Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0608-10133. The contractual start date was in April 2010. The final report began editorial review in September 2018 and was accepted for publication in January 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Clive Ballard reports grants from Novo Nordisk (Bagsværd, Denmark); grants and personal fees from Acadia Pharmaceuticals (San Diego, CA, USA) and from Lundbeck Pharmaceuticals (Copenhagen, Denmark); and personal fees from Pfizer Inc. (New York, NY, USA), Roche Holding AG (Basel, Switzerland), Eli Lilly and Company (Indianapolis, IN, USA), Heptares Therapeutics (Hertford, UK), Otsuka Pharmaceutical (Tokyo Japan), Novartis (Basel, Switzerland) and Allergan (Dublin, Ireland), outside the submitted work. Esme Moniz-Cook held National Institute for Health Research (NIHR) funding for a programme grant during this period (RP-PG-0606-106). Anne Corbett reports personal fees from Lundbeck Pharmaceuticals, Novartis, Bial (Trofa, Portugal) and Acadia Pharmaceuticals, outside the submitted work, and a NIHR Programme Development Grant held during the study period (RP-DG-1212-10004). Dag Aarsland has received research support and/or honoraria from AstraZeneca (Cambridge, UK), Lundbeck Pharmaceuticals, Novartis and GE Healthcare (Chicago, IL, USA), and serves as a paid consultant for Lundbeck Pharmaceuticals and Axovant Gene Therapies (Hamilton, Bermuda), outside the submitted work. Zoe Hoare reports associate membership of the Health Services and Delivery Research programme board (2016–20). Jane Fossey reports a grant from the Alzheimer’s Society (London, UK) outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Ballard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
SYNOPSIS
Background
Dementia is characterised by the progressive loss of cogition and function, leading to the loss of independence and communication ability, and eventually to death. Dementia exerts an enormous impact on the individuals affected and their families, many of whom provide informal care for long periods of time. In the context of an ageing population, dementia is also a critical public health issue with considerable financial implications at a societal level. There are 46 million people with dementia worldwide, including 850,000 people in the UK, of whom an estimated 250,000 live in care homes. 1,2 In the USA, 64% of people receiving Medicare in nursing homes have dementia. 3 Care homes provide full-time residential and nursing care, and represent a unique challenge for health care. There is an increasing focus in research and policy on the improvement of institutionalised care and the need for evidence-based interventions for people with dementia that are tailored to this setting.
Dementia in health-care provision and policy
Dementia has a vast impact on health and social care services in the UK. The direct cost of dementia is £42B per year,1 which is higher than the cost of stroke, heart disease and cancer combined. This was the basis of the UK’s national dementia strategy for England, developed by the Department of Health and Social Care in partnership with stakeholder organisations, such as the Alzheimer’s Society (London, UK). 4,5 The strategy laid out a unique vision for people with dementia and provided a 5-year plan for building health and social services for people with dementia that are fit for the 21st century. 4
The strategy included two key objectives with particular relevance to care home settings, with strategies to achieve them. ‘Improving the quality of care for people with dementia in care homes’ (Contains public sector information licensed under the Open Government Licence v3.0)4 – objective 11 – involved a government directive to include specialist mental health teams in dementia care within a range of other measures. ‘The development of an informed and effective workforce for people with dementia’ (Contains public sector information licensed under the Open Government Licence v3.0)4 – objective 13 – aimed to provide training and skills for health and social care staff to enable them to deliver the best possible care.
The importance of providing high-quality care for people with dementia who are living in residential and nursing homes received less attention in the subsequent Prime Minister’s Challenge on Dementia 20205 and the related implementation plan. 6 However, the value of an adequately trained workforce and the provision of evidence-based training was highlighted, and the focus on minimising unnecessary prescribing of antipsychotic medications to people with dementia was maintained,6 in line with international recommendations. 7 A subsequent report from The King’s Fund, Social Care for Older People: Home Truths,8 has shown the substantial pressures facing the care home sector and the urgent need to use limited resources more effectively to achieve high-quality care.
People with dementia have complex care needs, particularly those residing in care homes. The majority of these individuals have moderate or severe dementia and their care needs are influenced by a combination of cognitive, functional and communication impairments, and medical comorbidities, and a high frequency of behavioural and psychological symptoms of dementia (BPSD). 3,7,9 The NHS provides for these complex health needs largely through primary care and specialist service consultancy. Some specialist teams have introduced more proactive liaison services, but these are not widespread. An effective solution is needed to enable consistent and effective NHS support for people with dementia and staff, particularly in care home settings where specialist staff are rarely available. Such an NHS service would need to improve the general quality of care, the skill base of care staff and the availability of non-pharmacological interventions and reduce the frequency of potentially harmful antipsychotic prescribing to improve health and quality of life (QoL) for people with dementia in care homes.
The Well-being and Health for people with Dementia (WHELD) programme grant was run in parallel with the national dementia strategy for England and the Prime Minister’s challenge on dementia implementation activities, and within the context of a national drive to improve health-care service provision and care home services for people with dementia. The programme represents an important example of the development of a robust evidence-base for dementia care and the improvement of services in care homes.
Behavioural and psyschological symptoms in dementia
Neuropsychiatric symptoms, often referred to by consensus as BPSD, include aggression, agitation and restlessness, psychosis, depression, anxiety, elation, disinhibition, sleep disturbance and apathy. BPSD affect 90% of people with dementia at some point during the course of their condition. 10 These BPSD are critical indicators of QoL and well-being, and have clinically significant effects on the individual and their caregivers. They present a substantial challenge for health and care professionals, as there are limited treatment options. 10
Current best practice guidelines promote non-pharmacological interventions as the first-line approach for the treatment of BPSD, and there is a growing evidence base to support the value of this approach. 11 However, there is little guidance on how to implement non-drug treatments and no structured framework for their widespread use in practice.
The use of antipsychotics in people with dementia
Current best practice guidance limits the use of antipsychotics to short-term prescription (up to 12 weeks) of risperidone (Risperdal®, Janssen Pharmaceutica, Beerse, Belgium) in intractable cases that are causing risk to the person or others.
Clinical effectiveness of antipsychotic medications
Systematic reviews have analysed the clinical effectiveness of antipsychotics in people with dementia. Most studies have focused on the treatment of agitation, aggression, psychosis or overall BPSD. 12,13 These analyses are based on 18 placebo-controlled randomised trials, most of which were conducted over a 10- to 13-week period; however, many of these trials have not been published in full. The best evidence base exists for risperidone, with five fully published good-quality randomised controlled trials (RCTs) and a total of 1761 participants. 12,13 Adverse events were comprehensively reported in all five studies. 12,13 A meta-analysis reported a significant advantage for risperidone in the treatment of aggression {–0.84 points on the Behaviour in Alzheimer’s Disease (BEHAV-AD) scale [95% confidence interval (CI) –1.28 to 0.40 points] at a dose of 1 mg and –1.5 points (95% CI –2.05 to –0.95 points) at a dose of 2 mg}. 12 This threshold of change indicates a statistically significant difference but only borderline clinically meaningful benefit at the 2-mg dose of risperidone. Evidence of a clinically meaningful benefit is restricted to aggression; no clinically meaningful benefit was seen for non-aggressive symptoms of agitation. Effectiveness is even more limited for the treatment of psychosis with risperidone: statistically significant benefit but no clinically meaningful benefit was reported in one trial only at 1 mg of risperidone (BEHAV-AD mean difference –0.14. points, 95% CI –0.25 to –0.03 points). 12,13
There are important issues to highlight in the evidence base that relates to antipsychotic drug use in dementia. First, the evidence for benefit is not equal for all antipsychotic drugs. For example, a meta-analysis of published trials found no evidence that quetiapine (Seroquel, AstraZeneca, Cambridge, UK) confers benefit in the treatment of BPSD. 13 A meta-analysis of risperidone studies indicates that there is statistically significant benefit in risperidone treatment, but with a small effect size [Cohen’s d effect size (ES) of 0.18 for the treatment of psychosis and 0.2 for treating aggression]. 12,13 Olanzapine (Zyprexa, Eli Lilly) and aripiprazole (Abilify, Otsuka) appear to have similar effect sizes, but based on a smaller number of studies. Other atypical antipsychotic drugs have not been evaluated in RCTs. Second, all published antipsychotic trials have reported a high placebo response rate (e.g. 45% vs. 55% for risperidone), indicating that benefit is often related to the general benefits of good clinical practice.
Safety concerns for antipsychotics
The evidence of modest clinical effectiveness of antipsychotics must be balanced against the considerable risk of adverse events. There are established safety concerns associated with these medications, including worsening of cognitive decline, cerebrovascular events, sedation, falls and an increased risk of mortality. 14 A systematic review15 of 15 RCTs of antipsychotics in people with Alzheimer’s disease (AD) reported a 1% attributable risk of mortality over 12 weeks of treatment (risk difference 0.01, 95% CI 0.004 to 0.02; p = 0.01). A 12-month double-blind RCT16 examining antipsychotic discontinuation in people with AD, with a follow-up of participants for up to 5 years, found a significant reduction in mortality associated with discontinuation (hazard ratio 0.58, 95% CI 0.36 to 0.92), with a risk difference in mortality of 29% after 36 months. 16 A subsequent meta-analysis13,17 of all 18 RCTs of atypical antipsychotics for the treatment of BPSD also found a clinically significant acceleration of cognitive decline in people taking antipsychotics (Mini Mental State Examination mean difference 0.73 points, 95% CI 0.38 to 1.09 points; p < 0.0001) over 12 weeks.
Reporting of adverse events is the most complete for risperidone. Full data are not available for other antipsychotics as a result of the large number of unpublished studies. Of particular concern, meta-analyses have established a threefold increased risk of cerebrovascular events in people with AD who take risperidone compared with those taking placebo (odds ratio 3.43, 95% CI 1.60 to 7.32; z = 3.18; p = 0.001), with a risk difference of 3.1% compared with 1.0% in a pooled analysis. 11 Another important risk is an increased frequency of extrapyramidal symptoms (risk difference by meta-analysis 0.06, 95% CI 0.03 to 0.09, over 12 weeks). Peripheral oedema, sedation, prolonged QTc interval, infections and abnormal gait have also been identified as potential problems of atypical antipsychotics. 13 None of the RCTs of antipsychotic treatment for people with AD reported the impact on well-being or QoL. However, the relationship between the prescription of antipsychotics and well-being has been examined in one cohort study18 of 209 people, which suggested that there is lower well-being in people taking these medications in an analysis controlling for BPSD. 18
Long-term use of antipsychotics
Although more limited, evidence on the long-term use of antipsychotic drugs is also beginning to emerge. Only three trials12,16,19 have evaluated a range of atypical antipsychotic drugs over periods of 6 months and longer. Adverse events are more marked with longer-term use, with one RCT16 reporting 59% mortality in an intervention group compared with 30% in a placebo group after 36 months. In another 9-month RCT12 of 421 people with AD, 18% of patients receiving risperidone withdrew from the trial because of adverse events compared with 5% of those patients receiving placebo. Only one RCT19 has directly evaluated the impact of an antipsychotic (quetiapine) on agitation, reporting no benefit of the antipsychotic compared with placebo over 6 months. Overall, these studies have reported no benefit or very modest benefit in the treatment of BPSD over 6–12 months. The exception is one recent trial20 that compared the effect of withdrawal with the continuation of haloperidol, which indicated ongoing benefit of continuation in people who had initially responded to haloperidol treatment. A Cochrane review21 of nine randomised placebo-controlled trials concludes that long-term prescriptions of antipsychotics can be discontinued without a detrimental effect on BPSD. 21
The changing landscape of antipsychotic use
The lack of efficacy and the established safety concerns associated with antipsychotics is the basis of recommendations that these drugs are not to be used in people with AD. Only risperidone is licensed for use in this patient group, for cases of severe BPSD that are causing significant distress or risk to the individual and where other treatment approaches have failed. 7 Despite this, antipsychotic prescribing has been very common because of the lack of other treatments and the considerable pressure that is experienced by health professionals to prescribe medication. To address this issue, many governments and health authorities have promoted initiatives to reduce the unnecessary prescribing of antipsychotics, including the national dementia strategy for England. 7,17,22 These initiatives were supported by evidence that showed that there were no detrimental effects of withdrawing prescriptions on BPSD. 21 As a result, there has been a marked reduction in antipsychotic use in people with AD and dementia. An audit23 conducted in the UK in 2012 showed a 50% reduction in use of antipsychotics, with 16% of people with dementia continuing to receive antipsychotics. Similar trends have been documented across Europe and the USA. 24–26 It will now be imperative for authorities to support this success in reducing the unnecessary prescribing by adapting and updating guidance to reflect the altered landscape of antipsychotic use in dementia. For example, automatic withdrawal of antipsychotics may not be the best course of action given that the larger proportion of existing prescriptions may be appropriate for the individuals. Instead, it will be essential for guidance to focus on continued review of prescriptions and careful monitoring. The change in policy and pressure to reduce prescriptions also shows the importance of training for staff in care home settings and the need to enable their access to other effective therapies.
Non-pharmacological treatments for behavioural and psychological symptoms of dementia
All best practice guidance for the management of BPSD recommend the first-line use of non-pharmacological approaches unless the symptoms are so severe that they are causing great distress or risk. 11 Although more severe and challenging agitation and aggression often require pharmacological intervention, this level of BPSD does also respond to intense psychological approaches when tailored to the individual.
Person-centred care
Person-centred care dictates that all care planning and therapies are embedded within a framework that is tailored to the needs and wishes of each individual. This personalised approach involves taking a full history and record of the person’s interests, preferences and abilities, including their hobbies, culture and religious beliefs, previous employment and their medical and physical status. When working with people with dementia it is important to involve family members or close friends in discussions about the person to ensure that a rich life history is created. Person-centred care also requires a comprehensive medical review to ensure that the care and treatment is appropriate to their current and ongoing status (see the review by Fazio et al. 27 for an excellent overview of person-centred care). The value of a person-centred care approach is clearly established in the literature;28–31 however, a systematic review32 has shown a critical issue in how person-centred care is delivered. Of the 170 identified person-centred care training manuals available for use in care settings, only 30 met quality criteria and of these only four were supported by evidence of effectiveness. 32 This disconnect between available programmes and evidence is concerning and emphasises the importance of a standardised approach to person-centredness and the use of non-pharmacological approaches. All of the person-centred training programmes supported by a robust evidence have shown improvement in outcomes related to BPSD. In one RCT28 of an intensive 9-month training programme, the Focused Intervention for Training and Support (FITS) programme showed a significant 50% reduction in antipsychotic prescriptions without worsening of BPSD in 347 care home residents. A trial30 of dementia care mapping, an in-depth care-planning approach that is based on observation of care followed by planning to optimise it, reported improvements in agitation (Cohen’s d ES of > 0.5) but no reduction in antipsychotic use in 298 residents with dementia in 15 care homes. Finally, a cluster RCT33 of an enriched person-centred care programme that focused on improving QoL for care home residents with dementia reported significant improvements in mood and numerical benefit in QoL that did not reach statistical significance in > 200 residents in 10 care homes. The evidence base also supports the use of focused interventions using an ‘antecedent, behaviour, consequence’ approach to create individualised charts for care; these focused interventions are usually delivered by a clinical psychologist. 34 A meta-analysis of these studies highlighted significant improvements in agitation and a significant reduction in antipsychotic use, but no overall significant benefit in mood or QoL. 32
Specific non-pharmacological interventions
In addition to person-centred training interventions, which promote a holistic approach to person-centred care, there are a number of simple approaches available that can be used by health and care professionals or even family members. These include the Seattle Protocols,35 an approach that focuses on increasing physical and cognitive activities for people with dementia, such as balance exercises, gardening or walking; personalised social interaction, an approach that aims to ensure that each individual has dedicated time when they are interacting with other people through a personalised programme; and simulated presence therapy, an approach that uses recordings of conversations or events to promote conversation. More complex approaches include the Needs, Environment, Stimulation and Techniques (NEST) intervention, which is usually delivered by a recreational therapist, and the brief psychosocial treatment, an approach that creates a tailored programme of social interaction, which has been used as a lead-in phase in a clinical trial to effectively reduce placebo response to a drug treatment. 29,36
Non-drug approaches to improve well-being are frequently used as part of a person’s care plan. When non-drug approaches are used within a person-centred care framework these interventions provide an effective means to control for any unmet need that may lead to BPSD. Some specific interventions have also been evaluated for their effect on BPSD. Most of these interventions focus on promoting ‘pleasant activities’, either with or without elements of social interaction. The majority of trials of these approaches have shown improvements in both agitation and depression;37–41 for example, reminiscence therapy involves working with an individual to recall life events and memories, often through the use of props such as photographs or mementoes. Reminiscence therapy may also be enhanced through the use of audio- or video-recordings of events or family members, as in the simulated presence therapy described above. 42 For this reason, the reminiscence therapy approach also involves a great deal of social interaction. There is good evidence to support this approach in addressing symptoms of depression, with six out of seven published studies showing benefit. 43 A similar approach is used in validation therapy, although the evidence base for this is more limited. However, two small trials have reported beneficial effects on behaviour, including agitation, apathy and sleep disturbance. 44 The effect on other BPSD is less clear. The use of music in the care of people with dementia has shown some success in a number of trials that report improvements in both agitation and anxiety, but not depression. 43,45,46 A number of studies have also examined the use of physical exercise, although few have reported significant improvements in BPSD. 44
This emerging evidence base suggests that a number of interventions confer some benefit, but no single intervention has achieved improvement of mental health and reduction of antipsychotic use and none of the interventions conferred a direct benefit to the QoL of people with dementia in care homes.
Rationale for the WHELD programme
Key NHS priorities include improving mental health and the treatment of mental health problems to further reduce antipsychotic use and improve the QoL for people with dementia in care home settings. There is strong evidence that a number of specific person-centred care interventions confer some benefit, but no single intervention has achieved both an improvement of mental health and a reduction in psychotropic drug use in people with dementia. Furthermore, none of the interventions evaluated prior to this programme conferred a direct benefit to the QoL for people with dementia who were living in care homes. Most importantly, none of these interventions had achieved widespread implementation in a clinical or care setting as part of routine NHS practice.
Further research is, therefore, urgently needed to address these key issues. Such research will need to develop an optimised therapy that combines the most effective elements of the currently available evidence-based interventions to maximise the breadth of benefit. The intervention needs to be conceptually integrated, practical to implement in an NHS and care home setting and be cost-effective.
Achieving these objectives will require an integrated programme of research. We tackled this using the Medical Research Council (MRC)’s framework for complex interventions to model, develop, adapt, evaluate and disseminate the intervention.
The final stage of the MRC pathway, overcoming the barriers to enable widespread implementation, is also a major challenge, which cannot be overstated, as this has never been achieved for any therapy in a care home setting. Research is needed to tailor an optimised therapy to the needs of NHS staff, care home staff and people with dementia who are living in care homes and their families. In addition, research is also imperative to understand and overcome the potential obstacles to implementation and to refine the intervention model through an extensive period of testing in the field. Although much is known about the effectiveness of interventions, which benefit specific aspects of health and mental health, any intervention is of limited value unless it is practical and can be implemented routinely in clinical practice. A comprehensive programme of research is essential to overcome these barriers and to develop and implement an effective intervention that can be rolled out nationally as an NHS service to people with dementia in care homes, conferring the benefits to real people in everyday care settings. This is the basis for the design and delivery of the WHELD programme, which was funded by the National Institute for Health Research (NIHR) Programme Grants for Applied Research programme in 2012.
Aims and objectives
The overarching aim of the WHELD programme was to improve mental health and reduce the prescription of antipsychotic drugs for people with dementia living in care homes, by developing and evaluating an optimised intervention that is based on the most effective currently available therapies that can provide a broad range of benefits and can be routinely implemented as part of NHS care. We will also determine whether or not the intervention improves QoL.
To achieve these aims, the specific obectives for the WHELD programme were to:
-
update current systematic reviews regarding the most effective interventions to reduce BPSD experienced by people with dementia in care homes
-
optimise the most promising interventions and adapt them for an NHS and care home context through expert consenus and consultation with health and care professionals
-
determine the specific benefits of the interventions in incremental evaluation studies
-
develop an optimised intervention that is supported by an operationalised manual, with the specific aim of maximising the breadth of benefit while remaining practical and cost-effective
-
evaluate the optimised intervention and determie the cost-effectiveness in a well-powered, cluster RCT
-
understand the factors that contribute to feasible implementation and sustainability through consultation with study participants, and to field test the intervention to customise it as a practical NHS intervention
-
proactively disseminate the findings and intervention to enable widespread implementation.
In addition, the WHELD programme aimed to support capacity development within dementia reserach by providing research training to postdoctoral researchers to enable them to develop careers as independent researchers, and to build skills and knowledge among NHS care staff through study participation.
Guiding principles for the design of the WHELD programme
The design and approach taken for the WHELD programme followed a number of guiding principles to maximise the impact and relevance of the reserach to current practice and evidence. These guiding principles were as follows:
-
Development of interventions – it would have been inefficient and unneccesary to develop new interventions to promote mental health from basic principles, as effective interventions already existed at the commencement of the programme. However, these interventions required adaption for a UK NHS and care home setting and optimisation to improve the breadth of benefit.
-
Evaluation of QoL – previous trials had not been able to demonstrate improvement of care home residents’ QoL. The WHELD programme was, therefore, adequately designed and powered for such an effect to be demonstrated.
-
Meaningful evaluation of interventions – prior to a large RCT, incremental evaluation was prioritised as a means of determining the effective elements within the overall WHELD programme intervention, how these elements improve the breadth of benefits and which of these key elements deliver value for money.
-
Importance of field testing – it was deemed essential to undertake an extensive period of field testing to ensure that the WHELD programme intervention was fully tailored to the needs of the NHS and the care home sector, and that an intervention was delivered that could be a nationally implemented cornerstone of NHS treatment for people with dementia who are residing in care homes. This was particularly important, as it has not previously been possible to achieve widespread implementation of any intervention in a care home setting.
-
Dovetailing with national strategy and ongoing research – the WHELD programme recognises the need to work within the dynamic climate in UK health-care provision and alongside ongoing complementary research. The programme was designed to address the priorities published in the national dementia strategy for England and dovetails with studies examining specialist approaches for the psychological and pharmacological management of BPSD and other physical and mental health problems.
Work plan
The WHELD programme followed a pathway through modelling, feasibility, evaluation, dissemination and implementation, as illustrated in the MRC framework for complex interventions. The programme, which involved six work packages (WPs), is summarised in Figure 1.
FIGURE 1.
Research pathway diagram.
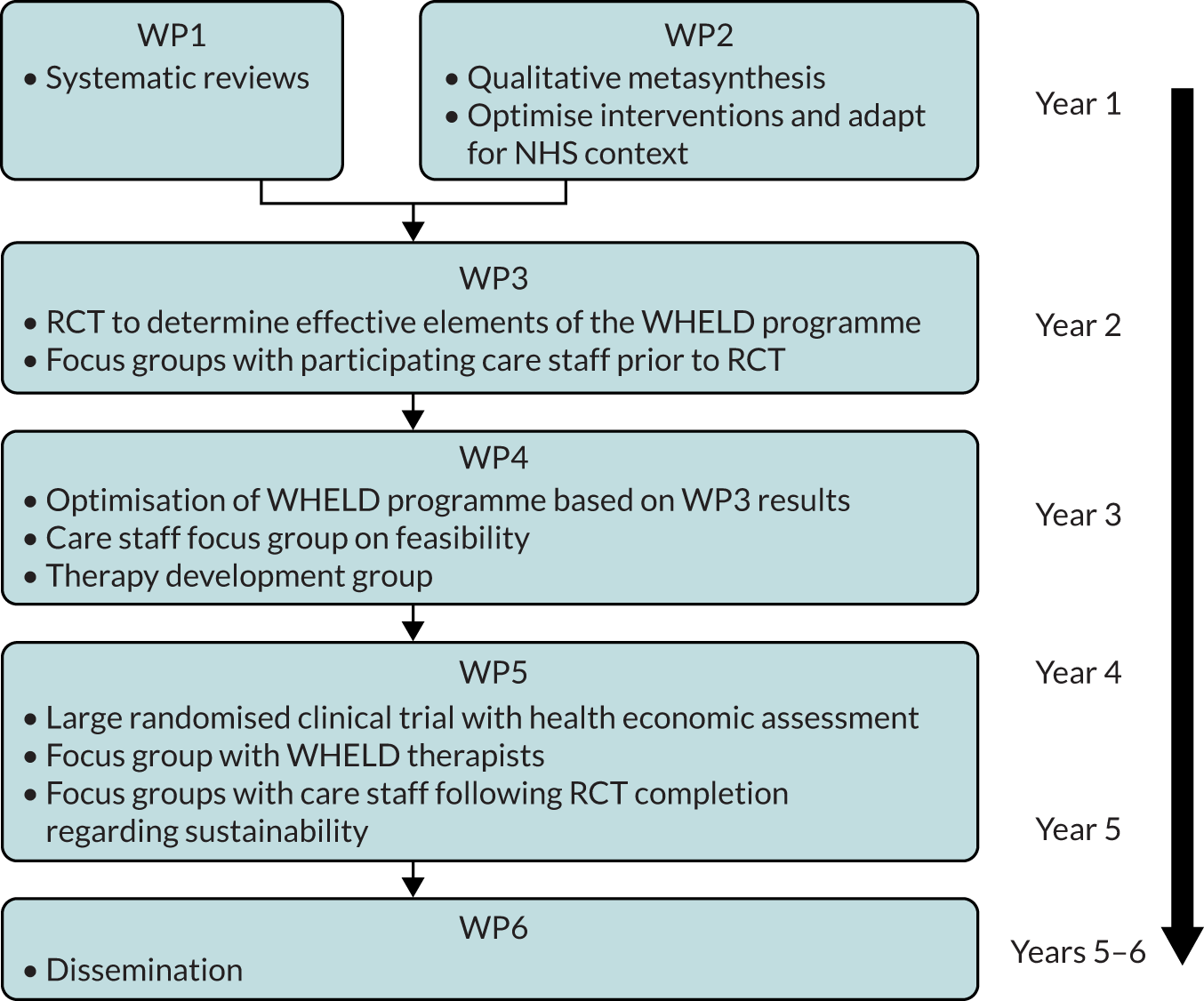
A description of each WP is as follows:
-
WP1 – update and publish a systematic review to show the service models and interventions for people with dementia living in care homes with the best available evidence base.
-
WP2 – model and adapt exisiting effective interventions to a UK NHS context.
-
WP3 – evaluate the breadth of benefits in an incremental design to establish the most effective and cost-effective combination of intervention elements (factorial study).
-
WP4 – develop an optimised intervention (WHELD), ‘welding together’ the most effective elements of the best available interventions, and develop a standardised manual and training programme for care staff.
-
WP5 – evaluate the full breadth of benefit conferred by the intervention through a large cluster RCT with parallel cost-effectiveness and process evaluations.
-
WP6 – disseminate the findings and the WHELD programme to enable widespread national implementation.
Limitations in design
In designing the study, we were mindful of the challenges of measuring QoL in people with moderate to severe dementia and, therefore, relied predominantly on a proxy measure. Within any RCT of a non-pharmacological intervention, it is a difficult decision whether to opt for treatment as usual or for an active control in the comparision arm. We used an active control (person-centred care) in WP3, which involved 16 care homes, but opted for a treatment as usual control in WP5, as a result of the scale of the study in 69 care homes. This may have inflated the magnitude of observed benefits with the WHELD programme in WP5.
The training and supervision in the intervention was delivered directly to care staff, who participated in qualitative work. However, we recognise that residents with dementia who received the intervention that was delivered by the staff were not included in the qualitative studies and could provide a valuable perspective.
The details of each WP are discussed in the following sections.
Work package 1: systematic reviews of psychosocial and person-centred care interventions for people with dementia living in care homes
Abstract
Objectives: WP1 sought to ask two specific research questions: (1) what is the evidence supporting the use of psychosocial interventions for BPSD? and (2) what is the quality and efficacy of existing psychosocial interventions and staff training programmes in person-centred care in improving BPSD and antipsychotic use?
Methods: Two systematic reviews were conducted. One examined publications relating to psychosocial interventions and the other conducted a search to identify person-centred care training manuals. Quality criteria were applied and the efficacy of manuals was considered.
Results: The systematic review of psychosocial interventions identified 40 studies and highlighted the evidence supporting the use of reminiscence therapy (ES 0.33), personalised pleasant activities (ES 0.46) and training in person-centred care. The modest number of adequately powered RCTs was highlighted. The efficacy and quality review identified 30 manuals meeting the quality criteria, of which only four were supported by RCT evidence. The studies reported benefit to agitation, depression, overall BPSD and antipsychotic use.
Conclusions: The reviews showed the limitations of the evidence base related to person-centred care training, but the combined reviews indicate that there are some benefits in the use of psychosocial interventions for people with dementia living in care homes.
Background
Behavioural and psychological symptoms of dementia represent a major challenge to dementia care and are associated with important clinical outcomes. 14,47 There is a high level of unmet need, particularly for individuals living in care homes. 2
A major element of dementia care is the development of safe, effective psychosocial interventions that improve residents’ care, reduce behavioural and psychological symptoms in people with dementia and offer an alternative to antipsychotic medication. There is a growing focus on a personalised approach to these interventions. Another element of dementia care is the need for high-quality training, in addition to effective support for clinicians. These requirements are highlighted in government directives worldwide. 7,17,48
As the starting point for the WHELD programme, WP1 aimed to synthesise the landscape of non-pharmacological treatment approaches and the resources that are currently available. This work directly informed decisions regarding the design and delivery of the WHELD programme.
Aim and objectives
The aim was to address and update gaps in the evidence base to inform the development of the WHELD programme.
The two major objectives were to:
-
update a systematic review highlighting service models and interventions for people with dementia living in care homes
-
conduct a two-pronged systematic review of quality and efficacy of existing person-centred care interventions, with a focus on the impact on BPSD and antipsychotic use.
Systematic review of psychosocial interventions to address behavioural and psychological symptoms experienced by people with dementia living in care homes
Rationale
The relevant literature includes a substantial number of studies investigating treatment approaches for BPSD. However, the majority of these studies focus on pharmacological intervention and of those evaluating psychosocial approaches only a fraction use robust, good-quality methodology to investigate effectiveness. 49–52 Of the recent systematic reviews, only O’Neil et al. 53 focused on the treatment of individual BPSD and none focused on the benefits of personalised psychosocial interventions. Despite the high profile of antipsychotic use as an issue in clinical practice, to our knowledge, no systematic reviews have considered this outcome. 28 A considerable number of new studies have been published since the most recent systematic reviews, which include studies published before 2009. 53
Methods
The work followed a systematic review protocol43 and focused on studies published from 1 January 2012 to 31 December 2012.
Development of review criteria
A preliminary search was undertaken to identify studies that examined the impact of psychosocial interventions on BPSD and antipsychotic use in people with dementia living in care homes. The search was subsequently refined by a specialist panel to focus on personalised psychosocial interventions.
Selection of included studies
A qualified librarian and one investigator performed the initial search. Two investigators examined these results and excluded irrelevant articles. Personalised psychosocial interventions were grouped into six categories of interventions.
Quality of included studies
A traffic-light system was used to describe a risk-of-bias analysis, using an adaptation of the 2008 Cochrane review framework (as reported in 2012 by Corbett et al. 54). The six criteria (adequate sequence selection, allocation concealment, blinding, incomplete outcome data, freedom from selective reporting and freedom from other bias) were each rated as green, amber or red to produce an overall rating.
Data analysis
Quality assessment used operationalised Cochrane criteria54 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidance. A descriptive summary was developed for the impact of each type of intervention. Where available, the ES was included.
Results
Studies included in the review
In total, 641 studies were identified in the initial search, of which 597 were excluded. A total of 40 studies were included, of which nine were rated green, eight were rated amber and 23 were rated red. None of the included studies contained a health economic analysis.
Description of studies
The 40 studies comprised 26 RCTs and 14 studies with quasi-experimental designs. The studies were performed in 13 different countries, using 66 different outcome measures. A total of 20 (50%) were published in the last 4 years. The studies were distributed into the six categories: reminiscence (n = 6), personalised music (n = 7), personalised pleasant activities with or without social interaction (n = 10), validation therapy (n = 2), personalised exercise/physical activities (n = 12) and person-centred care training and practice development (n = 3).
Reminiscence
Six studies55–60 evaluated reminiscence. Two studies had a parallel-group RCT design. 55,58 There was substantial variability in sample size (range 30–115 participants, median 67 participants), duration (range 3–8 weeks, median 7 weeks), frequency of intervention and session length (30–60 minutes). Results consistently showed significant benefit to depression (ES 0.33). 56–59 Other outcomes were less consistently evaluated and benefit was variable. One of two studies examining BPSD reported benefit. 60 None evaluated agitation.
Personalised music
Personalised music was examined in seven studies,45,46,61–65 including four parallel-group RCTs. 45,46,61,65 The studies included 20–104 participants (median 55.5 participants) and lasted 4–42 weeks (median 6 weeks), with variable frequency and session length (30–50 minutes). Benefit was identified in three studies that examined agitation,45,46,64 including two RCTs. 46,64 There was no difference in treatment ‘dose’. The ES of personalised music on agitation ranged from 0.43 (in favour of the control) to 0.66 (median 0.21). There was some evidence of benefit to overall BPSD46 and anxiety,45 but not depression. 46 Subsequent work has suggested that there is a possible impact on depression. 66
Personalised activities
Ten studies were included,37–39,41,67–72 of which seven were RCTs. The studies had 37–231 participants (median 147.5 participants) and lasted 1–36 weeks (median 4 weeks), with variable frequency and session length (30–240 minutes). Four studies37–39,67 reported benefit to agitation (ES 0.24–0.91, median 0.46). One study41 reported benefit to depression and four studies37,38,69,71 reported benefit to mood.
Validation therapy
Two studies44,73 analysed validation therapy. One 12-week trial of 30 patients (session length 45–60 minutes) showed benefit to BPSD in combination with reminiscence therapy. 73 Another 16-week trial of 50 patients also saw benefit to agitation, apathy, irritability and night-time behaviour. 44
Personalised exercise
In total, 12 trials of exercise interventions were identified,74–85 including 11 RCTs. Only four74,76,78,82 met criteria for personalised approaches; these involved 56–682 participants (median 205 participants) over 5 weeks (median 15 weeks), varied in frequency and session length (30–60 minutes) and showed limited benefit.
Person-centred care training and practice development
Three cluster RCTs focused on person-centred care training,28,30,33 which included 289–349 participants (median 293 participants) and lasted 4–18 months. Two evaluated the impact on antipsychotic use, with one indicating a significant reduction in use. 28 One study30 reported benefit to agitation. All examined mood, but only one33 reported significant benefit.
Further evaluations were undertaken for interventions that showed significant benefit to explore factors associated with favourable treatment response, defined as at least four studies examining the same outcome, with > 50% of studies indicating benefit. Reminiscence had a beneficial impact on mood in all studies, indicating a broad spectrum of benefit; however, further work is needed to clarify whether or not reminiscence benefits people living at home, younger individuals and those with severe dementia. Studies of personalised pleasant activities, with or without social interaction, indicated significant benefit for people with moderate to severe dementia who were living in nursing homes. Further work is needed to clarify whether or not there are benefits in other settings, in people with severe dementia or in younger individuals.
Discussion
This WP successfully completed and published a systematic review of the evidence supporting the use of psychosocial interventions for BPSD in care home settings. 43
This review has progressed the understanding of the potential differential benefits of interventions on specific BPSD, in particular through reminiscence on mood and depression (ES 0.33)55–57 and personalised pleasant activities on agitation (ES 0.46). 37–39,67 This provides valuable new evidence on the best use of psychosocial interventions for BPSD.
Only three RCTs28,30,33 were identified that evaluated person-centred care training interventions. Although all three RCTs reported benefit in at least one key outcome, benefits were inconsistent. One RCT reported reduced antipsychotic use,28 one improved agitation30 and one improved mood. 33 Further work is needed to optimise these interventions. Evidence regarding personalised music was inconsistent and mainly focused on the treatment of agitation, although interpretation was challenging and further work is needed. Despite the larger evidence base for exercise, only four studies43 focused on personalised approaches to exercise and the impact on BPSD and showed that there was limited benefit. Importantly, there is no evidence that any of the interventions specifically conferred benefit to psychosis.
Limitations
The outcome measures and trial duration in the included studies varied considerably, precluding a meta-analysis. In addition, we did not examine health economic outcomes. Only one study43 examined continued benefit beyond the period of the intervention, which is a key consideration for the development of the WHELD protocol.
It should also be noted that the study limited the period of inclusion to studies published after 2000, to focus on more recent and relevant studies. However, pre-2000 studies were consistent with our conclusions; for example, benefits were reported for social interaction,86 personalised pleasant activities without social interaction,86 personalised physical activity,87 an educational programme88 and reminiscence. 89
An additional limitation is the variety in theoretical frameworks underpinning the interventions and their frequency, session length and duration. Despite this, the review presents clear information to inform clinical practice and the next phases of the programme. This work is valuable in improving the targeting of individual BPSD with specific personalised psychosocial interventions, and in driving key research questions.
Implications for delivery of the WHELD programme
This review demonstrated valuable differential effects between different personalised psychosocial interventions that could have implications for the tailoring of care packages according to symptoms. It showed the importance of person-centred care interventions, pleasant activities for the treatment of agitation and the use of reminiscence therapy for mood. These findings were pulled through to WP2 and directly informed the development of the WHELD programme.
In addition, the review indicated areas of need for the design of the WHELD programme and protocol, including the need to focus on health economics, to improve the understanding of the impact of duration and frequency of specific interventions on ES and to develop a more detailed understanding of the impact of the conceptual frameworks on the outcome, and whether or not this can be used to optimise interventions. The review also emphasised the importance of understanding the level of care staff education that is needed to deliver specific interventions effectively, as well as the effect of the care environment and leadership on implementation.
The review was conducted based on a literature search up to December 2012, as part of the WHELD programme and to inform therapy development. A rapid updating search in October 2019 and citation tracking from out-published papers did not identify any relevant new studies that would change our conclusions.
Systematic review of person-centred interventions and training manuals for care home staff working with people with dementia
Rationale
Global government initiatives emphasise the importance of training for care staff and the need to improve access to effective psychosocial therapies for people with dementia. 5–7,48 These recommendations have resulted in a proliferation of training programmes that are promoted to care providers; however, the evidence to support the effectiveness of these programmes is unclear.
In the UK, increasing the skills of the workforce through training would cost an estimated £546M. Therefore, it is vital to have a clear understanding of the available training courses and their evidence base to support clinical and care interventions.
In line with the overall WHELD objectives, we focused on the implications for BPSD and antipsychotic use for this element of WP1.
Methods
See the published output for the full methodology. 32
The review had two objectives:
-
quality review – to review the quality of the available training manuals for person-centred care of people with dementia
-
efficacy review – to undertake a systematic review of RCTs that deliver training interventions to improve person-centred care.
Quality review
Manuals and training packages were identified through electronic searches, screened for eligibility and scored for comprehensiveness and degree of operationalisation. Studies scoring ≥ 3 for both criteria were deemed to provide broad person-centred interventions to address BPSD and/or antipsychotic use and were suitable for implementation. All papers and manuals that were published up to 30 June 2012 were included.
Data were extracted and manuals were separated into categories according to the type of intervention or training. They were rated independently by three investigators. The type of research evidence available was noted and summarised as anecdotal, qualitative study, open trials, quasi-experimental or RCTs.
Efficacy review
All RCTs and quasi-experimental studies that had a control group that addressed BPSD and/or antipsychotic use were included in the efficacy review. The methodological quality of studies was assessed applying the Cochrane system. 54
Data that pertained to BPSD or antipsychotic prescribing were extracted for meta-analysis. The meta-analysis was undertaken with the Comprehensive Meta-analysis package (version 2, Hewlett Packard, Palo Alto, CA, USA) for key outcomes (agitation, depression and total neuropsychiatric inventory) reporting standardised mean differences with 95% CIs and for antipsychotic drugs when data were available from two or more studies.
Results
Quality review
In total, 170 packages were identified. In total, 63 met the screening criteria and 30 were shortlisted, having obtained sufficient criteria scores. Of these, four were supported by RCT evidence.
Efficacy review
Seven studies were identified,28,30,37,90–93 of which five were parallel-group RCTs. Three studies28,30,93 evaluated the impact of person-centred care training on antipsychotic use, with two studies indicating significant reductions of 12.8%93 and 21.5%,28 which was confirmed by a meta-analysis (standard difference in means 1.08, z = 2.97; p = 0.003). Quantitative evaluation of agitation was available for four studies,28,30,37,92 with an overall significant benefit. Benefit to depression was reported in assisted living environments, but not in care home settings. One trial reported global impact on BPSD and showed a significant 8.7-point improvement. All six studies received a ‘green’ score for quality and risk of bias using the Cochrane quality review process. 54
Excluded studies
Several other interventions did not meet the inclusion criteria, including Reducing Disability in Alzheimer’s Disease (RDAD)31 and cognitive stimulation therapy. 94 Reasons for exclusion included focusing on specific domains, not focusing on BPSD or antipsychotic use, being in non-care home settings or interventions delivered directly to people.
Outcome of combined quality and efficacy review
Only four manuals met the quality criteria and had published clinical trial evidence of efficacy:
-
Focused Intervention of Training for Staff28,95 – a 10-month person-centred care training package that was delivered by a mental health professional, conferring a 19.1% reduction in antipsychotic use (95% CI 0.5% to 37.7%).
-
A collection of evidence-based protocols for non-drug strategies – NEST29,91 and the manual ‘Simple Pleasures’,90 conferring improvements in agitation [Cohen-Mansfield Agitation Inventory (CMAI); p = 0.01] and depression (Geriatric Depression Scale; p = 0.001). 91
-
Dementia Care Mapping – a detailed observational and care planning approach for care homes that showed a significant reduction in agitation (CMAI; p = 0.01) and falls (p = 0.02). 30
-
Improving Dementia Care96 – a practical training and staff development resource that showed a reduction in agitation, although the outcomes varied between sites (CMAI; p = 0.01). 30
Discussion
This review identified evidence that demonstrated the benefits of person-centred care interventions on agitation and antipsychotic use in people with dementia living in care homes. However, this was based on intervention studies that were performed on only a fraction of the training programmes that are currently available. Only 30 (18%) of the manuals followed good educational and person-centred care principles and only four (2.3%) had clinical trial evidence. Thus, over 80% of the available training packages are of variable quality and 98% are not evidence based; this is extremely concerning. Health care and care home sectors are investing in training following government directives, largely in programmes that carry no evidence of benefit. There is a need for person-centred care intervention training to be evidence based if it is to provide better social and medical care. Of note, interventions for which there is evidence of benefit were delivered over a period of at least 4 months, and involved ongoing clinical supervision or support to embed implementation into care home practice. This suggests that one-off training packages or classroom-based training would probably be ineffective.
The literature does not currently provide any evidence for effectiveness on psychosis, depression and QoL. This is an important priority for further research, as highlighted by the Department of Health and Social Care. 97
Limitations
Although the review incorporated national and international manuals, the review was limited to the English language. The search for published manuals was complemented by a search for RCTs, focusing on training and activity-based trials, thereby mitigating the limitations and ensuring a broad international perspective. In addition, training programmes without available manuals were excluded. Several manuals had a broader framework for care delivery rather than a specific focus on BPSD. It is, therefore, likely that wider benefits were not captured.
Implications for delivery of the WHELD programme
This review showed the major disconnect between the interventions that are routinely available and commissioned and the evidence base. This added further weight to the overall rationale of the WHELD programme.
The review provided key indicators of elements that are required for a successful person-centred intervention, including the importance of consistent, long-term support for staff. It indicated the need to evaluate effectiveness on psychosis, depression and QoL, in addition to agitation and antipsychotic use.
The limited number of available training manuals without direct clinical trial evidence of benefit for people with dementia is alarming, and emphasises the importance of a feasible, effective training programme.
Concluding remarks from work package 1
Work package 1 successfully delivered the objective of updating the current evidence base with systematic reviews. It highlighted pleasant activities and social intervention as priority areas for addressing agitation, and reinforced our view that augmentation of existing interventions is needed to deliver comprehensive benefit and improve QoL.
Finally, WP1 identified an alarming number of available training manuals that are not evidence based, and strongly emphasised the need for an evidence-based manual. These findings were taken forward by the Therapy Development Group (TDG) in WP2.
Work package 2: modelling and adaptation of interventions for use within an NHS context
Abstract
Objectives: WP2 sought to identify the factors that influence implementation of psychosocial interventions in care home settings and to develop the WHELD programme for evaluation, based on the outputs and findings from WP1.
Methods: A metasynthesis approach was used to conduct a review of studies that examine the implementation of psychosocial interventions in care homes. Data were analysed by thematic analysis using an interpretive method of metadata synthesis, grouping themes where they had the greatest explanatory power. These findings and WP1 results were used by a TDG to create and protocolise a WHELD programme package.
Results: The meta-analysis revealed key issues in promoting the use of interventions in care homes, including the core involvement of staff, buy-in by family members, flexibility of care home working arrangements, training, supervision and support for staff and the need for cultural change. These findings, combined with WP1, informed development of the intervention. The WHELD programme involved four key elements: person-centred care training that was based on adapted versions of published manuals, antipsychotic review by general practitioners (GPs) that was based on national best practice guidelines, social interaction and exercise, both of which were adapted from published interventions.
Conclusions: The work completed in WP2 follows a clear pathway, from theoretical basis to conceptual framework to a tangible operational intervention. The WHELD programme represents a synthesis of knowledge that was drawn from clinical trial data, existing resources and qualitative analysis, and was collated for use by a broad expert consensus process.
Background and rationale
Work package 1 illustrated the strength of evidence for several psychosocial interventions and manualised person-centred care programmes that confer benefit on mental health and antipsychotic medication use for people with dementia living in care homes. Promising interventions were identified, including staff training, person-centred care and structured social interaction approaches and exercise. Importantly, none of the interventions directly improved QoL for care home residents or achieved widespread implementation as part of routine care practice. WP1 also showed the lack of evidence-based training programmes and the importance of an integrated approach to ongoing support for care staff.
The work carried out in WP1 pointed towards the need for a whole-systems approach to improve person-centredness in care homes with integration of the most effective individual interventions, and to develop an understanding of the factors that influence implementation.
Therefore, WP2 encompassed a further review to gather qualitative data on aspects of psychosocial intervention design, followed by a series of therapy development steps to adapt and combine the key components of established effective interventions.
Aim and objectives
The overall aim of WP2 was to create a conceptually integrated evidence-based intervention that was practical to implement in UK care home settings.
The objectives of WP2 were to conduct a:
-
systematic review and metasynthesis of qualitative research to identify factors that influence the implementation of psychosocial interventions.
-
series of expert panel and TDG sessions to develop a person-centred care intervention by combining the best elements of existing interventions in a way that is both conceptually integrated and practical.
Implementation of psychosocial interventions: systematic review and meta-analysis
Rationale
Qualitative methods are the most appropriate approach for addressing complex questions regarding the implementation and acceptability of psychosocial interventions in care homes. Individual studies provide insight into how different psychosocial interventions are used and experienced within residential settings. Integrating these findings in a qualitative metasynthesis promises to enhance their implementation and impact on health policy and clinical practice. 98,99 WP2 used this approach to help to understand and overcome the potential obstacles to implementing psychosocial interventions as part of routine practice. To our knowledge, this is the first systematic review and metasynthesis of the qualitative evidence in this field.
Methods
Full details of the methodology are provided in a published output. 100 Systematic review methodology was used to identify relevant research regarding the use and effectiveness of psychosocial interventions that were designed to improve outcomes for people with dementia in care homes, from the perspective of people with dementia, relatives or care staff. 101 Papers published between 1 January 1995 and 31 January 2011 were included in the review.
Two investigators independently assessed relevant papers for methodological quality using the Critical Appraisal Skills Programme (CASP) checklist. 102 We opted for an inclusive strategy103 and, in common with another synthesis,99 the quality appraisal process was used as a criterion to judge the value of papers with respect to their contribution to the synthesis. Themes were included in the meta-synthesis if they were supported by data from at least one article that was judged as being of reasonable quality.
A description of the main concepts derived from each paper was recorded,104 and shared constructs across studies and areas of discordance were noted. The themes were combined using an interpretive method of metadata synthesis by grouping themes where they had the greatest explanatory power. A taxonomy was constructed that categorised findings in three domains, which are discussed below.
Results
The review identified 39 papers (34 studies),105–143 of which 29 were rated as being of reasonable quality or better (CASP ≥ 7), with a good level of agreement between reviewers (weighted Cohen’s kappa = 0.66). The 10 papers that did not meet the quality criteria were primarily rated as weak, owing to a lack of rigour in data analysis or a failure to comment on the bias in the study design. 108,116,117,120,123,124,126,128,133,134
The 34 studies spanned a broad range of psychosocial intervention types, which included exercise and other therapeutic activities (n = 6),110–112,117,125,138 music (n = 5),129–132,136 reminiscence (n = 4),113,121,128,135 communication strategies (n = 3),106,108,141 models of dementia care (n = 3),114,133,143 methods of orientation (n = 2),109,124 animal interventions (n = 2),122,123 staff training and supervision (n = 3),118,127,140 and other (n = 6). 105,115,119,137,139,142 Common themes were identified in three overarching categories: elements of a successful intervention, conditions required for a successful intervention and challenges to a successful intervention.
Elements of a successful intervention
Aspects that predicted successful interventions were categorised by whether they elicited benefit to people with dementia or to care staff.
People with dementia
Beneficial interventions often supported people with dementia in ‘connecting with others’ by enhancing communication. Conversation was stimulated between residents, staff and family through various means, including animals, dolls, music, reminiscence items or ‘memory boxes’. 107,116,122–124,128,136,139 Studies reported particular success with volunteer-led approaches. 122,123,137 Music and dance interventions were useful in supporting people to better express emotions,107,111,112 with group activities improving co-operation and a sense of inclusion. 125,131,139,143
Successful interventions also focused on ensuring that participants felt that they were making a meaningful contribution to an activity, for example through taking care of an animal. 107,113,122,128,135,137,139,143 Structured or spontaneous reminiscence was also a valuable element. 112,116,122,123,132,135,139
Care staff
There was a consensus that care staff find psychosocial approaches valuable in enabling them to build better relationships with individuals and their families. 113,115,118,119,128 Closeness between staff and residents was fostered by experiential learning, which encouraged staff to consider the perspectives of a person with dementia114,118,143 and gave them the opportunity to reflect on their approach to residents and the likely impact on them. Reflection reportedly led to changes in care-giving behaviour,118,127,128 particularly when supervision from senior staff was available. 113,127,140
Conditions required for a successful intervention
All of the studies illustrated the reliance on staff to support people with dementia to access the psychosocial interventions. 107,125,126,134 Person-centredness and the importance of relationship building was a major theme, as both a benefit and a determinant of being able to individualise their approach. 107,111,112,117,121,125,132,134,135,138
The involvement of family members was also key to personalising care107,136,142 and a source of mutual appreciation and respect. 121,127,142 A number of studies described successful approaches to stimulate collaboration between relatives and staff. 111,114,122,125,141
Challenges to a successful intervention
A number of challenges were identified, many of which related to the additional work, the requirement for flexibility of team rotas111,136,142 and low levels of staffing as significant barriers to implementation. 119,120,136,140,142 However, with the exception of two studies,126,134 the benefits conferred were thought to over-ride the difficulties. 111,120,136,140,142
The wide range of staff responsibilities and reporting requirements were frequently cited as a barrier to the implementation of psychosocial approaches. 119,133 Staff reported a perceived pressure to focus on responding to challenging behaviour, physical care and safety, rather than supporting individual interactions. 106,115,119,134,138
Finally, a number of challenges were described regarding negative attitudes towards psychosocial interventions and a general lack of knowledge about their potential benefit. 111,126,129,134,142
Discussion
Overall, the metasynthesis identified how important positive benefits from psychosocial interventions can be achieved. The major learning outputs that were taken forward were as follows:
-
It is critical to involve staff and care home managers as active participants and to ensure that they have personal investment in the intervention through collaborative, sympathetic approaches that openly accept the burden and challenges that they experience.
-
Buy-in by family members is also critical – workshops and group events are effective in promoting this.
-
Flexibility is an essential aspect – ensuring that the intervention is adaptable to different home structures and staffing levels, and tailored to resident abilities and interests.
-
Organisational support is required to sustain learning and change in practice, particularly given that training and mentoring involve considerable time investment by staff. It is important to make a clear health economics argument to support this.
-
Cultural change is required to encourage staff to think about residents individually and to address concerns about a new intervention alongside identifying benefits.
-
Person-centred care is fully accepted as the framework for intervention, with established means of tailoring interventions to an individual’s needs.
-
Training is critical and particularly effective when it includes scenario-based, interactive elements and the opportunity for staff to reflect on their practice.
Limitations
Qualitative metasynthesis offers a systematic, relevant overview of international qualitative research, while retaining much of the detail that individual studies provide. However, there is a risk that synthesising across qualitative studies could compromise the integrity of the individual projects, as well as their emphasis on context and holism. 98 We are mindful that this review identified a heterogeneous set of studies that varied in care setting, intervention type and methodological design. To convey the context of the study, comprehensive details are published in Lawrence et al. 100
We also recognise that the sample size is large for a metasynthesis, but are satisfied that the scope of the review was sufficiently focused and believe that the range of data assisted in identifying the properties and key concepts that can be applied across groups and settings.
Implications for the WHELD programme
The recommendations outlined above were taken forward to the intervention development phase of WP2 (described below). The identification of key elements of successful interventions, combined with the outputs of the review in WP1, indicated the need for incremental evaluation of individual elements of psychosocial interventions to enable an examination of the breadth of their benefits.
The WHELD therapy development
Rationale
The work completed in WPs 1 and 2 provided a comprehensive picture of the requirements and existing resources that are available for the creation of an optimised person-centred care intervention. The next phase of the work sought to operationalise this information through a period of collaborative development work with a TDG and associated expert panel.
Aim and objectives
The aim was to develop an optimised person-centred care intervention for people with dementia living in care homes, which would include a training programme for care staff.
Specific objectives within this aim were to:
-
co-ordinate a large-scale expert consultation and TDG to gather informed opinion on the most appropriate design and content for the WHELD programme
-
operationalise the WHELD programme by developing a manual for use in care homes
-
finalise the design of the evaluation of the WHELD programme for WP3.
Methods
Selection and scrutiny of the interventions
During October and November 2010, a TDG was convened that consisted of the WHELD investigators, lay representatives and care home experts, including providers and inspectors.
The expert panel reviewed existing programmes that had evidence of efficacy, to identify elements to be included in a person-centred care programme. The FITS28 and NEST,29 including the Simple Pleasures90 and Improving Dementia Care,30 interventions were scrutinised to identify which elements had proven to be the most effective, which could also be combined into a consistent conceptual framework for person-centred care. The manuals were also reviewed against the emerging literature identified in WP1 and the metasynthesis in WP2. The language and style of the interventions were reviewed, with consideration of the UK context of delivery.
Expert consultation: workshops
A series of workshops was co-ordinated to gather opinions and experience from a wide audience. In total there were 89 workshop participants, including national and international experts from research backgrounds; clinicians from nursing, occupational therapy, physiotherapy, clinical psychology and psychiatry; care home managers and practitioners; and family carers. Two group workshops were conducted with 40 participants in each group to achieve a robust consensus.
The meetings commenced with a presentation of work from WP1 and an overview of the evidence base. Participants were asked to consider the evidence and provide recommendations regarding the selection of interventions to include in the WHELD programme. In a second session, participants developed recommendations for refinement of the person-centred care training that was outlined in the FITS programme,28 to improve implementation. Suggestions were prioritised by consensus, working to create a list that informed the manual development for the intervention.
Intervention creation
The TDG collated and operationalised the outcome of the expert panels to produce the WHELD programme. Manuals of the four selected interventions and overall protocol were produced. Members of the TDG also liaised with three key external experts (Linda Buettner, Jiska Cohen-Mansfield and Linda Teri) regarding their existing interventions and how they might be adapted for the WHELD programme. 29,31,37 The formative materials were then edited to reflect UK practices where necessary. When there was overlap between materials, a decision was made based on the workshop feedback about which material would best meet the criteria for being effective and feasible for a UK care home setting. The full intervention was circulated to all WHELD study investigators for comment and, finally, to members of the TDG. The final version of the manual was approved by the WHELD Programme Management Group (PMG).
Results
Intervention selection
Four key interventions were to be used within the WHELD programme (person-centred care training for staff), which acted as the underpinning framework for the delivery of all interventions, social interaction, physical exercise and review of antipsychotic medication.
Four existing evidence-based interventions were identified for the adaptation to create the WHELD programme. These were the FITS person-centred care manual,28 a non-drug protocol focusing on pleasant activities,37 the NEST intervention29 and the Seattle Protocols. 35 Specific recommendations were made for refinements to individual elements based on safety and UK context-specific considerations.
Adaptation of interventions for the WHELD programme
The TDG developed a manual of the four interventions and the overarching delivery protocol, which are outlined below.
Person-centred care training for staff
This intervention combines elements of several effective interventions that promote person-centred care. These included the FITS person-centred care training package28 and opportunities for experiential learning about adapting care practice to meet the needs of someone with dementia. Using residents’ life stories was a foundation to understand individuals’ needs and preferences. A structured assessment of unmet needs29 underpinned positive individualised care planning by using specific, measurable, achievable, relevant, time-bound (i.e. SMART) goals to implement activities based on a person’s preferences. 90
Review of antipsychotic medication
Previous studies28 that have achieved a reduction in antipsychotic use have incorporated a formal review of antipsychotic medications according to standardised protocols. The National Service Framework recommend that reviews take place every 3 months. The TDG agreed that a review would be undertaken using best practice guidelines for the prescription of antipsychotics to people with dementia. 27 The aim was for care staff to understand the need for regular review and develop a process that was led by the care home that enabled this to be undertaken by the resident GPs. Workshops were also designed for GPs to support care staff in following the best practice guidelines.
Social interaction
This intervention was based on the Positive Events Schedule, the NEST intervention, the Seattle Protocols and Cohen-Mansfield’s ‘toolbox’ of psychosocial interventions, which were individualised to the needs of a particular individual. 29,35,144,145 A version of the ‘toolbox’ approach had been evaluated in several small pilot studies, as well as in a large, open, 4-week trial as part of the CALM-AD clinical trial146 and as part of the FITS study. 28 Activities promoting social interaction were adapted for a UK care setting, with the aim that the activities should be delivered for 60 minutes per week or that there would be a 20% increase in activity if the resident already engaged in this level of activity.
Exercise
The RDAD programme,147 which focuses on activities, strength, balance and flexibility training, was identified as the best evaluated programme for people with dementia, showing significant benefits for activity, mood and health in a RCT. 31 RDAD exercises were adapted to be simply and safely delivered by care staff who had no formal physiotherapy or exercise training. Walking or pleasant activities of the residents’ choice, including seated exercise or circle dance, were incorporated from the exercise section of the NEST protocols. 29 The aim was that this should be delivered for 60 minutes per week or that there would be a 20% increase in activity if the resident already engaged in this amount of exercise.
Operationalisation of the WHELD programme
The TDG produced a manual for the delivery of the WHELD programme as an incremental intervention for evaluation. The delivery was designed as a training programme that was co-ordinated by a central WHELD therapist, who then provided support and supervision for care home staff. Care homes also nominated WHELD dementia champions to act as internal mentors who had responsibility for the implementation of interventions within the care homes. The TDG, therefore, produced one detailed therapist manual, which provided detailed information on the delivery of each intervention, and one dementia champion manual, which was for care home staff. The manuals aimed to maintain distinctiveness between the elements that were delivered, but did not preclude homes from developing activities and interventions of their own volition.
Discussion
The work completed in WP2 follows a clear pathway, from a theoretical basis through a conceptual framework to a tangible operational intervention. The WHELD programme represents a synthesis of knowledge that is drawn from clinical trial data, existing resources and qualitative analysis, and is collated for use by a broad expert consensus process.
Concluding remarks for work package 2
Work package 2 successfully collated evidence from different sources, including systematic reviews, qualitative reviews, expert consensus and existing operationalised interventions, to prioritise interventions for the new WHELD programme. The large consensus process gives additional validation to the final intervention, which was taken forward for evaluation in WP3.
Major outputs of work package 2
The WHELD programme materials
The major outputs of WP2 were the WHELD Therapist Manual and the WHELD Dementia Champions Manual.
Work package 3: factorial pilot evaluation of non-pharmacological interventions in combination with person-centred care training in care homes
Abstract
Objectives: To establish the feasibility, effectiveness and cost-effectiveness of person-centred care training for care staff alone and in combination with antipsychotic review, social interaction and exercise.
Methods: A 9-month, cluster, factorial RCT of the WHELD programme in 16 care homes. All care homes received person-centred care. Eight care homes were randomly assigned to receive antipsychotic review, social interaction or exercise. The primary outcome measure was antipsychotic use. Secondary outcome measures were BPSD and mortality. The costs of care were defined and cost-effectiveness was analysed using the Client Service Receipt Inventory (CSRI). Focus group discussions with staff talked about their expectations.
Results: Antipsychotic review reduced antipsychotic use by 50% [odds ratio (OR) 0.17, 95% CI 0.05 to 0.6]. Antipsychotic review plus social interaction significantly reduced mortality but increased BPSD compared with receiving neither antipsychotic review nor social interaction (score difference 7.37, 95% CI 1.53 to 13.22). This detrimental impact was mitigated by concurrent delivery of social interaction (–0.44, 95% CI –4.39 to 3.52). The exercise intervention significantly improved neuropsychiatric symptoms (–3.58 symptoms, 95% CI –7.08 to –0.09 symptoms) but not depression (–1.21, 95% CI –4.35 to 1.93). The focus groups emphasised that successful training must acknowledge and respond to ‘whole-home’ issues.
Conclusions: Person-centred care, antipsychotic review and social interaction showed benefit to QoL, antipsychotic use and mortality and have indications of cost-effectiveness.
Background and rationale
The work that was completed in WPs 1 and 2 of the WHELD programme builds on a growing body of literature. 32 Our meta-analysis showed the benefit conferred by social interaction and pleasant activities on both BPSD and antipsychotic use,43 and of physical activity through personalised exercise on mood. 31 This suggested that augmented person-centred care with social interaction, pleasant activities and physical activity would be effective.
Until 2008, cohort studies and audits in the USA and Europe reported that 40% of people with dementia in care homes were receiving an antipsychotic drug. 148–150 In recent years, the concerted effort to reduce unnecessary prescribing has led to a 15–50% reduction in prescriptions of antipsychotic drugs across the USA and Europe. 23–25 Although recent randomised antipsychotic discontinuation studies have reported benefits following the withdrawal of antipsychotics,16 there have been no randomised trials of rigorous antipsychotic review.
Working papers detailing the work plan and analysis plan are in Appendix 1.
Aim and objectives
Work package 3 reports a factorial evaluation of the WHELD programme, to evaluate the effectiveness of person-centred care training for care staff alone and in combination with antipsychotic review, social interaction and exercise interventions.
Factorial randomised controlled trial of the WHELD programme
Rationale
Our work had raised clear questions regarding the potential to build an effective, feasible real-world intervention to manage antipsychotic use and BPSD in care homes.
Hypotheses
-
Person-centred care plus antipsychotic review will result in the reduction of programme prescribing.
-
Person-centred care and social interaction will result in reductions in BPSD, particularly agitation and aggression.
-
Person-centred care and exercise will improve mood.
Methods
Full details of the methodology for WP3 are published in outputs. 151–153
A 9-month, cluster, factorial RCT of the WHELD programme in 16 care homes was carried out. All care homes received person-centred care. Eight care homes were randomly assigned to each of antipsychotic review, social interaction or exercise, so that 2 of the 16 homes had each possible combination of treatments. The eight care homes that were assigned to each type of intervention (factor) were compared with the eight care homes that were not assigned to that intervention (factor), to determine the impact of each intervention. A further analysis was undertaken to examine whether or not there were any additional benefits of receiving more than one type of intervention.
Inclusion criteria
The participants were people with dementia, as defined by the Clinical Dementia Rating (CDR)154 (i.e. a score of ≥ 1) and Functional Assessment Staging (i.e. a stage ≥ 4). 155
Interventions
The person-centred care intervention used tools that were developed for the FITS programme. 28 Supplementary materials were drawn from the best available training manuals32 and were augmented by leadership training using principles from our systematic qualitative review. 100
Antipsychotic prescriptions were reviewed by primary care physicians or psychiatry specialists, based on existing guidance. 7,27
The objective was to use the evidence-based protocols that were described in WP2 to provide 1 hour of social interaction per week for each resident, through positive, planned social interactions. Personalised activities were developed based on conversation with each resident, their life history and interests, to ensure that the activities and interactions were individually tailored. The aim was to enhance resident interactions with staff, family and volunteers, which were delivered through individual or group sessions based on their preferences. 29,31,37
Outcome measures
The primary outcome measure was antipsychotic use, which was recorded according to the British National Formulary156 classification.
Secondary outcome measures were:
-
depression – measured using the Cornell Scale for Depression in Dementia157
-
agitation – measured using the 29-item CMAI158
-
BPSD – measured through the 10 domains of the Neuropsychiatric Inventory, care home version (NPI-NH)159
-
health-related QoL –measured by the Dementia Quality of Life Scale (DEMQoL) – Proxy. 160,161
Mortality
The severity of dementia was measured by the CDR154 and the Functional Assessment Staging instrument. 155
All assessments were undertaken based on informant interviews with members of care home staff, which were conducted by trained members of the WHELD programme research team.
It should be noted that there are some differences between the secondary outcomes that are reported in the protocol and the secondary outcomes that are described in the narrative of the text and reported in papers. As stated in the protocol, mental health (depression, agitation, overall neuropsychiatric symptoms and apathy)151,162 and QoL were also reported as secondary outcomes in line with the protocol. 153 Physical morbidity was complicated and inconsistently described; therefore, we adopted mortality as a secondary outcome as a proxy for severe physical morbidity. Mortality is presented in the text and in a published output. 151 Falls was another secondary outcome in the protocol; this was inconsistently reported and, therefore, we did not feel that falls could be reliably included in the report. The coding complexities of recording non-antipsychotic psychotropic medication made it difficult to incorporate a meaningful analysis in the trial report; however, we have made these data available to an ongoing NIHR systematic review. One of the original goals was to also examine mediating factors that are related to delivery implementation. The large number of data made this impractical, but it was routinely used to guide supervision and improve the fidelity of intervention delivery. These changes were approved by the Trial Steering Committee (TSC).
Power calculation
Based on PASS 11 Version 11.0.10 2012 (NCSS, Kaysville, UT, USA) (power analysis and sample size software) sample sizes, 96 in each group would give 82% power to detect a difference between the two proportions of –0.20. The test statistic used is the two-sided z-test to compare two independent proportions for a cluster randomised trial in which the intracluster correlation is assumed to be 0.05. The significance level of the test is 0.05. After adjusting for a drop-out rate of 25%, the sample size required was 128 per trial arm or about 16 participants per home. Based on the ES (> 0.50) seen in the Caring for Aged Dementia Care REsident Study (CADRES) study,30 the study was designed to detect an ES of 0.5 for the other outcomes. A total of 128 participants for each of the group comparisons gives 80% power to detect a treatment difference at a two-sided 0.05 significance level, if the true ES is 0.5. Cluster randomisation reduces efficiency and leads to loss of power but was essential, as the intervention has to be implemented throughout individual care homes. The design effect, otherwise known as the inflation factor, is defined as the ratio of the total number of participants required using cluster randomisation to the number of participants required using individual randomisation. Statistical theory leads to the following formula:
called the intracluster correlation coefficient (ICC), where s2b is the between-cluster variance and s2w is the within-cluster variance. ICCs for authentic resident outcome measures (rather than process outcomes) rarely exceed 0.03. An estimated average of 16 eligible participants per cluster leads to an inflation factor of 1.45. Therefore, 186 partipants were required to give this level of power for each outcome. Given the frailty of the population and the estimated mortality rate, a total sample size of 240 participants was stipulated to allow for mortality and dropout. This sample size does not give power to correct for multiple testing with respect to the three primary hypotheses.
Statistical analysis
Analyses accounted for intervention, baseline demographic and clinical characteristics, and site as covariates and exposure variables. The analysis used multiple linear regression models for continuous outcome measures and logistic regression models for binary outcome measures. 163–165
The primary outcome was an intention-to-treat analysis, with age, gender and severity of dementia included as covariates.
Although our protocol paper does not document our planned between-group analyses, it was always the intention to report these analyses. The prespecified primary outcomes for each comparison of antipsychotic review, social interaction and exercise were antipsychotic use, agitation and depression, respectively. Of the three comparisons, we were in greatest need of statistical power for the antipsychotic outcome. Therefore, we sought a sample size of 96 participants per group to show a 20% difference in this outcome with 82% power.
This approach was approved by the TSC. The statistical analysis plan was agreed by the TSC, Data Monitoring Committee and trial statistician prior to data lock. The statistical analysis plan is available in Appendix 1.
Results
Factorial randomised controlled trial
The main factorial study demonstrated that antipsychotic review significantly reduced antipsychotic use by 50% (OR 0.17, 95% CI 0.05 to 0.60). The intervention of antipsychotic review plus social interaction significantly reduced mortality (OR 0.26, 95% CI 0.13 to 0.51) compared with the group receiving neither antipsychotic review nor social interaction; however, the antipsychotic review plus social interaction intervention showed significantly worse outcome in neuropsychiatric symptoms than the group receiving neither of these (score difference 7.37, 95% CI 1.53 to 13.22). This detrimental affect was mitigated by concurrent delivery of the social intervention (mean difference –0.44, 95% CI –4.39 to 3.52). The exercise intervention significantly improved neuropsychiatric symptoms (mean difference –3.58, 95 % CI –7.08 to –0.09) but not depression (mean difference –1.21, 95% CI –4.35 to 1.93). None of the interventions had a significant impact on agitation specifically. The cohort characteristics and results are described in detail in published outputs. 151,166
Additional economic analysis
An economic analysis was conducted using baseline data to understand the main cost drivers for people with dementia in care homes. This was an additional output that was not stated in the hypotheses. 166
Discussion
The WHELD programme conferred a significant 50% reduction in antipsychotic use, even in a population with a baseline antipsychotic use < 20%. Exercise conferred significant benefits to overall BPSD. In addition, the group who received antipsychotic review in combination with social interaction had a significant reduction in mortality. Social interaction alone also conferred benefit to health-related QoL.
The detrimental affect of antipsychotic review on BPSD and health-related QoL was an important finding, which was probably explained by the changed landscape of antipsychotic prescribing. 17,23–25 The antipsychotic review intervention was based on guidance that was created before the substantial reductions in antipsychotic use over the last 5 years. 27 Although this has achieved significant benefits, it has meant that the severity of BPSD in people who are now receiving antipsychotics is probably much higher than it was previously.
The mortality figures also have important implications, particularly given that mortality risk has been a key driver in the campaign to reduce antipsychotic use. 15 Although antipsychotic review alone reduced mortality by > 30%, this became statistically significant only in combination with social interaction.
Implications for the WHELD programme
Work package 3 clearly demonstrated the feasibility of the WHELD programme to reduce antipsychotic use in people with dementia and suggested the potential for benefit in QoL.
Qualitative analysis: implementation of psychosocial interventions in care homes
Rationale
This qualitative analysis collated the opinions of care home staff about the interventions and their view on the most effective means of implementing psychosocial interventions. The aim was to glean practical and theoretical factors that could then be integrated into the WHELD programme and optimisation in WP4.
Methods
Focus group discussions were conducted with care home staff prior to the factorial study. Purposive sampling was conducted to obtain the perspectives of staff in a variety of roles who had a range of experience and expertise. Focus group discussions were held with 8–12 members of the care team, where possible, to explore successful working practices, challenges and priorities within the care home, as well as specific attitudes and beliefs surrounding psychosocial interventions and the support that would be required to deliver them. The discussions were recorded and transcribed verbatim, and observations and impressions were routinely noted at the end of each group. Transcripts were subjected to thematic analysis through the constant comparison method. 167
Results
Participants in the focus groups comprised 53 care assistants (45%), 30 senior care assistants (25%), 13 activity therapists (11%), six registered nurses (6%), five deputy managers (4%), two managers (2%) and 10 other staff (8%). Three key themes emerged: ‘undervalued and understaffed’, ‘centrality of relationships’ and ‘existing practices and desire for support’. 168
The work related to the implementation of a specific social activity was published as a separate report. 169
Discussion
The outcomes of the thematic analysis emphasised the importance of acknowledging ‘whole-home’ issues, such as environmental, care practice and attitudinal factors. 28 A collaborative approach that seeks the views of staff from the outset provides positive feedback, does not judge past or present care practices and offers an effective method of engagement. 170 The work revealed optimism among staff that the training programme might, first, enhance their status among relatives, managers and care commissioners and, second, encourage these parties to commit greater resources to delivering psychosocial care.
The review of non-pharmacological interventions conducted in WP2 chimes with the findings of this WP, indicating the critical barrier of limited resources in care homes. 171
The expressed enthusiasm for one-to-one time with the residents is a positive outcome that could be fostered to promote person-centred care and job satisfaction. 172 Research elsewhere has indicated that the more staff relate to residents as individuals, the less they perceive difficult behaviour as challenging. 173 However, training and support interventions need to acknowledge that empathising with residents and becoming involved in their lives can contribute to burnout if not accompanied by appropriate support. 174 Participants cited the value of peer support in this respect. More generally, the importance of teamwork in improving quality of care is well documented,175 as is the role of leadership in promoting communication and relationships among staff. 176 Training and support programmes need to recognise the impact of these dynamics and assist managers to promote information flow among staff, facilitate inclusive discussions about care delivery, incorporate diverse points of view and build positive relationships among all those living and working in the care home. 177 This should extend to family members, who were frequently criticised for being unduly critical of staff. 178
Implications for the WHELD programme
Qualitative work conducted with care staff clearly demonstrated the need to involve the entire staff team in any new intervention to have complete ‘buy-in’, as well as ensuring that trainers fully acknowledge the cultural and resource challenges inherent in care home settings and strive to work with staff to overcome them.
Concluding remarks for work package 3
Work package 3 was successfully delivered and demonstrated an impact on QoL, antipsychotic use and mortality with the combination of person-centred care, social interaction and antipsychotic review. These, therefore, appeared to be the key components to integrate into a single optimised intervention in WP4.
The qualitative work provides clear insights into the needs for delivery of the intervention to support future implementation. In practical terms, this includes effective supervision and support for therapists to enable ‘on-the-job’ learning, and an understanding of the drivers for motivation and engagement among care home staff.
Based on qualitative feedback from this WP and WP4 (see Work package 4: evidence-based and user-driven optimisation of the WHELD programme for use in a real-world setting), the decision was made to develop a lower cost champion model for intervention delivery.
Success indicator
One of the care homes involved in the factorial study won Care Home of the Year at the National Care awards for the year that the home was involved in the WHELD programme.
Work package 4: evidence-based and user-driven optimisation of the WHELD programme for use in a real-world setting
Abstract
Objectives: WP4 sought to optimise the WHELD programme through a series of review and consultation steps.
Methods: WP4 involved (1) review of the outcomes of WP3 with expert and governance groups, (2) review of the study materials and their usage with WHELD programme therapists and (3) focus group discussions with 41 care staff who were involved in the factorial trial in WP3 to understand their experience of involvement in research and use of the WHELD programme. The intervention was then refined according to the outputs.
Results: A number of key changes were made to the intervention. The optimised intervention consisted of person-centred care and social interaction, with activities elements from the exercise package and a cascade version of the antipsychotic review intervention in which staff prompted GPs for review. The delivery model was adapted for implementation and cost-effectiveness, replacing intensive therapist time with a champions model. The focus group discussions relating to both the overall research experience and the use of WHELD materials reported a generally positive experience for staff, and beneficial aspects of WP3 were identified. However, there were issues identified in relation to the extra burden of data collection for research and the perceived pressure of time to deliver the intervention alongside routine care activities.
Conclusions: The adaptations of the intervention and protocols ensured that the WHELD programme was fit for full evaluation in WP5.
Background and rationale
The factorial trial completed in WP3 provided robust, meaningful data regarding the value of the four elements of the WHELD programme, in addition to critical information regarding the most valuable outcome measures for a full evaluation and the importance of user involvement in intervention development.
This work was completed in the context of a considerable literature and academic consensus that supports the need and rationale for an intervention that draws together the best available interventions to create an optimised ‘real-life’ approach, which is both effective and feasible for implementation. WP3 provided key evidence of effectiveness, but showed the need to optimise the intervention further to ensure that it could be feasibly rolled out. A critical factor in this was the need to involve end users in the refinement process. Existing literature consistently reports the need for a collaborative approach that seeks the views of staff from the outset, provides positive feedback, does not judge past or present care practices and offers an effective method of engagement. 170
Cost-effectiveness is also a critical aspect in the prospective success of any health service intervention, particularly in the care sector, in which resources are limited and there is considerable competition for commissioning. This aspect was clearly shown in the cost–function analysis performed in WP3, which indicated the need to integrate meaningful cost-effectiveness measures into large-scale trials, as well as improving needs-based care for people with dementia.
Work package 4, therefore, collated the findings of the programme to date, and consisted of a collaborative final optimisation stage to create a final WHELD programme described in a manual for full evaluation in WP5.
Aim and objectives
The overall aim of WP4 was to optimise the overall WHELD programme for full RCT evaluation.
This was achieved through the following objectives:
-
conduct a review of the materials used in WP3 and collate learning points from supervision conducted with WHELD therapists regarding feasibility of intervention delivery
-
conduct a consultation with key groups (PMG and TDG) to agree on the requirements for adaptation of the WHELD programme
-
conduct a series of focus group discussions with care staff, with the aim of ensuring a collaborative approach to the intervention optimisation
-
adapt the intervention manuals to reflect the approved amendments.
Methods
Review of use of WHELD programme materials
A full review of the WHELD manuals was conducted by a subgroup of the PMG. The review focused on determining where resources could be adapted and combined as a means of streamlining the intervention. This process involved three main steps:
-
reviewing feedback from therapists collected during the factorial study in WP3
-
collation of notes taken during supervisions with WHELD therapists in WP3
-
co-ordination of an end-of-study meeting with therapists who were involved in WP3.
Consultation with expert groups
Expert consultation was conducted in two ways. First, a meeting was convened with the PMG, in which the study statistician presented the statistical outputs from the factorial trial in WP3. The group then discussed the implications for the recruitment strategy and intervention design, and made recommendations for refinement. Second, consultations were conducted with selected members of the TDG (specifically Professor Dawn Brooker, Professor Graham Stokes and care home managers). These discussions focused on the most effective way to streamline the intervention to make it deliverable in a real-world setting. The outputs of these consultations were collated and used to inform the intervention optimisation.
Focus group discussions with care staff
Six focus group discussions were conducted with 41 care home staff, who were selected from six of the care homes (two from each site) from the factorial trial in WP3. The focus group discussions aimed to explore staff’s experiences of being involved in a research trial and which elements of the research and intervention they felt had been successful. The discussions also explored any unhelpful or challenging aspects and any learning points for staff members. Focus group discussions were recorded and transcribed verbatim; observations and impressions were noted at the end of each group. Inductive thematic analysis was used to identify themes and interpret the data.
Intervention optimisation
The outputs of the consultation and focus group discussions were collated by two investigators (CB and JF) and one therapist (LG). This subgroup adapted the existing manuals from each individual intervention element to create the optimised WHELD programme manual.
Results
Review of materials in work package 3
Review of the patterns of usage and therapist feedback regarding the WHELD programme manuals and resources highlighted key points for adaptation. These were:
-
The need to emphasise the purpose of the materials to be used as templates for adaptation by individual homes according to their processes and structures. It was felt that homes had not fully appreciated this flexibility in the resources.
-
The adaptation of the language and terminology in activities derived from the NEST manual, which required further adaptation for a UK audience.
-
A number of NEST manual activities had not been used as a result of the perceived cultural differences in UK settings. These were recommended for removal.
-
Staff time required to set up and deliver interventions was highlighted as an important factor in the likelihood of the usage of these activities. Time-intensive activities were deprioritised in favour of less intensive options.
-
Staff skills in delivering group activities were reported to often be lacking. Improved training and support in developing and applying these skills were, therefore, prioritised.
-
Peer support and supervision for the WHELD programme therapists was highlighted.
Feedback clearly described the need for a launch event at each care home to ensure that there was buy-in and engagement of all staff, and to promote motivation within the study. Certification of training and involvement was also considered to be a driver for continued engagement.
Consultation with expert groups
The PMG discussion resulted in three main recommendations for optimisation of the WHELD programme. These were:
-
To retain the social interaction intervention as a key element owing to the significant benefit conferred in the factorial study.
-
To include a reduced selection of activities taken from the exercise intervention as part of the pleasant activities component of the social interaction intervention.
-
To reduce the focus on intensive antipsychotic review due to the negative impact on BPSD. The group recommended that all participants should be reviewed, but that this should be prompted by trained care staff. The group suggested that the active GP education element of the intervention was removed to ensure that the intervention was streamlined for a real-world setting.
The consultation with members of the TDG resulted in one main recommendation that related to the implementation and delivery of the intervention. The group agreed that, although it provided consistency and support, the delivery model utilised in WP3, in which a therapist provided training and intensive support, was highly resource intensive and, therefore, not feasible or cost-effective for a real-world setting. To provide an intervention that required less intensive support from the WHELD therapist, the dementia champions role was extended, with more emphasis on a ‘train-the-trainer’ model with supervision from the therapist. This approach has been increasingly used in practice in recent years, and is regarded as cost-effective and a means of skill-building within services, although published evidence is limited.
Focus groups with care staff
Focus group discussion demographics
Six focus groups involving 41 staff were conducted across 6 of the 16 care homes in the UK that had participated in a 9-month RCT of psychosocial interventions for people with dementia, as described in WP3. Participants included 24 care assistants (59%), six senior care assistants (15%), five activity therapists (12%), one registered nurse (2%), two managers (5%) and three housekeeping staff (7%). The time they had spent working at the home ranged from 3 months to 20 years, with the median being 12 staff who had worked in the home for 3–5 years. In total, 41% of staff reported no formal care qualification, 20% had their highest level of National Vocational Qualification (NVQ) at level 2 and 24% at NVQ level 3.
Thematic outcomes related to the WHELD research aims and process
The focus group discussions were transcribed verbatim and analysed using thematic analysis. This led to the identification of three key themes relating to the research experience: ‘recognising preparedness’, ‘working together’ and ‘learning more than expected’. These are detailed in a published output. 179
The learning for the research team in relation to optimising the approach and materials for WP5 is summarised here. The majority of staff felt that the study had value and was a good means of raising the profile of dementia care practice. The research team reported that it had been valuable to include a range of staff because of the differing perceptions of care between staff seniorities and shifts, particularly including both day and night staff. Staff also felt that it had been valuable for the WHELD therapist to get to know the home in the early phase of the study and be able to respond to the staff’s hopes and concerns about the research. Knowing residents’ needs was key to being able to implement the ideas, and could be best achieved by care staff themselves. Some of the WHELD materials were highlighted as being particularly helpful in facilitating staff to collect and record information about residents. In terms of the process of delivery, focus group discussions highlighted the benefits of the therapist working alongside dementia champions in the care homes for a sustained period to build trust and confidence in adopting the WHELD approach.
Staff appreciated feedback and positive affirmation of their good practice and had found this motivating in several respects, including the development of person-centred protocolled activities. In addition, staff felt that they developed skills in team working and educating colleagues, involving residents’ families more actively in care planning and in systematically recording information. Families’ knowledge was highlighted as particularly valuable in understanding a resident’s distress and in helping to resolve challenging situations.
Staff reported some barriers to effective person-centred care. Situations were described in which staff opinions of best practice conflicted with family wishes and their understanding of dementia, and staff believed that some families were reluctant to be closely involved with activities. Despite this, instances were also identified in which family involvement had been helpful. This theme demonstrated a need to provide better operationalisation for family working in the WHELD manual. A second issue in person-centred care was the involvement of volunteers. Conflicting views were held regarding the benefit of additional capacity balanced with the burden of increased administration and support from staff.
The social interaction and exercise interventions were described positively by staff, although time constraints were cited as a considerable barrier, in addition to the reluctance of residents to take part on occasions. Staff reported that it was, therefore, very helpful that the goals for recommended activity time were weekly, allowing for flexibility. These were regarded as helpful, realistic and achievable, and staff clearly articulated their understanding of the value of the approach. Several staff also reported that taking part in the activities had been personally rewarding and benefited residents, with identifiable positive emotional and personal impacts.
Focus group discussions were positive about the antipsychotic review intervention, showing an understanding of the importance of judicious antipsychotic use. Staff particularly valued the closer working with GPs and felt that this collaboration worked well in that it motivated staff to be more proactive in this aspect of the residents’ care.
Refinement of intervention protocol
Two investigators (CB and JF) and a WHELD therapist (LG) optimised the intervention manual to reflect the recommendations from the consultations. This involved combining the individual manuals for person-centred care, social interaction and exercise into one WHELD therapist manual. The work also involved the creation of a new WHELD champions manual to support the new delivery model.
The newly adapted manuals and associated protocols were circulated and approved by all members of the PMG.
Finalisation of the randomised controlled trial protocol
Based on the evidence in the three review activities, refinements were made to the protocol for the future RCT evaluation of the WHELD programme and were approved by the PMG. These included:
-
The success of recruiting care homes in WP3 confirmed the feasibility of a ‘cold contact’ approach to recruitment directly from Care Quality Commission registers.
-
Recruitment protocols were adapted to use a stepwise approach across geographical areas within each site to be more timely and cost-effective. A care home demographic screening questionnaire was included earlier in the recruitment process. Home information was adapted to provide clarity on the time commitment involved for homes.
-
The number of assessment measures was reduced by removing the Camberwell Assessment of Need in the Elderly (CANE) scale from the protocol. Detailed process monitoring with WHELD therapists in a larger RCT was not feasible. For this reason, a qualitative package was planned in WP5 to capture this information and to reduce burden on the therapists.
Discussion and implications for the WHELD programme
Work package 4 drew on consultations with a range of stakeholders who had been involved in WP3 to understand how best to adapt the intervention, delivery model and evaluation protocol. It was important to consider the overall experience of staff in the whole research process in recognition that these elements may have an indirect impact on staff motivation and effectiveness in engaging with the project. This approach proved helpful in triangulating information about which elements had been effective and which required revision, both from a process and a content perspective. The resulting adaptations of the intervention and protocols ensured that the WHELD programme was fit for full evaluation in WP5.
Limitations
This work was pragmatic and resulted in an optimised intervention for WP5. However, it would have been helpful to engage with residents and family members directly about their experiences. Unfortunately, owing to the nature of the intervention delivery and the extent of impairment of residents, this was not deemed feasible within the scope of WP3 or WP4.
Concluding remarks for work package 4
This was a largely operational WP, combining consultation with key groups with a period of refinement and optimisation of the WHELD programme. The process followed by the investigator team resulted in a fit-for-purpose intervention portfolio and trial protocol. This fulfilled the stated objectives of the WP.
Of note, this WP particularly benefited from user involvement and patient and public involvement (PPI) throughout, ensuring that the various discussions and alterations were directly informed by stakeholder feedback and opinion.
Major outputs from work package 4
-
Final WHELD programme manual.
-
Final WHELD champions manual.
Work package 5: randomised controlled trial and field testing of the WHELD programme in care homes
Abstract
Objectives: WP5 aimed to establish the clinical effectiveness and cost-effectiveness of the optimised WHELD programme and to determine factors influencing sustainability for use in UK care homes.
Methods: This was achieved through (1) a large RCT of the optimised intervention compared with usual care; (2) an integrated cost-effectiveness analysis of the optimised intervention; (3) a qualitative analysis of WHELD materials that were used by WHELD therapists and supervisors to determine factors that are associated with successful implementation; and (4) a qualitative analysis with care home staff to determine the sustainability of the WHELD programme.
Results: WP5 successfully delivered a robust and rigorous clinical evaluation of the optimised WHELD programme utilising a champion’s model. The intervention conferred a statistically significant improvement in QoL [DEMQoL – proxy z-score of 2.82, mean difference 2.54, standard error of measurement (SEM) 0.88, 95% CI 0.81 to 4.28, Cohen’s d ES of 0.24; p = 0.0042]. There were also statistically significant benefits on agitation (CMAI z-score of 2.68, mean difference –4.27, SEM 1.59, 95% CI –7.39 to –1.15, Cohen’s d ES of 0.23; p = 0.0076) and overall neuropsychiatric symptoms (NPI-NH z-score of 3.52, mean difference –4.55, SEM 1.28, 95% CI –7.07 to –2.02, Cohen’s d ES of 0.30; p < 0.001). Benefits were greatest in people with moderately severe dementia.
Conclusions: These findings suggest that the WHELD programme confers benefits to QoL, agitation and neuropsychiatric symptoms, albeit with relatively small ESs, as well as cost saving in a model that can readily be implemented into nursing homes. Future work should consider how to facilitate sustainability of the intervention in these settings.
Background and rationale
Work package 5 represents a culmination of extensive evaluation, consultation and optimisation work, leading to an optimised WHELD champion intervention.
Aim and objectives
Work package 5 aimed to establish the effectiveness and cost-effectiveness of the optimised WHELD programme and to determine which factors influence sustainability for use in UK care homes.
This was achieved through the following main objectives:
-
A large RCT of the optimised intervention compared with usual care.
-
An integrated cost-effectiveness analysis of the optimised intervention. To determine whether or not the intervention is cost-effective is an important factor in whether or not commissioners and private payers are likely to pay for the training.
-
A qualitative analysis of WHELD materials used by WHELD therapists and supervisors to determine factors that are associated with successful implementation.
-
A qualitative analysis with care home staff to determine the sustainability of the WHELD programme.
Randomised controlled trial of the optimised WHELD programme in care homes in the UK
The first phase of WP5 was a large-scale multicentre RCT of the optimised WHELD programme, as described in a published output. 180
Aim
The goal was to evaluate the impact of the WHELD programme on QoL, agitation, neuropsychiatric symptoms, antipsychotic use, global deterioration, mood, unmet needs, mortality, quality of interactions, pain and cost in comparison with treatment as usual (TAU).
Methods
Study design
This study was a 9-month, cluster, two-arm RCT conducted following Consolidated Standards of Reporting Trials guidelines in 69 care homes in the UK. There were three recruiting hubs that were based in north and south London and Buckinghamshire. Each cluster was randomised to receive either the WHELD programme or TAU for 9 months. All residents with dementia (defined as a score of ≥ 1 on the CDR scale) were eligible. Rating assessments were conducted at two time points: baseline (prior to randomisation) and after 9 months. This is described in a published output. 181
Interventions
The WHELD programme focused on training in person-centred care for care staff, promoting tailored person-centred activities and social interactions and the development of a system for triggering appropriate review of antipsychotic medications by the prescribing physician. The intervention is described in more detail in a published output. 180
Outcome measures
The primary outcome was QoL, which was measured by the DEMQoL – proxy. 182
The secondary outcome measures included:
-
agitation – assessed using the CMAI158
-
overall neuropsychiatric symptoms – assessed using the NPI-NH159,183
-
global severity of dementia – assessed using the CDR154
-
mood – assessed using the Cornell Scale for Depression in Dementia (CSSD)157
-
antipsychotic use
-
unmet needs – assessed using the CANE scale184
-
quality of interactions – assessed using the Quality of Interactions Scale (QUIS)185
-
pain – assessed using the Abbey Pain Scale186
-
mortality
-
cost.
Economic data for each individual in the study were collected using an adapted version of the CSRI. 187,188
All assessments were undertaken based on informant interviews with members of care home staff that were conducted by trained members of the WHELD research team.
Randomisation and blinding
Nursing homes were allocated to receive either the WHELD programme or TAU using secure web access [North Wales Organisation for Randomised Trials (NWORTH) Clinical Trials Unit at Bangor University]. 189
Sample size
Previous studies have indicated that intra-home correlation coefficients rarely exceed 0.05. Taking this into account, a sample size of 640 participants at 9 months gives 90% power to a p-value of < 0.05 to detect a standardised ES of 0.3 standard deviations (SDs), which is generally accepted as the lowest threshold of a clinically meaningful benefit. The recruitment of a minimum of 840 participants allowed for loss of 200 through mortality or withdrawal.
Data analysis
Outcome measures were assessed at baseline and at 9 months. The primary outcome measure (DEMQoL – proxy) and the secondary outcome measures were analysed using the multilevel modelling approach to analysis of covariance (ANCOVA), with the value at 9 months as the response. The baseline value was the covariate.
Mortality and antipsychotic use were compared between treatment groups using relative risk with 95% CIs. QUIS used the care home-level data and was compared between treatment groups using ANCOVA, but as a result of the smaller sample size did not use baseline covariates.
Cohen’s d is defined as the difference between two means divided by the SD of the data, that is the difference in the means of WHELD and TAU divided by their pooled SD.
Cost analysis
Costs were derived from service use data collected over the 3-month period prior to the intervention (baseline) and the 9 months of the intervention (follow-up) and consisted of three main cost categories: intervention costs, accommodation charges, and health and social care costs. Data on each nursing home resident’s use of health care (obtained from the CSRI) were multiplied by the appropriate unit costs to calculate health and social care costs for each participant at each time point. The mean differences in costs and 95% CIs were obtained by non-parametric bootstrapped regression (1000 repetitions), modelling to account for non-normal distributions. A multilevel mixed model controlling for baseline costs, site and age at entry into the study was used. The health economic analysis followed Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines.
Results
Cohort characteristics
In total, 1006 participants were consented to the study, with 847 randomised to either TAU (n = 443) or the WHELD programme (n = 404). Follow-up assessments were available for 553 participants (TAU, n = 296; WHELD, n = 257), the majority of whom had moderately severe or severe dementia. Mortality accounted for the majority of participants who did not complete follow-up. Anonymised data are available in an online registry. 180
Outcomes
The WHELD programme conferred a statistically significant 2.54-point (SEM 0.88-point) improvement in QoL compared with TAU (95% CI 0.81 to 4.28 points, Cohen’s d ES of 0.24; p = 0.0042), as measured by the DEMQoL – proxy over 9 months. The WHELD programme also conferred a statistically significant 4.27-point (z-score 2.68, mean difference –4.27 points, SEM 1.59 points, 95% CI –7.39 to –1.15 points, Cohen’s d ES of 0.23; p = 0.0076) benefit on the CMAI compared with TAU with respect to agitation, and conferred a statistically significant 4.55-point (z-score 3.52, mean difference –4.55 points, SEM 1.28 points, 95% CI –7.07 to –2.02 points, Cohen’s d ES of 0.30; p < 0.001) benefit on the total NPI-NH compared with TAU. The main results are summarised in Table 1. The full results are described in a published output. 180
| Outcome measure | Adjusted effecta | p-value | Mean difference (95% CI) |
|---|---|---|---|
| DEMQoL – proxy (n = 553) | R = 0.12 | 0.0042 | 2.54 (0.81 to 4.28) |
| CMAI (n = 553) | R = 0.11 | 0.0076 | –4.27 (–7.23 to –1.15) |
Prescriptions of antipsychotic medications were stable across the study in both treatment groups [change in antipsychotic use: WHELD –0.1% (SEM 0.1%); TAU –0.2% (SEM 0.1%); p = 0.60]. There were no statistically significant differences between the WHELD programme and TAU groups for change in global severity of dementia, unmet needs, pain, mood or mortality. There was a statistically significant 19.7% greater increase in the proportion of positive care interactions from baseline to 9 months in the WHELD group than in the TAU group (SEM 8.94%, 95% CI 2.12% to 37.16%, Cohen’s d ES of 0.55; p = 0.03).
Adverse events
A total of 549 serious adverse events were recorded during the period of the trial. The events were balanced between the two treatment groups, with no statistically significant differences.
Cost analysis
The direct cost of delivering the intervention compared with TAU was £8627 more per care home. After adding the intervention to social and health-care costs, the cost per participant receiving the WHELD programme was £4740 lower than the cost of those participants receiving usual care over the 9 months of the study (Table 2).
| Cost category | Cost (£), mean (SD) | Intervention vs. TAU, unadjusted mean cost (£) difference (95% CI) | |
|---|---|---|---|
| Intervention | TAU | ||
| The WHELD programme | 2713 (121) | 0 (–) | 2713 (2701 to 2724) |
| Baseline (n = 887) | |||
| Accommodation charges | 9480 (2010) | 10,233 (3675) | –753 (–1128 to –365) |
| Hospital | 387 (1759) | 407 (2413) | –20 (283 to 242) |
| Primary care | 96 (126) | 98 (148) | –2 (19 to 14) |
| Community health | 23 (80) | 19 (79) | 4 (–7 to 14) |
| Emergency | 12 (37) | 9 (34) | 3 (–1 to 7) |
| Total health and social care costs | 9998 (2601) | 10,766 (4396) | –768 (–1249 to –338) |
| 9 months’ follow-up (n = 553) | |||
| Accommodation charges | 28,606 (10,863) | 33,005 (12,428) | –4399 (–5725 to –2898) |
| Hospital | 269 (1166) | 262 (1267) | 7 (–183 to 188) |
| Primary care | 700 (294) | 1,020 (301) | –320 (–364 to –277) |
| Community health | 78 (260) | 70 (206) | 8 (–23 to 44) |
| Emergency | 49 (133) | 85 (244) | –36 (–68 to –10) |
| Total health and social care costs | 29,702 (8774) | 34,442 (11,106) | –4740 (–6129 to –3156) |
For the participants with a CMAI score of > 40, the relative treatment effect for health-related QoL (as measured via the DEMQoL – proxy) increased at the 5% level (1.96, 95% CI 0.42 to 3.39) and the benefit on the CMAI scale improved by more than 1 point compared with the base case (3.72; 95% CI 1.28 to 6.51). However, the difference in costs was around £400 less than in the ordinary least squares model, with a part of the bootstrapped CI stretching to the positive part of the number line. The health economic analysis is presented in more detail in a published output. 190
Discussion
The WHELD programme conferred a statistically significant improvement in QoL over 9 months. There was also a statistically significant benefit regarding agitation and overall neuropsychiatric symptoms, and a significant increase in the proportion of positive care interactions between care staff and residents with dementia. Importantly, the benefits were achieved in the context of cost saving with a model that can readily be implemented in nursing homes.
Although comparable to atypical antipsychotics, the standardised ESs of benefit are modest in the context of a clinical intervention. The benefits do, however, also include improvements in QoL, which have not been shown with pharmacological interventions or other person-centred care interventions. Although there is no established threshold for a clinically meaningful benefit in QoL or quality of care, any statistically significant benefit is important given the lack of any benefit in previous RCTs. In addition, the intervention was not just delivered to people with clinically significant neuropsychiatric symptoms, but conferred benefit among the broader population of people with dementia living in care homes and could, therefore, be considered as a health and well-being intervention rather than a clinical intervention per se. Although the ES would be considered marginal in terms of a clinically significant benefit, we do believe that the benefits to the broader population of people with dementia living in care homes make this a meaningful benefit in the quality of care. The benefits were also smaller than hypothesised.
Agitation is a frequent and distressing symptom for people with dementia. 191,192 The findings are favourable when compared with trials of antipsychotic medications, which show only very modest benefits over 12 weeks in the treatment of agitation in the context of significant harms. 13,16 Our results compare favourably with the small number of previous studies that focused on person-centred care, in which benefits in agitation are inconsistent and none of which has reported benefits in QoL. 28,30 In addition, this study shows cost advantages over usual care, which have not been demonstrated with any previous drug or non-drug intervention.
Recent studies have begun to suggest that other pharmacological therapies, such as citalopram (Cipramil, Lundbeck)193 and dextromethorphan (Neudexta, Avanir),194 may confer benefit for the treatment of agitation; however, further studies are needed.
Elements of the WHELD programme, such as social interaction and pleasant events, have previously been demonstrated to improve agitation in modest-sized RCTs. 49,195 Incorporating these elements within a coherent framework, such as WHELD, enables straightforward and affordable implementation.
In contrast to our previous factorial RCT of the WHELD programme, no significant reduction in antipsychotics prescriptions was achieved. This is probably attributable to a combination of a small number of baseline antipsychotic prescriptions and the more limited education programme for primary care physicians. It should also be noted that the ICCs were higher than the 0.03 that was identified from previous studies, which may have limited statistical power of the trial. Other limitations include the challenges of measuring QoL for people with moderately severe and severe dementia and the potential challenges of data analysis in the context of a frail group of individuals with high rates of mortality.
Pain was a prespecified secondary outcome for the WP5 WHELD RCT. Given that several members of our research group were particularly interested in pain in people with dementia, which we consider to be a key factor for health and well-being, we made these data available for a more detailed evaluation. This was an added value to the outputs from the programme and led to a secondary paper that focused on pain experienced by people with dementia. The baseline prevalence of pain was 35.3% among residents with dementia. Pain severity was significantly correlated with dementia severity, neuropsychiatric symptoms, depression, agitation and QoL at baseline and at 9 months. Regular treatment with analgesics was associated with reduced pain severity. Pain was significantly associated with more antipsychotic prescriptions and with all-cause mortality during follow-up (OR 1.48, 95% CI 1.18 to 1.85). See the published output for the full paper. 196
Field testing and implementation of the WHELD programme in care homes in the UK
Aim and objectives
This evaluation gathered information on the experiences of the WHELD therapists and supervisors at the end of the intervention period, and from care home staff after a period of 9–12 months following study completion.
Methods
Focus group discussions were conducted with the 12 members of the therapy team who had delivered or supported the intervention in the WP5 RCT to explore the therapist and supervisor experiences of delivering the intervention. The detailed findings are reported in a published output. 197
Results
The WHELD therapist focus group discussions
Feedback on therapist training and supervision
The therapists reported that they had received comprehensive training and that the content of the interventions and the expectations of how they would be delivered was clear.
The participants identified areas that they felt required particular support. This included ensuring that they had the skills that were required to train dementia champions. The participants particularly supported the use of experiential exercises for successful learning. Therapists felt that the development of their coaching skills during supervision was particularly valuable, as well as ensuring that there was promotion of a multicultural attitude and awareness of differences in language and needs among staff. The importance of ongoing supervision within the homes and for the WHELD therapists themselves was also seen as an essential feature.
Developing relationships with care homes and staff
Therapists strongly articulated the importance of establishing a good working relationship with care home managers and staff early in the study. This required a genuine interest in the care team as individuals to build relationships and support the dementia champions. All therapists acknowledged that a flexible approach was imperative. Some therapists felt that, on occasion, they were adding pressures on staff, who were trying to find a balance between fidelity to the intervention and other work duties.
Optimising success of the champion role
There was a strong consensus for advocating self-selection of dementia champions rather than nominating people for the role. Having multiple champions in each home was also strongly supported, as this provided peer support and built complementary skills. Buy-in from the home manager was a critical driver for success, as it enabled staff to dedicate time to the role. The therapists perceived that many dementia champions enjoyed the role and felt empowered in promoting activities and ideas with residents. For many dementia champions, reflecting on their practice was a new and appreciated way of working.
Therapists spoke positively about the effect of the WHELD programme on care home residents. They spoke with enthusiasm about the many individualised activities that care home staff enabled and how staff became creative in responding to people with diverse needs.
Focus group discussions with care home staff
Care homes receiving the WHELD programme were invited to take part in focus groups 9–12 months after the intervention had concluded. The results are reported in detail in a published output. 198
Demographics
In total, 47 care home staff participated in the focus groups. Almost half of the participants were carers or senior carers, three were nurses (6%), six were activity co-ordinators (13%), three were managers (6%), six were assistant managers (13%) and six were traditionally non-direct care roles (13%). A total of 32% of focus group participants were WHELD dementia champions during WP5. The remaining participants started work in the home after the intervention had finished.
Value of the WHELD programme
The first theme that emerged related to staff recognising the value of the research for different groups of people. They described benefits in the way that they recognised the individual and interpersonal needs of residents and acknowledged that some of the methods allowed them to get to know residents better, which led to positive responses from the residents that were rewarding for staff. Staff also saw value for themselves in forming relationships within the team and enhancing their ability to provide care. During the study, some homes had adapted their organisational routines and found this helpful in developing a team approach to sharing person-centred care principles with the wider staff group. Many homes felt that participating in the WHELD programme had also changed their perspective on working with families.
Skills development in care staff: ‘being well practised’
The second theme related to the skills staff felt that they developed. All care homes felt that the person-centred philosophy was a feature that had endured following the research. A notable element in achieving this was the development of empathy and being able to identify more closely with residents. Experiential learning exercises, which were run by dementia champions with their colleagues, were particularly helpful. The WHELD programme also helped care home staff to develop new communication skills and improve their ability to tailor their approach to individuals. The sustained change in perception that activities could be carried out at ‘any time’ and could be incorporated into physical care tasks through the use of conversations and short individual activities was particularly beneficial.
Sustainable practice: ‘taking ownership’
The third theme that emerged was being able to adopt the ‘WHELD’ approach as the ‘home’s own’. Where there was a strong local leadership from managers and peers to develop creative ways of working with residents, the care homes found ways to continue with the approach and ensure that the underlying philosophy and valued practice was not lost.
Perspective on research involvement
The staff generally held a positive view of research and a sense of pride at having contributed to a major study.
Discussion
Discussion with care home staff represented a unique opportunity to explore their perceptions of the long-term impacts of the WHELD programme and the factors influencing its sustainability. The focus group discussions highlighted that the ethos of person-centred care and associated approaches can be embedded and that a number of the WHELD materials continue to be used after study involvement has ceased.
Concluding remarks from work package 5
Work package 5 successfully delivered a robust and rigorous clinical evaluation of the optimised WHELD programme utilising a champion’s model. Significant benefits were seen in the treatment of agitation, overall scores of neuropsychiatric symptoms and health-related QoL in individuals with agitation compared with TAU.
There are also economic benefits from a low-resource multicomponent intervention that is aimed at people with dementia living in a care home setting.
Feedback from therapists and staff involved in the trial also confirmed a positive and empowering experience.
Work package 6: dissemination of the study outcomes and impacts
Abstract
Objectives: WP6 sought to effectively disseminate the findings of the programme to impact on practice and maximise the output of the research.
Methods: A dedicated dissemination phase involved a series of tailored activities to maximise the impact of the WHELD programme. Work included academic publication and presentations, outreach to GPs, updating of national best practice guidelines and additional events for care homes.
Results: The dissemination activities were successfully completed. Key activities included regional workshops for GPs, which received excellent feedback and response, and the creation of an e-learning module for GPs with the British Medical Journal learning portal. The national guidelines on BPSD management were updated and endorsed by NHS England. Additional events and workshops were held with care home staff, and investigators presented the findings at numerous national and international conferences. All findings were prepared for publication in peer-reviewed journals.
Conclusions: WP6 constituted an integrated and broad approach to ensure that the outputs of the WHELD programme were effectively communicated in a targeted manner. WP6 exemplifies the success of the WHELD programme and highlights the importance of the key findings from the clinical evaluations that were conducted throughout the study. Involvement of key health-care professionals, particularly GPs and care home staff, was purposely selected to ensure that the learning from the programme informs future practice in a meaningful way.
Background and rationale
The WHELD programme consisted of a series of high-quality research elements that sought to understand the UK care home landscape, develop and optimise a complex care intervention, evaluate it in a real-world environment and fully understand its implications for care home residents and staff. Ultimately, the aim of this programme was to improve the care that is provided to people with dementia, with a focus on improving management of BPSD, increasing QoL and promoting the judicious use of antipsychotic medications.
Dissemination activity is an integral aspect of a successful research programme and an essential step in ensuring that research informs improvements to clinical and care practice. There was, therefore, a need to dedicate time and resources to the final adaptations of the intervention, inform peripheral resources to support ongoing implementation and move towards the roll-out of WHELD as an intervention.
Aim and objectives
Work package 6 aimed to maximise the impact and success of the WHELD programme through a dedicated dissemination package. This was achieved through the following specific objectives:
-
academic publication of the findings collated throughout the WHELD programme
-
population of a dedicated website to promote the WHELD outcomes
-
delivery of workshops to GPs across the UK to disseminate the findings
-
updating of the national BPSD management guidance resource, in partnership with NHS England
-
creating an e-learning module for GPs in partnership with the British Medical Journal
-
seeking additional opportunities for presentations and workshops to target audiences
-
disseminating findings to care home audiences through events and tailored communications
-
seeking opportunities for further funding to promote implementation of the WHELD programme.
Dissemination activity
Publications
The outputs from each WP in the WHELD programme have been successfully published, with some additional publications in writing or in press at the time of publication of the final report (see Publications).
The WHELD programme website
A succinct online presence was created for the WHELD programme to provide information about the study and its impacts. The site is hosted by Oxford Health NHS Foundation Trust at www.oxfordhealth.nhs.uk/research/making-a-difference/improving-wellbeing-and-health-for-people-with-dementia-wheld/ (accessed 4 November 2019).
The website does not aim to provide detailed information about the intervention. Instead, it is tailored to an online audience, providing snapshots of its impacts and YouTube (YouTube, LLC, San Bruno, CA, USA) video footage of care home staff describing their experience of the study. There are also plans to produce additional pieces of video footage in the future as part of an ongoing resource to support training.
General practitioner learning workshops: optimising the use of antipsychotic medication and non-pharmacological treatment for people with dementia
A major dissemination activity for WP6 was a series of GP workshops that ran across the UK from November 2015 to January 2016. The GP training resources used in the factorial study in WP3 were accredited and used as the basis for these events. The 4-hour workshops were held in York, Exeter, Bicester (Oxfordshire), Manchester and London. In addition to information provision, workshops were delivered with additional time for GPs to explore how they could implement best practice in antipsychotic prescribing and support care home staff to adopt non-pharmacological approaches in response to BPSD. All the workshops were facilitated by a core group from the WHELD team, with other members of the project team and local figureheads supporting individual workshops in each locality.
Feedback from the 118 attendees at the GP workshops was overwhelmingly positive, with all members rating their learning as ‘very effective’ or ‘effective’, and stating that the event had met their learning objectives. Testimonial statements from attendees included the following:
I was surprised to discover that antipsychotics are not very effective and glad to discover that social interaction has evidence to support its recommendation.
I obtained more information on antipsychotic medication use in people with dementia.
Understood the risks around prescribing antipsychotic medication. Makes complete sense to use a person-centred approach.
Became aware of non-pharmacological methods of managing behavioural problems in dementia.
Excellent, very interesting and relevant afternoon.
Definitely think harder about risks and harm of prescribing drugs and benefits of not.
This programme has now been accredited by the Royal College of General Practitioners for further training workshops.
Creation of a British Medical Journal e-learning module for general practitioners
Outreach to primary care and eliciting change in these settings is known to be challenging as a result of the competing priorities that GPs experience in their role. An online resource for GPs was also created to increase the reach of the learning for GPs, inform judicious antipsychotic prescription and promote the use of non-drug treatment approaches. An e-learning module was created in partnership with the British Medical Journal that collated the key clinical messages arising from the WHELD programme.
The e-learning module was created through the following steps:
-
An initial discussion between British Medical Journal authors and investigators (AC, CB and JF) to establish the format and timelines for delivery.
-
The creation of 12 learning objectives. Objectives were designed to have a practical focus with relevance to commonly encountered situations in clinical practice.
-
Joint working between the British Medical Journal writing team and the investigators to create 12 scenarios, which were based on the learning objectives. Each scenario included four possible outcomes, with one correct answer, two partially correct and one incorrect answer. Each answer given was accompanied by further information to support understanding.
-
The final version of the module was created by the British Medical Journal and approved by the investigator team (CB, JF and AC).
The WHELD e-learning module has now been launched as part of the British Medical Journal portfolio of online health-care education materials, British Medical Journal Learning. Sign Up Form – Dementia Assessment Module.
Update of best practice guidance for management of behavioural and psychological symptoms of dementia
A core element of the antipsychotic review component of the WHELD programme was derived from an existing best practice guide published by the Department of Health and Social Care and the Alzheimer’s Society in 2012. Authorship of the original version of the guide involved several of the WHELD programme investigators, which has become widely used in the UK and worldwide. The outcomes of the WHELD programme, in addition to newly published evidence from related studies,21–23,25,27,149 resulted in the existing guide becoming outdated.
A key dissemination activity was, therefore, to produce an updated version of the best practice guide as a means of promoting evidence-based practice in addition to disseminating the outcomes of the WHELD programme. This process involved the following steps:
-
review of the existing manual by the three leading authors (CB, AC and Professor Alistair Burns)
-
involvement in a Delphi consensus meeting led by the International Psychogeriatrics Association in 2015, which discussed recent evidence pertaining to BPSD management and antipsychotic use
-
collation of the relevant WHELD outcomes with the Delphi outcomes
-
creation of detailed changes to the content and format of the best practice guide
-
liaison with a professional designer to adapt the guide
-
approval of the updated guide by all of the related authors.
The guide is endorsed by NHS England (primary endorser), the Alzheimer’s Society and the Royal Colleges and is available online (URL: http://medicine.exeter.ac.uk/apex/research/#tab2; accessed 27 May 2020).
Additional outreach activities
The WHELD investigator team has also contributed to a number of events, presentations and publicity opportunities as part of WP6 and developed some further research. These are detailed in Appendix 1.
Concluding remarks from work package 6
Work package 6 constituted an integrated and broad approach to ensure that the outputs of the WHELD programme were effectively communicated in a targeted manner. WP6 exemplifies the success of the WHELD programme and highlights the importance of the key findings from the clinical evaluations conducted throughout the study.
Furthermore, the involvement of key health-care professionals, particularly GPs and care home staff, was purposely selected to ensure that the learning from the programme informs future practice in a meaningful way.
Overall summary and discussion
Patient and public involvement in the WHELD programme: discussion and reflections on the programme by our patient and public involvement co-investigator
Contributed by Barbara Woodward-Carlton (lay co-investigator)
I was very pleased to be invited to be part of the WHELD project funded by NIHR some 6 years ago. I can remember saying to Clive Ballard ‘do you really think I can do this?’. After this length of time I can say, sincerely, it has been gratifying to have an involvement in this research. I had worked with the lead researchers as a Research Network Volunteer, Alzheimer’s Society, on the Focused Intervention for Training of Staff project which had demonstrated that, beyond doubt, quality of life could be greatly improved for people with dementia in care homes by implementing the evidenced based findings of that project.
The WHELD research which followed on from that project has made a huge difference to the lives of those with dementia in care homes. The WHELD Therapist and Dementia Champions Manuals are models of thoroughness based as they are on person-centred care enabling successful psychosocial interventions in care homes. The training for the therapists and other members of staff enables knowledge to be translated into practice and the enhancement of skills.
To deliver planned personalised interaction to residents involving staff, families, and volunteers necessitates training. The resources provided are truly comprehensive and if properly used in care homes transform the lives of both those with dementia and those who care for them and so give a greatly enhanced quality of life to both groups.
I am totally convinced of the value of this research, the results are simply stunning. I hope it can be successfully implemented in care homes across the UK and even beyond. As someone who has been involved in PPI for many years now, knowing that such interventions can make a huge difference to lives of those with dementia, it is my fervent hope that these findings will be adopted throughout the care system. It has been my pleasure to have had an involvement with such a group of talented, caring researchers who are so committed to making life better for those with dementia. It would be such a waste of money, expertise and time if such planned personalised interactions did not become compulsory in all care homes.
Barbara Woodward-Carlton, lay co-investigator
Additional PPI is summarised in Table 3, reported in line with the Guidance for Reporting Involvement of Patients and the Public 2 (GRIPP2) framework. 199
| Section and topic | Item |
|---|---|
| Aim | The aim was to integrate PPI in a meaningful way in the WHELD programme, focusing on the views, experiences and perspectives of people affected by dementia and people who support them in care homes |
| Methods | PPI in the WHELD study involved:
|
| Study results | Outputs relating to PPI in the WHELD programme were:
|
| Discussion and conclusions | The PPI activity involved some family carers and predominantly people working in care homes, who contributed their perspectives on the overall research process and advice on the development of the research intervention. These participants had the most relevant experience related to delivering the intervention and so their views were prioritised. Their contribution shaped the overall project development, shared between participating centres and has also been documented through ENRICH to enhance learning more widely |
| Reflections/critical perspective |
Stakeholder views were sought for each WP and dissemination of the project and have contributed to the overall results Advice was not sought directly from people with dementia living in care homes as part of the process and could have been a valuable addition to the PPI process. Many care home residents have advanced communication difficulties and engaging them in the process would require creative and adapted strategies. Our reflections on this have led to the development of a project to improve communication with people with these needs, which is being led by the University of Exeter |
Investigator team discussion and reflections on the WHELD programme
The WHELD programme built on a series of systematic reviews to design and optimise a person-centred care training package by augmenting person-centred care with personalised pleasant activities/social interaction, exercise and antipsychotic review. This was evaluated in a factorial RCT that demonstrated tangible added benefits, including improvements in QoL and reduced mortality as well as a reduction in antipsychotic use. Further optimisation of the intervention developed a training approach that used a champion model to make the programme more pragmatic: an essential component for successful implementation. The definitive RCT of the WHELD champion intervention conducted in WP5 showed significant benefits in QoL and neuropsychiatric symptoms (including agitation) for people with dementia at a lower cost than usual care. This provides an evidence-based platform to enable effective implementation in care home settings for people with dementia.
Although the programme had a number of methodological strengths, there were also limitations. The qualitative work undertaken to inform the optimisation of the intervention was focused largely on the participating staff who were responsible for delivering the intervention, but we recognise that residents with dementia who received the intervention delivered by the staff were not included in the qualitative studies and could provide a valuable perspective. In designing the study, we carefully considered the challenges of measuring QoL in people with moderate to severe dementia and, therefore, relied predominantly on a proxy measure. In addition, we opted for a TAU control in WP5 because of the scale of the study (i.e. 69 care homes), but this may have inflated the magnitude of observed benefits.
The intervention in WP5 was optimised not just for efficacy, but to also to design an intervention that was more suitable for practical implementation in real-world settings. However, as part of the adaptation of the intervention there was a less proactive approach to GP education as part of antipsychotic review and the modified WHELD programme did not achieve an overall reduction in antipsychotic use in this RCT. The GP intervention has been developed as a British Medical Journal educational module and in practice it should, therefore, be possible to implement the WHELD programme directly in care homes and promote the GP educational component in parallel.
A key question is whether or not the benefits are meaningful. The standardised ESs of benefit for the WHELD programme were small in the context of a clinical intervention (Cohen’s d standardised ESs 0.23–0.3). However, the benefits included improvements in QoL, which have not been demonstrated with other pharmacological or non-pharmacological treatments. The benefit in QoL was modest but is important given the absence of any benefit in previous studies. The findings are particularly favourable when compared with those of trials of antipsychotic medications that show only modest benefits in treating agitation over 12 weeks and with the potential of significant harms.
Discussion of implications for practice
First, with regard to antipsychotic use, the clinical trials in the programme provide evidence that advocates the continued judicious prescribing of antipsychotics that follows the changing landscape of their use in the UK and worldwide. Given the findings related to antipsychotic review, prescribers should balance the potential impacts of antipsychotic withdrawal and carefully balance the harm-to-benefit ratio associated with antipsychotics.
Second, the WHELD programme has clearly demonstrated the value of social interaction and individualised pleasant activities in the treatment and care of people with dementia. Clinicians and care staff should consider these approaches as part of usual care.
Furthermore, the qualitative work conducted in this programme has identified opportunities and challenges in implementation of psychosocial approaches in care homes.
Recommendations for future research
The WHELD investigator team would like to make recommendations for future research in this field.
First, there is a need to examine the sustainability of the WHELD programme in care homes. The programme has demonstrated effectiveness over 9 months and this raises the question of whether or not this impact would be translated to longer-term effects for residents.
Future research might also examine cost-effective models of delivery to achieve sustained benefit. A pilot is under way to develop an e-learning version of the WHELD programme and it will be important to evaluate the effectiveness of this approach for care staff and its impact on residents.
An interesting secondary outcome from the RCT in WP5 was the significant impact of the intervention on pain in people who had significant pain at baseline. Pain is a major health concern in people with dementia and is a common underlying factor in BPSD. Therefore, it will be interesting to consider the effectiveness of training and tailored non-drug interventions on this key clinical factor.
Acknowledgements
Contributions of authors
Clive Ballard (https://orcid.org/0000-0003-0022-5632) (Professor of Age-Related Diseases at the University of Exeter) was the chief investigator for this programme and was a member of the TDG and PMG.
Martin Orrell (https://orcid.org/0000-0002-1169-3530) (Director of the Institute of Mental Health at the University of Nottingham) co-led WP2 and was a centre lead for the RCT in WP5. He was a member of the TDG and PMG.
Esme Moniz-Cook (https://orcid.org/0000-0002-7232-4632) (Professor of Clinical Psychology at the University of Hull) co-led WP2 and the TDG, and was centre lead for the RCT in WP5. She was a member of the PMG.
Robert Woods (https://orcid.org/0000-0002-6781-651X) (Director of Dementia Service Development Centre) chaired the PMG.
Rhiannon Whitaker (https://orcid.org/0000-0003-4305-3236) (Senior Statistician at Bangor University and is CEO of Whitaker Research Ltd) led the statistics and statistical design of the programme and was a member of the PMG.
Anne Corbett (https://orcid.org/0000-0003-2015-0316) (Senior Lecturer in Dementia Research at the University of Exeter) led WP6.
Dag Aarsland (https://orcid.org/0000-0001-6314-216X) (Chairperson of Old Age Psychiatry at King’s College London) led work in WP1 and was a member of the TDG and PMG.
Joanna Murray (https://orcid.org/0000-0002-7348-7505) (Senior Lecturer in Qualitative Research at King’s College London) led the qualitative research analysis in WP3 and was a member of the PMG.
Vanessa Lawrence (https://orcid.org/0000-0001-7852-2018) (Lecturer in Qualitative Research at King’s College London) led the qualitative research work in WP2 and co-led WP3.
Ingelin Testad (https://orcid.org/0000-0002-0534-6575) [Director of SESAM (Centre for Age-related Medicine) at Stavanger University and Professor of Nursing at the University of Exeter] led recruitment to the RCTs in WP3 and WP5.
Martin Knapp (https://orcid.org/0000-0003-1427-0215) (Professor of Social Policy at the London School of Economics) led the health economics in WP3 and WP4 and was a member of the PMG.
Renee Romeo (https://orcid.org/0000-0003-3871-9697) (Senior Lecturer in Health Economics at King’s College London) managed the health economics analyses for data arising from WP3 and WP5.
Darshan Zala (https://orcid.org/0000-0001-5020-2354) (Research Associate at King’s College London) supported health economics data analysis from WP3 and WP5.
Jane Stafford (https://orcid.org/0000-0002-4964-075X) (Programme Manager at Oxford Health NHS Foundation Trust) was the programme manager for WHELD.
Zoe Hoare (https://orcid.org/0000-0003-1803-5482) is a Senior Statistician at NWORTH in the University of Bangor. She supported statistical analysis for WP5.
Lucy Garrod (https://orcid.org/0000-0003-4378-1372) (Research Therapist at Oxford Health NHS Foundation Trust) was a WHELD therapist in WP3 and therapy supervisor in WP5 and contributed to the qualitative evaluation in WP5.
Yongzhong Sun (https://orcid.org/0000-0003-0659-9811) (Statistician at NWORTH at Bangor University) performed statistical analysis for WPs 3 and 5.
Eddie McLaughlin (Director of Adult Mental Health Services at Oxford Health NHS Foundation Trust) was the NHS manager for the programme.
Barbara Woodward-Carlton (Research Network Volunteer at Alzheimer’s Society and a former carer of a person with dementia) provided a lay perspective throughout the programme.
Gareth Williams (Lead Bioinformatician, Wolfson Centre for Age Related Diseases, King’s College London) contributed to statistical analysis and interpretation and co-writing of WP5.
Jane Fossey (https://orcid.org/0000-0002-8533-3263) (Associate Director of Psychological Services at Oxford Health NHS Foundation Trust) led one of the systematic reviews in WP1, led WP3 and the qualitative work in WP5 and co-led WPs 2 and 4, supervised the programme manager, provided NHS management and was a member of the TDG and PMG.
Publications
Major outputs of work package 1
Fossey J, Masson S, Stafford J, Lawrence V, Corbett A, Ballard C. The disconnect between evidence and practice: a systematic review of person-centred interventions and training manuals for care home staff working with people with dementia. Int J Geriatr Psychiatry 2014;29:797–807.
Testad I, Corbett A, Aarsland D, Lexow KO, Fossey J, Woods B, et al. The value of personalised psychosocial interventions to address behavioural and psychological symptoms in people with dementia living in care home settings: a systematic review. Int Psychogeriatr 2014;26:1083–98.
Major outputs of work package 2
Lawrence V, Fossey J, Ballard C, Moniz-Cook E, Murray J. Improving quality of life for people with dementia in care homes: making psychosocial interventions work. Br J Psychiatry 2012;201:344–51.
Major outputs of work package 3
Fossey J, Lawrence, V. Staff views on the involvement of animals in care home life: an exploratory study. J Sociol Soc Welf 2013;40:305–33.
Whitaker R, Ballard C, Stafford J, Orrell M, Moniz-Cook E, Woods R, et al. Feasibility study of an optimised person-centred intervention to improve mental health and reduce antipsychotics among people with dementia in care homes: study protocol for a randomised controlled trial. Trials 2013;14:13.
Ballard C, Orrell M, YongZhong S, Moniz-Cook E, Stafford J, Whitaker R, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomised controlled trial by the well-being and health for people with dementia (WHELD) program. Am J Psychiatry 2016;173:252–62.
Lawrence V, Fossey J, Ballard C, Ferreira N, Murray J. Helping staff to implement psychosocial interventions in care homes: augmenting existing practices and meeting needs for support. Int J Geriatr Psychiatry 2016;31:284–93.
Rajkumar A, Ballard C, Fossey J, Corbett A, Woods R, Orrell M, et al. Apathy and its response to antipsychotic review and non-pharmacological interventions in people with dementia living in nursing homes: WHELD, a factorial cluster randomised controlled trial. J Am Med Dir Assoc 2016;17:741–7.
Romeo R, Knapp M, Salverda S, Orrell M, Fossey J, Ballard C. The cost of care homes for people with dementia in England: a modelling approach. Int J Geriatr Psychiatry 2016;32.
Ballard C, Orrell M, Sun Y, Moniz-Cook E, Stafford J, Whitaker R, et al. Impact of antipsychotic review and non-pharmacological intervention on health-related quality of life in people with dementia living in care homes: WHELD – a factorial cluster randomised controlled trial. Int J Geriatr Psychiatry 2017;32:1094–103.
Major outputs of work package 4
Fossey J, Garrod L, Lawrence V, Testad I, Stafford J, Murray J. ‘We should see her like part of the team’: an investigation into care home staff’s experiences of being part of a RCT of a complex psychosocial intervention. Aging Ment Health 2020;24:178–85.
Major outputs of work package 5
Whitaker R, Fossey J, Ballard C, Orrell M, Moniz-Cook E, Woods RT, et al. Improving well-being and health for people with dementia (WHELD): study protocol for a randomised controlled trial. Trials 2014;15:284.
Rajkumar AP, Ballard C, Fossey J, Orrell M, Moniz-Cook E, Woods R, et al. Epidemiology of pain in people with dementia living in care homes: longitudinal course, prevalence, and treatment implications. J Am Med Dir Assoc 2017;18:453.e1–453.e6.
Ballard C, Corbett A, Orrell M, Williams G, Moniz-Cook E, Romeo R, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLOS Med 2018;15:e1002500.
Fossey J, Garrod L, Tolbol Froiland C, Ballard C, Lawrence V, Testad I. What influences the sustainability of an effective psychosocial intervention for people with dementia living in care homes? A 9 to 12-month follow-up of the perceptions of staff in care homes involved in the WHELD randomised controlled trial. Int J Geriatr Psychiatry 2019;34:674–82.
Romeo R, Zala D, Knapp M, Orrell M, Fossey J, Ballard C. Improving the quality of life of care home residents with dementia: cost-effectiveness of an optimised intervention for residents with clinically significant agitation in dementia. Alzheimers Demen 2019;15:282–91.
Fossey J, Garrod L, Garcia A, Testad I. A qualitative analysis of trainer/coach experiences of changing care home practice in the Well-being and Health in Dementia randomised control trial. Dementia 2020;19:237–52.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review and appropriate agreements being in place. Data from the RCT described in WP5 is in the Dryad repository (URL: https://datadryad.org/resource/doi:10.5061/dryad.75373; accessed 1 November 2019).
The authors will be happy to discuss potential collaborations regarding other data and will make anonymised data available on that basis.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Alzheimer’s Disease International . World Alzheimer Report 2009. www.alz.co.uk/research/files/WorldAlzheimerReport.pdf (accessed 5 November 2019).
- Alzheimer’s Disease International . World Alzheimer’s Report: Journey of Caring. An Analysis of Long-Term Care in Dementia 2013.
- Centers for Medicare & Medicaid Services Center of Clinical Standards and Quality . CMS 2012 Nursing Home Action Plan: Action Plan for Further Improvement of Nursing Home Quality 2012.
- Department of Health and Social Care (DHSC) . Living Well With Dementia: A National Dementia Strategy 2009.
- Department of Health and Social Care . Prime Minister’s Challenge on Dementia 2020 2015. www.gov.uk/government/publications/prime-ministers-challenge-on-dementia-2020 (accessed 4 November 2019).
- Department of Health and Social Care . Prime Minister’s Challenge on Dementia 2020: Implementation Plan 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/507981/PM_Dementia-main_acc.pdf (accessed 6 November 2019).
- Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry 2016;173:543-6. https://doi.org/10.1176/appi.ajp.2015.173501.
- The King’s Fund . Social Care for Older People: Home Truths 2016. www.kingsfund.org.uk/publications/social-care-older-people (accessed 5 November 2019).
- Corbett A, Nunez K, Thomas A. Coping with dementia in care homes. Maturitas 2013;76:3-4. https://doi.org/10.1016/j.maturitas.2013.06.002.
- Lyketsos CG, Colenda CC, Beck C, Blank K, Doraiswamy MP, Kalunian DA, et al. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry 2006;14:561-72. https://doi.org/10.1097/01.JGP.0000221334.65330.55.
- Corbett A, Smith J, Creese B, Ballard C. Treatment of behavioural and psychological symptoms of Alzheimer’s disease. Curr Treat Options Neurol 2012;14:113-25. https://doi.org/10.1007/s11940-012-0166-9.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210. https://doi.org/10.1097/01.JGP.0000200589.01396.6d.
- Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.CD003476.pub2.
- Corbett A, Ballard C. Antipsychotics and mortality in dementia. Am J Psychiatry 2012;169:7-9. https://doi.org/10.1176/appi.ajp.2011.11101488.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43. https://doi.org/10.1001/jama.294.15.1934.
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 2009;8:151-7. https://doi.org/10.1016/S1474-4422(08)70295-3.
- US Food and Drug Administration . FDA Public Health Advisory: Deaths With Antipsychotics in Elderly Patients With Behavioural Disturbances 2005. http://psychrights.org/drugs/FDAantipsychotics4elderlywarning.htm (accessed 20 May 2020).
- Ballard C, O’Brien J, James I, Mynt P, Lana M, Potkins D, et al. Quality of life for people with dementia living in residential and nursing home care: the impact of performance on activities of daily living, behavioral and psychological symptoms, language skills, and psychotropic drugs. Int Psychogeriatr 2001;13:93-106. https://doi.org/10.1017/s1041610201007499.
- Ballard C, Margallo-Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. BMJ 2005;330. https://doi.org/10.1136/bmj.38369.459988.8F.
- Devanand DP, Pelton GH, Cunqueiro K, Sackeim HA, Marder K. A 6-month, randomized, double-blind, placebo-controlled pilot discontinuation trial following response to haloperidol treatment of psychosis and agitation in Alzheimer’s disease. Int J Geriatr Psychiatry 2011;26:937-43. https://doi.org/10.1002/gps.2630.
- Declercq T, Petrovic M, Azermai M, Vander Stichele R, De Sutter AI, van Driel ML, et al. Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia. Cochrane Database Syst Rev 2013;3. https://doi.org/10.1002/14651858.CD007726.pub2.
- European Medicines Agency . European Medicines Agency 2013 Priorities for Drug Safety Research: Long Term Safety Effects of Antipsychotics in Patients With Dementia 2012.
- Barnes TR, Banerjee S, Collins N, Treloar A, McIntyre SM, Paton C. Antipsychotics in dementia: prevalence and quality of antipsychotic drug prescribing in UK mental health services. Br J Psychiatry 2012;201:221-6. https://doi.org/10.1192/bjp.bp.111.107631.
- NHS Digital . National Dementia and Antipsychotic Prescribing Audit n.d. https://digital.nhs.uk/data-and-information/clinical-audits-and-registries/national-dementia-and-antipsychotic-prescribing-audit (accessed 6 November 2019).
- Gallini A, Andrieu S, Donohue JM, Oumouhou N, Lapeyre-Mestre M, Gardette V. Trends in use of antipsychotics in elderly patients with dementia: impact of national safety warnings. Eur Neuropsychopharmacol 2014;24:95-104. https://doi.org/10.1016/j.euroneuro.2013.09.003.
- CMS.gov . New Data Show Antipsychotic Drug Use Is Down in Nursing Homes Nationwide 2012.
- Fazio S, Pace D, Flinner J, Kallmyer B. The fundamentals of person-centered care for individuals with dementia. Gerontologist 2018;58:S10-S19. https://doi.org/10.1093/geront/gnx122.
- Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006;332:756-61. https://doi.org/10.1136/bmj.38782.575868.7C.
- Buettner L, Fitzsimmons S. NEST Approach. Dementia Practice Guidelines for Disturbing Behaviours. Needs, Environment, Stimulation, Techniques. State College, PA: Venture Publishing Inc.; 2009.
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein-Parbury J, Norman R, et al. Caring for Aged Dementia Care Resident Study (CADRES) of person-centred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trial. Lancet Neurol 2009;8:317-25. https://doi.org/10.1016/S1474-4422(09)70045-6.
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 2003;290:2015-22. https://doi.org/10.1001/jama.290.15.2015.
- Fossey J, Masson S, Stafford J, Lawrence V, Corbett A, Ballard C. The disconnect between evidence and practice: a systematic review of person-centred interventions and training manuals for care home staff working with people with dementia. Int J Geriatr Psychiatry 2014;29:797-80. https://doi.org/10.1002/gps.4072.
- Brooker DJ, Argyle E, Scally AJ, Clancy D. The enriched opportunities programme for people with dementia: a cluster-randomised controlled trial in 10 extra care housing schemes. Aging Ment Health 2011;15:1008-17. https://doi.org/10.1080/13607863.2011.583628.
- Bird M. Psychosocial Approaches to Challenging Behaviour in Dementia: A Controlled Trial. Report to the Commonwealth Department of Health and Ageing 2002.
- Teri L, Logsdon RG, McCurry SM. Exercise interventions for dementia and cognitive impairment: the Seattle Protocols. J Nutr Health Aging 2008;12:391-4. https://doi.org/10.1007/BF02982672.
- Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014;383:533-40. https://doi.org/10.1016/S0140-6736(13)62106-6.
- Cohen-Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: a controlled trial of systematic individualized intervention. J Gerontol A Biol Sci Med Sci 2007;62:908-16. https://doi.org/10.1093/gerona/62.8.908.
- Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M, Freedman L. Efficacy of nonpharmacologic interventions for agitation in advanced dementia: a randomized, placebo-controlled trial. J Clin Psychiatry 2012;73:1255-61. https://doi.org/10.4088/JCP.12m07918.
- Kovach CR, Taneli Y, Dohearty P, Schlidt AM, Cashin S, Silva-Smith AL. Effect of the BACE intervention on agitation of people with dementia. Gerontologist 2004;44:797-806. https://doi.org/10.1093/geront/44.6.797.
- Fitzsimmons S, Buettner LL. Therapeutic recreation interventions for need-driven dementia-compromised behaviors in community-dwelling elders. Am J Alzheimers Dis Other Demen 2002;17:367-81. https://doi.org/10.1177/153331750201700603.
- Buettner LL, Fitzsimmons S. AD-venture program: therapeutic biking for the treatment of depression in long-term care residents with dementia. Am J Alzheimers Dis Other Demen 2002;17:121-7. https://doi.org/10.1177/153331750201700205.
- Dempsey L, Murphy K, Cooney A, Casey D, O’Shea E, Devane D, et al. Reminiscence in dementia: a concept analysis. Dementia 2014;13:176-92. https://doi.org/10.1177/1471301212456277.
- Testad I, Corbett A, Aarsland D, Lexow KO, Fossey J, Woods B, et al. The value of personalized psychosocial interventions to address behavioural and psychological symptoms in people with dementia living in care home settings: a systematic review. Int Psychogeriatr 2014;26:1083-98. https://doi.org/10.1017/S1041610214000131.
- Tondi L, Ribani L, Bottazzi M, Viscomi G, Vulcano V. Validation therapy (VT) in nursing home: a case-control study. Arch Gerontol Geriatr 2007;44:407-11. https://doi.org/10.1016/j.archger.2007.01.057.
- Sung HC, Chang AM, Abbey J. The effects of preferred music on agitation of older people with dementia in Taiwan. Int J Geriatr Psychiatry 2006;21:999-1000. https://doi.org/10.1002/gps.1585.
- Choi AN, Lee MS, Cheong KJ, Lee JS. Effects of group music intervention on behavioural and psychological symptoms in patients with dementia: a pilot-controlled trial. Int J Neurosci 2009;119:471-81. https://doi.org/10.1080/00207450802328136.
- Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 2009;5:245-55. https://doi.org/10.1038/nrneurol.2009.39.
- Banerjee S. Living well with dementia – development of the national dementia strategy for England. Int J Geriatr Psychiatry 2010;25:917-22. https://doi.org/10.1002/gps.2598.
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Old Age Task Force of the World Federation of Biological Psychiatry . Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021. https://doi.org/10.1176/appi.ajp.162.11.1996.
- Aylward S, Stolee P, Keat N, Johncox V. Effectiveness of continuing education in long-term care: a literature review. Gerontologist 2003;43:259-71. https://doi.org/10.1093/geront/43.2.259.
- Kverno KS, Black BS, Nolan MT, Rabins PV. Research on treating neuropsychiatric symptoms of advanced dementia with non-pharmacological strategies, 1998–2008: a systematic literature review. Int Psychogeriatr 2009;21:825-43. https://doi.org/10.1017/S1041610209990196.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord 2010;30:161-78. https://doi.org/10.1159/000316119.
- O’Neil ME, Freeman M, Christensen V, Telerant R, Addleman A, Kansagara D. A Systematic Evidence Review of Non-pharmacological Interventions for Behavioural Symptoms of Dementia. Washington, DC: Department of Veterans Affairs; 2011.
- Corbett A, Stevens J, Aarsland D, Day S, Moniz-Cook E, Woods R, et al. Systematic review of services providing information and/or advice to people with dementia and/or their caregivers. Int J Geriatr Psychiatry 2012;27:628-36. https://doi.org/10.1002/gps.2762.
- Haslam C, Haslam SA, Jetten J, Bevins A, Ravenscroft S, Tonks J. The social treatment: the benefits of group interventions in residential care settings. Psychol Aging 2010;25:157-67. https://doi.org/10.1037/a0018256.
- Hsu YC, Wang JJ. Physical, affective, and behavioural effects of group reminiscence on depressed institutionalized elders in Taiwan. Nurs Res 2009;58:294-9. https://doi.org/10.1097/NNR.0b013e3181a308ee.
- Jones ED. Reminiscence therapy for older women with depression. Effects of nursing intervention classification in assisted-living long-term care. J Gerontol Nurs 2003;29:26-33. https://doi.org/10.3928/0098-9134-20030701-07.
- Karimi H, Dolatshahee B, Momeni K, Khodabakhshi A, Rezaei M, Kamrani AA. Effectiveness of integrative and instrumental reminiscence therapies on depression symptoms reduction in institutionalized older adults: an empirical study. Aging Ment Health 2010;14:881-7. https://doi.org/10.1080/13607861003801037.
- Wang JJ. Group reminiscence therapy for cognitive and affective function of demented elderly in Taiwan. Int J Geriatr Psychiatry 2007;22:1235-40. https://doi.org/10.1002/gps.1821.
- Wang JJ, Yen M, OuYang WC. Group reminiscence intervention in Taiwanese elders with dementia. Arch Gerontol Geriatr 2009;49:227-32. https://doi.org/10.1016/j.archger.2008.08.007.
- Sung HC, Lee WL, Li TL, Watson R. A group music intervention using percussion instruments with familiar music to reduce anxiety and agitation of institutionalized older adults with dementia. Int J Geriatr Psychiatry 2012;27:621-7. https://doi.org/10.1002/gps.2761.
- Sung HC, Chang AM, Lee WL. A preferred music listening intervention to reduce anxiety in older adults with dementia in nursing homes. J Clin Nurs 2010;19:1056-64. https://doi.org/10.1111/j.1365-2702.2009.03016.x.
- Ledger AJ, Baker FA. An investigation of long-term effects of group music therapy on agitation levels of people with Alzheimer’s Disease. Aging Ment Health 2007;11:330-8. https://doi.org/10.1080/13607860600963406.
- Lin Y, Chu H, Yang CY, Chen CH, Chen SG, Chang HJ, et al. Effectiveness of group music intervention against agitated behavior in elderly persons with dementia. Int J Geriatr Psychiatry 2011;26:670-8. https://doi.org/10.1002/gps.2580.
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Villani D, et al. Efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Dis Assoc Disord 2008;22:158-62. https://doi.org/10.1097/WAD.0b013e3181630b6f.
- van der Steen JT, van Soest-Poortvliet MC, van der Wouden JC, Bruinsma MS, Scholten RJPM, Vink AC. Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev 2017;5. https://doi.org/10.1002/14651858.CD003477.pub3.
- Cohen-Mansfield J, Marx MS, Dakheel-Ali M, Regier NG, Thein K, Freedman L. Can agitated behavior of nursing home residents with dementia be prevented with the use of standardized stimuli?. J Am Geriatr Soc 2010;58:1459-64. https://doi.org/10.1111/j.1532-5415.2010.02951.x.
- Finnema E, Dröes RM, Ettema T, Ooms M, Adèr H, Ribbe M, et al. The effect of integrated emotion-oriented care versus usual care on elderly persons with dementia in the nursing home and on nursing assistants: a randomized clinical trial. Int J Geriatr Psychiatry 2005;20:330-43. https://doi.org/10.1002/gps.1286.
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT. A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. J Am Geriatr Soc 2011;59:1032-41. https://doi.org/10.1111/j.1532-5415.2011.03449.x.
- Kovach CR, Logan BR, Noonan PE, Schlidt AM, Smerz J, Simpson M, et al. Effects of the Serial Trial Intervention on discomfort and behavior of nursing home residents with dementia. Am J Alzheimers Dis Other Demen 2006;21:147-55. https://doi.org/10.1177/1533317506288949.
- Phillips LJ, Reid-Arndt SA, Pak Y. Effects of a creative expression intervention on emotions, communication, and quality of life in persons with dementia. Nurs Res 2010;59:417-25. https://doi.org/10.1097/NNR.0b013e3181faff52.
- Politis AM, Vozzella S, Mayer LS, Onyike CU, Baker AS, Lyketsos CG. A randomized, controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long-term care. Int J Geriatr Psychiatry 2004;19:1087-94. https://doi.org/10.1002/gps.1215.
- Deponte A, Missan R. Effectiveness of validation therapy (VT) in group: preliminary results. Arch Gerontol Geriatr 2007;44:113-17. https://doi.org/10.1016/j.archger.2006.04.001.
- Brittle N, Patel S, Wright C, Baral S, Versfeld P, Sackley C. An exploratory cluster randomized controlled trial of group exercise on mobility and depression in care home residents. Clin Rehabil 2009;23:146-54. https://doi.org/10.1177/0269215508098891.
- Conradsson M, Littbrand H, Lindelof N, Gustafson Y, Rosendahl E. Effects of a high-intensity functional exercise programme on depressive symptoms and psychological well-being among older people living in residential care facilities: a cluster-randomized controlled trial. Aging Ment Health 2010;14:565-76. https://doi.org/10.1080/13607860903483078.
- Dechamps A, Diolez P, Thiaudière E, Tulon A, Onifade C, Vuong T, et al. Effects of exercise programs to prevent decline in health-related quality of life in highly deconditioned institutionalized elderly persons: a randomized controlled trial. Arch Intern Med 2010;170:162-9. https://doi.org/10.1001/archinternmed.2009.489.
- Eggermont LH, Knol DL, Hol EM, Swaab DF, Scherder EJ. Hand motor activity, cognition, mood, and the rest-activity rhythm in dementia: a clustered RCT. Behav Brain Res 2009;196:271-8. https://doi.org/10.1016/j.bbr.2008.09.012.
- Kerse N, Peri K, Robinson E, Wilkinson T, von Randow M, Kiata L, et al. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ 2008;337. https://doi.org/10.1136/bmj.a1445.
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc 2007;55:158-65. https://doi.org/10.1111/j.1532-5415.2007.01035.x.
- Rosendahl E, Gustafson Y, Nordin E, Lundin-Olsson L, Nyberg L. A randomized controlled trial of fall prevention by a high-intensity functional exercise program for older people living in residential care facilities. Aging Clin Exp Res 2008;20:67-75. https://doi.org/10.1007/BF03324750.
- Rosendahl E, Lindelöf N, Littbrand H, Yifter-Lindgren E, Lundin-Olsson L, Håglin L, et al. High-intensity functional exercise program and protein-enriched energy supplement for older persons dependent in activities of daily living: a randomised controlled trial. Aust J Physiother 2006;52:105-13. https://doi.org/10.1016/S0004-9514(06)70045-9.
- Sackley CM, van den Berg ME, Lett K, Patel S, Hollands K, Wright CC, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ 2009;339. https://doi.org/10.1136/bmj.b3123.
- Williams CL, Tappen RM. Effect of exercise on mood in nursing home residents with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2007;22:389-97. https://doi.org/10.1177/1533317507305588.
- Williams CL, Tappen RM. Exercise training for depressed older adults with Alzheimer’s disease. Aging Ment Health 2008;12:72-80. https://doi.org/10.1080/13607860701529932.
- Fan JT, Chen KM. Using silver yoga exercises to promote physical and mental health of elders with dementia in long-term care facilities. Int Psychogeriatr 2011;23:1222-30. https://doi.org/10.1017/S1041610211000287.
- Cohen-Mansfield J, Werner P. Management of verbally disruptive behaviors in nursing home residents. J Gerontol A Biol Sci Med Sci 1997;52:M369-77. https://doi.org/10.1093/gerona/52a.6.m369.
- Buettner LL, Lundegren HM, Lago D, Farrell P, Smith RW. Therapeutic recreation as an intervention for persons with dementia and agitation: an efficacy study. Am J Alz Dis 1996;11:4-12. https://doi.org/10.1177/153331759601100503.
- Proctor R, Burns A, Powell HS, Tarrier N, Faragher B, Richardson G, et al. Behavioural management in nursing and residential homes: a randomised controlled trial. Lancet 1999;354:26-9. https://doi.org/10.1016/S0140-6736(98)08237-3.
- Goldwasser AN, Auerbach SM, Harkins SW. Cognitive, affective, and behavioural effects of reminiscence group therapy on demented elderly. Int J Aging Hum Dev 1987;25:209-22. https://doi.org/10.2190/8UX8-68VC-RDYF-VK4F.
- Buettner LL. Simple pleasures: a multilevel sensorimotor intervention for nursing home residents with dementia. Am J Alz Dis 1999;14. https://doi.org/10.1177/153331759901400103.
- Buettner L, Ferranio J. Therapeutic recreation-nursing team: a therapeutic intervention for nursing home residents with dementia. Ann Ther Recreat 1998;7:21-8.
- Burgio LD, Stevens A, Burgio KL, Roth DL, Paul P, Gerstle J. Teaching and maintaining behavior management skills in the nursing home. Gerontologist 2002;42:487-96. https://doi.org/10.1093/geront/42.4.487.
- Rovner BW, Steele CD, Shmuely Y, Folstein MF. A randomized trial of dementia care in nursing homes. J Am Geriatr Soc 1996;44:7-13. https://doi.org/10.1111/j.1532-5415.1996.tb05631.x.
- Spector A, Thorgrimsen L, Woods R, Orrell M. Making a difference: an evidence-based group programme to offer cognitive stimulation therapy (CST) to people with dementia: the manual for group leaders. London: Hawker Publications Ltd; 2006.
- Fossey J, James I. Evidence Based Approaches to Improving Dementia Care in Care Homes. London: Alzheimer’s Society; 2009.
- Loveday B, Kitwood T, Bowe B. Improving Dementia Care: a Resource for Training and Professional Development. London: Hawker Publications Ltd; 1998.
- NHS Improvement . An Economic Evaluation of Alternatives to Antipsychotic Drugs for Individuals Living With Dementia 2011.
- Sandelowski M, Docherty S, Emden C. Focus on qualitative methods. Qualitative metasynthesis: issues and techniques. Res Nurs Health 1997;20:365-71. https://doi.org/10.1002/(SICI)1098-240X(199708)20:4<365::AID-NUR9>3.0.CO;2-E.
- Campbell R, Pound P, Pope C, Britten N, Pill R, Morgan M, et al. Evaluating meta-ethnography: a synthesis of qualitative research on lay experiences of diabetes and diabetes care. Soc Sci Med 2003;56:671-84. https://doi.org/10.1016/S0277-9536(02)00064-3.
- Lawrence V, Fossey J, Ballard C, Moniz-Cook E, Murray J. Improving quality of life for people with dementia in care homes: making psychosocial interventions work. Br J Psychiatry 2012;201:344-51. https://doi.org/10.1192/bjp.bp.111.101402.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30. https://doi.org/10.1371/journal.pmed.1000097.
- Critical Appraisal Skills Programme (CASP) . Collaboration for Qualitative Methodologies 1998.
- Dixon-Woods M, Bonas S, Booth A, Jones DR, Miller T, Sutton AJ, et al. How can systematic reviews incorporate qualitative research? A critical perspective. Qual Res 2006;6:27-44. https://doi.org/10.1177/1468794106058867.
- Britten N, Campbell R, Pope C, Donovan J, Morgan M, Pill R. Using meta ethnography to synthesise qualitative research: a worked example. J Health Serv Res Policy 2002;7:209-15. https://doi.org/10.1258/135581902320432732.
- Spalding M, Khalsa P. Aging matters: humanistic and transpersonal approaches to psychotherapy with elders with dementia. J Humanist Psychol 2010;50:142-74. https://doi.org/10.1177/0022167809341995.
- Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: video recorded morning care from different care units. J Clin Nurs 2003;12:888-98. https://doi.org/10.1046/j.1365-2702.2003.00809.x.
- Sixsmith A, Gibson G. Music and the wellbeing of people with dementia. Ageing Soc 2007;27:127-45. https://doi.org/10.1017/S0144686X06005228.
- Perry J, Galloway S, Bottorff JL, Nixon S. Nurse-patient communication in dementia: improving the odds. J Gerontol Nurs 2005;31:43-52. https://doi.org/10.3928/0098-9134-20050401-10.
- Patton D. Reality orientation: its use and effectiveness within older person mental health care. J Clin Nurs 2006;15:1440-9. https://doi.org/10.1111/j.1365-2702.2005.01450.x.
- Palo-Bengtsson L, Ekman S. Emotional response to social dancing and walks in persons with dementia. Am J Alzheimer Dis Other Demen 2002;17:149-53. https://doi.org/10.1177/153331750201700308.
- Palo-Bengtsson L, Ekman SL. Dance events as a caregiver intervention for persons with dementia. Nurs Inq 2000;7:156-65. https://doi.org/10.1046/j.1440-1800.2000.00064.x.
- Palo-Bengtsson L, Winblad B, Ekman SL. Social dancing: a way to support intellectual, emotional and motor functions in persons with dementia. J Psychiatr Ment Health Nurs 1998;5:545-54. https://doi.org/10.1046/j.1365-2850.1998.560545.x.
- McKeown J, Clarke A, Ingleton C, Ryan T, Repper J. The use of life story work with people with dementia to enhance person-centred care. Int J Older People Nurs 2010;5:148-58. https://doi.org/10.1111/j.1748-3743.2010.00219.x.
- McAllister CL, Silverman MA. Community formation and community roles among persons with Alzheimer’s disease: a comparative study of experiences in a residential Alzheimer’s facility and a traditional nursing home. Qual Health Res 1999;9:65-8. https://doi.org/https://doi.org/10.1177/104973299129121703.
- Long Foley K, Sudha S, Sloane PD, Gold DT. Staff perceptions of successful management of severe behavioural problems in dementia special care units. Dementia 2003;2:105-24. https://doi.org/10.1177/1471301203002001998.
- Kydd P. Using music therapy to help a client with Alzheimer’s disease adapt to long-term care. Am J Alzheimers Dis Other Demen 2001;16:103-8. https://doi.org/10.1177/153331750101600209.
- Kovach CR, Henschel H. Planning activities for patients with dementia: a descriptive study of therapeutic activities on special care units. J Gerontol Nurs 1996;22:33-8. https://doi.org/10.3928/0098-9134-19960901-10.
- Kontos PC, Mitchell GJ, Mistry B, Ballon B. Using drama to improve person-centred dementia care. Int J Older People Nurs 2010;5:159-68. https://doi.org/10.1111/j.1748-3743.2010.00221.x.
- Kolanowski A, Fick D, Frazer C, Penrod J. It’s about time: use of nonpharmacological interventions in the nursing home. J Nurs Scholarsh 2010;42:214-22. https://doi.org/10.1111/j.1547-5069.2010.01338.x.
- Kemeny B, Boettcher IF, Deshon RP, Stevens AB. Postintervention focus groups: toward sustaining care. J Gerontol Nurs 2004;30:4-9. https://doi.org/10.3928/0098-9134-20040801-04.
- Kellett U, Moyle W, McAllister M, King C, Gallagher F. Life stories and biography: a means of connecting family and staff to people with dementia. J Clin Nurs 2010;19:1707-15. https://doi.org/10.1111/j.1365-2702.2009.03116.x.
- Kawamura N, Niiyama M, Niiyama H. Animal-assisted activity: experiences of institutionalized Japanese older adults. J Psychosoc Nurs Ment Health Serv 2009;47:41-7. https://doi.org/10.3928/02793695-20090101-08.
- Katsinas RP. The use and implications of a canine companion in a therapeutic day program for nursing home residents with dementia. Act Adapt Aging 2000;25:13-30. https://doi.org/10.1300/J016v25n01_02.
- Johnson JR. Effectiveness of memory notebooks upon problematic behaviour of residents with Alzheimer’s disease. Phys Occup Ther Geriatr 1997;15:15-32. https://doi.org/10.1080/J148v15n02_02.
- Holthe T, Thorsen K, Josephsson S. Occupational patterns of people with dementia in residential care: an ethnographic study. Scand J Occup Ther 2007;14:96-107. https://doi.org/10.1080/11038120600963796.
- Hernandez RO. Effects of therapeutic gardens in special care units for people with dementia: two case studies. J Hous Elderly 2007;21:117-52.
- Hansebo G, Kihlgren M. Patient life stories and current situation as told by carers in nursing home wards. Clin Nurs Res 2000;9:260-79. https://doi.org/10.1177/10547730022158582.
- Hagens C, Beaman A, Ryan EB. Reminiscing, poetry writing, and remembering boxes: personhood-centered communication with cognitively impaired older adults. Act Adapt Aging 2003;27:97-112. https://doi.org/10.1300/J016v27n03_07.
- Götell E, Brown S, Ekman SL. The influence of caregiver singing and background music on vocally expressed emotions and moods in dementia care: a qualitative analysis. Int J Nurs Stud 2009;46:422-30. https://doi.org/10.1016/j.ijnurstu.2007.11.001.
- Götell E, Brown S, Ekman SL. Influence of caregiver singing and background music on posture, movement, and sensory awareness in dementia care. Int Psychogeriatr 2003;15:411-30. https://doi.org/10.1017/S1041610203009657.
- Götell E, Brown S, Ekman SL. Caregiver singing and background music in dementia care. West J Nurs Res 2002;24:195-216. https://doi.org/10.1177/019394590202400208.
- Götell E, Brown S, Ekman SL. Caregiver-assisted music events in psychogeriatric care. J Psychiatr Ment Health Nurs 2000;7:119-25. https://doi.org/10.1046/j.1365-2850.2000.00271.x.
- Gnaedinger N. Changes in long-term care for elderly people with dementia: a report from the front lines in British Columbia, Canada. J Soc Work in Long Term Care 2003;2:355-71. https://doi.org/10.1300/J181v02n03_12.
- Gibson G, Chalfont GE, Clarke PD, Torrington JM, Sixsmith AJ. Housing and connection to nature for people with dementia. J Hou Elderly 2007;21:55-72. https://doi.org/10.1300/J081v21n01_04.
- Gibb H, Morris CT, Gleisberg J. A therapeutic programme for people with dementia. Int J Nurs Pract 1997;3:191-9. https://doi.org/10.1111/j.1440-172x.1997.tb00099.x.
- Gerdner LA. Use of individualized music by trained staff and family: translating research into practice. J Gerontol Nurs 2005;31:22-30. https://doi.org/10.3928/0098-9134-20050601-08.
- George DR. Intergenerational volunteering and quality of life: mixed methods evaluation of a randomized control trial involving persons with mild to moderate dementia. Qual Life Res 2011;20:987-95. https://doi.org/10.1007/s11136-010-9837-8.
- Galik EM, Resnick B, Pretzer-Aboff I. ‘Knowing what makes them tick’: motivating cognitively impaired older adults to participate in restorative care. Int J Nurs Pract 2009;15:48-55. https://doi.org/10.1111/j.1440-172X.2008.01721.x.
- Fraser F, James I. Why does doll therapy improve the well-being of some older adults with dementia. PSIEGE Newsl 2008;105:55-63.
- Emilsson UM. Supervision as pedagogy and support in the Swedish eldercare – a developmental project. J Gerontol Soc Work 2006;47:83-102. https://doi.org/10.1300/J083v47n03_06.
- Eggers T, Norberg A, Ekman SL. Counteracting fragmentation in the care of people with moderate and severe dementia. Clin Nurs Res 2005;14:343-69. https://doi.org/10.1177/1054773805277957.
- Barry A, Davies S. Moving forward together: evaluation of an action group involving staff and relatives within a nursing home for older people with dementia. Int J Older People Nurs 2006;1:95-104. https://doi.org/10.1111/j.1748-3743.2006.00005.x.
- Dupuis M, Dobbelsteyn J, Ericson P. Special care units for residents with Alzheimer’s (investigating the perceptions of families and staff). Can Nurs Home 1996;7.
- Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviours in dementia: a review, summary, and critique. Am J Geriatr Psychiatry 2001;9:361-81. https://doi.org/10.1097/00019442-200111000-00005.
- Teri L, Logsdon RG, McCurry SM. Nonpharmacologic treatment of behavioural disturbance in dementia. Med Clin North Am 2002;86:641-56. https://doi.org/10.1016/S0025-7125(02)00006-8.
- Ballard C, Brown R, Fossey J, Douglas S, Bradley P, Hancock J, et al. Brief psychosocial therapy for the treatment of agitation in Alzheimer disease (the CALM-AD trial). Am J Geriatr Psychiatry 2009;17:726-33. https://doi.org/10.1097/JGP.0b013e3181b0f8c0.
- Teri L, McCurry SM, Logsdon RG, Moore MS, Tyll T, Ebel S, et al. Reducing Disability in Alzheimer’s Disease (RDAD). A Manual for Therapists. Seattle, WA: University of Washington; 2003.
- All Party Parliamentary Group on Dementia . Always a Last Resort: Inquiry into the Prescription of Antipsychotic Drugs to People With Dementia Living in Care Homes 2008.
- Overprescribed: The Human and Taxpayers’ Costs of Antipsychotics in Nursing Homes n.d. www.govinfo.gov/content/pkg/CHRG-112shrg72764/html/CHRG-112shrg72764.htm (accessed 5 November 2019).
- Galeotti F, Giusti A, Meduri F, Raschetti R, Scardetta P, Vanacore N. Alzheimer Cooperative Valuation in Europe, ALCOVE Surveys on the Exposure to Antipsychotics in People Living With Dementia n.d. www.alcove-project.eu (accessed 5 November 2019).
- Ballard C, Orrell M, YongZhong S, Moniz-Cook E, Stafford J, Whittaker R, et al. Impact of antipsychotic review and non-pharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the well-being and health for people with dementia (WHELD) program. Am J Psychiatry 2016;173:252-62. https://doi.org/10.1176/appi.ajp.2015.15010130.
- Whitaker R, Ballard C, Stafford J, Orrell M, Moniz-Cook E, Woods RT, et al. Feasibility study of an optimised person-centred intervention to improve mental health and reduce antipsychotics amongst people with dementia in care homes: study protocol for a randomised controlled trial. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-13.
- Ballard C, Orrell M, Sun Y, Moniz-Cook E, Stafford J, Whitaker R, et al. Impact of antipsychotic review and non-pharmacological intervention on health-related quality of life in people with dementia living in care homes: WHELD – a factorial cluster randomised controlled trial. Int J Geriatr Psychiatry 2017;32:1094-103. https://doi.org/10.1002/gps.4572.
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-14. https://doi.org/10.1212/wnl.43.11.2412-a.
- Reisberg B. Functional assessment staging (FAST). Psychopharmacol Bull 1988;24:653-9. https://doi.org/10.1037/t08620-000.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed July 2020).
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for depression in dementia. Biol Psychiatry 1988;23:271-84. https://doi.org/10.1016/0006-3223(88)90038-8.
- Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol 1989;44:M77-84. https://doi.org/10.1093/geronj/44.3.m77.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. https://doi.org/10.1212/wnl.44.12.2308.
- Mulhern B, Rowen D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17050.
- Mulhern B, Smith SC, Rowen D, Brazier JE, Knapp M, Lamping DL, et al. Improving the measurement of QALYs in dementia: developing patient- and carer-reported health state classification systems using Rasch analysis. Value Health 2012;15:323-33. https://doi.org/10.1016/j.jval.2011.09.006.
- Rajkumar A, Ballard C, Fossey J, Corbett A, Woods R, Orrell M, et al. Apathy and its response to antipsychotic review and non-pharmacological interventions in people with dementia living in nursing homes: WHELD, a factorial cluster randomised controlled trial. J Am Med Dir Assoc 2016;17:741-7. https://doi.org/10.1016/j.jamda.2016.04.006.
- Huber PJ. Robust Statistics. New York, NY: John Wiley & Sons; 1981.
- White HL. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817-38. https://doi.org/10.2307/1912934.
- Rogers WH. Regression standard errors in clustered samples. Stata Tech Bull 1993;13:19-23.
- Romeo R, Knapp M, Salverda S, Orrell M, Fossey J, Ballard C. The cost of care homes for people with dementia in England: a modelling approach. Int J Geriatr Psychiatry 2016;32:1466-75. https://doi.org/10.1002/gps.4637.
- Glaser BG. Theoretical Sensitivity: Advances in the Methodology of Grounded Theory. Mill Valley, CA: Sociology Press; 1978.
- Lawrence V, Fossey J, Ballard C, Ferreira N, Murray J. Helping staff to implement psychosocial interventions in care homes: augmenting existing practices and meeting needs for support. Int J Geriatr Psychiatry 2016;31:284-93. https://doi.org/10.1002/gps.4322.
- Fossey J, Lawrence V. Staff views on the involvement of animals in care home life: an exploratory study. J Soc and Soc Welfare 2013;4:305-33.
- Lawrence V, Banerjee S. Improving care in care homes: a qualitative evaluation of the Croydon care home support team. Aging Ment Health 2010;14:416-24. https://doi.org/10.1080/13607860903586144.
- Seitz DP, Brisbin S, Herrmann N, Rapoport MJ, Wilson K, Gill SS, et al. Efficacy and feasibility of nonpharmacological interventions for neuropsychiatric symptoms of dementia in long term care: a systematic review. J Am Med Dir Assoc 2012;13:503-6.e2. https://doi.org/10.1016/j.jamda.2011.12.059.
- Zimmerman S, Sloane PD, Williams CS, Reed PS, Preisser JS, Eckert JK, et al. Dementia care and quality of life in assisted living and nursing homes. Gerontologist 2005;45:133-46. https://doi.org/10.1093/geront/45.suppl_1.133.
- Moniz-Cook E, Woods RT, Gardiner E. Staff factors associated with perception of behaviour as ‘challenging’ in residential and nursing homes. Aging Ment Health 2000;4:48-55. https://doi.org/10.1080/13607860055973.
- Brodaty H, Draper B, Low LF. Nursing home staff attitudes towards residents with dementia: strain and satisfaction with work. J Adv Nurs 2003;44:583-90. https://doi.org/10.1046/j.0309-2402.2003.02848.x.
- Berlowitz DR, Young GJ, Hickey EC, Saliba D, Mittman BS, Czarnowski E, et al. Quality improvement implementation in the nursing home. Health Serv Res 2003;38:65-83. https://doi.org/10.1111/1475-6773.00105.
- Scott-Cawiezell J, Schenkman M, Moore L, Vojir C, Connolly RP, Pratt M, et al. Exploring nursing home staff’s perceptions of communication and leadership to facilitate quality improvement. J Nurs Care Qual 2004;19:242-52. https://doi.org/10.1097/00001786-200407000-00011.
- Rantz MJ, Zwygart-Stauffacher M, Flesner M, Hicks L, Mehr D, Russell T, et al. The influence of teams to sustain quality improvement in nursing homes that ‘need improvement’. J Am Med Dir Assoc 2013;14:48-52. https://doi.org/10.1016/j.jamda.2012.09.008.
- Robison J, Curry L, Gruman C, Porter M, Henderson CR, Pillemer K. Partners in caregiving in a special care environment: cooperative communication between staff and families on dementia units. Gerontologist 2007;47:504-15. https://doi.org/10.1093/geront/47.4.504.
- Fossey J, Garrod L, Lawrence V, Testad I, Stafford J, Murray J. ‘We should see her like part of the team’: an investigation into care home staff’s experiences of being part of a RCT of a complex psychosocial intervention. Aging Ment Health 2020;24:178-85. https://doi.org/10.1080/13607863.2018.1525603.
- Ballard C, Corbett A, Orrell M, Williams G, Moniz-Cook E, Romeo R, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLoS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002500.
- Whitaker R, Fossey J, Ballard C, Orrell M, Moniz-Cook E, Woods RT, et al. Improving Well-being and Health for People with Dementia (WHELD): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-284.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry 2000;8:75-83. https://doi.org/10.1097/00019442-200002000-00010.
- Reynolds T, Thornicroft G, Abas M, Woods B, Hoe J, Leese M, et al. Camberwell Assessment of Need for the Elderly (CANE). Development, validity and reliability. Br J Psychiatry 2000;176:444-52. https://doi.org/10.1192/bjp.176.5.444.
- Dean R, Proudfoot R, Lindesay J. The quality of interactions schedule (QUIS): development, reliability and use in the evaluation of two domus units. Int J Geriatr Psychiatry 1993;8:819-26. https://doi.org/10.1002/gps.930081004.
- Abbey J, Piller N, De Bellis A, Esterman A, Parker D, Giles L, et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs 2004;10:6-13. https://doi.org/10.12968/ijpn.2004.10.1.12013.
- Romeo R, Knapp M, Hellier J, Dewey M, Ballard C, Baldwin R, et al. Cost-effectiveness analyses for mirtazapine and sertraline in dementia: randomised controlled trial. Br J Psychiatry 2013;202:121-8. https://doi.org/10.1192/bjp.bp.112.115212.
- Knapp M, Iemmi V, Romeo R. Dementia care costs and outcomes: a systematic review. Int J Geriatr Psychiatry 2013;28:551-61. https://doi.org/10.1002/gps.3864.
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. https://doi.org/10.1002/sim.4175.
- Romeo R, Zala D, Knapp M, Orrell M, Fossey J, Ballard C. Improving the quality of life of care home residents with dementia: cost-effectiveness of an optimized intervention for residents with clinically significant agitation in dementia. Alzheimers Demen 2019;15:282-91.
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006;355:1525-38. https://doi.org/10.1056/NEJMoa061240.
- Wetzels RB, Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Determinants of quality of life in nursing home residents with dementia. Dement Geriatr Cogn Disord 2010;29:189-97. https://doi.org/10.1159/000280437.
- Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014;311:682-91. https://doi.org/10.1001/jama.2014.93.
- Cummings JL, Lyketsos CG, Peskind ER, Porsteinsson AP, Mintzer JE, Scharre DW, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015;314:1242-54. https://doi.org/10.1001/jama.2015.10214.
- Teri L, Logsdon RG, Uomoto J, McCurry SM. Behavioral treatment of depression in dementia patients: a controlled clinical trial. J Gerontol B Psychol Sci Soc Sci 1997;52:P159-66. https://doi.org/10.1093/geronb/52b.4.p159.
- Rajkumar AP, Ballard C, Fossey J, Orrell M, Moniz-Cook E, Woods R, et al. Epidemiology of pain in people with dementia living in care homes: longitudinal course, prevalence, and treatment implications. J Am Med Dir Assoc 2017;18:453.e1-6. https://doi.org/10.1016/j.jamda.2017.01.024.
- Fossey J, Garrod L, Garcia A, Testad I. A qualitative analysis of trainer/coach experiences of changing care home practice in the Well-being and Health in Dementia randomised control trial. Dementia 2020;19:237-52. https://doi.org/10.1177/1471301218772178.
- Fossey J, Garrod L, Tolbol Froiland C, Ballard C, Lawrence V, Testad I. What influences the sustainability of an effective psychosocial intervention for people with dementia living in care homes? A 9 to 12-month follow-up of the perceptions of staff in care homes involved in the WHELD randomised controlled trial. Int J Geriatr Psychiatry 2019;34:674-82. https://doi.org/10.1002/gps.5066.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
Appendix 1 Working papers in respect of work package 3
Agreed work plan
Analysis plan
List of abbreviations
- AD
- Alzheimer’s disease
- ANCOVA
- analysis of covariance
- BEHAV-AD
- Behaviour in Alzheimer’s disease
- BPSD
- behavioural and psychological symptoms of dementia
- CANE
- Camberwell Assessment of Need in the Elderly
- CASP
- Critical Appraisal Skills Programme
- CDR
- Clinical Dementia Rating
- CI
- confidence interval
- CMAI
- Cohen-Mansfield Agitation Inventory
- CSRI
- Client Service Receipt Inventory
- DEMQoL
- Dementia Quality of Life Scale
- ES
- effect size
- FITS
- Focused Intervention for Training and Support
- GP
- general practitioner
- GRIPP2
- Guidance for Reporting Involvement of Patients and the Public 2
- ICC
- intracluster correlation coefficient
- MRC
- Medical Research Council
- NEST
- Needs, Environment, Stimulation and Techniques
- NIHR
- National Institute for Health Research
- NPI-NH
- Neuropsychiatric Inventory (nursing home version)
- NVQ
- National Vocational Qualification
- NWORTH
- North Wales Organisation for Randomised Trials
- OR
- odds ratio
- PMG
- Programme Management Group
- PPI
- patient and public involvement
- QoL
- quality of life
- QUIS
- Quality of Interactions Scale
- RCT
- randomised controlled trial
- RDAD
- Reducing Disability in Alzheimer’s Disease
- SD
- standard deviation
- SEM
- standard error of measurement
- TAU
- treatment as usual
- TDG
- Therapy Development Group
- TSC
- Trial Steering Committee
- WHELD
- Well-being and Health for people with Dementia
- WP
- work package
