Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0609-10162. The contractual start date was in June 2011. The final report began editorial review in June 2017 and was accepted for publication in February 2019. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paolo Deluca acknowledges past and current research funding from the National Institute for Health Research (NIHR), the Medical Research Council (MRC) and the European Commission. Paolo Deluca is also supported by South London and Maudsley NHS Foundation Trust (SlaM) and by the NIHR Biomedical Research Centre (BRC) for Mental Health at King’s College London and SlaM. Simon Coulton acknowledges past and current research funding as chief investigator and co-investigator from NIHR, Alcohol Research UK, Dunhill Medical Trust, MRC, Lundbeck Ltd (St Albans, UK) and Kent County Council. Mohammed Fasihul Alam acknowledges past and current research funding from Qatar University Internal Grant, NIHR and Community Pharmacy Wales. Kim Donoghue acknowledges past and current research funding from NIHR. Eilish Gilvarry acknowledges grants from NIHR during the conduct of the study. Eileen Kaner is a senior scientist in the NIHR School of Primary Care Research and NIHR School of Public Health Research as part of Fuse, a UK Clinical Research Collaboration (UKCRC) Centre of Excellence in Translation Public Health Research. Eileen Kaner also acknowledges past and current research funding as chief investigator and co-investigator from NIHR, the MRC Public Health Intervention Development Scheme (PHIND), the Department of Health and Social Care, The British Academy, Public Health England, European Research Area Network on Illicit Drugs (ERANID), Policing Research Partnership, North Yorkshire County Council, the Institute of Local Governance, Alcohol Research UK, MRC, the European Commission, Sunderland Clinical Commissioning Group (CCG), the Health Foundation, Research Capability Funding, Diabetes UK and Newcastle upon Tyne Hospitals NHS Foundation Trust. Ian Maconochie acknowledges grants from NIHR during the conduct of the study. Paul McArdle reports grants from NIHR during the conduct of the study. Ruth McGovern acknowledges past and current research from NIHR, Public Health England, North East and North Cumbria, North Yorkshire County Council, ERANID, the Department of Health and Social Care, the Institute of Local Governance, N8 Policing Research Partnership, Alcohol Research UK, The Children’s Society, Mental Health Research Network – North East Hub and Sunderland CCG. Dorothy Newbury-Birch acknowledges past and current research funding from Public Health England, North Yorkshire County Council, Healum, Alcohol Research UK, County Durham and Darlington NHS Foundation Trust, The Children’s Foundation, NIHR, MRC PHIND, Forces in Mind Trust, the Joseph Rowntree Foundation, The Children’s Society, the European Commission, British Skin Foundation Small Grant and Newcastle upon Tyne Hospitals NHS Foundation Trust. Robert Patton acknowledges past and current research funding from NIHR, Surrey County Council, the Software Sustainability Institute, Alcohol Research UK and the Higher Education Academy. Ceri Phillips acknowledges past and current research funding from the National Institute for Social Care and Health Research, NIHR, United European Gastroenterology and Asthma UK. Thomas Phillips was funded by a NIHR Clinical Doctoral Research Fellowship. Ian T Russell acknowledges grants from NIHR during the conduct of the study and personal fees from Swansea University outside the submitted work. John Strang reports grants and other funding from Martindale Pharma (Ashton Gate, UK), grants and other funding from Mundipharma (Cambridge, UK), and grants and other funding from Braeburn (Plymouth Meeting, PA, USA) outside the submitted work. In addition, John Strang has a patent Euro-Celtique issued and a patent King’s College London pending, is supported by the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London, and is in receipt of a NIHR Senior Investigator Award. He has also worked with a range of governmental and non-governmental organisations and with pharmaceutical companies to seek to identify new or improved treatments from which he and his employer (King’s College London) have received honoraria, travel costs and/or consultancy payments. This includes work with, during the past 3 years, Martindale, Reckitt Benckiser/Indivior (Slough, UK), Mundipharma and Braeburn/Medpace (Cincinnati, OH, USA) and trial medication supply from iGen Networks Corp. (Las Vegas, NV, USA) (iGen/Atral-Cipan, Castanheira do Ribatejo, Portugal). His employer, King’s College London, has registered intellectual property on a novel buccal naloxone formulation, and he has also been named in a patent registration by a pharmaceutical company as inventor of a concentrated nasal naloxone spray. John Strang also acknowledges past and current research funding as chief investigator and co-investigator from NIHR, Mundipharma, MRC, The Pilgrim Trust, Martindale Pharma, the Alcohol and Education Research Council, the Institute of Social Psychiatry and the University of London Central Research Fund. Colin Drummond is partly funded by the NIHR Biomedical Research Centre for Mental Health at SLaM and King’s College London, and partly funded by the NIHR Collaborations for Leadership in Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust. In addition, Colin Drummond is in receipt of a NIHR Senior Investigator Award. Colin Drummond acknowledges past and current research funding as chief investigator and co-investigator from NIHR, MRC, Guy’s and St Thomas’ Charity, Nuffield Foundation, European Union Directorate-General for Justice and Consumers (JUST), Alcohol Research UK, NHS England, the Department of Health Policy Research Programme, World Health Organization, the European Commission and the Alcohol Education and Research Council.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Deluca et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
SYNOPSIS
Setting the scene
The excessive consumption of alcohol is a major global public health issue,1 and, in Europe, alcohol accounted for 6.5% of deaths and 11.6% of disability-adjusted life-years in 2004. 2 Although the main burden of chronic alcohol-related disease is in adults, its foundations often lie in adolescence. 3 The proportion of young people in England aged between 11 and 15 years who reported that they had drunk alcohol decreased from 62% to 54% between 1988 and 2007, but the mean amount consumed by those who drank doubled (from 6.4 to 12.7 units of alcohol per week) between 1994 and 2007. 4 About 10% of 11- to 15-year-olds and 33% of 15- to 16-year-olds in England report alcohol intoxication in the past month. 5,6 Adolescents in the UK are now among the heaviest drinkers in Europe. 6 The Chief Medical Officer for England provided recommendations on alcohol consumption in young people in 2009,7 based on an evidence review. 8 These advise that children abstain from alcohol before the age of 15 years and that 15- to 17-year-olds should not drink, but, if they do drink, then they should consume no more than the recommended limits for adults (currently 14 units per week). 7
Alcohol consumption and related harm increase steeply from the age of 12 to 20 years. 9 In early adolescence, alcohol use and alcohol use disorders (AUDs) (alcohol abuse, harmful alcohol use and alcohol dependence) are relatively uncommon. However, alcohol has a disproportionate effect on younger adolescents, for example by predisposing them to alcohol dependence in later life10,11 and damage to the developing brain. 12 In middle adolescence (ages 15–17 years), binge drinking emerges. Although binge drinking does not necessarily meet the criteria for AUDs, it is associated with increased risk of unprotected or regretted sexual activity, criminal and disorderly behaviour, suicidality and self-harm, injury, drink driving, alcohol poisoning and accidental death. 6,13–16
Alcohol screening
Opportunistic alcohol screening and brief interventions (SBIs) in emergency departments (EDs) capitalise on the ‘teachable moment’ when a connection can be made between alcohol consumption and ED attendance. 17–20 Alcohol SBI in EDs has shown efficacy in adults20 and adolescents,17,18,21 with evidence of cost-effectiveness in adults. 22 Over the past 15 years, the World Health Organization, the US Surgeon General, the American Medical Association and the American Academy of Pediatrics have called for practitioners to carry out SBIs for adolescent drinkers. 23–26 The alcohol strategies for both England and Scotland identify adolescents as a key target group in which to reduce alcohol consumption and related harm. 27,28 However, although there has been an increase in alcohol SBIs for adults, adolescents remain a neglected group. A recent audit of EDs in Scotland found that only 5% of alcohol-related attenders aged < 18 years receive an alcohol intervention before discharge, and that ED staff focus more on those young people presenting with acute intoxication or self-harm. 29 Of the 12 EDs in the north-east of England and London approached during our research programme, none used routine alcohol screening in 10- to 17-year-olds and only three did so in adults.
Several alcohol screening methods have been developed in the USA but have not been evaluated in the UK. A recent systematic review of alcohol SBIs in young people (aged 10–17 years) and adults (aged ≥ 18 years), conducted for the National Institute for Health and Care Excellence (NICE),30 examined 51 studies of alcohol screening. Questionnaires were found to perform better than blood markers or breath alcohol concentration in all age groups. In adolescents ,the Alcohol Use Disorders Identification Test (AUDIT) questionnaire was found to have greater sensitivity and specificity than other questionnaires, including CAGE (Cut Down, Annoyed, Guilty, Eye Opener), TWEAK (Tolerance, Worried, Eye-opener, Amnesia, K/Cut Down), CRAFFT (Car, Relax, Alone, Forget, Friends, Trouble), RAPS4-QF (Rapid Alcohol Problems Screen – Quantity Frequency), FAST (Fast Alcohol Screening Test), RUFT (Cut-Riding, Unable, Family/Friends, Trouble, Cut down) and POSIT (Problem Oriented Screening Instrument for Teenagers). AUDIT sensitivities for adolescents range from 54% to 87% and specificities range from 65% to 97%. 31 However, the majority were at the lower end of these ranges and are therefore suboptimal for effective screening.
Additional shortcomings of existing alcohol screening methods for adolescents have been identified. 31 Existing approaches do not sufficiently take into account the age and developmental stage of adolescents. Any alcohol consumption under 15 years of age is of concern, whereas the identification of AUDs is more relevant in older adolescents. There is therefore a need for screening methods that are sensitive to the developmental stage of the adolescent to maximise opportunities for intervention. Alcohol screening has been mostly studied in older adolescents and young adults of college age (18–24 years). Therefore, the validity of alcohol screening methods in younger adolescents is unclear. Questionnaires such as the AUDIT may be too lengthy (10 items) to implement in busy EDs, pointing to the need for briefer tools for routine clinical practice. Methods to increase compliance, particularly by younger adolescents, are also needed. The use of computer screening and interviewing adolescents confidentially and separately from parents has shown some promise in the USA. 32,33
Alcohol brief interventions in health settings
Several systematic reviews have noted the effectiveness of SBIs in adults in health settings. 34–38 Less research in this area has been conducted in adolescents. A systematic review of brief alcohol interventions for young people attending health settings identified nine randomised controlled trials (RCTs) between 1999 and 2008. 30 Eight were based in the USA17–19,21,39–41 and one was based in Australia. 42 Most trials were considered to be methodologically sound, although two were considered to be weak in randomisation and allocation concealment. 40,42 Sample sizes ranged from 34 to 655 and ages ranged from 12 to 24 years. Three trials40–42 targeted socioeconomically disadvantaged groups among whom drug and alcohol misuse were more prevalent. Four trials17–19,21 were based in EDs to maximise the potential for ‘teachable moments’ when the connection between alcohol consumption and its adverse consequences can be more readily highlighted. Two studies39,40 recruited adolescents during routine general check-ups in primary care and one43 recruited in a university health centre. The remaining trials targeted homeless adolescents41 and those attending a youth centre that delivered health services. 42
Six trials17,18,21,40,41,43 tested brief interventions based on one or two sessions of motivational interviewing (MI) that lasted between 20 and 45 minutes. Delivery was carried out by a range of trained professionals, including physicians, nurse practitioners, psychologists, addiction clinicians and youth workers. One trial tested a more intensive programme of four MI sessions over 1 month. 42 Two studies used information technology to deliver brief interventions, one using an audio programme in primary care39 and the other using an interactive computer program in a minor injury unit. 19 The length of follow-up ranged from 2 to 12 months. Loss to follow-up was generally low (0–20%), although the authors of one study40 reported that 34% of their study population were lost to follow-up.
Five trials17,18,21,42,43 reported significant positive effects of brief interventions on a range of alcohol consumption measures. Bailey et al. 42 reported that brief intervention participants showed increased readiness to reduce alcohol consumption, an initial reduction in alcohol consumption and an improvement in knowledge of alcohol and related problems, compared with control subjects. Schaus et al. 43 also reported reductions in blood alcohol concentration, number of drinks per week and risk-taking behaviour. Monti et al. 18 reported that brief intervention subjects were less likely than control subjects to drink and drive or to experience alcohol-related injury, although both treatment groups significantly reduced their alcohol consumption. A subsequent trial, conducted by the same research group,17 reported that alcohol consumption also significantly decreased in both the brief intervention group and the control group. Last, Spirito et al. 21 reported a significant reduction in alcohol consumption at follow-up in both the brief intervention group and the control group. However, adolescents who screened positive for alcohol problems at baseline reported more change after MI than the control subjects.
Three trials reported null effects after brief intervention. 19,40,41 One trial that used an audio-taped programme with 12- to 17-year-old adolescents39 reported an increase in alcohol use and binge drinking among brief intervention subjects, representing a possible adverse effect of this type of intervention.
Summary
In summary, there is a need to develop more effective alcohol screening tools for adolescents in the ED, which are age appropriate and cover a wider range of alcohol consumption and alcohol-related problems than do existing methods. Furthermore, as most of the existing research has been conducted in the USA, screening methods appropriate to EDs are needed in the UK context of the NHS.
Moreover, the majority of alcohol SBI studies among adolescents in health-care settings were conducted in EDs and reported positive outcomes. However, three trials reported alcohol consumption reductions in both the intervention group and the control group, and three more trials reported no effect of brief intervention. None of these trials was in the UK and few studies were conducted in young adolescents. Thus, although there is evidence to suggest that brief intervention may be beneficial for adolescents, particularly in EDs, there is a clear need for a UK trial of this.
This monograph describes the results of our findings linked to the original programme objectives (a full list of publications arising from our programme of work can be found in Overall conclusions, Dissemination).
Work package 1: screening prevalence study of alcohol consumption and alcohol use disorders in adolescents aged 10–17 years attending emergency departments
Introduction
Adolescence is a critical period of development, during which the initiation and continuing use of alcohol may have detrimental consequences for the young person. 44 Several adverse health and social consequences of alcohol use in young people are widely reported in research and health policy, including an increase in depressive feelings, an increase in sexual risk taking, a reduction in educational performance, difficulties in maintaining relationships with peers and friends, and an increase in vulnerability to becoming a victim of crime. 8 Although it is difficult to establish a direct causal relationship between alcohol use in adolescents and social and behavioural problems, several studies have shown that earlier consumption is associated with alcohol-related problems in later life. 45–51 A recent review52 recommended further research to establish the advantages of delaying the onset in drinking when establishing guidelines for drinking in adolescence.
The identification of adolescents who consume alcohol at problematic levels is a key element of any screening and intervention strategy. To offer such interventions, practitioners need access to screening tools that are high in both sensitivity and specificity and are quick and easy to apply at minimal cost. Biochemical markers of alcohol use, such as gamma-glutamyl transferase, aspartate aminotransferase, erythrocyte mean cell volume and carbohydrate-deficient transferrin, are impractical and of little use in this population, and have been found to be inferior to short screening questionnaires in adult populations. 53
The AUDIT54 is a 10-item self-completion questionnaire with established diagnostic properties for hazardous and harmful alcohol use in adults. It addresses three domains: alcohol consumption, harmful consequences and symptoms of dependence. AUDIT is one of the few screening instruments that specifically incorporates consumption into the scoring algorithm and may be particularly suitable for adolescents who are more likely to experience a range of alcohol-related harms as a result of consumption rather than experiencing symptoms of alcohol dependence. Furthermore, it may be the case that the three specific alcohol consumption questions constituting the Alcohol Use Disorders Identification Test, Consumption (3 items) (AUDIT-C) may be as efficient and brief a screening instrument as the full AUDIT. Previous studies suggest that the AUDIT may be more useful than other brief screening instruments in adolescent populations, but there is limited evidence regarding appropriate cut-off points for different severities of alcohol misuse,55–60 and no previous research has compared the relative effectiveness of AUDIT with that of AUDIT-C in adolescent populations.
Aims
This work package had three principal aims:
-
to estimate and compare the sensitivity, specificity and diagnostic odds ratios (ORs) of the AUDIT and AUDIT-C in identifying at-risk alcohol use, monthly heavy episodic alcohol use, alcohol abuse and alcohol dependence in the context of an opportunistic screening programme for adolescents attending EDs in England
-
to examine the prevalence of alcohol consumption among adolescents (aged 10–17 years) presenting to hospital EDs in England
-
to determine the association between alcohol consumption and age at onset of alcohol consumption with health and social consequences among adolescents presenting to EDs in England.
Findings from aim 1 have been published in Coulton et al. 61 and findings covering aims 2 and 3 have been published in Donoghue et al. 62 These are summarised here and reproduced in full in the appendices.
Methods
Patient and public involvement in work package 1
For work package 1, we collaborated with three organisations to ensure that both parents and young people were engaged in the development of our methodology and materials (the British Youth Council, Parenting UK and the Family and Parenting Institute). We organised focus groups in the north and south of England, at which we presented our planned protocols and then engaged the public to critique our plans and to make suggestions for change. Our work with the parent groups helped to shape the study protocol in terms of the optimal way to introduce the study and obtain informed consent. Consultation with the young people indicated that electronic data capture methods would be better received than interview or paper-and-pencil approaches and, as a consequence, we developed an iPad-based screening and data collection tool (Apple Inc., Cupertino, CA, USA) that we utilised throughout the entire programme of research.
The initial intention of the prevalence study was to examine the prevalence of alcohol consumption and AUDs among adolescents presenting to EDs. The questionnaire included demographic and lifestyle questions, attitude scales and a range of alcohol measures to determine which to use in the main trial, and it was expected to be around 30 pages in length. The patient and public involvement showed that adolescents were unlikely to consent to such a survey and, if consent was given, completion was not likely. A tablet interface and shorter questionnaire were more acceptable and would encourage participation in the study.
iPad data collection tool
Following a consultation stage with the target groups, we decided to develop an iPad application to better engage adolescents in the prevalence study and to facilitate data collection and improve data quality. The iPad application for the prevalence study has been developed for this research by the software developer Codeface Ltd (Hove, UK) in collaboration with the research team. Codeface Ltd, study investigators and target groups have all been actively involved in its development, testing and piloting. The application provided a flexible approach to conduct the prevalence study and was an innovative method to administer a relatively long battery of measures to this target group (Figure 1). It also had the advantage of automating the routing through the questionnaire, showing the respondent only applicable questions in an engaging and clear layout. Moreover, encrypted data were uploaded securely onto a secure server and could be monitored by the co-ordinating centre in real time. This allowed the research team to check daily when quotas for each year group had been reached. It also reduced the time needed for data entry and cleaning, negating the need for manual data entry for most of the data collected. This data collection application program (app) was further developed and adapted for the data collection and randomisation of participants in the RCTs as part of work package 3.
FIGURE 1.
Screenshot of the data collection app developed for the programme and available from the authors.
Participants
Data collection took place between December 2012 and May 2013. Participants were aged between their 10th and 18th birthdays and were attending 1 of 10 participating EDs across England: in the North East, Yorkshire and The Humber, and London. To be eligible for inclusion in the research, the participant had to be alert and orientated and able to speak sufficient English to complete the research assessments. Participants were not eligible for inclusion if they had a severe injury, were suffering from a serious mental health problem or were grossly intoxicated (as determined by ED staff). Participants were also not eligible to take part if they or their parent or guardian (as applicable) were unable or unwilling to provide informed consent.
We excluded grossly intoxicated patients on the basis that they would not be able to provide informed consent. Clinical protocols for young people presenting to accident and emergency (A&E) departments in a grossly intoxicated state would involve escalation to consider safeguarding concerns and potentially referral to specialist services in most of the hospitals involved in this programme of research. Therefore, the brief interventions being studied in this programme would have been less than the minimal intervention considered necessary for this group.
However, if those patients sobered up during their stay in A&E, then they were approached at a later stage about participating in the research, provided that there were no other clinical concerns or reasons for exclusion.
Procedure
Following clearance by ED staff, a researcher approached consecutive ED attenders meeting the study criteria every day of the week between 8 a.m. and midnight. For those participants aged < 16 years and unaccompanied by a parent or guardian, Gillick competence was assessed by a member of ED staff. Those assessed as Gillick competent were approached by the researcher and invited to provide informed consent for participation. 63
We extended Gillick competency to consent for participation in research on the grounds of minimal/no risk in taking part in this prevalence study. 64
Those aged 16 or 17 years provided informed consent without recourse to a parent or guardian.
Participants completed the study questionnaires independently in a private area of the ED. The researcher was available in case clarification of questions or help with the software program was required. The study data were anonymised and collected using an iPad electronic tablet device, with the exception of the Timeline Followback questionnaire, which was manually administered by the researcher. A £5 gift voucher was given to all participants at the end of the interview to thank them for their time. All young people participating in the study were also given age-appropriate material containing information on alcohol and local services and helplines providing further support.
Measures
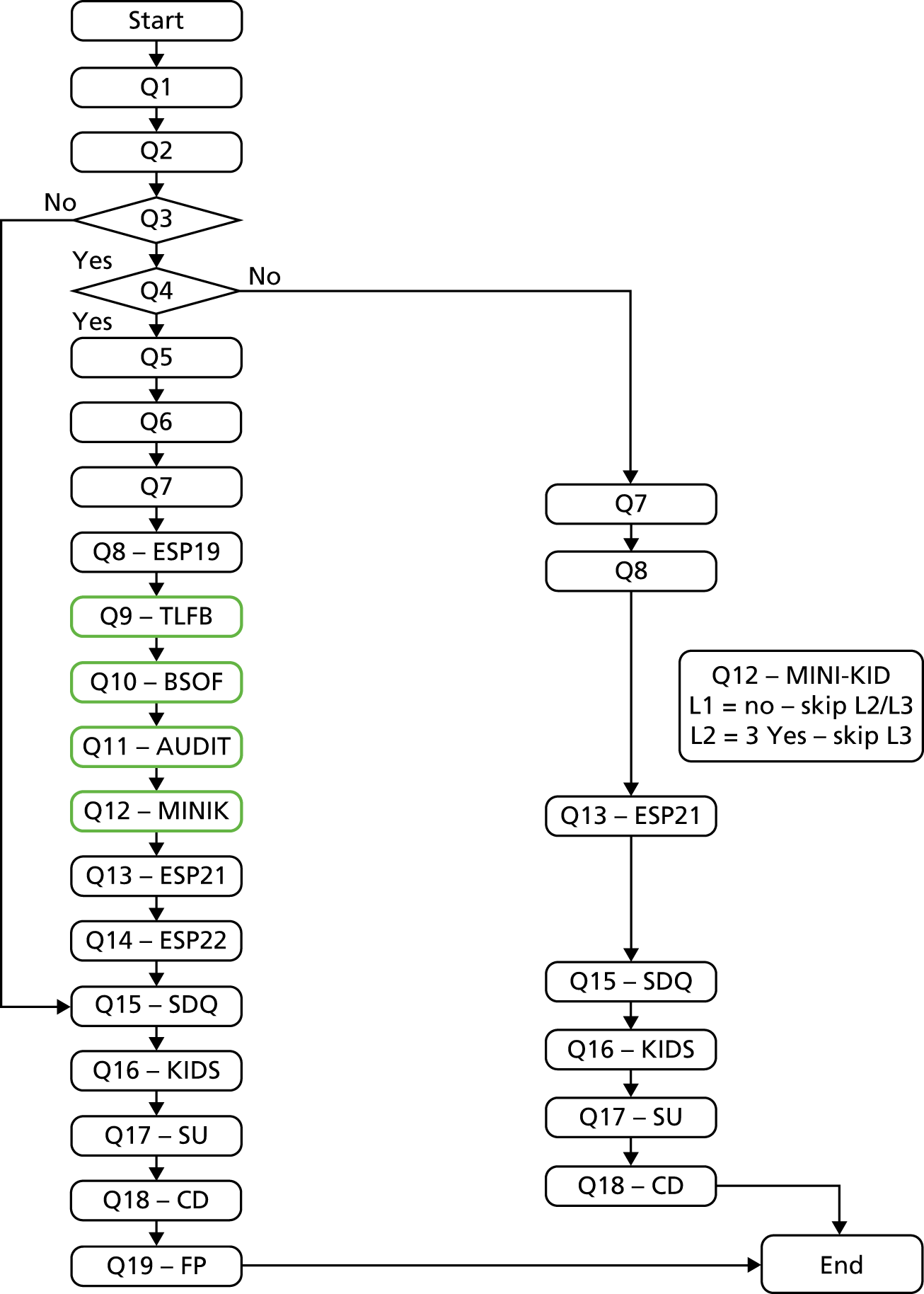
Figure 2 illustrates the flow of research questions. Demographic data, including age, gender and ethnicity, were collected for all participants, as was information on general health behaviours and lifestyle, including tobacco smoking. Health-related quality of life was assessed using the health-related quality-of-life questionnaire for children and young people and their parents (Kidscreen);65 this is a 10-item generic health-related quality-of-life measure, with established validity and reliability in this population. Behavioural and emotional functioning was measured using the Strengths and Difficulties Questionnaire. 66,67 In addition, several questions relating to age-relevant service use, including questions on previous use of health and social services, school attendance and contact with the criminal justice system, were asked.
FIGURE 2.
Flow of research questions. Q1, Demographics. Q2, Health and Lifestyle questionnaire. Q3, Filter question 1: have you ever drunk alcohol? Do not include just a sip of somebody else’s drink. Q4, Filter question 2: have you ever drunk alcohol in the past 3 months? Do not include just a sip of somebody else’s drink. Q5, Have you had a drink of alcohol in the past 24 hours? Q6, Have you consumed any alcohol prior to attendance at ED? Q7, How old were you when you had your first sip of alcohol (beer, cider, alcopops, wine, etc.)? Q8, European School Survey Project on Alcohol and Other Drugs Q19 (alcohol intoxication). Q9, Timeline Followback 90. a,c Q10, Beverage Specific Quantity Frequency Questionnaire. a,c Q11, AUDIT. b,c Q12, Mini International Neuropsychiatry Interview for Children and Adolescents (MINI-KID) Alcohol. b,c Q13, European School Survey Project on Alcohol and Other Drugs Q21. Q14, European School Survey Project on Alcohol and Other Drugs Q22. Q15, Strengths and Difficulties Questionnaire (SDQ). Q16, health-related quality of life questionnaire for children and young people and their parents (Kidscreen). Q17, service utilisation. Q18, cognitive debrief. Q19, future participation details. BSQF, Beverage Specific Quantity Frequency; ESP19, European School Survey Project on Alcohol and Other Drugs Q19; L, Level; Q, Question; SDQ, Strengths and Difficulties Questionnaire; TLFB, Timeline Followback. a, The order of presentation of quantity–frequency measures (Timeline Followback and Beverage Specific Quantity Frequency Questionnaire) were allocated at random, stratified by age group. b, The order of presentation of diagnostic measures (AUDIT and MINI-KID) were allocated at random, stratified by age group. c, The order of presentation of quantity–frequency measures and diagnostic measures were allocated at random, stratified by age group.
Results
Among participants who reported any alcohol consumption, the age of first consumption in years was recorded using a single question [‘how old were you when you had your first drink of alcohol (beer, cider, alcopops wine, etc.)?’], and further questions about whether or not they had consumed alcohol in the past 3 months and past 24 hours were asked. In addition, all participants who had ever drunk alcohol were asked question 19 (‘experienced alcohol intoxication in your lifetime?’) and question 21 (‘personal experience of alcohol?’) from the European School Survey Project on Alcohol and other Drugs (ESPAD). 68 Further questions were included to assess the feasibility of conducting a future alcohol intervention study, including whether or not the participant wanted further information or advice about alcohol, and whether or not they were willing to participate in an intervention and follow-up study, if this was offered. They were also asked how easy they had found it to complete the questionnaire electronically.
Those participants who indicated that they had consumed alcohol that was ‘more than a sip’ in the past 3 months were asked additional questions about alcohol use. Hazardous alcohol use, harmful alcohol use and harmful alcohol dependence were assessed using the three-item AUDIT-C,54 the full 10-item AUDIT and the alcohol section of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID), respectively. 69 Quantity of alcohol consumed in the past 90 days was derived from the Timeline Followback Form 9070 and converted to standard units, for which one unit was the equivalent of 8 g of pure ethanol. In addition, beverage-specific quantity and frequency questions were asked for consumption of beer, cider, alcopops, spirits and wine. This is an ad hoc tool developed for this study. The Beverage Specific Quantity Frequency Questionnaire’s measure of alcohol consumption is derived from methods used to measure consumption in adolescent populations6 and conforms with European guidance on the standardisation of measurement of consumption. This questionnaire measures total quantity and frequency of consumption of specific beverages and episodes of excessive consumption over a 90-day period.
The AUDIT has been validated in adolescent populations in EDs in the USA. 56,58 As part of the current programme of research, the shorter, three-question AUDIT-C was validated with a cut-off point of 3 [see Characteristics analysis of screening tools (aim 1)]. The Timeline Followback Form 90 has been validated for use among this population. 71–73 Perceived consequences of alcohol consumption were assessed by question 22 of ESPAD: ‘because of your own alcohol use, how often during the last 12 months have you experienced the following?’. 68
Overall, 5781 participants were asked to participate in the survey, of whom 5377 (93%) consented to participate across the 10 EDs. The mean age of participants was 13.3 [standard deviation (SD 2.1)] years, with similar proportions of male (53.7%) and female (46.3%) participants and a majority of white participants (72.6%). Overall, 2112 (39.3%) participants had consumed alcohol at some time in the past and 1378 (25.6%) participants had consumed alcohol in the past 3 months. Those who had consumed alcohol tended to be older (14.8 years vs. 12.3 years) and were more likely to be white (83.4% vs. 65.6%).
Characteristics analysis of screening tools (aim 1)
A significant positive correlation was identified for AUDIT score for the total number of standard drinks consumed in the past 3 months [Spearman’s r = 0.72, 95% confidence interval (CI) 0.71 to 0.73; p < 0.001] and a similar correlation was identified for AUDIT-C score (Spearman’s r = 0.69, 95% CI 0.68 to 0.70; p < 0.001).
Screening properties of the AUDIT-C and the 10-item AUDIT questionnaire were tested against the gold-standard criteria for at-risk drinking, heavy episodic alcohol consumption, alcohol abuse and alcohol dependence, and appropriate cut-off points were identified for each instrument.
The optimum cut-off point for AUDIT in identifying either at-risk drinking, monthly heavy episodic drinking or alcohol abuse was a score of ≥ 4; this provided acceptable sensitivity, specificity and diagnostic odds. An AUDIT-C score of ≥ 3 demonstrated almost identical diagnostic properties but with a significantly better sensitivity for at-risk drinking.
An AUDIT score of ≥ 7 provided a significantly more effective cut-off point for alcohol dependence than any other cut-off point, and demonstrated significantly better diagnostic properties than an AUDIT-C score of ≥ 5.
Sensitivity analysis that incorporated age, gender and ED into the analysis as covariates indicated no influence of these covariates on the observed outcomes.
Prevalence of alcohol consumption (aim 2)
A total of 2112 (39.3%) of the 5377 participants who consented to take part in the research reported having had a drink of alcohol that was more than a sip in their lifetime, with prevalence increasing steadily with age (Figure 3).
FIGURE 3.
Percentage of participants who had a drink of alcohol that was more than a sip in their lifetime by current age. Reproduced from Donoghue et al. 62 Reprinted from Journal of Adolescent Health, vol. 60, Donoghue K, Rose H, Boniface S, Deluca P, Coulton S, Alam MF, et al. Alcohol consumption, early-onset drinking, and health-related consequences in adolescents presenting at emergency departments in England pp. 438–446, 2017, with permission from Society for Adolescent Health and Medicine.
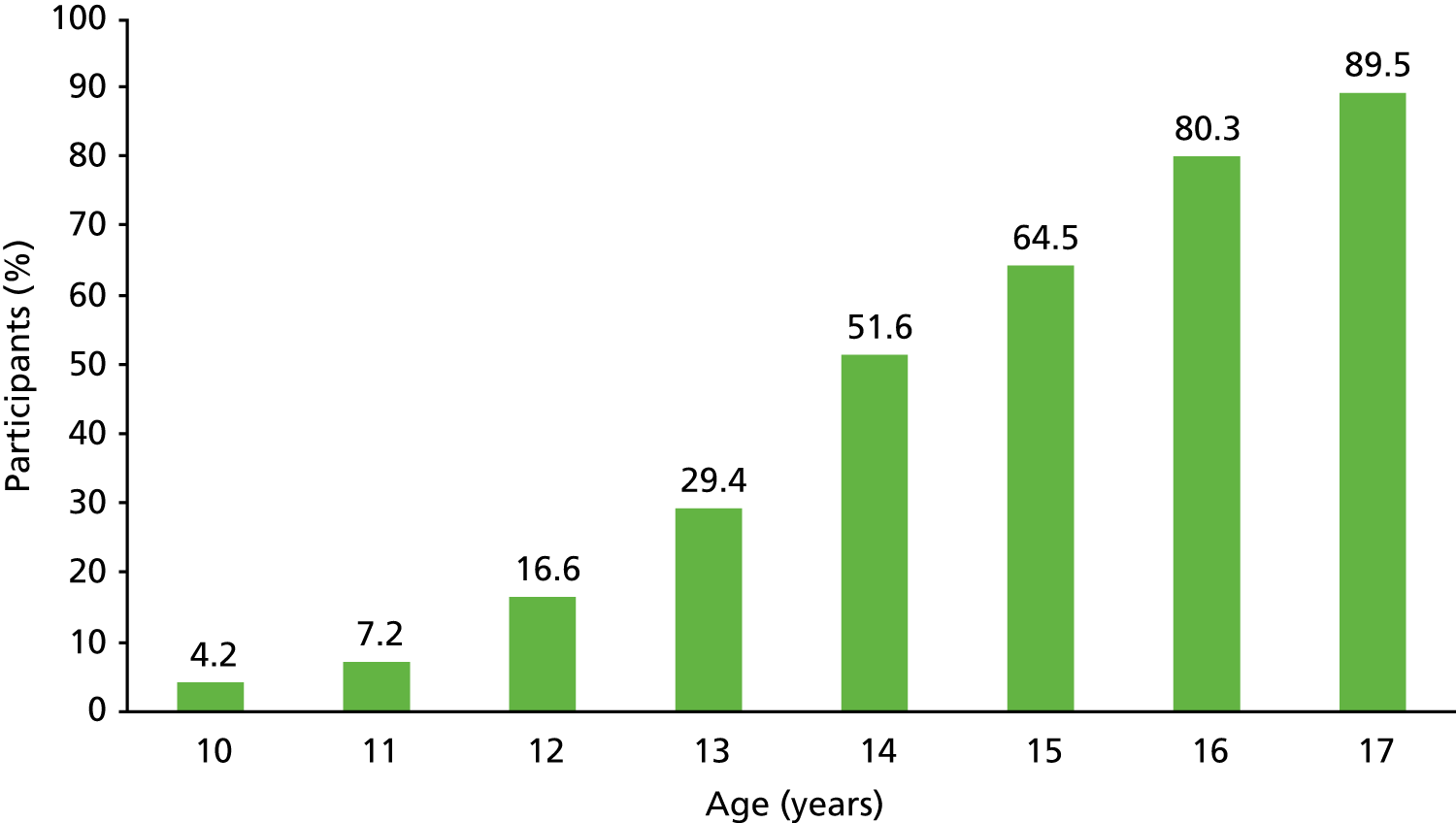
A total of 1374 participants (25.6% of the whole sample) reported drinking more than a sip of alcohol in the previous 3 months. The average age of first alcoholic drink was 12.9 years, ranging from 5 to 17 years of age (17 years was the upper limit for inclusion in this study). The prevalence of at-risk drinking was 14.8% (95% CI 13.9% to 15.8%), of monthly heavy episodic alcohol use was 10.6% (95% CI 9.8% to 11.4%), of alcohol abuse was 2.4% (95% CI 2.0% to 2.8%) and of alcohol dependence was 1.2% (95% CI 0.9% to 1.5%). Among the sample of those who had consumed alcohol in the past 3 months, the prevalence of these behaviours was significantly higher.
Relationship between alcohol consumption and harm (aim 3)
Alcohol consumption in the previous 3 months was associated with older age, being female, being white and having smoked tobacco. In addition, those who had consumed alcohol within the previous 3 months were more likely to report a lower quality of life and to have peer and social problems.
We also found that total alcohol consumed in the previous 90-day period was associated with tobacco use, lower quality of life, poorer general social functioning (conduct and hyperactivity), and the ESPAD questions on health and social problems.
Further analysis investigated the association between age of first alcohol consumption and psychological and social problems. Only participants aged 16 or 17 years who had consumed alcohol in the past 3 months (N = 609, n = 316 female) were included in this analysis. This analysis showed that consumption of alcohol before the age of 15 years was associated with an increased risk of a number of health and social problems. These included a greater risk of smoking tobacco (p < 0.001), lower quality of life (p = 0.003) and a diagnosis of an AUD, as indicated by the MINI-KID (p = 0.002). Consumption of alcohol before the age of 15 years was also associated with a greater risk of experiencing conduct (p = 0.001) and hyperactivity problems (p = 0.001), and more alcohol-related social problems, including having an accident (p = 0.046), problems with a parent (p = 0.017), school problems (p = 0.0117) and experiencing problems with the police (p = 0.012).
Discussion
In this work package, we investigated for the first time the screening properties of a short tool, the prevalence of alcohol consumption, the relationship with emotional and behavioural problems, and alcohol-related harms in adolescents presenting to the ED. The strengths of this study include the large sample size, the wide age range of those studied who were not seeking alcohol treatment and the broad spread of study across 10 EDs in England.
We found that a simple, short three-item self-completed screening instrument, the AUDIT-C, is overall more effective than the longer 10-item AUDIT in identifying adolescents who engage in at-risk alcohol consumption, monthly heavy episodic alcohol use and fulfil the ICD-10 criteria for alcohol abuse. Furthermore, the AUDIT with a cut-off score of 7 is more efficient than the AUDIT-C in identifying adolescents with alcohol dependence. In addition, the AUDIT-C and the AUDIT are widely employed as screening tools for adults in clinical and non-clinical settings and these can be applied equally to adolescent populations with these lower cut-off scores. We conclude that the AUDIT-C should be employed with this population with a cut-off score of 3 as a positive screen for at-risk drinking, monthly heavy episodic alcohol use and alcohol abuse. For those who score ≥ 5 on the AUDIT-C, we recommend that the additional seven questions constituting the full AUDIT be administered. Those scoring ≥ 7 should be clinically assessed for alcohol dependence.
We also found that nearly 40% of the adolescents presenting to the study EDs in England reported that they had consumed a drink of alcohol that was more than a sip in their lifetime. Rates of consumption increased considerably with age, ranging from just 4% for those aged 10 years to 90% for those aged 17 years. Among adolescents who had consumed alcohol in the past 3 months, 14.8% of drinkers screened positive for hazardous alcohol use (≥ 3 on the AUDIT-C).
This work package shows an association between earlier alcohol consumption and harm in adolescents. The prevalence of a diagnosis of harmful alcohol use or dependence was considerably higher among participants who started drinking before the age of 15 years, but it remains to be established whether or not these persist into adulthood. Although the results of this work package do not establish causality, effective interventions to reduce alcohol consumption in this population could potentially mitigate the harmful consequences related to alcohol that are experienced from a young age in this group.
This study identified a high prevalence of AUDs in adolescents attending EDs; we suggest that this setting is relevant for research on alcohol screening in young people. The ED also has a high level of staff expertise, which is well placed to initiate safeguarding procedures when required and provide a good point of onward referral to specialist services. The possibility of conducting alcohol screening among adolescents presenting to the ED and the potential for providing interventions to help reduce alcohol consumption in this population was investigated further in the following work packages of this programme.
The use of technology to collect data was successful in this study, and it is known that technology shows promise as a tool to deliver interventions.
Work package 2: exploratory modelling of the interventions
This work package focuses on the development of age-appropriate alcohol interventions for adolescents. These interventions have been developed with extensive patient and public involvement through a series of focus groups and evaluation work; a review of reviews to explore the evidence base on alcohol SBI for adolescents to determine age-appropriate screening tools; and a systematic review of electronic alcohol interventions.
Systematic review of electronic alcohol interventions
We conducted a systematic review and meta-analysis of the available literature to determine the effectiveness of electronic screening and brief interventions (eSBIs) over time in non-treatment-seeking hazardous/harmful drinkers.
This systematic review has been published in Donoghue et al. 74
The widespread use of computers, the internet and smartphones has led to the development of electronic systems to deliver alcohol SBIs that can potentially address some of the barriers to implementation of traditional face-to-face SBIs. eSBIs have the potential to offer greater flexibility and anonymity for the individual and to reach a larger proportion of the in-need population. For both adults and adolescents, eSBIs (computer, web and phone based) can offer effective delivery of interventions in both educational and health-care settings, which may prove to be more acceptable than more traditional (face-to-face) approaches. 75–77 In addition, eSBIs could offer a more cost-effective alternative to face-to-face interventions.
A systematic search of the literature was conducted in May 2013 (with no restriction on publication date) to identify RCTs investigating the effectiveness of eSBIs to reduce alcohol consumption through searching the electronic databases PsycINFO, MEDLINE and EMBASE. Two members of the study team independently screened studies for inclusion criteria and extracted data. Studies reporting data that could be transformed into grams of ethanol per week were included in the meta-analysis. The mean difference in grams of ethanol per week between eSBI and control groups was weighted using the random-effects method based on the inverse-variance approach to control for differences in sample size between studies.
We defined an eSBI as an electronic intervention aimed at providing information and advice designed to achieve a reduction in hazardous/harmful alcohol consumption, with no substantial face-to-face therapeutic component. A SBI was defined as screening followed by a brief intervention composed of a single session, ranging from 5 to 45 minutes in duration, and up to a maximum of four sessions aimed at providing information and advice designed to achieve a reduction in hazardous/harmful alcohol consumption. Studies were not deemed eligible for inclusion if participants were alcohol dependent, mandated to complete eSBIs or part of a preselected specific group (e.g. pregnant women). There were no restrictions on age.
A total of 23 studies78–101 were deemed eligible for inclusion in this systematic review. All study interventions were either computer or web based. The content of the interventions included an assessment followed by personalised and/or normative feedback. Control conditions generally consisted of an assessment with no further feedback, but four studies82,85,90,91 included general information on alcohol consumption for those in the control conditions. There was some variation in the dose of the intervention, with the reported time taken to complete the intervention ranging from < 5 minutes91 to 45 minutes. 94 The dose of exposure to the intervention could also be increased through repeated access during the study period81 and/or a printed copy of the personalised feedback provided. 83,88,93,95,97,100 The attrition rate was highly variable between studies, ranging from 1% or 2%87 to > 50%. 99
We found that there was a statistically significant mean difference in grams of ethanol consumed per week between those receiving an eSBI and those in the control group at up to 3 months (mean difference –32.74, 95% CI –56.80 to –8.68), from 3 months’ to < 6 months’ (mean difference –17.33, 95% CI –31.82 to –2.84), and from 6 months’ to < 12 months’ follow-up (mean difference –14.91, 95% CI –25.56 to –4.26). No statistically significant difference was found at a follow-up period of ≥ 12 months (mean difference –7.46, 95% CI –25.34 to 10.43).
The results of this systematic review and meta-analysis suggest that eSBIs are effective in reducing alcohol consumption in the follow-up post-intervention period between 3 months and < 12 months, but not in the longer-term follow-up period of ≥ 12 months.
A review of alcohol screening and brief interventions for adolescents
In addition to the systematic review, we conducted a review of reviews to explore the evidence base on alcohol SBIs for adolescents, and to determine age-appropriate screening tools, effective brief interventions and appropriate locations to undertake these activities, in order to address the lack of consensus about the most effective components of effective interventions. This review of reviews has been published102 and is reproduced in full in Appendix 4.
We conducted a review of reviews based on publications from 2003 to 2013 identified through a search of electronic databases (e.g. PubMed, Web of Science). These were judged to capture all trials of alcohol SBIs in an adolescent population. Thirteen review papers76,77,103–113 were identified and summarised. We also found five additional studies114–118 of alcohol SBIs for adolescents (all published between 2010 and 2012) that were not included in any of the published systematic reviews, and these were also included in this review. Studies that focused on primary prevention of alcohol use were excluded from this review.
Various alcohol screening methods for adolescents have been developed in the USA but have not been evaluated in the UK. Questionnaires were found to perform better than blood markers or breath alcohol concentration in all age groups. The CRAFFT and AUDIT tools are recommended for identification of ‘at-risk’ adolescents. In particular, the AUDIT questionnaire54 was found to have greater sensitivity and specificity than other tools. AUDIT sensitivities for adolescents ranged from 54% to 87% and specificities ranged from 65% to 97%. 31
A number of reviews on effective interventions for adolescents identified as being in need of help or advice about their drinking have now been published; the most recent of these have focused on the use of internet, computer and mobile phone technologies, collectively referred to as electronic brief interventions (eBIs). These reviews present limited evidence that eBIs significantly reduce alcohol consumption compared with minimal or no intervention controls,76,77,104 and our review presented in the previous section extends this work, indicating effectiveness of eBIs in a meta-analysis. 74 However, some caution should be exercised when interpreting these findings, as an earlier meta-analysis by Carey et al. ,119 which compared eBIs with a more traditional face-to-face delivery of interventions, concluded that face-to-face delivery was superior. Indeed, motivational interventions delivered over one or more sessions and based in health-care or educational settings are effective in reducing levels of consumption and alcohol-related harm. 107
Further research to develop age-appropriate screening tools needs to be undertaken. The effect of SBI activity should be investigated in settings in which young people are likely to present; further assessment at venues such as paediatric EDs, sexual health clinics and youth offending teams should be evaluated. The use of electronic (web-/smartphone-based) screening and intervention shows promise and should be another focus of future research.
Overall, this review of reviews and recent RCTs suggests that, despite an increasing interest in applying SBIs to an adolescent population, there are no clear indications of which target population, setting, screening tool or intervention approach can be recommended. The relationship between age, alcohol consumption and harm is complex, and further research is required to establish guidelines for consumption and thresholds of harm for different age groups.
Patient and public involvement in work package 2
In addition to the reviews described above, we engaged with a number of youth organisations (British Youth Council, The Well Centre and Redthread) to help refine our methodology and interventions. There was a clear indication that the stepped care motivational enhancement therapy approach that we had proposed during the application stage was not well received by our target group. As a result, we adopted their suggestions to undertake brief ED-based interaction and to use technology, and we developed a smartphone-based intervention app and a personalised feedback and brief advice (PFBA) (leaflet-based) condition for use in the intervention trials. We have involved young people in the design and content of the app and the leaflet, and have found this to be a particularly useful exercise that has helped us to achieve credibility with young people and to engage young people with our proposed interventions.
The second phase of the patient and public involvement was conducted to develop the interventions. Initially, we had planned to screen adolescents (using the optimal screening method from work package 1) and invite them to participate in a prospective RCT, using therapist-guided brief interventions and, where indicated, intensive motivational enhancement therapy (stepped care intervention). These interventions were to be compared with treatment as usual. The patient and public involvement work showed that young people felt electronic screening and consent was acceptable. A face-to-face brief intervention was acceptable in the ED, but any form of extensive intervention was not. An educational app was recommended by our focus group participants. Furthermore, the prevalence study showed that the questionnaire was too long to be acceptable to participants.
As a result, the iPad screening tool was shortened and refined to include the consent procedure to improve participant management. Participants and parents were e-mailed the information leaflets instead of being given paper copies, with laminated versions kept in the ED for reference. The iPad app was also developed to randomise participants to the different arms of the trial and to record a random sample of brief interventions for fidelity purposes.
The study design was revised so that the intervention comprised a brief intervention (PFBA) with a web-enabled smartphone app (eBI). The smartphone app was not in the initial plan for the trial but was included on the basis of the patient and public involvement, as young people had said that an educative app would be better received than our planned interventions. Adolescents had a preference for images over text, and it was suggested to make the app look and feel like a game.
The eBI takes the form of an app called ‘SIPS City’ [Screening and Intervention to Promote Sensible drinking (SIPS)]. The app home screen is a cartoon street with different places for young people to visit (and learn facts about alcohol), and includes gamification features that encourage participants to find and collect coins. It is designed to be engaging and educational, and to provide ongoing feedback and advice about alcohol consumption. It is loosely based on the FRAMES (Feedback of personalized risks: Responsibility, Advice, Menu of options, Empathy, Self-efficacy) motivational brief intervention approach. 120 A demonstration version of the SIPS City app was installed on iPads in the EDs to show to participants randomised to the eSBI arm of the trial, who were not able to access the app on their own smartphone while in the ED. Participants without a smartphone were asked to use an online web browser version of the app; participants who did have a smartphone but were not able to use it while in the ED were sent a link to download the app later.
The final phase of the patient and public involvement was conducted to develop an online self-completion form of the retrospective Timeline Followback-28 (alcohol consumption in the past 28 days), which was later modified in favour of a shorter outcome measure (AUDIT-C).
Work package 3: linked randomised controlled trials of face-to-face and electronic brief intervention methods to prevent alcohol-related harm in young people aged 14–17 years presenting to emergency departments
Background
A number of trials17,18,42,43,117,118 focusing on young people (aged 12–21 years) have reported significant positive effects of brief interventions on a range of alcohol consumption measures. Our systematic review (reported in Work package 2: exploratory modelling of the interventions) suggested that eBIs can significantly reduce alcohol consumption compared with minimal or no intervention controls, and have the added advantage of being more acceptable and easier to implement than more traditional face-to-face interventions. Our study of the prevalence of risky drinking among an adolescent population (aged 10–17 years) reported in Work package 1: screening prevalence study of alcohol consumption and alcohol use disorders in adolescents aged 10–17 years attending emergency departments found that about one in four young people presenting to EDs was consuming three or more drinks on one or more occasion over the preceding month, and that this level of consumption was associated with increased physical, social and educational adverse consequences. We also observed a steep transition in drinking prevalence between 13 and 17 years of age.
Several school-based interventions121 that target non-drinking adolescents have been found to delay the onset of drinking behaviours, and a recent study of adolescents122 found lower rates of substance misuse initiation among those exposed to a web-based intervention. Web-based alcohol interventions for adolescents also demonstrated significantly greater reductions in consumption and harm among ‘high-risk’ drinkers. 123 However, changes in risk status at follow-up for non-drinkers or low-risk drinkers have not been assessed in controlled trials of brief intervention.
Recruitment of both ‘high-risk’ and ‘low-risk’ drinkers has the additional benefit of addressing a major concern among both young people and parents, namely that participation in a trial of this nature may identify the young person as drinking at a level that warrants concern and intervention. Young people interviewed as part of our patient and public involvement work in work package 2 indicated that they would prefer to take part in a trial if there was no implication that they had an ‘alcohol problem’ and were assured that information about their drinking would not be disclosed to parents or health-care staff. Recruitment of both high- and low-risk-drinking young people was more acceptable to both young people and their parents, as was emphasising participant confidentiality.
Thus, we conducted two linked RCTs that included both high- and low-risk drinkers and abstainers, informing them that the study sought to prevent alcohol-related harm in young people. In addition, embedded within the proposed study was an internal feasibility study conducted prior to proceeding to the main trial.
The trials protocol has been published in Deluca et al. 124 and parts of this section have been reproduced from Deluca et al. 124 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Objectives
Primary objective
The primary objective was to conduct two linked RCTs to evaluate the clinical effectiveness and cost-effectiveness of brief intervention strategies compared with screening alone. One trial focused on high-risk adolescent drinkers attending EDs and the other focused on those identified as low risk or abstinent from alcohol. In both trials our primary outcome measure was quantity of alcohol consumed at 12 months after randomisation.
Secondary objectives
The secondary objectives of each study were:
-
to identify key predictors of recruitment to the trials
-
to explore the process of intervention through key psychological constructs that may lead to further refinement of the proposed interventions
-
to identify prognostic factors related to better outcomes
-
to explore interactions between participant factors, setting factors, treatment allocation and outcomes.
Our primary (null) hypothesis was similar for both trials: PFBA and personalised feedback plus eBIs is no more effective than screening alone in reducing alcohol consumed at 12 months after randomisation as measured with the AUDIT-C. Our secondary (null) hypothesis relating to health economics states that PFBA and eBIs are no more cost-effective than screening alone.
Methods
The linked trials were granted ethics approval by the National Research Ethics Service London – Fulham (reference 14/LO/0721). The trials comply with the Declaration of Helsinki125 and Good Clinical Practice126 and have been registered as ISRCTN45300218.
Study setting and participants
The trials were carried out in 10 EDs across three regions of England: North East, Yorkshire and The Humber, and London. Data collection was carried out from 10 a.m. to 10 p.m., 7 days per week, over an 8-month period (October 2014–May 2015). During these screening hours, consecutive ED attenders who were between their 14th and 18th birthdays and who met the inclusion criteria but none of the exclusion criteria were approached by a researcher and invited to participate in the study once cleared by ED staff to do so.
Eligibility criteria
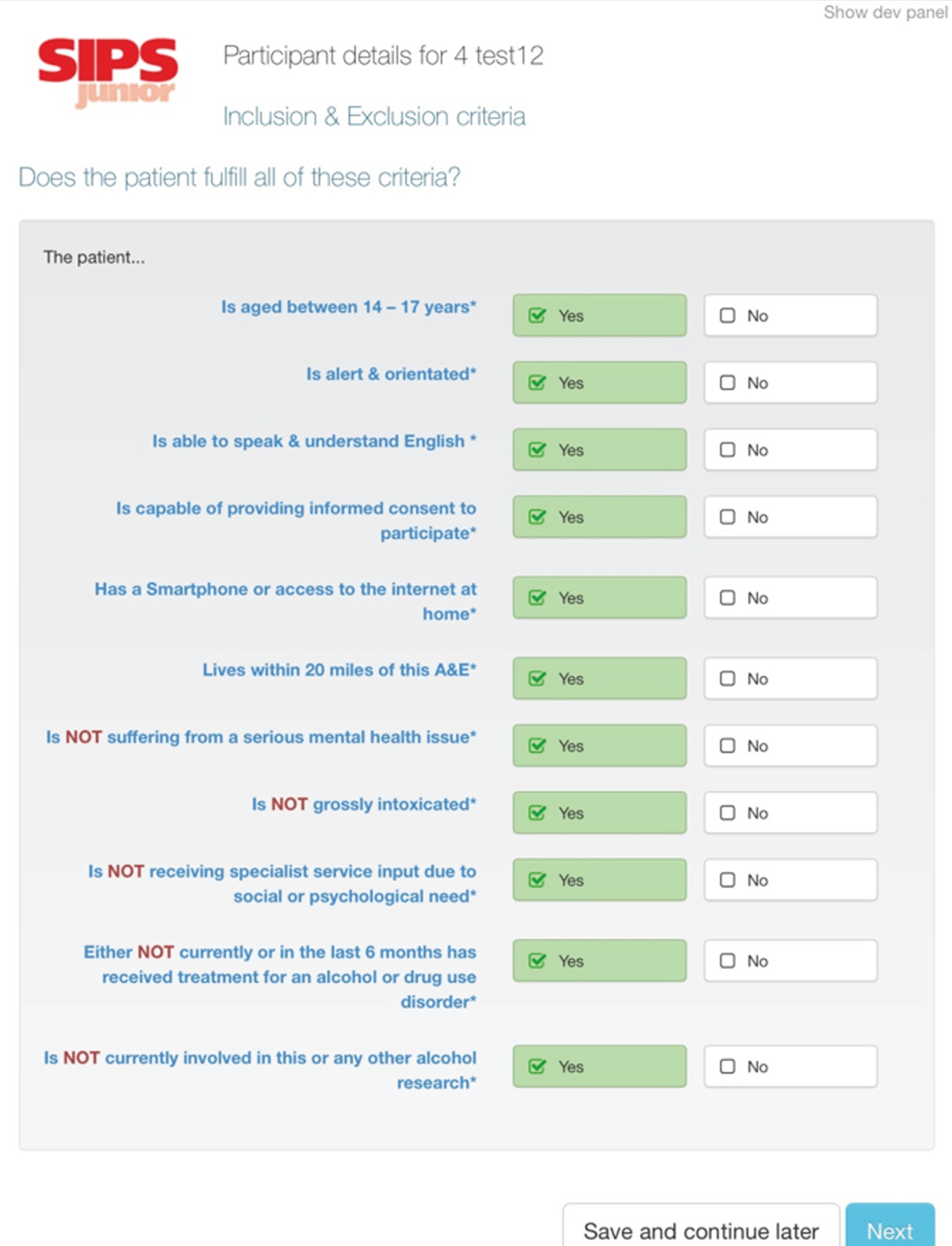
Inclusion and exclusion criteria were chosen to maintain a balance between ensuring the sample was representative of the ED population while also able to engage with both the relevant interventions and follow-up.
Inclusion criteria
The inclusion criteria were being aged between 14 and 17 years inclusive; being alert and orientated; being able to speak English sufficiently well to complete the research assessment; living within 20 miles of the ED; being able and willing to provide informed consent to screening, intervention and follow-up; if under aged < 16 years, being ‘Gillick competent’ or having a parent or guardian who was able and willing to provide informed consent; and owning a smartphone or having access to the internet at home.
Exclusion criteria
The exclusion criteria were having a severe injury; suffering from a serious mental health problem; being grossly intoxicated; specialist services being involved because of social or psychological needs; receiving treatment for an AUD or substance use disorder within the past 6 months; or currently participating in other alcohol-related research.
The inclusion and exclusion criteria were discussed with hospital nurses/doctors before a potential participant was approached and after clinical staff assessed the participant. We relied on their knowledge and professional judgement.
Those who were grossly intoxicated on attendance were not the population of interest. The study addressed those who consumed alcohol at levels at risk to their health, rather than alcohol-related attendances. Although it is possible that these two groups overlapped, we were mindful of the issue of informed consent for those who presented as grossly intoxicated; however, if their intoxicated state reduced to an acceptable level while they were in the ED, they were approached.
Those who met the inclusion criteria and none of the exclusion criteria and scored ≥ 3 on the screening questionnaire, AUDIT-C, were eligible for the high-risk study; those who scored < 3 on AUDIT-C were eligible for the low-risk study.
Consent procedure
The study was introduced to patients, and to their parent or guardian if they were aged < 16 years, as a study about alcohol, lifestyle and health, with the focus on preventing alcohol-related harm in all young people attending ED irrespective of their alcohol consumption. Patients aged < 16 years attending the ED without their parent or guardian were also approached to take part if ED staff confirmed that they were ‘Gillick competent’. We extended Gillick competency to consenting for participation in research on the grounds of minimal/no risk in taking part in this study, the potential direct benefit that they would gain from the advice received and the potential benefit to the wider society in the roll-out of the findings. 64
The study was first introduced by ED staff and then explained in more detail by research staff, both verbally and using the patient information sheet. If the patient was under the age of 16 years and accompanied by a parent or guardian, the parent or guardian would also receive the patient information sheet. Patients, and parents or guardians if applicable, had up to 4 hours to ask any questions about the study and to decide whether or not to take part. To obtain the most valid self-report data, patients were told as part of the informed consent procedure that their answers, including those on alcohol consumption, would not be disclosed to their parent or guardian or the ED staff without their consent (Figure 4).
FIGURE 4.
Decision tree for consent. PIS, patient information sheet.
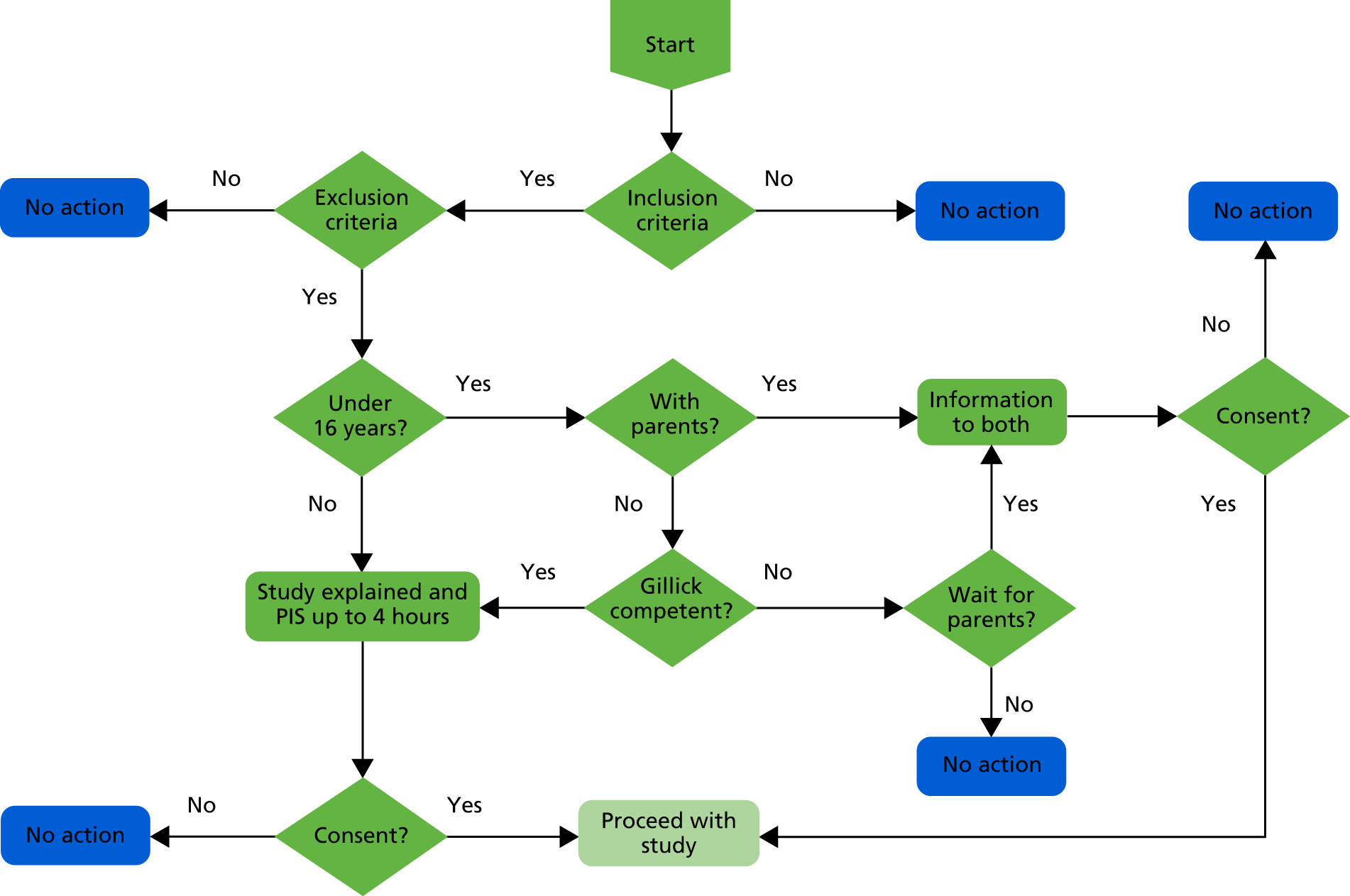
If patients agreed to participate, their informed consent was recorded using an electronic device (iPad), overseen by a research assistant who also introduced and delivered the allocated intervention to each patient in a private area of the ED. Consent to participate included permission to give the patient’s data and contact details to the research staff, to provide the research team with access to the patient’s ED records, and to participate in follow-up at 6 and 12 months after recruitment.
Screening and baseline assessment
After consent was given by the patient or their parent or guardian, as appropriate, the participant completed a screening and baseline assessment (Figure 5 shows the sequence of tools administration). All participants scoring ≥ 3 on the AUDIT-C (high-risk drinkers) were randomised between three groups [two intervention groups (PFBA and eBIs) and the control group receiving screening alone]. Of those scoring < 3 on the AUDIT-C (low-risk drinkers or abstainers), one in three was randomly selected to continue with the study and then randomised between three analogous groups. Participants who scored < 3 but were not selected for the trial were thanked for their participation, given a £5 voucher and returned to the care of the ED staff.
FIGURE 5.
Baseline sequence. APP, SIPS Jr City app; BA, brief advice; EQ-5D-5L, EuroQol-5 Dimensions, five-level version; ESP19, European School Survey Project on Alcohol and Other Drugs Q19; ESP19-C, European School Survey Project on Alcohol and Other Drugs Q19c; ESP21, European School Survey Project on Alcohol and Other Drugs Q21; ESP22, European School Survey Project on Alcohol and Other Drugs Q22; Q, question; SDQ, Strengths and Difficulties Questionnaire; SU, Service Use Questionnaire.
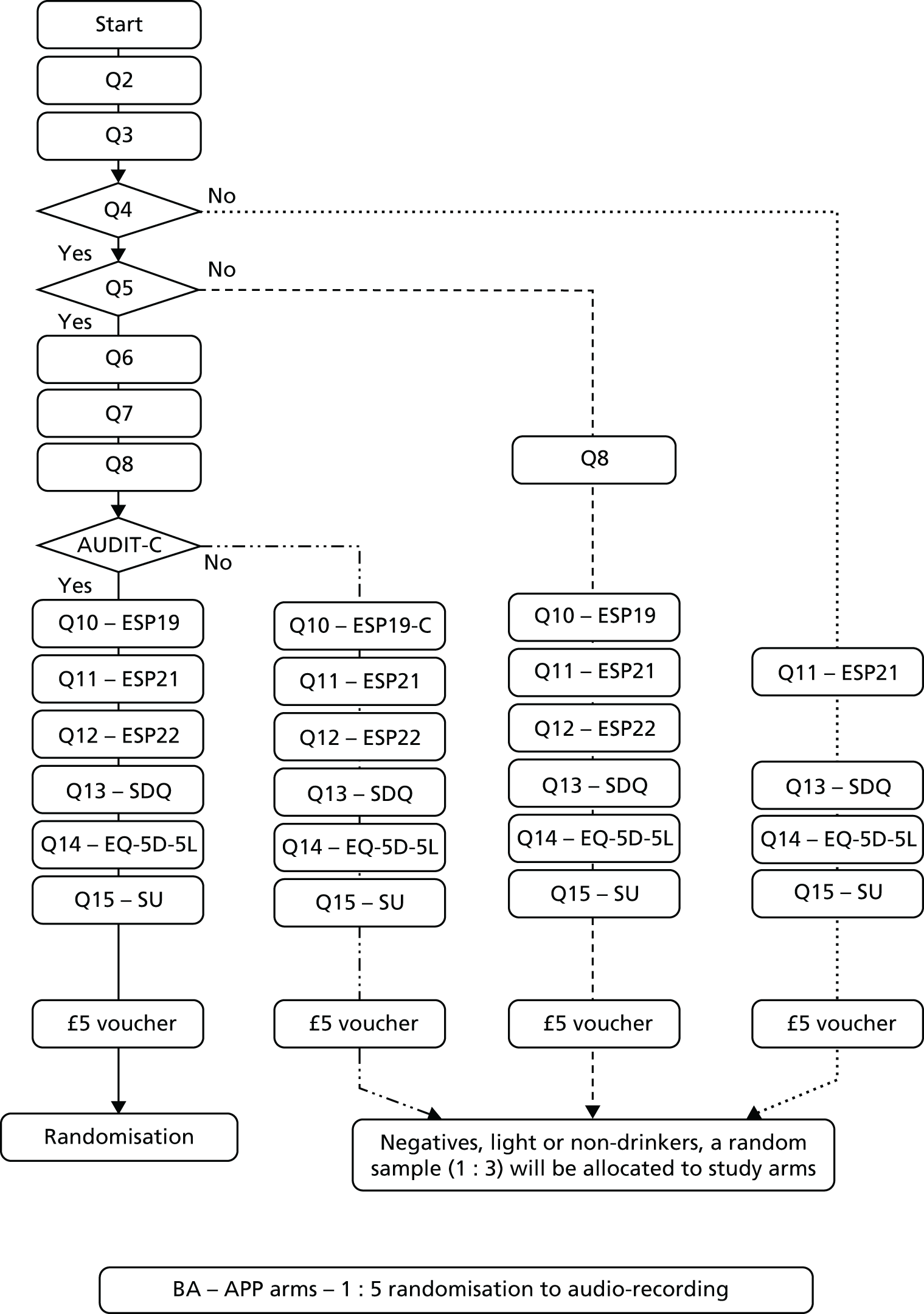
The screening and baseline assessment includes demographic information and contact details; health and lifestyle questions; the AUDIT-C;54 questions 19, 21 and 22 from ESPAD;68 the Strengths and Difficulties Questionnaire,127 the EuroQol-5 Dimensions, five-level version (EQ-5D-5L);128 and a short service use questionnaire. 129 This took approximately 10 minutes to complete.
To simplify and enhance data collection, we used a bespoke electronic interface (developed in work package 1), which automated question routing, showing participants only relevant questions. To maximise completion rates, we used an attractive graphical interface. Participants were able to skip questions or withdraw consent at any stage. All of the instruments have been designed and validated for those aged 14–17 years. The screening and baseline assessment was conducted by trained researchers with experience of working with adolescents, and all researchers had completed enhanced Disclosure and Barring Service checks prior to working in the ED. All information that participants gave was treated in confidence.
Participants were remotely randomised with equal probability, stratified by centre, between a screening only control group and one of the two interventions: a single session of face-to-face PFBA or personalised feedback plus a smartphone- or web-based brief intervention (eBI). All participants were eligible to receive treatment as usual in addition to any trial intervention.
Randomisation
Randomisation to trial participant or non-participant was conducted using a simple block randomisation, with a one in three probability of selection. For those selected as participants, randomisation to study group was conducted using strings of randomly selected block sizes, three or six, stratified by ED and gender. Each iPad within a centre had a separate pre-programmed allocation sequence derived by an independent party and made secure using encryption. Researchers engaged in the baseline assessment were not aware of allocated group until after outcomes had been completed. Participants were not blind to allocated group.
Interventions
Screening only group: treatment as usual
After completing the baseline assessment, participants in the screening arm were thanked for their participation, reminded that a member of the research team would contact them in 6 and 12 months to conduct a follow-up interview and returned to the care of the ED staff for usual care.
Personalised feedback and brief advice
The PFBA intervention is structured brief advice that takes approximately 5 minutes to deliver (Figure 6) in one session. It is based on an advice leaflet adapted for the target age group in this study from the SIPS brief advice about alcohol risk intervention. 130,131 It is based on the FRAMES model:132
-
Feedback: Give feedback on the risks and negative consequences of alcohol use. Seek the patient’s reaction and listen.
-
Responsibility: Emphasise that the individual is responsible for making his or her own decision about his/her alcohol use.
-
Advice: Give straightforward advice on modifying alcohol use.
-
Menu of options: Give menus of options to choose from, fostering the patient’s involvement in decision-making.
-
Empathy: Be empathic, respectful and non-judgemental.
-
Self-efficacy: Express optimism that the individual can modify his or her alcohol use if they choose. Self-efficacy is one’s ability to produce a desired result or effect.
FIGURE 6.
Brief advice leaflet.

It is conveyed verbally to the participant by trained research assistants or nurses and tailored to their risk status (high or low). It was delivered in a quiet room in the ED.
The advice covers recommended levels of alcohol consumption for young people; gives feedback on the screening results and their meaning; provides normative comparison information on prevalence rates of high- and low-risk drinking in young people; summarises the risks of drinking and highlights the benefits of stopping or reducing alcohol consumption; outlines strategies that they might employ to help stop or reduce alcohol consumption; highlights goals they might wish to consider; and indicates where to obtain further help if they are unsuccessful or need more support.
Each participant received a copy of the leaflet, which included additional information about alcohol intoxication, alcohol poisoning, and alcohol and the law.
Personalised feedback plus a smartphone- or web-based brief intervention
The eBI smartphone intervention SIPS City is an offline-capable mobile web application that works on a variety of platforms but is optimised for recent iPhone (Apple Inc., Cupertino, CA, USA) and Android (Google Inc., Mountain View, CA, USA) phones (Figure 7). It was developed for this research by the software developer Codeface Ltd (Hove, UK) in collaboration with the research team. It followed the recommendations from patient and public involvement, and it was developed using the concept of gamification so that users can navigate, explore, learn facts and figures about alcohol, receive personalised feedback and set goals in an engaging format. The content was adapted to provide the most pertinent information and advice for high- or low-risk drinkers and was similar in content to what was provided in the PFBA intervention arm described above in Personalised feedback and brief advice. Games components of the web application supported high-risk drinkers to reduce or stop their alcohol consumption and low-risk users to maintain abstinence or low-risk drinking.
FIGURE 7.
SIPS Jr Street app with full view of East and West Streets.



The SIPS City app was formatted into a virtual reality of two streets, west and east, in which there were multiple buildings such as a general practice, a pub and a youth centre. To gain access to some buildings, participants had to collect a certain number of coins, which could be obtained from talking to characters on the street or by answering questions correctly. When interacting with people on the street, participants were directed to certain buildings depending on the problem that person was encountering, for example the doctor for alcohol poisoning. It was also possible to drive in the car of ‘Rod McDuff’s School of Motoring’, and facts regarding the risks of alcohol and drinking were portrayed while inside the car.
The first building was the participants’ home, where they could fill out a drinking diary and receive feedback from this. It was also possible to view information on units and a letter from the local A&E about the participant’s drinking. Interaction with a health worker at the general practice allowed a user to follow-up the A&E letter and set personal alcohol goals. There was a sexual health clinic building that provided information on the increase of sexual health risks with increased alcohol intake. After two coins had been obtained, access to East Street was granted. The pharmacy was here, which provided information on how to reduce the effects of a hangover. The school provided information on the harmful effects of alcohol in relation to education, which provided relatable information to those in the age group in this study.
Whenever possible, the app was installed, with the help of a research assistant/nurse, on the participant’s smartphone while they were attending A&E and the participant was encouraged to use it. In the instances when they did not have access to their phone (e.g. flat battery, left at home, no data plan), patients were introduced to a demonstration version of the app on a study device (iPad) and allowed to play with it while in A&E. An e-mail and short message service (SMS) were also sent to the patient within 24 hours with instructions on how to download and install the app on their smartphone once they were at home.
Two further remainders (e-mail and SMS) were sent in the following 2 weeks to those who had failed to install the app on their smartphone.
For participants without access to a smartphone but with access to the internet through other computerised devices, access to a web-based version of the application was provided along with appropriate instructions for its use.
After receiving their allocated intervention (including the screening only group), all participants were thanked for their participation, reminded that a member of the research team would contact them in 6 and 12 months to conduct a follow-up interview, given a £5 voucher to thank them for their time and returned to the care of the ED staff.
Intervention fidelity
Research assistants were responsible for recruiting participants and delivering the interventions. The research assistants were trained during a 2-hour training session, which covered the rationale and procedures of the trial, the importance of reducing alcohol consumption and the correct delivery of the interventions. Filmed examples of delivery were presented and discussed, and role-play sessions were undertaken.
During the trial, we assessed fidelity of the delivery of the PFBA interventions by audio-recording a random sample of 20% of intervention sessions for each researcher. Each recording was assessed by a senior clinician member of the team on whether or not key aspects of the intervention were delivered as intended against a predefined checklist. When necessary, feedback was provided to researchers to improve fidelity. These recordings were prespecified in the protocol analysis plan.
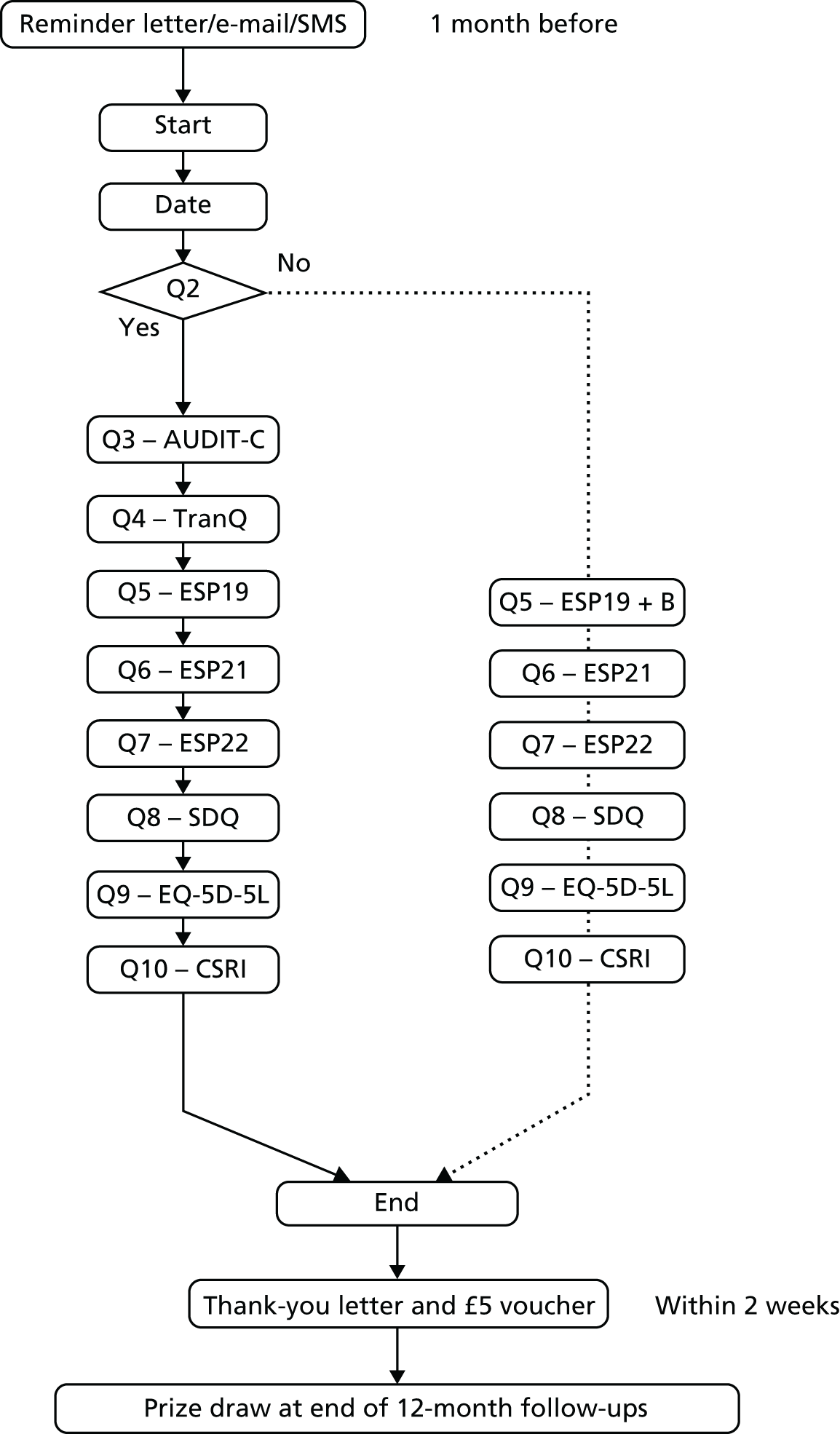
Follow-up assessments
All participants were followed up with a brief set of questions at 6 months after randomisation (Figure 8), and then at 12 months for a full assessment (Figure 9). Follow-up interviews were conducted over the telephone, face to face or electronically via self-completion web survey, as preferred by the participant. The telephone and face-to-face follow-ups were conducted by research assistants trained in the administration of the assessment tools and blinded to the group allocation of the participants. Letters of thanks were sent to participants after each follow-up stage. On completion of each follow-up interview, participants were sent a gift token for £5 by post in recognition of their participation. On completion of the 12-month follow-up, participants were additionally entered in to a prize draw to win an iPad Air (Apple Inc., Cupertino, CA, USA), iPad mini (Apple Inc., Cupertino, CA, USA) or iPod (Apple Inc., Cupertino, CA, USA).
FIGURE 8.
List of tools and order of presentation at 6-month follow-up. Q, question; SU, service utilisation.
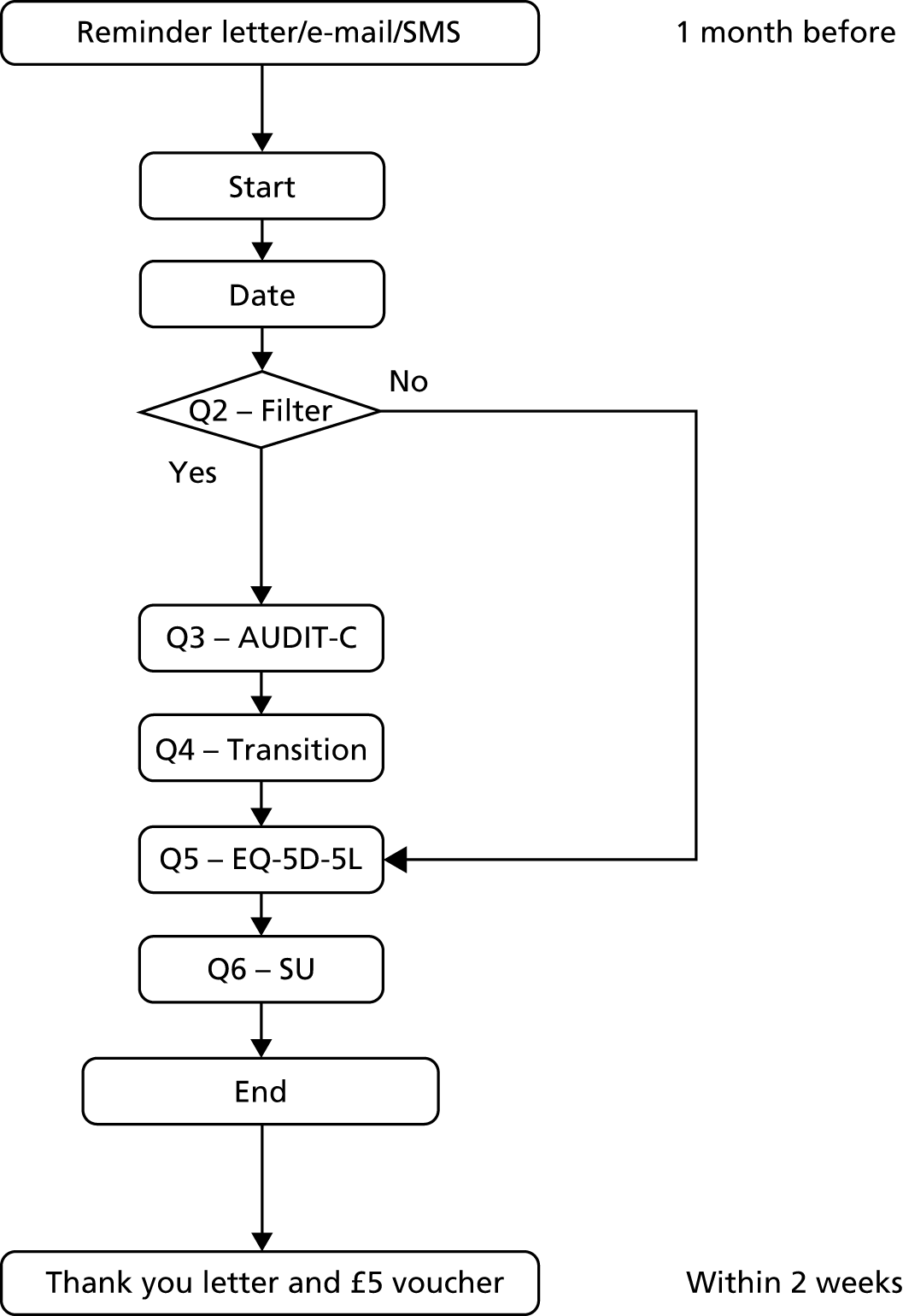
FIGURE 9.
List of tools and order of presentation at 12-month follow-up. B, items A (lifetime) and B (last 12 months) in question 19; CSRI, Client Service Receipt Inventory; EQ-5D-5L, EuroQol-5 Dimensions, five-level version; ESP19, European School Survey Project on Alcohol and Other Drugs Q19; ESP21, European School Survey Project on Alcohol and Other Drugs Q21; ESP22, European School Survey Project on Alcohol and Other Drugs Q22; Q, question; SDQ, Strengths and Difficulties Questionnaire; TranQ, transition question.
Outcome measures
Primary outcome measure
The primary outcome was the total amount of alcohol consumed in standard UK units (1 unit = 8 g of ethanol) over the previous 3 months, measured at the 12-month follow-up using the AUDIT-C, which was either self-completed by web survey or administered by researchers blinded to treatment allocation.
In the published protocol124 we intended to use the Timeline Followback interview (28-day version). However, this was subsequently changed to the AUDIT-C to facilitate completion rate at follow-up. The AUDIT-C is a much shorter tool (three items) and can be self-administered.
Calculation of weekly units from the AUDIT-C was conducted as follows. The extended AUDIT-C asked two questions regarding frequency and quantity of alcohol consumed. Question 1 asks about frequency, and these values are converted to weekly frequency using the following algorithm: never (0), monthly (0.25), two to four times per month (0.75), two or three times per week (2.5), four or five times per week (4.5) and six or more times per week (6.6). Question 2 asks about quantity on each drinking occasion and is converted to standard units using the following algorithm: none (0), one or two (1.5), three or four (3.5), five or six (5.5), seven to nine (8), ten to twelve (11), 13 to 15 (14) and 15 or more (15). Weekly units are calculated by multiplying converted values for frequency and quantity.
This value allocates participants to 1 of 35 categories of consumption. An ordinal is one in which values are ranked, A is greater than B, but the relative magnitude of A relative to B is unknown. The weekly consumption calculation not only ranks participants but also allows a derivation of the relative difference between participant drinking levels. The large number of data points and the ability to assess relative magnitude means that the weekly consumption can be taken as a continuous measurement variable. This implicit assumption was tested as part of the overall analysis.
Moreover, any ordinal scale with > 11 data points can be treated as continuous. 133
Secondary outcome measures
Participants were also asked questions about the consequences of alcohol consumption using questions 19, 21 and 22 from ESPAD. 68 Hazardous alcohol use was assessed using the extended AUDIT-C questionnaire54 at baseline and after 6 and 12 months. General health and functioning was measured using the Strengths and Difficulties Questionnaire127 at baseline and 12 months.
Economic outcome measures
The primary outcome measure for the economic evaluation in the trial was a preference-based measure calculated from the EQ-5D-5L. The EQ-5D-5L quality-of-life instrument is preferred by NICE for the economic evaluation of NHS interventions. The tool focuses on five dimensions of health: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. 128 The original EuroQol-5 Dimensions had three response categories (EuroQol-5 Dimensions, three-level version) for each dimension. A newly released validated version with five response categories (EQ-5D-5L) for each dimension, providing enhanced discriminatory power, was used in the study. 134 EQ-5D-5L requires no more than a few minutes to complete and thus imposes minimal burden on participants.
The EQ-5D-5L scores were converted to health utilities (1 = perfect health, 0 = equivalent to dead) using a tariff provided by the EuroQol group derived from UK social preference surveys. Resulting utilities were combined with survival data (unlikely to be affected by the service) and expressed in quality-adjusted life-years (QALYs). The estimated incremental cost per QALY from the service can be compared with the willingness-to-pay (WTP) threshold of £20,000–30,000 per extra QALY currently used by NICE to determine whether or not an intervention is ‘cost-effective’ and hence recommended for use in the NHS. 135
Process outcome measures
Expectancy was measured using the ESPAD question 2168 at baseline and 12 months after randomisation. Adherence to the eBI was assessed by monitoring remotely either when the smartphone device was connected to the internet or when the web application was accessed.
Analysis
Sample size calculation
For both studies, the sample size addresses the effect of interventions on the primary outcome measure (alcohol consumption at 12 months after randomisation). We aimed to detect a meaningful effect size difference of ≥ 0.3, based on literature relating to adults and similar to differences observed for adolescents; this would equate with a difference in weekly consumption between intervention and control of 0.1 units in the low-risk trial and 2 units in the high-risk trial. 136 To detect this with a significance level of 5% and statistical power of 80% when using a two-sided continuity-corrected test requires 175 in each of the three groups, yielding a target of 525 analysable participants in each of the two trials.
As there was little prior research in this specific area, our sample size calculation was based on similar UK RCTs137,145 addressing alcohol use in primary care populations. These RCTs reported effect size differences between brief interventions and minimal intervention of 0.36 and 0.27. 138,145 Similar effects have been reported from studies in the USA, and an effect size of 0.3 is considered clinically important for alcohol brief intervention studies. 139 As there is no literature on what might be a clinically important difference for the low-risk trial, we hypothesised that a small effect size difference, of a similar magnitude to or greater than that for the high-risk trial, could be interpreted as an important effect.
Predicting that follow-up at 12 months would be 70%, we needed to randomise 750 high-risk drinkers and 750 low-risk drinkers. Based on the estimated prevalence of 24.2% for high-risk drinking (namely AUDIT-C ≥ 3) from our earlier survey, and a consent rate of 60% (see Work package 1: screening prevalence study of alcohol consumption and alcohol use disorders in adolescents aged 10–17 years attending emergency departments), we estimated a number needed to approach of 5165 potential participants over the recruitment period. Of these participants, our survey predicts that 2350 will be low-risk drinkers consenting to the study.
Statistical analysis
The outcomes for both trials were analysed in a similar manner. Analysis was conducted using an intention-to-treat principle, whereby participants were analysed as members of their allocated group irrespective of treatment received. All analysis was conducted using SAS® software 9.4 (SAS Institute Inc., Cary, NC, USA) and conducted blind to allocated group.
The analytical approach employed a mixed-effects model, with a fixed effect for allocated group and a random effect for ED. The covariates age, gender and baseline alcohol consumption were included as baseline covariates, as these are known to influence outcome. The distribution of the primary outcome was assessed prior to analysis and, if necessary, appropriate transformations were undertaken. A sensitivity analysis was undertaken using a non-parametric approach and assessed change in consumption. Wilcoxon rank-sum indices were generated and analysed using a similar mixed-effects approach. The influence of missing data was assessed using a series of multiple imputation models, and these were synthesised to assess the sensitivity of the observed results to missing data. Secondary outcomes were assessed using a similar mixed-model approach and adjusting for respective baseline values. To explore the value of the findings, we performed a post hoc analysis and calculated the Bayes’ factor of the primary outcome, comparing eBI and PFBA with control.
Two exploratory analyses were undertaken. The first was to investigate the relationship between potential prognostic pre-randomisation factors and alcohol consumption at 12 months. The factors included were alcohol expectancy, alcohol-related problems and demographics, and any interaction between these factors and intervention group. An initial analysis explored the relationship between alcohol consumption and each factor individually, with factors or interaction terms with a p-value of < 0.2 combined to create a full model. Backward elimination was used, retaining factors with a p-value of < 0.2 in the final model. If an interaction term had a p-value of < 0.2 but the p-value for the main effect was > 0.2, both terms were retained in the model. A second exploratory analysis explored the relationship between eBI usage and alcohol consumption at month 12 for those allocated to eBI using a linear regression approach, controlling for baseline alcohol consumption and gender.
We estimated in a sample size calculation that we would assess 70% of those allocated at baseline and we achieved this end. In our analysis, we explored the nature of missing data at 12 months post randomisation using multiple imputation and assessed the impact of these imputation models on the observed outcome using sensitivity analyses. The derived models, which assume potential bias in loss to follow-up, had no effect on the outcomes observed, so these data without imputation were employed for the primary analysis.
Cost-effectiveness analysis
Individual-level data were used to estimate mean differential costs between interventions. As data were not normally distributed, 95% CIs were calculated using a non-parametric bootstrapped method. 140 This was also done for effects, the EQ-5D-5L score and QALYs at 6- and 12-month follow-up. Difference in QALYs was estimated using the area under the curve method.
Sensitivity analysis
Cost-effectiveness results [mean total costs and effects, hence the incremental cost-effectiveness ratio (ICER)] are subject to uncertainty or sampling error. A joint uncertainty in costs and effects was investigated via a stochastic sensitivity analysis. Using a large number of non-parametric bootstrapped replications (n = 10,000) of costs and effects (jointly), this uncertainty was quantified through a 95% CI of the ICER. 141,142 Based on the above bootstrapped replications, a two-dimensional cost-effectiveness plane was created, plotting the joint uncertainty in costs and effects between two groups. Furthermore, a cost-effectiveness acceptability curve was undertaken to show the probability that an intervention was cost-effective at a range of WTP values (£20,000 and £30,000 per QALY gain in the UK).
Valuation of resource use
All NHS resource use was reported in appropriate physical units and valued using relevant unit costs. 143 All figures were based on 2014 costs. As costs were incurred only over 12 months, discounting was not necessary. The cost of screening and delivering the two interventions were ascertained by prospectively monitoring resource inputs to each arm of the trial at 6- and 12-month follow-up, including training, and valued using standard methods. 141
Training costs
All resources involved in training were costed, including:
-
trainer time in preparing for training sessions, in travelling to training sessions and in delivering the training sessions (and anything else); this was costed by using the number of trainers and their salary or university/NHS grade/band
-
trainee time in travelling to training session and in attending training session; costed accordingly as in (a)
-
expenses incurred by trainers or trainees (e.g. train/bus fares, taxis, parking); for car travel, the travel time reported above was be converted into motoring costs
-
cost of any materials used (either described or in pounds sterling spent).
NHS and non-NHS costs
Effects on NHS and non-NHS costs was based on information gathered on patient contact with primary care, secondary care, specialist health services, social service and criminal justice, and other resources. These were collected prospectively using the appropriately modified version of the Client Service Receipt Inventory (CSRI). The CSRI captures any resource implication for the last 6 months. Service utilisation in CSRI was valued using local costs and, when possible, supplemented by national resources and information from previous alcohol studies. 130,144,145 Appropriate unit costs were used to derive a cost of any NHS resource [e.g. hospitalisation, general practitioner (GP) visit] or non-NHS resource (e.g. cost of criminal offence) use. 143
Missing data
Multiple imputation was used to handle missing values related to individual EQ-5D-5L input variables, with EQ-5D-5L utility values calculated from the imputed variables. Ten imputations were calculated. For missing costs, it was first determined whether costs were truly missing or truly zero, and for the truly missing costs the average costs for each intervention were imputed.
Results
Low-risk drinkers trial
Participant flow
Participant progress throughout the trial is presented in the CONSORT (Consolidated Standards of Reporting Trials) flow diagram (Figure 10). Of the 7854 attendees, 5016 were approached (63.9%). All reasons for exclusion are reported in Figure 10. Approximately 1% (n = 83) were intoxicated at the time of presentation and not approached for participation in the study. Twenty-five patients were excluded, because they did not own a smartphone or have internet access to receive the intervention. Of the patients approached, a total of 3326 met all of the inclusion criteria and consented to be screened (66%). Of these patients, 2571 (77.3%) scored < 3 on the AUDIT-C and were eligible for the low-risk study. One-third of these potential participants (n = 884) were selected at random and randomly allocated into one of the three groups.
FIGURE 10.
The CONSORT flow diagram of the linked trials.
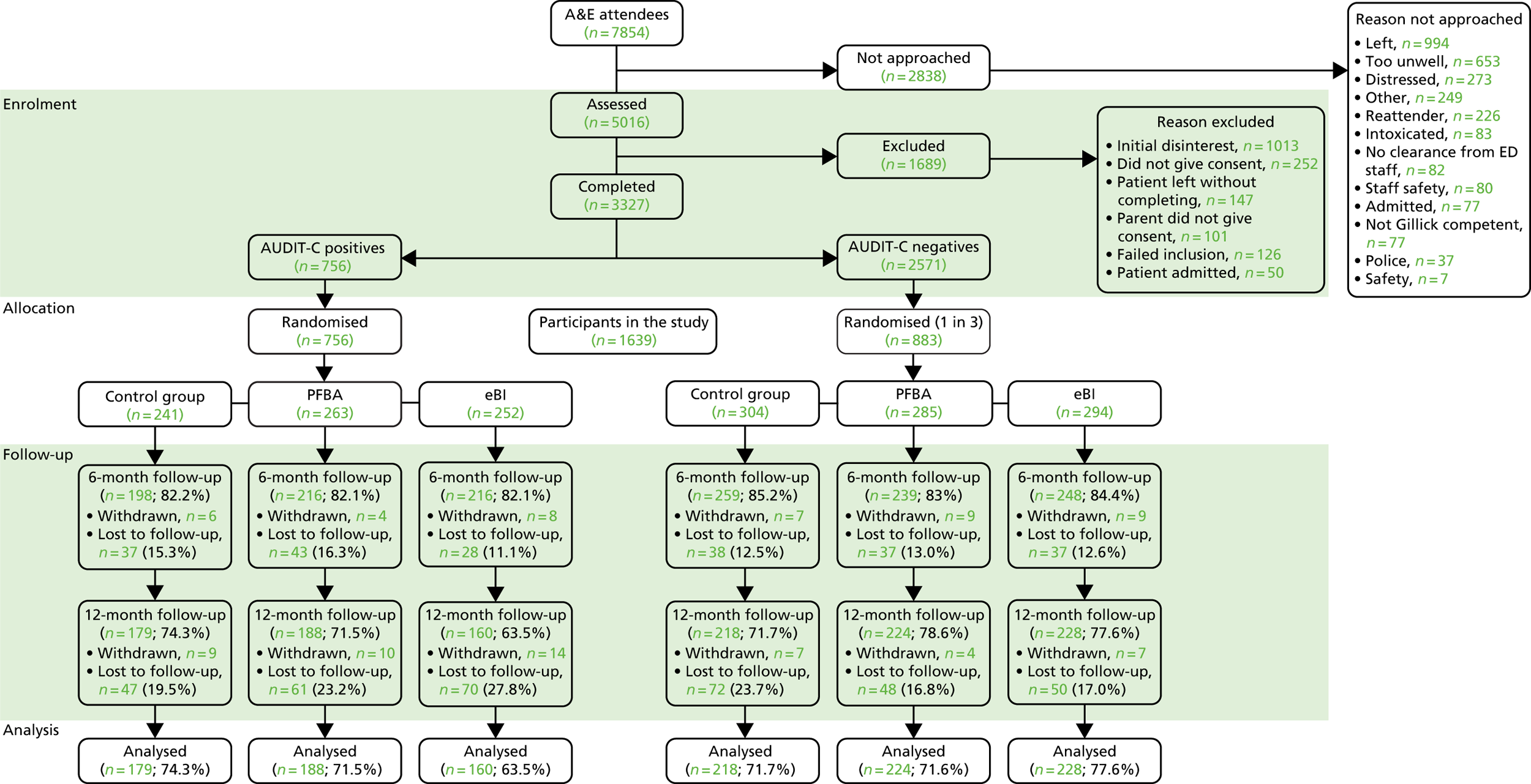
Sample characteristics
Demographic and outcome variables were similar across all three groups at baseline (Table 1). Overall, the mean age of those participating in the study was 15.1 years, 51% were female and 62.5% of the sample classified their ethnicity as white. Participants’ mean age at the time of first drink was 13.8 years and mean weekly alcohol consumption was low at 0.14 units of alcohol.
| Demographic | Control (n = 304) | PFBA (n = 285) | eBI (n = 294) |
|---|---|---|---|
| Mean age in years (SD) | 15.2 (1.1) | 15.1 (1.0) | 15.2 (1.0) |
| Mean age (years) of first drink (SD) | 13.8 (1.7) | 13.6 (1.9) | 13.9 (1.8) |
| Male (%) | 47.4 | 50.9 | 48.6 |
| White (%) | 61.5 | 64.9 | 61.2 |
| Smoker (%) | 10.3 | 7.1 | 8.7 |
| Alcohol use | |||
| Mean weekly alcohol consumption (SD)a | 0.14 (0.28) | 0.14 (0.28) | 0.15 (0.29) |
| Mean AUDIT-C score (SD) | 0.38 (0.66) | 0.40 (0.71) | 0.43 (0.72) |
| Monthly episodic alcohol use (%)b | 2.6 | 7.4 | 6.4 |
| Ever intoxicated (%)c | 34.7 | 35.4 | 34.7 |
| Intoxicated in past 12 months (%)c | 25.0 | 26.6 | 26.0 |
| Intoxicated in past 30 days (%)c | 6.0 | 7.5 | 8.0 |
| Alcohol-related problem (%) | |||
| Ever fighting | 10.9 | 9.2 | 5.4 |
| Ever accident or injury | 16.7 | 14.1 | 10.9 |
| Ever parent problem | 10.9 | 7.0 | 6.1 |
| Ever peer problem | 10.3 | 11.3 | 6.8 |
| Ever school problem | 9.0 | 9.2 | 6.8 |
| Ever victim of theft | 5.8 | 2.1 | 4.1 |
| Ever police problem | 3.8 | 4.9 | 5.4 |
| Ever hospitalised | 10.8 | 7.9 | 6.8 |
| Ever unprotected sex | 4.5 | 2.8 | 6.1 |
| Ever regretted sex | 2.5 | 2.1 | 3.4 |
| Strengths and difficulties, mean (SD) | |||
| Total score | 10.9 (5.7) | 10.7 (5.5) | 11.0 (5.7) |
| Emotional symptom score | 3.0 (2.2) | 3.1 (2.3) | 3.2 (2.4) |
| Conduct problem score | 2.0 (1.7) | 1.9 (1.6) | 2.0 (1.6) |
| Hyperactivity score | 3.7 (2.3) | 3.8 (2.3) | 3.7 (2.2) |
| Peer problem score | 2.1 (1.6) | 1.9 (1.6) | 2.1 (1.6) |
| Prosocial behaviour score | 7.8 (1.8) | 7.9 (1.8) | 7.8 (1.7) |
Main outcomes in the low-risk trial
The primary outcome, weekly alcohol units consumed at month 12, was derived from the AUDIT-C. As consumption was positively skewed, we explored transformations using the Box–Cox transformation approach and identified a cube-root transformation as appropriate to fit the data.
Outcomes at 6 and 12 months were back-transformed and are presented in Table 2. Mean differences and associated 95% CIs are presented in Table 3. No differences were observed between the groups for the primary outcome at 6 or 12 months. A sensitivity analysis employing the Wilcoxon rank-sum of the change score demonstrated similar results, as did an assessment of multiple imputation of missing values. A similar pattern was observed for secondary outcomes.
| Measure | Control (n = 304) | PFBA (n = 285) | eBI (n = 294) |
|---|---|---|---|
| Alcohol use | |||
| Weekly alcohol consumptiona | |||
| Month 6 | 0.06 (0.03 to 0.10) | 0.04 (0.02 to 0.07) | 0.05 (0.03 to 0.09) |
| Month 12 | 0.10 (0.05 to 0.18) | 0.12 (0.06 to 0.21) | 0.10 (0.05 to 0.19) |
| AUDIT-C score | |||
| Month 6 | 0.14 (0.08 to 0.22) | 0.08 (0.04 to 0.14) | 0.06 (0.19 to 0.21) |
| Month 12 | 0.22 (0.12 to 0.36) | 0.21 (0.11 to 0.35) | 0.21 (0.11 to 0.35) |
| Strengths and difficulties (12 months) | |||
| Total score | 10.8 (10.2 to 11.4) | 10.2 (9.58 to 10.8) | 10.4 (9.76 to 11.0) |
| Emotional symptom score | 3.32 (3.07 to 3.57) | 3.06 (2.81 to 3.30) | 3.14 (2.90 to 3.39) |
| Conduct problem score | 1.58 (1.40 to 1.77) | 1.59 (1.41 to 1.77) | 1.75 (1.57 to 1.93) |
| Hyperactivity score | 3.48 (3.21 to 3.75) | 3.35 (3.08 to 3.61) | 3.23 (2.97 to 3.50) |
| Peer problem score | 2.41 (2.20 to 2.61) | 2.17 (1.96 to 2.37) | 2.29 (2.08 to 2.49) |
| Prosocial behaviour score | 7.95 (7.71 to 8.19) | 7.98 (7.74 to 8.22) | 7.75 (7.51 to 7.99) |
| Measure | PFBA | eBI |
|---|---|---|
| Alcohol use | ||
| Weekly alcohol consumptiona | ||
| Month 6 | –0.06 (–0.14 to 0.03) | –0.02 (–0.10 to 0.06) |
| Month 12 | 0.03 (–0.07 to 0.13) | 0.01 (–0.10 to 0.11) |
| AUDIT-C score | ||
| Month 6 | –0.08 (–0.18 to 0.02) | –0.03 (–0.13 to 0.07) |
| Month 12, Bayes’ factor (SE) | –0.01 (–0.12 to 0.11), 0.05 (0.13) | –0.01 (–0.12 to 0.11), 0.05 (0.18) |
| Strengths and difficulties (12 months) | ||
| Total score | –0.58 (–1.45 to 0.28) | –0.40 (–1.26 to 0.46) |
| Emotional symptom score | –0.27 (–0.62 to 0.09) | –0.18 (–0.53 to 0.17) |
| Conduct problem score | 0 (–0.25 to 0.26) | 0.16 (–0.09 to 0.42) |
| Hyperactivity score | –0.14 (–0.52 to 0.24) | –0.25 (–0.63 to 0.13) |
| Peer problem score | –0.24 (–0.50 to 0.03) | –0.12 (–0.38 to 0.15) |
| Prosocial behaviour score | 0.03 (–0.27 to 0.33) | –0.20 (–0.51 to 0.10) |
A post hoc analysis was also performed for the Bayes’ factor comparing eBI and PFBA with control: 0.05 [standard error (SE) 0.13] and 0.05 (SE 0.18), respectively. These results indicate that the null result is a true null finding of no effect of either intervention.
An analysis exploring potential interactions between quantity of alcohol consumption at baseline and allocated group found no significant interactions for the low-risk study (F = 1.78; p = 0.17).
Our exploratory analysis of prognostic factors that may impact on alcohol consumption at month 12 identified a number of significant positive predictors: higher baseline consumption, lower age of first drink, older age, being female, greater positive alcohol expectancy and greater alcohol-related problems (see Table 15, Appendix 1).
For those allocated to eBI, 103 (35.0%) participants actually engaged with the intervention after leaving the ED. No relationship was identified between engagement with the intervention and alcohol consumption at month 12.
Cost-effectiveness analysis in the low-risk trial
Cost-effectiveness analysis compared both the eBI and PFBA intervention groups with the control group for all societal costs (Table 4) and for NHS/Personal and Social Services (PSS) costs only (Table 5). The analyses show that, for both the societal cost perspective and the narrower NHS/PSS perspective, the eBI is dominated by the control, whereas the PFBA intervention generates ICERs of £130,822 (societal) and £120,693 (NHS/PSS) per QALY gained, respectively.
| Control | eBI | Difference | |
|---|---|---|---|
| Total costs (£) | 1132 | 1884 | 751 |
| Total QALYs | 0.90 | 0.89 | –0.01 |
| ICER (£/QALY gained) | eBI dominated | ||
| Control | PFBA | Difference | |
| Total costs (£) | 1132 | 1735 | 603 |
| Total QALYs | 0.90 | 0.91 | 0.005 |
| ICER (£/QALY gained) | 130,822 | ||
| Control | eBI | Difference | |
|---|---|---|---|
| Total costs (£) | 912 | 1683 | 771 |
| Total QALYs | 0.90 | 0.89 | –0.01 |
| ICER (£/QALY gained) | eBI dominated | ||
| Control | PFBA | Difference | |
| Total costs (£) | 912 | 1468 | 556 |
| Total QALYs | 0.90 | 0.91 | 0.005 |
| ICER (£/QALY gained) | 120,693 | ||
From the societal cost perspective, probabilistic sensitivity analysis (PSA) indicated that approximately 9% of the simulations for eBI compared with control were cost-effective at both the £20,000 and the £30,000 WTP thresholds, whereas approximately 26% and 30% of the simulations for PFBA compared with control were cost-effective at the £20,000 and £30,000 WTP thresholds, respectively (Table 6).
| WTP | ||
|---|---|---|
| £20,000 | £30,000 | |
| eBI vs. control (%) | 8.7 | 8.8 |
| PFBA vs. control (%) | 26.4 | 30.2 |
From the NHS/PSS cost perspective, PSA again indicated that approximately 9% of the simulations for eBI compared with control were cost-effective at both the £20,000 and the £30,000 WTP thresholds, whereas approximately 31% and 33% of the simulations for PFBA compared with control were cost-effective at the £20,000 and £30,000 WTP thresholds, respectively (Table 7).
| WTP | ||
|---|---|---|
| £20,000 | £30,000 | |
| eBI vs. control (%) | 9.1 | 8.8 |
| PFBA vs. control (%) | 30.6 | 33.2 |
The deterministic analyses and PSA show that it is highly unlikely that either intervention is cost-effective at either the £20,000 or the £30,000 WTP threshold when compared with the control intervention in low-risk patients.
High-risk drinkers trial
Participant flow: high-risk trial
Participant progress throughout the trial is presented in the flow diagram (see Figure 10). Of the 7854 attendees, 5016 (63.9%) were approached. A total of 3326 participants consented to be screened (66.0%) and, of these, 756 (22.7%) participants scored ≥ 3 on the AUDIT-C and were eligible for the high-risk study.
Sample characteristics: high-risk trial
Demographic and outcome variables were similar across all three groups at baseline (Table 8). Overall, the mean age of those participating into the high-risk study was 16.1 years, 50.2% were female and 84.9% of the sample classified their ethnicity as white. Mean age at first drink was 13.5 years and mean weekly alcohol consumption was higher than in the low-risk trial, at 4.7 units of alcohol.
| Demographic | Control (n = 241) | PFBA (n = 263) | eBI (n = 252) |
|---|---|---|---|
| Mean age in years (SD) | 16.1 (0.9) | 16.0 (0.9) | 16.1 (0.9) |
| Mean age (years) at first drink (SD) | 13.4 (2.1) | 13.7 (1.7) | 13.3 (2.2) |
| Male (%) | 51.9 | 48.3 | 49.2 |
| White (%) | 85.9 | 84.8 | 84.1 |
| Smoker (%) | 40.3 | 36.1 | 38.2 |
| Alcohol use | |||
| Mean weekly alcohol consumption (SD)a | 5.01 (7.82) | 4.33 (8.96) | 4.55 (7.43) |
| Mean AUDIT-C score (SD) | 4.86 (1.80) | 4.77 (1.93) | 4.87 (1.88) |
| Monthly episodic alcohol use (%)b | 37.8 | 34.6 | 42.1 |
| Ever intoxicated (%)c | 80.7 | 80.8 | 82.5 |
| Intoxicated in past 12 months (%)c | 70.6 | 70.9 | 72.4 |
| Intoxicated in past 30 days (%)c | 31.4 | 30.7 | 27.2 |
| Alcohol-related problem (%) | |||
| Ever fighting | 17.1 | 17.6 | 22.6 |
| Ever accident or injury | 32.8 | 32.4 | 33.3 |
| Ever parent problem | 17.0 | 15.0 | 19.7 |
| Ever peer problem | 22.8 | 23.4 | 28.3 |
| Ever school problem | 10.0 | 17.9 | 15.1 |
| Ever victim of theft | 15.9 | 17.6 | 17.5 |
| Ever police problem | 7.5 | 11.8 | 15.5 |
| Ever hospitalised | 14.9 | 13.3 | 12.4 |
| Ever unprotected sex | 19.1 | 14.9 | 24.3 |
| Ever regretted sex | 13.4 | 14.8 | 18.8 |
| Strengths and difficulties, mean (SD) | |||
| Total score | 12.0 (5.6) | 11.9 (6.1) | 12.6 (5.9) |
| Emotional symptom score | 3.4 (2.4) | 3.3 (2.4) | 3.4 (2.5) |
| Conduct problem score | 2.3 (1.7) | 2.3 (1.7) | 2.6 (1.8) |
| Hyperactivity score | 4.2 (2.2) | 4.3 (2.3) | 4.4 (2.3) |
| Peer problem score | 2.2 (1.7) | 2.0 (1.7) | 2.3 (1.6) |
| Prosocial behaviour score | 7.3 (1.9) | 7.3 (2.0) | 7.5 (2.0) |
Main outcomes in the high-risk trial
The primary outcome, weekly alcohol units consumed at month 12, was derived from the AUDIT-C. As consumption was positively skewed, we explored transformations using the Box–Cox transformation approach and identified a cube-root transformation as appropriate to fit the data.
Outcomes at 6 and 12 months were back-transformed and are presented in Table 9. Mean differences and associated 95% CIs are presented in Table 10. No differences were observed between the groups for the primary outcome at 6 or 12 months. A sensitivity analysis employing the Wilcoxon rank-sum of the change score demonstrated similar results, as did an assessment of multiple imputation of missing values. A similar pattern was observed for secondary outcomes.
| Measure | Control | PFBA | eBI |
|---|---|---|---|
| Alcohol use | |||
| Weekly alcohol consumptiona | |||
| Month 6 | 2.42 (1.84 to 3.11) | 2.13 (1.62 to 2.74) | 2.33 (1.77 to 3.00) |
| Month 12 | 2.99 (2.38 to 3.70) | 3.56 (2.90 to 4.32) | 3.18 (2.50 to 3.97) |
| AUDIT-C score | |||
| Month 6 | 4.64 (4.17 to 5.11) | 4.30 (3.85 to 4.75) | 4.64 (4.18 to 5.11) |
| Month 12 | 5.04 (4.65 to 5.44) | 5.25 (4.87 to 5.63) | 5.12 (4.70 to 5.54) |
| Strengths and difficulties (12 months) | |||
| Total score | 11.0 (10.2 to 11.7) | 10.9 (10.2 to 11.6) | 10.9 (10.1 to 11.6) |
| Emotional symptom score | 3.14 (2.82 to 3.46) | 3.23 (2.91 to 3.54) | 3.09 (2.75 to 3.43) |
| Conduct problem score | 1.90 (1.70 to 2.10) | 1.74 (1.55 to 1.94) | 1.86 (1.65 to 2.07) |
| Hyperactivity score | 3.54 (3.23 to 3.84) | 3.73 (3.43 to 4.02) | 3.87 (3.55 to 4.19) |
| Peer problem score | 2.30 (2.06 to 2.54) | 2.21 (1.97 to 2.44) | 2.05 (1.80 to 2.30) |
| Prosocial behaviour score | 7.91 (7.66 to 8.16) | 8.21 (7.97 to 8.45) | 7.75 (7.49 to 8.01) |
| Measure | PFBA | eBI |
|---|---|---|
| Alcohol use | ||
| Weekly alcohol consumptiona | ||
| Month 6 | –0.286 (–0.903 to 0.478) | –0.0886 (–0.756 to 0.737) |
| Month 12 | 0.570 (–0.362 to 1.70) | 0.186 (–0.714 to 1.30) |
| AUDIT-C score | ||
| Month 6 | –0.334 (–0.858 to 0.189) | 0.00685 (–0.528 to 0.542) |
| Month 12 | 0.206 (–0.334 to 0.747) | 0.0818 (–0.488 to 0.652) |
| Strengths and difficulties (12 months) | ||
| Total score | –0.0170 (–1.02 to 0.981) | –0.0998 (–1.14 to 0.945) |
| Emotional symptom score | 0.0891 (–0.340 to 0.518) | –0.0523 (–0.501 to 0.396) |
| Conduct problem score | –0.161 (–0.436 to 0.113) | –0.0426 (–0.330 to 0.245) |
| Hyperactivity score | 0.193 (–0.232 to 0.618) | 0.334 (–0.111 to 0.779) |
| Peer problem score | –0.0901 (–0.386 to 0.206) | –0.249 (–0.559 to 0.0608) |
| Prosocial behaviour score | 0.293 (–0.0406 to 0.626) | –0.165 (–0.514 to 0.183) |
We computed the Bayes’ factor comparing eBI and PFBA with control: 0.08 (SE 0.16) and 0.08 (SE 0.36), respectively. These results indicate that the null result is a true null finding of no effect of either intervention.
An analysis exploring potential interactions between quantity of alcohol consumption at baseline and allocated group found no significant interactions for the high-risk study (F = 0.27; p = 0.76).
Our exploratory analysis of prognostic factors that may impact on alcohol consumption at month 12 identified a number of significant positive predictors: higher baseline consumption, lower age of first drink, older age, being female, greater positive alcohol expectancy and greater alcohol-related problems (see Table 15, Appendix 1).
For those allocated to eBI, 84 (33.3%) actually engaged with the intervention after leaving the ED. No relationship was identified between engagement with the intervention and alcohol consumption at month 12.
Cost-effectiveness analysis
Cost-effectiveness analysis compared both the eBI and PFBA intervention groups with the control group for all societal costs (Table 11) and for NHS/PSS costs only (Table 12). The analyses show that, for both the societal cost perspective and the narrower NHS/PSS perspective, the eBI is dominated by the control, whereas the PFBA intervention generates ICERs of £7580 (societal) and £6213 (NHS/PSS) per QALY gained, respectively.
| Screening only | eBI | Differencea | |
|---|---|---|---|
| Total costs | £1703 (SD £6049) | £2110 (SD £7040) | £406 (95% CI –£1334 to £2331) |
| Total QALYs | 0.900 (SD 0.096) | 0.892 (SD 0.105) | –0.008 (95% CI –0.038 to 0.021) |
| ICER (£/QALY gained) | Screening dominates eBI | ||
| Control | PFBA | Differencea | |
| Total costs | £1703.65 (SD £6049) | £1726.39 (SD £6152) | £22.74 (95% CI –£1860 to £1663) |
| Total QALYs | 0.900 (SD 0.096) | 0.903 (SD 0.089) | 0.003 (95% CI –0.023 to 0.028) |
| ICER (£/QALY gained) | £7580 (95% CI from –£1,088,865 to £794,373) (not at all significant) | ||
| Screening only | eBI | Difference | |
|---|---|---|---|
| Bootstrapped mean (bootstrapped SD) | Intervention – control (bootstrapped 95% CI) | ||
| Total costs | £1552 (SD £6019) | £1953 (SD £6960) | £401 (95% CI –£1424 to +£2346) |
| Total QALYs | 0.900 (SD 0.096) | 0.892 (SD 0.105) | –0.008 (95% CI –0.037 to +0.019) |
| ICER (£/QALY gained) | Screening dominates eBI | ||
| Control | PFBA | Differencea | |
| Total costs | £1552.69 (SD £6019) | £1571.33 (SD £6114) | £18.64 (95% CI –£1752 to +£1586) |
| Total QALYs | 0.900 (SD 0.096) | 0.903 (SD 0.089) | 0.003 (95% CI –0.023 to +0.026) |
| ICER (£/QALY gained) | £6213 (95% CI from –£736,843 to £812,884) (not at all significant) | ||
From the societal cost perspective, PSA indicated that approximately 28% of the simulations for eBI compared with control were cost-effective at the £20,000 WTP threshold and 27% at the £30,000 WTP threshold; whereas approximately 54% and 55% of the simulations for PFBA compared with control were cost-effective at the £20,000 and £30,000 WTP thresholds, respectively (Table 13). Although PFBA has a chance of being cost-effective when compared with control, the distribution of the bootstrapped ICERs show that there is a wide distribution (Figure 11).
| WTP | ||
|---|---|---|
| £20,000 | £30,000 | |
| eBI vs. control (%) | 28.3 | 26.9 |
| PFBA vs. control (%) | 54.2 | 55.2 |
FIGURE 11.
Cost-effectiveness plane: PFBA vs. control, societal perspective, in the high-risk trial.
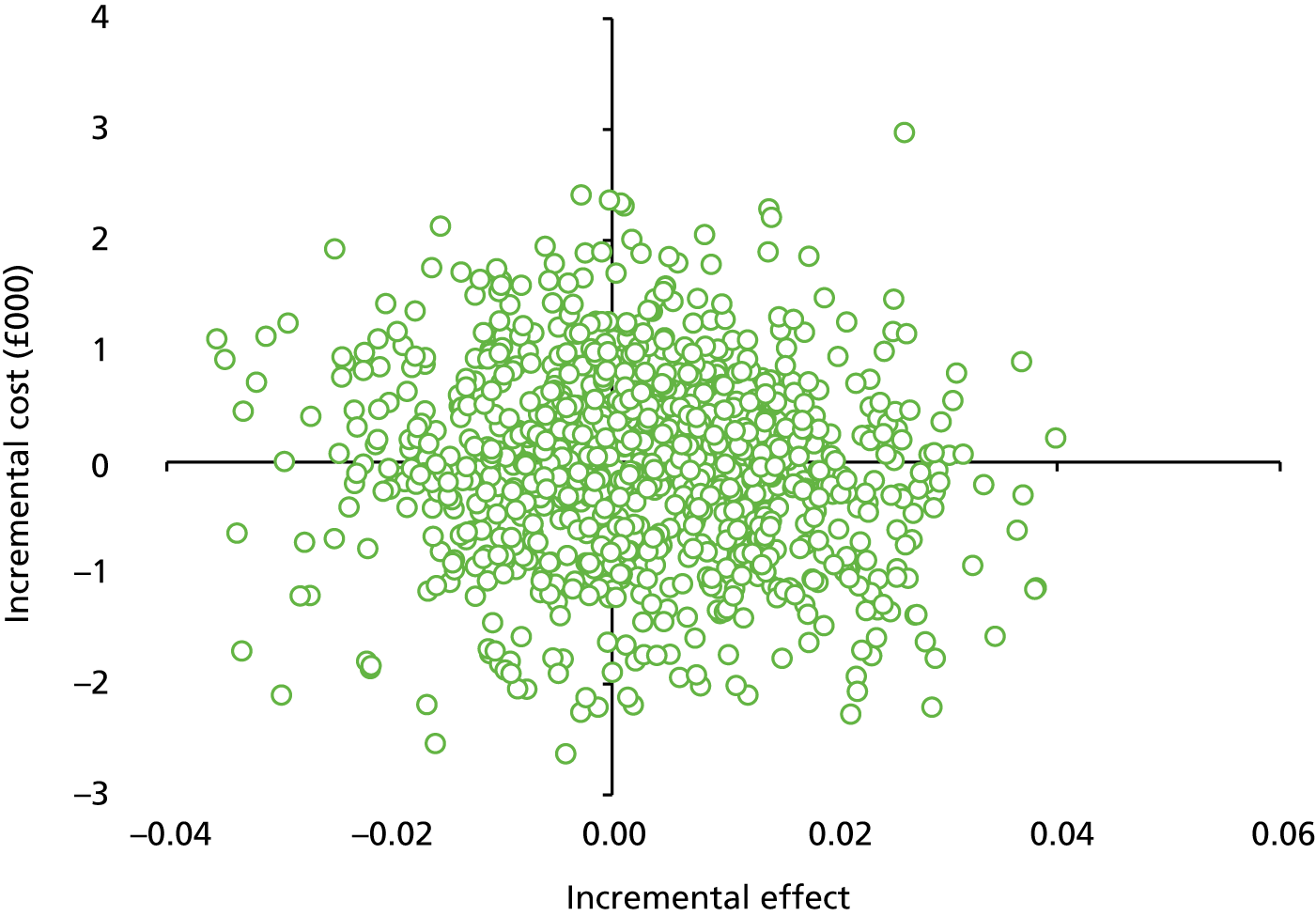
From the NHS/PSS cost perspective, PSA again indicated that approximately 30% of the simulations for eBI compared with control were cost-effective at the £20,000 WTP threshold and 29% at the £30,000 threshold; whereas approximately 54% and 56% of the simulations for PFBA compared with control were cost-effective at the £20,000 and £30,000 WTP thresholds, respectively (Table 14). Again, although PFBA has a chance of being cost-effective when compared with control, the distribution of the bootstrapped ICERs show that there is a wide distribution (Figure 12).
| WTP | ||
|---|---|---|
| £20,000 | £30,000 | |
| eBI vs. control (%) | 29.9 | 29.4 |
| PFBA vs. control (%) | 54.0 | 55.6 |
FIGURE 12.
Cost-effectiveness plane: PFBA vs. control, NHS/PSS perspective, in the high-risk trial.
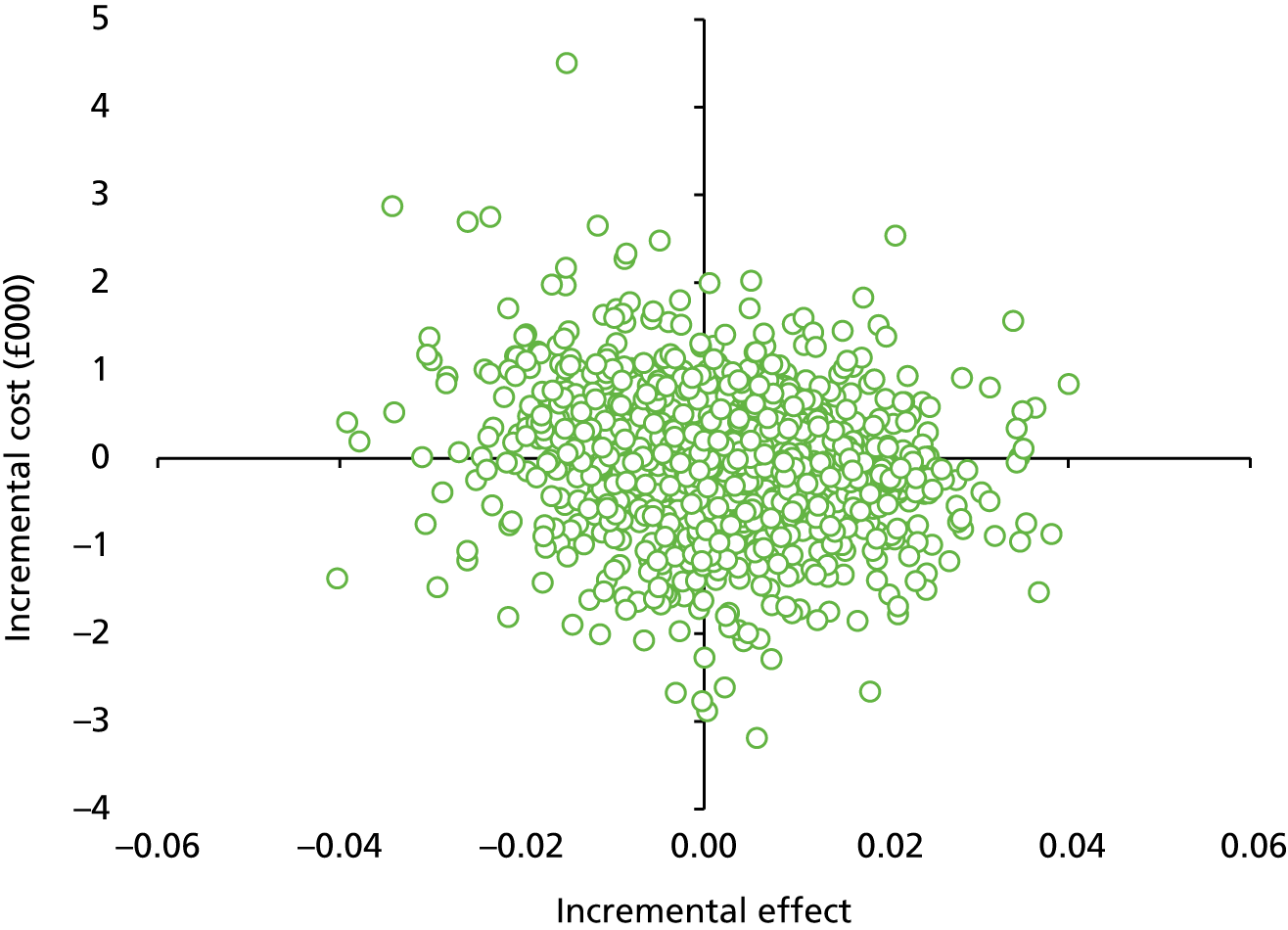
The deterministic analyses and PSA show that it is highly unlikely that the eBI is cost-effective at either the £20,000 or the £30,000 WTP threshold when compared with the control intervention in high-risk patients, although there is an approximately 55% chance that the PFBA intervention is cost-effective compared with the control.
Discussion
The results of both the low- and the high-risk trials showed that we were able to recruit a sufficient number of participants to each trial to meet our target recruitment informed by the power calculation. We were also able to exceed the minimum follow-up targets in both trials. Both trials were therefore adequately powered to detect significant differences between intervention groups on our primary outcome measure.
Analyses of baseline characteristics comparing different intervention groups showed that the groups were well matched based on our randomisation methods reducing the risk of bias in detecting effects in both trials. To further mitigate potential bias, we also controlled for key baseline characteristics in outcome analyses.
In the low-risk trial, no significant differences in outcome were found between groups on either primary or secondary outcome measures. The additional post hoc calculation of the Bayes’ factor supported the null hypothesis that PFBA and eBI are no more effective in reducing alcohol consumption in low-risk drinkers than screening alone. However, we did find that higher baseline consumption, lower age of first drink, older age, being female, greater positive alcohol expectancy and greater alcohol-related problems at baseline predicted higher levels of drinking at 12 months follow-up, which is similar to previous research findings.
The economic analysis undertaken for the low-risk cohort showed that, from both the societal and the NHS/PSS perspectives, the eBI generated more costs and slightly fewer QALYs and was thus dominated by the control, whereas the PFBA intervention generated higher costs and slightly more QALYs than the control, resulting in unacceptably large ICERs.
Probabilistic sensitivity analysis showed that the eBI was cost-effective in between 8% and 9% of simulations at the £20,000 and £30,000 WTP thresholds, respectively, for both the social and the NHS/PSS perspectives, and between 26% and 33% of simulations at the £20,000 and £30,000 WTP thresholds, respectively, for the PFBA intervention.
As there was very little difference observed in utility between the interventions and the control, the differences observed in the cost-effectiveness analyses were driven by costs. There was also very little difference observed in resource use between the intervention groups and the control group, therefore the difference observed in costs was due to the cost of the intervention. The health economic analysis supported the null hypothesis that neither PFBA nor eBI is more cost-effective than screening alone in low-risk drinkers.
In the high-risk trial, no significant differences in outcome were found between the intervention groups on either primary or secondary outcome measures. The additional post hoc calculation of the Bayes’ factor supported the null hypothesis that PFBA and eBI are not more effective in reducing alcohol consumption in high-risk drinkers than screening alone. The health economic analysis also supported the null hypothesis that PFBA and eBI are not more cost-effective than screening alone in high-risk drinkers. Predictors of increased alcohol consumption at 12 months were similar to those in the low-risk study: higher baseline consumption, being female, older age, younger age at first drink, greater alcohol-related positive expectancy and greater alcohol-related problems.
The economic analysis undertaken for the high-risk cohort showed that, from both the societal and NHS/PSS perspectives, the eBI generated more costs and slightly less QALYs and was thus dominated by the control; whereas the PFBA intervention generated higher costs and QALYs of negligible difference than the control, resulting in ICERs of £7580 and £6213 per QALY, respectively.
Probabilistic sensitivity analysis showed that the eBI was cost-effective in between 26% and 30% of simulations at the £20,000 and £30,000 WTP thresholds, respectively, for both the social and the NHS/PSS perspectives, and between 54% and 56% of simulations at the £20,000 and £30,000 WTP thresholds, respectively, for the PFBA intervention.
Although the analyses showed that the PFBA intervention has a chance of being cost-effective when compared with control, the distribution of the bootstrapped ICERs showed that there is a wide distribution due to a large level of variability and uncertainty in the model parameters.
As there was very little difference observed in utility between the interventions and the control, the differences observed in the cost-effectiveness analyses were, again, driven by costs. In general, very little difference was observed in the resource use between the intervention groups and the control group, though social care resulted in some large observed costs.
In both trials, we found that engagement with the eBI was low in participants randomised to eBI. One-third of participants engaged with the eBI platform after leaving EDs. This may have limited the impact of the eBI compared with the control intervention. However, as these were pragmatic trials, this is likely to be the level of engagement expected in the typical patient recruited from an ED.
Low app usage or engagement is a common issue. The vast majority of apps, and other online interventions, are not used 1 month after they are downloaded. 146 We also know that patients are less likely to engage in extended interventions when the onus to engage is on them. 131,147,148
A large proportion of the literature based on eBI has focused on the provision of websites as opposed to smartphone apps. 74 Arguably, the most important problem with developing an effective eBI app is engaging participants enough for them to find it useful.
Engagement can be defined as how a user interacts with and experiences the technology in question. 149 The method of measuring engagement varies depending on what subjects are being engaged with. For example, engagement and usage patterns of smartphone apps can be measured using the pattern of downloads, number of page visits and average session lengths. Usage patterns can be used to determine the most and least useful features of an app and identify user’s preferences to improve engagement and outcomes. Bewick et al. 150 demonstrated the value of participant engagement with a web based electronic intervention in achieving a reduction in the consumption of alcohol. Further evidence has emerged on user preferences for content, features and style, and strategies to improve engagement. 149,151,152 However, recent findings from two personalised alcohol interventions apps [Drinks Metre (version 2.5.0, Global Drug Survey Ltd, London, UK) and OneTooMany] for young adults have found no impact on their risky drinking. 153
Exploring adolescent perspectives on participation in alcohol intervention research based in emergency care (the SIPS Junior trials): a qualitative study
The aim of this part of the work programme was to explore the acceptability of trial tools and processes to young people presenting to EDs. Although assessments of acceptability have received increasing focus in recent years,154 there is no clear consensus as to how acceptability should be defined and measured. 154 In this work the authors consider that, to be acceptable, research and intervention processes should be not only appropriate, comprehensible, effective and well received by participants but also ethical. The latter is particularly key in work with young adolescents, as there is debate about the extent to which they can and should participate in treatment and also in research about treatment and care. 155–157 In addition, the issue of whether or not adolescents should rely on parental consent to participate in research (or indeed treatment per se) is increasingly contested, with a growing view that adolescents should have the opportunity to contribute to these decisions themselves, albeit with due consideration of their developmental capacity to understand the implication that participation might have for them. 157,158
Although there is a distinction between clinical and research ethics,159 recent work that has argued that the distinction between what is research and what is care can be overemphasised,160 especially when it comes to pragmatic effectiveness research,161,162 which is embedded in clinical practice and ultimately aims to achieve practical, timely care improvement. 161 As this study was based in routine practice, the contemporary view that clinical and research ethics are relatively indistinct was taken and the widely accepted four principles of biomedical ethics outlined by Beauchamp and Childress163 were deemed to provide a suitable and crucially simple framework to guide our analysis and data interpretation.
As such, this section draws on the four key principles of research ethics – autonomy, beneficence, non-maleficence and justice163 – to explore adolescents’ perspectives about their participation in two linked alcohol intervention trials described above. We considered these principles in relation to two key aspects of the research process:
-
consent and enrolment procedures, which governed how young people were approached, how they experienced explanation of the study and how consent was understood and given for individuals to become research participants
-
SBI activities, which related to the actual study procedures that were delivered to and experienced by young participants and which varied according to the trial arm to which they had been randomly allocated.
Four principles of research ethics outlined by Beauchamp and Childress:163
-
autonomy – the right of an individual to make their own choice
-
beneficence – the principle of acting with the best interest of the other in mind
-
non-maleficence – do no harm, as stated in the Hippocratic Oath
-
justice – a concept that emphasises fairness and equality among individuals.
A purposive sample of participants was selected from the pool of participants in the two linked trials, across the three intervention arms, for interviews about their experience of being involved in the research and the acceptability of receiving the interventions.
We used semistructured interviews to structure the conversations, covering issues relating to consent by young people aged 14–17 years; alcohol screening; the baseline questionnaire and the burden on emergency care; and young people’s experiences of intervention delivery.
Individual interviews were preferred to focus groups for practical reasons, as it would have been too difficult to organise a focus group meeting with participants from 10 different geographical areas.
An initial framework for coding based on participants’ experience and understanding of the different stages of the research process (approach, screening, intervention and follow-up) was developed and employed to analyse interview transcripts. The four guiding principles of biomedical ethics were then employed to structure coding, data analysis and interpretation and to provide a framework for discussing the findings.
A paper incorporating an extended analysis has been submitted for publication164 and is reproduced in full in Appendix 2.
Our analysis shows that the majority of participants understood the context of the study, including the fact that participation was voluntary, and they seemed to grasp most aspects of the complex SBI procedures. Participant reports focused on the ease of process as well as the benefits of employing technology. Furthermore, the study interventions were generally seen as acceptable, relevant and helpful to participants, who welcomed having something to do while waiting for treatment in the ED.
Although findings do not differ greatly from those we would expect in adult populations, interviews were often fairly brief, with more curtailed responses than one might expect when working with adult participants. Harden et al. 165 query the assumption that a young person who talks less during an interview has provided fewer data, suggesting instead that this young person has provided an account in their own words. In this case, the shorter responses are interpreted as offering an authentic account of adolescents’ participation in the research and also a demonstration of the lack of demand characteristics influencing interview responses. Indeed, comparison across interviews reveal that older participants, who were approaching adulthood, tended to provide longer, more in-depth responses than younger participants. For example, when asked how clearly the researcher had explained the study and if they knew that taking part was voluntary, a 17-year-old participant responded:
Yes, everything was made clear to me. All the ethical considerations were made clear to me, that I could pull out at any point.
Male,17, south, high-risk, control
By contrast, a 14-year-old simply stated, ‘Yeah’ (female, 14, north, high-risk, control), and repeated this response when probes such as ‘did you know that you didn’t have to take part if you didn’t want to?’ were used to explore understanding in more depth.
The findings have implications for future trials. Although the majority of participants showed good understanding of the study, there were some instances of a lack of recall about the study and understanding of the randomisation procedure was particularly limited. Thus, it is imperative that information be provided in a clear succinct manner, that opportunities for participants to seek clarification are provided and that researchers check participants’ understanding before proceeding. When complex research designs are employed, this may be just as important when working with adults as it is with young people.
All participants seemed to be in favour of young people participating in research, even on a potentially sensitive topic such as alcohol use. It is possible that participants who agreed to be interviewed were those who were more engaged with the research and may have held better informed or more positive views about our trial or research participation in general. Nevertheless, it was striking across our range of narrative accounts that the young people in our study displayed good understanding about research participation and the ability to assess the scope for benefit and harm, not only for themselves but also potentially for other people. This should offer reassurance for those seeking to conduct research with adolescents.
There are also implications for future intervention research. Previous research in the field of brief interventions, and especially those related to alcohol use, has tended to focus on adult populations. 166 The need for more research in this area is illustrated by the fact that over half (n = 15) of our participants were identified as drinking at risky levels and enrolled in the high-risk trial, and young people who had not started to consume alcohol accepted the intervention as useful for ‘when’ (not ‘if’) they eventually started drinking alcohol. That alcohol consumption was already framed as inevitable at the time of screening in our trial makes it seem crucial that young people have access to high-quality information and advice that can enable them to take steps to reduce potential health risk in the future.
We found no indication that brief intervention methods cannot or should not be employed with young people. The participants interviewed were able to understand what was being proposed and were able to provide informed consent, and welcomed being treated with respect for their autonomy. There was no evidence to support fears that talking about alcohol with young people will necessarily lead to early initiation of alcohol consumption or have a negative effect on existing drinking behaviours. On the contrary, a number of participants identified that education in this area is important because alcohol is a part of adolescents’ lives. Most of our participants already appeared to possess a clear sense that alcohol use could pose a health risk to them. Davison et al. 167 coined the term ‘lay epidemiology’ to describe the way in which beliefs and values about health and causes of disease are gained from one’s own experiences, as well as what is witnessed through popular media, family and social networks. 168 Although such knowledge may have some utility, it is important that young people are provided with accurate information from reputable sources to combine with the knowledge gained from other areas, to allow them to make informed decisions about their own health behaviour.
However, parental presence during screening and intervention may limit disclosure or lead to socially desirable responding. The involvement of parents or carers in the process was accepted by many, especially if the young person was not currently drinking or if their alcohol use was already known. Yet some young people were less comfortable about parental involvement or gate-keeping within the study and felt this might inhibit both their own and others’ ability to speak or participate freely. Although parental involvement could create a home environment with increased parental supervision and a clear awareness of alcohol risk, which has been shown to influence adolescent alcohol consumption169,170 where the presence of parents restricts disclosure, it may also restrict intervention delivery for those who could benefit most. The fact that young people were able to provide consent under Gillick competence means that intervention procedures need not always involve parents, which should encourage more confidence when working with young people.
Even in the absence of parents, there was indication that some participants may have felt the need to provide socially desirable responses or to hide the reason they were attending the ED. Therefore, researchers need to find ways of explaining (or reassuring adolescents) that being open in the research process will not lead to them being judged and, wherever possible, provide a private environment in which confidentiality can be assured.
Young people aged 14–17 years seemed to be enthusiastic about receiving information about alcohol use that they felt to be relevant to their current and future lifestyles. The participants in this study also reported that the ED was a suitable context both for the delivery of interventions and for the conduction of research. Young people welcomed the invitation to participate in research, especially when it specifically related to their age group. They demonstrated the ability to understand the implications of participation, making informed decisions by weighing the costs and benefits both to themselves and to wider society. Further research exploring the effectiveness of brief interventions for alcohol use in adolescent populations is recommended.
Overall conclusions
This research programme was designed to address key gaps in the evidence base for the most effective and cost-effective screening, and brief interventions for at-risk adolescent heavy drinkers in EDs. As a consequence of extensive engagement with young people and parents, we changed the direction of the research considerably from that originally planned. In particular, this patient and public involvement identified the greater acceptability and potential of electronic data collection and intervention delivery with adolescents, which had not been considered at the application stage. Although eSBIs have been previously studied, they have received limited attention to date in younger adolescents. A systematic review and meta-analysis conducted as part of this programme revealed promising findings about the efficacy of eSBIs.
Consequently, in collaboration with application developers, we developed two electronic applications for use in this programme: (1) a bespoke data collection and trial management tool and (2) an eBI, SIPS City app. The data collection and trial management tool much reduced the cost of conducting several aspects of the research, which in turn allowed us to carry out two linked RCTs that were larger than planned in the original budget.
Our first work package carried out a large-scale prevalence study of 5376 10- to 17-year-olds attending 10 EDs across England. Again, the patient and public involvement work facilitated successful implementation. It influenced our informed consent procedures for adolescents aged < 16 years and was helpful in obtaining NHS research ethics approval. We followed up this issue in work package 3, a qualitative study with a sample of participants in the RCTs, to explore participants’ experience of taking part in research and investigate whether or not we achieved the ethics requirements for clinical research. We found that young people welcomed invitations to participate in research, especially when it related to their own age group. They clearly understood the implications of participation, making informed decisions by weighing the costs and benefits both to themselves and to wider society. This will be helpful in informing future research in this age group.
In work package 1, we established the prevalence of hazardous drinking and AUDs in young people attending EDs, using validated research tools delivered mostly by an electronic application. We were further able to validate short alcohol screening tools, AUDIT and AUDIT-C, against standard research interview methods (Timeline Followback), and establish age-appropriate cut-off points. These tools will have wide application in the NHS, as the validity of these short screening approaches in this population was previously unknown. Indeed, Public Health England has already issued clinical guidance to EDs on alcohol screening in adolescents based on this research. 171
Beyond validation of short screening tools against research standard methods, we were also able to explore the clinical significance of hazardous and harmful drinking levels in this population. We found that the clinical cut off for hazardous drinking derived from this research was strongly associated with a wide range of adverse health and social consequences. This research also provided support for the Chief Medical Officer’s guidance on alcohol consumption before the age of 15 years, showing that any consumption before this age was strongly associated with increased risk of a wide range of health and social adverse consequences. 7
In work package 2, we again made extensive use of our partnerships with national and local organisations to develop age-appropriate and acceptable interventions for this population. This radically changed the approaches proposed in the original application through feedback from young people and parents. In particular, we were able to develop an age-appropriate electronic alcohol brief intervention application in collaboration with young people and specialist application developers. We produced an age-appropriate brief intervention that could be disseminated for use in the NHS. We also developed a bespoke SIPS Trial Management app (version 3.14, King’s College London, London, UK) for use on iPads and other electronic platforms; this was acceptable to patients and much reduced the cost of conducting the clinical trials. It also allowed us to conduct two linked randomised trials in work package 3, which were considerably larger than planned in the original application. The application also allowed us to manage trial recruitment and data collection much more efficiently in real time by uploading trial data to a central server.
In work package 3, we had the benefit of our previous prevalence study in refining our recruitment methods to include 14- to 17-year-olds. The trial management application we developed for work package 3, built on our screening application from work package 1. Questionnaire content was adapted for the trials, and we developed additional consent and randomisation features and the capability to record a random sample of interventions to examine the fidelity of delivering the interventions. We extensively piloted this with patient and public involvement representatives before the trial went live, thus achieving effective implementation of the trial protocol across the 10 EDs. In total, we recruited 1640 participants to the two trials: 756 high-risk drinkers and 884 low-risk drinkers or abstainers. Together with higher follow-up than predicted at 6 and 12 months, this provided two adequately powered RCTs, both of which compared two forms of brief intervention with screening alone. To our knowledge, these were the first large effectiveness trials in this population, and the study design addressed many of the shortcomings of previous trials. However, face-to-face PFBA and eBI were no more clinically effective or cost-effective than screening alone. We are conducting further analysis of both the application usage data and qualitative interviews with participants to gain greater understanding of how the interventions were perceived and used by participants. This will inform further development of the interventions for study in future research.
Hence, the research programme has advanced our understanding of the nature and prevalence of AUDs in adolescents and provided a firm foundation for future research to improve care for this population. Based on this work, we have conducted a new programme of research on eSBI and young adults and developed a new alcohol screening and intervention app (BRANCH). This was conducted as part of the National Institute for Health Research Collaborations for Leadership in Applied Health Research South London. This research was informed by our earlier work on eSBI in this programme and explored strategies to enhance engagement with digital interventions in young adults.
Recommendations for future research
-
We found that the risks of drinking are not restricted to those with an early onset. Future studies should explore how the risks associated with drinking alcohol vary by age at onset in more detail.
-
A limitation of the eBI was that only one-third of participants engaged with the application after leaving the ED; this is likely to have limited the effect of the eBI. We recommend that future research focus on methods to maximise engagement with digital interventions and evaluate the effect of such engagement on clinical outcomes.
-
Our study identified only a small proportion of young people who attended EDs in an intoxicated state, about 1%, and in workstream 1 a proportion of those surveyed exhibited symptoms associated with alcohol dependence. Although this proportion is smaller than similarly intoxicated adults presenting to ED, they have substantial alcohol-related health, psychological and social problems and consume more health and social care resources than young people in general. Research is needed to identify clear treatment pathways and liaison with external agencies to address the needs of this population.
-
There is a paucity of research addressing the longitudinal epidemiology of alcohol using young people, and research that employs large-scale longitudinal data sets has the potential to provide additional information on the relationship between drinking in adolescence and future health, social and economic costs.
-
Our qualitative research showed that young people welcome invitations to participate in research. This should encourage greater clinical research in this population rather than speculatively extrapolating research findings from adult populations to adolescents.
-
A greater consensus in the reporting of outcome measures and more uniform reporting of the content and theoretical basis of eSBIs would generate more robust conclusions on the effectiveness of eSBIs in reducing alcohol consumption and alcohol-related harms in the longer term.
Implication for practice
A simple three-item self-completed screening instrument, the AUDIT-C, is generally more effective than the full 10-item AUDIT in identifying adolescents who engage in at-risk alcohol consumption or heavy episodic alcohol use and fulfil the ICD-10 criteria for harmful alcohol use. Furthermore, the 10-item AUDIT with a cut-off score of 7 is more efficient than the AUDIT-C in identifying adolescents with alcohol dependence. In addition, the AUDIT-C and AUDIT are widely employed as screening tools for adults in clinical and non-clinical settings and these can be applied to adolescent populations with lower cut-off scores. We conclude that the AUDIT-C could be used with this population with a cut-off score of 3 as a positive screen for at-risk drinking, monthly heavy episodic alcohol use and harmful alcohol use. For those who score ≥ 5 on the AUDIT-C, we recommend the use of the additional seven questions constituting the full AUDIT. Those scoring ≥ 7 should be clinically assessed for alcohol dependence.
We also found that face-to-face PFBA and eBIs were no more clinically effective or cost-effective than screening alone in reducing alcohol consumption in the high-risk group and preventing it in the low-risk group. Hence, the roll-out of these interventions is not supported by evidence.
Dissemination
A range of dissemination activities occurred throughout the programme to facilitate the progress of the programme. These included presentations to national and international conferences and the setting up of a dedicated website at URL: www.sipsjunior.net (accessed 17 May 2019).
The main public/policy and academic dissemination activities are listed in Acknowledgements, Publications.
Acknowledgements
The authors thank the NHS clinical and nursing staff at our study sites, study participants and their families for their help and support in the prosecution of this research programme, as well as those who contributed to the production of this report.
Patient and public involvement organisations
Parenting UK, Family and Parenting Institute, British Youth Council and The Well Centre/Redthread.
Participating emergency departments
Prevalence study in work package 1
St Thomas’ Hospital, London; King’s College Hospital, London; University Hospital Lewisham, London; Ealing Hospital, London; Hull Royal Infirmary, Hull; Darlington Memorial Hospital, Darlington; Queen Elizabeth Hospital, Gateshead; University Hospital of North Tees, Hartlepool; South Tyneside District Hospital, South Shields; and James Cook University Hospital, Middlesbrough.
Randomised controlled trials in work package 3
St Thomas’ Hospital, London; King’s College Hospital, London; Ealing Hospital, London; Croydon University Hospital, Croydon; Hull Royal Infirmary, Hull; Darlington Memorial Hospital, Darlington; Queen Elizabeth Hospital, Gateshead; University Hospital of North Tees, Hartlepool; South Tyneside District Hospital, South Shields; and Sunderland Royal Hospital, Sunderland.
List of researchers who participated in the data collection of work package 1
Sadie Boniface, Zoe Davey, Kideshini Widyaratna, Ashley Brewer, Natasha Muzengi, Victoria Brooks, Catherine Elzerbi, Jurate Dudley, Natalie Wood, Andri Kyriacou, Kwame Appiah-Dwomoh, Hannah Boardman, Charlene Colegate, Miriam Adesokan, Mohamed Pujeh, Ruth Brennen, Rachelle Tipler, Lewis Owusu-afriyie, Adrianne Appiah-dwomoh, Carlene Parchment, Natalie Appiah-Dwomoh, Lorraine Omari-Asor, Amy Wolstenholme, Abimbola Alabi, Helen Rand, Victoria Ratti, Melissa Ashe, Adeyinka Kumuyui, Angelica Kakouratos, Theodorah Nago, Miriam Cottle, Alissa Williams, Hazel Harrop, Abel Jalloh, Shari Chitando, Jade Jones, Fiona Watson, Caroline Mckenna, James Martin, Clare Gray, Andrea Mulligan, Alison Cairns, Lucy Adamson, Cherry Raymond, Rebecca Reed, Joanne Boyd, Dot Turton, Jane Weston, Carly Forbes, Christine Mayne, Lynne Hayward, Liz Fowles, Louisa Atkinson, Gillian Lathan, Leanne Collins, Monica Jullian, Michelle Allen, Anne Gourlay, Karen Lindsau, Jacqui Jones, Jill Mcmillan, Susan Beech, Sarah Dean, Rebecca Collinson, Stephanie Harrison, Arantxa Baglietto, Karen Bibbings, Ruth Mcgovern, Dee Lewis, Graeme Wilson, Amy O’Donnell, Julie Dickinson, Steph O’Neil, Kath Parkinson, Thomas Phillips, Rachel Simpson, Cara Gates, Emma Medford, Laura Hermann, Meg Beadle, Kelly Nelson, Paul Burnett, Sian Copeland, John Tomlinson, Marie Burdett, Paul Bryan, Julie Caldwell, Steve Lundie, Tim Turner, Sarah Hurst, Angie Newman, Paul Williams, James Tovell, Isaac Narh, Vicky Taylor, Heather Marley, Sharon Stevens, Wendy Cheadle, Tarn Nozedar, Amanda Cowton, Andrea Whitehouse, Elaine Garrett, Jill Deane, Sheila Blenkin, Lorna Morgan, Glynis Rose, Gil Horner, Julie Colarossi, Claire Martin, Irene Sambath, Sue Leach, Philippa Laverick, Linda Mcnamee, Linder Tinkler, Carly Brown, Paul Coyne, Jackie Stove, Shelia Blenkin, Natasha Newell, Lisa Wayman, Sarah Welsh, Karen Woode, Lisa Godwin, Tracey Gore, Amy Bennison, Marie Presgrave, Dorothy Newbury-Birch, June Battram, Lindsey Hines, Georgina Forden, Delah Akomah, Simon Flynn, Megan Lawrence, Carole Milburn, Gemma Musson and Ursula Balderson.
List of researchers who have participated in the data collection and follow-up stages of work package 3
Ruth McGovern, Jen Bradley, Matt Breckons, Emma Simpson, Hayley Alderson, Kirsten Hall, Jen Birch, Paul Corrigan, Jamie Rea, Emily Clare, Carly Brown, Andrea Mulligan, Lisa McDougall, Kenn Walker, Jan Milner, Steph Ogilvie, Steven Elliott, Gillian Lathan, Ashley Lowe, Nicola Connor, Lisa Dingwall, Samantha Nesbitt, Joanne Firman, Alice Hunt, Amanpreet Banga, Amy Wolstenholme, Delah Akomah, Ellen McDonald, Hannah Rose, Jon Jezak, Jordan Quinn, Kim Mihaljevic, Lauren Schumacher, Melissa Ashe, Rebecca McDonald, Sadi Boniface, Saira Shamim, Sarah Feehan, Tajinder Rai, Vera Forjaz, Vicky Brooks, Michelle Lee, Abel Jalloh, Paul Burnett, Carol Taylor, Helen Garvey, Lydia Bromley, Kate Cheung, Paul Williams, Hilary Thornton, Thomas Phillips, Gayle Clifford, Madeleine Duffy, Lyndsey Dixon, Sue Leach, Deborah Smart, Liam Spencer, Anne Marie Ianzito, Louise Carr, Mohamed Pujeh, Sarah Chamberlin, Chidimma Onyejiaka, Tara Harvey, Antionette McNulty, Pat Daly, Val Dun-Toroosian, Lorraine O’Connell, Lesley Haley, Danielle Walker, Anthony Kennedy, Lesley Alderton, Sheila Blenkin, Jill Deane, Amanda Cowton, Tarn Nozedar, Emma Grey, Chloe Barclay, Mandy Porritt, Natasha Newell, Elaine Garett, Kirsty Banham, Julie Gray, Susan Crawford, Debbie Wilson, Wendy Cheadle, June Battram, Julie Colarossi, Claire Irish, Gabrielle Osborne, Heather Marley, Jason Pickering, Karolina Bogdanowicz, Catherine Elzerbi, Susan Kelsey, Hannah Kaner, Andrea Mulligan, Rebecca Reed, Stephanie Ogilvie, Erin Graybill, Sasha Taylor, Wendy Hall, Louise Tam, Naomi Bateman, Liz Jacques, Khatiba Raja, Tom Bramhall, Samantha Lovely and Simon Flynn.
Members of the Independent Steering Committee
Chairperson, Professor Paul Wallace; members, Professor Ian White, Dr Lynn Owens, Professor Jim McCambridge, Professor Mat Hickman and Professor Jonathan Chick.
Software developer
We also thank Richard McGregor and Danny Berzon at Codeface Ltd (Hove, UK) for developing the trial management app and the eBI app (SIPS City).
Contributions of authors
Paolo Deluca (Senior Research Fellow/Senior Lecturer) was a co-applicant and the programme manager. He contributed to study design and protocol writing. He was responsible for study management, oversight of study conduct, and the writing and final editing of the report. He co-led the development of the SIPS Jr City app, as well as the trial management app, and is the corresponding author.
Simon Coulton (Professor of Health Services Studies) was a co-applicant. He contributed to study design and writing the protocol. He contributed to study management as a member of the Programme Management Group (PMG) and contributed to the writing and final editing of the report. He led the methodological and statistical aspects, wrote the statistical analysis plan, provided statistical support and performed the statistical analysis of the study.
Mohammed Fasihul Alam (Research Fellow, Health Economist) contributed to study design, particularly the health economics, and writing the protocol. He contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Sadie Boniface (Research Fellow) was the local trial co-ordinator. She contributed to data collection and study management as a member of the PMG, and to the writing and final editing of the report.
Kim Donoghue (Research Fellow) contributed to study design and writing the protocol, contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Eilish Gilvarry (Consultant Psychiatrist in Addictions) was a co-applicant. She contributed to study design and writing the protocol, provided clinical advice, contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Eileen Kaner (Professor of Public Health and Primary Care Research) was a co-applicant. She contributed to study design and writing the protocol, contributed to study management as a member of the PMG, contributed to the writing and final editing of the report, and led on the qualitative components.
Ellen Lynch (Research Fellow) was the local trial co-ordinator. She contributed to data collection and study management as a member of the PMG, and to the writing and final editing of the report.
Ian Maconochie (Consultant in Paediatric Emergency Medicine) was a co-applicant. He contributed to study design and writing the protocol, provided clinical advice, contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Paul McArdle (Child and Adolescent Psychiatrist) was a co-applicant. He contributed to study design and writing the protocol, provided clinical advice, contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Ruth McGovern (Senior Research Associate) was the local trial co-ordinator. She contributed to data collection and study management as a member of the PMG, contributed to the writing and final editing of the report, and led on patient and public involvement and engagement activities in Newcastle.
Dorothy Newbury-Birch (Professor of Alcohol and Public Research) was a co-applicant. She contributed to study design and writing the protocol, contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Robert Patton (Research Fellow/Lecturer in Clinical Psychology) was a co-applicant and local trial co-ordinator. He contributed to study design and writing the protocol, contributed to study management as a member of the PMG, and to the writing and final editing of the report. He led on patient and public involvement and engagement activities in London and co-led the development of the SIPS Jr City app, as well as the Trial Management app.
Tracy Pellatt-Higgins (Senior Research Fellow and Statistician) was responsible for the statistical analysis and reporting of the data. This included presenting summaries of the data and primary and secondary analysis results and the interpretation of results. She contributed to the writing and final editing of the report.
Ceri Phillips (Professor of Health Economics) contributed to study design and writing the protocol, contributed to study management as a member of the PMG, and to the writing and final editing of the report. She led the health economics components of the programme and provided health economic supervision and advice.
Thomas Phillips (National Institute for Health Research Research Fellow and Deputy Director for Nursing and Quality) was a co-applicant. He contributed to study design and writing the protocol, to study management as a member of the PMG, and to the writing and final editing of the report. He co-led the development of the SIPS Jr City app, as well as the Trial Management app, and provided clinical advice.
Rhys Pockett (Epidemiologist) contributed to the health economic components and the health economic analysis plan, and conducted the health economic analysis. He contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Ian T Russell (Professor of Clinical Trials) was a co-applicant. He contributed to study design and writing the protocol, to study management as a member of the PMG, and to the writing and final editing of the report.
John Strang (Professor of Psychiatry of Addictions) was a co-applicant. He contributed to study design and writing the protocol, contributed to study management as a member of the PMG, and to the writing and final editing of the report.
Colin Drummond (Professor of Addiction Psychiatry) was the chief investigator for this programme. He contributed to study design and protocol writing, study management, oversight of study conduct, and to the initial writing and final editing of the report.
All authors also provided a critical review and final approval of the report. They all agreed to be accountable for all aspects of the work.
Publications
Donoghue K, Patton R, Phillips T, Deluca P, Drummond C. The effectiveness of electronic screening and brief intervention for reducing levels of alcohol consumption: a systematic review and meta-analysis. J Med Internet Res 2014;16:e142.
Patton R, Deluca P, Kaner E, Newbury-Birch D, Phillips T, Drummond C. Alcohol screening and brief intervention for adolescents: the how, what and where of reducing alcohol consumption and related harm among young people. Alcohol Alcohol 2014;49:207–12.
Deluca P, Coulton S, Alam MF, Cohen D, Donoghue K, Gilvarry E, et al. Linked randomised controlled trials of face-to-face and electronic brief intervention methods to prevent alcohol related harm in young people aged 14-17 years presenting to Emergency Departments (SIPS junior). BMC Public Health 2015;15:345.
Elzerbi C, Donoghue K, Drummond C. A comparison of outcomes for brief interventions to reduce hazardous and harmful alcohol consumption between European and non-European countries: a systematic review and meta-analysis of randomised controlled trials. Addiction 2015;110:1082–91.
Rose H, Coulton S, Lynskey M, Drummond C. Adolescent alcohol beverage preferences and related harms: a latent class analysis. Alcohol Clin Exp Res 2015;39:139A.
Deluca P, Drummond C, Coulton S, Boniface S, Donoghue K, Gilvarry E, et al. Optimal screening tool for underage drinking: findings from the SIPS Jr programme. Alcohol Clin Exp Res 2016;40:237A.
Milward J, Deluca P, Khadjesari Z, Watson R, Fincham-Campbell S, Drummond C. Making electronic interventions engaging: Development of a smartphone app targeting harmful drinking in young adults. Addict Sci Clin Pract 2016;11:A27.
Milward J, Khadjesari Z, Fincham-Campbell S, Deluca P, Watson R, Drummond C. User preferences for content, features, and style for an app to reduce harmful drinking in young adults: analysis of user feedback in app stores and focus group interviews. JMIR Mhealth Uhealth 2016;4:e47.
Deluca P, Coulton S, Alam F, Cohen D, Donoghue K, Gilvarry E, et al. Effectiveness and Cost effectiveness of a Smartphone Based Electronic Alcohol Intervention for Adolescents. Findings From the SIPS JR Trials. International Network on Brief Interventions for Alcohol and Other Drugs, 13–15 September 2017, New York, NY, USA, conference abstract.
Donoghue K, Rose H, Boniface S, Deluca P, Coulton S, Alam M, et al. Risk of alcohol and other health-related consequences with increased consumption and early onset of drinking among adolescents presenting at emergency departments. J Adolesc Health 2017;60:438–46.
Elzerbi C, Donoghue K, Boniface S, Drummond C. Variance in the efficacy of brief interventions in emergency departments to reduce hazardous and harmful alcohol consumption between injury and non-injury patients: a systematic review and meta-analysis of randomised controlled trials. Ann Emerg Med 2017;70:714–23.
Coulton S, Alam MF, Boniface S, Deluca P, Donoghue K, Gilvarry E, et al. Opportunistic screening for alcohol use problems in adolescents attending emergency departments: an evaluation of screening tools. J Public Health 2019;41:e53–60.
Lynch E, McGovern R, Elzerbi C, Breckons M, Deluca P, Drummond C, et al. Adolescent perspectives about their participation in alcohol intervention research in emergency care: a qualitative exploration using ethical principles as an analytical framework. PLOS ONE 2019;14:e0217855.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Exclusive use will be retained until the publication of major outputs.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Ofori-Adjei D, Casswell S, Drummond DC, Wei H, Medina-Mora ME, Ranganathan S, et al. World Health Organization Expert Committee on Problems Related to Alcohol Consumption. Second Report 2007.
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223-33. https://doi.org/10.1016/S0140-6736(09)60746-7.
- Bellis MA, Phillips-Howard PA, Hughes K, Hughes S, Cook PA, Morleo M, et al. Teenage drinking, alcohol availability and pricing: a cross-sectional study of risk and protective factors for alcohol-related harms in school children. BMC Public Health 2009;9. https://doi.org/10.1186/1471-2458-9-380.
- Fuller E. Drug Use, Smoking and Drinking Among Young People in England in 2007. London: National Centre for Social Research, National Foundation for Educational Research; 2008.
- Fuller E. Smoking, Drinking and Drug Use Among Young People in England in 2011. NHS Digital: Leeds; 2012.
- Hibbell B, Guttormsson U, Ahlstrom S, Balakireva O, Bjarnason T, Kokkevi A, et al. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries 2009.
- Donaldson L. Guidance on the Consumption of Alcohol by Children and Young People. London: Department of Health and Social Care; 2009.
- Newbury-Birch D, Gilvarry E, McArdle P, Stewart S, Walker J, Lock C, et al. The Impact of Alcohol Consumption on Young People: A Review of Reviews. London: Department of Children Schools and Families; 2009.
- NHS Digital . Statistics on Alcohol: England, 2008 2008. www.digital.nhs.uk/data-and-information/publications/statistical/statistics-on-alcohol/2008 (accessed 20 May 2019).
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res 2008;32:2149-60. https://doi.org/10.1111/j.1530-0277.2008.00806.x.
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med 2006;160:739-46. https://doi.org/10.1001/archpedi.160.7.739.
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, et al. American Medical Association . The neurocognitive effects of alcohol on adolescents and college students. Prev Med 2005;40:23-32. https://doi.org/10.1016/j.ypmed.2004.04.044.
- McCloud A, Barnaby B, Omu N, Drummond C, Aboud A. Relationship between alcohol use disorders and suicidality in a psychiatric population: in-patient prevalence study. Br J Psychiatry 2004;184:439-45. https://doi.org/10.1192/bjp.184.5.439.
- Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, et al. Alcohol as a risk factor for global burden of disease. Eur Addict Res 2003;9:157-64. https://doi.org/10.1159/000072222.
- Thunström M. The alcohol intoxicated child and its prognosis. Acta Paediatr Scand 1988;77:3-9. https://doi.org/10.1111/j.1651-2227.1988.tb10589.x.
- Rogers PD, Harris J, Jarmuskewicz J. Alcohol and adolescence. Pediatr Clin North Am 1987;34:289-303. https://doi.org/10.1016/S0031-3955(16)36215-0.
- Monti PM, Barnett NP, Colby SM, Gwaltney CJ, Spirito A, Rohsenow DJ, et al. Motivational interviewing versus feedback only in emergency care for young adult problem drinking. Addiction 2007;102:1234-43. https://doi.org/10.1111/j.1360-0443.2007.01878.x.
- Monti PM, Colby SM, Barnett NP, Spirito A, Rohsenow DJ, Myers M, et al. Brief intervention for harm reduction with alcohol-positive older adolescents in a hospital emergency department. J Consult Clin Psychol 1999;67:989-94. https://doi.org/10.1037/0022-006X.67.6.989.
- Maio RF, Shope JT, Blow FC, Gregor MA, Zakrajsek JS, Weber JE, et al. A randomized controlled trial of an emergency department-based interactive computer program to prevent alcohol misuse among injured adolescents. Ann Emerg Med 2005;45:420-9. https://doi.org/10.1016/j.annemergmed.2004.10.013.
- Crawford MJ, Patton R, Touquet R, Drummond C, Byford S, Barrett B, et al. Screening and referral for brief intervention of alcohol-misusing patients in an emergency department: a pragmatic randomised controlled trial. Lancet 2004;364:1334-9. https://doi.org/10.1016/S0140-6736(04)17190-0.
- Spirito A, Monti PM, Barnett NP, Colby SM, Sindelar H, Rohsenow DJ, et al. A randomized clinical trial of a brief motivational intervention for alcohol-positive adolescents treated in an emergency department. J Pediatr 2004;145:396-402. https://doi.org/10.1016/j.jpeds.2004.04.057.
- Barrett B, Byford S, Crawford M, Patton R, Drummond C, Henry J, et al. Cost-effectiveness of screening and referral to an alcohol health worker in alcohol misusing patients attending an accident and emergency department: a decision-making approach. Drug Alcohol Depen 2006;81:47-54. https://doi.org/10.1016/j.drugalcdep.2005.05.015.
- Stevens NG, Lyle S. Guidelines for adolescent preventive services: a critical review. The American Medical Association Department of Adolescent Health. J Am Board Fam Pract 1994;7:421-30.
- Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: a review. J Stud Alcohol Drugs 2014;75:158-69. https://doi.org/10.15288/jsad.2014.75.158.
- World Health Organization . Orientation Programme on Adolescent Health for Healthcare Providers 2006.
- American Academy of Pediatrics Committee on Substance Abuse . Alcohol Use and Abuse 2001.
- Department of Health and Social Care . Safe, Sensible, Social – Consultation on Further Action 2008.
- Scottish Government . Changing Scotland’s Relationship With Alcohol: A Framework for Action 2013.
- NHS Scotland . Understanding Alcohol Misuse in Scotland. Harmful Drinking. Five: Alcohol and Young People 2008.
- Jackson R, Johnson M, Campbell F, Messina J, Guillaume L, Purshouse R, et al. Screening and Brief Interventions: Effectiveness Review to the National Institute for Health & Clinical Excellence. Sheffield: School of Health and Related Research, University of Sheffield; 2009.
- Clark DB, Moss HB. Providing alcohol-related screening and brief interventions to adolescents through health care systems: obstacles and solutions. PLOS Med 2010;7. https://doi.org/10.1371/journal.pmed.1000214.
- Gregor MA, Shope JT, Blow FC, Maio RF, Weber JE, Nypaver MM. Feasibility of using an interactive laptop program in the emergency department to prevent alcohol misuse among adolescents. Ann Emerg Med 2003;42:276-84. https://doi.org/10.1067/mem.2003.265.
- Ford CA, Millstein SG, Halpern-Felsher BL, Irwin CE. Influence of physician confidentiality assurances on adolescents’ willingness to disclose information and seek future health care. A randomized controlled trial. JAMA 1997;278:1029-34. https://doi.org/10.1001/jama.1997.03550120089044.
- Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD004148.pub3.
- Poikolainen K. Effectiveness of brief interventions to reduce alcohol intake in primary health care populations: a meta-analysis. Prev Med 1999;28:503-9. https://doi.org/10.1006/pmed.1999.0467.
- Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med 2005;165:986-95. https://doi.org/10.1001/archinte.165.9.986.
- Ballesteros JA, Duffy JC, Querejeta I, Arino J, Gonzalez-Pinto A. Efficacy of brief interventions for hazardous drinkers in primary care: systematic review and meta-analysis. Alcohol Clin Exp Res 2004;28:608-18. https://doi.org/10.1097/01.ALC.0000122106.84718.67.
- Carter MA. Review: brief multicontact behavioural counselling interventions in primary care reduce risky or harmful alcohol use. Evid Based Nurs 2004;7. https://doi.org/10.1136/ebn.7.4.108.
- Boekeloo BO, Jerry J, Lee-Ougo WI, Worrell KD, Hamburger EK, Russek-Cohen E, et al. Randomized trial of brief office-based interventions to reduce adolescent alcohol use. Arch Pediatr Adolesc Med 2004;158:635-42. https://doi.org/10.1001/archpedi.158.7.635.
- D’Amico EJ, Miles JN, Stern SA, Meredith LS. Brief motivational interviewing for teens at risk of substance use consequences: a randomized pilot study in a primary care clinic. J Subst Abuse Treat 2008;35:53-61. https://doi.org/10.1016/j.jsat.2007.08.008.
- Peterson PL, Baer JS, Wells EA, Ginzler JA, Garrett SB. Short-term effects of a brief motivational intervention to reduce alcohol and drug risk among homeless adolescents. Psychol Addict Behav 2006;20:254-64. https://doi.org/10.1037/0893-164X.20.3.254.
- Bailey KA, Baker AL, Webster RA, Lewin TJ. Pilot randomized controlled trial of a brief alcohol intervention group for adolescents. Drug Alcohol Rev 2004;23:157-66. https://doi.org/10.1080/09595230410001704136.
- Schaus JF, Sole ML, McCoy TP, Mullett N, O’Brien MC. Alcohol screening and brief intervention in a college student health center: a randomized controlled trial. J Stud Alcohol Drugs Suppl 2009;16:131-41. https://doi.org/10.15288/jsads.2009.s16.131.
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLOS Med 2008;5. https://doi.org/10.1371/journal.pmed.0050141.
- Baird AA, Gruber SA, Fein DA, Maas LC, Steingard RJ, Renshaw PF, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38:195-9. https://doi.org/10.1097/00004583-199902000-00019.
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry 2000;157:745-50. https://doi.org/10.1176/appi.ajp.157.5.745.
- Hingson RW, Edwards EM, Heeren T, Rosenbloom D. Age of drinking onset and injuries, motor vehicle crashes, and physical fights after drinking and when not drinking. Alcohol Clin Exp Res 2009;33:783-90. https://doi.org/10.1111/j.1530-0277.2009.00896.x.
- Guttmannova K, Bailey JA, Hill KG, Lee JO, Hawkins JD, Woods ML, et al. Sensitive periods for adolescent alcohol use initiation: predicting the lifetime occurrence and chronicity of alcohol problems in adulthood. J Stud Alcohol Drugs 2011;72:221-31. https://doi.org/10.15288/jsad.2011.72.221.
- Odgers CL, Caspi A, Nagin DS, Piquero AR, Slutske WS, Milne BJ, et al. Is it important to prevent early exposure to drugs and alcohol among adolescents?. Psychol Sci 2008;19:1037-44. https://doi.org/10.1111/j.1467-9280.2008.02196.x.
- Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol 2002;70:1224-39. https://doi.org/10.1037/0022-006X.70.6.1224.
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J Subst Abuse 2001;13:493-504. https://doi.org/10.1016/S0899-3289(01)00096-7.
- Maimaris W, McCambridge J. Age of first drinking and adult alcohol problems: systematic review of prospective cohort studies. J Epidemiol Community Health 2014;68:268-74. https://doi.org/10.1136/jech-2013-203402.
- Coulton S, Drummond C, James D, Godfrey C, Bland JM, Parrott S, et al. Stepwice Research Team . Opportunistic screening for alcohol use disorders in primary care: comparative study. BMJ 2006;332:511-17. https://doi.org/10.1136/bmj.38743.421574.7C.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Aertgeerts B, Buntinx F, Bande-Knops J, Vandermeulen C, Roelants M, Ansoms S, et al. The value of CAGE, CUGE, and AUDIT in screening for alcohol abuse and dependence among college freshmen. Alcohol Clin Exp Res 2000;24:53-7. https://doi.org/10.1111/j.1530-0277.2000.tb04553.x.
- Chung T, Colby SM, Barnett NP, Rohsenow DJ, Spirito A, Monti PM. Screening adolescents for problem drinking: performance of brief screens against DSM-IV alcohol diagnoses. J Stud Alcohol 2000;61:579-87. https://doi.org/10.15288/jsa.2000.61.579.
- Demartini KS, Carey KB. Optimizing the use of the AUDIT for alcohol screening in college students. Psychol Assess 2012;24:954-63. https://doi.org/10.1037/a0028519.
- Kelly TM, Donovan JE, Kinnane JM, Taylor DM. A comparison of alcohol screening instruments among under-aged drinkers treated in emergency departments. Alcohol Alcohol 2002;37:444-50. https://doi.org/10.1093/alcalc/37.5.444.
- Knight JR, Sherritt L, Harris SK, Gates EC, Chang G. Validity of brief alcohol screening tests among adolescents: a comparison of the AUDIT, POSIT, CAGE, and CRAFFT. Alcohol Clin Exp Res 2003;27:67-73. https://doi.org/10.1097/01.ALC.0000046598.59317.3A.
- Santis R, Garmendia ML, Acuña G, Alvarado ME, Arteaga O. The Alcohol Use Disorders Identification Test (AUDIT) as a screening instrument for adolescents. Drug Alcohol Depend 2009;103:155-8. https://doi.org/10.1016/j.drugalcdep.2009.01.017.
- Coulton S, Alam MF, Boniface S, Deluca P, Donoghue K, Gilvarry E, et al. Opportunistic screening for alcohol use problems in adolescents attending emergency departments: an evaluation of screening tools. J Public Health 2019;41:e53-60. https://doi.org/10.1093/pubmed/fdy049.
- Donoghue K, Rose H, Boniface S, Deluca P, Coulton S, Alam M, et al. Risk of alcohol and other health-related consequences with increased consumption and early onset of drinking among adolescents presenting at emergency departments. J Adolesc Health 2017;60:438-46. https://doi.org/10.1016/j.jadohealth.2016.11.017.
- Gillick v West Norfolk and Wisbech Area Health Authority. All ER 1985;3. www.hrcr.org/safrica/childrens_rights/Gillick_WestNorfolk.htm (accessed 20 May 2019).
- Hunter D, Pierscionek BK. Children, Gillick competency and consent for involvement in research. J Med Ethics 2007;33:659-62. https://doi.org/10.1136/jme.2006.018853.
- Ravens-Sieberer U, Erhart M, Rajmil L, Herdman M, Auquier P, Bruil J, et al. Reliability, construct and criterion validity of the KIDSCREEN-10 score: a short measure for children and adolescents’ well-being and health-related quality of life. Qual Life Res 2010;19:1487-500. https://doi.org/10.1007/s11136-010-9706-5.
- Goodman R, Scott S. Comparing the Strengths and Difficulties Questionnaire and the child behavior checklist: is small beautiful?. J Abnorm Child Psychol 1999;27:17-24.
- Muris P, Meesters C, Eijkelenboom A, Vincken M. The self-report version of the Strengths and Difficulties Questionnaire: its psychometric properties in 8- to 13-year-old non-clinical children. Br J Clin Psychol 2004;43:437-48. https://doi.org/10.1348/0144665042388982.
- Hibell B, Guttormsson U, Ahstom S, . The 2011 ESPAD Report; Substance Use Among Students in 36 European Countries n.d. www.espad.org/sites/espad.org/files/The_2011_ESPAD_Report_FULL_2012_10_29.pdf (accessed 20 May 2019).
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry 2010;71:313-26. https://doi.org/10.4088/JCP.09m05305whi.
- Sobell LC, Sobell B, Litten Z, Allen P. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Human Press; 1992.
- Waldron HB, Slesnick N, Brody JL, Turner CW, Peterson TR. Treatment outcomes for adolescent substance abuse at 4- and 7-month assessments. J Consult Clin Psychol 2001;69:802-13. https://doi.org/10.1037/0022-006X.69.5.802.
- Brown SA, Tapert SF, Tate SR, Abrantes AM. The role of alcohol in adolescent relapse and outcome. J Psychoactive Drugs 2000;32:107-15. https://doi.org/10.1080/02791072.2000.10400216.
- Donohue B, Azrin NH, Strada MJ, Silver NC, Teichner G, Murphy H. Psychometric evaluation of self- and collateral timeline follow-back reports of drug and alcohol use in a sample of drug-abusing and conduct-disordered adolescents and their parents. Psychol Addict Behav 2004;18:184-9. https://doi.org/10.1037/0893-164X.18.2.184.
- Donoghue K, Patton R, Phillips T, Deluca P, Drummond C. The effectiveness of electronic screening and brief intervention for reducing levels of alcohol consumption: a systematic review and meta-analysis. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.3193.
- Bewick BM, Trusler K, Barkham M, Hill AJ, Cahill J, Mulhern B. The effectiveness of web-based interventions designed to decrease alcohol consumption – a systematic review. Prev Med 2008;47:17-26. https://doi.org/10.1016/j.ypmed.2008.01.005.
- Champion KE, Newton NC, Barrett EL, Teesson M. A systematic review of school-based alcohol and other drug prevention programs facilitated by computers or the internet. Drug Alcohol Rev 2013;32:115-23. https://doi.org/10.1111/j.1465-3362.2012.00517.x.
- Newton AS, Dong K, Mabood N, Ata N, Ali S, Gokiert R, et al. Brief emergency department interventions for youth who use alcohol and other drugs: a systematic review. Pediatr Emerg Care 2013;29:673-84. https://doi.org/10.1097/PEC.0b013e31828ed325.
- Cunningham JA, Wild TC, Cordingley J, van Mierlo T, Humphreys K. A randomized controlled trial of an internet-based intervention for alcohol abusers. Addiction 2009;104:2023-32. https://doi.org/10.1111/j.1360-0443.2009.02726.x.
- Cunningham JA, Wild TC, Cordingley J, Van Mierlo T, Humphreys K. Twelve-month follow-up results from a randomized controlled trial of a brief personalized feedback intervention for problem drinkers. Alcohol Alcohol 2010;45:258-62. https://doi.org/10.1093/alcalc/agq009.
- Araki I, Hashimoto H, Kono K, Matsuki H, Yano E. Controlled trial of worksite health education through face-to-face counseling vs. e-mail on drinking behavior modification. J Occup Health 2006;48:239-45. https://doi.org/10.1539/joh.48.239.
- Blankers M, Koeter MW, Schippers GM. Internet therapy versus internet self-help versus no treatment for problematic alcohol use: a randomized controlled trial. J Consult Clin Psychol 2011;79:330-41. https://doi.org/10.1037/a0023498.
- Boon B, Risselada A, Huiberts A, Riper H, Smit F. Curbing alcohol use in male adults through computer generated personalized advice: randomized controlled trial. J Med Internet Res 2011;13. https://doi.org/10.2196/jmir.1695.
- Butler LH, Correia CJ. Brief alcohol intervention with college student drinkers: face-to-face versus computerized feedback. Psychol Addict Behav 2009;23:163-7. https://doi.org/10.1037/a0014892.
- Cunningham JA, Hendershot CS, Murphy M, Neighbors C. Pragmatic randomized controlled trial of providing access to a brief personalized alcohol feedback intervention in university students. Addict Sci Clin Pract 2012;7. https://doi.org/10.1186/1940-0640-7-21.
- Ekman DS, Andersson A, Nilsen P, Ståhlbrandt H, Johansson AL, Bendtsen P. Electronic screening and brief intervention for risky drinking in Swedish university students – a randomized controlled trial. Addict Behav 2011;36:654-9. https://doi.org/10.1016/j.addbeh.2011.01.015.
- Hansen AB, Becker U, Nielsen AS, Grønbæk M, Tolstrup JS, Thygesen LC. Internet-based brief personalized feedback intervention in a non-treatment-seeking population of adult heavy drinkers: a randomized controlled trial. J Med Internet Res 2012;14. https://doi.org/10.2196/jmir.1883.
- Hester RK, Delaney HD, Campbell W. The college drinker’s check-up: outcomes of two randomized clinical trials of a computer-delivered intervention. Psychol Addict Behav 2012;26:1-12. https://doi.org/10.1037/a0024753.
- Hester RK, Squires DD, Delaney HD. The Drinker’s Check-up: 12-month outcomes of a controlled clinical trial of a stand-alone software program for problem drinkers. J Subst Abuse Treat 2005;28:159-69. https://doi.org/10.1016/j.jsat.2004.12.002.
- Kypri K, Hallett J, Howat P, McManus A, Maycock B, Bowe S, et al. Randomized controlled trial of proactive web-based alcohol screening and brief intervention for university students. Arch Intern Med 2009;169:1508-14. https://doi.org/10.1001/archinternmed.2009.249.
- Kypri K, Langley JD, Saunders JB, Cashell-Smith ML, Herbison P. Randomized controlled trial of web-based alcohol screening and brief intervention in primary care. Arch Intern Med 2008;168:530-6. https://doi.org/10.1001/archinternmed.2007.109.
- Kypri K, McCambridge J, Vater T, Bowe SJ, Saunders JB, Cunningham JA, et al. Web-based alcohol intervention for Māori university students: double-blind, multi-site randomized controlled trial. Addiction 2013;108:331-8. https://doi.org/10.1111/j.1360-0443.2012.04067.x.
- Kypri K, Saunders JB, Williams SM, McGee RO, Langley JD, Cashell-Smith ML, et al. Web-based screening and brief intervention for hazardous drinking: a double-blind randomized controlled trial. Addiction 2004;99:1410-17. https://doi.org/10.1111/j.1360-0443.2004.00847.x.
- Lewis MA, Neighbors C, Oster-Aaland LO, Kirkeby BS, Larimer ME. Indicated prevention for incoming freshmen: personalised normative feedback and high risk drinking. Addict Behav 2007;32:2495-508. https://doi.org/10.1016/j.addbeh.2007.06.019.
- Murphy JG, Dennhardt AA, Skidmore JR, Martens MP, McDevitt-Murphy ME. Computerized versus motivational interviewing alcohol interventions: impact on discrepancy, motivation, and drinking. Psychol Addict Behav 2010;24:628-39. https://doi.org/10.1037/a0021347.
- Neighbors C, Larimer ME, Lewis MA. Targeting misperceptions of descriptive drinking norms: efficacy of a computer-delivered personalized normative feedback intervention. J Consult Clin Psychol 2004;72:434-47. https://doi.org/10.1037/0022-006X.72.3.434.
- Neighbors C, Lewis MA, Atkins DC, Jensen MM, Walter T, Fossos N, et al. Efficacy of web-based personalized normative feedback: a two-year randomized controlled trial. J Consult Clin Psychol 2010;78:898-911. https://doi.org/10.1037/a0020766.
- Neumann T, Neuner B, Weiss-Gerlach E, Tønnesen H, Gentilello LM, Wernecke KD, et al. The effect of computerized tailored brief advice on at-risk drinking in subcritically injured trauma patients. J Trauma 2006;61:805-14. https://doi.org/10.1097/01.ta.0000196399.29893.52.
- Palfai TP, Zisserson R, Saitz R. Using personalized feedback to reduce alcohol use among hazardous drinking college students: the moderating effect of alcohol-related negative consequences. Addict Behav 2011;36:539-42. https://doi.org/10.1016/j.addbeh.2011.01.005.
- Spijkerman R, Roek MA, Vermulst A, Lemmers L, Huiberts A, Engels RC. Effectiveness of a web-based brief alcohol intervention and added value of normative feedback in reducing underage drinking: a randomized controlled trial. J Med Internet Res 2010;12. https://doi.org/10.2196/jmir.1465.
- Wagener TL, Leffingwell TR, Mignogna J, Mignogna MR, Weaver CC, Cooney NJ, et al. Randomized trial comparing computer-delivered and face-to-face personalized feedback interventions for high-risk drinking among college students. J Subst Abuse Treat 2012;43:260-7. https://doi.org/10.1016/j.jsat.2011.11.001.
- Walters ST, Vader AM, Harris TR, Field CA, Jouriles EN. Dismantling motivational interviewing and feedback for college drinkers: a randomized clinical trial. J Consult Clin Psychol 2009;77:64-73. https://doi.org/10.1037/a0014472.
- Patton R, Deluca P, Kaner E, Newbury-Birch D, Phillips T, Drummond C. Alcohol screening and brief intervention for adolescents: the how, what and where of reducing alcohol consumption and related harm among young people. Alcohol Alcohol 2014;49:207-12. https://doi.org/10.1093/alcalc/agt165.
- Pilowsky DJ, Wu LT. Screening instruments for substance use and brief interventions targeting adolescents in primary care: a literature review. Addict Behav 2013;38:2146-53. https://doi.org/10.1016/j.addbeh.2013.01.015.
- Mitchell SG, Gryczynski J, O’Grady KE, Schwartz RP. SBIRT for adolescent drug and alcohol use: current status and future directions. J Subst Abuse Treat 2013;44:463-72. https://doi.org/10.1016/j.jsat.2012.11.005.
- Haug S, Sannemann J, Meyer C, John U. Internet and mobile phone interventions to decrease alcohol consumption and to support smoking cessation in adolescents: a review. Gesundheitswesen 2012;74:160-77. https://doi.org/10.1055/s-0030-1268446.
- Yuma-Guerrero PJ, Lawson KA, Velasquez MM, von Sternberg K, Maxson T, Garcia N. Screening, brief intervention, and referral for alcohol use in adolescents: a systematic review. Pediatrics 2012;130:115-22. https://doi.org/10.1542/peds.2011-1589.
- Carney T, Myers B. Effectiveness of early interventions for substance-using adolescents: findings from a systematic review and meta-analysis. Subst Abuse Treat Prev Policy 2012;7. https://doi.org/10.1186/1747-597X-7-25.
- Jackson C, Geddes R, Haw S, Frank J. Interventions to prevent substance use and risky sexual behaviour in young people: a systematic review. Addiction 2012;107:733-47. https://doi.org/10.1111/j.1360-0443.2011.03751.x.
- Calabria B, Shakeshaft AP, Havard A. A systematic and methodological review of interventions for young people experiencing alcohol-related harm. Addiction 2011;106:1406-18. https://doi.org/10.1111/j.1360-0443.2011.03418.x.
- Lemstra M, Bennett N, Nannapaneni U, Neudorf C, Warren L, Kershaw T, et al. A systematic review of school-based marijuana and alcohol prevention programs targeting adolescents aged 10–15. Addict Res Theory 2010;18:84-96. https://doi.org/10.3109/16066350802673224.
- Wachtel T, Staniford M. The effectiveness of brief interventions in the clinical setting in reducing alcohol misuse and binge drinking in adolescents: a critical review of the literature. J Clin Nurs 2010;19:605-20. https://doi.org/10.1111/j.1365-2702.2009.03060.x.
- Deas D. Evidence-based treatments for alcohol use disorders in adolescents. Pediatrics 2008;121:348-54. https://doi.org/10.1542/peds.2007-2243G.
- Carey KB, Henson JM, Carey MP, Maisto SA. Which heavy drinking college students benefit from a brief motivational intervention?. J Consult Clin Psychol 2007;75:663-9. https://doi.org/10.1037/0022-006X.75.4.663.
- Gmel G, Venzin V, Marmet K, Danko G, Labhart F. A quasi-randomized group trial of a brief alcohol intervention on risky single occasion drinking among secondary school students. Int J Public Health 2012;57:935-44. https://doi.org/10.1007/s00038-012-0419-0.
- Winters KC, Fahnhorst T, Botzet A, Lee S, Lalone B. Brief intervention for drug-abusing adolescents in a school setting: outcomes and mediating factors. J Subst Abuse Treat 2012;42:279-88. https://doi.org/10.1016/j.jsat.2011.08.005.
- Segatto ML, Andreoni S, de Souza e Silva R, Diehl A, Pinsky I. Brief motivational interview and educational brochure in emergency room settings for adolescents and young adults with alcohol-related problems: a randomized single-blind clinical trial. Braz J Psychiatry 2011;33:225-33. https://doi.org/10.1590/S1516-44462011000300004.
- Walton MA, Chermack ST, Shope JT, Bingham CR, Zimmerman MA, Blow FC, et al. Effects of a brief intervention for reducing violence and alcohol misuse among adolescents: a randomized controlled trial. JAMA 2010;304:527-35. https://doi.org/10.1001/jama.2010.1066.
- Bernstein J, Heeren T, Edward E, Dorfman D, Bliss C, Winter M, et al. A brief motivational interview in a pediatric emergency department, plus 10-day telephone follow-up, increases attempts to quit drinking among youth and young adults who screen positive for problematic drinking. Acad Emerg Med 2010;17:890-902. https://doi.org/10.1111/j.1553-2712.2010.00818.x.
- Carey KB, Scott-Sheldon LA, Elliott JC, Garey L, Carey MP. Face-to-face versus computer-delivered alcohol interventions for college drinkers: a meta-analytic review, 1998 to 2010. Clin Psychol Rev 2012;32:690-703. https://doi.org/10.1016/j.cpr.2012.08.001.
- Enhancing Motivation for Change in Substance Abuse Treatment. Rockville, MD: Substance Abuse and Mental Health Services Administration (US); 1999.
- Conrod PJ, Castellanos N, Mackie C. Personality-targeted interventions delay the growth of adolescent drinking and binge drinking. J Child Psychol Psychiatry 2008;49:181-90. https://doi.org/10.1111/j.1469-7610.2007.01826.x.
- Walton MA, Resko S, Chermack ST, Bingham CR, Shope J, Zimmerman MA, et al. Computerized screening and brief intervention for alcohol use and violence among adolescents in the inner-city emergency department. Acad Emerg Med 2009;16:1193-207. https://doi.org/10.1111/j.1553-2712.2009.00513.x.
- Doumas DM, Andersen LL. Reducing alcohol use in first-year university students: evaluation of a web-based personalized feedback program. J Coll Couns 2009;12:18-32. https://doi.org/10.1002/j.2161-1882.2009.tb00037.x.
- Deluca P, Coulton S, Alam MF, Cohen D, Donoghue K, Gilvarry E, et al. Linked randomised controlled trials of face-to-face and electronic brief intervention methods to prevent alcohol related harm in young people aged 14–17 years presenting to emergency departments (SIPS junior). BMC Public Health 2015;15. https://doi.org/10.1186/s12889-015-1679-4.
- World Medical Association . Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects 1964. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 11 June 2019).
- Medical Research Council . Ethics Series. Good Research Practice: Principles and Guidelines n.d. https://mrc.ukri.org/research/policies-and-guidance-for-researchers/good-research-practice/ (accessed 11 June 2019).
- Goodman R, Meltzer H, Bailey V. The Strengths and Difficulties Questionnaire: a pilot study on the validity of the self-report version. Eur Child Adolesc Psychiatry 1998;7:125-30. https://doi.org/10.1007/s007870050057.
- EuroQol Group . EQ-5D-5L 2013.
- Chisholm D, Knapp MR, Knudsen HC, Amaddeo F, Gaite L, van Wijngaarden B. Client socio-demographic and service receipt inventory – European version: development of an instrument for international research. EPSILON study 5. European psychiatric services: inputs linked to outcome domains and needs. Br J Psychiatry Suppl 2000;39:s28-33. https://doi.org/10.1192/bjp.177.39.s28.
- Coulton S, Perryman K, Bland M, Cassidy P, Crawford M, Deluca P, et al. Screening and brief interventions for hazardous alcohol use in accident and emergency departments: a randomised controlled trial protocol. BMC Health Serv Res 2009;9. https://doi.org/10.1186/1472-6963-9-114.
- Drummond C, Deluca P, Coulton S, Bland M, Cassidy P, Crawford M, et al. The effectiveness of alcohol screening and brief intervention in emergency departments: a multicentre pragmatic cluster randomized controlled trial. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0099463.
- Hester RK, Miller WR. Handbook of Alcoholism Treatment Approaches. Boston, MA: Allyn and Bacon; 1995.
- Nunnally JC, Bernstein IH. Psychometric Theory. New York, NY: McGraw-Hill College; 1994.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013.
- Tanner-Smith EE, Lipsey MW. Brief alcohol interventions for adolescents and young adults: a systematic review and meta-analysis. J Subst Abuse Treat 2015;51:1-18. https://doi.org/10.1016/j.jsat.2014.09.001.
- Wallace P, Cutler S, Haines A. Randomized controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988;297:663-68. https://doi.org/10.1136/bmj.297.6649.663.
- Cutler S, Haines A. Randomized controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988;297:663-68.
- Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction 2002;97:279-92. https://doi.org/10.1046/j.1360-0443.2002.00018.x.
- Tibshirani EA. An Introduction to the Bootstrap. New York, NY: Chapman and Hall; 1993.
- Drummond M, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1999.
- Briggs A. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford: Oxford University Press; 2001.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Coulton S, Watson J, Bland M, Drummond C, Kaner E, Godfrey C, et al. The effectiveness and cost-effectiveness of opportunistic screening and stepped care interventions for older hazardous alcohol users in primary care (AESOPS) – a randomised control trial protocol. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472-6963-8-129.
- Drummond C, Coulton S, James D, Godfrey C, Parrott S, Baxter J, et al. Effectiveness and cost-effectiveness of a stepped care intervention for alcohol use disorders in primary care: pilot study. Br J Psychiatry 2009;195:448-56. https://doi.org/10.1192/bjp.bp.108.056697.
- Kohl LF, Crutzen R, de Vries NK. Online prevention aimed at lifestyle behaviors: a systematic review of reviews. J Med Internet Res 2013;15. https://doi.org/10.2196/jmir.2665.
- Kaner E, Bland M, Cassidy P, Coulton S, Dale V, Deluca P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.e8501.
- Khadjesari Z, Murray E, Kalaitzaki E, White IR, McCambridge J, Thompson SG, et al. Impact and costs of incentives to reduce attrition in online trials: two randomized controlled trials. J Med Internet Res 2011;13. https://doi.org/10.2196/jmir.1523.
- Alkhaldi G, Hamilton FL, Lau R, Webster R, Michie S, Murray E. The effectiveness of prompts to promote engagement with digital interventions: a systematic review. J Med Internet Res 2016;18. https://doi.org/10.2196/jmir.4790.
- Bewick BM, West R, Gill J, O’May F, Mulhern B, Barkham M, et al. Providing web-based feedback and social norms information to reduce student alcohol intake: a multisite investigation. J Med Internet Res 2010;12. https://doi.org/10.2196/jmir.1461.
- Milward J, Khadjesari Z, Fincham-Campbell S, Deluca P, Watson R, Drummond C. User preferences for content, features, and style for an app to reduce harmful drinking in young adults: analysis of user feedback in app stores and focus group interviews. JMIR Mhealth Uhealth 2016;4. https://doi.org/10.2196/mhealth.5242.
- Crane D, Garnett C, Brown J, West R, Michie S. Behavior change techniques in popular alcohol reduction apps: content analysis. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4060.
- Davies EL, Lonsdale AJ, Hennelly SE, Winstock AR, Foxcroft DR. Personalized digital interventions showed no impact on risky drinking in young adults: a pilot randomized controlled trial. Alcohol Alcohol 2017;52:671-6. https://doi.org/10.1093/alcalc/agx051.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Christensen PM, James A. Research with Children: Perspectives and Practices. New York, NY: Routledge; 2008.
- Jans M. Children as citizens: towards a contemporary notion of child participation. Childhood 2004;11:27-44. https://doi.org/10.1177/0907568204040182.
- Sanci LA, Sawyer SM, Weller PJ, Bond LM, Patton GC. Youth health research ethics: time for a mature-minor clause?. Med J Aust 2004;180:336-8.
- Nuffield Council on Bioethics . Children and Clinical Research: Ethical Issues 2015. http://nuffieldbioethics.org/wp-content/uploads/Children-and-clinical-research-full-report.pdf (accessed 26 April 2018).
- Levine RJ. Ethics and Regulation of Clinical Research: Second Edition. New Haven, CT: Yale University Press; 1988.
- Beauchamp TL, Saghai Y. The historical foundations of the research-practice distinction in bioethics. Theor Med Bioeth 2012;33:45-56. https://doi.org/10.1007/s11017-011-9207-8.
- Faden RR, Kass NE, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. Hastings Cent Rep 2013;Spec:S16-27. https://doi.org/10.1002/hast.134.
- Kass NE, Faden RR, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. The research-treatment distinction: a problematic approach for determining which activities should have ethical oversight. Hastings Cent Rep 2013;Spec:S4-S15. https://doi.org/10.1002/hast.133.
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. New York, NY: Oxford University Press; 2001.
- Lynch E, McGovern R, Elzerbi C, Breckons M, Deluca P, Drummond C, et al. Adolescent perspectives about their participation in alcohol intervention research in emergency care: a qualitative exploration using ethical principles as an analytical framework. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0217855.
- Harden J, Scott S, Backett-Milburn K, Jackson S. Can’t talk, won’t talk?: Methodological issues in researching children. Sociol Res Online 2000;5:1-12. https://doi.org/10.5153/sro.486.
- National Institute for Health and Care Excellence . Alcohol-Use Disorders – Preventing the Development of Hazardous and Harmful Drinking 2010.
- Davison C, Smith GD, Frankel S. Lay epidemiology and the prevention paradox: the implications of coronary candidacy for health education. Sociol Health Illn 1991;13:1-19. https://doi.org/10.1111/j.1467-9566.1991.tb00085.x.
- Allmark P, Tod A. How should public health professionals engage with lay epidemiology?. J Med Ethics 2006;32:460-3. https://doi.org/10.1136/jme.2005.014035.
- Clark DB, Neighbors BD, Lesnick LA, Lynch KG, Donovan JE. Family functioning and adolescent alcohol use disorders. J Fam Psychol 1998;12. https://doi.org/10.1037/0893-3200.12.1.81.
- Clark DB, Winters KC. Measuring risks and outcomes in substance use disorders prevention research. J Consult Clin Psychol 2002;70:1207-23. https://doi.org/10.1037/0022-006X.70.6.1207.
- Public Health England . Young People’s Hospital Alcohol Pathways. Support Pack for A&Amp;E Departments 2014.
- Department of Health and Social Care . Reference Costs 2013–14 n.d. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 18 June 2019).
- Department for Education . Exclusion from Maintained Schools, Academies and Pupil Referral Units in England: Statutory Guidance for Those With Legal Responsibilities in Relation to Exclusion 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/641418/20170831_Exclusion_Stat_guidance_Web_version.pdf (accessed 20 May 2019).
- Journalism.co.uk . Private Tuition Fees: New Data on UK Tutor Rates n.d. www.journalism.co.uk/press-releases/private-tuition-fees-new-data-on-uk-tutor-rates/s66/a604769/ (accessed 9 May 2019).
- Department for Education . Teaching Salaries n.d. https://getintoteaching.education.gov.uk/teachers-salary-and-teaching-benefits/teacher-salaries (accessed 9 May 2019).
- The National Police Chiefs’ Council . National Policing Guidelines on Charging for Police Services: Mutual Aid Cost Recovery n.d. www.npcc.police.uk/documents/finance/2018/Charging%20for%20Police%20Services_July_2018.pdf (accessed 20 May 2019).
- Crown Prosecution Service . Application for Costs Against Convicted Defendants – Scales of Cost n.d. www.cps.gov.uk/legal/a_to_c/costs/annex_1_-_scales_of_cost/ (accessed 9 May 2019).
- Alexander S. Night in a prison cell costs more than a night at the Ritz hotel. Mirror, 20 April 2015 n.d. www.mirror.co.uk/news/uk-news/night-prison-cell-costs-more-5555031 (accessed 9 May 2019).
Appendix 1 Unpublished tables
| Effect | Residual | p-value |
|---|---|---|
| Baseline alcohol consumption | 20.42 | <.001 |
| Age in years | 64.37 | <.001 |
| Sex | 3.97 | 0.05 |
| Ethnicity | 2.06 | 0.06 |
| Smoking status | 1.77 | 0.13 |
| Consume fruit | 1.15 | 0.33 |
| Age (years) at first drink | 2.14 | 0.01 |
| Alcohol-related problems | ||
| Fighting | 1.93 | 0.10 |
| Parents | 1.40 | 0.22 |
| Friends | 2.53 | 0.04 |
| Police | 2.14 | 0.10 |
| Alcohol expectancy | ||
| Feel more relaxed | 5.60 | 0.01 |
| More trouble with police | 2.51 | 0.04 |
| Forget problems | 0.80 | 0.53 |
| Unable to stop drinking | 3.37 | 0.01 |
| More friendly | 3.09 | 0.02 |
| Regretful activity | 2.39 | 0.05 |
Appendix 2 Published research content
Reproduced from Lynch et al. 164 © 2019 Lynch et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix 3 Incremental cost-effectiveness ratios tables
Table 16 outlines each of the resources costed for use within the cost-effectiveness analysis. It shows the cost identified for each resource, any assumption of calculation used and the reference for the costs. All costs were recorded in 2014 UK sterling prices. Resource use, their subsequent costs and any benefits observed were modelled over a 12-month time horizon. A discount rate of 3.5% was used.
| Contact with health professional | Unit cost (£) | Details of cost component(s) | Reference |
|---|---|---|---|
| Consultation with GP | 46.00 | 11.7 minutes of consultation, including direct care staff costs, with qualification costs (p. 195) | Curtis143 |
| Seen the practice nurse | 13.69 | £53 per hour for face-to-face consultation, including qualification costs. Average consultations last 15.5 minutes. Cost calculated as follows: £53/60 × 15.5 (p. 192) | Curtis143 |
| Seen the health visitor | 19.69 | £76 per hour of patient-related work, including qualification costs. Average consultation time for a practice nurse (15.5 minutes). So, £1.27 per minute × 15.5 = £19.69 (pp. 187, 189, 192) | Curtis143 |
| Hospital inpatient elective stay | 3375.42 | National average unit cost | Department of Health and Social Care172 |
| Hospital inpatient elective stay: excess bed days | 326.90 | National average unit cost | Department of Health and Social Care172 |
| Hospital inpatient non-elective short stay (1–3 days) | 602.52 | National average unit cost | Department of Health and Social Care172 |
| Hospital inpatient non-elective long stay (4–6 days) | 2837.31 | National average unit cost | Department of Health and Social Care172 |
| Hospital inpatient non-elective excess bed-days (incurred for stays > 6 days) | 275.05 | National average unit cost | Department of Health and Social Care172 |
| Hospital day case | 697.55 | National average unit cost | Department of Health and Social Care172 |
| Regular day/night admission | 400.23 | National average unit cost | Department of Health and Social Care172 |
| A&E visit | 123.67 | National average unit cost | Department of Health and Social Care172 |
| Outpatient department visit | 109.00 | Weighted average of all outpatient attendances (p. 111) | Curtis143 |
| Consulted or visited other health-care professional | 34.67 | Calculated average of hospital-based health-care staff, including qualification costs: physiotherapist (£37), occupational therapist (£36), SALT (£37), dietitian (£37), radiographer (£38), allied health professional support worker (£23) = £34.67 (pp. 235–241). Assumed 1-hour consultation, given that there is no average consultation time | Curtis143 |
| Community services | |||
| Visited optician | 21.00 | Average Specsavers cost | Curtis143 |
| Visited family therapist | 45.83 | £50 per hour. Average consultations last 55 minutes. Cost calculated as follows: (£50/60) × 55 = £45.83 (p. 51) | Curtis143 |
| Visited individual therapist | 45.83 | £50 per hour. Average consultations last 55 minutes. Cost calculated as follows: (£50/60) × 55 = £45.83 (p. 51) | Curtis143 |
| Visited psychiatrist/psychologist | 69.00 | £138 per hour for client-related work, including qualification costs. Average consultations last 30 minutes (assumption). Cost calculated as follows: (£138/60) × 30 = £69.00 (p. 183) | Curtis143 |
| Visited social worker | 39.50 | £79 per hour for client-related work, including qualification costs. Average consultations last 30 minutes (assumption). Cost calculated as follows: (£79/60) × 30 = £39.50 (p. 207) | Curtis143 |
| Home visit optician | 31.00 | Estimated cost of optician visit at £21.00 + £10.00 travel costs | |
| Home visit family therapist | 70.83 | £50 per hour. Average consultations last 55 minutes + 15 minutes each way travel costs (assumption). Cost calculated as follows: (£50/60) × 85 = £70.83 (p. 51) | Curtis143 |
| Home visit individual therapist | 70.83 | £50 per hour. Average consultations last 55 minutes + 15 minutes each way travel costs (assumption). Cost calculated as follows: (£50/60) × 85 = £70.83 (p. 51) | Curtis143 |
| Home visit psychiatrist/psychologist | 138.00 | £138 per hour for client-related work, including qualification costs. Average consultations last 30 minutes + 15 minutes each way travel (assumption). Cost calculated as follows: (£138/60) × 60 = £138.00 (p. 183) | Curtis143 |
| Home visit social worker | 39.50 | £79 per hour for client-related work, including qualification costs. Average consultations last 30 minutes (assumption). Cost calculated as follows: (£79/60) × 30 = £39.50 (p. 207) | Curtis143 |
| Home visits | |||
| GP | 91.26 | 11.4-minutes home visit, plus 12-minutes travel time per visit on average. 1 minute of GP time is costed at £3.90 (including direct care staff costs with qualification costs) (p. 195), this is multiplied by 23.4 to estimate cost of home visit (pp. 194–5) | Curtis143 |
| Community nurse | 30.25 | £66 per hour of patient-related work, including qualification costs. Average consultation time for a practice nurse (15.5 minutes) and average travel time for a GP (12 minutes) used to calculate cost per visit. So, (£1.10 per minute × 15.5 = £17.05) + (£1.10 × 12 = £13.20) = £30.25 (pp. 187, 192, 194–5) | Curtis143 |
| Practice nurse | 24.29 | £53 per hour for face-to-face consultation at GP surgery, including qualification costs. Average consultations last 15.5 minutes (assumed to be the same for home visits). Average travel time for a GP (12 minutes) used to calculate travel costs. So, (53/60 × 15.5 = £13.69) + (53/60 × 12 = £10.60) = £24.29 (pp. 192, 194–5) | Curtis143 |
| Health visitor | 34.93 | £76 per hour of patient-related work, including qualification costs. Average consultation time for a practice nurse (15.5 minutes) and average travel time for a GP (12 minutes) used to calculate cost per visit. So, (£1.27 per minute × 15.5 = £19.69) + (£1.27 × 12 = £15.24) = £34.93 (pp. 187, 189, 192, 194–5) | Curtis143 |
| Other health-care professional | 48.48 | Calculated average of community-based health-care staff, including qualification costs: physiotherapist (£36), occupational therapist (£36), SALT (£36), palliative care nurse specialist (£74), clinical support worker (£20) = £40.40. Then added travel costs, estimated by using GP travel time of 12 minutes. So, additional travel cost = (40.40/60 × 12 = £8.08) (pp. 235–241). Assumed 1-hour consultation, given that there is no average consultation time | Curtis143 |
| Drug and alcohol services | |||
| CAMHS face to face | 84.00 | CAMHS cost per hour ranges between £84 and £115 per hour of face-to-face contact, depending on case mix. Average face-to-face meeting lasts 60 minutes (assumption) (pp. 222–5) | Curtis143 |
| CAMHS telephone | 16.38 | 11.7-minute consultation (average GP telephone consultation time) as a proportion of CAMHS cost | Curtis143 |
| Other face to face | 84.00 | Assumed same as CAMHS | Curtis143 |
| Other telephone | 16.38 | Assumed same as CAMHS | Curtis143 |
| Sick/truancy days | |||
| School exclusion (permanent) | 4000.00 | Department for Education173 | |
| Educational help | |||
| Individual tuition at home | 35.00 | Fees average between £29 and £41 per hour | Journalism.co.uk174 |
| Individual tuition in some classes | 14.26 | £14.26 per hour, based on an average teaching salary of £27,813.5 and 37.5 hours per week of working time | Department for Education175 |
| Lessons in a special unit in school | 14.26 | Assumed same as individual tuition in some classes | Department for Education175 |
| School professionals | |||
| School nurse (per contact) | 53.00 | £53 per contact, school-based children’s health-care (other) services (p. 85) | Curtis143 |
| Educational psychologist (per contact) | 41.00 | £41 per contact, educational psychologist (p. 156) | Curtis143 |
| Educational welfare officer (per contact) | 22.50 | £22.50 per contact, EWO. Checklist completed by EWO £18 + TAC meeting attended by EWO £27. Average calculated (p. 155) | Curtis143 |
| School counsellor/health advisor (per contact) | 41.00 | Assumed same as educational psychologist | Curtis143 |
| Additional meetings with tutors (per minute) | 0.24 | £0.24 per minute, based on an average teaching salary of £27,813.5 and 37.5 hours per week of working time | Department for Education175 |
| Other care | |||
| Foster care (days) | 427.86 | £2995 establishment costs per week/7days = £427.86 per day (p. 86) | Curtis143 |
| Residential care (days) | 90.43 | £633 establishment costs per week/7days = £90.43 per day (p. 64) | Curtis143 |
| Supported accommodation (days) | 90.43 | Assumed same as residential care. £633 establishment costs per week/7days = £90.43 per day (p. 64) | Curtis143 |
| Other (days) | 90.43 | Assumed same as residential care. £633 establishment costs per week/7days = £90.43 per day (p. 64) | Curtis143 |
| Policing and crime | |||
| Police contact (spoken to by) | 21.85 | Cost of police constable, per hour (p. 16) | NPCC176 |
| Court appearance | 100.00 | Costs vary massively depending on type of court and whether the defendant pleads guilty or goes to trial. We have calculated the cost based on the lowest costed court attendance at a magistrates court. Costs exclude lawyers | Crown Prosecution Service177 |
| Custody (day) | 418.00 | Alexander178 | |
Incremental cost-effectiveness ratios for interventions compared with control for the low- and high-risk populations are given in Tables 17 and 18.
| Perspective | Trial group | Total | Difference | ICER (£/QALY) | ||
|---|---|---|---|---|---|---|
| QALY | Cost (£) | QALY | Cost (£) | |||
| Societal | Control | 0.904 | 1132 | – | – | – |
| PFBA | 0.909 | 1735 | 0.005 | 603 | 130,822 | |
| eBI | 0.894 | 1884 | –0.013 | 751 | eBI dominated | |
| NHS/PSS | Control | 0.904 | 912 | – | – | – |
| PFBA | 0.909 | 1468 | 0.005 | 556 | 120,693 | |
| eBI | 0.894 | 1683 | –0.013 | 771 | eBI dominated | |
| Population | Trial group | Total | Difference | ICER (£/QALY) | ||
|---|---|---|---|---|---|---|
| QALY | Cost (£) | QALY | Cost (£) | |||
| Societal | Control | 0.900 | 1704 | – | – | – |
| PFBA | 0.903 | 1726 | 0.003 | 23 | 8683 | |
| eBI | 0.892 | 2110 | –0.008 | 407 | eBI dominated | |
| NHS/PSS | Control | 0.900 | 1553 | – | – | – |
| PFBA | 0.903 | 1571 | 0.003 | 19 | 7115 | |
| eBI | 0.892 | 1953 | –0.008 | 401 | eBI dominated | |
Appendix 4 Copies of previously published papers
Reproduced from Coulton et al. 61 © The Author(s) 2018. Published by Oxford University Press on behalf of Faculty of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
Reproduced from Donoghue et al. 74 © Kim Donoghue, Robert Patton, Thomas Phillips, Paolo Deluca, Colin Drummond. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 02.06.2014. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on http://www.jmir.org/, as well as this copyright and license information must be included.
Reproduced from Donoghue et al. 62 © 2016 Society for Adolescent Health and Medicine. All rights reserved. Reprinted from Journal of Adolescent Health, vol. 60, Donoghue K, Rose H, Boniface S, Deluca P, Coulton S, Alam MF, et al. Alcohol consumption, early-onset drinking, and health-related consequences in adolescents presenting at emergency departments in England pp. 438–446, 2017, with permission from Elsevier.
Reproduced from Patton et al. 102 © The Author 2013. Published by Oxford University Press on behalf of the Medical Council on Alcohol. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http://creativecommons.org/licenses/by-nc-nd/3.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
Reproduced from Deluca et al. 124 © Deluca et al. ; licensee BioMed Central. 2015. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
List of abbreviations
- A&E
- accident and emergency
- app
- application program
- AUD
- alcohol use disorder
- AUDIT
- Alcohol Use Disorders Identification Test
- AUDIT-C
- Alcohol Use Disorders Identification Test, Consumption (3 items)
- CI
- confidence interval
- CRAFFT
- Car, Relax, Alone, Forget, Friends, Trouble
- CSRI
- Client Service Receipt Inventory
- eBI
- electronic brief intervention
- ED
- emergency department
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- eSBI
- electronic screening and brief intervention
- ESPAD
- European School Survey Project on Alcohol and Other Drugs
- GP
- general practitioner
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- MI
- motivational interviewing
- MINI-KID
- Mini International Neuropsychiatric Interview for Children and Adolescents
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PFBA
- personalised feedback and brief advice
- PMG
- Programme Management Group
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal and Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SBI
- screening and brief intervention
- SD
- standard deviation
- SE
- standard error
- SIPS
- Screening and Intervention to Promote Sensible drinking
- SMS
- short message service
- WTP
- willingness to pay
