Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1210-12016. The contractual start date was in February 2013. The final report began editorial review in March 2020 and was accepted for publication in June 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Harding et al. This work was produced by Harding et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Harding et al.
Synopsis
Background
A recent report by the World Health Organization (WHO) shows that diabetic retinopathy (DR) remains one of the most common causes of visual loss in adults worldwide. 1 Screening for DR aims to detect sight-threatening DR (STDR) and refer people with diabetes (PWD) to the hospital eye service (HES) for timely treatment. Systematic programmes of screening are universally recognised to be important in preventing visual impairment (VI). 2
In England, annual eye screening for STDR for PWD over the age of 12 years commenced in the early 1990s and developed into the National Diabetic Eye Screening Programme (NDESP), with complete coverage achieved by 2008. 3
As in many other countries, the programme screening interval was set at 12 months in England and Wales for all PWD,4 while in the HES follow-up intervals are variable. Evidence to support the delivery of the pathway is limited to screening research cohorts and with minimal input from users.
There has been a rapid increase in the prevalence of diabetes, increasing faster in low- and middle-income countries than high-income countries,5 and resources are stretched. Much evidence supporting extended intervals suggests that it is safe to screen low-risk people at longer intervals6–8 and this has been introduced in some countries. However, the underpinning evidence for this change comes from observational studies in areas with low incidence rates6,9–11 and from modelling studies,12–14 and is not conclusive. In England and Wales, extended intervals have not been adopted, largely because of safety concerns highlighted by a recent systematic review calling for a randomised controlled trial (RCT) and cost-effectiveness data,8 and recent problems in cancer screening programmes. 15
The emerging technologies of digital data linkage and risk prediction offer opportunities to personalise the approach to screening. By varying the frequency of screening depending on a person’s own risk of progression, an individualised approach can be developed with further potential improvements in effectiveness and cost-effectiveness.
There are potential opportunities for reallocation of resources within the NHS by varying the screen interval, but no cost-effectiveness research data other than from limited modelling. The safety and acceptability of extending the screen interval in low-risk groups have not been investigated.
Estimates of the incidence and prevalence of DR are important in designing screening programmes and clinical services. The landmark epidemiological studies on DR that have provided these data are over 30 years old. 16,17 Since then, there have been changes in diagnostic criteria for diabetes,18,19 and diabetes and blood pressure (BP) control have improved in some populations. 20 At the time of the design of this programme, there were few data on incidence and prevalence in screening populations, mainly those from our own work in the 1990s. 6 As systematic programmes have been introduced and detected early disease, incidence in screened populations has changed, characterised as the ‘first pass effect’. Clinical studies of interventions aimed at early disease utilise cohorts, such as those undergoing systematic screening, and require prevalence and incidence data for study design and comparison.
Aims and objectives of the ISDR programme of applied research
Our aim in this National Institute for Health and Care Research (NIHR) Programme Grant for Applied Research (PGfAR) on individualised screening for DR (ISDR) was to conduct a mixed quantitative and qualitative programme of research to strengthen the evidence base for DR screening in the United Kingdom (UK) and beyond. We structured the programme in work packages, reordered here to aid the narrative in our report. A very active patient and public involvement (PPI) group was involved throughout, so we describe their involvement first, with additional detail in Supplementary Material 1.
Data warehouse (work package B1)
The aim of work package B1 was to collate, link and store patient data from routinely collected sources in a study data warehouse (DW) to support the development of a risk-calculation engine (RCE), the RCT and epidemiological, qualitative and cost-effectiveness studies.
The Liverpool Risk Calculation Engine (work package C)
The aim of work package C was to develop an individualised (personalised) method of calculating screen intervals using a RCE based on individual risk factors (see Eleuteri et al. 21).
RCT to evaluate the safety, efficacy and cost-effectiveness of individualised variable-interval risk-based screening (work package E)
The aim of work package E was to investigate the safety, efficacy and cost-effectiveness of longer screening intervals in low-risk PWD and shorter intervals in high-risk PWD (see Broadbent et al. 22,23).
Health economics (work package D)
The aim of work package D was to estimate the likelihood that individualised screening is more cost-effective than current practice (see Broadbent et al. 23).
Qualitative study with patients and healthcare professionals on changing screening intervals (work package F)
The aim of work package F was to explore the underlying perceptions of screening and early detection, and to test the acceptability of our new method of screening to PWD and healthcare professionals (HCPs) (see Byrne et al. 24).
Observational cohort study in people attending DR screening (work package B2)
The aim of work package B2 was to conduct a whole-population longitudinal observational cohort study to estimate up-to-date rates of incidence of DR and STDR in PWD in Liverpool (see Cheyne et al. 25).
Knowledge transfer and preparation for implementation (work package G)
To disseminate the research findings to clinical and academic beneficiaries, develop an NHS implementation plan through a dissemination event and develop any intellectual property (IP).
Figure 1 shows the stages and development of the work packages, how the work packages interconnect and the contribution of each work package (WP) to the whole programme.
FIGURE 1.
Pathway diagram showing the work flow in the ISDR Programme Grant for Applied Research, the work packages and their interrelationships. WP B1 DW development; WP C RCE; PPI (involvement shown in dashed boxes); WP E RCT; WP D Health economics; WP B2 Observational cohort study; WP F Qualitative study; WP G Dissemination. Resources were reallocated to the RCT from the cohort study in year 4 to support delayed recruitment (white hexagon and arrow). Arrows indicate input into specific item(s) of a WP. ADA, American Diabetes Association; ARVO, Association for Research in Vision and Ophthalmology; EASD, European Association for the Study of Diabetes; EASDec, European Association for the Study of Diabetes eye complications study group; LCCG, Liverpool Clinical Commissioning Group; NSC, National Screening Committee.
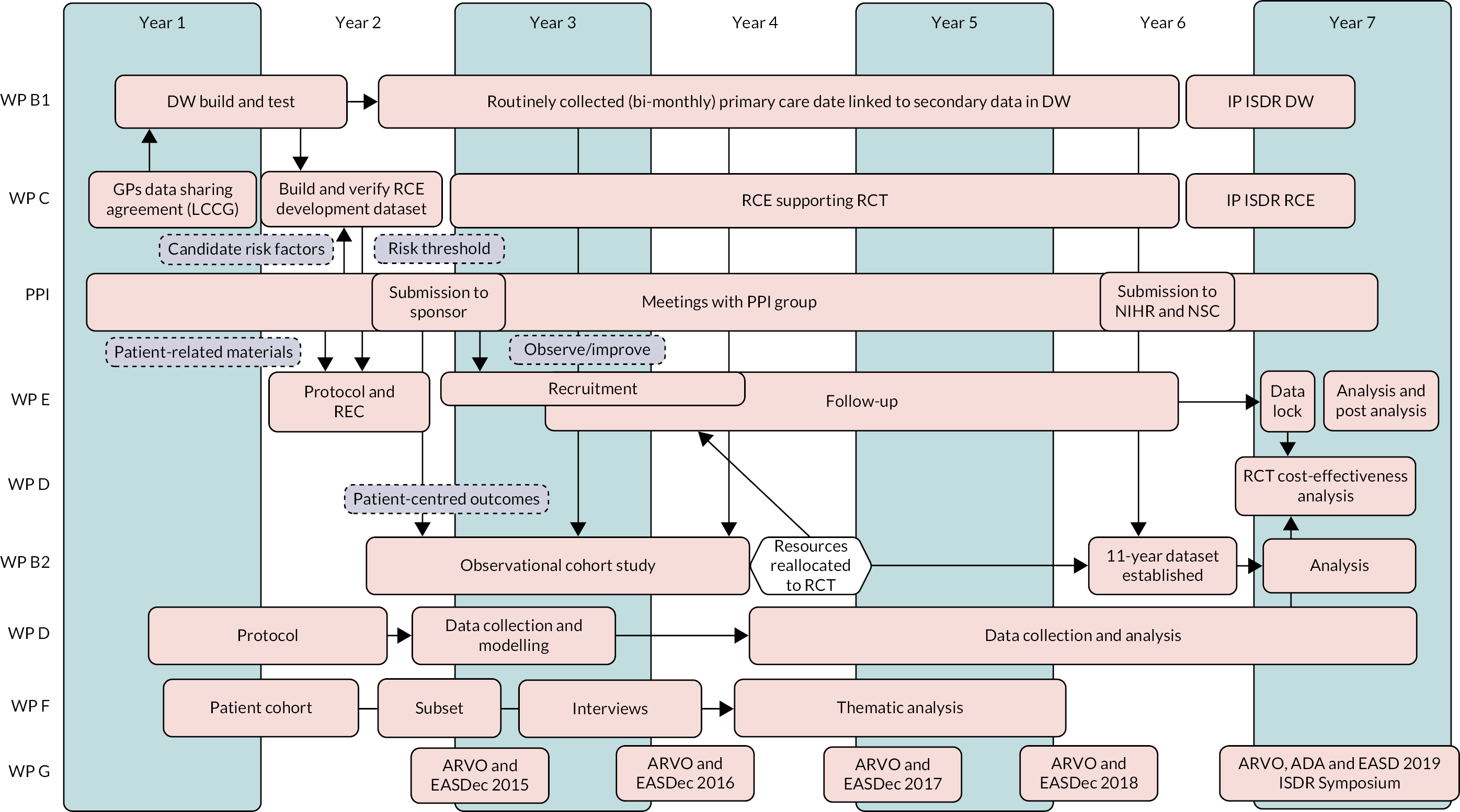
Changes to the programme
A number of changes to the planned programme of work occurred in the early years of the programme:
-
We planned to conduct a systematic review (WP A in the grant application). However, a systematic review in this area by Taylor-Phillips et al. 8 was published early in the programme in 2016. To replicate this was inefficient and, therefore, we conducted a literature review instead. Taylor-Phillips et al. 8 concluded that there was insufficient evidence to recommend a move to extended interval screening and mixed and poorly designed cost-effectiveness studies. They provided further confirmation of the need for a RCT with a robust economic analysis.
-
A decision was made by the NIHR PGfAR team in May 2016 to move resources from the cohort study to support the RCT (WP E), which was behind with recruitment. This meant that the cohort study needed to be scaled back. We were able to report findings in an 11-year cohort of PWD being screened in Liverpool, but not the people attending the HES.
-
The observational cohort study (WP B2) utilised data prospectively collected from 2005. However, we were unable to obtain data on people who had died prior to the start of the programme. This resulted in the cohort needing to be considered as partly retrospective, making analysis more difficult to interpret.
-
We were unable to recruit non-attenders in the screening programme, a secondary aim in our qualitative study (WP F), despite multiple attempts in an identified cohort.
-
We had intended to interview participants longitudinally in the qualitative study. However, insufficient participants responded to our invitation, so we revised our sample to reach 60 single interviews.
Patient and public involvement in the ISDR programme
Public engagement is considered a key element in healthcare research by UK and international funders to ensure that research is relevant. Effective engagement empowers patients, ensures research is fit for purpose and develops patient-centred outcomes. NIHR describes involvement of the public in research under its ‘INVOLVE’ guidance. 26
Aims
We set out to involve members of the public in all aspects of our programme, the RCT in particular, and to support the research team to make the research relevant, attainable and applicable to patients and HCPs.
Methods
Seven PWD were recruited to the programme with the help of local and national service-user groups. Some members were recruited during the development phase of the programme, with all of the current group retaining membership during the course of the research grant.
The methods developed for the PPI group within ISDR are listed below:
-
We held regular meetings preceded by lunch in a venue located within the university campus. The location helped to create a relaxed and social atmosphere.
-
We sent the agenda prior to meetings, and any information related to agenda items with items signposted for discussion with and decisions by the group.
-
We made significant efforts to ‘translate’ any scientific or medical information.
-
Our discussions of items often created more questions from our PPI members and a need for other information. We realised early on in the meetings that we often needed at least two (and sometimes three) sessions to discuss agenda items and arrive at a considered position. Much of the information that we gave was technical, scientific or medical and there was often an associated high amount of material. We were asking our PPI group to make significant research decisions, such as the level of risk for the RCE, that would affect other PWD.
-
We worked with the PPI group in their decision-making to ensure that they were able to arrive at decisions without any doubts around their understanding or favouring a decision. This involved the following:
-
The chief investigator (CI) of the programme attended every PPI meeting and gave an update on the RCT.
-
We created a ‘research translator’, a member of the team who checked on the PPI group’s understanding and expectations.
-
We invited experts from the ISDR research team to give presentations and answer any queries from members of the PPI group.
-
We fed back at every meeting the progress of the PPI group’s decisions on every aspect of the RCT.
-
-
Members of the PPI group were also on the Programme Steering Committee, Programme Investigators Committee and Trial Steering Committee and had a macro view of the research process. Before each of these meetings, a pre meeting took place between the PPI member and one of the investigators.
-
Operational, logistical or political issues were discussed with the group for potential strategies.
Results: summary of patient and public involvement contributions to the ISDR programme
We met with our PPI group 19 times between 2013 and 2019, as detailed in Supplementary Material 1. In summary, they:
-
contributed to the programme development grant and research and programme grant application
-
developed with the research team candidate risk factors associated with progression of DR (WP C)
-
prioritised research questions by identifying the most important patient-centred outcomes (WP B2)
-
were heavily involved in setting the acceptable risk threshold in the RCE to be tested in the RCT (WPs C and E)
-
assisted in the preparation of patient-related materials, such as consent forms and patient information booklets (WP E)
-
suggested measures to improve study recruitment by observing screening clinics and the ISDR RCT consent process (WP E)
-
made suggestions to the clinical director of the Liverpool Diabetic Eye Screening Programme (LDESP) to improve screening attendance
-
served on oversight committees and helped to resolve issues during the lifetime of the grant
-
participated in cost-effectiveness analyses and suggested ways in which savings could be used to improve future services (WP D)
-
commented on this report and draft papers from the RCT and a paper on the acceptability of variable-interval risk-based screening to HCPs and PWD (WP F)
-
were involved in a PPI session in the dissemination conference (WP G).
Discussion: reflections/critical perspective
Members of our PPI group had a very positive impact on all aspects of the programme and strongly supported the research team. Their activities have been wide-ranging, not only in the technical details of the RCT, but also in their lobbying for variable-interval screening. As PWD, they were primarily concerned about the safety of the trial and improving the health of others, and mindful of the potential consequences of making the wrong decision and its impact on others’ lives. Our key lessons on successful PPI are set out in Box 1.
-
Co-investigators and preferably the CI to attend all PPI meetings.
-
Identify a ‘research translator’ or ‘research connector’ with sufficient knowledge of the research objectives and empowered to constructively challenge the research team.
-
Identify critical decisions in the research design to be taken by the group and develop a process to reach a clear conclusion. Needs to extend beyond reviewing patient information sheets and consent forms.
-
Avoid overload – plan to address two to three topics at each meeting and develop over a series of meetings.
-
Go slowly – give time for PPI members to express their opinions.
-
Assess levels of knowledge at all stages.
-
Hold regular meetings in a relaxed environment.
-
Ensure an appropriate spread of ages, experience and, where relevant, disease type.
-
Work hard at maintaining engagement over the duration of the research.
-
Ensure adequate funding is available.
-
Patients and members of the public can become ‘patient experts’.
-
Complex nuanced decisions can be reached.
-
Patient-centred outcomes can change research questions.
-
Well-argued patient opinions that are much more powerful than views of academics.
-
A research team with greatly improved communication skills and a clearer insight of the potential impact of their research.
Over the extended duration of our research programme, our PPI members developed wide expertise and became ‘patient experts’. Further work to develop systems to retain and build on this expertise could provide a useful resource for funding bodies and policy-makers.
Interrelation to other work packages and overall aims of the programme
The PPI group members supported most work packages of the programme, including B2 (observational cohort study), C (RCE), D (health economics), E (RCT) and F (qualitative study).
The ISDR study data warehouse
Research aims
To design and develop a purpose-built DW to support the development of the RCE, the delivery of the RCT, and the economic, qualitative and cohort studies.
Methods
Using Structured System Analysis and Design Methodology,27 the ISDR business intelligence (BI) team designed and built the ISDR DW and supporting architecture. The DW centralises data into a single Microsoft SQL (structured query language) server data repository hosted on a secure private local area network within the Department of Medical Physics and Clinical Engineering at the Royal Liverpool and Broadgreen University Hospital Trust (RLBUHT). All incoming and outgoing file transfers were carried out using secure networks and encryption methods, and received local information governance approvals (Privacy Impact Assessment, Data Governance Committee, Caldicott Guardian).
The following data sources interacted with the ISDR DW:
-
Demographic and systemic risk factor data from general practices (GPs) via the Liverpool Clinical Commissioning Group (LCCG) (Egton Medical Information Systems, EMIS Web, EMIS Health; www.emishealth.com).
-
Demographic, retinopathy grading and visual acuity (VA) data from –
-
the LDESP, collected in the Orion/Digital healthcare database between 1995 and 2013 and in OptoMize from 2013
-
HES-based screen-positive assessment clinics, collected in a bespoke Microsoft Access database (Diabolos) (1991–2015) and the hospital patient electronic notes system (PENS) (from 2016).
-
-
Appointment and treatment records from the hospital’s integrated patient-management system.
-
An ‘opt-out register’. PWD who were registered with GPs in Liverpool were informed of the project in a letter addressed from the screening programme. Their consent for inclusion in the DW was obtained via an ‘opt-out’ method and a record of those patients who opted out was kept in a register.
The flow of data in the programme is illustrated in Figure 2.
FIGURE 2.
Diagrammatic representation of ISDR DW data flows.
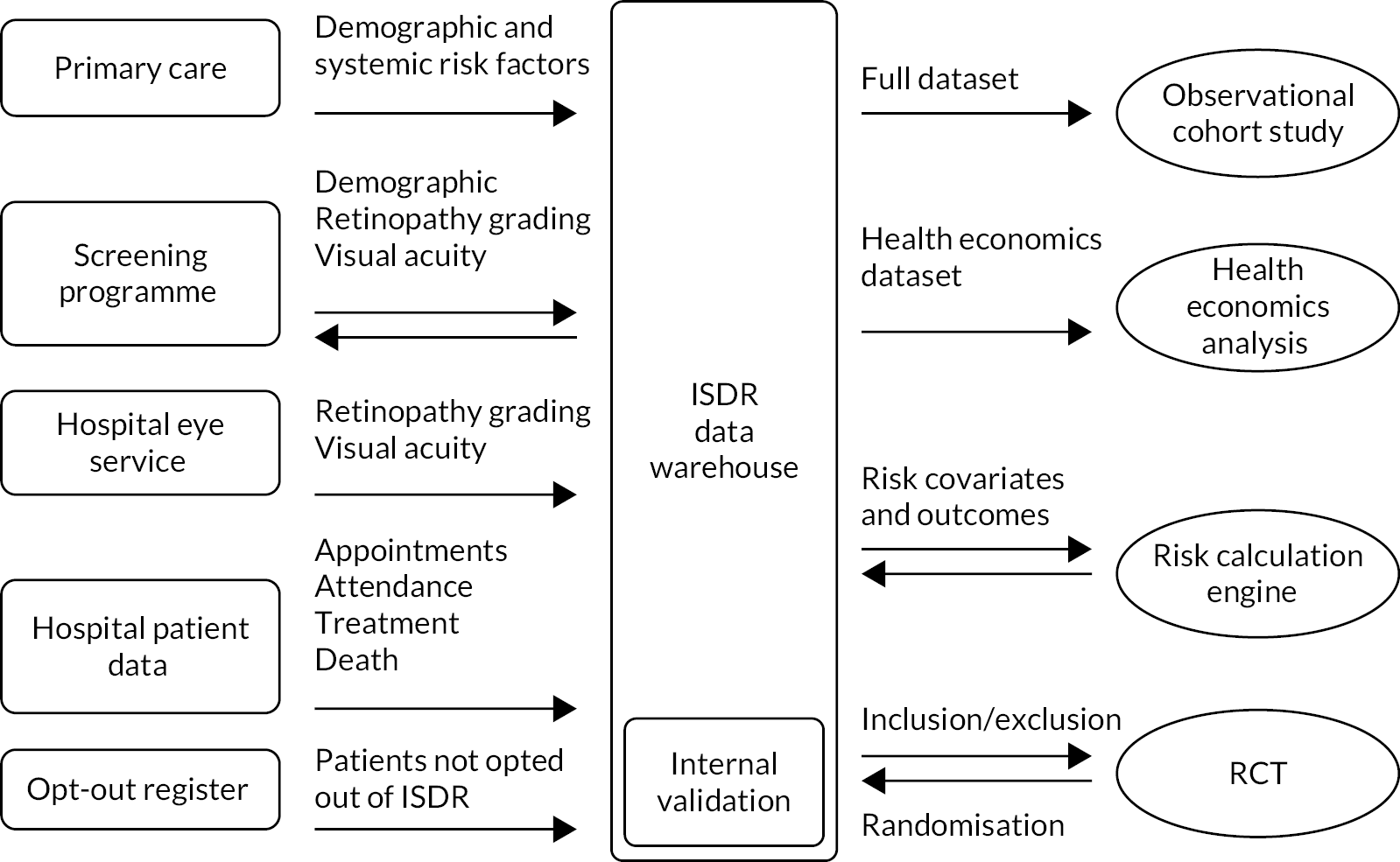
The DW received all relevant data from PWD streamed from primary and secondary care. The RCE was fed directly from the DW. Risk factors included were demographic (e.g. age, deprivation and ethnicity), ocular (e.g. previous and current retinopathy severity, previous treatment and VA) and systemic (e.g. diabetes control and duration, BP, and lipids). The data fields are listed in more detail in Supplementary Material 2, Table 1.
To ensure the credibility of data coming from multiple sources, which contained data with varying quality, covariates in the source data were examined for outliers and distribution inconsistencies. Credibility limits were developed using a statistical methodology. This set of scripts cleansed and validated incoming data for the DW. Input and output schemas (see Supplementary Material 2, Table 2) were developed for each data transfer element, as indicated by the arrows in Figure 2.
Results
The ISDR DW held data from 2009 on 28,556 participants as of January 2020 (end of the programme) over 11 screening cycles. Data linkage was achieved for all participants. The ISDR DW received test and preliminary data sets from early 2013. One of the main data sets was the ‘ISDR Cohort Study Dataset’, which supported WP B2. This contained data for 28,384 participants in October 2017, equivalent to 318,053,075 data points; a data point is a discrete unit of information,28 an example being a single recording of weight for a patient.
Discussion: challenges in the DW
The ISDR programme collated and linked large-scale routinely collected clinical data across different domains in a single repository. It successfully supported all aspects of the programme and routine patient management in the local screening programme. The DW is dynamic in that it updates on a regular basis. It demonstrated resilience and the potential to be developed into a routine health informatics tool in the NHS for personalised medicine.
We faced a number of challenges. No existing ‘off the shelf’ databases existed. The data were complex and came from multiple data sources. The Liverpool CCG had to set up and run a set of manual processes throughout the lifetime of the project to link the various domains within primary care. There was variability in data quality, which required bespoke processing and the development and application of credibility limits before the data could be considered adequate for a clinical application. Systems were of widely differing design: some were commercial (OptoMize) and some were developed in house (Diabolos, PENS). Systems had to be ‘cracked’ before the relevant data could be extracted. Data labels had to be investigated and tested. Several clinical system upgrades took place during the lifetime of the programme, which tended to occur unannounced, resulting in some lengthy delays and wasted effort.
The multidisciplinary nature of the programme meant that several members of the team had very little understanding of the requirements of database development and engineering, and similarly the database team struggled with adequate engagement with the clinical teams. There appears to be a lack of bioinformatic expertise to act as a bridge between the technical BI and computer science teams on the one hand and the clinical/research end-users on the other. This needs urgently addressing.
We learnt several important lessons. Establishing a real-time data repository within an NHS environment to underpin personalised medicine requires:
-
sufficient time for interdisciplinary team working on data sanity checks, outlier/credibility handling protocols, logic rule development and imputation strategies
-
investment in developing cross-disciplinary bioinformatics expertise to link technical teams and clinical/research end-users.
Interrelation to other work packages and overall aims of the programme
In addition to the underpinning role of the DW across much of the programme, it has led to development of new IP covered in WP G. Imputation strategies needed to be developed in several WPs to deal with missing data.
The Liverpool Risk-Calculation Engine
A peer-reviewed publication21 reports additional information on the continuous Markov process, imputation, covariate selection, threshold setting and model checking.
Parts of this chapter are reproduced or adapted from Broadbent et al. 22 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Research aims
To develop and internally validate a RCE to estimate the risk of progression to screen-positive or referable DR and assign individualised screening intervals (WP C).
Methods
Data set
Data from established screening and primary care systems were combined in the ISDR DW. A set of candidate covariates was selected for the model in partnership with our PPI group. A review of the literature around the known risk factors was presented by the research team, and additional candidate covariates were proposed by the PPI team to create a ‘long list’. The ISDR DW was explored with this set of candidates in mind, and a RCE development data set was extracted containing covariates with ≥ 80% completeness in PWD who were screen-negative at the first of at least two episodes that occurred in a 5-year sample period.
Model
We selected a continuous-time Markov process to allow for a set of individuals to transition among states over time. 29 The state at each time point was defined by the level of retinopathy, including separation by one or both eyes (Figure 3):30 state 1 – no DR detected; state 2 – non-referable DR in one eye only; state 3 – non-referable DR in both eyes; and state 4 – referable DR (screen-positive for at least one eye).
FIGURE 3.
States and transitions in the continuous-time Markov process in the Liverpool Risk-Calculation Engine.
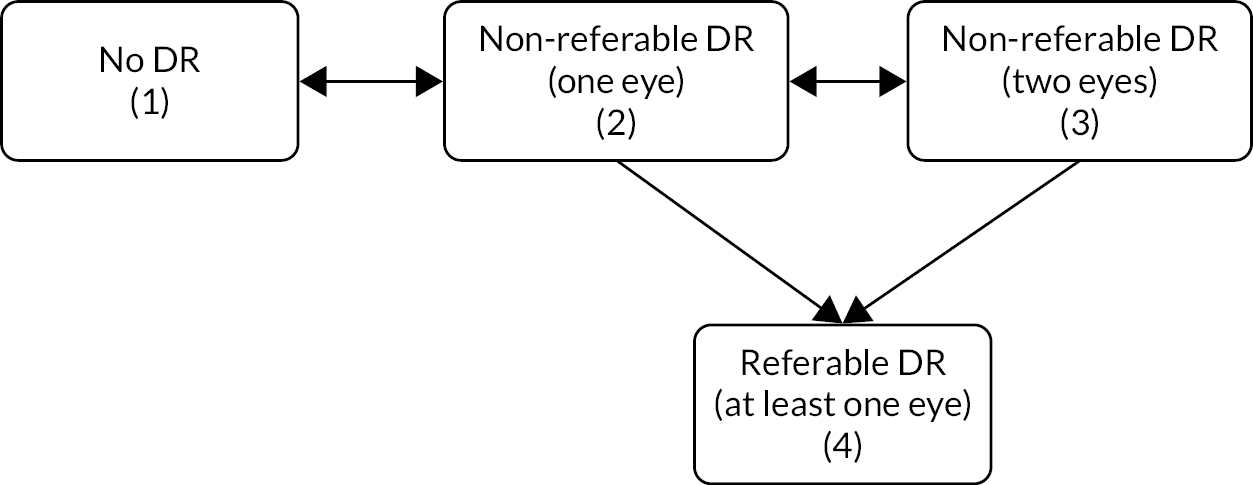
Only one baseline screening event was used. The risks or intensities for each of six transitions between these states were entered into the model within a probability matrix. The interval censoring seen in screening data required special methodology. Missing clinical data were handled using multiple imputation.
Ten covariates met the entry criteria and were ranked using Wald statistics. A set of nested models were built to estimate corrected Akaike’s information criterion (AICc). The model with the smallest AICc was chosen to give the best fit to the data.
We checked the RCE development data set using random samples of event vectors. The model was checked for the influence of outliers, regression and distributional assumptions; Pearson-type goodness of fit and corrected C-index were calculated. Bootstrapping and fourfold cross-validation were used for internal validation. Further internal validation was conducted using a geographical split based on deprivation index. 31
Implementation
The effect of a set of risk thresholds (5%, 2.5%, 1%) on screening interval allocation was investigated and a final threshold was selected in discussion with the PPI group.
A small sample of cases assigned by the RCE to 6-, 12- and 24-month intervals were independently checked against patient records for clinical credibility.
After design and testing, the RCE was implemented within the Liverpool Clinical Trials Centre for testing within the RCT (see Randomised controlled trial to evaluate the safety and cost-effectiveness of individualised variable-interval risk-based screening). Data were received from the DW electronically on a daily basis.
Results
Data extracted into the RCE development data set were from 11,806 PWD attending the LDESP between 20 February 2009 and 4 February 2014.
Covariates meeting the selection criteria are listed in Table 1. Those that gave the best fit and were included in the final model were disease duration, glycated haemoglobin (HbA1c), age at diagnosis, systolic BP and total cholesterol. Although the retinopathy stage is not technically a covariate, it is included in the table to show the improvement in predictive power when covariates are added to the model.
| Covariates | Wald statistic | Rescaled AICc | Explained likelihood (%) |
|---|---|---|---|
| Retinopathy (baseline) | 893.65 | – | |
| + Disease duration (years) | 293.4 | 423.23 | 48 |
| + HbA1c (mmol/mol) | 201.2 | 68.61 | 85 |
| + Age at diagnosis (years) | 44.2 | 10.85 | 92 |
| + Systolic BP (mmHg) | 18.9 | 6.61 | 94 |
| + Total cholesterol (mmol/l) | 18.7 | 0 | 96 |
| + Disease type | 15.2 | 0.99 | 97.5 |
| + Diastolic BP (mmHg) | 8.2 | 5.61 | 98.6 |
| + eGFR (ml/min–1 1.73 m2 –1) | 5.4 | 13.63 | 99.4 |
| + Sex | 5.1 | 24.95 | 99.9 |
| + HDL cholesterol (mmol/l) | 0.73 | 40.99 | – |
The risk model is summarised in three equations. The first gives the hazard rates (or intensities or ‘risks’) of going from one state to another for each transition:
where i, j = {1, 2, 3, 4} and βCij is the model parameter for covariate C (or baseline intensity when C = 0); AgeD is age at diagnosis and DiseaseD is disease duration. From this equation, a transition intensity matrix is derived for the four states described above:
Probabilities of transition occurring at a specific time are obtained by using the third equation:
Tables 2 and 3 show the estimated baseline hazard ratios and probabilities of each transition state. Further details are available elsewhere. 30
| Transition | HR for transition (95% CI) | ||||
|---|---|---|---|---|---|
| Age at diagnosis | Disease duration | HbA1c | Cholesterol | SBP | |
| 1 to > 2 | 1.00450 (1.00115 to 1.00787) | 1.0280 (1.0213 to 1.0348) | 1.0101 (1.00743 to 1.0128) | 0.963 [0.923 to 1.00521] | 1.00409 (1.00104 to 0.0073) |
| 2 to > 1 | 1.00580 (1.00237 to 1.00919) | 0.983 (0.975 to 0.992) | 0.998 (0.995 to 1.00140) | 1.0153 [0.973 to 1.0592] | 0.999 (0.996 to 1.00244) |
| 2 to > 3 | 0.989 (0.984 to 0.994) | 1.0261 (1.0173 to 1.0350) | 1.00621 (1.00221 to 1.0102) | 0.965 [0.901 to 1.0333] | 0.998 (0.993 to 1.00255) |
| 2 to > 4 | 1.0245 (0.990 to 1.0605) | 0.989 (0.931 to 1.0510) | 1.00554 (0.983 to 1.0285) | 1.0231 [–0.27 to 0.37] | 1.00342 (0.977 to 1.0310) |
| 3 to > 2 | 1.00839 (1.00329 to 1.0135) | 0.959 (0.949 to 0.968) | 0.990 (0.985 to 0.994) | 1.0836 [1.0147 to 1.157] | 0.997 (0.993 to 1.00126) |
| 3 to > 4 | 0.986 (0.977 to 0.995) | 1.00420 (0.989 to 1.0200) | 1.0164 (1.00888 to 1.0239) | 1.0346 [0.918 to 1.166] | 1.00501 (0.996 to 1.0141) |
| Transition | Probability (95% CI) |
|---|---|
| 1 to > 2 | 0.114 (0.111 to 0.118) |
| 2 to > 1 | 0.552 (0.541 to 0.565) |
| 2 to > 3 | 0.141 (0.134 to 0.148) |
| 2 to > 4 | 0.0163 (0.0139 to 0.0202) |
| 3 to > 2 | 0.283 (0.272 to 0.294) |
| 3 to > 4 | 0.0574 (0.0485 to 0.0678) |
Data and model checking
Homogeneity in the RCE was checked by smoothed summary residuals compared with follow-up time with 95% CI. The calibration curves for the Cox–Snell residuals were close to the theoretical optimal calibration. The Pearson-type statistic denoted not enough evidence to reject the null hypothesis of good fit. Cross validation showed that the training and test performance measures were essentially the same. Fitting the model to the most deprived subjects produced only very small changes in risk allocation of the non-deprived group.
Setting the risk threshold
The PPI group identified acceptable screen intervals of 6, 12 and 24 months, and risks of 1% and 2.5% of missing screen-positive disease at any future screen episode. Exploration of the effect of different risk thresholds on allocation to the three different screen intervals showed that, as the threshold decreased, the proportion of incorrect allocations decreased for screen-positives (overestimation) and increased for negatives (underestimation). For all three, there was a reduction in the overall number of screening episodes required. The research team and PPI group considered that a 2.5% criterion showed a satisfactory distribution across the three screening intervals and a reasonable reduction in episodes, and this was selected for implementation.
Discussion
We have developed and tested a RCE in which an individual’s risk can be predicted from routinely collected clinical data, referenced to the clinical histories of the local population, and using covariates of local relevance. The risk can be reassessed at each screening episode as new clinical information is acquired.
Strengths of our model include the strongly embedded PPI group, which allowed us to develop an appropriate preliminary covariate list, acceptable screen intervals and risk threshold. The model internally handles interval censoring, inherent to retinopathy data in screening. 29 It predicts the probabilities of transition for all patient states and embeds a model for multiple imputation of missing covariates.
Potential limitations include not adjusting for misclassification of retinopathy during grading. This could be addressed by adding a misclassification model but at the cost of substantially more observations and computational complexity. Some potentially useful covariates were not informative in the Liverpool setting: ethnic diversity and prevalence of abnormal estimated glomerular filtration rate levels were low, and ‘type of diabetes’ may not have been accurately recorded. We used the date of the first HbA1c test to improve data on ‘duration of diabetes’. This was helpful in people with long durations but less reliable since the introduction of HbA1c as a primary screening test.
The model consistently showed good levels of prediction for the 2.5% risk threshold. The numbers of screen-positive cases with overestimated screening dates and screen-negative cases with underestimated screening dates were smaller. The majority of people were correctly allocated (78% of screen-positives, 80% of screen-negatives), with a reasonable allocation across the 6-, 12- and 24-month intervals (approximately 10% : 10% : 80%). The number of patients who had a screen-positive event before the allocated screening date reduced by > 50% and the overall number of screening episodes by 30%.
Our RCE development process is suitable for a wide range of geographical locations and populations, with a minimum prerequisite of a disease register with adequate historical data. Revision/addition of covariates can be accommodated based on the strength that they add to a locally developed model. We give the key steps to developing and building such a system in Box 2.
-
Approvals and systems for regular data transfers.
-
Patient and professional groups for covariate selection.
-
First iteration data set.
-
Explore data and verify.
-
Set data range criteria for local relevance.
-
Lock risk engine development data set.
-
Handle missingness with multiple imputation.
-
Select preliminary Markov model using all available covariates selection (model fitting).
-
Assign informedness to covariates.
-
Review with local patient and professional groups.
-
Finalise covariates and fix model structure.
-
Build test model.
-
Agree choice of screen intervals.
-
Run diagnostics and validation.
-
Final model version (revise if required).
-
Secure domain for implementation model.
-
Determine frequency to update data (recommend 2 monthly).
-
Re-tune model (recommend 3 yearly).
The use of near real-time data and a model developed from local data in our approach is novel. Aspelund et al. 13 reported on a risk-estimating model using retinopathy data collected in Iceland between 1994 and 1997, and risks for covariates estimated from data published in the 1980s. This was tested in a prospective cohort of people with type 2 diabetes. 32 Discriminatory ability was good (C-statistic 0.83) but 67 out of 76 people (88.2%) who developed STDR developed it after the time predicted by the model. This overestimation of risk highlights the weakness of using historical data. In 2020, van der Heijden et al. 33 reported a systematic review of risk-prediction models in DR screening. Discrimination was reported in seven studies with C-statistics ranging from 0.55 to 0.84. Our study21 was published subsequently and, therefore, was not included. The corrected C-index in the Liverpool RCE was 0.687.
Access to clinical information is not routinely available. We had to overcome significant challenges in developing a near real-time data flow; this may be too difficult in some populations. However, we determined that including clinical data would aid acceptance among the professional community, offer better prospects for generalisability and allow inclusion of more frequent screening for high-risk individuals. Our view is supported by our own data,6 those of others34 and our PPI group. We recognise that estimates of resource requirements for introduction of our type of RCE are not available.
External validation of models is required before general implementation. 32,35 An implementation phase will include model updating (temporal validation and model tuning) and the opportunity for comparative cross-population (external) validation to correct for potential over-performance. 36
Conclusions
This research programme indicates that the Liverpool RCE is feasible, reliable, safe and acceptable to patients. Our evidence suggests that a RCE could offer potential significant transfer of resources into targeting high-risk and hard-to-reach groups, as well as improved cost-effectiveness. Based on the internal validations we performed, it showed sufficient performance for a local introduction and testing of safety and acceptability within a RCT. However, we did not provide evidence of external validation.
Interrelation with other parts of the programme and overall aims of the programme
The RCE was developed using data from primary and secondary care linked through the ISDR DW developed specifically for this programme. It was required for the development of the RCT and is part of the developed IP. It will form part of any potential future benefit from the programme if deployed within the NHS and other countries. The health economics team worked with the RCE team ensuring that the economic model followed the structure and content of the RCE and could be fully integrated alongside it.
Randomised controlled trial to evaluate the safety and cost-effectiveness of individualised variable-interval risk-based screening
Peer-reviewed publications are available on the ISDR RCT and its protocol. 22,23 Additional information is available in these publications on trial methods, sample size revisions, withdrawals, and additional secondary safety and efficacy data. Our economic analysis is described in Cost-effectiveness of individualised variable-interval risk-based screening for diabetic retinopathy.
We followed the CONSORT 2016 reporting guidelines for non-inferiority and equivalence trials.
Parts of this chapter are reproduced or adapted from Byrne et al. 24 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Research aims
We designed a RCT to investigate the safety, feasibility, efficacy and cost-effectiveness of extending screening intervals in low-risk PWD and reducing screening intervals in high-risk PWD (WP E). We utilised the emerging methodology and technologies of personalised risk prediction37,38 and an innovative data linkage system to develop an individualised variable-interval risk-based screening approach. Individualised clinical care offers opportunities for improved patient engagement.
We tested the hypothesis of equivalence between the attendance rates as a primary measure of safety, for individualised and annual screening. An equivalence design was selected, instead of a superiority trial, because the aim was to demonstrate equivalence between attendance rates rather than difference. Our PPI group was involved in all aspects of design, delivery and interpretation (see Patient and public involvement in the ISDR programme).
Methods
This was a single site, two-arm, parallel assignment, equivalence RCT conducted in all screening clinics in the LDESP, part of the NDESP. The trial was conducted with the Liverpool Clinical Trials Centre.
The main inclusion and exclusion criteria were: registered with a GP whose postcode was within the city boundaries of Liverpool, aged ≥ 12 years, attending screening for DR and had not opted out of data-sharing. Age-appropriate patient information leaflets were sent to eligible patients with their screening appointment. Participants provided written informed consent. For children aged 12–15 years, proxy consent by the parent/guardian was provided and, where appropriate, assent from the child. Preston Research Ethics Committee approved the trial (14/NW/0034). The trial registry number is ISRCTN87561257.
Allocation was randomly 1 : 1 to individualised variable-interval risk-based screening recall at 6, 12 or 24 months (intervention arm, high-, medium-, low-risk respectively), or annual screening (control arm, current routine care).
The ISDR DW automatically populated the majority of the fields in the baseline and follow-up electronic CRFs, including data for randomisation. Screening staff and clinical assessors were masked to intervention arm, risk-calculation and interval.
Procedures
Allocation of participants by the RCE (see The Liverpool Risk-Calculation Engine) at baseline and each follow-up was against the risk of becoming screen-positive at 6-, 12- and 24-month intervals. The interval was allocated against the criterion deemed appropriate by the PPI group of less than 2.5% risk of becoming screen-positive before the next appointment. Independent risk factor covariates were retinopathy levels in both eyes, age, duration of diabetes, HbA1c, systolic BP and total cholesterol. Participants in the fixed-interval control arm continued with annual screening. For those in the individualised arm, the interval could change at each visit.
Outcomes
The primary outcome of attendance rate at the first follow-up visit assessed the safety of individualised variable-interval risk-based screening. Non-attendance was defined as failure to attend any appointment within 90 days of the follow-up invitation date. Secondary outcomes measuring efficacy and safety were screen-positive disease, number of cases of STDR detected, median VA [logarithm of the minimum angle of resolution (logMAR)] and rates of VI (VA ≥ 0.30 logMAR). All cases of STDR that occurred between baseline and 24 months (+ 90 days) were included in the analysis. A medical retina specialist determined if referred STDR was true- or false-positive using slit-lamp biomicroscopy at a dedicated HES clinic.
Statistical analyses and sample size calculations
The primary analysis was to test for equivalence in attendance rates between individualised and annual screening. The estimated minimum number of patients required was 4460 (90% power, 2.5% one-sided significance level, 5% equivalence margin, 6% loss to follow-up). Primary analysis was per protocol (PP). Intention-to-treat (ITT) and multiple imputation analyses were also conducted.
A secondary aim was to investigate whether or not personalised screening could be considered as non-inferior for the detection of STDR when compared with annual screening; this used a 1.5% non-inferiority margin.
Within the three risk groups, equivalence in attendance rates between the two arms and non-inferiority in the detection of STDR in the individualised arm were explored. For this analysis, participants in the control arm were allocated to risk groups based on the RCE risks at baseline. Generalised linear models were fitted with arm, level of risk and their interaction added as factors.
Results
The trial opened on 1 May 2014, randomisation took place from 12 November 2014 to 14 June 2016 and last follow-up was 16 August 2018. In total, 4069 out of 8607 eligible people were excluded, which meant that there were 4538 individuals who were randomised and allocated to a trial arm (2269 fixed interval, 2265 individualised, 4 withdrawals). In the individualised arm, at baseline 198 participants were allocated their first screening recall at 6 months, 211 at 12 months and 1856 at 24 months, which reflects the allocation to high, medium and low risk by the RCE. The trial profile is shown in Figure 4.
FIGURE 4.
CONSORT 2010 flow diagram showing the numbers for eligibility, allocation, withdrawals in the PP and ITT data sets for the primary analysis.
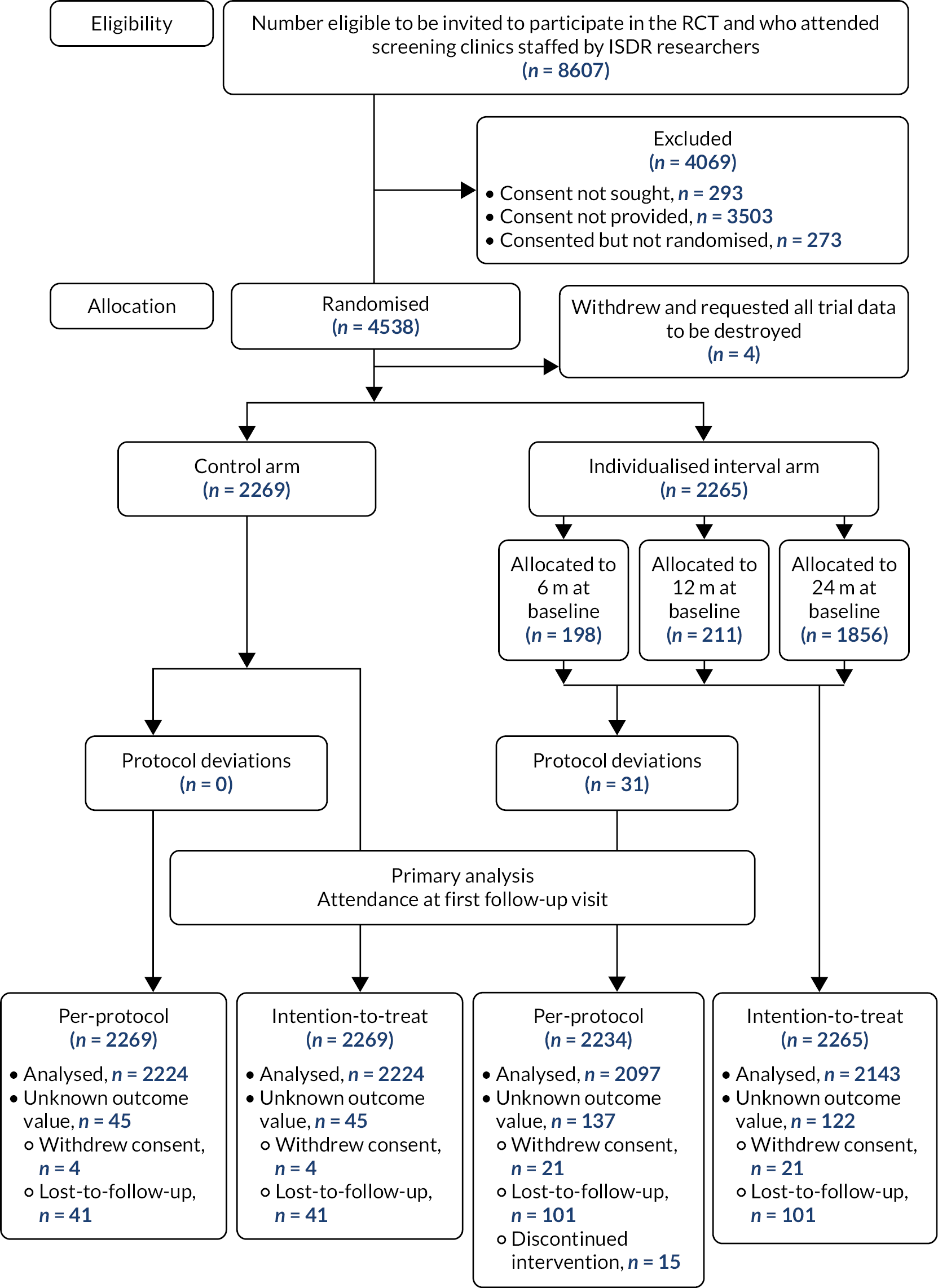
Baseline characteristics in the PP data set are shown in Table 4; the ITT data set had similar characteristics. Participants had a median age of 63 years (range 14–100 years), 60.4% were male, 94.6% were white and 88.5% had type 2 diabetes. Those in the high-risk group were more likely to have type 1 diabetes, a longer diabetes diagnosis and higher HbA1c, and less likely to have ever smoked, when compared to those in the medium- and low-risk groups.
| Baseline characteristic | Arm | Baseline risk group | Overall total (N = 4503) | |||
|---|---|---|---|---|---|---|
| Fixed (12 m) (N = 2269) | Individualised (N = 2234) | High (N = 197) | Medium (N = 211) | Low (N = 1826) | ||
| Gender, n (%) | ||||||
| Male | 1358 (59.9) | 1360 (60.9) | 124 (62.9) | 135 (64.0) | 1101 (60.3) | 2718 (60.4) |
| Female | 911 (40.1) | 874 (39.1) | 73 (37.1) | 76 (36.0) | 725 (39.7) | 1785 (39.6) |
| Ethnicity, n (%) | ||||||
| White | 2140 (94.3) | 2120 (94.9) | 180 (91.4) | 204 (96.7) | 1736 (95.1) | 4260 (94.6) |
| Asian | 48 (2.1) | 30 (1.3) | 2 (1.0) | 3 (1.4) | 25 (1.4) | 78 (1.7) |
| Black | 40 (1.8) | 43 (1.9) | 6 (3.0) | 3 (1.4) | 34 (1.9) | 83 (1.8) |
| Chinese | 7 (0.3) | 6 (0.3) | 1 (0.5) | 1 (0.5) | 4 (0.2) | 13 (0.3) |
| Other | 25 (1.1) | 29 (1.3) | 8 (4.1) | 0 (0.0) | 21 (1.2) | 54 (1.2) |
| Unknown | 9 (0.4) | 6 (0.3) | 0 (0.0) | 0 (0.0) | 6 (0.3) | 15 (0.3) |
| Smoking status, n (%) | ||||||
| Smoker | 419 (18.5) | 364 (16.3) | 26 (13.2) | 39 (18.5) | 299 (16.4) | 783 (17.4) |
| Ex-smoker | 877 (38.7) | 899 (40.2) | 69 (35.0) | 76 (36.0) | 754 (41.3) | 1776 (39.4) |
| Non-smoker | 965 (42.5) | 967 (43.3) | 102 (51.8) | 96 (45.5) | 769 (42.1) | 1932 (42.9) |
| Unknown | 8 (0.4) | 4 (0.2) | 0 (0.0) | 0 (0.0) | 4 (0.2) | 12 (0.3) |
| Diabetes type, n (%) | ||||||
| Type 1 | 80 (3.5) | 99 (4.4) | 38 (19.3) | 14 (6.6) | 47 (2.6) | 179 (4.0) |
| Type 2 | 2024 (89.2) | 1962 (87.8) | 140 (71.1) | 180 (85.3) | 1642 (89.9) | 3986 (88.5) |
| Unknown | 165 (7.3) | 173 (7.7) | 19 (9.6) | 17 (8.1) | 137 (7.5) | 338 (7.5) |
| Age (years) | ||||||
| Observed, n | 2269 | 2234 | 197 | 211 | 1826 | 4503 |
| Median (IQR) | 63.3 (55.0–71.0) | 62.8 (54.8–70.3) | 58.3 (49.9–66.2) | 60.9 (53.4–69.8) | 63.7 (55.9–70.8) | 63.1 (54.9–70.7) |
| Range | 14.1–100.7 | 15.4–91.3 | 17.5–86.8 | 15.4–86.8 | 16.8–91.3 | 14.1–100.7 |
| Disease duration (years) | ||||||
| Observed, n | 2267 | 2231 | 197 | 209 | 1825 | 4498 |
| Unknown, n | 2 | 3 | 0 | 2 | 1 | 5 |
| Median (IQR) | 6.9 (4.2–10.9) | 7.0 (4.2–11.2) | 11.1 (7.3–16.1) | 9.8 (6.3–13.7) | 6.4 (4.0–10.1) | 7.0 (4.2–11.0) |
| Range | 0.6–66.4 | 1.0–44.7 | 1.2–44.7 | 1.1–37.2 | 1.0–39.1 | 0.6–66.4 |
| HbA1c | ||||||
| Observed, n | 2269 | 2232 | 197 | 211 | 1824 | 4501 |
| Unknown, n | 0 | 2 | 0 | 0 | 2 | 2 |
| Median (IQR) (mmol/mol) | 51 (44–61) | 52 (44–63) | 67 (53–84) | 58 (51–67) | 50 (44–60) | 51 (44–62) |
| Range |(mmol/mol) | 26–146 | 28–155 | 33–134 | 34–155 | 28–104 | 26–155 |
| Median (IQR) (%) | 6.8 (6.2–7.7) | 6.9 (6.2–7.9) | 8.3 (7.0–9.8) | 7.5 (6.8–8.3) | 6.7 (6.2–7.6) | 6.8 (6.2–8.8) |
| Range (%) | 4.5–15.5 | 4.7–16.3 | 5.2–14.4 | 5.3–16.3 | 4.7–11.7 | 4.5–16.3 |
| Systolic BP | ||||||
| Observed, n | 2268 | 2234 | 197 | 211 | 1826 | 4502 |
| Unknown, n | 1 | 0 | 0 | 0 | 0 | 1 |
| Median (IQR) (mmHg) | 130.0 (121.0–138.0) | 130.0 (122.0–138.0) | 130.0 (124.0–138.0) | 132.0 (124.0–140.0) | 130.0 (122.0–138.0) | 130.0 (122.0–138.0) |
| Range (mmHg) | 84.0–213.0 | 90.0–204.0 | 93.0–175.0 | 95.0–204.0 | 90.0–200.0 | 84.0–213.0 |
| Diastolic BP | ||||||
| Observed, n | 2208 | 2180 | 193 | 201 | 1786 | 4388 |
| Unknown, n | 61 | 54 | 4 | 10 | 40 | 115 |
| Median (IQR) (mmHg) | 76.0 (70.0–80.0) | 76.0 (70.0–80.0) | 77.0 (70.0–80.0) | 77.0 (70.0–80.0) | 76.0 (70.0–80.0) | 76.0 (70.0–80.0) |
| Range (mmHg) | 46.0–140.0 | 46.0–130.0 | 54.0–105.0 | 57.0–130.0 | 46.0–110.0 | 46.0–140.0 |
| Total cholesterol | ||||||
| Observed, n | 2258 | 2224 | 196 | 209 | 1819 | 4482 |
| Unknown, n | 11 | 10 | 1 | 2 | 7 | 21 |
| Median (IQR) (mmol/l) | 4.0 (3.4–4.7) | 4.0 (3.4–4.7) | 4.0 (3.4–4.9) | 4.0 (3.4–4.6) | 4.0 (3.5–4.7) | 4.0 (3.4–4.7) |
| Range (mmol/l) | 1.4–8.1 | 1.8–9.7 | 2.0–9.0 | 2.2–7.6 | 1.8–9.7 | 1.4–9.7 |
| Retinopathy level, n (%) | ||||||
| R0 R0 | 1857 (81.8) | 1800 (80.6) | 1 (0.5) | 44 (20.9) | 1755 (96.1) | 3657 (81.2) |
| R1 R0 | 262 (11.5) | 296 (13.2) | 58 (29.4) | 167 (79.1) | 71 (3.9) | 558 (12.4) |
| R1 R1 | 146 (6.4) | 137 (6.1) | 137 (69.5) | 0 (0.0) | 0 (0.0) | 283 (6.3) |
Primary safety findings
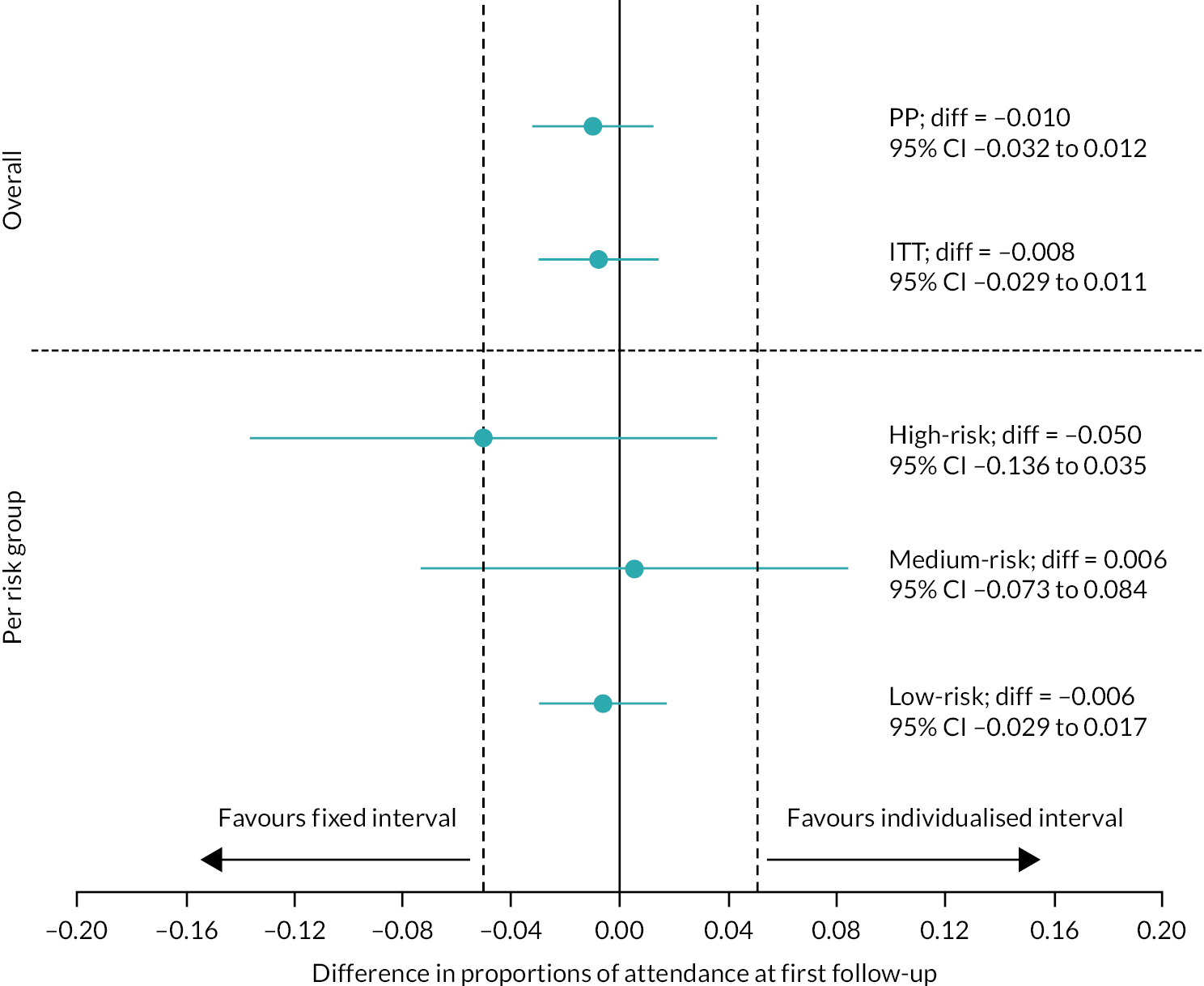
Attendance rates at the first follow-up visit for the control and individualised arms (primary outcome) were 84.7% (1883/2224) and 83.6% (1754/2097), respectively (difference –1.0%, 95% CI –3.2% to 1.2%, PP analysis). Against an acceptability range of 0.05, these attendance rates were equivalent (Figure 5 upper panel, Table 5). PP and ITT analyses with multiple and simple imputation confirmed equivalence in attendance rates between the two arms.
FIGURE 5.
Plot showing point estimates and 90% CIs for participants attending the first follow-up visit in the two arms. Upper panel: primary analysis. Lower panel: high-, medium- and low-risk groups in the individualised arm.
| Approach | Control arm | Individualised arm | Difference in proportions (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| N | Attended/STDR detected (n) | Proportion | N | Attended/STDR detected (n) | Proportion | |||
| Primary outcome: attendance at first follow-up | ||||||||
| PP | ||||||||
| Overall | 2224 | 1883 | 0.847 | 2097 | 1754 | 0.836 | –0.010 (–0.032 to 0.012) | |
| High risk | 203 | 157 | 0.773 | 195 | 141 | 0.723 | –0.050 (–0.136 to 0.035) | |
| Medium risk | 169 | 138 | 0.817 | 208 | 171 | 0.822 | 0.006 (–0.073 to 0.084) | |
| Low risk | 1852 | 1588 | 0.857 | 1694 | 1442 | 0.851 | –0.006 (–0.029 to 0.017) | |
| Multiple imputation | 2269 | 1910 | 0.842 | 2234 | 1870 | 0.837 | –0.005 (–0.026 to 0.017) | |
| ITT | ||||||||
| Overall | 2224 | 1883 | 0.847 | 2143 | 1798 | 0.839 | –0.008 (–0.029 to 0.014) | |
| Multiple imputation | 2269 | 1913 | 0.843 | 2265 | 1903 | 0.840 | –0.004 (–0.025 to 0.018) | |
| Secondary outcome: STDR within 24 months | ||||||||
| PP | ||||||||
| Overall | 2042 | 35 | 0.017 | 1956 | 28 | 0.014 | –0.003 (–0.011 to 0.005) | |
| High risk | 176 | 20 | 0.114 | 127 | 17 | 0.134 | 0.020 (–0.053 to 0.100) | |
| Medium risk | 157 | 5 | 0.032 | 179 | 7 | 0.039 | 0.007 (–0.038 to 0.051) | |
| Low risk | 1709 | 10 | 0.006 | 1650 | 4 | 0.002 | –0.003 (–0.009 to 0.001) | |
| Multiple imputation | 2269 | 39 | 0.017 | 2234 | 36 | 0.016 | –0.001 (–0.009 to 0.007) | |
| ITT | ||||||||
| Overall | 2042 | 35 | 0.017 | 2056 | 32 | 0.016 | –0.002 (–0.010 to 0.006) | |
| Multiple imputation | 2269 | 39 | 0.017 | 2265 | 39 | 0.017 | –0.001 (–0.008 to 0.008) | |
Equivalence in the analysis of the three risk groups in the individualised arm (see Figure 5, lower panel, and Table 5) was found for the low-risk group (control 85.7%, individualised 85.1%, difference –0.6%, 95% CI –2.9% to 1.7%). For the medium-risk group, equivalence was not confirmed (control 81.7%, individualised 82.2%, difference 0.6%, 95% CI –7.3% to 8.4%); differences were very small but equivalence was not confirmed because of the relatively wide CIs. Attendance rates were lower in the high-risk group (control 77.3%, individualised 72.3%, difference 5.0%, 95% CI –13.6% to 3.5%) than in the medium- and low-risk groups. Although the hypothesis of equivalence was not supported in this group, the attendance rates observed over 12 months (percentage with at least one attended appointment) were higher in the individualised arm (89.1%) than in the control arm (77.3%).
Secondary safety findings
There was no evidence of a loss of ability to detect STDR in the individualised arm (1.4% vs. 1.7%, difference –0.3%, 95% CI –1.1% to 0.5%, prespecified non-inferiority margin 1.5%) (see Table 5). Similar results were obtained in the ITT and imputation analyses. Non-inferiority was found for the low-risk group (control 0.6%, individualised 0.2%, difference –0.3%, 95% CI –0.9% to 0.1%), but not the high- and medium-risk groups; this was probably explained by the small numbers.
We did not detect any clinically significant worsening of diabetes control. There was no difference in the proportions of participants in each group who had a significant (≥ 11 mmol/mol) increase in HbA1c during the trial: control 15.2%, individualised 14.6%, high 13.4%, medium 15.0% and low 14.7%. We did not detect any differences in logMAR VA (p = 0.64) or in rates of VI in the better eye at the last attended visit between the two arms. Findings for worse eye VA and VI were similar.
Efficacy
In total, 43.2% fewer screening attendances were required in the individualised arm than in the control arm (2008 vs. 3536). Higher rates of screen-positive by screen episode attended were seen in the individualised arm than in the control arm [control 4.52% (160/3536), individualised 5.08% (102/2008)]. Within the individualised arm, the high-risk group had the highest screen-positive rate [high 10.72% (34/317), medium 6.02% (15/249), low 3.7% (53/1442)]. In the low-risk group, most of the screen-positive cases were a result of other eye disease; the rates of screen-positive for DR were low at 0.5% (7/1442). Screening episodes that detected STDR were earlier in the individualised arm than in the control arm: 6–12 months 17.9% (5/28) versus 2.9% (1/35), 12–18 months 32.1% (9/28) versus 60.0% (21/35), respectively.
The number of appointments over the 2 years per participant in the individualised arm varied by allocated group at baseline: 1.83, 1.06 and 0.85 in the high-, medium- and low-risk groups, respectively. The RCE was stable throughout the RCT. A total of 132 participants were switched by the RCE to a longer screening interval and 176 to a shorter screening interval.
Discussion
Our study, which, to the best of our knowledge, is the largest ophthalmic RCT performed in individualised DR screening to date, shows that all parties involved in diabetes care can be reassured that extended and individualised (personalised) interval screening can safely be introduced in an established screening programme. We have shown that variable-interval risk-based screening, as developed by us in Liverpool, is equivalent to the current standard of annual screening, in our setting. Our secondary safety findings support this primary result. In the individualised arm, we did not detect any drop in rates of detection of STDR, worse VA or higher rates of VI, or worsening glycaemic control. Attendance levels were maintained throughout the study.
We have also shown improved efficacy for our personalised approach, reducing the number of appointments by 43.2%. In the individualised arm, there were higher proportions of screening episodes that were screen-positive and screening episodes that detected STDR were earlier than in the control arm. In the setting of a RCT, the RCE showed feasibility and reliability with good discrimination.
A move to longer screening intervals for people at low risk of DR has been suggested in the literature prior to39 and during40–42 our programme, but without convincing evidence on safety. 8 Overall, 19.0% of people invited to take part in our study explicitly stated that they wished to remain on annual screening or did not want a change of interval. We did not detect a worsening in glycaemic control. Our findings give substantial reassurance that a 24-month interval for low-risk individuals with diabetes is safe in a setting such as ours. However, for resource-poor or rural settings in low- and middle-income countries, further research is required before longer intervals can be contemplated.
Our findings have some limitations when considering wider application. We developed and applied our approach to screening in a single centre with relatively low rates of baseline DR and low rates of progression to sight-threatening disease. Our programme has been running for over 30 years. Participants’ glycaemia and BP control were relatively good. Our results should be generalised with caution to other settings with higher prevalence, poorer control of diabetes, wider ethnic group representation, or in programmes in set-up. Our study has important strengths including the RCT design, its size, independent oversight, and involvement of an expert patient group.
Our approach allowed us to target high-risk people using a 6-month screening interval. Attendance rates at first visit in this group were lower in the individualised arm (72.3% vs. 77.3% in the control group) but disease was detected earlier through more frequent screening (1.7 visits, 89.1% attendance over the first 12 months vs. 77.3% estimated in controls) than in the control arm. The rates of detection of STDR were higher in the high- and medium-risk individualised groups (13.4% and 3.9% vs. 1.7% in controls). The proportion of screening episodes that were positive was higher in the individualised arm at 5.1% than in the control arm at 4.5%. By contrast, there very low rates of screen-positive episodes in the low-risk group (0.2%). These rates are similar if slightly lower than the 0.3–1.0% reported over 2 years in people with no retinopathy in either eye in a study of seven screening programmes in England. 41
The value of adding in clinical data is a matter of debate. Our PPI group advised strongly during the design phase of the study that including clinical data was important to them. It allows the introduction of a high-risk group and better targeting than stratification (see Further analysis for post-RCT analysis). 30 We would suggest that the advantages for patient and clinician engagement outweigh the added cost and complexity. Around 15% of people had at least one change in risk-based interval: 59% people allocated to the medium (annual) group experienced a change of interval, adding further evidence to move away from a fixed interval for all.
Interrelation to other work packages and overall aims of the programme
Data collated in the ISDR DW (WP B1) informed the RCE (WP C), which in turn supported the RCT. The PPI group developed the risk criteria, actively supported with the production of patient-related materials and observed clinics to improve recruitment.
Cost-effectiveness of individualised variable-interval risk-based screening for diabetic retinopathy
A peer-reviewed publication24 describes the cost-effectiveness study24 and additional information is available in this publication on methods and results. We followed the CHEERS reporting guidance.
Parts of this chapter are reproduced or adapted from Bryne et al. 24 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Research aims
We conducted an economic analysis within our RCT to investigate the cost-effectiveness of individualised variable-interval risk-based screening from an NHS and a societal perspective (WP D).
Our review of the literature showed limited evidence on cost-effectiveness of screening. Study designs have been heterogeneous and relied on modelling rather than direct observation. One systematic review of screening called specifically for cost-effectiveness studies. 8
Methods
Within-trial analysis
The costs of routine screening were directly measured using a mixed micro-costing and observational health economics analysis. Societal costs, including participant and companion costs, which were collected using a bespoke questionnaire, comprised time lost from work (productivity losses) and travel and parking costs. A detailed workplace analysis, measuring resources and staff time to deliver the screening programme, was observed at each screening centre. This ingredient-based bottom-up approach enabled a current resource-based cost to be attributed to the individual patient cost of screening, taking into account both attendees and the related cost of non-attendance. We estimated the additional costs of running the RCE using a screen population of size of 22,000 (Liverpool). Treatment costs were excluded because the design was a within-trial analysis. The study was not designed to make economic inference of eye screening in general.
The first 868 participants enrolled into the RCT completed the EuroQol-5 Dimensions, five-level version (EQ-5D-5L),43 and Health Utilities Index Mark 3 (HUI3)44 instruments at baseline and follow-up visits. Health state utilities were mapped45 from the EQ-5D-5L to the EQ-5D three-level version and used a UK population tariff. 46 We applied a relevant Canadian tariff 47 to health state classifications of the HUI3 in the absence of an English or UK valuation set. Discounting was not applied as both costs and quality-adjusted life-years (QALYs) were assumed to be assigned and incurred on an annual basis.
A 90-day attendance window was utilised with a further 90 days added at 24 months to allow for the compounding lag in scheduling.
QALYs were constructed using AUC, and incremental QALYs estimated through ordinary least squares regression (for the univariate distributions of complete cases) and seemingly unrelated regressions (for the joint distributions of multiply imputed sets) on baseline utilities. Unadjusted estimates were used as sensitivity analyses. We bootstrapped these regressions to characterise sampling distributions and derive 95% bias-corrected CIs around trial arm means and mean differences. 48 ITT analyses were conducted in Stata/SE (Release 16; StataCorp LP, College Station, TX, USA) from an NHS/societal perspective, and post-multiple imputation analyses followed Rubin’s combination rules for estimation within multiply imputed sets. 49 Incremental cost-effectiveness ratios (ICERs) were generated from mean differences in QALYs and NHS screening costs, as well as incremental net monetary benefit (INMB) derived from a £20,000 threshold. 50 Multiple imputation of chained equations was run using available case data. Bootstrapping was used to characterise sampling distributions and derive 95% CIs around mean differences.
Results
The costs of screening are shown in Table 6. Costs per attendance (n = 16,736) were £11.73 for programme costs, £10.00 for photography and £7.00 for grading, with a total NHS cost of £28.73. The cost for non-attendance at £12.73 was principally the programme cost, with a small contribution for lost photographer time. Additional productivity losses and out-of-pocket payments by the patient accounted for £9.00.
| Variable | Top-down annual total (£) | Per attendance (n = 16,736) (£) | Per non-attendance (n = 6179) (£) |
|---|---|---|---|
| Programme costs | |||
| Staff (including oncosts) | 242,715 | 10.59 | 10.59 |
| Stationery | 3056 | 0.13 | 0.13 |
| IT | 22,988 | 1.00 | 1.00 |
| Total | 268,759 | 11.73 | 11.73 |
| Photography | |||
| Staff | 157,528 | 9.08 | 0.91 |
| Cameras and equipment | 11,777 | 0.68 | 0.07 |
| Medical consumables | 4229 | 0.25 | 0.00 |
| Total | 173,534 | 10.00 | 1.00 |
| Grading (P3–P6) | |||
| Staff | 117,234 | 7.00 | 0.00 |
| Total NHS cost | 559,528 | 28.73 | 12.73 |
| Societal costs associated with screening attendance | |||
| Patient-borne costs | – | 2.64 | – |
| Productivity loss | – | 6.36 | – |
| Total societal cost | – | 9.00 | – |
| Estimated Liverpool RCE costs | Annual total | Per patient | |
| Database administrator | 34,000 | 1.54 | |
| CCG administrator (20% FTE) | 6000 | 0.27 | |
| RCE total | 40,000 | 1.81 | |
The estimated cost of running the RCE was £40,000 per annum for a total screen population of 22,099 in Liverpool allocated to the intervention arm as £1.81 per person.
Table 7 shows summary health economic and cost-effectiveness data. There was no statistically significant difference in QALY scores between the trial arms (EQ-5D 0.006, 95% CI −0.039 to 0.06; EuroQol Visual Analogue Score 0.004, 95% CI −0.049 to 0.052; and HUI3 −0.017, 95% CI −0.083 to 0.04). We observed incremental cost savings per participant of £17.34 (£17.02 to £17.67) from an NHS perspective (not including treatment costs), corresponding to a potential reduction in total programme costs of 20% (95% CI from £193,983 to £154,386), and £23.11 (95% CI £22.73 to £23.53) from a societal perspective, and an INMB for the EQ-5D-5L of £736 (95% CI –£239 to £1718) and £178 (95% CI –£919 to £1338) for the HUI3 £26.19 (95% CI £24.41 to £27.87).
| Variable (n/N) | Mean difference (95% CI) | |
|---|---|---|
| Complete case | Multiple imputed | |
| EQ-5D (539/868) | ||
| Unadjusted | 0.012 (–0.097 to 0.119) | 0.043 (0.032 to 0.055) |
| Baseline adjusted | 0.006 (–0.039 to 0.06) | 0.044 (0.038 to 0.05) |
| EQ-VAS (548/868) | ||
| Unadjusted | –0.033 (–0.109 to 0.044) | 0.013 (0.005 to 0.022) |
| Baseline adjusted | 0.004 (–0.049 to 0.052) | 0.022 (0.017 to 0.028) |
| HUI3 (408/868) | ||
| Unadjusted | –0.016 (–0.135 to 0.116) | 0.068 (0.056 to 0.081) |
| Baseline adjusted | –0.017 (–0.083 to 0.04) | 0.051 (0.045 to 0.058) |
| Costs (4389/4534) | ||
| NHS screening | –17.44 (–18.57 to –16.31) | –17.34 (–17.67 to –17.02) |
| Societal | –23.26 (–24.65 to –21.92) | –23.11 (–23.53 to –22.73) |
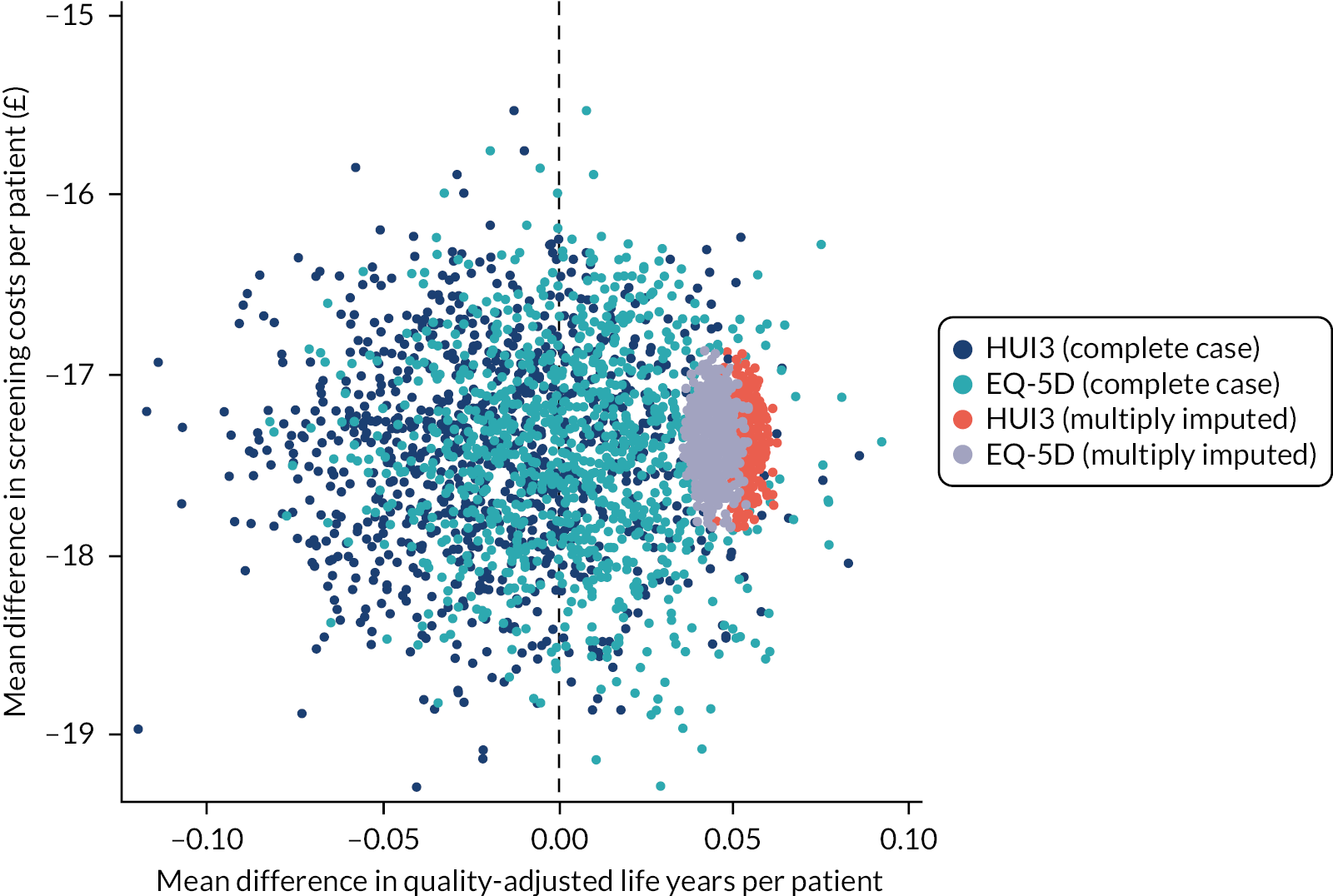
Results following multiple imputation indicated the dominance of the individualised arm in both QALY gain (EQ-5D-5L and HUI3) and cost savings (screening and societal) (Figure 6). All ICER point estimates fell in the south-east and south-west quadrants of the cost-effectiveness plane, signifying the dominance in cost savings, with mean differences in QALYs clustered around the zero threshold, EQ-5D-5L reporting a marginal benefit, and the HUI3 reporting an incremental distribution of near equivalence between the arms.
FIGURE 6.
Baseline adjusted EQ-5D, HUI3, and NHS perspective incremental cost-effectiveness of individualised vs. annual screening from 1000 iteration bootstraps.
Economic model
Risk-based cohort model
We worked with the RCE team (WS C) to develop a risk-based economic model that closely followed the structure and content of the RCE. The economic model was designed to be fully integrated and usable with the risk engine. We evaluated three alternative screening programmes: (1) current practice, (2) biennial stratification (as proposed by the English NDESP) and (3) ISDR individualisation using the ISDR RCE.
A microsimulation state transition (semi-Markov) model was developed based on DR disease states, as identified through photographic screening, with time- and event-dependent transition probabilities. The patient-level simulation (an individual sampling model) tracked individual characteristics through time, so that their changing risk of disease onset could be used to determine their pathways through the model. The simulation incorporates both disease states and events that determine individuals’ pathways through the model, which are observed in discrete units of time. Parameters used in this analysis were drawn from both published literature and local data.
Model structure
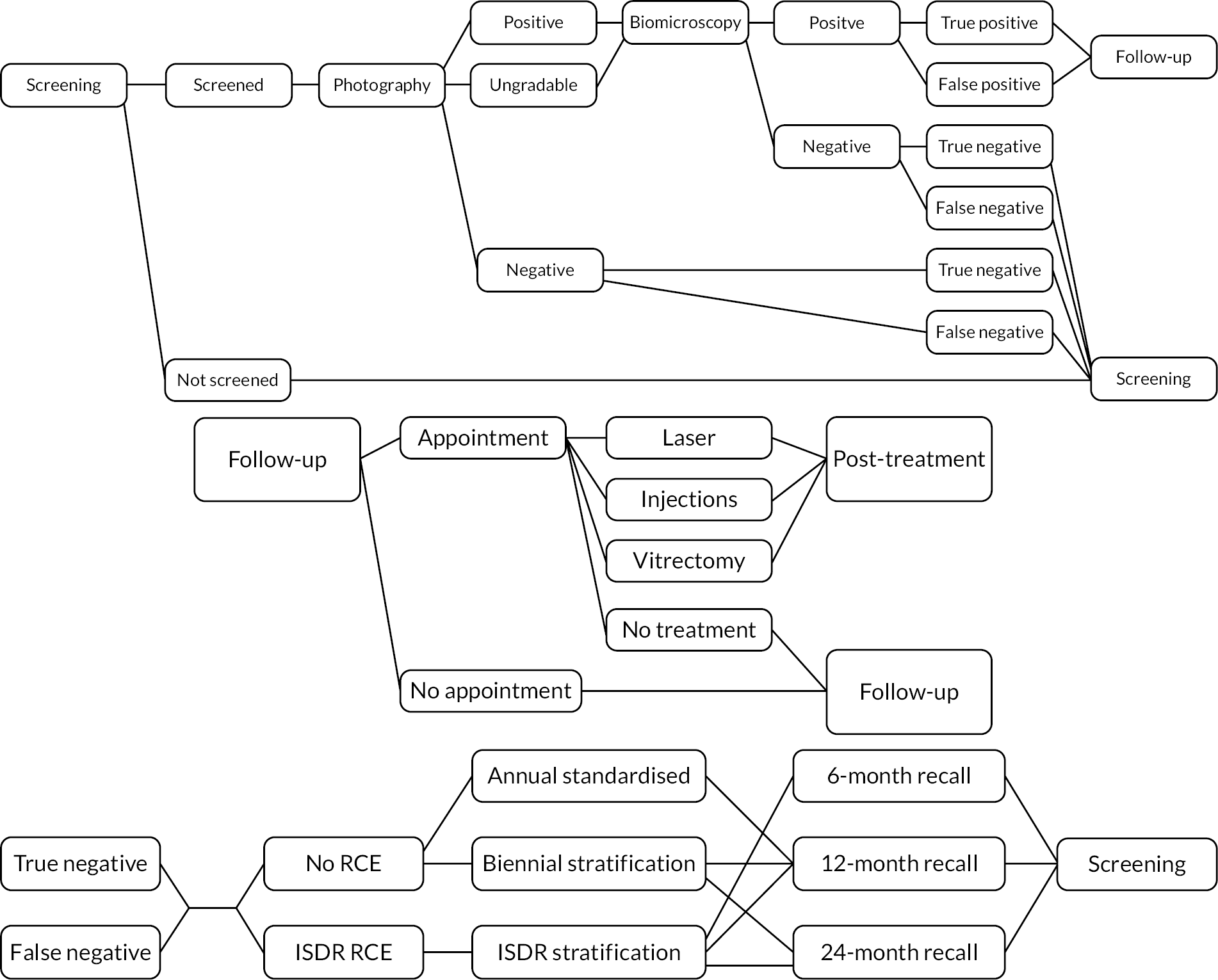
The disease states used in the model were defined in the terms used by the NDESP, namely ‘R0M0’ gradings. Retinopathy is graded as R0 (no retinopathy), R1 (background), R2 (pre proliferative) or R3 (proliferative). Maculopathy is graded as either M0 (absent) or M1 (present).
All individuals were in the screening programme, except for those who had experienced a screen-positive event and were, therefore, in follow-up and may have been receiving treatment. However, those who receive treatment that was successful may have been referred back to the screening programme. Therefore, the model allows for individuals to be in either screening or follow-up, whether or not they received treatment.
Individuals who went on to receive treatment were subject to different progression rates. Therefore, it was necessary to divide the model further based on the stages of the treatment pathway. The model allowed for four separate groups of individuals: (1) those in screening who had not received treatment, (2) those in screening who had previously received treatment, (3) those in follow-up who had not yet received treatment and (4) those in follow-up who had previously received treatment. This increases the number of disease-specific states fourfold, giving 38 states.
The comparators for analysis (described above) are similarly included as an additional event process, applied to all people whose screening outcome is negative and who are referred back to screening. These event pathways are outlined in Figure 7.
FIGURE 7.
Event pathways in the economic model developed within the ISDR programme of applied research. Upper panel: pathway within screening programme; middle panel: pathway within HES for screen-positive individuals; lower panel: pathway showing comparison between three screening models.
The model was developed using Microsoft Excel 2013. The intent was to run the model from NHS and Personal Social Services perspectives over a life-time horizon with cycles occurring monthly, and transitions and events occurring at the end of each cycle. The computational and mathematical requirements of such a large-scale model proved non-operational by the end of the programme, requiring around 4 days of processing. Work continued to produce a final version of the model based on 10,000 people.
Discussion
Our data provide evidence that an individualised screening approach may provide cost savings within the programme. We feel that the assumptions and costs applied throughout were conservative and took care not to overstate the benefits of this approach or underplay any of the costs, for example providing a realistic estimate of replicating the cost of a risk engine elsewhere.
By moving to variable-interval risk-based screening, patients were not compromised on safety or quality of life. We calculated potential incremental cost savings over the 2-year time horizon within the trial of £17.34 NHS costs, rising to £23.11 per patient from a societal perspective. In a population such as in Liverpool, this may amount to potential annual savings in the region of £199,000 in NHS screening programme costs. For England [screening population 2.76M (2018–19)51], this could amount to around £23.9M in the NHS, not including treatment costs, rising to £31.9M from a societal perspective. Such resources could be used to target groups that are hard to reach and those at high risk of VI, and more cost-efficiently screen the expanding population of PWD. Individualised screening (on average) reduces the number of attendances required, given that patients in low-risk groups would be spared the inconvenience and additional personal cost of attending non-essential appointments. In brief, fewer visits reduce both patient and hospital costs and the trial shows that this can happen without negative clinical consequences.
Strengths of this within-trial cost-effectiveness study are the detailed characterisation of the costs to the health service and society of a person attending screening and the large number of observations. Our work is limited by the 2-year time horizon, which is short for a long-term disease, such as diabetes. Our work could have been further strengthened by taking a long-term time horizon to capture lifetime costing differentials and years of sight loss averted. Treatment costs were excluded as per the within-trial analysis and the study objectives of comparing screening options rather than of a lifetime analysis of screening. We did explore adding a post-screening analysis to include treatment costs but considered it very unlikely to be informative because the numbers progressing to treatment were very small (two in each group).
It may have been useful to collect utility data for the entire cohort. We elected not to undertake this to minimise disruption at screening clinics. Multiple imputation indicated that quality-of-life (QoL) data were robust. At a 2-year time horizon, the effects of screening intervals on QoL appear to be close to negligible, where our between-arm utilities and incremental QALYs demonstrated near-equivalence.
Although the intention had been to report cost-effectiveness acceptability curves, the dominance in cost reduction of variable-interval risk-based screening and little fluctuation in QALYs across all instruments rendered this metric uninformative, as the proportion cost-effective was inelastic to varying thresholds. See Strengths and limitations for a further discussion of strengths and limitations.
In 2015, Scanlon et al. 42 reported a cost-effectiveness model developed in a historical data set of 10,942 people in Gloucestershire with screening and clinical data over at least 3 years and validated it in three other English programmes. They reported a 3-yearly screening interval to be the most cost-effective in the absence of personalised risk-based intervals. Using their risk-based strategy, the most cost-effective options were to screen those at low risk every 5 years and those at medium and high risk every 3 and every 2 years, respectively. We now add robust RCT evidence to support the introduction of variable-interval risk-based screening.
Interrelation to other work packages and overall aims of the programme
The health economics and RCE teams (WS C, WS D) worked closely together. The economic model was designed to be fully integrated and usable with the RCE. This is an important element should the Liverpool approach be rolled out into wider practice. Co-ordination occurred with WP F to enable a more inclusive and comprehensive understanding of valuation in QoL in DR screening. This was important as this group was under-represented in the trial itself.
Qualitative study with patients and healthcare professionals on changing screening intervals
A peer-reviewed publication is available24 and includes example narratives of the key findings. COREQ reporting guidelines were followed.
Parts of this chapter are reproduced or adapted from Byrne et al. 24 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Research aims
We aimed to explore perceptions of changing from annual to individualised variable-interval risk-based screening and to gain wider insights into users’ perceptions to enable successful implementation (WP F). We also wanted to explore the wider aspects of DR screening in general.
Methods
Setting
We conducted our qualitative studies within the setting of our RCT. This allowed for the first time a real rather than theoretical investigation of the perceptions around the acceptability of implementation of varying intervals and the use of a risk calculator in a population from an established screening programme and in a geographical location where annual fixed-interval screening was already established.
Design
Semi-structured interviews were conducted after informed consent was obtained to gather views on variable-interval risk-based screening. Interviews with PWD were conducted before the start of the RCT (phase 1, baseline) and subsequently with a second group (phase 2) during the RCT. All interviews with HCPs took place prior to commencement of the RCT. The research team and PPI group created interview topic guides (see Supplementary Material 3) covering participants’ background, beliefs and attitudes towards diabetes, management of diabetes, medical management and contact, and future management of screening. For HCP interviews, discussions included participants’ background in diabetes, types of patients they see and potential issues with changing screening intervals. Interviews lasted 30–90 minutes, with most lasting around 45 minutes.
Participants
Participants aged over 16 years who were PWD attending the eye screening programme were identified in two GPs in Liverpool. The practices were approached by a research nurse. The practices reflected the range of socioeconomic status, with one located in a disadvantaged location and the other in an affluent location.
We used purposive sampling to identify potential participants aiming to reflect the characteristics of the local diabetic population and of HCPs involved in eye screening. Suitability of potential PWD participants was reviewed by a GP in the practice prior to contact by the research team.
All participants received a brief overview of individualised variable-interval risk-based screening. Most patient interviews were conducted in participants’ homes, although some chose to complete them in a university office and one completed their interview in their own office.
In total, 60 PWD were recruited: 30 to phase 1 (baseline) and 30 to phase 2 (post implementation of variable-interval risk-based screening). Further details are given in Table 8. Interviews took place between October 2014 and April 2015 in private NHS offices (with the exception of one joint interview completed at a participant’s home). Allocations within phase 2 were balanced to the overall allocations within the RCT.
| Variable | n |
|---|---|
| PWD (n = 60) | |
| Male | 34 |
| Age | 19–83 years |
| Type 1 | 8 |
| Time since diagnosis | 1–40 years |
| 1–5 years | 10 |
| 6–10 years | 7 |
| 11–15 years | 4 |
| > 15 years | 9 |
| Risk-based screening interval baseline allocation (phase 2) | |
| 6 months | 4 |
| 12 months | 5 |
| 24 months | 21 |
| HCPs ( n = 21) | |
| Professional role | |
| Screener/grader | 7 |
| Consultant ophthalmologist (retina) | 5 |
| Eye screening service manager | 4 |
| Optometrist providing DR screening | 2 |
| Public health specialist | 2 |
| GP | 1 |
The PWD participants had a range of social situations; the occupations described by the sample were very mixed, including a range of professionals as well as students and manual workers, retired and unemployed, and one person who was unable to work owing to long-term ill health. A majority described their ethnicity as white British, two as white European and one each of mixed race and Asian.
A total of 21 HCPs involved in eye screening were recruited through existing knowledge networks, building on our 2011–12 programme development work. A small number of these were recruited through ‘snowballing’ (identified as potential participants by other participating HCPs). In total, 16 out of 21 participated in interviews (six in groups of three), while five completed interview questions via e-mail (see Table 8 for further details).
We attempted in parallel to access and interview a purposive sample of PWD who had been offered and did not attend two consecutive appointments. Despite many efforts to recruit, we were unable to interview non-attenders.
Analysis
Interviews were audio-recorded and transcribed verbatim for detailed analysis. Semi-structured case summaries were produced by a researcher on completion of each interview to provide a summary of key themes and enable identification of emerging themes to inform further data collection and analysis. Thematic analysis was used. Members of the research team (CT, PB and MG) read and coded separately the first three interviews to ensure that emerging themes were captured and had agreement. Further coding was then undertaken at several time points and regular meetings were conducted between the researchers for consistency.
After reading and re-reading the transcripts, data were analysed to identify sections of text that informed understandings of the issues. 52–54 Each concept was assigned a descriptive or analytical code, which was then combined into conceptual categories and broader themes using NViVO software, which enabled searching and retrieval of specific data.
Results
Here we summarise key findings from interviews with PWD and HCPs. Further details included patient narratives, in line with standards for reporting qualitative research,55 and are reported elsewhere. 24 The narratives give a compelling insight into the perceptions and views of users of screening services.
Acceptability of changing screening intervals
The majority of PWD expressed the view before and during the ISDR RCT that risk-based variable-interval screening was potentially acceptable. They focused on concepts of pragmatism and diverting any cost savings towards other PWD who may need to be seen more often. For HCPs, the majority were also in favour of introducing the proposed new approach to screening. However, implementing such changes to eye screening was accompanied by a range of caveats, which are discussed in the next subsections below.
Safety of the risk calculation engine
Many of the PWD and HCPs indicated that they would be supportive of the introduction of risk-based allocation to variable screening intervals, provided that particular safeguards or service enhancements were introduced. Safeguards around missed STDR needed to be visible and obvious to both groups. Specifically, for a PWD on a 24-month screening interval, if they had any disruptions to their diabetes self-care, then they wanted to be able to self-refer back into the screening programme. Similarly, for HCPs, they also wanted to refer a PWD back into the screening programme if there were changes that they felt were clinically relevant.
Acceptability of 6-month interval
Within the variable screening model, allocation to a 6-month interval meant that a person with diabetes was considered to be at high risk of developing STDR. There were some tensions within PWD’s understandings about the 6-month interval, with it being seen as clinical surveillance and reassurance against the clinical reality that being allocated to this interval means that there is a high risk of developing STDR. In addition, there was conflation about the purpose of eye screening, where it was perceived as a preventative measure against DR. For HCPs, the shorter interval was not only welcomed, but also represented an operational issue of managing resources.
Acceptability of 12-month interval with conditions
As the current annual screening interval is established and embedded into practice, it was foreseeable that PWD felt that this was an appropriate length of time for their eye screening. However, discussions also highlighted misunderstandings about the purpose of eye screening as a preventative measure against the development of DR and related to diabetes control.
Acceptability of 24-month interval with conditions
For PWD and HCPs, there were a range of responses to extending screening intervals to 2 years. For some PWD, an extension was welcome because it reflected good diabetes self-care, contrasting with outright rejection from others over concerns about developing eye disease in the extended time period. For HCPs, the 2-year interval was acceptable in the context of many patients having minimal or no disease. There was some apprehension about the perceptual impact on patients of changing screening intervals, with PWD feeling that screening was not as important if it was changed to a 2-year interval.
Macro impact of changing screening intervals
Although our participants discussed the impact on themselves of changing screening intervals, they also reflected on the consequences in a much broader societal viewpoint, for which we have used the term ‘macro’. There was a recognition that against a backdrop of increasing numbers of PWD, current screening intervals are unsustainable. In addition, it was also seen as an inefficient use of finite resources, which would be better deployed in targeting PWD who do not attend eye screening appointments. It was recognised that the variable screening may well save money, but this was also seen as a restrictive practice in accessing healthcare services. There was considerable conflation and misunderstanding about different eye-related appointments within secondary care and at opticians.
Discussion
To the best of our knowledge, our qualitative study was the first to explore perceptions of changing screening intervals, with our findings showing general support from PWD and HCPs for the introduction of variable-interval risk-based screening. This is reassuring for policy-makers and service providers who are considering introducing variable-interval screening, stratified based on either retinal grading or variable intervals based on risk estimation. Support was more clearly expressed by HCPs who had a better understanding of the aims and current pressures in screening. Our findings also have relevance to other screening programmes with fixed intervals and help to mitigate in part the concerns raised recently in cancer screening. 15,56
For HCPs, and to a lesser extent PWD, the safety of the RCE was crucial to it being accepted into clinical practice. Safeguards for the RCE had to be made visible and obvious to HCPs and PWD, including the opportunity for HCPs or PWD to refer or self-refer back into the eye screening programme.
In terms of variable screening intervals, there were some misunderstandings among PWD participants about the 6-month interval, whereas HCPs welcomed the opportunity for increased surveillance for high-risk groups. PWD and HCPs were supportive of the current 12-month interval, while recognising that NHS resources are finite.
There were widely differing views on extending screening intervals to 24 months, with both groups of participants having significant concerns about safety. There were worries that any changes in a person’s diabetes eye health would be missed, which could lead to developing STDR in this time, leading to poor quality of life for a patient and increased costs to the NHS.
There was a recognition by both groups of participants that against a backdrop of increasing numbers of PWD, current screening intervals are unsustainable and resources could be better used to target high-risk groups. The considerable conflation and misunderstanding about different eye-related appointments has been reported elsewhere in the literature. 57,58 Changing the message to PWD from regular annual check-ups to extended eye screening will be a challenging message to convey positively.
We attempted to recruit PWD who had a history of multiple non-attendance for screening. Our research team tried contacting over 30 people in this group without success. Poor attendance, known to be a risk factor for STDR,59,60 is a major issue for all stakeholders involved in screening. Novel ways need to be developed to approach these individuals so that the reasons for non-attendance can be investigated and addressed.
We had intended to investigate changes in attitudes through sampling prior to and during the RCT but follow-up interviews proved to be difficult to obtain. However, we did not identify any themes suggesting differences in perceptions in those who had already been enrolled in the RCT compared with the pre-trial group.
Among the multiple healthcare-related appointments for diabetes, there are misunderstandings by PWD of NHS systems and other appointments with HCPs. Understanding the frequency of screening required to prevent the development of DR61 was another barrier for PWD and was echoed by HCPs. Allied to appointments related to eye care, there can often be misinterpretations by PWD about the relationship between diabetes and DR, and differences between DR screening and routine eye tests, which in turn led to beliefs that screening appointments had been fulfilled when they had not,62 as well as related anxiety about screening. 63 A study by Strutton et al. 64 concluded that screening attendance could be improved by the sharing of pertinent information between healthcare providers and a greater understanding of patients’ circumstances and barriers.
Conclusions
It is reassuring that PWD and HCPs appear to be supportive of variable intervals in DR screening. However, for successful implementation, important caveats and misconceptions must be addressed. Interpretable and clear safeguards for individual PWD are required against increasing non-attendance, loss of diabetes control and system failures. Alternative referral pathways are required for those lost to follow-up or whose risk factors change substantially over longer intervals. For risk-calculation systems, reliable monitoring and clear communication are necessary.
Interrelation with other parts of the programme and overall aims of the programme
The implication of our evidence is that a clear and sustained communication strategy around eye screening and intervals is needed both in the current screening programme and in any future revision. These were considered in WP G.
Observational cohort study in people attending screening for diabetic retinopathy
A peer-reviewed publication25 provides additional detail on methods and a discussion on the censoring effect. STROBE reporting guidelines were followed.
Parts of this chapter are reproduced or adapted from Cheyne et al. 25 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Research aims
We conducted an observational cohort study to estimate the prevalence and incidence of DR and STDR in the established screening programme in Liverpool (WP B). We wanted to provide up-to-date data to inform clinical services and screening programmes, and for clinical research programmes, especially in early disease.
Up-to-date epidemiological data on DR are limited. Much of the population data comes from landmark epidemiological studies in the 1970s and 1980s16,17 before systematic screening was introduced. Data from screening programmes are limited and reported inconsistently. When programmes are introduced, those in the community with long-standing disease are detected for the first time and referred into the HES; as this ‘first pass effect’ wanes, the prevalence of referable DR and STDR in the screening population falls. Populations attending for systematic screening are dynamic and subject to several long-term influences. In recent years, there have been changes in the diagnosis of diabetes,18 and improvements in some populations in glycaemic and BP control. On the other hand, there has been a long-term increase in prevalence of diabetes.
Methods
The cohort study was approved separately by the Preston North West NHS Ethics Committee (13/NW/0196), the local governance committees and Caldicott Guardian. The Liverpool Local Medical Committee (LMC) and Primary Care Research Group and LCCG supported Liverpool general practices who were invited to participate in the study. Data-sharing agreements were prepared with each practice, the RLBUHT and LCCG to include all retrospective and prospective demographic and clinical records collected by practice electronic records systems (EMIS web, EMIS Health, www.emishealth.com).
Data for the cohort study were held in the DW described in The ISDR study data warehouse. Individuals were identified from the patient register of all PWD in Liverpool held by the LDESP. Consent for data collection and analysis was through an ‘opt-out’ process (see page 13). Eligible participants comprised all PWD aged ≥ 12 years diagnosed with diabetes who were registered with a practice in Liverpool. PWD aged 12–15 years received a letter and booklet written specifically for young people. Recruitment of general practices commenced in 2013; by 2016, all had agreed to participate. Once a practice had been recruited, newly diagnosed PWD and those moving into the area continued to be invited to the programme.
The following data sets were available: Diabolos (from 1995), a bespoke screening-management software developed in Liverpool; Orion (2005–13), the first iteration of the English NDESP standardised screening-management software; and OptoMize (from 2013), the current NDESP system.
Data underwent credibility checks using MATLAB (version 8.3, Mathworks, Inc., Natick, Massachusetts, USA) before being merged for analysis. The values of the time-dependent clinical variables closest to the time of the screen episodes were used.
Data from the HES were used to investigate the outcomes of a positive screen event. Data from the first biomicroscopy recorded within 1 year of the positive screen event were used to provide the final outcome for that event. The first recorded attended screening appointment was defined as the first recorded attended appointment at which there were no earlier recorded attended screening appointments or biomicroscopies for that individual.
Confounders
Demographic and clinical data from primary care exhibited a censoring effect prior to 2013; data governance rules removed data from PWD who had died prior to a practice joining the study. This effect phased out during the 3-year period of recruitment of practices.
Data on annual incidence were adjusted for the 2265 participants who were allocated to individualised screening in the ISDR RCT (see Randomised controlled trial to evaluate the safety and cost-effectiveness of individualised variable-interval risk-based screening). Those who were assigned to 24 months would under normal circumstances have been screened annually in these years. All were assumed to be screen-negative. The attendance rate was assumed to be 0.85.
Results
Data set
Data collection commenced on 31 October 2013. Data presented here come from an analysis data set including all relevant data extracted up to at least 31 March 2017. Data were extracted on 25 September 2017.
For the primary analysis, we censored the data until 1 April 2006. EMIS web systems started to be introduced into GPs only in 2009, and the data quality was very variable in the first 12 months.
The size of the data set varied throughout the 11 years studied, with year-end effects and variation owing to sampling time. On 25 September 2017, 42,334 people were registered as having diabetes with a Liverpool general practice. After exclusions/ineligibility, the number of people eligible to be invited to join the ISDR cohort study was 30,771. Data distribution is shown in Figure 8; data from 28,384 PWD were available for analysis.
FIGURE 8.
Data flows in the ISDR cohort study with figures from an illustrative timepoint close to the mid-point of the last full year (25 September 2017).
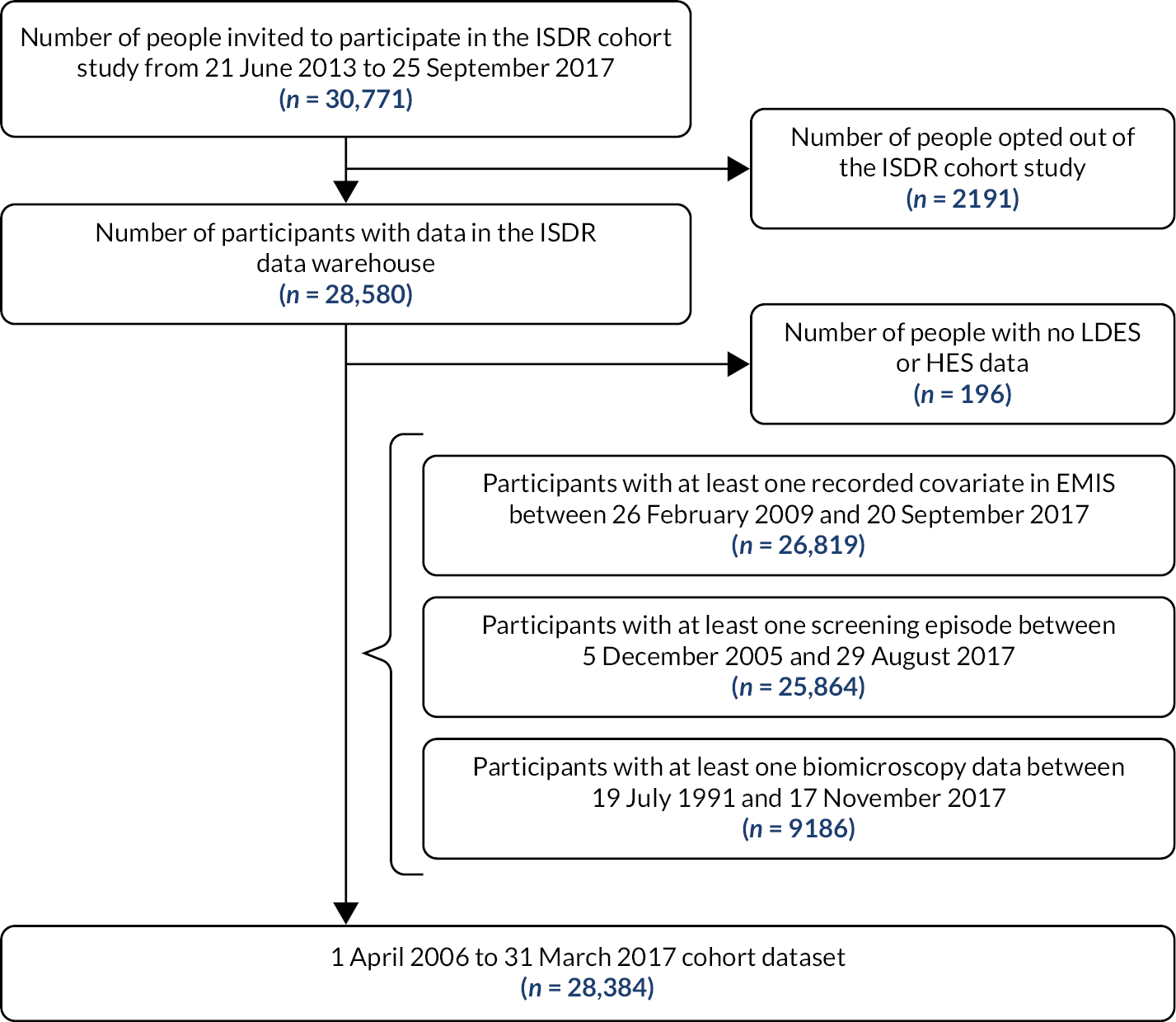
Demographics
Overall demographics are shown in Table 9. The number of individuals with at least one screening appointment recorded each year increased steadily between 2006/07 and 2014/15 to 15,518, and with adjustment for the effect of the ISDR RCT to 16,001 by 2016/17. Over the 11 years, the median age increased steadily from 60.3 to 64.5 years, and the median disease duration increased from 2.3 to 7.0 years, which was most likely because of the censoring effect prior to 2013/14. Gender proportions were relatively stable across all screening years (55–58% male, 40–43% female, 1–3% unknown). The proportion with type 2 diabetes rose steadily from 76.1% to 81.4%, whereas the median HbA1c was stable (50–52 mmol/mol). Data on ethnicity appeared to be subject to some inconsistencies, mainly around data quality.
| Variable | Screening yeara | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006/07 (N = 6637) | 2007/08 (N = 8088) | 2008/09 (N = 8664) | 2009/10 (N = 10,266) | 2010/11 (N = 11,214) | 2011/12 (N = 13,124) | 2012/13 (N = 13,518) | 2013/14 (N = 15,115) | 2014/15 (N = 15,552) | 2015/16 (N = 15,054) | 2016/17 (N = 14,864) | |
| Gender, n (%)b | |||||||||||
| Male | 3647 (54.9) | 4508 (55.7) | 4804 (55.4) | 5740 (55.9) | 6367 (56.8) | 7444 (56.7) | 7713 (57.1) | 8660 (57.3) | 8956 (57.6) | 8569 (56.9) | 8243 (55.5) |
| Female | 2823 (42.5) | 3367 (41.6) | 3670 (42.4) | 4287 (41.8) | 4603 (41.0) | 5406 (41.2) | 5533 (40.9) | 6265 (41.4) | 6478 (41.7) | 6171 (41.0) | 5997 (40.3) |
| Unknown | 167 (2.5) | 213 (2.6) | 190 (2.2) | 239 (2.3) | 244 (2.2) | 274 (2.1) | 272 (2.0) | 190 (1.3) | 118 (0.8) | 314 (2.1) | 624 (4.2) |
| Diabetes type, n (%)b | |||||||||||
| Type 1 | 441 (6.7) | 537 (6.6) | 536 (6.2) | 576 (5.6) | 579 (5.2) | 687 (5.1) | 684 (5.1) | 754 (5.0) | 795 (5.1) | 750 (5.0) | 731 (4.9) |
| Type 2 | 5053 (76.1) | 6241 (77.2) | 6784 (78.3) | 8194 (79.8) | 9081 (81.0) | 10,645 (81.1) | 11,078 (81.9) | 12,486 (82.6) | 12,962 (83.3) | 12,417 (82.5) | 12,104 (81.4) |
| Unknown | 1143 (17.2) | 1310 (16.2) | 1344 (15.5) | 1496 (14.6) | 1554 (13.9) | 1792 (13.7) | 1756 (13.0) | 1875 (12.4) | 1795 (11.5) | 1887 (12.5) | 2029 (13.7) |
| Ethnicity, n (%)b | |||||||||||
| White | 5327 (80.3) | 6456 (79.8) | 6920 (79.9) | 8094 (78.8) | 8729 (77.8) | 9940 (75.7) | 10,156 (75.1) | 11,258 (74.5) | 11,478 (73.8) | 10,811 (71.8) | 10,252 (69.0) |
| Asian | 158 (2.4) | 183 (2.3) | 224 (2.6) | 256 (2.5) | 271 (2.4) | 353 (2.7) | 369 (2.7) | 428 (2.8) | 460 (3.0) | 438 (2.9) | 456 (3.1) |
| Black | 105 (1.6) | 116 (1.4) | 139 (1.6) | 175 (1.7) | 197 (1.8) | 243 (1.9) | 253 (1.9) | 293 (1.9) | 286 (1.8) | 305 (2.0) | 309 (2.1) |
| Chinese | 57 (0.9) | 75 (0.9) | 85 (1.0) | 79 (0.8) | 85 (0.8) | 108 (0.8) | 121 (0.9) | 125 (0.8) | 144 (0.9) | 150 (1.0) | 160 (1.1) |
| Other | 88 (1.3) | 103 (1.3) | 108 (1.2) | 127 (1.2) | 135 (1.2) | 169 (1.3) | 166 (1.2) | 201 (1.3) | 210 (1.4) | 202 (1.3) | 199 (1.3) |
| Unknown | 902 (13.6) | 1155 (14.3) | 1188 (13.7) | 1535 (15.0) | 1797 (16.0) | 2311 (17.6) | 2453 (18.1) | 2810 (18.6) | 2974 (19.1) | 3148 (20.9) | 3488 (23.5) |
| Age (years) | |||||||||||
| Observed (n) | 6470 | 7875 | 8474 | 10,027 | 10,970 | 12,850 | 13,246 | 14,925 | 15,434 | 14,740 | 14,240 |
| Unknown (n) | 167 | 213 | 190 | 239 | 244 | 274 | 272 | 190 | 118 | 314 | 624 |
| Median | 60.3 | 61.0 | 61.5 | 62.3 | 62.6 | 62.9 | 63.3 | 63.9 | 64.2 | 64.5 | 64.5 |
| IQR | 51.2–68.7 | 51.9–69.4 | 52.4–70.1 | 53.1–70.6 | 53.4–71.1 | 53.6–71.5 | 54.0–72.1 | 54.4–72.6 | 54.6–73.0 | 55.0–73.1 | 55.1–73.3 |
| Range | 12.0–92.1 | 12.0–93.4 | 12.0–94.4 | 12.1–94.5 | 9.9–96.5 | 11.9–98.1 | 12.0–97.7 | 12.1–98.7 | 12.2–112.9 | 13.3–113.6 | 13.7–114.6 |
| Disease duration (years) | |||||||||||
| Observed (n) | 6637 | 8088 | 8664 | 10,266 | 11,214 | 13,124 | 13,518 | 15,115 | 15,552 | 15,054 | 14,864 |
| Unknown (n) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Median | 2.3 | 2.6 | 2.9 | 3.5 | 4.2 | 4.9 | 5.5 | 6.1 | 6.4 | 6.7 | 7.0 |
| IQR | 0.2–5.6 | 1.1–6.1 | 1.7–6.5 | 2.1–6.9 | 2.1–7.2 | 2.2–7.7 | 2.6–8.2 | 3.1–8.9 | 3.3–9.4 | 3.5–9.9 | 3.1–10.5 |
| Range | 0.0–53.7 | 0.0–54.7 | 0.0–55.4 | 0.0–56.6 | 0.0–57.7 | 0.0–55.2 | 0.0–59.3 | 0.0–60.4 | 0.0–61.6 | 0.0–62.6 | 0.0–63.7 |
| HbA1c (mmol/mol) | |||||||||||
| Observed (n) | 0 | 0 | 123 | 6216 | 8495 | 11,188 | 11,972 | 13,869 | 14,638 | 14,006 | 13,540 |
| Unknown (n) | 6637 | 8088 | 8541 | 4050 | 2719 | 1936 | 1546 | 1246 | 914 | 1048 | 1324 |
| Median | – | – | 50 | 50 | 51 | 51 | 52 | 51 | 51 | 51 | 52 |
| IQR | – | – | 43–60 | 44–60 | 45–61 | 45–61 | 45–62 | 44–61 | 44–61 | 45–62 | 46–62 |
| Range | – | – | 33–117 | 23–157 | 21–160 | 24–172 | 22–168 | 22–166 | 22–174 | 20–156 | 22–140 |
Incidence
Data on incidences are shown in Table 10. There was some variation of rates of screen-positive across the 11 years, with an overall reduction from 7.9–10.6% in the early years to 4.4–6.7% in the later years.
| Variable | Screening year*, n (%^) | Overall, n (%^; 95% CI^^^) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006/07 | 2007/08 | 2008/09 | 2009/10 | 2010/11 | 2011/12 | 2012/13 | 2013/14 | 2014/15 | 2015/16 | 2016/17 | ||
| Number of individuals with at least one attended screening appointment | 6637 (100.0) | 8088 (100.0) | 8664 (100.0) | 10266 (100.0) | 11214 (100.0) | 13124 (100.0) | 13518 (100.0) | 15115 (100.0) | 15552 (100.0) | 15280^^ (100.0) | 16096^^ (100.0) | 133554 (100.0) |
| Screen-positive | 527 (7.9) | 714 (8.8) | 922 (10.6) | 854 (8.3) | 695 (6.2) | 921 (7.0) | 774 (5.7) | 669 (4.4) | 805 (5.2) | 1031 (6.7) | 994 (6.2) | 8906 (6.7; 6.5 to 6.8) |
| Screen-positive for DR | 271 (4.1) | 355 (4.4) | 401 (4.6) | 360 (3.5) | 327 (2.9) | 419 (3.2) | 330 (2.4) | 345 (2.3) | 387 (2.5) | 441 (2.9) | 437 (2.7) | 4073 (3.0; 3.0 to 3.1) |
| Screen-positive for unassessable images | 201 (3.0) | 255 (3.2) | 358 (4.1) | 372 (3.6) | 283 (2.5) | 425 (3.2) | 322 (2.4) | 179 (1.2) | 258 (1.7) | 408 (2.7) | 383 (2.4) | 3444 (2.6; 2.5 to 2.7) |
| Screen-positive for other eye disease requiring HES** | 55 (0.8) | 104 (1.3) | 163 (1.9) | 122 (1.2) | 85 (0.8) | 77 (0.6) | 122 (0.9) | 145 (1.0) | 160 (1.0) | 182 (1.2) | 174 (1.1) | 1389 (1.0; 1.0 to 1.1) |
| Biomicroscopy recorded*** | 446 (6.7) | 618 (7.6) | 794 (9.2) | 712 (6.9) | 612 (5.5) | 774 (5.9) | 650 (4.8) | 621 (4.1) | 742 (4.8) | 965 (6.3) | 868 (5.4) | 7802 (5.8; 5.7 to 6.0) |
| STDR | 116 (1.7) | 145 (1.8) | 192 (2.2) | 178 (1.7) | 160 (1.4) | 223 (1.7) | 179 (1.3) | 212 (1.4) | 246 (1.6) | 251 (1.6) | 290 (1.8) | 2192 (1.6; 1.6 to 1.7) |
| STR$ | 41 (0.6) | 44 (0.5) | 81 (0.9) | 65 (0.6) | 56 (0.5) | 75 (0.6) | 57 (0.4) | 69 (0.5) | 98 (0.6) | 85 (0.6) | 110 (0.7) | 781 (0.6; 0.5 to 0.6) |
| STM$ | 91 (1.4) | 119 (1.5) | 157 (1.8) | 135 (1.3) | 130 (1.2) | 197 (1.5) | 149 (1.1) | 179 (1.2) | 191 (1.2) | 216 (1.4) | 237 (1.5) | 1801 (1.3; 1.3 to 1.4) |
| Unknown STR & unknown STM | 0 (0.0) | 0 (0.0) | 2 (< 0.1) | 2 (< 0.1) | 4 (< 0.1) | 0 (0.0) | 4 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 0 (0.0) | 0 (0.0) | 14 (< 0.1; 0.0 to 0.0) |
| Not STDR | 330 (5.0) | 473 (5.8) | 602 (6.9) | 534 (5.2) | 452 (4.0) | 551 (4.2) | 471 (3.5) | 406 (2.7) | 496 (3.2) | 714 (4.7) | 555 (3.4) | 5584 (4.2; 4.1 to 4.3) |
| Unknown STDR status | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (< 0.1) | 0 (0.0) | 0 (0.0) | 23 (0.1) | 26 (< 0.1; 0.0 to 0.0) |
| No biomicroscopy recorded*** | 81 (1.2) | 96 (1.2) | 128 (1.5) | 142 (1.7) | 83 (0.7) | 147 (1.1) | 124 (0.9) | 48 (0.3) | 63 (0.4) | 66 (0.4) | 126 (0.8) | 1104 (0.8; 0.8 to 0.9) |
| Screen-negative | 6110 (92.1) | 7374 (91.2) | 7742 (89.4) | 9412 (91.7) | 10,519 (93.8) | 12,203 (93.0) | 12,744 (94.3) | 14,446 (95.6) | 14,747 (94.8) | 14,249 (93.3) | 15,102 (93.8) | 12,4648 (93.3; 93.2 to 93.5) |
The annual incidence of screen-positive owing to DR appeared to decrease from 4–5% (2006/07 to 2009/10) to 2–3% (2010/11 to 2016/17). The rates of screen-positive for unassessable images appeared to fluctuate by around 2–3% (highest proportion 4.1% in 2008/09, lowest proportion 1.2% in 2013/14) and for other eye disease was stable (around 1%) apart from a peak in 2008/9 (1.9%). Rates of STDR (true-positive, examined by a medical retina specialist using biomicroscopy) were relatively stable at around 1.5–2% [sight-threatening retinopathy (STR) around 0.6%, sight-threatening maculopathy (STM) 1.0–1.5%]. Figure 9 illustrates these trends.
FIGURE 9.
Plot of overall screen-positive/STDR/STR annual incidences 2006/07–2016/17 (out of number of people with at least one attended screening appointment) by screening year (with 95% wilson score cis). STM not shown.
Of the 133,554 screening episodes across the 11 years studied, 6.7% (8906) were screen-positive, 3.0% (4073) were screen-positive for DR, 2.6% (3444) due to ungradeable images and 1.0% (1389) due to other significant eye disease.
Of the 7802 screen-positive individuals who attended the HES and were examined by a medical retina specialist, only 28.1% (2192) were confirmed as having STDR [overall incidence 1.6% (2192/133,554)], with 71.6% (5584/7802) having unassessable images, other disease or a false positive. A total of 53.8% (2192/4073) of the people referred with screen-positive disease due to DR were true-positive. In total, 82.2% (1801/2192) of individuals had STM and 35.6% (781/2192) had STR.
People attending for their first visit
Annual incidences of screen-positive/negative and STDR for individuals attending their first ever recorded attended screening appointment were 2–4% higher (absolute difference) than the rates for the overall group (Tables 11 and 12). Screen-positive rates were 62.3% higher (9.9% vs. 6.1%) and STDR was 37.5% higher (2.2% vs. 1.6%) than the rates seen in those who had had at least one previous screening year. However, much of the screen-positive effect appears to have been due to unassessable images (78.3%, 4.1% vs. 2.3%) and other eye disease (200%, 2.4% vs. 0.8%).
| Variable | Screening year*, n (^) | Overall, n (^; 95% CI^^) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007/08 | 2008/09 | 2009/10 | 2010/11 | 2011/12 | 2012/13 | 2013/14 | 2014/15 | 2015/16 | 2016/17 | ||
| Number of Individuals with a first ever recorded attended screening appointment | 1883 (100.0) | 1542 (100.0) | 1566 (100.0) | 1665 (100.0) | 1941 (100.0) | 1503 (100.0) | 1588 (100.0) | 1634 (100.0) | 1629 (100.0) | 1877 (100.0) | 16828 (100.0) |
| Screen-positive | 230 (12.2) | 226 (14.7) | 169 (10.8) | 148 (8.9) | 197 (10.1) | 131 (8.7) | 112 (7.1) | 129 (7.9) | 157 (9.6) | 160 (8.5) | 1659 (9.9; 9.4 to 10.3) |
| Screen-positive for DR | 98 (5.2) | 67 (4.3) | 48 (3.1) | 52 (3.1) | 64 (3.3) | 42 (2.8) | 40 (2.5) | 49 (3.0) | 55 (3.4) | 56 (3.0) | 571 (3.4; 3.1 to 3.7) |
| Screen-positive for unassessable images | 83 (4.4) | 88 (5.7) | 87 (5.6) | 72 (4.3) | 103 (5.3) | 57 (3.8) | 33 (2.1) | 43 (2.6) | 55 (3.4) | 62 (3.3) | 683 (4.1; 3.8 to 4.4) |
| Screen-positive for other eye disease requiring HES** | 49 (2.6) | 71 (4.6) | 34 (2.2) | 24 (1.4) | 30 (1.5) | 32 (2.1) | 39 (2.5) | 37 (2.3) | 47 (2.9) | 42 (2.2) | 405 (2.4; 2.2 to 2.6) |
| Biomicroscopy recorded*** | 186 (9.9) | 197 (12.8) | 135 (8.6) | 127 (7.6) | 169 (8.7) | 114 (7.6) | 106 (6.7) | 119 (7.3) | 145 (8.9) | 144 (7.7) | 1442 (8.6; 8.2 to 9.0) |
| STDR | 39 (2.1) | 37 (2.4) | 31 (2.0) | 31 (1.9) | 42 (2.2) | 30 (2.0) | 33 (2.1) | 36 (2.2) | 40 (2.5) | 45 (2.4) | 364 (2.2; 2.0 to 2.4) |
| STR$ | 15 (0.8) | 26 (1.7) | 18 (1.1) | 14 (0.8) | 20 (1.0) | 13 (0.9) | 14 (0.9) | 23 (1.4) | 14 (0.9) | 23 (1.2) | 180 (1.1; 0.9 to 1.2) |
| STM$ | 35 (1.9) | 29 (1.9) | 21 (1.3) | 25 (1.5) | 39 (2.0) | 26 (1.7) | 28 (1.8) | 23 (1.4) | 35 (2.1) | 38 (2.0) | 299 (1.8; 1.6 to 2.0) |
| Unknown STR & unknown STM | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (< 0.1; 0.0 to 0.0) |
| Not STDR | 147 (7.8) | 160 (10.4) | 104 (6.6) | 96 (5.8) | 127 (6.5) | 84 (5.6) | 73 (4.6) | 83 (5.1) | 105 (6.4) | 96 (5.1) | 1075 (6.4; 6.0 to 6.8) |
| Unknown STDR status | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.2) | 3 (< 0.1; 0.0 to 0.1) |
| No biomicroscopy recorded*** | 44 (2.3) | 29 (1.9) | 34 (2.2) | 21 (1.3) | 28 (1.4) | 17 (1.1) | 6 (0.4) | 10 (0.6) | 12 (0.7) | 16 (0.9) | 217 (1.3; 1.1 to 1.5) |
| Screen-negative | 1653 (87.8) | 1316 (85.3) | 1397 (89.2) | 1517 (91.1) | 1744 (89.9) | 1372 (91.3) | 1476 (92.9) | 1505 (92.1) | 1472 (90.4) | 1717 (91.5) | 15,169 (90.1; 89.7 to 90.6) |
| Variable | Screening year*, n (%^) | Overall, n (%^; 95% CI^^^) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2007/08 | 2008/09 | 2009/10 | 2010/11 | 2011/12 | 2012/13 | 2013/14 | 2014/15 | 2015/16 | 2016/17 | ||
| Number of Individuals with an attended screening appointment (not first ever recorded) | 6349 (100.0) | 7155 (100.0) | 8769 (100.0) | 9585 (100.0) | 11,248 (100.0) | 12,037 (100.0) | 13,561 (100.0) | 13,935 (100.0) | 13,656^^ (100.0) | 14,230^^ (100.0) | 11,2173 (100.0) |
| Screen-positive | 486 (7.7) | 698 (9.8) | 686 (7.8) | 549 (5.7) | 724 (6.4) | 644 (5.4) | 557 (4.1) | 676 (4.9) | 875 (6.4) | 835 (5.9) | 6929 (6.2; 6.0 to 6.3) |
| Screen-positive for DR | 258 (4.1) | 334 (4.7) | 313 (3.6) | 276 (2.9) | 355 (3.2) | 289 (2.4) | 305 (2.2) | 338 (2.4) | 387 (2.8) | 382 (2.7) | 3351 (3.0; 2.9 to 3.1) |
| Screen-positive for unassessable images | 173 (2.7) | 271 (3.8) | 285 (3.3) | 212 (2.2) | 322 (2.9) | 265 (2.2) | 146 (1.1) | 215 (1.5) | 353 (2.6) | 321 (2.3) | 2632 (2.3; 2.3 to 2.4) |
| Screen-positive for other eye disease requiring HES** | 55 (0.9) | 93 (1.3) | 88 (1.0) | 61 (0.6) | 47 (0.4) | 90 (0.7) | 106 (0.8) | 123 (0.9) | 135 (1.0) | 132 (0.9) | 946 (0.8; 0.8 to 0.9) |
| Biomicroscopy recorded*** | 432 (6.8) | 598 (8.4) | 578 (6.6) | 485 (5.1) | 605 (5.4) | 537 (4.5) | 515 (3.8) | 623 (4.5) | 820 (6.0) | 724 (5.1) | 6082 (5.4; 5.3 to 5.6) |
| STDR | 106 (1.7) | 155 (2.2) | 148 (1.7) | 129 (1.3) | 181 (1.6) | 149 (1.2) | 179 (1.3) | 210 (1.5) | 211 (1.5) | 245 (1.7) | 1766 (1.6; 1.5 to 1.6) |
| STR$ | 29 (0.5) | 55 (0.8) | 47 (0.5) | 42 (0.4) | 55 (0.5) | 44 (0.4) | 55 (0.4) | 75 (0.5) | 71 (0.5) | 87 (0.6) | 579 (0.5; 0.5 to 0.6) |
| STM$ | 84 (1.3) | 128 (1.8) | 115 (1.3) | 105 (1.1) | 158 (1.4) | 123 (1.0) | 151 (1.1) | 168 (1.2) | 181 (1.3) | 199 (1.4) | 1453 (1.3; 1.2 to 1.4) |
| Unknown STR & unknown STM | 0 (0.0) | 2 (< 0.1) | 1 (< 0.1) | 3 (< 0.1) | 0 (0.0) | 4 (< 0.1) | 1 (< 0.1) | 1 (< 0.1) | 0 (0.0) | 0 (0.0) | 12 (< 0.1; 0.0 to 0.0) |
| Not STDR | 326 (5.1) | 443 (6.2) | 430 (4.9) | 356 (3.7) | 424 (3.8) | 388 (3.2) | 333 (2.5) | 413 (3.0) | 609 (4.5) | 459 (3.2) | 4293 (3.8; 3.7 to 3.9) |
| Unknown STDR status | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (< 0.1) | 0 (0.0) | 0 (0.0) | 20 (0.1) | 23 (< 0.1; 0.0 to 0.0) |
| No biomicroscopy recorded*** | 54 (0.9) | 100 (1.4) | 108 (1.2) | 64 (0.7) | 119 (1.1) | 107 (0.9) | 42 (0.3) | 53 (0.4) | 55 (0.4) | 111 (0.8) | 847 (0.8; 0.7 to 0.8) |
| Screen-negative | 5863 (92.3) | 6457 (90.2) | 8083 (92.2) | 9036 (94.3) | 10,524 (93.6) | 11,393 (94.6) | 13,004 (95.9) | 13,259 (95.1) | 12,781 (93.6) | 13,395 (94.1) | 105,244 (93.8; 93.7 to 94.0) |
Type 1 and type 2 diabetes
Rates of screen-positive (11.9% vs. 6.0%) and screen-positive because of DR (10.7% vs. 2.3%) were higher in people with type 1 diabetes. Rates of STDR were much higher (6.4% vs. 1.2%) in people with type 1 diabetes and a higher proportion of STDR was because of STR (47% vs. 33%).
Discussion
Our data from an established screening programme give up-to-date estimates of current incidences of key stages in the diabetes care pathway of importance to patients, namely progression to screen-positive, screen-positive because of DR and STDR. The annual incidence of screen-positive cases showed a consistent fall over a decade from 8–10% to around 5–6%. The rates of screen-positive attributed to DR also fell over time to around 3%, slightly lower but consistent with a referable retinopathy rate (equivalent to our screen-positive because of DR) of 4.3% reported in 2013 from Scotland. 9 Both of these trends occurred against a background of stable rates of true-positive STDR. These reductions require further investigation in other screening programmes. They may represent improvements in grading.
The rates of STDR were stable and consistently under 2.0% apart from 1 year early in the study. These low rates have also been reported in 2015 from Gloucestershire, a programme similar to ours in Liverpool. 42
The strengths of our study include the size of the data set, the duration of data collection and the robust analysis. Previous studies were from several decades ago, when levels of diabetes control were poorer. HbA1c levels were generally well controlled in our population.
The main limitation of this study is the nature of data availability, which meant that the data set was partly retrospective. Around 5% of the population died or moved away each year and without consent we were unable to access their historical data. This censoring could have affected our estimates of DR and STDR between 2006/7 and 2012/13.
The ISDR RCT commenced in 2014, resulting in a proportion of patients moving to 2-yearly screening. We corrected for this effect in the last two years of our analysis.
In Liverpool in 2017/18, 9.4% (2054/21853) people were attending the hospital for management of their diabetes or slit-lamp-based screening. Nonetheless we are able to estimate a rate of STDR in people attending screening of 164/10,000 PWD/annum (2192/133,554*10,000), useful data for people running established screening programmes and in designing future RCTs. Around 82% of these people had STM and 36% STR.
We detected a shift in ethnicity over the 11 years of the study but the data were difficult to interpret due to the increase in unknown data on ethnicity caused by changes in the methods of collection.
Fewer than half of people graded as screen-positive were considered to have DR by the grading teams and around half of these were judged as true-positive in medical retina clinics. A debate needs to take place about these levels of positivity, preferably with patient groups and other stakeholders. This is especially clear in new screenees, where only a 1/5th (21.7%) of people identified as screen-positive had true-positive disease. Much of the screen-positive disease is due to unassessable images, most likely due to cataract, and other significant eye disease, none of which are the objectives of DR screening.
In spite of the high levels of previously undetected cataract and other eye disease (80% of screen-positives due to unassessable images or other significant eye disease), people undergoing their first screening visit did have higher rates (37.5%) of STDR, suggesting that these individuals might be targeted specifically in screening programmes. We detected screen-positive rates in these new screenees of 9.9%. In Hong Kong, which introduced screening in 2015, a similar 10% of screen-positives were detected in their first year of screening a population that had not been screened previously. 65
Conclusions
In an established screening programme with a stable population, rates of screen-positive overall and due to DR show a consistent fall over time to a low level. The annual incidence of STDR is under 2%, suggesting future work on defining screen-positive and supporting extended intervals for people at low risk. Current screening definitions and pathways were established over 15 years ago at the introduction of screening and were consensus-based and large numbers of people appear to be being referred unnecessarily to the HES. Higher rates of disease are seen in new screenees, especially those with a history of non-attendance, suggesting specific targeting of this group. Our data are useful for the design of future epidemiological and intervention studies. For epidemiological data from screening programmes to be interpretable, future reporting should clearly differentiate between screen-positive, screen-positive due to DR, ungradeable and other eye disease, as well as STDR.
Interrelation to other work packages and overall aims of the programme
This work package was supported by work from the PPI group around patient-centred outcomes as well as data collected in the ISDR DW (WP B1).
Knowledge transfer
Research outputs
Programme outputs (work package G) as of 30 September 2021 are listed in Peer reviewed, full publications and Other publications.
Dissemination activities
Simon Harding, programme CI, is a member of the Research Advisory Committee, English NDESP and reports on findings and policy implications of ISDR. He has attended UK and international conferences 2017–2020 raising awareness of individualised screening.
We held a dissemination event (25 September 2019) for the screening community and commissioners from the home nations and outside the UK and to involve patient groups.
There was general support on safety of switching to extended intervals in stratified and/or personalised/individualised screening. Scale-up through a whole population ‘pilot’ was considered reasonable. Failsafe and communication needed revising.
Facilitated discussion identified a number of issues in the ISDR data set to be addressed:
-
3503 people did not consent for the RCT during recruitment
-
external validation was needed in another UK programme
-
further analysis was recommended: non-consented group, model cohort data set through RCE, compare with the stratification model to be used in the NDESP.
Further analysis
The team conducted further analyses within the programme in response to the symposium (see Supplementary Material 4).
People who did not consent to recruitment in the RCT were more likely to be female, older, have longer disease duration, be smokers (all p < 0.001) and had slightly higher screen-positive rates (p = 0.11). They had similar other clinical risk factors. This is likely to reflect older people being less likely to agree to participate in research.
Allocations by the RCE to 6-, 12- and 24-month intervals in the non-consenting group in the RCT (n = 3180) were similar to those who consented. For the ISDR cohort study data set there was a slightly higher (4.5%) allocation to 6 months (high-risk) and a corresponding reduction in the 24-month (low-risk) group. In scaling-up to whole populations there may be a small reduction in resources being freed up and a small increase in the high-risk group.
We compared IDSR variable-interval risk-based screening with the planned English NDESP stratified screening30 by applying the proposed rules to the RCT data set. We estimated that the NDESP stratification will allocate 33.1% of the RCT group to 12-months rescreening and 66.9% to 24-months (ISDR 82.3%), a 26% reduction in the move to an extended interval.
We modelled a ‘virtual RCE annual review’ as a failsafe for people being allocated to extended interval to run at 12 months using updated clinical data and baseline grading. We ran the RCE in the 24-month RCT group (n = 1776) at 12 months; 97.1% (1725/1776) remained in the low-risk group. Two people (0.1%) were reallocated to 6 months and 49 (2.8%) to 12 months.
The Liverpool RCE was ‘re-run’ at the end of the study to act as an internal validation and check of robustness of the model, which showed the RCE model is ‘stable’ in the Liverpool population.
An executable RCE package has been produced. Independent external validation will be completed (at the time of writing, under way in partnership with the Diabetes Research Unit Cymru). We have designed a pilot phase IV whole-population scale-up in Liverpool to start in 2022 (at the time of writing under review by the UK National Screening Committee).
Intellectual property
The team has worked with the RLBUHT Innovation Team’s partner, 2Bio Ltd, throughout the programme. Two components have been assessed as having potential for IP, the RCE and the DW.
RCE
-
mathematics, covariate selection, import scripts, imputation protocols
-
potential to function as a stand-alone clinical tool.
DW
-
SQL server integration service protocols, real-time DW, imputation and credibility systems
-
potential to be developed as a NHS clinical tool.
In any future commercialisation or deployment of these systems re-writing in a more portable and commercially attractive programming language will be required. The original code base built in MATLAB and MatSoap for the RCE has been ported into Python and validated using risk-calculations from the RCT.
The RLBUHT Innovation team will support the process of commercialisation, including discussions and negotiations with potential partners and licensees.
Conclusions and recommendations
Reflections on the programme
A PGfAR offers long-term funding for a large piece of work. Much can be achieved. We completed a large RCT and produced evidence of global significance which will underpin the next 10–15 years of development of DR screening. We completed a large qualitative study that will underpin the future implementation of screening and diabetes services, with relevance outside ophthalmology. We undertook micro-costing and economic analyses using real-world resource data rather than modelling. We overcame the challenges of data access and variability to develop a data linkage and integration system implemented into NHS clinical care. We built and tested a RCE for ophthalmology, tuneable for local populations.
Our findings have given new impetus to the introduction of extended screening in England, due to be introduced in 2022/23. Our RCT has given impetus to the development of variable-interval risk-based screening which is being rolled out in other countries, including Hong Kong, Denmark and Singapore. Our team are engaged in global DR screening research in Malawi, Malaysia and China. Our expertise has been utilised in 2020 by WHO Europe in developing guidance for policy-makers on DR screening. 66
We were not able to deliver on all aspects of the planned work. The recruitment of such a large sample required screening of over 8000 individuals, each requiring identification, information and consenting. It took time to mobilise recruitment teams and we were about 12 months late in delivering the recruitment milestone. The teams that we had originally planned for proved unavailable at a key stage. Accessing the data proved more challenging than expected. The multiple data platforms and the poor or absent documentation prevented us from accessing all the planned data types. We received substantial additional research capacity and sustainability funds from our sponsor at two key stages, to expand the recruitment teams and for the data engineering, both of which we had underestimated in our funding model. We also needed to reallocate resources from our observational cohort study to support the RCT. This meant that we cut the section in WP B on collection of VA and grading data from the HES, to the significant disappointment of the PPI team. Nonetheless we did complete a longitudinal cohort study on over 28,000 individuals attending the screening programme.
Our development grant phase was essential. It allowed us to develop a comprehensive and effective team of co-investigators including a patient representative and a local manager and develop relationships with key stakeholders especially in primary care. We addressed key areas of uncertainty:
-
We were able to identify and extract sample data from the widely varying domains in primary care, the screening programme and the hospital.
-
We explored and made progress with the challenging governance and data-protection environments in the NHS.
-
We built a demonstration version of the RCE.
Managing a long and complex programme of work must not be underestimated. It requires substantial commitment from the CI and an effective and able programme manager. The role of the WP leads is critical. Regular investigator meetings are essential to maintain enthusiasm and engagement. Inevitably co-investigators’ priorities can change or there can be extended periods of leave. Effective support for the CI from senior members of research departments can help maintain momentum or input additional resources and this needs to happen early. An effective Programme Steering Committee is a ‘critical friend’ at challenging times.
Strengths and limitations
Our programme provides for the first time RCT evidence on individualised DR screening with a full economic evaluation with the quality assured by a clinical trials centre. Recommendations are supported by qualitative evidence, not previously reported, and an active PPI group was embedded throughout. Our findings do come with a number of limitations, which have been described above. Here we briefly summarise the most important of these.
Participants were enrolled from a long-established programme with low rates of DR and progression to STDR. Good glycaemic and BP control and a relatively low proportion of type 1 diabetes (4.0%) might have biased the sample. Findings may not be generaliseable across other programme in the UK with differing ethnicity. Low rates of DR have been reported in other similar settings, but our results should be treated with caution in areas with a higher prevalence, poorer control of diabetes or wider ethnic mix, or in programmes during the set-up process.
Our trial had only a 2-year time horizon, which is short in the context of a life-long condition. With a move to extended-interval screening in several countries, we wanted to provide high-quality RCT evidence on how people act when given variable-interval risk-based screening. The study was designed to compare safety and effectiveness of two screening regimens, not the cost-effectiveness of screening versus no screening. To allow for two cycles for the low-risk group would have extended the study duration from 4 to 6 years. The low rates of retinopathy and STDR in the low-risk group suggest that our findings on effectiveness would be unlikely to change; most disease was detected in the high- and medium-risk groups. Our relatively short time horizon could have been mitigated by an extensive risk-based economic model based on the RCE (described in Economic model) but the model ran into computational difficulties. It will be important in future testing to recognise this limitation and ensure robust monitoring of attendance rates. It may also be informative to extend the cost-effectiveness analysis to include the whole care pathway, including downstream costs and outcomes.
Some of our secondary analyses within the individualised group relied on small numbers, especially in the high- and medium-risk groups. Conclusions based on these analyses need to be treated with some caution.
We were unable to recruit a cohort of regular non-attenders so cannot address this important issue. We were unable to complete the component of the cohort study in the HES, so were only able to provide data on incidences in the screening population.
Conclusions
Here we summarise the key findings:
-
Evidence from the ISDR RCT shows that extended and personalised interval screening can safely be introduced in established screening programmes.
-
Our data provide evidence that an individualised variable-interval risk-based approach has the potential to give improvements in cost-effectiveness without compromising safety or quality of life, but we did not include treatment costs in our economic analyses.
-
Our qualitative evidence shows that PWD and HCPs appear to be supportive of variable intervals in screening but with important caveats. For successful implementation interpretable and clear safeguards against increasing non-attendance, loss of diabetes control and system failures are required. Alternative referral pathways are required for those lost to follow-up or whose risk factors change substantially over longer intervals.
-
Variable-interval screening supported by clinical data allows 6-monthly targeting of a high-risk group, detecting disease earlier, while substantially reducing the number of appointments for a low-risk group.
-
The rates of STDR in our long-established screening programme have dropped to a very low level, supported by other limited UK data. Higher rates are seen in those attending screening for the first time and with type 1 diabetes. The majority of cases classed as screen-positive do not have sight-threatening disease.
-
Robust monitoring of attendance and retinopathy rates should be included in any wider testing. Our trial was over a 2-year time horizon when the disease has a long time-frame, and our findings come from a single centre with a relatively stable mainly white population.
-
Findings from this research show that PPI can produce key decisions to underpin critical parts of the design and delivery of applied research. Strong commitment of the senior investigators is essential.
-
The evidence from our programme and elsewhere shows that RCEs can be feasible, reliable, safe and acceptable to patients with the caveat that reliable monitoring and clear communication to stakeholders are necessary.
-
Evidence from our programme has demonstrated that real-time integration of data from primary and secondary care sectors can be achieved, and indeed is necessary for the RCE, but relies on successful engagement of key stakeholders. This may not be feasible in some settings.
Recommendations for future research
Our recommendations are based on the evidence from the ISDR programme and elsewhere.
-
Individualised variable-interval risk-based screening is ready to be upscaled in a phase IV whole-population pilot. Evidence of repeatable results as seen in ISDR should be collected before considering wider dissemination.
-
Further health economics research is required to support potential adoption of individualised/personalised screening, including modelling beyond the 2-year time horizon and extending to include lifetime costs of treatment and care, and blindness averted.
-
The Liverpool RCE requires external validation with at least one other screening programme before it could be considered for adoption.
-
Further qualitative research is required around the potential adoption of extended and variable-interval screening to develop and implement safeguards and monitoring systems interpretable by end-users.
-
Evidence on efficacy in populations with higher rates of diabetes and different mixes of ethnicity, in newly commencing programmes and in low/middle-income countries is required.
-
New research is needed on methods to address the misunderstandings and conflation in PWD around their diabetes and eye care.
-
Evidence of current rates of VI should be collected in line with this being a key patient-centred outcome.
-
Novel approaches to recruit non-attenders to future qualitative research need to be developed for research into non-attendance.
-
Research is needed to establish more appropriate referral thresholds in DR screening.
The knowledge base in the field of screening for DR is changing in some areas. Technology is developing around automated grading and artificial intelligence. Some work is under way to integrate within screening but progress is not rapid. Digital healthcare is being developed with initiatives on big data and data integration which will support future personalisation. However, these approaches are still yet to be embedded in large health systems such as the NHS. Large epidemiological studies especially on important functional patient-centred outcomes are few, with much of the data being outdated and with limited current and future work planned. Pilot studies on introduction of new technologies are being considered and may move the field forwards in the medium term. The qualitative evidence base is small with little of relevance to screening and is unlikely to move forward rapidly.
Acknowledgements
The authors are grateful to the ISDR PPI Group for essential input into design and review, to the Liverpool Clinical Commissioning Group (LCCG) for data extraction and transfer, and to the Liverpool Local Medical Committee and local GPs for support with establishing patient lists and consent. We would like to acknowledge the outstanding attention to detail from the ISDR administrative team, the Clinical Trials Research Centre, the North West Coast Clinical Research Network and our other trained researchers for their immeasurable help in recruiting to target, and the Liverpool Diabetic Eye Screening Programme (LDESP) team for their support and assistance throughout the RCT and cohort study. We received considerable support from our sponsors, The Royal Liverpool and Broadgreen University Hospitals NHS Trust, now Liverpool University Hospital NHS Foundation Trust, throughout the programme, including additional funding to support recruitment and the DW.
Members of the ISDR Study Group who are not included as primary authors are listed in Appendix 1.
Contributions of authors
Simon Harding (https://orcid.org/0000-0003-4676-1158) (Chair Professor, Clinical Ophthalmology) was CI on the programme and drafted and produced the final report including revisions.
Duncan Appelbe (https://orcid.org/0000-0003-1493-0391) (Senior Research Information Specialist) was the information-systems manager for the CTRC.
Ayesh Alshukri (https://orcid.org/0000-0003-0852-2068) (Research Associate, Computer Science) was part of the observational cohort, RCE and RCT teams.
Deborah Broadbent (https://orcid.org/0000-0002-3195-5024) (Honorary Senior Lecturer, Ophthalmology) was an applicant on the grant and led the RCT.
Philip Burgess (https://orcid.org/0000-0002-3959-2299) (Clinical Lecturer, Ophthalmology) was part of the observational cohort team.
Paula Byrne (https://orcid.org/0000-0001-8090-6184) (Senior Lecturer, Sociology of Health and Illness) led the qualitative team.
Christopher Cheyne (https://orcid.org/0000-0002-2330-9559) (Research Associate, Biostatistics) was part of the team that conducted the statistical analysis.
Antonio Eleuteri (https://orcid.org/0000-0003-0718-6672) (Honorary Lecturer, Medical Physics and Clinical Engineering) built the RCE.
Anthony Fisher (https://orcid.org/0000-0001-9086-8812) (Consultant Clinical Scientist, Medical Physics and Clinical Engineering) was an applicant on the grant and led the RCE team.
Marta García-Fiñana (https://orcid.org/0000-0003-4939-0575) (Professor of Biostatistics) was an applicant on the grant and led the statistics team.
Mark Gabbay (https://orcid.org/0000-0002-0126-8485) (Professor of General Practice, Health Services Research) was an applicant on the grant and contributed to the qualitative work package.
Marilyn James (https://orcid.org/0000-0003-0408-7898) (Professor of Health Economics) was an applicant on the grant and led the health economics team.
James Lathe (https://orcid.org/0000-0002-5416-0873) (Health Economist) was part of the team that conducted the cost-effectiveness analysis.
Tracy Moitt (https://orcid.org/0000-0002-5579-996X) (Senior Trials Manager) was an applicant on the grant and led the trial management.
Mehrdad Mobayen Rahni (https://orcid.org/0000-0002-1278-8558) (Lead Database Analyst, Business Intelligence) was the data warehouse designer and manager.
John Roberts (https://orcid.org/0000-0003-3392-7243) (Patient representative) was an applicant on the grant and led the involvement of the PPI group.
Christopher Sampson (https://orcid.org/0000-0001-9470-2369) (Health Economist) was part of the team that conducted the cost-effectiveness analysis.
Daniel Seddon (https://orcid.org/0000-0002-0296-6234) (GP, NHS England) was an applicant on the grant, represented the end-user and contributed to several work packages
Irene Stratton (https://orcid.org/0000-0003-1172-7865) (Senior Statistician, Medical Statistics) was an applicant on the grant and provided senior advice and input to the statistical team and all aspects of the programme.
Clare Thetford (https://orcid.org/0000-0003-2188-3052) (Research Fellow) was part of the qualitative team.
Pilar Vazquez-Arango (https://orcid.org/0000-0002-4117-7676) (Research Coordinator, Ophthalmology) was responsible for project management (2018–20) and with Simon Harding produced the final report.
Jiten Vora (https://orcid.org/0000-0002-3488-5287) (Professor/Consultant Endocrinologist) was an applicant on the grant and led the observational cohort study.
Amu Wang (https://orcid.org/0000-0002-1764-6524) (Honorary Research Associate) was responsible for project management (2013–18).
Paula Williamson (https://orcid.org/0000-0001-9802-6636) (Professor of Medical Statistics) was an applicant on the grant and Director of the Liverpool CTRC.
Publications
Cheyne CP, Burgess PI, Broadbent DM, García-Fiñana M, Stratton IM, Criddle T, et al. Incidence of sight-threatening diabetic retinopathy in an established urban screening programme: an 11-year cohort study. Diabet Med 2021;38:e14583.
Eleuteri A, Fisher AC, Broadbent DM, García-Fiñana M, Cheyne CP, Wang A, et al. Individualised variable-interval risk-based screening for sight-threatening diabetic retinopathy: the Liverpool Risk Calculation Engine. Diabetologia 2017;60:2174–82.
Byrne P, Thetford C, Gabbay M, Clarke P, Doncaster E, Harding SP, ISDR Study Group. Personalising screening of sight-threatening diabetic retinopathy – qualitative evidence to inform effective implementation. BMC Public Health 2020;20:881.
Broadbent DM, Wang A, Cheyne CP, James M, Lathe J, Stratton IM, et al. Safety and cost-effectiveness of individualised screening for diabetic retinopathy: the ISDR open-label, equivalence RCT. Diabetologia 2021;64:56–69.
Peer-reviewed, full publications
| Author | Title | Year | Output type | Work package | URL link |
|---|---|---|---|---|---|
| Sampson CJ et al. | Health state utility values for diabetic retinopathy: protocol for a systematic review and meta-analysis | 2015 | Peer reviewed publication – Systematic Reviews | D | https://doi.org/10.1186/s13643–015–0006–6 |
| Eleuteri A et al. | Individualised variable interval risk-based screening for sight threatening diabetic retinopathy – the Liverpool Risk Calculation Engine | 2017 | Peer reviewed publication – Diabetologia | C | https://doi.org/10.1007/s00125-017-4386-0 |
| García-Fiñana M et al. | Personalised risk-based screening for diabetic retinopathy: a multivariate approach versus the use of stratification rules | 2018 | Peer reviewed publication – Diabetes, Obesity and Metabolism | E | https://doi.org/10.1111/dom.13552 |
| Broadbent et al. | Individualised screening for diabetic retinopathy: the ISDR study – rationale, design and methodology for a randomised controlled trial comparing annual and individualised risk-based variable-interval screening | 2019 | Peer reviewed publication – BMJ Open | E | http://dx.doi.org/10.1136/bmjopen-2018–025788 |
| Byrne et al. | Personalising screening of sight-threatening diabetic retinopathy – qualitative evidence to inform effective implementation | 2020 | Peer reviewed publication – BMC Public Health | F | https://doi.org/10.1186/s12889–020–08974–1 |
| Broadbent et al. | Safety and cost-effectiveness of individualised screening for diabetic retinopathy: the ISDR open-label, equivalence randomised controlled trial | 2020 | Peer reviewed publication – Diabetologia | D,E | https://doi.org/10.1007/s00125–020–05313–2 |
| Cheyne et al. | Incidence of sight-threatening diabetic retinopathy in an established urban screening programme: an 11-year cohort study | 2021 | Peer reviewed publication – Diabetic Medicine | B | https://doi.org/10.1111/dme.14583 |
Other publications and outputs
| Author | Title | Year | Output type | Work package | URL link |
|---|---|---|---|---|---|
| Thetford C et al. | Patient perspectives of diabetic retinopathy screening and models of diabetes care | 2014 | Conference presentation – SAPC | F | N/A |
| Sampson C et al. | Individualised cost-effectiveness analysis in risk-based screening: practical and ethical? | 2015 | Conference presentation – Personalised Medicine and Resource Allocation | D | N/A |
| Harding S et al. | 10-year observational study of patient related outcomes during expansion to full population coverage of a systematic diabetes eye care programme – the Liverpool Diabetic Eye Study | 2016 | Conference presentation – ARVO | B | https://iovs.arvojournals.org/article.aspx?articleid=2559561&resultClick=1 |
| Appelbe D et al. | E-trials within the UKCRC CTU network – opportunities and challenges | 2017 | Conference presentation – NIHR e-trial | E | N/A |
| García-Fiñana M et al. | A novel multivariate discriminant approach to predict sight threatening diabetic retinopathy (STDR) cases – data from the Liverpool Diabetic Eye Study | 2017 | Conference presentation – ARVO | E | https://iovs.arvojournals.org/article.aspx?articleid=2641391&resultClick=1 |
| Harding SP | The UK as a clinical trials destination – 10 years of the National Institute for Health Research. Personalised medicine in the NHS | 2017 | Conference presentation – NIHR CRN Ophthalmology symposium, ARVO | E | N/A |
| Harding SP et al. | Detecting sight threatening diabetic retinopathy in the 21st century – lessons from 20 years of screening in Liverpool | 2017 | Conference presentation – Macula Society | B2,C-F | N/A |
| Harding SP | Personalising the interval in screening for sight threatening diabetic retinopathy | 2017 | Presentation – Royal Society of Medicine | E | N/A |
| Sampson CJ et al. | Systematic review and meta-analysis of health state utility values for diabetic retinopathy: implications for model-based economic evaluation | 2017 | Conference presentation – HEG | D | N/A |
| Harding S et al. | The costs of screening for sight-threatening diabetic retinopathy | 2019 | Conference presentation – ARVO | D | https://iovs.arvojournals.org/article.aspx?articleid=2744254&resultClick=1 |
| Broadbent DM | Developments in individualised risk based screening, applicable to large local populations | 2019 | Conference presentation – EASD | E | N/A |
| Harding SP | Safety and efficacy of individualised compared with annual screening for diabetic retinopathy in the ISDR randomised controlled trial | 2019 | Conference presentation – EASD | E | N/A |
| James M | Cost-effectiveness of personalised screening and scaling up to address a worldwide ‘pandemic’ | 2019 | Conference presentation – EASD | D | N/A |
| Harding et al. | Safety, efficacy and cost-effectiveness of individualised screening for diabetic retinopathy: the ISDR open-label, equivalence randomised controlled trial | 2020 | Conference presentation – Macula Society | D,E | N/A |
| Broadbent et al. | Safety and cost-effectiveness of individualised screening for diabetic retinopathy: the ISDR open-label, equivalence randomised controlled trial | 2020 | Conference presentation – EASDec | D,E | N/A |
| Harding SP et al. | Individualised risk-based screening for sight threatening diabetic retinopathy – the Liverpool Risk Calculation Engine | 2015 | Published abstract – ARVO | C | http://iovs.arvojournals.org/article.aspx?articleid=2331769 |
| Appelbe D et al. | Introducing personalised risk-based intervals in screening for diabetic retinopathy: development, implementation and assessment of safety, cost-effectiveness and patient experience (ISDR): a case study in the use of automated systems in trials | 2015 | Published abstract – International Clinical Trials Methodology Conference | E | https://doi.org/10.1186/1745–6215–16-S2-O59 |
| Broadbent DM et al. | Introducing personalised risk-based intervals in screening for diabetic retinopathy: the ISDR study | 2015 | Published abstract – EASDec | E | https://doi.org/10.5301/ejo.5000612 |
| Byrne P et al. | Patient perspectives of acceptability of risk-based individualised diabetic retinopathy | 2015 | Published abstract – EASDec | F | https://doi.org/10.5301/ejo.5000612 |
| Harding SP et al. | Individualised risk-based screening for diabetic retinopathy: the Liverpool Risk Calculation Engine | 2015 | Published abstract – EASDec | C | https://doi.org/10.5301/ejo.5000612 |
| Sampson CJ et al. | Cost-effectiveness of a risk-based screening programme for diabetic retinopathy: a modelling approach | 2015 | Published abstract – EASDec | D | https://doi.org/10.5301/ejo.5000612 |
| Harding SP et al. | Individualised risk-based screening for sight threatening diabetic retinopathy – the Liverpool Risk Calculation Engine | 2015 | Published abstract – ARVO | C | https://iovs.arvojournals.org/article.aspx?articleid=2331769 |
| Harding SP et al. | 10-year observational study of patient related outcomes during expansion to full population coverage of a systematic diabetes eye care programme – the Liverpool Diabetic Eye Study | 2016 | Published abstract – EASDec | B | https://doi.org/10.5301/ejo.5000799 |
| James M et al. | Micro-costing diabetic eye screening: estimation of personal expense, attendance and health care resource use | 2016 | Published abstract – EASDec | D | https://doi.org/10.5301/ejo.5000799 |
| Sampson CJ et al. | Health-related quality of life of people attending screening for diabetic retinopathy within a trial setting | 2016 | Published abstract – EASDec | D | https://doi.org/10.5301/ejo.5000799 |
| Thetford C et al. | Comparing health professionals’ and patient perspectives of individualised screening intervals for diabetic retinopathy screening | 2016 | Published abstract – EASDec | F | https://doi.org/10.5301/ejo.5000799 |
| Wang A et al. | Building a dynamic functional clinical data warehouse (CDW) for personalised health care – lessons from the Individualised Screening for Diabetic Retinopathy (ISDR) study | 2016 | Published abstract – EASDec | B | https://doi.org/10.5301/ejo.5000799 |
| Harding SP et al. | Safety of individualised variable interval screening for referable diabetic retinopathy – baseline data from the ISDR randomised controlled study | 2017 | Published abstract – EASDec | E | https://doi.org/10.5301/ejo.5000979 |
| Byrne P et al. | Putting patients’ eyes back into their bodies: implications of a social science study of screening and diabetes eye care | 2018 | Published abstract – EASDec | F | https://doi.org/10.1177/1120672118773246 |
| Vazquez-Arango P et al. | Visual impairment in a population-based evaluation of people with diabetes | 2018 | Published abstract – EASDec | B | https://doi.org/10.1177/1120672118773246 |
| Wang A et al. | ISDR PPI group. Enabling patients to be partners in research – how to ensure publicly funded applied health services research is fit-for-purpose | 2018 | Published abstract – EASDec | F | https://doi.org/10.1177/1120672118773246 |
| Broadbent DM et al. | Individualised screening for diabetic retinopathy: the ISDR study – a randomised controlled trial of safety, efficacy and cost-effectiveness | 2019 | Published abstract – ADA | E | https://doi.org/10.2337/db19–39-LB |
| Harding SP et al. | Safety and efficacy of individualised compared to annual screening for diabetic retinopathy in the ISDR randomised controlled trial | 2019 | Published abstract – EASD | E | https://link.springer.com/content/pdf/10.1007%2Fs00125–019–4946–6.pdf |
| James M et al. | Cost-effectiveness of individualised versus annual screening for diabetic retinopathy in the ISDR randomised controlled trial | 2019 | Published abstract – EASD | D | https://link.springer.com/content/pdf/10.1007%2Fs00125–019–4946–6.pdf |
| Stratton IM et al. | Individualised screening for diabetic retinopathy. A randomised controlled clinical trial of precision medicine | 2019 | Poster – ADA Research symposium | E | N/A |
Data-sharing statement
All requests for data should be sent to the corresponding author. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- World Health Organization (WHO) . World Report on Vision 2019. www.who.int/publications-detail/world-report-on-vision (accessed 10 December 2019).
- The Saint Vincent Declaration on diabetes care and research in Europe. Acta Diabetologia 1989;10:143-4.
- Scanlon PH. The English national screening programme for sight-threatening diabetic retinopathy. J Med Screen 2008;15:1-4. https://doi.org/10.1258/jms.2008.008015.
- Taylor R, Broadbent DM, Greenwood R, Hepburn D, Owens DR, Simpson H. Mobile retinal screening in Britain. Diabet Med 1998;15:344-7. https://doi.org/10.1002/(SICI)1096-9136(199804)15:4<344::AID-DIA588>3.0.CO;2-O.
- NCD Risk Factor Collaboration . Worldwide trends in diabetes since 1980: a pooled analysis of 752 population-based studies with 4.4 million participants. Lancet 2016;387:1513-30.
- Younis N, Broadbent DM, Vora JP, Harding SP. Incidence of sight-threatening retinopathy in type 2 diabetes in a systematic screening programme. Lancet 2003;361:195-200.
- Echouffo-Tcheugui JB, Ali MK, Roglic G, Hayward RA, Narayan KM. Screening intervals for diabetic retinopathy and incidence of visual loss: a systematic review. Diabet Med 2013;30:1272-92. https://doi.org/10.1111/dme.12274.
- Taylor-Phillips S, Mistry H, Leslie R, Todkill D, Tsertsvadze A, Connock M, et al. Extending the diabetic retinopathy screening interval beyond 1 year: systematic review. Br J Ophthalmol 2016;100:105-14. https://doi.org/10.1136/bjophthalmol-2014-305938.
- Looker HC, Nyangoma SO, Cromie DT, Olson JA, Leese GP, Philip S, et al. Predicted impact of extending the screening interval for diabetic retinopathy: the Scottish Diabetic Retinopathy Screening programme. Diabetologia 2013;56:1716-25.
- Agardh E, Tababat-Khani P. Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care 2011;34:1318-9. https://doi.org/10.2337/dc10-2308.
- Grauslund J, Andersen N, Andresen J, Flesner P, Haamann P, Heegaard S, et al. Evidence-based Danish guidelines for screening of diabetic retinopathy. Acta Ophthalmol 2018;96:763-9. https://doi.org/10.1111/aos.13936.
- Chalk D, Pitt M, Vaidya B, Stein K. Can the retinal screening interval be safely increased to 2 years for type 2 diabetic patients without retinopathy?. Diabetes Care 2012;35:1663-8.
- Aspelund T, Þórisdóttir O, Ólafsdottir E, Gudmundsdottir A, Einarsdóttir AB, Mehlsen J, et al. Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia 2011;54:2525-32.
- Lund SH, Aspelund T, Kirby P, Russell G, Einarsson S, Palsson O, et al. Individualised risk assessment for diabetic retinopathy and optimisation of screening intervals: a scientific approach to reducing health-care costs. Br J Ophthalmol 2016;100:683-7. https://doi.org/10.1136/bjophthalmol-2015-307341.
- National Health Executive . Backlog of 150,000 Cervical Screenings Revealed As All Major Health Screenings Failing to Hit Targets 2019. www.nationalhealthexecutive.com/News/backlog-of-150000-cervical-screenings-revealed-as-all-major-health-screenings-failing-to-hit-targets-/220549 (accessed 9 May 2019).
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of diabetic retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol 1994;112:1217-28. https://doi.org/10.1001/archopht.1994.01090210105023.
- Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156-63. https://doi.org/10.1007/s001250051594.
- Wareham NJ, O’Rahilly S. The changing classification and diagnosis of diabetes. Br Med J 1998;317:359-60.
- World Health Organization (WHO) . Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus 2011.
- Rahimi K, Emdin CA, MacMahon S. The epidemiology of blood pressure and its worldwide management. Circ Res 2015;116:925-36. https://doi.org/10.1161/CIRCRESAHA.116.304723.
- Eleuteri A, Fisher AC, Broadbent DM, García-Fiñana M, Cheyne CP, Wang A, et al. Individualised variable-interval risk-based screening for sight-threatening diabetic retinopathy: the Liverpool Risk Calculation Engine. Diabetologia 2017;60:2174-82. https://doi.org/10.1007/s00125-017-4386-0.
- Broadbent DM, Sampson CJ, Wang A, Howard L, Williams AE, Howlin SU, et al. Individualised screening for diabetic retinopathy: the ISDR study-rationale, design and methodology for a randomised controlled trial comparing annual and individualised risk-based variable-interval screening. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-025788.
- Broadbent DM, Wang A, Cheyne CP, James MJ, Lathe J, Stratton IM, et al. Safety and cost-effectiveness of individualised screening for diabetic retinopathy: the ISDR open-label, equivalence randomised controlled trial. Diabetologia 2021;64:56-69.
- Byrne P, Thetford C, Gabbay M, Clarke P, Doncaster E, Harding SP. ISDR Study Group . Personalising screening of sight-threatening diabetic retinopathy – qualitative evidence to inform effective implementation. BMC Public Health 2020;20. https://doi.org/10.1186/s12889-020-08974-1.
- Cheyne CP, Burgess PI, Broadbent DM, García-Fiñana M, Stratton IM, Criddle T, et al. Incidence of sight-threatening diabetic retinopathy in an established urban screening programme: An 11-year cohort study. Diabet Med 2021;38. https://doi.org/10.1111/dme.14583.
- INVOLVE . INVOLVE: Briefing Notes for Researchers 2012. www.invo.org.uk/wp-content/uploads/2014/11/9938_INVOLVE_Briefing_Notes_WEB.pdf (accessed 26 August 2019).
- techopedia.com . Structured Systems Analysis and Design Method (SSADM) n.d. www.techopedia.com/definition/3983/structured-systems-analysis-and-design-method-ssadm (accessed 5 November 2019).
- What Is? Data Point n.d. https://whatis.techtarget.com/definition/data-point (accessed 427 September 2019).
- Papoulis A. Probability, Random Variables, and Stochastic Processes. New York: McGraw-Hill; 1991.
- Stratton IM, Aldington SJ, Taylor DJ, Adler AI, Scanlon PH. A simple risk stratification for time to development of sight-threatening diabetic retinopathy. Diabetes Care 2013;36:580-5. https://doi.org/10.2337/dc12-0625.
- Austin PC, van Klaveren D, Vergouwe Y, Nieboer D, Lee DS, Steyerberg EW. Geographic and temporal validity of prediction models: different approaches were useful to examine model performance. J Clin Epidemiol 2016;79:76-85.
- van der Heijden AA, Walraven I, van ‘t Riet E, Aspelund T, Lund SH, Elders P, et al. Validation of a model to estimate personalised screening frequency to monitor diabetic retinopathy. Diabetologia 2014;57:1332-8. https://doi.org/10.1007/s00125-014-3246-4.
- van der Heijden AA, Nijpels G, Badloe F, Lovejoy HL, Peelen LM, Feenstra TL, et al. Prediction models for development of retinopathy in people with type 2 diabetes: systematic review and external validation in a Dutch primary care setting. Diabetologia 2020;63:1110-9. https://doi.org/10.1007/s00125-020-05134-3.
- Porta M, Maurino M, Severini S, Lamarmora E, Trento M, Sitia E, et al. Clinical characteristics influence screening intervals for diabetic retinopathy. Diabetologia 2013;56:2147-52. https://doi.org/10.1007/s00125-013-2989-7.
- Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012;98:683-90. https://doi.org/10.1136/heartjnl-2011-301246.
- Moons KG, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012;98:691-8. https://doi.org/10.1136/heartjnl-2011-301247.
- Klonoff DC. Personalised medicine for diabetes. J Diabetes Sci Technol 2008;2:335-41.
- Grey JA. The shift to personalised and population medicine. Lancet 2013;382:200-1.
- Olafsdóttir E, Stefánsson E. Biennial eye screening in patients with diabetes without retinopathy: 10-year experience. Br J Ophthalmol 2007;91:1599-601.
- Basu S, Sussman JB, Berkowitz SA, Hayward RA, Bertoni AG, Correa A, et al. Validation of risk equations for complications of type 2 diabetes (RECODe) using individual participant data from diverse longitudinal cohorts in the US. Diabetes Care 2018;41:586-95.
- Leese GP, Stratton IM, Land M, Bachmann MO, Jones C, Scanlon P, et al. Progression of diabetes retinal status within community screening programs and potential implications for screening intervals. Diabetes Care 2015;38:488-94. https://doi.org/10.2337/dc14-1778.
- Scanlon PH, Aldington SJ, Leal J, Luengo-Fernandez R, Oke J, Sivaprasad S, et al. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19740.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems. Health Utilities Index. PharmacoEconomics 1995;7:490-502. https://doi.org/10.2165/00019053-199507060-00004.
- Hernandez-Alava M, Pudney S. eq5dmap: a command for mapping between EQ-5D-3L and EQ-5D-5L. Stata J 2018;18:395-41.
- Dolan P. Modelling valuations for EuroQol health states. Med Care 1997;35:1095-108.
- Furlong W, Feeny D, Torrance GW, Goldsmith CH, DePauw S, Zhu Z, et al. Multiplicative Multiattribute Utility Function for the Health Utilities Index Mark 3 (HUI3) System: A Technical Report 1998.
- Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018;37:2252-66. https://doi.org/10.1002/sim.7654.
- Rubin DB. Multiple imputation after 18 + years. J Am Stat Assoc 1996;91:473-89.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/article/pmg9/chapter/Foreword (accessed 23 January 2019).
- Public Health England . Report of the Independent Review of Adult Screening Programmes in England n.d. www.england.nhs.uk/wp-content/uploads/2019/02/report-of-the-independent-review-of-adult-screening-programme-in-england.pdf (accessed 28 January 2020).
- Flick U. An Introduction to Qualitative Research. London: Sage; 2018.
- Galletta A. Mastering the Semi-structured Interview and Beyond. New York, NY: NYU Press; 2013.
- Coffey A, Atkinson P. Making Sense of Qualitative Data: Complementary Research Strategies. London: Sage; 2006.
- Silverman D. Interpreting Qualitative Data. London: Sage; 2011.
- Duffy SW, Tabar L, Olsen AH, Vitak B, Allgood PC, Chen TH, et al. Absolute numbers of lives saved and overdiagnosis in breast cancer screening, from a randomised trial and from the Breast Screening Programme in England. J Med Screen 2010;17:25-30. https://doi.org/10.1258/jms.2009.009094.
- Hipwell AE, Sturt J, Lindenmeyer A, Stratton I, Gadsby R, O’Hare P, et al. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005498.
- Moss SE, Klein R, Klein BE. Factors associated with having eye examinations in persons with diabetes. Arch Fam Med 1995;4:529-34. https://doi.org/10.1001/archfami.4.6.529.
- Grey RH, Blades C, Jobson T. Screening clinic non-attendance and the risk of sight threatening retinopathy. Eur J Ophthalmol 2009;19.
- Zoega GM, Gunnarsdóttir T, Björnsdóttir S, Hreietharsson AB, Viggósson G, Stefánsson E. Screening compliance and visual outcome in diabetes. Acta Ophthalmol Scand 2005;83:687-90.
- Applebee E. The Barriers and Enables That Affect Access to Primary and Secondary Eye Care Services – Bradford Site Report. A Report to RNIB by Shared 2012. www.rnib.org.uk/knowledge-and-research-hub/research-reports/prevention-sightloss/access-eye-care (accessed 5 March 2019).
- Hurrell DD, Donohue S. The Barriers and Enablers That Affect Access to Primary and Secondary Eye Care Services – Glasgow Site Report 2012.
- Sachdeva A, Stratton IM, Unwin J, Moreton R, Scanlon PH. Diabetic retinopathy screening; study to determine risk factors for non-attendance. Diabetes Prim Care 2012;14:308-16.
- Strutton R, Du Chemin A, Stratton IM, Forster AS. System-level and patient-level explanations for non-attendance at diabetic retinopathy screening in Sutton and Merton (London, UK): a qualitative analysis of a service evaluation. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010952.
- Lian JX, Gangwani RA, McGhee SM, Chan CK, Lam CL, Wong DS. Primary Health-care Group . Systematic screening for diabetic retinopathy (DR) in Hong Kong: prevalence of DR and visual impairment among diabetic population. Br J Ophthalmol 2016;100:151-5. https://doi.org/10.1136/bjophthalmol-2015-307382.
- World Health Organization (WHO) . Diabetic Retinopathy Screening: A Short Guide 2020. https://apps.who.int/iris/bitstream/handle/10665/336660/9789289055321-eng.pdf (accessed 21 January 2021).
Appendix 1 ISDR Study Group
Catey Bunce, School of Population Health and Environmental Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, UK
Helen Cooper, Alder Hey Children’s NHS Foundation Trust, Liverpool, UK
John Collins, ISDR patient expert group, Liverpool, UK
Ticiana Criddle, Liverpool Diabetic Eye Screening Programme, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK
Darsy Darssan, Clinical Trials Research Centre and Department of Biostatistics, University of Liverpool, Liverpool, UK
Emily Doncaster, ISDR patient expert group, Liverpool, UK
Paula Grey, Liverpool Primary Care Trust, Liverpool UK
Christopher Grierson, St. Pauls Eye Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK
Lola Howard, Clinical Trials Research Centre and Department of Biostatistics, University of Liverpool, Liverpool, UK
Susan Howlin, Clinical Trials Research Centre and Department of Biostatistics, University of Liverpool, Liverpool, UK
Lisa Jones, Liverpool Clinical Commissioning Group, Liverpool, UK
John Kelly, ISDR patient expert group, Liverpool, UK
Vineeth Kumar, Arrowe Park Hospital, Wirral, UK
Peter Lees, ISDR patient expert group, Liverpool, UK
Sandra Lees, ISDR patient expert group, Liverpool, UK
Nathalie Massat, Centre for Cancer Prevention, Wolfson Institute of Preventive Medicine, Queen Mary University of London, London, UK
Andy Ovens, Clinical Trials Research Centre and Department of Biostatistics, University of Liverpool, Liverpool, UK
Stephanie Perrett, Liverpool Diabetic Eye Screening Programme, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK
Chris Rogers, University of Bristol, Bristol, UK
Alison Rowlands, Arrowe Park Hospital, Wirral, UK
Kate Silvera, Clinical Trials Research Centre and Department of Biostatistics, University of Liverpool, Liverpool, UK
Gideon Smith, NHS Tameside & Glossop, Ashton-under-Lyne, UK
David Szmyt, St. Pauls Eye Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK
Julia West, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK
Abigail Williams, Clinical Trials Research Centre, University of Liverpool, Liverpool, UK
Lilian Williams, ISDR patient expert group, Liverpool, UK
Naveed Younis, Wythenshawe Hospital, Manchester, UK
List of abbreviations
- ADA
- American Diabetes Association
- AICc
- corrected Akaike’s information criterion
- ARVO
- Association for Research in Vision and Ophthalmology
- BI
- business intelligence
- BMJ
- British Medical Journal
- BP
- blood pressure
- CI
- chief investigator
- CRF
- case record form
- CTRC
- clinical trials research centre
- DR
- diabetic retinopathy
- DW
- data warehouse
- EASD
- European Association for the Study of Diabetes
- EASDec
- European Association for the Study of Diabetes eye complications study group
- EMIS
- Egton Medical Information Systems
- EQ-5D-5L
- EuroQol five dimension five level instrument
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HCP
- healthcare professional
- HDL
- high-density lipoprotein
- HES
- hospital eye service
- HUI3
- Health Utilities Index Mark 3
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- IP
- intellectual property
- ISDR
- individualised screening for diabetic retinopathy
- ITT
- intention-to-treat
- LCCG
- Liverpool Clinical Commissioning Group
- LDESP
- Liverpool Diabetes Eye Screening Programme
- logMAR
- logarithm of the minimum angle of resolution
- NDESP
- National Diabetic Eye Screening Programme
- NIHR
- National Institute for Health Research
- PENS
- patient electronic notes system
- PGfAR
- Programme Grant for Applied Research
- PP
- per-protocol
- PPI
- patient and public involvement
- PWD
- people with diabetes
- QALY
- quality-adjusted life year
- RCE
- risk calculation engine
- RCT
- randomised controlled trial
- RLBUHT
- Royal Liverpool and Broadgreen University Hospital Trust
- SQL
- structured query language
- STDR
- sight-threatening diabetic retinopathy
- STM
- sight-threatening maculopathy
- STR
- sight-threatening retinopathy
- VA
- visual acuity
- VI
- visual impairment
- WHO
- World Health Organization
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/HRFA3155).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
