, (2) the interventions were reassuring, motivating and enjoyable to use, (3) the research studies were accessible and feasible for participants to take part in and (4) the research was more likely to lead to sustainable change. Our PPI contributors were an integral part of all stages of the research cycle, including designing and undertaking the research, and interpreting and disseminating the findings. We worked closely with PPI contributors one to one and during group meetings throughout the programme, as we sought written input on research documents and interventions.
Patient and public involvement contributors provided significant and valuable input throughout the DIPSS research programme and we gained important generalisable learning about how to combine PPI with our theory-, evidence- and person-based approaches. Boxes 1 and 2 share insights into our PPI process. Box 1 gives specific examples of how our HOME BP PPI contributor Cathy Rice improved our research and Box 2 presents Cathy Rice’s own reflections on the process of contributing to the HOME BP intervention.
-
Improving clarity of statements in the participant information sheet for the main trial to avoid ambiguity for the patient.
-
Optimising the e-mail to notify usual-care patients which group they are in to ensure that patients felt valued and understood the next steps.
-
Optimising the e-mail used to invite trial participants to take part in a qualitative process interview to increase uptake and to ensure that all the relevant information was provided to help patients make a decision about whether or not they want to be interviewed.
-
Informing decisions about a new process to share a study flow chart with patients in the trial, in response to patient feedback indicating confusion about the order of trial procedures.
-
Improving the communication of key findings from the patient qualitative process interviews in a newsletter designed for study participants.
-
Revising the letter sent to participants when participants withdraw from the study to improve clarity and reassurance for the patient (see Report Supplementary Material 3 for examples of the letter before and after and it was revised).
-
Modifying the content of intervention training sessions (e.g. how to explain taking readings in the morning).
-
Testing the Getting Active intervention for physical activity and providing feedback on how it could be improved to optimise patient experience.
-
Working closely with the research team to interpret the qualitative process interviews with patients about their experiences of using the HOME BP intervention, and reading the draft manuscript prior to submission.
-
Inputting to discussions about dissemination of the intervention at an interactive stakeholder workshop.
My involvement started in April 2016 when I phoned Kate Morton in response to an ad[vert] on the INVOLVE Research website for a member of the public to join the team.
It made a big difference having an informal pre-meeting with Kate each time I attended a management meeting in person. The first time, we spoke for perhaps an hour and a half, discussing my comments on the patient information leaflet, filling me in on the context of the study and what had happened so far, as well as more social getting to know each other. I remember being particularly impressed that Kate had offered to meet me at the train station, at the end of her working day. I felt this was a clear statement that the research team wanted to make the trip as easy as possible for me, and that members of the public are appreciated.
September 2016 management meeting. This time I suggested we meet at the B&B [bed and breakfast] as I was happy to get the taxi alone, and we went to the same pub for a chat, again perhaps one and a half hours. To me this socialising was important, as it developed our relationship to the extent that we were then easily able to discuss lots of aspects of the study over the phone. (Academics have ‘corridor conversations’ all the time. Even if they’re not based at the same institution, they meet up at conferences, etc., and develop relationships that make it more feasible to collaborate. It’s much harder for public contributors to build up working relationships when we’re on the fringes.)
February 2017 management meeting was the final one I attended in person. Since then, most of the meetings have been by teleconference only, and that has worked well for me. But I wouldn’t have had the confidence to chip in during these meetings if I hadn’t already met Lucy Yardley at the previous meetings, and built a rapport with her and Kate.
My involvement throughout has centred around giving e-mail feedback to documents Kate e-mailed me, and phone conversations with Kate. Because Kate sometimes showed me several iterations of a document, I could quickly see that my comments were taken seriously, and documents changed as a result. This is tremendously good feedback to be receiving, and it makes it feel worthwhile to put the effort in.
Phone conversations have often been useful to tease out different options— I always feel, at the end of a conversation with Kate, that we have ended up at a place that neither of us would have reached alone. It’s so rewarding. She has always been extremely accommodating in arranging for calls to be at a time to suit me, and has always left me to make the choice whether I wanted the conversation, as opposed to email. Last, but certainly not least, Lucy Yardley has made clear by her own behaviour that she believes public contributors should be treated with respect, consideration and appreciation.
Strengths and limitations
Strengths
This programme of work benefited from the inclusion of two diverse patient populations (i.e. asthma and hypertension), which helped to compare findings between these different contexts and further developed our understanding of how self-management DIs can facilitate long-term condition management. In-depth PPI was a strength of the programme, with both projects working closely with dedicated PPI contributors throughout all phases of research, enhancing the relevance and value of the research. Furthermore, rigorous methods included RCTs with nested process evaluation studies, using mixed-methods analysis to develop a more holistic understanding of how patients and HCPs experienced the interventions, including which factors influenced adherence, to better inform the potential for further implementation.
This programme used the PBA to develop the two interventions. A strength of this approach is its use of in-depth qualitative research to provide a detailed understanding of the target population’s beliefs about their health condition and the target behaviours. The PBA helped inform a rigorous intervention optimisation process to ensure that the interventions were as feasible, persuasive and enjoyable for participants to use as possible. Another strength of the PBA is that it is a highly flexible approach that can be used alongside other intervention development approaches to complement the use of theory and evidence, which are important for identifying effective intervention content and features.
Limitations
Although we endeavoured to recruit participants across varied demographics, participants were generally white (asthma study, 96%; hypertension study, 94%; compared with 86% of the population of England and Wales as a whole). 90 Participants in the asthma feasibility study were generally older (median age 61 years; compared with a median age of 39 years for the population of England and Wales as a whole)90 and participants in the process evaluation interview study had high levels of educational attainment (55% of participants had an undergraduate qualification or higher). Furthermore, people invited to the HOME BP study who declined to take part and participants lost to follow-up in the My Breathing Matters study were more likely to be from deprived areas, suggesting a bias towards higher socioeconomic status. These differences suggest that although we sought to make the interventions accessible to as much of the population as possible we were only partially successful. One of the most common reasons for declining to take part in the HOME BP study was lack of internet access, which suggests technological barriers remain an issue, despite steady increases in online access. Although the reach of DIs improves as digital literacy increases nationally, care must be taken to ensure that these DIs do not further facilitate health-care inequalities. Further research is required to investigate if and how it may be possible to overcome barriers to engagement with digital support among people with higher socioeconomic deprivation to ensure that DIs can improve health-care outcomes across the population.
Another limitation of this research in terms of wider implementation is the challenge of integrating DIs with existing clinical systems in primary care. Using a separate digital system increases the burden on HCPs and reduces the feasibility of long-term maintenance of the intervention. It is recommended that wider dissemination of evidence-based effective DIs be supported in primary care.
As is commonly the case in trials of complex behavioural interventions, patients in both RCTs were not blinded and would have known that they were allocated to the intervention rather than the usual-care control. However, our researchers and statisticians were blind to group allocation.
The digital aspects of the HOME BP intervention were challenging to cost accurately. The cost of the DI may be overstated in the trial, as its potential for scale means that it could be used for many more patients at very low marginal cost. Although this is, to some extent, inevitable, the results indicated that such interventions can be provided at a modest cost per patient, which would be very likely to show economies of scale and reduced cost per patient if made widely available.
Summary and recommendations for future research
In summary, the DIPSS research programme achieved the objectives of developing highly acceptable and feasible DIs for the contrasting conditions of asthma and hypertension. As intended, we showed that the intervention for hypertension was both clinically effective and cost-effective, and the intervention for asthma had good potential for a full effectiveness trial. Our research is already beginning to influence future clinical research and practice through further implementation. In addition, the theory-, evidence- and person-based approaches to intervention development that we refined through this research programme were shown to be successful in enabling us to identify and address important contextual barriers to and facilitators of engagement with the intervention by HCPs and patients. Therefore, we have documented, reported and are very actively disseminating these methods to the wider community of intervention developers in the public and private sector.
Recommendations for future research
-
A fully powered RCT of the My Breathing Matters intervention should be carried out to assess clinical effectiveness and cost-effectiveness. To ensure adequate data quality, the health-care utilisation data should be collected from the patients’ medical records by a trained member of the study team (e.g. a trained research nurse), as this has been successful in previous studies. 66
-
For the HOME BP intervention, more comprehensive modelling of the long-term effects of BP reduction would appear to be useful, perhaps supported by a meta-analysis of the relevant trials.
-
Further research is required to investigate if and how it may be possible to overcome barriers to engagement with digital support among people with higher socioeconomic deprivation.
-
For the My Breathing Matters intervention, a process evaluation nested in a fully powered RCT study should assess if quantitative process measures, such as perceptions of asthma, pre-intervention levels of medication adherence and time since diagnosis, are associated with user engagement and asthma outcomes.
-
Intervention evaluations should explore perceived benefits, as well as burdens, for patients using DIs to better understand how to optimise the experience. Developing suitable measures to capture the emotional benefits and burdens of using DIs could complement the existing measures of structural burden, and further enhance our understanding of patients’ experiences.
-
Self-monitoring interventions can be empowering and reassuring, but more research is needed to consider how to sustain engagement when patients are not well controlled and may find self-monitoring a stressful experience.
Implications for health care
-
Our HOME BP study findings suggest that the use of digital support to help patients self-manage their hypertension is not only clinically effective but also cost-effective, and both feasible and acceptable for clinicians and patients. The HOME BP intervention is potentially scalable for use in the NHS.
-
Our asthma feasibility trial findings suggest that there is potential for a benefit in patient-reported outcomes of an order of magnitude within the range of that seen from commonly used pharmacological treatments. However, a definite fully powered RCT is required to confirm this.
-
Careful consideration about how to optimally feedback self-monitored data from DIs to HCPs is needed to promote more feasible integrated digital systems and to reduce the workload in primary care.
-
Using a PBA to complement evidence and theory in developing behaviour change interventions can help ensure that an intervention is feasible, persuasive and enjoyable for the target population, and that key behavioural barriers are addressed.
-
Providing training for patients online rather than face to face minimised the burden on HCPs, and could be a cost-effective option for DIs, provided that the training is developed with thorough patient input to ensure it meets people’s needs.
Acknowledgements
Contributions of authors
Lucy Yardley (https://orcid.org/0000-0002-3853-883X) (Professor, Health Psychology) was the chief investigator, and initiated, led and had overall responsibility for the programme.
Kate Morton (https://orcid.org/0000-0002-6674-0314) (Senior Research Assistant, Health Psychology) was lead researcher on the qualitative meta-ethnography, the qualitative and mixed-methods hypertension process analyses and on PPI engagement for the HOME BP intervention.
Kate Greenwell (https://orcid.org/0000-0002-3662-1488) (Senior Research Fellow, Health Psychology) was lead researcher on the asthma mixed-methods process evaluation.
Beth Stuart (https://orcid.org/0000-0001-5432-7437) (Associate Professor, Medical Statistics) led on the quantitative statistical analysis for both trials and was a member of the Programme Management Group.
Cathy Rice (https://orcid.org/0000-0001-5961-2413) (PPI contributor) provided PPI input throughout the research programme, and drafted and revised the relevant PPI sections of this report (see Plain English summary and Chapter 6).
Katherine Bradbury (https://orcid.org/0000-0001-5513-7571) (Senior Research Fellow, Health Psychology) co-led the hypertension intervention development and supervised the qualitative HOME BP process analyses.
Ben Ainsworth (https://orcid.org/0000-0002-5098-1092) (Lecturer, Health Psychology) was lead researcher on the development of the My Breathing Matters intervention and the asthma feasibility trial.
Rebecca Band (https://orcid.org/0000-0001-5403-1708) (Senior Research Fellow, Health Psychology) co-led the development of the HOME BP intervention and the HOME BP quantitative process evaluation.
Elizabeth Murray (https://orcid.org/0000-0002-8932-3695) (Professor, e-Health and Primary Care) led the hypertension systematic review and was a member of overall the Programme Management Group, contributing expertise in implementing DIs in primary care.
Frances Mair (https://orcid.org/0000-0001-9780-1135) (Professor, General Practice and Primary Care) led the asthma systematic review and was a member of overall the Programme Management Group, contributing expertise in implementing DIs in primary care.
Carl May (https://orcid.org/0000-0002-0451-2690) (Professor, Medical Sociology) was a member of the Programme Management Group, contributing expertise in NPT and implementation of DIs.
Susan Michie (https://orcid.org/0000-0003-0063-6378) (Professor, Health Psychology) was a member of the Programme Management Group, contributing expertise in BCTs.
Samantha Richards-Hall (https://orcid.org/0000-0002-7665-4268) (PPI contributor) was a member of the Programme Management Group, providing public contributor perspectives throughout the research programme.
Peter Smith (https://orcid.org/0000-0003-4423-5410) (Professor, Social Statistics) was a member of the Programme Management Group, providing senior expertise in statistical methods.
Anne Bruton (https://orcid.org/0000-0002-4550-2536) (Professor, Respiratory Rehabilitation) was a member of the Programme Management Group and co-led the asthma workstream, including the intervention development.
James Raftery (https://orcid.org/0000-0003-1094-8578) (Professor, HTA) was a member of the Programme Management Group and led the health economics analysis.
Shihua Zhu (https://orcid.org/0000-0002-1430-713X) (Senior Research Fellow, Health Economics) assisted with the health economics analysis.
Mike Thomas (https://orcid.org/0000-0001-5939-1155) (Professor, Primary Care Research) was a member of the Programme Management Group and led the asthma workstream.
Richard J McManus (https://orcid.org/0000-0003-3638-028X) (Professor, Primary Care) was a member of the Programme Management Group and led the hypertension workstream.
Paul Little (https://orcid.org/0000-0003-3664-1873) (Professor, Primary Care Research) was a member of the Programme Management Group, helped initiate and oversee the programme, and co-led the hypertension workstream.
All authors made substantial contributions to design, or acquisition of data, analysis and interpretation of data, all authors were involved in the drafting of the manuscript or revising it critically for important intellectual content, and all authors approved the final version of the manuscript to be published.
Funding
The HOME BP and My Breathing Matters interventions were developed using LifeGuide software, which was partly funded by the NIHR Southampton Biomedical Research Centre (BRC).
Publications
McLean G, Murray E, Band R, Saunderson K, Hanlon P, Little P, et al. Digital interventions to promote self-management in adults with hypertension: protocol for systematic review and meta-analysis. JMIR Res Protoc 2015;4:e133. https://doi.org/10.2196/resprot.4648
Band R, Morton K, Stuart B, Raftery J, Bradbury K, Yao GL, et al. Home and Online Management and Evaluation of Blood Pressure (HOME BP) digital intervention for self-management of uncontrolled, essential hypertension: a protocol for the randomised controlled HOME BP trial. BMJ Open 2016;6:e012684. https://doi.org/10.1136/bmjopen-2016-012684
McLean G, Band R, Saunderson K, Hanlon P, Murray E, Little P, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens 2016;34:600–12. https://doi.org/10.1097/HJH.0000000000000859
McLean G, Murray E, Band R, Moffat KR, Hanlon P, Bruton A, et al. Interactive digital interventions to promote self-management in adults with asthma: systematic review and meta-analysis. BMC Pulm Med 2016;16:83. https://doi.org/10.1186/s12890-016-0248-7
Band R, Bradbury K, Morton K, May C, Michie S, Mair FS, et al. Intervention planning for a digital intervention for self-management of hypertension: a theory-, evidence- and person-based approach. Implement Sci 2017;12:25. https://doi.org/10.1186/s13012-017-0553-4
Bradbury K, Morton K, Band R, May C, McManus R, Little P, Yardley L. Understanding how primary care practitioners perceive an online intervention for the management of hypertension. BMC Med Inform Decis Mak 2017;17:5. https://doi.org/10.1186/s12911-016-0397-x
Morton K, Dennison L, May C, Murray E, Little P, McManus RJ, Yardley L. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns 2017;100:616–35. https://doi.org/10.1016/j.pec.2016.10.019
Bradbury K, Morton K, Band R, van Woezik A, Grist R, McManus RJ, et al. Using the Person-Based Approach to optimise a digital intervention for the management of hypertension. PLOS ONE 2018;13:e0196868. https://doi.org/10.1371/journal.pone.0196868
Morton K, Dennison L, Bradbury K, Band RJ, May C, Raftery J, et al. Qualitative process study to explore the perceived burdens and benefits of a digital intervention for self-managing high blood pressure in Primary Care in the UK. BMJ Open 2018;8:e020843. https://doi.org/10.1136/bmjopen-2017-020843
Ainsworth B, Greenwell K, Stuart B, Raftery J, Mair F, Bruton A, et al. Feasibility trial of a digital self-management intervention ‘My Breathing Matters’ to improve asthma-related quality of life for UK primary care patients with asthma. BMJ Open 2019;9:e032465. https://doi.org/10.1136/bmjopen-2019-032465
Greenwell K, Ainsworth B, Bruton A, Murray E, Russell D, Thomas M, Yardley L. Mixed methods process evaluation of my breathing matters, a digital intervention to support self-management of asthma. NPJ Prim Care Respir Med 2021;31:35. https://doi.org/10.1038/s41533-021-00248-6
McManus RJ, Little P, Stuart B, Morton K, Raftery J, Kelly J, et al. Home and Online Management and Evaluation of Blood Pressure (HOME BP) using a digital intervention in poorly controlled hypertension: randomised controlled trial. BMJ 2021;372:m4858. https://doi.org/10.1136/bmj.m4858
Morton K, Dennison L, Band R, Stuart B, Wilde L, Cheetham-Blake T, et al. Implementing a digital intervention for managing uncontrolled hypertension in Primary Care: a mixed methods process evaluation. Implement Sci 2021;16:57. https://doi.org/10.1186/s13012-021-01123-1
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Office for National Statistics . Internet Users, UK: 2019 2019. www.ons.gov.uk/businessindustryandtrade/itandinternetindustry/bulletins/internetusers/2019 (accessed 3 June 2019).
- European Parliament . General Data Protection Regulation 2018.
- National Institute for Health and Care Excellence . Evidence Standards Framework for Digital Health Technologies 2019. www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies (accessed 3 June 2019).
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217-23. https://doi.org/10.1016/S0140-6736(05)17741-1.
- NHS Digital . Health Survey for England 2017 [NS] 2018. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2017 (accessed 26 July 2022).
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338. https://doi.org/10.1136/bmj.b1665.
- Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens 2008;10:644-6. https://doi.org/10.1111/j.1751-7176.2008.08329.x.
- Guthrie B, Inkster M, Fahey T. Tackling therapeutic inertia: role of treatment data in quality indicators. BMJ 2007;335:542-4. https://doi.org/10.1136/bmj.39259.400069.AD.
- Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens 2006;8:174-80. https://doi.org/10.1111/j.1524-6175.2006.04872.x.
- Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009;151:687-95. https://doi.org/10.7326/0003-4819-151-10-200911170-00148.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011;57:29-38. https://doi.org/10.1161/HYPERTENSIONAHA.110.160911.
- AbuDagga A, Resnick HE, Alwan M. Impact of blood pressure telemonitoring on hypertension outcomes: a literature review. Telemed J E Health 2010;16:830-8. https://doi.org/10.1089/tmj.2010.0015.
- Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev 2010;3. https://doi.org/10.1002/14651858.CD005182.pub4.
- Bosworth HB, Olsen MK, McCant F, Harrelson M, Gentry P, Rose C, et al. Hypertension Intervention Nurse Telemedicine Study (HINTS): testing a multifactorial tailored behavioral/educational and a medication management intervention for blood pressure control. Am Heart J 2007;153:918-24. https://doi.org/10.1016/j.ahj.2007.03.004.
- McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet 2010;376:163-72. https://doi.org/10.1016/S0140-6736(10)60964-6.
- Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002389.
- National Institute for Health and Care Excellence . Hypertension in Adults: Diagnosis and Management 2019. www.nice.org.uk/guidance/cg127 (accessed 27 July 2021).
- Department of Health and Social Care . An Outcomes Strategy for People With Chronic Obstructive Pulmonary Disease (COPD) and Asthma in England 2011. www.gov.uk/government/publications/an-outcomes-strategy-for-people-with-chronic-obstructive-pulmonary-disease-copd-and-asthma-in-england (accessed 27 July 2021).
- Levy M, Andrews R, Buckingham R, Evans H, Francis C, Houston R, et al. Why Asthma Still Kills. The National Review of Asthma Deaths (NRAD) 2014. www.asthma.org.uk/globalassets/campaigns/nrad-full-report.pdf (accessed 27 July 2021).
- Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003;1. https://doi.org/10.1002/14651858.CD001117.
- British Thoracic Society . British Guideline on the Management of Asthma 2012. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (accessed 27 July 2021).
- Adams RJ, Smith BJ, Ruffin RE. Factors associated with hospital admissions and repeat emergency department visits for adults with asthma. Thorax 2000;55:566-73. https://doi.org/10.1136/thorax.55.7.566.
- Royal College Physicians . Why Asthma Still Kills. The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report 2014. www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills (accessed 27 October 2022).
- Thomas M, Price D. Impact of comorbidities on asthma. Expert Rev Clin Immunol 2008;4:731-42. https://doi.org/10.1586/1744666X.4.6.731.
- O’Cathain A, Croot E, Duncan E, Rousseau N, Sworn K, Turner K, et al. Guidance on how to develop complex interventions to improve health and health care. BMJ 2019;9. https://doi.org/10.1136/bmjopen-2019-029954.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Cargo M, Harris J, Pantoja T, Booth A, Harden A, Hannes K, et al. Cochrane Qualitative and Implementation Methods Group guidance series-paper 4: methods for assessing evidence on intervention implementation. J Clin Epidemiol 2018;97:59-6. https://doi.org/10.1016/j.jclinepi.2017.11.028.
- McLean G, Band R, Saunderson K, Hanlon P, Murray E, Little P, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens 2016;34:600-12. https://doi.org/10.1097/HJH.0000000000000859.
- Morton K, Dennison L, May C, Murray E, Little P, McManus RJ, et al. Using digital interventions for self-management of chronic physical health conditions: a meta-ethnography review of published studies. Patient Educ Couns 2017;100:616-35. https://doi.org/10.1016/j.pec.2016.10.019.
- Band R, Bradbury K, Morton K, May C, Michie S, Mair FS, et al. Intervention planning for a digital intervention for self-management of hypertension: a theory-, evidence- and person-based approach. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0553-4.
- Bradbury K, Morton K, Band R, van Woezik A, Grist R, McManus RJ, et al. Using the person-based approach to optimise a digital intervention for the management of hypertension. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0196868.
- Bradbury K, Morton K, Band R, May C, McManus R, Little P, et al. Understanding how primary care practitioners perceive an online intervention for the management of hypertension. BMC Med Inform Decis Mak 2017;17. https://doi.org/10.1186/s12911-016-0397-x.
- McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014;312:799-808. https://doi.org/10.1001/jama.2014.10057.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4055.
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827-38. https://doi.org/10.1016/0140-6736(90)90944-Z.
- Noblit GW, Hare RD. Meta-Ethnography: Synthesizing Qualitative Studies. Newbury Park, CA: SAGE Publications Ltd; 1988.
- Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Decis Process 1991;50:248-87. https://doi.org/10.1016/0749-5978(91)90022-L.
- Little P, Stuart B, Hobbs FR, Kelly J, Smith ER, Bradbury KJ, et al. An internet-based intervention with brief nurse support to manage obesity in primary care (POWeR+): a pragmatic, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:821-8. https://doi.org/10.1016/S2213-8587(16)30099-7.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Smith E, Bradbury K, Scott L, Steele M, Little P, Yardley L. Providing online weight management in primary care: a mixed methods process evaluation of healthcare practitioners’ experiences of using and supporting patients using POWeR. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0596-6.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Baxter S, Killoran A, Kelly MP, Goyder E. Synthesizing diverse evidence: the use of primary qualitative data analysis methods and logic models in public health reviews. Public Health 2010;124:99-106. https://doi.org/10.1016/j.puhe.2010.01.002.
- Leventhal H, Brissette I, Cameron LD, Leventhal H. The Self-Regulation of Health and Illness behaviour. New York, NY: Routledge; 2012.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- McManus RJ, Little P, Stuart B, Morton K, Raftery J, Kelly J, et al. Home and Online Management and Evaluation of Blood Pressure (HOME BP) using a digital intervention in poorly controlled hypertension: randomised controlled trial. BMJ 2021;372. https://doi.org/10.1136/bmj.m4858.
- Morton K, Dennison L, Bradbury K, Band RJ, May C, Raftery J, et al. Qualitative process study to explore the perceived burdens and benefits of a digital intervention for self-managing high blood pressure in primary care in the UK. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020843.
- Morton K, Dennison L, Band R, Stuart B, Wilde L, Cheetham-Blake T, et al. Implementing a digital intervention for managing uncontrolled hypertension in primary care: a mixed methods process evaluation. Implement Sci 2021;16. https://doi.org/10.1186/s13012-021-01123-1.
- Monahan M, Jowett S, Nickless A, Franssen M, Grant S, Greenfield S, et al. Cost-effectiveness of telemonitoring and self-monitoring of blood pressure for antihypertensive titration in primary care (TASMINH4). Hypertension 2019;73:1231-9. https://doi.org/10.1161/HYPERTENSIONAHA.118.12415.
- Kaambwa B, Bryan S, Jowett S, Mant J, Bray EP, Hobbs FD, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a cost-effectiveness analysis. Eur J Prev Cardiol 2014;21:1517-30. https://doi.org/10.1177/2047487313501886.
- Charmaz K. Constructing Grounded Theory. London: SAGE Publications Ltd; 2014.
- Eton DT, Yost KJ, Lai JS, Ridgeway JL, Egginton JS, Rosedahl JK, et al. Development and validation of the Patient Experience with Treatment and Self-management (PETS): a patient-reported measure of treatment burden. Qual Life Res 2017;26:489-503. https://doi.org/10.1007/s11136-016-1397-0.
- Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res 2000;42:241-7. https://doi.org/10.1016/S0920-9964(99)00130-9.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1-24. https://doi.org/10.1080/08870449908407311.
- Bandura A, Urdan T, Pajares F. Self-efficacy Beliefs of Adolescents. Greenwich: Age Information Publishing; 2006.
- Howie JG, Heaney DJ, Maxwell M, Walker JJ. A comparison of a Patient Enablement Instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam Pract 1998;15:165-71. https://doi.org/10.1093/fampra/15.2.165.
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341. https://doi.org/10.1136/bmj.c4587.
- NHS England . GP Contract Documentation 2018 19 2018. www.england.nhs.uk/gp/investment/gp-contract/gp-contract-documentation-2018-19/ (accessed 30 September 2022).
- McLean G, Murray E, Band R, Moffat KR, Hanlon P, Bruton A, et al. Interactive digital interventions to promote self-management in adults with asthma: systematic review and meta-analysis. BMC Pulm Med 2016;16. https://doi.org/10.1186/s12890-016-0248-7.
- Yardley L, Ainsworth B, Arden-Close E, Muller I. The person-based approach to enhancing the acceptability and feasibility of interventions. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0033-z.
- Greenwell K, Ainsworth B, Bruton A, Murray E, Russell D, Thomas M, et al. Mixed methods process evaluation of my breathing matters, a digital intervention to support self-management of asthma. NPJ Prim Care Respir Med 2021;31. https://doi.org/10.1038/s41533-021-00248-6.
- Anhøj J, Nielsen L. Quantitative and qualitative usage data of an internet-based asthma monitoring tool. J Med Internet Res 2004;6. https://doi.org/10.2196/jmir.6.3.e23.
- Morrison D, Mair FS, Chaudhuri R, McGee-Lennon M, Thomas M, Thomson NC, et al. Details of development of the resource for adults with asthma in the RAISIN (Randomized trial of an Asthma Internet Self-management InterventioN) study. BMC Med Inform Decis Mak 2015;15. https://doi.org/10.1186/s12911-015-0177-z.
- Morrison D, Wyke S, Saunderson K, McConnachie A, Agur K, Chaudhuri R, et al. Findings from a pilot Randomised trial of an Asthma Internet Self-management Intervention (RAISIN). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-009254.
- Bruton A, Lee A, Yardley L, Raftery J, Arden-Close E, Kirby S, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med 2018;6:19-28. https://doi.org/10.1016/S2213-2600(17)30474-5.
- Brown J, Michie S, Geraghty AW, Yardley L, Gardner B, Shahab L, et al. Internet-based intervention for smoking cessation (StopAdvisor) in people with low and high socioeconomic status: a randomised controlled trial. Lancet Respir Med 2014;2:997-1006. https://doi.org/10.1016/S2213-2600(14)70195-X.
- Essery R, Pollet S, Smith KA, Mowbray F, Slodkowska-Barabasz J, Denison-Day J, et al. Planning and optimising a digital intervention to reduce older adults’ cognitive decline. Pilot Feasibility Stud 2021;7. https://doi.org/10.21203/rs.3.rs-20513/v1.
- Little P, Stuart B, Hobbs FD, Moore M, Barnett J, Popoola D, et al. An internet-delivered handwashing intervention to modify influenza-like illness and respiratory infection transmission (PRIMIT): a primary care randomised trial. Lancet 2015;386:1631-9. https://doi.org/10.1016/S0140-6736(15)60127-1.
- Ainsworth B, Greenwell K, Stuart B, Raftery J, Mair F, Bruton A, et al. Feasibility trial of a digital self-management intervention ‘My Breathing Matters’ to improve asthma-related quality of life for UK primary care patients with asthma. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-032465.
- Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14:32-8. https://doi.org/10.1034/j.1399-3003.1999.14a08.x.
- Smith T, Noble M, Noble S, Wright G, McLennan D, Plunkett E. The English Indices of Deprivation 2015 2015. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/464485/English_Indices_of_Deprivation_2015_-_Technical-Report.pdf (accessed 27 July 2021).
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902-7. https://doi.org/10.1034/j.1399-3003.1999.14d29.x.
- NHS . Guidance on Implementing the Friends and Family Test 2019. www.england.nhs.uk/fft/fft-guidance/ (accessed 27 July 2021).
- Yardley L, Miller S, Teasdale E, Little P. Primit Team . Using mixed methods to design a web-based behavioural intervention to reduce transmission of colds and flu. J Health Psychol 2011;16:353-64. https://doi.org/10.1177/1359105310377538.
- Yardley L, Williams S, Bradbury K, Garip G, Renouf S, Ware L, et al. Integrating user perspectives into the development of a web-based weight management intervention. Clin Obes 2012;2:132-41. https://doi.org/10.1111/cob.12001.
- Anthierens S, Tonkin-Crine S, Douglas E, Fernandez-Vandellos P, Krawczyk J, Llor C, et al. General practitioners’ views on the acceptability and applicability of a web-based intervention to reduce antibiotic prescribing for acute cough in multiple European countries: a qualitative study prior to a randomised trial. BMC Fam Pract 2012;13. https://doi.org/10.1186/1471-2296-13-101.
- Yardley L, Morrison LG, Andreou P, Joseph J, Little P. Understanding reactions to an internet-delivered health-care intervention: accommodating user preferences for information provision. BMC Med Inform Decis Mak 2010;10. https://doi.org/10.1186/1472-6947-10-52.
- Rowsell A, Muller I, Murray E, Little P, Byrne CD, Ganahl K, et al. Views of people with high and low levels of health literacy about a digital intervention to promote physical activity for diabetes: a qualitative study in five countries. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4999.
- Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet 2013;382:1175-82. https://doi.org/10.1016/S0140-6736(13)60994-0.
- Ainsworth B, Steele M, Stuart B, Joseph J, Miller S, Morrison L, et al. Using an analysis of behavior change to inform effective digital intervention design: how did the PRIMIT website change hand hygiene behavior across 8993 users?. Ann Behav Med 2016;51:423-31. https://doi.org/10.1007/s12160-016-9866-9.
- Yardley L, Douglas E, Anthierens S, Tonkin-Crine S, O’Reilly G, Stuart B, et al. Evaluation of a web-based intervention to reduce antibiotic prescribing for LRTI in six European countries: quantitative process analysis of the GRACE/INTRO randomised controlled trial. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-134.
- Bradbury K, Watts S, Arden-Close E, Yardley L, Lewith G. Developing digital interventions: a methodological guide. Evid Based Complement Alternat Med 2014;2014. https://doi.org/10.1155/2014/561320.
- Clegg D, Barker R. Case Method Fast-Track: A RAD Approach. Boston, MA: Addison-Wesley Longman Publishing Co., Inc.; 1994.
- Public Health England . Improving People’s Health: Applying Behavioural and Social Sciences 2018. www.gov.uk/government/publications/improving-peoples-health-applying-behavioural-and-social-sciences (accessed 27 July 2021).
- Mohr DC, Schueller SM, Riley WT, Brown CH, Cuijpers P, Duan N, et al. Trials of intervention principles: evaluation methods for evolving behavioral intervention technologies. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4391.
- McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet 2018;391:949-59.
- Rai T, Morton K, Roman C, Doogue R, Rice C, Williams M, et al. Optimizing a digital intervention for managing blood pressure in stroke patients using a diverse sample: integrating the person-based approach and patient and public involvement. Health Expect 2021;24:327-40. https://doi.org/10.1111/hex.13173.
- Bateman ED, Esser D, Chirila C, Fernandez M, Fowler A, Moroni-Zentgraf P, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol 2015;136:914-22. https://doi.org/10.1016/j.jaci.2015.03.023.
- GOV.UK . Age Groups 2020. www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/age-groups/latest (accessed 27 July 2021).
- NHS Digital . Health Survey for England 2016. http://digital.nhs.uk/catalogue/PUB30169 (accessed 27 July 2021).
- Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003423.
- NHS . High Blood Pressure (Hypertension) 2016. www.nhs.uk/conditions/high-blood-pressure-hypertension/ (accessed 3 March 2019).
- NHS Business Services Authority . Drug Tariff June 2018 n.d. www.nhsbsa.nhs.uk/sites/default/files/2018-05/Drug%20Tariff%20June%202018.pdf (accessed 8 August 2022).
- Joint Formulary Committee . British National Formulary 2018.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury; 1998.
- NHS England . 201819 National Cost Collection Data Publication 2021. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication (accessed 28 July 2021).
- McKinstry B, Hanley J, Wild S, Pagliari C, Paterson M, Lewis S, et al. Telemonitoring based service redesign for the management of uncontrolled hypertension: multicentre randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f3030.
- Stoddart A, Hanley J, Wild S, Pagliari C, Paterson M, Lewis S, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): cost and cost-effectiveness analysis of a randomised controlled trial. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002681.
Appendix 1 Template for Intervention Description and Replication: the HOME BP intervention
This appendix describes the HOME BP intervention using the TIDieR checklist. 46
Brief name
The HOME BP intervention.
Why?
High BP or hypertension is a common condition in the UK, affecting approximately one in four adults, with many patients taking medication for their high BP remaining above national targets. 91,92 Raised BP is a key risk factor for cardiovascular events, including heart attack and stroke. 93 Clinical inertia is a recognised contributor to uncontrolled BP,8 and this occurs when a HCP chooses not to increase antihypertensive medication during an appointment, despite the patient’s BP reading being above target. Reasons for clinical inertia can include uncertainty about the accuracy of readings taken in clinic, concerns about side effects and a patient’s reluctance to take more medication. Previous evidence15,33 has shown that non-DIs can reduce BP by encouraging patients and GPs to make medication changes in accordance with a pre-agreed plan, based on an algorithm for home BP readings. The HOME BP intervention aimed to provide a cost-effective feasible digital solution for managing uncontrolled BP by translating these effective procedures into an online intervention.
The target behaviours to reduce BP included self-monitoring BP at home, changing medication when BP was above-target and optional healthy behaviour changes. Social cognitive theory39 and NPT35 were used to explain how the intervention would change the target behaviours.
What materials?
The HOME BP intervention comprised three online training sessions for patients, monthly online BP entry and feedback pages, two online training sessions for HCPs and automated e-mails that acted as reminders and prompts for action for patients and HCPs.
Each participant received an OMRON M3 BP monitor (Milton Keynes, UK) to use for self-monitoring.
The online materials were as follows.
HOME BP patient session 1
Patient session 1 took approximately 20 minutes to complete and provided information about the health consequences of raised BP in the form of a motivational quiz, with click-through pages for participants who wanted to read more. The session explained the rationale for home monitoring in terms of increased accuracy, and sought to promote engagement by letting patients know how self-monitoring would help their GP find the right medicine for them. At the end of the session, a question and answer section was provided to address common concerns and increase self-efficacy, for example explaining what support would be available from the GP and nurse during the intervention, and reassuring people about side effects.
HOME BP patient session 2
Patient session 2 took approximately 30 minutes to complete and sought to build patients’ skills to self-monitor BP and their self-efficacy to do this accurately. The session included a video, step-by-step written instructions with diagrams showing how to use the monitor, and clear explanations of when to self-monitor and how to enter the readings online. The session also covered what to do in the event of a very high or very low reading, and explained the targets for BP. At the end of the session, there was the option to read short stories from people who had used the intervention, which aimed to increase self-efficacy by showing that the intervention had worked for people.
Sessions 1 and 2 were compulsory to complete before the patient could enter any readings online. The sessions were tunnelled so that session 2 was available only after session 1 had been completed.
HOME BP patient session 3
Patient session 3 was optional and became available 9 weeks after randomisation. The session explained the benefits of engaging in healthy behaviours for health in general, and specifically for managing BP. The patient could choose to view more information about each of the following health behaviours: reducing salt, reducing alcohol, healthy diet, increasing physical activity and losing weight (for those with a BMI > 25 kg/m2). If patients chose to try one of the healthy behaviour changes, then they received an e-mail with a link to register on a standalone online intervention to support the behaviour change of their choice.
Blood pressure entry and feedback pages
When patients logged in, having completed the compulsory training, they were prompted to enter seven home BP readings to receive instant feedback. This option was available only once every 4 weeks, and patients received e-mails to notify them when it was time to start monitoring and time to enter their readings on the HOME BP intervention. Tailored feedback was shown immediately after patients submitted their readings, based on the average of their readings. Patients could choose to receive their feedback as an e-mail and, in some cases (e.g. when a medication change was recommended, or had been recommended last month), patients could send an e-mail to their GP via the intervention.
Tools, Ask The Nurse and frequently asked questions
The home page, which patients saw every time they logged in after completing the compulsory training, showed a menu with options that included ‘Tools’ (which provided links to various key sections of the intervention), ‘Ask the Nurse’ (which enabled the patient to send an e-mail to the nurse at their general practice about the intervention) and ‘FAQs’ (frequently asked questions; which provided answers to frequently asked questions about the intervention).
Prescriber training
Prescribers in the intervention could be GPs or nurse prescribers. The online training session was compulsory for each prescriber prior to recruiting patients to the intervention. The training session took approximately 20 minutes to complete and included a rationale for the intervention and supporting evidence, which sought to change prescribers’ perceptions of the likely outcomes of changing patients’ medication. The online training also explained how to plan three medication changes for each patient (with examples given to increase self-efficacy) and how to implement medication changes when needed. Common concerns were addressed using evidence to show that, for example, patients using this kind of intervention did not need more consultations and only rarely had very high or low readings. The team who created the HOME BP intervention were also introduced at the start of the training to increase perceived credibility of the intervention.
Supporter training
Supporters in the intervention could be practice nurses or health-care assistants. The online training was compulsory for each supporter prior to recruiting patients to the intervention. The training took approximately 20 minutes to complete, and the first few pages were the same as the prescriber training, including a rationale for the intervention, supporting evidence and the opportunity to see who had created the intervention. Subsequently, the supporter training explained how to deliver face-to-face support for patients in the intervention using the CARE approach,32 including a rationale for why this approach was effective, quotes from patients and HCPs to increase confidence in the approach and examples of how to implement CARE for both types of patient appointment that could occur within the intervention. The supporter training also explained how to e-mail patients via the intervention to provide remote support for self-monitoring and medication change.
e-mails
The HOME BP intervention included a large number of tailored e-mails for patients and HCPs. The e-mails included prompts for when to start monitoring, prompts to enter readings online, and tailored feedback for the patient and HCPs on the patient’s BP readings and recommended actions.
What: procedures
The intervention procedures are shown in Figure 6.
FIGURE 6.
HOME BP intervention procedures.
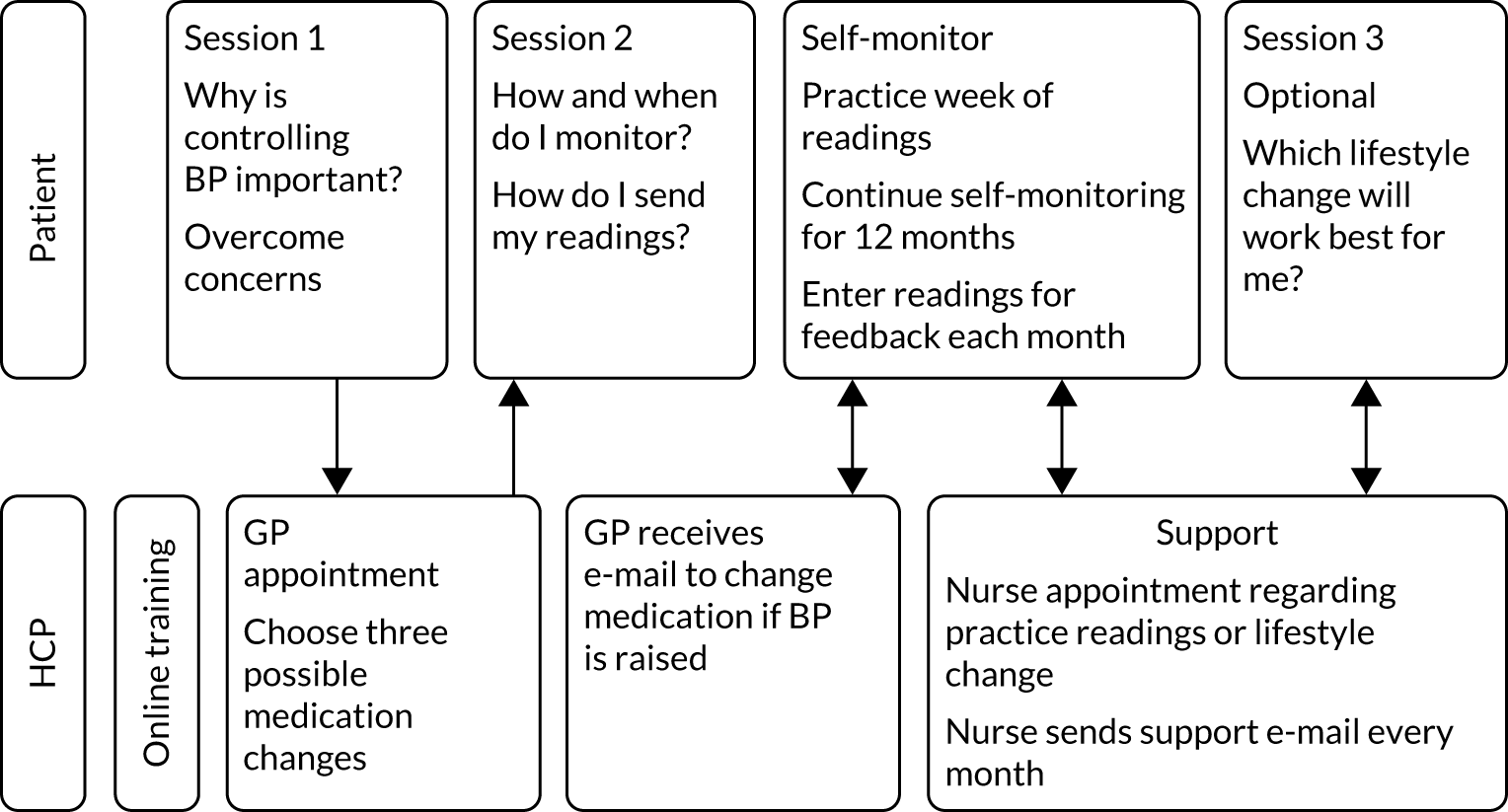
Figure 6 shows that patients completed the first training session online and then had an appointment with their prescriber to agree three potential medication changes. The prescriber recorded the three planned medication changes in a template, which was saved to the patient’s notes. At this time, the patient also collected their BP monitor at their general practice. At home, the patient could then login to the HOME BP intervention and complete session 2, which trained patients to take two morning readings for 7 days, record these on paper and then enter the second reading from each day on to the HOME BP intervention.
Following completion of session 2, patients were prompted to take 1 week of practise home readings and enter these on the HOME BP intervention. The intervention offered the opportunity for patients to send their nurse a message via the HOME BP intervention about their practise readings if they wanted to. Patients also received an e-mail that reminded them that they could make an appointment to see their nurse if they wanted to talk about how to use the BP monitor. These steps were taken to help promote patients’ self-efficacy in home readings and to ensure that patients felt confident to take their own BP going forwards.
After entering their week of practise readings, patients received reminders to monitor their BP every 4 weeks during the 12 months, except after 3 consecutive months of well-controlled readings at which point the reminders dropped to once every 8 weeks. Patients received automated feedback on their readings as soon as the readings were entered, and the readings were also shared with the prescriber and supporter. The algorithm calculated an average, and a medication change was recommended if the average was above-target for 2 consecutive months. Prescribers were trained in the procedure for changing medication during their online training, and the steps were reinforced in the e-mail they received at the time a change was needed. The steps involved checking that the planned change was still appropriate for the patient and issuing the new prescription along with a template letter that included instructions for the patient on how to make the change and any blood tests that might be needed.
Throughout the 12-month intervention, supporters were asked to send monthly e-mails to patients using predesigned templates, which could be edited to personalise the e-mail. This monthly remote support was designed to help patients feel supported when self-monitoring their BP at home and to reinforce the benefits of adhering to self-monitoring and medication change.
Nine weeks from when the patient was randomised, an automated e-mail was sent to alert patients that the optional healthy behaviour change session was now available, and there was also an option to view session 3 from their homepage when they logged into the HOME BP intervention. If patients wanted, they could book an appointment with the supporter at this point to discuss their choice of healthy behaviour change.
Who provided?
The intervention was delivered by a prescriber (i.e. a GP or nurse prescriber) and a supporter (i.e. a practice nurse or a health-care assistant) at each general practice. Each prescriber and supporter was required to complete an online training session prior to recruiting any patients to the intervention, the content of which is described in Chapter 3. General practices were reimbursed for taking part in the research study.
How?
The majority of the intervention was delivered online individually. Some components of the intervention involved face-to-face or telephone appointments with a HCP, which could be initiated by the patient or the HCP.
Where?
The intervention was implemented in a primary care context in the south of England from 2015 to 2018. Online components were completed by patients at home or by HCPs at their general practice. Patients needed to have internet access to be able to take part. Any general practice was eligible to sign up to the study.
When and how much?
The intervention was designed to last 12 months. Patients were asked to take their BP readings for 7 days, twice every morning. Patients would then have 3 weeks off before starting again. Patients record their 7 days of readings online at the end of the week. If their BP average was well controlled for 3consecutive months, then their self-monitoring frequency dropped to once every 8 weeks.
It was anticipated that each online training session for patients would take approximately 30 minutes to complete.
Tailoring
Blood pressure targets were tailored to take account of age and diabetes as follows:
-
Patients aged < 80 years without diabetes: 135/85 mmHg.
-
Patients with diabetes: 135/75 mmHg.
-
Patients aged ≥ 80 years without diabetes: 145/85 mmHg.
The optional healthy behaviour session (i.e. session 3) was tailored to only offer the fifth option of weight loss if the patient’s BMI was > 25 kg/m2.
Modifications
The intervention procedures and materials were only minimally modified during the course of the study. Small changes were made in line with feedback from process interviews during the internal pilot trial. The changes included the addition of a stress-related quiz question in session 1 because of the prevalent perception among participants that stress was the main cause of their high BP, additional e-mails about the benefits of healthy behaviour changes to increase uptake, and the option to launch a healthy behaviour change module from the intervention homepage, rather than needing to complete the optional healthy behaviour change session.
How well: planned?
Online usage data were captured automatically by the intervention software LifeGuide:
-
The number of patients completing the core training (i.e. sessions 1 and 2).
-
The number of patients completing session 3 (optional).
-
The number of patients completing 7 days of practise readings.
-
The number of BP entries made by patients.
-
The number of prescribers completing the core training.
-
The number of supporters completing the core training.
-
The number of recommendations made to change patients’ medication.
The software enabled patient and HCP engagement to be assessed. In addition, reviews were conducted of the patients’ medical notes at the end of the study to explore prescribers’ adherence to planning three medication changes in advance and implementing recommended medication changes.
Qualitative process evaluations were undertaken to explore patients’ and HCPs’ experiences of implementing the intervention.
How well: actual?
Two detailed mixed-methods process evaluation studies were undertaken to explore patients’ and HCPs’ adherence and experiences of implementing the HOME BP intervention. The findings are described in detail in Chapter 3.
Appendix 2 Health economic evaluation
This appendix was authored by James Raftery, Sue Jowett, Shihua Zhu and Richard McManus.
Introduction
The HOME BP trial aimed to assess the cost-effectiveness of the HOME BP DI relative to usual care. Within-trial results are reported in a cost-effectiveness analysis in terms of cost per unit of BP reduction. As the intended effects are reduced risk of stroke, heart failure and unstable angina, these longer-term effects were estimated in a model that was previously developed for that purpose. 1 The analysis is expressed in terms of incremental cost per QALY gained. The perspective in both short- and long-term analyses is that of the NHS, but the role of non-NHS costs on patients was explored in the within-trial analysis. Costing was based on unit costs in 2018/19. Bootstrapping was used to estimate mean values for costs and QALYs.
The main results of the trial are reported fully elsewhere,47 as are details of the longer-term model. 50 Although key elements are summarised here, please see these publications47,50 for fuller details.
The next section reports on the within-trial analysis of the HOME BP trial. A later section reports on the long-term economic modelling, along with exploration of various scenarios, before making comparisons with other similar analyses and drawing some general conclusions.
Costing
Costing was based on various resource use headings (Table 13). Data were collected from a review of case notes at the end of the trial. The cost estimates derived were for each patient and were included as a cost variable in the statistical analysis. Only service use data relating to BP were included. These data covered cardiovascular disease and possible side effects of antihypertensive medications, including dizziness and falls. Clinical advice was used to adjudicate on the inclusion/exclusion decisions. The number of patients using each service is shown along with costs of BP-related services (see Table 13).
| Heading | Data | Costing | Number of patients | Comments |
|---|---|---|---|---|
| Drugs at baseline | Drug by name, dose and duration | NHS Drugs Tariff June 2018,94 with BNF95 for drugs not on Tariff | All (n = 575) | Assumptions needed for a few drugs with doses not listed in Tariff/BNF94,95 |
| Drugs changed during the trial | Drug by name, dose and duration | NHS Drugs Tariff June 2018,94 with BNF95 for drugs not on Tariff | 304 | Assumptions needed for a few drugs with doses not listed in Tariff/BNF94,95 |
| Costs relating to changes in drugs | Type of consultation (letter, telephone, face to face, etc.) | Unit cost for face-to-face consultations, with estimates for other types of consultation | 304 | Lacking staff linked to face to face. Assumed captured in 4 to avoid double counting. Scope for detailed cross-referencing in future94,95 |
| Other BP-related primary care contacts | Contacts by staff (GP, practice nurse, health-care assistant) by type (face to face, telephone) | Six unit costs were estimated and applied | 544 | Based on PSSRU unit costs96 for GP, applied to practice nurse and to band 4 (i.e. health-care assistant), with pro rata for GP telephone cost |
| A&E visits relating to BP | Data by reason for visit | £160/visit from NHS Improvement | 9 (92 all) | Included only if visit was after a medication change |
| Outpatient attendances relating to BP | Data by specialty and reason for attendance | £125 per attendance from NHS Improvement97 | 13 (673 all) | Included only if visit was after a medication change |
| Inpatient admissions | Reason for admission | By HRG, National Tariff | 5 (108 all) | Included only if visit was after a medication change |
| Total | ||||
| Intervention cost (not included above) | Ongoing cost of running website. Any instruction time to practice or patients | Based on trial | £39.72 | See relevant section below |
As the trial ran between 2015 and 2018, the year for costing was taken as 2018. Discounting of costs was not considered necessary as the follow-up period was limited to 12 months.
Few patients recorded use of A&E, outpatient or inpatient departments in relation to BP. Inpatient admissions received particular attention because of their relatively high cost. Five inpatient admissions that were potentially related to BP were recorded. A review, carried out blind to which arm patients were in, showed that the dates on which all these admissions occurred were all before any changes in medication by patients in the trial. These admissions were, therefore, taken to reflect decisions made, or conditions in place, before the start of the trial. For that reason, these admissions were excluded from the base case, but were included in a sensitivity analysis.
The same logic of excluding service use that occurred before any medication changes in the trial was applied to outpatient and A&E visits. Therefore, the base or preferred case costing included only inpatient, outpatient and A&E use that occurred after any medication changes in the trial. Again, this decision was made and applied before the data were unblinded. The sensitivity analysis included all BP-related use of these services, regardless of their timing in relation to medication changes.
Costing drugs
Data were collected on all BP-related drugs that patients were taking at baseline, including name, dose and duration, along with subsequent consultations and changes in medication. BP-related drugs were defined as antihypertensives, broadly defined (see list available on request). Clinical advice was sought in unclear instances. We assumed that patients who did not have a change of medication during the trial continued their baseline medication unchanged for the duration of the trial. When data were missing with regard to duration of medications for patients who had changes in their medications, the durations were assumed to continue unchanged to the next change of medication or to the end of the trial, whichever was reached first. These assumptions involved a minority of patients (around 100–150 patients), most of whom had no change in their drugs.
The NHS Drugs Tariff June 201894 was copied for each BP drug mentioned in the trial. Each patient’s use of drugs was costed, from baseline and through changes during the trial, at the dose-specific prices listed in the NHS Drugs Tariff June 2018. 94 When branded rather than generic drugs were recorded, the generic rather than the brand price was used. This reflected common NHS prescribing practice, and also avoided bias due to the occasional prescription of brand rather than generic drugs. As very few brand names were listed, this assumption made little difference. The few drugs that did not have a generic version were costed using the stated price for the brand by dose.
If a drug was not included in the NHS Drugs Tariff June 2018,94 then the British National Formulary (BNF)95 was used; however, this applied in only a small number of cases.
When the dose recorded in the trial was not listed in the NHS Drugs Tariff June 2018or the BNF,94,95 then it was assumed that the dose could be made up by several dosages. This meant patients might have to use two pills rather than one, but in a few cases it involved up to four drugs (e.g. 4 × 25 mg of atenolol for 100 mg of atenolol).
Costing primary care contacts
The data recorded were in response to the following two questions. First, ‘Who did the patient speak to’, which was followed by three options: (1) GP, (2) practice nurse or (3) health-care assistant. Second, ‘How’, which was followed by four options: (1) face to face, (2) telephone, (3) letter or (4) e-mail. This led to 12 options (GP-face to face, GP-telephone, practice nurse-face to face, practice nurse-telephone, health-care assistant-face to face, health-care assistant-telephone, etc.). A unit cost (Table 14) was estimated for each option and was applied to all BP-related contacts.
| Who did the patient speak to? | Unit cost (£) | |||
|---|---|---|---|---|
| Face to face | Telephone | Letter | ||
| GP | 34.30 | 8.10 | 1.67 | 1.00 |
| Practice nurse | 6.45 | 1.52 | 1.67 | 1.00 |
| Health-care assistant | 4.00 | 0.94 | 1.67 | 1.00 |
Personal Social Services Research Unit (PSSRU) unit costs for 2017/18 (p. 12796) put the cost per hour of GP time from £181 to £243 (depending on in/exclusion of direct care costs and with/out qualification costs). A cost per consultation is provided for only GPs and not for other staff and this varied from £28.30 to £37.40 for an average 9.22-minute consultation.
The costing in Table 14 used the cost per GP consultation of £34.30, that is, including qualification but not direct costs. The higher figure that included direct costs was not considered appropriate as it appeared to include the overhead costs, which are also included in the estimate of the cost of the practice nurse.
The cost per hour of a practice nurse in the PSSRU data96 ranged from £36 to £42 per hour, depending on qualifications. We used the higher nurse cost per hour, including qualification (as with the GP estimate). Assuming the same duration of consultation as for a GP, this put the cost per face-to-face nurse consultation at £6.50.
As the term ‘health-care assistant’ is not listed by the PSSRU,96 this was costed as a band 4 nurse (i.e. the lowest grade for which the PSSRU provided a unit cost).
The PSSRU provide an estimate of £8.10 for a GP telephone call, based on a small study to do with triage in general practice, and this seems a reasonable proportion, as £8.10 of £34.3 is approximately 25%. The same proportion was applied to the cost per face-to-face consultation for practice nurses and HCAs.
A small number of letter and e-mail contacts were recorded. These were costed at £1.67 and £1.00 each, respectively, as a fixed cost, regardless of who they were from. A small number of non-BP-related primary care contacts were recorded and these were reviewed and included/excluded prior to unblinding by two clinicians (RMcM and PL).
All patients in the trial had an initial GP consultation to do with entering the trial. However, this consultation was not included in the costing, as it was considered a research cost and applied equally to both arms of the trial. If the intervention were to become routine practice, then the assumption implied here is that discussion of patients’ use of the intervention would occur in a routine consultation.
Costing inpatient episodes
The data collected showed all BP-related inpatient admissions by specialty and reason (Table 15). Checking the dates of these admissions showed that all inpatient admissions occurred before any changes of medication occurred in the trial. A decision was made pre-unblinding that these admissions should be omitted from the base-case cost, but are included as part of a sensitivity analysis (see Sensitivity analysis).
| Reason for secondary care stay | BP related or not: comments | Classified as |
|---|---|---|
| NSTEMI coronary angioplasty | Had chest pain outpatient attendance and was admitted same day | Angioplasty, most common HRG |
| Angioplasty and stent insertion | Outpatient attendance for chest pain day after operation | Angioplasty, most common HRG |
| Pacemaker insertion | No other consultation | Pacemaker insertion, most common HRG |
| Falls due to postural hypotension | Preceded by same-day outpatient attendance for fall, admitted for 9 days, had subsequent admission for a UTI 2 months later | Fall-related HRG, most common |
| Falls team review | Followed same-day outpatient attendance for contusion. Discharged same day | As above but short stay |
The two angioplasty admissions were allocated to an angioplasty Healthcare Resource Group (HRG) using the National Tariff, which puts the cost in 2017/18 at £2404 for the most common HRG (EY41D Standard Percutaneous Transluminal Coronary Angioplasty with CC score 0–3).
Similarly with pacemaker insertion, the cost of the most common HRG was put at £2814 (HRG EY06E Dual Chamber Pacemaker Insertion with CC score 0–2). 97
The two admissions linked to falls were classified under HRG WHO9G (Tendency to Fall, Senility or Other Conditions Affecting Cognitive Functions, without Interventions with CC score 0–1). One admission was classed as routine with a cost of £1844 and the other was classed as a short stay with a cost of £533.
Costing accident and emergency attendances
Data were collected on all attendances at A&E by reason. Attendances deemed most likely to be BP related are listed in Table 16. The most common reason was chest pain. As discussed above, A&E attendances occurring before any change in medication were excluded from the base-case costing following the logic outlined above for inpatients. The cost per A&E attendance was put at £160 in 2017/18 by NHS Improvement. 97
| Patient ID number | Reason 1 | Reason 2 |
|---|---|---|
| 27526 | Fall – hypotension | UTI |
| 25925 | To exclude DVT | Swollen legs secondary to drug |
| 22225 | Chest pain | |
| 28005 | Chest pain | |
| 24952 | Chest pain diagnosis unstable angina | |
| 27861 | Chest pain, gastritis | |
| 23994 | Chest pain | |
| 25673 | Chest pain | |
| 27295 | A.F. |
Costing outpatient attendances
Data were collected on all outpatient attendances. Outpatient attendances most likely relating to BP are shown in Table 17.
| NHS service | HRG code | HRG | Cost (£) |
|---|---|---|---|
| Angioplasty | EY41D | Standard Percutaneous Transluminal Coronary Angioplasty with CC score 0–3 | 1707 |
| Pacemaker | EY06d | Implantation of Dual-Chamber Pacemaker with CC score 3–5 | 2909 |
| Falls | WHO9G | Tendency to Fall, Senility or Other Conditions Affecting Cognitive Functions, without Interventions with CC score 0–1 | 1844 |
| Falls (short stay) | WHO9G short stay | Tendency to Fall, Senility or Other Conditions Affecting Cognitive Functions, without Interventions with CC score 0–1 | 533 |
| Outpatient | Cardiology | 125 | |
| A&E | VB08Z | Emergency Medicine, Category 2 Investigation with Category 1 Treatment | 160 |
Outpatient visits occurring before any change in medication for trial patients were excluded, following the logic outlined in Costing inpatient episodes. Excluding outpatient visits in this way led to only a small number of outpatient visits being included. The cost per outpatient attendance was put at £125 in 2017/18 by NHS Improvement. 97 More detailed unit costs are available for A&E, outpatients and inpatients. The higher-level averages have been used as a first cut, but see more detailed unit costs below for inpatients. Data are not available to disaggregate A&E attendances, but may be applicable to outpatient attendances depending on inclusion/exclusion criteria.
Sensitivity analysis
As discussed above, the base-case scenario was costed on the basis of BP-related service use in primary care, including outpatients and A&E attendances that occurred after a medication change in the trial. This latter criterion excluded the five inpatient admissions that might have been BP related.
An alternative scenario was costed, based on including those inpatient, outpatient and A&E visits plausibly related to BP.
A third scenario explored costing only primary care costs, that is, excluding the relatively small numbers of outpatient and A&E attendances that occurred after a medication change in the trial, and this gave results almost identical to the base-case scenario, reflecting the paucity of use of these services.
Non-NHS costs
Although data were collected on the amount of time patients spent using the website, we decided not to cost it because of the finding from the process evaluation that showed that patients valued the DI in terms of perceived benefits (e.g. reassurance and improved health), but also experienced burdens (e.g. worry about health). Time did not appear to be an important feature. Furthermore, patients’ perceptions of illness and treatment perceptions about hypertension appeared to influence perception of benefit or burden. Valuation of such issues would require going well beyond estimates of time spent online.
We offer suggestions under Recommendations for research on how these matters might be developed.
On diet and lifestyle, very few patients recorded any changes, and this finding was consistent with the overall finding that the key changes arising from the intervention were to do with changes in prescribed drugs. Therefore, no further costing was deemed necessary.
The result was that we are able to present only cost-effectiveness estimates from an NHS perspective. We note that this is the perspective required by NICE.
Costing the HOME BP intervention
Blood pressure monitors were provided by OMRON at a lower cost than those sold commercially. The cost to the trial was £23, whereas the commercially available units cost around £65 (Boots UK Limited, Nottingham, UK, 2018 price). As these monitors last for several years, usually taken as 4 or 5 years, an estimate of the cost for 1 year, that is, the same as for other cost headings, is required. This costing can be calculated in two ways: straight-line depreciation (offset the same amount each year, e.g. 25% for each year if over 4 years) and the annuity method. The £23 cost incurred in the study is shown in annual terms using both methods and in time frames of 4 and 5 years in Table 18. The methods make fairly little difference, ranging from £4.60 to £6.26.
| Cost (£) | Annuity method | Straight-line depreciation | ||
|---|---|---|---|---|
| 4 years | 5 years | 4 years | 5 years | |
| Monitor | 6.26 | 5.09 | 5.75 | 4.60 |
| Programer | 23.90 | 23.00 | 23.90 | 23.90 |
| Total | 30.16 | 28.09 | 29.65 | 28.50 |
| Programmer with 40% overhead costs | ||||
| Monitor | 6.26 | 5.09 | 5.75 | 4.60 |
| Programer | 33.46 | 33.46 | 33.46 | 33.46 |
| Total | 39.72 | 38.55 | 39.21 | 38.06 |
The other element of the intervention had to do with the website. Following advice from the principal investigator, the cost of the website was based on the cost of the employee who programed and maintained LifeGuide. This cost was estimated based on the programer’s salary and the proportion of time spent on maintaining server, spread over then current 10 projects:
-
5% of £48,677 (level 5/spine point 43) for 3 years (HOME BP was live from 2015 to 2018) = £7300.
-
Divided by the number of participants in the intervention arm (n = 305) = £23.90.
This gives the figure of £23.90 (see Table 18). As this figure does not appear to include overhead costs (rent, utilities, etc.), a 40% overhead has been added (see Table 18).
Depending on the overhead issue, as well as writing off the monitor over 4 years, puts the cost of the intervention at £30.16 or £39.72 (see Table 18). Slightly different results due to different assumptions are also shown in Table 18.
The higher cost of £39.72 was chosen for the base case on the grounds that using the higher cost meant that estimates of cost-effectiveness would err on the high rather than the low side.
Quality-adjusted life-years
The data from EuroQol-5 Dimensions (EQ-5D), which were collected at baseline and at 6 and 12 months, were used to estimate QALYs. Although the health gain from reduced BP has to do with long-term effects of reduced risk of cardiovascular events and death, these data help indicate whether or not any short-term changes might result from the intervention. The more relevant long-term cost-effectiveness of the intervention was estimated in long-term modelling, which is in Long-term cost-effectiveness. For completeness, the within-trial results for incremental cost per QALY are reported here.
Data collected using EQ-5D were used to estimate QALYs via preferences based on a survey of the public. The results indicated a very small QALY, statistically non-significant, loss in the intervention group relative to the control group (Table 19).
| Group | Time point | Mean EQ-5D score (SD), n |
|---|---|---|
| Usual care (N = 277) | Baseline | 0.84 (0.16), 277 |
| 6 months | 0.88 (0.14), 190 | |
| 12 months | 0.85 (0.14), 183 | |
| Intervention (N = 266) | Baseline | 0.85 (0.17), 266 |
| 6 months | 0.85 (0.17), 243 | |
| 12 months | 0.85 (0.17,) 209 |
Some EQ-5D scores were missing. Full values were available for 89% of patients, with no difference by arm. 47 The principal analysis of the primary outcome used raw and adjusted data, and was agreed in a statistical analysis plan before final data lock. 47 The primary analysis used general linear modelling to compare systolic BP in the intervention and usual-care groups at follow-up, adjusting for baseline BP, practice (as a random effect to take into account clustering), BP target levels and sex. Analyses were on an intention-to-treat basis and used 100 multiple imputations by chained equations for missing data. The imputation model included all outcome and stratification variables.
The QALY values were then imputed based on BP and these values were used in both the within-trial analysis and in the longer-term modelling.
Results
This within-trial cost-effectiveness analysis was conducted from an NHS perspective using data collected on use of services and on the intervention. The reduction in BP of 3.45 mmHg, combined with increased cost in the intervention arm of £38, led to an incremental cost per unit of BP reduction in the base case of £11 (95% CI £5 to £29) (see Table 5). The increased cost per patient of £38 in the intervention arm was due almost entirely to that of the intervention (£39.73).
Complete-case and imputed analyses (see Table 5) gave almost identical results, with only very small differences. The uncertainty around this estimate was simulated probabilistically using 10,000 runs (Figure 7).
FIGURE 7.
Scatterplot of joint distribution of incremental mean cost from NHS perspective and mean BP reduction from baseline (mmHg) over 12 months.
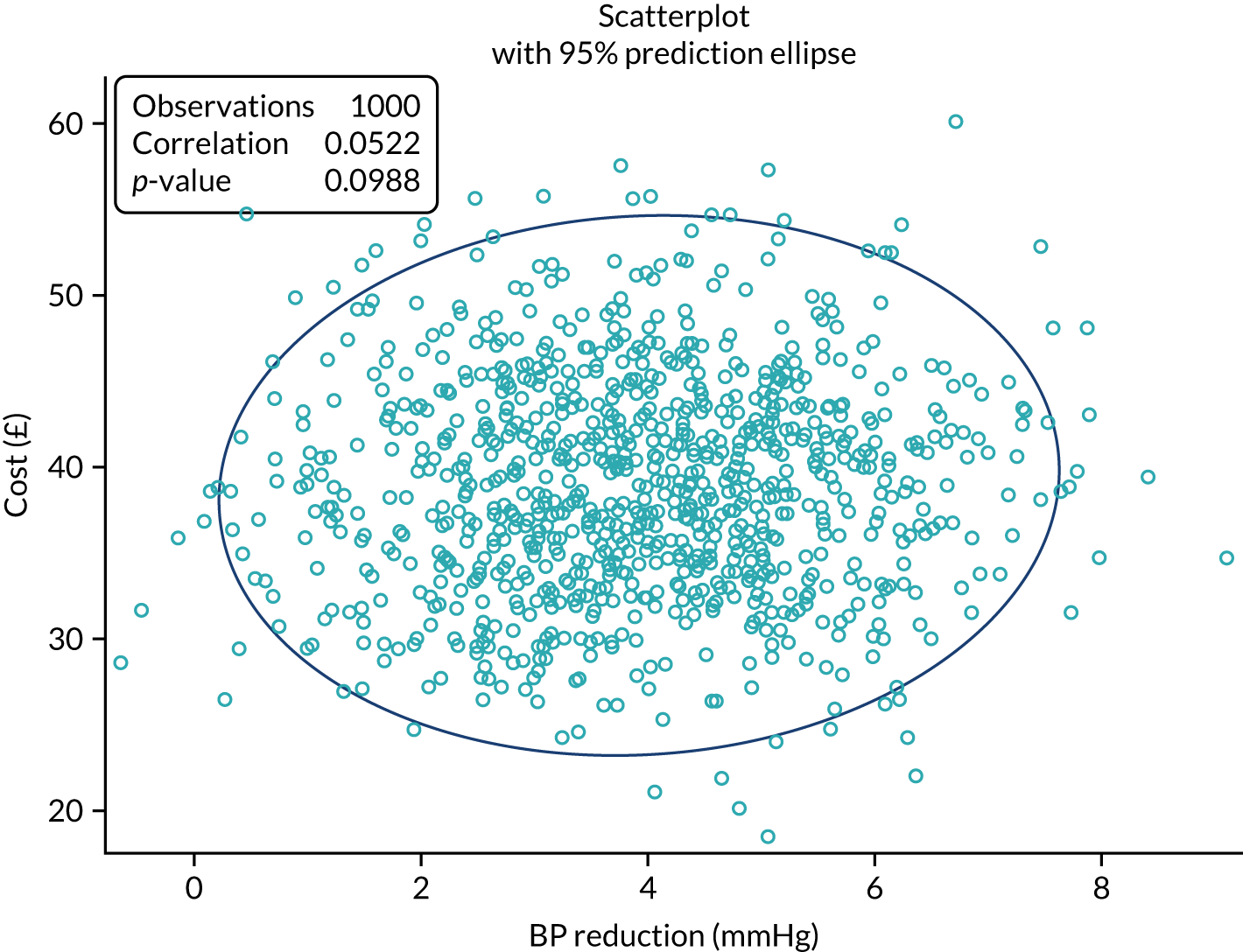
The base case included use of NHS services relating to BP. This included the full range of NHS services, including hospital admissions. Although few such admissions were recorded, some were elective procedures that had to have been planned before entry to the trial. Consequently, only those hospital admissions that occurred after a change of medication were included in the base-case costing. To test the sensitivity of results to this assumption, a scenario was costed that included all hospital BP-related service use, regardless of timing. This scenario made little difference overall, but reduced both the cost difference and the incremental cost-effectiveness.
A small, non statistically significant decrement in QALYs was observed in the intervention arm (see Table 5), which, when combined with its higher cost, meant that the intervention arm was dominated by the usual-care arm. However, as QALY differences to do with improved BP control at 12 months are of little interest compared with those in the longer term, the results of the lifelong modelling provide a more robust and plausible estimate of cost effectiveness. The mean cost per patient in primary care was similar to the base-case cost, indicating that primary care accounted for almost all the costs (see Table 6). Within primary care, costs were split roughly 60 : 40 between costs attributable to consultations and costs for prescriptions. Patients in the intervention arm had slightly higher prescription costs, associated with changes in medication and/or dose. However, these costs did not increase the cost of primary care consultations because of the role of the DI. These trends were as might be expected. Further analysis of these changes is planned for a separate publication, which will include changes in the time spent by patients in managing their hypertension.
The resulting cost effectivness acceptability curve (Figure 8) indicatated that the probabilities of being cost-effective for the intervention against the usual care are 51%, 90% and 98% at thresholds of £10, £20 and £50 per unit of BP, respectively.
FIGURE 8.
Cost-effectiveness acceptability curve of the intervention and usual-care groups based on BP from baseline over 12 months.
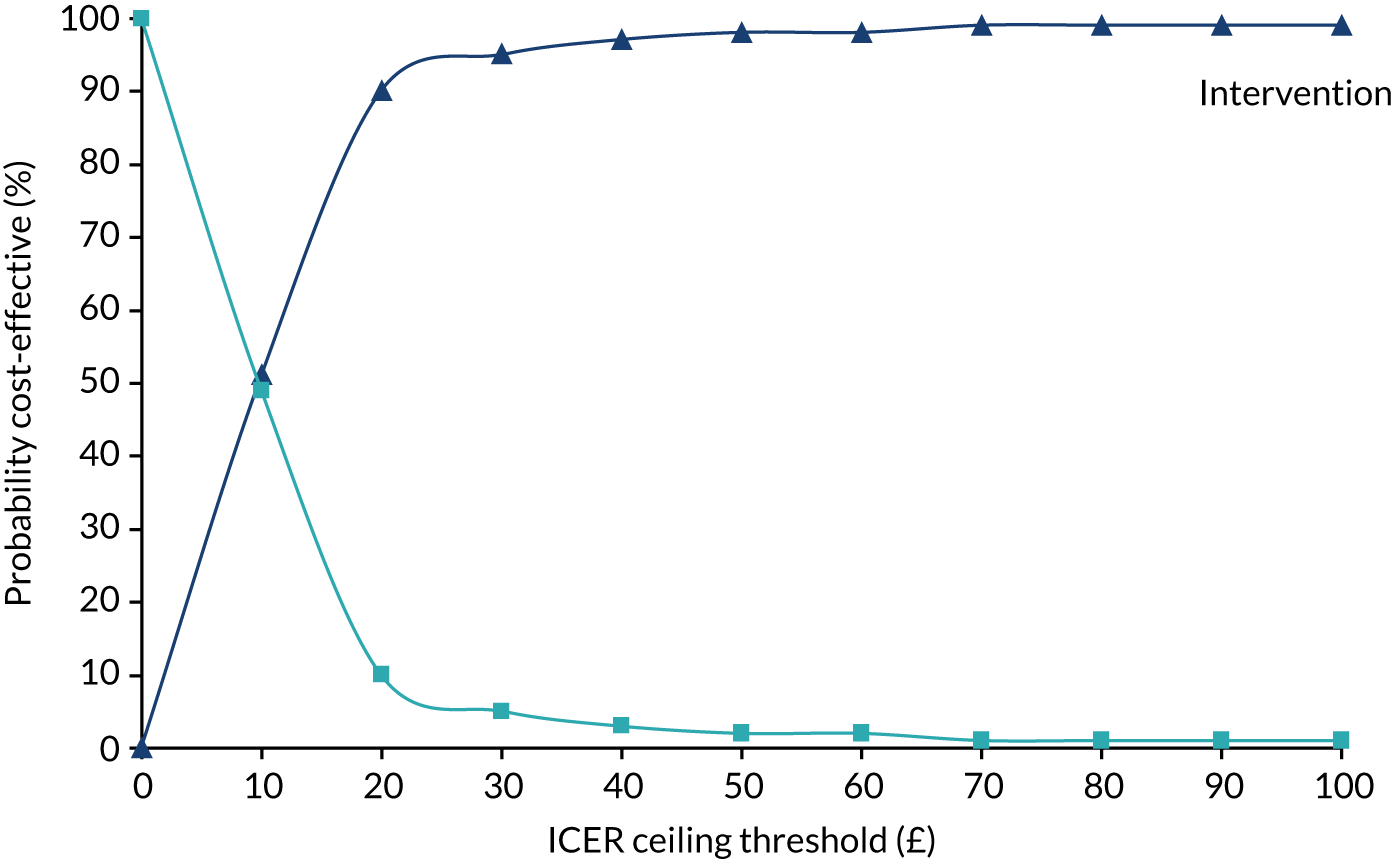
Long-term cost-effectiveness
A published long-term cost-effectiveness study1 of self-management of BP with and without telephone support, broadly similar to the HOME BP intervention, published in 2019, provided a relevant long-term model. Use of this model1 was facilitated by the overlap of investigators (RMcM) in these two trials.
The model, hereafter referred to as the TASMINH4 model, was a Markov patient-level simulation undertaken in TreeAge to model the different strategies. This type of Markov model tracks the costs and consequences of individual patients passing through the model, with characteristics (taken from the trial) free to vary between patients. The model was run over the maximum lifetime of the patients (maximum of 65 years; minimum trial inclusion criteria was age 35 years), a time horizon sufficient to capture all relevant long-term costs and consequences.
Each patient had characteristics created by randomly sampling the trial patient-level data by means of a uniform distribution. These characteristics affected their probability of subsequent model events. For instance, males had a higher risk of cardiovascular disease than females. The model was run with a large number of simulated patients (n = 50,000) to account for interpatient variability and to adequately model a representative clinical population.
Model structure
All patients started in the well/no event health state. Within a 6-month time cycle, a patient had a risk of suffering a fatal or non-fatal cardiovascular event or dying from other causes. The possible cardiovascular events in the model were stable angina, unstable angina, stroke, myocardial infarction and transient ischaemic attack. Ten-year cardiovascular risk was calculated for each individual patient, with the distribution of coronary heart disease and stroke events dependent on age and sex. Patients who suffered a non-fatal cardiovascular event transitioned to a post-event cardiovascular health state and additional clinical events were not modelled. Once a cardiovascular event had occurred, mortality risk was adjusted accordingly. The impact of each intervention in terms of event reduction was applied as a relative risk, taking into account the mean differences in systolic BP observed in the HOME BP trial.
Results (long term)
Owing to a cost difference of £402 and a QALY difference of 0.044, the results from inputting the HOME BP trial results into the long-term TASMIN4 cost-effectiveness model put the ICER at just over £9000 (Table 20). The key inputs from the HOME BP trial were a 3.45 mmHg difference in BP and a cost difference of £38. The small QALY decrement in the intervention arm in the trial was offset in the longer-term modelling by reductions in cardiovascular events and deaths (see Table 20).
| Strategy | Total cost (£) | Incremental cost (£) (90% CrI) | QALY | Incremental effect (90% CrI) | Incremental cost/effect |
|---|---|---|---|---|---|
| Usual care | 2685 | 11.562 | |||
| HOME BP intervention | 3087 | 402 (–2379 to 3936) | 11.606 | 0.044 (0.01 to 0.09) | 9107 |
The range of the increments in the different runs of the model are shown in a scattergram form in Figure 9. The cost-effectiveness acceptability curves (Figure 10) show a 66% probability of the intervention being cost-effective at £20,000 per QALY, rising to 72% at £30,000 (note that these relatively low probabilities imply considerable noisiness or wide CIs).
FIGURE 9.
Scattergram of repeated runs of long-term model.
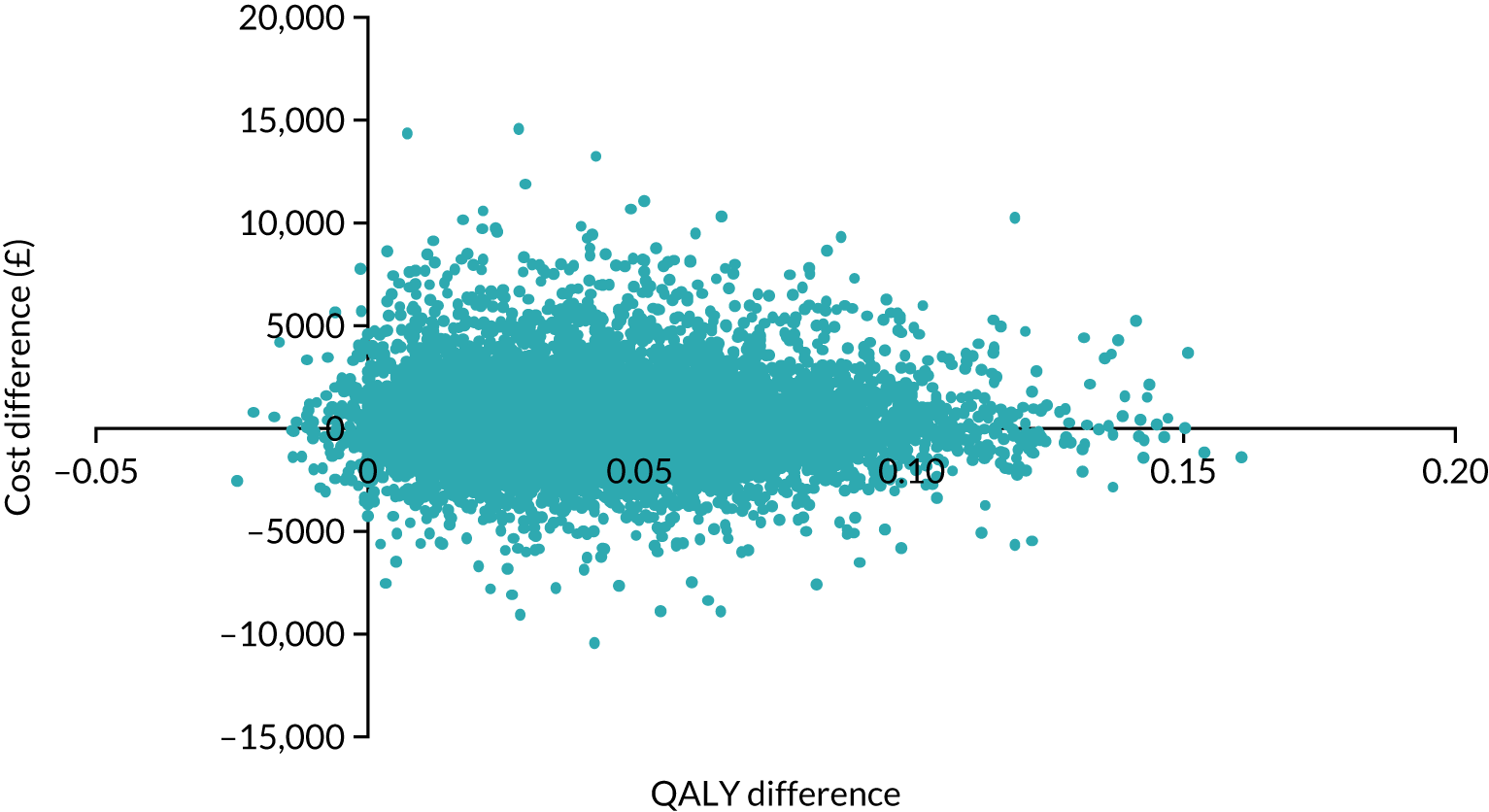
FIGURE 10.
Cost-effectiveness acceptability curve of long-term cost per QALY.
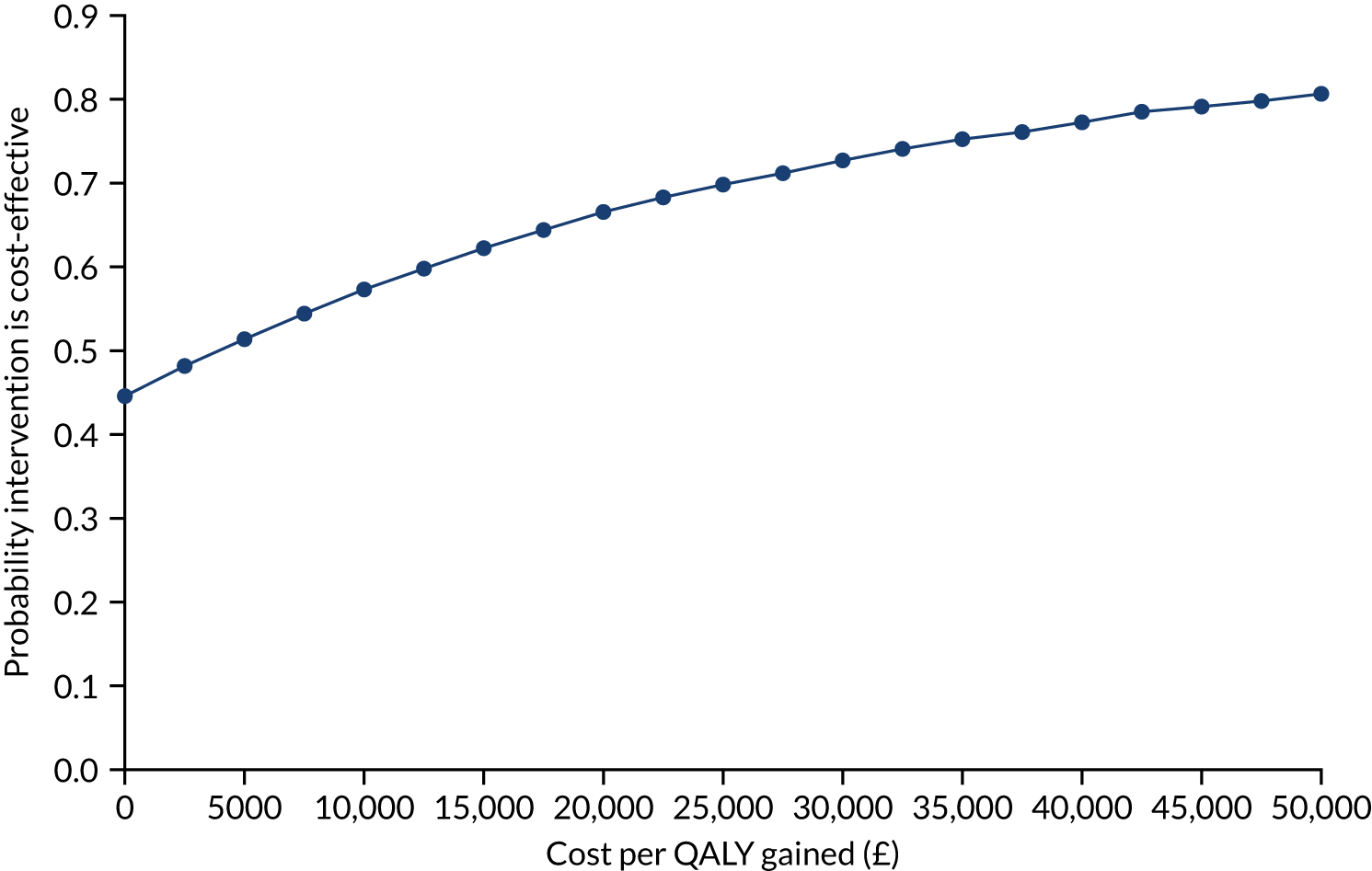
Scenario analyses
Different scenarios explored the implications of varying the inputs to the model, including the cost difference and the timescale (Table 21), as well as the number of events averted (Table 22).
| Strategy | Total cost (£) | Incremental cost (£) | QALY | Incremental effect | Incremental cost/effect |
|---|---|---|---|---|---|
| £50 difference (£92 vs. £142) | |||||
| Usual care | 2685 | 11.562 | |||
| HOME BP intervention | 3235 | 550 | 11.606 | 0.044 | 12,460a |
| £75 difference (£92 vs. £167) | |||||
| Usual care | 2685 | 11.562 | |||
| HOME BP intervention | 3543 | 858 | 11.606 | 0.044 | 19,446 |
| £100 difference (£92 vs. £192) | |||||
| Usual care | 2685 | 11.562 | |||
| HOME BP intervention | 3851 | 1166 | 11.606 | 0.044 | 26,432 |
| Cost difference for £20,000/QALY | |||||
| Usual care | 2685 | 11.562 | |||
| HOME BP intervention | 3568 | 882 | 11.606 | 0.044 | 20,005 |
| Model time horizon 5 years | |||||
| Usual care | 694 | 3.872 | |||
| HOME BP intervention | 844 | 150 | 3.874 | 0.002 | 66,768 |
| Event | Usual care (n) | HOME BP (n) | Usual care (proportion) | HOME BP (proportion) | Difference |
|---|---|---|---|---|---|
| CHD death | 1149 | 1138 | 0.0230 | 0.0228 | 0.0002 |
| CVD death (stroke) | 888 | 801 | 0.0178 | 0.0160 | 0.0017 |
| Non-fatal MI | 2814 | 2760 | 0.0563 | 0.0552 | 0.0011 |
| Non-fatal stroke | 3711 | 3461 | 0.0742 | 0.0692 | 0.0050 |
| TIA | 761 | 714 | 0.0152 | 0.0143 | 0.0009 |
| Stable angina | 3806 | 3706 | 0.0761 | 0.0741 | 0.0020 |
| Unstable angina | 1312 | 1303 | 0.0262 | 0.0261 | 0.0002 |
| Any event | ∫ | 13,883 | 0.2888 | 0.2777 | 0.0112 |
As expected, when the cost difference in the base case (£38/year) was increased, so too did the QALY ICER. When the difference was increased to £100, the ICER rose to £26,432. A cost difference of £77 led to an ICER of just under £20,000, taken by some as a willingness-to-pay threshold.
In addition, when the time frame was reduced from lifetime in the base case, the ICER increased (see Table 23) from just over £9000 to just under £67,000. This implies that the health gains occur mainly after 5 years.
The long-term cost-effectiveness of the intervention depends on the number of cardiovascular events averted. Table 22 shows the number of events predicted in the model for each arm along with the proportions.
A total of 7.4% of individuals in the usual-care strategy had a non-fatal stroke, compared with 6.9% of individuals in the HOME BP arm. The majority of people (approximately 71–72%) did not have an event and died from other causes. Differences in outcomes were from the 28% of individuals who had an event.
The cost per major event avoided was about £36,000 (£402/0.0112).
Discounting made a difference, as might be expected. When costs were discounted 3.5%, then the base-case QALY ICER was £4508 per QALY and the difference in QALYs is 0.0891. When neither costs nor QALYs were discounted, then the ICER was £6573 per QALY.
Comparisons with other similar studies
Two studies were considered to be relevant: (1) TASMINH450 and (2) HITS a Scottish randomised trial of telemonitoring to control BP. 98,99 The TASMINH450 and HITS98,99 studies are briefly summarised in Table 23.
| Scenarios using a range of cost differences | Input | Output | |||
|---|---|---|---|---|---|
| BP difference (mmHg) at 6 months/12months | NHS cost (£) difference at 12 months | Cost difference (£) | QALY difference lifetime | Cost/QALY ICER | |
| TASMINH4:50 self-management vs. usual care | 3.5 | 8 | 124 | 0.0407 | 3035 |
| TASMINH4:50 telephone supporta vs. self-management | 4.7 | 14 | 302 | 0.0137 | 17,424 |
| HOME BP | 3.45 | 38 | 402 | 0.044 | 9107 |
| Not included in modelling | |||||
| HITS98,99 | 4.5 | 109 | |||
The reported comparisons from TASMINH450 were self-management compared with usual care and telemonitoring compared with self-management, and these are summarised along with HOME BP and HITS in Table 23. Several points are noted.
First, the reductions in BP are broadly similar across the three trials, ranging from 3.5 to 4.7 mmHg. The differences are more apparent in terms of their costs, which varied by a factor of £3 between TASMINH450 and HOME BP, with the HITS trial98,99 having a much higher cost.
Second, the relevant comparison between TASMINH450 and HOME BP would be that between telephone and usual care; however, this was not presented in the published analysis, which followed the standard practice of reporting interventions in terms of next best. However, the relevant ICER for that comparison was just over £3000, which is well below that for HOME BP, and this prompts the question of how the relative cost compared.
The costs in Table 23 refer to the differences between TASMINH450 and HOME BP in total cost after 12 months, including both the cost of the intervention and of services used. Neither TASMINH450 nor HOME BP provide precise estimates of the intervention for several reasons. Costs of interventions in trials reflect those incurred during the trial, which might well be different if those interventions were used in routine practice. The emphasis in trials is on delivering a new untried intervention, which may incur costs that would not otherwise be incurred. Furthermore, if used in routine practice, most interventions, and particularly interventions that are digital, would benefit from economies of scale. With the interventions under discussion, separation of the intervention and service use cost was difficult, with some elements of the intervention, such as follow-up reminders or telephone calls, being recorded as service use, rather than part of the intervention. For these reasons, we do not consider that the cost differences between the various interventions shown in Table 23 for TASMINH450 and HOME BP should be treated as precise. Rather, the cost differences provide an indication of the likely low cost of the interventions.
Turning to the HITS trial,98,99 two points are worth making. The HITS trial98,99 showed a similar reduction in BP as TASMINH450 and HOME BP, but a higher per patient cost (£109 vs. £38 for HOME BP) (see Table 23). This higher cost may reflect the HITS trial’s98,99 reliance on telemonitoring, which may have been more expensive at that time. Although we have not analysed the cost difference in detail, the long-term scenario analyses reported above included one scenario with a cost difference of £100, close to that in the HITS trial. 98,99 The scenario resulted in an incremental cost per QALY of £26,432. Although well above the incremental cost per QALY in the two other trials (i.e. TASMINH450 and HOME BP), this might still be considered as worthwhile value for money, particularly if the cost of providing the intervention might have declined since then.
Limitations
The usual limitations to do with randomised trials apply in that every trial is a specific one-off experiment. Against that, two trials50,98,99 have come to similar conclusions. All three trials (i.e. TASMINH4,50 the HITS trial98,99 and HOME BP) were fairly big and recruited from general practice in different locations. The overall conclusion must be that self-monitoring, supported to some extent by telemonitoring or a website, can lead to improvements in BP. Although the improvements are modest, as might be expected given the populations (i.e. most patients were already on medication for BP), they seem to be clinically worthwhile and reasonably cost-effective.
All three trials (i.e. TASMINH4,50 the HITS trial98,99 and HOME BP) shared a limitation to do with the costing of the intervention and its knock-on cost impact in the short run. Although this limitation is, to some extent, inevitable, the results indicated that such interventions can be provided at a modest cost per patient, which would be very likely to show economies of scale and reduced cost per patient if made widely available.
Recommendations for research
First, more comprehensive modelling of the long-term effects of BP reduction would appear to be useful, perhaps supported by an individual-level meta-analysis of the relevant trials. The informal review of the most relevant trials50,98,99 showed that the interventions trialled resulted in QALY gains, which seemed to be mainly in the longer term, a finding consistent with cost-effectiveness modelling carried out for NICE. Modelling might also be improved by more detailed comparisons between the relevant models and by monitoring the extent to which the projected reductions in cardiovascular events are confirmed in practice.
Second, more attention to the costing of the range of relevant interventions would be helpful. As discussed above, randomised trials providing a novel intervention designed only for that trial are not an ideal way to study what such interventions might cost in routine care, particularly if provided to large numbers of people.
Third, as noted above, although we aimed to include costs incurred beyond the NHS, we were unable to do so. Very few patients reported cost effects due to changes in lifestyle, which is consistent with the overall finding that the main impact of the intervention was on use of the appropriate medications. Although we collected data on the amount of time patients ‘spent’ using the web support, the process evaluation found that patients using he website experienced benefits, as well as costs. Research might usefully explore improved ways of measuring these contrasting elements.
Finally, research is needed on how digital aids for treating hypertension can best be located within the range of other self-management aids in other diseases and conditions. Following the COVID-19 pandemic, a shift to remote consultation seems likely to continue, as does increased provision of digital supports.
Conclusions
The reduction in BP in the HOME BP intervention was similar to that in other comparable trials. 50,98,99 The cost of the intervention was modest at just under £40 per patient. Although this is probably an overestimate, given that it was based on providing a novel service for relatively few people, it, nonetheless, delivered benefits that would be considered cost-effective in terms of NICE and the NHS. Long-term modelling puts the incremental cost per QALY at just over £9000. If included in a suite of DIs, which seems increasingly possible, the cost per patient would probably reduce.
More generally, post-COVID-19 and in line with demographic trends, self-management seems likely to become more widely used in modernised health services. The work reported here provides evidence of both its clinical effectiveness and cost-effectiveness.
Contributions
James Raftery designed, costed and wrote this appendix, with input from Richard J McManus. Shihua Zhu provided input on the within-trial analysis and Sue Jowett and Richard J McManus provided input on the long-term modelling.
Appendix 3 Predictors of systolic blood pressure at 12 months and engagement with self-reporting blood pressure readings
Predictors of systolic blood pressure at 12 months
The number of BP entries a patient made, the number of medication changes recommended and medication necessity beliefs to predict systolic BP at 12 months are provided in Tables 24–26.
| Predictor variable | β | SE | p-value |
|---|---|---|---|
| Number of BP entries | –0.908 | 0.26 | 0.001 |
| Baseline systolic BP | 0.323 | 0.077 | < 0.001 |
| Age | 0.151 | 0.105 | 0.344 |
| Predictor variable | β | SE | p-value |
|---|---|---|---|
| Number of recommended changes | –1.527 | 0.463 | 0.001 |
| Baseline systolic BP | 0.347 | 0.078 | < 0.001 |
| Age | 0.061 | 0.106 | 0.566 |
| Predictor variable | β | SE | p-value |
|---|---|---|---|
| Baseline medication necessity | –4.104 | 1.74 | 0.018 |
| Baseline systolic BP | 0.347 | 0.078 | < 0.001 |
| Age | 0.124 | 0.107 | 0.246 |
Predictors of number of blood pressure entries
Patients’ self-reported medication adherence (MARS), patients’ perceived concerns and patients’ perceived necessity of BP medication (BMQ) predicted the number of BP entries they made during the study, controlling for age and baseline BP (Tables 27–29).
| Predictor variable | β | SE | p-value |
|---|---|---|---|
| Baseline MARS | 3.874 | 0.416 | < 0.001 |
| Baseline systolic BP | –0.007 | 0.016 | 0.654 |
| Age | 0.047 | 0.022 | 0.032 |
| Predictor variable | β | SE | p-value |
|---|---|---|---|
| Baseline medication concerns | 2.851 | 0.345 | < 0.001 |
| Baseline systolic BP | 0.0006 | 0.017 | 0.968 |
| Age | 0.037 | 0.023 | 0.098 |
| Predictor variable | β | SE | p-value |
|---|---|---|---|
| Baseline medication necessity | 2.604 | 0.331 | < 0.001 |
| Baseline systolic BP | –0.013 | 0.016 | 0.442 |
| Age | 0.041 | 0.022 | 0.068 |
Appendix 4 Behaviour change constructs and techniques in the My Breathing Matters intervention
| Key barrier identified | Intervention component | Target construct (BCW) | Intervention function (BCW) | BCT (BCTv1) | Target construct (NPT) |
|---|---|---|---|---|---|
| Key behaviour: improved preventer medication adherence | |||||
| Participants do not know the difference between preventers and relievers, and do not associate symptom improvement with use of preventer | Simple information about different types of medication (including preventer vs. reliever), including noted normalisation of incorrect technique and videos of correct technique | Physical capability | Education; training |
4.1. Instructions on how to perform the behaviour 5.1. Information about health consequences 6.1. Demonstration of behaviour |
Collective action (skill set workability) |
| Information and advice is often too complex and large because of the large range of reasons for non-adherence | Tailored information about how to improve adherence based on current inhaler use | Psychological capability | Enablement; education |
5.1. Information about health consequences 2.2. Feedback on behaviour |
Coherence (individual specification) |
| User stories demonstrating how others benefited from more appropriate medication use | Reflective motivation | Persuasion |
5.1. Information about health consequences 6.2 Social comparison |
Cognitive participation (legitimation) Coherence (communal specification) |
|
| Information about identification of asthma triggers and appropriate management |
Psychological capability Physical capability |
Education; training | 4.1. Instructions on how to perform the behaviour | Collective action (skill set workability) | |
| Information about asthma symptoms and appropriate management of them |
Psychological capability Physical capability |
Education; training | 4.1. Instructions on how to perform the behaviour | ||
| Education and information about asthma medication and answers to common concerns | Psychological capability | Education |
5.1. Information about health consequences 9.1. Credible source 9.2. Pros and cons |
||
| Irregular schedule/too busy/run out of medicine/forget | Information on initial barriers to beginning challenge and how to overcome them |
Psychological capability Physical capability |
Education; enablement |
1.2. Problem-solving 8.6. Generalisation of a target behaviour 12.1. Restructuring the physical environment |
Cognitive participation (enrolment) Collective action (contextual integration) |
| Patients lack motivation to use inhaler regularly | Set goal dates of 1-month medication adherence | Physical opportunity | Enablement; environmental restructuring |
1.1. Goal-setting (behaviour) 1.4. Action planning |
Cognitive participation (enrolment/activation/initiation) |
| e-mails throughout challenge to remind user of commitment | Physical opportunity | Environmental restructuring |
7.1. Prompts/cues 8.1. Behavioural practice/rehearsal 8.3. Habit formation 12.5. Adding objects to the environment |
Collective action (contextual integration) | |
| User stories of benefits of 4-week adherence to medication | Automatic motivation | Persuasion; modelling |
5.1. Information about health consequences 5.6. Information about emotional consequences 6.2. Social comparison |
Reflexive monitoring (communal appraisal) | |
| Patients do not associate slow improvement of symptoms with increased adherence | Self-reporting of subjective symptoms to establish benefit | Reflective motivation | Enablement; persuasion |
2.2. Feedback on behaviour 2.4. Self-monitoring of outcome of behaviour |
Reflexive monitoring (systematisation, individual appraisal, reconfiguration) |
| Key behaviour: engagement with a PAAP | |||||
| Patients have not heard of an action plan | Information about benefits of an action plan to provide motivation to create a PAAP with the HCP | Psychological capability | Education |
4.1. Instructions on how to perform the behaviour 5.1. Information about health consequences |
Coherence (internalisation) |
| Patients want to make their PAAP without input from a HCP | Robust evidence that PAAPs work best when created in conjunction with HCPs | Reflective motivation | Persuasion | 5.1. Information about health consequences |
Coherence (individual specification) Collective action (contextual integration) |
| User stories of benefits of creating a PAAP |
Reflective motivation Automatic motivation |
Persuasion; modelling |
5.1. Information about health consequences 5.6. Information about emotional consequences |
Coherence (communal specification) | |
| Patients do not schedule a HCP appointment to make their PAAP | Blank PAAP to facilitate HCP consultation |
Physical capability Physical opportunity |
Enablement; environmental restructuring |
1.4. Action planning 12.1. Restructuring the physical environment |
Cognitive participation (initiation/enrolment) Collective action (contextual integration) |
| Reminder to facilitate booking appointment with a HCP | Physical opportunity | Environmental restructuring | 7.1. Prompts and cues | ||
| PAAP may not be used once made | Online storage and access of the PAAP | Physical opportunity | Environmental restructuring | 12.1. Restructuring the physical environment | Cognitive participation (activation) |
| Option to review the PAAP if having noticeable asthma symptoms | Physical opportunity | Environmental restructuring |
1.4. Action planning 2.7. Feedback on outcomes of behaviour |
||
| Key behaviour: attendance at annual asthma reviews | |||||
| Patients do not believe that their quality of life can be improved | Information about relevance of asthma review to improve quality of life |
Psychological capability Reflective motivation |
Education; persuasion |
5.1. Information about health consequences 5.6. Information about emotional consequences |
Coherence (internalisation/communal specification/individual specification) Cognitive participation (legitimation) |
| User stories from patients and GPs about the benefits of asthma review |
Reflective motivation Automatic motivation |
Persuasion; modelling |
5.1. Information about health consequences 5.6. Information about emotional consequences 6.2. Social comparison |
||
| Patients do not schedule a HCP appointment for a review | Facility to schedule reminder before asthma review and encouragement to book | Physical opportunity | Environmental restructuring |
7.1. Prompts/cues 12.5. Adding objects to the environment |
Cognitive participation (initiation/activation) Collective action (contextual integration) |
| Key behaviour: engagement with breathing retraining | |||||
| Patients do not believe that breathing retraining is as effective as medicine | Information regarding rationale behind breathing retraining and potential benefits | Reflective motivation | Education; persuasion |
5.1. Information about health consequences 5.6 Information about emotional consequences |
Coherence (internalisation) |
| Assessment of current breathing habits and tailored feedback regarding opportunities to improve | Psychological capability | Education; enablement |
1.1. Goal-setting (behaviour) 2.2. Feedback on behaviour |
Reflexive monitoring (individual appraisal) | |
| User stories emphasising benefits of breathing retraining | Automatic motivation | Persuasion; modelling |
5.1. Information about health consequences 5.6. Information about emotional consequences 6.2. Social comparison |
Coherence (communal specification) | |
| Patients find breathing retraining time-consuming and difficult, and lose motivation | Facility to plan times to practise to facilitate regular practise at convenient times |
Physical opportunity Physical capability |
Enablement; environmental restructuring |
1.1. Goal-setting (behaviour) 1.4. Action planning 4.1. Instruction on how to perform a behaviour |
Coherence (individual specification) Cognitive participation (initiation) |
| e-mails to remind user of ongoing practise and to facilitate proactive overcoming of barriers | Physical opportunity | Environmental restructuring; enablement | 7.1. Prompts/cues | Reflexive monitoring (individual appraisal) | |
| Examples of positive HCP views of breathing retraining |
Psychological capability Reflective motivation |
Education; enablement |
6.3. Information about others’ approval 9.1. Credible source |
Cognitive participation (legitimation) Collective action (relational integration) |
|
| Videos to demonstrate breathing retraining techniques (and text descriptions) | Psychological capability | Education; training |
4.1 Instructions on how to perform the behaviour 6.1. Demonstration of behaviour 8.1. Behavioural practice/rehearsal |
Collective action (skill set workability) | |
| Examples of effectively integrating techniques into everyday life | Psychological capability | Education; enablement |
1.2. Problem-solving 1.4. Action planning 4.1. Instructions on how to perform the behaviour 8.3. Habit formation |
Collective action (interactional workability) | |
| Patients do not associate slow improvement with breathing retraining practice | Self-monitoring of progress, including ability to track slow breathing and breath holds | Psychological capability | Training | 2.4. Self-monitoring of outcome of behaviour | Reflexive monitoring (individual appraisal) |
| Self-report symptom change over last month to establish benefit | Reflective motivation | Education | 5.2. Salience of consequences | Reflexive monitoring (systematising) | |
| Key behaviour: engagement with cognitive–behavioural stress management practice | |||||
| Patients do not consider asthma to impact quality of life/stress | Information about how stress impacts on quality of life | Reflective motivation | Education; persuasion |
5.1. Information about health consequences 5.6. Information about emotional consequences 6.3. Information about others’ approval 9.1. Credible source |
Coherence (individual specification/internalisation) |
| Information about prevalence in of stress in asthma population | Psychological capability | Education | 5.1. Information about health consequences | Coherence (individual specification) | |
| User stories on how to select appropriate stress reduction method and about impact on asthma quality of life |
Reflective motivation Automatic motivation |
Persuasion; modelling |
5.1. Information about health consequences 6.2. Social comparison |
Cognitive participation (legitimation) | |
| Information about unhelpful thought patterns that maintain and exacerbate disease | Psychological capability | Training | 5.6. Information about emotional consequences | Reflexive monitoring (individual appraisal) | |
| Stress occurs for many reasons and can be ‘just my breathing’ | Provide instruction on relaxation methods to reduce stress | Psychological capability | Training |
4.1. Instructions on how to perform the behaviour 6.1. Demonstration of behaviour |
Collective action (skill set workability) Reflexive monitoring (reconfiguration) |
| Stress management through planning, time management and self-care | Psychological capability | Training |
4.1. Instructions on how to perform the behaviour 6.1. Demonstration of behaviour |
||
| Patients have trouble finding time/continuing motivation for destress activities | Help planning time for practice and advice on when to schedule |
Psychological capability Physical opportunity |
Enablement; environmental restructuring |
1.4. Action planning 4.1. Instructions on how to perform the behaviour |
Cognitive participation (enrolment) |
| Reminder e-mails to facilitate practice | Physical opportunity | Environmental restructuring | 7.1. Prompts/cues | Collective action (contextual integration) | |
| Subsidiary behaviour: effective engagement with DI and its target behaviours | |||||
| Patients do not believe that their quality of life can be improved | Information about awareness of symptom prevalence in population, and impact of symptoms on quality of life | Psychological capability | Education |
5.1. Information about health consequences 5.6 Information about emotional consequences |
Coherence (internalisation) |
| Robust evidence that others have benefited/could benefit (e.g. scientific studies, individual success stories) | Reflective motivation | Education; persuasion |
5.1. Information about health consequences 6.3. Information about others’ approval 9.1. Credible source |
Coherence (communal specification) | |
| Evidence of development team expertise | Automatic motivation | Persuasion |
6.3. Information about others’ approval 9.1. Credible source |
Collective action (relational integration) | |
| Informing participants of necessary support provided by Asthma UK alongside DI | Physical opportunity | Enablement |
3.1. Social support (unspecified) 9.1. Credible source |
Cognitive participation (legitimation) | |
| Patients do not view asthma as a chronic disease (‘no symptoms, no asthma’) | Subjective self-monitoring of quality of life over last 2 weeks |
Reflective motivation Psychological capability |
Enablement | 5.2. Salience of consequences | Reflexive monitoring (systematisation/individual appraisal) |
| Tailored feedback to increase intervention relevance |
Psychological capability Reflective motivation |
Education |
5.1. Information about health consequences 2.2. Feedback on behaviour |
Coherence (individual specification) | |
| Education and information on asthma management and the My Breathing Matters intervention |
Psychological capability Reflective motivation |
Education; persuasion; environmental restructuring |
7.1. Prompts/cues 12.5. Adding objects to the environment |
Cognitive participation (activation) | |
| Patients are not motivated to engage family/friends in asthma management | Information about how social support can improve asthma quality of life |
Psychological capability Reflective motivation |
Education |
5.1. Information about health consequences 5.6. Information about emotional consequences |
Coherence (individual specification/communal specification) |
| User stories of successfully involving social support in asthma management | Automatic motivation | Persuasion; modelling |
5.1. Information about health consequences 5.6. Information about emotional consequences 3.1. Social support (unspecified) |
Cognitive participation (activation) Collective participation (legitimation) |
|
| Patients find it difficult to approach family and friends in care management | e-mail link to friends and family to involve them in asthma management | Physical opportunity | Enablement | 3.1. Social support (unspecified) |
Cognitive participation (enrolment) Collective action (contextual integration) |
| Family/friends do not understand maintenance treatment | Information for friends and family about asthma treatment and impact on quality of life | Social opportunity | Environmental restructuring |
5.1. Information about health consequences 6.3. Information about others’ approval |
Coherence (communal specification) |
Appendix 5 TIDieR report of the My Breathing Matters intervention
| Intervention item | Description |
|---|---|
| Name | My Breathing Matters. A DI to support self-management of asthma for patients in primary care |
| Rationale | The aim of the My Breathing Matters intervention was to improve functional quality of life of primary care patients with asthma, by supporting illness self-management by both pharmacological and non-pharmacological means |
| Materials |
The My Breathing Matters intervention contained several components, each with content designed to address specific behavioural targets. After an introductory session, which intended to improve motivation and engagement, session content was tailored for users based on their self-reported asthma-related quality-of-life scores. Users also received motivating e-mails specific to content that they had accessed (or were now able to access), as well as general ‘reminder e-mails’ intended to facilitate adaptive behaviour change independent of online My Breathing Matters intervention usage. All of the content was released over a period that ranged from 4 days to 1 month, depending on their tailored content and choices during the online program Pharmacological content All pharmacological content was tailored according to (1) frequency of current medication use and (2) self-reported medication behaviours. Based on these, users were recommended the following: |
|
|
| Procedures | Users needed to sign up to the My Breathing Matters intervention online using an access code that allows the study team to monitor their engagement and intervention usage. The My Breathing Matters intervention was designed to be unobtrusive to users’ lives. Users were encouraged to use the My Breathing Matters intervention as frequently as they saw necessary, consistent with their perceived quality of life impairment (although intervention content aimed to increase motivation and engagement with adaptive behaviours). For example, if users were to have no current symptoms, low My Breathing Matters engagement would be expected. e-mail reminders were sent out biweekly/monthly to facilitate engagement should symptom severity increase |
| Provision | The intervention was created by a collaborative multidisciplinary team of respiratory clinicians, physiotherapists, behaviour change and DI development experts, and two patient representatives (adults with asthma). The My Breathing Matters intervention was developed and is hosted using the opensource LifeGuide platform at the University of Southampton |
| Delivery | My Breathing Matters is designed to be delivered entirely online and by email. Optional external support for the intervention was provided by trained research nurses through the Asthma UK helpline |
| Location | The My Breathing Matters intervention was designed to be accessible entirely online. The website could be accessed wherever was convenient for the user |
| Timing | The My Breathing Matters intervention is designed to facilitate effective engagement with adaptive behaviours through a time course that suits users, reflecting the varied symptom severity that is characteristic of asthma patients. For example, some patients may immediately engage with support for improved medication adherence or healthy behaviour change, whereas others may sign up to the intervention but not engage until their symptoms noticeably affect their quality of life |
| Tailoring | The My Breathing Matters intervention was tailored at several points to facilitate tailored information for users. Techniques to do this included:
|
| Modifications | The My Breathing Matters intervention was not modified during the course of the study |
| Planned fidelity | Data on intervention engagement of individual users is available to the research team through usage data generated by the LifeGuide platform. The data were collected on 44 participants who were randomised to the intervention arm of the feasibility trial. Chapter 5 details the methods for the trial and usage analysis in full |
| Actual fidelity | Of the participants in the intervention group (n = 44), 81.8% (n = 36) logged into the My Breathing Matters intervention at least once. Participants logged in between 1 and 25 times (median = 4; interquartile range = 8) and participants using the intervention more than once (n = 27) used it between 1.89 to 337.85 days (median = 120.96 days; interquartile range = 148.23 days). Chapter 5 reports the usage analysis in full |
List of abbreviations
- ACQ
- Asthma Control Questionnaire
- A&E
- accident and emergency
- BCT
- behaviour change technique
- BCW
- behaviour change wheel
- BMI
- body mass index
- BMQ
- Beliefs about Medication Questionnaire
- BNF
- British National Formulary
- BP
- blood pressure
- BREATHE
- Breathing Retraining for Asthma – a Trial of Home Exercises
- CARE
- Congratulate, Ask, Reassure, Encourage
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- DI
- digital intervention
- DIPSS
- Integrating Digital Interventions into Patient Self-Management Support
- DVD
- digital versatile disc
- EQ-5D
- EuroQol-5 Dimensions
- GP
- general practitioner
- HCP
- health-care professional
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- INDEX
- IdentifyiNg and assessing different approaches to DEveloping compleX interventions
- MARS
- Medication Adherence Scale
- Mini AQLQ
- Mini Asthma Quality of Life Questionnaire
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NPT
- normalisation process theory
- PBA
- person-based approach
- PPI
- patient and public involvement
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RAISIN
- Randomized trial of an Asthma Internet Self-management InterventioN
- RCT
- randomised controlled trial
- SD
- standard deviation
- TASMINH2
- Telemonitoring and Self-Management in the Control of Hypertension
- TASMINH4
- Telemonitoring and Self-Monitoring of Blood Pressure for Antihypertensive Titration in Primary Care
- TASMIN-SR
- Targets and Self-Management for the Control of Blood Pressure in Stroke and at Risk Groups
- TIDieR
- Template for Intervention Description and Replication
- WS1
- workstream 1
- WS2
- workstream 2
- WS3
- workstream 3
Notes
-
Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist
-
Withdrawal letter before and after patient and public involvement input
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/BWFI7321).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
