Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number DTC-RP-PG-0311-12003. The contractual start date was in September 2013. The final report began editorial review in April 2020 and was accepted for publication in February 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Clarkson et al. This work was produced by Clarkson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Clarkson et al.
SYNOPSIS
Background
‘Living well with dementia’
With the population ageing, dementia represents a significant public health and care challenge. 1 It is a major cause of disability and high-cost care in older people. 2 Finding cost-effective ways to improve care to people with dementia and their families has been termed the £20B question. 3 More recent figures suggest that the cost is around £24B a year in England. 4 Almost half of this cost is attributed to unpaid family care. Social care costs are three times higher than health-care costs. There is a growing body of research that addresses this question, including work on primary prevention (i.e. preventing the development of dementia) and secondary prevention (i.e. offering early treatment). 5 The immediate priority, reflected in policy,6 is one of helping people to ‘live well with dementia’, tertiary prevention, ameliorating difficulties and enhancing well-being. This translates to enabling those with dementia and their family carers to live as well as they can from a humane perspective.
Approximately 60% of people with dementia live at home. Helping people with dementia to live well necessitates establishing appropriate and effective home or personal support, including that from the NHS and social care (often in combination) and taking into account the wishes and views of carers. There is insufficient overview of the different forms of support available, including their relative effects and cost-effectiveness. 2 Studies of psychosocial interventions have identified potentially effective approaches,7 but there has been little or no work translating these into routine home support provided by NHS and social care organisations, nor in evaluating these. For care and support, the evidence base regarding how components (‘active ingredients’) of interventions could be combined into different models of support, and the likely costs and effects of adopting these, is weak compared with treatment for dementia. Translating this evidence into models of support that could benefit the NHS and social care is therefore compromised.
The clinical characteristics of dementia render individuals less able to care for themselves, more prone to emotional and behavioural problems and more likely to have poor physical health. 8,9 Support at home has to respond to these needs appropriately, including enhancing existing coping skills of people with dementia and their carers. Medicines management is one area that can be confusing and burdensome. The needs of individuals and families in accessing appropriate help over the course of life with dementia are changeable and diverse. 10 Support required also needs to be compatible with, and take heed of, existing support networks of people with dementia and their families. 11 Therefore, people with dementia require specialist support from a range of sources, including family, friends, professional health and social care agencies and also charitable organisations. 12
Service reviews have testified to a lack of appropriate home support services for people with dementia. The National Institute for Health and Care Excellence (NICE)/Social Care Institute for Excellence drew attention to the lack of robust data on the organisation and delivery of services for people with dementia and their carers, and the need for specialist support at home. The Association of Directors of Adult Social Services13 reported fragmented services and a lack of clarity about what preventative services are most effective. The National Audit Office10 highlighted the paucity of data about the costs and benefits of home support, causing local decisions on priorities to be ill-informed. We know very little of the range of specialist home care (domiciliary care) for dementia commissioned by local authorities and this is an under-researched area as far as costs and benefits of provision are concerned.
Evidence suggests that although specialist home support for older people with dementia exists in some localities, it is often underdeveloped. Older people with dementia receive a higher level of support than those without. However, this is often less than expected given their level of impairment. 14 Home support to those with dementia is often underdeveloped in comparison with services for older people generally. 15 This is despite the fact that specialist home support is perceived by service users, carers and care workers to deliver better-quality care than standard services. 16
Evaluating home support for dementia
Both the NHS and social care providers provide home support to people with dementia and their families. We appear to know more about unhelpful approaches to supporting people with dementia and carers at home than we do about effective forms of home support. Home support from social care (‘home care’) is provided largely in a ‘generic’ manner to older people, in general, rather than tailored specifically to the needs of people with dementia. This is largely task based, with little heed paid to the particular nuances of individual presentation of the condition,17 and may be indicative of the ‘old culture’ of dementia care. 18 Within the NHS, home support for people with dementia in later stages is provided via Community Mental Health Teams (CMHTs), for example through support workers. This appears not to be co-ordinated well and, in some areas, teams do not provide this support. 10 There appears, therefore, to be scope for more specialist and person-centred approaches to the care of people with dementia at home. The recent Lancet commission on dementia5 articulated principles for such approaches. There is no magic bullet. Interventions should be multicomponent and individualised to need, with support for carers to develop their own coping skills and to modify the environment around the person with dementia.
There are challenges to evaluating such approaches to home support, where they might exist. Existing meta-analyses of studies investigating home support to older people, in general,19–21 have argued for more precise descriptions of the actual components employed (i.e. ‘who, did what, where and how’). An analysis of such components, where these exist within more specialist models of support,22 would be beneficial. For example, for people in the early stages of dementia, early identification through memory clinics has been one development,23 but we know little of the way in which follow-up at home is conducted allied to this. 24 Preliminary studies testify to potential benefits of a home-based information and memory management component at this stage. 25 There is therefore a need for more robust evidence, building on this work. Research also suggests that specialist domiciliary care for people with dementia at later stages can reduce the likelihood of requiring, or delay entry into, long-term care26,27 and enable carers to care for longer. 26 For the NHS, reduction in hospital admissions, which can have a deleterious effect for people with dementia, may be one benefit of developing more intensive or specialist models of home support.
This National Institute for Health Research (NIHR) programme was framed in response to these challenges. It drew on and aimed to extend previous Department of Health and Social Care-funded work17 in marshalling evidence and primary research into the most cost-effective home and personal support approaches for people with dementia.
Effective home support in dementia care programme overview
The programme aimed to discern different models of home support, systematise them, survey their current operation in England and evaluate their cost-effectiveness in providing care for people with dementia and their carers. It also aimed to disseminate findings in the form of guidance for managers and commissioners.
The research was undertaken between September 2013 and March 2020. The main output was to develop evidenced-based guidance on home support models for dementia care, with direct applicability by NHS trusts and partner organisations. Importantly, we included social care and the third sector. We aimed to understand the benefits of different forms of home support in terms of more efficient and effective care and how they might enhance the patient and carer experience.
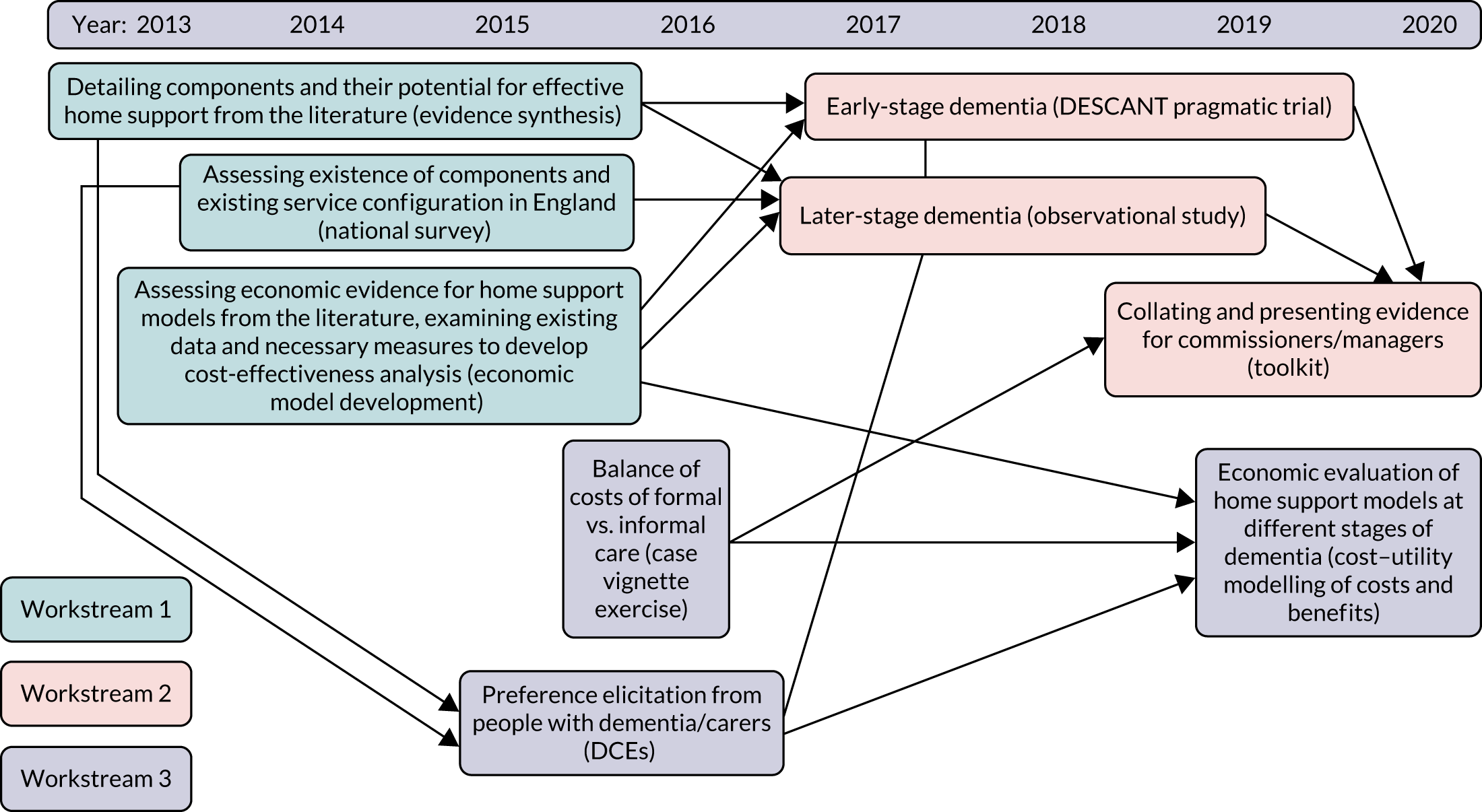
Addressing issues in developing more cost-effective home support approaches in dementia care required a multiphase work programme. Figure 1 outlines the research programme, its constituent parts (i.e. workstreams) and individual projects within them. The programme consisted of three interconnected thematic workstreams that contained nine projects designed to generate an integrated understanding of effective home support for older people with dementia. Each workstream sought to enhance evaluation of one of the three core features of effective home support for people with dementia: (1) the components of high-quality home support, (2) its impact and (3) the costs and consequences of service delivery. The workstreams were thematic and so individual projects within them were not undertaken sequentially. A research pathway diagram of the stages and development of the interconnecting workstreams and how they contributed to the whole programme is shown in Figure 2.
FIGURE 1.
Effective home support in dementia care: workstreams, projects and their relationships. DCE, discrete choice experiment.
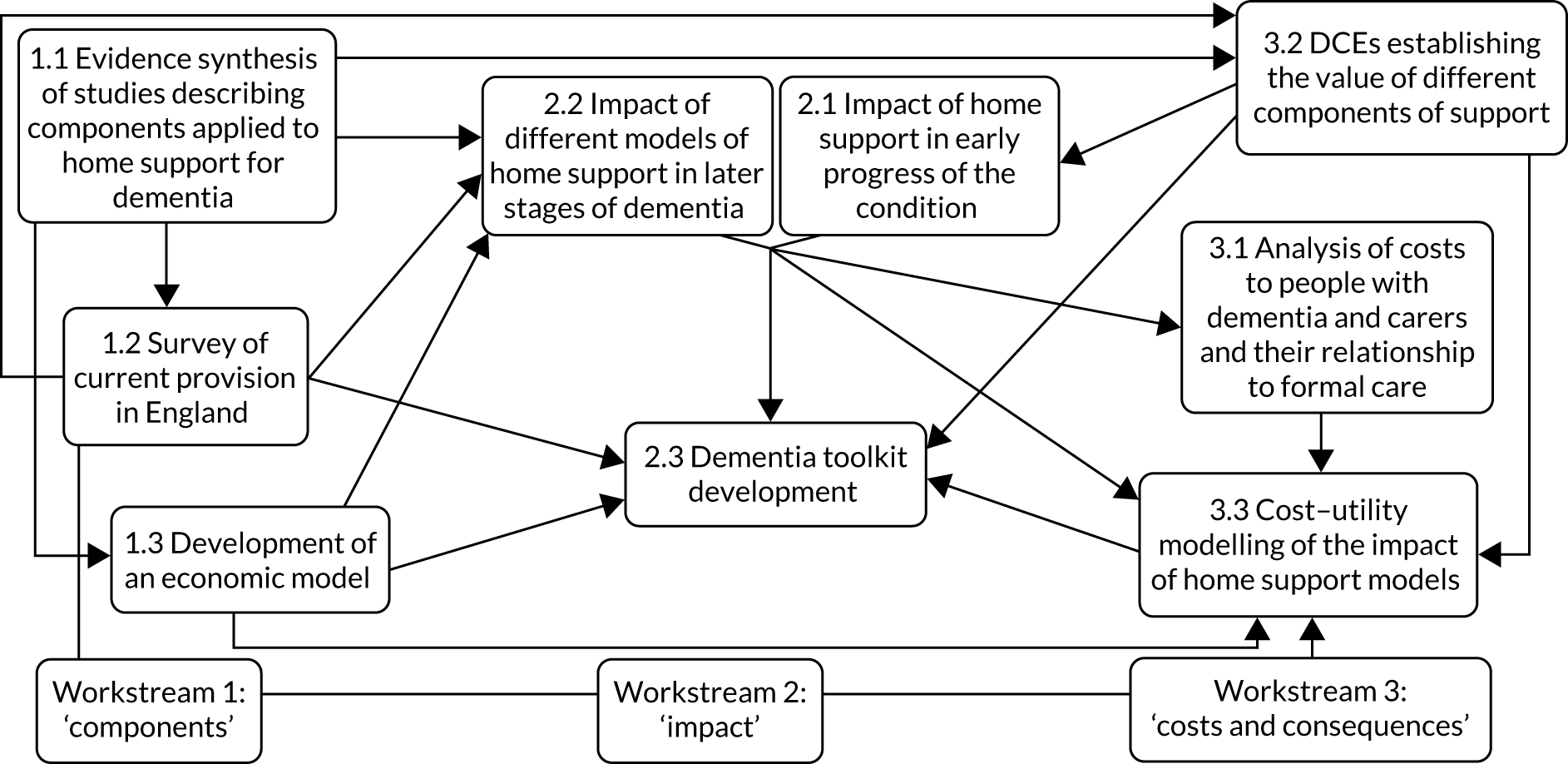
FIGURE 2.
Research pathway diagram of stages and development of workstreams. DCE, discrete choice experiment.
Workstream 1
In workstream 1, we identified the components of home support that may benefit people with dementia and their carers through three projects. First, we undertook a narrative synthesis of two literature reviews (an overview of reviews and a systematic review). Second, we conducted a national survey of different components of existing provision in England. Third, we developed an economic model to inform analyses later in the programme.
Workstream 2
In workstream 2, we assessed the impact of two forms of home support. First, a new intervention that was funded at the inception of the programme offered people with early-stage dementia support and guidance on the use of memory aids through dementia support practitioners (DSPs) from memory clinics. We tested the clinical effectiveness and cost-effectiveness of this intervention in a pragmatic randomised trial and a process evaluation to investigate fidelity and practice. Second, we examined through a prospective observational study naturally occurring packages of home support for people with later-stage dementia and their carers. Again, we tested the clinical effectiveness and cost-effectiveness of these approaches, but this time through multivariate models of effects and costs. The evidence from both these primary studies and other evidence from across the programme was used to develop a toolkit to guide managers and commissioners in how best to provide home support.
Workstream 3
In workstream 3 we evaluated the costs and consequences of different approaches to home support through three projects. First, we undertook an analysis of the balance between costs of informal carer and statutory support by using case vignettes and costing methodology. Second, we examined the preferences of people with dementia and carers through two discrete choice experiments (DCEs) in early- and later-stage dementia. Third, we conducted economic modelling to bring together results from the two primary studies, above, and allied this with data from the economic development work in workstream 1.
Within each workstream, patient, public and carer involvement (PPCI) was crucial to the effective collection and dissemination of evidence. We worked together with established groups in framing the individual studies, designing methods of data collection and in the analysis, interpretation and dissemination of findings.
Programme management
A Programme Steering Committee oversaw the entire programme with a Data Monitoring and Ethics Committee (DMEC) as a subcommittee, approved by NIHR. The Programme Steering Committee included members with expertise in patient and public involvement, old-age psychiatry, health services research, psychology and biostatistics. The Programme Steering Committee and DMEC met biannually in the early phases of the programme and then maintained e-mail and telephone correspondence once the trial on the programme was underway. A PPCI Reference Group was established and led by two of the investigators (BR and Jean Tottie). Varying numbers of members resident in North West England joined the group, dependent on changing circumstances, and met regularly (at least twice a year) with investigators BR and PC in Liverpool throughout the programme. A Lay Advisory Panel (LAP) of 20 members (carers of those with dementia) was established for consultation and comment, permitting a larger number of carers to contribute to the research across a wide geographical area. Members contributed by e-mail, although paper, telephone or face-to-face communication were also used. The LAP was aligned initially with Uniting Carers (London, UK), part of Dementia UK (London, UK), through the chairperson Jean Tottie, a programme investigator. However, from April 2015, this was hosted by Together in Dementia Everyday (TIDE) (Liverpool, UK), a national community interest company (and now charity), as Uniting Carers ceased operation. Operationally, the entire programme was managed through a Programme Management Group that comprised all investigators. This group met initially every 3 months and then communicated regularly by e-mail and telephone. A Trial Management Group at Manchester University (Manchester, UK) met monthly and liaised with the Clinical Trials Unit at Swansea University (Swansea, UK) [URL: www.swanseatrialsunit.org (accessed 5 April 2021)] and the DMEC on all management matters relating to the trial.
Summary of alterations to the programme
There were no alterations to the original aims and design of the programme. However, recruitment to the trial and observational study suffered from delays caused by Health Research Authority governance changes, recruitment difficulties with this population and staffing changes. We had two extensions to our programme, in May 2017 and November 2018, to deal with these delays. We adapted the original economic model structure, summarising the costs and benefits of home support approaches, from the initial model development (see Workstream 1, Development of an economic model). The form of the model changed in later work because of the lack of appropriate data with which to populate more complex mathematical models. We also took the opportunity afforded to us by the rich primary data from the trial and observational study to implement the model differently.
Workstream 1: identifying components of home support that may benefit people with dementia and their carers
Workstream 1, undertaken during the first 2 years of the programme (September 2013 to September 2015), had the following aims:
-
to investigate the evidence for effective components of psychosocial interventions for dementia in any setting (via an overview of systematic reviews) and to assess the extent to which they can be combined into multicomponent approaches to support people with dementia and their carers at home (via a systematic review)
-
to assess the presence of different components of home support in existing provision in England through a national survey of NHS and social care (local authority) services
-
to develop an economic model to inform later analyses in the programme.
Findings from the systematic reviews were brought together to inform other programme studies [i.e. the national survey and economic model development, evaluation of home support models in later-stage dementia (workstream 2) and DCEs investigating people with dementia and carer preferences (workstream 3)]. The national survey, in turn, informed the choice of which home support models to evaluate in workstream 2 and definitions of attributes for the DCEs in workstream 3. Likewise, the economic model development informed the methods used to evaluate home support models in workstream 2 and was consolidated in workstream 3.
Evidence synthesis of studies describing components applied to home support for dementia
We addressed the difficulties in eliciting firm evidence of the effectiveness of home support by evidence synthesis. This was a review with a specific purpose, that is to examine the components (‘active ingredients’) that may be responsible for the effectiveness of home support approaches for people with dementia or their carers. This informed later stages of the programme and enabled us to discern potential and existing models of home support and to systematise them. The overall aim of the review was to identify, describe, classify and analyse models for delivering home support to people with dementia and their carers in terms of their effectiveness, how and to whom effects are directed, and their cost-effectiveness and acceptability in ameliorating difficulties and improving well-being.
We undertook two systematic reviews to address this aim. First, an overview of systematic reviews to identify components of psychosocial interventions to people with dementia in any setting (e.g. care homes, day care or at home). Second, a systematic review of published studies of support interventions delivered at home, in which we used the components identified in the overview to discern distinct (multicomponent) approaches to home support. Full publications of this work were published in the Journal of Advanced Nursing (the protocol for the evidence synthesis in 201628 and the overview of reviews29 and systematic review30 in 2017). The outputs from these papers are in Appendix 1.
The overview of reviews drew on systematic reviews of randomised controlled trials in any setting. We undertook a narrative synthesis of the evidence because of the heterogeneity of interventions and outcome measures.
We searched CDSR (Cochrane Database of Systematic Reviews), DARE (Database of Abstracts of Reviews of Effects) and EPPI-Centre (Evidence for Policy and Practice Information and Co-ordinating Centre) between September 2013 and April 2014 for published systematic reviews in English. We appraised these reviews against Cochrane Collaboration levels of effectiveness. Components of psychosocial interventions were then identified along with their theoretical rationale. Components were defined as the ‘constituents’ or ‘active ingredients’ of interventions that may have an effect on quality of life (including neuropsychiatric symptoms), hospital admissions or time to care home admission. ‘Components’ were conceptualised as ‘common and distinctive techniques across evaluated interventions’. 66 We explored the findings from this identification of components with our PPCI group.
Thirty-six reviews7,31–65 were included in the overview, from 279 references. Over half (148; 53%) of excluded studies were of pharmacological interventions. Of the included reviews, 21 (58%) were of specific interventions (e.g. physical activity programmes), whereas 15 (42%) were reviews of a range of interventions. The reviewed interventions were set predominantly within nursing/care homes (n = 18 reviews) and not at home. The synthesis identified 14 components employed as part of interventions, nine for people with dementia and five for carers (see Appendix 2, Table 2). Our PPCI group articulated that these components could be summarised in a typology referred to as SITE (support, information, therapy or education). Components could reflect the general aims of support, information, therapy or education. For people with dementia, there was evidence of effectiveness for cognitive support, but less evidence for sensory stimulation, emotional support (i.e. reminiscence), behaviour management and daily living assistance [i.e. help with activities of daily living (ADL)]. For carers, there was evidence of effectiveness for behaviour management (i.e. education and training) and emotional support (i.e. psychotherapy and counselling).
Review limitations were that the detail available to describe interventions was variable. The content from which data on components were extracted was based predominantly on descriptions of interventions in the reviews and not in the primary studies on which they were based. Details of who provided the interventions were sometimes partial and there was a lack of evidence about whether or not interventions were undertaken as intended.
This overview provided evidence of several components that may be effective if integrated into home support interventions. However, most reviews investigated were undertaken for studies in settings other than at home. An important evidence gap was therefore identified that could guide practitioners (i.e. nurses, social workers, occupational therapists and voluntary sector support workers) who co-ordinate long-term support to people with dementia at home. This was taken forward to the next stage of the evidence synthesis to discern the clinical effectiveness of different multicomponent approaches to home support for people with dementia and their carers. This was a systematic review of studies, again with a narrative synthesis of the evidence owing to the heterogeneity of interventions, methods and outcome measures. Both quantitative and qualitative studies were included.
PubMed, CENTRAL (Cochrane Central Register of Controlled Trials), PsycInfo, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Applied Social Science Index (ASSIA) and CSA Sociological Abstracts were searched for studies of support interventions delivered at home to people with dementia or their carers. Databases were searched from inception to April 2014, with no date restrictions to locate studies. Data across studies were synthesised using the 14 components of care for people with dementia and their carers identified previously. We grouped interventions to capture the most prevalent approaches and described them as models of home support, which relied on one or more of the components. We then applied effectiveness ratings to them. Qualitative studies were synthesised using key themes.
Seventy studies16,26,67–134 (including four qualitative studies) were included in the review from 603 references. There were 52 multicomponent studies for carers, and 15 multicomponent and 21 single-component studies for people with dementia. We identified nine home support models, seven for carers and two for people with dementia, covering 81% of studies (see table 4 in Clarkson et al. 30). Home support models for carers were based on five components: (1) behaviour management, (2) education or advice, (3) social support, (4) emotional support and (5) respite. Home support models for people with dementia were based on two components: (1) environmental modifications and (2) care co-ordination. Three components identified from our overview (i.e. daily living assistance, cognitive support and physical activity for people with dementia) were absent from these home support models. Models containing education, social support and behaviour management appeared most effective.
Limitations of this review were that the interventions presented in studies were not always described with sufficient detail. Data on the stage of dementia (i.e. early progress of the condition vs. later stages) were sometimes not available. The rating of effectiveness of the different home support models was challenging. The number of included studies (n = 70) made it difficult to appraise the effectiveness of each intervention, particularly when they contained multiple components. Data limitations from some studies, in particular, made it difficult to calculate effect sizes. This made it difficult to rate effectiveness comprehensively.
This review provided evidence of potential home support models for people with dementia or their carers. Nine models combined components in different ways. Predominantly, these were to provide support to carers, with environmental modifications and care co-ordination being central components of care delivered to people with dementia. Importantly, we identified a gap in the literature relating to components of daily living assistance, cognitive support and physical activity for people with dementia living at home. This informed the design of the primary studies in the programme (workstream 2). One of the primary studies, the Dementia Early Stage Cognitive Aids New Trial (DESCANT), was designed at inception of the programme, building on an existing Cochrane review (see Appendix 5), but other evidence from this programme review was used to guide the manual for support workers used in the intervention.
Survey of current provision in England
As well as the lack of sufficient evidence of different home support models, collated in our evidence synthesis, there is also little knowledge across the country about the availability of home support to people with dementia. In particular, there is a lack of knowledge about what components are provided by specialist health and social care (local authority) services. We addressed this by undertaking national surveys of NHS and social care services in England in 2014/15. These surveys informed later stages of this programme and contributed to the choice of which home support models to evaluate and the definitions of attributes for the DCEs. The aim of the survey was to assess the presence of different components of home support in existing provision for people with dementia in England and who provides them.
We undertook two surveys. First, to investigate staff roles and tasks provided in specialist NHS services, memory clinics and CMHTs providing early diagnosis and long-term support for older people with dementia in England. Second, to explore the commissioning of social care services for people with dementia living at home. The studies were published in the International Journal of Geriatric Psychiatry (the NHS survey in 2018135 and the local authority survey in 2019136). The outputs from these papers are in Appendix 3.
The NHS survey135 investigated how staff in CMHTs and memory clinics support people with dementia in their own homes. We collected data in 2015 through a cross-sectional survey of the 68 NHS trusts that provide mental health services in England. The questionnaire classified home support interventions according to the SITE typology from our evidence synthesis (see Evidence synthesis of studies describing components applied to home support for dementia). We obtained approval for the study from the University of Manchester Ethics Committee on 24 June 2014 (reference 14209). We also received the support of the Research Group of the Association of Directors of Adult Social Services on 22 August 2014 (reference RG14–016). We posted or e-mailed questionnaires to NHS trusts’ chief executives and research and development departments to encourage completion and we registered the survey on the NIHR portfolio in March 2015.
We received responses from 51 (75%) NHS trusts that provided data on 120 (79%) of the 151 local authority areas in England. The support provided by CMHTs and memory services were categorised into 14 indicators of service provision within the SITE typology. Both CMHTs and memory services offered information and advice about dementia, access to relaxation and assistance in managing challenging behaviours. In particular, 110 (92%) of memory clinics and 108 (90%) of CMHTs provided advice on using memory aids. Limitations of this survey included the variation of response rates by region, the need for a single response for all CMHTs and memory clinics within each geographical area, and the danger that, although findings described NHS services for patients in detail, they may have understated services for carers.
The local authority survey136 explored the provision of social care support to people with dementia through a survey to commissioners in English local authorities. An exploratory cluster analysis of nominal data identified similar groups of local commissioning practices. 137 The survey, undertaken in 2014/15, received responses from 122 (81%) of the 151 local authorities. Respite care (in 83% of local authority areas) and day care (in 81% of local authority areas) were the most frequently reported services. Specialist home care, usually provided to people at later stages of dementia, was available in 28% of areas. This showed that joint working between health and social care organisations resulted in a greater range of services for people with dementia, complementing services available to all older people. It confirmed the significant role of health providers in the delivery of social care services for those with dementia. Limitations of this survey included that the data relied on self-reported activities of commissioners working within local authorities, the survey did not identify how specialist services for people with dementia differed from generic services offered to all older people and the potential for changes in practice since the data were collected.
We analysed data from both surveys to produce a ‘service mix score’ for site selection in the observational study of later dementia (see Workstream 2, Effectiveness of home support models in later dementia). We analysed 43 matched local authority and NHS trust areas to create a set of 16 indicators (nine indicators about local authorities and seven indicators about the NHS) to reflect the broad range of services available. We favoured items that provided a good split of the data and for which there was face validity. We could then score areas on the presence or absence of each indicator so that the total score represented the scope for residents to receive a range of different services. No area scored the maximum of 16 points, and the minimum was 3 points. Most areas were in the middle of the distribution, with scores of 8 to 10 points. The Kolmogorov–Smirnov test showed that the service mix score followed a normal distribution, with a mean of 8.9 and a standard deviation (SD) of 2.3.
Development of an economic model
As part of workstream 1 (i.e. gathering evidence of different approaches to home support), we began to develop an economic model. This was to be used to synthesise the economic evidence (costs and consequences) for different home support approaches in dementia gathered during the programme. We focused on (1) what data might be currently available concerning typical and potential packages of home support and (2) how we could extrapolate from these approaches to examine the consequences of subsequent pathways of care. In particular, we intended that this developmental work would frame the approaches and methodology used in a full economic model of dementia home support towards the end of the programme, drawing on data from our programme projects, particularly the two primary studies, projects 2.1 and 2.2, in early and later stages of dementia, respectively.
There were therefore two stages: (1) a systematic review to identify current evidence about the cost-effectiveness of home support services for dementia and (2) economic model development, examining the choice of model, the types of data to be used (and any data shortfalls) and what types of home support approaches could have potential benefits versus costs for testing later.
The systematic economic review was a review of full and partial economic evaluations using the NHS Economic Evaluation Database supplemented by additional references. Study characteristics and findings, including incremental cost-effectiveness ratios (ICERs) when available, were summarised narratively. We appraised study quality using the NHS Economic Evaluation Database critical appraisal criteria and independent ratings, agreed by two reviewers. Studies were located on a permutation matrix, describing their mix of incremental costs and effects to aid decision-making.
Of the 151 articles retrieved, 14 studies met the inclusion criteria (eight concerning support to people with dementia and six concerning support to carers). Five studies were incremental cost–utility analyses, seven were cost-effectiveness analyses and two were cost–consequences analyses. Five studies expressed ICERs as cost per quality-adjusted life-year (QALY) (£6696–207,942/QALY). In four studies, home support interventions were dominant over usual care. Two interventions were more costly but more beneficial and were favourable against current acceptability thresholds. Occupational therapy,138 home-based exercise139 and a carers’ coping intervention140 emerged as potentially cost-effective approaches for which there was better evidence. These interventions used environmental modifications, behaviour management, physical activity and emotional support as active components. There were limitations in the availability of cost or benefits data from which to judge some interventions. This review therefore signalled that more robust evidence was required to judge the value of these and other approaches across the dementia care pathway. This work was published in Value in Health in 2017. 141 The output is in Appendix 4.
A small project team was responsible for the development of the economic model. We concentrated on characterising ‘care as usual’ for people with early- and later-stage dementia, respectively, as a yardstick for the relative effects of a range of models of home support. 142 Our PPCI LAP commented on care at different stages of dementia and the range of different services available. This assisted in establishing what characterised usual care for people with dementia and their families and our search for data to inform this in economic analysis. One conclusion was that usual care varied, both by geography and by dementia stage. Our decisions on the choice of model were informed by existing guidance. 143 We reviewed discrete event simulation models,144,145 which concentrate on sending virtual cases through the care system and analysing the costs and consequences of them receiving different combinations of care. However, we decided, after testing a selection of data from the economic review in a discrete event simulation model, that this model form was too complex to estimate from the data available. There were insufficient data from other sources (e.g. national reports and guidance) to populate more complex decision-analytic models. There were data on service receipt and costs from some studies, but a lack of longitudinal data, particularly on health-related quality-of-life outcomes. This also meant that it was not feasible to model longer-term changes in resources and outcomes for dementia care in our subsequent work. Therefore, we decided to rely on primary data from the two studies, projects 2.1 and 2.2, later in the programme. We concentrated on ensuring that data collection instruments for these studies included the necessary information to generate data for modelling costs and benefits. Cost data needed to include resources consumed from multiple perspectives. These were the NHS, social care, voluntary sector organisations and people with dementia/their carers (i.e. the key actors affected by home support services). The instrument for collecting these data included questions to elicit these perspectives. The measure of benefit for the analyses was the QALY and measures to generate utility values to calculate QALYs were needed.
The eventual economic analyses (described in Cost–utility modelling of the impact of home support models and detailed in Appendix 11) comprised cost-effectiveness acceptability analyses to estimate the incremental cost per QALY gained and the probability that home support models were cost-effective compared with usual care. We aimed to assess this probability against a range of threshold values, reflecting the opportunity costs faced by the NHS and social care in deciding whether or not to fund any approaches to home support for which different organisations are responsible (i.e. whether or not the benefits accrued by new approaches offset the benefits forgone elsewhere). 146 The eventual model incorporated both probabilistic and deterministic sensitivity analyses to quantify uncertainty in the evidence and structural uncertainty. 147 These included varying the source of unit cost and direct cost data, perspective and scope of the analysis (e.g. including impact on families) and alternative measures of benefit. We decided on a time horizon for the primary economic analyses of 6 months.
Summary
Building up the evidence collated in this workstream led to the following summary conclusions. Most approaches where there was evidence of cost-effectiveness data concerned home support at moderate to severe stages of dementia and later in the care pathway. Only two approaches138,148 concerned people in early-stage dementia and it is here that approaches may offer more sustainable benefits to people in terms of them ‘living well’ with the condition. However, the economic evidence around these interventions was far from robust. Three approaches showed more positive cost-effectiveness evidence (i.e. occupational therapy, home-based exercise and a carers’ coping intervention). These approaches relied on the active components of environmental modifications, behaviour management, physical activity and emotional support. Better economic evidence of approaches in early-stage dementia, such as home-based cognitive support, and in late-stage dementia, such as palliative care, is required.
These conclusions were taken forward in designing and analysing our two primary studies in early- and later-stage dementia, which are summarised in workstream 2 in the next section.
Workstream 2: impact of different forms of home support
Workstream 2 was conducted throughout years 4–6 of the programme. We aimed to:
-
evaluate memory aids in early-stage dementia through a pragmatic trial
-
estimate the effectiveness of different emerging models of home support in later-stage dementia
-
disseminate evidence-based guidance through a toolkit developed in one trust area and roll out with commissioners, managers and practitioners.
Effectiveness of home support in early dementia: the DESCANT
Building on the results of our evidence synthesis (see Workstream 1, Evidence synthesis of studies describing components applied to home support for dementia) and economic review (see Workstream 1, Development of an economic model), we identified evidence gaps for the home support of people with early-stage dementia. There was existing preliminary evidence for the potential of memory aids in this population and in our original programme plan we sought to evaluate the success of a novel home-based cognitive support intervention. Our evidence synthesis helped in drafting a protocol that included provision of the aids themselves and also in providing guidance in their use. Despite being widely recommended in practice (see Workstream 1, Survey of current provision in England), the use of aids to assist cognition [e.g. calendars, clocks, whiteboards with electric timers and Post-it® (3M, Saint Paul, MN, USA)] dispensers by those diagnosed with early dementia has not been evaluated robustly. 149,150 We therefore undertook DESCANT, a multisite, pragmatic, randomised trial to evaluate the clinical effectiveness and cost-effectiveness of memory aids delivered by DSPs to people with early-stage dementia relative to treatment as usual (TAU).
We published the trial protocol in Trials in 2018. 151 This included the sample size estimation for the primary outcome of the ADL for people with dementia, as measured by the Bristol Activities of Daily Living Scale (BADLS),152 and a description of the intervention and of all trial procedures. The methods of the trial and findings on clinical effectiveness are summarised below. More detail on the trial rationale and findings and a link to the protocol paper are in Appendix 5.
Methods
We calculated that an analysable sample of 360 participants (180 participants in each group) across participating trusts would yield 80% power to detect an effect size (standardised mean difference) of 0.30 on BADLS when using a two-sided significance level of 5%. To allow for 25% attrition (estimated from previous similar studies) between baseline and final interviews, we aimed to recruit 480 randomised pairs of people with mild to moderate dementia and their identified carers. Our prespecified effect size, used in this calculation, corresponds to a 3.5 minimum clinically important difference on BADLS, with a SD of 8.7. 153
We successfully delivered the trial by recruiting participants (people with early-stage dementia and their informal carers) from memory services in nine NHS trusts across England and one health board in Wales. The trial received a favourable Research Ethics Committee opinion on 13/05/16 (reference 16/NW/0389). We negotiated processes to allow the trial to proceed, initially, in two host NHS trusts, with a recruitment start date of 25 November 2016. The first participant was enrolled on 6 December 2016. We then added sites in NHS trusts with the support of their local Clinical Research Network (CRN) teams (and the equivalent in Wales). The trial eventually used 10 local CRNs across England and Wales for consent and recruitment. Capacity to consent for people with dementia was determined by research nurses from the local CRNs who received specific training for research interviews and measures from the University of Manchester research team (i.e. the sponsor), in accordance with the principles of the Mental Capacity Act 2005. 154 Where people with dementia were judged as having capacity, we obtained their informed consent. If people with dementia were assessed as lacking capacity, we asked the primary carer or a personal consultee about the participant’s wishes regarding taking part in research and the primary carer/personal consultee was asked to provide consent. Consent was not assumed at follow-up and additional verbal consent was obtained and recorded at the follow-up interviews. We also recruited from the NIHR Join Dementia Research platform where people with dementia and their carers could register their interest in participating. We randomised participants (i.e. people with early-stage dementia and their informal carers) between the comparator group receiving TAU plus an existing dementia guide155 and the intervention group receiving TAU plus the DESCANT intervention. The rationale for adding a general dementia guide to TAU for the comparator group was informed by our public engagement work. It was thought that additional material, as well as usual care from memory services, would help encourage people with dementia and carers to take part.
In a comprehensive portfolio of outcomes for people with dementia and their carers, the primary outcome was the BADLS, measured at baseline and at 13 and 26 weeks (i.e. the primary end point) after baseline. Secondary outcomes for people with dementia were CASP-19 (Control, Autonomy, Self-Realization and Pleasure) (quality of life), CDRS (Clinical Dementia Rating Scale), DEMQOL (Dementia Quality of Life), ICECAP-O (ICEpop CAPability measure for Older people) (capability measure) and EQ-5D-5L (EuroQol-5 Dimensions, five-level version) (both of which are health-related quality-of-life measures used in the economic evaluation), LSNS-R (Lubben Social Network Scale-Revised), R-IDDD (Revised Interview for Deterioration in Daily Living Activities in Dementia) and S-MMSE (Standardised Mini Mental State Examination). Secondary outcomes for carers were the GHQ-12 (General Health Questionnaire-12 items), which assessed their psychological health, and the SSCQ (Short Sense of Competence Questionnaire), which assessed their sense of competence.
To ensure that the intervention and methods worked in practice, we conducted internal feasibility and pilot studies with 40 participants recruited from the two initial host trusts in equal numbers, completed in August 2017. We adapted the ACCEPT (Acceptance Checklist for Clinical Effectiveness Pilot Trials) criteria156 to assess whether or not the intervention and trial protocol worked in practice and the DMEC accepted these criteria. The only adjustment suggested by the pilot was to reduce the length of follow-up from the initially planned 12 months to 6 months in the main study. This enabled us to meet recruitment targets and tackle the more realistic goal of improving BADLS scores over 6 months. Therefore, we were able to include the pilot data, adjusted to reflect this change, in the main analysis.
We manualised the intervention, which added specialist equipment and advice by trained DSPs to TAU. Training for DSPs was provided by the University of Manchester research team and investigator NK. The intervention provided up to 6 hours’ contact with a DSP for the person with dementia and the identified carer. DSPs designed and delivered a package of memory aids up to a maximum cost of £150 for the person with dementia to use at home. The package for each depended on their needs, preferences and existing use of memory aids. DSPs also advised on improving everyday memory skills and on using these aids to reduce memory lapses. The follow-up sessions addressed queries from participants and recorded whether or not aids were appropriate to identified goals and needs and used accordingly. A concurrent process evaluation provided further insight into the implementation and acceptability of the intervention and details of this are provided in Appendix 6.
Interviewers, trial statisticians, the University of Manchester research team, Programme Steering Committee and DMEC were masked to participants’ allocations. However, masking participants and DSPs was not possible. We took precautions to minimise the risk of bias,157 including randomising after collecting baseline data and asking research interviewers to record after each interview to which group they judged participants belonged and with how much confidence.
We undertook a qualitative analysis, embedded within the trial research interviews, by audio-recording the incidental conversations and comments made by a sample of participants. This analysis was published in Dementia in June 2019158 and the output of the paper is in Appendix 5. The aim was to collect contextual and conversational data from participants (n = 28) during structured interviews for the main study to provide evidence about their experiences and use of memory aids.
Results
We recruited and randomised 468 people with dementia and their carers at baseline, with 234 pairs in each arm of the study. This was slightly below the 480 people we estimated we would need to achieve our target of analysing 360 participants after allowing for the attrition characteristic of recruitment in this vulnerable population. Attrition at 6 months was in line with that expected (26% rather than 25%) and so the total number of analysable participants with data at baseline and 6 months was 347, also slightly lower than the target.
A multilevel mixed-effects model enabled us to adjust for differences at baseline, notably in age, sex and ethnicity. We used mixed models to examine treatment effect, as these take better account of missing data, particularly if missing at random, and explicitly account for correlations between repeated measurements within each participant. 159 This showed no significant differences between arms over time. Outcomes in both arms reflected increasing dependency by people with dementia, notably in the ADL. In particular, BADLS scores, which range from 0 to 60 with higher scores showing greater dependence, showed a mean difference of only 0.38 at 6 months, slightly but not significantly favouring the comparator group receiving TAU. The 95% confidence interval (CI) ran from –0.89 to 1.65 (p = 0.56). This (non-significant) mean difference of 0.38 was substantially lower than the smallest difference of 3.5 considered important for patient management (see Methods).
A total of 43 serious adverse events (SAEs) were reported for 42 people; one participant had two SAEs. There were more, but not significantly more, SAEs in the intervention arm (n = 24) than in the TAU arm (n = 19). We have no evidence that any SAE was related to the study intervention.
Our process evaluation (see Appendix 6) showed good engagement, with almost all participants completing the intervention, which was delivered as planned with packages individually tailored to participants. Misplacement of items and orientation to date and time were common areas of need. Memory aids that were frequently supplied or supported participants included orientation clocks, whiteboards, calendars and notebooks, as well as bespoke items. These findings suggested a potentially positive impact of the intervention on the well-being of people with dementia and their carers. We identified facilitators of implementation and wider roll-out, barriers to both and strategies to overcome challenges.
Findings from our qualitative analysis highlighted issues concerning the research interview itself and the recruitment of people with dementia to trials. The context and content of the interviews often posed difficulties for participants. People in early-stage dementia struggled with the structured and standardised nature of the research interviews, finding them a linguistic and cognitive challenge. Research interviews addressed sensitive issues that could be distressing for people with dementia and their carers and difficult for interviewers to manage. There was the added tension of the interviewer often having to negotiate the relationships between people with dementia and their carers, and determining whose perspective was being addressed by the questionnaire responses. We return to these issues in Conclusions from the whole programme.
Conclusions
Dementia support practitioners were successfully trained in the DESCANT intervention and delivered it to 98% of participants in the intervention arm. This finding was supported through qualitative findings, which show that implementation was successful and the intervention was well received. However, our main trial failed to show any significant effect of the intervention on the participant outcome measures. The intervention, although well received, did not maintain the ADL or improve other outcomes for people with dementia or carers. Within an expectation of increasing dependency in the ADL over time for dementia,160 any slowing of that dependency is a legitimate aim, with BADLS chosen as the primary outcome to test efficacy of the intervention. However, our intervention did not achieve that aim sufficiently.
Effectiveness of home support models in later dementia
As dementia proceeds in individuals, challenges to everyday living become more apparent and the role of non-health support takes precedence. Informal care by family and friends and social care commissioned or provided by local authorities begins to assume more importance. 161 Our evidence synthesis (see Workstream 1, Evidence synthesis of studies describing components applied to home support for dementia) found a paucity of evidence on home support models addressing daily living activities to help individuals and their families at this stage. We therefore undertook a naturalistic, observational study to discern the naturally occurring home support available in England to people in later-stage dementia and their carers. We then measured the relative effectiveness of these different models. An associated aim of this study was to examine whether or not the models described in the evidence synthesis, and the components thereof, existed in the real world of service delivery across local authority areas of England.
We published the full protocol for the study in International Psychogeriatrics in 2017. The protocol included the final sample size calculation for the primary outcome BADLS152 and a description of data collection and analytic procedures. The output of this paper is in Appendix 7. We summarise below the methods and results of the study in terms of effectiveness of different home support models in later-stage dementia. More detail on the background and findings are in Appendix 7.
Methods
This was a prospective observational study that examined outcomes for people with dementia and their carers after receiving different packages of home support. The outcomes studied were BADLS, DEMQOL, SSCQ and place of residence at 12 months. The analysis plan, contained in the protocol, was to discern the different combinations of home support services received by participants and to aggregate these into separate care package groups (i.e. naturally occurring mixtures of different components of support). 162
Our sampling strategy allowed for potential variation in service mix received by people with dementia living at home. We approached sites (local authority-designated areas) for recruitment with potentially different intensities of service provision, using data from our national surveys (see Workstream 1, Survey of current provision in England). The project received a favourable Research Ethics Committee opinion on 18 December 2015 (reference 15/NW/0822) but Health Research Authority approval was not given until 9 May 2016. Our planned date of recruitment therefore had to be delayed. We negotiated access to participants through home care and respite services, NHS CMHTs and local carer support services. We began recruiting on 10 May 2016, with the first participant enrolled on 31 May 2016. We collaborated with 17 local authority areas and their local CRN teams (following NHS trust boundaries not local authorities) for consent and recruitment. Consent procedures at baseline and follow-up mirrored those in the trial, described above.
The analysis deviated from that described in the protocol for creating the care package groups. We initially planned to use data reduction techniques163,164 on service receipt data to create the packages of care empirically. However, before the end of recruitment, we decided with our statistician that this approach would yield too many groups for analysis and would fail to reflect real-world service mix. This would have compromised the objectives of the analysis (i.e. to examine the approaches occurring naturally to support people in later-stage dementia and their carers at home). We therefore created groups substantively, investigating how the service receipt data mapped on to the approaches identified from our evidence synthesis (see Workstream 1, Evidence synthesis of studies describing components applied to home support for dementia) and how service combinations (‘care packages’) were configured in the real world, described by our PPCI group.
We evaluated the relative effectiveness of the care packages through a multivariate model. We used propensity scores165 to minimise the risk of confounding (i.e. the error of not accounting for variables associated with both receipt of a care package and outcomes). These scores combined the effects of baseline characteristics on receipt of different care packages into one composite measure used to adjust for this in the multivariate models. 166
We undertook an embedded qualitative analysis similar to that in the trial (see Effectiveness of home support in early dementia: the DESCANT) by audio-recording the conversations and comments made by a sample of participants. This analysis was published in BMC Geriatrics in 2019 and the link to the paper is in Appendix 7. Again, the aim was to collect contextual and conversational data from participants (n = 17 carers) during structured interviews. This provided evidence about experiences of the research process and of daily caring for someone with dementia at this late stage.
Results
We recruited 518 people with later-stage dementia and their carers at baseline, well above our target sample size of 400, which allowed for attrition. At 6-month follow-up, there was inevitably some attrition, with 389 participants (pairs of people with dementia and their carers) interviewed at both baseline and follow-up to provide data on circumstances, service receipt and outcomes. This sample with follow-up data was still above our initial sample size target of 310 people.
Creating separate care package groups from the data was a challenge. At baseline, service receipt differed between agencies and professional groups. Packages that grouped these data together, overlapped for many participants. This meant that packages including particular components, for example focused on social care or supporting daily living, were not distinct from other packages. Participants tended to receive these services, but also others that could have been grouped into other packages. Therefore, attempting to create distinct care package groups resulted in groups with fewer than 30 participants and the loss of those who belonged to no particular group. To simplify the eventual grouping of services into care packages relied on a measure of service intensity in line with that guiding our sampling strategy from the national survey. We used a subset of eight dementia-specific home support services to create ‘service intensity’ care package groups: basic care (none or one service), intermediate care (two or three services) and advanced care (four or more services). Effectiveness analysis through the multivariate models therefore examined outcomes for each of the intermediate and advanced groups, compared with a reference group of basic care. The models revealed no significant effects of the advanced or intermediate care packages on the primary outcome (i.e. BADLS) or secondary outcomes (see Appendix 7, Tables 8–11). However, participants with more home care visits were more likely, and those receiving advanced care were less likely, to be living at home at 12 months. Whether or not participants changed care package had no effect on these outcomes.
Conclusions
A complex picture emerged of the care packages received routinely by people with later-stage dementia and their carers across 17 areas of England. Home support mixed social care, NHS professional support and voluntary sector contributions, focusing on all components identified in our evidence synthesis for the person with dementia, with the exception of behaviour management. Although the analysis did not show evidence of effectiveness, the data enabled us to investigate the natural patterns of support and how it was targeted on the most vulnerable. The intensity of most people’s care packages did not change over time. Those that did change mostly decreased in care intensity. The study generated a large and comprehensive data set that could be used to examine naturally occurring support in this vulnerable group.
Toolkit to improve management and commissioning
Evidence from across the whole programme was included in the toolkit developed from 2018 to 2020. Its purpose was to present evidence from the programme in a way that was easily accessible to managers and commissioners. We extracted data from the projects across the programme and consulted stakeholders regarding content and presentation. The toolkit provides evidence to inform service specification and to redesign and benchmark practice. The toolkit is available in an accessible web format with the link: https://sites.manchester.ac.uk/home-support-dementia/ (accessed 6 April 2021).
The toolkit had six modules (Table 1). It was designed for the use of commissioners and providers within the statutory and non-statutory sectors, including:
-
Clinical Commissioning Groups (i.e. the NHS bodies responsible for the planning and commissioning of health-care services for their local area)
-
local authorities as commissioners of social care for older people
-
joint commissioners of older people’s services (i.e. commissioners whose responsibilities span Clinical Commissioning Groups and local authorities)
-
provider units within NHS trusts
-
adult social care providers within the statutory and non-statutory sectors
-
commissioners within provider organisations who assume a lead/strategic role in commissioning services within their own organisation and other providers.
| Module | Title | Data source (project number)a | Research question |
|---|---|---|---|
| 1 | Scoping the evidence | Literature review (1.1) | What do we know? |
| 2 | Evaluating the service landscape | Survey of current provision (1.2) | What is the service landscape? |
| 3 | Bridging the memory gap | Trial of home support in early stage dementia (2.1) | What new evidence is there for commissioners and providers to help people in early-stage dementia? |
| 4 | Maintaining well-being at home | Study of impact of different models of home support in late-stage dementia (2.2) | What new evidence is there for commissioners and providers to help people in late-stage dementia? |
| 5 | Preferences for care and support |
Analysis of costs to people with dementia and carers (3.1) Establishing the value of different components of support (3.2) |
What services do consumers (patients/service users/carers) want? |
| 6 | Costs and benefits |
Development of economic model (1.3) Cost–utility modelling of home support models (3.3) |
What is the cost of improving care? |
A small project team, including a stakeholder representative, was responsible for developing the modules for the toolkit by reviewing relevant programme publications and findings, extracting salient data, presenting findings in an accessible format and reviewing each module. To enhance the accessibility of the research findings to commissioners and providers, the material was professionally written in ‘plain English’. Subsequently, a smaller project team designed a set of infographics and web-based tools through Visme™ [2021 Easy WebContent, Inc. (DBA Visme), Derwood, MD, USA] for the toolkit website. A full description of the design and development of the toolkit and a summary of the data within each module is in Appendix 8.
Workstream 3: evaluation of the costs and consequences of different approaches to home support
In this workstream, we aimed to identify the costs of models of home support to public agencies, people with dementia and carers, and their cost-effectiveness. We elicited data on costs and consequences through examining the preferences of staff, carers and people with dementia. This work supported the overall programme aim of examining the success of tertiary prevention for dementia care at home and maintaining well-being, if possible, by minimising people with dementia’s reduced function and ameliorating negative impacts (e.g. by reducing unplanned hospital admissions). Such actions are thought to reduce costs,167 but policy-makers need to understand the consequences for different parties of different forms of home support and their interactions with care provided by carers. To justify the NHS and social care providing more individually tailored care, improvements in the patient–carer experience is required. This workstream generated data on these issues and therefore enabled the economic model, already developed, to evaluate the home support models emerging from the programme.
Analysis of costs to people with dementia and carers and their relationship to formal care
We sought in this study to provide evidence about the transition from informal to formal home support at moderate and later stages of dementia. The relative balance between the costs of formal and informal care has been previously explored,168 but there was a need for evidence about the costs of home support models. This project aimed to do this through the participation of diverse groups of carers of people with dementia recruited via local voluntary organisations, and groups of professional staff in the host NHS trust. The research aims were to investigate which inputs from health and social care and which informal support carers and professional staff considered important to support people with dementia at home effectively. What are the costs of these inputs? What is the relative balance between informal and formal support?
We consulted panels of experts in two senses – carers expert by experience and staff expert by training – between July 2015 and January 2016. The consultations were undertaken through simulation exercises where participants were asked to outline the components they saw as necessary to support people in different circumstances identified through five ‘case vignettes’. These vignettes described circumstances of real cases drawn from the English sample of a European dementia programme169 that were representative of people with dementia at risk of entering care homes. Through the consultations using these vignettes, we collected data on the inputs seen as necessary to support people with dementia at home effectively. We asked participants to consider both formal paid help (e.g. from the NHS or social services) and informal care (i.e. specific inputs from the person living with or offering support to the person with dementia). These inputs were costed using nationally available unit cost data. From this, we analysed the balance of expertly assessed costs between informal care and formal (NHS/social care) support.
The project received a favourable Research Ethics Committee opinion on 29 June 2015 (reference 15/LO/1137). We recruited 14 informal carers of people with dementia via two local community centres and consulted an additional minority ethnic group for guidance. We also recruited 14 professional staff from the host trust through a senior manager who was a member of the programme team. These covered a range of professions in health and social care, including occupational therapists, community psychiatric nurses, social workers and managers. This work was published in Dementia in 2019,170 and is now in Appendix 9.
The vignettes used to collect data represented 42% of people with dementia living at home but at risk of entering care homes in England. The inputs suggested most frequently by both paid staff and informal carers were informal care, personal home care and day-care centres. However, staff suggested an average of 66 hours per week of support across the five case types, whereas informal carers suggested an average of 51 hours. Translating these inputs into average costs at 2014/15 prices, formal care would cost a mean of £719 per week when recommended by staff and a mean of £634 per week when recommended by informal carers. Informal care would cost a mean of £632 per week when recommended by staff and a mean of £391 per week when recommended by informal carers. Therefore, staff recommended informal care costing 88% of formal care, whereas for informal carers the recommended ratio was 62%. Taking recommendations for formal care costs from staff and for informal care costs from informal carers yielded a ratio of 54%.
The limitations of this work included the small sample of 28 participants consulted. We based the case vignettes on a range of people with moderate or advanced dementia judged to be on the margins of care home entry, rather than people with mild or early-stage dementia who may require little or no home support. We derived indicative costs from participants’ judgements. Therefore, they do not represent full societal costs. In particular, they do not include accommodation costs and other social costs, particularly by informal carers.
Nevertheless, data from this study offer insights into the preferences of key actors – informal carers and professional staff – for the inputs and, therefore, costs needed to support people with dementia at home. Informal carers offered different recommendations from those of staff, more frequently identifying provision of hot meals, day care and increased support for carers.
Staff recommended more personal and domestic services than informal carers, probably reflecting carers’ experiences of providing the majority of care. 171 Staff were also more likely to suggest support by speech and language therapists and dieticians, reflecting greater awareness of these services. Carers and people with dementia frequently lack awareness of services, as well as the knowledge of how to access these. 172 Therefore, these data suggest that dementia home support could be more individualised, with the balance between formal and informal care, depending on the needs of the person with dementia.
Discrete choice experiments establishing the value of different components of support
The aim of these two studies was to examine the preferences of people with early- and late-stage dementia and their carers between different home support services. Separate DCEs for early- and later-stage dementia elicited these preferences. Attributes for the DCEs were drawn from the components of care investigated in other parts of the programme (see Appendix 2) and informed by the evidence synthesis and lay consultation with our PPCI group.
We recruited participants to complete DCE questionnaires by a variety of means. For early-stage dementia, we recruited 44 people with dementia and 103 carers through memory clinics and used an online questionnaire. For later-stage dementia, we recruited 100 carers through discussion groups of family carers and used a questionnaire, both online and by post. Analysis used a conditional logistic regression model that examined the strength of preferences for different attributes of home support packages. The project received a favourable Research Ethics Committee opinion on 17 July 2014 (reference 14/NW/1044). The work was published in Ageing & Mental Health for early-stage dementia173 and for later-stage dementia. 161 These outputs are contained in Appendix 10.
We found that the most preferred components for a home support package in early-stage dementia were support with personal feelings and concerns (coefficient 0.67; p ≤ 0.001) and information on coping with dementia provided by a trained worker at home (coefficient 0.59; p ≤ 0.001). For people with dementia, however, opportunities for social and recreational activities were most preferred (coefficient 0.48; p ≤ 0.001). For carers of those in later-stage dementia, the most preferred attributes were regular respite care (coefficient 1.29; p ≤ 0.001) and regular home care (coefficient 0.93; p ≤ 0.001). Cost also had a significant effect, with lower cost packages preferred, and respite care was the most important attribute for all carers. Most carers reported that completing the DCE had been a positive experience.
Cost–utility modelling of the impact of home support models
From the development of the economic model earlier in the programme (see Workstream 1, Development of an economic model), we experienced challenges in evaluating the cost-effectiveness of home support models in dementia. Most approaches we reviewed addressed home support at moderate to severe stages of dementia later in the care pathway, but there were few data about support services offered to those in early-stage dementia. Even the studies identified in our evidence synthesis and economic review lacked reliable data on the costs and benefits of home support models. This was particularly true of social care.
We therefore modelled the economic costs and benefits (cost–utility analysis) of home support models from data collected in our two primary studies (see Workstream 2, Effectiveness of home support in early dementia: the DESCANT, and Workstream 2, Effectiveness of home support models in later dementia). The methods and results of this are summarised below for early- and later-stage dementia, respectively. More details of the methods and findings are in Appendix 11.
Early-stage dementia: within-trial economic analysis
Cost-effectiveness analysis followed an agreed statistical analysis plan, which was summarised in the trial protocol paper in Trials in 2018. 151 The aim was to evaluate whether or not DSPs with guidance in using memory aids were cost-effective compared with TAU for a range of values of willingness to pay (WTP) for a QALY gained. The perspective of the primary analysis was public (NHS and social care), carer (costs) and people with dementia and their carer (health benefits), but multiple perspectives were also presented (see Appendix 11). We estimated QALYs from the EQ-5D-5L completed at baseline, 3 and 6 months, and associated utility tariffs recommended by NICE at the time of analysis. 174–176 We also used dementia-specific utility values from the DEMQOL177 to estimate QALYs, in a sensitivity analysis to examine alternative measures of benefit. We estimated total QALYs from the usual formula:(1)QALY=Σ[(Ui+Ui+1)/2] ×(ti+1−ti)over i=0&1,178
where U is utility and t is time at assessment. The time between assessments is the time from baseline to 3-month follow-up (i = 0), and from 3- to 6-month follow-up (i = 1).
We estimated the direct costs of services used by participants by summing the cost of each resource used to provide health and social care. We collected data from participants and carers on the resources used through the Client Service Receipt Inventory (CSRI)179 to include equipment, adaptations and ambulance use. The Resource Utilisation in Dementia questionnaire (RUD)180 complemented the CSRI by identifying and estimating the volume, duration and cost to participants of support from formal and informal carers. We documented the resources used to provide the DESCANT intervention (e.g. staff time, training and materials) and added them to the services used by participants to estimate the total cost of the intervention. We used national average unit cost data181 to estimate the costs of formal health and social care for each person. The price year for all costs was 2017/18. Costs and effects were not discounted, as the evaluation period was < 1 year.
In summary, the results showed that the intervention was, on average, more costly but slightly more effective than TAU. The bootstrapped results, allowing for uncertainty, showed that the intervention had a mean incremental cost (over TAU plus dementia guide) of £412 (standard error £2745, 95% CI –£496 to £5792) and mean incremental QALYs of 0.004 (standard error 0.005, 95% CI –0.006 to 0.014). The intervention was probably not cost-effective at a range of WTP values. Sensitivity analyses, using different measures of utility, did not alter these findings to any degree. At our £15,000 threshold, the intervention had a 42–44% probability of being cost-effective, depending on the measure chosen.
Later-stage dementia: analysing different models of support
Cost-effectiveness analysis again followed an agreed statistical analysis plan. We calculated all resources, costs and QALYs in the same way as in the within-trial analysis above. However, for later-stage dementia, all data from the observational study of home support (see Workstream 2, Effectiveness of home support models in later dementia) related to ‘care as usual’. Therefore, the aim was to examine the cost-effectiveness of different intensities of home support against a minimal model or viable low-cost alternative182 constituting home support from one or no dementia-specific services (e.g. visits only by social workers, community nurses or voluntary sector support workers). We evaluated the incremental costs and benefits of more complex models representing different combinations of services of intermediate and advanced intensity, relative to this minimal model. The perspective of the primary analysis was again public costs to providers (i.e. NHS, social care and third sector), carers (costs) and people with dementia and their carer (health benefits), but multiple perspectives were also presented (see Appendix 11). Again, costs and effects were not discounted, as the evaluation period was < 1 year.
In summary, the bootstrapped results, allowing for uncertainty, showed intermediate intensity home support packages as having a mean incremental cost (over basic care) of £7121 (standard error £4261, 95% CI –£1194 to £15,593) and mean incremental QALYs of –0.01 (standard error 0.01, 95% CI –0.03 to 0.007), with an ICER of –£712,100. The advanced intensity package had a mean incremental cost of £3556 (standard error £5720, 95% CI –£7677 to £14,742) and mean incremental QALYs of –0.05 (standard error 0.02, 95% CI –0.09 to –0.02), with an ICER of –£71,120. Neither care package was probably cost-effective against a range of WTP values. However, care packages were more likely to be cost-effective from the perspective of particular stakeholders. From a third-sector perspective, intermediate intensity home support packages had a mean incremental cost (over basic care) of –£428 (standard error £149, 95% CI –£713 to –£134) and mean incremental QALYs of –0.01 (standard error 0.01, 95% CI –0.34 to 0.006), with an ICER of £42,800. For this sector, the intermediate care package had a 84% probability of being cost-effective at a WTP threshold of £15,000. From an informal carer perspective, advanced intensity home support packages had a mean incremental cost (over basic care) of –£1895 (standard error £5609, 95% CI –£13,012 to £9248) and mean incremental QALYs of –0.05 (standard error 0.01, 95% CI –0.09 to –0.02), with an ICER of £37,900. For this sector, the advanced care package had a 59% probability of being cost-effective at the same WTP threshold of £15,000.
Sensitivity analyses, using DEMQOL-generated utility values, did not change the position of either care package on the cost-effectiveness plane or their probability of cost-effectiveness.
Involvement of patients or the public
Public, patient and carer involvement was integral to the development and operation of the programme. The PPCI Reference Group and the LAP of carers of those with dementia contributed to the design, methods and interpretation of the programme. The PPCI Reference Group contributed to the original bid to NIHR, when members met with investigators PC, BR and JT in Liverpool to advise on the design of the individual projects in the programme. This meeting was facilitated by Greater Manchester CRN and was made possible by a small grant from the Research Design Service North West (Lancaster, UK).
Throughout the programme, the PPCI Reference Group advised and contributed to examining the following:
-
What are the effective components of services? This information was used to inform the evidence synthesis in workstream 1.
-
In what ways could home support services help? This information was used to assist with literature on the components in Appendix 1.
-
The economic model: what works? What are the disadvantages of certain services? This information was used in the development of the economic model in workstream 1.
-
Materials for the proposed DESCANT (memory aids package and intervention manual) and encouragement of participation through the addition of an appropriate dementia guide. The group commented on all materials ready for the pilot phase of the trial in workstream 2.
-
Diagnosis and usual care in memory clinics. This information was used to help describe usual care and determine the follow-up period for the DESCANT.
-
Strategies to maximise recruitment to the trial in response to shortfall of potential participants through memory clinics in November 2016. This included editing of participant-facing materials (e.g. information sheets) to stimulate recruitment.
-
Research questions for the qualitative study within the trial and interpretation of the analysis.
-
Co-production of the Plain English summary for this report.
The LAP were involved in assisting in the design and piloting of the questionnaires for the vignette exercise (see Workstream 3, Analysis of costs to people with dementia and carers and their relationship to formal care) and DCEs (see Workstream 3, Discrete choice experiments establishing the value of different components of support).
Our PPCI work was evaluated continuously as we progressed through the programme. Members were asked about what they thought were the strengths and limitations of their involvement, and what they saw as the benefits to themselves and to the programme. The PPCI Reference Group co-wrote a paper outlining this public engagement work on the programme. The published output183 from this element of the programme is in Appendix 12.
A variety of methods of en5gagement were used. The PPCI Reference Group met face to face and also corresponded with the research team by e-mail and telephone. The LAP ‘met’ electronically through e-mail. The positive feedback from PPCI members was that this mix of approaches worked well. There were unforeseen circumstances and challenges around the operation of this element of the programme. Maintaining groups of people over an extended time in a complex research programme was such a challenge. Members were absent through illness at points and two members sadly died during the programme. Hence, the PPCI Reference Group was replenished with new members at various points, made possible by the involvement of TIDE.
An unforeseen challenge was the COVID-19 pandemic and the need to respond accordingly. NHS and local authority advice led us to cancel our very last PPCI Reference Group meeting in Liverpool on 13 March 2020 and communicate findings electronically. Other PPCI groups representing this vulnerable population may benefit from our experiences.
Reflections on what was and what was not successful in the programme
We envisaged in the original bid that data would enable us to construct economic models that could generalise to the national picture regarding the most cost-effective home support approaches. However, in developing our economic model (workstream 1), we found that the available data were of limited quality and were missing in some important areas, for example in early-stage dementia. It was not possible therefore to construct reliable mathematical models to inform judgements of cost-effectiveness as we originally intended. The programme was successful in collecting primary data from participants in both early- and later-stage dementia to judge the cost-effectiveness of different approaches (see Workstream 3, Cost–utility modelling of the impact of home support models). Indeed, these primary studies collected comprehensive data, often from very vulnerable people with dementia and their carers, with large sample sizes, which was a testament to the skill mix of the research team and also the sustained support of colleagues in the CRN.
Our observational study in later-stage dementia was successful in achieving a large sample size, permitting the planned analysis of the effectiveness of different intensity care package groups. It generated a unique and comprehensive data set on the routine care received by people with dementia at later stages and their carers living at home. Such a comprehensive array of data on what usual care consists of (including social care as well as NHS care) is, to the best of our knowledge, not available elsewhere in the UK. Although we found no evidence of effectiveness in more intensive support, according to our planned analysis, the data provide an opportunity for further work on the effects of different forms of care available, permitting secondary analyses of the data in different ways. The data offer potential evidence from everyday real-world dementia care at home, including evidence of relative costs and outcomes of different service mixes for different groups. Such data would be useful for social and health-care commissioners who would like to see more detailed information, for example on the costs/benefits of specific services within care packages, such as dementia support workers and home care. Evidence on subgroups, such as those living alone or with comorbidities, would also help to target resources to areas of need.
The embedded qualitative studies hosted within the trial and observational studies were successful in two ways. First, they demonstrate the feasibility of collecting rich qualitative data without additional burden to participants. Second, the data provided new insights into the challenges of undertaking, and people with dementia participating in, research interviews using standardised measures. However, the embedded qualitative work from the trial was intended to provide data on participants’ assessments of the impact of memory aids in combating memory loss to enhance understanding of the intervention. Instead, the findings shed light more on the specific challenges for people with mild to moderate dementia of being a research participant. In this respect, this work was unsuccessful in achieving its original aim, despite producing insightful data on people’s responses to the research process itself, principally the trial. These insights, particularly how standardised measures might be received and responded to by participants, should inform future feasibility studies, particularly trials, in this population.
Limitations relating to the method or execution of the research
All projects on the programme, apart from the exact form of the final economic model (see Reflections on what was and what was not successful in the programme), were undertaken as originally intended. However, several limitations emerged, with respect to both the methodology and the execution of the research.
Robust methods, both quantitative and qualitative, were used throughout, which was a strength of the programme. However, methodologies offered by particular studies do raise limitations in the findings from other parts of the programme. For example, from the reviews, the home support models containing education, social support and behaviour management appeared most effective. In addition, the DCEs did not point to memory aids as the most preferred intervention by people with dementia and their carers. It is possible, therefore, that the intervention evaluated in our trial was not what people wanted or needed, which may have limited its efficacy.
In our analysis of costs to people with dementia and carers and their relationships to formal care (see Workstream 3, Analysis of costs to people with dementia and carers and their relationship to formal care), the sample of 28 people consulted to provide feedback on the type and hours of their own and formal care was small. However, our decision to focus on people with moderate to severe dementia was appropriate, as a group with milder dementia would be likely to have minimal care needs. However, it would have been interesting and important to explore wider personal and societal costs that were not part of the original study design; for example, the costs of informal care, such as informal carers being unable to work or have their own leisure time, and reduced hospital admissions for patients with dementia.
The outcome measures chosen for both the trial and the observational study were ones used previously in other dementia care studies, and for which there were data already available to assist in sample size calculations. Particularly for ADL (i.e. BADLS, the primary outcome in both studies), there are data available on the minimum clinically important difference to estimate expected effects for trial sample sizes. There are no such data available for other outcomes that may be important, such as quality of life (DEMQOL), and certainly none for other outcomes of value that have been developed more recently. This is a current challenge in trial science for dementia studies. Although improvements in, or at least maintenance of, ADL is an appropriate outcome measure to investigate effectiveness of care in later-stage dementia, it may not have been sensitive to potential effects of the trial intervention in early stages. In addition, in a wider sense, a measure of functioning like BADLS may not reflect an outcome that is viewed as important by people with dementia and their carers. BADLS is also limited in being an informant-rated measure. One of our inclusion criteria for entry to the trial was that the person with dementia should have a carer in touch with them who could comment on their functioning, using the BADLS. This carer need not have been living with the participant and in our trial a large proportion (37%) were not. Nevertheless, using BADLS as an outcome would exclude participants with dementia in particular circumstances, such as those without any contact with friends or family. This could compromise future trial designs for more vulnerable and isolated people with dementia and other outcome measures may be more appropriate.
There were challenges in executing the research. Most of the participants in the trial were white. It is possible that there was recruitment bias here in that people with dementia from certain cultural backgrounds may not access mainstream services, such as memory clinics, which was the access point for the trial. Non-white participants may therefore have been harder to recruit, but could have possibly benefited from the intervention. There were delays and difficulties in recruitment to both the trial and the observational study. These led to delays in data collection and some limitations in the data available for the final analyses. For example, the DESCANT did not recruit to its target sample size of number of patients randomised or followed up. There was significant attrition (26%), slightly more than anticipated, and so there were missing follow-up data for outcome measures. For the observational study, attrition was 25%, which was in line with expectations in this more vulnerable and frail population. Although the follow-up period for both studies was relatively short (6 months), this can be a long time in the lives of people with dementia and their carers. Circumstances change and the condition fluctuates. Participants did drop out or refuse follow-up interviews, as is their right to do. These delays and difficulties are common in dementia research, particularly in trials, where recruitment is often difficult. Nevertheless, we achieved large target sample sizes, particularly for the observational study, which recruited from a more vulnerable population. However, to achieve this necessitated extensions to the programme. For the trial, this also needed negotiations around excess treatment costs, initially with Clinical Commissioning Groups and then local CRNs, as governance arrangements changed during the life of the programme.
The research was mainly undertaken in England (with one trial site being in Wales), which may limit the generalisability of the findings to other countries with different health and social care systems. However, the reviews examined international literature that were designed to achieve similar aims to those explored in this programme, as a whole, and had findings relevant to international developments in home support for dementia.
Conclusions from the whole programme
This was an integrated programme, with the whole being greater than the sum of its parts. The results from the individual projects paint a picture of the components that may make up effective home support, their impact, and their potential costs and effects. Bringing all results together, we conclude the following:
-
Our literature searches identified several components, in varied combinations, that could potentially be implemented to support people with dementia and their carers at home. For carers, these were behaviour management, education and advice, social support, emotional support and respite. For people with dementia, these were environmental modifications and care co-ordination. There was little evidence in the literature on the effectiveness of cognitive support, daily living assistance and physical activity for people with dementia living at home. Home support interventions of potential benefit are ones combining education, social support and behaviour management, particularly to carers. For people with dementia, modifying the immediate environment and co-ordinating care delivered by different agencies emerged as potentially the most effective.
-
Our national surveys identified a range of currently available services that might use these components in different ways. The NHS provides services (e.g. CMHTs and memory services) that include information and advice, relaxation and behaviour management. Most memory clinics and CMHTs also provide advice on memory aids, but there was a need identified for rigorous evidence of their effectiveness. Social care provides respite care and day care (the most frequently reported services) and specialist home care (to a lesser extent), which includes support with daily living activities as a central component and occasionally social and emotional support.
-
Our DCEs and vignette study generated evidence about the preferences of people with dementia, carers and professional staff for components of home support packages. The method, although demanding for participants to complete, was well received and enabled people to state what approaches they valued as part of a package of care. Advice on memory aids and cognitive support was judged of value by people with dementia and carers (but not as highly relative to other services), but was considered less important by professionals, perhaps because evidence as to their effectiveness was lacking. Carers suggested many potential components, whereas people with dementia valued advice on memory aids, emotional support, access to community facilities, health promotion, information and relaxation. Professionals were mixed in their preferences. They tended to value access to health services, home care, carer support, respite care and support with daily living activities.
-
Our two primary studies in early- and later-stage dementia aimed to evaluate the effectiveness of some of these components. For early-stage dementia, our pragmatic randomised trial found no evidence of effects on daily living activities through the use of memory aids and guidance by DSPs or of effects on other secondary outcomes (i.e. cognition, quality of life, social networks or carers’ psychological health and competence). The intervention was implemented successfully and qualitative evidence suggests that it was well received and people felt engaged with it. However, the qualitative work also raised issues of the research interview itself and the use of standardised measures in trials that may be cognitively demanding for participants to respond to. Although standardised measures are necessary, the research interview is not a neutral encounter. People with dementia and their carers often had to negotiate between them and the interviewer as to what responses captured their experiences in the most reliable way. This raised challenges, and it is important in the planning of trials that investigators consider the nature and number of outcome measures chosen to help recruitment.
-
For later-stage dementia, our naturalistic experiment found no evidence, overall, that more intensive packages of care were more effective than basic care at home. However, it is useful to highlight specific data that may be driving these results. For example, data from the observational study suggested that home care is successfully targeted in everyday conditions. More home care visits were associated with a greater likelihood of people with dementia remaining at home. This positive finding demonstrates that the objective of home care (domiciliary care commissioned by local authorities) was achieved successfully for people with dementia across 17 areas in England. People with dementia receiving more intensive care were less likely to remain at home. This finding demonstrates evidence of successful targeting, in that more intensive care was for more vulnerable people likely to move on to nursing or residential care, an interpretation supported by the evidence at baseline. Such findings, as above, would be interesting and useful in informing social care commissioning decisions. The qualitative work for this study revealed that carers were often trying, sometimes successfully, to maintain a ‘normal’ life for themselves and the person with dementia within the confines of the condition. They tried to arrange care in line with families’ expectations for services that were compassionate and convenient for their needs. The context of the research interview was, again, raised. The standardised measures used, intended to measure functioning and well-being, focused on difficulties and challenges for people and used words that did not necessarily align with participants’ outlooks.
-
The literature has little robust evidence about the cost-effectiveness of different home support models. Occupational therapy, home-based exercise and carers’ coping interventions are potentially cost-effective. However, we need better economic evidence of home-based cognitive support in early dementia (which our trial has provided) and of later palliative care.
-
Our two primary studies generated new cost-effectiveness evidence. In early-stage dementia, our DESCANT intervention is probably not cost-effective. In later-stage dementia, more intensive care packages at home are less likely to be cost-effective (i.e. they were more costly and less effective than basic care). However, from the perspective of the third sector, packages of intermediate intensity were less costly but also less effective, with these cost reductions predominantly leading to a potential for cost-effectiveness at higher cost thresholds. Again, it is useful to look at how particular data may be driving these results. For example, in our report of mean cost per item differences across the three care packages (see Appendix 11, Tables 23 and 24), the reduction in third-sector costs from more intensive packages was predominantly from reduced costs of carer groups. Such findings, of the cost consequences of different configurations of care from different sources, could be used by commissioners to decide how to allocate funding across sectors to achieve desired effects. As stated above, there is an opportunity to further use these rich data from the observational study to inform resource and policy decisions.
-
Our toolkit [URL: https://sites.manchester.ac.uk/home-support-dementia/ (accessed 6 April 2021)] is a tangible product from the study and brings together all the evidence from the programme in one place in a manner useful for managers and commissioners. Our programme findings imply that health and social care decision-makers should direct their attention to tailoring services to the expressed preferences of people with dementia and their carers. People with dementia most value emotional support, access to community facilities, health promotion advice, information and signposting, and relaxation techniques. Carers value aids and adaptations, home care, support in coping with difficult behaviour and agitation, emotional support, access to community facilities, health promotion, information and signposting, relaxation and respite care. In commissioning services, decision-makers should seek to combine these services in different ways, depending on changing needs. Although the NHS and social care already provide many of these approaches to helping people with dementia ‘live well’, there might be more benefit in concentrating on approaches that are particularly effective (i.e. those combining education, social support and helping carers cope with difficult behaviour). Modifying the immediate environment around the person with dementia is particularly valuable, as is ensuring that services are co-ordinated. Home care, commissioned by local authority social care, is particularly effective at helping people with dementia remain at home and appears to operate appropriately in providing more intensive support to achieve this. It should be prioritised, despite the resource constraints currently operating within social care as carers value it. Providing memory aids to people at early stages of dementia, although valued, does not help maintain their daily living activities to a sufficient extent, and scarce resources might be better directed elsewhere. In later-stage dementia, combining more services together to help the person stay at home and support carers is not necessarily better. Much depends on people’s specific needs. It would be useful to continue to commission care from the third sector (e.g. dementia support workers, drop-in centres and tailored support to carers). The evidence is that the third sector may provide this more cheaply, but that this is not, however, necessarily more effective in maintaining quality of life.
Recommendations for future research
Building on these conclusions, we have four main research recommendations for future work: (1) research on more sensitive outcome measures for dementia trials, (2) further research on home support interventions, (3) innovative methods of recruitment for dementia intervention studies and (4) expanding methodological boundaries for cost-effectiveness analysis, particularly where social care is a large component. We describe these below in priority order.
-
There is a need to examine more sensitive outcome measures in dementia trials. Our two primary studies, at different stages of dementia, were essentially negative. Our trial showed no evidence of effectiveness on the chosen outcomes. However, the intervention was well received and qualitative research suggested that it might have led to other changes of value to participants, for example greater engagement, independence and other more subtle effects, which, to the best of our knowledge, have not been measured in trials up to now. 184,185 Other recent but unpublished dementia trials mirror these findings. There is therefore an emerging opinion in the dementia research community that more sensitive outcome measures are required. Outcomes that can measure more personal effects of value to people with dementia and their carers. We judge that the time is ripe to draw on more recently developed core outcome sets186 for use in planning future dementia trials. Aiming to use these emerging core outcomes will necessitate trial science investigations, for example analyses determining the minimum clinically important difference for promising outcomes (e.g. the Engagement and Independence in Dementia Questionnaire). 184 Such analyses would be a prerequisite to calculating appropriate sample sizes for trials using these outcomes and there is scope for undertaking these in feasibility studies before a full trial.
-
There is a need for research to elicit further evidence of multicomponent home support packages for people with dementia at different stages. 187 The knowledge base, particularly of non-pharmacological interventions and those in social care settings, is growing rapidly. Research on interventions from social care, for example those from home (domiciliary) care commissioned by local authorities, focusing on providing daily living assistance, would provide evidence in a hitherto neglected area. More recently, especially towards the end of this programme, with the limitations brought about by the COVID-19 pandemic, emerging evidence has shown a reduction in access to home support for people with dementia and their carers, which may have contributed to worsening quality of life. 188 To continue providing support, services will need to be adjusted to operate remotely, in the light of restrictions. Therefore, future research could examine how some of the home support models we have identified as showing promise, such as those with components of carer support, behaviour management and emotional support, could be adapted appropriately to be delivered remotely. Examining the preferences of different stakeholders, including people with dementia themselves, for such interventions is also a fruitful area for further research and our programme has shown that such perspectives can be elicited and people respond well.
-
There is a need for dedicated work, including intensive qualitative analysis, on the effectiveness of novel recruitment strategies to support dementia intervention studies. Data collection and recruitment for our primary studies in the programme was challenging. Although we piloted our approach in our pragmatic trial, dementia research is difficult, particularly the recruitment and retention of enough participants for analysis. These are vulnerable participants, and there are many challenges in ensuring participants’ awareness of research, supporting them in approaches to take part, informed consent, and arrangements for research interviews and data collection. The infrastructure is there, particularly through local CRNs to support with these challenges. However, there are still potential barriers to achieving sufficient numbers of participants, especially those participants who are hard to reach. Examples include those with dementia living alone or without much support from informal carers. Recruiting within social care settings, for example from home care, day care or through third-sector organisations providing support, is also an area where the CRN support infrastructure is only just developing. Although issues with recruitment for dementia trials have been researched, most of the difficulties identified, and discussion of possible strategies to ameliorate them, relate to pharmacological trials rather than non-pharmacological support interventions, such as those evidenced in this programme. 189 There is also little evidence from controlled comparison of recruitment strategies190 and therefore detailed feasibility studies testing different ways of recruiting and the benefits accrued would be potentially useful.
-
Finally, our cost-effectiveness analyses raised fruitful areas to explore methodologically. The economic evaluation of social care, a major part of this programme, was the subject of a recent review. 191 Issues include developing appropriate measures of benefit, particularly for long-term conditions like dementia where the aim is maintenance rather than cure; how to estimate costs and benefits that are the responsibility of different stakeholders (e.g. the third sector, contracted with local authorities); the appropriate threshold value with which to compare interventions, as resource responsibilities are shared between the NHS and local authorities; and the important role of informal carers, notably their contribution to utility, costs and how best to measure their inputs.
Acknowledgements
Contributions of others
This was a large and complex programme with several interlocking projects within the workstreams. It would not have been possible to undertake it successfully without the effort and commitment of a number of people, whom we thank here.
We thank our successive NIHR programme managers, throughout the programme period, for their guidance and advice and our colleagues in the host NHS trust, Pennine Care NHS Foundation Trust (Ashton-under-Lyne, UK), for its support to several projects within the programme, in particular Carol Ainsworth, Carol Harper, Kim Bennett, Brenda Pimlott and Reagan Blyth. We thank colleagues in other NHS trusts for their support, particularly to the trial and observational study, in particular Pat Mottram and Theresa Whittingham of Cheshire and Wirral Partnership NHS Foundation Trust (Chester, UK) and Ritchard Ledgerd of North East London NHS Foundation Trust (London, UK). We thank Penny Lane and Janine Smith of NAViGO Health and Social Care CIC (Grimsby, UK) for their generous support in the recruitment of patients for DESCANT.
We thank members of the DMEC for their oversight of DESCANT: Peter Crome (chairperson), Gill Manthorpe, Linda Clare, Zoe Hoare and Anthony Hodgson.
We would like to thank researchers who undertook research interviews and analysis for individual projects, some of whom are authors on published outputs from the programme: Amy Roberts, Jude Wells, Megan Patterson, Matthew Larbey, Chengqiu Xie, Nik Loynes, Alexandra Feast, Eleni Kampanellou, Karen Davies and Karen Stewart. We also thank staff in each of the local CRNs involved in recruitment of participants and research interviews for their hard work and commitment. We would particularly like to thank staff at the Greater Manchester CRN for steering projects through to final completion: Angela Aldridge, Angela Parker and Emma Oughton.
We thank Professor Peter Bower, Professor of Health Sciences, Division of Population Health, University of Manchester, for mentorship to Dr Paul Clarkson during the last 14 months of the programme. Claire Evans at the Swansea Trials Unit and Simon Kaye and Amy Kennon at Pennine Care NHS Foundation Trust provided support and advice concerning contractual matters during this period and we thank them for their important contribution.
We thank the following people for their contributions to the stakeholder consultation to develop the programme toolkit: Professor Alistair Burns, University of Manchester; Dr Georgina Charlesworth, University College London, Strategic and Clinical Lead for Older People Psychology, North East London NHS Foundation Trust; Neil Jones, Borough Lead, Pennine Care NHS Foundation Trust; and Alison Kendall, Programme Manager Implementation of Mental Health Strategy, Pennine Care NHS Foundation Trust.
Programme investigators who do not constitute authors of this report but who wish to be acknowledged include Professor Christopher Roberts, Statistician, University of Manchester, a programme investigator until retirement in August 2016, provided expertise in design and sample size calculation for the observational study. Subsequently, Dr Mark Hann, Statistician, University of Manchester, provided support for statistical analysis of the observational study. Jean Tottie, chairperson of TIDE was an original investigator, co-leading the PPCI work across the programme. We would like to thank her and her organisation for the sustained support given to us throughout the programme timeline.
Contributions of authors
Paul Clarkson (https://orcid.org/0000-0002-0778-312X) (Senior Lecturer in Social Care) was the chief investigator (2019–20) and managed the programme from its inception. He led on the production of this report.
David Challis (https://orcid.org/0000-0002-6464-2286) (Professor of Social Care) was the chief investigator (2013–18).
Jane Hughes (https://orcid.org/0000-0002-6158-1211) (Senior Research Fellow) co-investigator, led on the national survey and toolkit development work.
Brenda Roe (https://orcid.org/0000-0001-8227-0116) (Professor of Health Research) co-investigator, led on the evidence synthesis and PPCI activities.
Linda Davies (https://orcid.org/0000-0001-8801-3559) (Professor of Health Economics) co-investigator, led on the health economic work on the programme.
Ian Russell (https://orcid.org/0000-0002-0069-479X) (Emeritus Professor of Clinical Trials) co-investigator, led on trial design and statistics.
Martin Orrell (https://orcid.org/0000-0002-1169-3530) (Professor of Psychiatry) co-investigator, led on design of the trial intervention and contributed to the process evaluation, NHS survey and evidence synthesis.
Fiona Poland (https://orcid.org/0000-0003-0003-6911) (Professor of Social Research Methodology) co-investigator, led on the qualitative work for the trial and observational study.
David Jolley (https://orcid.org/0000-0001-5534-5637) (Honorary Reader) co-investigator, contributed to the evidence synthesis, and trial and observational study development.
Narinder Kapur (https://orcid.org/0000-0002-8854-1754) (Visiting Professor of Neuropsychology) co-investigator, led on trial intervention training and trial review of literature.
Catherine Robinson (https://orcid.org/0000-0001-7240-7107) (Professor of Social Care Research) was co-chief investigator (2019–20) and contributed to the production of this report.
Helen Chester (https://orcid.org/0000-0001-6587-6618) (Senior Research Fellow) was trial manager until July 2019 and led on the trial process evaluation.
Sue Davies (https://orcid.org/0000-0002-7818-3228) (Research Associate) led on analysis for the health economic work and the national (local authority) survey, and contributed to data collection for the preference studies.
Caroline Sutcliffe (https://orcid.org/0000-0002-4626-7750) (Research Fellow) was study manager for the observational study and contributed to the preference studies.
Julie Peconi (https://orcid.org/0000-0003-1221-827X) (Data Manager, Assistant Trial Manager) managed the trial data and also contributed to data management for the observational study.
Rosa Pitts (https://orcid.org/0000-0002-8526-7390) (Research Assistant) assisted with data management for the trial, and contributed to the process evaluation and to toolkit development.
Greg Fegan (https://orcid.org/0000-0002-2663-2765) (Professor) managed the trials unit and led on data supervision for the trial and observational study.
Saiful Islam (https://orcid.org/0000-0003-3182-8487) (Statistician) led on the statistical analysis for the trial and contributed to the design of the observational study.
Vincent Gillan (https://orcid.org/0000-0002-7927-9387) (Research Assistant) managed data for the trial and contributed to the trial process evaluation.
Charlotte Entwistle (https://orcid.org/0000-0002-2739-2644) (Research Assistant) analysed data for the observational study, managed data for the trial and contributed to the trial process evaluation.
Rebecca Beresford (https://orcid.org/0000-0002-8163-1351) (Research Assistant) led on analysis and production of the toolkit from April 2019, assisted with data management for the trial and contributed to trial process evaluation.
Michele Abendstern (https://orcid.org/0000-0002-8672-7387) (Research Fellow) led on the qualitative analysis for the trial and observational study.
Clarissa Giebel (https://orcid.org/0000-0002-0746-0566) (Research Fellow) led on analysis for the PPCI element of the programme, and analysed data for the evidence synthesis, case vignette and preference studies.
Saima Ahmed (https://orcid.org/0000-0002-6206-3184) (Research Associate) led on planning and analysis for the national (NHS) survey and contributed to planning and data collection for the observational study.
Rowan Jasper (https://orcid.org/0000-0003-1328-2812) (Research Associate) analysed data for the economic literature review and contributed to toolkit design and development.
Adeela Usman (https://orcid.org/0000-0003-1517-0080) (Research Associate) led on the statistical analysis for the observational study.
Baber Malik (https://orcid.org/0000-0002-7153-7003) (Honorary Research Fellow) was trial and programme manager from August 2019.
Karen Hayhurst (https://orcid.org/0000-0002-8976-2356) (Research Fellow) analysed data for the economic development work.
Publications
Workstream 1
Clarkson P, Giebel CM, Larbey M, Roe B, Challis D, Hughes J, et al. A protocol for a systematic review of effective home support to people with dementia and their carers: components and impacts. J Adv Nurs 2016;72:186–96. https://doi.org/10.1111/jan.12737
Clarkson P, Davies L, Jasper R, Loynes N, Challis D. A systematic review of the economic evidence for home support interventions in dementia. Value Health 2017;20:1198–209. https://doi.org/10.1016/j.jval.2017.04.004
Clarkson P, Hughes J, Xie C, Larbey M, Roe B, Giebel CM, et al. Overview of systematic reviews: Effective home support in dementia care, components and impacts – stage 1, psychosocial interventions for dementia. J Adv Nurs 2017;73:2845–63. https://doi.org/10.1111/jan.13362
Ahmed S, Hughes J, Davies S, Stewart K, Orrell M, Clarkson P, Challis D. Specialist services in early diagnosis and support for older people with dementia in England: staff roles and service mix. Int J Geriatr Psychiatry 2018;33:1280–5. https://doi.org/10.1002/gps.4925
Clarkson P, Hughes J, Roe B, Giebel CM, Jolley D, Poland F, et al. Systematic review: effective home support in dementia care, components and impacts – stage 2, effectiveness of home support interventions. J Adv Nurs 2018;74:507–27. https://doi.org/10.1111/jan.13460
Davies S, Hughes J, Ahmed S, Clarkson P, Challis D. Commissioning social care for people with dementia living at home: findings from a national survey. Int J Geriatr Psychiatry 2019;53:53–9. https://doi.org/10.1002/gps.5214
Workstream 2
Chester H, Clarkson P, Hughes J, Russell I, Beresford J, Davies L, et al. Evaluating the effectiveness of different approaches to home support for people in later stage dementia: a protocol for an observational study. IntPsychogeriatr 2017;29:1213–21. https://doi.org/10.1017/s1041610217000291
Chester H, Clarkson P, Davies L, Hughes J, Islam M, Kapur N, et al. Cognitive aids for people with early stage dementia versus treatment as usual (Dementia Early Stage Cognitive Aids New Trial (DESCANT)): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2933-8
Abendstern M, Davies K, Chester H, Clarkson P, Hughes J, Sutcliffe C, et al. Applying a new concept of embedding qualitative research: an example from a quantitative study of carers of people in later stage dementia. BMC Geriatr 2019;19:227. https://doi.org/10.1186/s12877-019-1240-x
Abendstern M, Davies K, Poland F, Chester H, Clarkson P, Hughes J, et al. Reflecting on the research encounter for people in the early stages of dementia: lessons from an embedded qualitative study. Dementia 2020;19:2732–49. https://doi.org/10.1177/1471301219855295
Workstream 3
Chester H, Clarkson P, Davies L, Sutcliffe C, Davies S, Feast A, et al. People with dementia and carer preferences for home support services in early-stage dementia. Aging Ment Health 2018;22;270–9. https://doi.org/10.1080/13607863.2016.1247424
Giebel CM, Davies S, Clarkson P, Sutcliffe C, Challis D. Costs of formal and informal care at home for people with dementia: ‘expert panel’ opinions from staff and informal carers. Dementia 2019;18:210–27. https://doi.org/10.1177/1471301216665705
Kampanellou E, Chester H, Davies L, Davies S, Giebel C, Hughes J, et al. Carer preferences for home support services in later stage dementia. Aging Ment Health 2019;23:60–8. https://doi.org/10.1080/13607863.2017.1394441
Involvement of patients and the public
Giebel C, Roe B, Hodgson A, Britt D, Clarkson P, Members of the Home Support in Dementia Programme Management Group and Patient Public and Carer Involvement Groups. Effective public involvement in the HoST-D Programme for dementia home care support: from proposal and design to methods of data collection (innovative practice). Dementia 2019;18:3173–86. https://doi.org/10.1177/1471301216687698
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112-17. https://doi.org/10.1016/S0140-6736(05)67889-0.
- Knapp M, Prince M, Albanese E, Banerjee S, Dhanasiri S, Fernandez J-L, et al. A Report Into the Prevalence and Cost of Dementia Prepared by the Personal Social Services Research Unit (PSSRU) at the London School of Economics and the Institute of Psychiatry at King’s College London, for the Alzheimer’s Society and Dementia UK 2007.
- House of Commons All-Party Parliamentary Group on Dementia . The £20 Billion Question: An Enquiry into Improving Lives through Cost-Effective Dementia Services 2011.
- Wittenberg R, Knapp M, Hu B, Comas-Herrera A, King D, Rehill A, et al. The costs of dementia in England. Int J Geriatr Psychiatry 2019;34:1095-103. https://doi.org/10.1002/gps.5113.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734. https://doi.org/10.1016/S0140-6736(17)31363-6.
- Department of Health and Social Care (DHSC) . Living Well With Dementia: A National Dementia Strategy 2009.
- Olazaran J, Reisberg B, Clare L, Cruz I, Pena-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord 2010;30:161-78. https://doi.org/10.1159/000316119.
- Burns A, O’Brien J, Ames D. Dementia. London: Hodder Arnold; 2005.
- MacKnight C, Rockwood K. Use of the chronic disease score to measure comorbidity in the Canadian Study of Health and Aging. Int Psychogeriatr 2001;13:137-42. https://doi.org/10.1017/s1041610202008074.
- National Audit Office . Improving Services and Support for People With Dementia 2007.
- Charlesworth G, Shepstone L, Wilson E, Reynolds S, Mugford M, Price D, et al. Befriending carers of people with dementia: randomised controlled trial. BMJ 2008;336:1295-7. https://doi.org/10.1136/bmj.39549.548831.AE.
- National Institute for Health and Care Excellence/Social Care Institute for Excellence . Dementia: Supporting People With Dementia and Their Carers in Health and Social Care. National Clinical Guideline Number 42 2006.
- Association of Directors of Adult Social Services . Response by the Association of Directors of Adult Social Services (ADASS) to 2011 Inquiry of the All-Party Parliamentary Group on Dementia: How to Save Money in Dementia Care and Deliver Better Outcomes for People With Dementia? 2011.
- Burholt V, Wenger G, Scott A. Dementia, disability and contact with formal services: a comparison of dementia sufferers and non-sufferers in rural and urban settings. Health Soc Care Community 1997;5:384-97. https://doi.org/10.1111/j.1365-2524.1997.tb00136.x.
- McDonald A, Heath B. Developing services for people with dementia: findings from research in a rural area. Qual Ageing Older Adults 2008;9:9-18. https://doi.org/10.1108/14717794200800023.
- Rothera I, Jones R, Harwood R, Avery AJ, Fisher K, James V, et al. An evaluation of a specialist multiagency home support service for older people with dementia using qualitative methods. Int J Geriatr Psychiatry 2008;23:65-72. https://doi.org/10.1002/gps.1841.
- Challis D, Clarkson P, Hughes J, Chester H, Davies S, Sutcliffe C, et al. Community Support Services for People with Dementia: The Relative Costs and Benefits of Specialist and Generic Domiciliary Care Services Discussion Paper M245-3. Manchester: Personal Social Services Research Unit; 2010.
- Kitwood T. Dementia Reconsidered: The Person Comes First. Buckingham: Open University Press; 1997.
- Elkan R, Kendrick D, Dewey M, Hewitt M, Robinson J, Blair M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ 2001;323:719-25. https://doi.org/10.1136/bmj.323.7315.719.
- Hedrick SC, Koepsell TD, Inui T. Meta-analysis of home-care effects on mortality and nursing-home placement. Med Care 1989;27:1015-26. https://doi.org/10.1097/00005650-198911000-00003.
- Hughes SL, Ulasevich A, Weaver FM, Henderson W, Manheim L, Kubal JD, et al. Impact of home care on hospital days: a meta analysis. Health Serv Res 1997;32:415-32.
- Stevenson G, Ewing H, Herschell J, Keith D. An enhanced assessment and support team (EAST) for dementing elders – review of a Scottish regional initiative. J Ment Health 2006;15:251-8. https://doi.org/10.1080/09638230600609006.
- Banerjee S, Willis R, Matthews D, Contell F, Chan J, Murray J. Improving the quality of care for mild to moderate dementia: an evaluation of the Croydon Memory Service Model. Int J Geriatr Psychiatry 2007;22:782-8. https://doi.org/10.1002/gps.1741.
- Jolley D, Moniz-Cook E. Memory clinics in context. Indian J Psychiatry 2009;51:S70-6.
- Moniz-Cook E, Agar S, Gibson G, Win T, Wang M. A preliminary study of the effects of early intervention with people with dementia and their families in a memory clinic. Aging Ment Health 1998;2:199-211. https://doi.org/10.1080/13607869856687.
- Riordan J, Bennett A. An evaluation of an augmented domiciliary service to older people with dementia and their carers. Aging Ment Health 1998;2:137-43. https://doi.org/10.1080/13607869856821.
- Andrew T, Moriarty J, Levin E, Webb S. Outcome of referral to social services departments for people with cognitive impairment. Int J Geriatr Psychiatry 2000;15:406-14. https://doi.org/10.1002/(SICI)1099-1166(200005)15:5<406::AID-GPS122>3.0.CO;2-F.
- Clarkson P, Giebel CM, Larbey M, Roe B, Challis D, Hughes J, et al. A protocol for a systematic review of effective home support to people with dementia and their carers: components and impacts. J Adv Nurs 2016;72:186-96. https://doi.org/10.1111/jan.12737.
- Clarkson P, Hughes J, Xie C, Larbey M, Roe B, Giebel CM, et al. Overview of systematic reviews: effective home support in dementia care, components and impacts – stage 1, psychosocial interventions for dementia. J Adv Nurs 2017;73:2845-63. https://doi.org/10.1111/jan.13362.
- Clarkson P, Hughes J, Roe B, Giebel CM, Jolley D, Poland F, et al. Systematic review: effective home support in dementia care, components and impacts – stage 2, effectiveness of home support interventions. J Adv Nurs 2018;74:507-27. https://doi.org/10.1111/jan.13460.
- Elvish R, Lever SJ, Johnstone J, Cawley R, Keady J. Psychological interventions for carers of people with dementia: a systematic review of quantitative and qualitative evidence. Couns Psychother Res 2013;13:106-25. https://doi.org/10.1080/14733145.2012.739632.
- Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD006489.pub2.
- Forbes D, Culum I, Lischka AR, Morgan DG, Peacock S, Forbes J, et al. Light therapy for managing cognitive, sleep, functional, behavioural, or psychiatric disturbances in dementia. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD003946.pub3.
- Hall S, Kolliakou A, Petkova H, Froggatt K, Higginson IJ. Interventions for improving palliative care for older people living in nursing care homes. Cochrane Database Syst Rev 2011;3. https://doi.org/10.1002/14651858.CD007132.pub2.
- Hansen NV, Jørgensen T, Ørtenblad L. Massage and touch for dementia. Cochrane Database Syst Rev 2006;4. https://doi.org/10.1002/14651858.CD004989.pub2.
- Hermans DG, Htay UH, McShane R. Non-pharmacological interventions for wandering of people with dementia in the domestic setting. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD005994.pub2.
- Lee H, Cameron M. Respite care for people with dementia and their carers. Cochrane Database Syst Rev 2004;2. https://doi.org/10.1002/14651858.CD004396.pub2.
- Li R, Cooper C, Austin A, Livingston G. Do changes in coping style explain the effectiveness of interventions for psychological morbidity in family carers of people with dementia? A systematic review and meta-analysis. Int Psychogeriatr 2013;25:204-14. https://doi.org/10.1017/S1041610212001755.
- Littbrand H, Stenvall M, Rosendahl E. Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: a systematic review. Am J Phys Med Rehabil 2011;90:495-518. https://doi.org/10.1097/PHM.0b013e318214de26.
- Lou MF. The use of music to decrease agitated behaviour of the demented elderly: the state of the science. Scand J Caring Sci 2001;15:165-73. https://doi.org/10.1046/j.1471-6712.2001.00021.x.
- Martin S, Kelly G, Kernohan WG, McCreight B, Nugent C. Smart home technologies for health and social care support. Cochrane Database Syst Rev 2008;4. https://doi.org/10.1002/14651858.CD006412.pub2.
- Neal M, Barton Wright P. Validation therapy for dementia. Cochrane Database Syst Rev 2003;3. https://doi.org/10.1002/14651858.CD001394.
- Price JD, Hermans D, Grimley Evans J. Subjective barriers to prevent wandering of cognitively impaired people. Cochrane Database Syst Rev 2001;1. https://doi.org/10.1002/14651858.CD001932.
- Sánchez A, Millán-Calenti JC, Lorenzo-López L, Maseda A. Multisensory stimulation for people with dementia: a review of the literature. Am J Alzheimers Dis Other Demen 2013;28:7-14. https://doi.org/10.1177/1533317512466693.
- Sitzer DI, Twamley EW, Jeste DV. Cognitive training in Alzheimer’s disease: a meta-analysis of the literature. Acta Psychiatr Scand 2006;114:75-90. https://doi.org/10.1111/j.1600-0447.2006.00789.x.
- Spector A, Davies S, Woods B, Orrell M. Reality orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist 2000;40:206-12. https://doi.org/10.1093/geront/40.2.206.
- Subramaniam P, Woods B. The impact of individual reminiscence therapy for people with dementia: systematic review. Expert Rev Neurother 2012;12:545-55. https://doi.org/10.1586/ern.12.35.
- Vernooij-Dassen M, Draskovic I, McCleery J, Downs M. Cognitive reframing for carers of people with dementia. Cochrane Database Syst Rev 2011;11. https://doi.org/10.1002/14651858.CD005318.pub2.
- Vink AC, Bruinsma MS, Scholten RJPM. Music therapy for people with dementia. Cochrane Database Syst Rev 2004;3. https://doi.org/10.1002/14651858.CD003477.pub2.
- Woods B, Spector AE, Jones CA, Orrell M, Davies SP. Reminiscence therapy for dementia. Cochrane Database Syst Rev 2005;2. https://doi.org/10.1002/14651858.CD001120.pub2.
- Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2012;2. https://doi.org/10.1002/14651858.CD005562.pub2.
- Ayalon L, Gum AM, Feliciano L, Areán PA. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia: a systematic review. Arch Intern Med 2006;166:2182-8. https://doi.org/10.1001/archinte.166.20.2182.
- Basu A, Brinson D. The effectiveness of non-pharmacological interventions for behavioural and psychological symptom management for people with dementia in residential care settings. HSAC Report 2010;3:1-216.
- Brodaty H, Burns K. Nonpharmacological management of apathy in dementia: a systematic review. Am J Geriatr Psychiatry 2012;20:549-64. https://doi.org/10.1097/JGP.0b013e31822be242.
- Cooper C, Balamurali TB, Selwood A, Livingston G. A systematic review of intervention studies about anxiety in carers of people with dementia. Int J Geriatr Psychiatry 2007;22:181-8. https://doi.org/10.1002/gps.1656.
- Cooper C, Mukadam N, Katona C, Lyketsos CG, Ames D, Rabins P, et al. Systematic review of the effectiveness of non-pharmacological interventions to improve quality of life of people with dementia. Int Psychogeriatr 2012;24:856-70. https://doi.org/10.1017/S1041610211002614.
- Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family carers of older adults. Psychol Aging 2007;22:37-51. https://doi.org/10.1037/0882-7974.22.1.37.
- Kim SY, Yoo EY, Jung MY, Park SH, Park JH. A systematic review of the effects of occupational therapy for persons with dementia: a meta-analysis of randomized controlled trials. NeuroRehabilitation 2012;31:107-15. https://doi.org/10.3233/NRE-2012-0779.
- Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health 2009;13:512-20. https://doi.org/10.1080/13607860902774394.
- Kverno KS, Black BS, Nolan MT, Rabins PV. Research on treating neuropsychiatric symptoms of advanced dementia with non-pharmacological strategies, 1998–2008: a systematic literature review. Int Psychogeriatr 2009;21:825-43. https://doi.org/10.1017/S1041610209990196.
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Old Age Task Force of the World Federation of Biological Psychiatry . Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021. https://doi.org/10.1176/appi.ajp.162.11.1996.
- Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging 2007;22:28-36. https://doi.org/10.1037/0882-7974.22.1.28.
- Pieper MJ, van Dalen-Kok AH, Francke AL, van der Steen JT, Scherder EJ, Husebø BS, et al. Interventions targeting pain or behaviour in dementia: a systematic review. Ageing Res Rev 2013;12:1042-55. https://doi.org/10.1016/j.arr.2013.05.002.
- Spijker A, Vernooij-Dassen M, Vasse E, Adang E, Wollersheim H, Grol R, et al. Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: a meta-analysis. J Am Geriatr Soc 2008;56:1116-28. https://doi.org/10.1111/j.1532-5415.2008.01705.x.
- Spira AP, Edelstein BA. Behavioral interventions for agitation in older adults with dementia: an evaluative review. Int Psychogeriatr 2006;18:195-22. https://doi.org/10.1017/S1041610205002747.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. https://doi.org/10.1037/0278-6133.27.3.379.
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med 2006;145:727-38. https://doi.org/10.7326/0003-4819-145-10-200611210-00005.
- Bourgeois MS, Burgio LD, Schulz R, Beach S, Palmer B. Modifying repetitive verbalizations of community-dwelling patients with AD. Gerontologist 1997;37:30-9. https://doi.org/10.1093/geront/37.1.30.
- Bourgeois MS, Schulz R, Burgio L, Beach S. Skills training for spouses of patients with Alzheimer’s disease: outcomes of an intervention study. J Clin Geropsychol 2002;8:53-7. https://doi.org/10.1023/A:1013098124765.
- Buckwalter KC, Gerdner L, Kohout F, Hall GR, Kelly A, Richards B, et al. A nursing intervention to decrease depression in family caregivers of persons with dementia. Arch Psychiatr Nurs 1999;13:80-8. https://doi.org/10.1016/S0883-9417(99)80024-7.
- Burgio L, Stevens A, Guy D, Roth DL, Haley WE. Impact of two psychosocial interventions on white and African American family caregivers of individuals with dementia. Gerontologist 2003;43:568-79. https://doi.org/10.1093/geront/43.4.568.
- Colvez A, Joël ME, Ponton-Sanchez A, Royer AC. Health status and work burden of Alzheimer patients’ informal caregivers: comparisons of five different care programs in the European Union. Health Policy 2002;60:219-33. https://doi.org/10.1016/S0168-8510(01)00215-9.
- Drentea P, Clay OJ, Roth DL, Mittelman MS. Predictors of improvement in social support: Five-year effects of a structured intervention for caregivers of spouses with Alzheimer’s disease. Soc Sci Med 2006;63:957-67. https://doi.org/10.1016/j.socscimed.2006.02.020.
- Eisdorfer C, Czaja SJ, Loewenstein DA, Rubert MP, Argüelles S, Mitrani VB, et al. The effect of a family therapy and technology-based intervention on caregiver depression. Gerontologist 2003;43:521-31. https://doi.org/10.1093/geront/43.4.521.
- Farran CJ, Gilley DW, McCann JJ, Bienias JL, Lindeman DA, Evans DA. Efficacy of behavioral interventions for dementia caregivers. West J Nurs Res 2007;29:944-60. https://doi.org/10.1177/0193945907303084.
- Finkel S, Czaja SJ, Schulz R, Martinovich Z, Harris C, Pezzuto D. E-care: a telecommunications technology intervention for family caregivers of dementia patients. Am J Geriatr Psychiatry 2007;15:443-8. https://doi.org/10.1097/JGP.0b013e3180437d87.
- Gallagher-Thompson D, Gray HL, Tang PC, Pu CY, Leung LY, Wang PC, et al. Impact of in-home behavioral management versus telephone support to reduce depressive symptoms and perceived stress in Chinese caregivers: results of a pilot study. Am J Geriatr Psychiatry 2007;15:425-34. https://doi.org/10.1097/JGP.0b013e3180312028.
- Gavrilova SI, Ferri CP, Mikhaylova N, Sokolova O, Banerjee S, Prince M. Helping carers to care – the 10/66 dementia research group’s randomized control trial of a caregiver intervention in Russia. Int J Geriatr Psychiatry 2009;24:347-54. https://doi.org/10.1002/gps.2126.
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill-building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH Initiative. Gerontologist 2003;43:532-46. https://doi.org/10.1093/geront/43.4.532.
- Guerra M, Ferri CP, Fonseca M, Banerjee S, Prince M. Helping carers to care: the 10/66 dementia research group’s randomized control trial of a caregiver intervention in Peru. Braz J Psychiatry 2011;33:47-54. https://doi.org/10.1590/S1516-44462010005000017.
- Horvath KJ, Trudeau SA, Rudolph JL, Trudeau PA, Duffy ME, Berlowitz D. Clinical trial of a home safety toolkit for Alzheimer’s disease. Int J Alzheimers Dis 2013;2013. https://doi.org/10.1155/2013/913606.
- Kelly AW, Buckwalter KC, Hall G, Weaver AL, Butcher HK. The caregivers’ story: Home caregiving for persons with dementia. Home Health Care Manag Pract 2002;14:99-109. https://doi.org/10.1177/1084822302014002004.
- Kosloski K, Montgomery RJV. The effects of respite on caregivers of Alzheimer’s patients: One-year evaluation of the Michigan model respite programs. J Appl Gerontol 1993;12:4-17. https://doi.org/10.1177/073346489301200102.
- Kuo LM, Huang HL, Huang HL, Liang J, Chiu YC, Chen ST, et al. A home-based training program improves Taiwanese family caregivers’ quality of life and decreases their risk for depression: a randomized controlled trial. Int J Geriatr Psychiatry 2013;28:504-13. https://doi.org/10.1002/gps.3853.
- Kwok T, Wong B, Ip I, Chui K, Young D, Ho F. Telephone-delivered psychoeducational intervention for Hong Kong Chinese dementia caregivers: a single-blinded randomized controlled trial. Clin Interv Aging 2013;8:1191-7. https://doi.org/10.2147/CIA.S48264.
- Lawton MP, Brody EM, Saperstein AR. A controlled study of respite service for caregivers of Alzheimer’s patients. Gerontologist 1989;29:8-16. https://doi.org/10.1093/geront/29.1.8.
- Livingston G, Barber J, Rapaport P, Knapp M, Griffin M, King D, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of family carers of people with dementia: pragmatic randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f6276.
- Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from the REACH for TLC intervention study. Gerontologist 2003;43:556-67. https://doi.org/10.1093/geront/43.4.556.
- Mittelman MS, Ferris SH, Steinberg G, Shulman E, Mackell JA, Ambinder A, et al. An intervention that delays institutionalization of Alzheimer’s disease patients: treatment of spouse-caregivers. Gerontologist 1993;33:730-40. https://doi.org/10.1093/geront/33.6.730.
- Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. Am J Psychiatry 2004;161:850-6. https://doi.org/10.1176/appi.ajp.161.5.850.
- Mittelman MS, Roth DL, Haley WE, Zarit SH. Effects of a caregiver intervention on negative caregiver appraisals of behaviour problems in patients with Alzheimer’s disease: results of a randomized trial. J Gerontol B Psychol Sci Soc Sci 2004;59:27-34. https://doi.org/10.1093/geronb/59.1.P27.
- Mohide EA, Pringle DM, Streiner DL, Gilbert JR, Muir G, Tew M. A randomized trial of family caregiver support in the home management of dementia. J Am Geriatr Soc 1990;38:446-54. https://doi.org/10.1111/j.1532-5415.1990.tb03544.x.
- Moniz-Cook E, Elston C, Gardiner E, Agar S, Silver M, Win T, et al. Can training community mental health nurses to support family carers reduce behavioural problems in dementia? An exploratory pragmatic randomised controlled trial. Int J Geriatr Psychiatry 2008;23:185-91. https://doi.org/10.1002/gps.1860.
- Sussman T, Regehr C. The influence of community-based services on the burden of spouses caring for their partners with dementia. Health Soc Work 2009;34:29-3. https://doi.org/10.1093/hsw/34.1.29.
- Sutcliffe C, Larner S. Counselling carers of the elderly at home: a preliminary study. Br J Clin Psychol 1988;27:177-8. https://doi.org/10.1111/j.2044-8260.1988.tb00768.x.
- Torkamani M, McDonald L, Saez Aguayo I, Kanios C, Katsanou MN, Madeley L, et al. A randomized controlled pilot study to evaluate a technology platform for the assisted living of people with dementia and their carers. J Alzheimers Dis 2014;41:515-23. https://doi.org/10.3233/JAD-132156.
- Vernooij-Dassen M, Lamers C, Bor J, Felling A, Grol R. Prognostic factors of effectiveness of a support program for caregivers of dementia patients. Int J Aging Hum Dev 2000;51:259-74. https://doi.org/10.2190/P8L1-N8QD-VTJ4-EURT.
- Winter L, Gitlin LN. Evaluation of a telephone-based support group intervention for female caregivers of community-dwelling individuals with dementia. Am J Alzheimers Dis Other Demen 2006;21:391-7. https://doi.org/10.1177/1533317506291371.
- Woods RT, Wills W, Higginson IJ, Hobbins J, Whitby M. Support in the community for people with dementia and their carers: a comparative outcome study of specialist mental health service interventions. Int J Geriatr Psychiatry 2003;18:298-307. https://doi.org/10.1002/gps.822.
- Askham J, Thompson C. Dementia and Home Care: A Research Report on a Home Support Scheme for Dementia Sufferers 1990.
- Burgener SC, Bakas T, Murray C, Dunahee J, Tossey S. Effective caregiving approaches for patients with Alzheimer’s disease. Geriatr Nurs 1998;19:121-6. https://doi.org/10.1016/S0197-4572(98)90055-6.
- Challis D, Sutcliffe C, Hughes J, von Abendorff R, Brown P, Chesterman J. Supporting People with Dementia at Home. Farnham: Ashgate; 2009.
- Chang BL. Cognitive-behavioural intervention for homebound caregivers of persons with dementia. Nurs Res 1999;48:173-82. https://doi.org/10.1097/00006199-199905000-00007.
- Chien WT, Lee YM. A disease management program for families of persons in Hong Kong with dementia. Psychiatr Serv 2008;59:433-6. https://doi.org/10.1176/ps.2008.59.4.433.
- Chien WT, Lee IY. Randomized controlled trial of a dementia care programme for families of home-resided older people with dementia. J Adv Nurs 2011;67:774-87. https://doi.org/10.1111/j.1365-2648.2010.05537.x.
- Chu P, Edwards J, Levin J, Thompson J. The use of clinical case management for early stage Alzheimer’s patients and their families. Am J Alzheimer’s Dis Other Dement 2000;15:284-92. https://doi.org/10.1177/153331750001500506.
- Dias A, Dewey ME, D’Souza J, Dhume R, Motghare DD, Shaji KS, et al. The effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: a randomised controlled trial from Goa, India. PLOS ONE 2008;3. https://doi.org/10.1371/journal.pone.0002333.
- Eloniemi-Sulkava U, Notkola IL, Hentinen M, Kivelä SL, Sivenius J, Sulkava R. Effects of supporting community-living demented patients and their caregivers: a randomized trial. J Am Geriatr Soc 2001;49:1282-7. https://doi.org/10.1046/j.1532-5415.2001.49255.x.
- Eloniemi-Sulkava U, Saarenheimo M, Laakkonen ML, Pietilä M, Savikko N, Kautiainen H, et al. Family care as collaboration: effectiveness of a multicomponent support program for elderly couples with dementia. Randomized controlled intervention study. J Am Geriatr Soc 2009;57:2200-8. https://doi.org/10.1111/j.1532-5415.2009.02564.x.
- Engelhardt JB, Kisiel T, Nicholson J, Mulichak L, DeMatteis J, Tobin DR. Impact of a care coordination and support strategic partnership on clinical outcomes. Home Healthc Nurse 2008;26:166-72. https://doi.org/10.1097/01.NHH.0000313348.85980.1e.
- Farran CJ, Loukissa D, Perraud S, Paun O. Alzheimer’s disease caregiving information and skills. Part I: care recipient issues and concerns. Res Nurs Health 2003;26:366-75. https://doi.org/10.1002/nur.10101.
- Farran CJ, Loukissa D, Perraud S, Paun O. Alzheimer’s disease caregiving information and skills. Part II: family caregiver issues and concerns. Res Nurs Health 2004;27:40-51. https://doi.org/10.1002/nur.20006.
- Gitlin LN, Corcoran M, Winter L, Boyce A, Hauck W. A randomized, controlled trial of a home environmental intervention. Gerontologist 2001;41:4-14. https://doi.org/10.1093/geront/41.1.4.
- Gitlin LN, Hauck WW, Dennis MP, Winter L. Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer’s disease and related disorders. J Gerontol A Biol Sci Med Sci 2005;60:368-74. https://doi.org/10.1093/gerona/60.3.368.
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry 2008;16:229-39. https://doi.org/10.1097/01.JGP.0000300629.35408.94.
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA 2010;304:983-91. https://doi.org/10.1001/jama.2010.1253.
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. J Am Geriatr Soc 2010;58:1465-74. https://doi.org/10.1111/j.1532-5415.2010.02971.x.
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ 2006;333. https://doi.org/10.1136/bmj.39001.688843.BE.
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Olderikkert MG. Effects of community occupational therapy on quality of life, mood, and health status in dementia patients and their caregivers: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2007;62:1002-9. https://doi.org/10.1093/gerona/62.9.1002.
- Hinchliffe AC, Hyman IL, Blizard B, Livingston G. Behavioral complications of dementia – can they be treated. Int J Geriatr Psychiatry 1995;10:839-47. https://doi.org/10.1002/gps.930101005.
- Huang HL, Shyu YI, Chen MC, Chen ST, Lin LC. A pilot study on a home-based caregiver training program for improving caregiver self-efficacy and decreasing the behavioral problems of elders with dementia in Taiwan. Int J Geriatr Psychiatry 2003;18:337-45. https://doi.org/10.1002/gps.835.
- Johnson DK, Niedens M, Wilson JR, Swartzendruber L, Yeager A, Jones K. Treatment outcomes of a crisis intervention program for dementia with severe psychiatric complications: the Kansas bridge project. Gerontologist 2013;53:102-12. https://doi.org/10.1093/geront/gns104.
- Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology 2006;67:1592-9. https://doi.org/10.1212/01.wnl.0000242727.81172.91.
- Newcomer R, Yordi C, DuNah R, Fox P, Wilkinson A. Effects of the Medicare Alzheimer’s Disease Demonstration on caregiver burden and depression. Health Serv Res 1999;34:669-89.
- O’Connor D, Pollitt P, Brook C, Reiss B, Roth M. Does early intervention reduce the number of elderly people with dementia admitted to institutions for long term care?. BMJ 1991;302:871-5. https://doi.org/10.1136/bmj.302.6781.871.
- Phung KT, Waldorff FB, Buss DV, Eckermann A, Keiding N, Rishøj S, et al. A three-year follow-up on the efficacy of psychosocial interventions for patients with mild dementia and their caregivers: the multicentre, rater-blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003584.
- Quayhagen MP, Quayhagen M, Corbeil RR, Hendrix RC, Jackson JE, Snyder L, et al. Coping with dementia: evaluation of four nonpharmacologic interventions. Int Psychogeriatr 2000;12:249-65. https://doi.org/10.1017/S1041610200006360.
- Samus QM, Johnston D, Black BS, Hess E, Lyman C, Vavilikolanu A, et al. A multidimensional home-based care coordination intervention for elders with memory disorders: the maximizing independence at home (MIND) pilot randomized trial. Am J Geriatr Psychiatry 2014;22:398-414. https://doi.org/10.1016/j.jagp.2013.12.175.
- Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 2003;290:2015-22. https://doi.org/10.1001/jama.290.15.2015.
- Teri L, McCurry SM, Logsdon R, Gibbons LE. Training community consultants to help family members improve dementia care: a randomized controlled trial. Gerontologist 2005;45:802-11. https://doi.org/10.1093/geront/45.6.802.
- Tibaldi V, Aimonino N, Ponzetto M, Stasi MF, Amati D, Raspo S, et al. A randomized controlled trial of a home hospital intervention for frail elderly demented patients: behavioral disturbances and caregiver’s stress. Arch Gerontol Geriatr Suppl 2004;9:431-6. https://doi.org/10.1016/j.archger.2004.04.055.
- Vickrey BG, Mittman BS, Connor KI, Pearson ML, Della Penna RD, Ganiats TG, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med 2006;145:713-26. https://doi.org/10.7326/0003-4819-145-10-200611210-00004.
- Davis RN, Massman PJ, Doody RS. Cognitive intervention in Alzheimer disease: a randomized placebo-controlled study. Alzheimer Dis Assoc Disord 2001;15:1-9. https://doi.org/10.1097/00002093-200101000-00001.
- Steinberg M, Leoutsakos JM, Podewils LJ, Lyketsos CG. Evaluation of a home-based exercise program in the treatment of Alzheimer’s disease: the Maximizing Independence in Dementia (MIND) study. Int J Geriatr Psychiatry 2009;24:680-5. https://doi.org/10.1002/gps.2175.
- Ahmed S, Hughes J, Davies S, Stewart K, Orrell M, Clarkson P, et al. Members of the HoSt-D (Home Support in Dementia) Programme Management Group . Specialist services in early diagnosis and support for older people with dementia in England: staff roles and service mix. Int J Geriatr Psychiatry 2018;33:1280-5. https://doi.org/10.1002/gps.4925.
- Davies S, Hughes J, Ahmed S, Clarkson P, Challis D. Members of the HoSt-D (Home Support in Dementia) Programme Management Group . Commissioning social care for people with dementia living at home: findings from a national survey. Int J Geriatr Psychiatry 2020;35:53-9. https://doi.org/10.1002/gps.5214.
- Care Quality Commission . Beyond Barriers – How Older People Move Between Health and Social Care in England 2018. www.cqc.org.uk/sites/default/files/20180702_beyond_barriers.pdf (accessed 17 September 2019).
- Graff MJ, Adang EM, Vernooij-Dassen MJ, Dekker J, Jönsson L, Thijssen M, et al. Community occupational therapy for older patients with dementia and their care givers: cost effectiveness study. BMJ 2008;336:134-8. https://doi.org/10.1136/bmj.39408.481898.BE.
- Pitkälä KH, Pöysti MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med 2013;173:894-901. https://doi.org/10.1001/jamainternmed.2013.359.
- Knapp M, King D, Romeo R, Schehl B, Barber J, Griffin M, et al. Cost effectiveness of a manual based coping strategy programme in promoting the mental health of family carers of people with dementia [the START (STrAtegies for RelaTives) study]: a pragmatic randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f6342.
- Clarkson P, Davies L, Jasper R, Loynes N, Challis D. Home Support in Dementia (HoSt-D) Programme Management Group . A systematic review of the economic evidence for home support interventions in dementia. Value Health 2017;20:1198-209. https://doi.org/10.1016/j.jval.2017.04.004.
- Dias S, Welton N, Sutton A, Ades A. NICE DSU Technical Support Document 1: Introduction to Evidence Synthesis for Decision Making. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2012.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Green C. Modelling disease progression in Alzheimer’s disease: a review of modelling methods used for cost-effectiveness analysis. PharmacoEconomics 2007;25:735-50. https://doi.org/10.2165/00019053-200725090-00003.
- Cohen JT, Neumann PJ. Decision analytic models for Alzheimer’s disease: state of the art and future directions. Alzheimers Dement 2008;4:212-22. https://doi.org/10.1016/j.jalz.2008.02.003.
- Eckermann S, Pekarsky B. Can the real opportunity cost stand up: displaced services, the straw man outside the room. PharmacoEconomics 2014;32:319-25. https://doi.org/10.1007/s40273-014-0140-3.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Duru OK, Ettner SL, Vassar SD, Chodosh J, Vickrey BG. Cost evaluation of a coordinated care management intervention for dementia. Am J Manag Care 2009;15:521-8.
- Gillespie A, Best C, O’Neill B. Cognitive function and assistive technology for cognition: a systematic review. J Int Neuropsychol Soc 2012;18:1-19. https://doi.org/10.1017/S1355617711001548.
- O’Neill B, Gillespie A. Assistive Technology for Cognition: A Handbook for Clinicians and Developers. Hove: Taylor & Francis Ltd; 2014.
- Chester H, Clarkson P, Davies L, Hughes J, Islam MS, Kapur N, et al. Cognitive aids for people with early stage dementia versus treatment as usual [Dementia Early Stage Cognitive Aids New Trial (DESCANT)]: study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2933-8.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing 1996;25:113-20. https://doi.org/10.1093/ageing/25.2.113.
- Howard R, Phillips P, Johnson T, O’Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. Int J Geriatr Psychiatry 2011;26:812-17. https://doi.org/10.1002/gps.2607.
- Great Britain . Mental Capacity Act 2005 2005.
- Society As . The Memory Handbook: A Practical Guide to Living With Memory Problems 2014.
- Charlesworth G, Burnell K, Hoe J, Orrell M, Russell I. Acceptance checklist for clinical effectiveness pilot trials: a systematic approach. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-78.
- Minns Lowe CJ, Wilson MS, Sackley CM, Barker KL. Blind outcome assessment: the development and use of procedures to maintain and describe blinding in a pragmatic physiotherapy rehabilitation trial. Clin Rehabil 2011;25:264-74. https://doi.org/10.1177/0269215510380824.
- Abendstern M, Davies K, Poland F, Chester H, Clarkson P, Hughes J, et al. Reflecting on the research encounter for people in the early stages of dementia: lessons from an embedded qualitative study. Dementia 2020;19:2732-49. https://doi.org/10.1177/1471301219855295.
- Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA 2016;315:407-8. https://doi.org/10.1001/jama.2015.19394.
- Giebel CM, Sutcliffe C, Challis D. Activities of daily living and quality of life across different stages of dementia: a UK study. Aging Ment Health 2015;19:63-71. https://doi.org/10.1080/13607863.2014.915920.
- Kampanellou E, Chester H, Davies L, Davies S, Giebel C, Hughes J, et al. Carer preferences for home support services in later stage dementia. Aging Ment Health 2019;23:60-8. https://doi.org/10.1080/13607863.2017.1394441.
- Diwan S, Berger C, Manns EK. Composition of the home care service package: predictors of type, volume, and mix of services provided to poor and frail older people 1. Gerontologist 1997;37:169-81. https://doi.org/10.1093/geront/37.2.169.
- Rogerson P. Statistical Methods for Geography. London: SAGE Publications Ltd; 2001.
- Kline P. An Easy Guide to Factor Analysis. London: Routledge; 1994.
- Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Di Renzo V. Observational studies: propensity score analysis of non-randomized data. Int MS J 2009;16:90-7.
- Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. https://doi.org/10.1080/00273171.2011.568786.
- House of Commons: Committee of Public Accounts . Improving Services and Support for People With Dementia 2008. https://publications.parliament.uk/pa/cm200708/cmselect/cmpubacc/228/228.pdf (accessed 7 April 2021).
- Schneider J, Hallam A, Murray J, Foley B, Atkin L, Banerjee S, et al. Formal and informal care for people with dementia: factors associated with service receipt. Aging Ment Health 2002;6:255-65. https://doi.org/10.1080/13607860220142486.
- Verbeek H, Meyer G, Leino-Kilpi H, Zabalegui A, Hallberg IR, Saks K, et al. A European study investigating patterns of transition from home care towards institutional dementia care: the protocol of a RightTimePlaceCare study. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-68.
- Giebel CM, Davies S, Clarkson P, Sutcliffe C, Challis D. Members of the HoSt-D (Home Support in Dementia) Programme Management Group . Costs of formal and informal care at home for people with dementia: ‘expert panel’ opinions from staff and informal carers. Dementia 2019;18:210-27. https://doi.org/10.1177/1471301216665705.
- Hajek A, Brettschneider C, Ernst A, Posselt T, Wiese B, Prokein J, et al. Longitudinal predictors of informal and formal caregiving time in community-dwelling dementia patients. Soc Psychiatry Psychiatr Epidemiol 2016;51:607-16. https://doi.org/10.1007/s00127-015-1138-7.
- Sutcliffe CL, Roe B, Jasper R, Jolley D, Challis DJ. People with dementia and carers’ experiences of dementia care and services: outcomes of a focus group study. Dementia 2015;14:769-87. https://doi.org/10.1177/1471301213511957.
- Chester H, Clarkson P, Davies L, Sutcliffe C, Davies S, Feast A, et al. People with dementia and carer preferences for home support services in early-stage dementia. Aging Ment Health 2018;22:270-9. https://doi.org/10.1080/13607863.2016.1247424.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019). n.d. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 7 April 2021).
- Mulhern B, Rowen D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17050.
- Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan 2006;21:402-8. https://doi.org/10.1093/heapol/czl018.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell; 1992.
- Wimo A, Wetterholm AL, Mastey V, Winblad B, Wimo A, Jonsson B, et al. Health Economics of Dementia. Chichester: Wiley; 1998.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Drummond MF, Sculpher M, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Giebel C, Roe B, Hodgson A, Britt D, Clarkson P. Members of the HoSt-D (Home Support in Dementia) Programme Management Group . Effective public involvement in the HoST-D Programme for dementia home care support: from proposal and design to methods of data collection (innovative practice). Dementia 2019;18:3173-86. https://doi.org/10.1177/1471301216687698.
- Stoner CR, Orrell M, Spector A. Psychometric properties and factor analysis of the Engagement and Independence in Dementia Questionnaire (EID-Q). Dement Geriatr Cogn Disord 2018;46:119-27. https://doi.org/10.1159/000488484.
- Harding AJE, Morbey H, Ahmed F, Opdebeeck C, Elvish R, Leroi I, et al. Core outcome set for nonpharmacological community-based interventions for people living with dementia at home: a systematic review of outcome measurement instruments. Gerontologist 2020. https://doi.org/10.1093/geront/gnaa071.
- Harding AJE, Morbey H, Ahmed F, Opdebeeck C, Wang YY, Williamson P, et al. Developing a core outcome set for people living with dementia at home in their neighbourhoods and communities: study protocol for use in the evaluation of non-pharmacological community-based health and social care interventions. Trials 2018;19. https://doi.org/10.1186/s13063-018-2584-9.
- Keogh F, Pierce M, Neylon K, Fleming P. Intensive home care packages for people with dementia: a realist evaluation protocol. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-3630-8.
- Giebel C, Lord K, Cooper C, Shenton J, Cannon J, Pulford D, et al. A UK survey of COVID-19 related social support closures and their effects on older people, people with dementia, and carers. Int J Geriatr Psychiatry 2020;36:393-402. https://doi.org/10.1002/gps.5434.
- Morrison K, Winter L, Gitlin LN. Recruiting community-based dementia patients and caregivers in a nonpharmacologic randomized trial: what works and how much does it cost?. J Appl Gerontol 2016;35:788-800. https://doi.org/10.1177/0733464814532012.
- Grill JD, Galvin JE. Facilitating Alzheimer disease research recruitment. Alzheimer Dis Assoc Disord 2014;28:1-8. https://doi.org/10.1097/wad.0000000000000016.
- Weatherly H, Faria R, Berg B, Sculpher M, O’Neill P, Nolan K, et al. Scoping Review on Social Care Economic Evaluation Methods 2017. https://ideas.repec.org/p/chy/respap/150cherp.html (accessed 7 April 2021).
- Goertz G. Social Science Concepts: A User’s Guide. Princeton, NJ: Princeton University Press; 2006.
- Fukui H, Toyoshima K. Music facilitate the neurogenesis, regeneration and repair of neurons. Med Hypotheses 2008;71:765-9. https://doi.org/10.1016/j.mehy.2008.06.019.
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol 1979;109:186-204. https://doi.org/10.1093/oxfordjournals.aje.a112674.
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull 1985;98:310-57. https://doi.org/10.1037/0033-2909.98.2.310.
- Roth D, Mittelman M, Clay O, Madan A, Haley W. Changes in social support as mediators of the impact of a psychosocial intervention for spouse caregivers of persons with Alzheimer’s disease. Psychol Aging 2005;20:634-44. https://doi.org/10.1037/0882-7974.20.4.634.
- Swaab D, Dubelaar E, Hofman M, Scherder E, van Someren E, Verwer R. Brain aging and Alzheimer’s disease; use it or lose it. Prog Brain Res 2002;138:343-73. https://doi.org/10.1016/s0079-6123(02)38086-5.
- Hall L, Orrell M, Stott J, Spector A. Cognitive stimulation therapy (CST): neuropsychological mechanisms of change. Int Psychogeriatr 2013;25:479-89. https://doi.org/10.1017/S1041610212001822.
- Folkman S, Lazarus RS. An analysis of coping in a middle-aged community sample. J Health Soc Behav 1980;21:219-39. https://doi.org/10.2307/2136617.
- Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement 2007;3:30-7. https://doi.org/10.1016/j.jalz.2007.01.013.
- Lawton M, Nahemow L, Eisdorfer C, Lawton M. The Psychology of Adult Development and Aging. Washington, DC: American Psychological Association; 1973.
- Jacobson N, Martell C, Dimidjian S. Behavioural activation treatment for depression: returning to contextual roots. Clin Psychol Sci Pract 2001;8:255-70. https://doi.org/10.1093/clipsy.8.3.255.
- Hall G, Buckwalter K. Progressively lowered stress threshold: a conceptual model for care of adults with Alzheimer’s disease. Arch Psychiatr Nurs 1987;1:399-406.
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963;185:914-19. https://doi.org/10.1001/jama.1963.03060120024016.
- Reid R, Haggerty J, McKendry R. Defusing the Confusion: Concepts and Measures of Continuity of Healthcare. Ottawa, ON: Canadian Health Services Research Foundation; 2002.
- Fisher J, Fisher W. Changing AIDS-risk behavior. Psychol Bull 1992;111:455-74. https://doi.org/10.1037/0033-2909.111.3.455.
- Losada A, Márquez-González M, Romero-Moreno R. Mechanisms of action of a psychological intervention for dementia caregivers: effects of behavioral activation and modification of dysfunctional thoughts. Int J Geriatr Psychiatry 2011;26:1119-27. https://doi.org/10.1002/gps.2648.
- Maslow A. A theory of human motivation. Psychol Rev 1943;50:370-97. https://doi.org/10.1037/h0054346.
- Stirling CM, Dwan CA, McKenzie AR. Why carers use adult day respite: a mixed method case study. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-245.
- Chester H, Clarkson P, Hughes J, Russell I, Beresford J, Davies L, et al. Evaluating the effectiveness of different approaches to home support for people in later stage dementia: a protocol for an observational study. Int Psychogeriatr 2017;29:1213-21. https://doi.org/10.1017/S1041610217000291.
- Kilian R, Angermeyer MC. The effects of antipsychotic treatment on quality of life of schizophrenic patients under naturalistic treatment conditions: an application of random effect regression models and propensity scores in an observational prospective trial. Qual Life Res 2005;14:1275-89. https://doi.org/10.1007/s11136-004-5533-x.
- Office for National Statistics . English Indices of Deprivation 2015. Statistics on Relative Deprivation in Small Areas in England n.d. www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (accessed 18 May 2021).
- NHS Digital . Adult Social Care Activity and Finance Report, England – 2017–18 [PAS]. n.d. https://digital.nhs.uk/data-and-information/publications/statistical/adult-social-care-activity-and-finance-report/2017-18 (accessed 18 May 2021).
- Clarkson P, Brand C, Hughes J, Challis D. Integrating assessments of older people: examining evidence and impact from a randomised controlled trial. Age Ageing 2011;40:388-91. https://doi.org/10.1093/ageing/afr015.
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 2013;4:133-42. https://doi.org/10.1111/j.2041-210x.2012.00261.x.
- Swan J, Clarke A, Nicolini D, Powell J, Scarbrough H, Roginski C, et al. Evidence Management Decisions (EMD) – Advancing Knowledge Utilization in Healthcare Management Final Report 2012.
- Wye L, Brangan E, Cameron A, Gabbay J, Klein J, Pope C. Knowledge exchange in health-care commissioning: case studies of the use of commercial, not-for-profit and public sector agencies 2011–14. Health Serv Deliv Res 2015;3. https://doi.org/10.3310/hsdr03190.
- Dopson S, Bennett C, Fitzgerald L, Ferlie E, Fischer M, Ledger J, et al. Health Care Managers’ Access and Use of Management Research. Final Report 2013.
- Edwards C, Fox R, Gillard S, Gourlay S, Guven P, Jackson C, et al. Explaining Health Managers’ Information Seeking Behaviour and Use. Final Report 2013. https://doi.org/10.1186/1472-6963-14-S2-P34.
- Wye L, Brangan E, Cameron A, Gabbay J, Klein JH, Pope C. Evidence based policy making and the ‘art’ of commissioning – how English healthcare commissioners access and use information and academic research in ‘real life’ decision-making: an empirical qualitative study. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-1091-x.
- Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A. ‘Psychological Theory’ Group. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26-33. https://doi.org/10.1136/qshc.2004.011155.
- Turner-Stokes L, Harding R, Sergeant J, Lupton C, McPherson K. Generating the evidence base for the national service framework for long term conditions: a new research typology. Clin Med 2006;6:91-7. https://doi.org/10.7861/clinmedicine.6-1-91.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 2018;361. https://doi.org/10.1136/bmj.k1675.
- NHS Improvement . NHS Reference Costs 2017 18 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 8 April 2021).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2009. Canterbury: PSSRU, University of Kent; 2009.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Berg B, Brouwer W, Koopmanschap M. Economic valuation of informal care. Eur J Health Econ 2004;5:36-45. https://doi.org/10.1007/s10198-003-0189-y.
- Hoefman RJ, van Exel J, Brouwer W. How to include informal care in economic evaluations. PharmacoEconomics 2013;31:1105-19. https://doi.org/10.1007/s40273-013-0104-z.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Complete Care Shop . Indiana Rise and Recline Chair – Biscuit n.d. www.completecareshop.co.uk/chairs-and-seating/single-motor-riser-recliner-chairs/indiana-rise-and-recline-chair-biscuit (accessed 24 September 2019).
- Complete Care Shop . Bed Grab Rails n.d. www.completecareshop.co.uk/beds-and-bedding/bed-grab-rails/ (accessed 24 September 2019).
- Complete Care Shop . Key Safe n.d. www.completecareshop.co.uk/household-aids/key-safes/key-safe (accessed 24 September 2019).
- Complete Care Shop . Oxford Midi 180 Mobile Hoist n.d. www.completecareshop.co.uk/oxford-hoists-and-slings/oxford-mobile-hoists/oxford-midi-180-mobile-hoist (accessed 24 September 2019).
- Complete Care Shop . Atlas Transfer Disc n.d. www.completecareshop.co.uk/moving-and-handling/patient-turners/atlas-transfer-disc (accessed 24 September 2019).
- Complete Care Shop . Medelert Automatic Pill Dispense n.d. www.completecareshop.co.uk/medical-aids/medication-dispensers/medelert-automatic-pill-dispenser (accessed 24 September 2019).
- Complete Care Shop . Interlude Incontinence Pads – Case n.d. www.completecareshop.co.uk/continence-care/incontinence-pads/interlude-incontinence-pads-case (accessed 24 September 2019).
- Complete Care Shop . V Shaped Back Support Pillow n.d. www.completecareshop.co.uk/dressing-and-comfort-aids/support-pillows/v-shaped-back-support-pillow (accessed 24 September 2019).
- Complete Care Shop . Apollo 5 Premium Airflow Mattress n.d. www.completecareshop.co.uk/beds-and-bedding/airflow-mattresses/apollo-5-premium-airflow-mattress (accessed 24 September 2019).
- Complete Care Shop . Essential Care Pressure Mattress n.d. www.completecareshop.co.uk/beds-and-bedding/pressure-mattresses/essential-care-pressure-mattress (accessed 24 September 2019).
- Complete Care Shop . Medium Risk Chaier Pressure Cushion n.d. www.completecareshop.co.uk/chairs-and-seating/chair-pressure-cushions-medium-risk/medium-risk-chair-pressure-cushion (accessed 24 September 2019).
- Complete Care Shop . One Way Slide Sheet n.d. www.completecareshop.co.uk/moving-and-handling/glide-and-slide-sheets/one-way-slide-sheet (accessed 24 September 2019).
- Complete Care Shop . Crash Mat n.d. www.completecareshop.co.uk/beds-and-bedding/bed-cot-bumpers/crash-mat (accessed 24 September 2019).
- Complete Care Shop . Mattress Genie – Double Bed n.d. www.completecareshop.co.uk/beds-and-bedding/bed-supports-and-hoists/mattress-genie-double-bed (accessed 24 September 2019).
- Complete Care Shop . Mangar Elk Emergency Lifting Cushion n.d. www.completecareshop.co.uk/moving-and-handling/emergency-lifting-cushions/mangar-elk-emergency-lifting-cushion (accessed 24 September 2019).
- Complete Care Shop . Leg and Footstool n.d. www.completecareshop.co.uk/chairs-and-seating/footstools/leg-and-footstool (accessed 24 September 2019).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Complete Care Shop . Motion Sensor Light n.d. www.completecareshop.co.uk/household-aids/night-lights-and-safety-lights/motion-sensor-light (accessed 24 September 2019).
- Complete Care Shop . Dementia Care Day and Night Clock n.d. www.completecareshop.co.uk/sensory-aids/big-digit-and-talking-clocks/dementia-care-day-and-night-clock (accessed 24 September 2019).
- Complete Care Shop . Big Button Phone n.d. www.completecareshop.co.uk/household-aids/phones/big-button-phone (accessed 24 September 2019).
- Live Better With Dementia . Forgetfulness n.d. https://dementia.livebetterwith.com/products/yepzon-one-simple-gps-locator-tracker (accessed 24 September 2019).
Appendix 1 Systematic review of effective home support to people with dementia and their carers: components and impacts
Protocol: Clarkson et al. (2016)
Clarkson P, Giebel CM, Larbey M, Roe B, Challis D, Hughes J, et al. A protocol for a systematic review of effective home support to people with dementia and their carers: components and impacts. J Adv Nurs 2016;72:186–96. https://doi.org/10.1111/jan.12737
Overview of reviews: Clarkson et al. (2017)
Clarkson P, Hughes J, Xie C, Larbey M, Roe B, Giebel CM, et al. Overview of systematic reviews: effective home support in dementia care, components and impacts – stage 1, psychosocial interventions for dementia. J Adv Nurs 2017;73:2845–63. https://doi.org/10.1111/jan.13362
Systematic review: Clarkson et al. (2018)
Clarkson P, Hughes J, Roe B, Giebel CM, Jolley D, Poland F, et al. Systematic review: effective home support in dementia care, components and impacts – stage 2, effectiveness of home support interventions. J Adv Nurs 2018;74:507–27. https://doi.org/10.1111/jan.13460
Appendix 2 Components drawn from the evidence synthesis
The components in Table 2, derived from the overview of reviews, were used throughout the programme to help design and guide analyses of other subsequent projects.
| Component definition (basic level)192 | Constituent elements/mechanisms of action (secondary level)192 | Examples (indicator level)192 |
|---|---|---|
| For the person with dementia | ||
| Sensory enhancement/relaxation |
To increase or relax the overall level of sensory stimulation in the environment to counterbalance the negative impact of sensory deprivation/stimulation common in dementia Mechanism of action: facilitation of neurogenesis, the regeneration and repair of cerebral nerves193 |
Relaxation therapy; massage; music |
| Social engagement |
To provide access to different forms of social contact to counterbalance the limited contact with others that may be characteristic of the experience of dementia. This social contact may be real or simulated Mechanism of action: social support/social network theory194–196 |
Social support group; befriending service |
| Cognitive training (support) |
To provide enhancement and stimulation of cognitive functions through guided practice on a set of standard tasks, reflecting memory, attention or problem-solving Mechanism of action: improving neuronal functioning hypothesis197,198 |
Memory aids; memory training |
| Emotional support |
To address feelings and emotional needs through prompts, discussion or by stimulating memories and enabling the person to share their experiences. Undertaken to counterbalance and help people manage difficult feelings and emotions Mechanism of action: coping mediating strategies,199 including changing the meanings attached to events or circumstances |
‘Life story’ books; memory wallets; reminiscence sessions |
| Physical activity |
To provide structured activities and/or exercise to provide meaningful and engaging experiences that can be a useful counterbalance to difficult behaviours Mechanism of action: up-regulation of growth factors, increased neurogenesis and improved learning and memory200 |
Exercise programme |
| Environmental modifications |
To modify the living environment, including the visual environment, to lessen agitation and/or wandering and promote safety Mechanism of action: Competence–Environmental Press Framework201 – adapting the physical and social environment with declining competency can lead to fewer problem behaviours and disabilities |
Aids; adaptations; assistive technologies; signage |
| Behaviour management |
To increase pleasant events and/or to identify and modify factors that lead to difficult behaviours or their consequences through distraction or communication Mechanisms of action: behavioural activation,202 engaging in more pleasant and constructive activities aimed at increasing positive reinforcement; progressively lowered threshold model,203 identifying the antecedents and consequences of problem behaviour to remove or modify environmental demands |
Distraction; skills training; pain management |
| Daily living assistance |
To assist with basic care (e.g. provision of laundry services, basic nutrition and help with ADL) Mechanism of action: maintaining primary biological and psychosocial function. 204 Declining neurological and locomotor responses can lead to difficulties with tasks such as feeding, bathing and dressing. Therefore, cognitive deficits underlie certain functional deficits |
Home care; personal care; meals; nutrition advice |
| Care co-ordination |
Connecting and bringing together different services around the person. Advising on and negotiating the delivery of services from multiple providers on behalf of the person to provide benefit Mechanism of action: continuity and integration of care. 205 Delivering care in a coherent and complementary manner to achieve major goals, such as the awareness of and access to required services |
‘Case worker’; ‘care manager’; key worker |
| For the carer | ||
| Education/advice |
Structured presentation of information concerning the condition and carer-related issues (e.g. legal issues, carer’s health), including an active role for carers (e.g. role-playing) Mechanism of action: information–motivation–behavioural skills model206 |
Information/advice service; web-based information |
| Social support |
The opportunity to share personal feelings and concerns and overcome feelings of social isolation Mechanism of action: social support/social network theory194–196 |
Support group; befriending |
| Behaviour management |
Education on techniques to identify and modify beliefs and develop new repertoires of behaviour to deal with behavioural challenges of the person with dementia Mechanism of action: cognitive restructuring – identifying, analysing and correcting maladaptive beliefs207 |
Carer education/skills training |
| Emotional support |
To resolve pre-existing personal problems that can complicate caregiving and that can reduce conflicts between caregiver and person with dementia Mechanism of action: emotion-orientated coping strategies,199 managing the emotions that accompany stress (disclaiming, escape-avoidance, accepting responsibility or blame, exercising self-control and positive reappraisal) |
Counselling |
| Respite |
Planned, temporary relief through the provision of substitute care (e.g. day care, in-home sitting, residential care for the person with dementia) Mechanism of action: hierarchy of needs,208 addressing lower-level needs of the person with dementia, including everyday functioning, and higher-level needs, including emotional and social support, can provide relief from caring through improved sleeping for instance209 |
Sitting service; short-term residential care |
Appendix 3 Survey of current provision in England
NHS survey: Ahmed et al. (2018)
Ahmed S, Hughes J, Davies S, Stewart K, Orrell M, Clarkson P, Challis D, Members of the HoSt-D (Home Support in Dementia) Programme Management Group. Specialist services in early diagnosis and support for older people with dementia in England: Staff roles and service mix. Int J Geriatr Psychiatry 2018;33:1280–5. https://doi.org/10.1002/gps.4925
Local authority survey: Davies et al. (2019)
Davies S, Hughes J, Ahmed S, Clarkson P, Challis D, Members of the HoSt-D (Home Support in Dementia) Programme Management Group. Commissioning social care for people with dementia living at home: findings from a national survey. Int J Geriatr Psychiatry 2020;35:53–9. https://doi.org/10.1002/gps.5214
Appendix 4 Development of an economic model
Economic review: Clarkson et al. (2017)
Clarkson P, Davies L, Jasper R, Loynes N, Challis D, Home Support in Dementia (HoSt-D) Programme Management Group. A systematic review of the economic evidence for home support interventions in dementia. Value Health 2017;20:1198–209. https://doi.org/10.1016/j.jval.2017.04.004
Appendix 5 DESCANT: evaluation of a support model in early-stage dementia
Protocol: Chester et al. (2018)
Chester H, Clarkson P, Davies L, Hughes J, Islam M, Kapur N, et al. Cognitive aids for people with early stage dementia versus treatment as usual (Dementia Early Stage Cognitive Aids New Trial (DESCANT)): study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-018-2933-8
Repository (gold open access)
URL: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-018-2933-8
Qualitative study: Abendstern et al. (2019)
Abendstern M, Davies K, Poland F, Chester H, Clarkson P, Hughes J, et al. Reflecting on the research encounter for people in the early stages of dementia: lessons from an embedded qualitative study. Dementia 2020;19:2732–49. https://doi.org/10.1177/1471301219855295
Appendix 6 DESCANT process evaluation: examining the implementation of dementia support practitioners and guidance with memory aids for people in early-stage dementia
Chester H, Beresford R, Clarkson P, Entwistle C, Gillan V, Huges J, et al. Implementing the Dementia Early Stage Cognitive Aids New Trial (DESCANT) intervention: mixed-method process evaluation alongside a pragmatic randomised trial. Aging Ment Health 2021;1–13. https://doi.org/10.1080/13607863.2020.1870204
Appendix 7 Observational study of effectiveness of home support approaches in later-stage dementia
Protocol: Chester et al. (2017)
Chester H, Clarkson P, Hughes J, Russell I, Beresford J, Davies L, et al. Evaluating the effectiveness of different approaches to home support for people in later stage dementia: a protocol for an observational study. Int Psychogeriatr 2017;29:1213–21. https://doi.org/10.1017/S1041610217000291
Qualitative study: Abendstern et al. (2019)
Abendstern M, Davies K, Chester H, Clarkson P, Hughes J, Sutcliffe C, et al. Applying a new concept of embedding qualitative research: an example from a quantitative study of carers of people in later stage dementia. BMC Geriatrics 2019;19:227. https://doi.org/10.1186/s12877-019-1240-x
Repository (gold open access)
URL: https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-019-1240-x
Observational study
Introduction
Our evidence synthesis (see Workstream 1, Evidence synthesis of studies describing components applied to home support for dementia) found evidence on home support models at later stages of dementia to be lacking. It is not known what combinations of services routinely received are most effective, along several dimensions, including helping to maintain daily living activity, enhancing well-being, reducing carers’ stress and keeping people with dementia at home for longer.
We undertook a naturalistic study of home support packages received by people in later-stage dementia and their carers in local authority areas in England. There are limited studies of the effects of service mix in this population. We drew on US work in devising care package groups for analysis162 with robust statistical methods allowing for potential confounding.
Methods
This was a prospective observational study that examined outcomes for people with dementia and their carers after receiving different packages of home support in 17 areas of England. The analysis plan, in the protocol,210 was to discern different combinations of home support services received by participants and aggregate these into care package162 groups (i.e. naturally occurring mixes of support relying on different components). We then tested the clinical effectiveness and cost-effectiveness of each of these groups against minimal, basic, care.
We based the sample size calculation (conducted a priori) on a regression model, exploring relationships between group membership, covariates and outcomes. To take account of attrition between baseline and follow-up, the target sample size at baseline interview was 400 participants.
We described naturally occurring packages of care received by people with dementia and their carers, using descriptions of service receipt from the baseline interview schedule. This identified service clusters or types of service mix. We did this initially without reference to the data and so did not construct care package types empirically by data reduction or clustering techniques. We first constructed the groups, detailing different care packages, based on our systematic literature review. This review indicated that support to carers was important and potentially effective, with packages containing environmental modifications and care co-ordination also being important. Help with daily living assistance was also important, but there was limited evidence. The resultant care packages were checked against baseline data to determine numbers populating each group. Participants were divided into no more than four care package types. This descriptive analysis was repeated using different mixes of support. We attempted to ensure sufficient participant numbers in each package, while covering as much of the data as possible.
We recruited participants according to a sampling strategy that allowed for potential variation in service mix received by people with dementia. We approached sites (local authority designated areas) with potentially different intensities of services, determined by the service mix score data from our national surveys (see Workstream 1, Survey of current provision in England). We assessed participants at baseline and 26 weeks (6 months). The primary outcome was the BADLS. Other participant outcomes included quality of life (DEMQOL), carer competence (SSCQ) and destination at 12 months (whether or not remaining at home).
In observational studies, the assignment of participants to the ‘treatment groups’ in question (here, care package types) may be influenced by factors also affecting outcomes. That is, there is a risk of confounding (i.e. the systematic error of not accounting for variables associated with both receipt of a particular care package and the outcomes under study). 165 Therefore, before the effects of different care package types were assessed, we constructed propensity scores to control for this. 166 The aim was to reduce the effects of baseline characteristics on receipt of care packages to a series of composite measures. These scores were then used to adjust for this bias in multivariate models of effects of the care package types. 211
Propensity scores were calculated as the predicted probabilities of being assigned to a care package, depending on a set of independent variables at baseline. These probabilities were estimated from a multinomial logit model. Therefore, category of care package type, at baseline, was regressed on the following variables (Table 3).
| Variable | Data source |
|---|---|
| Hours of informal care | Baseline interview questionnaire |
| Index of Multiple Deprivation score for each of the geographical sites | Office for National Statistics212 |
| Service mix scores for each of the geographical sites | Calculated from the national survey data (see Workstream 1, Survey of current provision in England) |
| Living alone | Baseline interview questionnaire |
| ADL using BADLS | Baseline interview questionnaire |
| Community home care expenditure for each geographical region | Adult social care finance return213 |
Multivariate models (linear mixed models) were then used to test the relationship between outcome measures, care package type and covariates. Propensity scores were included as covariates, as well as several other variables (Box 1). We estimated the relative effectiveness of each approach to home support against basic care, controlling for these variables. The dependent variable in each was the change in outcome (time point 2 – time point 1) over 6 months against the independent variables, including receipt of either intermediate or advanced care packages.
-
Age.
-
Sex.
-
Duration of home care visits 6 months before baseline (in hours).
-
Propensity score (of each care package type compared with the reference group).
It was possible that between baseline and follow-up there would be a change in services received by participants and hence their membership of care package groups. Therefore, we conducted a sensitivity analysis to account for this change over time. First, we compared baseline characteristics of those whose care packages had changed at follow-up with those whose care packages had not. Second, we conducted a subgroup analysis to assess any outcome differences between those whose care package had and those whose care package had not changed at follow-up.
Results
We achieved a data set and completed baseline interviews with 518 participants (pairs of people with dementia and their carers), with 389 of these participants also completing 6-month interviews, allowing measurements of effect. Therefore, the sample for analysis exceeded the minimum required. Attrition was 25% of baseline, which is in line with expectations from both our professional knowledge and other similar studies of this population. 214
The creation of care package groups from the data was challenging. A complex picture of service receipt from different agencies and professions characterised baseline service receipt. Membership of proposed packages, when grouping these data together, overlapped for many participants. Packages relying on particular components (e.g. social care-focused daily living support) were therefore not distinct from others. Participants tended to receive these services but also others included as part of other packages. Attempting to create care packages, employing only selected components, resulted in groups with very small numbers of participants (< 30) and residual numbers in no particular group, which would have meant loss of data. To simplify, eventual care package groupings relied on a measure of service intensity, which was in line with that guiding our sampling strategy from the national survey. We used a subset of eight dementia-specific home support services (Box 2) to create ‘service intensity’ care package groups [i.e. basic care (none or one service), intermediate care (two or three services), advanced care (four or more services)], which allowed us to use all the data. Effectiveness analysis, through the multivariate models, therefore examined predicted outcomes for each of the intermediate and advanced groups compared with a reference group of basic care.
-
Home care (domiciliary care).
-
Community mental health nurse.
-
Dementia support worker.
-
Day/respite care.
-
Social worker or case manager.
-
Occupational therapist.
-
Admiral Nurse.
-
Home-delivered meals.
Services chosen from indicators in the national survey (see Workstream 1, Survey of current provision in England) and after discussion with our PPCI group on the services delivered to people with dementia/their carers at different stages.
Figure 3 shows the range of areas (local authority boundaries) where we recruited participants. We recruited and interviewed in collaboration with CRN research teams in each area. The sample eventually recruited exemplified the breadth of different service arrangements from the NHS, social care and independent agencies that formed the basis of naturally occurring home support models.
FIGURE 3.
Locations of participating recruitment sites.
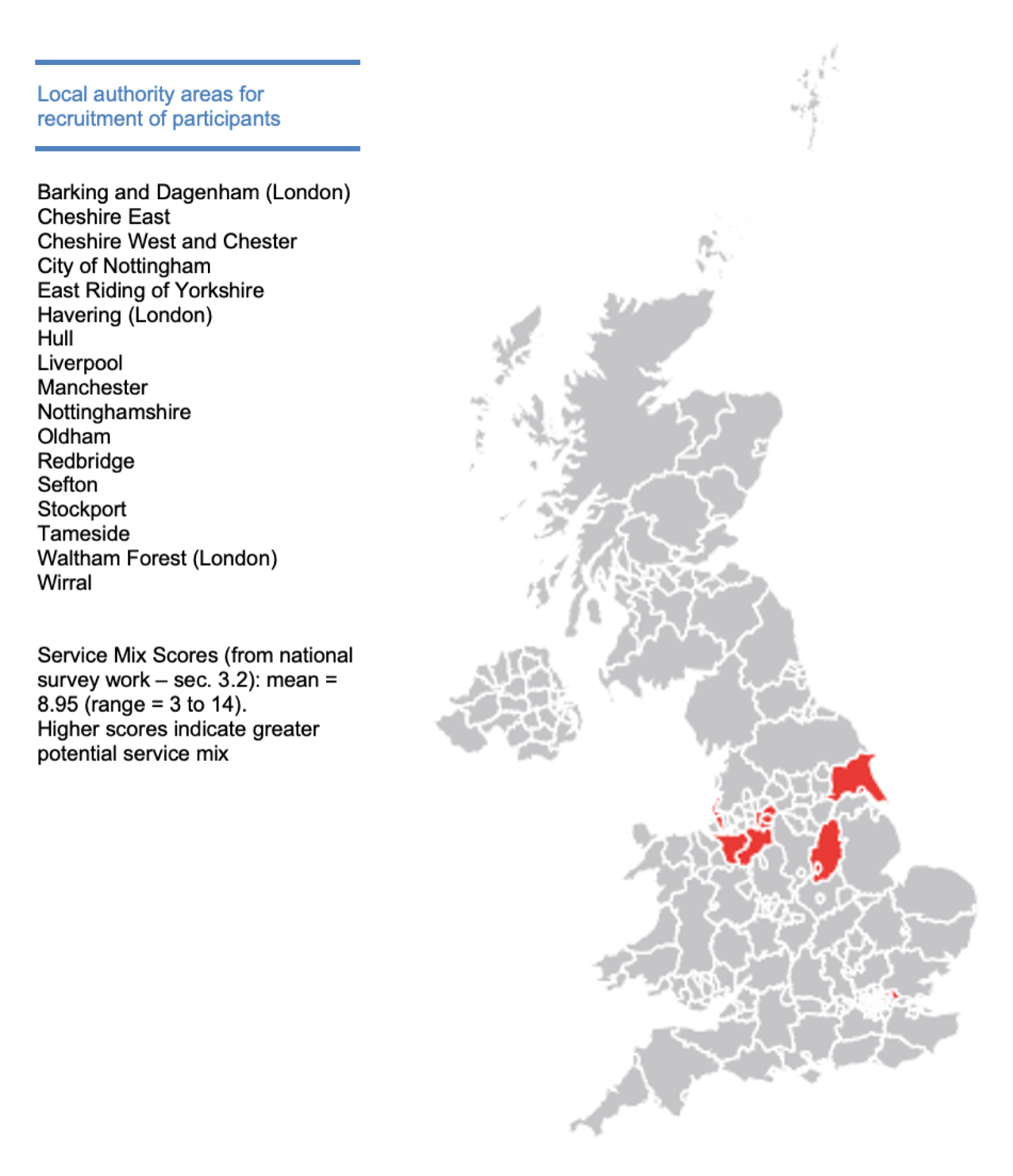
Table 4 shows descriptive data for each care package group at baseline. Results of post hoc tests revealed that carers receiving basic intensity care packages were older and that they and their families received more home care visits. Differences in outcome measures at baseline indicated that those receiving advanced intensity packages were more dependent in ADL (BADLS) and their carers experienced more psychiatric symptoms (GHQ-12). Those receiving basic packages were more cognitively able.
| Characteristic | Basic care (N = 154) | Intermediate care (N = 268) | Advanced care (N = 96) | Post hoc test |
|---|---|---|---|---|
| PwD: age (years), mean (SD) | 80.49 (7.19) | 80.86 (7.74) | 78.95 (8.07) | p = 0.10 |
| PwD: sex, n (%) | p = 0.86 | |||
| Male | 71 (46.1) | 126 (47.0) | 42 (43.8) | |
| Female | 83 (53.9) | 142 (53.0) | 54 (56.3) | |
| PwD: ethnicity, n (%) | p = 0.69 | |||
| White | 147 (95.4) | 257 (97.1) | 91 (94.7) | |
| Mixed/multiple ethnic groups | 0 (0.0) | 1 (0.4) | 1 (1.0) | |
| Asian/Asian British | 4 (2.5) | 4 (1.6) | 1 (1.0) | |
| Black/African/Caribbean/black British | 3 (1.9) | 3 (1.1) | 3 (3.1) | |
| PwD: marital status, n (%) | p = 0.64 | |||
| Single | 2 (1.3) | 7 (2.6) | 3 (3.2) | |
| Married/cohabiting | 108 (71.1) | 166 (62.2) | 58 (61.1) | |
| Separated | 4 (2.6) | 9 (3.4) | 3 (3.2) | |
| Divorced | 0 (0.0) | 2 (0.7) | 0 (0.0) | |
| Widowed | 38 (25.0) | 83 (31.1) | 31 (32.6) | |
| Carer: age (years), mean (SD) | 69.37 (11.2) | 66.15 (12.0) | 66.78 (11.0) | p = 0.02 |
| Carer: sex, n (%) | p = 0.46 | |||
| Male | 48 (31.2) | 69 (25.7) | 25 (26.0) | |
| Female | 106 (68.8) | 199 (74.3) | 71 (74.0) | |
| Carer: ethnicity, n (%) | p = 0.74 | |||
| White | 147 (95.4) | 258 (96.3) | 92 (95.8) | |
| Mixed/multiple ethnic groups | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Asian/Asian British | 4 (2.5) | 7 (2.6) | 1 (1.0) | |
| Black/African/Caribbean/black British | 3 (1.9) | 3 (1.1) | 3 (3.1) | |
| Carer: marital status, n (%) | p = 0.46 | |||
| Single | 7 (4.6) | 24 (9.0) | 9 (9.4) | |
| Married/cohabiting | 130 (85.0) | 223 (83.5) | 80 (83.3) | |
| Separated | 14 (9.2) | 13 (4.9) | 5 (5.2) | |
| Divorced | 0 (0.0) | 2 (0.7) | 1 (1.0) | |
| Widowed | 2 (1.3) | 5 (1.9) | 1 (1.0) | |
| Carer relationship to PwD, n (%) | p = 0.550 | |||
| Spouse/partner | 98 (63.6) | 150 (56.0) | 56 (58.3) | |
| Grandchild | 0 (0.0) | 4 (1.5) | 0 (0.0) | |
| Son/daughter | 50 (32.5) | 100 (37.3) | 33 (34.4) | |
| Brother/sister | 0 (0.0) | 1 (0.4) | 1 (1.0) | |
| Other relative | 3 (1.9) | 8 (3.0) | 2 (2.1) | |
| Friend | 2 (1.3) | 2 (0.7) | 3 (3.1) | |
| Other | 1 (0.6) | 3 (1.1) | 1 (1.0) | |
| Number of home care visits (previous 6 months), mean (SD) | 326.1 (242.3) | 311.8 (246.9) | 229.4 (220.7) | p = 0.04 |
| Duration of home care visits in hours (previous 6 months), mean (SD) | 1.06 (.85) | 1.29 (1.80) | 1.34 (2.87) | p = 0.82 |
| Informal care in hours (previous 6 months), mean (SD) | 386.0 (723.8) | 546.2 (825.4) | 622.6 (838.6) | p = 0.05 |
| Service mix scores, mean (SD) | 9.16 (3.46) | 9.24 (3.29) | 9.85 (2.80) | p = 0.21 |
| PwD: place of residence at baseline, n (%) | p = 0.29 | |||
| Living with carer | 98 (63.6) | 157 (58.6) | 61 (63.5) | |
| Living in own home with relative | 22 (14.3) | 44 (16.4) | 9 (9.4) | |
| Living in own home alone | 30 (19.5) | 50 (18.7) | 21 (21.9) | |
| Supported accommodation (e.g. sheltered) | 3 (1.9) | 17 (6.3) | 5 (5.2) | |
| Other | 1 (0.6) | 0 (0.0) | 0 (0.0) | |
| BADLS score, mean (SD) | 23.17 (12.71) | 31.02 (12.86) | 32.35 (11.48) | p < 0.001 |
| PwD: DEMQOL score, mean (SD) | 89.70 (11.24) | 88.10 (11.63) | 88.11 (11.87) | p = 0.57 |
| S-MMSE score, mean (SD) | 18.11 (7.19) | 15.74 (6.87) | 16.20 (6.29) | p = 0.03 |
| Carer: GHQ-12, mean (SD) | 3.19 (310) | 3.97 (3.34) | 5.06 (3.39) | p < 0.001 |
Table 5 shows a breakdown of the number of participants (people with dementia or carers) receiving each dementia-specific service at baseline. Each care package group combined these services in different ways. There was a rich mix of services belonging to each care package, with the difference being the intensity with which these were combined.
| Service type | Basic care (N = 154), n | Intermediate care (N = 268), n | Advanced care (N = 96), n |
|---|---|---|---|
| Home care | 31 | 141 | 75 |
| Community mental health nurse | 22 | 103 | 59 |
| Dementia support worker | 22 | 86 | 54 |
| Day/respite care | 22 | 138 | 75 |
| Social worker or case manager | 12 | 112 | 76 |
| Occupational therapist | 6 | 40 | 47 |
| Admiral Nurse | 1 | 5 | 9 |
| Home-delivered meals | 1 | 20 | 11 |
At baseline, the dementia-specific services provided several components of care (Table 6). Descriptions of components, drawn from our evidence synthesis, were included in the research interview questionnaire. Participants identified a range of components that were received across all care package groups. From our evidence synthesis (see Appendix 2), all components were received, with the exception of behaviour management directed at the person with dementia. Daily living assistance and care co-ordination were frequently described components across all groups.
| Componenta | Basic care (none or one service), n | Intermediate care (two or three services), n | Advanced care (four or more services), n |
|---|---|---|---|
| Person with dementia | |||
| Daily living assistance | |||
| Personal care | 25 | 125 | 63 |
| Meal preparation | 14 | 63 | 30 |
| Nutrition | 9 | 27 | 12 |
| Emotional support | 3 | 15 | 9 |
| Care co-ordination | 11 | 133 | 104 |
| Sensory enhancement | 3 | 13 | 3 |
| Cognitive training/support | 8 | 22 | 10 |
| Physical activity | 4 | 36 | 18 |
| Environmental modifications | 3 | 13 | 16 |
| Carer | |||
| Education/advice | 33 | 153 | 94 |
| Respite | 4 | 44 | 19 |
| Social support | 5 | 31 | 22 |
| Emotional support | 4 | 37 | 29 |
| Behaviour management | 2 | 21 | 16 |
Table 7 shows the results of the multinomial logit model for predicting care package groups at baseline, creating propensity scores used in the final multivariate models. The coefficients indicate the change in logarithmic probability of getting a particular care package group compared with basic care. Looking at significant estimates, three variables stood out as predicting either intermediate or advanced care package receipt. In comparison with basic care, those who lived in more deprived areas (site deprivation score) had a lower probability of getting either care package, those more dependent in the ADL (BADLS) had a greater probability of receiving either package, and those participants living in local authorities with higher expenditure on home care had a greater probability of receiving either package. As shown by the pseudo-R2 (0.13), the model explained 13% of the variance in care package receipt.
| Care package group | Independent variable | β | SE | p-value | 95% CI |
|---|---|---|---|---|---|
| Intermediate care | Site service mix scores | –0.05 | 0.040 | 0.17 | 0.89 to 1.02 |
| Informal care (hours per week) | –0.001 | 0.004 | 0.85 | 0.99 to 1.01 | |
| Site deprivation score | –0.04 | 0.01 | 0.01 | 0.93 to 0.99 | |
| Living alone | –0.04 | 0.27 | 0.87 | 0.56 to 1.638 | |
| BADLS scores (ADL) | 0.05 | 0.01 | < .001 | 1.033 to 1.072 | |
| Site community home care expenditure | 0.00 | 0.00 | 0.008 | 1.000 to 1.000 | |
| Constant | 0.03 | 0.58 | 0.95 | ||
| Propensity score ‘intermediate care’ (mean/SD) | 0.52 (0.10) | ||||
| Advanced care | Site service mix scores | 0.01 | 0.05 | 0.82 | 0.92 to 1.112 |
| Informal care (hours per week) | 0.00 | 0.005 | 0.924 | 0.99 to 1.010 | |
| Site deprivation score | –0.04 | 0.02 | 0.04 | 0.92 to 0.99 | |
| Living alone | –0.29 | 0.34 | 0.39 | 0.38 to 1.454 | |
| BADLS scores (ADL) | 0.06 | 0.01 | < .001 | 1.035 to 1.085 | |
| Site community home care expenditure | 0.000 | 0.000 | 0.02 | 1.000 to 1.000 | |
| Constant | –1.844 | 0.76 | 0.01 | ||
| Propensity score ‘advanced care’ (mean/SD) | 0.19 (0.06) | ||||
| Model fit | Log-likelihood | 976.02 | |||
| χ2 LR/p > χ2 | 60.347 | ||||
| Pseudo-R2 (Nagelkerke) | 0.13 | ||||
Tables 8–11 show the results of the predicated outcomes from the multivariate models. These tables describe the influence of each of the intermediate and advanced care packages on outcomes, controlling for several other variables, including propensity scores.
| Independent variable | β | SE | p-value | 95% CI |
|---|---|---|---|---|
| Age | 0.07 | 0.06 | 0.28 | –0.003 to 0.002 |
| Sex (0 = female, 1 = male) | 1.229 | 1.148 | .286 | –1.036 to 3.495 |
| Duration of care visits in hours (previous 6 months) | –0.001 | 0.001 | 0.641 | –0.003 to 0.002 |
| Care package type | ||||
| Intermediate care | 0.316 | 1.412 | 0.823 | –2.471 to 3.103 |
| Advanced care | 2.463 | 1.611 | 0.128 | –0.716 to 5.642 |
| Propensity scores | ||||
| Intermediate care | –11.704 | 7.432 | 0.117 | –26.369 to 2.961 |
| Advanced care | –24.747 | 11.132 | 0.027 | –46.711 to –2.782 |
| Intercept | 8.315 | 6.815 | 0.224 | –5.133 to 21.762 |
| n | 189 | |||
| Independent variable | β | SE | p-value | 95% CI |
|---|---|---|---|---|
| Age | 0.034 | 0.127 | 0.789 | –0.216 to 0.284 |
| Sex (0 = female, 1 = male) | 1.358 | 2.273 | 0.551 | –3.130 to 5.846 |
| Duration of care visits in hours (previous 6 months) | –7.134 | 0.003 | 0.977 | –0.005 to 0.005 |
| Care package type | ||||
| Intermediate care | –2.209 | 2.752 | 0.423 | –7.642 to 3.225 |
| Advanced care | –0.242 | 3.217 | 0.940 | –6.596 to 6.111 |
| Propensity scores | ||||
| Intermediate care | 2.405 | 14.463 | 0.868 | –26.154 to 30.964 |
| Advanced care | 5.245 | 21.991 | 0.812 | –38.179 to 48.670 |
| Intercept | –2.246 | 13.171 | 0.865 | –28.254 to 23.763 |
| n | 171 | |||
| Independent variable | β | SE | p-value | 95% CI |
|---|---|---|---|---|
| Age | 0.020 | 0.017 | 0.234 | –0.013 to 0.052 |
| Sex (0 = female, 1 = male) | 0.300 | 0.290 | 0.303 | –0.273 to 0.873 |
| Duration of care visits in hours (previous 6 months) | < –0.001 | < 0.001 | 0.424 | –0.001 to < 0.001 |
| Care package type | ||||
| Intermediate care | 0.063 | 0.348 | 0.857 | –0.625 to 0.751 |
| Advanced care | 0.415 | 0.400 | 0.302 | –0.375 to 1.205 |
| Propensity scores | ||||
| Intermediate care | 1.895 | 1.866 | 0.311 | –1.788 to 5.578 |
| Advanced care | –0.472 | 2.735 | 0.863 | –5.870 to 4.927 |
| Intercept | –2.665 | 1.737 | 0.127 | –6.094 to 0.764 |
| n | 178 | |||
| Independent variable | β | SE | Exp(β) | p-value | 95% CI |
|---|---|---|---|---|---|
| Age | –0.030 | 0.022 | 0.97 | 0.176 | 0.929 to 1.014 |
| Sex (0 = female, 1 = male) | 0.105 | 0.379 | 1.11 | 0.782 | 0.528 to 2.332 |
| Duration of home care visits in hours (previous 6 months) | 0.002 | 0.001 | 1.00 | 0.018 | 1.000 to 1.004 |
| Care package type | |||||
| Intermediate care | –0.43 | 0.506 | 0.65 | 0.396 | 0.241 to 1.754 |
| Advanced care | –1.09 | 0.548 | 0.33 | 0.046 | 0.114 to 0.981 |
| Propensity scores | |||||
| Intermediate care | 0.823 | 2.596 | 2.28 | 0.751 | 0.014 to 369.2 |
| Advanced care | 3.711 | 3.830 | 40.89 | 0.333 | 0.022 to 74467.6 |
| Constant | 1.950 | 2.308 | 7.03 | 0.398 | |
| Pseudo-R2 | 0.151 | ||||
| N | 172 | ||||
| χ2 LR/p > χ2 | 9.476 | ||||
The regression coefficients reveal no significant effects for participants receiving either advanced or intermediate care packages on the primary outcome (i.e. BADLS) or secondary outcomes. There was a significant coefficient for the advanced care propensity score on BADLS. However, for destination at 12 months, there were significant predictors for number of home care visits and advanced care at baseline. Participants with more home care visits were more likely to be living at home at 12 months. Those receiving advanced care were less likely to be living at home at 12 months.
Owing to multiple error terms, linear mixed models do not routinely produce R2 statistics to estimate the degree of variance explained. We used the approach of Nakagawa and Schielzeth,215 implemented in the R package ‘MuMIn’ (The R Foundation for Statistical Computing, Vienna, Austria), to produce marginal R2 estimates from these models. These R2 values were 0.11 for BADLS, 0.003 for DEMQOL and 0.02 for SSCQ. Comparing models without and with propensity scores showed that including propensity scores increased the explained variance, apart from for DEMQOL (BALDS from 0.03 to 0.11, DEMQOL from 0.004 to 0.003 and SSCQ from 0.01 to 0.02).
Sensitivity analysis that examined changes in care packages showed that there were changes in membership between baseline and follow-up (Table 12). A total of 171 (44%) participants changed (most to lower intensity packages). There were, however, 218 (56%) participants who received the same intensity package at follow-up. However, there were no outcome differences between those with the same, with higher or with lower intensity packages for DEMQOL (analysis of variance; p = 0.73), SSCQ (analysis of variance; p = 0.13), BADLS (analysis of variance; p = 0.73) or destination (chi-square 4.63; p = 0.09).
| Care package intensity | Care package intensity, follow-up, n | Total, n | ||
|---|---|---|---|---|
| Basic | Intermediate | Advanced | ||
| Basic | 19 | 35 | 14 | 68 |
| Intermediate | 27 | 111 | 58 | 196 |
| Advanced | 5 | 32 | 88 | 125 |
| Totals | 51 | 178 | 160 | 389 |
Conclusions
We examined, naturalistically, the effects of different intensity care packages on outcomes for people with later-stage dementia and their carers. This was accomplished using multivariate models with propensity scores used to control for confounding. Using propensity scores in models explained greater variance than those without, apart from for DEMQOL. The variance explained was low. However, models were specified a priori to avoid data mining.
The study generated a large and comprehensive data set. A complex mix of services characterised support received by participants across 17 areas of England. We found no significant effects for either intermediate or advanced care packages on the primary or most secondary outcomes. However, there were significant findings for destination at 12 months (i.e. those with more home care visits were more likely, and those receiving advanced care were less likely, to be living at home at 12 months). These findings raise interesting issues of targeting. A primary aim of home care is to enable people to have a greater chance of remaining at home, but more intense support, in terms of a greater mix of services, tends to be delivered to those more vulnerable. Scores at baseline confirmed this, with more intense care packages being received by participants at greater dependency who were receiving more hours of support from their informal carers. These participants are at greater risk of entering care homes or hospital care. We provided new evidence of home support and its potential effects in this frail population.
Appendix 8 Toolkit design and development
A challenge of this programme was to disseminate research findings in a manner useful for commissioners and providers. This is a complex process and academic findings do not necessarily affect commissioning decisions directly. Rather, local knowledge is used to inform service development. 216,217 Often, commissioning and service decisions are made using information from professional relationships, personal experience, best practice examples, internal data and strategy/policy directives. 218–220 The commissioning and policy-making context is also fast-paced, with changing priorities, which often does not fit with academic research contexts. Especially in the social care setting, most of the behaviour change methods associated with knowledge transfer have been intuitive or educational, including printed materials, audio and feedback. 221 We developed the toolkit within this context.
Development was facilitated by the use of the SITE concept that is used elsewhere in the programme. 30,135 We derived the toolkit presentation from the work of Goertz192 who proposed a three-level structure, with a basic, secondary and indicator level. The basic level is the central theoretical aspect of a concept, for example democracy. The secondary level then gives the constitutive dimensions of the basic level. For example, one secondary dimension of democracy would be competitive elections. The indicator level then operationalises the theoretical aspects of a concept by giving suitable data to analyse it.
The advantages in using this structure were as follows.
-
It describes how to build and thereby define concepts for audiences, articulated as attributes. This was of use in presenting research evidence for non-academic audiences.
-
Each domain consists of a number of attributes, which were operationalised by indicators relevant to supporting people with dementia at home. We used elements of each of three levels of the concept in presenting findings for the toolkit.
-
It permits the extension of coverage by changing the structure of attributes. We extended the SITE concept to include additional aspects of home support, for example health promotion.
For programme findings to be of most use to commissioners and providers, the toolkit should be relevant to policy guidance. We created a database with existing guidance and policy on home support for older people. Documents included were government guidance from England, Wales and Scotland and non-academic reports since 2010. The latter included reports from statutory bodies and non-government organisations from which we extracted information. Documents were found through a number of search strategies:
-
a Google search (Google Inc., Mountain View, CA, USA) of ‘home support older people’, ‘home support older people guidelines’, ‘dementia guidelines’, ‘dementia home support’ and ‘domiciliary care older people’
-
a search of relevant websites, including those of NICE and Social Care Institute for Excellence, for ‘dementia’, ‘homecare’, ‘assistive technology’, ‘respite care’, ‘day care’ and ‘carers’
-
a hand-search of previous literature reviews for relevant policy documents and guidelines.
Initially, the title, contents page and executive summaries documents were read and judgements made on whether or not they were worth interrogating further. These documents were downloaded, saved and the relevant date accessed through a data extraction tool. To extract relevant data, SITE attributes were used as data extraction points. Each attribute had a string of search terms that were used to search the document (Table 13).
| Service domain/attribute | Search terms |
|---|---|
| Support/social engagement | Support, socialise, engagement, community, facilities, contact, link, friend, network |
| Support/emotional support | Emotion, feeling, stress, psych, mental |
| Support/physical activity | Physical, activity, outdoor, mobility, exercise |
| Support/environmental modifications | Environment, build, built, housing, modification, wander, sensor, safety, adaption |
| Support/daily living assistance | Daily, nutrition, food, clean, toilet, assistance, eat, bath, dress, bed, day-to-day, housework, shopping |
| Information/fact and advice | Information, facts, advice, sign |
| Therapy/behaviour management | Behaviour, challenge, anger, angry, medication, difficult |
| Education/cognitive support | Cognitive, training, aids, memory, education |
| Education/sensory enhancement/relaxation | Sensor, sense, enhancement, stimulation, relaxation, deprivation |
We also undertook a website review with assistance from the University of Manchester’s Web Team. The purpose of this was to identify feasible and effective webpage design and structure by which to present the toolkit. Sites reviewed included research institutes at the University of Manchester, organisations presenting research to lay audiences (e.g. The King’s Fund website) and other publicly available toolkits. From this, the use of visual cues, links, navigation bars and images were highlighted.
The design of the toolkit involved (1) the appraisal of the quality of the publications from the programme, (2) a consultation with stakeholders about the nature and (3) presentation of the findings and extraction of key findings.
The research questions were as follows:
-
How are these research findings best communicated to stakeholders, commissioners, providers and policy-makers?
-
What findings from the programme are most useful in service re-design?
To appraise the quality of the programme publications from which data for the toolkit were extracted, we used a research typology developed by Turner-Stokes et al. 222 This was selected as a ‘simple assessment of both qualitative and quantitative research evidence in terms of design, quality and applicability, and is practical for use by clinicians’. 222 Each paper was classified using the typology’s three main criteria: (1) design, (2) quality rating and (3) applicability. Overall, research papers were of high quality.
To inform the development of the toolkit, we conducted a stakeholder consultation exercise. We recruited participants from two NHS trusts and a senior government advisor (see Acknowledgements). Their views were obtained through a series of semistructured interviews. We elicited stakeholder views to:
-
scope the knowledge requirements of commissioners and providers
-
identify the knowledge gap(s) that findings from the programme could address
-
explore how the materials from the research findings might be presented to best effect.
Semistructured interviews were undertaken. The interviews used a schedule to provide direction, while allowing other issues of importance to interviewees to emerge (Box 3). In the context of home support for people with dementia, questions focused on:
-
information currently used to commission services
-
the source of this information
-
additional information that might be useful
-
the balance between data and interpretation of the findings.
Prior to interview, define ‘effective home support for people with dementia and their carers’.
-
Please summarise your current roles and responsibilities.
-
To what extent do you focus on community support for people with dementia and their carers?
-
What proportion of your time do you estimate you spend on this?
-
-
How do you define evidence in the context of commissioning services?
-
Type of evidence: staffing, services, costs, effectiveness, outcomes, impact, social value
-
Type of presentation: facts and figures, opinions, case studies, (national) research reports, local research, other?
-
Source: from where do you generally source this? Which source of information/evidence do you prefer and why?
-
-
What influences commissioning and service re-design?
-
How is evidence used in this context? (Other issues likely to influence changes in service delivery are crises and funding.)
-
What evidence is likely to prompt managers to change how they deliver services?
-
How significant are the requirements of commissioners in motivating managers to deliver services differently?
-
-
What evidence do you use currently in decision-making?
-
Purpose: what decisions do you use evidence for?
-
Frequency: how often do you access data to inform decision-making?
-
-
What evidence/information would you need to re-design services to support people with dementia at home?
-
What information would you like to help you do your job better?
-
What kind of evidence do you value/need? (Empirical/qualitative.)
-
Focus on person with dementia or carer?
-
How might you use research evidence to influence service re-design?
-
-
How should research evidence be presented to facilitate its use by commissioners and managers?
-
What format would work for you? (Case studies, tables, text, etc.)
-
Length.
-
Type of document? (Expert briefing, research summary, policy and practice update, something else?)
-
Each interview lasted approximately 45 minutes and was audio-recorded and professionally transcribed. The interviews were read and summarised (see Box 4 for a summary of findings). From this, implications for the toolkit were derived following discussion in the research team (Table 14).
-
Current problems in translating evidence to practice.
-
Difference in timings/priorities of services and academia.
-
Anecdotal and local evidence is sometimes prioritised.
-
Issues with accurate outcome and financial data.
-
Interventions not fitting with current structures.
-
-
How is evidence best presented?
-
Online, for example blog, WebEx™ (Cisco systems, Milpitas, CA, USA), high on Google search, web page.
-
E-mail bulletins (to local networks).
-
Face to face (to local forums).
-
Briefing/executive summary format.
-
Signposting to academic references.
-
NICE/CQUINs.
-
-
What kind of evidence is deemed useful?
-
Local/non-academic literature.
-
Short and snappy summaries.
-
Simple cost information.
-
Outcome information.
-
Fit with current service and staff.
-
Light-touch academic information.
-
-
Target context.
-
Commissioners/providers.
-
Operational staff.
-
Policy and strategy.
-
Service improvement and cost reduction.
-
CQUIN, Commissioning for Quality and Innovation.
| Facilitator | Implications for toolkit |
|---|---|
| Use snappy, short summaries of findings | Exemplars, standardisation of terminology, hyperlinks and consider video guide |
| Use non-academic local knowledge/data/outputs | Language relevant to current concepts and thinking, use plain English |
| Include relevant outcomes | Link findings to standardised outcomes, where possible |
| Include information on bottom-line costs | Link cost data to scope and scale of intervention, where possible |
| Align findings with service/staff structures | Link findings to existing structures, for example CMHT and IAPT, and use standardised staffing descriptions |
| Align findings with guidance | Scrutinise NICE guidelines and other relevant documents for links |
| Target relevant people/use relevant networks | Disseminate findings through the Association of Directors of Adults Social Services and NHS England. Also Department of Health and Social Care in Wales |
| Signpost to academic literature | Hyperlinks to DOI of articles |
In the development of the modules within the toolkit, we made a distinction between findings relating to people with dementia and their carers and to the stage of dementia, wherever possible. For each module, a bespoke approach to data extraction was undertaken, which is summarised below.
Module 1: scoping the evidence
This module presented data from the systematic literature reviews. Nine potential approaches to home support for older people with dementia and their carers were identified (see Table 4 in Clarkson et al. 30). These approaches employed components in different ways. Using the findings from the stakeholder consultation, each was modified for the toolkit.
For each approach, preparation of the research findings comprised the following:
-
Components of the interventions from the synthesis of home support interventions were aggregated and standardised.
-
Descriptions of staffing were standardised, reflecting UK practice.
-
Intervention outcomes were aggregated and standardised. These were grouped to reflect those relating to people with dementia and their carers (Table 15).
-
The measure of effectiveness used to appraise the evidence base in the publication was replaced with an evaluation using the same research typology generated by Turner-Stokes et al. 222 This was also employed to appraise the quality of programme publications.
| Outcomea | First-level aggregation (cluster generalisation) | Second-level aggregation (influence on) |
|---|---|---|
| Physical health and functioning (C1); care recipient physical function (C3) | Physical health | Influence physical functioning of people with dementia |
| ADLs (person with dementia) (C1); ADLs (C2); ADLs (C7); IADLs/ADLs (P1) | ADLs | |
| Affective symptoms (person with dementia); depression and anxiety (C4); depression and anxiety (C4); person with dementia mood (C6); person with dementia mood (C7) | People with dementia affective symptoms/mood | Influence on people with dementia emotional well-being and functioning |
| Quality of life of care recipient (C3); quality of life of care recipient (C4) | Quality of life of care recipient | |
| Affective symptoms/mood (C1); caregiver’s self-efficacy/perceived stress (C1); carers’ sense of competence/well-being (C2); caregiver neuroticism (C2); caregiver well-being and mood (C3); caregiver skills/efficacy (C3); carer self-efficacy (C3); carer self-efficacy (C4); depression and anxiety of carer (C4); carer mood (C5); carer mood (C6); carer mood (P1) | Carers’ sense of competence/well-being | Influence on carer emotional well-being and functioning |
| Quality of life (C1); quality of life of carer (C4); carer quality of life (C5); carer quality of life (P1): carer quality of life (P2) | Quality of life of carer | |
| Caregiver burden (C2); caregiver objective and subjective burden (C3); carer burden (C5); carer burden (C6); carer burden (C7); carer burden (P1); carer burden (P2) | Carer burden | |
| Carer social support/social network (C2) | Social support/social network | |
| Admissions to care home (C2); nursing home admission (C5); nursing home admission (C6); nursing home admission (P2) | Nursing home admission | Influence on likelihood of remaining at home |
| Time at community tenure (C4) | Time at community tenure | |
| Dementia severity (person with dementia) (C2) | Dementia severity | Influence on dementia symptoms (people with dementia) |
| Frequency of repetitive verbalisation (C1) | Frequency of repetitive verbalisation | |
| Behavioural problems (C1); behavioural problems (C2); care recipient problem behaviours (C3); behaviour (person with dementia) (C4); behaviour (C5); behaviour (C7); behaviour (P2) | Behaviour | Influence problem behaviours (people with dementia) |
| Potentially abusive behaviour by carer towards care recipient (C4) | Abusive behaviour by carer | Influence abusive behaviours by carer |
In addition, for each approach, the toolkit included a time frame and an exemplar, from which indicative costs were developed.
-
Exemplars, from the evidence synthesis, were selected from each of the nine approaches and described. For this, researchers used the following criteria: overall effectiveness rating, staff group, fidelity, location of study and real-world usefulness.
-
Measures (minimum, average, maximum) of staff time involved within each approach were identified to give examples of the duration of interventions.
-
Indicative costings were applied to the exemplar for each approach. A cost per case was calculated for each exemplar intervention, based on the amount of time spent by practitioners multiplied by their unit cost. 223 Estimates of travel and administrative time were collaboratively agreed by the project team and included in the calculation. Total costs for each intervention per case were calculated based on a notional caseload.
In Figure 4 we summarise one example of an approach that used components of home support in different ways: ‘education and advice, behaviour management and emotional support’.
FIGURE 4.
Description for toolkit: ‘education and advice, behaviour management and emotional support’.
Module 2: evaluating the service landscape
This module explored a means of describing services using indicators designed to differentiate between different health and social care dementia services in localities. Using our national survey results, we created indicators capturing variation in services.
Two published papers informed the module. 135,136 One provided a snapshot of NHS service provision in England through a cross-sectional survey conducted in 2015, achieving a response rate of 79%. 135 The second was similar in design, but focused on social care provision commissioned by local authorities in England. It was conducted in 2014/15 and achieved a response rate of 81%. 136 We presented these data in an accessible format for service commissioners and providers through:
-
descriptions of indicators, reviewed against plain English guidelines for ease of access and understanding
-
additional information provided from the survey papers to provide clearer and more complete service descriptions, where appropriate
-
examples of the application of the survey data (Table 16 and Figure 5).
| Indicator | n (%) |
|---|---|
| Organisational characteristics | |
| Old age mental health services jointly commissioned | 65 (68) |
| Old age mental health services jointly provided | 20 (21) |
| Service characteristics | |
| Social worker | 64 (67) |
| Generic support worker | 46 (48) |
| Occupational therapist assistant | 31 (32) |
| Admiral Nurse | 16 (16) |
| Services provided by NHS (health care) | |
| Early-stage dementia servicesa | 70 (73) |
| Later-stage dementia servicesb | 66 (69) |
| Support for carers of people with dementiac | 48 (50) |
| Services funded by local authorities (social care) | |
| Home care specialist for older people with dementia | 25 (26) |
| Home care night-time | 75 (78) |
| Specialist respite cared | 88 (92) |
| Specialist day caree | 86 (90) |
| Respite care, family placement | 38 (40) |
| Hospital discharge services, specialist for older people with dementia | 46 (48) |
| Assistive technologies, specialist for older people with dementia | 77 (80) |
FIGURE 5.
Distribution of service mix score by geographical areas (n = 96). Bold lines show quartile divisions. Score fits normal distribution: Kolmogorov–Smirnov test, 0.1546; p = 0.017.
For this module, these descriptions of services, used to define the indicators and the data from our surveys, were used in an infographic that outlined the variation in services potentially available in localities in England. The infographic also included output from our service mix score to provide a snapshot of the distribution of potential service availability across geographical areas of England (see Figure 5).
Module 3: bridging the memory gap
This module presented findings from DESCANT in a readily accessible format for consumption by service managers and commissioners. We created a description and infographic, which was based on a similar format presented for a dementia trial in the British Medical Journal. 224 Figure 6 shows the infographic from the toolkit summarising the main findings.
FIGURE 6.
Description for toolkit: DESCANT in early-stage dementia.
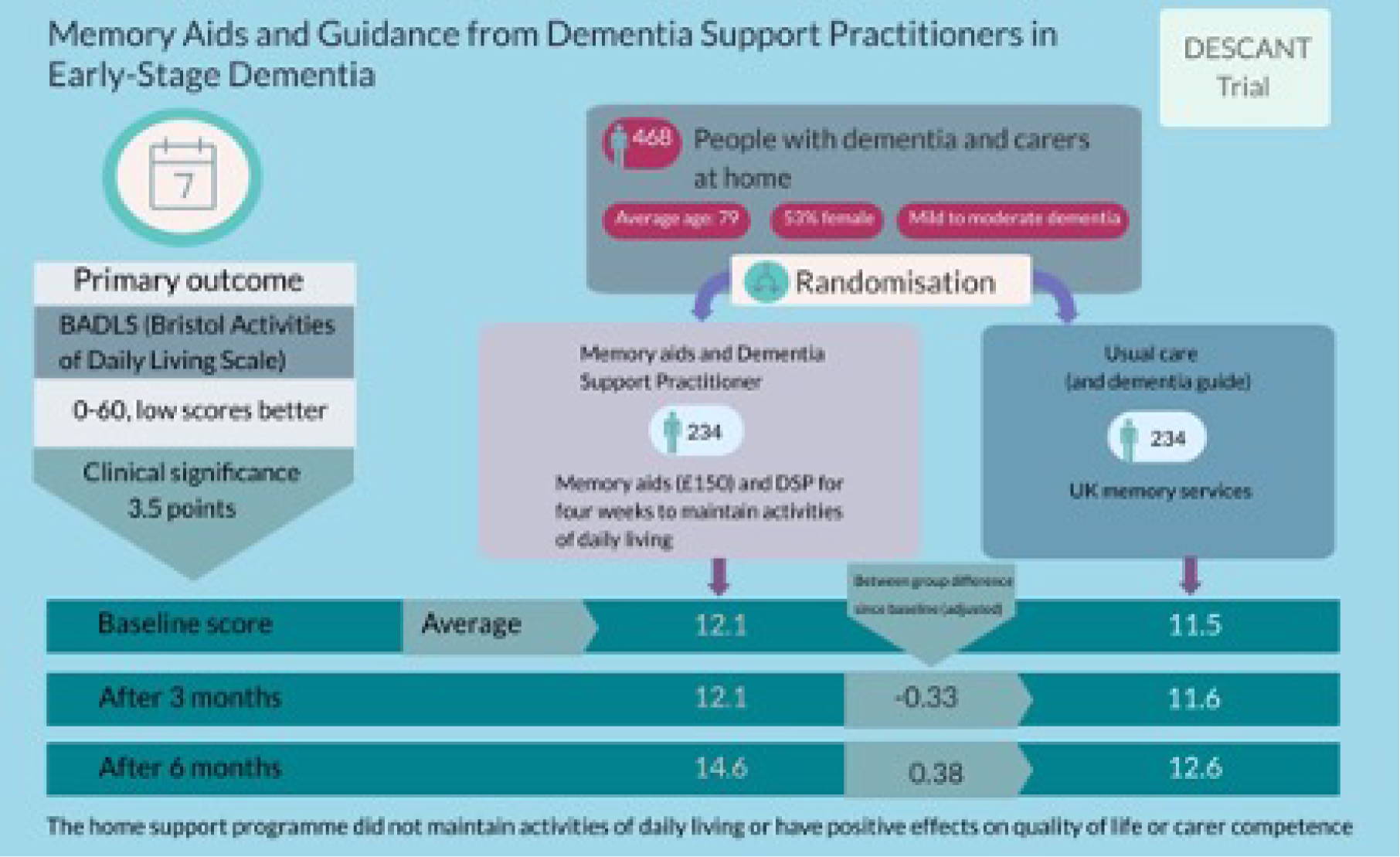
Module 4: maintaining well-being at home
This module presented findings from our observational study in later-stage dementia. The aim here was to present quite complex methods and findings in a readily accessible format. Figure 7 shows the infographic summarising the findings.
FIGURE 7.
Description for toolkit: observational study in later-stage dementia.
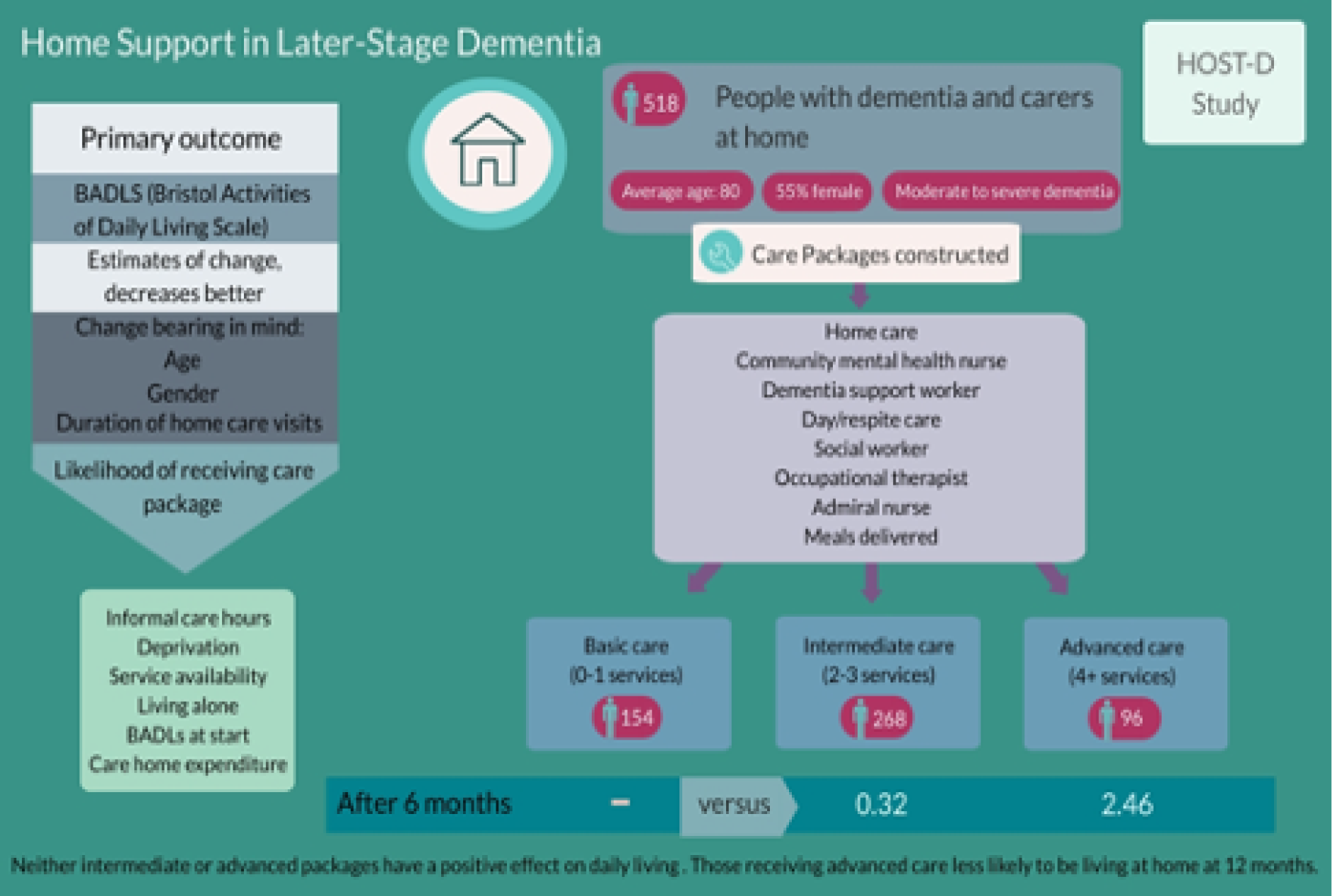
Module 5: preferences for care and support
This module explored the preferences of carers, people with early-stage dementia and professionals for services providing care and support at home. Their preferences were extracted from three papers. 161,170,173
The first two papers161,173 explored the preferences of carers and people with early-stage dementia through DCEs. This is a questionnaire that identified individual preferences for different aspects of a hypothetical intervention or service. It presented participants with a series of choices and asked them to select groups of attributes they preferred. The third paper170 used case vignettes to explore preferences for formal and informal care from carers and professionals. This comprised different professionals with backgrounds in health and social care, for example community psychiatric nurses and social workers.
Each DCE explored stated preferences from a list of seven attributes. 161,173 From both, only significant attributes were extracted as preferences.
The case vignette paper170 explored preferences for 23 potential services comprising both formal and informal care to support older people with dementia at home. The data extraction process was as follows:
-
We grouped individual services using the broad attributes from the DCEs and SITE, supplemented by our own original descriptions (see Box 5 for an example). All subsequent extraction was based on these aggregations.
-
From the results section,170 we excluded those services that were least recommended by staff and carers from the summary findings for the module.
-
Using text in the results section, a judgement was made as to which services were recommended by carers, professionals or both groups.
Speech and language therapist for assistance and training.
Continence advisor for assistance and advice.
Dietitian for assistance and advice.
Nursing care, for example wound dressing.
↓
Access to community health services (e.g. continence advisor, speech therapist, dietitian, community nurse) available to the general population.
From these three papers,161,170,173 we formed separate lists of preferences for people with early-stage dementia and professionals. For carers, data were available from all three papers. 161,170,173 For people with early-stage dementia173 and professionals,170 data were available from one paper for each. The results, as presented in the toolkit, are shown in Figure 8.
FIGURE 8.
Preferences by stakeholder group.
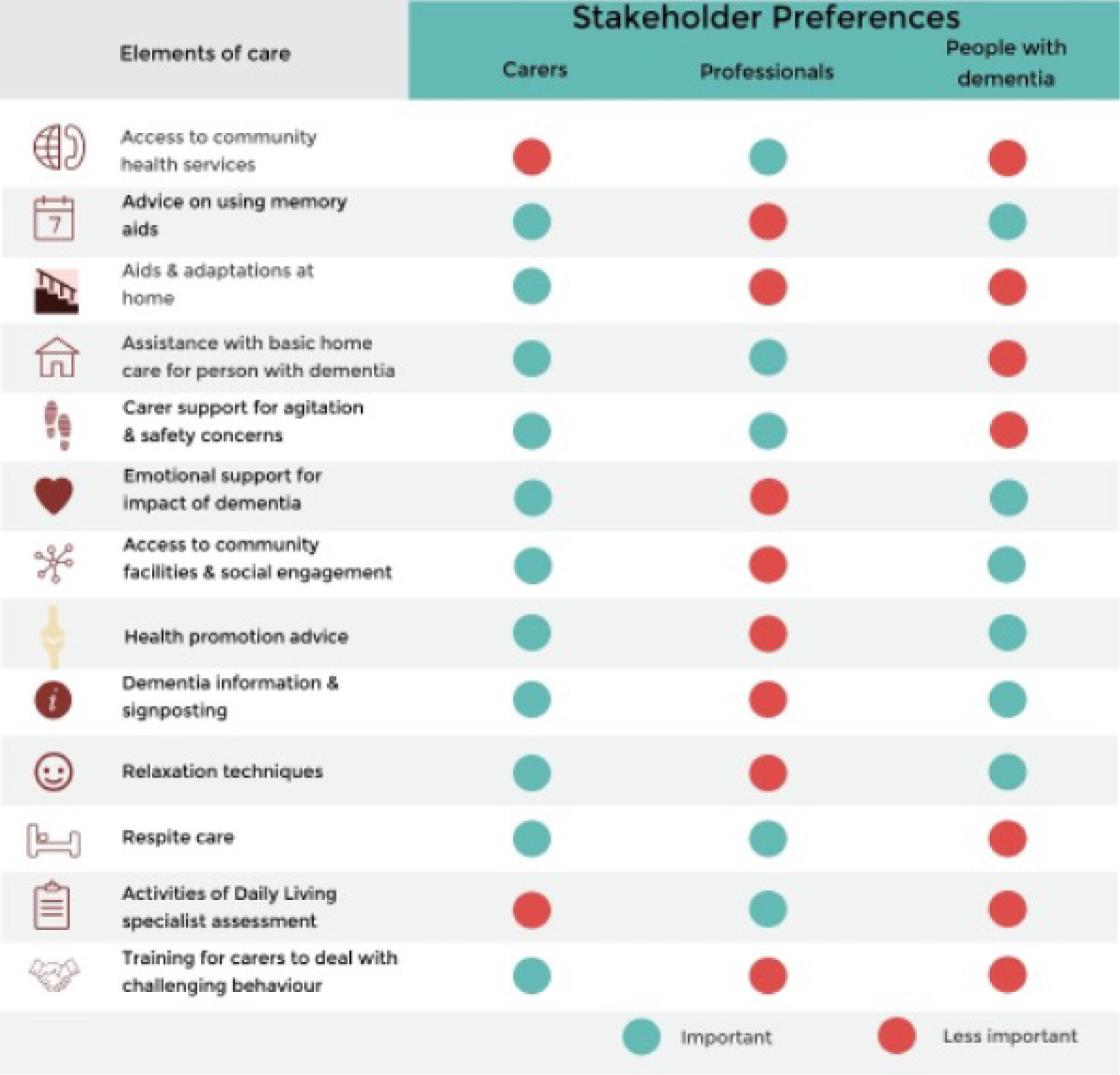
Module 6: costs and benefits
This module explored the results of the cost-effectiveness work (see Appendix 11), examining costs and benefits of different approaches to home support for carers, people with dementia at different stages and society. We aimed to provide information on costs to different parties that would be of particular use to commissioners. In addition, we aimed to determine if costs to informal carers could be offset by the delivery of support at home by more formal means (e.g. professional help or health and social care services). Examples of these outputs are online as part of the toolkit [URL: https://sites.manchester.ac.uk/home-support-dementia/ (accessed 6 April 2021)].
Appendix 9 Analysis of costs to people with dementia and carers and their relationship to formal care
Giebel et al. (2019)
Giebel CM, Davies S, Clarkson P, Sutcliffe C, Challis D, Members of the HoSt-D (Home Support in Dementia) Programme Management Group. Costs of formal and informal care at home for people with dementia: ‘Expert panel’ opinions from staff and informal carers. Dementia 2019;18:210–27. https://doi.org/10.1177/1471301216665705
Appendix 10 Discrete choice experiments establishing the value of different components of support
Chester et al. (2018)
Chester H, Clarkson P, Davies L, Sutcliffe C, Davies S, Feast A, et al. People with dementia and carer preferences for home support services in early-stage dementia. Aging Ment Health 2018;22:270–9. https://doi.org/10.1080/13607863.2016.1247424
Kampanellou et al. (2019)
Kampanellou E, Chester H, Davies L, Davies S, Giebel C, Hughes J, et al. Carer preferences for home support services in later stage dementia. Aging Ment Health 2019;23:60–8. https://doi.org/10.1080/13607863.2017.1394441
Appendix 11 Cost-effectiveness impact of home support approaches
Introduction
Our findings from economic model development (see Workstream 1, Development of an economic model) signalled a lack of recent and reliable data to model home support approaches at different stages of dementia. The later studies, projects 2.1 and 2.2, presented an opportunity to use primary data from research participants to generate cost-effectiveness acceptability analyses, using participant-level data. These analyses, summarised below, estimated the incremental cost per QALY and the probability that home support models were cost-effective, compared with usual care (or, for later-stage dementia, viable, low-cost, basic care), for people with dementia at different stages.
Methods
We estimated cost-effectiveness from the DESCANT intervention and two approaches, specifying intermediate and advanced intensity support packages, for the observational study. From both studies, we analysed person with dementia-/carer-level data at baseline and during follow-up. The perspective of the primary (‘base case’) analysis was public (NHS and social care), informal carer/person with dementia costs and people with dementia/carer health benefits.
The objectives were to:
-
estimate the costs in intervention and comparator groups (and assess whether or not there were differences)
-
estimate participants’ QALYs in intervention and comparator groups (and assess differences)
-
assess whether or not any additional benefit was worth any additional cost.
The time horizon of the primary analyses was 6 months, which was the scheduled end of follow-ups. We estimated costs and QALYs from baseline to 26 weeks to estimate incremental cost-effectiveness of home support approaches. We compared home support approaches with usual or basic care: the DESCANT intervention (plus TAU) compared with TAU (plus dementia guide) for the trial; and intermediate and advanced intensity packages compared with basic care for the observational study.
For the primary analysis, we measured health benefit using QALYs estimated from the EQ-5D-5L, which compares outcomes across diseases. Without such a generic measure of benefit, it would be impossible to compare condition-specific outcomes, such as for dementia, with those of other conditions. NICE recommends the QALY and the EQ-5D-5L as measures for economic evaluations. We estimated QALYs from the EQ-5D-5L completed at baseline and through follow-up and associated utility tariffs recommended by NICE at the time of analysis. QALYs from the early-stage dementia trial pertained to people with dementia. QALYs from the later-stage observational study pertained to carers. The rationale for this was that in early stage the intervention was to people with dementia, whereas in later stage the approaches mainly supported carers. Research interviews for the observational study were with carers and cognitive functioning (S-MMSE) and quality of life (DEMQOL) were assessed, if possible, in only people with dementia.
We estimated the direct costs of services used by participants by summing the cost of each resource used to provide health and social care. We collected data from participants on the resources they used through two validated questionnaires: the CSRI (measuring formal service use) and the RUD (identifying and estimating the volume, duration and cost of support from formal and informal carers). Services covered included community health and social care services, hospital and emergency care, formal and informal carer support, and equipment, adaptations and ambulance use.
We documented the resources used to provide the intervention/approaches (e.g. staff contact time, training and materials) and added them to the services used by participants to estimate the total cost of each home support approach. We used national average unit cost data to estimate costs of formal health and social care for each person, using the NHS reference costs database225 and the Unit Costs of Health and Social Care. 226–230 The price year for all costs was that of the most recent published unit costs at the time of analysis (2017/18).
For informal carer costs, important particularly in later stages, we estimated time assisting relatives and time lost from work (if appropriate), valued by the cost of a home care worker as a proxy good. The categories of questions to elicit these data in the RUD meant that the issue of joint production (i.e. doing two or more activities at the same time, for example bathing and supervising) was not sufficiently accounted for. This resulted in some carers responding that they spent over 24 hours a day in caring for their relative. We accounted for the potential inaccuracy in these data by costing this contribution as 18 hours per day if the response was ≥ 18 hours, as recommended by Berg et al. 231 and Hoefman et al. 232 A further informal carer cost was the direct costs of aids and adaptations received by the household, if the carer paid for these privately.
We analysed data ‘by treatment allocated’ (or ‘approach adopted’) and included available data for all participants, whether or not they completed planned care. We accounted for missing data using imputation. We used single imputation for missing baseline measures of cost, utility and clinical indicators, but not missing demographic data. If data approximated missing at random or missing completely at random then we used multiple imputation from available data, as recommended. 233 We imputed for each time point, by category of cost and EQ-5D-5L, to make best use of available data.
The primary measure for the economic analysis was the ICER. Rather than considering cost and outcomes separately, the ICER combines them by dividing the difference between intervention/control in costs (net costs) by the difference in QALYs (net QALYs). Therefore, it estimated the additional cost per additional QALY gained by the approaches:
We bootstrapped these estimates of costs and outcomes to replicate 10,000 pairs of incremental costs/QALYs. This characterises the distribution of pairs of net costs/QALYs on the cost-effectiveness plane and therefore summarised parametric uncertainty in our modelling. Analysis for the trial used regression-based estimates of net costs and QALYs and that for the observational data used unadjusted costs and QALYs.
Incremental cost-effectiveness ratios estimate the marginal cost per QALY of an intervention and raise the question of whether or not that cost is worth paying. To address this, one compares ICERs with how much decision-makers may be willing to pay for an additional QALY. However, the UK has no universally agreed cost-effectiveness threshold and this value is debated, even more for social care support. 191 Although NICE have suggested a threshold of £20,000–30,000 per QALY, this may have decreased recently alongside constrained expenditure. Accordingly, Claxton et al. 234 tentatively estimated that the threshold was £18,317 per QALY by comparing NHS expenditure with corresponding mortality. In February 2015, Claxton et al. 234 updated this estimate to £13,000 per QALY. Reflecting this lack of consensus, we varied the monetary value of our simulated QALYs from £0 to £30,000. This recognises that decision-makers may not be willing to pay for an additional QALY (i.e. they may seek only the lowest cost option), but they could be willing to pay up to £30,000 for an extra QALY. To estimate the likelihood that home support approaches were cost-effective, we used a WTP threshold of £15,000 (the mid-point of the £0 to £30,000 range).
We valued each of the bootstrapped net QALY estimates by multiplying by an appropriate WTP threshold, therefore estimating the net benefit (NB) from each pair of simulated net costs and outcomes as:
where O is net outcome score (QALY) and C is net cost.
By repeating this calculation across the range of plausible WTP thresholds, we generated a cost-effectiveness acceptability curve that showed the probability of interventions generating a positive net benefit at each WTP threshold and hence being cost-effective. As decision-makers increase what they are willing to pay for an extra QALY, the additional benefits from an intervention become more valuable and it achieves net benefit in a bigger proportion of the 10,000 replicates.
We used sensitivity analysis to assess how our study design affected estimates of ICERs and the shape of the cost-effectiveness acceptability curve. The following were used in supplementary analyses to judge sensitivity of results against different assumptions:
-
different condition-specific preference-based measures of benefit, rather than generic EQ-5D-5L, utility values generated from the DEMQOL (trial and observational study) and ICECAP-O and carer-rated EQ-5D-5L (trial)
-
comparing results from multiple perspectives: overall (base case) with NHS, social care, third sector and informal carers (observational study).
Results
We summarise our findings in each of early- and later-stage dementia.
Intervention in early-stage dementia
The primary analyses were undertaken on data from participants with imputed missing values and regression-based estimates of costs/QALYs.
Tables 17–19 show resource use, unit costs and differences between intervention and TAU arms for average costs and QALYs per participant, using different utility measures.
| Service used | Baseline period | Follow-up period | ||||
|---|---|---|---|---|---|---|
| TAU (N = 234), n (%) | Intervention groups (N = 234), n (%) | p-valuea | TAU (N = 229), n (%) | Intervention groups (N = 226), n (%) | p-valuea | |
| Home care worker | 35 (15.0) | 40 (17.1) | 0.61 | 35 (15.3) | 40 (17.7) | 0.53 |
| Case/care manager | 13 (5.6) | 9 (3.8) | 0.51 | 12 (5.3) | 4 (9.0) | 0.66 |
| Social worker | 22 (9.4) | 21 (9.0) | 1.00 | 8 (3.5) | 18 (8.0) | 0.05* |
| Dementia advice worker | 48 (20.5) | 32 (13.7) | 0.07 | 24 (10.5) | 26 (11.5) | 0.77 |
| DSP | 39 (16.7) | 42 (17.9) | 0.81 | 26 (11.4) | 39 (17.3) | 0.08 |
| Support worker | 16 (6.8) | 13 (5.6) | 0.70 | 17 (7.4) | 20 (8.8) | 0.61 |
| Voluntary worker | 48 (20.5) | 53 (22.6) | 0.65 | 60 (26.3) | 52 (23.0) | 0.45 |
| Community mental health nurse | 61 (26.1) | 60 (25.6) | 1.00 | 25 (10.9) | 28 (12.4) | 0.66 |
| Community district nurse | 29 (12.4) | 31 (13.2) | 0.89 | 30 (13.1) | 24 (10.6) | 0.47 |
| GP | 178 (76.1) | 178 (76.1) | 1.00 | 156 (68.1) | 168 (74.3) | 0.15 |
| General practice nurse | 109 (46.6) | 114 (48.7) | 0.71 | 125 (54.6) | 122 (54.0) | 0.93 |
| Community pharmacist | 66 (28.2) | 61 (26.1) | 0.68 | 82 (35.8) | 97 (42.9) | 0.13 |
| Psychologist | 12 (5.1) | 8 (3.4) | 0.49 | 11 (4.8) | 13 (5.8) | 0.40 |
| Physiotherapist | 33 (14.1) | 25 (10.7) | 0.33 | 37 (16.2) | 34 (15.0) | 0.80 |
| Dietitian | 7 (3.0) | 3 (1.3) | 0.34 | 3 (1.3) | 5 (2.2) | 0.36 |
| Health visitor | 0 (0.0) | 1 (0.4) | 1.00 | 0 (0.0) | 4 (1.8) | 0.06 |
| Chiropodist | 69 (29.5) | 76 (32.5) | 0.55 | 73 (31.9) | 87 (38.5) | 0.14 |
| Benefits adviser | 13 (5.6) | 10 (4.3) | 0.67 | 21 (9.2) | 15 (6.6) | 0.39 |
| Short-term respite care | 1 (0.4) | 1 (0.4) | 1.00 | 3 (1.3) | 4 (1.8) | 0.72 |
| Transport | 15 (6.4) | 18 (7.7) | 0.72 | 18 (7.9) | 22 (9.7) | 0.51 |
| Drop-in centre | 17 (7.3) | 10 (4.3) | 0.23 | 15 (6.6) | 16 (7.1) | 0.85 |
| Day-care centre | 10 (4.3) | 10 (4.3) | 1.00 | 0 (0.0) | 1 (0.4) | 0.50 |
| All outpatient visits | 164 (70.1) | 167 (71.4) | 0.84 | 145 (63.3) | 151 (66.8) | 0.49 |
| All inpatient visits | 22 (9.4) | 36 (15.4) | 0.07 | 29 (12.6) | 38 (16.7) | 0.24 |
| Inpatient: A&E visit | 16 (6.8) | 16 (6.8) | 1.00 | 10 (4.4) | 16 (7.1) | 0.23 |
| Inpatient: general medical ward | 7 (3.0) | 21 (9.0) | 0.01* | 10 (4.4) | 21 (9.3) | 0.03* |
| Inpatient: geriatric ward | 0 (0.0) | 3 (1.3) | 0.25 | 4 (1.7) | 0 (0.0) | 0.12 |
| Inpatient: surgical ward | 5 (2.1) | 8 (3.4) | 0.58 | 12 (5.2) | 12 (5.3) | 1.00 |
| All aids and adaptations | 148 (63.2) | 151 (64.5) | 0.85 | 106 (46.3) | 61 (27.0) | 0.00* |
| Adaptations | 32 (13.7) | 32 (13.7) | 1.00 | 10 (4.4) | 17 (7.5) | 0.17 |
| Equipment | 125 (53.4) | 131 (56.0) | 0.64 | 101 (44.1) | 49 (21.7) | 0.00* |
| Technological aidsb | 60 (26.5) | 59 (25.5) | 1.00 | 47 (20.5) | 9 (4.0) | 0.00* |
| Memory aid: clockb | 44 (18.8) | 45 (19.2) | 1.00 | 61 (26.6) | 2 (0.9) | 0.00* |
| Memory aid: medication reminderb | 81 (34.6) | 75 (32.1) | 0.62 | 89 (38.9) | 0 (0.0) | 0.00* |
| Service | Costed as | Unit cost (£) | Sourcea |
|---|---|---|---|
| Social care related | |||
| Home care worker | Face-to-face contact, day time: independent sector care provided for social services | 27/hour | Unit Cost of Health and Social Care 2018 181 |
| Care/case manager | Social worker: client-related work, including qualifications | 84/hour | Unit Cost of Health and Social Care 2018 (p. 139)181 |
| Social worker | Client-related work, including qualifications | 84/hour | Unit Cost of Health and Social Care 2018 181 |
| Reablement | Reablement service, face-to-face contact | 46/hour | Unit Cost of Health and Social Care 2018 181 |
| Transportation | Voluntary day care for older people vehicle and transport costs | 8.25/client day | Unit Cost of Health and Social Care 2009 226 |
| Home delivered meals | Meals on Wheels, average cost | 49.11/week | Unit Cost of Health and Social Care 2014 227 |
| Short-term respite care in residential/nursing home | Establishment cost plus personal living expenses and external services | 708/week | Unit Cost of Health and Social Care 2018 181 |
| NHS/health related | |||
| Community mental health nurse | Nurse band 6: client-related work, including qualifications | 81.50/hour | Unit Cost of Health and Social Care 2018 181 |
| Occupational therapist | Community occupational therapist, including qualifications | 47/hour | Unit Cost of Health and Social Care 2018 181 |
| Community/district nurse | Nurse band 6: client-related work, including qualifications | 81.50/hour | Unit Cost of Health and Social Care 2018 181 |
| Speech therapist | Band 6, including qualifications | 47.50/hour | Unit Cost of Health and Social Care 2018 181 |
| Outpatient | Weighted average of all outpatient attendances | 134/attendance | Unit Cost of Health and Social Care 2018 181 |
| Psychologist | Band 7 clinical psychologist | 53/hour | Unit Cost of Health and Social Care 2018 181 |
| Physiotherapist | Band 6, including qualifications | 49.40/hour | Unit Cost of Health and Social Care 2018 181 |
| Chiropodist/podiatrist | Band 6 | 46/hour | Unit Cost of Health and Social Care 2018 181 |
| Dietitian | Band 6, including qualifications | 49.50/hour | Unit Cost of Health and Social Care 2018 181 |
| Health visitor | Band 6, including qualifications | 81.50/hour | Unit Cost of Health and Social Care 2018 181 |
| Optician | Band 6 | 46/hour | Unit Cost of Health and Social Care 2018 181 |
| Dentist | NHS dentist: performer only | 133/hour | Unit Cost of Health and Social Care 2018 181 |
| GP | Including qualifications | 37/consultation | Unit Cost of Health and Social Care 2018 181 |
| General practice nurse | Including qualifications | 42/hour | Unit Cost of Health and Social Care 2018 181 |
| Community pharmacist | Band 6, including qualifications | 51.20/hour | Unit Cost of Health and Social Care 2018 181 |
| Accident and emergency (ward admission) | Emergency medicine, average cost of admitted living patient | 154/admittance | NHS Reference Costs 2017/18 225 |
| Inpatient: psychologist | Inpatient, mental health – elderly | 256/bed-day | Unit Cost of Health and Social Care 2010 228 |
| Inpatient: geriatric | Inpatient, geriatric | 221/bed-day | Unit Cost of Health and Social Care 2010 228 |
| Inpatient: surgical ward | Inpatient, general surgery | 110/bed-day | Unit Cost of Health and Social Care 2010 228 |
| Inpatient: general medicine | Inpatient, general medicine | 141/bed-day | Unit Cost of Health and Social Care 2010 228 |
| Inpatient: cardiology | Inpatient, cardiology | 119/bed-day | Unit Cost of Health and Social Care 2010 228 |
| Inpatient: rehabilitation ward | Inpatient, rehabilitation | 176/bed-day | Unit Cost of Health and Social Care 2010 228 |
| Inpatient: ICU | All adult critical care (average) | 1395/admittance | NHS Reference Costs 2017/18 225 |
| Ambulance | Emergency ambulance, routine transport ambulance (average) | 120/journey | Unit Cost of Health and Social Care 2018 181 |
| Outpatient visit | Average cost per outpatient attendance | 134/visit | Unit Cost of Health and Social Care 2018 181 |
| Third sector | |||
| Benefits advisor (e.g. Citizens Advice Bureau) | Support and outreach worker | 23/hour | Unit Cost of Health and Social Care 2018 181 |
| Drop-in centre | Health action area community programme | 37.70/client session | Unit Cost of Health and Social Care 2016 229 |
| Day-care centre (including respite day care) | Local authority day care for older people (aged ≥ 65 years) | 13/client hour | Unit Cost of Health and Social Care 2018 181 |
| Carer group | Health action area community programme | 37.70/client session | Unit Cost of Health and Social Care 2016 229 |
| Admiral Nurse | Nurse band 6, client-related work, including qualifications | 81.50/hour | Unit Cost of Health and Social Care 2018 181 |
| Dementia advice worker | Support and outreach worker | 23/hour | Unit Cost of Health and Social Care 2018 181 |
| Counsellor | Band 6, including qualifications | 51.40/hour | Unit Cost of Health and Social Care 2018 181 |
| Voluntary organisation worker (e.g. Age UK, Alzheimer’s Society) | Support and outreach worker | 23/hour | Unit Cost of Health and Social Care 2018 181 |
| Support worker | Support and outreach worker | 23/hour | Unit Cost of Health and Social Care 2018 181 |
| Aids/adaptations | |||
| Accessible shower/wet room | Major adaptation materials and installation: level-access shower | 5078 | Unit Cost of Health and Social Care 2018 181 |
| Converted room for downstairs WC/washroom | Major adaptation materials and installation: convert room downstairs for WC/washroom | 10,761 | Unit Cost of Health and Social Care 2018 181 |
| Extension for downstairs WC/washroom | Major adaptation materials and installation: build downstairs extension for WC/washroom | 24,635 | Unit Cost of Health and Social Care 2018 181 |
| Extension bedroom and bathroom | Major adaptation materials and installation: build downstairs extension for bedroom and en suite | 36,729 | Unit Cost of Health and Social Care 2018 181 |
| Shower over bath | Minor adaptation materials and installation: over bath shower | 1394 | Unit Cost of Health and Social Care 2018 181 |
| Create ramp | Minor adaptation materials and installation: ramp to front/back door | 657 | Unit Cost of Health and Social Care 2018 181 |
| Create step | Minor adaptation materials and installation: step to front/back door | 775 | Unit Cost of Health and Social Care 2018 181 |
| Stair lift | Major adaptation materials and installation: stair lift, straight | 2046 | Unit Cost of Health and Social Care 2018 181 |
| Modified doorways | Minor adaptation materials and installation: widen doorway for wheelchair access | 323 | Unit Cost of Health and Social Care 2018 181 |
| Move bed downstairs/upstairs | Minor adaptation materials and installation: move bed to downstairs room | 41 | Unit Cost of Health and Social Care 2018 181 |
| Commode | Simple aids for daily living: commode (minimum cost) | 31.49 | Unit Cost of Health and Social Care 2013 230 |
| Toilet adaptations | Simple aids for daily living: toilet frame and seat | 32.57 | Unit Cost of Health and Social Care 2013 230 |
| Handrail: bathroom | Minor adaptation material and installation: fit handrail to bath | 22.60 | Unit Cost of Health and Social Care 2018 181 |
| Handrail: internal | Minor adaptation material and installation: fit handrail internal | 33.90 | Unit Cost of Health and Social Care 2018 181 |
| Handrail: external | Minor adaptation material and installation: fit handrail internal | 23.80 | Unit Cost of Health and Social Care 2018 181 |
| Wheelchair | NHS wheelchair (attendant powered) and maintenance | 322.50 | Unit Cost of Health and Social Care 2018 181 |
| Bath step | Simple aids for daily living: bath step | 21.47 | Unit Cost of Health and Social Care 2013 230 |
| Walking aid | Simple aids for daily living: walking stick (minimum cost) | 23.89 | Unit Cost of Health and Social Care 2013 230 |
| Trolley | Simple aids for daily living: trolley | 36.92 | Unit Cost of Health and Social Care 2013 230 |
| Perch stool | Simple aids for daily living: perching stool with arms and or back | 24.97 | Unit Cost of Health and Social Care 2013 230 |
| Bath/shower seat | Simple aids for daily living: mobile shower chair | 59.72 | Unit Cost of Health and Social Care 2013 230 |
| Specialist chair | Simple aids for daily living: high-back chair/specialist chair | 131.38 | Unit Cost of Health and Social Care 2013 230 |
| Electronic/hospital bed | Variable posture bed (minimum) | 679.71 | Unit Cost of Health and Social Care 2013 230 |
| Riser recliner/electronic chair | Indiana rise and recline chair | 399.95 | Complete Care Shop235 |
| Bed rail | Economy bed grab rail | 14.95 | Complete Care Shop236 |
| Key box | Key safe | 15.49 | Complete Care Shop237 |
| Hoist: mobile | Oxford midi 180 mobile hoist | 939 | Complete Care Shop238 |
| Hoist: ceiling | Major adaptation materials and installation: stair lift, straight | 2046 | Unit Cost of Health and Social Care 2018 181 |
| Hoist: bath | Minor adaptation materials and installation: standard bath lift (minimum cost) | 329 | Unit Cost of Health and Social Care 2013 230 |
| Turner | Atlas transfer disc | 178.45 | Complete Care Shop239 |
| Medication box/reminder | Medelert automatic pill dispenser | 62.95 | Complete Care Shop240 |
| Incontinence pads/sheets | Incontinence pads 144 pack (six packs) | 113.70/6 months | Complete Care Shop241 |
| Support pillow/sheets | V-shaped pillow | 16.25 | Complete Care Shop242 |
| Air-flow mattress/blow up bed | Apollo 5 airflow mattress | 293.95 | Complete Care Shop243 |
| Pressure mattress | Essential care pressure mattress | 84.95 | Complete Care Shop244 |
| Pressure cushion/pad | Medium-risk chair pressure cushion | 31.45 | Complete Care Shop245 |
| Slide sheet | One-way slide sheet | 104.95 | Complete Care Shop246 |
| Fall mat | Crash matt | 37.95 | Complete Care Shop247 |
| Inflatable bed support | Mattress genie | 146.95 | Complete Care Shop248 |
| Mangar elk | Lifting cushions | 1148.95 | Complete Care Shop249 |
| Table | Trolley | 36.92 | Unit Cost of Health and Social Care 2013 230 |
| Scooter | Wheelchair (electric), equipment and maintenance | 1592.5 | Unit Cost of Health and Social Care 2018 181 |
| Footstool | Leg and footstool | 31.45 | Complete Care Shop250 |
| Technology | |||
| Telecare, personal alarm with networked sensors/alarms | Second-generation telecare, equipment and support | 660.16 | Unit Cost of Health and Social Care 2013 230 |
| Telecare, personal alarm without networked sensors/alarms | First-generation telecare, installation and materials | 428.04 | Unit Cost of Health and Social Care 2012 251 |
| Light sensor | Automated lights | 12.54 | Complete Care Shop252 |
| Date/time aid | Dementia day and night clock | 31.43 | Complete Care Shop253 |
| Accessible telephone | Big button telephone | 25.14 | Complete Care Shop254 |
| Tracking device | GPS locator | 70.00 | Live Better With Dementia255 |
| DESCANT intervention |
DSP: band 6 NHS practitioner (at rate of £26.63 per hour assisting for 10 hours per participant, including travel time) plus e-mail/telephone support (band 3; £12.93 per hour at 4 hours per participant); records, photocopying/transfer of documents DSP travel allowance to appointments [assumption of 24 miles per home visit (two home visits) at 56p per mile] Plus a package of memory aids at £150 per participant |
464.90 | Agreed NHS excess treatment costs with each local CRN |
| Variable | Group | Mean | SE | 95% CI |
|---|---|---|---|---|
| Total cost (£) | Comparator, TAU | 37,775 | 2323 | 33,179 to 42,372 |
| Intervention | 38,372 | 2123 | 34,191 to 42,554 | |
| QALY, EQ-5D-5L | Comparator, TAU | 0.370 | 0.008 | 0.355 to 0.385 |
| Intervention | 0.375 | 0.007 | 0.361 to 0.388 | |
| QALY carer: EQ-5D-5L | Comparator, TAU | 0.605 | 0.005 | 0.595 to 0.614 |
| Intervention | 0.608 | 0.004 | 0.599 to 0.616 | |
| QALY ICECAP-O | Comparator, TAU | 0.204 | 0.007 | 0.191 to 0.217 |
| Intervention | 0.212 | 0.007 | 0.199 to 0.226 | |
| QALY DEMQOL | Comparator, TAU | 0.430 | 0.004 | 0.422 to 0.437 |
| Intervention | 0.433 | 0.004 | 0.426 to 0.441 |
Results from the ICER analysis, based on different measures of utility (Table 20), show that the intervention was, on average, more costly but slightly more effective than TAU, necessitating consideration of ICER values against threshold values of WTP. The mean ICER for our primary analysis, using the EQ-5D-5L for the person with dementia, was £103,000 per QALY.
| Observed bootstrap | Incremental cost or QALY, over comparator | SE | z-value | Normal based | |
|---|---|---|---|---|---|
| p > z | 95% CI | ||||
| Net cost (£) | 412 | 2745 | 0 | 1 | –4969 to 5792 |
| Net QALY, EQ-5D-5L | 0.004 | 0.005 | 0.730 | 0.466 | –0.006 to 0.014 |
| Net QALY carer, EQ-5D-5L | 0.002 | 0.003 | 0.730 | 0.466 | –0.004 to 0.009 |
| Net QALY DEMQOL | 0.003 | 0.004 | 0.640 | 0.523 | –0.005 to 0.010 |
| Net QALY ICECAP-O | 0.009 | 0.006 | 1.650 | 0.100 | –0.002 to 0.020 |
The bootstrapped results, allowing for uncertainty, showed that the intervention had a mean incremental cost (over TAU) of £412 (standard error £2745, 95% CI –£496 to £5792) and mean incremental QALYs of 0.004 (standard error 0.005, 95% CI –0.006 to 0.014).
The statistical uncertainty surrounding the costs, QALYs and incremental cost per QALYs are illustrated in Figures 9 and 10 (cost-effectiveness acceptability). These results show that the intervention is likely not to be cost-effective over a range of WTP thresholds. At our £15,000 threshold, the intervention has approximately 42–44% probability of being cost-effective, depending on the QALY values generated by our different preference measures. The different measures used to generate QALYs showed little variation in terms of the probability of cost-effectiveness with only the ICECAP-O showing a marginally higher probability.
FIGURE 9.
Early-stage dementia: base-case analysis (overall) ICERs – 10,000 bootstrapped replicates.
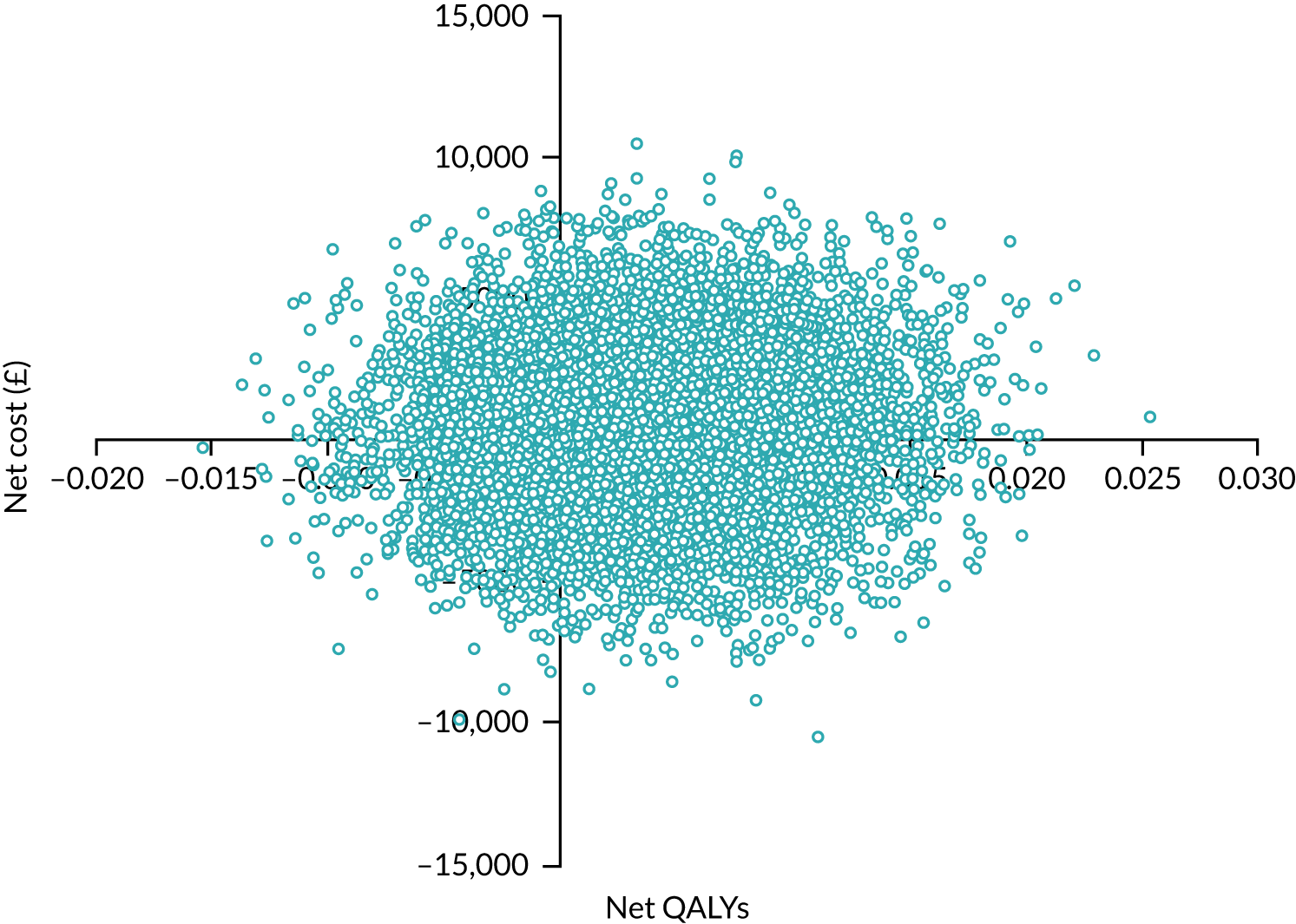
FIGURE 10.
Early-stage dementia: cost-effectiveness acceptability curves.
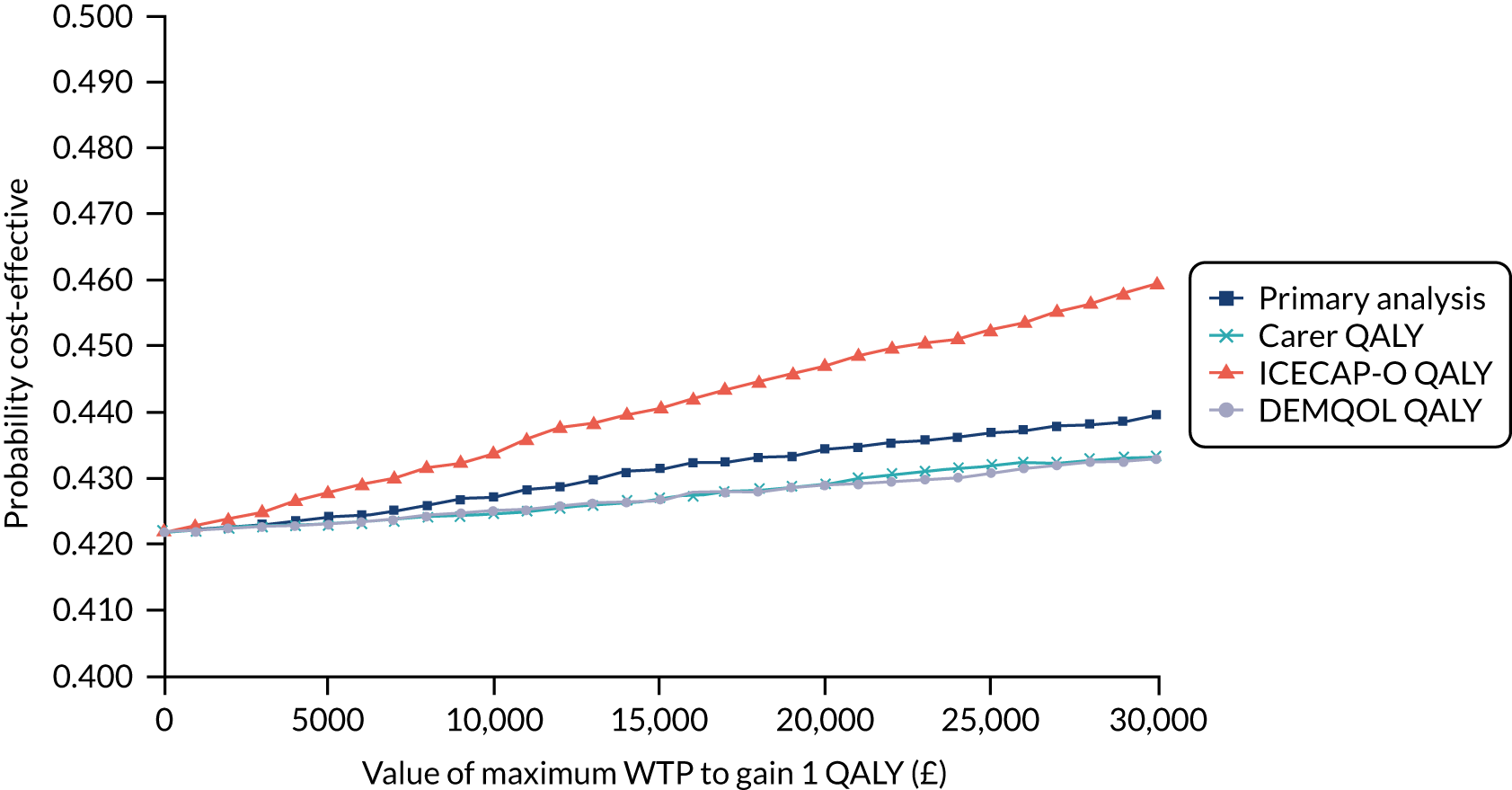
Naturally occurring care packages in later-stage dementia
The primary analyses were conducted on data from participants responding at both baseline and 6-month follow-up (n = 389), although for the EQ-5D-5L proxy there were three missing responses, meaning that data for 386 participants were analysed. Tables 21 and 22 compare resource use for each care package group.
| Service used | Baseline period | Follow-up period | ||||
|---|---|---|---|---|---|---|
| Basic (N = 154), n (%) | Intermediate (N = 268), n (%) | p-valuea | Basic (N = 125), n (%) | Intermediate (N = 196), n (%) | p-valuea | |
| Home care worker | 33 (21.4) | 153 (57.1) | 0.00* | 39 (31.2) | 118 (60.2) | 0.00* |
| Case/care manager | 9 (5.8) | 47 (17.5) | 0.00* | 10 (8.0) | 26 (13.3) | 0.20 |
| Social worker | 3 (1.9) | 75 (28.0) | 0.00* | 12 (9.6) | 48 (24.5) | 0.00* |
| Dementia advice worker | 4 (2.6) | 8 (3.0) | 1.00 | 1 (0.8) | 5 (2.6) | 0.41 |
| Support worker | 4 (2.6) | 38 (14.2) | 0.00* | 5 (4.0) | 22 (11.2) | 0.02* |
| Voluntary worker | 17 (11) | 47 (17.5) | 0.09 | 16 (12.8) | 21 (10.7) | 0.60 |
| Community mental health nurse | 22 (14.3) | 98 (36.6) | 0.00* | 20 (16.0) | 55 (28.1) | 0.02* |
| Occupational therapist | 6 (3.9) | 39 (14.6) | 0.00* | 13 (10.4) | 32 (16.3) | 0.14 |
| Admiral Nurse | 1 (0.6) | 5 (1.9) | 0.42 | 2 (1.6) | 5 (2.6) | 0.71 |
| Councillor | 2 (1.3) | 2 (0.7) | 0.63 | 2 (1.6) | 0 (0.0) | 0.15 |
| Community district nurse | 23 (14.9) | 75 (28.0) | 0.00* | 26 (20.8) | 58 (29.6) | 0.09 |
| GP | 136 (88.3) | 239 (89.2) | 0.87 | 111 (88.8) | 174 (88.8) | 1.00 |
| General practice nurse | 134 (87.0) | 224 (83.6) | 0.40 | 109 (87.2) | 166 (84.7) | 0.63 |
| Community pharmacist | 51 (33.1) | 80 (29.9) | 0.51 | 53 (42.4) | 67 (34.2) | 0.16 |
| Speech and language therapist | 1 (0.6) | 5 (1.9) | 0.42 | 4 (3.2) | 3 (1.5) | 0.44 |
| Psychologist | 7 (4.5) | 6 (2.2) | 0.24 | 6 (4.8) | 0 (0.0) | 0.00* |
| Physiotherapist | 21 (13.6) | 45 (16.8) | 0.41 | 18 (14.4) | 26 (13.3) | 0.87 |
| Dietitian | 9 (5.8) | 19 (7.1) | 0.69 | 4 (3.2) | 17 (8.7) | 0.64 |
| Health visitor | 0 (0.00) | 2 (0.7) | 0.54 | 1 (0.8) | 2 (1.0) | 1.00 |
| Chiropodist | 78 (50.6) | 144 (53.7) | 0.55 | 69 (55.2) | 107 (54.6) | 1.00 |
| Benefits adviser | 7 (4.5) | 16 (6.0) | 0.66 | 5 (4.0) | 10 (5.1) | 0.79 |
| Optician | 13 (8.4) | 11 (4.1) | 0.08 | 7 (5.6) | 5 (2.6) | 0.23 |
| Dentist | 9 (5.8) | 8 (3.0) | 0.20 | 9 (7.2) | 6 (3.1) | 0.11 |
| Meals on Wheels | 1 (0.6) | 20 (7.5) | 0.00* | 2 (1.6) | 11 (5.6) | 0.09 |
| Short-term respite care | 4 (2.6) | 0 (0.0) | 0.00* | 4 (3.2) | 32 (16.3) | 0.00* |
| Transport | 9 (5.8) | 40 (14.9) | 0.00* | 3 (2.4) | 26 (13.3) | 0.00* |
| Carer group | 67 (43.5) | 70 (26.1) | 0.00* | 50 (40.0) | 46 (23.5) | 0.00* |
| Drop-in centre | 18 (11.7) | 15 (5.6) | 0.04* | 11 (8.8) | 10 (5.1) | 0.25 |
| Day-care centre | 21 (13.6) | 119 (44.4) | 0.00* | 26 (20.8) | 74 (37.8) | 0.00* |
| All outpatient visits | 119 (77.3) | 198 (73.9) | 0.49 | 93 (74.4) | 132 (67.3) | 0.21 |
| All inpatient visits | 27 (17.5) | 74 (27.6) | 0.02* | 21 (16.8) | 41 (20.9) | 0.39 |
| Inpatient: A&E visit | 14 (9.1) | 34 (12.7) | 0.34 | 11 (8.8) | 19 (9.7) | 0.85 |
| Inpatient: general medical ward | 10 (6.5) | 33 (12.3) | 0.07 | 10 (8.0) | 24 (12.2) | 0.67 |
| Inpatient: geriatric ward | 3 (1.9) | 15 (5.6) | 0.08 | 5 (4.0) | 6 (3.1) | 0.76 |
| Inpatient: surgical ward | 5 (3.2) | 11 (4.1) | 0.79 | 3 (2.4) | 5 (2.6) | 1.00 |
| Inpatient: psychiatric ward | 0 (0.0) | 1 (0.4) | 1.00 | 1 (0.8) | 3 (1.5) | 1.00 |
| Inpatient: cardiology ward | 0 (0.0) | 10 (3.7) | 0.02* | 1 (0.8) | 3 (1.5) | 1.00 |
| Inpatient: ICU | 0 (0.0) | 2 (0.7) | 0.54 | 0 (0.0) | 2 (1.0) | 0.52 |
| Inpatient: rehabilitation | 1 (0.6) | 2 (0.7) | 1.00 | 1 (0.8) | 1 (0.5) | 1.00 |
| Ambulance | 17 (11.0) | 88 (32.8) | 0.00* | 29 (23.2) | 60 (30.6) | 0.16 |
| All aids and adaptations | 107 (69.5) | 219 (81.7) | 0.01* | 44 (35.2) | 78 (39.8) | 0.48 |
| Adaptations | 41 (26.6) | 104 (38.8) | 0.01* | 9 (7.2) | 17 (8.7) | 0.68 |
| Equipment | 98 (63.6) | 204 (76.1) | 0.01* | 40 (32.0) | 69 (35.2) | 0.63 |
| Technological aids | 35 (22.7) | 92 (34.3) | 0.02* | 12 (9.6) | 14 (7.1) | 0.53 |
| Memory aid: clock | 4 (2.6) | 12 (4.5) | 0.43 | 3 (1.9) | 2 (0.7) | 0.38 |
| Memory aid: medication reminder | 1 (0.6) | 6 (2.2) | 0.43 | 0 (0.0) | 0 (0.0) | |
| Service used | Baseline period | Follow-up period | ||||
|---|---|---|---|---|---|---|
| Basic (N = 154), n (%) | Advanced (N = 96), n (%) | p-valuea | Basic (N = 125), n (%) | Advanced (N = 68), n (%) | p-valuea | |
| Home care worker | 33 (21.4) | 81 (84.4) | 0.00* | 39 (31.2) | 47 (69.1) | 0.00* |
| Case/care manager | 9 (5.8) | 27 (28.1) | 0.00* | 10 (8.0) | 15 (22.1) | 0.01* |
| Social worker | 3 (1.9) | 64 (66.7) | 0.00* | 12 (9.6) | 21 (30.9) | 0.00* |
| Dementia advice worker | 4 (2.6) | 7 (7.3) | 0.11 | 1 (0.8) | 1 (1.5) | 1.00 |
| Support worker | 4 (2.6) | 19 (19.8) | 0.00* | 5 (4.0) | 11 (16.2) | 0.01* |
| Voluntary worker | 17 (11) | 32 (33.3) | 0.00* | 16 (12.8) | 11 (16.2) | 0.52 |
| Community mental health nurse | 22 (14.3) | 58 (60.4) | 0.00* | 20 (16.0) | 18 (26.5) | 0.09 |
| Occupational therapist | 6 (3.9) | 46 (47.9) | 0.00* | 13 (10.4) | 11 (16.2) | 0.26 |
| Admiral Nurse | 1 (0.6) | 9 (9.4) | 0.00* | 2 (1.6) | 6 (8.8) | 0.02* |
| Councillor | 2 (1.3) | 4 (4.2) | 0.21 | 2 (1.6) | 3 (4.4) | 0.35 |
| Community district nurse | 23 (14.9) | 27 (28.1) | 0.02* | 26 (20.8) | 17 (25.0) | 0.59 |
| GP | 136 (88.3) | 84 (87.5) | 0.84 | 111 (88.8) | 59 (86.8) | 0.65 |
| General practice nurse | 134 (87.0) | 80 (83.3) | 0.46 | 109 (87.2) | 56 (82.4) | 0.40 |
| Community pharmacist | 51 (33.1) | 33 (34.4) | 0.89 | 53 (42.4) | 27 (39.7) | 0.76 |
| Speech and language therapist | 1 (0.6) | 2 (2.1) | 0.56 | 4 (3.2) | 1 (1.5) | 0.66 |
| Psychologist | 7 (4.5) | 2 (2.1) | 0.49 | 6 (4.8) | 3 (4.4) | 1.00 |
| Physiotherapist | 21 (13.6) | 23 (24.0) | 0.04* | 18 (14.4) | 16 (23.5) | 0.12 |
| Dietitian | 9 (5.8) | 9 (9.4) | 0.32 | 4 (3.2) | 4 (5.9) | 0.46 |
| Health visitor | 0 (0.0) | 1 (1.0) | 0.38 | 1 (0.8) | 0 (0.0) | 1.00 |
| Chiropodist | 78 (50.6) | 45 (46.9) | 0.60 | 69 (55.2) | 33 (48.5) | 0.45 |
| Benefits adviser | 7 (4.5) | 12 (12.5) | 0.03* | 5 (4.0) | 4 (5.9) | 0.72 |
| Optician | 13 (8.4) | 6 (6.3) | 0.63 | 7 (5.6) | 5 (7.4) | 0.76 |
| Dentist | 9 (5.8) | 1 (1.0) | 0.09 | 9 (7.2) | 4 (5.9) | 1.00 |
| Meals on Wheels | 1 (0.6) | 11 (11.5) | 0.00* | 2 (1.6) | 7 (10.3) | 0.01* |
| Short-term respite care | 4 (2.6) | 32 (33.3) | 0.00* | 4 (3.2) | 14 (20.6) | 0.00* |
| Transport | 9 (5.8) | 16 (16.7) | 0.01* | 3 (2.4) | 12 (17.6) | 0.00* |
| Carer group | 67 (43.5) | 21 (21.9) | 0.01* | 50 (40.0) | 15 (22.1) | 0.02* |
| Drop-in centre | 18 (11.7) | 3 (3.1) | 0.02* | 11 (8.8) | 4 (5.9) | 0.58 |
| Day-care centre | 21 (13.6) | 60 (62.5) | 0.00* | 26 (20.8) | 33 (48.5) | 0.00* |
| All outpatient visits | 119 (77.3) | 70 (72.9) | 0.45 | 93 (74.4) | 46 (67.6) | 0.32 |
| All inpatient visits | 27 (17.5) | 31 (32.3) | 0.01* | 21 (16.8) | 24 (35.3) | 0.01* |
| Inpatient: A&E visit | 15 (9.7) | 17 (17.7) | 0.08 | 11 (8.8) | 14 (20.6) | 0.03* |
| Inpatient: general medical ward | 10 (6.5) | 17 (17.7) | 0.01* | 10 (8.0) | 13 (19.1) | 0.04* |
| Inpatient: geriatric ward | 3 (1.9) | 1 (1.0) | 1.00 | 5 (4.0) | 2 (2.9) | 1.00 |
| Inpatient: surgical ward | 5 (3.2) | 3 (3.1) | 1.00 | 3 (2.4) | 0 (0.0) | 0.55 |
| Inpatient: psychiatric ward | 0 (0.0) | 2 (2.1) | 0.15 | 1 (0.8) | 3 (4.4) | 0.13 |
| Inpatient: cardiology ward | 0 (0.0) | 1 (1.0) | 0.38 | 1 (0.8) | 1 (1.5) | 1.00 |
| Inpatient: ICU | 0 (0.0) | 2 (2.1) | 0.15 | 0 (0.0) | 0 (0.0) | |
| Inpatient: rehabilitation | 1 (0.6) | 2 (2.1) | 0.56 | 1 (0.8) | 1 (1.5) | 1.00 |
| Ambulance | 17 (11.0) | 37 (38.5) | 0.00* | 29 (23.2) | 30 (44.1) | 0.00* |
| All aids and adaptations | 107 (69.5) | 78 (81.3) | 0.05 | 44 (35.2) | 29 (42.6) | 0.35 |
| Adaptations | 41 (26.6) | 26 (27.1) | 1.00 | 9 (7.2) | 7 (10.3) | 0.59 |
| Equipment | 98 (63.6) | 73 (76.0) | 0.05* | 40 (32.0) | 23 (33.8) | 0.87 |
| Technological aids | 35 (22.7) | 38 (39.6) | 0.01* | 12 (9.6) | 7 (10.3) | 1.00 |
| Memory aid: clock | 4 (2.6) | 1 (1.0) | 0.65 | 3 (1.9) | 0 (0.0) | 0.55 |
| Memory aid: medication reminder | 1 (0.6) | 2 (2.1) | 0.56 | 0 (0.0) | 0 (0.0) | |
Tables 23 and 24 show differences between care package groups for average costs per participant (carer–person with dementia dyad) from different perspectives.
| Cost item | Basic intensity (none or one service) | Intermediate intensity (two or three services) | Advanced intensity (four or more services) | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | n | Mean (SD) (£) | |
| Aids and adaptations | 125 | 17 (108) | 194 | 22 (117) | 67 | 22 (132) |
| Community mental health nurse | 125 | 74 (418) | 194 | 106 (498) | 67 | 54 (136) |
| Occupational therapist | 125 | 6 (20) | 194 | 10 (32) | 67 | 26 (81) |
| Community district nurse | 125 | 61 (242) | 194 | 239 (1581) | 67 | 149 (586) |
| Speech and language therapist | 125 | 5 (37) | 194 | 1 (6) | 67 | 0 (1) |
| GP | 125 | 332 (164) | 194 | 322 (162) | 67 | 347 (174) |
| General practice nurse | 125 | 203 (104) | 194 | 205 (102) | 67 | 200 (109) |
| Pharmacy | 125 | 35 (44) | 194 | 28 (41) | 67 | 34 (45) |
| Psychologist | 125 | 3 (16) | 194 | 0 (0) | 67 | 2 (11) |
| Physiotherapist | 125 | 7 (17) | 194 | 6 (16) | 67 | 12 (21) |
| Dietitian | 125 | 2 (9) | 194 | 5 (15) | 67 | 3 (12) |
| Health visitor | 125 | 1 (7) | 194 | 1 (13) | 67 | 0 (0) |
| Chiropodist | 125 | 32 (32) | 194 | 32 (33) | 67 | 30 (34) |
| Optician | 125 | 3 (15) | 194 | 1 (9) | 67 | 5 (18) |
| Dentist | 125 | 14 (53) | 194 | 5 (28) | 67 | 10 (42) |
| All outpatient visits | 125 | 328 (437) | 194 | 323 (766) | 67 | 276 (290) |
| A&E admittance | 125 | 23 (88) | 194 | 21 (75) | 67 | 37 (85) |
| ICU inpatient stay | 125 | 0 (0) | 194 | 14 (141) | 67 | 0 (0) |
| Inpatient general medical ward | 125 | 96 (387) | 194 | 412 (1831) | 67 | 278 (861) |
| Psychiatric inpatient stay | 125 | 43 (481) | 194 | 96 (845) | 67 | 928 (4365) |
| Geriatric inpatient stay | 125 | 253 (1409) | 194 | 133 (906) | 67 | 231 (1709) |
| Surgical inpatient stay | 125 | 33 (289) | 194 | 15 (128) | 67 | 0 (0) |
| Cardiology inpatient stay | 125 | 8 (85) | 194 | 21 (242) | 67 | 9 (73) |
| Rehab inpatient stay | 125 | 14 (157) | 194 | 19 (265) | 67 | 8 (65) |
| Total inpatient | 125 | 499 (1871) | 194 | 775 (2317) | 67 | 1583 (4673) |
| Ambulance (emergency and routine) | 125 | 42 (93) | 194 | 88 (195) | 67 | 116 (182) |
| Total NHS | 125 | 1501 (2115) | 194 | 2000 (3104) | 67 | 2708 (4644) |
| Cost item | Basic intensity (none or one service) | Intermediate intensity (two or three services) | Advanced intensity (four or more services) | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) (£) | n | Mean (SD) (£) | n | Mean (SD) (£) | |
| Social care perspective | ||||||
| Equipment | 125 | 113 (646) | 194 | 98 (453) | 67 | 234 (885) |
| Home care | 125 | 1829 (4287) | 194 | 5488 (10,715) | 67 | 5437 (9840) |
| Support worker | 125 | 67 (559) | 194 | 96 (500) | 67 | 253 (879) |
| Case manager | 125 | 6 (25) | 194 | 33 (197) | 67 | 42 (138) |
| Social worker | 125 | 19 (79) | 194 | 45 (149) | 67 | 51 (101) |
| Day care | 125 | 3 (6) | 194 | 5 (6) | 67 | 7 (7) |
| Meals | 125 | 2 (17) | 194 | 31 (216) | 67 | 111 (572) |
| Respite care | 125 | 16 (125) | 194 | 302 (1241) | 67 | 412 (1061) |
| Transport | 125 | 12 (125) | 194 | 91 (318) | 67 | 107 (363) |
| Total social care | 125 | 2067 (4352) | 194 | 6190 (10,837) | 67 | 6653 (10,165) |
| Third-sector perspective | ||||||
| Equipment | 125 | 0 (4) | 194 | 0 (3) | 67 | 50 (270) |
| Admiral Nurse | 125 | 7 (62) | 194 | 3 (26) | 67 | 18 (85) |
| Dementia advice worker | 125 | 0 (1) | 194 | 5 (60) | 67 | 1 (11) |
| Councillor | 125 | 4 (46) | 194 | 0 (0) | 67 | 16 (85) |
| Voluntary worker | 125 | 63 (349) | 194 | 79 (780) | 67 | 83 (295) |
| Benefits adviser | 125 | 1 (7) | 194 | 1 (5) | 67 | 2 (9) |
| Carer group | 125 | 739 (1159) | 194 | 362 (1023) | 67 | 404 (1194) |
| Drop in | 125 | 111 (450) | 194 | 47 (285) | 67 | 9 (57) |
| Total third sector | 125 | 927 (1281) | 194 | 498 (1335) | 67 | 584 (1353) |
| Informal carer perspective | ||||||
| Informal carer time | 125 | 62,772 (35,401) | 194 | 65,802 (35,508) | 67 | 60,903 (37,998) |
| Equipment | 125 | 615 (3236) | 194 | 512 (2915) | 67 | 589 (3076) |
| Total informal care | 125 | 63,388 (35,559) | 194 | 66,315 (35,823) | 67 | 61,492 (38,239) |
| Total all costs (societal) | 125 | 67,882 (36,258) | 194 | 75,003 (37,664) | 67 | 71,438 (39,479) |
Results from the ICER analysis, based on the EQ-5D-5L (Table 25), show that, overall, both intermediate and advanced care packages were, on average, more costly and less effective than basic care. From this perspective, both approaches were dominated by basic intensity care. This pattern was similar for social care.
| Perspective | Incremental costs (£) | Incremental effects (QALYs) | ICER (£/QALY) | Probability of approach being cost-effective for different threshold values of society’s WTP for a QALY | ||
|---|---|---|---|---|---|---|
| £0 | £15,000 | £30,000 | ||||
| Overall societal | ||||||
| Intermediate care package | 7121 | –0.01 | –712,100 | 0.04 | 0.04 | 0.03 |
| Advanced care package | 3556 | –0.05 | –71,120 | 0.26 | 0.24 | 0.19 |
| NHS | ||||||
| Intermediate care package | 500 | –0.01 | –50,000 | 0.04 | 0.02 | 0.02 |
| Advanced care package | 1208 | –0.05 | –24,160 | 0.01 | 0.00 | 0.00 |
| Social care | ||||||
| Intermediate care package | 4123 | –0.01 | –412,300 | 0.00 | 0.00 | 0.00 |
| Advanced care package | 4586 | –0.05 | –91,700 | 0.00 | 0.00 | 0.00 |
| Third sector | ||||||
| Intermediate care package | –428 | –0.01 | 42,800 | 1.00 | 0.82 | 0.50 |
| Advanced care package | –342 | –0.05 | 6560 | 0.96 | 0.09 | 0.05 |
| Informal carer | ||||||
| Intermediate care package | 2927 | –0.01 | –292,700 | 0.24 | 0.23 | 0.21 |
| Advanced care package | –1895 | –0.05 | 37,900 | 0.62 | 0.59 | 0.52 |
However, from a third-sector perspective, both intermediate and advanced intensity packages were less costly but less effective than basic care. Incremental cost per QALY gain for the intermediate package was £42,800 and for the advanced package it was £6560. The results from the informal carers’ perspective were mixed. Intermediate care packages were, on average, more costly and less effective (dominated), but advanced packages mirrored those of the third sector (i.e. they were less costly but also less effective).
The statistical uncertainty surrounding these estimates is illustrated in Figure 11 (bootstrapped analyses). From an overall perspective, most iterations are in the north-west quadrant (i.e. care packages are likely to be more expensive but less effective than basic care). Therefore, both intermediate and advanced care packages are unlikely to be cost-effective over a range of WTP thresholds (at our £15,000 threshold, intermediate care and advanced care packages have approximately 5% and 25% probability of being cost-effective, respectively).
FIGURE 11.
Later-stage dementia care packages: primary analysis (overall) ICERs – 10,000 bootstrapped replicates. (a) Intermediate vs. basic care; and (b) advanced vs. basic care.
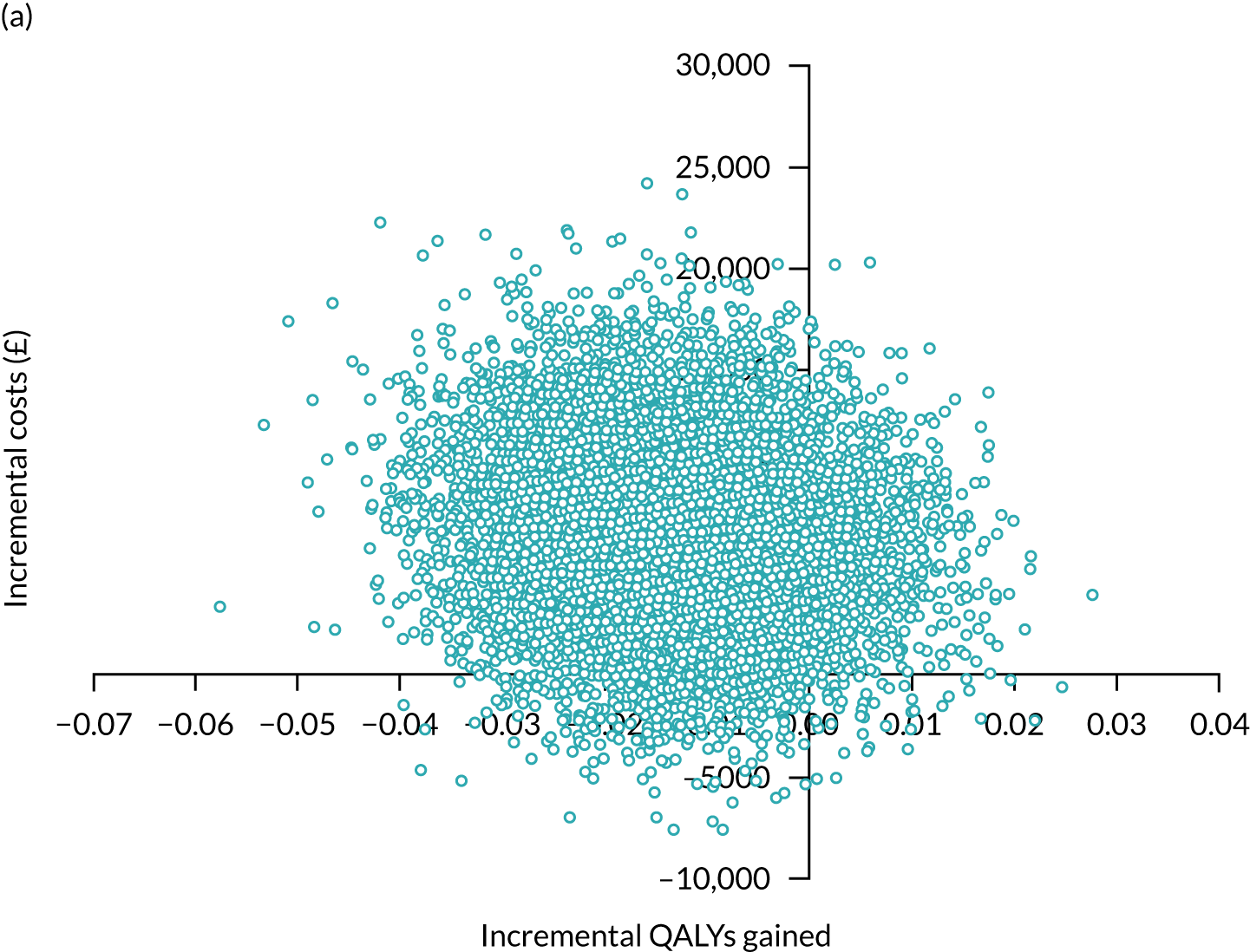
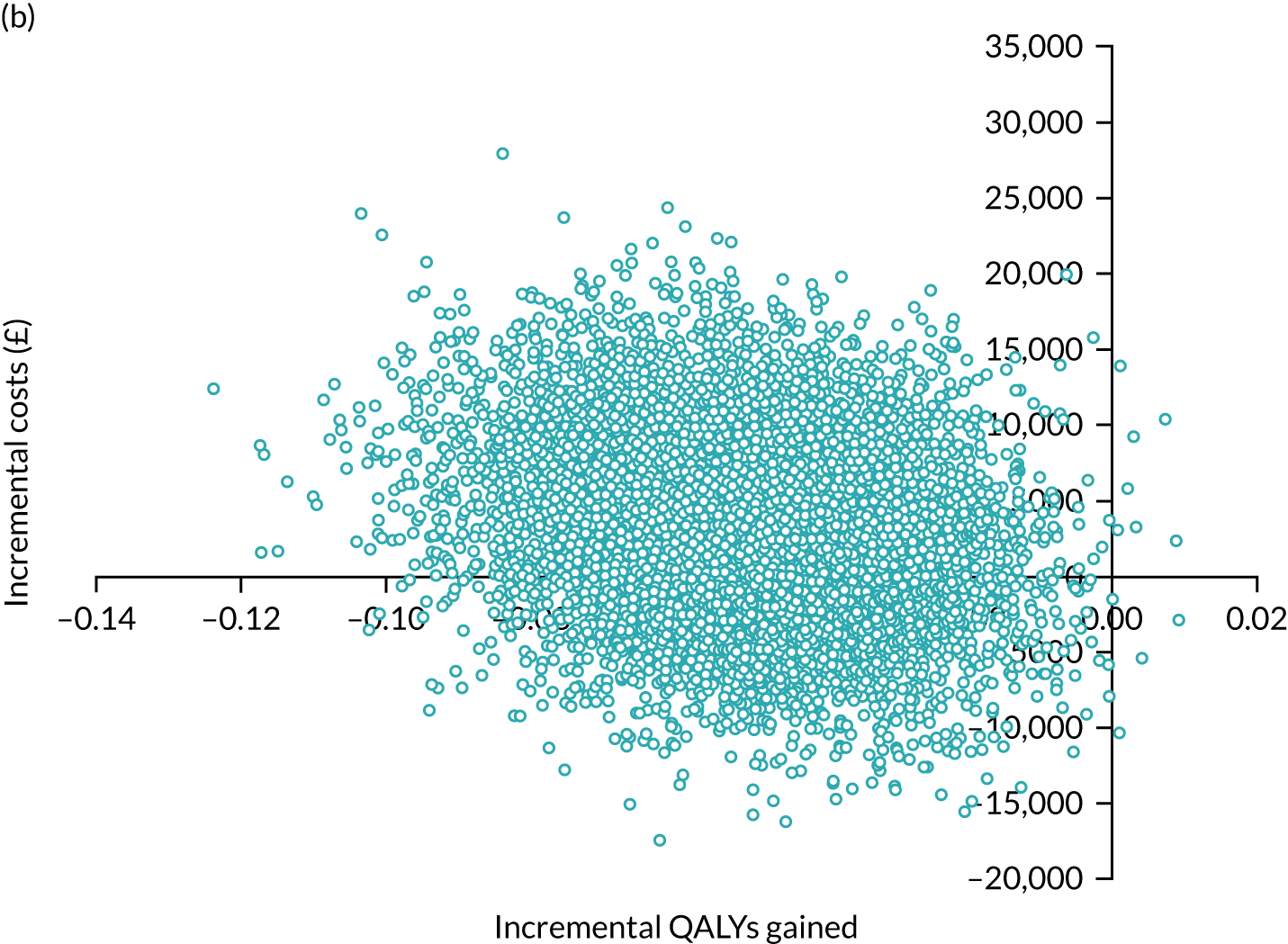
From a third-sector perspective, cost-effectiveness reduced at higher thresholds for both packages. However, for intermediate care, the probability of cost-effectiveness was 84% at our £15,000 WTP threshold (Figure 12). For informal care, advanced care had a 59% probability of being cost-effective.
FIGURE 12.
Intermediate care package: third-sector perspective. (a) ICERs; and (b) cost-effectiveness acceptability curve.
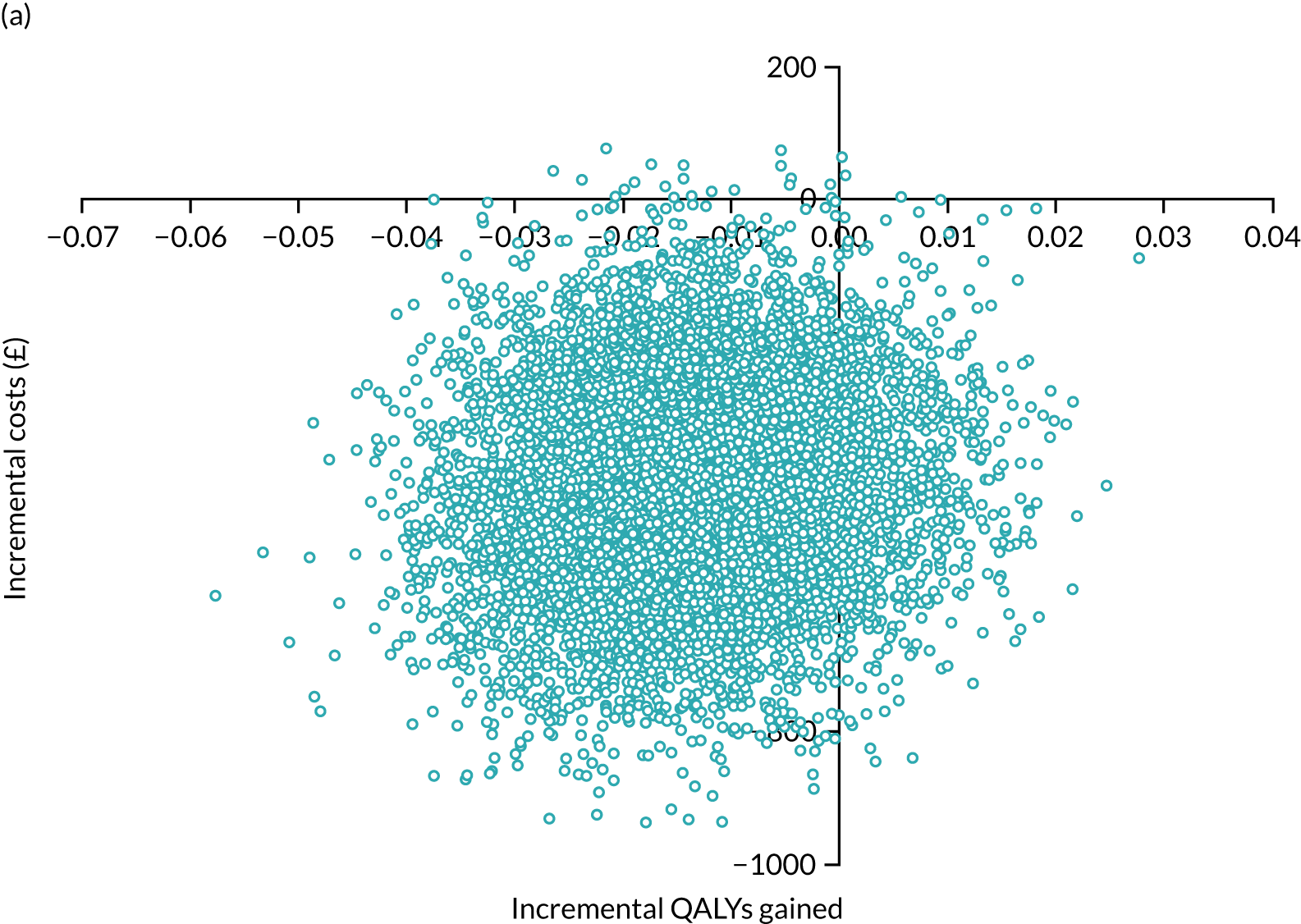
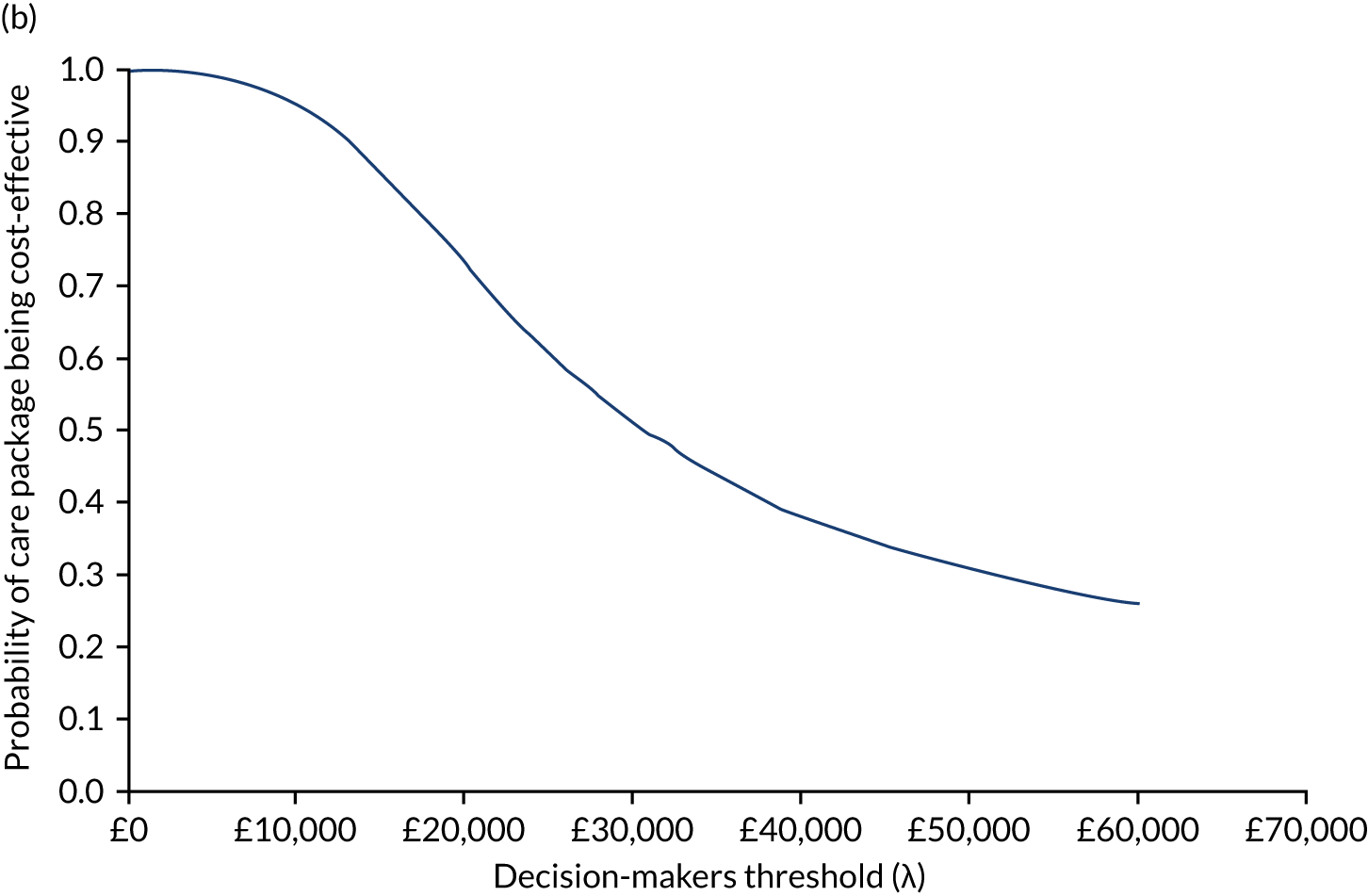
In sensitivity analyses, using DEMQOL-generated utility values did not change the position of either care package on the cost-effectiveness plane or their probability of cost-effectiveness.
Conclusions
In early-stage dementia, our intervention of provision and guidance with memory aids is likely not to be cost-effective. It was modestly more costly but slightly more effective than TAU. In later-stage dementia, more intensive care packages are less likely to be cost-effective, as they were more costly and less effective than basic care. However, from a third-sector perspective, intermediate intensity packages were cheaper but less effective.
Appendix 12 Patient, public and carer involvement
Giebel et al. (2019)
Giebel C, Roe B, Hodgson A, Britt D, Clarkson P, Members of the HoSt-D (Home Support in Dementia) Programme Management Group. Effective public involvement in the HoST-D Programme for dementia home care support: from proposal and design to methods of data collection (innovative practice). Dementia 2019;18:3173–86. https://doi.org/10.1177/1471301216687698
List of abbreviations
- ADL
- activities of daily living
- BADLS
- Bristol Activities of Daily Living Scale
- CI
- confidence interval
- CMHT
- Community Mental Health Team
- CRN
- Clinical Research Network
- CSRI
- Client Service Receipt Inventory
- DCE
- discrete choice experiment
- DEMQOL
- Dementia Quality of Life
- DESCANT
- Dementia Early Stage Cognitive Aids New Trial
- DMEC
- Data Monitoring and Ethics Committee
- DSP
- dementia support practitioner
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GHQ-12
- General Health Questionnaire-12 items
- ICECAP-O
- ICEpop CAPability measure for Older people
- ICER
- incremental cost-effectiveness ratio
- LAP
- Lay Advisory Panel
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PPCI
- patient, public and carer involvement
- QALY
- quality-adjusted life-year
- RUD
- Resource Utilisation in Dementia questionnaire
- SAE
- serious adverse event
- SD
- standard deviation
- SITE
- support, information, therapy or education
- S-MMSE
- Standardised Mini Mental State Examination
- SSCQ
- Short Sense of Competence Questionnaire
- TAU
- treatment as usual
- TIDE
- Together in Dementia Everyday
- WTP
- willingness to pay
