Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1212-20015. The contractual start date was in March 2015. The final report began editorial review in February 2020 and was accepted for publication in December 2020. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Wildman et al. This work was produced by Wildman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Wildman et al.
SYNOPSIS
Background
The clinical problem
Cystic fibrosis (CF) is the commonest inherited, life-limiting disease in white populations. In 2018, 222 new cases of CF were diagnosed, totalling 10,509 people with cystic fibrosis (PWCF) in the UK, with a median age at diagnosis of 2 months. 1 Although CF is a multisystem disorder, the upper and lower airways and digestive system are more commonly affected. 2,3 Gene mutations lead to clinical manifestation of the disease whereby secretions in the lungs become congealed and sticky. 4 In > 80% of cases, PWCF experience respiratory failure due to lung damage. 5 In the UK, the median predicted survival for a child born with CF is currently 47.3 years. 1
Cystic fibrosis management
Preventative medications both preserve lung function and reduce pulmonary exacerbations. 6–12 Typical treatments for CF target airway clearance, reduction in bacterial infection and inflammation. Patients self-manage their disease through a complex, time-consuming daily regimen of treatments that include inhaled therapies delivered via a nebuliser. 13,14 As with other long-term conditions, adherence levels to medication are low. Estimates by the World Health Organization suggest that 30–50% of prescribed medicines are not taken as intended. 15 In CF, observed objective adherence rates to nebulised therapies are 36% in adults16 and 67% in children,17 despite self-reported rates of adherence of 80%. 16 Treatments work only if they are taken; poor adherence predicts periods of exacerbation requiring intravenous antibiotics (IVAB). 18,19 Exacerbations carry both the burden of systemic side effects and significant mortality;20,21 frequent exacerbations are linked with accelerated decline in lung function and increased 3-year risk of lung transplant or death. 22
The cost of non-adherence
The cost of pulmonary exacerbations is high. The total UK spend in 2012 for CF was estimated to be £100 million, £30 million of which was spent on inhaled antibiotics and mucolytics (Paul McManus, Lead Pharmacist, South Yorkshire and Bassetlaw Area Team, NHS England, 2015, personal communication). In the UK, CF patients received 171,907 days of IVAB, with 93,455 days occurring in hospital, at an estimated cost of £27 million (Stephanie McNeil, Chief Statistician, UK Cystic Fibrosis Registry, 2016, personal communication). Medicines possession ratio (MPR) data suggests that PWCF with good adherence (MPR > 80%) have lower health-care costs than PWCF with poor adherence (MPR < 50%). Hospital admissions for IVAB are responsible for most of the excess costs. 23,24 At the heart of enabling preventative therapy is the task of making adherence visible and allowing this key metric to be available to both PWCF and their clinical teams to move from rescue to prevention.
Potential for cost savings
In the early stages of the programme grant reported here, Tappenden et al. 24 conducted an early, model-based cost–utility analysis in adults with CF with chronic Pseudomonas aeruginosa infection in a project funded separately from the programme grant. Therapies included nebulised or dry powder inhaled antibiotics prescribed by the NHS and Personal Social Services (PSS) over a lifetime horizon. The analysis suggested that an intervention such as ‘CFHealthHub’ had the potential to generate cost savings.
Aims
We developed a complex intervention with several components25 to support adherence to medication for PWCF, in line with identified research priorities. 26,27
Objectives
Our specific objectives map to three overarching work packages (WPs):
-
to develop a mechanism for objective measurement of adherence through (1) development of a data-capture and transfer infrastructure that could collect time- and date-stamped data and (2) display this data both on a CFHealthHub website, for use by both PWCF and their clinicians, and on a CFHealthHub mobile application (app), for PWCF, and (3) develop the CFHealthHub web interface with patients and clinicians
-
to develop an evidence-based behaviour change intervention (BCI) to increase adherence to nebulised CF medications that works synonymously with the CFHealthHub digital platform
-
to evaluate the CFHealthHub intervention (digital platform plus BCI) in terms of (1) clinical effectiveness, (2) acceptability and (3) cost-effectiveness and to examine the processes that drive these outcomes.
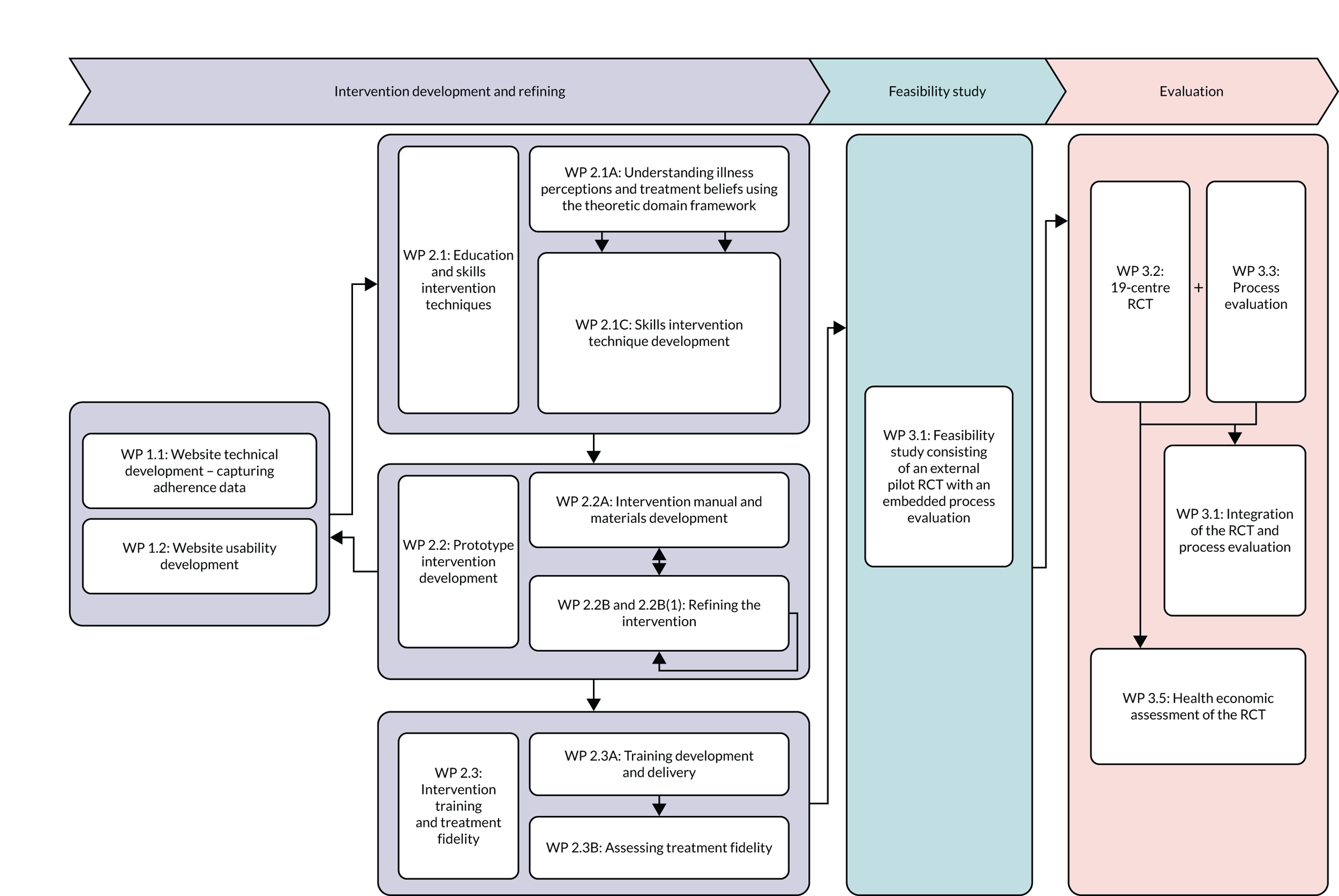
These objectives were met through three WPs from intervention development to evaluation, details of which are outlined in Figure 1.
FIGURE 1.
Work packages in the research programme. RCT, randomised controlled trial.
Key changes to the original programme
Interventionists
We adapted our initial plans to employ physiotherapists as interventionists because, early in the programme, CF clinicians identified that it was challenging to recruit physiotherapists because of workforce shortages. Intervention training was adapted to ensure that a range of health-care professionals could deliver the intervention competently through the use of tests and certification. Consequently, any member of the multidisciplinary team (MDT) could be trained to deliver the CFHealthHub intervention. Additional funding from NHS England allowed us to increase the amount of interventionist time from 0.8 to 1.0 whole time equivalent at randomised controlled trial (RCT) sites in England.
Primary outcome
The definition of the primary outcome – number of exacerbations – was changed from the standard Fuchs criteria (IVAB plus 4/10 symptoms)28 to the modified Fuchs criteria (IVAB plus 1/10 symptoms) to increase the sensitivity of the primary outcome, in line with other, recent CF research. 29
Number of centres and treatment costs
As a way of paying treatment costs, the NHS England patient activation quality improvement scheme authorised the CF Commissioning for Quality and Innovation (CQUIN). The CQUIN was offered to all 26 adult CF centres in the UK, adoption of which was a means of engaging with quality improvement by delivering the full-scale RCT in our programme. A total of 19 sites participated in the RCT, nine of which were funded by the CQUIN and 10 by a combination of local and central Department of Health and Social Care commissioning.
Fidelity
Fidelity checklists together with audio-recording sessions were used to assess treatment fidelity. CFHealthHub also records exactly what intervention components have been prescribed at each session, and the extent of participants’ independent engagement with the intervention. Interventionists were trained to audio-record all intervention delivery sessions of which a purposive sample were assessed based on the type and time point (phase) of the session administered. Data were analysed and rated for fidelity by two independent assessors for inter-rater reliability.
Changes to work packages
The WPs completed are shown in Figure 1. Changes to WPs included:
-
Combining distinct WPs together – WP 2.1B was completed as part of integrating the findings of WP 2.1A with WP 1 to develop the CFHealthHub portal.
-
Creating a smaller, distinct WP [WP 2.2B(1)] within WP 2.2B to allow us to recruit an initial set of participants to start refining the intervention.
Patient and public involvement
Active involvement of the patient and public involvement (PPI) panel was integral to the design and implementation of key aspects of the ACtiF (Development and evaluation of an intervention to support Adherence to treatment in adults with Cystic Fibrosis) programme. PPI was embedded in the early intervention development phase, the feasibility and RCT phases and the dissemination of the programme findings. As outlined by Shippee et al. ,30 patient and service user involvement was considered in each of the three phases of our research: preparatory, execution and translational.
Specific objectives were to:
-
seek iterative cycles of advice and feedback from PWCF and the wider clinical community during the development of the CFHealthHub intervention, including from our PPI panel
-
receive guidance on key research procedures and materials used in the feasibility study and full-scale RCT
-
contribute to discussions on a dissemination strategy, including the most effective methods of communicating programme findings to the CF community.
Service user initiation
Dan Beever, a co-applicant on the ACtiF programme, is a PWCF and was the PPI panel chairperson throughout the programme. Dan Beever was involved in providing patient input to research design at the bid stage. The remaining panel consisted of five PWCF and two parents of PWCF. The initial PPI panel consisted of PWCF members from the Sheffield CF centre who were involved during the intervention development phase. To establish a more diverse panel, membership was extended to PWCF from across the country. A leaflet campaign was used to recruit patients, carers and other people with an interest in CF through sites involved in WP 2.1C. In addition, the opportunity was advertised on the People in Research website (URL: www.peopleinresearch.org/; accessed 12 May 2021), and contact made with some individuals known to Dan Beever. Roles and responsibilities of the group were discussed at the first meeting. During the course of the programme, the study manager provided regular updates on programme progress to allow co-learning30 and maintain engagement.
Development phase (work packages 1.1 and 2.2)
A number of frameworks and approaches that were used during the intervention development phase necessitated input from the PPI panel. Use of the agile software development approach required iterative cycles of development and feedback from both the research team and the PPI panel. This included giving feedback on the proposed input of the educational content, the CFHealthHub landing page and demo versions of the CFHealthHub website and giving advice on practical issues of sharing data on the platform. The PPI panel ensured that the CFHealthHub user guide was accessible and coherent. Frameworks to guide decision-making and prioritisation of work implementation in this process required PPI input to understand which tasks were the most important and relevant to PWCF.
Our PPI panel also contributed to discussions on the themes identified in the qualitative research conducted in WP 2.1 to provide context and additional insights. Feedback on the content and nature of PPI meetings was continuously sought from the panel. Alterations were made to agenda items to include agenda items that the panel wished to discuss, for example ongoing progress.
Feasibility and randomised controlled trial phases (work packages 3.1 and 3.2)
The PPI panel advised on research procedures, in preparation for both the feasibility study and the full-scale RCT. This included input on the best way to contact participants to arrange consent visits and collect prescription data as well as discussions on the most appropriate terminology and communicative approaches to use during intervention sessions and on the CFHealthHub digital platform. The panel advised and provided input on the essential trial documents and their administration with participants. One important element was testing the participant-administered questionnaires, with feedback on completion times and appropriateness of both questionnaire and text message wordings to participants.
Dissemination phase
The PPI panel advised the research team on dissemination strategies across the clinical community and participants. Co-learning facilitated sharing knowledge in a multidirectional way between the study management team and the PPI panel. The RCT findings were presented to the PPI panel members on the Trial Steering Committee (TSC), who provided input on the way in which these findings should be presented to PWCF. One of the PPI panel members with expertise in managing communications assisted with the development of a formal dissemination strategy for feedback of results to multiple stakeholders in multiple ways. The PPI panel agreed the dissemination plan.
Challenges and successes
We had regular contact with the PPI panel but input was limited by the inability of the group to attend face-to-face meetings because of infection control requirements. Visual conferencing facilities were a challenge to use in practice and this was not pursued owing to technical issues. Recruitment of the PPI panel was a challenge but we were able to expand recruitment from the initial Sheffield CF centre to several other CF centres through our leaflet campaign. Involvement was much more frequent in the earlier intervention development phase than during the evaluation phase. Two members of the PPI panel also became members of the TSC and, as such, were able to provide oversight to the programme as a whole.
One of the key successes of our PPI panel was their continued input and engagement, which was sustained throughout the duration of the programme. This is in part a result of having a patient co-applicant on the ACtiF grant who acted as the PPI panel chairperson. Dan Beever’s contributions were of particular value in the recruitment of the PPI panel, as well as in maintaining the interest and engagement of the panel throughout the duration of the programme. A short survey was distributed to members of the panel at the end of the RCT phase to seek their views on their involvement during the course of programme. Three completed responses were received, all reflecting positively on involvement, particularly in terms of contributions being valued by the research team.
Specific impacts
-
When PPI panel members used the prototype website they found it difficult to locate appropriate patient videos, so suggested that these be tagged with labels for ease of use (change recommended 14 September 2016). This change was made to the intervention.
-
We asked for advice from the panel around contacting potential RCT participants to arrange consent visits. The group recommended sending a text message to the potential participant the day before the planned telephone contact to prepare them, followed by a telephone call the next day, leaving a voicemail message if there was no answer (discussions held 23 June 2017 and 2 October 2017). This approach was used.
-
A guide was produced for people using the intervention. The panel recommended a number of changes, for example referring to it as a ‘guide’ rather than as a ‘how to guide’ and being clear that the ‘problem-solving’ related to solving problems with adherence and not with using the intervention (changes recommended 13 November 2017). These changes were made to documentation.
Work package 1: developing the technology and infrastructure to collect adherence data
A digital platform – CFHealthHub – was created to provide the technical components of the complex intervention to support adherence to treatment in PWCF. CFHealthHub provided the front-end user-facing components of the intervention (a website and mobile app) and the back-end server infrastructure for secure receipt and storage of adherence data. The technical development was defined by two high-level phases: (1) technical scoping and configuration of the infrastructure and (2) development of the digital components of the BCI.
The digital platform was developed iteratively over an 18-month period, with the first phase conducted with 27 participants from August 2015 to April 2016, followed by a two-centre feasibility study conducted with 64 participants across two CF centres in 2016–2017. The digital platform was finalised and became feature complete for the full-scale RCT launch in October 2017. The first phase of the technical development involved high-level requirements capture for the project. The top priority identified was to enable the display of objective adherence data to the interventionists and PWCF through the CFHealthHub website. Discussions with the nebuliser supplier led to the configuration of a secure data-transfer mechanism, enabling nebuliser devices to submit data in real-time to the study server, hosted at the University of Manchester (Manchester, UK). To display the adherence data in CFHealthHub it was necessary to develop a manual entry point for the prescription information through the CFHealthHub website. The website was developed to display the objective adherence data in a variety of graphical and tabular formats.
After the website was designed to display the objective adherence data, an intensive co-design process was launched to refine the display of data and to incorporate, develop and refine the BCI components. This involved multiple project stakeholders reviewing successive prototypes and wireframes of designs, and iterative website software releases. Input from the PPI panel and from the qualitative research contributed to the design and refinement process. Development of the mobile app drew heavily on the earlier website development reviews, but designs were tailored for smaller screen sizes. Mobile-specific engagement strategies (e.g. push notifications to encourage engagement with the CFHealthHub digital platform) were also incorporated.
The technical architecture of the digital component of the intervention is shown in Figure 2.
FIGURE 2.
Technical architecture of the digital component of the intervention.
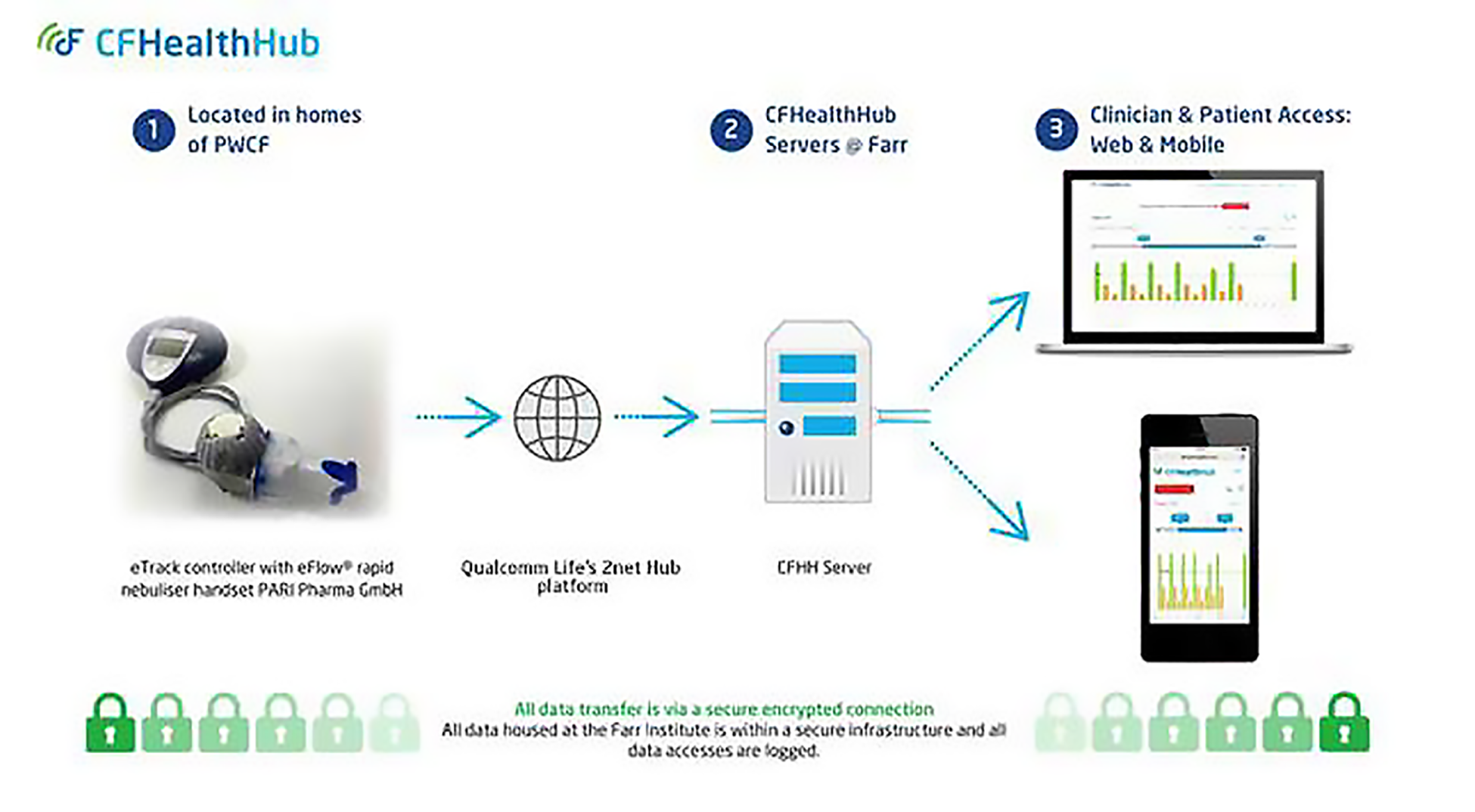
The software development was conducted by the mobile health (mHealth) team at the Health eResearch Centre, University of Manchester, with design expertise provided by user experience (UX) company Keep It Usable (Manchester, UK). Later phases of the design work were completed in-house at the Health eResearch Centre.
For more details on WP 1, see Appendix 1.
Work packages 2.1A and 2.1B: a qualitative study – understanding the illness perceptions and treatment beliefs of people with cystic fibrosis
Aims
The aims of WPs 2.1A and 2.1B were to explore PWCF’s perceptions of their illness and their treatments, perceptions of barriers to and facilitators of medication adherence using nebulisers, and the acceptability of visual displays of their recent medication adherence.
Methods
Sarah J Drabble undertook 18 face-to-face semistructured qualitative interviews with PWCF in the Sheffield CF centre. Sarah J Drabble is a female research associate with a doctoral degree, psychology training and experience in qualitative research. The proposal stated that 20–24 interviews would be undertaken. Our research physiotherapist Marlene Hutchings approached people who met our sampling strategy criteria to obtain permission to pass on their details to our qualitative researchers. She did this in a variety of ways, such as face to face and by e-mail. A total of 21 PWCF agreed to have their details passed on, 20 of whom gave informed consent for an interview. A total of 18 of these were available for interview. We used purposive sampling to identify adults with different objectively measured adherence levels. We knew their objectively measured adherence rates because the Sheffield CF centre had introduced measurement using chipped nebulisers. In addition, we undertook maximum diversity sampling by asking the research physiotherapist to approach adults of different genders, ages and socioeconomic backgrounds.
The stance taken was phenomenological in that we were interested in patient experiences of CF and adherence. The participants did not know Sarah J Drabble prior to the research. She was clear with participants that she had no prior experience of CF. Interviews were undertaken in people’s homes. Usually only the participant was present, although in two interviews the participant’s partner was present. The topic guide used for the interviews is included as Report Supplementary Material 7. This developed over time after the first couple of interviews. We audio-recorded the interviews and made field notes after each interview. Interviews lasted 65–147 minutes (mean 99 minutes).
We used framework analysis31 deductively to code high and low levels of adherence behaviour into the theoretical domains framework (TDF)32 and inductively to identify perceptions of CF and treatments and to explore the acceptability of the adherence graphs. Two researchers coded the data using NVivo (QSR International, Warrington, UK). There was no participant checking but the findings were discussed with the PPI panel.
We followed consolidated criteria for reporting qualitative research (COREQ) guidance (see Report Supplementary Material 4).
Findings
The detailed findings were published in two journal articles33,34 and are briefly summarised here with illustrative quotations.
Perceptions of cystic fibrosis and treatments
Key contextual issues
-
Being normal: PWCF wanted to fit in and be like other peopleParticipant (P)3, very low level of adherence
I just think I’m not doing it today just because I just want to be normal for a day or I just don’t want to do it.
-
Openness about having CF and adherence: some PWCF discussed having CF with friends but others did notP4, very low level of adherence P17, high level of adherence
I do tell people you know. I’m quite open minded I like to let people know if I’m not OK.
I think the telling of people – I have this big thing about they’d treat me differently.
-
Forgetting: talk of ‘forgetting to take medication’ could be used to exercise control in the context of the moral obligation to adhere to treatment (see Drabble et al. 33)P4, very low level of adherence
[. . .] probably when I’m socialising and going out and things you know so if I go out an earlier time I might forget to have it.
-
Health belief: some PWCF were driven to keep healthy until a cure was found (high-level adherers), whereas others experimented on themselves and actively decided which treatments worked for themP16, high level of adherence
My nebulisers. I’ve just sort of tested them. Just not took them until next time I’ve been in clinic just to see what effect it would have. Like my lung capacity and stuff like that.
-
Treatment burden: varied within the sample and could be all consuming for those with more severe CF symptoms and other health conditionsP10, low level of adherence
This time my weight had gone up but my lung function had gone down, so I am trying to find that balance.
-
Tiredness: CF and daily life activities caused fatigue, leading to non-adherenceP11, moderate level of adherence
When you’re using a lot of effort just to breathe, then it takes away your efforts on other places, other parts.
-
Emotions: some of our interviewees described how adherence behaviour was often emotionally driven depending on their feelings about aspects of their lives. How they felt about their CF could affect their motivation to adhere to treatmentsP2, very low level of adherence
If I get up and it’s one of those days I can’t be [bothered] to do anything, I just won’t do it.
Barriers to and facilitators of medication adherence using the theoretical domains framework
Factors influencing adherence to treatment included all 14 domains of the TDF,34 most of which varied by adherence level:
-
skills
-
memory and decision-making
-
behavioural regulation
-
environmental context and resources
-
social influences
-
beliefs about consequences
-
beliefs about capability
-
reinforcement
-
social role and identity
-
intentions
-
optimism
-
emotions.
Factors identified by PWCF in higher and lower adherence level categories are summarised in Table 1.
| Factor | Quotation |
|---|---|
| Identified by PWCF in higher adherence level categories | |
| Believing that complete adherence was unachievable | In a way you sort of think you can’t remember every timeP18, high level of adherence |
| Concerns about bacterial resistance to treatment | I’m on antibiotics. Sort of the more you take them, the less effective they getP16, high level of adherence |
| Having a habit for treatment | It has got to a stage since that that in my head now, routine. I don’t need a checklistP15, high level of adherence |
| Fear about becoming ill | Just that fear, know I could be unwell some days. Just sort of striving to do it reallyP16, high level of adherence |
| Identified by PWCF in lower adherence level categories | |
| Feeling worse as a result of treatment | What runs through my head like especially the Promixin [Profile Pharma Ltd, Chichester, UK; colistimethate sodium] is that 3 months where I took it and still end up on [i.v. therapy]. And then end up having a reaction to the i.v. [therapy] from of itP5, very low level of adherence |
| Being rebellious or disorganised | If I don’t see no point in doing it, I won’t do it no matter what anybody saysP7, low level of adherence |
| Feeling that treatment was difficult when tired | I think Fridays I’m always tired from the whole week so I just always went to bed early or so I might just put it offP6, low level of adherence |
| Finding stressful events a barrier to and being in hospital a facilitator of adherence | I was really good at taking it then [in hospital] because I have got a watchful eye over meP5, very low level of adherence |
| Forgetting | When I remembered to tick it off it were alright but ‘cos I put it in the cupboard at side of me bed I just forgot about itP3, very low level of adherence |
| Avoiding thinking about CF | I know it will all affect me in the long run but I just don’t think about itP6, low level of adherence |
| Declining support | He does look after me but when I’m alright and walking around and stuff I don’t want him to have to do thatP3, very low level of adherence |
| Feeling in conflict with health-care professionals | I’ve had a doctor tell me before [laughs] if you took your nebuliser you wouldn’t be on [i.v. therapy]. But he said it so confrontationalP5, very low level of adherence |
Acceptability of adherence graphs
People with CF in the sample generally found the charts interesting and easy to understand. They found the different ways of looking at adherence, overall, by week or by time of day, useful because they could see different patterns in their adherence:
I like the graph idea, if it gives you a visual aid. This is basically what you’ve done since your last visit.
P11, moderate adherence
They identified ways of improving the visual attractiveness of the charts. We also identified that there was a need for the research team to identify an approach to measuring adherence in the context of multiple and changing prescriptions.
Conclusions
The findings from this WP shaped the intervention in three ways. First, the findings on the perceptions of CF and treatment identified aspects of any intervention that were important to attend to, and in particular that an intervention should not increase treatment burden. Second, the findings from the TDF domains identified how different people faced different barriers so that it was important to tailor the intervention to individuals; these findings also helped to identify key content for different components of the intervention. Third, the findings on acceptability of the graphs offered confidence that PWCF welcomed the graphs and identified ways of improving the presentation of data in these graphs.
Work package 2.1C: patient story video interviews for use in the CFHealthHub intervention
Aim
The aim of this qualitative study was to develop a series of patient story videos capturing PWCF talking about their experiences of managing nebulised medication as part of their overall CF treatment. The videos produced would be part of the BCI on the website.
Design
A qualitative study was undertaken by the Health Experiences Research Group at the University of Oxford (Oxford, UK). They used purposive sampling based on objectively measured adherence to nebulised medication, lung function and sociodemographic characteristics. Inclusion criteria for PWCF were as follows:
-
age ≥ 16 years
-
not on the active transplant list
-
not post lung transplant or in the palliative phase of the disease.
All participants had high and/or improved levels of adherence.
Methods
In-depth qualitative interviews were carried out with 14 PWCF from five UK CF centres. A total of 14 participants (aged 20–57 years) were interviewed between November 2015 and August 2016 by a female senior qualitative researcher from the Health Experiences Research Group (Susan Kirkpatrick). The researcher did not establish a relationship with participants prior to the research. Participants knew that the aim of the research was to create videos. The approach taken was phenomenological in that participants talked about their experiences. Face-to-face interviews were conducted in participants’ homes and video-recorded. Interviews lasted between 45 and 60 minutes. A topic guide was used to explore participants’ experiences of living with CF, to reflect on times when their nebuliser adherence level had been lower and to reflect on how and why they had improved their use of nebulised medication. Written consent was obtained by the researcher for participation in an interview and for selected clips being used on the website prior to editing and publication.
Analysis
Interview transcripts were analysed thematically using NVivo. The research team reviewed each transcript to select suitable content for inclusion on the CFHealthHub website. Criteria for inclusion of content was the identification of talk about making positive changes to adherence or reflecting on times of difficulties and how these had been overcome. These video clips were checked by participants.
Results
We created a library of short ‘talking heads’ videos that are hosted on the CFHealthHub website as part of the complex intervention. Emerging topics from the transcripts were identified and those selected were arranged into video categories, for example ‘Coping with feeling low’ and ‘Juggling treatment and life’. There was a total of 66 videos across 16 different categories (Table 2). The descriptions of the categories were created by the PPI panel.
| Categories | Examples of video descriptions created by PPI panel |
|---|---|
| Advice to younger self | PWCF talks openly about the risks of underestimating his condition, missing treatment and how you are the person who can make the biggest positive change |
| Coping with feeling low | There will always be peaks and troughs with CF; it’s important to keep motivated |
| Juggling treatment and life | PWCF talks about how seeing adherence data motivates him and supports what he wants to achieve in life |
| Going to university | PWCF talks about what motivated him to do his treatment and not rely on i.v. therapy |
| The importance of nebulisers | PWCF discusses thinking long term and doing your treatment to help you live a normal life |
| Keeping motivation up | Treatments are not a chore; you should look at them like drinking water – just something you have to do |
| Having a routine | PWCF explains that the key to doing her treatment is organisation and accepting that everyone slips once in a while |
| Finding support | PWCF shares her views on why it’s important to talk to others with CF |
| Nebuliser tips | Not cleaning your nebuliser will increase your treatment times |
| Having a normal life | PWCF talks about what routine works for her, being a mum with CF |
| Being normal | PWCF explains that doing her nebulisers allows her to be well enough to do the fun things in life, rather than just seeing them as a burden |
| Advice to others | PWCF talks about how to find a way to make nebulisers fit into your life |
| Late diagnosis | PWCF explains that, after discovering that he had CF later in life, the nebulised medicines he started to take completely changed his life for the better. The stark improvement in health after beginning his treatment is all the motivation he needs to take them |
| Growing up with CF | PWCF talks about the positive relationship with his CF team. Even though he was critical of them when he was younger, he reflects that they were only trying to help him |
| General tips | PWCF provides a tip to help when coughing in a public place |
| Talking to others | PWCF talks about the benefit of talking to other people with CF – being able to offer your experience. Hearing stories from those who are much older with CF has also made him feel much more positive about life expectancy |
Conclusions
This WP produced a series of ‘talking head’ video clips from qualitative interviews with PWCF in which PWCF shared their experiences and passed on knowledge to others about how they adhered to their treatments. These were used as part of the intervention.
Work package 2.2: development and refinement of the CFHealthHub intervention
This section outlines the process of planning, designing and creating, refining and documenting the intervention35 that the team undertook with input from the PPI panel and alongside the technical development of the digital platform (see Work package 1: developing the technology and infrastructure to collect adherence data).
Intervention development combined a ‘theory- and evidence-based’ approach36 [the behaviour change wheel (BCW) approach]37 with a ‘target population-based’ approach [the person-based approach (PBA)]. 38 The BCW considers capability, opportunity and motivation in relation to behaviour (i.e. nebuliser adherence) and, through a series of stages, systematically selects intervention functions and behaviour change techniques. The PBA utilises mixed methods with people from the target population (i.e. adults with CF) to inform all of the intervention development stages in an iterative process.
Stage 1: planning the intervention
We undertook a needs analysis informed by the qualitative research undertaken in WP 2, a literature review and PPI panel input and determined which capability, opportunity and motivation barriers the intervention should address. The qualitative research helped us to understand the context of PWCF’s lives.
Stage 2: designing and creating the intervention
We used the BCW approach to identify what ways to enact the intervention, and identified suitable behaviour change techniques, drawing on relevant theory and evidence. We embedded these in a prototype CFHealthHub website. We considered how the intervention could be tailored to meet the needs of patients with different needs. We considered the competencies required by interventionists and developed job descriptions and a prototype training manual.
Stage 3: refining the intervention
Refinement of the prototype intervention took place iteratively based on feedback from users. We undertook two studies with participants who were PWCF aged ≥ 16 years and on the CF registry. Participants were provided with an eTrack (PARI GmbH, Starnberg, Germany) nebuliser and Qualcomm (San Diego, CA, USA) hub and were given access to the CFHealthHub digital platform. Five participants took part in the first study, which assessed the ability of the system to successfully record and display nebulisations. Participants were interviewed 1 month later about their experiences and views. We made changes to the intervention based on this feedback. A total of 22 participants took part in the second study. During the intervention development phase our research physiotherapist delivered the intervention of four sessions to each participant. We conducted 18 semistructured telephone interviews with participants in different cycles of the software development to ask about acceptability, appearance and functionality of the digital platform and suggestions for improvements. We identified during this work the need for ‘talking heads’ videos of other PWCF to help participants learn how to improve their adherence (see Work package 2.1C: patient story video interviews for use in the CFHealthHub intervention). We also conducted six in-depth think-aloud interviews with participants while they were using the platform and these were screen- and audio-captured. We interviewed the physiotherapist delivering the intervention about their views. All of this feedback was used to improve the prototype digital platform and training manual.
Stage 4: documenting the intervention
At the end of this process we created an intervention manual that outlined the components of the intervention, the features and functions of the CFHealthHub digital platform and the proposed structure of delivery by health-care professionals/interventionists. An associated training programme for interventionists was also developed.
The CFHealthHub intervention
The CFHealthHub intervention is described in detail in Appendix 2 using template for intervention description and replication (TIDieR) guidelines. 39 The intervention comprises a web platform and app, which display graphs and tables of objectively measured nebuliser adherence and include modules of behaviour change techniques designed to increase motivation for adherence, to address capability and opportunity barriers and to build habits for treatment taking, and an intervention manual including procedures and worksheets for delivery by a health-care professional (interventionist).
The content of the website/app is tailored to individual participants’ needs based on their nebuliser medication prescription and their responses to the Beliefs about Medicines Questionnaire40 and displays individual real-time adherence data and personalised information in a ‘Toolkit’ area.
Participants are supported to interact with the digital platform content and tools in sessions by trained interventionists following a manualised delivery procedure, with a person-centred communication style. Six sessions (one ‘first intervention’ visit, 40–60 minutes; two ‘intermediate’ reviews, 5–15 minutes each; two ‘main’ reviews, 30–45 minutes each; one ‘phase’ review, 20–30 minutes) were usually delivered over a 12-week phase of delivery, with phase reviews every 12 weeks thereafter, or every 6 weeks thereafter for participants with objectively measured adherence of < 25%. Participants with a high level of adherence during the baseline period of the study (> 80%) received two sessions (one first intervention visit and one phase review), with phase reviews every 12 weeks thereafter. Additional blocks of sessions (a ‘phase’) were offered when (1) participants requested further support, (2) participant’s adherence was reduced by 20% in a 4-week period or (3) participants received intravenous (i.v.) therapy for an exacerbation. First intervention sessions were always delivered face to face, whereas review sessions were delivered either face-to-face or by telephone, allowing the interventionist to intervene with one set of sessions or repeat further sets of sessions under certain circumstances. This means that, if used in the real world, the interventionist-delivered component of the intervention may be used throughout a person’s lifetime if their adherence drops for any reason.
A full description of the intervention is available in Appendix 2.
Work package 3.1: feasibility study comprising an external pilot randomised controlled trial and process evaluation
Parts of this section are reproduced or adapted with permission from Hind et al. 41 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Aim
The aim was to assess the feasibility of RCT procedures and the intervention.
Methods
Design
This was a pilot, open-label, parallel-group RCT with concurrent mixed-methods process evaluation. The pilot RCT has been reported in Hind et al. 41 The qualitative research from the process evaluation has been reported in Drabble et al. 42 and Hind et al. 43
Randomised controlled trial
Participants were PWCF at two CF centres. Inclusion criteria: aged ≥ 16 years and on the CF registry. Exclusion criteria: post lung transplant or on the active list, unable to consent, or using dry-powder inhalers.
Qualitative research
Sarah J Drabble, Samuel Keating and Alexander Scott interviewed intervention (n = 14) and control (n = 5) participants, interventionists (n = 3 on two occasions) and CF team members (n = 5) at the two CF sites. See Work packages 2.1A and 2.1B: a qualitative study – understanding the illness perceptions and treatment beliefs of people with cystic fibrosis for Sarah J Drabble’s credentials and experience. Participants were identified from RCT records and sampled purposively in a similar way to WP 2.1. A total of 49 patients agreed to be approached for interview. We were unable to contact 14 patients and did not actively invite seven, leaving a sample of 28, among whom 23 consented. People declined because they were too busy, had withdrawn from the intervention or did not show up for the interview. Patients were interviewed mainly at home and staff in their workplace or by telephone. The patient sample comprised nine male and five female patients, aged 17–69 years, across all deprivation levels and had fewer people with low levels of adherence than we wanted. The topic guide was used in the process evaluation of the full RCT is shown in Report Supplementary Material 8. All interviews were audio-recorded and field notes were taken and these were analysed using NVivo. Interviews lasted between 11 and 102 minutes (mean 56 minutes).
Interventions
Central randomisation using a computer-generated pseudo-random list and random permuted blocks of varying sizes (2, 4 and 6), stratified by site and number of IVAB days in the previous 12 months (≤ 14 days and > 14 days) on a 1 : 1 allocation to (1) the intervention arm, which involved linking a nebuliser with data recording and transfer capability to a software platform and strategies to support self-management with trained interventionists (n = 32), or (2) the control arm, which involved typically face-to-face meetings every 3 months with the CF team (n = 32).
Outcomes and processes
Trial feasibility was defined as recruitment of > 48 participants (75% of target) in 4 months (pilot primary outcome), valid exacerbation data available for > 85% of those randomised (future RCT primary outcome), change in percentage of medication adherence (secondary outcome in future RCT), use of CFHealthHub and positive perceptions of the intervention from qualitative interviews.
Results
The pilot RCT recruited to target, randomising 33 participants to the intervention arm and 31 participants to the control arm during the 4-month period June to September 2016. At study completion (30 April 2017), 60 (94%) participants (intervention arm, n = 32; control arm, n = 28) contributed good-quality exacerbation data. Five serious adverse events occurred, none of which was related to the intervention. The process evaluation identified problems with data connectivity, which affected adherence data. The mean change in adherence was 10% (95% CI –5.2% to 25.2%) higher in the intervention arm. Data from CFHealthHub identified that interventionists delivered insufficient numbers of review sessions to identify and address low levels of motivation. Concentration on participant recruitment left site interventionists insufficient time for key intervention procedures.
In the qualitative interview study there was evidence of the expected behaviour change mechanisms of action. Mechanisms of action were similar to those associated with effective telehealth interventions for self-management support: relationships, visibility and fit. 42 PWCF described how building a relationship with the interventionist helped them to consider ways of increasing their level of adherence to medication. PWCF in this sample found having their data visible to themselves and others motivating, particularly if they received praise from others about the progress they had made. The intervention was tailored to individuals but there were challenges in how the intervention fitted into some patients’ busy lives when delivered through a desktop computer. Interventionists identified that patients with moderate adherence rates were more likely to benefit from the intervention. PWCF in the control arm had not seen their adherence data or other parts of the intervention, indicating that the level of contamination was low.
The feasibility study led to 25 key changes to RCT procedures and the intervention. 43 Plans to develop an app for mobile phones were progressed. Changes were made to CFHealthHub to make it easier for interventionists to view and edit prescription data and to handle alternating treatment regimens. Other changes to CFHealthHub included making graphs more easily interpretable and adding descriptions to videos so that they could be found more easily by PWCF. Changes to the interventionist manual increased the emphasis on ‘active ingredients’, introduced intervention triggers for reduced adherence or exacerbations and introduced new habit formation sessions. The need for increased numbers of protocolised intervention review sessions arose because a focus on RCT recruitment targets gave interventionists inadequate time to deliver review visits, which were critical for updating personalised action plans and updating coping plans. For the same reason, we planned for the subsequent full-scale RCT to have a longer accrual window. Training and job specifications were modified to suit interventionists from different disciplines and manage expectations about the need for travel and flexible working.
Conclusions
We concluded that, with improved intervention/research processes and lower monthly participant recruitment targets, a full-scale RCT and the intervention were feasible.
Work packages 3.2, 3.3 and 3.4: full-scale randomised controlled trial with concurrent process evaluation
Parts of this section are reproduced or adapted with permission from Wildman et al. 44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Having integrated the identified changes into the RCT and intervention protocols, we conducted a full-scale RCT to determine the efficacy of the CFHealthHub intervention (WP 3.2). Concurrently, we undertook a process evaluation to explore implementation (including fidelity), mechanisms of action and context (WP 3.3). 45 We report the independent streams of research and the triangulation of the results (WP 3.4).
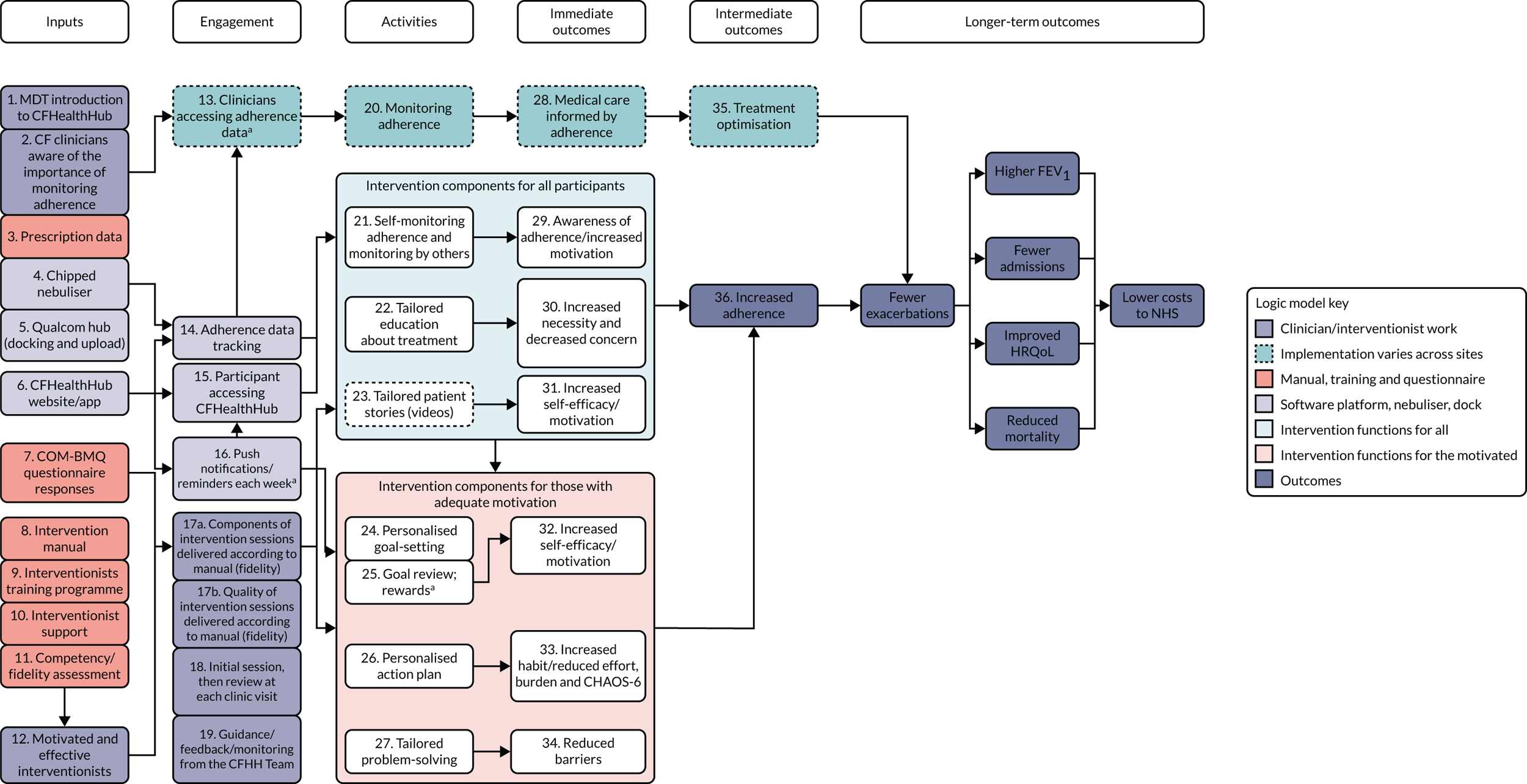
A logic model was constructed early in the programme and refined throughout to show how the intervention could affect outcomes (Figure 3).
FIGURE 3.
Logic model. a, Optional; depends on participant consent. CHAOS-6, Confusion, Hubbub and Order Scale-6 item; COM-BMQ, capability opportunity motivation – behaviour beliefs about medicines questionnaire; FEV1, forced expiratory volume in first second; HRQoL, health-related quality of life.
Design
This was a two-armed, parallel-group, open-labelled, efficacy superiority RCT comparing intervention with usual care, with concurrent process evaluation. The protocol is available online. 46
Methods
Objective
The objective of the RCT was to determine the effect of the CFHealthHub intervention on clinical and participant-reported outcomes.
Sample size
Sample size estimation was conducted using a between-group difference in mean exacerbations of 0.5 over the 12-month follow-up period, a standard deviation (SD) of 1.5, a design effect of 1.16 to allow for clustering, an alpha level of 5% and 90% power. After adjusting for 20% loss to follow-up, the recruitment target was 556 participants (278 per arm). The sample size was predicated on 2.0 exacerbations per year and reducing this by 0.5 to 1.5 per year. This is equivalent to an incidence rate ratio (IRR) of 0.75 (2.0 ÷ 1.5).
Participants
Potential participants were identified using the UK Cystic Fibrosis Registry. Eligible participants were aged ≥ 16 years and willing to take inhaled mucolytics and antibiotics via the eTrack nebuliser. Participants were ineligible if they were post lung transplant, on the lung transplant list, receiving palliative care, lacking capacity for informed consent or using dry-powder devices to take mucolytics or antibiotics.
Intervention and allocation
Intervention participants received the intervention described in Work package 2.2: development and refinement of the CFHealthHub intervention and Appendix 2. The intervention was delivered by full-time interventionists employed specifically for the research study to deliver both the intervention and the RCT (recruitment and some data collection). They were physiotherapists in 13 of the 19 centres and nurses, psychologists, a pharmacist and a dietitian in other centres. Some centres had two interventionists that shared the role, sometimes from different clinical disciplines. Control participants were given an eTrack controller and Qualcomm (San Diego, CA, USA) hub to enable accurate recording of inhalation data and calculation of adherence levels. They did not have access to CFHealthHub, that is, its adherence data, behaviour-change tools, educational content and visits from interventionists. Control arm participants received usual care.
Participants were allocated 1 : 1 to the intervention arm or control arm using a computer-generated pseudo-random list with random-permuted blocks of randomly varying sizes, via a central, web-based randomisation system. The allocation sequence was hosted by the Clinical Trials Research Unit at the University of Sheffield (Sheffield, UK), with the sequence created by a statistician (not otherwise involved with trial) and held on a secure server. The recruiting health-care professional logged into the server and entered basic demographic information, then the allocation was revealed. Stratification was by centre and number of past-year i.v. antibiotic days (≤ 14 days and > 14 days) – a predictor of current-year i.v. days. 21 The trial statistician remained blind to treatment allocation until database freeze. Participants and health-care professionals collecting primary outcome data were not blind to treatment allocation. The trial statistician remained blind to treatment allocation until database freeze; analyses were conducted unblinded.
An intention-to-treat approach was used, with all participants included in the arm to which they were randomised and exclusions being made only in the event of insufficient data for inclusion in the model for a given outcome. In addition, per-protocol and complier average causal effect (CACE) analyses were conducted, with protocol compliers defined as participants participating in both a first intervention visit and a review visit during which adherence graphs and/or charts were accessed.
Outcome measures
The primary analysis consisted of a between-group comparison of pulmonary exacerbation rates over the 12-month period from consent, with exacerbations defined as meeting at least one of the 12 Fuchs criteria and being treated by i.v. antibiotics. 47 The following sensitivity analyses were conducted to assess the robustness of the findings, applying the same model as for primary analysis: inclusion of all (including those not treated with i.v. antibiotics) exacerbations, multiple imputation for missing outcome data, best-case imputation, per-protocol analysis and CACE analysis (see Report Supplementary Material 1 and 2).
Key secondary outcomes included weekly medication adherence, forced expiratory volume in first second (per cent) (FEV1%) predicted and body mass index (BMI). To calculate numerator-adjusted normative adherence, daily doses taken were recorded, capped at the number of doses prescribed if the participant took more than the prescribed dose, divided by the appropriate daily dose given the participant’s disease status and treatment regimen, and summarised as weekly means. Lung function and BMI were measured at baseline and 12-month follow-up visits. Health-related quality of life (HRQoL), beliefs and perceived behaviours were assessed by way of the following patient-reported measures:
-
generic health status – EuroQol-5 Dimensions, five-level version (EQ-5D-5L)
-
Patient Activation Measure-13 item (PAM-13)
-
Confusion, Hubbub and Order Scale-6 item (CHAOS-6)
-
perceptions of treatment adherence – Medication Adherence Data-3 item (MAD-3)
-
Self-Report Behavioural Automaticity Index (SRBAI)
-
Cystic Fibrosis Questionnaire-Revised (CFQ-R)
-
Generalised Anxiety Disorder-7 (GAD-7)
-
specific concerns and necessities – Capability Opportunity Motivation – Behaviour Beliefs About Medicines Questionnaire (COM-BMQ)
-
Patient Health Questionnaire-8 item (PHQ-8).
Patient-reported outcomes were recorded at baseline and 12 months.
Participant safety was assessed by way of adverse and serious adverse event reporting. All randomised participants were included in safety summaries.
Statistical analysis
The statistical analysis plan is detailed in Report Supplementary Material 1. Analysis is summarised in this section.
Baseline and safety data were reported using summary statistics.
For the primary outcome (and associated sensitivity analyses), pulmonary exacerbation rates were compared using the IRR from a negative binomial model adjusted for stratification factors and including an offset for follow-up time.
Weekly numerator-adjusted normative adherence data were analysed using a longitudinal mixed model with random slopes and intercepts and adjustment for stratification factors and ‘baseline’ (weeks 1 and 2 post consent) adherence. The treatment effect was quantified using the adjusted between-group difference in mean normative adherence. Other secondary outcomes were analysed using 12-month follow-up data adjusted for baseline values and stratification factors. Treatment effects were determined by adjusted between-group differences in means.
For all models, treatment effects were reported with corresponding 95% confidence intervals (CIs). No adjustments were made for multiplicity. Adjustment for multiplicity was not specified in the statistical analysis plan, which was written, in accordance with the Clinical Trials Research Unit at the University of Sheffield (Sheffield, UK) standard operating procedures, before the data were analysed and was reviewed and approved by the independent members (which included two statisticians) on the TSC. There is no consensus on what procedure to adopt to allow for multiple comparisons. 48 Therefore, we followed Altman et al. ’s49 recommendation of reporting unadjusted p-values (to three decimal places/significant figures) and confidence limits, with a suitable note of caution with respect to interpretation. As Perneger concludes: ‘simply describing what tests of significance have been performed, and why, is generally the best way of dealing with multiple comparisons.’50
Analyses were conducted in R v3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria) and SAS® v9.4. (SAS Institute Inc., Cary, NC, USA).
We followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines (see Report Supplementary Material 5).
Process evaluation methods
There were six components in the process evaluation. Each component was undertaken and analysed separately. The findings from each component were brought together using a triangulation protocol51 adapted for use with qualitative research and RCTs. 52 Experts in process evaluation recommend that the process evaluation is analysed before the RCT results are known. 53 All components, except the mediation analysis, were reported to the team before the RCT results were known, although further analysis continued on some components after the RCT results were revealed.
Fidelity
The aim was to explore the fidelity of the intervention in practice. We used the Borrelli checklist54 as the framework to assess and monitor the fidelity of the intervention delivered in the RCT. Intervention sessions were assessed for fidelity at certification and for drift. CFHealthHub data on use of different parts of the website (click analytics) were included in this assessment. Details of the fidelity assessment methods, together with the fidelity results, are available in Appendix 3.
Usual-care survey
The aim was to understand usual care in each of the RCT sites, and how it changed over the time of the RCT, to assess how different the intervention was from usual care. We used an 11-item survey at baseline and at 12 months at each RCT site. Questionnaires were completed by the site interventionist and/or other members of the MDT. Questions included a mixture of items requiring five-point nominal scale and free-text responses. Medians, interquartile ranges and percentages by response were used to summarise categorical items. Free-text responses were summarised by identifying key themes. To examine change in usual care at sites over the course of the 12-month follow-up, change scores were calculated. All sites responded to the survey. Details of methods are reported in Appendix 4.
User acceptability survey
The aim was to measure the acceptability of different components of the intervention. We asked 11 questions about the perceived helpfulness of different components of the intervention in the 12-month follow-up questionnaire for those in the intervention arm. The questionnaire was either posted and handed to PWCF who had had the intervention for completion in the presence of the interventionist. A total of 257 out of 305 (84%) participants in the intervention arm responded. Details of methods are reported in Appendix 5.
Trial monitoring data
The aim was to monitor RCT progress in terms of numbers of people approached, reasons for not agreeing to participate in the RCT and numbers withdrawing from the intervention. This allowed us to consider reach and engagement.
Qualitative research
The aim was to explore perceptions of the intervention in practice. We sampled patients purposively using a similar approach to WP 2.3. A total of 84 patients agreed to be approached for interview. We were unable to contact 37 patients, and did not approach 12, leaving a sample of 35. A total of 32 patients consented and 22 were interviewed. Some patients declined and others said that they were too busy to participate; three were unwell on the day of the interview and one died. We approached and interviewed all 26 interventionists. We approached nine MDT members and did not get a response from four, so interviewed five.
We undertook face-to-face interviews with 22 intervention users in seven CF centres, 26 interventionists (some sites had more than one) and five members of the MDT who acted as principal investigators for the study at five RCT sites. Patients comprised 10 male and 12 female patients, aged 19–58 years, across all deprivation levels and all adherence levels.
The interviews were undertaken by Sarah J Drabble (see Work packages 2.1A and 2.1B: a qualitative study – understanding the illness perceptions and treatment beliefs of people with cystic fibrosis for her credentials) and Elizabeth Lumley, a female clinically trained qualitative researcher educated to master’s level with no experience of CF research. The relationship between researchers and participants, and the approach taken, was similar to those in WPs 2.1 and 2.3. The topic guide for PWCF included questions relating to acceptability of different aspects of the intervention and what aspects of the intervention, if any, helped them to increase their adherence. The topic guide for interventionists included questions on the delivery of the intervention, the trial processes and aspects of the context (see Report Supplementary Material 2 for both topic guides). Interviews were audio-recorded and field notes taken. Interviews lasted between 17 and 83 minutes (mean 42 minutes).
We used framework analysis,31 deductively coding to the TDF,32 mechanisms of action including Vassilev’s telehealth mechanisms of action,55 different components of the intervention and its delivery, and inductively to context. Three researchers (SJD, EL, AS) coded the data in NVivo. No participant checking occurred; the findings were discussed with the PPI panel.
Mediation analysis
Structural equation modelling was undertaken on the RCT data to identify the mechanisms by which the CFHealthHub intervention could have influenced medication adherence. Prior to analysis, a logic model (see Figure 3) was constructed to map the anticipated mechanistic pathway, along with potential effect moderators (including two-way interactions) from which a provisional directed acyclic graph (DAG) was created. The factors were further screened for inclusion prior to fitting the model by graphically assessing two-way associations and, for potential mediators, by calculating mean differences between the randomised arms. Factors with little apparent association (defined as an absolute correlation of < 0.1 or a mean difference < 0.1 SDs) were removed from the DAG prior to model fitting. Factors identified as potential mediator–outcome confounders were included in the model as fixed-effect covariates. Pearson’s correlation coefficients with their 95% CI (calculated using Fisher’s z transformation) were used as a guide to identify relationships between mediators. Model fit statistics comparative fit index (CFI) and root mean squared error of approximation (RMSEA) were used to select the final model. In addition, 95% bootstrap CIs were used to estimate the indirect effect of the chosen mediators as well as the direct and total effect of the intervention on medication adherence. Sensitivity analysis was carried out, removing intervention arm participants whose follow-up overlapped with the intervention system being unavailable owing to technical difficulties (i.e. from 20 March to 23 April 2019).
Results
Key results are described in this section. Further results from the statistical analysis plan are reported in Report Supplementary Material 2. Extra analyses were undertaken that were not specified in the statistical analysis plan and these are reported in Report Supplementary Material 3.
Participant flow
Participants were recruited from October 2017 to June 2018. Participants were followed up until trial completion in June 2019. Participant recruitment and disposition is shown in Figure 4.
FIGURE 4.
The CONSORT flow diagram. a, Exclusions due to missing covariates. Reproduced with permission from Wildman et al. 44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
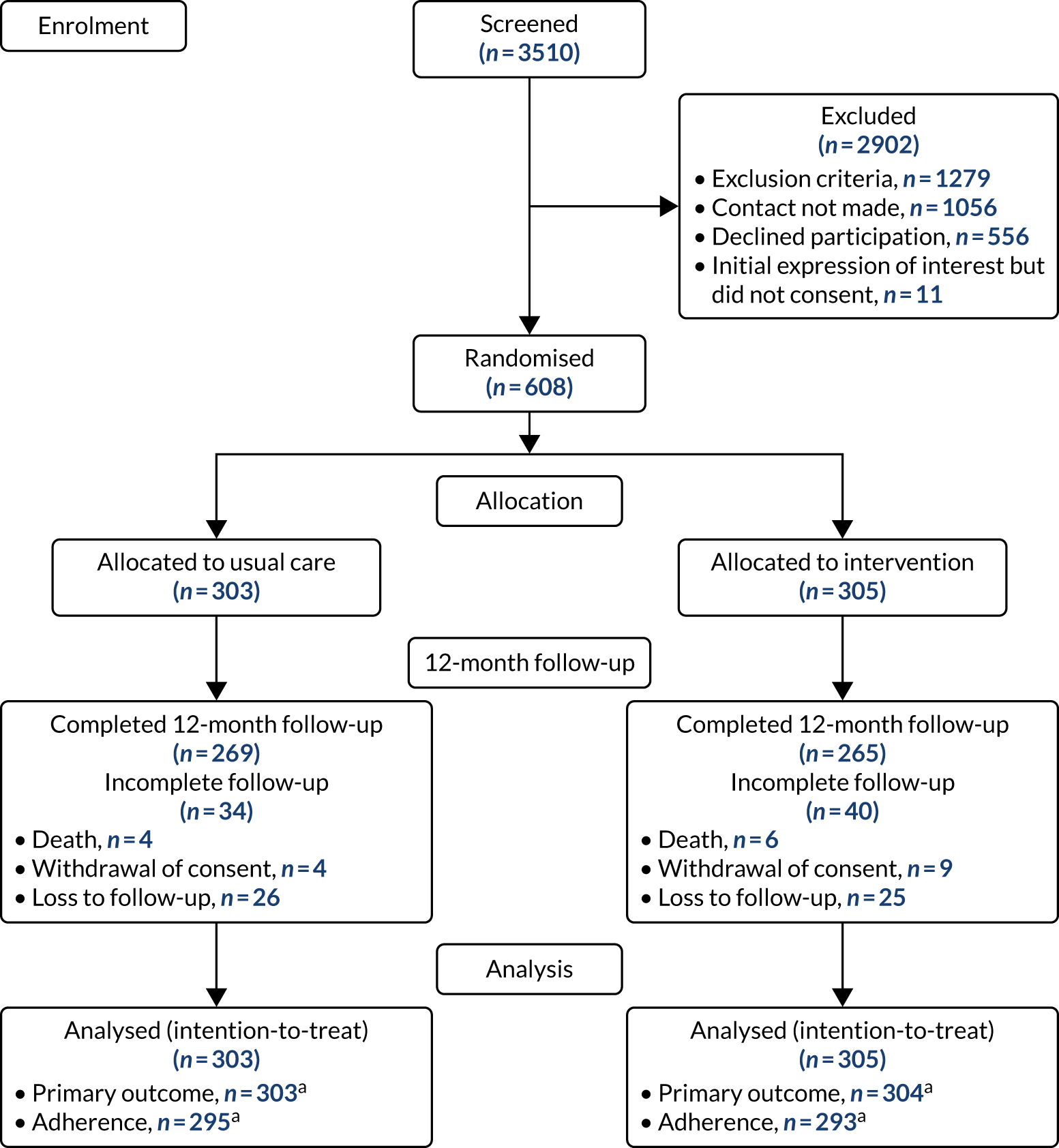
Reasons for declining participation and premature discontinuation of intervention
Common reasons for declining to participate were unwillingness to change nebuliser (125/566) and that the trial would be too time-consuming (118/556). There were 54 premature discontinuations of adherence data collection (control arm, n = 29; intervention arm, n = 25) and 32 premature discontinuations of intervention delivery. Unhappiness with the device/nebuliser or a preference for a previous device was reported as a reason for discontinuation (see Report Supplementary Material 2).
Baseline characteristics
One participant withdrew prior to baseline data collection. Participant characteristics at baseline are shown in Table 3. There were no discernible between-group differences in baseline demographic characteristics. A difference was observed in ‘baseline’ numerator-adjusted normative adherence, which was measured in the first 2 weeks post consent. Participants in the intervention arm had slightly higher FEV1% predicted and fewer i.v. therapy-days in the prior year. In accordance with CONSORT reporting guidelines, we did not carry out any significance tests of baseline differences. We carried out an analysis adjusted for covariates. We describe this analysis briefly in the statistical analysis section and in more detail in the statistical analysis plan. In summary, we adjusted for baseline stratification factors (site and previous years’ i.v. therapy-days) and baseline value of the outcome (where available) in all statistical models.
| Variable | Control arm | Intervention arm | Overall | |||
|---|---|---|---|---|---|---|
| N | Mean (SD)/n (%) | N | Mean (SD)/n (%) | N | Mean (SD)/n (%) | |
| Demographic data: numeric | ||||||
| Age (years) | 303 | 30.3 (10.8) | 304 | 31.1 (10.6) | 607 | 30.7 (10.7) |
| Weight (kg) | 303 | 63.2 (14.2) | 304 | 64.1 (14.1) | 607 | 63.7 (14.1) |
| Height (cm) | 303 | 167.2 (9.2) | 304 | 167.7 (9.5) | 607 | 167.5 (9.4) |
| BMI (kg/m2) | 303 | 22.5 (4.2) | 304 | 22.7 (4.2) | 607 | 22.6 (4.2) |
| Demographic data: categorical | ||||||
| Gender | ||||||
| Female | 303 | 154 (50.8) | 304 | 156 (51.3) | 607 | 310 (51.1) |
| Male | 303 | 149 (49.2) | 304 | 148 (48.7) | 607 | 297 (48.9) |
| Deprivation | ||||||
| 1st quintile | 302 | 51 (16.9) | 302 | 50 (16.6) | 604 | 101 (16.7) |
| 2nd quintile | 302 | 71 (23.5) | 302 | 59 (19.5) | 604 | 130 (21.5) |
| 3rd quintile | 302 | 66 (21.9) | 302 | 63 (20.9) | 604 | 129 (21.4) |
| 4th quintile | 302 | 67 (22.2) | 302 | 63 (20.9) | 604 | 130 (21.5) |
| 5th quintile | 302 | 47 (15.6) | 302 | 67 (22.2) | 604 | 114 (18.9) |
| Clinical characteristics: numeric | ||||||
| FEV1% predicted | 302 | 58.3 (22.6) | 304 | 60.7 (23.5) | 606 | 59.5 (23.1) |
| i.v. therapy-days in previous 12 months | 303 | 27.7 (33) | 304 | 24.2 (27.9) | 607 | 25.9 (30.6) |
| Subjective adherence (%) | 298 | 69 (30.8) | 300 | 69.9 (31) | 598 | 69.4 (30.9) |
| Numerator-adjusted normative adherence (weeks 1 and 2 post consent) (%) | 295 | 45.6 (34.2) | 296 | 54.0 (32.9) | 591 | 49.8 (33.8) |
| Clinical characteristics: categorical | ||||||
| Chronic Pseudomonas infection | 299 | 175 (58.5) | 304 | 174 (57.2) | 603 | 349 (57.9) |
| Non-chronic Pseudomonas infection | 299 | 124 (41.5) | 304 | 130 (42.8) | 603 | 254 (42.1) |
Primary outcome
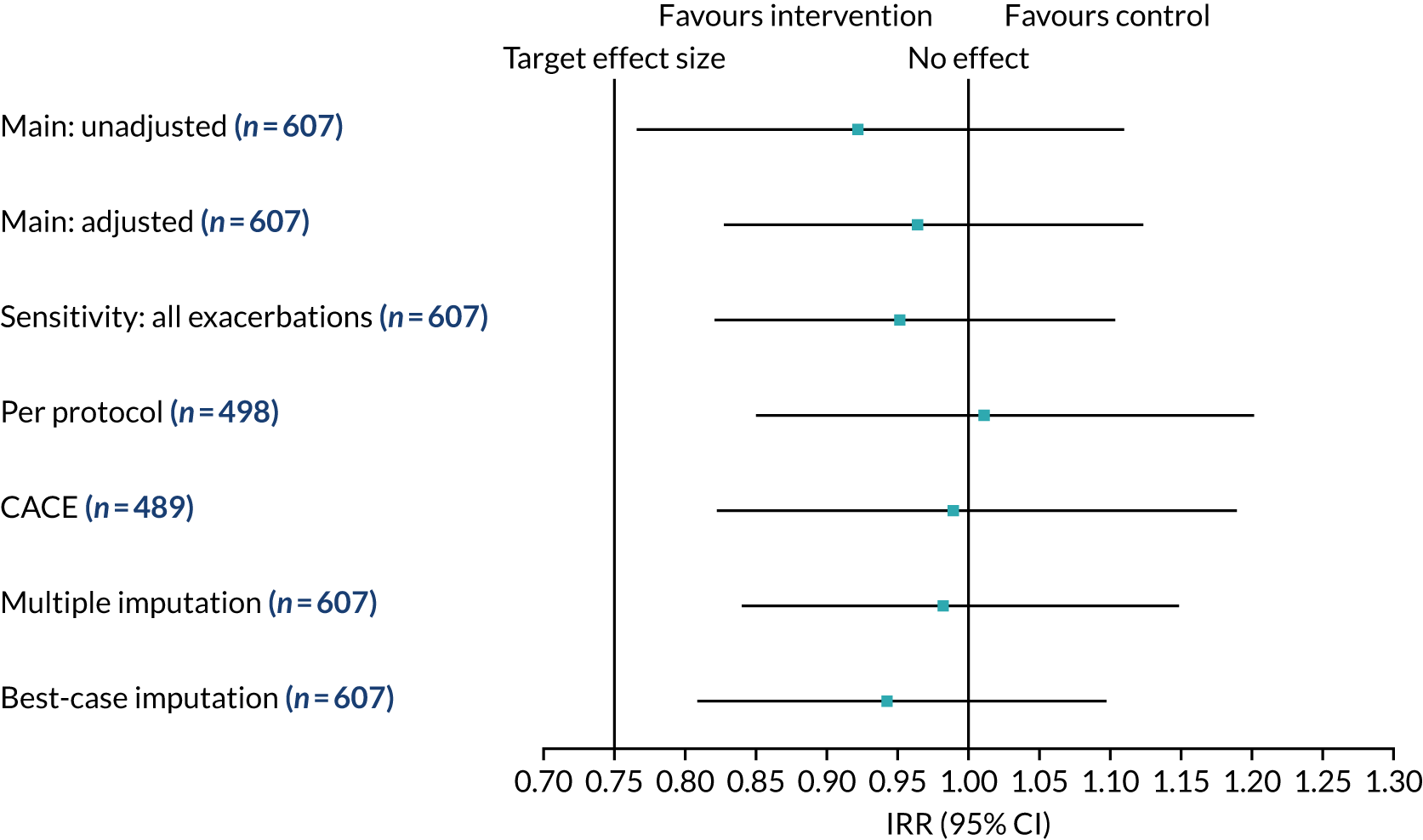
The IRRs from the primary and sensitivity analyses comparing exacerbation rates between the intervention and control arms are presented in Figure 5. The IRR for the main primary analysis was 0.96 (95% CI 0.83 to 1.12; p = 0.638). The point estimate of the IRR is < 1, which favours the intervention arm. However, the 95% CI for the treatment effect included 1, which is consistent with no overall difference in exacerbation rates between the two randomised arms. The sample size was predicted on the assumption of 2.0 exacerbations per year prior to intervention, with a reduction of 0.5 exacerbations to 1.5 per year. This is equivalent to an IRR of 0.75 (2.0 ÷ 1.5). We observed 1.77 exacerbations in the control arm and 1.63 in the intervention arm. If we are looking for a 0.5 reduction in exacerbations from 1.8 to 1.3 then this gives an IRR of 0.72 (1.8 ÷ 1.3). Based on our sample size calculation, a clinically important IRR is between 0.65 and 0.75. Because the lower limit of the estimate (i.e. 0.83) is above this, our result is not statistically significant, and not clinically significant if we believe an important IRR is ≤ 0.75. Findings from sensitivity analyses were consistent with the primary analysis, with 95% CIs encapsulating the null IRR value of 1.
Further details about the exacerbation analysis can be found in Report Supplementary Material 2.
FIGURE 5.
Pulmonary exacerbation IRR from primary and sensitivity analyses. Reproduced with permission from Wildman et al. 44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Secondary outcomes
Adherence
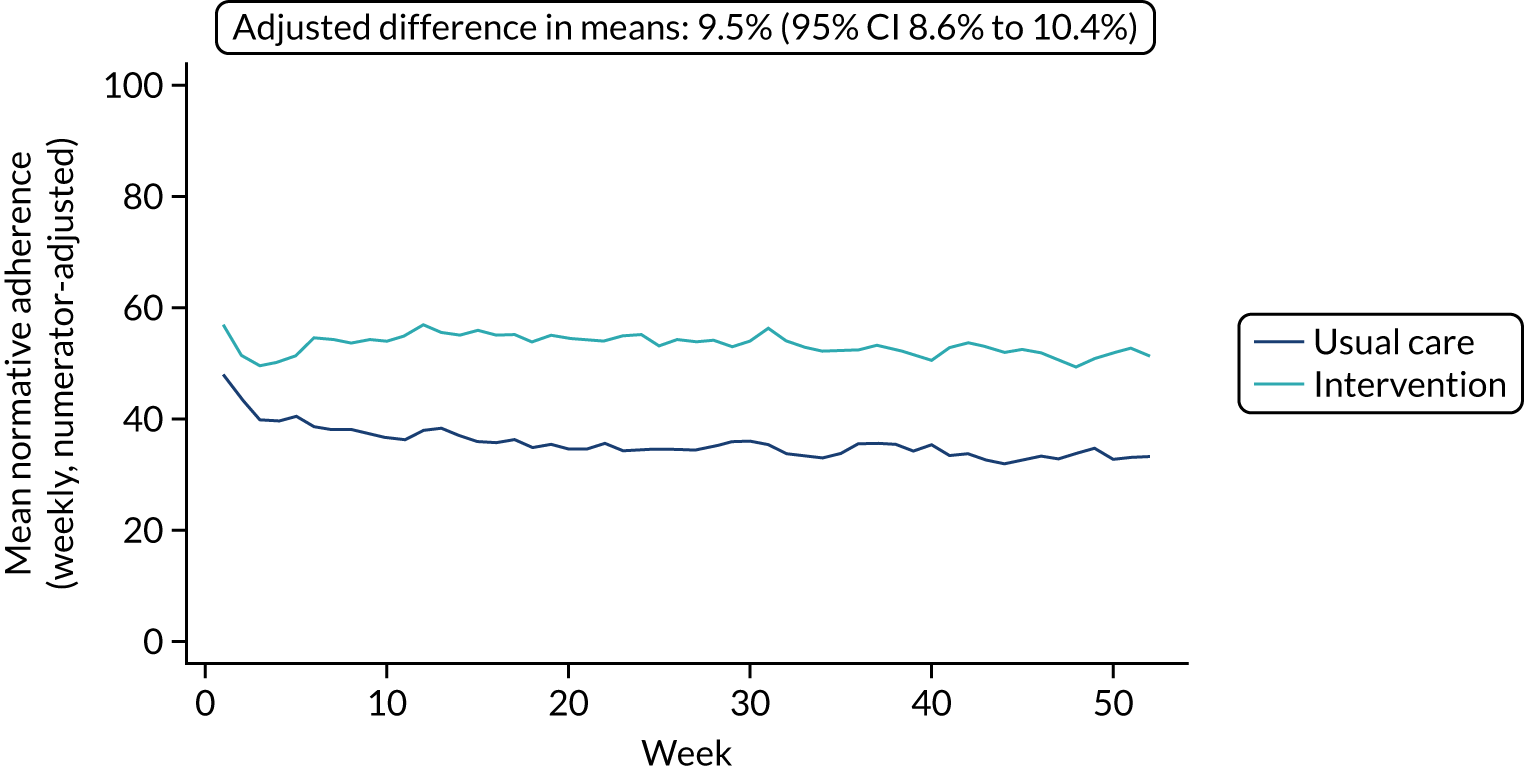
The mean weekly numerator-adjusted normative adherence over the course of the RCT is shown in Figure 6. The adjusted between-group difference in mean weekly adherence was 9.5 (95% CI 8.6 to 10.4; p < 0.001) percentage points in favour of the intervention arm. Further details about adherence can be found in Report Supplementary Material 2.
FIGURE 6.
Mean weekly numerator-adjusted normative adherence. Reproduced with permission from Wildman et al. 44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Other secondary outcomes
The adjusted mean FEV1% predicted at 12-month follow-up was 1.4 (95% CI –0.2 to 3.0; p = 0.082) percentage points higher in the intervention arm. The adjusted mean BMI was 0.3 kg/m2 (95% CI 0.1 kg/m2 to 0.6 kg/m2; p = 0.008) higher in the intervention arm. Effect sizes were modest for the remaining secondary outcomes, but all excluding the patient-reported measure of anxiety (GAD-7) showed a direction of effect favouring the intervention. Observed follow-up means and adjusted between-group differences for all outcomes are presented in Tables 4 and 5. Further detail can be found in Report Supplementary Material 2.
| Primary outcome | Control arm | Intervention arm | IRR (95% CI)a | Direction of positive effect | ||||
|---|---|---|---|---|---|---|---|---|
| N | Exacerbations/person-years | Exacerbation rate/year | N | Exacerbations/person-years | Exacerbation rate/year | |||
| Exacerbations | 303 | 526/297.2 | 1.77 | 304 | 482/294.9 | 1.63 | 0.96 (0.83 to 1.12) | IRR < 1 |
| Secondary outcome | Control arm | Intervention arm | Adjusted difference in means (95% CI)a | Direction of positive effect | Standardised effect size | ||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||||
| Key secondary outcomes | |||||||
| Normative adherence (%) | 295 | 34.9 (31.7) | 293 | 52.9 (31.4) | 9.5 (8.6 to 10.4) | Increase | 0.29 |
| FEV1% predicted (%) | 282 | 56.9 (23.0) | 274 | 60.6 (24.2) | 1.4 (–0.2 to 3.0) | Increase | 0.06 |
| BMI (kg/m2) | 282 | 22.6 (4.1) | 273 | 23.1 (4.4) | 0.3 (0.1 to 0.6) | Increase | 0.07 |
| Patient-reported outcomes | |||||||
| Beliefs about medication | |||||||
| Concerns | 271 | 2.1 (0.5) | 263 | 2.0 (0.5) | –0.2 (–0.2 to –0.1) | Decrease | 0.29 |
| Necessities | 271 | 3.5 (0.7) | 263 | 3.7 (0.8) | 0.1 (0.0 to 0.2) | Increase | 0.18 |
| SRBAI (habit) | 271 | 11.7 (4.9) | 261 | 12.9 (4.9) | 1.2 (0.5 to 1.8) | Increase | 0.24 |
| CFQ-R | |||||||
| Physical | 274 | 52.6 (30.6) | 264 | 55.8 (30.2) | 2.3 (–1.0 to 5.6) | Increase | 0.08 |
| Emotion | 274 | 66.5 (24.7) | 264 | 66.6 (22.9) | 0.2 (–2.9 to 3.2) | Increase | 0.01 |
| Social | 274 | 59.6 (20.0) | 264 | 60.5 (20.0) | 0.3 (–2.2 to 2.7) | Increase | 0.01 |
| Eating | 274 | 81.0 (23.2) | 264 | 84.0 (21.5) | 1.9 (–1.3 to 5.2) | Increase | 0.09 |
| Body image | 274 | 65.1 (29.3) | 264 | 67.2 (27.3) | 1.7 (–1.4 to 4.8) | Increase | 0.06 |
| Treatment burden | 274 | 51.6 (19.7) | 265 | 56.6 (19.5) | 3.9 (1.2 to 6.7) | Increase | 0.20 |
| Respiratory | 271 | 56.6 (21.9) | 263 | 58.0 (22.5) | 0.7 (–2.4 to 3.8) | Increase | 0.03 |
| Digestion | 272 | 80.2 (21.6) | 263 | 80.4 (19.4) | 1.1 (–1.7 to 3.9) | Increase | 0.05 |
| MAD-3 (medication adherence) | 245 | 9.9 (3.6) | 237 | 10.8 (3.3) | 0.7 (0.2 to 1.2) | Increase | 0.20 |
| Behavioural question (effort) | 270 | 3.0 (1.2) | 260 | 3.3 (1.3) | 0.3 (0.1 to 0.5) | Increase | 0.22 |
| Subjective adherence | 267 | 65.6 (32.8) | 258 | 68.6 (31.3) | 1.9 (–2.8 to 6.6) | – | 0.06 |
| CHAOS-6 (routine) | 272 | 9.6 (3.2) | 263 | 9.4 (3.4) | –0.2 (–0.6 to 0.3) | Decrease | 0.05 |
| PAM-13 (health-style assessment) | 274 | 64.9 (13.0) | 265 | 68.1 (15.6) | 3.4 (1.3 to 5.4) | Increase | 0.23 |
| PHQ-8 (depression) | 272 | 6.4 (5.0) | 262 | 6.3 (5.6) | –0.1 (–0.8 to 0.7) | Decrease | 0.01 |
| GAD-7 (anxiety) | 273 | 4.5 (4.8) | 262 | 4.9 (5.3) | 0.3 (–0.4 to 1.0) | Decrease | 0.05 |
| EQ-5D-5L | 272 | 0.81 (0.18) | 264 | 0.84 (0.15) | 0.01 (–0.01 to 0.04) | Increase | 0.09 |
Adverse event data from the 12-month follow-up period are presented in Table 6. A full list is presented in Appendix 6. There were no serious adverse events deemed related to the intervention.
| Adverse event | Control arm (N = 303), n (%) | Intervention arm (N = 305), n (%) | Overall (N = 608), n (%) |
|---|---|---|---|
| Non-serious adverse events | |||
| All AEs | 301 (46.9) | 341 (53.1) | 642 (100.0) |
| Participants experiencing at least one AE | 125 (41.3) | 139 (45.6) | 264 (43.4) |
| AEs by category | |||
| Expected | 242 (80.4) | 263 (77.1) | 505 (78.7) |
| New depression requiring treatment | 1 (0.3) | 5 (1.5) | 6 (0.9) |
| Other | 58 (19.3) | 73 (21.4) | 131 (20.4) |
| Serious adverse events | |||
| All SAEs | 64 (47.4) | 71 (52.6) | 135 (100.0) |
| Participants experiencing at least one SAE | 43 (14.2) | 56 (18.4) | 99 (16.3) |
| SAEs by category | |||
| Expected | 21 (32.8) | 28 (39.4) | 49 (36.3) |
| New depression requiring treatment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 41 (64.1) | 42 (59.2) | 83 (61.5) |
| Unknown | 2 (3.1) | 1 (1.4) | 3 (2.2) |
Subgroup analyses
See later in Implications for randomised controlled trial subgroup and sensitivity analyses.
Extra analyses: longer-term outcomes
The above analysis is based on 12 months; however, some PWCF stayed in the RCT for up to 21 months. Analysis of the longer-term outcomes up to 21 months post consent back up the results of the primary analysis using 12-month data [no difference in exacerbations or forced expiratory volume in first second (FEV1)]. There is a difference in adherence of a similar order to the 12-month analysis.
For exacerbations, there was a total of 1326 exacerbations (control, n = 693; intervention, n = 633) during that time. The observed exacerbation rate in the extended post-consent follow-up was 1.70 per year in the control arm and 1.58 per year in the intervention arm (compared with an exacerbation rate of 1.77 per year in the control arm and 1.63 in the intervention arm in the primary analysis). The primary analysis model included adjustments for the previous year’s i.v. therapy-days and site, which were stratifying factors in the randomisation schedule. The estimated treatment effect for this analysis, the IRR, was 0.97 (95% CI 0.84 to 1.12), which is < 1, favouring the intervention arm. However, the 95% CI for the treatment effect included 1, which is consistent with no overall difference in exacerbation rates between the two arms. The estimated treatment effect for the longer-term follow-up is very similar to the primary analysis, with 12-month post-consent follow-up of 0.96 (95% CI 0.83 to 1.12). For FEV1, the estimated treatment effect was 0.6 (95% CI –0.2 to 1.4) percentage points and the time effect was –0.1 percentage points (95% CI –0.2 to –0.0 percentage points) decline in FEV1 per month. The direction of effect favoured the intervention arm, but the 95% CI included zero, consistent with there being no difference between arms. There was a small trend for decreasing FEV1% predicted over time and no significant interaction between randomised treatment arm and time. For adherence, the original mixed-effects adherence model was applied to the extended follow-up data. Increasing the follow-up time from 12 to 21 months increased the estimated treatment effect from 9.5 to 11.9 percentage points (95% CI 11.1 to 12.7 percentage points). The time coefficient was –0.2 percentage points (95% CI –0.2 to –0.1 percentage points), suggestive of a slight decreasing trend in adherence levels over time.
Process evaluation results
Fidelity (work package 2.3)
For both certification and drift, two persons independently assessed each intervention and the level of agreement between assessors was high. All interventionists were successfully certified as competent to deliver the intervention, including the first visit, review visit and phase review. A total of 110 assessments were assessed to explore drift in fidelity over the duration of the trial and a pass mark threshold of 80% was set for drift assessments. Among all paired assessments during the RCT, there was 97.2% agreement when comparing pass/fail decisions at the 80% threshold (207/213 assessments in agreement). That is, the RCT had good fidelity (overall fidelity by site, range 79–97%), with only one site not achieving over the mean threshold (> 80%) on drift assessments. See Appendix 3 for further details of the fidelity assessment methods and findings.
Usual-care survey
Although most CF centres reported using objective adherence data at baseline, this was described as ad hoc or infrequent at most sites, indicating that our intervention’s systematic approach to measurement was different from usual care. Change scores indicated that usual care within the sites was consistent from baseline to follow-up. There was variation in usual care between RCT sites. See Appendix 4 for further details.
User satisfaction
Among those intervention users completing the satisfaction survey, most rated the following intervention components as ‘very helpful’: the first intervention meeting (77.4%), adherence graphs/tables (68.5%), interventionist support to solve problems (60%) and telephone (58.7%) and face-to-face (67.2%) follow-up visits with the interventionist (Table 7). Videos of other PWCF were rated as less helpful. There was variation between RCT sites. See Appendix 5 for further details.
| How helpful did you find the different parts of your intervention? | |||||
|---|---|---|---|---|---|
| Subquestion | n | Percentage | |||
| Very helpful | Quite helpful | Not very helpful | Not helpful at all | ||
| 1. The first meeting with the interventionist when they showed you CFHealthHub | 257 | 77.4 | 20.6 | 1.9 | 0.0 |
| 2. Graphs and tables of your adherence data | 257 | 68.5 | 28.0 | 2.7 | 0.8 |
| 3. ‘My Toolkit’ | 255 | 39.6 | 44.3 | 14.1 | 2.0 |
| 4. Video about how your treatment works | 254 | 37.8 | 36.6 | 20.1 | 5.5 |
| 5. Video clips of other people with CF | 252 | 29.0 | 36.9 | 25.8 | 8.3 |
| 6. My treatment on CFHealthHub | 252 | 48.4 | 40.5 | 8.3 | 2.8 |
| 7. ‘Problem-solving’ on CFHealthHub | 251 | 33.5 | 47.8 | 15.1 | 3.6 |
| 8. Making action plans | 255 | 38.8 | 41.6 | 16.1 | 3.5 |
| 9. Interventionist support to solve problems | 255 | 60.0 | 33.3 | 4.7 | 2.0 |
| 10. Follow-up telephone calls | 254 | 58.7 | 33.5 | 6.3 | 1.6 |
| 11. Follow-up visits (face to face) with the interventionist | 256 | 67.2 | 29.3 | 3.1 | 0.4 |
Trial monitoring
Among the participants approached for the RCT, 48% were recruited. A total of 566 declined recruitment, citing reasons such as not wanting to change the type of nebuliser they used and being too busy (see Report Supplementary Material 2).
The CFHealthHub digital platform was taken down for emergency technical work for 5 weeks (from 20 March to 23 April 2019). It was not available to participants in the RCT in that period. This affected the delivery of the intervention for a minority of PWCF in the intervention arm. This was taken into consideration in the mediation analysis and in a sensitivity analysis for the RCT.
Qualitative research
Process evaluations focus on mechanisms of action, implementation/delivery of the intervention and context. 45 The qualitative research focused on these issues. We report the findings in three parts: one focusing on a single mechanism of action (‘objective adherence data as proof’) (see Appendix 7), one focusing on the range of mechanisms of action operating in practice in the intervention, including some that were not identified in the feasibility study (see Appendix 8), and one focusing on variation in context and implementation between RCT sites/CF centres (see Appendix 9).
During data collection one of the qualitative researchers noted that PWCF and interventionists sometimes talked about the adherence data as ‘proof’. This mechanism of action was explored in detail by analysing codes related to mechanisms of action and identifying different aspects of this mechanism. The objective adherence data were described as offering proof to both self and others about adherence behaviour. PWCF perceived that this could offer benefits, including improving their relationships with their clinical team and their families, if objective adherence was higher than believed by these external parties (see Appendix 7 for further details about the role of proof in improving adherence).
During the feasibility study, we explored the range of mechanisms of action of the intervention. During the evaluation phase we continued to be interested in this. We had coded data to expected mechanisms of action and mechanisms of action associated with effective telehealth intervention and analysed these codes. There was evidence to support expected mechanisms of action around self-monitoring and self-regulation. Other mechanisms of action were also apparent, for example being monitored by others, which some interventionists believed affected control participants as well as intervention participants who mistakenly believed that clinicians could see their adherence data. The relationship between interventionists and PWCF in the intervention arm appeared to be an important mechanism, as found in the feasibility study. Open communication, home visits, continuity of relationship and time helped to build trust between interventionists and PWCF. This trust helped PWCF to talk openly and honestly about the challenges that they faced adhering to treatment. This meant that the interventionists understood more about the real-life challenges faced by PWCF and could help them to find ways to address those challenges. PWCF with high levels of baseline adherence reported gaining reassurance from the intervention. PWCF with very low levels of baseline adherence had challenging life situations that made improvement difficult. PWCF with low to moderate levels of adherence could improve adherence, with action plans to help establish treatment habits, especially if the time was right in their lives. PWCF found the components of the intervention acceptable, but some did not like the patient video clips and some struggled with setting formal action plans. (See Appendix 8 for further details about mechanisms of action).
There was considerable variation between the different RCT sites/CF centres in terms of the backgrounds of the interventionists delivering the intervention at each site, and the way in which the MDT engaged with the intervention and interventionist. That is, the context in which the intervention was delivered varied in the RCT, with the potential to affect implementation. See Appendix 9 for further details.
Mediation analysis
Awareness, habit, concerns, self-efficacy and effort were selected as mediators from the logic model (see Figure 3) because these were associated with both intervention and normative medication adherence. The standardised mean differences of the mediator between the intervention and control arms ranged from 0.6 to 0.2 and mediator–medication adherence correlation ranged from 0.1 to 0.5. Two-way interaction graphs showed that treatment effect had a stronger effect on awareness as baseline prescription increased, and treatment effect on effort was different for men and women. Hence, baseline prescription and gender were included as moderators. Age, gender and baseline measures of FEV1, number of nebulisers prescribed, depression score, i.v. therapy-days (binary), medication adherence, awareness, motivation and chaos were included as fixed-effect covariates. All selected mediators except awareness had a statistically significant association between each other.
The final mediation analysis model included all the selected mediators, moderators and fixed-effect covariates, demonstrating a good model fit (CFI 1.00, RMSEA 0.00, 95% CI 0.00 to 0.01). The total effect of the intervention on mean normative medication adherence was 13.3 (95% CI 9.6 to 17.0). The direct effect on adherence (i.e. not explained via the selected mediators) was 6.1 (95% CI 2.7 to 9.5). The overall indirect effect of awareness was 5 (95% CI 3.2 to 6.8) and mediated 37.3% of the total effect, but interacted linearly with mean baseline prescription: awareness mediated 18% of the total effect for patients using a nebuliser on alternate days and mediated 58% of the effect for those using six per day. The indirect effect of habit was 1.3 (95% CI 0.4 to 2.1; 9.4% mediated), of self-efficacy was 0.2 (95% CI –0.3 to 0.7; 1.6% mediated) and of concerns was 0.2 (95% CI –0.3 to 0.7; 1.5% mediated). The indirect effect of ease of effort was 0.2 (95% CI –0.1 to 0.5; 1.4% mediated) but interacted with sex: effort had some mediation for women at 0.4 (95% CI –0.2 to 1.0; 2.8% mediated) but little mediation for men at –0.03 (95% CI –0.3 to 0.3; –0.2% mediated). Results from the sensitivity analysis showed a similar trend but with a slightly higher mediating effect as mean baseline prescription increased (14% to 63% for the baseline prescription range 0.5 to 6.0).
The results from the mediation analysis suggests that the intervention helped improve patient’s awareness of their medication usage. This increased awareness contributed to an increased medication adherence, with some evidence to suggest that this effect was more pronounced for patients who used several nebulisers at baseline. Another pathway in which the intervention could have affected medication adherence was by facilitating habit formation, resulting in decreased effort required to take medication, thereby reducing concerns and improving self-efficacy. The total mediated effect of all these mediators was 51%.
See Appendix 10 for further information.
Triangulation for process evaluation
The triangulation grid bringing together all the components of the process evaluation is reported in Appendix 11. This grid was used to draw key conclusions from the process evaluation. The synthesised conclusions were as follows:
-
Implementation was very good. The intervention was delivered with good fidelity at all RCT sites with the exception of one. There was one period of 5 weeks towards the end of the RCT when the CFHealthHub platform was not available; this was considered in both the mediation analysis and the RCT sensitivity analysis.
-
The intervention was acceptable. The majority of PWCF who completed the survey found it very helpful, particularly the graphs of adherence and the interventionists’ visits. Some caution is appropriate because not all PWCF completed the survey and PWCF may have wanted to please the interventionist, who might have been present at the time the questionnaire was completed.
-
The expected mechanisms of action were evident (e.g. self-monitoring), and further mechanisms of action were identified for improvements in adherence to nebulisers. Changes in people’s calibration of their perceived adherence rates affected improvements in objectively measured adherence rates, showing the importance of the objective adherence data.
-
The intervention was different from usual care.
-
The context in which the intervention was delivered differed by RCT site owing to the differing strengths of the interventionists and the different levels of engagement of MDTs with the intervention.
Implications for randomised controlled trial subgroup and sensitivity analyses
Our proposed RCT subgroup analyses identified from the process evaluation were specified prior to analysis of the RCT data. Based on the process evaluation, we were keen to understand if RCT results differed by:
-
good versus poor implementation at different sets of sites
-
whether or not the CFHealthHub intervention was available (i.e. where it was not available for 5 weeks owing to technical difficulties)
-
levels of baseline adherence.
In practice it was not possible to identify a set of RCT sites with poorer implementation because contextual issues varied greatly between sites. Therefore, we did not undertake this subgroup analysis. The issue about the lack of availability of the intervention for a few weeks was addressed in a sensitivity analysis and the mediation analysis (see Report Supplementary Material 2). The lack of availability of the intervention for 5 weeks did not affect the RCT results.
The baseline adherence subgroup analysis had been specified in our set of a priori RCT subgroup analyses but these planned subgroup analyses were undertaken on the primary outcome only. After our process evaluation we were interested in the relationship between baseline adherence and the key secondary outcomes of change in adherence rates and FEV1.
Subgroup analyses are shown in Report Supplementary Material 2. There were no statistically significant subgroup differences in number of exacerbations (primary outcome). Post hoc additional subgroup analyses are also shown in Report Supplementary Material 2. The only statistically significant subgroup difference was related to improvement in adherence rates differing by baseline objective adherence (p < 0.001). PWCF with low and moderate levels of baseline adherence had the biggest improvement (18% and 15%, respectively) and high-level adherers had the least (3%) improvement and very low-level adherers had a 10% improvement. Adherence graphs by baseline adherence are displayed in Report Supplementary Material 2.
Triangulation of randomised controlled trial results and process evaluation findings (work package 3.4)
Work package 3.4 brings together the findings from the RCT and process evaluation related to outcomes. See Appendix 12 for more details. The process evaluation cannot identify changes in outcomes compared with control but can offer insights to support (or otherwise) RCT results, as well as explain how outcomes might have been achieved. The overall conclusions of this triangulation process were:
-
The intervention did not statistically significantly reduce the primary outcome of numbers of exacerbations. This was not due to implementation problems because the intervention was implemented with high levels of fidelity.
-
The intervention increased adherence rates. This finding was supported by multiple components of the process evaluation, showing the importance of the objective adherence data to this improvement as well as habit forming and the relationship with interventionists. Improvements in adherence rates were greater among those with low to moderate levels of baseline adherence than among those with high levels of baseline adherence (where ceiling effects may have operated) or those with very low levels of baseline adherence (who may have struggled with complex lives).
Work package 3.5: health economic evaluation
Introduction
This section presents the methods and results of economic analyses undertaken to assess the cost-effectiveness of the CFHealthHub intervention evaluated within the RCT (Dr Martin Wildman, Sheffield Teaching Hospitals, 2019, personal communication).
We undertook two related economic analyses to assess whether or not the intervention represents good value for money for the NHS: (1) a short-term ‘within-trial’ economic evaluation alongside a clinical trial (EEACT) that compares health gains and costs for the intervention and usual-care arms using individual patient-level data (IPD) from the trial (Dr Martin Wildman, personal communication) only, and (2) a model-based analysis that compares the intervention with usual care over a lifetime horizon. The scope of the analyses is summarised in Table 8. Both analyses evaluate the cost-effectiveness of the intervention in a population of PWCF aged ≥ 16 years who are taking inhaled mucolytics or antibiotics from the perspective of the NHS and PSS. The within-trial analysis is restricted to the health outcomes and costs accrued in the trial follow-up period, whereas the model-based analysis includes the extrapolation of health outcomes and costs over a lifetime horizon and includes the use of additional evidence beyond that collected in the RCT.
| Element of analysis | Within-trial EEACT | Model-based analysis |
|---|---|---|
| Population | ACtiF trial population: people with diagnosed CF aged ≥ 16 years who are taking inhaled mucolytics or antibiotics | |
| Intervention | CFHealthHub intervention as per trial | CFHealthHub intervention as it would be rolled out to the NHS |
| Comparator | Usual care (no adherence intervention) | |
| Economic outcome | Incremental cost per QALY gained | |
| Time horizon | 1 year (primary outcome window) | Lifetime (70 years) |
| Perspective | NHS and PSS | |
| Discount rate | 3.5% for health outcomes and costs | |
| Price year | 2017/18 (£) | |
We used Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guidance (see Report Supplementary Material 6). 56
Within-trial economic evaluation alongside a clinical trial
Within-trial economic evaluation alongside a clinical trial methods
The EEACT was undertaken using IPD from the RCT (Dr Martin Wildman, personal communication) Quality-adjusted life-year (QALY) gains for each patient were estimated as the ‘background QALYs’ accrued based on EQ-5D-5L assessments measured during routine clinic visits, less health losses associated with exacerbations (days spent receiving i.v. antibiotics). EQ-5D-5L measurements were mapped to the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), using van Hout et al. 57
Background QALYs for each patient were calculated under four assumptions:
-
Mean utility was calculated using an area under the curve approach via the trapezium rule, assuming a constant rate of improvement/worsening in HRQoL between successive EQ-5D-5L measurements.
-
For patients with a final EQ-5D-5L visit beyond the primary outcome cut-off, the patient’s EQ-5D-5L score was estimated at day 365 based on assumption 1.
-
For patients with a final EQ-5D-5L visit before the primary outcome cut-off, the patient’s mean utility during the observed period was assumed to apply during the unobserved period (i.e. mean utility is ‘stretched’ over the whole primary outcome window).
-
Patients with missing baseline EQ-5D-5L measurements and those with fewer than two EQ-5D-5L assessments over the primary outcome window were treated as missing.
All QALY losses associated with i.v. days were estimated as the mean loss of utility between the first and last exacerbation-related EQ-5D-5L measurements (‘visit E3’, which took place approximately 4 weeks after the start of the exacerbation, minus ‘visit E1’, which took place at the start of the exacerbation) for the whole patient population multiplied by the number of i.v. therapy-days incurred by each individual patient. Total QALYs gained per patient were calculated by subtracting i.v.-related QALY losses from the background QALYs.
The EEACT includes the following resource costs: the CFHealthHub adherence intervention (applied to the intervention arm only); i.v. therapy-days in hospital; i.v. therapy-days at home; i.v drugs and consumables; visits from health-care practitioners; and nebuliser devices. Resource use estimates were derived from data collected using a standardised resource use form in the trial as well as expert opinion. The costs of nebulised drugs were not included in this analysis. Unit costs were taken from NHS Reference Costs 2017/18,58 the Personal Social Services Research Unit (PSSRU)59 and personal communication [Misbah Tahir, Clinical Pharmacist, Sheffield Teaching Hospitals NHS Foundation Trust, 2019; Carola Fuchs, PARI GmbH, 2019; Pauline Whelan, Farr Institute, and ACtiF Project Management Group (PMG), 2019]. The resource and cost inputs applied in the EEACT are presented in Appendix 13.
Missing data were handled using multiple imputation by chained equations over 10 iterations. 60 A complete-case analysis was undertaken as a secondary analysis. Total QALY gains and costs (with or without imputation) were included in a seemingly unrelated regression (SUR) model,61 which adjusts for baseline imbalances between the two groups in terms of baseline EQ-5D-5L and i.v. therapy-days in the previous 12 months (a stratification factor in the ACtiF RCT).
Within-trial economic evaluation alongside a clinical trial results
The results of the EEACT are summarised in Table 9. The base-case results for the EEACT suggest that the intervention generated 0.01 additional QALYs at an additional cost of £865.91 per patient; the corresponding incremental cost-effectiveness ratio (ICER) is £71,136 per QALY gained. The complete-case analysis suggests a higher ICER of £109,754 per QALY gained.
| Option | Descriptive statisticsa | SUR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| QALYs | SE | 95% CI QALYs | Costs (£) | SE | 95% CI costs (£) | Incremental QALYs | 95% CI incremental QALYs | Incremental costs (£) | 95% CI incremental costs (£) | ICER (£) | |
| Complete-case analysis (N = 587) | |||||||||||
| CFHealthHub | 0.77008 | 0.01002 | 0.7504104 to 0.7897539 | 12,709.19 | 723.93 | 11,287.39 to 14,131.00 | 0.0113299 | –0.19729272 to 0.21995255 | 1243.50 | 1174.39 to 1312.62 | 109,754 |
| Usual care | 0.75015 | 0.01051 | 0.7295104 to 0.7707954 | 11,760.74 | 825.24 | 10,139.95 to 13,381.54 | – | – | – | – | – |
| Imputed (N = 608) | |||||||||||
| CFHealthHub | 0.771179 | 0.0098649 | 0.7518052 to 0.7905527 | 12,399.96 | 707.36 | 11,010.77 to 13,789.14 | 0.0121726 | –0.0057556 to 0.0301008 | 865.91 | –1082.972 to 2814.792 | 71,136 |
| Usual care | 0.7509504 | 0.0103025 | 0.7307169 to 0.771184 | 11,921.04 | 814.43 | 10,321.59 to 13,520.48 | – | – | – | – | – |
The results of this analysis should be interpreted with caution for two reasons. First, the short time horizon for the EEACT incorporates all of the upfront costs of the intervention but does not include the potential longer-term benefits that accrue as a result of these. Second, the allocation of the intervention costs across the trial group is unlikely to reflect the actual per-patient costs of delivering the intervention in practice, further inflating costs and associated ICERs.
Model-based analysis
Model structure and assumptions
The model-based analysis uses evidence from the RCT (Dr Martin Wildman, personal communication) and other external sources to estimate the cost-effectiveness of the intervention versus usual care over a lifetime horizon. The model structure was based on the model used to inform the National Institute for Health and Care Excellence (NICE) technology appraisal of dry powder antibiotics. 62 The model adopts a state transition approach, defined according to five health states: (1) FEV1 ≥ 70% predicted, (2) FEV1 40–69% predicted, (3) FEV1< 40% predicted, (4) post transplant and (5) death (Figure 7). During each annual model cycle, patients may remain in their current FEV1% predicted state, transition to an improved or worsened FEV1% predicted state or die. A small proportion of patients in the worst lung function state (FEV1 < 40%) may undergo lung (or heart and lung) transplantation and do not subsequently receive further nebulised treatment. HRQoL is modelled according to FEV1% predicted stratum and transplant history, with QALY losses applied according to the proportion of time spent receiving i.v. therapy. The model uses a 12-month cycle length over 70 cycles; a half-cycle correction was applied to account for the timing of events. Total QALYs are calculated as the sojourn time in each health state weighted by state-specific utility scores, less any i.v.-related QALY losses. The model includes the costs associated with (1) the adherence intervention (intervention arm only), (2) health state resource use conditional on FEV1% predicted stratum (based on the trial resource use form used in the trial), (3) i.v. therapy-days at home or in hospital (including hospital inpatient stays, i.v. drugs and consumables), (4) nebuliser devices, (5) nebulised drugs and (6) lung or heart and lung transplant.
FIGURE 7.
State transition model structure.
The model employs the following key structural assumptions:
-
At model entry, patients are assumed to be aged 30 years, based on the mean age of patients at entry into the RCT (Dr Martin Wildman, personal communication).
-
Patients in any FEV1% predicted stratum can progress/regress to any other FEV1% predicted stratum during any model cycle.
-
Patients who undergo transplantation cannot revert back to other alive health states; hence, the only remaining event for these patients is death.
-
Mortality risk is conditional on model health state. General population and CF-specific mortality constraints63,64 are applied to ensure that the survival projection is plausible over the entire time horizon.
-
Exacerbation risk is dependent on FEV1% predicted stratum.
-
HRQoL is dependent on lung function and whether or not the patient is receiving i.v. therapy for exacerbations.
-
The impact of the adherence intervention is assumed to manifest through two mechanisms: (1) risk ratios (RRs) of switching FEV1% predicted strata in a given year and (2) a relative rate ratio (RRR) for the number of i.v. therapy-days incurred (which is, in turn, assumed to be constant across all three FEV1% predicted states):
-
The model assumes that the treatment effects and costs related to the intervention accrue only in the years in which the intervention is given.
-
In the base-case analysis, the intervention is assumed to be given for 10 years; this assumption is tested in sensitivity analyses.
-
The intervention is not assumed to directly affect the probability of undergoing transplantation or death.
-
The costs of antibiotics and ‘high-cost therapies’ are independent of adherence to those therapies. This assumption is tested extensively in sensitivity analyses.
Evidence used to inform the model’s parameters
The evidence sources used to inform the model’s parameters are summarised in Table 10; these are briefly described below. All model parameters are presented in detail in Appendix 15.
| Parameter | Source |
|---|---|
| Initial patient characteristics (age, sex, baseline FEV1% predicted) | RCT (Dr Martin Wildman, personal communication) (data pooled across arms) |
| Transition probabilities, including transplant and death: usual care | Multistate models fitted to CF registry data65 (including general population63 and CF-specific64 mortality constraints) |
| Exacerbation risk (FEV1% predicted) | CF registry data set65 |
| Treatment effects: RRs transitioning out of FEV1 states | RCT cumulative logit model |
| Treatment effects: RRR i.v. therapy-days | RCT zero-inflated negative binomial model |
| Utility by FEV1% predicted strata | Mixture model fitted to EQ-5D-5L data1 |
| Exacerbation i.v. therapy day disutility | RCT EQ-5D data (mapped from EQ-5D-5L to EQ-5D-3L) |
| i.v. therapy-days frequency (FEV1% predicted) | CF registry data set65 |
| Utility post transplant | Anyanwu et al.66 |
| Health state costs | RCT resource use form (Dr Martin Wildman, personal communication) |
| Nebulised treatment costs | CF registry data set65 |
| i.v. therapy day (hospital) cost | NHS Reference Costs 2017/1858 (bronchiectasis) |
| i.v. drugs (hospital/home) costs | Misbah Tahir, Sheffield Teaching Hospitals NHS Foundation Trust, personal communication |
| Nebuliser costs | Carola Fuchs, PARI GmbH, personal communication |
| Adherence intervention costs | RCT (Dr Martin Wildman, personal communication) and assumptions (see Appendix 13) |
Baseline characteristics
Initial patient characteristics (age, sex and the distribution of patients across FEV1% predicted strata at baseline) were based on pooled data for both arms of the RCT (Dr Martin Wildman, personal communication).
Transition probabilities: usual care
The probabilities of transitioning between the model health states under usual care were informed by analyses of a bespoke registry data set provided by the Cystic Fibrosis Trust. 65 The data set contained patient-level records for all patients included in the CF registry aged ≥ 16 years who had been prescribed nebulised mucolytics or antibiotics at any time point (data years 2006–15). The overall data set included 44,464 records across 7144 patients. We fitted a series of homogenous continuous time multistate models to the available data using the msm package in R. This method assumes that event hazards are constant with respect to time; hence, fitting a single model to the available data would have failed to reflect the increasing risk of death for older patients. To address this structural limitation in the approach, we fitted five separate multistate models to subsets of the data relating to patients in the following age categories: 30–34 years, 35–39 years, 40–44 years, 45–49 years and ≥ 50 years. Annual transition probabilities for each age category were estimated using the msm pmatrix function. In the economic model, a constraint was applied to ensure that the risk of death for any CF patient in the model is at least as high as that for the general population. 63 A further constraint was applied to all cycles from the age of 60 years to ensure that the risk of death from any state is at least as high as that estimated by the flexible parametric survival models reported by Keogh et al. ;64 this constraint was applied to prevent underestimation of the mortality rate caused by limited numbers of older patients in the registry data set.
Frequency of intravenous therapy-days: usual care
The expected number of i.v. therapy-days per year conditional on FEV1% predicted stratum under usual care was estimated directly from the overall CF registry data set. 65
Treatment effect models, risk ratios of transitioning between states
The FEV1% predicted states were modelled using a cumulative link logit model with a nominal treatment group effect, baseline FEV1% predicted state and adjustment for the previous year’s i.v. therapy-days, fitted to data from the RCT. The model was used to generate predicted probabilities for each follow-up FEV1% predicted state, given baseline state and treatment allocation, from which RRs for between-state transitions by treatment group were derived. A bootstrap approach was used to estimate the standard errors (SEs) of the ratios on a log normal scale.
Treatment effect models, relative rate ratio for intravenous therapy-days
The i.v. therapy day counts in the 12-month period following consent into the RCT were modelled using zero-inflated negative binomial regression with a treatment group factor, adjustment for baseline FEV1 status and the previous year’s i.v. therapy-days, and an offset for follow-up time. The treatment coefficient was exponentiated to give the IRR and the SE estimated using the delta method.
Health-related quality of life
Health utility values for the FEV1% predicted states were estimated using a de novo function developed to map from absolute FEV1% predicted scores to the EQ-5D-3L using data collected in the RCT (Dr Martin Wildman, personal communication). The data used to estimate the mapping model included repeated observations on 608 patients. Observations were not included in the analysis if EQ-5D-3L and FEV1% predicted were missing or not measured in the same day/visit (2308 excluded observations). The final number of observations used was 2386 (out of 4694), corresponding to 576 patients with a mean of 3.4 clinic visits (SD 2.07). The mapping function developed by van Hout et al. 57 was employed to transform the EQ-5D-5L descriptive system into EQ-5D-3L utility values. The mean EQ-5D-3L and FEV1% predicted values were 0.78 (SD 0.2) and 58.7 (SD 0.23). An adjusted limited dependent variable mixture model67,68 (ALDVMM) was used to estimate the relationship between FEV1% predicted and the mapped EQ-5D-3L values. The ALDVMM was developed specifically for mapping preference-based measures and has been shown to outperform other models. 69 Robust clustered SEs were used to take into account the presence of repeated observations per patient. Models using two to four components were estimated. On the basis the Akaike information criterion (AIC), the Bayesian Information Criterion (BIC) and the mean absolute error (MAE), a model with three components was selected for inclusion in the final economic model. 70 An assessment of the goodness of fit of the alternative models is presented in Appendix 15.
Utility values for patients undergoing transplantation and for patients receiving i.v. treatments were excluded from the mapping analysis. The utility value for the transplant state was taken from Anyanwu et al. 66 QALY losses associated with receiving i.v. treatments were estimated non-parametrically using the RCT data (using the same disutility applied in the EEACT; see Within-trial economic evaluation alongside a clinical trial methods). Health utility values were age-adjusted using Ara and Brazier. 71
Resource costs
The cost of the adherence intervention includes an initial cost associated with training and set-up (year 1 only), ongoing annual costs (including data transfer, monitoring, maintenance and hosting of the data platform), fidelity support and delivery of the intervention by interventionists. The model-based analysis assumes that 30 interventionists can support 5900 adult CF patients receiving nebulised therapies.
Resource use estimates and unit costs were taken from NHS Reference Costs 2017/18,58 the PSSRU,58 the British National Formulary (BNF),72 the ACtiF RCT (Dr Martin Wildman, personal communication) and personal communication (Misbah Tahir, Clinical Pharmacist, Sheffield Teaching Hospitals NHS Foundation Trust; Carola Fuchs, PARI GmbH; Pauline Whelan, Farr Institute, and the ACtiF PMG). Further details of the cost and resource use inputs are provided in Appendix 13.
Model evaluation methods
The model estimates the incremental cost-effectiveness of the intervention compared with usual care in terms of the incremental cost per QALY gained. Central estimates of cost-effectiveness were based on the expectation of the mean derived from the probabilistic version of the model, based on 2000 Monte Carlo samples. Parameter uncertainty was evaluated using both deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA). DSAs were used to identify key drivers of the cost-effectiveness. PSA was used to estimate the probability that the intervention is cost-effective across a range of willingness-to-pay (WTP) thresholds. Uncertainty was represented using cost-effectiveness planes and cost-effectiveness acceptability curves.
Validation
A number of activities were undertaken to ensure internal validity and credibility of the model; these are summarised in Appendix 16.
Model-based analysis: results
Table 11 presents the results of the base case analysis; the results of additional sensitivity analyses can be found in Appendix 17. Based on the probabilistic version of the model, the intervention is expected to generate 0.17 additional QALYs and cost savings of £1790 compared with usual care; therefore, the adherence intervention is expected to dominate usual care (i.e. it is expected to be more effective and less expensive). The results of the deterministic model are very similar. Assuming a WTP threshold of £20,000 per QALY gained, the probability that the intervention generates more net benefit than usual care is approximately 0.89.
| Option | LYGa | QALYs | Cost (£) | Incremental LYGa | Incremental QALYs | Incremental costs (£) | ICER |
|---|---|---|---|---|---|---|---|
| Probabilistic model | |||||||
| CFHealthHub adherence intervention | 23.42 | 11.01 | 297,872 | 0.40 | 0.17 | –1790 | Dominating |
| Usual care | 23.02 | 10.84 | 299,662 | – | – | – | – |
| Deterministic model | |||||||
| CFHealthHub adherence intervention | 23.37 | 10.96 | 299,107 | 0.40 | 0.17 | –1637 | Dominating |
| Usual care | 22.97 | 10.79 | 300,743 | – | – | – | – |
The DSAs indicate that the conclusions of the economic analysis are sensitive to assumptions regarding:
-
the duration over which health effects and costs of the adherence intervention apply
-
the impact of the adherence intervention on the patient’s FEV1% predicted stratum
-
increases in adherence to nebulised treatments and associated effects on drug-prescribing levels.
Discussion
Summary of findings
We successfully developed an intervention and refined it during a feasibility study. In a full-scale RCT of 608 PWCF, there was no statistically significant reduction in the primary outcome of incidence rate of pulmonary exacerbations over a 12-month follow-up period. However, the RCT demonstrated a clinically and statistically significant improvement in the secondary outcome of normative adherence for PWCF. The magnitude of the increase in adherence, at 10% on average, may not have been large enough to affect exacerbations. No significant difference was found between groups in lung function at 12 months. There were statistically significant improvements in secondary outcomes that supported the increase in adherence found, namely improvements in necessities and concerns, habit formation and treatment effort. Only one of the eight domains of quality of life showed statistically significant improvement: there was a reduction in treatment burden. There was a small average weight gain (typically a positive outcome in CF).
The process evaluation identified that the level of implementation fidelity was high and that some of the key expected mechanisms of action were in operation, including self-monitoring. PWCF in the RCT valued the objective adherence data and the relationship they built with the interventionists, which they perceived as helping them to change their behaviour. The process evaluation suggested that adherence improvement might occur more in the baseline middle adherence level groups than the high adherence level groups and the low adherence level groups. A statistical subgroup analysis supported these findings.
The health economic model suggested that the intervention is expected to generate additional health gains of 0.17 QALYs and cost-savings of £1790 over the patient’s remaining lifetime. In this modelling the intervention is assumed to be given for 10 years and that treatment effects and costs accrue only in the years the intervention is given.
Successes of the programme grant
The ACtiF programme set out to develop an intervention to help PWCF to increase adherence to nebuliser adherence and subsequently reduce pulmonary exacerbations. Between February 2015 and December 2019 objectives were achieved through a series of WPs. Each work package was delivered on time and to plan. The intervention was evaluated in what was, to our knowledge, the largest CF RCT conducted in the UK, which exceeded its participant recruitment targets and maintained a sufficient sample size at follow-up. This contrasts with similar RCTs conducted in the CF population that have failed to meet sample size requirements owing to research and intervention procedure difficulties at recruitment and over the duration of the study. 73–75 Extensive intervention development and feasibility testing allowed the programme to develop a professional, theory-based, person-based intervention that is both feasible and acceptable. Prior to this study there was insufficient evidence to support the use of any individual self-management support strategy. 76,77 A recent, large, well-conducted RCT of strategies to improve adherence in CF had null findings. 73 The legacy of the programme is a digital platform for measuring objective adherence in PWCF that is being used in the NHS now, and a manualised set of procedures for training and delivery of behaviour change techniques that improves adherence to nebuliser treatments but does not reduce exacerbations.
Challenges and methodological limitations
A major challenge for the whole programme was obtaining treatment costs for the RCT. Negotiating treatment costs in the context of UK clinical research is a well-documented problem. 78
The National Institute for Health Research (NIHR) funding panel members were keen that the RCT in this programme focused on improvements in patient health. The primary outcome in the RCT was a reduction in the number of exacerbations experienced by patients. The number of exacerbations is a valid and sensitive outcome for the detection of change in blinded drug trials. 79 However, we identified problems with its use as a primary outcome in this open-label RCT of a complex intervention based in routine care settings. The use of IVAB in clinical practice is essentially discretionary: about half of those meeting objective criteria, and < 10% of people suffering acutely poor FEV1, decline to receive them. 80,81 Conversely, uptake in i.v. rescue antibiotics appears to increase as an individual becomes more adherent with medication, with increased clinician contact allowing more exacerbations to be detected. 82,83 It is possible that our intervention and usual-care arms were subject to different levels of surveillance, with the intervention arm experiencing greater contact time and perhaps being more likely to self-present during exacerbations. Therefore, the estimated event rates may have been subject to ascertainment bias, with under-reporting of exacerbations in the usual-care arm.
Improvement in normative adherence was a key secondary outcome on the proposed causal pathway to reductions in exacerbations (see Figure 3). Some RCTs of adherence interventions use this as the primary outcome, but our goal was more challenging. There was an improvement of 10% in this outcome. It is clear (see Figure 6) that adherence rates were high in the first week of the RCT. Indeed, our baseline adherence level was high (≈ 45%) compared with the average observed adherence in UK CF centres (≈ 35%). 16,84 This reduced in both arms for a few weeks before increasing in the intervention arm. It was not possible to measure true baseline adherence; baseline adherence was measured when PWCF entered the RCT and it is likely that the Hawthorne effect occurred in the first week of the RCT as both intervention and usual-care arms reacted to being monitored in the RCT by trying to adhere to their medication. It is possible that this was followed by a return to normal adherence before the intervention began to help PWCF to improve their adherence.
The magnitude of the increase in adherence, at 10% on average and 15% in the low- to moderate-level adherers, may not have been large enough to affect exacerbations and FEV1. Alternatively, detection of change in exacerbations and FEV1 may be challenging within the time constraints of a 12-month follow-up period. As depicted in the CFHealthHub logic model (see Figure 3), compared with increased necessity, increased habit, decreased concern (immediate outcomes) and increased adherence (intermediate outcome), improvements in exacerbation rates and lung function (FEV1) are longer-term outcomes. It may be that further health benefits might be observed over longer periods of time, given the window over which habits are thought to develop,85 although our extra analysis of longer-term follow-up over 21 months for some RCT participants did not support this proposition. It is also possible that the number of exacerbations is not a good indicator of patient health, and that quality of life may be a better outcome in future studies, although other secondary outcomes such as quality of life also showed little or no improvement.
A key limitation of the economic analysis is that the data collection mechanisms in the CFHealthHub trial did not allow us to quantify the impact of the intervention on the number of packs of medicines prescribed. As a consequence, there remains uncertainty regarding the relationship between improving adherence on overall treatment costs incurred by the NHS. We undertook sensitivity analyses to explore the potential impact of this factor on the cost-effectiveness of the intervention.
Implications for practice and lessons learned
Effective chronic disease management with accurate diagnosis and medicines optimisation is reliant on accurate adherence measures. 15 Using state-of-the-art measurement tools to collect time- and date-stamped inhalation data, this study has evidenced that level of objective adherence to nebulised medications is lower than the patient themselves often realise. 16 Poor calibration between subjective and objective adherence may lead to inefficient clinical care for patients and consequent deteriorating health. Clinicians may be unable to distinguish between patients who are deteriorating because of untreated CF and patients whose CF is treated but who have complications requiring a change in treatment. The digital platform component of the intervention provides objective adherence data that may be useful in this context. A total of 19 UK CF centres now have access to the CFHealthHub platform. The joint Chief Investigator Martin Wildman has established the CFHealthHub Data Observatory and Improvement Collaborative (registered as ISRCTN14464661), which is using the infrastructure and learning from the ACTIF programme to enable access to the CFHealthHub platform for all PWCF and members of the MDT in routine care.
If used in practice, the expectation would be that the real-time measurement of adherence would be continuous and that the interventionist-delivered part of the intervention would be used once or more than once when someone’s adherence drops for any reason over their lifetime.
Recommendations for future research (in order of priority)
-
Given the non-significant difference in the primary outcome, further research is required to explore why an increase in objective normative adherence did not reduce exacerbations, and to develop interventions that reduce exacerbations.
-
The existing intervention could be adapted or tailored to address the needs of PWCF with different levels of baseline adherence, including those with low levels of baseline adherence who often have complex problems. That is, PWCF with low levels of motivation, who are disengaged from their CF team or who inhabit adverse social situations as indicated by high self-reported life chaos and low income. An enhanced, health and social care professional-led intervention to address the wider complexity of individuals’ lives, as a precursor to adherence support, could be developed.
-
The economic modelling indicates that the intervention is expected to dominate current care. Future research would be valuable to better understand whether or not and how the effects of the intervention change over time and whether or not improving adherence leads to increases in prescribed CF medicines.
Conclusions
The CFHealthHub was successfully developed. In the full-scale RCT there was no statistically significant reduction in the primary outcome of the number of pulmonary exacerbations at 12 months. Clinically and statistically significant improvements in the key secondary outcome of normative adherence were observed. The magnitude of the increase in adherence, at 10% on average, may not have been large enough to affect exacerbations. The intervention was delivered with fidelity and key mechanisms of action, including self-monitoring, were observed. The health economic model suggests that the intervention is expected to generate additional health gains of 0.17 QALYs and cost-savings of £1790 over the patient’s remaining lifetime. This finding is dependent on assumptions regarding the duration over which costs and effects of the intervention apply, the impact of the intervention on FEV1% predicted and the relationship between increased adherence and drug-prescribing levels.
Acknowledgements
We thank all participants who have taken part throughout the programme; Kathy Rowan as independent chairperson of both the NIHR Programme Grant Committee and Trial Steering Committee for the ACtiF Programme; the members of the Independent Trial Steering Committee (including David Torgerson); the members of the PPI panel (including Caroline Greenslade, Martin Cameron, Karen Leyland and Ruth Day); trial support officers (Louise Turner and Helen Wakefield); the data management support team (Amanda Loban and Chris Turtle); the CFHealthHub support team (including Steven Antrobus); the PARI GmbH support team; the Sheffield Teaching Hospital as sponsor; Carla Girling for support in the PMGs; Hannah Cantrill for contributions as a research assistant; Susan Kirkpatrick for her contribution to WP 2.1C; Alexander Scott and Samuel Keating for their contributions to the qualitative work in WP 3.1; Saleema Rex for her contribution to the mediation analysis in WP 3.3; Kate Ennis for her contribution to the health economic evaluation in WP 3.5; pilot trial principal investigators (Julia Nightingale, Jane Dewar and Mark Allenby) and interventionists (including Claire Oliver); and RCT principal investigators (Edward Nash, Jo Whitehouse, Ian Ketchall, Jackie Rendall, Helen Rodgers, Caroline Elston, Stephen Bourke, William Flight, Angela McGowen, Danie Watson, Rebecca Thomas, Dejene Shiferaw, Kathryn Bateman, Nick Bell, Nick Withers, Chris Sheldon, David Derry, David Waine, Uta Hill, Jonathan Myers, Chandra Ohri, Giles Fitch and Susan Madge) and interventionists (Katherine Miles, Lisa Kent, Valerie Mills, Peter Moran, Georgia King, Lucy Funnell, Jocelyn Choyce, Jessica Williams, Cally Evans, Kamilla Dack, Jayne Trott, Annant Damani, Shehnaz Raniwalla, Darren Tature, Alan Anderson, Penny Galey, Louise Warnock, Jayne Faulkner, Kathryn Barbour, Michelle Thomas, Harriet Gledhill, Katherine Donohue, Heather Seabridge, Michael Martin,, Katy Lee, Corina Weir, Laura Barlow, Sarah Salberg and Caroline Whitton).
Contributions of authors
Martin J Wildman (https://orcid.org/0000-0002-1658-881X) (Consultant in Respiratory Medicine and Adult Cystic Fibrosis) was the joint chief investigator of the ACtiF programme.
Alicia O’Cathain (https://orcid.org/0000-0003-4033-506X) (Professor of Health Services Research) was the joint chief investigator of the ACtiF programme and led the qualitative research and process evaluations
Daniel Hind (https://orcid.org/0000-0002-6409-4793) (Professor in Complex Interventions) was a co-applicant, led the feasibility study and provided oversight for the management of the programme.
Chin Maguire (https://orcid.org/0000-0002-9397-7608) (Study Manager) was the programme manager.
Madelynne A Arden (https://orcid.org/0000-0002-6199-717X) (Professor of Health Psychology) was a co-applicant and led the design of the CFHealthHub intervention and development and delivery of the intervention training programme and input into the design and analysis of the fidelity assessment.
Marlene Hutchings (https://orcid.org/0000-0002-4710-657X) (Lead Physiotherapist) led WP 2.1C and worked closely with Madelynne Arden in the design of the CFHealthHub intervention and development and delivery of the intervention training programme and fidelity assessment.
Judy Bradley (https://orcid.org/0000-0002-7423-135X) (Professor of Respiratory Health) led the design and analysis of the fidelity assessment and worked with Madelynne A Arden and Marlene Hutchings to deliver the intervention training programme.
Stephen J Walters (https://orcid.org/0000-0001-9000-8126) (Professor of Medical Statistics and Clinical Trials) led the statistical analysis throughout the programme.
Pauline Whelan (https://orcid.org/0000-0001-8689-3919) (Information Systems Manager) led the technical development of the CFHealthHub digital platform throughout the programme.
John Ainsworth (https://orcid.org/0000-0002-2187-9195) (Professor of Health Informatics) was a co-applicant and provided oversight of the technical development of the CFHealthHub digital platform.
Paul Tappenden (https://orcid.org/0000-0001-6612-2332) (Professor of Health Economic Modelling) was a co-applicant and led the health economic evaluation in WP 3.5.
Iain Buchan (https://orcid.org/0000-0003-3392-1650) (Chair in Public Health and Clinical Informatics for Population Health Sciences) was a co-applicant and advised on the digital aspects of data acquisition.
Rachel Elliott (https://orcid.org/0000-0002-3650-0168) (Professor in Health Economics) was a co-applicant and provided input to the health economic evaluation.
John Nicholl (https://orcid.org/0000-0001-5436-1264) (Professor of Health Services Research) was a co-applicant and advised on the RCT design.
Stuart Elborn (https://orcid.org/0000-0002-2323-442X) (Professor of Respiratory Medicine) was a co-applicant and advised on interpretation of the results from a clinical CF perspective.
Susan Michie (https://orcid.org/0000-0003-0063-6378) (Professor of Health Psychology) was a co-applicant and advised on developing the BCI.
Laura Mandefield (https://orcid.org/0000-0002-4219-5673) (Research Fellow/Statistician) conducted statistical analyses.
Laura Sutton (https://orcid.org/0000-0003-3327-5927) (Research Associate/Statistician) conducted statistical analyses.
Zhe Hui Hoo (https://orcid.org/0000-0002-7067-3783) (Clinical Research Fellow) provided ongoing clinical expertise.
Sarah J Drabble (https://orcid.org/0000-0001-7183-6321) (Research Fellow) led and conducted the qualitative data collection and analysis.
Elizabeth Lumley (https://orcid.org/0000-0002-8962-7568) (Research Associate) conducted qualitative data collection and analysis in WP 3.
Daniel Beever (https://orcid.org/0000-0001-9063-3677) (Study Manager and PPI lead) was a co-applicant and stood as PPI chairperson throughout the programme.
Aline Navega Biz (https://orcid.org/0000-0002-6923-4556) (Research Associate) conducted the health economic evaluation with Paul Tappenden in WP 3.5.
Anne Scott (https://orcid.org/0000-0003-4396-438X) (Research Assistant) led the context analysis conducted in WP 3.3, contributed to qualitative analyses and worked in the programme management team.
Simon Waterhouse (https://orcid.org/0000-0002-6303-9610) (Data Specialist) led data management activity and critical analyses that enabled the calculation of adherence outcomes.
Louisa Robinson (https://orcid.org/0000-0001-5680-0734) (Research Assistant) led the drafting of the report, worked in the programme management team and provided analyses for WP 2.3 and 3.3.
Mónica Hernández Alava (https://orcid.org/0000-0003-4474-5883) (Reader in Health Econometrics) conducted the health economic evaluation with Paul Tappenden in WP 3.5.
Alessandro Sasso (https://orcid.org/0000-0001-8909-926X) (Research Associate, Health Econometrician) conducted the health economic evaluation with Paul Tappenden in WP 3.5.
All authors contributed to the drafting of this report and had the opportunity to approve the final version.
Publications
Peer-reviewed journal articles published
Hoo ZH, Curley R, Campbell MJ, Walters SJ, Hind D, Wildman MJ. Accurate reporting of adherence to inhaled therapies in adults with cystic fibrosis: methods to calculate ‘normative adherence’. Patient Prefer Adherence 2016;10:887–900.
Tappenden P, Sadler S, Wildman M. An early health economic analysis of the potential cost effectiveness of an adherence intervention to improve outcomes for patients with cystic fibrosis. PharmacoEconomics 2017;35:647–59.
Hoo ZH, Coates E, Maguire C, Cantrill H, Shafi N, Nash EF, et al. Pragmatic criteria to define chronic pseudomonas aeruginosa infection among adults with cystic fibrosis. Eur J Clin Microbiol Infect Dis 2018;37:2219–22.
Arden MA, Drabble S, O’Cathain A, Hutchings M, Wildman M. Adherence to medication in adults with cystic fibrosis: an investigation using objective adherence data and the theoretical domains framework. Br J Health Psychol 2019;24:357–80.
Drabble SJ, O’Cathain A, Arden MA, Hutchings M, Beever D, Wildman M. When is forgetting not forgetting? A discursive analysis of differences in forgetting talk between adults with cystic fibrosis with different levels of adherence to nebulizer treatments. QualHealthRes 2019;29:2119–31.
Gao J, Arden M, Hoo ZH, Wildman M. Understanding patient activation and adherence to nebuliser treatment in adults with cystic fibrosis: responses to the UK version of PAM-13 and a think aloud study. BMC Health Serv Res 2019;19:420.
Hind D, Drabble SJ, Arden MA, Mandefield L, Waterhouse S, Maguire C, et al. Supporting medication adherence for adults with cystic fibrosis: a randomised feasibility study. BMC Pulm Med 2019;19:77.
Hoo ZH, Gardner B, Arden MA, Waterhouse S, Walters SJ, Campbell MJ, et al. Role of habit in treatment adherence among adults with cystic fibrosis. Thorax 2019;74:197–9.
Hoo ZH, Wildman MJ, Campbell MJ, Walters SJ, Gardner B. A pragmatic behavior-based habit index for adherence to nebulized treatments among adults with cystic fibrosis. Patient Prefer Adherence 2019;13:283–94.
Drabble SJ, O’Cathain A, Scott AJ, Arden MA, Keating S, Hutchings M, et al. Mechanisms of action of a web-based intervention with health professional support to increase adherence to nebulizer treatments in adults with cystic fibrosis: qualitative interview study. J Med Internet Res 2020;22:e16782.
Hind D, Drabble SJ, Arden MA, Mandefield L, Waterhouse S, Maguire C, et al. Feasibility study for supporting medication adherence for adults with cystic fibrosis: mixed-methods process evaluation. BMJ Open 2020;10:e039089.
Arden M, Hutchings M, Whelan P, Drabble SJ, Beever D, Bradley JM, et al. Development of an intervention to increase adherence to nebuliser treatment in adults with cystic fibrosis: CFHealthHub. Pilot and Feasibility Stud 2021;7:PMC7780635.
Wildman MJ, O’Cathain A, Maguire C, Arden MA, Hutchings M, Bradley J, et al. Self-management intervention to reduce pulmonary exacerbations by supporting treatment adherence in adults with cystic fibrosis: a randomised controlled trial [published online ahead of print September 23 2021]. Thorax 2021.
Conference abstracts
Arden MA, Drabble SJ, O’Cathain A, Hutchings M, Wildman M. ACtiF study: understanding adherence to nebuliser treatment in adults with cystic fibrosis using the theoretical domains framework. J Cyst Fibros 2016;15:S26.
Arden MA, Drabble SJ, O’Cathain A, Hutchings M, Wildman M. Applying the theoretical domains framework to adherence to nebuliser treatment in adults with cystic fibrosis. Eur Heal Psychol 2016;18:689.
Sadler S, Wildman M, Tappenden P. Cost-effectiveness of a complex adherence intervention to improve prevention in cystic fibrosis by empowering effective self-management: an exploratory health economic evaluation using UK Registry data. J Cyst Fibros 2016;15:S119–20.
Whelan P. Co-producing Digital Platforms to Support an Ageing Population, Employing Information and Communications Technologies in Homes and Cities for the Health and Well-Being of Older People, Sichuan University, Chengdu, China, 15–17 August 2016.
Whelan P. Using Digital Platforms to Improve the Lives of People with Chronic Conditions: A Connected Health Case Study of CFHealthHub, Mobile Apps & Behaviour Change for Connected Health Seminar, University of Manchester, Manchester, UK, 27 September 2016.
Wildman M, Buchan I, Ainsworth J, Whelan, P. CFHealthHub: Complex Intervention to Support Self-Management in People with Cystic Fibrosis. A Case Study for the Farr Institute ‘100 Ways of Using Data to Make Lives Better’. West De Moines, IA: Farr Institute. 2016.
Arden M, Drabble S, O’Cathain A, Hind D, Hutchings M, Whelan P, et al. CFHealthHub: The Development of a Digital Intervention to Provide Feedback on Objective Nebuliser Adherence Data for Adults with Cystic Fibrosis (CF). 3rd UCL Centre for Behaviour Change Digital Health Conference 2017: Harnessing Digital Technology for Behaviour Change, 22–23 February 2017, London, UK.
Arden MA, Hutchings M, Whelan P, Wildman MJ, CFHealthHub group. Understanding Nebuliser Adherence in Adults with Cystic Fibrosis: Comparing High, Medium and Low Adherers. 31st European Health Psychologist conference, 31 August to 2 September 2017, Padua, Italy.
Hind D, Maguire C, Cantrill H, O’Cathain A, Wildman M. CFHealthHub: complex intervention to support adherence to treatment in adults with cystic fibrosis: external pilot trial. J Cyst Fibros 2017;16:S40–1.
Hoo ZH, Curley RE, Campbell SMJ, Wildman MJ. Cluster analysis of objective nebuliser adherence data among adults with CF: change in adherence over time and association with clinical outcomes. J Cyst Fibros 2017;16:S60–1.
Hoo ZH, Waterhouse S, Nightingale JA, Dewar J, Allenby M, Haynes F, et al. CFHealthHub: using Leeds criteria and clinicians’ decision to determine the pseudomonas status among the 64 adults with cystic fibrosis in the two centre CFHealthHub (CFHealthHub) pilot study. J Cyst Fibros 2017;16:S105–6.
Horspool K, Whelan P, Wildman M. CFHealthHub: iterative co-production of a data feedback platform providing patient level adherence data to multi-disciplinary teams to optimise individual care and centre level adherence data to centre directors allowing benchmarking to enable centre level quality improvement cycles in a 3 centre improvement collaborative. J Cyst Fibros 2017;16:S155.
Kirkpatrick S, Arden M, Beever D, Bradley J, Cantrill H, Daniels T, et al. CFHealthHub: development and evaluation of videos incorporating peer description of successful self-management with inhaled therapies in adults with CF used to build self-efficacy to support self-care within the CFHealthHub complex intervention. J Cyst Fibros 2017;16:S155–6.
Whelan P. Data and mHealth Technologies. Digital Health Outlook, UK and Italy Experiences in a Global Context, 20 March 2017, Milan, Italy.
Whelan P, Hassan L, Fox S, Tempest E. Developing Mobile Applications for Health. Informatics for Health Congress, 24–26 April 2017, Manchester, UK.
Arden MA, Gao J, Hoo ZH, Wildman M. The Patient Activation Measure and Adherence in Adults with Cystic Fibrosis: A Think-Aloud Study. BPS Division of Health Psychology Annual Conference, 5–7 September 2018, Newcastle upon Tyne, UK.
Wildman M, Hutchings M, Arden M, O’Cathain A, Drabble S, Walters SJ, et al. Development and Evaluation of an Intervention to Support Adherence to Treatment in Adults with Cystic Fibrosis: A Randomised Controlled Trial and Parallel Process Evaluation Protocol. Sheffield: Sheffield Teaching Hospitals NHS Foundation Trust; 2018.
Arden M. Health and habits. Psychologist 2019;32:7.
Whelan P, Stockton-Powdrell C, van Velsen L, Tabak M, Wolf K. Interdisciplinary Perspectives on Connected Health Research: Facilitators and Barriers for Sustainable Platform Development. MedInfo: 17th World Congress of Medical and Health Informatics, 25–30 August 2019, Lyon, France.
Other publications
Beever DA, Wildman M. A personal reflection on being a co-applicant in the ACtiF study. Res Involv Engagem 2017;3:12.
Data-sharing statement
Requests for patient-level data and statistical code should be made to the corresponding author and will be considered by members of the original trial management group, including the chief investigators and members of the Clinical Trials Research Unit (CTRU), who will release data on a case-by-case basis. Data will be shared following the principles for sharing patient-level data as described by Smith et al. 86 The data will not contain any direct identifiers, we will minimise indirect identifiers and we will remove free-text data to minimise the risk of identification.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- UK Cystic Fibrosis Registry . UK Cystic Fibrosis Registry Annual Data Report 2018 2019.
- Davies JC, Alton EWFW, Bush A. Cystic fibrosis. BMJ 2007;335. https://doi.org/10.1136/bmj.39391.713229.AD.
- Villanueva G, Marceniuk G, Murphy MS, Walshaw M, Cosulich R. Guideline Committee . Diagnosis and management of cystic fibrosis: summary of NICE guidance. BMJ 2017;359. https://doi.org/10.1136/bmj.j4574.
- Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis. BMJ 2016;352. https://doi.org/10.1136/bmj.i859.
- O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009;373:1891-904. https://doi.org/10.1016/S0140-6736(09)60327-5.
- Southern KW, Barker PM, Solis-Moya A, Patel L. Cochrane Cystic Fibrosis and Genetic Disorders Group . Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev 2012;11. https://doi.org/10.1002/14651858.CD002203.pub4.
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72. https://doi.org/10.1056/NEJMoa1105185.
- McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med 2008;178:921-8. https://doi.org/10.1164/rccm.200712-1804OC.
- Ryan G, Mukhopadhyay S, Singh M. Nebulised anti-pseudomonal antibiotics for cystic fibrosis. Cochrane Database Syst Rev 2003;3. https://doi.org/10.1002/14651858.CD001021.
- Jones AP, Wallis C. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev 2010;3. https://doi.org/10.1002/14651858.CD001127.pub2.
- Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2009;2. https://doi.org/10.1002/14651858.CD001506.pub3.
- Ryan G, Singh M, Dwan K. Inhaled antibiotics for long-term therapy in cystic fibrosis. Cochrane Database Syst Rev 2011;3. https://doi.org/10.1002/14651858.CD001021.pub2.
- Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009;8:91-6. https://doi.org/10.1016/j.jcf.2008.09.007.
- Sawicki GS, Tiddens H. Managing treatment complexity in cystic fibrosis: challenges and opportunities. Pediatr Pulmonol 2012;47:523-33. https://doi.org/10.1002/ppul.22546.
- World Health Organization (WHO) . Adherence to Long-Term Therapies: Evidence for Action 2003.
- Daniels T, Goodacre L, Sutton C, Pollard K, Conway S, Peckham D. Accurate assessment of adherence: self-report and clinician report vs electronic monitoring of nebulizers. Chest 2011;140:425-32. https://doi.org/10.1378/chest.09-3074.
- McNamara PS, McCormack P, McDonald AJ, Heaf L, Southern KW. Open adherence monitoring using routine data download from an adaptive aerosol delivery nebuliser in children with cystic fibrosis. J Cyst Fibros 2009;8:258-63. https://doi.org/10.1016/j.jcf.2009.04.006.
- Eakin MN, Bilderback A, Boyle MP, Mogayzel PJ, Riekert KA. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros 2011;10:258-64. https://doi.org/10.1016/j.jcf.2011.03.005.
- Briesacher BA, Quittner AL, Saiman L, Sacco P, Fouayzi H, Quittell LM. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med 2011;11. https://doi.org/10.1186/1471-2466-11-5.
- Smyth A, Lewis S, Bertenshaw C, Choonara I, McGaw J, Watson A. Case-control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax 2008;63:532-5. https://doi.org/10.1136/thx.2007.088757.
- Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med 2002;166:1550-5. https://doi.org/10.1164/rccm.200202-087OC.
- de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011;66:680-5. https://doi.org/10.1136/thx.2011.161117.
- Quittner AL, Zhang J, Marynchenko M, Chopra PA, Signorovitch J, Yushkina Y, et al. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest 2014;146:142-51. https://doi.org/10.1378/chest.13-1926.
- Tappenden P, Sadler S, Wildman M. An early health economic analysis of the potential cost effectiveness of an adherence intervention to improve outcomes for patients with cystic fibrosis. PharmacoEconomics 2017;35:647-59. https://doi.org/10.1007/s40273-017-0500-x.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Horne RW, Barber N, Elliot R, Morgan M. Concordance, Adherence and Compliance in Medicine Taking. Report for the National Co-Ordinating Centre for NHS Service Delivery and Organisational R&Amp;D (NCCSDO) 2005.
- James Lind Alliance . Cystic Fibrosis Top 10 n.d. www.jla.nihr.ac.uk/priority-setting-partnerships/cystic-fibrosis/top-10-priorities.htm.
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994;331:637-42. https://doi.org/10.1056/NEJM199409083311003.
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 2006;354:229-40. https://doi.org/10.1056/NEJMoa043900.
- Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, Nabhan M, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect 2015;18:1151-66. https://doi.org/10.1111/hex.12090.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analysing Qualitative Data. London: Routledge; 1994.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Drabble SJ, O’Cathain A, Arden MA, Hutchings M, Beever D, Wildman M. When Is forgetting not forgetting? a discursive analysis of differences in forgetting talk between adults with cystic fibrosis with different levels of adherence to nebulizer treatments. Qual Health Res 2019;29:2119-31. https://doi.org/10.1177/1049732319856580.
- Arden MA, Drabble S, O’Cathain A, Hutchings M, Wildman M. Adherence to medication in adults with Cystic Fibrosis: an investigation using objective adherence data and the Theoretical Domains Framework. Br J Health Psychol 2019;24:357-80. https://doi.org/10.1111/bjhp.12357.
- O’Cathain A, Croot L, Sworn K, Duncan E, Rousseau N, Turner K, et al. Taxonomy of approaches to developing interventions to improve health: a systematic methods overview. Pilot Feasibility Stud 2019;5. https://doi.org/10.1186/s40814-019-0425-6.
- O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029954.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4055.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1-24. https://doi.org/10.1080/08870449908407311.
- Hind D, Drabble SJ, Arden MA, Mandefield L, Waterhouse S, Maguire C, et al. Supporting medication adherence for adults with cystic fibrosis: a randomised feasibility study. BMC Pulm Med 2019;19. https://doi.org/10.1186/s12890-019-0834-6.
- Drabble SJ, O’Cathain A, Scott AJ, Arden MA, Keating S, Hutchings M, et al. Mechanisms of action of a web-based intervention with health professional support to increase adherence to nebulizer treatments in adults with cystic fibrosis: qualitative interview study. J Med Internet Res 2020;22. https://doi.org/10.2196/16782.
- Hind D, Drabble SJ, Arden MA, Mandefield L, Waterhouse S, Maguire C, et al. Feasibility study for supporting medication adherence for adults with cystic fibrosis: mixed-methods process evaluation. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-039089.
- Wildman MJ, O’Cathain A, Maguire C, Arden MA, Hutchings M, Bradley J, et al. Self-management intervention to reduce pulmonary exacerbations by supporting treatment adherence in adults with cystic fibrosis: a randomised controlled trial [published online ahead of print September 23 2021]. Thorax 2021. https://doi.org/10.1136/thoraxjnl-2021-217594.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Wildman M, Hutchings M, Arden M, O’Cathain A, Drabble S, Walters SJ, et al. Development and Evaluation of an Intervention to Support Adherence to Treatment in Adults with Cystic Fibrosis: A Randomised Controlled Trial and Parallel Process Evaluation Protocol. Sheffield: Sheffield Teaching Hospitals NHS Foundation Trust; 2018.
- Ratjen F, Durham T, Navratil T, Schaberg A, Accurso FJ, Wainwright C, et al. Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J Cyst Fibros 2012;11:539-49. https://doi.org/10.1016/j.jcf.2012.05.003.
- Altman D, Machin D, Bryant T, Gardner M. Statistics with Confidence: Confidence Intervals and Statistical Guidelines. London: BMJ Books; 2000.
- Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with confidence. London: BMJ; 2000.
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ 1998;316:1236-8. https://doi.org/10.1136/bmj.316.7139.1236.
- Farmer T, Robinson K, Elliott SJ, Eyles J. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res 2006;16:377-94. https://doi.org/10.1177/1049732305285708.
- O’Cathain A. A Practical Guide to Using Qualitative Research with Randomized Controlled Trials. Oxford: Oxford University Press; 2018.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. https://doi.org/10.1136/bmj.332.7538.413.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:52-63. https://doi.org/10.1111/j.1752-7325.2011.00233.x.
- Vassilev I, Rowsell A, Pope C, Kennedy A, O’Cathain A, Salisbury C, et al. Assessing the implementability of telehealth interventions for self-management support: a realist review. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0238-9.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- NHS Improvement . NHS Reference Costs 2017 18 2018.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit; 2018.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Willan AR, Lin DY, Manca A. Regression methods for cost-effectiveness analysis with censored data. Stat Med 2005;24:131-45. https://doi.org/10.1002/sim.1794.
- Tappenden P, Harnan S, Uttley L, Mildred M, Carroll C, Cantrell A. Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis: systematic review and economic model. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17560.
- Office for National Statistics (ONS) . National Life Tables: England 2019.
- Keogh RH, Szczesniak R, Taylor-Robinson D, Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: A longitudinal study using UK patient registry data. J Cyst Fibros 2018;17:218-27. https://doi.org/10.1016/j.jcf.2017.11.019.
- Cystic Fibrosis Trust . CF Registry Bespoke Dataset (Years 2006–2015; Data Held on File) n.d. www.cysticfibrosis.org.uk/the-work-we-do/uk-cf-registry/reporting-and-resources (accessed 20 July 2021).
- Anyanwu AC, McGuire A, Rogers CA, Murday AJ. Assessment of quality of life in lung transplantation using a simple generic tool. Thorax 2001;56:218-22. https://doi.org/10.1136/thorax.56.3.218.
- Hernández Alava M, Wailoo A. Fitting adjusted limited dependent variable mixture models to EQ-5D. Stata J 2015;15:737-50. https://doi.org/10.1177/1536867X1501500307.
- Hernández Alava M, Wailoo AJ, Ara R. Tails from the peak district: adjusted limited dependent variable mixture models of EQ-5D questionnaire health state utility values. Value Health 2012;15:550-61. https://doi.org/10.1016/j.jval.2011.12.014.
- Hernández Alava M, Wailoo A, Pudney S, Gray L, Manca A. Modelling generic preference-based outcome measures: development and comparison of methods. Health Technol Assess 2019;24. https://doi.org/10.3310/hta24340.
- Wailoo AJ, Hernandez-Alava M, Manca A, Mejia A, Ray J, Crawford B, et al. Mapping to estimate health-state utility from non-preference-based outcome measures: an ISPOR Good Practices for Outcomes Research Task Force Report. Value Health 2017;20:18-27. https://doi.org/10.1016/j.jval.2016.11.006.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed May 2021).
- Quittner AL, Eakin MN, Alpern AN, Ridge AK, McLean KA, Bilderback A, et al. Clustered randomized controlled trial of a clinic-based problem-solving intervention to improve adherence in adolescents with cystic fibrosis. J Cyst Fibros 2019;18:879-85. https://doi.org/10.1016/j.jcf.2019.05.004.
- Sagel SD, Khan U, Jain R, Graff G, Daines CL, Dunitz JM, et al. Effects of an antioxidant-enriched multivitamin in cystic fibrosis. A randomized, controlled, multicenter clinical trial. Am J Respir Crit Care Med 2018;198:639-47. https://doi.org/10.1164/rccm.201801-0105OC.
- Bruzzese E, Raia V, Ruberto E, Scotto R, Giannattasio A, Bruzzese D, et al. Lack of efficacy of Lactobacillus GG in reducing pulmonary exacerbations and hospital admissions in children with cystic fibrosis: a randomised placebo controlled trial. J Cyst Fibros 2018;17:375-82. https://doi.org/10.1016/j.jcf.2017.10.014.
- Goldbeck L, Fidika A, Herle M, Quittner AL. Psychological interventions for individuals with cystic fibrosis and their families. Cochrane Database Syst Rev 2014;6. https://doi.org/10.1002/14651858.CD003148.pub3.
- Savage E, Beirne PV, Ni Chroinin M, Duff A, Fitzgerald T, Farrell D. Self-management education for cystic fibrosis. Cochrane Database Syst Rev 2014;9. https://doi.org/10.1002/14651858.CD007641.pub3.
- Palmer R, Harrison M, Cross E, Enderby P. Negotiating excess treatment costs in a clinical research trial: the good, the bad and the innovative. Trials 2016;17. https://doi.org/10.1186/s13063-016-1208-5.
- Vandevanter DR, Yegin A, Morgan WJ, Millar SJ, Pasta DJ, Konstan MW. Design and powering of cystic fibrosis clinical trials using pulmonary exacerbation as an efficacy endpoint. J Cyst Fibros 2011;10:453-9. https://doi.org/10.1016/j.jcf.2011.07.003.
- Schechter MS, Regelmann WE, Sawicki GS, Rasouliyan L, VanDevanter DR, Rosenfeld M, et al. Antibiotic treatment of signs and symptoms of pulmonary exacerbations: a comparison by care site. Pediatr Pulmonol 2015;50:431-40. https://doi.org/10.1002/ppul.23147.
- Morgan WJ, Wagener JS, Pasta DJ, Millar SJ, VanDevanter DR, Konstan MW. Scientific Advisory Group, Investigators, and Coordinators of the Epidemiologic Study of Cystic Fibrosis . Relationship of antibiotic treatment to recovery after acute FEV1 decline in children with cystic fibrosis. Ann Am Thorac Soc 2017;14:937-42. https://doi.org/10.1513/AnnalsATS.201608-615OC.
- Hoo ZH, Bramley NR, Curley R, Edenborough FP, Walters SJ, Campbell MJ, et al. Intravenous antibiotic use and exacerbation events in an adult cystic fibrosis centre: a prospective observational study. Respir Med 2019;154:109-15. https://doi.org/10.1016/j.rmed.2019.06.017.
- Lechtzin N, Mayer-Hamblett N, West NE, Allgood S, Wilhelm E, Khan U, et al. Home monitoring of patients with cystic fibrosis to identify and treat acute pulmonary exacerbations. eICE Study results. Am J Respir Crit Care Med 2017;196:1144-51. https://doi.org/10.1164/rccm.201610-2172OC.
- Hoo ZH, Curley R, Campbell MJ, Walters SJ, Hind D, Wildman MJ. Accurate reporting of adherence to inhaled therapies in adults with cystic fibrosis: methods to calculate ‘normative adherence’. Patient Prefer Adherence 2016;10:887-900. https://doi.org/10.2147/PPA.S105530.
- Lally P, van Jaarsveld CHM, Potts HWW, Wardle J. How are habits formed: modelling habit formation in the real world. Eur J Soc Psyc 2010;40:998-1009. https://doi.org/10.1002/ejsp.674.
- Tudur Smith C, Hopkins C, Sydes MR, Woolfall K, Clarke M, Murray G, et al. How should individual participant data (IPD) from publicly funded clinical trials be shared?. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0532-z.
- Martin RC. Agile Software Development Principles, Patterns, and Practices. Harlow: Pearson Education Inc.; 2003.
- Beck K, Beedle M, van Bennekum A, Cockburn A, Cunningham W, Fowler M, et al. Manifesto for Agile Software Development. Corryton, TN: Agile Alliance; 2001.
- Rubin KS. Essential Scrum: A Practical Guide to the Most Popular Agile Process. London: Addison-Wesley Professional; 2012.
- Schwaber K. Agile Project Management with Scrum. Redmond, WA: Microsoft Press; 2004.
- Clegg D, Barker R. Case Method Fast-Track: A RAD Approach. Boston, MA: Addison-Wesley Longman Publishing Co. Inc.; 1994.
- Bittner K, Spence I. Use Case Modeling. Boston, MA: Addison-Wesley Longman Publishing Co. Inc.; 2002.
- International Organisation for Standardization . ISO/IEC/27000/Family/–/Information/Security/Management/Systems 2013.
- Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Decis Process 1991;50:248-87. https://doi.org/10.1016/0749-5978(91)90022-L.
- Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality–social, clinical, and health psychology. Psych Bulletin 1982;92. https://doi.org/10.1037/0033-2909.92.1.111.
- Gardner B. A review and analysis of the use of ‘habit’ in understanding, predicting and influencing health-related behaviour. Health Psych Rev 2015;9:277-95. https://doi.org/10.1080/17437199.2013.876238.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555-67. https://doi.org/10.1016/S0022-3999(99)00057-4.
- Wood W, Neal DT. The habitual consumer. J Consumer Psych 2009;19:579-92. https://doi.org/10.1016/j.jcps.2009.08.003.
- Lally P, Wardle J, Gardner B. Experiences of habit formation: a qualitative study. Psych Health Med 2011;16:484-9. https://doi.org/10.1080/13548506.2011.555774.
- Rothman AJ, Sheeran P, Wood W. Reflective and automatic processes in the initiation and maintenance of dietary change. Ann Behaviour Med 2009;38:4-17. https://doi.org/10.1007/s12160-009-9118-3.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behaviour change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behaviour change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Craig P, Di Ruggiero E, Frohlich KL, Mykhalovskiy E. Taking Account of Context in Population Health Intervention Research: Guidance for Producers, Users and Funders of Research. Southampton: NIHR Evaluation, Trials and Studies Coordinating Centre; 2018.
- Booth A, Noyes J, Flemming K, Moore G, Tunçalp Ö, Shakibazadeh E. Formulating questions to explore complex interventions within qualitative evidence synthesis. BMJ Glob Heal 2019;4. https://doi.org/10.1136/bmjgh-2018-001107.
- Pfadenhauer LM, Gerhardus A, Mozygemba K, Lysdahl KB, Booth A, Hofmann B, et al. Making sense of complexity in context and implementation: the Context and Implementation of Complex Interventions (CICI) framework. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0552-5.
- Yin RK. Case Study Research: Design and Methods. Thousand Oaks, CA: SAGE Publications; 2003.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Drummond M, Sculpher M, Claxton K, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. ISPOR Task Force on Good Research Practices – Modeling Studies . Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health 2003;6:9-17. https://doi.org/10.1046/j.1524-4733.2003.00234.x.
- Bradley J, Blume S, Stafford M. Quality of Life and Health Utility in Patients With Cystic Fibrosis n.d.
Appendix 1 Work package 1: developing the technology and infrastructure to collect adherence data
Introduction
A digital platform, CFHealthHub, was created to provide the technical components of the complex intervention to support adherence to treatment in PWCF. CFHealthHub provided the front-end, user-facing components of the intervention (a website and mobile app) and the back-end server infrastructure for secure receipt and storage of adherence data. The technical development was defined by two high-level phases: (1) technical scoping and configuration of the infrastructure and (2) development of the digital BCI.
The platform was developed iteratively over an 18-month period, with a pre-pilot phase conducted with 27 participants from August 2015 to April 2016, followed by a two-centre feasibility study conducted with 64 participants across two CF centres in 2016–2017. The digital platform was finalised and became feature complete for the main RCT launch in October 2017. The first phase of the technical development involved high-level requirements capture for the project. The top priority identified was to enable the display of objective adherence data to the interventionists and PWCF through the CFHealthHub website. Discussions with the nebuliser supplier led to the configuration of a secure data transfer mechanism, enabling the devices to submit data in real-time through to the study server, hosted at the University of Manchester. To display the adherence data in CFHealthHub it was necessary to develop a manual entry point for the prescription information through the CFHealthHub website. The website was developed to display the objective adherence data in a variety of graphical and tabular formats.
After the website was scaffolded to display the objective adherence data, an intensive co-design process was launched to refine the display of data and to incorporate, develop and refine the BCI components. This involved multiple project stakeholders reviewing successive prototypes and wireframes of designs, and iterative website software releases. Input from the PPI group and from the qualitative research contributed to the design and refinement process. Development of the mobile app drew heavily on the earlier website development reviews, but designs were tailored for smaller screen sizes. Mobile-specific engagement strategies (e.g. push notifications to encourage engagement with the CFHealthHub digital platform) were also incorporated.
The software development was conducted by the mHealth team at the Health eResearch Centre, University of Manchester, with design expertise provided by the Manchester-based user experience company KeepItUsable. Later phases of the design work were completed in-house at the Health eResearch Centre.
Methods
Agile software methodologies were used during the development cycles of the software. 87,88 The software team worked to regular sprint cycles, delivering successive software releases approximately every 3 weeks. These iterative releases were reviewed by members of the research team, PWCF and other project stakeholders for refinement and feedback. An intensive phase of co-design ran from August 2015 until the launch of the feasibility study and involved weekly meetings with the software team lead, external design lead, the health psychologist and the research physiotherapist leading the intervention design. During these meetings key features were designed and refined through prototyping and wire-framing until project stakeholders were satisfied. PPI and feedback using qualitative research with users were fed into these design meetings by research team members and if areas were identified where further input was required from PWCF, these were highlighted to the PPI lead of the project and taken to the PPI group for consultation.
Internally, the software team used agile scrum methodology, meeting at daily stand-ups to report and plan progress, and delivering software in regular three-weekly sprint cycles. 89,90 This agile process allowed the software team to be responsive to changing requirements and flexible to the changing needs of the platform as identified through PPI and qualitative feedback.
The MoSCoW91 (must have, should have, could have, won’t have) prioritisation system was used in the later stages of the project to ensure that technical resources were directed to the critical areas of feature development and enhancements. 92 Using the MoSCoW91 system the team categorised each feature or change request into must have, should have, could have or will not have at this time. This was supported by effort estimates provided by the technical team and priorities as identified in the PPI and qualitative feedback.
The digital platform CFHealthHub is hosted in a secure, ISO 27001-certified environment at the University of Manchester. 93
Results
Figure 2 outlines the technical architecture of the digital components of the intervention delivery and shows (1) the nebulisers used by PWCF, which have electronic monitoring capabilities and transfer data to the Qualcomm hub located in the participant’s home (nebuliser and hub provided by PARI GmbH), (2) how data are received by the CFHealthHub server located at the University of Manchester, which stores these inhalation data securely and combines them with prescription data entered manually through the website, and (3) the user-facing components, website and mobile apps that are used by the clinicians and participants in the trial to view adherence data and display the behaviour change components.
The website was designed to support role-based access to clinicians/interventionists (for viewing adherence data), PWCF in the trial and members of the research team, who required access to the data for further analysis. Features developed for the website and mobile app included the following:
-
Adherence display in graphical (Figure 8) and tabular (Figure 9) format, supported by manual prescription entry through the web interface and automatic real-time data transfer from the nebulisers to the CFHealthHub.
-
‘My Education’ and ‘Problem-solving’ information, included for PWCF to view information and support (e.g. to read about the reasons for taking medication, clarify concerns about antibiotic use or to view information about how to clean the nebuliser) (Figure 10).
-
‘My Toolkit’ – a personalised space in the platform for PWCF to view information that had been tailored specifically to them.
-
Screening tool to support interventionists tailor the content delivered in the ‘My Toolkit’ section towards the individual person with CF (Figure 11).
-
Peer videos – an important aspect of our iterative response to patient feedback included the incorporation of videos in which patients tell their own stories. The additional complexity of indexing in the region of 20 interviews cut into short segments and tagged by identifiers (e.g. managing CF at university) was likely to lead to around 80 or more individual video clips that needed to be easily found on the CFHealthHub digital platform. Making this part of the website compelling and easy to use required programming and graphic design input. Following consultation with the Medicines and Healthcare products Regulatory Agency (MHRA) it was confirmed we did not need to pay MHRA fees to support the evaluation of CFHealthHub.
-
‘Action Planning’, ‘Coping Planning’, ‘Party Planning’ and ‘Day Planning’ to support the behaviour change components of the trial.
-
Push notifications to encourage engagement with the CFHealthHub tools.
-
Push notifications informing participants of their adherence rates and encouraging an increase in adherence to medication.
-
Click and touch analytics – embedded in both the website and mobile apps, enabling the research team to view engagement metrics (e.g. how often each element on the website and mobile apps were visited and by whom).
-
Export functionality to allow members of the quantitative research team to download the data in secure encrypted format for further analysis.
FIGURE 8.
Adherence display in tabular format.
FIGURE 9.
Adherence display in graphical format.
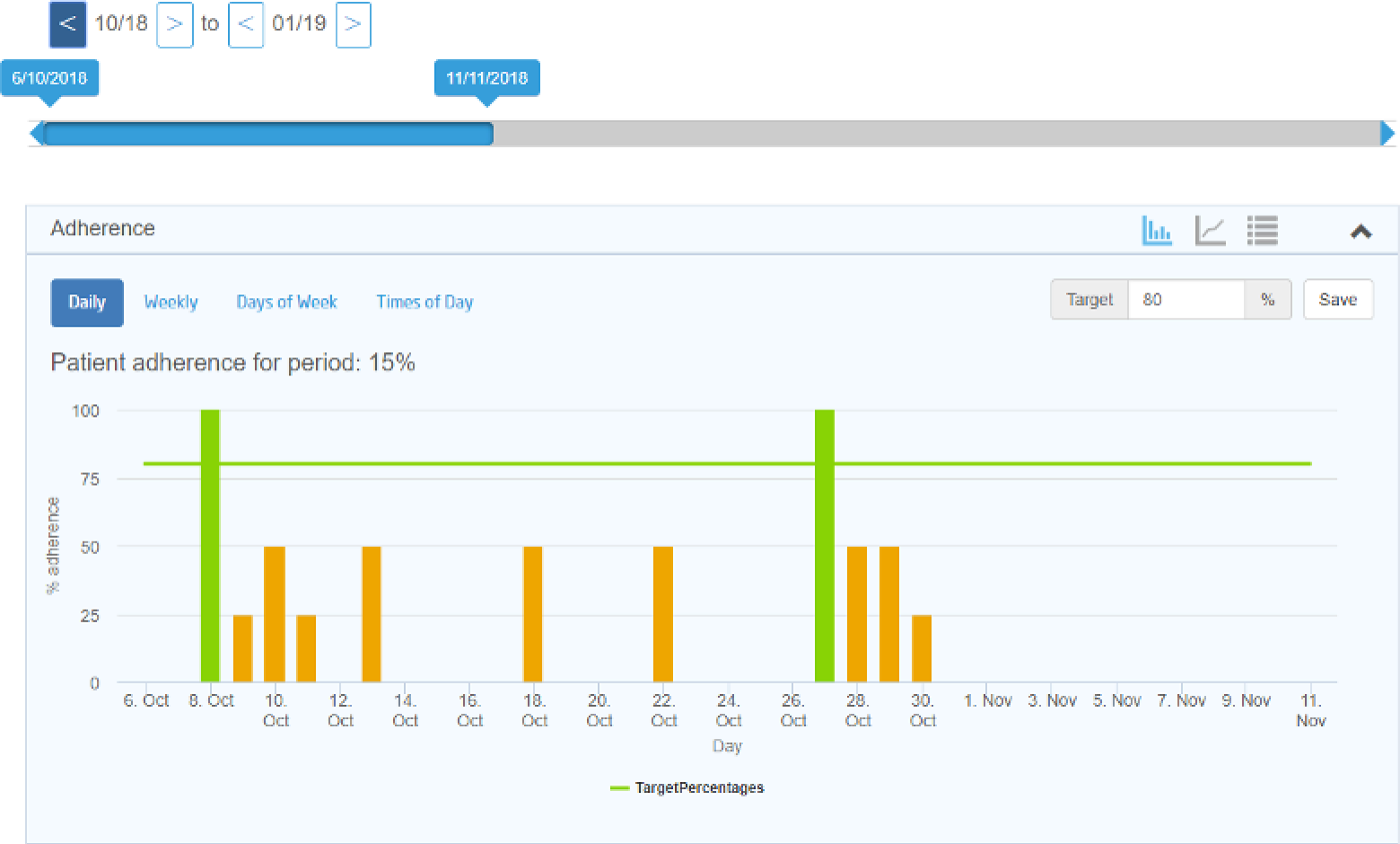
FIGURE 10.
‘My Education’ and ‘Problem-solving’ information on CFHealthHub.
FIGURE 11.
Screening tool to support interventionists to tailor CFHealthHub.
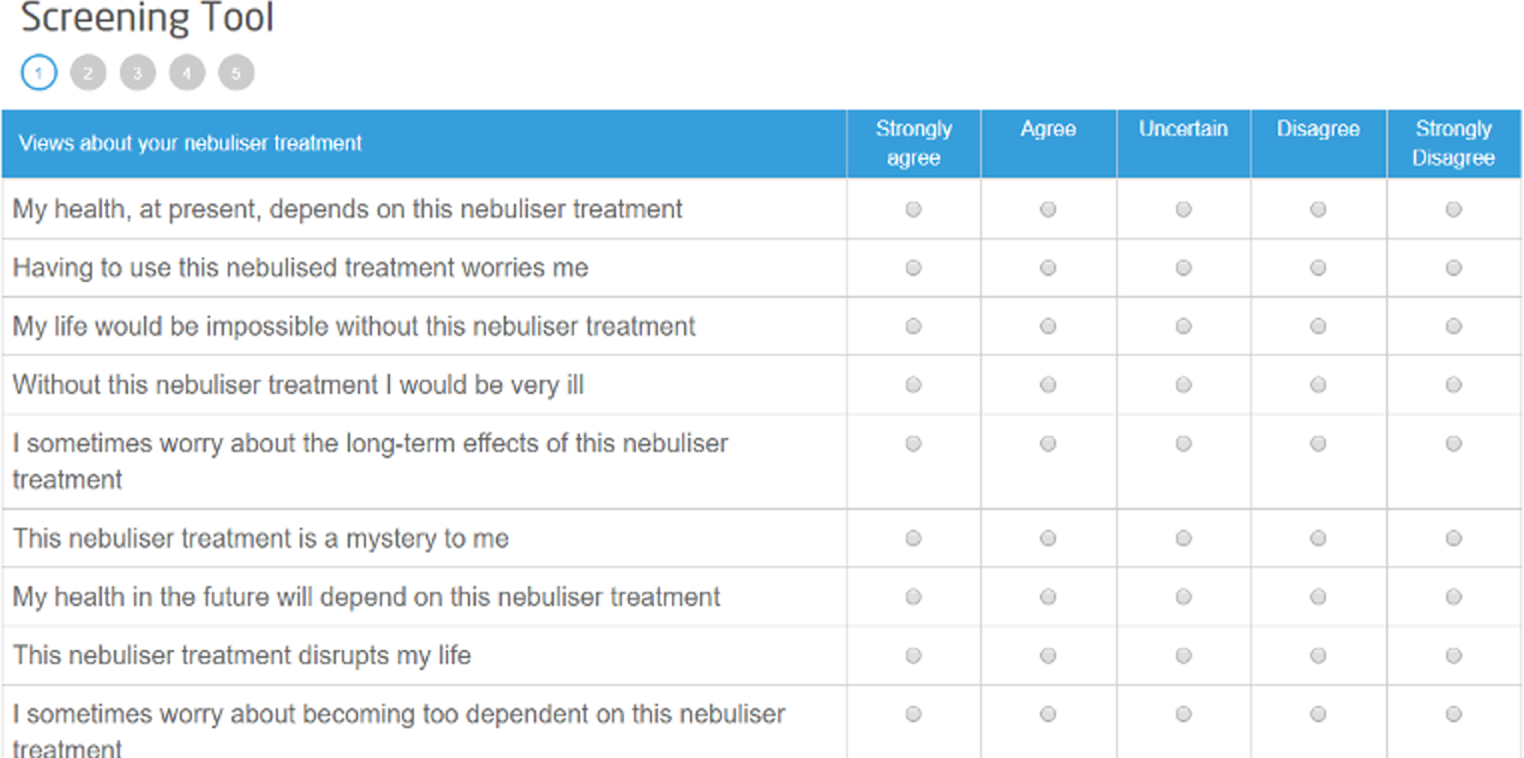
The technical design of the system employed a flexible, extensible, modular architecture to enable ease of integrating features as the requirements developed over the lifetime of the project, and to future-proof the system. The platform was designed to be as device agnostic as possible within the timelines and budget available to enable the future integration of other kinds of medical devices and nebulisers. (The system has subsequently integrated with another nebuliser device, demonstrating this capability.) Mobile app development used a cross-platform development technology, Apache Cordova (Apache Software Foundation, Forest Hill, MA, USA), and delivered both Android and iOS (Apple Inc., Cupertino, CA, USA) versions.
Conclusions
The co-design and delivery of a digital platform to capture and display adherence data and to include digital behaviour change tools was achieved for the launch of the main RCT. Agile software development methods supported a co-design process of the digital platform and allowed input from multiple stakeholders (PWCF, interventionists, members of the research team and members of the technical teams) to shape the development of the digital platform CFHealthHub. To ensure optimal use of resources, particularly in the later stages of the software refinement, the MoSCoW91 prioritisation system for software requirements proved useful for determining which elements of the software were critical to support the goals of the RCT and which elements could be saved for a future iteration of the software. Inevitably, additional features and enhancements were identified for the system that could have been implemented with additional time and resources. At the date of the main RCT launch, CFHealthHub provided a professionally-designed, feature-rich website and mobile apps that supported the digital components defined in the study protocol that were required by the complex intervention.
Appendix 2 Work package 2.2: description of the CFHealthHub intervention
This description follows the TIDieR checklist and guide. 39
Aim
The CFHealthHub intervention aims to support adults with CF to increase and maintain their adherence to prescribed nebulised medication to reduce exacerbations and improve or prevent decline in lung function.
Rationale
The CFHealthHub intervention is underpinned by the capability opportunity motivation – behaviour (COM-B) model. 37 It has been developed using the BCW approach alongside a PBA to intervention development. This process is described in detail elsewhere but broadly consisted of the following stages:
-
identification of barriers to and facilitators of nebuliser adherence using the TDF
-
identification of appropriate intervention functions and behaviour change techniques to address barriers identified
-
iterative development of the CFHealthHub intervention with patients, using feedback from interviews and ‘think aloud’ to refine the intervention
-
creation of an intervention manual and training programme for interventionists
-
pilot and feasibility trial including a process evaluation which was used to further refine the intervention, manual and training process.
Conceptual framework and theory
The conceptual framework that describes the intervention is shown in Figure 12. Consistent with the COM-B model, the framework considers issues of capability, opportunity and motivation, each of which must be present for repetition of the behaviour (i.e. medication adherence) to occur. Initially, we anticipate that repetition will require effortful self-regulation, but with repetition and strategies to promote habit formation we aim for the behaviour to become more automatic.
FIGURE 12.
Sustained behaviour conceptual framework: self-regulation or habit.
The intervention addresses a range of different barriers and is tailored to meet the specific needs of the person. The intervention draws on key theories to address different parts of the proposed process: social cognitive theory,94 control theory95 and habit theory. 96
Social cognitive theory94 proposes that behaviour is influenced by two core constructs: (1) perceived self-efficacy (i.e. an individual’s beliefs in their capability to adhere to treatment) and (2) outcome expectancies (i.e. an individual’s beliefs about the likely consequences of their actions). Self-efficacy can be enhanced through (1) mastery, (2) vicarious experiences, where a role model similar to the individual successfully achieves behavioural change in a similar situation, or (3) verbal persuasion. Outcome expectancies include beliefs about the positive and negative and short- and long-term consequences of adherence, and in this context include perceived necessities and concerns. 97 According to social cognitive theory outcome expectancies may result in intentions to change ones behaviour. Self-efficacy then influences the translation of that intention into action through the pursuit of goals.
Control theory95 explains the processes of self-regulation. When a behavioural standard or goal has been set, an individual directs their attention through monitoring behaviour to the discrepancy between their current behaviour and their goal. They then use this feedback to regulate their behaviour to meet their goal through action control. This in the context of adherence; once an adherence goal is set, self-monitoring of treatment-taking provides the feedback to prompt action to enable self-regulation of behaviour.
A habit is where a behaviour is prompted automatically by a situational cue. Habits are created through the repetition of a behaviour in a specific context,85 which over time results in a learned cue–behaviour association. 98 In the context of adherence, the repeated taking of treatment in a specific context or in the presence of a specific cue should over time result in the formation of a habit. Habits are particularly advantageous because theory predicts that, once formed, they do not rely on motivational processes and, therefore, should persist even if motivation wanes. 99 Therefore, they may play a particularly important role in the promotion of long-term maintenance of behaviour,100 in this case adherence, which is a key aim of the programme.
Materials
The CFHealthHub intervention includes a range of materials as follows:
-
eTrack nebuliser
-
Qualcomm hub
-
research procedures manual
-
CFHealthHub website
-
CFHealthHub app (available for Android and iOS devices)
-
COM-BMQ screening tool
-
CFHealthHub participant manual
-
CFHealthHub interventionist manual, including worksheets for intervention delivery
-
training slides and online resources [via the virtual learning environment (VLE) Blackboard (University of Sheffield, Sheffield, UK)] for interventionist training
-
fidelity scoring sheets.
Intervention providers
Intervention providers were recruited from each site. The majority of sites recruited individuals who were already members of the MDTs working in CF at that site. Other sites recruited from other parts of the hospital or recruited externally. Thus, interventionists had a range of backgrounds and included:
-
physiotherapists working in CF or other respiratory conditions
-
nurses working in CF
-
psychologists
-
pharmacists
-
dietitians.
Procedure
Interventionist training, assessment and support
Interventionists received training in how to deliver the intervention in a variety of ways:
-
Training in use of equipment – interventionists received training in how to use the eTrack nebuliser and Qualcomm hub, how to pair the devices and how to register a new participant onto the CFHealthHub digital platform and eTrack system as part of their research procedures training. This was delivered face to face by the study manager and PARI GmbH and supported with a research procedures manual and ad hoc telephone support throughout the trial.
-
Training in delivery of CFHealthHub intervention – interventionists received training in how to use the CFHealthHub digital platform and how they deliver the CFHealthHub intervention. Training was delivered during a 2-day face-to-face training session followed by a schedule of online training to be completed over the equivalent of 4 days hosted on the VLE. Training consisted of presentations, with exercises in small groups or pairs, and supported use of CFHealthHub, role-play delivery of the intervention and discussion. A training version of the CFHealthHub platform was provided for use during training that included dummy data. Interventionists were paired to form buddies for support and additional role play during the online part of the training.
-
Competency assessment – interventionists undertook two competency assessments during the training period: (1) a theory test that assessed understanding of the content of the CFHealthHub digital platform content and data. This test was delivered through an online survey on the VLE and consisted of multiple choice and sort answer questions. The answers were marked according to a pre-determined marking schedule. Interventionists passed if they received a mark of ≥ 80%. Individual feedback was provided on the answers given and, where the first test was failed, additional tutorial support was provided and the test retaken until passed. (2) A practical test that assessed delivery of the first intervention visit of the CFHealthHub intervention. This was assessed through an audio-recorded role play. The part of the participant was played by a member of the study team and the interventionist role played their part. The intervention delivery was assessed using a competency assessment sheet that consisted of sections on preparation, delivery of intervention components and the quality of delivery. Two members of the training team looked at the completed worksheet for the session and listened to the accompanying audio-recording. They discussed the marks and agreed marks where there were any differences. Agreed marks for each section were averaged and the pass threshold was 90%. Interventionists received individual feedback on their performance and tutorial support where they had failed. The test was retaken until passed.
-
Competency to deliver a review visit and a phase review visit were assessed by listening to the first audio-recorded visit of that kind for each interventionist. Two members of the training team looked at the completed worksheet for the session and listened to the accompanying audio-recording. They discussed and agreed marks. Agreed marks for each section were averaged and the pass mark threshold was 90%. Interventionists received individual feedback on their performance and tutorial support where they had failed. The next audio-recorded visit of that kind was assessed where the assessment was failed.
-
Ongoing support – for interventionists this was delivered at a weekly teleconference, by e-mail and telephone support with the training team and through technical support by telephone and e-mail. The weekly teleconference provided a space where interventionists could discuss problems, successes and case studies (anonymised) to aid group learning. Individuals could also access members of the team individually and individual interventionists were targeted with support where they had failed their earlier competency assessment or where there were any problems identified.
Intervention schedule of delivery
The intervention schedule of delivery is described in Figure 13. The content of each kind of intervention session is described in this section. Within this schedule there were a number of different paths that were determined during delivery.
FIGURE 13.
Schedule of intervention delivery.
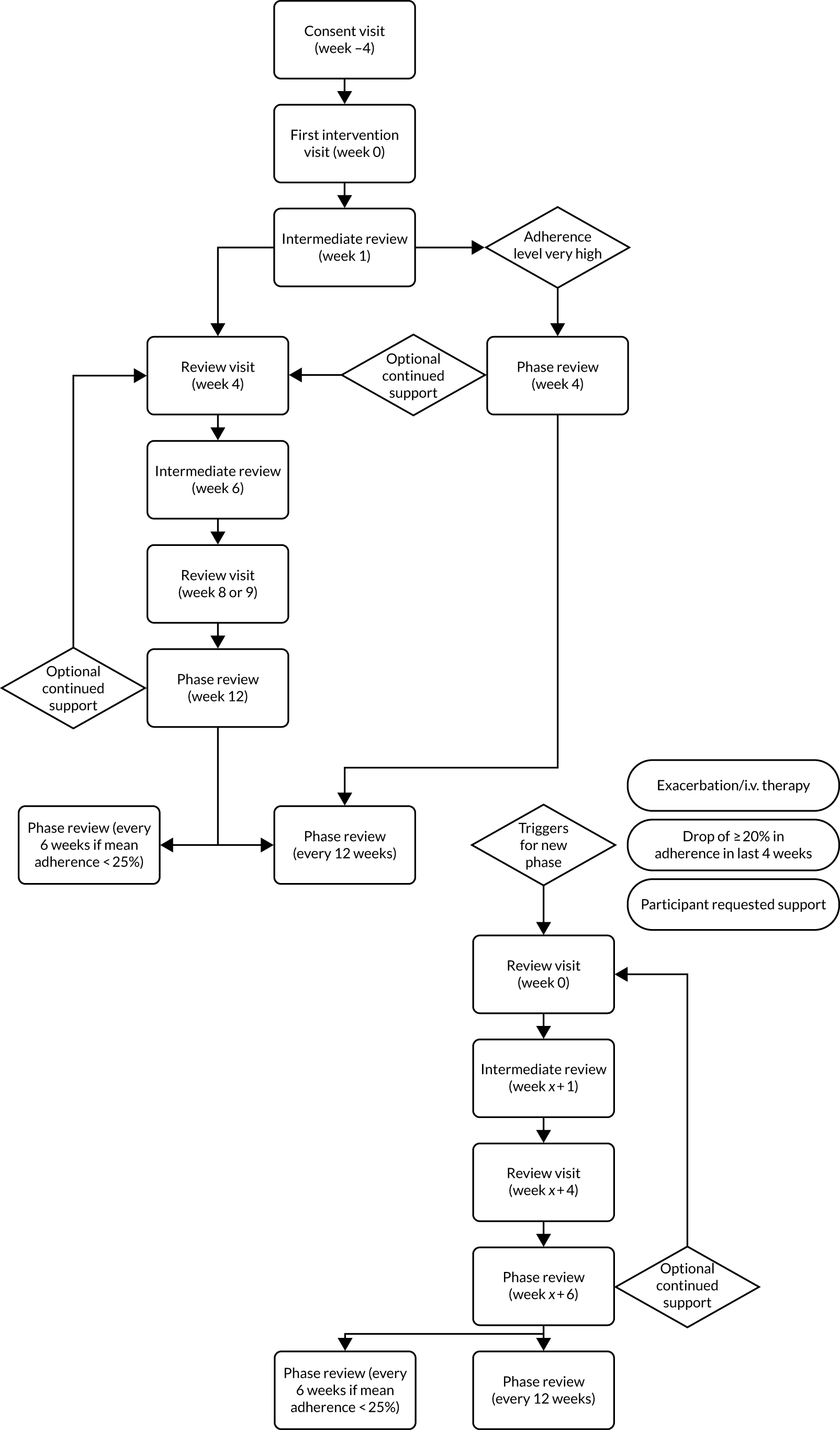
Consent visit and set-up
All participants receive their eTrack nebuliser and Qualcomm hub at the consent visit. They also complete the COM-BMQ screening tool at this visit. An account is created on CFHealthHub into which is added the current prescription data for the participant and the data from the COM-BMQ screening tool. The consent visit takes place at least 4 weeks prior to the first intervention visit. During this time adherence data are transmitted automatically from the eTrack nebuliser via the Qualcomm hub, which is plugged into their home, to the CFHealthHub platform. Figure 2 shows this process.
Intervention sessions received by all participants
All participants receive their first intervention visit at least 4 weeks following consent (so that the consultation is based on at least 4 weeks’ worth of objective adherence data). This visit is always done face to face, although it can be in a variety of locations, including at hospital (in-patient), a clinic or at home. All participants receive an intermediate review telephone call 1 week later. Subsequent visits depend on their adherence level. Participants with an objectively measured adherence level of ≥ 80% follow the ‘very high level of adherence’ path and those with an adherence level of < 80% follow the normal path.
Normal pathway (adherence level < 80%)
Participants on this pathway have intervention sessions over a 12-week period. In addition to the first intervention session (at week 0) and an intermediate review (at week 1) they receive a review session at week 4, an intermediate review at week 6, a second review session at weeks 8 or 9 and a phase review at week 12. This pattern of delivery constitutes a phase. They then receive a phase review session every 12 weeks, or every 6 weeks if their adherence level is < 25%.
Very high adherence level pathway (adherence level ≥ 80%)
Participants on this pathway have intervention sessions over a 4-week period. In addition to the first intervention session (at week 0) and an intermediate review (at week 1) they receive a phase review at week 4. They then receive a phase review session every 12 weeks.
Triggers
In addition to these pathways there are a number of criteria that, if met, trigger a new phase of intervention delivery. These are:
-
Participant-requested support. This can be a request for additional support at a phase review, in which case an additional intervention delivery period is triggered without a break, or at any other time. Additional periods of delivery are offered to participants if one or both of the following triggers occurs following the first phase review.
-
A drop of ≥ 20% in adherence since the phase review.
-
An exacerbation requiring i.v. treatment.
When there is a drop of ≥ 20% in adherence since the phase review or exacerbation requiring i.v. treatment, participants are contacted and additional support is offered. If participants agree then the triggered pathway commences with a review session at week 0, an intermediate review 1 week later, a review visit 4 weeks later and a phase review 6 weeks later.
Participants then revert back to phase reviews at 12-week intervals (or 6 weeks if adherence is < 25%).
Access to the CFHealthHub digital platform
Participants had an individual login providing access to the CFHealthHub digital platform throughout the intervention. It could be accessed on a laptop or through an app available for Android or iOS devices.
Participants were encouraged to access the site regularly and were provided with a participant guide with instructions on how to access and information about what to find where.
Intervention modules
The CFHealthHub contains a number of distinct modules each of which focuses on a different aspect using a range of specific behaviour change techniques (described using the definitions in the behaviour change taxonomy)101 and modes of delivery. These are described in Table 12, which shows which aspects of the intervention were delivered using the CFHealthHub digital platform and which were delivered by the interventionist.
| Module | BCTs101 | Mode of delivery |
|---|---|---|
| My treatment |
|
CFHealthHub:
|
| Self-monitoring |
|
CFHealthHub:
|
| Confidence building |
|
CFHealthHub:
|
| Goal setting and review |
|
CFHealthHub:
|
| Treatment plan |
|
CFHealthHub:
|
| Problem-solving |
|
CFHealthHub:
|
Tailoring and personalisation
The CFHealthHub intervention is not one size fits all and is designed to be tailored and personalised so that it can best meet the needs of a wide range of participants. Although the entire content of the CFHealthHub website is available for participants to browse, tailored aspects are emphasised or added into a specific personal ‘favourites’ area called ‘My Toolkit’. Table 13 described the ways in which the intervention is tailored.
| Tailored component | How non-tailored components are accessed | How version is determined |
|---|---|---|
| Contents of ‘My treatment’ and ‘Problem-solving’ focus on information relevant to current prescription drugs | All generic information in available to all participants to browse. Information on treatments not currently prescribed are available but minimised | Prescription is entered into CFHealthHub at consent and altered whenever there is a prescription change. CFHealthHub automatically tailors content based on this information |
| Modules of ‘My treatment’ are selected and placed into ‘My Toolkit’ based on the scores on the COM-BMQ questionnaire | Participants can browse all modules of ‘My treatment’ | Participants responses to the COM-BMQ questionnaire are entered into CFHealthHub at consent. CFHealthHub recommends the most relevant modules based on a scoring algorithm. If CFHealthHub recommends more than three modules then interventionists select three based on the scores and their judgement based on conversations with the participant. Modules can be changed throughout the intervention and these are recorded on CFHealthHub |
| Modules of ‘Problem-solving’ are selected and placed into My Toolkit based on the barriers identified in consultations with the interventionist | Participants can browse all modules of ‘Problem-solving’ | Interventionists can select modules of ‘Problem-solving’ content based on the barriers identified in consultations. Modules can be changed throughout the intervention and these are recorded on CFHealthHub |
| ‘Talking heads’ videos are selected to match key participant characteristics and placed into ‘My Toolkit’. This is optional | Participants can browse the entire ‘talking heads’ video library | Interventionists can select relevant videos that match key characteristics of the participant (e.g. age, gender, occupation, life role, problems experienced). Videos can be changed throughout the intervention and these are recorded on CFHealthHub |
| ‘Goal-setting and review’ and ‘Treatment planning’ are utilised only for participants who are motivated (want to) take more treatment. Participants with very low levels of motivation do not receive these parts of the intervention. Instead, they spend more time focusing on the content of ‘My treatment’ and relationship building with the interventionist | Participants can choose to set goals and make plans at any point in a consultation or by contacting the interventionist | A very low level of motivation is determined by a combination of a low score on the COM-BMQ motivation item and discussion with the participant in a consultation. The identification of a very low level of motivation is recorded where this applies |
A number of features of the CFHealthHub digital platform are individually personalised for each participant. These are described in Table 14.
| Personalised component | How personalisation is achieved |
|---|---|
| Graphs and charts show personal data | Participant’s eTrack nebuliser collects and send adherence data to CFHealthHub via the Qualcomm hub for display |
| Target line on graph | Participants determine their adherence goal in consultation with the interventionist. This is displayed on their charts |
| Plans | Participants make individual plans based on discussions with the interventionist. These are made using the tools on the CFHealthHub digital platform and recorded in ‘My Toolkit’. New plans can be added and CFHealthHub records all plans for each participant |
| Home page | Participants can select an image to display on their home page from a default selection or can upload their own image |
| Notifications | Participants can optionally choose to receive personalised notifications using the CFHealthHub app. A message is sent to the participant if they have met their goal in the previous week, or a messaging is sent to encourage them to keep going if they did not |
| Reminders | Participants can choose to receive reminders through the CFHealthHub app. A reminder message is sent to the participant if they have not accessed their CFHealthHub account for a period of 2 weeks |
| Reward messages | Participants can optionally choose to receive reward messages through the CFHealthHub app. A reward message is sent to the participant if they have met their goal in the last week, 2 weeks or month |
Types of intervention visit
The intervention visits all have broadly the same aim, that is, to enable participants to look at their data, reflect on why adherence is important, set goals to increase their adherence and make plans as to how they will achieve these, and problem-solve any barriers that are likely to get in the way. However, the intervention visit types do differ somewhat in their set-up, focus and how in-depth they are as follows. Detailed information about the structure of the delivery for each type of session is provided in the intervention manual and the relevant worksheets.
First intervention visit
This session always occurs face to face, although this can be in a hospital/clinic setting or at home. It lasts between 40 and 60 minutes. It is the first time that the participant accesses the CFHealthHub digital platform and sees their data. Interventionists must prepare for this session by entering the data from the COM-BMQ screening tool and checking that there is data coming through to CFHealthHub from the nebuliser.
The key things that happen in this session are:
-
participant receives their log-in details and accesses CFHealthHub
-
participant (optionally) downloads the CFHealthHub app onto their smartphone
-
the following modules are covered –
-
My treatment
-
Self-monitoring
-
Confidence building
-
-
the following modules are covered for those who want to increase their treatment adherence (sufficiently motivated) –
-
Goal-setting
-
Treatment plan.
-
Problem-solving intermediate review
The intermediate review is a short session that is designed to trouble-shoot ‘quick’ and easy-to-solve problems (e.g. an action plan that is not working). It is typically delivered by telephone and lasts 5–15 minutes. The review is less structured than other visits.
Ad hoc review
This follows the same structure as the intermediate review but is delivered where there is unplanned face-to-face contact with a participant (e.g. in a clinic).
Review visit
This session typically lasts 30–45 minutes and can be delivered face to face or by telephone. The session focuses on the data and what has happened in terms of adherence since the last visit. The precise focus will vary depending on the individual participant, for example a session with a participant who has met their goal would have a different focus to one with a participant who has not met their goal (or did not set one). The session will cover the following modules:
-
My treatment
-
Self-monitoring
-
Confidence building
-
Goal-setting and review
-
Treatment plan.
Problem-solving phase review
The focus of this appointment is to facilitate reflection on progress since the intervention (or the current phase of delivery) began and to consider whether continued support is required or the participant wishes to manage their adherence independently. Ideally, this should be delivered face to face but can be delivered by telephone. It typically lasts 20–30 minutes and covers the following modules:
-
My treatment
-
Self-monitoring
-
Confidence building
-
Problem-solving.
Appendix 3 Work package 2.3: intervention training and fidelity assessment
Introduction
The Borrelli checklist54 was used as the framework to assess and monitor fidelity. In the feasibility study the procedures for operationalising fidelity assessment in the main RCT were developed. Fidelity was assessed across five domains.
Design
This explores whether or not the groups received the expected treatment (i.e. the CFHealthHub intervention or usual care). For the intervention group, there is complexity in the flow of intervention delivery where the next step in the intervention flow could change in response to events during the trial. Therefore, we explored whether or not a participant received the minimum expected dose. This was also complex because it differed depending on whether or not the participant was a high- or low-level adherer. In general, there was reasonable agreement (74%) between expected versus actual intervention in the treatment group. The control group participants received usual care, which for the majority of sites did not include focused adherence support. For example, at trial initiation < 10% of the centres used objective data such as MPR or I-neb adherence data to inform care.
Training
A blended programme of training was delivered to all interventionists that was supplemented throughout the duration of the trial. Assessment of skill acquisition comprised two parts: (1) a written theoretical competency test and (2) a practical competency test. To be fully certified as competent to deliver this intervention, interventionists had to pass the theoretical competency test (pass mark threshold 80%) as well as pass the competency threshold (90%) on fidelity assessments in all three types of consultation: (1) first intervention visit, (2) review visit and (3) phase review visit. Certification to deliver the first intervention visit was conducted as part of the initial intervention training. Certification to deliver the review/phase review visits was conducted as they occurred in the main RCT. Those achieving < 90% were given individual feedback and tutoring as well as direction to specific learning materials provided on a VLE. All 30 interventionists completed the theory test. A total of 27 out of 30 interventionists achieved the pass mark (> 80%) on first attempt. Evaluation forms also demonstrated that interventionists rated their competence to deliver the intervention at the end of the training as high across a number of core domains involved in intervention delivery. A total of 27 interventionists completed first intervention certification prior to conducting any intervention sessions in the RCT. For the assessments in which the participant was certified the mean score was 96% (SD 4%) and the scores ranged from 88% to 100%. Each interventionist was given individual feedback, with interventionists who failed given individualised retraining before reassessment. For review and phase review certification 30 interventionists were certified. The mean score for the review was 96.2% (SD 3.7%, range 90.7–100%] and for the phase review was 96.0% (SD 3.13%, range 91.7–100%]. Interventionists who failed were given individualised retraining before reassessment and also were subsequently certified.
Delivery of treatment
Assessment and monitoring of provider skill maintenance over time (fidelity drift) was assessed by sampling from the different types of visits (first intervention visit, review visit or phase review visit). We attempted to assess 20% of all visits with clear criteria to inform choice of assessments for drift (18 targeted for high withdrawal rates, 37 for insufficient audio-recordings, 82 for having fewer visits or action/coping plans created than expected and nine at random to ensure a total assessment sample of ≥ 20% of all interventionist visits). A total of 110 assessments were assessed to explore drift in fidelity over the duration of the trial and a pass mark threshold of 80% was set was drift assessments. For both certification and drift, two people independently assessed each intervention, and agreement between assessors was high. Of all paired assessments during the trial there was 97.2% agreement when comparing pass/fail decisions at the 80% threshold (207 out of 213 assessments in agreement).
The trial had a high level of fidelity (overall fidelity by site, range 79–97%) with only one site not achieving over the mean threshold (> 80%) on drift assessments.
Receipt
Setting of action and coping plans were assessed as a proxy for fidelity receipt. Receipt in this trial was deemed to be satisfactory. Among the 265 participants who completed the 12-month trial, 205 (77%) completed at least one action plan and 160 completed at least one coping plan.
Enactment
A total of 268 participants used web/app click analytics outside intervention sessions. In addition, qualitative data provides evidence that participants were able to enact skills in real life settings.
Further details of fidelity findings are available in Report Supplementary Material 2.
Appendix 4 Work package 3.3: usual-care survey summary
A usual-care survey was conducted to examine the use and perceptions of the provision of adherence monitoring and support in sites taking part in the full-scale RCT (WP 3.2).
Methods
All RCT sites were asked to complete a usual-care survey at study start and at 12-months. This was an 11-item questionnaire.
Questionnaires were to be completed by the site interventionist and/or other members of the MDT.
Median and interquartile ranges were used to summarise questionnaire items requiring a response on 5-point nominal scales. Percentages by outcome response were also summarised for these items. Key themes were extracted and summarised from free-text responses. Change scores were calculated within sites by subtracting follow-up scores [time 2 (T2)] from baseline scores [time 1 (T1)] and summarised overall.
Results
A total of 20 centres responded to the questionnaire. (Two centres share a CF service across the two NHS trusts. For this reason, they were classed as a single site for the purposes of the full-scale RCT but, because usual care could vary between the two sites, we collected a usual-care survey from both sites.) Five sites responded outside the protocol window at baseline; all but one were within 3–18 days of the expected response window (study start plus 1 month) and have been included in analyses. One site was excluded at baseline and for comparison analyses between baseline and follow-up because the questionnaire was not completed until a full-time interventionist replacement was confirmed, 9 months post baseline.
Table 15 presents the results from nine questions, summarised quantitatively.
| Question | T1 | T2 | Change | Interpretation |
|---|---|---|---|---|
| 1. Do you use MPRa to understand a patient’s adherence during consultations? |
|
|
|
On average, sites never used MPR during consultations at baseline. Use of MPR was more frequent for 58% of sites at follow-up |
| 2. Do you reduce the target prescriptionb to promote adherence? |
|
|
|
On average, sites would sometimes reduce a target prescription to promote adherence at baseline. This was largely consistent with the approach used at follow-up, although 37% of sites reported using this technique more frequently |
| 4. How often do you ask about/discuss adherence with your patients? |
|
|
|
On average, sites always discussed adherence with their patients at baseline. There was a trend to discuss adherence less frequently in some sites at follow-up |
| 5. How important would you consider adherence support in your centre? |
|
|
|
On average, adherence support was considered to be very important at both baseline and follow-up |
|
6. In your centre which are priorities about improving CF? Please number 1–7, with 1 being the most important: 6(1) encouraging airway clearance 6(2) early treatment of exacerbations with IVAB 6(3) encouraging exercise 6(4) early detection and treatment of diabetes 6(5) adherence to inhaled therapies (nebulised antibiotics) 6(6) adherence to inhaled therapies (mucolytics) 6(7) nutritional support to maintain BMI at target |
|
|
|
On average, encouraging airway clearance was considered the most important; encouraging exercise was considered the least important. There was substantial variability between sites in responses to this question and many felt that they could not answer because they felt that these practices were of equal importance and their priority varied by patient |
| 8. Do you use the I-neb data to provide objectively recorded recent adherence data to inform your consultations (e.g. the percentages calculated per week or per month from the I-neb download)? |
|
|
|
An additional three sites acquired the use of I-nebs in usual care at follow-up. On average, sites sometimes used objective data downloaded from the I-neb to inform their consultations and this was largely consistent with practice at follow-up |
| 9. Do you use bespoke graphs plotted from the I-neb device that you have developed at your centre? |
|
|
|
The majority of sites at baseline (93%) or follow-up (82%) never used bespoke graphs plotting I-neb data |
| 10. Do you sit with the patient and use Insight Online (the Philips graphical plotter) [Koninklijke Philips N.V.]? |
|
|
|
On average, sites never used Insight Online with their patients at baseline or follow-up |
| 11. Do you have confidence that the percentage adherence from Insight Online is derived from the correct prescription (i.e. that it is based on the correct denominator)? |
|
|
|
Some sites felt this question was not applicable to them if they did not use I-neb data. Sites indicated that they sometimes had confidence in the adherence calculation derived from the prescription input. However, this varied between sites and between baseline and follow-up |
Change scores indicate consistency in all responses from baseline to follow-up, with the exception of more frequent use of MPR at follow-up than at baseline (see question 1). Change scores should be interpreted with caution because, in some cases, different members of the MDT completed questionnaires at baseline and follow-up.
Question 3
Do you use any other motivational or behavioural strategies to promote adherence? If so please specify
Use of behavioural strategies in routine care varied between sites. Some sites regularly used a range of behavioural strategies in practice. One site did not state the use of any behavioural strategies in routine care. At baseline, at least 63% of sites used MI or techniques derived from MI in usual care; a further five sites (totalling 17 out of 19 sites over the course of the study) reported using MI techniques with their patients at follow-up. The proportion of members of the MDT that were trained in MI varied from some to all, but for the most part this was not specified. Goal-setting or use of specific, measurable, achievable, relevant and time-bound (SMART) goals was used by at least five of 19 sites at baseline. At least 26% of sites stated that they discussed barriers and/or used problem-solving to overcome barriers with their patients, and at least 53% of sites had access to cognitive–behavioural therapy and/or support from a trained psychologist. One site referenced the use of the COM-B model and one site referenced discussing habit, both at follow-up.
Question 7
Do you use any objective measures of adherence? If so specify
Some method of objectively measuring adherence to inhaled medications was identified in 75% of sites. This included general practitioner/home care/pharmacist prescription collections, downloads of adherence data from the I-neb, checking of drug levels, visual analogue scales of adherence, dosset boxes and tick charts. Collection and utilisation of these data varied in frequency; most sites reported using objective measures on an ad hoc or relatively infrequent basis, often in selected groups of patients whom they had concerns about and only by specific members of the MDT. In 25% of the sites, no formal measures were identified over the course of the study.
Summary
Adherence support was mostly considered very important and adherence was discussed with patients at least sometimes, across all sites. Some method of objectively measuring adherence to inhaled medications was identified in 75% of sites. Qualitative data highlighted that implementation of adherence support varied between RCT sites. Collection and utilisation of adherence data varied in frequency and formality; many sites reported using objective measures on an ad hoc or relatively infrequent basis. Change scores indicated that, on the whole, practice was consistent in usual care over the follow-up period.
Appendix 5 Work package 3.3: user satisfaction survey
A survey was conducted to evaluate satisfaction with the CFHealthHub intervention, delivered in the full-scale RCT (WP 3.2).
Methods
All participants allocated to the intervention arm and who completed the 12-month follow-up were asked to complete an 11-item survey. This was completed as part of the 12-month follow-up questionnaire battery. Interventionists were usually present during the completion of the survey.
All questionnaire items asked participants to rate how helpful they found specific parts of the intervention. Each item required responses on a 4-point nominal scale, from very helpful to not helpful at all. Responses have been summarised as percentages for each response category.
Results
A total of 257 out of the 305 participants randomised to the intervention arm responded. Summaries by response category are presented in the main report (see Table 7). The intervention element rated as most helpful was the first intervention visit and exposure to CFHealthHub.
There was variation in overall satisfaction between sites. Summaries of scores averaged across all intervention elements by site are shown in Table 16.
| Site code | Very helpful (%) | Quite helpful (%) | Not very helpful (%) | Not helpful at all (%) |
|---|---|---|---|---|
| 1 | 52.3 | 41.5 | 6.2 | 0.0 |
| 2 | 70.0 | 25.0 | 5.0 | 0.0 |
| 3 | 33.0 | 53.7 | 12.7 | 0.6 |
| 4 | 59.4 | 34.3 | 6.3 | 0.0 |
| 5 | 34.9 | 44.1 | 11.8 | 9.1 |
| 6 | 41.6 | 45.5 | 8.4 | 4.5 |
| 7 | 55.8 | 22.4 | 8.5 | 13.3 |
| 8 | 66.2 | 27.3 | 6.5 | 0.0 |
| 9 | 53.0 | 32.0 | 12.4 | 2.7 |
| 10 | 48.1 | 34.5 | 15.2 | 2.3 |
| 11 | 45.5 | 41.6 | 10.4 | 2.6 |
| 12 | 32.8 | 52.3 | 9.6 | 5.2 |
| 13 | 24.2 | 48.5 | 18.2 | 9.1 |
| 14 | 55.2 | 30.1 | 9.8 | 4.9 |
| 15 | 41.1 | 38.2 | 20.8 | 0.0 |
| 16 | 70.8 | 23.0 | 4.3 | 1.9 |
| 17 | 44.9 | 46.4 | 8.7 | 0.0 |
| 18 | 28.2 | 28.7 | 40.0 | 3.0 |
| 19 | 73.3 | 24.4 | 2.3 | 0.0 |
Appendix 6 Work package 3.3: adverse events
| Adverse event | Usual care (n = 303), n (%) | Intervention (n = 305), n (%) | Overall (n = 608), n (%) |
|---|---|---|---|
| Non-serious adverse events | |||
| All AEs | 301 (46.9) | 341 (53.1) | 642 (100.0) |
| Participants experiencing at least one AE | 125 (41.3) | 139 (45.6) | 264 (43.4) |
| AEs by category | |||
| Expected or common eventa | 242 (80.4) | 263 (77.1) | 505 (78.7) |
| 01 Acute FEV1 drop > 15% after first dose of medication | 1 (0.4) | 2 (0.8) | 3 (0.6) |
| 02 Increased productive cough | 62 (25.6) | 73 (27.8) | 135 (26.7) |
| 03 Nasal congestion or stuffy nose | 12 (5.0) | 10 (3.8) | 22 (4.4) |
| 04 Chest congestion | 8 (3.3) | 15 (5.7) | 23 (4.6) |
| 05 Wheezing | 8 (3.3) | 10 (3.8) | 18 (3.6) |
| 06 Chest pain or chest discomfort | 18 (7.4) | 11 (4.2) | 29 (5.7) |
| 07 Voice alteration/change | 4 (1.7) | 2 (0.8) | 6 (1.2) |
| 08 Dyspnoea (breathlessness) | 17 (7) | 14 (5.3) | 31 (6.1) |
| 09 Haemoptysis (coughing blood) | 26 (10.7) | 40 (15.2) | 66 (13.1) |
| 10 Rhinitis | 1 (0.4) | 2 (0.8) | 3 (0.6) |
| 11 Headache | 3 (1.2) | 4 (1.5) | 7 (1.4) |
| 12 Crackles in lung | 9 (3.7) | 6 (2.3) | 15 (3.0) |
| 13 Throat irritation/sore throat | 15 (6.2) | 12 (4.6) | 27 (5.3) |
| 14 Upper respiratory tract infection | 6 (2.5) | 8 (3.0) | 14 (2.8) |
| 15 Sinusitis | 3 (1.2) | 3 (1.1) | 6 (1.2) |
| 16 Deafness | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| 17 Indigestion/reflux | 2 (0.8) | 0 (0.0) | 2 (0.4) |
| 18 Tonsillitis | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| 19 Joint pain | 2 (0.8) | 5 (1.9) | 7 (1.4) |
| 20 Decreased appetite | 5 (2.1) | 1 (0.4) | 6 (1.2) |
| 21 Fatigue | 12 (5.0) | 6 (2.3) | 18 (3.6) |
| 22 Headache | 0 (0.0) | 3 (1.1) | 3 (0.6) |
| 23 Distal intestinal obstructive syndrome | 0 (0.0) | 1 (0.4) | 1 (0.2) |
| 24 Fever | 2 (0.8) | 3 (1.1) | 5 (1.0) |
| 25 Otitis media or ear infection | 1 (0.4) | 2 (0.8) | 3 (0.6) |
| 26 Conjunctivitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 27 Pneumothorax | 2 (0.8) | 2 (0.8) | 4 (0.8) |
| 28 Decreased exercise tolerance | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| 29 Pyrexia | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| 30 Abdominal pain | 4 (1.7) | 14 (5.3) | 18 (3.6) |
| 31 Influenza | 3 (1.2) | 4 (1.5) | 7 (1.4) |
| 32 New Pseudomonas infection since recruitment in patient who was previously Pseudomonas free | 6 (2.5) | 2 (0.8) | 8 (1.6) |
| 33 Vomiting | 4 (1.7) | 4 (1.5) | 8 (1.6) |
| 34 New diagnosis of diabetes | 4 (1.7) | 1 (0.4) | 5 (1.0) |
| 35 Pneumonia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| New depression requiring treatment | 1 (0.3) | 5 (1.5) | 6 (0.9) |
| Other | 58 (19.3) | 73 (21.4) | 131 (20.4) |
| Serious adverse events | |||
| All SAEs | 64 (47.4) | 71 (52.6) | 135 (100.0) |
| Participants experiencing at least one SAE | 43 (14.2) | 56 (18.4) | 99 (16.3) |
| SAEs by category | |||
| Expected or common eventa | 21 (32.8) | 28 (39.4) | 49 (36.3) |
| 01 Acute FEV1 drop > 15% after first dose of medication | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 02 Increased productive cough | 2 (9.5) | 2 (7.1) | 4 (8.2) |
| 03 Nasal congestion or stuffy nose | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 04 Chest congestion | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 05 Wheezing | 0 (0.0) | 1 (3.6) | 1 (2.0) |
| 06 Chest pain or chest discomfort | 1 (4.8) | 1 (3.6) | 2 (4.1) |
| 07 Voice alteration/change | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 08 Dyspnoea (breathlessness) | 0 (0.0) | 3 (10.7) | 3 (6.1) |
| 09 Haemoptysis (coughing blood) | 1 (4.8) | 5 (17.9) | 6 (12.2) |
| 10 Rhinitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 11 Headache | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 Crackles in lung | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 13 Throat irritation/sore throat | 0 (0.0) | 1 (3.6) | 1 (2.0) |
| 14 Upper respiratory tract infection | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 15 Sinusitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 16 Deafness | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 17 Indigestion/reflux | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 18 Tonsillitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 19 Joint pain | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 20 Decreased appetite | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 21 Fatigue | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 22 Headache | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 23 Distal intestinal obstructive syndrome | 3 (14.3) | 5 (17.9) | 8 (16.3) |
| 24 Fever | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 25 Otitis media or ear infection | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 26 Conjunctivitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 27 Pneumothorax | 2 (9.5) | 0 (0.0) | 2 (4.1) |
| 28 Decreased exercise tolerance | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 29 Pyrexia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 30 Abdominal pain | 9 (42.9) | 2 (7.1) | 11 (22.4) |
| 31 Influenza | 1 (4.8) | 1 (3.6) | 2 (4.1) |
| 32 New Pseudomonas infection since recruitment in patient who was previously Pseudomonas free | 0 (0.0) | 1 (3.6) | 1 (2.0) |
| 33 Vomiting | 2 (9.5) | 5 (17.9) | 7 (14.3) |
| 34 New diagnosis of diabetes | 0 (0.0) | 1 (3.6) | 1 (2.0) |
| 35 Pneumonia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| New depression requiring treatment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 41 (64.1) | 42 (59.2) | 83 (61.5) |
| Unknown | 2 (3.1) | 1 (1.4) | 3 (2.2) |
Appendix 7 Work package 3.3: the benefits of objective nebuliser adherence data as ‘proof’ in the management of cystic fibrosis in adults – a qualitative study
Background
Low rates of adherence or non-adherence to nebuliser treatment in CF are linked to poor clinical outcomes. With advances in technology there has been an increase in electronic monitoring of nebuliser usage in CF clinical practice. There is little understanding of how objective data, when used as evidence of adherence, is perceived by PWCF and health-care professionals, and whether or not it can facilitate discussions surrounding the reasons for adherent or non-adherent behaviours.
Methods
A qualitative study as part of a RCT evaluating the effectiveness of a new intervention to promote adherence. The intervention comprised feedback of real-time, objective adherence data to PWCF and an interventionist – usually a health-care professional from the CF MDT – offering a BCI. During intervention sessions between interventionists and PWCF the objective adherence data were discussed and targets for improvement agreed on and set. A total of 22 PWCF and 26 interventionists took part in individual semistructured interviews; a framework approach was used for analysis.
Results
Objective adherence data were welcomed by both interventionists and PWCF in the intervention arm of the RCT. PWCF were able to choose how to display their data, for example different types of graphs (bar/line) and daily, weekly or specific times. Interventionists suggested that these easy-to-read graphs provided a focus for discussions with PWCF about their adherence patterns. Details about how the data were displayed were significant. In particular, using a traffic light system of red, amber and green enabled PWCF to see at a glance if they were meeting targets set by themselves and the interventionist. ‘Gamification’ of the data, encouraging PWCF to achieve green graphs signifying meeting targets, was key to engaging PWCF, acting as a motivator to meet targets. Technological issues did not appear to affect PWCFs’ trust in the data.
The objective data were used as ‘proof to self’, offering reassurance to high-level adherers who believed they were adhering to their nebuliser treatments but nevertheless appreciated external proof of this belief. By contrast, some PWCF with lower adherence levels could be shocked by the proof of the number of treatments they had missed in previous weeks and months. PWCF from all adherence groups used the data to keep track of their progress, and seeing patterns of adherence helped them to consolidate their routines to improve adherence. The data as ‘proof to self’ provided both motivation to improve for lower-level adherers and motivation to maintain high levels for higher-level adherers. The data were used as a platform to initiate sometimes difficult discussions about adherence, and were perceived as promoting honesty between PWCF and clinicians. When patients could see improvements, this increased their motivation to continue to meet targets set.
The objective data could also act as ‘proof to others’. The ability to prove what they were doing to others was very important to PWCF, regardless of adherence grouping, because they often perceived that their clinical team – and in some cases their close family members – did not believe their subjective reports of adherence. Some PWCF in the sample had objective adherence rates that matched their subjective adherence rates and were considerably higher than their clinical team or family members believed. In these cases the proof offered by the objective data could radically change the PWCF’s identity as a low-level adherer, improving relationships with clinicians and family. It could even affect future clinical care when clinicians understood that the health problems experienced by the PWCF could not be due to lack of adherence to nebuliser medication, and that alternative reasons needed to be investigated.
Most interventionists and PWCF viewed the objective data, and the proof it offered, positively. However, interventionists pointed out that it needed to be used carefully, particularly with those PWCF not achieving high levels of adherence. They stressed that it was important that this proof was used to instigate a discussion, not as a reason to castigate PWCF. At times there was disparity between the objective data, and PWCF’s perceptions of their adherence, which had to be addressed with sensitivity.
Conclusion
In this study of PWCF who chose to participate in a RCT of an intervention to improve adherence, objective nebuliser adherence data appeared to facilitate honest discussions around reasons for non-adherence, and helped with identification of strategies to resolve barriers to adherence. In addition, the data offered proof to both PWCF and their clinical team that both motivated and rewarded improvements in adherence.
Appendix 8 Work package 3.3: mechanisms of action based on the perceptions of people with cystic fibrosis and interventionists
Background
The logic model (see Figure 3) and prior research33 identified a number of mechanisms of action important to increasing adherence in the CFHealthHub intervention. We explored whether or not and how these mechanisms of action operated in patient and interventionist accounts of using the CFHealthHub intervention.
Methods
We analysed the perceived mechanisms of action identified in 22 patient and 26 interventionist interviews. We grouped patients into four categories of baseline levels of objectively measured adherence (very low: 0.0–24.9%; low: 25.0–49.9%; moderate: 50.0–74.9%; high: ≥ 75%) as part of this analysis.
Results
Perceived changes in adherence
Most patients in this sample (20/22) believed that they had increased their adherence. Some patients made connections between changes in health and doing their nebuliser treatments, which reinforced the need for treatment. Small adjustments to routines allowed high-level adherers to maintain adherence. Participants perceived that moderate-level adherers increased adherence by establishing routines for additional treatments by making informal or formal action plans with the interventionist. For those with lower levels of adherence, there were more barriers to overcome, which interventionists sometimes struggled to address.
Expected mechanisms of action in different groups of baseline adherence
Self-monitoring was acceptable in this sample of PWCF and was the most used aspect of the intervention, usually accessed through the app. Self-monitoring seemed less important to high-level adherers who knew how they were doing and very low-level adherers who sometimes did not see the need to look at their adherence.
Tailored education and problem-solving were used successfully by some interventionists and PWCF to address individual issues for low- and moderate-level adherers such as unhelpful beliefs about treatments. The videos about how treatments worked were seen as particularly useful to patients and interventionists, with the exception of high-level adherers.
Tailored patient videos were useful to some patients provided that they found someone like them. However, some patients disliked seeing other PWCF.
Personalised goal-setting, goal review and rewards were acceptable and used in visits to set targets acceptable to patients. Lower- and moderate-level adherers often lowered their targets initially to hit their target. Some then increased their target and maintained it, although this could be sporadic to begin with.
Personalised action plans were sometimes problematic as patients did not understand their purpose and struggled to write them in the intervention format. High-level adherers did not need action plans; however, these plans were sometimes successful in forming habits for low- and moderate-level adherers through trial and error and tackling assumptions about when treatments could happen.
Additional mechanisms of action
The patient–interventionist relationship gave PWCF someone to talk to about adherence issues, someone who believed in them, and made them accountable to someone about adherence progress. Most PWCF and interventionists in the sample believed that visits needed to happen face to face and outside clinic visits to enable time to talk through issues with someone who cared.
Others (interventionists and MDT members) monitoring adherence was reassuring and motivating for most participants in the sample, particularly when the interventionist or MDT members praised PWCF.
The right time to receive the intervention was important for some PWCF who increased their adherence; for example, during a time of change, such as a new job, house or relationship, a recent hospital admission or a sense of getting older.
Conclusion
Where the intervention helped PWCF to increase adherence, a combination of mechanisms of action came together at the right time for patients. Key mechanisms were self-monitoring, others monitoring nebuliser adherence, the relationship between the patient and the interventionist, and action plans. In some cases, the intervention was unable to address all the barriers in the individual’s life.
Appendix 9 Work package 3.3: variation in context between randomised controlled trial sites
Introduction
Understanding the relationship between intervention and context is essential to determine the factors involved in the success and failure of interventions, causal mechanisms and variation in effects. 102 In addition, decision-makers need to understand the role of context so that they can have confidence that the intervention will work well in other settings. 103
The UK Medical Research Council Guidance definition of context includes ‘anything external to the intervention that may act as a barrier or facilitator to its implementation, or its effects’. 45 It ‘interacts, influences, modifies and facilitates or constrains the intervention and its implementation’. 104
Methods
We considered context at the macro (CF community), meso (CF unit size, culture, attitude to medication adherence) and micro (interventionists’ skills, backgrounds, practices) levels. 104 We focused only on the meso and micro levels in our analysis because we were interested in variation between RCT sites. We brought together data from a range of sources in the process evaluation: qualitative interviews with interventionists (patients and MDT were not included because we did not have interviews for all RCT sites), trial management reports, fidelity scores, the usual-care survey and the acceptability of intervention survey (focusing on items relating to interventionists only). We displayed data in a grid, with sites in rows and variables in columns. We colour coded each cell as red if problems were identified and as blue if the site looked very good compared with other sites. We then undertook ‘pattern matching’ used in multiple case study analysis, with each site acting as a case. 105
Results
We found variation between sites in all the variables we included. For example, some sites used different nebulisers to the type used in the RCT, interventionists had different disciplinary backgrounds and experience with CF (e.g. physiotherapist, pharmacist), interventionists had different reasons to get involved in the RCT, interventionists had different levels of communication with the MDT about the intervention and about specific patients in the intervention arm, interventionists organised their work differently, some sites did not allow home visits and so all assessments had to occur on site when the intervention specified home, some sites had different cultural alignment with the intervention, MDT attitudes to the RCT varied from receptive to indifferent to hostile, and some interventionists found it easier to deliver patient-centred communication.
A key finding was that potential barriers did not hinder the intervention but potential facilitators could enhance the implementation of the intervention. The extent to which the interventionist was embedded in the MDT appeared to be very important to implementation of the intervention.
Conclusions
A key contextual factor of how embedded interventionists were in the MDT may have enhanced the implementation of the intervention.
Appendix 10 Work package 3.3: mediation analysis
A mediation analysis was conducted to examine the mechanisms of change in relation to those hypothesised in an a priori logic model.
The mean adherence between 6 to 12 months was primarily mediated by awareness of medication usage (37% of the total effect mediated, 95% CI 24% to 51%), with habit formation (9%, 95% CI 3% to 16%) the second most important factor (Table 18). Other mediating effects were reduced concerns, increased self-efficacy and ease of effort, resulting in a total mediated effect of 51%. There was some evidence that the intervention increased awareness among patients who used several nebulisers at baseline.
| Variablea | N | Correlation with medication adherence (95% CI) | Standardised mean difference, Cohen’s d (95% CI) | Mediator selected? |
|---|---|---|---|---|
| Awareness | 570 | 0.45 (0.38 to 0.51) | 0.61 (0.44 to 0.77) | Yes |
| Motivation | 534 | 0.06 (–0.03 to 0.14) | 0.11 (–0.06 to 0.28) | No |
| Habit | 532 | 0.17 (0.08 to 0.25) | 0.29 (0.12 to 0.46) | Yes |
| Concerns | 534 | 0.13 (0.05 to 0.22) | 0.40 (0.22 to 0.56) | Yes |
| Necessity | 534 | 0.04 (–0.05 to 0.12) | 0.19 (0.02 to 0.36) | No |
| Self-efficacy | 534 | 0.16 (0.078 to 0.25) | 0.17 (0.002 to 0.34) | Yes |
| Barriers | 607 | 0.10 (0.02 to 0.18) | –0.01 (–0.17 to 0.14) | No |
| Chaos | 535 | 0.10 (0.01 to 0.19) | 0.06 (–0.11 to 0.22) | No |
| Effort | 530 | 0.18 (0.1 to 0.27) | 0.25 (0.08 to 0.42) | Yes |
| Burden | 539 | 0.03 (–0.06 to 0.11) | 0.17 (–0.003 to 0.34) | No |
As shown in Figure 14, better awareness seems to be associated to better normative medication adherence.
FIGURE 14.
Normative medication adherence vs. baseline prescription by randomisation group: (a) usual care; and (b) intervention.
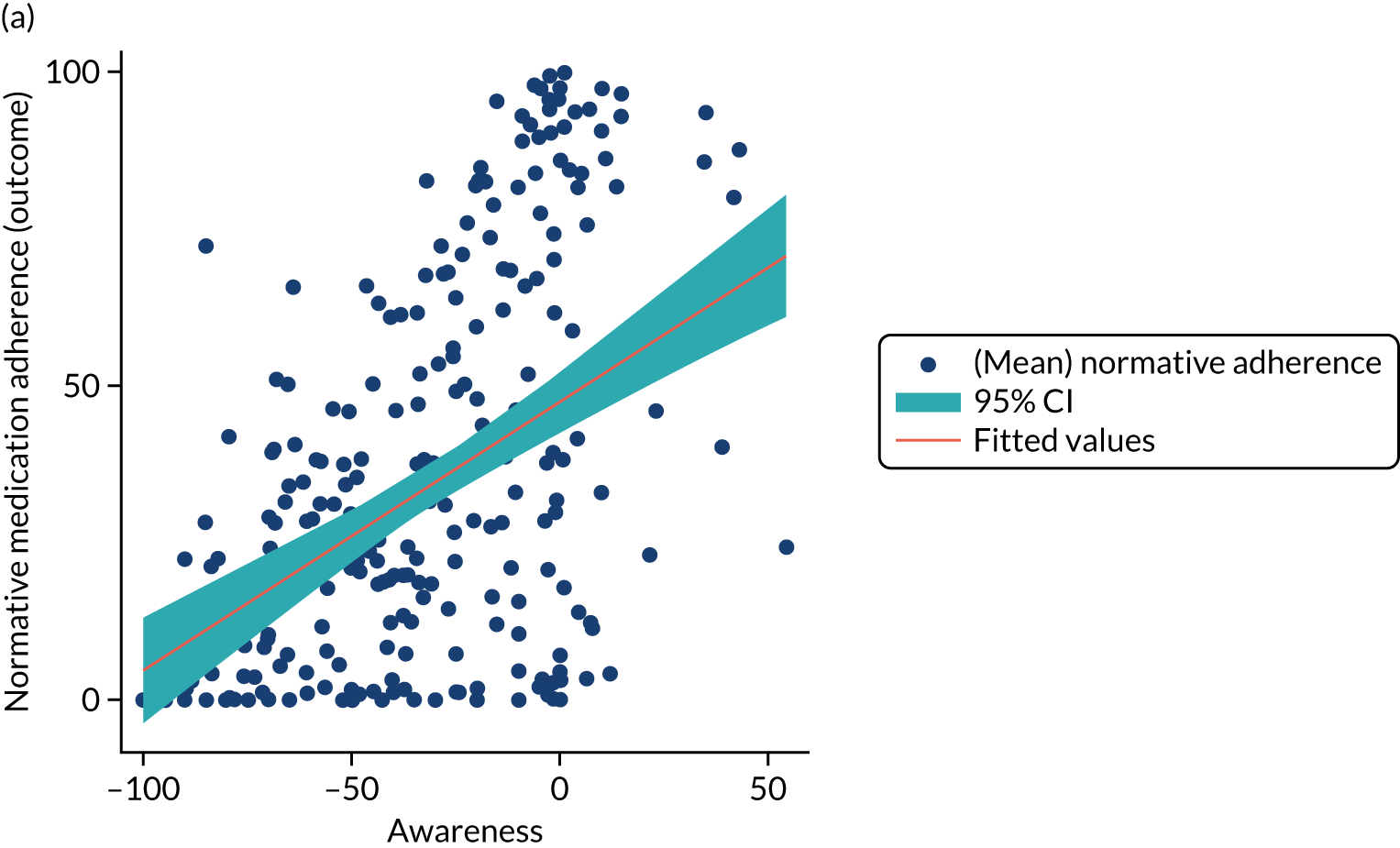
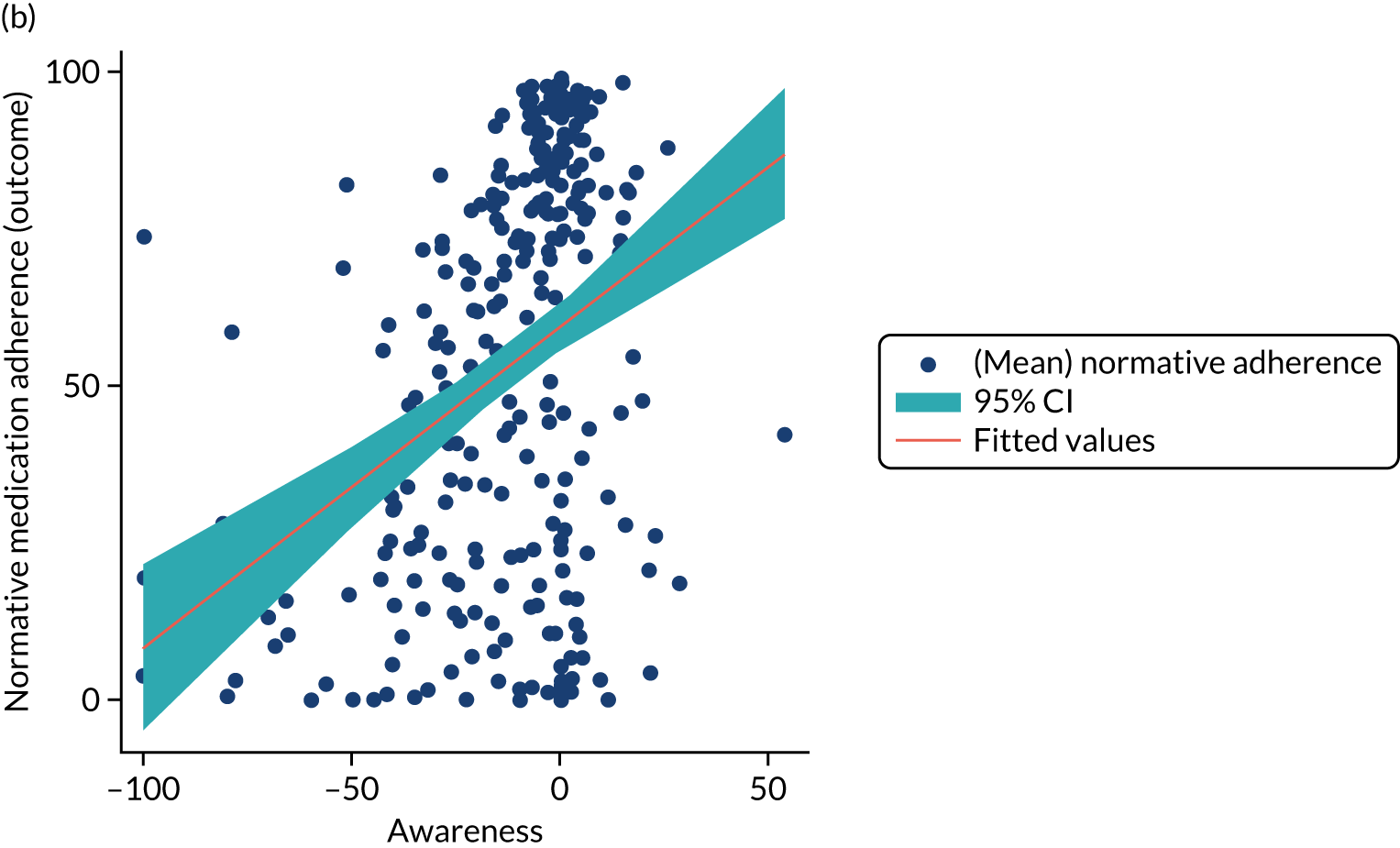
As shown in Figure 15, increase in baseline prescription seems to reduce awareness in the usual-care arm but not in the intervention group.
FIGURE 15.
Awareness vs. baseline prescription by randomisation group interaction graph: (a) usual care; and (b) intervention.
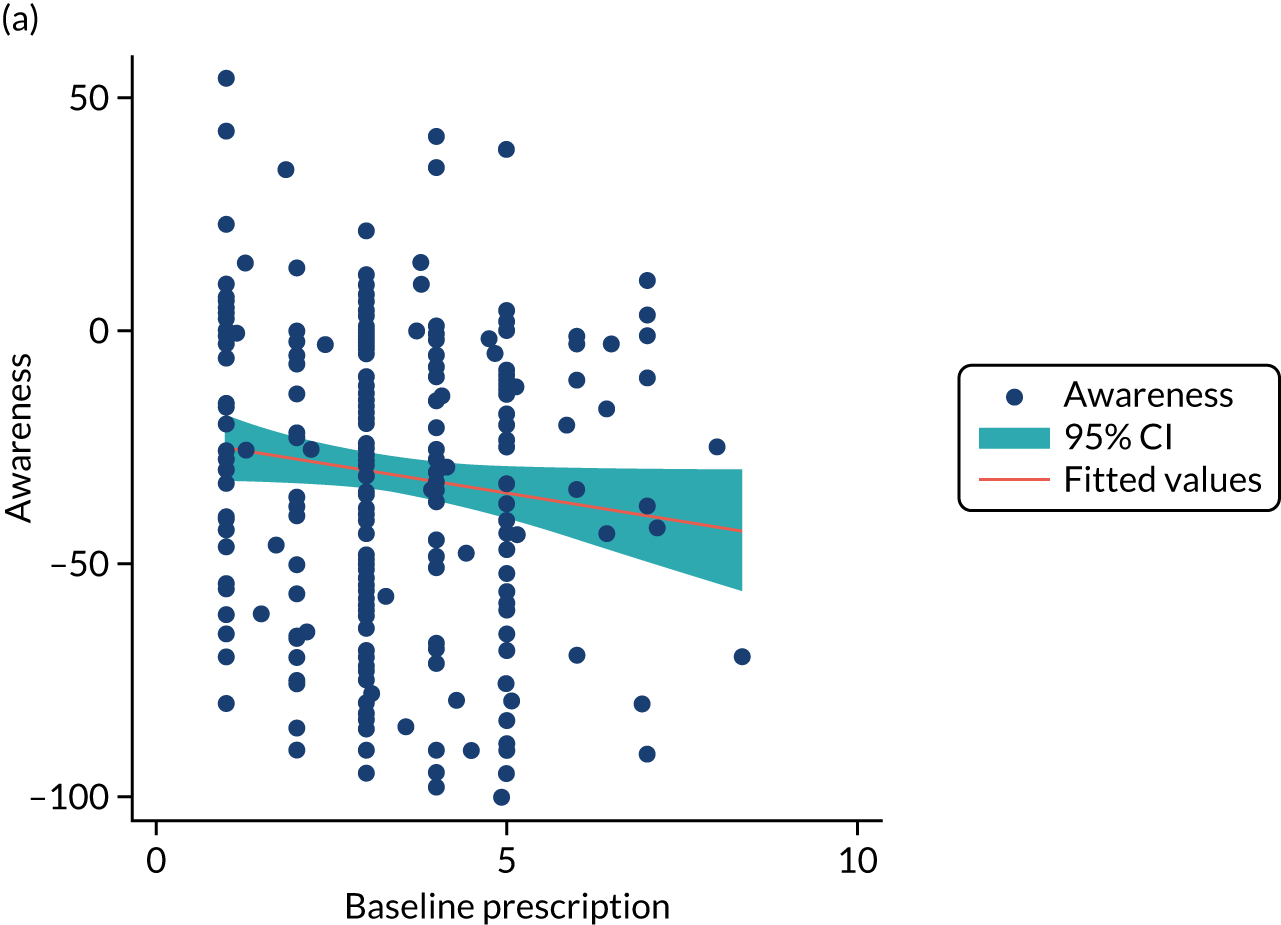
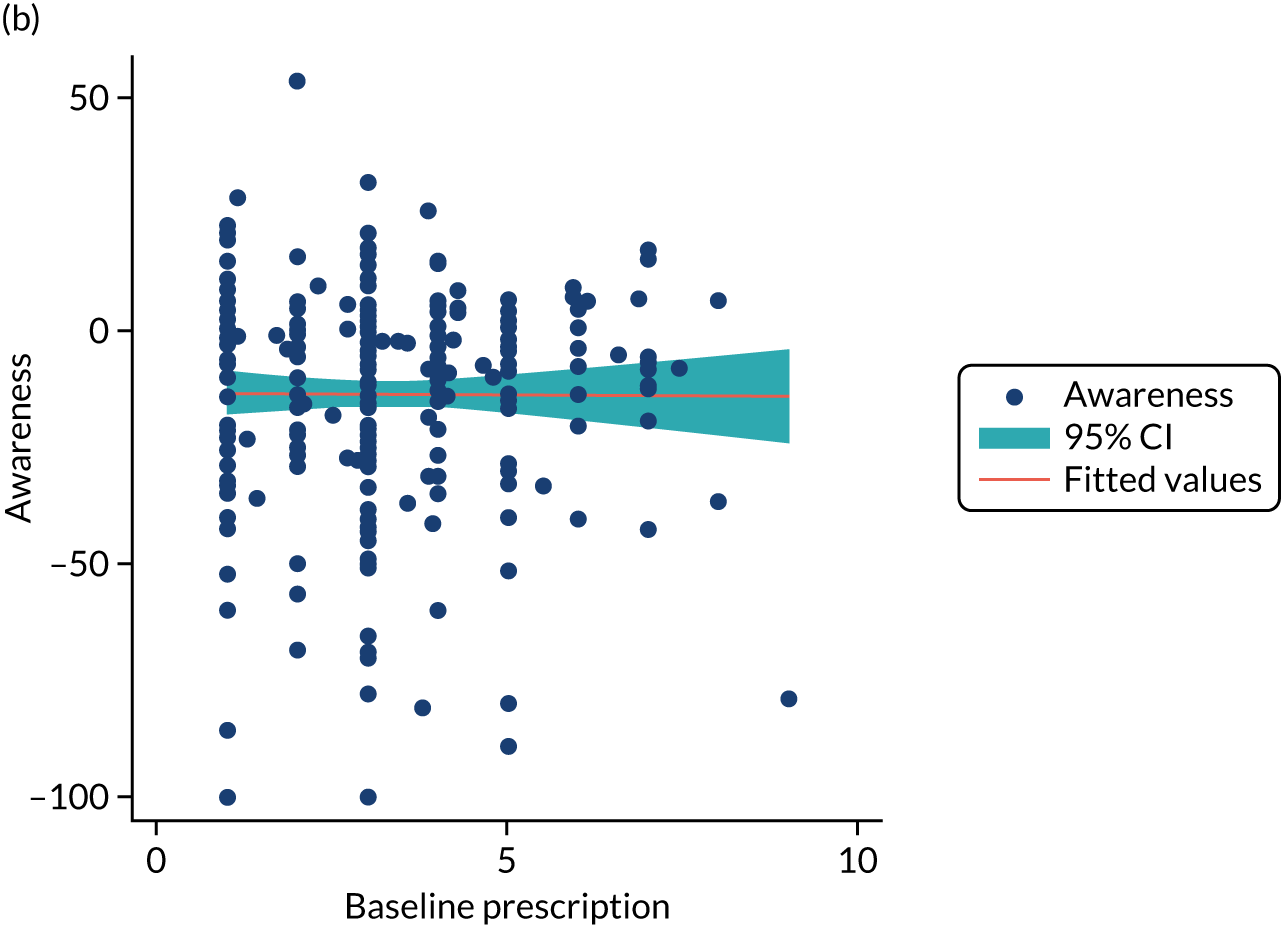
As shown in Figure 16, increase in effort seems to improve normative medication adherence but this effect varies for males and females.
FIGURE 16.
Effort vs. gender by randomisation group interaction graph: (a) usual care, female; (b) usual care, male; (c) intervention, female; and (d) intervention, male.
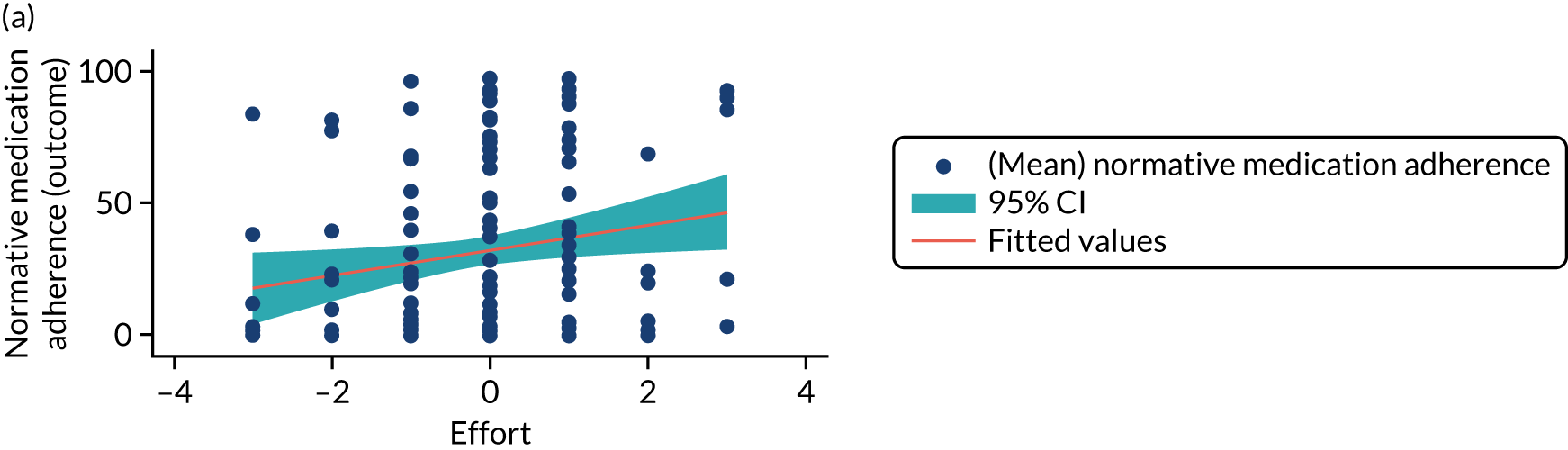
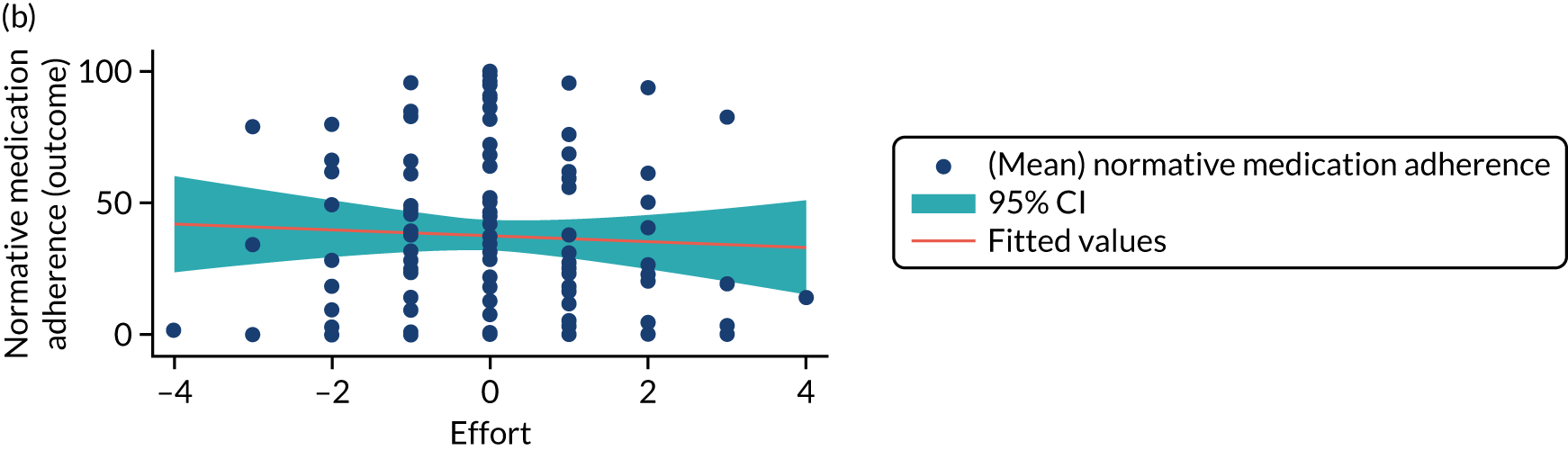
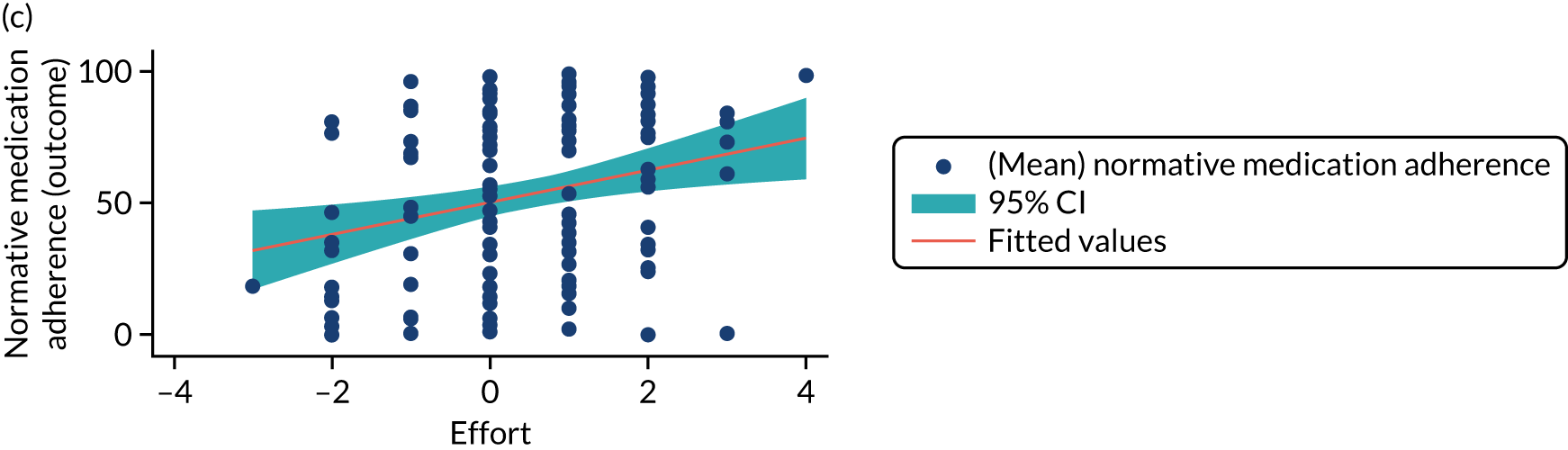
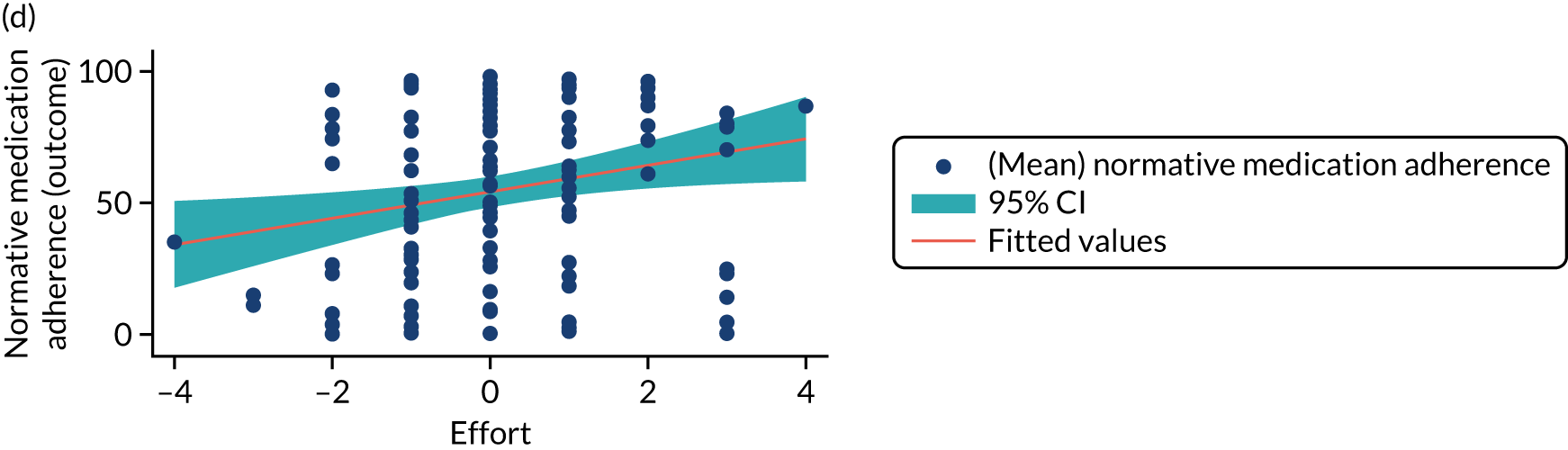
Figure 17 and Tables 19–21 and provide additional information and data from this analysis.
FIGURE 17.
Mediation pathway DAG. Model fit of the model with concerns affecting self-efficacy was similar to self-efficacy affecting concerns but both models produced similar estimates.
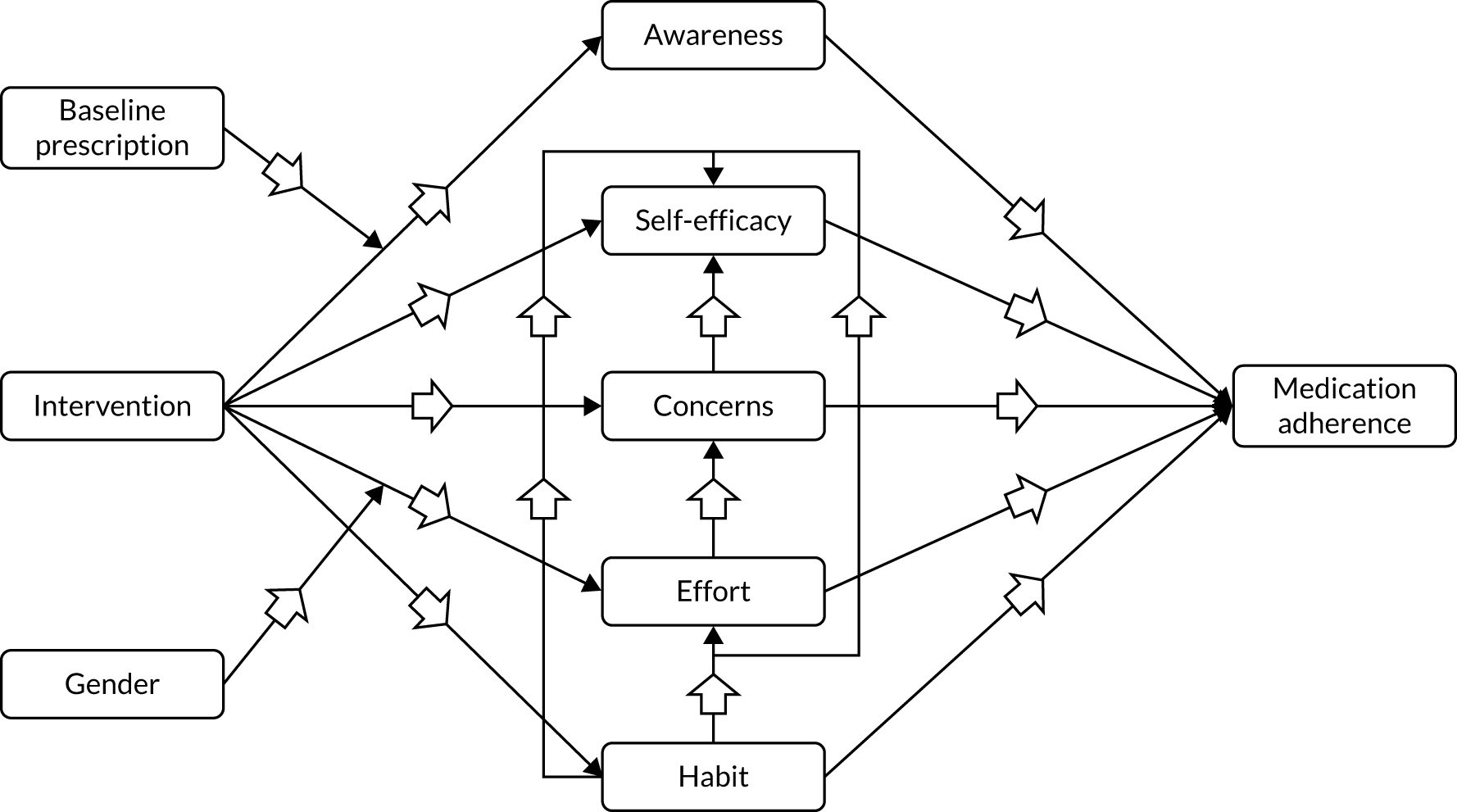
| Mediator 1a | Mediator 2a | Correlation (95% CI) |
|---|---|---|
| Awareness | Self efficacy | –0.01 (–0.10 to 0.08) |
| Awareness | Concerns | 0.06 (–0.03 to 0.14) |
| Awareness | Habit | –0.05 (–0.13 to 0.04) |
| Awareness | Effort | –0.05 (–0.14 to 0.04) |
| Self efficacy | Concerns | 0.16 (0.07 to 0.24) |
| Self efficacy | Effort | 0.20 (0.12 to 0.28) |
| Self efficacy | Habit | 0.20 (0.12 to 0.28) |
| Concerns | Habit | 0.21 (0.12 to 0.29) |
| Concerns | Effort | 0.23 (0.15 to 0.31) |
| Habit | Effort | 0.32 (0.24 to 0.39) |
| Effect | Mediator | Indirect effect estimate (95% CI) | Proportion mediated (%) |
|---|---|---|---|
| Indirect effects | Awareness (prescription 0.5) | 2.40 (–0.56 to 5.36) | 18.00 |
| Awareness (prescription 2) | 3.85 (1.79 to 5.91) | 28.90 | |
| Awareness (prescription 4) | 5.79 (3.76 to 7.81) | 43.43 | |
| Awareness (prescription 6) | 7.72 (4.45 to 10.99) | 57.97 | |
| Self-efficacy | 0.21 (–0.31 to 0.73) | 1.58 | |
| Concerns | 0.21 (–0.29 to 0.71) | 1.55 | |
| Habit | 1.26 (0.36 to 2.15) | 9.44 | |
| Effort (females) | 0.37 (–0.23 to 0.98) | 2.80 | |
| Effort (males) | –0.03 (–0.34 to 0.28) | –0.24 | |
| Direct effect | 6.07 (2.65 to 9.48) | ||
| Total effect | 13.32 (9.60 to 17.04) | ||
| Effect | Mediator | Indirect effect point estimate (95% CI) | Proportion mediated (%) |
|---|---|---|---|
| Indirect effects | Awareness (prescription 0.5) | 1.88 (–0.99 to 4.74) | 14.08 |
| Awareness (prescription 2) | 3.65 (1.64 to 5.67) | 27.43 | |
| Awareness (prescription 4) | 6.02 (4.08 to 7.97) | 45.23 | |
| Awareness (prescription 6) | 8.40 (5.30 to 11.49) | 63.03 | |
| Self-efficacy | 0.20 (–0.31 to 0.71) | 1.54 | |
| Concerns | 0.18 (–0.33 to 0.68) | 1.32 | |
| Habit | 1.26 (0.36 to 2.15) | 9.45 | |
| Effort (females) | 0.38 (–0.23 to 0.98) | 2.83 | |
| Effort (males) | –0.03 (–0.35 to 0.28) | –0.25 | |
| Direct effect | 6.17 (2.74 to 9.61) | ||
| Total effect | 13.33 (9.60 to 17.06) | ||
Appendix 11 Work package 3.3: process evaluation triangulation table
| Type/source of data | Qualitative | Quantitative | Acceptability questions in RCT intervention arm (n/N = 257/305)/usual-care surveys (n/N = 19/19)/CONSORT RCT monitoring (N = 305) | Convergence, complementary, disagree, silence (meta-inferences) | ||
|---|---|---|---|---|---|---|
| Interventionist and MDT interviews | PWCF in intervention arm of RCT interviews | Mediation analysis | Fidelity | |||
| Implementation | ||||||
| Fidelity: domains of fidelity |
|
|
– |
|
– |
|
| Adaptations |
|
|
– | – | – | – |
| Access to intervention |
|
|
– | – |
|
|
| Dose |
|
|
– |
|
– |
|
| Feasibility |
|
|
– | – | – |
|
| Reach |
|
|
– | – |
|
|
| Mechanisms of action/impact | ||||||
| Acceptability of interventionists |
|
|
– | – |
|
|
| Acceptability of adherence feedback graphs |
|
|
|
– |
|
|
| My toolkit |
|
|
– | – |
|
|
| Action plans/coping plans |
|
|
– | – |
|
|
| Treatment videos |
|
|
– | – |
|
|
| ‘Talking head’ videos |
|
|
– | – |
|
|
| My treatment | – | – | – | – |
|
|
| Problem-solving |
|
|
– | – |
|
|
| App | – |
|
– | – |
|
|
| Interaction with MDT |
|
|
– | – | – |
|
| Training in delivering the intervention |
|
– | – | – | – |
|
| Value/perceived benefits of intervention |
|
|
– | – | – |
|
| Unexpected pathways and consequences/safety |
|
|
– | – |
|
|
| Context | ||||||
| Differences between PWCF |
|
|
– | – | – |
|
| Usual care in RCT sites |
|
|
– | – |
|
|
| Nebuliser type |
|
|
– | – |
|
|
| RCT sites/CF centres |
|
– | – | – | – |
|
| RCT |
|
|
– | – | – |
|
Appendix 12 Work package 3.4: triangulation of randomised controlled trial and process evaluation findings
| Outcomes and processes | RCT | Process evaluation | Convergence, complementary, disagreement, silence (meta inferences) |
|---|---|---|---|
| Exacerbations |
|
|
The intervention may have helped some PWCF reduce exacerbations but the size of effect on average was not clinically or statistically significant |
| Adherence rates |
|
|
Adherence rates increased because of objective measurement of adherence and potentially a range of other mechanisms |
| FEV1 |
|
|
Possible small improvement in lung function |
| BMI |
|
|
Small improvement in weight gain |
| Medication beliefs |
|
|
Positive changes in beliefs about medication through discussion with interventionists and videos in CFHealthHub |
| Habit |
|
|
Improvement in habit forming |
| HRQoL |
|
|
Possibly some improvements in quality of life. Reduction in treatment burden caused by a range of issues |
| Effort |
|
|
Less perceived effort to take treatments |
| PAM |
|
|
Improvement in patient activation |
| Depression |
|
|
No change in depression |
| Anxiety |
|
|
No change in general anxiety |
| Implementation | – |
|
Intervention implemented as planned |
| Safety |
|
– | No serious adverse events |
Appendix 13 Work package 3.5: resource and cost inputs economic evaluation alongside a clinical trial
| Resource type | Resource component | Unit cost (£) | Assumptions | Source |
|---|---|---|---|---|
| i.v. drugs: hospitala | Ceftazidime, 3 g | 3.15 | 42 doses in 14 days | Misbah Tahir, Clinical Pharmacist, Sheffield Teaching Hospitals NHS Foundation Trust, personal communication |
| Tobramycin, 481–560 mg | 15.16 | 14 doses in 14 days | ||
| Sodium chloride, 0.9% | 0.06 | 28 doses in 14 days | ||
| Heparin, 50 units in 5 ml | 0.41 | 42 doses in 14 days | ||
| 30 l WIVA™ waste bin (Mauser UK T/A Daniels Healthcare, Littleborough, UK) | – | 1 unit | ||
| 1 × 100 Sani-cloth® wipes (PDI EMEA Ltd, Corby, UK) | – | 1 unit | ||
| Delivery | – | 1 unit | ||
| Total (per i.v. therapy day) | 25.96 | |||
| i.v. drugs: homecarea | Ceftazidime, 3 g | 30.68 | 42 doses in 14 days | Misbah Tahir, Clinical Pharmacist, Sheffield Teaching Hospitals NHS Foundation Trust, personal communication |
| Tobramycin, 481–560 mg | 44.82 | 14 doses in 14 days | ||
| Sodium chloride, 0.9% | 0.60 | 28 doses in 14 days | ||
| Heparin, 50 units in 5 ml | 3.00 | 42 doses in 14 days | ||
| 30 l WIVA™ waste bin | 5.79 | 1 unit | ||
| 1 × 100 Sani-cloth® wipes (PDI EMEA Ltd, Corby, UK) | 24.00 | 1 unit | ||
| Delivery | 75.00 | 1 unit | ||
| Total (per i.v. therapy day) | 154.55 | |||
| Nebulised drugs | – | 0.00 | Not included in the within-trial analysis | – |
| Adherence intervention | Data transfer | 133.77 | Conversion rate €1 = £0.88756 (1 October 2019) | Qualcomm |
| Monitoring | Confidential information has been removed | Conversion rate €1 = £0.88756 (1 October 2019) | PARI GmbH | |
| Data platform (management, maintenance and customer support) | 312.72 | Costs of running CFHealthHub for 12 months; 305 trial patients in the intervention arm | Farr Institute | |
| Data platform (hosting and penetration testing) | 72.13 | Costs for 12 months; 305 trial patients in the intervention arm | Farr Institute | |
| ScHARR research costs: staff costs | 34.93 | One programme manager (1 hour/3 weeks), one research assistant (1.5 hours/3 weeks), two data specialists (0.5 days/week + 6 weeks full and 1 hour/3 weeks); includes overheads for academic staff; 305 trial patients in the intervention arm | University of Sheffield (yearly ages); Chin Maguire, ACTIF Project Manager, University of Sheffield, 2020, personal communication (hours and personnel involved) | |
| ScHARR research costs: TMG meetings | 58.50 | 15 interventionists, one programme manager, one data specialist, two research assistants, one trainer; includes overheads for academic staff; 305 trial patients in the intervention arm | University of Sheffield (yearly ages); PSSRU; personal communication (hours and personnel involved) | |
| Interventionists training | 345.45 | Three trainers (2 × 180.5 hours and 61 hours for developing training, delivering face-to-face and competency assessment), 30 interventionists (grade 7 physiotherapists); includes overheads for academic staff; 305 trial patients in the intervention arm | University of Sheffield (yearly ages); PSSRU; personal communication (hours and personnel involved) | |
| Ongoing fidelity support | 58.50 | Assumed the same costs as TMG; telephone calls with all interventionists every 3 weeks; 305 trial patients in the intervention arm | University of Sheffield (yearly ages); PSSRU; personal communication (hours and personnel involved) | |
| Initial data set-up visit | 159.00 | Average of 3 hours per patient; grade 7 physiotherapists | PSSRU; personal communication | |
| Delivery of intervention | 257.00 | Data from the intervention log; 305 trial patients in the intervention arm | PSSRU; ACtiF trial | |
| Total (annual costs) | 1442.66 | |||
| Nebuliser: intervention | eTrack | Confidential information has been removed | Annualised cost per patient; equipment lifetime of 5 years and discount rate of 3.5% | PARI GmbH |
| eflow | Confidential information has been removed | Annualised cost per patient, equipment lifetime of 5 years, 20% of patients would receive a new nebuliser and discount rate of 3.5% | PARI GmbH | |
| Resource use | i.v. therapy in hospital (days) | 406.03 | Cost per non-elective bed-day, weighted by FCEs and average length of stay, assumed interventions for bronchiectasis (codes DZ12C to DZ12F) | NHS Reference Costs 2017/18 58 |
| Other hospitalisation i.v. therapy (total days) | 337.36 | Cost per non-elective excess bed-day weighted by FCEs; weighted average across all interventions | NHS Reference Costs 2017/18 58 | |
| GP (minutes) – surgery and at home | 4.06 | Assumed that each surgery consultation lasts 9.22 minutes; unit costs of GP includes direct care staff costs, qualification costs and carbon emissions | PSSRU report 2018106 | |
| Consultant hospital visits (n) | 165.98 | Costs of consultant-led services for respiratory medicine (service code 340) from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Non-consultant hospital visits (n) | 119.64 | Costs of non-consultant led services for respiratory medicine (service code 340) from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Nurse (minutes) | 0.75 | Unit costs of hospital or community-based nurses, assumed professional at band 6 (nurse specialist/team leader) | PSSRU report 2018106 | |
| Physiotherapist (n) | 54.91 | Costs of total services for physiotherapy (consultant and non-consultant led, service code 650) from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Dietitian (n) | 82.64 | Costs of total services for dietetics (consultant and non-consultant led, service code 654), from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Telephone contact (call) | 10.45 | Unit costs of telephone triage – assumed the average between the GP-led and nurse-led services | PSSRU report 2018106 | |
| Psychologist visits (n) | 170.27 | Costs of total services for clinical psychology (consultant and non-consultant led, service code 656) from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Occupational therapist visits (n) | 73.25 | Costs of total services for occupational therapy (consultant and non-consultant led, service code 651) from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Radiographer visits (n) | 57.73 | Assumed the costs of total services for diagnostic imaging (consultant and non-consultant led, service code 812) from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Social worker (minutes) | 1.00 | Unit costs of telephone triage – assumed the average between the GP-led and nurse-led services | PSSRU report 2018106 | |
| Other visits (n) | 121.34 | Assumed all interventions for total outpatient attendances, excluding paediatric (and related) items; costs were weighted by FCEs | NHS Reference Costs 2017/18 58 | |
| A&E (n) | 135.80 | Costs of total services for A&E (consultant and non-consultant led, service code 180) from total outpatient attendances data | NHS Reference Costs 2017/18 58 | |
| Transplant | 45,458.41 | Assumed the costs of total services for lung and heart and lung transplants (elective inpatient, elective inpatient excess bed-days and non-elective long stay, codes DZ01Z and ED01Z); costs weighted by FCEs | NHS Reference Costs 2017/18 58 |
Appendix 14 Work package 3.5: health economic model parameters
| Health state | FEV1 ≥ 70% | FEV1 ≥ 40–69% | FEV1 < 40% | Transplant | Dead |
|---|---|---|---|---|---|
| n | 212 | 242 | 152 | 0 | 0 |
| Probability | 0.35 | 0.40 | 0.25 | 0 | 0 |
Transition probabilities: usual care
| From/to | FEV1 ≥ 70% | FEV1 ≥ 40–69% | FEV1 < 40% | Transplant | Dead |
|---|---|---|---|---|---|
| Aged 30–34 years | |||||
| FEV1 ≥ 70% | 0.8749 | 0.1138 | 0.0074 | 0.0001 | 0.0038 |
| FEV1 ≥ 40–69% | 0.0806 | 0.8047 | 0.1045 | 0.0029 | 0.0073 |
| FEV1 < 40% | 0.0044 | 0.0879 | 0.7804 | 0.0441 | 0.0832 |
| Transplant | 0.0000 | 0.0000 | 0.0000 | 0.9450 | 0.0550 |
| Dead | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
| Aged 35–39 years | |||||
| FEV1 ≥ 70% | 0.8545 | 0.1322 | 0.0068 | 0.0001 | 0.0065 |
| FEV1 ≥ 40–69% | 0.0775 | 0.8291 | 0.0849 | 0.0015 | 0.0070 |
| FEV1 < 40% | 0.0045 | 0.0961 | 0.7906 | 0.0285 | 0.0803 |
| Transplant | 0.0000 | 0.0000 | 0.0000 | 0.9672 | 0.0328 |
| Dead | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
| Aged 40–44 years | |||||
| FEV1 ≥ 70% | 0.8599 | 0.1245 | 0.0081 | 0.0001 | 0.0074 |
| FEV1 ≥ 40–69% | 0.0624 | 0.8216 | 0.1064 | 0.0015 | 0.0081 |
| FEV1 < 40% | 0.0033 | 0.0879 | 0.7944 | 0.0222 | 0.0921 |
| Transplant | 0.0000 | 0.0000 | 0.0000 | 0.8875 | 0.1125 |
| Dead | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
| Aged 45–49 years | |||||
| FEV1 ≥ 70% | 0.8892 | 0.0927 | 0.0062 | 0.0000 | 0.0119 |
| FEV1 ≥ 40–69% | 0.0770 | 0.8025 | 0.1078 | 0.0010 | 0.0116 |
| FEV1 < 40% | 0.0045 | 0.0938 | 0.7792 | 0.0149 | 0.1075 |
| Transplant | 0.0000 | 0.0000 | 0.0000 | 0.9465 | 0.0535 |
| FEV1 ≥ 70% | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
| Age ≥ 50 years | |||||
| FEV1 ≥ 70% | 0.8291 | 0.1527 | 0.0129 | 0.0001 | 0.0052 |
| FEV1 ≥ 40–69% | 0.0837 | 0.7466 | 0.1271 | 0.0020 | 0.0406 |
| FEV1 < 40% | 0.0060 | 0.1077 | 0.7465 | 0.0241 | 0.1158 |
| Transplant | 0.0000 | 0.0000 | 0.0000 | 0.9558 | 0.0442 |
| Dead | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 1.0000 |
| Parameter | FEV1 ≥ 70% predicted | FEV1 40–69% predicted | FEV1 < 40% predicted |
|---|---|---|---|
| i.v. therapy-days in hospital | 72,891 | 187,183 | 195,998 |
| i.v. therapy-days at home | 79,617 | 192,393 | 179,325 |
| Total i.v. therapy-days | 152,508 | 379,576 | 375,323 |
| Patient-years in FEV1% category | 17,178 | 16,210 | 7964 |
| i.v. therapy-days per year | 8.88 | 23.42 | 47.13 |
| Proportion of year on i.v. therapy | 0.02 | 0.06 | 0.13 |
| Probability i.v. therapy given in hospital | 0.48 | 0.49 | 0.52 |
Treatment effects: risk ratios of switching state, intervention versus control
| Transition | p 1 | p 2 | Point estimate (95% CI) | Log SE | Bootstrap back-transformed mean |
|---|---|---|---|---|---|
| FEV1 ≥ 70% : FEV1 40–69% | 0.17 | 0.27 | 0.65 (0.40 to 1.04) | 0.25 | 0.64 |
| FEV1 ≥ 70% : FEV1 < 40% | 0.01 | 0.01 | 1.18 (0.69 to 2.04) | 0.28 | 1.18 |
| FEV1 40–69% : FEV1 ≥ 70% | 0.09 | 0.05 | 1.65 (0.95 to 2.94) | 0.29 | 1.67 |
| FEV1 40–69% : FEV1 < 40% | 0.24 | 0.21 | 1.14 (0.74 to 1.75) | 0.22 | 1.14 |
| FEV1 < 40% : FEV1 ≥ 70% | 0.01 | 0.00 | 1.71 (0.95 to 3.15) | 0.31 | 1.73 |
| FEV1 < 40% : FEV1 40–69% | 0.15 | 0.17 | 0.86 (0.53 to 1.36) | 0.24 | 0.85 |
Treatment effects: relative rate ratio for intravenous therapy-days, intervention versus control
| Parameter | Mean (95% CI) | SE |
|---|---|---|
| RRR i.v. therapy-days | 0.92 (0.80 to 1.06) | 0.07 |
Health-related quality of life mapping
| Component | EQ-5D | Coefficient | SE | z | p > |z|a | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|---|
| Comp_1 | FEV1% | 0.1499 | 0.0667 | 2.2484 | 0.0246 | 0.0192 | 0.2806 |
| Comp_1 | _cons | 0.7068 | 0.0360 | 19.6061 | 0.0000 | 0.6361 | 0.7774 |
| Comp_2 | FEV1% | 0.4843 | 0.1341 | 3.6127 | 0.0003 | 0.2216 | 0.7470 |
| Comp_2 | _cons | 0.4168 | 0.1479 | 2.8179 | 0.0048 | 0.1269 | 0.7066 |
| Comp_3 | FEV1% | 1.2016 | 0.3618 | 3.3213 | 0.0009 | 0.4925 | 1.9106 |
| Comp_3 | _cons | 0.3371 | 0.1102 | 3.0585 | 0.0022 | 0.1211 | 0.5532 |
| Prob_C1 | FEV1% | 4.9465 | 2.1909 | 2.2577 | 0.0240 | 0.6524 | 9.2407 |
| Prob_C1 | _cons | –1.4490 | 1.1952 | –1.2123 | 0.2254 | –3.7915 | 0.8936 |
| Prob_C2 | FEV1% | 7.1422 | 2.0871 | 3.4221 | 0.0006 | 3.0516 | 11.2329 |
| Prob_C2 | _cons | –3.8112 | 0.9412 | –4.0491 | 0.0001 | –5.6560 | –1.9664 |
| lns_1 | _cons | –1.9981 | 0.0737 | –27.1154 | 0.0000 | –2.1425 | –1.8537 |
| lns_2 | _cons | –1.1134 | 0.1014 | –10.9779 | 0.0000 | –1.3122 | –0.9146 |
| lns_3 | _cons | –1.6441 | 0.1257 | –13.0789 | 0.0000 | –1.8905 | –1.3977 |
| _diparm1 | sigma1 | 0.1356 | 0.0100 | – | – | 0.1174 | 0.1567 |
| _diparm1 | sigma2 | 0.3284 | 0.0333 | – | – | 0.2692 | 0.4007 |
| _diparm1 | sigma3 | 0.1932 | 0.0243 | – | – | 0.1510 | 0.2472 |
Costs
| Resource type | Resource component | Unit cost (£) | Assumptions | Source |
|---|---|---|---|---|
| i.v. drugs: hospitala | Ceftazidime, 3 g | 3.15 | 42 doses in 14 days | Misbah Tahir, Clinical Pharmacist, Sheffield Teaching Hospitals NHS Foundation Trust, personal communication |
| Tobramycin, 481–560 mg | 15.16 | 14 doses in 14 days | ||
| Sodium chloride, 0.9% | 0.06 | 28 doses in 14 days | ||
| Heparin, 50 units in 5 ml | 0.41 | 42 doses in 14 days | ||
| 30 l WIVA™ waste bin | – | 1 unit | ||
| 1 x 100 Sani-cloth® wipes | – | 1 unit | ||
| Delivery | – | 1 unit | ||
| Total (per i.v. therapy day) | 25.96 | |||
| i.v. drugs: homecarea | Ceftazidime, 3 g | 30.68 | 42 doses in 14 days | Misbah Tahir, Clinical Pharmacist, Sheffield Teaching Hospitals NHS Foundation Trust, personal communication |
| Tobramycin, 481–560 mg | 44.82 | 14 doses in 14 days | ||
| Sodium chloride, 0.9% | 0.60 | 28 doses in 14 days | ||
| Heparin, 50 units in 5 ml | 3.00 | 42 doses in 14 days | ||
| 30 l WIVA™ waste bin | 5.79 | 1 unit | ||
| 1 × 100 Sani-cloth® wipes | 24.00 | 1 unit | ||
| Delivery | 75.00 | 1 unit | ||
| Total (per i.v. therapy day) | 154.55 | |||
| Nebulised drugs | Tobramycin solution (per year) | 2380.45 | Daily dose of 600 mg; tobramycin, 300 mg/5 ml; drug tariff price; weighted by the proportion of patients in CF registry | BNF; CF registry; Misbah Tahir and Martin Wildman, Clinical Pharmacist, Sheffield Teaching Hospitals NHS Foundation Trust, personal communication |
| Other aminoglycoside | 5.89 | Daily dose of 160 mg; gentamicin, 80 ml/2 ml; NHS indicative price; weighted by the proportion of patients in CF registry | ||
| Colistin | 442.19 | Daily dose of 2 million units; colomycin, 2 million units powder; NHS indicative and drug tariff prices; weighted by the proportion of patients in CF registry | ||
| Colistimethate sodium (Promixin®, Zambon S.p.A., Bresso, Italy) | 1158.39 | Daily dose of 4 million units (average); Promixin, 1 million units powder; NHS indicative and drug tariff prices; weighted by the proportion of patients in CF registry | ||
| Aztreonam | 1506.06 | Daily dose of 225 mg; Cayston, 75 mg powder; NHS indicative and drug tariff prices; weighted by the proportion of patients in CF registry | ||
| Colistimethate dry powder | 923.62 | Daily dose of 3.32 million units; Colobreathe (Teva UK Ltd, Castleford, UK), 1.6 million units powder capsules; NHS indicative and drug tariff prices; weighted by the proportion of patients in CF registry | ||
| Tobramycin dry powder | 1212.22 | Daily dose of 224 mg; tobramycin, 28 mg powder capsules; drug tariff price; weighted by the proportion of patients in CF registry | ||
| Azithromycin | 69.44 | Daily dose of 250 mg; azithromycin, 250 mg tablets; drug tariff price; weighted by the proportion of patients in CF registry | ||
| Prophylactic flucloxacillin | 6.76 | Daily dose of 1 g; flucloxacillin 250 mg or 500 mg capsules; NHS indicative or drug tariff prices; weighted by the proportion of patients in CF registry | ||
| Mannitol | 341.18 | Daily dose of 800 mg; Bronchitol (Pharmaxis Europe Limited, Dublin, Ireland), 40 mg inhalation powder capsules; NHS indicative price; weighted by the proportion of patients in CF registry | ||
| DNase | 4160.35 | Daily dose of 2.5 mg; Pulmozyme (Roche Products Limited, Welwyn Garden City, UK), 2.5 mg; NHS indicative and drug tariff prices; weighted by the proportion of patients in CF registry | ||
| Hypertonic saline | 91.33 | Daily dose of 8 ml; 6% or 7% inhalation solution; NHS indicative price; weighted by the proportion of patients in CF registry | ||
| Total | 12,297.87 | – | ||
| Adherence intervention | Data transfer | 202.00 | Commercial value | Qualcomm |
| Monitoring | Confidential information has been removed | Same costs as in the trial; conversion rate €1 = £0.88756 (1 October 2019) | PARI GmbH | |
| Data platform (management, maintenance and customer support) | 16.17 | Same annual cost as in the trial; 5900 patients eligible for the intervention | Farr Institute | |
| Data platform (hosting and penetration testing) | 3.73 | Same annual cost as in the trial; 5900 patients eligible for the intervention | Farr Institute | |
| ScHARR research costs: staff costs | 0 | Not applicable | - | |
| ScHARR research costs: TMG meetings | 0 | Not applicable | – | |
| Interventionists training | 17.86 | Same number of trainers, interventionists and costs as in the trial; 305 trial patients in the intervention arm; applicable only for the first year of the model | University of Sheffield (yearly ages); PSSRU; personal communication (hours and personnel involved) | |
| Ongoing fidelity support | 3.02 | Assumed the same annual costs as the TMG meetings in the trial; 5900 patients eligible for the intervention | University of Sheffield (yearly ages); PSSRU; personal communication (hours and personnel involved) | |
| Initial data set-up visit | 159.00 | Same cost and assumptions as in the trial; applicable only for the first year of the model | PSSRU; personal communication | |
| Delivery of intervention | 257.00 | Assumed the same average per patient as in the trial | PSSRU; ACtiF trial | |
| Total first year (annual costs) | 669.43 | |||
| Total subsequent years (annual costs) | 492.57 | |||
| Nebuliser: intervention | eTrack | Confidential information has been removed | Annuitised cost per patient; equipment lifetime of 5 years and discount rate of 3.5% | |
| eflow | Confidential information has been removed | Annuitised cost per patient, equipment lifetime of 5 years and discount rate of 3.5% | ||
| Resource use | i.v. therapy in hospital (days) | 406.03 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 |
| Other hospitalisation i.v. therapy (total days) | 337.36 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| GP (minutes): surgery and at home | 4.06 | Same cost and assumptions as in the trial | PSSRU Report 2018 | |
| Consultant hospital visits (n) | 165.98 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Non-consultant hospital visits (n) | 119.64 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Nurse (minutes) | 0.75 | Same cost and assumptions as in the trial | PSSRU Report 2018 | |
| Physiotherapist (n) | 54.91 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Dietitian (n) | 82.64 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Phone contact (call) | 10.45 | Same cost and assumptions as in the trial | PSSRU Report 2018 | |
| Psychologist visits (n) | 170.27 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Occupational therapist visits (n) | 73.25 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Radiographer visits (n) | 57.73 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Social worker (minutes) | 1.00 | Same cost and assumptions as in the trial | PSSRU report 2018 | |
| Other visits (n) | 121.34 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| A&E (n) | 135.80 | Same cost and assumptions as in the trial | NHS Reference Costs 2017/18 58 | |
| Transplant | 45,458.41 | Same cost and assumptions as in the trial. Applied once on transition into the transplant health state | NHS Reference Costs 2017/18 58 |
Appendix 15 Work package 3.5: forced expiratory volume in first second (per cent) predicted to EuroQol-5 Dimensions, three-level version, mapping function – assessment of goodness of fit of alternative models
| Summary measure | Two components | Three components | Four components |
|---|---|---|---|
| AIC | 588.55 | 564.26 | 568.39 |
| BIC | 634.76 | 639.37 | 672.38 |
| MAE | 0.1495 | 0.1486 | 0.1487 |
| RMSEA | 0.1922 | 0.1909 | 0.1909 |
FIGURE 18.
Plots of mean prediction vs. data group means for ALDVMMs: (a) two components; (b) three components; and (c) four components.
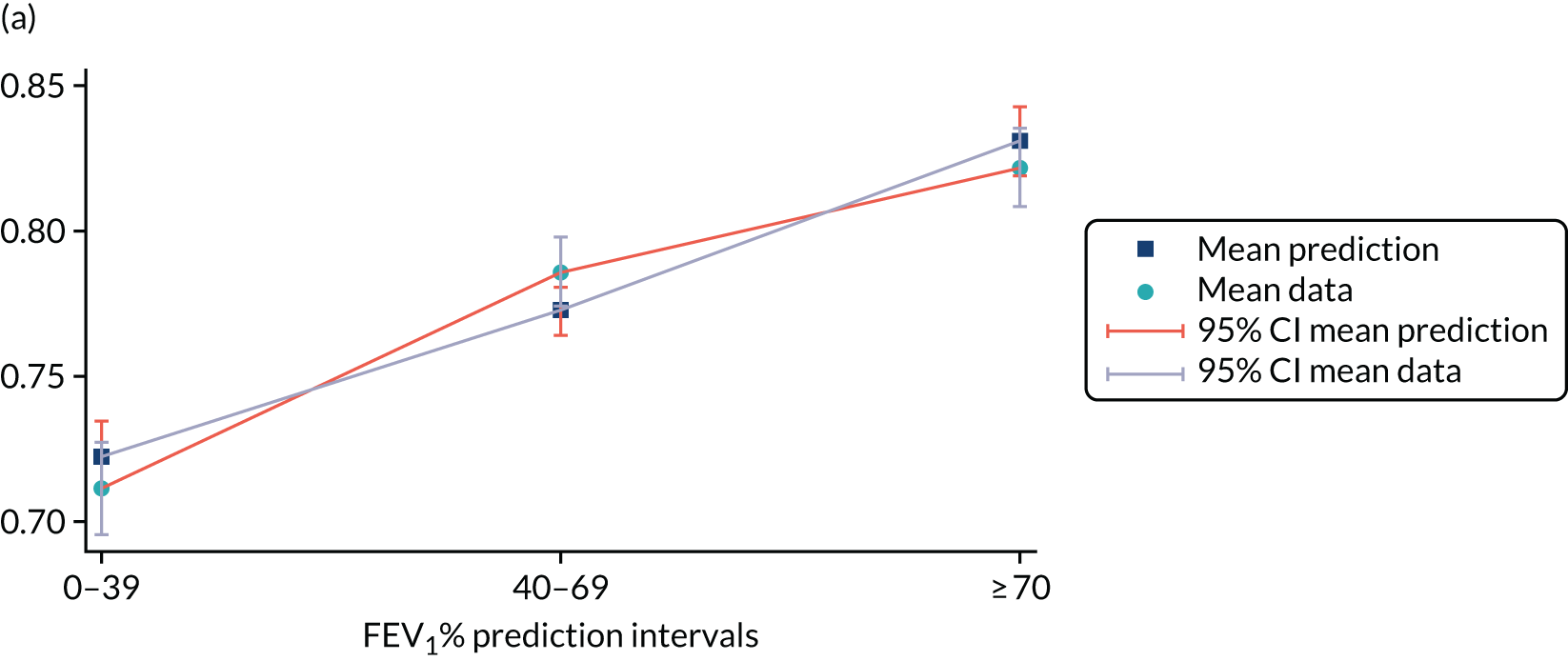
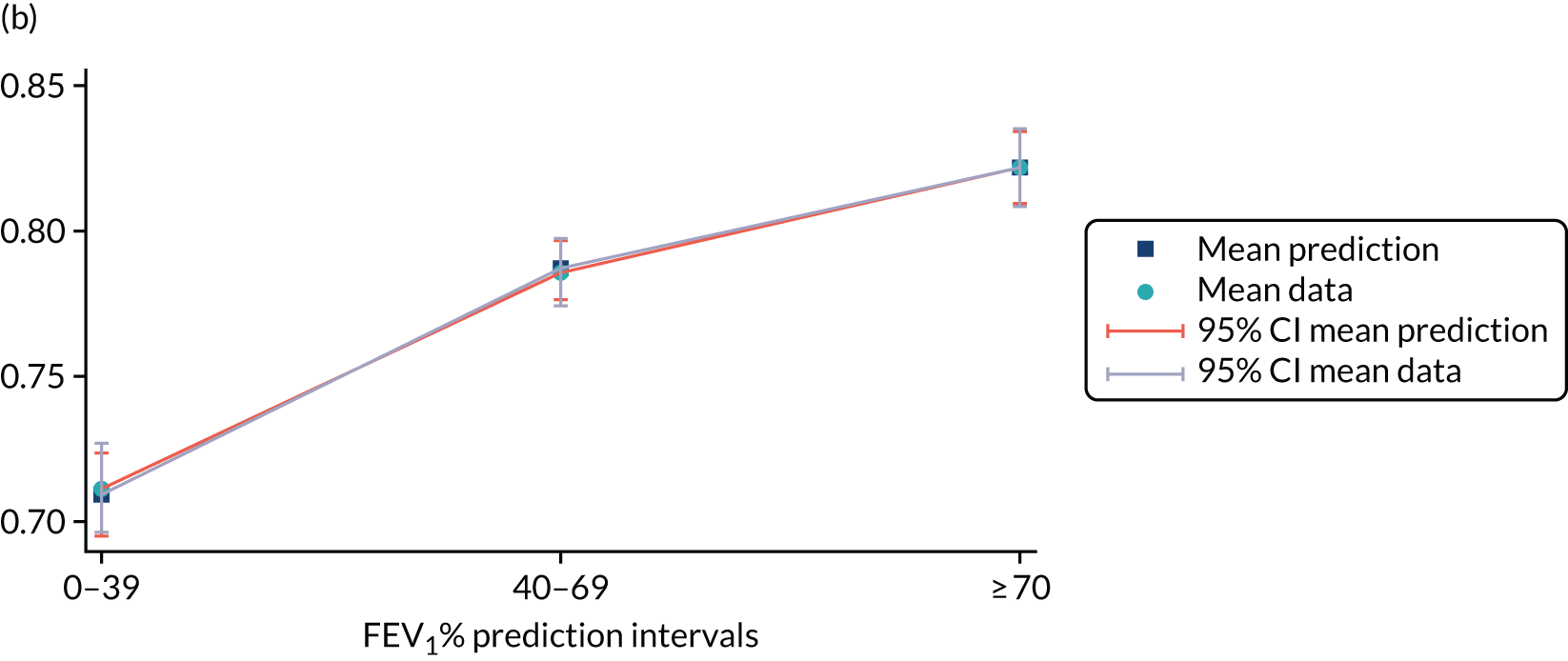
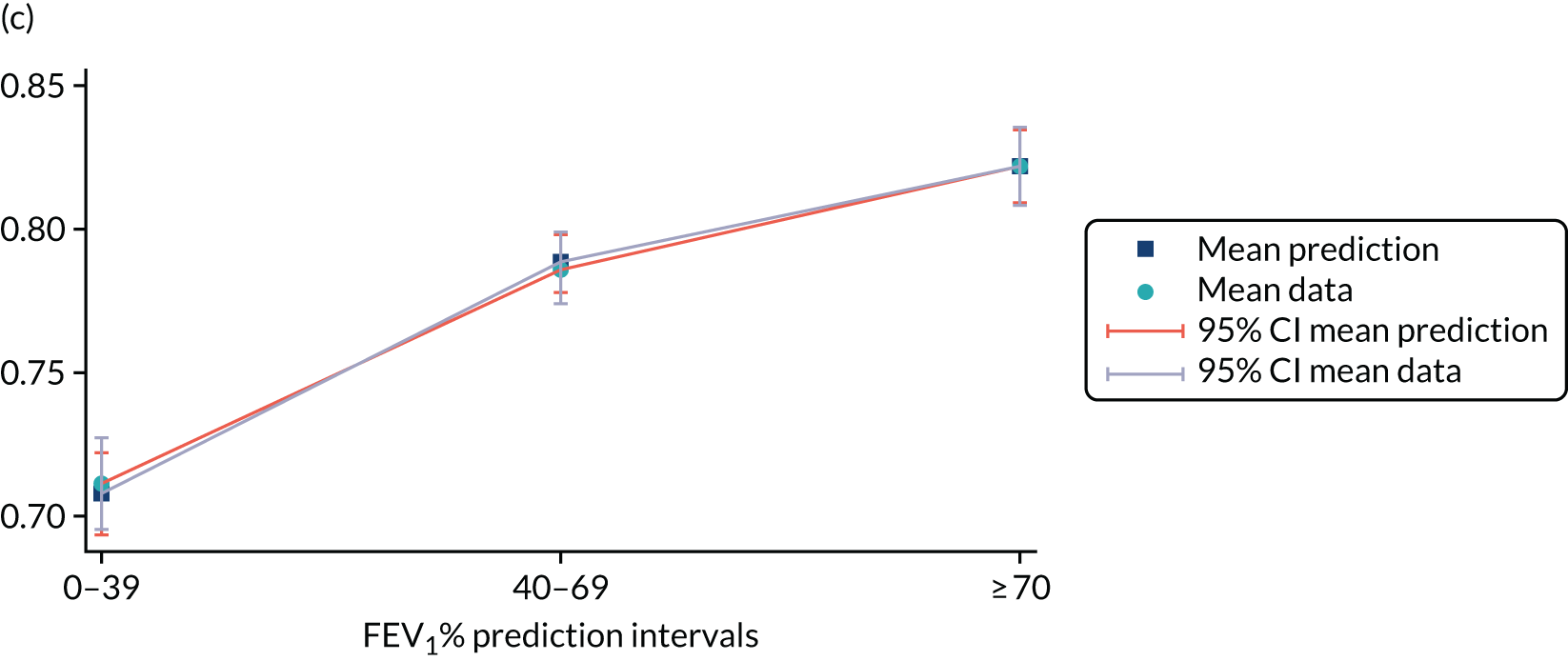
FIGURE 19.
Plots of the cumulative percentage of actual data vs. model for ALDVMMs: (a) two components; (b) three components; and (c) four components.
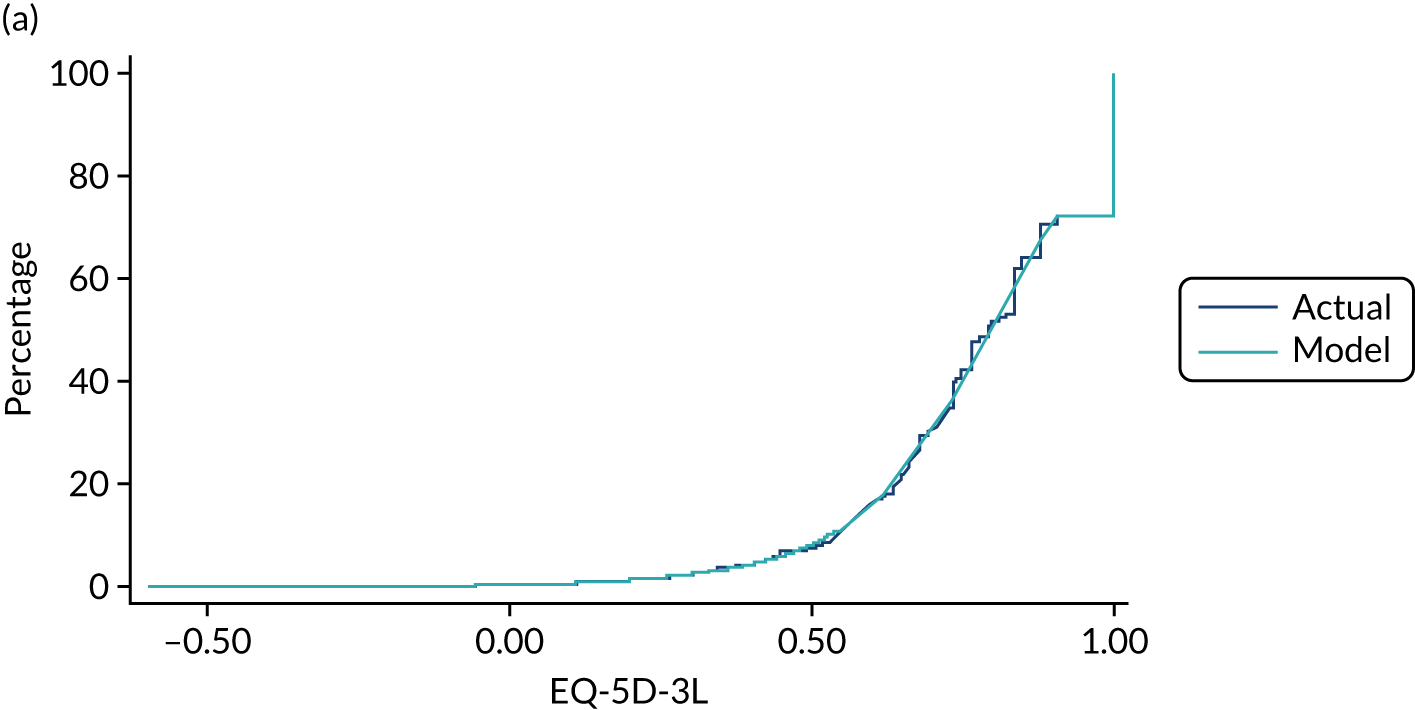
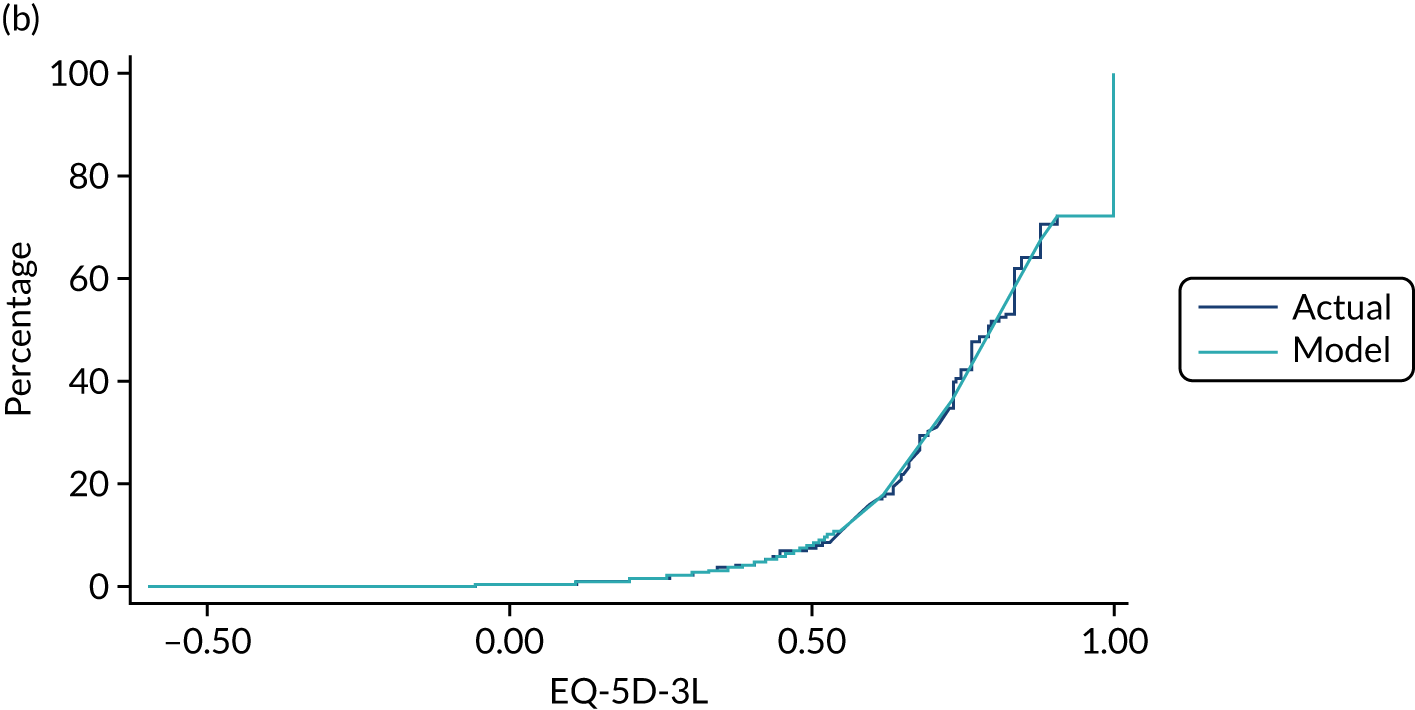
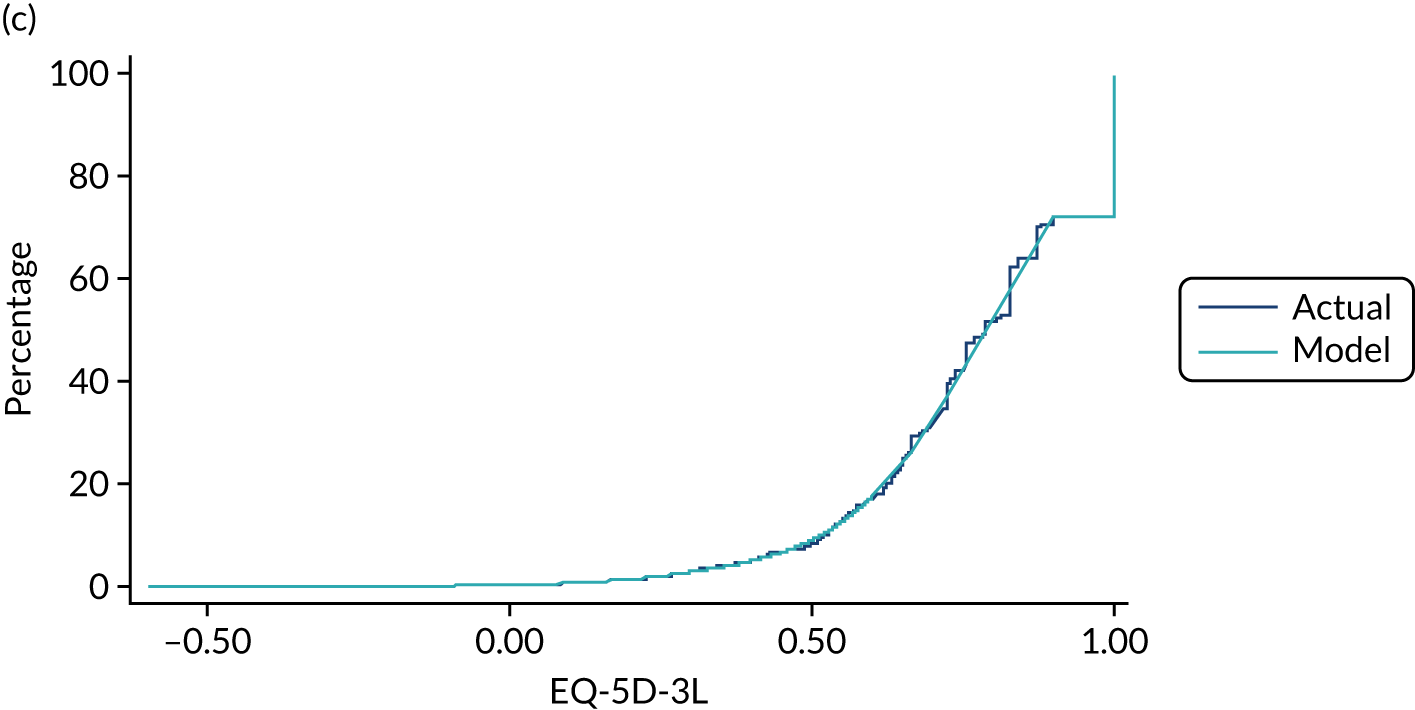
Appendix 16 Work package 3.5: validation of model outputs
The following activities were undertaken to ensure the internal validity and credibility of the model:
-
consideration of key items contained in published economic evaluation and health economic modelling checklists107,108
-
discussion of the proposed model structure with members of the ACtiF PMG
-
double programming of the deterministic version of the model to ensure that the model is not subject to programming errors
-
checking the model parameter values against their original data sources
-
checking the integrity of the distributions sampled in the PSA
-
using expert clinical input to determine alternative plausible assumptions
-
comparing the observed and model-predicted state sojourn times for the age-specific registry data sets (Figures 20–24)
-
comparing the model-predicted survival against previously published estimates (Figure 25)8
-
comparing the model-predicted health state occupancy estimates by age against recent estimates from the CF registry65 (Figure 26).
FIGURE 20.
msm prevalence plots, aged 30–34 years: (a) state 1; (b) state 2; (c) state 3; (d) state 4; and (e) state 5.
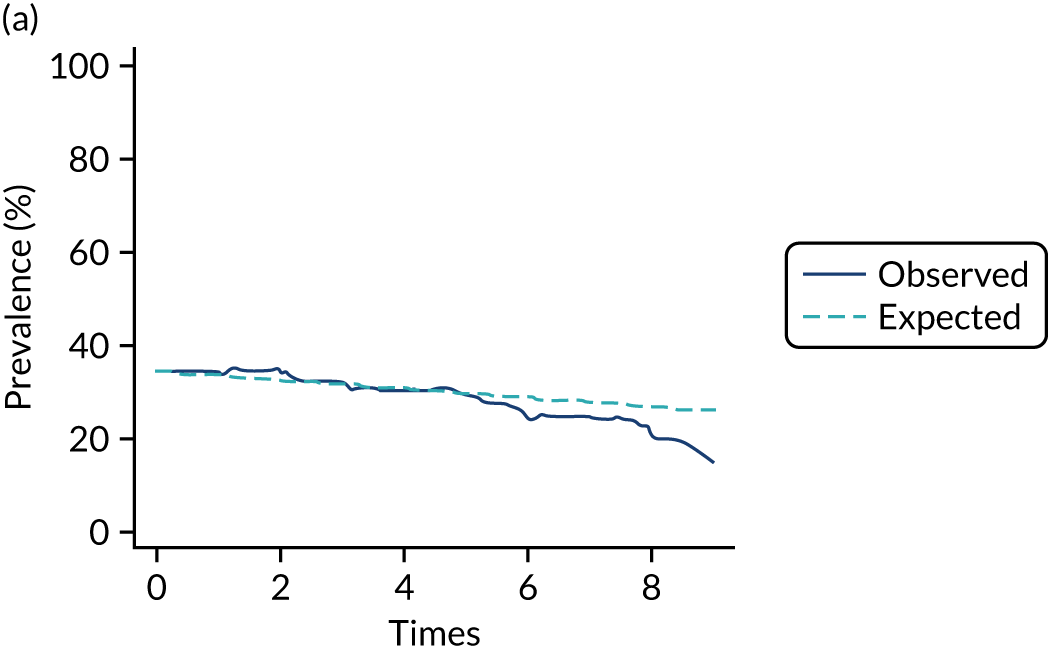
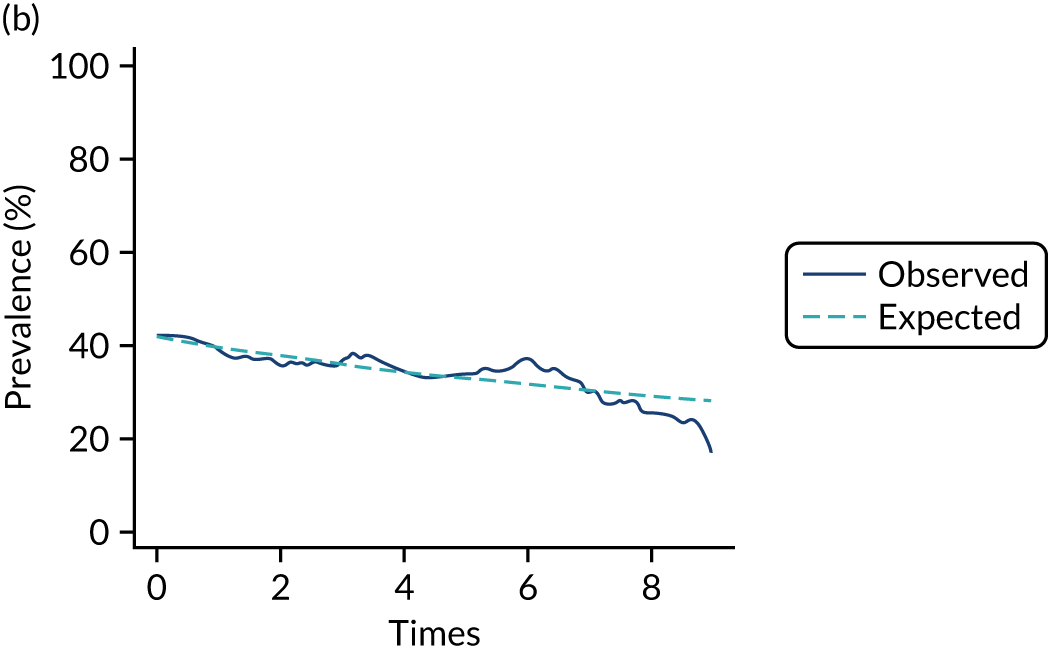
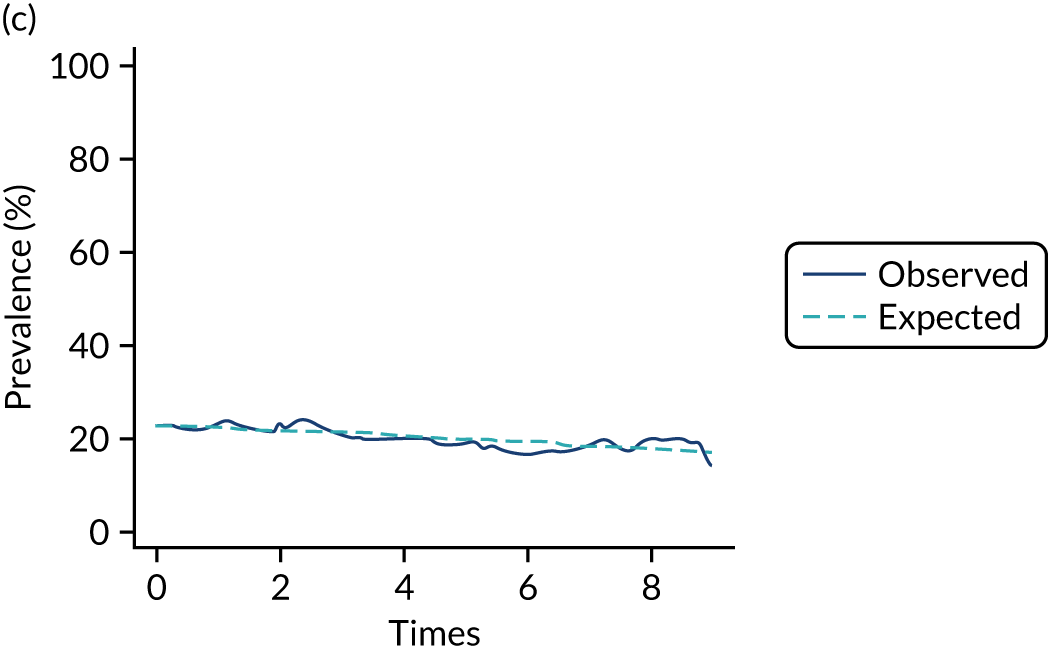
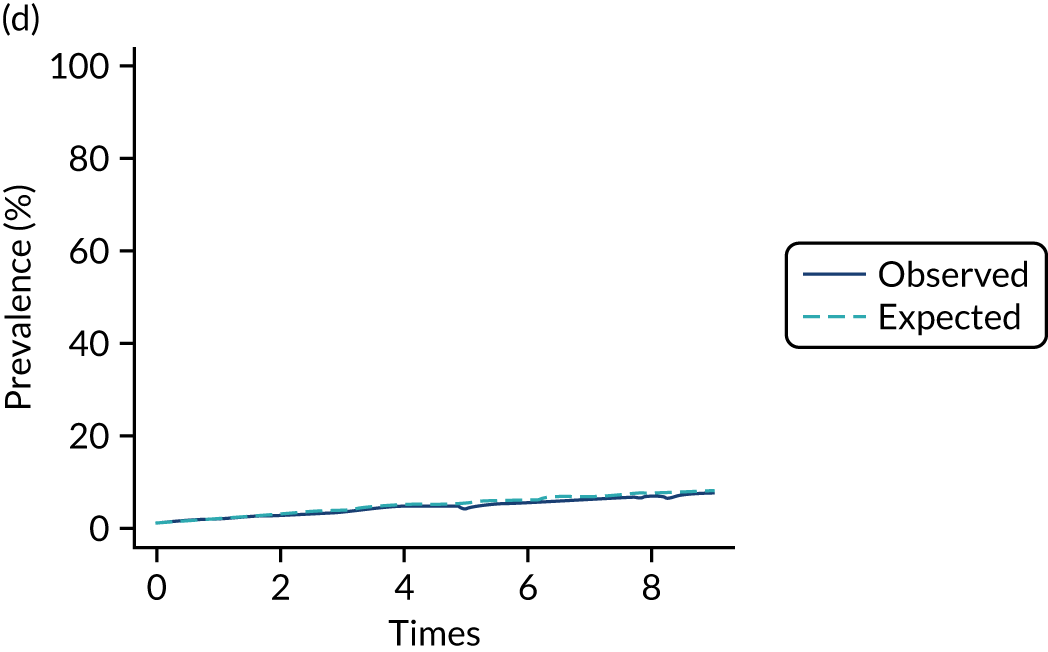
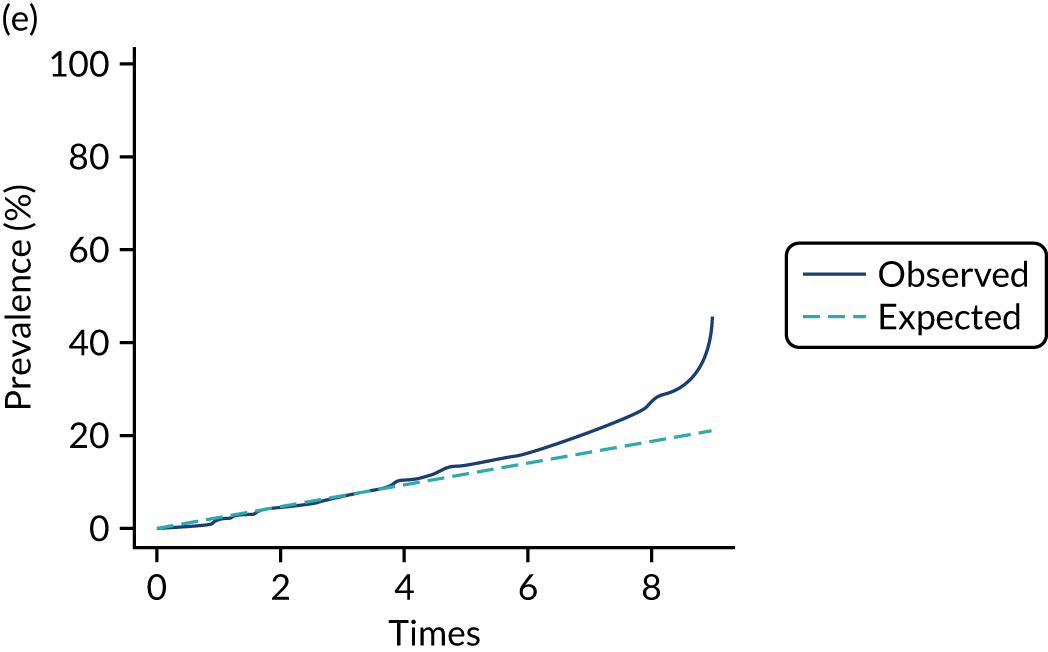
FIGURE 21.
msm prevalence plots, aged 35–39 years: (a) state 1; (b) state 2; (c) state 3; (d) state 4; and (e) state 5.
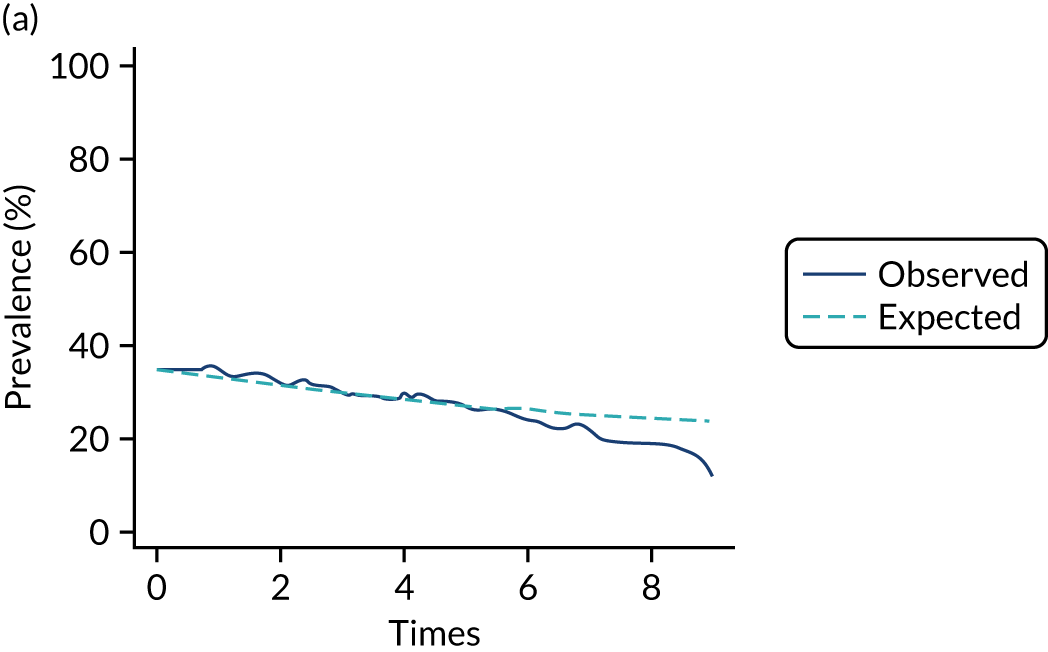
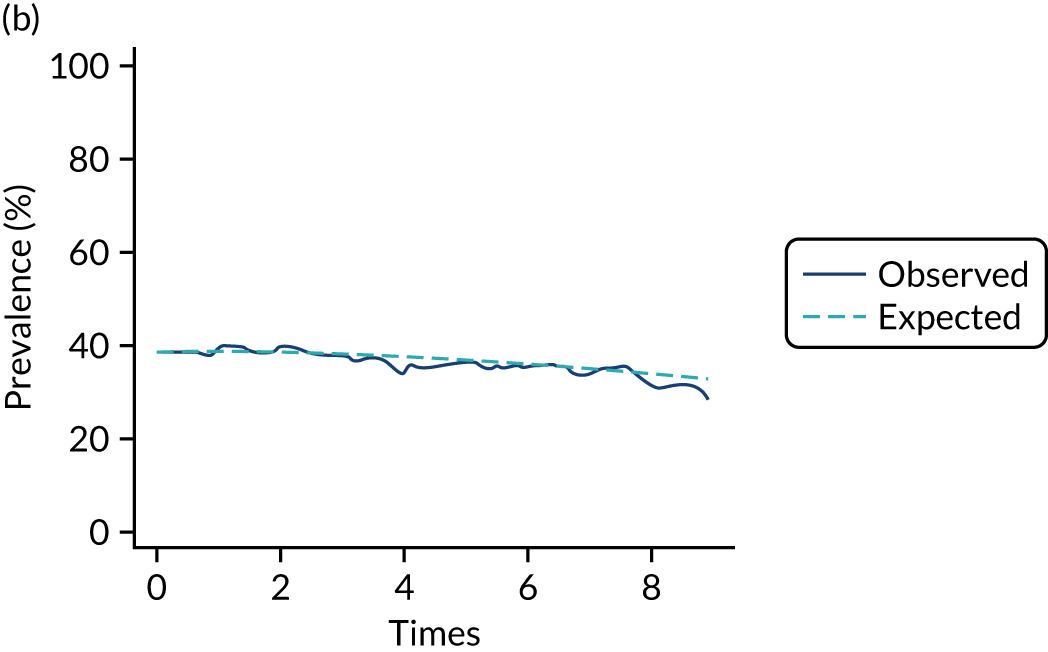
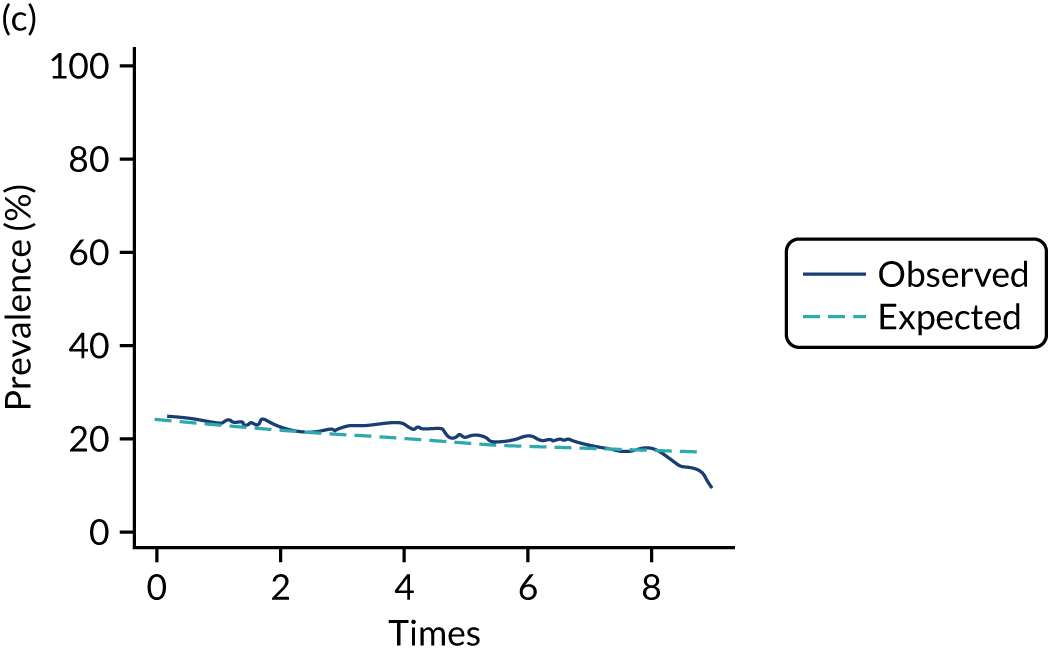
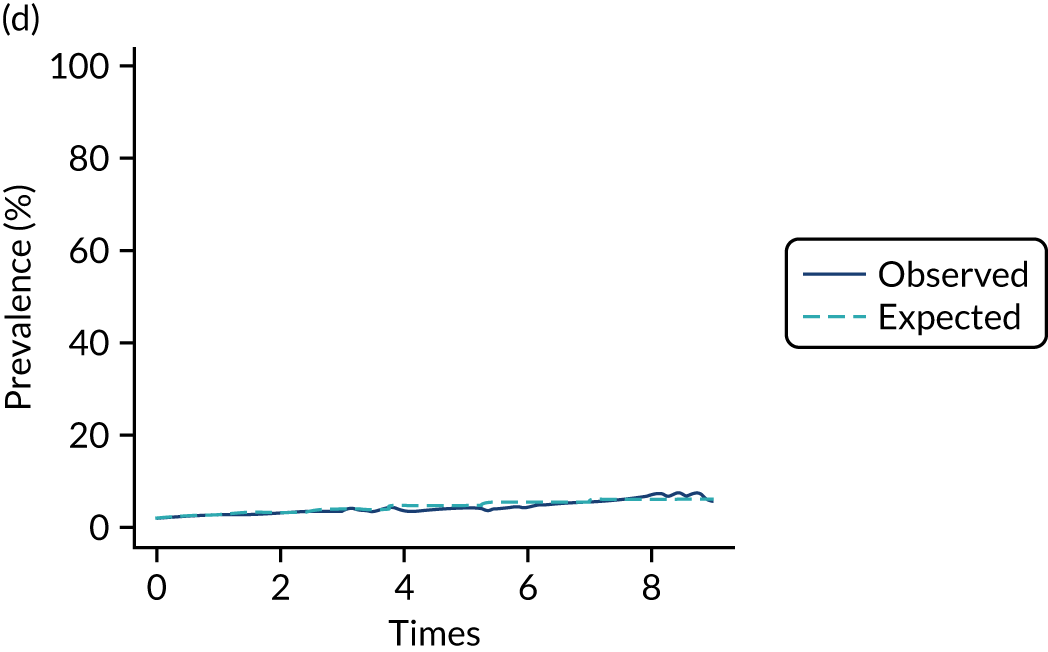
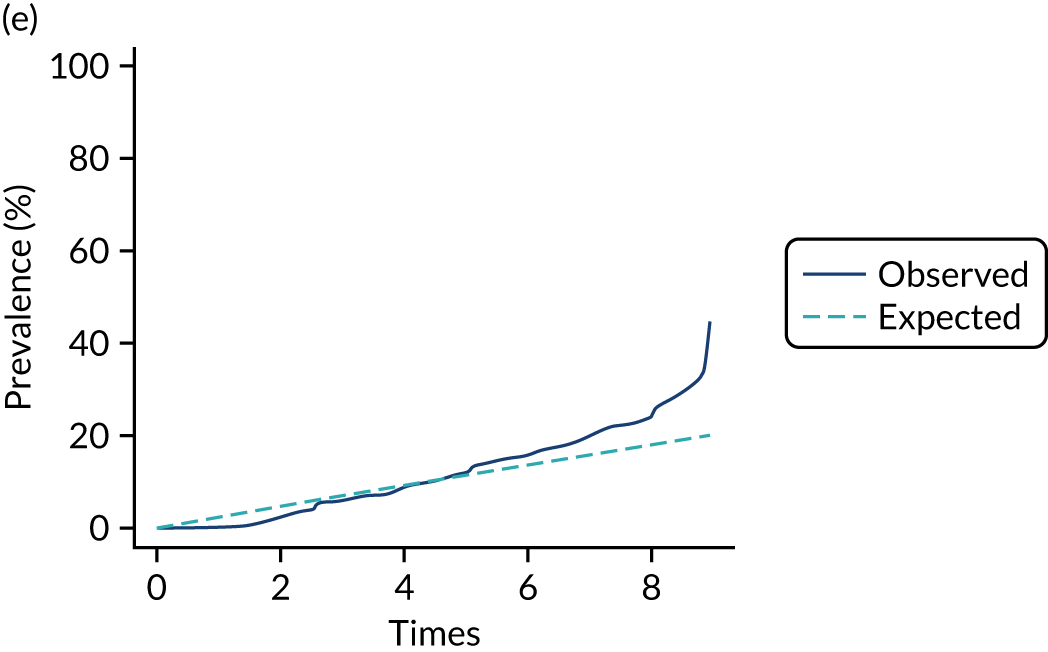
FIGURE 22.
msm prevalence plots, aged 40–44 years: (a) state 1; (b) state 2; (c) state 3; (d) state 4; and (e) state 5.
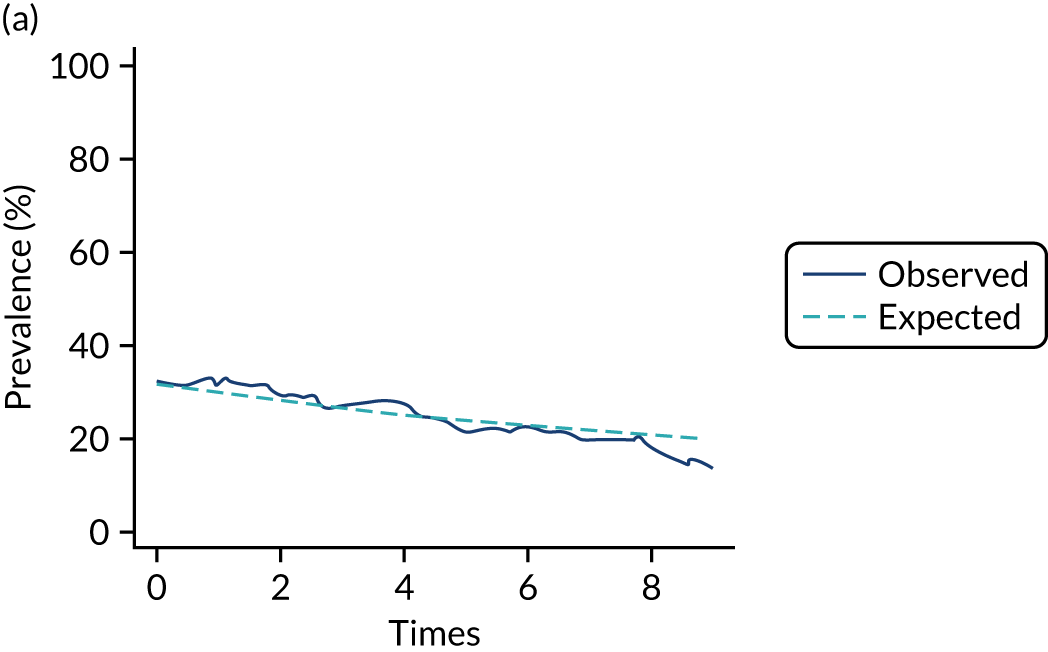
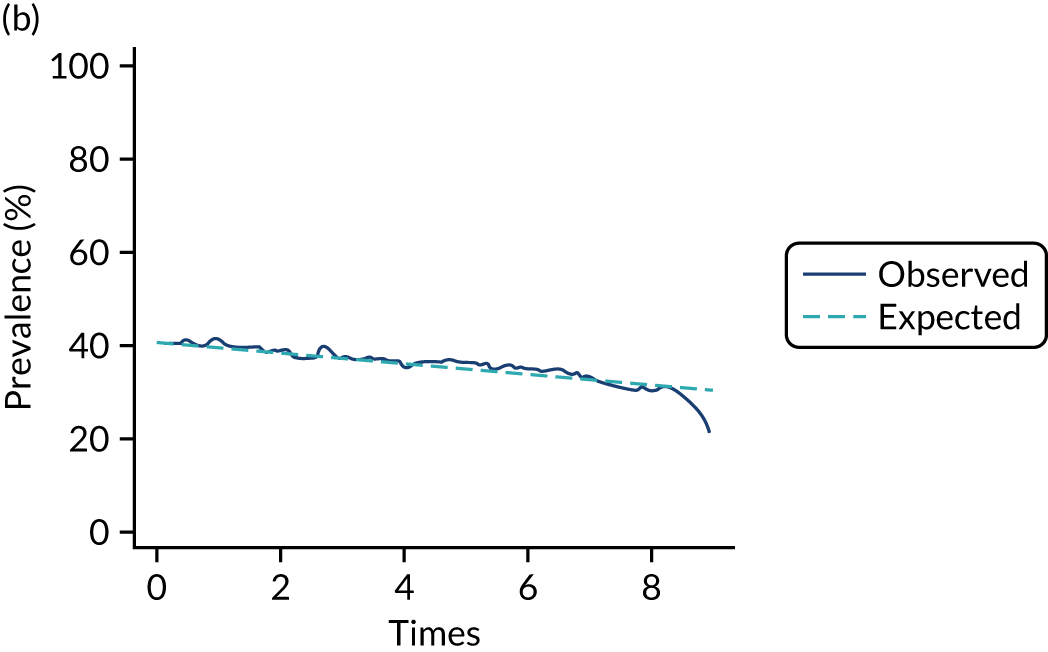
FIGURE 23.
msm prevalence plots, aged 45–49 years: (a) state 1; (b) state 2; (c) state 3; (d) state 4; and (e) state 5.
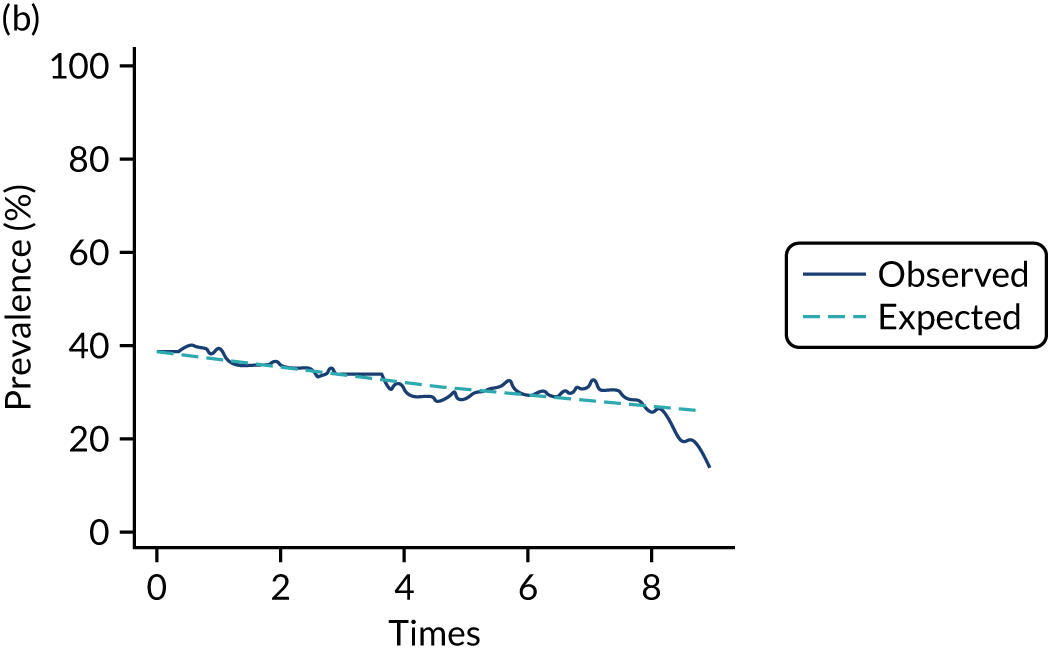
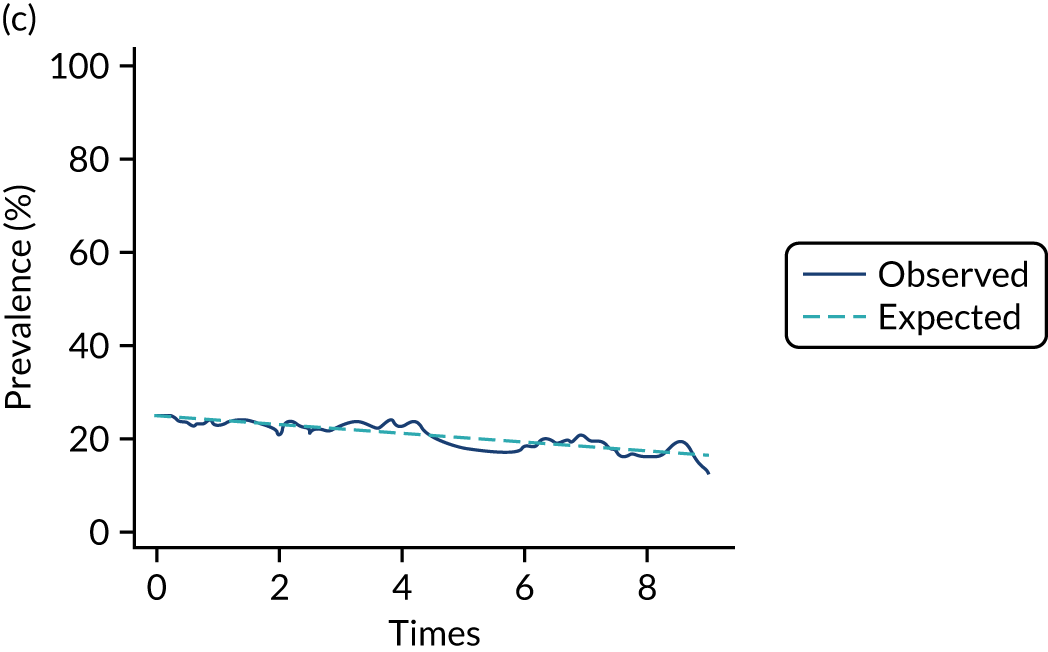
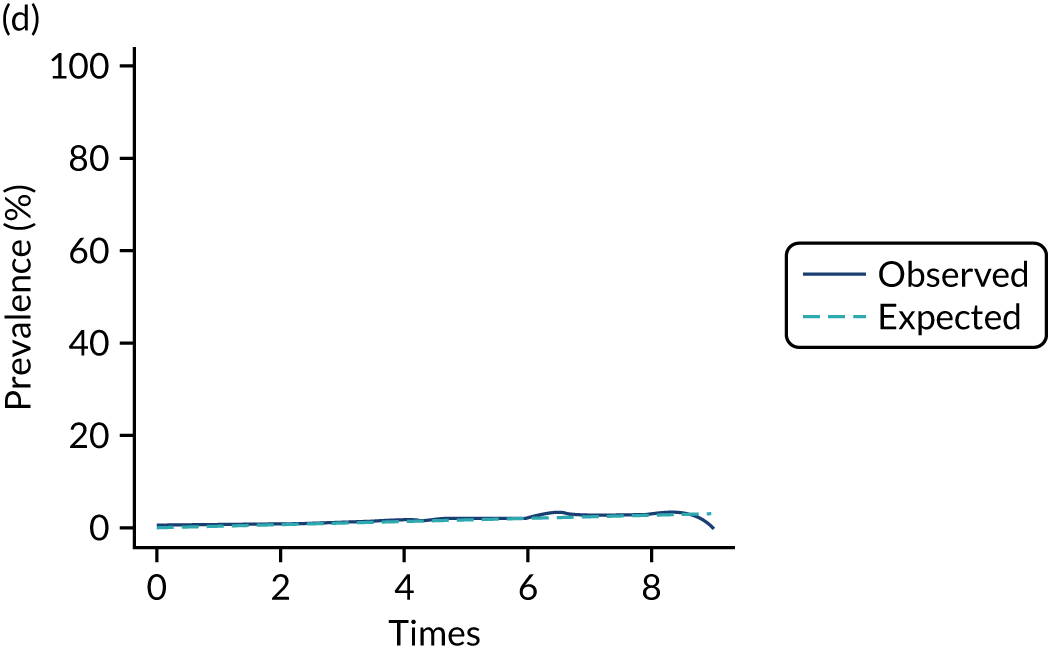
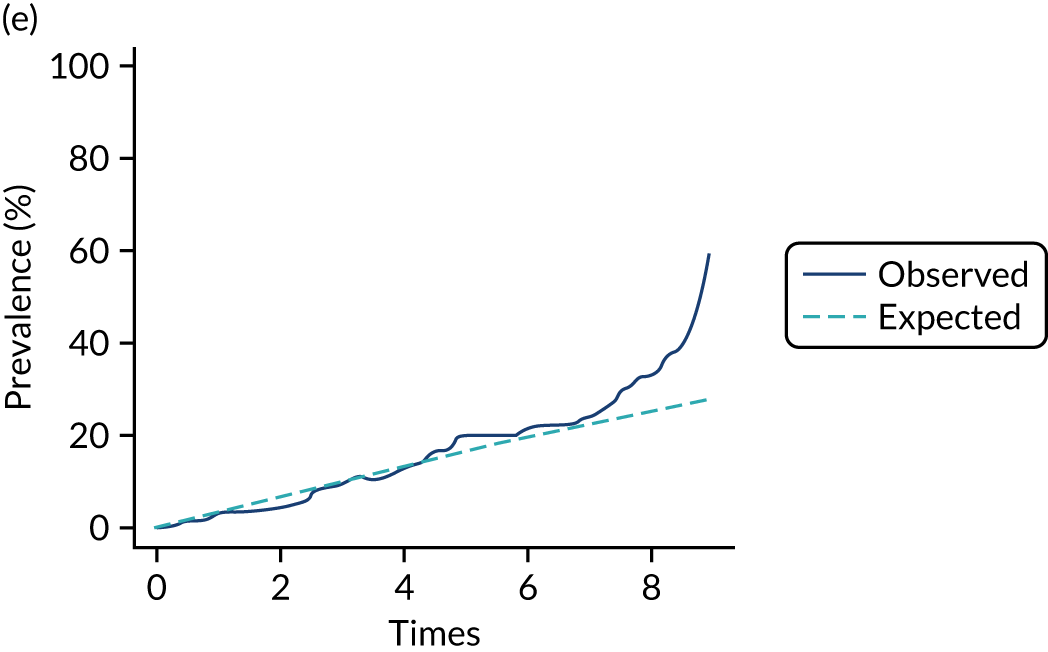
FIGURE 24.
msm prevalence plots, aged ≥ 50 years: (a) state 1; (b) state 2; (c) state 3; (d) state 4; and (e) state 5.
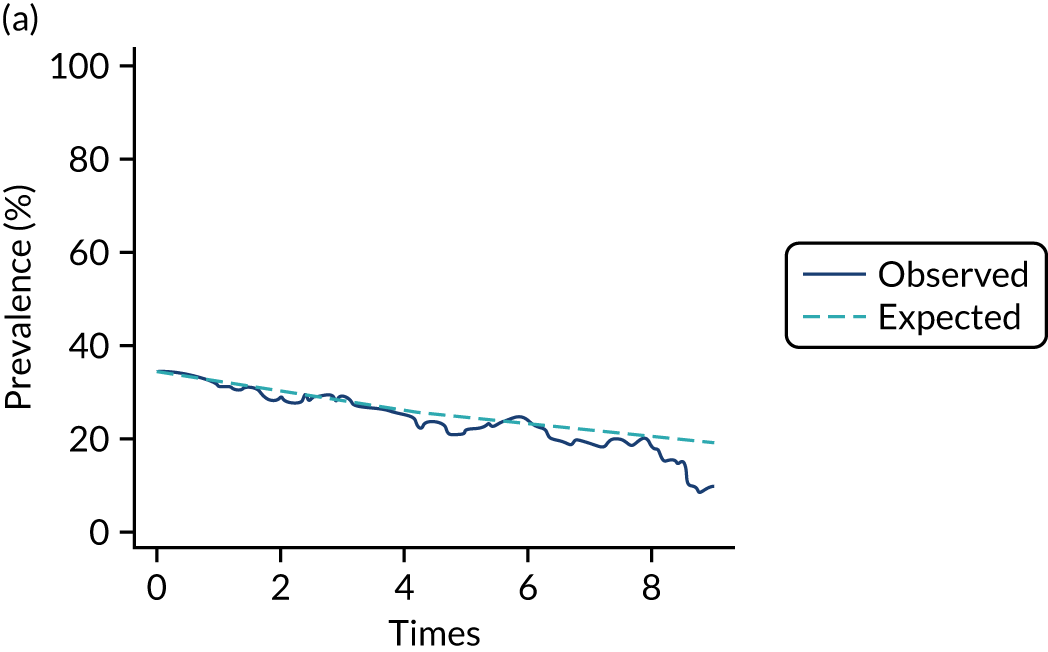
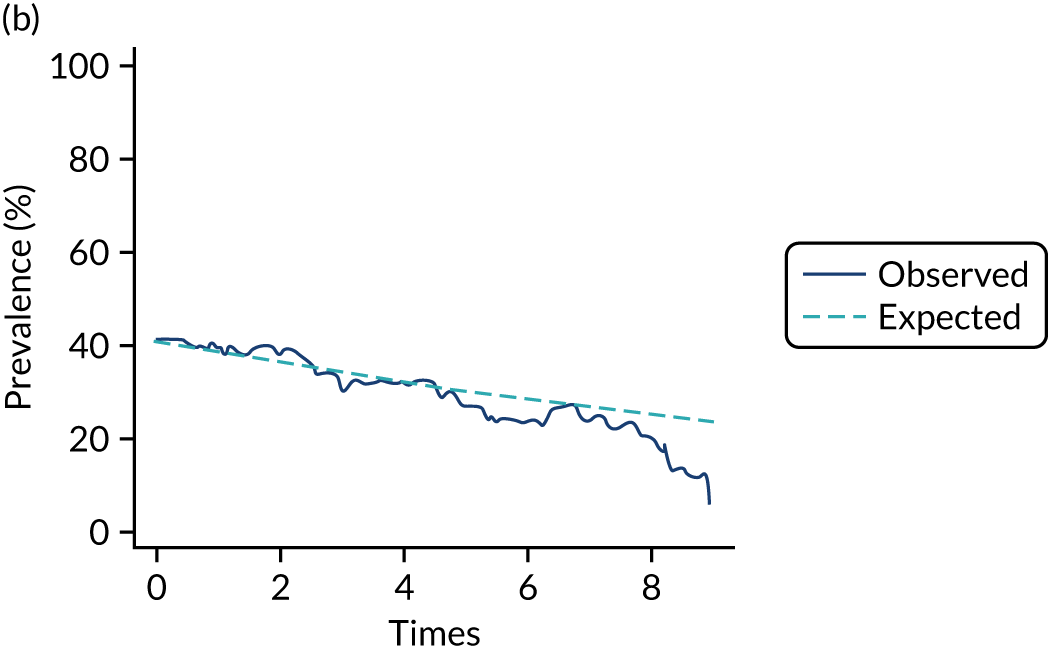
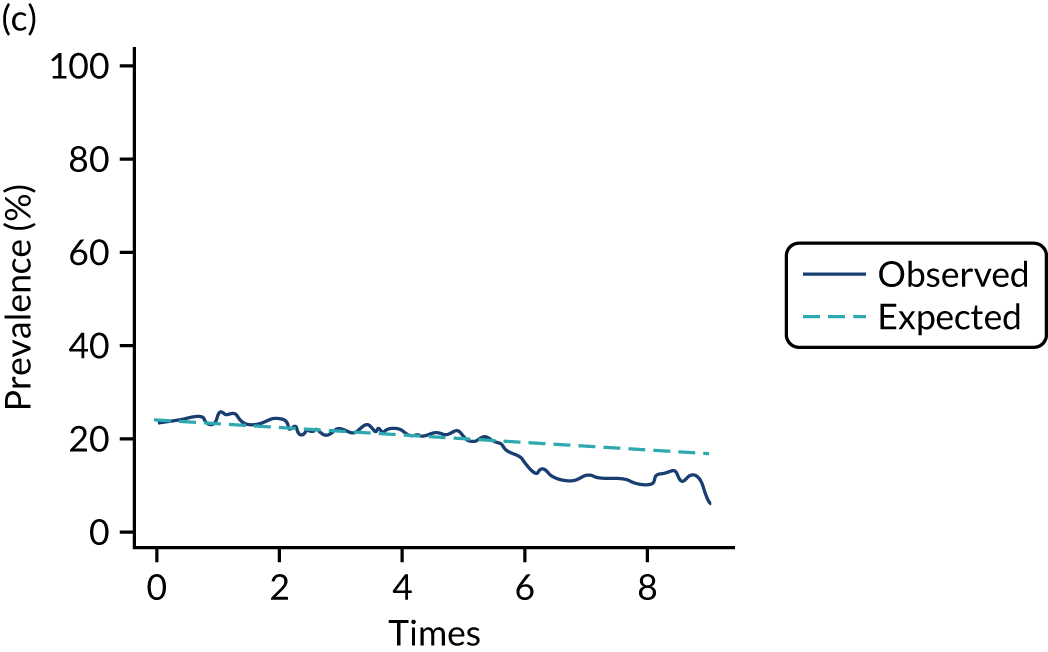
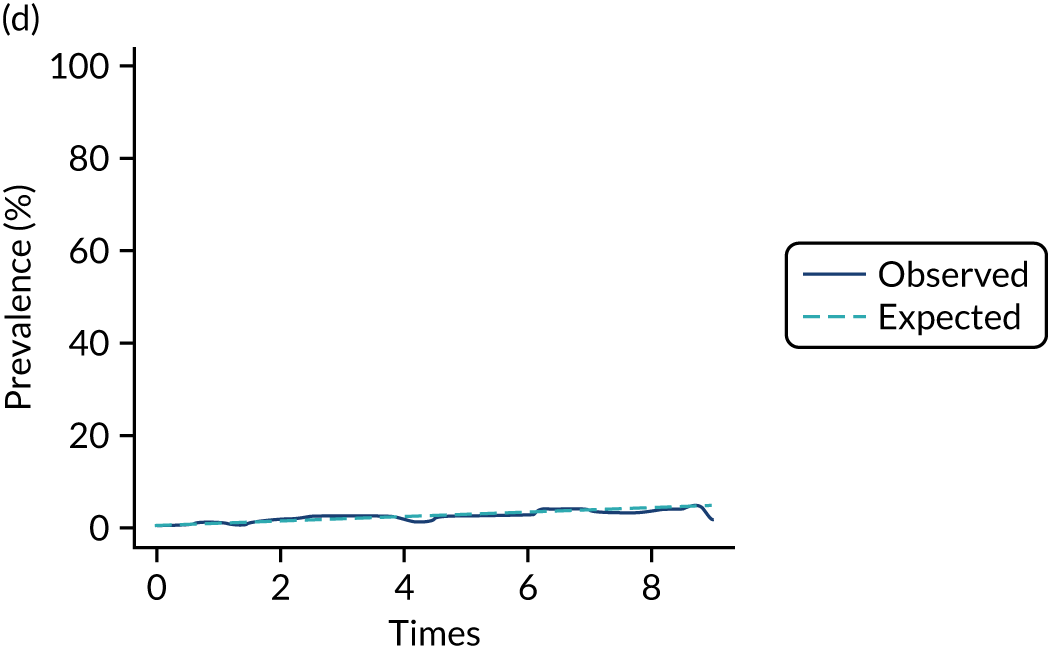
FIGURE 25.
Modelled overall survival versus Keogh et al. 64 spline model (weighted by prevalence of covariate groupings).
FIGURE 26.
The FEV1% predicted by age category: model predicted vs. 2015 CF registry estimates.
Appendix 17 Work package 3.5: model-based analysis sensitivity analysis results
FIGURE 27.
Cost-effectiveness plane: ACtiF adherence intervention vs. usual care, 10-year treatment effect duration.
FIGURE 28.
Cost-effectiveness acceptability curves: ACtiF adherence intervention vs. usual care, 10-year treatment effect duration.
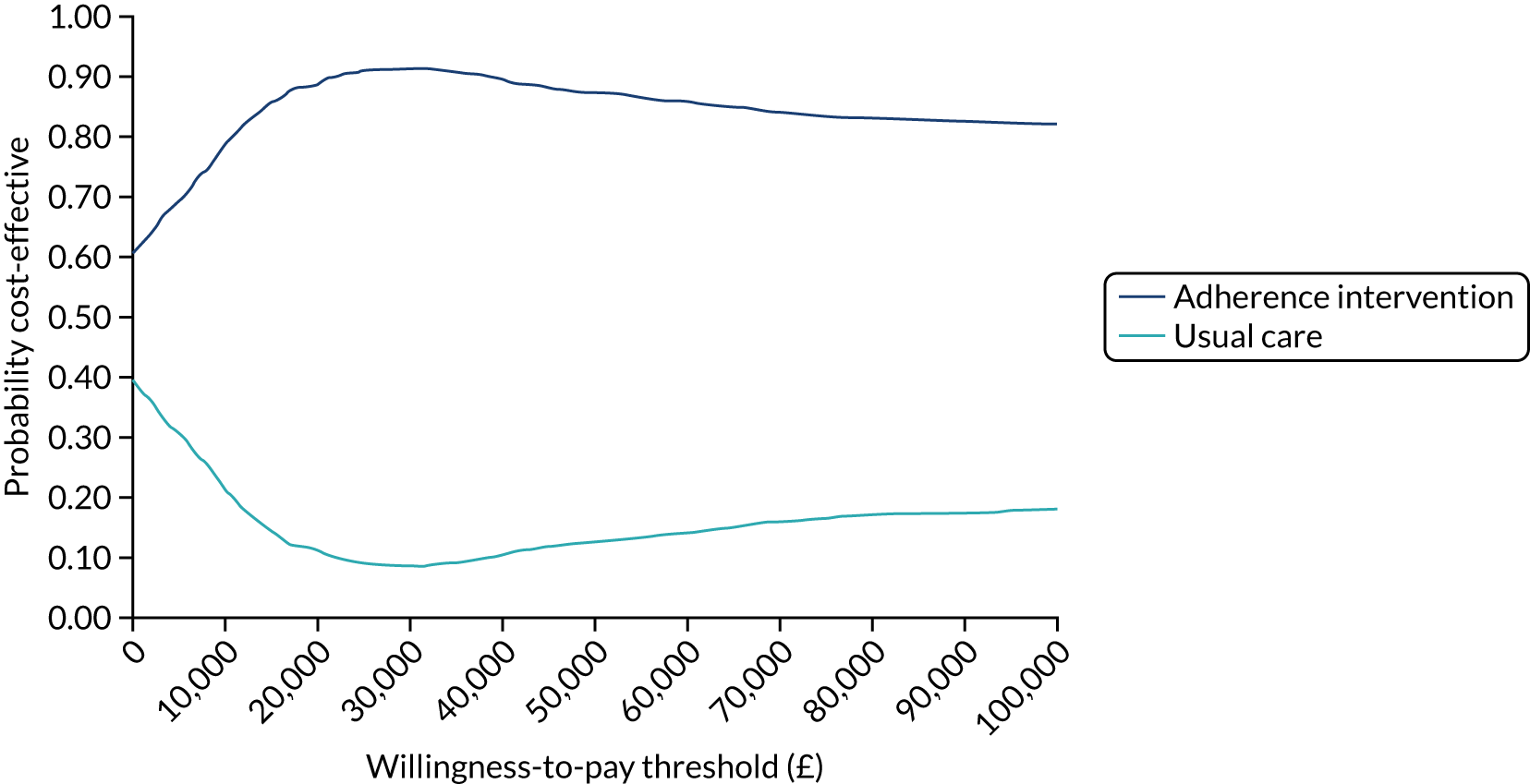
FIGURE 29.
Incremental net montary benefit by treatment effect duration: ACtiF adherence intervention vs. usual care (assuming a WTP threshold of £20,000 per QALY gained).
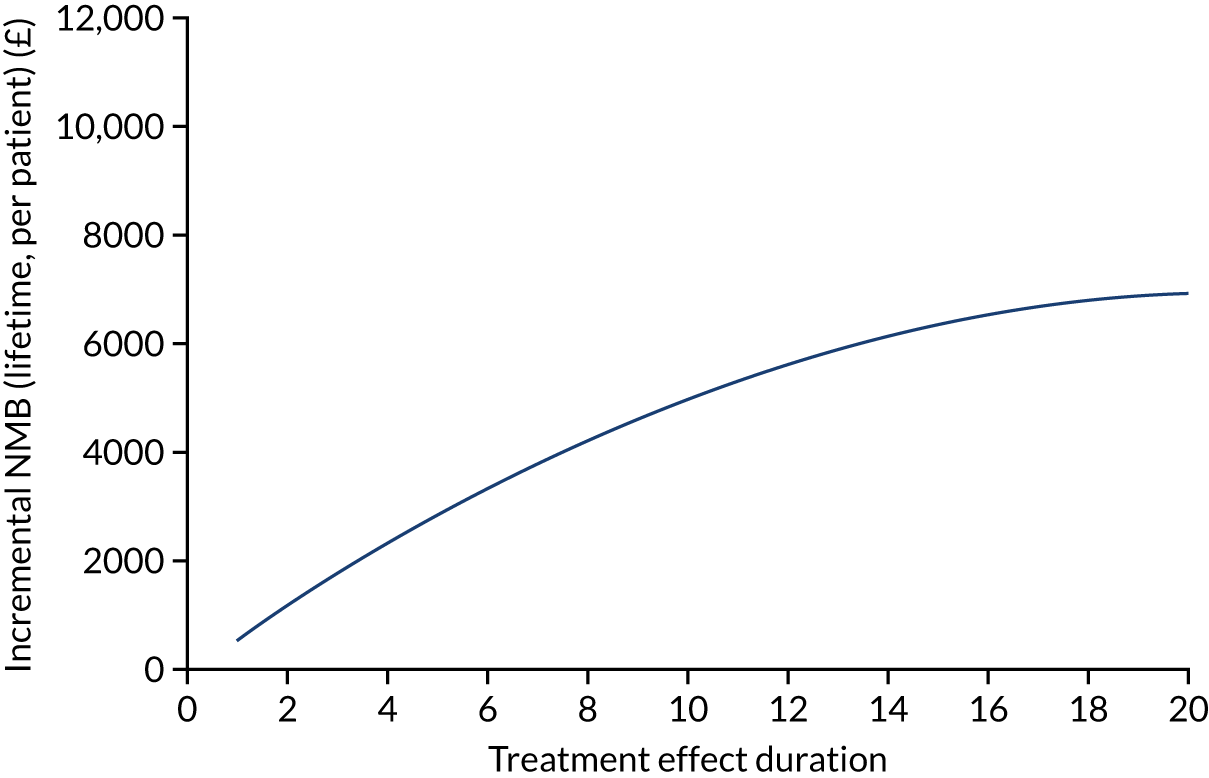
| Scenario | Incremental LYGa | Incremental QALYs | Incremental costs (£) | ICER | Incremental NMB (£)b |
|---|---|---|---|---|---|
| 5-year treatment duration | |||||
| 1. Discounted results | 0.20 | 0.09 | –1065 | Dominating | 2854 |
| 2. Undiscounted results | 0.20 | 0.18 | 815 | 4610 | 2721 |
| 3. Increase in adherence increases nebulised drug costs | 0.20 | 0.09 | 3198 | 35,749 | –1409 |
| 4. Nebulised drugs costs halved | 0.20 | 0.09 | –1660 | Dominating | 3449 |
| 5. Increase in adherence increases nebulised drug costs plus nebulised drugs costs halved | 0.20 | 0.09 | 472 | 5273 | 1317 |
| 6. No treatment effect on transition probabilities | 0.00 | 0.00 | 119 | 46,927 | –68 |
| 7. No treatment effect on i.v. therapy-days | 0.20 | 0.09 | 1207 | 13,868 | 534 |
| 8. i.v. therapy-days treatment effect doubled | 0.20 | 0.09 | –3338 | Dominating | 5175 |
| 9. Utilities based on Bradley et al.109 | 0.20 | 0.11 | –1065 | Dominating | 3283 |
| 10. i.v. disutility halved | 0.20 | 0.09 | –1065 | Dominating | 2804 |
| 11. i.v. disutility doubled | 0.20 | 0.09 | –1065 | Dominating | 2956 |
| 12. Health state resource costs halved | 0.20 | 0.09 | –1094 | Dominating | 2883 |
| 13. Health state resource costs doubled | 0.20 | 0.09 | –1009 | Dominating | 2798 |
| 14. Intervention cost 25% higher | 0.20 | 0.09 | –497 | Dominating | 2286 |
| 15. Intervention cost 25% lower | 0.20 | 0.09 | –1634 | Dominating | 3423 |
| 16. Transplant costs halved | 0.20 | 0.09 | –1033 | Dominating | 2822 |
| 17. Transplant costs doubled | 0.20 | 0.09 | –1130 | Dominating | 2919 |
| 18. Remove survival constraints | 0.24 | 0.09 | –892 | Dominating | 2782 |
| 10-year treatment effect duration | |||||
| 1. Discounted results | 0.40 | 0.17 | –1637 | Dominating | 4991 |
| 2. Undiscounted results | 0.40 | 0.35 | 2026 | 5729 | 5047 |
| 3. Increase in adherence increases nebulised drug costs | 0.40 | 0.17 | 5477 | 32,657 | –2123 |
| 4. Nebulised drugs costs halved | 0.40 | 0.17 | –2787 | Dominating | 6141 |
| 5. Increase in adherence increases nebulised drug costs plus nebulised drugs costs halved | 0.40 | 0.17 | 770 | 4592 | 2584 |
| 6. No treatment effect on transition probabilities | 0.00 | 0.00 | 93 | 21,671 | –7 |
| 7. No treatment effect on i.v. therapy-days | 0.40 | 0.16 | 2121 | 12,957 | 1153 |
| 8. i.v. therapy-days treatment effect doubled | 0.40 | 0.17 | –5394 | Dominating | 8829 |
| 9. Utilities based on Bradley et al.109 | 0.40 | 0.21 | –1637 | Dominating | 5772 |
| 10. i.v. disutility halved | 0.40 | 0.16 | –1637 | Dominating | 4904 |
| 11. i.v. disutility doubled | 0.40 | 0.18 | –1637 | Dominating | 5164 |
| 12. Health state resource costs halved | 0.40 | 0.17 | –1712 | Dominating | 5066 |
| 13. Health state resource costs doubled | 0.40 | 0.17 | –1487 | Dominating | 4841 |
| 14. Intervention cost 25% higher | 0.40 | 0.17 | –697 | Dominating | 4051 |
| 15. Intervention cost 25% lower | 0.40 | 0.17 | –2576 | Dominating | 5930 |
| 16. Transplant costs halved | 0.40 | 0.17 | –1578 | Dominating | 4932 |
| 17. Transplant costs doubled | 0.40 | 0.17 | –1753 | Dominating | 5107 |
| 18. Remove survival constraints | 0.50 | 0.18 | –1218 | Dominating | 4813 |
| 20-year treatment effect duration | |||||
| 1. Discounted results | 0.69 | 0.26 | –1812 | Dominating | 6937 |
| 2. Undiscounted results | 0.69 | 0.59 | 4360 | 7336 | 7526 |
| 3. Increase in adherence increases nebulised drug costs | 0.69 | 0.26 | 8841 | 34,499 | –3716 |
| 4. Nebulised drugs costs halved | 0.69 | 0.26 | –3624 | Dominating | 8750 |
| 5. Increase in adherence increases nebulised drug costs plus nebulised drugs costs halved | 0.69 | 0.26 | 1702 | 6643 | 3423 |
| 6. No treatment effect on transition probabilities | 0.00 | 0.01 | –7 | Dominating | 137 |
| 7. No treatment effect on i.v. therapy-days | 0.69 | 0.25 | 3751 | 14,985 | 1255 |
| 8. i.v. therapy-days treatment effect doubled | 0.69 | 0.26 | –7374 | Dominating | 12,619 |
| 9. Utilities based on Bradley et al.109 | 0.69 | 0.31 | –1812 | Dominating | 8065 |
| 10. i.v. disutility halved | 0.69 | 0.25 | –1812 | Dominating | 6812 |
| 11. i.v. disutility doubled | 0.69 | 0.27 | –1812 | Dominating | 7188 |
| 12. Health state resource costs halved | 0.69 | 0.26 | –1952 | Dominating | 7077 |
| 13. Health state resource costs doubled | 0.69 | 0.26 | –1532 | Dominating | 6657 |
| 14. Intervention cost 25% higher | 0.69 | 0.26 | –415 | Dominating | 5540 |
| 15. Intervention cost 25% lower | 0.69 | 0.26 | –3209 | Dominating | 8334 |
| 16. Transplant costs halved | 0.69 | 0.26 | –1727 | Dominating | 6853 |
| 17. Transplant costs doubled | 0.69 | 0.26 | –1981 | Dominating | 7107 |
| 18. Remove survival constraints | 0.93 | 0.29 | –806 | Dominating | 6507 |
List of abbreviations
- ACtiF
- Development and evaluation of an intervention to support Adherence to treatment in adults with Cystic Fibrosis
- AIC
- Akaike information criterion
- ALDVMM
- adjusted limited dependent variable mixture model
- app
- application
- BCI
- behaviour change intervention
- BCW
- behaviour change wheel
- BIC
- Bayesian information criterion
- BMI
- body mass index
- BNF
- British National Formulary
- CACE
- complier average causal effect
- CF
- cystic fibrosis
- CFI
- comparative fit index
- CFQ-R
- Cystic Fibrosis Questionnaire-Revised
- CHAOS-6
- Confusion, Hubbub and Order Scale-6 item
- CI
- confidence interval
- COM-B
- capability opportunity motivation – behaviour
- COM-BMQ
- capability opportunity motivation – behaviour beliefs about medicines questionnaire
- CQUIN
- Commissioning for Quality and Innovation
- CTRU
- Clinical Trials Research Unit
- DAG
- directed acyclic graph
- DSA
- deterministic sensitivity analysis
- EEACT
- economic evaluation alongside a clinical trial
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FEV1
- forced expiratory volume in first second
- FEV1%
- forced expiratory volume in first second (per cent)
- GAD-7
- Generalised Anxiety Disorder-7
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient-level data
- IRR
- incidence rate ratio
- i.v.
- intravenous
- IVAB
- intravenous antibiotics
- MAD-3
- Medication Adherence Data-3 item
- MAE
- mean absolute error
- MDT
- multidisciplinary team
- mHealth
- mobile health
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MPR
- medicines possession ratio
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PAM-13
- Patient Activation Measure-13 item
- PBA
- person-based approach
- PHQ-8
- Patient Health Questionnaire-8 item
- PMG
- Project Management Group
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- PWCF
- people with cystic fibrosis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RMSEA
- root mean squared error of approximation
- RR
- risk ratio
- RRR
- relative rate ratio
- SRBAI
- Self-Report Behavioural Automaticity Index
- SD
- standard deviation
- SE
- standard error
- SUR
- seemingly unrelated regression
- T1
- time 1
- T2
- time 2
- TDF
- theoretical domains framework
- TIDieR
- template for intervention description and replication
- TSC
- Trial Steering Committee
- VLE
- virtual learning environment
- WP
- work package
- WTP
- willingness-to-pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/pgfar09110).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
