Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1049. The contractual start date was in August 2007. The final report began editorial review in September 2018 and was accepted for publication in September 2019. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Fiona Gaughran reports support from Janssen Pharmaceutica (Johnson & Johnson, Beerse, Belgium), personal fees from Sunovion Pharmaceuticals Inc. (Marlborough, MA, USA), H. Lundbeck A/S (Copenhagen, Denmark), F. Hoffman-La Roche Ltd (Basel, Switzerland) and Otsuka Pharmaceutical Co., Ltd (Tokyo, Japan) outside the submitted work; and has a family member with professional links to Eli Lilly and Company (Indianapolis, IN, USA) and to GlaxoSmithKline plc (London, UK), including shares. She is in part supported by the Maudsley Charity and the National Institute for Health Research (NIHR) Applied Research Collaboration South London at King’s College Hospital NHS Foundation Trust. Khalida Ismail has been paid honorarium by Eli Lilly and Company, Janssen Pharmaceutica, Sanofi S.A. (Paris, France) and Novo Nordisk A/S (Bagsværd, Denmark) for educational lectures. David Hopkins reports personal fees from Sunovion Pharmaceuticals Inc., Eli Lilly and Company, Novo Nordisk A/S, AstraZeneca plc (Cambridge, UK), F. Hoffman-La Roche Ltd, Medtronic plc (Dublin, Ireland), Fractyl, Inc (Lexington MA, USA) and Sanofi, outside the submitted work. Robin M Murray has received honoraria for lectures from Lundbeck, Otsuka, Janssen and Sunovian. Marta Di Forti has received honoraria for lectures from Janssen and Sunovian.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Gaughran et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
SYNOPSIS
Background
The physical health of people with severe mental illness (SMI) is very poor overall and the rates of premature death in this population are markedly increased, mainly attributable to natural causes,1 similar to those in the ageing general population. Mortality rates from cardiovascular disease (CVD) in SMI are nearly threefold higher than in the general population. 2 The World Health Organization’s (WHO’s) definition of a ‘healthy life’ makes it clear that this encompasses physical, mental and social health, and, indeed, at the WHO World Mental Health Day in 2014, ‘living a healthy life with schizophrenia’ was highlighted (reproduced with permission from WHO3). Therefore, we should treat the physical health of people with psychosis alongside their mental health. The WHO’s Comprehensive Mental Health Action Plan,4 adopted in 2013 by the World Health Assembly,4 advises the development of policy to improve the physical and mental health of people with SMI with a particular focus on improving access to good-quality physical health-care services.
Although intrinsic risk factors such as age, familial traits and ethnicity may increase vulnerability to physical illness, people with SMI are also more likely to have lifestyles that increase the risk of preventable physical disease, such as smoking, obesity, poor diet and lack of exercise; in addition, they are more likely to live in poverty. 5–8 Although the wider determinants of health are challenging to change in a research setting, the above knowledge brings with it the hope that lifestyle choices could be modified, leading to better outcomes.
But which outcome to choose? There is evidence that the mortality rate is highest in people with psychosis who are not on antipsychotic medication;9,10 however, we also know that starting antipsychotic medication accelerates weight gain and diabetes risk. 11,12 Although it is important to minimise the side-effect burden of medication, to do so by inadequately treating the psychosis may well be counterproductive in terms of both short-term quality of life (QoL) and longer-term life expectancy. Taking a cardiovascular measure as one’s only outcome measure runs the risk of neglecting the whole person, just as much as concentrating only on a mental health measure. It seems that QoL may be the most important measure for assessing overall health, in addition to reporting cardiovascular risk factors and measures of psychopathology and substance use. We also need to gather evidence of the costs of the cardiovascular risk states and of the cost-effectiveness of interventions to address them.
Cardiovascular risk factors include weight, blood pressure (BP) and markers of lipid and glucose metabolism, as well as smoking rates. One way of conceptualising cardiovascular risk is the metabolic syndrome (MetS). MetS is a constellation of CVD risk factors (abdominal obesity, insulin resistance/glucose intolerance, hypertension and dyslipidaemia)13 that predict the chance of a person developing or dying from a cardiac event. Each risk factor alone almost doubles the risk of a CVD event occurring in the next decade. MetS is highly prevalent among people with psychosis,14 at a rate of 52%;15 in addition, the prevalence of obesity and dyslipidaemia is 50%, with diabetes at 14% and hypertension at 49.5%. 16–18 A rapid emergence of components of MetS is seen on treatment initiation. 19 Despite this, screening for cardiovascular risk factors remains poor. 20–24
Likewise, the US CATIE17 study found high rates of diabetes, obesity, hypertension and high cholesterol in people with SMI; this has since been confirmed in meta-analysis. 14 The emergence of cardiovascular risk and full-blown MetS can be prevented and reversed. At the time of applying for the programme, the CATIE study17 had suggested that smoking cessation, nutrition counselling and supervised exercise programmes could help to reduce cardiovascular mortality in this population. 17 However, practice changes had yet to consolidate. Despite government initiatives to improve better physical health monitoring for UK patients with SMI, a survey of 209 general practitioners (GPs) at that time revealed that only half provided lifestyle advice and fewer than one-third referred patients with SMI to specialist physical health support programmes. 25 Since then, Commissioning for Quality and Innovation (CQUIN) incentivisation targets have greatly improved physical health screening and intervening in mental health inpatient settings, but this has been more challenging to achieve in community settings. The recent National Audit of Schizophrenia26 audited whether or not clinicians assess five factors [smoking, elevated body mass index (BMI), blood glucose control, blood lipids and BP] annually and found that in only 33% of people with schizophrenia were all of these factors monitored.
To add to the burden, there is a clear excess of diabetes in schizophrenia. A recent meta-analysis27 from our wider group showed a prevalence of type 2 diabetes mellitus among 438,245 people with SMI of 11.3% [95% confidence interval (CI) 10.0% to 12.6%]. Notably, the corresponding figure in antipsychotic-naive patients was only 2.9% (95% CI 1.7% to 4.8%). We also reported27 that people who have experienced multiple episodes of psychosis (n = 133,470) were more likely in our comparative meta-analysis to have type 2 diabetes mellitus than were matched controls [n = 5,622,664, relative risk (RR) 1.85, 95% CI 1.45 to 2.37; p < 0.001] and women were more at risk than men (RR 1.43, 95% CI 1.20 to 1.69; p < 0.001).
The management of diabetes and CVD risk is particularly difficult in people with SMI, and carries massive projected long- and short-term cost implications. Worryingly, patients with diabetes and SMI are treated less aggressively for CVD risk than those without mental disorders. 28 Rates of non-treatment for conditions such as diabetes, hypertension and dyslipidaemia in psychosis are high, with the discrepancy most marked in non-white women. 29 The effectiveness of cardiovascular risk management in people with SMI who have diabetes is also poor. 30 These patients are less likely to be prescribed cholesterol-lowering statin medications, angiotensin-converting enzyme inhibitors and angiotensin receptor blocking agents than diabetes patients without SMI. 28 A recent study of Danish population registers found significantly fewer prescriptions for cardiovascular medications for patients with schizophrenia or bipolar disorder than for the general population. When the authors examined the records of people without previous myocardial infarction or cerebrovascular disease, those with schizophrenia or bipolar disorder had up to 6- and 15-fold increased mortality from all causes or unnatural causes, respectively, compared with the general population; this was most pronounced among those without CVD treatment (16-fold increase). This concurs with previous work suggesting that the treatment of CVD risk factors is relatively neglected in these patients, although the excess of unnatural deaths in the untreated group also suggests that the link between CVD treatment and mortality may be confounded by illness severity. 31
Unfortunately, evidence on optimal management of people with SMI and comorbid diabetes is lacking and there are many obstacles in practice. Poor motivation makes it difficult for people to make the lifestyle changes needed to avoid diabetic complications and episodes of acute illness can interrupt diabetic control. This cohort will put significant strain on NHS resources unless effective, evidence-based measures are developed to improve outcomes.
Those from black and ethnic minority (BME) communities face particular inequalities concerning both their physical and their mental health, and large-scale epidemiological work is under way in our wider group to specifically investigate CVD in BME groups with SMI. 32 BME groups have higher rates of not only SMI33 but also diabetes. 34 Our study was largely based at the South London and Maudsley (SLaM) NHS Trust, where BME groups are over-represented among those attending services. The improving physical health and reducing substance use in psychosis (IMPaCT) study population contains a high proportion of BME participants to ensure that the results will be relevant to the health needs of a multiethnic society.
As well as the poor diet and sedendary lifestyles common in so much of our society, and especially in those with psychosis, health outcomes are compromised still further by concomitant substance use. Tobacco-smoking, a major prognostic factor for health, is decreasing in the general population,35 but remains common among people using mental health services, adding to their longer-term cardiovascular risk. Alcohol has a deleterious effect on both physical and mental health and complicates the management of psychotic conditions. Use of illicit substances also introduces complexity and unpredictability into the lives of people with psychosis.
Cannabis use
Over the last two decades, there has been a justified interest in comorbid substance use by those with a psychotic illness because of its high prevalence and significant impact on clinical and social problems, as well as the heavy burden laid on the health services. According to the Health and Social Care Information Centre’s 2014 report36 on statistics in drug misuse in England, primary diagnoses of a drug-related mental health and behavioural disorder increased by 8.5% from 2012/13 to 2013/14. 36 The size of the problem becomes even more important when those with a psychotic illness use substances significantly more than the general population.
The interaction between a psychotic illness and substance use is complex and can have major detrimental effects on the course of the illness, including a patient’s risk of experiencing violence and being hospitalised, their ability to comply with treatment and even possibly the aetiology. 37,38 Such patients may also be at particular risk of deterioration in their mental health. A recent systematic meta-analysis of the outcomes associated with psychosis and comorbid substance use showed that current substance users with psychosis may have more severe positive symptoms than patients who have never used substances. 39
Aetiological links between cannabis and psychosis
Interestingly, cannabis was used 150 years ago to treat ‘insanity’, while also being recognised as increasing the risk of ‘madness’, especially among young persons at the Maudsley Hospital. 40 Apart from some experimental studies examining the effects of cannabis during the 1960s, no interest was shown in cannabis studies. In 1987, Andréasson et al. 41 published a longitudinal study involving > 50,000 Swedish conscripts and reported that those who used cannabis by the age of 18 years were twice as likely to develop schizophrenia as non-users. The risk increased to sixfold in heavy cannabis users. This study was criticised and ignored until the early 2000s, when Zammit et al. 42 reanalysed the Swedish data, coming up with similar findings. The subject of cannabis and psychosis once more attracted empirical interest and the subsequent publications indicated a causal link. 43–45 Other studies, including systematic reviews, concluded that using cannabis increased the risk of developing a psychotic illness by two to six times, particularly in those with a predisposition to the condition and in a dose-dependent manner. 37,46,47
Furthermore, experimental studies carried out on healthy volunteers have shown that cannabis can induce transient psychosis in some individuals, but not all. 48–50 At the same time, biochemical, imaging and genetic studies were carried out to examine the links between cannabis and psychosis, which have all added to the existing knowledge. For instance, we now know that the main psychoactive ingredient of cannabis, delta-9-tetrahydrocannabinol (THC), binds to CB1 receptors within the endocannabinoid system, and the very same brain regions are also implicated in psychoses, particularly in schizophrenia. 51–53
Cannabis use in first episode of psychosis patients, prevalence and increased risks
Cannabis is the most frequently used illicit substance in the world, with nearly half of 15- to 34-year-olds reported to be lifetime users in the USA, Australia and Canada. 54 According to the 2015 European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) report,55 lifetime use of cannabis among the same age group in the UK is reported to be 29.9%, whereas the last 12-month use was 11.2%. Cannabis is also the most commonly used substance after tobacco, according to epidemiological surveys of people with psychosis. 56–59 Perhaps unsurprisingly, we found that the use of substances other than cannabis among first episode of psychosis (FEP) patients was minimal. Given the changing attitudes towards cannabis use, reflected by its ever-increasing medicinal use or decriminalisation in some countries, its more widespread use by new users is expected. 60 It is, therefore, important that the health risks of cannabis use are sufficiently researched.
At the time of the original National Institute for Health Research (NIHR) Programme Grants for Applied Research application, we wrote that a higher percentage of people with psychosis used cannabis and other drugs than the general population61 and regular cannabis use increased the risk of schizophrenia two- to threefold. 44 We also highlighted that ongoing cannabis use in those with psychosis led to increased relapse and hospitalisation, lack of compliance with treatment and longer illness. 39
Indeed, a considerable number of studies since have further emphasised the impact of cannabis use on people with psychosis. First, the higher prevalence of cannabis use among people with FEP has been validated in numerous studies. 62,63 A recent meta-analysis64 on the prevalence of cannabis use in people with FEP pooled the data from 37 studies and concluded that cannabis use is highly prevalent in this group. The same study64 also reported that the pooled estimate from 10 studies showed that regular use of cannabis begins 6.3 years before the onset of psychosis. In fact, the same research group has previously shown in another meta-analysis65 that the age at the onset of psychosis among cannabis users was nearly 3 years earlier.
Additionally, prospective epidemiological studies and meta-analyses of such studies have concluded that using cannabis can indeed increase the risk of developing a psychotic illness and that there is a dose-related risk. 37,66–68 It should be noted that the main psychoactive compound of cannabis (i.e. THC) is nearly three times as concentrated in the cannabis used today as that used in earlier decades.
In a study in South East London, using ‘skunk-like’ cannabis was shown to triple the risk of psychosis and, when the results were extrapolated across the population, the use of high-potency cannabis was found to be associated with a 24% increase in new cases of psychosis. 67 It was also shown that both the frequency of use and the strength of the variety of cannabis used were associated with the increased risk. In another recent study with FEP patients carried out across Europe and Brazil in 11 sites, daily cannabis use with high-potency varieties increased the odds of psychotic disorder nearly five times in comparison with never users, and population attributable fractions calculations indicated that, if such high-THC cannabis had not been available, 12.2% of cases of FEP could have been prevented across these sites. 69
Why do first episode of psychosis patients use cannabis?
The questions of why people with FEP use cannabis significantly more than others and why some continue to use it despite its deleterious effects remain important and have been explored using various theories. One of the first proposed theories was ‘self-medication’ hypothesis or ‘reverse causality’, suggesting that psychiatric symptoms are alleviated by cannabis or that cannabis is used to counter the side effects of psychotropic medication. However, this theory has since been disputed by several studies. 46,47,70 The recent meta-analysis64 finding of a 6.3-year gap between the initiation of cannabis use and the onset of FEP is further evidence against this proposal.
However, some studies support the self-medication or reverse causality theory, as it is possible that some early dysphoric symptoms or anxiety make it more likely that some people will use cannabis. One such study carried out a comprehensive examination of experiences of cannabis use and reasons for using cannabis among people with psychosis and found that the primary reason given was not related to positive or negative symptoms or side effects of medication,71 as only 10% reported using it for these reasons. Instead, the most frequently cited motivations were to reduce boredom, to improve socialisation and to alleviate some symptoms, such as agitation (47%) and difficulty sleeping (43%). Participants also reported that cannabis reduced feelings of depression.
The increased use of cannabis among people with psychosis has also been attributed to certain personality traits/disorders. There is mounting evidence that high-scoring schizotypal people who use cannabis are more likely to experience psychotic symptoms. 72–74 Another study has shown that conduct disorder symptoms are significantly associated with use of cannabis, particularly if it is used by the age of 14 years, among people with FEP; conduct disorder symptoms have been suggested to independently increase the possibility of cannabis use, which then increases the risk of psychosis. 75
Finally, genetic studies have gained particular significance because most cannabis users do not develop psychosis; therefore, genetic factors need to be examined to find out what determines sensitivity to the psychosis-inducing effects of cannabis. 76 One of the earlier theories was genetic confounding: in other words, that cannabis use and psychosis risk shared genetic origins. However, more recently gene–environment interaction studies have gained more recognition; these refer to an environmental factor, such as the use of cannabis, being influenced by genetic factors. For instance, when there was a familial risk, taken as the measure of genetic loading, the non-psychotic siblings of people with psychosis were found to be significantly more vulnerable to mental health disturbances than the control participants. 77 Further support was provided by another study that showed increased sensitivity to the psychotogenic effects of cannabis to be associated with familial risk of psychosis. 78
In addition to familial risk factors, there have been a number of candidate gene studies. The first of these was carried out by Caspi et al.,79 who focused on a functional polymorphism of the valine allele of catechol-O-amyltransferase (COMT) gene in the New Zealand birth cohort study in examining the interaction between cannabis use and risk of psychosis. Later, a number of studies concentrated on this gene, leading to mixed results when further replication and validation studies were carried out. 80,81 However, one promising gene candidate is thought to be alpha serine/threonine-protein kinase (AKT1) as a variant of this gene displayed a twofold increased risk of a psychotic disorder after use of cannabis. 82 This finding was later independently replicated. 83 However, further studies need to be carried out because of the small evidence base.
In conclusion, the available evidence suggests that the interaction between the increased use of cannabis and the development of a psychotic illness is complex and may have both environmental and genetic causes.
What are the physical health effects of cannabis on first episode of psychosis patients?
Although the mental health risks of cannabis have been well studied, there is paucity of research on its possible health-care risks not only in psychiatric but also in general populations.
The question of why cannabis could affect physical health is linked to the knowledge that the endocannabinoid system where THC binds exists not only in the brain but also in most other organs, such as the heart, lungs, liver, kidneys, thyroid, bones and reproductive organs, as well as the immune system. 84–86
Although knowledge about the multiple functions of this widespread neurotransmitter system is evolving, the available evidence implies that the endocannabinoid system has varied roles ranging from regulating the metabolism, circulatory system, reproduction, sleep and pain to ocular pressure. 87–90 It would therefore be possible to assume that the interference of THC, by interrupting the normal functioning of this widespread system, may lead to physical health problems. Only during the last few years has there been an interest in studying the physical health-care effects of cannabis among non-psychiatric populations.
One possible risk is to the functioning of the respiratory system as a result of the fact that cannabis is usually inhaled. The use of both cannabis and tobacco is common,91 which also creates confounding effect problems in research. Findings so far show that there may be a dose-related risk, in that low levels of cannabis use (three to five joints per month) may increase respiratory function, whereas higher levels of use have the opposite effect. 59,92 So far, a conclusive association has not been established between cannabis use and lung cancer. 93
The endocannabinoid system plays an important role in the regulation of food intake and reduces energy expenditure. 94,95 Based on this knowledge, some cannabis compounds are used to stimulate appetite and encourage weight gain in some patients with HIV (human immunodeficiency virus), AIDS (acquired immunodeficiency syndrome) or cancer. Interestingly, however, some recent studies have shown that in the general population cannabis use is associated with lower body mass than in non-users. 96 This paradoxical finding may be due to various factors. For instance, there may be a difference between long-term use, as found in general population studies, and short-term use, as found in appetite stimulation studies. The other explanation would be the possibility of poly-drug use or food and drugs competing for the same reward sites in the brain. It is also suggested that cannabis may act as a regulatory compound, increasing weight in those with low weight but not in those who are already overweight. 97 Interestingly, however, most recent studies carried out not only on the general population98–100 but also on people with SMI101 report that cannabis use is associated with lower BMI, smaller waist circumference, lower diastolic BP and more severe psychotic symptoms.
Unfortunately, no established and practical treatment programmes target both improving physical health and reducing substance use among people with psychosis, and attending parallel treatment programmes is often impractical. Treatment programmes, such as MIDAS, for people with both psychosis and substance misuse appeared promising,102,103 but these are lengthy, complex and expensive.
The huge implications of these health gaps in terms of morbidity, mortality, QoL and projected future cost make addressing physical health in SMI a national priority. The National Service Framework and the National Institute for Health and Care Excellence (NICE) have emphasised the importance of good physical health in people with SMI and encourage primary and secondary care services to collaborate to improve physical outcomes in this population. 104,105 In 2004, the UK government emphased physical health promotion as a means of reducing CVD burden and endorsed the aims of programmes addressing physical health in people with SMI. 106 None of these programmes, however, was adequately tested in the UK to ensure that it could accurately quantify the problem in those with SMI, could identify those most at risk, could reduce physical health risk factors and was reliable and reproducible enough to be disseminated across the NHS.
This is a matter of equity. A decade ago, the UK Disability Rights Commission investigation stated overtly that people with mental health problems were more likely than others to experience major illness and develop serious health conditions earlier to and die earlier as a result. 107 Yet this group were less likely to receive some important treatments/health checks and faced real barriers to accessing services. The report called for a clear shift in approach to eliminate inequitable treatment and to target high-risk groups. The idea was that this would prevent the extra costs of serious ill health being passed on to other parts of the NHS and enable people with SMI to be healthier and participate fully in society. 107 Similar concerns were reflected in the Choosing Health: Making Healthy Choices Easier White Paper. 106 Since then, this topic has gathered momentum; there have been a number of high-profile policy documents, including No Health Without Mental Health,108 the Department of Health and Social Care report Closing The Gap: Priorities For Essential Change in Mental Health,109 Whole-person Care: from Rhetoric to Reality,110 the BMA report Recognising the Importance of Physical Health in Mental Health and Intellectual Disability,111 the Annual Report of the Chief Medical Officer 2013112 and the London Health Commission report Better Health for London. 113 Indeed, the London Health Commission’s ambition to reduce the gap in life expectancy between adults living with SMI and the rest of the population has led to the creation of a working group, ‘Stolen Years’, to work across health systems to make this a reality.
The challenge remains the evidence base. Most studies of health promotion in SMI to date have focused on selected groups of patients and achieved only modest outcomes. None has addressed comorbid substance misuse. Importantly, none has evaluated the cost-effectiveness of a combined lifestyle intervention across a service, work needed to inform health commissioning. The next logical step was to develop a more practical alternative to separate programmes, and so we set out to develop and evaluate the benefits of a well-defined, standardised, person-centred intervention, using modules, as appropriate, to target both lifestyle and substance use to maximise physical and mental health. We also undertook an evaluation of the costs of the cardiovascular burden and the cost-effectiveness of the intervention to reduce it.
Healthy living interventions should be an integral part of the care provided to people with SMI. This must become a priority for both primary and secondary care services, although it will require a cultural shift in terms of how care is provided to those with SMI, with a greater emphasis on a holistic approach. There is an urgent need for (1) a reliable way of identifying those most at risk, (2) guidelines on how best to screen for the emergence of MetS and (3) an effective intervention to reduce modifiable cardiovascular risk factors in this group.
Aims and objectives
The aim of IMPaCT was to use the available evidence to develop culturally appropriate, innovative and cost-effective programmes to achieve better physical and mental health in people with SMI by improving lifestyle choices and decreasing illicit drug use.
Programme plan (Figure 1)
Work package 1: PUMP
FIGURE 1.
Research design pathway.
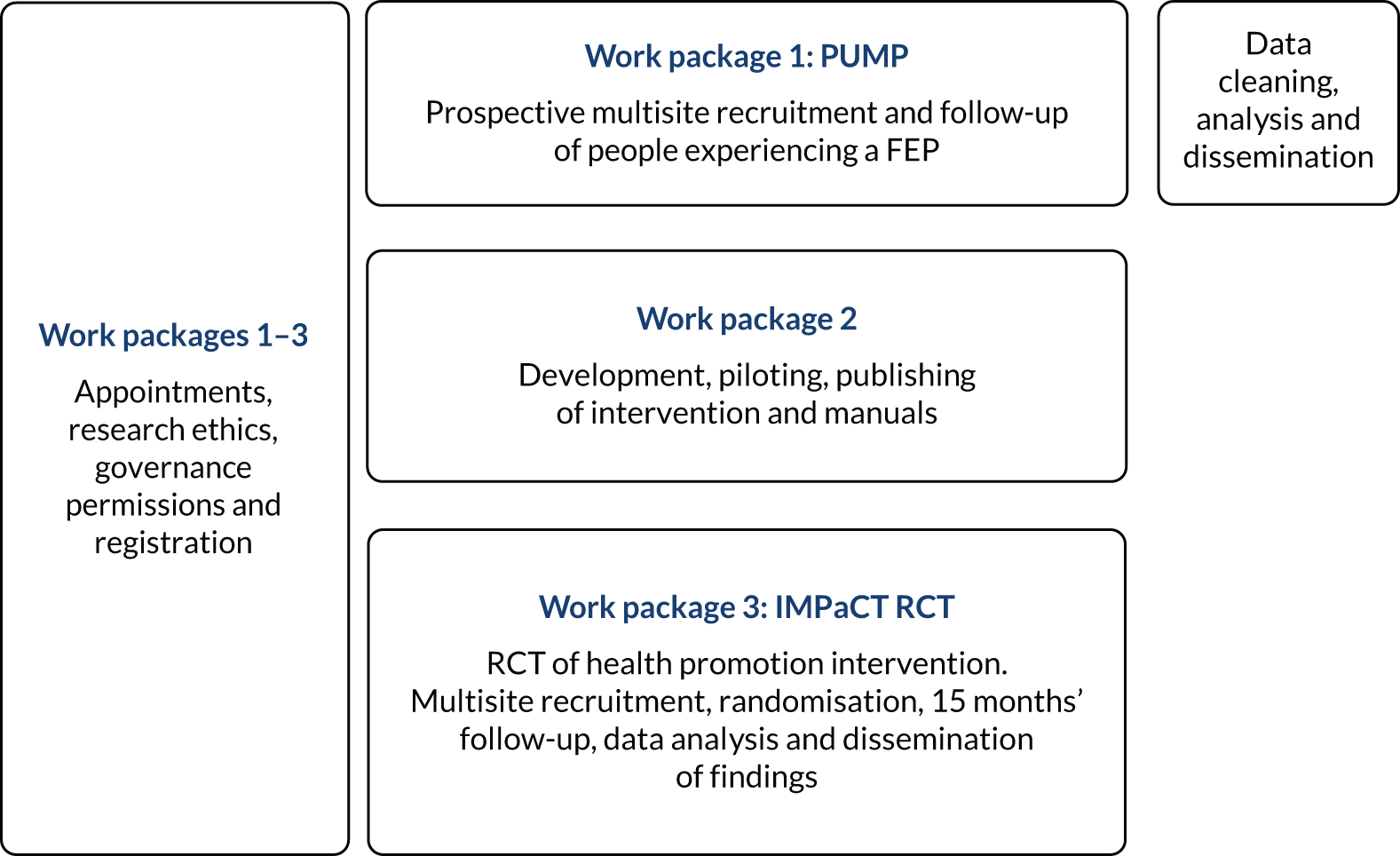
The Physical health and substance Use Measures in first episode of Psychosis (PUMP) study was a prospective observational cohort of patients presenting with their FEP. The participants were recruited from the four boroughs of the SLaM NHS Foundation Trust, two boroughs of the Oxleas NHS Foundation Trust and two boroughs of the Sussex Partnership NHS Foundation Trust. Patients from both inpatient and outpatient services were followed up for 12 months to observe health and service utilisation markers over time and to test the association between lifestyle (diet, exercise and substance use) and antipsychotic medication factors and the emergence of components of MetS.
In the first part of the IMPaCT programme, we also wanted to examine the extent of cannabis use among our group of FEP patients, as well as finding out the mental and physical health-care effects of this use at baseline and at 12 months’ follow-up, compared with non-users.
Work package 2: development of the health promotion intervention (IMPaCT therapy)
The available evidence was used to inform the development, validation and manualisation of a health promotion intervention (HPI) tailored to the needs of the individual, addressing a number of lifestyle choices, including substance use. The purpose of the development of the manual was to create a culturally appropriate, innovative and effective programme for use in routine care systems to achieve better physical and mental health in people with SMI by improving lifestyle choices and decreasing substance use. This intensive HPI (IMPaCT therapy) was designed to cover physical health, mental health and substance use using motivational interviewing (MI) and cognitive–behavioural therapy (CBT) and was designed to be sufficiently pragmatic to be deliverable within the NHS.
Work package 3: IMPaCT randomised controlled trial
A randomised controlled trial (RCT) assessing whether or not the addition of an intensive HPI (IMPaCT therapy) to usual mental health care delivered by care co-ordinators is more effective and more cost-effective than usual mental health care in improving metabolic outcomes and reducing substance use among people with SMI on completion of the intervention at 12 months and 15 months after baseline.
Work package 1: physical health and substance use in first episode of psychosis (PUMP)
Background
People with SMI experience a significant reduction in life expectancy of approximately 10 years. 1 There are a number of possible contributors to this premature death rate; for example, at the service level, risk factors such as unequal access to care followed by substandard treatment influence outcomes. At the individual level, a greater prevalence of CVD, and unhealthy lifestyle choices or substance use, may also increase risk. Some of these factors may interact; for example, lifestyle choices may have an impact on cardiometabolic status, thus reducing life expectancy. Although there is evidence surrounding the impact of unhealthy lifestyle choices, little is known about whether lifestyle choice or substance use predicts changes in cardiometabolic status. Investigating these factors among patients presenting with their FEP thus offers the opportunity to map the course and impact of lifestyle choices on cardiometabolic risk in people with psychosis. This section reports a longitudinal study that sought to observe health behaviours and the progression of cardiometabolic disease among patients with FEP and to investigate links between these factors over time.
Setting and method
A total of 293 patients with first-onset psychosis were recruited from inpatient units and community mental health teams (CMHTs) across eight boroughs in the south of England. Participants meeting the following inclusion criteria were consented to the study: (1) aged between 16 and 65 years, (2) experiencing a FEP [according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10),114 codes F20–29 and F30–33], (3) proficient in English with no requirement for an interpreter and (4) no diagnosis of severe learning difficulties.
A total of 118 participants completed the 3-month follow-up and 127 participants completed the 12-month follow-up.
Patients who were pregnant or had an organic cause of their psychosis, those with a major medical illness or neurological disease, and those with history of previous contact with health (GP or psychiatric) services for the presence of psychosis were excluded from participating in the study.
Measures
A range of measures were collected over three time points (baseline and 3 and 12 months), including the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impression Severity scale, Global Assessment of Functioning (GAF), Young Mania Rating Scale, Calgary Depression Scale and operational criteria checklist for psychotic and affective illness (OPCRIT) diagnostic scale, as well as anthropometric measures and blood sampling for cardiometabolic and inflammatory markers and measures of nicotine, alcohol and substance use, including the Nicotine Dependence questionnaire (Fagerström scale), the Alcohol Use Disorders Identification Test (AUDIT) and a modified Cannabis Experience Questionnaire (CEQ-4), along with the International Physical Activity Questionnaire (IPAQ). 72,115–121 All measures in the study were administered face to face by a trained researcher, except the OPCRIT, which was rated by a clinician based on the clinical notes.
Follow-up contacts
Clinical, sociodemographic and substance use data were also collected using the Follow-up Psychiatric and Personal History Schedule (FUPPHS). This records information about the patient’s mental state, general behaviour, substance use, events and personal history over a defined period using information obtained from patients, informants, case notes and other records. 122 Researchers rating the Psychiatric and Personal History Schedule (PPHS) at follow-up achieved an intraclass correlation of 0.90 on all PPHS items when duplicate ratings were compared. Information on medication adherence and remission during the 1-year follow-up was also extracted from the PPHS, where poor adherence is defined as 1 = lapses of ≥ 3 days more than once, and 2 = not taking any prescribed medication. Remission was operationally defined as the absence of positive, negative or disorganised symptoms for at least 30 days. In our study122 examining the effect of substance use and medication adherence on outcomes, the 1-year follow-up period was taken as the date of first contact with mental health services of the SLaM NHS Foundation Trust for psychosis to the date exactly 1 year later using the clinical records held on the SLaM electronic Patient Journey System (ePJS), with all of the PPHS measures completed by a researcher retrospectively using the electronic mental health records system (i.e. the SLaM electronic Patient Journey System).
Linear regression models were used to examine for the associations between baseline diet, alcohol use, sedentary behaviour and prescriptions of olanzapine with cardiometabolic risk both at baseline and at 12 months’ follow-up. Baseline cross-sectional associations between continuous scores for lifestyle and cardiometabolic factors were investigated using unadjusted and adjusted linear regression models, as was the relationship between baseline lifestyle choices and change in cardiometabolic factors at 12 months, investigating associations between use of dibenzodiazepine medications between baseline and 12 months (yes/no) and cardiometabolic factors in the same way. We used the mi impute chained command in Stata® version 15.1 (StataCorp LP, College Station, TX, USA) with 50 imputed data sets for each MI model to account for different patterns of missingness. To account for multiple testing, a stricter alpha of 0.01 was prespecified as the significance level. The assumptions of the regression analyses of constant variance and normal distribution were assessed with a visual inspection of residual plots.
We used univariate and multivariate analyses to determine the relationship between measures of cardiometabolic risk at baseline and admission, and home treatment costs in the following year (see Appendix 1).
Patient and public involvement
Service user and carer experts were part of the core planning and management throughout the project (see Programme patient and public involvement).
Results
Metabolic characteristics and changes over 12 months
There were significant levels of cardiometabolic risk at first presentation, with rates of obesity, raised levels of glycated haemoglobin (HbA1c), levels of C-reactive protein (CRP) and low levels of high-density lipoprotein (HDL) cholesterol increasing over the following 12 months. In the overall group, central obesity rates at baseline were higher in women (62.7%; n = 37/59; waist circumference ≥ 80 cm) than in men (35.3%; n = 36/102; waist circumference ≥ 94 cm; χ2 = 11.34; p = 0.001). The mean waist circumference in white men increased by 4.9 cm over the year, such that by 12 months white men had a waist measurement 7.3 cm greater than their counterparts of other ethnicities, despite having comparable measures at baseline. The average waist circumference of white women at baseline was 9.6 cm smaller than of women of other ethnicities.
The mean total cholesterol levels among those participants of white ethnicity (4.9 mmol/l) and those of other ethnicities (4.7 mmol/l) were comparable at baseline, but white patients had higher levels at 12 months (5.1 vs. 4.6 mmol/l; p = 0.04) (see Gaughran et al. 123).
Baseline antipsychotic use and associated metabolic impact
The median duration of treatment with antipsychotics at baseline was 21 days (interquartile range 9–55.5 days) (mean 33.7 days, SD 50.3 days), with 95% of the sample prescribed second-generation antipsychotics. The most frequently prescribed antipsychotic was olanzapine.
Patients who had been prescribed antipsychotic medication for ≥ 2 weeks had an average total cholesterol higher (0.5 mmol/l, 95% CI 0.1 to 0.8 mmol/l; p = 0.007) than those who had been prescribed antipsychotics for less than a fortnight. Participants medicated prior to baseline had a higher baseline average waist circumference (7.7 cm, 95% CI 2.0 to 13.4 cm; p = 0.009) than antipsychotic-naive patients.
Lifestyle choices and baseline cardiometabolic outcomes and changes over time
There was no association between any of the baseline or 12-month cardiometabolic outcomes and Dietary Instrument for Nutrition Education (DINE) fat scores, sedentary behaviour, AUDIT scores and pre-baseline olanzapine. The lack of association remained when adjusting for potential confounders (age, gender, ethnicity or pre-baseline days on medication) and in sensitivity analyses using all non-missing data from the full data set.
Subgroup analyses were run comparing those participants who took olanzapine between baseline and 12 months and those who did not. No differences were found between the two groups in the magnitude of associations between changes in cardiometabolic outcomes at 12 months’ follow-up and baseline lifestyle factors, nor were there any such associations within either of the two groups. No associations emerged from sensitivity analyses using the full data set (both unadjusted and adjusted for the same potential confounders) (see Gaughran et al. 123).
Obesity at baseline was associated with higher subsequent admission costs, low HDL cholesterol at baseline was associated with lower subsequent admission costs and higher HDL cholesterol at baseline was associated with a greater subsequent quality-adjusted life-year (QALY) gain (see Appendix 1).
Cannabis use in PUMP first episode of psychosis patients
Of 206 patients with FEP, 102 used cannabis at baseline (49.5%), with 23 out of 183 (12.6%) reporting current use of other recreational drugs. Current users at baseline were more likely to be men [59.4%, degrees of freedom (df) 1; p < 0.05] and most were younger, belonging to the 18–33 years age group (df 1; p < 0.05), than the non-users. They were also more likely to be single (df 4; p < 0.05). In terms of ethnicity, occupation, having close confidants, ICD-10 diagnoses from OPCRIT, drug naivety and seeing GP or health specialists, there were no significant differences between cannabis users and non-users. Cannabis users also differed from non-users in terms of using cigarettes (df 1; p < 0.0001) and other stimulants (df 3; p < 0.01). At 12 months’ follow-up, we had data on 105 patients and the number of current users dropped to 40 (38.1%). The corresponding figure at 12 months for those reporting use of other substances was 12 out of 102 (11.8%). From first data collection to 12 months, 12 (11.3%) participants started using cannabis, whereas 16 people (15.5%) stopped.
Effects of cannabis on outcomes in first episode of psychosis patients
In terms of physical health outcomes, non-cannabis users had higher cholesterol levels at 12 months’ follow-up than non-users (df 1; p <.045). Female users were thinner at baseline (df 1; p < 0.036) but male users were heavier at 12 months (df 1; p < 0.003) than non-users. Female users had smaller waistlines at baseline (df 1; p < 0.008) than female non-users and male users had larger waistlines at 12 months (df 1; p < 0.01) than male non-users.
There has been much speculation about why people with a diagnosis of psychosis may use cannabis; some suggest a self-medication hypothesis, whereas other evidence suggests that it is used to alleviate dysphoria. Kolliakou et al. 124 conducted a study nested within the PUMP study, which investigated the reasons for use reported by those participants who used cannabis and how these varied over time. Participants rated their motives at baseline (n = 69), at 3 months (n = 29) and at 12 months (n = 36) on the Reasons for Use Scale, which has five subscales (enhancement, social motive, coping with unpleasant affect, conformity and acceptance, and relief of positive symptoms and side effects). At all of the time points, ‘enhancement’ received most endorsement, followed by ‘coping with unpleasant affect’ and ‘social motive’. ‘Conformity and acceptance’ followed closely. The least endorsed motive was ‘relief of positive symptoms and side effects’. When participants continued to use cannabis at 3 months and 12 months, they endorsed these reasons less strongly than at baseline. We found little support for the theory that people were using cannabis for self-medication or to alleviate dysphoria. Rather, in keeping with the general population, the most common reason that people with their FEP gave for their use of cannabis was ‘enhancement’.
Colizzi et al. 122 demonstrated that substance use and poor medication adherence contribute to poor outcomes in the year following the FEP. Colizzi et al.’s work detailed the relative contributions of medication adherence and substance use to outcome over the year after the FEP. We had data on 205 patients on use of tobacco, alcohol, cannabis and stimulants at psychosis onset and at 1 year, along with data on medication adherence and symptom remission. Rates of overall substance use were high both before (37–65%) and after psychosis onset (45–66%). Nicotine dependence was reported in 53.2% of patients at baseline, whereas 40.5% reported premorbid problem drinking, 65.4% reported premorbid cannabis use and 36.6% reported premorbid stimulant use. A total of 44% of patients had poor medication adherence and 55% failed to reach remission from psychosis.
Both nicotine dependence and cannabis use after the onset of psychosis predicted poor medication adherence and non-remission significantly. Poor medication adherence occurred in 43.9% of patients at some point in the first year and also significantly predicted remission during the 1-year follow-up, with patients with poor medication adherence having a sixfold increased likelihood of psychosis non-remission when compared with those with good adherence. This association between medication adherence and remission was still significant when substance use in the 1-year follow-up period was added into this model. Sobel tests for mediation showed that medication adherence was a significant mediator of the relationship between nicotine dependence and remission (z = 2.02; p = 0.04) and of that between cannabis use and remission (z = 2.12; p = 0.03). 122
Discussion
Cardiovascular disease risk emerges very soon after presentation, consistent with recent US work. 125 These findings confirm previous literature that demonstrates people with FEP lead unhealthy lifestyles marked by poor diet, lack of physical activity and high rates of nicotine, alcohol and drug use. Our sample also presented with impaired metabolic status on first contact with services as indicated by glycose dysregulation and hypertension. They also had metabolic features consistent with the emergence of type 2 diabetes mellitus. Throughout the study, there was significant weight gain in both men and women. Interestingly, despite national stop-smoking campaigns that were running alongside our study, we did not find a significant reduction in smoking rates. Indeed, a high proportion of the sample smoked at baseline (76.8%), which had changed little at 12 months.
Strengths of this study include the diverse population recruited from both inpatient and community settings in the south of England. A limitation is that there were missing data at both time points. Although booklets were produced to ensure the consistent order of the administration of questionnaires, this order was not always followed for pragmatic reasons. However, we have no reason to believe that data were not missing at random. Another limitation is the level of follow-up, with only 125 of the 293 eligible participants completing 12 months’ follow-up and 140 dropping out after baseline. The largest group of non-completers were those who declined an individual assessment point, although a smaller number withdrew from the study. The reasons for this are likely to be manifold, and the main recruitment site, south London, has a very mobile population. There was recompense for time, but no overt financial incentivisation. Where we had missing measures of key variables, these were imputed using a commonly used statistical approach and sensitivity analyses was undertaken to demonstrate the robustness of the imputation process. We have no reason to believe that attrition was not random, with no differences between demographic or baseline clinical attributes between completers and non-completers, although they may have differed on other factors.
Despite concerns regarding the use of olanzapine as a first-line antipsychotic, over half of participants had been prescribed olanzapine by the time of the baseline assessment. Not all of these participants continued to be prescribed olanzapine over the 12 months of the study, and, although we controlled for days prescribed olanzapine at baseline, we would not have been able to identify whether or not cardiometabolic risk reduced once olanzapine had been discontinued.
These high and rising levels of CVD risk are worrying outcomes and it is hugely clinically important that both nicotine dependence and cannabis use after the onset of psychosis predicted both poor medication adherence and non-remission significantly. Poor medication adherence mediated the effects of substance use on non-remission, demonstrating that medication adherence is on the causal pathway between cannabis use and nicotine dependence and a non-remission outcome. 122
Planned future work
We plan to look further at the relationship between inflammatory markers and cardiovascular risk in this population. Preliminary work by our wider team126 has suggested that greater increases in inflammatory markers soon after first presentation with psychosis are associated with a greater risk of short-term metabolic abnormalities, in particular dyslipidaemia, which are independent of gains in weight. We are, therefore, interested in whether or not early evidence of inflammation may predict those most likely to develop cardiometabolic disease.
Mapping health promotion programmes
As part of work package 1, we also mapped local health promotion programmes (HPPs). Below is a summary of work published in O’Brien et al. 127
Background
The increased risk of physical health comorbidities combined with low socioeconomic status means that patients with SMI require higher levels of physical health care and in particular greater attention to cardiovascular risk factors. However, in practice, reports indicate that medical treatment rates for those factors are low among those with SMI and, if SMI patients receive medications, the choice of medication may be outdated. 29,31 The physical health of people with mental health problems has been set as a priority for improvement by the Department of Health and Social Care (DHSC). 128 The NHS and local authorities have been encouraged to develop HPPs, with particular emphasis on HPPs addressing modifiable health issues pertinent to SMI patients: diet, exercise and substance use. However, prior to our work, it was not clear what the distribution of such HPPs was or whether or not there were barriers to access for people with psychosis.
Aims and hypotheses
Because a systematic exploration of the provision of HPP available for SMI patients was lacking, the study objective was to assess the magnitude and type of HPPs that were available for or inclusive of people with SMI in four socially deprived boroughs in south London, UK.
Methods
The study looked at the four boroughs served by the SLaM NHS Foundation Trust: Southwark, Lewisham, Croydon and Lambeth. These boroughs (especially Lambeth, Lewisham and Southwark) have lower health-related sociodemographics than London and the national average.
The study design was a cross-sectional mapping of HPPs available to people with SMI across these four boroughs of London in 2008–9. These programmes covered a range of health promotion areas: sexual health, carer support, diabetes and nutrition, drug and alcohol use, physical activity and smoking cessation. The programmes were based in drop-in centres, outpatient services and day centres.
Mapping was stratified by local authority, the voluntary sector and the NHS. It was performed using standard scoping methodologies using internet searches of websites of the local authority, mental health charities, mental health trusts and primary care trusts. After this, confirmation was sought of the HPP identified, and further details (the costs, the access methods and the extent to which people with SMI were included in the programme) were requested from the programme convener or administrator.
Health sociodemographic details for each borough were obtained from GP registers.
The service user and carer experts in the IMPaCT Programme Management Group had input on the development of this work.
Results
The prevalence of people with SMI was 1.1% in Croydon and Southwark, 1.2% in Lewisham and 1.5% in Lambeth. A total of 145 HPPs available to people with SMI were identified: 38 in Lewisham (1 : 82 people with SMI), 50 in Southwark (1 : 60 people with SMI), 27 in Lambeth (1 : 149 people with SMI) and 30 in Croydon (1 : 121 people with SMI).
As for availability, 61 HPPs [15 in Lewisham (40%), 14 in Southwark (28%), 14 in Lambeth (52%) and 18 in Croydon (60%)] were targeted specifically at people with SMI and the rest were inclusive of people with SMI. No publicly funded local authority leisure centre across the four boroughs stated in response to direct questioning that they had the facility to perform risk assessments or had staff trained to run HPPs inclusive of this population.
A small proportion of HPPs stipulated eligibility criteria for people with SMI, which, if not satisfied, would preclude. These access to the service the programme’s included the patient being required concordance with prescribed medications or to attend with their care co-ordinator.
Looking at the focus of HPPs across boroughs, physical activity was most common (51 HPPs) and sexual health was least common (10 HPPs), with only two specifically provided for or inclusive of SMI patients.
Discussion
The number of HPPs accessible to people with SMI varied across the four boroughs; for example, Lambeth had the largest number of people with SMI but provided the fewest accessible HPPs (rate of 1 : 149), in contrast to Southwark, which had fewer people with SMI but more HPPs available to them (1 : 60).
Stipulating inclusion and exclusion criteria may impede access to HPPs and cause unnecessary stress and distress, as well as adding to the stigma surrounding SMI. This is likely to act as a deterrent to this population, who are in great need of health promotion services.
The mapping was time-consuming, which may account for the difficulties that clinicians and service users themselves experience when trying to identify available HPPs. These details should ideally be readily accessible from a single source to patients and staff alike. This action would have three foreseeable benefits. First, it would facilitate the integration of physical and mental health care. Second, it would be easier for health-care professionals to recommend certain programmes to service users. Third, it would support people with SMI and their carers who may have difficulty in navigating information from multiple resources to access HPPs themselves. However, such a resource would require to be updated regularly, which has cost implications.
Conclusion
This was the first attempt to map HPPs available for patients with SMI across these South London boroughs and demonstrated that the distribution of these was inequitable. This may not reflect demand, as some HPPs set exclusion criteria and provide inadequate support to staff. Accurate and readily available information on local HPPs would benefit the population with SMI who are in dire need of such services. 127
Work package 2: the IMPaCT therapy – development of a comprehensive, integrative manualised psychological intervention for physical health and substance use in severe mental illness
Introduction
People with psychosis experience significant and broad-ranging physical health problems, including CVD and diabetes, leading to lower QoL and a 10- to 25-year reduction in life expectancy compared with the general population. 129–131 Those with psychosis in the adult age group are 5.7 times more likely than the general population to be treated for diabetes. 132 Contributing factors include medication treatment regimes, lifestyle choices, inequities in health service access and substance use. 133–135 Despite the size of the problem, effective intervention models, tailored to the person’s needs, are few and are not used in clinical settings in a standardised manner. Studies examining the topic reinforce recommendations that people with co-occurring disorders require more intensive and integrated interventions. 136
Psychological interventions for physical health in psychosis have largely focused on a single aspect of health, such as smoking,137 diet and weight gain,138 exercise139–141 and substance use. 142,143 Yet clinical presentation and research evidence suggests that these health risks often co-exist, are inter-related and contribute exponentially to morbidity and mortality. In fact, a recently published 10-year follow-up of the ÆSOP frst-episode cohort study144 demonstrates the significant impact of substance use on both natural and unnatural causes of mortality. The authors conclude that early intervention and dual-diagnosis services may play a key role in achieving more rapid remission and carer involvement in addressing substance use problems to reduce excess mortality in psychosis. Indeed, there is a long overdue need for the development of comprehensive, person-centred intervention models tailored to individual needs to improve health and QoL and reduce the economic burden on services and society. 145
Psychological interventions have traditionally been denied to people with psychosis not only because of a lack of resources but also because of a belief that people with psychosis cannot benefit from these because of their illness, lack of insight and cognitive deficits.
The theory behind the cognitive model of psychopathology can be defined as how thoughts and perceptions or inner interpretations of specific situations can influence one’s behaviour and emotions and can even lead to physiological reactions. Cognitive–behavioural therapy is a well-researched and validated intervention model that aims to modify a person’s negative thoughts and interpretations. The evidence on the effect of CBT for psychosis has accumulated over only the last 15 years. 146 Meta-analyses studying the efficacy of CBT on positive symptoms of schizophrenia,147–149 as well as secondary outcome measures such as functioning levels, mood and social anxiety,148,150 have revealed medium beneficial effects. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study151 examined the decision-making capacity of patients with schizophrenia and found that negative symptoms had very little effect and positive symptoms had no effect on this. These studies have emphasised the need to develop and adapt for psychological interventions in patients with psychosis.
As a result of this renewed interest in psychological interventions in psychosis, models of psychological intervention used in non-psychotic populations, such as those with substance misuse, have been proposed for a psychosis population. One such model is MI, which is a ‘directive, client-centred counselling style for eliciting behaviour change by helping people to explore and resolve ambivalence’ in relation to health behaviour. 152 MI aims to build a person’s motivation for change by increasing their awareness of the impact of problem behaviour, while maintaining an empathic approach to encourage responsibility as part of an open, non-judgemental therapist–client interaction and with realistic expectations about what can be achieved. There is a robust evidence base for this type of intervention in alcohol, substance use and smoking, and emerging evidence also in diet, exercise and diabetes, in non-psychotic populations. 153,154
In a large population of university students who had been using substances, a 3-month follow-up revealed that a single session of MI had reduced their use of cigarettes, alcohol and cannabis, mainly through moderation rather than complete cessation. 155 However, it is unlikely that a single MI session would be sufficient for patients with psychosis. Indeed, a study156 involving patients who use substances suggests that modifications be made to standard MI, particularly taking into account possible cognitive deficits by simplifying open-ended questions, refining reflective thinking skills, heightening emphasis on affirmations and integrating psychiatric issues into personalised feedback.
A number of studies have examined the effectiveness of integrative models combining different interventions, particularly comorbid psychosis and substance use studies that have used a combination of MI and cognitive approaches, as well as family therapy,102,103,137,142 and most have found modest improvements, particularly in the medium term. This integrated approach is consistent with theoretical and aetiological models that link substance use with psychosis onset and maintenance,157 psychosis onset with deterioration in self-beliefs, motivation and insight,158–160 and psychosis treatments with physical health complications. However, interventions in psychosis that focus on only physical health or substance use have suffered from high attrition rates in addition to poor outcomes. 161 It is recommended that future research would need to take into account methodological standardisation, longitudinal perspectives, interventions of subgroups and stages, sequenced interventions and the changing realities of treatment systems. 162 Integrating of MI with CBT approaches provides more opportunities to address the complex mental and physical health presentations that are common in psychosis. 162
Previous group-based interventions for substance use in psychosis and brief CBT interventions that focus on aspects of mental health such as worry, sleep and self-esteem have comprised 6–8 sessions. 142,163 Furthermore, previous ‘integrated’ therapies for specific behaviours, such as substance use, in the context of psychosis extended interventions to 9 months. 161 Pre-clinical modelling demonstrates that HPIs are particularly crucial for people with psychosis and should be integrated within CMHTs, ideally in a cost-effective manner.
Although there has been some interest in promoting better physical health among patients with psychosis over the last few decades, only a small number of trials have sound methodologies and a limited range of treatment models tested, as shown by a recent systematic review on the topic. 164 Different groups have tried different approaches and have concentrated on pharmacological interventions and/or behavioural interventions. 164 For instance, some studies have targeted weight gain by promoting healthy diets and physical exercises. In another systematic meta-analysis, individual or group interventions, CBT and nutritional counselling were found to be effective in reducing antipsychotic-induced weight gain. 165 More recently, however, a number of studies have taken a holistic approach by targeting patients with psychosis and their multiple health behaviours in terms of their lifestyles. For instance, Baker et al. 166 in their RCT compared a 90-minute face-to-face ‘healthy lifestyles’ intervention for smoking and cardiovascular risk behaviours with a telephone-based intervention, in addition to providing both groups with nicotine replacement therapy. Both types of interventions resulted in improvement in 12 months, with no differences between conditions.
As part of the IMPaCT programme, we thought that by integrating the two well-established intervention methods, namely CBT for psychosis and MI, we would theoretically be focusing on increasing personal awareness of the impact of one’s thought processes on emotions and behaviour while applying MI principles, which would positively enhance the person’s autonomy in making choices. We thought that this approach would be beneficial to patients with psychosis, as well as meeting their individual needs, whether or not the person had physical health problems and/or used substances. To validate this intervention, we had to prepare manuals that described the actual intervention, as well as providing it in a modular format, so that the therapist could use the required modules with a particular person who needed to make certain lifestyle changes towards an improved healthier life. We adapted the actual intervention, which included a combination of MI and CBT, similar to the one used by James et al.,142 although applied in individual face-to-face contact.
Aims
The study was designed to develop, validate and manualise IMPaCT therapy, a HPI tailored to the needs of the individual, addressing unhealthy lifestyle factors including substance use and inter-related comorbid cognitive and mental health difficulties in psychosis. The aim of the intervention was to modify lifestyle factors that have a negative impact on health in SMI (i.e. substance use, cigarette smoking, weight gain, poor nutrition and lack of exercise). IMPaCT therapy followed a staged model of behavioural therapies development. 167–169
Method
Design
Initially, a consultation among experts in therapeutic interventions for mental and physical health, substance use and diabetes generated the key features and focus of the intervention. After this, IMPaCT therapy was developed through three stages of design and analysis: (1) therapy and training development, and manual writing; (2) piloting, evaluation and refining the training package with clinicians; and (3) a Delphi process to reach consensus on the therapy model and manual through initial consultation followed by two rounds of follow-up questionnaire feedback. The feedback was from each of three expert groups: (1) therapists, (2) clinician providers within CMHTs and (3) psychosis service users. The emphasis was on an iterative process leading to a therapy model and manual that would be well grounded both in theory and in feedback from experts in behavioural change therapies, clinician providers within CMHTs and psychosis service users.
The intervention was based on the transtheoretical behaviour change model. 170 The training and reference guide introduced this model of behaviour change and its link with the intervention approach. The intervention manual was then framed around this model. The manual was divided into separate sections for the distinct stages of behaviour change: (1) precontemplation, (2) contemplation, (3) preparation and contingency planning, (4) action and (5) maintenance and relapse prevention. At the start of each section was an overview that defined the stage and how to identify whether or not a client was in this stage. This was followed in each section by aims, objectives and between 3 and 12 specifically behaviour change ‘interventions’ that were consistent with the behaviour change taxonomy171,172 and applicable to that stage.
Participants
Participants in the phase 2 training development were eight staff selected from a range of in- and outpatient settings and backgrounds, working in the area of psychosis within the SLaM NHS Foundation Trust, but not eligible to participate in the subsequent IMPaCT RCT. Participants in the phase 3 Delphi consensus and manual development comprised three expert groups of (1) therapist/researchers, (2) clinicians and (3) service users, each of whom took part in two iterative review and feedback sessions. Therapist/researcher experts were recruited from the local and national community and were involved in the development, implementation or research evaluation of CBT and MI approaches in psychosis. Clinicians were staff who took part in the phase 3 training, and service users were people who were receiving a service from the relevant clinicians at the time and had provided informed consent to participate in the study.
Pilot training
All participants completed a 5-day pilot training, which comprised 10 sessions: (1) introduction to IMPaCT and the manual/basic CBT skills, (2) introduction to MI, (3) practical MI, (4) intermediate MI skills in psychosis I, (5) intermediate MI skills in psychosis II, (6) physical health awareness and monitoring, (7) physical health awareness and diabetes monitoring, (8) substance use awareness, (9) group work skills and (10) healthy living groups. The training was delivered by members of the research team (KG, SS, ZA and Manyara Mushore), as well as an expert in healthy living groups and an expert in MI for psychosis. All participants in the training self-rated of their knowledge and skills before and after the training, and their confidence in therapy delivery after training.
Delphi consultation
The Delphi process comprised three iterative stages of consultation revolving around an adapted questionnaire that incorporated feedback from each preceding stage, consistent with recommended Delphi methodology. The Delphi consultation on the model and manuals was collected in semistructured e-mail questionnaires for therapist/researchers and clinicians, and in face-to-face interviews with service users, in accordance with the algorithm presented by Jones and Hunter173 (see Appendix 2, Figure 2). The Delphi process allowed us to access the acceptability and face validity of the model, manuals and training package, using expert consensus. The Delphi process was an in-depth consultation requiring the expert clinician and patient consultants to undertake a selected practice intervention session in pairs, using the intervention, reference guide, manual and handbook, and provide iterative feedback. Feedback was obtained in the form of qualitative comments and Likert scale ratings. For each feedback session, each participant read specified sections of the manuals. Therapists/researchers read all sections of all manuals (the reference guide, the manual and the Better Health Handbook), whereas clinicians focused preferentially on the therapy manual and handbook sections. The service users took part in two selected intervention sessions with their trained clinician and reviewed preferentially the service user resource handbook. Questions considered user-friendliness, spirit of MI, integration with CBT, usefulness in routine NHS practice, length and complexity, and whether anything was missing or should be added. Feedback was obtained from numerical ratings on a Likert scale (0–10), where 0 was least positive and 10 was most positive, as well as qualitatively. After each round of feedback, changes were made to the content and structure of the manual before the next round of feedback. In follow-up consultations, the previous individual ratings and group rating were also fed back, and participants were encouraged to revise their ratings towards or away from the group mean to determine consensus within each group. Consensus across the group as a whole was based on threshold ratings for the acceptability and user-friendliness of key elements of the manual on Likert scales of ≥ 7 in the final stage.
Alongside the Delphi process, an informal presentation and consultation were conducted with a carers group for people with SMI. The perspectives gained from this presentation were used in the development of a brief carers’ section to support the therapy.
Results
Initial consultation to form key features and components of the intervention and to consider mechanisms of change
A consultation among experts in the field of MI and psychosocial interventions for physical health, substance use and diabetes and CBT for psychosis (KG, SS, ZA, KI and Gill Todd) yielded a set of broad features and foci of the intervention for consideration in subsequent stages of development. The initial therapy built on an adapted version of the physical health intervention used in the Well-Being Support Programme,140,174 incorporating a substance use intervention based on ‘Managing Mental Health and Drug Use’. 142 Key features of the therapy were that it had to be comprehensive, integrative, flexible and pragmatic, deliverable by mental health clinicians from a range of backgrounds, and able to be readily implemented in NHS settings. The focus of the components incorporated (1) mental health and psychosis, (2) alcohol use, (3) cannabis use, (4) smoking, (5) other drug use, (6) diabetes, (7) exercise and (8) healthy eating. It was initially proposed that, based on previous research, the intervention should be delivered in a group format.
Phase 1: initial therapy training development and manual writing
Extensive review of the Well-Being Support Programme and substance use intervention, as well as of relevant literature about mental health, physical health and substance use interventions incorporating CBT and/or MI in psychosis and non-psychosis populations, led to the development of a preliminary outline of the intervention and of the training package.
Phase 2: piloting, evaluation and refining the training package
The pilot training was delivered to clinicians (n = 8; 50% male) from CMHTs (n = 2), to specialist inpatient (n = 3) and psychiatric intensive care units (n = 3), and to individuals from a range of disciplines [psychiatric nurses (n = 5), occupational therapists (n = 2) and social workers (n = 1)].
The knowledge/skills ratings pre and post training are presented in Appendix 2, Figure 3. The mean self-rated knowledge scores increased from pre to post training on all core areas of training (physical health, substance use, running groups and using MI).
In addition, the mean post-training confidence ratings were all high for core components (physical health 81.3%, substance use 79.4%, running groups 86.3%, MI 83.8%) and the mean ratings of the importance of these aspects of support for psychosis were also all high (physical health, 86.9%; substance use, 86.9%; running groups, 87.5%; MI, 92.5%).
Clinicians rated the core training modules on various dimensions, including training quality, resources, level, value, applicability and impact on confidence, as well as on specific skills learnt. Ratings were on a scale from 1 (poor/strongly disagree) to 5 (excellent/strongly agree). The mean ratings and ranges for physical health, substance use, healthy living groups and MI training were 4.4 (4.0–4.9), 4.7 (4.09–5.0), 4.4 (4.0–5.0) and 4.3 (4.0–4.9), respectively.
Qualitatively, clinicians recommended that the primary mode for intervention delivery be individual sessions because of the difficulty in co-ordinating and engaging service users in groups. They also requested that basic CBT be included in the training programme and the manual. They reported that the physical health training was empowering, unleashing potential, and should be more available and that the substance use training should be longer, mandatory and focused more on individual substances. The training on healthy living groups seemed to fine-tune and enhance existing skills, whereas the MI training challenged preconceived ideas and a ‘nursing’ model, and was well shaped for community populations. It gave a structured approach to the intervention, had good links to theory, and provided an opportunity to try things out, but it should be more focused specifically on MI in mental health as opposed to general health.
Phase 3: the Delphi consultation
A total of five expert therapist/researchers from a range of backgrounds (two psychologists, two psychiatrists and one nurse) took part in two rounds of Delphi consultation on the reference book, the therapy manual and the service user handbook. All manuals were redrafted after each round of the process, based on numerical and qualitative feedback, and re-rated such that each underwent three iterations, involving substantial modification of content, format and language. Owing to time pressures in development, no Delphi rating was obtained for the final version. One expert service user/researcher also provided qualitative feedback in both rounds. [See www.amazon.co.uk/Impact-Reference-Improving-Physical-Substance/dp/095688850X (accessed 6 December 2019) for the final versions of the reference guide, the manual and the Better Health Handbook.]
Among those expert therapist/researchers who completed both rounds of the Delphi process, the mean ratings of the therapy manual for user-friendliness, spirit of MI, integration with CBT, usability in the NHS, length and complexity increased over time from 7.3 to 7.6 out of 10, with a rating of 7.4 out of 10 from four participants for the second rating. Similarly, ratings for the reference book increased over time from 6.8 to 7.1 out of 10, with a rating of 7.4 from four participants for the second rating. Finally, ratings for the handbook increased over time from 7.3 to 7.7 but with a lower second rating from four participants in the second iteration of 6.4. This lower rating was as a result of a low mean score from one expert, who also provided extensive feedback that was incorporated in the final handbook.
Two clinicians (one nurse and one occupational therapist) reviewed the therapy manual quantitatively and qualitatively while using it to deliver a ‘test’ session each with a service user (one male and one female). These service users in turn provided qualitative feedback on their experiences of the session. For the therapist who completed both rounds of the Delphi process, the mean rating for the therapy manual increased from 7.3 to 8.2. This related to its usability for day-to-day standard NHS work, including length, complexity and confidence provided.
The expert therapists/researchers generated a large amount of qualitative feedback. The therapy manual was seen as too long, and all manuals needed more simplified language. Specific core recommendations for change included developing the therapy into modules, including practice examples and case studies, providing greater clarity on how to manage interactions with mental health and issues with medication, having built-in guidelines on training and having supervised practice to support manualised therapy delivery. In terms of therapy content and structure, recommendations were made to include a clear definition of each stage of change, what a client might say or do in this stage, and the sessions associated with this stage; that a structure should be followed for each session to include a session number and why and when to do the session, followed by clear aims and objectives and then session content; and that therapists should be advised that they can dip into the sessions and materials relevant to their client and should not be expected to cover everything. This would allow flexibility and avoid overprescription. Explicit links were recommended to be made between the sessions and linked handbook resources. Greater CBT for Psychosis coverage was requested; some concern was raised about offering too brief a CBT for Psychosis section without a preliminary formulation. Another recommendation was to include the application of CBT to enable goals to be reached at the action stage in MI. The various flow charts were often viewed as rather complex. The general point was made that outside IMPaCT therapy in the NHS there would need to be some form of physical health assessment or other means to lead to a health target.
One therapist/researcher rightly suggested that the relatives session may be too short to reduce expressed emotion at home. The relatives session did not aim to be a therapy. At the suggestion of carers who were presented with the IMPaCT study and asked for comments, the session aimed to address some of the misconceptions about and heightened expectations for change that might be held by relatives following the offer of therapy. The session therefore aimed to present only the basic tenets of behaviour change and the notion that most change occurs at a cognitive level before any behavioural change occurs. It aimed to clarify that developing intrinsic motivation will lead to more long-lasting change, as opposed to a forced change that may be short lived.
Clinicians fed back qualitatively that the theory should be clearly separated from the therapy sessions as a separate reference book. The sessions themselves needed to be simplified and clearer in their aims, and more stand-alone as individual session guides, with less ‘jargon’. There was a need to ensure better cross-referencing between the therapy manual sessions and the handbook resources. The MI section was viewed very positively. Overall, echoing the view of the expert therapists/researchers, the consensus was that the initial therapy manual was too long.
The two service users provided only qualitative feedback over the two time points. They confirmed that they found the focus on health to be beneficial, leading to an increase in knowledge, motivation and ideas for improving health, and one reported a significant change in alcohol use (from heavy drinking to 2-week abstinence) following the session. Materials in the handbook were viewed positively. One expert service user/researcher provided specific and detailed feedback on the handbook. Key points included, again, using simpler language; giving greater explanation of how to use and complete the various resources, and what they are; including colour and pictures to liven up the handbook; and including positive language and hopeful messages about change. Consistent with expert therapists, a suggestion was made that terms such as ‘heavy’ or ‘light’ smoking/substance use needed to be defined; in addition, benchmarks and thresholds, for example for problematic waist circumference, should be provided for mainstream NHS use. In the research therapy, this will be provided as part of the IMPaCT assessment.
Discussion
The production of the training and manuals for the IMPaCT therapy followed multiple iterative phases of piloting, consultation and refinement. The process culminated in a 5-day training programme and a set of three manuals to support training and intervention delivery: the reference guide, the manual and the Better Health Handbook.
The final training retained all of the pilot training sections but extended the substance use training. The MI section was delivered with a greater focus on application for service users with comorbid physical and mental health difficulties. The training included sessions on basic skills in CBT for (1) mental health and (2) achieving goals in physical health during the action stage of behavioural change. Finally, it commenced with a specific session on how to use the reference guide, the manual and the Better Health Handbook as part of a therapeutic process.
The set of three manuals was designed to provide all of the basic information required to deliver IMPaCT therapy within routine NHS practice. The manuals were necessarily lengthy but significant effort was made to ensure that these were concise and used plain language.
The reference guide contained three sections:
-
An introduction to the IMPaCT study and the guiding ethos, including an overview and key information on the primary approaches of MI and CBT.
-
A section comprising the primary interventions. This encompassed an overview of the eight individual module plans for CBT in mental health, alcohol, cannabis, smoking, other drugs, diabetes, exercise and healthy eating; a chapter on working with carers in the IMPaCT approach; and a chapter on running groups. A specific emphasis here was on how to adapt the basic session and group plans for working with different target health issues, how to integrate MI and CBT for these issues, and how these issues might overlap with mental health. Outcome measurement and module-specific resources were introduced.
-
A section on setting up a service. This section contained information about how to run the training and to set up and deliver supervision as part of an IMPaCT therapy service. It also included information on how to map local community health provisions, to which service users could then be directed as part of the intervention. 127
Finally, a set of appendices included guidance on aspects of therapy delivery, such as flow charts to determine the starting module, for transition between modules and for the evaluation of module progress. Outcome measures and psychoeducation materials were also included.
The final manual comprised introductory materials on how to use the manual and the Better Health Handbook, followed by information on getting started and the introductory session. In response to feedback from the Delphi process, 37 individual intervention sessions followed, grouped into:
-
introductory sessions (1–3)
-
supplementary session (4–7) if initial sessions did not lead to a health goal
-
sessions for pre-contemplation (3, 4, 8–12)
-
sessions for contemplation (13–21)
-
sessions for preparation and contingency planning (22–29)
-
sessions for action (30–32)
-
sessions for maintenance and relapse (33–37).
This last section also included an extensive CBT session (36), which was itself broken down into eight subcomponents. Based on feedback from the Delphi process, each section commenced with a definition of the particular stage of change, what the client might say or do in this stage, and key aims and objectives for this stage, with a contents list. Then followed each individual session plan, which commenced with reasons to offer this session, when to offer this session and a guide to session content. The sessions were designed so that therapists could select the relevant sections based on the client’s individual needs and stage of change. Each session included links to appendices that provided example dialogues, derived from real-case examples, to show how to implement the intervention. Each session also provided a reference to all relevant resources in the Better Health Handbook.
Finally, the manual also included group session plans for (1) beginner’s exercise (six sessions), (2) maintaining exercise (six sessions), (3) healthy eating (nine sessions), and (4) social activities (10 sessions).
The Better Health Handbook comprised a set of useful resources that could be photocopied, separated according to stages of change and numbered according to the intervention session. They included diaries, worksheets, physical health summary sheets, questionnaires, and lists of coping strategies and alternative behaviours. The resources were colourful and included images, where appropriate, and could be used to develop a tailored therapy pack for each service user.
The intervention for any particular health issue was the delivery of a module of up to approximately eight sessions, lasting approximately 1 hour. Sessions were designed to be tailored to the individual’s needs, to be positively framed, and to encourage opportunities to replace unhealthy with alternative health and social behaviours. Mental health support was incorporated alongside support for physical health, and modules were designed so that individuals could work flexibly alone or in groups at their own pace and level.
We designed and developed the manualised approach and training package to provide registered mental health nurses and other care co-ordinators in routine clinical care with knowledge and skills so that they would feel more confident monitoring and supporting their clients with their physical health. Resources associated with the development and implementation of the HPI will be measured to estimate its costs and inform service providers. The implementation, fidelity to protocol and efficacy of the intervention was assessed in a large-scale RCT in SMI: the IMPaCT trial (see Work package 3: IMPaCT randomised controlled trial). 175
Work package 3: randomised controlled trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT)
This section provides a summary of work published in Gaughran et al. 175,176 and Heslin et al. 177
Parts of this text have been reproduced from Gaughran et al. 176 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Parts of this text have been reproduced from Heslin et al. 177 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Background
Living with a diagnosis of psychosis is associated with a reduced life expectancy of 10–20 years, largely because of CVD. As detailed in work package 2, we developed a HPI to help people to make healthier choices. We aimed to determine the effect of the HPI on improving health and reducing cardiovascular risk in people with psychosis compared with treatment as usual (TAU). Additionally, given the demands on health services, we planned to examine the costs associated with these patient groups and to evaluate the economic impact of the proposed interventions (see Work package 3: IMPaCT randomised controlled trial, Health economics). Therefore, this trial aimed to determine the clinical effectiveness and cost-effectiveness of a modular HPI in psychosis when compared with the established normal treatment.
Aims and hypothesis
The primary objective was to test the clinical effectiveness and cost-effectiveness (see Work package 3: IMPaCT randomised controlled trial, Health economics) of adding IMPaCT therapy, an intensive HPI designed to improve physical health and reduce substance use, to TAU delivered by care co-ordinators to people with psychosis.
Our primary hypothesis was that the addition of IMPaCT therapy to the normal mental health care delivered by care co-ordinators over 9 months would be more effective than TAU alone in improving patients’ QoL, as measured with the Short Form questionnaire-36 items (SF-36) mental health and physical health subscales178 at 12 months from baseline, and that this advantage would be maintained at a follow-up assessment 15 months from baseline. The 12-month post-randomisation period included an initial 3 months in which the care co-ordinators were trained to provide the HPI over the subsequent 9 months.
Our secondary hypothesis was that the HPI group would see a greater improvement in metabolic outcomes and substance use than the TAU group at both time points; specifically, (1) TAU plus IMPaCT therapy would result in a greater reduction of waist circumference by at least 1 cm at 12 months than TAU alone, (2) TAU plus IMPaCT therapy would be more effective in reducing weight at 12 months compared with TAU alone, (3) TAU plus IMPaCT therapy would result in a 50% reduction in the proportion of people using cannabis compared with TAU alone and (4) TAU plus IMPaCT therapy would be more effective in reducing the symptoms of psychosis than TAU alone.
Methods
A multicentre, two-arm, parallel-cluster RCT was conducted across five mental health UK NHS trusts. Care co-ordinators were randomly assigned to either receiving training, and ongoing supervision, in delivering IMPaCT therapy or delivering TAU to their current patients with psychosis (cluster).
A more detailed explanation of all methods can be found in the published study protocol, available open access;175 the methods are briefly described in the following sections.
Study design and setting
A pragmatic, multicentre, two-arm, parallel-cluster, RCT design was used and the HPI was integrated into routine services across five mental health trusts in South London, Kent, Sussex, Somerset and Staffordshire, including both urban and rural populations. The Consolidated Standards of Reporting Trials (CONSORT) cluster trial extension standards guided the planning and implementation of this study. Ethics approval was obtained from the joint South London and Maudsley and the Institute of Psychiatry NHS Ethics Committee (REC reference number 09/HO80/41). Colleagues with lived experience, including both service users and carers, were involved throughout the research, from contributing to funding applications, to managing steering groups, to coauthoring the papers arising from the work.
Participants
Recruitment took place between 1 March 2010 and 1 July 2014. Eligible participants were aged between 18 and 65 years and had an established diagnosis of a psychotic disorder (ICD-10114 diagnosis F20–29, F31.2 or F31.5). Patients were excluded if they (1) had a primary diagnosis of learning disability, (2) had a pre-existing physical health problem that would independently affect the metabolic measures (as judged by medical investigators), (3) were pregnant or < 6 months post partum or (4) had a life-threatening or terminal medical condition. We did not recruit from FEP services. Recruitment started on 1 March 2010, with first randomisation on 9 August 2010.
Study procedure
There were two waves of participant recruitment. First, all permanently employed community care co-ordinators with a minimum of four psychosis patients on their caseload in participating CMHTs were approached in random order (using a random number generator) and invited to participate. Following informed consent from individual care co-ordinators, patients from their caseload who met the inclusion criteria were identified. These patients were then approached and invited to participate, also in a random order, until either four participants consented or all eligible patients had been approached. Baseline measures were then taken from the consenting patients. After baseline assessments of all consenting patients on a care co-ordinator’s caseload were completed, care co-ordinators were randomised and stratified by borough using randomisation blocks of random sizes to deliver IMPaCT therapy or TAU alone to their own current patients (cluster). Both researchers and the statistician remained blind to treatment allocation for the duration of data collection.
In the treatment arm, the intervention (IMPaCT therapy) was provided by a patient’s usual community care co-ordinator. In the first 3 months following a patient’s randomisation, their community care co-ordinator received a 4-day IMPaCT training course. This covered physical health and substance misuse, MI, CBT techniques for running group sessions, and how to deliver IMPaCT therapy and health promotion, and it was given by trainers in MI and in CBT. An evaluation of this is reported in Appendix 3. Throughout the subsequent 9-month intervention, participating care co-ordinators were offered fortnightly supervision in IMPaCT therapy and all care co-ordinators were offered a 1-hour training session in best practice for physical health awareness to ensure more standardised TAU.
The intervention was rolled out into routine care by holding discussions with management, which confirmed their strong support, and then approaching local team management about the practicalities, and then informing the community teams about the RCT and offering the 1-hour education session as above. Further practicalities were addressed at the 4-day training sessions in the intervention group, including advice on planning group sessions if needed. Day-to-day practical issues that arose were dealt with in the fortnightly supervision sessions or, if these were at the team, borough or trust level, the relevant layer of management was involved.
Outcome measures
A change in outcome was defined as a difference from baseline and (1) on completion of the supervised intervention, at 12 months, and (2) 3 months after the end of treatment, at 15 months. Baseline and follow-up assessments were conducted during face-to-face interviews by researchers within established time windows (–6 weeks/+4 weeks at 12 months’ follow-up and ± 4 weeks for 15 months’ follow-up). Data collected outside these times were recorded but used only for sensitivity analyses and not for the main analysis.
Primary outcome
The primary outcomes were the physical and mental health component scores of the SF-36,178 measured at 12 and 15 months. We originally proposed the SF-36 as an integrated score but revised the protocol so that each of the two components was a primary outcome.
Secondary outcomes
Secondary outcome measures were (1) physical health measures (levels of total HDL and low-density lipoprotein cholesterol, triglycerides, HbA1c and CRP; anthropometric measurements, including waist circumference, BMI, BP; MetS defined according to the International Diabetes Federation criteria);13 (2) substance use measures [alcohol use recorded using the AUDIT,121 smoking prevalence and cigarettes per day using the Nicotine Dependence Questionnaire,120 use of cannabis and other illegal substances (e.g. opiates, methamphetamine, cocaine) using the Time Line Follow Back];72 (3) lifestyle measures (dietary patterns quantified using the DINE179 and physical activity using the short-form International Physical Activity Questionnaire);116,180 and (4) mental health status (using PANSS,115 GAF,117 SF-36 and Montgomery–Åsberg Depression Rating Scales). 181
Sample size
Power analyses were performed for the two subscales measures: physical and mental health components of the SF-36 QoL scale. 178 To detect a clinically significant reduction of 5 points (using 80% power, a 5% alpha level and two-tailed assumption), and to allow for a 20% loss of care co-ordinators and an additional 30% loss of patients to follow-up, a total sample size of 70 care co-ordinators, yielding 280 participants, was needed for the physical scale (d = 0.5), and 98 care co-ordinators, yielding 392 participants, were needed for the mental health scale (d = 0.42).
Statistical analysis
Primary statistical analyses was based on the intention-to-treat principle and aimed to estimate the difference in mean outcomes between participants randomised to HPI and those randomised to TAU at 12 and 15 months using mixed-effects models. Bias as a result of missing follow-up data was assessed by comparing the baseline characteristics of those with and those without complete data. In two linear mixed-effects models, the physical and mental health component scores at 12 months and 15 months constituted the dependent variable. ‘Treatment randomisation group’, ‘time’ (with two levels, i.e. 12 and 15 months post randomisation), and the interaction between ‘treatment group and time’, ‘centre’ and the ‘baseline values of physical and mental health component scores’ constitute the fixed part of the model. An unstructured covariance pattern model was used to model the dependency of the repeated observations of the same subject and to account for the dependency of the subjects within a cluster, and the care co-ordinator was included as a random factor. Model assumptions were assessed by visual inspection of the residuals. Standardised effect sizes, using pre-randomisation variability for standardisation, were also reported.
Secondary outcomes were analysed using the same methods as for the primary outcome. For all models, the interaction between treatment group and time was not significant and was removed from the final analyses. Treatment effects are, therefore, estimates for both time points. Logistic mixed models were used for binary outcomes (e.g. smoking) and Poisson mixed models were used for count data (e.g. number of cigarettes per day). Because of the large number of tests, significant results need to be treated as explorative.
Sensitivity analyses
Approximately 15% of the observations were collected outside the time window, so analyses using all available data were repeated as sensitivity analyses.
Handling of missing data
Models were rerun with predictors related to outcome missingness included as further covariates in the model. For the main outcomes, a second sensitivity analysis of missing outcome data, using multiple imputations by chained regression equations, was performed using all available clinical and demographic scores. This was done separately for each treatment group. A further sensitivity analysis was carried out to explore the impact of not missing at random patterns by adding positive or negative values to the imputed data sets.
Complier average effect analyses
In addition to the standard intention-to-treat (efficiency) analysis, we estimate the measure of the treatment impact for compliers only (treatment efficacy) using an instrumental variable approach with randomisation indicator as an instrumental variable. 182 Analyses were carried out using Stata’s® (StataCorp LP, College Station, TX, USA) ivregress package, with centre, treatment arm and baseline values of the outcome constituting the fixed part of the model and with cluster robust standard errors (SEs) to control for clustering effects of care co-ordinator.
Results
Key findings
A total of 104 care co-ordinators were recruited; 52 (with 213 patients) were randomised to deliver IMPaCT therapy and 52 (with 193 patients) were randomised to TAU. Of the 406 patients, 318 (78%) and 301 (74%) attended the 12- and 15-month follow-ups, respectively. The total sample size was reduced to 263 (64.8%) for 12 months (TAU, n = 132; HPI, n = 131) and to 238 (58.6%) for 15 months (TAU, n = 114; HPI, n = 124) because some of the patients who attended follow-up assessments were not seen within the required time frame. The required sample size based on the power analyses was achieved at both time points.
IMPaCT therapy showed no significant effect on the physical or mental health components of the SF-36 scores compared with TAU at 12 or 15 months. 176 Sensitivity analyses, including missing data analyses, did not alter any conclusion176 (see Appendix 5). A complier average casual effect analysis did not provide evidence of the efficacy of the intervention at 12 months for the physical health component (PHC) (mean difference –2.01, 95% CI –4.18 to 0.16; p = 0.07) or mental health component (MHC) (mean difference 0.02, 95% CI –2.79 to 2.83; p = 0.99).
No effect was observed for cardiovascular risk indicators, except for HDL cholesterol, which showed greater improvement following IMPaCT therapy than following TAU (treatment effect 0.09, 95% CI 0.007 to 0.16; p = 0.034). The 22% of patients who received > 180 minutes of IMPaCT therapy in addition to usual care achieved a greater reduction in waist circumference (–4.2 cm, 95% CI –7.2 to –1.2 cm; p = 0.006) than did control patients. This reduction is clinically significant. There was no difference in the rates of serious adverse events between the groups. The fidelity assessment is detailed in Appendix 3.
Discussion
Strengths of the trial
The main strength of this trial is that it was a pragmatic study: the design of the HPI was centred on creating an intervention accessible to the greatest possible number of people with psychosis receiving care in CMHTs. This is a traditionally hard-to-reach group; many people are unable or unwilling to attend standard group interventions, which is particularly problematic because of the high levels of cardiometabolic risk. 183
This study’s integrated personalised approach sought to maximise representativeness of the population studied. It addressed both lifestyle choices and substance use and avoided a piecemeal approach to behavioural change. By integrating IMPaCT therapy into established mental health care and by providing the care through a patient’s usual therapist, care could be adapted to the patient’s individual needs. To date, few large-scale, long-term RCTs have attempted to improve health in its widest sense in people with psychosis, instead focusing on a single target and often introducing new therapists to run the trial intervention.
The study recruited to target and was sufficiently powered to detect a difference between the two groups of at least 5 points in physical and mental health component scores (Cohen’s d = 0.42), had good follow-up rates and recruited a diverse, multiethnic sample of people with psychosis.
Limitations of the trial
Continuity of care was affected by multiple reorganisations of staff among the hospital trusts. Staff turnover sometimes delayed recruitment to the trial, as an inclusion criterion was that the staff members expected to be in post for the forthcoming year. Despite this, although not statistically different, staff turnover was slightly greater in the treatment arm than in the control group. However, when patients remained with the same trained care co-ordinator throughout the trial, there were indications that the intervention may result in significantly lower cholesterol levels and more exercise in the treatment group than in the control group. It is known that diminished continuity of care is associated with worse clinical outcomes. 184
Care co-ordinators struggled to deliver six or more 30-minute sessions of the HPI in addition to routine care, despite the training and ongoing supervision, and had limited fidelity to the MI model. This is in keeping with the recognised challenges of delivering targeted psychosocial interventions to people with psychosis that are faced by care co-ordinators in a busy secondary care environment. 185 It is also consistent with the findings of the Health Improvement Profile (HIP) RCT, which trained community mental health nurses to administer health checks to their patients and found that the volunteer nurses administered health checks to only a minority of participating patients. 186 However, given that the subset who received > 180 minutes of additional time devoted to IMPaCT therapy had a clinically significant reduction of –4.2 cm (95% CI –7.2 to –1.2 cm) in their waist circumference, a focus on implementation strategies in future work may be important.
A pilot trial was not conducted because the intervention was built on two pre-existing, successful, published interventions in this population, used a recognised behaviour change theory and incorporated additional recommendations for improving the preceding interventions. However, the longer combined intervention and the delivery model using non-expert care co-ordinators in a UK setting were not tested previously and raised logistical issues, which perhaps could have been identified and addressed more effectively in a large-scale pilot trial, although it is doubtful that they would have been identified in a small pilot.
The HPI was broad and participants self-selected, the target behaviours to focus on. It is conceivable that a more structured intervention targeting specific health behaviours, such as those described elsewhere,187,188 could be more successful in demonstrating statistically significant improvements in circumscribed aspects of the physical health of this population. Nonetheless, a longer-term, sustainable and integrated approach to overall health behaviours and health outcomes is urgently needed to allow successful and accessible routine care in the NHS.
Conclusions
Training and supervising community care co-ordinators to administer IMPaCT therapy to patients with psychosis was seen to be insufficient for significantly improving physical or mental health QoL. Front-line clinical staff, although willing to take part in training and supervision, struggled to consolidate training as part of applied MI therapy. However, the data suggest that continuity of care staff and protected time to deliver HPI work may potentially enhance outcomes.
Work package 3: economic evaluation section
Introduction
To address the key question of value for money in a resource-constrained health-care system, we conducted a comprehensive within-trial economic evaluation of the IMPaCT HPI to inform future decisions about its adoption into the NHS. This section summarises the work published in Heslin et al. ,177 with further unpublished data presented in Appendix 4.
Economic evaluation methods
Data collection
An adapted version of the Client Service Receipt Inventory (CSRI)189 was used to measure individual-level resource use. It covered the use of (all-cause) secondary and community-based health and social care services, prescription medication, time off work, and social security benefits received by participants and carers. It was administered as a retrospective, self-report, questionnaire-based interview conducted by assessors who were blind to treatment allocation. It covered the previous 6-month period at baseline and at the 12-month follow-up, and the previous 3-month period at the 15-month follow-up.
Health-related QoL was assessed with self-report questionnaires at baseline and at 12 and 15 months, and the SF-36 and EuroQol-5 Dimensions, three-level version (EQ-5D-3L),190 were used to estimate QALY gains.
Costs
Unit costs were applied to individual-level resource use data to calculate the total costs per participant from two cost perspectives: health and social care, and societal. All-cause health and social care costs included the trial interventions (HPI for the intervention group and standard information session for the control group), specialist accommodation, inpatient services, outpatient services, community-based services, community-based professionals and prescription medications. Societal costs included the same health and social care costs plus third-sector (charity) costs, lost productivity costs due to absence from work (if in employment during the assessment period), participant out-of-pocket expenditure on community-based day services and selected social security benefits. Lost productivity costs were capped at 5 days per week (maximum of 130 days for 6-month data and 65 days for 3-month data).
Unit costs are detailed for reference in Appendix 4, Table 9. In brief, the unit costs for most hospital and primary care services were obtained from the NHS Reference Costs 2010–11191 (inflated to 2011–12 prices using the Hospital and Community Health Services Pay and Prices Index or retail price index as appropriate) and the Unit Costs of Health and Social Care 2012 compilation. 192 Medication unit costs, taken from the British National Formulary,193 were converted into cost per milligram based on the most cost-efficient pack size, choosing maintenance doses over initial treatment doses and generic formulations over branded ones to obtain conservative estimates.
All costs are reported in Great British pounds (GBP) at 2011–12 prices. Discounting was applied to cost and outcome data related to the 12- to 15-month assessment period. Intervention costs were not discounted before being added to the 12- to 15-month costs because they were incurred in the first year. Discounting was applied at a rate of 3.5%. 194
Cost of the health promotion intervention
We included the following resource components to estimate the costs associated with the intervention:
-
the production of manuals (excluding the development work)
-
the training of care co-ordinators
-
the ongoing supervision of care co-ordinators
-
the implementation of the intervention by care co-ordinators with trial participants.
The resources and costs associated with each of these components are available for reference in Appendix 4, Table 9. The costliest of the four components was the implementation with patients, at a mean cost of £92.31 per case. To estimate the total participant-level intervention costs, we first averaged out general costs using a ‘top-down’ approach (i.e. we summed the costs of the manual, training and supervision, and divided this by the recommended number of patients under the care of each care co-ordinator). To this average we added the individual-level implementation costs.
Health promotion intervention manual costs
Care co-ordinators randomised to the intervention arm were provided with a copy of the IMPaCT manual, the IMPaCT reference guide and the Better Health Handbook. The time inputs to the intellectual development of these documents were considered a sunk cost and were, therefore, excluded. However, we included the reproduction costs.
Health promotion intervention training costs
Costs related to training care co-ordinators included the following components: the cost of trainer time (contact and non-contact), the cost of care co-ordinator attendance time and the cost of materials necessary to deliver the training courses. The training programme was delivered over 4 days by members of the research team in a standardised manner, with specific staff dedicated to implementing certain parts of the programme. The total number of hours and the number of sessions for each staff type were calculated as detailed in Appendix 4, Table 10. Preparation time was estimated by multiplying the number of training sessions by an assumed preparation time per session of 10 minutes. Costs were calculated by multiplying the appropriate unit costs by the number of minutes for each staff type.
Each of the care co-ordinator attendees was an NHS band 6 nurse. Each training programme took 32 hours including breaks (09.00 to 17.00 for 4 days). Costs were calculated by multiplying the appropriate unit costs by this time.
Materials were broken down into one-off costs and repeat costs. One-off costs included BP machine, weighing scales, tape measure, flip-chart stand and memory stick. Repeat costs included flip-chart paper, flip-chart pens, paper and pens.
Health promotion intervention supervision costs
At the outset of the study, it was envisaged that ongoing supervision of care co-ordinators would take place in a combination of individual and group sessions, led by a band 6 nurse trainer and a band 8 nurse trainer, for 9 months after attendance at the training programme. However, because of practical limitations, group supervision was unable to be provided, so all supervision took place at the individual level in 90-minute sessions. Costs were calculated by multiplying appropriate unit costs by the number of minutes spent in supervision by both the supervisors and the supervisees.
Health promotion intervention participant implementation costs
Similarly, it was originally envisaged that the intervention would be delivered in a combination of individual and group sessions led by care co-ordinators. However, group intervention sessions were not provided, largely because of practical limitations, so all implementation occurred in individual sessions. To estimate the costs of the intervention at the level of individual patient participants, we multiplied the cost of each individual therapy session by the care co-ordinator estimates of the proportion of time in each session spent delivering an aspect of the HPI.
Cost of the control intervention
Care co-ordinators in the control group of the trial were given a one-off information-giving session on mental and physical health issues. This involved a consultant psychiatrist delivering the session over a 1.5-hour period. The appropriate unit costs were applied and the cost per care co-ordinator was then divided by the recommended number of patients under the care of each care co-ordinator.
Outcome measures
Cost-effectiveness analyses were based on the joint primary outcome measures: the SF-36 MCS score and SF-36 PCS score. Cost–utility analyses were based on QALYs derived from the EQ-5D-3L and the SF-36 (US version 1) via the Short Form questionnaire-6 Dimensions (SF-6D). Appropriate utility weights were attached to health states for each measure at baseline and at 12 and 15 months. 178,190 QALY gains between 12 months and 15 months were then calculated using the total area under the curve approach with linear interpolation between assessment points. 195
Analyses, missing data and sensitivity analyses
For the economic evaluation analysis, see Appendix 4.
Key findings
The economic evaluation showed no evidence of a clear difference in health and social care costs between the two trial groups. Societal costs may be higher in the intervention group. Alongside a lack of additional benefit, there is no evidence that the intervention is cost-effective. The integrated mental and physical health-care intervention itself involved no significant additional resource use or associated costs. However, it is unclear to what extent this finding reflects a true lack of impact on costs and outcomes, or whether or not it reflects the fact that, because very few participants in the intervention group received the intervention as planned, both groups ultimately received similar care.
Discussion
Limitations
The economic evaluation had some limitations. Data on resource use and, therefore, on costs were collected using the self-reported CSRI. This makes the data subject to participant recall bias. However, there is evidence for the reliability of self-reported resource use data in similar populations196,197 and we considered the approach necessary given the multisite and multiperspective nature of this study; the lack of integration of all relevant health and social care sector client records necessitated such an approach to avoid restriction to a narrow definition of health and social care. Furthermore, at least some resource use would still have been needed to be collected via self-report (i.e. welfare benefits and employment losses) to enable a societal perspective to be considered, which we considered of particular relevance for a patient group whose health and care needs can have economic impacts on multiple sectors of society. Additionally, there is no reason to believe that any biases related to data collection would be unbalanced between the two trial groups, particularly because the CSRI was administered by blinded assessors.
A further limitation is that we may have double counted resource use associated with the HPI. We collected this information separately from care co-ordinators rather than from patient participants to avoid unblinding the assessors conducting the participant interviews. However, this carried the risk of participants reporting resource use related to the intervention in response to questions about their service use. Although this may skew absolute estimates of costs for the intervention arm, any bias in the calculation of treatment effects would work against the intervention group by overestimating the intervention cost.
There has been some discussion around the validity of using the SF-36 and EQ-5D-3L with study participants who have mental health problems, especially those with schizophrenia and other psychoses. 198 Brazier et al. 198 suggest that neither scale performs particularly well in terms of quantitative testing against psychometric criteria and that both have a limited coverage of domains identified as relevant by people with mental health problems. Thus, it is unclear whether or not the lack of QALY difference between the two trial groups reflects a lack of intervention effect or limitations associated with the measurement properties of these two health-related QoL measures. However, given the lack of effect based on the SF-36 MCS and PCS, and all other outcome measures, it is unlikely that there was a difference in QALYs that we have been unable to detect.
Finally, the time horizon of the evaluation is likely to have been insufficient to identify all relevant outcomes for this patient group, particularly given the longer-term impacts of physical health problems. However, it is unlikely that any effects of the intervention would transpire in the longer term if absent in the short term.
Conclusions
There was no evidence of a difference in costs or outcomes from a health and social care perspective. From a societal perspective, the intervention may incur higher costs for no associated benefit.
Programme discussion
People with SMI are at increased risk of poor health outcomes and have a marked premature mortality rate. The aim of the IMPaCT project was to develop culturally appropriate, innovative, practical and effective programmes to achieve better physical and mental health in patients with SMI by improving lifestyle choices and reducing illicit drug use. Three separate studies were conducted. Work package 1 (‘the PUMP study’) aimed to observe health behaviours, the progression of cardiometabolic disease among patients with their FEP, and the links between these factors over the course of 1 year. Consistent with previous studies, we found high rates of cardiometabolic risk at presentation, which increased over the 12-month period. No relationship was found between antipsychotic choice and emergence of cardiometabolic risk. Work package 2 saw the successful development of a HPI, IMPaCT therapy, based on MI and CBT techniques aimed at effecting behaviour change in people with established psychosis. Work package 3 was a large RCT of IMPaCT therapy among patients with established psychosis that was carried out in five NHS trusts throughout the UK. The intervention was integrated into NHS secondary care as care co-ordinators delivered it to their own patients. IMPaCT therapy was not found to affect QoL any more than did TAU, nor was it cost-effective. There was limited evidence of efficacy on secondary outcomes such as cardiovascular risk indicators, substance use or mental health measure compared with TAU alone.
Work package 1, PUMP, demonstrated that the use of substances such as tobacco and alcohol and less healthy dietary and exercise choices among people with FEP are common on presentation to clinical services and that cardiometabolic risk continues to increase over the course of the year, with rates of obesity rising from 17.8% to 23.7% in this relatively young population. These health profiles may also have an impact on service costs, with obesity at baseline showing an association with higher subsequent admission costs, low HDL cholesterol at baseline being associated with lower subsequent admission costs and higher HDL cholesterol at baseline being associated with a greater subsequent QALY gain. In the IMPaCT study group, who were older, half were obese; furthermore, 20% of our population with established psychosis had diabetes, and a further 30% had glucose dysregulation. 183 These findings both testify to the importance of preventing diabetes in the first instance and highlight the challenge in preventing diabetes among people with SMI. Management of diabetes is also difficult in this population; for example, people with diabetes and SMI have fewer routine eye checks and poorer glycaemic and lipid control,199 and perhaps ultimately have a 50% poorer survival rate, than those with a diagnosis of diabetes alone. 200
In both PUMP and the RCT study, we found high rates of physical inactivity alongside an unhealthy dietary intake typified by high saturated fat and low dietary fibre, which is consistent with previous literature. It is acknowledged that sedentary behaviour and unhealthy eating further increase vulnerability to ill health and early death. 201 Furthermore, we conducted a systematic review and meta-analysis of sedentary behaviour in people with psychosis, which showed that people with psychosis engage in very high levels of sedentary behaviour while awake. 202 We related sedentary behaviour in the IMPaCT population to an inflammatory marker of CVD, CRP. 203 We found that those with higher levels of sedentary behaviour have elevated CRP levels. Yet, despite the IMPaCT HPI addressing the above lifestyle behaviours, there were no significant improvements in physical activity or dietary consumption over the course of the study. The HPI exercise module aimed to increase the level of exercise and, specifically, the number of minutes patients engaged in physical activity but was unsuccessful. This is in keeping with the findings from our recent meta-analysis that people with schizophrenia engage in significantly less moderate and vigorous physical activity than do control participants. 204 The above finding suggests that although the overall aim is to make people more physically active, attention needs also to be directed at making people less sedentary. As a result of our findings, we are now running a trial of an intervention specifically to increase activity and reduce sedentary behaviour, Walk this Way. 202
We found that 62% of our patients with established psychosis smoke tobacco, and the rates were even higher among people presenting with their FEP. Although tobacco-smoking rates are known to be high in people with psychosis, our figures are thrown into sharp relief when compared with the current 18% prevalence rate among the general UK population. We are currently investigating in our sample of people with established psychosis the effect of cigarette smoking on both depressive and psychotic symptoms over time. The very high rates of tobacco-smoking in the first episode group changed little over the following year, especially so among those from black and minority ethnic groups. A potential explanation for this may be that national smoking campaigns and incentives for health-care practitioners to address smoking behaviours are not well targeted towards this population. The potential adverse effects of nicotine dependence among our sample highlight the need for effective smoking cessation strategies in psychosis. The findings may also help both patients and clinicians to view smoking cessation more positively by understanding the detrimental effects smoking can have both on physical health and on mental health symptoms.
We failed to identify differences in cardiometabolic risk in people with established psychosis who were prescribed antipsychotic medication of different types or in emergent cardiometabolic risk in FEP patients. 176 Despite the side effects, antipsychotic medication is important in optimising one’s mental health so that one is well enough to take charge of one’s physical health. In a large study of 66,881 patients with schizophrenia over a 10-year period, long-term treatment with antipsychotic medication was associated with lower mortality than receiving no antipsychotic medication. 9 Additionally, the longer people were prescribed antipsychotics, the smaller the mortality gap became. Furthermore, those prescribed with clozapine, the gold-standard treatment for refractory schizophrenia that is known to have a risk of rapid weight gain and glucose dysregulation,12 had the lowest mortality rate of all patients with schizophrenia. Therefore, antipsychotics, used thoughtfully at the lowest effective dose, should be seen as integral to mortality reduction strategies.
A relatively small proportion of our IMPaCT patients were prescribed cardiovascular medication, although further work is needed to determine the proportion of our sample for whom medications such as statins were indicated under current NICE guidelines, which are not based on blood test results alone. Nevertheless, it is an important area for attention. A Danish study31 has shown that people with schizophrenia with concomitant CVD are prescribed most cardiovascular drug classes less often than the general population, in particular lipid-lowering and antihypertensive medication. Additionally, in that study, patients with SMI were less likely to be prescribed more potent medications (such as angiotensin-converting enzyme inhibitors or beta-blockers), suggesting inadequate treatment of CVD.
It is not immediately clear why the IMPaCT therapy intervention conferred no advantage on recipients, relative to control treatment under intention-to-treat conditions. It is possible that potential efficacy was suppressed because of factors affecting consistent implementation of the intervention in an increasingly busy NHS setting, despite ongoing supervision. A key strength of the IMPaCT RCT was its pragmatic design; the trial was integrated into the existing workforce and conducted across several NHS foundation trusts, thus widening its generalisability. Significant practical challenges were overcome during implementation; for example, as services were reconfigured or contracted, there was some emergent resistance among team leaders to allowing care co-ordinators to fully participate in the study as planned. Once care co-ordinators were randomised to the IMPaCT therapy arm of the trial, they were required to attend a mandatory 4-day training course on how to deliver IMPaCT therapy, but difficulties were experienced in encouraging care co-ordinators to attend the planned course. For example, despite initial buy-in to the IMPaCT study from team leaders, there was a degree of resistance at team level to freeing the care co-ordinator from clinical duties to attend the training. Resistance may have arisen because, within each participating centre, several care co-ordinators could be recruited from one team. Given care co-ordinators’ already heavy workload, team leaders were understandably reluctant to allow to be absent from their regular duties for the full 4-day period. In anticipation of these concerns, the study team offered care co-ordinators the opportunity to attend 2-day training in any given week and to complete the remaining 2 days at the following training session. Feedback from participating care co-ordinators indicated that the training was well received. Although a reduction in training duration appeared to have improved the acceptability of the intervention among team managers, the shortened training session may have been less immersive and, thus, less effective than the planned 4-day session, which may have had an impact on the results.
Indeed, it is questionable whether or not intensive training courses are sufficient to equip care co-ordinators with the skills needed to deliver a given psychosocial intervention. Motivational interviewing requires that therapists reframe the way in which they approach discussions with their clients. Shifting from a didactic to a more client-centred approach probably requires that care co-ordinators reconsider their ingrained practices. The care co-ordinators recruited to the study had many years’ experience in their role, over which time they were likely to have formed habitual clinical practices. Indeed, no care co-ordinator ran group sessions with patients, despite receiving training in this and supervision to support group work; we noted that running groups had not been standard practice for any of the care co-ordinators before joining the study. Not only may care co-ordinators have lacked the time to organise and facilitate these sessions, but changing practice may have required them to break habits. Care co-ordinators may require support from team leaders to deliver new models of care and, although trust management and team leaders were supportive, future work may need to include an element addressing organisational barriers.
Care co-ordinators can be from a number of professions, but in the participating trusts they were predominantly from either nursing or social work backgrounds. We did not want to limit the evaluation to nurses only as the cardiometabolic risk was universal. Social workers would not have received training in physical health management as part of their professional training; additionally, in recent decades, mental health nurses will have received only limited training in physical health when completing their undergraduate course. The intervention was designed with this in mind and to address the gaps between physical and mental health care. Interventions such as the HIP RCT have also highlighted challenges in incorporating physical health management into mental health care. 186 There has also been recent consideration of other service models. The research evidence suggests that high-intensity interventions incorporating specialists in diet and exercise are most effective,187,205 although, in the UK, service providers may be hesitant owing to the cost of such initiatives.
Additional challenges were posed by shifts in the political landscape during the study period, specifically the implementation of the Health and Social Care Act 2012,206 accompanied by significant economic pressure and a large-scale restructuring of the NHS. The IMPaCT RCT study relied on the recruitment of CMHTs, which are embedded in mental health trusts. The main recruiting mental health trust experienced major restructuring and changes in remit during the study period, which in turn led to delays, as care co-ordinators could not commit to participation until this reorganisation was complete. The reorganisation was associated with a degree of uncertainty among staff about their job continuity, which had an impact on their ability to participate in the IMPaCT therapy training; specifically, many were unsure, because of restructuring, whether or not they would be with the team for the duration of the trial (15 months). Additionally, there may have been tensions within teams regarding care co-ordinators’ involvement in the study; although training staff members is perceived as beneficial to the team, freeing staff for training purposes inevitably means distributing their workload among other staff members. However, it is important that these challenges are recognised; this intervention was designed to be easily incorporated within standard care teams and the practicalities of doing so are part of the evaluation. This context should be considered when interpreting the costs associated with IMPaCT therapy. The evaluation suggested that the intervention itself involved no significant additional resource use or associated costs, nor an impact on broader overall costs, but it is unclear to what extent this reflects a true lack of impact on costs or merely low levels of implementation and, thus, limited potential to have an impact on resource use.
Additional methodological limitations must be acknowledged. Study procedures may have been conducive to selection biases. Studies of non-participation have typically found that those who decline to participate in psychosis research studies tend to be male, have severe symptoms, substance abuse problems, low educational attainment and socioeconomic status, and be unmarried and unemployed. Additionally, patients were required to provide informed consent to participate, which presupposes sufficient cognitive ability to read, interpret and deliberate over the information presented in a patient information sheet and to make decisions regarding whether or not to consent to all aspects of the research, while acknowledging the potential risk of doing so. Obtaining informed consent risks excluding the most impaired patients from a study.
Work package 1, PUMP, was designed so that all people presenting with their FEP were potentially eligible for inclusion, whereas the RCT was carefully designed to be as representative as possible, so that all patients with a primary diagnosis of a psychotic disorder allocated to the care of a participating care co-ordinator were potentially eligible for inclusion. To further explore representativeness of psychosocial research models, we decided to test the hypothesis that, even with such an inclusive RCT design, those who consented to participate in the trial would be less ill than those who did not. We did this by comparing the Health of the Nation Outcomes Scales (HoNOS) scores at baseline among the SLaM IMPaCT RCT participants (n = 293) with similarly timed HoNOS scores recorded as part of clinical care in the eligible population of patients (i.e. those eligible patients of the participating care co-ordinators, n = 774) using an anonymised case register. The mean total HoNOS score of the eligible comparator population was indeed significantly higher than that of the IMPaCT RCT participants (t = 3.810; p = 0.006), as was the degree of overall illness severity and functional impairment, as measured with HoNOS. We concluded that the patients who participated in the RCT had better mental health at entry to the trial than the total eligible population, although we found no difference in physical health needs. 207 This is important to bear in mind when interpreting the findings of RCTs of lifestyle interventions for use in service planning. We would further speculate that the greater the effort needed to participate in an intervention and evaluation thereof, the more selective recruitment and the less generalisable the results to the overall SMI population in need.
Furthermore, in the PUMP study, the mean duration of treatment was 35 days prior to consent to the study. This time lag was the product of waiting for participants to be well enough to provide informed consent to the study. Thus, our measure of baseline symptom severity is necessarily more representative of a less ill population. This highlights the difficulties that researchers face in recruiting participants at the time of presentation to psychosis services, while also illustrating the potential for results not to generalise to people with more severe psychosis at the point of presentation. Because of these problems, it is possible that our sample under-represents the severity of illness often witnessed in people with psychosis. Indeed, the relatively low total PANSS scores in the PUMP study suggest mild to moderate symptom severity. However, it is likely that we were examining a cohort of patients representative of FEP. For example, our study208 examining the longer-term follow-up of the same sample found that 34% of the sample were treatment resistant at 5 years, with 70% of those never achieving symptomatic remission from the time of first presentation.
Missing data also posed a challenge in the PUMP study. Partly, this may have been a result of attrition; one of the key difficulties of conducting both prospective cohort studies and RCTs is the time lag between study recruitment and follow-up, which can lead to attrition bias. 209 PUMP included a large number of assessments and so participants may have felt fatigued and unable to complete all of the tests. To minimise repetition, the PUMP study joined with the Genetics and Psychosis (GAP) study210 that was recruiting FEP patients concurrently; although this limited the number of repeat assessments, the overall assessment battery remained intensive. Additionally, participants living in inner-city areas are highly mobile, making it harder to locate them at follow-up. This, combined with the social deprivation faced by this population, may also increase the mobility of this sample, with many participants being frequently rehoused, which may result in limited traceability. 211 However, measures were employed to limit attrition. Contact details of the participant and their relatives/friends (e.g. e-mail and postal address, mobile and home phone numbers) were collected at the start of the study and contact was pursued using participants’ preferred contact mode. We also sought to maintain interest and remind participants of the study aims by sending newsletters to participants between baseline and follow-up assessments. Additionally, a courtesy call was made midway between the assessments to remind participants of their upcoming follow-up appointments. These communications also alerted the research team to barriers to contacting the participant (e.g. newsletter returned as undelivered), and other means of contact were used to maximise the likelihood of booking follow-up assessments. Nonetheless, in the PUMP study, only 125 of 293 participants completed the 12-month face-to-face assessment measures. However, we retrieved follow-up data from a number of sources including case notes and other records, thereby, not solely relying on face-to-face measures, which resulted in high completeness rates. 122 Thus, the potential impact of attrition was mitigated.
To minimise the impact of missing data on our research findings in the PUMP study, we utilised, with consent, data from a number of sources; these included anthropometric, metabolic and biochemistry data recorded in the patients’ electronic case records. However, we found low levels of documentation of physical health measures. This is in keeping with a series of national audits of schizophrenia, which have retrospectively examined the quality of physical health monitoring, including whether or not weight, BMI, BP, tobacco use, alcohol use, substance misuse, blood glucose, blood lipids and family history of CVD, diabetes, hypertension or dyslipidaemia were documented. Documented evidence in the case notes fell below agreed standards, with < 25% of patients having all nine parameters documented. 212 This highlights the need to regularly monitor and record these data systematically. However, it should be noted that this finding was before the introduction of CQUIN targets, which have since enhanced physical health data documentation substantially, particularly for those patients who have had an inpatient stay.
Finally, both studies relied heavily on self-report measures. Although these are validated for use with participants with psychosis, potential problems are associated with the accuracy of such measures, which must be acknowledged. Of note is that many participants chose not to self-administer the measures, largely because of the nature of the illness (which can result in lack of motivation, low mood, cognitive impairment), and low literacy levels. Instead, researchers were often required to read aloud each item and present the corresponding scale on which participants would indicate their response. Although researchers sought to read aloud questionnaire items in a non-biased manner, it is known that the way in which data collection is conducted, or by whom it is conducted, can have unintended potential consequences on study findings.
Recommendations for research
At the individual level, having schizophrenia makes it more difficult to engage in healthy behaviours. For example, social withdrawal reduces opportunities to participate in physical activity,213 and negative and cognitive symptoms make it even more difficult for people with schizophrenia to organise themselves to prepare healthy meals. We have not yet had the opportunity to fully explore the effect of negative and cognitive symptoms on outcomes in our study populations, but this is work we plan to do in the future.
At a biological level, genetic factors may also influence cardiometabolic risk. An identified locus is associated with both increased CVD risk factors and schizophrenia,214 while polymorphisms in a type 2 diabetes gene may increase susceptibility to schizophrenia. 215 We are examining the IMPaCT population to look for genetic predictors of metabolic status both in people with FEP and those with established psychosis. We have created a genetic risk score and are performing statistical analyses to establish the relationship between this score and MetS in psychosis.
Inflammatory mechanisms have been suggested as a possible driver of CVD in schizophrenia. 216 We examined the relationship between inflammation as measured by levels of CRP and metabolic risk factors in people with their FEP. We found that triglyceride dysregulation is associated with elevation of CRP levels early in the disease course of people with psychotic disorders. 126 This suggests that inflammation should be investigated as a therapeutic target in the future.
Sexual dysfunction is common in people with schizophrenia, with a reported prevalence of up to 80%. 217,218 In our service user focus group this was highlighted as a priority topic. Although sexual dysfunction is often attributed to the side effects of psychotropic medication, we have found that it exists even before the FEP in people not treated with antipsychotic medication. 219 Nevertheless, the development of sexual side effects contributes to non-adherence to psychotropic medication. This is a frequently overlooked aspect of general well-being in people with schizophrenia; it is important to QoL and should be actively enquired about by treating clinicians. Future clinical research is needed to determine how best to systematically identify and manage sexual dysfunction in people with a diagnosis of SMI, bearing in mind its multiple causes, many of which, such as medication choice, obesity, smoking and sedentary behaviour, are also relevant to cardiovascular health.
The search for effective evidence-based approaches to reduce the excess cardiometabolic risk experienced by people with SMI is proving a challenge for many research groups, following the negative results in all recent large European RCTs on the topic, namely IMPaCT, STEPWISE, PRIMROSE, HIP and CHANGE. 176,186,220–222 Although the interventions in all these studies were intuitively sensible and it was reasonable to believe that they should have been helpful, researchers are struggling to demonstrate evidence of additional effectiveness in the context of structured health-care systems. It may be that the effectiveness of more individualised long-term approaches to reverse cardiometabolic risk in this population needs to be evaluated, bearing in mind that reversing established cardiometabolic risk in the general population is also a challenge.
Conclusion
The IMPaCT programme has highlighted the high rates of cardiometabolic risk soon after first presentation with psychosis and the early emergence of additional risk. We did not find that baseline lifestyle choices and addiction behaviours identified those people most likely to increase their cardiometabolic risk in the first year, but we did note a differential pattern of emergence of cardiometabolic risk in relation to ethnicity.
We developed, in an iterative process, a modular HPI to be used in routine clinical care and implemented by the patient’s usual care co-ordinator and evaluated it in what is to our knowledge the first randomised trial of such an intervention. The RCT confirmed the challenges in targeting lifestyle and substance use to modify cardiometabolic risk and highlighted the difficulties of adding specific psychosocial interventions to the workloads of increasingly stretched clinical teams.
Our work also demonstrated that, even in the early stages of psychosis, there are high rates of vitamin D deficiency, which was associated with cardiometabolic risk markers in people with established psychosis. As a result of this, we have now started a RCT of vitamin D supplementation in FEP (URL: www.clinicaltrialsregister.eu/ctr-search/trial/2014-002639-32/GB; accessed 2 December 2019).
A better understanding of the role and interplay of exercise, nutrition, substance use and medication will allow for the development, delivery and evaluation of better and more efficient interventions, for example targeting the overall individual’s fitness instead of simple measure of their body shape. 223 Importantly, at a wider level, factors hindering access to care should also be addressed to improve the knowledge of service planners and to plan reasonable adjustments. The low efficacy of HPI interventions such as IMPaCT RCT in reversing established unhealthy behaviours is consistent with other emerging work. 220 In this context, our results underlined the importance of preventing the formation of (unhealthy) habits around physical exercise, alimentation, substance use and lifestyle choices rather than modifying them later.
The IMPaCT study provides important information to health service planners, suggesting that they need to be aware that training and supervising staff to work on lifestyle choices and substance use with their patients as part of routine care is not sufficient to reduce cardiometabolic risk or improve mental and physical QoL.
Programme patient and public involvement
This whole programme was, from the outset, co-produced with experts by experience. Ms Bee Harries was a co-applicant on the original grant application. Ms Harries was involved in the planning and development of IMPaCT and facilitated our patient and public consultation, starting in the borough of Lewisham. She led the focus groups held there, which informed our study design and emphasis; for example, the inclusion of work on sexual dysfunction derived from that focus group (Reis-Marques et al. 219). She helped generate solutions to enhance recruitment to the study and presented the work with us to collaborating services. Sadly, Ms Harries died during the course of the study. She contributed immensely to the development and accessibility of the intervention, which is dedicated to her memory.
A stakeholder engagement event was held in the Civic Suite in Lewisham on 20 March 2008 entitled ‘Our Bodies are Important Too . . . as well as our mental health’ (Box 1). The event included information, small-group work (up to six people in a group) and, at lunchtime, a well women’s group, hand massage, activity from Network Arts, information on physical activity and expert patients’ group. The aim of the day was to find out from people using mental health services what is important to them in their treatment around physical health care. We wanted to find out what people would like to see more of or change and whether or not people would like to get involved in planning or advising on health promotion services. The day was co-ordinated by Ms Miriam Mica, Mental Health Promotion Co-ordinator at SLaM NHS Foundation Trust, and the sessions were led by Bee Harries. The day was co-hosted with Family Health Isis, a local African and Caribbean mental health organisation.
10.00–10.30 Sign-in and registration
Delegates can leave their addresses if they want information from the day.
11.00 Introduction of the day
Miriam
11.15 Interview of Fiona Gaughran about the IMPaCT Project
Bee and Kristine
11.45 Group exercise – what one thing would you say about your health to your support worker, doctor, etc.
Collated and used in theatre after lunch
12.00 Small groups and consultation exercise around IMPaCT questions
13.00 Lunch – hot and cold food
14.00 Playback theatre around Bee’s poem – I am a person 1, 2 and 3, and group exercise results
14.15 Where do we go from here – next steps
Kristine/Miriam/Bee
Questions/areas for the group work included:
-
What has worked already for you within SLaM services for your health care?
-
Why did this work?
-
What would you like to see more of/different?
-
Talk about the health module – what is important to have on the health module (e.g. exercise, diet, etc.)?
-
Any interest in getting involved in the health modules, training on the health module?
-
Any interest in joining the advisory group for the IMPaCT Project?
On the untimely death of Bee Harries, Thomas Kabir from the Mental Health Research Network helped us with the essential patient and public involvement input to the programme and then put us in touch with Maurice Arbuthnott, who also has expertise by experience. Maurice Arbuthnott has contributed hugely as an equal partner to the running of all aspects of the programme.
We set up a partnership with carers through the Mental Health Research Network body FACTOR, co-ordinated by Dr Geraldine Mason. We set up a carers’ consultation group; this consisted of six to eight carers who met regularly with two IMPaCT team members to discuss the project. Two carer members of that group, Philippa Lowe and Diana Orr, started to attend the monthly IMPaCT management meetings and are important partners in the team, advising on recruitment and follow-up strategies as well as engagement and dissemination.
All our experts through lived experience have not just contributed to the development and implementation of the research, but also brought their own knowledge and expertise to enrich the quality and applicability of the work. Our carer involvement structures are described in detail in a case report by an anthropologist in Devon Partnership NHS Trust (see Report Supplementary Material 1; reproduced with permission from Dr Sarah Robens, Devon Partnership NHS Trust).
Expert service user input
My name is Maurice and I live in Central London. I have been using mental health services for just over 20 years. I became involved in IMPaCT through the Mental Health Research Network and I am pleased to help with representing service user interests in the IMPaCT research project. My main role is as a participant and observer at the monthly IMPaCT steering group meetings. I consider my participation at these meeting in three ways: 1. Offering advice where appropriate e.g. on how to encourage service users to get involved in the project. 2. Enquiring about the progress and methodology of the project for the sake of clarification. 3. Making constructive comments for general consideration. My perspective of the research in question comes not only as a service user experienced in psychosis and treatment but also as a lay person e.g. the proverbial man in the street so I aim to keep this perspective as wide as possible.
On occasion I have also talked to researchers about my own experiences of how my physical health needs have been met and my relationship with medical professionals, especially my community mental health team. I am very interested in being a source of reference to researchers and for them to enquire about my experiences as a service user. I also compiled a questionnaire for the service users to complete after their involvement in IMPaCT. This aimed to find out what service users found was important to them in participating in research and what they found interesting, useful or otherwise. We are soon to analyse these results.
I am particularly interested in how this project will benefit the maximum number of service users through helping them achieve the best possible physical health and I am looking forward to assisting with the dissemination of the eventual results and findings not only to the medical profession but also to my fellow service users and the lay person. I feel very positive about IMPaCT goals to empower people into better health especially as it is a well-documented observation that people with a mental illness also endure poor physical health.
Expert carer input
Written by Philippa Lowe:
There are many ways of coping as a carer for someone with mental illness and one of them is to get thoroughly involved in the subject and to take an interest in related research developments. This is the path I chose, so I was very pleased to be invited to be a carer adviser for the IMPaCT project.
The subject of the research was of particular interest to me as my son, who has had schizophrenia for nearly 20 years, is now very much better in respect of the more dramatic symptoms of the illness but has experienced considerable weight gain which puts him at risk of developing severe physical illnesses which may shorten his life. I was grateful for the opportunity to help shed light on the problem both for the sake of my own family and for others facing similar issues.
I found it a pleasure to work with a team of people from different relevant disciplines who were clearly dedicated to their subject and welcomed the various contributions with respect and interest. I found that I could make a useful input, for example, by suggesting ways of helping the research subjects cope with the various procedures which were required of them, by proposing the best ways of setting out clear information in guidance notes and newsletters and by urging the involvement of carers, where available, as the people who can offer support and encouragement for ongoing participation. Colleagues involved with the project gave a clear indication that this sort of input was of value to the project, which was very good to hear.
Acknowledgements
We would like to acknowledge the assistance of many people over the course of this study, including, but not limited to, the late Bee Harries, Susan Moore, Stefania Bonaccorso, Anna Kolliakou, Conan O’Brien, Ali Featherman, Catherine Fung, Keji Dalemo, Stella Anakwe-Umeh, Gill Todd, Manyara Mushore, Diana Orr, Oliver Howes, John Lally, Ruth Ohlsen, Evangelos Papanastasiou, Hannah Sallis, Irene Sambath, Guilia Di Clemente, Josefine Breedvelt, Candice Joseph, Jonas Eberhard, Andy Healy, Funda Sinan, Keerthana Rudhra, Hannah Kelly, Janet Treasure, Anthony Davis, Caroline Murphy, Joanna Kelly, Matthew Goldin, Hugh Williams, Rosemary Padley, Miriam Mica, the Genetics and Psychosis Team and the Mental Health Research Network along with patients, carers and staff of participating trusts.
We also wish to acknowledge the support of the Trial Steering Committee for their expert guidance: David Osborn (chairperson), Jack Gibson, the late Adrienne Reveley and Richard Drake.
As well as the funding from the NIHR under its IMPaCT programme, we also wish to acknowledge the support of the NIHR Biomedical Research Centre at SLaM NHS Foundation Trust and King’s College London. Fiona Gaughran and Brendon Stubbs are in part supported by the Maudsley Charity and the NIHR Applied Research Collaboration (ARC) South London at King’s College Hospital NHS Foundation Trust.
Contributions of authors
Fiona Gaughran (https://orcid.org/0000-0001-7414-5569), Daniel Stahl (https://orcid.org/0000-0001-7987-6619), Anita Patel (https://orcid.org/0000-0003-0769-1732), Khalida Ismail (https://orcid.org/0000-0001-6084-449X), Shubulade Smith (https://orcid.org/0000-0002-3797-6985), Kathryn Greenwood (https://orcid.org/0000-0001-7899-8980), Zerrin Atakan (https://orcid.org/0000-0001-9209-3993), David Hopkins (https://orcid.org/0000-0002-0451-0900), Anthony S David (https://orcid.org/0000-0003-0967-774X) and Robin M Murray (https://orcid.org/0000-0003-0829-0519) conceived the study design and objectives and interpreted the data.
Poonam Gardner-Sood (https://orcid.org/0000-0002-5875-4142) and Marta Di Forti (https://orcid.org/0000-0002-3218-6925) significantly contributed to the acquisition of data.
Poonam Gardner-Sood, Dominic Stringer (https://orcid.org/0000-0001-5624-1733), John Lally (https://orcid.org/0000-0003-3038-0625), Marta Di Forti, Brendon Stubbs (https://orcid.org/0000-0001-7387-3791), Philippa Lowe, Maurice Arbuthnott and Margaret Heslin (https://orcid.org/0000-0002-3094-9255) significantly contributed to the analysis and interpretation of the results of the work. All authors read and approved the final manuscript.
Fiona Gaughran is the guarantor for the manuscript and the data contained within.
Contributions of others
Bee Harries was an expert with lived experience who was involved in the development of the study design, was a co-applicant on the grant and worked with the team to implement the research. Diana Orr (expert by lived experience) was part of the trial management team and helped in the planning of the projects. Kurtis Stewart (research worker), Simone Ciufolini (clinical research fellow), Mary Aboasu (research worker), Niamh Lyons (research worker) and all of the Institute of Psychiatry, Psychology & Neuroscience, King’s College London, assisted in the preparation of the manuscript.
Publications
Please contact the first author for manuscript copies.
Work package 1: PUMP
Reis-Marques T, Smith S, Bonaccorso S, Gaughran F, Kolliakou A, Dazzan P, et al. Sexual dysfunction in people with prodromal or first-episode psychosis. Br J Psychiatry 2012;201:131–6.
Crews M, Lally J, Gardner-Sood P, Howes O, Smith S, Murray RM, et al. Vitamin D deficiency in first episode psychosis: case–control study. Schizophr Res 2013;150:533–7.
O’Brien C, Gardner-Sood P, Corlett SK, Ismail K, Smith S, Atakan Z, et al. Provision of health promotion programmes to people with serious mental illness: a mapping exercise of four South London boroughs. J Psychiatr Ment Health Nurs 2014;21:121–7.
Kolliakou A, Castle D, Sallis H, Joseph C, O’Connor J, Wiffen B, et al. Reasons for cannabis use in first-episode psychosis: does strength of endorsement change over 12 months? Eur Psychiatry 2015;30:152–9.
Russell A, Ciufolini S, Gardner-Sood P, Taylor H, Bonaccorso S, Gaughran F, et al. Inflammation and metabolic changes in first episode psychosis: preliminary results from a longitudinal study. Brain Behav Immun 2015;49:25–9.
Colizzi M, Carra E, Fraietta S, Lally J, Quattrone D, Bonaccorso S, et al. Substance use, medication adherence and outcome one year following a first episode of psychosis. Schizophr Res 2016;170:311–17.
Lally J, Ajnakina O, Stubbs B, Williams HR, Colizzi M, Carra E, et al. Hyperprolactinaemia in first episode psychosis – a longitudinal assessment. Schizophr Res 2017;189:117–25.
Theleritis C, Bonaccorso S, Habib N, Stahl D, Gaughran F, Vitoratou S, et al. Sexual dysfunction and central obesity in patients with first episode psychosis. Eur Psychiatry 2017;42:1–7.
Gaughran F, Stahl D, Stringer D, Hopkins D, Atakan Z, Greenwood K, et al. Effect of lifestyle, medication and ethnicity on cardiometabolic risk in the year following the first episode of psychosis: prospective cohort study. Br J Psychiatry 2019;215:712–19.
Work package 3: IMPaCT randomised controlled trial
Gaughran F, Stahl D, Ismail K, Atakan Z, Lally J, Gardner-Sood P, et al. Improving physical health and reducing substance use in psychosis – randomised control trial (IMPaCT RCT): study protocol for a cluster randomised control trial. BMC Psychiatry 2013;13:263.
Gardner-Sood P, Lally J, Smith S, Atakan Z, Ismail K, Greenwood KE, et al. Cardiovascular risk factors and metabolic syndrome in people with established psychotic illnesses: baseline data from the IMPaCT RCT study. Psychol Med 2015;45:2619–29.
Stubbs B, Gardner-Sood P, Smith S, Ismail K, Greenwood K, Farmer R, Gaughran F. Sedentary behaviour is associated with elevated C-reactive protein levels in people with psychosis. Schizophr Res 2015;168:461–4.
Stubbs B, Gardner-Sood P, Smith S, Ismail K, Greenwood K, Patel A, Farmer R, Gaughran F. Pain is independently associated with reduced health related quality of life in people with psychosis. Psychiatry Res 2015;230:585–91.
Lally J, Gardner-Sood P, Firdosi M, Iyegbe C, Stubbs B, Greenwood K, et al. Clinical correlates of vitamin D deficiency in established psychosis. BMC Psychiatry 2016;16:76.
Gaughran F, Stahl D, Ismail K, Greenwood K, Atakan Z, Gardner-Sood P, et al. Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT). BMC Psychiatry 2017;17:413.
Heslin M, Patel A, Stahl D, Gardner-Sood P, Mushore M, Smith S, et al. Randomised controlled trial to improve health and reduce substance use in established psychosis (IMPaCT): cost-effectiveness of integrated psychosocial health promotion. BMC Psychiatry 2017;17:407.
Harrison RNS, Gaughran F, Murray RM, Lee SH, Cano JP, Dempster D, et al. Development of multivariable models to predict change in body mass index within a clinical trial population of psychotic individuals. Sci Rep 2017;7:14738.
Onwumere J, Shiers D, Gaughran G. Physical health problems in psychosis: is it time to consider the views of family carers? Front Psychiatry 2018;9:668.
Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and position statement from the European Psychiatric Association (EPA), supported by the International Organisation of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry 2018;54:124–44.
Westman J, Eberhard J, Gaughran FP, Lundin L, Stenmark R, Edman G, et al. Outcome of a psychosocial health promotion intervention aimed at improving physical health and reducing alcohol use in patients with schizophrenia and psychotic disorders (MINT). Schizophr Res 2019;208:138–44.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334-41. https://doi.org/10.1001/jamapsychiatry.2014.2502.
- Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004;161:1334-49. https://doi.org/10.1176/appi.ajp.161.8.1334.
- Yasamy MT, Cross A, McDaniell E, Saxena S. World Mental Health Day 2014: Living with Schizophrenia. Geneva: WHO; 2014.
- World Health Organization (WHO) . Comprehensive Mental Health Action Plan 2013–20 2013.
- Strassnig M, Brar JS, Ganguli R. Body mass index and quality of life in community-dwelling patients with schizophrenia. Schizophr Res 2003;62:73-6. https://doi.org/10.1016/S0920-9964(02)00441-3.
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med 1999;29:697-701. https://doi.org/10.1017/s0033291798008186.
- McCreadie RG. Scottish Schizophrenia Lifestyle Group . Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry 2003;183:534-9. https://doi.org/10.1192/03-162.
- Bushe C, Haddad P, Peveler R, Pendlebury J. The role of lifestyle interventions and weight management in schizophrenia. J Psychopharmacol 2005;19:28-35. https://doi.org/10.1177/0269881105058682.
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009;374:620-7. https://doi.org/10.1016/S0140-6736(09)60742-X.
- Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 2011;168:603-9. https://doi.org/10.1176/appi.ajp.2011.10081224.
- Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 2011;68:609-16. https://doi.org/10.1001/archgenpsychiatry.2011.2.
- Howes OD, Gaughran FP, Amiel SA, Murray RM, Pilowsky LS. The effect of clozapine on factors controlling glucose homeostasis. J Clin Psychiatry 2004;65:1352-5. https://doi.org/10.4088/JCP.v65n1009.
- International Diabetes Federation (IDF) . IDF Consensus Worldwide Definition Of The Metabolic Syndrome n.d. www.idf.org/metabolic-syndrome (accessed September 2016).
- Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 2015;14:339-47. https://doi.org/10.1002/wps.20252.
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32. https://doi.org/10.1016/j.schres.2005.07.014.
- Citrome L, Yeomans D. Do guidelines for severe mental illness promote physical health and well-being?. J Psychopharmacol 2005;19:102-9. https://doi.org/10.1177/0269881105059505.
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 2005;80:45-53. https://doi.org/10.1016/j.schres.2005.08.010.
- Hägg S, Lindblom Y, Mjörndal T, Adolfsson R. High prevalence of the metabolic syndrome among a Swedish cohort of patients with schizophrenia. Int Clin Psychopharmacol 2006;21:93-8. https://doi.org/10.1097/01.yic.0000188215.84784.17.
- Zhang Z-J, Yao Z-J, Liu W, Fang Q, Reynolds GP. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Br J Psychiatry 2003;184:58-62. https://doi.org/10.1192/bjp.184.1.58.
- Kendrick T. Cardiovascular and respiratory risk factors and symptoms among general practice patients with long-term mental illness. Br J Psychiatry 1996;169:733-9. https://doi.org/10.1192/bjp.169.6.733.
- Lawrence DM, Holman CD, Jablensky AV, Hobbs MS. Death rate from ischaemic heart disease in Western Australian psychiatric patients 1980–1998. Br J Psychiatry 2003;182:31-6. https://doi.org/10.1192/bjp.182.1.31.
- Paton C, Esop R, Young C, Taylor D. Obesity, dyslipidaemias and smoking in an inpatient population treated with antipsychotic drugs. Acta Psychiatr Scand 2004;110:299-305. https://doi.org/10.1111/j.1600-0447.2004.00372.x.
- Cormac I. Improving the physical health of long-stay psychiatric in-patients. Adv Psychiatr Treat 2004;10:107-15. https://doi.org/10.1192/apt.10.2.107.
- Royal College of Psychiatrists . Report of the National Audit of Schizophrenia (NAS) 2012 2012.
- TNS Healthcare . Omnibus Survey Conducted Amongst 209 UK General Practitioners 2005. https://books.google.co.uk/books/about/Running_on_Empty.html?id=xvkwngAACAAJ&redir_esc=y (accessed 6 December 2019).
- Royal College of Psychiatrists . Report of the Second Round of the National Audit of Schizophrenia (NAS) 2014 2014.
- Vancampfort D, Correll CU, Galling B, Probst M, De Hert M, Ward PB, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry 2016;15:166-74. https://doi.org/10.1002/wps.20309.
- Kreyenbuhl J, Dickerson FB, Medoff DR, Brown CH, Goldberg RW, Fang L, et al. Extent and management of cardiovascular risk factors in patients with type 2 diabetes and serious mental illness. J Nerv Ment Dis 2006;194:404-10. https://doi.org/10.1097/01.nmd.0000221177.51089.7d.
- Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006;86:15-22. https://doi.org/10.1016/j.schres.2006.06.026.
- Weiss AP, Henderson DC, Weilburg JB, Goff DC, Meigs JB, Cagliero E, et al. Treatment of cardiac risk factors among patients with schizophrenia and diabetes. Psychiatr Serv 2006;57:1145-52. https://doi.org/10.1176/ps.2006.57.8.1145.
- Laursen TM, Mortensen PB, MacCabe JH, Cohen D, Gasse C. Cardiovascular drug use and mortality in patients with schizophrenia or bipolar disorder: a Danish population-based study. Psychol Med 2014;44:1625-37. https://doi.org/10.1017/S003329171300216X.
- Das-Munshi J, Ashworth M, Gaughran F, Hull S, Morgan C, Nazroo J, et al. Ethnicity and cardiovascular health inequalities in people with severe mental illnesses: protocol for the E-CHASM study. Soc Psychiatry Psychiatr Epidemiol 2016;51:627-38. https://doi.org/10.1007/s00127-016-1185-8.
- Fearon P, Kirkbride JB, Morgan C, Dazzan P, Morgan K, Lloyd T, et al. Incidence of schizophrenia and other psychoses in ethnic minority groups: results from the MRC AESOP Study. Psychol Med 2006;36:1541-50. https://doi.org/10.1017/S0033291706008774.
- Forouhi NG, Merrick D, Goyder E, Ferguson BA, Abbas J, Lachowycz K, et al. Diabetes prevalence in England, 2001 – estimates from an epidemiological model. Diabet Med 2006;23:189-97. https://doi.org/10.1111/j.1464-5491.2005.01787.x.
- Health and Social Care Information Centre . Statistics on Smoking: England 2016 2016.
- Health and Social Care Information Centre . Statistics on Drug Misuse: England 2014 2014.
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007;370:319-28. https://doi.org/10.1016/S0140-6736(07)61162-3.
- Grech A, Van Os J, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. Eur Psychiatry 2005;20:349-53. https://doi.org/10.1016/j.eurpsy.2004.09.013.
- Large M, Mullin K, Gupta P, Harris A, Nielssen O. Systematic meta-analysis of outcomes associated with psychosis and co-morbid substance use. Aust N Z J Psychiatry 2014;48:418-32. https://doi.org/10.1177/0004867414525838.
- Mills JH. Cannabis Britannica: Empire, Trade, And Prohibition 1800–1928. Oxford: Oxford University Press; 2003.
- Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet 1987;2:1483-6. https://doi.org/10.1016/S0140-6736(87)92620-1.
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7374.1199.
- Degenhardt L, Hall W, Lynskey M. Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend 2003;71:37-48. https://doi.org/10.1016/S0376-8716(03)00064-4.
- Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 2004;184:110-17. https://doi.org/10.1192/bjp.184.2.110.
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol 2002;156:319-27. https://doi.org/10.1093/aje/kwf043.
- Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med 2003;33:15-21. https://doi.org/10.1017/s0033291702006402.
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull 2005;31:608-12. https://doi.org/10.1093/schbul/sbi027.
- D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry 2005;57:594-608. https://doi.org/10.1016/j.biopsych.2004.12.006.
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O’Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry 2009;66:442-51. https://doi.org/10.1001/archgenpsychiatry.2009.17.
- Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Prata D, et al. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of δ-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry 2012;17:1152-5. https://doi.org/10.1038/mp.2011.187.
- van Os J, Kapur S. Psychosis: from diagnosis to syndrome. Ned Tijdschr Geneeskd 2010;154.
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, et al. Neuroimaging predictors of transition to psychosis – a systematic review and meta-analysis. Neurosci Biobehav Rev 2010;34:1207-22. https://doi.org/10.1016/j.neubiorev.2010.01.016.
- Stone JM. Glutamatergic antipsychotic drugs: a new dawn in the treatment of schizophrenia?. Ther Adv Psychopharmacol 2011;1:5-18. https://doi.org/10.1177/2045125311400779.
- European Monitoring Centre for Drugs and Drug Addiction . Annual Report 2011: The State of the Drugs Problem in Europe 2011.
- European Monitoring Centre for Drugs and Drug Addiction . European Drug Report 2015: Trends and Developments 2015.
- Sevy S, Robinson DG, Holloway S, Alvir JM, Woerner MG, Bilder R, et al. Correlates of substance misuse in patients with first-episode schizophrenia and schizoaffective disorder. Acta Psychiatr Scand 2001;104:367-74. https://doi.org/10.1111/j.1600-0447.2001.00452.x.
- Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull 2010;36:1115-30. https://doi.org/10.1093/schbul/sbp031.
- Hall WD, Pacula R. Cannabis Use and Dependence: Public Health and Public Policy. Cambridge: Cambridge University Press; 2010.
- Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use?. Addiction 2015;110:19-35. https://doi.org/10.1111/add.12703.
- Hall W, Weier M. Assessing the public health impacts of legalizing recreational cannabis use in the USA. Clin Pharmacol Ther 2015;97:607-15. https://doi.org/10.1002/cpt.110.
- Boydell J, van Os J, Caspi A, Kennedy N, Giouroukou E, Fearon P, et al. Trends in cannabis use prior to first presentation with schizophrenia, in South-East London between 1965 and 1999. Psychol Med 2006;36:1441-6. https://doi.org/10.1017/S0033291706008440.
- González-Pinto A, Alberich S, Barbeito S, Gutierrez M, Vega P, Ibáñez B, et al. Cannabis and first-episode psychosis: different long-term outcomes depending on continued or discontinued use. Schizophr Bull 2011;37:631-9. https://doi.org/10.1093/schbul/sbp126.
- Schimmelmann BG, Conus P, Cotton S, Kupferschmid S, McGorry PD, Lambert M. Prevalence and impact of cannabis use disorders in adolescents with early onset first episode psychosis. Eur Psychiatry 2012;27:463-9. https://doi.org/10.1016/j.eurpsy.2011.03.001.
- Myles H, Myles N, Large M. Cannabis use in first episode psychosis: meta-analysis of prevalence, and the time course of initiation and continued use. Aust N Z J Psychiatry 2016;50:208-19. https://doi.org/10.1177/0004867415599846.
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry 2011;68:555-61. https://doi.org/10.1001/archgenpsychiatry.2011.5.
- Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull 2014;40:1509-17. https://doi.org/10.1093/schbul/sbt181.
- Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry 2015;2:233-8. https://doi.org/10.1016/S2215-0366(14)00117-5.
- Smith N. High potency cannabis: the forgotten variable. Addiction 2005;100:1558-60. https://doi.org/10.1111/j.1360-0443.2005.01295.x.
- Di Forti M, Quattrone D, Freeman TP, Tripoli G, Gayer-Anderson C, Quigley H, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry 2019;6:427-36. https://doi.org/10.1016/S2215-0366(19)30048-3.
- Kuepper R, van Os J, Lieb R, Wittchen HU, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ 2011;342. https://doi.org/10.1136/bmj.d738.
- Schofield D, Tennant C, Nash L, Degenhardt L, Cornish A, Hobbs C, et al. Reasons for cannabis use in psychosis. Aust N Z J Psychiatry 2006;40:570-4. https://doi.org/10.1080/j.1440-1614.2006.01840.x.
- Barkus EJ, Stirling J, Hopkins RS, Lewis S. Cannabis-induced psychosis-like experiences are associated with high schizotypy. Psychopathology 2006;39:175-8. https://doi.org/10.1159/000092678.
- Stirling J, Barkus EJ, Nabosi L, Irshad S, Roemer G, Schreudergoidheijt B, et al. Cannabis-induced psychotic-like experiences are predicted by high schizotypy. Confirmation of preliminary results in a large cohort. Psychopathology 2008;41:371-8. https://doi.org/10.1159/000155215.
- Anglin DM, Corcoran CM, Brown AS, Chen H, Lighty Q, Brook JS, et al. Early cannabis use and schizotypal personality disorder symptoms from adolescence to middle adulthood. Schizophr Res 2012;137:45-9. https://doi.org/10.1016/j.schres.2012.01.019.
- Malcolm CP, Picchioni MM, DiForti M, Sugranyes G, Cooke E, Joseph C, et al. Pre-morbid conduct disorder symptoms are associated with cannabis use among individuals with a first episode of psychosis. Schizophr Res 2011;126:81-6. https://doi.org/10.1016/j.schres.2010.11.025.
- van Winkel R, Kuepper R. Epidemiological, neurobiological, and genetic clues to the mechanisms linking cannabis use to risk for nonaffective psychosis. Annu Rev Clin Psychol 2014;10:767-91. https://doi.org/10.1146/annurev-clinpsy-032813-153631.
- Hollis C, Groom MJ, Das D, Calton T, Bates AT, Andrews HK, et al. Different psychological effects of cannabis use in adolescents at genetic high risk for schizophrenia and with attention deficit/hyperactivity disorder (ADHD). Schizophr Res 2008;105:216-23. https://doi.org/10.1016/j.schres.2008.07.010.
- Genetic Risk and Outcome in Psychosis (GROUP) Investigators . Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient-sibling and sibling-control pairs. Arch Gen Psychiatry 2011;68:138-47. https://doi.org/10.1001/archgenpsychiatry.2010.132.
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 2005;57:1117-27. https://doi.org/10.1016/j.biopsych.2005.01.026.
- Zammit S, Spurlock G, Williams H, Norton N, Williams N, O’Donovan MC, et al. Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use. Br J Psychiatry 2007;191:402-7. https://doi.org/10.1192/bjp.bp.107.036129.
- Decoster J, van Os J, Myin-Germeys I, De Hert M, van Winkel R. Genetic variation underlying psychosis-inducing effects of cannabis: critical review and future directions. Curr Pharm Des 2012;18:5015-23. https://doi.org/10.2174/138161212802884591.
- van Winkel R. Genetic Risk and Outcome of Psychosis (GROUP) Investigators . Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry 2011;68:148-57. https://doi.org/10.1001/archgenpsychiatry.2010.152.
- Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry 2012;72:811-16. https://doi.org/10.1016/j.biopsych.2012.06.020.
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 2006;66:234-46. https://doi.org/10.1016/j.mehy.2005.08.026.
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 2006;147:163-71. https://doi.org/10.1038/sj.bjp.0706406.
- Taylor AH, Amoako AA, Bambang K, Karasu T, Gebeh A, Lam PM, et al. Endocannabinoids and pregnancy. Clin Chim Acta 2010;411:921-30. https://doi.org/10.1016/j.cca.2010.03.012.
- Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci 1999;40:2442-8.
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 2002;54:161-202. https://doi.org/10.1124/pr.54.2.161.
- Gazzerro P, Caruso MG, Notarnicola M, Misciagna G, Guerra V, Laezza C, et al. Association between cannabinoid type-1 receptor polymorphism and body mass index in a southern Italian population. Int J Obes 2007;31:908-12. https://doi.org/10.1038/sj.ijo.0803510.
- Chen J, Matias I, Dinh T, Lu T, Venezia S, Nieves A, et al. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun 2005;330:1062-7. https://doi.org/10.1016/j.bbrc.2005.03.095.
- Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol 2014;46:65-81. https://doi.org/10.1007/s12016-013-8374-y.
- Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA 2012;307:173-81. https://doi.org/10.1001/jama.2011.1961.
- Joshi M, Joshi A, Bartter T. Marijuana and lung diseases. Curr Opin Pulm Med 2014;20:173-9. https://doi.org/10.1097/MCP.0000000000000026.
- Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C, et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci 2010;30:6273-81. https://doi.org/10.1523/JNEUROSCI.2648-09.2010.
- Silvestri C, Ligresti A, Di Marzo V. Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocr Metab Disord 2011;12:153-62. https://doi.org/10.1007/s11154-011-9167-3.
- Ngueta G, Bélanger RE, Laouan-Sidi EA, Lucas M. Cannabis use in relation to obesity and insulin resistance in the Inuit population. Obesity 2015;23:290-5. https://doi.org/10.1002/oby.20973.
- Sansone RA, Sansone LA. Marijuana and body weight. Innov Clin Neurosci 2014;11:50-4.
- Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol 2011;174:929-33. https://doi.org/10.1093/aje/kwr200.
- Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 2013;126:583-9. https://doi.org/10.1016/j.amjmed.2013.03.002.
- Meier MH, Caspi A, Cerdá M, Hancox RJ, Harrington H, Houts R, et al. Associations between cannabis use and physical health problems in early midlife: a longitudinal comparison of persistent cannabis vs tobacco users. JAMA Psychiatry 2016;73:731-40. https://doi.org/10.1001/jamapsychiatry.2016.0637.
- Bruins J, Pijnenborg MG, Bartels-Velthuis AA, Visser E, van den Heuvel ER, Bruggeman R, et al. Cannabis use in people with severe mental illness: the association with physical and mental health – a cohort study. A Pharmacotherapy Monitoring and Outcome Survey study. J Psychopharmacol 2016;30:354-62. https://doi.org/10.1177/0269881116631652.
- Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O’Brien R, et al. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. Am J Psychiatry 2001;158:1706-13. https://doi.org/10.1176/appi.ajp.158.10.1706.
- Haddock G, Barrowclough C, Tarrier N, Moring J, O’Brien R, Schofield N, et al. Cognitive-behavioural therapy and motivational intervention for schizophrenia and substance misuse. 18-month outcomes of a randomised controlled trial. Br J Psychiatry 2003;183:418-26. https://doi.org/10.1192/bjp.183.5.418.
- Department of Health and Social Care . National Service Framework for Mental Health. Modern Standards and Service Models 1999.
- National Institute for Health and Care Excellence . Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care. Clinical Guideline 1 2002.
- Department of Health and Social Care . Choosing Health: Making Healthy Choices Easier 2004.
- Disability Rights Commission . Equal Treatment: Closing the Gap 2006.
- Department of Health and Social Care . No Health Without Mental Health: A Cross-Government Mental Health Outcomes Strategy for People of All Ages 2011.
- Local Government and Care Partnership Directorate . Closing the Gap: Priorities for Essential Change in Mental Health n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/281250/Closing_the_gap_V2_-_17_Feb_2014.pdf (accessed September 2016).
- Royal College of Psychiatrists . Whole-Person Care: From Rhetoric to Reality 2013.
- BMA Board of Science . Recognising the Importance of Physical Health in Mental Health and Intellectual Disability: Achieving Parity of Outcomes 2014. www.google.com/search?client=safari&rls=en&q=BMA+Board+of+Science.+Recognising+the+Importance+of+Physical+Health+in+Mental+Health+and+Intellectual+Disability:+Achieving+Parity+of+Outcomes.+2014&ie=UTF-8&oe=UTF-8 (accessed 6 December 2019).
- Docherty M, Thornicroft G. Specialist mental health services in England in 2014: overview of funding, access and levels of care. Int J Ment Health Syst 2015;9.
- London Health Commission . Better Health for London 2014. www.londonhealthcommission.org.uk/wp-content/uploads/London-Health-Commission_Better-Health-for-London.pdf (accessed 1 September 2016).
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems 2016. https://icd.who.int/browse10/2016/en (accessed 3 December 2019).
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. https://doi.org/10.1093/schbul/13.2.261.
- Guy W, Rush John A. Task Force for the Handbook of Psychiatric Measures. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000.
- Richard CW, Hall MD. Global assessment of functioning: a modified scale. Psychosomatics 1995;36:267-75. https://doi.org/10.1016/S0033-3182(95)71666-8.
- Craddock M, Asherson P, Owen MJ, Williams J, McGuffin P, Farmer AE. Concurrent validity of the OPCRIT diagnostic system. Comparison of OPCRIT diagnoses with consensus best-estimate lifetime diagnoses. Br J Psychiatry 1996;169:58-63. https://doi.org/10.1192/bjp.169.1.58.
- Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res 1990;3:247-51. https://doi.org/10.1016/0920-9964(90)90005-R.
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 1978;3:235-41. https://doi.org/10.1016/0306-4603(78)90024-2.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Colizzi M, Carra E, Fraietta S, Lally J, Quattrone D, Bonaccorso S, et al. Substance use, medication adherence and outcome one year following a first episode of psychosis. Schizophr Res 2016;170:311-17. https://doi.org/10.1016/j.schres.2015.11.016.
- Gaughran F, Stahl D, Stringer D, Hopkins D, Atakan Z, Greenwood K, et al. Effect of lifestyle, medication and ethnicity on cardiometabolic risk in the year following the first episode of psychosis: prospective cohort study. Br J Psychiatry 2019;215:712-19. https://doi.org/10.1192/bjp.2019.159.
- Kolliakou A, Castle D, Sallis H, Joseph C, O’Connor J, Wiffen B, et al. Reasons for cannabis use in first-episode psychosis: does strength of endorsement change over 12 months?. Eur Psychiatry 2015;30:152-9. https://doi.org/10.1016/j.eurpsy.2014.10.007.
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 2014;71:1350-63. https://doi.org/10.1001/jamapsychiatry.2014.1314.
- Russell A, Ciufolini S, Gardner-Sood P, Bonaccorso S, Gaughran F, Dazzan P, et al. Inflammation and metabolic changes in first episode psychosis: preliminary results from a longitudinal study. Brain Behav Immun 2015;49:25-9. https://doi.org/10.1016/j.bbi.2015.06.004.
- O’Brien C, Gardner-Sood P, Corlett SK, Ismail K, Smith S, Atakan Z, et al. Provision of health promotion programmes to people with serious mental illness: a mapping exercise of four South London boroughs. J Psychiatr Ment Health Nurs 2014;21:121-7. https://doi.org/10.1111/jpm.12057.
- Department of Health and Social Care . Choosing Health: Supporting The Physical Needs Of People With Severe Mental Illness – Commissioning Framework 2006.
- Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry 2012;25:83-8. https://doi.org/10.1097/YCO.0b013e32835035ca.
- Behan C, Doyle R, Masterson S, Shiers D, Clarke M. A double-edged sword: review of the interplay between physical health and mental health. Ir J Med Sci 2015;184:107-12. https://doi.org/10.1007/s11845-014-1205-1.
- Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease – a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry 2014;5. https://doi.org/10.3389/fpsyt.2014.00137.
- Foley DL, Mackinnon A, Watts GF, Shaw JE, Magliano DJ, Castle DJ, et al. Cardiometabolic risk indicators that distinguish adults with psychosis from the general population, by age and gender. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0082606.
- Okereke OI, Folsom DP. Medical comorbidity in geriatric psychiatry. Am J Geriatr Psychiatry 2005;13:177-9. https://doi.org/10.1097/00019442-200503000-00001.
- Iwata K, Strydom A, Osborn D. Insight and other predictors of physical examination refusal in psychotic illness. J Ment Health 2011;20:319-27. https://doi.org/10.3109/09638237.2011.556158.
- Nery FG, Miranda-Scippa A, Nery-Fernandes F, Kapczinski F, Lafer B. Prevalence and clinical correlates of alcohol use disorders among bipolar disorder patients: results from the Brazilian Bipolar Research Network. Compr Psychiatry 2014;55:1116-21. https://doi.org/10.1016/j.comppsych.2014.02.006.
- Baker KD, Lubman DI, Cosgrave EM, Killackey EJ, Yuen HP, Hides L, et al. Impact of co-occurring substance use on 6 month outcomes for young people seeking mental health treatment. Aust N Z J Psychiatry 2007;41:896-902. https://doi.org/10.1080/00048670701634986.
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, et al. A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. Am J Psychiatry 2006;163:1934-42. https://doi.org/10.1176/ajp.2006.163.11.1934.
- Alvarez-Jiménez M, Martínez-García O, Pérez-Iglesias R, Ramírez ML, Vázquez-Barquero JL, Crespo-Facorro B. Prevention of antipsychotic-induced weight gain with early behavioural intervention in first-episode psychosis: 2-year results of a randomized controlled trial. Schizophr Res 2010;116:16-9. https://doi.org/10.1016/j.schres.2009.10.012.
- Skrinar GS, Huxley NA, Hutchinson DS, Menninger E, Glew P. The role of a fitness intervention on people with serious psychiatric disabilities. Psychiatr Rehabil J 2005;29:122-7. https://doi.org/10.2975/29.2005.122.127.
- Ohlsen RI, Peacock G, Smith S. Developing a service to monitor and improve physical health in people with serious mental illness. J Psychiatr Ment Health Nurs 2005;12:614-19. https://doi.org/10.1111/j.1365-2850.2005.00884.x.
- Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med 2015;45:1343-61. https://doi.org/10.1017/S0033291714003110.
- James W, Preston NJ, Koh G, Spencer C, Kisely SR, Castle DJ. A group intervention which assists patients with dual diagnosis reduce their drug use: a randomized controlled trial. Psychol Med 2004;34:983-90. https://doi.org/10.1017/s0033291703001648.
- Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ 2010;341. https://doi.org/10.1136/bmj.c6325.
- Reininghaus U, Dutta R, Dazzan P, Doody GA, Fearon P, Lappin J, et al. Mortality in schizophrenia and other psychoses: a 10-year follow-up of the ÆSOP first-episode cohort. Schizophr Bull 2015;41:664-73. https://doi.org/10.1093/schbul/sbu138.
- Morgan VA, McGrath JJ, Jablensky A, Badcock JC, Waterreus A, Bush R, et al. Psychosis prevalence and physical, metabolic and cognitive co-morbidity: data from the second Australian national survey of psychosis. Psychol Med 2014;44:2163-76. https://doi.org/10.1017/S0033291713002973.
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012;36:427-40. https://doi.org/10.1007/s10608-012-9476-1.
- Gould RA, Mueser KT, Bolton E, Mays V, Goff D. Cognitive therapy for psychosis in schizophrenia: an effect size analysis. Schizophr Res 2001;48:335-42. https://doi.org/10.1016/S0920-9964(00)00145-6.
- Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523-37. https://doi.org/10.1093/schbul/sbm114.
- Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis 2001;189:278-87. https://doi.org/10.1097/00005053-200105000-00002.
- Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry 2014;171:523-38. https://doi.org/10.1176/appi.ajp.2013.13081159.
- Stroup S, Appelbaum P, Swartz M, Patel M, Davis S, Jeste D, et al. Decision-making capacity for research participation among individuals in the CATIE schizophrenia trial. Schizophr Res 2005;80:1-8. https://doi.org/10.1016/j.schres.2005.08.007.
- Miller SR, Rollnick S. Motivational Interviewing. New York, NY: Guildford Press; 1991.
- Wong EM, Cheng MM. Effects of motivational interviewing to promote weight loss in obese children. J Clin Nurs 2013;22:2519-30. https://doi.org/10.1111/jocn.12098.
- Wang D, Li X yun, Zhang L na, Zhou L, Zhang K jin. Effects of motivational interviewing on lifestyle modification and diabetes prevention in adults with pre-diabetes. Diabetes Res Clin Pract 2015;23.
- McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-site cluster randomized trial. Addiction 2004;99:39-52. https://doi.org/10.1111/j.1360-0443.2004.00564.x.
- Martino S, Carroll K, Kostas D, Perkins J, Rounsaville B. Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat 2002;23:297-308. https://doi.org/10.1016/S0740-5472(02)00295-7.
- Caspi A, Reichenberg A, Weiser M, Rabinowitz J, Shmushkevich M, Lubin G, et al. Premorbid behavioral and intellectual functioning in schizophrenia patients with poor response to treatment with antipsychotic drugs. Schizophr Res 2007;94:45-9. https://doi.org/10.1016/j.schres.2007.04.007.
- Cooke MA, Peters ER, Kuipers E, Kumari V. Disease, deficit or denial? Models of poor insight in psychosis. Acta Psychiatr Scand 2005;112:4-17.
- Cooke MA, Peters ER, Greenwood KE, Fisher PL, Kumari V, Kuipers E. Insight in psychosis: influence of cognitive ability and self-esteem. Br J Psychiatry 2007;191:234-7. https://doi.org/10.1192/bjp.bp.106.024653.
- Murray GK, Clark L, Corlett PR, Blackwell AD, Cools R, Jones PB, et al. Incentive motivation in first-episode psychosis: a behavioural study. BMC Psychiatry 2008;8. https://doi.org/10.1186/1471-244X-8-34.
- Barrowclough C, Haddock G, Beardmore R, Conrod P, Craig T, Davies L, et al. Evaluating integrated MI and CBT for people with psychosis and substance misuse: recruitment, retention and sample characteristics of the MIDAS trial. Addict Behav 2009;34:859-66. https://doi.org/10.1016/j.addbeh.2009.03.007.
- Drake RE, O’Neal EL, Wallach MA. A systematic review of psychosocial research on psychosocial interventions for people with co-occurring severe mental and substance use disorders. J Subst Abuse Treat 2008;34:123-38. https://doi.org/10.1016/j.jsat.2007.01.011.
- Freeman D, Pugh K, Dunn G, Evans N, Sheaves B, Waite F, et al. An early Phase II randomised controlled trial testing the effect on persecutory delusions of using CBT to reduce negative cognitions about the self: the potential benefits of enhancing self confidence. Schizophr Res 2014;160:186-92. https://doi.org/10.1016/j.schres.2014.10.038.
- Papanastasiou E. Interventions for the metabolic syndrome in schizophrenia: a review. Ther Adv Endocrinol Metab 2012;3:141-62. https://doi.org/10.1177/2042018812458697.
- Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O, Ramirez-Bonilla ML, Alvarez-Jimenez M, Pelayo-Teran JM, et al. Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naive population. Schizophr Res 2008;99:13-22. https://doi.org/10.1016/j.schres.2007.10.022.
- Baker AL, Richmond R, Kay-Lambkin FJ, Filia SL, Castle D, Williams JM, et al. Randomized controlled trial of a healthy lifestyle intervention among smokers with psychotic disorders. Nicotine Tob Res 2015;17:946-54. https://doi.org/10.1093/ntr/ntv039.
- Medical Research Council (MRC) . A Framework for the Development and Evaluation of Randomised Controlled Trials for Complex Interventions to Improve Health 2000.
- Medical Research Council (MRC) . Developing and Evaluating Complex Interventions: New Guidance 2006.
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: getting started and moving on from stage I. Clin Psychol Sci Pract 2001;8:133-42. https://doi.org/10.1093/clipsy.8.2.133.
- DiClemente CC, Prochaska JO. Self-change and therapy change of smoking behavior: a comparison of processes of change in cessation and maintenance. Addict Behav 1982;7:133-42. https://doi.org/10.1016/0306-4603(82)90038-7.
- Michie S. Designing and implementing behaviour change interventions to improve population health. J Health Serv Res Policy 2008;13:64-9. https://doi.org/10.1258/jhsrp.2008.008014.
- Lorencatto F, West R, Stavri Z, Michie S. How well is intervention content described in published reports of smoking cessation interventions?. Nicotine Tob Res 2013;15:1273-82. https://doi.org/10.1093/ntr/nts266.
- Jones J, Hunter D. Qualitative research: consensus methods for medical and health services research. BMJ 1995;311:376-80. https://doi.org/10.1136/bmj.311.7001.376.
- Smith S, Yeomans D, Bushe CJ, Eriksson C, Harrison T, Holmes R, et al. A well-being programme in severe mental illness. Baseline findings in a UK cohort. Int J Clin Pract 2007;61:1971-8. https://doi.org/10.1111/j.1742-1241.2007.01605.x.
- Gaughran F, Stahl D, Ismail K, Atakan Z, Lally J, Gardner-Sood P, et al. Improving physical health and reducing substance use in psychosis – randomised control trial (IMPACT RCT): study protocol for a cluster randomised controlled trial. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-263.
- Gaughran F, Stahl D, Ismail K, Greenwood K, Atakan Z, Gardner-Sood P, et al. Randomised control trial of the effectiveness of an integrated psychosocial health promotion intervention aimed at improving health and reducing substance use in established psychosis (IMPaCT). BMC Psychiatry 2017;17. https://doi.org/10.1186/s12888-017-1571-0.
- Heslin M, Patel A, Stahl D, Gardner-Sood P, Mushore M, Smith S, et al. Randomised controlled trial to improve health and reduce substance use in established psychosis (IMPaCT): cost-effectiveness of integrated psychosocial health promotion. BMC Psychiatry 2017;17. https://doi.org/10.1186/s12888-017-1570-1.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Roe L, Strong C, Whiteside C, Neil A, Mant D. Dietary intervention in primary care: validity of the DINE method for diet assessment. Fam Pract 1994;11:375-81. https://doi.org/10.1093/fampra/11.4.375.
- Faulkner G, Cohn T, Remington G. Validation of a physical activity assessment tool for individuals with schizophrenia. Schizophr Res 2006;82:225-31. https://doi.org/10.1016/j.schres.2005.10.020.
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9. https://doi.org/10.1192/bjp.134.4.382.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95. https://doi.org/10.1191/0962280205sm403oa.
- Gardner-Sood P, Lally J, Smith S, Atakan Z, Ismail K, Greenwood KE, et al. Cardiovascular risk factors and metabolic syndrome in people with established psychotic illnesses: baseline data from the IMPaCT randomized controlled trial. Psychol Med 2015;45:2619-29. https://doi.org/10.1017/S0033291715000562.
- Macdonald A, Adamis D, Craig T, Murray R. Continuity of care and clinical outcomes in the community for people with severe mental illness. Br J Psychiatry 2019;214:273-8. https://doi.org/10.1192/bjp.2018.261.
- Slade M, Bird V, Clarke E, Le Boutillier C, McCrone P, Macpherson R, et al. Supporting recovery in patients with psychosis through care by community-based adult mental health teams (REFOCUS): a multisite, cluster, randomised, controlled trial. Lancet Psychiatry 2015;2:503-14. https://doi.org/10.1016/S2215-0366(15)00086-3.
- White J, Lucas J, Swift L, Barton GR, Johnson H, Irvine L, et al. Nurse-facilitated health checks for persons with severe mental illness: a cluster-randomized controlled trial. Psychiatr Serv 2018;69:601-4. https://doi.org/10.1176/appi.ps.201700258.
- Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013;368:1594-602. https://doi.org/10.1056/NEJMoa1214530.
- Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Jue K, Wolfe RS, et al. Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv 2013;64:729-36. https://doi.org/10.1176/appi.ps.003622012.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for Euroqol: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Department of Health and Social Care . Reference Costs 2010–11 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140 (accessed 16 January 2013).
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Joint Formulary Committee . British National Formulary 2012.
- HM Treasury . The Green Book: Appraisal and Evaluation in Central Government 2003.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Calsyn RJ, Allen G, Morse GA, Smith R, Tempelhoff B. Can you trust self-report data provided by homeless mentally ill individuals?. Eval Rev 1993;17:353-66. https://doi.org/10.1177/0193841X9301700306.
- Goldberg RW, Seybolt DC, Lehman A. Reliable self-report of health service use by individuals with serious mental illness. Psychiatr Serv 2002;53:879-81. https://doi.org/10.1176/appi.ps.53.7.879.
- Brazier J, Connell J, Papaioannou D, Mukuria C, Mulhern B, Peasgood T, et al. A systematic review, psychometric analysis and qualitative assessment of generic preference-based measures of health in mental health populations and the estimation of mapping functions from widely used specific measures. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18340.
- Frayne SM, Halanych JH, Miller DR, Wang F, Lin H, Pogach L, et al. Disparities in diabetes care: impact of mental illness. Arch Intern Med 2005;165:2631-8. https://doi.org/10.1001/archinte.165.22.2631.
- Vinogradova Y, Coupland C, Hippisley-Cox J, Whyte S, Penny C. Effects of severe mental illness on survival of people with diabetes. Br J Psychiatry 2010;197:272-7. https://doi.org/10.1192/bjp.bp.109.074674.
- Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123-32. https://doi.org/10.7326/M14-1651.
- Williams J, Stubbs B, Gaughran F, Craig T. ‘Walk This Way’ – a pilot of a health coaching intervention to reduce sedentary behaviour and increase low intensity exercise in people with serious mental illness: study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1660-2.
- Stubbs B, Gardner-Sood P, Smith S, Ismail K, Greenwood K, Farmer R, et al. Sedentary behaviour is associated with elevated C-reactive protein levels in people with psychosis. Schizophr Res 2015;168:461-4. https://doi.org/10.1016/j.schres.2015.07.003.
- Stubbs B, Firth J, Berry A, Schuch FB, Rosenbaum S, Gaughran F, et al. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression. Schizophr Res 2016;176:431-40. https://doi.org/10.1016/j.schres.2016.05.017.
- Teasdale SB, Curtis J, Ward PB, Watkins A, Lederman O, Rosenbaum S, et al. The effectiveness of the Keeping the Body in Mind Xtend pilot lifestyle program on dietary intake in first-episode psychosis: two-year outcomes. Obes Res Clin Pract 2019;13:214-16. https://doi.org/10.1016/j.orcp.2019.02.003.
- Great Britain . Health and Social Care Act 2012 2012. https://doi.org/10.12968/eqhe.2012.1.7.5.
- Lally J, Watkins R, Nash S, Shetty H, Gardner-Sood P, Smith S, et al. The representativeness of participants with severe mental illness in a psychosocial clinical trial. Front Psychiatry 2018;9. https://doi.org/10.3389/fpsyt.2018.00654.
- Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med 2016;8:1-10.
- Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg 2010;126:2234-42. https://doi.org/10.1097/PRS.0b013e3181f44abc.
- Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry 2009;195:488-91. https://doi.org/10.1192/bjp.bp.109.064220.
- Ajnakina O, Morgan C, Gayer-Anderson C, Oduola S, Bourque F, Bramley S, et al. Only a small proportion of patients with first episode psychosis come via prodromal services: a retrospective survey of a large UK mental health programme. BMC Psychiatry 2017;17. https://doi.org/10.1186/s12888-017-1468-y.
- Crawford MJ, Jayakumar S, Lemmey SJ, Zalewska K, Patel MX, Cooper SJ, et al. Assessment and treatment of physical health problems among people with schizophrenia: national cross-sectional study. Br J Psychiatry 2014;205:473-7. https://doi.org/10.1192/bjp.bp.113.142521.
- Roick C, Fritz-Wieacker A, Matschinger H, Heider D, Schindler J, Riedel-Heller S, et al. Health habits of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol 2007;42:268-76. https://doi.org/10.1007/s00127-007-0164-5.
- Andreassen OA, Djurovic S, Thompson WK, Schork AJ, Kendler KS, O’Donovan MC, et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet 2013;92:197-209. https://doi.org/10.1016/j.ajhg.2013.01.001.
- Alkelai A, Greenbaum L, Lupoli S, Kohn Y, Sarner-Kanyas K, Ben-Asher E, et al. Association of the type 2 diabetes mellitus susceptibility gene, TCF7L2, with schizophrenia in an Arab-Israeli family sample. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0029228.
- Leonard BE, Schwarz M, Myint AM. The metabolic syndrome in schizophrenia: is inflammation a contributing cause?. J Psychopharmacol 2012;26:33-41. https://doi.org/10.1177/0269881111431622.
- Macdonald S, Halliday J, MacEwan T, Sharkey V, Farrington S, Wall S, et al. Nithsdale Schizophrenia Surveys 24: sexual dysfunction. Case-control study. Br J Psychiatry 2003;182:50-6. https://doi.org/10.1192/bjp.182.1.50.
- Raja M, Azzoni A. Sexual behavior and sexual problems among patients with severe chronic psychoses. Eur Psychiatry 2003;18:70-6. https://doi.org/10.1016/S0924-9338(03)00009-9.
- Reis-Marques T, Smith S, Bonaccorso S, Gaughran F, Kolliakou A, Dazzan P, et al. Sexual dysfunction in people with prodromal or first-episode psychosis. Br J Psychiatry 2012;201:131-6. https://doi.org/10.1192/bjp.bp.111.101220.
- Speyer H, Christian Brix Nørgaard H, Birk M, Karlsen M, Storch Jakobsen A, Pedersen K, et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry 2016;15:155-65. https://doi.org/10.1002/wps.20318.
- Holt RI, Hind D, Gossage-Worrall R, Bradburn MJ, Saxon D, McCrone P, et al. Structured lifestyle education to support weight loss for people with schizophrenia, schizoaffective disorder and first episode psychosis: the STEPWISE RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22650.
- Osborn D, Burton A, Hunter R, Marston L, Atkins L, Barnes T, et al. Clinical and cost-effectiveness of an intervention for reducing cholesterol and cardiovascular risk for people with severe mental illness in English primary care: a cluster randomised controlled trial. Lancet Psychiatry 2018;5:145-54. https://doi.org/10.1016/S2215-0366(18)30007-5.
- Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation 2011;124:2483-90. https://doi.org/10.1161/CIRCULATIONAHA.111.038422.
- Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 2005;165:2644-50. https://doi.org/10.1001/archinte.165.22.2644.
- Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, et al. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord 2009;7:305-14. https://doi.org/10.1089/met.2008.0070.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat 2005;28:19-26. https://doi.org/10.1016/j.jsat.2004.11.001.
- Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol 2004;72:1050-62. https://doi.org/10.1037/0022-006X.72.6.1050.
- Greenwood K, Smith S, Atakan Z. Impact – The Reference Guide: Improving Physical Health and Treating Substance Use in Mental Illness 2011. www.amazon.co.uk/Impact-Reference-Improving-Physical-Substance/dp/095688850X (accessed 6 December 2019).
- Madson MB, Loignon AC, Lane C. Training in motivational interviewing: a systematic review. J Subst Abuse Treat 2009;36:101-9. https://doi.org/10.1016/j.jsat.2008.05.005.
- NHS . NHS Trust Reference Cost Schedules 2010–11 n.d. www.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131113 (accessed 19 February 2013).
- Ministry of Justice . Costs Per Place and Costs Per Prisoner, 2012–13 n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/367551/cost-per-place-and-prisoner-2013-14-summary.pdf (accessed 4 April 2014).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Cancer Research UK . Music Therapy n.d. www.cancerresearchuk.org/cancer-help/about-cancer/treatment/complementary-alternative/therapies/music-therapy (accessed 16 May 2013).
- NHS Direct . NHS Direct Score Care n.d. www.nhsdirect.nhs.uk/about/∼/media/files/boardpapers/november2010/board%20scorecard%20-%20header%20sheet.ashx (accessed 10 June 2011).
- National Register of Personal Trainers . Common FAQs n.d. www.nrpt.co.uk/find/questions/index.htm#6 (accessed 26 November 2014).
- Life Coach Directory . Frequently Asked Questions n.d. www.lifecoach-directory.org.uk/content/industryfaqs.html?#islifecoachingexpensive (accessed 14 April 2014).
- Samaritans . Annual Report 2010–11 n.d. www.samaritans.org/sites/default/files/kcfinder/files/Annual%20Report%202011.pdf (accessed 16 April 2013).
- NHS Choices . Overview. Osteopathy n.d. www.nhs.uk/conditions/Osteopathy/Pages/Introduction.aspx (accessed 14 April 2014).
- Cancer Research UK . Reike n.d. www.cancerresearchuk.org/cancer-help/about-cancer/treatment/complementary-alternative/therapies/reiki?script=true (accessed 26 November 2014).
- Direct Gov . New Pension and Benefit Rates for 2012–13 n.d. http://webarchive.nationalarchives.gov.uk/20121015000000/https://www.direct.gov.uk/en/Nl1/Newsroom/DG_200634 (accessed 28 April 2014).
- Carpenter JR, Kenward MG. Randomised Controlled Trials: A Practical Guide. Health Technology 2007. https://researchonline.lshtm.ac.uk/id/eprint/4018500.
Appendix 1 Work package 1: PUMP – health economics
Background
Although there is clear evidence of the increased cardiometabolic risk among people with SMI14 and its likely consequences for long-term morbidity (diabetes, heart disease, stroke, amputation, renal failure, blindness) and care costs later in life, it is unclear whether or not there are more immediate impacts on service costs and QoL following first presentation to mental health services.
Aims and hypotheses
We aimed to investigate the relationship between metabolic factors/syndrome at first presentation to mental health services and (1) mental health hospital admission costs in the subsequent 1-year period and (2) QALYs in the subsequent 1-year period.
Method
Measures
At baseline, after giving informed, written consent, each participant was asked to complete a battery of measures. This included sociodemographics, clinical measures and health-related QoL, measured using the EQ-5D-3L. 190 Clinical measures relevant to these analyses were clinical indicators of MetS/cardiometabolic risk (obesity, triglycerides, HDL cholesterol, BP and fasting plasma glucose). 13 The EQ-5D-3L was again administered at the 12-month follow-up to enable the calculation of 1-year QALY gains.
Data on mental health care hospital admissions for the 12 months prior and 12 months following presentation for FEP were manually extracted from electronic patient record systems in each of the relevant NHS trusts using a pro forma specifically designed for the task to standardise the methodology and information that was collected. Core data included admission and discharge dates of each admission to enable the calculation of length of stay per admission, and ward type to enable more accurate estimation of costs.
Unit costs
Unit costs were applied to individual-level inpatient days according to ward type to calculate total mental health hospital admission costs per participant over 1 year. Unit costs were obtained from NHS Reference Costs for the year 2010–11 and inflated to 2011–12 prices using the Hospital and Community Health Services Pay and Prices Index. 192 We used the Reference Costs 2010–11191 because they offered more detailed cost data according to the type of mental health service, which is not offered in the 2011–12 Reference Costs. Unit costs are detailed in Table 1. All costs are reported in GBP at 2011–12 prices. Discounting was not necessary as all costs related to a 1-year period.
| Service | Unit | Unit cost (2010–11 prices)191 (£) | Inflated unit cost (2011–12 prices)192 (£) | Notes |
|---|---|---|---|---|
| Acute ward | Bed-day | 319 | 329 | MHIPA2 – adult: acute care |
| Assessment ward | Bed-day | 319 | 329 | MHIPA2 – adult: acute care |
| Accident and emergency | Bed-day | 108 | 112 | TA and EMSNA – accident and emergency services: not leading to admitted |
| Personality disorder | Bed-day | 553 | 571 | SCU44 – high-dependency secure provision: personality disorder |
| Mother and baby unit | Bed-day | 651 | 672 | MHIPMB – mother and baby units |
| Medium secure | Bed-day | 483 | 499 | SCU3 – medium-level secure services |
| Intensive assessment | Bed-day | 627 | 647 | MHIPA1 – adult: intensive care |
| Psychiatric intensive care | Bed-day | 627 | 647 | MHIPA1 – adult: intensive care |
| Low secure | Bed-day | 427 | 441 | SCU2 – low-level secure services |
| Adolescent inpatient | Bed-day | 602 | 622 | MHIPC1 – children |
| Elderly | Bed-day | 329 | 340 | MHIPE1 – elderly |
Analyses
For the purpose of univariate associations with costs and QALYs, individual metabolic factors were examined in both binary (present or not) and continuous form. Furthermore, the total number of metabolic factors present was investigated.
To estimate QALYs, we applied general population utility weights to EQ-5D-3L health state measurements at baseline and at 12 months. 124 QALY gains between baseline and 12 months were then calculated using the total area under the curve approach with linear interpolation between assessment points. 195
Data were analysed using Stata 11. Despite the skewed nature of the cost data, the mean and SDs for cost data are reported as recommended. 224 Associations with costs and QALYs were explored using the stepped approach. 225 Univariate associations of metabolic and sociodemographic variables with costs were examined using non-parametric bootstrap regressions (ordinary least squares); we conducted each regression both with and without a covariate for baseline costs. All independent variables that were associated (p < 0.1) with costs in these univariate analyses were then carried forward for inclusion in a multivariate non-parametric bootstrap regression. We examined the results of the multivariate regression to identify any variables that no longer remained significantly associated with costs (p > 0.1) and then reran the multivariate regression without those variables. Variables that did not appear significantly associated with costs in the univariate regressions were then added into the model and retained if they significantly improved the model or rejected if they did not. The same process was repeated for the analysis of associations with QALYs.
Results
Of 321 people initially consented, 28 were subsequently deemed ineligible, leaving 293 eligible participants recruited to the study.
Sample characteristics
Of the 293 participants, 190 (65%) were male and 136 (46.4%) were of white ethnic origin. The mean age was 30.6 years (SD 10.5 years). Seventy-six (31.8%) lived alone and 155 (66.2%) were unemployed.
Data completeness
A total of 274 out of 293 participants (93.5%) had full data on resource use/costs for both the 12 months prior to and the 12 months following presentation for FEP, which were gathered from electronic health records. However, fewer people had all relevant data on metabolic factors and QALYs. This resulted in 100 participants with all relevant data for the examination of associations with admission costs and 55 participants with all relevant data for the examination of associations with QALYs. Table 2 shows that the two subgroups available for analyses were similar to the full sample in terms of a range of baseline characteristics. However, both subgroups had lower admission costs at baseline than the full sample. The potential implications of missing data are explored in Discussion.
| Variable | Full sample (N = 293) | Subsample with full data for admission cost analyses (N = 100) | Subsample with full data for utility analyses (N = 54) |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 103 (35) | 40 (40) | 24 (44) |
| Male | 190 (65) | 60 (60) | 31 (57) |
| Ethnicity, n (%) | |||
| White | 136 (46) | 42 (42) | 23 (23) |
| Other | 157 (54) | 58 (58) | 32 (32) |
| Living alone (n = 239), n (%) | |||
| No | 217 (68) | 70 (74) | 40 (78) |
| Yes | 76 (32) | 25 (26) | 11 (22) |
| Employment status (n = 234), n (%) | |||
| Employed/student | 79 (34) | 29 (31) | 19 (37) |
| Unemployed | 155 (66) | 64 (69) | 32 (63) |
| Age (years), mean (SD) | 30.6 (10.5) | 29.8 (9.8) | 30.0 (10.1) |
| Baseline utility score (n = 178), mean (SD) | 0.79 (0.26) | 0.82 (0.25) | 0.83 (0.18) |
| Baseline admission costs (£) (n = 274), mean (SD) | 443 (5814) | 77 (276) | 83 (320) |
Resource use and costs
Among the 293 participants, we were able to extract data for 278 on the number of inpatient days for the year prior to FEP. Of these, 243 participants had no inpatient days prior to FEP. Of the 35 participants who had had a mental health admission, this was mostly because of a few (1–5) days admitted for symptoms during the prodromal period before a diagnosis of psychosis was assigned. Four participants had total admission days ranging between 8 and 172 days and the most likely reason for this was an evolving clinical picture before the diagnosis of psychosis became clear. The mean cost of admissions in the year prior to FEP was £443 (SD £5814, range £0–95,957).
We were able to extract data on the number of inpatient days for the year following presentation for FEP for all 279 participants. Twenty-eight participants had no admissions. The overall mean total was 63 days [SD 69 days, range 0–367 days (exceeds 365 as a result of accounting for movement between wards)]. The associated mean cost was £25,376 (SD £34,411, range £0–236,945).
The proportion of participants who had at least one admission and the mean number of inpatient days for the year prior to FEP were similar in the full sample and in the subsample who had all required data for the analysis of admission costs (i.e. they also had full metabolic and sociodemographic data) (Table 3). This was also true for the 1 year following presentation for FEP. However, the mean cost of admissions in the year prior to FEP was lower for the subsample with full data than for the full sample [£77 (SD £276) vs. £433 (SD £5814)].
| Full sample (N = 293) | Subsample with full data for admission cost analyses (N = 100) | |||
|---|---|---|---|---|
| Baseline | ||||
| Had at least one admission (%, n/valid n) | 13 | 35/278 | 12 | 12/100 |
| Mean number of days (mean, SD) | 1 | 10 | < 1 | 1 |
| Mean cost (£) (SD) | 433 | 5814 | 77 | 276 |
| Follow-up | ||||
| Had at least one admission (%, n/valid n) | 90 | 251/279 | 89 | 89/100 |
| Mean number of days (SD) | 63 | 69 | 55 | 64 |
| Mean cost (£) (SD) | 25,376 | 34,411 | 20,922 | 25,510 |
Metabolic syndrome factors and admission costs
Univariate analyses suggested that being male, obese or of non-white ethnicity was associated with higher admission costs during the 1-year follow-up (p < 0.1), whereas being older or having reduced HDL cholesterol (< 1.03 mmol/l for men and < 1.29 mmol/l for women) was associated with lower costs during the 1-year follow-up (p < 0.1) (Table 4). When these factors were carried through to a multivariate analysis, all except ethnicity remained significantly associated with costs (Table 5).
| Variable | Unadjusted coefficient | Unadjusted 95% CI | Adjusted coefficienta | Adjusted 95% CIa |
|---|---|---|---|---|
| Gender | ||||
| Female | – | – | – | – |
| Male | 11,613 | 3092 to 20,133* | 11,544 | 2861 to 20,226* |
| Ethnicity | ||||
| White | – | – | – | – |
| Other | 7990 | –1439 to 17,420* | 8437 | –1179 to 18,054* |
| Age | ||||
| –606 | –1078 to –134* | –631 | –1119 to –143* | |
| Binary MetS | ||||
| No | – | – | – | – |
| Yes | –4600 | –13,945 to 4744 | –4786 | –14,192 to 4621 |
| Binary obesity | ||||
| No | – | – | – | – |
| Yes | 15,350 | –408 to 311.09* | 15,378 | –352 to 31,108* |
| Binary high triglycerides | ||||
| No | – | – | – | – |
| Yes | –1850 | –12,742 to 9041 | –2150 | –13,106 to 8807 |
| Binary high BP | ||||
| No | – | – | – | – |
| Yes | –4224 | –13,452 to 5004 | –4642 | –14,114 to 4830 |
| Binary raised fasting glucose | ||||
| No | – | – | – | – |
| Yes | –7197 | –17,472 to 3078 | –7266 | –17,515 to 2983 |
| Binary reduced HDL cholesterol | ||||
| No | – | – | – | – |
| Yes | –9452 | –17,915 to –988* | –9524 | –18,069 to –979* |
| Triglycerides (continuous) | ||||
| 1693 | –3600 to 6985 | 1564 | –3762 to 6890 | |
| Systolic BP (continuous) | ||||
| 73 | –127 to 273 | 69 | –131 to 269 | |
| Diastolic BP (continuous) | ||||
| –26 | –486 to 434 | –41 | –510 to 427 | |
| Fasting glucose (continuous) | ||||
| –6843 | –16,718 to 3031 | –7172 | –17,302 to 2958 | |
| HDL cholesterol (continuous) | ||||
| –2466 | –12,471 to 7539 | –1992 | –12,467 to 8482 | |
| Number of metabolic factors (continuous) | ||||
| –1189 | –5322 to 2963 | –1307 | –5495 to 2880 | |
| Variable | Adjusted coefficient | SE | 95% CIa |
|---|---|---|---|
| Constant | 30,496 | 7854 | 15,102 to 45,890* |
| Gender | |||
| Female | – | – | – |
| Male | 12,227* | 4226 | 3945 to 20,509* |
| Age | |||
| –557* | 224 | –995 to –119* | |
| Binary obesity | |||
| No | – | – | – |
| Yes | 17,691* | 7173 | 3632 to 31,750* |
| Binary reduced HDL cholesterol | |||
| No | – | – | – |
| Yes | –10,399* | 4428 | –19,077 to –1720* |
Health state utility
Of the 293 participants in the full sample, 89 had full EQ-5D-3L data at both baseline and follow-up to enable the calculation of QALY gains for the year following presentation for FEP. The mean QALY gain for these 89 participants was 0.84 (SD 0.17). The subsample was further reduced to 55 when accounting for availability of relevant metabolic and sociodemographic data; however, this subsample appeared broadly representative of the full sample of participants, who had a comparable mean QALY gain of 0.86 (SD 0.14).
Metabolic syndrome factors and quality-adjusted life-years
Both unadjusted and adjusted (for baseline utility value) univariate analyses suggested that, at baseline, having a MetS, being obese, having reduced HDL cholesterol (binary variable), having higher HDL cholesterol (continuous variable) and having more metabolic indicators were associated with lower QALY gains (Table 6). Having raised fasting glucose or higher triglycerides (adjusted coefficient –0.03, 95% CI –0.07 to –0.00) was associated with lower QALY gains in adjusted analyses only.
| Variable | Unadjusted coefficient | Unadjusted 95% CI | Adjusted coefficienta | Adjusted 95% CIa |
|---|---|---|---|---|
| Gender | ||||
| Female | – | – | – | – |
| Male | –0.01 | –0.08 to 0.07 | –0.03 | –0.08 to 0.02 |
| Ethnicity | ||||
| White | – | – | – | – |
| Other | 0.00 | –0.07 to 0.07 | –0.02 | –0.07 to 0.03 |
| Age | ||||
| 0.00 | –0.00 to 0.00 | –0.00 | –0.00 to 0.00 | |
| Binary MetS | ||||
| No | – | – | – | – |
| Yes | –0.12 | –0.26 to 0.01* | –0.13 | –0.25 to –0.02* |
| Binary obesity | ||||
| No | – | – | – | – |
| Yes | –0.14 | –0.25 to –0.02* | –0.08 | –0.17 to 0.00* |
| Binary high triglycerides | ||||
| No | – | – | – | – |
| Yes | –0.01 | –0.09 to 0.06 | –0.03 | –0.10 to 0.03 |
| Binary high BP | ||||
| No | – | – | – | – |
| Yes | 0.00 | –0.08 to 0.09 | –0.03 | –0.09 to 0.04 |
| Binary raised fasting glucose | ||||
| No | – | – | – | – |
| Yes | –0.07 | –0.22 to 0.07 | –0.14 | –0.31 to 0.02* |
| Binary reduced HDL cholesterol | ||||
| No | – | – | – | – |
| Yes | –0.11 | –0.21 to –0.02* | –0.08 | –0.16 to –0.01* |
| Triglycerides (continuous) | ||||
| –0.01 | –0.06 to 0.04 | –0.03 | –0.07 to 0.00* | |
| Systolic BP (continuous) | ||||
| –0.00 | –0.00 to 0.00 | –0.00 | –0.00 to 0.00 | |
| Diastolic BP (continuous) | ||||
| –0.00 | –0.01 to 0.00 | –0.00 | –0.01 to 0.00 | |
| Fasting glucose (continuous) | ||||
| –0.01 | –0.07 to 0.09 | –0.05 | –0.11 to 0.01 | |
| HDL cholesterol (continuous) | ||||
| 0.14 | 0.06 to 0.21* | 0.09 | 0.02 to 0.17* | |
| Number of metabolic factors (continuous) | ||||
| –0.03 | –0.06 to –0.00* | –0.03 | –0.06 to –0.01* | |
When examining these seven variables in a multivariate analysis adjusted for baseline utility, only HDL level (as a continuous variable) remained statistically associated with QALYs, with higher HDL cholesterol associated with greater QALY gains (HDL 0.09, 95% CI 0.02 to 0.17; p = 0.013) (Table 7).
| Variable | Adjusted coefficient | SE | 95% CIa |
|---|---|---|---|
| Constant | 0.320 | 0.066 | 0.190 to 0.450* |
| HDL cholesterol (continuous) | 0.094 | 0.036 | 0.024 to 0.166* |
Discussion
The results suggest that obesity at baseline is associated with higher subsequent admission costs, that low levels of HDL cholesterol at baseline is associated with lower subsequent admission costs and that higher levels of HDL cholesterol at baseline is associated with a greater subsequent QALY gain.
Strengths and limitations
To our knowledge, this is the first study to examine the association between MetS indicators and admission costs and QALYs in patients with a FEP. However, the findings should be seen as exploratory and be interpreted with caution owing to several limitations. First, missing data resulted in small sample sizes for the analyses. Although the subsamples analysed were broadly representative of the full sample in terms of mean values for costs and QALYs, they may have differed on other factors and the small sample size in general increases the risk of spurious results given the number of independent factors that we sought to examine. Second, we examined only psychiatric admission costs and the impact of MetS in this patient group is on broader health-care costs remains unclear. However, because psychiatric inpatient costs are likely to be the main driver of total care costs in this patient group,226 the inclusion of other care costs is unlikely to substantially alter the findings.
Conclusion
Finally, this study examines cardiometabolic risk indicators that increase a person’s risk of heart disease, stroke and other conditions affecting blood vessels in the long term. 192 We have focused on only short-term mental health admission costs because we were interested in any additional impacts within the mental health care sector, but longer-term and broader data would be required to better assess the full additional impact of these high levels of cardiometabolic risk.
Appendix 2 Health promotion intervention development (work package 2)
FIGURE 2.
Model Delphi method adapted to our study, drawing on the method described by Jones and Hunter. 173
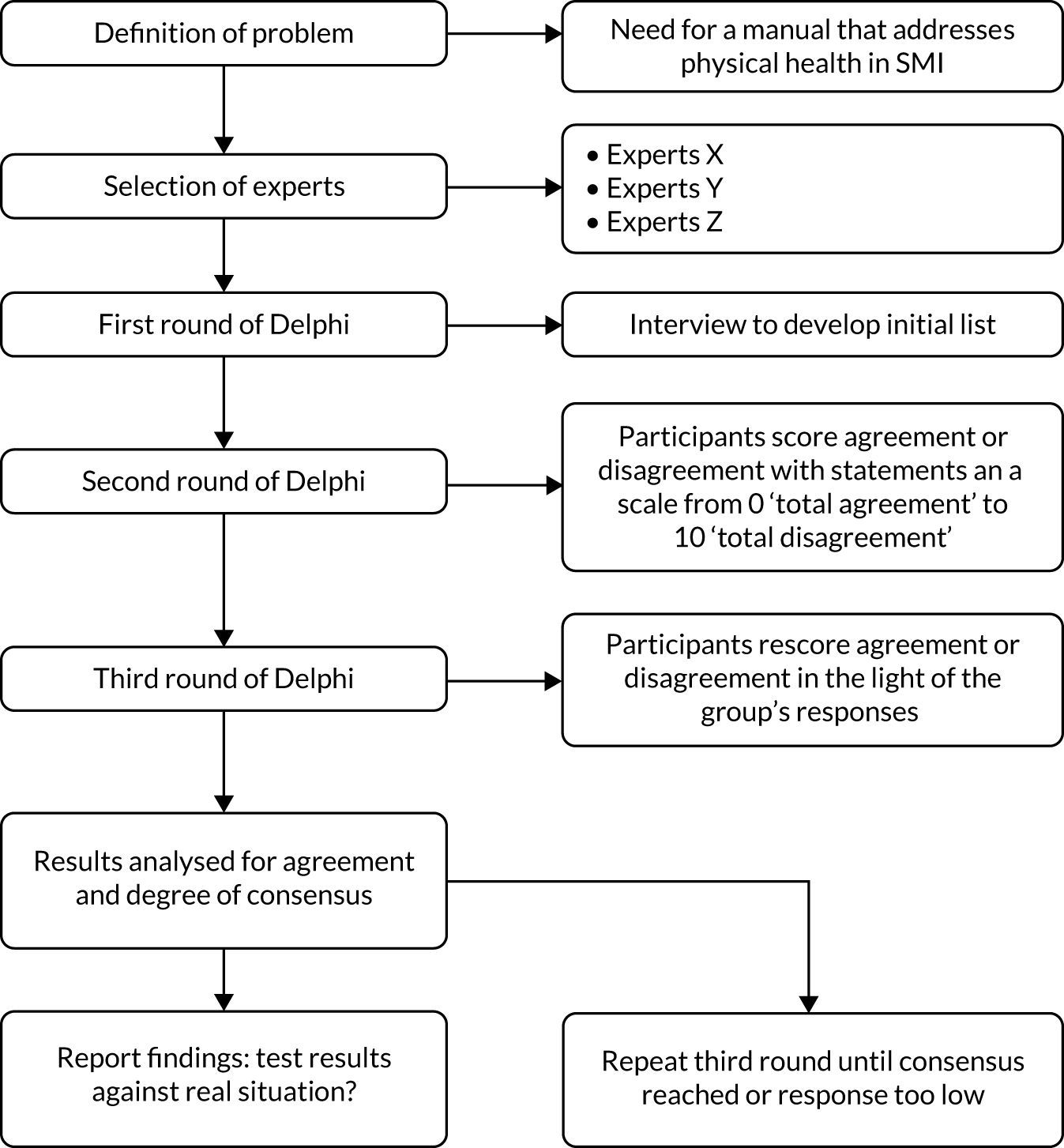
FIGURE 3.
Mean self-rated knowledge and skills scores pre and post pilot training for the eight pilot-trained clinicians.
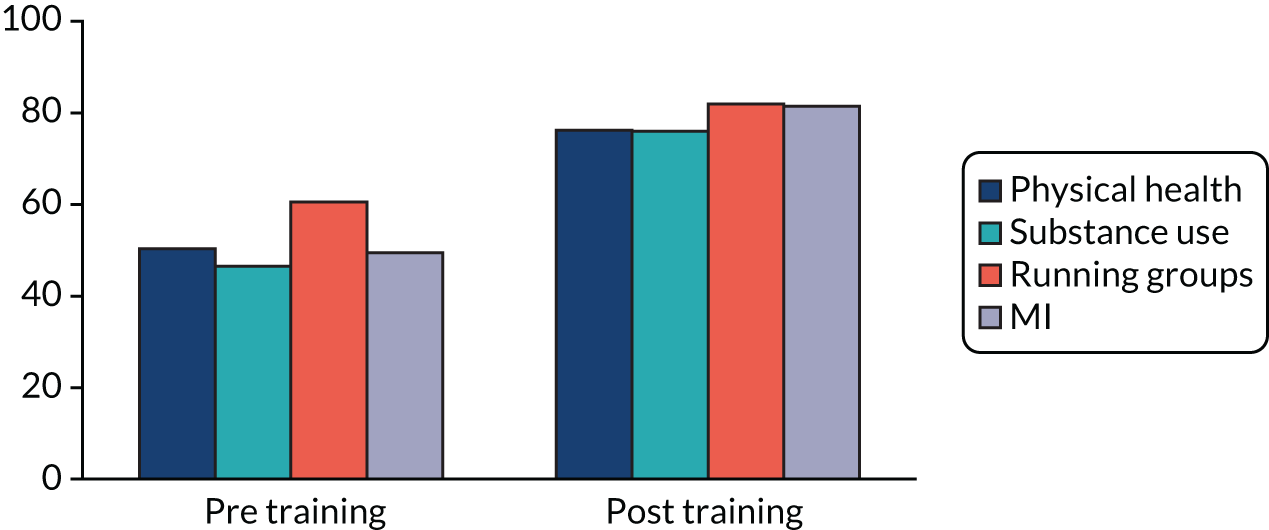
Appendix 3 Care co-ordinator fidelity to health promotion intervention (work package 3: IMPaCT randomised controlled trial)
Care co-ordinators recruited to the IMPaCT HPI trial were, importantly, randomly selected from within routine front-line NHS staff, and did not need to have prior therapy or research experience. Those randomised to deliver the intervention attended a 4-day training programme. As part of the training package, all care co-ordinators received their own copy of the IMPaCT HPI reference guide, manual and Better Health Handbook (described in work package 2), which outlined the guiding ethos, core therapeutic approaches and specific intervention adaptations for the different aspects of health behaviour, as well as providing all session plans, example dialogues and a full set of resources to support delivery.
A number of possible approaches were considered to determine therapeutic competence and fidelity to the HPI protocol. These included (1) the therapists’ own ratings of their perceived confidence and competence in delivering the different aspects of the IMPaCT intervention immediately before compared with immediately following the training delivery, thus providing both their final perceived confidence and competence and their change during training; (2) an individual role play by each therapist of a brief section of therapy delivery with a trained actor as the patient, to be conducted during the final afternoon of training, and audio-recorded; and (3) a set of three audio-recorded sessions to be obtained during an early, middle and late phase of therapy delivery from each care co-ordinator. All audio-recordings would be transcribed, with the central 20 minutes rated by professionals trained in the use of the Motivational Interviewing Treatment Integrity (MITI) scale (see Moyers et al. 227). This scale was selected as it has been well used as a research tool to determine fidelity to MI, which is the main therapeutic approach of the IMPaCT HPI. The MITI scale provides five summary scores that capture engagement with the spirit of MI, the process of MI (complex reflection, open questions, and the ratio between these) and overall MI adherence. The final approach considered was (4) in the spirit of the ‘learning to learn’ approach in MI, advocated by Miller and Moyers. Training alone has been shown to be insufficient to produce a change in therapeutic competence. Regular, supervised practice is also required to ensure that therapeutic competence is met and maintained (see Miller et al. 228). The IMPaCT therapy was designed to be supported, for each care co-ordinator, by a 1-hour session per fortnight (see Greenwood et al. 229).
On commencing the training with care co-ordinators, it was decided by consensus among the study team that for this research and therapy-naive population of clinicians, an assessment of competence through an audio-taped role play with an actor may be too threatening and may lead to therapist disengagement from the trial. As a result, this assessment was excluded from the fidelity evaluation.
Of the 52 care co-ordinators who completed the IMPaCT HPI training, 16 completed the training questionnaire both pre and post training. The questionnaire included ratings of knowledge across domains of physical health, substance use, running groups and MI therapy approaches. All ratings of knowledge and confidence post training were high (mean ratings of 73–80/100 for knowledge compared with 42–52/100 pre training and 78–81/100 for confidence). Knowledge increased significantly from pre training across all domains [physical health awareness t = –8.4 df(15), 95% CI –37 to –22; p < 001; substance use awareness t = –8.1 df(14) 95% CI –36 to –21; p < 001; running groups t = –4.4 df(15), 95% CI –42 to –15; p < 001; and MI t = –7.1 df(12), 95% CI –43 to –23; p < 001]. These outcomes are consistent with care co-ordinators having obtained stage 1 of Miller and Moyer’s eight stages of learning in MI, represented by their increased understanding and confidence in the ethos of MI delivery. However, confidence does not necessarily relate to competence in clinical practice and therapy and, as a result, additional approaches were explored to investigate the competence of the care co-ordinators to provide IMPaCT therapy.
Ten care co-ordinators each produced a single therapy audio-recording across sessions ranging from early to late therapy delivery. Each tape was transcribed and the MITI scale applied to the typed transcript by a trained professional and was independently validated by a second trained professional. The independent raters achieved 86% concordance on thresholds for proficiency and competence, and, in the case of different ratings, the higher rating was awarded. An analysis of the tapes revealed that most care co-ordinators reached beginning proficiency in at least one of the five domains scored, but only three (30%) reached this proficiency in more than two domains. These three had also obtained the higher threshold of competency in at least three domains. Of rated care co-ordinators, 50% obtained a rating that indicated adherence to a MI model. Table 8 summarises the level of care co-ordinator proficiency and competence in therapy delivery.
| MITI summary score | Care co-ordinators achieving beginning proficiency | Care co-ordinators achieving competence |
|---|---|---|
| Global spirit of MI | 4/10 | 4/10 |
| Complex reflections (%) | 8/10 | 7/10 |
| Open questions (%) | 4/10 | 1/10 |
| Reflection-to-question ratio | 3/10 | 1/10 |
| MI adherence (%) | 5/10 | 5/10 |
The training questionnaires revealed that care co-ordinators rated themselves as having a high level of knowledge and confidence in using the information about physical health and substance use, and in offering motivational approaches and groups to address these health behaviours in their clinical practice. This was a positive endorsement of the training from the care co-ordinator perspective and reflected at least their self-reported achievement of level 1 (understanding and confidence) in the stages of learning in MI.
Owing to concerns about acceptability, an assessment of initial competence using an audio-taped role play with an actor in the final training session was dropped. This concern would appear to have been borne out in the uptake of audio-taped therapy sessions, over which care the co-ordinators had control. Despite continued encouragement to produce three audio-taped sessions of their therapy from any of their clients over the course of therapy, only 10 care co-ordinators produced any audio tape, and none produced more than one tape. The tapes that were provided are, therefore, likely to derive from the smaller proportion of more enthusiastic and committed therapists in the IMPaCT trial. It would be important to learn more about other factors that differentiate between those therapists who adhered well to a MI model and those who were less able. Support for therapy delivery within the local NHS environment, pre-existing therapist skills and commitment are just some of the candidate factors that may mediate between therapy training and successful therapy delivery (Madson et al. 230). Although brief training sessions in MI have been shown to be effective in promoting MI therapy skills (Madson et al. 230), routine front-line clinical staff who work with people with psychosis, even when willing to take part in a 4-day training programme and when offered fortnightly supervision, struggle to consolidate training into meaningful adherence to MI therapy.
The MITI scale assesses the spirit and process of MI, which form the first five stages of learning in MI. It does not assess the later stages of transition to behaviour change, commitment and integration with other therapies such as CBT. However, based on the fidelity outcomes of these early MI phases of therapy delivery it seems possible that therapists would also struggle with these later stages of IMPaCT therapy.
Appendix 4 Economic evaluation of the IMPaCT randomised controlled trial (work package 3)
Analyses, missing data and sensitivity analyses
Data were analysed using Stata 11. Participants were analysed according to the group to which they were randomised regardless of intervention compliance.
Costs and outcomes were compared at baseline and at 12 and 15 months and are presented as mean values by arm with SDs. Mean differences and 95% CIs were obtained by non-parametric bootstrap regressions (1000 repetitions) to account for the non-normal distribution commonly found in economic data, with adjustment for clustering at the care co-ordinator level. To provide more relevant treatment-effect estimates,189 regressions to calculate mean differences in costs included covariates for the baseline value for the same cost category, baseline SF-36 MCS, baseline SF-36 PCS, baseline SF-36 utility and baseline EQ-5D-3L utility, plus baseline demographic variables expected to be associated with costs (gender, ethnicity, borough). Similarly, comparisons of outcome data included baseline SF-36 MCS, SF-36 PCS, SF-36 utility and EQ-5D-3L utility as covariates, plus baseline demographic variables that were expected to be associated with outcome (gender, age, ethnicity, place of birth and borough).
Individual item non-response for the CSRI was minimal. When this occurred, an item cost was imputed using the mean cost for the same item for other users in the same trial arm and at the same assessment point. When this was not possible, the overall cost component was imputed using the mean cost for the same cost component in the same trial arm at the same assessment point. For medication data, a series of assumptions and imputations were necessary depending on the nature of the missing information, as follows, making use of available data components when possible. If medication name was missing, we applied an average prescription cost [from DHSC prescription cost analyses (PCA)], accounting for the reported number of days on that medication and assuming that the prescription lasted for 1 month. If number of days on medication was missing, a PCA average item cost for that medication was used, with the assumption that the patient was prescribed that medication just once in that period. If dose was missing, a PCA average item cost was used, assuming that each prescription lasted 1 month but accounting for the number of days on the medication. If the dose unit was missing, a PCA average item cost was used assuming that each prescription lasted 1 month, with an account of the number of days on medication. If dose frequency was missing, a PCA average item cost was used, assuming that each prescription lasted 1 month, again accounting for number of days. Finally, if it was unknown whether or not the medication was administered as a depot, a PCA average item cost was used assuming that each prescription lasted 1 month, accounting for the number of days on medication.
The primary analysis was undertaken using cases with available relevant cost and/or outcome data (i.e. excluding those lost to follow-up for the CSRI, EQ-5D-3L or SF-36 assessments, as relevant).
Cost-effectiveness and cost–utility analyses
Cost-effectiveness and cost–utility analyses were conducted at 15 months to focus on the more pertinent question of whether or not any effect lasted beyond the end of the intervention, but 12-month cost and outcome data are also reported for information. The economic evaluation examined eight possible cost–outcome combinations (accounting for the two cost perspectives and the four outcomes). Incremental cost-effectiveness ratios (ICERs) were calculated for any combination showing both higher costs and better outcomes in either the intervention group or the control group (it was unnecessary to calculate ICERs for any combinations where one group showed both lower costs and better outcomes as this group was then considered to ‘dominate’ the other).
Uncertainty around cost-effectiveness/cost utility was explored using cost-effectiveness planes and cost-effectiveness acceptability curves based on the net-benefit approach. These curves are an alternative to CIs around ICERs and show the probability that one intervention is cost-effective compared with the other for a range of values that a decision-maker would be willing to pay for an additional unit of an outcome. Net benefits for each participant were calculated using the following formula, where λ is the willingness to pay for one additional unit of outcome: net benefit = (λ × outcome) – cost.
A series of net benefits were calculated for each individual for a λ range that would include any policy-making perspectives relevant at the time of analysis. After calculating net benefits for each participant for each value of λ, coefficients of differences in net benefits between the trial arms were obtained through a series of bootstrapped linear regressions (1000 repetitions) of group on net benefit that included the covariates used for the comparisons of mean costs and outcomes (i.e. baseline value of the same cost category, SF-36 MCS, SF-36 PCS, EQ-5D-3L utility score, SF-36 utility score, gender, age, ethnicity, place of birth and borough) and an adjustment for clustering by care co-ordinator. The resulting coefficients were then examined to calculate for each value of λ the proportion of times that the intervention group had a greater net benefit than the control group. These proportions were then plotted to generate cost-effectiveness acceptability curves for all eight cost–outcome combinations.
| Item | Unit | Cost (£) | Source | Notes |
|---|---|---|---|---|
| Accommodation | ||||
| Supported housing | Day | 100 | Curtis192 | Private-sector care home, excluding social services and benefits |
| Sheltered housing | Day | 100 | Curtis192 | Private-sector care home, excluding social services and benefits |
| Hostel/shelter | Day | 100 | Curtis192 | Private-sector care home, excluding social services and benefits |
| Inpatient servicesa | ||||
| Acute psychiatric ward | Bed-day | 329 | NHS231 | TMHIP (Mental Health Inpatients) tab, MHIPA2 – adult: acute care |
| Psychiatric intensive care | Bed-day | 647 | NHS231 | TMHIP (Mental Health Inpatients) tab, MHIPA1 – adult: intensive care |
| Medical ward | Bed-day | 440 | NHS231 | Using activity data to calculate a cost per bed-day |
| Mother and baby unit | Bed-day | 672 | NHS231 | TMHIPSS (Mental Health Inpatients: Specialist Services) tab, MHIPMB – mother and baby units |
| Accident and emergency department | Bed-day | 112 | NHS231 | TA and EMSNA – Accident and Emergency Services: Not Leading to Admitted |
| Surgical ward | Bed-day | 440 | NHS231 | Medical ward cost |
| Medium secure | Bed-day | 499 | NHS231 | TMHSU (Mental Health Secure Units) tab, SCU3 – medium level secure services |
| Low secure | Bed-day | 441 | NHS231 | TMHSU (Mental Health Secure Units) tab, SCU2 – low level secure services |
| High Down prison | Bed-day | 69 | Ministry of Justice232 | Based on £26,139 per prisoner per year (deflated to 2011–12 costs) |
| Psychiatric rehabilitation | Bed-day | 289 | NHS231 | TMHIP (Mental Health Inpatients) tab, MHIPA3 – adult: rehabilitation |
| Cardiology | Bed-day | 453 | NHS231 | Using activity data to calculate a cost per bed-day for HRG chapter E items (cardiology) |
| Ear, nose and throat | Bed-day | 440 | NHS231 | Medical ward cost |
| Older adults mental health | Bed-day | 340 | NHS231 | TMHIP (Mental Health Inpatients: Specialist Services) tab, MHIPE1 – elderly |
| Stroke unit | Bed-day | 325 | NHS231 | TNEI_L (Non-Elective Inpatient (Long Stay) HRG Data) tab, using activity data to calculate a cost per bed day for HRG items AA22A (Non-Transient Stroke or Cerebrovascular Accident, Nervous system infections or Encephalopathy with CC) +AA22B (Non-Transient Stroke or Cerebrovascular Accident, Nervous system infections or Encephalopathy without CC) |
| Acute medical ward | Bed-day | 440 | NHS231 | Medical ward cost |
| Respiratory ward | Bed-day | 326 | NHS231 | Using activity data to calculate a cost per bed-day for HRG chapter D items (respiratory system) |
| Emergency surgery | Bed-day | 440 | NHS231 | Medical ward cost |
| Respite care | Bed-day | 516 | NHS231 | TNEI_L (Non-Elective Inpatient (Long Stay) HRG Data) tab, PA55Z – respite care |
| Spinal injury unit | Bed-day | 440 | NHS231 | Medical ward cost |
| Outpatient servicesb | ||||
| Psychiatry | Visit | 171 | NHS231 | TMHCSOPFUAF tab (Mental Health Consultant Services (Outpatient Setting) – Follow-up Attendance Face to Face), MHOPFUA2 – adult other services |
| Non-psychiatric/general/medical | Visit | 108 | NHS231 | Total – OPATT (Outpatient Attendances) tab, Total – Outpatient Attendances |
| Diabetes clinic | Visit | 134 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 307 – diabetic medicine |
| Blood tests/phlebotomy | Visit | 3 | NHS231 | TDAPS (Direct Access: Pathology Services) tab, DAP839 – Phlebotomy |
| Psychiatric day hospital | Visit | 103 | NHS231 | TMHDCFRAD tab (Mental Health Day Care Facilities: Regular Attendances), DCF41 – Mental Health Patients Adult |
| Non-psychiatric/general/medical day hospital | Visit | 686 | NHS231 | Index tab: TDC – Day Cases HRG Data |
| Day surgery centre | Visit | 686 | NHS231 | Index tab: TDC – Day Cases HRG Data |
| Accident and emergency | Visit | 117 | NHS231 | Index tab, average of all A&E tabs – TAandEMSAD (Accident and Emergency Services: Leading to Admitted), TAandEMSNA (Accident and Emergency Services: Not Leading to Admitted), TAandEMinAD (Accident and Emergency Services: Minor Injury Service: Leading to Admitted), TAandEMinNA (Accident and Emergency Services: Minor Injury Service: Not Leading to Admitted), TAandEWiCAD (Accident and Emergency Services: Walk In Centres: Leading to Admitted), TAandEWiCNA (Accident and Emergency Services: Walk In Centres: Not Leading to Admitted), TNon24HRDEPAD (Non 24 hr AandE/Casualty Department: Leading to Admitted), TNon24HRDEPNA (Non 24 hr AandE/Casualty Department: Not Leading to Admitted) |
| X-ray (X-ray only) | Visit | 30 | NHS231 | Total – OPATT (Outpatient Attendances) Tab: DAPF – Direct Access Plain Film |
| Substance misuse clinic | Visit | 99 | NHS231 | TMHCSOPFUAF tab (Mental Health Consultant Services (Outpatient Setting) – Follow-up Attendance Face to Face), MHOPFUA1 – adult drug and alcohol services |
| Dietetics | Visit | 57 | NHS231 | Total – OPATT (Outpatient Attendances) Tab, Service Code 654 A – Adult dietetics |
| Electrocardiogram | Visit | 58 | NHS231 | TDADS (Direct Access: Diagnostic Services) tab, DA09 – 24-hour ECG/BP monitoring |
| Ambulance | Visit | 227 | NHS231 | Index tab, average of all paramedic activity – TPARA (Paramedic Services: Category A/Red), TPARB (Paramedic Services: Category B/Amber, TPARC (Paramedic Services: Category C/Green, TPARETU (Paramedic Services: Emergency Transfers/Urgents, TPARO (Paramedic Services: Other, TPARA(Act) (Paramedic Services: Category A/Red (Activity Data), TPARB(Act) (Paramedic Services: Category B/Amber (Activity Data), TPARC(Act) (Paramedic Services: Category C/Green (Activity Data), TPARETU(Act) (Paramedic Services: Emergency Transfers/Urgents (Activity Data), TPARO(Act) (Paramedic Services: Other (Activity Data) |
| Angiogram | Visit | 125 | NHS231 | TDIAGIM_DA (Diagnostic Imaging: Direct Access) tab, average of all the fluoroscopy – RA16Z (Contrast Fluoroscopy Procedures less than 20 minutes), RA17Z (Contrast fluoroscopy Procedures 20–40 minutes), RA18Z (Contrast fluoroscopy Procedures more than 40 minutes), RA19Z (Mobile/Intraoperative Contrast Fluoroscopy Procedures less than 20 minutes), RA20Z (Mobile/Intraoperative Contrast Fluoroscopy Procedures 20–40 minutes), RA21Z (Mobile/Intraoperative Contrast Fluoroscopy Procedures more than 40 minutes) |
| Computerised tomography scan | Visit | 93 | NHS231 | TDIAGIM_DA (Direct Access: Diagnostic Services) tab, average of all CT scans – RA08Z (Computerised Tomography Scan, one area, no contrast), RA09Z (Computerised Tomography Scan, one area with post contrast only), RA10Z (Computerised Tomography Scan, one area, pre and post contrast), RA11Z (Computerised Tomography Scan, two areas without contrast), RA12Z (Computerised Tomography Scan, two areas with contrast), RA13Z (Computerised Tomography Scan, three areas with contrast), RA14Z (Computerised Tomography Scan, more than three areas), RA50Z (Computerised Tomography Scan, three areas without contrast) |
| Chiropodist | Visit | 44 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 653 – podiatry |
| Colonoscopy | Visit | 360 | NHS231 | TOPROC (Outpatient Procedures) tab – mean of all colonoscopy procedures – FZ51Z (Diagnostic Colonoscopy 19 years and over), FZ52Z (Diagnostic Colonoscopy with biopsy 19 years and over), FZ53Z (Therapeutic Colonoscopy 19 years and over) |
| Chronic obstructive pulmonary disease clinic | Visit | 153 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 340 – respiratory medicine |
| Computerised tomography scan | Visit | 93 | NHS231 | TDIAGIM_DA (Direct Access: Diagnostic Services) tab, average of all CT scans – RA08Z (Computerised Tomography Scan, one area, no contrast), RA09Z (Computerised Tomography Scan, one area with post contrast only), RA10Z (Computerised Tomography Scan, one area, pre and post contrast), RA11Z (Computerised Tomography Scan, two areas without contrast), RA12Z (Computerised Tomography Scan, two areas with contrast), RA13Z (Computerised Tomography Scan, three areas with contrast), RA14Z (Computerised Tomography Scan, more than three areas), RA50Z (Computerised Tomography Scan, three areas without contrast) |
| Dental X-ray | Visit | 30 | NHS231 | Total – OPATT (Outpatient Attendances) tab, DAPF – Direct Access Plain Film |
| Dentist | Visit | 81 | NHS231 | Total – Other Currencies tab, CN20 row – community dental services |
| Diabetes eye test | Visit | 134 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 307 – diabetic medicine |
| Endocrinology/urology | Visit | 145 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 302 – endocrinology |
| Gynaecologist | Visit | 122 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 502 – gynaecology |
| Hearing aid clinic | Visit | 117 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 840 – audiology |
| Lung function clinic | Visit | 153 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 340 – respiratory medicine |
| Lupus clinic | Visit | 108 | NHS231 | Total – OPATT (Outpatient Attendances) tab, OPATT tab: Total – Outpatient Attendances |
| Magnetic resonance imaging | Visit | 180 | NHS231 | TDIAGIM_DA (Direct Access: Diagnostic Services) tab, mean of all MRI scans – RA01Z (Magnetic Resonance Imaging Scan, one area, no contrast), RA02Z (Magnetic Resonance Imaging Scan, one area, post contrast only), RA03Z (Magnetic Resonance Imaging Scan, one area, pre and post contrast), RA04Z (Magnetic Resonance Imaging Scan, two or three areas, no contrast), RA05Z (Magnetic Resonance Imaging Scan, two or three areas, with contrast), RA06Z (Magnetic Resonance Imaging Scan, more than three areas), RA07Z (Magnetic Resonance Imaging Scan, requiring extensive patient repositioning and/or more than one contrast agent) |
| Neurology assessment | Visit | 173 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 400 – neurology |
| Ophthalmologist | Visit | 86 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 130 – Ophthalmology |
| Physiotherapy | Visit | 39 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 650 A – Physiotherapy Total Attendances – Adult (19 and Over) |
| Psychologist | Visit | 136 | Curtis192 | £136 per hour of client contact – assumes 1-hour appointment, excludes qualification costs |
| Sexual health clinic | Visit | 67 | NHS231 | Total – OPATT (Outpatient Attendances) tab, FPC – Sexual and Reproductive Health Clinic (previously referred to as Family Planning Clinic) |
| Thyroid clinic | Visit | 108 | NHS231 | Total – OPATT (Outpatient Attendances) tab, OPATT tab: Total – Outpatient Attendances |
| Turning Point | Visit | 35 | Curtis192 | Cost as care co-ordinator/key worker – £67 per hour of face-to-face contact; assumes 30-minute appointment, excludes qualification costs |
| Ultrasound scan | Visit | 58 | NHS231 | TDIAGIM_DA (Direct Access: Diagnostic Services) tab, average of ultrasound under and over 20 minutes – RA23Z (Ultrasound Scan less than 20 minutes), RA24Z (Ultrasound Scan more than 20 minutes) |
| Community-based day services | ||||
| Individual therapy CMHT, etc. | Visit | 68 | NHS231 | TCSCT (community therapy services) tab, N6A1 – Community Occupational Therapy Services: Adult – One-to-One |
| Individual therapy at home | Visit | 95 | NHS231 | TCSCT (community therapy services) tab, N6A1 – Community Occupational Therapy Services: Adult – One-to-One - plus the proportion of district nurse home visit hour/clinic hour proportion from Curtis 2010233 |
| Group therapy, all locations | Visit | 73 | NHS231 | TCSCT (community therapy services) tab, N6A2 – Community Occupational Therapy Services: Adult – Group Services |
| Medication monitoring/administration, all locations | Visit | 34 | Curtis192 | Cost as care co-ordinator |
| Music therapy, all locations | Visit | 40 | Cancer Research UK234 | – |
| Art therapy, all locations | Visit | 30 | Curtis192 | Cost as occupational therapist |
| Community-based professionalsc | ||||
| Care co-ordinator at surgery | Occurrence | 34 | Curtis192 | £67 per hour of face-to-face contact; assumes 30-minute appointment, excludes qualification costs |
| Care co-ordinator at home | Occurrence | 47 | Curtis192 | As above, plus proportion of district nurse home visit hour/clinic hour proportion from Curtis 2010233 (139%) |
| Care co-ordinator over telephone | Occurrence | 10 | Curtis192 | As above; assumes same proportion of costs as a psychiatrist face to face vs. non-face to face (30%) |
| Home treatment team at surgery | Occurrence | 188 | NHS231 | TMHSTSAF tab (Mental Health Specialist Teams: Adult – Face to Face), MHST20 (Crisis resolution home treatment teams) |
| Home treatment team at home | Occurrence | 188 | NHS231 | As above |
| Home treatment team over telephone | Occurrence | 60 | NHS231 | TMHSTSANF tab (Mental Health Specialist Teams: Adult – Non-Face to Face), MHST20 (Crisis resolution home treatment teams) |
| Crisis resolution team at surgery | Occurrence | 188 | NHS231 | TMHSTSAF tab (Mental Health Specialist Teams: Adult – Face to Face), MHST20 (Crisis resolution home treatment teams) |
| Crisis resolution team at home | Occurrence | 188 | NHS231 | As above |
| Crisis resolution team over telephone | Occurrence | 60 | NHS231 | TMHSTSANF tab (Mental Health Specialist Teams: Adult – Non-Face to Face), MHST20 (Crisis resolution home treatment teams) |
| Early intervention team at surgery | Occurrence | 177 | NHS231 | TMHSTSAF tab (mental health specialist teams: adult – face to face), MHST22 (early intervention in psychosis studies) |
| Early intervention team at home | Occurrence | 177 | NHS231 | As above |
| Early intervention team over telephone | Occurrence | 57 | NHS231 | TMHSTSANF tab (Mental Health Specialist Teams: Adult – Non-Face to Face), MHST22 (Early intervention in psychosis studies) |
| Community psychiatric nurse at surgery | Occurrence | 34 | Curtis192 | Care co-ordinator – above |
| Community psychiatric nurse at home | Occurrence | 47 | Curtis192 | Care co-ordinator – above |
| Community psychiatric nurse over telephone | Occurrence | 10 | Curtis192 | Care co-ordinator – above |
| Social worker at surgery | Occurrence | 78 | Curtis192 | £156 per hour of face-to-face contact, assume 30-minute appointment, excludes qualifications |
| Social worker at home | Occurrence | 108 | Curtis192 | As above, with proportion of district nurse home visit hour/clinic hour proportion from PSSRU 2010 (139%) |
| Social worker over telephone | Occurrence | 23 | Curtis192 | As above, assume same proportion of costs as a psychiatrist face to face vs. non-face to face (30%) |
| Psychiatrist at surgery | Occurrence | 171 | NHS231 | TMHCSOPFUAF tab (Mental Health Consultant Services (Outpatient Setting) – Follow-up Attendance Face to Face), MHOPFUA2 (Adult other services) |
| Psychiatrist at home | Occurrence | 238 | NHS231 | As above, with the proportion of district nurse home visit hour/clinic hour proportion from PSSRU 2010 (139%) |
| Psychiatrist over telephone | Occurrence | 52 | NHS231 | TMHCSOPFUANF tab (Mental Health Consultant Services (Outpatient Setting) – Follow-up Attendance Non-Face to Face), MHOPFUA2 (Adult other services) |
| Psychologist at surgery | Occurrence | 136 | Curtis192 | £136 per hour of client contact, assume 1-hour appointment, excludes qualification costs |
| Psychologist at home | Occurrence | 189 | Curtis192 | As above, based on psychologist visit cost above but use the proportion of district nurse home visit hour/clinic hour proportion from PSSRU 2010 (139%) |
| Psychologist over telephone | Occurrence | 41 | Curtis192 | As above, assume same proportion of costs as a psychiatrist face to face vs. non face to face (30%) |
| Psychotherapist at surgery | Occurrence | 136 | Curtis192 | Assume same as a psychologist |
| Psychotherapist at home | Occurrence | 189 | Curtis192 | Assume same as a psychologist |
| Psychotherapist over telephone | Occurrence | 41 | Curtis192 | Assume same as a psychologist |
| Counsellor at surgery | Occurrence | 59 | Curtis192 | £59 per consultation |
| GP at surgery | Occurrence | 36 | Curtis192 | £36 per patient contact lasting 11.7 minutes, excludes qualification costs, including direct care staff costs |
| GP at home | Occurrence | 92 | Curtis192 | £92 per patient out of surgery visit lasting 23.4 minutes, excludes qualification costs, including direct care staff costs |
| GP over telephone | Occurrence | 22 | Curtis192 | £22 per telephone contact lasting 7.2 minutes, excludes qualification costs, including direct care staff costs |
| Blood test at GP surgery | Occurrence | 12 | Curtis192 | Assume practice nurse |
| Blood test at home | Occurrence | 16 | Curtis192 | Assume practice nurse |
| Diabetes nurse at surgery | Occurrence | 12 | Curtis192 | Assume practice nurse |
| Diabetes nurse at home | Occurrence | 16 | Curtis192 | Assume practice nurse |
| Diabetes nurse over telephone | Occurrence | 7 | Curtis192 | Assume practice nurse |
| Practice nurse at surgery | Occurrence | 12 | Curtis192 | £45 per hour of face-to-face contact, excluding qualifications assuming 15.5- (specified on p180) minute appointment |
| Practice nurse at home | Occurrence | 16 | Curtis192 | As above, with the proportion of district nurse home visit hour/clinic hour proportion from Curtis 2010233 (139%) |
| Practice nurse over telephone | Occurrence | 7 | Curtis192 | As above, with same proportion of costs as a GP telephone call (61%) |
| District nurse at surgery | Occurrence | 11 | Curtis192 | District nurse, with the proportion of clinic hour/home visit hour proportion from Curtis 2010233 (72%) |
| District nurse at home | Occurrence | 16 | Curtis192 | Community nurse including district, £61 per hour of home visiting including travel, excluding qualifications; assumes 15.5-minute appointment |
| District nurse over telephone | Occurrence | 10 | Curtis192 | Assume same proportion of costs as a GP telephone call (61%) |
| Occupational therapist at surgery | Occurrence | 30 | Curtis192 | NHS community occupational therapist – £30 per hour, assumes 1-hour meeting and excludes qualification costs |
| Occupational therapist at home | Occurrence | 55 | Curtis192 | As above, with proportions of client time set down in PSSRU 2009–10 (183%) excluding qualifications |
| Occupational therapist over telephone | Occurrence | 18 | Curtis192 | As above, with proportion of costs as a GP telephone call (61%) |
| Dietitian at surgery | Occurrence | 72 | NHS231 | TOCS (Other Community Services) tab – N800 Dietetics Services |
| Dietitian at home | Occurrence | 129 | NHS231 | As above, with proportions of client time set down in PSSRU 2009–10 (179%), excluding qualifications |
| Dietitian over telephone | Occurrence | 44 | NHS231 | As above, with proportion of costs as a GP telephone call (61%) |
| Home help/care worker at home | Occurrence | 12 | Curtis192 | Home care worker per hour of face-to-face contact, weighted average accounting for different rates for day/evening/weekday/weekends |
| Meals on wheels at home | Occurrence | 5 | Curtis192 | Average of £6 local authority meal and £4 independent sector cost per day |
| Pharmacist for advice at surgery | Occurrence | 4 | Curtis192 | £50, assuming 5-minute consultation; excludes qualification costs |
| Pharmacist for advice over telephone | Occurrence | 4 | Curtis192 | As above |
| NHS Direct over telephone | Occurrence | 16 | NHS Direct235 | Inflated to 2011/12 prices |
| Samaritans over telephone | Occurrence | 4 | National Register of Personal Trainers236 | Inflated to 2011/12 prices |
| Other community-based professionals | ||||
| Advocate at surgery | Occurrence | 76 | Curtis192 | Cost as an independent mental capacity advocates assessment |
| Advocate at home | Occurrence | 106 | Curtis192 | As above, with the proportion of district nurse home visit hour/clinic hour proportion from Curtis 2010233 (139%) |
| Podiatrist at surgery | Occurrence | 15 | Curtis192 | Community chiropodist – £30 per hour; assumes 30-minute appointment |
| Podiatrist at home | Occurrence | 42 | Curtis192 | As above, with proportion of district nurse home visit hour/clinic hour proportion from Curtis 2010233 (139%) |
| CMHT bloods at surgery | Occurrence | 12 | Curtis192 | Cost as GP surgery blood test |
| Emergency service over telephone | Occurrence | 16 | NHS Direct235 | Cost as NHS Direct |
| Depot at GP surgery | Occurrence | 12 | Curtis192 | Cost as practice nurse |
| Depot clinic surgery | Occurrence | 6 | Curtis192 | Cost as community psychiatric nurse for 5 minutes |
| Diabetes eye test at surgery | Occurrence | 134 | NHS231 | Total – OPATT (Outpatient Attendances) tab, 307 – diabetic medicine |
| Employment support worker at surgery | Occurrence | 30 | Curtis192 | Cost as occupational therapist |
| Forensic team at surgery | Occurrence | 34 | Curtis192 | Cost as care co-ordinator |
| Forensic team at home | Occurrence | 47 | Curtis192 | Cost as care co-ordinator |
| Gym practitioner (GP referral) at surgery | Occurrence | 60 | National Register of Personal Trainers236 | Costs between £20 and £100; chose mid-point – £60 |
| Human immunodeficiency virus clinic at surgery | Occurrence | 110 | NHS231 | TCSCNSN (community and outreach nursing services: specialist nursing) tab, CN208AF – band 8 – human immunodeficiency virus/acquired immunodeficiency syndrome nursing services: adult: face to face |
| Welfare benefits advisor at home | Occurrence | 78 | Curtis192 | Cost as social worker |
| Housing officer at home | Occurrence | 78 | Curtis192 | Cost as social worker |
| Lifestyle coach at surgery | Occurrence | 70 | Life Coach Directory237 | Costs ranged between £40 and £100; chose mid-point |
| Mental health helpline over telephone | Occurrence | 4 | Samaritans238 | Cost as Samaritans |
| Mind Advocates over telephone | Occurrence | 23 | Curtis192 | Cost as advocate, with proportion of costs as a psychiatrist face to face vs. non-face to face (30%) |
| Osteopath at surgery | Occurrence | 43 | NHS Choices239 | Costs ranged between £35 and £50 per 30- to 40-minute contact; chose mid-point |
| Probation officer at surgery | Occurrence | 78 | Curtis192 | Cost as social worker |
| Spirit Release Session at surgery | Occurrence | 38 | Cancer Research UK240 | Costs ranged between £15–60 per session; chose mid-point |
| Support worker at surgery | Occurrence | 11 | Curtis192 | Clinical support worker – assumes 30-minute appointment |
| Support worker at home | Occurrence | 15 | Curtis192 | As above, with proportion of district nurse home visit hour/clinic hour proportion from Curtis 2010233 (139%) |
| Benefits | ||||
| Patient social security benefits/tax credits | Week | 53.45 | Direct Gov241 | Employment and support personal allowance – assumes single < 25 years |
| Patient disability living allowance | ||||
| Higher | Week | 73.60 | Direct Gov241 | Assumes the care component |
| Middle | Week | 49.30 | Direct Gov241 | – |
| Lower | Week | 19.55 | Direct Gov241 | – |
| Carer benefit | ||||
| Higher | Week | 55.55 | Direct Gov241 | – |
| Middle | Week | 55.55 | Direct Gov241 | – |
| Lower | Week | 55.55 | Direct Gov241 | – |
| Component | Control | HPI | ||||
|---|---|---|---|---|---|---|
| Resources | Details | Unit cost (£) | Resources | Details | Mean cost (£) | |
| Manual | – | – | 0 |
Developmenta Printingb |
£35.68 per care co-ordinator, divided by a recommended average of 20 patients per care co-ordinator | 1.78 |
| Training | ||||||
| Trainer’s time | Consultant psychiatristc (90 minutes) | £186 divided by 25 attendees, divided by a recommended average of 20 patients per care co-ordinator | 0.37 |
Band 6 nursed Band 8a nursee Psychologist band 8df Consultant psychiatristg (total 29 hours) |
Cost of £2336 divided by an average of five care co-ordinators per training course, divided by a recommended average of 20 patients per care co-ordinator | 23.36 |
| Attendee’s time | Band 6 nurseh (90 minutes) | £63 per care co-ordinator, divided by a recommended average of 20 patients per care co-ordinator | 3.15 | Band 6 nursei (total 32 hours) | £1344 per care co-ordinator divided by a recommended average of 20 patients per care co-ordinator | 67.20 |
| Materials | – | None | 0 |
One-off items: BP machinej Weighing scalesk Tape measurel Flip-chart standm Memory stickn Consumables: Flip-chart papero Flip-chart pensp Paperq Pensr |
£20.97 divided by nine training courses, divided by five care co-ordinators per course, divided by a recommended average of 20 patients per care co-ordinator £6.49 per care co-ordinator, divided by a recommended average of 20 patients per care co-ordinator |
0.02 0.32 |
| Supervision | – | None | 0 |
Training supervisor: band 8 nurses Care co-ordinator: band 6 nurset (90 minutes) |
£150 per session, per care co-ordinator, divided by a recommended average of 20 patients per care co-ordinator | 41.41 |
| Implementation | – | Implemented into therapy as part of continuing care | 0 | Band 6 nurseu (individual times) | Individually calculated for each participant based on number and type of sessions attended | 92.31 |
| Mean total cost (£) | 3.52 per control participant | 226.40 per intervention participant | ||||
Sensitivity analyses
We conducted four sensitivity analyses to check the robustness of the base-case analyses defined above. First, we explored the potential impact of excluding those lost to follow-up. We examined key sociodemographic and clinical characteristics for those included and those excluded from the analyses and conducted an intention-to-treat analysis, which included those lost to follow-up by imputing missing total costs and outcomes using imputation in Stata 13. Imputations of costs and outcomes were based on variables that were expected to predict costs and outcomes. For cost imputations, these variables were baseline and 12-month values for the equivalent cost category, the SF-36 MCS, SF-36 PCS, EQ-5D-3L utility score, SF-36 utility score, plus gender, ethnicity, borough, age, place of birth and care co-ordinator. Imputation of outcomes was based on baseline and 12-month values of the SF-36 MCS, SF-36 PCS, EQ-5D-3L utility score, SF-36 utility score, plus gender, age, ethnicity, place of birth and borough, and care co-ordinator.
Second, to explore the potential impact of having follow-up interviews conducted more than 30 days before or after the follow-up date, we conducted a ‘correct time window’ analysis including only those trial participants whose data were collected within the correct window (within 30 days either side of the planned assessment date).
Third, to explore the potential impact of insufficient implementation of the HPI, we conducted a per-protocol analysis that included only those intervention arm trial participants who received the predefined minimum of six intervention sessions of at least 30 minutes each.
Finally, to explore the potential impact of care co-ordinator dropout, we conducted analyses that included only those trial participants whose care co-ordinator remained the same throughout the study.
For each of these sensitivity analyses, we examined whether or not the conclusions concerning the mean difference in costs or outcomes between the two trial arms differed from those drawn from the base-case analyses.
Economic evaluation results
In line with the analysis of the primary outcome measure and other clinical outcomes, the economic evaluation was based on all 406 participants who were randomised. We summarise the response rates in Tables 12–14.
| Trainer staff type | Number of sessions delivered | Total training time (minutes) | Total preparation time (minutes) |
|---|---|---|---|
| Nurse band 6 | 1 | 150 | 10 |
| Nurse band 8a | 4 | 810 | 40 |
| Psychologist band 8d | 2 | 270 | 20 |
| Consultant psychiatrist | 2 | 420 | 20 |
| Trial group | Time point, n (%) | All time points, n (%) | ||
|---|---|---|---|---|
| Baseline | 12 months | 15 months | ||
| Intervention (N = 213) | 212 (100) | 160 (75) | 152 (71) | 138 (65) |
| Control (N = 193) | 193 (100) | 159 (82) | 149 (77) | 143 (74) |
| Total (N = 406) | 405 (98) | 319 (79) | 301 (74) | 281 (69) |
| Outcome | Trial group | Time point, n (%) | All time points, n (%) | ||
|---|---|---|---|---|---|
| Baseline | 12 months | 15 months | |||
| SF-36 | Intervention (N = 213) | 210 (99) | 158 (74) | 149 (70) | 132 (62) |
| Control (N = 193) | 192 (100) | 155 (80) | 148 (77) | 138 (72) | |
| Total (N = 406) | 402 (99) | 313 (77) | 297 (73) | 270 (67) | |
| EQ-5D-3L | Intervention (N = 213) | 211 (99) | 159 (75) | 152 (71) | 136 (64) |
| Control (N = 193) | 193 (100) | 156 (81) | 149 (77) | 140 (73) | |
| Total (N = 406) | 404 (100) | 315 (78) | 301 (74) | 276 (68) | |
| Trial group | n (%) |
|---|---|
| Intervention (n = 213) | 213 (100) |
| Control (n = 193) | 193 (100) |
| Total (n = 406) | 406 (100) |
Table 15 summarises the joint availability of both cost and outcome data at 15 months (a requirement for the constructions of CEACs), by outcome measure. Equivalent data are also provided for the 12-month follow-up for information.
| Outcome | Trial group | Time point, n (%) | ||
|---|---|---|---|---|
| 12 months | 15 months | Both 12 and 15 months | ||
| SF-36 | Intervention (n = 213) | 157 (74) | 149 (70) | 131 (62) |
| Control (n = 193) | 154 (80) | 147 (76) | 137 (71) | |
| Total (n = 406) | 311 (77) | 296 (73) | 268 (66) | |
| EQ-5D-3L | Intervention (n = 213) | 158 (74) | 152 (71) | 135 (63) |
| Control (n = 193) | 155 (80) | 148 (77) | 139 (72) | |
| Total (n = 406) | 313 (77) | 300 (74) | 274 (68) | |
Tables 16 and 17 suggest that there were no notable differences in the baseline characteristics of the subsamples included in the base-case analyses of those with available data against the full sample.
| Characteristic | Full sample (N = 406) | Subsample | |
|---|---|---|---|
| With 12-month costs and SF-36 data (N = 311) | With 15-month costs and SF-36 data (N = 296) | ||
| Gender, n (%) | |||
| Male | 234 (58) | 183 (59) | 172 (58) |
| Female | 172 (42) | 128 (41) | 124 (42) |
| Ethnicity, n (%) | |||
| White | 222 (55) | 174 (56) | 170 (58) |
| Black | 137 (34) | 102 (33) | 97 (33) |
| Asian | 15 (4) | 11 (4) | 11 (4) |
| Mixed or other | 29 (7) | 23 (7) | 17 (6) |
| Place of birth, n (%) | |||
| UK | 318 (78) | 251 (81) | 237 (80) |
| Other | 88 (22) | 60 (19) | 59 (20) |
| Borough, n (%) | |||
| Croydon | 58 (14) | 51 (16) | 48 (16) |
| Lambeth | 44 (11) | 31 (10) | 31 (11) |
| Lewisham | 84 (21) | 62 (20) | 54 (18) |
| Southwark | 83 (20) | 66 (21) | 66 (22) |
| Greenwich | 30 (7) | 26 (8) | 26 (9) |
| Bromley | 34 (8) | 24 (8) | 25 (9) |
| Bexley | 23 (6) | 18 (6) | 18 (6) |
| East Sussex | 23 (6) | 20 (6) | 20 (7) |
| Somerset | 17 (4) | 5 (2) | 8 (3) |
| South Staffordshire | 10 (3) | 8 (3) | 0 (0) |
| Age (years), mean (SD) | 44 (10) | 45 (10) | 45 (10) |
| SF-36 MCS, mean score (SD) | 43 (13) | 43 (13) | 43 (13) |
| SF-36 PCS, mean score (SD) | 48 (11) | 48 (11) | 48 (11) |
| Characteristic | Full sample (N = 406) | Subsample | |
|---|---|---|---|
| With 12-month costs and EQ-5D-3L data (N = 313) | With 15-month costs and EQ-5D-3L data (N = 300) | ||
| Gender, n (%) | |||
| Male | 234 (58) | 183 (49) | 174 (58) |
| Female | 172 (42) | 130 (42) | 126 (42) |
| Ethnicity, n (%) | |||
| White | 222 (55) | 175 (56) | 172 (58) |
| Black | 137 (34) | 102 (33) | 98 (33) |
| Asian | 15 (4) | 12 (4) | 11 (4) |
| Mixed or other | 29 (7) | 23 (7) | 18 (60 |
| Place of birth, n (%) | |||
| UK | 318 (78) | 253 (81) | 241 (80) |
| Other | 88 (22) | 60 (19) | 59 (20) |
| Borough, n (%) | |||
| Croydon | 58 (14) | 51 (16) | 48 (16) |
| Lambeth | 44 (11) | 31 (10) | 31 (10) |
| Lewisham | 84 (21) | 63 (20) | 55 (18) |
| Southwark | 83 (20) | 66 (21) | 66 (22) |
| Greenwich | 30 (7) | 26 (8) | 27 (9) |
| Bromley | 34 (8) | 25 (8) | 25 (8) |
| Bexley | 23 (6) | 18 (6) | 18 (6) |
| East Sussex | 23 (6) | 20 (3) | 20 (7) |
| Somerset | 17 (4) | 5 (2) | 10 (3) |
| South Staffordshire | 10 (3) | 8 (3) | 0 (0) |
| Age (years), mean (SD) | 44 (10) | 45 (10) | 43 (11) |
| SF-36 MCS, mean score (SD) | 43 (13) | 43 (13) | 43 (13) |
| SF-36 PCS, mean score (SD) | 48 (11) | 48 (11) | 48 (11) |
Resource use
Resource use pattern data suggest that both arms were broadly balanced in their use of core services both before and during the study. These data are available for reference in Tables 18–20. As would be expected for this group of patients, service use is very broad in both nature and sector, illustrating the complexity of their care provision. These data were not compared statistically because the economic evaluation was focused on costs and cost-effectiveness/utility, and to avoid problems associated with multiple testing.
| Resource | Unit | Trial group | |||||
|---|---|---|---|---|---|---|---|
| Intervention (N = 212) | Control (N = 193) | ||||||
| Users (n) | Meana | SD | Users (n) | Meana | SD | ||
| Specialist accommodation | |||||||
| Supported housing/assisted living | Bed-day | 51 | 157 | 50 | 42 | 166 | 44 |
| Sheltered housing | Bed-day | 3 | 154 | 48 | 6 | 170 | 29 |
| Hostel/shelter | Bed-day | 7 | 120 | 83 | 6 | 182 | 0 |
| Hospital inpatient | |||||||
| Inpatient | Bed-day | 60 | 155 | 54 | 54 | 168 | 40 |
| Hospital outpatient | |||||||
| Psychiatric outpatient | Visit | 35 | 4 | 5 | 49 | 3 | 4 |
| Non-psychiatric/general/medical outpatient | Visit | 29 | 3 | 4 | 22 | 2 | 1 |
| Diabetes clinic | Visit | 15 | 2 | 2 | 13 | 2 | 1 |
| Blood tests | Visit | 95 | 5 | 4 | 84 | 4 | 3 |
| Psychiatric day hospital | Visit | 12 | 18 | 33 | 3 | 58 | 9 |
| Non-psychiatric/general/medical day hospital | Visit | 3 | 1 | 0 | 3 | 1 | 1 |
| Day surgery centre | Visit | 7 | 2 | 2 | 7 | 2 | 2 |
| Accident and emergency department | Visit | 32 | 2 | 2 | 23 | 1 | 1 |
| X-ray | Visit | 24 | 1 | 1 | 27 | 1 | 1 |
| Substance misuse clinic | Visit | 13 | 9 | 8 | 7 | 7 | 9 |
| Dietetics | Visit | 7 | 3 | 2 | 9 | 3 | 2 |
| Community-based day services | |||||||
| Community-based services | Visit | 72 | 34 | 32 | 73 | 29 | 31 |
| Community-based professionals | |||||||
| Care co-ordinator | Surgery visit | 129 | 10 | 8 | 132 | 9 | 6 |
| Care co-ordinator | Home visit | 107 | 10 | 9 | 83 | 8 | 7 |
| Care co-ordinator | Telephone call | 39 | 10 | 19 | 28 | 7 | 8 |
| Home treatment team | Surgery visit | 3 | 2 | 1 | 4 | 3 | 2 |
| Home treatment team | Home visit | 10 | 9 | 12 | 14 | 11 | 12 |
| Home treatment team | Telephone call | 1 | 1 | – | 2 | 3 | 1 |
| Crisis resolution team | Surgery visit | 0 | – | – | 0 | – | – |
| Crisis resolution team | Home visit | 8 | 7 | 7 | 3 | 3 | 1 |
| Crisis resolution team | Telephone call | 1 | 1 | – | 1 | 1 | – |
| Early intervention team | Surgery visit | 0 | – | – | 0 | – | – |
| Early intervention team | Home visit | 2 | 10 | 6 | 0 | – | – |
| Early intervention team | Telephone call | 0 | – | – | 0 | – | – |
| Community psychiatric nurse | Surgery visit | 13 | 7 | 7 | 6 | 5 | 4 |
| Community psychiatric nurse | Home visit | 5 | 5 | 3 | 1 | 4 | – |
| Community psychiatric nurse | Telephone call | 3 | 4 | 4 | 3 | 3 | 1 |
| Social worker | Surgery visit | 18 | 1 | 1 | 22 | 2 | 2 |
| Social worker | Home visit | 1 | 12 | – | 3 | 3 | 3 |
| Social worker | Telephone call | 2 | 4 | 4 | 1 | 1 | – |
| Psychiatrist | Surgery visit | 85 | 3 | 5 | 75 | 3 | 4 |
| Psychiatrist | Home visit | 6 | 5 | 9 | 9 | 2 | 1 |
| Psychiatrist | Telephone call | 0 | – | – | 0 | – | – |
| Psychologist | Surgery visit | 19 | 8 | 9 | 20 | 9 | 8 |
| Psychologist | Home visit | 3 | 11 | 2 | 2 | 12 | 0 |
| Psychologist | Telephone call | 0 | – | – | 0 | – | – |
| Psychotherapist | Surgery visit | 1 | 5 | – | 3 | 12 | 11 |
| Psychotherapist | Home visit | 1 | 12 | – | 1 | 6 | – |
| Psychotherapist | Telephone call | 0 | – | – | 0 | – | – |
| Counsellor | Surgery visit | 26 | 3 | 5 | 19 | 3 | 5 |
| Counsellor | Home visit | 0 | – | – | 1 | 1 | – |
| Counsellor | Telephone call | 0 | – | – | 0 | – | – |
| GP | Surgery visit | 119 | 4 | 7 | 108 | 3 | 3 |
| GP | Home visit | 3 | 2 | 1 | 2 | 2 | 1 |
| GP | Telephone call | 1 | 2 | – | 1 | 1 | – |
| Blood test at GP | Surgery visit | 47 | 2 | 2 | 47 | 2 | 1 |
| Diabetes nurse | Surgery visit | 11 | 2 | 1 | 10 | 1 | < 1 |
| Diabetes nurse | Home visit | 0 | – | – | 0 | – | – |
| Diabetes nurse | Telephone call | 0 | – | – | 2 | 1 | 0 |
| Practice nurse | Surgery visit | 27 | 3 | 5 | 31 | 2 | 2 |
| Practice nurse | Home visit | 0 | – | – | 0 | – | – |
| Practice nurse | Telephone call | 1 | 1 | – | 0 | – | – |
| District nurse | Surgery visit | 1 | 120 | – | 1 | 1 | – |
| District nurse | Home visit | 0 | – | – | 2 | 2 | 107 |
| District nurse | Telephone call | 0 | – | – | 0 | – | – |
| Occupational therapist | Surgery visit | 8 | 7 | 8 | 8 | 9 | 10 |
| Occupational therapist | Home visit | 0 | – | – | 2 | 5 | 4 |
| Occupational therapist | Telephone call | 0 | – | – | 0 | – | – |
| Dietitian | Surgery visit | 7 | 2 | 2 | 12 | 2 | 2 |
| Dietitian | Home visit | 0 | – | – | 1 | 1 | – |
| Dietitian | Telephone call | 0 | – | – | 0 | – | – |
| Home help | Home visit | 11 | 57 | 72 | 6 | 32 | 37 |
| Meals on wheels | Home visit | 0 | – | – | 2 | 2 | 1 |
| Pharmacist for advice | Surgery visit | 21 | 2 | 2 | 20 | 2 | 2 |
| Pharmacist for advice | Home visit | 1 | 2 | – | 0 | – | – |
| Pharmacist for advice | Telephone call | 0 | – | – | 1 | 2 | – |
| NHS Direct | Telephone call | 3 | 1 | 1 | 6 | 2 | 1 |
| Samaritans | Telephone call | 3 | 5 | 5 | 2 | 52 | 62 |
| Medication | 208 | – | – | 191 | – | – | |
| Resource | Unit | Trial group | |||||
|---|---|---|---|---|---|---|---|
| Intervention (N = 160) | Control (N = 159) | ||||||
| Users (n) | Meana | SD | Users (n) | Meana | SD | ||
| Specialist accommodation | |||||||
| Supported housing/assisted living | Bed-day | 37 | 182 | 1 | 30 | 179 | 12 |
| Sheltered housing | Bed-day | 1 | 182 | – | 6 | 158 | 60 |
| Hostel/shelter | Bed-day | 4 | 182 | 0 | 5 | 152 | 68 |
| Hospital inpatient | |||||||
| Inpatient | Bed-day | 42 | 182 | 1 | 41 | 173 | 34 |
| Hospital outpatient | |||||||
| Psychiatric outpatient | Visit | 13 | 4 | 2 | 6 | 2 | 1 |
| Non-psychiatric/general/medical outpatient | Visit | 14 | 3 | 2 | 16 | 2 | 2 |
| Diabetes clinic | Visit | 11 | 3 | 3 | 9 | 1 | 1 |
| Blood tests | Visit | 79 | 5 | 4 | 69 | 4 | 3 |
| Psychiatric day hospital | Visit | 2 | 2 | 1 | 1 | 6 | - |
| Non-psychiatric/general/medical day hospital | Visit | 2 | 1 | 0 | 2 | 4 | 4 |
| Day surgery centre | Visit | 4 | 2 | 1 | 6 | 1 | 0 |
| Accident and emergency department | Visit | 22 | 2 | 4 | 19 | 2 | 1 |
| X-ray | Visit | 23 | 1 | 1 | 14 | 1 | < 1 |
| Substance misuse clinic | Visit | 3 | 10 | 12 | 3 | 7 | 4 |
| Dietetics | Visit | 4 | 2 | 3 | 1 | 1 | – |
| Community-based day services | |||||||
| Community-based services | Visit | 70 | 44 | 40 | 63 | 40 | 36 |
| Community-based professionals | |||||||
| Care co-ordinator | Surgery visit | 95 | 9 | 7 | 98 | 7 | 7 |
| Care co-ordinator | Home visit | 67 | 9 | 8 | 64 | 8 | 8 |
| Care co-ordinator | Telephone call | 32 | 8 | 10 | 29 | 6 | 7 |
| Home treatment team | Surgery visit | 1 | 1 | – | 1 | 1 | – |
| Home treatment team | Home visit | 10 | 17 | 11 | 3 | 13 | 7 |
| Home treatment team | Telephone call | 1 | 2 | – | 0 | – | – |
| Crisis resolution team | Surgery visit | 1 | 3 | – | 0 | – | – |
| Crisis resolution team | Home visit | 1 | 2 | – | 0 | – | – |
| Crisis resolution team | Telephone call | 1 | 2 | – | 0 | – | – |
| Early intervention team | Surgery visit | 0 | – | – | 0 | – | – |
| Early intervention team | Home visit | 0 | – | – | 0 | – | – |
| Early intervention team | Telephone call | 0 | – | – | 0 | – | – |
| Community psychiatric nurse | Surgery visit | 1 | 6 | – | 2 | 4 | 4 |
| Community psychiatric nurse | Home visit | 2 | 5 | 2 | 3 | 3 | 3 |
| Community psychiatric nurse | Telephone call | 0 | – | – | 0 | – | – |
| Social worker | Surgery visit | 4 | 3 | 3 | 5 | 2 | 1 |
| Social worker | Home visit | 2 | 9 | 4 | 2 | 3 | 2 |
| Social worker | Telephone call | 0 | – | – | 0 | – | – |
| Psychiatrist | Surgery visit | 85 | 2 | 2 | 86 | 2 | 4 |
| Psychiatrist | Home visit | 7 | 5 | 9 | 8 | 6 | 8 |
| Psychiatrist | Telephone call | 0 | – | – | 0 | – | – |
| Psychologist | Surgery visit | 10 | 11 | 10 | 15 | 11 | 13 |
| Psychologist | Home visit | 1 | 14 | – | 1 | 24 | – |
| Psychologist | Telephone call | 0 | – | – | 1 | 1 | – |
| Psychotherapist | Surgery visit | 1 | 4 | – | 1 | 3 | – |
| Psychotherapist | Home visit | 0 | – | – | 0 | – | – |
| Psychotherapist | Telephone call | 0 | – | – | 0 | – | – |
| Counsellor | Surgery visit | 9 | 4 | 5 | 5 | 4 | 2 |
| Counsellor | Home visit | 0 | – | – | 0 | – | – |
| Counsellor | Telephone call | 0 | – | – | 0 | – | – |
| GP | Surgery visit | 110 | 3 | 3 | 104 | 3 | 4 |
| GP | Home visit | 1 | 3 | – | 2 | 3 | 2 |
| GP | Telephone call | 1 | 1 | – | 1 | 1 | – |
| Blood test at GP | Surgery visit | 38 | 2 | 1 | 44 | 2 | 2 |
| Diabetes nurse | Surgery visit | 9 | 2 | 4 | 6 | 2 | 1 |
| Diabetes nurse | Home visit | 0 | – | – | 0 | – | – |
| Diabetes nurse | Telephone call | 0 | – | – | 3 | 1 | 0 |
| Practice nurse | Surgery visit | 33 | 3 | 3 | 21 | 11 | 39 |
| Practice nurse | Home visit | 0 | – | – | 1 | 6 | – |
| Practice nurse | Telephone call | 1 | 2 | – | 0 | – | – |
| District nurse | Surgery visit | 2 | 6 | 6 | 0 | – | – |
| District nurse | Home visit | 0 | – | – | 0 | – | – |
| District nurse | Telephone call | 0 | – | – | 0 | – | – |
| Occupational therapist | Surgery visit | 4 | 6 | 7 | 2 | 6 | 4 |
| Occupational therapist | Home visit | 4 | 22 | 34 | 2 | 3 | 0 |
| Occupational therapist | Telephone call | 1 | 2 | – | 1 | 2 | – |
| Dietitian | Surgery visit | 3 | 1 | 0 | 6 | 3 | 3 |
| Dietitian | Home visit | 0 | – | – | 0 | – | – |
| Dietitian | Telephone call | 0 | – | – | 0 | – | – |
| Home help | Home visit | 11 | 53 | 52 | 7 | 61 | 59 |
| Meals on wheels | Home visit | 2 | 13 | 16 | 0 | – | – |
| Pharmacist for advice | Surgery visit | 16 | 2 | 2 | 14 | 3 | 2 |
| Pharmacist for advice | Home visit | 0 | – | – | 0 | – | – |
| Pharmacist for advice | Telephone call | 2 | 1 | 0 | 0 | – | – |
| NHS Direct | Telephone call | 0 | – | – | 2 | 2 | 1 |
| Samaritans | Telephone call | 5 | 79 | 90 | 4 | 24 | 45 |
| Medication | 159 | – | – | 158 | – | – | |
| Resource | Unit | Trial group | |||||
|---|---|---|---|---|---|---|---|
| Intervention (N = 152) | Control (N = 149) | ||||||
| Users (n) | Meana | SD | Users (n) | Meana | SD | ||
| Specialist accommodation | |||||||
| Supported housing/assisted living | Bed-day | 36 | 90 | 3 | 30 | 90 | 3 |
| Sheltered housing | Bed-day | 2 | 70 | 30 | 6 | 91 | 0 |
| Hostel/shelter | Bed-day | 1 | 81 | – | 5 | 91 | 0 |
| Hospital inpatient | |||||||
| Inpatient | Bed-day | 39 | 90 | 8 | 41 | 90 | 2 |
| Hospital outpatient | |||||||
| Psychiatric outpatient | Visit | 8 | 2 | 1 | 1 | 1 | – |
| Non-psychiatric/general/medical outpatient | Visit | 7 | 1 | < 1 | 10 | 2 | 2 |
| Diabetes clinic | Visit | 4 | 1 | 0 | 6 | 1 | 0 |
| Blood tests | Visit | 63 | 2 | 2 | 56 | 3 | 1 |
| Psychiatric day hospital | Visit | 2 | 5 | 1 | 0 | – | – |
| Non-psychiatric/general/medical day hospital | Visit | 1 | 2 | – | 3 | 1 | 0 |
| Day surgery centre | Visit | 3 | 1 | 0 | 2 | 1 | 0 |
| Accident and emergency department | Visit | 15 | 1 | 1 | 15 | 1 | 1 |
| X-ray | Visit | 10 | 1 | 0 | 12 | 1 | 1 |
| Substance misuse clinic | Visit | 2 | 24 | 17 | 1 | 1 | – |
| Dietetics | Visit | 2 | 1 | 0 | 2 | 1 | 0 |
| Community-based day services | |||||||
| Community-based services | Visit | 57 | 20 | 19 | 50 | 26 | 25 |
| Community-based professionals | |||||||
| Care co-ordinator | Surgery visit | 78 | 5 | 6 | 70 | 4 | 4 |
| Care co-ordinator | Home visit | 59 | 5 | 3 | 52 | 4 | 3 |
| Care co-ordinator | Telephone call | 28 | 5 | 6 | 26 | 5 | 5 |
| Home treatment team | Surgery visit | 2 | 2 | 1 | 1 | 8 | – |
| Home treatment team | Home visit | 7 | 9 | 8 | 4 | 12 | 13 |
| Home treatment team | Telephone call | 0 | – | – | 0 | – | – |
| Crisis resolution team | Surgery visit | 1 | 1 | – | 0 | – | – |
| Crisis resolution team | Home visit | 1 | 1 | – | 1 | 1 | – |
| Crisis resolution team | Telephone call | 0 | – | – | 0 | – | – |
| Early intervention team | Surgery visit | 1 | 36 | – | 0 | – | – |
| Early intervention team | Home visit | 0 | – | – | 0 | – | – |
| Early intervention team | Telephone call | 0 | – | – | 0 | – | – |
| Community psychiatric nurse | Surgery visit | 6 | 6 | 4 | 13 | 4 | 2 |
| Community psychiatric nurse | Home visit | 2 | 4 | 1 | 3 | 1 | 1 |
| Community psychiatric nurse | Telephone call | 3 | 8 | 10 | 4 | 5 | 5 |
| Social worker | Surgery visit | 2 | 8 | 6 | 4 | 5 | 5 |
| Social worker | Home visit | 0 | – | – | 2 | 7 | 7 |
| Social worker | Telephone call | 0 | – | – | 1 | 5 | – |
| Psychiatrist | Surgery visit | 65 | 1 | 1 | 60 | 1 | 1 |
| Psychiatrist | Home visit | 3 | 5 | 6 | 5 | 4 | 5 |
| Psychiatrist | Telephone call | 0 | – | – | 0 | – | – |
| Psychologist | Surgery visit | 14 | 6 | 5 | 8 | 5 | 5 |
| Psychologist | Home visit | 1 | 10 | – | 0 | – | – |
| Psychologist | Telephone call | 0 | – | – | 0 | – | – |
| Psychotherapist | Surgery visit | 2 | 10 | 3 | 0 | – | – |
| Psychotherapist | Home visit | 0 | – | – | 1 | 1 | – |
| Psychotherapist | Telephone call | 0 | – | – | 0 | – | – |
| Counsellor | Surgery visit | 3 | 2 | 2 | 1 | 2 | – |
| Counsellor | Home visit | 0 | – | – | 0 | – | – |
| Counsellor | Telephone call | 0 | – | – | 0 | – | – |
| GP | Surgery visit | 81 | 3 | 2 | 83 | 2 | 1 |
| GP | Home visit | 1 | 1 | – | 0 | – | – |
| GP | Telephone call | 1 | 2 | – | 1 | 4 | – |
| Blood test at GP | Surgery visit | 26 | 2 | 5 | 27 | 1 | 1 |
| Diabetes nurse | Surgery visit | 4 | 2 | 2 | 3 | 1 | 1 |
| Diabetes nurse | Home visit | 1 | 1 | – | 0 | – | – |
| Diabetes nurse | Telephone call | 0 | – | – | 0 | – | – |
| Practice nurse | Surgery visit | 16 | 2 | 2 | 21 | 2 | 1 |
| Practice nurse | Home visit | 0 | – | – | 0 | – | – |
| Practice nurse | Telephone call | 1 | 1 | – | 0 | – | – |
| District nurse | Surgery visit | 3 | 21 | 34 | 2 | 46 | 62 |
| District nurse | Home visit | 1 | 24 | – | 0 | – | – |
| District nurse | Telephone call | 0 | – | – | 0 | – | – |
| Occupational therapist | Surgery visit | 4 | 12 | 9 | 5 | 5 | 4 |
| Occupational therapist | Home visit | 2 | 13 | 16 | 3 | 12 | 0 |
| Occupational therapist | Telephone call | 1 | 3 | – | 0 | – | – |
| Dietitian | Surgery visit | 1 | 1 | – | 6 | 2 | 1 |
| Dietitian | Home visit | 0 | – | – | 1 | 12 | – |
| Dietitian | Telephone call | 0 | – | – | 0 | – | – |
| Home help | Home visit | 12 | 38 | 52 | 4 | 39 | 37 |
| Meals on wheels | Home visit | 4 | 47 | 33 | 1 | 15 | – |
| Pharmacist for advice | Surgery visit | 6 | 3 | 2 | 8 | 3 | 4 |
| Pharmacist for advice | Home visit | 0 | – | – | 0 | – | – |
| Pharmacist for advice | Telephone call | 2 | 2 | 1 | 1 | 1 | – |
| NHS Direct | Telephone call | 2 | 7 | 8 | 5 | 3 | 5 |
| Samaritans | Telephone call | 4 | 36 | 39 | 4 | 15 | 21 |
| Medication | 149 | 145 | |||||
Costs
There were 52 care co-ordinators in the intervention group and a total of 213 patient participants with whom they implemented the HPI. The mean number of trial participants per care co-ordinator was four (range 1–10 participants). The total average cost per case in the intervention arm was £226.40, compared with £3.52 in the control arm.
Health and social care and lost productivity formed the largest components of total societal costs. Costs at baseline, 12 months and 15 months are summarised for reference in Table 21. The mean costs of all summary components were similar between trial arms at all assessment points, except the cost of the intervention, which was naturally higher in the intervention group given the additional inputs required compared with the control group (adjusted mean difference £311, 95% CI £267 to £355), and costs borne by charities, which were higher in the intervention group at 12 months (adjusted mean difference £80, 95% CI £9 to £151).
| Costs | Valid (n) | Intervention costs (£) (N = 213) | Valid (n) | Control costs (£) (N = 193) | Unadjusted mean | 95% CIa | Adjusted mean | 95% CIb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Differencea | Differenceb | |||||
| Costs at baseline | ||||||||||
| Health and social care excluding interventionc | 212 | 10,242 | 13,374 | 193 | 9714 | 13,767 | 528 | –2953 to 4010 | 967 | –2442 to 4435 |
| Charityc | 212 | 83 | 611 | 193 | 80 | 435 | 3 | –109 to 115 | –22 | –137 to 94 |
| Lost productivityc | 212 | 8755 | 5964 | 193 | 7472 | 6311 | 1283 | –354 to 2920 | 456 | –894 to 1806 |
| Patientc | 212 | 72 | 433 | 193 | 188 | 188 | 35 | –31 to 102 | 33 | –37 to 104 |
| Benefitsc | 212 | 2211 | 1006 | 193 | 2009 | 940 | 202d | 13 to 391d | 127 | –70 to 324 |
| Costs at 12 months | ||||||||||
| Health and social care excluding interventionc | 160 | 10,220 | 12,341 | 159 | 10,196 | 16,987 | 24 | –4219 to 4267 | –1596 | –5145 to 1954 |
| Charityc | 160 | 120 | 369 | 159 | 61 | 256 | 60 | –6 to 125 | 80d | 9 to 151d |
| Lost productivityc | 160 | 8882 | 5998 | 159 | 7707 | 6333 | 1174 | –317 to 2665 | 1038 | –367 to 2443 |
| Patientc | 160 | 84 | 369 | 159 | 53 | 300 | 31 | –38 to 100 | 25 | –46 to 96 |
| Benefitc | 160 | 2328 | 931 | 159 | 2129 | 957 | 200 | –14 to 413 | 87 | –105 to 279 |
| Costs at 15 months | ||||||||||
| Health and social care excluding interventione | 152 | 4874 | 6317 | 149 | 4708 | 6383 | 166 | –1577 to 1910 | –231 | –1734 to 1272 |
| Charitye | 152 | 63 | 215 | 149 | 49 | 230 | 14 | –39 to 67 | 24 | –37 to 84 |
| Lost productivitye | 152 | 4731 | 2674 | 149 | 3880 | 3027 | 850d | 127 to 1573d | 608 | –25 to 1240 |
| Patiente | 152 | 24 | 141 | 149 | 30 | 162 | –6 | –38 to 27 | –6 | –37 to 25 |
| Benefitse | 152 | 1089 | 439 | 149 | 1049 | 441 | 40 | –70 to 150 | –24 | –125 to 76 |
| Intervention | 213 | 316 | 173 | 193 | 4 | 0 | 312d | 267 to 357d | 3142d | 268 to 359d |
Comparisons of the total costs from both health and social care and societal perspectives at 15 months suggested no difference between the trial arms, although the 95% CIs suggested a tendency for societal costs to be higher in the intervention arm (Table 22). Imputing the missing total costs at 15 months for the sensitivity analyses confirmed this (Table 23).
| Costs | Valid (n) | Intervention costs (£) (N = 213) | Valid (n) | Control costs (£) (N = 193) | Unadjusted mean differencea | 95% CIa | Adjusted mean differenceb | 95% CIb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||||
| Health and social care including interventionc | 152 | 5209 | 6326 | 149 | 4711 | 6383 | 498 | –1248 to 2244 | 95 | –1410 to 1599 |
| Societal perspective including interventionc | 152 | 11,116 | 7271 | 149 | 9720 | 7707 | 1396 | –684 to 3476 | 675 | –1039 to 2388 |
| Costs | Valid (n) | Intervention costs (£) (N = 213) | Valid (n) | Control costs (£) (N = 193) | Unadjusted mean differencea | 95% CIa | Adjusted mean differenceb | 95% CIb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||||
| Health and social care including interventionc | 213 | 5077 | 5652 | 193 | 4705 | 5848 | 372 | –1047 to 1791 | 51 | –1091 to 1192 |
| Societal perspective including interventionc | 213 | 10,830 | 6595 | 193 | 9683 | 7129 | 1147 | –551 to 2845 | 537 | –776 to 1850 |
Outcomes
There were no differences in any outcome at any time point (Table 24). As with cost data, imputing missing outcome data for those lost to follow-up (Table 25) did not alter the conclusions drawn from the base-case analyses of only those with available data.
| Time point | Intervention (N = 213) | Control (N = 193) | Unadjusted mean differencea | 95% CIa | Adjusted mean differenceb | 95% CIb | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Valid (n) | Mean | SD | Valid (n) | Mean | SD | |||||
| Baseline | ||||||||||
| SF-36 MCS | 213 | 41.37 | 13.26 | 193 | 42.25 | 11.81 | –0.88 | –3.44 to 1.68 | –0.26 | –1.55 to 1.02 |
| SF-36 PCS | 213 | 45.83 | 10.94 | 193 | 47.04 | 9.26 | –1.20 | –3.31 to 0.91 | –0.60 | –1.72 to 0.52 |
| SF-36 utility | 210 | 0.69 | 0.16 | 192 | 0.71 | 0.14 | –0.02 | –0.05 to 0.02 | 0.00 | –0.01 to 0.01 |
| EQ-5D-3L utility | 211 | 0.76 | 0.31 | 193 | 0.79 | 0.28 | –0.02 | –0.08 to 0.04 | 0.01 | –0.04 to 0.06 |
| 12 months | ||||||||||
| SF-36 MCS | 160 | 43.18 | 13.31 | 158 | 44.09 | 13.47 | –0.91 | –3.94 to 2.11 | –0.05 | –2.64 to 2.55 |
| SF-36 PCS | 160 | 46.76 | 11.23 | 158 | 49.02 | 10.55 | –2.27 | –4.74 to 0.21 | –1.45 | –3.56 to 0.66 |
| SF-36 utility | 158 | 0.70 | 0.16 | 155 | 0.71 | 0.15 | –0.02 | –0.05 to 0.02 | –0.00 | –0.03 to 0.02 |
| EQ-5D-3L utility | 159 | 0.80 | 0.25 | 156 | 0.80 | 0.28 | 0.00 | –0.06 to 0.06 | 0.03 | –0.03 to 0.08 |
| 15 months | ||||||||||
| SF-36 MCS | 152 | 42.47 | 13.58 | 149 | 45.01 | 13.65 | –2.54 | –6.00 to 0.92 | –0.80 | –3.66 to 2.06 |
| SF-36 PCS | 152 | 47.25 | 11.62 | 149 | 48.54 | 9.88 | –1.29 | –4.02 to 1.44 | –0.68 | –3.01 to 1.65 |
| SF-36 utility | 149 | 0.66 | 0.14 | 148 | 0.70 | 0.15 | –0.03c | –0.07 to –0.00c | –0.02 | –0.05 to 0.01 |
| SF-36-based QALY gain | 134 | 0.17 | 0.03 | 139 | 0.17 | 0.09 | –0.01 | –0.01 to 0.00 | –0.00 | –0.01 to 0.00 |
| EQ-5D-3L utility | 152 | 0.77 | 0.24 | 149 | 0.80 | 0.25 | –0.02 | –0.09 to 0.04 | 0.00 | –0.06 to 0.06 |
| EQ-5D-3L-based QALY gain | 137 | 0.19 | 0.05 | 140 | 0.20 | 0.06 | –0.00 | –0.02 to 0.01 | 0.00 | –0.01 to 0.02 |
| Outcome | Intervention (N = 213) | Control (N = 193) | Unadjusted mean differencea | 95% CI | Adjusted mean differenceb | 95% CIa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Valid (n) | Mean | SD | Valid (n) | Mean | SD | |||||
| SF-36 MCS | 213 | 42.95 | 12.59 | 193 | 44.84 | 12.52 | –1.88 | –4.75 to 0.98 | –0.64 | –2.77 to 1.49 |
| SF-36 PCS | 213 | 47.72 | 10.58 | 193 | 48.97 | 9.18 | –1.25 | –3.23 to 0.73 | –0.47 | –2.09 to 1.14 |
| SF-36-based QALY | 213 | 0.17 | 0.03 | 193 | 0.17 | 0.03 | –0.01 | –0.01 to 0.00 | –0.00 | –0.00 to 0.00 |
| EQ-5D-3L-based QALY | 213 | 0.19 | 0.05 | 193 | 0.20 | 0.05 | –0.00 | –0.01 to 0.01 | 0.00 | –0.01 to 0.01 |
Cost-effectiveness and cost–utility analyses
The two trial arms showed the same costs and same outcomes for all prespecified 15-month cost–outcome combinations using the base-case approach (Table 26); therefore, it was not necessary to compute any ICERs. Furthermore, none of the four sensitivity analyses altered the conclusions about between-group differences in total costs or outcomes.
| Costs | Cost per additional point improvement on the SF-36 PCS | Cost per additional point improvement on the SF-36 MCS | Cost per additional QALY (SF-36 based) | Cost per additional QALY (EQ-5D-3L based) |
|---|---|---|---|---|
| Control vs. intervention | Control vs. intervention | Control vs. intervention | Control vs. intervention | |
| Health and social costs including intervention costs | Same cost, same outcome | Same cost, same outcome | Same cost, same outcome | Same cost, same outcome |
| Societal perspective costs including intervention costs | Same cost, same outcome | Same cost, same outcome | Same cost, same outcome | Same cost, same outcome |
The cost-effectiveness planes from health/social care and societal perspectives for all four outcomes are in Figures 4–11, showing no difference between the groups in costs or outcomes. Although cost-effectiveness planes for EQ-5D-3L-based QALYs from the health and social perspective indicate a tendency for estimates to predominate in the north-east or south-east quadrants (indicating higher or lower costs and better outcomes), those for the SF-36 MCS and SF-36 PCS show more estimates falling into the north-west or south-west quadrants (indicating higher or lower costs and worse outcomes). Cost-effectiveness planes from the societal perspective (see Figures 8–11) all suggest that mean costs in the intervention arm are likely to be higher than in the control arm, as was suggested by the 95% CIs for estimates of mean cost differences (see Tables 22 and 23).
FIGURE 4.
Cost-effectiveness plane of mean differences in EQ-5D-3L-based QALYs and health and social care costs.
FIGURE 5.
Cost-effectiveness plane of mean differences in SF-36-based QALYs and health and social care costs.
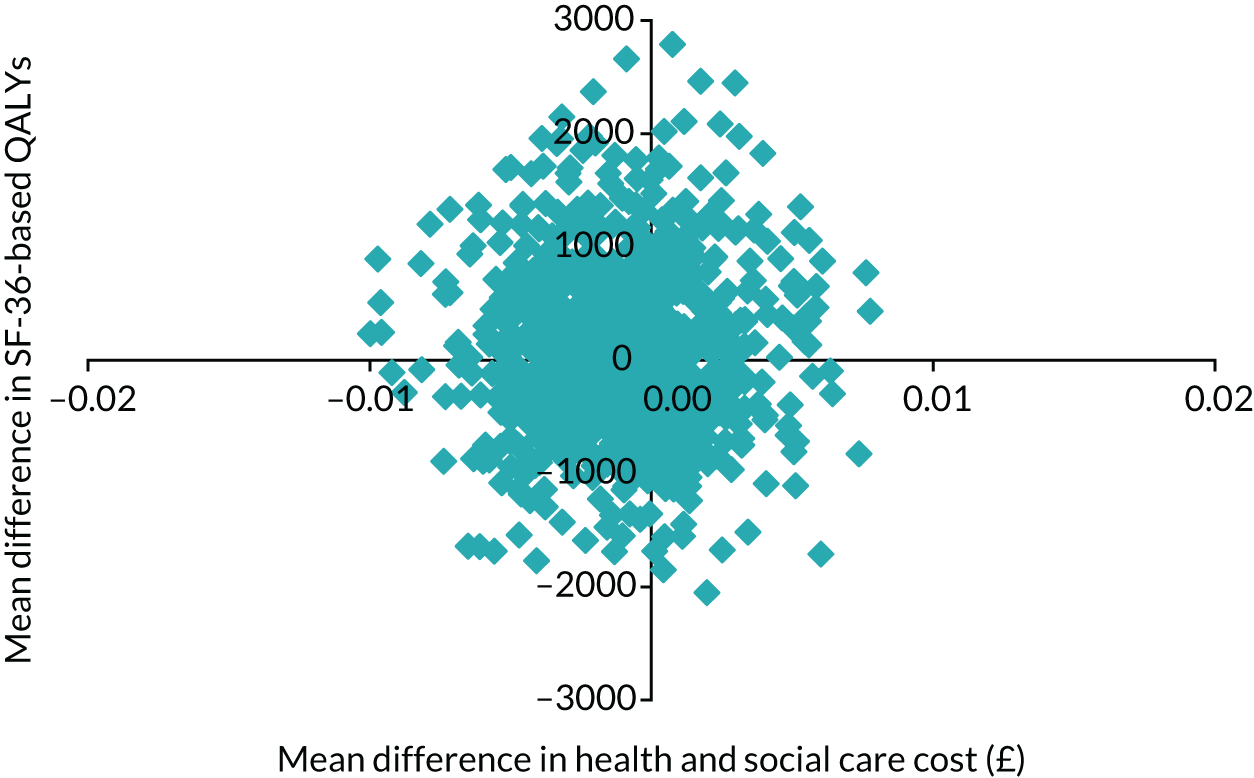
FIGURE 6.
Cost-effectiveness plane of mean differences in SF-36 MCS and health and social care costs.
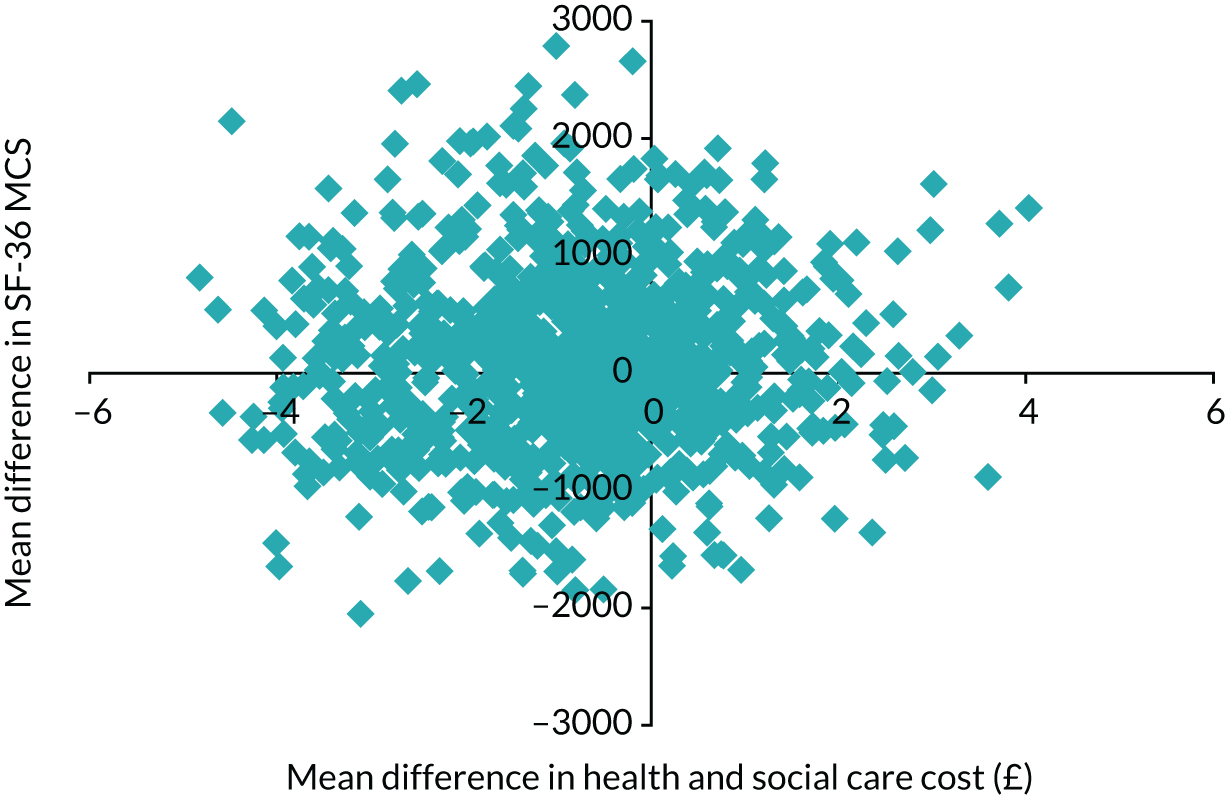
FIGURE 7.
Cost-effectiveness plane of mean differences in SF-36 PCS and health and social care costs.
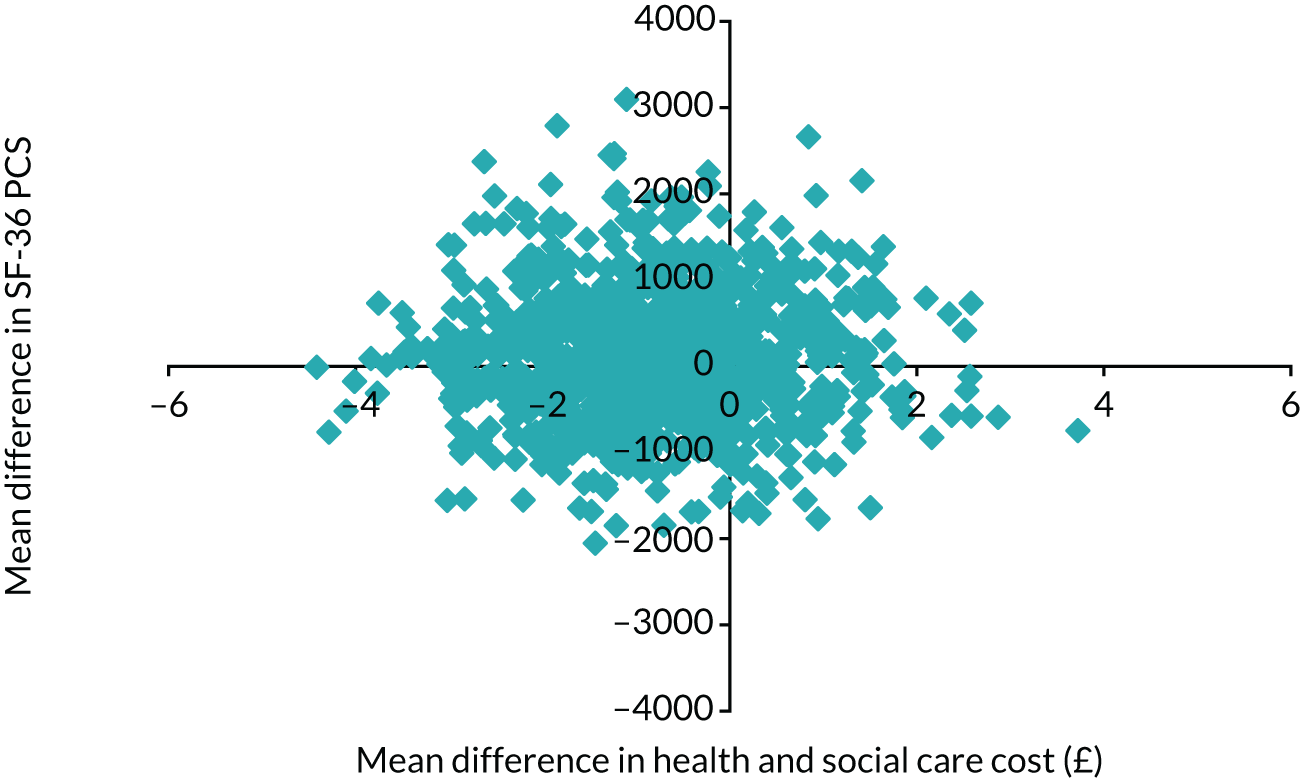
FIGURE 8.
Cost-effectiveness plane of mean differences in EQ-5D-3L-based QALYs and societal costs.
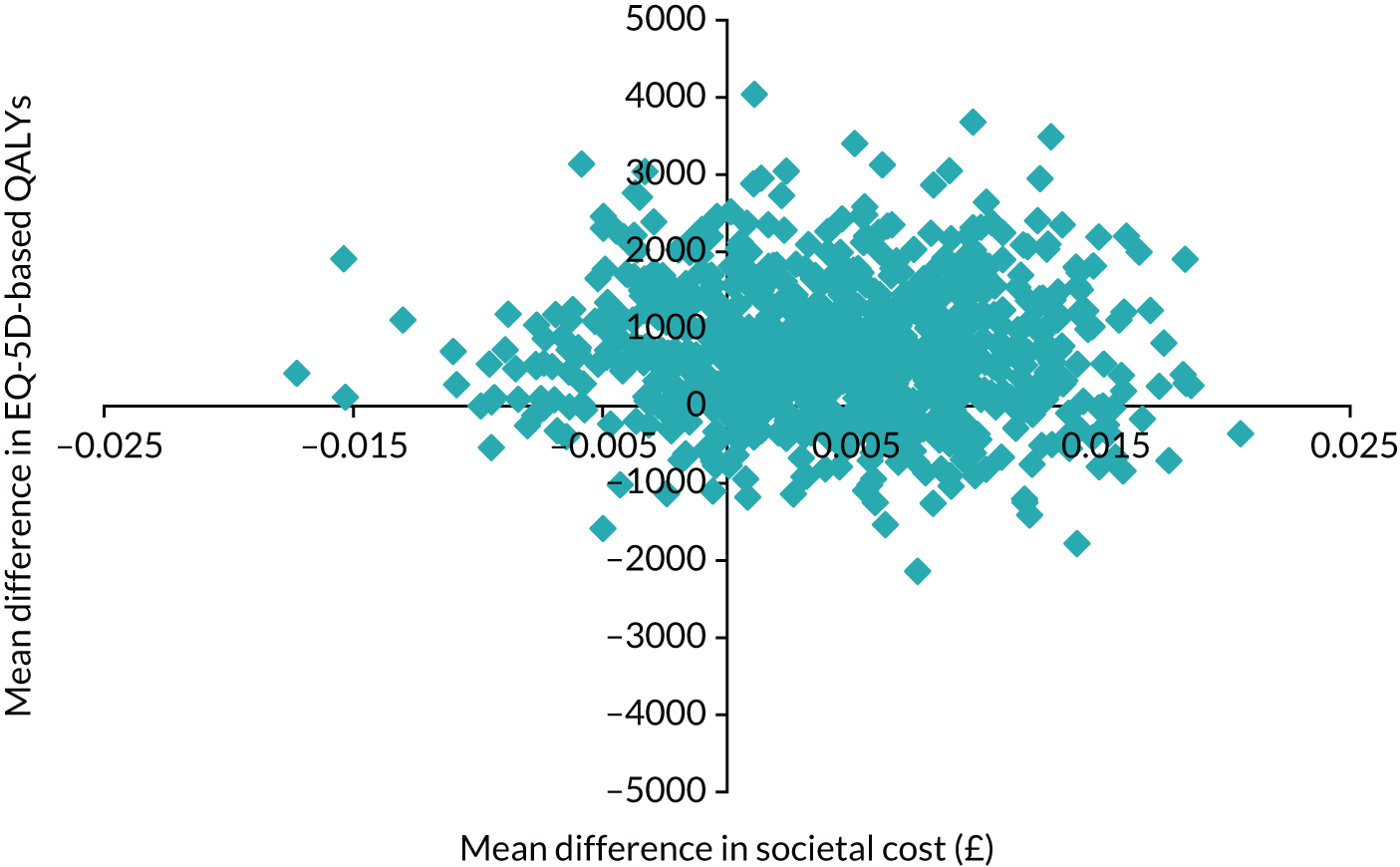
FIGURE 9.
Cost-effectiveness plane of mean differences in SF-36-based QALYs and societal costs.
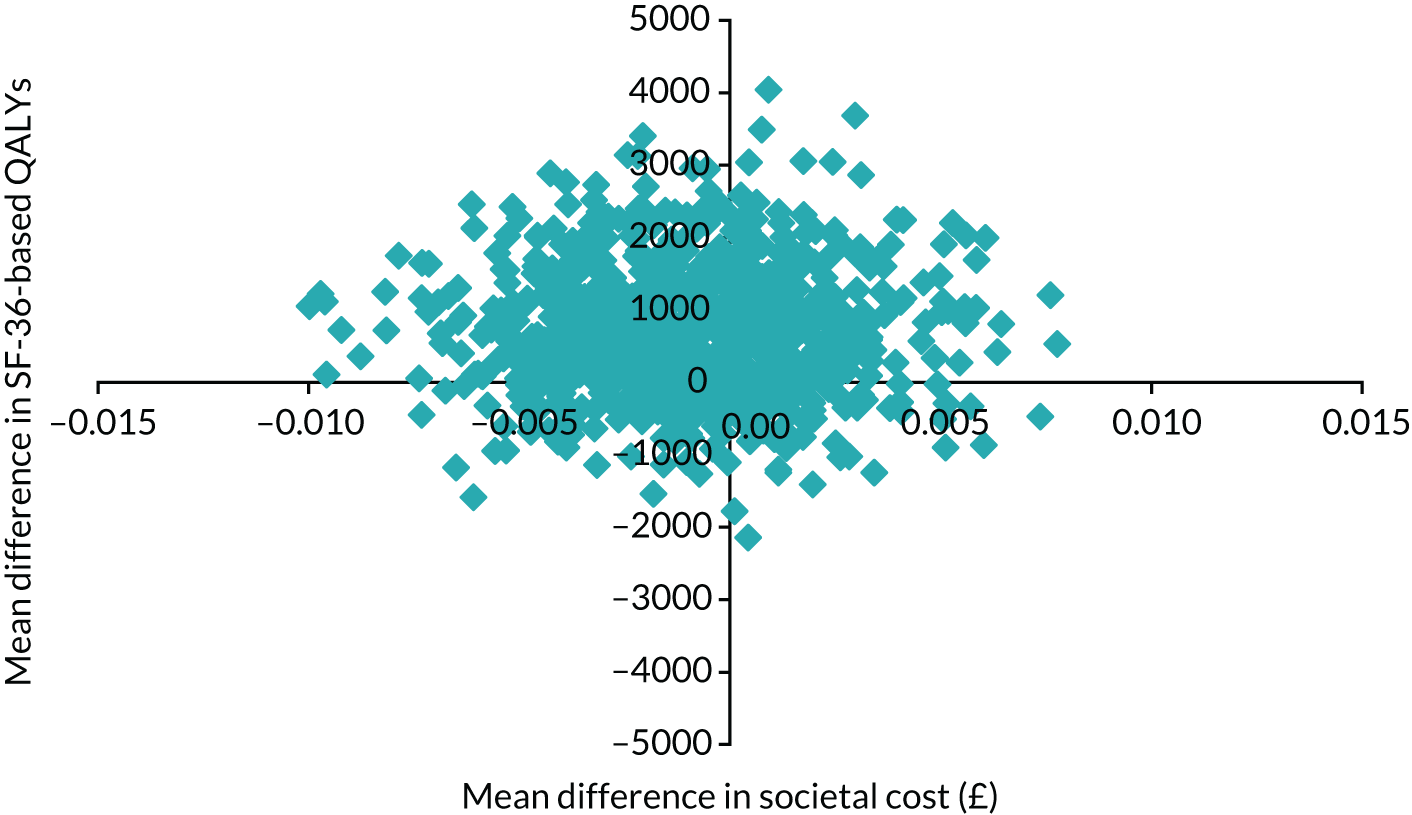
FIGURE 10.
Cost-effectiveness plane of mean differences in SF-36 MCS and societal costs.
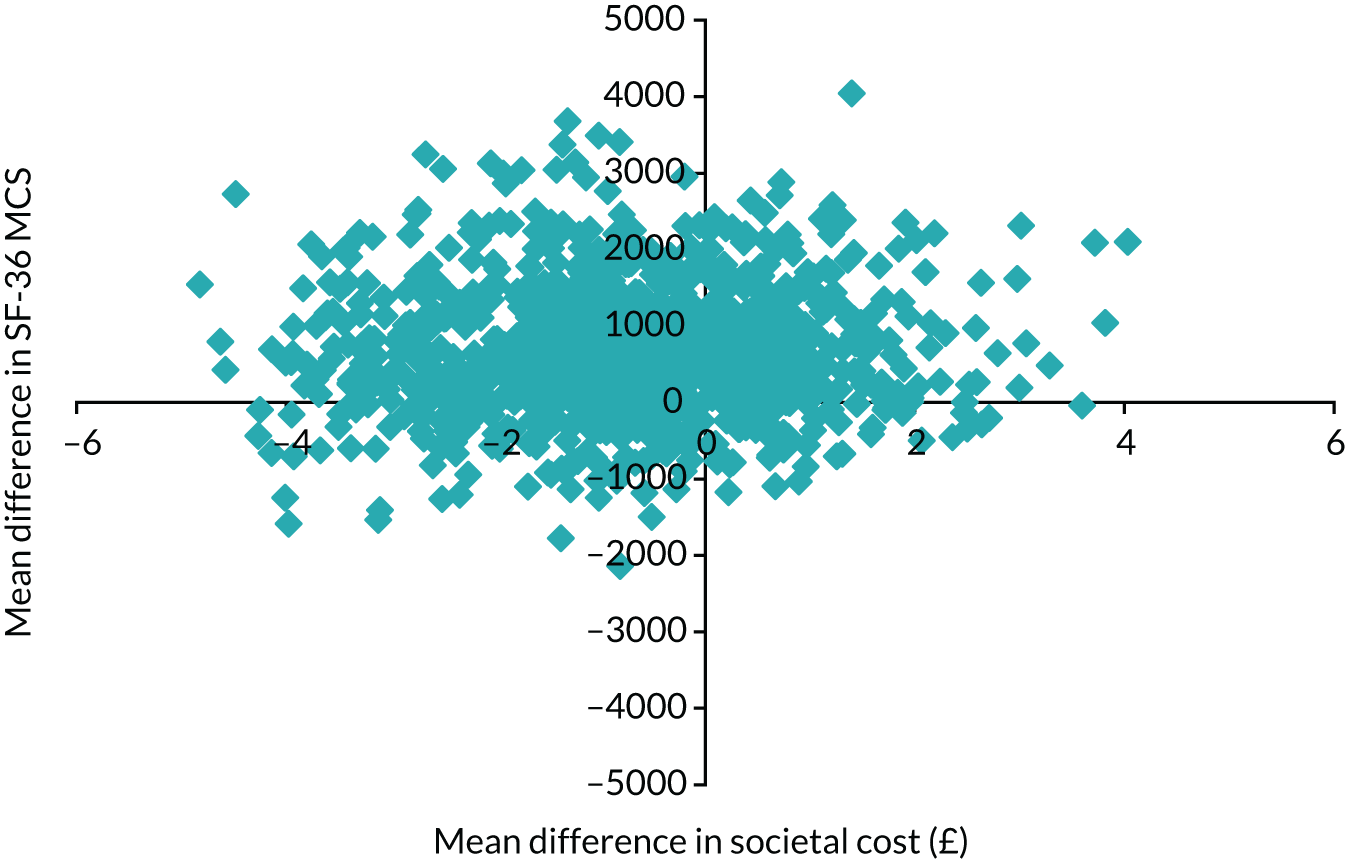
FIGURE 11.
Cost-effectiveness plane of mean differences in SF-36 PCS and societal costs.
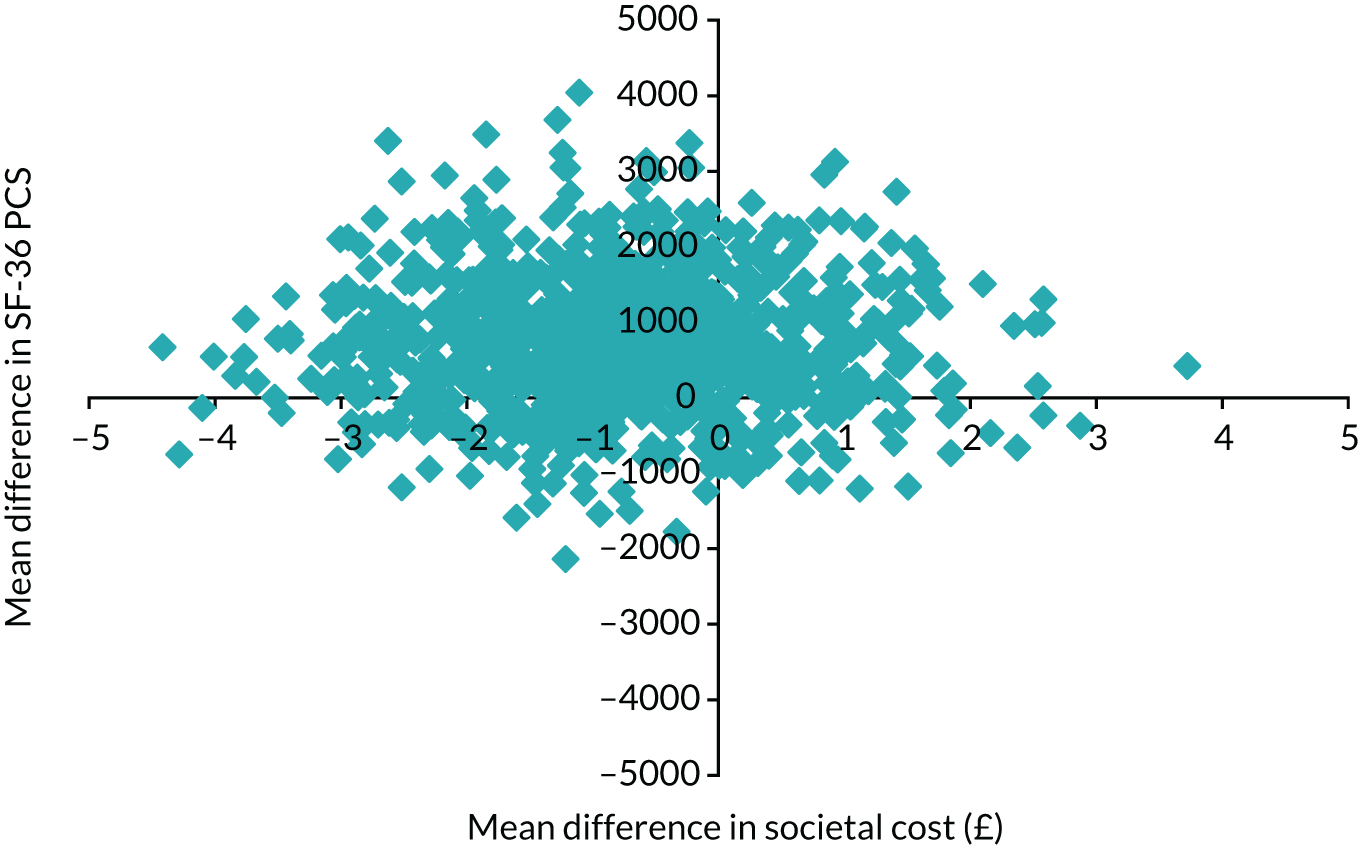
From a health and social care perspective, the probability that the HPI is cost-effective does not exceed 0.4 for any of the examined willingness-to-pay thresholds for QALY gains (based on either the SF-36 or the EQ-5D-3L). When considering willingness to pay for point improvements in the PCS and MCS of the SF-36, the probability of the intervention being cost-effective starts at 0.34 at a willingness to pay of £0 but reduces to around 0.2 for willingness-to-pay values between £5000 and £50,000 (Figures 12–15).
FIGURE 12.
Cost-effectiveness acceptability curves for SF-36- and EQ-5D-3L-based QALYs from a health and social care perspective.
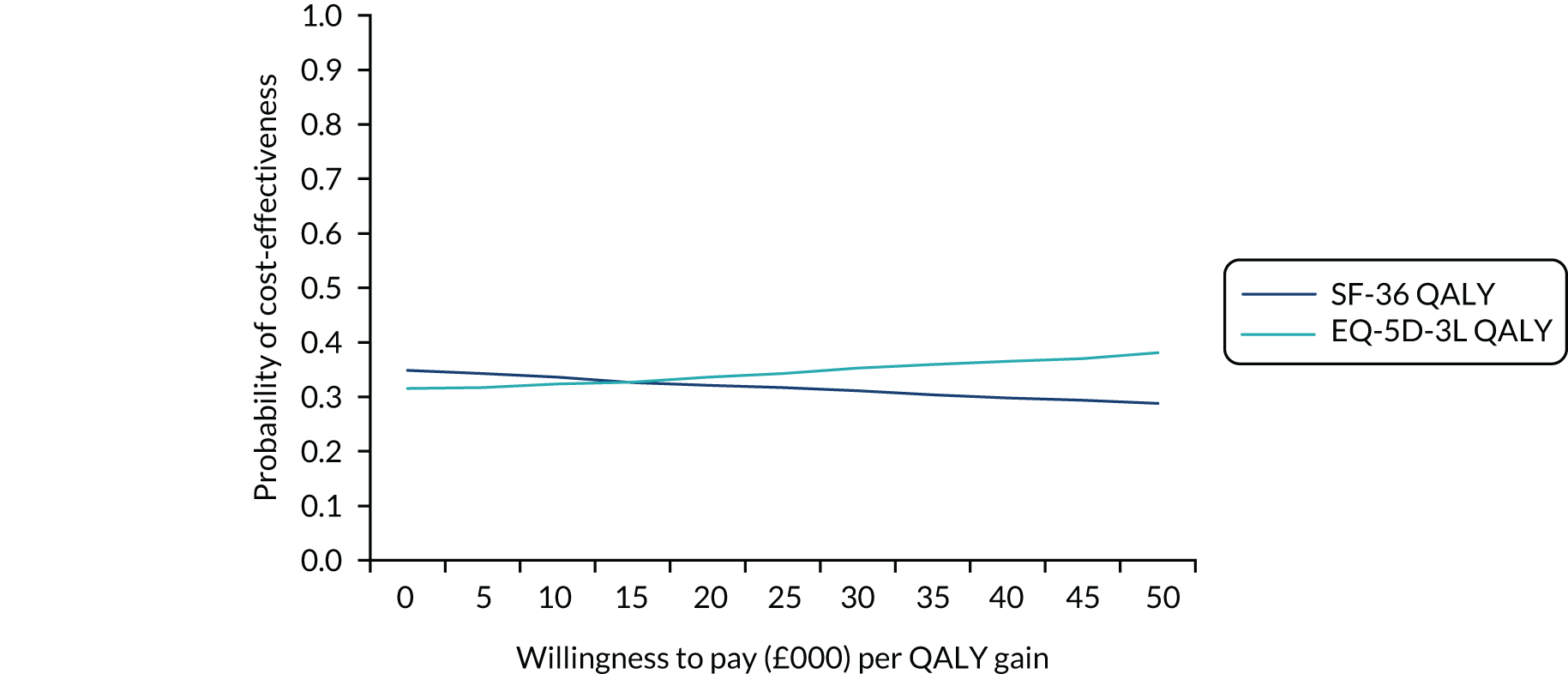
FIGURE 13.
Cost-effectiveness acceptability curves for the PCS and MCS of the SF-36 from a health and social care perspective.
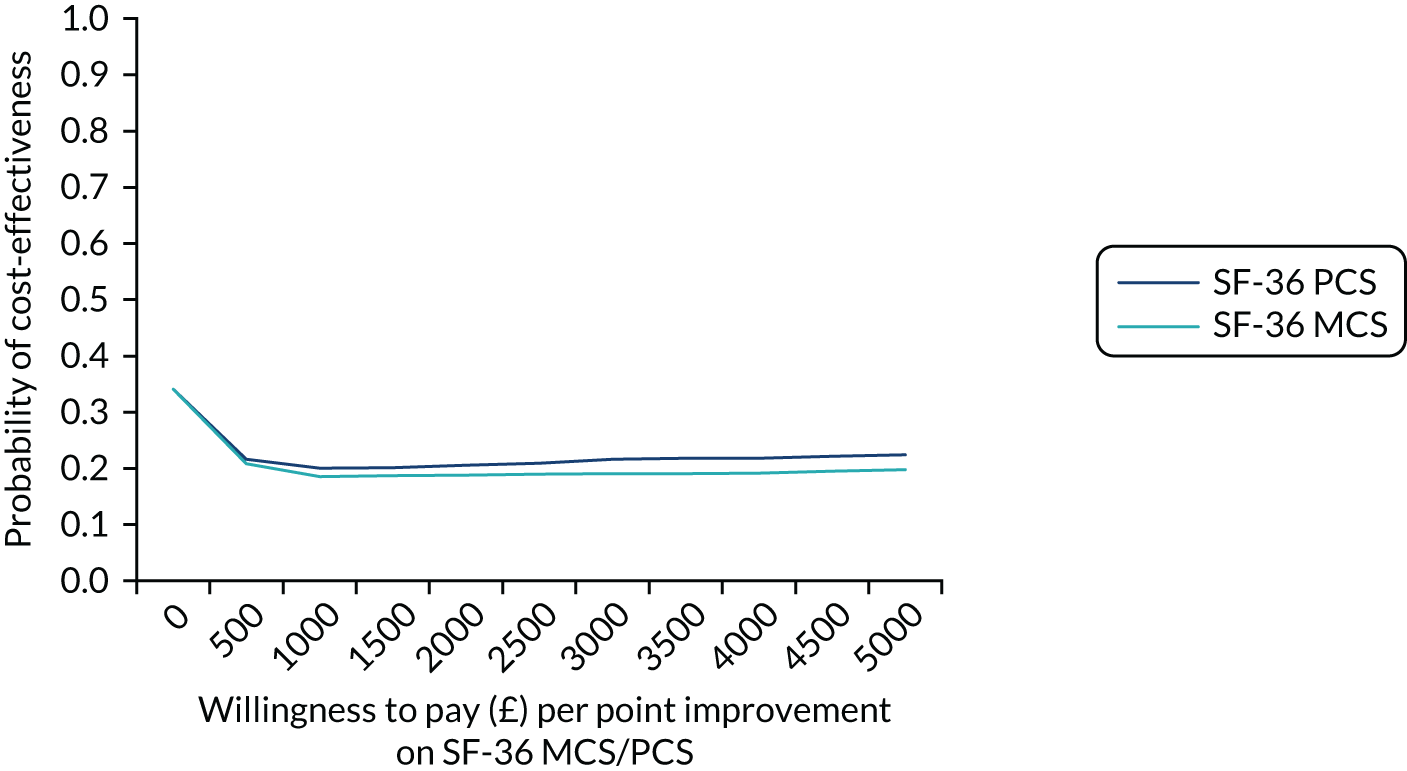
FIGURE 14.
Cost-effectiveness acceptability curves for SF-36- and EQ-5D-3L-based QALYs from a societal perspective.
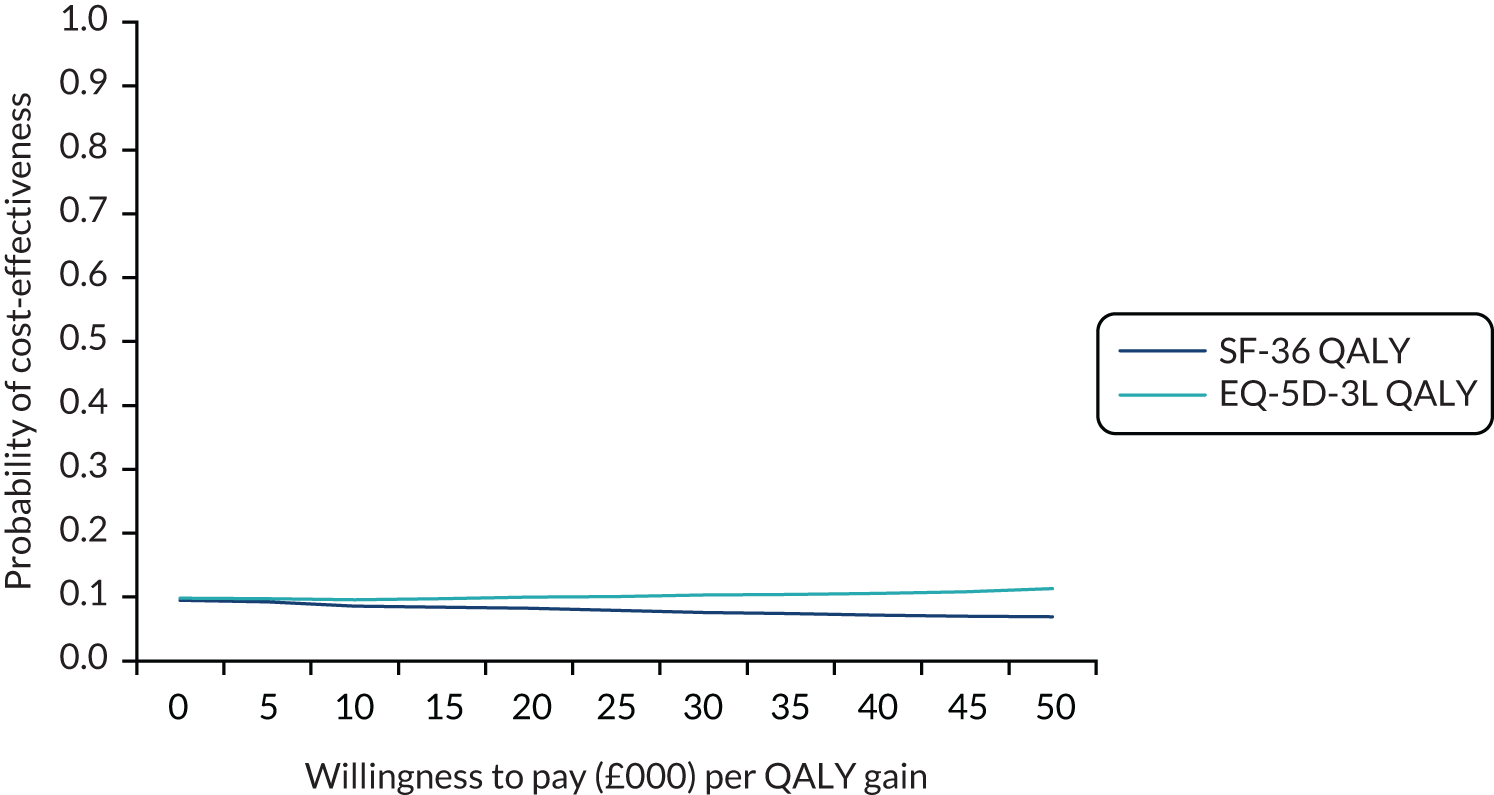
FIGURE 15.
Cost-effectiveness acceptability curves for the PCS and MCS of the SF-36 from a societal perspective.
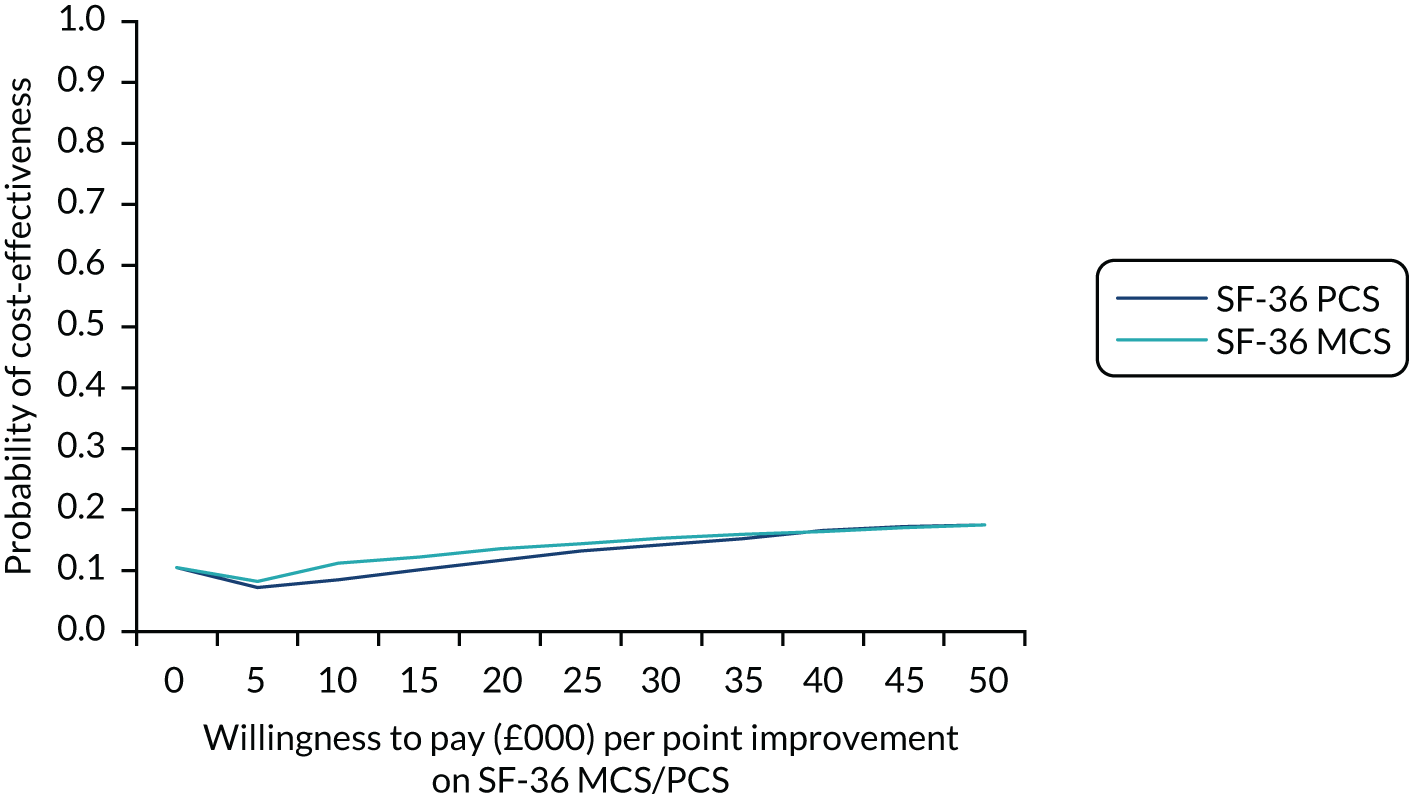
Conclusions are similar from a societal perspective: the probability that the HPI is cost-effective does not exceed 0.12 for any of the willingness-to-pay thresholds examined for QALY gains (calculated from either the SF-36 or the EQ-5D-3L), or 0.21 for the thresholds examined for the point improvements in the two SF-36 component scores.
Appendix 5 Primary outcome analyses
Appendix 4 shows that sensitivity analyses did not alter the conclusion that there was no treatment effect at any time point for the two main outcomes, PHC and MHC score. Furthermore, no changes over time could be detected. Tables 27 and 28 show the results of the main analyses presented in Gaughran et al. 176
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | –1.7 | 0.98 | –1.73 | 0.083 | –3.63 to 0.23 |
| Time (15 months = 1) | –0.23 | 0.72 | –0.32 | 0.746 | –1.64 to 1.17 |
| Arm × time | 1.1 | 1.02 | 1.08 | 0.282 | –0.9 to 3.1 |
| Borough | χ2(9) = 3.71; p = 0.93 | ||||
| Croydon | 0 | ||||
| Lambeth | 1.72 | 1.66 | 1.04 | 0.3 | –1.53 to 4.97 |
| Lewisham | 0.93 | 1.39 | 0.67 | 0.503 | –1.8 to 3.66 |
| Southwark | 2.02 | 1.36 | 1.49 | 0.136 | –0.64 to 4.68 |
| Greenwich | 2.09 | 1.74 | 1.2 | 0.229 | –1.32 to 5.51 |
| Bromley | 1.75 | 1.77 | 0.99 | 0.323 | –1.72 to 5.23 |
| Bexley | 1.24 | 2 | 0.62 | 0.536 | –2.68 to 5.15 |
| East Sussex | 1.69 | 1.9 | 0.89 | 0.375 | –2.04 to 5.41 |
| Somerset | 2.8 | 2.56 | 1.09 | 0.274 | –2.22 to 7.82 |
| South Staffordshire | 3.23 | 3.26 | 0.99 | 0.322 | –3.16 to 9.61 |
| PHC baseline | 0.61 | 0.04 | 15.77 | 0 | 0.53 to 0.69 |
| Constant | 18 | 2.22 | 8.12 | 0 | 13.66 to 22.35 |
| Pairwise comparison | Coefficient | SE | z | p-value | 95% CI |
| 12 months: TAU vs. HPI | –1.7 | 0.98 | –1.73 | 0.083 | –3.63 to 0.23 |
| 15 months: TAU vs. HPI | –0.6 | 0.99 | –0.61 | 0.541 | –2.54 to 1.33 |
| Group HPI: 15 months vs. 12 months | –0.23 | 0.72 | –0.32 | 0.746 | –1.64 to 1.17 |
| Group TAU: 15 months vs. 12 months | 0.87 | 0.72 | 1.19 | 0.232 | –0.55 to 2.29 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | 0 | 0 | |||
| Residual | |||||
| SD (12 months) | 8.58 | 0.34 | 7.94 to 9.27 | ||
| SD (15 months) | 8.54 | 0.35 | 7.89 to 9.25 | ||
| Correlation (12 months, 15 months) | 0.48 | 0.04 | 0.39 to 0.56 | ||
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | –0.41 | 1.29 | –0.32 | 0.751 | –2.95 to 2.13 |
| Time (15 months = 1) | 1 | 0.87 | 1.16 | 0.248 | –0.7 to 2.71 |
| Arm × time | –0.71 | 1.36 | –0.53 | 0.598 | –3.37 to 1.94 |
| Borough | χ2(9) = 14.69; p = 0.10 | ||||
| Croydon | 0 | ||||
| Lambeth | –1.91 | 2.17 | –0.88 | 0.378 | –6.16 to 2.34 |
| Lewisham | –1.97 | 1.85 | –1.06 | 0.288 | –5.6 to 1.66 |
| Southwark | –5.33 | 1.8 | –2.96 | 0.003 | –8.85 to –1.8 |
| Greenwich | –5.23 | 2.35 | –2.23 | 0.026 | –9.83 to –0.63 |
| Bromley | –3.7 | 2.34 | –1.58 | 0.113 | –8.28 to 0.88 |
| Bexley | –0.99 | 2.65 | –0.37 | 0.709 | –6.18 to 4.2 |
| East Sussex | –4.95 | 2.5 | –1.98 | 0.047 | –9.84 to –0.06 |
| Somerset | –6.91 | 3.37 | –2.05 | 0.04 | –13.53 to –0.3 |
| South Staffordshire | –2.09 | 4.44 | –0.47 | 0.637 | –10.79 to 6.6 |
| PHC baseline | 0.58 | 0.04 | 14.23 | 0 | 0.5 to 0.67 |
| Constant | 21.25 | 2.32 | 9.15 | 0 | 16.7 to 25.79 |
| Pairwise comparison | Coefficient | SE | z | p-value | 95% CI |
| 12 months: B vs. A | –0.41 | 1.29 | –0.32 | 0.751 | –2.95 to 2.13 |
| 15 months: B vs. A | –1.13 | 1.31 | –0.86 | 0.389 | –3.69 to 1.44 |
| Group TAU: 15 months vs. 12 months | 1 | 0.87 | 1.16 | 0.248 | –0.7 to 2.71 |
| Group HPI: 15 months vs. 12 months | 0.29 | 1.04 | 0.28 | 0.78 | –1.75 to 2.33 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | 1.68 | 1.3 | 0.37 to 7.65 | ||
| Residual | |||||
| Group TAU | |||||
| SD (12 months, 15 months) | 10.42 | 0.51 | 9.46 to 11.47 | ||
| Correlation (12 months, 15 months) | 0.49 | 0.07 | 0.34 to 0.61 | ||
| Group HPI | |||||
| SD (12 months, 15 months) | 11.22 | 0.52 | 10.24 to 12.3 | ||
| Correlation (12 months, 15 months) | 0.37 | 0.08 | 0.21 to 0.51 | ||
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | –1.86 | 1.07 | –1.74 | 0.082 | –3.95 to 0.24 |
| Time (15 months = 1) | –0.09 | 0.81 | –0.12 | 0.907 | –1.68 to 1.49 |
| Arm × time | 0.69 | 1.15 | 0.6 | 0.551 | –1.57 to 2.95 |
| Borough | χ2(9) = 8.46; p = 0.49 | ||||
| Croydon | |||||
| Lambeth | 1.3 | 1.76 | 0.74 | 0.461 | –2.15 to 4.74 |
| Lewisham | 1.48 | 1.5 | 0.99 | 0.324 | –1.46 to 4.41 |
| Southwark | 2.27 | 1.46 | 1.56 | 0.119 | –0.59 to 5.12 |
| Greenwich | 4.77 | 1.92 | 2.48 | 0.013 | 1.01 to 8.53 |
| Bromley | 1.2 | 1.96 | 0.61 | 0.539 | –2.63 to 5.04 |
| Bexley | 2.35 | 2.21 | 1.06 | 0.288 | –1.99 to 6.69 |
| East Sussex | 2.17 | 2.01 | 1.08 | 0.279 | –1.76 to 6.11 |
| Somerset | 3.58 | 2.77 | 1.29 | 0.197 | –1.85 to 9.01 |
| South Staffordshire | 5.49 | 3.7 | 1.48 | 0.138 | –1.76 to 12.75 |
| PHC baseline | 0.59 | 0.04 | 13.91 | 0 | 0.5 to 0.67 |
| Age at baseline | –0.11 | 0.05 | –2.4 | 0.016 | –0.2 to –0.02 |
| Constant | 23.85 | 3.51 | 6.8 | 0 | 16.97 to 30.72 |
| Pairwise comparison | Coefficient | SE | z | p-value | 95% CI |
| 12 months: TAU vs. HPI | –1.86 | 1.07 | –1.74 | 0.082 | –3.95 to 0.24 |
| 15 months: TAU vs. HPI | –1.17 | 1.08 | –1.08 | 0.28 | –3.29 to 0.95 |
| Group HPI: 15 months vs. 12 months | –0.09 | 0.81 | –0.12 | 0.907 | –1.68 to 1.49 |
| Group TAU: 15 months vs. 12 months | 0.59 | 0.83 | 0.72 | 0.474 | –1.03 to 2.21 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | 0.9 | 1.65 | 0.02 to 33.04 | ||
| Residual | |||||
| SD (12 months) | 8.32 | 0.39 | 7.58 to 9.13 | ||
| SD (15 months) | 8.13 | 0.43 | 7.34 to 9.01 | ||
| Correlation (12 months, 15 months) | 0.48 | 0.06 | 0.36 to 0.59 | ||
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | 0.38 | 1.42 | 0.27 | 0.789 | –2.4 to 3.15 |
| Time (15 months = 1) | 0.19 | 1 | 0.19 | 0.851 | –1.78 to 2.16 |
| Arm × time | –1 | 1.58 | –0.63 | 0.526 | –4.09 to 2.09 |
| Borough | χ2(9) = 8.79; p = 0.46 | ||||
| Croydon | 0 | ||||
| Lambeth | –1.68 | 2.32 | –0.73 | 0.468 | –6.23 to 2.86 |
| Lewisham | –2.17 | 2.03 | –1.07 | 0.286 | –6.15 to 1.81 |
| Southwark | –4.3 | 1.96 | –2.2 | 0.028 | –8.13 to –0.46 |
| Greenwich | –3.63 | 2.6 | –1.4 | 0.163 | –8.73 to 1.47 |
| Bromley | –4.22 | 2.62 | –1.61 | 0.107 | –9.35 to 0.91 |
| Bexley | –0.68 | 2.95 | –0.23 | 0.819 | –6.45 to 5.1 |
| East Sussex | –4.9 | 2.67 | –1.83 | 0.067 | –10.13 to 0.34 |
| Somerset | –6.15 | 3.69 | –1.67 | 0.095 | –13.38 to 1.08 |
| South Staffordshire | –3.34 | 5.01 | –0.67 | 0.505 | –13.16 to 6.47 |
| PHC baseline | 0.58 | 0.04 | 12.96 | 0 | 0.49 to 0.67 |
| Age at baseline | 0.06 | 0.06 | 1 | 0.316 | –0.05 to 0.17 |
| Constant | 18.59 | 3.54 | 5.26 | 0 | 11.66 to 25.52 |
| Pairwise comparison | Coefficient | SE | z | p-value | 95% CI |
| 12 months: B vs. A | 0.38 | 1.42 | 0.27 | 0.789 | –2.4 to 3.15 |
| 15 months: B vs. A | –0.62 | 1.47 | –0.42 | 0.671 | –3.49 to 2.25 |
| Group TAU: 15 months vs. 12 months | 0.19 | 1 | 0.19 | 0.851 | –1.78 to 2.16 |
| Group HPI: 15 months vs. 12 months | –0.81 | 1.22 | –0.67 | 0.506 | –3.2 to 1.58 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | 2.14 | 1.24 | 0.69 to 6.65 | ||
| Residual | |||||
| Group TAU | |||||
| SD (12 months, 15 months) | 10.23 | 0.56 | 9.19 to 11.38 | ||
| Correlation (12 months, 15 months) | 0.49 | 0.09 | 0.3 to 0.64 | ||
| Group HPI | |||||
| SD (12 months, 15 months) | 10.79 | 0.58 | 9.72 to 11.98 | ||
| Correlation (12 months, 15 months) | 0.32 | 0.1 | 0.12 to 0.5 | ||
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | –1.81 | 0.98 | –1.85 | 0.065 | –3.73 to 0.11 |
| Time (15 months = 1) | –0.22 | 0.72 | –0.31 | 0.756 | –1.63 to 1.18 |
| Arm × time | 1.07 | 1.02 | 1.05 | 0.294 | –0.93 to 3.07 |
| Borough | χ2(9) = 4.04; p = 0.91 | ||||
| Croydon | 0 | ||||
| Lambeth | 0.76 | 1.68 | 0.45 | 0.652 | –2.53 to 4.05 |
| Lewisham | 0.59 | 1.39 | 0.43 | 0.668 | –2.12 to 3.31 |
| Southwark | 1.89 | 1.34 | 1.4 | 0.161 | –0.75 to 4.52 |
| Greenwich | 2.07 | 1.72 | 1.2 | 0.229 | –1.31 to 5.45 |
| Bromley | 1.96 | 1.76 | 1.11 | 0.266 | –1.49 to 5.4 |
| Bexley | 1.33 | 1.98 | 0.67 | 0.503 | –2.55 to 5.2 |
| East Sussex | 1.31 | 1.89 | 0.7 | 0.486 | –2.38 to 5.01 |
| Somerset | 2.06 | 2.55 | 0.81 | 0.42 | –2.95 to 7.06 |
| South Staffordshire | 3.81 | 3.25 | 1.17 | 0.241 | –2.56 to 10.17 |
| PHC baseline | 0.59 | 0.04 | 14.94 | 0 | 0.51 to 0.66 |
| Age at baseline | –0.12 | 0.04 | –2.64 | 0.008 | –0.2 to –0.03 |
| Constant | 24.57 | 3.32 | 7.41 | 0 | 18.07 to 31.07 |
| Pairwise comparison | Coefficient | SE | z | p-value | 95% CI |
| 12 months: TAU vs. HPI | –1.81 | 0.98 | –1.85 | 0.065 | –3.73 to 0.11 |
| 15 months: TAU vs. HPI | –0.74 | 0.98 | –0.76 | 0.45 | –2.66 to 1.18 |
| Group HPI: 15 months vs. 12 months | –0.22 | 0.72 | –0.31 | 0.756 | –1.63 to 1.18 |
| Group TAU: 15 months vs. 12 months | 0.85 | 0.72 | 1.17 | 0.242 | –0.57 to 2.27 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | 0 | 0 | |||
| Residual | |||||
| SD (12 months) | 8.54 | 0.34 | 7.9 to 9.23 | ||
| SD (15 months) | 8.45 | 0.34 | 7.8 to 9.15 | ||
| Correlation (12 months, 15 months) | 0.47 | 0.04 | 0.38 to 0.56 | ||
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | –0.38 | 1.29 | –0.3 | 0.766 | –2.92 to 2.15 |
| Time (15 months = 1) | 1 | 0.87 | 1.15 | 0.251 | –0.7 to 2.7 |
| Arm × time | –0.7 | 1.36 | –0.52 | 0.603 | –3.36 to 1.95 |
| Borough | χ2(9) = 14.65; p = 0.10 | ||||
| Croydon | 0 | ||||
| Lambeth | –1.5 | 2.21 | –0.68 | 0.498 | –5.82 to 2.83 |
| Lewisham | –1.8 | 1.86 | –0.97 | 0.334 | –5.44 to 1.85 |
| Southwark | –5.25 | 1.8 | –2.92 | 0.003 | –8.77 to –1.73 |
| Greenwich | –5.19 | 2.34 | –2.22 | 0.027 | –9.79 to –0.6 |
| Bromley | –3.83 | 2.34 | –1.64 | 0.101 | –8.41 to 0.75 |
| Bexley | –1.06 | 2.64 | –0.4 | 0.689 | –6.24 to 4.12 |
| East Sussex | –4.83 | 2.5 | –1.93 | 0.053 | –9.72 to 0.06 |
| Somerset | –6.54 | 3.39 | –1.93 | 0.054 | –13.18 to 0.1 |
| South Staffordshire | –2.36 | 4.44 | –0.53 | 0.596 | –11.05 to 6.34 |
| PHC baseline | 0.58 | 0.04 | 14.09 | 0 | 0.5 to 0.66 |
| Age at baseline | 0.05 | 0.05 | 0.98 | 0.329 | –0.05 to 0.16 |
| Constant | 18.91 | 3.33 | 5.68 | 0 | 12.39 to 25.44 |
| Pairwise comparison | Coefficient | SE | z | p-value | 95% CI |
| 12 months: B vs. A | –0.38 | 1.29 | –0.3 | 0.766 | –2.92 to 2.15 |
| 15 months: B vs. A | –1.09 | 1.31 | –0.83 | 0.404 | –3.65 to 1.47 |
| Group TAU: 15 months vs. 12 months | 1 | 0.87 | 1.15 | 0.251 | –0.7 to 2.7 |
| Group HPI: 15 months vs. 12 months | 0.29 | 1.04 | 0.28 | 0.778 | –1.75 to 2.33 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | 1.68 | 1.3 | 0.37 to 7.67 | ||
| Residual | |||||
| Group TAU | |||||
| SD (12 months, 15 months) | 10.42 | 0.51 | 9.46 to 11.48 | ||
| Correlation (12 months, 15 months) | 0.49 | 0.07 | 0.34 to 0.61 | ||
| Group HPI | |||||
| SD (12 months, 15 months) | 11.2 | 0.52 | 10.22 to 12.28 | ||
| Correlation (12 months, 15 months) | 0.37 | 0.08 | 0.21 to 0.51 | ||
Multiple imputation
The following variables were used for the imputation model: relationship with two levels (yes or no, categorical), education as an ordinal variable, age at baseline, waist, BMI, systolic and diastolic BP, levels of HbA1c, GAF score, number of children, fasting glucose, PANNS total score, Montgomery–Åsberg Depression Rating Scales (MADRS), gender (categorical variable), attended 12 months within/outside follow-up (nominal variable), attended the 15-month follow-up within/outside time limits (nominal variable), MHC at baseline and at 12 and 24 months’ follow-up (log-transformed), PHC at baseline and at 12 and 24 months’ follow-up (log-transformed). Estimation was performed for each treatment arm ( = interaction between group TAU and all variables). Fifty data sets were imputed. MHC and PHC were log-transformed to be more nearly normal during the imputation process to avoid prediction outside the possible range. After imputation, the variables were back-transformed to the original scale. This procedure generally reduces bias and improves statistical properties. 242 The data were then analysed using the same model as in the main analyses.
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | –0.98 | 1.73 | –0.56 | 0.58 | –4.41 to 2.46 |
| Time (15 months = 1) | 0.36 | 1.47 | 0.24 | 0.81 | –2.55 to 3.26 |
| Arm × time | 0.45 | 2.01 | 0.23 | 0.82 | –3.52 to 4.42 |
| Borough | |||||
| Croydon | 0.00 | ||||
| Lambeth | 1.08 | 2.26 | 0.48 | 0.63 | –3.37 to 5.52 |
| Lewisham | 0.72 | 1.91 | 0.38 | 0.71 | –3.04 to 4.48 |
| Southwark | 1.56 | 1.86 | 0.84 | 0.40 | –2.10 to 5.21 |
| Greenwich | 2.11 | 2.43 | 0.87 | 0.39 | –2.66 to 6.87 |
| Bromley | 0.20 | 2.55 | 0.08 | 0.94 | –4.81 to 5.20 |
| Bexley | 1.50 | 2.74 | 0.55 | 0.59 | –3.89 to 6.89 |
| East Sussex | 2.08 | 2.60 | 0.80 | 0.43 | –3.02 to 7.17 |
| Somerset | 0.32 | 3.75 | 0.08 | 0.93 | –7.09 to 7.72 |
| South Staffordshire | 2.12 | 4.24 | 0.50 | 0.62 | –6.24 to 10.48 |
| PHC baseline | 0.61 | 0.06 | 10.34 | 0.00 | 0.49 to 0.72 |
| Constant | 17.44 | 3.36 | 5.20 | 0.00 | 10.83 to 24.06 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | –5.70 | 333.69 | –659.73 to 648.33 | ||
| Residual | |||||
| SD (12 months) | 2.32 | 0.09 | 2.14 to 2.50 | ||
| SD (15 months) | 0.34 | 0.11 | 0.12 to 0.56 | ||
| Correlation (12 months, 15 months) | 0.19 | 0.18 | –0.17 to 0.55 | ||
| Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|
| Arm (HPI = 1) | 0.57 | 2.10 | 0.27 | 0.79 | –3.55 to 4.69 |
| Time (15 months = 1) | –0.96 | 2.28 | –0.42 | 0.67 | –5.48 to 3.55 |
| Arm × time | –0.60 | 3.70 | –0.16 | 0.87 | –7.94 to 6.74 |
| Borough | |||||
| Croydon | 0.00 | ||||
| Lambeth | –1.71 | 3.23 | –0.53 | 0.60 | –8.06 to 4.64 |
| Lewisham | –3.06 | 2.73 | –1.12 | 0.26 | –8.44 to 2.32 |
| Southwark | –3.20 | 2.77 | –1.16 | 0.25 | –8.66 to 2.25 |
| Greenwich | –2.63 | 3.28 | –0.80 | 0.42 | –9.06 to 3.80 |
| Bromley | –3.06 | 3.52 | –0.87 | 0.38 | –9.99 to 3.86 |
| Bexley | –1.41 | 3.87 | –0.36 | 0.72 | –9.02 to 6.21 |
| East Sussex | –3.82 | 4.01 | –0.95 | 0.34 | –11.70 to 4.07 |
| Somerset | –6.72 | 4.99 | –1.35 | 0.18 | –16.58 to 3.13 |
| South Staffordshire | –0.19 | 6.44 | –0.03 | 0.98 | –12.89 to 12.52 |
| PHC baseline | 0.65 | 0.07 | 9.25 | 0.00 | 0.51 to 0.78 |
| Constant | 17.19 | 3.79 | 4.54 | 0.00 | 9.73 to 24.65 |
| Random-effects parameters | Estimate | SE | 95% CI | ||
| Care co-ordinator (SD) | |||||
| Residual | –5.10 | 195.33 | –387.94 to 377.75 | ||
| Group TAU | |||||
| ln [SD (12 months, 15 months)] | 2.76 | 0.17 | 2.43 to 3.09 | ||
| Correlation (12 months, 15 months) | 0.33 | 0.17 | 0.00 to 0.67 | ||
| Group HPI | |||||
| ln [SD (12 months, 15 months)] | –0.01 | 0.25 | –0.52 to 0.50 | ||
| Correlation (12 months, 15 months) | 0.18 | 0.12 | –0.05 to 0.42 | ||
Multiple imputation 2: sensitivity analysis for not missing at random pattern
Some large positive and large negative values were added to the multiple imputed missing values (values correspond to approximately ± 2 SDs and ± 1 SD of baseline measure of physical and mental health scores, respectively).
(Fifty imputed data sets; same imputation data sets used in multiple imputation analyses above were used.)
Only parameter estimates for arm (HPI = 1), time (15 months = 1) and arm × time interaction are shown.
| Value added to imputed missing values | Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|---|
| –20 for missing | ||||||
| Arm (HPI = 1) | –2.48 | 2.03 | –1.22 | 0.22 | –6.49 to 1.53 | |
| Time (15 months = 1) | –1.51 | 1.68 | –0.90 | 0.37 | –4.82 to 1.80 | |
| Arm × time | 1.38 | 2.31 | 0.60 | 0.55 | –3.16 to 5.92 | |
| –10 for missing | ||||||
| Arm (HPI = 1) | –1.74 | 1.83 | –0.95 | 0.34 | –5.36 to 1.87 | |
| Time (15 months = 1) | –0.58 | 1.53 | –0.38 | 0.71 | –3.59 to 2.44 | |
| Arm × time | 0.92 | 2.09 | 0.44 | 0.66 | –3.21 to 5.04 | |
| 0 for missing | ||||||
| Arm (HPI = 1) | –0.98 | 1.73 | –0.56 | 0.58 | –4.41 to 2.46 | |
| Time (15 months = 1) | 0.36 | 1.47 | 0.24 | 0.81 | –2.55 to 3.26 | |
| Arm × time | 0.45 | 2.01 | 0.23 | 0.82 | –3.52 to 4.42 | |
| +10 for missing | ||||||
| Arm (HPI = 1) | –0.19 | 1.77 | –0.11 | 0.92 | –3.69 to 3.31 | |
| Time (15 months = 1) | 1.29 | 1.52 | 0.85 | 0.40 | –1.71 to 4.29 | |
| Arm × time | –0.01 | 2.08 | –0.01 | 1.00 | –4.12 to 4.10 | |
| +20 for missing | ||||||
| Arm (HPI = 1) | 0.59 | 1.93 | 0.30 | 0.76 | –3.23 to 4.40 | |
| Time (15 months = 1) | 2.22 | 1.67 | 1.33 | 0.19 | –1.07 to 5.52 | |
| Arm × time | –0.47 | 2.29 | –0.21 | 0.84 | –4.99 to 4.04 | |
Conclusion: adding extreme values did not change major conclusions.
| Value added to imputed missing values | Variable | Coefficient | SE | z | p-value | 95% CI |
|---|---|---|---|---|---|---|
| –20 for missing | ||||||
| Arm (HPI = 1) | –1.28 | 2.38 | –0.54 | 0.59 | –5.96 to 3.40 | |
| Time (15 months = 1) | –3.30 | 2.53 | –1.30 | 0.20 | –8.29 to 1.70 | |
| Arm × time | 0.56 | 3.95 | 0.14 | 0.89 | –7.27 to 8.39 | |
| –10 for missing | ||||||
| Arm (HPI = 1) | –0.34 | 2.09 | –0.16 | 0.87 | –4.45 to 3.77 | |
| Time (15 months = 1) | –2.08 | 2.37 | –0.88 | 0.38 | –6.77 to 2.61 | |
| Arm × time | –0.04 | 3.76 | –0.01 | 0.99 | –7.50 to 7.42 | |
| 0 for missing | ||||||
| Arm (HPI = 1) | 0.57 | 2.10 | 0.27 | 0.79 | –3.55 to 4.69 | |
| Time (15 months = 1) | –0.96 | 2.28 | –0.42 | 0.67 | –5.48 to 3.55 | |
| Arm × time | –0.60 | 3.70 | –0.16 | 0.87 | –7.94 to 6.74 | |
| +10 for missing | ||||||
| Arm (HPI = 1) | 1.44 | 2.00 | 0.72 | 0.47 | –2.51 to 5.39 | |
| Time (15 months = 1) | 0.15 | 2.37 | 0.07 | 0.95 | –4.54 to 4.85 | |
| Arm × time | –1.15 | 3.76 | –0.31 | 0.76 | –8.62 to 6.31 | |
| +20 for missing | ||||||
| Arm (HPI = 1) | 2.38 | 2.23 | 1.07 | 0.29 | –1.99 to 6.76 | |
| Time (15 months = 1) | 1.37 | 2.53 | 0.54 | 0.59 | –3.63 to 6.37 | |
| Arm × time | –1.76 | 3.95 | –0.44 | 0.66 | –9.59 to 6.08 | |
Conclusion: adding extreme values did not change major conclusions.
List of abbreviations
- AUDIT
- Alcohol Use Disorders Identification Test
- BME
- black and ethnic minority
- BMI
- body mass index
- BP
- blood pressure
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- CMHT
- community mental health team
- CQUIN
- Commissioning for Quality and Innovation
- CRP
- C-reactive protein
- CSRI
- Client Service Receipt Inventory
- CVD
- cardiovascular disease
- df
- degrees of freedom
- DHSC
- Department of Health and Social Care
- DINE
- Dietary Instrument for Nutrition Education
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FEP
- first episode of psychosis
- GAF
- Global Assessment of Functioning
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HDL
- high-density lipoprotein
- HIP
- Health Improvement Profile
- HoNOS
- Health of the Nation Outcomes Scales
- HPI
- health promotion intervention
- HPP
- health promotion programme
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- IDF
- International Diabetes Federation
- IMPaCT
- improving physical health and reducing substance use in psychosis
- MCS
- mental component score
- MetS
- metabolic syndrome
- MHC
- mental health component
- MI
- motivational interviewing
- MITI
- Motivational Interviewing Treatment Integrity
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OPCRIT
- operational criteria checklist for psychotic and affective illness
- PANSS
- Positive and Negative Syndrome Scale
- PCA
- prescription cost analyses
- PCS
- physical component score
- PHC
- physical health component
- PPHS
- Psychiatric and Personal History Schedule
- PUMP
- physical health and substance use in first episode of psychosis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SLaM
- South London and Maudsley
- SMI
- severe mental illness
- TAU
- treatment as usual
- THC
- delta-9-tetrahydrocannabinol
- WHO
- World Health Organization
Notes
-
Case study on carer involvement in mental health research
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/pgfar/RP-PG-0606-1049/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
