Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1210-12005. The contractual start date was in February 2014. The final report began editorial review in June 2020 and was accepted for publication in May 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Blom et al. This work was produced by Blom et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Blom et al.
SYNOPSIS
Background to the INFORM programme
Joint replacement
Osteoarthritis is the most common form of joint disease, affecting nearly 10% of adults in the UK2 and about 23% of adults in the USA. 3 For people with advanced hip or knee osteoarthritis, as well as those with other joint conditions and injury, joint replacement aims to relieve pain and improve function.
In the UK in 2005, the lifetime risk of receiving a primary hip replacement was estimated to be 12% in women and 7% in men, and for primary knee replacement 11% in women and 8% in men. 4 Internationally, the lifetime risks of hip and knee replacement increased between 2003 and 2013. 5,6 In Spain in 2015, the lifetime risk in patients with osteoarthritis was 14% for hip replacement and 30% for knee replacement. 7
In the UK in 2019, > 200,000 primary hip and knee replacements were performed to treat diseased or damaged joints8,9 with about 98% a consequence of osteoarthritis. In the USA in 2020, an estimated 1.6 million hip and knee joints will have required replacement. 10
Prosthetic joint infection
For many people, joint replacement is a highly successful treatment leading to a reduction in pain and improvement in physical function. However, severe adverse events occur in a small number of patients, including infection, dislocation, fracture, thromboembolism and neurovascular damage. 11–16 Deep prosthetic joint infection is considered the most serious adverse event and, if untreated, can result in severe pain, disability and death. 17
Prosthetic joint infection occurring within 2 years of joint replacement is mainly surgically acquired18 and associated with wound inflammation, joint effusion, pain and loss of function. 19 Early infections are commonly caused by virulent bacteria with acute onset of pain, effusion, erythema and fever. Delayed infections typically present with symptoms similar to those after aseptic joint failure.
Bacteria adhere to implants, creating a glycocalyx biofilm and making treatment of established deep infection difficult. 20,21 Bacteria are introduced during the operation or later by haematogenous spread from other infected tissues and attach to the implant surface. Biofilm forms, matures and eventually disperses with spread of infection. Biofilm formation means that treatment with antibiotics alone is rarely effective, and, consequently, deep prosthetic joint infections are difficult to treat.
Deep surgical site infection after orthopaedic procedures has been described as ‘an event that inflicted deep suffering and changed the physical, emotional, social and economic aspects of life in extremely negative ways’. 22 People who develop prosthetic joint infection report high levels of pain and disability, and poor mental health and social functioning. 23 Even patients treated successfully have worse quality of life than those with uncomplicated joint replacement.
Incidence of prosthetic joint infection and rate of revision
In December 2019, we searched MEDLINE for cohort studies reporting incidence rates of hip and knee prosthetic joint infection. Fifteen studies were identified (see Appendix 1). Methodological quality was assessed, specifically concerns relating to selection bias (inclusion of consecutive patients and representativeness) and missing data (follow-up rate of < 80%). 24 Prospective studies had good follow-up rates. Concerns about generalisability in some studies arose from the inclusion of single centres and the retrospective identification of cases.
Hip prosthetic joint infection
Four studies had no methodological concerns. 25–28 In Finnish and Swedish joint registries, rates of prosthetic joint infection within 2 years of primary hip replacement were about 0.9%. 27,28 In other studies with no methodological concerns, rates of infection ranged from 0.78% within 2 years in a US multicentre cohort25 to 2.09% within 1 year in national surveillance data in the Republic of Korea. 26 Single-centre UK studies with prospective and retrospective designs reported infection rates of 0.57% at up to 15 years12 and 1.08% after 8 years,29 respectively.
Revision for hip prosthetic joint infection
In registry studies from Norway, the UK and Denmark that had no methodological concerns, revision to treat prosthetic joint infection after hip replacement was required in 0.55% of patients at 1 year,30 0.54% at 5 years31 and 0.62% at 10 years. 32 In a single-centre UK retrospective cohort, 0.45% of patients required revision for infection at 5- to 8-year review. 12
Knee prosthetic joint infection
In Finnish registry and Republic of Korea national surveillance data, neither of which had methodological concerns, rates of infection after knee replacement were 1.14% at 2 years27 and 1.9% at 1 year,26 respectively. In prospective single-centre Finnish and UK cohorts, the infection rates were 0.8% at 1 year33 and 0.86% at up to 15 years, respectively. 29 In a prospective single-centre Spanish cohort, the incidence of surgical site infection was 1.0% at 90 days. 34 Retrospective single-centre cohorts from the UK and Germany reported infection rates of 0.97% at a mean of 6.5 years11 and 3.08% at 1 year, respectively. 35
Revision for knee prosthetic joint infection
The rate of revision for treatment of knee prosthetic joint infection in a UK registry with no methodological concerns was 0.75% at 10 years. 36 In a retrospective single-centre cohort from Taiwan (Province of China), the rate of infection requiring surgical intervention was 1.19% at 2 years. 37
Treatment options
Patients with prosthetic joint infection and the surgical team face a difficult and protracted course of treatment and recovery. Multiple surgical procedures are frequently required to clear infection and reduce the need for joint excision or amputation. Treatment options include long-term suppressive antibiotic treatment, generally reserved for patients unsuitable for surgery because of comorbidities;38 debridement, antibiotics and implant retention (DAIR); revision with a single- or two-stage procedure; and temporary replacement with a functional articulating spacer.
Debridement, antibiotics and implant retention
Debridement, antibiotics and implant retention involving extensive debridement and prosthesis retention, but with replacement of modular components such as polyethylene liners, is a treatment option in early post-surgical and late acute infections. 39 DAIR is practical only if there is no evidence of prosthesis loosening or significant damage to soft tissue, and in infections that can be treated with antibiotics. 18 The need for long-term and possibly lifelong antibiotic treatment is acknowledged,40 and about 55% of patients treated with DAIR may subsequently need implant replacement. 41
Two-stage revision
Surgical revision involves prosthesis removal, debridement, antibiotic treatment and replacement. In the well-established two-stage procedure, the replacement of the prosthesis is delayed for a period of a few weeks to many months permitting localised antimicrobial strategies and monitoring of infection. However, mobility and quality of life are poor between surgeries. To reduce long-term problems resulting from an extended period without an implant, an antibiotic-impregnated cement ‘spacer’ may be used to maintain some function and a correct leg length, and to reduce long-term problems associated with non-use. Although spacers improve patient mobility, complications can arise, including spacer dislocations and fractures, and femoral fractures. 42,43
Single-stage revision
An alternative revision strategy with implant removal, debridement and replacement in one operation has been favoured in some centres44,45 and is increasingly used in the UK. Although single-stage revision surgery is considered by some surgeons to be appropriate for only a minority of patients, at the Hamburg ENDO-Klinik it has been used in the treatment of 85% of cases of hip prosthetic joint infection. 44
Functional articulating spacer
Some people who receive the first stage of a two-stage revision for hip prosthetic joint infection and have an articulating spacer fitted achieve a satisfactory outcome and prefer to keep this in place and not undergo the second-stage surgery. Systems have been refined with the aim of providing a potentially long-lasting functional joint with the need for only one operation but, if required, a straightforward second-stage total hip replacement. A custom-made articulating spacer (CUMARS) widely used in the UK has all-polyethylene acetabular components and the Exeter Universal stem (Stryker Orthopaedics, Mahwah, NJ, USA). 46 The stem is fixed in place with antibiotic loaded acrylic cement using a technique that allows for possible removal should a second-stage procedure be required.
Programme development
To support our programme application, we conducted a systematic review comparing single- and two-stage revision procedures in patients with hip prosthetic joint infection. 47 We found 2-year rates of reinfection or development of a new infection after single-stage revision of 8.6% [95% confidence interval (CI) 4.5% to 13.9%] and after two-stage revision of 10.2% (95% CI 7.7% to 12.9%). We limited bias by including series of consecutive patients with hip prosthetic joint infection treated exclusively by single- or two-stage revision. We concluded that randomised trials were needed to establish optimum management strategies. Furthermore, the evidence base was highly limited regarding health service, patient and economic significance, risk factors, patient and surgeon experiences, diagnosis, and patient preferences for treatments.
Programme development: patient and public involvement
Collaboration with patient-partners took place through meetings with our dedicated patient and public involvement (PPI) group, the Patient Experience Partnership in Research (PEP-R), which comprised nine people who had musculoskeletal conditions. 48 The group met every 6–8 weeks, providing input into project design and conduct. Through ongoing training and support from research staff and our patient involvement co-ordinator, group members were familiar with research designs, conduct and some of the barriers to successful research. We used National Institute for Health and Care Research (NIHR) INVOLVE guidance to ensure that PPI was appropriately organised49 and we were part of the South West ‘People in Research’ consortium.
In December 2010, February 2011 and September 2011 we discussed the planned research with PEP-R. The group believed that, although relatively few people developed an infection, the research was important. They noted the need for feasibility work to assess the acceptability of randomisation. They offered their full support for future work and we considered their ongoing involvement crucial to ensure that the project reflected the priorities of the public and was interwoven throughout the programme.
The INFORM programme
The overall aim of the INFORM (INFection ORthopaedic Management) programme was to identify methods that may improve treatments and outcomes for patients with deep prosthetic joint infection after total hip or knee replacement. Studies were grouped by research methodology into seven work packages, all supported by PPI (Figure 1):
-
Work package 1. We aimed to conduct systematic literature reviews of treatments for prosthetic joint infection after hip and knee replacement and conduct an individual patient data (IPD) meta-analysis to compare reinfection outcomes after single- and two-stage revision surgery. We also aimed to review risk factors, methods of diagnosis and costs.
-
Work package 2. We aimed to analyse the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man (NJR) to identify the predictors of prosthetic joint infection after hip and knee replacement and to compare outcomes according to health-care characteristics.
-
Work package 3. Through qualitative interviews, we aimed to assess the impact on patients of prosthetic joint infection and treatment strategies, surgeons’ views on treatment, and patients’ and surgeons’ views on a randomised trial.
-
Work package 4. In a randomised controlled trial (RCT) with embedded qualitative interviews, we aimed to investigate whether or not treating hip prosthetic joint infection with a single-stage revision rather than the traditionally used two-stage revision improved patients’ quality of life and was cost-effective.
-
Work package 5. In analyses of the NJR we aimed to assess the economic implications of prosthetic joint infection and treatment strategies. We also aimed to conduct a comprehensive economic evaluation within the RCT comparing single- and two-stage revision for treatment of hip prosthetic joint infection.
-
Work package 6. Through a discrete choice questionnaire, we aimed to assess the trade-offs that patients are willing to make between patient-reported and clinical outcomes and explore the degree to which treatment strategies change preferences for those outcomes.
-
Work package 7. Finally, we aimed to disseminate findings to patients, the public, clinicians and stakeholders.
FIGURE 1.
The INFORM programme. WP, work package.
Work packages were conducted in parallel. An exception was the work package 3 qualitative study looking at surgeon decision-making, which directly influenced the design of the INFORM RCT in work package 4. The study of patient preferences in work package 6 followed on from the INFORM trial but focused on patient experiences of surgical treatments independently of trial participation.
Changes to the programme and additional research
Planned research is published, has been submitted to a journal or is being written up. We have also received a NIHR Programme Development Grant to develop recommendations based on the INFORM findings and explore implementation.
An additional published systematic review of outcomes after DAIR is summarised. Further studies included systematic reviews of risk prediction tools, health-care needs and support, and treatment comparisons in ankle and shoulder infection; and a UK survey of care pathways.
We had originally planned to examine the accuracy, costs and cost-effectiveness of a broad range of methods for diagnosing and monitoring infection. Ultimately, in the programme, we focused on new promising biomarkers for the diagnosis of infection.
In NJR analyses, we planned to compare outcomes in ‘specialist’ and other centres. We were unable to define ‘specialist’ centre and have compared care according to different health-care characteristics.
The NJR and Hospital Episode Statistics (HES) linked analysis of economic burden of hip prosthetic joint infection was completed as planned. The study of knee prosthetic joint infection has been delayed as the COVID-19 pandemic prevented on-site access to NJR data.
INFORM: organisation and management
The INFORM programme was run and co-ordinated by the Musculoskeletal Research Unit at the University of Bristol and North Bristol NHS Trust.
Programme Steering Committee
The programme was overseen by a Programme Steering Committee chaired by Professor Rod Taylor, NIHR Senior Investigator and Professor of Health Services Research, University of Exeter. Other independent committee members were Martyn Porter, the past president of the British Orthopaedic Association and Medical Director of the NJR; Ali Heawood, Senior Research Fellow in Qualitative Primary Care Research; and Julie Chappell, a solicitor specialising in medico-legal negligence. The Programme Steering Committee met on nine occasions (every 6 months), with at least three independent members present, to monitor the progress of the programme and help the research team to meet agreed milestones. They expressed their satisfaction with progress in all work packages but noted the slow recruitment to the RCT and supported measures to enhance recruitment, in particular the inclusion of more sites.
Pan-programme group
The pan-programme group (research team, co-applicants, site principal investigators and sponsor representative) met on 24 occasions (every 3 months) to discuss progress, outputs and dissemination from each of the work packages.
Work packages
Each work package lead arranged an internal working group that met regularly to ensure the delivery of agreed outputs. Work package 4 was run by the Trial Management Group (chief investigator, site principal investigators, co-applicants, trial manager, statistician, health economist, qualitative researcher and methodologist from Bristol Randomised Trials Collaboration), which met monthly to design and deliver the randomised trial.
Data monitoring committee
For work package 4, an independent data monitoring committee was established, consisting of a trial methodologist and two orthopaedic surgeons. This committee met on four occasions and reported directly to the Programme Steering Committee.
Microbiology
A meeting was held in 2014 with the lead consultant clinical microbiologists from four of the trial sites (Bristol, Exeter, Oxford and Cardiff). The group assisted with trial design and participant recruitment processes and advised on the collection of microbiological, antibiotic and blood biomarker data.
Study sponsor
Good Clinical Practice, local research governance and finance were overseen by the study sponsor (North Bristol NHS Trust Research and Innovation Department) and the University of Bristol. NHS Research Ethics and Research and Development approvals were obtained for work packages 3, 4 and 6.
INFORM randomised controlled trial
Selection of centres
Four centres were specified in the application and surgeons from each centre were co-applicants. To address early low recruitment rates, more sites were added. Eligible sites were high-volume tertiary referral centres for infected joint replacements, or large NHS orthopaedic units. Participating surgeons had experience of and expertise in both single- and two-stage revision treatment for hip prosthetic joint infection. Surgeons from Sweden showed interest in the research and Swedish centres joined the trial in 2016.
Training of researchers
We held workshops with recruiting nurses to provide training and support for the identification and recruitment of patients. Audio-recordings of recruitment interviews were used to explore, critique and improve the role of research nurses in the recruitment process. Recruiters met on three occasions and held monthly teleconferences with the trial manager to discuss recruitment and share their experiences of recruitment interviews.
Publication committee
To achieve high publication standards, an INFORM publication policy was adhered to. Manuscripts and conference articles were approved by the committee prior to submission. The committee consisted of Professor Ashley Blom (chief investigator), Professor Rachael Gooberman-Hill (qualitative lead) and Mr Stephen Jones (orthopaedic surgeon).
Patient and public involvement in the INFORM programme
Aims
The aims of our PPI were to gain input into the design of patient recruitment and information literature, research processes and questionnaires; identification of outcomes of importance to patients; and dissemination strategies. PPI also helped to ensure that milestones were met and that input was provided into the interpretation of data and findings:
It feels that between us all we may make a difference to patients who may have an infection.
INFORM forum group member
Patient and public involvement
Patients have been crucial to the success of this research in the following ways:
The Patient Experience Partnership group
The PEP-R group is the dedicated patient involvement forum at the Musculoskeletal Research Unit, University of Bristol, which comprises nine people who have musculoskeletal conditions, most of whom have had joint replacement.
INFORM patient forum
To complement the PEP-R group’s activities, it had been proposed that each work package would have an oversight forum including patient-partners who had experience of infection following joint replacement. When talking to patients, it became apparent that they wanted to be involved in work package oversight through a forum that met regularly. Four patients agreed to take part and the first meeting was held in December 2013. An additional patient was keen to be involved but was house-bound while waiting for revision surgery. This patient joined the forum in April 2015:
It has been fantastic to have a group of patients with this rare condition who are happy to meet and discuss the project at regular intervals.
INFORM researcher
Arthritis Care/Versus Arthritis
In the development of the programme, we collaborated with Arthritis Care. It had been proposed that a representative from Arthritis Care would sit on the programme’s steering committee. Following a change in organisation within the charity, this was no longer possible. We plan to work with Versus Arthritis in disseminating details of the study to their members, their volunteers and the wider public.
Steering committee
A lay partner was a member of the Programme Steering Committee. This group received regular reports on the PPI activity.
Patient and public involvement meetings
Members of the PEP-R group worked on the grant application and were involved throughout the programme. A meeting in March 2020 to share results was postponed due to the COVID-19 pandemic. We will continue working with the group remotely. PEP-R group members helped to prepare the Plain English summary for this report.
The INFORM patient forum has met 33 times and provided input into all work packages. We are continuing to work with the forum remotely to complete work on writing an information leaflet for newly diagnosed patients, reviewing information on prosthetic joint infection on the internet, writing a summary of results for trial participants, creating infographics, finishing their conference presentation, and working with Versus Arthritis to support and inform patients who have prosthetic joint infection. The PPI activity is summarised in Table 1.
| Type | PPI activity and actions | Date of PPI activity |
|---|---|---|
| WP1 | ||
| INFORM | Discussed findings and public dissemination | April 2016, February 2017 |
| WP2 | ||
| INFORM | Discussed NJR | June 2014 |
| INFORM | Discussed the predictors of prosthetic joint infection | February 2016 |
| INFORM | Discussed The Lancet hip paper50 and public dissemination | June 2018 |
| INFORM | Reviewed The Lancet hip paper50 press release | September 2018 |
| INFORM | Discussed The Lancet knee paper51 | September 2018 |
| WP3 | ||
| INFORM | Discussed the design of the interview study and important interview questions | February 2014, April 2014 |
| INFORM | Discussed study summaries of findings for participants | August 2015 |
| INFORM | Discussed the findings from interviews with hip patients and the plans to interview knee patients | February 2016 |
| INFORM | Discussed a peer-support programme for patients having treatment for infection or other complication following joint replacement | November 2017 |
| INFORM | Discussed the findings of the interviews with surgeons | April 2018 |
| INFORM | Discussed the findings of the interviews with patients | April 2018, June 2018 |
| INFORM | Discussed peer-support programme for patients having treatment for infection or other complication following joint replacement | June 2018 |
| INFORM | Discussed the findings on information needs from the interviews with hip and knee patients | April 2019 |
| INFORM | Discussed public dissemination | April 2018, June 2018, September 2019, November 2019 |
| WP4 | ||
| INFORM | Discussed the design of the trial, the patient information leaflet, recruitment information and questionnaires | April 2014, September 2014, November 2014 |
| PEP-R | Reviewed patient information leaflet and questionnaires | September 2014 |
| INFORM | Discussed feedback and information for trial clinicians and researchers | June 2015 |
| INFORM | Discussed patient recruitment strategies | April 2016, January 2018 |
| INFORM | Discussed the extension to the trial | June 2017 |
| INFORM | Discussed a feasibility study of revision surgery for knee infections | October 2017 |
| INFORM | Discussed the design of an update leaflet for trial participants | February 2018, April 2018 |
| PEP-R | Discussed results and public dissemination | September 2019 |
| WP5 | ||
| INFORM | Discussed the resource use diaries and questionnaires | June 2014, November 2014 |
| WP6 | ||
| INFORM | Discussed the discrete choice questionnaire | February 2015, April 2015, September 2016, November 2016 |
| INFORM | Discussed the results | July 2019 |
| WP7 | ||
| INFORM | Discussed that findings should be written up as a patient information booklet in printed and electronic versions and short films | February 2015, November 2016, November 2018, February 2019, September 2018, ongoing |
| INFORM | Reviewed the film on the evaluation of the INFORM PPI group | September 2018 |
| INFORM | Discussed public dissemination of the trial | July 2019 |
| INFORM | Discussed the published papers to date | April 2019 |
| INFORM | Discussed important things to tell surgeons about PPI | September 2019 |
| INFORM | Discussed their conference presentation | January 2020, ongoing |
| INFORM | Discussed the conference invitations, timings, speakers, venue and agenda | September 2019, January 2020, ongoing |
| PEP-R | Discussed the conference | September 2019 |
| INFORM | Discussed the summary leaflet for participants/infographics | Ongoing |
| Final report | ||
| PEP-R | Read and revised draft versions of the final report Plain English summary | May 2020 |
Contributions to INFORM and impact on programme
The impact of the INFORM patient forum on programme conduct and dissemination is summarised in Tables 2 and 3. In Box 1 we summarise INFORM researcher comments on PPI impact as reported in evaluations in 2014 and 2019.
| What they told us | Impact |
|---|---|
| We should ‘take the research to the patient’ rather than ‘take the patient to the research’ | We established a new patient forum for the programme, and the researchers came to them to discuss all the WPs, rather than patients sitting on steering groups |
| This was the first time they had spoken to other patients who had experience of infection following joint replacement:When infection is diagnosed you feel as though it doesn’t happen to anyone else. Interesting to hear others’ experiencesINFORM PPI group member | Social time was built in to meetings |
| Infection following joint replacement has a considerable impact on their families as well as them |
The forum members felt that relatives and significant others should also be involved with the forum and it was decided that they should be invited to attend selected meetings. Two relatives joined the meeting in September 2014 at which we discussed the trial and the patient recruitment information The forum members suggested that 2020 conference attendees could invite someone to come with them |
| PPI needs to support patients with mobility issues | One forum member could attend by taxi only and there were times when others were unwell and unable to drive. We were fortunate that we had applied for funding to offer taxis to forum members |
| They wanted to know about the other PPI work and research being carried out by our unit | The forum members visited the Musculoskeletal Research Unit offices and had several opportunities to meet researchers outside the forum. In January 2015 we held an event so that the PEP-R forum members and the INFORM forum members could meet. Both groups enjoyed finding out about each other’s work and will have the opportunity to meet up at a planned INFORM conference when the COVID-19 pandemic allows |
| Forum members wanted to know about their impact on the programme:From your comments I feel sure that the group has helped you in your researchINFORM PPI group member | Forum members received verbal updates and feedback leaflets on previous meetings, including changes made by researchers following discussions with the group |
| Forum members were very positive about the PPI:I’d just like to say it’s been one of the most interesting things I’ve ever been involved inINFORM PPI group member | In 2014, forum members and researchers were given a questionnaire evaluating the group. In 2020, the forum members took part in an evaluation of the PPI work within the unit. The findings are being written up for publication |
| What they told us | Impact |
|---|---|
| All five members remain committed and enthusiastic members of the research team:I’ve just thoroughly enjoyed it; it gave me my confidence back againINFORM PPI group member | All forum members are keen to share their experiences of being involved in the patient forum and one member will speak on behalf of the group at the 2020 conference |
| They identified that there is a lack of support services for patients with infection following joint replacement:I’ve benefited from meeting other people in a similar situation to meINFORM PPI group member | Based on this we developed a new grant application; unfortunately, this was unsuccessful. We are now working with Versus Arthritis to see if it can be a signpost for patients to find existing support services |
| They identified the lack of patient information on infection following joint replacement. They suggested that a website or booklet with information would be insightful | We are working together to write up the findings as a patient information booklet to be given out in infection clinics. We plan to share this information with Versus Arthritis to update its website |
| They identified the importance of keeping participants informed when the trial was extended | We took their advice and sent update leaflets to participants to keep them engaged with the trial |
| They told us that the conference should be in plain English | We have taken their advice on conference invitations, timings, speakers, venue, inviting participants’ family members to attend and agenda. Twenty-five participants and their partners registered to attend the conference in May 2020 |
PPI was essential in the grant application . . . the views of patients were essential in development of diverse work packages . . . helped to shape the way I think about different methods used to treat infected joint replacement . . . the different responses and questions from patients compared with researchers and clinicians at scientific meetings.
Their advice greatly improved the patient information sheets and the planned ways of approaching and discussing the trial with potential participant.
The success of our ethics application is a reflection of the PPI work.
They valued the research and answered questions only they could answer. If you want to understand whether your research will work and if it is of value from a patient’s perspective, then ask a patient! It also helped me to very quickly gain an insight into the impact of the condition, giving me a head start, orientating me, and sharpening my focus in order to further develop our approach and design.
Having been through treatment themselves they were able to provide us information on home care and home changes that we had not previously known. This allowed us to amend and improve these sections (of the health economics questionnaires).
Public dissemination
Dissemination of the results from INFORM is ongoing. A 1-day conference to share results with study participants, patient-partners, carers and the general public was planned for May 2020 but has been delayed because of the COVID pandemic.
Our film about the INFORM randomised trial was featured on University of Bristol and NIHR websites in June 2018. By 22 February 2020 it had been viewed 378 times (www.youtube.com/watch?v=_AH1Nu3tWVch).
Our film about the PPI evaluation was tweeted about by the University of Bristol and featured in the INVOLVE September 2018 newsletter. By 22 February 2021 it had been viewed 231 times (www.youtube.com/watch?v=TrZfHfVaMZE).
The Musculoskeletal Research Unit website is at www.bristol.ac.uk/translational-health-sciences/research/musculoskeletal/orthopaedic/research/inform.html and INFORM Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) account is at https://twitter.com/BristolINFORM.
The significance of prosthetic joint infection to health care
Here we summarise our published NJR analyses,32,36,52 and an unpublished review is given in Appendix 2.
Aims
We aimed to describe the risk of revision due to prosthetic joint infection for patients undergoing primary and revision hip or knee replacement, the changes in risk over time and the overall significance of prosthetic joint infection to the NHS. Supported by a systematic review, we aimed to estimate the economic significance of revision for hip prosthetic joint infection.
Methods
Surgeon, patient and health-care implications of hip and knee prosthetic joint infection
We analysed revision surgeries performed as a result of a diagnosis of prosthetic joint infection linked to index procedures recorded in the NJR between 2003 and 2014. 8 Revision surgeries were a single stage or the first stage of a two-stage revision, hip excision or a DAIR procedure with modular exchange.
Prevalence, cumulative incidence functions and the burden of all procedures for the treatment of prosthetic joint infection were calculated. To investigate time trends in the risk of revision for hip and knee prosthetic joint infection, we plotted the time from index surgery (primary or aseptic revision) to revision for infection and compared rates within 3 months, between 3 and 6 months, 6 months to 1 year, 1–2 years, 2–3 years, 3–4 years and 5–6 years. Log-linear regression, using the year of the index knee replacement as a continuous independent factor, was used to explore overall linear trends between 2005 and 2013.
Health economic consequences of prosthetic joint infection treatment: systematic review
The protocol was registered on PROSPERO (CRD42017069526). We searched MEDLINE and EMBASE on Ovid from inception to 10 April 2019 with no language restrictions. Studies included in our other systematic reviews were inspected. Inclusion criteria were:
-
patients with hip or knee prosthetic joint infection after primary replacement or aseptic revision
-
intervention relating to revision surgery
-
comparator with no prosthetic joint infection or alternative surgical treatment
-
outcome of cost-effectiveness or comparative costs
-
full economic evaluation or cost comparison study.
After detailed screening by two reviewers, data were extracted on study setting, patient characteristics, treatments, dates, sources of cost information, currency, costs and issues relating to quality assessment.
Health economic consequences of prosthetic joint infection: NJR analysis
Patients with hip prosthetic joint infection who received a single- or two-stage revision procedure in England between 2006 and 2009 were matched 1 : 5 with patients who had neither infection nor revision. Matching was based on age, sex, American Society of Anesthesiologists (ASA) grade, total or resurfacing hip replacement, date of primary surgery and hospital. If fewer than five patients could be matched adequately with cases, then available patients with no infection were included.
Using patient health-care records from English Hospital Episode Statistics (HES) and values from 2014/15 UK national reference costs, overall hospital inpatient and day-case costs were calculated for the first 5 years after primary hip replacement. Costs were not limited to orthopaedic health care. Incremental differences in costs between those revised and those not revised for prosthetic joint infection were estimated using a two-part model (probit and generalised linear model).
Results
Surgeon, patient and health-care implications of hip prosthetic joint infection
The NJR cohort analysis included 623,253 primary hip replacements and 63,222 aseptic revision hip replacements. A total of 7040 patients required one or more courses of revision surgery to treat prosthetic joint infection (n = 7585 procedures). Of these, 3338 patients (n = 3546 procedures) had no first surgery recorded in the NJR. Thus, our analyses were limited to 3702 patients (n = 4039 procedures), of whom 2705 had an index primary hip replacement (n = 2926 procedures) and 997 had a revision hip replacement (n = 1113 procedures).
The cumulative incidence functions of revision for prosthetic joint infection following index primary and aseptic revision hip replacement are shown in Figure 2. The probability of revision for prosthetic joint infection after a primary hip replacement was 0.15% (95% CI 0.14% to 0.16%) at 1 year and 0.62% (95% CI 0.59% to 0.65%) at 10 years. The probability of revision for prosthetic joint infection following an aseptic revision was higher, with rates of 0.69% (95% CI 0.63% to 0.76%) at 1 year and 2.25% (95% CI 2.08% to 2.43%) at 10 years.
FIGURE 2.
Cumulative incidence function of revision for prosthetic joint infection following index primary and aseptic revision hip replacement.
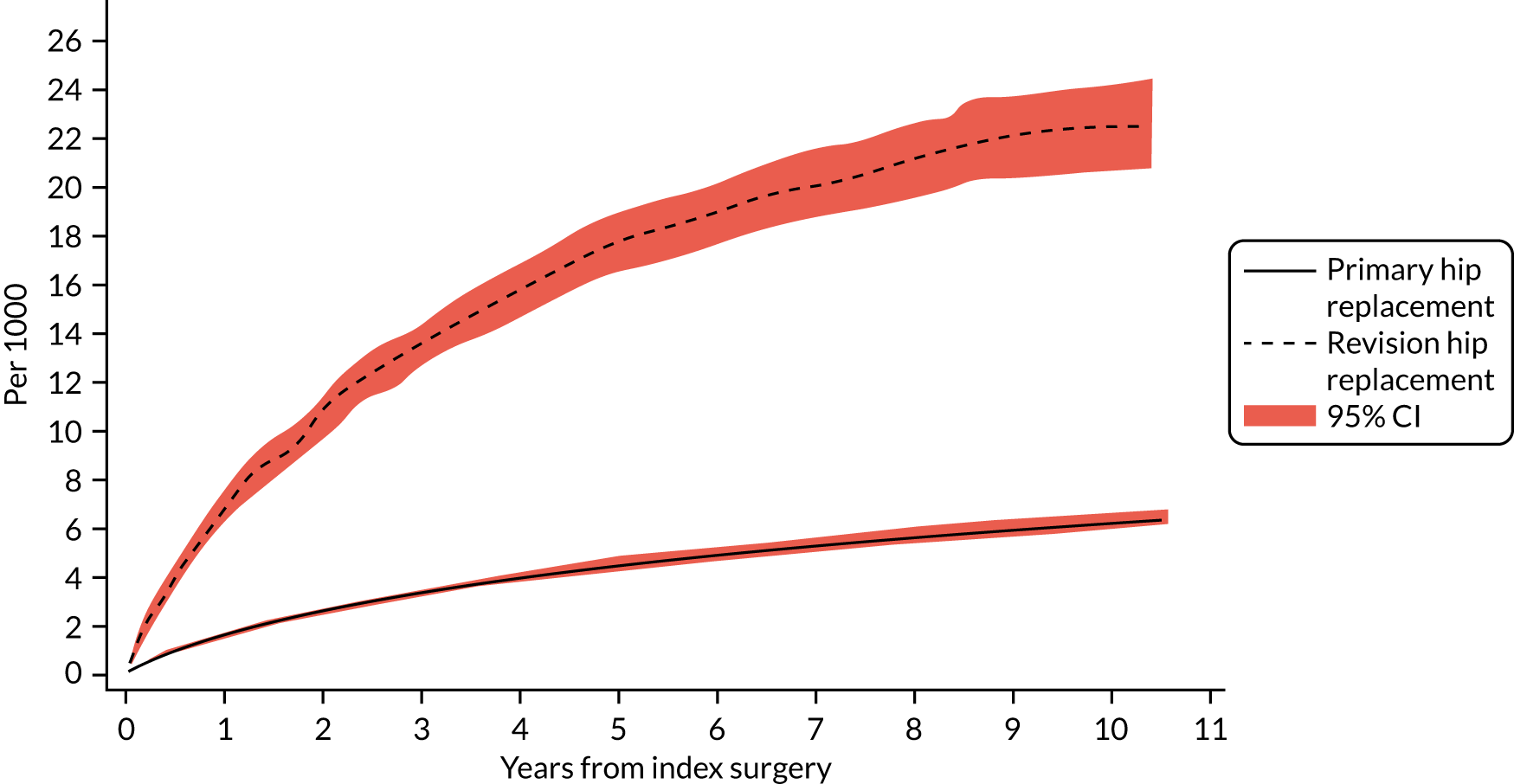
The prevalence of revision due to prosthetic joint infection in the 3 months following primary hip replacement more than doubled between 2005 and 2013 (rate ratio 2.29, 95% CI 1.28 to 4.08; time trend likelihood ratio test p < 0.0001). No time trends for revision for the time periods 3–6 months, 6 months to 1 year and 1–2 years were found. However, later rates of revision prosthetic joint infection decreased over time (between 2 and 3 years rate ratio 0.52, 95% CI 0.32 to 0.84, time trend p < 0.0001; between 3 and 4 years rate ratio 0.49, 95% CI 0.31 to 0.80, time trend p = 0.001; and between 4 and 5 years rate ratio 0.44, 95% CI 0.27 to 0.73, time trend p = 0.028).
The absolute number of procedures performed annually to treat infection increased from 384 in 2005 to 1002 in 2014, a 2.6-fold increase. This was greater than the twofold increase in primary procedures during this period.
Overall, 70% of revisions for prosthetic joint infection after hip replacement were two-stage procedures. However, in 2014, 60.7% of revision surgeries were carried out with a two-stage procedure and 29.7% were carried out with a single-stage procedure. The use of DAIR rose from < 1% of revision surgeries in 2005 to 7.6% in 2014.
Surgeon, patient and health-care implications of knee prosthetic joint infection
Between 2003 and 2014, 679,010 index primary knee replacements and 33,920 index revision knee replacements were registered in the NJR. A total of 8247 revision total knee replacements were performed as a result of a diagnosis of prosthetic joint infection. Our analyses of prevalence were limited to 3659 patients with a primary knee replacement (4004 procedures) and 717 patients with a revision knee replacement (785 procedures) as 3458 index operations had been conducted before the establishment of the NJR.
The cumulative incidence functions of revision for prosthetic joint infection following index primary and aseptic revision knee replacement are shown in Figure 3. The probability of revision knee replacement due to prosthetic joint infection was 0.17% (95% CI 0.16% to 0.18%) at 1 year and 0.75% (95% CI 0.72% to 0.78%) at 10 years. The probability of revision for prosthetic joint infection following an aseptic revision was higher, with rates of 0.76% (95% CI 0.68% to 0.86%) at 1 year and 3.13% (95% CI 2.81% to 3.49%) at 10 years.
FIGURE 3.
Cumulative incidence function of revision for prosthetic joint infection following index primary and aseptic revision knee replacement.
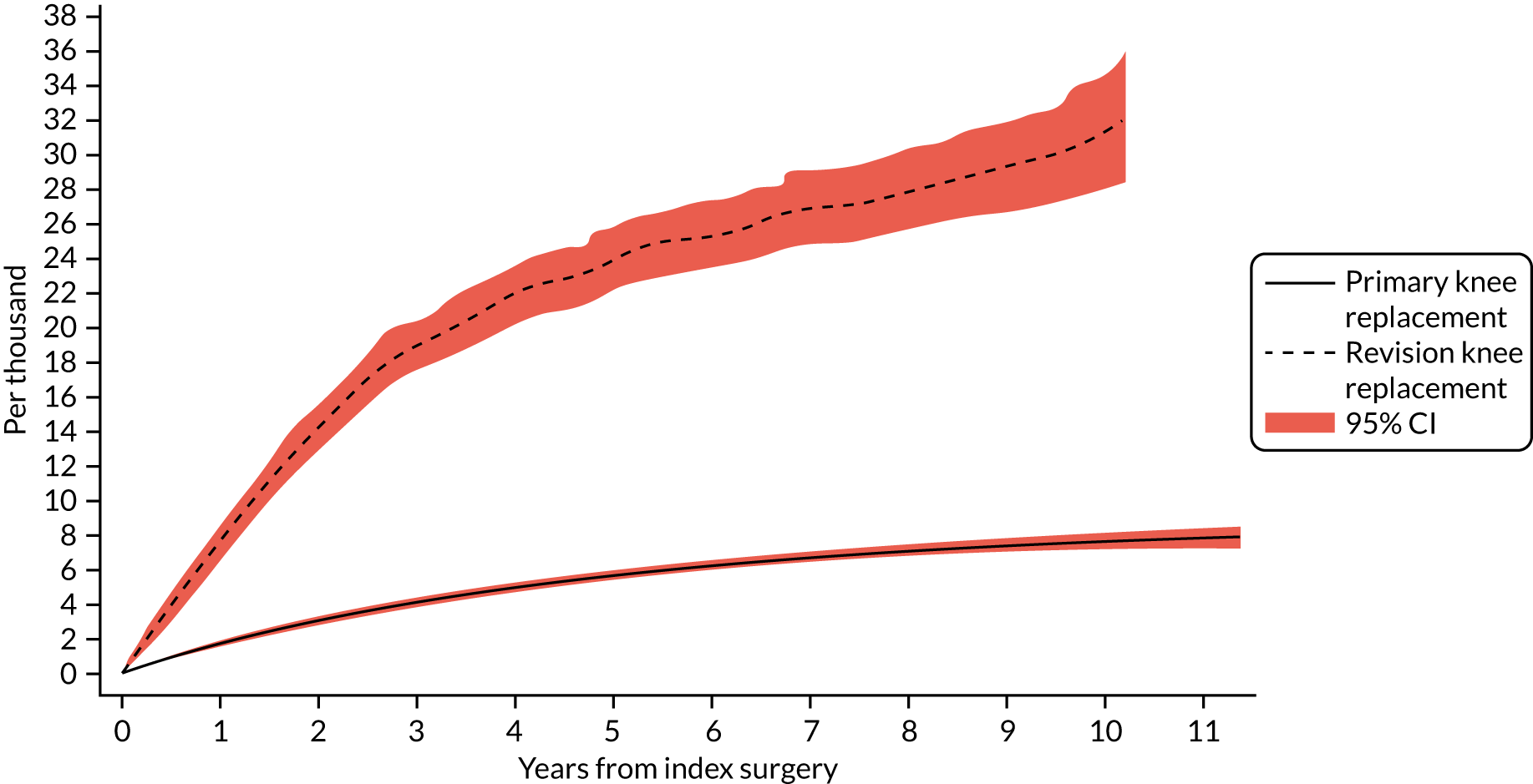
Revision rates within 3 months of the index knee replacement increased over time, with the prevalence rate in 2013 over twice that in 2005 (rate ratio 2.46, 95% CI 1.15 to 5.25; p < 0.0001). No time trends for revision for other time periods were apparent with the exception 5–6 years, which decreased over time (rate ratio 0.53, 95% CI 0.33 to 0.86; time trend p = 0.018).
In 2014, 1048 revision procedures were performed to treat knee prosthetic joint infection compared with 378 in 2005, a 2.8-fold increase. Overall, 75% of revision operations were conducted as a two-stage procedure, but there was an increase in use of single-stage revision from 7.9% in 2005 to 18.8% in 2014. The use of DAIR also increased, from 2.1% in 2005 to 9.1% in 2014.
Health economic consequences of prosthetic joint infection: systematic review
As shown in the flow diagram in Appendix 2, Figure 17, after detailed evaluation of 228 potentially relevant articles, 19 studies were included in the review, of which two were conducted in the UK. Fifteen studies reported cost comparisons of revision procedures for prosthetic joint infection with primary joint replacement or aseptic revision. Three studies presented cost comparisons between single-stage and two-stage revision procedures. In two studies, costs associated with different spacers were compared.
Cost of prosthetic joint infection treatment compared with primary hip or knee replacement
Eight studies reported comparisons between primary hip or knee replacement and first surgical treatments of infected hip or knee replacements other than DAIR. Treatments for infection cost on average 4.0 times (range 2.9–5.3 times) that of primary hip or knee replacement. DAIR treatment cost on average 3.0 times (range 2.9–3.1 times) that of primary hip or knee replacement. In one study that included costs of primary hip or knee replacement and subsequent first treatments for infection, including those occurring during primary admission, the costs were 2.9 times that of primary hip or knee replacement.
Only the cost of the first treatment for hip infection was measured in one study, and this was 3.6 times the cost of primary hip replacement.
In four studies the cost of revision for infection and any subsequent treatments relating to infection were calculated. On average, revision for infection treatment and further treatments cost 4.5 times (range 3.7–5.3 times) that of primary hip or knee replacement.
In two studies including costs of primary hip or knee replacement and all subsequent treatments for infection including persistent infection and reinfection, the costs reported were 3.5 and 4.1 times that of primary hip or knee replacement, respectively.
Cost of infection treatment compared with cost of aseptic revision
In seven studies, the average cost of revision for infection was 2.4 times (range 1.5–3.1 times) that for aseptic reasons and this was similar in the five studies exclusively reporting two-stage revision. In five studies, the cost of revision surgery was reported with no consideration of subsequent reoperations for failure of infection clearance. In these studies, the cost associated with treatment of infection was 2.3 times (range 1.5–3.1 times) that of aseptic revision. In five studies in patients with hip replacements, costs of revision for infection were 2.4 times those of aseptic revision (range 1.8–2.8 times). This difference was 3.1 and 1.5 in the two studies in patients with knee replacement. In two UK studies, the hospital costs of two-stage treatment of hip infection were 1.8 times greater, and for knee infection 3.1 times greater, than aseptic revision.
Costs of single-stage compared with cost of two-stage revision strategies
In two studies the cost of planned revision with no further treatment for persistent infection or reinfection using a two-stage procedure was higher than for a single-stage procedure, with a two-stage revision costing 1.7 times or 1.6 times that of a single-stage revision.
Considering all procedures, including planned operations and those required to treat persistent infection or reinfection, the relative costs differed in two studies reporting data. In one study, 3 out of 25 patients required additional treatment after single-stage revision and 1 out of 14 required this after two-stage revision. Considering the additional costs associated with these procedures, the overall cost of treatment with two-stage revision was about 1.6 times that of single-stage revision. In another study, five out of six single-stage revisions required additional operations and the overall cost of a two-stage revision was 0.6 times that of a single-stage revision.
Health economic consequences of hip prosthetic joint infection: NJR analyses
A total of 609 patients who had first revision surgery for hip prosthetic joint infection and whose primary operation had been recorded in the NJR between 2006 and 2009 met the inclusion criteria. Of these, 422 could be linked to HES for required variables and matched to a comparator patient with no revision surgery for hip prosthetic joint infection. Healthcare Resource Groups (HRGs) were available for 98% of hospital admissions, and weighted average costs of adult HRGs by admission type were applied to those remaining.
Our analysis included 422 patients with prosthetic joint infection after hip replacement and 1923 matched patients with no infection. Patients revised for hip prosthetic joint infection had an average of eight admissions during the 5 years after primary hip replacement compared with three admissions in those with no infection. Of the patients with no infection, 76% had an inpatient or day-case admission. In the 5 years following primary hip replacement, the average cost of inpatient and day-case admissions was £41,633 (95% CI £39,079 to £44,187) for patients with prosthetic joint infection and £8181 (95% CI £7614 to £8748) for those with no infection, a difference in costs of £33,452 (95% CI £30,828 to £36,077) (Table 4). The difference in costs between the groups decreased each year during the 5 years following primary hip replacement but was still nearly £3000 (95% CI £1999 to £3720) by the fifth year.
| Cases (n = 422), adjusted cost (£), mean (SE) | Controls (n = 1923), adjusted cost (£), mean (SE) | Adjusted difference in costs (£) (95% CI) | |
|---|---|---|---|
| First year post primary | 14,686 (816) | 1959 (111) | 12,727 (11,094 to 14,360) |
| Second year post primary | 10,575 (682) | 1503 (91) | 9071 (7719 to 10,424) |
| Third year post primary | 6974 (580) | 1512 (97) | 5462 (4306 to 6618) |
| Fourth year post primary | 5168 (501) | 1584 (131) | 3584 (2611 to 4557) |
| Fifth year post primary | 4427 (431) | 1568 (101) | 2859 (1999 to 3720) |
| Total over 5 years | 41,633 (1303) | 8181 (289) | 33,452 (30,828 to 36,077) |
Conclusions
The 10-year rates of revision for prosthetic joint infection after hip and knee replacement were 0.62% and 0.75%, which are lower than those in Scandinavian registries. 30,31 People receiving aseptic revision were at four times greater risk of hip or knee prosthetic joint infection.
There was a suggestion that prosthetic joint infection rates rose between 2005 and 2013. This was limited to the first 3 months after primary surgery and may reflect earlier identification and treatment of infection. The use of single-stage revision and DAIR has increased, and although the use of two-stage revision has decreased it is still the most widely used treatment.
Registry analyses showed fivefold higher health-care costs in the 5 years after primary surgery for people with prosthetic joint infection than for people with no infection. This was consistent with studies from Canada, France, Germany and the USA that reported costs of revision for infection and subsequent treatments ranging from 3.7 to 5.3 times that of primary hip or knee replacement. Comparative costs of treatment strategies are dependent on the success rate of treatments, which varied markedly in studies comparing single- and two-stage revision.
Limitations
In NJR analyses, we do not know how many people had an infection that was not treated surgically. However, the number treated without surgery is probably small as biofilm involvement means that antibiotic treatment alone can only suppress infection. Furthermore, arthroscopic management is not recommended. 53 In a Swedish cohort, 91% of people treated for prosthetic joint infection, and who subsequently received continuous outpatient antibiotic treatment, underwent a reoperation. 28 The UK Bone and Joint Infection Registry (BAJIR), established in 2018, collects information on all patients diagnosed with prosthetic joint infection in the UK and will provide accurate estimations of infection rates. 54
In the systematic review, the resources included in cost calculations varied considerably. In a comparison of costs and cost-effectiveness, future larger-scale and longer-term studies should assess costs to health service providers, social care and society, including those attributable to further treatments and revision operations.
In our NJR analysis, we estimated the burden of hip prosthetic joint infection with respect to inpatient and day-case admissions but did not consider costs relating to outpatient, primary and community care, and prescribed medications. Including these would have increased the overall costs in both patients who were and patients who were not treated for hip prosthetic joint infection and would probably have increased the cost difference between the two groups. For patients revised for hip prosthetic joint infection, the average cost of inpatient and day-case admissions in the 5 years following primary total hip replacement (including the cost of revision for prosthetic joint infection) was £41,633 using 2014–15 costs. Vanhegan et al. 55 reported that the cost of revision for hip prosthetic joint infection alone was £21,937 at a single UK hospital using costs from 2007–8. The authors found that this cost did not reflect NHS tariffs, which were considerably lower. Assuming that this underestimate is true and also holds for NHS reference costs (which were used in this study), the financial burden of hip prosthetic joint infection may have been underestimated.
As HES include hospital admissions at NHS hospitals in England only, the cost of admissions funded by the NHS outside England or in private facilities was not included. An estimated 83% of hip revision procedures are conducted in NHS hospitals. 56 With the need for involvement of an arthroplasty surgeon experienced in treating prosthetic joint infection and a well-co-ordinated multidisciplinary team, complex cases requiring revision surgery are conducted almost exclusively in NHS orthopaedic centres.
In the INFORM randomised trial, health service, social care and participant resource use data were collected and used in the cost-effectiveness analysis.
Patients’ experiences of prosthetic joint infection after hip or knee replacement and its treatment
Here we present an overview of two of our published articles. 57,58
Aims
We aimed to describe patients’ experiences and the impact of revision treatment for prosthetic joint infection after hip and knee replacement, and to compare patients’ experiences of single-stage and two-stage revision surgery.
Methods
We conducted qualitative semistructured interviews with patients who had received surgical revision for prosthetic joint infection after hip or knee replacement. Thirty-five patients (19 hip replacement and 16 knee replacement) from five NHS orthopaedic departments in England and Wales were interviewed between 2 weeks and 12 months after they had been discharged from hospital. Interview topic guides were developed in collaboration with the INFORM PPI group. The use of the topic guide was flexible to ensure that key topics were covered but that participants were able to discuss issues they considered important. The questions addressed included those on the experience of prosthetic joint infection, revision surgery and care, the impact of infection and treatment, and thoughts about recovery and the future. We asked participants who had received two-stage revision about their experiences of the time between operations. Data were audio-recorded, transcribed, anonymised and analysed thematically59 using the qualitative data management software NVivo 10 (QSR International, Warrington, UK).
Results
Prosthetic joint infection after hip replacement
Nineteen patients participated and gave consent. The patient group comprised 12 men and seven women with a mean age of 73 years (range 56–88 years); nine had received a single-stage revision and 10 had received a two-stage revision. Patients reported receiving 1–15 revision operations after their primary hip replacement. Analysis indicated that participants made sense of their experience through reference to three key phases: the period of symptom onset, the treatment period and protracted recovery after treatment. By conceptualising their experience in this way, and through themes that emerged in these periods, they conveyed the ordeal that prosthetic joint infection represented. Patients’ revision histories were often complex, extending over many years. Prosthetic joint infection and revision surgery affected all aspects of patients’ lives, physically, psychologically, socially and financially. Finally, considering the challenges of prosthetic joint infection, they described the need for support in all phases. Two-stage revision had a greater impact on participants’ mobility and resulted in additional complications.
Prosthetic joint infection after knee replacement
Interviews were conducted with 16 patients who consented to the study. The patient group comprised nine men and seven women with a mean age of 72 years (range 59–80 years); nine had received a single-stage and seven had received a two-stage revision. Participant experiences could be characterised according to three aspects of biographical disruption that we used to frame our analysis: onset and the problem of recognition; emerging disability and the problem of uncertainty; and chronic illness and the mobilisation of resources. Although the experiences of infection and treatment varied, all patients reported the devastating effects of infection and revision treatment. Participants described the use of social and health-care support and a need for more support. Some participants thought that the symptoms with which they had first presented had not been taken seriously enough.
Conclusions
Prosthetic joint infection is life-changing and has an impact on all aspects of a patient’s life. Among patients who had undergone revision surgery for prosthetic joint infection, a two-stage procedure had greater impact on participants’ well-being than a single-stage procedure because the time between revision procedures meant long periods of immobility, pain and related psychological distress. Participants expressed a need for more psychological and rehabilitative support during treatment and long-term recovery.
Surgeons’ decision-making for single-stage and two-stage revision surgery for prosthetic hip joint infection
Here we present an overview of our published article. 60
Aims
We aimed to explore decision-making by consultant orthopaedic surgeons about the use of single- or two-stage revision surgery for patients with hip prosthetic joint infection to inform our assessment of the feasibility of a randomised trial.
Methods
To guide the development of the INFORM multicentre RCT, we conducted semistructured interviews with 12 consultant surgeons performing revision surgery for hip prosthetic joint infection at five high-volume NHS orthopaedic departments in England and Wales. We analysed the data thematically.
Results
When choosing between single- and two-stage revision for hip prosthetic joint infection, surgeons considered multiple factors, including the patient context (age, ability to cope, carer responsibilities); patient preferences; primary prosthesis fixation; patient physiology (bone integrity and tissue damage, comorbidities, frailty); surgeon’s knowledge, training and peer influence; local infrastructure (availability of microbiology, resources, costs); the infecting organism (clinicians’ ability to identify, sensitivity to antibiotics); and duration of infection (acute or chronic). With evidence accruing on similar outcomes between surgical techniques, and observations of colleagues’ successful use of single-stage revision, surgeons questioned whether or not revision in two stages remained the best treatment for hip prosthetic joint infection, and some were increasingly willing to consider more revisions in a single stage. Some surgeons managed uncertainty about the choice of surgical technique by using a CUMARS. Although single-stage revision was considered to be, potentially, the best strategy to treat hip prosthetic joint infection, surgeons thought that a change in practice was not yet justified. To inform any change in practice, surgeons noted the need for evidence from randomised trials. If there was no clear best strategy based on patient factors, their own knowledge and expertise, the available infrastructure and the infecting organism, then surgeons believed that the patients they treated would be eligible for randomisation.
Conclusions
With growing evidence of the success of treatment of hip prosthetic joint infection in a single stage, the willingness of some surgeons to change practice has increased over time. By using a CUMARS, surgeons were able to manage uncertainty about the choice between single- and two-stage revision. To guide treatment, surgeons identified the need for high-quality evidence to support their choice of revision strategy. Surgeons believed that a RCT comparing single- and two-stage revision for hip prosthetic joint infection is needed. While recognising that patient, infection, surgeon and infrastructure factors may indicate the need for a particular strategy, surgeons considered that randomisation would be feasible and acceptable.
Impact of cases of prosthetic knee infection on surgeons’ personal and professional well-being
Here we present an overview of our published article. 61
Aims
Although prosthetic joint infection has been described as ‘an orthopaedic surgeon’s worst nightmare’,62 relatively little is known about how prosthetic joint infection affects surgeons professionally and personally. We explored the impact of cases of knee prosthetic joint infection on surgeons’ personal and professional well-being. The identification and acknowledgement of the emotional impact of prosthetic joint infection may help in developing support strategies and maintaining surgeons’ well-being,63 and a deeper understanding of the personal and professional impact of adverse events is needed. 64
Methods
We conducted qualitative telephone interviews with consultant orthopaedic surgeons who treated patients for prosthetic knee infection in one of six high-volume NHS orthopaedic departments. The interviews were audio-recorded, transcribed, anonymised and imported into the qualitative data management software NVivo 10 for thematic analysis. 59
Results
We interviewed 11 surgeons who had a mean of 9.5 years’ experience as an orthopaedic consultant (range 1 month to 20 years). Surgeons perceived that being required to treat knee prosthetic joint infection was inevitable at some time in their career and this was a major concern, irrespective of their years of experience.
In our analyses we identified three themes that characterised the views and experiences of surgeons treating knee prosthetic joint infection: at some point, infection is inevitable but surgeons still feel accountable; the profound emotional impact; and supporting each other.
Participating surgeons described dealing with a diagnosis of prosthetic joint infection as ‘devastating’, ‘soul-destroying’ and ‘deeply unpleasant’. Although they expected a diagnosis of prosthetic joint infection at some point in their career, its occurrence still made them question their practice, despite taking ‘every measure’, and left them feeling that they had let the patient down. Surgeons felt that empathy and honesty were important to patients who had received a diagnosis of prosthetic joint infection. They described how they needed to reflect carefully, questioning their practice, performance and surgical processes. To ensure that they maintained their confidence, surgeons felt that it was important to continue to perform surgery even after one of their patients had been diagnosed with prosthetic joint infection. Surgeons described their feelings of responsibility for patients’ well-being and how they were motivated by the potential for relieving a patient’s pain and improving quality of life, but that prosthetic joint infection could have serious consequences for a patient’s quality of life. When prosthetic joint infection does occur, surgeons highlighted the importance of acknowledging the diagnosis and being honest with patients. Our findings suggest that, in some departments, decisions about treatment may be made by surgeons individually and not always discussed with colleagues. Although the participants in the study felt supported by colleagues, one suggested that the occurrence of prosthetic joint infection could potentially be ‘isolating’ for a surgeon where such support did not exist.
Conclusion
Prosthetic joint infection has a considerable emotional impact on surgeons, who report a sense of devastation and personal ownership, particularly as they are largely unable to control its occurrence. Surgeons stressed the importance of a supportive multidisciplinary team in the management of prosthetic joint infection.
Risk factors for prosthetic hip or knee infection
Here we present an overview of five of our published articles. 50,51,65–67
Background
The risk of developing prosthetic joint infection may be influenced by patient characteristics, the surgical intervention and postoperative care.
Aims
We aimed to identify risk factors for prosthetic joint infection after hip or knee replacement in new systematic reviews of published research and joint registry analyses.
Methods
Systematic reviews
Systematic reviews were registered prospectively with PROSPERO (CRD42015023485, CRD42018106503 and CRD42018114592) and conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)68 and MOOSE (Meta-analysis Of Observational Studies in Epidemiology)69 guidelines. We searched MEDLINE, EMBASE, Cochrane Library and reference lists, with no language restrictions (see Appendices 3–5).
In risk factor reviews, eligible studies included those with the following:
-
patients with hip or knee replacement
-
people with a potential risk factor
-
comparator – non-exposed people
-
outcome – prosthetic joint infection with ≥ 1 year of follow-up
-
study – longitudinal study design.
In reviews of implant fixation, randomised trials with the following criteria were also included:
-
patients with hip or knee replacement
-
intervention – fixation
-
control – alternative fixation
-
outcome – prosthetic joint infection with ≥ 1 year of follow-up
-
study – randomised design.
Searches were carried out on 1 September 2016 (general risk factors), 24 April 2019 (hip implant fixation) and 1 November 2018 (knee implant fixation).
In systematic reviews of fixation, we also included RCTs. Two investigators extracted study information and assessed quality using the Newcastle–Ottawa Scale,70 with studies scoring ≥ 5 considered good quality. For RCTs, we used the Cochrane tool. 71 Study-specific relative risks (RRs) with 95% CIs were meta-analysed using random-effects models and grouped by study-level characteristics.
National Joint Registry analyses
We investigated the associations between potential risk factors and risk of revision for prosthetic joint infection after total hip and knee replacements recorded in the NJR. Primary procedures were performed between 2003 and 2013, with procedures subsequently revised for prosthetic joint infection up to 2014. Hospital activity and mortality were obtained through linkage with HES, the Patient Episode Database for Wales and the Office for National Statistics (ONS). Comorbidities were derived from the Charlson Comorbidity Index, as recorded in HES, using ICD-10 (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision) codes. 50,51
We investigated associations using Poisson multilevel models. 72 Regressions were adjusted for age, sex, ASA grade and body mass index (BMI). We reinvestigated associations in postoperative time periods using piecewise exponential multilevel models with period-specific effects. 73,74
Results: systematic reviews
General risk factors
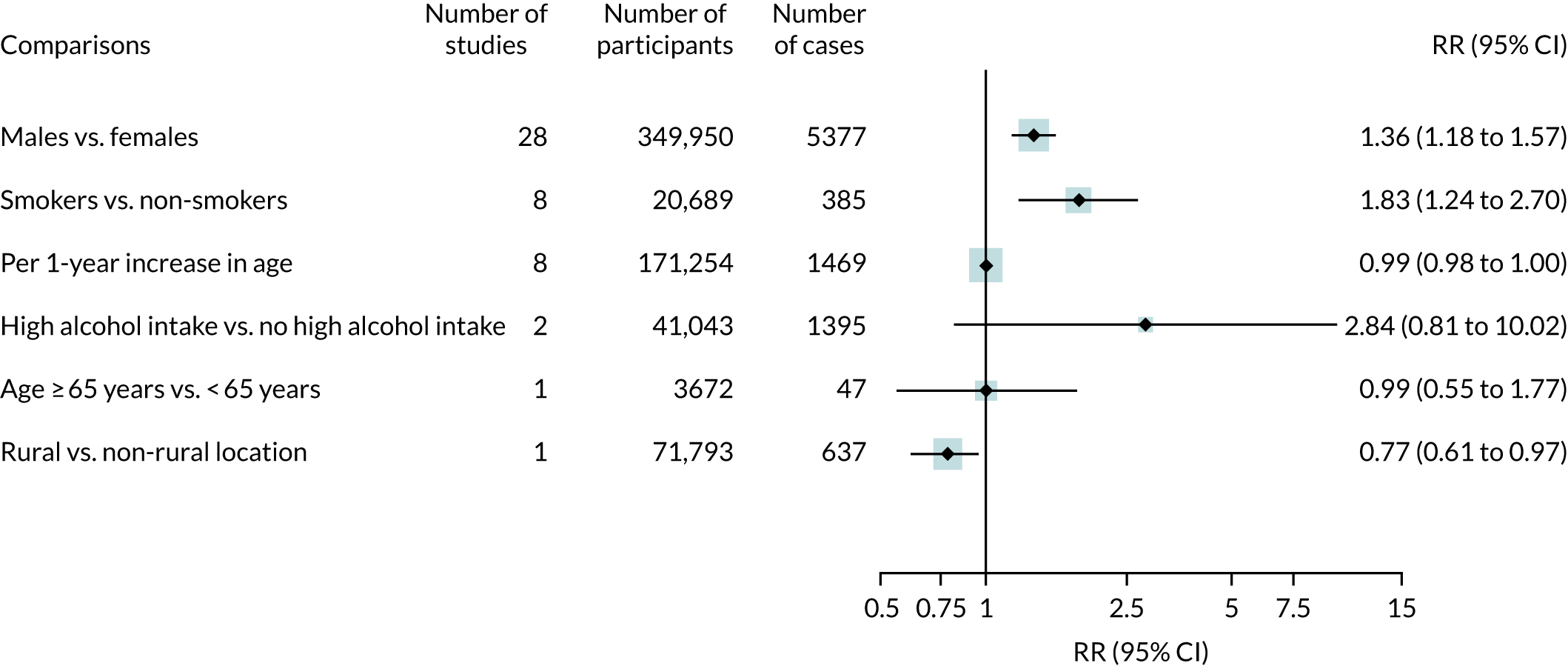
Searches identified 66 observational studies, including 512,508 participants with, predominantly, hip and knee replacement. The mean follow-up was 3.7 years (range 1–17 years). The risk of prosthetic joint infection was greater in men than in women (RR 1.36, 95% CI 1.18 to 1.57) and in smokers than in non-smokers (RR 1.83, 95% CI 1.24 to 2.70) (Figure 4). There were no associations between age or alcohol intake and the risk of prosthetic joint infection. One study reported a lower risk of prosthetic joint infection in patients living in rural locations than in those living in non-rural locations. The results of meta-analyses were consistent in higher-quality studies.
FIGURE 4.
Sociodemographic characteristics and risk of prosthetic joint infection.
For BMI, there were consistent positive associations for comparisons with cut-off points of ≥ 30 kg/m2 (Figure 5). Comparing BMI of ≥ 40 kg/m2 with < 40 kg/m2, the pooled RR was 3.68 (95% CI 2.25 to 6.01). In one study, people with BMI < 18.5 kg/m2 had a greater risk of prosthetic joint infection than those with BMI of 18.5–30.0 kg/m2.
FIGURE 5.
Body mass index and risk of prosthetic joint infection.
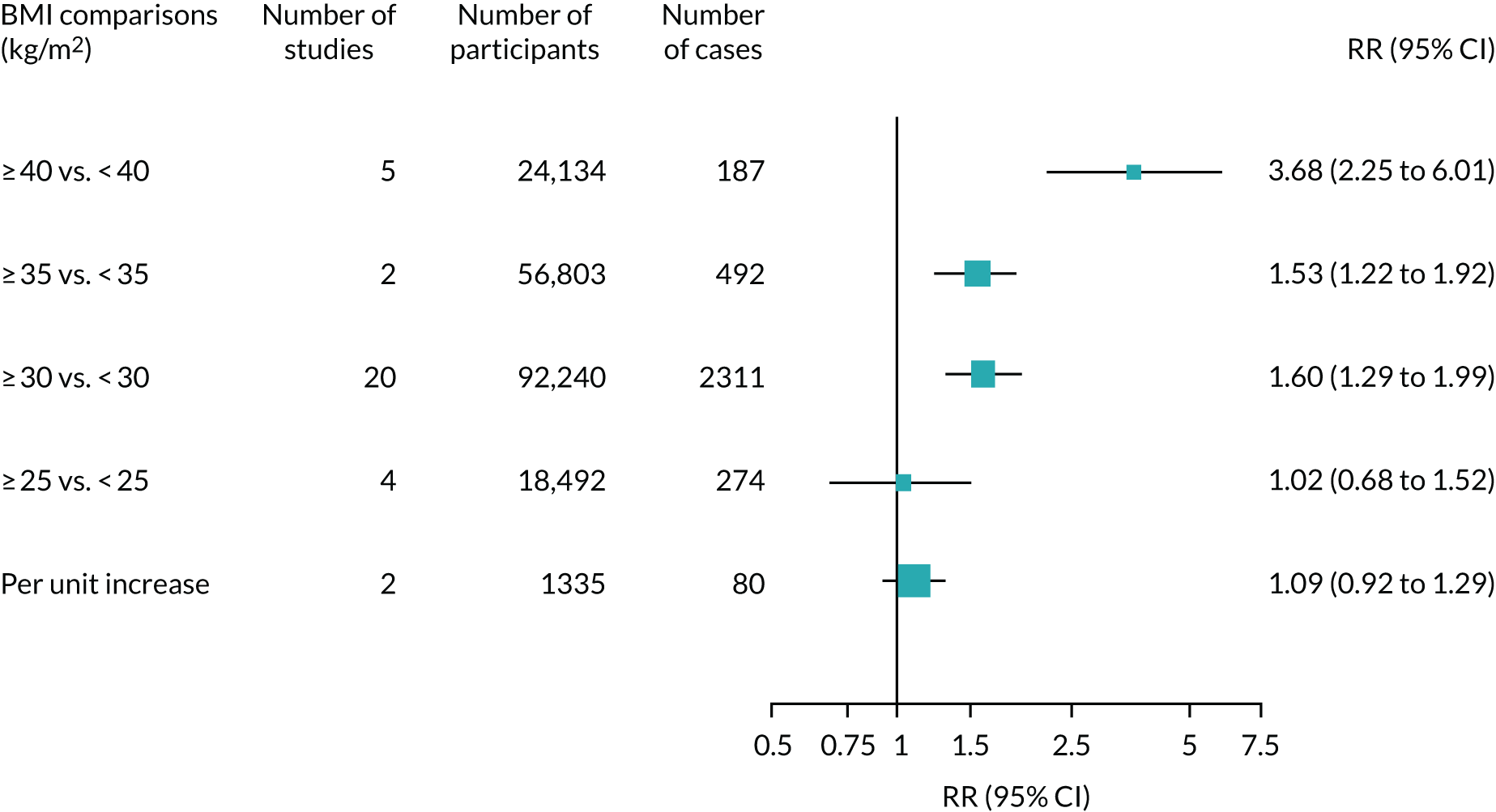
Medical and surgical risk factors for prosthetic joint infection were diabetes, rheumatoid arthritis, depression, history of steroid administration and previous joint surgery. There was no evidence of important associations with osteoarthritis, osteonecrosis, post-traumatic arthritis, cardiovascular disease, hypertension, cancer, high-risk dental procedures or intra-articular steroid injections.
Hip implant fixation
Searches identified four RCTs (945 hip replacements) and 11 observational cohorts (2,260,428 hip replacements).
All RCTs were rated as being at a low risk of bias for random sequence generation and incomplete outcome data but at an unclear risk of bias in ≥ 1 other area. There were no clear differences in the risk of prosthetic joint infection when cemented fixations were compared with uncemented or reverse hybrid fixations (Figure 6). In one RCT,75 the risk of prosthetic joint infection was lower in patients receiving implant fixation with antibiotic-loaded cement than in those receiving it with plain cement.
FIGURE 6.
Fixation types and risk of hip prosthetic joint infection in observational studies and RCTs.
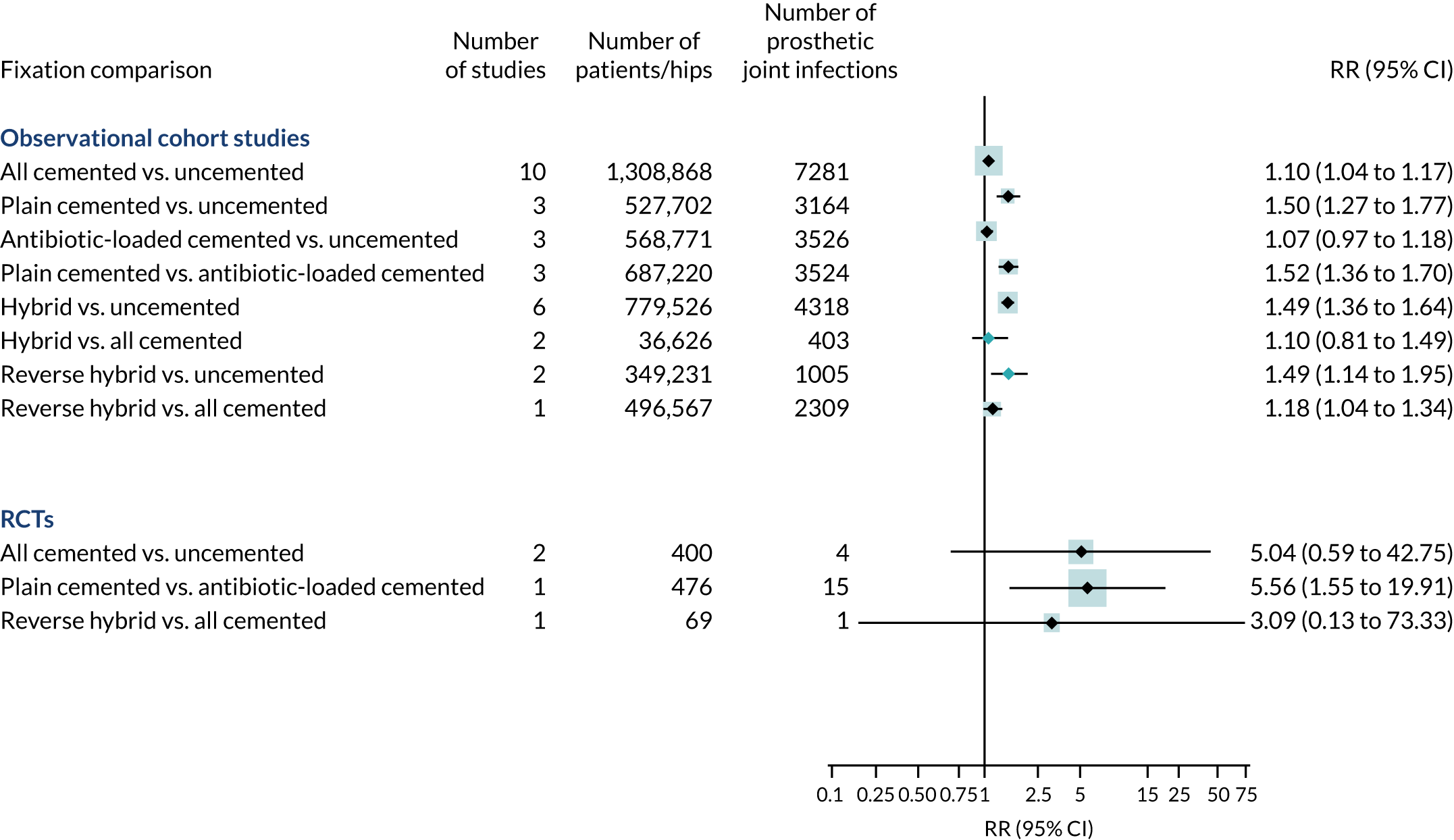
In a pooled analysis of observational studies, any fixation with cement was associated with an increased risk of prosthetic joint infection compared with uncemented fixation. In studies with higher quality scores, the difference favouring uncemented over cemented fixation was consistent (RR 1.09, 95% CI 1.03 to 1.16).
Compared with antibiotic-loaded cemented fixations, plain cemented fixations were associated with an increased risk of prosthetic joint infection (RR 1.52, 95% CI 1.36 to 1.70).
Knee implant fixation
Searches identified eight RCTs (4029 knee replacements) and 24 observational studies (1,161,292 knee replacements).
Randomised controlled trials were rated as being at low risk of bias for incomplete outcome data and selective reporting. Four were rated as being at an unclear risk of bias for allocation concealment. There was no difference in the risk of prosthetic joint infection when uncemented fixation was compared with cemented or hybrid fixation and when hybrid fixation was compared with cemented fixation (Figure 7). In one trial76 that randomised 2948 patients, there was no difference in the risk of prosthetic joint infection when antibiotic-loaded cemented fixation was compared with plain cemented fixation.
FIGURE 7.
Fixation types and risk of knee prosthetic joint infection in observational studies and RCTs.
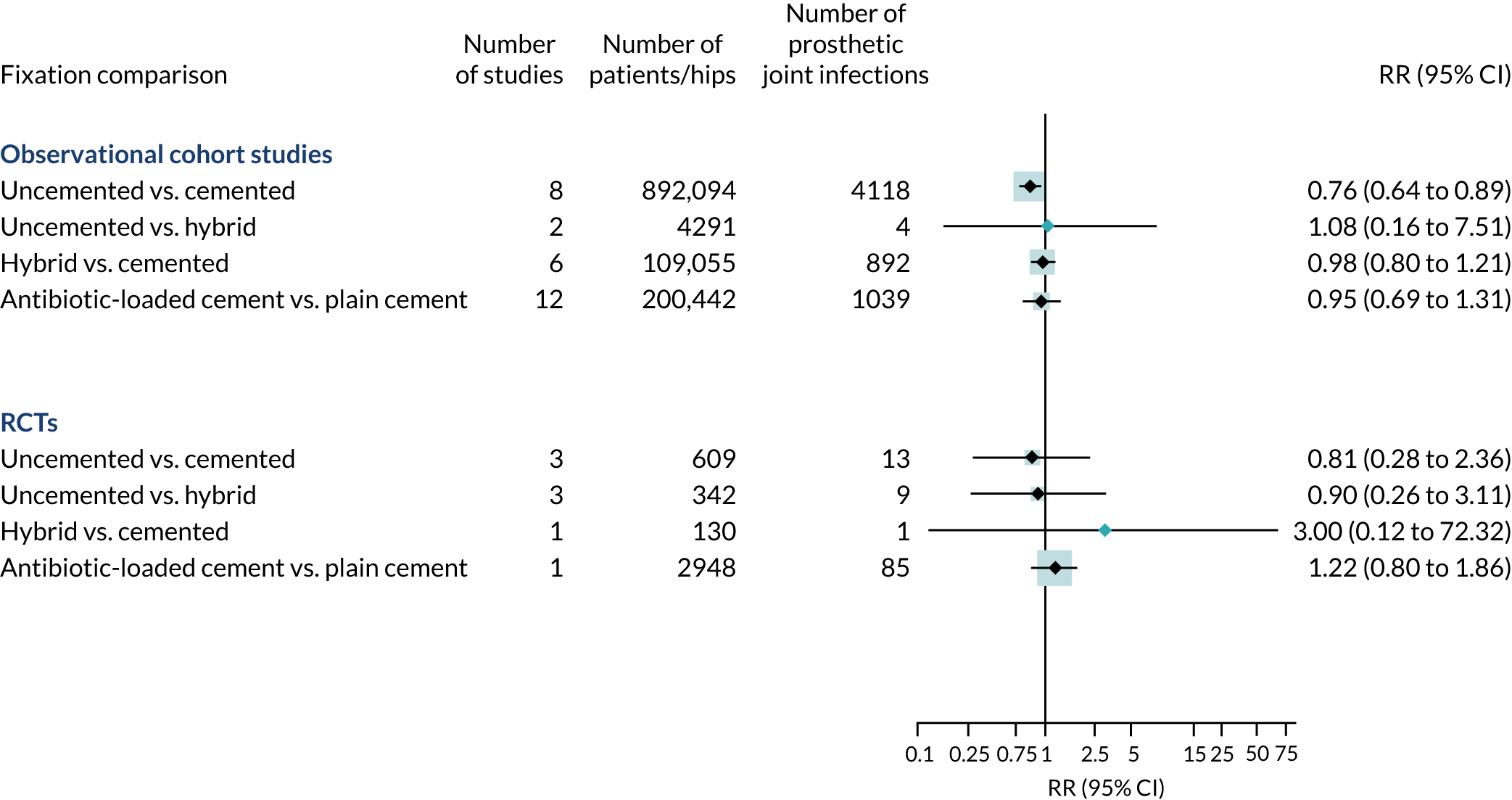
In a pooled analysis of observational studies, uncemented fixation was associated with a decreased overall risk of prosthetic joint infection compared with cemented fixation (RR 0.76, 95% CI 0.64 to 0.89). The results of meta-analyses were consistent in higher-quality studies. There was no difference in overall risk of prosthetic joint infection for antibiotic-loaded cemented fixation compared with plain cement.
Results: National Joint Registry analyses
Risk factors for revision of hip replacement for prosthetic joint infection
Of 623,253 primary hip replacements carried out between 2003 and 2013 and with ≥ 1 year of follow-up [median 4.6 years, interquartile range (IQR) 2.6–7.0 years], 2705 were revised for prosthetic joint infection. The incidence rate ratios for revision are summarised by patient, surgical and health system characteristics in Appendices 6–8.
Patient characteristics
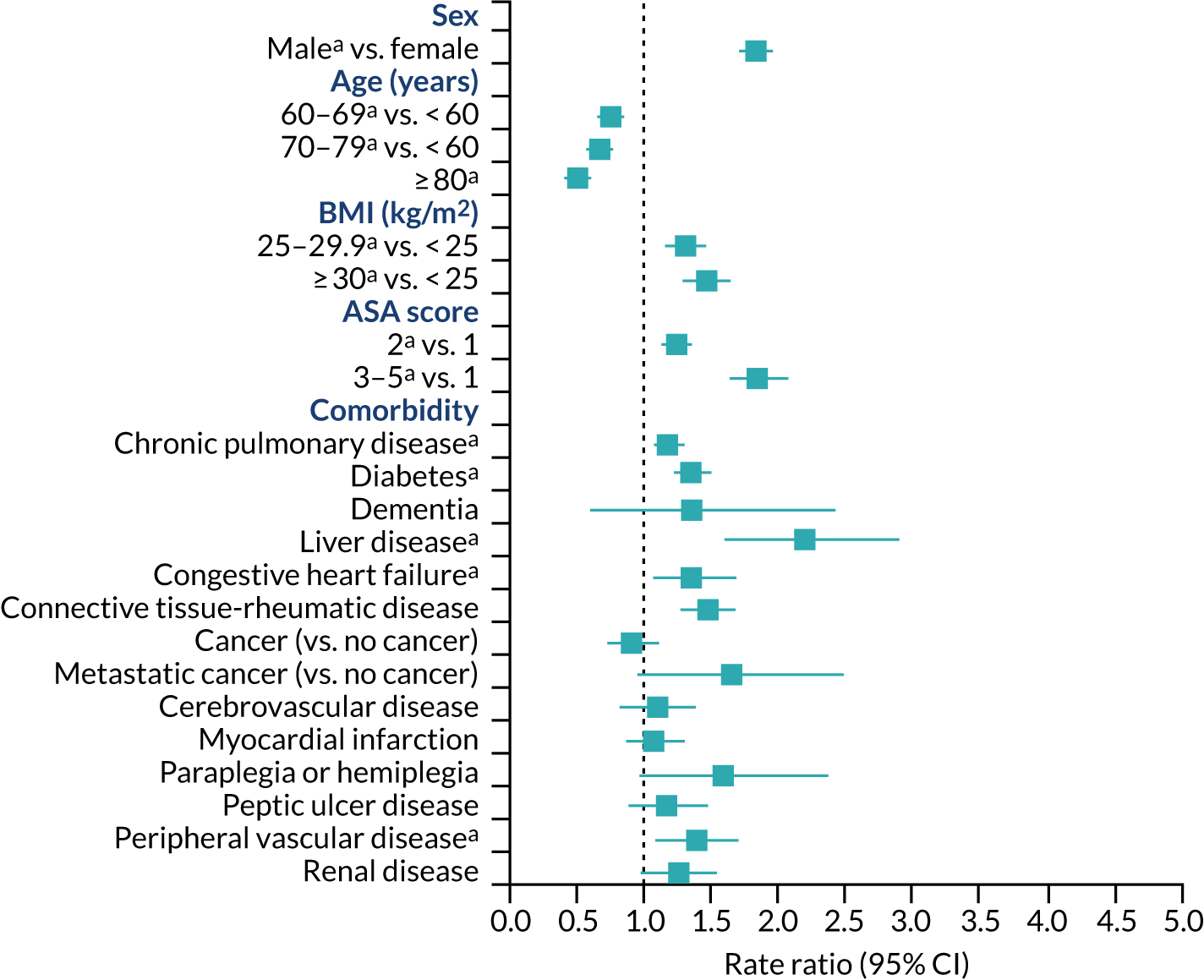
People aged ≥ 80 years were at lower risk of revision for hip prosthetic joint infection than people aged < 60 years (RR 0.66, 95% CI 0.56 to 0.76) (Figure 8). Men were at higher risk of revision of hip prosthetic joint infection than women (RR 1.68, 95% CI 1.56 to 1.81). The numbers of revisions in ethnic minority groups were small, and we were unable to investigate ethnicity as a possible risk factor. People with a BMI of ≥ 30 kg/m2 had a higher risk of revision for hip prosthetic joint infection than those with a BMI of < 25 kg/m2 (RR 1.92, 95% CI 1.72 to 2.15). People with diabetes, chronic pulmonary disease, congestive heart failure, connective tissue-rheumatic disease, previous septic arthritis or fractured neck of femur had a higher risk of revision for hip prosthetic joint infection. More generally, people with greater medical comorbidity had a higher risk of revision for hip prosthetic joint infection (ASA grades 3–5 vs. ASA grade 1: RR 1.63, 95% CI 1.42 to 1.87). In the first 3 months after primary hip replacement, the risk of revision for hip prosthetic joint infection was higher for people with dementia than for those without (RR 3.78, 95% CI 1.21 to 7.81). People with liver disease had an increased risk of revision for hip prosthetic joint infection but only at ≥ 2 years after primary hip replacement (RR 2.43, 95% CI 1.35 to 3.82). There were no clear associations between cardiovascular disease, cerebrovascular disease, myocardial infarction, peripheral vascular disease, cancer (except metastatic disease) and paraplegia or hemiplegia and the risk of revision for hip prosthetic joint infection.
FIGURE 8.
Patient characteristics and risk of revision for hip prosthetic joint infection during the whole postoperative period: NJR analysis. Reference category in parentheses. a, Adjusted p-value < 0.05.
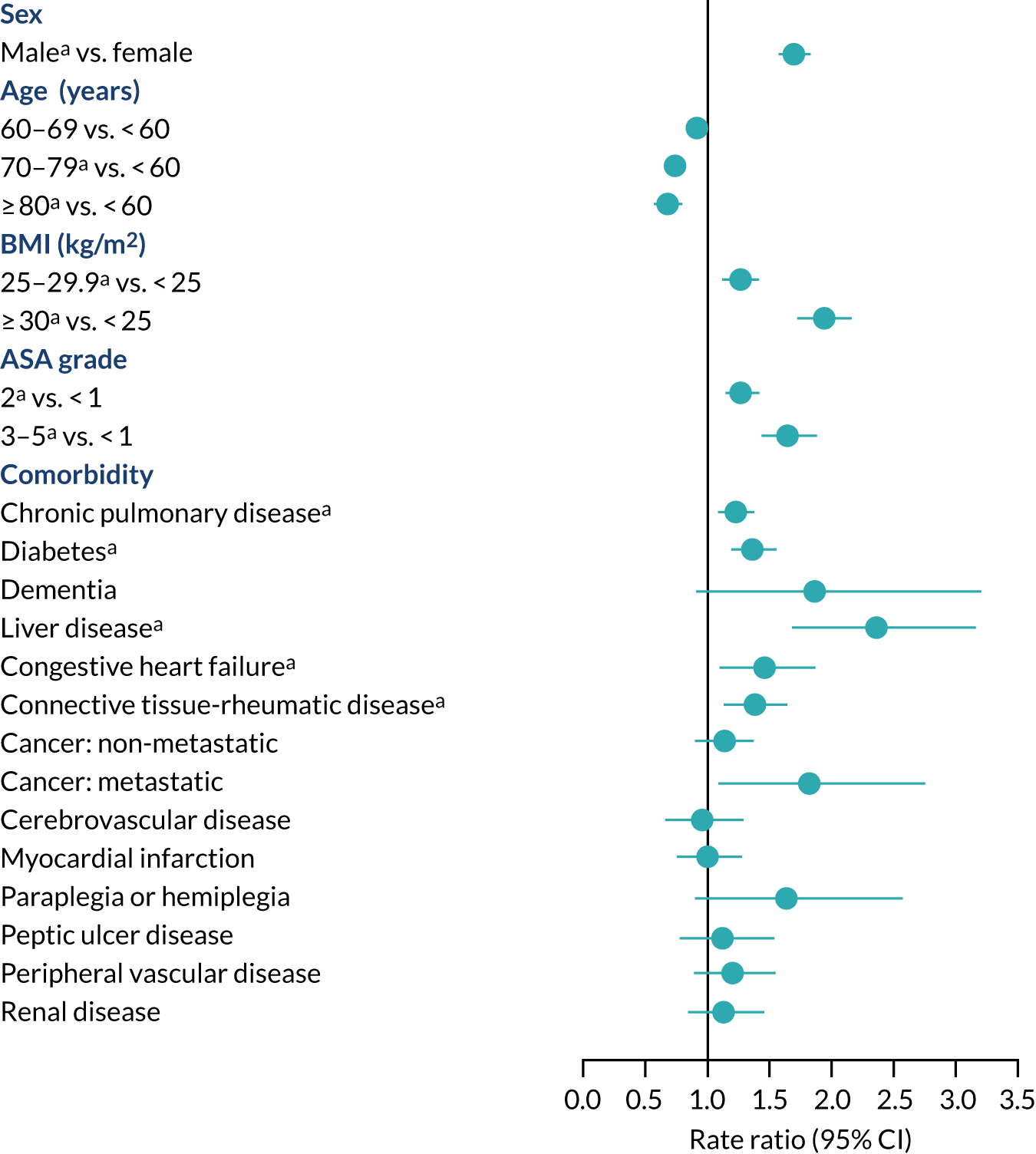
Surgical factors
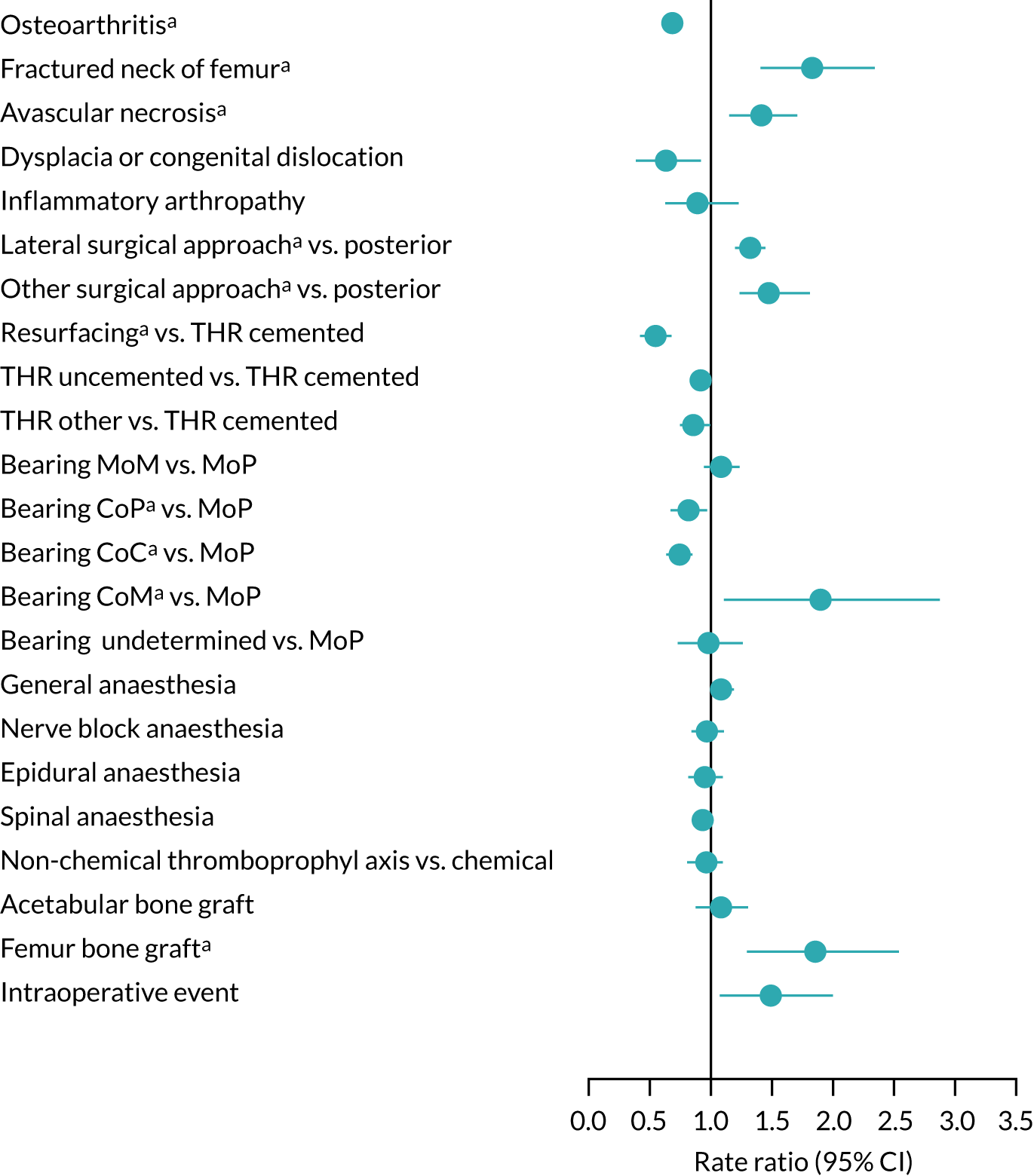
Compared with unaffected people, those with osteoarthritis or congenital hip dysplasia were at lower risk, while people treated for fractured neck of femur or osteonecrosis were at higher risk of revision for hip prosthetic joint infection (Figure 9). There was a particularly high risk of further infection in those who had experienced a previous hip infection (RR 6.69, 95% CI 4.18 to 9.80). The use of the lateral surgical approach was associated with an increased risk of revision for hip prosthetic joint infection compared with the posterior approach (RR 1.32, 95% CI 1.21 to 1.43). Up until 2 years after hip replacement, the risk with metal-on-metal bearing combinations was lower than or similar to that with metal-on-polyethylene, but at longer follow-up the risk was higher with metal-on-metal. Ceramic-on-ceramic and ceramic-on-polyethylene bearing combinations were associated with a lower risk of revision after 24 months than metal-on-polyethylene bearings. Little or no difference in the risk of revision for hip prosthetic joint infection was found for anaesthetic technique, thromboprophylaxis regime, the use of acetabular bone graft or intraoperative complication. Patients with a femoral bone graft during primary hip replacement were at higher risk of revision for hip prosthetic joint infection.
FIGURE 9.
Surgical factors and risk of revision for hip prosthetic joint infection during the whole postoperative period: NJR analysis. Reference category in parentheses. a, Adjusted p-value < 0.05. CoC, ceramic-on-ceramic; CoM, ceramic-on-metal; CoP, ceramic-on-polyethylene; MoM, metal-on-metal; MoP, metal-on-polyethylene; THR, total hip replacement.
Health system factors
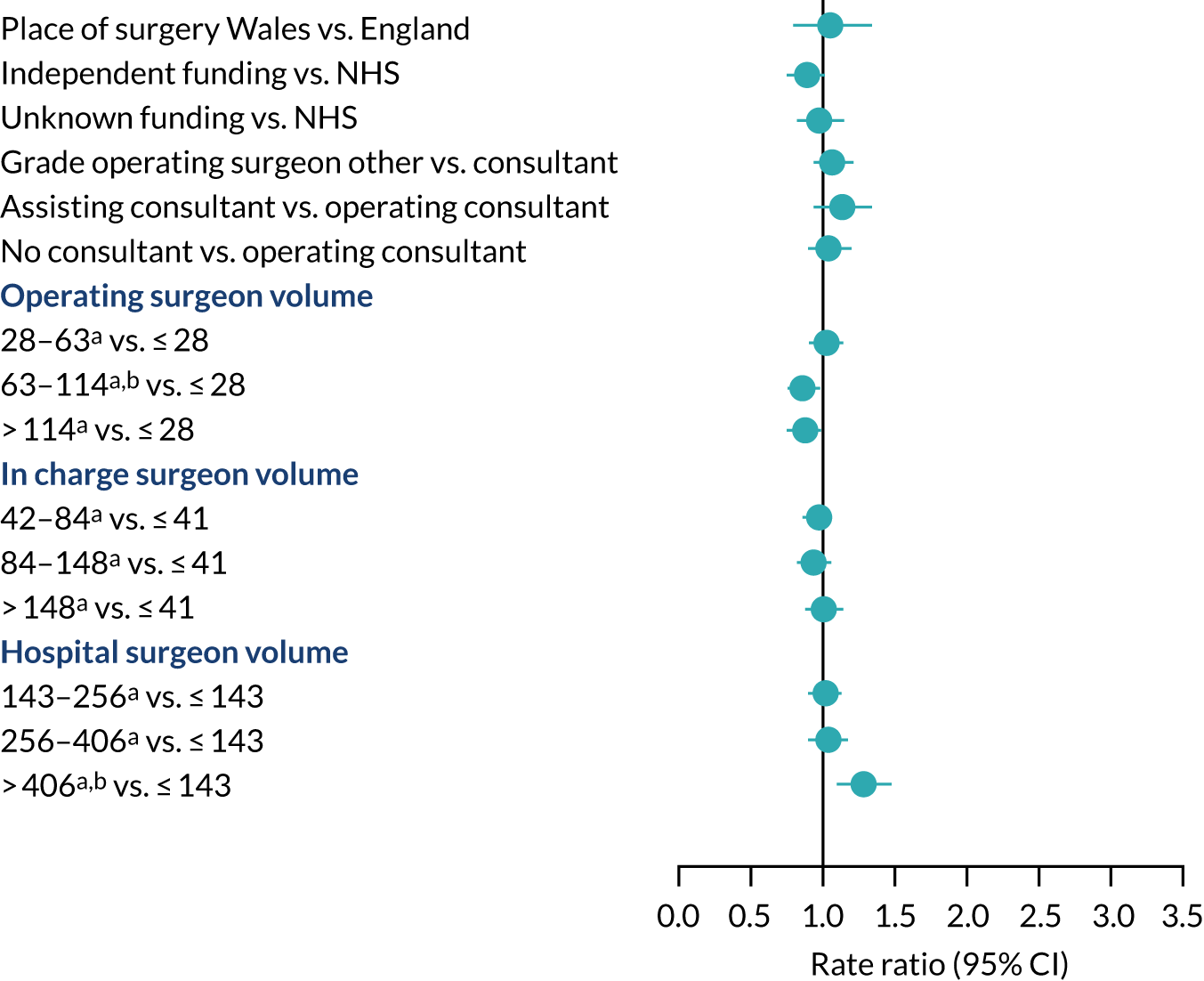
The risk of revision for hip prosthetic joint infection was similar in Wales and England and did not differ according to funding source, operating surgeon grade or consultant surgeon presence during surgery (Figure 10). Operating surgeons who performed more hip replacements annually had a lower risk of revision for hip prosthetic joint infection than surgeons with a lower volume. The volume of all hip procedures carried out by the surgeon in charge of the surgery did not affect the risk of revision. The risk of revision for hip prosthetic joint infection was higher in the first 3 months after primary surgery in hospitals that had performed over 255 hip procedures in the year before primary surgery than in hospitals with a small volume of activity. No specific difference in the rate ratios were found beyond this period or for units with lower volumes of hip procedures.
FIGURE 10.
Health system factors and risk of revision for hip prosthetic joint infection during the whole postoperative period: NJR analysis. Reference category in parentheses. a, Volume is the total number of hip replacements performed in the previous 12 months; b, adjusted p-value < 0.05.
Risk factors for revision of knee replacement for prosthetic joint infection
Between 2003 and 2015, 679,010 primary knee replacements were recorded in the NJR, of which 3659 were revised for prosthetic joint infection at ≥ 1 year follow-up (median 4.6 years, IQR 2.6–6.9 years). After primary knee replacement, incidence rate ratios of revision for prosthetic joint infection are summarised for different patient, surgical and health system characteristics in Appendices 9–11.
Patient characteristics
People aged ≥ 80 years were less likely to have a revision for knee prosthetic joint infection than those aged < 60 years (RR 0.50, 95% CI 0.43 to 0.57) (Figure 11). Men were more likely than women to have a revision for knee prosthetic joint infection (RR 1.83, 95% CI 1.71 to 1.96). There were too few revisions for knee prosthetic joint infection in ethnic minority groups to investigate ethnicity as a possible risk factor. The likelihood of revision for knee prosthetic joint infection was higher in people with a BMI of ≥ 30 kg/m2 than in those with a BMI of < 25 kg/m2 (RR 1.46, 95% CI 1.29 to 1.63). The risk of revision for knee prosthetic joint infection was higher in people with more medical comorbidities, and specifically in people with diabetes, chronic pulmonary disease, connective tissue and rheumatic diseases and peripheral vascular disease. An elevated risk in people with liver disease was specific to the period after 2 years.
FIGURE 11.
Patient characteristics and risk of revision for knee prosthetic joint infection during the whole postoperative period: NJR analysis. a, Adjusted p-value < 0.05.
Surgical factors
Aspects of surgery associated with an increased risk of revision for prosthetic joint infection after knee replacement were surgery for trauma, previous knee infection, inflammatory arthropathy, operation under general anaesthesia, requirement for tibial bone graft and use of posterior stabilised fixed-bearing prostheses compared with unconstrained fixed-bearing prostheses and constrained condylar prostheses (Figure 12). Compared with cemented total knee replacement, lower rates of prosthetic joint infection were seen in people who received uncemented, unicondylar or patellofemoral knee replacement.
FIGURE 12.
Surgical factors and risk of revision for knee prosthetic joint infection during the whole postoperative period: NJR analysis. a, Adjusted p-value < 0.05.
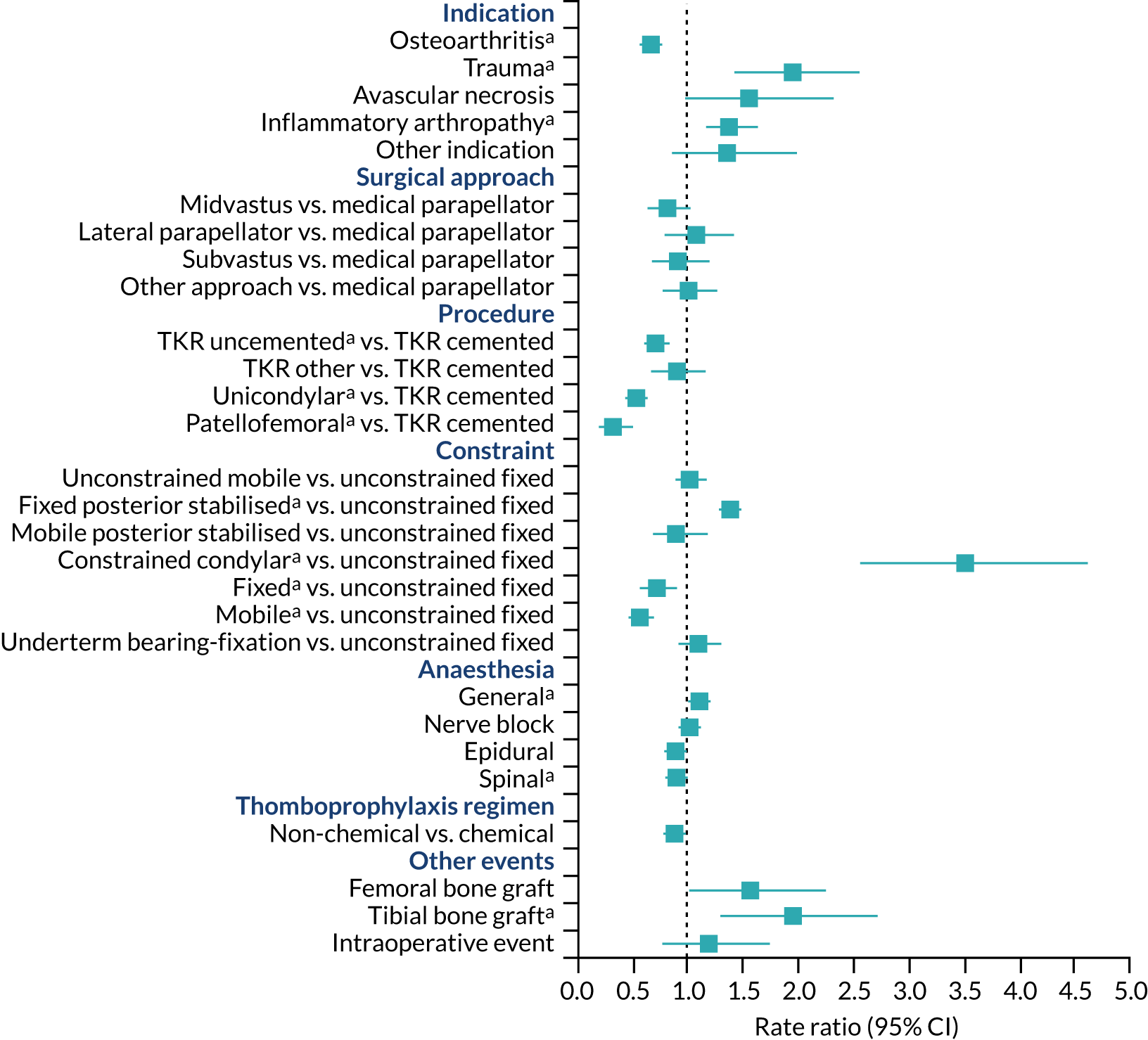
Health system factors
The risk of revision for prosthetic joint infection after knee replacement was similar in Wales and England (Figure 13). Privately funded procedures had a lower risk of revision than procedures funded by the NHS. Revision for knee prosthetic joint infection was not affected by the operating surgeon’s grade, the presence of a consultant surgeon during surgery, or the volume of knee procedures carried out by the operating surgeon or the surgeon in charge. The risk of revision for knee prosthetic joint infection was higher in high-volume hospitals than in low-volume hospitals. In hospitals that had carried out more than 440 knee procedures in the year preceding the index surgery, the risk of revision for prosthetic joint infection was higher in the first 3 months after surgery than in hospitals with a small volume of activity.
FIGURE 13.
Health service factors and risk of revision for knee prosthetic joint infection during the whole postoperative period: NJR analysis. a, Adjusted p-value < 0.05; b, volume is the total number of knee replacements performed in the previous 12 months.
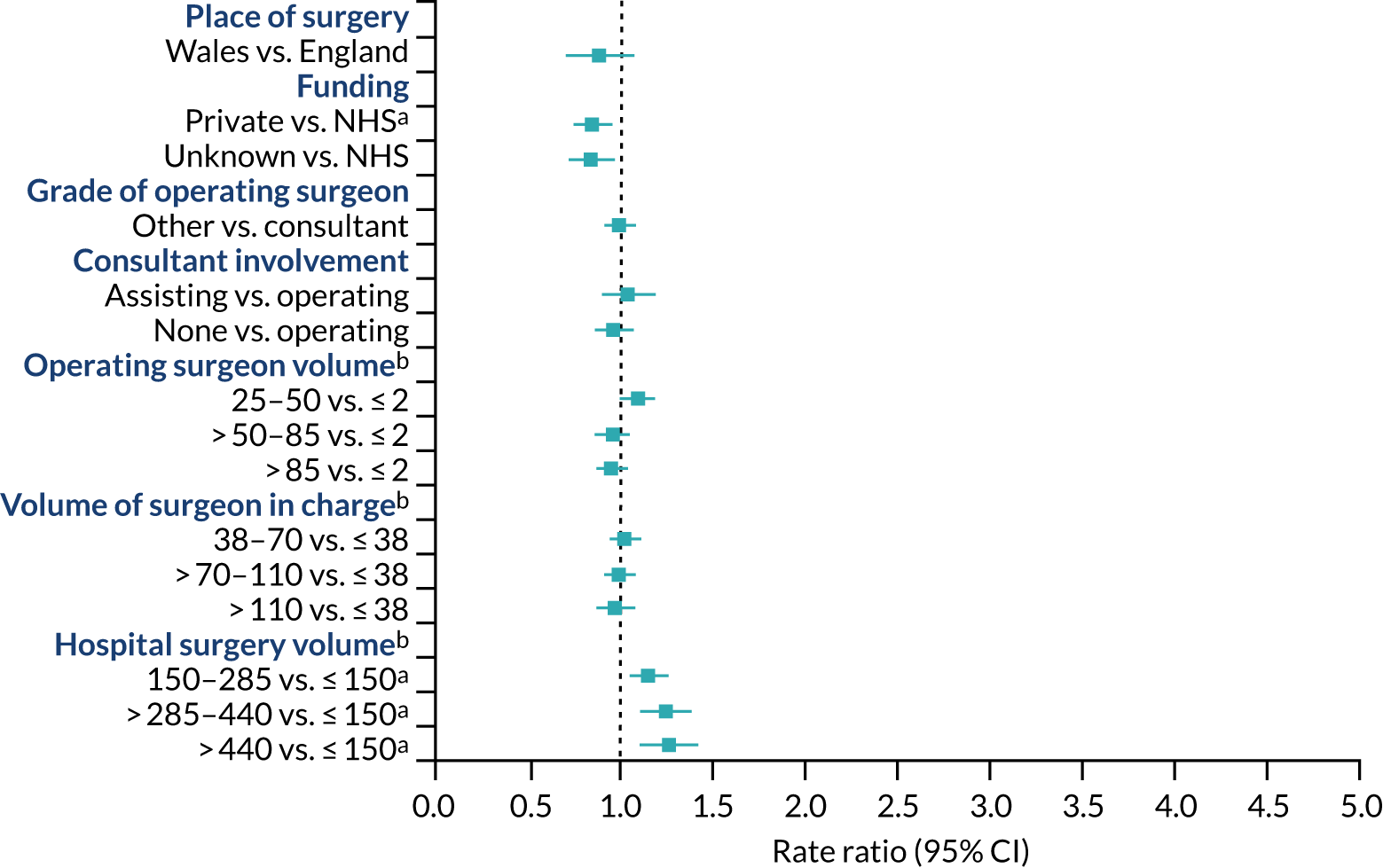
Discussion
The two research approaches complemented each other as cohort studies included in systematic reviews and the NJR reported associations between a diverse range of potential risk factors and risk of prosthetic joint infection and need for revision.
Risk of hip and knee prosthetic joint infection and need for revision is higher in men, people with a high BMI, people with diabetes and those receiving implants with cemented fixation. In the NJR an association was noted between dementia and increased early risk of revision for hip prosthetic joint infection.
Meta-analysis of cohort studies showed no association with age, but in the NJR, younger patients were at higher risk of revision for hip or knee prosthetic joint infection. This may be because the NJR had a longer follow-up with more time for joint infections to occur in younger patients. Older people at high risk of adverse outcomes and death from surgery may have received non-operative management. NJR data show not the risk of infection, but the risk of revision for infection. It may be that clinicians are more likely to treat older patients with suppressive antibiotics than with surgery. In NJR analyses, people with more medical comorbidities had an increased risk of revision for hip and knee prosthetic joint infection, and this was noted for specific conditions in meta-analyses and NJR analysis.
Analyses provide reassurance that many surgical and health system factors do not increase the risk of prosthetic joint infection. There was a suggestion that the posterior approach and use of ceramic-on-ceramic and ceramic-on-plastic bearings had a lower risk of infection after hip replacement.
Limitations
In meta-analyses, definitions of prosthetic joint infection and the adjustment for confounding factors differed between studies. The results of meta-analyses were consistent in higher-quality studies and we noted no differences in studies reporting univariable or multivariable analyses.
The NJR analyses relied on subjective diagnosis of prosthetic joint infection by individual surgeons. As with our meta-analyses, there may have been residual confounding factors and associations may vary with different causative pathogens. For example, older people may be at higher risk of infection with organisms that are difficult to treat. 77
With observational data such as those collected in the NJR and cohort studies included in our systematic reviews, we cannot establish for certain whether or not the relationships between risk factors and revision for prosthetic joint infection are causal.
Diagnosis of prosthetic joint infection: assessment of new methods
Here we present an overview of our published article. 78
Aims
We aimed to assess the diagnostic accuracy of promising synovial biomarkers for prosthetic joint infection: the alpha-defensin immunoassay and leucocyte esterase colorimetric strip test.
Methods
The systematic review was registered with PROSPERO (CRD42015023704) and conducted in accordance with PRISMA and diagnostic test accuracy guidelines. 68,79 We searched MEDLINE and EMBASE on Ovid from inception until 30 May 2015 (see Appendix 12).
Inclusion criteria were:
-
people with hip or knee replacement suspected of having a prosthetic joint infection but with true diagnostic uncertainty
-
measurements of synovial fluid alpha-defensin or leucocyte esterase
-
prosthetic joint infection according to Musculoskeletal Infection Society (MSIS) diagnostic criteria or confirmation during subsequent surgery
-
reports of diagnostic test accuracy.
Screening and data extraction were performed independently by two reviewers. Extracted data related to patient characteristics, hip or knee joint, diagnostic test, test cut-off points, reference standards and results (sensitivity, specificity and likelihood ratios). Sensitivity and specificity values from each evaluation were pooled using a bivariate meta-analysis framework. 80 A quality assessment of each study was performed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. 81
Results
We included 11 eligible studies. The mean QUADAS-2 score was 13 out of 14 (range 11–14), suggesting that the studies were of good quality. The pooled diagnostic sensitivity and specificity of synovial fluid alpha-defensin (six studies) for prosthetic joint infection were 1.00 (95% CI 0.82 to 1.00) and 0.96 (95% CI 0.89 to 0.99), respectively. The area under the curve (AUC) was 0.99 (95% CI 0.98 to 1.00).
The pooled diagnostic sensitivity and specificity of leucocyte esterase (five studies) for prosthetic joint infection were 0.81 (95% CI 0.49 to 0.95) and 0.97 (95% CI 0.82 to 0.99), respectively. The AUC was 0.97 (95% CI 0.95 to 0.98).
There was substantial heterogeneity among studies for both diagnostic tests. No studies reported cost-effectiveness.
Discussion
Synovial fluid alpha-defensin and leucocyte esterase showed high diagnostic accuracy for prosthetic joint infection. Alpha-defensin was extremely sensitive and specific in the identification of prosthetic joint infection. Leucocyte esterase was slightly less sensitive but was extremely specific in the identification of prosthetic joint infection.
A systematic review published in 2019 considered a broad range potential serum, synovial and tissue diagnostic tests for prosthetic joint infection. 82 Of 83 tests identified, 17 had enough data from studies to allow meta-analysis. The authors reached similar conclusions to ours in identifying synovial fluid alpha-defensin and leucocyte esterase as the best-performing diagnostic tests for prosthetic joint infection.
Cost implications of the tests vary considerably. In a UK report, the lateral flow tests for alpha-defensin cost £300 each when purchased in a pack of five. 83 The laboratory-based immunoassay costed within a suite of tests was £450. This compares with a cost of £0.11 for a leucocyte esterase test. 78
Since the publication of our study, the MSIS criteria have reported a new definition of prosthetic joint infection citing our systematic review (Figure 14). 84 These include a definition that a hip or knee is infected if synovial leucocyte esterase is elevated and alpha-defensin is positive.
FIGURE 14.
The 2018 MSIS criteria for prosthetic joint infection. a, For patients with inconclusive minor criteria, operative criteria can also be used to fulfill definition for PJI; b, consider further molecular diagnostics such as next-generation sequencing. Note: proceed with caution in adverse local tissue reaction, crystal deposition disease and slow-growing organisms. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LE, leukocyte esterase; PMN, polymorphonuclear; WBC, white blood cell. Reprinted from The Journal of Arthroplasty, Vol. 33, Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. pp. 1309–14. Copyright 2018, with permission from Elsevier.
Limitations
Our study was limited by the small number of studies in the systematic review. Thus, we were not able to assess publication bias, and four out of six studies of alpha-defensin were conducted by a research group who held patents relating to the test.
Surgical treatment of prosthetic joint infection: evidence from cohort studies
Here we summarise three of our systematic reviews,85–87 our IPD meta-analysis88 and our NJR analyses (see Appendix 13).
Aims
In systematic reviews and meta-analyses, we aimed to compare reinfection rates after single- and two-stage treatments of hip and knee prosthetic joint infection. In an IPD meta-analysis we aimed to compare reinfection outcomes after single- and two-stage treatment of hip prosthetic joint infection. In a meta-analysis we aimed to assess the risk of reinfection after treatment of prosthetic joint infection with DAIR.
Analyses of linked NJR/ONS data aimed to compare rates of reinfection and mortality for people with prosthetic joint infection treated with single- or two-stage revision.
Methods: systematic reviews
The systematic reviews were registered prospectively with PROSPERO (CRD42017057513, CRD42015016559 and CRD42015017327) and conducted according to PRISMA68 and MOOSE69 guidelines. We searched MEDLINE and EMBASE on Ovid and Web of Science from inception (see Appendices 14–16), and reference lists.
Eligible studies included:
-
unselected patients with hip or knee prosthetic joint infection
-
treatment with DAIR, or single- or two-stage revision
-
reinfection or new infection outcome within 2 years of single- or two-stage revision, or any time after DAIR
-
longitudinal design.
We excluded studies that reported case series of selected patients, for example those with a specific infection; studies with fewer than 10 patients; and, for studies of single- and two-stage revision, follow-up of < 2 years.
Searches were carried out on 1 May 2017 (DAIR), 1 March 2015 (hip single- or two-stage revision) and 1 August 2015 (knee single- or two-stage revision).
Methodological quality was assessed using MINORS (methodological index for non-randomized studies). 89 The rate of reinfection within 2 years was the primary outcome across studies. Pooled rates were calculated using the Freeman–Tukey variance-stabilising double arcsine transformation. 90 Publication bias was assessed using Egger’s regression symmetry tests. 91
Methods: individual patient data meta-analysis
The IPD meta-analysis protocol was registered on PROSPERO (CRD42015016664) and conducted as recommended by the Cochrane Collaboration,92 the guidance of Riley et al. 93 and the PRISMA-IPD guidelines. 94 The study was limited to studies reporting outcomes after single- and two-stage revision for hip prosthetic joint infection. Searches86 were updated in August 2016. Authors of eligible studies and well-known investigators in the field were contacted, provided with the study protocol and invited to join the Global INFORM collaboration. 95 Collaborators were provided with standardised spreadsheets that were combined into a single database. Data in published articles were also included.
The risks of reinfection were compared using Cox proportional shared frailty models. 96 Hazard ratios (HRs) with 95% CIs were calculated, with progressive adjustment for age, sex, Charlson Comorbidity Index, previous hip surgery and type of infecting organism (‘difficult to treat’ vs. ‘not difficult to treat’).
Methods: National Joint Registry analysis
As described in Appendix 13, we analysed NJR data for England and Wales collected between 2003 and 2014 linked with ONS data. We included patients with a primary hip or knee replacement that had subsequently been revised for prosthetic joint infection with either a single- or a two-stage procedure. Reinfection and mortality outcomes were compared between treatment of hip and knee prosthetic joint infection using single- and two-stage revision strategies, and for mortality also with primary hip and knee replacements, and aseptic revisions. Analyses were adjusted for age, sex and ASA grade.
Results: systematic reviews
DAIR
We included 99 observational studies with data on 4897 prosthetic joint infections treated with DAIR. The infection control rate for DAIR ranged from 11.1% to 100%, with an overall pooled estimate of 61.4% (95% CI 57.3% to 65.4%). Infection control rates were poorer in patients aged < 70 years, in knee compared with hip prosthetic joint infection, in late chronic infections and in DAIR conducted before 2000.
Single-stage compared with two-stage revision for hip prosthetic joint infection
No randomised trials were identified. An assessment of reinfection outcomes was reported after single-stage revision in 38 studies (n = 2536 patients) and after two-stage revision in 60 studies (n = 3288 patients). MINORS methodological quality scores ranged from 9 to 16. The median age of participants was 63 years in single-stage studies and 60 years in two-stage studies.
The rate of reinfection was 8.2% (95% CI 6.0% to 10.8%) after single-stage revision and 7.9% (95% CI 6.2% to 9.7%) after two-stage revision. Reinfection rates remained generally similar when grouped by several study- and population-level characteristics.
Single-stage compared with two-stage revision for knee prosthetic joint infection
Searches identified no RCTs. Reinfection outcomes were reported after single-stage revision in 10 studies (n = 423 patients) and after two-stage revision in 108 studies (n = 5129 patients). MINORS methodological quality scores ranged from 9 to 15. The median age of participants was 71 years in single-stage studies and 67 years in two-stage studies.
In single-stage cohorts, the rate of reinfection was 7.6% (95% CI 3.4% to 13.1%), compared with 8.8% (95% CI 7.2% to 10.6%) in two-stage cohorts. Reinfection rates remained generally similar in several study-level and clinical characteristic subgroups. There was evidence of publication bias in two-stage studies.
Results: individual patient data meta-analysis
Eighty-five articles were identified reporting 98 cohorts of patients with hip prosthetic joint infection published up to August 2016. Contact with authors of studies led to the Global INFORM collaboration, comprising 15 cohorts (n = 1383 patients) with author-provided data. Data from a further 29 studies (n = 473 patients) were extracted from published articles.
After a median follow-up of 3.7 years, 222 reinfections were recorded. Reinfection rates per 1000 person-years of follow-up were 16.8 (95% CI 13.6 to 20.7) and 32.3 (95% CI 27.3 to 38.3) for single- and two-stage procedures, respectively. Among individuals with available data, the age-adjusted HR for reinfection comparing two- with single-stage revision was 1.69 (95% CI 0.58 to 4.98; p = 0.338). Progressive adjustment for sex, previous hip surgery and comorbidities did not change the outcome.
Results: National Joint Registry analysis
Hip prosthetic joint infection
Between 2003 and 2014, 535 patients with hip prosthetic joint infection received a single-stage revision and 1605 received a two-stage revision. More patients who received a single-stage revision had a re-revision for prosthetic joint infection in the first 2 years (HR 1.70, 95% CI 1.19 to 2.44; p = 0.004). This difference was limited to the first 3 months after surgery (HR 1.81, 95% CI 1.22 to 2.68; p = 0.003). For all-cause re-revision, the 2-year HR was 1.54 (95% CI 1.15 to 2.07; p = 0.004), which again was limited to the first 3 months after surgery.
Patients who received an initial single-stage revision had, on average, fewer revision operations than those who received a two-stage revision (1.3 vs. 2.2; p < 0.0001). Mortality rates were comparable between single- and two-stage procedures (29/10,000 person-years vs. 33/10,000 person-years), but these rates were higher than those observed after primary hip replacement or aseptic revision.
Knee prosthetic joint infection
Between 2003 and 2013, 489 patients with knee prosthetic joint infection received a single-stage revision and 2377 received a two-stage revision. The rates of revision for reinfection in the first 2 years were similar (HR 1.09, 95% CI 0.76 to 1.57; p = 0.66), and this was consistent at all follow-up time points. There was weak evidence of an increased risk of re-revision for any cause after single-stage revision (HR 1.32, 95% CI 0.97 to 1.81; p = 0.08), and this was limited to the first 3 months after revision for knee prosthetic joint infection (HR 1.51, 95% CI 0.95 to 2.41; p = 0.08).
Patients who received an initial single-stage revision had, on average, fewer revision procedures than those who received a two-stage revision (1.2 vs. 2.2; p < 0.001). Mortality was lower for single-stage revision between 6 and 18 months post operation (adjusted HR at 6 months 0.51, 95% CI 0.25 to 1.00; p = 0.05).
Conclusion
DAIR may be effective for > 60% of patients, particularly if it is performed early. An analysis of the NJR showed a higher risk of re-revision following single-stage revision for hip prosthetic joint infection than following two-stage revision, but this was limited to the 3 months after revision surgery. Patients who received a a single-stage revision had a smaller number of operations. When considered alongside the results of meta-analyses, the single-stage revision strategy for hip prosthetic joint infection is a reasonable option, with acceptable rates of infection control. Re-infection rates after single- and two-stage revision for knee prosthetic joint infection were similar, and patients who received single-stage revision underwent considerably fewer operations.
Limitations
An important limitation is that people may have been selected for a revision strategy depending on patient factors, including health status. An IPD meta-analysis was limited by a lack of available data for clinically relevant subgroups, including BMI and outcomes such as early or late infection. Our comparison of outcomes in cohorts of knee prosthetic joint infection was limited because few studies have reported outcomes after single-stage revision. Few studies collected patient-reported outcomes.
In the NJR studies there were small differences in patient characteristics between groups, which may indicate a degree of selection bias, and this may differ between the hip and knee cohorts. Furthermore, there is no linkage with microbiological data, and patients may have received a revision strategy based on the virulence of the infecting organism.
Surgical treatment of prosthetic joint infection: the INFORM randomised controlled trial
Here we summarise the INFORM randomised trial methods97 and results (see Appendix 17).
Aims
Our aim was to determine whether or not there is a difference in patient-reported outcome 18 months after randomisation to single- or two-stage revision surgery for the treatment of hip prosthetic joint infection. Eighteen months was chosen because the median interval between the stages of a two-stage revision is about 105 days (IQR 70–173 days), and, therefore, most people receiving a two-stage surgery should have achieved maximum recovery. A longer follow-up may have limited recruitment and patient retention. Although the trial was not powered to compare rates of reinfection or occurrence of a new infection, adverse events were monitored.
Methods
INFORM was a multicentre, two-arm, parallel-group, participant and observer unblinded, randomised superiority trial (ISRCTN Registry number ISRCTN10956306). Full details are in the published protocol97 and at https://doi.org/10.5523/bris.2323q1mtahy6y2dy5ua3opivqf. Patients deemed by their treating surgeons and multidisciplinary prosthetic joint infection teams to have infected hip replacements were randomised to either a single- or a two-stage revision.
Trial oversight
The protocol was approved by the UK National Research Ethics Committee South West and the University of Gothenburg review board. An independent steering committee and a data monitoring committee oversaw the study.
Sample size
The study sample size was based on our previous RCT of hip replacement. 98 To show a 10-point difference (equivalent to a standard deviation of 0.5) in total Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC) score between revision strategies at 18 months, we calculated that a sample of 148 patients would give 80% power, assuming a two-sided type I error of 5% and attrition of 13% (128 participants with a primary end point).
Patients
We recruited 140 patients between March 2015 and September 2018 at 15 secondary care orthopaedic units (11 in England, one in Wales and three in Sweden). Eligible patients were aged ≥ 18 years, had a clinical diagnosis of hip prosthetic joint infection and required treatment with single- or two-stage revision surgery.
Randomisation
Randomisation was independently conducted by the Bristol Randomised Trials Collaboration. Randomisation was by a secure remote third party via an internet-based application or by telephone. Randomisation could not be carried out on the day of surgery because of the need to order equipment and for logistical planning in advance of surgery, but it occurred as close to the time of surgery as possible (≤ 12 weeks). Patients were randomly allocated in a 1 : 1 ratio to one of the intervention groups. Randomisation within blocks of randomly varying size (four or six) was stratified by hospital.
Interventions
Owing to the nature of the intervention and planning requirements, surgeons and patients were made aware of the assigned treatment shortly after the baseline assessment and randomisation had been carried out. Patients were assigned to either single- or two-stage revision surgery.
All other aspects of treatment (antibiotics, analgesia, investigations, implants, static or articulating spacers, surgical techniques and approach) were in accordance with the treating surgeon’s usual practice and in line with local policies and procedures. Usual clinical care continued throughout the study follow-up period.
Outcomes
Research assessments were carried out preoperatively and every 3 months until 18 months post randomisation. Data were collected from patient questionnaires and clinical performance tests and extracted from medical records.
The primary outcome measure was the total WOMAC score measured at 18 months post randomisation. The proposed follow-up duration of 24 months was changed to allow more time for recruitment and a lower risk of attrition. Eighteen months was chosen as the timing of primary outcome as maximum recovery from all surgeries should have been achieved and further health improvements would have been unlikely. Secondary outcomes measured every 6 months between 6 and 18 months post randomisation included the Brief Pain Inventory short form, the Oxford Hip Score, the Hip Dysfunction and Osteoarthritis Outcome and the Hospital Anxiety and Depression Score. WOMAC score measured at each 3-month follow-up was also a secondary outcome.
Statistical analysis
The study was reported as per CONSORT (Consolidated Standard of Reporting Trials) guidelines and the analyses were performed according to the predefined analysis plan agreed with the Trial Steering Committee.
The main analysis was based on a two-level linear mixed model regressing the repeated measures of total WOMAC score on allocation group, assessment time and their interaction, with adjustment for hospital and baseline total WOMAC score. The difference in mean total WOMAC score at 18 months post randomisation between patients who received single-stage revision and those who received a two-stage revision (reference) was identified using linear combination of the treatment effect, time of assessment and their interaction (contrast). Further analyses were conducted with imputation using chained equations99 and the following sensitivity analyses were conducted: imputation of missing primary outcome assessments; accommodation of the possible range of WOMAC scores; adjustment for further baseline variables; imputation plus adjustment; and restriction to centres where a majority of two-stage procedures did not use a CUMARS.
Results
A CONSORT flow diagram is shown in Figure 15. Of 186 patients eligible for inclusion in the trial, 65 were randomised to single-stage revision and 75 were randomised to two-stage revision. The baseline characteristics of participants in the randomised groups are shown in Appendix 17, Table 13, and these were balanced between the groups, except that patients in the single-stage group were more likely to be male, with ASA grade 1 or 2 and have received previous non-surgical infection management. The groups were similar in the number and type of organisms cultured, the rates of culture-negative infection and the presence of a sinus tract. A total of 126 (90%) patients had the primary total WOMAC score outcome at 18 months post randomisation and 133 (95%) patients had at least one postoperative total WOMAC score and were included in the main analysis.
FIGURE 15.
The INFORM RCT CONSORT flow diagram. PJI, prosthetic joint infection.
There was no evidence of a difference in the mean total WOMAC score at 18 months post randomisation between single- and two-stage management of hip prosthetic joint infection (mean difference favouring two-stage 0.13, 95% CI –8.20 to 8.46; p = 0.98) (Figure 16).
FIGURE 16.
Mean (95% CI) global WOMAC score by revision surgery for prosthetic hip infection groups. A linear mixed regression model adjusted for place of surgery and accounting for participant repeated measures was used to produce the mean score at each time point.
At 3 months post randomisation, participants in the single-stage procedure group had a better total WOMAC score (mean difference favouring single-stage 11.53, 95% CI 3.89 to 19.17; p = 0.003). This is greater than the minimal clinically important improvement of 7 (95% CI 4 to 10) reported by Bellamy et al. 100 in a before-and-after study in a general osteoarthritis population. Sensitivity analyses, with imputation of the 14 missing primary outcome assessments and accommodation of the possible range of WOMAC scores, adjusted for further baseline variables and imputation plus adjustment, and limited to centres where a majority of two-stage procedures did not use a CUMARS, supported the conclusions of the main analysis.
From 6 months post randomisation onwards, no difference was found between the two surgical procedures. Similar findings were found for all patient outcomes.
A higher rate of complications of surgery was seen among patients receiving a two-stage revision than among those receiving a single-stage revision (57.3% vs. 41.5%; p = 0.04) and this difference was marked for intraoperative events (26.7% vs. 7.7%; p = 0.01), predominantly calcar cracks and femoral shaft fractures (18 vs. 3). There was no evidence of a difference between the groups in length of hospital stay; other complications; rehospitalisation or reoperation due to prosthetic joint infection, the surgery or another cause; or serious adverse events or deaths (5 vs. 2). At 15–18 months post randomisation, 9 out of 59 (15%) patients who received single-stage revision had signs of infection in the operated hip, compared with 8 out of 67 (12%) who received two-stage revision (p = 0.59).
Conclusion
As shown by the similarity in WOMAC total scores at 18 months after randomisation, the INFORM trial demonstrated that single- and two-stage revision are equally efficacious in the treatment of hip prosthetic joint infection. There was, however, an early improvement in WOMAC total scores in the single- compared with the two-stage group, which was clinically important. Given that patients state a strong preference for a quick return to normal activity, this should be considered when selecting the intervention. Further research should explore the value of earlier improvement to patients and health care. Single-stage surgery accounted for 30% of revision procedures for the treatment of prosthetic joint infection in 2014, so there is scope for increased utilisation.
Limitations
The INFORM study was powered for a patient-reported outcome and the sample size precluded study of infection clearance. The numbers of patients in the sensitivity analysis were large, except those restricted to centres where a majority of two-stage procedures did not use a CUMARS, which was limited to 90 patients.
Surgical treatment of prosthetic joint infection: the INFORM randomised controlled trial health economic analysis
Here we summarise the health economic analysis of the INFORM RCT described in Appendix 18.
Aim
To compare the cost-effectiveness of single- and two-stage revision surgery in the treatment of hip prosthetic joint infection.
Methods
The primary economic analysis was from an NHS and Personal Social Services (PSS) perspective, with a secondary analysis from a societal perspective. Resource use data relating to the initial surgery and subsequent treatment for revision of hip prosthetic joint infection were collected by research staff at each hospital at 3, 6, 9, 12, 15 and 18 months post randomisation. Admissions for revision surgery were microcosted and valued using information from the finance department of a treating hospital and the NJR. All subsequent inpatient stays, day cases and outpatient procedures were allocated an appropriate HRG code. NHS reference costs were used to value these stays, subsequent outpatient appointments and emergency department attendances. A self-reported resource use questionnaire was posted to participants at 6, 12 and 18 months post randomisation to collect information relating to community-based health-care use, prescribed medications and emergency department attendances; PSS provision received; personal expenses, including travel and home changes; and time off work, usual activities and informal care. Resources were valued using 2018–19 UK reference costs. 101–103
The economic evaluation outcome measure was the quality-adjusted life-year (QALY), derived from the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) questionnaire at baseline and at 3, 6, 12 and 18 months post randomisation. These were converted to utility scores using the validated mapping function to the existing EuroQol-5 Dimensions, three-level version (EQ-5D-3L) societal UK utility tariffs. 104 Total QALYs were calculated by linear interpolation using the AUC approach, taking into account any deaths that occurred during the study period by allocating those who died a zero utility in the time periods following their death. 105 A 3.5% discount rate was applied to all costs and outcomes occurring after 1 year.
Multiple imputation by chained equations using predictive mean matching was used to address missing data. Simple imputation methods assuming zero resource use in a returned participant questionnaire when no responses were given for an item, and using mean imputation where a participant had indicated the use of a resource but did not provide further details, were used prior to multiple imputation. Missing values for baseline utility were also imputed using the mean baseline utility value106 prior to multiple imputation. The covariates in the imputation model were baseline utility, trial group, age, sex, ethnicity, education status, work status, hospital, ASA grade at first revision surgery and the latest surgical treatment for the management of prosthetic joint infection prior to study participation. Rubin’s rules were used to combine the 62 individual imputations, and a randomisation seed was used to enable reproducible imputations. 99
The adjusted mean costs and QALYs by trial group, the differences in adjusted mean costs and QALYs and the incremental net monetary benefit (iNMB) were estimated using the seemingly unrelated regression method, which accounts for the correlation between costs and QALYs. 107 Costs and QALYs were adjusted for study centre. QALYs were also adjusted for baseline utility. National Institute for Health and Care Excellence (NICE) willingness-to-pay thresholds of £20,000 and £30,000 per QALY were used. Sample uncertainty within the cost-effectiveness estimates was explored using cost-effectiveness acceptability curves (CEACs) derived from the estimates of the seemingly unrelated regression. Sensitivity analyses (see Appendix 18, Table 20) were undertaken to account for uncertainty in the parameters used in the cost-effectiveness analyses.
Results
Owing to differences in the collection of resource use data for UK and Swedish participants, the primary economic analysis was conducted on 128 participants from England and Wales using a multiply imputed data set.
Total theatre time was longer for those randomised to two-stage revision, and these patients spent longer in intensive care and recovery and had a longer overall hospital stay. They also had a greater number of subsequent inpatient stays and emergency department attendances. There were a greater number of primary care practice nurse visits for those randomised to a single-stage procedure, whereas those randomised to a two-stage procedure had a greater number of district nurse home visits, stays in residential homes and home care worker visits.
The EQ-5D-5L utility values at all time points are shown in Table 5. These illustrate the different pathways of the two procedures, with patients randomised to the single-stage procedure seeing a gradual improvement in their utility values from 3 months onwards, whereas for those randomised to the two-stage procedure the improvement begins only at 6 months.
| Time point | Single-stage revision surgery | Two-stage revision surgery | ||
|---|---|---|---|---|
| n | Mean utility (95% CI) | n | Mean utility (95% CI) | |
| Baseline | 60 | 0.32 (0.23 to 0.41) | 67 | 0.28 (0.20 to 0.36) |
| 3 months | 50 | 0.51 (0.41 to 0.61) | 62 | 0.33 (0.23 to 0.42) |
| 6 months | 45 | 0.54 (0.44 to 0.64) | 56 | 0.46 (0.37 to 0.55) |
| 12 months | 49 | 0.54 (0.44 to 0.64) | 56 | 0.50 (0.42 to 0.59) |
| 18 months | 52 | 0.60 (0.50 to 0.70) | 60 | 0.60 (0.52 to 0.68) |
Table 6 shows that the mean costs from the treating hospital were higher in the first year of follow-up in the single-stage group; however, in the last 6 months of the trial these costs were similar in the trial groups, indicating that the trial follow-up is sufficiently long to capture differences in costs.
| Single-stage revision surgery | Two-stage revision surgery | |||
|---|---|---|---|---|
| n | Mean cost (£) (95% CI) | n | Mean cost (£) (95% CI) | |
| Surgical admissions (0–6 months) | 58 | 21,287 (18,250 to 24,323) | 62 | 25,674 (21,696 to 29,652) |
| Follow-up outpatient visits and admissions (0–6 months) | 56 | 894 (123 to 1664) | 63 | 820 (427 to 1213) |
| Surgical admissions (6–12 months) | 58 | 2039 (613 to 3466) | 62 | 3660 (1688 to 5632) |
| Follow-up outpatient visits and admissions (6–12 months) | 56 | 437 (72 to 802) | 63 | 444 (181 to 707) |
| Surgical admissions (12–18 months) | 58 | 1593 (–426 to 3612) | 62 | 1489 (–76 to 3055) |
| Follow-up outpatient visits and admissions (12–18 months) | 56 | 484 (453 to 516) | 63 | 702 (443 to 962) |
The total adjusted mean costs in the single-stage group (£36,256) from the NHS/PSS perspective were lower than in the two-stage group (£46,312), a cost difference of –£10,055 (95% CI –£19,568 to –£542). The cost difference reduced slightly from the societal perspective (–£9450, 95% CI –£22,855 to £3956). Participants in the single-stage group had a greater number of adjusted mean QALYs (0.75) than those in the two-stage group (0.69), a difference of 0.06 (95% CI –0.07 to 0.18).
From the NHS/PSS perspective, the iNMB of single-stage revision compared with two-stage revision was £11,167 (95% CI £638 to £21,696) at £20,000 per QALY willingness-to-pay threshold, and at this threshold there was a 98% probability that the single-stage procedure was the cost-effective option. This reduced to 92% from the societal perspective. All sensitivity analyses conducted from the NHS/PSS perspective showed that the probability of the single-stage procedure being cost-effective was at least 98%.
Conclusion
The two hospital stays in a two-stage procedure led to the higher cost in this group. The EQ-5D-5L scores illustrate that while patients were waiting for their second-stage operation they had a poorer quality of life, which was reflected in the overall QALY score. The greater use of district nurse home visits and home care worker visits indicates that these patients were also less able to self-care and leave their home.
The within-trial economic evaluation has shown, with a great degree of certainty, that the single-stage procedure is the cost-effective option for patients with hip prosthetic joint infection.
Limitations
The analysis has limitations in relation to missing data, particularly for resource use as obtained from patient-completed questionnaires. A complete-case analysis could only be conducted from a treating hospital perspective, and indicated higher QALY values in both groups, indicating that those who did not complete all the questionnaires were likely to have poorer quality of life than those who did. Multiple imputation was used to account for the missing data, using data at each time point in the model, meaning that all available data were used. This meant that the multiple imputation could not be conducted by trial group. However, sensitivity analyses on different specifications of the multiple imputation model indicate that the model specification is unlikely to have affected the conclusions of the analysis.
Cost differences may extend beyond the 18-month trial follow-up. However, the randomised groups had similar hospital-based costs accruing between 12 and 18 months’ follow-up and had similar mean WOMAC and EQ-5D-5L scores at 18 months. There was also little to suggest a difference in reinfection rates between groups. Together, these suggest that patient outcomes and hospital costs may not differ in the longer term.
Surgeons’ views on the acceptability of trial information and recruitment
Aims
We aimed to explore surgeons’ experience and acceptance of the randomisation process, their understanding of equipoise, and their views on information about the trial and interventions.
Methods
We conducted telephone interviews with 12 surgeons from eight sites participating in the trial. We used a topic guide developed in collaboration with INFORM surgeons. Topics included surgeons’ experiences and acceptability of the randomisation process and their views on surgical equipoise, and information about the trial and interventions. Data were analysed using a descriptive qualitative approach and reported to the trial management group to inform the recruitment process and identify areas for improvement.
Results
Surgeons were happy with the information they received about the trial and the randomisation process. We identified a number of potential barriers to recruitment and areas for improvement. The complexity of cases often precluded patients from randomisation, and often this was because the surgeon lacked equipoise in that particular case. Some surgeons suggested that there was a lack of engagement at their centres and that their colleagues lacked equipoise about the trial. Recruitment of patients was difficult as prosthetic joint infection is a rare condition and we did not have the resources and staff to find eligible patients opportunistically at routine outpatient or emergency appointments. At one centre, a change in referral patterns meant that the number of patients being referred had reduced. We addressed these barriers in the following ways:
-
We originally planned to recruit patients from four centres but increased this number in the early stages of the trial when we realised that recruitment would be a challenge. The funding of additional sites was met from the existing budget and with UK Clinical Research Network portfolio support.
-
We continued to collect data on patients deemed ineligible.
-
We communicated anonymised case studies from two recruiting centres to surgeons in the other centres to demonstrate the range of patients randomised and the characteristics of individual cases. This resulted in debate and dialogue between surgeons regarding equipoise.
-
We communicated directly with all surgeons operating on patients with prosthetic joint infection within collaborating trusts to ensure that they remained engaged.
-
We communicated findings of other work packages to surgeons through newsletters to enhance understanding of the broad context of INFORM.
-
We held workshops with recruiting nurses to provide them with training and support on ways to identify and recruit patients and to ensure that potential participants were brought to the attention of participating surgeons.
-
The chief investigator and the clinical lead of work package 4 hosted information events at national conferences and visited collaborating centres to explore local service and recruitment pathways to ensure that eligible patients were identified and that surgeons remained engaged.
Conclusion
Data from interviews with surgeons in the early phase of the trial enabled us to identify barriers to recruitment and implement remedial strategies to improve recruitment.
Surgical treatment of prosthetic joint infection: patient experience of treatments in the INFORM randomised controlled trial
Here we summarise our published article. 108
Aims
We aimed to explore the early and long-term experiences of patients receiving single- and two-stage revision. In patients with two-stage revision we also aimed to explore experiences of patients with either a temporary cement spacer or CUMARS.
Methods
On two occasions, we interviewed 32 people participating in the INFORM RCT who had received single- or two-stage revision surgery for hip prosthetic joint infection. Patients were interviewed at 2–4 months and 18 months after their first revision operation.
Interview topic guides were developed with the INFORM PPI group but were flexible to allow participants to discuss areas that they felt were important. Themes were generated from the data inductively using the constant comparative method.
Results
Thirty people were interviewed 2–4 months after their first revision operation, and 17 were interviewed at the 18-month follow-up. The overall sample of 32 patients comprised 15 women and 17 men and had a mean age of 68.9 years (range 51 to 89 years). Eleven had received a single-stage and 21 had received a two-stage revision. Of those who had received a two-stage revision, seven had a cement spacer, 10 had a CUMARS and four had an excised hip with no spacer.
During their early recovery period, people who had received single- or two-stage revision for hip prosthetic joint infection experienced prolonged hospital stays of 2–4 weeks, difficult antibiotic regimens and only brief physiotherapy. People described the practical challenges and gains that they had experienced during day-to-day life while recovering from revision at home and identified their needs for information and support during recovery. At 18 months post revision, all patients described improvements in mobility and independence, but they also reported ongoing restrictions to walking and some functional limitations. People also described their emotional resilience and benefits from participating in the INFORM RCT.
The experiences of people who received a two-stage revision with a cement spacer or excised hip differed from those receiving single-stage revision or a two-stage revision with a CUMARS. Patients who received a single-stage revision or a two-stage revision with a CUMARS had greater mobility and independence in the early stages of recovery. However, patients who received a cement spacer or CUMARS perceived that their recovery was slow, and those with a CUMARS reported dislocations and uncertainty about the need for further surgery.
Conclusion
After treatment for prosthetic joint infection, important aspects of patient recovery relate to mobility, function, pain and independence. Patients who receive single- and two-stage revision experience different patterns of recovery and these are also influenced by use of a spacer and CUMARS. To be supported during treatment and recovery, patients require appropriate information and opportunities to talk and share experiences.
Limitations
The comparison of experiences of recovery at both the postoperative and the 18-month time points in the same patients was limited as we were not able to reinterview all 30 of the original participants. We interviewed other participants at 18 months to balance the characteristics of the sample and to ensure saturation. The participants who took part in the qualitative study may have been those who were most positive about trial participation.
Patient preferences for revision surgery after prosthetic joint infection
Here we summarise our published article. 109
Aims
Single- and two-stage revision options for treatment of prosthetic joint infection each have implications for the patient time course and experience of recovery. We aimed to assess the surgical preferences of patients who had received revision surgery for hip prosthetic joint infection.
Methods
We undertook a discrete choice experiment to quantify the surgical preferences of patients who had received a single- or two-stage revision for hip prosthetic joint infection. In a discrete choice experiment, participants choose between features of interventions or approaches to care. These are attributes that can have different levels. A checklist based on the guidance of Lancsar and Louviere110 is provided in Appendix 19.
Attributes
Attributes were developed from our INFORM qualitative patient interviews into the impact of hip prosthetic joint infection and revision surgery described in Patients’ experiences of prosthetic joint infection after hip or knee replacement and its treatment. 57 These data were analysed thematically, and attributes and levels were assigned. 111 We identified four attributes of revision surgery for hip prosthetic joint infection and defined levels (Table 7). Pairs of profiles were designed using a moduloarithmetic process, ensuring full balance and efficiency in the design. 112 To create a manageable questionnaire, the 32 feasible pairs of profiles were reduced to 16 using an orthogonal main effects plan to produce a fractional factorial design for the final questionnaire (see Appendix 20). The questionnaire was piloted and refined in collaboration with five PPI representatives.
| Attribute | Levels |
|---|---|
| Number of operations | One operation |
| Two operations | |
| Ability to engage in valued activities after new hip is fitted | Can do everything |
| Can do most things | |
| Cannot do most things | |
| Cannot do anything | |
| Time taken after surgical treatment starts to return to normal activities (months) | 3 |
| 6 | |
| 12 | |
| 18 | |
| Antibiotic side effects | Affect me a lot |
| Do not affect me much |
Sample size
Recognising the rarity of hip prosthetic joint infection, a sample size of ≥ 50 patients was considered appropriate.
Participants
Patients recruited to the INFORM RCT were invited to complete the questionnaire at their 18-month follow-up. The questionnaire was posted to participants for self-completion or completed with the assistance of a research nurse during a hospital clinic visit.
Data analysis
Questionnaire data were effects-coded113 and analysed using Stata SE version 15 (StataCorp LP, College Station, TX, USA). The influence of the four attributes on patient choices was analysed using a conditional logit model (pseudo R2 = 0.182). The effect-coding of attribute levels gives a mean coefficient of zero across each attribute.
Results
Questionnaires were sent to 80 patients at nine orthopaedic centres, and 57 were fully completed (71%). The mean age of patients was 70 years (range 51–90 years); 21 (37%) were female, 26 (46%) had received single-stage revision, 14 (25%) lived alone and 41 (72%) were retired. The questionnaire took between 20 and 40 minutes to complete.
Regression coefficients and results are shown in Table 8. Participants had the strongest preference for a surgical option that resulted in the least restrictions on engagement in valued activities after the new hip was fitted, illustrated by the largest preference weight. Less valued but important preferences were for a surgical treatment that would result in a shorter time to return to normal activities, few or no side effects from antibiotics, and only one operation. The results also suggest that the least restrictions on engaging in valued activities and the shortest time taken to return to normal activity are the individual attributes most valued by patients. This is indicated by the larger spread of coefficients. The most acceptable option was a time period of between 3 and 6 months to return to normal activity. There was no clear preference up to 12 months, but 18 months was considered unacceptable.
| Attribute | Coefficient | SE | 95% CI | p-value |
|---|---|---|---|---|
| Ability to engage in valued activities after new hip is fitted | ||||
| Can do everythinga | 0.70 | |||
| Can do most things | 0.49 | 0.08 | 0.33 to 0.64 | < 0.001 |
| Cannot do most things | –0.39 | 0.07 | –0.53 to –0.24 | < 0.001 |
| Cannot do anything | –0.80 | 0.13 | –1.05 to –0.55 | < 0.001 |
| Antibiotic side effects | ||||
| Do not affect me mucha | 0.22 | |||
| Affect me a lot | –0.22 | 0.05 | –0.33 to –0.12 | < 0.001 |
| Number of operations | ||||
| 1a | 0.20 | |||
| 2 | –0.20 | 0.07 | –0.35 to –0.06 | < 0.001 |
| Time taken after surgical treatment starts to return to normal activities (months) | ||||
| 3a | 0.20 | |||
| 6 | 0.31 | 0.09 | 0.14 to 0.48 | < 0.001 |
| 12 | –0.06 | 0.05 | –0.15 to 0.04 | 0.22 |
| 18 | –0.45 | 0.10 | –0.64 to –0.26 | < 0.001 |
Conclusion
The most valued factors in decisions about revision surgery for hip prosthetic joint infection were the ability to engage in valued activities and the time taken to return to normal activity.
Limitations
Feedback from early participants suggested that completing the questionnaire was difficult. However, after the instructions and format were altered and when nurse support was provided, participants were able to understand and complete the questionnaire. Our chosen analysis method of conditional logistic regression assumes preference homogeneity and it may be that participants exhibit a level of heterogeneity that cannot be captured using this method. The use of latent class analysis could further explore the patterns in choice data that suggest preference heterogeneity, but our sample size was too small for these explorations to be conducted.
Dissemination of the INFORM programme
Dissemination of our results has been extensive. Some activities have been more limited than planned owing to the COVID-19 pandemic. However, it is our intention to pursue activities further when group gatherings are permitted.
Publications
All our research is published or submitted, or analyses are in progress. Several articles have become highly cited in their area, including those relating to risk factors, the patient experience, diagnostic tests and treatments.
British Hip Society
The significance of the INFORM programme is recognised by the British Hip Society and an afternoon symposium was given over to presentation and discussion of results at the 2020 annual meeting in South Wales (see Appendix 21).
International Consensus Meeting on Periprosthetic Joint Infection
To address the wide international variation in prevention and treatment of prosthetic joint infection, the International Consensus Meeting on Periprosthetic Joint Infection was organised by Thorsten Gehrke and Javad Parvizi. The meeting took place in July 2018 and the INFORM programme was represented by Setor Kunutsor.
Conferences
The results from the INFORM programme have been presented at European Federation of National Associations of Orthopaedics and Traumatology (EFORT), International Society of Arthroplasty Registries (ISAR), European Orthopaedic Research Society (EORS), British Sociological Association (BSA), Combined Orthopaedic Associations (COMOC), British Pain Society (BPS), British Society for Rheumatology (BSR), British Orthopaedic Association (BOA) and British Hip Society (BHS) conferences.
M Shed
A 1-day conference was planned for May 2020 to present results to patient-partners, INFORM trial participants and representatives of recruitment centres. This will be rescheduled when feasible.
Implications of the INFORM programme
Overview
In the INFORM programme, we critically reviewed existing research and conducted new high-quality studies with the aim of improving treatment and outcomes for people with hip and knee prosthetic joint infection. Work packages either informed each other or triangulated results. Our studies were supported with extensive PPI.
Our NJR analyses showed that, in the UK, rates of revision surgery within 10 years of primary hip and knee replacement are 0.62% and 0.75%, respectively, but are about four times higher after aseptic revision. Further NJR analyses and meta-analyses of previous research identified multiple modifiable and non-modifiable patient and surgical risk factors, potentially allowing for better counselling of patients and the suggestion of preventative strategies.
Qualitative studies highlighted that infection and revision treatments have a devastating and long-lasting impact on patients and their families, who report that more psychosocial support is required to address unmet needs. Patients and surgeons described prolonged treatments and periods of uncertainty. Important attributes of a patient being considered for revision surgery for hip prosthetic joint infection were identified. In surgeon interviews specifically focusing on the INFORM trial, barriers to recruitment were identified and remedial strategies implemented.
The use of DAIR is effective in > 60% of cases and is more efficacious if performed early. Surgeons should be encouraged to act promptly when infection has occurred.
The INFORM randomised trial shows that revision for hip prosthetic joint infection is equally efficacious with a single- and a two-stage procedure, and good long-term patient outcomes are achieved in a wide range of patients. Two-stage revision is the more widely used treatment, but the use of single-stage revision has increased, with about 30% of revisions for hip and 19% for knee prosthetic joint infection having been performed in single operations in 2014. Single-stage revision gives better patient-reported outcomes in the first 3 months, but not thereafter.
In the INFORM trial, single-stage surgery was cost-effective in terms of QALYs compared with two-stage surgery for treatment of hip prosthetic joint infection, but infection is very expensive to treat.
Any greater risk of reinfection or new infection after a single-stage procedure than after a two-stage procedure seen in NJR analyses may be limited to the first few months after surgery. In this period, patients receiving a two-stage revision may also have infections that have not fully cleared, leading to delay in the second stage reimplantation. Thus, as with a two-stage revision, close monitoring of patients with a single-stage revision is warranted. Patients with a single-stage revision for treatment of hip prosthetic joint infection have a smaller number of operations overall. In meta-analysis and IPD meta-analysis, the single-stage revision strategy for hip prosthetic joint infection is a reasonable option with acceptable rates of infection control. Reinfection rates after single- and two-stage revision for knee prosthetic joint infection were similar and those patients receiving single-stage revision received considerably fewer operations.
Complementing the results of the INFORM trial, responses to the discrete choice experiment suggested that patients identify the ability to engage in valued activities and the time taken to return to normal activity as the most valued characteristics in decisions about revision surgery. Other preferences are less severe side effects from antibiotics and revision with only one operation. Recognising the different outcome trajectories of single- and two-stage revision, the choice of intervention should be based on the patient’s preference for the recovery profile that is important to them.
Limitations
Our research studies have limitations that may affect their generalisability.
General
-
Our studies mainly included people with joint replacement for osteoarthritis and the results may not be generalisable to other indications.
-
Some research was specific to people with hip prosthetic joint infection.
National Joint Registry and systematic reviews
-
Study populations may have been selected for specific treatments based on their health status and infecting organism.
-
In NJR studies, we were only able to report the outcome of revision for treatment of prosthetic joint infection and do not know how many people were treated non-surgically.
-
With observational data, we cannot establish for certain whether or not relationships between risk factors and revision for prosthetic joint infection are causal.
-
Individual patient data meta-analysis was limited by a lack of available data for some clinically relevant subgroups.
-
Few studies were identified reporting outcomes after single-stage revision for knee prosthetic joint infection.
-
Few studies collected patient-reported outcomes.
Randomised trial
-
The INFORM trial was not powered to study reinfection and was limited to 18 months’ follow-up.
-
Some centres used a CUMARS in the first stage of a two-stage revision, with the option of leaving in situ permanently.
Qualitative studies
-
Subgroups were small.
-
Patients reporting experiences of revision strategies may have been those most positive about trial participation.
Health economic studies
-
The resources included in cost calculations in the studies we reviewed varied considerably.
-
In NJR analyses, we did not consider costs relating to outpatient, primary and community care, prescribed medications and treatments received outside England.
-
In the INFORM trial, there were substantial missing data relating to resource use.
Review of diagnostic tests
-
There was an exclusive focus on two promising biomarkers.
-
A majority of studies of alpha defensin were conducted by a research group holding patents for related products.
Discrete choice questionnaire
-
Sample size limited options for analysis and was too small to allow an exploration of responses in different subgroups.
Research recommendations
-
In collaboration with patients and health-care professionals, develop clear information for people receiving treatments for prosthetic joint infection.
-
Develop, implement and evaluate enhanced care pathways for people with hip and knee prosthetic joint infection.
-
Develop counselling, peer support and supportive interventions in the revision surgery pathway and improve physiotherapy provision for patients with prosthetic joint infection.
-
Explore whether or not patient education and supportive care can lead to earlier recognition of signs and symptoms of infections.
-
Future work could look at preparedness for adverse outcomes, help-seeking in impactful situations, and information for health-care professionals about early signs and care for prosthetic joint infection.
-
Develop targeted preventative strategies for high-risk patients, for example better control of comorbidities such as diabetes and effective weight loss strategies in patients with very high BMI.
-
Explore the effectiveness of counselling, monitoring and targeted preventative strategies based on risk factors for prosthetic joint infection.
-
Use of the CUMARS has increased and research into their long-term survival is required.
-
The role of spacers in two-stage revisions needs to be appraised in view of the high prevalence of complications associated with their use.
-
The relative efficacy and cost-effectiveness of single- compared with two-stage revision surgery should be explored in certain high-risk subgroups, in particular those with difficult-to-treat organisms and culture-negative infections.
-
The use of a single-stage revision strategy in knee prosthetic joint infection has increased and a randomised evaluation is needed.
-
Further independent comparisons in UK representative cohorts should be made between synovial fluid alpha-defensin and leucocyte esterase, and traditional diagnostic tests to assess their relative clinical effectiveness and cost-effectiveness.
-
The majority of the minor criteria in the MSIS diagnostic criteria for prosthetic joint infection are not routinely available or used in the diagnosis of prosthetic joint infection in the NHS setting, and a set of diagnostic criteria relevant to contemporary NHS practice needs to be established and assessed.
Reflections on work packages
The involvement of our PPI group was valuable in all work packages. The group gave support to the INFORM RCT, which helped with the recruitment, retention and engagement of patients. The model of taking research to the patients was welcomed by patients, who found that inviting clinicians and researchers to meetings ‘owned’ and run by the PPI group was preferable to and less intimidating than patients attending research and management meetings. Patients felt happy to set the agenda and direct the meetings over which they had ownership. Researchers and clinicians found this refreshing and informative.
Members of the INFORM group carrying out secondary research felt that discussing the methods and results of the evidence synthesis with the PPI group helped them appreciate the significance of prosthetic joint infection and its treatment to patients. In the field of prosthetic joint infection, systematic reviews included many published studies that had small sample sizes. There is a need for more collaborative analyses. Investigators were very willing to share data for the IPD meta-analysis and we wish that we had done more IPD analysis on related topics. Further work using this approach is planned. Hopefully, in future, there will be more data from clinical trials that will benefit from meta-analysis.
Something that stands out is the value of the qualitative work in giving a small patient population a voice. The work was very important in the programme and we wish that we had done more qualitative work to further understand the experience of patients, their significant others and health-care workers treating infection. Although prosthetic joint infection affects only a small proportion of patients who undergo joint replacement, our work has shown just how devastating and life-changing its impact can be and has identified an important need for further supportive care pathways. It has also given surgeons a voice. While prosthetic joint infection has been identified as every orthopaedic surgeon’s ‘worst nightmare’,62 until now its impact on surgeons’ professional and personal well-being had not been addressed in the literature. We hope that this work in some ways helps surgeons to broach the topic with colleagues in a supportive fashion, rather than, as one surgeon suggested, ‘burying your head in the sand’.
Across all study designs, the significance of patient outcomes and not just reinfection was reinforced in interviews with patients and surgeons and meetings with the PPI group.
We enjoyed undertaking a discrete choice experiment in orthopaedics, even though it proved challenging for patients and, ultimately, quite labour intensive. The results gave novel insights into what was important to patients. In future studies, we will undertake training and methodological work to make this research easier for participants.
The NJR proved a rich resource for exploring the significance of prosthetic joint infection to the NHS. The sheer number and breadth of data afforded by NJR/HES linkage allowed an exploration of secular trends, risk factors and treatments that is not possible with other observational data sets. We are encouraged to see the establishment and development in the UK of similar large national audits for other conditions.
To guide decisions on appropriate care, the top level of evidence in the hierarchy of primary research is the RCT, which is acknowledged to be difficult when comparing surgical procedures. The INFORM multicentre trial was completed successfully and showed that equipoise and random allocation of treatments for prosthetic joint infection was acceptable to patients and surgeons. The trial was a difficult undertaking. For anyone replicating it, we recommend opening many sites early, spending time encouraging surgeons, and getting surgeons at different centres to talk to each other about cases to set their minds at ease. The use of a per-patient fee at research centres was critical in a trial focusing on a rare condition.
Challenges and successes
Having been involved in a number of NIHR Programme Grants, we are particularly pleased with the large volume of clear results of direct relevance to patients, clinicians and health-care providers that this programme has delivered. We now have new important knowledge on impact, risk factors and treatment that will inform care.
The qualitative work, PPI and evidence synthesis were particularly productive. The IPD analysis allowed us to internationalise the programme and it was pleasing to have such willing international co-operation leading to an important scientific publication. The evidence synthesis could, therefore, expand beyond the original aims and cover infection in a large range of joints.
Despite our extensive experience with NJR/HES, this work was initially more complex than we had anticipated, with lengthy data cleaning and the careful unpicking of the complex patient journey. For example, many patients do not simply received a single- or two-stage revision but have multiple first stages before the second stage. Despite this, the work package delivered excellent outputs, which are already influencing care and are highly cited.
Certainly, the most challenging aspect of the study was the randomised trial. Possibly quite predictably, we overestimated recruitment rates at some centres and had to open more centres than initially planned. This necessitated extending the programme to 6 years. Costs, however, were contained by introducing a per-patient fee with payments tied to milestones. We would encourage researchers to adopt this funding model, which incentivises centres and controls costs. It may be argued that a RCT focusing on the treatment of infection should be powered to study infection clearance. Our previous research, patient interviews and PPI indicated that patients, rather than being concerned with biomedical outcomes relating to prosthesis survival and further treatments, are concerned about pain, function and well-being after surgery. When data accrue from further RCTs, meta-analysis will allow a more thorough analysis of infection clearance after single- and two-stage revision.
In the programme, it was excellent to be able to approach important questions using a variety of methods and resources. When answers concurred, this was very reassuring.
Service developments
We have shown that infection is devastating and that patients lack the physical and psychological support that they have identified as necessary. We have identified the patients at highest risk, allowing adequate preoperative counselling and targeted measures to decease risks. We have delineated the efficacy of DAIR and shown that early intervention markedly improves the success rate of this treatment. Single- and two-stage revision appear equally efficacious, and both achieve good results; however, single-stage surgery gives better early results and is cost-effective in terms of QALYs. Surgeons can, therefore, feel confident to adopt this treatment.
Acknowledgements
We would like to thank all the patients, surgeons, health-care professionals, patient partners and researchers who have contributed to the INFORM programme. We acknowledge support from the NIHR Clinical Research Network.
Trial Steering Committee
We thank the members of the Trial Steering Committee for their support in the development, conduct and delivery of the INFORM RCT:
-
Professor Rod Taylor, University of Exeter
-
Martyn L Porter, Centre for Hip Surgery, Wrightington Hospital
-
Ali Heawood, Bristol Medical School: Population Health Sciences
-
Julie Chappell, DAC Beachcroft, Bristol.
INFORM group
The INFORM group comprises the following.
Lead surgeons recruiting patients to INFORM studies
Richard Baker, Tim Board, Ben Burston, Pedro Foguet, Ed Gardner, Peter Grant, Stephen Jones, Sanchit Mehendale, Jonathan Miles, Michael C Parry, Andrew J Porteous, Mike Reed, Jörg Schilcher, Olof Sköldenberg, Ian Stockley, Karin Svensson, Adrian H Taylor, Andrew D Toms, Jason CJ Webb, J Mark Wilkinson and Matthew J Wilson.
Study co-ordinator for centres in Sweden
Lotta Falkendahl.
Collaborators with specific expertise
Matthew C Barrett, Richard S Craig, Elizabeth Darley, Paul Dieppe, Tim J Peters, Caoimhe Rice, Michael C Wyatt and Vikki Wylde.
Patient partners
Edith Anderson, Lizzy Betts, Charlie Brimble, Pat Clark, Debby Collett, Sue Comley, Vernon Cooper, Geoff Dyer, Enid Edwards, Ken Kent, Mike Nicholson, David Peacock, Sheila Spence and Jean Welham-Clarke.
Support for recruitment, data collection and administration
Marie Burgess, Beverley Evanson, Louise Hawkins, Kristina Lewis and Makita Werrett.
Contributions of authors
Ashley W Blom (https://orcid.org/0000-0002-9940-1095) (Professor of Orthopaedic Surgery) was chief investigator with responsibility for the delivery of the overall programme and lead for work package 4. He is the corresponding author.
Andrew D Beswick (https://orcid.org/0000-0002-7032-7514) (Research Fellow in Evidence Synthesis) was a co-applicant and work package 1 lead. He provided support to the programme systematic reviewer and was responsible for drafting this report.
Amanda Burston (https://orcid.org/0000-0003-3480-4210) (Senior Research Associate in Public and Patient Involvement) was a co-applicant and led and co-ordinated PPI in the programme.
Fran E Carroll (https://orcid.org/0000-0002-7124-7719) (Research Fellow) developed and analysed the discrete choice questionnaire in work package 6.
Kirsty Garfield (https://orcid.org/0000-0002-8301-3602) (Research Associate in Health Economics) performed the health economic analysis of the NJR in work package 2.
Rachael Gooberman-Hill (https://orcid.org/0000-0003-3353-2882) (Professor of Health and Anthropology) was a co-applicant and overall lead for qualitative studies.
Shaun Harris (https://orcid.org/0000-0001-7724-6621) (Senior Research Associate in Health Economics) performed the health economic analysis of the INFORM RCT in work package 4.
Setor K Kunutsor (https://orcid.org/0000-0002-2625-0273) (Research Fellow in Evidence Synthesis) conducted the systematic reviews and meta-analyses in work package 1. He established and led the IPD meta-analysis.
Athene Lane (https://orcid.org/0000-0002-7578-4925) (Professor of Trials Research) provided expertise in randomised trial methods to support conduct of work package 4.
Erik Lenguerrand (https://orcid.org/0000-0002-0371-731X) (Senior Research Fellow in Medical Statistics) was a co-applicant and lead for work package 2. He was lead statistician in work package 4.
Alasdair MacGowan (https://orcid.org/0000-0002-6720-5268) (Professor of Antimicrobial Therapeutics) was a co-applicant and microbiology lead for the programme.
Charlotte Mallon (https://orcid.org/0000-0003-0179-0131) (Research Associate in Qualitative Research) carried out qualitative studies in work package 3.
Andrew J Moore (https://orcid.org/0000-0003-3185-1599) (Research Fellow in Qualitative Health Research) was qualitative lead for work packages 3 and 4 and provided support for work package 6.
Sian Noble (https://orcid.org/0000-0002-8011-0722) (Senior Lecturer in Health Economics) was a co-applicant and lead for work package 5.
Cecily K Palmer (https://orcid.org/0000-0002-2096-008X) (Senior Research Associate in Health Economics) carried out qualitative studies in work package 4.
Ola Rolfson (https://orcid.org/0000-0001-6534-1242) (Professor) was principal investigator for the Swedish work package 4 centres.
Simon Strange (https://orcid.org/0000-0002-4306-4640) was the programme manager and co-ordinated recruitment and data collection in work packages 4 and 6.
Michael R Whitehouse (https://orcid.org/0000-0003-2436-9024) (Reader and Consultant in Trauma and Orthopaedics) was work package 2 deputy lead and work package 4 co-lead.
Publications
Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD, INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected hip prosthesis: a systematic review and meta-analysis. PLOS ONE 2015;10:e0139166. https://doi.org/10.1371/journal.pone.0139166
Kunutsor SK, Whitehouse MR, Webb J, Toms A, Stockley I, Taylor A, et al. Re-infection outcomes following one- and two-stage surgical revision of infected hip prosthesis in unselected patients: protocol for a systematic review and an individual participant data meta-analysis. Syst Rev 2015;4:58. https://doi.org/10.1186/s13643-015-0044-0
Moore AJ, Blom AW, Whitehouse MR, Gooberman-Hill R. Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open 2015;5:e009495. https://doi.org/10.1136/bmjopen-2015-009495
Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD, INFORM Team. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLOS ONE 2016;11:e0150866. https://doi.org/10.1371/journal.pone.0150866
Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD, INFORM Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLOS ONE 2016;11:e0151537. https://doi.org/10.1371/journal.pone.0151537
Strange S, Whitehouse MR, Beswick AD, Board T, Burston A, Burston B, et al. One-stage or two-stage revision surgery for prosthetic hip joint infection – the INFORM trial: a study protocol for a randomised controlled trial. Trials 2016;17:90. https://doi.org/10.1186/s13063-016-1213-8
Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW. The alpha-defensin immunoassay and leucocyte esterase colormetric strip test as diagnostic tests in periprosthetic infection: a systematic review and meta-analysis. J Bone Joint Surg Am 2016;98:992–1000. https://doi.org/10.2106/JBJS.15.01142
Kunutsor SK, Beswick AD, Peters TJ, Gooberman-Hill R, Whitehouse MR, Blom AW, Moore AJ. Health care needs and support for patients undergoing treatment for prosthetic joint infection following hip or knee arthroplasty: a systematic review. PLOS ONE 2017;12:e0169068. https://doi.org/10.1371/journal.pone.0169068
Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD. Systematic review of risk prediction scores for surgical site infection or periprosthetic joint infection following joint arthroplasty. Epidemiol Infect 2017;145:1738–49. https://doi.org/10.1017/S0950268817000486
Lenguerrand E, Whitehouse MR, Beswick AD, Jones SA, Porter ML, Blom AW. Revision for prosthetic joint infection following hip arthroplasty: evidence from the National Joint Registry. Bone Joint Res 2017;6:391–8. https://doi.org/10.1302/2046-3758.66.BJR-2017-0003.R1
Lenguerrand E, Whitehouse MR, Beswick AD, Toms AD, Porter ML, Blom AW, National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open 2017;7:e014056. https://doi.org/10.1136/bmjopen-2016-014056
Moore AJ, Blom AW, Whitehouse MR, Gooberman-Hill R. Managing uncertainty – a qualitative study of surgeons’ decision-making for one-stage and two-stage revision surgery for prosthetic hip joint infection. BMC Musculoskelet Disord 2017;18:154. https://doi.org/10.1186/s12891-017-1499-z
Moore AJ, Whitehouse MR, Gooberman-Hill R, Heddington J, Beswick AD, Blom AW, Peters TJ. A UK national survey of care pathways and support offered to patients receiving revision surgery for prosthetic joint infection in the highest volume NHS orthopaedic centres. Musculoskeletal Care 2017;15:379–85. https://doi.org/10.1002/msc.1186
Kunutsor SK, Beswick AD, Whitehouse MR, Wylde V, Blom AW. Debridement, antibiotics and implant retention for periprosthetic joint infections: a systematic review and meta-analysis of treatment outcomes. J Infect 2018;77:479–88. https://doi.org/10.1016/j.jinf.2018.08.017
Kunutsor SK, Whitehouse MR, Blom AW, Board T, Kay P, Wroblewski BM, et al. One- and two-stage surgical revision of peri-prosthetic joint infection of the hip: a pooled individual participant data analysis of 44 cohort studies. Eur J Epidemiol 2018;33:933–46. https://doi.org/10.1007/s10654-018-0377-9
Lenguerrand E, Whitehouse MR, Beswick AD, Kunutsor SK, Burston B, Porter M, Blom AW. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis 2018;18:1004–14. https://doi.org/10.1016/S1473-3099(18)30345-1
Mallon C, Gooberman-Hill R, Blom A, Whitehouse M, Moore A. Surgeons are deeply affected when patients are diagnosed with prosthetic joint infection. PLOS ONE 2018;13:e0207260. https://doi.org/10.1371/journal.pone.0207260
Mallon CM, Gooberman-Hill R, Moore AJ. Infection after knee replacement: a qualitative study of impact of periprosthetic knee infection. BMC Musculoskelet Disord 2018;19:352. https://doi.org/10.1186/s12891-018-2264-7
Kunutsor SK, Beswick AD, Whitehouse MR, Blom AW. One- and two-stage surgical revision of infected elbow prostheses following total joint replacement: a systematic review. BMC Musculoskelet Disord 2019;20:467. https://doi.org/10.1186/s12891-019-2848-x
Kunutsor SK, Beswick AD, Whitehouse MR, Blom AW, Lenguerrand E. Implant fixation and risk of prosthetic joint infection following primary total hip replacement: meta-analysis of observational cohort and randomised intervention studies. J Clin Med 2019;8:722. https://doi.org/10.3390/jcm8050722
Kunutsor SK, Wylde V, Whitehouse MR, Beswick AD, Lenguerrand E, Blom AW. Influence of fixation methods on prosthetic joint infection following primary total knee replacement: meta-analysis of observational cohort and randomised intervention studies. J Clin Med 2019;8:828. https://doi.org/10.3390/jcm8060828
Lenguerrand E, Whitehouse MR, Beswick AD, Kunutsor SK, Foguet P, Porter M, et al. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis 2019;19:589–600. https://doi.org/10.1016/S1473-3099(18)30755-2
Carroll FE, Gooberman-Hill R, Strange S, Blom AW, Moore AJ. What are patients’ preferences for revision surgery after periprosthetic joint infection? A discrete choice experiment. BMJ Open 2020;10:e031645. https://doi.org/10.1136/bmjopen-2019-031645
Garfield K, Noble S, Lenguerrand E, Whitehouse MR, Sayers A, Reed MR, Blom AW. What are the inpatient and day case costs following primary total hip replacement of patients treated for prosthetic joint infection: a matched cohort study using linked data from the National Joint Registry and Hospital Episode Statistics. BMC Med 2020;18:335. https://doi.org/10.1186/s12916-020-01803-7
Kunutsor SK, Barrett MC, Whitehouse MR, Blom AW. Clinical effectiveness of treatment strategies for prosthetic joint infection following total ankle replacement: a systematic review and meta-analysis. J Foot Ankle Surg 2020;59:367–72. https://doi.org/10.1053/j.jfas.2019.04.016
Kunutsor SK, Barrett MC, Whitehouse MR, Craig RS, Lenguerrand E, Beswick AD, Blom AW. Incidence, temporal trends and potential risk factors for prosthetic joint infection after primary total shoulder and elbow replacement: systematic review and meta-analysis. J Infect 2020;80:426–36. https://doi.org/10.1016/j.jinf.2020.01.008
Palmer CK, Gooberman-Hill R, Blom AW, Whitehouse MR, Moore AJ. Post-surgery and recovery experiences following one- and two-stage revision for prosthetic joint infection – a qualitative study of patients’ experiences. PLOS ONE 2020;15:e0237047. https://doi.org/10.1371/journal.pone.0237047
Blom AW, Lenguerrand E, Strange S, Noble SM, Beswick AD, Burston A, et al. Clinical and cost effectiveness of single stage compared with two stage revision for hip prosthetic joint infection (INFORM): pragmatic, parallel group, open label, randomised controlled trial. BMJ 2022;379:e071281.
INFORM conference presentations
Beswick A, Strange S, Whitehouse M, Blom A. Prosthetic Joint Infection: The INFORM Programme. EORS; Bristol, UK, September 2015.
Gooberman-Hill R, Moore A. Impact and Preference for Treatment of Infection after Joint Replacement. EORS; Bristol, UK, September 2015.
Kunutsor S, Whitehouse M, Blom A, Beswick A. One- or Two-stage Surgical Revision of Infected Hip Prosthesis: A Meta-analysis. EORS; Bristol, UK, September 2015.
Moore A, Blom A, Whitehouse M, Gooberman-Hill R. Revision Surgery for Prosthetic Hip Joint Infection: Patients’ and Surgeons’ Views on Treatment and Research. EFORT; Prague, Czechia, May 2015.
Moore A, Gooberman-Hill R. Complications after Surgery for Painful Hip Osteoarthritis: A Qualitative Study with Patients and Surgeons. BPS; Glasgow, UK, April 2015.
Moore A, Gooberman-Hill R. Deep Prosthetic Joint Infection: Impact on Patient and Preferences for Treatment. BSR; Manchester, UK, May 2015.
Beswick A. Infection after Total Hip Replacement. The INFORM Programme. Zero Tolerance of Surgical Site Infection; Northumberland, UK, October 2016.
Beswick A, Strange S, Lenguerrand E, Moore A, Kunutsor S, Gooberman-Hill R, et al. One- or Two-stage Revision of Infected Hip Replacements: Is a Randomised Controlled Trial Possible? COMOC; Cape Town, South Africa, April 2016.
Lenguerrand E. Increasing Burden of Infection and Risk of Early Revision following Hip and Knee Replacement. Evidence from the National Joint Registry for England and Wales. EORS; Bologna, Italy, September 2016.
Lenguerrand E, Whitehouse M, Beswick A, Jones S, Porter M, Toms A, et al. Increasing Burden of Infection and Risk of Early Revision for Prosthetic Joint Replacement (PJI) following Primary and Revision Hip Replacements (HR) and Knee Replacements (KR). An Analysis of the National Joint Registry for England and Wales. International Society of Arthroplasty Registries (ISAR); Manchester, UK, April 2016.
Moore A, Whitehouse M, Blom A, Gooberman-Hill R. Managing Uncertainty – Surgeons’ Decision-making for One-stage and Two-stage Revision Arthroplasty for Prosthetic Joint Infection. EORS; Bologna, Italy, September 2016. https://doi.org/10.1186/s12891-017-1499-z
Whitehouse M, Carroll F, Moore A, Gooberman-Hill R. Development of a New ‘Paired Choice’ Questionnaire using Methods from Health Economics: Applying an Established Method to Deep Prosthetic Joint Infection after Hip Replacement. BOA; Belfast, UK, September 2016.
Lenguerrand E, Whitehouse M, Beswick A, Kunutsor S, Burston B, Porter M, et al. Patient, Perioperative and Healthcare System Risk Factors for Prosthetic Joint Infection following Primary Hip Replacement: Evidence from England and Wales. EORS; Munich, Germany, September 2017.
Mallon C, Gooberman-Hill R, Moore A. Understanding the Experience of Periprosthetic Knee Infection on Patients’ Sense of Identity. BSA; York, UK, September 2017.
Beswick A, Strange S, Mallon C, Lenguerrand E, Moore A, Kunutsor S, et al. One- or Two-stage Revision of Infected Knee Replacements: Is a Randomised Controlled Trial Feasible? EORS; Galway, Ireland, September 2018.
Beswick A, Whitehouse M, Lenguerrand E, Strange S, Blom A, Kunutsor S. Rates of Re-infection after One- and Two-stage Surgical Revision of Hip Prosthetic Joint Infection. A Systematic Review with Individual Patient Data Analysis. EORS; Galway, Ireland, September 2018.
Kunutsor S, Beswick A, Whitehouse M, Blom A, Lenguerrand E. Hip Replacement and Type of Fixation Method: Meta-analysis of Observational Cohort and Randomised Intervention Studies. EORS; Maastricht, the Netherlands, October 2019.
Kunutsor SK, Wylde V, Whitehouse M, Beswick A, Lenguerrand E, Blom A. Prosthetic Joint Infection following Primary Total Knee Replacement and Type of Fixation Method: Meta-analysis of Observational Cohort and Randomised Intervention Studies. EORS; Maastricht, the Netherlands, October 2019. https://doi.org/10.3390/jcm8060828
Lenguerrand E. Mortality and Re-revision following Single-stage and Two-stage Revision Surgery for the Management of Infected Primary Hip Replacement: A Study from the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. EORS; Maastricht, the Netherlands, October 2019.
Lenguerrand E. Mortality and Re-revision following Single-stage and Two-stage Revision Surgery for the Management of Infected Primary Knee Replacement: A Study from the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. EORS; Maastricht, the Netherlands, October 2019.
Moore A, Palmer C, Mallon C, Gooberman-Hill R, Whitehouse M, Blom A. Patient Experiences of Recovery from One- and Two-stage Revision Surgery for Prosthetic Hip Infection. EORS; Maastricht, the Netherlands, October 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Following review, access to available anonymised data may be granted.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Blom AW, Lenguerrand E, Strange S, Noble SM, Beswick AD, Burston A, et al. Clinical and cost effectiveness of single stage compared with two stage revision for hip prosthetic joint infection (INFORM): pragmatic, parallel group, open label, randomised controlled trial. BMJ 2022;379. https://doi.org/10.1136/bmj-2022-071281.
- Swain S, Sarmanova A, Mallen C, Kuo CF, Coupland C, Doherty M, et al. Trends in incidence and prevalence of osteoarthritis in the United Kingdom: findings from the Clinical Practice Research Datalink (CPRD). Osteoarthritis Cartilage 2020;28:792-801. https://doi.org/10.1016/j.joca.2020.03.004.
- Barbour KE, Moss S, Croft JB, Helmick CG, Theis KA, Brady TJ, et al. Geographic variations in arthritis prevalence, health-related characteristics, and management – United States, 2015. MMWR Surveill Summ 2018;67:1-28. https://doi.org/10.15585/mmwr.ss6704a1.
- Culliford DJ, Maskell J, Kiran A, Judge A, Javaid MK, Cooper C, et al. The lifetime risk of total hip and knee arthroplasty: results from the UK general practice research database. Osteoarthritis Cartilage 2012;20:519-24. https://doi.org/10.1016/j.joca.2012.02.636.
- Ackerman IN, Bohensky MA, de Steiger R, Brand CA, Eskelinen A, Fenstad AM, et al. Lifetime risk of primary total hip replacement surgery for osteoarthritis from 2003 to 2013: a multinational analysis using national registry data. Arthritis Care Res 2017;69:1659-67. https://doi.org/10.1002/acr.23197.
- Ackerman IN, Bohensky MA, de Steiger R, Brand CA, Eskelinen A, Fenstad AM, et al. Substantial rise in the lifetime risk of primary total knee replacement surgery for osteoarthritis from 2003 to 2013: an international, population-level analysis. Osteoarthritis Cartilage 2017;25:455-61. https://doi.org/10.1016/j.joca.2016.11.005.
- Burn E, Murray DW, Hawker GA, Pinedo-Villanueva R, Prieto-Alhambra D. Lifetime risk of knee and hip replacement following a GP diagnosis of osteoarthritis: a real-world cohort study. Osteoarthritis Cartilage 2019;27:1627-35. https://doi.org/10.1016/j.joca.2019.06.004.
- National Joint Registry (NJR) . 17th Annual Report 2020. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2017th%20Annual%20Report%202020.pdf (accessed 5 March 2021).
- Scottish Arthroplasty Project . Annual Report 2020 2020. https://readymag.com/PHIDigital/SAP-Annual-Report-2020/ (accessed 4 March 2021).
- Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020-2040 using the national inpatient sample. J Rheumatol 2019;46:1134-40. https://doi.org/10.3899/jrheum.170990.
- Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br 2004;86:688-91. https://doi.org/10.1302/0301-620x.86b5.14887.
- Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total hip arthroplasty: the Avon experience. J Bone Joint Surg Br 2003;85:956-9. https://doi.org/10.1302/0301-620x.85b7.14095.
- Blom AW, Rogers M, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Dislocation following total hip replacement: the Avon Orthopaedic Centre experience. Ann R Coll Surg Engl 2008;90:658-62. https://doi.org/10.1308/003588408X318156.
- Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am 1999;30:183-90. https://doi.org/10.1016/S0030-5898(05)70073-0.
- Lapidus LJ, Ponzer S, Pettersson H, de Bri E. Symptomatic venous thromboembolism and mortality in orthopaedic surgery – an observational study of 45 968 consecutive procedures. BMC Musculoskelet Disord 2013;14. https://doi.org/10.1186/1471-2474-14-177.
- Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop 2011;35:173-84. https://doi.org/10.1007/s00264-010-1075-8.
- Hunter G, Dandy D. The natural history of the patient with an infected total hip replacement. J Bone Joint Surg Br 1977;59:293-7. https://doi.org/10.1302/0301-620X.59B3.893507.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004;351:1645-54. https://doi.org/10.1056/NEJMra040181.
- Matthews PC, Berendt AR, McNally MA, Byren I. Diagnosis and management of prosthetic joint infection. BMJ 2009;338. https://doi.org/10.1136/bmj.b1773.
- Arnold WV, Shirtliff ME, Stoodley P. Bacterial biofilms and periprosthetic infections. J Bone Joint Surg Am 2013;95:2223-9.
- Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. Acta Orthop 2015;86:147-58. https://doi.org/10.3109/17453674.2014.966290.
- Andersson AE, Bergh I, Karlsson J, Nilsson K. Patients’ experiences of acquiring a deep surgical site infection: an interview study. Am J Infect Control 2010;38:711-17. https://doi.org/10.1016/j.ajic.2010.03.017.
- Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg 2008;16:58-65. https://doi.org/10.1177/230949900801600115.
- Wylde V, Beswick AD, Dennis J, Gooberman-Hill R. Post-operative patient-related risk factors for chronic pain after total knee replacement: a systematic review. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-018105.
- Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty 2009;24:105-9. https://doi.org/10.1016/j.arth.2009.04.027.
- Choi HJ, Adiyani L, Sung J, Choi JY, Kim HB, Kim YK, et al. Five-year decreased incidence of surgical site infections following gastrectomy and prosthetic joint replacement surgery through active surveillance by the Korean Nosocomial Infection Surveillance System. J Hosp Infect 2016;93:339-46. https://doi.org/10.1016/j.jhin.2015.12.021.
- Huotari K, Peltola M, Jämsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop 2015;86:321-5. https://doi.org/10.3109/17453674.2015.1035173.
- Lindgren V, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Deep infection after total hip replacement: a method for national incidence surveillance. Infect Control Hosp Epidemiol 2014;35:1491-6. https://doi.org/10.1086/678600.
- Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br 2006;88:943-8. https://doi.org/10.1302/0301-620X.88B7.17150.
- Gundtoft PH, Pedersen AB, Schønheyder HC, Møller JK, Overgaard S. One-year incidence of prosthetic joint infection in total hip arthroplasty: a cohort study with linkage of the Danish Hip Arthroplasty Register and Danish Microbiology Databases. Osteoarthritis Cartilage 2017;25:685-93. https://doi.org/10.1016/j.joca.2016.12.010.
- Dale H, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009;80:639-45. https://doi.org/10.3109/17453670903506658.
- Lenguerrand E, Whitehouse MR, Beswick AD, Jones SA, Porter ML, Blom AW. Revision for prosthetic joint infection following hip arthroplasty: evidence from the National Joint Registry. Bone Joint Res 2017;6:391-8. https://doi.org/10.1302/2046-3758.66.BJR-2017-0003.R1.
- Jämsen E, Varonen M, Huhtala H, Lehto MU, Lumio J, Konttinen YT, et al. Incidence of prosthetic joint infections after primary knee arthroplasty. J Arthroplasty 2010;25:87-92. https://doi.org/10.1016/j.arth.2008.10.013.
- Hijas-Gómez AI, Lucas WC, Checa-García A, Martínez-Martín J, Fahandezh-Saddi H, Gil-de-Miguel Á, et al. Surgical site infection incidence and risk factors in knee arthroplasty: a 9-year prospective cohort study at a university teaching hospital in Spain. Am J Infect Control 2018;46:1335-40. https://doi.org/10.1016/j.ajic.2018.06.010.
- Baier C, Adelmund S, Schwab F, Lassahn C, Chaberny IF, Gossé F, et al. Incidence and risk factors of surgical site infection after total knee arthroplasty: results of a retrospective cohort study. Am J Infect Control 2019;47:1270-2. https://doi.org/10.1016/j.ajic.2019.04.010.
- Lenguerrand E, Whitehouse MR, Beswick AD, Toms AD, Porter ML, Blom AW. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man . Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014056.
- Wang FD, Wang YP, Chen CF, Chen HP. The incidence rate, trend and microbiological aetiology of prosthetic joint infection after total knee arthroplasty: a 13 years’ experience from a tertiary medical center in Taiwan. J Microbiol Immunol Infect 2018;51:717-22. https://doi.org/10.1016/j.jmii.2018.08.011.
- Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. Instr Course Lect 2006;55:449-61.
- Giulieri SG, Graber P, Ochsner PE, Zimmerli W. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 2004;32:222-8. https://doi.org/10.1007/s15010-004-4020-1.
- Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, et al. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 2009;63:1264-71. https://doi.org/10.1093/jac/dkp107.
- Romanò C, Logoluso N, Drago L, Peccati A, Romanò D. Role for irrigation and debridement in periprosthetic infections. J Knee Surg 2014;27:267-72. https://doi.org/10.1055/s-0034-1373736.
- Yang FS, Lu YD, Wu CT, Blevins K, Lee MS, Kuo FC. Mechanical failure of articulating polymethylmethacrylate (PMMA) spacers in two-stage revision hip arthroplasty: the risk factors and the impact on interim function. BMC Musculoskelet Disord 2019;20. https://doi.org/10.1186/s12891-019-2759-x.
- Jung J, Schmid NV, Kelm J, Schmitt E, Anagnostakos K. Complications after spacer implantation in the treatment of hip joint infections. Int J Med Sci 2009;6:265-73. https://doi.org/10.7150/ijms.6.265.
- Gehrke T, Zahar A, Kendoff D. One-stage exchange: it all began here. Bone Joint J 2013;95–B:77-83. https://doi.org/10.1302/0301-620X.95B11.32646.
- Jenny JY, Lengert R, Diesinger Y, Gaudias J, Boeri C, Kempf JF. Routine one-stage exchange for chronic infection after total hip replacement. Int Orthop 2014;38:2477-81. https://doi.org/10.1007/s00264-014-2466-z.
- Tsung JD, Rohrsheim JA, Whitehouse SL, Wilson MJ, Howell JR. Management of periprosthetic joint infection after total hip arthroplasty using a custom made articulating spacer (CUMARS); the Exeter experience. J Arthroplasty 2014;29:1813-18. https://doi.org/10.1016/j.arth.2014.04.013.
- Beswick AD, Elvers KT, Smith AJ, Gooberman-Hill R, Lovering A, Blom AW. What is the evidence base to guide surgical treatment of infected hip prostheses? Systematic review of longitudinal studies in unselected patients. BMC Med 2012;10. https://doi.org/10.1186/1741-7015-10-18.
- Blom AW, Artz N, Beswick AD, Burston A, Dieppe P, Elvers KT, et al. Improving patients’ experience and outcome of total joint replacement: the RESTORE programme. Programme Grants Appl Res 2016;4. https://doi.org/10.3310/pgfar04120.
- INVOLVE . How to Involve People in Research 2015. www.invo.org.uk/find-out-more/how-to-involve-people/ (accessed 19 April 2021).
- Lenguerrand E, Whitehouse MR, Beswick AD, Kunutsor SK, Burston B, Porter M, et al. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis 2018;18:1004-14. https://doi.org/10.1016/S1473-3099(18)30345-1.
- Lenguerrand E, Whitehouse MR, Beswick AD, Kunutsor SK, Foguet P, Porter M, et al. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man . Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis 2019;19:589-600. https://doi.org/10.1016/S1473-3099(18)30755-2.
- Garfield K, Noble S, Lenguerrand E, Whitehouse MR, Sayers A, Reed MR, et al. What are the inpatient and day case costs following primary total hip replacement of patients treated for prosthetic joint infection: a matched cohort study using linked data from the National Joint Registry and Hospital Episode Statistics. BMC Med 2020;18. https://doi.org/10.1186/s12916-020-01803-7.
- Abouljoud MM, Backstein D, Battenberg A, Dietz M, Erice A, Freiberg AA, et al. Hip and knee section, treatment, surgical technique: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019;34:S445-51. https://doi.org/10.1016/j.arth.2018.09.029.
- Bone and Joint Infection Registry . BAJIR Bone and Joint Infection Registry 2020. https://bajir.org/ (accessed 16 February 2021).
- Vanhegan IS, Malik AK, Jayakumar P, Ul Islam S, Haddad FS. A financial analysis of revision hip arthroplasty: the economic burden in relation to the national tariff. J Bone Joint Surg Br 2012;94:619-23. https://doi.org/10.1302/0301-620X.94B5.27073.
- Graves N, Wloch C, Wilson J, Barnett A, Sutton A, Cooper N, et al. A cost-effectiveness modelling study of strategies to reduce risk of infection following primary hip replacement based on a systematic review. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20540.
- Moore AJ, Blom AW, Whitehouse MR, Gooberman-Hill R. Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009495.
- Mallon CM, Gooberman-Hill R, Moore AJ. Infection after knee replacement: a qualitative study of impact of periprosthetic knee infection. BMC Musculoskelet Disord 2018;19. https://doi.org/10.1186/s12891-018-2264-7.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Moore AJ, Blom AW, Whitehouse MR, Gooberman-Hill R. Managing uncertainty – a qualitative study of surgeons’ decision-making for one-stage and two-stage revision surgery for prosthetic hip joint infection. BMC Musculoskelet Disord 2017;18. https://doi.org/10.1186/s12891-017-1499-z.
- Mallon C, Gooberman-Hill R, Blom A, Whitehouse M, Moore A. Surgeons are deeply affected when patients are diagnosed with prosthetic joint infection. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0207260.
- Ruggieri P. Confessions of a Surgeon: The Good, The Bad and The Complicated. Life Behind the O.R. Doors. New York, NY: Penguin Group; 2012.
- Orri M, Farges O, Clavien PA, Barkun J, Revah-Lévy A. Being a surgeon – the myth and the reality: a meta-synthesis of surgeons’ perspectives about factors affecting their practice and well-being. Ann Surg 2014;260:721-8. https://doi.org/10.1097/SLA.0000000000000962.
- Scott SD, Hirschinger LE, Cox KR, McCoig M, Brandt J, Hall LW. The natural history of recovery for the healthcare provider ‘second victim’ after adverse patient events. Qual Saf Health Care 2009;18:325-30. https://doi.org/10.1136/qshc.2009.032870.
- Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD. INFORM Team . Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0150866.
- Kunutsor SK, Beswick AD, Whitehouse MR, Blom AW, Lenguerrand E. Implant fixation and risk of prosthetic joint infection following primary total hip replacement: meta-analysis of observational cohort and randomised intervention studies. J Clin Med 2019;8. https://doi.org/10.3390/jcm8050722.
- Kunutsor SK, Wylde V, Whitehouse MR, Beswick AD, Lenguerrand E, Blom AW. Influence of fixation methods on prosthetic joint infection following primary total knee replacement: meta-analysis of observational cohort and randomised intervention studies. J Clin Med 2019;8. https://doi.org/10.3390/jcm8060828.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30. https://doi.org/10.1371/journal.pmed.1000097.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. https://doi.org/10.1001/jama.283.15.2008.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses 2011. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 19 April 2021).
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Goldstein H, Browne W, Rasbash J. Multilevel modelling of medical data. Stat Med 2002;21:3291-315. https://doi.org/10.1002/sim.1264.
- Blossfeld H, Golsch K, Rohwer G, Blossfeld H, Golsch K, Rohwer G. Event History Analysis with Stata. Hove: Psychology Press; 2009.
- Sera F, Ferrari P. A multilevel model to estimate the within- and the between-center components of the exposure/disease association in the EPIC study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0117815.
- Hannske M, Tscherne H. Results of prophylactic use of Refobacin-Palacos in implantation of endoprostheses of the hip joint in Hannover. Aktuelle Probl Chir Orthop 1979;12:201-5.
- Hinarejos P, Guirro P, Leal J, Montserrat F, Pelfort X, Sorli ML, et al. The use of erythromycin and colistin-loaded cement in total knee arthroplasty does not reduce the incidence of infection: a prospective randomized study in 3000 knees. J Bone Joint Surg Am 2013;95:769-74. https://doi.org/10.2106/JBJS.L.00901.
- Hsieh PH, Lee MS, Hsu KY, Chang YH, Shih HN, Ueng SW. Gram-negative prosthetic joint infections: risk factors and outcome of treatment. Clin Infect Dis 2009;49:1036-43. https://doi.org/10.1086/605593.
- Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW. The alpha-defensin immunoassay and leucocyte esterase colormetric strip test as diagnostic tests in periprosthetic infection: a systematic review and meta-analysis. J Bone Joint Surg Am 2016;98:992-1000. https://doi.org/10.2106/JBJS.15.01142.
- Buntinx F, Aertgeerts B, Macaskill P, Knottnerus JA, Buntinx F. The Evidence Base of Clinical Diagnosis: Theory and Methods of Diagnostic Research. London: Wiley-Blackwell; 2009.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Carli AV, Abdelbary H, Ahmadzai N, Cheng W, Shea B, Hutton B, et al. Diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: a systematic review. J Bone Joint Surg Am 2019;101:635-49. https://doi.org/10.2106/JBJS.18.00632.
- Health Technology Wales . Synovasure® Alpha Defensin Test for Diagnosing Periprosthetic Joint Infection 2019. www.healthtechnology.wales/wp-content/uploads/2019/03/EAR008-Synovasure.pdf (accessed 19 April 2021).
- Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 2018;33:1309-14.e2. https://doi.org/10.1016/j.arth.2018.02.078.
- Kunutsor SK, Beswick AD, Whitehouse MR, Wylde V, Blom AW. Debridement, antibiotics and implant retention for periprosthetic joint infections: a systematic review and meta-analysis of treatment outcomes. J Infect 2018;77:479-88. https://doi.org/10.1016/j.jinf.2018.08.017.
- Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD. INFORM Team . Re-infection outcomes following one- and two-stage surgical revision of infected hip prosthesis: a systematic review and meta-analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0139166.
- Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD. INFORM Team . Re-Infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0151537.
- Kunutsor SK, Whitehouse MR, Blom AW, Board T, Kay P, Wroblewski BM, et al. One- and two-stage surgical revision of peri-prosthetic joint infection of the hip: a pooled individual participant data analysis of 44 cohort studies. Eur J Epidemiol 2018;33:933-46. https://doi.org/10.1007/s10654-018-0377-9.
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003;73:712-16. https://doi.org/10.1046/j.1445-2197.2003.02748.x.
- Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist 1950;21:607-11. https://doi.org/10.1214/aoms/1177729756.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. https://doi.org/10.1136/bmj.315.7109.629.
- Cochrane Collaboration Individual Patient Data Meta-analysis Methods Group . FAQs (Frequently Asked Questions) on IPD Meta-Analysis 2007. https://methods.cochrane.org/ipdma/frequently-asked-questions (accessed 4 March 2021).
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340. https://doi.org/10.1136/bmj.c221.
- Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. PRISMA-IPD Development Group . Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA 2015;313:1657-65. https://doi.org/10.1001/jama.2015.3656.
- Kunutsor SK, Whitehouse MR, Webb J, Toms A, Stockley I, Taylor A, et al. Re-infection outcomes following one- and two-stage surgical revision of infected hip prosthesis in unselected patients: protocol for a systematic review and an individual participant data meta-analysis. Syst Rev 2015;4. https://doi.org/10.1186/s13643-015-0044-0.
- Hougaard P. Frailty models for survival data. Lifetime Data Anal 1995;1:255-73. https://doi.org/10.1007/BF00985760.
- Strange S, Whitehouse MR, Beswick AD, Board T, Burston A, Burston B, et al. One-stage or two-stage revision surgery for prosthetic hip joint infection – the INFORM trial: a study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1213-8.
- Wylde V, Lenguerrand E, Gooberman-Hill R, Beswick AD, Marques E, Noble S, et al. Effect of local anaesthetic infiltration on chronic postsurgical pain after total hip and knee replacement: the APEX randomised controlled trials. Pain 2015;156:1161-70. https://doi.org/10.1097/j.pain.0000000000000114.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Bellamy N, Hochberg M, Tubach F, Martin-Mola E, Awada H, Bombardier C, et al. Development of multinational definitions of minimal clinically important improvement and patient acceptable symptomatic state in osteoarthritis. Arthritis Care Res 2015;67:972-80. https://doi.org/10.1002/acr.22538.
- NHS Improvement . NHS Reference Costs 2018 to 2019 2020. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication (accessed 19 November 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Office for National Statistics . Annual Survey of Hours and Earnings (ASHE) 2019. www.ons.gov.uk/surveys/informationforbusinesses/businesssurveys/annualsurveyofhoursandearningsashe (accessed 2 March 2021).
- EuroQol Research Foundation . EQ-5D-5L Valuation Crosswalk Index Value Calculator 2020. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/ (accessed 5 March 2021).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Greene WH. Econometric Analysis. Hoboken: NJ: Prentice Hall; 2002.
- Palmer CK, Gooberman-Hill R, Blom AW, Whitehouse MR, Moore AJ. Post-surgery and recovery experiences following one- and two-stage revision for prosthetic joint infection – a qualitative study of patients’ experiences. PLOS ONE 2020;15. https://doi.org/10.1371/journal.pone.0237047.
- Carroll FE, Gooberman-Hill R, Strange S, Blom AW, Moore AJ. What are patients’ preferences for revision surgery after periprosthetic joint infection? A discrete choice experiment. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-031645.
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. PharmacoEconomics 2008;26:661-77. https://doi.org/10.2165/00019053-200826080-00004.
- Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ 2012;21:730-41. https://doi.org/10.1002/hec.1739.
- Kuhfeld WF, Grover R, Vriens M. The Handbook of Marketing Research. Thousand Oaks, CA: SAGE Publications, Inc.; 2006.
- Bech M, Gyrd-Hansen D. Effects coding in discrete choice experiments. Health Econ 2005;14:1079-83. https://doi.org/10.1002/hec.984.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5. https://doi.org/10.1017/S0266462305050324.
- Alp E, Cevahir F, Ersoy S, Guney A. Incidence and economic burden of prosthetic joint infections in a university hospital: a report from a middle-income country. J Infect Public Health 2016;9:494-8. https://doi.org/10.1016/j.jiph.2015.12.014.
- Assmann G, Kasch R, Maher CG, Hofer A, Barz T, Merk H, et al. Comparison of health care costs between aseptic and two stage septic hip revision. J Arthroplasty 2014;29:1925-31. https://doi.org/10.1016/j.arth.2014.04.043.
- Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am 2005;87:1746-51. https://doi.org/10.2106/00004623-200508000-00012.
- Gow N, McGuinness C, Morris AJ, McLellan A, Morris JT, Roberts SA. Excess cost associated with primary hip and knee joint arthroplasty surgical site infections: a driver to support investment in quality improvement strategies to reduce infection rates. N Z Med J 2016;129:51-8.
- Haenle M, Skripitz C, Mittelmeier W, Skripitz R. Economic impact of infected total hip arthroplasty in the German diagnosis-related groups system. Orthopade 2012;41:467-76. https://doi.org/10.1007/s00132-012-1939-2.
- Haenle M, Skripitz C, Mittelmeier W, Skripitz R. Economic impact of infected total knee arthroplasty. ScientificWorldJournal 2012;2012. https://doi.org/10.1100/2012/196515.
- Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service?. Bone Joint J 2015;97–B:197-201. https://doi.org/10.1302/0301-620X.97B2.33707.
- Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty 2014;29:929-32. https://doi.org/10.1016/j.arth.2013.09.017.
- Kapadia BH, Banerjee S, Cherian JJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections after total hip arthroplasty at a specialized tertiary-care center. J Arthroplasty 2016;31:1422-6. https://doi.org/10.1016/j.arth.2016.01.021.
- Klouche S, Sariali E, Mamoudy P. Total hip arthroplasty revision due to infection: a cost analysis approach. Orthop Traumatol Surg Res 2010;96:124-32. https://doi.org/10.1016/j.rcot.2010.02.005.
- Merollini KM, Crawford RW, Graves N. Surgical treatment approaches and reimbursement costs of surgical site infections post hip arthroplasty in Australia: a retrospective analysis. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-91.
- Oduwole KO, Molony DC, Walls RJ, Bashir SP, Mulhall KJ. Increasing financial burden of revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2010;18:945-8. https://doi.org/10.1007/s00167-010-1074-8.
- Peel TN, Cheng AC, Lorenzo YP, Kong DC, Buising KL, Choong PF. Factors influencing the cost of prosthetic joint infection treatment. J Hosp Infect 2013;85:213-19. https://doi.org/10.1016/j.jhin.2013.07.012.
- Rennert-May ED, Conly J, Smith S, Puloski S, Henderson E, Au F, et al. The cost of managing complex surgical site infections following primary hip and knee arthroplasty: a population-based cohort study in Alberta, Canada. Infect Control Hosp Epidemiol 2018;39:1183-8. https://doi.org/10.1017/ice.2018.199.
- Romano CL, Romano D, Logoluso N, Meani E. Septic versus aseptic hip revision: how different?. J Orthop Traumatol 2010;11:167-74. https://doi.org/10.1007/s10195-010-0106-y.
- Peel TN, Dowsey MM, Buising KL, Liew D, Choong PF. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin Microbiol Infect 2013;19:181-6. https://doi.org/10.1111/j.1469-0691.2011.03758.x.
- Kalore NV, Maheshwari A, Sharma A, Cheng E, Gioe TJ. Is there a preferred articulating spacer technique for infected knee arthroplasty? A preliminary study. Clin Orthop Relat Res 2012;470:228-35. https://doi.org/10.1007/s11999-011-2037-1.
- Nodzo SR, Boyle KK, Spiro S, Nocon AA, Miller AO, Westrich GH. Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Knee 2017;24:1175-81. https://doi.org/10.1016/j.knee.2017.05.016.
- Jämsen E, Stogiannidis I, Malmivaara A, Pajamäki J, Puolakka T, Konttinen YT. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop 2009;80:67-7. https://doi.org/10.1080/17453670902805064.
- Melo L. REVISITS: Revision Single or Two Stage Surgery (REVISITS) 2018. https://clinicaltrials.gov/ct2/show/NCT03741296 (accessed 3 August 2022).
- Odum S, Cadd C. One Stage Versus Two Stage for Periprosthetic Hip and Knee Infection 2016. https://clinicaltrials.gov/ct2/show/NCT02734134 (accessed 3 August 2022).
- Scharfenberger A, Clark M, Lavoie G, O’Connor G, Masson E, Beaupre LA. Treatment of an infected total hip replacement with the PROSTALAC system. Part 2: health-related quality of life and function with the PROSTALAC implant in situ. Can J Surg 2007;50:29-33.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Turner J, O’Cathain A, Knowles E, Nicholl J, Tosh J, Sampson F, et al. Evaluation of NHS 111 Pilot Sites. Final Report 2012. http://data.parliament.uk/DepositedPapers/Files/DEP2012-1694/HL3309-LibDoc.pdf (accessed 9 September 2022).
- National Institute for Health and Care Excellence (NICE) . British National Formulary 2020. https://bnf.nice.org.uk/ (accessed 3 August 2022).
- NHS . Am I Entitled to Free Prescriptions? 2020. www.nhs.uk/using-the-nhs/help-with-health-costs/get-help-with-prescription-costs/ (accessed 3 August 2022).
- NRS Healthcare. Coalville: NRS Healthcare; 2020.
- Office for National Statistics . Annual Survey of Hours and Earnings (ASHE) 2020. www.ons.gov.uk/surveys/informationforbusinesses/businesssurveys/annualsurveyofhoursandearningsashe (accessed 3 August 2022).
- NHS Employers . NHS Terms and Conditions of Service Handbook 2019. www.nhsemployers.org/employershandbook/tchandbook/afc_tc_of_service_handbook_fb.pdf (accessed 16 August 2022).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Briggs AH. A Bayesian approach to stochastic cost-effectiveness analysis. Health Econ 1999;8:257-61. https://doi.org/10.1002/(SICI)1099-1050(199905)8:3<257::AID-HEC427>3.0.CO;2-E.
- Huber J, Zwerina K. The importance of utility balance in efficient choice designs. J Mark Res 1996;33:307-17. https://doi.org/10.1177/002224379603300305.
Appendix 1 Incidence of prosthetic joint infection after hip or knee replacement
| Authors, year; country; dates of data collection | Population | Outcome and rate of infection (follow-up period) | Methodological quality concerns and notes |
|---|---|---|---|
| Hip replacement | |||
| Infection outcome | |||
|
Ong et al. ,25 2009; USA; 1997–2006 Multicentre cohort study (prospective registration) |
n = 28,544 Elective THR Medicare 5% national sample administrative claims database Aged ≥ 65 years |
PJI (ICD-10 996.66) identified in claims records from inpatient, outpatient, carrier, skilled nursing facility, hospice care, home health, and durable medical equipment analytic data files 0.78% (2 years) 1.11% (10 years) |
None Including non-elective (bone cancer, joint infection, fracture) and elective THR (n = 39,929) the 2-year infection rate was 1.63%, 10-year rate 2.22% |
|
Choi et al. ,26 2016; Republic of Korea; 2008–12 National infection surveillance system |
n = 7656 THR including trauma and reoperation |
Infection monitoring during hospital admission and after discharge by surgeons, surgical nurses, infectious diseases physicians and infection control practitioners 2.09% (1 year) |
None 2.9% in 2008; 3.1% in 2009; 1.7% in 2010; 2.2% in 2011; 1.5% in 2012 |
|
Huotari et al. ,27 2015; Finland; 1998–2009 Joint registries |
n = 50,272 THR for osteoarthritis |
Revision and resection operations identified in joint registries and diagnosis of PJI or wound infection from hospital discharge register 0.71% (2 years) 0.92% (median 5 years; range 1–13 years) |
None |
|
Lindgren et al. ,28 2014; Sweden; 2005–8 Joint registry |
n = 45,531 (49,219 THRs) |
Patients with 4 weeks’ continuous outpatient antibiotic treatment within 2 years of THR in the Swedish Prescribed Drug Register 0.9% (95% CI 0.85% to 1.02%) (2 years) |
None |
|
Phillips et al. ,29 2006; UK; 1987–2001 Single-centre (20 consultants) prospective cohort |
n = 5947 Primary THR |
Positive cultures in microbiology laboratory 0.57% (up to 15 years) |
Single centre |
|
Blom et al. ,12 2003; UK; 1993–6 Single-centre retrospective cohort |
n = 1727 (91% followed up) Primary THR |
Postal questionnaire on infection. Hospital notes of patients who died 1.08% (reviewed 5–8 years after surgery) |
Single centre, retrospective |
| Revision for infection outcome | |||
|
Dale et al. ,31 2009; Norway; 1987–2007 Joint registry |
n = 97,344 Primary THR |
Revisions for deep infection recorded in joint registry 0.54% (5 years) |
None Risk of revision increased from 0.3% to 0.9% between 1987–92 and 2003–7 (adjusted risk ratio 3.0, 95% CI 2.2 to 4.0) |
|
Lenguerrand et al. ,32 2017; UK; 2003–14 Joint registry |
n = 623,253 Primary THR |
Revision surgery for PJI recorded in joint registry 0.15% (1 year) 0.26% (2 years) 0.44% (5 years) 0.62% (10 years) |
None |
|
Gundtoft et al. ,30 2017; Denmark; 2005–14 Region from joint registry |
n = 48,867 Primary THR |
Revision operation with ≥ 3 positive intraoperative cultures 0.55% (1 year) |
None 0.53% (95% CI 0.44 to 0.63) during 2005–9; 0.57% (95% CI 0.49 to 0.67) during 2010–14 |
|
Blom et al. ,12 2003; UK; 1993–6 Single-centre retrospective cohort |
n = 1727 (91% followed up) Primary THR |
Postal questionnaire on infection. Hospital notes of patients who died 0.45% (reviewed 5–8 years after surgery) |
Single centre, retrospective |
| Knee replacement | |||
| Infection outcome | |||
|
Huotari et al. ,27 2015; Finland; 1998–2009 Joint registries |
n = 62,436 TKR for osteoarthritis |
Revision and resection operations identified in joint registries and diagnosis of PJI or wound infection from hospital discharge register 1.14% (2 years) 1.41% (median 5 years; range 1–13 years) |
None |
|
Choi et al. ,26 2016; Republic of Korea; 2008–12 National infection surveillance system |
n = 7648 TKR including trauma and reoperation |
Infection monitoring during hospital admission and after discharge by surgeons, surgical nurses, infectious diseases physicians and infection control practitioners 1.9% (1 year) |
None 2.9% in 2008; 2.9% in 2009; 1.5% in 2010; 1.9% in 2011; 1.2% in 2012 |
|
Jämsen et al. ,33 2010; Finland; 2002–6 Single-centre prospective cohort |
n = 2647 Elective primary TKR |
Infections recorded in hospital infection register 0.80% (1 year) |
Single centre |
|
Phillips et al. ,29 2006; UK; 1987–2001 Single-centre prospective cohort |
n = 4788 Primary TKR |
Positive cultures in microbiology laboratory 0.86% (up to 15 years) |
Single centre |
|
Hijas-Gómez et al. ,34 2018; Spain; 2008–16 Single-centre prospective cohort |
n = 1969 Primary and revision TKR |
Surgical site infection surveillance (CDC criteria) 1.0% (90 days) |
Single centre, also includes revision TKR; 5% of infections superficial |
|
Blom et al. ,11 2004; UK; 1993–6 Single-centre retrospective cohort |
n = 931 (97% of 956 followed up) Primary TKR |
Postal questionnaire on infection. Hospital notes of patients who died 0.97% (mean 6.5 years) |
Single centre, retrospective |
|
Baier et al. ,35 2019; Germany; 2007–10 Single-centre retrospective cohort |
n = 2439 Elective TKR. Patients with existing knee infection who died within 3 days or had incomplete data excluded |
Periprosthetic joint infection (not including superficial incisional infections). Identified during hospital stay, outpatient visits and readmissions 3.08% (1 year) |
Single centre, retrospective Rate decreased from 4.4% in 2007 to 2.5% in 2010 (including superficial incisional infections) |
| Revision for infection outcome | |||
|
Lenguerrand et al. ,36 2017; UK; 2003–14 Joint registry |
n = 679,010 Primary TKR |
Revision surgery for PJI recorded in joint registry 0.32% (2 years) |
None |
|
Wang et al. ,37 2018; Taiwan (Province of China); 2002–14 Single-centre retrospective cohort |
n = 10,768 Primary TKR. Aged ≥ 18 years |
Infection requiring surgical intervention 0.41% (3 months); 0.54% (6 months); 0.79% (1 year); 1.19% (2 years); 1.54% (5 years) |
Single centre; 5-year follow-up included patients with TKR (2002–12) |
Appendix 2 Health economic consequences of prosthetic joint infection treatment: systematic review
In this appendix we provide further details of the systematic review described in The significance of prosthetic joint infection to health care.
Background
Different surgical methods to treat prosthetic joint infection have economic implications for health-care providers and patients. Health services and decision-makers need robust information identifying interventions that provide greatest value for money. Our aim was to identify and critically evaluate the available economic evidence to guide the surgical revision of prosthetic joint infection after hip and knee replacement.
Methods
The systematic review protocol was registered as PROSPERO CRD42017069526.
Eligibility criteria
Eligible studies satisfied the PICOS (population, intervention, comparison, outcomes and study) criteria:
-
patients with hip or knee prosthetic joint infection after primary replacement or aseptic revision
-
intervention relating to revision surgery
-
comparator with no prosthetic joint infection or alternative surgical treatment
-
outcome of cost-effectiveness or comparative costs
-
full economic evaluation or cost comparison study.
Database searches
We searched MEDLINE and EMBASE on Ovid to 10 April 2019 using the search strategy shown in Box 2. Studies included in our other systematic reviews were inspected. No language restrictions were applied, and translations were made as required. Studies reported only as abstracts or unobtainable by interlibrary loans and author contact were excluded.
Cost terms (modified from https://hiru.mcmaster.ca/hiru/HIRU_Hedges_MEDLINE_Strategies.aspx#Economics)
Screening and data extraction
We imported records into EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA). After an initial screen by one reviewer to exclude clearly irrelevant articles, abstracts were screened independently by at least two reviewers and reasons for exclusion were recorded.
Data were extracted into a Microsoft Excel spreadsheet by two reviewers, specifically country, dates, setting, participants, surgical intervention, currency, date of costs, source of cost data and costs included or excluded in the overall cost estimate. We included only studies that had attempted to calculate costings in their cohort.
Data analysis
The results were tabulated and comparative costs were calculated.
Quality assessment
To assess risk of bias in cohorts, we used a checklist to assess selection bias. 24 We considered inclusion of consecutive patients and representativeness, and completeness of both follow-up and cost information. To assess studies that had compared costs of revision strategies, we used the Consensus on Health Economic Criteria checklist (CHEC-list). 114
Results
Review progress is summarised in Figure 17. After detailed evaluation, 19 studies were included in the review. Fifteen studies reported cost comparisons of revision procedures for prosthetic joint infection with primary joint replacement or aseptic revision. One of these and two further studies presented different costs associated with single-stage and two-stage revision procedures. In two studies, costs associated with different spacers were compared. Quality assessment is summarised in Table 9.
FIGURE 17.
Economic evaluations of revision surgery for prosthetic hip or knee infection: systematic review flow diagram. PJI, prosthetic joint infection.
| Authors, year | Inclusion of consecutive patients | Representativeness | Completeness of follow-up | Completeness of cost information | Concerns |
|---|---|---|---|---|---|
| Alp et al.,115 2016 | Unclear | Single hospital | Unclear | Partial | No costs for surgeon, anaesthesiologist, infection specialist, physiotherapists, nurses, caregivers, physical therapy |
| Assmann et al.,116 2014 | Consecutive | Single hospital | None specified | Partial | No fixed hospital costs |
| Bozic and Ries,117 2005 | Consecutive | Single hospital (two surgeons) | None specified | Reasonable | |
| Gow et al.,118 2016 | Consecutive | Single hospital | None specified | Reasonable | |
| Haenle et al.,119 2012 | Consecutive | Single hospital | None specified | Reasonable | |
| Haenle et al.,120 2012 | Consecutive | Single hospital | None specified | Reasonable | |
| Kallala et al.,121 2015 | No (complete data only) | Single hospital | None specified | Reasonable | |
| Kapadia et al.,122 2014 | Consecutive | Single hospital | None specified | Reasonable | |
| Kapadia et al.,123 2016 | Consecutive | Single hospital | None specified | Reasonable | |
| Klouche et al.,124 2010 | Consecutive | Single hospital | None specified | Reasonable | |
| Merollini et al.,125 2013 | Consecutive | Regional database | None specified | Partial | No costs for antibiotics, primary care, travel, pharmaceuticals, indirect costs |
| Oduwole et al.,126 2010 | 95% of patients | Single hospital | None specified | Reasonable | |
| Peel et al.,127 2013 | Excluding patients who died and < 3 months follow-up | 10 hospitals | None specified | Reasonable | |
| Rennert-May et al.,128 2018 | Consecutive | Regional database | None specified | Reasonable | |
| Romano et al.,129 2010 | Unclear | Two hospitals | 3 out of 80 | Reasonable | |
| Vanhegan et al.,55 2012 | Consecutive | Single hospital | None specified | Reasonable |
Cost comparisons of prosthetic joint infection revision procedures with primary joint replacement or aseptic revision
Details of the 15 studies reporting cost comparisons of surgical treatments for infection after hip or knee replacement are summarised in Table 10. 55,115–124,126,128–130 Studies were conducted in Germany (n = 3),116,119,120 the USA (n = 3),117,122,123 and the UK (n = 2),55,121 with one each in Australia,130 Canada,128 France,124 Ireland,126 Italy,129 New Zealand118 and Turkey. 115 Thirteen studies were conducted in a single hospital site,55,115–124,126,130 one was conducted in two hospitals129 and one was conducted in hospitals contributing to a regional database. 128 Seven studies focused on hip infection,55,116,117,119,123,124,129 four focused on knee infection120–122,126 and four focused on both hip and knee infection. 115,118,128,130 In six studies the costs of any surgical treatment were reported,55,118–120,124,128 in seven studies treatment was by a two-stage revision,116,117,121–123,126,129 and in two studies treatment was with DAIR130 or predominantly with DAIR. 115
| Authors and year; country; recruitment dates; setting; risk-of-bias concerns | Indication; number of patients; follow-up; comparator group | Year (cost); currency; source of data | Costs included | Costs that studies state are not included | Costs with description of context |
|---|---|---|---|---|---|
| Alp et al.115 2016; Turkey; 2011–13; single hospital; none |
Hip and knee, deep infection, treatment with DAIR (n = 9), single-stage (n = 2) and two-stage (n = 5) n = 16 Hospital stay, mean 23.5 days (range 7–120 days) (n = 654 primary hip or knee replacement comparator group) |
2013; USD converted from Turkish lira; hospital accounting system | Antibiotics; laboratory tests; radiology; prosthesis; operation; bed stay; drugs, infusions | Surgeon; anaesthesiologist; infection specialist; nurses; physiotherapists; caregivers; physical therapy |
Median total cost of treating infection (predominantly DAIR) including primary joint replacement US$16,999 Median cost of primary hip or knee replacement US$5937 Total hospital costs 286% higher for infected than for uncomplicated hip or knee replacement |
| Assmann et al.116 2014; Germany; 2009–12; single hospital; none |
Hip, deep infection, two-stage revision n = 30 All cases Costs incurred during hospital stays (n = 114 aseptic revision comparator group) |
2013; USD (converted from euros); hospital purchase prices; documented nursing services; patient records; hospital pharmacy; laboratory, radiology and physical therapy services; clinical information system; human resources department; local ICU cost | Nursing staff; laboratory tests; radiology; physiotherapy; medication; surgeon; anaesthesiologist; anaesthesia nursing; implant; materials; sterilisation; transfusions; ICU | Fixed hospital costs |
Mean cost of infection treatment including additional operations to the planned two stages, US$14,380 (range US$7813–29,051) Mean cost of aseptic revision US$5487 (range US$3080–17,345) Cost of management of infection with two-stage revision 262% higher than aseptic revision |
| Bozic and Ries117 2005; USA; 2001–2; single hospital (two surgeons); none |
Hip, deep infection, two-stage revision n = 29 12 months (n = 29 primary hip replacement, n = 27 aseptic loosening comparator groups) |
2001–2; USD; hospital decision support cost-accounting system and outpatient billing system | Hospital costs; outpatient visits | Non-medical costs; patient and societal costs |
Mean cost of infection treatment with two-stage revision including treatment of reinfection US$96,166 (SD US$60,664) Mean costs of primary hip replacement US$21,654 (SD US$4291) and treatment of aseptic loosening US$34,866 (SD US$15,547) Management of infection with two-stage revision 276% greater than treatment of aseptic loosening and 444% greater than primary hip replacement |
| Gow et al.118 2016; New Zealand; 2013–14; single hospital; none |
Hip or knee (surgical site infection within 90 days of surgery), surgical procedure (median 2, range 1–4) n = 11 (9 deep infection) Costs incurred during hospital stays (n = 22 uncomplicated hip or knee replacement) |
2013; NZD; Auckland District Health Board clinical costing system | Laboratory tests; allied health input; radiology; drug therapy; bed costs | Outpatient visits; primary care visits |
Mean cost including first treatment and all admissions for infection NZ$61,157 (SD NZ$41,414) Mean cost of uncomplicated hip or knee replacement NZ$21,035 (SD NZ$6296) Cost of management of infection 291% greater than uncomplicated hip or knee replacement |
| Haenle et al.119 2012; Germany; 2004–7; single hospital; none |
Hip, deep infection, not single lavage debridement, surgical treatment n = 49 Costs incurred during hospital stays and reoperations (follow-up time not specified) (n = 21 primary hip replacements) |
2004–7; euros; patient records from hospital information system and data from the hospital central pharmacy and control centre | Implants; laboratory tests; radiology; blood products; antibiotics; pharmaceuticals; medical supplies; operating theatre personnel; anaesthesia; ward personnel; ICU | Patient factors: production, salary and social security taxes |
Mean cost for treatment of infection including additional operations €29,331 Mean cost of primary hip replacement €6264 Cost of hip replacement with subsequent treatment of infection 468% higher than primary hip replacement |
| Haenle et al.120 2012; Germany; 2004–7; single hospital; none |
Knee, deep infection, not single debridement and lavage, surgical treatment n = 28 Costs incurred during hospital stays (follow-up time not specified) (n = 21 primary knee replacements) |
2011; euros; health record and hospitals health information system; DRG relevant costs; federal employee tariff; hospital central controlling unit; local ICU costs | Implants; laboratory tests; radiology; blood products; antibiotics; pharmaceuticals; medical supplies; operating theatre personnel; anaesthesia; ward personnel; ICU | Patient factors: production, salary and social security taxes |
Mean cost for treatment of infection including additional operations €25,194 Mean cost of primary knee replacement €6889 Cost of treating knee infection 366% greater than primary knee replacement |
| Kallala et al.121 2015; UK; 2005–12; single hospital; none |
Knee, deep infection, two-stage revision n = 45 Costs incurred during hospital stays (n = 123 aseptic revision) |
2005–12; GBP; patient-level information and costing system, hospital, department of finance | Implants; materials and augments; operating theatres and recovery; ward care; physiotherapy; occupational therapy; pharmacy; radiology; laboratory tests | Readmissions for complications; patient costs; societal costs |
Mean cost of first treatment for infection with two-stage revision £30,011 (SD £4514) Mean cost of aseptic revision £9655 (SD £600) Cost of revision for knee infection 311% higher than aseptic revision |
| Kapadia et al.122 2014; USA; 2007–13; single hospital; none |
Knee, deep infection, two-stage revision n = 21 Minimum 1 year (n = 21 primary knee replacements) |
2007–13; USD; hospital financial system | Bed stay; operating room services; admission; pharmacy; laboratory tests; radiology; anaesthesia; blood transfusion; rehabilitation; consultation services; clinic visits; reoperation costs | Patient-incurred costs |
Including primary surgery, mean cost of first treatment of infected knee replacement with two-stage revision and subsequent reoperations US$116,383 (range US$44,416–269,914) Mean cost of primary knee replacement US$28,250 (range US$20,454–47,957) Cost of knee replacement with subsequent two-stage treatment of infection 412% higher than primary knee replacement |
| Kapadia et al.123 2016; USA; 2007–11; single hospital; none |
Hip, deep infection, two-stage revision n = 16 Minimum 1 year (n = 32 primary hip replacements) |
2007–11; USD; hospital financial system | Bed stay; operating room services; admission; pharmacy; laboratory tests; radiology; anaesthesia; blood transfusion; rehabilitation; consultation services; outpatient visits; reoperation costs | Patient-incurred costs |
Including primary surgery, mean cost of first treatment of infected knee replacement with two-stage revision and subsequent reoperations US$88,623 (range US$44,043–158,202) Mean cost of primary hip replacement US$25,659 (range US$13,595–48,631) Cost of hip replacement with subsequent two-stage treatment of infection 345% higher than primary hip replacement |
| Klouche et al.124 2010; France; 2006; single hospital; none |
Hip, deep infection, single- or two-stage revision n = 40 Costs incurred during hospital stays and home hospitalisation (n = 474 primary hip replacements and n = 57 aseptic revisions) |
2006; euros; analytic accounting system | Preoperative; hospital stay; staff costs; prescriptions; implants; general expenses; surgical unit; anaesthesia; physiotherapy; radiology; laboratory tests; day hospital; general services; additional hospital stay and rehabilitation; antibiotic therapy; home-based hospitalisation | Social expenses |
Mean cost of single or two-stage revision for infection €32,546 (SD €9587) Mean costs of primary hip replacement $9028 (SD €1924) and aseptic revision $12,409 (SD 2059) Cost of management of infection with single or two-stage revision 262% higher than aseptic revision and 361% higher than primary hip replacement |
| Oduwole et al.126 2010; Ireland; 1997–2001, 2002–6; single hospital; none |
Knee, deep infection, two-stage n = 20 (2002–6) Costs incurred during hospital stays (n = 99 aseptic revisions) |
Year not specified but costs adjusted for inflation; euros; insurer cost of semi-private patient; pharmacy records | Bed-cost; surgeon’s fees; implant; instrumentation; radiology; blood tests; transfusion; intravenous antibiotics; other medication | Bone scans; MRI |
2002–6 mean cost of treatment of infection with two-stage revision €23,113 (range €12,180–33,853) Mean cost of aseptic revision €15,174 (range €5837–24,777) Cost of two-stage treatment of knee infection 152% higher than aseptic revision |
| Peel et al.130 2013; Australia; 2008–10; single hospital; none |
DAIR for hip or knee PJI n = 21 Median 19 months (IQR 14–21 months) (n = 42 primary hip or knee replacements including two aseptic revisions) |
2008–10; AUD; St Vincent’s Hospital, Melbourne, administrative databases |
Government perspective Inpatient care (medical, nursing, operating theatre, prosthesis, ICU, CCU, allied health, medical imaging, pathology, pharmacy, hospital in the home) Total outpatient (medical, nursing, allied health, medical imaging, pathology, pharmacy) Total emergency |
Patient out-of-pocket expenses |
Mean cost of DAIR was AU$69,414 Mean cost of primary hip or knee replacement (includes 2/21 aseptic revisions) was AU$22,085 |
| Rennert-May et al.128 2018; Canada; 2012–15; hospitals in Canadian province; none |
Hip or knee, complex surgical site infection, surgical treatment n = 258 12 months (n = 24,409 primary hip or knee replacement) |
2016; CAD; Alberta health services microcosting | Admission; hospitalisation; emergency room; day surgery; day medicine visits | Other physicians; outpatient antibiotics; patient costs |
Mean cost of primary hip or knee replacement and subsequent infection treatment CA$70,144 (IQR CA$35,923–86,368) of which CA$14,071 (IQR CA$10,400–14,202) was initial treatment Mean cost of primary hip or knee replacement CA$13,195 (IQR CA$10,269–13,049) Cost of hip or knee replacement with treatment of subsequent infection 532% higher than primary hip or knee replacement |
| Romano et al.129 2010; Italy; 2001–6; two hospitals; none |
Hip, deep infection, two-stage n = 40 Minimum 2 years (n = 40 revision for aseptic loosening) |
2001–6; euros; hospital administrative decision support database | Operating room equipment; implants (including bone grafts and bone substitutes); operative staff; hospital stay in surgical and rehabilitation departments; blood; pharmacy; administrative costs | Readmissions; outpatient visits and charges; patient and societal costs |
Mean cost of initial infection treatment with two-stage revision €60,394 (SD €15,886) Mean cost of aseptic revision €27,194 (SD €5122) Cost of two-stage treatment of hip infection 222% higher than aseptic revision |
| Vanhegan et al.55 2012; UK; 1999–2008; single hospital; none |
Hip, deep infection, single or two-stage n = 76 Costs incurred during hospital stays (n = 194 aseptic revisions) |
2007–8; GBP; hospital costs – methods not described | Hospital stay; surgery time; inpatient nursing; blood loss; laboratory tests; radiology; drugs; implant; materials and augmentation; operating theatre; recovery room; physiotherapy; occupational therapy | Surgeon fees; readmissions; other direct and indirect patient and societal costs |
Mean cost of initial treatment of infection with two-stage revision £21,937 (SD £10,965) Mean cost of aseptic revision £11,897 (SD £4629) Cost of two-stage treatment of hip infection 184% higher than aseptic revision |
The resources included in cost calculations varied considerably, but no study reported the costs to patients. Generally, the focus was on in-hospital costs.
Risk of bias arose as many studies reported cost information limited to specific aspects of hospital care. As we only report cost comparisons between treatments within centres, this is not a major concern. Studies were mainly in single centres and reported consecutive patients.
Cost of infection treatment compared with cost of primary hip or knee replacement
Eight studies117–120,122–124,128 reported comparisons between primary hip or knee replacement and first surgical treatments of infected hip or knee replacements other than DAIR. None of the studies was conducted in the UK. The cost of treatments for infection was on average 4.0 times (range 2.9–5.3 times) that of primary hip or knee replacement. The cost of DAIR treatment was on average 3.0 times (range 2.9–3.1 times) that of primary hip or knee replacement.
In one study that included the costs of primary hip or knee replacement and subsequent first treatments for infection, including those occurring during primary admission, costs were 2.9 times those of primary hip or knee replacement. 118
Only the cost of the first treatment for hip infection was measured in one study,124 and this was 3.6 times the cost of primary hip replacement.
In four studies117,119,120,128 the revision costs of infection and any subsequent treatments relating to infection were calculated. On average, the cost of revision for infection treatment and further treatments was 4.5 times (range 3.7–5.3 times) that of primary hip or knee replacement. Only one study reported the length of follow-up. 117 In the 12 months after starting treatment of hip prosthetic joint infection with two-stage revision, costs were 4.4 times higher than those for primary hip replacement.
In two studies including costs of primary hip or knee replacement and all subsequent treatments for infection including persistent infection and reinfection after a minimum of 1 year, the costs reported were 3.5 and 4.1 times that of primary hip123 or knee122 replacement, respectively.
Cost of infection treatment compared with cost of aseptic revision
In seven studies,55,116,117,121,124,126,129 a comparison was made between the costs of revision for infection and those for aseptic indications. In two studies,55,124 patients were treated with either single- or two-stage revision, and in five studies116,117,121,126,129 patients were treated exclusively with two-stage revision. On average, the cost of revision for infection was 2.4 times (range 1.5–3.1 times) that of revision for aseptic reasons. In the five studies116,117,121,126,129 of patients with infection treated with two-stage revision, the cost of treatment was 2.5 times (range 1.5–3.1 times) that of aseptic revision. In two UK studies, the hospital cost of two-stage treatment of hip infection was 1.8 times greater55 and for knee infection 3.1 times greater121 than aseptic revision. Surgeries in the two studies were conducted between 1999 and 2008 and between 2005 and 2012, respectively.
In five studies, the cost of revision surgery was reported with no consideration of subsequent re-operations for failure of infection clearance. In these studies,55,121,124,126,129 the cost associated with the treatment of infection was 2.3 times (range 1.5 –3.1 times) that of aseptic revision.
In five studies55,116,117,124,129 in patients with hip replacements, the costs of revision for infection were 2.4 times (range 1.8–2.8 times) those of aseptic revision. This difference was 3.1 and 1.5 in the two studies121,126 of patients with knee replacement.
Costs of single-stage compared with costs of two-stage revision strategies
Three studies124,125,127 compared the costs of single- and two-stage revision strategies (Table 11). Two studies were conducted in Australia125,127 and one was conducted in France. 124 Two studies125,127 involved multiple centres and one study124 was at a single hospital. Two studies124,125 included patients with hip infection and one study127 included patients with both hip and knee infection. Key issues of concern were identified for each study based on the CHEC-list. In one study,124 the comparison of costs was between first treatments, and the effectiveness in relation to prevention of persistent infection or reinfection was not considered. In two studies, the costs of further treatments were included in cost estimates, but the numbers of patients were small in one study127 and no information on the selection of patients for treatments was provided in either study. 125,127
| Authors and year; country; recruitment dates; setting; risk-of-bias concerns | Indication; number of single-stage and two-stage; follow-up | Year (cost); currency; source of data | Costs included | Costs not included | Costs with description of context |
|---|---|---|---|---|---|
| Klouche et al.124 2010; France; 2006; single hospital; purely a comparison of first treatment costs |
Hip, deep infection n = 40 total Number of single- and two-stage revisions not specified Costs incurred during hospital stays and home hospitalisation |
2006; euros; analytic accounting system with internal criteria | Preoperative; hospital stay; staff costs; prescriptions; implants; general expenses; surgical unit; anaesthesia; physiotherapy; radiology; laboratory tests; day hospital; general services; additional hospital stay and rehabilitation; antibiotic therapy; home-based hospitalisation | Social expenses |
Mean cost of first treatment of infection: Single-stage €31,133 (SD €9733) Two-stage €54,098 (SD €12,700) Two-stage revision of infected hip replacement cost 1.74 times more than a single-stage revision |
| Merollini et al.125 2013; Australia; 2006–9; Queensland hospitals database; no information on reason for decision to perform single- or two-stage revision |
Hip, deep infection within 1 year of primary hip replacement n = 25; 14 Costs incurred during hospital stays Follow-up not reported |
2006–9; AUD; national hospital cost data collection | Surgery; prostheses; hospital | Antibiotics; primary care; travel; pharmaceuticals; indirect costs |
Mean cost of single-stage: AU$27,006 (range AU$8957–$36,408). Additional treatments in three patients mean AU$24,357 (range AU$15,801–36,408) Mean cost of two-stage: AU$42,772 (range AU$15,801–60,870). Total treatment costs in one patient requiring further revision: AU$70,381 Cost of successful treatment by two-stage 1.58 times single-stage. Cost of first two-stage treatment and reoperations 1.60 times that of single-stage |
| Peel et al.127 2013; Australia; 2006–8; 10 hospitals; no information on reason for decision to perform single- or two-stage revision, conclusions based on small number of patients |
Hip or knee, deep infection n = 6; 6 n = 108 DAIR comparator Mean 26 months (SD 19 months) |
2011–12; AUD; Australian Consumer Price Index 2012; Australian Refined Diagnosis Related Group; hospital costing data; Health Purchasing Victoria; Medicare Benefit Scheme | Surgery; prostheses; hospital; home care; antibiotics | Outpatient review; pathology; radiology; non-medical and societal costs |
Median cost of first treatment of infection and persistent infection AUD34,800 (5/6 single-stage required re-revision and these costs included) In multivariate model, single-stage revision and subsequent treatments for persistent infection cost 2.00 (95% CI 1.30 to 3.10) times DAIR Two-stage revision and subsequent treatments for persistent infection cost 1.20 (95% CI 0.83 to 1.73) times DAIR Estimate two-stage cost 0.6 times single-stage |
| Authors and year; country; recruitment dates; setting | Indication; number of different features | Year (cost); currency; source of data | Costs included | Costs not included | Costs with description of context |
|---|---|---|---|---|---|
| Kalore et al.131 2012; USA; 2001–9; two hospitals |
Two-stage revision of infected TKR with use of three antibiotic eluting spacer types. Reimplantation completed AOC, n = 15; NFC, n = 16; SMC, n = 22 |
Year not specified; USD | Implants; moulds; cement; antibiotics | Indirect costs related to operative time or the need for repeat hospitalisations or surgery |
Mean costs: AOC, US$932; NFC, NFC US$3589; SMC, US$3945 AOC technique 0.26 cost of NFC AOC technique 0.24 cost of SMC Mean follow-up (reinfection): AOC 73 months (33.3%); NFC 19 months (12.5%); SMC 32 months 35%) |
| Nodzo et al.132 2017; USA; 2005–14; single institution |
Two-stage revision of infected TKR with use of three articulating antibiotic eluting spacer types. Reimplantation completed AUTOCL, n = 39; PREFAB, n = 58; MOULD, n = 43 |
Year not specified; USD |
Cost of prefabricated spacer, commercial cement moulds, antibiotics added to cement, and cost of each bag of cement (off-the-shelf list pricing) Cost of a new femoral implant was calculated for the AUTOCL group as regulations do not permit reimplantation of explanted components |
Mean costs: AUTOCL, US$3764; PREFAB, US$4825; MOULD, commercial US$5439; MOULD, homemade US$1341 Mean follow-up (reinfection): AUTOCL 52.4 months (20.6% infection free); PREFAB 74.9 months (17.3% infection free); MOULD 43.7 months (11.6% infection free) No significant association between spacer type and reoperation in univariable or multivariable analysis |
In two studies,124,125 the cost of planned revision with no further treatment for persistent infection or reinfection using a two-stage procedure was higher than for a single-stage procedure, with two-stage revision costing 1.74 times124 or 1.58 times125 that of a single-stage revision.
Considering all procedures, including planned operations and those required to treat persistent infection or reinfection, the relative costs differed in two studies reporting data. 125,127 In one of the studies,125 3 out of 25 patients required additional treatment after single-stage revision and 1 out of 14 required additional treatment after two-stage revision. The follow-up time was not stated. Considering the additional costs associated with these procedures, the overall cost of treatment with two-stage revision was approximately 1.60 times that with single-stage revision. 125 In the other study,127 which had a mean follow-up of 26 months (standard deviation 19 months), five out of six single-stage revisions required additional operations and the overall cost of a two-stage revision was 0.6 times that of a single-stage revision.
Comparison of costs associated with different spacers
Two studies131,132 compared the costs associated with different spacers (Table 12). Both studies included a group with replacement of an autoclaved original femoral component. In one study this was included in the costing and, in combination with a cement spacer, the cost was about one-quarter of that when the femoral component was replaced and a polyethylene or cement spacer was used. 131 Infection rates were reported at widely different follow-up times, and it is not possible to compare rates between the groups and, thus, to determine whether or not the cost saving was associated with differences in reinfection. The autoclaving of components, particularly without sonication, is not believed to eradicate biofilm such that it can be used in contemporary practice. There was little difference in costs between polyethylene and cement spacers when the femoral component was replaced. 131,132 In a secondary analysis, the cost of homemade cement spacers was 25% of that of commercially available versions. 132 Again, follow-up rates varied between groups and no conclusion relating to reinfection rates can be drawn.
Discussion
The costs of planned revision of infection are, on average, approximately four times those of primary hip or knee replacement and 2.4 times those of aseptic revision. The costs of further treatments associated with treatment failure are highly dependent on the success rate of revision treatments in different studies, and this varied markedly in the studies we identified. Thus, in two studies with low rates of reinfection, two-stage revision was about 60–70% more costly than single-stage revision. However, in one study with a highly unfavourable reinfection rate after single-stage revision, a two-stage strategy was associated with 40% lower costs.
The studies we identified highlight the importance of knowing the clinical effectiveness of treatment strategies when assessing the costs associated with them. In the absence of randomised evaluations, in systematic reviews of effectiveness of revision strategies for treatment of prosthetic joint infection, an attempt to limit this has been to select and compare pure case-series in which all patients with infection have received a specific treatment. 47,86,87,133 The inclusion of studies with patients selected for a treatment strategy based on infection severity or their health may give misleading results. However, as surgeons must to some degree tailor surgical management to individual patients, this may not always be avoidable. Only through the collection of detailed cost data in randomised evaluations and treatment comparisons in large unselected cohorts can definitive information on the costs and cost-effectiveness of different treatment strategies be generated.
The treatment of infection after hip and knee replacement with a two-stage revision is costly, and the single-stage revision strategy and other alternatives potentially address the need of patients and health-care providers for a less invasive, time-consuming and expensive treatment for infection. Much emphasis has been placed on the use of DAIR as an alternative to single- or two-stage revision. This is less invasive, but overall infection clearance rates may be only 61.4%, with a wide variation in rates reported in case series (range of clearance rates 11.1–100%). 85 Another option for hip infection relies on the use of a CUMARS, with the option of postponing the second stage and retaining the CUMARS prosthesis. 46 As with DAIR, this is not the final treatment for many patients, but in a case series 45% of patients receiving this treatment chose to keep the temporary prosthesis rather than receive a new prosthesis in a second-stage operation. 46 The initial costs of DAIR and a CUMARS are lower than those of a two-stage revision but, with the need for further treatments, costs may, ultimately, be higher.
In the three studies we identified comparing single- and two-stage revision and considering the financial consequences of treatment failure, the rates of successful treatment differed markedly between studies. This led to contradictory conclusions that both the single-stage and the two-stage strategy are the lower-cost option. However, in these studies the numbers of patients receiving treatment were small.
With the reporting of ongoing RCTs comparing single- and two-stage revision of joints affected by prosthetic joint infection,97,134,135 associated economic analyses will provide both robust standalone data and information to populate decision models. Such analyses should be from a patient and societal perspective in addition to a health-care provider perspective. Until then, it is important that health-care providers are aware that the costs of treatment of prosthetic joint infection are high and vary considerably between individual patients, and that prior to the INFORM RCT, insufficient high-quality evidence was available to advise on choice of revision strategy.
Appendix 3 Literature search terms for risk factors for prosthetic joint infection as applied in MEDLINE on 1 September 2015 and described in Risk factors for prosthetic hip or knee infection
-
prosthetic joint infection.mp. or exp Surgical Wound Infection/ (30,119)
-
prosthetic infection.mp. (294)
-
wound infection.mp. or exp Wound Infection/ (45,531)
-
exp Sepsis/or sepsis.mp. (132,792)
-
exp Surgical Wound Infection/or surgical site infection.mp. (30,357)
-
arthroplasty.mp. or exp Arthroplasty, Replacement, Knee/or exp Arthroplasty, Replacement/or exp Arthroplasty/or exp Arthroplasty, Replacement, Hip/ (54,094)
-
joint replacement.mp. (4292)
-
exp Joint Prosthesis/or joint arthroplasty.mp. (37,545)
-
exp Arthroplasty, Replacement, Hip/or total arthroplasty.mp. (18,367)
-
risk factor.mp. or exp Risk Factors/ (661,103)
-
exp Biological Markers/or exp Risk Factors/or risk marker.mp. or exp Risk/or exp Diabetes Mellitus, Type 2/ (1,584,727)
-
predictor.mp. (109,243)
-
age.mp. (6,925,632)
-
sex.mp. or exp Sex/ (603,694)
-
body mass index.mp. or exp Body Mass Index/ (142,034)
-
body weight.mp. or exp Body Weight/ (451,315)
-
socioeconomic status.mp. or exp Social Class/ (51,072)
-
exp Smoking/or smoking.mp. (202,595)
-
exp “Tobacco Use”/or exp Tobacco/or tobacco.mp. (178,015)
-
alcohol.mp. (214,043)
-
diabetes.mp. or exp Diabetes Mellitus, Type 2/or exp Diabetes Mellitus/ (438,869)
-
exp Hypertension/or hypertension.mp. (365,828)
-
comorbidity.mp. or exp Comorbidity/ (91,604)
-
rheumatoid arthritis.mp. or exp Arthritis, Rheumatoid/ (114,884)
-
osteoarthritis.mp. or exp Osteoarthritis, Hip/or exp Osteoarthritis/or exp Osteoarthritis, Knee/ (55,616)
-
history of joint arthroplasty.mp. (5)
-
dental procedure.mp. or exp Tooth Extraction/ (17,354)
-
steroid.mp. or exp Steroids/ (779,238)
-
anticoagulant.mp. or exp Anticoagulants/ (192,581)
-
thromboprophylaxis.mp. (2762)
-
previous surgery.mp. (3074)
-
exp Femoral Fractures/or exp Hip Fractures/or exp Femoral Neck Fractures/or previous fracture surgery.mp. (30,456)
-
revision arthroplasty.mp. (973)
-
previous prosthetic joint infection.mp. (1)
-
1 or 2 or 3 or 4 or 5 (175,563)
-
6 or 7 or 8 or 9 (70,471)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 (9,860,110)
-
35 and 36 (3173)
-
37 and 38 (2441)
-
limit 39 to humans (2421)
Appendix 4 Literature search terms for hip implant fixation as a risk factor for prosthetic joint infection as applied in MEDLINE on 24 April 2019 and described in Risk factors for prosthetic hip or knee infection
-
exp Hip Prosthesis/ (21,604)
-
hip relacement.mp. (0)
-
exp Arthroplasty, Replacement, Hip/ (23,365)
-
exp Hip Joint/ (25,479)
-
fixation.mp. (195,733)
-
cemented.mp. (9687)
-
uncemented.mp. (2634)
-
hybrid.mp. (146,856)
-
reverse hybrid.mp. (32)
-
exp Prosthesis-Related Infections/ (10,732)
-
periprosthetic joint infection.mp. (900)
-
prosthetic joint infection.mp. (973)
-
prosthetic infection.mp. (396)
-
exp INFECTION/ (739,240)
-
exp Surgical Wound Infection/ (33,623)
-
surgical site infection.mp. (5502)
-
1 or 2 or 3 or 4 (55,289)
-
5 or 6 or 7 or 8 or 9 (350,215)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 (741,846)
-
17 and 18 and 19 (466)
-
limit 20 to humans (457)
Appendix 5 Literature search terms for knee implant fixation as a risk factor for prosthetic joint infection as applied in MEDLINE in November 2018 and described in Risk factors for prosthetic hip or knee infection
-
exp Knee Prosthesis/ (10,710)
-
exp Arthroplasty, Replacement, Knee/ (20,199)
-
exp Knee Joint/ (55,210)
-
fixation.mp. (197,432)
-
cement*.mp. (65,551)
-
uncemented.mp. (2661)
-
hybrid.mp. (149,302)
-
reverse hybrid.mp. (33)
-
stem.mp. (416,609)
-
exp Prosthesis-Related Infections/ (10,888)
-
prosthetic joint infection.mp. (1011)
-
prosthetic infection.mp. (399)
-
exp Wound Infection/ (44,055)
-
deep infection.mp. (2795)
-
exp SEPSIS/ (113,415)
-
surgical site infection*.mp. (8323)
-
1 or 2 or 3 (72,671)
-
4 or 5 or 6 or 7 or 8 or 9 (811,858)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 (170,789)
-
17 and 18 and 19 (534)
-
limit 20 to humans (529).
Appendix 6 Incidence rate ratios of revision for hip prosthetic joint infection and 95% credible intervals for patient characteristics as described in Risk factors for prosthetic hip or knee infection
| Characteristic | Total | ≤ 3 months | 3–6 months | 6–12 months | 12–24 months | ≥ 24 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | |
| Sex | ||||||||||||
| Male | 1.68 (1.56 to 1.81) | 6426 | 1.72 (1.39 to 2.10) | 4469 | 2.59 (1.93 to 3.44) | 3170 | 1.93 (1.57 to 2.37) | 3809 | 1.73 (1.47 to 2.03) | 4039 | 1.44 (1.28 to 1.62) | 4534 |
| Female | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Age group (years) | ||||||||||||
| ≥ 80 | 0.66 (0.56 to 0.76) | 6519 | 1.42 (0.98 to 1.99) | 2256 | 0.63 (0.32 to 1.10) | 3765 | 0.41 (0.25 to 0.63) | 4464 | 0.68 (0.49 to 0.91) | 4120 | 0.48 (0.37 to 0.61) | 5835 |
| 70–79 | 0.73 (0.66 to 0.81) | 4157 | 0.97 (0.70 to 1.30) | 2027 | 1.26 (0.84 to 1.82) | 1896 | 0.67 (0.49 to 0.89) | 2551 | 0.69 (0.55 to 0.86) | 2803 | 0.64 (0.54 to 0.74) | 3295 |
| 60–69 | 0.90 (0.81 to 0.99) | 4255 | 1.14 (0.84 to 1.52) | 2109 | 1.05 (0.69 to 1.54) | 2129 | 1.11 (0.85 to 1.43) | 2434 | 0.93 (0.75 to 1.14) | 2809 | 0.76 (0.66 to 0.88) | 3444 |
| < 60 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Ethnicity | ||||||||||||
| Black African origin | 0.80 (0.41 to 1.33) | 16,984 | 0.00 (0.00 to 0.00) | 0 | 0.99 (0.03 to 3.68) | 18,731 | 0.49 (0.01 to 1.82) | 18,739 | 0.57 (0.07 to 1.59) | 18,497 | 1.23 (0.53 to 2.26) | 17,161 |
| Other or mixed | 0.88 (0.48 to 1.40) | 17,820 | 0.00 (0.00 to 0.00) | 0 | 3.62 (0.98 to 8.04) | 17,595 | 1.74 (0.47 to 3.86) | 17,841 | 0.27 (0.01 to 1.00) | 18,615 | 0.75 (0.25 to 1.56) | 17,979 |
| South Asian | 0.70 (0.25 to 1.36) | 17,463 | 0.98 (0.02 to 3.62) | 18,738 | 1.70 (0.04 to 6.36) | 18,959 | 0.00 (0.00 to 0.00) | 0 | 1.02 (0.12 to 2.87) | 18,140 | 0.56 (0.07 to 1.57) | 18,580 |
| Unknown | 0.25 (0.16 to 0.36) | 17,484 | 0.43 (0.14 to 0.90) | 17,917 | 0.31 (0.04 to 0.87) | 18,126 | 0.23 (0.05 to 0.55) | 17,890 | 0.23 (0.08 to 0.48) | 17,986 | 0.21 (0.10 to 0.36) | 17,722 |
| White | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| BMI (kg/m2) | ||||||||||||
| ≥ 30 | 1.92 (1.72 to 2.15) | 1885 | 2.99 (2.12 to 4.13) | 842 | 2.41 (1.51 to 3.75) | 942 | 1.54 (1.14 to 2.04) | 1357 | 1.99 (1.56 to 2.53) | 1159 | 1.72 (1.45 to 2.03) | 1329 |
| 25–29.9 | 1.25 (1.11 to 1.40) | 2141 | 1.57 (1.09 to 2.22) | 1040 | 1.67 (1.03 to 2.61) | 1058 | 0.88 (0.64 to 1.19) | 1673 | 1.31 (1.01 to 1.68) | 1274 | 1.25 (1.05 to 1.49) | 1506 |
| < 25 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| ASA grade | ||||||||||||
| 3–5 | 1.63 (1.42 to 1.87) | 3434 | 2.40 (1.57 to 3.55) | 999 | 2.38 (1.26 to 4.15) | 975 | 2.43 (1.65 to 3.48) | 1452 | 1.64 (1.21 to 2.16) | 1980 | 1.12 (0.89 to 1.37) | 3620 |
| 2 | 1.26 (1.14 to 1.40) | 2211 | 1.64 (1.14 to 2.32) | 772 | 2.30 (1.38 to 3.73) | 685 | 1.49 (1.08 to 2.03) | 1029 | 1.25 (0.99 to 1.57) | 1306 | 1.04 (0.89 to 1.20) | 1954 |
| 1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Chronic pulmonary disease | 1.22 (1.08 to 1.37) | 14,760 | 1.41 (1.03 to 1.86) | 14,084 | 1.03 (0.62 to 1.55) | 14,798 | 1.28 (0.92 to 1.71) | 14,661 | 1.32 (1.03 to 1.65) | 14,179 | 0.96 (0.77 to 1.18) | 15,769 |
| Diabetes | 1.35 (1.18 to 1.54) | 15,265 | 1.53 (1.09 to 2.07) | 14,401 | 1.60 (1.01 to 2.35) | 14,781 | 1.52 (1.07 to 2.07) | 14,680 | 1.32 (0.99 to 1.71) | 14,937 | 1.00 (0.78 to 1.27) | 16,011 |
| Dementia | 1.85 (0.89 to 3.18) | 17,999 | 3.78 (1.21 to 7.81) | 17,553 | 1.80 (0.04 to 6.68) | 18,665 | 2.32 (0.28 to 6.51) | 18,082 | 0.66 (0.02 to 2.45) | 19,173 | 0.60 (0.02 to 2.22) | 18,863 |
| Liver disease | 2.35 (1.66 to 3.17) | 17,138 | 2.24 (0.81 to 4.38) | 17,260 | 1.39 (0.17 to 3.88) | 17,992 | 2.52 (1.01 to 4.75) | 17,781 | 1.90 (0.82 to 3.45) | 18,270 | 2.43 (1.35 to 3.82) | 17,701 |
| Congestive heart failure | 1.45 (1.09 to 1.86) | 16,891 | 1.65 (0.86 to 2.71) | 16,691 | 0.00 (0.00 to 0.00) | 0 | 2.34 (1.26 to 3.77) | 16,811 | 1.27 (0.67 to 2.07) | 17,427 | 1.27 (0.74 to 1.93) | 17,345 |
| Connective tissue-rheumatologic disease | 1.37 (1.12 to 1.64) | 16,585 | 1.49 (0.88 to 2.28) | 16,615 | 2.28 (1.22 to 3.72) | 15,762 | 1.60 (0.94 to 2.43) | 16,681 | 1.14 (0.71 to 1.67) | 16,701 | 1.12 (0.79 to 1.51) | 16,969 |
| Cancer | ||||||||||||
| Cancer | 1.12 (0.89 to 1.37) | 17,263 | 1.01 (0.53 to 1.66) | 16,623 | 1.14 (0.49 to 2.11) | 16,767 | 0.95 (0.47 to 1.61) | 17,302 | 0.95 (0.56 to 1.46) | 16,929 | 1.22 (0.84 to 1.67) | 17,255 |
| Metastatic | 1.81 (1.07 to 2.74) | 18,011 | 0.81 (0.10 to 2.26) | 18,367 | 1.85 (0.22 to 5.20) | 18,293 | 2.21 (0.60 to 4.86) | 17,883 | 1.53 (0.42 to 3.38) | 17,742 | 1.82 (0.66 to 3.55) | 18,064 |
| None | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Cerebrovascular disease | 0.94 (0.65 to 1.27) | 17,637 | 1.45 (0.68 to 2.52) | 17,183 | 0.87 (0.18 to 2.11) | 17,671 | 1.22 (0.49 to 2.31) | 17,928 | 0.62 (0.23 to 1.22) | 17,852 | 0.71 (0.34 to 1.21) | 17,492 |
| Myocardial infarction | 0.99 (0.75 to 1.26) | 16,677 | 1.17 (0.60 to 1.95) | 16,958 | 0.53 (0.11 to 1.28) | 17,940 | 0.65 (0.23 to 1.27) | 17,265 | 1.14 (0.64 to 1.78) | 17,442 | 1.00 (0.63 to 1.46) | 17,532 |
| Paraplegia and hemiplegia | 1.62 (0.88 to 2.57) | 17,787 | 0.70 (0.02 to 2.60) | 18,874 | 2.78 (0.33 to 7.80) | 18,227 | 1.51 (0.18 to 4.19) | 18,511 | 2.32 (0.74 to 4.81) | 16,973 | 1.18 (0.32 to 2.58) | 18,295 |
| Peptic ulcer disease | 1.12 (0.76 to 1.54) | 17,487 | 1.16 (0.37 to 2.40) | 17,851 | 0.88 (0.11 to 2.49) | 17,934 | 1.53 (0.55 to 2.99) | 17,734 | 0.76 (0.24 to 1.55) | 18,008 | 1.20 (0.66 to 1.92) | 17,854 |
| Peripheral vascular disease | 1.19 (0.87 to 1.55) | 17,278 | 1.25 (0.57 to 2.23) | 17,575 | 1.01 (0.27 to 2.24) | 17,968 | 1.76 (0.86 to 2.97) | 16,949 | 0.87 (0.40 to 1.54) | 17,734 | 1.00 (0.54 to 1.60) | 17,477 |
| Renal disease | 1.12 (0.84 to 1.45) | 17,256 | 1.21 (0.65 to 1.95) | 16,478 | 1.70 (0.76 to 3.05) | 16,088 | 0.93 (0.40 to 1.70) | 17,527 | 0.62 (0.28 to 1.10) | 17,310 | 0.97 (0.53 to 1.55) | 17,244 |
Appendix 7 Incidence rate ratios of revision for hip prosthetic joint infection and 95% credible intervals for surgical characteristics as described in Risk factors for prosthetic hip or knee infection
| Characteristic | Total | ≤ 3 months | 3–6 months | 6–12 months | 12–24 months | ≥ 24 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | |
| Osteoarthritis | 0.69 (0.60 to 0.79) | 927 | 0.67 (0.47 to 0.94) | 756 | 0.49 (0.32 to 0.75) | 935 | 0.57 (0.41 to 0.77) | 868 | 0.79 (0.59 to 1.05) | 589 | 0.80 (0.66 to 1.03) | 523 |
| Fractured neck of femur | 1.82 (1.40 to 2.30) | 16,934 | 3.00 (1.82 to 4.49) | 4997 | 2.07 (0.81 to 3.94) | 5272 | 1.38 (0.62 to 2.46) | 5800 | 1.32 (0.70 to 2.14) | 5787 | 1.30 (0.71 to 2.00) | 6067 |
| Previous hip infection | 6.69 (4.18 to 9.80) | 17,591 | 6.71 (1.38 to 16.31) | 10,601 | 23.3 (7.38 to 48.63) | 9769 | 6.45 (1.31 to 15.75) | 10,766 | 6.92 (2.25 to 14.28) | 10,718 | 4.30 (1.56 to 8.40) | 10,859 |
| Avascular necrosis | 1.42 (1.16 to 1.71) | 16,403 | 1.53 (0.84 to 2.44) | 4270 | 1.16 (0.42 to 2.30) | 4587 | 1.73 (1.02 to 2.66) | 4397 | 1.42 (0.90 to 2.07) | 4247 | 1.40 (0.99 to 1.81) | 4501 |
| Dysplasia/congenital dislocation | 0.64 (0.42 to 0.90) | 16,987 | 0.43 (0.05 to 1.21) | 12,128 | 0.89 (0.11 to 2.50) | 11,991 | 0.73 (0.20 to 1.61) | 11,699 | 0.52 (0.17 to 1.08) | 11,843 | 0.70 (0.38 to 1.09) | 11,419 |
| Inflammatory arthropathy | 0.90 (0.64 to 1.21) | 17,599 | 0.35 (0.04 to 1.00) | 12,160 | 1.81 (0.58 to 3.79) | 11,286 | 1.49 (0.67 to 2.64) | 11,412 | 0.83 (0.36 to 1.51) | 11,812 | 0.80 (0.48 to 1.31) | 11,645 |
| Surgical approach | ||||||||||||
| Lateral | 1.32 (1.21 to 1.43) | 5437 | 1.08 (0.86 to 1.35) | 2408 | 1.56 (1.16 to 2.06) | 2197 | 1.51 (1.21 to 1.87) | 2027 | 1.53 (1.28 to 1.81) | 1937 | 1.30 (1.16 to 1.50) | 1820 |
| Other | 1.48 (1.22 to 1.77) | 12,851 | 1.33 (0.79 to 2.03) | 4412 | 1.20 (0.53 to 2.18) | 4890 | 1.39 (0.80 to 2.16) | 4689 | 1.32 (0.85 to 1.89) | 4569 | 1.60 (1.15 to 2.06) | 4593 |
| Posterior | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Procedure | ||||||||||||
| Resurfacing | 0.56 (0.46 to 0.67) | 5451 | 0.68 (0.30 to 1.25) | 3296 | 0.32 (0.11 to 0.67) | 4234 | 0.39 (0.20 to 0.65) | 3366 | 0.53 (0.35 to 0.76) | 2445 | 0.70 (0.51 to 0.83) | 1674 |
| THR uncemented | 0.92 (0.83 to 1.01) | 3268 | 1.56 (1.17 to 2.05) | 986 | 0.67 (0.48 to 0.93) | 1894 | 0.66 (0.51 to 0.85) | 1601 | 0.79 (0.64 to 0.96) | 1408 | 0.90 (0.79 to 1.06) | 1351 |
| THR other | 0.87 (0.77 to 0.98) | 6898 | 1.60 (1.17 to 2.14) | 1720 | 0.61 (0.39 to 0.90) | 3829 | 0.95 (0.70 to 1.25) | 2824 | 0.60 (0.45 to 0.77) | 3287 | 0.80 (0.65 to 0.95) | 2870 |
| THR cemented | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Type of bearing | ||||||||||||
| MoM | 1.07 (0.95 to 1.21) | 3751 | 0.72 (0.45 to 1.08) | 5077 | 0.42 (0.21 to 0.72) | 6325 | 0.73 (0.49 to 1.02) | 4084 | 1.21 (0.93 to 1.54) | 2898 | 1.40 (1.21 to 1.69) | 2035 |
| CoP | 0.82 (0.71 to 0.95) | 10,206 | 1.08 (0.74 to 1.50) | 6672 | 0.61 (0.34 to 0.96) | 8286 | 0.97 (0.69 to 1.30) | 6081 | 0.72 (0.53 to 0.96) | 7474 | 0.70 (0.52 to 0.85) | 8093 |
| CoC | 0.76 (0.66 to 0.86) | 6054 | 1.21 (0.87 to 1.63) | 3171 | 0.78 (0.50 to 1.16) | 3893 | 0.64 (0.45 to 0.87) | 4268 | 0.77 (0.59 to 0.99) | 3791 | 0.60 (0.43 to 0.69) | 5446 |
| MoC/CoM | 1.88 (1.13 to 2.83) | 15,921 | 0.79 (0.02 to 2.95) | 12,309 | 1.11 (0.03 to 4.16) | 12,374 | 1.86 (0.38 to 4.55) | 11,499 | 1.45 (0.39 to 3.25) | 11,423 | 2.60 (1.26 to 4.38) | 10,362 |
| Other | 0.98 (0.74 to 1.25) | 16,134 | 1.81 (0.91 to 3.05) | 10,400 | 1.11 (0.36 to 2.33) | 11,201 | 1.33 (0.63 to 2.32) | 10,688 | 0.92 (0.46 to 1.56) | 11,505 | 0.80 (0.49 to 1.18) | 11,183 |
| MoP | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| General anaesthesia | 1.08 (0.99 to 1.18) | 5106 | 1.05 (0.83 to 1.30) | 3932 | 1.27 (0.95 to 1.67) | 4362 | 1.20 (0.97 to 1.48) | 3978 | 1.00 (0.84 to 1.18) | 4171 | 1.10 (0.97 to 1.25) | 3318 |
| Nerve block anaesthesia | 0.97 (0.85 to 1.10) | 13,240 | 1.14 (0.80 to 1.55) | 8668 | 1.15 (0.72 to 1.71) | 9899 | 0.81 (0.54 to 1.14) | 10,363 | 1.02 (0.77 to 1.32) | 9375 | 0.90 (0.76 to 1.13) | 9311 |
| Epidural anaesthesia | 0.96 (0.84 to 1.08) | 13,413 | 0.92 (0.61 to 1.31) | 9805 | 1.20 (0.72 to 1.82) | 9981 | 1.33 (0.94 to 1.80) | 9818 | 1.05 (0.78 to 1.35) | 9695 | 1.00 (0.80 to 1.16) | 9135 |
| Spinal anaesthesia | 0.93 (0.86 to 1.01) | 5365 | 0.88 (0.71 to 1.10) | 3698 | 0.77 (0.57 to 1.01) | 4611 | 0.95 (0.76 to 1.16) | 3771 | 0.96 (0.81 to 1.13) | 3556 | 0.90 (0.79 to 1.01) | 3953 |
| Thromboprophylaxis regime | ||||||||||||
| Not chemical | 0.96 (0.84 to 1.09) | 9422 | 0.85 (0.54 to 1.24) | 8245 | 1.17 (0.71 to 1.76) | 9633 | 1.25 (0.87 to 1.70) | 8786 | 1.31 (0.99 to 1.68) | 7667 | 1.00 (0.83 to 1.20) | 6879 |
| Chemical | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Acetabulum bone graft | 1.08 (0.89 to 1.29) | 16,221 | 0.75 (0.37 to 1.27) | 17,111 | 1.41 (0.69 to 2.40) | 16,596 | 1.50 (0.92 to 2.22) | 16,483 | 1.10 (0.71 to 1.57) | 16,893 | 1.00 (0.71 to 1.29) | 16,435 |
| Femur bone graft | 1.84 (1.29 to 2.50) | 16,220 | 2.35 (0.84 to 4.66) | 17,330 | 0.66 (0.02 to 2.47) | 18,866 | 2.09 (0.76 to 4.12) | 16,726 | 2.46 (1.21 to 4.17) | 16,903 | 1.50 (0.81 to 2.40) | 16,899 |
| Intraoperative event | 1.48 (1.06 to 1.98) | 17,144 | 1.93 (0.82 to 3.52) | 17,661 | 1.40 (0.29 to 3.40) | 18,255 | 2.08 (0.93 to 3.72) | 16,578 | 1.62 (0.80 to 2.73) | 17,208 | 1.00 (0.48 to 1.64) | 17,696 |
Appendix 8 Incidence rate ratios of revision for hip prosthetic joint infection and 95% credible intervals for health system characteristics as described in Risk factors for prosthetic hip or knee infection
| Characteristic | Total | ≤ 3 months | 3–6 months | 6–12 months | 12–24 months | ≥ 24 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | IRR (95% CrI) | ESS | |
| Place of surgery | ||||||||||||
| Wales | 1.04 (0.80 to 1.33) | 4126 | 1.07 (0.54 to 1.88) | 3496 | 0.91 (0.43 to 1.60) | 9274 | 0.76 (0.41 to 1.24) | 7665 | 1.37 (0.89 to 1.98) | 4454 | 1.03 (0.73 to 1.39) | 4541 |
| England | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Funding | ||||||||||||
| Independent | 0.88 (0.77 to 0.99) | 8853 | 0.94 (0.64 to 1.31) | 3396 | 1.10 (0.70 to 1.60) | 4391 | 1.19 (0.87 to 1.58) | 3711 | 0.83 (0.63 to 1.07) | 4023 | 0.81 (0.67 to 0.97) | 3396 |
| Unspecified | 0.98 (0.83 to 1.15) | 13,160 | 0.39 (0.15 to 0.75) | 5600 | 1.10 (0.49 to 1.98) | 5463 | 1.49 (0.91 to 2.24) | 5148 | 1.37 (0.95 to 1.88) | 4676 | 1.19 (0.95 to 1.46) | 4247 |
| NHS | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Grade of operating surgeon | ||||||||||||
| Other | 1.08 (0.96 to 1.20) | 11,347 | 1.03 (0.76 to 1.36) | 7753 | 1.10 (0.73 to 1.56) | 8728 | 1.18 (0.88 to 1.54) | 8516 | 1.25 (1.00 to 1.54) | 7636 | 1.02 (0.85 to 1.20) | 8148 |
| Consultant | Ref. | |||||||||||
| Consultant involved | ||||||||||||
| None involved | 1.04 (0.91 to 1.19) | 12,165 | 0.94 (0.64 to 1.32) | 8500 | 0.94 (0.55 to 1.46) | 10,075 | 1.02 (0.69 to 1.43) | 9460 | 1.25 (0.95 to 1.60) | 8457 | 1.07 (0.87 to 1.30) | 8431 |
| Assisting | 1.13 (0.95 to 1.33) | 14,681 | 1.19 (0.75 to 1.74) | 9795 | 1.38 (0.75 to 2.22) | 10,407 | 1.47 (0.95 to 2.11) | 10096 | 1.26 (0.90 to 1.71) | 9911 | 0.92 (0.68 to 1.20) | 10,403 |
| Operating | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Total volume: operating surgeona | ||||||||||||
| > 114 | 0.87 (0.77 to 0.98) | 5413 | 1.04 (0.75 to 1.39) | 3123 | 0.64 (0.41 to 0.94) | 4268 | 0.72 (0.53 to 0.97) | 3734 | 0.69 (0.54 to 0.87) | 3905 | 0.88 (0.73 to 1.05) | 3822 |
| 64–114 | 0.86 (0.77 to 0.96) | 5589 | 0.78 (0.56 to 1.04) | 3580 | 0.77 (0.51 to 1.11) | 4083 | 0.85 (0.63 to 1.11) | 3491 | 0.74 (0.59 to 0.92) | 3931 | 0.86 (0.73 to 1.02) | 4144 |
| 29–63 | 1.02 (0.92 to 1.13) | 5476 | 1.04 (0.78 to 1.35) | 3432 | 0.97 (0.67 to 1.37) | 3733 | 0.89 (0.67 to 1.16) | 3806 | 0.88 (0.71 to 1.08) | 3910 | 1.08 (0.93 to 1.25) | 3714 |
| ≤ 28 | Ref. | |||||||||||
| Total volume: surgeon in chargea | ||||||||||||
| > 148 | 1.00 (0.89 to 1.12) | 5163 | 1.13 (0.83 to 1.50) | 3130 | 0.69 (0.45 to 1.02) | 4259 | 1.01 (0.73 to 1.36) | 2856 | 0.75 (0.58 to 0.94) | 3688 | 1.01 (0.84 to 1.19) | 3698 |
| 85–148 | 0.93 (0.83 to 1.04) | 5403 | 0.85 (0.63 to 1.13) | 3652 | 0.72 (0.48 to 1.05) | 4226 | 1.13 (0.83 to 1.50) | 2717 | 0.92 (0.73 to 1.14) | 3438 | 0.83 (0.70 to 0.98) | 4222 |
| 42–84 | 0.97 (0.87 to 1.07) | 5748 | 0.84 (0.62 to 1.11) | 3843 | 0.95 (0.65 to 1.33) | 3833 | 1.18 (0.88 to 1.56) | 2801 | 0.83 (0.66 to 1.03) | 3688 | 0.96 (0.82 to 1.12) | 3974 |
| ≤ 41 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
| Total volume: hospitala | ||||||||||||
| > 406 | 1.28 (1.10 to 1.48) | 2830 | 1.91 (1.26 to 2.81) | 1301 | 1.19 (0.79 to 1.74) | 2845 | 1.01 (0.73 to 1.37) | 2716 | 0.88 (0.66 to 1.15) | 2516 | 1.12 (0.90 to 1.37) | 2737 |
| 257–406 | 1.03 (0.90 to 1.16) | 4096 | 1.57 (1.09 to 2.22) | 1516 | 0.90 (0.59 to 1.31) | 3361 | 0.74 (0.54 to 0.99) | 3665 | 0.86 (0.67 to 1.09) | 3052 | 0.89 (0.73 to 1.06) | 4217 |
| 144–256 | 1.01 (0.90 to 1.13) | 4592 | 1.23 (0.86 to 1.72) | 1916 | 0.76 (0.49 to 1.13) | 3604 | 0.72 (0.53 to 0.96) | 3951 | 0.95 (0.75 to 1.19) | 3252 | 1.01 (0.86 to 1.18) | 3784 |
| ≤ 143 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||
Appendix 9 Incidence rate ratios of revision for knee prosthetic joint infection and 95% credible intervals for patient characteristics as described in Risk factors for prosthetic hip or knee infection
| Characteristic | Total | ≤ 3 months | 3–6 months | 6–12 months | 12–24 months | ≥ 24 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | |
| Sex | ||||||||||||||||||
| Male | 1.83 (1.71 to 1.96) | < 0.0001 | < 0.0001 | 2.37 (1.81 to 3.06) | < 0.0001 | < 0.0001 | 2.17 (1.65 to 2.81) | < 0.0001 | < 0.0001 | 2.19 (1.86 to 2.57) | < 0.0001 | < 0.0001 | 1.85 (1.62 to 2.09) | < 0.0001 | < 0.0001 | 1.57 (1.42 to 1.74) | < 0.0001 | < 0.0001 |
| Female | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Age (years) | ||||||||||||||||||
| ≥ 80 | 0.50 (0.43 to 0.57) | < 0.0001 | < 0.0001 | 1.85 (1.14 to 2.85) | 0.01 | 0.05 | 0.64 (0.38 to 0.99) | 0.05 | 0.16 | 0.57 (0.40 to 0.77) | < 0.0001 | < 0.0001 | 0.48 (0.36 to 0.62) | < 0.0001 | < 0.0001 | 0.32 (0.25 to 0.40) | < 0.0001 | < 0.0001 |
| 70–79 | 0.65 (0.60 to 0.72) | < 0.0001 | < 0.0001 | 1.11 (0.73 to 1.63) | 0.69 | 0.87 | 0.70 (0.49 to 0.98) | 0.04 | 0.14 | 0.71 (0.56 to 0.88) | < 0.0001 | 0.01 | 0.75 (0.62 to 0.89) | < 0.0001 | 0.01 | 0.56 (0.49 to 0.64) | < 0.0001 | < 0.0001 |
| 60–69 | 0.76 (0.69 to 0.83) | < 0.0001 | < 0.0001 | 1.09 (0.72 to 1.59) | 0.76 | 0.90 | 0.61 (0.42 to 0.86) | 0.01 | 0.03 | 0.85 (0.68 to 1.05) | 0.13 | 0.31 | 0.87 (0.73 to 1.03) | 0.10 | 0.26 | 0.69 (0.60 to 0.78) | < 0.0001 | < 0.0001 |
| < 60 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Ethnicity | ||||||||||||||||||
| Black African origin | 1.67 (1.24 to 2.17) | < 0.0001 | < 0.0001 | 1.70 (0.35 to 4.20) | 0.58 | 0.79 | 2.05 (0.55 to 4.60) | 0.28 | 0.54 | 1.47 (0.63 to 2.69) | 0.38 | 0.63 | 1.79 (0.99 to 2.82) | 0.06 | 0.17 | 1.64 (1.02 to 2.41) | 0.03 | 0.12 |
| Other and mixed | 1.17 (0.84 to 1.57) | 0.36 | 0.61 | 1.43 (0.29 to 3.49) | 0.78 | 0.91 | 0.88 (0.10 to 2.48) | 0.62 | 0.81 | 1.59 (0.75 to 2.76) | 0.21 | 0.44 | 1.16 (0.57 to 1.96) | 0.74 | 0.89 | 0.98 (0.53 to 1.57) | 0.84 | 0.93 |
| South Asian | 1.17 (0.93 to 1.43) | 0.17 | 0.38 | 1.51 (0.59 to 2.90) | 0.41 | 0.65 | 1.89 (0.88 to 3.30) | 0.09 | 0.23 | 1.14 (0.64 to 1.78) | 0.72 | 0.88 | 1.33 (0.88 to 1.88) | 0.18 | 0.39 | 0.91 (0.61 to 1.26) | 0.54 | 0.75 |
| Unclear | 0.35 (0.23 to 0.48) | < 0.0001 | < 0.0001 | 0.20 (0.01 to 0.76) | 0.02 | 0.07 | 0.39 (0.05 to 1.09) | 0.13 | 0.31 | 0.39 (0.14 to 0.77) | 0.02 | 0.07 | 0.31 (0.12 to 0.58) | < 0.0001 | 0.01 | 0.35 (0.19 to 0.55) | < 0.0001 | < 0.0001 |
| White | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| BMI (kg/m2) | ||||||||||||||||||
| ≥ 30 | 1.46 (1.29 to 1.63) | < 0.0001 | < 0.0001 | 2.23 (1.35 to 3.60) | 0.02 | 0.09 | 1.34 (0.83 to 2.10) | 0.39 | 0.63 | 1.26 (0.96 to 1.64) | 0.12 | 0.31 | 1.52 (1.19 to 1.92) | < 0.0001 | 0.02 | 1.46 (1.22 to 1.73) | < 0.0001 | < 0.0001 |
| 25–29.9 | 1.30 (1.15 to 1.46) | < 0.0001 | < 0.0001 | 1.64 (0.96 to 2.67) | 0.25 | 0.49 | 1.54 (0.94 to 2.42) | 0.19 | 0.41 | 1.11 (0.84 to 1.46) | 0.53 | 0.75 | 1.39 (1.08 to 1.77) | 0.02 | 0.07 | 1.27 (1.05 to 1.53) | 0.02 | 0.09 |
| < 25 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| ASA grade | ||||||||||||||||||
| 3–5 | 1.84 (1.63 to 2.07) | < 0.0001 | < 0.0001 | 1.85 (1.11 to 2.95) | 0.02 | 0.08 | 1.89 (1.13 to 3.00) | 0.02 | 0.07 | 1.80 (1.34 to 2.39) | < 0.0001 | < 0.0001 | 1.71 (1.35 to 2.15) | < 0.0001 | < 0.0001 | 1.76 (1.46 to 2.11) | < 0.0001 | < 0.0001 |
| 2 | 1.23 (1.12 to 1.36) | < 0.0001 | < 0.0001 | 1.25 (0.81 to 1.89) | 0.36 | 0.61 | 1.29 (0.85 to 1.93) | 0.27 | 0.53 | 1.11 (0.87 to 1.41) | 0.43 | 0.67 | 1.11 (0.91 to 1.34) | 0.33 | 0.59 | 1.27 (1.10 to 1.47) | < 0.0001 | 0.01 |
| 1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Chronic pulmonary disease | 1.17 (1.05 to 1.29) | < 0.0001 | 0.02 | 1.33 (0.90 to 1.87) | 0.15 | 0.36 | 1.59 (1.10 to 2.19) | 0.01 | 0.05 | 1.04 (0.81 to 1.31) | 0.77 | 0.91 | 1.16 (0.96 to 1.39) | 0.13 | 0.31 | 1.03 (0.87 to 1.20) | 0.75 | 0.90 |
| Diabetes | 1.35 (1.22 to 1.48) | < 0.0001 | < 0.0001 | 0.99 (0.63 to 1.45) | 0.89 | 0.95 | 1.43 (0.96 to 2.01) | 0.07 | 0.21 | 1.27 (0.99 to 1.58) | 0.05 | 0.16 | 1.57 (1.31 to 1.86) | < 0.0001 | < 0.0001 | 1.17 (0.99 to 1.37) | 0.06 | 0.18 |
| Dementia | 1.35 (0.58 to 2.43) | 0.52 | 0.74 | 3.02 (0.36 to 8.52) | 0.30 | 0.56 | 3.39 (0.40 to 9.51) | 0.25 | 0.49 | 1.51 (0.31 to 3.64) | 0.72 | 0.88 | 0.54 (0.01 to 1.99) | 0.35 | 0.61 | |||
| Liver disease | 2.20 (1.60 to 2.90) | < 0.0001 | < 0.0001 | 1.36 (0.17 to 3.83) | 0.97 | 0.98 | 1.89 (0.38 to 4.59) | 0.48 | 0.70 | 2.09 (0.95 to 3.70) | 0.06 | 0.17 | 2.05 (1.08 to 3.31) | 0.02 | 0.08 | 2.32 (1.37 to 3.51) | < 0.0001 | 0.01 |
| Congestive heart failure | 1.34 (1.05 to 1.68) | 0.02 | 0.07 | 2.23 (1.04 to 3.90) | 0.03 | 0.10 | 1.21 (0.38 to 2.51) | 0.86 | 0.94 | 1.42 (0.79 to 2.25) | 0.23 | 0.47 | 1.15 (0.68 to 1.75) | 0.63 | 0.82 | 1.16 (0.73 to 1.69) | 0.56 | 0.77 |
| Rheumatologic disease | 1.47 (1.27 to 1.68) | < 0.0001 | < 0.0001 | 2.15 (1.26 to 3.31) | < 0.0001 | 0.02 | 1.95 (1.15 to 3.00) | 0.01 | 0.04 | 1.27 (0.87 to 1.75) | 0.22 | 0.44 | 1.33 (1.00 to 1.72) | 0.05 | 0.15 | 1.40 (1.13 to 1.72) | < 0.0001 | 0.01 |
| Cancer | ||||||||||||||||||
| Cancer | 0.90 (0.73 to 1.10) | 0.30 | 0.56 | 0.70 (0.25 to 1.39) | 0.31 | 0.56 | 0.76 (0.27 to 1.49) | 0.39 | 0.63 | 0.90 (0.54 to 1.36) | 0.58 | 0.79 | 0.80 (0.52 to 1.16) | 0.25 | 0.49 | 0.96 (0.68 to 1.30) | 0.76 | 0.91 |
| Metastatic | 1.64 (0.95 to 2.50) | 0.06 | 0.18 | 2.40 (0.29 to 6.77) | 0.46 | 0.70 | 3.60 (0.73 to 8.74) | 0.08 | 0.23 | 0.92 (0.11 to 2.55) | 0.66 | 0.84 | 1.24 (0.34 to 2.72) | 0.88 | 0.95 | 1.52 (0.56 to 2.95) | 0.44 | 0.68 |
| None | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Cerebrovascular disease | 1.08 (0.82 to 1.37) | 0.62 | 0.81 | 1.52 (0.60 to 2.88) | 0.39 | 0.63 | 1.63 (0.64 to 3.08) | 0.31 | 0.56 | 1.24 (0.68 to 1.98) | 0.51 | 0.74 | 0.84 (0.45 to 1.33) | 0.43 | 0.67 | 0.88 (0.53 to 1.33) | 0.52 | 0.74 |
| Myocardial infarction | 1.06 (0.86 to 1.29) | 0.58 | 0.79 | 1.17 (0.50 to 2.14) | 0.81 | 0.92 | 0.30 (0.04 to 0.83) | 0.06 | 0.18 | 1.09 (0.65 to 1.65) | 0.80 | 0.92 | 1.34 (0.91 to 1.85) | 0.13 | 0.31 | 0.95 (0.65 to 1.30) | 0.70 | 0.88 |
| Paraplegia and hemiplegia | 1.59 (0.96 to 2.37) | 0.06 | 0.18 | 3.59 (0.73 to 8.71) | 0.08 | 0.23 | 2.35 (0.28 to 6.56) | 0.47 | 0.70 | 1.78 (0.49 to 3.93) | 0.40 | 0.65 | 0.88 (0.18 to 2.13) | 0.63 | 0.82 | 1.45 (0.58 to 2.71) | 0.45 | 0.69 |
| Peptic ulcer disease | 1.15 (0.86 to 1.48) | 0.37 | 0.61 | 2.56 (1.09 to 4.70) | 0.02 | 0.08 | 0.63 (0.08 to 1.78) | 0.37 | 0.61 | 0.97 (0.42 to 1.76) | 0.80 | 0.92 | 1.44 (0.85 to 2.18) | 0.16 | 0.37 | 0.88 (0.51 to 1.35) | 0.52 | 0.74 |
| Peripheral vascular disease | 1.37 (1.08 to 1.71) | 0.01 | 0.04 | 0.87 (0.23 to 1.94) | 0.62 | 0.81 | 1.91 (0.82 to 3.50) | 0.12 | 0.29 | 1.15 (0.61 to 1.87) | 0.72 | 0.88 | 1.53 (0.98 to 2.20) | 0.06 | 0.16 | 1.22 (0.79 to 1.76) | 0.38 | 0.63 |
| Renal disease | 1.26 (1.00 to 1.55) | 0.04 | 0.14 | 1.23 (0.55 to 2.20) | 0.68 | 0.86 | 1.22 (0.52 to 2.24) | 0.73 | 0.89 | 1.13 (0.67 to 1.71) | 0.70 | 0.88 | 1.31 (0.87 to 1.84) | 0.18 | 0.40 | 0.90 (0.54 to 1.36) | 0.58 | 0.79 |
Appendix 10 Incidence rate ratios of revision for knee prosthetic joint infection and 95% credible intervals for surgical characteristics as described in Risk factors for prosthetic hip or knee infection
| Characteristic | Total | ≤ 3 months | 3–6 months | 6–12 months | 12–24 months | ≥ 24 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | |
| Osteoarthritis | 0.66 (0.56 to 0.77) | < 0.0001 | < 0.0001 | 0.61 (0.32 to 1.17) | 0.10 | 0.26 | 0.63 (0.33 to 1.17) | 0.11 | 0.28 | 0.58 (0.39 to 0.84) | < 0.0001 | 0.02 | 0.76 (0.54 to 1.07) | 0.10 | 0.26 | 0.68 (0.53 to 0.87) | < 0.0001 | 0.01 |
| Trauma | 1.94 (1.42 to 2.55) | < 0.0001 | < 0.0001 | 1.53 (0.18 to 4.33) | 0.85 | 0.93 | 1.26 (0.15 to 3.53) | 0.96 | 0.98 | 2.23 (1.01 to 3.94) | 0.03 | 0.12 | 2.16 (1.15 to 3.50) | 0.01 | 0.05 | 1.86 (1.13 to 2.78) | 0.01 | 0.04 |
| Previous knee infection | 4.86 (2.71 to 7.64) | < 0.0001 | < 0.0001 | 5.47 (0.14 to 20.34) | 0.38 | 0.63 | 9.71 (1.16 to 27.27) | 0.01 | 0.06 | 1.90 (0.05 to 7.04) | 0.96 | 0.98 | 5.00 (1.36 to 11.03) | 0.01 | 0.03 | 5.10 (2.04 to 9.52) | < 0.0001 | < 0.0001 |
| Avascular necrosis | 1.56 (0.97 to 2.33) | 0.06 | 0.17 | 1.15 (0.03 to 4.23) | 0.73 | 0.89 | 2.33 (0.28 to 6.51) | 0.48 | 0.70 | 3.15 (1.26 to 5.93) | 0.01 | 0.04 | 1.47 (0.48 to 3.02) | 0.55 | 0.77 | 1.03 (0.38 to 2.00) | 0.89 | 0.95 |
| Inflammatory arthropathy | 1.38 (1.15 to 1.65) | < 0.0001 | 0.01 | 2.04 (0.91 to 3.66) | 0.07 | 0.19 | 1.98 (0.93 to 3.45) | 0.06 | 0.18 | 1.24 (0.71 to 1.91) | 0.47 | 0.70 | 1.22 (0.79 to 1.74) | 0.37 | 0.61 | 1.43 (1.09 to 1.83) | 0.01 | 0.04 |
| Other indication | 1.36 (0.85 to 1.99) | 0.19 | 0.40 | 0.82 (0.02 to 3.06) | 0.54 | 0.75 | 1.45 (0.17 to 4.06) | 0.90 | 0.96 | 0.57 (0.07 to 1.60) | 0.31 | 0.56 | 1.18 (0.43 to 2.31) | 0.86 | 0.94 | 1.84 (0.94 to 3.05) | 0.06 | 0.18 |
| Surgical approach | ||||||||||||||||||
| Lateral parapatellar | 1.08 (0.79 to 1.42) | 0.64 | 0.83 | 1.66 (0.44 to 3.69) | 0.49 | 0.72 | 4.39 (2.16 to 7.45) | < 0.0001 | < 0.0001 | 1.32 (0.60 to 2.34) | 0.52 | 0.74 | 0.84 (0.38 to 1.48) | 0.51 | 0.74 | 0.68 (0.37 to 1.09) | 0.13 | 0.31 |
| Midvastus | 0.82 (0.64 to 1.02) | 0.08 | 0.23 | 0.93 (0.33 to 1.87) | 0.71 | 0.88 | 0.76 (0.24 to 1.58) | 0.42 | 0.66 | 0.45 (0.19 to 0.82) | 0.02 | 0.08 | 0.88 (0.56 to 1.28) | 0.48 | 0.70 | 0.86 (0.59 to 1.19) | 0.36 | 0.61 |
| Subvastus | 0.91 (0.67 to 1.19) | 0.47 | 0.70 | 0.82 (0.17 to 2.01) | 0.56 | 0.77 | 0.94 (0.19 to 2.28) | 0.71 | 0.88 | 1.35 (0.66 to 2.28) | 0.43 | 0.67 | 0.98 (0.52 to 1.59) | 0.82 | 0.93 | 0.78 (0.47 to 1.18) | 0.25 | 0.49 |
| Other | 1.01 (0.77 to 1.28) | 1.00 | 1.00 | 1.02 (0.32 to 2.17) | 0.85 | 0.93 | 1.16 (0.37 to 2.41) | 0.93 | 0.97 | 0.96 (0.47 to 1.63) | 0.77 | 0.91 | 0.98 (0.57 to 1.51) | 0.84 | 0.93 | 1.00 (0.66 to 1.43) | 0.92 | 0.97 |
| Medial parapatellar | Ref. | Ref. | Ref. | Ref. | Ref. | |||||||||||||
| Procedure | ||||||||||||||||||
| TKR uncemented | 0.71 (0.60 to 0.84) | < 0.0001 | < 0.0001 | 0.94 (0.45 to 1.63) | 0.72 | 0.88 | 1.56 (0.89 to 2.43) | 0.11 | 0.27 | 0.62 (0.37 to 0.92) | 0.03 | 0.10 | 0.86 (0.61 to 1.15) | 0.30 | 0.56 | 0.55 (0.41 to 0.71) | < 0.0001 | < 0.0001 |
| TKR other | 0.89 (0.66 to 1.16) | 0.38 | 0.63 | 1.15 (0.23 to 2.82) | 0.95 | 0.98 | 0.37 (0.01 to 1.38) | 0.22 | 0.45 | 1.30 (0.61 to 2.25) | 0.53 | 0.75 | 1.14 (0.63 to 1.81) | 0.72 | 0.88 | 0.81 (0.51 to 1.19) | 0.29 | 0.55 |
| Patellofemoral | 0.33 (0.19 to 0.50) | < 0.0001 | < 0.0001 | 0.35 (0.01 to 1.30) | 0.21 | 0.43 | 0.49 (0.13 to 1.09) | 0.12 | 0.29 | 0.15 (0.02 to 0.43) | 0.01 | 0.04 | 0.38 (0.18 to 0.68) | < 0.0001 | 0.02 | |||
| Unicondylar | 0.54 (0.46 to 0.61) | < 0.0001 | < 0.0001 | 0.53 (0.27 to 0.88) | 0.02 | 0.09 | 1.04 (0.63 to 1.55) | 0.97 | 0.98 | 0.42 (0.27 to 0.60) | < 0.0001 | < 0.0001 | 0.46 (0.33 to 0.61) | < 0.0001 | < 0.0001 | 0.56 (0.45 to 0.68) | < 0.0001 | < 0.0001 |
| TKR cemented | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Type of constraint | ||||||||||||||||||
| Unconstrained mobile | 1.02 (0.89 to 1.17) | 0.79 | 0.92 | 1.43 (0.82 to 2.24) | 0.20 | 0.42 | 1.29 (0.74 to 2.00) | 0.38 | 0.63 | 0.93 (0.65 to 1.27) | 0.63 | 0.82 | 1.16 (0.89 to 1.47) | 0.29 | 0.55 | 0.97 (0.79 to 1.17) | 0.71 | 0.88 |
| Posterior stabilised fixed | 1.38 (1.27 to 1.50) | < 0.0001 | < 0.0001 | 1.41 (1.01 to 1.90) | 0.04 | 0.14 | 1.34 (0.96 to 1.80) | 0.09 | 0.23 | 1.41 (1.16 to 1.69) | < 0.0001 | 0.01 | 1.32 (1.12 to 1.53) | < 0.0001 | 0.01 | 1.40 (1.24 to 1.59) | < 0.0001 | < 0.0001 |
| Posterior stabilised mobile | 0.90 (0.67 to 1.18) | 0.44 | 0.68 | 0.86 (0.17 to 2.14) | 0.60 | 0.81 | 0.91 (0.18 to 2.24) | 0.66 | 0.85 | 0.96 (0.43 to 1.71) | 0.78 | 0.91 | 0.85 (0.44 to 1.42) | 0.50 | 0.73 | 1.03 (0.67 to 1.48) | 0.96 | 0.98 |
| Constrained condylar | 3.50 (2.52 to 4.65) | < 0.0001 | < 0.0001 | 4.02 (1.07 to 9.02) | 0.02 | 0.09 | 3.30 (0.66 to 8.11) | 0.11 | 0.28 | 2.75 (1.10 to 5.20) | 0.02 | 0.08 | 3.60 (1.90 to 5.87) | < 0.0001 | < 0.0001 | 3.50 (1.99 to 5.46) | < 0.0001 | < 0.0001 |
| Fixed | 0.72 (0.56 to 0.92) | 0.01 | 0.04 | 1.06 (0.43 to 2.03) | 0.97 | 0.98 | 1.17 (0.46 to 2.25) | 0.85 | 0.93 | 0.69 (0.35 to 1.14) | 0.17 | 0.38 | 0.56 (0.30 to 0.90) | 0.03 | 0.10 | 0.70 (0.45 to 1.01) | 0.07 | 0.19 |
| Mobile | 0.57 (0.48 to 0.68) | < 0.0001 | < 0.0001 | 0.39 (0.12 to 0.83) | 0.03 | 0.12 | 1.10 (0.59 to 1.80) | 0.83 | 0.93 | 0.37 (0.20 to 0.59) | < 0.0001 | < 0.0001 | 0.50 (0.34 to 0.70) | < 0.0001 | < 0.0001 | 0.65 (0.50 to 0.81) | < 0.0001 | < 0.0001 |
| Undetermined | 1.11 (0.90 to 1.33) | 0.34 | 0.59 | 1.54 (0.65 to 2.86) | 0.34 | 0.59 | 1.64 (0.73 to 2.96) | 0.23 | 0.46 | 1.02 (0.57 to 1.60) | 0.95 | 0.98 | 1.21 (0.80 to 1.70) | 0.37 | 0.62 | 0.98 (0.71 to 1.30) | 0.85 | 0.93 |
| Unconstrained fixed | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| General anaesthesia | 1.11 (1.04 to 1.20) | < 0.0001 | 0.02 | 1.05 (0.79 to 1.36) | 0.79 | 0.92 | 1.27 (0.96 to 1.63) | 0.09 | 0.24 | 1.19 (1.00 to 1.40) | 0.05 | 0.16 | 1.12 (0.98 to 1.28) | 0.09 | 0.24 | 1.13 (1.01 to 1.25) | 0.03 | 0.11 |
| Nerve block anaesthesia | 1.02 (0.94 to 1.11) | 0.60 | 0.81 | 1.23 (0.87 to 1.67) | 0.24 | 0.48 | 1.00 (0.70 to 1.37) | 0.94 | 0.98 | 1.03 (0.84 to 1.26) | 0.78 | 0.91 | 1.02 (0.87 to 1.20) | 0.80 | 0.92 | 1.00 (0.88 to 1.14) | 0.97 | 0.98 |
| Epidural anaesthesia | 0.89 (0.79 to 0.99) | 0.04 | 0.14 | 0.55 (0.28 to 0.92) | 0.03 | 0.12 | 1.06 (0.64 to 1.59) | 0.90 | 0.96 | 0.82 (0.58 to 1.09) | 0.18 | 0.39 | 1.03 (0.82 to 1.27) | 0.83 | 0.93 | 0.99 (0.84 to 1.16) | 0.88 | 0.95 |
| Spinal anaesthesia | 0.90 (0.84 to 0.97) | < 0.0001 | 0.02 | 1.06 (0.80 to 1.37) | 0.75 | 0.90 | 0.77 (0.59 to 0.99) | 0.04 | 0.14 | 0.84 (0.71 to 0.99) | 0.04 | 0.14 | 0.89 (0.78 to 1.02) | 0.09 | 0.24 | 0.88 (0.79 to 0.97) | 0.01 | 0.06 |
| Thromboprophylaxis regimen | ||||||||||||||||||
| Not chemical | 0.88 (0.78 to 0.98) | 0.02 | 0.09 | 1.00 (0.62 to 1.49) | 0.92 | 0.97 | 0.96 (0.59 to 1.42) | 0.75 | 0.90 | 1.02 (0.76 to 1.32) | 0.95 | 0.98 | 0.96 (0.77 to 1.19) | 0.70 | 0.88 | 0.93 (0.79 to 1.08) | 0.33 | 0.59 |
| Chemical | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Tibial bone graft | 1.95 (1.31 to 2.71) | < 0.0001 | < 0.0001 | 2.92 (0.60 to 7.11) | 0.16 | 0.37 | 0.96 (0.02 to 3.59) | 0.63 | 0.82 | 1.50 (0.41 to 3.28) | 0.61 | 0.81 | 3.22 (1.72 to 5.21) | < 0.0001 | < 0.0001 | 1.35 (0.62 to 2.38) | 0.47 | 0.70 |
| Femoral bone graft | 1.56 (1.00 to 2.25) | 0.04 | 0.14 | 1.51 (0.18 to 4.25) | 0.86 | 0.94 | 0.87 (0.18 to 2.11) | 0.62 | 0.81 | 1.79 (0.81 to 3.15) | 0.13 | 0.31 | 1.82 (0.87 to 3.11) | 0.09 | 0.25 | |||
| Intraoperative event | 1.19 (0.75 to 1.73) | 0.48 | 0.70 | 2.40 (0.48 to 5.93) | 0.28 | 0.54 | 1.14 (0.31 to 2.53) | 1.00 | 1.00 | 1.46 (0.63 to 2.67) | 0.39 | 0.63 | 0.98 (0.42 to 1.77) | 0.82 | 0.93 | |||
Appendix 11 Incidence rate ratios of revision for knee prosthetic joint infection and 95% credible intervals for health system characteristics as described in Risk factors for prosthetic hip or knee infection
| Characteristic | Total | ≤ 3 months | 3–6 months | 6–12 months | 12–24 months | ≥ 24 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | IRR (95% CrI) | p-value | Adjusted p-value | |
| Place of surgery | ||||||||||||||||||
| Wales | 0.88 (0.70 to 1.08) | 0.21 | 0.43 | 1.22 (0.59 to 2.18) | 0.67 | 0.85 | 0.77 (0.37 to 1.34) | 0.34 | 0.59 | 0.66 (0.41 to 0.99) | 0.05 | 0.16 | 0.80 (0.56 to 1.10) | 0.17 | 0.38 | 1.00 (0.77 to 1.29) | 0.98 | 0.99 |
| England | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Funding | ||||||||||||||||||
| Independent | 0.85 (0.75 to 0.95) | 0.01 | 0.03 | 1.12 (0.71 to 1.64) | 0.67 | 0.86 | 1.36 (0.91 to 1.92) | 0.13 | 0.31 | 1.14 (0.87 to 1.46) | 0.34 | 0.59 | 0.76 (0.59 to 0.96) | 0.02 | 0.09 | 0.71 (0.59 to 0.85) | < 0.0001 | < 0.0001 |
| Unspecified | 0.84 (0.72 to 0.97) | 0.02 | 0.09 | 1.04 (0.48 to 1.84) | 0.96 | 0.98 | 0.81 (0.34 to 1.49) | 0.46 | 0.70 | 1.00 (0.64 to 1.44) | 0.92 | 0.97 | 1.03 (0.74 to 1.38) | 0.90 | 0.96 | 0.90 (0.73 to 1.09) | 0.29 | 0.55 |
| NHS | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Grade operating surgeon | ||||||||||||||||||
| Other | 0.99 (0.90 to 1.08) | 0.79 | 0.92 | 0.84 (0.55 to 1.19) | 0.32 | 0.57 | 1.39 (0.99 to 1.88) | 0.05 | 0.16 | 1.01 (0.80 to 1.25) | 0.95 | 0.98 | 1.14 (0.95 to 1.34) | 0.16 | 0.36 | 0.98 (0.85 to 1.12) | 0.78 | 0.91 |
| Consultant | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Consultant involved | ||||||||||||||||||
| None involved | 0.96 (0.86 to 1.07) | 0.47 | 0.70 | 0.67 (0.38 to 1.07) | 0.11 | 0.27 | 1.33 (0.86 to 1.92) | 0.20 | 0.41 | 1.08 (0.81 to 1.39) | 0.62 | 0.81 | 1.14 (0.92 to 1.39) | 0.22 | 0.44 | 0.96 (0.81 to 1.12) | 0.56 | 0.77 |
| Assisting | 1.04 (0.90 to 1.19) | 0.61 | 0.81 | 1.11 (0.61 to 1.76) | 0.81 | 0.92 | 1.49 (0.88 to 2.30) | 0.13 | 0.31 | 0.90 (0.61 to 1.26) | 0.52 | 0.74 | 1.12 (0.85 to 1.44) | 0.44 | 0.68 | 1.04 (0.83 to 1.27) | 0.77 | 0.91 |
| Operating | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Total volume of operating surgeona | ||||||||||||||||||
| > 85 | 0.95 (0.86 to 1.04) | 0.26 | 0.51 | 1.24 (0.83 to 1.78) | 0.31 | 0.57 | 0.91 (0.61 to 1.29) | 0.54 | 0.75 | 0.90 (0.72 to 1.12) | 0.34 | 0.59 | 0.74 (0.61 to 0.88) | < 0.0001 | 0.01 | 0.86 (0.73 to 0.99) | 0.04 | 0.14 |
| 51–85 | 0.96 (0.87 to 1.05) | 0.34 | 0.59 | 1.23 (0.83 to 1.75) | 0.33 | 0.59 | 0.86 (0.58 to 1.22) | 0.37 | 0.61 | 0.99 (0.79 to 1.22) | 0.85 | 0.93 | 0.78 (0.65 to 0.93) | 0.01 | 0.03 | 0.88 (0.76 to 1.00) | 0.06 | 0.18 |
| 26–50 | 1.09 (1.00 to 1.19) | 0.05 | 0.16 | 1.25 (0.85 to 1.78) | 0.28 | 0.54 | 1.18 (0.82 to 1.65) | 0.41 | 0.65 | 0.81 (0.64 to 1.01) | 0.06 | 0.19 | 1.00 (0.85 to 1.19) | 0.99 | 1.00 | 1.14 (1.01 to 1.29) | 0.04 | 0.14 |
| ≤ 25 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Total volume of surgeon in chargea | ||||||||||||||||||
| > 110 | 0.96 (0.87 to 1.06) | 0.46 | 0.70 | 1.21 (0.82 to 1.74) | 0.37 | 0.61 | 0.88 (0.60 to 1.25) | 0.45 | 0.69 | 0.94 (0.75 to 1.18) | 0.57 | 0.79 | 0.78 (0.64 to 0.93) | 0.01 | 0.03 | 0.88 (0.75 to 1.02) | 0.08 | 0.23 |
| 71–110 | 0.99 (0.90 to 1.09) | 0.84 | 0.93 | 1.13 (0.76 to 1.63) | 0.58 | 0.79 | 0.98 (0.67 to 1.39) | 0.84 | 0.93 | 0.99 (0.79 to 1.23) | 0.88 | 0.95 | 0.89 (0.74 to 1.05) | 0.17 | 0.38 | 0.86 (0.74 to 0.99) | 0.03 | 0.12 |
| 39–70 | 1.02 (0.94 to 1.11) | 0.64 | 0.83 | 1.29 (0.89 to 1.82) | 0.19 | 0.41 | 1.04 (0.72 to 1.46) | 0.88 | 0.95 | 0.90 (0.72 to 1.12) | 0.34 | 0.59 | 0.86 (0.72 to 1.01) | 0.07 | 0.19 | 1.04 (0.92 to 1.18) | 0.52 | 0.74 |
| ≤ 38 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
| Total volume of hospitala | ||||||||||||||||||
| > 440 | 1.25 (1.10 to 1.42) | < 0.0001 | 0.01 | 2.09 (1.31 to 3.18) | < 0.0001 | 0.01 | 0.95 (0.63 to 1.37) | 0.71 | 0.88 | 1.03 (0.79 to 1.32) | 0.87 | 0.94 | 1.00 (0.80 to 1.23) | 0.94 | 0.98 | 1.00 (0.84 to 1.19) | 0.97 | 0.98 |
| 286–440 | 1.24 (1.11 to 1.37) | < 0.0001 | < 0.0001 | 1.28 (0.82 to 1.90) | 0.30 | 0.56 | 0.98 (0.66 to 1.40) | 0.84 | 0.93 | 1.01 (0.79 to 1.27) | 1.00 | 1.00 | 1.16 (0.95 to 1.40) | 0.15 | 0.35 | 1.11 (0.96 to 1.29) | 0.17 | 0.38 |
| 151–285 | 1.15 (1.04 to 1.26) | 0.01 | 0.03 | 1.21 (0.79 to 1.77) | 0.42 | 0.66 | 0.98 (0.67 to 1.39) | 0.83 | 0.93 | 0.96 (0.76 to 1.20) | 0.68 | 0.86 | 1.14 (0.94 to 1.36) | 0.19 | 0.40 | 1.10 (0.96 to 1.25) | 0.20 | 0.41 |
| ≤ 150 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||||||||||||
Appendix 12 Literature search terms for use of alpha-defensin and leucocyte in the diagnosis of prosthetic joint infections as applied in MEDLINE on 30 May 2015 and described in Diagnosis of prosthetic joint infection: assessment of new methods
-
neutrophil antimicrobial peptide.mp. or alpha-defensins/
-
alpha defensin.mp. or alpha-defensins/
-
alpha-defensins/or peptide neutrophil antimicrobial.mp.
-
beta-defensins/or defensin.mp. or alpha-defensins/or defensins/
-
Arthroplasty, replacement/or knee joint/or arthroplasty, replacement, knee/or arthroplasty, replacement, hip/or hip prosthesis/or joint replacement.mp. or joint prosthesis/
-
Arthroplasty, replacement, knee/or arthroplasty, replacement, elbow/or arthroplasty, subchondral/or arthroplasty, replacement, ankle/or arthroplasty.mp. or arthroplasty, replacement, finger/or arthroplasty, replacement/or arthroplasty/or arthroplasty, replacement, hip/
-
Bacterial infections/or prosthesis-related infections/or prosthetic joint infection.mp. or surgical wound infection/
-
5 or 6 or 7
-
1 or 2 or 3 or 4
-
8 and 9
-
leukocyte esterase.mp.
-
leucocyte esterase.mp.
-
11 or 12
-
8 and 13
-
10 or 14
Appendix 13 Mortality and re-revision following single-stage and two-stage revision surgery for the management of infected primary hip or knee replacement in England and Wales as summarised in Surgical treatment of prosthetic joint infection: evidence from cohort studies
Introduction
We aimed to compare risk of re-revision and mortality after single- or two-stage revision procedures for treatment of hip or knee prosthetic joint infection in England and Wales.
Methods
Study design and data sources
Data for England and Wales from the NJR collected between 1 April 2003 and 31 December 2014 were linked with ONS data to provide the dates of death of patients who had died following single- or two-stage revision surgery. Patient consent was obtained for data collection and linkage by the NJR. According to the NHS Health Research Authority, separate consent and ethics approval were not required for this study.
Procedures and outcomes
We included patients with a primary hip or knee replacement that had subsequently been revised for prosthetic joint infection with a single- or two-stage procedure. NJR component-level data were used to identify the precise type of revision procedure. We considered all-cause re-revision and re-revision specifically for prosthetic joint infection.
In the mortality analysis, we compared primary hip or knee replacements with no revision, those revised for a non-septic indication and those revised for a prosthetic joint infection.
Age, sex and ASA grade at the time of the first revision for prosthetic joint infection were recorded.
Statistical methods
We compared re-revisions experienced by patients with infected primary hip or knee replacement treated with an initial single- or two-stage revision procedure using zero-truncated Poisson. Patients were followed up from the date of their first single-stage or stage one of a two-stage procedure until 31 December 2014, the date of their death or the date of a re-revision. A Kaplan–Meier failure function was produced to assess the cumulative re-revision incidence by study group. We used a Cox shared frailty model to compute the overall hazard ratio (HR) of re-revision for the first two years following single-stage revision performed for prosthetic joint infection (two-stage used as the reference). These time-averaged HRs were supplemented with time-dependent HRs to capture time-specific disparities between revision procedures. We used Poisson regression (time at risk modelled as an offset) adjusted for age, sex and ASA grade and modelled the baseline hazard function with restricted cubic splines. We used a similar approach to compare the incidence of re-revision for prosthetic joint infection (restricted cubic splines Poisson model with two degrees of freedom) and the risk of mortality (restricted cubic splines Poisson model with three degrees of freedom) revision procedure types.
We also compared the mortality rates of patients with unrevised primary procedures and patients with primary procedures revised for a non-septic indication with the mortality rates of patients revised for prosthetic joint infection.
Results: hip revision procedures
Between 2003 and 2014, 2140 primary hip replacements were revised for prosthetic joint infection: 535 with a single-stage procedure and 1605 with a two-stage procedure. Patients revised with single-stage procedure were on average older (68 years vs. 66 years) and less likely to be male (51% vs. 55%) and to have an ASA grade of > 2 (26% vs. 29%) than patients revised with a two-stage procedure.
Number of revision surgeries performed
The two-stage group underwent more operations than those managed with a single-stage procedure (mean number of procedures 2.2 vs. 1.3). Among patients receiving a single-stage revision, 16% required more than one procedure, with 8% re-revised three to five times. In patients receiving two-stage revision, 13% required more than two procedures with 5% re-revised four to nine times.
All-cause re-revision
Of the 2140 primary hip replacements revised for prosthetic joint infection, 311 underwent re-revision for any cause. The adjusted risk of re-revision in the first two postoperative years was higher following single-stage revision than two-stage revision (HR 1.54, 95% CI 1.15 to 2.07; p = 0.004).
Re-revision for prosthetic joint infection
Of the re-revisions, 187 (60%) were performed for an indication of prosthetic joint infection. The adjusted risk of re-revision for prosthetic joint infection in the first two postoperative years was higher following single-stage revision than two-stage revision (HR 1.70, 95% CI 1.19 to 2.44; p = 0.004). The increased incidence of re-revision for prosthetic joint infection after single-stage revision was apparent mainly in the first 3 months after the revision operation.
Mortality
Three-hundred and four patients who received revision surgery for hip prosthetic joint infection died. The adjusted risk of mortality in the first 2 years was comparable between single-stage and two-stage revision (HR 1.05, 95% CI 0.68 to 1.62; p = 0.814). No time-specific difference was noted.
Compared with patients who had undergone a primary hip replacement, mortality at 2 years was higher following both single- and two-stage revision for prosthetic joint infection (HR 1.40, 95% CI 0.95 to 2.06, p = 0.085, and HR 1.48, 95% CI 1.22 to 1.76, p < 0.001, respectively).
Compared with patients who had undergone a revision for a non-septic indication, mortality at 2 years was not different following single-stage revision for prosthetic joint infection (HR 1.30, 95% CI 0.88 to 1.94; p = 0.187) but was higher following two-stage revision (HR 1.37, 95% CI 1.11 to 1.70; p = 0.003).
Results: knee revision procedures
Between 2003 and 2014, 3369 primary knee replacements were revised for prosthetic joint infection. Of these, 489 were treated with a single-stage procedure and 2377 with both stages of a two-stage procedure. A further 503 patients received only the first stage of a planned two-stage procedure.
Number of revision surgeries performed
The two-stage group underwent more operations than those managed with a single-stage procedure (mean number of procedures 2.2 vs. 1.2). Among patients receiving a single-stage revision, 14.3% required more than one revision procedure, with 7.5% re-revised three to five times. In patients receiving two-stage revision, 11.5% required more than two procedures, with 6% re-revised four to eight times.
All-cause re-revision
Of the 3369 primary knee replacements revised for prosthetic joint infection, 397 subsequently underwent re-revision for any cause. The analysis provided weak evidence that the risk of re-revision was higher in the first two postoperative years after single-stage than after two-stage revision (HR 1.32, 95% CI 0.97 to 1.81; p = 0.08) and this was mainly apparent in the first 3 months postoperatively (HR 1.51, 95% CI 0.95 to 2.41; p = 0.08).
Re-revision for prosthetic joint infection
Of the re-revisions, 291 (73%) were performed for an indication of prosthetic joint infection. The adjusted risk of re-revision for prosthetic joint infection in the first two postoperative years was similar between single- and two-stage revision (HR 1.09, 95% CI 0.76 to 1.57; p = 0.66).
Mortality
Three-hundred and sixty-nine patients who received revision surgery for knee prosthetic joint infection died. The adjusted risk of mortality in the first 2 years was comparable between single-stage and two-stage revision (HR 1.21, 95% CI 0.72 to 2.03; p = 0.47). There was evidence that mortality was lower between 6 and 18 months in patients who had undergone a single-stage revision.
Compared with patients who had undergone a primary knee replacement, mortality at 2 years was similar following single-stage revision for prosthetic joint infection (HR 1.37, 95% CI 0.84 to 2.23; p = 0.21) but higher following two-stage revision (HR 1.66, 95% CI 1.40 to 1.96; p < 0.001).
Compared with patients who had undergone a revision for a non-septic indication, mortality at 2 years was similar following single-stage revision for prosthetic joint infection (HR 1.40, 95% CI 0.84 to 2.31; p = 0.19) but higher for those who underwent a two-stage revision (HR 1.69, 95% CI 1.38 to 2.08; p = 0.001).
Discussion
Our study of over 2000 revisions for the management of infected primary hip replacements shows that single-stage revision is associated with a higher risk of unplanned re-revision for both all-cause and specifically for further prosthetic joint infection when compared with two-stage revision. This is particularly marked in the earlier post-operative period.
In the management of knee prosthetic joint infection with over 3000 patients studied, rates of re-revision for all causes and specifically for reinfection were similar after single- and two-stage revision procedures.
Compared with patients receiving two-stage revision, those with single-stage revision for the treatment of hip and knee prosthetic joint infection received an average of 41% and 45% fewer operations respectively.
Although mortality was higher than after primary hip replacement or aseptic revision, mortality rates were comparable between single- and two-stage revision treatments of hip prosthetic joint infection. After revision of knee prosthetic joint infection, there was a higher mortality in patients who received a two-stage revision compared with those receiving a primary hip replacement or aseptic revision. There was no difference in mortality after single-stage revision compared with primary hip replacement or aseptic revision. Overall, mortality after single- and two-stage revision for knee prosthetic joint infection was similar.
To our knowledge, this is the largest study to compare the incidence of re-revision after single-stage and two-stage revision for prosthetic joint infection. We used a standardised data collection process and adjustment approach, examining component-level data to precisely define and group comparable procedures. Only procedures in which an implant is added, removed or modified are recorded in the NJR. Thus, we were unable to explore the risks for prosthetic joint infection treated with antibiotics or incision and drainage alone, but the reoperation outcomes are substantially worse for this strategy. The NJR does not capture data on the presence of a sinus or the microorganism causing the prosthetic joint infection and there may be selection of patients with easier to treat infections for a particular revision strategy.
Our research shows some advantage for a two-stage strategy over the single-stage revision with regard to preventing re-revision. However, with a two-stage strategy, the treatment burden for patients and families due to the greater number of surgeries, complications associated with the interim period and prolonged periods of immobility is considerable.
Appendix 14 Literature search terms for longitudinal studies of debridement, antibiotics and implant retention as applied in MEDLINE on 30 September 2017 and described in Surgical treatment of prosthetic joint infection: evidence from cohort studies
-
peri-prosthetic joint infection.mp. (11)
-
exp Prosthesis-Related Infections/1416, 1377, 1346, 1390, 1398 (10,156)
-
prosthetic joint infection.mp. (790)
-
peri-prosthetic infection.mp. (45)
-
deep infection.mp. (2634)
-
exp Wound Infection/1416, 1377, 1346, 1390, 1398 (42,315)
-
exp Sepsis/1416, 1377, 1346, 1390, 1398 (108,817)
-
exp Surgical Wound Infection/1416, 1377, 1346, 1390, 1398 (32,495)
-
DAIR.mp. (187)
-
exp Debridement/1416, 1377, 1346, 1390, 1398 (14,239)
-
implant retention.mp. (148)
-
exp Arthroplasty, Replacement/1416, 1377, 1346, 1390, 1398 (43,299)
-
exp Arthroplasty/1416, 1377, 1346, 1390, 1398 (54,112)
-
exp Arthroplasty, Replacement, Hip/1416, 1377, 1346, 1390, 1398 (21,936)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (160,263)
-
9 or 10 or 11 (14,507)
-
12 or 13 or 14 (54,112)
-
15 and 16 and 17 (356)
-
limit 18 to humans (353)
Appendix 15 Literature search terms for longitudinal studies of hip prosthetic joint infection as applied in MEDLINE in March 2015 and described in Surgical treatment of prosthetic joint infection: evidence from cohort studies
-
exp Prosthesis-Related Infections/or prosthesis-related infection*.mp. or exp Sepsis/ (101,971)
-
exp Infection/or exp Wound Infection/or exp Surgical Wound Infection/or infection*.mp. (1,603,015)
-
wound infection.mp. or exp Wound Infection/ (44,687)
-
arthroplasty.mp. or exp Arthroplasty, Replacement/or exp Arthroplasty/or exp Arthroplasty, Replacement, Hip/ (52,003)
-
exp Arthroplasty, Replacement, Hip/or exp Arthroplasty, Replacement/or Replacement.mp. (204,405)
-
exp Hip/or exp Arthroplasty, Replacement, Hip/or hip.mp. (107,123)
-
exp Hip Prosthesis/or exp Arthroplasty, Replacement, Hip/or hip replacement.mp. (31,655)
-
exp Hip Prosthesis/or total hip.mp. or exp Arthroplasty, Replacement, Hip/ (34,732)
-
hip arthroplasty.mp. (12,045)
-
total hip replacement.mp. or exp Arthroplasty, Replacement, Hip/ (20,706)
-
exp Arthroplasty, Replacement, Hip/or total hip arthroplasty.mp. (20,637)
-
exp Arthroplasty, Replacement, Hip/or exp Hip Prosthesis/or hip prosthes*.mp. (30,397)
-
1-stage.mp. (1576)
-
2-stage.mp. (2828)
-
one stage.mp. (8705)
-
two stage.mp. (15,977)
-
one-stage.mp. (8705)
-
two-stage.mp. (15,977)
-
single stage.mp. (4349)
-
single-stage.mp. (4349)
-
prosthesis exchange.mp. (15)
-
direct exchange.mp. (153)
-
direct-exchange.mp. (153)
-
Arthroplasty, Replacement, Hip/or revision arthroplasty.mp. (18,017)
-
exp Arthroplasty, Replacement, Hip/or staged revision.mp. (17,490)
-
reoperation.mp. or exp Reoperation/ (77,278)
-
reimplantation.mp. or exp Replantation/ (10,449)
-
1 or 2 or 3 (1,606,087)
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 (295,528)
-
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 (129,343)
-
28 and 29 and 30 (5795)
Appendix 16 Literature search terms for longitudinal studies of knee prosthetic joint infection as applied in MEDLINE on 31 August 2015 and described in Surgical treatment of prosthetic joint infection: evidence from cohort studies
-
exp Prosthesis-Related Infections/or prosthesis-related infection*.mp. (8340)
-
exp Infection/or infection.mp. (1,209,021)
-
wound infection.mp. or exp Wound Infection/ (44,719)
-
exp Surgical Wound Infection/or surgical infection.mp. (29,431)
-
exp Sepsis/or sepsis.mp. (129,398)
-
1-stage.mp. (1578)
-
2-stage.mp. (2833)
-
one stage.mp. (8710)
-
two stage.mp. (15,993)
-
one-stage.mp. (8710)
-
two-stage.mp. (15,993)
-
single stage.mp. (4354)
-
single-stage.mp. (4354)
-
exchange.mp. (231,326)
-
exp Prosthesis-Related Infections/or direct exchange.mp. (8465)
-
direct-exchange.mp. (153)
-
revision arthroplasty.mp. or exp Knee Prosthesis/ (9737)
-
staged revision.mp. (41)
-
reoperation.mp. or exp Reoperation/ (77,363)
-
reimplantation.mp. or exp Replantation/ (10,457)
-
reimplant*.mp. (6348)
-
1 or 2 or 3 or 4 or 5 (1,235,959)
-
6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (359,475)
-
arthroplasty.mp. or exp Arthroplasty, Replacement, Knee/or exp Arthroplasty, Replacement/or exp Arthroplasty/ (52,051)
-
exp Arthroplasty, Replacement/or Replacement.mp. or exp Arthroplasty, Replacement, Knee/ (204,563)
-
exp Knee/or exp Arthroplasty, Replacement, Knee/or knee.mp. (105,895)
-
exp Knee Joint/or exp Arthroplasty, Replacement, Knee/or exp Joint Prosthesis/or knee replacement.mp. or exp Knee Prosthesis/ (83,294)
-
exp Joint Prosthesis/or exp Knee Prosthesis/or exp Arthroplasty, Replacement, Knee/or Total knee.mp. or exp Knee Joint/ (83,497)
-
Knee arthroplasty.mp. or exp Arthroplasty, Replacement, Knee/ (16,575)
-
Knee prosthesis.mp. or exp Knee Prosthesis/ (9392)
-
Total knee replacement.mp. or exp Arthroplasty, Replacement, Knee/ (14,877)
-
exp Arthroplasty/or exp Knee Joint/or exp Arthroplasty, Replacement, Knee/or Total knee arthroplasty.mp. or exp Knee Prosthesis/ (83,930)
-
24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 (312,674)
-
22 and 23 and 33 (7306)
-
limit 34 to humans (7197)
Appendix 17 Surgical treatment of prosthetic joint infection: the INFORM randomised controlled trial as summarised in Surgical treatment of prosthetic joint infection: the INFORM randomised controlled trial
Aims
Our aim was to determine whether or not there is a difference in patient-reported outcome measures 18 months after randomisation to single- or two-stage revision surgery for the treatment of hip prosthetic joint infection. Patients deemed to have infected hip replacements by their treating surgeons and multidisciplinary prosthetic joint infection teams were randomised to either single- or two-stage revision. Although the study was not powered to compare rates of reinfection or occurrence of new infection, adverse events including infection were monitored.
Methods
The INFORM trial was a multicentre, two-arm, parallel-group, participant and observer unblinded, randomised, superiority trial with 1 : 1 treatment allocation (ISRCTN10956306). Full details of the study design, methods and statistical analysis, along with the associated cost-effectiveness and qualitative studies are available in the published protocol. 97
Trial oversight
The protocol was approved by the UK National Research Ethics Committee South West and the University of Gothenburg review board. The study was organised and co-ordinated by the Musculoskeletal Research Unit, University of Bristol, UK. An Independent Steering Committee and a Data Monitoring Committee oversaw the study.
Patients
A total of 140 patients were recruited from March 2015 to September 2018 from 15 secondary care orthopaedic units (11 in England, one in Wales and three in Sweden). Eligible patients were aged ≥ 18 years, had a clinical diagnosis of prosthetic hip joint infection and required treatment with single- or two-stage revision surgery.
Randomisation
Randomisation was conducted independently by the Bristol Randomised Trials Collaboration, a UK Clinical Research Collaboration-registered Clinical Trials Unit hosted by the University of Bristol. Randomisation was by secure remote third party, either via an internet-based application or by telephone. Randomisation was carried out as close to the time of surgery as possible, and no more than 12 weeks before surgery. It could not be carried out on the day of surgery due to the need to order equipment and for logistical planning in advance of surgery for these patients.
Allocation concealment was ensured by not releasing the treatment allocation until the patient had been recruited into the trial and all baseline measurements completed. Eligible participants who consented to the study were registered on the central trial database, were issued with a unique study identification number and had their baseline measures collected before the treatment allocation was generated, thereby ensuring that judgments about eligibility were made without knowledge of the next allocation.
Patients were randomly allocated in a 1 : 1 ratio to one of the intervention groups. Randomisation within blocks of randomly varying size (four or six) was stratified by hospital.
Interventions
Owing to the nature of the intervention and planning requirements, surgeons and patients were made aware of the assigned treatment shortly after the baseline assessment and randomisation had been carried out. Patients were assigned to either single- or two-stage revision surgery.
All other aspects of treatment, including antibiotics, analgesia, investigations, implants, static or articulating spacers, and surgical techniques and approach, were according to the treating surgeon’s usual practice and in line with local policies and procedures. Usual clinical care continued throughout the study follow-up period.
Outcomes
Research assessments were carried out preoperatively and every 3 months until 18 months post randomisation. Data were collected from patient questionnaires and clinical performance tests and extracted from medical records.
The primary outcome measure was the WOMAC total score, measured at 18 months post randomisation. Eighteen months was chosen as the timing of the primary outcome as maximum recovery from all surgeries was expected to have been achieved and further health improvements were thought unlikely. The WOMAC index is a patient-reported outcome questionnaire divided into three subscales consisting of five pain, two stiffness and 17 physical function items. Response options are in a five-point Likert scale (0–4) format. Subscales of pain (score range 0–20), stiffness (score range 0–8) and function (score range 0–68) are summed to form a total score ranging from 0 (worst) to 96 (best). Raw scores were normalised to produces scores ranging from 0 (worst) to 100 (best).
As secondary outcomes, WOMAC scores were also measured every 3 months between 3 and 15 months post randomisation. Other patient-reported outcomes included the Brief Pain Inventory short form, Oxford Hip Score, Hip Dysfunction and Osteoarthritis Outcome and Hospital Anxiety and Depression Score. These were measured every 6 months between 6 and 18 months post randomisation. Patients were also invited to complete a 20-metre timed walk test after 18 months. Complications related to surgery, prosthetic joint infection or other reasons were identified from medical notes. Rehospitalisation and reoperation related to these complications were also identified. The presence of an infection between 15 and 18 months post randomisation was also considered as well as the serious adverse event.
Sample size
A sample size of 148 patients provided 80% power, with an attrition rate of 13%, to test that one surgical strategy was superior to the other strategy at 18 months post randomisation by 10 points on the WOMAC index, equivalent to a difference of 0.5 standard deviations. The significance level of this superiority hypothesis was set at 5% (two-sided). Although it is known that infection following total joint replacement reduces patient satisfaction and seriously impairs functional health and quality of life, there is no published research on the likely difference in patient-reported outcome between patients undergoing single-stage and those undergoing two-stage revision for prosthetic joint infection. The standard deviations observed prior to single-stage or two-stage revision surgery for total WOMAC score and subscores range between 18 and 25. 129,136 The attrition rate observed in a surgical trial involving total hip replacement recently conducted in the co-ordinating centre was 13%. 98
Statistical analysis
The study was reported as per CONSORT guidelines and the analyses were performed according to the predefined analysis plan agreed with the Trial Steering Committee.
The main analysis was based on a two-level linear mixed-model regressing total WOMAC scores measured between 3 and 18 months post randomisation on the treatment effect and its interaction with a polynomial function of the time of assessment (first and second degrees). The model was also adjusted for baseline WOMAC and hospitals and had random effects at the patient level (measurement nested within participant). The difference in mean total WOMAC score at 18 months post randomisation between patients who received single-stage revision and those who received a two-stage revision (reference) was identified using linear combination of the treatment effect, the time of assessment and their interaction (contrast).
Although this modelling approach is robust to missing data when they are missing at random, multiple imputations with chained equations (n = 33) were conducted to include all randomised participants in the final analysis (imputed analysis). The imputation process was stratified by group.
To account for the fact that the WOMAC score is subject to a ceiling effect, the main analysis was replicated using a two-level tobit mixed model (Tobit analysis). Other sensitivity analyses included adjusting for sex, latest surgical treatment for the management of prosthetic joint infection prior to study participation and ASA grade at baseline, which were imbalanced between the groups (adjusted analysis, adjusted and imputed analysis). All of these analyses were based on the intention-to-treat principle.
A sensitivity analysis was performed on all randomised participants undergoing operations in centres in which the single-stage or two-stage procedure was not systematically performed with a CUMARS.
The secondary outcomes were modelled using a similar strategy and appropriate generalised linear mixed model depending on their distribution. Dichotomous outcomes such as complication, rehospitalisation or reoperation status with no repeated measurements were analysed with a generalised linear model, such as the log-binomial regression.
Results
A CONSORT flow diagram is shown in Figure 15. Of 186 patients eligible for inclusion in the trial, 65 were randomised to single-stage revision and 75 were randomised to two-stage revision. The baseline characteristics of participants in the randomised groups, shown in Table 13, were balanced between the groups, except that patients in the single-stage group were more likely to be male, to have ASA grade one or two and to have received previous non-surgical management for their infection. The groups were similar in the number and type of organisms cultured, rates of culture-negative infection and presence of a sinus tract. A total of 126 (90%) patients had the primary total WOMAC score outcome at 18 months post randomisation, and 133 (95%) patients had at least one postoperative total WOMAC score and were included in the main analysis.
| Characteristic | Category | Single-stage | Two-stage | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age at inclusion (years) | Mean (SD) | 65 | 70.0 (9) | 75 | 72 (10) |
| BMI (kg/m2) | Mean (SD) | 65 | 29.0 (7) | 75 | 29 (5) |
| Sex | Female | 20 | 30.8 | 31 | 41.3 |
| Male | 45 | 69.2 | 44 | 58.7 | |
| Ethnicity | Non-white | 1 | 1.5 | 2 | 2.7 |
| White | 64 | 98.5 | 73 | 97.3 | |
| ASA grade | 1 | 2 | 3.1 | 1 | 1.3 |
| 2 | 28 | 43.1 | 29 | 38.7 | |
| 3 | 26 | 40.0 | 38 | 50.7 | |
| 4 | 2 | 3.1 | 0 | 0.0 | |
| Missing | 7 | 10.8 | 7 | 9.3 | |
| Work status | Receiving sick pay | 6 | 9.2 | 3 | 4.0 |
| Retired | 44 | 67.7 | 58 | 77.3 | |
| Unemployed | 2 | 3.1 | 3 | 4.0 | |
| Working full time | 9 | 13.9 | 9 | 12.0 | |
| Working part time | 4 | 6.2 | 2 | 2.7 | |
| Number of comorbidities | None | 7 | 10.8 | 10 | 13.3 |
| One | 18 | 27.7 | 18 | 24.0 | |
| Three | 9 | 13.9 | 10 | 13.3 | |
| Two | 16 | 24.6 | 21 | 28.0 | |
| Four or more | 15 | 23.1 | 16 | 21.3 | |
| Home status | Alone | 18 | 27.7 | 24 | 32.0 |
| With husband/wife/partner | 47 | 72.3 | 45 | 60.0 | |
| With somebody else | 0 | 0.0 | 6 | 8.0 | |
| Marital status | Divorced/separated | 6 | 9.2 | 8 | 10.7 |
| Married/partner | 49 | 75.4 | 46 | 61.3 | |
| Single | 7 | 10.8 | 5 | 6.7 | |
| Widowed | 3 | 4.6 | 16 | 21.3 | |
In the single-stage group, 55 (84.6%) patients received their assigned intervention, eight received a two-stage procedure and one did not receive a revision. Four patients withdrew during follow-up and two died. In the two-stage group, 68 (90.7%) patients received their assigned intervention, five received an alternative revision procedure and one did not receive a revision. Three patients withdrew during follow-up and five died. The median time between stages in the two-stage group was 3.7 months (IQR 2.6–6.1 months).
Primary outcome
There was no evidence of a difference in the mean total WOMAC score at 18 months post randomisation between single- and two-stage management of hip prosthetic joint infection (mean difference favouring two stage 0.13, 95% CI –8.20 to 8.46; p = 0.98).
Secondary outcomes
At 3 months post randomisation, participants in the single-stage group had a better total WOMAC score (mean difference favouring single stage 11.53, 95% CI 3.89 to 19.17; p = 0.003). From 6 months post randomisation onwards, no evidence of a difference was found between the two surgical procedures. Similar findings were found for all patient outcomes.
There was a higher rate of complications of surgery in patients receiving a two-stage revision than in those receiving a single-stage revision (57.3% vs. 41.5%; p = 0.04) and this difference was marked for intraoperative events (26.7% vs. 7.7%; p = 0.01), predominantly calcar cracks and femoral shaft fractures (18 vs. 3). There was no evidence of a difference between the groups in length of stay; other complications; rehospitalisation or reoperation due to prosthetic joint infection, the surgery or another cause; serious adverse events; or deaths (5 vs. 2). Rates of serious adverse events, rehospitalisations, reoperations and deaths were similar between the groups. At 15–18 months post randomisation, 9 out of 59 (15%) patients who received single-stage revision had signs of infection in the operated hip, compared with 8 out of 67 (12%) people who received two-stage revision (p = 0.59).
Rates of serious adverse events and deaths were similar in the groups.
Conclusion
The INFORM trial demonstrated that single- and two-stage revisions are equally efficacious for the treatment of prosthetic joint infection. However, an early functional benefit was seen in the single-stage group and, given that patients stated a strong preference for a quick return to normal activity, this should be considered when selecting the intervention. Single-stage surgery accounted for 30% of revision procedures for the treatment of prosthetic joint infection in 2014, so there is scope for increased utilisation.
Limitation
The INFORM sample size precluded study of reinfection outcome.
Appendix 18 Surgical treatment of prosthetic joint infection: the INFORM randomised controlled trial cost-effectiveness analysis as described in Surgical treatment of prosthetic joint infection: the INFORM randomised controlled trial health economic analysis
Background
In the INFORM RCT we incorporated an economic evaluation to determine the cost-effectiveness of a single-stage compared with a two-stage revision strategy in patients with hip prosthetic joint infection.
Methods
Resource use measurement and valuation
Data on health service, PSS and participant resource use relating to the revision surgery for hip prosthetic joint infection and any subsequent treatments were collected over 18 months from randomisation from trial case report forms and patient-completed questionnaires.
Study research nurses from treating hospitals recorded resource use information on case report forms for the revision surgeries, postoperative inpatient stays and outpatient visits relating to hip infection. This included surgery time, types and brands of implants used, local and systemic antibiotics, time spent and resources used on postoperative wards. Case report forms were completed at 3, 6, 9, 12, 15 and 18 months post randomisation.
The self-reported resource use questionnaire was posted to participants at the 6-, 12- and 18-month follow-ups and was used to collect the following information concerning their prosthetic joint infection: community-based health-care use (e.g. general practitioner appointments and district nurse attendances), prescribed medications and emergency department attendances; social service provision received; personal expenses, including travel and home changes; informal care; and time off work and usual activities.
Resources used and the unit costs (GBP in 2018/19 prices) used to value them are shown in Table 14.
| Resource | Unit cost (£) | Source of cost |
|---|---|---|
| Theatre time | 29.91 per minutea | Finance department of treating hospital |
| Implants | Variesb | National Joint Registry8 |
| HDU/ICU | 42.12 per hour | Finance department of treating hospital |
| Ward | 12.62 per hour | Finance department of treating hospital |
| Radiological tests | Varies | NHS Reference Costs 2018 to 2019 101 |
| Biochemistry tests | 1.11 | NHS Reference Costs 2018 to 2019 101 |
| Haematology tests | 2.51 | NHS Reference Costs 2018 to 2019 101 |
| Microbiology tests | 7.59 | NHS Reference Costs 2018 to 2019 101 |
| Overheads | 9.60 per hour on ward | Finance department of treating hospital |
| Subsequent inpatient admissions | Variesc | NHS Reference Costs 2018 to 2019 101 |
| Outpatient appointments | Variesd | NHS Reference Costs 2018 to 2019 101 |
| Outpatient procedures | Varies | NHS Reference Costs 2018 to 2019 101 |
| Accident and emergency attendances (no admission) | 132.88 | NHS Reference Costs 2018 to 2019 101 |
| General practitioner (surgery) | 39.23 | Unit Costs of Health and Social Care 2019 102 |
| General practitioner (home) | 124.51 | Unit Costs of Health and Social Care 2013 137 |
| General practitioner (telephone) | 8.00 | Unit Costs of Health and Social Care 2019 102 |
| Nurse (surgery) | 10.85 | Unit Costs of Health and Social Care 2019 102 |
| Nurse (home) | 21.98 | Unit Costs of Health and Social Care 2019 102 |
| Nurse (telephone) | 5.53 | Unit Costs of Health and Social Care 2019 102 |
| GP receptionist | 7.80 | Unit Costs of Health and Social Care 2019 102 |
| Phlebotomist | 3.51e | Unit Costs of Health and Social Care 2018 138 |
| District nurse | 40.16e | Unit Costs of Health and Social Care 2015 139 |
| Occupational therapist | 34.00 | Unit Costs of Health and Social Care 2019 102 |
| Community physiotherapist | 54.00 | Unit Costs of Health and Social Care 2019 102 |
| NHS 111 service | 13.39 per call | Turner et al.140 |
| Other community care | Varies | Unit Costs of Health and Social Care 2019,102 NHS Reference Costs 2018 to 2019101 |
| Medications | Variesf | British National Formulary 141 |
| Prescription charges | 9.15 per item | NHS142 |
| Residential home stay |
Aged 18–64 years: £135 per day Aged ≥ 65 years: £146 per day |
Unit Costs of Health and Social Care 2019 102 |
| Home care worker | 28.29 per hourg | Unit Costs of Health and Social Care 2019 102 |
| Home changes and equipment | Varies | NRS Healthcare,143 Unit Costs of Health and Social Care 2019102 |
| Wage rate | 14.67 per hourh | ONS Annual Survey of Hours and Earnings144 |
| Travel cost per journey | Varies | The patient’s reported mode of transport to each health-care facility and the fare/milesi reported were used to create a mean unit cost for travel to each health facility |
Outcome measurement
The primary outcome of the economic analysis was the QALY as recommended by NICE. 146 The baseline EQ-5D-5L was completed at a clinic or home visit. At 3 months the questionnaire was completed over the telephone, and at 6, 12 and 18 months postal questionnaires were completed.
Utility values were estimated from the EQ-5D-5L using the validated mapping function to the existing EQ-5D-3L societal UK utility tariffs. 104 QALYs were calculated by linear interpolation using the area-under-the-curve approach, taking into account any deaths that occurred during the study period by allocating those who died a zero utility in the time period following their death. 105
Missing data
To account for missing data, multiple imputation by chained equations using predictive mean matching was used. Simple imputation methods assuming zero resource use within a returned participant questionnaire for item non-response, and using mean imputation where a participant had indicated use of a resource with no further details, were used prior to multiple imputation for the imputation to converge. Missing values for baseline utility were also imputed using the mean baseline utility value,106 prior to multiple imputation. The covariates in the imputation model were baseline utility, trial group, age, sex, ethnicity, education status, work status, centre, ASA grade of first revision surgery, and the latest surgical treatment for the management of prosthetic joint infection prior to study participation. Rubin’s rules were used to combine the 68 individual imputations, and a randomisation seed was used to enable reproducible imputations. 99
Analysis
Analyses were conducted using an intention-to-treat approach, analysing the groups as they were randomised. Stata version 15.1 (StataCorp LP, College Station, TX, USA) was used for statistical analysis. Any costs occurring between 12 and 18 months post randomisation were discounted at a rate of 3.5% per year as recommended by NICE. 146
Items were grouped into resource use categories and summed for each participant. Unadjusted costs from a treating hospital perspective were estimated by group for three time periods (0–6 months, 6 months to 1 year and 1 year to 18 months). The adjusted mean costs and QALYs by trial group and the differences in adjusted mean costs and QALYs (with 95% CIs) between the trial groups were estimated using the seemingly unrelated regressions method, which accounts for the correlation between costs and QALYs. 107 Costs and QALYs were adjusted for study centre. QALYs were additionally adjusted for baseline utility.
The iNMB was calculated parametrically using seemingly unrelated regression outputs, which represent the value of the intervention in monetary terms where a willingness-to-pay threshold for a unit of benefit is known. The NICE willingness-to-pay thresholds of £20,000 and £30,000 per QALY were used. 146 When the iNMB is positive, the single-stage revision surgery can be identified as cost-effective relative to two-stage revision surgery.
Allowance for uncertainty
Sample uncertainty in the cost-effectiveness estimates was explored using CEACs. 147 These were calculated from the net monetary benefit values at each threshold of willingness-to-pay per QALY (£0 to £100,000 in £1000 increments). The CEAC shows the probability that the single-stage is the cost-effective option compared with two-stage at different thresholds of willingness to pay per QALY.
Deterministic one-way sensitivity analyses were undertaken to examine the impact of changes in key parameters on the iNMB by modifying the value of one parameter at a time within a plausible range:
-
Including Swedish participants. These participants were excluded from the primary analysis because of differences in data collection and health-care jurisdiction.
-
Applying HRG costs to all intervention and subsequent surgeries as an alternative to the microcosting approach applied in the primary analysis to address the generalisability of health-care costs.
-
Using alternative discount rates of 2% and 5%.
-
Conducting an analysis adjusting for sex, latest surgical treatment for the management of prosthetic joint infection prior to study participation and ASA grade at baseline, which were imbalanced between the groups.
-
Conducting a complete-case analysis from a treating hospital perspective.
-
Conducting two analyses from a treating hospital perspective with two different specifications of the multiple imputation model to examine the impact of model structure on the results: one in which the multiple imputation model was conducted by group and the other in which this was not the case.
Results
The differences in the collection of data for UK and Swedish participants, and the differences in the health-care jurisdictions of the two countries, meant that the primary economic analysis was conducted on 128 UK participants (91% of the total sample). Resource use and cost data from the treating hospitals were available for 114 UK participants (89%). With the inclusion of patient questionnaire information, the proportion of participants with complete data from an NHS/PSS perspective was 41%. Thus, multiple imputation was used to create the data set comprising all 128 UK participants on whom the analyses of costs, QALYs and cost-effectiveness were conducted. Table 15 shows the proportion of missing data for each variable in the multiple imputation model.
| Variable | Components | Missing (n) (N = 128) | Missing (%) |
|---|---|---|---|
| Surgery total costs | Surgery antibiotics, surgery anaesthetics, theatre time | 4 | 3 |
| Implant total costs | Implant costs, cement costs | 5 | 4 |
| Ward total costs | Ward costs and overhead costs | 5 | 4 |
| HDU/ICU costs | HDU and ICU costs | 6 | 5 |
| Hospital antibiotic costs | Hospital-prescribed antibiotics | 5 | 4 |
| Surgery follow-up admissions | Inpatient stays for follow-up surgery (not single- or two-stage surgeries) | 3 | 2 |
| Hospital follow-up costs (treating hospital) | Outpatient appointments, outpatient procedures, inpatient stays | 9 | 7 |
| Non-treating-hospital follow-up admissions | Inpatient stays in hospitals other than where the revision surgery took place | 36 | 28 |
| Non-treating hospital outpatient visits | Outpatient visits to hospitals other than where the revision surgery took place | 36 | 28 |
| A&E total costs (6 months) | A&E visits | 24 | 19 |
| A&E total costs (12 months) | A&E visits | 23 | 18 |
| A&E total costs (18 months) | A&E visits | 25 | 20 |
| GP and community-based health care (6 months) | GP practice, GP home visits, GP telephone calls, GP practice nurse visits, GP practice nurse telephone calls, phlebotomist visits, GP receptionist visits, district nurse home visits, occupational therapist home visits, physiotherapist home visits, physiotherapist practice visits, NHS Direct telephone calls, other community care | 25 | 20 |
| GP total (12 months) | Components as for GP and community-based health care | 24 | 19 |
| GP total (18 months) | Components as for GP and community-based health care | 23 | 18 |
| Medications total costs (6 months) | GP-prescribed medication costs | 42 | 33 |
| Medications total costs (6 months) | GP-prescribed medication costs | 44 | 34 |
| Medications total costs (12 months) | GP-prescribed medication costs | 39 | 30 |
| Residential and home care costs (6 months) | Residential/nursing home costs, home care worker costs, home changes and equipment | 33 | 26 |
| Residential and home care costs (12 months) | Residential/nursing home costs, home care worker costs, home changes and equipment | 33 | 26 |
| Residential and home care costs (18 months) | Residential/nursing home costs, home care worker costs, home changes and equipment | 35 | 27 |
| EQ-5D utility (3 months) | EQ-5D utility | 16 | 13 |
| EQ-5D utility (6 months) | EQ-5D utility | 27 | 21 |
| EQ-5D utility (12 months) | EQ-5D utility | 23 | 18 |
| EQ-5D utility (18 months) | EQ-5D utility | 16 | 13 |
| Prescription and one-off expenses (6 months) | Prescribed medications, major one-off expenses | 29 | 23 |
| Prescription and one-off expenses (12 months) | Prescribed medications, major one-off expenses | 30 | 23 |
| Prescription and one-off expenses (18 months) | Prescribed medications, major one-off expenses | 32 | 25 |
| Lost work (6 months) | Lost work hours, paid leave, unpaid leave | 7 | 5 |
| Lost work (12 months) | Lost work hours, paid leave, unpaid leave | 3 | 2 |
| Lost work (18 months) | Lost work hours, paid leave, unpaid leave | 6 | 5 |
| Additional support (6 months) | Additional support hours required | 28 | 22 |
| Additional support (12 months) | Additional support hours required | 26 | 20 |
| Additional support (18 months) | Additional support hours required | 26 | 20 |
| Patient home care costs (6 months) | Home care changes and home care costs: patient contributions | 32 | 25 |
| Patient home care costs (12 months) | Home care changes and home care costs: patient contributions | 33 | 26 |
| Patient home care costs (18 months) | Home care changes and home care costs: patient contributions | 35 | 27 |
| Usual activities (6 months) | Usual activity hours lost | 25 | 20 |
| Usual activities (12 months) | Usual activity hours lost | 23 | 18 |
| Usual activities (18 months) | Usual activity hours lost | 25 | 20 |
| Hospital travel | Costs of travel to treating hospital | 21 | 16 |
| Hospital travel | Costs of travel to non-treating hospital | 36 | 28 |
| GP and community-based travel (6 months) | Travel costs to GP and community-based services | 28 | 22 |
| GP and community-based travel (12 months) | Travel costs to GP and community-based services | 27 | 21 |
| GP and community-based travel (18 months) | Travel costs to GP and community-based services | 29 | 23 |
Total theatre time was approximately 76 minutes longer for those randomised to two-stage revision surgery, and these patients spent a longer time in high-dependency or intensive therapy units and recovery and had a longer hospital stay (Table 16). They also had a greater number of subsequent inpatient stays and emergency department attendances. Those randomised to a single-stage procedure had a greater number of primary care practice nurse visits, whereas those randomised to a two-stage procedure had a greater number of district nurse home visits, stays in residential homes and home care worker visits. Participants randomised to two-stage revision took a greater number of hours of paid leave and lost a greater number of hours from usual activities, whereas those randomised to a single-stage revision lost more working hours in terms of permanently giving up work and permanent reduction in hours worked.
| Resource use category (unit of measurement) | Single-stage revision surgery | Two-stage revision surgery | ||
|---|---|---|---|---|
| n | Mean resource use (95% CI) | n | Mean resource use (95% CI) | |
| Theatre time (minutes)a | 59 | 333.85 (294.29 to 373.41) | 65 | 409.62 (364.81 to 454.43) |
| Recovery ward (minutes)b | 56 | 487.16 (–52.01 to 1026.33) | 61 | 716.75 (454.90 to 978.62) |
| Total high-dependency/intensive therapy units (hours) | 58 | 25.43 (12.22 to 38.63) | 64 | 30.06 (16.15 to 43.97) |
| Implant costs (£)c | 59 | 1948.41 (1644.54 to 2252.28) | 64 | 2391.68 (2033.27 to 2750.09) |
| Total preoperative stay (hours) | 59 | 17.42 (8.44 to 26.41) | 65 | 15.21 (11.14 to 19.27) |
| Total postoperative stay (days) | 59 | 20.83 (16.09 to 25.57) | 64 | 27.11 (20.90 to 33.33) |
| Total inpatient tests (radiology, microbiology, biochemisty, haematology) (n) | 59 | 44.73 (33.42 to 56.03) | 65 | 49.05 (37.86 to 60.24) |
| Subsequent non-surgical inpatient stays (number of stays) | 56 | 0.20 (0.05 to 0.34) | 63 | 0.32 (0.18 to 0.50) |
| Subsequent outpatient visits (number of visits) | 56 | 4.79 (4.16 to 5.41) | 63 | 5.40 (4.65 to 6.14) |
| Outpatient procedure-only visits (number of procedures) | 56 | 0.41 (0.13 to 0.69) | 63 | 0.24 (0.10 to 0.38) |
| Non-treating-hospital inpatient stays (number of stays) | 41 | 0.10 (–0.02 to 0.22) | 51 | 0.35 (0.14 to 0.57) |
| Non-treating-hospital outpatient visits (number of visits) | 41 | 0.54 (0.08 to 0.99) | 51 | 0.25 (0.05 to 0.46) |
| Emergency department (number of non-admission visits) | 41 | 0.12 (–0.04 to 0.28) | 51 | 0.33 (0.12 to 0.55) |
| Primary care clinician: surgery (number of visits)d | 41 | 2.30 (0.38 to 4.22) | 51 | 1.94 (0.85 to 3.04) |
| Primary care clinician: home (number of visits)d | 41 | 0.17 (–0.73 to 0.42) | 51 | 0.32 (0.01 to 0.63) |
| Telephone calls with primary care clinician (number of telephone calls)d | 41 | 0.61 (0.18 to 1.04) | 51 | 1.51 (0.38 to 2.63) |
| Primary care practice nurse visits (number of visits)d | 41 | 3.82 (1.14 to 6.51) | 51 | 1.77 (0.87 to 2.68) |
| Telephone calls with primary care practice nurse (number of telephone calls)d | 41 | 0.14 (–0.04 to 0.32) | 51 | 0.36 (0.11 to 0.61) |
| District nurse (number of home visits)d | 41 | 2.23 (–0.37 to 4.84) | 51 | 8.93 (2.38 to 15.49) |
| Other community-based health service contacts (number of contacts)d | 41 | 15.53 (5.57 to 25.49) | 51 | 16.64 (3.35 to 30.92) |
| Antibiotic medications (number of medications prescribed by hospital)b | 59 | 6.00 (4.98 to 7.02) | 65 | 6.12 (5.29 to 6.95) |
| Medications (number of medications prescribed by primary care clinician) | 39 | 1.59 (0.86 to 2.32) | 46 | 2.72 (1.66 to 3.78) |
| Residential home/nursing home (number of nights) | 41 | 2.61 (–1.16 to 6.38) | 51 | 11.96 (–0.75 to 24.68) |
| Home changes/equipment: NHS/PSS-provided (number of home changes/equipment) | 39 | 2.31 (1.23 to 3.38) | 46 | 2.50 (1.75 to 3.25) |
| Home changes/equipment: privately purchased (number of home changes/equipment) | 39 | 0.56 (0.07 to 1.06) | 46 | 1.52 (0.67 to 2.37) |
| Home care worker (number of visits) | 38 | 19.58 (–2.71 to 41.87) | 46 | 51.22 (4.54 to 97.90) |
| Prescription charges (number of charges) | 39 | 0.05 (–0.05 to 0.16) | 46 | 0.30 (–0.09 to 0.70) |
| One-off expenses (number of items) | 39 | 0.23 (0.09 to 0.37) | 46 | 0.33 (0.12 to 0.53) |
| Working hours lost (number of hours)e | 53 | 101.89 (–17.11 to 220.89) | 66 | 80.70 (–1.30 to 162.69) |
| Hours of paid leave (number of hours) | 53 | 17.97 (–5.64 to 41.59) | 66 | 44.31 (–14.87 to 103.51) |
| Hours of unpaid leave (number of hours) | 53 | 23.63 (–5.07 to 52.33) | 66 | 10.45 (–4.88 to 25.79) |
| Usual activity hours lost (number of hours)f | 40 | 274.96 (154.36 to 395.55) | 51 | 625.86 (337.78 to 913.95) |
| Additional support/care hours (number of hours)f | 38 | 274.08 (153.18 to 394.98) | 48 | 610.56 (400.33 to 820.79) |
The EQ-5D-5L utility values in Table 17 illustrate the different pathways of the two procedures, with those randomised to the single-stage procedure seeing a gradual improvement in their utility values from 3 months onwards, whereas for those randomised to the two-stage procedure the improvement only began at 6 months.
| EQ-5D utility | Single-stage revision surgery | Two-stage revision surgery | ||
|---|---|---|---|---|
| n | Mean utility (95% CI) | n | Mean utility (95% CI) | |
| EQ-5D utility (baseline) | 60 | 0.32 (0.23 to 0.41) | 67 | 0.28 (0.20 to 0.36) |
| EQ-5D utility (3 months) | 50 | 0.51 (0.41 to 0.61) | 62 | 0.33 (0.23 to 0.42) |
| EQ-5D utility (6 months) | 45 | 0.54 (0.44 to 0.64) | 56 | 0.46 (0.37 to 0.55) |
| EQ-5D utility (12 months) | 49 | 0.54 (0.44 to 0.64) | 56 | 0.50 (0.42 to 0.59) |
| EQ-5D utility (18 months) | 52 | 0.60 (0.50 to 0.70) | 60 | 0.60 (0.52 to 0.68) |
As shown in Table 18, the mean costs of surgical admissions were higher in the first year of follow-up in the single-stage group; however, in the last 6 months of the trial these costs were similar in both trial groups, with these being slightly more costly in the single-stage group (£1593 vs. £1489). The costs of follow-up outpatient visits and non-surgical admissions were similar in the first year, with these being more expensive in the two-stage revision surgery group in the final 6 months (£702 vs. £484).
| Surgical admissions and follow-up outpatient visits | Single-stage revision surgery | Two-stage revision surgery | ||
|---|---|---|---|---|
| n | Mean cost (£) (95% CI) | n | Mean cost (£) (95% CI) | |
| Surgical admissions (0–6 months) | 58 | 21,287 (18,250 to 24,323) | 62 | 25,674 (21,696 to 29,652) |
| Follow-up outpatient visits and admissions (0–6 months) | 56 | 894 (123 to 1664) | 63 | 820 (427 to 1213) |
| Surgical admissions (6–12 months) | 58 | 2039 (613 to 3466) | 62 | 3660 (1688 to 5632) |
| Follow-up outpatient visits and admissions (6–12 months) | 56 | 437 (72 to 802) | 63 | 444 (181 to 707) |
| Surgical admissions (12–18 months) | 58 | 1593 (–426 to 3612) | 62 | 1489 (–76 to 3055) |
| Follow-up outpatient visits and admissions (12–18 months) | 56 | 484 (453 to 516) | 63 | 702 (443 to 962) |
The total adjusted mean costs from the NHS/PSS perspective in the single-stage group (£36,256) were lower than in the two-stage group (£46,312), a cost difference of –£10,055 (95% CI –£19,568 to –£542) (Table 19). The cost difference reduced slightly from the societal perspective (–£9450, 95% CI –£22,855 to £3956).
| Treatment | n | Adjusted costsa (£), mean (95% CI) | Adjusted QALYs,a mean (95% CI) | Incremental costs (95% CI) | Incremental QALYs (95% CI) | iNMB (£) at £20,000/QALY (95% CI) | iNMB (£) at £30,000/QALY (95% CI) |
|---|---|---|---|---|---|---|---|
| NHS and PSS perspective | |||||||
| Single stage | 60 | 36,256 (29,344 to 43,169) | 0.75 (0.65 to 0.84) | ||||
| Two stage | 68 | 46,312 (39,876 to 52,747) | 0.69 (0.61 to 0.77) | –10,055 (–19,568 to –542) | 0.06 (–0.07 to 0.18) | 11,167 (638 to 21,696) | 11,723 (507 to 22,938) |
| Societal perspective | |||||||
| Single stage | 60 | 51,420 (41,551 to 61,288) | 0.75 (0.65 to 0.84) | ||||
| Two stage | 68 | 60,870 (51,864 to 69,878) | 0.69 (0.61 to 0.77) | –9450 (–22,855 to 3956) | 0.06 (–0.07 to 0.18) | 10,589 (–3855 to 25,033) | 11,158 (–3936 to 26,252) |
Participants in the single-stage group also had a greater number of adjusted mean QALYs (0.75) than those in the two-stage group (0.69), a difference of 0.06 (95% CI–0.07 to 0.18) (Table 20).
| Treatment | n | Adjusted mean costsa (£), mean (95% CI) | Adjusted QALYs,a mean (95% CI) | Incremental costs (95% CI) | Incremental QALYs (95% CI) | iNMB (£) at £20,000/QALY (95% CI) | iNMB (£) at £30,000/QALY (95% CI) | Probabiity cost-effective at £20,000 per QALY |
|---|---|---|---|---|---|---|---|---|
| Inclusion of Swedish patients | ||||||||
| Single stage | 65 | 34,758 (28,413 to 41,102) | 0.78 (0.69 to 0.86) | |||||
| Two stage | 75 | 44,896 (39,118 to 50,675) | 0.71 (0.63 to 0.79) | –10,138 (–18,738 to –1539) | 0.07 (–0.05 to 0.19) | 11,515 (1936 to 21,093) | 12,203 (1955 to 22,452) | 0.99 |
| Using HRG costs rather than microcosting for the surgical admissions | ||||||||
| Single stage | 60 | 26,072 (21,260 to 30,883) | 0.75 (0.65 to 0.84) | |||||
| Two stage | 68 | 37,270 (32,851 to 41,690) | 0.69 (0.61 to 0.77) | –11,199 (–17,779 to –4619) | 0.06 (–0.07 to 0.18) | 12,304 (4707 to 19,902) | 12,857 (4510 to 21,205) | 0.99 |
| 2% discount rate | ||||||||
| Single stage | 60 | 35,953 (29,074 to 42,833) | 0.75 (0.66 to 0.85) | |||||
| Two stage | 68 | 45,760 (39,277 to 52,242) | 0.69 (0.61 to 0.77) | –9806 (–19,234 to –379) | 0.06 (–0.06 to 0.19) | 11,084 (543 to 21,625) | 11,723 (446 to 23,001) | 0.98 |
| 5% discount rate | ||||||||
| Single stage | 60 | 35,945 (29,036 to 42,854) | 0.74 (0.65 to 0.84) | |||||
| Two stage | 68 | 46,106 (39,569 to 52,643) | 0.69 (0.60 to 0.77) | –10,161 (–19,646 to 677) | 0.06 (–0.07 to 0.18) | 11,313 (740 to 21,886) | 11,889 (597 to 23,181) | 0.98 |
| Adjusted analysis (adjusting for sex, latest surgical treatment for the management of PJI prior to study participation and ASA grade at baseline) | ||||||||
| Single stage | 60 | 36,586 (29,747 to 43,426) | ||||||
| Two stage | 68 | 46,021 (39,684 to 52,357) | 0.69 (0.60 to 0.77) | –9434 (–18,929 to 61) | 0.06 (–0.06 to 0.19) | 10,694 (215 to 21,173) | 11,324 (173 to 22,474) | 0.98 |
| Treating hospital perspective: complete case | ||||||||
| Single stage | 38 | 27,316 (22,548 to 32,084) | 0.78 (0.68 to 0.88) | |||||
| Two stage | 44 | 36,699 (32,282 to 41,116) | 0.72 (0.63 to 0.81) | –9383 (–16,022 to –2744) | 0.06 (–0.08 to 0.20) | 10,584 (2961 to 18,207) | 11,184 (2761 to 19,608) | 1.00 |
| Treating hospital perspective: MI (by group) | ||||||||
| Single stage | 60 | 29,855 (25,011 to 34,700) | 0.77 (0.67 to 0.88) | |||||
| Two stage | 68 | 34,894 (30,321 to 39,467) | 0.71 (0.61 to 0.80) | –5038 (–11,748 to 1671) | 0.07 (–0.07 to 0.20) | 6371 (–1312 to 14,053) | 7037 (–1420 to 15,494) | 0.95 |
| Treating hospital perspective: MI (not by group) | ||||||||
| Single stage | 60 | 29,728 (24,875 to 34,581) | 0.78 (0.66 to 0.89) | |||||
| Two stage | 68 | 34,972 (30,421 to 39,523) | 0.73 (0.64 to 0.82) | –5243 (–11,932 to 1445) | 0.05 (–0.10 to 0.21) | 6263 (–1392 to 13,918) | 6773 (–1746 to 15,292) | 0.95 |
From the NHS/PSS perspective, the iNMB of single-stage compared with two-stage was £11,167 (95% CI £638 to £21,696) at the willingness-to-pay threshold of £20,000 per QALY and £11,723 (95% CI £507 to £22,938) at the willingness-to-pay threshold of £30,000 per QALY. The iNMB was slightly smaller from the societal perspective, at £10,589 (95% CI –£3855 to £25,033) at the threshold of £20,000 per QALY.
Figure 18 shows the CEACs from both perspectives and indicates that, at a willingness-to-pay threshold of £20,000 per QALY, the probability that a single-stage procedure is the cost-effective treatment compared with a two-stage procedure is 98% from the NHS/PSS perspective and 92% from the societal perspective.
FIGURE 18.
The CEAC at 18 months.
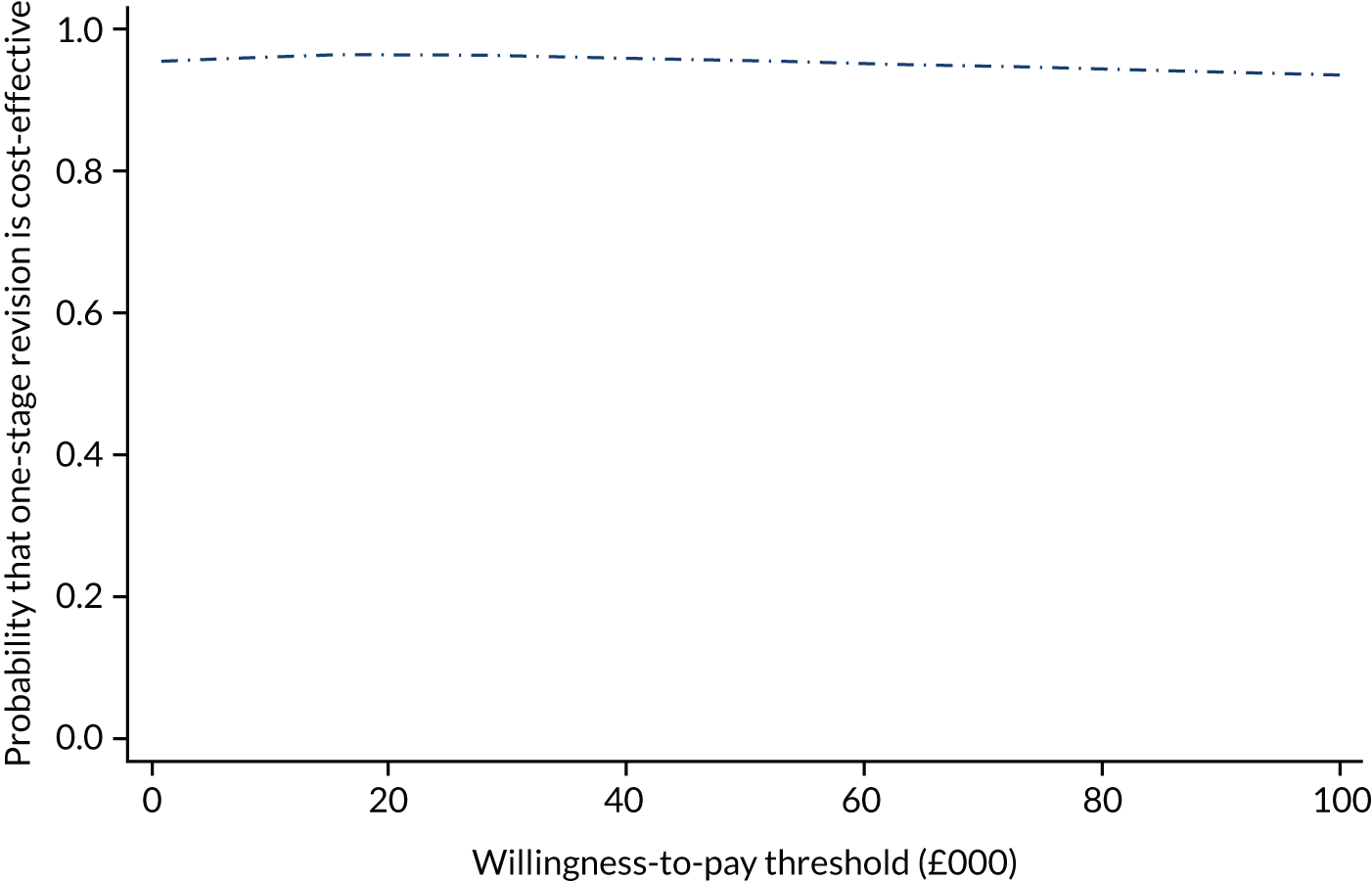
The sensitivity analyses (see Table 20) conducted from the NHS/PSS perspective did not show any reduction in the probability that the single-stage procedure was cost-effective. Including participants from Sweden, using HRG costs and a 5% discount rate led to a slight increase in the difference between mean costs, whereas the adjusted analysis led to a slight decrease in the difference between the groups. Using HRG costs led to substantial reductions in costs for both groups. The difference in QALYs remained the same, apart from when patients from Sweden were included, which resulted in an increase in the difference from 0.06 to 0.07. From the treating hospital perspective, the complete-case analysis showed an increase in the probability of the single stage being cost-effective to 99.7%. There was a larger difference in cost between the two groups compared with both multiple imputation analyses from this perspective. The results of the two multiple imputation analyses were similar, and the probability that the single-stage treatment was cost-effective reduced to just under 95%.
Discussion
From all perspectives, participants randomised to a single-stage procedure had both lower costs and more QALYs than those randomised to a two-stage procedure. A difference in QALYs of 0.06 equates to an extra 33 days in best imaginable health during the 18 months in favour of participants who had a single-stage procedure.
The two hospital stays involved with a two-stage procedure led to the higher cost in this arm. The EQ-5D-5L scores illustrate that while the participants in this group were awaiting their second-stage operation they had a poorer quality of life, which was reflected in the overall QALY score. The greater use of district nurse home visits and home care worker visits indicates that these participants were also less able to self-care and leave their home during this time.
The analysis has limitations, mainly relating to the resource use data obtained from the self-completed questionnaire. A complete case could be conducted only from a treating hospital perspective, and indicated higher QALY values in both groups, indicating that those who did not complete all of the questionnaires were likely to have poorer quality of life than those who did. Multiple imputation was therefore needed because only 41% of patients had complete resource use data from an NHS/PSS perspective and only 36% had these from a societal perspective. It was not possible to run multiple imputation by group from either the NHS/PSS or the societal perspective. Maximising the number of complete data by using data at each time point in the model had to be traded against the sample size. Imputation by group was not possible without a substantial loss of information. To gain insight into whether or not using imputation by group was likely to bias the results, both specifications were used in relation to a treating hospital perspective. The iNMBs were similar, and the probability that single stage treatment was cost-effective was 94.8% for the ‘by group’ model and 94.6% for the ‘not by group’ model, indicating that the model specification is unlikely to affect the conclusions of the analysis.
We used information from the finance department of a local trust to microcost the revision surgeries for hip prosthetic joint infection. The use of reference costs rather than microcosting to value HRGs reduced the costs in both groups, potentially indicating that reference costs may underestimate the true costs to health-care providers. This was also found by Vanhegan et al. 55 in relation to tariffs.
Cost differences may extend beyond the 18-month trial follow-up. However, the randomised groups had similar hospital costs between 12 and 18 months’ follow-up and had similar mean WOMAC and EQ-5D-5L scores at 18 months, and there was also little to suggest a difference in reinfection rates between the groups. Together, these factors suggest that patient costs and outcomes may not differ in the longer term.
In conclusion, the within-trial economic evaluation has shown that the single-stage procedure is the cost-effective option for patients with deep prosthetic hip joint infection.
Appendix 19 Checklist of factors to consider in undertaking and assessing the quality of a discrete choice experiment (using methods described by Lancsar and Louviere110)
| 1 | Conceptualising the choice process | |
|---|---|---|
| Was a choice rather than ranking, rating task used? | Yes | |
| What type of choice was used: binary response, pairs, multiple options? | Binary response (from a pair) | |
| Was a generic or labelled choice used? | Generic (unlabelled) | |
| Was an opt-out, neither or status quo option included? | No | |
| If a forced choice was used, was a justification provided? | N/A | |
| Was the task incentive compatible? | Yes | |
| 2 | Attribute selection | |
| How were they derived and validated? | Derived from qualitative interviews and validated in discussions with PPI group | |
| Was the number of attributes appropriate? | Yes | |
| Was the coverage appropriate? | Yes | |
| What form was used: generic or alternative specific? | Generic | |
| Was price included? If so, was an appropriate payment vehicle used? | No | |
| Was risk included? If so, was it appropriately communicated? | No | |
| 3 | Level selection | |
| How were they derived and validated? | Derived from qualitative interviews and validated in discussions with PPI group | |
| Was the number of levels per attribute appropriate? | Yes | |
| Was an appropriate range used? | Yes | |
| Were the levels evenly spaced? | Yes – derived using qualitative findings | |
| 4 | Experimental design | |
| What type of design was used? Full factorial? Fractional factorial? If fractional, which effects are identified: main effects; main effects + higher-order interactions? | Orthogonal main effects, fractional factorial design | |
| How were the profiles generated and allocated to choice sets? | To avoid order effects, both attributes and choice sets were randomised for profile 1. A moduloarithmetic method was used to generate the second profile | |
| What are the properties of the design? | Orthogonality, level and utility balance and minimum overlap of levels148 | |
| What is the efficiency of the design? | Unknown | |
| Was identification checked (e.g. is the variance-covariance matrix block diagonal)? | Yes | |
| Was the design blocked into versions? If so, how were choice sets allocated to versions? Were the resulting properties of the versions checked? | No blocking | |
| Were respondents randomly allocated to versions? | N/A | |
| How many choice sets were considered per respondent? | 16 | |
| If some profiles were implausible – how was implausibility defined and how was it addressed? | N/A | |
| 5 | Questionnaire design | |
| Was an appropriate level of background and contextual information provided? | Yes – designed and agreed with the PPI group | |
| Were the task instructions appropriate? | Yes – approved by PPI group | |
| Was the medium used to communicate attribute/level information (e.g. words, pictures, multimedia) appropriate? | Yes – approved by PPI group | |
| 6 | Piloting | |
| Was coverage of attributes and levels checked? | Yes | |
| Was understanding and complexity checked? | Yes | |
| Was the length and timing checked? | Yes | |
| 7 | Population/study perspective | |
| Appropriate for research question? | Yes | |
| 8 | Sample and sample size | |
| Were inclusion/exclusion criteria explicit? | Yes | |
| Was sample size appropriate for model estimation? | Yes | |
| 9 | Data collection | |
| What recruitment method was used? | Participants had been recruited into the INFORM RCT | |
| How were data collected (e.g. mail, personal interview, web survey)? | Questionnaire | |
| What was the response rate? | 57/80 (71%) were returned fully complete | |
| Were incentives used to enhance response rates? | No | |
| 10 | Coding of data | |
| Was coding explicitly discussed? | Yes – effects coding used | |
| Was the coding appropriate for effects to be estimated? | Yes | |
| 11 | Econometric analysis | |
| Were the estimation methods appropriate given experimental design and type of choice response? | Yes | |
| Was the functional form of the indirect utility functions appropriate given the experimental design? | Yes | |
| Were alternative specific constants included? | No | |
| Were sociodemographics and other covariates included? | No | |
| Was goodness of fit considered? | The analysis method provides a pseudo R2 value that can be used to inform goodness of fit | |
| 12 | Validity | |
| Was internal or external validity investigated? | Due to the topic of this programme, and the decision-making associated with this area of health care, there were no revealed preference data to compare with. Responses were in line with expectations and do not suggest any issue with validity of the questions or design | |
| Were answers for any respondents deleted and, if so, on what basis? | No | |
| 13 | Interpretation | |
| Was the interpretation appropriate given coding of data? | Yes | |
| Were results in line with a priori expectations? | Yes | |
| Were relative attribute effects compared using a common and comparable metric? | No | |
| 14 | Welfare and policy analysis | |
| Was willingness to pay estimated using welfare theoretic compensating variation? | No | |
| Was probability analysis undertaken? | No | |
| Were marginal rates of substitution calculated? | No |
Appendix 20 INFORM discrete choice questionnaire
Appendix 21 British Hip Society symposium
List of abbreviations
- ASA
- American Society of Anesthesiologists
- AUC
- area under the curve
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CHEC-list
- Consensus on Health Economic Criteria checklist
- CI
- confidence interval
- CONSORT
- Consolidated Standard of Report Trials
- CUMARS
- custom-made articulating spacer
- DAIR
- debridement, antibiotics and implant retention
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- INFORM
- INFection ORthopaedic Management
- iNMB
- incremental net monetary benefit
- IPD
- individual patient data
- IQR
- interquartile range
- MINORS
- methodological index for non-randomized studies
- MOOSE
- Meta-analysis Of Observational Studies in Epidemiology
- MSIS
- Musculoskeletal Infection Society
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NJR
- National Joint Registry for England, Wales, Northern Ireland and the Isle of Man
- ONS
- Office for National Statistics
- PEP-R
- Patient Experience Partnership in Research
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies-2
- RCT
- randomised controlled trial
- RR
- relative risk
- WOMAC
- Western Ontario and McMasters Universities Osteoarthritis Index
