Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0612-20001. The contractual start date was in July 2014. The final report began editorial review in October 2020 and was accepted for publication in May 2021. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Knowles et al. This work was produced by Knowles et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Knowles et al.
SYNOPSIS
Background
Burden of disease
Constipation is common in adults and children, and up to 20% of the population (2–28% of adults and 0.7–30.0% children) report this symptom depending on the definition used,1–3 with a much higher prevalence in women. 4–6 Chronic constipation (CC), usually defined as > 6 months of symptoms, is less common7 but results in 0.5 million UK general practitioner (GP) consultations per annum. A proportion (1–2%)8 of the population suffer more disabling symptoms, are very frequently female9 and are usually referred to secondary care, with many progressing to tertiary specialist investigation. Patient dissatisfaction levels are high in this group, with ≈ 80% feeling that laxative therapy is unsatisfactory;10 furthermore, the effect of symptoms on quality of life (QoL) is significant. 11 CC consumes significant health-care resources. In the USA in 2004, a primary complaint of constipation was responsible for 8 million physician visits,12 resulting in (direct and indirect) costs of US$1.7B. Although detailed figures are lacking in the UK, it is estimated that ≈ 10% of district nursing time is spent on constipation13 and that the annual spend on laxatives exceeds £100M. 14
Pathophysiological basis of chronic constipation
The act of defaecation is dependent on the co-ordinated functions of the colon, rectum and anus. Considering the complexity of neuromuscular (sensory and motor) functions required to achieve planned, conscious and effective defaecation,15 it is no surprise that disturbances to perceived ‘normal’ function occur commonly at all stages of life. Clinically, such problems commonly lead to de facto symptoms of obstructed defaecation (e.g. straining; incomplete, unsuccessful or painful evacuation; bowel infrequency), but symptoms such as abdominal pain and bloating are also very common. After exclusion of a multitude of secondary causes (e.g. obstructing colonic lesions; neurological, metabolic and endocrine disorders), the pathophysiology of CC can broadly be divided into problems of colonic contractile activity, and thus stool transit, and problems of the pelvic floor. Thus, with specialist physiological testing, patients may be divided into those who have slow colonic transit, evacuation disorder, both or neither (e.g. no abnormality found with current tests). Evacuation disorders can be subdivided into those in which a structurally significant pelvic floor abnormality is evident, such as rectocele or internal prolapse (e.g. intussusception), and those in which there is a dynamic failure of evacuation without structural abnormality, most commonly termed functional defaecation disorder.
Management of chronic constipation
Management of CC is a major problem because of its high prevalence and lack of widespread specialist expertise. In general, a step-wise approach is undertaken, with first-line conservative treatment such as lifestyle advice and laxatives (primary care) followed by nurse-led bowel retraining programmes, sometimes including focused biofeedback and psychosocial support (secondary/tertiary care). Although these treatments may improve symptoms in more than half of patients, they are far from universally successful. Thus, patients with intractable symptoms and impaired QoL may be offered a range of costly, irreversible surgical interventions with unpredictable results, sometimes resulting in major adverse events (AEs) or a permanent stoma.
The research programme
An evidence-based pathway for the management of CC in adults is currently lacking, although National Institute for Health and Care Excellence (NICE) guidance exists for the management of CC in children16–18 and for allied conditions (e.g. faecal incontinence) in adults. This arguably leads to variations in practice, particularly in specialist services. With a number of new drugs gaining NHS approval19–22 and new technologies at a horizon-scanning stage,23–26 it is timely that the currently limited evidence base is developed for resource-constrained NHS providers to have confidence that new and sometimes expensive investigations and therapies are appropriate and cost-effective. A cost-conscious pathway of care may help reduce health-care expenditures by appropriately sequencing the care provided while targeting more expensive therapies at those most likely to benefit from them. Such data could inform the development and commissioning of integrated care pathways. 27
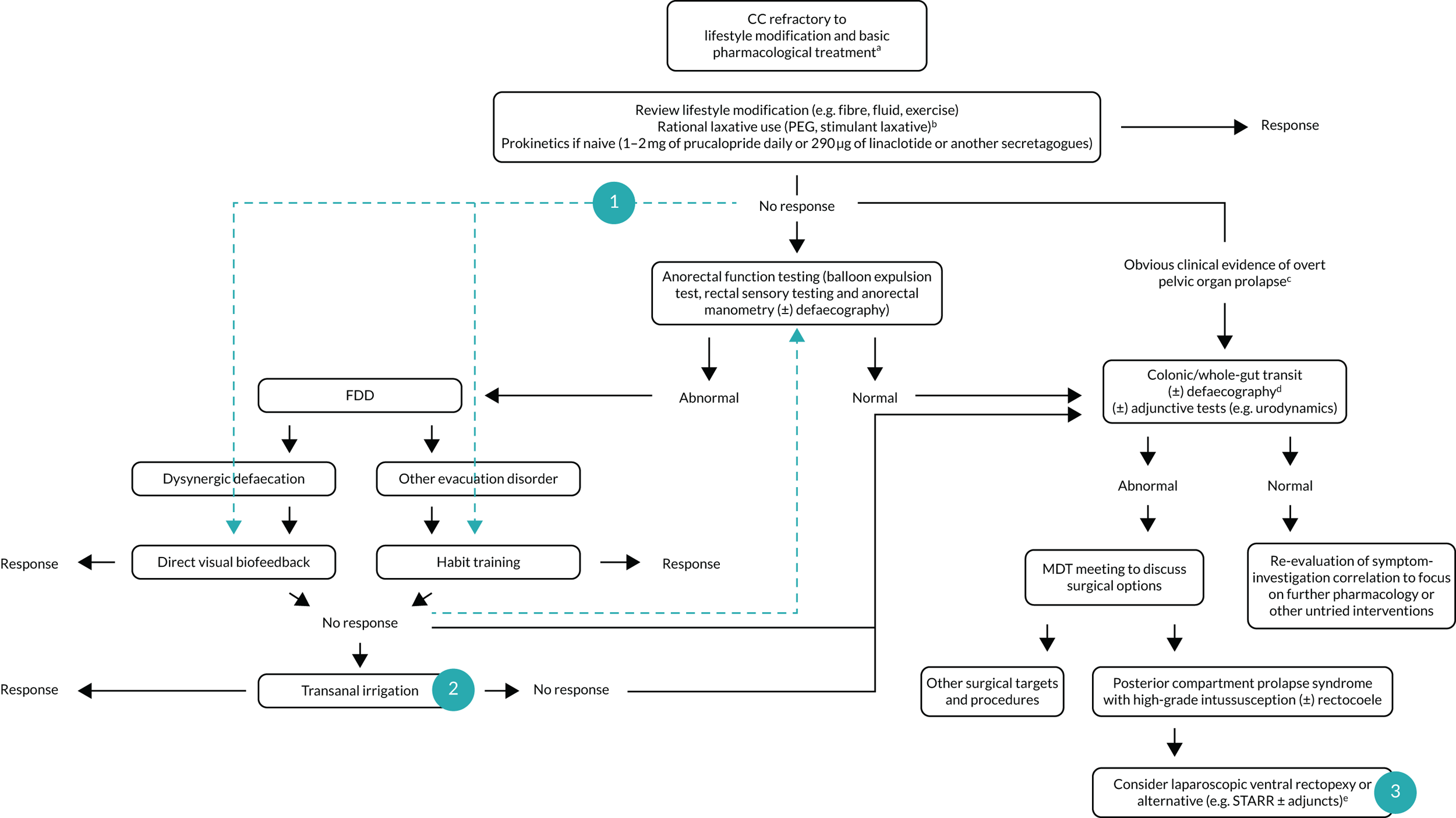
The overall rationale of the Chronic Constipation Treatment Pathway (CapaCiTY) research programme, therefore, was to develop an evidence base for CC management through a series of three randomised controlled trials (RCTs) that answered some of the important questions for sequenced patient care (Figure 1). For each, the focus was on generating real-life evidence based on valid clinical outcome measures, patient acceptability and cost.
FIGURE 1.
Overview of the CapaCiTY research programme. Green arrows indicate studied pathways in CapaCiTY trials 1–3 (numbered circles). a, Alarm features excluded and secondary causes treated appropriately; b, in IBS-C, consider antispasmodics or neuromodulators in case constipation improves but abdominal pain persists and is dominant symptom; c, examples of overt prolapse include anterior (stage 3 cystocele), middle (stage 3 rectocele, uterovaginal) and posterior compartments (grade IV/V intussusception); d, if not performed previously; e, common adjuncts include sacrocolpopexy, hysterectomy, transvaginal tape and cystocele repair. FFD, functional defaecation disorder; IBS-C, constipation-predominant irritable bowel syndrome; MDT, multidisciplinary team; PEG, polyethylene glycol[0]; STARR, stapled rectal resection.
The specific objectives were as follows:
-
work programme (WP)1
-
to develop a common methodological framework for subsequent studies
-
to recruit a UK cohort of adults with CC based on strict eligibility criteria and detail the baseline phenotype to identify disease risk factors, symptom burden, QoL and psychological morbidity.
-
-
WP2 (CapaCiTY trial 1)
-
to determine whether or not standardised specialist-led habit training plus pelvic floor retraining using computer-assisted direct visual biofeedback (HTBF) is more clinically effective than standardised specialist-led habit training alone (HT)
-
to determine whether or not outcomes of such specialist-led interventions are improved by stratification to HTBF or HT based on prior knowledge of anorectal and colonic pathophysiology using standardised radiophysiological investigations (INVEST).
-
-
WP3 (CapaCiTY trial 2)
-
to compare the impact of transanal irrigation (TAI) initiated with a low-volume and a high-volume system on patient disease-specific QoL after 3 months of treatment.
-
-
WP4 (CapaCiTY trial 3)
-
to systematically review the evidence for all common surgical procedures used for adults with CC
-
to determine the clinical efficacy of laparoscopic ventral mesh rectopexy (lapVMR) compared with controls at short-term follow-up (24 weeks).
-
-
WP5
-
to synthesise clinical outcome, patient acceptability and cost data from CapaCiTY trials and to develop an NHS pathway for the management of CC in adults based on data synthesis.
-
Work programme 1: common methodological framework and participant recruitment
Common methodological framework
To permit the synthesis of all data at the end of the programme, we defined eligibility criteria, outcome measures and analytic methods that were common across all three trials. In addition, at the outset it was envisaged that some participants could move sequentially through more than one trial if a prior treatment had failed (although in practice this happened very infrequently because of delays in recruitment to all studies).
Setting
Following scoping during the programme development phase, we pre-identified 10 UK specialist centres that geographically encompass the north and south of England with a mix of urban and rural referral bases. Other centres were recruited for specific studies, especially in CapaCiTY trial 3.
Target population
The programme addressed the NHS management of CC in secondary and tertiary care, rather than the broader patient population with relatively short-lived or mild symptoms, receiving self-care or primary care management. This focus was pragmatic, given the concentration of expertise, diagnostics and biofeedback equipment in hospital settings. Based on well-established epidemiological data28,29 (see SYNOPSIS, Background), the main target population was women of mean age 50 years.
Ethics approvals
The three trials had the following registration details and approvals:
-
CapaCiTY trial 1
-
Research Ethics Committee (REC) reference 14/LO/1786
-
Integrated Research Application System (IRAS) 160709
-
ISRCTN11791740
-
date of REC approval: 6 December 2014.
-
-
CapaCiTY trial 2
-
REC reference 15/LO/0732
-
IRAS 172401
-
ISRCTN11093872
-
date of REC approval: 6 July 2015.
-
-
CapaCiTY trial 3
-
REC reference 15/LO/0609
-
IRAS 171006
-
ISRCTN11747152
-
date of REC approval: 6 July 2015.
-
Eligibility assessments
Patients were recruited at the time of clinical consultation at physician- and nurse-led clinics, and also at the time of investigation in gastrointestinal physiology units. All patients expressing initial interest were referred to the local lead investigator to screen case notes for eligibility.
Good clinical practice-trained local investigators determined eligibility by interview on the basis of defined inclusion/exclusion criteria. Participants were recorded on a screening log and each allocated a unique participant identifier (ID) number. Eligible subjects were then provided with an adequate explanation of the aims, methods, anticipated benefits and risks of the programme, and a participant information sheet. Special emphasis was placed on the long-term nature of the programme, time commitments and number of assessments required. Patients were telephoned 1 week later (or given an appointment) to allow appropriate time for them to consider their participation.
Inclusion and exclusion criteria
Inclusion criteria
Chronic constipation was defined according to pragmatic criteria, broadly, those employed for recent pivotal trials of prokinetics19,22 and US guidance:30
-
age 18–70 years
-
patient self-reported problematic constipation
-
symptom onset > 6 months prior to recruitment
-
symptoms meeting American College of Gastroenterology definition of constipation (‘unsatisfactory defaecation characterized by infrequent stool, difficult stool passage or both for at least previous 3 months’)30
-
constipation that has failed treatment to a minimum basic standard according to the NHS Map of Medicine31 (lifestyle and dietary measures and two or more laxatives or prokinetics tried)
-
ability to understand written and spoken English (for questionnaire validity).
Exclusion criteria
Exclusions included major causes of secondary constipation and specific factors precluding participation in study interventions:
-
significant organic colonic disease (red flag symptoms e.g. rectal bleeding previously investigated); inflammatory bowel disease; megacolon or megarectum (if diagnosed beforehand); or severe diverticulosis, bowel stricture or birth defects deemed to contribute to symptoms (incidental diverticulosis not an exclusion criterion)
-
major colorectal resection surgery
-
current overt pelvic organ prolapse (e.g. bladder, uterus, rectum) or disease requiring obvious surgical intervention
-
previous pelvic floor surgery to address defaecatory problems [posterior vaginal repair, stapled rectal resection (STARR) and rectopexy] or previous sacral nerve stimulation
-
rectal impaction (as defined by digital and abdominal examination, which form part of the NHS Map of Medicine basic standard)31
-
significant neurological disease deemed to be causative of constipation (e.g. Parkinson’s disease), spinal injury, multiple sclerosis or diabetic neuropathy (uncomplicated diabetes alone not an exclusion criterion)
-
significant connective tissue disease – scleroderma, systemic sclerosis and systemic lupus erythematosus (hypermobility alone not an exclusion criterion)
-
significant medical comorbidities and activity of daily living impairment (based on a Barthel index32 score of > 11 in apparently frail patients)
-
major active psychiatric diagnosis (e.g. schizophrenia, major depressive illness and mania)
-
chronic regular opioid use (at least once-daily use) where this is deemed to be the cause of constipation based on temporal association of symptoms with onset of therapy, and all regular strong opioid use
-
pregnancy or intention to become pregnant during study period
-
previous experience of specific therapies included in the programme.
Trial-specific inclusion criteria
-
CapaCiTY trial 1:
-
vision sufficient to undertake visual biofeedback.
-
-
CapaCiTY trial 2:
-
sufficient manual dexterity of patient/carer to use TAI.
-
-
CapaCiTY trial 3:
-
failure of non-surgical interventions (minimum of nurse-led behavioural therapy)
-
internal rectal prolapse, as determined by clinical examination and INVEST, fulfilling two diagnostic criteria – (1) intra-anal or intrarectal intussusception with or without other dynamic pelvic floor abnormalities (e.g. rectocele, enterocele, perineal descent) and (2) deemed (by expert review) to be obstructing on defaecating proctogram (i.e. trapping contrast and/or associated with protracted or incomplete contrast evacuation using normal ranges). 33
-
Baseline clinical evaluation
In addition to screening questions, clinical examination and information obtained by baseline standardised outcome assessments, participants completed a structured interview to document other comorbidities and risk factors (e.g. metabolic, endocrine and neurological disease; obstetric and gynaecological history; joint hypermobility; past surgical history). Clinical examination of the perineum/anus/rectum/vagina was carried forward to baseline from the last clinical consultation to prevent unnecessary repetition of intimate examinations.
Specialist radiophysiological investigations
All three trials required, at least in part (depending on the trial), the results of a number of well-established specialist investigations of anorectal and colonic functions. These investigations were nationally standardised during National Institute for Health Research (NIHR) programme development work, including a consensus meeting (London 2013) highlighting universally discordant current practice; remarkably, no UK centre at that time had the same protocol for any of the four main tests:34
-
Anorectal manometry using high-resolution methods35–37 to determine defined abnormalities of rectoanal pressure gradient during simulated evacuation. 38–40
-
Balloon sensory testing using standardised methods41,42 (2 ml of air per second to a maximum of 360 ml) to determine volume inflated to first constant sensation, defaecatory desire and maximum tolerated volumes. Rectal hyposensation and hypersensation defined in accordance with sex-specific normative data on 91 healthy adults. 43 The rectoanal inhibitory reflex elicited by 50 ml rapid inflation (if necessary, in 50-ml aliquots up to 150 ml).
-
Fixed-volume (50 ml) water-filled rectal balloon expulsion test38,39,44,45 in the seated position on a commode. Abnormal expulsion is defined as failure to expel within 1.0 minute of effort for men and 1.5 minutes of effort for women. 46
-
Whole-gut transit study using serial (different shaped) radio-opaque markers over 3 days, with a single, plain radiograph at 120 hours. 47,48
-
Fluoroscopic evacuation proctography using rectal installation of barium porridge to defaecatory desire threshold (or a maximum of 300 ml) and evacuation on a radiolucent commode33,35–37,49 with pre-opacification of the small bowel (for enterocele). Radiation dose, proportion of contrast evacuated and time taken are recorded, as well as ‘functional’ features (i.e. pelvic floor dyssynergia) and ‘structural’ features (e.g. rectocele, enterocele and intussusception) deemed obstructive to defaecation. 38,43 Although magnetic resonance proctography is now used in some UK centres, with the advantage of no radiation dose, it was not widespread at the time of developing the CapaCiTY programme. There are also differences in sensitivity between fluoroscopic and magnetic resonance proctography, especially if the latter is carried out on a supine patient,50 that could prove difficult for standardisation.
Standardised radiophysiological investigations were performed if results were not already available for participants from tests in the preceding 12 months. In some participants, individual missing investigations were performed. Routine NHS practice (e.g. 10-day NHS rule related to menstrual cycle) was applied in respect of women between menarche and menopause. Participants who could potentially be pregnant had a serum or urine pregnancy test performed as per routine care. Participants were given the results of investigations by the physiologist or radiologist.
Programme outcomes
A common ‘standardised outcome framework’ was used throughout. All questionnaires contained written instructions to be completed by the participant in an undisturbed environment without prompting. Online and postal options were provided for participants who chose not to attend in person.
The plan at the start of the programme was that all outcomes would be recorded for each intervention at baseline, 3 months and 6 months before participants could progress to a further intervention WP. For participants not changing therapy, further outcomes were recorded at 6-month intervals to the end of the programme (to a theoretical maximum of 4 years). Participants who progressed from one intervention to another had a new ‘baseline’ recorded on recruitment. In practice, few participants progressed from one WP to another and follow-up was limited by delays in recruitment to a maximum of about 18 months.
Primary clinical outcome
Patients with CC complain of a multitude of symptoms including infrequent defaecation, pain, bloating, straining, passage of hard stool, incomplete evacuation and systemic symptoms. The relative importance of these varies between patients so that a single symptom, such as bowel frequency, does not adequately describe treatment effect in a population. 51 Symptom diaries have a poor record as primary outcome measures, suffering from incomplete data, retrospective completion, tolerance or sensitisation. 52,53 A number of composite scoring systems have been developed, but only one, the Patient Assessment of Constipation Quality Of Life (PAC-QoL), has been robustly developed and psychometrically validated to a high level, including a comprehensive assessment of effect size. 54–56 The PAC-QoL includes 28 items covering four domains, each item is scored (0–4 points) and items and domains are aggregated to a composite score (0–4 points).
Minimum clinically important differences have been defined for PAC-QoL scores and informed an analysis of responders to treatment. Treatment effects have been characterised using cumulative distribution curves and a 1.0-point reduction has been confirmed as a robust measure of a responder. 57 Furthermore, a minimum clinically important difference can be defined by a 10% change (i.e. 0.4 points in the scale), as reported broadly in the literature. These definitions were used to ensure that the primary outcome measure in each trial was based on magnitude, risk and cost of intervention. Thus, for trials 1 and 2, a ≥ 0.4-point reduction in PAC-QoL score was considered to be a minimally important mean difference between groups, whereas in CapaCiTY trial 3 (surgery) a ≥ 1.0-point difference was chosen to reflect the more costly and potentially harmful intervention posed by surgery.
Secondary clinical outcomes
Given the questionnaire format of most available CC outcome assessments and multiple time points of assessment, we carefully selected and justified each outcome to keep the number and length of assessments to an achievable level (for compliance). The choice of Patient Assessment of Constipation Symptoms (PAC-SYM) and Measure Yourself Medical Outcome Profile 2 (MyMOP2) was specifically informed by qualitative research performed during our NIHR programme development stage on 50 participants with CC:
-
PAC-QoL score – binary responder analyses using 0.4-point and 1.0-point cut-off points
-
PAC-SYM score – individual domains and total score (as continuous variables)
-
2-week patient diary (for 2 weeks prior to each assessment) to record bowel frequency and whether or not each evacuation was ‘spontaneous (no use of laxatives) and/or complete’; the journal also captured concurrent medication, health contacts and time away from normal activities (including work) since the patient’s last visit
-
a validated patient problem-specific measure, MyMOP2, which incorporates the two worst volunteered symptoms and a measure of well-being58 (lower scores represent less symptomatology)
-
a validated QoL cost-effectiveness questionnaire – EuroQol-5 Dimensions (EQ-5D), EuroQol-5 Dimensions, five-level version (EQ-5D-5L), and EuroQol Visual Analogue Scale (EQ-VAS)59 (higher scores indicate better QoL)
-
Patient Health Questionnaire-9 items (PHQ-9)60 – nine items measuring level of depression (lower scores indicate less depression)
-
Generalised Anxiety Disorder-7 (GAD-7)61 scale (lower scores indicate less anxiety)
-
avoidant and ‘all or nothing’ behaviour subscales of the Chronic Constipation Behavioural Response to Illness Questionnaire (CC-BRQ)62 and Brief Illness Perception Questionnaire for Chronic Constipation (BIPQ-CC)63 (specific analyses required for interpretation)
-
global participant satisfaction (five-point scale from ‘not at all satisfied’ to ‘completely satisfied’) and a global participant improvement score (0–100% visual analogue scale) comparing how the participant feels today compared with before the study (higher scores indicate greater satisfaction).
Health economic outcomes
Resource use at the participant level was captured using trial case report forms (CRFs) at scheduled clinical visits and contacts. Intervention costing was specific to each trial and was detailed in the reporting of findings. Assessments were carried out at 0, 3, 6 and 12 months’ follow-up, augmented by telephone calls (every 12 weeks for the CapaCiTY trial 3). Participant use of prescription drugs related to their condition was recorded and costed using Prescription Cost Analysis (PCA) data. 64 Health service contacts were recorded by asking participants to recall GP, district nurse, pharmacy, accident and emergency department (A&E), outpatient and inpatient visits. Health-care resource use was costed using published national reference costs. Individual patient costs were estimated in 2018 Great British pounds (GBP) as the sum of resources used weighted by their reference costs. Time away from work or usual activities was recorded and costed using national average weekly earnings,65 contributing to a broader societal costing (Table 1).
| Resource | Unit | Cost (£) | Source |
|---|---|---|---|
| Health-care contacts | |||
| GP | Per visit | 39.00 | PSSRU66 |
| District nurse | Per visit | 41.00 | aPSSRU67 |
| Pharmacist | Per visit | 14.00 | NHS CPCS68 |
| A&E | Per visit | 114.00 | NHS Improvement69 |
| Outpatient | Per visit | 135.00 | PSSRU66 |
| Inpatient | Per visit | 631.00 | PSSRU66 |
| Other resources | |||
| Prescription drugs | Per item | b | NHSD-PCA70 |
| Time off work | Per day | 92.00 | ONS65 |
| CapaCiTY trial 1 | |||
| INVEST | Per procedure | 462.00 | |
| Staff time | Per procedure | 192.00 | NHS Improvement69 |
| Anal manometry | Per test | 50.00 | Personal communicationc |
| Rectal sensation | Per test | 4.00 | Personal communicationc |
| Balloon expulsion test | Per test | 4.00 | Personal communicationc |
| Gut transit study | Per study | 66.00 | NHS Improvement69 and personal commmunicationc |
| Evacuation proctography | Per test | 141.00 | NHS Improvement69 and personal commmunicationc |
| Cleaning/sterilisation | Per procedure | 5.00 | Personal communicationc |
| Habit training (with/without biofeedback) | |||
| Training sessions | Per hour | 135.00 | PSSRU66 |
| Biofeedback | Per session | 70.00 | d |
| CapaCiTY trial 2 | |||
| Low-volume TAI | Per use | 3.93 | eNHSD-PCA70 |
| Per 30-day use | 118.00 | eNHSD-PCA70 | |
| High-volume TAI | Per use | 7.57 | fNHSD-PCA70 |
| Per 30-day use | 227.00 | fNHSD-PCA70 | |
| Training/support sessions | Per hour | 135.00 | PSSRU66 |
| CapaCiTY trial 3 | |||
| LapVMR surgery | Per procedure | 4941.00 | NHS Improvement69 |
Generic health-related QoL was assessed using the EQ-5D questionnaire: a participant-completed two-page questionnaire consisting of the EQ-5D-5L and the EQ-VAS. The EQ-5D-5L includes five questions addressing mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with each dimension assessed at five levels, from ‘no problems’ to ‘extreme problems’. EQ-5D-5L scores were converted to health status scores using the mapping function developed by van Hout et al. ,71 providing a single health-related index including 0 (death) and 1 (perfect health), for which negative scores are possible for some health states. Scores were captured in trial CRFs during clinic visits or contacts at baseline, 3 months, 6 months and 12 months for CapaCiTY trials 1 and 2, and at 12-week intervals from the screening visit up to 72 weeks for CapaCiTY trial 3. Using the trapezoidal rule, the area under the curve (AUC) of health status scores was calculated, providing participant-level quality-adjusted life-years (QALYs) estimates for the cost-effectiveness analyses. Because AUC estimates are predicted to correlate with baseline scores (and thus potential baseline imbalances), AUC estimates were adjusted for baseline scores in regression analyses.
Patient and health-care professional experience
Qualitative data were obtained to aid the interpretation of outcomes and the development of an authoritative clinical pathway that recognised informational needs of both clinicians and patients. Face-to-face, digitally recorded, semistructured interviews (duration up to 1 hour) were conducted by an experienced nurse with a social science background and involved a purposive, diverse sample of patients and health-care professionals throughout the programme, with participant recruitment reflecting a range of ages, geographical locations and, when possible, other pertinent attributes such as ethnicity and sex, continuing until data saturation when no new themes emerged. All participants were told that they might be invited for interview when they were informed about each trial, but provided separate informed consent for interview (in those agreeing to be approached). A topic guide for each interview, informed by existing literature and patient advisors, was developed and piloted prior to commencement.
Adverse events and other work package-specific data
Adverse events were recorded throughout the programme using an AE log to record the nature, seriousness, causality, expectedness, severity, relatedness and outcome. All serious adverse events (SAEs) that were related or unexpected were reported to the sponsor, REC, quality assurance manager and local research and development (R&D) departments and the participant followed-up until conclusion. Safety was monitored by the Data Monitoring and Ethics Committee (DMEC) and reported to the REC in the annual progress reports. Other important data such as treatment logs and perioperative courses were WP specific.
Common analytical framework
General
The Pragmatic Clinical Trials Unit (PCTU; a UK Clinical Research Collaboration registered and NIHR-funded clinical trials unit in the Institute of Population Health Sciences, Queen Mary University of London) managed the three trials, including the development and management of secure databases in accordance with standard operating procedures. Automated validation checks were carried out at source data entry and further checks were performed on receipt by the study statistician and data manager. Data queries were addressed to the data manager and participating centres as appropriate. The centrally held database was locked for analysis once the data quality and completeness were assured and on sign-off of the final statistical analysis plan (SAP).
Sample size calculations
Detailed individual justifications for each trial are included in Appendix 1. In brief, sample sizes were calculated using the primary clinical outcome: change in PAC-QoL score based on pre-defined scalar changes. All calculations were performed at 90% power at a 5% significance level and, thence, allowed for 10% drop-out after randomisation.
Baseline characteristics
Numbers and percentages of patients with important baseline characteristics are presented by trial group. Continuous variables (e.g. age) are summarised by treatment group using mean and SD [median and interquartile range (IQR) if non-normally distributed]. No statistical testing was performed.
Clinical outcomes
All analyses were performed by the study statistician after the SAP was reviewed by the DMEC and signed off by the chief investigator and senior statistician. Although exact analyses for each trial differed according to study design, primary outcomes were analysed on an intention-to-treat (ITT) basis at defined time points (3 or 6 months) using linear mixed regression models [using Stata 14.2 (Stata Corp LP, College Station, TX, USA) xtmixed] with a random effect for centre and fixed effects for intervention, sex, baseline PAC-QoL score and breakthrough medication. Secondary outcomes were analysed on an ITT basis. For each outcome, descriptive statistics (as appropriate) by trial group are presented; continuous variables (e.g. PAC-QoL score) are summarised by treatment group using mean, SD, median and IQR. For categorical variables, numbers and percentages of patients reporting each response option were presented by trial group. Given the lower than target number of patients recruited to the trials, adjusted analyses were performed on PAC-QoL-derived secondary outcomes in CapaCiTY trial 1 only (logistic regression models for categorical outcomes). Unadjusted treatment differences and respective 95% confidence intervals (CIs) are presented for all other secondary outcomes. Results obtained using the CC-BRQ and BIPQ-CC have been omitted pending further analysis.
Subgroup analyses
Originally planned subanalyses were restricted after poor recruitment to a small number of specific analyses for major baseline characteristics in the CapaCiTY trial 1 only. A p-value of < 0.05 was taken to indicate statistical significance.
Cost-effectiveness
The economic analysis of the three interlinked CapaCiTY trials followed ITT principles and a prospectively agreed analysis plan. Using the standardised outcome framework for each trial, treatment effects were summarised at the patient level as overall cost and QALYs. Within-trial patient cost-effectiveness analyses were conducted comparing alternative treatments in each trial. Primary analyses took an NHS perspective. 72
Follow-up of trial participants is problematic, particularly over longer periods, making incomplete data a routine challenge. Consequently, the planned base-case analysis for each trial assumed the use of multiple imputation to account for missing data. For each trial, the base-case analysis is presented as the imputed and adjusted within-trial incremental costs and QALYs gained. Supportive sensitivity analyses included participants with complete data; unplanned sensitivity analyses are added if informative. Imputation and estimation were conducted according to good practice guidance73 using the multiple imputation framework in Stata. Multiple imputation provides unbiased estimates of treatment effects if data are missing at random (MAR) (i.e. causes of missingness are captured in observed variables). This assumption was explored in the data using logistic regression of the missingness of costs and QALYs against baseline variables. 74 Patient prognostic variables and trial stratification variables were assessed as potential missingness predictors for use in the imputation, which included outcome measures and costs (at each time point) as predictors and imputed variables. Imputation models used fully conditional (Markov Chain Monte Carlo) methods (multiple imputation by chained equations), which are appropriate when correlation occurs between variables. Multiple imputation ‘draws’ each provide a complete data set, which probabilistically reflects the distributions and correlations between variables. Burn-in traces for imputation variables were visualised to assess the independence of draws. Predictive mean matching drawn from the five k-nearest neighbours (k-NN) was used to enhance the plausibility and robustness of imputed values, as normality may not be assumed. Analysis of multiple draws was conducted with Stata’s multiple imputation framework providing estimation adjusted for Rubin’s rule. 75 In the imputation, missing costs and EQ-5D-5L scores were imputed for each period of follow-up and aggregated to overall patient costs and QALYs for each draw. All imputed variables acted as predictive variables, supplemented by trial baseline variables if significant and plausible predictors of missingness. Analysis of costs and QALYs was conducted primarily using bivariate regression. Multiple imputation estimation models were bootstrapped to provide non-parametric estimates. To minimise the information loss of finite imputation sampling, the fraction of missing information (FMI) was used to ensure that the number of imputed draws exceeded the FMI percentage. The distributions of imputed and observed values were compared to establish the consequences of estimation.
The (bootstrapped) median incremental cost-effectiveness ratio (ICER) and CI were estimated from the bivariate analysis. Value for money was determined by comparing the ICER with two willingness-to-pay (WTP) thresholds: the NICE-recommended upper threshold for ‘regular’ approvals of £30,000 per QALY76 and a lower value of £15,000 per QALY, reflecting uncertainty about the true value appropriate to the NHS. 77 The chosen threshold represents the WTP for an additional QALY: an intervention with a lower ICER value than the threshold could be considered cost-effective for use in the NHS. To assess the robustness of findings, base-case assumptions were explored using a range of supportive sensitivity analyses.
Net monetary benefit (NMB) succinctly describes the resource gain (or loss) when investing in a new treatment when resources can be used elsewhere at (or up to) the same WTP threshold. It is calculated as:
where NMB is estimated across a range of WTP thresholds such as from £0 per QALY to £100,000 per QALY. Where an intervention ICER is cost-effective (i.e. lower than the WTP threshold) the incremental NMB will be positive. NMB is routinely estimated from a bivariate regression to help generate a cost-effectiveness acceptability curve (CEAC). The CEAC visualises the likelihood that treatments are cost-effective as the WTP threshold varies. 78 Univariate regression of NMB has several advantages over the bivariate approach: it may be more robust approach with sparse data; it transforms the cost/outcomes data from a ratio into a continuous variable, allowing for easier manipulation and interpretation; it manages the correlation between costs and QALYs; it easily manages covariate imbalances; and repeated estimations explore the WTP threshold. 79 However, it does not allow the cost-effectiveness plane to be visualised, and the univariate distribution varies by threshold. Univariate regression was used as a confirmatory analysis where patient numbers were low or distributions were highly non-normal.
The expected value of perfect information (EVPI) is the upper limit of the value to a health-care system of further research to eliminate uncertainty. 80 Findings from cost-effectiveness analyses remain uncertain because of the imperfect information they use. If a wrong adoption decision (e.g. to make a treatment available) is made, this will bring with it costs in terms of health benefit forgone: the NMB framework allows this expected cost of uncertainty to be determined and guide whether or not further research should be conducted to reduce uncertainty.
Analyses and modelling were undertaken in Stata 16.1 using the portal provided at Queen Mary University of London. Reporting follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 81
Patient experience
Interviews were digitally recorded, anonymised, transcribed verbatim and analysed using a pragmatic thematic analysis. Data analysis was developed as outlined by Fereday and Muir-Cochrane82 in the first instance by mapping key concepts derived from the transcripts (‘charting’) and extracting emergent themes from the transcripts. The topic guide was used for the pre-determined codes and supplemented with additional codes that arose from the data. The final analysis combined inductively and deductively derived codes. The qualitative researcher (Tiffany Wade) conducted independent analyses and then compared and refined resulting codes and themes in discussion with the qualitative research study lead (CN). Three members of the patient and public involvement (PPI) panel were shown the qualitative results and participated in an online discussion to refine the messages. Emergent themes formed the basis of analytical interpretation. Challenges to delivering the three trials were also enquired about during the qualitative interviews with participants and research staff, using the qualitative methods described.
Patient recruitment
Challenges to recruitment
Recruitment was poor and negatively affected the whole programme (i.e. all three trials). This reflected several issues with the process and some of the design. Thus, the programme as a whole suffered from the following:
-
Long-term staff [i.e. specialist NHS nurses and Clinical Research Network (CRN)-funded research nurses] sickness and retirements and difficulty in recruiting staff led to shortage of staff to deliver interventions. This was reflected by significant recruitment only at sites where research staff from the directly funded core research team took over delivery (e.g. Barts and the London School of Medicine and Dentistry, Queen Mary University of London, in CapaCiTY trial 1 and County Durham and Darlington NHS Foundation Trust in CapaCiTY trial 2).
-
R&D delays to get the study up and running at many sites (range 6–9 months), with five sites never opening.
-
Lack of funding to cover excess treatment costs (ETCs) [e.g. high-resolution anorectal manometry (HRAM) devices; Clinical Commissioning Groups (CCGs) to allow prescription of irrigation kits].
-
Recruitment targets too high, demotivating staff and pressurising them to start recruitment before they were prepared (according to interviews).
-
Some treatments were available as part of clinical practice immediately if patients refused entry into research, and with some delay if patients agreed to participate. Patients often had a significant burden of symptoms and were unwilling to delay treatment.
Other issues were trial specific. In CapaCiTY trial 1, delays due to infection control variations in approval of HRAM protocol and device cleaning (e.g. local views differed from published national standards and would require the purchase of expensive sterilisation equipment) hindered INVEST. Trial 1 also suffered criticism that the number of outcome questionnaires may have been off-putting to some patients (see Work programme 5, Patient and public involvement). In CapaCiTY trial 2, we underestimated the number of patients who would not be willing to try the irrigation device and the additional staff time above clinical need, making staff reluctant to recruit (both points highlighted in interviews). CapaCiTY trial 3 was seriously hindered by the evolving serious mesh controversy on national and international media. 83 Patients became unwilling to try the intervention and some hospitals were instructed by their lawyers to stop the intervention. A further problem in CapaCiTY trial 3 related to NHS bed pressures: the stepped-wedge design meant that one group of patients was randomised to have surgery caried out in 4 weeks. Some sites were unable to secure a bed in this time frame even with proscribed tolerance.
We took a number of measures to try to mitigate these issues:
-
We provided almost all sites with refresher site initiation visits and guidance in reviewing referral letters to identify suitable patients.
-
We provided all sites with a refresher investigator meeting in December 2017 to get all research staff together from all our sites with the aim to provide them with refresher training on protocol, exchange experience on overcoming recruitment barriers and encourage everyone to recruit.
-
We changed the design of the studies in January 2016 to shorten the time between the follow-up visits from 24 months to 12 months to lessen the burden of the follow-up visits on both patients and research staff, and removed two lengthy questionnaires to reduce file size.
-
We made protocol amendments to make the protocol more compatible with routine practice where possible.
-
We provided sites with support in data entry and administrative work, which would free up the research nurse to do the screening and recruitment.
-
We launched an advertising campaign in summer 2016 to find suitable patients through newspapers.
-
We provided HRAM devices to sites on loan in an effort to help sites meet the requirements of study device (CapaCiTY trial 1). We undertook competitive procurement and assisted sites with business cases to secure funding for HRAM equipment.
-
We secured funding from irrigation device company McGregor Healthcare Ltd (Macmerry, UK) to sponsor sites affected by the lack of prescription funding (CapaCiTY trial 2).
-
We engaged with NIHR to try to encourage CCGs to meet their obligations to fund ETCs. We had letters from the Department of Health and Social Care in this regard but both it and the CRN were unable to mandate ETCs (CapaCiTY trial 2).
-
We requested recruitment extensions for all three trials in an effort to boost recruitment.
-
We regularly presented at national speciality meetings (e.g. the Pelvic Floor Society).
-
We made use of incentives – prizes, a newsletter, social media and professional development opportunities.
Final programme recruitment
A total of 275 patients was recruited across three trials, representing a major shortfall in relation to the required cumulative sample size (n = 808). These patients were recruited from a total of 733 screened (37.1%); this recruitment rate was lower than the 50% recruitment rate we anticipated. About half of screen failures were due to failed eligibility and about half were due to the patient declining. There was also a problem of patient retention in all trials, with higher than anticipated drop-out rates before primary outcome (actual range 11–43% vs. anticipated range 20%).
A total of 90% of participants were female (100% in CapaCiTY trial 3) and participants had a mean age 45 years (IQR 33–57 years). The majority (70%) of participants were of white ethnicity. Baseline phenotyping indicated high levels of comorbidity in > 70% of patients and a history of previous abdominal and pelvic surgery in > 50%. In terms of other risk factors, psychiatric diagnoses (insufficient for exclusion) and joint hypermobility were each present in ≈ 20% of patients. Approximately two-thirds of participating women were parous. Although the criteria for chronicity of constipation was 6 months’ duration, mean duration was 6 years and almost all patients with CC had constipation that proved intractable to lifestyle modification and laxatives, which was reflected by referral pattern: 80% of referrals were from secondary or tertiary care. Almost 20% had also failed prokinetic therapy. Symptom burden was high, with mean PAC-QoL and PAC-SYM scores of > 2.0 points at baseline. In addition, > 50% of patients had faecal incontinence symptoms, > 30% had urinary symptoms and > 20% had pelvic organ prolapse symptoms (100% in CapaCiTY trial 3).
In CapaCiTY trial 1, data from the PHQ-9, which measures depression, showed that 32.9% of participants scored > 9 points, suggesting that one-third of the sample would meet case criteria for major depressive disorder (MDD). A further 33% had scores indicative of mild depression, suggesting that, overall, around two-thirds of the sample were distressed or depressed. This compares with a point prevalence of MDD in the general population of 5%. The prevalence of MDD is, on average, at least twice as high in people with chronic medical diseases than in those without a chronic medical condition, but, even taking this into account, the rates in the current study are at the high end. 84 The findings for anxiety were similar, with one-third of the cohort (33.6%) scoring above the GAD-7 cut-off point for an anxiety disorder, and a further 25.1% reporting mild symptoms of anxiety.
Interview participants
A total of 45 patients and 23 staff members were interviewed:
-
CapaCiTY trial 1. A total of 24 participants (men, n = 3; women, n = 21) were interviewed: nine participants were allocated to INVEST and 15 participants were allocated to no INVEST, to elucidate the experience of undergoing tests and being given an explanation of results or their feelings about not being tested; 11 participants were allocated to HT and 13 participants were allocated to HTBF, both improved and not improved (at 6 months) for perceptions of treatment. A total of 15 staff members were interviewed: five therapists involved in HT/HTBF to determine the comparative ease of delivery of the two therapies, three research nurses, four CapaCiTY research team members, one biofeedback physiologist, one clinical trials associate and one trainee clinical scientist.
-
CapaCiTY trial 2. A total of 11 patients (men, n = 4; women, n = 7) undergoing TAI were interviewed: seven received high-volume TAI, three received low-volume TAI and one received both high-volume and low-volume TAI. Most (n = 10) patients were continuing to use TAI at the time of interview; however, one had discontinued use by the interview date.
-
CapaCiTY trial 3. A total of 10 patients (all female) were interviewed. Nine patients were interviewed ≈ 1 year after their lapVMR to gain an understanding of what their experiences were prior to, immediately after and 1 year after the operation. One patient who declined surgery was also interviewed. A total of eight staff members (i.e. three surgeons, three research nurses, one gastroenterologist and one research team member) were also interviewed about both the interventional and research element of the study.
Work programme 2: CapaCiTY trial 1 – randomised trial of habit training compared with habit training with direct visual biofeedback in adults with chronic constipation
Specific background and rationale
In most UK practices, patients with CC are first referred to specialist nurses for a variety of nurse-led behavioural interventions to improve defaecatory function. A range of cohort studies,85 RCTs,86–91 reviews,92 guidelines93 and meta-analyses94 attest to the general success of this approach. However, opinion varies greatly concerning the complexity of intervention required and UK survey evidence (performed as part of this programme) indicates that there is remarkable variability of practice. 95
A simple form of behavioural therapy is habit training. This involves optimising dietary patterns to maximise gastrocolic response and the morning clustering of high-amplitude propagated colonic contractions that propel contents towards the rectum for subsequent evacuation. Dietary advice to optimise intake of liquid and fibre is given, as well as advice about frequency and length of toilet visits and posture. Patients are also instructed on basic gut anatomy and function, and gain an appreciation of how psychological and social stresses may influence gut functioning. Simple pelvic floor and balloon expulsion exercises are often included.
More complex forms of therapy include instrument-based biofeedback learning techniques. 85–91 Favoured in the USA and by about half of UK centres, these provide direct visual computer-based biofeedback of pelvic floor activity. Although small RCTs suggest an additive value of biofeedback over habit training alone in the management of selected patient subgroups of CC (e.g. dyssynergic defaecation),87,96–98 there has been no multicentre or adequately powered RCT in unselected patients, despite the uncertainty having significant resource implications. Aside from the issue that there is now considerable disagreement regarding the very diagnosis of dyssynergic defaecation (including from our own study using HRAM),99 most publications advocating biofeedback have come from specialist centres with considerable ‘investment’ in these techniques, with reports much less favourable when biofeedback is the ‘de-vested’ comparator. 100,101 The controversy following a Cochrane review that drew attention to the limitations of the evidence base102 attests to the polarity of opinion on this subject and the need for a more definitive (i.e. a larger, higher-quality) RCT.
This underpinned our first trial-specific research question:
-
In unselected (i.e. no-INVEST group) adults with CC, is HTBF more clinically effective than HT as measured by PAC-QoL score at 6 months’ follow-up?
A further unanswered question regards the utility of behavioural therapies in different subgroups of patients with CC. Well-regarded international consensus (e.g. the Rome VI Criteria)103 supports a view that biofeedback significantly benefits only a subgroup of patients with a form of functional defaecation disorder termed ‘dyssynergic defaecation’ (see Figure 1). This poses the question whether or not further tests are required at an early stage (i.e. before behavioural therapy to select patients for biofeedback). There are significant differences of expert opinion on this subject: some advocate early complex and expensive investigations to guide treatment in most patients,8 whereas others advocate undertaking such tests only in resistant cases or in those progressing to surgery. 104 The advantage of guiding treatment105,106 is balanced against the invasive nature of some tests, radiation exposure, embarrassment and cost (≈ £600–1200 NHS tariff); most also necessitate an escalation of care (i.e. from secondary to tertiary centre). The need to resolve this question has been consistently highlighted38,107 but, to our knowledge, prior to the programme no RCT had stratified treatment selection on this basis.
This underpinned our second trial-specific research question:
-
Is the impact of such specialist-led interventions improved by stratification to HTBF or HT based on prior knowledge of anorectal and colonic pathophysiology using INVEST as measured by PAC-QoL score at 6 months follow-up?
Both research questions were embedded in a single experimental design with three parallel trial groups and required a total sample size of 394 patients. For a full description of this trial, including interventions, trial-specific design procedures and all results and analyses, see Appendix 1. Only the main results and conclusions are summarised in this report.
Results
Recruitment started on 26 March 2015 (first intervention 21 May 2015) and ended 30 June 2018. A total of 182 participants (of the target 394 participants) were randomised out of 502 screened (36.3%) from 10 sites. Two sites opened but failed to recruit; the remainder randomised between 7 and 71 participants. A total of 68 participants were randomised to HT, 68 participants were randomised to HTBF and 46 participants underwent INVEST-guided therapy (HT or HTBF based on results) [the Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 2]. A total of 178 participants provided PAC-QoL data at one or more time points (Figure 3). The primary outcome (PAC-QoL score) was available at both baseline and 6 months for only 103 participants. There was no evidence of an additive effect of HTBF over and above HT (Table 2).
FIGURE 2.
The CapaCiTY trial 1 CONSORT flow diagram.
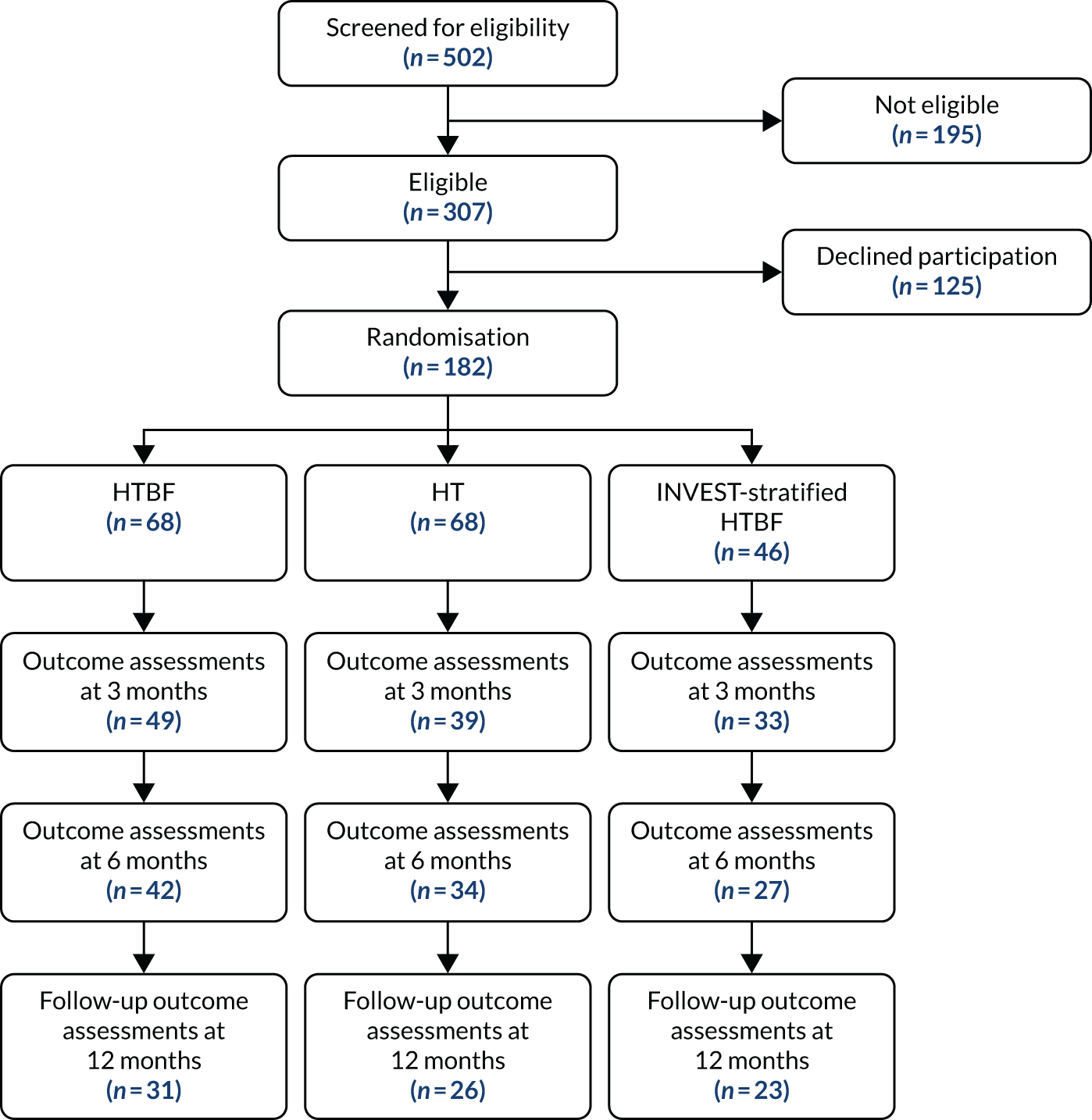
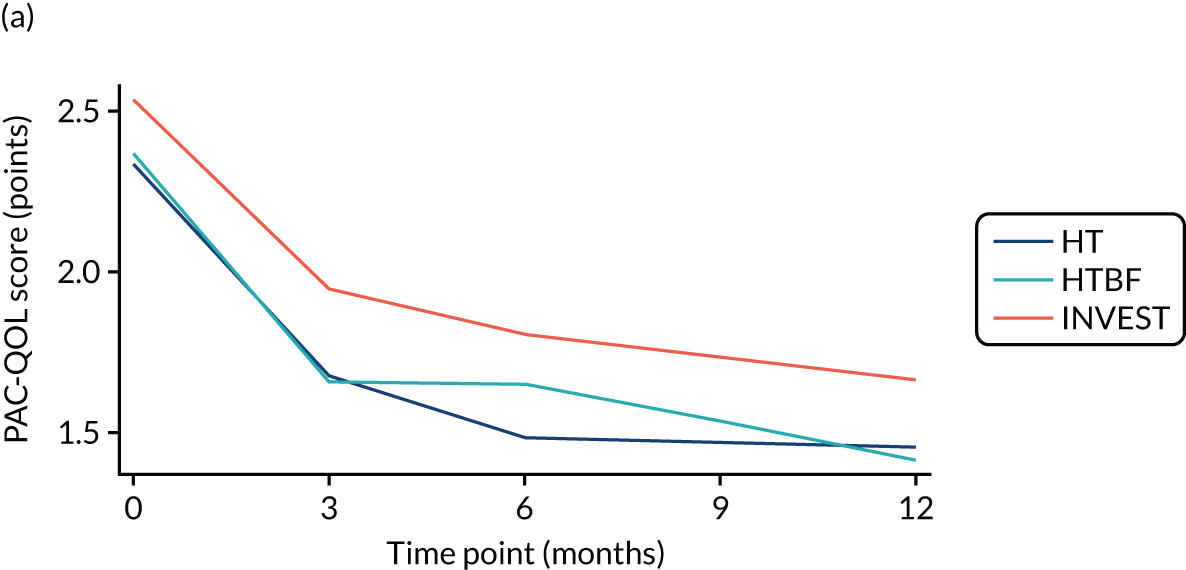
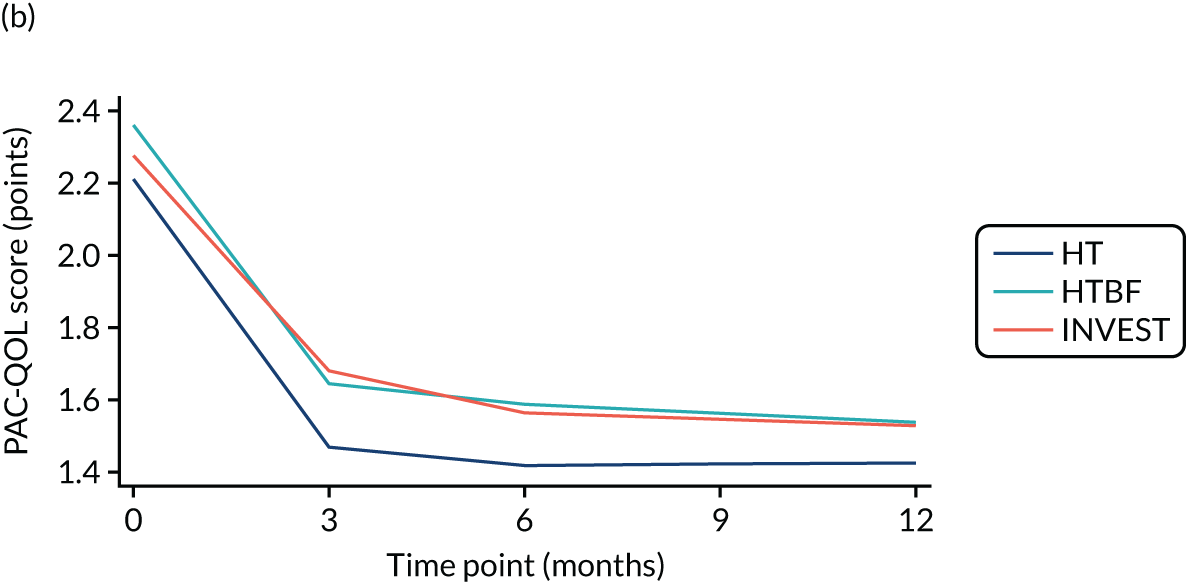
FIGURE 3.
Overall PAC-QoL scores. (a) All participants (n = 178 at baseline); and (b) participants with no missing data to 12 months (n = 54). Both figure parts show reductions in score (i.e. improvement) over time.
| Intervention | PAC-QoL score (points), mean (SD) | Treatment differencea (95% CI) | p-value | |
|---|---|---|---|---|
| Baseline | 6 months | |||
| HT vs. HTBF | ||||
| HT (n = 38) | 2.26 (0.69) | 1.49 (0.85) | Reference | |
| HTBF (n = 30) | 2.41 (0.81) | 1.65 (1.03) | –0.03 (–0.33 to 0.27) | 0.8445 |
| No INVEST vs. INVEST | ||||
| No INVEST (n = 68) | 2.33 (0.74) | 1.56 (0.93) | Reference | |
| INVEST (n = 22) | 2.36 (0.78) | 1.81 (1.03) | 0.22 (–0.11 to 0.55) | 0.1871 |
A range of secondary outcomes covering symptoms and QoL improved in both the HT group and the HTBF group (e.g. mean PAC-SYM score reduced from 2.2 points at baseline to 1.5 points at 6 months and weekly laxative use reduced fourfold). Interventions led to small reductions in depression but no significant differences between the intervention types. Overall, about 65% of participants were globally satisfied or very satisfied with both interventions and this was reflected by participant experience reported at interview (i.e. similar proportions of participants liked, and a minority disliked, both interventions for a number of reasons).
Similar results were obtained for INVEST versus no-INVEST, with no evidence of a difference in primary outcome (see Table 2): participants provided reasons for liking INVEST (e.g. greater knowledge of their condition and knowing that it was not ‘all in their mind’) and disliking the invasiveness and embarrassment of the tests.
Given similar changes in EQ-5D-5L scores for all interventions, cost-effectiveness analyses favoured the simpler strategies (i.e. HT and no INVEST) as the dominant strategies. In both instances, cost increases were significant (HTBF vs. HT: £239, 95% CI £133 to £354; INVEST vs. no INVEST: £543, 95% CI £403 to £685) and QoL reduced (HTBF: –0.010 QALYs, 95% CI –0.053 to 0.03 QALYs; INVEST: –0.047 QALYs, 95% CI –0.093 to –0.001 QALYs). The probability that HT is cost-effective was a p-value of 0.83 at a WTP threshold of £30,000 per QALY.
Conclusions
Taking together the results from clinical effectiveness, cost-effectiveness and patient experience data, the following conclusions may be drawn while accepting the major caveat of under-recruitment:
-
Included adults with CC had high levels of symptom burden and long durations of symptoms that had been refractory to previous treatments and could therefore be considered ‘hard to treat’. These symptoms were associated with a substantive effect on QoL and psychological well-being. Patient experience reflected the misery of the condition and fear that treatments would be ineffective.
-
Our analysis of clinical effectiveness showed that all interventions trialled (i.e. HT, HTBF with INVEST and HTBF with no INVEST) reduced symptom burden and improved disease-specific QoL. The observed magnitude of these PAC-QoL score changes (≈ 0.8 points) can be considered to be clinically meaningful and represented a greater reduction than the minimum clinically important difference sought between groups by design (mean change 0.4 points). The findings from the primary outcome were coherent with a panel of secondary outcomes, and, overall, such improvements are unlikely to have occurred spontaneously in a condition that is generally considered chronic and stable.
-
Confidence intervals rule out clinically important differences between HT and HTBF for the primary outcome and all main secondary outcomes. The same was true of INVEST and no INVEST.
-
Standardised specialist-led habit training alone and no INVEST are strongly supported by cost-effectiveness analyses. Despite under-recruitment (182 out of a planned 394 participants), participant-level data provided the most robust evidence to date on the first step of care for patients referred to hospital for CC. Neither a complex specialist-led intervention (e.g. pelvic floor retraining using biofeedback) nor stratification to complex or standardised therapy based on prior knowledge of anorectal and colonic pathophysiology (INVEST) were more cost-effective than HT. Analysis suggests that HT is the dominant strategy (i.e. lower cost and greater QoL) at a WTP threshold of £30,000 per QALY.
-
All procedures were safe and well tolerated by patients.
-
Interviews suggested that patient experience was mixed. Some regretted not being allocated to INVEST or HTBF (because they believed that they would have less knowledge of their condition and less likelihood of treatment success), whereas others felt that the tests and biofeedback were embarrassing and intrusive. Most participants reported a positive interaction with staff and at least some symptom benefit. Most would recommend trying the intervention they received to other people with CC.
-
Staff were mostly supportive, but some found that adhering to the agreed intervention protocol constrained their clinical flexibility and they would have preferred to individualise the intervention. The biofeedback element added time to consultations, or limited what they could cover in HT.
-
Because these patients had significant psychological comorbidity, and there is evidence that psychological treatments such as cognitive–behavioural therapy have significant and sustained benefits on symptoms, mood and QoL in people with irritable bowel syndrome (many of whom also experience severe constipation), future work should focus on incorporating psychological methods alongside HT. 108,109
-
Taken together, the cost-effectiveness data (in the absence of differences in clinical effectiveness, patient experience and safety) promote the adoption of the simpler pathway (i.e. HT without INVEST). A revised prototype pathway is provided on this basis in the final conclusions of the synopsis (see Figure 8).
Reflections on work programme 2
This WP was severely hampered by poor recruitment, with many of the general challenges listed in SYNOPSIS. It is not the place here to repeat well-rehearsed arguments about the frailties of delivering a complex research intervention in a resource-strained NHS, but these certainly affected this trial. That noted, with the benefit of hindsight, and even after simplifying the trial design at the award approval stages, the study was probably overambitious in design. We tried to answer two research questions concurrently with one experimental design (HT vs. HTBF and INVEST-stratified vs. no INVEST-stratified treatment). This was laudable and based very clearly on nationally agreed research priorities at the time (e.g. from the American Gastroenterology Association). 93 However, it might have been better to have simplified the design to one that compared only HT with HTBF in a two-group trial without INVEST. This would have not only reduced the sample size (and general complexity) but also avoided some of the issues of equipment shortages and approvals (including infection control procedures) that delayed many centres from opening for as long as 1 year (with some never opening). We considered this option at the second review stage but felt that it was too great a departure from our original application. An alternative design (based on our qualitative data) might have been preference based. Another issue was the complexity of inclusion criteria and the number and complexity of outcome instruments. Despite PPI input at the outset, and changes made (with PPI) during the programme, outcome collection was still burdensome. Although the type of strict inclusion criteria and outcome panel we employed seem to still be the norm in recent pivotal trials, a more pragmatic (relaxed) approach to inclusion and a smaller outcome booklet would (with hindsight) have been preferable.
Despite these challenges, the trial undoubtedly brought together a fragmented community of experts from around the UK and led to the standardisation of practice. Two publications from early in the programme highlight the previous lack of understanding of what actually constitutes biofeedback95 and what investigations tests should be performed and how. 34,110 The former was borne out by our qualitative data (i.e. many practitioners preferred to tailor their approach to each patient). The latter led to an international effort to introduce technical standards on performing and interpreting diagnostic tests of anorectal function, in which the CapaCiTY team were leaders, and these have been published. 111
Finally, it must be recognised that, despite under-recruitment, CapaCiTY trial 1 is, to our knowledge, nevertheless the largest RCT to date in which biofeedback is one of the trialled interventions. A total of 182 patients were randomised; sample sizes in 17 previous RCTs included in a Cochrane review102 ranged from 21 to 119 (and it is noted that the trial with 119 patients101 was focused on surgery with biofeedback as the comparator). Therefore, it is acknowledged that among many UK practitioners there will be a sentiment that the results of CapaCiTY trial 1 do still provide an answer to a major question – namely, that the sort of specialist-led biofeedback practice advocated by a small but very vocal group of experts in the USA is less likely to work in an NHS pathway. The main points are as follows: (1) there is insufficient human resource (trained specialist nurses) and equipment (manometry catheters) to provide it (CapaCiTY trial 1 had three to four sessions; in the USA five to seven sessions are prescribed); and (2) there is a general disbelief (reinforced by the findings of the study and a prior Cochrane review)102 that direct visual biofeedback offers much to patients over and above the panoply of approaches encompassed by HT (including holistic elements of patient support as well as didactic training) when it comes to the average patient (i.e. widespread adoption would be protracted if possible at all).
Research recommendations
The primary outcome effect size was almost identical for all groups and it is unlikely that another trial, even with a much greater sample size, would detect a clinically important difference between interventions. Certainly, the CI for the effect comparing HT and HTBF excludes the difference we initially set as the minimum clinically important difference in our sample size calculation. Thus, it is hard to recommend further investment, even with a more simplified trial design. We cannot comment on whether or not the US expert view is correct; perhaps limitation to highly selected patients with proven dyssynergic defaecation and specialist equipment in the hands of very expert medical practitioners (undertaking multiple sessions of therapy) does result in better outcomes (we did not trial this). However, we believe that this would be difficult to trial successfully in the NHS for the reasons outlined, and were it to produce the same results as CapaCiTY trial 1 it would still be unlikely to influence international opinion owing to the financial reimbursement drivers that promote the US approach. As noted, there may be rationale to include some form of psychological therapy alongside habit training for the large proportion of patients with significant psychological morbidity (akin to trials for patients with irritable bowel syndrome). This is an area for further research.
Work programme 3: trial 2 – pragmatic randomised trial of low-volume compared with high-volume initiated transanal irrigation therapy in adults with chronic constipation
Specific background and rationale
Transanal irrigation, for which there are a variety of commercially available devices, has been rapidly disseminated internationally since about 2000, first in CC patients with neurological injury112,113 and subsequently in other groups CC groups. 114,115 Despite a lack of published data other than from small selected case series, TAI is now available on drug tariff (at a cost of ≈ £2500 per patient per annum) and is generally considered to be the next step for patients failing other nurse-led interventions. TAI has permeated the UK market without robust efficacy data and with ongoing concerns regarding longevity of treatment and complications. 112,116 Retrospective clinical audit data and review116,117 by the applicants suggest a continued response rate after 1 year of ≈ 50%, with patients thus avoiding or delaying surgical intervention, but an accurate assessment of response rate and acceptability of this intervention required confirmation in a trial. In addition, two alternative systems for delivery of TAI exist: low-volume systems delivering ≈ 70 ml per TAI, and high-volume systems delivering up to 2 l of TAI (although typically only 0.5–1.5 l is required per TAI). The theoretical benefit of higher-volume TAI is greater efficacy – simply put, more washout. However, the low-volume system is cheaper, costing ≈£750 per annum, based on alternate-day use, compared with a cost of £1400–1900 per annum for high-volume TAI, and it may also be less traumatic and more acceptable to patients.
This underpinned our first specific research question:
-
In patients with CC who have failed conservative treatment (HT or HTBF), what is the impact on disease-specific QoL of TAI initiated with a low-volume and a high-volume system measured by PAC-QoL score at 3 months’ follow-up?
Transanal irrigation is an invasive therapy that requires, every day or every couple of days, insertion of the device into the anus followed by a period spent filling the rectum with fluid and then evacuating it. It is reasonable to suppose that patients would discontinue therapy that they felt was ineffective or unacceptable, or switch between low-volume and high-volume systems (permissible in the trial design).
This underpinned our second trial-specific research question:
-
What is the survival rate of therapy of TAI initiated with a low-volume and a high-volume system and do patients prefer one system to another?
Both research questions were embedded in a single, pragmatic, two-group parallel design (Figure 4) and required a total sample size of 300 patients (150 per group). Patients used one system only (plus defined ‘rescue therapies’) for a minimum of 3 months. After this time point they could switch to the other system if their initial therapy was ineffective/unsatisfactory. This allowed identification of response rates to each system in the short term (3 months) and, thereafter, a comparison between treatment strategies (TAI initiated with low-volume therapy or high-volume therapy) rather than a pure comparison of the two techniques. This was a patient-centred study design aiming to limit the time that patients spent using ineffective therapy without being allowed to try an alternative. Reasons for switching were captured qualitatively.
FIGURE 4.
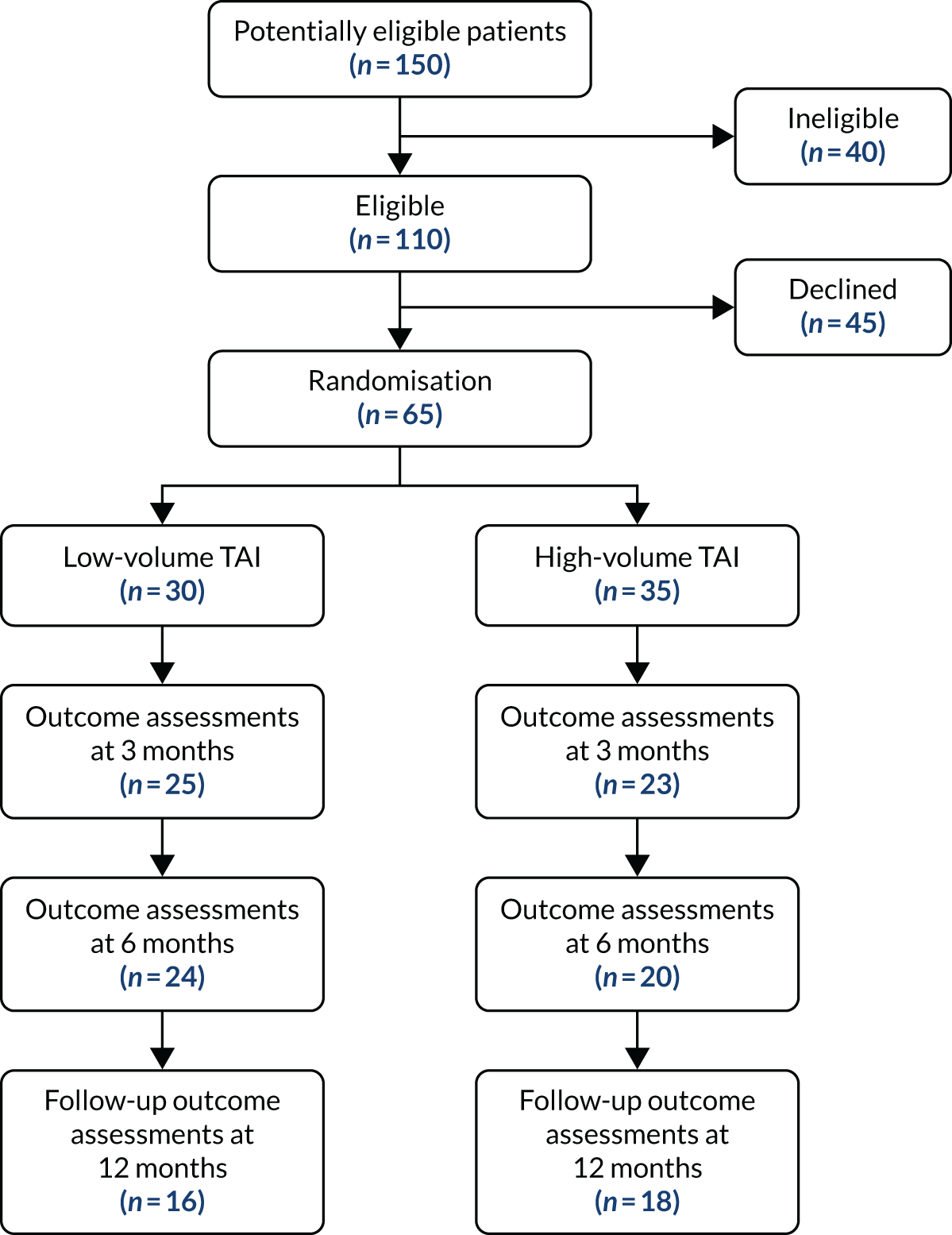
The CapaCiTY trial 2 CONSORT flow diagram.
For a full description of this trial, including interventions, trial-specific design procedures and all results and analyses, see Appendix 1. Only the main results and conclusions are summarised in this report.
Results
High-volume compared with low-volume transanal irrigation
First recruitment and intervention took place on 11 November 2015; recruitment ended 30 June 2018. A total of 65 patients (target 300 patients) were randomised from 150 screened (21.7%) from seven sites. Three sites opened but failed to recruit; the remainder randomised between 1 and 33 patients, with approximately half of the patients recruited from secondary care and half recruited from tertiary care. A total of 30 patients were randomised to low-volume TAI and 35 to high-volume TAI (see Figure 4). The primary outcome (PAC-QoL score) was available at baseline and 3 months for only 43 patients.
At 3 months there was a modest reduction in mean PAC-QoL score from 2.4 points to 2.2 points (SD –0.2 points) in the low-volume TAI group and a larger reduction of –0.5 points in the high-volume TAI group (Table 3). Although this difference was not large there was consistency of findings across some of the other outcome measures. For example, global satisfaction score, global improvement score and EQ-VAS score showed greater improvement in the high-volume TAI group. These results were based on an ITT analysis even though two patients crossed over (from low-volume to high-volume TAI) before the 3-month outcome (possibly diluting the difference in effect).
| Intervention | Baseline | 3 months | Difference in means (95% CI) | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Low-volume TAI (n = 19) | 2.4 (0.8) | 2.4 (1.9–2.9) | 2.2 (0.8) | 2.0 (1.5–2.8) | Reference |
| High-volume TAI (n = 25) | 2.3 (0.7) | 2.4 (1.8–2.8) | 1.8 (0.9) | 1.8 (1.1–2.3) | –0.37 (–0.89 to 0.15) |
Some further evidence of the greater benefit of high-volume TAI could be inferred from the fact that, over the follow-up period (with the majority after 3 months), 18 patients switched from low-volume to high-volume TAI but only six patients switched from high-volume to low-volume TAI, and two of these six switched back again to high-volume TAI.
Despite under-recruitment, patient-level data from the trial still provided the most robust evidence available to inform the pathway of care. Despite differences in initial purchase prices of the basic devices, initiating high-volume TAI did not increase study cost but resulted in higher patient QoL, suggesting a dominant interventional strategy (cost-effective at a WTP threshold of £30,000 per QALY; p = 0.99).
The interventions were generally well tolerated considering the invasive nature of the procedure. A total of 16 out of the 65 patients (25%) reported AEs, with a total of 68 AEs. Of these, 40 AEs (59%) were mild and 20 (29%) were moderate. There were six SAEs, of which three were expected. All resolved with no sequelae.
Survival rate of transanal irrigation therapy
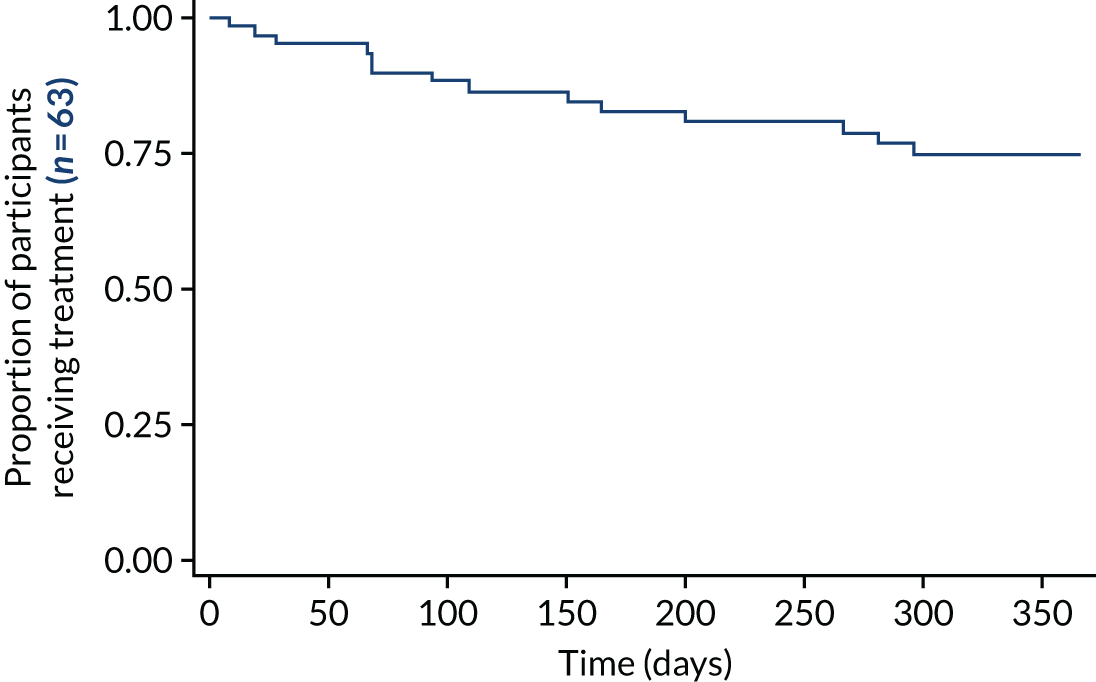
In the absence of a sham control (impossible to devise), and with the inability to compare with standard therapy, it was reasonable to use treatment continuation as a marker of efficacy. The treatment is burdensome, and it can be argued that patients who are not receiving benefit would not continue with it. There was no encouragement from research or clinical staff for patients to continue to use ineffective therapy. The 1-year survival rate of the treatment of 76% (Figure 5) implied significant continued effect. A comparison of survival rate plots of low-volume and high-volume TAI that allows for crossover has not been presented at the time of publication. This analysis is planned but was outside the SAP. 118
FIGURE 5.
The CapaCiTY trial 2 survival rate of treatment as a surrogate of ongoing benefit: Kaplan–Meier plot for time to cessation of treatment.
Conclusions
Taking together the results from clinical, cost-effectiveness and patient experience data, the following conclusions may be drawn while accepting the major caveat of under-recruitment:
-
The population studied represented a typical hospital-derived cohort of patients with severe CC that had not responded to conservative therapies.
-
We did not carry out statistical significance tests owing to under-recruitment. Despite this, there is preliminary evidence that high-volume TAI may be more effective than low-volume TAI. Although effect sizes were small for both primary and secondary outcome measures (especially global improvement scores), dominant crossover from low-volume to high-volume TAI and health economic analysis point in the same direction of favouring high-volume TAI.
-
Survival rate data were suggestive of a persisting benefit in the majority of patients, with three-quarters of patients still using TAI at 12 months. Overall, there were fewer patients on low-volume TAI at 12 months than on high-volume TAI. A survival rate analysis allowing for crossover is yet to be undertaken.
-
Some AEs were reported, but, of six SAEs, none was related or life threatening. The most common AEs were rectal bleeding and anal pain (48 events in 16 patients).
-
Despite under-recruitment, patient-level data from the trial still provided the most robust evidence currently available to inform the pathway of care. Initiating high-volume TAI did not increase overall cost (despite slightly higher basic device pricing) and resulted in higher patient QoL, suggesting a dominant interventional strategy (cost-effective at a WTP threshold of £30,000 per QALY; p = 0.99).
-
Although cost-effectiveness base-case findings were robust to the complete-case and sensitivity analyses conducted, the findings should be treated with caution. Small absolute numbers of patients and high levels of missing data as follow-up progressed placed great reliance on the imputation process and output. As with CapaCiTY trial 1, although substantial efforts were made to deliver a robust imputation process, it not possible to prove that a MAR process has been attained. Actual level of TAI use (the predominant cost) was not directly recorded and was instead approximated from available variables.
-
A final consideration in support of high-volume TAI concerns convergence. Health-care costs are similar comparing the two groups, suggesting that differences in health-care costs beyond 12 months are small. However, QoL diverges between groups over 12 months, suggesting that QALY gains may continue to accrue for some time beyond 12 months. Although speculative, any modelling of future cost and benefits would further increase the cost-effectiveness of high-volume TAI.
-
Transanal irrigation often caused initial anxiety, was not always easy to learn and was time-consuming. Ongoing staff support was much appreciated, especially as results were not always immediate. Some found it of no benefit, but many persevered and found that, although not necessarily a pleasant experience, it had an impact (sometimes large) on their symptoms and QoL. Staff were possibly more enthusiastic than patients initially, and this enthusiasm was picked up by patients in some cases and helped them to persevere.
Reflections on work programme 3
Poor recruitment (65/300; 22%) was a disappointment and reduced the inferential quality of the study. As well as the usual difficulties as discussed for CapaCiTY trial 1, we failed to recognise an important constituent: that the patients offered TAI had (1) a severe burden of illness and (2) already tried all the usual conservative measures (which had failed). They were resorting to TAI often in a state of desperation. The difficulty, then, was that recruitment to the trial resulted in a delay in treatment while baseline assessments and investigations were undertaken. At the same time, patients were aware that they could access the treatment immediately if they remained outside the trial. With hindsight, it should have been clear that this would lead to difficulties. To overcome this would have required a waiting list for non-trial treatment or a pragmatic approach in which investigations and baseline diaries were dispensed with (as they are dispensed with in emergency medicine trials). Further issues, such as the failure to obtain funding for the therapy in several UK regions, proved beyond our control despite extensive discussion with relevant CRNs and at a national level. Ironically, we received letters stating that funding would not be provided because the therapy lacked evidence from a randomised trial.
Despite gross under-recruitment, we believe that the trial provided some meaningful evidence that high-volume TAI may be more effective than low-volume TAI for the majority of patients. The CI for the effect size appeared to rule out a clinically important difference of 0.4 in favour of low-volume TAI and not in favour of high-volume TAI. This finding promotes a care pathway in which high-volume TAI might be initiated as a default for patients when there is not a compelling reason or preference for low-volume TAI. This is a useful conclusion, but it lacks identification of a more definite means of stratifying patients to one treatment or the other based on baseline phenotype. Ideally, we would have liked to study the efficacy of TAI per se, but there was no way of designing a sham control. A waiting list comparison was considered but rejected because waiting times were not long enough to provide meaningful data. A cohort design was also proposed, but the grant review panel encouraged a RCT. The appeal of considering low-volume and high-volume TAI was the ability to relate responders in the different treatment cohorts to symptoms (urge vs. no urge) and selected physiology results (notably, presence or absence of blunted rectal sensation). We could potentially have learned something about the physiology and mechanism of action of these treatments by doing this. However, with very low recruitment numbers, these additional analyses (covariates of response to each or both therapies) could not be undertaken.
It was interesting to note from the staff interviews that specialist nurses providing the treatment already had strong views that high-volume TAI was a better treatment. It is possible that some bias might have been affected by these opinions.
Research recommendations
It does not seem necessary to recommend repeating the trial on the basis of inadequate recruitment because any future trial will face many of the same challenges, even with the changes proposed here. Furthermore, a more basic question still remains: what is the value of any type of anal irrigation? There have been a significant number of retrospective observational studies and occasional prospective ones. The difficulty would be in establishing a control, as already stated here. It may be possible to develop a prospective cohort study of patients with CC that, while being monitored prospectively, might adopt TAI as an alternative to usual care. A trials within cohorts (TwiCs) design could be considered if patients would consider secondary randomisation against continuing usual care.
The qualitative evidence has been very illuminating and further analysis of this will be undertaken to understand drivers for continuing and discontinuing therapy (to guide patient selection and encourage compliance). As new equipment is developed (including devices activated by mobile telephones) there may be a desire to assess equivalence and safety. In addition, there is a need to understand the merits of contraindications such as mild colitis and pregnancy.
Work programme 4: CapaCiTY trial 3 – stepped-wedge randomised trial of laparoscopic ventral mesh rectopexy in adults with chronic constipation
Specific background and rationale
When non-surgical therapies fail, a decision must be made about whether or not to offer surgical interventions. Decision-making is greatly influenced by local expertise, commissioning and personal enthusiasm for particular interventions25,119,120 balanced against poor results in some patients. 25 Currently, there is large and difficult-to-justify variation in surgical practice according to need and type of procedure. The need to reduce variations in practice, based on available evidence, is a perpetual theme of national specialty group discussions, with various initiatives proposed. Multidisciplinary team (MDT) processes have been established in the UK with the aim of reducing potential for inadequately informed and potentially harmful interventions in poor surgical candidates. 25 However, when these MDT processes occur, decision-making is not helped by a near void of high-quality evidence.
At the time of starting the CapaCiTY research programme, lapVMR was considered a very effective procedure for the management of selected patients with CC. This procedure was first described for the treatment of external rectal prolapse in 2000121–123 and then for patients with internal prolapse and/or rectocele presenting with CC. 124–131 Its popularity, based on its lesser invasiveness and perceived small detrimental effect on bowel function,132 soared 15-fold in the UK between 2001 and 2012133 but rapidly declined during the CapaCiTY research programme (2016 to present) when concerns regarding the use of pelvic mesh surfaced in the media83 and the courts. 134
The evidence for efficacy of lapVMR was limited to observational data, the majority of which derived from single-centre case series. It is clear that complications can be limited by good technique and perhaps choice of mesh, but complications will not be eradicated. Thus, it can be argued that the future of lapVMR depends not on the very small observed differences in long-term mesh complications (e.g. in 1.0–2.0% of patients) but on a fundamental evaluation of whether or not the procedure is actually clinically beneficial (i.e. whether or not these complication rates would be deemed acceptable, provided patients are consented to the risk, if the patient benefit was sufficiently large).
This underpinned the specific research question:
-
In selected adults with CC who have failed conservative treatment (i.e. HT or HTBF with or without TAI) and who have high-grade internal rectal prolapse, what is the clinical efficacy of lapVMR compared with controls at short-term (i.e. 24-week) follow-up.
In addition, this WP included the delivery of a series of high-quality systematic reviews for all main surgical procedures employed to treat CC to define benefits and harms and provide prototype graded practice recommendations (including for lapVMR).
We used a stepped-wedge randomised trial design to permit observer-masked data comparisons between patients awaiting intervention and patients who have undergone surgery. Contrary to most stepped-wedge trials, individual patients (as opposed to clusters) were randomised. In brief, eligible participants were randomised to three groups with different delays before surgery (Figure 6). In all groups there was a period of 4 weeks post eligibility assessment to arrange the logistics of surgery [time (weeks) since randomisation (T) –4 (T–4) to T0] and ensure that patients have returned to their normal life routine after various assessments. lapVMR was performed in group 1 at T0, in group 2 at T12 and in group 3 at T24. Unavoidably, participants were aware when surgery was undertaken and thus met one of the assumptions of a stepped-wedge design (i.e. no effect of treatment expected until surgery has been performed). Efficacy outcome data were collected at equally stepped time points (T0, T12, T24, T36 and T48). PAC-QoL and PAC-SYM total scores were analysed using a mixed linear regression model, adjusting for a random effect of participant and a fixed effect of time since randomisation (see Figure 6). The effects of the intervention at 12, 24, 36, 48, 60 and 72 weeks post surgery were modelled as fixed effects. For example, the intervention effect at 12 weeks post surgery was included in the regression model using a dummy variable set to 1 in those cells of Figure 6 where surgery occurred 12 weeks previously [i.e. at T12 in group 1 (when surgery was at T0), T24 in group 2 (when surgery was at T12) and T36 in group 3 (when surgery was at T24). This was the expected difference in outcome between a patient who had surgery 12 weeks previously and a patient who had not had surgery. This was similar for intervention effects at T24, T36, T48, T60 and T72 weeks post surgery. The analysis included a Kenward–Roger correction to correct for the small sample size, and was performed in Stata using the ‘mixed’ command with the options ‘reml dfmethod(kroger)’.
FIGURE 6.
The CapaCiTY trial 3 schematic stepped-wedge design.
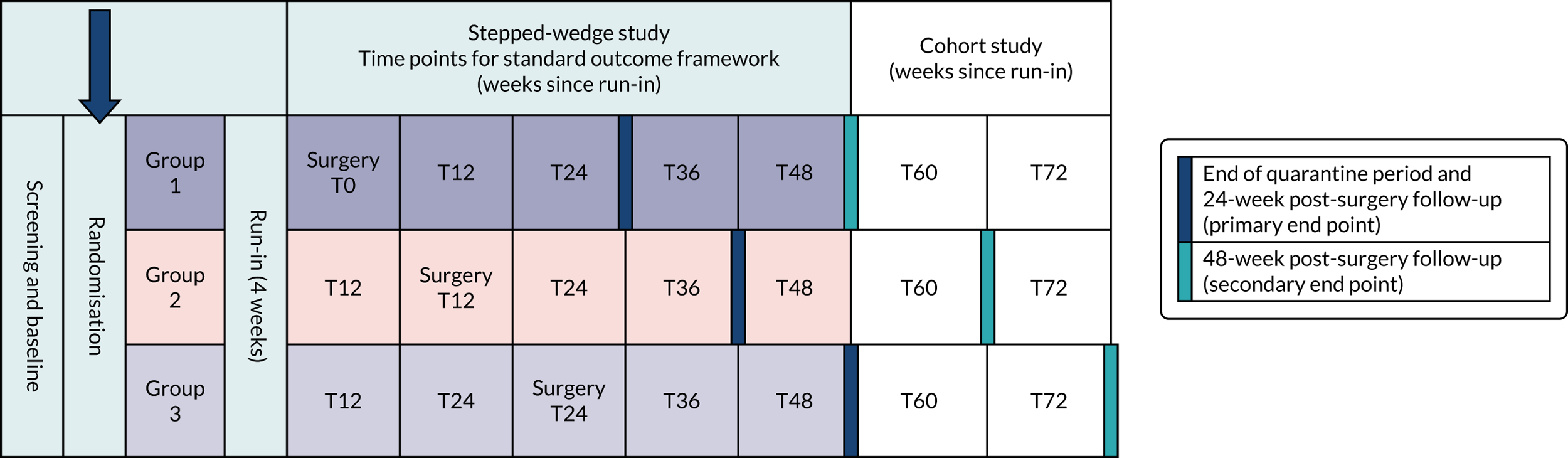
The choice of design (in effect a modification of a standard, parallel-group, waiting-list control design) had several advantages. First, a stepped-wedge design is more efficient and thus improves recruitment feasibility (the major hurdle of nearly all surgical trials). Despite the multicentre approach of this study, the problems of recruitment were manifest. Simulation demonstrated that a parallel-group design required a much larger sample size than that proposed for the current study at the same power. Second, the trial design meant that there was only a one in three chance (rather than a one in two chance as in a parallel-group design) of waiting almost 6 months for surgery, which was more acceptable to patients (according to a 100-patient survey during the programme development phase). Using this design required a sample of 114 patients with 95% power (purposely chosen to reflect the magnitude and risk of intervention) at a 5% significance level.
For a full description of this trial, including interventions, trial-specific design procedures and all results and analyses, see Appendix 1. Only the main results and conclusions are summarised in this report.
Results
Stepped-wedge randomised trial
The first recruitment was on 1 March 2016, with the first intervention on 15 June 2016. Recruitment ended on 31 January 2019. A total of 28 patients (target 114 patients) were randomised out of 81 screened (34.5%) from nine sites. Two sites opened but failed to recruit and one site failed to randomise; the remainder (n = 6) randomised 1–11 patients each. Nine patients were randomised to immediate surgery, 10 were randomised to a 12 weeks’ waiting time and nine were randomised to a 24 weeks’ waiting time. The CONSORT flow diagram is shown in Figure 7. One patient was randomised but did not undergo surgery. However, this patient remained in the study on ITT principles. Two patients dropped out of the study before the primary end point and a further five failed to complete the primary outcome (PAC-QoL score at 24 weeks), which was therefore undertaken in 19 patients.
FIGURE 7.
The CapaCiTY trial 3 CONSORT flow diagram. a, One patient did not undergo surgery; this patient continued to participate and was included in analysis on ITT principles.
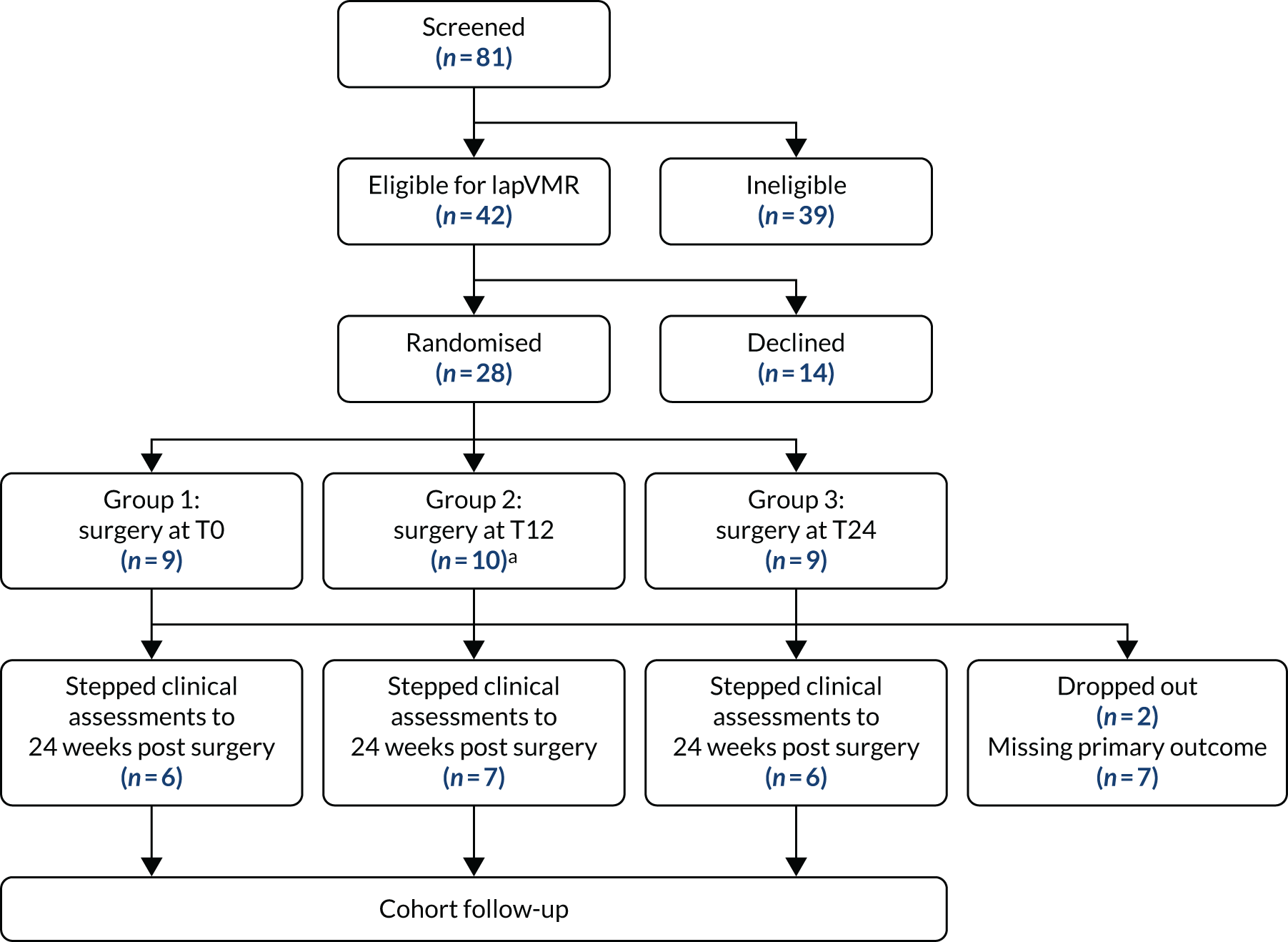
Laparoscopic ventral mesh rectopexy resulted in substantial improvement in symptoms, with the mean PAC-QoL score reducing from 2.63 points at baseline to 1.26 points at 24 weeks, and a similar reduction in PAC-SYM score from 2.24 points at baseline to 1.19 points at 24 weeks (Table 4). Secondary outcomes also indicated improvement over time, with PAC-QoL and PAC–SYM scores showing maintained reductions compared with baseline up to the 72-week time point (accepting potential attrition bias). Improvements in total score reflected improved scores across all domains of the instruments. Positive directional effects were observed for nearly all other secondary outcomes, with some quite substantial improvements in measures [e.g. > 25% scalar improvements in psychological measures (e.g. PHQ-9, GAD-7), St Mark’s Incontinence Score and EQ-VAS]. Global patient satisfaction was 2.7 points at 24 weeks (nearest to ‘very satisfied’), although this dropped to 2.2 points (‘moderately satisfied’) at 48 weeks. This result was mirrored in the global patient improvement score (EQ-VAS score 0–100 points between ‘no effect’ and ‘complete cure’), which was 72.2 points at 24 weeks and 56.5 points at 48 weeks.
| Time point | Number completing outcome evaluations | Observed mean score (points) | Estimated change from baseline score (points) | 95% CI for change in score (points) | p-value for change in score (points) |
|---|---|---|---|---|---|
| PAC-QoL | |||||
| Baseline | 26 | 2.63 | – | – | – |
| 12 weeks | 23 | 1.35 | –1.04 | –1.54 to –0.55 | 0.0001 |
| 24 weeks | 19 | 1.26 | –1.09 | –1.76 to –0.41 | 0.0019 |
| 36 weeks | 19 | 1.47 | –0.98 | –1.87 to –0.10 | 0.0296 |
| 48 weeks | 17 | 1.43 | –1.07 | –2.16 to 0.02 | 0.0552 |
| 60 weeks | 9 | 1.22 | –1.26 | –2.56 to 0.05 | 0.0587 |
| 72 weeks | 5 | 1.11 | –1.38 | –2.94 to 0.19 | 0.0840 |
| PAC-SYM | |||||
| Baseline | 26 | 2.24 | – | – | – |
| 12 weeks | 23 | 1.15 | –0.97 | –1.41 to –0.53 | 0.0000 |
| 24 weeks | 18 | 1.19 | –0.92 | –1.52 to –0.32 | 0.0029 |
| 36 weeks | 19 | 1.25 | –1.03 | –1.80 to –0.26 | 0.0094 |
| 48 weeks | 17 | 1.36 | –0.97 | –1.92 to –0.02 | 0.0444 |
| 60 weeks | 9 | 1.19 | –1.16 | –2.28 to –0.03 | 0.0448 |
| 72 weeks | 5 | 0.82 | –1.51 | –2.87 to –0.16 | 0.0289 |
Comparing pre-surgery and post-surgery periods, intervention led to a significant increase in cost (£5012, 95% CI £4446 to £5322), similar to the cost of surgery, and a modest and imprecise increase in QoL (0.043 QALYs, 95% CI –0.005 to 0.093 QALYs). The ICER was £115,512 per QALY. At the NICE threshold of £30,000 per QALY, the probability that surgery is cost-effective within 48 weeks of surgical intervention is 0%. The EVPI (per subject) reflects the opportunity loss of ongoing uncertainty and suggests no requirement for further research.
Laparoscopic ventral mesh rectopexy was shown to be a safe procedure. There were 30 AEs reported by 16 patients, 20 of which were considered to have possible causality related to surgery and none of which had any long-term sequelae. There were five SAEs, of which four were deemed to be related to surgery. Three of these were for postoperative pain, which was entirely to be expected in a proportion of patients; one was for a chest infection and none resulted in long-term patient harm.
Patient experience of lapVMR was, on the whole, positive. Some patients did not find surgery to be the miracle cure that they were looking for and some reported negative experiences in the perioperative period, but for the most part these were not related to the operation itself. Some patients also found benefit from the dietary and behavioural changes that they initiated as a result of advice that they were given as part of the perioperative care package.
The media scrutiny of the use of mesh undoubtedly affected both patient and surgeon perception and willingness to take part in the study. Some centres paused or abandoned lapVMR totally in the light of the mesh scandal, and there was a perception that for others the heightened scrutiny of practice in the protocol also negatively affected recruitment.
Systematic reviews
An aim of the programme was to systematically review outcomes from current surgical approaches to CC, with the aim of integrating new data with old data at the programme conclusion. On this basis, a series of workshops and rounds of Delphi consensus were undertaken, leading to seven major open access publications; these included an introduction and methods paper,135 five reviews of major procedure classes136–140 and a summary paper with European graded practice recommendations covering patient selection, benefits and harms of all procedures. 141 These papers have been cited 102 times in the scientific literature and also referenced in major textbooks and the national press; they represent a unique, contemporary and valuable open access resource for clinicians and researchers worldwide. The review of rectopexy confirmed that lapVMR evidence was confined to 18 studies, all of which contained poor-quality (Oxford Centre for Evidence-Based Medicine grade IV) observational data (case series and poor-quality cohort studies); these data provided the rationale for CapaCiTY trial 3.
Conclusions
Taking together the results from clinical effectiveness, cost-effectiveness and patient experience data, the following conclusions may be drawn while accepting the major caveat of under-recruitment:
-
Included patients had a high symptom burden and long duration of symptoms that had been refractory to previous treatments, including a minimum of bowel habit training by a specialist practitioner. Patients had been thoroughly investigated and, therefore, could be considered both ‘hard to treat’ and ‘carefully selected’ for surgical intervention.
-
Our analysis of clinical effectiveness showed a reduced symptom burden and improved disease-specific QoL. The magnitude of the effect of surgery (estimated reduction of 1.09 points in PAC-QoL at 24 weeks) was greater than the minimum clinically important difference sought by design (mean change 1.0 points) and this change was statistically significant. In addition, significant and clinically important improvements in PAC-SYM score and bowel frequency provided further evidence of the benefit of surgery.
-
The findings of the primary outcome showed a continued improvement for the duration of the study period (estimated 1.38-point reduction in PAC-QoL at 72 weeks), and this finding was supported by a panel of secondary outcome measures, accepting inferential limitations posed by potential attrition bias.
-
Serious under-recruitment limited the scope to conduct economic analysis. To explore the potential cost-effectiveness of lapVMR some assumptions were necessary: (1) the effect of surgery was not affected by allocation (and thus delay to surgery); (2) growth curve analysis is a reliable method to characterise pre-surgery and post-surgery costs and QoL; (3) pre-surgery non-surgery costs and EQ-5D-5L scores are stable, making it possible to construct a single pre-surgery follow-up covering the 48 weeks before surgery; and (4) benefits following surgery are limited to the first 48 weeks following surgery. The weakest of these assumptions might be limiting benefit from surgery to 48 weeks; any future QALY gain and reduced burden from decreased use of the health-care system for symptoms of CC would make surgery more cost-effective. Potential longer-term complications of mesh insertion and removal were not considered, but if emergent would reduce cost-effectiveness.
-
The base case suggests that lapVMR is not cost-effective (p = 0.00 at a WTP threshold of £30,000 per QALY). However, because this finding comes from an observational analysis of a stepped-wedge design, and for the reasons described, it remains vulnerable to bias that might increase or decrease cost-effectiveness.
-
Although some adverse effects were reported, lapVMR was safe and well tolerated overall.
-
Some patients described how their bowel function was better immediately after surgery and had continued success, and these patients noted a better QoL both mentally and physically; others found the benefit to be short-lived or did not feel any change. Pain was a significant issue for some patients. The mesh controversy dominated staff experience.
Reflections on work programme 4
The study was severely hampered by under-recruitment. Difficulties in attracting centres to recruit pre-dated the zenith of the mesh scandal and reflected wide variation in practice across the UK in terms of both patient selection and lapVMR operative technique. The mesh scandal undoubtedly compounded the recruitment problems faced by the study and yet paradoxically emphasised the need for such a study to take place. It was, for instance, very clear that the attention we paid to strict inclusion and exclusion criteria [actually based on guidance from the Pelvic Floor Society (London, UK)134] led to increased scrutiny in many centres where such surgery was being undertaken without rigorous application of these criteria. This regrettably (for the trial but not the patients concerned) led to a rapid revision (manifest as a drop-off) in the number of patients available at several centres for recruitment. Unfortunately, with the evolution of the media storm against placement of pelvic mesh and worldwide class actions against surgeons and manufacturers of mesh, the issue of patient selection has now made its way to the courts. Therefore, although it might be argued that the study design was overzealous in its approach to inclusion and exclusion, the decision of our expert panel to rigidly maintain the selection and procedural standards laid out in the protocol was undoubtedly the right one.
With the media storm blowing up and the Cumberlege report142 in preparation, there was never a time when the results of this trial were more needed, and we were very disappointed by our failure to keep the trial going. This was not for want of trying. With the strong support of the Association of Coloproctology of Great Britain and Ireland, the Pelvic Floor Society wrote on our behalf to NHS England seeking its support for a mandate to surgeons in England and Wales to continue surgery only in the context of research indemnity. This request, if granted, would have helped push recruitment and prevented some hospitals from abandoning the operation, thus denying patients access to lapVMR and the scientific community an answer to the main trial questions.
However, despite these setbacks, the main aim of the trial, namely to determine the effect size of surgery for the first time in a high-quality experimental design, and thus improve on the level IV evidence provided by 18 case series (as outlined in our systematic review),140 was addressed, albeit at a lower than desirable level of statistical power. Being to our knowledge the only high-quality evidence in the field, our results show substantial symptomatic benefit (more than we sought by design) to a cohort of highly selected patients from lapVMR performed to a standardised technique.
Cost-effectiveness analysis was disappointing and, as with all expensive (surgical) interventions, we did not show a cost benefit in the short term (to 48 weeks). Enthusiasts will say that the benefit of mesh placement is economically apparent only in the long term. It is also difficult to demonstrate cost-effectiveness in our multiply comorbid patients whose overall sense of well-being (as measured by the EQ-5D-5L) is only partially affected by improvement in bowel-associated symptoms.
Research recommendations
The current COVID-19 pandemic has created a natural hiatus in the provision of nearly all non-urgent benign surgery. This has coincided with the publication of the Cumberlege report142 and together these provide a ‘pause for thought’ on the future use of lapVMR in patients with CC. Despite the underpowering of our study, it is unlikely that such a trial will be repeated (at least in the UK). Rather, it is likely that enthusiasts will cite the effect size in our study as mirroring that in observational trials, balancing this against the very real risk of harm (notably the small proportion of patients with long-term mesh complications). The reduction in anxiety surrounding the use of mesh as time passes and the production of updated consensus guidance on patient selection and operative technique may make further study in this area feasible.
Work programme 5: synthesis of trial data and conclusions
A stated aim of the programme was to develop an NHS pathway for the management of CC in adults based on data synthesis. Specifically, we stated that ‘findings will be assimilated and synthesised with previous research, and a national working group convened to develop a new treatment pathway for management of CC in adults’. To this end each participant had a unique ID so that they could be followed through each of the trials. Considerable under-recruitment, reflecting the difficulties of recruiting the patient population, severely limited the scope for an integrated analysis or pathway. However, with all the caveats noted, evidence from the CapaCiTY trials supports the value of standard-care habit training and high-volume TAI and questions the value of habit-training with biofeedback, INVEST procedures and low-volume TAI. The findings as they pertain to lapVMR are mixed and lend some support to this procedure in a highly defined population with informed consent.
With these conclusions in mind, Figure 8 provides a revision of the pathway schematic presented in SYNOPSIS. Here, patients receive a standardised intervention with habit training, and only if this fails do they progress to INVEST. Thence, selected patients with the specific diagnosis of dyssynergic defaecation can undergo direct visual biofeedback, and others can undergo TAI, this being initiated by default with a high-volume device. Patients progressing to a MDT meeting for consideration of surgery may be offered lapVMR if they meet the strict selection criteria, but the consent process must include realistic data on the immediate and longer-term benefits (or otherwise) of this procedure, noting that enhanced consent procedures with standardised proformas and patient information leaflets (provided by the Pelvic Floor Society) are already best practice in relation to detailing long-term harms. Surgeons could, for instance, cite a conservative interpretation of our data in relation to reduced bowel symptoms but falling overall satisfaction with surgery over time, as well as little or no improvement in general QoL.
FIGURE 8.
Revised prototype pathways of care informed by results of the CapaCiTY research programme. a, Alarm features excluded and secondary causes treated appropriately; b, in IBS-C, consider antispasmodics or neuromodulators in case constipation improves but abdominal pain persists and is dominant symptom; c, examples of overt prolapse include anterior (stage 3 cystocele), middle (stage 3 rectocele, uterovaginal prolapse) and posterior compartments (grade IV/V intussusception); d, if not performed previously; e, unless patient preference is for low-volume TAI or there are specific contraindications to high-volume TAI; f, may reduce specific symptoms but not have overall effect on QoL; g, common adjuncts include sacrocolpopexy, hysterectomy, transvaginal tape and cystocele repair. IBS-C, constipation-predominant irritable bowel syndrome; PEG, polyethylene glycol[0].
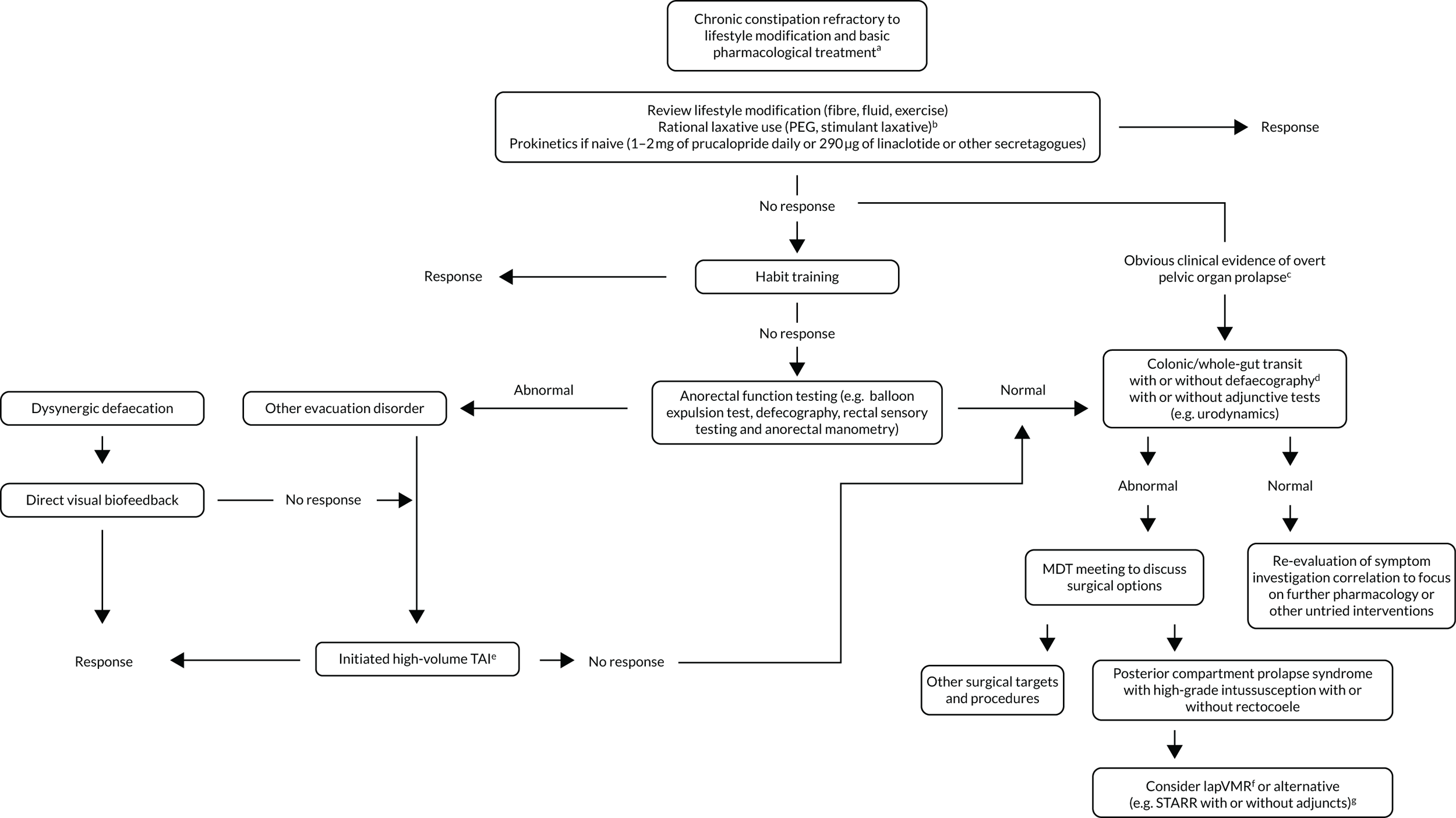
Reflections on the whole research programme and overall conclusions
How will the international community receive such a pathway? We feel that the answer to this in the UK is with general positivity. Over the 6 years that the CapaCiTY research programme has run, from initial meetings at national society conferences to the present, we have witnessed hugely positive engagement from experts in the field through to more general audiences. Our ability to bring together the specialist community, both specialists whose centres recruited and specialists whose centres were unable, has proved a major triumph of the programme. Although this is not measurable in adequately powered clinical-effectiveness data, it can be inferred by how many units have standardised their approach to diagnostics and therapy by education through the programme. Furthermore, conclusions from our systematic reviews have already entered major textbooks and have received national and international platforms for presentation. Dissemination activity is ongoing, and there is no doubt that we will be invited for national and international presentation of the conclusions of the three CapaCiTY trials. These ‘softer’ end points will be the main enduring legacy of the CapaCiTY research programme and the lives of people living with CC will be the better for it.
Lessons learned for future research
The considerable challenges to recruitment for all three trials have been noted. Table 5 outlines some insights about how these problems might be mitigated in the future.
| Problem | Mitigation | Notes |
|---|---|---|
| Design: three concurrent RCTs of complex interventions |
Limit to one or two major RCTs in a programme recommended Alternative design |
Although the team made considerable efforts to support each trial and we had sequential start dates, the dilution of focus and effort was probably a factor, especially with trying to ‘market’ more than one trial per recruitment centre. Disruption to the original planned sequence occurred because of set-up delays. The three-group design of study 1 was certainly overambitious, with INVEST leading to delays in recruitment to any group. With hindsight, an alternative design that incorporates experimental evaluations in a longitudinal cohort of patients would have been more appropriate, such as a TwiCs design. TwiCs designs have become increasingly popular during the period of the research programme, including pragmatic evaluations of complex interventions such as surgery. A TwiCs design would have provided a much greater cohort of patients with longitudinal observations of symptoms, QoL data and data on the effect of ongoing routine clinical care; this would have proved a valuable source of new learning in its own right |
| ‘Fat file’ as a deterrent to patients and local investigators | Reduce outcome frequency and number. Adhere to ‘one trial, one question’ | One problem of clinical programmes is trying to appease everyone’s interests, thus trying to rigorously cover aspects of qualitative and quantitative outcomes relevant to specialist clinical, QoL, health economic and psychological outcomes. This enthusiasm must be tempered with the reality of what is possible |
| Protocol compatibility with routine clinical practice | Increase pragmatism. Avoid or reduce standardisation of complex interventions | We tried (rightly) to answer the ‘important questions’, but the complexity of these led to the need for standardisation. This constrained clinicians to a point where many did not buy in to the agreed protocol. A better balance of standardisation vs. clinician-decisive care might have improved uptake, particularly in the nursing community |
| Choice of trial interventions: attribution of costs and NHS resources | Choose NHS interventions that reduce cost and complexity. Think ‘what research question can we answer’ before ‘what do we want to answer’ | We would advise future investigators to think very carefully about embarking on a RCT that requires complex interventions over and above routine care. The staffing to deliver complex research interventions in the NHS (even pre COVID-19) is fragile and most of our recruitment came care of directly funded research staff. ETCs make this even less tenable. In our experience, CRNs proved unable to resolve issues when individual trusts viewed research as a low priority compared with clinical delivery. Surgery added the additional problem of waiting-time inflexibility |
| Set-up time in multiple centres | Perform pre-trial recruitment feasibility studies rather than relying on site surveys | We grossly underestimated delays in R&D approval processes, with some experienced sites (with a willing principal investigator) taking up to 1 year to open from receiving all study documentation, and this despite intervention by the host CRN on our behalf. Several never opened |
| Failure of early learning from qualitative data | Perform some interviews (e.g. of staff) early in programme | We did not include a process evaluation in our programme but could have used some of the eventual qualitative analysis from staff and patients to troubleshoot some of the recruitment issues that we encountered |
Patient and public involvement
Patient representation in the CapaCiTY research programme supported all research activities. The focus of the CapaCiTY research programme from a PPI perspective was to convene a Constipation Research Advisory Group (CRAG) comprising eight patients and two carers from London, UK, and Durham, UK. This group had geographical diversity (covering both north and south England) and a disease-appropriate demographic (eight female and two male participants). PPI was managed by two co-applicants and the CRAG had an active ‘contributory’ rather than ‘representative’ role. The overarching functions of the CRAG included:
-
managing the research (e.g. steering/advisory group)
-
developing participant information resources
-
advising on protocol revisions (in particular on recruitment and participant burden)
-
disseminating of research findings.
Although CRAG members had patient knowledge of living with severe CC, they did not have the same knowledge of clinical trial and medical research terminology as the research team. Because CRAG members were invited (in rotation) to sit on the Programme Steering Committee (PSC), this put them at a disadvantage. Therefore, training was provided to CRAG members at the start of the project in 2015 that included taking part in an activity in identifying barriers to recruitment in clinical trials and potential solutions. This enabled the CRAG members to review all patient-facing documents including the patient information sheet, patient diaries and patient journals at the beginning of the programme for all three trials. It also enabled them to feel more comfortable when they attended a PSC meeting.
The CRAG was actively involved and effective in providing lay advice and guidance in areas of trial feasibility, design, management, marketing, analysis, recruitment and reporting. Furthermore, CRAG members reviewed all patient-facing material during the substantial amendment in 2016. The CRAG was also consulted about reducing the length of follow-up from 24 to 12 months.
The CRAG reported and made recommendations to the PSC. It was agreed that normally CRAG meetings would be held every 6 months prior to the PSC (ideally 1 month before). The CRAG would feed recommendations back to the PSC. Therefore, the CRAG had a significant role in strategic decision-making. For example, in 2017 the CRAG was provided with a detailed presentation as per the PSC’s recommendation. The PSC asked that the CRAG answer whether or not the CRAG deemed CapaCiTY trial 2 futile owing to under-recruitment. Although it was noted that the inclination of the PSC was to continue, they were keen to consult with the CRAG. The group discussed at length the key areas under consideration, which included the following: was it safe for the patients participating in the trial? Was it cost-effective to continue? Would the research questions be answered?
Though the group accepted that, ideally, more patients should be recruited to improve the accuracy and validity of the data results, it was generally agreed that 100 patients was an acceptable milestone.
The CRAG also discussed financial matters. However, the main focus of the in-depth discussion was whether or not the research questions would be answered. Owing to the smaller sample size it was recognised that the primary research question could be compromised. However, the CRAG recognised the importance of being able to answer many other questions in relation to high-volume and low-volume TAI treatments. The CRAG discussed the value and importance of the secondary data that had been captured to date and data that would continue to be collected if the trial was to continue. It was evident that from a patient perspective these data were of significance. It was noted that the CRAG wished to avoid any negative messaging and in particular did not wish to put off future patients from entering a trial because of the possibility of cancellation.
This consultative approach between the PSC and CRAG, seeking to direct and support the three trials, ensured that both researchers and patients had a mutual understanding of what was required. This ensured that the findings of the three trials would be of benefit to participants in those clinical trials. In addition, three members of the CRAG held a teleconference with Christine Norton and Shiva Taheri to contribute to the interpretation of the interview data and to agree and support the main messages for dissemination.
Overall, patient representation made a major contribution to design, conduct and recruitment of the CapaCiTY research programme. The CRAG was involved in all major decisions that were made during the programme, which meant that patient benefit was always put first in everything we did. The CRAG will continue to support the CapaCiTY research programme in dissemination activities, including at the Bowel Research UK annual Big Bowel Event.
Acknowledgements
The CapaCiTY research programme team gratefully acknowledges the assistance of:
-
Sybil Bannister for her recruitment of patients at Barts Health NHS Trust
-
Susan E Frost (Sales, Marketing and Clinical Director, MacGregor Healthcare Ltd, Tranent, UK) for her help in understanding the use and costing of NHS-prescribed colonic irrigation
-
Melanie Smuk and Vichithranie Madurasinghe for trial statistical support
-
Gian Luca Di Tanna for statistical support to the CapaCiTY trial 3
-
Tiffany Wade for interviews and qualitative analysis.
Programme Steering Committee
Ian McCurrach and Louisa Smalley (PPI representatives) and Daniel Altman and Melanie Smuk (programme statisticians).
Data Monitoring Committee
David Jayne (chairperson), Rupert Pearse (independent clinician) and Neil Corrigan (independent statistician).
Contributions of authors
Charles H Knowles (https://orcid.org/0000-0001-9854-6754) (Professor of Surgery) was chief investigator of the CapaCiTY programme
Lesley Booth (https://orcid.org/0000-0001-5812-4549) (Patient and Bowel Research UK Charity Research Director) was lead for PPI.
Steve R Brown (https://orcid.org/0000-0002-0980-2793) (Professor of Colorectal Surgery) was co-principal investigator for CapaCiTY trial 3.
Samantha Cross (https://orcid.org/0000-0002-0501-0289) (Lecturer in Medical Statistics) conducted statistical analyses and summarised results in tables for all three CapaCiTY trials.
Sandra Eldridge (https://orcid.org/0000-0001-5638-2317) (Professor of Statistics) provided senior oversight of trial design and all statistical analyses.
Christopher Emmett (https://orcid.org/0000-0002-6022-8475) (Clinical Research Fellow, Gastroenterology) assisted with recruitment and provided clinical support for CapaCiTY trial 2.
Ugo Grossi (https://orcid.org/0000-0001-5372-2873) (Clinical Research Fellow, Surgery) assisted with recruitment and provided clinical support for CapaCiTY trials 1 and 3.
Mary Jordan (https://orcid.org/0000-0002-0497-8634) (Senior Research Fellow, Health Economics) conducted the analysis of economic models.
Jon Lacy-Colson (https://orcid.org/0000-0002-5151-8358) (Consultant Colorectal Surgeon) was co-principal investigator for CapaCiTY trial 3.
James Mason (https://orcid.org/0000-0001-9210-4082) (Professor of Health Economics) provided senior oversight of health economic analyses and modelling.
John McLaughlin (https://orcid.org/0000-0001-6158-5135) (Professor of Gastroenterology) served as chairperson of the PSC and contributed to the study write up.
Rona Moss-Morris (https://orcid.org/0000-0002-2927-3446) (Professor of Psychology) conducted analyses and write-up of all psychological outcomes.
Christine Norton (https://orcid.org/0000-0003-2259-0948) (Professor of Nursing) was co-principal investigator for CapaCiTY trial 1 and oversaw all qualitative studies.
S Mark Scott (https://orcid.org/0000-0002-7997-1533) (Senior Health Scientist) was co-principal investigator for CapaCiTY trial 1 and oversaw analysis of all INVEST interventions.
Natasha Stevens (https://orcid.org/0000-0002-3910-7863) (Senior Trials Manager) provided programme management for all three trials (during years 1 and 2) and assisted development of the CapaCiTY research programme.
Shiva Taheri (https://orcid.org/0000-0003-0879-0601) (Trials Manager) provided programme management for all three trials (during years 3–5).
Yan Yiannakou (https://orcid.org/0000-0001-8437-2925) (Professor of Gastroenterology) was principal investigator for CapaCiTY trial 2.
Publications
Original research
Etherson KJ, Horrocks EJ, Scott SM, Knowles CH, Yiannakou Y. A national biofeedback practitioners service evaluation: focus on chronic idiopathic constipation. Frontline Gastroenterol 2017;8:62–7. https://doi.org/10.1136/flgastro-2015-100660
Trial protocols
Emmett C, Close H, Mason J, Taheri S, Stevens N, Eldridge S, et al. Low-volume versus high-volume initiated trans-anal irrigation therapy in adults with chronic constipation: study protocol for a randomised controlled trial. Trials 2017;18:151. https://doi.org/10.1186/s13063-017-1882-y
Norton C, Emmanuel A, Stevens N, Scott SM, Grossi U, Banister S, et al. Habit training versus habit training with direct visual biofeedback in adults with chronic constipation: study protocol for a randomised controlled trial. Trials 2017;18:139. https://doi.org/10.1186/s13063-017-1880-0
Grossi U, Stevens N, McAlees E, Lacy-Colson J, Brown S, Dixon A, et al. Stepped-wedge randomised trial of laparoscopic ventral mesh rectopexy in adults with chronic constipation: study protocol for a randomised controlled trial. Trials 2018;19:90. https://doi.org/10.1186/s13063-018-2456-3
Systematic reviews
Grossi U, Horrocks EJ, Mason J, Knowles CH, Williams AB, NIHR CapaCiTY working group. Surgery for constipation: systematic review and practice recommendations. Results IV: recto-vaginal reinforcement procedures. Colorectal Dis 2017;19(Suppl. 3):73–91. https://doi.org/10.1111/codi.13781
Grossi U, Knowles CH, Mason J, Lacy-Colson J, Brown SR, NIHR CapaCiTY working group. Surgery for constipation: systematic review and practice recommendations. Results II: hitching procedures for the rectum (rectal suspension). Colorectal Dis 2017;19(Suppl. 3):37–48. https://doi.org/10.1111/codi.13773
Knowles CH, Grossi U, Chapman M, Mason J, NIHR CapaCiTY working group, Pelvic Floor Society. Surgery for constipation: systematic review and practice recommendations. Results I: colonic resection. Colorectal Dis 2017;19(Suppl. 3):17–36. https://doi.org/10.1111/codi.13779
Knowles CH, Grossi U, Horrocks EJ, Pares D, Vollebregt PF, Chapman M, et al. Surgery for constipation: systematic review and clinical guidance. Paper 1: introduction & methods. Colorectal Dis 2017;19(Suppl. 3):5–16. https://doi.org/10.1111/codi.13774
Knowles CH, Grossi U, Horrocks EJ, Pares D, Vollebregt PF, Chapman M, et al. Surgery for constipation: systematic review and practice recommendations – graded practice and future research recommendations. Colorectal Dis 2017;19(Suppl. 3):101–13. https://doi.org/10.1111/codi.13775
Mercer-Jones M, Grossi U, Pares D, Vollebregt PF, Mason J, Knowles CH, NIHR CapaCiTY working group. Surgery for constipation: systematic review and practice recommendations. Results III: rectal wall excisional procedures (rectal excision). Colorectal Dis 2017;19(Suppl. 3):49–72. https://doi.org/10.1111/codi.13772
Pilkington SA, Emmett C, Knowles CH, Mason J, Yiannakou Y, NIHR CapaCiTY working group. Surgery for constipation: systematic review and practice recommendations. Results V: sacral nerve stimulation. Colorectal Dis 2017;19(Suppl. 3):92–100. https://doi.org/10.1111/codi.13780
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health and Social Care.
References
- Stewart WF, Liberman JN, Sandler RS, Woods MS, Stemhagen A, Chee E, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol 1999;94:3530-40. https://doi.org/10.1111/j.1572-0241.1999.01642.x.
- van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol 2006;101:2401-9. https://doi.org/10.1111/j.1572-0241.2006.00771.x.
- Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci 1989;34:606-11. https://doi.org/10.1007/BF01536339.
- McCrea GL, Miaskowski C, Stotts NA, Macera L, Paul SM, Varma MG. Gender differences in self-reported constipation characteristics, symptoms, and bowel and dietary habits among patients attending a specialty clinic for constipation. Gend Med 2009;6:259-71. https://doi.org/10.1016/j.genm.2009.04.007.
- Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011;25:3-18. https://doi.org/10.1016/j.bpg.2010.12.010.
- Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum 1989;32:1-8. https://doi.org/10.1007/BF02554713.
- Probert CS, Emmett PM, Heaton KW. Some determinants of whole-gut transit time: a population-based study. QJM 1995;88:311-15.
- Cook IJ, Talley NJ, Benninga MA, Rao SS, Scott SM. Chronic constipation: overview and challenges. Neurogastroenterol Motil 2009;21:1-8. https://doi.org/10.1111/j.1365-2982.2009.01399.x.
- Knowles CH, Scott SM, Rayner C, Glia A, Lindberg G, Kamm MA, et al. Idiopathic slow-transit constipation: an almost exclusively female disorder. Dis Colon Rectum 2003;46:1716-17. https://doi.org/10.1007/BF02660783.
- Wald A, Scarpignato C, Mueller-Lissner S, Kamm MA, Hinkel U, Helfrich I, et al. A multinational survey of prevalence and patterns of laxative use among adults with self-defined constipation. Aliment Pharmacol Ther 2008;28:917-30. https://doi.org/10.1111/j.1365-2036.2008.03806.x.
- Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol 2002;97:1986-93. https://doi.org/10.1111/j.1572-0241.2002.05843.x.
- Shah ND, Chitkara DK, Locke GR, Meek PD, Talley NJ. Ambulatory care for constipation in the United States, 1993-2004. Am J Gastroenterol 2008;103:1746-53.
- Poulton B, Thomas S. The nursing cost of constipation. Primary Health Care 1999;9:17-22. https://doi.org/10.7748/phc.9.9.17.s9.
- NHS Business Services Authority . Prescription Cost Analysis: England n.d. https://nhsbsa-opendata.s3.eu-west-2.amazonaws.com/pca/pca_summary_tables_2020_21_v001.xlsx (accessed 20 August 2021).
- Scott SM, van den Berg MM, Benninga MA. Rectal sensorimotor dysfunction in constipation: best practice and research. Best Pract Res Clin Gastroenterol 2011;25:103-18. https://doi.org/10.1016/j.bpg.2011.01.001.
- National Institute for Health and Care Excellence . Constipation in Children and Young People: Diagnosis and Management n.d. www.nice.org.uk/guidance/cg99 (accessed 3 September 2020).
- Bardisa-Ezcurra L, Ullman R, Gordon J. Guideline Development Group . Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ 2010;340. https://doi.org/10.1136/bmj.c2585.
- Hooban S. NICE’s first guideline on idiopathic childhood constipation aims to standardise practice. Nurs Times 2010;106.
- Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 2008;358:2344-54. https://doi.org/10.1056/NEJMoa0800670.
- Lembo AJ, Johanson JF, Parkman HP, Rao SS, Miner PB, Ueno R. Long-term safety and effectiveness of lubiprostone, a chloride channel (ClC-2) activator, in patients with chronic idiopathic constipation. Dig Dis Sci 2011;56:2639-45. https://doi.org/10.1007/s10620-011-1801-0.
- Lembo AJ, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, et al. Efficacy of linaclotide for patients with chronic constipation. Gastroenterology 2010;138:886-95.e1. https://doi.org/10.1053/j.gastro.2009.12.050.
- Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med 2011;365:527-36. https://doi.org/10.1056/NEJMoa1010863.
- Kamm MA, Dudding TC, Melenhorst J, Jarrett M, Wang Z, Buntzen S, et al. Sacral nerve stimulation for intractable constipation. Gut 2010;59:333-40. https://doi.org/10.1136/gut.2009.187989.
- Maeda Y, Lundby L, Buntzen S, Laurberg S. Sacral nerve stimulation for constipation: suboptimal outcome and adverse events. Dis Colon Rectum 2010;53:995-9. https://doi.org/10.1007/DCR.0b013e3181d64207.
- Knowles CH, Dinning PG, Pescatori M, Rintala R, Rosen H. Surgical management of constipation. Neurogastroenterol Motil 2009;21:62-71. https://doi.org/10.1111/j.1365-2982.2009.01405.x.
- Knowles CH, Thin N, Gill K, Bhan C, Grimmer K, Lunniss PJ, et al. Prospective randomized double-blind study of temporary sacral nerve stimulation in patients with rectal evacuatory dysfunction and rectal hyposensitivity. Ann Surg 2012;255:643-9. https://doi.org/10.1097/SLA.0b013e318247d49f.
- Campbell H, Hotchkiss R, Bradshaw N, Porteous M. Integrated care pathways. BMJ 1998;316. https://doi.org/10.1136/bmj.316.7125.133.
- Vollebregt PF, Burgell RE, Hooper RL, Knowles CH, Scott SM. Clinical impact of rectal hyposensitivity: a cross-sectional study of 2,876 patients with refractory functional constipation. Am J Gastroenterol 2021;116:758-68. https://doi.org/10.14309/ajg.0000000000001039.
- Vollebregt PF, Wiklendt L, Dinning PG, Knowles CH, Scott SM. Coexistent faecal incontinence and constipation: a cross-sectional study of 4027 adults undergoing specialist assessment. EClinicalMedicine 2020;27. https://doi.org/10.1016/j.eclinm.2020.100572.
- American College of Gastroenterology Chronic Constipation Task F . An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100:S1-4. https://doi.org/10.1111/j.1572-0241.2005.50613_1.x.
- Map of Medicine . Constipation in Adults and the Elderly n.d. http://healthguides.mapofmedicine.com/choices/map/constipation_in_adults_and_the_elderly1.html (accessed 3 September 2020).
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability?. Intl Disabil Stud 1988;10:64-7. https://doi.org/10.3109/09638288809164105.
- Palit S, Bhan C, Lunniss PJ, Boyle DJ, Gladman MA, Knowles CH, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal Dis 2014;16:538-46. https://doi.org/10.1111/codi.12595.
- Carrington EV, Heinrich H, Knowles CH, Rao SS, Fox M, Scott SM, et al. Methods of anorectal manometry vary widely in clinical practice: results from an international survey. Neurogastroenterol Moti 2017;29. https://doi.org/10.1111/nmo.13016.
- Mahieu P, Pringot J, Bodart P. Defecography: II. Contribution to the diagnosis of defecation disorders. Gastrointest Radiol 1984;9:253-61. https://doi.org/10.1007/BF01887846.
- Roberts JP, Womack NR, Hallan RI, Thorpe AC, Williams NS. Evidence from dynamic integrated proctography to redefine anismus. Br J Surg 1992;79:1213-15. https://doi.org/10.1002/bjs.1800791140.
- Womack NR, Williams NS, Holmfield JH, Morrison JF, Simpkins KC. New method for the dynamic assessment of anorectal function in constipation. Br J Surg 1985;72:994-8. https://doi.org/10.1002/bjs.1800721221.
- Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association . American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144:211-17. https://doi.org/10.1053/j.gastro.2012.10.029.
- Rao SS, Mudipalli RS, Stessman M, Zimmerman B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol Motil 2004;16:589-96. https://doi.org/10.1111/j.1365-2982.2004.00526.x.
- Ratuapli SK, Bharucha AE, Noelting J, Harvey DM, Zinsmeister AR. Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology 2013;144:314-22.e2. https://doi.org/10.1053/j.gastro.2012.10.049.
- Farthing MJ, Lennard-jones JE. Sensibility of the rectum to distension and the anorectal distension reflex in ulcerative colitis. Gut 1978;19:64-9. https://doi.org/10.1136/gut.19.1.64.
- Jameson JS, Chia YW, Kamm MA, Speakman CT, Chye YH, Henry MM. Effect of age, sex and parity on anorectal function. Br J Surg 1994;81:1689-92. https://doi.org/10.1002/bjs.1800811143.
- Zarate N, Knowles CH, Newell M, Garvie NW, Gladman MA, Lunniss PJ, et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol 2008;103:427-34. https://doi.org/10.1111/j.1572-0241.2007.01675.x.
- Preston DM, Lennard-Jones JE. Anismus in chronic constipation. Dig Dis Sci 1985;30:413-18. https://doi.org/10.1007/BF01318172.
- Barnes PR, Lennard-Jones JE. Balloon expulsion from the rectum in constipation of different types. Gut 1985;26:1049-52. https://doi.org/10.1136/gut.26.10.1049.
- Öncü K, Özel AM, Demırtürk L, Gürbüz AK, Yazgan Y, Kizilkaya E. Determination of the frequency of dyssynergic defecation and patient characteristics in patients with functional constipation. Turk J Gastroenterol 2010;21:372-80. https://doi.org/10.4318/tjg.2010.0123.
- Hinton JM, Lennard-Jones JE. Constipation: definition and classification. Postgrad Med J 1968;44:720-3. https://doi.org/10.1136/pgmj.44.515.720.
- Evans RC, Kamm MA, Hinton JM, Lennard-Jones JE. The normal range and a simple diagram for recording whole gut transit time. Int J Colorectal Dis 1992;7:15-7. https://doi.org/10.1007/BF01647654.
- Mahieu P, Pringot J, Bodart P. Defecography: I. Description of a new procedure and results in normal patients. Gastrointest Radiol 1984;9:247-51. https://doi.org/10.1007/BF01887845.
- Pilkington SA, Nugent KP, Brenner J, Harris S, Clarke A, Lamparelli M, et al. Barium proctography vs magnetic resonance proctography for pelvic floor disorders: a comparative study. Colorectal Dis 2012;14:1224-30. https://doi.org/10.1111/j.1463-1318.2012.02945.x.
- McColl E. Best practice in symptom assessment: a review. Gut 2004;53:iv49-54. https://doi.org/10.1136/gut.2003.034355.
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. BMJ 2002;324:1193-4. https://doi.org/10.1136/bmj.324.7347.1193.
- Verbrugge LM. Health diaries. Med Care 1980;18:73-95. https://doi.org/10.1097/00005650-198001000-00006.
- Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540-51. https://doi.org/10.1080/00365520510012208.
- Cowlam S, Milburn S, McColl E, Yiannakou Y. Preliminary validation of the PAC-QOL questionnaire for chronic idiopathic constipation in patients in the UK. Gastroenterology 2007;132.
- Yiannakou Y, Etherson K, Close H, Kasim A, Mercer-Jones M, Plusa S, et al. A randomized double-blinded sham-controlled cross-over trial of tined-lead sacral nerve stimulation testing for chronic constipation. Eur J Gastroenterol Hepatol 2019;31:653-60. https://doi.org/10.1097/MEG.0000000000001379.
- Dubois D, Gilet H, Viala-Danten M, Tack J. Psychometric performance and clinical meaningfulness of the Patient Assessment of Constipation-Quality of Life questionnaire in prucalopride (RESOLOR) trials for chronic constipation. Neurogastroenterol Motil 2010;22:e54-63. https://doi.org/10.1111/j.1365-2982.2009.01408.x.
- Paterson C. Measuring outcomes in primary care: a patient generated measure, MYMOP, compared with the SF-36 health survey. BMJ 1996;312:1016-20. https://doi.org/10.1136/bmj.312.7037.1016.
- Curtis L. Unit Costs of Health and Social Care 2006. Canterbury: PSSRU, University of Kent; 2006.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiat Ann 2002;32:509-15. https://doi.org/10.3928/0048-5713-20020901-06.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Spence M, Moss-Morris R, Chalder T. The Behavioural Responses to Illness Questionnaire (BRIQ): a new predictive measure of medically unexplained symptoms following acute infection. Psychol Med 2005;35:583-93. https://doi.org/10.1017/s0033291704003484.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- NHS Digital . Prescription Cost Analysis – England, 2018 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed 24 August 2021).
- Office for National Statistics (ONS) . Average Weekly Earnings in Great Britain: February 2020 2020.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- NHS England . NHS Community Pharmacist Consultation Service: Toolkit for Pharmacy Staff 2019. www.england.nhs.uk/publication/nhs-community-pharmacist-consultation-service-toolkit-for-pharmacy-staff (accessed 3 July 2020).
- NHS England . 2018/19/National/Cost/Collection/Publication n.d. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication (accessed 3 July 2020).
- NHS Digital . Prescription Cost Analysis – England, 2018 [PAS] n.d. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed 3 June 2019).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Val Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 3 July 2020).
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Claxton K, Sculpher M, Palmer S, Culyer AJ. Causes for concern: is NICE failing to uphold its responsibilities to all NHS patients?. Health Econ 2015;24:1-7. https://doi.org/10.1002/hec.3130.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002;360:711-15. https://doi.org/10.1016/S0140-6736(02)09832-X.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ 2013;14:367-72. https://doi.org/10.1007/s10198-013-0471-6.
- Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Meth 2006;5. https://doi.org/10.1177/160940690600500107.
- Riley E. NHS surgeon is sacked for performing controversial bowel mesh operations that left dozens of patients in severe pain. Daily Mail 2019.
- Gold SM, Köhler-Forsberg O, Moss-Morris R, Mehnert A, Miranda JJ, Bullinger M, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers 2020;6. https://doi.org/10.1038/s41572-020-0200-2.
- Chiotakakou-Faliakou E, Kamm MA, Roy AJ, Storrie JB, Turner IC. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut 1998;42:517-21. https://doi.org/10.1136/gut.42.4.517.
- Bleijenberg G, Kuijpers HC. Biofeedback treatment of constipation: a comparison of two methods. Am J Gastroenterol 1994;89:1021-6.
- Koutsomanis D, Lennard-Jones JE, Roy AJ, Kamm MA. Controlled randomised trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut 1995;37:95-9. https://doi.org/10.1136/gut.37.1.95.
- Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead WE. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum 2007;50:428-41. https://doi.org/10.1007/s10350-006-0814-9.
- Heymen S, Wexner SD, Vickers D, Nogueras JJ, Weiss EG, Pikarsky AJ. Prospective, randomized trial comparing four biofeedback techniques for patients with constipation. Dis Colon Rectum 1999;42:1388-93. https://doi.org/10.1007/BF02235034.
- Glia A, Gylin M, Gullberg K, Lindberg G. Biofeedback retraining in patients with functional constipation and paradoxical puborectalis contraction: comparison of anal manometry and sphincter electromyography for feedback. Dis Colon Rectum 1997;40:889-95. https://doi.org/10.1007/BF02051194.
- Chiarioni G, Whitehead WE, Pezza V, Morelli A, Bassotti G. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130:657-64. https://doi.org/10.1053/j.gastro.2005.11.014.
- Rao SS. Biofeedback therapy for constipation in adults. Best Pract Res Clin Gastroenterology 2011;25:159-66. https://doi.org/10.1016/j.bpg.2011.01.004.
- Bharucha AE, Pemberton JH, Locke GR. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218-38. https://doi.org/10.1053/j.gastro.2012.10.028.
- Enck P, Van der Voort IR, Klosterhalfen S. Biofeedback therapy in fecal incontinence and constipation. Neurogastroenterol Motil 2009;21:1133-41. https://doi.org/10.1111/j.1365-2982.2009.01345.x.
- Etherson KJ, Horrocks EJ, Scott SM, Knowles CH, Yiannakou Y. A national biofeedback practitioners service evaluation: focus on chronic idiopathic constipation. Frontline Gastroenterol 2017;8:62-7. https://doi.org/10.1136/flgastro-2015-100660.
- Rao SS, Seaton K, Miller M, Brown K, Nygaard I, Stumbo P, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5:331-8. https://doi.org/10.1016/j.cgh.2006.12.023.
- Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129:86-97. https://doi.org/10.1053/j.gastro.2005.05.015.
- Simón MA, Bueno AM. Behavioural treatment of the dyssynergic defecation in chronically constipated elderly patients: a randomized controlled trial. Appl Psychophysiol Biofeedback 2009;34:273-7. https://doi.org/10.1007/s10484-009-9100-7.
- Grossi U, Carrington EV, Bharucha AE, Horrocks EJ, Scott SM, Knowles CH. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut 2016;65:447-55. https://doi.org/10.1136/gutjnl-2014-308835.
- Farid M, El Monem HA, Omar W, El Nakeeb A, Fikry A, Youssef T, et al. Comparative study between biofeedback retraining and botulinum neurotoxin in the treatment of anismus patients. Int J Colorectal Dis 2009;24:115-20. https://doi.org/10.1007/s00384-008-0567-0.
- Lehur PA, Stuto A, Fantoli M, Villani RD, Queralto M, Lazorthes F, et al. Outcomes of stapled transanal rectal resection vs. biofeedback for the treatment of outlet obstruction associated with rectal intussusception and rectocele: a multicenter, randomized, controlled trial. Dis Colon Rectum 2008;51:1611-18. https://doi.org/10.1007/s10350-008-9378-1.
- Woodward S, Norton C, Chiarelli P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database Syst Rev 2014;3. https://doi.org/10.1002/14651858.CD008486.pub2.
- Rome Foundation . Rome IV Criteria n.d. https://theromefoundation.org/rome-iv/rome-iv-criteria/ (accessed 24 August 2021).
- Kamm MA. Clinical case: chronic constipation. Gastroenterology 2006;131:233-9. https://doi.org/10.1053/j.gastro.2006.05.027.
- Rao SS, Ozturk R, Laine L. Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am J Gastroenterol 2005;100:1605-15. https://doi.org/10.1111/j.1572-0241.2005.41845.x.
- Rao SS. Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol Clin North Am 2007;36. https://doi.org/10.1016/j.gtc.2007.07.013.
- Locke GR, Pemberton JH, Phillips SF. American Gastroenterological Association Medical Position Statement: guidelines on constipation. Gastroenterology 2000;119:1761-6. https://doi.org/10.1053/gast.2000.20390.
- Everitt HA, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. Assessing telephone-delivered cognitive-behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut 2019;68:1613-23. https://doi.org/10.1136/gutjnl-2018-317805.
- Everitt HA, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. Cognitive behavioural therapy for irritable bowel syndrome: 24-month follow-up of participants in the ACTIB randomised trial. Lancet Gastroenterol Hepatol 2019;4:863-72. https://doi.org/10.1016/S2468-1253(19)30243-2.
- Carrington EV, Grossi U, Knowles CH, Scott SM. Normal values for high-resolution anorectal manometry: a time for consensus and collaboration. Neurogastroenterol Motil 2014;26:1356-7. https://doi.org/10.1111/nmo.12364.
- Carrington EV, Heinrich H, Knowles CH, Fox M, Rao S, Altomare DF, et al. The international anorectal physiology working group (IAPWG) recommendations: standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil 2020;32. https://doi.org/10.1111/nmo.13679.
- Christensen P, Krogh K, Buntzen S, Payandeh F, Laurberg S. Long-term outcome and safety of transanal irrigation for constipation and fecal incontinence. Dis Colon Rectum 2009;52:286-92. https://doi.org/10.1007/DCR.0b013e3181979341.
- Ausili E, Focarelli B, Tabacco F, Murolo D, Sigismondi M, Gasbarrini A, et al. Transanal irrigation in myelomeningocele children: an alternative, safe and valid approach for neurogenic constipation. Spinal Cord 2010;48:560-5. https://doi.org/10.1038/sc.2009.186.
- Krogh K, Kvitzau B, Jørgensen TM, Laurberg S. Treatment of anal incontinence and constipation with transanal irrigation. Ugeskr Laeger 1999;161:253-6.
- Preziosi G, Gosling J, Raeburn A, Storrie J, Panicker J, Emmanuel A. Transanal irrigation for bowel symptoms in patients with multiple sclerosis. Dis Colon Rectum 2012;55:1066-73. https://doi.org/10.1097/DCR.0b013e3182653bd1.
- Emmanuel A. Review of the efficacy and safety of transanal irrigation for neurogenic bowel dysfunction. Spinal Cord 2010;48:664-73. https://doi.org/10.1038/sc.2010.5.
- Etherson KJ, Minty I, Bain IM, Cundall J, Yiannakou Y. Transanal irrigation for refractory chronic idiopathic constipation: patients perceive a safe and effective therapy. Gastroenterol Res Pract 2017;2017. https://doi.org/10.1155/2017/3826087.
- Morden JP, Lambert PC, Latimer N, Abrams KR, Wailoo AJ. Assessing methods for dealing with treatment switching in randomised controlled trials: a simulation study. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-4.
- Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg 1999;230:627-38. https://doi.org/10.1097/00000658-199911000-00004.
- Lindsey I, Knowles C. Abdominal surgery for chronic constipation. Colorectal Dis 2011;13. https://doi.org/10.1111/j.1463-1318.2011.02836.x.
- D’Hoore A, Cadoni R, Penninckx F. Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg 2004;91:1500-5. https://doi.org/10.1002/bjs.4779.
- Boons P, Collinson R, Cunningham C, Lindsey I. Laparoscopic ventral rectopexy for external rectal prolapse improves constipation and avoids de novo constipation. Colorectal Dis 2010;12:526-32. https://doi.org/10.1111/j.1463-1318.2009.01859.x.
- Wijffels N, Cunningham C, Dixon A, Greenslade G, Lindsey I. Laparoscopic ventral rectopexy for external rectal prolapse is safe and effective in the elderly. Does this make perineal procedures obsolete?. Colorectal Dis 2011;13:561-6. https://doi.org/10.1111/j.1463-1318.2010.02242.x.
- Badrek-Amoudi AH, Roe T, Mabey K, Carter H, Mills A, Dixon AR. Laparoscopic ventral mesh rectopexy in the management of solitary rectal ulcer syndrome: a cause for optimism?. Colorectal Dis 2013;15:575-81. https://doi.org/10.1111/codi.12077.
- Formijne Jonkers HA, Poierrié N, Draaisma WA, Broeders IA, Consten EC. Laparoscopic ventral rectopexy for rectal prolapse and symptomatic rectocele: an analysis of 245 consecutive patients. Colorectal Dis 2013;15:695-9. https://doi.org/10.1111/codi.12113.
- Owais AE, Sumrien H, Mabey K, McCarthy K, Greenslade GL, Dixon AR. Laparoscopic ventral mesh rectopexy in male patients with internal or external rectal prolapse. Colorectal Dis 2014;16:995-1000. https://doi.org/10.1111/codi.12763.
- Portier G, Kirzin S, Cabarrot P, Queralto M, Lazorthes F. The effect of abdominal ventral rectopexy on faecal incontinence and constipation in patients with internal intra-anal rectal intussusception. Colorectal Dis 2011;13:914-17. https://doi.org/10.1111/j.1463-1318.2010.02327.x.
- Slawik S, Soulsby R, Carter H, Payne H, Dixon AR. Laparoscopic ventral rectopexy, posterior colporrhaphy and vaginal sacrocolpopexy for the treatment of recto-genital prolapse and mechanical outlet obstruction. Colorectal Dis 2008;10:138-43.
- Wong M, Meurette G, Abet E, Podevin J, Lehur PA. Safety and efficacy of laparoscopic ventral mesh rectopexy for complex rectocele. Colorectal Dis 2011;13:1019-23. https://doi.org/10.1111/j.1463-1318.2010.02349.x.
- Collinson R, Wijffels N, Cunningham C, Lindsey I. Laparoscopic ventral rectopexy for internal rectal prolapse: short-term functional results. Colorectal Dis 2010;12:97-104. https://doi.org/10.1111/j.1463-1318.2009.02049.x.
- Gosselink MP, Adusumilli S, Harmston C, Wijffels NA, Jones OM, Cunningham C, et al. Impact of slow transit constipation on the outcome of laparoscopic ventral rectopexy for obstructed defaecation associated with high grade internal rectal prolapse. Colorectal Dis 2013;15:e749-56. https://doi.org/10.1111/codi.12443.
- Hidaka J, Elfeki H, Duelund-Jakobsen J, Laurberg S, Lundby L. Functional outcome after laparoscopic posterior sutured rectopexy versus ventral mesh rectopexy for rectal rolapse: six-year follow-up of a double-blind, randomized single-center study. EClinicalMedicine 2019;16:18-22. https://doi.org/10.1016/j.eclinm.2019.08.014.
- El-Dhuwaib Y, Pandyan A, Knowles CH. Epidemiological trends in surgery for rectal prolapse in England 2001–2012: an adult hospital population-based study. Colorectal Dis 2020;22:1359-66. https://doi.org/10.1111/codi.15094.
- Mercer-Jones MA, Brown SR, Knowles CH, Williams AB. Position Statement by The Pelvic Floor Society on behalf of The Association of Coloproctology of Great Britain and Ireland on the use of mesh in ventral mesh rectopexy (VMR). Colorectal Dis 2020;22:1429-35. https://doi.org/10.1111/codi.13893.
- Knowles CH, Grossi U, Horrocks EJ, Pares D, Vollebregt PF, Chapman M, et al. Surgery for constipation: systematic review and clinical guidance. Paper 1: Introduction & Methods. Colorectal Dis 2017;19:5-16. https://doi.org/10.1111/codi.13774.
- Grossi U, Horrocks EJ, Mason J, Knowles CH, Williams AB. NIHR CapaCiTY working group . Surgery for constipation: systematic review and practice recommendations. Results IV: recto-vaginal reinforcement procedures. Colorectal Dis 2017;19:73-91. https://doi.org/10.1111/codi.13781.
- Mercer-Jones M, Grossi U, Pares D, Vollebregt PF, Mason J, Knowles CH. NIHR CapaCiTY working group . Surgery for constipation: systematic review and practice recommendations. Results III: rectal wall excisional procedures (Rectal Excision). Colorectal Dis 2017;19:49-72. https://doi.org/10.1111/codi.13772.
- Knowles CH, Grossi U, Chapman M, Mason J. Pelvic Floor Society . Surgery for constipation: systematic review and practice recommendations. Results I: colonic resection. Colorectal Dis 2017;19:17-36. https://doi.org/10.1111/codi.13779.
- Pilkington SA, Emmett C, Knowles CH, Mason J, Yiannakou Y. NIHR CapaCiTY working group . Surgery for constipation: systematic review and practice recommendations. Results V: sacral nerve stimulation. Colorectal Dis 2017;19:92-100. https://doi.org/10.1111/codi.13780.
- Grossi U, Knowles CH, Mason J, Lacy-Colson J, Brown SR. NIHR CapaCiTY working group . Surgery for constipation: systematic review and practice recommendations. Results II: hitching procedures for the rectum (rectal suspension). Colorectal Dis 2017;19:37-48. https://doi.org/10.1111/codi.13773.
- Knowles CH, Grossi U, Horrocks EJ, Pares D, Vollebregt PF, Chapman M, et al. Surgery for constipation: systematic review and practice recommendations – graded practice and future research recommendations. Colorectal Dis 2017;19:101-13. https://doi.org/10.1111/codi.13775.
- Independent Medicines and Medical Devices Safety Review . First Do No Harm: The Report of the Independent Medicines and Medical Devices Safety Review 2020.
- Baron GR, Briggs AH, Fenwick EAL. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. https://doi.org/10.1111/j.1524-4733.2008.00358.x.
- D’Hoore A, Penninckx F. Laparoscopic ventral recto(colpo)pexy for rectal prolapse: surgical technique and outcome for 109 patients. Surg Endosc 2006;20:1919-23. https://doi.org/10.1007/s00464-005-0485-y.
- Haskell H. Cumberlege review exposes stubborn and dangerous flaws in healthcare. BMJ 2020;370. https://doi.org/10.1136/bmj.m3099.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 1 August 2021).
Appendix 1 Reports of studies not yet published
CapaCiTY trial 1: habit training compared with habit training with direct visual biofeedback in adults with chronic constipation – randomised controlled trial
Intervention
Habit training was provided by a trained specialist (nurse or physiotherapist with clinical experience) who had undertaken a standard 1-day (study-specific) training session. A standardised approach and intervention were provided using an intervention manual (see Appendix 2, CapaCiTY trial 1: intervention protocols), and at least one random observation visit was performed early in the study by lead researchers for quality control.
The course of therapy included three or four sessions (with interval tolerance of every 3–5 weeks). The first and last sessions were always face to face. Sessions were delivered by the same therapist if at all possible and tailored to a participant’s individual needs.
Habit training with biofeedback included the steps of habit training plus direct visual biofeedback using a portable HRAM manometry catheter connected to a computer monitor. Calibration, validation and maintenance of the equipment were built in to the programme and training manuals were provided. Training on the standardised system and protocol was performed and documented in the investigator site file prior to sites commencing treatment. The outcomes of each session, including the ability to expel the balloon, generate propulsion, increase rectal pressure, relax the anal canal and sense the balloon at lower or higher volumes (relevant to hyposensate and hypersensate patients) over successive sessions were recorded on an intervention CRF.
Trial design features
Basic design
A parallel, three-group, randomised trial design permitted two randomised comparisons: an overall evaluation of the performance of a panel of INVEST in improving the selection of treatment and an evaluation of treatment options (HT vs. HTBF) without INVEST. Thus, the overall evaluation addressed whether or not INVEST-guided care led to more favourable outcomes than randomised allocation.
Sample size calculation
Sample size was calculated using the primary clinical outcome, change in PAC-QoL score. A 10% scale difference or 0.4-point reduction in PAC-QoL score with a variance estimate conservatively set at SD = 1 was considered clinically relevant. To detect a mean change of 0.4 points in PAC-QoL score (SD = 1) with 90% power and 5% significance level, 132 participants per group or 264 participants in total was required for the comparison of HT and HTBF (no-INVEST group).
For the secondary comparison of INVEST and no INVEST, a reduction of 0.4 points (SD = 1) was also considered clinically meaningful. To detect an effect size of 0.4 points with 90% power and 5% significance level required 90 participants in the INVEST group, assuming 264 participants were recruited to the no-INVEST group.
Allowing for 10% loss to follow-up, a sample size of 147 participants was needed in both the HT and HTBF groups and 100 participants in the INVEST group. A total sample size of 394 participants across the three groups was required.
Randomisation procedure
Randomisation (with an allocation ratio of 3 : 3 : 2 to HT, HTBF and INVEST, respectively) occurred at one point in time following recruitment (after eligibility and baseline assessments). Randomisation was stratified by sex (and female participants by centre) with (block size 8) randomisation implemented via an online randomisation system developed by the PCTU. Randomisation was conducted by suitably trained and delegated researchers at recruiting sites and followed PCTU-validated standard operating procedures for the study.
Blinding
Patients and clinicians were necessarily aware of both INVEST and treatment allocations. To minimise bias, where possible, a blinded researcher collected outcome data. If a blinded researcher was not available, the participant completed the questionnaires and placed them in a sealed envelope. Participants were trained in completing questionnaires prior to randomisation and received a visual aid with standardised script and training for completing questionnaires. Quantitative (but not qualitative) outcome assessments were analysed by investigators and statisticians who were blind to allocation status and index intervention.
Concomitant medications
It was inevitable that participants would seek recourse to laxatives and other dietary supplements during the course of the programme. Experience shows that complete prohibition can lead to unreported laxative use, which might confound findings. Although we strongly discouraged ad libitum medication and specified a defined breakthrough regimen, we also recorded (by diary) cotreatment with sufficient fidelity and integrity to enable use as covariates in analyses.
Statistical methods
The primary outcome was analysed on an ITT basis at the 6-month time point. Descriptive statistics (i.e. mean, SD, median and IQR) are presented by trial group. The average reduction in PAC-QoL score was analysed using a linear mixed regression model. The two stratification variables (sex and study site) were included in the model, with site as a random effect and sex as a fixed-effect covariate. Other fixed-effect covariates included in the model were intervention, baseline PAC-QoL score and breakthrough medication (i.e. use of oral and/or rectal laxatives).
Secondary outcomes were also analysed on an ITT basis and presented as descriptive statistics by trial group: continuous variables (e.g. PAC-QoL score) were summarised by treatment group (using mean, SD, median and IQR). For categorical variables, numbers and percentages of patients reporting each response option were presented by trial group. Given the much smaller than target number of patients recruited to the trial, it was agreed that adjusted analysis would be performed on secondary outcomes 1 to 3 (i.e. PAC-QoL score) only, with unadjusted treatment differences and 95% CIs for other secondary outcomes. Results obtained using the CC-BRQ and BIPQ-CC have been omitted pending further analysis.
For cost-effectiveness, patients were randomised in parallel trial groups to (1) HT, (2) HTBF or (3) INVEST-recommended treatment using (1) or (2). The three-way cost-effectiveness comparison required the presentation of the cost-effectiveness acceptability frontier (CEAF) in addition to CEACs for each trial group, as the CEAF correctly identifies the optimal decision across the range of WTP thresholds when more than two options are being considered. 143 For this trial, the baseline (0 months) was set after completion of treatment, at the beginning of 12 months of follow-up. Treatment began an average of 3 months before this baseline. Consequently, the duration of follow-up is 15 months, and discounting of costs and QALYs (r = 0.035) has been applied to the last 3 months.
Recruitment
Recruitment started on 26 March 2015 (first intervention 21 May 2015) and ended 30 June 2018. A total of 182 patients (target 394 patients) were randomised out of 502 patients screened (36.3%) from 10 sites. Reasons for screen failure are shown in Figure 9. Two sites opened but failed to recruit; the remainder randomised between 7 and 71 patients. A total of 182 patients were randomised: 68 to HT, 68 to HTBF and 46 to INVEST-guided therapy (HT or HTBF based on results). A total of 79 participants dropped out of the study before the primary end point, leaving 103 participants at 6 months. The CONSORT flow diagram is shown in Figure 2.
FIGURE 9.
The CapaCiTY trial 1 screen failures. SNS, sacral nerve stimulation.
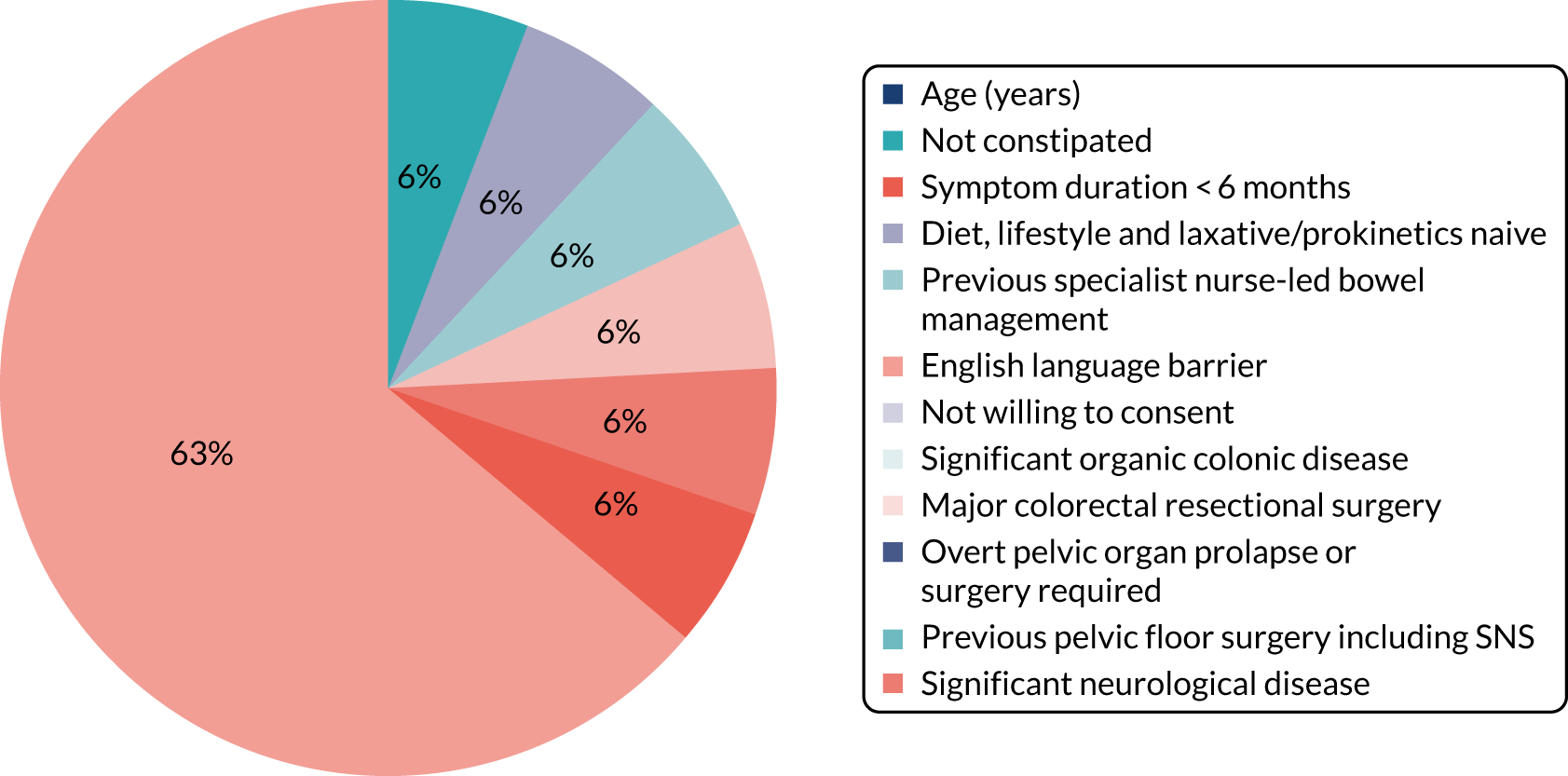
Baseline characteristics
Table 6 shows the numbers and percentages of patients with baseline characteristics presented by trial group. Continuous variables (e.g. age) were summarised by treatment group using mean and SD (and median and IQR if non-normally distributed). Table 7 shows the data for outcome measures at baseline. These were comparable between three trial groups for all major characteristics.
| Characteristic | HT (N = 68) | HTBF (N = 68) | No INVESTa (N = 136) | INVEST (N = 46) | Total (N = 182) |
|---|---|---|---|---|---|
| Referral method, n (%) | |||||
| Primary care | 18 (26.5) | 8 (11.8) | 26 (19.1) | 11 (23.9) | 37 (20.3) |
| Secondary care | 24 (35.3) | 32 (47.1) | 56 (41.2) | 16 (34.8) | 72 (39.6) |
| Tertiary care | 14 (20.6) | 15 (22.1) | 29 (21.3) | 10 (21.7) | 39 (21.4) |
| Other | 11 (16.2) | 12 (17.6) | 23 (16.9) | 8 (17.4) | 31 (17.0) |
| Missing | 1 (1.5) | 1 (1.5) | 2 (1.5) | 1 (2.2) | 3 (1.6) |
| Demographic characteristic | |||||
| Sex, n (%) | |||||
| Male | 8 (11.8) | 8 (11.8) | 16 (11.8) | 6 (13.0) | 22 (12.1) |
| Female | 60 (88.2) | 59 (86.8) | 119 (87.5) | 39 (84.8) | 158 (86.8) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Ethnicity, n (%) | |||||
| Asian | 11 (16.2) | 10 (14.7) | 21 (15.4) | 5 (10.9) | 26 (14.3) |
| Black | 7 (10.3) | 8 (11.8) | 15 (11.0) | 5 (10.9) | 20 (11.0) |
| Mixed | 1 (1.5) | 0 (0.0) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| White | 48 (70.6) | 49 (72.1) | 97 (71.3) | 33 (71.7) | 130 (71.4) |
| Other | 1 (1.5) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Age (years) | |||||
| Mean (SD) | 45.2 (13.6) | 45.7 (15.6) | 45.4 (14.6) | 43.6 (12.2) | 45.0 (14.0) |
| Median (IQR) | 47.5 (34.5–57.0) | 44.0 (31.0–62.0) | 46.0 (33.0–57.0) | 42.0 (32.0–53.0) | 44.5 (33.0–57.0) |
| Missing, n (%) | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Past medical history, n (%) | |||||
| Total | 50 (73.5) | 52 (76.5) | 102 (75.0) | 35 (76.1) | 137 (75.3) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Cardiovascular condition | 13 (19.1) | 10 (14.7) | 23 (16.9) | 7 (15.2) | 30 (16.5) |
| Heart disease | 1 (1.5) | 2 (2.9) | 3 (2.2) | 0 (0.0) | 3 (1.6) |
| Hypertension | 9 (13.2) | 6 (8.8) | 15 (11.0) | 4 (8.7) | 19 (10.4) |
| Hypercholesterolaemia | 8 (11.8) | 7 (10.3) | 15 (11.0) | 4 (8.7) | 19 (10.4) |
| Other | 3 (4.4) | 1 (1.5) | 4 (2.9) | 2 (4.3) | 6 (3.3) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Respiratory condition | 15 (22.1) | 12 (17.6) | 27 (19.9) | 9 (19.6) | 36 (19.8) |
| Asthma | 13 (19.1) | 11 (16.2) | 24 (17.6) | 9 (19.6) | 33 (18.1) |
| COPD | 5 (7.4) | 2 (2.9) | 7 (5.1) | 0 (0.0) | 7 (3.8) |
| Other | 2 (2.9) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 2 (1.1) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Gastrointestinal condition | 23 (33.8) | 19 (27.9) | 42 (30.9) | 20 (43.5) | 62 (34.1) |
| IBS | 11 (16.2) | 14 (20.6) | 25 (18.4) | 16 (34.8) | 41 (22.5) |
| Crohn’s disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ulcerative colitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cancer | 1 (1.5) | 0 (0.0) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Colonic polyps | 7 (10.3) | 2 (2.9) | 9 (6.6) | 4 (8.7) | 13 (7.1) |
| Other | 8 (11.8) | 5 (7.4) | 13 (9.6) | 5 (10.9) | 18 (9.9) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Metabolic condition | 9 (13.2) | 8 (11.8) | 17 (12.5) | 7 (15.2) | 24 (13.2) |
| Diabetes | 5 (7.4) | 1 (1.5) | 6 (4.4) | 1 (2.2) | 7 (3.8) |
| Hypothyroidism | 2 (2.9) | 6 (8.8) | 8 (5.9) | 4 (8.7) | 12 (6.6) |
| Hyperthyroidism | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Other | 2 (2.9) | 0 (0.0) | 2 (1.5) | 1 (2.2) | 3 (1.6) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Haematological condition | 3 (4.4) | 4 (5.9) | 7 (5.1) | 3 (6.5) | 10 (5.5) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Hepatic condition | 1 (1.5) | 1 (1.5) | 2 (1.5) | 0 (0.0) | 2 (1.1) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Renal disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1 (0.5) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Genito-urinary condition | 10 (14.7) | 12 (17.6) | 22 (16.2) | 4 (8.7) | 26 (14.3) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Neurological/CNS condition | 14 (20.6) | 14 (20.6) | 28 (20.6) | 6 (13.0) | 34 (18.7) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Psychiatric condition | 15 (22.1) | 10 (14.7) | 25 (18.4) | 12 (26.1) | 37 (20.3) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Dermatological condition | 12 (17.6) | 11 (16.2) | 23 (16.9) | 5 (10.9) | 28 (15.4) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Musculoskeletal condition | 6 (8.8) | 12 (17.6) | 18 (13.2) | 5 (10.9) | 23 (12.6) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Other condition | 11 (16.2) | 16 (23.5) | 27 (19.9) | 9 (19.6) | 36 (19.8) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Past surgical history, n (%) | |||||
| Total | 36 (52.9) | 35 (51.5) | 71 (52.2) | 22 (47.8) | 93 (51.1) |
| Abdominal operation | 13 (19.1) | 11 (16.2) | 24 (17.6) | 7 (15.2) | 31 (17.0) |
| Gynaecological procedure | 23 (33.8) | 24 (35.3) | 47 (34.6) | 17 (37.0) | 64 (35.2) |
| Proctological or perineal procedure | 10 (14.7) | 8 (11.8) | 18 (13.2) | 6 (13.0) | 24 (13.2) |
| Neuromodulation | 1 (1.5) | 1 (1.5) | 2 (1.5) | 0 (0.0) | 2 (1.1) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Duration (months) of constipation symptoms | |||||
| Mean (SD) | 72.1 (51.9) | 76.8 (56.3) | 74.5 (54.0) | 75.7 (55.2) | 74.8 (54.1) |
| Missing, n (%) | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Previous lifestyle modifications, n (%) | |||||
| Total | 67 (98.5) | 67 (98.5) | 134 (98.5) | 45 (97.8) | 179 (98.4) |
| Diet | 67 (98.5) | 65 (95.6) | 132 (97.1) | 44 (95.7) | 176 (96.7) |
| Fluid | 62 (91.2) | 64 (94.1) | 126 (92.6) | 41 (89.1) | 167 (91.8) |
| Exercise | 54 (79.4) | 58 (85.3) | 112 (82.4) | 39 (84.8) | 151 (83.0) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Alternative therapies, n (%) | |||||
| Total | 7 (10.3) | 1 (1.5) | 8 (5.9) | 1 (2.2) | 9 (4.9) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Laxatives, n (%) | |||||
| Total | 67 (98.5) | 65 (95.6) | 132 (97.1) | 45 (97.8) | 177 (97.3) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Antidiarrhoeals, n (%) | |||||
| Total | 3 (4.4) | 4 (5.9) | 7 (5.1) | 2 (4.3) | 9 (4.9) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Prokinetics, n (%) | |||||
| Total | 9 (13.2) | 15 (22.1) | 24 (17.6) | 7 (15.2) | 31 (17.0) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Nurse-led bowel management, n (%) | |||||
| Total | 12 (17.6) | 11 (16.2) | 23 (16.9) | 12 (26.1) | 35 (19.2) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Family history of bowel disease, n (%) | |||||
| IBS | 15 (22.1) | 11 (16.2) | 26 (19.1) | 10 (21.7) | 36 (19.8) |
| IBD | 1 (1.5) | 3 (4.4) | 4 (2.9) | 5 (10.9) | 9 (4.9) |
| Gastrointestinal cancer | 3 (4.4) | 7 (10.3) | 10 (7.4) | 4 (8.7) | 14 (7.7) |
| Other | 2 (2.9) | 5 (7.4) | 7 (5.1) | 2 (4.3) | 9 (4.9) |
| Missing | 1 (1.5) | 2 (2.9) | 3 (2.2) | 2 (4.3) | 5 (2.7) |
| Sexual history (female participants only), n (%) | |||||
| Sexually active | 43 (71.7) | 40 (67.8) | 83 (69.7) | 29 (74.4) | 112 (70.9) |
| Child-bearing potential | 38 (63.3) | 32 (54.2) | 70 (58.8) | 25 (64.1) | 95 (60.1) |
| > 1 year post menopausal | 21 (35.0) | 24 (40.7) | 45 (37.8) | 9 (23.1) | 54 (34.2) |
| Surgically sterile | 13 (21.7) | 13 (22.0) | 26 (21.8) | 8 (20.5) | 34 (21.5) |
| Contraceptive use (female participants only), n (%) | |||||
| Total | 25 (41.7) | 19 (32.2) | 44 (37.0) | 18 (46.2) | 62 (39.2) |
| Barrier | 10 (16.7) | 8 (13.6) | 18 (15.1) | 6 (15.4) | 24 (15.2) |
| Non-barrier | 15 (25.0) | 12 (20.3) | 27 (22.7) | 12 (30.8) | 39 (24.7) |
| Missing | 2 (3.3) | 4 (6.8) | 6 (5.0) | 5 (12.8) | 11 (7.0) |
| Past obstetric history (female participants only) | |||||
| Total, n (%) | 38 (63.3) | 37 (62.7) | 75 (63.0) | 27 (69.2) | 102 (64.6) |
| Number of vaginal deliveries, mean (SD) | 1.9 (1.4) | 1.8 (1.3) | 1.8 (1.4) | 1.7 (1.3) | 1.8 (1.4) |
| Number of caesareans, mean (SD) | 0.2 (0.5) | 0.4 (0.6) | 0.3 (0.6) | 0.4 (0.8) | 0.3 (0.6) |
| Number of forceps/ventouse deliveries, mean (SD) | 0.1 (0.3) | 0.2 (0.4) | 0.2 (0.4) | 0.1 (0.6) | 0.1 (0.4) |
| Number of episiotomies, mean (SD) | 0.5 (0.8) | 0.4 (0.8) | 0.4 (0.8) | 0.6 (0.7) | 0.5 (0.8) |
| Number of obstetric tears, mean (SD) | 0.6 (0.8) | 0.5 (0.7) | 0.5 (0.8) | 0.8 (0.9) | 0.6 (0.8) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Faecal incontinence symptoms, n (%) | |||||
| Total | 38 (55.9) | 33 (48.5) | 71 (52.2) | 29 (63.0) | 100 (54.9) |
| Faecal urgency | 30 (44.1) | 20 (29.4) | 50 (36.8) | 19 (41.3) | 69 (37.9) |
| Urge faecal incontinence | 14 (20.6) | 9 (13.2) | 23 (16.9) | 11 (23.9) | 34 (18.7) |
| Passive faecal incontinence | 9 (13.2) | 10 (14.7) | 19 (14.0) | 10 (21.7) | 29 (15.9) |
| Postdefaecation leakage | 10 (14.7) | 10 (14.7) | 20 (14.7) | 15 (32.6) | 35 (19.2) |
| Difficulty wiping clean | 24 (35.3) | 16 (23.5) | 40 (29.4) | 24 (52.2) | 64 (35.2) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Pelvic organ prolapse symptoms, n (%) | |||||
| Total | 13 (19.1) | 14 (20.6) | 27 (19.9) | 14 (30.4) | 41 (22.5) |
| Vaginal bulging | 11 (16.2) | 14 (20.6) | 25 (18.4) | 13 (28.3) | 38 (20.9) |
| External rectal prolapse | 1 (1.5) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.5) |
| External uterine prolapse | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Urinary symptoms, n (%) | |||||
| Total | 26 (38.2) | 17 (25.0) | 43 (31.6) | 16 (34.8) | 59 (32.4) |
| Urinary incontinence | 19 (27.9) | 7 (10.3) | 26 (19.1) | 14 (30.4) | 40 (22.0) |
| Urinary urgency | 16 (23.5) | 13 (19.1) | 29 (21.3) | 13 (28.3) | 42 (23.1) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 1 (2.2) | 2 (1.1) |
| Joint hypermobility,b n (%) | |||||
| Total | 17 (25.0) | 15 (22.1) | 32 (23.5) | 12 (26.1) | 44 (24.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Outcome measure | HT (N = 68) | HTBF (N = 68) | No INVESTa (N = 136) | INVEST (N = 46) | Total (N = 182) |
|---|---|---|---|---|---|
| PAC-QoL score (points) | |||||
| Overall, mean (SD) | 2.3 (0.7) | 2.4 (0.9) | 2.4 (0.8) | 2.5 (0.8) | 2.4 (0.8) |
| Missing, n (%) | 0 (0.0) | 2 (2.9) | 2 (1.5) | 2 (4.3) | 4 (2.2) |
| Dissatisfaction, mean (SD) | 3.1 (0.6) | 3.2 (0.8) | 3.1 (0.7) | 3.3 (0.7) | 3.2 (0.7) |
| Missing, n (%) | 0 (0.0) | 2 (2.9) | 2 (1.5) | 2 (4.3) | 4 (2.2) |
| Physical discomfort, mean (SD) | 2.5 (0.8) | 2.4 (1.0) | 2.4 (0.9) | 2.6 (0.8) | 2.5 (0.9) |
| Missing, n (%) | 0 (0.0) | 2 (2.9) | 2 (1.5) | 2 (4.3) | 4 (2.2) |
| Psychosocial discomfort, mean (SD) | 1.8 (1.0) | 1.8 (1.0) | 1.8 (1.0) | 2.0 (1.0) | 1.8 (1.0) |
| Missing, n (%) | 0 (0.0) | 2 (2.9) | 2 (1.5) | 2 (4.3) | 4 (2.2) |
| Worries and concerns, mean (SD) | 2.3 (1.0) | 2.4 (1.0) | 2.4 (1.0) | 2.6 (0.9) | 2.4 (1.0) |
| Missing, n (%) | 0 (0.0) | 2 (2.9) | 2 (1.5) | 2 (4.3) | 4 (2.2) |
| PAC-SYM score (points) | |||||
| Overall, mean (SD) | 2.1 (0.8) | 2.0 (0.8) | 2.1 (0.8) | 2.2 (0.8) | 2.1 (0.8) |
| Missing, n (%) | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Stool symptoms, mean (SD) | 2.5 (1.0) | 2.4 (0.9) | 2.4 (0.9) | 2.6 (0.9) | 2.5 (0.9) |
| Missing, n (%) | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Abdominal symptoms, mean (SD) | 2.2 (1.0) | 2.0 (1.0) | 2.1 (1.0) | 2.2 (1.1) | 2.1 (1.0) |
| Missing, n (%) | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Rectal symptoms, mean (SD) | 1.4 (1.1) | 1.5 (1.1) | 1.5 (1.1) | 1.7 (1.2) | 1.5 (1.1) |
| Missing, n (%) | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Bowel frequency, number reported over 14 days (diary data) | |||||
| Attempts to empty bowels, mean (SD) | 26.1 (19.8) | 24.9 (16.4) | 25.5 (18.1) | 26.3 (15.1) | 25.7 (17.4) |
| Missing, n (%) | 11 (16.2) | 14 (20.6) | 25 (18.4) | 12 (26.1) | 37 (20.3) |
| Times stool was actually passed, mean (SD) | 14.8 (10.2) | 14.7 (9.9) | 14.8 (10.0) | 14.3 (8.5) | 14.7 (9.7) |
| Missing, n (%) | 11 (16.2) | 14 (20.6) | 25 (18.4) | 11 (23.9) | 36 (19.8) |
| Nature of bowel movement, number of days out of 14 (diary data) | |||||
| Laxatives used, mean (SD) | 4.4 (5.4) | 3.3 (5.0) | 3.9 (5.2) | 5.9 (6.1) | 4.3 (5.5) |
| Missing, n (%) | 12 (17.6) | 14 (20.6) | 26 (19.1) | 12 (26.1) | 38 (20.9) |
| Glycerine suppositories used, mean (SD) | 0.8 (2.6) | 0.7 (1.7) | 0.7 (2.2) | 1.1 (2.8) | 0.8 (2.4) |
| Missing, n (%) | 12 (17.6) | 14 (20.6) | 26 (19.1) | 13 (28.3) | 39 (21.4) |
| EQ-5D-5L: problems indicated, n (%) | |||||
| Mobility | 16 (23.5) | 21 (31.3) | 37 (27.4) | 11 (25.0) | 48 (26.8) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Self-care | 8 (11.8) | 5 (7.5) | 13 (9.6) | 6 (13.6) | 19 (10.6) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Usual activities | 20 (29.4) | 24 (36.4) | 44 (32.8) | 23 (52.3) | 67 (37.6) |
| Missing | 0 (0.0) | 2 (2.9) | 2 (1.5) | 2 (4.3) | 4 (2.2) |
| Pain/discomfort | 56 (82.4) | 55 (82.1) | 111 (82.2) | 39 (88.6) | 150 (83.8) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| Anxiety/depression | 42 (61.8) | 33 (49.3) | 75 (55.6) | 28 (63.6) | 103 (57.5) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| EQ-VAS score (points) | |||||
| Total, mean (SD) | 67.1 (20.8) | 66.6 (18.6) | 66.8 (19.7) | 61.9 (18.2) | 65.6 (19.4) |
| Missing, n (%) | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| PHQ-9 depression severity, n (%) | |||||
| None | 25 (36.8) | 26 (38.8) | 51 (37.8) | 10 (22.7) | 61 (34.1) |
| Mild | 22 (32.4) | 20 (29.9) | 42 (31.1) | 17 (38.6) | 59 (33.0) |
| Moderate | 11 (16.2) | 11 (16.4) | 22 (16.3) | 11 (25.0) | 33 (18.4) |
| Moderately severe | 4 (5.9) | 7 (10.4) | 11 (8.1) | 3 (6.8) | 14 (7.8) |
| Severe | 6 (8.8) | 3 (4.5) | 9 (6.7) | 3 (6.8) | 12 (6.7) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
| GAD-7 anxiety severity, n (%) | |||||
| None | 29 (42.6) | 28 (41.8) | 57 (42.2) | 17 (38.6) | 74 (41.3) |
| Mild | 21 (30.9) | 16 (23.9) | 37 (27.4) | 8 (18.2) | 45 (25.1) |
| Moderate | 7 (10.3) | 13 (19.4) | 20 (14.8) | 10 (22.7) | 30 (16.8) |
| Severe | 11 (16.2) | 10 (14.9) | 21 (15.6) | 9 (20.5) | 30 (16.8) |
| Missing | 0 (0.0) | 1 (1.5) | 1 (0.7) | 2 (4.3) | 3 (1.6) |
Results: clinical effectiveness
Primary outcome
Table 8 shows the results for the primary outcome.
| Intervention | Mean score (points) | Treatment differencea (95% CI) | p-value | |
|---|---|---|---|---|
| Baseline (SD) | 6 months (SD) | |||
| HT vs. HTBF | ||||
| HT (n = 38) | 2.26 (0.69) | 1.49 (0.85) | Reference | |
| HTBF (n = 30) | 2.41 (0.81) | 1.65 (1.03) | –0.03 (–0.33 to 0.27) | 0.8445 |
| No INVEST vs. INVEST | ||||
| No INVEST (n = 68) | 2.33 (0.74) | 1.56 (0.93) | Reference | |
| INVEST (n = 22) | 2.36 (0.78) | 1.81 (1.03) | 0.22 (–0.11 to 0.55) | 0.1871 |
Secondary outcomes
Table 9 shows changes in PAC-QoL score analysed as binary variables (adjusted analyses) and Table 10 shows data for individual PAC-QoL domains. All other secondary outcomes are shown in Tables 11 and 12. Figure 10 provides a summary of the similar decrease in PAC-QoL score in all intervention groups.
| Intervention | PAC-QoL score (points), mean (SD) | Patients with ≥ 0.4-point reduction, n (%) | OR for ≥ 0.4-point reductiona (95% CI) | Patients with ≥ 1.0-point reduction, n (%) | OR for ≥ 1.0-point reductiona (95% CI) | |
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| 3 months | ||||||
| HT (N = 43) | 2.3 (0.7) | 1.7 (0.8) | 26 (60.5) | Reference | 15 (34.9) | Reference |
| HTBF (N = 34) | 2.3 (0.9) | 1.7 (1.0) | 18 (52.9) | 0.74 (0.3 to 1.9) | 11 (32.4) | 0.94 (0.3 to 2.7) |
| No INVEST (N = 77) | 2.3 (0.8) | 1.7 (0.9) | 44 (57.1) | Reference | 15 (34.9) | Reference |
| INVEST (N = 26) | 2.5 (0.8) | 1.9 (1.0) | 15 (57.7) | 0.95 (0.4 to 2.4) | 11 (32.4) | 0.34 (0.1 to 1.1) |
| 6 months | ||||||
| HT (N = 38) | 2.3 (0.7) | 1.5 (0.8) | 26 (68.4) | Reference | 14 (36.8) | Reference |
| HTBF (N = 30) | 2.4 (0.8) | 1.7 (1.0) | 21 (70.0) | 1.09 (0.4 to 3.2) | 10 (33.3) | 0.91 (0.3 to 2.8) |
| No INVEST (N = 68) | 2.3 (0.7) | 1.6 (0.9) | 47 (69.1) | Reference | 14 (36.8) | Reference |
| INVEST (N = 22) | 2.4 (0.8) | 1.8 (1.0) | 12 (54.5) | 0.48 (0.2 to 1.4) | 10 (33.3) | 0.66 (0.2 to 1.9) |
| 12 months | ||||||
| HT (N = 29) | 2.2 (0.7) | 1.5 (0.8) | 16 (55.2) | Reference | 9 (31.0) | Reference |
| HTBF (N = 23) | 2.2 (0.9) | 1.4 (1.2) | 16 (69.6) | 1.68 (0.5 to 5.6) | 7 (30.4) | 0.84 (0.2 to 3.0) |
| No INVEST (N = 52) | 2.2 (0.8) | 1.4 (1.0) | 32 (61.5) | Reference | 9 (31.0) | Reference |
| INVEST (N = 13) | 2.4 (0.8) | 1.7 (0.9) | 9 (69.2) | 1.60 (0.4 to 6.3) | 7 (30.4) | 1.37 (0.3 to 6.5) |
| Intervention | 3 months | 6 months | 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD)a | Follow-up, mean (SD) | Treatment differenceb (95% CI) | n | Baseline, mean (SD)a | Follow-up, mean (SD) | Treatment differenceb (95% CI) | n | Baseline, mean (SD)a | Follow-up, mean (SD) | Treatment differenceb (95% CI) | |
| Overall score (points) | ||||||||||||
| HT | 43 | 2.3 (0.1) | 1.7 (0.1) | Reference | 38 | 2.3 (0.1) | 1.5 (0.1) | Reference | 29 | 2.2 (0.1) | 1.5 (0.1) | Reference |
| HTBF | 34 | 2.3 (0.2) | 1.7 (0.1) | –0.02 (–0.3 to 0.3) | 30 | 2.4 (0.1) | 1.7 (0.1) | –0.03 (–0.3 to 0.3) | 23 | 2.2 (0.2) | 1.4 (0.2) | –0.01 (–0.4 to 0.4) |
| No INVEST | 77 | 2.3 (0.1) | 1.7 (0.1) | Reference | 68 | 2.3 (0.1) | 1.6 (0.1) | Reference | 52 | 2.2 (0.1) | 1.4 (0.1) | Reference |
| INVEST | 26 | 2.5 (0.2) | 1.9 (0.1) | 0.09 (–0.2 to 0.4) | 22 | 2.4 (0.2) | 1.8 (0.1) | 0.22 (–0.1 to 0.5) | 13 | 2.4 (0.2) | 1.7 (0.2) | –0.02 (–0.4 to 0.4) |
| Dissatisfaction score (points) | ||||||||||||
| HT | 43 | 3.1 (0.1) | 2.1 (0.1) | Reference | 38 | 3.1 (0.1) | 2.2 (0.0) | Reference | 29 | 3.1 (0.1) | 2.2 (0.0) | Reference |
| HTBF | 34 | 3.1 (0.1) | 2.2 (0.1) | 0.17 (–0.3 to 0.6) | 30 | 3.3 (0.1) | 2.3 (0.1) | –0.04 (–0.5 to 0.4) | 22 | 3.2 (0.1) | 2.0 (0.1) | –0.17 (–0.7 to 0.3) |
| No INVEST | 77 | 3.1 (0.1) | 2.2 (0.0) | Reference | 68 | 3.2 (0.1) | 2.2 (0.1) | Reference | 51 | 3.2 (0.1) | 2.1 (0.1) | Reference |
| INVEST | 26 | 3.4 (0.1) | 2.4 (0.1) | 0.09 (–0.3 to 0.5) | 22 | 3.3 (0.1) | 2.3 (0.1) | 0.04 (–0.4 to 0.5) | 13 | 3.2 (0.2) | 2.0 (0.2) | –0.31 (–0.9 to 0.2) |
| Physical discomfort score (points) | ||||||||||||
| HT | 43 | 2.5 (0.1) | 1.8 (0.1) | Reference | 38 | 2.5 (0.1) | 1.7 (0.1) | Reference | 29 | 2.4 (0.1) | 1.6 (0.1) | Reference |
| HTBF | 34 | 2.3 (0.2) | 1.8 (0.2) | 0.10 (–0.3 to 0.5) | 30 | 2.4 (0.2) | 1.7 (0.2) | 0.06 (–0.3 to 0.5) | 23 | 2.2 (0.2) | 1.5 (0.2) | 0.14 (–0.4 to 0.7) |
| No INVEST | 77 | 2.4 (0.1) | 1.8 (0.1) | Reference | 68 | 2.5 (0.1) | 1.7 (0.1) | Reference | 52 | 2.3 (0.1) | 1.6 (0.1) | Reference |
| INVEST | 26 | 2.5 (0.2) | 2.1 (0.1) | 0.14 (–0.2 to 0.5) | 22 | 2.4 (0.2) | 1.9 (0.2) | 0.27 (–0.1 to 0.7) | 13 | 2.3 (0.3) | 1.8 (0.2) | 0.10 (–0.4 to 0.6) |
| Psychosocial discomfort score (points) | ||||||||||||
| HT | 43 | 1.8 (0.1) | 1.3 (0.1) | Reference | 38 | 1.7 (0.2) | 1.1 (0.1) | Reference | 29 | 1.5 (0.2) | 1.1 (0.1) | Reference |
| HTBF | 34 | 1.8 (0.2) | 1.3 (0.2) | –0.05 (–0.4 to 0.3) | 30 | 1.9 (0.2) | 1.4 (0.1) | 0.07 (–0.3 to 0.4) | 23 | 1.8 (0.2) | 1.1 (0.2) | –0.17 (–0.6 to 0.2) |
| No INVEST | 77 | 1.8 (0.1) | 1.3 (0.1) | Reference | 68 | 1.8 (0.1) | 1.2 (0.1) | Reference | 52 | 1.6 (0.1) | 1.1 (0.1) | Reference |
| INVEST | 26 | 1.9 (0.2) | 1.6 (0.2) | 0.21 (–0.1 to 0.6) | 22 | 1.7 (0.2) | 1.4 (0.2) | 0.24 (–0.1 to 0.6) | 13 | 1.9 (0.3) | 1.3 (0.3) | 0.02 (–0.4 to 0.5) |
| Worries and concerns score (points) | ||||||||||||
| HT | 43 | 2.3 (0.1) | 1.7 (0.1) | Reference | 38 | 2.2 (0.2) | 1.4 (0.1) | Reference | 29 | 2.3 (0.2) | 1.3 (0.1) | Reference |
| HTBF | 34 | 2.3 (0.2) | 1.6 (0.1) | –0.08 (–0.4 to 0.3) | 30 | 2.4 (0.2) | 1.6 (0.2) | –0.01 (–0.4 to 0.4) | 23 | 2.2 (0.2) | 1.4 (0.2) | 0.15 (–0.3 to 0.6) |
| No INVEST | 77 | 2.3 (0.1) | 1.7 (0.1) | Reference | 68 | 2.3 (0.1) | 1.5 (0.1) | Reference | 52 | 2.2 (0.1) | 1.4 (0.1) | Reference |
| INVEST | 26 | 2.5 (0.2) | 2.0 (0.2) | 0.08 (–0.3 to 0.4) | 22 | 2.4 (0.2) | 1.9 (0.2) | 0.33 (–0.1 to 0.7) | 13 | 2.4 (0.3) | 1.7 (0.2) | 0.12 (–0.4 to 0.6) |
| Continuous outcome | 3 months | 6 months | 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Follow-up, mean (SD) | Treatment difference (95% CI) | n | Baseline, mean (SD) | Follow-up, mean (SD) | Treatment difference (95% CI) | n | Baseline, mean (SD) | Follow-up, mean (SD) | Treatment difference (95% CI) | |
| PAC-SYM score (points) | ||||||||||||
| Overall | ||||||||||||
| HT | 43 | 2.2 (0.8) | 1.6 (0.8) | Reference | 38 | 2.2 (0.8) | 1.5 (0.8) | Reference | 29 | 2.0 (0.8) | 1.3 (0.7) | Reference |
| HTBF | 34 | 2.1 (0.9) | 1.7 (1.1) | –0.1 (–0.5 to 0.3) | 30 | 2.1 (0.8) | 1.5 (1.1) | –0.1 (–0.5 to 0.3) | 23 | 2.1 (0.9) | 1.3 (1.1) | 0.0 (–0.5 to 0.5) |
| No INVEST | 77 | 2.2 (0.8) | 1.6 (0.9) | Reference | 68 | 2.1 (0.8) | 1.5 (0.9) | Reference | 52 | 2.1 (0.8) | 1.3 (0.9) | Reference |
| INVEST | 26 | 2.3 (0.9) | 1.7 (0.9) | –0.1 (–0.5 to 0.3) | 22 | 2.2 (0.9) | 1.7 (0.8) | –0.2 (–0.6 to 0.2) | 13 | 2.0 (0.9) | 1.4 (0.8) | –0.1 (–0.6 to 0.4) |
| Stool symptoms | ||||||||||||
| HT | 43 | 2.6 (0.9) | 1.8 (0.9) | Reference | 38 | 2.5 (0.9) | 1.8 (1.0) | Reference | 29 | 2.5 (0.7) | 1.5 (0.7) | Reference |
| HTBF | 34 | 2.5 (0.8) | 1.8 (1.2) | –0.0 (–0.5 to 0.5) | 30 | 2.5 (0.9) | 1.7 (1.3) | 0.1 (–0.4 to 0.6) | 23 | 2.5 (0.9) | 1.4 (1.2) | 0.1 (–0.4 to 0.6) |
| No INVEST | 77 | 2.6 (0.9) | 1.8 (1.0) | Reference | 68 | 2.5 (0.9) | 1.8 (1.1) | Reference | 52 | 2.5 (0.8) | 1.5 (0.9) | Reference |
| INVEST | 26 | 2.7 (1.0) | 2.0 (1.1) | –0.2 (–0.7 to 0.3) | 22 | 2.6 (1.0) | 2.0 (1.0) | –0.3 (–0.8 to 0.2) | 13 | 2.4 (1.2) | 1.6 (0.8) | –0.1 (–0.7 to 0.5) |
| Abdominal symptoms | ||||||||||||
| HT | 43 | 2.2 (1.0) | 1.6 (1.1) | Reference | 38 | 2.2 (0.9) | 1.3 (0.9) | Reference | 29 | 2.0 (1.0) | 1.4 (1.0) | Reference |
| HTBF | 34 | 2.0 (1.1) | 1.7 (1.1) | –0.1 (–0.6 to 0.4) | 30 | 2.0 (1.0) | 1.5 (1.2) | –0.2 (–0.7 to 0.3) | 23 | 1.9 (1.1) | 1.3 (1.3) | 0.1 (–0.6 to 0.8) |
| No INVEST | 77 | 2.1 (1.0) | 1.6 (1.1) | Reference | 68 | 2.1 (1.0) | 1.4 (1.0) | Reference | 52 | 2.0 (1.0) | 1.4 (1.1) | Reference |
| INVEST | 26 | 2.2 (1.0) | 1.9 (1.0) | –0.2 (–0.7 to 0.3) | 22 | 2.0 (1.1) | 1.7 (1.0) | –0.3 (–0.8 to 0.2) | 13 | 1.7 (0.9) | 1.3 (1.0) | 0.0 (–0.7 to 0.7) |
| Rectal symptoms | ||||||||||||
| HT | 42 | 1.5 (1.1) | 1.2 (0.9) | Reference | 37 | 1.5 (1.1) | 1.0 (0.8) | Reference | 29 | 1.3 (1.0) | 0.7 (0.7) | Reference |
| HTBF | 34 | 1.6 (1.2) | 1.3 (1.3) | –0.1 (–0.6 to 0.4) | 30 | 1.6 (1.1) | 1.2 (1.2) | –0.2 (–0.7 to 0.3) | 23 | 1.5 (1.1) | 0.9 (1.0) | –0.1 (–0.6 to 0.4) |
| No INVEST | 76 | 1.5 (1.2) | 1.2 (1.1) | Reference | 67 | 1.5 (1.1) | 1.1 (1.0) | Reference | 52 | 1.4 (1.0) | 0.8 (0.8) | Reference |
| INVEST | 26 | 1.8 (1.2) | 1.2 (1.0) | 0.0 (–0.5 to 0.5) | 22 | 1.7 (1.3) | 1.1 (0.9) | 0.0 (–0.5 to 0.5) | 13 | 1.7 (1.1) | 1.1 (1.0) | –0.3 (–0.8 to 0.2) |
| Diary data | ||||||||||||
| Bowel frequency: mean number of attempts to empty bowels over 2 weeks | ||||||||||||
| HT | 42 | 27.9 (22.4) | 22.4 (11.9) | Reference | 34 | 29.0 (24.1) | 21.8 (11.0) | Reference | 25 | 31.6 (26.1) | 24.2 (12.7) | Reference |
| HTBF | 33 | 23.4 (12.6) | 21.7 (12.9) | 0.7 (–5.0 to 6.4) | 29 | 26.6 (18.1) | 22.6 (13.8) | –0.7 (–7.0 to 5.6) | 22 | 27.4 (19.7) | 24.5 (15.0) | –0.3 (–8.4 to 7.8) |
| No INVEST | 75 | 25.9 (18.7) | 22.1 (12.3) | Reference | 63 | 27.9 (21.4) | 22.2 (12.3) | Reference | 47 | 29.6 (23.2) | 24.4 (13.7) | Reference |
| INVEST | 26 | 29.9 (15.0) | 26.3 (13.6) | –4.2 (–9.9 to 1.5) | 22 | 28.4 (15.6) | 23.0 (11.5) | –0.8 (–6.8 to 5.2) | 12 | 24.4 (12.4) | 23.0 (11.2) | 1.4 (–7.2 to 10.0) |
| Bowel frequency: mean number of times stool was passed over 2 weeks | ||||||||||||
| HT | 42 | 15.1 (10.9) | 15.9 (10.8) | Reference | 34 | 15.8 (11.7) | 14.2 (8.3) | Reference | 25 | 16.2 (11.9) | 17.6 (11.1) | Reference |
| HTBF | 33 | 14.8 (7.6) | 16.5 (10.8) | –0.6 (–5.6 to 4.4) | 28 | 14.8 (8.9) | 15.4 (11.3) | –1.2 (–6.2 to 3.8) | 22 | 16.2 (9.1) | 18.6 (13.0) | –1.0 (–8.1 to 6.1) |
| No INVEST | 75 | 14.9 (9.5) | 16.1 (10.7) | Reference | 62 | 15.3 (10.4) | 14.8 (9.7) | Reference | 47 | 16.2 (10.6) | 18.1 (11.9) | Reference |
| INVEST | 26 | 15.0 (9.7) | 16.7 (10.9) | –0.5 (–5.4 to 4.4) | 22 | 13.8 (7.7) | 14.3 (8.2) | 0.5 (–4.1 to 5.1) | 12 | 15.2 (11.4) | 17.0 (10.7) | 1.1 (–6.4 to 8.6) |
| Nature of bowel movement: mean number of days (out of 14) laxatives used | ||||||||||||
| HT | 42 | 4.3 (5.2) | 1.0 (3.0) | Reference | 34 | 3.6 (4.5) | 0.5 (1.6) | Reference | 25 | 3.8 (4.9) | 1.3 (2.5) | Reference |
| HTBF | 33 | 3.6 (5.0) | 1.0 (3.1) | –0.0 (–1.4 to 1.4) | 29 | 3.5 (4.8) | 1.0 (2.7) | –0.4 (–1.5 to 0.7) | 22 | 3.6 (5.0) | 0.9 (2.2) | 0.4 (–1.0 to 1.8) |
| No INVEST | 75 | 4.0 (5.1) | 1.0 (3.0) | Reference | 63 | 3.5 (4.6) | 0.7 (2.2) | Reference | 47 | 3.7 (4.9) | 1.1 (2.3) | Reference |
| INVEST | 26 | 6.2 (6.0) | 1.7 (4.1) | –0.6 (–2.1 to 0.9) | 22 | 6.2 (6.1) | 0.6 (3.0) | 0.1 (–1.1 to 1.3) | 12 | 5.1 (5.9) | 2.6 (5.4) | –1.5 (–3.5 to 0.5) |
| Nature of bowel movement: mean number of days (out of 14) glycerine suppositories used | ||||||||||||
| HT | 42 | 0.8 (2.5) | 1.1 (2.3) | Reference | 34 | 0.2 (0.7) | 0.8 (2.0) | Reference | 25 | 0.1 (0.3) | 0.5 (1.1) | Reference |
| HTBF | 32 | 0.5 (1.5) | 0.9 (2.3) | 0.2 (–0.9 to 1.3) | 29 | 0.9 (2.1) | 1.2 (2.8) | –0.4 (–1.6 to 0.8) | 22 | 0.6 (1.9) | 0.1 (0.3) | 0.4 (–0.1 to 0.9) |
| No INVEST | 74 | 0.7 (2.1) | 1.0 (2.3) | Reference | 63 | 0.5 (1.5) | 1.0 (2.4) | Reference | 47 | 0.4 (1.3) | 0.3 (0.9) | Reference |
| INVEST | 26 | 1.2 (3.3) | 0.8 (2.0) | 0.2 (–0.8 to 1.2) | 22 | 1.3 (3.6) | 1.2 (3.1) | –0.3 (–1.6 to 1.0) | 12 | 1.3 (4.2) | 2.2 (4.0) | –1.8 (–3.0,–0.6) |
| EQ-VAS score (points) | ||||||||||||
| HT | 43 | 68.4 (21.0) | 71.0 (21.6) | Reference | 39 | 69.9 (19.7) | 69.0 (23.8) | Reference | 29 | 69.7 (18.1) | 73.8 (20.2) | Reference |
| HTBF | 34 | 66.7 (20.1) | 71.2 (21.1) | –0.2 (–10.0 to 9.6) | 29 | 66.1 (20.1) | 71.0 (20.1) | –2.0 (–12.9 to 8.9) | 23 | 68.8 (17.4) | 74.7 (18.8) | –0.9 (–11.9 to 10.1) |
| No INVEST | 77 | 67.6 (20.5) | 71.1 (21.2) | Reference | 68 | 68.3 (19.8) | 69.8 (22.1) | Reference | 52 | 69.3 (17.6) | 74.2 (19.4) | Reference |
| INVEST | 26 | 65.3 (17.4) | 66.8 (17.6) | 4.3 (–4.9 to 13.5) | 22 | 68.1 (16.6) | 71.1 (17.3) | –1.3 (–11.6 to 9.0) | 13 | 71.5 (17.4) | 71.2 (19.9) | 3.0 (–9.1 to 15.1) |
| PHQ-9 score (points) | ||||||||||||
| HT | 43 | 8.4 (7.0) | 7.8 (7.1) | Reference | 38 | 7.5 (6.5) | 7.7 (7.4) | Reference | 29 | 6.7 (6.8) | 7.2 (6.5) | Reference |
| HTBF | 34 | 6.8 (6.4) | 6.4 (6.2) | 1.4 (–1.7 to 4.5) | 30 | 6.8 (6.4) | 7.2 (6.9) | 0.5 (–3.0 to 4.0) | 23 | 6.4 (6.8) | 5.9 (7.6) | 1.3 (–2.6 to 5.2) |
| No INVEST | 77 | 7.7 (6.8) | 7.2 (6.7) | Reference | 68 | 7.2 (6.4) | 7.5 (7.1) | Reference | 52 | 6.6 (6.8) | 6.6 (6.9) | Reference |
| INVEST | 26 | 10.0 (6.0) | 9.5 (7.4) | –2.3 (–5.4 to 0.8) | 22 | 8.8 (4.8) | 8.8 (5.6) | –1.4 (–4.7 to 1.9) | 13 | 8.2 (7.0) | 8.2 (7.1) | –1.6 (–5.9 to 2.7) |
| GAD-7 score (points) | ||||||||||||
| HT | 43 | 7.4 (6.8) | 7.1 (6.9) | Reference | 38 | 6.9 (6.4) | 5.8 (6.3) | Reference | 29 | 6.4 (6.4) | 6.5 (6.5) | Reference |
| HTBF | 34 | 7.6 (6.5) | 6.6 (6.1) | 0.6 (–2.4 to 3.6) | 30 | 7.0 (6.4) | 6.4 (6.0) | –0.6 (–3.6 to 2.4) | 23 | 6.2 (6.0) | 5.1 (6.3) | 1.4 (–2.2 to 5.0) |
| No INVEST | 77 | 7.5 (6.6) | 6.9 (6.5) | Reference | 68 | 7.0 (6.4) | 6.1 (6.1) | Reference | 52 | 6.3 (6.2) | 5.9 (6.4) | Reference |
| INVEST | 26 | 8.8 (6.5) | 9.2 (6.5) | –2.3 (–5.2 to 0.6) | 22 | 8.4 (6.0) | 8.7 (6.4) | –2.6 (–5.6 to 0.4) | 13 | 6.8 (6.1) | 7.4 (7.1) | –1.5 (–5.6 to 2.6) |
| Global patient satisfaction score (points) | ||||||||||||
| HT | 42 | – | 2.5 (1.0) | Reference | 37 | – | 2.8 (0.9) | Reference | 29 | – | 2.7 (0.8) | Reference |
| HTBF | 32 | – | 2.4 (1.1) | 0.1 (–0.4 to 0.6) | 29 | – | 2.3 (1.3) | 0.4 (–0.1 to 0.9) | 23 | – | 2.5 (1.5) | 0.2 (–0.5 to 0.9) |
| No INVEST | 74 | – | 2.5 (1.0) | Reference | 66 | – | 2.6 (1.1) | Reference | 52 | – | 2.6 (1.2) | Reference |
| INVEST | 25 | – | 2.3 (1.4) | 0.2 (–0.3 to 0.7) | 21 | – | 2.5 (1.1) | 0.1 (–0.4 to 0.6) | 13 | – | 2.6 (1.1) | –0.0 (–0.7 to 0.7) |
| Global patient improvement score (points) | ||||||||||||
| HT | 43 | – | 61.5 (27.8) | Reference | 38 | – | 65.8 (23.1) | Reference | 29 | – | 69.8 (23.2) | Reference |
| HTBF | 34 | – | 44.9 (34.3) | 16.7 (2.6 to 30.8) | 28 | – | 52.7 (35.2) | 13.0 (–1.4 to 27.4) | 23 | – | 59.8 (38.0) | 10.0 (–7.2 to 27.2) |
| No INVEST | 77 | – | 54.2 (31.7) | Reference | 66 | – | 60.2 (29.4) | Reference | 52 | – | 65.4 (30.7) | Reference |
| INVEST | 26 | – | 45.0 (30.8) | 9.2 (–5.0 to 23.4) | 22 | – | 45.7 (30.2) | 14.5 (0.0 to 29.0) | 13 | – | 62.2 (32.3) | 3.2 (–16.0 to 22.4) |
| Binary outcome | N | Indicating problems at baseline, n (%) | 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicating problems at follow-up, n (%) | Difference in proportions (95% CI) | N | Indicating problems at baseline, n (%) | Indicating problems at follow-up, n (%) | Difference in proportions (95% CI) | N | Indicating problems at baseline, n (%) | Indicating problems at follow-up, n (%) | Difference in proportions (95% CI) | |||
| Mobility | ||||||||||||
| HT | 43 | 14 (32.6) | 12 (27.9) | Reference | 38 | 11 (28.9) | 12 (31.6) | Reference | 29 | 7 (24.1) | 5 (17.2) | Reference |
| HTBF | 34 | 9 (26.5) | 12 (35.3) | –0.1 (–0.3 to 0.1) | 30 | 9 (30.0) | 12 (40.0) | –0.1 (–0.3 to 0.1) | 23 | 6 (26.1) | 8 (34.8) | –0.2 (–0.4 to 0.1) |
| No INVEST | 77 | 23 (29.9) | 24 (31.2) | Reference | 68 | 20 (29.4) | 24 (35.3) | Reference | 52 | 13 (25.0) | 13 (25.0) | Reference |
| INVEST | 26 | 9 (34.6) | 8 (30.8) | 0.0 (–0.2 to 0.2) | 22 | 8 (36.4) | 7 (31.8) | 0.0 (–0.2 to 0.3) | 13 | 5 (38.5) | 4 (30.8) | –0.1 (–0.3 to 0.2) |
| Self-care | ||||||||||||
| HT | 43 | 8 (18.6) | 8 (18.6) | Reference | 38 | 4 (10.5) | 4 (10.5) | Reference | 29 | 2 (6.9) | 4 (13.8) | Reference |
| HTBF | 34 | 4 (11.8) | 7 (20.6) | –0.0 (–0.2 to 0.2) | 29 | 4 (13.8) | 5 (17.2) | –0.1 (–0.2 to 0.1) | 23 | 4 (17.4) | 3 (13.0) | 0.0 (–0.2 to 0.2) |
| No INVEST | 77 | 12 (15.6) | 15 (19.5) | Reference | 67 | 8 (11.9) | 9 (13.4) | Reference | 52 | 6 (11.5) | 7 (13.5) | Reference |
| INVEST | 26 | 5 (19.2) | 4 (15.4) | 0.0 (–0.1 to 0.2) | 22 | 4 (18.2) | 3 (13.6) | –0.0 (–0.2 to 0.2) | 13 | 1 (7.7) | 0 (0.0) | 0.1 (0.0 to 0.2) |
| Usual activities | ||||||||||||
| HT | 43 | 14 (32.6) | 20 (46.5) | Reference | 38 | 10 (26.3) | 15 (39.5) | Reference | 29 | 6 (20.7) | 8 (27.6) | Reference |
| HTBF | 34 | 14 (41.2) | 15 (44.1) | 0.0 (–0.2 to 0.2) | 30 | 13 (43.3) | 11 (36.7) | 0.0 (–0.2 to 0.3) | 22 | 8 (36.4) | 9 (40.9) | –0.1 (–0.4 to 0.1) |
| No INVEST | 77 | 28 (36.4) | 35 (45.5) | Reference | 68 | 23 (33.8) | 26 (38.2) | Reference | 51 | 14 (27.5) | 17 (33.3) | Reference |
| INVEST | 26 | 11 (42.3) | 14 (53.8) | –0.1 (–0.3 to 0.1) | 22 | 8 (36.4) | 8 (36.4) | 0.0 (–0.2 to 0.3) | 13 | 6 (46.2) | 3 (23.1) | 0.1 (–0.2 to 0.4) |
| Pain/discomfort | ||||||||||||
| HT | 43 | 37 (86.0) | 32 (74.4) | Reference | 38 | 33 (86.8) | 27 (71.1) | Reference | 29 | 25 (86.2) | 20 (69.0) | Reference |
| HTBF | 34 | 28 (82.4) | 27 (79.4) | –0.0 (–0.2 to 0.1) | 30 | 25 (83.3) | 24 (80.0) | –0.1 (–0.3 to 0.1) | 23 | 18 (78.3) | 13 (56.5) | 0.1 (–0.1 to 0.4) |
| No INVEST | 77 | 65 (84.4) | 59 (76.6) | Reference | 68 | 58 (85.3) | 51 (75.0) | Reference | 52 | 43 (82.7) | 33 (63.5) | Reference |
| INVEST | 26 | 24 (92.3) | 22 (84.6) | –0.1 (–0.2 to 0.1) | 22 | 19 (86.4) | 17 (77.3) | –0.0 (–0.2 to 0.2) | 13 | 12 (92.3) | 12 (92.3) | –0.3 (–0.5 to –0.1) |
| Anxiety/depression | ||||||||||||
| HT | 43 | 26 (60.5) | 24 (55.8) | Reference | 38 | 23 (60.5) | 20 (52.6) | Reference | 29 | 18 (62.1) | 16 (55.2) | Reference |
| HTBF | 33 | 15 (45.5) | 14 (42.4) | 0.1 (–0.1 to 0.4) | 30 | 12 (40.0) | 14 (46.7) | 0.1 (–0.2 to 0.3) | 23 | 8 (34.8) | 11 (47.8) | 0.1 (–0.2 to 0.3) |
| No INVEST | 76 | 41 (53.9) | 38 (50.0) | Reference | 68 | 35 (51.5) | 34 (50.0) | Reference | 52 | 26 (50.0) | 27 (51.9) | Reference |
| INVEST | 26 | 17 (65.4) | 17 (65.4) | –0.2 (–0.4 to 0.1) | 22 | 15 (68.2) | 16 (72.7) | –0.2 (–0.4 to –0.0) | 13 | 8 (61.5) | 8 (61.5) | –0.1 (–0.4 to 0.2) |
FIGURE 10.
Average PAC-QoL score over time in months for (a) all participants (n = 178); and (b) those with no missing data (n = 54). Both show reductions in score (i.e. improvement) over time.
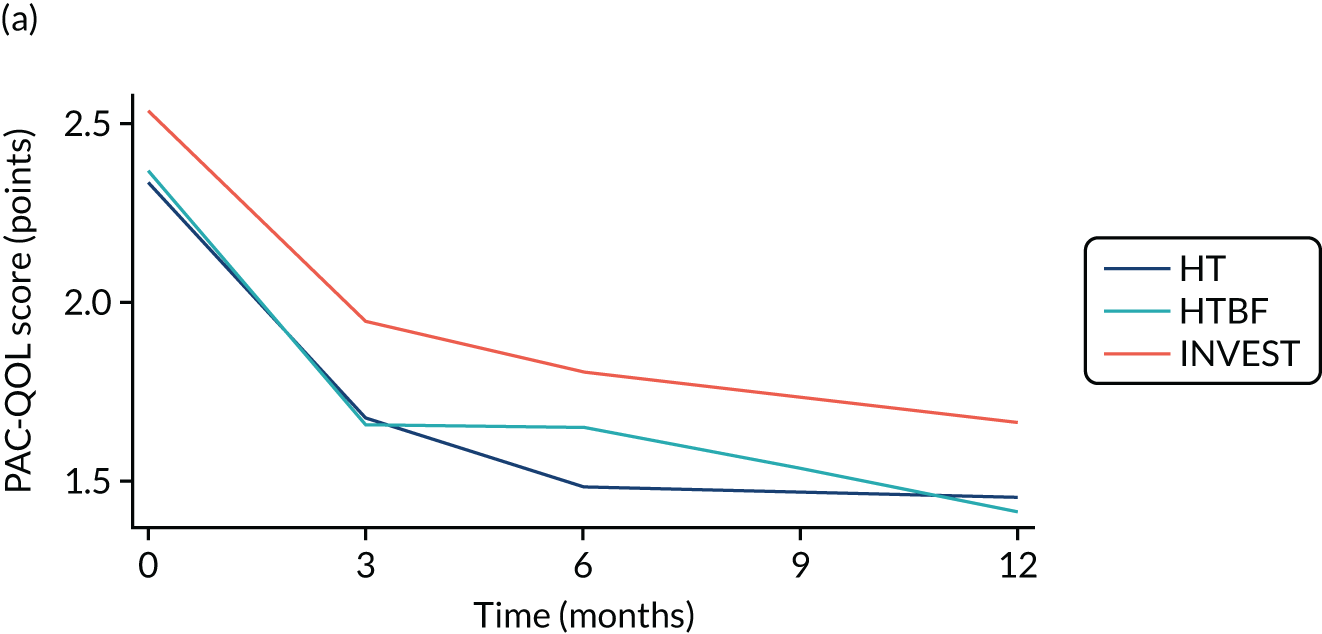
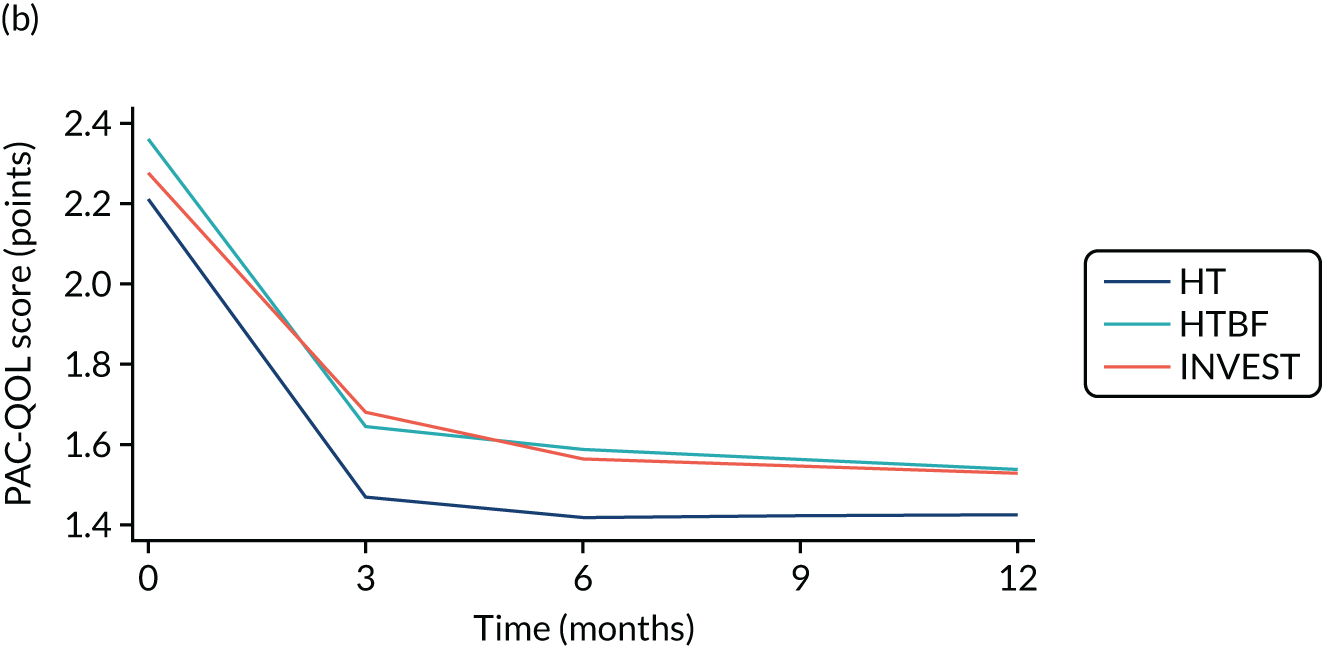
Supplementary analyses for secondary outcomes included:
-
Binary responses (i.e. responder vs. non-responder) to treatment defined as a ≥ 0.4-point reduction in PAC-QoL score (at 3, 6 and 12 months post end of treatment). A logistic regression model was fitted using the ‘xtmelogit’ (with logit link) command in Stata. Two stratification variables (sex and study site) are included in the model; sex was included as a fixed-effect covariate in the regression model and site as a random effect. Other covariates in the model were fixed effects for intervention, baseline PAC-QoL score and breakthrough medication (i.e. use of oral and/or rectal laxatives).
-
Binary responses to treatment defined as a ≥ 1.0-point reduction in PAC-QoL score (at 3, 6 and 12 months post end of treatment). A logistic regression model was fitted in Stata using the ‘xtmelogit’ (with logit link) command. Two stratification variables (sex and study site) were included in the model; sex was included as a fixed-effect covariate in the regression model and site as random effect. Other covariates included the model were fixed effects for intervention, baseline PAC-QoL score and breakthrough medication (i.e. use of oral and/or rectal laxatives).
Subgroup analyses
Planned subgroup analyses showed no difference in response (e.g. reduction in PAC-QoL score) between patients with or without depression (baseline PHQ-9 score ≥ 5 points), anxiety (baseline GAD-7 score ≥ 4.5 points) or joint hypermobility (baseline joint hypermobility syndrome score ≥ 2 points). However, there was a significant interaction between depression and the INVEST treatment and anxiety and the INVEST treatment.
Safety analyses
The study (not being of a medicinal product) did not record unrelated AEs. Serious adverse events (SAEs) and related AEs were small in number, with only two (both unrelated) SAEs reported (Table 13).
| Variable | HT (N = 68) | HTBF (N = 68) | No INVEST (N = 136) | INVEST (N = 46) | Total (N = 182) |
|---|---|---|---|---|---|
| Related AEs | |||||
| Number of patients reporting AE | 4 | 4 | 8 | 5 | 13 |
| Number of AEs reported by category | |||||
| Abdominal pain | 0 | 0 | 0 | 0 | 0 |
| Anal/rectal pain or discomfort | 0 | 2 | 2 | 1 | 3 |
| Bloating | 0 | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 |
| Haemorrhoids | 0 | 0 | 0 | 0 | 0 |
| Loose motions | 0 | 2 | 2 | 0 | 2 |
| Rectal bleeding | 3 | 2 | 5 | 5 | 10 |
| Vaginal/perineal bulge | 0 | 0 | 0 | 0 | 0 |
| Miscellaneous | 1 | 0 | 1 | 2 | 3 |
| Severity | |||||
| Mild | 2 | 2 | 4 | 5 | 9 |
| Moderate | 2 | 4 | 6 | 3 | 9 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| Action | |||||
| No action taken | 1 | 3 | 4 | 5 | 9 |
| Withdrawal | 0 | 0 | 0 | 1 | 1 |
| Concomitant medication | 1 | 1 | 2 | 2 | 4 |
| Non-drug therapy | 1 | 2 | 3 | 0 | 3 |
| SAEsa | |||||
| Number of patients reporting SAE | 0 | 0 | 0 | 2 | 2 |
| Number of SAEs reported that require hospitalisation or prolongation of existing hospitalisation | 0 | 0 | 0 | 2 | 2 |
| Causality | |||||
| Unlikely to be related | 0 | 0 | 0 | 2 | 2 |
| Related | 0 | 0 | 0 | 0 | 0 |
| Expectedness | |||||
| Expected | 0 | 0 | 0 | 0 | 0 |
| Unexpected | 0 | 0 | 0 | 2 | 2 |
| Action taken | |||||
| Withdrawal | 0 | 0 | 0 | 1 | 1 |
| None | 0 | 0 | 0 | 1 | 1 |
Results: cost-effectiveness
Summary and completeness of data
Participants were allocated randomly to HT, HTBF or INVEST-guided treatment. The average duration of training and support visits for HTBF was greater than for HT, as expected, with the INVEST group falling between these two, reflecting the split allocation. Patients allocated to HTBF completed an average of 2.1 biofeedback training sessions; 43 (93%) patients allocated to INVEST completed the INVEST procedure. The cost of intervention procedures was the predominant determinant of overall cost (Table 14) and the only variable differing significantly between groups [one-way analysis of variance (ANOVA), p < 0.001]. Use of prescription drugs and community health-care was relatively low and similar between groups. Hence, cost differences were driven by the intervention costs. Societal costs reflected a minority of patients (≈ 20%) reporting substantial time away from work. Completeness of cost data diminished as follow-up progressed from 86% in the first period to 36% in the last period. Quality-of-life scores at each follow-up point and summary QALYs were similar between groups. Similarly, completeness of EQ-5D-5L data diminished as follow-up proceeded from 98% to 35%. An apparent trend of improving quality-of-life in all groups may be confounded by increasing missingness of data.
| Variable | HT (N = 68 patients) | HTBF (N = 68 patients) | INVEST (N = 46 patients) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| Resource use | |||||||||
| Intervention | |||||||||
| Duration of visits (minutes) | 140 | 80 | 57 | 152 | 102 | 61 | 143 | 85 | 39 |
| Number of biofeedback sessions | – | – | – | 2.09 | 1.43 | 67 | 0.93 | 1.35 | 44 |
| Invest procedure completed | – | – | – | – | – | – | 0.93 | 0.33 | 46 |
| Number of prescriptions | 2.00 | 1.98 | 48 | 1.43 | 1.97 | 46 | 1.53 | 1.62 | 40 |
| 0–3 months | |||||||||
| Number of prescriptions | 0.48 | 0.70 | 44 | 0.83 | 1.42 | 36 | 0.52 | 1.09 | 27 |
| GP visits | 0.02 | 0.15 | 44 | 0.14 | 0.35 | 36 | 0.11 | 0.32 | 27 |
| District nurse visits | 0.02 | 0.15 | 44 | 0.00 | 0.00 | 36 | 0.00 | 0.00 | 27 |
| Pharmacist visits | 0.05 | 0.21 | 44 | 0.06 | 0.23 | 36 | 0.15 | 0.46 | 27 |
| A&E visits | 0.00 | 0.00 | 44 | 0.03 | 0.17 | 36 | 0.00 | 0.00 | 27 |
| Outpatient visits | 0.20 | 0.51 | 44 | 0.08 | 0.28 | 36 | 0.15 | 0.46 | 27 |
| Days from work | 0.98 | 2.65 | 44 | 0.64 | 1.73 | 36 | 2.59 | 5.80 | 27 |
| Inpatient visits | 0.00 | 0.00 | 43 | 0.11 | 0.68 | 35 | 0.00 | 0.00 | 27 |
| 4–6 months | |||||||||
| Number of prescriptions | 0.54 | 1.00 | 39 | 0.37 | 0.61 | 30 | 0.32 | 0.78 | 22 |
| GP visits | 0.18 | 0.60 | 39 | 0.17 | 0.59 | 30 | 0.14 | 0.47 | 22 |
| District nurse visits | 0.00 | 0.00 | 39 | 0.00 | 0.00 | 30 | 0.00 | 0.00 | 22 |
| Pharmacist visits | 0.08 | 0.35 | 39 | 0.17 | 0.59 | 30 | 0.05 | 0.21 | 22 |
| A&E visits | 0.00 | 0.00 | 39 | 0.00 | 0.00 | 30 | 0.00 | 0.00 | 22 |
| Outpatient visits | 0.18 | 0.72 | 39 | 0.10 | 0.31 | 30 | 0.14 | 0.35 | 22 |
| Days from work | 3.51 | 12.72 | 39 | 0.86 | 2.26 | 29 | 1.95 | 5.72 | 22 |
| Inpatient visits | 0.03 | 0.16 | 39 | 0.00 | 0.00 | 30 | 0.00 | 0.00 | 21 |
| 7–12 months | |||||||||
| Number of prescriptions | 0.48 | 0.89 | 31 | 0.33 | 0.64 | 24 | 1.15 | 1.46 | 13 |
| GP visits | 0.27 | 0.64 | 30 | 0.29 | 0.91 | 24 | 0.46 | 1.66 | 13 |
| District nurse visits | 0.07 | 0.37 | 30 | 0.00 | 0.00 | 24 | 0.00 | 0.00 | 13 |
| Pharmacist visits | 0.03 | 0.18 | 30 | 0.00 | 0.00 | 24 | 0.54 | 1.66 | 13 |
| A&E visits | 0.10 | 0.55 | 30 | 0.00 | 0.00 | 24 | 0.08 | 0.28 | 13 |
| Outpatient visits | 0.10 | 0.55 | 30 | 0.42 | 1.32 | 24 | 0.38 | 1.39 | 13 |
| Days from work | 2.30 | 6.72 | 30 | 1.08 | 3.08 | 24 | 4.00 | 11.48 | 13 |
| Inpatient visits | 0.00 | 0.00 | 30 | 0.04 | 0.21 | 23 | 0.00 | 0.00 | 13 |
| Health-care cost | |||||||||
| –3 to 0 months (intervention) | 326 | 186 | 57 | 493 | 322 | 61 | 804 | 351 | 39 |
| 0–3 months | 42 | 87 | 43 | 55 | 137 | 35 | 35 | 75 | 27 |
| 4–6 months | 59 | 152 | 39 | 26 | 51 | 30 | 25 | 51 | 21 |
| 7–12 months | 57 | 142 | 30 | 132 | 320 | 23 | 147 | 283 | 13 |
| Overall | 506 | 205 | 19 | 815 | 411 | 17 | 988 | 269 | 9 |
| Societal cost | |||||||||
| Overall | 1187 | 1866 | 19 | 1231 | 1016 | 17 | 1312 | 665 | 9 |
| EQ-5D-5L | |||||||||
| –3 months | 0.664 | 0.308 | 68 | 0.693 | 0.247 | 66 | 0.679 | 0.223 | 45 |
| 3 months | 0.652 | 0.329 | 43 | 0.708 | 0.261 | 33 | 0.665 | 0.211 | 26 |
| 6 months | 0.733 | 0.251 | 38 | 0.681 | 0.272 | 29 | 0.642 | 0.256 | 22 |
| 12 months | 0.737 | 0.262 | 29 | 0.734 | 0.289 | 22 | 0.713 | 0.136 | 13 |
| QALYs | |||||||||
| –3 to 12 months | 0.874 | 0.357 | 23 | 0.842 | 0.345 | 19 | 0.897 | 0.171 | 11 |
Cost-effectiveness analyses
The base-case model is reported in Table 15. Compared with HT, both HTBF and INVEST-guided treatment were estimated to be associated with higher costs and lower QoL, making HT the dominant strategy. In both instances cost increases were significant (HTBF vs. HT: £239, 95% CI £133 to £354; INVEST vs. HT: £543, 95% CI £403 to £685), and QoL decrements were small for HTBF (–0.010 QALYs, 95% CI –0.053 to 0.03 QALYs) and more significant for INVEST (–0.047 QALYs, 95% CI –0.093 to –0.001 QALYs), compared with HT. These findings are presented in Figure 11. On the ICER plane, credible regions are plotted around median ICERs reflecting where 95% of bootstrapped estimates lie. Incremental differences comparing HTBF and INVEST are not presented because both are dominated by HT. NMB values were negative for HTBF and INVEST across the commonly used range of WTP thresholds, indicating lack of cost-effectiveness (see Table 15). These findings are presented as CEACs at varying WTP thresholds, showing the probability of each intervention being cost-effective. At the NICE threshold of £30,000 per QALY, the probability that HT is the most cost-effective alternative is 83%. The EVPI values the opportunity loss of ongoing uncertainty. At a WTP threshold of £30,000 per QALY, the EVPI per subject is £59. There are no reliable British figures for the incidence, prevalence or chronic idiopathic constipation requiring hospital management or a future timeline for relevant new research. Assuming that an approximate cost of a further definitive RCT is £1M, the research would need to benefit only ≈ 17,000 patients, which might indicate that further research is merited, particularly if the findings have international relevance.
| Variable | ICER (95% CI) | IC (95% CI) | IQ (95% CI) | CEAC p-valuea | CEAC p-valueb | NMBa (95% CI) | NMBb (95% CI) | EVPIb |
|---|---|---|---|---|---|---|---|---|
| Base case | ||||||||
| HTBF vs. HT | c (7506 to c) | 239 (133 to 354) | –0.010 (–0.053 to 0.03) | 0.098 | 0.168 | –402 (–1058 to 219) | –565 (–1858 to 665) | 59 |
| INVEST vs. HT | c (c to c) | 543 (403 to 685) | –0.047 (–0.093 to –0.001) | 0.001 | 0.002 | –1241 (–1962 to –539) | –1939 (–3338 to –564) | – |
| Complete case | ||||||||
| HTBF vs. HT | c (3777 to c) | 340 (135 to 578) | –0.016 (–0.121 to 0.076) | 0.142 | 0.174 | –620 (–2206 to 798) | –897 (–4026 to 1906) | 609 |
| INVEST vs. HT | c (3848 to c) | 542 (351 to 764) | –0.005 (–0.156 to 0.124) | 0.230 | 0.304 | –670 (–2934 to 1357) | –793 (–5262 to 3259) | – |
| Sensitivity analysis 1d | ||||||||
| HTBF vs. HT | c (73,105 to c) | 615 (536 to 695) | –0.031 (–0.074 to 0.008) | 0.001 | 0.005 | –1092 (–1761 to –468) | –1570 (–2885 to –330) | 1 |
| INVEST vs. HT | c (c to c) | 744 (624 to 875) | –0.069 (–0.113 to –0.021) | 0.000 | 0.000 | –1777 (–2454 to –1079) | –2809 (–4149 to –1394) | – |
| Sensitivity analysis 2e | ||||||||
| HTBF vs. HT | c (11,439 to c) | 246 (136 to 365) | –0.024 (–0.073 to 0.019) | 0.041 | 0.081 | –621 (–1382 to 64) | –996 (–2468 to 335) | 25 |
| INVEST vs. HT | c (c to c) | 532 (407 to 652) | –0.053 (–0.102 to –0.002) | 0.000 | 0.001 | –1325 (–2077 to –566) | –2119 (–3609 to –617) | – |
FIGURE 11.
The CapaCiTY trial 1 cost-effectiveness analysis: base case. (a) ICER plane [the average ICER for each intervention (compared with HT) is shown as a line from the origin and the credible regions (dashed circles) show where 95% of estimates lie]; (b) incremental NMB (the shaded regions are the corresponding 95% CIs); (c) CEACs and CEAF; and (d) EVPI.
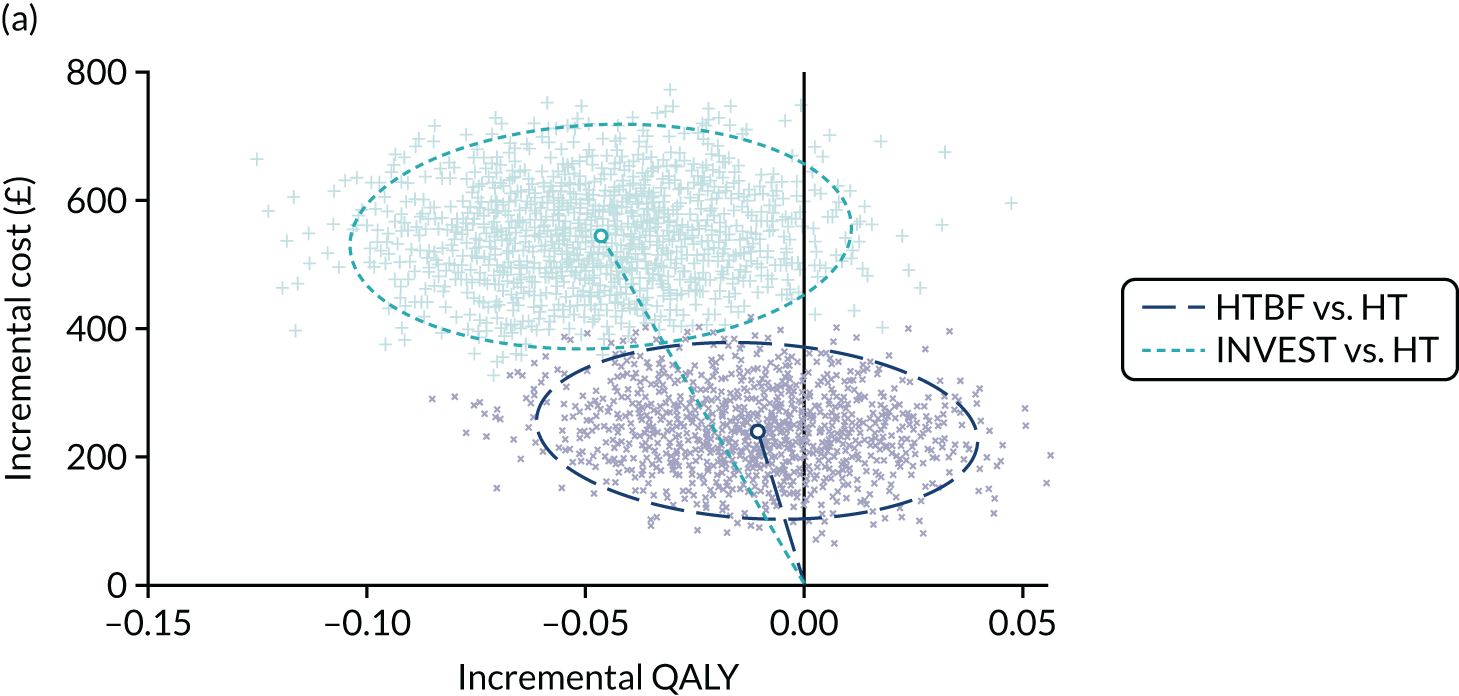
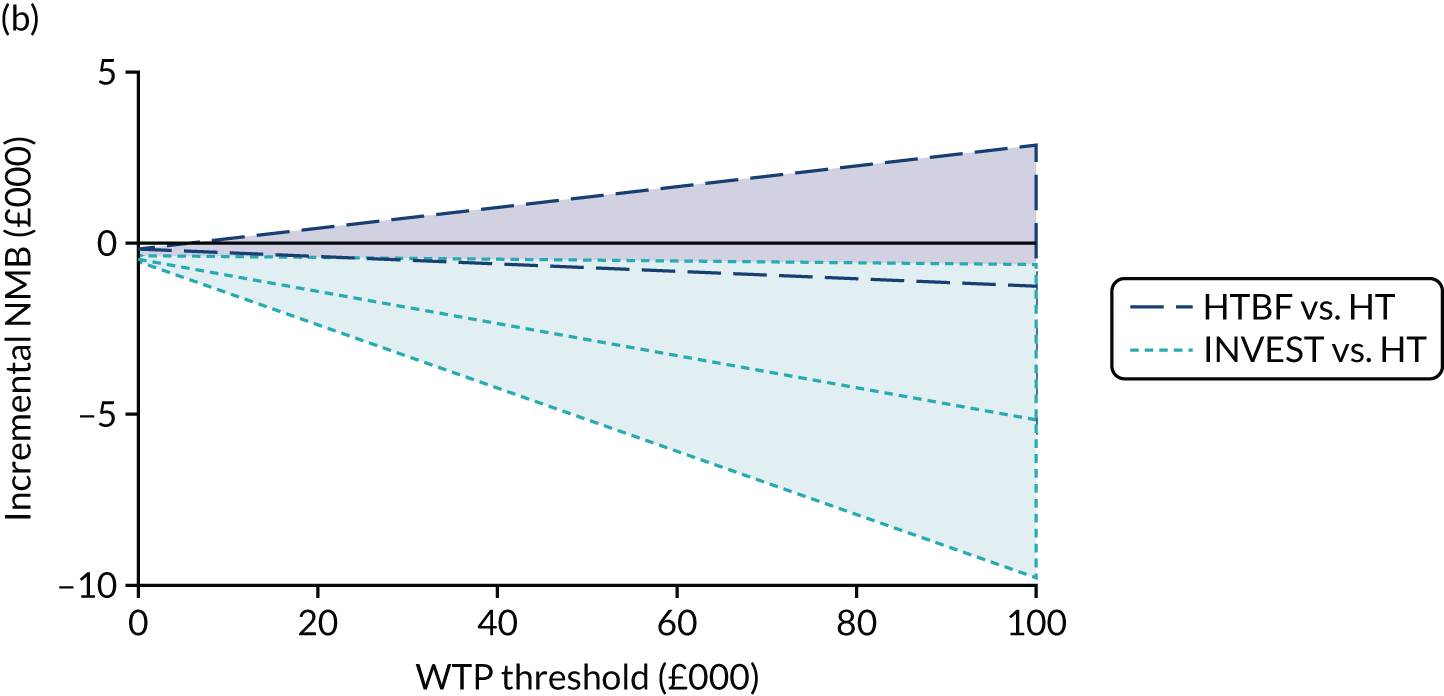
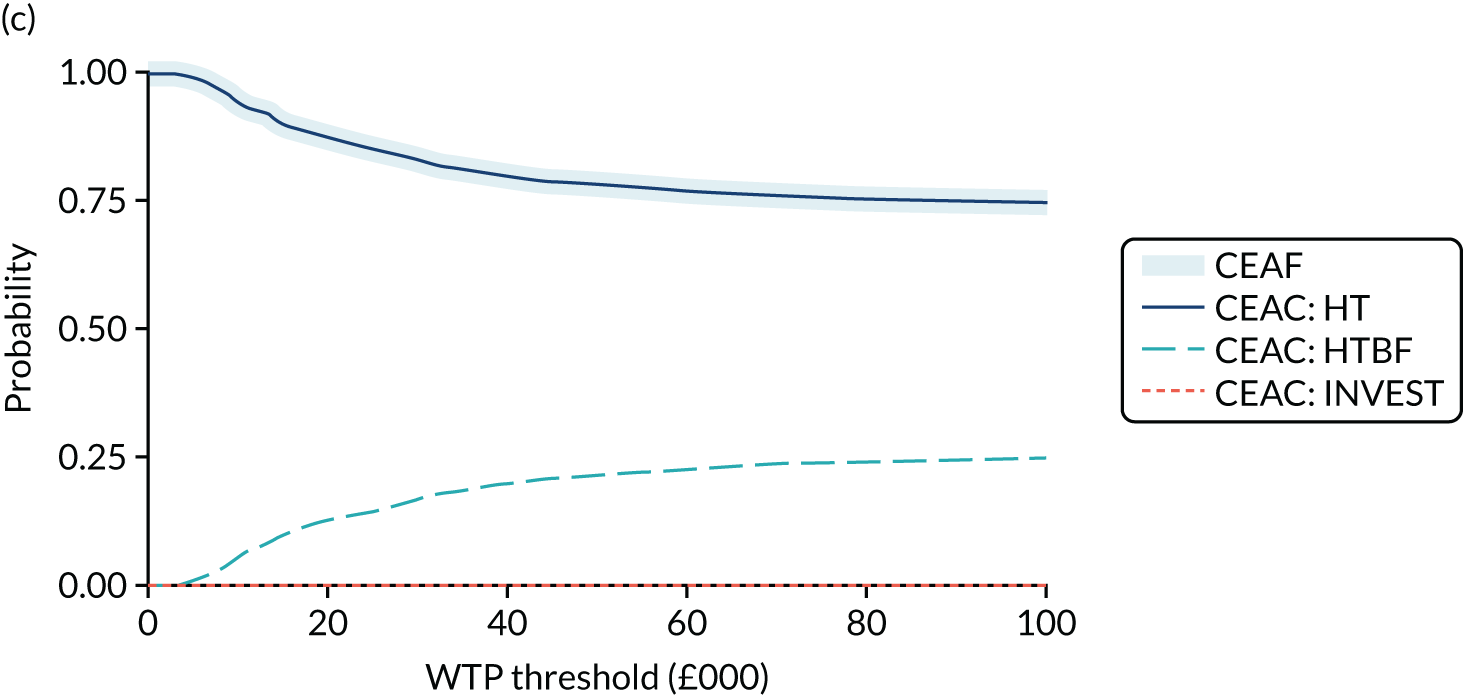
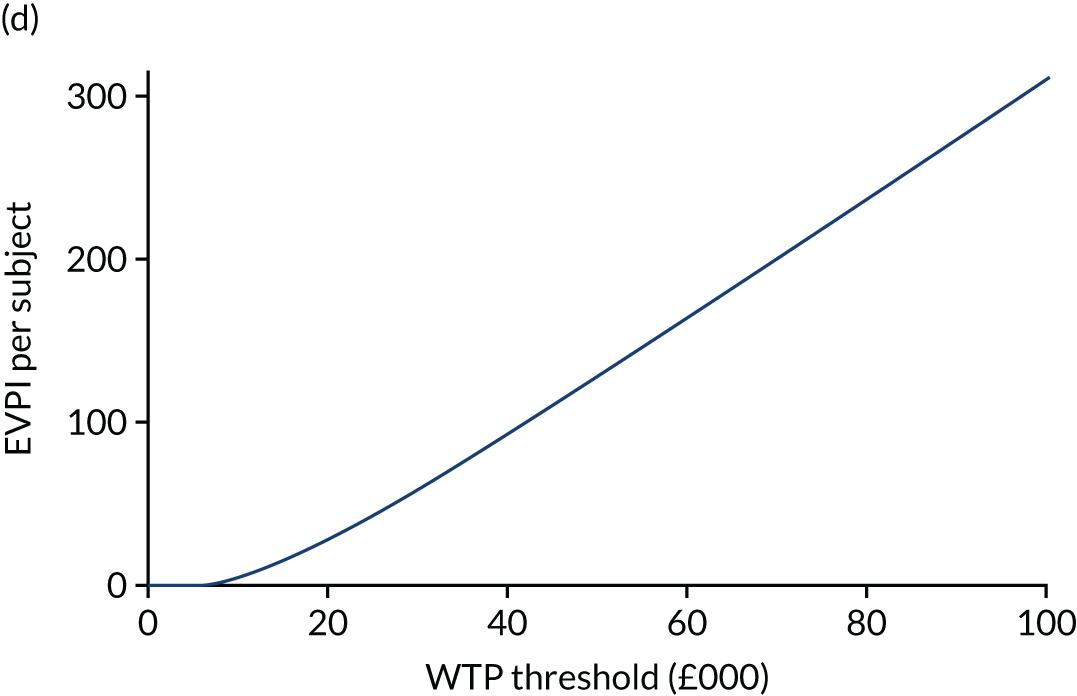
In Figure 11a the ICER plane shows the bootstrapped distribution of estimated incremental costs and QALYs comparing HTBF or INVEST with HT. Bootstrapping is a method of resampling the trial data to mimic conducting many trials; the range of findings reflects the uncertainty in the trial. The average ICER for each intervention (compared with HT) is shown as a line from the origin. The credible regions (dashed circles) show where 95% of estimates lie. Compared with HT, average costs increase for both HTBF and INVEST and QALYs decrease, meaning that HT is the dominant treatment strategy. In Figure 11b the incremental NMB describes the resource gain (or loss) when investing in HTBF or INVEST. The shaded regions are the corresponding 95% CIs. Investing in HTBF or INVEST at any threshold WTP would impose a loss to the health system when compared with other alternative choices. In Figure 11c the presentation of CEACs re-presents the incremental NMB findings as the probability that each intervention is cost-effective at different WTP thresholds. The CEAF is a necessary further analysis when three or more interventions are compared. Although HT is the dominant strategy the finding is uncertain, being estimated to be correct with a probability of 83% at a WTP threshold of £30,000 per QALY. In Figure 11d the EVPI indicates the value to a health-care system of further research to eliminate uncertainty, as WTP varies.
The complete-case analysis is also presented in Table 15. Although the numbers contributing are small, the findings are similar to the base case, if less precise. The base-case analysis costed the intervention received by each participant in terms of the number of scheduled visits attended, time taken and equipment used. This assumes no resource losses due to non-attendance or cancellation. A first post hoc sensitivity analysis costed each intervention fully per protocol, assuming that these were non-recoverable costs. The consequence is that the incremental cost of HTBF and INVEST increases, increasing the probability that HT is cost-effective (p > 99% at a WTP threshold of £30,000 per QALY). A minority of patients (12%) had no record of engaging in the trial intervention. In a second post hoc sensitivity analysis, these subjects were removed to explore a participant analysis and reduce the imputation burden. Findings were similar to and thus supportive of the base-case findings.
Results: patient experience and qualitative analysis
Emergent themes are presented in this section. Numbers in square brackets refer to the numbered quotations in Appendix 2, Quotations from participants contributing to qualitative analysis in all three trials, CapaCiTY trial 1: habit training with or without biofeedback with or without standardised radiophysiological investigations.
INVEST compared with no INVEST
For those randomised to the INVEST tests, some felt embarrassment, especially if the health-care professional was of a different sex from the patient. However, some patients did not seem to mind the tests, particularly because they felt that the health-care team managed the investigations professionally and with dignity. Several were relieved to have been in the INVEST group because they felt that the tests provided more information about potential sources of their constipation. The tests also gave some reassurance that no underlying diseases were the root cause of their constipation, providing a sense of closure and peace of mind [1].
Many patients randomised to the no-INVEST group were disappointed not to have received the tests because they felt that the investigations would aid in pinpointing underlying issues that were contributing to their CC. In addition, some patients thought that having visualisation of their gut would help them to better understand their bodily issues. The desire of patients to have the tests was also noticed by staff delivering the intervention.
For a few patients, not having the prior testing was not an issue. Some patients had mixed feelings in terms of not being allocated to the INVEST group; some were pleased that they did not have to experience the embarrassment or invasiveness of the procedures. They also initially believed that they would have received better care had they been in the INVEST group [2]. Others felt some regret at not having tests but appeared to accept it and move on.
Habit training only
Some patients were initially disappointed when they discovered that they were in the HT group because they did not believe that HT on its own would be of benefit. For others, being placed in this group provided a sense of encouragement. For many patients the best thing about HT was being able to talk to an interested professional during the sessions about their constipation, particularly because they considered this a taboo subject [3]. Being able to speak about their issues helped to normalise the experience of CC [4].
A number of patients found that the HT helped them to better understand how diet and fluids influence their constipation [5]. Those who had the additional biofeedback element often felt that the HT elements were the most important [6]. Patients reported better knowledge of the types of foods that helped or worsened constipation. However, a few patients did not find changes in their diet beneficial or their diet already followed the HT advice. Some patients noted that learning to breathe and push correctly during defaecation helped them to pass stools more easily [7]. However, a few struggled with utilising this breathing technique.
Some patients also noted that learning how their bowels worked allowed them to better understand their own bodies [8]. Learning was enhanced by visual aids in the booklet; staff echoed the usefulness of the booklet [9]. HT also allowed more time than HTBF for education during the sessions. Some patients stated that being taught how to sit on the toilet and for how long, and/or using a stool to elevate their legs, was helpful [9]. Others did not find the advice about sitting worked for them, or they did not comply with the advice.
Several patients stated how helpful and supportive they found the staff. Many found that the programme helped them to focus on their constipation and the potential solutions without reverting to over-reliance on laxatives [11]. Some felt that HT took considerable time and dedication to start seeing improvements; others stated that carrying out the HT methods on a regular basis was difficult or painful to maintain without laxatives. For those who found HT helpful, benefits ranged from some improvement [12] to substantial improvement [13].
Habit training with biofeedback
For patients who had the additional biofeedback element, some stated that they found the visual information on the screen helpful [13]; some staff echoed the visual benefit of biofeedback. Some patients in the HTBF group saw a substantial improvement, whereas others did not appear to see much benefit.
Staff opinions were mixed regarding the HTBF intervention, with some suggesting that biofeedback helped patients improve more than HT. However, others felt that biofeedback machines offered little in terms of improving patient symptoms [14]. Some patients found the HTBF sessions a little embarrassing because a probe needed to inserted into the anus [15]. For others the worst thing about biofeedback was that they did not find that it helped their symptoms. Therefore, a few of the patients seem to believe that their constipation would never improve. For clinical staff, seeing patients’ constipation problems improve was the most satisfying aspect for them, whether that was with HT or HTBF. However, it was frustrating for staff when interventions did not work, particularly for patients who had had symptoms for many years.
Maintaining learning
Many patients who saw benefits from HT or HTBF are continuing to implement what they learned, whether that is diet and fluid maintenance, using a footstool and/or utilising breathing exercises. A few patients reported that they still took laxatives either on occasion or regularly to help their constipation. However, others reported that they no longer needed laxatives [16].
When asked whether or not they would recommend HT, many stated that they would. Some people would recommend HTBF [17] and a few would recommend HT but not HTBF. A few would recommend one HT session but not multiple sessions because they did not find it beneficial enough. For others, even if the programme did not work for them in the way that they had hoped, they would still recommend to others with constipation that they try it.
Staff experience of delivering the intervention
Many staff indicated that the intervention was not difficult to deliver, particularly the HT aspect for nursing staff, because this was their main clinical role prior to the study. However, time spent filling in paperwork, setting up rooms, arranging biofeedback machines and organising a clinical scientist to be present (if one was needed to help with setting up and operating the biofeedback machine) had an impact on their workload, adding 10–20 minutes to their usual consultation time per patient [18].
Regarding the use of biofeedback machines, staff ranged in their confidence levels depending on whether or not they had:
-
additional professional support such as a clinical scientist
-
enough previous experience using the same biofeedback machine [19]
-
used the machine on a regular basis
-
received formal training.
Although training sessions were provided to staff, a few staff did not receive formal biofeedback training at their site and had to rely on the protocol description. Missing formal training may have contributed to the reduction in confidence levels among some staff members. Staff lack of confidence utilising the machines may have affected the patient experience during biofeedback.
Feedback on the study/treatment design
Some patients felt that the study/treatment plan was well designed and did not believe that the intervention could be improved in any way, even if it did not address their needs specifically. Many patients stated that the number and duration of sessions were right. However, some reported that the time between sessions felt like long periods without support. A few mentioned that they would have liked more information about their INVEST results.
Many patients and staff mentioned stress and psychological issues related to constipation, and specific recommendations were made about addressing this aspect more directly in future roll-outs of the intervention. A number of patients and staff mentioned that lying down during the biofeedback sessions seemed counterintuitive to retraining their muscles for a bowel emptying process that is carried out sitting up. Some patients found the information in the HT sessions too basic for their situation or felt patronised and would have preferred a more tailored approach.
Patient discontinuation
Four of the 24 patients interviewed for CapaCiTY trial 1 reported that they had discontinued their participation in the intervention because of:
-
unsuitability for the study (e.g. having an anal fissure)
-
misunderstanding what the study could provide, such as expecting to be placed into a group for surgery
-
the intervention not appearing to work/constipation symptoms returning
-
fear of stopping laxative and suppository use.
CapaCiTY trial 2: pragmatic randomised trial of low-volume compared with high-volume initiated transanal irrigation therapy in adults with chronic constipation
Intervention
Transanal irrigation training was provided by a trained nurse or physiotherapist with experience in delivering care for CC. A standardised approach and intervention was provided via use of an intervention manual.
The course of therapy included a nurse-led training session (or multiple sessions if required to ensure that the device was being used effectively) followed by patient-led home TAI therapy. The low-volume system commonly used in practice was Qufora® IrriSedo Mini (MacGregor Healthcare Ltd, Tranent, UK). Various commercially available high-volume systems could be used, including Peristeen® Anal Irrigation System (Coloplast A/S, Humlebæk, Denmark) and Qufora IrriSedo Balloon (MacGregor Healthcare Ltd).
Low-volume transanal irrigation
This system consists of a small reservoir attached to a cone. The reservoir holds approximately 70 ml of water and is squeezed to inject water into the rectum. The regimen used will be as follows: initial TAI once daily for 14 days using one–three insufflations (each of ≈ 70 ml). This may then be reduced to alternate days depending on response. Patients may then adjust the frequency and volume depending on response. They may irrigate as much and as often as they feel is necessary to give them benefit and this information should be captured on the CRF, with the aid of an irrigation journal.
High-volume transanal irrigation
High-volume systems consist of an irrigation bag connected to a tube. The water flows into the rectum either by gravity or using a pump. Some systems employ a balloon to hold the device in place during irrigation; others require the patient to hold it in place. The mechanism of action is the same for all systems. Initial frequency of TAI is the same as for low-volume TAI (i.e. daily for 14 days, then on alternate days). Patients will commence with irrigations of 300 ml and increase this by 100 ml every 2 days until satisfactory defaecation is achieved or the procedure becomes uncomfortable, up to a maximum of 1500 ml. Patients may adjust therapy depending on response, as for low-volume TAI.
Design features
Basic design
Robust data for the use of irrigation therapy in chronic (idiopathic) constipation are lacking. In addition, there are no data demonstrating superiority of high-volume over low-volume systems. Given differences in cost between the two systems, a randomised study of well-characterised patients comparing the two methods would provide useful information on whether or not one system holds a clear advantage over the other. In addition, the short- and long-term efficacy and acceptability of therapy in CC could be evaluated.
Patients used one system only (plus defined ‘rescue therapies’) for a minimum of 3 months. After this time point they could switch to the other system if their initial therapy was ineffective/unsatisfactory. Thus, consenting patients were randomised to initiate therapy with one of these systems but had the option of switching to the other after an initial 3-month period. This allowed identification of response rates to each system in the short term (3 months) and thereafter a comparison between treatment strategies (low-volume vs. high-volume initiated therapy) rather than a pure comparison of the two techniques. This was a patient-centred study design aiming to limit the time patients spent using ineffective therapy without being allowed to try an alternative. Reasons for switching were captured qualitatively (see Patient experience).
Switching TAI systems before completing the 3-month waiting period was discouraged and, if it occurred, was documented as a protocol violation, with the timing and reason documented. If symptoms were severe despite the use of TAI and rescue therapies then other medications could be used on compassionate grounds, but these were also recorded in the CRF/concomitant medications log.
Sample size calculation
The PAC-QoL is a 28-item disease-specific measure, with each item scoring 0–4 points, providing an aggregate score 0.0–4.0 points. Superiority of either low-volume or high-volume TAI could be demonstrated by a difference in effect size of 0.4 points, with a variance estimate conservatively set at SD = 1 point from the published literature. To detect an effect size of 0.4 points (mean/SD = 0.4 points) between the two groups with 90% power and 5% significance at 3 months requires 133 patients per group and 266 in total. Allowing for an anticipated 10% loss to follow-up, we planned to recruit 300 patients.
Randomisation procedure
Patients were randomised 1 : 1 into two groups: those to commence therapy with a low-volume device and those to commence therapy with a high-volume device. Because male patients represent a minority of patients with CC (≈ 10%) and there may be differences in pathophysiology (and therefore possibly response) between male and females patients, patients were stratified by sex and female patients by centre.
Blinding
Patients and clinicians were necessarily aware of treatment allocations. The need to collect data on frequency and volume of TAI, as well as reasons for discontinuing or switching between systems, meant that assessor blinding was not possible with respect to these outcomes. Any researcher collecting CRFs or handling journals was therefore unblinded. However, the primary outcome (PAC-QoL score at 3 months) was concealed: patients completed this questionnaire without a researcher present and placed it in a sealed envelope.
Concomitant medications
Methods as in CapaCiTY trial 1, with recorded ad libitum usage.
Statistical methods
The primary outcome, PAC-QoL score, was analysed as a continuous variable on an ITT basis; that is, all patients for whom an outcome was available at 3 months were included in the analysis, and analysed according to the treatment group to which they were originally randomised (even though two patients crossed over before the primary outcome was measured).
We present descriptive statistics (i.e. mean, SD, median and IQR) by trial group. Owing to the small numbers of patients providing the outcome data at follow-up, no statistical comparisons were performed.
All secondary outcomes were also analysed on an ITT basis. For each outcome we present descriptive statistics (as appropriate) by trial group; continuous variables (e.g. PAC-QoL score) have been summarised by treatment group using mean, SD, median and IQR. For categorical variables, numbers and percentages of patients reporting each response option have been presented by trial group. Note that irrigation volume transgresses the ITT rule, being reported as that actually being used despite original allocation. Results obtained using the CC-BRQ and BIPQ-CC have been omitted pending further analysis.
Time to cessation of TAI is presented using Kaplan–Meier graphs, and where available reasons for cessation of each system presented.
For cost-effectiveness, patients were randomised in parallel trial groups to low-volume or high-volume TAI therapy. Days of use of low-volume and high-volume TAI were not recorded directly but approximated from available data for using evidence of use in each follow-up period, frequency of use (days per week) in each period and withdrawal data. No discounting was applied to economic data reflecting the follow-up period of 1 year.
Recruitment
First recruitment and intervention was on 11 November 2015; recruitment ended 30 June 2018. A total of 65 patients (target 300 patients) were randomised out of 150 screened (21.7%) from seven sites. Reasons for screen failure are shown in Figure 12. Three sites opened but failed to recruit; the remainder randomised 1–33 patients, with approximately half of the patients recruited from secondary care and half from tertiary care. A total of 65 patients were randomised. A total of 14 patients withdrew from the study before the primary end point (51 completed the 3-month follow-up). A total of 30 patients were randomised to low-volume TAI and 35 to high-volume TAI. The CONSORT flow diagram is shown in Figure 4.
FIGURE 12.
The CapaCiTY trial 2 screen failures. SNS, sacral nerve stimulation.
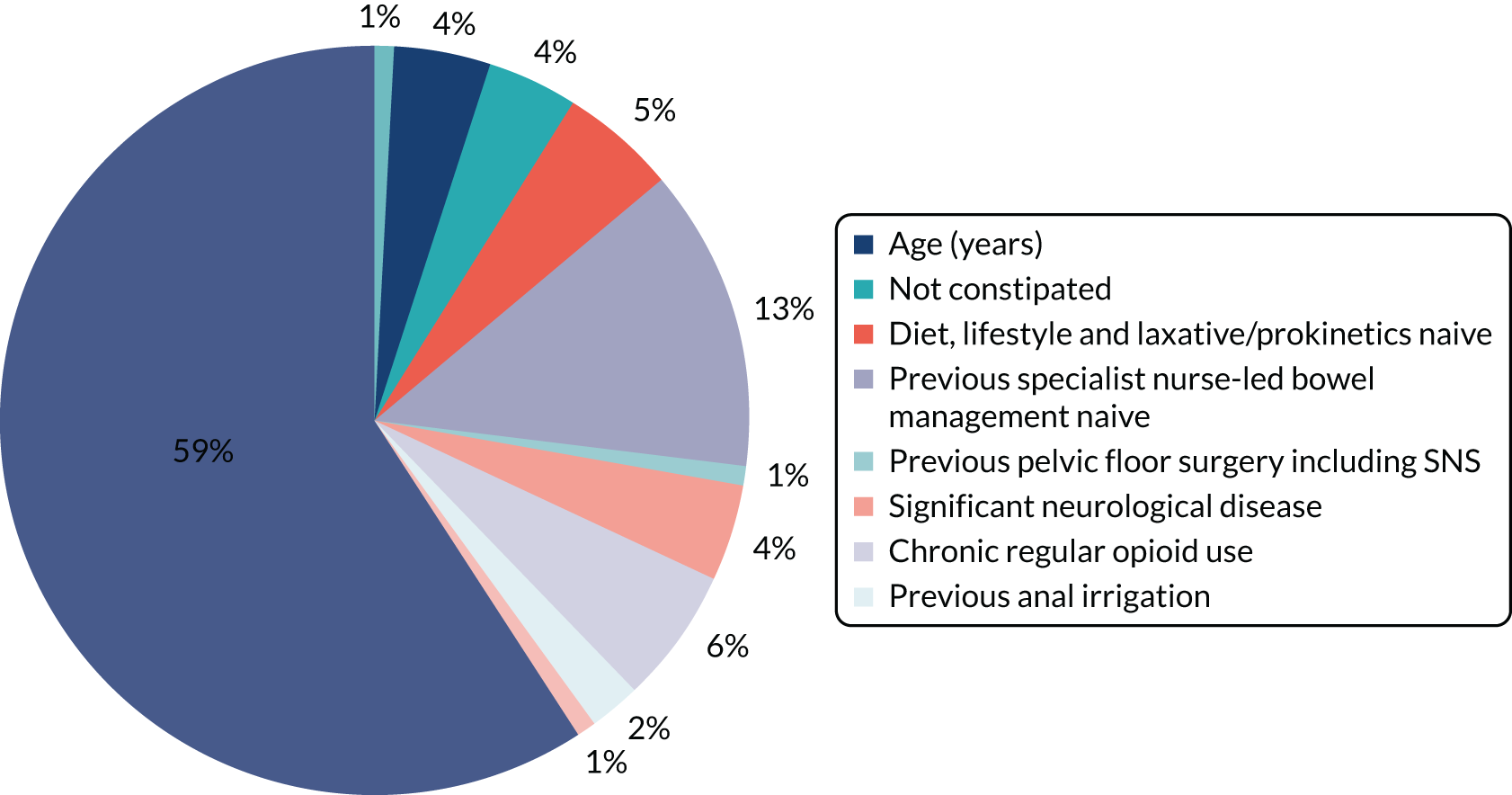
Baseline characteristics
Table 16 shows the numbers and percentages of patients with baseline characteristics presented by trial group. Continuous variables (e.g. age) were summarised by treatment group using mean and SD (median and IQR if non-normally distributed). The data show that half of the patients were in tertiary care and that, as would be expected, all had tried lifestyle measures and laxatives. The age and sex distribution are as would be expected from a hospital-based constipation cohort. The prevalence of other medical conditions was fairly typical for any hospital-attending patient group of this age range with a chronic illness. One-quarter of patients had hypermobility, higher than reported in the general population.
| Characteristic | Low-volume TAI (N = 30) | High-volume TAI (N = 35) | Total (N = 65) |
|---|---|---|---|
| Referral method, n (%) | |||
| Secondary care | 11 (36.7) | 16 (45.7) | 27 (41.5) |
| Tertiary care | 16 (53.3) | 18 (51.4) | 34 (52.3) |
| Other | 3 (10.0) | 1 (2.9) | 4 (6.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Demographic characteristic | |||
| Sex, n (%) | |||
| Male | 3 (10.0) | 4 (11.4) | 7 (10.8) |
| Female | 27 (90.0) | 31 (88.6) | 58 (89.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity, n (%) | |||
| Asian | 1 (3.3) | 1 (2.9) | 2 (3.1) |
| Black | 2 (6.7) | 1 (2.9) | 3 (4.6) |
| Mixed | 0 (0.0) | 2 (5.7) | 2 (3.1) |
| White | 26 (86.7) | 31 (88.6) | 57 (87.7) |
| Missing | 1 (3.3) | 0 (0.0) | 1 (1.5) |
| Age (years) | |||
| Mean (SD) | 43.3 (15.0) | 44.9 (11.8) | 44.2 (13.3) |
| Median (IQR) | 41.5 (37.0–55.0) | 43.0 (40.0–53.0) | 43.0 (39.0–54.0) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Past medical history, n (%) | |||
| Total | 27 (90.0) | 28 (80.0) | 55 (84.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cardiovascular condition | 8 (26.7) | 6 (17.1) | 14 (21.5) |
| Heart disease | 1 (3.3) | 0 (0.0) | 1 (1.5) |
| Hypertension | 5 (16.7) | 4 (11.4) | 9 (13.8) |
| Hypercholesterolaemia | 5 (16.7) | 3 (8.6) | 8 (12.3) |
| Other | 1 (3.3) | 1 (2.9) | 2 (3.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Respiratory condition | 10 (33.3) | 7 (20.0) | 17 (26.2) |
| Asthma | 9 (30.0) | 7 (20.0) | 16 (24.6) |
| COPD | 3 (10.0) | 1 (2.9) | 4 (6.2) |
| Other | 1 (3.3) | 1 (2.9) | 2 (3.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal condition | 15 (50.0) | 12 (34.3) | 27 (41.5) |
| IBS | 11 (36.7) | 7 (20.0) | 18 (27.7) |
| Crohn’s disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ulcerative colitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cancer | 0 (0.0) | 1 (2.9) | 1 (1.5) |
| Colonic polyps | 3 (10.0) | 3 (8.6) | 6 (9.2) |
| Other | 4 (13.3) | 3 (8.6) | 7 (10.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Metabolic condition | 8 (26.7) | 4 (11.4) | 12 (18.5) |
| Diabetes | 5 (16.7) | 2 (5.7) | 7 (10.8) |
| Hypothyroidism | 2 (6.7) | 3 (8.6) | 5 (7.7) |
| Hyperthyroidism | 1 (3.3) | 0 (0.0) | 1 (1.5) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Haematological condition | 2 (6.7) | 0 (0.0) | 2 (3.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hepatic condition | 2 (6.7) | 3 (8.6) | 5 (7.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal disease | 1 (3.3) | 0 (0.0) | 1 (1.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Genito-urinary condition | 6 (20.0) | 7 (20.0) | 13 (20.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neurological/CNS condition | 10 (33.3) | 7 (20.0) | 17 (26.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Psychiatric condition | 12 (40.0) | 12 (34.3) | 24 (36.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dermatological condition | 6 (20.0) | 6 (17.1) | 12 (18.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal condition | 4 (13.3) | 5 (14.3) | 9 (13.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other condition | 3 (10.0) | 1 (2.9) | 4 (6.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Past surgical history, n (%) | |||
| Total | 17 (56.7) | 17 (48.6) | 34 (52.3) |
| Abdominal operation | 10 (33.3) | 7 (20.0) | 17 (26.2) |
| Gynaecological procedure | 10 (33.3) | 11 (31.4) | 21 (32.3) |
| Proctological or perineal procedure | 2 (6.7) | 5 (14.3) | 7 (10.8) |
| Neuromodulation | 1 (3.3) | 0 (0.0) | 1 (1.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Duration (months) of constipation symptoms | |||
| Mean (SD) | 90.4 (79.1) | 120.7 (76.3) | 106.7 (78.5) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Previous lifestyle modifications, n (%) | |||
| Total | 30 (100.0) | 35 (100.0) | 65 (100.0) |
| Diet | 30 (100.0) | 35 (100.0) | 65 (100.0) |
| Fluid | 30 (100.0) | 35 (100.0) | 65 (100.0) |
| Exercise | 27 (90.0) | 31 (88.6) | 58 (89.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alternative therapies, n (%) | |||
| Total | 5 (16.7) | 8 (22.9) | 13 (20.0) |
| Missing | 0 (0.0) | 1 (2.9) | 1 (1.5) |
| Laxatives, n (%) | |||
| Total | 30 (100.0) | 34 (97.1) | 64 (98.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antidiarrhoeals, n (%) | |||
| Total | 1 (3.3) | 2 (5.7) | 3 (4.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prokinetics, n (%) | |||
| Total | 18 (60.0) | 23 (65.7) | 41 (63.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nurse-led bowel management, n (%) | |||
| Total | 30 (100.0) | 35 (100.0) | 65 (100.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Family history of bowel disease, n (%) | |||
| IBS | 7 (23.3) | 5 (14.3) | 12 (18.5) |
| IBD | 2 (6.7) | 3 (8.6) | 5 (7.7) |
| Gastrointestinal cancer | 7 (23.3) | 5 (14.3) | 12 (18.5) |
| Other | 4 (13.3) | 5 (14.3) | 9 (13.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sexual history (female participants only), n (%) | |||
| Sexually active | 14 (51.9) | 23 (74.2) | 37 (63.8) |
| Child-bearing potential | 18 (66.7) | 19 (61.3) | 37 (63.8) |
| > 1 year post menopausal | 4 (14.8) | 7 (22.6) | 11 (19.0) |
| Surgically sterile | 7 (25.9) | 9 (29.0) | 16 (27.6) |
| Contraceptive use (female participants only), n (%) | |||
| Barrier | 12 (44.4) | 14 (45.2) | 26 (44.8) |
| Non-barrier | 2 (7.4) | 6 (19.4) | 8 (13.8) |
| Missing | 11 (40.7) | 8 (25.8) | 19 (32.8) |
| Past obstetric history (female participants only) | |||
| Total, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number of vaginal deliveries, mean (SD) | 19 (70.4) | 25 (80.6) | 44 (75.9) |
| Number of caesareans, mean (SD) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number of forceps/ventouse deliveries, mean (SD) | 1.9 (1.1) | 2.3 (1.3) | 2.1 (1.3) |
| Number of episiotomies, mean (SD) | 0.2 (0.4) | 0.2 (0.5) | 0.2 (0.4) |
| Number of obstetric tears, mean (SD) | 0.5 (0.7) | 0.4 (0.9) | 0.4 (0.8) |
| Missing, n (%) | 0.7 (0.9) | 0.3 (0.5) | 0.5 (0.7) |
| Faecal incontinence symptoms, n (%) | |||
| Total | 0.6 (1.0) | 0.6 (0.9) | 0.6 (0.9) |
| Faecal urgency | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urge faecal incontinence | 17 (56.7) | 17 (48.6) | 34 (52.3) |
| Passive faecal incontinence | 9 (30.0) | 14 (40.0) | 23 (35.4) |
| Post defaecation leakage | 5 (16.7) | 7 (20.0) | 12 (18.5) |
| Difficulty wiping clean | 2 (6.7) | 8 (22.9) | 10 (15.4) |
| Missing | 4 (13.3) | 8 (22.9) | 12 (18.5) |
| Pelvic organ prolapse symptoms, n (%) | |||
| Total | 7 (23.3) | 9 (25.7) | 16 (24.6) |
| Vaginal bulging | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| External rectal prolapse | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| External uterine prolapse | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| In what proportion of bowel motions does the patient experience spontaneous rectal urge to defaecate (without laxatives)?, n (%) | |||
| Never | 10 (33.3) | 9 (25.7) | 19 (29.2) |
| < 25% | 10 (33.3) | 12 (34.3) | 22 (33.8) |
| 25–75% | 5 (16.7) | 7 (20.0) | 12 (18.5) |
| > 75% | 2 (6.7) | 1 (2.9) | 3 (4.6) |
| Always | 2 (6.7) | 5 (14.3) | 7 (10.8) |
| Missing | 1 (3.3) | 1 (2.9) | 2 (3.1) |
| Joint hypermobility,a n (%) | |||
| Total | 8 (26.7) | 9 (25.7) | 17 (26.2) |
| Missing | 1 (3.3) | 2 (5.7) | 3 (4.6) |
Table 17 shows the data for outcome measures at baseline. These were comparable between the two groups for all major characteristics.
| Outcome measure | Low-volume TAI (N = 30) | High-volume TAI (N = 35) | Total (N = 65) |
|---|---|---|---|
| PAC-QoL score (points) | |||
| Overall, mean (SD) | 2.5 (0.8) | 2.4 (0.7) | 2.4 (0.8) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Dissatisfaction, mean (SD) | 3.3 (0.6) | 3.2 (0.6) | 3.3 (0.6) |
| Missing, n (%) | 2 (6.7) | 3 (8.6) | 5 (7.7) |
| Physical discomfort, mean (SD) | 2.7 (0.9) | 2.7 (0.8) | 2.7 (0.8) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Psychosocial discomfort, mean (SD) | 1.8 (1.0) | 1.8 (1.0) | 1.8 (1.0) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Worries and concerns, mean (SD) | 2.5 (1.0) | 2.5 (0.9) | 2.5 (0.9) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| PAC-SYM score (points) | |||
| Overall, mean (SD) | 2.1 (0.8) | 2.1 (0.7) | 2.1 (0.8) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Stool symptoms, mean (SD) | 2.3 (1.0) | 2.4 (1.1) | 2.3 (1.0) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Abdominal symptoms, mean (SD) | 2.4 (1.2) | 2.4 (0.8) | 2.4 (1.0) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Rectal symptoms, mean (SD) | 1.3 (1.2) | 1.3 (0.8) | 1.3 (1.0) |
| Missing, n (%) | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Bowel frequency, number reported over 14 days (diary data) | |||
| Attempts to empty bowels, mean (SD) | 20.3 (10.6) | 21.2 (13.2) | 20.8 (12.0) |
| Missing, n (%) | 6 (20.0) | 6 (17.1) | 12 (18.5) |
| Times stool was actually passed, mean (SD) | 12.1 (9.5) | 11.3 (9.9) | 11.6 (9.6) |
| Missing, n (%) | 7 (23.3) | 6 (17.1) | 13 (20.0) |
| Nature of bowel movement, number of days out of 14 (diary data) | |||
| Laxatives used, mean (SD) | 21.7 (5.8) | 23.7 (5.2) | 22.8 (5.6) |
| Missing, n (%) | 6 (20.0) | 6 (17.1) | 12 (18.5) |
| Glycerine suppositories used, mean (SD) | 26.0 (4.0) | 26.5 (3.3) | 26.3 (3.6) |
| Missing, n (%) | 7 (23.3) | 6 (17.1) | 13 (20.0) |
| EQ-5D-5L: ‘no problem’ indicated, n (%) | |||
| Mobility | 6 (20.0) | 9 (25.7) | 15 (23.1) |
| Missing | 3 (10.0) | 2 (5.7) | 5 (7.7) |
| Self-care | 4 (13.3) | 4 (11.4) | 8 (12.3) |
| Missing | 3 (10.0) | 2 (5.7) | 5 (7.7) |
| Usual activities | 11 (36.7) | 17 (48.6) | 28 (43.1) |
| Missing | 3 (10.0) | 2 (5.7) | 5 (7.7) |
| Pain/discomfort | 24 (80.0) | 30 (85.7) | 54 (83.1) |
| Missing | 3 (10.0) | 2 (5.7) | 5 (7.7) |
| Anxiety/depression | 15 (50.0) | 20 (57.1) | 35 (53.8) |
| Missing | 3 (10.0) | 2 (5.7) | 5 (7.7) |
| EQ-VAS score (points) | |||
| Total, mean (SD) | 63.9 (20.3) | 70.9 (19.1) | 67.8 (19.8) |
| Missing, n (%) | 3 (10.0) | 2 (5.7) | 5 (7.7) |
| PHQ-9 depression severity, n (%) | |||
| None | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| Mild | 14 (46.7) | 16 (45.7) | 30 (46.2) |
| Moderate | 5 (16.7) | 8 (22.9) | 13 (20.0) |
| Moderately severe | 3 (10.0) | 2 (5.7) | 5 (7.7) |
| Severe | 6 (20.0) | 7 (20.0) | 13 (20.0) |
| Missing | 2 (6.7) | 2 (5.7) | 4 (6.2) |
| GAD-7 anxiety severity, n (%) | |||
| None | 8 (26.7) | 15 (42.9) | 23 (35.4) |
| Mild | 8 (26.7) | 5 (14.3) | 13 (20.0) |
| Moderate | 6 (20.0) | 9 (25.7) | 15 (23.1) |
| Severe | 3 (10.0) | 1 (2.9) | 4 (6.2) |
| Missing | 3 (10.0) | 3 (8.6) | 6 (9.2) |
Results: clinical effectiveness
Owing to the low numbers of patients recruited, only descriptive statistics are presented (Table 18). At 3 months there was a modest reduction of mean PAC-QoL score from 2.4 to 2.2 points (0.2-point reduction) in the low-volume irrigation group and a larger reduction of 0.5 points in the high-volume TAI group. Although the difference is not large there was consistency of findings across some of the other outcome measures. Secondary outcomes are shown in Tables 19 and 20. For example, global satisfaction, global improvement and EQ-VAS scores were more improved in the high-volume TAI group. These results were based on an ITT analysis and, therefore, included two patients who crossed over before 3 months (possibly diluting the difference in effect).
| Intervention | Baseline, mean (SD) | Baseline, median (IQR) | 3 months, mean (SD) | 3 months, median (IQR) | Difference in means (95% CI) |
|---|---|---|---|---|---|
| Low-volume TAI (n = 19) | 2.4 (0.8) | 2.4 (1.9–2.9) | 2.2 (0.8) | 2.0 (1.5–2.8) | Reference |
| High-volume TAI (n = 25) | 2.3 (0.7) | 2.4 (1.8–2.8) | 1.8 (0.9) | 1.8 (1.1–2.3) | –0.37 (–0.89 to 0.15) |
| Intervention | 3 months | 6 months | 12 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline, mean (SD) | Follow-up, mean (SD) | Baseline, median (IQR) | Follow-up, median (IQR) | n | Baseline, mean (SD) | Follow-up, mean (SD) | Baseline, median (IQR) | Follow-up, median (IQR) | n | Baseline, mean (SD) | Follow-up, mean (SD) | Baseline, median (IQR) | Follow-up, median (IQR) | |
| PAC-QoL score (points) | |||||||||||||||
| Overall | |||||||||||||||
| Low-volume TAI | 19 | 2.4 (0.8) | 2.2 (0.8) | 2.4 (1.9–2.9) | 2.0 (1.5–2.8) | 19 | 2.4 (0.7) | 1.8 (0.9) | 2.4 (1.9–2.9) | 1.4 (1.1–2.6) | 14 | 2.5 (0.7) | 2.1 (0.9) | 2.5 (2.0–2.9) | 1.9 (1.4–2.7) |
| High-volume TAI | 25 | 2.3 (0.7) | 1.8 (0.9) | 2.4 (1.8–2.8) | 1.8 (1.1–2.3) | 21 | 2.2 (0.7) | 1.4 (0.8) | 2.4 (1.8–2.8) | 1.2 (0.9–1.9) | 18 | 2.3 (0.7) | 1.5 (0.9) | 2.3 (1.8–2.8) | 1.1 (0.9–2.1) |
| Dissatisfaction | |||||||||||||||
| Low-volume TAI | 19 | 3.4 (0.4) | 2.8 (0.7) | 3.4 (3.2–3.8) | 3.0 (2.2–3.4) | 19 | 3.4 (0.4) | 2.2 (0.9) | 3.4 (3.2–3.6) | 2.2 (1.8–2.8) | 14 | 3.4 (0.4) | 2.5 (1.0) | 3.4 (3.2–3.6) | 2.5 (2.0–3.2) |
| High-volume TAI | 24 | 3.1 (0.7) | 2.5 (0.9) | 3.2 (2.8–3.6) | 2.8 (2.1–3.1) | 20 | 3.0 (0.7) | 2.1 (1.1) | 3.1 (2.6–3.6) | 2.1 (1.3–3.0) | 17 | 3.1 (0.7) | 2.2 (1.1) | 3.2 (2.8–3.6) | 2.2 (1.4–2.4) |
| Physical discomfort | |||||||||||||||
| Low-volume TAI | 18 | 2.7 (0.9) | 2.4 (0.9) | 2.9 (2.3–3.3) | 2.3 (2.0–3.0) | 19 | 2.6 (0.9) | 1.8 (1.0) | 2.8 (2.0–3.3) | 1.8 (1.0–2.8) | 14 | 2.6 (0.9) | 2.1 (1.2) | 2.9 (2.3–3.3) | 2.5 (1.3–2.8) |
| High-volume TAI | 25 | 2.6 (0.7) | 1.9 (0.9) | 2.5 (2.3–3.0) | 1.8 (1.3–2.3) | 20 | 2.4 (0.6) | 1.3 (0.9) | 2.5 (2.1–2.8) | 1.3 (0.6–2.0) | 18 | 2.4 (0.6) | 1.6 (0.9) | 2.5 (2.3–2.8) | 1.3 (1.0–2.0) |
| Psychosocial discomfort | |||||||||||||||
| Low-volume TAI | 19 | 1.6 (1.0) | 1.5 (1.1) | 1.5 (0.8–2.8) | 1.3 (0.8–2.4) | 19 | 1.6 (1.0) | 1.3 (1.1) | 1.5 (0.9–2.3) | 0.9 (0.5–2.3) | 14 | 2.0 (1.1) | 1.7 (1.3) | 1.9 (1.1–2.9) | 1.6 (0.5–3.1) |
| High-volume TAI | 25 | 1.7 (1.0) | 1.2 (0.9) | 1.8 (0.8–2.6) | 0.9 (0.6–1.9) | 21 | 1.5 (1.1) | 0.8 (0.8) | 1.3 (0.6–2.4) | 0.6 (0.3–1.1) | 18 | 1.5 (1.1) | 1.0 (0.9) | 1.2 (0.6–2.5) | 0.6 (0.4–1.8) |
| Worries and concerns | |||||||||||||||
| Low-volume TAI | 19 | 2.4 (1.0) | 2.3 (1.0) | 2.6 (1.5–3.1) | 2.2 (1.6–2.8) | 19 | 2.4 (0.9) | 1.8 (1.1) | 2.6 (1.9–3.1) | 1.5 (0.9–2.5) | 14 | 2.5 (0.8) | 2.0 (1.1) | 2.8 (2.1–3.1) | 2.0 (1.2–2.5) |
| High-volume TAI | 25 | 2.4 (1.0) | 1.9 (1.1) | 2.4 (1.8–3.1) | 1.8 (1.0–2.4) | 21 | 2.3 (0.9) | 1.4 (1.1) | 2.3 (1.8–3.0) | 1.0 (0.6–1.9) | 18 | 2.4 (0.9) | 1.5 (1.1) | 2.2 (1.8–3.0) | 1.0 (0.6–2.6) |
| PAC-SYM score (points) | |||||||||||||||
| Overall | |||||||||||||||
| Low-volume TAI | 19 | 2.0 (0.8) | 1.8 (0.8) | 2.0 (1.4–2.6) | 1.8 (1.2–2.1) | 19 | 2.0 (0.8) | 1.4 (0.8) | 2.0 (1.4–2.4) | 1.4 (0.8–1.7) | 14 | 2.0 (0.9) | 1.6 (0.8) | 1.8 (1.3–2.4) | 1.5 (0.8–2.3) |
| High-volume TAI | 25 | 2.0 (0.7) | 1.4 (0.7) | 2.1 (1.4–2.5) | 1.4 (1.0–1.8) | 21 | 1.9 (0.6) | 1.2 (0.6) | 1.9 (1.4–2.4) | 1.3 (0.7–1.7) | 18 | 1.9 (0.6) | 1.3 (0.6) | 1.9 (1.4–2.4) | 1.3 (0.8–1.7) |
| Stool symptoms | |||||||||||||||
| Low-volume TAI | 19 | 2.2 (1.0) | 2.0 (1.0) | 2.2 (1.6–3.0) | 2.0 (1.0–2.4) | 19 | 2.1 (1.0) | 1.5 (0.9) | 2.2 (1.4–3.0) | 1.6 (0.8–2.0) | 14 | 2.0 (0.8) | 1.7 (0.9) | 2.0 (1.4–2.4) | 1.6 (1.2–2.4) |
| High-volume TAI | 25 | 2.2 (1.1) | 1.6 (0.8) | 2.4 (1.6–3.0) | 1.8 (1.2–2.0) | 21 | 2.1 (1.1) | 1.4 (0.8) | 2.4 (1.4–3.0) | 1.4 (0.8–2.0) | 18 | 2.1 (1.0) | 1.6 (0.9) | 2.3 (1.4–3.0) | 1.8 (0.8–2.4) |
| Abdominal symptoms | |||||||||||||||
| Low-volume TAI | 19 | 2.3 (1.2) | 2.0 (0.9) | 2.0 (2.0–3.5) | 2.0 (1.3–2.8) | 19 | 2.5 (1.1) | 1.7 (1.0) | 2.3 (2.0–3.5) | 1.5 (0.8–2.3) | 14 | 2.5 (1.2) | 1.9 (1.1) | 2.4 (2.0–4.0) | 2.0 (0.8–2.8) |
| High-volume TAI | 25 | 2.3 (0.7) | 1.8 (0.9) | 2.5 (1.8–2.8) | 2.0 (1.3–2.3) | 21 | 2.1 (0.7) | 1.4 (0.7) | 2.0 (1.8–2.8) | 1.5 (1.0–2.0) | 18 | 2.2 (0.8) | 1.4 (0.8) | 2.4 (1.8–2.8) | 1.5 (0.8–2.3) |
| Rectal symptoms | |||||||||||||||
| Low-volume TAI | 19 | 1.1 (1.1) | 1.0 (1.3) | 1.0 (0.3–1.7) | 0.3 (0.3–1.7) | 19 | 1.1 (1.0) | 0.8 (0.8) | 1.0 (0.3–1.7) | 1.0 (0.0–1.3) | 14 | 1.2 (1.1) | 1.0 (1.1) | 0.8 (0.3–1.7) | 0.7 (0.3–1.3) |
| High-volume TAI | 25 | 1.1 (0.7) | 0.6 (0.7) | 1.0 (0.7–1.7) | 0.3 (0.0–1.0) | 21 | 1.0 (0.7) | 0.5 (0.6) | 1.0 (0.3–1.3) | 0.3 (0.0–1.0) | 18 | 1.1 (0.7) | 0.8 (0.9) | 1.2 (0.3–1.7) | 0.3 (0.0–1.3) |
| Irrigation journal | |||||||||||||||
| Average number of times per week that the patient has used their anal irrigation | |||||||||||||||
| Low-volume TAI | 19 | – | 5.2 (7.5) | – | 4.0 (1.0–7.0) | 19 | – | 3.3 (2.5) | – | 3.0 (2.0–6.0) | 17 | – | 2.3 (2.1) | – | 2.0 (1.0–3.0) |
| High-volume TAI | 21 | – | 3.1 (2.1) | – | 3.0 (2.0–4.0) | 22 | – | 2.8 (2.5) | – | 3.0 (0.0–4.0) | 19 | – | 2.6 (2.1) | – | 3.0 (0.0–4.0) |
| Average volume (ml) of water used per irrigationa | |||||||||||||||
| Low-volume TAI | 16 | – | 147.8 (94.2) | – | 143.5 (70.0–210.0) | 13 | – | 97.8 (92.6) | – | 70.0 (0.0–170.0) | 11 | – | 83.2 (102.9) | – | 70.0 (0.0–210.0) |
| High-volume TAI | 26 | – | 579.8 (296.0) | – | 570.0 (400.0–750.0) | 32 | – | 518.3 (316.4) | – | 500.0 (376.5–745.0) | 25 | – | 535.7 (381.7) | – | 500.0 (373.0–703.0) |
| Diary data | |||||||||||||||
| Bowel frequency: mean number of attempts to empty bowels over 2 weeks | |||||||||||||||
| Low-volume TAI | 19 | 19.7 (11.3) | 17.3 (10.6) | 18.0 (11.0–28.0) | 20.0 (7.0–23.0) | 15 | 18.1 (8.3) | 19.5 (22.6) | 18.0 (11.0–22.0) | 11.0 (10.0–22.0) | 12 | 21.8 (12.2) | 19.2 (13.7) | 20.5 (12.0–30.0) | 17.5 (10.5–23.0) |
| High-volume TAI | 18 | 23.1 (14.1) | 19.3 (9.8) | 21.5 (13.0–33.0) | 16.5 (12.0–26.0) | 17 | 25.0 (13.8) | 16.6 (9.1) | 23.0 (17.0–34.0) | 16.0 (10.0–22.0) | 13 | 24.1 (16.5) | 18.4 (12.8) | 20.0 (13.0–37.0) | 13.0 (7.0–25.0) |
| Bowel frequency: mean number of times stool was passed over 2 weeks | |||||||||||||||
| Low-volume TAI | 18 | 11.8 (10.2) | 10.9 (6.9) | 9.5 (5.0–15.0) | 10.0 (7.0–13.0) | 16 | 8.8 (5.3) | 12.8 (13.9) | 8.0 (4.5–13.0) | 9.5 (6.0–15.0) | 13 | 12.2 (11.6) | 11.2 (8.7) | 9.0 (5.0–13.0) | 10.0 (5.0–15.0) |
| High-volume TAI | 18 | 13.1 (11.2) | 13.3 (8.2) | 10.0 (6.0–17.0) | 11.5 (8.0–16.0) | 17 | 14.2 (11.0) | 11.7 (5.3) | 11.0 (7.0–17.0) | 9.0 (8.0–15.0) | 13 | 12.2 (12.6) | 12.5 (9.0) | 10.0 (4.0–15.0) | 9.0 (7.0–18.0) |
| Nature of bowel movement: mean number of days (out of 14) laxatives used | |||||||||||||||
| Low-volume TAI | 19 | 21.7 (6.2) | 22.7 (5.5) | 23.0 (14.0–28.0) | 25.0 (17.0–28.0) | 16 | 22.4 (5.7) | 22.4 (5.5) | 24.0 (16.5–28.0) | 23.0 (18.5–28.0) | 13 | 20.8 (6.0) | 22.8 (6.5) | 23.0 (14.0–26.0) | 25.0 (20.0–28.0) |
| High-volume TAI | 18 | 22.7 (5.6) | 23.1 (5.7) | 25.0 (17.0–28.0) | 26.0 (20.0–28.0) | 17 | 24.1 (5.2) | 23.4 (6.0) | 27.0 (22.0–28.0) | 27.0 (17.0–28.0) | 13 | 22.0 (6.0) | 24.4 (5.7) | 23.0 (16.0–28.0) | 28.0 (23.0–28.0) |
| Nature of bowel movement: mean number of days (out of 14) glycerine suppositories used | |||||||||||||||
| Low-volume TAI | 18 | 26.5 (3.5) | 26.7 (3.7) | 28.0 (27.0–28.0) | 28.0 (28.0–28.0) | 15 | 26.7 (3.6) | 27.5 (1.4) | 28.0 (27.0–28.0) | 28.0 (28.0–28.0) | 12 | 26.3 (4.2) | 26.1 (5.3) | 28.0 (27.5–28.0) | 28.0 (28.0–28.0) |
| High-volume TAI | 18 | 26.6 (2.7) | 26.8 (3.3) | 28.0 (26.0–28.0) | 28.0 (27.0–28.0) | 17 | 25.6 (4.1) | 27.4 (1.5) | 28.0 (24.0–28.0) | 28.0 (28.0–28.0) | 13 | 26.7 (2.9) | 27.6 (1.0) | 28.0 (27.0–28.0) | 28.0 (28.0–28.0) |
| EQ-VAS score (points) | |||||||||||||||
| Low-volume TAI | 19 | 60.8 (22.7) | 56.1 (26.0) | 65.0 (45.0–75.0) | 60.0 (40.0–75.0) | 18 | 61.1 (21.2) | 63.3 (22.4) | 67.5 (50.0–75.0) | 68.5 (60.0–80.0) | 13 | 62.7 (21.9) | 49.6 (19.6) | 70.0 (60.0–75.0) | 50.0 (40.0–64.0) |
| High-volume TAI | 25 | 72.5 (19.0) | 70.0 (25.9) | 80.0 (65.0–90.0) | 80.0 (62.0–90.0) | 21 | 69.7 (19.2) | 67.7 (22.4) | 80.0 (50.0–80.0) | 70.0 (49.0–85.0) | 17 | 67.2 (20.4) | 69.2 (22.1) | 70.0 (50.0–80.0) | 80.0 (50.0–85.0) |
| PHQ-9 score (points) | |||||||||||||||
| Low-volume TAI | 19 | 9.7 (7.1) | 9.1 (6.9) | 10.0 (3.0–17.0) | 6.0 (4.0–15.0) | 19 | 9.4 (7.0) | 9.3 (7.6) | 8.0 (3.0–17.0) | 5.0 (4.0–11.0) | 14 | 9.6 (8.3) | 11.4 (8.5) | 6.5 (3.0–17.0) | 9.5 (4.0–21.0) |
| High-volume TAI | 24 | 6.6 (6.4) | 8.1 (8.1) | 5.0 (1.5–11.0) | 4.0 (1.0–17.0) | 21 | 6.0 (5.8) | 6.6 (6.8) | 3.0 (2.0–10.0) | 4.0 (1.0–9.0) | 18 | 6.4 (5.7) | 7.1 (6.7) | 5.0 (2.0–10.0) | 6.0 (2.0–10.0) |
| GAD-7 score (points) | |||||||||||||||
| Low-volume TAI | 19 | 7.8 (6.5) | 6.9 (6.5) | 6.0 (3.0–13.0) | 6.0 (2.0–12.0) | 19 | 7.6 (6.4) | 7.4 (6.2) | 5.0 (3.0–13.0) | 7.0 (3.0–9.0) | 14 | 7.7 (7.5) | 9.9 (8.1) | 4.5 (1.0–13.0) | 9.0 (3.0–18.0) |
| High-volume TAI | 24 | 6.0 (5.8) | 6.9 (6.9) | 4.0 (2.0–8.0) | 4.0 (1.0–13.1) | 21 | 4.8 (4.3) | 5.9 (6.7) | 4.0 (2.0–6.0) | 4.0 (0.0–12.0) | 18 | 4.9 (4.5) | 6.6 (7.4) | 4.0 (2.0–6.0) | 3.5 (0.0–14.0) |
| Global patient satisfaction score (points) | |||||||||||||||
| Low-volume TAI | 19 | – | 2.0 (1.2) | – | 2.0 (1.0–3.0) | 19 | – | 2.5 (1.0) | – | 3.0 (2.0–3.0) | 14 | – | 2.0 (1.2) | – | 2.0 (2.0–3.0) |
| High-volume TAI | 24 | – | 2.6 (1.1) | – | 2.5 (2.0–3.5) | 21 | – | 2.9 (0.9) | – | 3.0 (3.0–3.0) | 18 | – | 2.8 (0.9) | – | 3.0 (3.0–3.0) |
| Global patient improvement score (points) | |||||||||||||||
| Low-volume TAI | 19 | – | 40.4 (31.0) | – | 40.0 (11.0–70.0) | 19 | – | 51.7 (30.1) | – | 56.0 (20.0–80.0) | 14 | – | 46.4 (31.2) | – | 50.0 (20.0–70.0) |
| High-volume TAI | 25 | – | 53.6 (28.7) | – | 60.0 (35.0–70.0) | 21 | – | 70.2 (25.7) | – | 80.0 (70.0–85.0) | 18 | – | 66.1 (24.6) | – | 75.0 (65.0–80.0) |
| Intervention | 3 months | 6 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Indicating problems at baseline, n (%) | Indicating problems at follow-up, n (%) | N | Indicating problems at baseline, n (%) | Indicating problems at follow-up, n (%) | N | Indicating problems at baseline, n (%) | Indicating problems at follow-up, n (%) | |
| Mobility | |||||||||
| Low-volume TAI | 19 | 3 (15.8) | 4 (21.1) | 18 | 3 (16.7) | 4 (22.2) | 13 | 4 (30.8) | 3 (23.1) |
| High-volume TAI | 25 | 8 (32.0) | 6 (24.0) | 21 | 7 (33.3) | 6 (28.6) | 17 | 6 (35.3) | 4 (23.5) |
| Self-care | |||||||||
| Low-volume TAI | 19 | 4 (21.1) | 2 (10.5) | 18 | 3 (16.7) | 3 (16.7) | 13 | 3 (23.1) | 2 (15.4) |
| High-volume TAI | 25 | 4 (16.0) | 3 (12.0) | 21 | 4 (19.0) | 5 (23.8) | 17 | 4 (23.5) | 4 (23.5) |
| Usual activities | |||||||||
| Low-volume TAI | 19 | 9 (47.4) | 7 (36.8) | 18 | 8 (44.4) | 6 (33.3) | 13 | 8 (61.5) | 8 (61.5) |
| High-volume TAI | 25 | 12 (48.0) | 9 (36.0) | 21 | 10 (47.6) | 7 (33.3) | 17 | 7 (41.2) | 6 (35.3) |
| Pain/discomfort | |||||||||
| Low-volume TAI | 19 | 16 (84.2) | 19 (100.0) | 18 | 15 (83.3) | 14 (77.8) | 13 | 11 (84.6) | 10 (76.9) |
| High-volume TAI | 25 | 22 (88.0) | 18 (72.0) | 21 | 18 (85.7) | 13 (61.9) | 17 | 14 (82.4) | 10 (58.8) |
| Anxiety/depression | |||||||||
| Low-volume TAI | 19 | 12 (63.2) | 12 (63.2) | 18 | 11 (61.1) | 10 (55.6) | 13 | 6 (46.2) | 11 (84.6) |
| High-volume TAI | 24 | 13 (54.2) | 13 (54.2) | 21 | 11 (52.4) | 10 (47.6) | 17 | 9 (52.9) | 9 (52.9) |
Further evidence of the greater benefit of high-volume TAI could be inferred from the fact that 15 patients switched from low-volume to high-volume TAI but only four patients switched from high-volume to low-volume TAI and two of these six switched back again to high-volume TAI (Table 21).
| Characteristic, n | Assigned to low-volume TAI (N = 30) | Assigned to high-volume TAI (N = 35) | Total (N = 65) |
|---|---|---|---|
| Number of patients who switched from low-volume to high-volume TAI | 15 | 0 | 15 |
| Number of patients who switched from high-volume to low-volume TAI | 4a | 4 | 8 |
| Number of patients who discontinued therapy | 4 | 11 | 15 |
| Numbers of patients who switched and/or discontinued therapy | 17 | 12 | 29 |
| Reason for withdrawal | |||
| Sensation of incomplete evacuation | 1 | 3 | 4 |
| Frequent trips to the toilet | 0 | 2 | 2 |
| Satisfactory defaecation achieved with very low volumes (< 300 ml) | 0 | 1 | 1 |
| No significant effect on symptoms or QoL | 2 | 5 | 7 |
| Treatment not acceptable to patient | 0 | 1 | 1 |
| Excess bloating | 0 | 1 | 1 |
| Excess pain | 0 | 3 | 3 |
| Other AE | 0 | 1 | 1 |
| Other reason | 1 | 1 | 2 |
| Total | 4 | 18 | 22 |
Many of the treatment responses improved over time, suggesting a possible beneficial effect of TAI. This improvement cannot be definitively assigned to the treatment owing to confounding factors such as regression to the mean, loss of participants and the absence of a sham control. However, it is reasonable to postulate that survival rate of therapy (being both cumbersome and unpleasant) indicated ongoing patient perception that it had clinical benefit.
A secondary aim of CapaCiTY trial 2 was to observe the survival rate of TAI therapy against time, this being a surrogate of ongoing perceived patient benefit (given the invasive and time-consuming nature of self-administering the therapy). Figure 13 shows that three-quarters of patients were still using TAI at 12 months – an impressively high number. Concomitant medicine usage was roughly equal in both groups (Table 22).
FIGURE 13.
Survival rate of TAI therapy: Kaplan–Meier plot for time to cessation of treatment (all participants).
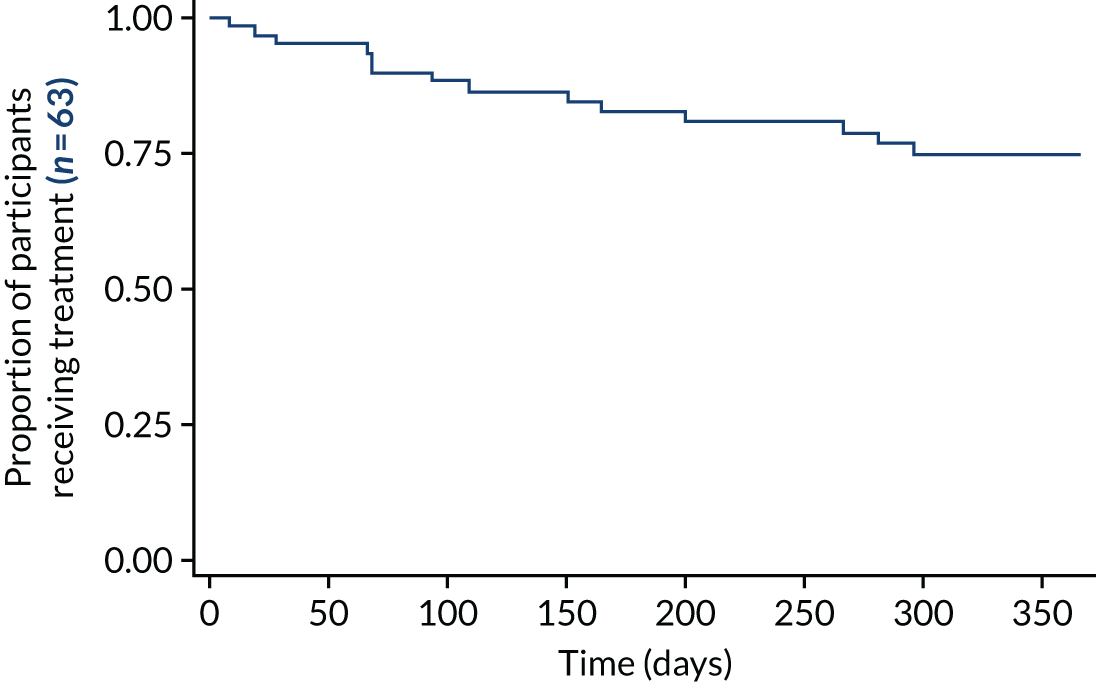
| Characteristic (n) | Low-volume TAI (N = 30) | High-volume TAI (N = 35) | Total (N = 65) |
|---|---|---|---|
| Number of patients reporting the use of concomitant medications | 28 | 28 | 56 |
| Number of concomitant medications reported | 171 | 156 | 327 |
Safety analyses
The anal irrigation systems used in CapaCiTY trial 2 are widespread and established in clinical use throughout the NHS, with known AEs (22%) being mostly low grade and reversible. All trial interventions were as per the standard care provided in the NHS for CC. Related AEs and SAEs were recorded on the CRF and in the medical notes to enable assessment and reporting in line with sponsor and regulatory requirements. Causality was at the discretion of the health-care provider (e.g. research nurse, physiotherapist, principal investigator, delegated member of team).
Serious adverse events that were considered ‘related’ and ‘unexpected’ were reported to the sponsor within 24 hours of learning of the event and to the main REC within 15 days, in line with the required time frame. The number of patients reporting AEs/SAEs was presented by trial group.
The study (not being of a medicinal product) did not record unrelated AEs. SAEs and related AEs were small in number, with six SAEs reported (Table 23). None of these was related to trial treatment and all were resolved.
| Characteristic (n) | Low-volume TAI (N = 30) | High-volume TAI (N = 35) | Total (N = 65) |
|---|---|---|---|
| Related AEs | |||
| Number of patients reporting AE | 11 | 5 | 16 |
| Number of AEs reported | |||
| Abdominal cramping | 1 | 0 | 1 |
| Abdominal pain | 3 | 3 | 6 |
| Anal/rectal pain or discomfort | 17 | 9 | 26 |
| Constipation | 1 | 0 | 1 |
| Loose motions | 1 | 0 | 1 |
| Pain | 1 | 4 | 5 |
| Rectal bleeding | 10 | 12 | 22 |
| Miscellaneous | 2 | 4 | 6 |
| Severity | |||
| Mild | 22 | 18 | 40 |
| Moderate | 8 | 12 | 20 |
| Severe | 4 | 2 | 6 |
| Life-threatening | 2 | 0 | 2 |
| Action | |||
| No action taken | 23 | 22 | 45 |
| Withdrawal | 0 | 3 | 3 |
| Concomitant medication | 5 | 5 | 10 |
| Non-drug therapy | 6 | 2 | 8 |
| SAE, n | |||
| Number of patients reporting SAE | 6 | 0 | 6 |
| Number of SAEs reported | |||
| Life-threatening, unlikely to be related | |||
| Life-threatening, related | |||
| Required hospitalisation or prolongation of existing hospitalisation, unlikely to be related | 6 | 0 | 6 |
| Required hospitalisation or prolongation of existing hospitalisation, related | |||
| Expectedness | |||
| Expected | 3 | 0 | 3 |
| Unexpected | 3 | 0 | 3 |
| Action taken | |||
| No action taken | 1 | 0 | 1 |
| Non-drug therapy | 1 | 0 | 1 |
| Hospitalisation | 4 | 0 | 4 |
Results: cost-effectiveness
Summary and completeness of data
Participants were allocated randomly to low-volume or high-volume TAI. Variables used to estimate costs and QALYs are described in Table 24. The average training time for the two methods was similar. Participants were instructed to make daily use of their allocated method for the first 14 days, reducing to alternate days depending on response. Estimated use of TAI was similar in the two groups during the first month, but thereafter a difference emerged. Average daily use in the high-volume group diminished over time, due primarily to the number of users diminishing, and there was very little crossover to low-volume TAI. There was a similar decrease in low-volume TAI use but substantial crossover to high-volume TAI over the duration of follow-up. The cost of TAI was the predominant determinant of overall cost in each period. TAI treatment was provided as a prescription and the baseline analysis estimated the number of prescriptions of TAI equipment received (see Table 24). Levels of use of (non-irrigation) prescription drugs and community health-care were relatively low and similar comparing groups. Hence, cost differences were driven by the intervention costs. Societal costs reflected a minority (≈ 20%) of patients reporting substantial time away from work. Reported days away from work in the periods 4–6 and 7–12 months suggest more return to work in the high-volume group, contributing to a lower societal cost, although the cost difference is not statistically significant (p = 0.34). Completeness of cost data diminishes as follow-up progresses from 86% in the first period to 48% in the last period. Comparing QoL scores, there is an apparent trend of improving QoL in the high-volume TAI group not apparent in the low-volume TAI group, although this may be confounded by increasing missingness of data. Again, completeness of EQ-5D-5L data decreases as follow-up proceeds, from 94% to 62%.
| Characteristic | Low-volume TAI (N = 30) | High-volume TAI (N = 35) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | |
| Resource use | ||||||
| 0–1 months | ||||||
| Training time, hours | 1.53 | 0.51 | 29 | 1.60 | 0.46 | 34 |
| Low-volume TAI, days | 21.90 | 0.00 | 24 | 0.00 | 0.00 | 32 |
| High-volume TAI, days | 0.00 | 0.00 | 30 | 19.29 | 6.70 | 35 |
| Number of prescriptions | 1.85 | 1.83 | 26 | 1.19 | 1.20 | 32 |
| 1–3 months | ||||||
| Low-volume TAI, days | 22.23 | 14.41 | 21 | 0.00 | 0.00 | 26 |
| High-volume TAI, days | 3.54 | 9.17 | 30 | 14.30 | 12.86 | 35 |
| Number of prescriptions | 0.91 | 1.11 | 22 | 0.96 | 0.93 | 23 |
| GP visits | 0.05 | 0.22 | 21 | 0.00 | 0.00 | 22 |
| District nurse visits | 0.00 | 0.00 | 21 | 0.00 | 0.00 | 22 |
| Pharmacist visits | 0.05 | 0.22 | 21 | 0.50 | 1.47 | 22 |
| A&E visits | 0.19 | 0.68 | 21 | 0.00 | 0.00 | 22 |
| Outpatient visits | 0.05 | 0.22 | 21 | 0.18 | 0.39 | 22 |
| Days from work | 3.52 | 9.87 | 21 | 4.59 | 21.09 | 22 |
| Inpatient visits | 0.00 | 0.00 | 20 | 0.00 | 0.00 | 22 |
| 4–6 months | ||||||
| Low-volume TAI, days | 16.82 | 20.88 | 22 | 0.33 | 1.60 | 32 |
| High-volume TAI, days | 12.87 | 17.24 | 30 | 15.28 | 17.90 | 35 |
| Number of prescriptions | 1.36 | 2.48 | 22 | 0.71 | 1.30 | 24 |
| GP visits | 0.05 | 0.22 | 20 | 0.04 | 0.21 | 23 |
| District nurse visits | 0.00 | 0.00 | 20 | 0.00 | 0.00 | 23 |
| Pharmacist visits | 0.40 | 1.19 | 20 | 0.00 | 0.00 | 23 |
| A&E visits | 0.05 | 0.22 | 20 | 0.04 | 0.21 | 23 |
| Outpatient visits | 0.55 | 0.60 | 20 | 0.17 | 0.39 | 23 |
| Days from work | 8.20 | 21.47 | 20 | 0.68 | 2.01 | 22 |
| Inpatient visits | 0.15 | 0.67 | 20 | 0.00 | 0.00 | 22 |
| 7–12 months | ||||||
| Low-volume TAI, days | 10.50 | 22.50 | 20 | 1.84 | 10.55 | 33 |
| High-volume TAI, days | 16.23 | 29.92 | 30 | 22.20 | 31.84 | 35 |
| Number of prescriptions | 0.62 | 1.07 | 21 | 0.65 | 1.11 | 23 |
| GP visits | 0.29 | 0.69 | 17 | 0.05 | 0.23 | 19 |
| District nurse visits | 0.00 | 0.00 | 17 | 0.00 | 0.00 | 19 |
| Pharmacist visits | 0.06 | 0.24 | 17 | 0.05 | 0.23 | 19 |
| A&E visits | 0.00 | 0.00 | 17 | 0.00 | 0.00 | 19 |
| Outpatient visits | 0.24 | 0.44 | 17 | 0.26 | 0.65 | 19 |
| Days from work | 7.24 | 23.93 | 17 | 1.16 | 4.00 | 19 |
| Inpatient visits | 0.00 | 0.00 | 17 | 0.00 | 0.00 | 18 |
| NHS cost (£) | ||||||
| Treatment period (0–1 months) | 370 | 110 | 24 | 462 | 69 | 32 |
| 1–3 months | 294 | 132 | 18 | 284 | 152 | 21 |
| 4–6 months | 525 | 340 | 19 | 435 | 277 | 20 |
| 7–12 months | 556 | 359 | 14 | 565 | 305 | 17 |
| Overall | 1995 | 796 | 10 | 1816 | 629 | 15 |
| Societal cost (£) | ||||||
| Overall | 4985 | 6425 | 10 | 2803 | 3246 | 14 |
| EQ-5D-5L | ||||||
| Baseline (0 months) | 0.64 | 0.28 | 27 | 0.63 | 0.29 | 34 |
| 3 months | 0.68 | 0.24 | 24 | 0.73 | 0.26 | 29 |
| 6 months | 0.58 | 0.31 | 20 | 0.70 | 0.31 | 24 |
| 12 months | 0.65 | 0.35 | 19 | 0.74 | 0.23 | 21 |
| QALYs | ||||||
| 0–12 months | 0.62 | 0.37 | 10 | 0.72 | 0.26 | 15 |
Cost-effectiveness analyses
The base-case model is reported in Table 25. Compared with low-volume TAI, high-volume TAI featured similar costs (median difference –£8, 95% CI –£240 to £221) and significantly higher QoL (0.093 QALYs, 95% CI 0.016 to 0.175 QALYs); these findings are visualised in Figure 14. Incremental NMB values are positive across the commonly used range of WTP thresholds, indicating that high-volume TAI is cost-effective and appears to deliver a gain to the health system compared with alternative choices. These findings are presented as a CEAC showing the probability of being cost-effective at varying WTP thresholds. At the NICE-recommended WTP threshold of £30,000 per QALY, the probability that high-volume TAI is a cost-effective alternative to low-volume TAI is 99%. The EVPI (per subject) reflects the opportunity loss of ongoing uncertainty. At a WTP threshold of £30,000 per QALY, EVPI per subject is just £2, reflecting the certainty of the finding. Assuming that the finding is robust, this would indicate that further research is not merited.
| High-volume vs. low-volume TAI | ICER (95% CI) | IC (95% CI) | IQ (95% CI) | CEAC p-valuea | CEAC p-valueb | NMBa (95% CI) | NMBb (95% CI) | EVPIb |
|---|---|---|---|---|---|---|---|---|
| Base case | c (c to 3409) | –8 (–240 to 221) | 0.093 (0.016 to 0.175) | 0.993 | 0.993 | 1399 (266 to 2648) | 2781 (523 to 5252) | 2 |
| Complete case | c (c to 4837) | –335 (–907 to 313) | 0.087 (–0.082 to 0.264) | 0.907 | 0.887 | 1643 (–888 to 4428) | 2947 (–2091 to 8365) | 160 |
| Sensitivity analysis 1d | 910 (c to 7119) | 83 (–120 to 255) | 0.091 (0.01 to 0.179) | 0.983 | 0.985 | 1277 (105 to 2600) | 2651 (276 to 5281) | 6 |
| Sensitivity analysis 2e | – | – | – | 0.881 | 0.907 | 2097 (–1387 to 5543) | 2378 (–1049 to 5988) | 73 |
FIGURE 14.
The CapaCiTY trial 2 cost-effectiveness analysis: base case. (a) ICER plane [the credible region (dashed circle) shows where 95% of estimates lie]; (b) incremental NMB; (c) CEAC; and (d) EVPI.
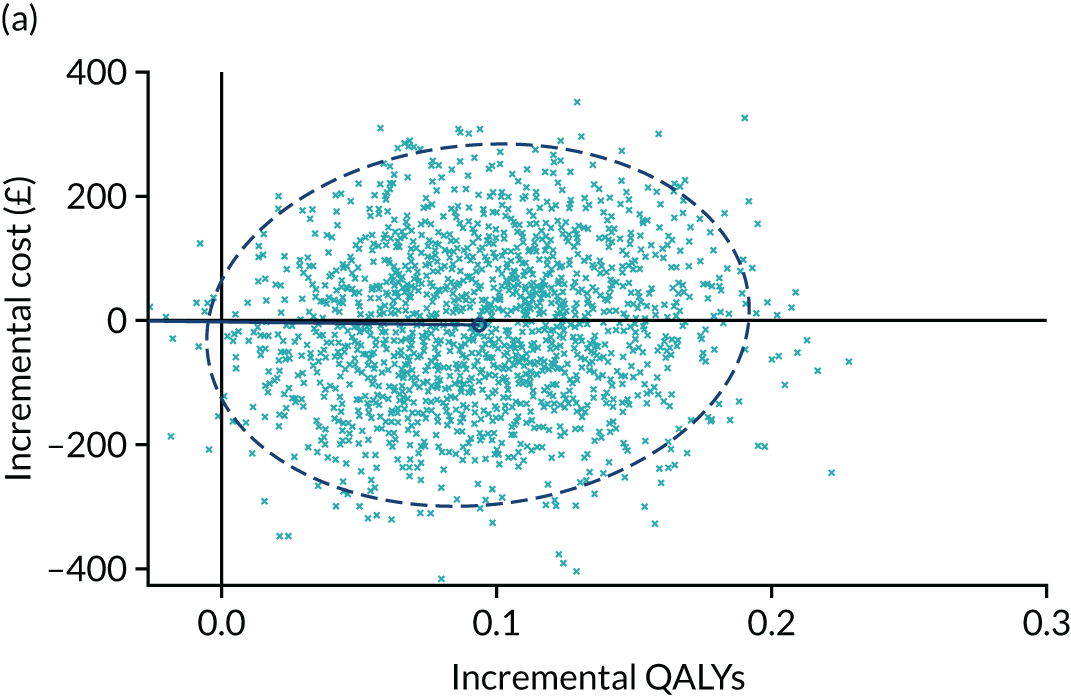
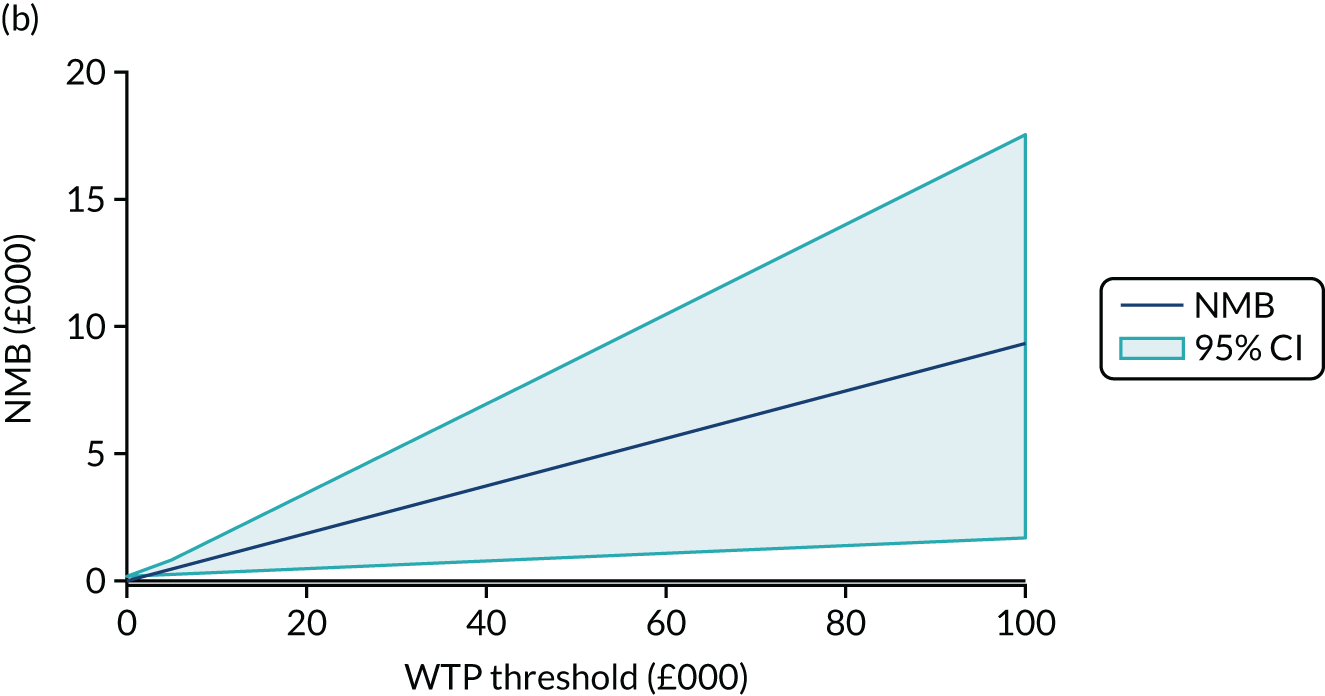
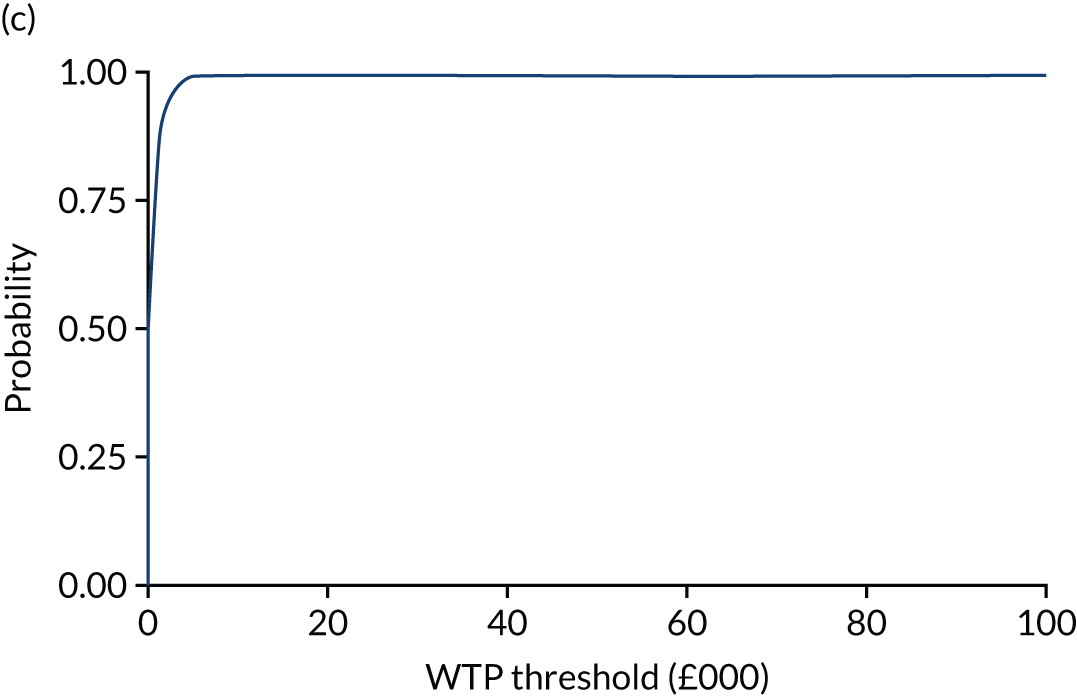
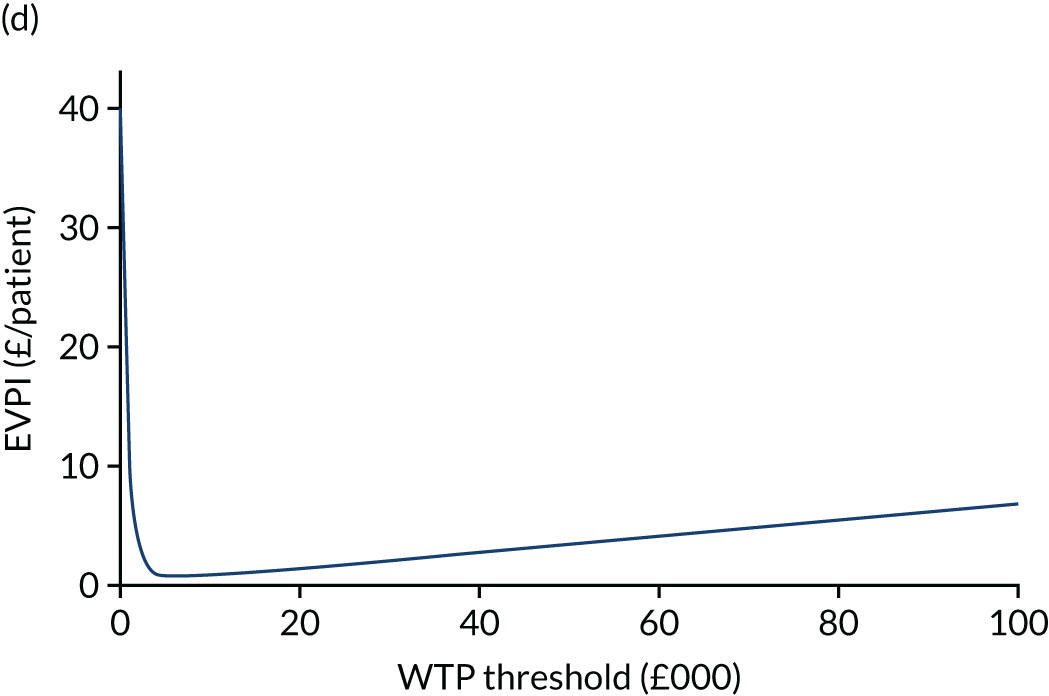
The complete-case analysis is also presented (see Table 26). Although numbers contributing are small, the findings are similar to the base case, if less precise. The base-case analysis costed the number of irrigation prescriptions received by each participant, assuming that unused portions of prescriptions were lost. As a sensitivity analysis the number of daily uses was costed to provide greater granularity to the costing. This marginally increased the incremental costs, but the overall finding remained robust. Given the small numbers of participants in the trial, as a second sensitivity analysis the base-case model was re-estimated as a univariate regression (NMB at varying WTP thresholds) to explore distributional assumptions. Qualitatively, the finding was similar, although at a WTP threshold of £30,000 per QALY the probability that high-volume TAI is cost-effective reduced to 91%.
Results
Patient experience and qualitative analysis
Emergent themes are presented in this section. Numbers in square brackets refer to the numbered quotations in Appendix 2, Quotations from participants contributing to qualitative analysis in all three trials, CapaCiTY trial 2: transanal irrigation.
Rapport with nurse
Several patients mentioned the importance of the positive rapport that they had with the TAI nurse. Staff echoed the importance of rapport and also indicated that demonstrating enthusiasm and belief in the TAI system appeared to assist patients [1].
Transanal irrigation training
Patients recalled the TAI training as a positive experience and particularly liked that they had the option to try the device either in clinic (for the first time) with the nurse close to hand or at home on their own [2]. Patients also found the telephone follow-up support reassuring [3, 4]. Staff noted the importance of these follow-up support telephone calls and how they helped instil confidence in the patients.
Although the information about how to use the TAI system was clear, for some patients it was still a lot of information to take in at once [5]. Therefore, the follow-up telephone calls allowed patients to troubleshoot with the TAI nurse. Staff and patients both felt that the follow-up timings seemed appropriate. For some patients it took a little getting used to the device [6]. However, several found both high-volume and low-volume TAI devices relatively easy to use [7].
Initial expectations of transanal irrigation compared with actual experience
A number of patients spoke about their initial fears about whether or not TAI would be painful, and several found that the device was more comfortable and easier to use than they initially thought. For a few, the TAI system could be uncomfortable, particularly at the beginning [8].
Patient views on randomisation group
Patients views about which group they were randomised to varied. For some, because they had struggled with constipation for years they wanted to have what they considered to be the most effective intervention (i.e. high-volume TAI). Others, particularly if they had a fear of TAI being painful, were pleased to have been in the low-volume TAI group [9]. However, initial trepidation often transformed after discussing the situation with their nurse.
Practical experiences of using transanal irrigation
The time taken to use the TAI device ranged from 10 minutes with the low-volume device to 20–30 minutes with the high-volume device. Patients reported using the TAI systems from once per week to several times per week. Many appeared to have a relatively easy time obtaining the devices.
Some people found TAI relatively easy to fit into their daily routine [10], whereas for others it was more difficult to factor into daily living [11]. A few patients noticed an improvement in their symptoms immediately on using TAI, whereas for others the process took longer (up to a few months) to see results [12]. Some (particularly in the high-volume TAI group) needed to get to grips with the technical elements of using the device before they started to see results.
Impact of transanal irrigation on quality of life
Several patients chose to continue using their TAI device and have found it to be of great benefit in terms of improving their overall health, mood and activity levels [13, 14]. They noted the psychological impact of not having to worry about when they use the toilet, as they now have more control over their bowels through using the TAI device. This means that they are able to plan their days more than they could prior to TAI use. Some patients have noticed that if they did not use the device their constipation symptoms began to return. Some stated that, because of their continued use of TAI, they no longer considered themselves chronically constipated [15]. Others mentioned that, although they did not feel that their bowels were ‘normal’, the TAI device had brought them back to their own sense of ‘normal’. Irrigating daily allowed them to remove a little stool each day, relieving many of the debilitating effects of CC.
For many, the freedom from the ongoing discomfort of CC was the best thing about using the TAI system. Many stated that they would recommend TAI to someone experiencing CC. Some specifically stated that TAI was preferrable to laxatives because of the side effects from these medicines (e.g. bloating, uncontrolled bowels). A few had concerns about their ability to use the device in their later years, whereas others were happy to continue use it indefinitely.
Frustrations with transanal irrigation
Although the devices appeared to work for the most part, there were occasions when TAI was not as effective, which was frustrating for some. Many patients described that, at times, using the device felt like a bit of a chore, but ultimately a chore worth doing because of the beneficial effects. Some indicated that the most difficult part of the treatment was leakage [16]. For others the most difficult part was finding a routine that worked for them.
One patient with no success with low-volume TAI switched to high-volume TAU. The high-volume TAI system did not appear to relieve another patient’s symptoms and she chose to discontinue usage.
Suggestions from patients
Suggestions included:
-
additional pages in the booklet to fill in thoughts and experiences of usage
-
putting the kit together themselves at clinic
-
having the same sex as the intervention nurse.
Staff experience of delivering the intervention
Intervention staff found TAI intervention delivery relatively easy because they typically provided similar TAI support in their clinic. However, they also noted that they needed to be mindful of the way that they delivered even the most seemingly straightforward information because assumptions and miscommunication could contribute towards patients misusing the devices.
Some nurses indicated that they believed that the low-volume TAI is easier for patients to learn and usually quicker to use, but for this particular patient group it may be less effective. By contrast, the high-volume system may seem more onerous, with more steps to learn, and may require perseverance, but is usually more effective for this patient cohort [17].
For nurses, the most satisfying element of the intervention was when it benefited the patient. Likewise, it was frustrating when nurses felt restricted to the research protocol.
These views highlight the clash between clinical agendas (to manage patient concerns with the most suitable treatment) and research agendas (to test which treatments are most effective through randomisation, which may not always be in the best interest of the individual patient).
Staff were often under the impression that high-volume TAI was the most appropriate and most effective treatment for patients.
It was particularly difficult to convince patients who were allocated to low-volume TAI to ‘stick with’ a treatment that nurses believed would not work. Specifically, it was difficult to persuade frustrated patients with a long history of constipation to maintain an intervention that was not currently providing benefits. However, a few nurses did indicate that it may have been helpful to start some patients on low-volume TAI first, because the kit is somewhat easier to use, and then graduate patients up to high-volume TAI after their confidence has increased.
At times participants were removed from the study in the best interest of the patient (e.g. if interventions were not working). Furthermore, at times patients and/or staff did not believe that they could delay treatment any longer, which affected recruitment rates.
CapaCiTY trial 3: stepped-wedge randomised trial of laparoscopic ventral mesh rectopexy in adults with chronic constipation
Intervention
Participants attended for surgery at their allocated time, with admissions procedures as per routine clinical care with normal preparation, for example bowel cleansing.
Surgical procedure
Perioperative care used normal adjuncts [including informed NHS consent, World Health Organization surgical checklists, appropriate broad-spectrum antibiotic prophylaxis, venous thromboembolism prevention, patient warming and urinary catheter insertion]. Surgery could be performed as a day case procedure in an enhanced recovery programme, although most patients had an overnight stay. Consent included discussion of the risks of conversion to open surgery and specific complications. A phosphate enema or similar (optional) could be used to clear the rectum.
Figure 15 illustrates lapVMR surgical technique. Exact surgical technique was surgeon specific (i.e. based on individual preference) but in accord with expert guidance and training. All participating surgeons required sign-off by a delegated surgical team provided by the Pelvic Floor Society, an affiliate of the Association of Coloproctology of Great Britain and Ireland. Where required, preceptorship was provided to meet sign-off requirements.
FIGURE 15.
Laparoscopic ventral mesh rectopexy surgical technique. Reprinted by permission from Springer Nature: Surgical Endoscopy, Laparoscopic ventral recto(colpo)pexy for rectal prolapse: surgical technique and outcome for 109 patients, D’Hoore et al. 144 © 2006.
In brief, after positioning the patient (in a modified lithotomy position on a non-slip mat) and port site insertion (using standard equipment and technique), the rectosigmoid junction is retracted to the left and a peritoneal incision is made over the right side of the sacral promontory and extended in an inverted J form along the rectum and over the deepest part of the pouch of Douglas. Special care is taken not to damage the right hypogastric nerve. Denonvillier’s fascia is incised and the rectovaginal septum is broadly opened. Limited rectal mobilisation and lateral dissection are performed as required to expose the distal rectum and pelvic floor. A strip of trimmed mesh (biologic or synthetic) is inserted. Using slowly absorbable sutures (polydioxanone recommended), the mesh is sutured to the ventral aspect of the distal rectum and further fixed to the lateral seromuscular borders of the rectum proximal and distal to the incised pouch of Douglas and/or pelvic floor. The proximal end of the mesh is fixed on the sacral promontory using either sutures or an endofascia stapler. Limited traction is exerted on the rectum as required to obliterate the intussusception and/or rectocele. If deemed necessary, the posterior vaginal fornix may be elevated and sutured to the anterior aspect of the mesh; this allows closure of the rectovaginal septum and correction of a mid-compartment prolapse, if present. The lateral borders of the incised peritoneum are then closed over the mesh. This elevates the new pouch of Douglas over the colpopexy and completely covers the mesh with peritoneum. No drain is usually required. Ports should be closed directly [using an Endoclose™ device (Medtronic Ltd, Watford, UK) for lateral ports] owing to the high risk of early and late port site hernias in this group of patients with potential connective tissue laxity.
Postoperative management
Postoperative management was as per routine clinical care, usually an overnight hospital stay followed by urinary catheter removal, mobilisation and discharge. Postoperative laxative use was standardised to a weaning course of Movicol (Macrogol, Norgine) or Laxido (Macrogol, Galen) three times per day immediately postoperatively for 1 day, then reduced according to ease of bowel movements. This prevented postoperative constipation from immobility, narcotics or general anaesthesia, which if left untreated may cause painful straining on the mesh and thus protraction of the sacral promontory periosteum, potentially leading to readmission. Surgeons aimed to discharge patients 1 day postoperatively. However, length of stay was determined by clinical evaluation and could be longer if required.
Laparoscopic ventral mesh rectopexy 30-day follow-up
At 30 days, clinical recurrence of rectal prolapse was determined based on physical examination. Morbidity and mortality data were collected and treatment administered for any complications arising from lapVMR surgery. The 30-day readmission rates were also recorded. A CRF was used to capture intra-operative and postoperative data for surgery-specific outcomes.
Surgical quality assessment
Monitoring and quality control were conducted remotely via video submission and assessed against the standardised lapVMR protocol and defined assessment criteria. Monitoring took the form of planned, random and triggered sessions.
Potential principal investigators had to record and submit two unedited and anonymised videos of lapVMR performed in non-study patients. Each video was allocated to two peer reviewers of a three-member expert panel. Based on blinded assessment of unedited and anonymised videos by expert review, the panel then decided whether or not the principal investigator was ‘adherent’ to the standardised technique. Any disagreement was resolved by consensus after consulting a third independent expert. If deemed ‘non-adherent’ to the standardised technique, the site was notified that a step needed to be corrected and invited to submit another video for similar review. An unedited video of the first patient at each site enrolled in CapaCiTY trial 3 was also reviewed in this manner. Any ‘failure’ to comply with the standardised surgical technique for lapVMR in any submitted video of a study patient would trigger the request for a further submission of the next recruited patient from that centre. A second judgement of ‘non-adherence’ to the standardised technique would trigger an onsite training and monitoring session for the site. Monitoring would continue until adherence was achieved. A third ‘non-adherence’ or ‘failure’ would result in withdrawal of the site/principal investigator from the trial.
Random monitoring
All principal investigators had to record and submit the unedited and anonymised video of the lapVMR performed in a randomly selected patient enrolled in CapaCiTY trial 3 (one in five at site level). The adherence to the standardised technique was established by consensus as described for the planned monitoring.
Triggered monitoring
The DMC reviewed the morbidity and mortality rates and AEs and SAEs from all sites. Safety concerns could trigger additional monitoring or onsite training and mentorship visits to take place by expert panel. Repeated ‘non adherence’ or ‘failure’ to comply would result in withdrawal of the site/principal investigator from the trial.
Standardisation of UK patient selection practice for surgery and laparoscopic ventral mesh rectopexy technique
The CapaCiTY trial 3 required the recruitment of 80 patients to undergo lapVMR. At the time of starting the programme this seemed easily achievable, with several UK centres performing > 100 such operations per annum. It became abundantly clear, however, that many centres’ view of selecting patients for surgery did not agree with expert opinion (even at that time) in terms of defining surgical target pathophysiology (defined by INVEST) or in excluding patients with relative contraindications to surgery. The development of consensus in respect to this and other surgical approaches to patients with CC was timely because it coincided with the rapid evolution of a media storm against the placement of pelvic mesh and worldwide class actions against surgeons and manufacturers of mesh. Although detrimental to recruitment, the increased rigour invoked by the programme in patient selection probably protected some patients (and their surgeons) from further harm.
Selection of surgeons to participate in CapaCiTY trial 3 also required submission of two videos for quality assurance of surgical technique. This highlighted not only unnecessary variation in technique but also some practices that could promote harm from mesh (e.g. use of polyester sutures). Again, although this actually delayed the study, it was probably beneficial to patients.
In regard to subsequent national and international scrutiny of mesh, work performed as part of this programme has featured in national guidance by learned bodies,134 patient-facing information on consent for surgery and several news reports. 145
Design features
Basic design
We used a stepped-wedge randomised trial design to permit observer-masked data comparisons between patients awaiting intervention and those who had undergone surgery. Contrary to most stepped-wedge trials, individual patients rather than clusters were randomised. In brief, eligible participants were randomised to three groups, with different delays before surgery (see Figure 6). In all groups there was a period of 4 weeks post eligibility to arrange the logistics of surgery (T–4 weeks to T0) and ensure that patients had returned to their normal life routine after various assessments. LapVMR was performed at T0 in group 1, T12 in group 2 and T24 in group 3. Unavoidably, participants were aware when surgery was undertaken; however, this fortuitously met the assumptions of the stepped-wedge design (i.e. no effect of treatment is expected until surgery has been performed). Efficacy outcome data were collected at equally stepped time points (T0, T12, T24, T36 and T48).
This was, in effect, a modification of a standard parallel-group, waiting-list control design, but with several advantages. First, a stepped-wedge design is more efficient and thus improves recruitment feasibility (the major hurdle of nearly all surgical trials). Despite the multicentre approach of this study, the problems of recruitment were manifest. Simulation demonstrated that a parallel-group design required a much larger sample size than that proposed for the current study at the same power. Second, the trial design meant that there was only a one-in-three chance (rather than one-in-two chance for a parallel group) of waiting 6 months for surgery, which was more acceptable to patients (see Work programme 5, Patient and public involvement).
Sample size calculation
Sample size was calculated using the primary clinical outcome (PAC-QoL score), but with a 1.0-point change deemed clinically important and sufficient to justify the cost and invasive nature of lapVMR. Previous surgical trials had shown a 1-point decrease in PAC-QoL score from pre surgery to 48 weeks (approximately 1 year) post surgery. 131 Using a stepped-wedge design, we hypothesised that PAC-QoL score at any time point during follow-up would be approximately 1.0 points lower than in preoperative participants.
Sample size was calculated by simulation using the ‘simsam’ package in Stata. We assumed that PAC-QoL score followed a normal distribution over all time points with a SD of 1.5 points and with a correlation between repeated assessments equal to 0.5 points. Simulation showed that detection of a 1.0-point difference in 6-month PAC-QoL score, with 95% power (purposely chosen to reflect the magnitude and risk of intervention) at the 5% significance level, required 34 participants in each of the three groups. Allowing for a 10% loss to follow-up, a sample size of 38 was needed per group (i.e. a total sample size of 114 patients across the three groups). Should the correlation between repeated assessments be < 0.5 points, a sample size of 114 will still provide at least 90% power for the study. This was calculated using the same simulation procedure with correlations of 0.3 and 0.1 points.
Randomisation procedure
Randomisation to the three trial groups (1 : 1 : 1) was stratified by sex, and female participants further stratified by centre (for the reasons outlined for CapaCiTY trial 2; in fact, only females were recruited).
Blinding
Patients and clinicians were necessarily aware of allocation to different waiting times. Quantitative outcomes were collected by investigators unaware of the before or after status of the patient in respect of surgery, and analysis was performed blind to allocation status.
Concomitant medications
Methods as for CapaCiTY trial 1, with recorded ad libitum use.
Statistical methods
Primary outcome
Total PAC-QoL scores at the time points T0, T12, T24, T36, T48, T60 and T72 in the three groups were analysed using a mixed linear regression model, with random effects for participants and a fixed effect of time since randomisation (potentially considering a random effect for time as well to relax the assumption of same time trend for each participant) to estimate mean differences between PAC-QoL score before and after lapVMR. The comparison of primary interest was between PAC-QoL score at 24 weeks after surgery and PAC-QoL score at baseline. A correction was included in the model to account for the small sample size. Missing data were imputed through multiple imputation by chained equations.
Secondary outcomes
Total PAC-SYM scores were analysed using the approach described in the paragraph above. All other secondary clinical outcomes derived from the standardised outcome framework were analysed only at 24 and 48 weeks post operatively. We present mostly descriptive statistics, with simple regression models used to obtain CIs for the results. Results obtained using the CC-BRQ and BIPQ-CC have been omitted pending further analysis.
Because of the nature of the intervention (i.e. pelvic surgery), a further outcome was included in CapaCiTY trial 3 (not included in CapaCiTY trials 1 and 2). The Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire-12 (PISQ-12) is a self-administered questionnaire that evaluates sexual function in heterosexual women with urinary incontinence and/or pelvic organ prolapse. It has 12 items; responses are graded on a 5-point Likert scale from ‘never’ to ‘always’. Scores are calculated by totalling the scores for each question, with 0 indicating never and 4 indicating always. Reverse scoring is used for items 1–4. It can be used with up to two missing responses. To handle missing values, the sum is calculated by multiplying the number of items by the mean of answered items. The PISQ-12 scores can be reported only as a total or on a per-item basis.
All participants randomised to the three groups were analysed according to their allocation ± 2 weeks from the scheduled intervention date.
Cost-effectiveness
Patients were randomised in a stepped-wedge design to (1) immediate lapVMR, (2) lapVMR after a 12-week delay or (3) lapVMR after a 24-week delay. The design was chosen because of efficiency considerations and the logistical constraint of participants wanting surgery. The stepped-wedge design we adopted has three steps and features a closed cohort in which all patients spend time as controls and treated patients. Although the within-patient design is efficient, the step ‘placing’ creates particular challenges for the cost-effectiveness analysis. Economic analysis requires balanced time receiving alternatives to estimate incremental costs and QALYs. The value of surgery may be demonstrated over time frames of 1 or 2 years, and require a balanced pre-surgery period. Patient follow-ups were between 48 and 72 weeks post surgery. However, even using the screening visit as an approximate –12-week time point, each patient had between only 12 and 36 weeks’ pre-surgery follow-up. As has been reported, this trial was also severely hampered by under-recruitment. In an exploratory analysis, the trial was analysed as a simple pre–post within-patient design. Data were too sparse to use multiple imputation to cover an adequate pre-surgery period or postsurgery period beyond 48 weeks. Consequently, growth curve analyses (hierarchical generalised linear model) were used to describe and model pre-surgery and postsurgery trends. These models suggest that pre-surgery EQ-5D-5L scores and costs were constant and could reasonably be used to estimate EQ-5D-5L scores and costs at 48 weeks pre surgery using a ‘first observation carried back’ (FOCB) approach. The pre-surgery period was then conflated into a single period in subsequent analyses to reduce spurious precision. Postsurgery growth curve analysis found stable (non-surgery) costs and that EQ-5D-5L scores, although initially improving, returned to pre-surgery levels by 48 weeks, obviating the need for further extrapolation post surgery. An assumption of the approach taken is that there is no confounding with time (secular change). Although delay to surgery was to be explored in regression of costs and QALYs, the available sample is too underpowered to detect differences. No discounting was applied to economic data in CapaCiTY trial 3, reflecting the within-patient comparison of 48 weeks pre surgery and 48 weeks post surgery.
Recruitment
First recruitment occurred on 1 March 2016 and first intervention on 15 June 2016. Recruitment ended on 31 January 2019. A total of 28 (target 114) patients were randomised out of 81 screened (30.9%) from six sites. Reasons for screen failure are shown in Figure 16. Two sites opened but failed to recruit and one site failed to randomise; the remaining sites (n = 6) randomised 1–11 patients. Among the 28 patients randomised, nine patients were randomised to immediate surgery, 10 patients were randomised to a 12-week waiting list and nine patients were randomised to a 24-week waiting list. Six patients failed to complete the primary outcome at 24 weeks, among whom two dropped out of the study, which was therefore completed by 19 patients. The CONSORT flow diagram is shown in Figure 7.
FIGURE 16.
The CapaCiTY trial 3 screen failures. SNS, sacral nerve stimulation.
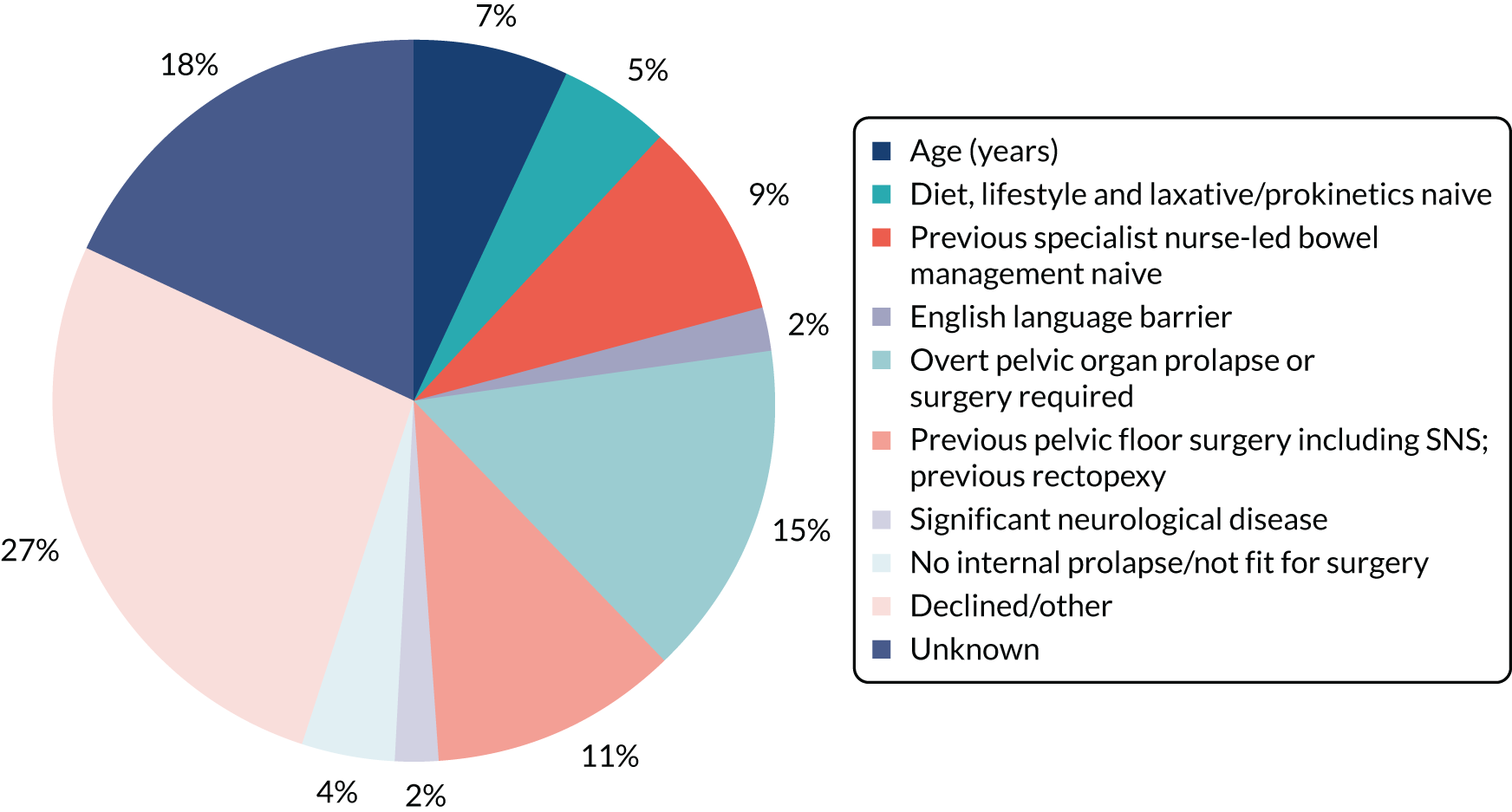
Baseline characteristics
Table 26 shows the numbers and percentages of patients with baseline characteristics by trial group. Continuous variables (e.g. age) were summarised by treatment group using mean and SD (median and IQR if non-normally distributed). Table 27 shows the data for outcome measures at baseline. These were comparable between the two groups for all major characteristics.
| Characteristic | Group 1: lapVMR performed at T0 (N = 9) | Group 2: lapVMR performed at T12 (N = 10)a | Group 3: lapVMR performed at T24 (N = 9) | Total (N = 28) |
|---|---|---|---|---|
| Referral method, n (%) | ||||
| Secondary care | 5 (55.6) | 6 (60.0) | 4 (44.4) | 15 (53.6) |
| Tertiary care | 3 (33.3) | 4 (40.0) | 5 (55.6) | 12 (42.9) |
| Other | 1 (11.1) | 0 (0.0) | 0 (0.0) | 1 (3.6) |
| Demographic characteristic | ||||
| Sex, n (%) | ||||
| Female | 9 (100.0) | 10 (100.0) | 9 (100.0) | 28 (100.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ethnicity, n (%) | ||||
| Asian | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (3.6) |
| Black | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (3.6) |
| Mixed | 1 (11.1) | 0 (0.0) | 0 (0.0) | 1 (3.6) |
| White | 8 (88.9) | 9 (90.0) | 8 (88.9) | 25 (89.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Age (years) | ||||
| Mean (SD) | 52.7 (14.3) | 52.9 (14.4) | 53.4 (9.8) | 53.0 (12.6) |
| Median (IQR) | 59.0 (39.0–66.0) | 56.0 (42.0–64.0) | 55.0 (49.0–58.0) | 55.0 (42.0–64.5) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Past medical history, n (%) | ||||
| Total | 7 (77.8) | 9 (90.0) | 6 (66.7) | 22 (78.6) |
| Cardiovascular condition | 4 (44.4) | 3 (30.0) | 1 (11.1) | 8 (28.6) |
| Heart disease | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (3.6) |
| Hypertension | 3 (33.3) | 2 (20.0) | 1 (11.1) | 6 (21.4) |
| Hypercholesterolaemia | 2 (22.2) | 1 (10.0) | 1 (11.1) | 4 (14.3) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Respiratory condition | 0 (0.0) | 2 (20.0) | 1 (11.1) | 3 (10.7) |
| Asthma | 0 (0.0) | 2 (20.0) | 1 (11.1) | 3 (10.7) |
| COPD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal condition | 5 (55.6) | 3 (30.0) | 2 (22.2) | 10 (35.7) |
| IBS | 3 (33.3) | 3 (30.0) | 1 (11.1) | 7 (25.0) |
| Crohn’s disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ulcerative colitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Colonic polyps | 1 (11.1) | 0 (0.0) | 0 (0.0) | 1 (3.6) |
| Other | 2 (22.2) | 0 (0.0) | 1 (11.1) | 3 (10.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Metabolic condition | 0 (0.0) | 3 (30.0) | 4 (44.4) | 7 (25.0) |
| Diabetes | 0 (0.0) | 1 (10.0) | 1 (11.1) | 2 (7.1) |
| Hypothyroidism | 0 (0.0) | 2 (20.0) | 3 (33.3) | 5 (17.9) |
| Hyperthyroidism | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (3.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Haematological condition | 2 (22.2) | 1 (10.0) | 0 (0.0) | 3 (10.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hepatic condition | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Genito-urinary condition | 2 (22.2) | 0 (0.0) | 2 (22.2) | 4 (14.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neurological/CNS condition | 2 (22.2) | 4 (40.0) | 3 (33.3) | 9 (32.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Psychiatric condition | 2 (22.2) | 5 (50.0) | 4 (44.4) | 11 (39.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dermatological condition | 1 (11.1) | 3 (30.0) | 1 (11.1) | 5 (17.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal condition | 3 (33.3) | 2 (20.0) | 3 (33.3) | 8 (28.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other condition | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (3.6) |
| Past surgical history, n (%) | ||||
| Total | 5 (55.6) | 10 (100.0) | 7 (77.8) | 22 (78.6) |
| Abdominal operation | 2 (22.2) | 3 (30.0) | 2 (22.2) | 7 (25.0) |
| Gynaecological procedure | 4 (44.4) | 9 (90.0) | 5 (55.6) | 18 (64.3) |
| Proctological or perineal procedure | 0 (0.0) | 4 (40.0) | 1 (11.1) | 5 (17.9) |
| Neuromodulation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Duration (months) of constipation symptoms | ||||
| Mean (SD) | 68.7 (36.9) | 63.3 (31.6) | 76.6 (55.4) | 69.5 (41.3) |
| Missing, n (%) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (3.6) |
| Previous lifestyle modifications, n (%) | ||||
| Total | 9 (100.0) | 10 (100.0) | 9 (100.0) | 28 (100.0) |
| Diet | 9 (100.0) | 10 (100.0) | 9 (100.0) | 28 (100.0) |
| Fluid | 9 (100.0) | 10 (100.0) | 9 (100.0) | 28 (100.0) |
| Exercise | 7 (77.8) | 10 (100.0) | 7 (77.8) | 24 (85.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alternative therapies, n (%) | ||||
| Total | 1 (11.1) | 1 (10.0) | 1 (11.1) | 3 (10.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Laxatives, n (%) | ||||
| Total | 9 (100.0) | 10 (100.0) | 9 (100.0) | 28 (100.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antidiarrhoeals, n (%) | ||||
| Total | 0 (0.0) | 2 (20.0) | 1 (11.1) | 3 (10.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prokinetics, n (%) | ||||
| Total | 1 (11.1) | 3 (30.0) | 2 (22.2) | 6 (21.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nurse-led bowel management, n (%) | ||||
| Total | 9 (100.0) | 10 (100.0) | 9 (100.0) | 28 (100.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Family history of bowel disease, n (%) | ||||
| IBS | 2 (22.2) | 2 (20.0) | 2 (22.2) | 6 (21.4) |
| IBD | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (3.6) |
| Gastrointestinal cancer | 1 (11.1) | 3 (30.0) | 2 (22.2) | 6 (21.4) |
| Other | 1 (11.1) | 2 (20.0) | 0 (0.0) | 3 (10.7) |
| Missing | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (3.6) |
| Sexual history (female participants only), n (%) | ||||
| Sexually active | 5 (55.6) | 6 (60.0) | 3 (33.3) | 14 (50.0) |
| Child-bearing potential | 4 (44.4) | 4 (40.0) | 4 (44.4) | 12 (42.9) |
| > 1 year post menopausal | 3 (33.3) | 6 (60.0) | 4 (44.4) | 13 (46.4) |
| Surgically sterile | 3 (33.3) | 5 (50.0) | 4 (44.4) | 12 (42.9) |
| Contraceptive use (female participants only), n (%) | ||||
| Total | 2 (22.2) | 2 (20.0) | 3 (33.3) | 7 (25.0) |
| Barrier | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (3.6) |
| Non-barrier | 2 (22.2) | 1 (10.0) | 3 (33.3) | 6 (21.4) |
| Missing | 4 (44.4) | 3 (30.0) | 1 (11.1) | 8 (28.6) |
| Past obstetric history (female participants only) | ||||
| Total, n (%) | 9 (100.0) | 10 (100.0) | 9 (100.0) | 28 (100.0) |
| Number of vaginal deliveries, mean (SD) | 2.1 (1.1) | 2.5 (1.1) | 2.7 (1.0) | 2.4 (1.0) |
| Number of caesareans, mean (SD) | 1.0 (1.1) | 0.1 (0.3) | 0.4 (1.0) | 0.5 (0.9) |
| Number of forceps/ventouse deliveries, mean (SD) | 0.2 (0.7) | 0.5 (0.7) | 0.3 (0.7) | 0.4 (0.7) |
| Number of episiotomies, mean (SD) | 1.1 (1.1) | 0.2 (0.4) | 1.0 (1.2) | 0.8 (1.0) |
| Number of obstetric tears, mean (SD) | 0.6 (0.7) | 0.4 (0.5) | 0.6 (0.5) | 0.5 (0.6) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Faecal incontinence symptoms, n (%) | ||||
| Total | 7 (77.8) | 9 (90.0) | 7 (77.8) | 23 (82.1) |
| Faecal urgency | 4 (44.4) | 8 (80.0) | 5 (55.6) | 17 (60.7) |
| Urge faecal incontinence | 5 (55.6) | 6 (60.0) | 3 (33.3) | 14 (50.0) |
| Passive faecal incontinence | 4 (44.4) | 6 (60.0) | 3 (33.3) | 13 (46.4) |
| Postdefaecation leakage | 5 (55.6) | 4 (40.0) | 4 (44.4) | 13 (46.4) |
| Difficulty wiping clean | 6 (66.7) | 5 (50.0) | 4 (44.4) | 15 (53.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pelvic organ prolapse symptoms, n (%) | ||||
| Total | 6 (66.7) | 5 (50.0) | 7 (77.8) | 18 (64.3) |
| Vaginal bulging | 6 (66.7) | 5 (50.0) | 7 (77.8) | 18 (64.3) |
| External rectal prolapse | 1 (11.1) | 0 (0.0) | 0 (0.0) | 1 (3.6) |
| External uterine prolapse | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Outcome measure | Group 1: lapVMR performed at T0 (N = 9) | Group 2: lapVMR performed at T12 (N = 10) | Group 3: lapVMR performed at T24 (N = 9) | Total (N = 28) |
|---|---|---|---|---|
| PAC-QoL score (points) | ||||
| Overall, mean (SD) | 2.7 (0.6) | 2.7 (0.6) | 2.5 (0.8) | 2.6 (0.6) |
| Missing, n (%) | 1 (11.1) | 0 (0.0) | 1 (11.1) | 2 (7.1) |
| Dissatisfaction, mean (SD) | 3.1 (0.6) | 3.1 (0.4) | 3.2 (0.9) | 3.1 (0.6) |
| Missing, n (%) | 1 (11.1) | 0 (0.0) | 1 (11.1) | 2 (7.1) |
| Physical discomfort, mean (SD) | 2.7 (0.6) | 2.9 (0.6) | 2.7 (0.5) | 2.8 (0.6) |
| Missing, n (%) | 1 (11.1) | 0 (0.0) | 1 (11.1) | 2 (7.1) |
| Psychosocial discomfort, mean (SD) | 2.3 (0.9) | 2.3 (0.9) | 2.1 (0.9) | 2.2 (0.9) |
| Missing, n (%) | 1 (11.1) | 0 (0.0) | 1 (11.1) | 2 (7.1) |
| Worries and concerns, mean (SD) | 2.7 (0.7) | 2.8 (0.7) | 2.5 (1.0) | 2.7 (0.8) |
| Missing, n (%) | 1 (11.1) | 0 (0.0) | 1 (11.1) | 2 (7.1) |
| PAC-SYM score (points) | ||||
| Overall, mean (SD) | 2.3 (0.4) | 2.1 (0.6) | 2.3 (0.7) | 2.2 (0.6) |
| Missing, n (%) | 1 (11.1) | 1 (10.0) | 0 (0.0) | 2 (7.1) |
| Stool symptoms, mean (SD) | 2.7 (0.7) | 2.1 (0.9) | 2.5 (1.3) | 2.4 (1.0) |
| Missing, n (%) | 1 (11.1) | 1 (10.0) | 0 (0.0) | 2 (7.1) |
| Abdominal symptoms, mean (SD) | 2.3 (0.6) | 2.5 (0.8) | 2.4 (0.8) | 2.4 (0.7) |
| Missing, n (%) | 1 (11.1) | 1 (10.0) | 0 (0.0) | 2 (7.1) |
| Rectal symptoms, mean (SD) | 1.5 (0.7) | 1.6 (1.2) | 2.0 (1.2) | 1.7 (1.0) |
| Missing, n (%) | 1 (11.1) | 1 (10.0) | 0 (0.0) | 2 (7.1) |
| Bowel frequency, number reported over 14 days (diary data) | ||||
| Attempts to empty bowels, mean (SD) | 41.3 (16.2) | 43.9 (27.6) | 45.1 (23.2) | 43.5 (22.0) |
| Missing, n (%) | 2 (22.2) | 2 (20.0) | 2 (22.2) | 6 (21.4) |
| Times stool was actually passed, mean (SD) | 21.3 (14.5) | 36.5 (18.7) | 24.4 (20.8) | 27.8 (18.6) |
| Missing, n (%) | 2 (22.2) | 2 (20.0) | 2 (22.2) | 6 (21.4) |
| Nature of bowel movement, number of days out of 14 (diary data) | ||||
| Laxatives used, mean (SD) | 20.4 (5.7) | 24.1 (6.6) | 22.3 (6.6) | 22.3 (6.2) |
| Missing, n (%) | 2 (22.2) | 3 (30.0) | 2 (22.2) | 7 (25.0) |
| Glycerine suppositories used, mean (SD) | 27.7 (0.8) | 27.7 (0.8) | 27.6 (0.8) | 27.7 (0.7) |
| Missing, n (%) | 2 (22.2) | 3 (30.0) | 2 (22.2) | 7 (25.0) |
| EQ-5D-5L: ‘no problem’ indicated, n (%) | ||||
| Mobility | 4 (44.4) | 2 (20.0) | 4 (44.4) | 10 (35.7) |
| Missing | 2 (22.2) | 1 (10.0) | 0 (0.0) | 3 (10.7) |
| Self-care | 1 (11.1) | 1 (10.0) | 1 (11.1) | 3 (10.7) |
| Missing | 2 (22.2) | 1 (10.0) | 0 (0.0) | 3 (10.7) |
| Usual activities | 5 (55.6) | 7 (70.0) | 6 (66.7) | 18 (64.3) |
| Missing | 2 (22.2) | 1 (10.0) | 0 (0.0) | 3 (10.7) |
| Pain/discomfort | 7 (77.8) | 9 (90.0) | 9 (100.0) | 25 (89.3) |
| Missing | 2 (22.2) | 1 (10.0) | 0 (0.0) | 3 (10.7) |
| Anxiety/depression | 6 (66.7) | 4 (40.0) | 6 (66.7) | 16 (57.1) |
| Missing | 2 (22.2) | 1 (10.0) | 0 (0.0) | 3 (10.7) |
| EQ-VAS score (points) | ||||
| Total, mean (SD) | 59.3 (14.3) | 53.4 (23.0) | 63.3 (17.3) | 58.6 (18.6) |
| Missing, n (%) | 2 (22.2) | 1 (10.0) | 0 (0.0) | 3 (10.7) |
| PHQ-9 depression severity, n (%) | ||||
| None | 5 (55.6) | 2 (20.0) | 3 (33.3) | 10 (35.7) |
| Mild | 1 (11.1) | 4 (40.0) | 3 (33.3) | 8 (28.6) |
| Moderate | 2 (22.2) | 1 (10.0) | 1 (11.1) | 4 (14.3) |
| Moderately severe | 0 (0.0) | 0 (0.0) | 2 (22.2) | 2 (7.1) |
| Severe | 0 (0.0) | 2 (20.0) | 0 (0.0) | 2 (7.1) |
| Missing | 1 (11.1) | 1 (10.0) | 0 (0.0) | 2 (7.1) |
| GAD-7 anxiety severity, n (%) | ||||
| None | 4 (44.4) | 4 (40.0) | 4 (44.4) | 12 (42.9) |
| Mild | 3 (33.3) | 1 (10.0) | 3 (33.3) | 7 (25.0) |
| Moderate | 0 (0.0) | 2 (20.0) | 1 (11.1) | 3 (10.7) |
| Severe | 1 (11.1) | 2 (20.0) | 1 (11.1) | 4 (14.3) |
| Missing | 1 (11.1) | 1 (10.0) | 0 (0.0) | 2 (7.1) |
| St Mark’s Incontinence Score (points) | ||||
| Total, mean (SD) | 12.4 (3.6) | 11.7 (5.9) | 11.3 (4.7) | 11.8 (4.7) |
| Missing, n (%) | 1 (11.1) | 0 (0.0) | 1 (11.1) | 2 (7.1) |
| PISQ-12 score (points) | ||||
| Total, mean (SD) | 19.3 (7.1) | 21.4 (6.7) | 20.8 (5.4) | 20.5 (6.1) |
| Missing, n (%) | 2 (22.2) | 3 (30.0) | 0 (0.0) | 5 (17.9) |
Results: clinical effectiveness
Results for the primary outcome (i.e. PAC-QoL score) at 24 weeks (the primary end point) and at other time points are shown in Table 28. There was a substantive reduction in estimated PAC-QoL score at 24 weeks compared with the baseline of 1.09 points (p = 0.0019), exceeding that sought by design (1.0 points). A similar magnitude of change was observed for the modelled secondary outcome (i.e. PAC-SYM score). Reductions in scores were sustained at later time points, accepting a strong chance of attrition bias.
| Time point | Number completing outcome evaluations | Observed mean score (points) | Estimated change from baseline score (points) | 95% CI for change in score (points) | p-value for change in score (points) |
|---|---|---|---|---|---|
| PAC-QoL | |||||
| Baseline | 26 | 2.63 | – | – | – |
| 12 weeks | 23 | 1.35 | –1.04 | –1.54 to –0.55 | 0.0001 |
| 24 weeks | 19 | 1.26 | –1.09 | –1.76 to –0.41 | 0.0019 |
| 36 weeks | 19 | 1.47 | –0.98 | –1.87 to –0.10 | 0.0296 |
| 48 weeks | 17 | 1.43 | –1.07 | –2.16 to 0.02 | 0.0552 |
| 60 weeks | 9 | 1.22 | –1.26 | –2.56 to 0.05 | 0.0587 |
| 72 weeks | 5 | 1.11 | –1.38 | –2.94 to 0.19 | 0.0840 |
| PAC-SYM | |||||
| Baseline | 26 | 2.24 | – | – | – |
| 12 weeks | 23 | 1.15 | –0.97 | –1.41 to –0.53 | 0.0000 |
| 24 weeks | 18 | 1.19 | –0.92 | –1.52 to –0.32 | 0.0029 |
| 36 weeks | 19 | 1.25 | –1.03 | –1.80 to –0.26 | 0.0094 |
| 48 weeks | 17 | 1.36 | –0.97 | –1.92 to –0.02 | 0.0444 |
| 60 weeks | 9 | 1.19 | –1.16 | –2.28 to –0.03 | 0.0448 |
| 72 weeks | 5 | 0.82 | –1.51 | –2.87 to –0.16 | 0.0289 |
Secondary outcomes are shown in Tables 29 (continuous) and 30 (binary). These show positive directional effects for nearly all outcomes, with some quite substantial improvements in measures, including > 25% scalar improvements in psychological measures (PHQ-9 score, GAD-7 score, St Mark’s Incontinence Score and EQ-VAS score). Global patient satisfaction was 2.7 points at 24 weeks (i.e. closest to ‘very satisfied’), although this dropped to 2.2 points (i.e. closest to ‘moderately satisfied’) at 48 weeks. This result was mirrored in the global patient improvement score (EQ-VAS score 0–100 points between ‘no effect’ and ‘complete cure’), which was 72.2 points at 24 weeks and 56.5 points at 48 weeks.
| Time | n | Mean (SD) | Median (IQR) | Difference in means (95% CI) |
|---|---|---|---|---|
| PAC-QoL score (points) | ||||
| Dissatisfaction | ||||
| Baseline | 26 | 3.1 (0.6) | 3.1 (2.8–3.6) | Reference |
| 24 weeks | 19 | 1.8 (1.0) | 1.8 (1.0–2.4) | –1.3 (–1.8 to –0.8) |
| 48 weeks | 17 | 2.1 (1.0) | 2.0 (1.4–2.8) | –1.0 (–1.5 to –0.5) |
| Physical discomfort | ||||
| Baseline | 26 | 2.8 (0.6) | 2.8 (2.5–3.0) | Reference |
| 24 weeks | 19 | 1.3 (0.9) | 1.3 (0.5–1.8) | –1.5 (–2.0 to –1.0) |
| 48 weeks | 17 | 1.6 (1.1) | 1.5 (0.8–2.0) | –1.1 (–1.6 to –0.6) |
| Psychosocial discomfort | ||||
| Baseline | 26 | 2.2 (0.9) | 2.2 (1.5–3.0) | Reference |
| 24 weeks | 19 | 0.9 (0.7) | 0.9 (0.3–1.5) | –1.3 (–1.8 to –0.8) |
| 48 weeks | 17 | 1.0 (0.9) | 0.6 (0.1–1.4) | –1.2 (–1.8 to –0.7) |
| Worries and concerns | ||||
| Baseline | 26 | 2.7 (0.8) | 2.9 (2.1–3.3) | Reference |
| 24 weeks | 19 | 1.3 (1.0) | 1.2 (0.5–2.0) | –1.4 (–2.0 to –0.8) |
| 48 weeks | 17 | 1.4 (1.2) | 1.0 (0.5–1.7) | –1.3 (–1.9 to –0.7) |
| Stool symptoms | ||||
| Baseline | 26 | 2.4 (1.0) | 2.6 (2.0–3.2) | Reference |
| 24 weeks | 18 | 1.2 (0.7) | 1.3 (0.8–1.6) | –1.2 (–1.8 to –0.6) |
| 48 weeks | 17 | 1.6 (1.0) | 1.4 (0.8–2.4) | –0.9 (–1.5 to –0.3) |
| Abdominal symptoms | ||||
| Baseline | 26 | 2.4 (0.7) | 2.4 (2.0–2.8) | Reference |
| 24 weeks | 18 | 1.4 (1.0) | 1.3 (0.8–1.8) | –1.0 (–1.5 to –0.5) |
| 48 weeks | 17 | 1.5 (0.8) | 1.5 (1.0–2.0) | –0.9 (–1.4 to –0.4) |
| Rectal symptoms | ||||
| Baseline | 26 | 1.7 (1.0) | 1.7 (1.0–2.0) | Reference |
| 24 weeks | 18 | 0.8 (0.5) | 0.7 (0.3–1.0) | –0.9 (–1.4 to –0.3) |
| 48 weeks | 17 | 0.8 (1.0) | 0.7 (0.3–1.0) | –0.9 (–1.4 to –0.3) |
| Diary data | ||||
| Bowel frequency: mean number of attempts to empty bowels over 2 weeks | ||||
| Baseline | 22 | 43.5 (22.0) | 45.5 (28.0–61.0) | Reference |
| 24 weeks | 20 | 22.9 (18.1) | 19.0 (11.5–25.0) | –20.5 (–32.5 to –8.5) |
| 48 weeks | 15 | 30.6 (16.6) | 30.0 (19.0–44.0) | –12.9 (–25.9 to 0.1) |
| Bowel frequency: mean number of times stool was passed over 2 weeks | ||||
| Baseline | 22 | 27.8 (18.6) | 19.5 (15.0–46.0) | Reference |
| 24 weeks | 21 | 17.3 (12.2) | 14.0 (8.0–22.0) | –10.5 (–20.1 to –0.9) |
| 48 weeks | 15 | 21.3 (15.3) | 19.0 (10.0–26.0) | –6.6 (–17.1 to 4.0) |
| Nature of bowel movement: mean number of days (out of 14) laxatives used | ||||
| Baseline | 21 | 22.3 (6.2) | 26.0 (15.0–28.0) | Reference |
| 24 weeks | 21 | 23.7 (4.7) | 24.0 (21.0–28.0) | 1.4 (–2.0 to 4.7) |
| 48 weeks | 15 | 22.7 (5.3) | 25.0 (18.0–28.0) | 0.4 (–3.3 to 4.1) |
| Nature of bowel movement: mean number of days (out of 14) glycerine suppositories used | ||||
| Baseline | 21 | 27.7 (0.7) | 28.0 (28.0–28.0) | Reference |
| 24 weeks | 21 | 26.5 (2.7) | 28.0 (26.0–28.0) | –1.1 (–2.2 to –0.0) |
| 48 weeks | 15 | 27.4 (1.1) | 28.0 (27.0–28.0) | –0.3 (–1.5 to 0.9) |
| EQ-VAS score (points) | ||||
| Baseline | 25 | 58.6 (18.6) | 60.0 (40.0–75.0) | Reference |
| 24 weeks | 20 | 73.7 (17.1) | 77.0 (60.0–90.0) | 15.1 (4.1 to 26.1) |
| 48 weeks | 17 | 68.2 (19.3) | 70.0 (60.0–80.0) | 9.6 (–1.9 to 21.1) |
| PHQ-9 score (points) | ||||
| Baseline | 26 | 8.0 (6.5) | 5.0 (4.0–11.0) | Reference |
| 24 weeks | 18 | 6.1 (6.0) | 4.5 (2.0–9.0) | –2.0 (–6.0 to 2.0) |
| 48 weeks | 17 | 6.7 (7.0) | 3.0 (2.0–10.0) | –1.3 (–5.4 to 2.7) |
| GAD-7 score (points) | ||||
| Baseline | 26 | 7.1 (6.4) | 6.5 (2.0–10.0) | Reference |
| 24 weeks | 18 | 5.0 (6.1) | 2.5 (0.0–7.0) | –2.1 (–5.9 to 1.6) |
| 48 weeks | 17 | 4.4 (5.7) | 2.0 (1.0–6.0) | –2.8 (–6.6 to 1.1) |
| Global patient satisfaction score (points) | ||||
| Baseline | NA | NA | NA | NA |
| 24 weeks | 18 | 2.7 (0.8) | 3.0 (2.0–3.0) | NA |
| 48 weeks | 17 | 2.2 (1.3) | 3.0 (1.0–3.0) | NA |
| Global patient improvement score (points) | ||||
| Baseline | NA | NA | NA | NA |
| 24 weeks | 18 | 72.2 (25.0) | 80.0 (67.0–88.0) | NA |
| 48 weeks | 17 | 56.5 (34.6) | 75.0 (25.0–80.0) | NA |
| St Mark’s Incontinence Score (points) | ||||
| Baseline | 26 | 11.8 (4.7) | 13.0 (8.0–16.0) | Reference |
| 24 weeks | 16 | 8.7 (4.5) | 8.5 (4.5–13.0) | –3.1 (–6.3 to 0.1) |
| 48 weeks | 17 | 8.7 (5.8) | 8.0 (3.0–15.0) | –3.1 (–6.2 to 0.1) |
| PISQ-12 score (points) | ||||
| Baseline | 23 | 20.5 (6.1) | 21.0 (15.0–25.0) | Reference |
| 24 weeks | 12 | 18.8 (5.9) | 18.0 (15.5–22.5) | –1.7 (–5.7 to 2.4) |
| 48 weeks | 12 | 17.3 (4.5) | 17.0 (14.5–19.0) | –3.2 (–7.3 to 0.9) |
| Time point | n/N (%) | OR (95% CI) |
|---|---|---|
| PAC-QoL score, ≥ 1-point reduction | ||
| Baseline | NA/NA | NA |
| 24 weeks | 11/18 (61.1) | NA |
| 48 weeks | 7/16 (43.8) | NA |
| EQ-5D-5L, problems indicated | ||
| Mobility | ||
| Baseline | 10/25 (40.0) | NA |
| 24 weeks | 7/20 (35.0) | 0.81 (0.24 to 2.73) |
| 48 weeks | 5/17 (35.0) | 0.63 (0.17 to 2.33) |
| Self-care | ||
| Baseline | 3/25 (12.0) | NA |
| 24 weeks | 4/20 (20.0) | 1.83 (0.36 to 9.35) |
| 48 weeks | 2/17 (11.8) | 0.98 (0.15 to 6.58) |
| Usual activities | ||
| Baseline | 18/25 (72.0) | NA |
| 24 weeks | 10/20 (50.0) | 0.39 (0.11 to 1.34) |
| 48 weeks | 8/17 (47.1) | 0.35 (0.09 to 1.26) |
| Pain/discomfort | ||
| Baseline | 25/25 (100.0) | NA |
| 24 weeks | 18/20 (90.0) | 1.00a |
| 48 weeks | 17/17 (100.0) | 1.00a |
| Anxiety/depression | ||
| Baseline | 16/25 (64.0) | NA |
| 24 weeks | 11/20 (55.0) | 0.69 (0.21 to 2.29) |
| 48 weeks | 9/17 (52.9) | 0.63 (0.18 to 2.22) |
Adherence to treatment
The numbers of patients (1) undergoing surgery as planned and (2) reporting that they were taking confounding medications, including those bought over the counter and numbers of confounding medications used, are presented by trial group in Table 31. As shown, concomitant medication use was high (i.e. by almost all patients).
| Characteristic | Group 1: lapVMR performed at T0 (N = 9) | Group 2: lapVMR performed at T12 (N = 10) | Group 3: lapVMR performed at T24 (N = 9) | Total (N = 28) |
|---|---|---|---|---|
| Number of patients who underwent surgery | 9 | 9 | 9 | 27 |
| Number of patients reporting the use of concomitant medications | 9 | 8 | 7 | 24 |
| Number of concomitant medications reported | 64 | 80 | 56 | 200 |
Safety analyses
Laparoscopic ventral mesh rectopexy has a number of specific complications in addition to the general risks of surgery. Data on these complications are in the public domain and can be considered to be expected events. However, these were still recorded for outcome reporting. The study (not being of a medicinal product) did not record unrelated AEs, and related AEs and SAEs were small in number, with only two related SAEs reported (Table 32).
| Variable | Group 1: lapVMR performed at T0 (N = 9) | Group 2: lapVMR performed at T12 (N = 10) | Group 3: lapVMR performed at T24 (N = 9) | Total (N = 28) |
|---|---|---|---|---|
| Related AEs | ||||
| Number of patients reporting AE | 8 | 7 | 1 | 16 |
| Number of AEs reported by category | ||||
| Abdominal pain | 2 | 2 | 0 | 4 |
| Anal/rectal pain or discomfort | 1 | 3 | 0 | 4 |
| Bloating | 0 | 1 | 0 | 1 |
| Constipation | 1 | 0 | 0 | 1 |
| Haemorrhoids | 1 | 0 | 0 | 1 |
| Loose motions | 0 | 1 | 0 | 1 |
| Rectal bleeding | 1 | 1 | 0 | 2 |
| Vaginal/perineal bulge | 2 | 1 | 0 | 3 |
| Miscellaneous | 4 | 8 | 0 | 12 |
| Severity | ||||
| Mild | 5 | 5 | 0 | 10 |
| Moderate | 7 | 12 | 1 | 20 |
| Causality | ||||
| Unlikely to be related | 3 | 4 | 1 | 8 |
| Possibly related | 4 | 7 | 0 | 11 |
| Definitely related | 3 | 6 | 0 | 9 |
| Action | ||||
| No action taken | 8 | 7 | 1 | 16 |
| Withdrawal | 1 | 0 | 0 | 1 |
| Concomitant medication | 2 | 9 | 0 | 11 |
| Non-drug therapy | 1 | 0 | 0 | 1 |
| Hospitalisation | 0 | 1 | 0 | 1 |
| SAEsa | ||||
| Number of patients reporting SAEs | 2 | 3 | 0 | 5 |
| Number of SAEs reported that require hospitalisation or prolongation of existing hospitalisation | ||||
| Unlikely to be related | 1 | 0 | 0 | 1 |
| Possibly related | 0 | 2 | 0 | 2 |
| Definitely related | 1 | 1 | 0 | 2 |
| Expectedness | ||||
| Expected | 1 | 2 | 0 | 3 |
| Unexpected | 1 | 1 | 0 | 2 |
| Action taken | ||||
| No action | 0 | 1 | 0 | 1 |
| Concomitant medication | 1 | 1 | 0 | 2 |
| Non-drug therapy | 1 | 0 | 0 | 1 |
| Hospitalisation | 0 | 1 | 0 | 1 |
Results: cost-effectiveness
Summary and completeness of data
Patients were allocated randomly to lapVMR with either immediate surgery or surgery after a 12-week or 24-week delay. The trial under-recruited (25 out of a planned 114 patients), making analysis exploratory. Variables used to estimate costs and QALYs are described in Table 33. For brevity, prescription and community health-care resources are shown costed and aggregated by period before and after surgery. Non-interventional costs were small and stable in the periods before and after surgery, compared with the cost of surgery itself (£4941 per patient). All patients had a minimum follow-up period from 12 weeks pre surgery to 48 weeks post surgery. Pre-surgery and postsurgery follow-up could be extended by up to 24 weeks depending on the delay in allocation to surgery. Completeness of cost and QALY data was reasonable over the period of common follow-up (–12 to 48 weeks) but diminished outside this follow-up window, reflecting a combination of allocation and missed visits.
| Cost (£, 2018) | QoL, EQ-5D-5L score (points) | ||||||
|---|---|---|---|---|---|---|---|
| Perioda | Mean (SD) | SD | n | Perioda | Mean (SD) | SD | n |
| –48 to –36 weeksb | – | – | 0 | –48 weeksb | – | – | 0 |
| –36 to –24 weeksc | 39 | 66 | 7 | –36 weeksc | 0.664 | 0.145 | 9 |
| –24 to –12 weeksc | 138 | 259 | 15 | –24 weeksc | 0.596 | 0.224 | 17 |
| –12 to 0 weeks | 82 | 147 | 23 | –12 weeks | 0.637 | 0.198 | 22 |
| 0 to 12 weeks | 85 | 126 | 22 | 0 weeks | 0.613 | 0.286 | 23 |
| 12 to 24 weeks | 126 | 264 | 21 | 12 weeks | 0.731 | 0.150 | 24 |
| 24 to 36 weeks | 64 | 80 | 17 | 24 weeks | 0.671 | 0.235 | 20 |
| 36 to 48 weeks | 146 | 194 | 16 | 36 weeks | 0.701 | 0.196 | 19 |
| 48 to 60 weeks | 120 | 95 | 7 | 48 weeks | 0.618 | 0.279 | 17 |
| 60 to 72 weeks | 126 | 127 | 6 | 60 weeks | 0.719 | 0.225 | 10 |
| – | – | 72 weeks | 0.809 | 0.118 | 5 | ||
| FOCB | |||||||
| –48 to –36 weeks | 71 | 191 | 27 | –48 weeks | 0.637 | 0.172 | 26 |
| –36 to –24 weeks | 71 | 191 | 27 | –36 weeks | 0.637 | 0.172 | 26 |
| –24 to –12 weeks | 90 | 210 | 27 | –24 weeks | 0.629 | 0.192 | 25 |
| LOCF | |||||||
| 36 to 48 weeks | 141 | 189 | 17 | 48 weeks | 0.627 | 0.257 | 20 |
| 48 to 60 weeks | 130 | 137 | 17 | 60 weeks | 0.627 | 0.283 | 20 |
| 60 to 72 weeks | 121 | 124 | 18 | 72 weeks | 0.635 | 0.278 | 21 |
| Health-care cost | |||||||
| –48 to 0 weeksd | 239 | 442 | 23 | –48 to 0 weeksd | 0.571 | 0.164 | 19 |
| 0 to 48 weeks | 468 | 452 | 15 | 0 to 48 weeks | 0.639 | 0.184 | 12 |
| Δe | 5252 | 564 | 14 | Δe | 0.018 | 0.121 | 10 |
| Societal costf | |||||||
| –48 to 0 weeks | 1131 | 1762 | 23 | – | – | – | – |
| 0 to 48 weeks | 2835 | 4474 | 15 | – | – | – | – |
| Δg | 6540 | 4863 | 14 | – | – | – | – |
To construct an informative exploratory analysis, a simple pre–post design was employed, with the three trial groups aligned and the time of surgery of each group becoming T0. Exploratory growth curve analysis of the postsurgery period found a stable cost consistent with a constant value over each period but an increasing and then decreasing EQ-5D-5L score over the 48-week period following surgery. Using last observation carried forward (LOCF) from 48 weeks to compensate for diminishing data, the EQ-5D-5L score was consistent with a constant value beyond 48 weeks. An incremental analysis would then require costs and QALYs estimated 48 weeks before to 48 weeks after surgery. Comparison of observed data and growth curve analysis using FOCB from –12 weeks found that costs and EQ-5D-5L scores were stable and consistent with a constant value over the pre-intervention period. Consequently, costs and EQ-5D-5L scores were estimated for the entire pre-surgery period (–48 to 0 weeks) using the FOCB approach because data were too sparse to attempt imputation. Multiple imputation was used in the base-case model to manage missing values in the pre-surgery period and the postsurgery periods from 0 to 48 weeks.
Cost-effectiveness analyses
The base-case model is reported in Table 34. Comparing pre-surgery and postsurgery periods, intervention led to a significant increase in cost (£5012, 95% CI £4446 to £5322), similar to the cost of surgery, and a modest and imprecise increase in QoL (0.043 QALYs, 95% CI –0.005 to 0.093 QALYs). The ICER of £115,512 per QALY is visualised using the ICER plane in Figure 17. NMB values were negative for surgery across the commonly used range of WTP thresholds, indicating a lack of cost-effectiveness. These findings are presented as a CEAC showing the probability of being cost-effective at various WTP thresholds. At the NICE-recommended WTP of £30,000 per QALY, the probability that surgery is cost-effective is 0%. The EVPI (per subject) reflects the opportunity loss of ongoing uncertainty and suggests that there is no requirement for further research.
| lapVMR vs. no lapVMR | ICER (95% CI) | IC (95% CI) | IQ (95% CI) | CEAC p-valuea | CEAC p-valueb | NMBa (95% CI) | NMBb (95% CI) | EVPIb |
|---|---|---|---|---|---|---|---|---|
| Base case | 115,512 (53295 to c) | 5012 (4446 to 5322) | 0.043 (–0.005 to 0.093) | 0.000 | 0.000 | –4346 (–5115 to –3517) | –3693 (–5098 to –2166) | 0 |
| Complete case | 204,374 (49308 to c) | 5020 (4757 to 5288) | 0.025 (–0.056 to 0.101) | 0.000 | 0.000 | –4668 (–5915 to –3436) | –4308 (–6760 to –1939) | 0 |
| Sensitivity analysisd | – | – | – | 0.000 | 0.000 | –4354 (–5351 to –3378) | –3681 (–5181 to –2087) | 0 |
FIGURE 17.
The CapaCiTY trial 3 cost-effectiveness analysis: base case. (a) ICER plane [the credible region (dashed circle) shows where 95% of estimates lie]; (b) incremental NMB; (c) CEAC; and (d) EVPI.
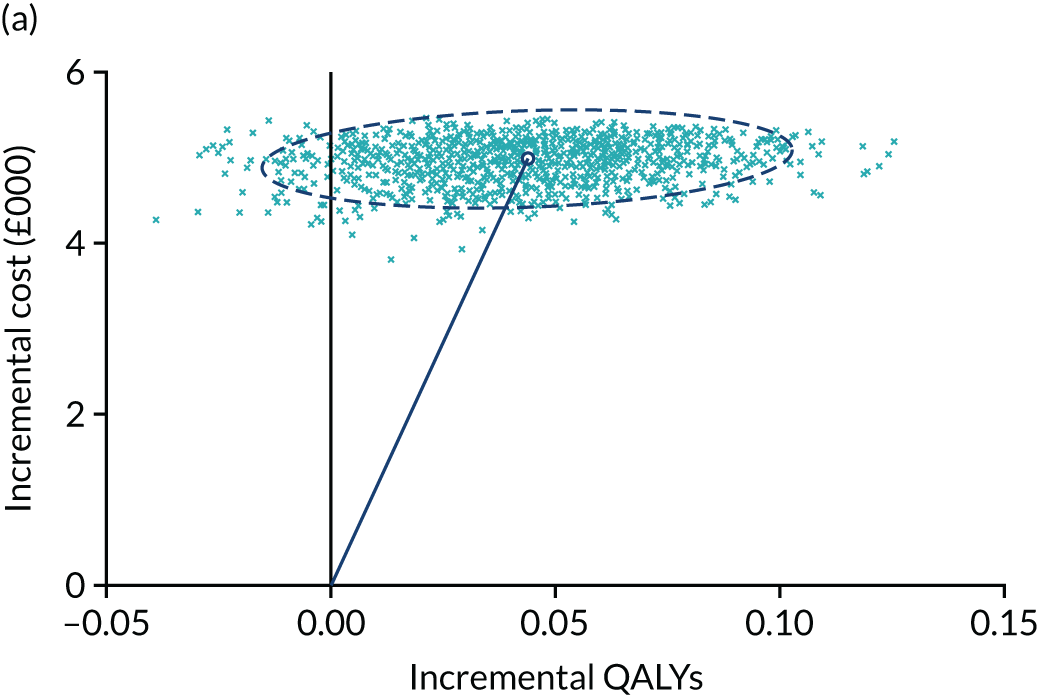
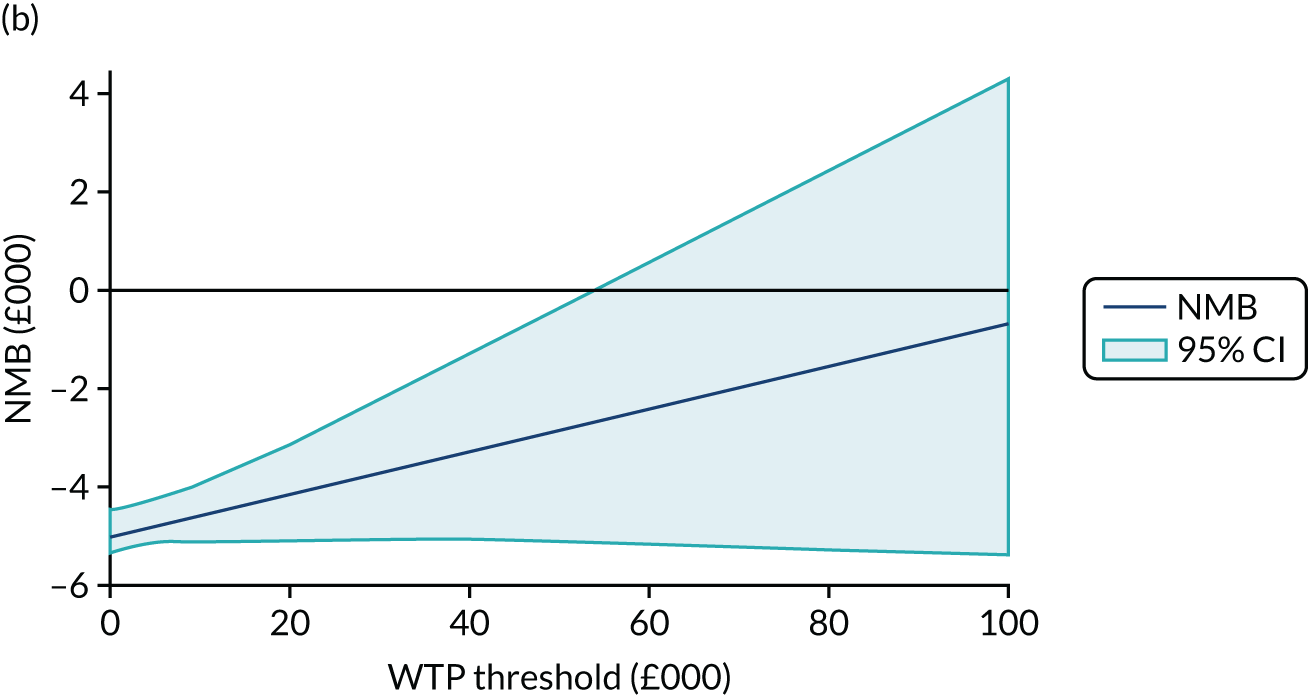
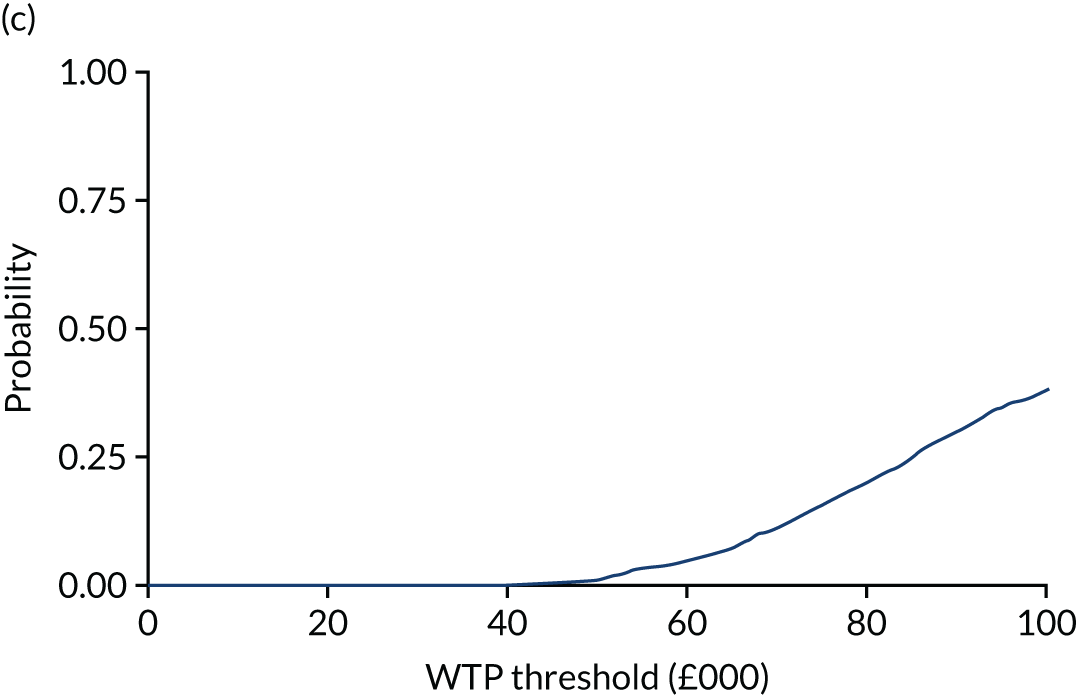
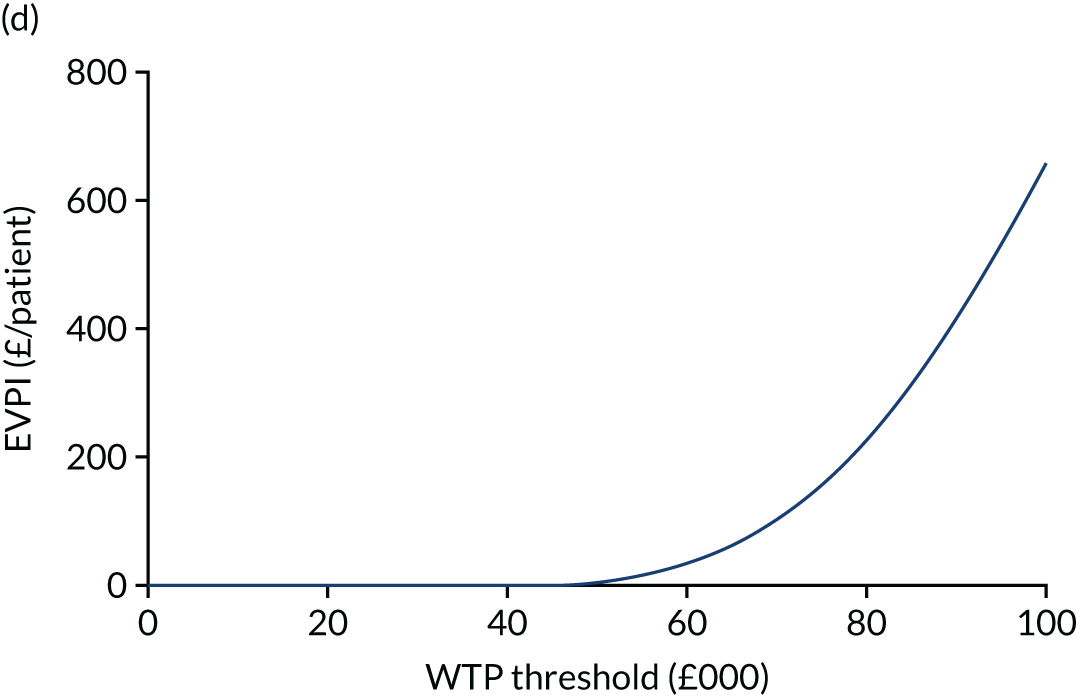
In Figure 17a, the ICER plane shows that lapVMR resulted in a significant increase in costs but modest and imprecise increase in QALYs. In Figure 17b, the incremental NMB shows that investing in lapVMR at commonly used WTP thresholds appears to deliver a loss to the health-care system compared with alternatives. In Figure 17c, the CEAC shows that there is a very low probability that lapVMR is cost-effective. In Figure 17d, the EVPI suggests that there is little value to the health-care system of further research in this field unless the WTP threshold was considerably higher than commonly used thresholds.
The complete-case analysis (using the FOCB estimate of pre-surgery cost) is also presented in Table 34. Although the numbers contributing are small, the findings are statistically similar to the base case, if less precise. Given the very small numbers in the trial, as a sensitivity analysis the base-case model was re-estimated as a univariate regression (NMB at various WTP thresholds). The findings were very similar: at a WTP of £30,000 per QALY, the probability that surgery was cost-effective was 0%.
Results: patient experience and qualitative analysis
Emergent themes are presented in this section. Numbers in square brackets refer to the numbered quotations in Appendix 2, Quotations from participants contributing to qualitative analysis in all three trials, CapaCiTY trial 3: laparoscopic ventral mesh rectopexy.
Making the decision for surgery
Nine out of the 10 interviewees opted for lapVMR surgery. For some, the decision to undergo surgery was a much-welcomed pragmatic option [1]. Others appeared to go through a longer deliberation process about potential adverse effects of the surgery, including the recently publicised issues around mesh usage in surgery [2]. The health-care professional’s detailed explanation of the surgery, as well as trust in the surgeon and wider bowel team, seemed to mitigate these concerns. For one patient, the reasons for not opting for surgery at this time included several current physical and mental health issues. This patient had chosen to manage her CC with a change in diet, TAI, suppositories and laxatives, as advised by health-care professionals.
Patient surgical experience
A few patients experienced surgical delays, cancellations and pathway difficulties due to administration or cross-departmental communication issues. A number reported a very good hospital stay experience and described how they felt supported by the surgical and wider health-care team. Some patients reported that the surgery was better than they had initially expected [3]. However, a few patients did report negative experiences during their hospital stay. These did not appear to be related to the surgery itself, but rather to the before and after care provided on the wards.
Patient postoperative experience
The level of postsurgical pain seemed to vary. For some, pain was not much of an issue [4], whereas for others postoperative pain was a significant feature [5]. Recovery times also varied, with a couple of patients going back to work within weeks. However, for several it took between 6 weeks and 9 months to fully recover postoperatively.
Surgical results
Some patients described how their bowel functioning was better immediately after surgery [6]. For others, it took a few weeks to start seeing improvement. However, for a few patients the surgery did not seem to improve their constipation [7]. Some patients have reported continued success and a better QoL both mentally and physically [8] and would recommend the surgery to someone who was experiencing similar bowel issues.
For others, the surgery has corrected some but not all issues, such as being able to pass stools in a timely manner. Thus, for some, it was not the ‘miracle cure’ they were hoping for [9]. A few patients reported that, although surgery initially seemed to be addressing their problematic bowel issues, they were now re-experiencing these problems [10].
Postoperative behavioural changes
Some patients changed their diet and fluid intake after surgery to better manage their bowels and to promote continued success, and others had a healthy diet prior to surgery and continued to maintain this. Some patients reported using laxatives from time to time after surgery, but that this use was much less frequent than before.
Patient concerns
A few patients, particularly those who were re-experiencing their previous symptoms, reported concerns about a prolapse recurring as well as new defaecation pain. For a few patients, there had been additional concerns about whether or not mesh use played a role in continued pain following surgery.
Mesh in the media
Although the study had the full backing from national expert groups such as the Pelvic Floor Society, negative coverage of mesh use in the media coincided with the start of the study [11]. This affected the wider elements of the study in terms of patient participation.
Staff experiences
Because of the media coverage there was reluctance from surgeons to recruit patients for the study and to surgery in general, or surgeons decided to no longer perform lapVMR at all. These media concerns have led to surgeons directly expressing anxieties [12]. In addition, some hospitals erred on overemphasising the risks involved, providing patients with multiple consent forms, which potentially exacerbated patient anxieties about lapVMR. Equally, entire organisations chose to no longer offer the surgery because of litigation concerns.
Documents outlining the differences between the gynaecological mesh issues (in the media) and the current lapVMR surgery helped to clarify some patient concerns, as did surgical mentoring and patient tracking databases. However, recruitment continued to be severely affected by the negative media coverage [13]. Developing one nationally recognised information sheet and lapVMR surgical certification may assist with patient and surgeon anxieties in future.
Some staff stated that CapaCiTY trial 3 did not appear to affect current clinical practice. However, others did acknowledge that the research affected practice and/or current practice affected the research procedure owing to protocol restrictions and scheduling issues.
Surgeons noted that lapVMR surgery is technically challenging, particularly if the patient has additional medical complications. Furthermore, owing to the technical nature of the operation, extensive experience of carrying out lapVMR surgeries is deemed necessary to perform the procedure, limiting the number of surgeons able to offer this operation. The research team attempted to mitigate any potential surgical variation by reviewing operation videos; however, disparity still existed.
Although the surgical video review embedded in the current study attempted to solve the issue of operational variation, it presented additional challenges around:
-
patient consent deviations
-
potential reluctance of sites/surgeons participating owing to feeling scrutinised
-
professional feedback difficulties, which may have contributed towards administrative video review delays.
Anxieties around potential legal issues, lack of experienced lapVMR surgeons and surgeons possibly feeling scrutinised by video review could have contributed towards the difficulty of recruiting surgical staff to take part in CapaCiTY trial 3 interviews (in addition to the patient recruitment issues). However, the general sense from the interviewed staff was that lapVMR is helpful for the majority of patients, although staff also recognise that some patients (particularly those who have multiple medical issues) may not experience lasting beneficial results.
Appendix 2 Other outputs arising from the programme
CapaCiTY trial 1: intervention protocols
Habit training
Sessions (minimum 3, maximum 4; ≈ 60 minutes each).
This will use a standardised pro forma.
-
Participants receive a written information leaflet covering normal bowel function, causes of constipation, diet and fluid advice, and getting into a good bowel habit.
-
Participants receive a review of written information using locally available teaching tools such as models or diagrams.
-
Participants receive advice to stop using all laxatives, including drugs that the British National Formulary146 describes as having a laxative effect or over-the-counter herbal teas that contain strong purgatives. Glycerine suppositories (one or two) as rescue if there is no stool for 3 days is allowed. No use of irrigation devices or enemas.
-
Participants encouraged to follow a daily routine: sit on the toilet 20–30 minutes after first meal and/or hot drinks (sooner if urge felt).
-
Participants instructed that they can attempt defaecation after meals or when urge is felt, but no more than three times per day.
-
Participants instructed that they should sit on the toilet with knees bent in a 45-degree position, with feet elevated on stool or equivalent, and abdominal brace and breathe while performing anal relaxation.
-
Participants instructed that they must attempt to push for only 5–10 minutes maximum.
-
Defaecation manoeuvres taught while the patient is positioned sitting on a chair, with verbal coaching to breathe while pushing.
-
Participants strongly discouraged from multiple attempts and prolonged straining.
-
Participants instructed that there must be no digitation anally.
-
Where appropriate, participants taught rectocele (vaginal), perineal and perianal splinting.
-
Therapists prohibited from using digital rectal exam to train manoeuvres.
-
Participants receive diet and lifestyle advice (e.g. moderate but not excessive fibre; moderate but not excessive fluid intake; increase exercise, such as walking, if possible).
-
Only participants with evacuation difficulty and/or perineal descent taught pelvic floor exercises.
-
Participants receive optimistic encouragement and personal attention.
-
Participants receive suggestions about what to work on until next meeting.
-
Therapists to complete relevant sections in participant booklet.
Habit training and direct visual biofeedback
-
Biofeedback balloon and catheter/probe connected to computer monitor. Patient lying in lateral position facing computer screen (supine if unable to lie in lateral position). Probe taped or held into position.
-
Resting pressure noted (using event recorder). Squeeze pressure noted.
-
Rectal balloon inflated with air (2 ml/second) to assess first sensation and urge volume. Volumes noted. Maximum = 360 ml.
-
Rectoanal inhibitory reflex elicited with 50-ml aliquots and rapid inflation with air (30 ml/second); volume to first urge and the effect on resting pressure noted. Maximum = 150 ml.
-
Coaching to evacuate with 60 ml of water (one syringe full) in the balloon. Participant attempts balloon expulsion and the effect on anal pressure noted.
-
Attempts to relax while pushing to expel balloon are monitored. Participant instructed to push and breathe, with emphasis on needing to push from the waist while relaxing the anus. Propulsive effort noted. A minimum of three and no more than 10 attempts in total, with coaching (therapist observes abdominal and anal activity and advises), or until balloon is expelled (not essential). Correct pushing technique ensured.
-
HRAM can be used to coach pelvic floor exercises if indicated.
-
Participants undergoing biofeedback may have rectal hypersensitivity or hyposensitivity. At each interventional visit, these participants will undergo sensitivity training. The goal will be to increase (hypersensitive) or decrease (hyposensitive) tolerated balloon volume by gentle progressive distension or depression of air.
Quotations from participants contributing to qualitative analysis in all three trials
Synonyms and group allocation are indicated after each quotation.
CapaCiTY trial 1: habit training with or without biofeedback with or without standardised radiophysiological investigations
-
Brian, HTBF with INVEST
It was good; indeed, to know that quite early on, that there was nothing serious [after INVEST], that has helped.
-
Beth, HT
Well, relief in one way because they didn’t sound like a lot of fun. But also, perhaps, a little regret because I thought I might get better treatment if I had. So a mixture.
-
Francesca, HT
My constipation is stress related and psychologically related and talking to somebody about it really helped.
-
Helen, HT
Meeting people that it was OK to be like this around, and being OK to talk about it, that they understood and not just thinking ‘is this just me?’, ‘is this just me and am I going to live like this for the rest of my life?’
-
Anne-Marie, HT with INVEST
I’d got into a very bad routine and I learned more about improving . . . the dietary side of things . . . and that includes fluids, which I’ve always been very bad at having enough [of].
-
Edward, HTBF
I’ve introduced fibre . . . fibre is more the crucial thing.
-
Anne-Marie, HT with INVEST
One really good thing for me was becoming aware, after all these years, that I’d actually been working against nature, in that I was given advice to be breathing – which, to me, was very, very useful, because I had been doing the opposite, which basically means tightening . . . the rectum instead of relaxing it.
-
Teresa, HT with INVEST
Having the nurse talk to me about how the bowels actually work, how the problems that my bowels actually have – I mean that’s really helped me. It has reassured me and I don’t really get as stressed out as I used to. I don’t have any accidents [faecal incontinence] like I used to either.
-
Bill, clinical intervention nurse
I find [the booklet] very informative, especially to the patient and also to the clinician as well, especially if they are not, you know, they are not so familiar yet with the normal evacuation process.
-
Lauren, HTBF with INVEST
It’s much easier for me when I use a [foot]stool. So what I even did – I bought myself a portable stool, and took it with me when we went on holidays.
-
Samantha, HT
I guess it’s the reiteration, because a lot of the stuff that he’s telling me is stuff that I kind of know already, insoluble and soluble fibre stuff, prune juice, I’ve been doing that for ages now. Exercise. But again it’s just the reiteration and kind of focusing on what I need to concentrate on.
-
Linda, HT
I certainly feel that there’s been some improvement.
-
Becky, physiologist
I did have some patients that really grasped using the screen and the images and that really helped them understand their condition and enabled them to be able to use the map in the right way and improve, so I found that really interesting to see.
-
Maria, clinical intervention nurse
I personally felt that when the way that I treated the patients before CapaCiTY came along, worked exceptionally well and patients were improving. I don’t think the machine added any benefit at all.
-
Kate, HTBF
It was a little bit undignified . . . but I’m normally very OK about that sort of thing, I just get on with it.
-
Lauren, HTBF with INVEST
The fact that I’m off the [laxatives] is the best thing. I’m still having interventions and it’s not perfect, but it’s not like it used to be.
-
Edward, HTBF
I have no qualms about recommending it to absolutely anybody.
-
Shaunett, clinical intervention nurse
It takes longer setting up and takes another 15–20 minutes.
-
Maria, clinical intervention nurse
I just didn’t like using [the biofeedback machine] and that might be because I’m not great with change or adopting something new.
CapaCiTY trial 2: transanal irrigation
-
Bernadette, clinical intervention nurse
We’re very enthusiastic because we’ve seen the results and we know that it works. So if we’re really positive about it, I think that really helps. And bringing the patient along with you as well, so you’ve got to build a good rapport with the patient and bring them along with you.
-
Warren, high-volume TAI, continuing use
I think the opportunity to practice during that visit with the nurse was extremely helpful, because there were some initial ‘so I do it this way, do I do it that way?’ things I could get immediate feedback on so that was very helpful.
-
Derek, high-volume TAI, continuing use
She offered me the chance to do it [in the clinic] but I just did it at home, it seemed quite straightforward . . . it’s still something that I feel a bit embarrassed talking about, or doing, so I just preferred to do it at home.
-
Rita, high-volume TAI, continuing use
I was still worried about doing it, because you’re doing it once with the nurses and then you’re coming home and you’re on your own. But as I say, I knew they were on the end of the phone, and I knew I could phone them.
-
Warren, high-volume TAI, continuing use
You’re trying to take in a lot of information all at once with a procedure that feels really unnatural, so I think there’s lots of kind of sequential things that could go wrong . . . not wrong, but just kind of, are not entirely clear. So even with all that really good explanation and opportunity [to practice in clinic] there was still a lot of confusion for a wee while.
-
Josephine, high-volume TAI, continuing use
It does take a bit of time to get used to it, because it’s a strange sensation, but once you do it’s quite straightforward to do.
-
Lacey, low-volume TAI, continuing use
It was fine; easy to do.
-
Laurella, high-volume TAI, continuing use
In the first 3 or 4 days I got what felt like period pain, in my abdomen, just the pressure of the water being pumped up, but then after, nothing in my abdomen after that. It just feels strange. I wouldn’t say that it’s painful, it just feels strange using a catheter.
-
Lacey, low-volume TAI, continuing use
[I was] quite happy to do the low[-volume] one . . . I thought the higher[-volume] one would be more like the colonic irrigation, more painful or more intense.
-
Elizabeth, high-volume TAI, discontinued use
You fit it into your daily routine easily, it’s quick to use.
-
Lacey, low-volume TAI, continuing use
Sometimes it’s quite restrictive, in that it’s hard to fit it into my daily life. Sometimes I get into a routine with the children and sort of forget about doing it.
-
Laurella, high-volume TAI, continuing use
I think for a couple of months, possibly a bit longer, maybe for a month after using it or a bit longer, it was still hit or miss, but more recently it has been absolutely fantastic and it does work every time.
-
Pamela, low-volume TAI, continuing use
I am absolutely over the moon with it and I’m happy with how it’s all ended [up]. Compared to how I am now to how I was I’m like a different person because I feel clean all the time.
-
Derek, high-volume TAI, continuing use
100% satisfied. I’ll go on holidays now . . . I’m alive again, I feel like I’m bubbly inside . . . I can go outside, I can do a lot of walks.
-
Pamela, low-volume TAI, continuing use
I don’t suffer from chronic constipation anymore. So I used to go maybe once a week and that’s how my life was. I used to joke that every Wednesday was my poo day. So this has helped me so much, I can only thank the team for transforming my life.
-
Rebecca, high-volume TAI, continuing use
It’s the leakage, you have to change once or twice until it stops and then after that it’s fine, but there’s always that bit of leakage coming out after and with me.
-
Joyce, clinical intervention nurse
Sometimes this group of patients are able to get results straight away, particularly with the high-volume kit; however, some do need to persevere in order to see the results as it may take time to adapt the system to meet the person’s particular needs.
CapaCiTY trial 3: laparoscopic ventral mesh rectopexy
-
Rachel, post surgery
I sort of jumped at the chance . . . I just thought if I could just empty naturally, then it would make life a lot easier.
-
Camilla, post surgery
Obviously [I had] fears, lots of fears about having it done, whether it was the right thing to do . . . it was all the publicity about mesh.
-
Rita, post surgery
I think the actual surgery – afterwards, it was better because I thought I was going to be all scarred down below but it was all done in my tummy, which was better than what I expected . . . The actual recovery from the surgery, that went quite quickly.
-
Peggy, post surgery
There wasn’t a lot of pain with it.
-
Esther, post surgery
I did have a lot of pain post op. I had quite a severe nerve pain on my right-hand side; that gave me more problems than the actual pain in my bottom area. That took about 3 or 4 months to calm down.
-
Lilian, post surgery
The treatment was excellent and, as I say, the bowel sensation down below literally disappeared immediately and I don’t have to use gloves and a finger to get all the poo out.
-
Camilla, post surgery
[The surgery] didn’t change anything really.
-
Amanda, post surgery
I used to get very down before the operation and then afterwards even my son noticed and said ‘you seem cheerful mum’, and I said ‘yeah, I feel quite good actually’.
-
Rachel, post surgery
It wasn’t the miracle cure that I thought it might be, although [the surgeon] didn’t promise me a miracle cure. But he said, you know, that it should improve my quality of life. I don’t think it has really.
-
Esther, post surgery
My prolapse is back, the pain’s back, it was about 10 months after the surgery that my prolapse came back and I’m waiting to see the surgeon again. I was told that it should last about 10 years, but it lasted 10 months.
-
Angela, research team member
There’s been a massive coverage in the media about the negatives of using mesh and how it introduces long-term issues for the patient.
-
Christopher, surgeon
It’s a dangerous place being a pelvic floor surgeon. But you’ve got to keep yourself buoyed up by the fact that the majority of patients do well. Otherwise you wouldn’t do it at all. There is a danger that we all stop doing this because of the small minority of patients that are not happy.
-
Marshall, surgeon
We were just . . . getting [the study] off the ground when this all blew up and sabotaged it. And of course, the question is more relevant than ever now with the mesh problems. The study aimed to address [this], which is the sort of horrendous irony of it really.
List of abbreviations
- A&E
- accident and emergency department
- AE
- adverse event
- AUC
- area under the curve
- BIPQ-CC
- Brief Illness Perception Questionnaire for Chronic Constipation
- CapaCiTY
- Chronic Constipation Treatment Pathway
- CC
- chronic constipation
- CC-BRQ
- Chronic Constipation Behavioural Response to Illness Questionnaire
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRAG
- Constipation Research Advisory Group
- CRF
- case report form
- CRN
- Clinical Research Network
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-VAS
- EuroQol Visual Analogue Scale
- ETC
- excess treatment cost
- EVPI
- expected value of perfect information
- FMI
- fraction of missing information
- FOCB
- first observation carried back
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- HRAM
- high-resolution anorectal manometry
- HT
- standardised specialist-led habit training alone
- HTBF
- standardised specialist-led habit training plus pelvic floor retraining using computer-assisted direct visual biofeedback
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- INVEST
- standardised radiophysiological investigations
- IQR
- interquartile range
- IRAS
- Integrated Research Application System
- ITT
- intention to treat
- lapVMR
- laparoscopic ventral mesh rectopexy
- LOCF
- last observation carried forward
- MAR
- missing at random
- MDD
- major depressive disorder
- MDT
- multidisciplinary team
- MyMOP2
- Measure Yourself Medical Outcome Profile 2
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PAC-QoL
- Patient Assessment of Constipation Quality of Life
- PAC-SYM
- Patient Assessment of Constipation Symptoms
- PCA
- Prescription Cost Analysis
- PCTU
- Pragmatic Clinical Trials Unit
- PHQ-9
- Patient Health Questionnaire-9 items
- PISQ-12
- Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire-12
- PPI
- patient and public involvement
- PSC
- Programme Steering Committee
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- STARR
- stapled rectal resection
- T
- time (weeks) since randomisation
- TAI
- transanal irrigation
- TwiCs
- trials within cohorts
- WP
- work programme
- WTP
- willingness to pay
