Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0611-20005. The contractual start date was in October 2013. The final report began editorial review in May 2019 and was accepted for publication in May 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Louise Robinson was a member of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme primary care themed call board from 1 October 2012 to 18 February 2014. Claire Goodman was a trustee of the Order of St John Care Trust from 2016 to 2019, a NIHR Health Services and Delivery Research (HSDR) Programme Commissioning Board member from February 2012 to 30 June 2015 and a NHS Service Delivery and Organisation (SDO) Commissioning Board member from 1 April 2009 to 1 January 2012; she is currently a NIHR senior investigator (since 2016). Denise Howel was a member of the NIHR SDO Commissioning Board from April 2009 to December 2011, a member of the NIHR HSDR Programme Commissioning Board from January 2012 to November 2015 and a member of the NIHR Programme Grants for Applied Research (PGfAR) Programme funding subpanel from February 2016 to present. Luke Vale was a member of the NIHR HTA Clinical Evaluation and Trials funding panel from 2014 to 2018 and a member of the NIHR PGfAR Programme funding subpanel from 2008 to 2016.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Robinson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
SYNOPSIS
Background
Our ageing societies and prevalence of dementia
The number of people living with dementia is predicted to double by 2040. 1,2 Dementia has the greatest disease burden of all long-term illnesses. 1,3 Nationally, the cost of dementia care is estimated to be £26B, with community care costs accounting for almost half of this. 2,4 More older people are experiencing a slower, more unpredictable, dying pathway5,6 as a result of multimorbidity,7 age-related illnesses such as dementia8,9 and frailty, leading to an increased need for better-integrated community care, especially if the preferred final outcome is death in the usual place of care. 5,10–12
Dying with dementia
In the UK, the National Institute for Health and Care Excellence (NICE) considers end-of-life care (EOLC) to include all health and social care provided in all settings to the following groups of people: those who are likely to die within 12 months, those with advanced, progressive, incurable conditions, and those with life-threatening acute conditions. 13–15 EOLC also covers support for families and carers. More recently, NICE has provided separate evidence-based care recommendations for patients whom professionals consider to be in the last few days of their life, when more intensive support is needed. 13,14 Because of the unpredictable dying trajectory in dementia, professionals often find it difficult to predict when a person is dying. 16,17 EOLC can, therefore, be considered more than the last few days of life: the term ‘supportive care’ was coined to reflect the need for sustained care throughout the illness trajectory. 18 In terms of the quality of care, evidence consistently shows that people with advanced dementia experience poorer EOLC than those with cancer, with increased hospitalisation, inadequate pain control and fewer palliative care interventions. 19–21 In addition, family carers of people with advanced dementia require more emotional support prior to the person’s death than afterwards;22 many do not consider dementia as a terminal illness and know little about the symptoms and prognosis of the advanced illness. 23 With respect to place of death for people with dementia in the UK, few people die at home. Nearly half die in care homes and around one-third die in hospital;24–26 very few people with a primary diagnosis of dementia use hospice care. 27
Palliative care in dementia
Palliative care is defined as:
An approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual.
World Health Organization. 28 © Copyright World Health Organization (WHO), 2020. All Rights Reserved. URL: www.who.int/cancer/palliative/definition/en/
People with advanced dementia experience symptoms that are comparable to the symptoms of those dying with cancer. 12 Professionals experience a number of difficulties in meeting the palliative care needs of people with advanced dementia; for example, pain and symptom management is particularly difficult, as people with dementia may not be able to verbalise their symptoms. Despite this, however, the use of evidence-based pain assessment tools in community settings is low. 29 In addition, some professionals do not consider dementia a terminal illness30 and find prognostication difficult. 20,21,31 Both medical and nursing home staff consistently overestimate prognosis in advanced dementia. In one US study,31 only 1% of care home residents at admission were thought to have a life expectancy of < 6 months, yet 71% died in that period. A palliative care approach as dementia progresses is recommended both nationally29,32 and internationally;33 however, the evidence base to inform translation of these recommendations into practice is still limited.
End-of-life care in dementia in the UK: current service provision and commissioning
In the UK, access to specialist palliative care by families caring for people with advanced dementia is limited. 34 The quality of EOLC, in general, has been strongly influenced by the introduction of a national End of Life Care Strategy and programme,35 with associated quality markers to measure care outcomes. 36 These include the use of advance care planning (ACP), to promote patient choice, and EOLC pathways, for example the Liverpool Care Pathway for the Dying Patient (LCP)37 and the Gold Standards Framework (GSF). 38 However, the national End of Life Care Strategy35 was developed from cancer care, and directly transferring interventions may not be appropriate in dementia, for which the dying trajectory is longer and more unpredictable. 39,40 In a UK population, median survival time in dementia is 4.1 years from diagnosis,41 but this can be up to 7–10 years in younger age groups (60–69 years),42 or as low as 1.3 years in an older care home population. 43 Evidence has been slow to emerge of the effectiveness of ACP in dementia care44–46 in terms of reducing potentially harmful interventions such as hospitalisation, but few families living with dementia seem to want to complete formal ACP documents. 47–49
In England, guidance on commissioning dementia services is available from several sources,50 and NICE reiterated the need to follow other relevant existing guidance. 32,51 Currently, health and well-being boards in England do not prioritise EOLC in their strategies. 52 The Department of Health has developed dementia commissioning resources on early diagnosis and intervention, better care in acute hospitals and support for people in the community,53 but none of these documents covers EOLC in detail.
End-of-life care in dementia in the UK: research to date
Despite increasing international research, there has been little UK research in this area,20,54–56 even though EOLC was highlighted as a national research priority. 57 The Marie Curie, Alzheimer’s Society James Lind Alliance Priority Setting partnerships, which worked with the public, have also prioritised research in this area. 58,59
One UK-based study explored what constituted good EOLC in dementia with bereaved carers and professionals. 47 Family carers felt that people with dementia should die free from pain and surrounded by their relatives, whereas professional carers identified physical needs, emotional and spiritual issues, and care planning. However, general practitioners (GPs), who provide most of the EOLC in dementia,60 were not included.
England still has a high rate of hospital death in dementia (40%),61 and few people with dementia die at home. 35,62 Avoiding care transitions is important, but policy has focused mainly on promoting death in the usual place of care, rather than on the quality of dying. 35 Carers and people with dementia report comfort and QoL as the main goals of care. 43 Updated NICE dementia guidance states that people with dementia should receive flexible, needs-based palliative care that addresses the unpredictable progression,29 but models of how to achieve this in practice are lacking. Recent (2013–18) UK research has found high levels of persistent pain in care home residents with severe dementia,63 increasing anguish for carers,64,65 and rising numbers of people with dementia attending emergency departments in the last year of life. 66 Realist methods have increased understanding of integration67 and the barriers to and facilitators of good dementia palliative care,68,69 but significant research gaps remain. These include the following:
Programme aim and objectives
The overall aim of the Supporting Excellence in End-of-life care in Dementia (SEED) programme was to support professionals to deliver good-quality, community-based care towards, and at, the end of life (EOL) for people living with dementia and their families. Specific objectives were to:
-
identify which aspects of existing care towards, and at, EOL in dementia are effective and efficient
-
develop, implement and evaluate an evidence-based intervention, and associated resources, to support good-quality care towards, and at, EOL in dementia
-
determine how community-based EOLC in dementia should be organised and commissioned.
Programme design and methods
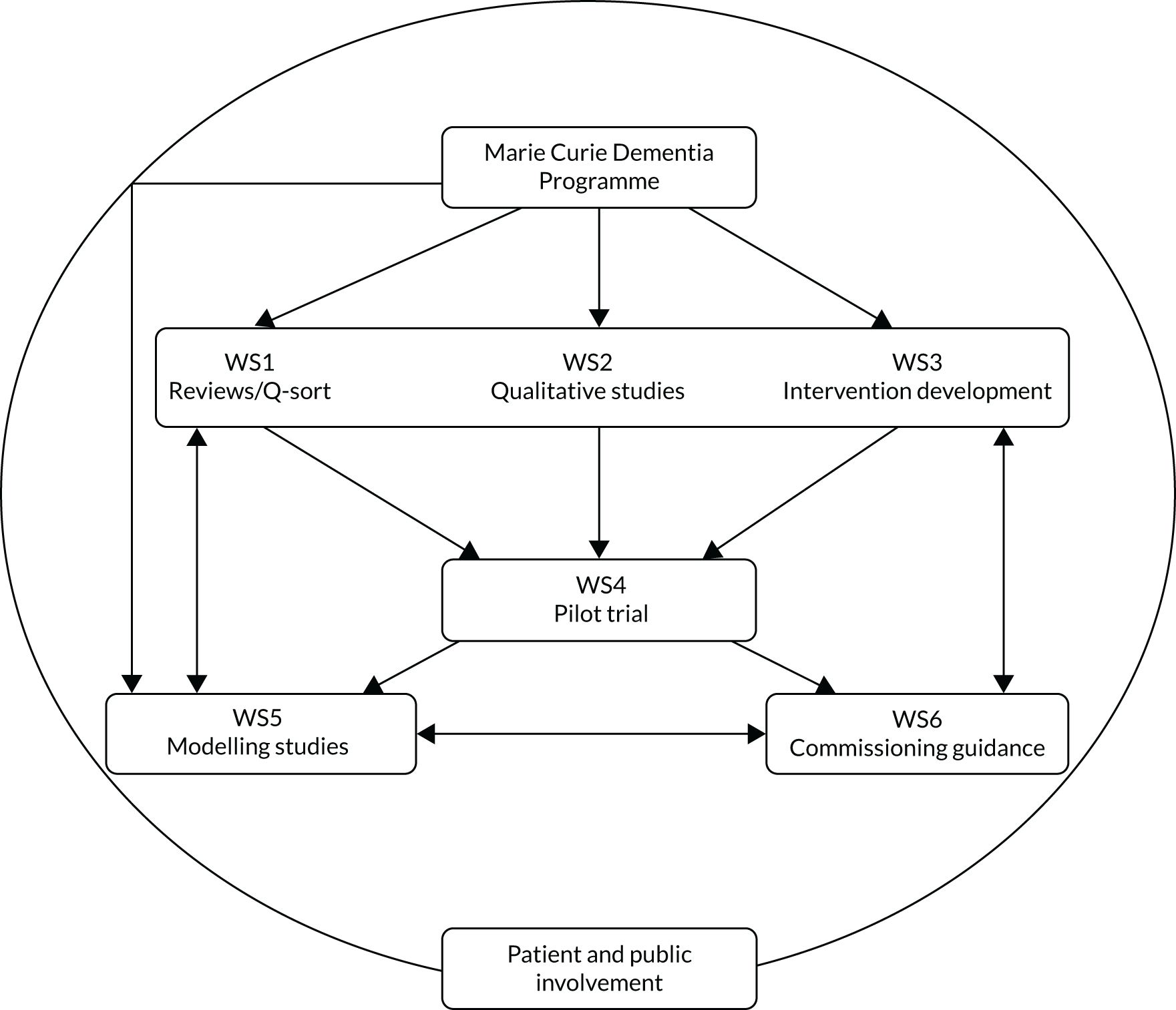
The SEED programme comprised six separate and interlinked workstreams (WSs); Figure 1 illustrates the relationship between individual WSs. We utilised the Medical Research Council (MRC) guidance on the development, piloting, implementation and evaluation of complex interventions. 72–74 The intervention development phrase comprised evidence synthesis (WS1) and a qualitative exploration of current care (WS2) to describe core processes, structures and outcomes. Individual WSs comprised the following:
-
WS1 – mapping current evidence and identifying quality indicators and/or outcome measures for EOLC in dementia
-
WS2 – qualitative studies to identify components of good EOLC in dementia
-
WS3 – development of the SEED intervention using data from WSs 1 and 2 and the Marie Curie Dementia Programme (MCDP)
-
WS4 – pilot trial of the SEED intervention, with process evaluation, to ascertain feasibility and acceptability
-
WS5 – economic modelling of the SEED intervention, including a willingness-to-pay (WTP) exercise to explore cost versus consequences
-
WS6 – commissioning good-quality, community-based EOLC in dementia.
FIGURE 1.
Inter-relationship of WSs in the SEED programme.
Patient and public involvement
Patient and public involvement (PPI) has been pivotal to the creation and subsequent development of this programme. The initial idea for this research originated from Alzheimer’s Society’s Research Network carer groups. A series of collaborative workshops between several representatives from these carer groups and the programme leads ensued to further develop the programme. To ensure that this level of PPI continued throughout the programme, one of the members of the original Alzheimer’s Society Research Network carer groups (ST) became programme co-lead for PPI. In addition, some of the carer group members also joined the external patient and public advisory board (PPAB).
The PPAB met a total of seven times throughout the programme: the initial two meetings were held in the first 6 months, subsequent meetings were held annually and two meetings were held in the final year. Alzheimer’s Society Research Network volunteers already receive training in basic dementia science, research methodologies, ethics and governance and reviewing funding bids. The first meeting was an educational session on EOLC in dementia and the purpose of the group. The second meeting focused on enhancing knowledge and the PPAB management role in terms of:
-
individual members’ roles and responsibilities
-
monitoring individual WSs
-
provision of ongoing support for members.
As required by the National Institute for Health Research (NIHR), we established an External Steering Committee (ESC), which also included PPI representation. This group met a total of five times during the programme to review progress and provide critical advice. We also had a local PPI group, which provided more in-depth ongoing input, for example providing constructive comments on topic guides. Therefore, each WS benefited from regular insightful feedback grounded in the views and experiences of families living with dementia. The PPI contributions to individual WSs are described in the specific WS sections. The continuing engagement and enthusiasm of PPAB and PPI group members is demonstrated by their contributions to the development and piloting of the massive open online course (MOOC) and their agreement to join new projects.
Major changes to the proposed programme
Workstream 3
The original proposal aimed to develop and evaluate an integrated care pathway (ICP) for professionals to use with people dying from, or with, dementia in community settings. This was grounded in the palliative care intervention, the LCP,37 which provided professionals with advice on how to provide better care in the last days of life. The LCP had been widely implemented on a national level. However, following the recommendation of a national review in 2013, the LCP was withdrawn from practice. 75 The SEED programme team, advised by the ESC, thus avoided any future use of the phrase ‘care pathway for the dying’, or ICP, and developed the intervention from the empirical data.
Workstream 6
In 2015, NICE announced that its dementia care guidelines were to be updated. As this national guidance was anticipated to include evidence-based recommendations on care towards, and at, the EOL in dementia, the ESC recommended postponing the development of any guidance for commissioners based on the SEED intervention findings until the updated guidance was published (in June 2018). 29
Workstream 1: mapping current evidence and identifying quality indicators and/or outcome measures for end-of-life care in dementia
Overview
We utilised the MRC guidance on the development and evaluation of complex interventions72–74 to inform intervention development, beginning with evidence synthesis (WS1). This WS comprised (1) updated systematic reviews to identify existing relevant guidelines, quality indicators and outcome measures, (2) a national online survey to identify current examples of good and sustainable practice (to inform WS2 sampling), and (3) a Q-sort study, with people with dementia, current carers and bereaved carers (n = 57), to explore their views on important outcomes for EOLC. Existing guidelines recommended that care towards, and at, the EOL for people with dementia be community based for as long as possible. As we had identified a number of existing, recent reviews of outcome measures for EOLC in dementia, we did not repeat this work; our article on the systematic review of quality indicators has now been published. 76 No dementia guidelines included any quality indicators to measure, and thus drive, improvement in this area of care. However, current palliative care quality indicators are not entirely suitable as they do not incorporate key aspects of dementia, such as person-centred care or behaviours that challenge. Results from the national survey showed that examples of current good practice rely heavily on (1) non-commissioned, non-recurrent funding and (2) leadership from an interested clinician or ‘local champion’. People with dementia and their families consider compassionate care and informed shared decision-making as important outcomes of good-quality EOLC.
Patient and public involvement
Significant PPI contributions to WS1 included discussion of the content and format of the relevance of existing outcome measures and/or quality indicators, testing the Q-methodology approach and advising on statements to be used, and informing the participant sampling frame for the Q-sort.
Research aim
To inform the development of the SEED intervention, WS1 sought to determine what is already known about the organisation and provision of EOLC for people with dementia and their families. Specific objectives were to:
-
map the evidence base for existing EOLC guidance and care pathways in dementia
-
identify national examples of good and sustainable practice
-
identify outcome measures and indicators to measure good-quality EOLC in dementia
-
elicit the views of people with mild dementia and carers on the elements of the care pathway(s) and outcomes important to them.
Existing end-of-life care guidance and models of care in dementia
Further details of existing EOLC guidance and models of care in dementia are provided in Appendix 1.
Methods
We built on a previous systematic review of dementia practice guidelines. 77 The original review retrieved 27 sets of dementia practice guidelines, 12 of which were eligible for inclusion [i.e. scored at least four on the Appraisal of Guidelines for REsearch and Evaluation II (AGREE II) instrument78]. Of these, five guidelines specifically addressed palliative care and EOLC:
-
Clinical Practice Guidelines and Care Pathways for People with Dementia Living in the Community79 (Queensland University of Technology, 2008)
-
guideline on supporting people with dementia and their carers in health and social care80 [NICE–Social Care Institute for Excellence (SCIE), 2007]
-
Guideline for Alzheimer’s Disease Management81 (California Workgroup on Guidelines for Alzheimer’s Disease Management, 2008)
-
Ministry of Health’s Dementia: MOH Clinical Practice Guidelines82 (Singapore, 2013)
-
Ministry of Health’s Clinical Practice Guidelines, Management of Dementia, 2nd edition83 (Malaysia, 2009).
In collaboration with WS5, we further examined the content in each guideline, looking specifically at setting, content, timing, care model(s), staff and resource implications, and clinical audit parameters. This exercise helped inform WS5 in terms of cost estimation and subsequent modelling.
Key findings
Existing guidelines recommended that people with dementia be managed as far as possible in the community. Recommendations varied as to when palliative care for people with dementia should be introduced, ranging from as early as diagnosis through to < 6 months to live. Only UK guidelines did not make any clear recommendations on the timing of the introduction of palliative care. Guidelines covered a range of aspects of palliative dementia care, including assessment, access to services, ACP and symptom management. Guidelines varied in the level of primary care involvement and support, and included shared care and case management models. Finally, no guideline incorporated any quality indicators/outcomes specific to the palliative care phase of the dementia care trajectory.
Mapping UK end-of-life care services in dementia
This study has been published as Amador et al. 84 For a full-text version of this paper see Appendix 2.
Methods
To map national initiatives, including examples of good and sustainable practice in EOLC in dementia, we updated and repeated the National Council for Palliative Care 2008 local practice online survey from October 2014 to the end of February 2015. We enquired about (1) general information regarding the service (i.e. title, contact information and location), (2) service activities and referral criteria, (3) team size and composition, (4) situation, funding mechanisms and sectors of operation and (5) dissemination and evaluation activities. 84 More than 60 services, set up specifically to provide EOLC to people with dementia, were purposively sampled via targeted e-mail invitation, in addition to open-call invitations.
Key findings
Fifteen respondents representing discrete service initiatives responded. Two-thirds of returns were received in response to targeted e-mail invitations, and one-third in response to open calls. Initiatives engaged in a wide range of activities, predominantly providing direct care and workforce development/advisory or educational activities. Findings suggested that sustainability of services was reliant on (1) enthusiastic clinicians with a leadership role, (2) wider system support through reliable funding mechanisms and (3) a minimum level of integration with normal service provision. More recent initiatives were largely built on the expertise of the nursing professions, and driven mainly by charity and hospice sector funding.
Identifying quality indicators/outcome measures to measure good-quality care
This work has been published as Amador et al. 76 (see Acknowledgements, Publications).
Quality indicators are defined as measurable elements of work/practice performance for which there is evidence or consensus that they can be used for assessing and changing the quality of care being provided. Quality indicators can be related to three key elements of care: process, outcomes and structure. 85,86 Outcome measures, more specifically patient- or public-related outcome measures, assess changes at an individual level in terms of health status or health-related QoL. Both types of measures were considered to be equally important when assessing the impact of a complex, community-based intervention that could potentially affect service users (i.e. patients and their families) as well as service providers and commissioners.
Methods
We had previously identified a number of existing systematic reviews of outcome measures for EOLC in dementia and, therefore, did not repeat this work. 87–89 To identify quality indicators to measure good-quality EOLC in dementia, we built on a previous systematic review of quality indicators for palliative care by de Roo et al. 90 The original review identified 17 sets of quality indicators for palliative care, containing 326 unique indicators. After screening, we excluded over half of the indicators because they were not applicable to long-term care settings, lacked procedural relevance or were specific to a particular scale. In addition, other indicators excluded at this stage were not applicable to UK care settings or lacked conceptual clarity. The remaining indicators (n = 156) were mapped against the European Association for Palliative Care (EAPC) framework for optimal palliative care in older people with dementia, which was developed through a rigorous international consensus process. 33 The framework comprises 11 domains:
-
applicability of palliative care
-
person-centred care, communication and shared decision-making
-
setting care goals and ACP
-
continuity of care
-
prognostication and timely recognition of dying
-
avoid aggressive treatment
-
comfort and optimal symptom treatment
-
psychosocial and spiritual support
-
family carer involvement
-
education of the health-care team
-
societal and ethical issues.
Key findings
Overall, quality indicators available to assess optimal palliative care in older people with dementia covered some of the EAPC domains, including ACP (domain 3), continuity of care (domain 4), prognostication and timely recognition of dying phase (domain 5) and family carer involvement (domain 9). However, existing indicators would need to be further developed in order for each to comprise the necessary elements (i.e. numerator, denominator and performance standard) and have its fundamental properties assessed (i.e. feasibility, acceptability, reliability, sensitivity to change and predictive validity).
There were major gaps in existing quality indicators in the following areas: (1) person-centred care, especially in specific aspects of dementia care (behaviour that challenges), (2) non-pharmacological interventions, (3) the appropriateness of pharmacological and other interventions at EOL (i.e. use of restraints, tube nutrition and the use of antibiotics), (4) the need for appropriate skill mix in health-care teams, including specialist nursing care and dementia care to support optimal symptom management, and (5) the quality of the dying environment.
Developing person-centred outcome measures: views of people with mild/moderate dementia and carers
This work has been published as Hill et al. 91 (see Acknowledgements, Publications).
Methods
Q-methodology is a mixed-methods approach combining qualitative and quantitative techniques to study subjectivity. 92 In this study, it was used to identify the views of people with mild dementia, family carers and bereaved carers on what is important (or unimportant) to them about the care provided to people with dementia approaching the EOL. In the first stage, participants ranked in order, from the most important to the least important, a set of 24 cards printed with statements about the type of care patients could receive (the statements are available in Report Supplementary Material 1). By-person factor analysis was used to identify clusters of respondents who completed the Q-sort in a similar way,92 and these clusters helped define the different factors. Short interviews were conducted following the card sort to provide additional information to aid interpretation of the factors.
Key findings
Four distinct viewpoints were identified:
-
Family involvement – decisions should be made by, and with, the family, and the wishes of people with dementia should be documented in advance to help families with this process. Family carers do not see caring for their relative as a burden: it is more important to keep the person with dementia in their own home and have the family with them at the EOL.
-
Living in the present – people with dementia live life day by day, and carers are more concerned with ensuring the comfort and safety of the person with dementia at that moment in time rather than planning ahead.
-
Pragmatic expectations – carers acknowledge their limits as carers for their relative with dementia and give high priority to having processes in place to provide the best possible care. This may include moving the person with dementia to a care home.
-
Autonomy and individuality – people with dementia want a significant level of autonomy and individuality, with their opinions and choices respected and integrated into their EOLC plans.
These findings reveal several different views on what is important about EOLC for people with dementia; therefore, a one-size-fits-all approach to care is unlikely to be the most appropriate. However, areas of consensus across all views did emerge, including the provision of compassionate care and ensuring that relevant information was available to people with dementia and their families when making decisions.
Workstream 1 conclusions
Existing guidelines recommend that people with dementia be cared for as long as possible in the community. These guidelines include key aspects of care, such as access to key services, ACP and optimal symptom management. Examples of sustainable national good practice are dependent on reliable funding streams, and local clinical leadership, hospice and charity sectors play a key role in the development and sustainability of such services.
No guidelines provided any quality improvement indicators specific to the palliative care phase of dementia. Current palliative care quality indicators may not be entirely suitable for use as they do not include key aspects of dementia care, such as behaviours that challenge and person-centred care. In the design of future services for EOLC, the Q-methodology study highlighted that there is no single way of providing care that will suit everyone. Outcomes for measuring EOLC that are important to people with dementia and their families include the provision of compassionate care and facilitating informed, shared decision-making.
Reflections on workstream 1
There persists a lack of empirical data to inform policy and clinical guidelines in this area of dementia care. Although a consensus framework has been developed, which identifies 11 domains for optimal palliative care for people with dementia, further research is needed to develop appropriate outcome measures or quality indicators to better assess both the quality of EOLC in dementia and outcomes that are important to people with dementia and their families. Q-methodology has the potential to identify person-centred outcomes; unfortunately, this study was limited to a small, selective sample of people with mild dementia and carers who were recruited from a dementia research network. To be generalisable, the study should be replicated with a larger and broader sample to capture additional viewpoints.
Workstream 2: defining and delivering good practice for care towards, and at, end of life in dementia
This work has been published as Bamford et al. ,93 Lee et al. 94,95 (see Acknowledgements, Publications) and Poole et al. 96 (see Appendix 2).
Overview
We used the MRC guidance on the development and evaluation of complex interventions72–74 to inform intervention development. The evidence synthesis (WS1) was followed by a qualitative exploration of current care delivery (WS2). This provided new insights into the key components that are essential for good-quality EOLC in dementia by using qualitative methods (i.e. interviews, focus groups and observation) to explore and compare the perspectives of different stakeholder groups. Three published papers from this workstream separately describe the views of key groups: national experts,94 service managers and front-line staff,95 (see Acknowledgements, Publications) and people with dementia and family carers96 (see Appendix 2). These individual WS2 studies contributed to a final data set that comprised 119 interviews, 12 focus groups, 256 hours of observation and three case studies. The findings of the integrative analysis are summarised in this section, with full details available in the published paper93 (see Acknowledgements, Publications), which drew together the findings of the three studies to identify seven key components of good EOLC:
-
timely planning discussions
-
recognising EOL and providing supportive care
-
co-ordinating care
-
working effectively with primary care
-
managing hospitalisation
-
continuing care after death
-
valuing staff and ongoing learning.
These key components then informed intervention development (described in Workstream 3: development of the SEED intervention).
The integrative analysis highlighted discrepancies between the data, policy objectives and existing literature. Although policy, national experts and service managers often emphasised ACP as crucial to delivering good EOLC,35,44,45 whereas many people with dementia and their families preferred to focus on the present or considered future planning only in relation to wills and funeral arrangements. 96 Providing timely opportunities to discuss future care preferences is challenging in a context in which people with dementia generally receive little support during the mid-stage of the illness trajectory. 97,98 The uncertainty of the dying trajectory in dementia has been identified as a key barrier to good EOLC. 20,21,31,99 However, care home staff did not necessarily view uncertainty as problematic, partly because they were comfortable with the lack of a clear trajectory and partly because they felt that they were often able to identify when individuals were approaching the EOL, but also because following the principles of person-centred care would ensure that needs were recognised and met at all stages of the illness.
Patient and public involvement
Members contributed to WS2 by advising on recruitment approaches and materials, discussing sampling for services to be included in focus groups and the comparative case studies, and reviewing emerging themes from the qualitative analyses.
Research aim
The aim of WS2 was to develop a detailed understanding of good practice in EOLC in dementia to inform development of an intervention (WS3), which would subsequently be tested (WS4). This was achieved through a series of qualitative substudies, with the objectives of:
-
defining good practice from the perspectives of key stakeholders, including national experts, service managers, front-line staff and people with dementia and their family carers
-
understanding existing approaches to EOLC in dementia
-
exploring challenges and unmet need in EOLC in dementia
-
exploring the value and relevance of current tools for EOLC in dementia.
Methods
Qualitative methods were used throughout WS2, including semistructured interviews (face to face and telephone), focus groups, informal discussions and non-participant observation. Data were collected between October 2013 and January 2016 for four substudies that explored:
-
the range of approaches to EOLC in dementia with national experts (WS2.1)
-
service manager approaches to providing EOLC in dementia (WS2.2)
-
the views and experiences of EOLC from the perspectives of people with dementia, family carers and front-line staff (WS2.3)
-
day-to-day practice in EOLC in dementia (WS2.4).
Topic guides are available in Report Supplementary Material 1. The principles of purposive sampling were used in all substudies. 100 Interviews and focus groups were transcribed verbatim and analysed thematically. 101 Episodes of observation were recorded in anonymised field notes. Analysis was iterative and interspersed with data collection. To avoid imposing ideas from one group of stakeholders onto subsequent groups, data sets from individual studies were initially analysed independently. Further details of methods and participants are available in the publications of this work. 93–96 The subsequent integrative analysis involved reconceptualising and developing themes to reflect the nuances in the data from different stakeholders. 93
Key findings
The integrative analysis led to the identification of seven key components of good EOLC for people with dementia. These were central to the development of the intervention in WS3. Table 1 illustrates how themes from different data sets were combined and reconceptualised to produce the seven key components. The original themes from individual data sets often contributed to more than one of the seven components. For example, the theme ‘planning for EOL’ from the comparative case studies was relevant to both timely planning discussion and managing hospitalisation. The mapping was sometimes less intuitive, reflecting nuances within themes that were not necessarily reflected in the overall theme title.
| Seven key components | Interviews and focus groups with | Comparative case studies | ||
|---|---|---|---|---|
| National experts | Service managers and front-line staff | People with dementia and family carers | ||
| Timely planning discussions | Leadership and management | Communicating with families | Uncertainty about planning ahead/difficulties planning ahead | Planning for EOL |
| Continuity of care | Expectations about decisions and decision-makers | |||
| Recognising EOL and providing appropriate care | ||||
| Recognising EOL and providing supportive care | Use of guidelines | Supporting families | The value of practical support | Recognising EOL and providing physical care |
| Integrating clinical expertise | Ensuring comfort at the EOL | Emotional support towards and after EOL | Planning for EOL | |
| Continuity of care | Communicating with families | Reliance on family at EOL | Access to clinical care | |
| Leadership and management | Recognising EOL and providing appropriate care | Confidence in standards of future care | Emotional work at EOL | |
| Continuity of care | ||||
| Co-ordinating care | Integrating clinical expertise | Collaborative working | Reliance on family at EOL | Access to clinical support |
| Continuity of care | Continuity of care | Challenges in accessing and co-ordinating care | Planning for EOL | |
| Recognising EOL and providing appropriate care | Equipping staff with appropriate skills and knowledge | |||
| Ensuring comfort at the EOL | ||||
| Working effectively with primary care | Integrating clinical expertise | Collaborative working | Challenges in accessing and co-ordinating care | Access to clinical support |
| Continuity of care | Planning for EOL | |||
| Recognising EOL and providing appropriate care | Equipping staff with appropriate skills and knowledge | |||
| Ensuring comfort at the EOL | ||||
| Developing and supporting staff | ||||
| Managing hospitalisation | Continuity of care | Recognising EOL and providing appropriate care | The value of practical support | Access to clinical support |
| Collaborative working | Reliance on family at EOL | Planning for EOL | ||
| Continuity of care | ||||
| Communication with families | ||||
| Continuing care after death | Supporting families | The value of practical support | Emotional work at the EOL | |
| Ensuring comfort at EOL | Emotional support towards and after EOL | Recognising EOL and providing physical care | ||
| Developing and supporting staff | ||||
| Valuing staff and ongoing learning | Leadership and management | Developing and supporting staff | Confidence in standards of future care | Equipping staff with appropriate skills and knowledge |
| Continuity of care | Recognising EOL and providing appropriate care | Skilled and empathic staff | Emotional work at the EOL | |
| Use of guidelines | Continuity of care | Access to clinical support | ||
| Communicating with families | ||||
| Ensuring comfort at EOL | ||||
Although there were differences in emphasis between data sets, the relevance of the seven components to all stakeholder groups is largely demonstrated in Table 1. The integrative analysis was also helpful in refining minor themes within data sets. For example, the emotional work in providing EOLC for front-line staff was a strong theme in the comparative case studies, but was not identified as an explicit theme in other data sets. The integrative analysis drew attention to the presence of this theme in other data sets and helped to ensure that it was embedded in the theme of valuing staff and ongoing learning.
Workstream 2 conclusions
There were some important discrepancies between the findings, policy objectives and existing literature. Although planning for EOL is promoted as best practice,33,35,45 the findings confirmed that people with dementia often prefer to live in the moment, and some had strong reservations about planning for the future. 47,49,62 In terms of practical implications, this suggests the need for planning discussions to be conducted with a professional who has time to get to know the individuals, understands the barriers to planning and is able to approach topics over a period of time (while recognising that it may never be appropriate for some families). Although national experts emphasised skills and training, they paid less attention to the relational context needed to support discussions about future care. 94 The integrative analysis promoted a more detailed understanding and provided insights into how to translate the components into practice. Seven key components were identified as being core to the provision of good-quality EOLC in dementia: (1) timely planning discussions, (2) recognising EOL and providing supportive care, (3) co-ordinating care, (4) working effectively with primary care, (5) managing hospitalisation, (6) continuing care after death, and (7) valuing staff and ongoing learning.
The uncertainty of the dementia trajectory is often cited as a key barrier to good EOLC in dementia. 20,21,31,99,102 However, data from front-line staff, particularly care home staff, suggested that uncertainty may be less relevant than was previously thought. Many staff anticipated and were accepting of fluctuations in people with dementia, and were able to explain these to family carers. Experienced staff often used a combination of their personal experience and knowledge of the individual, subjective changes (e.g. seeming more withdrawn) and objective measures (e.g. weight loss and decreased appetite) to identify people potentially approaching the EOL. 95
Reflections on workstream 2
The integrative analysis promoted a more detailed understanding of key components of EOLC in dementia than would have been achieved through individual data sets. Observation provided valuable insights into how to translate these components into practice. The findings, therefore, highlight the value of including multiple stakeholder groups and different methods to inform complex interventions. A key limitation was the relatively small numbers of people with dementia involved in interviews. It proved difficult to recruit participants through the services taking part in the focus groups; this may have been because of workload, desire to ‘protect’ people with dementia or a lack of confidence in the research team. These difficulties were offset, to some extent, by the involvement of a considerable number of people with dementia in the observations, which often included informal conversations about the care they received and their views on the components of good care.
Workstream 3: development of the SEED intervention
This work has been published as Macdonald et al. 103 (see Acknowledgements, Publications). A detailed description of the SEED intervention is provided in Appendix 3.
Overview
In WS3, we developed an intervention that is grounded in the key findings of WS1 and WS2, and that builds on the results of the MCDP. Using a co-design approach, the seven key components identified in WS2 as underpinning good-quality EOLC in dementia were operationalised into a primary care-based intervention to be piloted in WS4. The primary care-based intervention, delivered by a DNS, targets two key groups of people: (1) those in the earlier stages of the dementia trajectory, with mental capacity to address future care planning; and (2) those in the more advanced stages of dementia, who would benefit from a palliative approach to their care. Findings also suggested the need for a care resource kit, containing current and possibly new resources, targeting the seven key components. As an integral part of the SEED intervention, the resource kit supports intervention delivery, enables effective working with people with dementia and their families, and improves the knowledge and skill set of community-based health and social care professionals. WS3 used an inclusive design-led approach103–105 to co-develop blueprints for a number of new EOL resources that could be included in the care resource kit.
Patient and public involvement
This was integrated throughout WS3. Specific PPI contributions included discussing and advising on the emerging intervention, testing workshop-based activities to inform future stakeholder involvement methods, advising on acceptability of existing resources for the care resource kit, providing detailed feedback on the draft care plan guide and advising on the concept of developing a MOOC.
Research aims
The research aims were to:
-
develop an evidence-based intervention to support professionals to provide good-quality EOLC in dementia
-
co-develop new resources to support implementation of the intervention
-
identify key determinants of costs and outcome to inform WS5 (to prevent duplication, this element is described in Workstream 5: economic modelling study, Valuing the consequences of the SEED intervention).
Developing an evidence-based intervention (see Appendix 3)
The MRC guidance on developing complex interventions includes three key activities relating to intervention development: (1) identifying the evidence base, (2) identifying/developing theory and (3) modelling process and outcome. 74 In the SEED programme, the first activity took place in a series of workshops with the full SEED programme team to review the evidence from WSs 1 and 2 and to generate and prioritise ideas for possible intervention. The broad concept of the intervention was then operationalised through small group co-design workshops, which included modelling process and outcome using the theory of change. 106 Although we had intended to develop the intervention within the team and then conduct task groups with stakeholders, this process was adopted to ensure more integrated involvement of stakeholders (i.e. PPI members, clinical specialists and service providers) throughout the second phase of intervention development.
Phase 1: generating and prioritising ideas
A series of five workshops with the full SEED programme team was undertaken (November 2014 – December 2015). The initial five workshops involved early and ongoing discussions of the existing evidence base (WS1 reviews) and qualitative findings (WS2). The focus was to identify possible frameworks for the intervention, and appropriate methods and processes to inform its development. A brief summary of each workshop is provided in Appendix 3, Developing an evidence-based intervention. Following this series of workshops, the co-design process then continued in a smaller group to develop the intervention in more detail and facilitate translation into practice.
Phase 2: prototype development
The smaller group met every few weeks over a 12-month period, with a 5-month gap between months 4 and 9 (when Sandra Neves was on maternity leave). Members included the design team, PPI members, key researchers, clinical experts and service providers. The main focus of the smaller group was to consider how the ideas identified in the workshops linked to existing theoretical frameworks and could be operationalised in terms of what the intervention would comprise, who it would be targeted at, where it would be based, who would deliver it and how intervention delivery could best be supported. A summary of each of these areas is provided in the following sections.
What theoretical approaches could inform the SEED intervention?
We did not have an explicit theoretical framework to inform the intervention at the outset. Relevant theoretical frameworks to inform the intervention were identified from the literature reviews, qualitative interviews and case studies. These included extending the ideas of person-centred care107,108 to person-centred death and to other key individuals (family members and professionals) involved in EOLC. 109,110 We also drew on ethnomethodological ideas about the social organisation of death. 111 Other aspects of the intervention were informed by complexity theory,112,113 recognising the need for the intervention not only to address individual needs but to enhance systems to support EOLC. The SEED intervention was, therefore, informed by a range of blended theories. With the exception of the social organisation of death, each of the remaining theories informed all of the seven components comprising the intervention.
We subsequently used the framework of normalisation process theory (NPT)114,115 to understand whether or not and how the intervention was implemented (see Workstream 4: pilot trial of the SEED intervention, with process evaluation, to ascertain feasibility and acceptability).
What components would comprise the SEED intervention?
It was agreed that the SEED intervention would focus on the seven key components of care identified in WS2 and would consist of:
-
direct work to support people with dementia and carers towards, and at, the EOL
-
developing a supportive context for EOLC in dementia by –
-
mapping and co-ordinating local services
-
developing the workforce through co-working, training and development
-
improving systems to enhance EOLC, for example improving use of the general practice palliative care register, or improving links between general practices and local care homes
-
a care resource kit.
-
Who would the SEED intervention focus on?
As the aim of the programme was on improving care towards, and at, the EOL in dementia, the consensus of the small co-design group was that the intervention would focus on two groups of people with dementia and their families: (1) those in the earlier stages of the dementia trajectory with mental capacity, to address future care planning; and (2) those in the more advanced stages of dementia, who would benefit from a palliative approach to their care. The ambition was that the intervention would also focus on improving the delivery of EOLC to people with dementia through more strategic work. Although this would be tailored to the local context, we anticipated that this systems-level work might include building capacity of existing staff or enhancing use of the general practice palliative care register for people with dementia.
Where would the SEED intervention be based?
Alzheimer's Disease International has urged implementation of a task-shifted model of dementia care whereby the majority of post-diagnostic care is delivered in community settings by a generalist workforce such as primary care teams. 116,117 In England, this approach has been widely implemented for a range of long-term conditions, usually with a specialist nurse co-ordinating care and facilitating links and knowledge exchange between the general practice and secondary care. 118 To date, dementia has generally been excluded from this model. The findings of WS2 suggested that an intervention based in primary care could address a number of existing shortcomings in EOLC in dementia. For example, better community support was needed to facilitate care in place and obtaining timely support from primary care was a recurrent issue for some care homes. Although the MCDP tested a care home-based intervention,119 this excluded those people with dementia living in their own homes. Basing the intervention in primary care was therefore supported both by the empirical data and the increasing policy emphasis on a primary care-led model of dementia care.
Who would deliver the SEED intervention?
As the seven key components of care largely involved clinically related duties such as future care planning, care co-ordination, working effectively with primary care and supporting/training generalist staff, it was agreed that the SEED intervention should be delivered by a professional with clinical knowledge and expertise. The recently completed MCDP intervention study had tested a non-clinical care co-ordinator role. 119 However, as the SEED intervention would comprise independent working in the community, it was considered that it should be delivered by an experienced nurse, particularly as this was consistent with the role of specialist/nurse practitioners in delivering most chronic care in the community. The post was termed a DNS.
Further discussion led to the development of a job description and person specification outlining the prior knowledge and expertise required and responsibilities of the role (see Appendix 3, Job description provided to NHS trusts and person specification). Existing job specifications for similar community-based, specialist nurse roles (e.g. Macmillan nurse for cancer and Admiral nurse for care of people with dementia and their families) were used to inform the job description.
What support will be needed to deliver the SEED intervention?
To enable the DNS to deliver the SEED intervention, a range of support needs were identified:
-
training and supervision
-
practical support in negotiating the new role
-
an accessible manual describing the SEED intervention [see Report Supplementary Material 2 and Appendix 3, Example of SEED activities and outcomes for one key component (timely planning discussions), and Example of SEED activity checklists for one key component (timely planning discussions)]
-
care resource kit [see Appendix 3, Example of resources for one key component (timely planning discussions)].
Practical issues included secondment arrangements, equipment required, induction and training, and anticipated caseload. Training needs were identified and prioritised using an educational needs assessment (see Appendix 3, Educational needs assessment for dementia nurse specialist). An induction period of 4–6 weeks was agreed to enable the DNSs to meet key professionals in the locality and to build relationships with their general practice. A bespoke SEED manual was developed to introduce the DNSs to the research programme and the intervention, and to help guide them through the role (see Report Supplementary Material 2). This included a description of the intervention using the Template for Intervention Description and Replication (TIDieR) checklist. 120
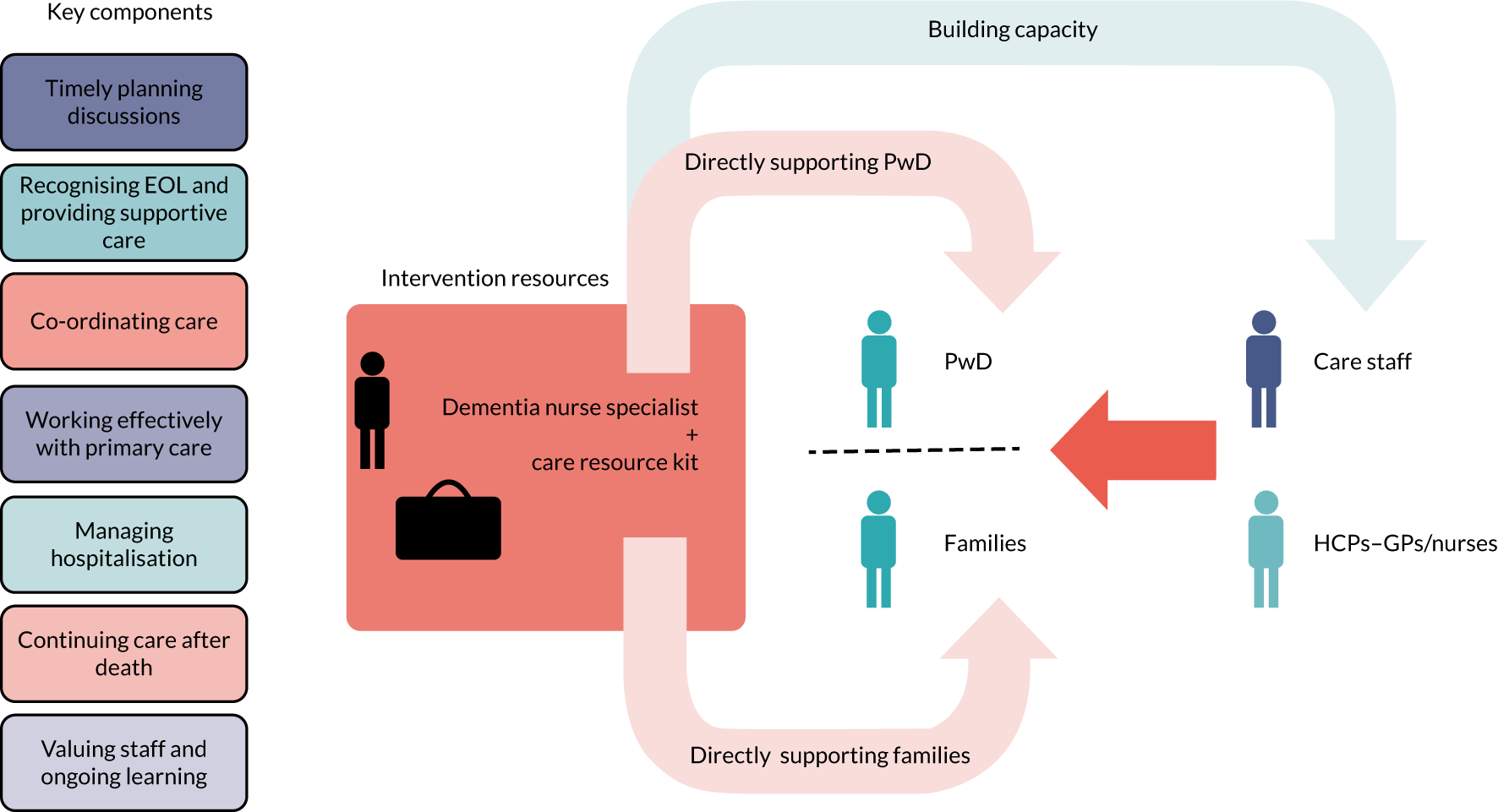
The SEED intervention
The SEED intervention comprised a DNS to focus on the seven key components of good EOLC, identified in WS2, through working with individual people with dementia and family carers and working at a more strategic level to build capacity. A conceptual model of the SEED intervention is provided in Figure 2.
FIGURE 2.
The SEED intervention. HCP, health-care professional; PwD, people with dementia. Reproduced from Macdonald et al. 103 with permission from The Journal of Dementia Care.
Using the theory of change to translate theory into practice
The next stage of intervention development involved translating the conceptual model into practical activities. For each of the seven components, we began by identifying objectives for working (1) with individual people with dementia and their families and (2) at a strategic level (Box 1). The activities were also presented as checklists that could be used by the DNSs to document activities and plan their work. These documents were included in an appendix to the SEED manual [see examples in Appendix 3, Example of SEED activities and outcomes for one key component (timely planning discussions), and Example of SEED activity checklists for one key component (timely planning discussions)].
-
To provide opportunities for discussions about EOLC with patients and families.
-
To provide opportunities for documenting preferences for EOLC.
-
To ensure appropriate dissemination of completed documents.
-
To ensure the timely review of completed documents.
-
To identify people with dementia approaching the EOL and add them to the palliative care register.
-
To share prognosis with families and prepare them for EOL.
-
To ensure the timely recognition and management of pain and discomfort at EOL.
-
To review EOL planning documents.
-
To ensure that all staff are aware of, and follow, relevant documentation.
-
To improve co-ordination between multiple services and agencies.
-
To improve communication within and between services.
-
To improve access to continuing health-care funding.
-
To refer appropriately to specialist services.
-
To provide a conducive environment in care homes for GP visits.
-
To have a named GP and alternate identified.
-
To ensure regular (e.g. 3-monthly) proactive clinical review of people with dementia.
-
To review medications towards EOL.
-
To ensure that a clear rationale is provided for hospital admissions.
-
To ensure that preferences regarding hospitalisation are reviewed and documented.
-
To identify a range of options to support families and care home staff in the event of unanticipated changes.
-
To ensure that professionals who do not know the patient have access to key information.
-
To prepare families for what will happen following the death of the person with dementia.
-
To support families in the immediate post-death period.
-
To assess the need for ongoing bereavement support.
-
To value the emotional work involved in EOLC.
-
To recognise the personal strengths of staff.
-
To establish routine post-death reviews.
We then summarised the intervention using the theory of change. 106,121 This was used because it focuses on desired outcomes, adopts a collaborative approach and explicitly explores the rationale underlying interventions. Developing a theory of change for the SEED intervention involved an iterative and collaborative process between the research team and key local stakeholders, including a palliative care clinical lead, who would support the DNSs, and a specialist dementia nurse (who was subsequently seconded to the role of DNS for the pilot trial).
The stages involved in developing the theory of change included:
-
identifying a realistic and definite goal for the SEED intervention (Figure 3 presents the ultimate goal)
-
working backwards from the goal to identify outcomes that would contribute to achieving the goal
-
summarising activities needed to achieve these outcomes and the intended changes through which the outcomes would be achieved
-
identifying resources required for the intervention.
FIGURE 3.
Theory of change for the SEED intervention.
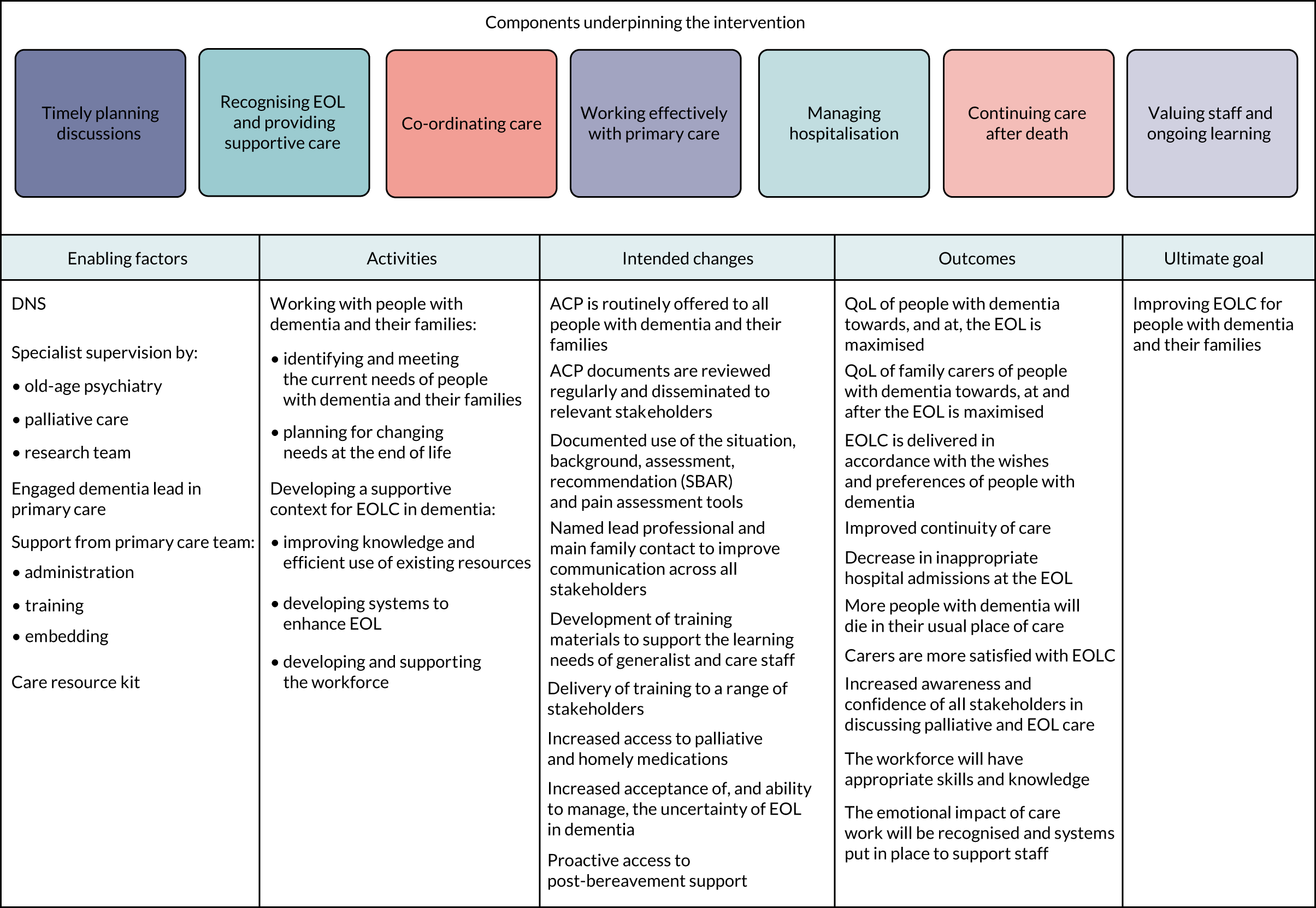
In addition, we explored the assumptions underlying the links between activities and the overall goal. The theory of change aimed to provide an overview of the intervention. Individual activities included in Figure 3 are, therefore, not specifically linked to individual components of the SEED intervention (e.g. planning for changing needs at the EOL is clearly linked to timely planning discussions, but is also likely to affect other components, such as providing supportive care, working effectively with primary care and managing hospitalisation). Similarly, the intended changes may result from one or more of the activities (e.g. increased acceptance of, and ability to manage, the uncertainty of EOL in dementia may result from developing and supporting the workforce and/or from planning for changing needs at the EOL). The outcomes and ultimate goal are, therefore, achieved through a combination of activities, rather than being directly linked to a specific activity.
Co-development of the care resource kit
A key role of the DNSs was to provide appropriate information to the right people at the right time. Therefore, we developed a care resource kit containing examples of existing resources to facilitate intervention delivery, and identified potential new resources to address any gaps.
Existing resources
Existing resources were identified through online searches, targeting key websites (e.g. Alzheimer’s Society, Alzheimer’s Association and the National Council for Palliative Care) and using the keywords ‘end-of-life care’ and ‘dementia’. A small group of SEED programme team members with personal and professional experience of EOLC in dementia reviewed the suitability of resources for (1) people with dementia and their families and (2) professional carers. Selected resources were grouped according to:
-
relevance to one or more of the seven key components
-
whether the resource focused on EOLC in general or was specific to dementia
-
target audience (people with dementia, family carers and professionals)
-
country of origin.
The accessibility of the resources was also considered in terms of format and availability (e.g. downloadable, free or paid for). The quality of the identified resources was then reviewed by considering whether and how research evidence had informed their development. In view of the large number of resources identified, many of which covered similar areas, we then selected the most appropriate existing resources for inclusion in the care resource kit, based on the following criteria: dementia-specific, freely available, UK based and evidence based. International resources for professionals were selected if they were of high quality with a strong evidence base. International resources for people with dementia and their families were included if they were accurate, of high quality and judged acceptable by the PPI member of the SEED team.
This process resulted in a detailed table of resources, which was included in the appendices to the SEED manual [see Appendix 3, Example of resources for one key component (timely planning discussions), for an example]. Different ways of enabling the DNSs to quickly identify and access the resources were considered. One suggestion was to develop a web portal to facilitate searching and retrieval of documents, which could be used by people with dementia and their families, as well as professionals. A preliminary structure for the web portal was agreed, in which resources would be organised by their intended audience, the seven components and format (e.g. booklet or video). Although a blueprint for the portal was developed, there were insufficient resources to develop it further.
New resources
The review of existing resources highlighted gaps in three key areas:
-
a simple introductory guide to planning for the future
-
clinical scenarios illustrating common issues in EOLC in dementia and strategies to address these
-
online training for both family carers and professional carers focused on advanced dementia and EOLC.
The rationale for selecting these areas and description of the progress made in developing new resources are described in detail in Appendix 3, Development of new SEED resources. Only one of these new resources, a MOOC on advanced dementia, was successfully completed and marketed.
Massive open online course
This work has been published in Poole et al. 122,123 (see Acknowledgements, Publications and Appendix 2).
The major gap identified by the review of existing resources was the absence of educational resources for both family and professional carers on advanced dementia and the provision of care as the illness progresses. A MOOC was seen as the most appropriate way of addressing this gap. A MOOC is an online course aimed at unlimited participation and open access via the web. In addition to traditional course materials, such as filmed lectures, many MOOCs provide an interactive forum for users.
The SEED-based MOOC, Dementia Care: Living Well as Dementia Progresses, aims to help family carers of people with advancing dementia to feel prepared and supported towards, and at, the EOL. Although primarily designed for family carers, the MOOC was a useful resource for professional carers, particularly care home staff. The content is underpinned by the seven key components that informed the SEED intervention, and addresses three main areas:
-
understanding the progression of dementia and planning for the future
-
working together to ensure the care and comfort of the person with dementia
-
looking after yourself as a carer.
Participants involved in the MOOC included members of the SEED project team (Marie Poole and Louise Robinson), DNSs, family carers, a range of health-care professionals who participated in SEED and additional professionals to ensure that key organisations were represented. The resources included in the MOOC aim to be engaging and informative, and comprise short videos, articles, images, quizzes, animations and interactive forums. Dementia Care: Living Well as Dementia Progresses was launched in March 2019; the 3-week course is now delivered twice-yearly and has been completed by > 3000 participants from 130 countries. To date, the MOOC has been promoted at a range of national and international conferences and with organisations including Dementia UK, Health Education England, local NHS foundation trusts and local dementia services.
Workstream 3 conclusions
Following an extensive co-design process involving all key stakeholders, the seven key components identified in WS2 as underpinning good-quality EOLC in dementia were operationalised as a primary care-based, nurse-led intervention. From a theoretical perspective, we utilised the theory of change106,121 as it allows a collaborative and iterative process and focuses on desired outcomes. A training and supervision programme was developed along with an intervention manual. Findings also indicated the need for a care resource kit to help the DNSs deliver the intervention, work more effectively with people with dementia and their families, and improve the knowledge and skills of family carers and community-based care professionals. An extensive review was undertaken of existing resources, information and tools focused on supporting the delivery of good-quality dementia care towards, and at, the EOL. This indicated a number of gaps, in particular the absence of educational resources for both family carers and professional carers on advanced dementia and the provision of care as the illness progresses. Using the empirical data from earlier WSs, we developed a MOOC to address this gap. The MOOC, Dementia Care: Living Well as Dementia Progresses, was launched in March 2019.
Reflections on workstream 3
Development of the intervention required considerable time and effort that, in hindsight, we underestimated. In addition, the length of the process was increased because of (1) the design researcher undertaking maternity leave mid-way through WS3 and (2) a recommendation from our ESC to extend intervention development time to ensure that key aspects that were essential for operationalisation in practice were completed. Developing new, relevant and innovative educational resources was another key challenge during WS3. Considerable effort was put into searching for and retrieving existing resources, tools and information on EOLC in dementia. However, even once we had identified potential areas for the development of new resources, it was difficult to determine if there were any resources already under development that would address these gaps. Consequently, considerable time and effort were spent creating potential new resources and tools, only for updated review searches, which also required considerable time and human power, to identify new resources that had just been published.
Workstream 4: pilot trial of the SEED intervention, with process evaluation, to ascertain feasibility and acceptability
Further details on WS4 are provided in Appendix 4.
Overview
Workstream 4 comprised a pilot trial to assess the feasibility and acceptability of recruitment and retention, the SEED intervention and outcome measures. Key success criteria for recruitment and retention were generally achieved, but operationalising the eligibility criteria was time-consuming. Many stakeholders thought that all people with dementia would benefit from the intervention and that this would offer a potential strategy for meeting the NICE recommendation for a named care co-ordinator for all people with dementia throughout the illness trajectory. 29 Despite the complexity of the SEED intervention and the requirement for the DNSs to adapt it to the local context, it proved both feasible and acceptable. The DNSs made significant changes at a strategic level (e.g. introducing a template for the annual dementia review in primary care), in addition to working with individuals and their families. None of the outcome measures was considered suitable as the primary outcome measure for a future trial. In the light of these uncertainties, we do not intend to proceed to a definitive trial of the SEED intervention at this stage.
Patient and public involvement
The views of PPI members were sought on progression to a future trial. In particular, we explored extending the intervention to all people with dementia, advantages and disadvantages of alternatives to the current model of one DNS for each general practice, and their views on appropriate outcome measures.
Research aim
The aim of WS4 was to investigate the feasibility of a definitive multicentre RCT of the SEED intervention. Specific objectives focused on three areas:
-
recruitment and retention of people with dementia, family carers and key informants, specifically to –
-
test the feasibility of recruiting 66 people with dementia (with at least 11 from each practice)
-
ascertain whether or not we could collect 12-month follow-up data from at least half (n = 33) of the people with dementia who were recruited
-
-
the implementation of the SEED intervention, specifically to –
-
explore the feasibility and acceptability of the SEED intervention and supporting educational resources
-
explore how and to what extent the intervention was implemented in practice
-
identify, describe and explain factors influencing the implementation of the SEED intervention
-
-
capturing outcome data, specifically to –
-
investigate the feasibility and acceptability of available outcome measures
-
assess the feasibility of collecting resource use data and health-related QoL for people with dementia and family carers
-
explore ways of capturing data on future care planning.
-
Methods
The MRC guidance on developing and evaluating complex interventions emphasises the importance of pilot work to address key uncertainties before progressing to a full trial. 74 Key functions of feasibility/pilot studies are estimating recruitment/retention, testing procedures and estimating sample size. 74 In the present study, there was a high level of uncertainty over each of these areas, in particular over whether or not the intervention could be delivered in practice and whether or not available outcome measures would prove feasible and acceptable in a UK community context. These uncertainties indicated that a pilot trial with an embedded process evaluation was required to inform whether or not progression to a full trial was appropriate.
The strategic focus of the intervention could potentially lead to changes in general practices, local care homes and joint working arrangements with other professionals. Because these changes would affect all participants regardless of their allocation, randomising individual participants was not appropriate. Therefore, we used a cluster design, with clusters comprising individual general practices. Each cluster contained two general practices from North East England, one of which was allocated to receive the intervention, whereas the other acted as a control, providing usual care. The trial methods are described in full in Appendix 4, Pilot trial methods, including key areas from the relevant reporting guidelines. 124–127 Details of approvals and trial management by the Newcastle Clinical Trials Unit are provided in Appendix 4, Trial management. Data collection tools (e.g. activity logs and topic guides) are available in Report Supplementary Material 1.
Recruitment
Allowing for 10% attrition, we aimed to recruit a total of 66 people with dementia, to meet the recommended minimum sample size of 30 participants per trial arm. 128 To test the feasibility of recruitment, we aimed to recruit at least 11 people with dementia from each practice. People with dementia were initially identified from the practice dementia register and were screened by a GP to ascertain whether or not:
-
they had been diagnosed within 2 years (hereafter termed ‘recently diagnosed’)
-
they were on the palliative care register
-
they were thought to be approaching EOL, as judged by the question ‘Would you be surprised if this patient were to die in the next 12 months?’129 (hereafter termed ‘potentially approaching EOL’).
Those on the palliative care register were assumed to be approaching EOL and were, therefore, combined with the third group. We anticipated that the intervention would focus on future care preferences with the recently diagnosed group and on the co-ordination of care and supporting non-specialists caring for those potentially approaching EOL. Full eligibility criteria are provided in Appendix 4, Pilot trial methods.
Following screening, selected eligible participants were sent a participant information sheet (PIS) giving them the opportunity to opt out of further contact. The remainder were contacted by a member of the practice team to seek verbal consent to pass their contact details to the research team. The researchers then telephoned potential participants to discuss the study further, and, if appropriate, arranged a home visit to take formal consent and complete the baseline outcome measures. We followed the provisions of the Mental Capacity Act 2005130 for those people with dementia thought to be unable to give informed consent; in such cases, we approached either a personal or a nominated consultee.
Although not essential to participation, for each person with dementia we sought to recruit a family carer and, for those living in care homes, a key informant. They were identified by the person with dementia, the general practice or the care home managers. Family carers and key informants were provided with a PIS, then followed up by the research team, as described previously. We analysed the numbers of eligible participants seen over the recruitment period, and the resulting rates of recruitment, and retention, both by intervention arm and by practice.
Process evaluation
Although the primary focus of the process evaluation was on the implementation, feasibility and acceptability of the intervention, it also provided insights into recruitment and outcome measures. The consent form for people with dementia, family carers and key informants asked if they were also willing to participate in the process evaluation. Additional health and social care professionals for the process evaluation were identified through the DNSs, lead GP and/or practice manager and non-participant observation. The principles of purposive sampling100 were used to obtain a maximum variation sample of people with dementia and family carers in terms of demographic factors, social arrangements, stage of dementia and types of engagement with the DNS. Health and social care professionals (e.g. social workers, members of community palliative care and mental health teams, home care and care home staff) were sampled in terms of level and type of involvement with the DNS. We also interviewed both DNSs at different time points during the study and members of the supervisory team.
All potential process evaluation participants were sent a PIS and followed up by the researcher, and consent was sought prior to data collection. People with dementia who lacked mental capacity to consent, as judged by the researcher and in line with guidance,130 were eligible to participate in observation.
Interviews with professionals were informed by NPT,114 for example by asking about whether or not and how the SEED intervention was distinct from existing services, whether or not and how host general practices supported the DNSs, skills displayed and required by the DNSs, and ways in which the intervention evolved over time. All interviews and informal discussions covered selected areas from the following list, tailored for different types of participant:
-
recruitment processes
-
views on outcome measures and perceived impacts
-
feasibility and acceptability of the SEED intervention
-
fit with existing services
-
factors influencing implementation.
For the process evaluation, we continued data collection until data saturation was reached; we estimated that this would be achieved with up to 10 people with dementia, 15 family carers and 30 professionals.
We also captured intervention delivery through intervention supervision and activity logs (see Appendix 4, Delivery of the SEED intervention). Initially, the DNSs kept weekly activity logs using a predefined list of categories. As the role evolved, additional activity logs were introduced to capture, in more detail, interactions with people with dementia and family carers, care home staff and other professionals.
Details of data management for the process evaluation are provided in Appendix 4, Pilot trial methods. Data were analysed thematically. 101 The team discussed emerging issues and themes in data workshops and drafted a coding frame; this was then applied to new transcripts and modified until a final version was agreed. All qualitative data were coded in NVivo version 10 (QSR International, Warrington, UK). Team members wrote narrative summaries for each code for discussion in further data workshops. Data relating to implementation of the SEED intervention were subsequently mapped to the key constructs of NPT. This framework has been used extensively in exploring the implementation of complex health-care interventions,115 and focuses on the individual and collective work of implementation. 114
We iteratively developed and piloted a coding frame with each type of activity log (weekly, individual, care home, professional), until a final coding frame was agreed. All logs were then coded in a bespoke Microsoft Access® database (Microsoft Corporation, Redmond, WA, USA). Data from different logs were cross-referenced to ensure that data were as complete as possible and to avoid double counting. Data were transferred to IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA) for simple descriptive analysis.
We also developed a series of vignettes to provide insight into the SEED intervention and the ways in which the seven components were enacted in practice. We purposefully sampled four people with dementia (and their family carers and/or key informants) to showcase the range of activities, settings and ways in which the intervention was tailored to the needs of individuals and services. The individuals varied in terms of their eligibility criteria (recently diagnosed or potentially approaching EOL), informal support and living arrangements. Data from interviews, observation and activity logs were cross-referenced to develop a comprehensive account of the content and delivery of the SEED intervention for each selected participant. To capture the more strategic components of the intervention, we used similar methods to develop a vignette from a care home perspective. A care home was selected where the DNS had worked with individual study participants and at a more strategic level to improve EOLC.
Collection of outcome data
A detailed description of all outcome measures and their interpretation is provided in Appendix 4, Table 12. In brief, measures relating to the person with dementia explored neuropsychiatric symptoms, physical health, pain and QoL. Family carer measures included anxiety and depression, views on care provided and QoL. Key informants completed measures relating to the care provided. Data on resource use by people with dementia and carers were collected using a bespoke questionnaire. Family carers and/or key informants completed a proxy version of the EuroQol-5 Dimensions, five level version (EQ-5D-5L),131 for all people with dementia at all time points to ensure that full data were available in case the person with dementia was unable or unwilling to complete this measure. When data were provided by both a family carer and a key informant, the latter was given precedence because key informants usually had more consistent daily contact with the person with dementia.
Most outcome measures were completed at baseline and at 4, 8 and 12 months (Table 2). Demographic data were collected during baseline study visits. A post-death study visit was completed at either 2 months (family carers) or 2 weeks (key informants) after the death of the person with dementia. These time periods were selected to avoid the interview request clashing with the NHS survey typically sent out 3 months after death (for family carers) and to maximise recall (for key informants). Data on comorbidities, ACP and, when appropriate, cause of death were collected from general practice and/or care home records at baseline and either at 12 months or post death (see Table 2).
| Outcome measure | Completed by | Completed at | ||||
|---|---|---|---|---|---|---|
| Baseline | 4 months | 8 months | 12 months | Follow-up after death | ||
| HADS132 | Family carer | ✓ | ✓ | ✓ | ✓ | ✓ |
| NPI-NH133 | Key informant | ✓ | ✓ | ✓ | ✓ | |
| NPI134 | Family carer | ✓ | ✓ | ✓ | ✓ | |
| QUALID135 | Key informant/family carer | ✓ | ✓ | ✓ | ✓ | |
| BANS-S136 | Key informant | ✓ | ✓ | ✓ | ✓ | |
| PAINAD137 | Researchers (observation) | ✓ | ✓ | ✓ | ✓ | |
| SWC-EOLD138 | Key informant/family carer | ✓ | ✓ | ✓ | ✓ | ✓ |
| SM-EOLD138 | Key informant/family carer | ✓ | ✓ | ✓ | ✓ | |
| CAD-EOLD138 | Key informant/family carer | ✓ | ||||
| EQ-5D-5L131 | Person with dementia | ✓ | ✓ | ✓ | ✓ | |
| Family carer | ✓ | ✓ | ✓ | ✓ | ✓ | |
| EQ-5D-5L Proxy131 | Key informant/family carer | ✓ | ✓ | ✓ | ✓ | |
| Resource utilisation questionnaire (person with dementia)a | Key informant/family carer/care home records | ✓ | ✓ | ✓ | ✓ | |
| Resource utilisation questionnaire (family carer)a,b | Family carer (about own service use) | ✓ | ✓ | |||
| Advance care plan | Researchers | ✓ | N/A | N/A | ✓ | ✓ |
| DNACPR | Researchers | ✓ | N/A | N/A | ✓ | ✓ |
| Emergency Healthcare Plan | Researchers | ✓ | N/A | N/A | ✓ | ✓ |
| Prescription of anticipatory medications | Researchers | ✓ | N/A | N/A | ✓ | ✓ |
| Views on hospitalisation | Researchers | ✓ | N/A | N/A | ✓ | ✓ |
| Demographics | Researchers | ✓ | N/A | N/A | N/A | N/A |
| CCI140,141 | Researchers | ✓ | N/A | N/A | ✓ | ✓ |
| Cause of death | Researchers | N/A | N/A | N/A | N/A | ✓ |
The feasibility and acceptability of outcome measures were assessed by examining:
-
the proportion of outcome measures completed within data collection windows
-
the proportion of people with dementia for whom outcome data were collected
-
data completion rates for each outcome measure
-
the views of people with dementia, family carers, key informants and the researchers administering the outcome measures.
Statistical analyses of outcome measures focused on data completeness of outcome measures and any potential bias in the completion of follow-up data (see Appendix 4, Pilot trial methods). When statistical analysis indicated larger numbers of missing data, or when findings were inconsistent with data from other studies [e.g. Pain Assessment in Advanced Dementia (PAINAD)], we drew on data from reflective field notes by the researchers and interviews with people with dementia, family carers and key informants to explore reasons for missing data.
Integrating data from different methods, data sources and respondents
We designed the pilot trial to provide both methodological and data triangulation142 for each of the broad research objectives (Table 3). With the exception of the planned development of vignettes, because of the large number of data available and limited time, we adopted a pragmatic, problem-solving approach, using triangulation to explore emerging issues.
| Objective | Data collection techniques | Data sources and/or respondent groups |
|---|---|---|
| Recruitment and retention |
|
|
| Intervention delivery |
|
|
| Outcome measures |
|
|
Recruitment and retention of people with dementia, family carers and key informants
Recruitment
We achieved the minimum target of 11 people with dementia per practice, but fell marginally short of the overall target (62/66). Recruitment and retention of people with dementia are summarised in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 4). CONSORT diagrams for family carers and key informants are provided in Appendix 4, Figures 5 and 6. Overall, 82% of patients who were screened met the eligibility criteria. Either a family carer or a key informant was recruited for at least part of the study for all but three people with dementia.
FIGURE 4.
The CONSORT flow diagram for people with dementia. P, practice.
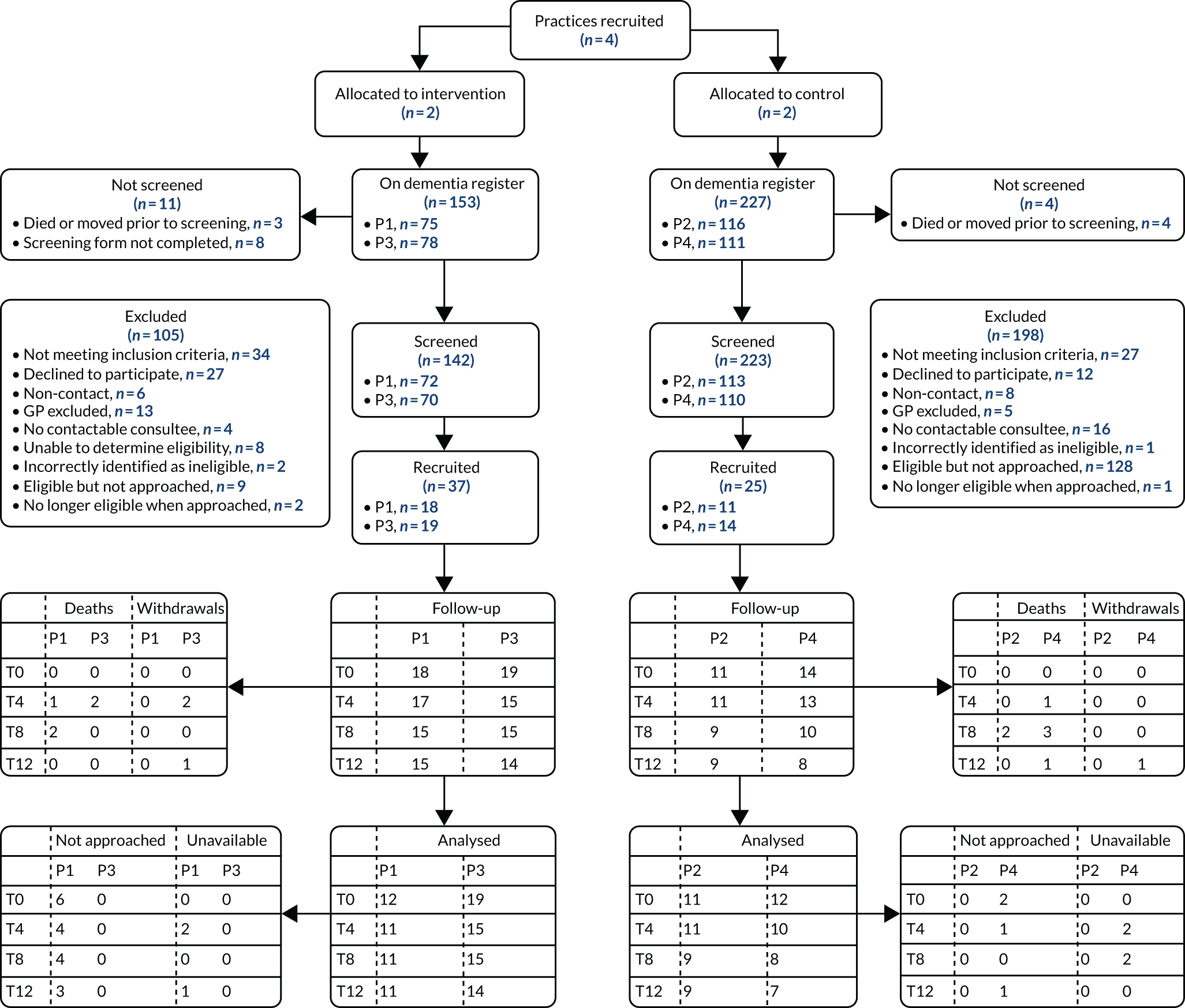
There was some variation in screening and recruitment processes between practices (see Figure 4). The proportion of people on the dementia register who were screened ranged from 89.3% to 91.1%, and the proportion of those screened who were eligible ranged from 73.1% to 91.2%. Lack of resources in control practices meant that almost three-quarters (74%) of people with dementia who were eligible and potentially contactable (i.e. had not been excluded by the GP and, if appropriate, had a contactable consultee) were not approached, compared with only 11% in intervention practices. This explains the better recruitment rates observed in intervention practices (where 36.7% and 38.8% of eligible patients were recruited, compared with 10.7% and 14.3% of those eligible in control practices).
Three-quarters (75%) of the 56 family carers identified were recruited; only one person with dementia requested that we did not approach a family carer. Key informants were identified, and agreed to take part, for 26 of the 33 (79%) people with dementia who lived in a care home at some point during the study. Although key informants were not recruited for the remaining seven people with dementia, this was at the request of either the person with dementia or their family carer, with the latter providing information instead.
Retention
Retention was better than expected: 12-month data were successfully collected for 41 (66%) people with dementia, exceeding the target of 33 people. Mortality was lower than expected: 12 people with dementia died during the study, five in the intervention and seven in the control arm. All of those who died had been identified as potentially approaching the EOL by a GP during screening. Post-death interviews were completed for 11 out of the 12 people with dementia who died; one family carer declined. Four people with dementia withdrew from the study: three moved to a care home and changed general practice, and one was withdrawn because of the ill health of his spouse.
No family carers died during the study. Four family carers withdrew: two when the person with dementia moved care home and changed general practice, one because of ill health and one did not give a reason. Post-death information was provided by five of the six family carers who were the main informants for people with dementia who died.
Three key informants were withdrawn when the person with dementia moved care home. There was considerable continuity of key informant, with 18 key informants providing information at all time points; two key informants were involved for seven people with dementia, and information on one person with dementia was provided by three different key informants. An additional 10 key informants were recruited during the study: eight replaced key informants who were unavailable at follow-up and another two provided data for people with dementia who moved into a care home or moved between care homes. Post-death interviews were completed with key informants for six of the eight people with dementia who died while in a care home (the family carer provided post-death information for the remaining two people).
We collected data at the end of the study to examine the accuracy of the ‘surprise’ question by reviewing the status of all patients who had been on the dementia register at the time of screening. Patients for whom the surprise question was not answered (n = 28) and those whose status was not known at the end of the study (e.g. because they had moved practice; n = 35) were excluded (Table 4).
| ‘Surprise’ question | Status at study end, n (%) | ||
|---|---|---|---|
| Dead | Alive | Total | |
| Would you be surprised if this patient were to die in next 12 months? | |||
| Yes, surprised | 8 (9.1) | 80 (90.9) | 88 (100) |
| No, not surprised | 86 (36.8) | 148 (63.2) | 234 (100) |
Only 9% of patients not thought to be potentially approaching the EOL had died by the end of the study, compared with 37% of those thought by the GP to be potentially approaching the EOL. These figures nevertheless confirm the difficulties of identifying people with dementia approaching the EOL, as > 60% of those thought to be potentially approaching the EOL were still alive at the end of the study.
Participant characteristics
Participant characteristics were reasonably well balanced between intervention and control arms, given the relatively small study. Full details are provided in Appendix 4, Tables 19–22.
As previously described, we aimed to recruit two distinct groups of patients: those recently diagnosed and those potentially approaching the EOL. Patients who met both criteria have been classed as potentially approaching EOL in Table 5. This shows that the majority of patients screened (85%) were considered to be potentially approaching the EOL. Those not considered to be potentially approaching EOL were almost four times as likely to be recruited as those potentially approaching EOL (42% vs. 11%). Despite the unequal recruitment of patients from the two eligible groups, the majority of those recruited (69.4%) were nevertheless thought to be potentially approaching the EOL. We are, therefore, confident that the intervention was provided to both groups of eligible patients.
| Recruited to trial | Screened as potentially approaching EOL | Total, n | |
|---|---|---|---|
| No, n (%) | Yes, n (%) | ||
| No | 26 (57.8) | 208 (88.9) | 235 |
| Yes | 19 (42.2) | 43 (11.1) | 62 |
| Total | 45 (100) | 251 (100) | 296 |
Data from the qualitative interviews suggest that reservations over approaching patients and their families thought to be approaching the EOL and concerns over what the intervention would involve or could offer this patient group contributed to the under-recruitment of people with dementia potentially approaching the EOL. Similar issues with access to patients approaching the EOL and their carers were experienced in the process evaluation, whereby the DNSs could be protective towards potential participants thought to be at the EOL.
Recruitment to the process evaluation
We completed a total of 55 interviews with 59 interviewees in the process evaluation. Interviewees included people with dementia; family carers; DNSs; the intervention supervisory team; general practice staff (intervention and control sites), including GPs, practice managers, administrative staff and pharmacists; and a range of health-care and social care professionals who worked in care homes, community mental health teams, palliative care teams, primary care, sheltered housing and hospices. On average, interviews lasted 33 minutes.
Non-participant observation was conducted on 23 occasions (total time: 1243 minutes, mean 54 minutes, range 15–120 minutes). Observations were predominantly of intervention delivery, including training, working with other practitioners and working with people with dementia and their families. Observation settings included care homes, hospitals, GP surgeries and participants’ homes. DNS training and intervention supervision sessions provided by the research team were also observed and audio-recorded with the consent of all participants.
Views on screening and recruitment
Key findings from the qualitative data are summarised in this section; full details with illustrative quotations are provided in Appendix 4, Feasibility and acceptability of recruitment and retention. Feedback from practice staff and the DNSs indicated that the process of screening patients and recruitment was more challenging than anticipated. This was partly because of difficulties in answering the screening questions, with some GPs questioning the validity of the ‘surprise’ question. Recruitment of family carers was hindered by poor documentation of next of kin in GP records. Relatively few potential participants opted out of further contact from the research team, suggesting that this approach was acceptable to this group of patients and carers. Few comments were made by people with dementia and carers about recruitment processes. Some people with dementia and their carers who had been recently diagnosed did not see the SEED intervention as relevant to their situation. However, some of those who initially thought that the intervention had little to offer were subsequently surprised by how much they gained from the intervention. Key issues relating to recruitment and potential strategies to maximise recruitment to a future trial are summarised in Appendix 4, Table 23.
Feasibility and acceptability of the SEED intervention
An overview of DNS activity, including new resources developed and implemented during the study, is summarised in Table 6. This shows that interventions relating to all seven components of the SEED intervention were delivered to participants living at home and in care homes, at both individual and systems levels. Although all activity logs, except for the weekly log, provided space to map activities to the seven key components of the SEED intervention, this aspect of the logs was inconsistently completed and there was a significant number of missing data. We could not, therefore, analyse the extent to which different components were covered by the intervention. Instead, we focused on the activities listed in the theory of change and considered:
-
the proportion of days on which specific activities were recorded
-
the focus of work with participating dyads
-
collaborative working.
| Component | Individual work | Systems-level work | Resources |
|---|---|---|---|
| Timely planning discussions |
|
|
|
| Recognising EOL and providing supportive care |
|
|
|
| Co-ordinating care |
|
|
|
| Working effectively with primary care |
|
|
|
| Managing hospitalisation |
|
|
|
| Continuing care after death |
|
|
|
| Valuing staff and ongoing learning |
|
|
Findings from the activity analysis showed that the intervention focused on the current needs of people with dementia and family carers, networking and service mapping (see Appendix 4, Figure 7 and Table 26). The vignettes (see Appendix 4, Boxes 2–6) illustrate how the seven components were operationalised. Four examples for individual dyads and one for a care home are provided in Appendix 4, Boxes 2–6. Bespoke training and supervision arrangements were provided for the DNSs by the research team, an old-age psychiatrist and a palliative care clinical lead (see Appendix 4, Tables 24 and 25). The DNSs also met regularly for peer support. Only one DNS received supervision from the dementia lead GP; without this, the role was potentially isolating. Administrative and information technology (IT) support from the host general practices were necessary to navigate primary care systems effectively. The different backgrounds of the DNSs (in palliative care and mental health) proved useful in joint working and mutual support. Although we did not succeed in establishing a multidisciplinary team (MDT) to support the DNSs, they did not feel that their case load was complex enough to merit this.
Stakeholder views on the SEED intervention
Key issues relating to the feasibility and acceptability of the SEED intervention concerned the location of the SEED intervention in primary care, relevance of the seven components to real-world practice, appropriateness of working with individuals and at a more strategic level, qualifications and training needed to fulfil the DNS role, and the fit of the SEED intervention with existing services. These issues are summarised in this section, with further elaboration and illustrative quotations provided in Appendix 4, Feasibility and acceptability of the SEED intervention.
Basing the intervention in primary care was valued because this facilitated access for people with dementia and family carers, face-to-face contact with GPs, access to patient records and links with other services.
Each of the seven components was thought to be relevant, and no additional components were identified. Although the DNSs were initially more comfortable working with individuals, through intervention supervision and the growing familiarity with their role, they became enthusiastic about working at a systems level (see examples in Table 6). The DNS role in co-ordinating care (across primary and secondary care) was valued by family carers and enhanced continuity of care. From a GP perspective, a proactive approach was thought to avoid crises, and reduce hospitalisation and demands on their time. The emphasis on timely planning discussions was valued by health-care professionals, as no existing professionals had a clear responsibility for these. Some of the barriers to planning ahead that were identified in our earlier work,96 for example reluctance to talk about the future and a preference to focus on the present, were encountered by the DNSs. The opportunity to invest time in relationship-building and to embed such discussions in a broader context of talking about changes in circumstances were key to facilitating ACP.
The nursing background of the DNSs was valued by both family carers and care home staff. Others considered professional background less important, but emphasised personal attributes, such as inspiring confidence and being reliable and approachable. Most stakeholders viewed the SEED intervention as complementary to existing services and tensions over role boundaries were rare.
We drew on the framework of NPT114 to identify factors influencing the implementation of the SEED intervention (see Appendix 4, Feasibility and acceptability of the SEED intervention). The NPT analysis suggested that the individual and collective work required for successful implementation of a new intervention was largely achieved. In terms of coherence (whether or not the intervention was easy to grasp and made sense), the DNSs and practice staff contested the focus on EOLC and were keen to extend the intervention to all people with dementia. Although the perceived lack of clarity over the focus and content of the SEED intervention was initially a barrier to implementation, as the DNSs became more confident with the components underpinning the intervention they valued the opportunity to develop the intervention autonomously.
Cognitive participation or investment in the SEED intervention was high for the DNSs, but varied between the two host general practices; this affected the extent to which the DNSs were successfully embedded and limited the scope for improving systems in one practice. The temporary nature of the intervention influenced buy-in of all stakeholders and required a strong focus on relationship-building by the DNSs to successfully engage people with dementia, family carers and care homes.
The work involved in delivering the SEED intervention (collective action) required experienced staff who were used to working autonomously. Supervision arrangements ensured access to specialist knowledge and support relating to different aspects of their role. Intervention supervision was particularly important because of the initial uncertainties of the DNSs over the scope of the intervention, and to encourage working at a more strategic level.
Although the SEED intervention was piloted for only 12 months, there was evidence of reflexive monitoring: the process of reflecting on, and adapting, the intervention. Access to different perspectives through supervision facilitated reflection on the intervention. A number of pieces of work were undertaken iteratively with care home managers, GPs and other local professionals to maximise likelihood of integration into practice. System-level changes likely to be sustained after the study included the annual dementia review template in one general practice, comfort care planning and revised EOL documentation in some care homes.
Feasibility and acceptability of outcome measures
Completion of outcome measures
All data were collected within the target window with one exception, confirming that the time parameters set for data collection were achievable. Aggregating data across all time points for current study participants (i.e. excluding those who had died or withdrawn), data were collected from 86% of people with dementia, 97% of family carers and 100% of key informants. The main reason for missing data was that some people with dementia were not approached for data collection at the request of their family carer or key informant, because the family carer or key informant either thought that the person would not be able to provide data and/or thought that data collection would have a negative impact (e.g. creating anxiety). Data were occasionally missing because of ill health or holidays. Data for family carers were missing because of unavailability, typically because of holidays, their own ill health or difficult circumstances.
The proportion of fully completed measures (i.e. those with no missing items) at each time point by type of respondent ranged from 9.1% to 100%. All but three measures were fully completed on > 80% of occasions. The three measures with poor completion rates were as follows:
-
the PAINAD during movement, with a full completion rate of 53.5% across all time points and respondents (range 43.8–68.4%; see Appendix 4, Tables 30–33)
-
the EQ-5D-5L completed by people with dementia, with a full completion rate of 65.1% across all time points (range 54.2–86.7%; see Appendix 4, Table 36)
-
the Satisfaction with Care at the End of Life in Dementia (SWC-EOLD), with full completion rates of 40.7% for family carers (range 11.8–100%) and 35.4% for key informants (range 9.1–66.7%; see Appendix 4, Tables 30–33 and Table 35).
Qualitative data and further analyses suggested that the reasons for missing data were specific to individual measures. Missing data on the PAINAD were because of the inability of researchers to rate pain during movement when people with dementia remained seated (or asleep). Difficulties on the EQ-5D-5L appeared to reflect the difficulties experienced by some people with dementia in understanding and grasping the task. Given the large number of missing data on the SWC-EOLD, completion rates for individual items were reviewed. This suggested particular difficulties with two items (questions 4 and 10; see Appendix 4, Table 34, for details). The finding that completion of this measure improved at the post-death interview (see Appendix 4, Table 35) may suggest that the items are more relevant at the EOL, although interpretation is difficult because numbers are small. The validity of the SWC-EOLD was disputed by key informants who commented that they were unlikely to criticise their own care; this may contribute to the large numbers of missing data.
Detailed scores for numeric outcome measures are tabulated by time point and intervention arm in Appendix 4, Tables 40–61.
Capturing data on advance care planning
Although the intention had been to collect data from both general practice and care home records at baseline, 12 months and post death to ascertain whether or not plans were filed in both places, only one set of records was routinely checked. Data were available for all people with dementia at baseline and for all but four individuals at follow-up, most commonly because they had withdrawn (see Appendix 4, Table 38). Further analyses indicated wide variation between practices in the proportion of people with dementia for whom plans were available in either general practice or care home records (see Appendix 4, Table 39).
Stakeholder view on outcome measures
A detailed analysis of the qualitative data relating to outcome measures, including quotations from participants, is provided in Appendix 4, Feasibility and acceptability of outcome measures. In summary, all participants thought that the set of measures was acceptable. The relevance of some measures was questioned, for example whether or not the End of Life in Dementia (EOLD) measures were appropriate for recently diagnosed people with dementia, and whether or not rating satisfaction with care was appropriate for care home staff. Concerns were raised over the reliability of some measures; in particular, some family carers and key informants queried the responses of the person with dementia to the EQ-5D-5L. Some measures [e.g. Neuropsychiatric Inventory (NPI), Comfort Assessment in Dying with Dementia (CAD-EOLD)] were potentially distressing for family carers, either because they raised awareness of the range of symptoms that might occur in the future or because they highlighted discrepancies between desired and actual care. A number of potential modifications to the resource use questionnaire were identified to reduce respondent burden.
Key implications for a future trial of the SEED intervention
Recruitment to a future trial
Given the high proportion (82%) of patients on the dementia register who were eligible, the rationale for excluding the remainder was questioned. Broadening the eligibility criteria would ensure equitable provision to all people with dementia. It would also streamline recruitment processes. A range of practical barriers to recruitment were identified. One successful strategy used in the pilot trial was to pay for a locum to free up GP time for screening; although the screening process may be less onerous in a future trial (if the eligibility criteria change), this strategy should still be considered. The acceptability of an opt-out approach was demonstrated for this patient group. Training other practice staff to make the follow-up telephone calls could also reduce the burden on GPs. The lack of information routinely recorded on next of kin created additional work and caused delays in recruitment. Working with practices prior to recruitment to improve recording of next of kin is recommended in a future trial. The pilot trial demonstrated the importance of close, formal monitoring of recruitment processes, and this should be planned from the outset. Detailed recommendations for maximising recruitment to a future trial are provided in Appendix 4, Table 23.
Changing the eligibility criteria would, however, have implications for the intervention that was developed rigorously using extensive data from WS2 and the previous experience of the MCDP to improve EOLC. Additional components are likely to be relevant at other stages of the illness trajectory and further work would be needed to identify these and to consider how best to address them if the intervention were to be extended to cover the entire dementia trajectory.
Implementation of the SEED intervention in a future trial
Basing the intervention in general practices was successful, but more explicit negotiation about the required level of engagement from GPs and practice staff is needed. Intervention supervision facilitated an ongoing dialogue about the boundaries of the intervention, how to operationalise the seven key components and opportunities for making strategic changes. It is, therefore, recommended that similar arrangements are made in a future trial. Although we did not succeed in establishing a MDT to support the DNSs, the clinical supervision from old-age psychiatry and palliative care met their needs.
Data from the pilot trial could be used to further refine the intervention manual and resources (e.g. by providing examples of the range of activities). This should enable DNSs in a future trial to feel clearer and more confident about their new role. Assessing the fidelity of a complex, tailored intervention was inevitably challenging. Involving the DNSs in the development of activity analysis tools and iteratively monitoring and refining their use is recommended in a future trial. Despite clear areas of overlap with existing services, particularly Admiral nurses, most stakeholders saw this as a benefit, rather than a problem. Each intervention practice was allocated a full-time DNS in the pilot trial; alternative, less costly, ways of providing the intervention should be explored.
Outcomes for a future trial
Many of the outcome measures appeared to work well in the pilot trial in terms of acceptability to participants and completion rates. However, we did not clearly identify an appropriate primary outcome measure for a future trial, particularly if the intervention was extended to include the full illness trajectory. Despite the previous validation and successful use of the SWC-EOLD in the USA138 and the UK,64,119,143 it was poorly completed and criticised by respondents and researchers in this study. Identifying measures that focus on well-being, not just deficits, is also recommended because the intervention may foster a positive sense of well-being.
We demonstrated the feasibility of collecting data on resource use and health-related QoL. Given the relatively large numbers of missing data on the EQ-5D-5L for people with dementia, collecting proxy data at all times is recommended in a future trial. People with dementia may also find the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), easier to complete. Further investigation of how to prioritise or combine data from proxy respondents is needed. A number of recommendations for modifying the resource use questionnaire were made to reduce respondent burden. Data on ACP were successfully captured and suggest that the percentage change in documentation in practices may be a more appropriate way of analysing data in a future study. Although we focused on whether a range of plans was present or absent from the notes, content analysis of such plans may provide a more nuanced approach. Given the emphasis on staff training, the inclusion of measures of staff knowledge, skill and confidence could be considered in a future trial.
Workstream 4 conclusions
The pilot trial achieved the key success criteria of recruiting at least 11 people with dementia per practice, collecting outcome data for at least 33 people with dementia at 12 months, and demonstrating the feasibility and acceptability of the SEED intervention. The data and insights from the pilot trial will enable us to revise intervention materials and provide more practical guidance on future implementation. Extending the intervention to all people with dementia was widely recommended by stakeholders and could offer one model for providing the named care co-ordinator recommended by NICE. 29 The feasibility of collecting outcome data on ACP and resource use was demonstrated, although further adaptations to data collection are recommended. None of the outcome measures used, however, was found to be suitable as the primary outcome measure for a future trial.
Reflections on workstream 4
In the pilot trial, we allocated one DNS to each intervention practice; this is unlikely to be sustainable. Alternative ways of delivering the intervention by using teams with a range of qualifications and experience (including dementia advisors) across a number of practices may be more cost-effective. However, this would need to be managed in a way that facilitates relationship-building in the host general practice, with individual people with dementia and family carers, and with local care homes.
Key limitations related to recruitment processes and the difficulties in identifying a primary outcome measure. Lack of support for recruitment meant that only a small proportion of eligible people with dementia were approached in control practices, highlighting the need to adequately resource recruitment in a future trial. Further work is needed to identify appropriate outcome measures to capture the impacts of the intervention on people with dementia and family carers, with consideration also given to evaluating the impact of the SEED intervention on care home staff. In the light of the remaining uncertainties over eligibility and outcome measures, we do not intend to proceed to a definitive trial of the SEED intervention at this stage.
Workstream 5: economic modelling study
Further details on the economic modelling study are provided in Appendices 5 and 6.
Overview
As health and social care resources are limited, decision-makers need information about whether or not the benefits an intervention provides are worth its costs. 144 This information can be provided by an economic evaluation. An economic evaluation involves the comparative analysis of alternative courses of action in terms of both costs and effects. 145 In this section, we compare the SEED intervention, in which a DNS based in a general practice focuses their efforts on seven key components of EOLC, with alternative ways of providing care, including an example of current practice. The potential value of the SEED intervention was assessed using a contingent valuation survey of 1002 members of the general public. These data were used in an economic decision model. The economic model describes what happens to a person who has been diagnosed with dementia over time and how the SEED intervention might change this. Findings are presented in terms of costs and consequences (e.g. hospitalisations) and, using the contingent valuation data, a cost–benefit analysis. We found that the general population perceived the SEED intervention as having real value in economic terms. This was particularly the case for individuals with some experience of dementia in their close family members, colleagues or relatives, and by those with higher income levels.
Research aim
The aim of WS5 was to estimate the relative efficiency of the SEED intervention. WS5 was conducted between October 2013 and May 2018. The specific objectives were to:
-
value the consequences of the SEED intervention using contingent valuation methods
-
develop an economic model of the usual care pathway and new alternative pathways, including the SEED intervention developed in WS3
-
conduct a cost–consequence analysis of the SEED intervention compared with usual care
-
conduct a cost–benefit analysis of the SEED intervention compared with usual care by incorporating the results of the contingent valuation into the economic model.
Work relating to the first objective is described in the following section; the remaining objectives are addressed collectively in Economic evaluation of the SEED intervention. The economic evaluation was conducted following best-practice guidelines conforming to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 146
Valuing the consequences of the SEED intervention
See Appendix 5, Valuing the consequences of the SEED intervention, for supplementary data. This work has been published as Bhattarai et al. 147 (see Acknowledgements, Publications).
Measures typically used in economic evaluations to quantify the benefits of interventions, such as quality-adjusted life-years (QALYs), may not adequately capture individual preferences for how services are organised and their associated outcomes. An alternative approach to determine the benefits that an individual derives from an intervention is to determine their maximum WTP for it using a contingent valuation study. Maximum WTP represents the maximum amount, expressed in monetary terms or in terms of other goods, an individual is willing to give up (or sacrifice) to gain the benefits of the intervention. 148
Methods
Five scenarios were developed that described different combinations of the seven key components to support good EOLC identified from WS2. These five scenarios mirrored the comparators used in the economic model. The contingent valuation survey took a community perspective, with respondents asked to give their WTP for the SEED intervention to be available in the NHS, even though they would not (necessarily) benefit from it themselves. Given this perspective, respondents were asked their WTP in the form of an additional tax per month that they would pay for the next 10 years. The survey comprised three sections: background information on current provision of dementia care towards the EOL and on the SEED intervention, the WTP questions and the participant demographics (see Report Supplementary Material 1). The survey was pre-piloted with Newcastle University staff using the ‘think-aloud’ technique. 149 Piloting of the full web survey was conducted in a subsample of the target general population. For the pilot and final surveys, the sample of the general population was recruited from the online panel managed by a marketing company (ResearchNow, London, UK). For the main survey, a sample of 1000 respondents was targeted, with quotas on age, gender and employment status to be representative of the UK general population.
Key findings
Data were collected from 1002 members of the general public (see Appendix 5, Valuing the consequences of the SEED intervention for details of the sample). Table 7 reports the mean and median WTP values across the scenarios for both the untrimmed and the trimmed data sets. The trimmed data set excluded the top 1% of responses. The mean WTP computed from the untrimmed data set for the alternative scenarios was much higher than the mean WTP value for the main scenario, and the very wide 95% confidence intervals (CIs) indicate the presence of very high outlier values. When the top 1% of WTP values were trimmed,150 the mean WTP for the main scenario was higher than for the alternatives and the CIs were narrower. The medians for both the trimmed and the untrimmed data sets generally remained the same. The number of zero responses per scenario ranged from 19% to 35% of the total sample, of which 10–13% could be classified as protest zeros, that is respondents indicated that they were not willing to pay because they believe that should not have to pay for health care. The protest zero responses were removed from the analysis of mean/median WTP.
| WTP | Main | Alternative | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Mean (95% CI) | 40.13 (26.25 to 54.01) | 2357.20 (23 to 14,006) | 257.47 (28 to 1391) | 810.22 (27 to 4700) | 2313.69 (22 to 13,750) |
| Mean (95% CI)a | 24.19 (21.85 to 26.52) | 18.38 (15.95 to 20.82) | 16.18 (13.59 to 18.76) | 18.36 (15.72 to 21.00) | 16.99 (14.15 to 19.83) |
| Median (95% CI) | 10.0 (10.0 to 15.0) | 10.0 (7.5 to 10.0) | 7.5 (5.0 to 8.0) | 9.25 (7.5 to 10.0) | 6.0 (5.0 to 9.0) |
| Median (95% CI)a | 10.0 (10.0 to 12.5) | 10.0 (7.5 to 10.0) | 7.5 (5.0 to 8.0) | 8.0 (7.5 to 10.0) | 6.0 (5.0 to 8.0) |
Compared with individuals with no experience of dementia, individuals who have seen their close family members, friends or relatives with dementia placed a higher value on the tailored support provided by the DNS and the provision of high-quality EOLC to people with dementia (see Appendix 5, Table 65). Respondent characteristics, such as age, gender, household size or health utility score, did not influence the WTP value (see Appendix 5, Table 66). However, some higher-income groups had WTP values that were significantly higher than the WTP values of those on the lowest income level, which is consistent with economic theory. These findings suggest that members of the general public do value the care provided by the SEED intervention. Moreover, a higher WTP value for the main scenario indicated that it was valued more than packages with selected features only.
Economic evaluation of the SEED intervention
See Appendix 5, Economic evaluation of the SEED intervention, and Appendix 6, Economic modelling study (workstream 5): additional explanatory text, for supplementary data.
The economic evaluation aimed to estimate the potential relative efficiency of the SEED intervention. As the way in which this intervention will be implemented is not, as yet, precisely known, an early economic model was developed. 151 In such models, plausible ranges for model parameters are specified and the impact on cost–benefit outcomes of varying these model parameters over these plausible ranges is estimated. A cost–consequences analysis was designed in which multiple health and non-health consequences were estimated separately. 152,153 A cost–benefit analysis was then conducted, incorporating the results of the contingent valuation into the economic model, and valuing the benefits of the intervention collectively in commensurate units (money). 145
Methods
A decision-analytic model was developed to estimate the impact of the SEED intervention activities on relevant service outputs and related outcomes for people with dementia (see Appendix 5, Economic evaluation of the SEED intervention), as well as health and societal costs. We modelled the main elements of a patient’s journey through care, as well as how dementia may progress over time. The literature was reviewed for economic evaluations to inform the model structure (see Appendix 5, Economic evaluation of the SEED intervention). In the model, care and health events occur based on probabilities derived from different sources. Following the diagnosis, dementia progresses in three severity domains (see Appendix 5, Event probabilities, and Appendix 6, Dementia progression). The setting of care for an individual may change between home, care home and hospital (see Appendix 5, Event probabilities, and Appendix 6, Transition probabilities between care settings). An individual eventually requires palliative care, and there is a risk of death at any stage of the model (see Appendix 6, Mortality and palliative care). The likelihood of care and health events were influenced by patient characteristics (see Appendix 6, Baseline population) and by the prevalence of specific dementia care services (see Appendix 6, Care services and their effects). Finally, costs were assigned to the SEED intervention, the care settings and the care services (see Appendix 6, People with dementia: care-setting costs).
The cost–consequence analysis compared the presence of the SEED intervention with usual care. The effect of the SEED intervention on service outputs and related outcomes for people with dementia is unknown. Therefore, a set of scenarios was designed to estimate the relative impact of all activities combined and each activity individually:
-
the maximum and minimum expected provision of all activities are set in a favourable and conservative SEED scenario, respectively
-
the minimum and maximum expected provision of each activity was set, while the remaining four activities were set at the average level of provision.
In the cost–benefit analysis, the net monetary benefit of providing a SEED intervention over an illustrative 5-year period was estimated for each of the scenarios described in Appendix 5, Valuing the consequences of the SEED intervention, compared with a scenario in which these services are absent. The net monetary benefit is the difference between the WTP for the services and the additional costs associated with the services.
Key findings
Cost–consequence analysis
The SEED intervention had, on average, the following effects: reduced rates of hospitalisation; reduced length of hospital stay; increased number discharged from hospital to usual place of care; and increased access to, and duration of, palliative care (Table 8). However, the health-care and social care costs increased. The 95% CIs indicate some uncertainty in the direction of the effect for all outcomes, except for duration of palliative care and number of hospitalisations avoided.
| Scenario | Estimate | Mean estimatea (95% CI) | ||||
|---|---|---|---|---|---|---|
| Cost (£) | Length of stay (days) | Time in palliative care (months) | Discharge to usual place of care (per 1000 discharges) | Avoided hospitalisations (per 1000 possible hospitalisations) | ||
| Favourable SEED scenario | 9930 (–3174 to 23,553) | –1.4 (–2.8 to 0) | 1.91 (1.07 to 2.86) | 25 (–31 to 75) | 161 (119 to 201) | |
| Conservative SEED scenario | 2007 (–10,701 to 14,189) | –1.3 (–2.8 to 0.1) | 0.46 (0.09 to 0.9) | 0 (–50 to 44) | 81 (57 to 109) | |
| Reviews | Minimum | 5833 (–7946 to 18,342) | –1.35 (–2.8 to –0.1) | 1.17 (0.52 to 1.91) | 13 (–38 to 61) | 120 (82 to 164) |
| Maximum | 6669 (–6512 to 19,907) | –1.36 (–2.7 to 0) | 1.87 (0.99 to 2.94) | 13 (–40 to 63) | 121 (84 to 165) | |
| ACP | Minimum | 4590 (–8362 to 17,295) | –1.32 (–2.8 to 0) | 1.83 (0.96 to 2.95) | 3 (–47 to 51) | 103 (73 to 136) |
| Maximum | 8481 (–4339 to 21,854) | –1.33 (–2.8 to 0) | 1.84 (1.01 to 2.97) | 22 (–30 to 73) | 138 (99 to 180) | |
| SBAR technique | Minimum | 6067 (–6552 to 19,160) | –1.3 (–2.8 to 0) | 1.85 (1.01 to 2.96) | 13 (–39 to 61) | 106 (72 to 142) |
| Maximum | 7202 (–6176 to 19,945) | –1.32 (–2.8 to 0) | 1.89 (1.04 to 3) | 13 (–39 to 64) | 135 (96 to 177) | |
| Transfer sheets | Minimum | 6553 (–6392 to 19,493) | –1.35 (–2.8 to 0) | 1.85 (0.99 to 2.93) | 13 (–42 to 65) | 121 (84 to 164) |
| Maximum | 6586 (–6222 to 19,496) | –1.43 (–3.1 to 0) | 1.87 (0.98 to 2.97) | 13 (–42 to 65) | 121 (84 to 164) | |
| Discharge planning | Minimum | 6475 (–6102 to 19,756) | –1.32 (–2.9 to 0) | 1.87 (0.96 to 2.98) | 7 (–48 to 57) | 121 (84 to 164) |
| Maximum | 6788 (–5321 to 20,159) | –1.32 (–2.9 to 0) | 1.87 (1.01 to 2.94) | 13 (–42 to 66) | 121 (84 to 166) | |
The SEED conservative scenario is, on average, less costly than the SEED favourable scenario because the expected provision of the SEED intervention activities is not as great in the conservative scenario.
Cost–benefit analysis
The cost–benefit analysis compared the different variants of the SEED intervention, described in Appendix 5, Valuing the consequences of the SEED intervention (main scenario and alternative scenarios 1–4; see Boxes 7–11). Every WTP scenario is associated with a positive net benefit (column D in Table 9). Scenarios in Table 9 are ordered from lowest to highest net benefits. The main scenario, which incorporates all of the activities of the SEED intervention, has the greatest net benefit; alternative 4 has the lowest net benefit. These net benefits are all relative to the provision of usual care, showing that it would be efficient to provide the SEED intervention. Column F in Table 9 shows the probability that each scenario provides the greatest net benefit. The main scenario has the highest probability of being the most cost-effective (30%). However, no scenario clearly stands out because of the considerable uncertainty in the cost and WTP estimates.
| Scenario | (A) Mean cost (95% CI) | (B) Mean incremental cost compared with usual care (95% CI) | (C) Mean incremental monetary benefit (95% CI) | (D) Net benefit (95% CI) (C – B) | (E) Incremental net benefit | (F) Probability of being the optimal strategy (%) |
|---|---|---|---|---|---|---|
| Alternative 4 | 58,999 (53,295 to 63,153) | –30 (–1048 to 968) | 8263 (280 to 31,629) | 8293 (–63 to 31,602) | 8293 | 10 |
| Alternative 1 | 58,587 (53,408 to 62,750) | –443 (–1675 to 466) | 8372 (732 to 27,407) | 8815 (960 to 28,164) | 522 | 16 |
| Alternative 2 | 54,455 (49,578 to 58,434) | –4574 (–10,556 to 1332) | 7221 (432 to 28,743) | 11,795 (1458 to 33,186) | 2980 | 19 |
| Alternative 3 | 54,593 (50,090 to 58,446) | –4437 (–10,622 to 2267) | 8832 (426 to 34,220) | 13,269 (976 to 38,614) | 1474 | 24 |
| Main scenario | 53,841 (49,931 to 57,118) | –5188 (–10,471 to 931) | 11,313 (491 to 43,849) | 16,501 (2994 to 49,612) | 3232 | 30 |
The results of the cost–consequence analysis suggest that the SEED intervention is likely to increase costs, overall, related to changes in the care services. These services are expected to benefit people with dementia and family carers. These findings are reinforced by the cost–benefit analysis, which suggests that the value of the benefits of the SEED intervention is likely to be greater than the increased cost of care services. The results are imprecise (the CIs are wide) because they are based on an early economic model; further research is needed to obtain more evidence for the model inputs, particularly of the effects of SEED intervention activities.
Workstream 5 conclusions
This WS describes several innovative economic evaluations, namely the first contingent valuation of a specialist dementia service, the first detailed economic model for a non-pharmacological intervention in dementia from diagnosis to EOL and the first economic evaluation model that incorporates the results of a contingent valuation into a probabilistic economic model. Its methodology, as a minimum, meets internationally accepted best-practice recommendations for contingent valuation, economic evaluation and economic modelling. 154
A key finding is that the SEED intervention is perceived by the general population as having real value in economic terms, in particular by individuals with some experience of dementia in their close family members, colleagues or relatives and by those with higher income levels.
Despite being highly valued by the general public, the SEED intervention is unlikely to reduce costs, but it may change service use in ways that benefit people with dementia and their families. These changes may relieve pressure on some NHS services (e.g. hospital beds), but may increase demand on other NHS services that are overstretched (e.g. palliative care services).
Reflections on workstream 5
The results of the contingent valuation study are based on a large sample thought to represent the UK general population, but the validity of the responses could have been affected by biases arising out of the construction of the WTP survey or by the interpretation and understanding of the scenarios by the respondents. Using the internet survey panels could have introduced bias by failing to include major consumers of health-care services who are not internet users.
The economic analyses are based on an early economic model; therefore, there is considerable uncertainty surrounding both the model inputs and the underlying structure of the model. The effects of this uncertainty are that estimates for model outputs may be imprecise (i.e. CIs are wide) and important costs and benefits may not be accurately captured. Nevertheless, rigorous approaches were taken to use the best evidence available to ensure that the model captured key aspects.
The economic evaluation allowed us to explore the contribution of each component of the SEED intervention to relative efficiency. The reliability of these estimates is directly related to the trustworthiness of the structural assumptions of the model. The individual components of the SEED intervention do not change outcomes in an additive way; rather there appear to be diminishing returns from adding each component. This phenomenon has been observed in many studies investigating complex multicomponent interventions. 155,156 However, the precise nature of correlation between components is unclear. Should new data and understanding become available, consideration should be given to refining the model and the data inputs. Further details of data reported here are presented in Appendices 5 and 6.
Workstream 6: commissioning good-quality, community-based end-of-life care in dementia
Overview
Specific evidence-based guidance to inform the commissioning of co-ordinated EOLC for people with dementia is limited;51 WS6 aimed to develop and disseminate evidence-based guidance for the commissioning of better-quality EOLC in dementia. Initially, a narrative review was undertaken to better understand the organisation of commissioning in dementia and EOLC; this found considerable gaps in the existing guidance. 157 The review was complemented by in-depth interviews with commissioners, which revealed an experiential picture different from the ideal commissioning scenario outlined in policy. 157 In 2015, NICE announced an update of national dementia care guidance. As this would include EOLC, specific SEED guidance development was postponed; instead, additional interviews and an updated review were performed while awaiting release of the revised guidance. Updated NICE guidance (2018) showed little new evidence underlying EOLC, but recommended the provision of a ‘single named health or social care co-ordinator’ [reproduced with permission from NICE. 158 © NICE 2018 Resource Impact Report: Dementia: Assessment, Management and Support for People Living with Dementia and their Carers (NG97). Available from www.nice.org.uk/guidance/ng97/resources/resource-impact-report-pdf-4897901485. All rights reserved. Subject to Notice of rights] from point of diagnosis to death. 158 The SEED intervention has been proposed to commissioners as a potential method for implementing the nationally recommended care co-ordinator role.
Patient and public involvement
Contributors advised the team whether to pause or amend planned work owing to the revision of the NICE guidelines. They also contributed ideas for the dissemination event.
Research aims
-
To summarise how EOLC for people with dementia was commissioned and organised.
-
To produce guidance that summarises the evidence, and case for change, to commission good-quality, community-based EOLC in dementia.
-
To facilitate national dissemination of this guidance.
Workstream 6 was conducted between October 2014 and September 2018.
Commissioning end-of-life care in dementia: mapping the status quo
This study has been published as Gotts et al. 157 (see Acknowledgements, Publications).
Methods
A mixed-methods approach was used, combining a narrative review and qualitative interviews with commissioners of EOLC for people with dementia. The former examined current guidance and policy (national and international) and academic literature with initial web-based searches in January 2014, repeated in January 2016. Academic papers were included if they focused on commissioners’ experiences of the commissioning process, service providers’ experiences of the commissioning process or factors that enable or inhibit the commissioning process, or if they compared commissioning arrangements. Using review findings, a semistructured topic guide was developed (see Report Supplementary Material 1). Interviews with professionals responsible for commissioning EOLC for people living with dementia (n = 20) took place between October 2014 and January 2016. All interviews were audio-recorded and transcribed verbatim, then checked and anonymised prior to analysis.
Policy and guidance documents included in the narrative review were categorised as follows:
-
guidance (a guide to commissioning or clinical practice)
-
policy (documents concerned with aspirations and aims issued by the Department of Health and Social Care, NHS England and other government agencies)
-
strategy (e.g. national EOLC strategy)
-
non-governmental organisation position papers (e.g. Alzheimer’s Society, Nuffield Trust and The King’s Fund).
Findings from the academic literature were summarised and grouped thematically. Interview transcripts were analysed using a thematic approach. 101
Key findings
The review found major gaps in commissioning guidance for EOLC, specifically for people with dementia. Findings from the academic literature mainly focused on commissioning at a general level, with little on condition-specific commissioning for EOLC. Three key themes emerged from the triangulated findings of the narrative review and first round of commissioner interviews:157
-
the importance of joint commissioning
-
a lack of clarity in commissioning processes
-
facilitators of and barriers to commissioning.
Commissioners faced several challenges, not least a constantly changing policy landscape. Broader policy change, for example the introduction of sustainability and transformation plans and constantly changing commissioning structures, led to commissioners working in a context of persistent uncertainty. In exploring health professionals’ perceptions of the commissioning process, uncertainty emerged as an overarching theme. In terms of expertise, commissioners need succinct evidence summaries, knowledge of local resources and an understanding of how health-care organisations function at a national level. New guidance could focus on assisting commissioners to address day-to-day practical problems and contain concise evidence to inform activities such as contract specification (a structured description of what the commissioning organisation requires from a provider).
The narrative review was updated with new searches conducted between February and June 2017 using the same search strategy and search terms as the original review. Only studies published since 2015 were considered for inclusion. Fifteen potentially relevant articles were retrieved, of which eight met the inclusion criteria. Three studies explored dementia and EOLC,159–161 with the other five focused on general commissioning of health services. 30,162–165 A summary of the studies included in the updated narrative review is provided in Appendix 7, Table 95. Key findings revealed increasing complexity and persistent lack of clarity in processes, particularly in three areas: the role of the GP, contracting models and the commissioning of palliative care and EOLC in general.
Clinical Commissioning Groups and the role of the general practitioner
Clinical Commissioning Groups are very complex, varying both in size (population coverage ranges from 90,000 to 855,000) and organisation. 166 There was a lack of clarity as to how other health-care organisations and governance structures related to CCGs and whether or not any formal relationships had been established. In this new commissioning environment, the role of the GP was complicated; GPs held various and diverse roles (e.g. account officers and CCG lead) in addition to their clinical provider role.
Contracting models and care integration
The contracting models used by CCGs varied, for example (1) a prime provider model whereby one prime provider undertakes responsibility for parts, or all, of care agreed, (2) a prime contractor model whereby an organisation manages other providers that directly provide care services and (3) an alliance contracts model whereby separate providers share responsibility. The main aim of the contractual frameworks was, ultimately, to attain greater care integration, but it was unclear how the different models were successfully operationalised in practice.
Commissioning of palliative and end-of-life care in general
Often service provision was not consistent with population need, with great variability in the funding provided from local authorities; this was particularly so for palliative care services. In terms of dementia, there was an imbalance in service commissioning, with the main focus of resource allocation dedicated to early diagnosis and intervention (i.e. memory clinics) and early disease management (i.e. day care services), rather than care at the EOL. 160,161 Moreover, some commissioners do not have an in-depth understanding of the needs of people with advanced dementia, expressing uncertainty as to whether or not people with dementia need EOLC provision that is different from that for other patients. 161
Follow-up interviews
Further commissioner interviews (n = 7) were conducted between January and August 2017, to explore any subsequent changes to commissioning processes and information needs. There were a number of parallels with the thematic analysis conducted for the first round of interviews. A theme of continuous organisational change with persistent lack of clarity in processes prevailed.
Organisation of commissioning
Participants commented on the continued re-organisation of commissioning services alongside complex top-heavy commissioning structures and new initiatives (such as sustainability and transformation plans), which created additional upheaval. There was also a lack of clarity around commissioning processes and where responsibility lay for commissioning. Notwithstanding a new proposed local organisational structure, an Accountable Care Organisation was considered an opportunity for more efficient, integrated working.
End-of-life care and dementia: lack of integrated guidance
Some progress has occurred regarding integrated EOLC in dementia, but, generally, systems remained separate. For example, it was highlighted that the EOL guidelines do not include dementia, and dementia commissioning guidelines do not include the EOL.
Specification as an emerging art form
Participants still considered specification, a structured description of what the commissioning organisation requires from the service provider, a useful tool. However, this too was a constantly changing process to adapt to a shifting political and organisational landscape. It did, however, provide an opportunity to clearly define the detail of service provision, and its evidence base, and to incorporate performance measurement.
Evidence-based guidance for commissioners: comparison of the updated National Institute for Health and Care Excellence guideline with the SEED intervention findings
Further details are provided in Appendix 7.
Methods
Following a decision to suspend the development of SEED specific guidance in view of the updated NICE guidance in dementia care, the new 2018 guidance, once published, was scrutinised to identify new recommendations and/or changes in guidance specific to EOLC in dementia, for example care planning, review and co-ordination, and involving people with dementia in decisions about their care. Sections of the original32 and updated29 clinical guideline were extracted and entered into a structured data table. The content along with the strength and quality of the evidence underpinning the recommendations was compared.
Key findings
Although very similar in terms of the recommendations for practice, the evidence base underpinning the 2018 NICE recommendations had strengthened considerably, owing to an increase in both the quantity and the quality of available research. The one exception to this was for palliative care and EOLC, for which there was still limited empirical research. Three key components were relevant to improving EOLC in dementia; these are outlined below.
Involving people with dementia in decisions about their care
In terms of involving people with dementia in decisions about their care, 13 recommendations are made, which are grouped into three themes: providing information, ACP and involving people in decision-making. There is a clear emphasis on providing ongoing opportunities throughout the illness trajectory to discuss and make advance decisions. Both the person with dementia and the staff should be supported to engage in discussions about future care preferences.
Care planning
In terms of care planning, six recommendations are made, with a core emphasis on the provision of a single named health-care or social care professional who is responsible for co-ordinating the care of a person with dementia from diagnosis to the EOL. Guidance is also provided about the roles and responsibilities of the care co-ordinator role in practice, and the involvement of the person with dementia in care planning.
Palliative care
For the delivery of palliative care, the 2018 dementia recommendations29 refer the user to the NICE guidelines on (1) palliative care15 and (2) care of adults in their last days of life. 14 The emphasis is on a person-centred approach that includes the use of anticipatory health-care planning, ACP and structured observational tools.
Comparison with the SEED intervention findings
Table 10 shows how the seven SEED components, identified as essential to the delivery of good EOLC for people with dementia, closely align with several elements of the NICE recommendations29 (e.g. providing ongoing opportunities throughout the illness trajectory, use of a person-centred approach, co-ordination of care). To ‘[p]rovide people living with dementia with a single named health or social care professional who is responsible for co-ordinating their care’ [reproduced with permission from NICE. 158 © NICE 2018 Resource Impact Report: Dementia: Assessment, Management and Support for People Living with Dementia and their Carers (NG97). Available from www.nice.org.uk/guidance/ng97/resources/resource-impact-report-pdf-4897901485. All rights reserved. Subject to Notice of rights] was one of three original (clinical guideline 4232) NICE recommendations that was identified as not having been fully implemented, and for which there was evidence of wide variation in practice. 158 The SEED intervention, developed with the seven factors at its core, provides a potential solution to the uptake of this recommendation.
| SEED intervention component | NICE recommendation |
|---|---|
| Timely planning discussions | 1.1 Involving people living with dementia in decisions about their care |
| Co-ordinating care | 1.3 Care co-ordination |
| Working effectively with primary care | 1.3 Care co-ordination |
| Managing hospitalisations | 1.1 Involving people living with dementia in decisions about their care |
| 1.3 Care co-ordination | |
| 1.10 Palliative care | |
| Recognising EOL and providing supportive care | 1.10 Palliative care |
| Continuing care after death | 1.11 Supporting carers |
| Valuing staff and ongoing learning | 1.13 Staff training and education |
Dissemination and refinement of guidance on end-of-life care in dementia
A series of local and national dissemination activities for commissioners and service providers was undertaken to publicise both the NICE 2018 dementia guidance specific to EOLC and the SEED intervention.
Local dissemination: North East Dementia Alliance presentation
Twelve members were in attendance at the North East Dementia Alliance presentation, representing a range of regional organisations, including hospice and care homes, charities, the NHS, local authorities and research. A further presentation was made to regional dementia leads in October 2019.
National workshop: commissioning for excellence in end-of-life care in dementia
A half-day interactive event was held in London (in December 2018), attended by 52 delegates (commissioners and providers of dementia services). The majority of delegates indicated that they were familiar with the 2018 NICE dementia guidance. 29 Commissioning care co-ordination through the dementia trajectory was very difficult. Dementia and EOLC are not joined up: there is no ‘connectivity’ between guidance for each area of care and there is ‘not enough cross-talk’ between the two specialties. No one has ultimate responsibility (accountability and authority) for the total dementia care pathway or for the joining up or integration of services. Rather than being a strategic process, commissioning appeared to be guided by personal interests and ‘who knows who’. The DNS who was included in the SEED intervention as a named care co-ordinator would require expertise in palliative care and dementia. Concerns were raised that such a specialist role could lead to increased silo-working and duplication, and about whether or not such a new role (or person) was necessary. It would be important to look at the current system and existing resources to see whether or not a new role is needed. Delegates considered whether or not the SEED intervention should be a care pathway (from diagnosis to EOL), rather than a person. Generally, delegates concluded that there is an abundance of good, evidence-based guidance for both commissioners of dementia and EOLC, but what is missing is guidance that joins/integrates the two.
Workstream 6 conclusions
Service commissioners work in a context of persistent uncertainty, because a constantly changing policy landscape, with little national guidance or training regarding their role. Currently, dementia and EOLC are commissioned separately; a more integrated, joined-up commissioning approach is required. Updated 2018 NICE guidance29 showed little new empirical evidence underlying EOLC recommendations; however, it recommends the provision of a ‘single named health or social care professional’ [reproduced with permission from NICE. 158 © NICE 2018 Dementia: Assessment, Management and Support for People Living with Dementia and their Carers. Available from www.nice.org.uk/guidance/ng97 All rights reserved. Subject to Notice of rights] responsible for co-ordinating care from diagnosis to EOL. The SEED intervention is a potential method for implementing this new role.
Reflections on workstream 6
The approach to participant sampling for the commissioner interviews was a mixture of purposive and convenience. We sought diversity in geographical area, urban/rural setting and between clinical organisations and adult services. However, the introduction of both new commissioning structures during the programme and new national service models for older people’s care (e.g. vanguard sites167) led to difficulty in undertaking repeat interviews with first-round participants. Thus, the second-round participants included a mixture of repeat interviewees and new participants who were part of newly formed structures, such as vanguard site representatives.
Programme grants offer a unique opportunity to undertake health service research over a 5-year period. However, the biggest challenge to undertaking and completing such research was the constantly changing health-care and social care landscape at both local and national levels.
Overall conclusions
Updated national guidelines on dementia care29 revealed that, although there has been a considerable increase in dementia research, there has been little UK-based empirical research to inform evidence-based practice in EOLC. Extending existing evidence and using new empirical data, we followed the MRC framework for complex interventions73 to co-design and pilot a primary care-led DNS intervention to enable community-based professionals deliver co-ordinated, proactive EOLC to people with dementia and their families. The intervention was acceptable, feasible and shown to integrate well with existing care. The DNS role was highly valued by all stakeholders, both in real life and hypothetically in the contingent valuation study. Seven components of care were key to the DNS role: timely planning discussions, recognising EOL and providing supportive care, co-ordinating care, working effectively with primary care, managing hospitalisation, continuing care after death, and valuing staff and ongoing learning. The economic evaluation, cost–consequence analysis and cost–benefits analysis showed that the DNS intervention is unlikely to reduce costs; however, it was highly valued by all stakeholders.
National policy recommends that older people be cared for in their usual place of care. In addition, there has been a sustained shift of chronic illness management to primary care. Notwithstanding, nearly 40% of people with dementia in England still die in acute hospitals and very few die in their own homes. 61,62 In addition, a recent UK cohort study found that > 50% of participants with severe dementia in care homes had persistent pain and distressing agitation over a 9-month follow-up period,63 that family stress increased as the dementia advanced64,65 and that large numbers of people with dementia attended emergency departments in the last year of life. 66 This evidence suggests the need for an enhanced care model as dementia progresses that targets comfort and QoL for people with dementia,43 proactive care planning, care co-ordination and carer support, to address these findings and ensure the provision of person-centred care throughout the illness from diagnosis to death. 29
These findings and the proposed intervention are timely from multiple perspectives. First, the persistent lack of specific, integrated commissioning guidance for people living with dementia as they approach EOL. 15 Nationally, examples of local good practice were limited and usually reliant on enthusiastic service providers and short-term funding, thus leading to unacceptable inequalities in care. This is surprising considering the persistent evidence of suboptimal care, compared with the care provided to people with cancer. 12,31,44,48,54,63,168–170 Caring for people with advanced dementia is especially challenging, for both families and professional carers, because of a loss of both communication skills and mental capacity, which makes needs assessment and decision-making complex. Second, updated national dementia guidance recommends that all people with dementia have a named health-care/social care co-ordinator from the point of diagnosis to the time of death. 29 Finally, and perhaps most importantly, there is a rapidly increasing service demand: dementia is now the most common cause of death in women aged > 65 years4,5,171 and the number of people with the illness is predicted to double in the next two decades. 172,173
Comparison with national and international models of care
A recent national cohort study of people with advanced dementia showed that symptom management was still suboptimal, with high levels of pain and agitation. 63 However, even in European countries where national quality improvement policies for palliative care have been introduced, there is still a need for added intervention(s) to improve EOLC for people with dementia and older people in care homes. 174 National and international studies have demonstrated care deficiencies in many of the seven components of good practice that underpin our intervention, namely timely planning discussions,175,176 co-ordinating care,177 effective working with primary care,178,179 recognising EOL and providing supportive care,63,174,175,180 and educating and supporting families in areas of conflict and decision-making. 175,180–182 Recent reviews have also highlighted the need for studies that explore (1) how to best implement ACP in practice, via an ongoing process of communication with a trusted professional, and (2) the use of more informal proactive planning processes, rather than formal written documentation. 183,184 The DNS role has considerable potential in both of these areas. In addition, this is the first primary care-based intervention to specifically target both people with dementia living in their own homes and those living in care homes; most studies have focused only on care/nursing home settings.
Strengths and key challenges/limitations
In a complex and highly sensitive area of care for which there is very limited research to date, we have undertaken and successfully completed a number of innovative research ‘firsts’. These are as follows:
-
the development of an evidence-based primary care intervention, addressing key areas of need identified via new empirical research, and aimed at improving the quality of EOLC in dementia
-
a successful pilot trial achieving predicted recruitment rates
-
the completion of novel health economic evaluations, for example the first economic model for a non-pharmacological intervention that attempts to model disease progression from diagnosis to EOL in detail and that incorporates a contingent valuation study. 146,154
Key methodological limitations have already been outlined and discussed in the individual WS sections. Ultimately, the biggest challenge to the successful delivery and completion of this programme was the translation of the theoretically co-developed intervention to real-world practice in a constantly changing policy and service organisational landscape at both national and local levels. Early in the programme, a well-established, but non-evidence-based, palliative care intervention, the LCP, was removed from practice as a consequence of a national investigation. 75 This led to confusion and greater variation in the definition of usual care. The introduction of new commissioning structures, especially in primary and community care, with a considerable and continuous period of change and reorganisation, led to difficulty identifying and recruiting participants (WSs 2 and 6) and delays in securing governance approvals. However, the most significant change that we could not have foreseen was an unplanned shift in the provision of post-diagnostic dementia services to primary care. Local memory clinics moved to a ‘one-stop shop’ diagnostic service, with loss of any specialist post-diagnostic follow-up for the majority of newly diagnosed patients. If such a shift is happening on a national basis, there is considerable potential for the DNS role to be available throughout the entire post-diagnostic dementia care pathway and/or fulfil the recent NICE-recommended care co-ordinator role. 29
A further major limitation, especially for a future trial, is the lack of a valid and relevant primary outcome measure to evaluate the effectiveness of such complex interventions to improve care at EOL in dementia, which targets both patient- and system-level outcomes. Two of the potential future primary outcome measures performed well [Symptom Management at the End of Life in Dementia (SM-EOLD) and CAD-EOLD]; however, the SWC-EOLD measure, which was the proposed primary outcome measure for a future trial, was criticised by participants. A 2018 systematic review177 confirmed the need for further research in this area. It evaluated the applicability and psychometric properties of 67 tools to measure (1) quality of care at the EOL and (2) quality of dying and death. No single tool was found to be adequate across all the properties assessed. However, for quality of care, two measures, the Care of the Dying Evaluation (CODE)185 and the SWC-EOLD,138 performed very well psychometrically, and, for quality of dying, two measures, Quality of Dying and Death186 and Staff Perception of their patient’s End of Life Experience (SPELE),187 performed moderately well. Despite the SWC-EOLD performing well in their systematic review, the authors177 concluded that it required further testing in different settings as its use to date had been limited to research studies in care home settings. The review noted that some of the newer and promising outcome measures, such as CODE and SPELE, included a comprehensive range of assessment criteria, for example environment, symptom management, communication and decision-making. 177 It may be that, for dementia care in general, new measures need to be developed that better reflect outcomes that are important to people with dementia and their families, in terms of evaluating the success of new interventions188 or more accurately reflecting the symptom burden of dying with, or from, dementia, by incorporating both the symptoms of advanced dementia and general EOL symptoms and outcomes. 189,190 In addition, emerging research on effective quality indicators that measure practice performance and/or changes in the processes, outcomes and structure of community care systems may be more relevant. 191,192
Future research recommendations and implications for practice
Based on these key findings, we do not plan to progress to a full randomised trial of the SEED intervention in its current form. In view of the introduction of updated NICE dementia guidance, and a steady and unplanned shift of post-diagnostic dementia care to primary care, the priorities for future research are to:
-
Determine the feasibility of providing the SEED intervention throughout the illness trajectory, that is to all people with dementia from point of diagnosis to death, and if, and how, it would need to be adapted.
-
Identify appropriate, and/or develop new, outcomes to evaluate the effectiveness of such a complex intervention in real-world settings, including (1) patient- and carer-relevant outcome measures and (2) quality indicators to assess/measure quality of care. When possible, the latter should focus on processes, outcomes and structure of care.
In addition, we also think that there is a need for further health economics-related research to:
-
Refine estimates for the cost of the SEED intervention and of its consequences based on actual data from its implementation. Ideally, as its relative impact is not known, these data should come from a study with a strong study design.
-
Cross-validate estimates of WTP from the public with those obtained from a sample of patients and carers to understand whether or not views and preferences for care differ substantially between these different groups.
-
Revise WTP estimates based on the data obtained from any future rigorous evaluation (WTP for an intervention will depend on what respondents understand that intervention provides, and this should come from a more rigorous prospective evaluation).
Notwithstanding, in the absence of the proposed future research above, it would be worth exploring whether or not, from a commissioning and service provider perspective, specialist micro- and macro-simulation economic modelling techniques, as used in the Modelling Outcome and cost impacts of interventions for DEMentia (MODEM) programme,193 could help inform translation of the SEED intervention into an efficient model for future practice. MODEM is using a suite of techniques to model the costs and outcomes of care from the point of diagnosis and how these can be influenced by particular interventions. It has already produced an online dementia guidance toolkit and is developing a legacy model for commissioners to use to inform service provision at a local level. 190 Such an approach may facilitate how the SEED intervention may be more efficiently implemented while containing costs but improving quality.
Acknowledgements
Thank you to all of the professional support staff who have contributed to the smooth running of the programme.
We are extremely grateful to the sustained efforts of the PPAB, whose enthusiasm and insightful contribution to this programme was unwavering through the entire 5 years: Frank Arrojo, Lynne Chambers, Monica Cheeseman, Angela Clayton-Turner, Elspeth Gould, Deidre Harding, Gillian Harrison, Uhla Htay, Elizabeth Hughes, Jo Johnston, Lynne Ramsay, Geoff Redman, Judith Webster and Barbara Woodward-Carlton.
The ESC provided invaluable advice and constructive criticism; sincere thanks go to the ESC chairperson Professor Murna Downs and the ESC members: Dr Clare Abley, Professor Amanda Farrin, Dr Christina Faull, Dr Katherine Froggatt, Ms Elspeth Gould, Professor Rowan Harwood, Professor Claire Hulme, Miss Rachel Hutchings, Dr Alice Jordan, Dr Elizabeth Kendrick and Dr Jill Rasmussen.
Other contributors
-
Dr Nicole Baur (Research Associate): data collection and analysis for WS6.
-
Dr Sonya Carnell (Senior Trials Manager).
-
Lucy Devapal (Research Associate): data collection and analysis for WS2.
-
Dr Sean Gill (Clinical Trials Database Manager).
-
Iona Hughes (Clinical Trials Database Manager).
-
Mrs Angela Mattison (Senior Research Administrator): programme management.
-
Claire O’Toole (DNS).
-
Jonathan Prichard (Clinical Trials Database Manager).
-
Pam Ransom (Palliative Care Clinical Lead): WS3 – co-design workshops, supervised DNSs.
-
Dr Laura Ternent (Senior Lecturer, Health Economics) assisted in the design of WS5.2.
-
Jared Thornton (Senior Trials Manager).
-
Jenn Walker (Senior Trials Manager).
-
Julie Young (DNS).
Contributions of authors
Louise Robinson (https://orcid.org/0000-0003-0209-2503) (Professor of Primary Care and Ageing) was the chief investigator and the co-lead for WSs 3, 4 and 6.
Marie Poole (https://orcid.org/0000-0001-8379-7462) (Research Associate, Ageing) collected and analysed qualitative data for WSs 1.3, 2 and 4; led on the process evaluation (WS4); led on the development of the MOOC; contributed to WSs 3 and 5; co-authored publications for WSs 1.3, 2, 3 and 4; and wrote content for the WS4 section of the final report.
Emma McLellan (https://orcid.org/0000-0003-1310-1436) (Research Assistant, Ageing) was a researcher for WSs 2, 3, 4 and 6; was responsible for data collection and analysis, and review and curation of resources in WS3; and contributed to WS papers.
Richard Lee (https://orcid.org/0000-0003-3854-601X) (Senior Lecturer, Public Health) was a researcher for WSs 2 and 6, and was responsible for data collection and analysis, and writing of WS papers.
Sarah Amador (https://orcid.org/0000-0003-4196-6410) (Research Associate, Systematic Reviewer) was a researcher for WS1: she conducted the survey and review of quality indicators for dementia palliative care.
Nawaraj Bhattarai (https://orcid.org/0000-0002-1894-2499) (Research Assistant, Health Economics) was involved in WS5.2: he designed, conducted and analysed data, and prepared text for the final report.
Andrew Bryant (https://orcid.org/0000-0003-4351-8865) (Research Associate, Statistics) carried out statistical analyses for WS4.
Dorothy Coe (https://orcid.org/0000-0002-9982-1567) (Research Associate, Ageing) was a researcher for WS4 and contributed to the design, collection and analysis of WS4 outcome data.
Anne Corbett (https://orcid.org/0000-0003-2015-0316) (Senior Lecturer in Dementia Research) was a co-lead for PPI for the first 2 years of the programme.
Catherine Exley (https://orcid.org/0000-0002-3570-7503) (Dean of Applied Health Research and Professor of Qualitative Health Research) was a co-lead for WS2, and was responsible for the qualitative analysis and writing for WS2 papers.
Claire Goodman (https://orcid.org/0000-0002-8938-4893) (Professor of Health Care Research) was a co-lead for WSs 1 and 6; she was responsible for the analysis of WS1 systematic reviews and critical review, and contributed writing for WS1 papers. For WS6, she was responsible for the critical interpretation of data.
Zoe Gotts (https://orcid.org/0000-0003-0040-1720) (Research Associate) was responsible for WS6 data collection and analysis and was lead author of the WS6 paper.
Karen Harrison-Dening (https://orcid.org/0000-0001-7635-644X) (Head of Research and Publications, Dementia UK) provided nursing expertise throughout the programme and expert advice on recruitment for WSs 2 and 4; was responsible for co-development of the intervention (WS3); and provided expert advice on operationalisation in WS4.
Sarah Hill (https://orcid.org/0000-0002-5408-2473) (Research Assistant, Health Economics) designed WS1.3, was responsible for data collection and analysis, was the lead author for the WS1.3 paper and provided critical comments on the WS1 section of the final report.
Denise Howel (https://orcid.org/0000-0002-0033-548X) (Senior Lecturer, Statistics) was the senior statistician and supervised the WS4 data analysis.
Susan Hrisos (https://orcid.org/0000-0003-2877-692X) (Senior Research Associate, Ageing) contributed to the analysis of qualitative data for WS4; led on WS6.2 and the dissemination activities for WS6; and contributed to the writing of WSs 2, 3 and 6 sections of the final report.
Julian Hughes (https://orcid.org/0000-0002-9863-0478) (Professor of Old Age Psychiatry) was responsible for the study design for WS2 and provided ethics expertise.
Ashleigh Kernohan (https://orcid.org/0000-0002-5514-3186) (Research Associate, Health Economics) helped design and conduct WS5.2, and provided critical comment to final WS5.2 narrative.
Alastair Macdonald (https://orcid.org/0000-0001-9282-6229) (Senior Researcher, Design) was a WS3 co-lead, was the lead for co-design and co-production work and intervention development, was the lead author for the WS3 paper and was the lead on development of potential resources for WS4.
Helen Mason (https://orcid.org/0000-0002-9303-2794) (Senior Lecturer, Health Economics) was the WS5 lead.
Christopher Massey (https://orcid.org/0000-0002-2396-8909) (Consultant in Old Age Psychiatry) was responsible for co-designing the WS3 workshops, was a site lead for WS4 and was responsible for the mentoring and training of the DNSs.
Sandra Neves (https://orcid.org/0000-0002-1679-6002) (Research Associate, Design) was the researcher for WS3 for co-design and co-production work and intervention development.
Paul Paes (https://orcid.org/0000-0003-3544-1976) (Palliative Medicine Consultant) was a site lead for WS4, and was responsible for the mentoring and training of the DNSs.
Katherine Rennie (https://orcid.org/0000-0003-1703-3768) (Trial Manager, Clinical Trials) was the trial manager, with lead responsibility for WS4 conduct and governance.
Stephen Rice (https://orcid.org/0000-0002-6767-0813) (Senior Research Associate, Health Economics) designed, conducted and wrote up WS5.1, including the text for final report.
Tomos Robinson (https://orcid.org/0000-0001-8695-9738) (Research Associate, Health Economics) was responsible for WS5 health economics data analysis and writing.
Elizabeth Sampson (https://orcid.org/0000-0001-8929-7362) (Reader, Old Age Psychiatry) provided WS1 leadership, and supervision of systematic reviews and WS1 survey; she was also responsible for data analysis and was a lead for WS1 papers. She also provided expert methodological advice for WS4 regarding the conduct and outcome measures.
Susan Tucker (https://orcid.org/0000-0001-9221-306X) (PPI) was a co-lead PPI member; she facilitated PPI recruitment, training and mentoring.
Dimitrios Tzelis (https://orcid.org/0000-0002-6524-9048) (Research Assistant, Health Economics) designed, conducted and wrote up WS5.1, including text for the final report.
Luke Vale (https://orcid.org/0000-0001-8574-8429) (Health Foundation Chairperson in Health Economics) was lead for WSs 1.3 and 5, supervised the health economics data collection and analysis, and was lead for the WS5 text for the final report.
Claire Bamford (https://orcid.org/0000-0003-2885-801X) (Senior Research Associate, Gerontology) was a WS2 co-lead; was a senior researcher for WSs 3 and 4; was responsible for the design, conduct and analysis of WSs 2 and 4 data; wrote WS papers; and drafted the WS2 and WS4 sections of the final report.
All authors provided critical review and final approval of the report.
Publications
Workstream 1
Amador S, Goodman C, Robinson L, Sampson EL. UK end-of-life care services in dementia, initiatives and sustainability: results of a national online survey. BMJ Support Palliat Care 2016;8:424–7.
Hill SR, Mason H, Poole M, Vale L, Robinson L. What is important at the end of life for people with dementia? The views of people with dementia and their carers. Int J Geriatr Psychiatry 2017;32:1037–45.
Amador S, Sampson EL, Goodman C, Robinson L. A systematic review and critical appraisal of quality indicators to assess optimal palliative care for older people with dementia. Palliat Med 2019;33:415–29.
Workstream 2
Lee RP, Bamford C, Exley C, Robinson L. Expert views on the factors enabling good end of life care for people with dementia a qualitative study. BMC Palliat Care 2015;14:1–10.
Lee RP, Bamford C, Poole M, McLellan E, Exley C, Robinson L. End of life care for people with dementia: the views of service managers and frontline staff on key requirements for good practice. PLOS ONE 2017;12:1–19.
Bamford C, Lee RP, McLellan E, Poole M, Harrison-Dening K, Robinson L, et al. What enables good end of life care for people with dementia? A multi-method qualitative study with key stakeholders. BMC Geriatr 2018;18:302.
Poole M, Bamford C, McLellan E, Lee RP, Exley C, Hughes J, et al. End-of-life care: a qualitative study comparing the views of people with dementia and family carers. Palliat Med 2018;32:631–42.
Workstream 3
Macdonald A, Neves S, McLellan E, Poole M, Harrison-Dening K, Tucker S, et al. End of life care: resources to strengthen support. J Dement Care 2018;26:28–31.
Workstream 5
Bhattarai N, Mason H, Kernohan A, Poole M, Bamford C, Robinson L, Vale L. The value of dementia care towards the end of life – a contingent valuation study. Int J Geriatr Psychiatry 2020;35:489–97.
Workstream 6
Gotts ZM, Baur N, McLellan E, Goodman C, Robinson L, Lee RP. Commissioning care for people with dementia at the end of life: a mixed methods study. BMJ Open 2016;6:e013554.
Massive open online course
Poole M, Yemm H, Young J, Davis M, Robinson L. Living well as dementia progresses: a MOOC for all. J Dement Care 2019;27:20–3.
Poole M, Davis N, Robinson L. Massive Open Online Courses: enhancing caregiver education and support about dementia care towards and at end of life. Age Ageing 2020;49:171–4.
Other outputs
Conference presentations
Bamford C, Lee R, Exley C, Robinson L. Expert Views on the Factors Enabling Good End of Life Care for People with Dementia. International Psychogeriatric Association European Regional Meeting, Brussels, 3–5 December 2014.
Poole M, Bamford C, Lee R, McLellan E, Exley C, Robinson L. Good End of Life Care for People with Dementia, the Perspectives of Managers and Frontline Staff from Services in England’. Part of symposium Dementia and Dying, Perspectives on Good End of Life Care. British Society of Gerontology 44th annual conference, Newcastle, 3 July 2015.
Lee R, Goodman C, McLellan E, Robinson L. The Commissioning Process for End of Life Care for People with Dementia. Part of symposium Dementia and Dying, Perspectives on Good End of Life Care. British Society of Gerontology 44th annual conference, Newcastle, July 2015.
Amador S, Goodman C, Sampson L, Robinson L. Assessing Quality in End-of-Life Care in Dementia. Part of symposium Dementia and Dying, Perspectives on Good End of Life Care. British Society of Gerontology 44th annual conference, Newcastle, July 2015.
Goodman C, Amador S, Froggatt, K, Mayrhofer A, Mathie E. Managing Uncertainty for People Living and Dying with Dementia in Care Homes. British Society of Gerontology 44th annual conference, Newcastle, July 2015.
Hill S, Mason H, Poole M, Vale L. Conducting Q-sorts with Participants with Dementia: A Case Study from the SEED Project. 31st Annual Conference of the International Society for the Scientific Study of Subjectivity, Ancona, Italy, 16 September 2015.
Macdonald AS, Robinson L. Design Priorities for People with Dementia. Innovation in Medicine and Healthcare 2015 Conference, Kyoto, Japan, 11 September 2015.
Robinson L. Supporting Excellence in End of Life Care – SEED – How can Family Doctors Support End of Life Care for People with Dementia and their Families? 21st World Organization of Family Doctors (WONCA) Europe Conference, Copenhagen, 15–18 June 2016.
Robinson L. Care Commissioning for End of Life Care and Dementia: How Evidence Based is Current Practice. Society of Academic Primary Care conference, Dublin, 8 July 2016.
Bamford C, Poole M, Lee R, McLellan E, Exley C, Robinson L. Improving End of Life Care in Dementia: Key Areas for Improvement. Part of symposium, chaired by Jenny van der Steen, End of Life Care in Dementia: Developing and Testing Interventions to Improving Quality of Care. The 21st International Association of Gerontology and Geriatrics (IAGG) World Congress of Gerontology and Geriatrics, hosted by the Gerontological Society of America, San Francisco,CA, 23–7 July 2017.
Macdonald AS, Neves S. Co-developing End of Life Resources: An Equal and Reciprocal Relationship. The 27th Alzheimer Europe Conference, Berlin, 2–4 October 2017.
Sampson L, Bamford C, Coe D, McLellan E, Poole M, Robinson L. Supporting Specialists to Deliver Excellent End of Life Care in Dementia: The SEED Programme’. Alzheimer’s Society conference, London, 23 May 2018.
Bamford C, Coe D, McLellan E, Poole M, Robinson L. Improving End of Life Care Through Dementia Nurse Specialists in Primary Care: Findings from a Feasibility Study. British Geriatrics Society spring meeting, Nottingham, 11–13 April 2018.
Bamford C, Coe D, McLellan E, Poole M, Robinson L. Supporting Specialists to Deliver Excellent End of Life Care in Dementia: The SEED Programme. Symposium at the 24th Nordic Congress of Gerontology, Oslo, 2–4 May 2018.
Robinson L. What Enables Good End of Life Care for People with Dementia? A Multi-method Study with Key Stakeholders. The 47th annual scientific meeting of the Society of Academic Primary Care, London, 10–12 July 2018.
Robinson L. Supporting Professionals to Deliver Excellent End of Life Care in Dementia: The SEED Programme. The 28th Alzheimer Europe Conference, Barcelona, 29–31 October 2018.
Conference posters
Amador S, Goodman C, Robinson L, Sampson L. Development of a Conceptual Framework to Assess Quality in End of Life Care in Dementia: Contextual, Structural, Process and Outcome Variables. Congress of the European Association for Palliative Care, Copenhagen, 8–10 May 2015.
Robinson L, Bamford C, McLellan E, Lee R, Poole M, Exley C. Developing an Evidence, and Experience, Based Intervention for Professionals. Alzheimer’s Association International Conference, London, 17 July 2017.
Bhattarai N, Mason H, Vale L. The Value of Dementia Care Towards the End of Life – A Contingent Valuation Study. European Health Economics Association conference, Maastricht, 11–14 July 2018.
Other presentations
Lee R. End of Life Care for People with Dementia: Practices, Policies and Problems. Sheffield University, Risk, Policy and the Law ‘Health Inequalities’ Seminar Series, Sheffield, December 2014.
Poole M, McLellan E, Bamford C, Lee R, Exley C, Robinson L. Supporting Excellence in End of Life Care in Dementia (SEED) – What is Important to People with Dementia and Carers? Old Age Psychiatry Faculty’s annual conference, Bristol, March 2017.
Macdonald AS, Neves S. Co-designing Tools for End of Life Care for People with Dementia. University of Glasgow’s End-of-life Studies End of Life Research, Glasgow, April 2017.
Macdonald AS, Neves S. Co-designing a new resource to support end-of-life care for people with dementia. Glasgow School of Art Health and Wellbeing event, Glasgow, October 2017.
Bamford C, on behalf of the SEED research team. Supporting Excellence in End of Life Care in Dementia (SEED). Dementia Study Day, Nottingham, December 2017.
Bamford C. Supporting Excellence in End of Life Care in Dementia (SEED): Developing an evidence-based intervention. Northern Region Dementia Clinical Networks event, Newcastle, October 2017.
Poole M. Dementia Nurse Specialists: A New Primary Care Resource for End of Life Care. North East Palliative Care event, Newcastle UK, March 2018.
Bamford C. Improving End of Life Care in Dementia. Admiral Nurse Forum, Nottingham, September 2018.
Book chapters
Robinson L, Tang EYH. Caring for People with Dementia, Towards, and at, the End of Life. In Chew-Graham C, Ray M, editors. Mental Health and Older People: A Guide for Primary Care Practitioners. Cham: Springer; 2016. pp. 319–27.
Robinson L. Caring for People with Dementia Towards and at the End of Life. In Waldemar G, Burns A, editors. Alzheimer’s Disease. 2nd edition. Oxford: Oxford University Press; 2016. pp. 85–92.
Macdonald AS, Robinson L. Care at the End of Life: Design Priorities for People with Dementia. In Chen YW, Torro C, Tanaka S, Howlett RJ, Jain LC, editors. Innovation in Medicine and Healthcare 2015. 2015;45:519–526. Cham: Springer International Publishing; 2015.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Alzheimer’s Disease International . World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends 2015.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Second Edition – Overview. London: Alzheimer’s Society; 2014.
- Alzheimer’s Disease International . World Alzheimer Report 2010. The Global Economic Impact of Dementia 2010.
- House of Commons All-Party Parliamentary Group on Dementia . The £20 Billion Question – An Inquiry into Improving Lives Through Cost-Effective Dementia Services 2011.
- World Health Organization . Palliative Care for Older People: Better Practices 2011. www.euro.who.int/__data/assets/pdf_file/0017/143153/e95052.pdf (accessed 7 November 2016).
- Goodman C, Froggatt K, Amador S, Mathie E, Mayrhofer A. End of life care interventions for people with dementia in care homes: addressing uncertainty within a framework for service delivery and evaluation. BMC Palliat Care 2015;14. https://doi.org/10.1186/s12904-015-0040-0.
- Collerton J, Davies K, Jagger C, Kingston A, Bond J, Eccles MP, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ 2009;339. https://doi.org/10.1136/bmj.b4904.
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. Medical Research Council Cognitive Function and Ageing Collaboration . A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405-12. https://doi.org/10.1016/S0140-6736(13)61570-6.
- Matthews FE, Stephan BC, Robinson L, Jagger C, Barnes LE, Arthur A, et al. Cognitive Function and Ageing Studies (CFAS) Collaboration . A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun 2016;7. https://doi.org/10.1038/ncomms11398.
- Amador S, Goodman C, Mathie E, Nicholson C. Evaluation of an organisational intervention to promote integrated working between health services and care homes in the delivery of end-of-life care for people with dementia: understanding the change process using a social identity approach. Int J Integr Care 2016;16. https://doi.org/10.5334/ijic.2426.
- Bennett HQ, Norton S, Bunn F, Robinson L, Rait G, Goodman C, et al. The impact of dementia on service use by individuals with a comorbid health condition: a comparison of two cross-sectional analyses conducted approximately 10 years apart. BMC Med 2018;16. https://doi.org/10.1186/s12916-018-1105-8.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734. https://doi.org/10.1016/S0140-6736(17)31363-6.
- National Institute for Health and Care Excellence . Care of Dying Adults in the Last Days of Life 2017. www.nice.org.uk/guidance/qs144 (accessed 27 April 2020).
- National Institute for Health and Care Excellence . End of Life Care for Adults 2011. www.nice.org.uk/guidance/qs13 (accessed 27 April 2020).
- National Institute for Health and Care Excellence . Care of Dying Adults in the Last Days of Life 2015. www.nice.org.uk/guidance/ng31.
- Mathie E, Goodman C, Crang C, Froggatt K, Iliffe S, Manthorpe J, et al. An uncertain future: the unchanging views of care home residents about living and dying. Palliat Med 2012;26:734-43. https://doi.org/10.1177/0269216311412233.
- Goodwin B, Walters H. In solitary confinement: planning end of life well being with people with advanced dementia; their family and professional carers. Mortality 2009;14:265-85. https://doi.org/10.1080/13576270903056840.
- Hughes JC, Lloyd-Williams M, Sacks GA, Hughes JC, Lloyd-Williams M, Sachs C. Supportive Care for the Person with Dementia. Oxford: Oxford University Press; 2009.
- Davies N, Maio L, Rait G, Iliffe S. Quality end-of-life care for dementia: what have family carers told us so far? A narrative synthesis. Palliat Med 2014;28:919-30. https://doi.org/10.1177/0269216314526766.
- van der Steen JT. Dying with dementia: what we know after more than a decade of research. J Alzheimers Dis 2010;22:37-55. https://doi.org/10.3233/JAD-2010-100744.
- van der Steen JT, Sternberg S, Volicer L. Palliative care in dementia 1986–2016: progress and remaining challenges. J Am Med Dir Assoc 2017;18:190-1. https://doi.org/10.1016/j.jamda.2016.11.009.
- Albinsson L, Strang P. Differences in supporting families of dementia patients and cancer patients: a palliative perspective. Palliat Med 2003;17:359-67. https://doi.org/10.1191/0269216303pm669oa.
- Thune-Boyle I, Sampson EL, Jones L, King M, Lee DR, Blanchard MR. Challenges to improving end of life care of people with advanced dementia in the UK. Dementia 2010;9:259-84. https://doi.org/10.1177/1471301209354026.
- Houttekier D, Cohen J, Bilsen J, Addington-Hall J, Onwuteaka-Philipsen BD, Deliens L. Place of death of older persons with dementia. A study in five European countries. J Am Geriatr Soc 2010;58:751-6. https://doi.org/10.1111/j.1532-5415.2010.02771.x.
- Ho D, Harris S, Verne J. Deaths from Alzheimer’s Disease, Dementia and Senility in England. London: National End of Life Care Intelligence Network; 2010.
- Public Health England . Dying With Dementia. National Dementia Intelligence Network and National End of Life Care Intelligence Network Briefing 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/611463/Dying_with_dementia_briefing.pdf (accessed 2 May 2018).
- McCarthy M, Addington-Hall J, Altmann D. The experience of dying with dementia: a retrospective study. Int J Geriatr Psychiatry 1997;12:404-9. https://doi.org/10.1002/(SICI)1099-1166(199703)12:3<404::AID-GPS529>3.0.CO;2-2.
- World Health Organization . WHO Definition of Palliative Care 1998. www.who.int/cancer/palliative/definition/en/ (accessed 27 April 2020).
- National Institute for Health and Care Excellence . Dementia – Assessment, Management and Support for People Living With Dementia and Their Carers 2018. www.nice.org.uk/guidance/ng97 (accessed 2 May 2018).
- Sachs GA, Shega JW, Cox-Hayley D. Barriers to excellent end-of-life care for patients with dementia. J Gen Intern Med 2004;19:1057-63. https://doi.org/10.1111/j.1525-1497.2004.30329.x.
- Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med 2004;164:321-6. https://doi.org/10.1001/archinte.164.3.321.
- National Institute for Health and Care Excellence . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care 2006. www.nice.org.uk/Guidance/CG42 (accessed 2 May 2018).
- van der Steen JT, Radbruch L, Hertogh CM, de Boer ME, Hughes JC, Larkin P, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med 2014;28:197-209. https://doi.org/10.1177/0269216313493685.
- Scott S, Pace V. The first 50 patients: a brief report on the initial findings from the Palliative Care in Dementia Project. Dementia 2009;8:435-41. https://doi.org/10.1177/14713012090080030705.
- Department of Health and Social Care . End of Life Care Strategy: Promoting High Quality Care for Adults at the End of Their Life 2008.
- Department of Health and Social Care . End of Life Care Strategy: Quality Markers and Measures for End of Life Care 2009.
- Ellershaw JE, Wilkinson S. Care of the Dying. A Pathway to Excellence. Oxford: Oxford University Press; 2003.
- Thomas K. The GSF prognostic indicator guidance. End of Life Care 2010;4:62-4.
- Hughes JC, Robinson L, Volicer L. Specialist palliative care in dementia. BMJ 2005;330:57-8. https://doi.org/10.1136/bmj.330.7482.57.
- Sampson EL, Burns A, Richards M. Improving end-of-life care for people with dementia. Br J Psychiatry 2011;199:357-9. https://doi.org/10.1192/bjp.bp.111.097030.
- Xie J, Brayne C, Matthews FE. Medical Research Council Cognitive Function and Ageing Study collaborators . Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 2008;336:258-62. https://doi.org/10.1136/bmj.39433.616678.25.
- Rait G, Walters K, Bottomley C, Petersen I, Iliffe S, Nazareth I. Survival of people with clinical diagnosis of dementia in primary care: cohort study. BMJ 2010;341. https://doi.org/10.1136/bmj.c3584.
- Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med 2009;361:1529-38. https://doi.org/10.1056/NEJMoa0902234.
- Harrison-Dening K, Jones L, Sampson EL. Advance care planning for people with dementia. A review. Int Psychogeriatr 2011;23:1535-51. https://doi.org/10.1017/S1041610211001608.
- Dixon J, Karagiannidou M, Knapp M. The effectiveness of advance care planning in improving end-of-life outcomes for people with dementia and their carers: a systematic review and critical discussion. J Pain Symptom Manage 2018;55:132-50.e1. https://doi.org/10.1016/j.jpainsymman.2017.04.009.
- Dixon J, King D, Matosevic T, Clark M, Knapp M. Equity in the Provision of Palliative Care in the UK: Review of Evidence (PSSRU Discussion Paper 2894). London: London School of Economics; 2015.
- Lawrence V, Samsi K, Murray J, Harari D, Banerjee S. Dying well with dementia: qualitative examination of end-of-life care. Br J Psychiatry 2011;199:417-22. https://doi.org/10.1192/bjp.bp.111.093989.
- Sampson EL, Jones L, Thuné-Boyle IC, Kukkastenvehmas R, King M, Leurent B, et al. Palliative assessment and advance care planning in severe dementia: an exploratory randomized controlled trial of a complex intervention. Palliat Med 2011;25:197-209. https://doi.org/10.1177/0269216310391691.
- Dickinson C, Bamford C, Exley C, Emmett C, Hughes J, Robinson L. Planning for tomorrow whilst living for today: the views of people with dementia and their families on advance care planning. Int Psychogeriatr 2013;25:2011-21. https://doi.org/10.1017/S1041610213001531.
- Joint Commissioning Panel for Mental Health . Guidance for Commissioners of Dementia Services: Two: Practical Mental Health Commissioning 2012. www.jcpmh.info/wp-content/uploads/jcpmh-dementia-guide.pdf (accessed 27 April 2020).
- National Institute for Health and Care Excellence (NICE) . Improving Supportive and Palliative Care for Adults With Cancer 2004.
- Sleeman KE, Leniz J, Higginson IJ, Bristowe K. Is end-of-life care a priority for policymakers? Qualitative documentary analysis of health care strategies. Palliat Med 2018;32:1474-86. https://doi.org/10.1177/0269216318786333.
- Department of Health . Dementia Commissioning Pack 2011. https://webarchive.nationalarchives.gov.uk/20130104165614/https://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/Browsable/DH_127381 (accessed 9 June 2020).
- Sampson EL, Gould V, Lee D, Blanchard MR. Differences in care received by patients with and without dementia who died during acute hospital admission: a retrospective case note study. Age Ageing 2006;35:187-9. https://doi.org/10.1093/ageing/afj025.
- Robinson L, Hughes JC, Daley S, Keady J, Ballard C, Volicer L. End-of-life care and dementia. Rev Clin Gerontol 2005;15:135-48. https://doi.org/10.1017/S0959259806001833.
- Goodman C, Evans C, Wilcock J, Froggatt K, Drennan V, Sampson E, et al. End of life care for community dwelling older people with dementia: an integrated review. Int J Geriatr Psychiatry 2010;25:329-37. https://doi.org/10.1002/gps.2343.
- Department of Health and Social Care . Ministerial Advisory Group on Dementia Research: Headline Report 2011.
- James Lind Alliance Priority Setting Partnerships . Palliative and End of Life Care n.d. www.jla.nihr.ac.uk/priority-setting-partnerships/palliative-and-end-of-life-care/ (accessed 25 April 2019).
- James Lind Alliance Priority Setting Partnerships . Dementia n.d. www.jla.nihr.ac.uk/priority-setting-partnerships/dementia/ (accessed 25 April 2019).
- Munday D, Petrova M, Dale J. Exploring preferences for place of death with terminally ill patients: qualitative study of experiences of general practitioners and community nurses in England. BMJ 2009;339. https://doi.org/10.1136/bmj.b2391.
- Sleeman KE, Ho YK, Verne J, Gao W, Higginson IJ. GUIDE_Care project . Reversal of English trend towards hospital death in dementia: a population-based study of place of death and associated individual and regional factors, 2001–2010. BMC Neurol 2014;14. https://doi.org/10.1186/1471-2377-14-59.
- Dening KH, Jones L, Sampson EL. Preferences for end-of-life care: a nominal group study of people with dementia and their family carers. Palliat Med 2013;27:409-17. https://doi.org/10.1177/0269216312464094.
- Sampson EL, Candy B, Davis S, Gola AB, Harrington J, King M, et al. Living and dying with advanced dementia: a prospective cohort study of symptoms, service use and care at the end of life. Palliat Med 2018;32:668-81. https://doi.org/10.1177/0269216317726443.
- Moore KJ, Davis S, Gola A, Harrington J, Kupeli N, Vickerstaff V, et al. Experiences of end of life amongst family carers of people with advanced dementia: longitudinal cohort study with mixed methods. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0523-3.
- Chan D, Livingston G, Jones L, Sampson EL. Grief reactions in dementia carers: a systematic review. Int J Geriatr Psychiatry 2013;28:1-17. https://doi.org/10.1002/gps.3795.
- Sleeman KE, Perera G, Stewart R, Higginson IJ. Predictors of emergency department attendance by people with dementia in their last year of life: retrospective cohort study using linked clinical and administrative data. Alzheimers Dement 2018;14:20-7. https://doi.org/10.1016/j.jalz.2017.06.2267.
- Kupeli N, Leavey G, Harrington J, Lord K, King M, Nazareth I, et al. What are the barriers to care integration for those at the advanced stages of dementia living in care homes in the UK? Health care professional perspective. Dementia 2018;17:164-79. https://doi.org/10.1177/1471301216636302.
- Kupeli N, Leavey G, Moore K, Harrington J, Lord K, King M, et al. Context, mechanisms and outcomes in end of life care for people with advanced dementia. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0103-x.
- Iliffe S, Davies N, Vernooij-Dassen M, van Riet Paap J, Sommerbakk R, Mariani E, et al. Modelling the landscape of palliative care for people with dementia: a European mixed methods study. BMC Palliat Care 2013;12. https://doi.org/10.1186/1472-684X-12-30.
- van der Steen JT, Goodman C. What research we no longer need in neurodegenerative disease at the end of life: the case of research in dementia. Palliat Med 2015;29:189-92. https://doi.org/10.1177/0269216315569998.
- Hendriks SA, Smalbrugge M, Deliens L, Koopmans RTCM, Onwuteaka-Philipsen BD, Hertogh CMPM, et al. End-of-life treatment decisions in nursing home residents dying with dementia in the Netherlands. Int J Geriatr Psychiatry 2017;32:e43-e49. https://doi.org/10.1002/gps.4650.
- Vanhaecht K, De Witte K, Panella M, Sermeus W. Do pathways lead to better organised care processes?. J Eval Clin Pract 2009;15:782-8. https://doi.org/10.1111/j.1365-2753.2008.01068.x.
- Medical Research Council (MRC) . A Framework for the Development and Evaluation of Randomised Controlled Trials for Complex Interventions to Improve Health 2000.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Neuberger J, Aaronwitch D, Bonser T, Charlesworth-Smith D, Cox D, Guthrie C, et al. More Care, Less Pathway. A Review of the Liverpool Care Pathway 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/212450/Liverpool_Care_Pathway.pdf (accessed 9 June 2020).
- Amador S, Sampson EL, Goodman C, Robinson L. SEED Research Team . A systematic review and critical appraisal of quality indicators to assess optimal palliative care for older people with dementia. Palliat Med 2019;33:415-29. https://doi.org/10.1177/0269216319834227.
- Ngo J, Holroyd-Leduc JM. Systematic review of recent dementia practice guidelines. Age Ageing 2015;44:25-33. https://doi.org/10.1093/ageing/afu143.
- Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J 2010;182:E839-42. https://doi.org/10.1503/cmaj.090449.
- Palk E, Carlson L, Parker D, Abbey JA. Clinical Practice Guidelines and Care Pathways for People With Dementia Living in the Community 2008. www.eprints.qut.edu.au/17393/ (accessed 25 April 2019).
- National Collaborating Centre for Mental Health . Dementia: The NICE SCIE Guideline on Supporting People With Dementia and Their Carers in Health and Social Care. National Clinical Practice Guideline No. 42 2007.
- California Workgroup on Guidelines for Alzheimer’s Disease Management . Guideline for Alzheimer’s Disease Management 2008. www.alzheimersla.org/wp-content/uploads/2016/01/Professionals-Guideline-FullReport-CA.pdf (accessed 25 April 2019).
- Ministry of Health, Singapore . Dementia: MOH Clinical Practice Guidelines 2013. www.moh.gov.sg/docs/librariesprovider4/guidelines/dementia-10-jul-2013---booklet.pdf (accessed 25 April 2019).
- Ministry of Health Malaysia . Clinical Practice Guidelines. Management of Dementia 2009. www.acadmed.org.my/index.cfm?%26menuid=67#Mental_Health (accessed 25 April 2019).
- Amador S, Goodman C, Robinson L, Sampson EL. SEED Research Team . BMJ Support Palliat Care 2018;8:424-7. https://doi.org/10.1136/bmjspcare-2016-001138.
- Jones R, Westert GP, Schellevis FG. Morbidity, Performance and Quality in Primary Care: Dutch General Practice on Stage. Oxford: Oxford University Press; 2006.
- Donabedian A. The quality of care: how can it be assessed?. JAMA 1988;260:1743-8. https://doi.org/10.1001/jama.1988.03410120089033.
- van Soest-Poortvliet MC, van der Steen JT, Zimmerman S, Cohen LW, Klapwijk MS, Bezemer M, et al. Psychometric properties of instruments to measure the quality of end-of-life care and dying for long-term care residents with dementia. Qual Life Res 2012;21:671-84. https://doi.org/10.1007/s11136-011-9978-4.
- Zimmerman S, Cohen L, van der Steen JT, Reed D, van Soest-Poortvliet MC, Hanson LC, et al. Measuring end-of-life care and outcomes in residential care/assisted living and nursing homes. J Pain Symptom Manage 2015;49:666-79. https://doi.org/10.1016/j.jpainsymman.2014.08.009.
- Volicer L, van der Steen JT. Adv Geriatr 2014;2014. https://doi.org/10.1155/2014/346485.
- De Roo ML, Leemans K, Claessen SJ, Cohen J, Pasman HR, Deliens L, et al. EURO IMPACT . Quality indicators for palliative care: update of a systematic review. J Pain Symptom Manage 2013;46:556-72. https://doi.org/10.1016/j.jpainsymman.2012.09.013.
- Hill SR, Mason H, Poole M, Vale L, Robinson L. SEED team . What is important at the end of life for people with dementia? The views of people with dementia and their carers. Int J Geriatr Psychiatry 2017;32:1037-45. https://doi.org/10.1002/gps.4564.
- Watts S, Stenner P. Doing Q Methodological Research: Theory, Method and Interpretation. London: SAGE Publications Ltd; 2012.
- Bamford C, Lee R, McLellan E, Poole M, Harrison-Dening K, Hughes J, et al. What enables good end of life care for people with dementia? A multi-method qualitative study with key stakeholders. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0983-0.
- Lee RP, Bamford C, Exley C, Robinson L. Expert views on the factors enabling good end of life care for people with dementia: a qualitative study. BMC Palliat Care 2015;14. https://doi.org/10.1186/s12904-015-0028-9.
- Lee RP, Bamford C, Poole M, McLellan E, Exley C, Robinson L. End of life care for people with dementia: the views of health professionals, social care service managers and frontline staff on key requirements for good practice. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0179355.
- Poole M, Bamford C, McLellan E, Lee RP, Exley C, Hughes JC, et al. End-of-life care: a qualitative study comparing the views of people with dementia and family carers. Palliat Med 2018;32:631-42. https://doi.org/10.1177/0269216317736033.
- Alzheimer’s Society . Dementia 2015: Aiming Higher to Transform Lives 2015.
- National Audit Office . Improving Dementia Services in England – An Interim Report 2010.
- Barclay S, Froggatt K, Crang C, Mathie E, Handley M, Iliffe S, et al. Living in uncertain times: trajectories to death in residential care homes. Br J Gen Pract 2014;64:e576-83. https://doi.org/10.3399/bjgp14X681397.
- Silverman D. Doing Qualitative Research: A Practical Handbook. London: SAGE Publications Ltd; 2013.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Boogaard JA, de Vet HCW, van Soest-Poortvliet MC, Anema JR, Achterberg WP, van der Steen JT. Effects of two feedback interventions on end-of-life outcomes in nursing home residents with dementia: a cluster-randomized controlled three-armed trial. Palliat Med 2018;32:693-702. https://doi.org/10.1177/0269216317750071.
- Macdonald A, Neves S, McLellan E, Poole M, Harrison-Dening K, Tucker S, et al. End of life care: resources to strengthen support. J Dement Care 2018;26:28-31.
- Cottam H, Leadbeater C. Health: Co-Creating Services 2001. www.designcouncil.org.uk/sites/default/files/asset/document/red-paper-health.pdf.
- Robert G, Macdonald AS, Sangiorgi D, Prendiville A. Designing for Service: Contemporary Issues and Novel Spaces. London: Bloomsbury Publishing plc; 2017.
- Taplin DH, Clark H. Theory of Change Basics: A Primer on Theory of Change. New York, NY: ActKnowledge; 2012.
- Kitwood T. Dementia Reconsidered: The Person Comes First. Buckingham: Open University Press; 1997.
- Brooker D. What is person-centred care in dementia?. Rev Clin Gerontol 2003;13:215-22. https://doi.org/10.1017/S095925980400108X.
- Nolan M, Davies S, Brown J. Transitions in care homes: towards relationship-centred care using the ‘senses framework’. Qual Ageing 2006;7:5-14. https://doi.org/10.1108/14717794200600015.
- Watson J. Developing the Senses Framework to support relationship-centred care for people with advanced dementia until the end of life in care homes. Dementia 2019;18:545-66. https://doi.org/10.1177/1471301216682880.
- Sudnow D. Passing On: The Social Organization of Death. Englewoods Cliff, NJ: Prentice Hall; 1967.
- Thompson DS, Fazio X, Kustra E, Patrick L, Stanley D. Scoping review of complexity theory in health services research. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1343-4.
- Plsek P. Complexity and the Adoption of Innovation in Health Care. Accelerating Quality Improvement in Health Care: Strategies to Accelerate the Diffusion of Evidence-based Innovations. Washington, DC: National Institute for Healthcare Management Foundation and National Committee for Quality in Health Care; 2003.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM, et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- Alzheimer’s Disease International . World Alzheimer Report 2011. The Benefits of Early Diagnosis and Intervention 2011.
- Alzheimer’s Disease International . World Alzheimer Report 2016. Improving Healthcare for People Living With Dementia: Coverage, Quality and Costs Now and in the Future 2016.
- Department of Health and Social Care . The NHS Improvement Plan: Putting People at the Heart of Public Services 2004.
- Moore KJ, Candy B, Davis S, Gola A, Harrington J, Kupeli N, et al. Implementing the compassion intervention, a model for integrated care for people with advanced dementia towards the end of life in nursing homes: a naturalistic feasibility study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015515.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- W.K. Kellogg Foundation . Logic Model Development Guide 2004.
- Poole M, Davis N, Robinson L. Massive open online courses: enhancing caregiver education and support about dementia care towards and at end of life. Age Ageing 2020;49:171-4. https://doi.org/10.1093/ageing/afz150.
- Poole M, Yemm H, Young J, Davis N, Robinson L. Living well as dementia progresses: a MOOC for all. J Dement Care 2019;27:20-3.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355. https://doi.org/10.1136/bmj.i5239.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345. https://doi.org/10.1136/bmj.e5661.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-111.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- Thomas K, Armstrong-Wilson J. GSF Team . Proactive Identification Guidance (PIG) National Gold Standards Framework Centre in End of Life Care 2016. www.goldstandardsframework.org.uk/ (accessed 25 April 2019).
- Department for Constitutional Affairs . Mental Capacity Act 2005: Code of Practice 2007.
- Rabin R, de Charro CF. EQ-5D: a measure of health status from the EuroQuol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry 2000;8:75-83. https://doi.org/10.1097/00019442-200002000-00010.
- Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997;48:10-6. https://doi.org/10.1212/wnl.48.5_suppl_6.10s.
- Weiner MF, Martin-Cook K, Svetlik DA, Saine K, Foster B, Fontaine CS. The quality of life in late-stage dementia (QUALID) scale. J Am Med Dir Assoc 2000;1:114-16. https://doi.org/10.1037/t00432-000.
- Bellelli G, Frisoni GB, Bianchetti A, Trabucci M. The Bedford Alzheimer Nursing Severity Scale for the severley demented: validation study. Alzheimer Dis Assoc Disord 1997;11:71-7. https://doi.org/10.1097/00002093-199706000-00003.
- Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc 2003;4:9-15. https://doi.org/10.1097/01.JAM.0000043422.31640.F7.
- Volicer L, Hurley AC, Blasi ZV. Scales for evaluation of end-of-life care in dementia. Alzheimer Dis Assoc Disord 2001;15:194-200. https://doi.org/10.1097/00002093-200110000-00005.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Royal College of Psychiatrists; 2001.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. https://doi.org/10.1097/01.mlr.0000182534.19832.83.
- Denzin NK. The Research Act: A Theoretical Introduction to Sociological Methods. New York, NY: McGraw-Hill; 1978.
- Evans SC, Harrison-Dening K, Read K. Towards the end of life: an in-depth exploration of the role of Admiral Nursing in dementia care (innovative practice). Dementia 2018;17:244-51. https://doi.org/10.1177/1471301216636485.
- National Institute for Health and Care Excellence (NICE) . The Guidelines Manual: Process and Methods 2012.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Cost Eff Resour Alloc 2013;11. https://doi.org/10.1186/1478-7547-11-6.
- Bhattarai N, Mason H, Kernohan A, Poole M, Bamford C, Robinson L, et al. The value of dementia care towards the end of life – a contingent valuation study. Int J Geriatr Psychiatry 2020;35:489-97. https://doi.org/10.1002/gps.5259.
- Pauly M, Sloan F. Valuing Health Care: Costs, Benefits and Effectiveness of Pharmaceuticals and Other Medical Technologies. Cambridge: Cambridge University Press; 1995.
- Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: SAGE Publications, Inc.; 2005.
- Pennington M, Baker R, Brouwer W, Mason H, Hansen DG, Robinson A, et al. EuroVaQ Team . Comparing WTP values of different types of QALY gain elicited from the general public. Health Econ 2015;24:280-93. https://doi.org/10.1002/hec.3018.
- Brennan A, Akehurst R. Modelling in health economic evaluation. What is its place? What is its value?. PharmacoEconomics 2000;17:445-59. https://doi.org/10.2165/00019053-200017050-00004.
- Thorsen T, Mäkelä M. Changing Professional Practice: Theory and Practice of Clinical Guidelines Implementation. Copenhagen: Danish Institute for Health Services Research and Development; 1999.
- McIntosh E, Thorsen T, Mäkelä M. Changing Professional Practice: Theory and Practice of Clinical Guidelines Implementation. Copenhagen: Danish Institute for Health Services Research and Development; 1999.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Lawrenson JG, Graham-Rowe E, Lorencatto F, Burr J, Bunce C, Francis JJ, et al. Interventions to increase attendance for diabetic retinopathy screening. Cochrane Database Syst Rev 2018;1. https://doi.org/10.1002/14651858.CD012054.pub2.
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8060.
- Gotts ZM, Baur N, McLellan E, Goodman C, Robinson L, Lee RP. Commissioning care for people with dementia at the end of life: a mixed-methods study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-013554.
- National Institute for Health and Care Excellence . Putting NICE Guidance into Practice. Resource Impact Report: Dementia: Assessment, Management and Support for People Living With Dementia and Their Carers (NG97) 2018. www.nice.org.uk/guidance/ng97/resources/resource-impact-report-pdf-4897901485 (accessed 27 April 2020).
- Davies S, Goodman C. Supporting quality improvement in care homes for older people: the contribution of nurses working in primary care. J Nurs Manag 2008;16:115-20. https://doi.org/10.1111/j.1365-2834.2007.00838.x.
- Steves CJ, Schiff R, Martin FC. Geriatricians and care homes: perspectives from geriatric medicine departments and primary care trusts. Clin Med 2009;9:528-33. https://doi.org/10.7861/clinmedicine.9-6-528.
- Gladman JRF. Provision of Medical Care in Care Homes in the UK 2010. www.nottingham.ac.uk/mcop/documents/papers/issue1-mcop-issn2044-4230.pdf (accessed 27 April 2020).
- Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs 2008;17:1144-63. https://doi.org/10.1111/j.1365-2702.2007.02220.x.
- Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA. Management of noncancer pain in community-dwelling persons with dementia. J Am Geriatr Soc 2006;54:1892-7. https://doi.org/10.1111/j.1532-5415.2006.00986.x.
- Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manage 2006;31:170-92. https://doi.org/10.1016/j.jpainsymman.2005.07.001.
- Zwakhalen SM, Hamers JP, Abu-Saad HH, Berger MP. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr 2006;6. https://doi.org/10.1186/1471-2318-6-3.
- Checkland K, McDermott I, Coleman A, Perkins N. Complexity in the new NHS: longitudinal case studies of CCGs in England. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010199.
- NHS England . Models of Care n.d. www.england.nhs.uk/ourwork/new-care-models/vanguards/about-vanguards/ (accessed 15 June 2020).
- Scherder E, Oosterman J, Swaab D, Herr K, Ooms M, Ribbe M, et al. Recent developments in pain in dementia. BMJ 2005;330:461-4. https://doi.org/10.1136/bmj.330.7489.461.
- Froggatt K, Vaughan S, Bernard C, Wild D. Advance care planning in care homes for older people: an English perspective. Palliat Med 2009;23:332-8. https://doi.org/10.1177/0269216309103802.
- Livingston G, Pitfield C, Morris J, Manela M, Lewis-Holmes E, Jacobs H. Care at the end of life for people with dementia living in a care home: a qualitative study of staff experience and attitudes. Int J Geriatr Psychiatry 2012;27:643-50. https://doi.org/10.1002/gps.2772.
- Brayne C, Gao L, Dewey M, Matthews FE. Medical Research Council Cognitive Function and Ageing Study Investigators . Dementia before death in ageing societies – the promise of prevention and the reality. PLOS Med 2006;3. https://doi.org/10.1371/journal.pmed.0030397.
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112-17. https://doi.org/10.1016/S0140-6736(05)67889-0.
- Alzheimer’s Society . Dementia UK: The Full Report 2007.
- Pivodic L, Smets T, Van den Noortgate N, Onwuteaka-Philipsen BD, Engels Y, Szczerbińska K, et al. Quality of dying and quality of end-of-life care of nursing home residents in six countries: an epidemiological study. Palliat Med 2018;32:1584-95. https://doi.org/10.1177/0269216318800610.
- Lamahewa K, Mathew R, Iliffe S, Wilcock J, Manthorpe J, Sampson EL, et al. A qualitative study exploring the difficulties influencing decision making at the end of life for people with dementia. Health Expect 2018;21:118-27. https://doi.org/10.1111/hex.12593.
- Rietjens JAC, Sudore RL, Connolly M, van Delden JJ, Drickamer MA, Droger M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol 2017;18:e543-51. https://doi.org/10.1016/S1470-2045(17)30582-X.
- Kupeli N, Candy B, Tamura-Rose G, Schofield G, Webber N, Hicks SE, et al. Tools measuring quality of death, dying, and care, completed after death: systematic review of psychometric properties. Patient 2019;12:183-97. https://doi.org/10.1007/s40271-018-0328-2.
- Greenwood N, Menzies-Gow E, Nilsson D, Aubrey D, Emery CL, Richardson A. Experiences of older people dying in nursing homes: a narrative systematic review of qualitative studies. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-021285.
- Penders YWH, Albers G, Deliens L, Miccinesi G, Vega Alonso T, Miralles M, et al. End-of-life care for people dying with dementia in general practice in Belgium, Italy and Spain: a cross-sectional, retrospective study. Geriatr Gerontol Int 2017;17:1667-76. https://doi.org/10.1111/ggi.12948.
- Tilburgs B, Vernooij-Dassen M, Koopmans R, van Gennip H, Engels Y, Perry M. Barriers and facilitators for GPs in dementia advance care planning: a systematic integrative review. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0198535.
- Davies N, Mathew R, Wilcock J, Manthorpe J, Sampson EL, Lamahewa K, et al. A co-design process developing heuristics for practitioners providing end of life care for people with dementia. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0146-z.
- Davies N, Rait G, Maio L, Iliffe S. Family caregivers’ conceptualisation of quality end-of-life care for people with dementia: a qualitative study. Palliat Med 2017;31:726-33. https://doi.org/10.1177/0269216316673552.
- Van den Block L. Advancing research on advance care planning in dementia. Palliat Med 2019;33:259-61. https://doi.org/10.1177/0269216319826411.
- Bryant J, Turon H, Waller A, Freund M, Mansfield E, Sanson-Fisher R. Effectiveness of interventions to increase participation in advance care planning for people with a diagnosis of dementia: a systematic review. Palliat Med 2019;33:262-73. https://doi.org/10.1177/0269216318801750.
- Mayland CR, Lees C, Germain A, Jack BA, Cox TF, Mason SR, et al. Caring for those who die at home: the use and validation of ‘Care Of the Dying Evaluation’ (CODE) with bereaved relatives. BMJ Support Palliat Care 2014;4:167-74. https://doi.org/10.1136/bmjspcare-2013-000596.
- Downey L, Curtis JR, Lafferty WE, Herting JR, Engelberg RA. The Quality of Dying and Death Questionnaire (QODD): empirical domains and theoretical perspectives. J Pain Symptom Manage 2010;39:9-22. https://doi.org/10.1016/j.jpainsymman.2009.05.012.
- Cornally N, Coffey A, Daly E, McGlade C, Weathers E, O’Herlihy E, et al. Measuring staff perception of end-of-life experience of older adults in long-term care. Appl Nurs Res 2016;30:245-51. https://doi.org/10.1016/j.apnr.2015.05.015.
- Tochel C, Smith M, Baldwin H, Gustavsson A, Ly A, Bexelius C, et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? A systematic review. Alzheimers Dement 2019;11:231-47. https://doi.org/10.1016/j.dadm.2018.12.003.
- Ellis-Smith C, Evans CJ, Murtagh FE, Henson LA, Firth AM, Higginson IJ, et al. BuildCARE . Development of a caregiver-reported measure to support systematic assessment of people with dementia in long-term care: the Integrated Palliative care Outcome Scale for Dementia. Palliat Med 2017;31:651-60. https://doi.org/10.1177/0269216316675096.
- De Schreye R, Houttekier D, Deliens L, Cohen J. Developing indicators of appropriate and inappropriate end-of-life care in people with Alzheimer’s disease, cancer or chronic obstructive pulmonary disease for population-level administrative databases: a RAND/UCLA appropriateness study. Palliat Med 2017;31:932-45. https://doi.org/10.1177/0269216317705099.
- Joling KJ, van Eenoo L, Vetrano DL, Smaardijk VR, Declercq A, Onder G, et al. Quality indicators for community care for older people: a systematic review. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0190298.
- Virdun C, Luckett T, Lorenz KA, Phillips J. National quality indicators and policies from 15 countries leading in adult end-of-life care: a systematic environmental scan. BMJ Support Palliat Care 2018;8:145-54. https://doi.org/10.1136/bmjspcare-2017-001432.
- MODEM Project . MODEM: Modelling Outcome and Cost Implications of Interventions for Dementia n.d. www.modem-dementia.org.uk/ (accessed 15 June 2020).
- The AGREE Collaboration . Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care 2003;12:18-23. https://doi.org/10.1136/qhc.12.1.18.
- Reisberg B, Sclan SG, Franssen E, Kluger A, Ferris S. Dementia staging in chronic care populations. Alzheimer Dis Assoc Disord 1994;8:188-205.
- Abbey J, Piller N, De Bellis A, Esterman A, Parker D, Giles L, et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs 2004;10:6-13. https://doi.org/10.12968/ijpn.2004.10.1.12013.
- World Health Organization . WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents 2018.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons . Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009;57:1331-46. https://doi.org/10.1111/j.1532-5415.2009.02376.x.
- Luckett T, Phillips J, Agar M, Virdun C, Green A, Davidson PM. Elements of effective palliative care models: a rapid review. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-136.
- Great Britain . Mental Health Act 1983 1983.
- Bate P, Robert G. Bringing User Experience to Healthcare Improvement. Abingdon: Radcliffe Publishing Ltd; 2007.
- Department of Health and Social Care . Liberating the NHS: Legislative Framework and Next Steps 2011. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_123853.pdf (accessed 25 April 2019).
- NHS . End of Life Care n.d. www.nhs.uk/conditions/end-of-life-care/ (accessed 15 June 2020).
- The Sound Doctor n.d. www.thesounddoctor.org (accessed 15 June 2020).
- Youtube . Breech Decisions Film 2016. www.youtube.com/watch?v=7wB_2o784oI (accessed 15 June 2020).
- Donaldson C, Mason H, Shackley P, Jones A. The Elgar Companion to Health Economics. Cheltenham: Edward Elgar; 2006.
- Rowe RD, Schulze WD, Breffle WS. A test for payment card biases. J Environ Econ Manage 1996;31:178-85. https://doi.org/https://doi.org/10.1006/jeem.1996.0039.
- R Core Team . R: A Language and Environment for Statistical Computing 2016. www.R-project.org/ (accessed 25 April 2019).
- Bateman IJ, Carson RT, Day B, Hanemann M, Hanleys N, Hett T, et al. Economic Valuation with Stated Preference Techniques: A Manual. Cheltenham: Edward Elgar; 2002.
- Donaldson C, Jones AM, Mapp TJ, Olson JA. Limited dependent variables in willingness to pay studies: applications in health care. Appl Econ 1998;30:667-77. https://doi.org/10.1080/000368498325651.
- König M, Wettstein A. Caring for relatives with dementia: willingness-to-pay for a reduction in caregiver’s burden. Expert Rev Pharmacoecon Outcomes Res 2002;2:535-47. https://doi.org/10.1586/14737167.2.6.535.
- O’Brien B, Gafni A. When do the ‘dollars’ make sense? Toward a conceptual framework for contingent valuation studies in health care. Med Decis Making 1996;16:288-99. https://doi.org/10.1177/0272989X9601600314.
- Klose T. The contingent valuation method in health care. Health Policy 1999;47:97-123. https://doi.org/10.1016/S0168-8510(99)00010-X.
- Kielhorn A, Graf von der Schulenburg J. The Health Economics Handbook. Chester: Adis International Ltd; 2000.
- Renz SM, Boltz MP, Wagner LM, Capezuti EA, Lawrence TE. Examining the feasibility and utility of an SBAR protocol in long-term care. Geriatr Nurs 2013;34:295-301. https://doi.org/10.1016/j.gerinurse.2013.04.010.
- Green C, Shearer J, Ritchie CW, Zajicek JP. Model-based economic evaluation in Alzheimer’s disease: a review of the methods available to model Alzheimer’s disease progression. Value Health 2011;14:621-30. https://doi.org/10.1016/j.jval.2010.12.008.
- Green C, Zhang S. Predicting the progression of Alzheimer’s disease dementia: a multidomain health policy model. Alzheimers Dement 2016;12:776-85. https://doi.org/10.1016/j.jalz.2016.01.011.
- Knapp M, Chua KC, Broadbent M, Chang CK, Fernandez JL, Milea D, et al. Predictors of care home and hospital admissions and their costs for older people with Alzheimer’s disease: findings from a large London case register. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-013591.
- Kasteridis P, Mason A, Goddard M, Jacobs R, Santos R, Rodriguez-Sanchez B, et al. Risk of care home placement following acute hospital admission: effects of a pay-for-performance scheme for dementia. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0155850.
- Purdy S, Griffin T, Salisbury C, Sharp D. Ambulatory care sensitive conditions: terminology and disease coding need to be more specific to aid policy makers and clinicians. Public Health 2009;123:169-73. https://doi.org/10.1016/j.puhe.2008.11.001.
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevelance and mortality. Br J Psychiatry 2009;195:61-6. https://doi.org/10.1192/bjp.bp.108.055335.
- Zheng LF, Finucane AM, Oxenham D, McLoughlin P, McCutheon H, Murray S. How good is UK primary care at identifying patients for generalist and specialist palliative care: a mixed methods study. Eur J Palliat 2013;20:216-22.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Department for Business, Energy and Industrial Strategy . Ethncity Facts and Figures: Average Hourly Pay 2018. www.ethnicity-facts-figures.service.gov.uk/work-pay-and-benefits/pay-and-income/average-hourly-pay/latest (accessed 25 April 2019).
- CHKS . An Economic Analysis of the Excess Costs for Acute Care for Patients With Dementia 2013.
- Department of Health and Social Care . NHS Reference Costs 2015–16 2016.
- RStudio T . RStudio: Intergrated Dvelopment for R. Studio, Inc n.d. www.rstudio.com/ (accessed 25 April 2019).
- Schlosser RW, Wendt O, Bhavnani S, Nail-Chiwetalu B. Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: the application of traditional and comprehensive Pearl Growing. A review. Int J Lang Commun Disord 2006;41:567-82. https://doi.org/10.1080/13682820600742190.
- Gonçalves-Bradley DC, Lannin NA, Clemson LM, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev 2016;1. https://doi.org/10.1002/14651858.CD000313.pub5.
- Getsios D, Caro JJ, Caro G, Ishak K. AHEAD Study Group . Assessment of Health Economics in Alzheimer’s Disease (AHEAD): galantamine treatment in Canada. Neurology 2001;57:972-8. https://doi.org/10.1212/wnl.57.6.972.
- McDonnell J, Redekop WK, van der Roer N, Goes E, Ruitenberg A, Busschbach JJ, et al. The cost of treatment of Alzheimer’s disease in The Netherlands: a regression-based simulation model. PharmacoEconomics 2001;19:379-90. https://doi.org/10.2165/00019053-200119040-00005.
- Banerjee S, Murray J, Foley B, Atkins L, Schneider J, Mann A. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry 2003;74:1315-16. https://doi.org/10.1136/jnnp.74.9.1315.
- Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16210.
- Jones RW, McCrone P, Guilhaume C. Cost effectiveness of memantine in Alzheimer’s disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Aging 2004;21:607-20. https://doi.org/10.2165/00002512-200421090-00005.
- Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, Leon J, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology 1999;52:1138-45. https://doi.org/10.1212/wnl.52.6.1138.
- Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, et al. Predicting time to nursing home care and death in individuals with Alzheimer’s disease. JAMA 1997;277:806-12. https://doi.org/10.1001/jama.1997.03540340040030.
- Getsios D, Blume S, Ishak KJ, Maclaine GD. Cost effectiveness of donepezil in the treatment of mild to moderate Alzheimer’s disease: a UK evaluation using discrete-event simulation. PharmacoEconomics 2010;28:411-27. https://doi.org/10.2165/11531870-000000000-00000.
- Guo S, Getsios D, Revankar N, Xu P, Thompson G, Bobula J, et al. Evaluating disease-modifying agents: a simulation framework for Alzheimer’s disease. PharmacoEconomics 2014;32:1129-39. https://doi.org/10.1007/s40273-014-0203-5.
- Stallard E, Kinosian B, Zbrozek AS, Yashin AI, Glick HA, Stern Y. Estimation and validation of a multiattribute model of Alzheimer disease progression. Med Decis Making 2010;30:625-38. https://doi.org/10.1177/0272989X10363479.
- Emilien G, Beyreuther K, Masters CL, Maloteaux JM. Prospects for pharmacological intervention in Alzheimer disease. Arch Neurol 2000;57:454-9. https://doi.org/10.1001/archneur.57.4.454.
- Burns A, Brayne C, Folstein MF. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. M. Folstein, S. Folstein and P. McHugh, Journal of Psychiatric Research (1975) 12, 189–198. Int J Geriatr Psychiatry 1998;13:285-94. https://doi.org/10.1002/(SICI)1099-1166(199805)13:5<285::AID-GPS753>3.0.CO;2-V.
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12:233-9. https://doi.org/10.1176/jnp.12.2.233.
- Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323-9. https://doi.org/10.1093/geronj/37.3.323.
- Cepoiu-Martin M, Tam-Tham H, Patten S, Maxwell CJ, Hogan DB. Predictors of long-term care placement in persons with dementia: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2016;31:1151-71. https://doi.org/10.1002/gps.4449.
- Wing JK, Beevor AS, Curtis RH, Park SB, Hadden S, Burns A. Health of the Nation Outcome Scales (HoNOS). Research and development. Br J Psychiatry 1998;172:11-8. https://doi.org/10.1192/bjp.172.1.11.
- Thomas K. The GSF Prognostic Indicator Guidance. The National GSF Centre’s Guidance for Clinicians to Support Earlier Recognition of Patients Nearing the End of Life. London: The Gold Standards Framework Centre in End of Life Care; 2011.
- van Riet Paap J, Mariani E, Chattat R, Koopmans R, Kerhervé H, Leppert W, et al. Identification of the palliative phase in people with dementia: a variety of opinions between healthcare professionals. BMC Palliat Care 2015;14. https://doi.org/10.1186/s12904-015-0053-8.
- General Medical Council . Treatment and Care Towards the End of Life: Good Practice in Decision Making: General Medical Council 2010. www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/treatment-and-care-towards-the-end-of-life (accessed 25 April 2019).
- National Institute for Health and Care Excellence . Dementia: Assessment, Management and Support for People Living With Dementia and Their Carers 2018. www.nice.org.uk/guidance/ng97 (accessed 22 June 2020).
- Livingston G, Lewis-Holmes E, Pitfield C, Manela M, Chan D, Constant E, et al. Improving the end-of-life for people with dementia living in a care home: an intervention study. Int Psychogeriatr 2013;25:1849-58. https://doi.org/10.1017/S1041610213001221.
- Morrison RS, Chichin E, Carter J, Burack O, Lantz M, Meier DE. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc 2005;53:290-4. https://doi.org/10.1111/j.1532-5415.2005.53116.x.
- NHS Digital . GP and GP Practice Related Data 2018. https://digital.nhs.uk/services/organisation-data-service/data-downloads/gp-and-gp-practice-related-data (accessed 25 April 2019).
- Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986;149:698-709. https://doi.org/10.1192/bjp.149.6.698.
- Sheehan B. Assessment scales in dementia. Ther Adv Neurol Disord 2012;5:349-58. https://doi.org/10.1177/1756285612455733.
- Bank of England . Inflation Calculator 2018. www.bankofengland.co.uk/monetary-policy/inflation/inflation-calculator (accessed 25 April 2019).
- Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ 2006;15:1295-310. https://doi.org/10.1002/hec.1148.
- Office for National Statistics . Income Tax Liabilities Statistics: Tax Year 2015 to 2016 to Tax Year 2018 to 2019 2018. www.gov.uk/government/statistics/income-tax-liabilities-statistics-tax-year-2015-to-2016-to-tax-year-2018-to-2019 (accessed September 2018).
- Addicott R. Challenges of commissioning and contracting for integrated care in the National Health Service (NHS) in England. Aust J Prim Health 2016;22:50-4. https://doi.org/10.1071/PY15067.
- Jones L, Candy B, Davis S, Elliott M, Gola A, Harrington J, et al. Development of a model for integrated care at the end of life in advanced dementia: a whole systems UK-wide approach. Palliat Med 2016;30:279-95. https://doi.org/10.1177/0269216315605447.
- Lancaster H, Finlay I, Downman M, Dumas J. Commissioning of specialist palliative care services in England. BMJ Support Palliat Care 2018;8:93-101. https://doi.org/10.1136/bmjspcare-2016-001119.
- McDermott I, Checkland K, Coleman A, Osipovič D, Petsoulas C, Perkins N. Engaging GPs in commissioning: realist evaluation of the early experiences of Clinical Commissioning Groups in the English NHS. J Health Serv Res Policy 2017;22:4-11. https://doi.org/10.1177/1355819616648352.
- Moran V, Checkland K, Coleman A, Spooner S, Gibson J, Sutton M. General practitioners’ views of clinically led commissioning: cross-sectional survey in England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015464.
Appendix 1 Supplementary data relating to workstream 1: evidence synthesis
Existing end-of-life care guidance and models of care in dementia
Key WS1 deliverables were to provide a summary of existing recommendations in EOLC in dementia, and of the quality of the evidence base underpinning them, and to identify resource implications of existing pathways to inform subsequent modelling. This work builds on a 2015 systematic review,77 which employed a methodology identical to that described in the SEED protocol to provide (1) a systematic review of current guidelines/pathways in dementia and (2) an assessment of the quality of guidelines/pathways using the AGREE instrument. 194
Five dementia practice guidelines that scored ≥ 4 on the AGREE-II instrument address issues related to palliative care:
-
Clinical Practice Guidelines and Care Pathways for People with Dementia Living in the Community79 (Queensland University of Technology, 2008)
-
guideline on supporting people with dementia and their carers in health and social care80 [NICE–Social Care Institute for Excellence (SCIE), 2007]
-
Guideline for Alzheimer’s Disease Management81 (California Workgroup on Guidelines for Alzheimer’s Disease Management, 2008)
-
Ministry of Health’s Dementia: MOH Clinical Practice Guidelines82 (Singapore, 2013)
-
Ministry of Health’s Clinical Practice Guidelines, Management of Dementia, 2nd edition83 (Malaysia, 2009).
To identify resource implications of existing pathways, we assessed the key components and content of guidelines/care pathways (i.e. setting, timing and content of each pathway, care model or guideline, who ‘leads’ it, staff/resource implications and how adherence and variance are documented), to begin cost estimation for WS5, in collaboration with WS5 lead Luke Vale.
Setting
Overall, guidelines recommend that people with dementia be managed, as far as possible, in the community. UK guidelines80 do, however, recognise that admission to acute or general inpatient services/psychiatric admission may sometimes be required. Only Malaysian guidelines83 recommend assessment and treatment in outpatient and inpatient, as well as community, settings.
Timing
Recommendations vary as to when palliative care for people with dementia should be introduced (Table 11). Only one set of guidelines (UK) does not make a clear recommendation as to the timing of palliative care. Both Australian79 and Singaporean82 guidelines tie the introduction of palliative care to the severity of dementia as assessed by the Functional Assessment Staging Tool (FAST),195 in addition to specified dementia-related comorbidities. Californian81 guidelines also recommend the introduction of palliative care when the person with dementia becomes eligible for hospice care (mortality predicted within 6 months). Finally, Malaysian83 guidelines suggest that palliative care begins from the time of diagnosis to death.
| Clinical area | Queensland, Australia79 | UK80 | California, USA81 | Singapore82 | Malaysia83 |
|---|---|---|---|---|---|
| Timing of palliative care | Prognosis of ≤ 6 months | Not specified | Not specified, although notes that hospice care requires a prognosis of mortality within 6 months | Provides severity indicators, which indicate that palliative approach should be considered | Palliative care begins at diagnosis and ends at death |
| Assessment of palliative stage and review |
|
Primary care teams should ensure that the palliative care needs of people with dementia are assessed and communicated |
|
||
| Palliative care approaches in dementia | Health and social care professionals should adopt a palliative approach | ||||
| Access to palliative care services | People with dementia who are dying should have the same access to palliative care services as people without dementia | Provide appropriate EOLC, including palliative care as needed | |||
| Hydration and nutrition | Artificial (tube-) feeding is not recommended | Health and social care staff should encourage people with dementia to eat and drink by mouth for as long as possible. Specialist assessment and advice concerning swallowing and feeding should be available. Nutritional support, including artificial (tube-) feeding should be considered if dysphagia is thought to be a transient phenomenon, but should not generally be used in people with severe dementia for whom dysphagia or disinclination to eat is a manifestation of disease severity | Decisions on tube-feeding should be individualised given the lack of evidence for its efficacy in advanced dementia |
|
|
| Management of fever and infection | Antibiotics for fever management | Following a clinical assessment, simple analgesics, antipyretics and mechanical means of cooling the person may suffice. Antibiotics may be considered as a palliative measure | Decisions on the use of antibiotics in advanced dementia should be individualised to the patient by weighing the risk and benefit of antibiotic treatment | The use of antibiotics for treatment of infections and pneumonia in severe and late-stage dementia should be individualised, taking into consideration the severity of dementia, comorbidity, nutritional status, mobility status and virility of the organism | |
| Pain management |
|
|
|
||
| ACP, including decisions to resuscitate |
|
|
|
ACP with regard to CPR should be encouraged, given the poor outcomes of CPR in advanced dementia |
|
| Assessment of carer needs (including support system, grief and loss) |
|
The right of carers to receive an assessment of their needs should be upheld by health and social care managers | Identify the primary caregiver and assess the adequacy of family and other health support systems, paying particular attention to the caregiver’s own mental and physical health |
Content
Guidelines are a mix of evidence- and consensus-based recommendations (note that Asian guideline recommendations are graded according to the strength of the evidence underpinning them, which may include workgroup consensus only). Guidelines outline anywhere between 5 and 11 recommendations, covering the following issues (see Table 11):
-
assessment (including staging) and review
-
palliative care approaches in dementia
-
access to palliative care services and communication within and across services
-
hydration and nutrition (including tube-feeding)
-
management of fever and infection
-
pain management
-
ACP (including decisions to resuscitate)
-
assessment of carers needs (including support system, grief and loss).
Two topics are not included in Table 11, as each appears in only a single guideline. Australian guidelines79 include two recommendations on admission to residential care (when care outside the home is needed, staff should be aware of the wishes of the person with dementia; a carer may benefit from the help of health professionals in planning for residential care). Malaysian guidelines83 cover the use of restraints (physical restraint should be used sparingly and should be individualised).
In addition, Australian79 and Singaporean82 guidelines provide context-specific contact information for local programmes, helplines and services termed ‘practice tips’ and ‘community resources’, respectively. Californian,81 Malaysian83 and Singaporean82 guidelines provide copies of some of the assessment tools (e.g. functional and nutrition assessment), which are cited in the text. Finally, Australian79 guidelines include three advanced phase pathways for care workers, allied health professionals and GPs.
Care lead/model
The model of care outlined by each set of recommendations has been identified and categorised using Luckett et al.’s199 definitions of models of palliative care.
Both Singaporean82 and Malaysian83 guidelines appear to recommend a ‘consultation model’ approach to care, in as much as neither outlines mechanisms for collaboration between the health-care professionals involved. Luckett et al. 199 define a consultation model as:
An approach to care by which specialist advice is provided on assessment and treatment of symptoms, communication about goals of care and support for complex medical decision-making, provision of practical and psychosocial support, care coordination and continuity, and bereavement services when appropriate. Advice is provided without necessarily assuming primary responsibility for care, although there is negotiation of the level of palliative care involvement.
Luckett et al. 199 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated
UK80 and Californian81 guidelines recommend a ‘case management model’, led by care managers/co-ordinators and primary care practitioners. Luckett et al. 199 define a case management model as:
. . . a collaborative process of assessment, planning, facilitation and advocacy for options and services to meet an individual’s holistic needs through communication and available resources to promote quality cost effective outcomes. The definition of case management notes the focus upon the meeting of a client’s health needs. Case management can be placed within a social model of health, within which improvement in health and well-being are achieved by directing efforts towards addressing the social and environmental determinants of health, in tandem with biological and medical factors.
Luckett et al. 199 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated
Finally, Australian79 guidelines recommend a ‘shared-care model’ approach to palliative care involving GPs, allied health professionals/care managers and care workers. Luckett et al. 199 provide one definition of shared care as:
. . . an approach to care which uses the skills and knowledge of a range of health professionals who share joint responsibility in relation to an individual’s care. This also implies monitoring and exchanging patient data and sharing skills and knowledge between disciplines.
Luckett et al. 199 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated
Staffing/resourcing
Overall, guidelines outline the type of resourcing required, rather than the quantity of the resource use that is needed, for example how much of a care worker’s time is required.
Clinical audit parameters/indicators for quality improvement
Only Singaporean82 and Malaysian83 guidelines have a set of indicators for quality improvement, although neither provides a set of indicators that is specific to the palliative care phase. Remaining guidelines either do not address clinical audit and/or quality improvement, or recommend that inspection standards be developed separately within a more broadly based implementation strategy (i.e. UK80 guidance).
Appendix 2 Full texts of publications that are not open access
Amador et al.84
Reproduced from UK end-of-life care services in dementia, initiatives and sustainability: results of a national online survey. Amador S, Goodman C, Robinson L, Sampson EL. Vol. 8, pp. 424–7, 2016, with permission from BMJ Publishing Group Ltd.
Hill et al.91
Reproduced from What is important at the end of life for people with dementia? The views of people with dementia and their carers, with permission from John Wiley & Sons, Ltd. Copyright © 2016 John Wiley & Sons, Ltd.
Appendix 3 The SEED intervention (workstream 3)
Overview
In this appendix we present additional information on how the intervention was developed and operationalised. Further details are available in the SEED manual and appendices available as Report Supplementary Material 2. Key areas included in this appendix are:
-
overview of phase 1 workshops
-
recruitment of the DNSs –
-
job description for DNS
-
person specification for DNS
-
-
materials for the DNSs –
-
example of resources for one key component (timely planning discussions)
-
educational needs assessment
-
example of SEED activities and outcomes for one key component (timely planning discussions)
-
example of SEED activity checklists for one key component (timely planning discussions)
-
-
development of new SEED resources.
Developing an evidence-based intervention
Overview of phase 1 workshops to generate and prioritise ideas
-
Workshop 1 comprised small group activities to discuss the purpose of the intervention and potential uses and users, and to clarify the boundaries of the intervention.
-
In workshops 2 and 3, care trajectories from the MCDP cohort data and clinical scenarios from WS2 data were used to prompt discussion of the possible content of, and resources needed to support, the intervention.
-
Workshop 4 considered how existing national guidance could inform the intervention and reviewed available educational/training resources.
-
In workshop 5, key findings from WSs 1 and 2 were presented, followed by team activities to identify gaps in existing care and identify possible ideas for the intervention.
Job description for dementia nurse specialist
| Job details | |||
| Job title: | Dementia nurse/care facilitator | ||
| Business unit: | Community | ||
| Department/ward: | Palliative care | ||
| Location: | North Tyneside Community | ||
| Pay band: | Band 6 | ||
| CAJE no: | NUR1199 | KSF no: | |
| Main purpose of the job | |||
|---|---|---|---|
| The SEED programme is funded by NIHR and is led by Professor Louise Robinson at the University of Newcastle, in collaboration with partner organisations. This is a 5-year research programme, which will explore in detail what constitutes ‘good practice’ care; first by interviewing key groups and observing real-world practice. From these data, we will develop a good-practice intervention to test out and compare with usual care. This post, dementia nurse care facilitator, was developed from the study findings. This fixed-term post for 12 months will aim to support professionals, both commissioning and providers, to deliver good-quality community-based EOLC in dementia through the development of an integrated care pathway | |||
| Dimensions | |||
| The post-holder will be recognised as an expert in either dementia and/or palliative care and will act as a lead (1) provider of information and (2) facilitator of care/services for people with dementia and their carers | |||
| In addition, their role will influence, initiate and manage change to influence local health agenda in relation to dementia and EOLC. To be aware of the constantly changing local and national political agenda and respond appropriately, for example national service frameworks. To contribute to the development of clinical governance and quality frameworks and lead as appropriate on the implementation of policies in and across professions and teams | |||
| Organisation chart | |||
|
|||
| 1. Communications and relationships | |||
|
|||
| 2. Knowledge, skills, training and experience | |||
|
|||
| 3. Analytical skills | |||
|
|||
| 4. Planning and organisational skills | |||
|
|||
| 5. Physical skills | |||
|
|||
| 6. Patient/client care | |||
|
|||
| 7. Policy and service development | |||
|
|||
| 8. Financial and physical resources | |||
|
|||
| 9. Human resources | |||
|
|||
| 10. Information resources | |||
| The postholder will have responsibility for ensuring that patient records are up to date, as required | |||
| 11. Research and development | |||
| A key focus of this role is for the postholder to participate in the SEED research programme as a self-directed professional in collaboration with other members of the multiprofessional team. Encourage and support other professional involvement in the relevant research and contribute to the evidence base for the nursing and medical interventions | |||
| 12. Freedom to act | |||
| The postholder of this fixed-term contract will be an autonomous practitioner who will liaise closely with the palliative care CNS/senior consultant in liaison with the research team. The postholder will be accountable for their own actions | |||
Person specification for dementia nurse specialist
Job title: dementia nurse/care facilitator.
Department: palliative care.
Location: North Tyneside Community.
| Specification | Essential | Desirable |
|---|---|---|
| Qualifications/professional registration |
|
|
| Experience and knowledge | Autonomous professional with experience of working with people with dementia at EOL across primary care settings, including specialist knowledge of Mental Health Act 1983200 legislation | |
| Skills and abilities |
|
|
| Personal attributes | Committed to:
|
|
| Other requirements |
|
Example of resources for one key component (timely planning discussions)
Timely planning discussions
| Scene-setting (illness trajectory) | People with dementia | Family | Staff |
|---|---|---|---|
| ACP and advance health-care directives with a person with dementiaa (68-page guidance) | ✓ | ||
| Advanced dementiaa (online information) | ✓ | ||
| EOLCa (12-page fact sheet) | ✓ | ||
| Facilitating discussions on future and EOLC with a person with dementiaa (52-page guidance) | ✓ | ||
| Later stages of dementiaa (eight-page fact sheet) | ✓ | ✓ | |
| Progression of Alzheimer’s diseasea (two-page fact sheet) | ✓ | ✓ |
| Planning (value of, types of, who to involve) | People with dementia | Family | Staff |
|---|---|---|---|
| ACP and advance health-care directives with a person with dementiaa (68-page guidance) | ✓ | ||
| ACP and advance health-care directives with a person with dementiaa (two-page fact sheet) | ✓ | ||
| ACPb (online information) | ✓ | ||
| Advance decisions and advance statementsa (11-page fact sheet) | ✓ | (✓) | |
| Arranging for someone to make decisions on your behalfb (42-page fact sheet) | ✓ | (✓) | |
| Before you go: planning and support for EOLb (48-page information guide) | ✓ | (✓) | |
| Caring for someone with dementiaa (two-page practical guide) | ✓ | ||
| Create an Advance Decisionb,c (online tool, £10) | ✓ | (✓) | |
| Create an Advance Statementb,c (online tool, £5) | ✓ | (✓) | |
| Deciding Right Appb,c (support guide app) | ✓ | ||
| Dementia and decision-makinga (online information) | ✓ | ||
| Dementia and EOL planninga (online information) | ✓ | ✓ | |
| Early planninga (two-page fact sheet) | ✓ | ✓ | |
| EOLC: what matters to the person who’s dyingb (11-minute video) | ✓ | ||
| EOLC: why talking about death and dying mattersb,c (10-minute video) | ✓ | ||
| Exercising choice and control through a living willb (online information) | ✓ | (✓) | |
| Financial and legal affairsa (13-page fact sheet) | ✓ | ✓ | |
| I have dementia, how do I plan for the future?a (32-page booklet) | ✓ | (✓) | |
| Lasting power of attorneya (14-page fact sheet) | ✓ | (✓) | |
| Making a willb (14-page fact sheet) | ✓ | (✓) | |
| Making decisions and planning your careb (online information) | ✓ | ✓ | |
| Planning for a funeralb (32-page fact sheet) | ✓ | (✓) | |
| Planning for your future careb (16-page guide) | ✓ | (✓) | |
| Powers of attorneyb (40-page information guide) | ✓ | (✓) | |
| Wills and estate-planningb (32-page information guide) | ✓ | (✓) |
| Process of planning (skills to facilitate discussions) | People with dementia | Family | Staff |
|---|---|---|---|
| Before you go: planning and support for end of lifeb (48-page information guide) | ✓ | (✓) | |
| Dementia and decision-makinga (online information) | ✓ | ||
| EOLC: what matters to the person who’s dyingb (11-minute video) | ✓ | ||
| Facilitating discussions on future and EOLC with a person with dementiaa (52-page guidance) | ✓ | ||
| Facilitating discussions on future and EOLC with a person with dementiaa (two-page fact sheet) | ✓ | ||
| Five things to do before I die!b (trifold leaflet) | ✓ | ||
| I have dementia . . . how do I plan for the future?a (32-page booklet) | ✓ | (✓) | |
| Looking aheada,c (four-page tool) | ✓ | ||
| My future well-being toola,c (12-minute demonstration video) | ✓ | ✓ | ✓ |
| One last thing . . .b (trifold leaflet) | ✓ | ||
| Remember when we . . .b (trifold leaflet) | ✓ | ||
| Thinking ahead: ACP discussionsb,c (two-page discussion document) | ✓ | ||
| Thinking aheada,c (four-page tool) | ✓ | ||
| Time to talk?a (eight-page leaflet) | ✓ | ||
| Time to talk, Doc?a (4-minute video) | ✓ | ||
| To-do listb (trifold leaflet) | ✓ |
Educational needs assessment for dementia nurse specialist
| Thinking about . . . | Specific skills/knowledge | Confident about this | Need to learn about this |
|---|---|---|---|
| Timely planning discussions | Able to establish relationships and communicate effectively with people with dementia and their families | ||
| Aware of how to introduce ACP and other possible planning/decisions | |||
| Understand the legal and clinical status of different approaches to planning (e.g. LPA and DNACPR) | |||
| Able to work collaboratively with existing staff responsible for discussing EOL planning (e.g. in hospices or with cancer patients) to share skills and knowledge | |||
| Able to mentor and support staff to take on additional responsibilities related to timely planning discussions with people with dementia | |||
| Able to advocate on behalf of the people with dementia if family members have reservations about his/her preferences | |||
| Able to resolve conflict effectively when people with dementia and family members disagree | |||
| Recognition of EOL and provision of supportive care | Understand common symptoms that may arise at EOL in dementia and how to identify and manage these (including use of appropriate assessment tools) | ||
| Able to elicit and address fears and concerns of people with dementia /families/professionals about management of crisis, distress and pain | |||
| Co-ordination of care | Able to support community staff in analysing and responding to behavioural and psychological symptoms of dementia | ||
| Able to support professionals/people with dementia/families to plan for crisis/deterioration | |||
| Able to identify and analyse support networks of people with dementia and families, and to develop or sustain support | |||
| Well informed about sources of support locally (including out-of-hours services) | |||
| Able to negotiate effectively with multiple health and social care agencies | |||
| Able to foster links between day and night staff to improve integration | |||
| Effective working relationships with primary care | Able to develop good working relationships with members of an established team | ||
| Able to provide training on dementia and EOL needs to primary care colleagues as required | |||
| Able to contribute effectively to existing primary care meetings (e.g. district nurse meetings and ‘virtual ward rounds’) | |||
| Able to use new systems for recording medical and nursing records effectively | |||
| Able to review existing systems within primary care relating to EOLC (e.g. prescription of anticipatory medicines) and identify strategies for reducing unwarranted variations in practice | |||
| Managing hospitalisation | Able to command confidence and exhibit negotiation skills in liaison with a MDT | ||
| Able to advocate on the person’s behalf or support them in self-advocacy | |||
| Able to work with multiple agencies to develop pathways for aspects of EOLC for people with dementia that will minimise unnecessary hospitalisation | |||
| Continuing care after death | Able to offer support to bereaved carers and other members of the support network | ||
| Understand systems and policies to ensure appropriate care of the deceased (including involvement of services such as the police/coroner) | |||
| Valuing staff and ongoing learning | Able to deliver training at an appropriate level for a range of community staff | ||
| Able to facilitate detailed case review discussions with a range of community staff to identify successes and areas for development | |||
| Identify own support needs and those of other health and social care staff involved in EOLC for people with dementia, and identify ways of addressing these | |||
| Able to raise awareness of the emotional work involved in EOLC for people with dementia and ways of supporting staff | |||
| Additional skills | Well informed about the range of dying trajectories in dementia | ||
| Able to contribute to development of interventions by using the theory of change | |||
| Understand the roles and responsibilities of different individuals and organisations in clinical research | |||
| Understand the process of receiving informed consent and the roles and responsibilities of those involved in this process | |||
| Understand the Mental Capacity Act 2005130 and be able to assess the capacity of people with dementia who are eligible for the pilot trial |
Example of SEED activities and outcomes for one key component (timely planning discussions)
| Activities: individual level | Outcomes | |
|---|---|---|
| 1.1 | Discussions about:
|
Documented in patient notes |
| 1.2 | Assessment of capacity, when relevant, prior to completion of formal documentation | Documented in patient notes |
Documentation completed on:
|
|
|
| 1.3 | Documentation disseminated to:
|
Review of care home and GP records |
| 1.4 | Timely review of documents above | Review of care home and GP records |
| Conditions: system level | Outcomes | |
|---|---|---|
| 1.1 |
|
|
| 1.2 |
|
|
| 1.3 | Staff aware of documentation and requirements for completion (including time frame for review) |
|
| 1.4 | A protocol for appropriate dissemination of completed documents | Protocol exists, is accessible and has date for review |
| 1.5 | Protocol that sets out appropriate intervals and trigger points for reviewing EOL documentation | Protocol exists, is accessible and has date for review |
Example of SEED activity checklists for one key component (timely planning discussions)
1. Timely planning discussions to:
-
1.1 provide opportunities for discussions about EOLC with patients and families
-
1.2 provide opportunities for documenting preferences for EOLC
-
1.3 ensure appropriate dissemination of completed documents
-
1.4 ensure timely review of completed documents.
| Activities: individual level | Achieved (yes/no) | Date | Plan of action | |
|---|---|---|---|---|
| 1.1 |
|
|||
| Discussions about personal values | ||||
| Discussions about preferred decision-makers (including LPA) | ||||
| Discussions about comfort care planning | ||||
| Discussions about unwanted treatments and interventions (including hospitalisation and DNACPR) | ||||
| 1.2 | Assessment of capacity, when relevant, prior to completion of formal documentation | |||
| Documentation completed on preferred decision-makers (including LPA) | ||||
| Documentation completed about comfort care planning | ||||
| Documentation completed about unwanted treatments and interventions (including hospitalisation and DNACPR) | ||||
| 1.3 | Documentation disseminated to care home | |||
| Documentation disseminated to out-of-hours service | ||||
| Documentation disseminated to care home ambulance service | ||||
| 1.4 | Timely review of documents |
| Conditions: system level | Achieved (yes/no) | Date | Plan of action | |
|---|---|---|---|---|
| 1.1 |
|
|||
| 1.2 |
|
|||
| 1.3 | Staff aware of documentation and requirements for completion (including time frame for review) | |||
| 1.4 | A protocol for appropriate dissemination of completed documents | |||
| 1.5 | Protocol that sets out appropriate intervals and trigger points for reviewing EOL documentation |
Development of new SEED resources
The review of existing resources highlighted gaps in three key areas:
-
a simple introductory guide to planning for the future
-
clinical scenarios illustrating common issues in EOLC in dementia and strategies to address these
-
online training focused on advanced dementia and EOLC.
The rationale for selecting these areas and a description of the progress made in developing new resources are described below.
Introductory guide to planning for the future
Although a wide range of resources addressed planning for the EOL, there was no simple, introductory overview of the different options available or that provided key information on completed plans (e.g. where such plans were stored and whether or not they had been discussed with the GP). WS2 highlighted the lack of knowledge of ACP and misconceptions over the validity and status of completed documents. For example, even when preferences for invasive treatment had been discussed, these had not necessarily been shared with the GP or documented in a way that would ensure that these preferences were followed in the event of an emergency. A simple document, outlining the range of plans to consider, prompting discussion of key plans with the GP and indicating where the plans were stored, was, therefore considered a potentially useful resource.
A co-design approach was seen as integral to the development of a meaningful and useful care planning guide (CPG) to meet the needs outlined above. 103,104 A key tenet of co-design is that users, as experts of their own experience, become central to, and embedded within, the design process. 105,201 The process was led by researchers from the Glasgow School of Art who were experienced in using co-design and stakeholder engagement for co-developing health-care interventions. A combination of approaches was used, beginning with full project team workshops and then moving onto small task group work202 with potential users of the CPG (i.e. people with dementia, families and professionals). The development of the prototype involved two stages:
-
initial ideas and prototypes were developed during internal project workshops with the multidisciplinary SEED team, which included PPI, and the PPAB convened to support the SEED programme
-
refinement of the prototype CPG through three external workshops involving 20 newly recruited participants from key stakeholder groups (people with dementia, family carers, paid carers, doctors, nurses, support workers and occupational therapists).
These external workshops resulted in a mock-up of the CPG in paper format and diagrams to illustrate a potential digital/app format. The latter was subsequently developed into a demonstration prototype app. However, it became apparent that different stakeholder groups had different needs and wishes. Meanwhile, the NHS created a new EOLC website,203 which was widely recommended for national use. Consequently, further development of the SEED CPG was considered unnecessary as this new resource covered much of the same ground.
Clinical scenarios to illustrate common issues in end-of-life care in dementia
Workstream 2 findings highlighted a number of areas in which user-friendly resources were needed to support good EOLC. The comparative case studies suggested that developing clinical scenarios to illustrate common issues and facilitate discussion would be valued. Such resources could either be used for staff training or be used with people with dementia and carers as a way of opening up discussions. Using electronic learning (e-learning) resources was identified as a key way of reaching a large number of participants, with limited input required post development. Examples that illustrated common clinical situations and areas of difficult decision-making that are likely to be encountered towards, and at, the EOL in dementia were identified. One example, relating to continuing care after death, was developed (see Appendix 3, Clinical scenario: continuing care after death). The WS2 team developed the initial content using WS2 data. This was then refined and further developed by the WS3 team. The presentation was informed by the review of existing resources, including The Sound Doctor (films focused on a wide variety of long-term illnesses, including dementia)204 and Breech Birth (an animated film exploring decision-making). 205 The Sound Doctor resource for dementia comprises a series of films for people with dementia and their families, which cover the trajectory from diagnosis to the later stages of the illness. The films are designed for people with dementia and carers, but are also relevant to health-care and social care staff. Owing to resource constraints, it was not feasible to develop the clinical scenarios further; however, the work was subsequently used in the development of the MOOC (see below).
Massive open online course
Newcastle University had already developed a MOOC that focused on the earlier stages of dementia: Dementia Care: Living Well and Staying Connected. This MOOC was hosted by FutureLearn (part of the Open University) and had attracted > 7000 active learners across 168 countries since its launch in 2016. Having presented the findings of the resource review and a demonstration of the dementia MOOC to the PPAB, it recommended that a second dementia MOOC should be developed focusing on the more advanced stages of dementia and based on the key findings from the SEED programme. Additional funding from Newcastle University was secured for the development of the MOOC. Further details of the MOOC are provided in Workstream 3: development of the SEED intervention.
Clinical scenario: continuing care after death
Appendix 4 Pilot trial of the SEED intervention with process evaluation to ascertain feasibility and acceptability (workstream 4)
Pilot trial methods
The aim of WS4 was to investigate whether or not a definitive multicentre RCT of the SEED intervention is feasible. This appendix provides additional details of the methods, including key areas from the relevant reporting guidelines. 124–127
The study was set in North East England in two urban sites (in North Tyneside/Newcastle) and two rural sites (in Northumberland). North Tyneside’s older population is representative of the general population, whereas Northumberland has a higher percentage of older people than the national average. 7 All practices were invited to use the Dementia Quality Toolkit to review their dementia register and ensure that it was as complete as possible prior to the start of the trial.
The sample size of 66 participants was estimated to provide standard errors around the recruitment rate of ≈ 4.7%, and standard errors around the completion of outcome data no larger than 6.2% at 4 and 8 months, rising to 8.7% at 12 months, assuming a 50% mortality rate.
Inclusion criteria
People with dementia on the dementia register who:
-
received a diagnosis of dementia in the previous 2 years
-
were on the palliative care register
-
were considered to be within 12 months of EOL as judged by a member of the clinical care team (e.g. GP, district nurse, care home nurse) who knows them well using the ‘surprise’ question ‘Would you be surprised if this patient were to die in the next 12 months?’.
Family carers:
-
main family carer of the participating person with dementia
-
aged ≥ 18 years.
Key informants:
-
health or social care professionals who know the person well, who are in direct service provision to the person with dementia and are able to report on QoL, behavioural and psychological symptoms of dementia, symptom management and so on.
Exclusion criteria for all participants
-
Potential participants who refuse consent.
-
Individuals aged < 18 years.
-
Potential participants who are judged as inappropriate for the study by a member of the primary care team (e.g. because of concurrent life events such as bereavement).
-
Participants who are not fluent English speakers, because they would be unable to complete the standardised outcome measures (people with dementia, family carers and key informants) and are likely to have difficulties in participating in a qualitative interview (people with dementia, family carers and professionals).
Process evaluation
The process evaluation aimed to understand the implementation, feasibility and acceptability of the intervention. The aim was also to explore stakeholder views on recruitment processes and outcome measures (in terms of burden, ease of completion and perceived relevance). Inclusion criteria for the process evaluation were as follows:
-
people with dementia who have consented to participate in the pilot trial and agreed to contact from the qualitative research team
-
family carers of people with dementia recruited to the study who have consented to participate in the pilot trial and agreed to contact from the qualitative research team
-
health and social care professionals linked to intervention sites who provide EOLC to people with dementia and their families
-
members of the intervention supervision team
-
members of the primary care team most closely involved in screening and study recruitment.
People with dementia and/or family carers who did not consent to contact from the qualitative team during the initial trial consent process were not eligible for the process evaluation. Potential participants who refused consent were excluded, as were any individuals aged < 18 years.
Interviews, training and supervision were audio-recorded, transcribed verbatim, anonymised and checked prior to analysis. Field notes were written during or soon after events were observed, and were anonymised and checked.
Outcome data
As this is a pilot trial, the main outcomes were feasibility outcomes. We ascertained data completeness of the instruments and any potential bias in the completion of follow-up data to inform the choice of instruments in a future trial. Complete responses were defined as participants completing all items on the questionnaire, and partial responses were defined as participants completing at least 80% of the items but not fully completing the questionnaire. Missing was defined as answering < 80% of the questionnaire items. Details of scoring procedures are provided in Table 12. Data were analysed using Stata® version 14 (StataCorp LP, College Station, TX, USA).
| Questionnaire | Scale/subscale details | Question scoring | Overall score | Notes |
|---|---|---|---|---|
| HADS | 14 questions in total; seven questions each for anxiety and depression subdomains | Questions scored 0–3 and 3–0 (ranging from not at all/never/hardly at all, etc. to very often/most of the time, etc.) | 0–21 for each domain; overall score range is 0–42 when scores from each question are added | If ≥ 80% (at least 12/14 questions) of questions have been answered, then the median value for each participant’s questionnaire score will be ascribed to any missing questions |
| Higher scores indicate greater impact of anxiety and depression | ||||
| CAD-EOLD | 14 symptoms on comfort assessment scale | Each question is scored 1–3 | 14–42 when scores from each question are added | Symptoms listed are discomfort, pain, restlessness, shortness of breath, choking, gurgling, difficulty swallowing, fear, anxiety, crying, moaning, serenity, peace and calm. Lower scores indicate greater symptom burden |
| The last three items are reverse coded (3–1), so higher score is positive. A score of 1 represents ‘a lot’, 2 represents ‘somewhat’ and 3 represents ‘not at all’ | ||||
| CCI | 17 comorbidity disease/condition categories | Each question scored as 1 | 0–17 when scores from each question are added | If no condition is identified, score would be 0; if all conditions indicated, then total score would be 17, which is merely the total number of comorbidities |
| NPI and NPI-NH | 12 symptom domains form total score | Each domain is screened; if no, then scored zero, if yes, then additional questions explore frequency (scored 1–4 where 1, ‘rarely’; 2, ‘sometimes’; and 3, ‘often’; and 4, ‘very often’) severity (1–3, where 1 ‘mild’; 2, ‘moderate’; and 3, ‘severe’) and distress (for family carers) or occupational disruptiveness (for key informants) both scored 0–5, where 0, ‘not at all’; 1, ‘minimal’; 2, ‘mild’; 3, ‘moderate’; 4, ‘severe’; and 5, ‘very severe or extreme’ |
|
|
The overall score can be recoded into three categories:
|
||||
| Higher scores indicate greater symptom burden | ||||
|
Five indicator categories for each PAINAD domain | Each indicator is scored 0–2. Indicator categories roughly correspond to 0, representing ‘no, normal or none’; 1, representing ‘occasional, some, etc.’; and 2, representing ‘a lot, repeated, severe, etc.’ | Total scored by adding all five indicators, so total score range is 0–10 on each PAINAD domain | |
| Higher scores indicate greater impact of symptoms | ||||
| QUALID | 11 responses that best describe person with dementia over the previous week | Each response is scored 1–5 | Total scored by adding all 11 indicators, so total score range is 11–55 | The questionnaire includes options on smiles, appears sad, cries, facial expression/discomfort, physically uncomfortable, discontent/unhappiness, irritable/aggressive, eating, touching, interacting and emotions |
| Lower scores represent higher QoL | ||||
| There are also options for quality of interview (scored 0–2) and knowledge of caregiver (0–2), with lower scores indicating more favourable outcome | ||||
| SM-EOLD | Nine domains on symptom management at the EOL in dementia | Each response is scored 5–0; the calm domain is reverse-coded, 0–5 | Total scored by adding all nine indicators, so total score range is 0–45 | Domains include pain, shortness of breath, skin breakdown, calm, depression, fear, anxiety, agitation and resistiveness to care |
| Higher scores indicate greater comfort | ||||
SWC-EOLD
|
10 domains on satisfaction with care at the EOL in dementia | Each response is scored from 1 to 4 (1 = strongly disagree, 2 = disagree, 3 = agree, 4 = strongly agree). Items 2, 5 and 10 are reverse-scored | Total scored by adding all 10 questions, so total score range is 10–40 | Each domain includes just one question about satisfaction with care at EOL and includes questions such as feelings on getting all necessary nursing assistance to feeling that better medical care at the EOL is/was needed |
| Higher scores indicate more satisfaction | ||||
| BANS-S | Seven items on dressing, sleeping, speech, eating, mobility, muscles and eye contact | Each item is scored on a four-point scale (1–4). The scoring system is specified [e.g. for speech: (a) completely intact ability to speak, (b) somewhat decreased ability to speak, (c) moderately decreased ability to speak, (d) totally mute]. Absence of impairment in a given item is credited with 1 point, whereas 4 points are given for complete impairment | Total scored by adding all seven items; thus, the total score ranges from 7 (no deficit in any item) to 28 (complete impairment in all items) | |
| Higher scores indicate greater impairment | ||||
| EQ-5D-5L, proxy EQ-5D-5L | Five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression | Responses to each dimension are scored on a five-point scale (1–5) where: 1 = no problems, 2 = slight problems, 3 = moderate problems, 4 = severe problems and 5 = extreme problems | No total score is calculated across the five items |
|
|
Resource utilisation questionnaire (person with dementia) Resource utilisation questionnaire (family carer) |
12 broad domains covering respondent characteristics, use of health and social services, and use of the welfare system | Descriptive framework. Responses are reported either categorically (0, absent; 1, present) or cardinal 0 to ∞ | No total score is calculated | Data are descriptive. Data can be used to estimate costs. This will not be considered in this study |
The majority of the outcome data are presented in simple descriptive tables with percentages, means and standard deviations and/or a five number summary (as appropriate), for each arm of the study. This information will inform the design, choice of primary outcome, necessary sample size and approach to the analysis of a future definitive trial.
Trial management
Research ethics and governance
The study received ethics approval from the Newcastle and North Tyneside 1 Research Ethics Committee (REC) on 16 January 2017 (reference number 16/NE/0356). Health Research Authority approval was granted on 18 January 2017. Scheduled reports were submitted to the REC as planned: an annual progress report was submitted on 15 January 2018 and the end-of-study notification was submitted on 8 January 2018. No concerns or queries were received from the REC after report submissions. The SEED WS4 study was sponsored by Northumbria Healthcare NHS Foundation Trust [reference number Integrated Research Application System (IRAS) 211291]. Study management, including database management, was delegated to the Newcastle Clinical Trials Unit; full details, including amendments and protocol deviations, are provided below.
Key site dates
Site initiation visits were conducted at each site by the study senior research associate and trial manager. Key site dates are shown in Table 13. The date of the ‘last patient, last visit’ was 6 August 2018.
| Site | Date | ||
|---|---|---|---|
| Of site initiation visit | Opened | First person with dementia participant recruited | |
| Practice 1 (intervention) | 2 March 2017 | 15 March 2017 | 10 May 2017 |
| Practice 2 (control) | 15 February 2017 | 1 March 2017 | 30 March 2017 |
| Practice 3 (intervention) | 14 March 2017 | 12 April 2017 | 15 May 2017 |
| Practice 4 (control) | 5 April 2017 | 24 April 2017 | 5 July 2017 |
Clinical Research Network
Discussions with primary care sites have indicated that they often struggle to find the capacity to support some research administration tasks, for example maintenance of essential documentation in the investigator site file. With this in mind, assistance was requested from the local Clinical Research Network. Facilitators from Clinical Research Network North East and North Cumbria provided support to sites by ensuring that the investigator site file was complete, particularly when new documentation was implemented as a result of substantial amendments.
Protocol amendments
Protocol amendments are listed in Table 14.
| Amendment reference | Summary | REC approval | Health Research Authority approval |
|---|---|---|---|
| SA01 (re-categorised as non-substantial) | Replacement of site (listed in error) | N/A | 4 April 2017 |
| SA02 | Increase in participant recruitment to allow either a family carer and/or a key informant to be recruited for each person with dementia | 18 April 2017 | 2 May 2017 |
| SA03 | Removal of SEED manual from list of REC-approved documents | 15 May 2017 | 15 May 2017 |
| SA04 | Update to consultee declaration form | 11 August 2017 | 23 August 2017 |
| SA05 | Collecting aggregated data on date of death from screened person with dementia without consent | 25 May 2018 | 25 May 2018 |
Trial management group
The trial management group was scheduled to meet monthly from the study set-up period until the first draft of the study final report. In total, over 32 months, 24 trial management group meetings were held.
Trial Oversight Committee
An independent Trial Oversight Committee provided external oversight of the WS4 clinical trial. Members comprised four independent members of the SEED ESC (dementia care physicians and a statistician), and an independent patient representative. The Trial Oversight Committee met three times during the trial, and no concerns relating to the safety of the participants or scientific integrity of the trial were raised.
Safety
Adverse events were not recorded in this study. Practice managers were contacted on a quarterly basis and asked to review the practice patient complaints log. There were no complaints from participants, and no concerns were raised by the DNSs or by the SEED research team regarding participant safety and well-being as a result of their participation in the study.
Deviations
Three protocol deviations took place; all were reviewed by the Newcastle Clinical Trials Unit quality assurance team and the sponsor. Full details of the deviations and sites affected are provided in Table 15.
| Protocol deviation | Details |
|---|---|
| Not allowing the participant at least 1 week to read the PIS before a member of the primary care team makes a follow-up telephone call to seek consent to pass the details of the person with dementia on to the university research team |
|
| Screening person with dementia without signing delegation log | Two GPs from an intervention practice signed screening forms for people with dementia without being delegated to do so on the site delegation log |
| Participants moved from a SEED GP site to a non-SEED GP and remained in the study |
|
Trial monitoring
Monitoring was conducted according to the trial monitoring plan. As well as the site initiation visits, the trial manager carried out planned on-site monitoring at each site at the end of the recruitment window. The SEED WS4 research team also received two on-site monitoring visits, primarily to monitor original consent and case report forms. In total, 100% of the eligibility criteria for 100% of the participants with dementia were monitored. No ineligible participants were identified. A total of 100% of the informed consent and consultee declaration forms for the participants with dementia were monitored. No deviations from the informed consent process were identified. After the research team confirmed that data collection was complete, a close-out monitoring visit was conducted by the trial manager at each site.
Feasibility and acceptability of recruitment and retention
Recruitment and retention of family carers and key informants are summarised in CONSORT flow diagrams (Figures 5 and 6).
FIGURE 5.
The CONSORT flow diagram for family carers. FFC, family and friend carers; P, practice; PwD, people with dementia.
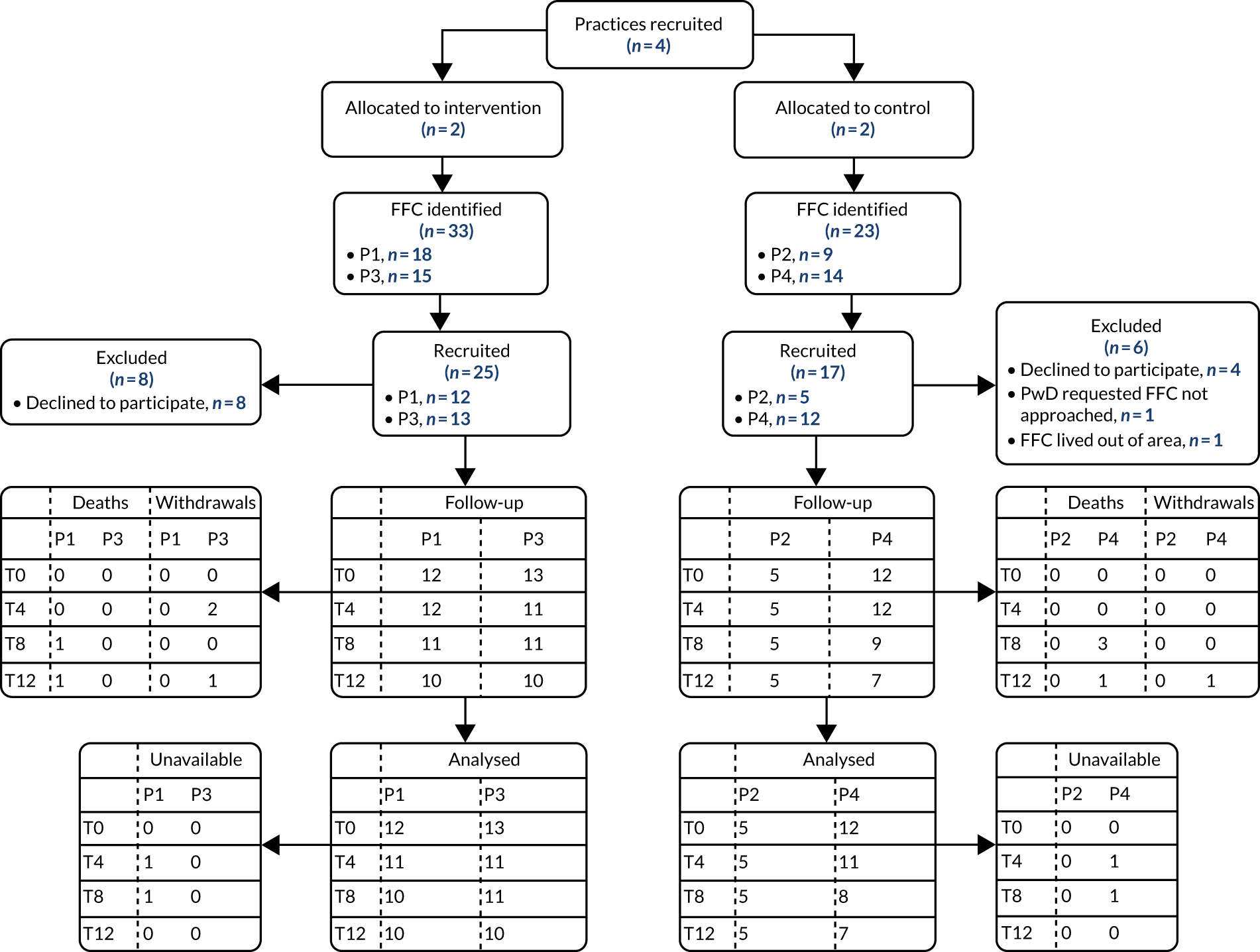
FIGURE 6.
The CONSORT flow diagram for key informants. FFC, family and friend carers; P, practice; PwD, people with dementia.
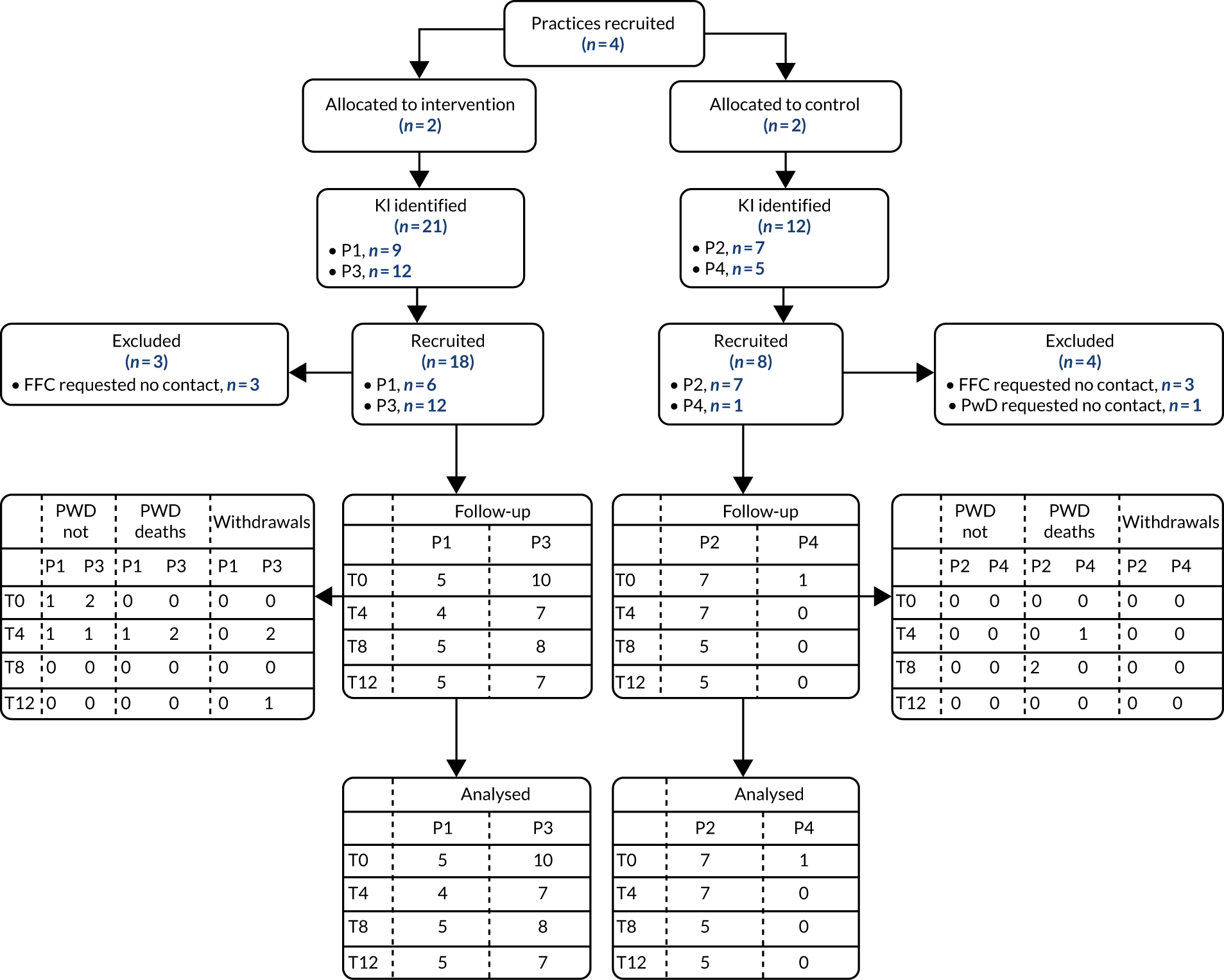
Participant characteristics
The characteristics of all participants were reasonably well balanced between the intervention and the control arms, given the relatively small study (see Tables 16–22). Participating people with dementia were predominantly female, and more than one-third owned and lived in their own home (Table 16). More people with dementia in the intervention arm than in the control arm lived in a dementia-specific care home (10 intervention, compared with one control). The average age was around 85 years (Table 17). In terms of the eligibility criteria, 32 people with dementia were recently diagnosed and 43 were potentially approaching EOL (13 met both eligibility criteria). Place of residence was fairly stable during the study: three people with dementia moved house, five moved into a care home and one changed care home. Most people with dementia had at least two comorbidities according to the Charlson Comorbidity Index questionnaire at baseline (Table 18).
| Categorical variable | Trial arm, n (%) | Total (N = 62), n (%) | |
|---|---|---|---|
| Intervention (N = 37) | Control (N = 25) | ||
| Gender | |||
| Male | 17 (46) | 9 (36) | 26 (42) |
| Female | 20 (54) | 16 (64) | 36 (58) |
| Type of living accommodation | |||
| Owner–occupier housing | 13 (35) | 12 (48) | 25 (40) |
| Privately rented housing | 1 (3) | 1 (4) | 2 (3) |
| Local authority housing | 1 (3) | 1 (4) | 2 (3) |
| Care home (not dementia specific) | 7 (19) | 9 (36) | 16 (26) |
| Dementia-specific care home | 10 (27) | 1 (4) | 11 (18) |
| Sheltered housing/warden control | 3 (8) | 1 (4) | 4 (6) |
| Other/not otherwise specified | 2 (5) | 0 (0) | 2 (3) |
| Continuous variable | Intervention arm (n = 37) | Control arm (n = 25) | Total (n = 62) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD) | Median (IQR) | Range | Participants (n) | Mean (SD) | Median (IQR) | Range | Participants (n) | Mean (SD) | Median (IQR) | Range | |
| Age (years) | 37 | 85.0 (6.7) | 84.8 (80.9–88.5) | 72.0–98.1 | 25 | 84.8 (7.2) | 87.2 (80.4–89.2) | 66.9–96.7 | 62 | 84.9 (6.8) | 85.0 (80.7–89.2) | 66.9–98.1 |
| CCI | Baseline, n (%) | 12 months, n (%) | ||
|---|---|---|---|---|
| Intervention (N = 37) | Control (N = 25) | Intervention (N = 37) | Control (N = 25) | |
| CCI score | ||||
| 1 | 11 (30) | 4 (16) | 11 (30) | 3 (12) |
| 2 | 10 (28) | 7 (28) | 7 (19) | 6 (24) |
| 3 | 11 (30) | 4 (16) | 6 (16) | 4 (16) |
| 4 | 3 (8) | 6 (24) | 4 (11) | 3 (12) |
| 5 | 2 (5) | 3 (12) | 2 (5) | 1 (4) |
| 6 | 0 (0) | 1 (4) | 0 (0) | 1 (4) |
| Dead | 0 (0) | 0 (0) | 5 (14) | 7 (28) |
| Missing | 0 (0) | 0 (0) | 2 (5) | 0 (0) |
Most family carers were female and more than half lived in the same household as the person with dementia (Table 19). Most family carers had frequent contact with the person with dementia (60% had contact for 6 days of the week). Although average contact time per week appeared to be more extensive in the control arm, and the control arm had slightly more males and family carers living in the same home as the people with dementia (see Table 19), these slight imbalances were to be expected given the relatively small number of family carers and the non-randomised nature of the study. On average, family carers had known the person with dementia for > 56 years and the average contact time with them in the previous week was substantial (mean of 15 hours per week; Table 20).
| Categorical variable | Trial arm, n (%) | Total (N = 42), n (%) | |
|---|---|---|---|
| Intervention (N = 25) | Control (N = 17) | ||
| Gender | |||
| Male | 4 (16) | 6 (35) | 10 (24) |
| Female | 21 (84) | 11 (65) | 32 (76) |
| Live in same house as person with dementia | |||
| Yes | 12 (48) | 12 (71) | 24 (57) |
| No | 13 (52) | 5 (29) | 18 (43) |
| Relationship with person with dementia | |||
| Spouse | 11 (44) | 8 (47) | 19 (45) |
| Child | 13 (52) | 8 (47) | 21 (50) |
| Other family member | 1 (4) | 1 (6) | 2 (5) |
| Friend | 0 (0) | 0 (0) | 0 (0) |
| Neighbour | 0 (0) | 0 (0) | 0 (0) |
| Paid carer | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 0 (0) | 0 (0) |
| Contact in previous week with person with dementia (0–7 days) | |||
| 0 | 0 (0) | 0 (0) | 0 (0) |
| 1 | 1 (4) | 0 (0) | 1 (2) |
| 2 | 1 (4) | 2 (12) | 3 (7) |
| 3 | 6 (24) | 1 (6) | 7 (16) |
| 4 | 0 (0) | 0 (0) | 0 (0) |
| 5 | 5 (20) | 0 (0) | 5 (12) |
| 6 | 0 (0) | 1 (6) | 1 (2) |
| 7 | 12 (48) | 13 (76) | 25 (60) |
| Continuous variable | Intervention (N = 25) | Control (N = 17) | Total (N = 42) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD) | Median (IQR) | Range | Participants (n) | Mean (SD) | Median (IQR) | Range | Participants (n) | Mean (SD) | Median (IQR) | Range | |
| Age (years) | 23 | 67.4 (11.6) | 65.8 (58.8–78.4) | 44.6–85.9 | 15 | 67.0 (12.4) | 64.1 (58.6–77.8) | 48.1–87.1 | 38 | 67.2 (11.7) | 65.7 (58.8–77.8) | 44.6–87.1 |
| Length of time known to person with dementia (years) | 25 | 56.2 (10.6) | 58 (51–65) | 30–69 | 17 | 58.0 (6.9) | 60 (51–62) | 45–70 | 42 | 56.9 (9.2) | 59 (51–64) | 30–70 |
| Average contact time with person with dementia in previous week (hours/day) | 25 | 13.0 (11.1) | 14 (1.5–24) | 0–24 | 17 | 18.1 (9.5) | 24 (5–24) | 2–24 | 42 | 15.0 (10.7) | 24 (2.5–24) | 0–24 |
Family carers of people with dementia in care homes were more likely to decline to participate in the trial (n = 11) than family carers of people living at home (n = 1). Typically, carers were happy for the person with dementia to take part and for care home staff to act as key informants, but did not wish to be involved themselves.
Because the number of key informants fluctuated during the study, data are presented for the 23 key informants recruited at baseline. Key informants were predominantly female and had mixed working roles, but most were managers (Table 21). Key informants had at least 3 days’ contact with the person with dementia per week (average contact time per week was 7.3 hours; Table 22). The average length of time key informants had known the person with dementia was 30.2 months, but there was an apparent difference between arms, with key informants in the intervention arm having known the person with dementia for longer, although numbers are sparse.
| Categorical variable | Trial arm, n (%) | Total (N = 23), n (%) | |
|---|---|---|---|
| Intervention (N = 15) | Control (N = 8) | ||
| Gender | |||
| Male | 1 (7) | 1 (13) | 2 (9) |
| Female | 14 (93) | 7 (88) | 21 (91) |
| Role | |||
| Care assistant | 0 (0) | 3 (38) | 3 (13) |
| Senior care assistant | 2 (13) | 0 (0) | 2 (9) |
| Nurse | 3 (20) | 2 (25) | 5 (22) |
| Deputy manager | 0 (0) | 1 (13) | 1 (4) |
| Manager | 10 (67) | 2 (25) | 12 (52) |
| Contact in previous week with person with dementia (0–7 days) | |||
| 0 | 0 (0) | 0 (0) | 0 (0) |
| 1 | 0 (0) | 0 (0) | 0 (0) |
| 2 | 0 (0) | 0 (0) | 0 (0) |
| 3 | 1 (7) | 2 (25) | 3 (13) |
| 4 | 3 (20) | 4 (50) | 7 (30) |
| 5 | 11 (73) | 2 (25) | 13 (57) |
| 6 | 0 (0) | 0 (0) | 0 (0) |
| 7 | 0 (0) | 0 (0) | 0 (0) |
| Continuous variables | Intervention (n = 15) | Control (n = 8) | Total (n = 23) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (n) | Mean (SD) | Median (IQR) | Range | Participants (n) | Mean (SD) | Median (IQR) | Range | Participants (n) | Mean (SD) | Median (IQR) | Range | |
| Length of time known to person with dementia (months) | 15 | 40.8 (34.8) | 30 (30–39) | 12–156 | 8 | 10.4 (2.5) | 10.5 (9–12.5) | 6–13 | 23 | 30.2 (31.5) | 30 (12–30) | 6–156 |
| Average contact time with person with dementia in previous week (hours/day) | 14 | 6.7 (3.8) | 8 (2.5–8) | 1–12 | 8 | 8.5 (3.6) | 9.75 (7–10.75) | 1–12 | 22 | 7.3 (3.7) | 8 (6–10.5) | 1–12 |
Qualitative data on recruitment processes
The process of completing the screening forms was generally considered too onerous, particularly by one control practice. In this practice, the situation was resolved by paying for a locum to enable a senior GP to spend half a day screening the forms. A researcher checked the screening forms as they were completed, and this proved an efficient approach as it minimised missing data and subsequent queries. The approach taken by GPs to the ‘surprise’ question varied. The question was sometimes left blank, for example when the GP had not seen the person with dementia for some time, or when the person with dementia was new to the practice. Some GPs found it difficult to make a judgement and questioned the validity of the ‘surprise’ question:
It was almost impossible to answer that [surprise] question . . . we’ve got lots of our patients that are old and frail that go on for a longer period of time than we might expect. It’s just a very difficult – if you ask 10 GPs about the same patient ‘would you be surprised?’, you might not get the same 10 answers.
Interview, GP 3.2, site 3
To try to manage the recruitment process, practices staggered recruitment, approaching a few people at a time, rather than approaching all of those who were eligible. This may have led to some ‘cherry-picking’ of potential participants. Although the intention was for the researchers to assist with the selection of people with dementia to be approached, this was feasible in only one practice, with other practices preferring to make the decisions themselves of who to approach.
The invitation letters gave potential participants the opportunity to opt out of further contact by telephoning the general practice. Only nine potential participants opted out and no complaints were received about this approach, suggesting that it is acceptable to this group of patients and carers. One control practice would have preferred to have used an opt-in approach, whereby potential participants contacted the research team directly. This would have reduced the workload for GPs and DNSs (who made the follow-up telephone calls), but would also probably have reduced recruitment.
Those who did not opt out were followed up by telephone by either the DNS (intervention practices) or a GP (control practices). The DNSs found these calls challenging, partly because they saw them as a research activity (and of which they had no previous experience), but also because the telephone calls were made at the outset of the study when they were uncertain about what the intervention would entail:
The actual recruiting bit wasn’t particularly comfortable . . . you didn’t know who you were ringing . . . And also because I didn’t really know what the role was, we were trying to persuade people to become involved in something that I didn’t actually know what it was going to be.
Interview, DNS1
Although concerns were expressed that the follow-up telephone calls might turn into ‘mini consultations’, they proved less time-consuming than anticipated. Relatively few people with dementia or carers raised additional issues during the telephone calls, and one GP viewed the opportunity to deal with urgent problems as a benefit:
We would never have known she had problems because she was just trying to carry on on her own. But it meant we got her sorted and it was great. That was a really nice thing to do. There were only a couple of those or ‘I can get the nurse to come out to you. That’s not a problem while I’m on the phone to you’. There has been a benefit for the practice from that point of view.
Interview, GP 4.1, site 4
One GP in a control practice commented that recruitment might have been easier if it had taken place prior to allocation to trial arm:
I think it might have made it easier to, I think ‘sell’ is a strong word, but to promote to patients if we thought that there may have been a possible gain in terms of a nurse specialist being involved with them. . . . obviously it’s a control practice, we were going in and saying ‘we are not going to do anything to help your relative, we are really just going to sort of ask questions and things for a trial’, you know. I think it’s harder to market or promote to patients than if you are saying ‘I don’t know which arm you are going to. It’s possible that you might get extra support from a nurse specialist.’
Interview, GP 2.1, site 2
Few comments specifically about recruitment processes were made by people with dementia and carers. Consistent with previous studies, the primary motivation for participating was altruism, with people with dementia and carers hoping that their involvement would help others in the future:
I’m happy to get involved in any sort of research that’s going to help people. I mean, I might be one of these people in the future, who knows. So I think it’s a really good thing.
Interview, family carer 3018
Some people with dementia who had been recently diagnosed, and their family carers, did not see the SEED intervention as appropriate to their situation. Although they felt that the immediate post-diagnostic period was too early for the intervention, they found it difficult to identify the most appropriate time:
(What is the best point at which to offer some kind of nurse specialist role?) When you need help. I’m sorry, but that is ‘How long is a piece of string?’.
Interview, family carer 1070
The key issues relating to recruitment and potential strategies to maximise recruitment to a future trial are summarised in Table 23.
| Issue | Proposed strategy |
|---|---|
| Pre-trial work to facilitate recruitment | |
| Poor-quality information on next of kin (i.e. often no address, telephone numbers out of date and little information on full name/relationship) | Provide funding for practice administrative staff to update information on next of kin for all patients on the dementia register prior to the start of recruitment |
| Clear contract with general practices to clarify responsibilities and timelines | |
| Practices agreed to participate but did not necessarily have the resources or will to deliver | Providing a written contract with explicit responsibilities and timelines may be helpful |
| Reducing workload and responsibility of GPs | |
GPs found various aspects of screening and recruitment onerous
|
To fund a locum (to free up GP time) or a research nurse in each practice to lead on screening and recruitment. Their role would be to:
|
| Maximising buy-in for practices | |
| One control practice had limited engagement with the study and GPs were not clear what they were getting out of participation | Recruitment prior to randomisation should help, as practices will not know whether or not they will be in the intervention arm until after recruitment is complete |
| We offered a teaching session on the SEED study as an incentive – can we make this more attractive (e.g. CPD points)? | |
| Emphasise that, generally, patients in trials tend to have better outcomes even if they are in the control arm; contact with researchers may be beneficial | |
| Recruitment staff to be blinded to trial arm | |
| As practices knew which arm of the study they were in, this may have influenced their approach to recruitment | The research nurses and practices should be blind to randomisation until recruitment is complete |
| The DNSs in the pilot trial were known to some potential participants from their previous role and this probably increased consent rates | The research nurse responsible for recruitment would not be the person delivering the intervention |
| Streamlining of screening log | |
| Screening log was considered to be both too ‘big’ and missing key information (e.g. whether or not patient on palliative care register, patient address) | Delegating responsibility for most of the screening log to a research nurse should help this, as GPs would no longer be expected to complete any sections of the form |
| Despite inclusion of stop/go criteria, there were still inconsistencies in completion of the forms | Amend the form in the light of feedback |
| Consider alternative formats [e.g. SurveyMonkey® (Palo Alto, CA, USA)] with built-in controls to prevent screening errors | |
| Provide a detailed protocol and training for the research nurses | |
| Increase monitoring and supervision to identify errors at an early stage and provide additional training | |
| Transferring data from the screening logs for analysis was time-consuming | Moving to an electronic system (e.g. SurveyMonkey) would avoid the need to transfer data and data entry errors |
| Clarification of inclusion/exclusion criteria | |
| Some GPs found the ‘surprise’ question difficult, because they did not know the patient, the patient had not consulted recently or they felt that it was ‘giving them a death sentence’ | Consider whether to continue to try to target the intervention on specific groups of patients to whom it might be more relevant, or to include all patients on the dementia register |
| Some GPs automatically selected that they would not be surprised if the patient died in the next 12 months for any patients living in care homes | Consider whether to treat all people with dementia living in care homes as eligible for the study, rather than completing the ‘surprise’ question for these patients |
| Some GPs in control practices thought that it was inappropriate to contact those on the palliative care register as the study was thought too burdensome for no return | This may be less of an issue if patients are recruited prior to randomisation; providing feedback from the ‘pilot’ study may also help in demonstrating that patients and carers found participation acceptable regardless of which arm they were in |
| Clear procedures for sampling from completed screening logs | |
| We lost control of the process of selecting eligible patients for approach in all but one practice. This may have led to oversampling of people within 2 years of diagnosis, as they were typically easier (and quicker) to recruit because they could usually give consent and, therefore, a consultee did not have to be identified and approached | Provision of clear guidelines as to how to sample patients and additional training and supervision should ensure a more consistent approach whereby the sample is selected to include people with dementia within 2 years of diagnosis, those thought to be approaching the EOL, those with capacity to consent for themselves, those requiring a consultee, those living in their own home and those living in care homes |
| Streamlining recruitment processes | |
| The protocol was too specific about who would make the follow-up telephone call and when the call would be made | Be less precise about the time period between sending the letter and making the follow-up call, for example ‘a few days’ |
| The protocol should state that an appropriate member of the primary care team will make the follow-up telephone call | |
| Completion of the section on the timing of follow-up calls was poor | Ensure that the research nurse understands the rationale for providing this information and increase monitoring and supervision to identify errors at an early stage |
| Managing workload for care homes | |
| The process of being a key informant required a fairly significant time commitment (30 minutes for each participant on four occasions over 1 year). To avoid overburdening individual care homes, should we set a maximum number of patients to be recruited from each home? | Agree a maximum number of participants per home, for intervention sites; other residents may still benefit from the intervention as changes may be to systems or staff training |
| Monitoring of screening and recruitment process | |
| The screening logs were systematically reviewed by a member of the research team in only one practice; this lack of oversight led to errors, inconsistencies and missing information | To have an explicit, formal monitoring plan and to ensure that this is implemented so that additional training/support can be provided to practices at an early stage |
Feasibility and acceptability of the SEED intervention
Training and supervision of the dementia nurse specialists
Table 24 provides an overview of training and supervision arrangements. The initial 3-day training and induction programme was provided by the research team and clinical leads (Table 25 contains the agenda) and included an educational needs assessment (see Appendix 3, Educational needs assessment for dementia nurse specialist). Additional training needs were met through supervision, tailored training sessions and self-directed learning using the care resource kit and e-learning. One general practice provided a 1-week induction programme to help the DNSs integrate with the primary care teams.
| Characteristic | Training and induction | Intervention supervision | Clinical supervision (old-age psychiatry/palliative care) | Clinical supervision (GP dementia lead) |
|---|---|---|---|---|
| Frequency | Once at start of study | Monthly | Monthly | Approximately monthly |
| Number of sessions | 6 | 12 | 27 individual and 4 joint sessions | 9 |
| Duration | 3 full days | 90–120 minutes | Unknown | 120 minutes |
| Content | Introduction to the SEED intervention, advanced dementia, palliative care, primary care, study recruitment, process evaluation | Review training needs, progress with individual and systems work, use of the seven components. Additional issues raised by the DNSs | Additional specialist training, case-based discussions | Case-based discussions, development of dementia review template, implementing changes in general practice |
| Main aims | To familiarise the DNSs with the SEED intervention and build confidence to start their new role | To monitor intervention delivery and address emerging training needs | To ensure that the DNSs felt supported within their existing discipline and provide additional training | To facilitate embedding in practice and maximise impact of the DNSs |
| Day | Agenda |
|---|---|
| 1 | Introduction to the SEED programme |
| Key findings to date | |
| The SEED intervention | |
| The care resource kit | |
| Advanced dementia: signs, symptoms and challenges | |
| Mental Capacity Act | |
| Primary care and dementia | |
| 2 | Primary and community care |
| Local service mapping | |
| Developing a clinical support network | |
| Getting started with the SEED intervention | |
| Ongoing support and supervision | |
| 3 | Understanding the study design
|
| Screening and approaching people with dementia | |
| Accessing existing resources | |
| Managing a new role |
To address the issues of anxiety and deskilling, which were inevitably associated with taking on a new role, bespoke supervision arrangements provided support with recruitment processes, the content and delivery of the intervention, palliative care and dementia. Establishing a MDT to provide supervision was not feasible. Instead, support for the first two areas was provided through monthly intervention supervision sessions led by the research team and a palliative care clinical lead. The palliative care clinical lead and an old-age psychiatrist provided clinical support and case supervision. In addition, both DNSs continued to receive supervision from their previous work teams. The DNSs also met regularly for peer support. Their different backgrounds (in palliative care and mental health) proved useful in joint working and mutual support.
Both DNSs found the monthly supervisions with the research team a helpful way of working through ideas, identifying problems and sharing solutions. From the lead researcher’s perspective, these meetings were useful in clarifying boundaries, promoting systems work and obtaining feedback on the intervention. More administrative and IT support from the host general practices would have been useful. Only one DNS received supervision from the dementia lead GP; without this, the role was potentially isolating and scope for improving systems within the practice was limited. In addition to formal supervision arrangements, both DNSs valued informal contact with the researchers.
Delivery of the SEED intervention
In this section, we illustrate how the SEED intervention was translated into practice using data from activity logs, the vignettes and the resources developed by the DNSs.
Activity analysis
The activity logs enabled us to examine the proportion of days on which prespecified activities were recorded, the focus of direct work with patient–carer dyads, and collaborative working. The initial analysis focused on the number of days on which specific activities were recorded (Figure 7 and Table 26). Details of activities were recorded for 206 days for DNS1 and 180 days for DNS2, reflecting their different working hours and patterns. In terms of the proportion of days on which specific activities were recorded, major components of the intervention were addressing the current needs of people with dementia, current needs of family carers, and networking and service mapping (with all of these activities being recorded on > 50% of working days). The proportion of days on which different activities were recorded was similar for both DNSs, with the exception of developing and implementing systems and research activities. DNS1 had more scope for developing systems because she worked with a larger number of care homes and in a practice that was more open to change. The difference in research activities reflects the different approach to recruitment in the two practices. DNS1 attended daily MDT meetings in the practice and discussed a few potential participants each day, whereas DNS2 distributed screening forms to the most appropriate GP and then collated the completed forms, which was less time-consuming.
FIGURE 7.
Proportion of days on which activities were recorded by DNSs.
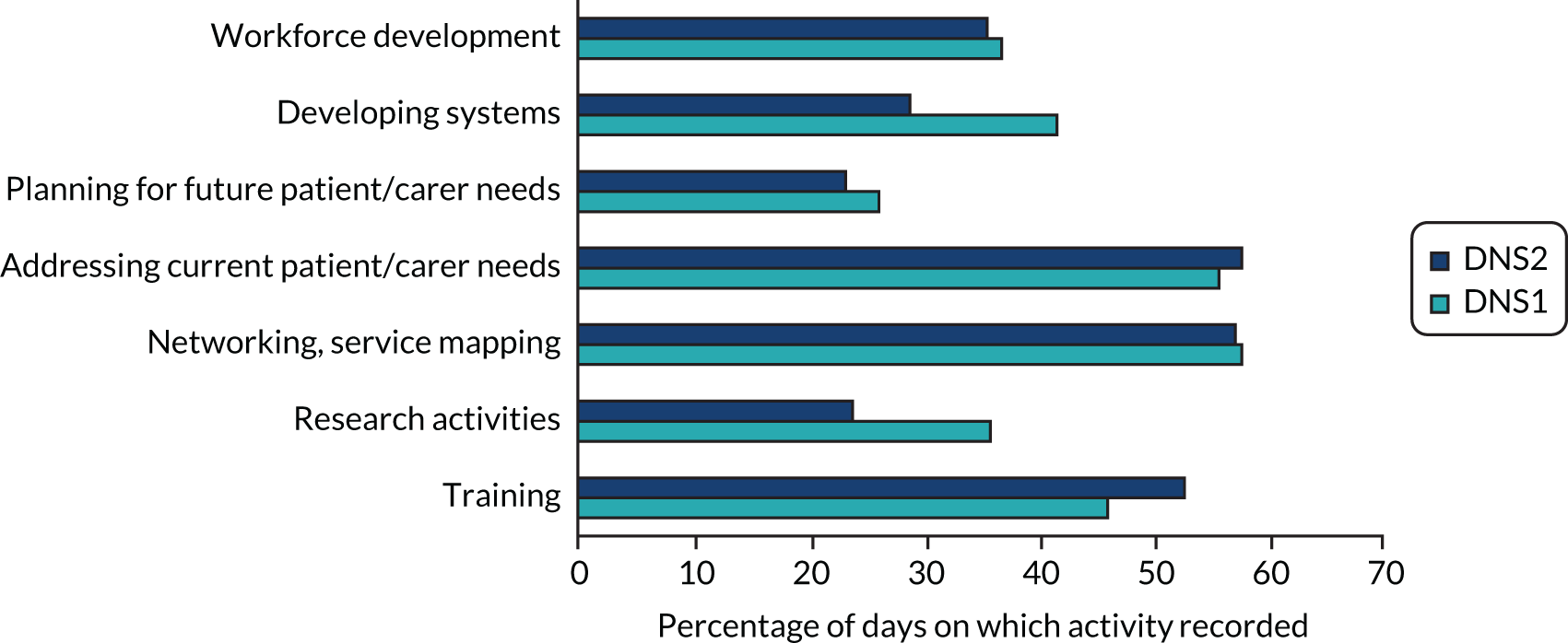
| Activity | Days, n (%) | Total days, n (%) | |
|---|---|---|---|
| DNS1 | DNS2 | ||
| Training | 95 (46.1) | 95 (52.8) | 190 (49.2) |
| Study activities | 74 (35.9) | 43 (23.9) | 117 (30.3) |
| Networking, service mapping | 119 (57.8) | 103 (57.2) | 222 (57.5) |
| Addressing current needs of person with dementia/carer | 115 (55.8) | 104 (57.8) | 219 (56.7) |
| Planning for future with person with dementia/carer | 54 (26.2) | 42 (23.3) | 96 (24.9) |
| Developing and implementing systems to improve EOLC | 86 (41.7) | 52 (28.9) | 138 (35.8) |
| Workforce development | 76 (36.9) | 64 (35.6) | 140 (36.3) |
| Total days | 206 (100) | 180 (100) | 386 (100) |
We also examined the number and type of direct contacts (i.e. face-to-face meetings and telephone calls) with individual patient–carer dyads. The number of such contacts ranged from 0 to 18 (median 7) for people with dementia and from 0 to 31 for carers (median 4). Nearly all contacts were in their place of residence, with only seven contacts elsewhere. The most common activities with participating dyads related to the well-being of the person with dementia (97%), family carer well-being (35%) and future planning (33%). With the exception of future planning, people with dementia living in care homes and their family carers tended to receive fewer contacts relating to all other activities than those living in their own homes (Figure 8 and Table 27).
FIGURE 8.
Focus of direct work with participating dyads by place of residence.
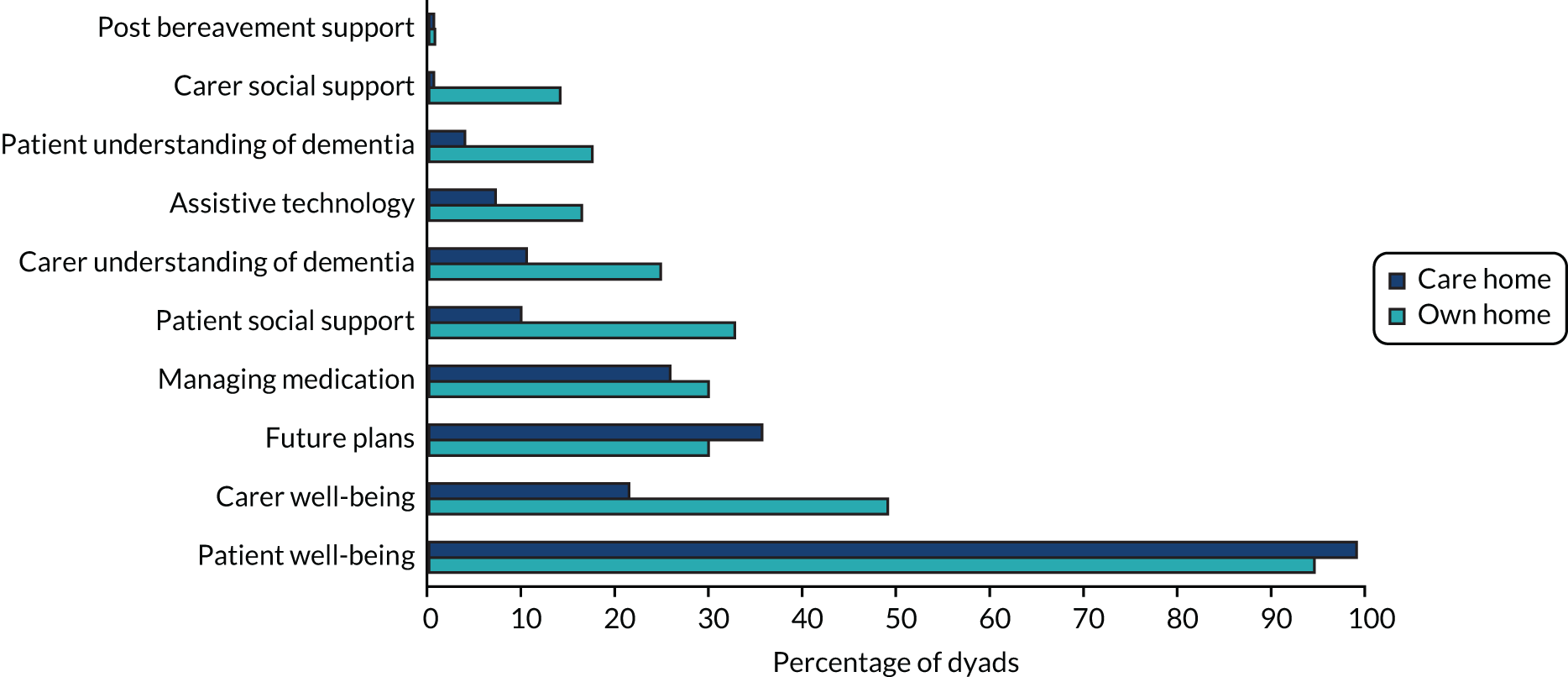
| Activity | Number of direct contacts, n (%) | ||
|---|---|---|---|
| Community dwelling (n = 178) | Care home residents (n = 183) | All (n = 361) | |
| Patient well-being | 168 (94.4) | 181 (98.9) | 96.7 (361) |
| Carer well-being | 87 (48.9) | 39 (21.3) | 126 (34.9) |
| Future plans | 53 (29.8) | 65 (35.5) | 118 (32.7) |
| Managing medication | 53 (29.8) | 47 (25.7) | 100 (27.7) |
| Patient informal networks and social support | 58 (32.6) | 18 (9.8) | 76 (21.1) |
| Carer informal networks and social support | 25 (14.0) | 1 (0.5) | 26 (7.2) |
| Patient understanding of dementia | 31 (17.4) | 7 (3.8) | 38 (10.5) |
| Carer understanding of dementia | 44 (24.7) | 19 (10.4) | 63 (17.5) |
| Environment and assistive technology | 29 (16.3) | 13 (7.1) | 42 (11.6) |
| Post-bereavement support | 1 (0.6) | 1 (0.5) | 2 (0.6) |
Further analysis of aggregated data for individual patient–carer dyads showed high levels of collaborative working or informal discussions (83.8% of dyads) and indicated that just over half (51.4%) were referred to another service, most frequently the third sector (Figure 9 and Table 28). Collaborative working was most common with colleagues in primary care, care home staff and MDTs. The high proportion of dyads (70%) discussed with primary care colleagues is likely to have been facilitated by physical proximity, which enabled brief informal discussions with GPs. Further analyses showed that people with dementia living in care homes and their family carers were referred less frequently (33.3%) than those living in their own homes (75%), but no differences were found in the frequency of informal discussions according to place of residence.
FIGURE 9.
Referrals and collaborative working with participating dyads.
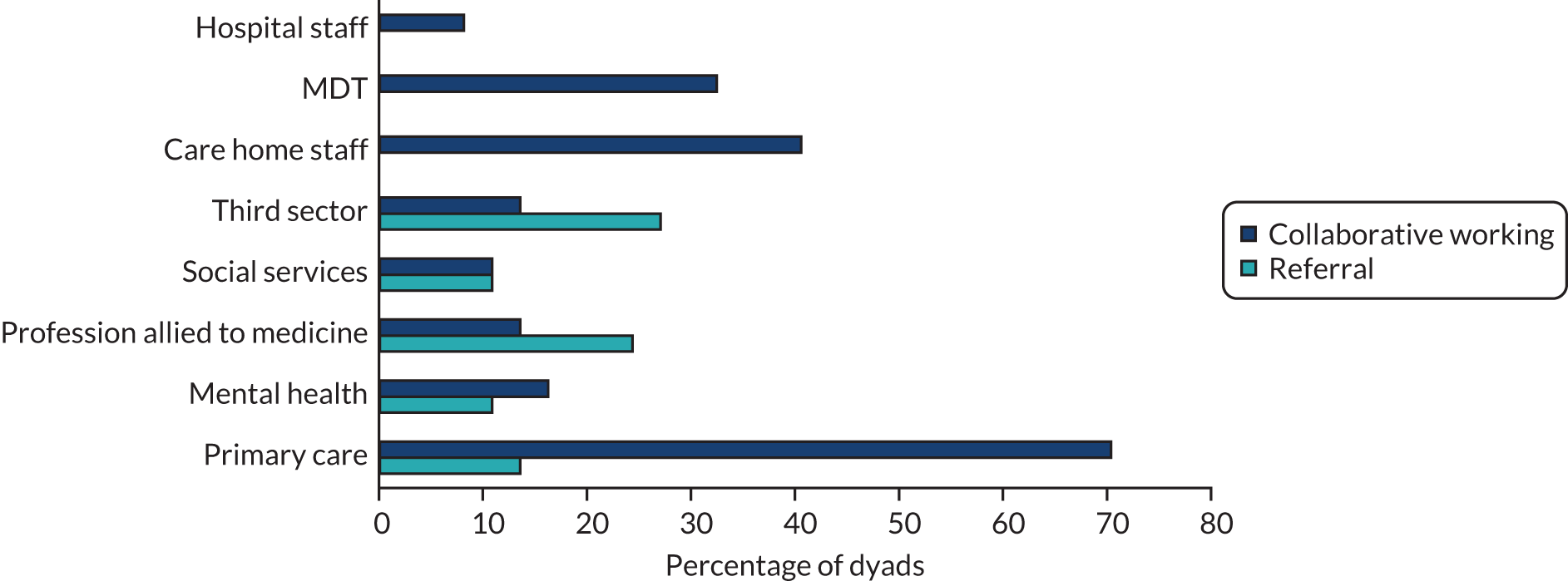
| Sector/professional | n (%) | |
|---|---|---|
| Referrals (N = 37) | Collaborative working (N = 37) | |
| Primary care | 5 (13.5) | 26 (70.3) |
| Mental health | 4 (10.8) | 6 (16.2) |
| Profession allied to medicine | 9 (24.3) | 5 (13.5) |
| Social services | 4 (10.8) | 4 (10.8) |
| Third sector | 10 (27) | 5 (13.5) |
| Care home staff | 0 (0) | 15 (40.5) |
| MDT | 0 (0) | 12 (32.4) |
| Hospital staff | 0 (0) | 3 (8.1) |
Despite the challenges of recording and analysing activity data, the analyses were generally consistent with the findings of observation and interviews, suggesting that the data were reasonably robust. For example, the DNS who was more embedded in the general practice discussed a higher proportion (89%) of dyads with primary care colleagues than the DNS based in the less engaged practice (53%).
Vignettes illustrating the SEED intervention
Greater insight into the SEED intervention and the way in which the seven components were translated into practice is provided by the vignettes in Boxes 2–6. Pseudonyms were used for the individuals to ensure confidentiality. The key components covered by different vignettes are:
-
timely planning discussions (vignettes 3, 4 and 5)
-
recognising EOL and providing supportive care (vignettes 1, 2, 4 and 5)
-
co-ordinating care (vignettes 1, 2, 3, 4 and 5)
-
working effectively with primary care (vignettes 3, 4 and 5)
-
managing hospitalisation (vignettes 2 and 5)
-
continuing care after death (vignette 2)
-
valuing staff and ongoing learning (vignettes 1 and 5).
Mr Jennings has been in a nursing home for 4 years. He has had dementia for around 10 years and is considered by his GP to be approaching the EOL. His wife is in another care home and his daughter visits regularly. The DNS reviews Mr Jennings’ care needs with his daughter and care home staff. All agree that he seems to be having more difficulties with communication, and his mobility and ability to self-care have declined. Mr Jennings is frustrated by these changes. The care home staff are keen to provide the best care possible, but feel that they are currently struggling to meet his needs.
The DNS suggests using the comfort care plan to explore and address Mr Jennings’ changing needs. Using Mr Jennings as an example, she delivers a training session at the care home for staff involved in Mr Jennings’ care (including the care home manager and senior staff). Prior to the session, the DNS met with Mr Jennings’ daughter to talk about her father’s care needs, and whether or not and how these were changing as his dementia progressed. This enabled the DNS to include these insights from his daughter in the training session for care home staff. In the training session, staff considered his likes and dislikes, identified ways of enhancing his physical and emotional well-being and then developed a draft plan. The DNS encourages the care home staff to think about comfort care planning for others in the care home.
The DNS discusses the plan with Mr Jennings’ daughter, who supports the suggested changes. She works with the care home to implement small changes in her father’s care, such as improving access to, and variety in, his favourite music and television programmes, and interventions to calm Mr Jennings during his personal care.
One month later, the DNS reviews the comfort care plan with the staff and Mr Jennings’ daughter. They are pleased with the changes and Mr Jennings is now calmer and more relaxed. This makes his daughter’s visits more enjoyable and the staff feel that Mr Jennings is less distressed and that they are now meeting his needs around comfort. The care home manager and the DNS work on more comfort care plans for other residents and have implemented a system of ‘flash meetings’ to ensure that as many staff as possible are aware of individual comfort care plans for residents.
Mr Thompson and his wife live in their family home. Their daughter and son-in-law live locally and visit regularly, supporting their parents with some everyday tasks, such as shopping. Their daughter feels that her mum struggles to cope with her dad and would benefit from some extra support; however, Mrs Thompson is reluctant to accept help. Using the newly developed template, the DNS completes a comprehensive annual dementia review with the couple. Although the couple seemed to be coping well on the surface, the assessment reveals that the couple need support with managing Mr Thompson’s physical health, particularly his continence needs. Mrs Thompson confides in the DNS about her own emotional struggles. Mrs Thompson had previously refused to engage with services, but the DNS persuades Mrs Thompson to accept support. She refers Mr Thompson to the Community Mental Health Team and Mrs Thompson to psychology, to meet their individual psychological needs. The DNS also feeds back her findings to the GP, highlighting concerns around safeguarding for the couple.
Mr and Mrs Thompson engage with the mental health services and receive support. However Mr Thompson is taken ill with an infection and is admitted to hospital. While in hospital, he becomes ill with viral gastroenteritis, and is increasingly frail. The DNS discusses Mr Thompson’s health with the hospital MDT. Mr and Mrs Thompson’s daughter is concerned that her mother will no longer be able to cope with her dad on discharge from hospital, but Mrs Thompson is adamant that she wants her husband to come home when he is able. The DNS has built up a relationship of trust with Mrs Thompson and asks her to consider a placement in a local care home to help her husband to recuperate. The DNS visits Mr Thompson in hospital and keeps the hospital MDT and family informed of each other’s wishes and preferences around the best care for Mr Thompson.
Mr Thompson deteriorates further and the hospital staff and his daughter believes that he would be best cared for in hospital. He is moved to a side ward, and dies a few days later. The DNS visits Mrs Thompson and her daughter soon after Mr Thompson’s death to continue to support them. They feel that Mr Thompson died peacefully and comfortably. The DNS stays in touch, and refers Mrs Thompson for bereavement support. Her daughter feels that this is very helpful for her mother.
Mrs Robertson has had a recent diagnosis of dementia. She lives alone in a flat and her son visits every day on the way home from work. She is in good physical health and loves going out in town, but recognises that changes in her short-term memory are starting to affect her ability to get out and about.
Again, using the new dementia review template, the DNS completes a comprehensive assessment of Mrs Robertson’s physical and emotional well-being. The DNS realises that Mrs Robertson is fit and active, and very sociable, but needs support in maintaining a good social network.
Owing to the severity of Mrs Robertson’s short-term memory loss, the DNS feels that it is appropriate to discuss planning future care and decision-making while she is able to do so. The DNS broaches this cautiously over several visits. Mrs Robertson is initially reluctant, shrugging off the need to think about the future. The DNS uses documentation, such as ‘This is me’, as a way of discussing future preferences and establishes that her son already has lasting power of attorney for health and welfare in place.
The DNS also suggests different day centres and clubs that Mrs Robertson may be interested in. She agrees to attend the day hospice at the local hospital, but is less keen on local interest groups, such as a choir. The DNS arranges a referral to the day hospice and ensures transport is arranged. The DNS telephones Mrs Robertson’s son to discuss the new service and decision-making around future care. Her son is open to discussions about planning for the future.
The DNS follows up with Mrs Robertson and also liaises with the day hospice team. Mrs Robertson and the hospice team are very positive, both describing how she enjoys her weekly visits to the centre. However, the hospice team are concerned that they currently provide only short-term placements and will have to discharge Mrs Robertson from the service after 3 months. They discuss options for re-referral if the service has capacity. Providing a service for Mrs Robertson is unusual for the hospice. It has led them to question whether or not their service is appropriately organised and equipped for people with dementia. They plan to implement changes to adapt the service for people with conditions that have uncertain trajectories, and to develop staff training on dementia. The DNS and Mrs Robertson continue to explore alternative local organisations and groups to meet Mrs Robertson’s psychosocial needs.
Mrs O’Shea is a widow and lives alone with the support of her daughter and son-in-law. They visit frequently and provide support around housework, shopping, home maintenance and some personal care. They also have other family support commitments and Mrs O’Shea sometimes goes into respite care at a local care home when her daughter and son-in-law are away. They already have support from social work and the local Admiral Nursing team.
Mrs O’Shea is in her 90s, but her diagnosis of dementia is recent. Her daughter is concerned that her mother is unable to appreciate the amount of support she now needs, and she is uncertain whether or not she and her husband can continue to meet this increasing need. Although she has lasting power of attorney for her mother’s health and welfare, and finance, she is worried about making decisions on behalf of her mother because of complications with a sibling who lives abroad.
During a follow-up visit, the DNS reviews these concerns with Mrs O’Shea, her daughter and her son-in-law, together. Mrs O’Shea’s daughter is keen for her mother to be involved in her own decision-making as much as possible. They talk about future plans together, but choose to fill in documentation together at a time when they can focus on this as a family. Her daughter finds it helpful that the DNS is involved in these discussions so that she cannot be accused of overinfluencing her mother. They also discuss managing Mrs O’Shea’s increasing needs and her family’s need for respite. This is a tricky issue and, again, the DNS acts as a third party, facilitating a discussion about why respite care is necessary and explaining carefully how it can be mutually beneficial in meeting both their needs. Mrs O’Shea’s daughter finds it helpful to have these conversations jointly. Although she values the support from the Admiral nurse, the nurse has never met with her mother and is there to support her and her husband.
Some of the residents in care home 2 were participants in the research. As well as visiting the individual residents, the DNS met with the manager and senior staff to explore ways of improving the care provided to benefit other residents and the care home. The DNS supported the care home staff to identify and make changes in ways that enabled staff to take ownership and maintain changes once the study was over.
The manager and clinical lead were keen to secure outstanding status on EOLC from the independent regulators. They identified that improvements were needed in their ACP documentation before this could be achieved. The DNS worked with the senior staff, a community matron and the local hospice to improve their documentation so it both captured key information and was more user friendly. The manager subsequently shared the revised documentation with the senior management team of the care home group, of which her home was a part; they were keen to adopt the documents in their other homes. In addition, the community matron discussed the new documentation at a meeting of local care homes with a view to wider adoption in the area.
Ad hoc opportunities for systems change also arose. On one visit to review a resident, the clinical lead confided in the DNS that some of the local GPs seemed unclear about which forms to use for Emergency Healthcare Planning, and how to personalise the content for individual residents. The DNS was able to discuss this with practice staff at a multidisciplinary meeting, and developed a flow chart for the GPs to use when completing documentation with the care home staff.
Through joint visits with GPs, the DNS was able to introduce staff and residents to her colleagues and facilitate stronger links between the care home and the general practice. The DNS and the practice pharmacist also worked together on medication reviews for residents in the study. This paved the way for the pharmacist to work with the clinical lead to monitor and review medications for all residents in the home.
Resources developed by the dementia nurse specialists to support end-of-life care
Further insight into the delivery of the intervention is provided by the resources developed by the DNSs. To improve the context for EOLC, they developed and implemented several new resources during the study (Table 29). These resources highlight the breadth of their work, and the potential benefits of the intervention for care homes and general practices. Interview data consistently indicated the value of these resources to stakeholders (see Table 29).
| Characteristic | Mapping local resources | Comfort care planning | Training | Template for annual dementia review |
|---|---|---|---|---|
| Contributors | DNSs | DNSs | DNSs | DNSs and dementia lead GP |
| Activities | Networking and mapping local services |
|
Developing and delivering training on:
|
|
| Outputs | Summary sheet for general practice |
|
|
Template to improve consistency of annual reviews, which includes prompts to discuss next of kin and ACP |
| Illustrative stakeholder feedback | [DNS2] put together information sheets for us about local sources of other help, which has been probably one of the most helpful thingsInterview, GP 3.3, site 3 | Well the main thing she’s done is to develop a comfort care plan for my dad, which I think was really lovely, actually. I think not only has she listened to what I was saying, which she clearly did, but she’d obviously been out to the care home a couple of times and had spoken to staff . . . I felt reading it, that it was yes, this is what my dad is like now and what he needs nowInterview, family carer 1058 | One of the nursing homes, when I did the pain assessment [training], one of the carers said she would like more end-of-life training, so I’ll work on that as a form of education and awarenessIntervention supervision, DNS2 | I’ve done a few [dementia annual reviews] myself and I find it a really user-friendly template. We need to go around the care homes and do all of those . . . So I’m going to be putting [it] into a lot more practice within the next couple of months. But I think it’s fantasticInterview, GP 1.1, site 1 |
Stakeholder views on feasibility and acceptability of the SEED intervention
Location in primary care
All participants agreed that primary care was the right location for the DNS, with the majority seeing the general practice as the most appropriate. However, a small number of health-care professionals suggested that the DNS could be located with other primary care teams (e.g. district nurses or palliative care teams).
Key benefits of the DNS being based in a general practice were ease of access for people with dementia and carers, opportunities for face-to-face contact with GPs, access to patient records and the established links to other services:
We used to have district nurses based on-site, we used to have health visitors based on-site. Now the health visitors are based in their own little silo away, and the district nurses are based in their silo off-site. But you can’t beat that interaction, with a GP, down a corridor. ‘Actually Mrs Smith is struggling, can we do something about this?’. So yes you could probably do it off-site, but you’re not going to get that quality input.
Interview, practice manager 3.1, site 3
As most people with dementia receive most of their care from primary care, this was seen as an appropriate location, although the need for the DNS to liaise with, and co-ordinate, other services as needed was emphasised:
Many patients will go through long periods of the dementia illness, without being in touch with secondary care services. So, from that point alone it makes sense to be embedded with primary care, one of the core roles . . . is developing links with other parts of the wider system.
Interview, consultant old-age psychiatrist, intervention supervision team
To maximise the benefits of co-location in the general practice, being recognised and valued as a member of the team was essential. This was facilitated by providing a planned induction, regular supervision meetings with the dementia lead GP, and attending key meetings. The frequent changes in the host general practices (e.g. merging with a neighbouring practice, managing staff shortages) highlighted the need for DNSs to be adept at managing change. Furthermore, their confidence in their specialist knowledge and ability to develop their role in the practice influenced the extent to which they were successfully embedded:
. . . but the challenges were sitting in a group of people that I don’t know, like the MDT, and thinking, ‘right, I’m going to have to speak up here, I’ve got to fight for some recognition’. I don’t want to just be somebody who disappears into the background, otherwise I’m not going to make a difference. Having to sort of steel myself to go and speak to GPs about people
Interview, DNS 1
Relevance of the seven key components to real-world practice
The DNSs delivered interventions relating to all seven components for participants living at home and in care homes, at both an individual and a systems level (see Table 6 and the vignettes in Boxes 2–6). Components that were most commonly reported in interviews with people with dementia and carers were co-ordinating care, working effectively with primary care and timely planning discussions. It was through these activities that other components were often addressed, for example recognising EOL and providing supportive care, and managing hospitalisation. No additional components were identified.
Carers valued the continuity of care provided by their DNS, which was in marked contrast to their experience of other services, where they sometimes needed to recount events multiple times. Continuity was also identified as important by other health-care professionals:
Well you don’t have to start from scratch. I mean, obviously they’ve got notes, but you feel like they know you. You don’t feel like it’s a stranger and you’re having to say ‘Well my dad doesn’t know this’, because she knows.
Interview, family carer 3014
When there are lots of people involved, for the family, they don’t obviously know which nurse belongs to which service, and that family might have had a crisis and are run ragged and they’ve got different nurses going in, and they think ‘Oh, who’s been in?’. When lots of services are involved, it’s sometimes difficult, and obviously difficult for families as well to interpret who is who.
Interview, district nurse HCP 5, site 3
Some of the barriers to planning ahead identified in our earlier work,96 for example reluctance to talk about the future and a preference to focus on the present, were encountered by the DNSs:
To be honest, I haven’t given it a thought, because I thought ‘just take things as they come’. That’s the only way I think of it. You can’t plan ahead, you just don’t know what’s going to happen. So, you just take one day at a time.
Interview, person with dementia 3035
Both DNSs developed a range of strategies to encourage discussions around planning future care, including providing documentation to allow people to digest information in their own time, exploring planning future care as part of annual dementia reviews, or using changes in circumstances to prompt ‘what if’ discussions:
. . . you have that opening to start on that conversation. For example, they noticed a deterioration so that was the perfect opportunity to say ‘If we don’t get you help and start planning ahead now, you’re not going to be able to look after him at home, and I assume that is your ultimate wish’. ‘Oh, yes’.
Intervention supervision, DNS1
The ability of the DNSs to address future planning was particularly important, as health-care professionals described a lack of responsibility around discussing and documenting future care preferences with people with dementia:
. . . well we don’t really do any [ACP] as part of the memory service because we don’t really have time, but who does in effect? Actually that might be a role of the support.
Interview, consultant old-age psychiatrist HCP 6 site 3
Opportunities for continuing care after death were limited owing to the small number of deaths during the study. Only two contacts with family carers relating to continuing care after death were recorded in the activity logs, suggesting that relatively little attention was paid to this component. Discussion in an intervention supervision meeting highlighted a mismatch between the intention of one DNS to use a structured checklist for post-death review meetings in care homes and the intended focus on a more discursive exploration of the positive aspects of EOLC, identifying areas for improvement, and considering the emotional impact on staff. However, a more reflective approach was subsequently used:
I did one [post-death review] with the lady that died in the home. Similar to DNS2, I highlighted who was the best person to do this. They came up with some interesting stuff, because there were a couple of new staff there, and they’d had quite a few deaths.
Intervention supervision, DNS1
Feasibility and acceptability of individual- and systems-level work
The DNSs were assigned a small caseload to enable tailored proactive working with individuals and to secure time for strategic and systems-level working. The DNSs were experienced in working with individual patients in their previous roles and were confident in identifying the needs of people with dementia and family carers and then providing tailored interventions (see the vignettes in Boxes 2–6). In this aspect of their role, the DNSs worked autonomously, seeking clinical supervision as needed. Most families viewed the frequency and duration of visits as appropriate. The small case load was, however, questioned by some primary care staff:
The only downside, I suppose, for me and the practice is the numbers. Providing that very expensive nurse for a few people isn’t something that we’re used to in primary care . . . We are a large practice, we’ve got a multiskilled workforce, I’ve got a practice pharmacist, I’ve just employed a paramedic, so we’re looking at how we can really skill-mix across the practice and [DNS1] fitted in with that. If it was a full time role, we’d have to look at how that worked with the numbers.
Interview, practice manager 1.1, site 1
At a systems level, the role offered freedom to work autonomously and proactively, and to make changes to benefit service users more broadly. Despite some anxieties about working at this more strategic level, both DNSs grew in confidence and successfully developed a range of systems primarily, although not exclusively, in the general practices and care homes. Working at a more strategic level required support through intervention supervision, but the DNSs enjoyed the chance to make changes that would have a wider impact on EOLC for people with dementia. A key example of systems-level work was the development of a template for the annual dementia reviews. This work was encouraged by the dementia lead GP in one of the practices:
So when [DNS1] and I sat down when she started her job, in terms of systems within the practice, what we looked at were ways that we could improve the quality of the dementia reviews, to ensure that they were done to a high standard, but also done consistently throughout the practice.
Interview, GP 1.1, site 1
Qualifications and experience needed for the dementia nurse specialist role
Some professionals considered that the knowledge and skills required to fulfil the role effectively were facilitated by a nursing background. The medical knowledge of the DNSs was particularly valued by carers and care home staff. Some carers described how both they and the person with dementia were more likely to accept help and support when it was framed medically and advice was given from a professional:
I think it’s quite good that she has got the nursing background because when we were talking about him having problems with his incontinence . . . he would go ‘There is nothing wrong’. I said ‘Well actually, [DNS2] is a nurse’. I think when I said ‘She’s actually from the doctors. She’s medical’, then he’ll go ‘Oh.’. I think he feels a bit better then.
Interview, family carer 3014
Others considered professional background less important, but emphasised the need for knowledge and skills in managing the physical and psychological needs of people with dementia and carers. Other personal attributes, such as inspiring confidence, being reliable, being tenacious, being approachable and putting people with dementia and their families at ease, were also emphasised:
I think [DNS1] is such a professional, very knowledgeable. She just makes you feel at ease. She’s just really experienced.
Interview, care home manager CH 13, site 1
Carers commonly regarded the DNS as having expertise to meet both their own needs and those of the person with dementia. They were confident that the DNS would be able to respond to a range of problems and issues, seeking input from others as necessary and even helping with problems beyond the scope of their remit (e.g. identifying relevant social groups, facilitating parking at the practice when attending for appointments).
Fit with existing models of care
The majority of professionals and carers considered the DNS role as complementary to existing services, and valued their inclusion as part of the multidisciplinary care team. When a degree of overlap with existing services was identified, this was generally considered beneficial rather than problematic:
. . . I think, undoubtedly, there’s overlap with our roles, but very much, they’re complementary. We find that throughout palliative care, that there’s quite a lot of blurring of roles, but it is about teamwork.
Interview, Macmillan Nurse HCP 5, site 1
The role perceived as having the most in common with the DNS was Admiral nursing. However, a key difference was the integration of the DNS in general practice, which, as already described, had a number of benefits for all stakeholders. Furthermore, Admiral nurses are not universally available to people with dementia and carers, and they were perceived by some participants as being primarily focused on carers, with less attention to the needs of people with dementia. Carers and professionals acknowledged a significant gap around post-diagnostic support in dementia, and felt that the DNSs were well placed to address this need and provide ongoing support:
. . . my experience of the GPs basically ‘Here’s the diagnosis and see you.’. That’s it, because there’s no medication and you don’t get offered anything at all, that was it.
Interview, family carer 1058
Tensions over role boundaries were experienced only in relation to a local hospice seeking to expand its services to include people with dementia.
Factors influencing implementation of the SEED intervention
The framework of NPT114 was used to identify factors influencing the implementation of the SEED intervention.
-
Coherence
This considers whether or not the SEED intervention made sense to all stakeholders, was easy to understand and was distinct from existing services. The intervention was not perceived as relevant to some people with dementia and family carers because of the focus on EOLC; this aspect of the intervention was also contested by the DNSs and other stakeholders who were keen to expand the intervention to all people with dementia. The location of the intervention in primary care was valued by nearly all stakeholders. The fact that the SEED intervention was seen as overlapping with existing interventions was nearly always viewed positively, although tensions occasionally arose over role boundaries.
A recurring theme in early intervention supervision and initial interviews with the DNSs was uncertainty over their new role, suggesting a lack of coherence:Interview, DNS1
From the outside looking in, people probably think it’s quite a straightforward role. Often, I feel like a duck that looks quite calm on the outside, but paddling like mad underneath, thinking ‘What on earth am I supposed to be doing?’. I think there’s a lot of self-motivation and discipline required around mapping out what the role is.
Many of these anxieties were resolved over time as they began working with individual people with dementia and carers, established their role in the practice and gained confidence around the seven components.
-
Cognitive participation
Cognitive participation focuses on whether or not stakeholders engaged with, and invested in, the SEED intervention. The temporary nature of the intervention influenced the buy-in of all stakeholders. Relationship-building was key to engaging people with dementia, family carers and care homes. Investing time in getting to know study participants often led to the identification of unmet needs, opportunities to intervene and the potential avoidance of crises. Differing levels of engagement and commitment were observed in the two general practices:Interview, DNS2Interview, DNS1
Well, I think I’ve been unlucky in the fact that there hasn’t been a dementia lead GP with a vested interest in dementia. I think that’s been an obstacle, really, from the start, because none of the GPs have shone out with a great enthusiasm for dementia, and even end of life. I think they are so under pressure just doing their job. But, I think it does need to start with a GP who wants to invest in care of people with dementia.
I had a 2-week induction at the surgery, which was really well planned, so [name], who is the sort of head administrator come everything really, she had organised for me to go out with different people, and that was great.
-
Collective action
Intervention implementation depends on whether or not work is allocated to the right individuals and whether or not they are adequately trained and resourced. The different professional backgrounds of the DNSs were complementary, enabling them to share specialist knowledge and work together effectively:Interview, GP 1.1, site 1
I think the key thing, really, about [DNS1] being in role is the fact that she is a mental health nurse. It’s the fact that she’s got that knowledge and those links that have made it work . . . Because a lot of the focus hasn’t been on the last few days of somebody’s life, it’s not been on that palliative, necessarily, care, it’s been on their future care and planning, and an understanding around dementia diagnosis and things.
Supervision arrangements proved successful in ensuring access to specialist knowledge and support relating to different aspects of their role. Intervention supervision was particularly important because of the initial uncertainties of the DNSs over the scope of the intervention. The relationships that people with dementia and family carers developed with the DNSs were valued and were key to engaging them in ACP:Field notes, site 3
She gave a very emotional account of having discussed it [do not attempt cardiopulmonary resuscitation (DNACPR)] with DNS2, but didn’t feel ready. However, soon after she read an article in the Sunday newspaper (which she found quite harrowing) about the ambulance service ‘forcing’ people to do CPR [cardiopulmonary resuscitation] on their relatives who were clearly already dead (she became tearful when talking about this), and drew on her knowledge as a prior first aider, knowing that performing the procedure could often break bones, and decided that DNACPR was probably right for her mother . . . At a later visit, the wish to complete DNACPR was discussed with DNS2 who signposted to the GP and this was arranged with the GP over the telephone. So it seemed that DNS2 had ‘planted the seed’, and then given the carer space to make her decisions in the time frame appropriate to her.
The extent to which the host general practices supported the intervention varied. This was evident through the level of involvement of the lead dementia GP, but was also demonstrated through the lack of support provided with practice IT systems.
-
Reflexive monitoring
A key aspect of successful implementation is the extent to which stakeholders are aware of the difference made by a new intervention, whether this is through formal or informal means. The ability to reflect on, and adapt, the intervention in the light of this appraisal is also crucial. Although no formal mechanisms for evaluating impacts were in place, informal feedback from study participants, including care home staff and some GPs, was positive:Interview, family carer 1053Interview, GP 1.1, site 1Interview, clinical lead, care home 2, site 1
I mentioned it to [DNS1], I said ‘I’m having a real struggle for them to realise that dad can’t physically get to the surgery any more’. Then within a day, 2 days, the district nurses were there, taking his samples and giving him the blood.
She’s been proactive in picking up medical problems. So she would be speaking to them, and relatives would maybe mention something, and before I know it, she’s coming back to me, saying ‘I wonder whether this person might actually have an underlying physical health problem. We need to do a blood test.’. So that proactive work, I’m sure, has decreased the burden on secondary care.
She’s also helped to implement emergency health-care plans and DNRs [do not resuscitate], which we previously didn’t do, which has been a big help, because the GPs have been understaffed and struggling, and we haven’t been able to get them, basically, since the community matron left post.
Although such comments were not necessarily directly fed back to the DNSs, the benefits identified often led to ongoing work with individual patient–carer dyads and requests from care homes for further input confirming the value of the DNS input. Access to different perspectives through supervision also facilitated reflection on the intervention.
Overall, the individual and collective work required for successful implementation of a new intervention was largely achieved. Although investment in the intervention was relatively poor in one GP practice, and other stakeholders were, understandably, reluctant to engage with a short-term intervention, the interpersonal skills of the DNSs and having time to develop relationships enabled them to build engagement throughout the trial.
Feasibility and acceptability of outcome measures
Data completion of outcome measures
Analysis of outcome measures focused initially on missing data. Imputed scores for partially completed responses made little difference to overall attrition rates, with the exception of the SWC-EOLD questionnaire, for which there were predominantly partial responses. As there was little evidence that completion rates varied between intervention arms, summary figures provide an overview of baseline data, and tables present the full data for intervention and control arms at each time point.
Outcome measures relating to symptoms and well-being of people with dementia and family carers
Completion rates for these outcome measures were generally good (Figure 10, Tables 30–33). Several family carers were unable to complete the Quality of Life in Late-stage Dementia (QUALID) because they had not spent the required time with the person with dementia in the previous week. Data on the PAINAD during movement were missing for the significant number of people with dementia who remained seated throughout the assessment.
FIGURE 10.
Data completion of outcome measures relating to symptoms and well-being of people with dementia and family carers at baseline. a, Unavailable includes those who were not approached or were unwell. BANS-S, Bedford Alzheimer Nursing Severity Scale; HADS, Hospital Anxiety and Depression Scale; KI, key informant; NPI-NH, Neuropsychiatric Inventory-Nursing Home; QUALID, Quality of Life in Late-stage Dementia.
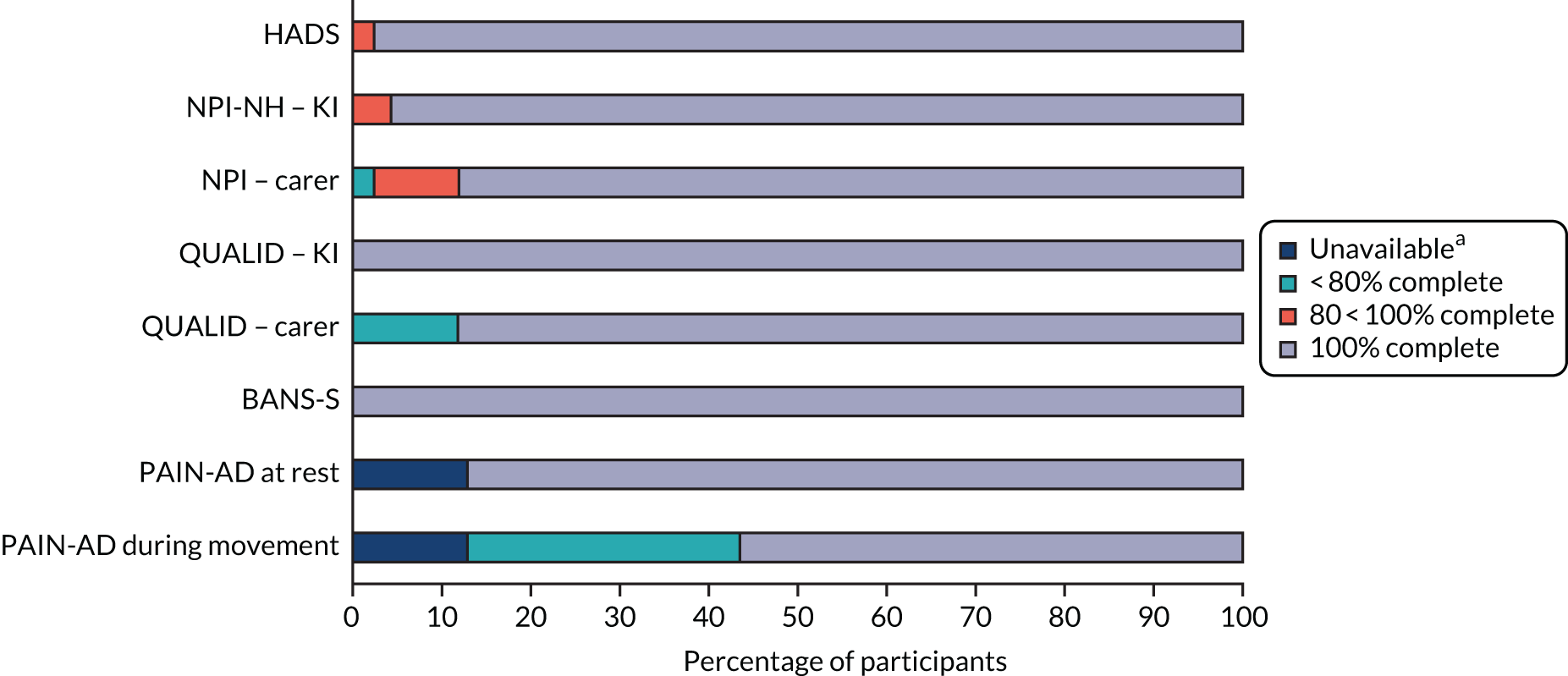
| Questionnaire | Baseline, n (%) | 4 months, n (%) | ||||
|---|---|---|---|---|---|---|
| Missinga | Partialb | Completec | Missinga | Partialb | Completec | |
| Questionnaires completed by family carers (n = 25) | (n = 23)d | |||||
| HADS | ||||||
| Anxiety | 0 (0) | 0 (0) | 25 (100) | 1 (4) | 0 (0) | 22 (96) |
| Depression | 0 (0) | 0 (0) | 25 (100) | 1 (4) | 0 (0) | 22 (96) |
| Overall score | 0 (0) | 0 (0) | 25 (100) | 1 (4) | 0 (0) | 22 (96) |
| NPI | 0 (0) | 4 (16) | 21 (84) | 1 (4) | 3 (13) | 19 (83) |
| QUALID | 3 (12) | 0 (0) | 22 (88) | 3 (13) | 0 (0) | 20 (87) |
| SM-EOLD | 1 (4) | 1 (4) | 23 (92) | 2 (9) | 2 (9) | 19 (83) |
| SWC-EOLD | 3 (12) | 10 (40) | 12 (48) | 1 (4) | 14 (61) | 8 (35) |
| Questionnaires completed by key informants (n = 15) | (n = 11)d | |||||
| NPI-NH | 0 (0) | 1 (7) | 14 (93) | 2 (18) | 1 (9) | 8 (72) |
| BANS-S | 0 (0) | 0 (0) | 15 (100) | 0 (0) | 0 (0) | 11 (100) |
| QUALID | 0 (0) | 0 (0) | 15 (100) | 1 (9) | 1 (9) | 9 (81) |
| SM-EOLD | 0 (0) | 0 (0) | 15 (100) | 0 () | 0 (0) | 11 (100) |
| SWC-EOLD | 1 (7) | 7 (47) | 7 (47) | 1 (9) | 9 (81) | 1 (9) |
| Questionnaires completed by both key informant and family carere (n = 37) | (n = 30)d | |||||
| QUALID | 3 (8) | 0 (0) | 34 (92) | 2 (7) | 1 (3) | 27 (90) |
| SM-EOLD | 2 (5) | 1 (3) | 34 (92) | 2 (7) | 0 (0) | 28 (93) |
| SWC-EOLD | 5 (14) | 17 (46) | 15 (41) | 2 (7) | 19 (63) | 9 (32) |
| Questionnaire completed by research team (n = 37) | (n = 32)d | |||||
| PAINAD | ||||||
| At rest | 6 (16) | 0 (0) | 31 (84) | 7 (22) | 0 (0) | 25 (78) |
| During movement | 18 (49) | 0 (0) | 19 (51) | 18 (56) | 0 (0) | 14 (44) |
| Questionnaire | Baseline, n (%) | 4 months, n (%) | ||||
|---|---|---|---|---|---|---|
| Missinga | Partialb | Completec | Missinga | Partialb | Completec | |
| Questionnaires completed by family carers (n = 17) | (n = 17) | |||||
| HADS | ||||||
| Anxiety | 0 (0) | 0 (0) | 17 (100) | 1 (6) | 0 (0) | 16 (94) |
| Depression | 0 (0) | 1 (6) | 16 (94) | 1 (6) | 0 (0) | 16 (94) |
| Overall score | 0 (0) | 1 (6) | 16 (94) | 1 (6) | 0 (0) | 16 (94) |
| NPI | 1 (6) | 0 (0) | 16 (94) | 1 (6) | 1 (6) | 15 (88) |
| QUALID | 2 (12) | 0 (0) | 15 (88) | 3 (18) | 0 (0) | 14 (82) |
| SM-EOLD | 0 (0) | 2 (12) | 15 (88) | 1 (6) | 0 (0) | 16 (94) |
| SWC-EOLD | 5 (29) | 10 (59) | 2 (12) | 3 (18) | 8 (47) | 6 (35) |
| Questionnaires completed by key informants (n = 8) | (n = 7)d | |||||
| NPI-NH | 0 (0) | 0 (0) | 8 (100) | 0 (0) | 0 (0) | 7 (100) |
| BANS-S | 0 (0) | 0 (0) | 8 (100) | 0 (0) | 0 (0) | 7 (100) |
| QUALID – key informant | 0 (0) | 0 (0) | 8 (100) | 2 (29) | 0 (0) | 5 (71) |
| SM-EOLD – key informant | 0 (0) | 0 (0) | 8 (100) | 0 (0) | 0 (0) | 7 (100) |
| SWC-EOLD – key informant | 1 (12) | 5 (63) | 2 (25) | 1 (14) | 4 (57) | 2 (29) |
| Questionnaires completed by both key informant and family carere (n = 25) | (n = 23)d | |||||
| QUALID | 2 (8) | 0 (0) | 23 (92) | 4 (17) | 0 (0) | 19 (83) |
| SM-EOLD | 1 (4) | 2 (8) | 22 (88) | 1 (4) | 0 (0) | 22 (96) |
| SWC-EOLD | 7 (28) | 14 (56) | 4 (16) | 4 (17) | 11 (48) | 8 (35) |
| Questionnaire completed by research team (n = 25) | (n = 24)d | |||||
| PAINAD | ||||||
| At rest | 2 (8) | 0 (0) | 23 (92) | 3 (12) | 0 (0) | 21 (88) |
| During movement | 9 (36) | 0 (0) | 16 (64) | 12 (50) | 0 (0) | 12 (50) |
| Questionnaire | 8 months, n (%) | 12 months, n (%) | ||||
|---|---|---|---|---|---|---|
| Missinga | Partialb | Completec | Missinga | Partialb | Completec | |
| Questionnaires completed by family carers (n = 22)d | (n = 20)e | |||||
| HADS | ||||||
| Anxiety | 0 (0) | 0 (0) | 22 (88) | 0 (0) | 0 (0) | 20 (100) |
| Depression | 0 (0) | 0 (0) | 22 (88) | 0 (0) | 0 (0) | 20 (100) |
| Overall score | 0 (0) | 0 (0) | 22 (88) | 0 (0) | 0 (0) | 20 (100) |
| NPI | 0 (0) | 5 (23) | 17 (77) | 0 (0) | 5 (25) | 15 (75) |
| QUALID | 3 (14) | 0 (0) | 19 (86) | 5 (25) | 0 (0) | 15 (75) |
| SM-EOLD | 1 (5) | 0 (0) | 21 (95) | 1 (5) | 0 (0) | 19 (95) |
| SWC-EOLD | 1 (5) | 13 (59) | 8 (36) | 0 (0) | 10 (50) | 10 (50) |
| Questionnaires completed by key informants (n = 13)d | (n = 12)e | |||||
| NPI-NH | 0 (0) | 1 (8) | 12 (92) | 0 (0) | 1 (8) | 11 (92) |
| BANS-S | 0 (0) | 1 (8) | 12 (92) | 0 (0) | 1 (8) | 11 (92) |
| QUALID | 1 (8) | 0 (0) | 12 (92) | 1 (8) | 0 (0) | 11 (92) |
| SM-EOLD | 0 (0) | 0 (0) | 13 (100) | 0 (0) | 0 (0) | 12 (100) |
| SWC-EOLD | 0 (0) | 8 (62) | 5 (38) | 2 (17) | 5 (42) | 5 (42) |
| Questionnaires completed by both key informant and family carerf (n = 30)d | (n = 29)e | |||||
| QUALID | 2 (7) | 0 (0) | 28 (93) | 5 (17) | 0 (0) | 24 (83) |
| SM-EOLD | 0 (0) | 0 (0) | 30 (100) | 1 (3) | 0 (0) | 28 (97) |
| SWC-EOLD | 1 (3) | 19 (63) | 10 (33) | 2 (7) | 12 (41) | 15 (52) |
| Questionnaire completed by research team (n = 30)d | (n = 29)e | |||||
| PAINAD | ||||||
| At rest | 5 (17) | 0 (0) | 25 (83) | 3 (10) | 0 (0) | 26 (90) |
| During movement | 14 (47) | 0 (0) | 16 (53) | 15 (52) | 0 (0) | 14 (48) |
| Questionnaire | 8 months, n (%) | 12 months, n (%) | ||||
|---|---|---|---|---|---|---|
| Missinga | Partialb | Completec | Missinga | Partialb | Completec | |
| Questionnaires completed by family carers (n = 14)d | (n = 12)e | |||||
| HADS | ||||||
| Anxiety | 1 (7) | 0 (0) | 13 (93) | 0 (0) | 0 (0) | 12 (100) |
| Depression | 1 (7) | 0 (0) | 13 (93) | 0 (0) | 0 (0) | 12 (100) |
| Overall score | 1 (7) | 0 (0) | 13 (93) | 0 (0) | 0 (0) | 12 (100) |
| NPI | 1 (7) | 2 (14) | 11 (79) | 0 (0) | 1 (8) | 11 (92) |
| QUALID | 3 (21) | 0 (0) | 11 (79) | 2 (17) | 0 (0) | 10 (83) |
| SM-EOLD | 1 (7) | 0 (0) | 13 (93) | 0 (0) | 0 (0) | 12 (100) |
| SWC-EOLD | 5 (36) | 4 (29) | 5 (36) | 3 (25) | 4 (33) | 5 (42) |
| Questionnaires completed by key informants (n = 5)d | (n = 5)e | |||||
| NPI-NH | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 1 (20) | 4 (80) |
| BANS-S | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | 5 (100) |
| QUALID | 1 (20) | 0 (0) | 4 (80) | 1 (20) | 0 (0) | 4 (80) |
| SM-EOLD | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | 5 (100) |
| SWC-EOLD | 1 (20) | 2 (40) | 2 (40) | 0 (0) | 3 (60) | 2 (40) |
| Questionnaires completed by both key informant and family carerf (n = 17)d | (n = 16)e | |||||
| QUALID | 2 (12) | 0 (0) | 15 (88) | 2 (13) | 0 (0) | 14 (88) |
| SM-EOLD | 0 (0) | 0 (0) | 17 (100) | 0 (0) | 0 (0) | 16 (100) |
| SWC-EOLD | 5 (29) | 6 (35) | 6 (35) | 3 (19) | 6 (38) | 7 (44) |
| Questionnaire completed by research team (n = 19)d | (n = 17)e | |||||
| PAINAD | ||||||
| At rest | 3 (16) | 0 (0) | 16 (84) | 1 (6) | 0 (0) | 16 (94) |
| During movement | 6 (32) | 0 (0) | 13 (68) | 7 (41) | 0 (0) | 10 (59) |
Outcome measures relating to end-of-life care
Although completion rates for the SM-EOLD were good, completion rates were poor for the SWC-EOLD (Figure 11) (see Tables 30–33). Given the high numbers of missing data on the SWC-EOLD, completion rates for individual items were examined (Table 34). This indicated that responses to the following two items were particularly low:
FIGURE 11.
Data completion of outcome measures relating to EOLC at baseline. KI, key informant.
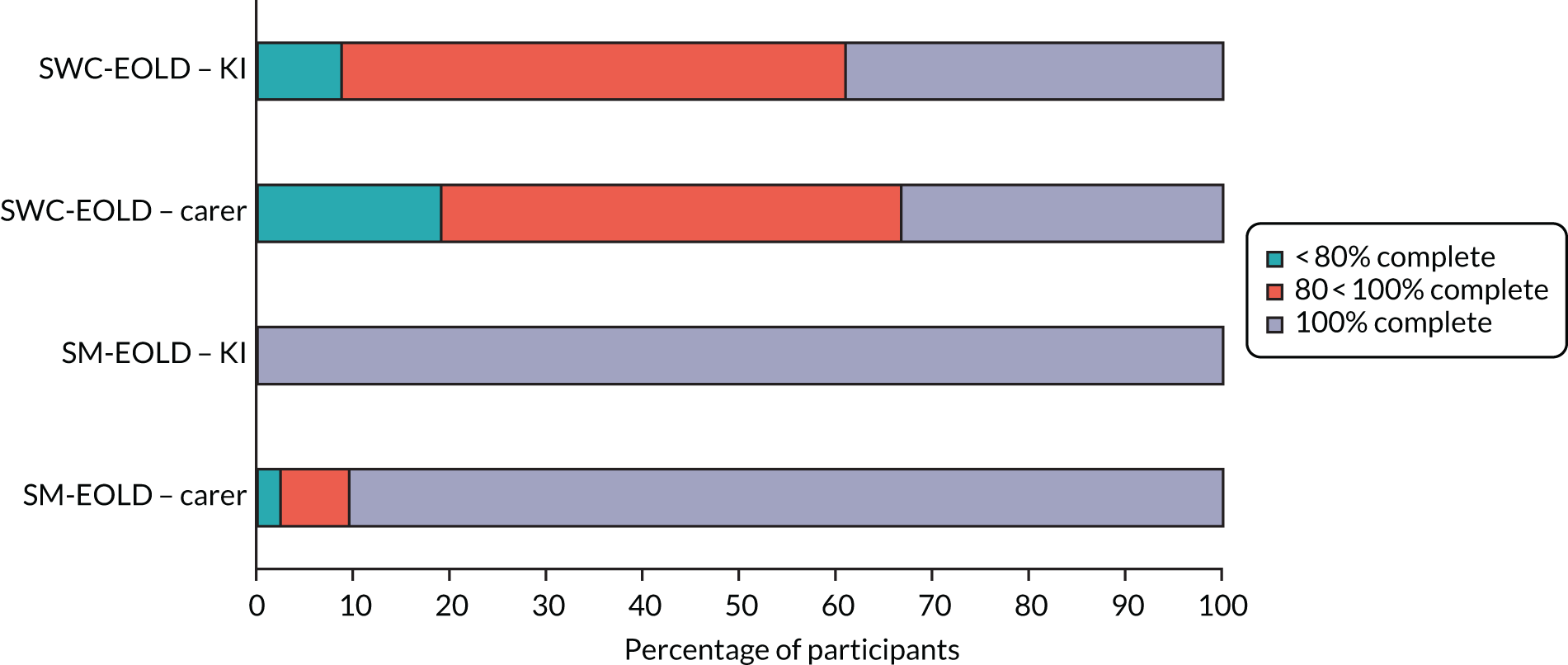
-
The health-care team are sensitive to my needs and feelings (Q4).
-
I feel that my relative needs better medical care at the end of his or her life (Q10).
| SWC-EOLD question | Intervention arm, n (%) | Control arm, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (N = 37) | 4 months (N = 30) | 8 months (N = 30) | 12 months (N = 29) | Baseline (N = 25) | 4 months (N = 23) | 8 months (N = 17) | 12 months (N = 16) | |
| Nursing assistance (Q1) | 35 (95) | 25 (83) | 29 (97) | 27 (93) | 17 (68) | 18 (78) | 11 (65) | 13 (81) |
| Treatments (Q2) | 35 (95) | 29 (97) | 29 (97) | 27 (93) | 21 (84) | 21 (91) | 16 (94) | 14 (88) |
| Comfortable (Q3) | 34 (92) | 28 (93) | 30 (100) | 28 (97) | 21 (84) | 20 (87) | 16 (94) | 16 (100) |
| Sensitive (Q4) | 26 (70) | 20 (67) | 22 (73) | 19 (66) | 15 (60) | 16 (70) | 12 (71) | 14 (88) |
| Decision-making (Q5) | 32 (86) | 28 (93) | 26 (87) | 26 (90) | 23 (92) | 19 (83) | 16 (94) | 15 (94) |
| Not understand (Q6) | 34 (92) | 27 (90) | 30 (100) | 28 (97) | 23 (92) | 21 (91) | 16 (94) | 16 (100) |
| Different (Q7) | 33 (89) | 28 (93) | 29 (97) | 27 (93) | 22 (88) | 22 (96) | 14 (82) | 16 (100) |
| Doctor/nurse (Q8) | 33 (89) | 29 (97) | 30 (100) | 27 (93) | 22 (88) | 22 (96) | 17 (100) | 16 (100) |
| Medication (Q9) | 34 (92) | 29 (97) | 30 (100) | 27 (93) | 22 (88) | 21 (91) | 15 (88) | 14 (88) |
| Medical care (Q10) | 25 (68) | 19 (63) | 21 (70) | 20 (69) | 7 (28) | 15 (65) | 10 (43) | 7 (44) |
Further exploration of the pattern of missing data for family carers and key informants is warranted as it may be that questions are not equally relevant to both; this is certainly suggested by the qualitative data on the SWC-EOLD (see Qualitative data on outcome measures). The SWC-EOLD and CAD-EOLD were also administered to family carers and key informants at the post-death interviews (Table 35). All participating family carers completed all items on both measures (SWC-EOLD and CAD-EOLD). Key informants completed the CAD-EOLD with no missing data but, consistent with the results in Figure 11, only half provided complete data on the SWC-EOLD. The reasons for better completion by family carers at the post-death interview are unclear, but better completion at the post-death interview suggests that the items may be more relevant to family carers when the person with dementia is approaching the EOL. Table 35 shows the completion of the questionnaires relating to EOL by family carers and key informants of people with dementia who died, by intervention arm.
| Questionnaire | Intervention arm, n (%) (N = 5) | Control arm, n (%) (N = 7) | ||||
|---|---|---|---|---|---|---|
| Missinga | Partialb | Completec | Missinga | Partialb | Completec | |
| Questionnaires completed by family carers (n = 6) | ||||||
| HADS | ||||||
| Anxiety | 0 (0) | 0 (0) | 2 (100) | 1 (25) | 0 (0) | 3 (75) |
| Depression | 0 (0) | 0 (0) | 2 (100) | 1 (25) | 0 (0) | 3 (75) |
| Overall score | 0 (0) | 0 (0) | 2 (100) | 1 (25) | 0 (0) | 3 (75) |
| CAD-EOLD | 0 (0) | 0 (0) | 2 (100) | 1 (25) | 0 (0) | 3 (75) |
| SWC-EOLD | 0 (0) | 0 (0) | 2 (100) | 1 (25) | 0 (0) | 3 (75) |
| Questionnaires completed by key informants (n = 6) | ||||||
| CAD-EOLD | 0 (0) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 3 (100) |
| SWC-EOLD | 0 (0) | 2 (67) | 1 (33) | 0 (0) | 1 (33) | 2 (67) |
| Questionnaires completed by both key informants and family carersd (n = 12) | ||||||
| SWC-EOLD | 0 (0) | 2 (40) | 3 (60) | 1 (14) | 1 (14) | 5 (72) |
| CAD-EOLD | 0 (0) | 0 (0) | 5 (100) | 1 (14) | 0 (0) | 6 (86) |
Feasibility of collecting data on health-related quality of life and resource use
Completion rates for the EQ-5D-5L varied by type of respondent. Only baseline data are shown in Figure 12 for clarity; full details by time point and intervention arm are provided in Table 36. The results show that, although there were few missing data for the family carer (when rating their own QoL) or for proxy respondents, there were substantial missing data for people with dementia, only 57% of whom were able to complete all items at baseline (see Figure 12). Some people with dementia were not approached at the request of their family carer or key informant and, therefore, did not have the opportunity to try to complete the questionnaire; these are classed as ‘missing not at random’. However, the majority of missing data were missing because of the inability of people with dementia to respond to the questions (from a statistical perspective, this might be classed as ‘missing at random’). Distinguishing between different types of missing data is important because it dictates the approaches that might be used to impute missing data in a full analysis.
FIGURE 12.
Data completion of EQ-5D-5L at baseline by respondent. a, Unavailable includes those who were not approached or were unwell.
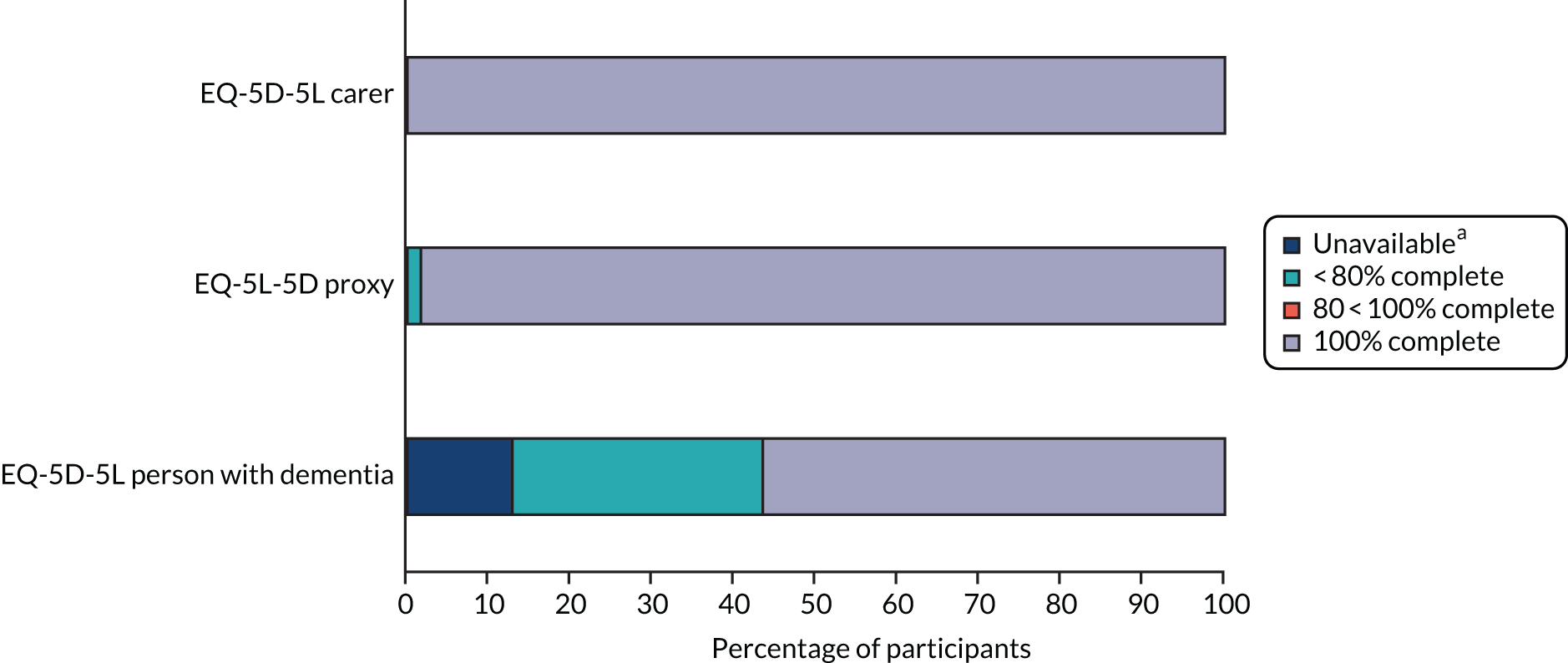
| Respondent | Time point (n) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 months | 8 months | 12 months | Follow-up after death | ||||||||||||||||
| Dead/witha | Missingb (too illc) | Partiald | Completee | Dead/witha | Missingb (too illc) | Partiald | Completee | Dead/witha | Missingb (too illc) | Partiald | Completee | Dead/witha | Missingb (too illc) | Partiald | Completee | Dead/witha | Missingb (too illc) | Partiald | Completee | |
| Intervention arm | ||||||||||||||||||||
| Carer | 0 | 0 (0) | 0 | 25 | 0 | 1 (0) | 0 | 22 | 0 | 0 (0) | 0 | 22 | 0 | 0 (0) | 0 | 20 | 0 | 0 (0) | 0 | 2 |
| Proxy | 0 | 2 (0) | 0 | 35 | 5 | 3 (0) | 0 | 29 | 2 | 0 (0) | 1 | 29 | 1 | 2 (0) | 0 | 27 | 0 | 0 (0) | 0 | 0 |
| Person with dementia | 0 | 20 (14) | 0 | 17 | 5 | 14 (8) | 3 | 15 | 2 | 12 (7) | 0 | 18 | 1 | 14 (9) | 2 | 13 | N/A | N/A | N/A | N/A |
| Control arm | ||||||||||||||||||||
| Carer | 0 | 0 (0) | 0 | 17 | 0 | 1 (0) | 0 | 16 | 0 | 1 (0) | 0 | 13 | 0 | 0 (0) | 0 | 12 | 0 | 0 (0) | 0 | 3 |
| Proxy | 0 | 1 (0) | 0 | 24 | 1 | 2 (0) | 0 | 22 | 5 | 2 (0) | 1 | 16 | 2 | 1 (0) | 0 | 16 | 0 | 0 (0) | 0 | 0 |
| PWD | 0 | 7 (6) | 0 | 18 | 1 | 8 (6) | 0 | 16 | 5 | 5 (2) | 1 | 13 | 2 | 4 (3) | 1 | 12 | N/A | N/A | N/A | N/A |
Completion rates for the resource use questionnaire relating to the person with dementia at 4, 8 and 12 months and after death are provided in Table 37. The majority of the questions relating to the person with dementia were answered, with two exceptions: weekly charge of the care home (if applicable) and the total income of the person with dementia. We also piloted the resource use questionnaire with five family carers regarding their own use of services; this pilot suggested that the questions were acceptable and feasible for respondents, although researchers commented on the additional burden.
| Resource | Time point, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 4 months | 8 months | 12 months | After deathb | |||||
| Intervention (N = 34) | Control (N = 23) | Intervention (N = 35) | Control (N = 18) | Intervention (N = 32) | Control (N = 17) | Intervention (N = 5) | Control (N = 6) | |
| Patient’s living accommodation | 32 (94) | 23 (100) | 33 (94) | 18 (100) | 32 (100) | 17 (100) | 4 (80) | 6 (100) |
| Usual living accommodation | 32 (94) | 22 (96) | 33 (94) | 18 (100) | 32 (100) | 17 (100) | 4 (80) | 6 (100) |
| Temporary accommodation (previous 3 months) | 32 (94) | 23 (100) | 34 (97) | 18 (100) | 32 (100) | 16 (94) | 4 (80) | 6 (100) |
| Organisation managing care facility (if applicable)c | 11 (73) | 2 (66) | 12 (80) | 2 (100) | 14 (100) | 1 (100) | 2 (66) | 3 (100) |
| Patient’s weekly charge (if applicable)c | 3 (20) | 2 (66) | 4 (27) | 0 (0) | 4 (29) | 0 (0) | 0 (0) | 1 (33) |
| Inpatient health services used | 32 (94) | 23 (100) | 34 (97) | 18 (100) | 32 (100) | 16 (94) | 4 (80) | 6 (100) |
| Outpatient health services used | 32 (94) | 23 (100) | 34 (97) | 18 (100) | 31 (97) | 15 (88) | 4 (80) | 6 (100) |
| Day activity services used | 32 (94) | 23 (100) | 34 (97) | 18 (100) | 31 (97) | 17 (100) | 4 (80) | 6 (100) |
| Community care services used | 31 (91) | 23 (100) | 31 (89) | 18 (100) | 29 (91) | 15 (88) | 4 (80) | 5 (83) |
| Patient’s main employment status | 33 (97) | 23 (100) | 34 (97) | 18 (100) | 32 (100) | 17 (100) | 4 (80) | 6 (100) |
| Carer asked about patient’s income | 33 (97) | 23 (100) | 34 (97) | 18 (100) | 32 (100) | 17 (100) | 4 (80) | 6 (100) |
| Main source of patient’s income (if applicable)c | 15 (100) | 8 (100) | 12 (80) | 6 (86) | 14 (100) | 4 (100) | N/Ad | 1 (100) |
| Type of benefit patient receives (if applicable)c | 5 (100) | 3 (100) | 6 (100) | 6 (100) | 7 (100) | 4 (100) | N/Ad | N/Ad |
| Patient’s total income (if applicable)c | 10 (67) | 8 (100) | 12 (80) | 6 (86) | 12 (92) | 4 (100) | N/Ad | 0 (0) |
Capturing data on advance care planning
Details of the proportions of people with dementia for whom a range of planning documents were filed in either GP or care home records are summarised in Figure 13 and provided in detail in Table 38.
FIGURE 13.
Percentage of GP and/or care home records containing information on advance care plans by intervention arm and time point. EHCP, Emergency Healthcare Plan.
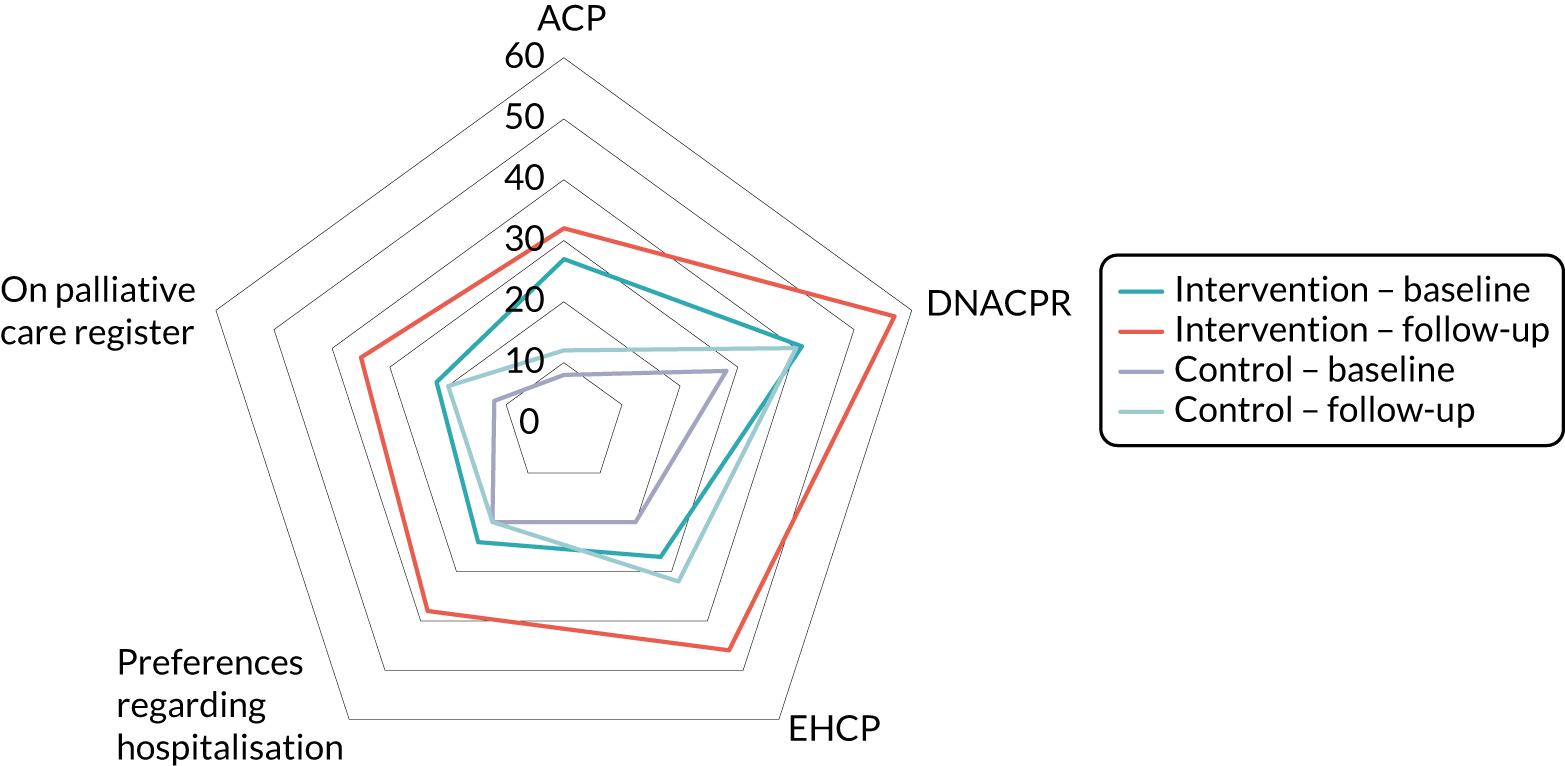
| Outcome | Baseline, n (%) | 12 months or after death, n (%) | ||
|---|---|---|---|---|
| Intervention (N = 37) | Control (N = 25) | Intervention (N = 37) | Control (N = 25) | |
| Advance care plan | ||||
| No | 27 (73) | 23 (92) | 22 (60) | 21 (84) |
| Yes | 10 (27) | 2 (8) | 12 (32) | 3 (12) |
| Missing | 0 (0) | 0 (0) | 3 (8) | 1 (4) |
| DNACPR | ||||
| No | 22 (59) | 18 (72) | 13 (35) | 14 (56) |
| Yes | 15 (41) | 7 (28) | 21 (57) | 10 (40) |
| Missing | 0 (0) | 0 (0) | 3 (8) | 1 (4) |
| Emergency Healthcare Plan | ||||
| No | 27 (73) | 20 (80) | 17 (46) | 16 (64) |
| Yes | 10 (27) | 5 (20) | 17 (46) | 8 (32) |
| Missing | 0 (0) | 0 (0) | 3 (8) | 1 (4) |
| Views on hospitalisation | ||||
| No | 28 (76) | 20 (80) | 20 (54) | 19 (76) |
| Yes | 9 (24) | 5 (20) | 14 (38) | 5 (20) |
| Missing | 0 (0) | 0 (0) | 3 (8) | 1 (4) |
| On palliative care register | ||||
| No | 29 (78) | 22 (88) | 21 (57) | 19 (76) |
| Yes | 8 (22) | 3 (12) | 13 (35) | 5 (20) |
| Missing | 0 (0) | 0 (0) | 3 (8) | 1 (4) |
With the exception of DNACPRs, documents relating to future care were generally available in either care home or GP records for fewer than half of the study participants (see Table 38). DNACPR was the most commonly documented aspect of ACP: it was recorded for 57% of people with dementia in the intervention arm at the 12-month follow-up or after death (see Table 38). Advance care plans were least likely to be documented at all time points. There was a tendency for all types of documents to increase between baseline and 12 months (or after death) in both trial arms (see Figure 13).
Further investigation of responses indicated wide variation in documentation recorded for patients registered with different practices: those registered with practice 4 had very low rates of completion of the various ACP documents, particularly at baseline (Table 39). This suggests that the percentage change in documentation within practices may be a more appropriate way of analysing data. However, numbers are generally sparse, so it is difficult to make any firm inferences.
| Documentation | Time point | Practice 1 (n = 18)a,b | Practice 2 (n = 11)a,b | Practice 3 (n = 19)a (n = 16)b | Practice 4 (n = 14)a (n = 13)b | All (n = 62)a (n = 58)b |
|---|---|---|---|---|---|---|
| ACP, n (%) | Baseline | 2 (11) | 2 (18) | 8 (42) | 0 (0) | 12 (19) |
| Follow-up | 5 (28) | 4 (36) | 9 (56) | 5 (39) | 23 (40) | |
| DNACPR, n (%) | Baseline | 7 (39) | 5 (46) | 8 (42) | 2 (14) | 22 (36) |
| Follow-up | 10 (56) | 6 (55) | 9 (56) | 0 (0) | 25 (43) | |
| EHCP, n (%) | Baseline | 2 (11) | 5 (46) | 8 (42) | 0 (0) | 14 (24) |
| Follow-up | 8 (44) | 5 (46) | 10 (63) | 3 (23) | 26 (45) | |
| Hospitalisation, n (%) | Baseline | 1 (6) | 5 (46) | 8 (42) | 0 (0) | 14 (23) |
| Follow-up | 6 (33) | 4 (36) | 8 (50) | 2 (15) | 20 (35) |
Outcome measure scores
Questionnaire scores were tabulated by intervention arm and time point. To investigate the shape of the distributions, the mean and standard deviation, as well as median, interquartile range and range, in each arm were examined (Tables 40–47). There were no notable differences in any of the questionnaire scores between the arms at the 8- and 12-month follow-ups, for which numbers were small (especially in questionnaires completed by key informants only). For the purpose of this pilot trial, the actual scores at these time points are of limited interest because the primary purpose was to assess attrition. The findings are not described in detail, although attention is drawn to the skewed responses noted on the PAINAD (see Tables 46 and 47), on which people with dementia were usually assessed as being in no pain, with only an occasional non-zero score. This raises the issue as to who is the most appropriate person to complete this questionnaire; it was generally accepted that a family carer or key informant may be better placed to complete it than a member of the research team, owing to their better knowledge of the person with dementia and opportunity to spend more time with him/her.
| Questionnaire | Intervention arm | Control arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 25) | 4 months (n = 23) | Baseline (n = 17) | 4 months (n = 17) | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| HADS | ||||||||||||
| Anxiety | 5.4 (4.4) | 4 (2–9) | 0–15 | 5.5 (4.1) | 5 (3–7) | 0–17 | 5.4 (3.3) | 5 (3–7) | 0–11 | 5.5 (3.8) | 5 (2–7.5) | 1–15 |
| Depression | 4.5 (3.3) | 3 (2–7) | 0–11 | 4.1 (3.3) | 3.5 (2–5) | 0–13 | 4.4 (3.6) | 4 (1–6) | 0–11 | 5.3 (4.0) | 5.5 (2–8) | 0–15 |
| Overall score | 9.8 (7.6) | 7 (3–17) | 0–23 | 9.5 (7.2) | 8 (5–13) | 0–30 | 9.8 (6.6) | 9 (4–14) | 0–22 | 10.8 (7.4) | 10.5 (4–14.5) | 2–27 |
| NPI | ||||||||||||
| Overall score | 22.4 (19.9) | 20 (9–30) | 1–90 | 19.0 (15.5) | 19.5 (5–28) | 0–59 | 22.0 (25.5) | 15.5 (3–27.5) | 0–99 | 26.1 (30.0) | 18.5 (9–35.5) | 0–126 |
| Distress domain | 10.4 (7.5) | 10 (5–15) | 0–31 | 8.3 (7.5) | 7 (2–12) | 0–24 | 9.4 (11.5) | 6.5 (1–12) | 0–44 | 10.1 (14.5) | 4.5 (2.5–10.5) | 0–55 |
| Questionnaire | Intervention arm | Control arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 months (n = 22) | 12 months (n = 20) | 8 months (n = 14) | 12 months (n = 12) | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| HADS | ||||||||||||
| Anxiety | 4.8 (4.4) | 3.5 (2–8) | 0–18 | 6.7 (4.9) | 6 (3–9) | 0–17 | 7.5 (4.1) | 8 (4–10) | 2–14 | 6.0 (3.7) | 5.5 (2–10) | 1–11 |
| Depression | 4.7 (3.2) | 4 (3–7) | 0–12 | 6.0 (3.6) | 5.5 (3–8.5) | 0–13 | 7.4 (3.6) | 7 (6–10) | 2–13 | 5.4 (2.8) | 4.5 (3–7.5) | 2–10 |
| Overall score | 9.5 (7.3) | 8 (3–14) | 1–30 | 12.6 (8.2) | 13 (6–16) | 1–29 | 14.8 (7.6) | 14 (9–20) | 4–26 | 11.4 (6.3) | 10.5 (5–18) | 4–20 |
| NPI | ||||||||||||
| Overall score | 22.5 (20.2) | 21.5 (5–32) | 0–88 | 22.6 (15.0) | 22.5 (12.5–36) | 0–49 | 29.8 (29.3) | 26 (8–34) | 1–112 | 27.4 (25.6) | 16.5 (10–41.5) | 4–80 |
| Distress domain | 9.7 (10.0) | 7 (2–15) | 0–35 | 10.0 (8.9) | 6 (4–14.5) | 0–32 | 9.9 (11.0) | 7 (4–11) | 0–42 | 9.3 (11.5) | 5 (0–17) | 0–38 |
| Questionnaire | Intervention arm | Control arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 15) | 4 months (n = 11) | Baseline (n = 8) | 4 months (n = 7) | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| BANS-S | 15.9 (3.7) | 15 (13–19) | 10–22 | 16.2 (4.3) | 17 (13–18) | 9–23 | 13.1 (3.8) | 13.5 (10–15.5) | 8–19 | 13.9 (4.5) | 14 (11–16) | 8–22 |
| NPI | ||||||||||||
| Overall score | 28.5 (19.4) | 21 (12–48) | 4–62 | 29.9 (17.1) | 30 (20–42) | 2–52 | 24.6 (22.6) | 22.5 (5–38.5) | 0–65 | 35.3 (28.3) | 35 (11–55) | 4–84 |
| OD domain | 9.7 (9.4) | 6 (2–16) | 0–28 | 9.1 (7.6) | 12 (2–15) | 0–19 | 8.1 (8.0) | 7.5 (0.5–15.5) | 0–18 | 9.1 (12.2) | 5 (0–12) | 0–35 |
| Questionnaire | Intervention | Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 months (n = 13) | 12 months (n = 12) | 8 months (n = 5) | 12 months (n = 5) | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| BANS-S | 16.9 (3.1) | 17 (15–19) | 12–23 | 17.8 (3.7) | 18 (15.5–21) | 11–23 | 12 (3.2) | 12 (12–13) | 7–16 | 12.6 (4.0) | 14 (11–15) | 7–17 |
| NPI-NH | ||||||||||||
| Overall score | 24.5 (21.1) | 23 (9–29) | 1–61 | 26.7 (26.4) | 16.5 (9–40) | 0–88 | 22.6 (24.0) | 20 (6–24) | 1–62 | 24 (16.4) | 16 (16–21) | 14–53 |
| OD domain | 6.4 (6.8) | 5 (0–10) | 0–20 | 7.5 (9.2) | 5.5 (0–10.5) | 0–26 | 4 (3.4) | 4 (2–5) | 0–9 | 7.4 (7.7) | 6 (3–8) | 0–20 |
| Questionnairea | Intervention arm | Control arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 months | Baseline | 4 months | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| QUALID | 22.0 (9.3) | 20.5 (14–28) | 11–47 | 21.8 (8.2) | 20 (15–28) | 11–37 | 21.2 (9.4) | 18 (13–29) | 11–40 | 22.1 (9.9) | 18.0 (15–29) | 11–46 |
| SM-EOLD | 29.6 (7.8) | 27 (24–38) | 17–40 | 32.8 (6.1) | 34.5 (29.5–38) | 19–40 | 28.0 (7.7) | 29.5 (20.5–35) | 13–40 | 26.0 (9.2) | 29.5 (18–32) | 7–40 |
| SWC-EOLD | 26.3 (3.1) | 27 (25–28) | 18–32 | 26.2 (2.7) | 26 (25–28) | 20–33 | 26.1 (2.8) | 26.5 (24–28) | 20–31 | 25.2 (1.7) | 25 (24–26) | 20–28 |
| Questionnairea | Intervention arm | Control arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 months | 12 months | 8 months | 12 months | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| QUALID | 22.4 (7.2) | 22 (16.5–28.5) | 13–38 | 21.5 (7.8) | 19 (16–24.5) | 11–39 | 24.1 (8.8) | 20 (19–29) | 13–47 | 24.5 (8.5) | 21.5 (18–32) | 15–41 |
| SM-EOLD | 31.7 (6.3) | 32.5 (27–37) | 17–40 | 29.9 (8.5) | 30.5 (28–36) | 11–40 | 25.1 (8.1) | 24 (21–31) | 8–40 | 28.6 (8.2) | 29.5 (21.5–35) | 9–40 |
| SWC-EOLD | 26.9 (2.0) | 27 (26–28) | 24–31 | 25.8 (2.6) | 26 (24–28) | 20–32 | 25 (3.2) | 26 (24.5–27) | 18–28 | 25.5 (1.5) | 26 (24–26) | 23–28 |
| Questionnaire | Intervention arm | Control arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 months | Baseline | 4 months | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| PAINADa during | ||||||||||||
| 1. Rest | 0 (0) | 0 (0–0) | 0–0 | 0 (0) | 0 (0–0) | 0–0 | 0.1 (0.6) | 0 (0–0) | 0–3 | 0.1 (0.7) | 0 (0–0) | 0–3 |
| 2. Movement | 0 (0) | 0 (0–0) | 0–0 | 0 (0) | 0 (0–0) | 0–0 | 0.4 (1.2) | 0 (0–0) | 0–4 | 0 (0) | 0 (0–0) | 0–0 |
| Questionnaire | Intervention arm | Control arm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 months | 12 months | 8 months | 12 months | |||||||||
| Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | |
| PAINADa during | ||||||||||||
| 1. Rest | 0 (0) | 0 (0–0) | 0–0 | 0.1 (0.3) | 0 (0–0) | 0–1 | 0 (0) | 0 (0–0) | 0–0 | 0.1 (0.3) | 0 (0–0) | 0–1 |
| 2. Movement | 0 (0) | 0 (0–0) | 0–0 | 0.6 (1.9) | 0 (0–0) | 0–7 | 0 (0) | 0 (0–0) | 0–0 | 0.3 (0.7) | 0 (0–0) | 0–2 |
The detailed responses to each domain of the EQ-5D-5L (Tables 48–50) highlight differences between types of respondent. There was a tendency for respondents with dementia (see Table 48) to report ‘no’ or ‘slight’ problems, with relatively few respondents reporting that problems were ‘severe’ or that they were ‘unable to do’ the activity. This pattern was relatively consistent across the intervention and control arms. This is consistent with the qualitative data that suggest that people with dementia may overestimate their abilities on the EQ-5D-5L, although it could be that people with dementia able to complete the questionnaire have fewer health problems.
| EQ-5D-5L domain and time point | Intervention arm (number of respondents) | Control arm (number of respondents) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Slight | Moderate | Severe | Unable to do | No | Slight | Moderate | Severe | Unable to do | |
| Mobility | ||||||||||
| Baseline | 11 | 4 | 3 | 2 | 0 | 10 | 3 | 3 | 3 | 1 |
| 4 months | 10 | 4 | 2 | 2 | 1 | 8 | 1 | 5 | 3 | 0 |
| 8 months | 12 | 3 | 1 | 1 | 1 | 7 | 3 | 3 | 1 | 0 |
| 12 months | 8 | 2 | 2 | 2 | 2 | 6 | 3 | 3 | 1 | 0 |
| Self-care | ||||||||||
| Baseline | 16 | 2 | 2 | 0 | 0 | 13 | 4 | 1 | 1 | 0 |
| 4 months | 15 | 3 | 1 | 0 | 1 | 10 | 2 | 2 | 0 | 2 |
| 8 months | 14 | 1 | 3 | 0 | 0 | 9 | 3 | 1 | 0 | 0 |
| 12 months | 9 | 3 | 3 | 0 | 1 | 10 | 3 | 1 | 0 | 0 |
| Usual activities | ||||||||||
| Baseline | 12 | 4 | 3 | 0 | 0 | 12 | 3 | 3 | 0 | 0 |
| 4 months | 12 | 3 | 1 | 0 | 1 | 5 | 3 | 6 | 1 | 1 |
| 8 months | 14 | 1 | 2 | 0 | 1 | 6 | 5 | 3 | 0 | 0 |
| 12 months | 9 | 2 | 3 | 0 | 1 | 7 | 2 | 3 | 0 | 0 |
| Pain and discomfort | ||||||||||
| Baseline | 10 | 4 | 5 | 0 | 0 | 13 | 0 | 3 | 2 | 0 |
| 4 months | 12 | 3 | 3 | 1 | 1 | 10 | 3 | 2 | 2 | 0 |
| 8 months | 12 | 2 | 5 | 0 | 0 | 8 | 4 | 1 | 1 | 0 |
| 12 months | 6 | 5 | 4 | 0 | 0 | 8 | 2 | 4 | 0 | 0 |
| Anxiety and depression | ||||||||||
| Baseline | 12 | 3 | 2 | 0 | 0 | 9 | 5 | 4 | 1 | 0 |
| 4 months | 11 | 6 | 1 | 0 | 0 | 7 | 5 | 1 | 2 | 1 |
| 8 months | 11 | 4 | 4 | 0 | 0 | 7 | 5 | 1 | 1 | 0 |
| 12 months | 7 | 4 | 2 | 0 | 0 | 8 | 1 | 4 | 0 | 0 |
| EQ-5D-5L domain and time point | Intervention arm (number of respondents) | Control arm (number of respondents) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Slight | Moderate | Severe | Unable to do | No | Slight | Moderate | Severe | Unable to do | |
| Mobility | ||||||||||
| Baseline | 12 | 7 | 4 | 2 | 0 | 11 | 2 | 1 | 3 | 0 |
| 4 months | 12 | 5 | 3 | 2 | 0 | 9 | 4 | 2 | 1 | 0 |
| 8 months | 13 | 6 | 2 | 1 | 0 | 6 | 3 | 2 | 2 | 0 |
| 12 months | 13 | 5 | 1 | 1 | 0 | 6 | 3 | 1 | 2 | 0 |
| Follow-up after death | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Self-care | ||||||||||
| Baseline | 21 | 2 | 1 | 1 | 0 | 15 | 0 | 2 | 0 | 0 |
| 4 months | 19 | 2 | 0 | 1 | 0 | 15 | 0 | 1 | 0 | 0 |
| 8 months | 18 | 2 | 1 | 1 | 0 | 11 | 0 | 1 | 1 | 0 |
| 12 months | 19 | 0 | 0 | 1 | 0 | 10 | 1 | 0 | 1 | 0 |
| Follow-up after death | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| Usual activities | ||||||||||
| Baseline | 17 | 3 | 4 | 1 | 0 | 13 | 1 | 3 | 0 | 0 |
| 4 months | 13 | 6 | 2 | 1 | 0 | 10 | 2 | 2 | 2 | 0 |
| 8 months | 17 | 2 | 2 | 1 | 0 | 8 | 0 | 2 | 3 | 0 |
| 12 months | 16 | 1 | 2 | 1 | 0 | 7 | 1 | 2 | 2 | 0 |
| Follow-up after death | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 |
| Pain and discomfort | ||||||||||
| Baseline | 10 | 7 | 5 | 1 | 2 | 5 | 6 | 4 | 2 | 0 |
| 4 months | 11 | 5 | 4 | 1 | 1 | 7 | 4 | 3 | 2 | 0 |
| 8 months | 10 | 7 | 3 | 2 | 0 | 3 | 5 | 2 | 3 | 0 |
| 12 months | 9 | 7 | 2 | 1 | 1 | 5 | 2 | 5 | 0 | 0 |
| Follow-up after death | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Anxiety and depression | ||||||||||
| Baseline | 12 | 7 | 4 | 2 | 0 | 8 | 8 | 1 | 0 | 0 |
| 4 months | 9 | 7 | 6 | 0 | 0 | 7 | 6 | 2 | 1 | 0 |
| 8 months | 8 | 11 | 3 | 0 | 0 | 3 | 6 | 4 | 0 | 0 |
| 12 months | 6 | 6 | 7 | 1 | 0 | 3 | 6 | 3 | 0 | 0 |
| Follow-up after death | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| EQ-5D-5L domain and time point | Intervention arm (number of respondents) | Control arm (number of respondents) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Slight | Moderate | Severe | Unable to do | No | Slight | Moderate | Severe | Unable to do | |
| Mobility | ||||||||||
| Baseline | 6 | 9 | 10 | 4 | 7 | 4 | 6 | 5 | 8 | 1 |
| 4 months | 5 | 4 | 5 | 9 | 6 | 3 | 6 | 7 | 4 | 2 |
| 8 months | 5 | 7 | 7 | 3 | 8 | 2 | 3 | 6 | 3 | 3 |
| 12 months | 4 | 5 | 6 | 3 | 10 | 2 | 5 | 5 | 2 | 2 |
| Self-care | ||||||||||
| Baseline | 4 | 7 | 7 | 2 | 16 | 3 | 6 | 8 | 5 | 2 |
| 4 months | 6 | 4 | 4 | 0 | 15 | 4 | 1 | 8 | 4 | 5 |
| 8 months | 5 | 4 | 4 | 3 | 14 | 2 | 2 | 6 | 2 | 5 |
| 12 months | 6 | 2 | 3 | 4 | 13 | 0 | 0 | 9 | 5 | 2 |
| Usual activities | ||||||||||
| Baseline | 3 | 8 | 2 | 6 | 17 | 2 | 2 | 6 | 10 | 4 |
| 4 months | 3 | 3 | 7 | 5 | 11 | 3 | 3 | 2 | 7 | 7 |
| 8 months | 4 | 4 | 7 | 5 | 10 | 0 | 4 | 4 | 3 | 6 |
| 12 months | 5 | 1 | 5 | 5 | 12 | 0 | 1 | 3 | 7 | 5 |
| Pain and discomfort | ||||||||||
| Baseline | 12 | 14 | 7 | 2 | 0 | 6 | 6 | 8 | 4 | 0 |
| 4 months | 14 | 5 | 6 | 2 | 2 | 6 | 5 | 8 | 2 | 1 |
| 8 months | 11 | 9 | 6 | 3 | 0 | 7 | 2 | 3 | 3 | 1 |
| 12 months | 10 | 8 | 5 | 4 | 0 | 6 | 2 | 4 | 4 | 0 |
| Anxiety and depression | ||||||||||
| Baseline | 12 | 11 | 9 | 2 | 1 | 4 | 7 | 9 | 3 | 1 |
| 4 months | 12 | 10 | 5 | 1 | 1 | 6 | 3 | 9 | 2 | 2 |
| 8 months | 9 | 9 | 11 | 1 | 0 | 1 | 5 | 9 | 2 | 0 |
| 12 months | 12 | 6 | 6 | 2 | 1 | 4 | 3 | 6 | 2 | 1 |
Comparing responses between the person with dementia and the proxy, a striking finding is the very different number of proxy respondents reporting that the person with dementia had severe restrictions on mobility or were unable to perform self-care or usual activities (see Tables 48–50). Responses for family carers and key informants have been combined, with precedence given to key informant responses when both were available. Further analysis could explore whether or not there are systematic differences between the two types of respondent when acting as proxies for the person with dementia. The differences between responses from the person with dementia and responses from the proxy merit further investigation. Typically, responses from the person with dementia would be used in the main analysis, with proxy responses used in a sensitivity analyses, but the differences are so large that further exploration is needed.
Responses to the resource use questionnaire for people with dementia at 12 months are displayed in Tables 51–61. (Owing to space limitations, data are not presented for all time points; similarly, because the questionnaire was piloted on only five family carers regarding their own service use, these results are not included.) Several responses are worth noting. Few individuals used inpatient services and, as a group, they were more likely to use outpatient services. Few individuals used day activity services but, as a group, they were much more likely to use community care services, such as the chiropodist, district nurse or GP. None of the patients across the trial was in employment. Carer responses to the questions regarding the patient’s source of income were limited.
| Living with | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| Alone | 3 (9) | 1 (6) |
| Husband/wife (without children) | 6 (19) | 4 (24) |
| Husband/wife (with children) | 2 (6) | 0 (0) |
| Partner as a couple or with siblings | 0 (0) | 0 (0) |
| Children | 2 (6) | 3 (18) |
| Other relatives | 1 (3) | 0 (0) |
| Care home | 18 (56) | 9 (53) |
| Usual accommodation | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| Domestic/family | ||
| Own home/flat | 10 (31) | 5 (29) |
| Privately rented home | 1 (3) | 0 (0) |
| Housing association rented home | 3 (9) | 3 (18) |
| Community (non-hospital) | ||
| Residential/nursing home | 4 (13) | 8 (47) |
| Dementia-specific care home | 14 (44) | 1 (6) |
| If living in community | Intervention (N = 18) | Control (N = 9) |
| Care facility organisation | ||
| Local authority social services | 1 (6) | 0 (0) |
| NHS | 0 (0) | 0 (0) |
| Private organisation | 17 (94) | 8 (88) |
| Voluntary organisation | 0 (0) | 1 (12) |
| Organisation | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 18) | Control (N = 9) | |
| Department of Social Security | 1 (6) | 0 (0) |
| NHS | 6 (33) | 0 (0) |
| Local authority | 8 (44) | 1 (13) |
| Private or voluntary organisation | 0 (0) | 0 (0) |
| Patient | 7 (39) | 1 (13) |
| Patient’s family | 1 (6) | 0 (7) |
| Insurance policy | 0 (0) | 0 (0) |
| Not known | 2 (11) | 2 (25) |
| Use of temporary accommodation over the previous 3 months | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| No | 30 (94) | 15 (88) |
| Yes | 2 (6) | 1 (6) |
| Missing | 0 (0) | 1 (6) |
| Type of temporary accommodation | Intervention (N = 2) | Control (N = 1) |
| Nursing home | 1 (50) | 0 (0) |
| Hospital | 1 (50) | 0 (0) |
| Dementia-specific care home | 0 (0) | 1 (100) |
| Use of inpatient services | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| No | 28 (87) | 16 (94) |
| Yes | 4 (13) | 0 (0) |
| Missing | 0 (0) | 1 (6) |
| Type of inpatient service used | Intervention (N = 4) | Control (N = 0) |
| Acute medical unit | 2 (50) | 0 (0) |
| Accident and emergency | 2 (50) | 0 (0) |
| Fracture ward | 1 (25) | 0 (0) |
| Use of outpatient services | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| No | 26 (81) | 14 (82) |
| Yes | 5 (16) | 1 (6) |
| Missing | 1 (3) | 2 (12) |
| Type of outpatient service used | Intervention (N = 5) | Control (N = 1) |
| Psychiatric outpatient visit | 1 (20) | 0 (0) |
| Dermatology | 1 (20) | 0 (0) |
| Orthopaedic | 1 (20) | 0 (0) |
| Scan appointments | 1 (20) | 0 (0) |
| Ultrasonography appointment | 1 (20) | 0 (0) |
| Urinary and bowel | 0 (0) | 1 (100) |
| Vascular | 0 (0) | 1 (100) |
| Use of day activity services | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| No | 26 (81) | 15 (88) |
| Yes | 5 (16) | 2 (12) |
| Missing | 1 (3) | 0 (0) |
| Type of day service used | Intervention (N = 5) | Control (N = 2) |
| Voluntary organisation | 0 (0) | 2 (100) |
| Lunch club | 1 (20) | 0 (0) |
| Gardening group | 1 (20) | 0 (0) |
| Private day centre | 3 (60) | 0 (0) |
| Use of community services | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| No | 4 (13) | 2 (13) |
| Yes | 25 (78) | 12 (75) |
| Missing | 3 (9) | 3 (18) |
| Type of community service used | Intervention (N = 25) | Control (N = 12) |
| Care manager | 0 (0) | 1 (8) |
| Chiropodist | 13 (52) | 0 (0) |
| Community matron | 1 (4) | 0 (0) |
| Community psychiatric nurse | 0 (0) | 1 (8) |
| Dentist | 5 (20) | 0 (0) |
| District nurse | 4 (16) | 1 (8) |
| DoLS assessment | 1 (4) | 0 (0) |
| DoLS service | 1 (4) | 0 (0) |
| Ear check | 0 (0) | 1 (8) |
| GP | 20 (80) | 10 (83) |
| General practice nurse | 6 (24) | 2 (17) |
| Home care worker | 1 (4) | 0 (0) |
| Meals on wheels | 1 (4) | 0 (0) |
| Occupational therapist | 1 (4) | 0 (0) |
| Optician | 7 (28) | 1 (8) |
| Physiotherapist | 0 (0) | 1 (8) |
| Podiatrist | 0 (0) | 2 (17) |
| Specialist nurse | 1 (4) | 0 (0) |
| Social worker | 2 (8) | 0 (0) |
| Employment status | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| Paid or self-employed | 0 (0) | 0 (0) |
| Voluntary work | 0 (0) | 0 (0) |
| Unemployed | 0 (0) | 0 (0) |
| Housewife/househusband | 0 (0) | 0 (0) |
| Retired | 32 (100) | 17 (100) |
| Exempt through disability | 0 (0) | 0 (0) |
| Patient income | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 32) | Control (N = 17) | |
| Carer asked about patient’s income | ||
| No | 18 (56) | 13 (76) |
| Yes | 14 (44) | 4 (24) |
| Source of patient’s incomea | Intervention (N = 14) | Control (N = 4) |
| Wage | 0 (0) | 0 (0) |
| State pension | 14 (100) | 3 (75) |
| Private pension scheme | 6 (43) | 2 (50) |
| Benefits | 7 (50) | 4 (100) |
| Income bond | 1 (7) | 0 (0) |
| NHS pension | 0 (0) | 1 (25) |
| Type of benefits receivedb | Intervention (N = 7) | Control (N = 4) |
| Council tax benefit | 1 (14) | 0 (0) |
| Pension credit guarantee | 1 (14) | 0 (0) |
| Pension credit savings | 1 (14) | 1 (14) |
| Disability living allowance: care | 0 (0) | 1 (14) |
| Disability living allowance: mobility | 0 (0) | 1 (14) |
| Severe disability premium | 0 (0) | 1 (14) |
| Attendance allowance: low rate | 2 (29) | 1 (14) |
| Attendance allowance: high rate | 2 (29) | 2 (29) |
| Carer’s allowance | 2 (29) | 1 (14) |
| Total patient income (£) | Trial arm, n (%) | |
|---|---|---|
| Intervention (N = 14) | Control (N = 4) | |
| Gross | ||
| ≤ 277 | 2 (14) | 2 (50) |
| 278–379 | 0 (0) | 0 (0) |
| 380–518 | 0 (0) | 0 (0) |
| 519–728 | 0 (0) | 0 (0) |
| > 728 | 0 (0) | 0 (0) |
| Net | ||
| ≤ 277 | 6 (43) | 0 (0) |
| 278–379 | 3 (21) | 2 (50) |
| 380–518 | 1 (7) | 0 (0) |
| 519–728 | 1 (7) | 0 (0) |
| > 728 | 0 (0) | 0 (0) |
| Not known | 1 (7) | 0 (0) |
Qualitative data on outcome measures
Stakeholder feedback on the outcome measures used in the pilot trial is summarised below. We also consider the extent to which the measures captured the types of outcomes described by study participants in interviews. Detailed field notes were made by the researchers on the resource use questionnaire and we suggest how this might be adapted to be more suitable for people with dementia and family carers. Five themes were identified from the analysis of data from people with dementia, carers, key informants and researchers regarding the study outcome measures; each is briefly explored in the following sections.
Perceived burden of the outcome measures
Despite our concerns about the burden of the outcome measures, no people with dementia or carers made any negative comments about the duration or frequency of the assessments, and most found them acceptable. Similarly, care home staff reported that participation had not been too onerous, even in homes with a number of study participants:
It’s been no bother. It hasn’t taken long. It’s just been ticking things off, me and my dad. It’s no problem. It’s been fine.
Interview, family carer 3014
I was a bit worried about the impact on staff time and resources. But it’s been very, very minimal in regard to SEED . . . [Researcher] was very efficient. She would sit with the staff and get the information she needed; didn’t have a major impact at all.
Interview, care home manager CH2, site 1
Relevance of some outcome measures
The researchers queried the relevance of some measures to recently diagnosed participants. Self-reported pain was considered more accurate and appropriate than the PAINAD for people recently diagnosed with dementia, most of whom would be able to articulate pain themselves. Similarly, for carers of people recently diagnosed with dementia, questions relating to symptom management and satisfaction with EOLC were considered inappropriate and potentially anxiety-provoking for carers.
Although not explicitly discussed by stakeholders, data from interviews suggest that the outcome measures used may not capture the types of changes resulting from the SEED intervention. One GP felt that the proactive work undertaken by the DNS with people with dementia and carers had resulted in considerable benefits:
Some of the patients that [DNS1] had, and especially the social situations and the problems that she has just tackled head-on, before it’s reached crisis point, I’m sure, has prevented some people going into care homes. It’s prevented some safeguarding issues. It’s prevented, even, admissions to hospitals; she’s been proactive in picking up medical problems. So she would be speaking to [relatives] . . . and before I know it, [DNS1’s] coming back to me, saying ‘I wonder whether this person might actually have an underlying physical health problem. We need to do a blood test.’ . . . that proactive work, I’m sure, has decreased the burden on secondary care.
Interview, GP 1.1, site 1
This extract highlights the potential impacts of the SEED intervention on service use. Although a resource use questionnaire was used, a number of shortcomings were identified (see below); developing a more robust and user-friendly tool (or alternative ways of collecting data on resource use) will be essential to capture these potential changes.
Perceived reliability of outcome measures
Carers, key informants and the researchers all questioned the reliability of some outcome measures. The extent to which standardised questionnaires captured the reality of living with dementia was disputed:
They don’t show a true picture of what’s going on . . . You just think to yourself ‘Well, how can you rate that between 1 and 10 when sometimes that might be a nice 10 for you and other times it’s down to a 2, the same question?’.
Interview, family carer 1054
Key informants explicitly commented on the inherent bias in the SWC-EOLD, as they were effectively being asked to rate the quality of their own service:
I thought this was a bit biased, because I’m the one looking after her, so I feel she’s getting the necessary nursing assistance. I wasn’t going to tick ‘no . . . I strongly disagree, she’s getting rubbish care’.
Interview, care home clinical lead, CH2S2, site 1
Concerns were raised by carers and researchers on the reliability of information provided by people with dementia on the EQ-5D-5L. Carers commented on the discrepancy between self-reported ratings and their knowledge of the difficulties experienced by the person with dementia in their day-to-day life:
One of the questions was ‘How do you feel physically, your physical things’, something like that. ‘100%’, he said . . . ‘I’m as fit as a lop’. I said ‘Well, you can’t walk’. He can’t even get out of bed in the morning, never mind walk.
Interview, family carer 1054
The researchers similarly suggested that some people with dementia appeared to overestimate their abilities and QoL rating on the EQ-5L-5D. In addition, they were concerned that carers sometimes minimised problems when completing the NPI.
The low scores on the PAINAD were queried by members of the Trial Oversight Committee, who felt that the scores were inconsistent with the prevalence of pain shown in previous studies. They suggested that more accurate ratings would be obtained from a family carer or key informant who knew the person with dementia well. The researchers acknowledged that their ratings related to a defined, short period of time and that more familiarity with the person with dementia and contact over a longer period of time may have given a different picture (and would probably have decreased the number of missing data relating to pain during movement).
Potential negative emotional impact of some measures on carers
Some carers were distressed by some outcome measures, in particular the NPI and HADS. The researchers were also aware that the NPI could potentially provide new insights into the illness trajectory and the range of symptoms that might be experienced in future. During the post-death interviews, some carers appeared to find the measures focusing on EOLC cathartic, but, for others, the discrepancy between actual and desired EOLC was distressing. The majority of outcome measures used focused on deficits and problems; finding ways to capture positive outcomes may improve the experience of data collection, as well as ensuring a more holistic picture of the impacts of the SEED intervention.
Perceived duplication of items
From the perspectives of the researchers, there was duplication in items across different outcome measures. Although the researchers acknowledged that the wording was not identical and questions on different measures covered different time periods, the process of completing the measures could, nevertheless, feel repetitive. Agitation and aggression were covered in the NPI, the QUALID and the SM-EOLD questionnaires, and anxiety and depression were addressed in the EQ-5D-5L, the NPI, the QUALID and the SM-EOLD questionnaires. Because the NPI includes a series of in-depth questions about different symptom areas, carers and key informants often covered similar items included in subsequent measures.
Suggested modifications to outcome measures
The resource use questionnaire enabled the use of services to be captured, but would not be suitable for use in its current format in a definitive study. The researchers who administered the resource use questionnaire found it unwieldy and onerous for participants and researchers. Currently, the questionnaire asks about contacts with multiple named services (many of which participants struggled to distinguish between). Restructuring the questionnaire, to take a narrative sequential approach to key (health or social care-related) events over the period of interest, may be a more user-friendly way of collecting the data. The researcher could explore each episode of health or social care use in detail. This would enable a narrative, contextualised approach to be used, which is likely to be easier for participants. Other suggested changes to this questionnaire to reduce respondent burden and potentially increase the accuracy of responses include:
-
Tailoring response categories so that they are more appropriate for this patient group, for example by –
-
including categories for types of accommodation (e.g. residential/nursing/care homes, sheltered accommodation, extra care facilities, specialist dementia care units)
-
including district nurse in the list of services potentially used
-
removing questions on employment as they are largely irrelevant.
-
-
Removing those questions that few respondents were able to answer and finding alternative ways of collecting the data. For example, many family carers were unsure of the providers of residential/nursing/care homes or sheltered accommodation and of the funding arrangements for such care. However, if they provided basic information (e.g. the name and address of the care home), researchers could be responsible for identifying the provider. Similarly, with appropriate consent, researchers could explore funding arrangements if this information is needed to ascertain who bears the cost.
-
Simplifying the questionnaire, for example by asking about the number of hospital outpatient appointments, rather than trying to establish additional details, such as specialty. Similarly, focusing on details such as length of inpatient stays, without collecting additional information on the type of ward and transitions between wards.
-
Data on total income help in understanding the impacts of income inequalities. However, some respondents found it easier and more acceptable to provide weekly income. Allowing respondents to give income in the format that is easiest for them (i.e. weekly, monthly or annual income) is suggested.
Appendix 5 Economic modelling study (workstream 5)
Economic modelling summary
As health and social care resources are limited, decision-makers need information about whether or not the benefits that an intervention provides are worth its costs. 144 This information can be provided by an economic evaluation. An economic evaluation involves the comparative analysis of alternative courses of action. 145 In this appendix, the SEED intervention developed in WS3 is compared with alternative ways of providing care, including an example of current practice. The SEED intervention involves a DNS based in a general practice focusing their efforts on seven key components of EOLC.
The potential value of the SEED intervention was assessed using a contingent valuation survey of 1002 members of the general public. These data were used in an economic evaluation decision model. This economic model describes what happens to a person who has been diagnosed with dementia over time and how the SEED intervention might change this. The results of the model were presented in terms of the costs and consequences (e.g. hospitalisations) and, using the contingent valuation data, a cost–benefit analysis.
The contingent valuation showed that the SEED intervention was valued and that a wider package of care was valued more than selected features in isolation. Individuals with experience of dementia placed a higher value on the SEED intervention than those without such experience, but there was no evidence of a difference in the value by gender, household size or health status. The SEED intervention is unlikely to reduce costs, but these may be offset by the value placed on the SEED intervention by the general public. The SEED intervention is expected to improve the well-being of people with dementia and carers, but the impact on services is mixed.
Patient and public involvement perspectives were sought on the economic modelling study, and on the findings of economic modelling and the WTP survey.
Research aim
The aim of WS5 was to estimate the relative efficiency of the SEED intervention. Specific objectives were to:
-
Value the consequences of the SEED intervention using contingent valuation methods.
-
Develop an economic model of the usual care pathway and new alternative pathways including the SEED intervention developed in WS3.
-
Conduct a cost–consequences analysis of the SEED intervention compared with usual care.
-
Conduct a cost–benefit analysis of the SEED intervention that compared the scenarios used in the contingent valuation. These scenarios described variations in how the SEED intervention might be provided and they were valued by the contingent valuation.
The next section describes work relating to the first objective; the remaining objectives are addressed in Economic evaluation of the SEED intervention.
Valuing the consequences of the SEED intervention
The measures typically used in economic evaluations to quantify the benefits of interventions, such as QALYs, may not adequately capture individual preferences for how services are organised and their associated outcomes.
An alternative approach to determine the benefits an individual derives from an intervention is to determine their maximum WTP for it using a contingent valuation study. Maximum WTP represents the maximum amount, expressed in monetary terms or other goods, an individual is willing to give up (or sacrifice) to gain the benefits of the intervention. 148 In the absence of conventional markets to observe individuals’ monetary valuations of the benefits of health-care interventions, it is necessary to directly elicit such values. The contingent valuation method involves setting up a hypothetical market and asking individuals to state their WTP for the interventions in question. 206 The resulting WTP values can then be compared with the cost of providing the intervention in a cost–benefit analysis. This approach was used to estimate a WTP value from members of the general public for the different care packages that could be provided by the SEED intervention using the contingent valuation method.
Methods
Survey development
For the contingent valuation survey, an internet questionnaire was developed. The questionnaire incorporated five WTP scenarios, each of which represent an alternative package of care that could be provided by variations of the SEED intervention. The content of the WTP scenarios was based on the seven key components to support good EOLC identified from WS2 and synthesised into the SEED intervention in WS3.
The main scenario was designed to include all seven key features identified in WS2, but we also recognised that, for many reasons, such as budget or staff availability, there could be situations in which it was not possible to provide a service that would include all seven components. Therefore, it was important to determine if members of the public would value a service that included fewer components and also how these would affect the net benefits estimated in the cost–benefit analysis. The decision on which components to group together to create the four alternative scenarios was based on discussion with the wider SEED team as to what components would be compatible together.
These WTP scenarios were mirrored by comparators used in the economic model (see Economic evaluation of the SEED intervention). Prior to the pilot survey, each WTP scenario was tested with the SEED project team and the wording was revised to ensure that the components of the SEED intervention could be understood by members of the public. The final WTP scenarios are presented in Boxes 7–11 and are summarised, to facilitate comparison, in Table 62.
The DNS provides tailored support to enable the provision of high-quality EOLC to people with dementia. The support from the DNS is expected to result in the following:
-
Developing confidence in people with dementia, their family and carers, and doctors (GPs) to make timely and early decisions about EOLC and the arrangements after death.
-
Documenting the wishes of the person with dementia to help everyone involved in their care to quickly access and understand their preferences and needs.
-
Timely co-ordination of care with multiple services to reduce the burden on carers.
-
Regular involvement of and visits from the same doctor (GP), nurse or care workers, meaning that the values, medical need and history of the person with dementia are well understood.
-
Early recognition of the person nearing the EOL well in advance to help care providers recognise changes indicating that the person with dementia is nearing the EOL so that pain and discomfort are easily detected and managed responsively with the appropriate medication.
-
Avoidance of unnecessary hospitalisations but, if admission to the hospital is needed, helps to assist discharge and prevent excessive length of stay.
-
Ensuring that health-care workers possess the right skills to provide compassionate care to people with dementia.
The DNS provides tailored support to enable the provision of high-quality EOLC to people with dementia. The support from a DNS is expected to result in the following:
-
Timely co-ordination of care with multiple services to reduce burden on carers.
The DNS provides tailored support to enable the provision of high-quality EOLC to people with dementia. The support from a DNS is expected to result in the following:
-
Developing confidence in people with dementia and their family, carers and doctors (GPs) to make timely and early decisions about EOLC and the arrangements after death.
-
Documenting the wishes of the person with dementia to help everyone involved in their care to quickly access and understand their preferences and needs.
The DNS provides tailored support to enable the provision of high-quality EOLC to people with dementia. The support from the DNS is expected to result in the following:
-
Regular involvement of and visits from the same doctor (GP), nurse or care workers, meaning that the values, medical need and history of the person with dementia are well understood.
-
Early recognition of the person nearing the EOL well in advance to help care providers recognise changes indicating that the person with dementia is nearing the EOL, so that pain and discomfort are easily detected and managed responsively with the appropriate medication.
-
Avoidance of unnecessary hospitalisations but, if admission to the hospital is needed, helps to assist discharge and prevent excessive length of stay.
The DNS provides tailored support to enable the provision of high-quality EOLC to people with dementia. The support from the DNS is expected to result in the following:
-
Ensuring that health-care workers possess the right skills to provide compassionate care to people with dementia.
| Option | Scenario | ||||
|---|---|---|---|---|---|
| Main | Alternative 1 | Alternative 2 | Alternative 3 | Alternative 4 | |
| 1. People with dementia and their family, carers and doctors (GPs) are confident to make timely and early decisions about EOLC and the arrangements after death | ✓ | ✓ | |||
| 2. The wishes of the person with dementia are documented to help everyone involved in their care to quickly access and understand their preferences and needs | ✓ | ✓ | |||
| 3. A timely co-ordination of care with multiple services will reduce the burden on carers | ✓ | ✓ | |||
| 4. The values, medical need and history of the person with dementia is well understood because of regular involvement of and visits from the same doctor (GP) | ✓ | ✓ | |||
| 5. The care providers recognise changes indicating that the person is nearing EOL well in advance, so that pain and discomfort are easily detected and managed responsively with the appropriate medication | ✓ | ✓ | |||
| 6. Unnecessary hospitalisations are avoided, but, if admission to the hospital is needed, discharge is assisted and excessive length of stay is prevented | ✓ | ✓ | |||
| 7. It is ensured that health and social care professionals possess the right skills to provide compassionate care | ✓ | ✓ | |||
The contingent valuation study took a community perspective, with respondents asked to give their WTP for the SEED intervention to be available in the NHS, even though they would not (necessarily) benefit from it themselves. Given this perspective, respondents were asked their WTP in the form of an additional tax per month that they would pay for the next 10 years. The 10-year duration was chosen as a meaningful time scale for respondents and was also representative of how long a policy intervention might exist before it was redesigned.
The contingent valuation questionnaire comprised three sections. The first section provided a background on current provision of dementia care towards the EOL and the SEED intervention. The second section presented the five hypothetical scenarios described in Boxes 7–11, and respondents were asked whether or not they were willing to pay for each of the scenarios. The steps involved in eliciting WTP values were as follows:
-
All respondents were presented with the ‘main’ WTP scenario and then randomly assigned to receive two of the four remaining WTP scenarios.
-
If they answered ‘yes’ to a question saying that they would be willing to pay, then they were presented with a series of payment cards selected at random on the screen. For each, they were asked to state their WTP for the proposed scenario with a question ‘Would you be willing to pay £X for the scenario described?’.
-
Twelve payment cards with amounts ranging from 50p to £100 were used.
-
Respondents were asked to sort the payment cards by dragging and dropping (using the computer mouse) the WTP amount in the appropriate box (‘definitely would pay’, ‘maybe’, ‘definitely would not pay’), depending on their answers.
-
The respondents were presented with a summary of the maximum card value that they were definitely willing to pay and the minimum card value that they were definitely not willing to pay and were again asked an open-ended question to state their maximum WTP.
This approach used to present the WTP questions was expected to minimise the potential starting point bias (bidding games) and range bias (payment scales). 207 Respondents answering ‘no’ to the WTP question on the scenario presented were asked to indicate a reason for not being willing to pay from a set of reasons or using a free-text option. The third section of the survey elicited respondents’ socioeconomic and demographic characteristics (age, gender, income, education, etc.). The final questionnaire is available in Report Supplementary Material 1.
Survey administration
Pre-piloting of the contingent valuation component of the survey was conducted to test usability and ease of understanding the scenarios. The pre-piloting work was undertaken as ‘think-aloud’ interviews with seven members of the Institute of Health & Society at Newcastle University. 149
Piloting of the full web survey was conducted in a subsample of the target general population. For both the pilot and final surveys, the sample of the general population was recruited from the online panel managed by a marketing company (ResearchNow). Respondents were offered a small (£1–2) incentive in the form of shopping vouchers, as per their normal procedures. The pilot sample size (n = 270) was considered large enough to conduct preliminary analysis and resulted in small amendments to the response options in the ‘No, I am not willing to pay for the scenario’. This study was approved by the Faculty of Medical Sciences REC, part of Newcastle University’s REC (approval code number 1410/136).
Data analysis
Data were analysed in statistical programming language R208 (The R Foundation for Statistical Computing, Vienna, Austria) and reported as the mean and median WTP for each of the five scenarios. Protest responses that indicated that respondents were not willing to pay anything, with a reason ‘I don’t think I should have to pay for health care’ or ‘the government should pay’, were excluded as per conventional practice in WTP studies. 209 All other reasons for not being willing to pay anything were interpreted as a true zero value and were included in the analysis. To reduce the effect on means of extreme upper-end WTP responses, means and medians were trimmed by excluding responses from the top 1% of WTP values. 150 Given a large proportion of zero WTP values and left-skewed data expected meant that standard regression methods such as ordinary least squares would yield biased and inconsistent estimates. In such a circumstance, a tobit model is the preferred alternative;210,211 the impact of respondent characteristics (e.g. gender, age, income, education, family size and experience of dementia) on WTP values was investigated using this model for the trimmed sample.
Results
A total of 1002 respondents completed the online survey. Table 63 presents the number of responses per scenario. The number of protest responses and the reasons for not being willing to pay anything for each of the scenarios are also presented in Table 63.
| Item | Scenario | ||||
|---|---|---|---|---|---|
| Main | Alternative 1 | Alternative 2 | Alternative 3 | Alternative 4 | |
| Initial sample (N) | 1002 | 496 | 506 | 500 | 502 |
| Number of yes, positive WTP values, n (%) | 807 (80.5) | 335 (67.5) | 327 (64.6) | 359 (71.8) | 324 (64.5) |
| Number of no, zero WTP values, n (%) | 195 (19.5) | 161 (32.5) | 179 (35.4) | 141 (28.2) | 178 (35.5) |
| Number of protest zeros,a n (%) | 104 (10.4) | 67 (13.5) | 62 (12.3) | 57 (11.4) | 65 (12.9) |
| Reasons for not being willing to pay (n) | |||||
| I do value the improvement in dementia care, but I cannot afford to pay anything for it | 62 | 49 | 45 | 54 | 41 |
| I do not think I should have to pay for health care | 94 | 61 | 55 | 54 | 60 |
| I think the dementia care without the nurse involvement would be satisfactory | 19 | 29 | 41 | 19 | 35 |
| Other | 20 | 22 | 38 | 14 | 42 |
Table 64 reports the mean and median WTP values across the scenarios for both the trimmed and the untrimmed data sets. The mean WTP values computed from the untrimmed data set for the alternative scenarios were much higher than the mean WTP values for the main scenario and the very wide 95% CIs indicates the presence of very high outlier values. When the top 1% of WTP values were trimmed, the mean WTP was higher for the main scenario than for the alternatives. The medians for both the trimmed and the untrimmed data sets generally remained the same.
| WTP | Scenario | ||||
|---|---|---|---|---|---|
| Main | Alternative 1 | Alternative 2 | Alternative 3 | Alternative 4 | |
| Mean (95% CI) | 40.13 (26.25 to 54.01) | 2357.20 (23 to 14,006) | 257.47 (28 to 1391) | 810.22 (27 to 4700) | 2313.69 (22 to 13,750) |
| Mean (95% CI)a | 24.19 (21.85 to 26.52) | 18.38 (15.95 to 20.82) | 16.18 (13.59 to 18.76) | 18.36 (15.72 to 21.00) | 16.99 (14.15 to 19.83) |
| Median (95% CI) | 10 (10 to 15) | 10 (7.5 to 10) | 7.5 (5 to 8) | 9.25 (7.5 to 10) | 6 (5 to 9) |
| Median (95% CI)a | 10 (10 to 12.5) | 10 (7.5 to 10) | 7.5 (5 to 8) | 8 (7.5 to 10) | 6 (5 to 8) |
Table 65 summarises the mean WTP values by experience of dementia (i.e. who have seen their family, friends or colleagues with dementia). Across all the scenarios, individuals with some experience of dementia were willing to pay more for the improved dementia care service than those with no experience of dementia. However, there is no evidence of a statistically significance difference for alternative 2.
| Subgroup | Mean WTP (95% CI) | ||||
|---|---|---|---|---|---|
| Main | Alternative 1 | Alternative 2 | Alternative 3 | Alternative 4 | |
| Dementia experience | 29.26 (25.72 to 32.79) | 21.87 (18.33 to 25.41) | 17.21 (13.72 to 20.70) | 22.15 (18.14 to 26.16) | 19.99 (15.75 to 24.23) |
| No dementia experience | 17.14 (14.67 to 19.60) | 13.32 (10.40 to 16.24) | 14.79 (10.94 to 18.65) | 13.25 (10.33 to 16.17) | 12.41 (9.41 to 15.42) |
| Difference in mean WTPa | 12.12 (7.81 to 16.42) | 8.55 (3.98 to 13.12) | 2.42 (–2.76 to 7.60) | 9.25 (3.95 to 13.85) | 7.58 (2.40 to 12.76) |
| p-value | 0.0000 | 0.0003 | 0.36 | 0.0004 | 0.0042 |
The results of the regression analysis of WTP values on selected respondent characteristics for each of the scenarios is presented in Table 66. There was no evidence to suggest that respondent characteristics such as age, gender, family size, health utility or education status influenced the WTP values.
| Covariate | Coefficient (SE) | ||||
|---|---|---|---|---|---|
| Main | Alternative 1 | Alternative 2 | Alternative 3 | Alternative 4 | |
| Age | 0.16 (0.09) | –0.08 (0.10) | 0.14 (0.11) | 0.08 (0.1) | –0.16 (0.13) |
| Male | 4.45 (2.73) | 6.16 (3.20) | 1.7 (3.31) | 4.08 (3.19) | 4.56 (4.08) |
| No dementia experience | –11.65 (2.77)*** | –10.71 (3.24)*** | 1.38 (3.40) | –6.59 (3.25)* | –9.22 (4.11)* |
| Family size | 0.58 (0.96) | –0.50 (0.99) | –0.71 (0.85) | –0.65 (1.0) | –1.0 (1.02) |
| Health score | 0.11 (0.08) | 0.26 (0.10)* | 0.21 (0.10)* | 0.23 (0.1)* | –0.001 (0.123) |
| Utility | –11.24 (6.95) | –13.28 (8.26) | –10.25 (8.59) | –8.0 (7.66) | –3.90 (10.36) |
| Household income (£) | |||||
| < 10,000 | – | – | – | – | – |
| 10,000–19,999 | –1.14 (6.25) | –3.78 (7.32) | 11.24 (7.7) | –3.27 (7.02) | 4.46 (9.52) |
| 20,000–29,999 | 3.54 (6.20) | –1.23 (7.23) | 11.91 (7.5) | –1.33 (6.81) | 9.90 (9.58) |
| 30,000–39,999 | 3.49 (6.20) | –2.25 (7.25) | 6.53 (7.56) | 3.92 (6.95) | 2.68 (9.46) |
| 40,000–49,999 | 4.15 (6.50) | –2.02 (7.52) | 7.33 (8.11) | –1.94 (7.24) | –5.46 (10.14) |
| 50,000–59,999 | 9.23 (7.09) | 7.96 (8.28) | 17.98 (8.6)* | 3.73 (8.58) | 8.7 (10.38) |
| 60,000–69,999 | 17.83 (8.24)* | 8.51 (9.55) | 20.04 (10.06)* | 1.59 (8.88) | 11.80 (13.58) |
| 70,000–79,999 | 6.68 (8.66) | 0.65 (10.18) | 27.69 (10.06)** | 6.45 (9.62) | 8.29 (13.21) |
| 80,000–89,999 | 13.67 (8.53) | 2.90 (9.85) | 23.32 (10.10)* | 8.15 (8.36) | 25.14 (15.75) |
| 90,000–99,999 | 19.69 (9.80)* | 17.87 (11.20) | 7.46 (12.41) | 2.18 (11.6) | 18.39 (13.77) |
| 100,000–149,999 | 24.82 (9.03)** | 17.48 (9.44) | 41.97 (1.77)*** | 49.10 (11.98)*** | 16.65 (12.1) |
| 150,000–199,999 | 7.82 (15.34) | 9.63 (16.44) | 0.89 (21.07) | –22.97 (25.37) | 28.64 (22.88) |
| 200,000–499,999 | 27.29 (19.41) | 13.21 (29.95) | 71.49 (19.18)*** | 50.4 (22.04)* | 87.92 (26.99)** |
| ≥ 500,000 | 44.26 (11.76)*** | 28.43 (12.15)* | 12.19 (17.98) | 57.55 (13.59)*** | 22.28 (17.56) |
| Prefer not to answer | 2.11 (7.12) | –9.87 (8.88) | 5.17 (8.53) | –4.18 (7.98) | 2.09 (10.94) |
| Education | |||||
| Incomplete secondary education (below GCSE/O level) | – | – | – | – | – |
| Do not want to disclose | 0.16 (18.7) | –25.42 (22.42) | 5.20 (27.26) | 19.09 (19.39) | 2.73 (29.26) |
| Doctorate, post doctorate or equivalent | 12.61 (9.39) | –6.65 (10.83) | 8.11 (12.75) | 7.34 (11.06) | 2.55 (13.86) |
| Postgraduate education completed (e.g. masters) | 0.009 (7.84) | –2.71 (8.79) | 19.37 (10.56) | –0.36 (9.39) | 3.85 (11.27) |
| Secondary education completed (A level or equivalent) | –1.0 (7.55) | –5.35 (8.47) | 15.29 (10.15) | –2.86 (8.78) | 4.27 (10.96) |
| Secondary education completed (GCSE/O level/CSE or equivalent) | 1.82 (7.47) | –2.35 (8.52) | 17.10 (9.83) | 1.09 (8.58) | –2.54 (10.92) |
| Some vocational or technical qualifications | 8.79 (13.35) | 0.1 (13.85) | 29.65 (21.48) | –4.34 (19.98) | 19.44 (17.44) |
| University education completed (first degree) | 4.72 (7.17) | –3.24 (8.22) | 11.28 (9.71) | –4.96 (8.51) | 2.83 (10.42) |
| Vocational or technical qualifications completed (e.g. HND, NVQ) | 4.12 (7.38) | 1.69 (8.59) | 21.25 (9.77)* | 1.50 (8.66) | 6.04 (10.74) |
Summary of results of the contingent valuation study
Members of the general public do value the care provided by the SEED intervention. Moreover, a higher WTP value for the main scenario indicated that it was valued more than packages with selected features only. The subgroup analysis showed that individuals who have seen their family, friends or colleagues with dementia place a higher value on the tailored support provided by the SEED intervention and the provision of high-quality EOLC to people with dementia than individuals with no experience of dementia.
There was no evidence of a relationship between the WTP value placed on the improvement of dementia care services with age of the respondent, gender, household size or the health utility score. This indicates that the value of quality dementia care towards, and at, the EOL is of importance to all, irrespective of these respondent characteristics.
In line with economic welfare theory, it is expected that individuals with higher ability to pay would give higher WTP values. 212,213 The WTP values were significantly higher for high-income groups than for those on the lowest income level, which corroborates with economic welfare theory; however, there was no evidence of a simple linear relationship with WTP values.
Economic evaluation of the SEED intervention
The economic evaluation aimed to estimate the relative efficiency of the SEED intervention. As the precise form by which this intervention would be implemented is not known at this stage, an early economic model was developed for this purpose. 151 In such models, plausible ranges for model parameters are specified and the impact on a set of specified modelled outcomes (e.g. predicted costs or effectiveness measures) of varying these model parameters over this plausible range is estimated.
During the design stage for the evaluation, and as noted in Valuing the consequences of the SEED intervention, there was a concern that QALYs may not capture the full benefit of the SEED intervention. Therefore, the economic evaluation was designed as a cost–consequence analysis, which does not seek to aggregate impacts on patients’ and carers’ health and well-being into a single measure, such as QALYs. Instead, it reports each impact in units that make sense for that impact (e.g. for hospitalisations, the impact could be reported as number of hospitalisations or days in hospital). The purpose of reporting impacts like this is to highlight choices and trade-offs between costs and impacts. The cost–consequences analysis approach is useful because it can incorporate health and non-health impacts, something that QALYs cannot do. 214
A cost–consequences analysis can also be thought of as a step along the way to the most comprehensive form of economic evaluation: a cost–benefit analysis. 152,153 In a cost–benefit analysis, costs and benefits are valued in commensurate units, normally money. 145 Following the completion of the cost–consequences analysis, we use cost–benefit analysis approach here, in a novel analysis, to incorporate the results of the contingent valuation into the economic model.
Methods
Cost–consequences analysis
For the cost–consequences analysis, a comparison was drawn between the SEED intervention and usual care. The SEED intervention affects several activities, which directly or indirectly affect the care and well-being of the person with dementia and their family/carers. These activities, service outputs and outcomes are described in detail in Appendix 6, Dementia care services. The main activities included in this model were as follows:
-
regular clinical reviews
-
ACP discussions and documentation
-
liaison between services before hospitalisation [proxy: use of the situation, background, assessment, recommendation (SBAR)215 technique]
-
liaison between services during hospitalisation (proxy: use of transfer sheets)
-
liaison between services after hospitalisation (proxy: discharge planning).
The extent to which the SEED intervention will affect these activities is unknown until a prospective evaluation is conducted. Therefore, a set of exploratory scenarios were produced, whereby:
-
In the SEED intervention arm, the provision of all care activities was set to its expected maximum and was compared with the control arm (usual practice), for which the provision of all care activities was set to its expected minimum. This scenario was named the ‘favourable scenario’.
-
In the SEED intervention arm, the provision of all care activities was set to its expected minimum and was compared with the control arm (usual practice), for which the provision of all care activities was set to its expected maximum. This scenario was named the ‘conservative scenario’.
-
The expected provision of a single care activity was set to its expected maximum, while the other four activities were set at their average level of provision. This analysis was repeated for each care activity.
-
The expected provision of one care activity only was set to its expected minimum, while the other four activities were set at their average level of provision. This analysis was repeated for each care activity.
Scenarios 1 and 2 allowed the estimation of the boundaries of the plausible cost and consequences associated with the SEED intervention, compared with usual care. Scenarios 3 and 4 provided an estimate of the relative impact of each activity alone, which forms part of the SEED intervention on total costs and consequences compared with usual care. The data for scenarios 3 and 4 can be used to refine the SEED intervention by identifying those activities of the intervention that are likely to be more cost-effective and, hence, more worthwhile in terms of both intervention development and intervention implementation.
Cost–benefit analysis
The contingent valuation study valued five example scenarios (the main scenario and alternative scenarios 1–4; see Boxes 7–11) for the SEED intervention, defined in terms of the differing impacts that the SEED intervention may have. For analysis purposes, a sixth scenario was used to provide a common baseline for comparison: a do-nothing option in which no aspects of the SEED intervention were provided. This is in contrast to the cost–consequence analysis, in which usual care was used as a comparator. As noted earlier, this option represents the complete absence of care activities, service outputs and patient outcomes that could be caused by the SEED intervention. The rationale for this change in comparator stems from the way in which the WTP scenarios and questions were formulated in the contingent valuation study. In the contingent valuation, respondents were asked to reveal their WTP for a package of care services that would always be present following the introduction of the SEED intervention, compared with a situation in which these services would be absent.
Development of the model structure
Initial model scoping work
The literature was reviewed to identify existing economic models comparing interventions for dementia (see Appendix 6, Model structure). No existing economic evaluation model was identified that was suitable for this analysis. Therefore, a new early economic model was needed.
The economic model seeks to describe the key elements of how dementia may develop over time. The model starts at the point when the individual receives a diagnosis of dementia, and then follows them until death. Thus, the model took a lifetime time horizon for a person with dementia and their families; bereavement services shortly after death were also included in the model.
The model is a simplification of a complex situation and it sought to include those changes in services that were deemed most important and that might occur because of the five activities defined in the cost–consequence analysis. Importance was judged based on where the largest impacts on costs and care preferences might occur. This was informed by a review of the literature on cost-effectiveness, epidemiological and cost studies that had been conducted in this area. These studies were selected based on searches of the bibliographic databases and discussion with the project team, along with their relevance to the UK. Further detail is provided in Appendix 6, Model structure and Dementia progression.
The core components of the model are presented in Figure 14. In this model, following diagnosis, the disease continues to progress until, eventually, palliative care is considered appropriate, or until the person with dementia dies. The place where care is provided (the care setting) at any point in time could be home, care home or hospital. The model assumes that a person may move between these care settings over time.
FIGURE 14.
Model of disease progression and care setting.
Defining model parameters
To use the model in Figure 14, data on event probabilities and cost are required; how these were derived is described in the following section.
Event probabilities
Dementia progression and transitions between places of care were modelled separately (see Appendix 6, Dementia progression and Transition probabilities between care settings). We reviewed the literature for data on dementia progression and care transitions, for example from home to care home and from care home to hospital (further detail is provided in Appendix 6, Transition probabilities between care settings). The target population was all dementia subtypes; the aim was to examine the relationship between a change in care setting and disease progression. Selected data had to be relevant to current service delivery in the UK.
No disease progression data for all dementia subtypes suitable for this purpose were identified. There is considerable uncertainty in the disease trajectory;6 however, a systematic review was identified that provided a framework for the modelling. 216 Data from a health policy model in Alzheimer’s disease were used as a proxy for all dementia subtypes and to derive the probability of moving from one severity level to another (called transition probabilities). 217 Appendix 6, Dementia progression, provides details of how this was done.
Data on movement between setting of care were mainly based on an econometric model that predicted changes in care setting and rate of hospitalisation in the UK. 218 Further details about this model are provided in Appendix 6, Transition probabilities between care settings.
To use the information on disease progression and on changes in place of care in our model, a mapping exercise was conducted. This mapping exercise ensured that the definitions used for severity of disease in the health policy model217 (and described in Appendix 6, Transition probabilities between care settings) were consistent with those used in the setting of care prediction study. 218 Details of the probabilities of disease progression and changes in the place of care and hospitalisation required in the model are described below:
-
Change of care setting from home to care home and vice versa (further detail is provided in Appendix 6, Transition probabilities between care settings).
The average monthly probability of making the transition from the home of the person with dementia to a care home was estimated as 0.0137. 218 A probability of 0.0005 was assumed, based on expert opinion, for the transition from care home to home.
-
Hospital admission and hospital deaths.
A weighted average of the general and psychiatric hospital probabilities was used for the probability of hospitalisation (further detail is provided in Appendix 6, Transition probabilities between care settings and Educational needs assessment for dementia nurse specialist).
-
Discharge from hospital to previous place of care.
Two predictive logistic models for the probability of discharge to a care home from hospital for patients admitted from home for England and Wales were used; these came from the same study. 219 These models covered two populations with dementia: (1) patients with a primary diagnosis of dementia and (2) patients with dementia, but a primary diagnosis of an ambulatory care sensitive condition,220 for which hospitalisation may be preventable with timely care (e.g. bacterial pneumonia). A weighted average from these two models was calculated. 219 For all people admitted from home and subsequently discharged, the probability of being discharged to a care home was, on average, 0.149 (further detail is provided in Appendix 6, Transition probabilities between care settings). We assumed that remaining patients either returned home or died during the index admission. For patients admitted to hospital from a care home, an assumption was made that only 0.5% are discharged to their home.
Mortality
Estimates of mortality rates by age and gender for people with dementia were obtained from the MRC Cognitive Function and Ageing Studies (CFAS) multicentre longitudinal prospective study in the UK. 41 These data may overestimate the risk of death at the earlier stages of dementia and underestimate it for more severe stages. For those people with dementia cared for in hospital, it was assumed that 18% would die during their index admission, regardless of their age and disease severity221 (see Appendix 6, Mortality and palliative care).
Palliative care
Based on the GSF,129 we assumed that a person with dementia would receive dementia-related palliative care only once they had severe cognitive decline. The probability of receiving palliative care in usual practice was estimated by subtracting the time spent on the primary care palliative care register from the individual’s time to death following the diagnosis. The distribution of time spent on the palliative care register was based on published data on the proportion of people with dementia and frailty placed on the palliative care register before their death (20%; 32/160 individuals) and their time on the palliative care register prior to death (median 2.42 weeks, interquartile range 0.43–13.14 weeks). 222 How this was done is described in more detail in Appendix 6, Mortality and palliative care.
Modelled population characteristics
At entry to the model, the characteristics of the hypothetical individuals modelled varied in terms of gender, age, cognitive function, functional ability and behavioural symptoms. With the exception of gender, these characteristics were allowed to change over time as the individual journeyed through the model. The data for these characteristics were for the UK,2,9 except for the distribution of dementia severity, which was based on data from the USA, as no suitable UK data were available. 217 It was assumed that people with dementia eligible for the intervention would have mild to moderately severe dementia at the time of diagnosis (further detail is provided in Appendix 6, Baseline population).
Estimation of costs
The estimation of costs has two components. First, the relative impact of the SEED intervention on the use of dementia care services was estimated. Second, information on the use of services was combined with information on the cost of a single use of each service (the unit cost) to generate the overall costs of these dementia care services.
Impact of the SEED intervention on dementia care services
How the introduction of the SEED intervention might influence the use of other services and costs is shown in the influence diagram (Figure 15). The influence diagram was informed by the theory of change developed in WS3 (see Box 1). For example, the SEED intervention is expected to improve the liaison between services before hospitalisation through increased and more effective use of the SBAR technique. 215 Care home staff training and increased use of SBAR (which are part of the activities that form part of the SEED intervention), in turn, help to avoid unnecessary hospitalisations (a potential impact of using the SEED intervention). The cost of staff training and the use of SBAR were taken as the cost of providing the SEED intervention and the reduction in hospitalisation was used to estimate the cost saving that might be produced by the using the SEED intervention.
FIGURE 15.
Influence diagram of the expected key effects of the SEED intervention components on other services and clinical outcomes. PwD, person with dementia.
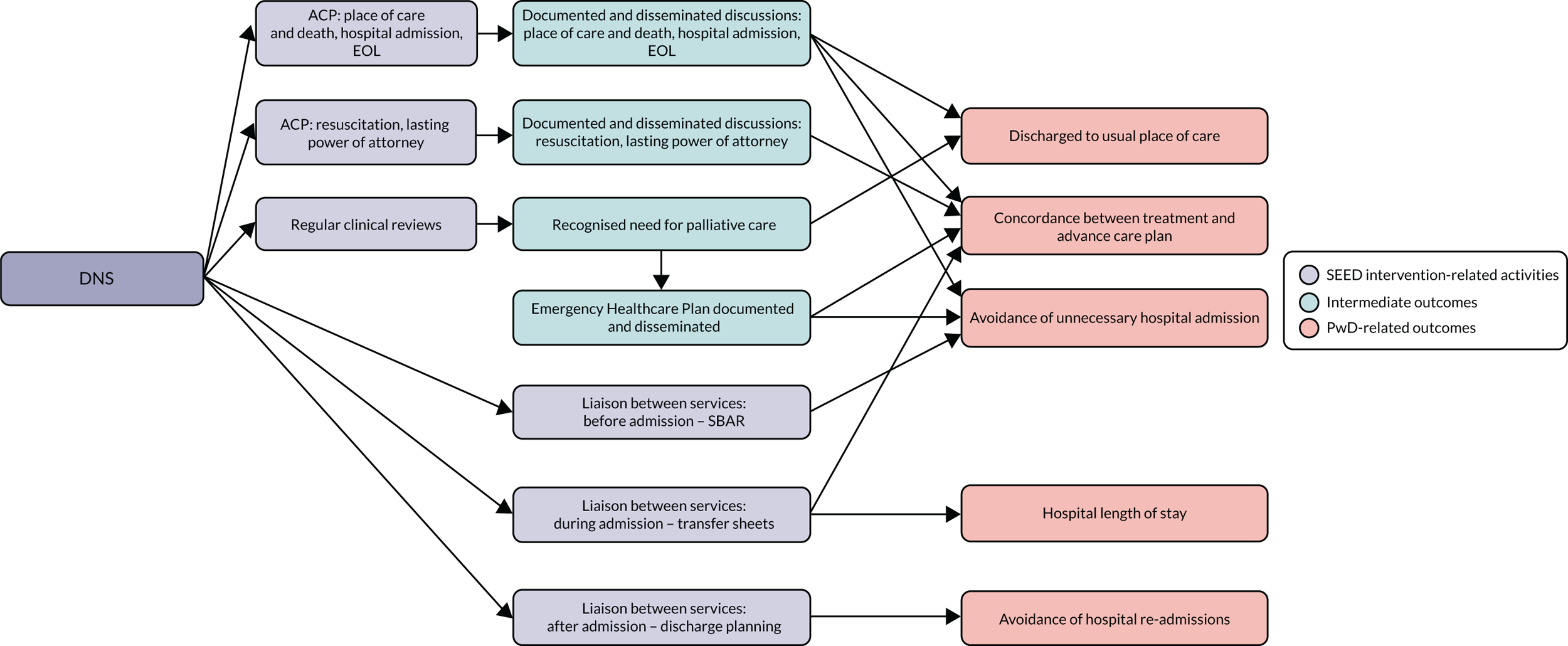
To estimate a possible range of outcomes associated with the SEED intervention, minimum and maximum values for a care activity were specified. These were informed by relevant data identified from literature searches and on plausible assumptions made about the frequency of individual activities and impacts of services (see Appendix 6, Care services and their effects).
Costs (see Appendix 6, Cost of the SEED intervention)
Costs were assessed from a societal perspective, as the SEED intervention could have an impact on costs that fall outside the NHS. The type of costs included in the model relate to the SEED intervention itself, SEED intervention-related activities (e.g. addition of patient’s name to palliative care register and more regular clinical reviews) and service outputs (e.g. care-setting costs and hospitalisation).
The SEED intervention costs
The costs associated with the SEED intervention are estimated to be £20.40 per person with dementia, per month. These are associated with the fixed time and cost of the DNS, supervised by clinical specialists. Consequently, the operating cost of the intervention is assumed to be independent of the number and level of care activities the SEED team will be involved in. A detailed breakdown of the SEED intervention operating costs is presented in Appendix 6, Cost of the SEED intervention.
The SEED intervention-related activities and service outputs
There are costs associated with the care services presented in Figure 15. The resource use estimates were based on expert opinion from the project team. Unit costs were obtained from a routine source223 for each unit of resource use. Family carer time is costed according to the latest government labour force survey, at a median of £11.34 per hour. 224
Care-setting cost
The cost of care at home or in a care home varied according to the severity of dementia. 2 These costs cover services such as inpatient stays, day-care visits and contacts with GPs and other community-based professionals. Adjustments were made to the care-setting costs to avoid double counting the costs of hospitalisations, regular clinical reviews and ACP (see Appendix 6, People with dementia: care-setting costs).
Hospitalisation
The cost of a hospitalisation was calculated as a weighted average of the cost of a hospital episode for the four main reasons (hip fracture, kidney or urinary tract infections, pneumonia and stroke) for hospitalisation in this population. 225 The cost per episode came from the NHS Reference Costs 2015–16,226 using the weighted average of relevant health-care resource group codes. The estimated average cost was £282 per day (further detail is provided in Appendix 6, People with dementia: care-setting costs).
Data analysis (see Appendix 6, Analyses)
A patient-level simulation was conducted in R statistical software. 227 In this model, hypothetical cohorts of 380 people with dementia were considered. The size of cohort was a balance between representing heterogeneity between individuals and the computing time needed for analysis. As described above, each individual in this hypothetical cohort was defined in terms of a unique set of characteristics at the time of diagnosis, when they entered the model. 218,219 Some of these individual characteristics changed over an individual’s journey through the model, such as the age of an individual, whereas others were fixed, such as gender.
The individual characteristics were used to derive the likelihood that a person with dementia moved between care settings (see Appendix 6, Transition probabilities between care settings). To capture the uncertainty in the association between the characteristics of an individual and their movement between care setting and disease progression, transition probabilities were randomly sampled for 800 cohorts (with each cohort including 380 people with dementia). Again, the choice of number of cohorts was pragmatic and represented a trade-off between the computing time required for analysis and ensuring that we could capture uncertainty around the model parameters. Average costs and consequences were estimated for each cohort. The model was run for 120 cycles; each cycle represented 1 month (in total 120 cycles represents 10 years). After 10 years, it was expected that 95% of the simulated patients would have died. Costs are reported in 2017 Great British pounds. Costs and benefits were discounted at an annual rate of 3.5%. 154 This process was repeated for each comparator considered in the cost–consequences analysis and for each of the WTP scenarios and which were compared in the cost–benefit analysis.
Cost–consequences analysis (see Appendix 6, Analyses)
The primary outcomes in this analysis were total cost, length of stay in hospital, time receiving palliative care, the number of people with dementia discharged to their usual place of care from hospital per 1000 patients and the number of avoided hospitalisations per 1000 possible hospitalisations. The total cost is the sum of the discounted costs over the 10-year duration of the model.
Cost–benefit analysis (see Appendix 6, Analyses)
The net benefit of providing a SEED intervention to new incident cohorts over a 5-year period was estimated for each of the scenarios described in Valuing the consequences of the SEED intervention. In the cost–benefit analysis, the WTP scenario with the highest net benefit is the most efficient. By modelling 800 cohorts, a distribution of net benefits was produced for each WTP scenario, and, from this, the mean net benefit and 95% CIs were produced for each scenario. The probability that any given scenario would be most efficient compared with the other scenarios was also estimated.
For the cost–benefit analysis, it was assumed that the intervention would be made available to people diagnosed with early to mild dementia over an illustrative 5-year period. This means that these people receive the intervention (or relevant components) for the rest of their lives, but that anyone diagnosed with early to mild dementia outside this 5-year window would not be offered the SEED intervention. This approach was taken because the implementation would affect the costs and outcomes of those who receive it over their entire lifetime (through the upskilling of community staff), but patients beyond this 5-year period might receive a different intervention as the SEED intervention itself may be replaced after a period of time. However, possible changes in the delivery of health care beyond this 5 years is out of the scope of this analysis and does not influence the results of the cost–benefit analysis. Five years was chosen as this is consistent with the minimum likely time scale before the NICE guidelines might be revised. This is a shorter period than was considered in the contingent valuation work, and so the analysis may overestimate benefits, but, by the same token, total cost for 5 years is likely to be lower than total costs estimated over 10 years.
The contingent valuation work, reported in Valuing the consequences of the SEED intervention, expressed WTP as monthly tax contributions by individual tax payers for all or some components of the SEED intervention to be delivered nationally. Therefore, to make the modelled cost and the WTP data comparable, the cumulative costs and benefits were estimated at the national level. Further details of how this was conducted are described in Appendix 6, Analyses.
Results
Cost–consequence analysis
Table 67 reports the results for two scenarios: scenario one describes a situation in which the data and assumptions used are all more favourable to the SEED intervention (favourable SEED scenario) than to usual care. The second scenario is one in which the data and assumptions are less favourable to the SEED intervention (conservative SEED scenario). For each scenario, the mean difference for each outcome between the SEED intervention and a do-nothing option is presented.
| Analysis | Mean (95% CI) | ||||
|---|---|---|---|---|---|
| Cost (£) per patienta | Length of hospital staya (days) | Duration of palliative carea (months) | Discharge from hospital to usual place per 1000 dischargesa | Avoided hospitalisations per 1000 hospitalisationsa | |
| Favourable SEED scenario | 9930 (–3174 to 23,553) | –1.4 (–2.8 to 0) | 1.91 (1.07 to 2.86) | 25 (–31 to 75) | 161 (119 to 201) |
| Conservative SEED scenario | 2007 (–10,701 to 14,189) | –1.3 (–2.8 to 0.1) | 0.46 (0.09 to 0.9) | 0 (–50 to 44) | 81 (57 to 109) |
As Table 67 shows, the SEED intervention had, on average, reduced rates of hospitalisation, a reduced length of hospital stay and an increased number who were discharged from hospital to usual place of care. There was also increased access to, and duration of, palliative care. However, there was an increased cost. There is considerable uncertainty surrounding these values, with CIs including zero for all outcomes except for duration of palliative care and number of hospitalisations avoided. The CIs for costs, and discharge to usual place of care, are sufficiently wide to include clinically and economically important differences that could favour either the SEED intervention or usual practice.
The SEED conservative scenario is, on average, less costly than the SEED favourable scenario (but still, on average, more costly than usual practice). This is because the SEED favourable scenario increases service use, and hence costs, but is also expected to improve outcomes for people with dementia. These increased costs for the SEED favourable scenario are not fully offset by the reduced need for other services such as hospitalisations.
Further analysis explored the impact of high and low levels of provision of individual SEED intervention-related activities (Table 68). The ACP component was treated as one service in this analysis. As Table 68 illustrates, the individual components of the SEED intervention are not assumed to be additive. Furthermore, some components have no expected impact on some outcomes; for example, there is little to no impact of ACP service provision on length of stay.
| SEED intervention component | Intervention level | Mean (95% CI) | ||||
|---|---|---|---|---|---|---|
| Cost (£) per patienta | Length of hospital staya (days) | Duration of palliative carea (months) | Discharge from hospital to usual place per 1000 dischargesa | Avoided hospitalisations per 1000 hospitalisationsa | ||
| Reviews | Minimum | 5833 (–7946 to 18,342) | –1.35 (–2.8 to –0.1) | 1.17 (0.52 to 1.91) | 13 (–38 to 61) | 120 (82 to 164) |
| Maximum | 6669 (–6512 to 19,907) | –1.36 (–2.7 to 0) | 1.87 (0.99 to 2.94) | 13 (–40 to 63) | 121 (84 to 165) | |
| ACP | Minimum | 4590 (–8362 to 17,295) | –1.32 (–2.8 to 0) | 1.83 (0.96 to 2.95) | 3 (–47 to 51) | 103 (73 to 136) |
| Maximum | 8481 (–4339 to 21,854) | –1.33 (–2.8 to 0) | 1.84 (1.01 to 2.97) | 22 (–30 to 73) | 138 (99 to 180) | |
| SBAR | Minimum | 6067 (–6552 to 19,160) | –1.3 (–2.8 to 0) | 1.85 (1.01 to 2.96) | 13 (–39 to 61) | 106 (72 to 142) |
| Maximum | 7202 (–6176 to 19,945) | –1.32 (–2.8 to 0) | 1.89 (1.04 to 3) | 13 (–39 to 64) | 135 (96 to 177) | |
| Transfer sheets | Minimum | 6553 (–6392 to 19,493) | –1.35 (–2.8 to 0) | 1.85 (0.99 to 2.93) | 13 (–42 to 65) | 121 (84 to 164) |
| Maximum | 6586 (–6222 to 19,496) | –1.43 (–3.1 to 0) | 1.87 (0.98 to 2.97) | 13 (–42 to 65) | 121 (84 to 164) | |
| Discharge planning | Minimum | 6475 (–6102 to 19,756) | –1.32 (–2.9 to 0) | 1.87 (0.96 to 2.98) | 7 (–48 to 57) | 121 (84 to 164) |
| Maximum | 6788 (–5321 to 20,159) | –1.32 (–2.9 to 0) | 1.87 (1.01 to 2.94) | 13 (–42 to 66) | 121 (84 to 166) | |
Cost–benefit analysis
The cost–benefit analysis compares the different variants of the SEED intervention (main scenario and alternative scenarios 1–4). Every WTP scenario is associated with a positive net benefit (column D in Table 9). The scenarios in Table 9 are ordered from lowest to highest net benefits. The main scenario, which incorporates all the activities of the SEED intervention, has the greatest net benefit; alternative scenario 4 has the lowest net benefit. Although there is considerable uncertainty in the net benefit estimates, the 95% CI includes zero for only one scenario (alternative 4). These net benefits are all relative to the provision of no SEED intervention, that is an absence of care activities and service outputs.
Column E in Table 9 shows the incremental net benefit. This illustrates the gain from moving to a scenario that provides more benefits. Column F shows the probability that each scenario provides the greatest net benefit, namely that it is the most efficient. The main scenario has the highest probability of being the most efficient (30%) out of the five compared. However, no scenario clearly stands out because of the considerable uncertainty in the cost and WTP estimates.
Appendix 6 Economic modelling study (workstream 5): additional explanatory text
This appendix provides further details of the methods and results of the economic evaluation.
Dementia care services
The activities of the SEED intervention, the service outputs and the outcomes related to people with dementia are described in detail in Table 69.
| Services and outcomes | Description |
|---|---|
| SEED intervention activities | |
| Regular clinical reviews to support recognition of the need for palliative care input towards the EOL (short name: regular clinical reviews) | Regular clinical review by GP or care home nurse to identify changes in activities of daily living, physical health and cognitive functioning that indicate an increase in care needs as the person with dementia approaches the EOL, and may suggest that they should be added to the primary care palliative care register. The process could be facilitated by the use of formal assessment tools, GSF criteria or the ‘surprise question’. This may improve the recognition of patients approaching the EOL and help to ensure that appropriate care plans are in place |
| Planning discussions offered | The provision of opportunities for timely discussions involving the person with dementia and/or key family members about future care preferences, including EOLC. Systems are in place to prompt primary care and care home staff to prompt the process and to review at specified trigger points (e.g. annual dementia review) |
| Discussions can be around the preferred place of care and death, hospitalisation, DNACPR, tube-feeding, lasting power of attorney and/or preferred decision-maker, and EHCPs | |
| Planning discussions outcome is categorised in two different categories because each category is anticipated to affect different groups of outcomes, and, in some discussion sessions, some topics might not be covered | |
| EHCP documented and disseminated | Ensuring that family carers and professionals are aware of the care plan for the person with dementia, which includes contact details for the most appropriate service to contact in case of an emergency or change in condition. Information is accessible via the EHCP to services potentially involved (e.g. out-of-hours services, paramedics). It is expected that this will result in appropriate care being provided by the most appropriate person, rather than by generic services. Additional information on how to deal with expected acute events that might arise is provided in the EHCP document |
| ACP discussions documented and disseminated | Documentation is completed to an appropriate standard and is relevant to the person with dementia’s current clinical needs. This outcome has been split into the following categories: preferred place of care and death, hospitalisation, DNACPR, tube-feeding, lasting power of attorney and/or preferred decision-maker and EHCPs |
| Each of these refers to the documentation of the corresponding discussion topics (mentioned in planning discussions offered) that reflect the preferences of the patient with respect to each of these topics | |
| ACP documents are expected to have also been flagged and disseminated to all key stakeholders (i.e. care home, out-of-hours services, ambulance service) | |
| Liaison between hospitals, community services and families before admission (short name: SBAR) | Documentation of reasons for referral (e.g. by using a structured tool such as SBAR) to clarify the purpose of the referral and desired outcomes and to prompt staff to consider the referral in the broader context of the patient’s overall condition and where they are on the illness trajectory |
| Improving communication through the use of structured documents might facilitate decision-making. The approach may also help professionals to identify alternative ways of achieving the desired outcomes, which could help to avoid a potential hospitalisation | |
| Liaison between hospitals, community services and families during admission (short name: transfer sheets) | Accessible information provided to hospital staff in the event of hospitalisation to facilitate person-centred care towards, and at, and at the EOL (e.g. through documents such as ‘TOP 5’ or ‘this is me’) |
| Liaison between hospitals, community services and families after discharge (short name: discharge planning) | Transparent communication between hospitals, community services and families to develop a shared understanding of the patient’s needs and how best to meet them and facilitate timely discharge |
| Service outputs and patient-related outcomes | |
| Recognised as being in need of palliative care (proxy: entering patient in palliative care register) | This is a state that there is an anticipated change in care type. The major care transition that happens at the point of palliative care recognition is to stop regular/preventative medication (e.g. statins), and to even stop the dementia-modifying drugs when condition deteriorates significantly. The approach to care in this state is palliative. To capture the palliative care state, we are using the palliative care register as a proxy |
| Practical and emotional support offered to carers prior to and after patient’s death (proxy: bereavement services) | Information is given to families prior to or at the time of death containing practical advice and listing legal requirements (e.g. details of local undertakers, how to register a death), emotional support is offered to families in the period around the death and practical support is offered with immediate tasks (e.g. contacting other relatives). This outcome captures both emotional and practical support before death |
| Meeting the needs of the person with dementia for comfort at the EOL | Comfort at the EOL is promoted through comfort care planning and review. This encompasses physical, emotional and spiritual comfort. Comfort may be promoted through the use of pain assessment scales, which help both in identifying pain and in evaluating the response to pain-relieving medications |
| This is expected to improve care and the QoL and death of individuals by tailoring care to their individual preferences, but is not captured in the model | |
| Concordance between treatment and advance care plan | There is agreement between the patient’s preferences (when recorded in an ACP document) and the actual treatment received. Because ACP is separated in different components now in the interactions table, concordance applies to different categories. One is concordance for aggressive treatments, such as CPR and feeding tube, and the other is for hospitalisations |
| This outcome also describes avoiding overly aggressive, burdensome or futile treatment when a person with dementia is considered to be in a palliative care state. The unwanted interventions or treatments in our case include CPR, tube-feeding and hospitalisations. This is expected to improve the QoL and death of individuals by tailoring care to their preferences, but it is not an outcome captured in the model | |
| Length of hospital stay | Length of inpatient hospital stay which might vary according to the presence or absence of other outcomes |
| Reduced unnecessary hospitalisations and re-admissions | Reduction of hospitalisations for conditions that could have been effectively treated or managed in the community by utilising existing alternative care services (e.g. district nurse). This applies for ambulatory care-sensitive conditions, but not for hospitalisations that are necessary |
| Discharged to usual place of care | The individual patient is discharged to the place of care from which he/she was admitted to the hospital |
| Deaths in preferred place of care | Individual patient dies in the preferred place of care/death |
| Time spent in preferred place of care | Duration for which individuals are being cared in their preferred place of care |
| Hospital deaths | Number of deaths in hospital |
Model structure
The literature was reviewed for cost-effectiveness, epidemiological and cost studies, preferably relevant to the UK. A focused search (Table 70) was run in the MEDLINE database to identify reviews of studies that model Alzheimer’s disease or dementia progression and economic impacts. A review of studies by Green et al. 216 was identified, which described the analytical approach and health outcomes of previously published model-based economic evaluations in Alzheimer’s disease. Using pearl-growing techniques228 to search for more recent evidence, an update of this review was identified. 229 The search strategy used by this review216 was replicated to explore further evidence not captured in the review from 2013 to 2016. The search strategy can be found in Green and Zhang. 217
| # | Searches: MEDLINE® In-Process & Other Non-Indexed Citations (via Ovid) | Results |
|---|---|---|
| 1 | Alzheimer Disease/di, ec [Diagnosis, Economics] | 14,297 |
| 2 | Dementia/di, ec, ep [Diagnosis, Economics, Epidemiology] | 16,096 |
| 3 | Cost-Benefit Analysis/ | 74,599 |
| 4 | “Costs and Cost Analysis”/or “Cost of Illness”/ | 69,980 |
| 5 | Decision Support Techniques/or Decision Making, Computer-Assisted/ | 20,509 |
| 6 | Health Care Costs/ | 35,880 |
| 7 | models, economic/ | 9029 |
| 8 | Models, Statistical/ | 85,192 |
| 9 | 1 or 2 | 28,123 |
| 10 | 3 or 4 or 5 or 6 or 7 or 8 | 267,740 |
| 11 | 9 and 10 | 1316 |
| 12 | “review”/ | 2,421,662 |
| 13 | 11 and 12 | 200 |
The SEED intervention is not expected to slow down the progression of dementia, but rather to support the continuity of care and transitions between settings of care. Therefore, a model structure to account for transitions between settings of care, rather than clinical outcomes, was considered most appropriate. In the identified review, the Assessment of Health Economics in Alzheimer’s Disease model230 and the McDonnell model231 had adopted this approach. Many studies have applied these two models, or modified them, to conduct economic evaluations in Alzheimer’s disease and dementia. 216,217
Our economic model sought to incorporate the costs and benefits to the people with dementia, their family and their carers, as well as to the NHS and Personal Social Services. It sought to describe the key elements of the ‘typical’ illness trajectory of an individual with dementia from the point of diagnosis through progression until death, this being the period during which the effects of SEED intervention are anticipated to occur. Consequently, the model took a lifetime time horizon for a person with dementia. The model structure was selected to provide the best estimates of the most significant cost and care preference consequences of an improvement in the provision of a selection of dementia care services.
The core components of the model are presented in Figure 14. In this model, following diagnosis, the disease continues to progress until eventually palliative care is considered appropriate, or until the person with dementia dies. The care setting at any point in time could be at home, in a care home or in hospital. The model assumes that a person may move between these care settings over time. The structure enables the estimation of the key costs and consequences of dementia care services.
Dementia progression
A focused search was conducted in January 2017 to identify studies that model disease progression in dementia or Alzheimer’s disease. This was required to inform the change in severity of the individual in the model over time, which is a known predictor of changing between settings of care. 218,219,231–236 The search was conducted in MEDLINE® In-Process & Other Non-Indexed Citations, via Ovid, and is presented in Table 71.
| # | Searches | Results (n) |
|---|---|---|
| 1 | disease progression/ | 143,345 |
| 2 | Humans/ | 17,379,680 |
| 3 | DEMENTIA/ | 45,912 |
| 4 | Alzheimer Disease/di [Diagnosis] | 13,680 |
| 5 | 3 or 4 | 57,213 |
| 6 | 2 and 3 and 5 | 45,327 |
| 7 | models, statistical/ | 85,143 |
| 8 | 1 and 2 and 5 and 7 | 36 |
Of the 36 studies identified from the search strategy, a systematic review and a multidomain health policy model by the same author were used to identify further relevant studies. 216,217 Several methods of modelling disease progression were identified in this focused review of evidence. 217,237–239 Our criteria for the selection of method to model progression in dementia were relevance to current UK treatment and care practice, size of the analysis data set and the use of multiple disease domains (e.g. functional capacity) that enable disease progression to be linked to change of care setting and risk of hospitalisation.
Data from Green and Zhang217 were used to model disease progression. Although patients included in this study were diagnosed with Alzheimer’s dementia subtype, the choice was based on the size of the cohort (n = 3009), the recent (2016) publication date of the study and the ability to map the dementia severity characteristics to the characteristics used in Knapp et al. 218 The relatively recent date of the study was considered an important factor in the disease progression model, as it ensures that the cohort is receiving care that is contemporaneous with current practice. Furthermore, in this study, dementia was described using three severity domains: cognitive capacity, behavioural symptoms and functional ability. Each domain had three severity levels: cognition (mild, moderate and severe), behaviour (no problem/mild, moderate and severe) and functional ability (no problem, mild and severe). The definitions are provided in Table 72.
| Domain | Severity level | Definition |
|---|---|---|
| Cognitive function | Mild | 21 ≥ MMSE ≥ 26 |
| Moderate | 10 ≥ MMSE ≥ 20 | |
| Severe | 0 ≥ MMSE ≥ 9 | |
| Behaviour and mood | No problem/mild | NPI-Q: each item ≥ 1 |
| Moderate | NPI-Q: each item ≥ 2, with at least one item equal to 2 | |
| Severe | NPI-Q: at least one item equal to 3 | |
| Functional ability | No problem | 0 ≥ FAQ total ≥ 8 |
| Moderate | 9 ≥ FAQ total ≥ 23 | |
| Severe | 24 ≥ FAQ total ≥ 30 |
Although more methodologically sophisticated models of disease progression were available in the literature,216 such as the Getsios et al. 237 microsimulation model or the Peninsula Technology Assessment Group (PenTAG) model,233 these were based on cohorts of patients recruited before 2000, when the disease-modifying acetylcholinesterase inhibitors were not yet adopted into current practice. 240
In addition, in a more modern cohort, the type of care received is also expected to be more representative. Other studies, such as the one by Stallard et al. ,239 that modelled disease progression in Alzheimer’s were considered; however, to model disease progression according to their suggested methodology would be computationally burdensome, and would be technically challenging to use when secondary data are the main type of data available.
As described previously, in Green and Zhang,217 which was used to model disease progression, the severity of the condition was described using three severity domains: cognitive capacity, functional ability and behavioural symptoms. These three domains were measured using the Mini Mental State Examination (MMSE), the Functional Activities Questionnaire (FAQ) and the Neuropsychiatric Inventory Questionnaire (NPI-Q), respectively. These instruments are presented in Table 72.
The MMSE is a 30-question questionnaire, widely used in clinical settings to measure the global cognitive ability of an individual. 241 To measure the progression in behavioural symptoms, data collected using the NPI-Q were used. The NPI-Q is a self-administered questionnaire that identifies the presence and measures the severity of neuropsychiatric symptoms in patients with dementia and their informants. 242 Functional abilities were measured using the FAQ. The FAQ measures the activities of daily living and can accurately discriminate individuals according to their functional levels. 243
The transition probabilities from each severity level to the other severity levels for each severity domain are reported in Table 73. These were derived from the transition matrix reported in Green and Zhang. 217 These probabilities are a simplified version of the Green and Zhang217 disease progression model which estimates the probability of multidomain severity transitions over 1 year. Green and Zhang217 defined a severity state as the combined severity in cognition, functional ability and behavioural symptoms, resulting in a 20-state disease progression model. For example, if an individual had mild cognitive decline, mild functional impairment and mild behavioural symptoms, then this individual is in state ‘1–1–1’. If an individual had the same characteristics but severe cognitive decline instead, then the individual would be in state ‘3–1–1’, where ‘1’ stands for mild, ‘2’ for moderate and ‘3’ for severe.
| Dementia severity | Cognitive function | Functional ability | Behavioural symptoms |
|---|---|---|---|
| Mild to mild | 0.96 | 0.9699 | 0.9359 |
| Mild to moderate | 0.0349 | 0.0233 | 0.0598 |
| Mild to severe | 0.0011 | 0.0068 | 0.0043 |
| Moderate to mild | 0.011 | 0.0386 | 0.0032 |
| Moderate to moderate | 0.9726 | 0.9421 | 0.9631 |
| Moderate to severe | 0.0164 | 0.0194 | 0.0336 |
| Severe to mild | 0 | 0 | 0 |
| Severe to moderate | 0.0067 | 0.0411 | 0.00702 |
| Severe to severe | 0.9933 | 0.96 | 0.9929 |
In contrast to Green and Zhang,217 it was assumed in our model that progression within a severity domain was independent from the severity stage in other domains. Consequently, the 20-state model reported by Green and Zhang217 was reduced to a three-state model for all domains. To estimate the probabilities of progressing to the next severity stage for each severity domain in our model, the number of patients in a specific severity level at the beginning of the study was multiplied by the sum of the transition probabilities to the next severity levels. This resulted in a 3 × 3 transition matrix for each severity domain. Finally, it was assumed that the probability of the next transition between severity levels does not depend on how much time had already elapsed. This means that the probability of progression to the next severity level was the same for someone who had spent 1 month at the current severity level as someone who had spent 12 months at that same level. As the model allowed transitions between severity levels on a monthly basis, annual probabilities of disease progression were transformed into monthly probabilities.
Transition probabilities between care settings
A predictive logistic model for the probability of making the transition from the home of the person with dementia to a care home for the UK setting estimated by Knapp et al. 218 was used. The precise coefficient values and the CIs were provided to us directly by personal communication with the authors (Professor Martin Knapp, London School of Economics and Political Science, London, 17 November 2017, personal communication; Kia–Chong Chua, King’s College London, London, 17 November 2017, personal communication); these are reported in Table 74 (reported to two decimal places only; more precise estimates can be found elsewhere). The results of this model were used to estimate the probability of a patient being admitted to a care home within a 6-month period.
| Coefficients | OR | CI |
|---|---|---|
| MMSE | 1.08 | 1.00 to 1.16 |
| MMSE (squared) | 1 | 0.99 to 0.99 |
| Year (reference: 2006 or earlier) | ||
| 2007 | 1.00 | 0.69 to 1.46 |
| 2008 | 1.27 | 0.89 to 1.81 |
| 2009 | 1.08 | 0.75 to 1.55 |
| 2010 or later | 1.00 | 0.71 to 1.41 |
| Prior 12 months: general hospital inpatient care (reference: no history) | 1.54 | 1.22 to 1.94 |
| Prior 12 months: mental health inpatient care (reference: no history) | 2.59 | 1.42 to 4.74 |
| Age | 1.04 | 1.02 to 1.06 |
| Gender (0 = female, 1 = male) | 1.11 | 0.86 to 1.44 |
| Ethnicity (reference: white) | ||
| Caribbean/African | 0.57 | 0.38 to 0.86 |
| East/South Asian | 0.57 | 0.24 to 1.34 |
| Mixed/unknown | 0.30 | 0.12 to 0.74 |
| Partner (reference: no partner) | 0.60 | 0.45 to 0.80 |
| Living alone (reference: not) | 1.29 | 0.99 to 1.67 |
| Living conditions (HoNOS11) | ||
| Minor problems only | 1.59 | 1.19 to 2.12 |
| Significant problems | 1.65 | 1.18 to 2.32 |
| Activities of daily living (HoNOS10) | ||
| Minor problems only | 1.17 | 0.72 to 1.91 |
| Significant problems | 1.87 | 1.21 to 2.90 |
| Physical illness (HoNOS5) | ||
| Minor problems only | 1.10 | 0.81 to 1.51 |
| Significant problems | 1.23 | 0.91 to 1.68 |
| Agitated (HoNOS1) | ||
| Minor problems only | 1.41 | 1.05 to 1.89 |
| Significant problems | 1.98 | 1.45 to 2.70 |
| Depression (HoNOS7) | ||
| Minor problems only | 0.94 | 0.72 to 1.24 |
| Significant problems | 1.13 | 0.79 to 1.60 |
| Relationship (HoNOS9) | ||
| Minor problems only | 1.10 | 0.82 to 1.48 |
| Significant problems | 1.22 | 0.88 to 1.69 |
| Constant term | 0.002 | < 0.01 to < 0.01 |
To transform the 6-month probabilities derived by the logistic regression to the monthly probabilities required in our model, it was assumed that the rate of moving from home to a care home is constant over the 6-month period. Therefore, for the monthly probability of moving to a care home, first, the rate (r) was calculated by:
This rate was used to derive the monthly probability (p):
The probability of a care home admission differed between patients according to a patient’s characteristics (e.g. gender, ethnicity). Moreover, the probability of a patient being moved to a care home varied over time, as some of the patient’s characteristics (e.g. age, severity) were updated in every cycle of the model. For patient characteristics, the average value of the covariates were assigned to each patient. The average monthly probability of moving from the home of the person with dementia to a care home was estimated to be 0.0137.
When additional effects on the probability of admission to a care home were available in the literature, we applied these on the probability estimated from the logistic regression model. For example, a hazard ratio of 1.12 (from Cepoiu-Martin et al. 244) of having a different dementia subtype to Alzheimer’s disease was applied to the logistic regression before the probability of moving from the home of the person with dementia to a care home was estimated. An assumption was made that the probability of moving from a care home to the person’s own home was 0.0005 per month, as experts from the project team suggested that this event is rare.
As described in Table 74, disease severity in terms of cognitive capacity, functional ability and behavioural symptoms is expected to have an impact on the likelihood of moving between settings of care. In the logistic regressions used to estimate the likelihood of moving between settings of care (see Table 74), dementia severity in these three domains is captured by MMSE scores (for cognitive capacity), activities of daily living [Health of the Nation Outcome Scales (HoNOS 10)] (for functional ability), and agitation (HoNOS 1) and depression (HoNOs 7) (for behavioural symptoms). However, in our model, severity in these three domains is assumed to change over time, and this will have an impact on the probability of moving between settings of care. Because different instruments are used by Green and Zhang,217 whose data we are using to model disease progression, and Knapp et al. ,218 whose data we are using to model transitions between settings of care, the severity levels used in Green and Zhang217 were mapped onto severity levels in Knapp et al. ,218 as outlined below, for the three domains: cognitive decline, functional ability and behavioural symptoms.
Cognitive decline was measured in both studies using the MMSE instrument. Green and Zhang217 classified patients in three discrete MMSE severity categories (Table 75). However, in the logistic regression models estimated by Knapp et al. ,218 the MMSE was analysed as a continuous measure. In our economic evaluation model, it was assumed that patients who had mild, moderate or severe cognitive decline were assigned a MMSE value of 24, 15 and 5, respectively.
| Cognitive decline (MMSE severity) | MMSE score | ||
|---|---|---|---|
| Green and Zhang217 | Knapp et al.218 | Model | |
| Mild | 21–26 | 21–30 | 24 |
| Moderate | 10–20 | 11–20 | 15 |
| Severe | 0–9 | 0–10 | 5 |
Progression in functional ability was modelled in Green and Zhang217 using the FAQ. This questionnaire measures instrumental activities of daily living (IADLs). Knapp et al. 218 use the 10th item from the HoNOS245 to measure functional ability. The HoNOS describes problems with both basic activities of daily living and more complex activities implicitly (IADLs), and is categorised in three severity levels, as described in Table 76. In the logistic regression, reported by Knapp et al. ,218 functional ability was reported by three discrete categories: ‘no problems’, ‘minor problems’ and ‘significant problems’ (see Table 76). An assumption was made that the ‘minor problems’ category in the HoNOS reflects moderate severity of functional impairment, according to the FAQ, and that the ‘significant problems’ category in the HoNOS10 reflects severe impairment in function, according to the FAQ (see Table 76).
| Green and Zhang217 | Knapp et al.218 |
|---|---|
| No problems, 0 ≤ FAQ ≤ 8 | Baseline, (HoNOS10) = 0 |
| Moderate, 9 ≤ FAQ ≤ 23 | Minor problems, (HoNOS10) = 1 |
| Severe, 24 ≤ FAQ ≤ 30 | Significant problems, (HoNOS10) = 2, 3 and 4 |
Behavioural symptoms in Green and Zhang217 are captured by the NPI-Q, whereas, in the logistic regression by Knapp et al. ,218 behavioural symptoms are captured by HoNOS1 (agitation) and HoNOS7 (depression). In the logistic regression, Knapp et al. 218 categorise the HoNOS1 (agitation) and HoNOS7 (depression) in three discrete categories (‘no problems’, ‘minor problems’ and ‘significant problems’), as shown in Table 77. The behavioural disturbance categories were mapped between Knapp et al. 218 and Green and Zhang,217 as described in Table 77. In our model, if a patient is considered to have mild behavioural symptoms by the NPI-Q, then this would match to ‘no HoNOS1 agitation problems’ and ‘no HoNOS7 depression problems’. Moderate behavioural symptoms as measured by the NPI-Q would match to ‘minor HoNOS1 agitation problems’ and ‘minor HoNOS7 depression problems’ in the logistic regression used by Knapp et al. 218
| Green and Zhang217 (NPI-Q) | Knapp et al.218 | |
|---|---|---|
| HoNOS1 (agitation) | HoNOS7 (depression) | |
| No problem/mild NPI-Q: each item ≤ 1 | Baseline, (Honos1) = 0 | Baseline, (HoNOS7) = 0 |
| Moderate NPI-Q: each item ≤ 2, with at least one item = 2 | Minor problems, (HoNOS 1) = 1 | Minor problems, (HoNOS7) = 1 |
| Severe NPI-Q: at least one item = 3 | Significant problems, (HoNOS 1) = 2, 3 and 4 | Significant problems, (HoNOS7) = 2, 3 and 4 |
Hospitalisation
The predictive logistic model for the probability of hospitalisation was based on a UK population. 218 The precise coefficient values and the CIs were provided to us directly by personal communication with the authors (Professor Martin Knapp, London School of Economics and Political Science, London, 17 November 2017, personal communication; Kia–Chong Chua, King’s College London, London, 17 November 2017, personal communication), and are reported to two decimal places in Table 78 (more precise estimates are reported elsewhere). 218
| Coefficient | General hospital admission | Psychiatric hospital admission | ||
|---|---|---|---|---|
| OR | CI | OR | CI | |
| MMSE | 0.93 | 0.88 to 0.97 | 0.94 | 0.86 to 1.02 |
| MMSE (squared) | 1.00 | 0.10 to 1.00 | 1.00 | 0.99 to 1.00 |
| Year (reference: 2006 or earlier) | ||||
| 2007 | 1.05 | 0.83 to 1.33 | 0.86 | 0.55 to 1.36 |
| 2008 | 1.33 | 1.07 to 1.67 | 0.90 | 0.58 to 1.39 |
| 2009 | 1.23 | 0.98 to 1.54 | 0.50 | 0.31 to 0.83 |
| 2010 or later | 1.16 | 0.93 to 1.43 | 0.36 | 0.22 to 0.58 |
| Prior 12 months: general hospital inpatient care (reference: no history) | 2.21 | 1.92 to 2.55 | 2.40 | 1.75 to 3.29 |
| Prior 12 months: mental health inpatient care (reference: no history) | 0.83 | 0.48 to 1.42 | 7.73 | 4.47 to 13.35 |
| Age | 1.04 | 1.03 to 1.05 | 0.96 | 0.94 to 0.98 |
| Gender (0 = female, 1 = male) | 1.37 | 1.16 to 1.61 | 1.16 | 0.83 to 1.63 |
| Ethnicity (reference: white) | ||||
| Caribbean/African | 0.68 | 0.53 to 0.88 | 0.89 | 0.54 to 1.47 |
| East/South Asian | 0.43 | 0.25 to 0.79 | 1.21 | 0.53 to 2.75 |
| Mixed/unknown | 1.35 | 0.93 to 1.96 | 0.87 | 0.36 to 2.09 |
| Partner (reference: no partner) | 0.77 | 0.65 to 0.93 | 1.63 | 1.11 to 2.39 |
| Living alone (reference: not) | 1.25 | 1.05 to 1.49 | 2.56 | 1.76 to 3.71 |
| Living conditions (HoNOS11) | ||||
| Minor problems only | 1.48 | 1.22 to 1.79 | 1.90 | 1.28 to 2.82 |
| Significant problems | 1.75 | 1.37 to 2.22 | 2.06 | 1.32 to 3.21 |
| Activities of daily living (HoNOS10) | ||||
| Minor problems only | 1.15 | 0.91 to 1.46 | 1.23 | 0.69 to 2.19 |
| Significant problems | 1.25 | 0.99 to 1.57 | 0.97 | 0.56 to 1.68 |
| Physical illness (HoNOS5) | ||||
| Minor problems only | 1.30 | 1.07 to 1.57 | 1.76 | 1.13 to 2.73 |
| Significant problems | 2.15 | 1.78 to 2.60 | 1.70 | 1.10 to 2.64 |
| Agitated (HoNOS1) | ||||
| Minor problems only | 1.11 | 0.92 to 1.35 | 1.86 | 1.23 to 2.82 |
| Significant problems | 1.50 | 1.21 to 1.88 | 3.56 | 2.36 to 5.45 |
| Depression (HoNOS7) | ||||
| Minor problems only | 0.96 | 0.81 to 1.14 | 0.92 | 0.63 to 1.34 |
| Significant problems | 1.49 | 1.18 to 1.88 | 2.04 | 1.35 to 3.09 |
| Relationship (HoNOS9) | ||||
| Minor problems only | 1.01 | 0.84 to 1.22 | 1.20 | 0.80 to 1.81 |
| Significant problems | 0.98 | 0.78 to 1.24 | 1.71 | 1.13 to 2.60 |
| Constant term | 0.01 | < 0.01 to 0.03 | 0.42 | 0.07 to 2.67 |
A logistic regression of general inpatient hospitalisations and a logistic regression for mental care hospitals were available. To estimate the probability of any type of hospitalisation, we used a weighted average of the probabilities of these logistic regressions. The proportion of patients admitted to a mental care hospital and the proportion of patients admitted to a general care hospital in Knapp et al. 218 were applied as weights as:
An assumption was made that the probability of being hospitalised from home and from a care home is derived from the same predictive logistic model. This predictive regression model provided the probability of a patient being admitted to a care home within a 6-month period. To transform the 6-month probabilities derived by the logistic regression to monthly probabilities, the same approach described above for transition between home and care home was used. Logistic regressions of a general hospital admission from any care setting produced an average monthly probability of 0.0331 for general hospitals and 0.00121 for psychiatric hospital admissions.
Hospital length of stay
Length of stay in hospital was estimated by combining two different sources. A median of 11 days in hospital for patients with dementia has been reported in a previous study. 221 However, a non-parametric distribution of length of stay in hospitals was provided directly to us by personal communication with Sampson and colleagues (Professor Elizabeth Sampson and Dr Victoria Vickerstaff, University College London, London, 14 November 2017, personal communication). These data reported the length of stay of 805 patients with dementia admitted to an acute general hospital in London, for > 2 days. Because we wanted to capture all acute inpatient hospitalisations, the proportion of patients with dementia being admitted overnight, but for < 2 days, was used to update the probability distribution of length of stays. This was carried out by estimating the proportion of patients with dementia admitted for 1 day and those admitted for > 1 day. These figures were available in a report based on Hospital Episode Statistics for 2010–11. 225 According to this report,225 16.9% of inpatient non-elective hospitalisations were admitted to hospital for 1 day, whereas 88.1% of patients were admitted for > 1 day. The distribution of length of stay can be seen in Figure 16.
FIGURE 16.
Distribution of days in hospital for non-elective hospitalisations of people with dementia (length of stay for unplanned admissions of > 1 day).
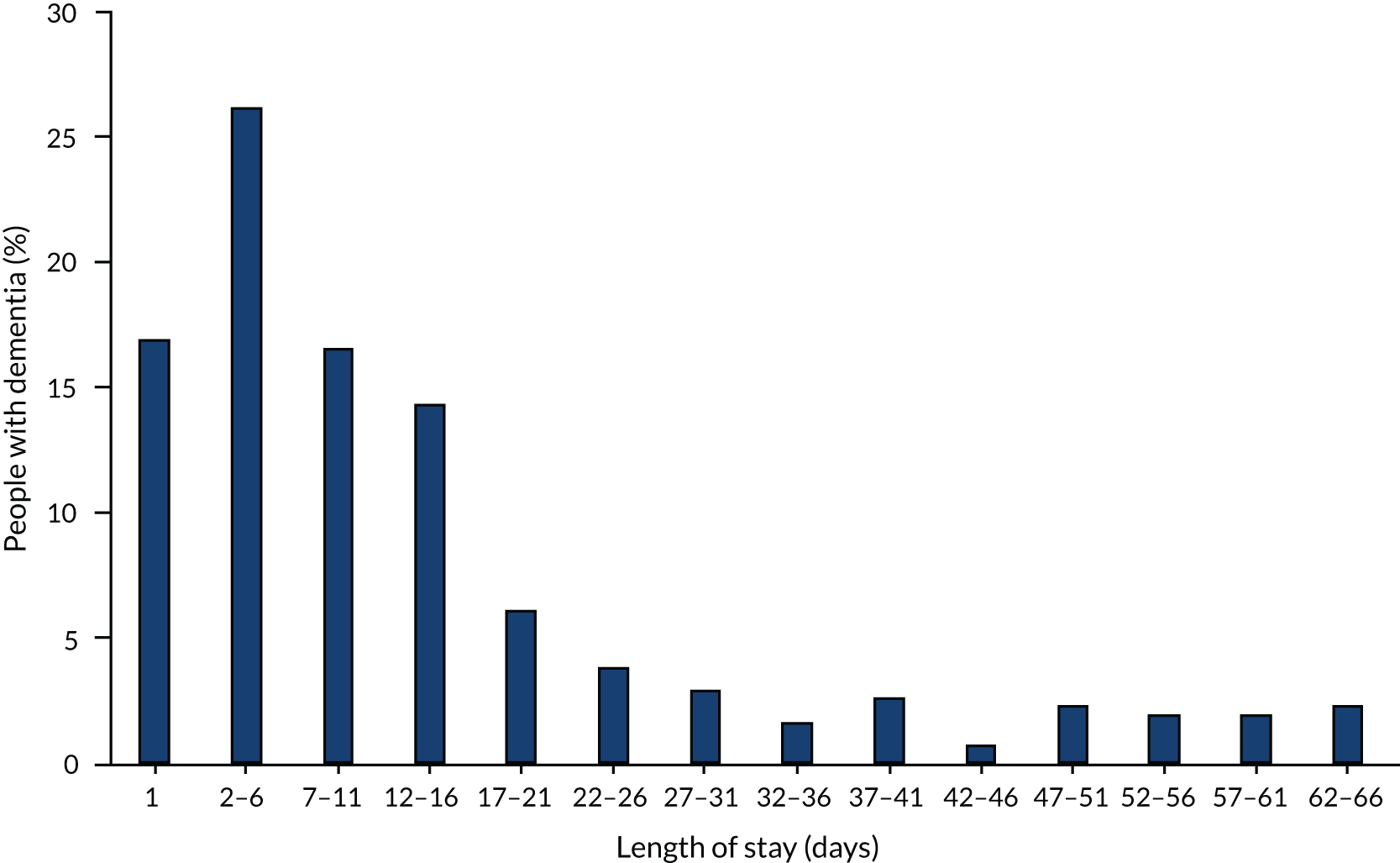
In contrast to settings-of-care states, the ‘hospital state’ in the model varied according to the length of time individuals spend in hospital. A length of stay for each patient who was hospitalised was sampled from the distribution reported in Figure 16. Progression in age and disease severity in the ‘hospital state’ were automatically adjusted in the model to account for different length of stay.
To transform the monthly probabilities of progressing to a different severity level for cognition to probabilities considering a different duration of hospitalisation, it was assumed that the rate of progressing to a different severity level was constant over time. Therefore, to estimate the probability of progressing to a different severity level for different length of stay, the rate was calculated by:
This rate was used to derive the probability that is conditional on different lengths of stay in the model:
where ‘LoS’ is length of stay.
Probability of discharge from hospital
A probability of discharge to the previous place of care was assigned to patients who were admitted to hospital. Two predictive logistic models for the probability of discharge to a care home from hospital for patients admitted from home for the England/Wales setting were used (Table 79). 219 The precise coefficient values and the CIs were provided to us directly by the author (Dr Panos Kasteridis and Mrs Anne Mason, University of York, York, 14 November 2017, personal communication). However, only two decimal places for the estimates are reported in Table 79. The more precise values can be found elsewhere. 218 These models differed by their populations: patients with a primary diagnosis of dementia and patients with dementia with a primary diagnosis of ambulatory care sensitive condition. 220 Using a weighted average, for patients admitted from home, the probability of being discharged to a care home was, on average, 0.149. It was then assumed that the rest of the patients returned home or died during the index admission.
| Explanatory variable | Admissions for dementia | Admissions for ambulatory care sensitive condition | ||
|---|---|---|---|---|
| OR | CI | OR | CI | |
| QOF dementia score (%) | 1.0 | 0.998 to 1.00 | 0.99 | 0.99 to 1 |
| Age | 1.0 | 1.02 to 1.03 | 1.03 | 1.02 to 1.03 |
| Male | 0.86 | 0.80 to 0.91 | 0.88 | 0.84 to 0.91 |
| White ethnicity | 0.99 | 0.91 to 1.09 | 1.09 | 1.04 to 1.15 |
| Alzheimer’s disease | 1.19 | 1.11 to 1.28 | 1.04 | 0.99 to 1.09 |
| Vascular dementia | 1.19 | 1.11 to 1.28 | 1.10 | 1.05 to 1.15 |
| Urinary incontinence | 1.24 | 1.07 to 1.44 | 1.23 | 1.13 to 1.34 |
| Faecal incontinence | 1.28 | 1.05 to 1.56 | 1.32 | 1.19 to 1.48 |
| Fall (excludes hip fracture cases) | 1.16 | 1.05 to 1.30 | 1.20 | 1.13 to 1.27 |
| Hip fracture (excludes falls) | 1.48 | 1.00 to 2.17 | 1.44 | 1.28 to 1.63 |
| Cancer | 1.38 | 1.15 to 1.65 | 1.05 | 0.96 to 1.15 |
| Myocardial infarction | 0.99 | 0.76 to 1.27 | 1.11 | 0.99 to 1.23 |
| Peripheral vascular disease | 0.85 | 0.68 to 1.06 | 0.87 | 0.79 to 0.95 |
| Cerebrovascular disease | 1.10 | 1.01 to 1.20 | 1.25 | 1.19 to 1.32 |
| Delirium | 1.04 | 0.91 to 1.19 | 1.22 | 1.04 to 1.42 |
| Senility | 1.30 | 1.17 to 1.45 | 1.20 | 1.13 to 1.28 |
| Total diagnoses (count) | 1.13 | 1.11 to 1.14 | 1.11 | 1.10 to 1.11 |
| ACSC: acute (reference) | ||||
| ACSC: chronic d | 0.71 | 0.68 to 0.74 | ||
| ACSC: vaccine | 1.09 | 1.04 to 1.14 | ||
| Carer 1: % of LSOA population providing 1–19 hours per week of unpaid care | 1.04 | 1.01 to 1.06 | 1.02 | 1.00 to 1.03 |
| Carer 2: % of LSOA population providing 20–49 hours per week of unpaid care | 0.92 | 0.84 to 1.00 | 0.96 | 0.91 to 1.00 |
| Carer 3: % of LSOA population providing ≥ 50 hours per week of unpaid care | 0.93 | 0.89 to 0.98 | 0.90 | 0.88 to 0.93 |
| Living alone: % of LSOA population aged ≥ 60 years living alone | 0.99 | 0.99 to 1.00 | 0.99 | 0.99 to 0.99 |
| Deprivation 1: % of LSOA population aged ≥ 60 years claiming guarantee credit | 0.99 | 0.98 to 0.99 | 0.99 | 0.99 to 0.99 |
| Deprivation 2: % of LSOA population aged ≥ 60 years claiming savings credit | 1.02 | 1.00 to 1.04 | 1.02 | 1.00 to 1.03 |
| Deprivation 3: % of LSOA population aged ≥ 60 years claiming both types of pension credit | 1.02 | 1.01 to 1.03 | 1.03 | 1.02 to 1.03 |
| Care home beds: beds per 100 population aged ≥ 60 years within 10 km of LSOA centroid | 1.01 | 0.99 to 1.04 | 1 | 0.98 to 1.02 |
| Urban residential area (population of > 10,000) | 1.06 | 0.97 to 1.16 | 1.10 | 1.04 to 1.16 |
| Year = 2006/7 | 1 | 0.90 to 1.01 | ||
| Year = 2007/8 | 0.95 | 0.86 to 1.05 | 0.95 | |
| Year = 2008/9 | 0.93 | 0.84 to 1.03 | 0.91 | 0.86 to 0.96 |
| Year = 2009/10 | 0.85 | 0.78 to 0.94 | 0.73 | 0.69 to 0.77 |
| Year = 2010/11 | 0.67 | 0.61 to 0.74 | 0.61 | 0.58 to 0.65 |
| Constant | < 0.01 | < 0.01 to < 0.01 | < 0.01 | < 0.01 to < 0.01 |
The probability of returning to home if a patient was admitted from home is a joint probability, calculated as:
For patients admitted from a care home, an assumption was made that only 0.5% are discharged to their home.
Mortality and palliative care
Estimates of mortality rates, conditional on age and gender, for people with dementia were obtained from a study based on the MRC CFAS multicentre longitudinal prospective study in the UK. 41 These are reported in Table 80. Because the risk of mortality is not conditional on stage of dementia, the mortality risk may be overestimated at the earlier stages of dementia and underestimated at the later stages of dementia. Based on the mean time to death, we fitted exponential distributions to derive the monthly probability of death for each age group and gender.
| Age group (years) | Mortality | Probability of entering the register | ||||
|---|---|---|---|---|---|---|
| Control | SEED | |||||
| Women | Men | Women | Men | Women | Men | |
| 65–69 | 0.00716 | 0.00488 | 0.0075 | 0.0051 | 0.0082 | 0.0053 |
| 70–79 | 0.0096 | 0.01070 | 0.0103 | 0.0115 | 0.0116 | 0.0131 |
| 80–89 | 0.01202 | 0.01369 | 0.0131 | 0.0151 | 0.0152 | 0.0179 |
| ≥ 90 | 0.01468 | 0.01682 | 0.0161 | 0.0191 | 0.0197 | 0.0237 |
According to the World Health Organization, palliative care is a care approach that focuses on improving the QoL of patients with a life-threatening disease such as dementia, by preventing and relieving suffering by assessing timely care, assessing and treating pain and other psychosocial and spiritual problems. 28 Although an individual is expected to receive well-co-ordinated, high-quality care,246 there is no clear time point at which palliative care should replace curative treatment. 247 This should vary from patient to patient, but it is important to identify people nearing the EOL to ensure that they are entered in a register to facilitate better planning and care co-ordination. 246 According to the GSF, individuals are approaching the EOL when they are likely to die in the next 12 months. 248 To be in line with the GSF criteria for providing EOLC, it was assumed that a person with dementia would receive dementia-related palliative care only once they had a level of dementia severity defined by severe cognitive decline.
No good evidence on time to palliative care or the probability of receiving palliative care was identified. The probability of receiving palliative care in current practice was derived using simulation methods by subtracting the time spent on the palliative care register from the time to death for age and gender categories. The distribution of time spent on the palliative care register was provided by Zheng et al. ,222 who reported the proportion of patients with dementia and frailty who are placed in the palliative care register before their death. The median time on the palliative care register for older people with dementia and frailty was reported to be 2.42 weeks (interquartile range 0.43–13.14 weeks).
According to Zheng et al. ,222 20% (32/160) of those diagnosed with frailty and dementia spent some time on the palliative care register. In our model, we assumed that individuals would be at a different risk of dying according to whether or not they are entering palliative care. The 20% of patients who were expected to spend some time in palliative care were assigned the general age- and gender-specific mortality risk until they had the dementia severity level of severe cognition. From that point, they were assigned a probability of entering palliative care. Once they were receiving palliative care, they were assigned a time to death from the distribution of time spent on the palliative care register before death. The 80% of people who never spent any time in palliative care were assumed to have a mortality risk associated with their age and gender based on data from Xie et al. 41 The probabilities of entering the palliative care register in each cycle for the control arm and the SEED intervention arm are provided in Table 80.
In-hospital mortality
The probability of dying in the hospital was taken from a study by Sampson et al. ;221 it was 18.1% for patients with dementia. When patients with dementia were in the ‘hospital state’, they were subject to dying with a probability of 0.18, rather than a probability presented in Table 80. This may lead to an overestimation of mortality in the early stages of dementia.
Baseline population
When entering the model, each patient was assigned multiple characteristics that would influence disease progression, the transition between settings of care and the time to death. The population characteristics that are used as covariates in the predictive logistic models to estimate the likelihood of changing setting of care are reported in Tables 81 and 82. These patient characteristics are also used to estimate the time to death of each individual entering the model.
| Characteristic | Probability or % | |
|---|---|---|
| Age (years) | Female | Male |
| 65–69 | 8.7 | 10.7 |
| 70–74 | 11.2 | 16.3 |
| 75–79 | 20.1 | 21.4 |
| 80–84 | 27.8 | 28.7 |
| ≥ 85 | 32.2 | 22.9 |
| Cognitive capacity severity (%) | Incident cohort | Prevalent cohort |
| Mild | 80 | 58.8 |
| Moderate | 15 | 36.8 |
| Severe | 5 | 4.4 |
| Functional ability severity (%) | Incident cohort | Prevalent cohort |
| Mild | 75 | 57.9 |
| Moderate | 20 | 31.3 |
| Severe | 5 | 10.8 |
| Behavioural symptoms severity (%) | Incident cohort | Prevalent cohort |
| Mild | 65 | 22.7 |
| Moderate | 25 | 54.8 |
| Severe | 10 | 22.5 |
| Characteristic | % |
|---|---|
| Dementia subtype | |
| Alzheimer’s disease | 62 |
| Vascular | 17 |
| Mixed | 10 |
| Lewy bodies | 4 |
| Frontotemporal | 2 |
| Parkinson’s disease | 2 |
| Other | 3 |
| Ethnicity | |
| White | 83 |
| Caribbean/African | 10 |
| East/South Asian | 3 |
| Mixed/unknown | 4 |
The proportion of incident cases of people with dementia belonging to different age groups was derived from Matthews et al. 8 This study provided separate estimates by gender. Information on the severity of patients at the time of diagnosis was also sought. However, evidence was not available to inform the baseline severity of all three severity domains (cognitive decline, functional ability and behavioural symptoms) for patients at the point of diagnosis. The baseline severity of a prevalent cohort for the three different severity domains was derived by Green and Zhang. 217 To reflect a milder severity profile of patients at the point of diagnosis, an assumption was made that almost half of the patients in the severe and moderate stages in each severity domain would be in the mild stage at the point of diagnosis. Information on dementia subtype and the ethnicity of people with dementia was derived from the 2014 dementia report by the Alzheimer’s Society2 and Knapp et al. ,218 respectively.
Additional patient characteristics were also assigned to patients in the model. These characteristics were required to inform the covariates in the logistic regression used to estimate transitions between settings of care. The characteristics of patients required to estimate the probability of moving to a care home, and being admitted to hospital, are reported in Table 83. 218 To estimate the probability of being discharged back to home after a hospitalisation, patient characteristics from Kasteridis et al. 219 were used to inform the covariates in the logistic regression for discharge back home. These patient characteristics, reported in Table 84, were the weighted average of the four groups of patients described in the study. 219
| Patient characteristic | Percentage of individuals (%) |
|---|---|
| Partner | 36 |
| Living alone | 74 |
| Living conditions | |
| Minor problems only | 15 |
| Significant problems | 10 |
| Activities of daily living | |
| Minor problems only | 25 |
| Significant problems | 52 |
| Physical illness | |
| Minor problems only | 30 |
| Significant problems | 37 |
| Agitated | |
| Minor problems only | 19 |
| Significant problems | 15 |
| Depression | |
| Minor problems only | 26 |
| Significant problems | 11 |
| Relationship | |
| Minor problems only | 20 |
| Significant problems | 15 |
| Patient characteristic | Percentage of individuals (%) |
|---|---|
| QOF dementia score | 73.6 |
| Urinary incontinence | 5 |
| Faecal incontinence | 3.1 |
| Fall | 7.6 |
| Hip fracture | 1.1 |
| Cancer | 3 |
| Myocardial infarction | 2.1 |
| Peripheral vascular disease | 3 |
| Cerebrovascular disease | 10.6 |
| Delirium | 1 |
| Senility | 7.4 |
| Total diagnoses (count) | 6.3 |
| ACSC: acute | 0.566 |
| ACSC: chronic disease related | 0.279 |
| ACSC: vaccine | 0.156 |
| Enabling factors | |
| Carer 1: % of LSOA population providing 1–19 hours per week of unpaid care | 6.97 |
| Carer 2: % of LSOA population providing 20–49 hours per week of unpaid care | 1.12 |
| Carer 3: % of LSOA population providing ≥ 50 hours per week of unpaid care | 2.1 |
| Living alone: % of LSOA population aged ≥ 60 years living alone | 7.1 |
| Deprivation 1: % of LSOA population aged ≥ 60 years claiming guarantee credit | 10.1 |
| Deprivation 2: % of LSOA population aged ≥ 60 years claiming savings credit | 5.5 |
| Deprivation 3: % of LSOA population aged ≥ 60 years claiming both types of pension credit | 11.9 |
| Care home beds: beds per 100 population aged ≥ 60 years within 10 km of LSOA centroid | 84 |
| Urban residential area (population of > 10,000) | 84.3 |
| Year = 2006/7 | 0 |
| Year = 2007/8 | 0 |
| Year = 2008/9 | 0 |
| Year = 2009/10 | 0 |
| Year = 2010/11 | 1 |
Care services and their effects
The first set of analyses compare the SEED intervention with current practice. Current practice comparator describes the pattern of dementia care services provided at the time of writing. These are described by recently published dementia care guidance. 249 This supersedes earlier guidance,32 the implementation of which was suboptimal. 158 The expected effect of SEED intervention on service outputs and outcomes in many cases is an increase in the prevalence of care services described as good practice by the dementia care guidance. 158
The model parameters associated with the prevalence of services in the current practice, and the effects described in Figure 15, were too many to allow the use of systematic review methodology to identify evidence to inform them. Therefore, ranges of plausible values for model-effect parameters were specified so that we could estimate a possible range of outcomes associated with the SEED intervention. This involved defining maximum and minimum values for model parameters, with uniform distributions used to characterise the uncertainty in the value of these parameters. The potential minimum and maximum values of these parameters were informed by relevant data identified from focused literature searches for individual activities and service outputs. When evidence was not available, assumptions were made on the possible values that these parameters could be. Uncertainty in parameters associated with the prevalence of services are described in Table 85. (See Table 86 for the uncertainty in parameters’ associated effects.)
| Prevalence and frequency of service components | Current practice | SEED intervention |
|---|---|---|
| Prevalence of ACP discussions: hospitalisation, preferred place of care and death, and EOL | 13%250 to 39%250 | 46%250 to 100% |
| Prevalence of ACP discussions: resuscitation, tube-feeding, lasting power of attorney | 13%250 to 39%250 | 46% to 100% |
| Prevalence of liaison between services: before admission (SBAR) per admission | 10% to 40% | 60% to 100% |
| Prevalence of liaison between services: during admission (transfer sheets) per admission | 10% to 40% | 60% to 100% |
| Prevalence of liaison between services: after admission (discharge planning) per discharge | 10% to 40% | 60% to 100% |
| Likelihood of documenting an ACP discussion | 70% to 90% | 80% to 100% |
| Likelihood of having an EHCP in place if a patient enters the palliative care register | 70% to 90% | 80% to 100% |
| Likelihood of concordance between treatment and advance care plans | 50% to 82%251 | 100% |
| Prevalence of bereavement services offered to carers if SEED intervention is present | 30% to 60% | 60% to 100% |
| Number of clinical reviews per year to identify need for palliative care | 1 review | 2–6 reviews |
| Proportion of patients entering palliative care register | 0.2 (IQR 0.5–13)222 | 1 |
| Duration on palliative care register | 2.4 weeks222 | 1 year |
The values presented in Table 85 show the proportion of patients receiving a particular service. For instance, in current practice, 13–39% of patients are expected to be offered the opportunity for ACP discussions regarding their preferences on hospitalisations, place of care, and death. The equivalent value in the presence of the SEED intervention is expected to be between 46% and 100%. Some of these care services were conditional on other aspects of care; for example, Emergency Healthcare Plans were available only to people who were in the palliative care register. For instance, 70–90% of the people in the control group in the model would be eligible to have an Emergency Healthcare Plan once they entered palliative care register. The equivalent proportion of patients eligible for an emergency health-care plan once on the palliative care register for the SEED arm was 80–100%.
It was assumed that people in current practice have one clinical review by a GP per year, whereas, in the SEED intervention, this would vary from two to six reviews annually. In addition, in current practice, 20% of patients enter the palliative care register before they die, whereas, with the SEED intervention, it was assumed that all people having severe dementia would enter the palliative care register. The time spent on the palliative care register in current practice is considered to be 2.4 weeks,222 whereas it was assumed that, with SEED intervention, people should be entered in the palliative care register 1 year before death.
For the parameters reported in Table 85, when sources were listed, these same sources informed the choice of the minimum and maximum values of these parameters.
Table 86 presents the assumptions around the effects of the service outputs on patient-related outcomes. These effects, presented as relative risks, were applied to the probabilities of the associated patient outcomes. For instance, improved liaison between hospitals and care services through the provision of transfer sheets was expected to reduce the length of stay of a person with dementia by 8%. Similarly, being on the palliative care register was assumed to increase the likelihood of being discharged to the previous place of care by 5% to 10%.
| Effects of service components and other effects | Possible range of relative risks (RR) |
|---|---|
| Relative effect of transfer sheets on length of hospital stay | 0.92229 |
| Relative effect of liaison between services before an admission (SBAR) on the likelihood of a hospital admission | 0.85 to 0.95 |
| Relative effect of an advance care plan regarding preferences on place of care and hospitalisation on the likelihood of a hospital admission if an advance care plan is documented | 0.85 to 0.95 |
| Relative effect of EHCP on likelihood of a hospital admission during palliative care stage | 0.85 to 0.95 |
| Relative maximum effect of liaison between services after admission (discharge planning) on the likelihood of a re-admission in the next 3 months | 0.46 to 0.79229 |
| Relative effect of being on the palliative care register on the likelihood of being discharged to the previous place of care (home) | 1.05 to 1.10 |
| Relative effect of an ACP document on the preferred place of care and death and hospital admission on the likelihood of being discharged to the previous place of care (home) | 1.05 to 1.10 |
In the model, we sought to capture the main impact of the SEED intervention on the care service outputs, and on the outcomes for people with dementia (see Figure 15). The process of exploring how the introduction of the SEED intervention might influence the use of other services was an iterative process in which the SEED project team was involved. The SEED project team was presented with all the possible interactions between the SEED intervention’s activities, service outputs and patient outcomes. These were presented in a form of an interaction table. The team members were then asked which service outputs would be influenced by each of the SEED intervention’s main activities. The service outputs and effects were then reduced according to (1) the magnitude of the impact on costs and patient outcomes, and (2) when a service output was indicated as affecting final outcomes directly and indirectly via another care service, only the indirect links were retained, as described in Figure 17.
FIGURE 17.
Indirect and direct effects. Scenario B was retained; scenario A was not retained.
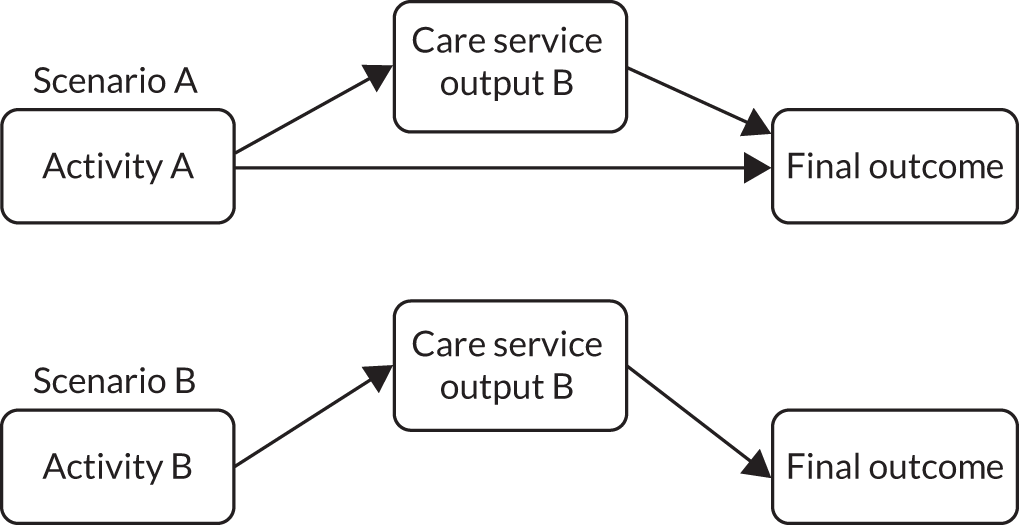
Following this methodology, in a stepwise approach, the influence diagram in Figure 15 was developed jointly with the SEED project team. The diagram illustrates the SEED intervention activities, service outputs, where a priori there would be a clear direct effect on costs and patient-related outcomes. An example of how the model works is as follows: the SEED intervention is expected to improve the liaison between services’ activity through increased use of the SBAR technique. The liaison between services affects the avoidance of unnecessary hospitalisations outcome. The effect of SEED is accounted for in the model by assuming that the effective use of the SBAR technique is increased through training, and that the increased use of the SBAR technique increases the avoidance of unnecessary hospitalisations. There is a cost attached to the SEED intervention and the use of the SBAR technique, and there is an impact on cost associated with avoiding hospitalisation.
Cost of the SEED intervention
Cost parameters in the model included costs associated with the SEED intervention, SEED intervention-related activities, service outputs, care-setting costs and hospitalisation. The SEED intervention is expected to occur an additional £20.40 for each patient per month. The intervention is designed to consist of nurse specialists trained in dementia care with a team consisting of two band 3 nurses, one band 5 nurse and one band 7 nurse working full time on training staff and operating care activities that improve the care service outputs and patient-related outcomes. This team will be led and supervised by the band-7 nurse, with additional supervision provided by an old-age psychiatrist who is expected to spend 2 hours per month on the SEED intervention. The band 7 nurse is expected to also supervise the rest of the team. This team is expected cover, on average, four registered practices. We have assumed that, on average, the estimated 773,502 patients with dementia2 in the UK will be distributed evenly between the 7271 registered practices. Therefore, a SEED intervention team is expected to cover, on average, 106 patients per practice, or 424 dementia patients in total (Table 87).
| Cost of SEED intervention (per patient per month) | Resource use per four practices | Unit cost (£) | Total monthly cost (£) |
|---|---|---|---|
| Band 3 nurses | Two nurses full time | 18,333 per annum | 7.20 |
| Band 5 nurse | One nurse full time | 26,038 per annum | 5.10 |
| Band 7 nurse | One nurse full time | 38,801 per annum | 7.60 |
| Old-age psychiatrist | 2 hours per month | 108 per hour | 0.50 |
| 773,502 dementia patients aged > 65 years,2 distributed equally over 7271 practices252 (424 patients per four practices) | 20.40 | ||
People with dementia: care-setting costs
These costs included costs for home care, care home care and hospitalisation. The cost of care at home or in a care home varied according dementia severity (mild, moderate or severe). 2 The instrument used in this study to categorise the severity of dementia was the Cambridge Mental Disorders of the Elderly Examination (CAMDEX),253 which, according to a review of assessment scales in dementia,254 performs well against the MMSE used in the present analysis. Therefore, these costs were linked in the model to the cognitive capacity severity domain (MMSE) of each individual (Table 88).
| Dementia severity | Care cost (£) | ||
|---|---|---|---|
| Home | Care home | Hospital | |
| Mild | 2183 | 2615 | 282 |
| Moderate | 3829 | 3168 | |
| Severe | 4818 | 3067 | |
The costs of home and care home services that are presented in Table 88 and include costs associated with health care, social care, unpaid care, and other costs. This is presented in Box 12.
-
Day hospital treatment.
-
Social club visits.
-
Day-care visits.
-
Time spent with community-based professionals.
-
Community psychologists.
-
Community psychiatrists.
-
GPs.
-
Practice nurses.
-
District nurses.
-
Social workers.
-
Occupational therapist.
-
Home care workers.
-
Physiotherapists.
The breakdown of costs for community care at home and at a care home were taken from the 2014 Alzheimer’s Society report. 2 These included costs associated with health care, social care, unpaid care and other costs. To account for hospital admissions and to avoid double counting, a table from this report was used to estimate the proportion of health-care costs that are attributed to hospitalisations. We estimated that 32.8–80.1% of the health-care costs are due to hospitalisations. Therefore, we estimated the minimum and maximum societal costs associated with being cared for at home and in a care home, as shown in Table 89. Then the average of these costs, reported in Table 88, were used in the model. It is worth noticing that the total cost at home are driven by the unpaid care costs.
| Dementia severity | Cost (£) | Societal cost minus hospital cost (£) | ||||
|---|---|---|---|---|---|---|
| Health care | Social care | Unpaid care | Other | Minimum | Maximum | |
| People with dementia living in the community (average cost) | ||||||
| Mild | 248 | 282 | 1780 | 12 | 2124 | 2241 |
| Moderate | 243 | 702 | 2911 | 12 | 3789 | 3869 |
| Severe | 1017 | 932 | 3024 | 12 | 4651 | 4985 |
| People with dementia living in residential care homes (average cost) | ||||||
| Mild | 407 | 2234 | 96 | 12 | 2423 | 2616 |
| Moderate | 852 | 2322 | 262 | 12 | 2765 | 3169 |
| Severe | 785 | 2337 | 191 | 12 | 2696 | 3067 |
Adjustments were made to avoid double counting the costs of hospitalisations from the summary estimates of state costs in Table 88. A distribution of costs of care between services was provided by the 2014 Alzheimer’s Society report. 2 Information on the proportion of costs associated with hospital services out of total health-care costs was available from this distribution. It was estimated that, on average, 57% of the health-care costs were attributed to hospital services. Therefore, the health-care costs for each severity and setting of care were reduced by 57% to exclude the cost of hospital services from the state costs.
Finally, the costs reported in the study2 were reported in 2012/13 prices. To estimate the value of these costs in 2017, an inflation index of 0.020198 was used from the Bank of England. 255 The final costs were therefore increased by z, where:
Care service costs
In addition to the state costs, the model accounts for costs associated with the care services reported. The resource use estimates were obtained by expert opinion from the project team. The unit costs associated with formal care were obtained from Curtis and Burns,223 and those associated with informal care were informed by the Department for Business, Energy and Industrial Strategy. 224 The costs of SEED intervention activities and service outputs are reported in Table 90.
| Service delivery factors | Resource use | Unit cost (£) | Total cost (£) |
|---|---|---|---|
| Cost of regular clinical reviews for EOLC (per review) | 30 minutes of GP time | 4 per minute | 120 |
| Cost of ACP discussions (per year) | 30–60 minutes GP time | 4 per minute | 146.67–393.34 |
| 30–60 minutes of nurse time | 42 per hour | ||
| 30–60 minutes of carer time | 11.34 per hour | ||
| Documentation of ACP discussions | 30 minutes of nurse time per year | 42 per hour | 21 |
| Liaison between services before admission (SBAR) | 10 minutes of care home staff time per admission | 59 per hour | 9.83 |
| Liaison between services during admission (transfer sheets) | 15 minutes of care home staff time per admission | 59 per hour | 14.75 |
| Recognised need for palliative care (inclusion in the palliative care register) | Direction of the cost unknown. The extra cost associated with palliative care drugs is considered insignificant compared with the scale of the rest of the costs | ||
| EHCP documented and disseminated (per EHCP) | 30 minutes of GP time per plan | 4 per minute | 120 |
| Bereavement services offered (per service) | 30 minutes of home care manager time | 39 per hour | 19.5 |
| Avoided hospital admission (per admission avoided) | Supervision: 10 minutes per hour for 5 days of district nurse (band 6) | 44 per hour | 1212 |
| 2 visits of 30 minutes of GP time | 4 per hour | ||
| 2 hours of Macmillan nurse time (band 7) | 53 per hour | ||
| 2 hours of Admiral nurse time (band 7) | 53 per hour |
Despite the adjustments made to avoid double counting, costs associated with the care settings may be slightly overestimated as it is possible that low-cost activities, such as the use of transfer sheets, may be captured in the care-setting costs. To further avoid double counting, the expected prevalence of a care activity and resource output on a monthly basis was multiplied by its cost and was subtracted by the costs associated with the setting of care.
Cost of hospitalisation
To estimate the cost of hospitalisation, the most common reasons for hospitalisation for people with dementia were first identified. According to Curtis and Burns,223,225 these were hip fracture, kidney or urinary tract infections, pneumonia and stroke. The cost of an episode was identified from NHS Reference Costs 2015–16226 using the weighted average of relevant HRG codes. Table 91 shows the data from the NHS reference costs used to estimate the daily cost of a hospitalisation. To estimate the value of each cost in 2017 prices, an inflation index of 0.017 was used from the Bank of England. 255
| Healthcare Resource Group code | Code description |
|---|---|
| HD39 | Pathological fractures |
| HE11 | Hip fracture with multiple interventions |
| HE12 | Other injury of hip with interventions |
| HT12 | Very major hip procedures for trauma |
| HT13 | Major hip procedures for trauma |
| HT14 | Intermediate hip procedures for trauma |
| HT15Z | Minor hip procedures for trauma |
| HT81 | Complex hip or knee procedures for trauma, with CC score |
| LA04 | Kidney or urinary tract infections, with interventions and without interventions |
| DZ22 | Unspecified acute lower respiratory infection, with and without interventions |
| DZ23 | Bronchopneumonia without, with single and with multiple interventions |
| AA22 | Cerebrovascular accident, nervous system infections or encephalopathy |
| AA23 | Haemorrhagic cerebrovascular disorders |
| AA29 | Transient ischaemic attack |
| AA35 | Stroke |
| WD11Z | All patients aged ≥ 70 years with a mental health primary diagnosis, treated by a non-specialist mental health service provider |
The daily cost of a patient with a hip fracture or injury was, on average, £291. For urinary tract infections, pneumonia and stroke, the daily costs were £257, £263 and £295, respectively. The weighted average according to the activity of these estimates was used to derive the pooled average daily cost in hospital of £282 per day.
Analyses
Two sets of comparisons were made:
-
A DNS who facilitates the provision of services aimed at improving the quality of care of a person with dementia and their carers (i.e. the SEED intervention) compared with current practice.
-
Comparison of the different care scenarios valued in the contingent valuation study.
Comparison 1
For this comparison, a cost–consequences analysis was conducted. 145
The comparator: a DNS who facilitates the provision of services aimed at improving the QoL of a person with dementia and their carer(s) is hereafter called the SEED intervention for brevity. The potential cost–consequences of the SEED intervention were evaluated by conducting two scenario analyses: (1) the ‘favourable analysis’ utilising the maximum parameter estimates for SEED intervention and the minimum for the control, and (2) the ‘conservative analysis’ utilising the minimum parameter estimates for SEED intervention and the maximum for the control.
The cost–consequences of individual care service outputs of the SEED intervention were also included in the analysis. The individual service outputs considered were as follows:
-
regular clinical reviews
-
ACP discussions and documentation
-
liaison between services before admission (proxy: use of the SBAR technique)
-
liaison between services during admission (proxy: use of transfer sheets)
-
liaison between services after admission (proxy: discharge planning).
For each of these service outputs, the analysis considered two alternative situations: one for which there was the maximum expected provision of the care service and one for which there was the minimum expected provision of the care service. When the effect of one of these care services was explored, the other services were assumed to have a level of provision in between the maximum and minimum values. It was assumed that it was equally probable that the level of provision of these services could take any value between (and including) the maximum and minimum value (i.e. the level of provision for these services was sampled from uniform distributions, with an upper and lower value given by the maximum and minimum value).
In addition, the 2018 NICE guidance for care services158 for dementia was reviewed to identify which care services included in the model were recommended in the guidance. All the SEED intervention care activities, and service outputs, appeared to feature in the NICE guidance (Table 92); consequently, no further analysis was conducted to evaluate the cost–consequences of the NICE guidance recommendations, as this analysis is reflected in the scenario of maximum provision of the care services under evaluation.
| SEED service/outcomes | NICE guidance249 corresponding section |
|---|---|
| Facilitator and team of DNSs |
1.3 Care co-ordination 1.13 Staff training and education |
| Regular reviews | 1.3 Care co-ordination |
| ACP discussions and documentation | 1.1 Involving people living with dementia in decisions about their care |
| Liaison between services to avoid hospital admission: SBAR | 1.9 Risks during hospital admission |
| Liaison between services: transfer sheets |
1.3 Care co-ordination 1.12 Moving to different care settings |
| Liaison between services: discharge planning | 1.12 Moving to different care settings |
| Emergency Healthcare Plan completed | 1.10 Palliative care |
| Bereavement support | 1.10 Palliative care |
Comparison 2
An analysis was conducted for each of the five scenarios valued in the contingent valuation study (see Boxes 7–11) and a control scenario where none of the services included in the contingent valuation scenarios are provided. These scenarios are referred to as control, scenario 1, scenario 2, scenario 3, scenario 4 and main scenario.
Analysis methods
A patient-level simulation was conducted in R statistical software. 227 Discrete-event simulations can be more computationally efficient, but some of the transition probabilities utilised in the model are based on logistic regressions with covariates that change over time. Despite the computationally intense approach, discrete time periods were retained. This allowed us to keep track of previous events that occurred in the model that would affect the likelihood of future events occurring. For instance, prior hospitalisations are a predictor of a future hospital admission, according to predictive logistic models. 218
In this model, hypothetical cohorts of people with dementia were considered. Each individual in this hypothetical cohort was defined in terms of a unique set of characteristics at the time of diagnosis, when they entered the model. Some of these characteristics changed over the course of the model, for example the age of the individual, whereas others were fixed, such as gender. These patient characteristics, among others that were assumed to be constant over time, were used as inputs in logistic regressions to derive the likelihood that a patient moves between settings of care.
Each cohort consisted of 380 people with dementia and a total of 800 cohorts were run through the model with different sampled values of parameter distributions representing uncertainty in the mean estimate. The simulation sample size is small for a patient-level simulation,256 but was adopted as this is an early economic model that aims to provide guidance on relative efficiency, rather than obtain precise estimates. To improve comparability between the analyses of the different service packages and the assumptions, the same random number seed was used in each analysis. The model was run for 120 cycles; each cycle was 1 month long. At the end of 120 cycles, it was estimated that 95% of the simulated patients would have died. Costs and benefits were discounted at an annual rate of 3.5% according to NICE guidelines. 154
Analysis for comparison 1, the analyses of the SEED dementia services
A patient-level simulation was conducted in R statistical software using the RStudio interface. 227 A cohort of people with dementia had different characteristics at diagnosis. These characteristics were sampled from distribution characteristics for patients with dementia. The characteristics that were allowed to change over time in the model were age, cognitive function, functional ability and behavioural symptoms.
The patient characteristics were characteristics that changed over the course of the model plus the gender of the person with dementia. For each modelled patient, the other patient characteristics included in logistic regressions in the model were assigned the average value reported in the source publication. One cohort consisted of 380 people with dementia; 800 cohorts of this size were run through the model with different sampled values of parameter distributions representing uncertainty in the mean estimate. A probabilistic analysis accounted for any non-linearity in the model design.
The simulation sample size adopted in our analysis is small for a patient-level simulation. This was done to minimise computation time, and, as this is an early economic model, the model is not estimating precise cost-effectiveness estimates. To improve comparability between the analyses of the different service packages and the assumptions, the same random number seed was used in each analysis. As described above, the model was run for 120 cycles of monthly length. Costs and benefits were discounted at an annual rate of 3.5%.
In the analyses of the SEED dementia services, the primary outcomes were total cost, length of stay in hospital, time receiving palliative care, the number of people with dementia discharged to the usual place of care from hospital per 1000 patients and the number of avoided admissions per 1000 potential admissions. The total cost is the sum of the discounted costs over the duration of the model.
Analysis for comparison 2, comparison of scenarios used in the contingent valuation survey
To conduct the analysis for comparison of the scenarios valued in the contingent valuation survey, we first had to determine how these scenarios would be represented in the model by the existing model structure and outcomes. To achieve this, a mapping exercise between care activities and service outputs captured in our model and those that were broadly described in each scenario were identified. This mapping exercise was undertaken by three members from the project team independently. These were provided with the descriptions of care activities, service outputs and outcomes related to people with dementia (see Table 69), the description of WTP scenarios and a table to checkmark which care activities were described by each WTP scenario (Table 93). Overall, the results of this exercise between the three individuals were similar. For elements of Table 93 for which there was no agreement, the majority view of the three individuals was considered as the result.
| Elements appearing in the scenarios | Alternative scenario | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Care activities | ||||
| Regular clinical reviews to identify need for palliative care | ✓ | ✓ | ||
| ACP discussions offered | ✓ | ✓ | ||
| Improved liaison between services: before admission (SBAR) | ✓ | ✓ | ||
| Improved liaison between services: during admission (transfer sheets) | ✓ | |||
| Improved liaison between services: on discharge (discharge planning) | ✓ | ✓ | ||
| Care service outputs | ||||
| ACP documented and disseminated | ✓ | ✓ | ||
| Recognised need for palliative care | ✓ | ✓ | ||
| EHCP documented and disseminated | ✓ | |||
| Bereavement services offered | ✓ | ✓ | ||
| Outcomes related to people with dementia | ||||
| Length of hospital stay | ✓ | |||
| Reduced unnecessary/avoidable hospital admissions and re-admissions | ✓ | |||
| Discharged to the usual place of care after hospitalisation | ||||
| Other outcomes (not included in the model) | ||||
| Dying in preferred place of care | ✓ | ✓ | ||
| Time spent in preferred place of care | ✓ | |||
| Hospital deaths | ✓ | |||
| Meeting needs for comfort at the EOL | ✓ | |||
| Concordance between treatment and advance care plan | ✓ | |||
The main scenario, which assumes perfect prevalence of care activities, care service outputs and outcomes related to people with dementia, and the scenario for which none of these was provided were not included in the exercise. For care activities, services outputs and outcomes related to people with dementia in Table 93 that are checkmarked for a scenario, perfect provision was assumed in the model. When a WTP scenario was not describing one of these elements, complete absence was assumed in the model.
The net benefit of providing a dementia service package to new incident cohorts over a 5-year programme was estimated for each of the scenarios. Incident cohorts were selected for the estimation of the net benefit rather than prevalent cohorts because the service package may affect costs in later years. The model was used to calculate a distribution of present values of the costs of a 5-year programme for the cumulative dementia cohort by year.
The average cost per person with dementia of the model in year 1 is denoted as CM1. The cost of the national programme in year 1 is denoted as CP1. For an incident cohort N, the costs for each year of a programme delivered to new incident annual cohorts, when the cumulative cohort increases each year of the 5 years, is as follows:
The present value of the costs over a 5-year course is the sum of the annual discounted programme costs.
The WTP estimates are monthly tax contributions made by individual taxpayers for a programme delivered nationally, that is to all people living with dementia. If b is the average WTP estimate for a service package, y is the total number of taxpayers, ni is the size of the cumulative cohort in year i, and x is the number of people living with dementia in a given year (prevalent cases), then the aggregate WTP estimate across all taxpayers for each year (Bi) is as follows:
The total numbers of people with dementia alive each year were obtained from the economic model. The parameters of a log-normal distribution were calculated from the WTP median and mean estimates. A distribution of the present value of the aggregate WTP estimates for each year of the 5-year programme was simulated.
The net benefit of a service package is the total value of the benefit B minus the cost of the service package C. The cost of a service package is the cost of the dementia services with the package Cd + p minus the cost of dementia services without the package Cd. The net benefit (NB) was calculated as:
For 800 samples, 800 net benefit estimates were derived and the mean and 95% CIs were produced.
A WTP scenario model was run to estimate Cd + p and a control model was run to estimate Cd.
For each WTP scenario, the included services were assumed to be delivered to every person with dementia. It is therefore assumed that the prevalence of a care service described in a WTP scenario exceeds the one that is expected to be accomplished by the SEED intervention. When an activity or service output was absent from a WTP scenario, then the complete absence of this service was assumed.
The incident dementia cases N, the prevalent dementia cases x, and the number of eligible taxpayers y are presented in Table 94.
Summary for economic evaluation
The results of the cost–consequence analysis suggest that the SEED intervention is unlikely to reduce costs, but that it may result in changes in the use of services expected to improve the well-being of people with dementia and family carers. These findings are reinforced by the cost–benefit analysis, which suggest that the SEED intervention is likely to be more efficient than not implementing any aspect of the SEED intervention.
However, the results are imprecise (the CIs are wide); further research is needed to obtain more precise estimates of the probabilities, and to ensure that all clinically and economically important costs and outcomes are included.
Overall workstream 5 conclusions
This WS (in Appendices 5 and 6) describes several innovative economic components, namely the first contingent valuation of a specialist dementia service, the first detailed economic model for a non-pharmacological intervention in dementia from diagnosis to EOL and the first economic evaluation model that incorporates the results of a contingent valuation into a probabilistic economic model. Its methodology, as a minimum, meets internationally accepted best-practice recommendations for contingent valuation, economic evaluation and economic modelling. 146,154
A key finding is that the SEED intervention is perceived by the general population as having real value in economic terms, in particular by individuals with some experience of dementia in their close family members, colleagues or relatives and by those with higher income levels.
Despite the high value of the SEED intervention to the general public, it is unlikely to reduce costs, but it may change service use in ways that improve the well-being of people with dementia and their families. These changes may relieve pressure on some NHS services, for example hospital beds, but may increase demand on others that are overstretched, for example palliative care services.
Reflections on workstream 5
The results of the contingent valuation study are based on a large sample thought to represent the UK general population, but the validity of the responses could have been affected by biases arising out of the construction of the WTP survey or by the interpretation and understanding of the scenarios by the respondents. Using the internet survey panels could have introduced bias by failing to include major consumers of health-care services who are not internet users.
The economic analyses are based on an early economic model; therefore, there is considerable uncertainty surrounding both the model inputs and the underlying structure of the model. The effect of this is that estimates for model outputs may be imprecise (i.e. CIs are wide) and important costs and benefits may not be accurately captured. Nevertheless, rigorous approaches were undertaken to use the best evidence available at the time to ensure that the model captured key aspects.
The economic evaluation allowed us to explore the contribution of each component of the SEED intervention to relative efficiency. The reliability of these estimates is directly related to the trustworthiness of the structural assumptions of the model. The individual components of the SEED intervention do not change outcomes in an additive way; rather, there appear to be diminishing returns from adding each component. This phenomenon has been observed in many studies investigating complex multicomponent interventions. 155,156 However, the precise nature of correlation between components is unclear. Should new data and understanding become available, consideration should be given to refining the model and the data inputs.
Appendix 7 Commissioning good-quality, community-based end-of-life care in dementia (workstream 6)
| Study | Title | Aims/objectives | Participants | Method | Results/conclusions |
|---|---|---|---|---|---|
| Addicott258 | Challenges of commissioning and contracting for integrated care in the NHS in England | Explore challenges in developing, commissioning and contracting models to stimulate greater integration between providers of NHS care | n = 31, of which 14 providers and 17 commissioners |
|
|
| Checkland et al.166 | Complexity in the new NHS: longitudinal case studies of CCGs in England | How CCGs are set up, structured and what is the role of the GP in a CCG | Phase 1:
|
Longitudinal design ongoing study, eight case studies/interviews, meeting observations, and two online surveys |
|
Phase 2:
|
|||||
| Jones et al.259 | Development of a model for integrated care at the EOL in advanced dementia: a whole-systems UK-wide approach | Develop an evidence-based intervention (COMPASSION) in order to improve EOLC for patients with advanced dementia and their carers | Dementia patients, carers and social care professionals (actual sample sizes not mentioned) | Realistic method: qualitative and quantitative, literature review, RAND/UCLA appropriateness method | Relevant qualitative results:
|
| Kupeli et al.67 | What are the barriers to care integration for those at the advanced stages of dementia living in care homes in the UK? Health-care professional perspective | To identify barriers to providing integrated dementia care | n = 14 health professionals, including two commissioners working in dementia care | Qualitative interviews, thematic analysis | Relevant qualitative results:
|
| Kupeli et al.68 | Context, mechanisms and outcomes in EOLC for people with advanced dementia | To explore the context, mechanisms and outcomes for providing good palliative care to people with advanced dementia residing in UK care homes | n = 14 health social care, nursing staff, home managers and commissioners for older adult services | Realistic evaluation framework/qualitative interviews |
Relevant results: CCGs not certain whether or not dementia-specific palliative care is required (beliefs that palliative care may not need to differentiate for dementia patients) |
| Lancaster et al.260 | Commissioning of specialist palliative care services in England | Explore variation in commissioning of palliative care in England | n = 176 CCGs | Freedom of information 3-wave survey |
|
| McDermott et al.261 | Engaging GPs in commissioning: realist evaluation of the early experiences of CCGs in the English NHS | Explore development of CCGs and what GPs add to the CCG process and in what ways they add value to the CCG |
|
Realist evaluation: longitudinal qualitative interviews/seven case study sites/theories were compared with observational data |
|
| Moran et al.262 | GPs’ views of clinically led commissioning: cross-sectional survey in England | To explore GP attitudes to involvement in commissioning and future intentions for engagement | National sample of GPs, n = 2611 | Survey | GPs believe that they can contribute to commissioning; however, the majority do perceive it to be important part of their role. Current leaders consider quitting their commissioning role in the next 5 years. Few would consider taking up commissioning responsibilities in the future |
List of abbreviations
- ACP
- advance care planning
- CAD-EOLD
- Comfort Assessment in Dying with Dementia
- CCG
- Clinical Commissioning Group
- CFAS
- Cognitive Function and Ageing Studies
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPG
- care planning guide
- DNACPR
- do not attempt cardiopulmonary resuscitation
- DNS
- dementia nurse specialist
- EAPC
- European Association for Palliative Care
- EOL
- end of life
- EOLC
- end-of-life care
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESC
- External Steering Committee
- FAQ
- Functional Activities Questionnaire
- FAST
- Functional Assessment Staging Tool
- GP
- general practitioner
- GSF
- Gold Standards Framework
- HADS
- Hospital Anxiety and Depression Scale
- HoNOS
- Health of the Nation Outcome Scales
- ICP
- integrated care pathway
- IT
- information technology
- LCP
- Liverpool Care Pathway for the Dying Patient
- MCDP
- Marie Curie Dementia Programme
- MDT
- multidisciplinary team
- MMSE
- Mini Mental State Examination
- MOOC
- massive open online course
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPI
- Neuropsychiatric Inventory
- NPI-Q
- Neuropsychiatric Inventory Questionnaire
- NPT
- normalisation process theory
- PAINAD
- Pain Assessment in Advanced Dementia
- PIS
- participant information sheet
- PPAB
- patient and public advisory board
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QUALID
- Quality of Life in Late-stage Dementia
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SBAR
- situation, background, assessment, recommendation
- SEED
- Supporting Excellence in End-of-life care in Dementia
- SM-EOLD
- Symptom Management at the End Of Life in Dementia
- SWC-EOLD
- Satisfaction with Care at the End of Life in Dementia
- WS
- workstream
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar08080).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
