Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1210-12012. The contractual start date was in October 2013. The final report began editorial review in January 2021 and was accepted for publication in June 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Wang et al. This work was produced by Wang et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Wang et al.
Synopsis
There were no substantial changes to the research plan proposed in the submitted grant application and all work packages were completed. However, as work packages D and E were reliant on recruitment to work package C, which was significantly below the calculated sample size of 650 participants with only 271 randomised, the analyses it was possible to conduct for these work packages were limited.
Work package E1 (the development of risk reduction scores) was further restricted by a lower than anticipated rate of primary end points (reconsultation owing to clinical deterioration) being met in the clinical trial. As a result, data with which to calculate factors that may lead to increased risk of reconsultation were extremely limited.
The following is a summary of the objectives and methods used for each work package.
Work package A (pre clinical trial)
Objectives
-
Identify risk factors and assess the reliability of published prognostic models for influenza-related complications in children (objective 1).
Methods
We carried out a systematic review of the existing literature to identify risk factors and assess existing models.
Work package B (pre and concurrent with clinical trial)
Objectives
-
Understand what factors influence general practitioners’ (GPs’) decisions about antibiotic prescribing for at-risk children with influenza/influenza-like illness (ILI) (objective 2).
-
Explore the experiences of parents of at-risk children who have previously become unwell owing to influenza/ILI (objective 3).
-
Explore parental consulting attitudes in relation to influenza/ILI (objective 4).
Methods
We conducted qualitative research via interviews with both GPs and parents/guardians of children with underlying medical conditions who had experienced an influenza/ILI episode.
Work package C: clinical trial
Objective
-
Determine the effectiveness of early co-amoxiclav use in at-risk children with influenza/ILI (objective 5).
Methods
We carried out randomised double-blind placebo-controlled clinical trial to determine if early treatment with co-amoxiclav reduces risk of reconsultation owing to clinical deterioration compared with placebo in at-risk children who present with ILI.
Work package D (concurrent with and post clinical trial)
Objectives
-
Examine the impact on antibiotic resistance of early co-amoxiclav use in at-risk children with influenza/ILI (objective 6).
-
Determine the impact on long-term respiratory bacterial carriage of early co-amoxiclav use in at-risk children with influenza/ILI (objective 7).
Methods
We conducted a nested substudy within the clinical trial to assess if there was an impact on antibiotic resistance or long-term respiratory bacterial carriage in participants randomised to co-amoxiclav compared with those randomised to placebo. Participants who were consented to the substudy were invited for additional throat swabs at 3, 6 and 12 months.
Work package E (post clinical trial)
Objectives
-
Develop and validate risk scores for influenza-related clinical deterioration and complications for use in children with influenza/ILI (objective 8, work package E1).
-
Explore the cost-effectiveness of different potential strategies for early antibiotic use in at-risk children with influenza/ILI (objective 9, work package E2).
Methods
To address objective 8, data collected during the clinical trial were used to compile risk reduction scores where sufficient data were available. To address objective 9, a within-trial health economics cost analysis was conducted.
Background
Influenza in children places a significant burden on health-care resources each year. The 2017 Global Burden of Disease study1 estimated that over 2.2 million children younger than 5 years were hospitalised owing to influenza-related lower respiratory tract infections that year. Around 1.1 million general practice consultations and 850,000 hospitalisations owing to influenza/ILI take place in England each year, of which children younger than 15 years account for 39% of consultations and 37% of hospitalisations. 2 During the 5-week period in winter 2017-8 when influenza was circulating above the seasonal threshold, influenza activity among children under 15 years of age in England was calculated as being medium to high, with an average of 30 children per 100,000 being assessed by their GP for influenza-like illness each week. 3
Although for most children influenza/ILI is a mild and relatively short illness, it is widely considered a predisposing factor for secondary complications, including bacterial infections. Previous studies have demonstrated synergistic adverse effects on illness outcome if the respiratory tract is colonised with influenza and bacteria. 4–7 Secondary complications may result in children consulting a clinician more than once during the same illness episode owing to clinical deterioration, putting additional strain on the NHS and families during the winter months.
Risk of influenza complications
Children who are at risk, by which we mean they already have an underlying health condition (e.g. asthma, diabetes mellitus or Down syndrome) or other risk factor (e.g. prematurity), are more prone to developing secondary complications than otherwise healthy children. Influenza-related complications such as otitis media and pneumonia occur in 18% of at-risk children versus 13% of otherwise healthy children within 30 days of initial presentation. 8
Hospitalisation owing to influenza/ILI is estimated to be five times more likely in at-risk children than otherwise healthy children aged 0–4 years (214.4 vs. 41.8 children per 1000) and 12 times more likely in at-risk children than otherwise healthy children aged 5–14 years (67.1 vs. 5.6 children per 1000). 9
In the most severe cases, children with influenza/ILI may additionally become infected with Streptococcus sp. and/or Staphylococcus aureus. Co-infection with these pathogens has been associated with an increased risk of influenza-related death in children. 10
Seasonal influenza vaccination programmes and their limitations
Prior to the introduction of the live attenuated influenza vaccine (LAIV), uptake of the seasonal vaccine in at-risk children varied between groups, for example 13.4% in children aged 6 months to 2 years with immunosuppression and 22.6% in children aged 2–16 years with degenerative neurological disease in the 2009–10 season. 11
In preparation for the 2013–4 winter season the UK introduced the newly licenced LAIV. This vaccine is intranasal and, in addition to delivery in primary care, is delivered in schools as part of an incremental universal vaccine programme. By the 2017–8 winter season children aged 2–8 years old across the UK were being offered the LAIV with higher uptake than previous seasons indicating how school programmes have successfully steadily improved uptake rates in this key demographic. 12
Children not yet of school age or classed as ‘at risk’ by their general practice continue to be invited to primary care for vaccination. Primary care influenza vaccination uptake rates among at-risk children were only around 50% in children aged 2–16 years and around 20% in children aged between 6 months and 2 years during the 2017/18 and 2018/19 seasons. 13
The vaccine efficacy (VE) of the LAIV, as is the case with the inactivated vaccine used to inoculate children under 2 years and adults, varies from year to year and is dependent on the correct circulating strains being identified pre production. The LAIV did not provide adequate protection from influenza in North America during the 2015–6 season (3% VE), for example, although offered better protection to children in Europe (57.6% VE). 14 Concerns have continued to be voiced in North America, where the LAIV has been used for longer, regarding its long-term effectiveness. 12
The mutating nature of influenza makes the development of a ‘universal’ vaccine difficult, necessitating that alternative strategies, particularly in the event of a pandemic, are considered.
Antiviral and antibiotic strategies
The use of neuraminidase inhibitors (NAIs), to treat both seasonal and pandemic influenza is one potential strategy. Evidence of the role of NAIs in at-risk children is currently weak. A Cochrane review of published trials of NAIs for the treatment and prevention of influenza in children before the 2009 pandemic15 found that NAIs only conferred modest clinical benefit, reducing the duration of symptoms in otherwise healthy children with influenza by about 1 day. None of the included trials was sufficiently powered to look at influenza-related pneumonia or hospitalisation. Only one trial involved children with asthma16 and found that oseltamivir (Tamiflu®, Roche) did not reduce asthma exacerbations or improve peak flow. Oseltamivir is also not licenced for children under the age of 1 year. 17 Zanamivir (Relenza®, GlaxoSmithKline UK) is not recommended in individuals with an underlying airways disease (such as asthma) owing to the risk of serious bronchospasm. 18
In recognition of the potential serious clinical and socioeconomic consequences of bacterial complications of influenza, and the limitation of vaccination and NAI strategies, the UK government stockpiles the antibiotic co-amoxiclav for use during influenza epidemics and pandemics.
Although antibiotics are not routinely recommended to treat viral respiratory tract infections (RTIs),19–24 the findings of a small trial conducted in children with ILI during an influenza epidemic suggest that early treatment with the antibiotic sultamicillin (Unasyn®, Pfizer) may reduce the incidence of pneumonia. 25 Published observational data have also previously demonstrated that duration of fever was significantly shorter in children with laboratory-confirmed influenza who had received antibiotics (mostly amoxicillin) at an early stage during their illness. This finding was not observed in children with any other type of viral infection. 26
Although antibiotics may be beneficial in treating influenza, the continuing efficacy of antibiotics is a crucial global health concern. Antibiotic resistance has been identified by the World Health Organization as a threat ‘to the very core of modern medicine and the sustainability of an effective, overall public health response to the enduring threat from infectious diseases’ (Global Action Plan on Antimicrobial Resistance. Geneva: World Health Organization; 2015. Licence: CC BY-NC-SA 3.0 IGO). 27
Research programme
An effective, evidence-based, policy on antibiotic use in at-risk children during influenza season is needed to ensure that national antibiotic stockpiles are used in the most clinically appropriate and cost-effective way while adhering to responsible antimicrobial stewardship.
The ARCHIE programme (early use of Antibiotics for at-Risk CHildren with InfluEnza) consists of five inter-related work packages, shown in Figure 1, and nine objectives. We present a summary of each work package that inform our conclusions and their implications.
FIGURE 1.
Inter-relationship of work packages.
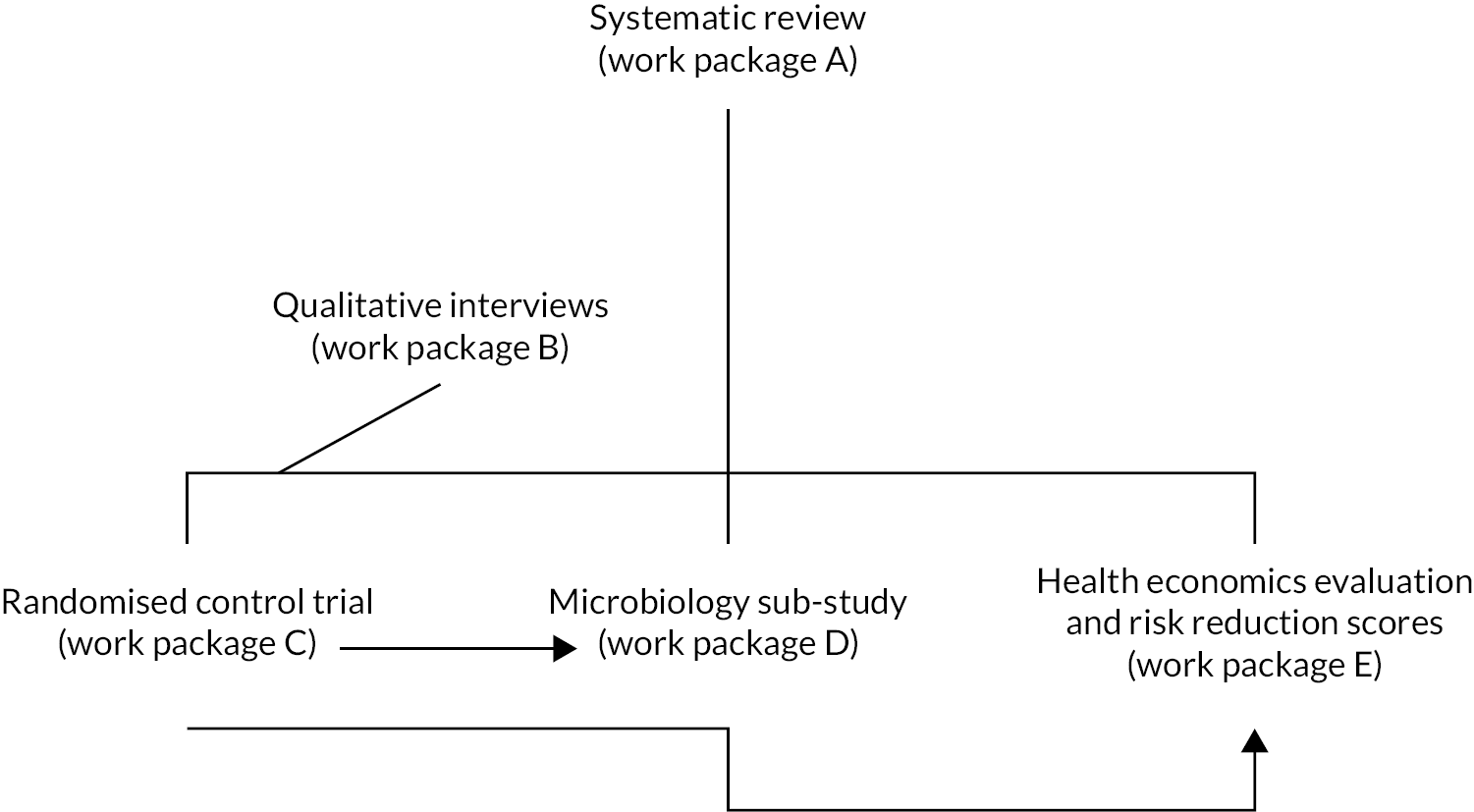
At the end of each work package summary is further detail on how it links to the others and how each work package contributed to the main implications of our work.
The management arrangements for the programme are detailed in Appendix 1.
Work package A: systematic review
-
Objective 1: identify risk factors and assess the reliability of published prognostic models for influenza-related complications in children.
Work package B: qualitative work
-
Objective 2: understand what factors influence GPs’ decisions about antibiotic prescribing for at-risk children with influenza/ILI.
-
Objective 3: explore the experiences of parents of at-risk children who have previously become unwell owing to influenza/ILI and
-
Objective 4: explore parental consulting attitudes in relation to influenza/ILI and involve parents of at-risk children in the development of trial materials.
Work package C: randomised controlled trial
-
Objective 5: determine the effectiveness of early co-amoxiclav use in at-risk children with influenza/ILI.
Work package D: microbiology
-
Objective 6 examine the impact on antibiotic resistance of early co-amoxiclav use in at-risk children with influenza/ILI.
-
Objective 7 determine the impact on long-term respiratory bacterial carriage of early co-amoxiclav use in at-risk children with influenza/ILI.
Work package E: risk reduction scores and health economics
-
Objective 8: develop and validate risk scores for influenza-related clinical deterioration and complications for use in children with influenza/ILI (work package E1).
-
Objective 9: explore the cost-effectiveness of different potential strategies for early antibiotic use in at-risk children with influenza/ILI (work package E2).
Addressing objective 1: identify risk factors and assess the reliability of published prognostic models for influenza-related complications in children
We began our research by conducting a systematic review to better understand and identify the risk factors associated with clinical deterioration in children with influenza/ILI. An understanding of risk factors in children is important, in view of the different comorbidity profiles encountered in paediatric versus adult populations and the high burden of disease associated with influenza in children. 28
We aimed to provide an evidence-based definition of which children presenting with influenza or ILI in primary or ambulatory care are at increased risk of developing influenza-related complications through a systematic review of published and unpublished data.
This work has been published as a separate paper, Gill et al. 29
Aim
Our aim was to provide an evidence-based definition of which children are at greater risk of influenza/ILI-related clinical deterioration via a systematic review of published and unpublished data.
Methods
We searched the MEDLINE, MEDLINE In-Process, EMBASE, Science Citation Index, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases for studies published between inception and 3 April 2013. Included studies reported data for underlying disorders and complications in children presenting in primary or ambulatory care with influenza or ILI. Our search used a validated child filter30 and did not include any language restrictions. We also reviewed reference lists of included articles, relevant reviews and guidelines.
We requested unpublished data from authors whose studies had been obtained but not yet published, which we sought to include in our analysis.
We included studies that recruited children up to 18 years of age who had influenza confirmed on laboratory or near-patient testing or ILI diagnosed on clinical grounds. We primarily looked for cohort studies to include in our review but to be as inclusive as possible we also included case–control studies if published or unpublished data relating to these studies were sufficient for us to construct 2 × 2 tables.
We excluded cross-sectional surveillance studies that did not report clinical outcomes. We also excluded case series of hospitalised children, since the main purpose of our study was to provide a definition of ‘at risk’ that would be applicable to primary care.
Standard forms were developed to assess risk of bias (based on previously published assessment tools31–33) and to extract data. We included 28 articles that reported data from 27 studies (14,086 children). The primary outcome was admission to hospital as a proxy for complications of influenza or ILI.
Analysis
Our main analysis was a meta-analysis of univariable results from all included studies to examine the association between different conditions and hospitalisation. However, owing to the potential for confounding in this type of analysis, we also performed a multivariable analysis using individual patient data from studies where the authors provided these data. This individual patient data analysis enabled us to account for multiple medical conditions and age.
Our analysis used a one-step approach whereby the summary odds ratio (OR) for each condition is generated directly from the data provided by each study rather than from study-specific summary ORs. This approach is considered preferable when there is a high risk of comparisons being distorted by low event rates and high numbers of zero cells. This was the case in our review, since we were focusing specifically on primary and ambulatory care populations where hospitalisation is a rare event and certain conditions are uncommon. Underlying conditions were well defined in most studies, as were the methods used for diagnosing influenza. Study quality was variable in terms of patient selection and definition of clinical outcomes. More than half of studies failed to provide a clear definition of hospitalisation. Nine studies involved prospectively recruited cohorts and there were two case–control studies. The remaining studies were retrospective database reviews.
We calculated ORs with 95% confidence intervals (CIs) for individual risk factors in relation to hospital admission as a proxy for influenza-related complications.
Strengths and limitations
A specific focus on children, an extensive search, use of univariable and individual patient data, and multivariable analyses including unpublished data were all strengths of our systematic review. A limitation was that most studies took place in hospitals or ambulatory care, possibly affecting the generalisability of our findings. Risk factor definitions were also often unclear particularly in relation to premature birth.
Key findings
-
Premature birth, neurological disorders, sickle cell disease, immunosuppression, diabetes and age <2 years were all risk factors for hospital admission in children who presented with influenza or ILI in primary or ambulatory care. In our individual patient data analysis, only 11 of the 48 children identified as having been born prematurely were aged ≥2 years. We were therefore unable to assess whether premature birth was still a risk factor for children older than 2 years of age.
-
Unlike the other factors identified in this systematic review, premature birth is not defined as a risk factor in any existing guidelines. We included premature birth in children <2 years of age as a risk factor in our clinical trial based on the evidence of this systematic review.
-
Respiratory disorders (including reactive airways disease such as asthma), obesity and older age groups (i.e. age 2–5 years and age >5–18 years) were not identified as risk factors.
-
The presence of more than one coexisting condition significantly increased the risk of hospital admission, from 52% (one condition only) to 74% (more than one condition) when aged <2 years is included as a risk condition.
Inter-relationship with other parts of the programme
An understanding of risk factors in children is important, in view of the different comorbidity profiles encountered in paediatric versus adult populations and the high burden of disease associated with influenza in children. 28 We included premature birth in children <2 years of age as a risk factor in our clinical trial based on the evidence of this systematic review. The systematic review also contributed to the pre-trial cost prediction model that was conducted as part of work package E.
Addressing objective 2: understand what factors influence GPs’ decisions about antibiotic prescribing for at-risk children with influenza/influenza-like illness
Microbiological and/or point-of-care testing for influenza is uncommon in UK practice and GPs are largely reliant on clinical features in their assessment of children with ILI. This takes place in a climate of increasing concern regarding antibiotic overuse but with the knowledge that at-risk children can deteriorate rapidly. We investigated GPs’ accounts of factors influencing their decision-making about antibiotic prescribing in the management of at-risk children with influenza/ILI.
This work has been published as a separate paper by Ashdown et al. 34 Work package B interview schedules and GP regional demographics are available as Report Supplementary Material 1.
Aim
Our aim was to identify and explore the key factors likely to influence implementation of our research findings by gathering a representative range of GP perspectives on diagnosis and treatment decisions when at-risk children with influenza/ILI present in general practice.
Methods
Interview participants were recruited via a range of methods including the former local primary care trust lists and Primary Care Research Network. Data on deprivation and prescribing were obtained via the NHS Information Centre in October 2012. 35,36 We aimed for maximum variation in our sample according to duration of experience in general practice and local factors. Interviews took place over telephone, over Skype™ (Microsoft Corporation, Redmond, WA, USA) or face to face between March 2013 and March 2014. A GP academic registrar conducted the interviews and aimed to complete each interview in around 20 minutes in view of GPs’ working schedules.
Our semistructured interviews included a mock case vignette, which GPs were sent by e-mail prior to their interview. Additional clinical findings were revealed during the course of the interview to simulate information gathering during GP consultations.
The research team developed the interview topic guide. It was then piloted with two GPs who did not participate in interviews for the study.
Analysis
We conducted thematic analysis using constant comparison, deriving our coding scheme from the interview data. 37 Two researchers coded the data using NVivo version 10 (QSR International, Warrington, UK) until agreement was reached, after which coding was completed by one researcher. During the latter interviews, applying the coding structure to the data set helped facilitate revision of the topic guide and establish when data saturation had been achieved. Analytic and conceptual categories were developed by grouping codes. The ‘comorbidity’ category was analysed by mind mapping to explore patterns in the data and deviant cases. 37
Strengths and limitations
We interviewed a diverse sample of GPs, thereby providing a comprehensive and varied range of views on clinical decision-making in at-risk children with ILI. The case vignette encouraged GPs to discuss their decision in real time, as if in a consultation. Interview participants’ responses may have been influenced by knowing that the researcher conducting their interview was a GP trainee, but considerable uncertainty was still evident. A qualitative researcher also reviewed early interview transcripts to ensure that the interview did not come across as testing participants.
Key findings
-
GPs expressed uncertainty and different views on the potential impact of comorbidities on risk of clinical deterioration and how this risk should be managed.
-
Factors that GPs considered included the child’s history, appearance, the GP–parent relationship and the GP’s own confidence in local arrangements for out-of-hours care.
-
Neither clinical suspicion of influenza nor immunisation status were considered important factors.
-
GPs did not view influenza differently from other causes of RTI in children, despite the greater risk of bacterial infections.
Inter-relationship with other parts of the programme
This work informed the relevance and acceptability of work carried out as part of work packages C, D and E to a key stakeholder group, in this case GPs. The uncertainty surrounding when to prescribe an antibiotic when an at-risk child presents with ILI highlights the need for more evidence for clinicians to base decisions on.
Addressing objective 3: explore the experiences of parents of at-risk children who have previously become unwell owing to influenza/influenza-like illness and
Addressing objective 4: explore parental consulting attitudes in relation to influenza/influenza-like illness and involve parents of at-risk children in the development of trial materials
We aimed to examine parents’ attitudes towards contacting their doctor when their child develops ILI and understand the experiences of parents of at-risk children who have previously become unwell owing to ILI. In addition, we explored parents’ beliefs regarding the relative necessity of antibiotics versus concerns that antibiotics may cause harm to their child, as general attitudes towards antimicrobial stewardship have significant implications for our findings.
The work specifically pertaining to the experiences of parents with at-risk children has been published online. 38 We have also published an additional paper on general parental attitudes towards antimicrobial stewardship as a separate paper by Van Hecke et al. 39
Aim
Our aim was to identify and explore the key factors likely to influence implementation of our research findings by gathering a representative range of parent perspectives on influenza/ILI in at-risk children.
Methods
For our article on Healthtalk.org (The Dipex Charity, Oxford, UK),38 parents were recruited to take part in interviews and/or focus groups using a range of strategies and sources. These included a national network of GPs, advertising on online support groups and forums, local newspapers and snowballing. We also recruited parents via the Health Experiences Research Group (Nuffield Department of Primary Care Health Sciences, University of Oxford) expert advisory panel members, which includes patients, health-care professionals and researchers with relevant content and research expertise.
We conducted in-depth qualitative interviews with 31 individual parents/carers and two pairs of parents of at-risk children across England for Healthtalk.org. Interviews were video- and audio-recorded with consent, exploring families’ experiences of their child falling ill with ILI. This included discussion of background information and previous experience of ILI; the consulting process and experience; the impact within family life; life afterwards/reflections; past experiences of clinical trials; and practical, social and ethical factors influencing attitudes.
The PAUSE (Parents' perceptions of Antibiotic USE and antibiotic resistance) study interviewed 23 parents of pre-school children in Oxfordshire who had a RTI in the past 3 months to explore their attitudes, perceptions and understanding of antibiotic use and resistance.
Analysis
Data analysis transcripts were entered into NVivo software (version 6) for our Healthtalk.org analysis and used to help organise and analyse anticipated and emergent themes using constant comparison. This ensured that we identified issues that are important to parents and children, not just those that health professionals and researchers perceive as being important. Data collection proceeded simultaneously and continued until data saturation was reached. NVivo software (version 11) was also used to analyse the PAUSE interviews. Ten PAUSE interviews were coded and categorised to create themes and subthemes and applied to subsequent interviews. Agreement on themes and subthemes was sought between members of the research team and 20% of the transcripts were coded by two members of the research team to ensure consistency.
Strength and limitations
Key strengths of this work were that it allowed parents to speak of their own experiences directly and that Healthtalk.org represents parents of children with varying ages and health conditions living in a range of geographical locations. PAUSE is the first UK study that specifically focuses on parents’ perceptions of antibiotic resistance, its relevance to them and the strategies that might work to change behaviour and reduce antibiotic use for children with acute RTIs. By understanding parents’ beliefs we can better understand the acceptability of our findings to them.
A limitation was that parents willing to be interviewed about their experiences may be self-selecting and not truly representative. PAUSE was biased towards white British mothers and did not focus on at-risk children. However, the attitudes and beliefs of those who do not need antibiotics routinely impacts those who do, making the PAUSE findings relevant to our overall objectives.
Key findings
-
Many parents discussed how quickly their child could deteriorate with an episode of ILI and that they were not always prepared for this consequence of their child’s underlying health condition.
-
Parents on the whole expressed positive attitudes towards the use of antibiotics while expressing awareness of the necessity of antimicrobial stewardship and that antibiotics were not typically useful against viral infections.
-
Some parents were aware that antibiotics can be used prophylactically to prevent rather than treat infection. They were supportive of this approach.
Inter-relationship with other parts of the programme
This work informs the relevance and acceptability of work carried out as part of work packages C, D and E to a key stakeholder group, in this case parents, including parents of children at risk from complications of ILI. It also allows issues of concern and interest to parents to be further explored rather than those of researchers only. See Patient and public involvement for details on parents’ involvement in the development of trial materials.
Addressing objective 5: determine the effectiveness of early co-amoxiclav use in at-risk children with influenza/influenza-like illness
Parts of this section have been adapted with permission from Wang et al. 40 The text below includes minor additions and formatting changes to the original text.
The UK government stockpiles co-amoxiclav for treating bacterial complications during influenza pandemics because consistently high susceptibility to co-amoxiclav has been demonstrated in lower respiratory tract bacterial isolates associated with influenza. Immediate antibiotic treatment is recommended for RTIs in individuals with significant underlying disease21–24 but general practitioners report considerable uncertainty about prescribing antibiotics to children with mild or moderate risk factors. 34 To assist in decision-making surrounding appropriate use of antibiotics in this cohort of children we conducted a double blind randomised placebo-controlled trial: ARCHIE (The early use of Antibiotics for at-risk children with InfluEnza-like illness). ARCHIE aimed to determine the effectiveness of early co-amoxiclav use in at-risk children with ILI.
The trial protocol has been published as Wang et al. 41 and the trial main results have been published as Wang et al. 40
Aims
The trial aimed to determine whether or not early co-amoxiclav use reduces risk of reconsultation owing to clinical deterioration in at-risk children who present with ILI.
Methods
Participants were recruited from general practices and other ambulatory care settings in England and Wales. Recruitment began on 11 February 2015. Subsequent recruitment seasons commenced in October and continued until the end of the following March or later if data from the Royal College of General Practitioners Research and Surveillance Centre indicated that the weekly ILI GP consultation rate was still above the baseline seasonal threshold calculated each season using the Moving Epidemic Method. 42 In total, we opened 151 general practices, 42 hospitals and 2 walk-in centres for recruitment.
We recruited at-risk children with known risk factors for influenza-related complications aged 6 months to 12 years (inclusive) who presented within the first 5 days of ILI onset. Risk factors included chronic underlying conditions, hospital admission with bronchiolitis, pneumonia or an acute asthma exacerbation within the last year, recurrent viral wheeze, or premature birth in children aged 6–23 months. ILI was defined as the presence of a cough and fever; the fever could be child-reported, parent/guardian-reported, or an axillary or tympanic temperature >37.8 °C. We excluded children with known contraindications to co-amoxiclav and children who required immediate antibiotics or hospital admission based on their clinician’s judgement. We also excluded children who had previously participated in the ARCHIE trial at any time or another medicinal trial within the last 90 days. Children with known cystic fibrosis were excluded because immediate antibiotic treatment for acute respiratory tract infections is recommended in these children.
Participants were randomly assigned (1 : 1) to receive co-amoxiclav 400/57 (amoxicillin 400 mg as trihydrate and clavulanic acid 57 mg as potassium salt/5 ml when reconstituted with water) or placebo suspension using a validated, web-based randomisation system (Sortition®; Clinical Trials Unit, Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK). Randomisation was stratified by geographical region and minimised, using a non-deterministic algorithm, for age (6–23 months or 2–12 years) and seasonal influenza vaccination status (yes or otherwise). The chance of being allocated to the minimising group was set to 80%. Each site was sent study medication in blocks of eight. Allocations were computer generated using block randomisation (block sizes of two and four) by the trial statistician using Stata® version 13.1 (StataCorp LP, College Station, TX, USA).
Health-care professionals, the study team, participants and parents/guardians were blinded to treatment allocation. Participants were asked to take study medication orally twice daily for 5 days at the British National Formulary (British National Formulary for Children 2017-2018)-recommended dosage for their age/weight. Nasal swabs were obtained, placed in viral transport medium and analysed by real-time polymerase chain reaction (PCR) analysis at Alder Hey Children’s Hospital to detect influenza and other respiratory viruses.
At the baseline visit we collected data on age, sex, comorbidities, household smoking status, influenza vaccination status, medications given during the current illness episode, duration of fever, duration of symptoms, heart rate and respiratory rate.
To determine the primary outcome (reconsultation owing to deterioration), data on reconsultations, medication prescriptions, investigations, hospitalisations and deaths within 28 days of randomisation were later extracted from participants’ medical records. Data on medical conditions, regular medications, vaccinations, acute consultations during the 12-month period before randomisation and antibiotic prescriptions during the 3-month period before randomisation were also extracted.
To collect data on medication adherence, duration of fever and duration of symptoms, parents/guardians were given four 1-week diaries to record doses of study medication taken (week 1 diary only), axillary temperature, symptoms and adverse events. Parents/guardians were asked to record symptom data daily until the child had recovered but to resume data collection if symptoms relapsed. Parents/guardians were also asked to record the child’s temperature daily for 28 days or until it had been below 37.5 ○C for two consecutive days. Health-care professionals contacted parents/guardians by telephone 1 and 2 weeks after randomisation to collect data on adverse events, duration of fever and study medication doses taken in case diary data were not provided.
Outcomes
Our primary outcome was reconsultation owing to clinical deterioration within 28 days of randomisation. We defined clinical deterioration as worsening symptoms, development of new symptoms or development of complications requiring medication or hospitalisation. ‘Worsening symptoms’ were identified through documented evidence of deterioration in symptoms reported at the index consultation. ‘New symptoms’ included any symptoms not reported at the index consultation. Hospitalisations included hospital admissions following primary care referrals and direct admissions from hospital ambulatory care settings. To ensure accurate recording of clinical outcome data, a clinician independent from the study team reviewed a random selection of medical records.
Secondary outcomes were medication prescriptions and/or further investigations, adverse events, hospitalisations or deaths (all within 28 days of randomisation), as well as duration of fever and duration of symptoms. Our protocol did not require recruiting sites to report oral mucocutaneous candidosis (thrush), diarrhoea, nausea, vomiting or rash as adverse events if they were assessed as being of mild or moderate clinical severity and did not result in a serious adverse event, as these are already known common side-effects of co-amoxiclav.
Analysis
Our target sample size was 650 participants, including allowance for 25% loss to follow-up and an inflation factor of 1.041 to allow for potential clustering within recruiting sites owing to differences in physician care and prescribing rates. This would allow the detection of a reduction in the proportion of participants reconsulting owing to clinical deterioration from 40% to 26% (35% relative risk reduction) with 90% power and 5% two-tailed alpha error.
We performed an intention-to-treat analysis and participants were analysed in the groups to which they were allocated.
The proportions of children reconsulting owing to clinical deterioration in the two groups was compared using a chi-squared test and log-binomial regression model with adjustment for region, age and current seasonal influenza vaccination status. The treatment effect is reported as a relative risk with a 95% CI; the p-value is also presented. An unadjusted risk difference is also presented with 95% CI.
Durations of fever and symptoms were compared between groups using the Wilcoxon rank-sum test and quantile regression. Analyses performed using quantile regression were adjusted for region, age and current seasonal influenza vaccination status. Binary outcomes (proportions of children prescribed medication and/or requiring further investigations, children in whom adverse events are reported and children who were hospitalised or died within 28 days of randomisation) were compared using chi-squared/Fisher’s exact test for the unadjusted analysis and log-binomial regression, adjusting for region, age and current seasonal influenza vaccination status.
Exploratory subgroup analyses of the primary outcome were pre-specified in the statistical analysis plan to explore whether laboratory-confirmed influenza and treatment with antiviral medications during the index ILI episode moderated the treatment effect. The log-binomial regression model was fitted on the outcome of reconsultation owing to clinical deterioration and adjusted for region, age and current seasonal influenza vaccination status with an additional main effect for the subgroup variable and an interaction term for randomised group and subgroup variable.
Strength and limitations
A key strength of this work was that it is highly generalisable to community-based health-care settings where at-risk children may present with ILI during influenza season owing to our wide geographical coverage, recruitment from primary and other ambulatory care settings, pragmatic ILI case definition, and high retention rate (265/271) for our primary outcome.
Our main limitations were a lower than anticipated proportion of children reconsulting owing to clinical deterioration, failure to reach our recruitment target of 650 participants and a low proportion of participants testing positive for influenza, with seasonal influenza activity being comparatively low during the years of trial recruitment. 43,44,45 Our subgroup of participants with laboratory-confirmed influenza therefore did not have sufficient statistical power to allow us to determine whether or not early co-amoxiclav treatment is beneficial in these children.
We were only able to obtain data on medication adherence from 184/271 participants (co-amoxiclav: 95/136, 70%; placebo: 89/135, 66%). We were also only able to analyse diary data on duration of fever or other symptoms in around half of children. These findings should therefore be interpreted with caution.
Key findings
-
Figure 2 summarises recruitment and follow-up of participants. Between 11 February 2015 and 20 April 2018 we screened 756 children for eligibility and randomised 271 eligible children whose parent/guardian consented to their participation (co-amoxiclav n = 136, placebo n = 135). Primary outcome data were available for 265 participants (co-amoxiclav n = 133, placebo n = 132).
-
Nearly three-quarters of risk factors were in the respiratory category (198/271 participants, 73.1%), most commonly asthma (n = 99) and recurrent viral wheeze (n = 70). Around one-third of participants received the influenza vaccination relating to the season during which they were recruited. Laboratory-confirmed influenza was detected in 37/271 children (13.7%). However, rhino/enteroviruses were more commonly isolated (119/271 children, 43.9%). Throat swabs were obtained from 225 participants (co-amoxiclav n = 114, placebo n = 111). The most common bacterial isolate was Haemophilus influenzae (Lehmann and Neumann 1896) Winslow et al. 1917, which was detected in 52/225 throat swabs (23.1%) and 13/37 participants with laboratory-confirmed influenza (35.1%).
-
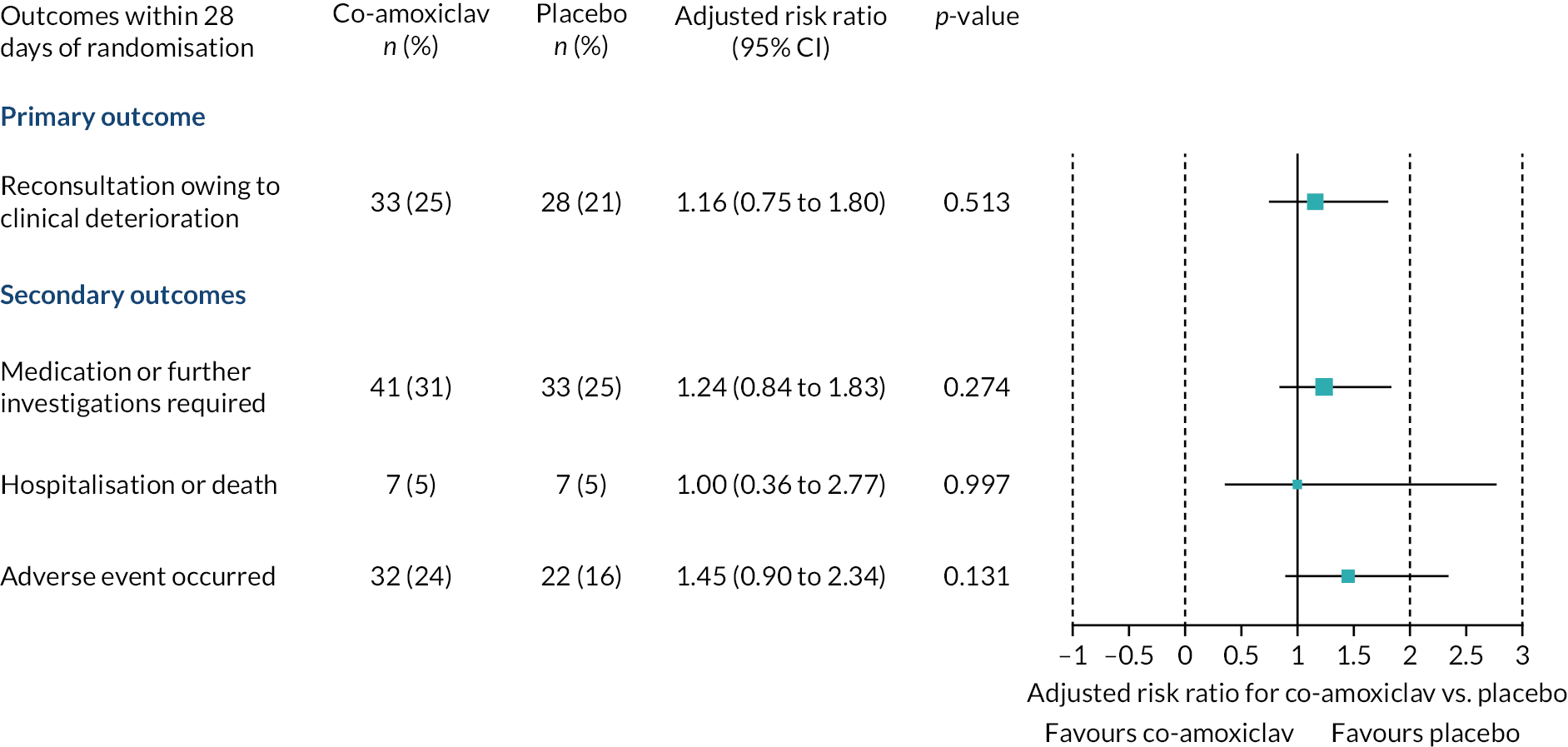
Figure 3 summarises reconsultations owing to clinical deterioration within 28 days of randomisation. At least one reconsultation was recorded in 33/133 children randomised to co-amoxiclav (24.8%) and 28/132 children randomised to placebo (21.2%). There was no evidence of a difference in clinical deterioration between groups after adjustment for stratification and minimisation factors [adjusted risk ratio (RR) 1.16, 95% CI 0.75 to 1.80; unadjusted RR 1.17, 95% CI 0.75 to 1.82, unadjusted risk difference 3.6%, 95% CI –6.5% to 13.7%]. No adjustment for clustering was performed because the average cluster size was only 1.4 (271 participants from 195 sites). No statistically significant differences were observed in the proportions of children requiring medication or further investigations, or hospitalisation (see Figure 2). No deaths were recorded.
-
Data on duration of fever were available for 136 participants (co-amoxiclav n = 71, placebo n = 65) and data on duration of symptoms were available for 134 participants (co-amoxiclav n = 63, placebo n = 71). Analyses performed using the Wilcoxon rank-sum test found that median duration of disturbed sleep was significantly shorter in children who received co-amoxiclav than those who received placebo [co-amoxiclav: median 4 days, interquartile range (IQR) 2–6 days; placebo: median 7 days, IQR 3–11 days; p = 0.021]. However, quantile regression analyses adjusted for region, age and current seasonal influenza vaccination status found that duration of shortness of breath was significantly shorter in the co-amoxiclav group [adjusted median difference –2.00 days, 95% CI –3.89 to –0.11 days; p = 0.038). No evidence of differences between groups was found for other symptoms or fever.
-
One or more adverse events were reported in 32/136 children in the co-amoxiclav group (24%) and 22/135 children in the placebo group (16%). Thirty-seven adverse events were reported in the co-amoxiclav group and 29 in the placebo group. Only 12 adverse events were reported as being possibly related to study medication (co-amoxiclav n = 5, placebo n = 7) and three as being probably related to study medication (co-amoxiclav n = 2, placebo n = 1). The most commonly reported adverse events were skin complaints and RTIs considered separate from the index ILI episode. Nine serious adverse events were reported in each group, none of which was considered related to study medication.
-
Our pre-specified subgroup analysis in children with laboratory-confirmed influenza found that the proportion of children who reconsulted owing to clinical deterioration was lower in the co-amoxiclav group (5/21, 23.8%) than in the placebo group (6/16, 37.5%). However, no statistically significant difference was demonstrated and there was no evidence of an interaction between treatment arm and laboratory-confirmed influenza status (p = 0.241). We did not perform our planned subgroup analysis in children who had been prescribed antiviral medication at or before their baseline visit, as no participants received antiviral medication.
FIGURE 2.
Participant recruitment and follow-up.
a, Protocol deviation: treating clinician withdrew study medication (medical notes available for review); b, parent left without study medication, withdrew consent after discussion with child’s father; c, child received study medication but parent subsequently withdrew consent for further contact and notes review; d, medical notes for primary outcome withheld by GP surgery for internal reasons but participant not withdrawn and diary data available for secondary outcomes.
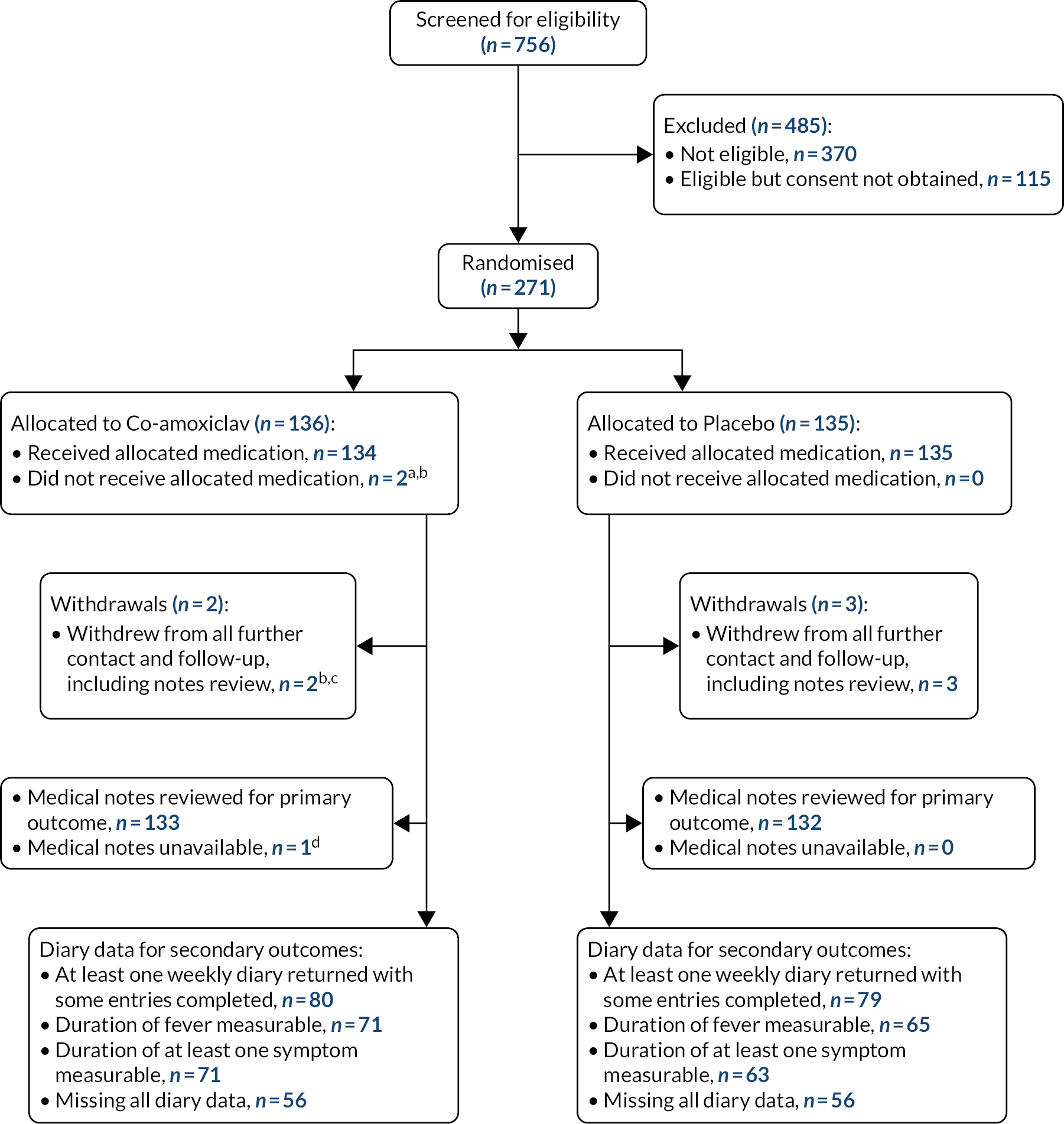
FIGURE 3.
Adjusted risk ratios with 95% CIs for primary and binary secondary outcomes.
Inter-relationship with other parts of the programme
This work addresses uncertainty around the benefits of prescribing antibiotics to at-risk children who present with ILI in community-based health-care settings, as discussed in work package B. Trial participants whose parents or guardians gave additional written informed consent also participated in our nested longitudinal cohort study to examine the impact of early co-amoxiclav use on long-term bacterial carriage and antibiotic resistance (work package D). Data collected through the clinical trial were used in work package E to calculate risk reduction scores to identify which children may benefit most from co-amoxiclav treatment when presenting with ILI and to perform a health economics analysis.
Addressing objectives 6 and 7: examine the impact on antibiotic resistance and long-term respiratory bacterial carriage of early co-amoxiclav use in at-risk children with influenza/influenza-like illness
Two of the most significant threats to global health systems are antibiotic resistance and emerging pathogens, such as new strains of respiratory viruses, with the potential to trigger pandemics. Concerns persist that overconsumption of antibiotics fuels antibiotic resistance both in the general population and specifically when children are prescribed antibiotics unnecessarily. At-risk children receive more courses of antibiotics than otherwise healthy children and as a result may carry a greater proportion of resistant species in their background respiratory tract flora that potentially put them at higher risk of not responding to future courses of antibiotics. Given the consequences to both individuals and communities of antibiotic resistance, it is vital that responsible antimicrobial stewardship is adopted and that the benefits of prescribing an antibiotic outweigh the potential risks.
Work exploring issues surrounding antibiotic resistance funded by the ARCHIE programme has been published by van Hecke et al. 46 and van Hecke et al. 47
The likelihood of fuelling antibiotic resistance has implications for our programme findings as a potential consideration in balancing the risk-to-benefit ratio of co-amoxiclav prescribing for ILI in at-risk children. This risk-to-benefit ratio is explored further in a nested microbiology substudy conducted as work package D of the ARCHIE programme.
Aims
We aimed to compare the group prevalence and resistance of Streptococcus pneumoniae and H. influenzae at 12 months between children in the co-amoxiclav and placebo arms of the ARCHIE trial. This was to examine the potential impact of a 5-day course of co-amoxiclav on long-term respiratory bacterial carriage and antibiotic resistance in at-risk children consulting with ILI.
Methods
Parents/guardians of participants recruited into work package C were invited to additionally give consent for their child to take part in a nested microbiology substudy. We estimated that a sample of 210 children would be sufficient to detect a 20% increase in the proportion of ampicillin-resistant S. pneumoniae and Haemophilus sp. in the co-amoxiclav group compared with the placebo group with 80% power and 5% alpha error. As we estimated a 40% loss to follow-up rate we planned to consent 360 children. Only 271 children were consented to the main trial. Baseline throat swabs were collected from all participants where possible as part of the main trial procedures with 225/271 baseline throat swabs being available and 201/271 children were consented to the nested substudy. Participants consented to the nested substudy were invited to have a follow-up throat swab at 3-, 6- and 12-month time points and a home visit for swab collection was offered where feasible. Table 1 provides a summary of completed swab data for all randomised children. Throat swabs were immediately placed into a 1 ml Amies [MWE (Medical Wire & Equipment, Potley Lane, Corsham, Wilts, SN13 9R)] liquid transport medium and posted by first class mail to Alder Hey Children’s Hospital’s NHS laboratory.
| Swabs obtained | Co-amoxiclav group (N = 136), n (%) | Placebo group (N = 135), n (%) |
|---|---|---|
| Swab taken at baseline | 114 (84) | 111 (82) |
| Consented to swab at 3, 6 and 12 months | 103 (76) | 98 (73) |
| Swab taken at 3 months | 55 (40) | 45 (33) |
| Swab taken at 6 months | 47 (35) | 45 (33) |
| Swab taken at 12 months | 47 (35) | 46 (34) |
Swabs [Swab kits components were provided by VWR International (Radnor, Pennsylvania, US).] were plated by application of 1 μl and 10 μl loops of transport media on blood and chocolate agar bacterial growth media both with and without ampicillin to be analysed for bacterial growth by species identified and resistance to common anti-microbial agents. We compared the group prevalence and resistance of H. influenzae, S. pneumoniae and Streptococcus species at 12 months among children in the co-amoxiclav versus those in the placebo arm. Parents/guardians gave consent for their children’s primary care records to be reviewed and data pertinent to antimicrobial prescribing in the 3 months prior to enrolment and during the study were collated and compared.
Analysis
We originally planned to apply a log transformation to minimum inhibitory concentration (MIC) measurements and summarise these data using geometric means and 95% CIs. We intended to compare the group prevalence of S. pneumoniae, H. influenzae and S. aureus at 12 months between children in the co-amoxiclav and placebo arms. Data on swabs at 3 and 6 months would help impute information on children without a 12-month swab.
Owing to limited available data caused by lower recruitment into work package C, a higher rate of follow-up attrition (66% vs. the estimated 40%) and fewer pathogens isolated (only two incidences of S. pneumoniae were cultured) than anticipated, analysis was descriptive as few statistical tests could be applied. However, enough data were available to perform a chi-squared test on the H. influenzae results and the prescribing pattern obtained from the medical notes review. Swabs were reported by bacterial species cultured. Where more than one isolate of the same species was present, only the isolate that had evidence of resistance was included in analysis. Where more than one bacterial species was present, these were analysed separately.
Strengths and limitations
A major strength of this work was that it recruited a cohort of at-risk children, who tend to be more frequent antibiotic consumers than their peers. The substudy was also embedded in a randomised controlled trial rather than merely observational. A limitation was that lower recruitment into work package C impacted recruitment into the nested substudy and retention in the nested substudy was lower than anticipated with approximately 66% attrition. A 12-month swab was obtained from only 93 of 225 participants who had a baseline swab with only 201/225 consenting to the nested substudy.
Key findings
-
Haemophilus influenzae was the most commonly isolated potential pathogen (23% of all pathogens identified) and no evidence was found to suggest that the 5-day course of co-amoxiclav impacted its susceptibility to antibiotics. Table 2 provides a summary of the prevalence of different isolates.
-
Only two isolates of S. pneumoniae were cultured. This may be indicative of the success of the pneumococcal vaccine programme, as the prevalence of S. pneumoniae was anticipated to be significantly higher in this cohort. No evidence was found to suggest that the additional course of co-amoxiclav leads to suppression of normal mixed flora or emergence of other resistant species such as methicillin-resistant Staphylococcus aureus (MRSA). Table 3 provides a summary of the prevalence of resistant isolates.
-
An unexpected observation was the substantial reduction in the number of antibiotics prescriptions issued to ARCHIE participants during the 12-month period of follow-up compared with the 3 months prior to study entry, suggesting a strong placebo effect to trial participation. This may reflect the confidence parents had in the regular safety monitoring provided by study participation.
-
The systematic review conducted by van Hecke et al. 48 showed that patients with laboratory-confirmed antibiotic-resistant RTIs are more likely to experience delays in clinical recovery after treatment with antibiotics.
-
The observational cohort study conducted by van Hecke et al. 49 showed that in children prescribed antibiotics for an acute RTI, the odds of response failure were greater for those who had already received two or more courses of antibiotics for RTIs in the preceding year.
| Bacterial isolates | Co-amoxiclav group (n) | Placebo group (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (N = 114) | 3 months (N = 55) | 6 months (N = 47) | 12 months (N = 47) | Baseline (N = 111) | 3 months (N = 45) | 6 months (N = 45) | 12 months (N = 46) | |
| H. influenzae | 20 | 11 | 7 | 6 | 32 | 10 | 12 | 6 |
| S. pneumoniae | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. aureus | 4 | 1 | 2 | 0 | 1 | 1 | 0 | 0 |
| Group A Streptococcus | 3 | 3 | 0 | 0 | 3 | 6 | 1 | 1 |
| Group C Streptococcus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group G Streptococcus | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| MRSA | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Mixed flora | 87 | 40 | 38 | 41 | 74 | 30 | 32 | 39 |
| No growth | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Antibiotic resistant isolates | Co-amoxiclav group, n/N (%) | Placebo group, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| Haemophilus-selective agar containing ampicillin | 6/20 (30) | 5/11 (46) | 1/7 (14) | 1/6 (17) | 7/32 (22) | 3/10 (30) | 4/12 (33) | 1/6 (17) |
| Streptococcus-selective agar containing ampicillin | 0/4 (0) | 0/4 (0) | 0/1 (0) | – | 0/6 (0) | 0/6 (0) | 0/1 (0) | 0/1 (0) |
| Methicillin-susceptible S. aureus: cefoxitin sensitive | 4/4 (100) | 1/1 (100) | 2/2 (100) | – | 1/2 (50) | 1/1 (100) | – | – |
| MRSA: cefoxitin resistant | 0/4 (0) | 0/1 (0) | 0/2 (0) | – | 1/2 (50) | 0/1 (0) | – | – |
Inter-relationship with other parts of the programme
This work addresses issues raised during our preliminary patient and public involvement (PPI) work and in work package B around parents’ beliefs concerning response failure, antibiotic resistance and general attitudes towards appropriate antibiotic use. It also addresses clinicians’ uncertainty about when to prescribe antibiotics. The substudy nested within our trial does not provide evidence that an additional course of co-amoxiclav impacts upon respiratory bacterial carriage or antibiotic resistance, which is important information for stakeholders such as parents and clinicians when discussing if antibiotic treatment would be appropriate when a child presents with ILI.
Appendix 2 provides a summary of our nested substudy findings.
Addressing objective 8: develop and validate risk scores for influenza-related clinical deterioration and complications for use in children with influenza/influenza-like illness (work package E1)
Our qualitative work (work package B) indicated that there is uncertainty over which at-risk children may benefit the most from early antibiotic treatment during ILI. Additionally, there is growing concern over prescribing antibiotics unnecessarily and driving antibiotic resistance. The development of risk reduction scores would assist with decision-making surrounding these issues.
Aims
We aimed to provide evidence-based guidance that clinicians can use to target early co-amoxiclav treatment at those children who may gain the greatest clinical benefit from it.
Methods
As part of work package C we collected baseline data from each participant on potential risk factors for influenza-related clinical deterioration and complications with the aim of developing four multivariable risk score models. Our chosen risk factors were age, type of co-morbidity, household smoking status, administration of the pneumococcal conjugate vaccine (PCV), duration of illness, heart rate, respiratory rate, laboratory-confirmed influenza, receipt of the current season’s influenza vaccination and receipt of the previous season’s influenza vaccination. For each risk score, our intention was to form three risk subgroups (low, medium and high risk). When estimating the sample size for work package C, we estimated that around 40% of children in the placebo group would reconsult owing to clinical deterioration. Based on this, we pre-specified that medium risk would be defined as 30–40%, low risk as <30% and high risk as >40%.
Analysis
The number of primary outcome events we observed in our trial population was not sufficient to produce risk scores as described above. Instead, we analysed risk factors using logistic regression in relation to reconsultation owing to clinical deterioration (our primary outcome).
A univariable analysis was performed on each risk factor and those that had an association with a p-value <0.1 were included in a multivariate model. Age was included in all evaluations, including ‘univariable’ evaluations.
Strengths and limitations
Strengths of this analysis included representation of a range of potential risk factors and the absence of confounding owing to indication in relation to antibiotic use, since co-amoxiclav and placebo were randomly allocated during our randomised controlled trial.
We were unable to calculate risk scores in relation to clinical intervention owing to an insufficient number of these outcomes in our trial population. The number of primary outcome events we observed was also not sufficient for us to calculate sufficiently robust multivariable risk scores to define the three risk groups we had previously specified.
Key findings
-
Only two variables showed statistically significant associations with reconsultation owing to clinical deterioration: higher respiratory rate was associated with greater risk of clinical deterioration, whereas presence of one of more smokers in the household was associated with decreased risk of clinical deterioration. However, these findings are based on a multivariable model that only included three baseline covariates (respiratory rate, household smoking status and age) and should be regarded as exploratory only.
-
No statistically significant associations with reconsultation owing to clinical deterioration were observed for treatment arm (co-amoxiclav vs. placebo), risk factor in respiratory category (vs. other risk factors) or recruitment in secondary care versus primary care.
Appendix 3 provides a summary of our findings.
Inter-relationship with other parts of the programme
This work supports GPs’ current emphasis on using a clinical impression to inform their decisions about antibiotic prescribing in at-risk children presenting with ILI, as described in work package B. The findings are also consistent with the main finding of work package C, which did not find evidence that early co-amoxiclav treatment reduces risk of reconsultation owing to clinical deterioration.
Addressing objective 9: explore the cost-effectiveness of different potential strategies for early antibiotic use in at-risk children with influenza/influenza-like illness (work package E2)
Influenza-like illness places a significant burden on both NHS resources and the resources of individual families, with lost school and work days being common and impacting quality of life. In addition to assessing if there is clinical benefit to prescribing co-amoxiclav to at-risk children presenting with ILI, it is also important to assess the overall benefits to the NHS, individuals and society of this intervention. A health economic analysis will contribute to this assessment.
Work to inform the design of this research has been previously published as Wolstenholme et al. 50 A separate paper discussing the final analysis has been published by Rombach et al. 51
Parts of this section have been reproduced from Rombach et al. 51 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/ The text below includes minor additions and formatting changes to the original text.
Aims
We aimed to explore different potential strategies for early antibiotic use in at-risk children with influenza/ILI in regard to the resource use, costs and health-related quality of life.
Methods
A pre-trial economic model was developed to estimate the cost–utility of being prescribed co-amoxiclav for 5 days compared with antipyretics. A literature review was conducted to evaluate the current evidence from economic evaluations on prescribing antibiotics for at-risk children for the treatment of influenza. A total of 911 articles were identified for the literature review, with 104 full articles read for relevance. Five publications were identified as relevant. A decision tree with a 1-year time horizon was developed using evidence from the literature review and the prognostic systematic review detailed in work package A to inform the model parameters.
In order to support the development of work package C and E2, a literature review was conducted to determine which instrument is most frequently used for child-based economic evaluations and whether child or proxy responses are preferred. Instruments were compared on dimensions, severity levels, elicitation and valuation methods, availability of value sets and validation studies, and the range of utility values generated. In addition, two focus groups of parents and young people (11–20 years), were convened to determine patient and proxy preferences across a number of suitable instruments. This qualitative work was undertaken as part of work package B and is summarised in Patient and public involvement.
The EuroQol-5 Dimensions, three-level youth version (EQ-5D-Y) and the Canadian Acute Respiratory Infection and Flu Scale (CARIFS) were the validated questionnaires selected for use in work package C based on the pre-trial findings. The EQ-5D-Y (descriptive system and visual analogue scale) was collected at baseline and days 4, 7, 14 and 28 and the CARIFS at baseline and day 7. Self-reported health-care use was collected in the study diaries with regards to medication use, hospital visits and admissions, and GP and nurse contacts. Information on the number of days children were unable to attend school or nursery and subsequent changes to childcare requirements was also collected. These data were collected in weekly diaries on days 7, 14, 21 and 28, requiring recall of health-care resource use and daily activities over the previous week. Data collected from the patients’ medical notes as part of work package C on the number of reconsultations, antibiotics and other drugs given, and chest radiograph and other investigations performed were also used in the estimation of health-care resource use. Where health-care resource use data were available from both self-report and medical notes reviews, the (more complete) data from the medical notes reviews were used to estimate the costs.
Analysis
Pre-trial decision model cost-effectiveness analysis
The incremental cost-effectiveness ratio (ICER) in terms of the cost per quality-adjusted life-year (QALY) of being prescribed co-amoxiclav for 5 days compared with antipyretics was estimated. A subgroup analysis was undertaken to explore the impact of age on the base-case results. Uncertainty was explored using one-way and probabilistic sensitivity analyses.
Within-trial analysis
Summaries were generated for all available cases and presented on an intention-to-treat basis for the within-trial analysis. EQ-5D-Y and CARIFS data were summarised descriptively by data collection time point. Differences between the trial arms at each time point were estimated using linear regression models, adjusted for the stratification factors age (used as continuous variable) and seasonal influenza vaccination status. Contacts with different health services were summarised using number and frequencies to indicate how often participants had used each service. The mean numbers of contacts with different health services were also calculated, and mean differences between the groups were generated as described above. Costs incurred for the different health service contacts were calculated by applying unit costs. The cost components and total costs of the trial were presented as mean costs by treatment arm; differences between the arms were calculated as described above.
Subgroup effects were explored by adding an interaction term between treatment allocation and laboratory-confirmed influenza at baseline to the primary analysis model for the total costs incurred over the trial follow-up period, and also separately for the costs incurred through medication use and the costs related to reconsultations, hospital admissions and referrals. Results from this investigation were presented as forest plots. As this analysis is exploratory, no p-values were displayed. A sensitivity analysis examined the effect of including costs associated with hospitalisations unrelated to the illness episode for which the child was recruited to ARCHIE. The costs were extrapolated to estimate the impact of either intervention on a national scale.
Strengths and limitations
A significant strength of the within-trial analysis was that the data were collected on a national scale throughout England and from a mixture of primary and ambulatory care settings. The availability of medical notes data enabled medical resource use to be calculated even when diary information was not available. A limitation, however, was that a full cost-effectiveness analysis could not be performed owing to the low availability of EQ-5D-Y data, with only 26.6% of diaries being available at day 28. This was a similar completion rate to other paediatric population studies. 52
A limitation of the pre-trial modelling was that the data from the literature review informing the model parameters were based on a general paediatric cohort, not those considered at risk, and included those presenting with a different disease, acute otitis media, rather than ILI. However this indicates the necessity for the ARCHIE Trial itself in order to gather more specific data on at-risk children presenting with ILI.
Key findings
-
The pre-trial cost-effectiveness decision model highlighted the need for a trial to be undertaken, owing to the paucity of evidence to inform the parameters in the model.
-
Despite the effort to ascertain the preferred outcome measure to use in this patient group, proxy completion rates for the EQ-5D-Y by day 28 were 26.6% of the sample. Parent proxies may have found some questions difficult to answer (e.g. a question asking if mobility has been affected may not have been applicable to children in whom limited mobility was to be expected owing to very young age or a pre-existing health condition).
-
When costs for reconsultations and hospital admissions related to the index ILI episode were included, the mean total cost per patient was £94 [standard deviation (SD) £480] in the co-amoxiclav group, and £122 (SD £539) in the placebo group (adjusted between group difference –£25, 95% CI –£113 to £62; p = 0.566). However, the difference between groups was not statistically significant.
-
The majority of costs were incurred in a secondary care setting.
-
A statistically non-significant trend towards lower non-medication costs and total costs for participants randomised to co-amoxiclav than those randomised to placebo was observed. However, larger studies would be needed to confirm this trend.
-
The laboratory-confirmed influenza subgroup analysis indicates that medication-related cost differences are very similar between the subgroups.
Inter-relationship with other parts of the programme
The pre-trial findings of work package E2 directly contributed to the design of the trial in work package C by selecting the most appropriate patient outcome measure to be used. The findings also contributed, alongside the findings of work package D, to the assessment of the risks and benefits of antibiotic prescription for work package C. This work package, specifically the pre-trial decision model, also drew on the systematic review that was conducted in work package A.
Patient and public involvement
Aims
The aims of involving patients and the public in the delivery of the ARCHIE programme were as follows:
-
Inform the design of patient-facing materials to be used during the trial. These included the parent and child participant information leaflets (PILs), recruitments posters, mascot, study diaries and quality-of-life questionnaires.
-
Inform the design of study procedures that children and parents taking part in the trial would find feasible and acceptable. This included informing the consent process and the communication of potentially sensitive topics, including views around placebo use and the participation of at-risk children.
-
Support the writing of plain English summaries and identify topics and findings that families find relevant to their experience.
Methods
We used the following methods to incorporate PPI in our programme:
-
Five discussion groups in Spring 2013 – four with parents (one being a very small initial discussion group with parents from Oxford) and one with young people. In total, we held discussion groups with 21 parents and 15 young people in four different locations: Oxford, London, Liverpool and Birmingham.
-
Inclusion of two PPI representatives in our Programme Steering Committee.
Results
Paperwork feedback
Mascot
The initial monkey mascot (see Figure 4) was quickly discarded. Although parents and young people felt a monkey was ‘child friendly’, they questioned its relevance to the study and a few parents and young people mentioned an association with animal studies. The penguin mascot (see Figure 5) was taken to three groups and the feedback was very positive: it was seen as ‘child friendly’, ‘cute’ and relevant to the seasonal winter theme. Further suggestions to the mascot to make it more ‘eye-catching’ included colour-coordinating the hat and scarf, making the colours brighter and making it more obvious within the ARCHIE logo that the mascot represented the letter ‘i’. Some preferred having the mascot out of the logo and placing it at the end for ease of reading. The young person group did not have the penguin logo to view but when asked they all stated that they preferred a penguin over a monkey logo.
FIGURE 4.
Feedback presentation from young person group including originally proposed monkey mascot. Used with permission.

FIGURE 5.
Archie finalised mascot.

Recruitment poster
Overall, parents found the amount of text on the recruitment poster to be acceptable but suggested that different sections of text should be separated more clearly. In general, parents wanted the poster to stand out more (they felt that it was ‘dull’) through the use of more creative font in the title (perhaps in a hand-written style), larger title font, colour, more images (i.e. penguins), and coloured paper. Views on the font varied but parents recommended that we consider people with dyslexia when adjusting the font and the colours. Red and green were thought to be appropriate colours for people with dyslexia. Both parents and young people disliked the font in the original ARCHIE logo. It was suggested that the URL on the poster was too long to remember and that a new URL should be secured, and also that the poster should include tear-off slips. The discussion groups thought that posters could be displayed in schools, nurseries, GPs, pharmacies, with community health visitors, community halls, children’s centres and hospital outpatients’ noticeboards.
Patient information leaflet and video
The patient information video narrated by Dr Wang (parts 1 and 2) was taken to three parent groups and received positively by all of them. They found that it answered their questions, felt that it was ‘reassuring’ and said that they would find watching it helpful when making decision about consent. It was suggested that the video was made available at the general practice (or in advance of an influenza like illness on a compact disc or via a web link) when making a decision about taking part. The video was made available on the trial website (www.archiestudy.com Most parents found the parent PIL too long and ‘wordy’ but struggled to point out what could be omitted. They stated they would prefer bullet points to long sentences. Suggestions for changes to the parent PIL included the following:
-
provide a clearer definition of ‘placebo’
-
give more detail, and in particular more reassurance, on the use of a placebo
-
provide details of the placebo and co-amoxiclav ingredients (to reassure parents that they are sugar-/gluten-/E-number-/colouring-free)
-
distinguish between type 1 and type 2: diabetes
-
increase the stated time it takes to fill in the study diary from 5 to 10 minutes
-
give more advice and details about what to do if your child deteriorates, what specific signs to look for and in what time frame
-
put a clearer emphasis on the fact that the antibiotic is for treating potential secondary bacterial infection and not ILI
-
provide more information about the possible risks of taking part (parents expressed concerns around penicillin allergies and the use of a placebo arm with at-risk children who can become unwell rapidly)
-
mention co-amoxiclav earlier in the PIL
-
explain what happens if the child does not complete the course of the study medication
-
in order to facilitate continued participation, more prominently acknowledge the time commitment of taking part in the trial but within the context of explaining why it is critical all these data are collected, why data is still collected when the child is already likely to have recovered and the individual/broader benefits of taking part.
The Young Person group did not like the child PILs and parents also felt that both the younger and older child-oriented versions of the PIL were too complicated for children, especially when ill. They wanted to see major changes to both child PILs. The leaflets were viewed as too long and overwhelming, plain and dull. Instead, the discussion groups suggested a story board, a comic strip or at least illustrations of the penguin at different stages of the trial. They suggested that the younger child version should be mostly picture-based with only a few words and the older child version should have illustrations but with slightly more text than the younger version. Parents suggested that children and young people should also have a patient information video specific to them and a ‘Where’s Archie’ picture game incorporated into the PIL. Overall, the content was viewed as reassuring (e.g. language such as ‘tickle’ and ‘easy to swallow’). It was pointed out (by the group coordinator) that as children would not be consenting there were fewer requirements for the child PILs and sections could be removed (e.g. the funding and complaints procedure section). The child PILs could then be adapted to focus on communicating what the study was about in a visual and text-light way.
Suggestions from the discussion groups for improvements to the child PILs included the following:
-
include shorter sentences, bullet points or speech bubbles (rather than prose), larger font, less text, more pictures, more colour and subheadings in a different colour
-
remove the current images (the younger child PIL logo was seen as ‘scary’) and replace with penguin illustrations (e.g. penguin taking liquid antibiotic, penguin receiving a swab and penguin thinking back to having the flu vaccine)
-
cut down on technical words, explain ‘antibiotics’ and ‘placebo’, and amend words seen as ‘babyish’
-
do not specify exact ages of the children in the PIL but instead label them as ‘older’ or ‘younger’ children
-
include further reassurance that regardless of whether the child receives the antibiotic or placebo they will still be safe, and if they become more unwell they will be closely monitored and receive good medical care.
Study diary
Overall, parents found filling in the paperwork one of the most challenging aspects of the trial, especially the expectation to continue after their child had recovered. Again, parents suggested that a clearer explanation of why this data collection was critical would be very helpful in encouraging parents to continue participating for the full 28 days. Parents suggested that the e-diary always be given to parents and most discussion group participants would have personally preferred this. Benefits of the e-diary over the paper diary included being easier to complete; being submitted after every week; providing a sense of completion; removing the possibility of the diary being stained, ripped or lost; not having to worry about posting; and a general preference of electronic forms over hard copies. If possible, parents preferred the diary to be smartphone-friendly.
Layout
It became clear early on that the initial 28-day diary was seen as confusing and cumbersome. We presented both the weekly and daily diaries to three parent focus groups and they all, without exception, preferred the daily layout for the diary. Benefits of the daily diary included being easier to fill in, making it more likely that parents would fill it in, being straightforward and streamlined, avoiding parents having to flick back and forth, and being as easy as possible to use when looking after an ill child.
Recording symptoms
The symptoms list was viewed as relevant. Some preferred to record the time of day of, and intervals between, doses of study medication rather than ‘morning’ and ‘evening’. Some found the first page too dense and preferred more spacing in the layout. Most parents had concerns about using an underarm thermometer because they felt they were unreliable, inaccurate and difficult to measure when the child is ill. They preferred an ear thermometer because they felt it was quick, familiar to both parents and children, and more accurate. Parents were unsure about using an infrared thermometer owing to being unfamiliar with them. All parents were happy to return any thermometer they were given after the study period had finished.
‘Other medication’
The discussion group felt that the ‘other medication’ section of the study diary needed an extra column for dosage and more rows for medications, ideally six to eight. It was also suggested that there could be a section for standard medications that their child may take regularly somewhere at the beginning of the document.
Quality-of-life questionnaire
Parents
Of the 21 parents, 14 preferred the EQ-5D-Y and seven preferred the Child Health Utility 9D (CHU9D). Discussed benefits to the EQ-5D-Y included having a better layout; being clearer, making it easier to assess the child and fill in; having better font; having fewer questions; and being more user-friendly, more applicable, more relevant, concise, more age-appropriate and more translatable across ages and symptoms than the CHU9D. Most parents liked the scale, although some found it hard to quantify their child’s well-being on the scale. Discussed benefits to the CHU9D included having more questions leading to more data, having more options with which to respond, being preferable for older children owing to more texture and detail, and being more relevant than the EQ-5D-Y. Drawbacks included too many options making it hard to differentiate between them, the questionnaire being too emotion focused, irrelevant content and the scoring of emotions being impossible for a baby or toddler. The frequency of completing the questionnaire on days 1, 4, 7, 14 and 28 was considered feasible and realistic. However, parents felt it needed to be made clearer why they would need to keep filling in the questionnaire even after their child had recovered.
Parents pointed out that it ought to be clearer in the instructions that the questions relate to the impact of the ILI on their child, not their overall well-being/state of mind. Parents felt children, especially when ill, would need support filling in either questionnaire.
Most parents found both questionnaires inappropriate for younger children (perhaps those under 3 or 5 years of age) and parents also noticed that the questions were not sensitive to the underlying problem that put their child at risk. In some cases (e.g. disabled children unable to speak or walk) the questionnaire results would be influenced more by the child’s underlying condition than by any deterioration in their well-being owing to ILI.
Young people
Of the 15 young people in the discussion group, nine preferred the EQ-5D-Y and six preferred the CHU9D. Those who preferred CHU9D said they would also be able to fill in the EQ-5D-Y. All the young people preferred the EQ-5D-Y. Discussed benefits to the EQ-5D-Y included being easier to complete on your own, having easier questions, placing more focus on practical and tangible experiences, being clearer owing to fewer and more straightforward options, being quicker to complete and requiring less thought, especially when ill, than the CHU9D. Some young people liked the scale, whereas others found it difficult to grasp.
Discussed benefits to the CHU9D included having more options than the EQ-5D-Y, allowing them to pinpoint how they were feeling more easily than the EQ-5D-Y (this feedback was from the older young people) and the reminder that the questionnaire is asking how they feel today, not generally. Younger members of the discussion group felt that the CHU9D was harder to understand than the EQ-5D-Y and would need to be completed with a parent.
Young people expressed no strong preference over whether they should complete the questionnaire themselves or their parents should complete it, although they felt that their parent may not know how to answer certain questions, such as questions on pain and moods. It was suggested that parents could have a note-taking/supportive capacity in the questionnaire completion. All young thought it was good to be given the option to fill it in themselves.
Canadian Acute Respiratory Infection and Flu Scale
Parents felt the CARIFS was very relevant and appropriate for assessing their child’s well-being when ill with ILI. Completing it on day 7 (and day 1 with the research nurse) was considered appropriate and feasible.
Health service contacts
Parents felt that details on health service contacts would be easier to fill in and keep track of at the end of each weekly diary. The early groups did not like the tick-box circles and these were quickly discarded in favour of the current format, which was suggested by one of the parents. Parents felt that sensitivity was needed around working options for parents who worked part-time, in shifts or from home, or were self-employed, as these would be harder to identify in the current health service contact form. Parents noted that part-time workers might get around losing a working day by changing their working day for that week or changing their shifts temporarily to look after the child.
Reminders
The views on reminders were split. On the whole, all parents felt that receiving a reminder on days 4, 7, 14 and 28 would be helpful because they would be unlikely to keep track of days or they would be likely to forget to complete the paperwork if their child was feeling better. They also felt that it was important that an interest was shown in them and their child and that contact with clinical staff such as a research nurse would provide them with an avenue for asking questions about their child’s health.
Very few parents felt comfortable with telephone reminders as they felt they would be inconvenient, time-consuming and intrusive. Many preferred text message reminders over e-mails and only a few felt happy to relay study data (i.e. temperatures) in a text message. This was seen by many to be inconvenient and potentially falsely reassuring (i.e. it would need to be made clear that there would be no clinical advice or follow-up to and that texting their child’s temperature was purely for data collection purposes, not for monitoring their child’s health). For some, daily text messages for the first week would be too much and the overall consensus was to give parents the choice of how they wished to be contacted (i.e. by text message, e-mail or telephone) and how often they wanted to receive reminders.
Other issues around study diaries
Parents queried what would happen if a child was hospitalised, leading to no data entries, and what would happens if a child recovers and then has a relapse within the 28 days. They felt that parents needed to be informed from the outset they could still continue filling in the diary if they missed a section and that they should not to make up data retrospectively.
Most parents suggested having a telephone number/contact details for a health care professional they could reach in case they had concerns over their child. If this was not possible, parents suggested giving clear guidance on who, why and when to contact if their child deteriorated. This was considered particularly important as a safety net for younger and newer parents who might not have established the ‘baseline’ for when their child was ill.
Parents suggested having an informal, ‘about me’ free-text box either at the beginning or end of the diary so that they could describe what their child was usually like (i.e. establish a baseline).
Trial practicalities/issues
Consent procedure
Out of three scenarios for the consent procedure that we presented to parents (making a decision during one visit, coming back to the surgery later in the day after having thought about it or having a research nurse come for a home visit), the least favoured option was coming back to the surgery later in the day. Reasons included wanting to get the ill child home to rest and give them medication, being exposed to germs at the surgery, and practicalities around transport, home life and other children). Views were split between the two other options. For some a home visit would be the deciding factor in being able to take part as it would allow more time to decide/discuss with a partner and make participation as easy as possible. Others wanted to be able to decide during their first visit to the surgery to avoid the inconvenience of an additional meeting. In the latter scenario, parents found it hard to estimate how long they would need to make a decision but emphasised the importance of having time to ask questions and clarify any concerns.
However, every group suggested that the best way to facilitate informed consent with the appropriate time and circumstances was by informing parents of the trial in advance of the flu season and enabling them to pre-register to take part if (and very often when) their child became unwell. Prior knowledge of the trial would also help parents to contact their GP early on with their child’s ILI when under normal circumstances they would choose to wait and manage the illness at home. Parents strongly felt that this option would give them time to read the trial information, discuss it with a partner and make a decision in principle in advance. They suggested being given access to the patient information video at this point.
Young people said that they would not want to wait in the surgery when ill for any longer than necessary. However, several expressed that they might feel a sense of pressure to agree to take part if a nurse came for a home visit. Preferences were split between these two options.
For young people the worst option was to come back to the surgery later in the day. Although young people said that their parents would ultimately make the decision to take part, they wanted to be heard and for it to be as much of a joint decision as possible. They felt the trial remit was such that a decision to take part would be easy and not seem daunting.
Placebo
Many parents expressed concerns about a placebo arm, despite being able to appreciate its necessity in the trial. The main concern was over the perception of taking a risk with their child’s health by entering a trial with a 50% chance of being given a placebo. Many already had access to early antibiotics through their GP or hospital and would not risk this for trial participation. It was also common that their child could become unwell very rapidly after their initial symptoms and parents felt that signing up for a trial might cost them these critical days while waiting to see if the study medication would help their child.
Parents suggested that more details of the placebo should be included in the PIL, with reassurance that explained how their child’s health would not be at risk in any way by enrolling (as from experience some felt that early antibiotics might be helpful).
Parents also recommended clearer and more detailed guidance over the safety-netting around deterioration, what exact symptoms to look out for, and who to contact and in what time frame. They felt this would be particularly important for newer parents. Many felt that it would be useful to have access to a telephone helpline with which to contact someone with an understanding of the trial, particularly in adverse situations.
The young person group’s concerns echoed the views by parents: they too wanted more reassurance in the PIL that their care and well-being would not be at risk at any point. They also pointed out that young people might place unhelpful connotations on the word ‘placebo’ (e.g. homeopathy) and felt that this could be more clearly explained in the child PILs.
Early antibiotics use
The aims of the trial would need to be explained carefully to parents, who are often told to avoid antibiotics. Parents were divided on their experience of accessing antibiotics; some had struggled in the past to get antibiotics prescribed for their children. Parents felt there was a risk of creating conflicting messages between warnings of the risks of antibiotic resistance and encouraging their early prescribing.
Swabs
Views were split on swabs. Some saw these as ‘an added bonus’ whereas others knew that they might not be able to participate in the trial because their child would refuse swabs. This was usually owing to either their disability (sensory sensitivities) or bad experiences and/or overexposure to swabs in the past.
The parent discussion groups suggested possible ways around this, including involving the parents in the process as much as possible (e.g. demonstrating to parents how to take the swabs and then asking them to take the swab from the child). They explained that their child often received injections or medications from a parent and would allow a medical intervention from their parent that they would not from a doctor. Parents also suggested sending a pack with swabs in advance to interested/pre-registered parents so that the child could familiarise themselves with the equipment and the process. If this was not possible, they suggested using parents or the Archie penguin as a medium to demonstrate the process of taking the swabs to make the child feel at ease.
Overall comments
Trial incentives
Parents suggested incentives, particularly for the children taking part. These included Archie penguin stickers, a sticker book, a key ring, a cuddly toy and certificates of participation. Colouring pages, puzzles for older children and a participation certificate were introduced and examples can be viewed in Report Supplementary Material 2.
Updates on trial progress and findings
Being kept up to date on the trial was considered very important. Parents wanted to be updated with the progress of the trial as well as its findings. The best format for this was considered to be a website or a newsletter (concerns were expressed over unmonitored social media platforms). This was felt to contribute to parents’ sense of worth and purpose for taking part.
Discussion and conclusions
The contribution of parents and young people to the design of work packages C, D and E provided invaluable feedback, ensuring the trial was more accessible to families. Their comments led to the adoption of the EQ-5D-Y questionnaire over the CHU9D and the use of weekly diaries rather than one 28-day diary. Contributors also supported the adoption of a home visit model to facilitate recruitment, although this was not feasible to introduce into ARCHIE until the 2016–7 season. It was not possible to introduce every suggestion; for example, a clinical contact number for trial participants was not appropriate because the trial team could not assume responsibility for clinical care and software to enable online diary completion could not be sourced in time for study commencement.
Reflections
Our PPI discussion groups were extremely informative and highlighted points and suggestions that we as a research team would not otherwise have considered and greatly facilitated the delivery of our study. These included advice about the trial mascot (which enabled us to avoid a character that some might have viewed as offensive), publicity materials and the use of weekly diaries instead of one 28-day diary. The latter most likely resulted in us being able to collect more diary data during the first week after children entered the study (the most relevant period for ILI episodes) than would have otherwise been possible had we relied upon participants to complete and return one 28-day diary. On reflection, our discussion groups may have been more productive if we had made clearer from the outset what the research team was in a position to provide, as some suggestions were beyond the scope of the resources available to us. We might also have gained additional benefit from holding some further discussion groups later in the programme to help us identify potential strategies for supporting recruitment and retention during the trial. Although we identified two patient representatives to join our Programme Steering Committee (one of whom provided us with very helpful advice during the entire course of the trial), a wider range of perspectives from both parents and young people may have been helpful in providing us with more options to consider.
Discussion and conclusions
Key findings
In this programme of research, we identified risk factors for influenza-related clinical deterioration in children, explored GPs’ decision-making around whether or not to prescribe antibiotics to at-risk children presenting with ILI in primary care and identified issues that are important to parents and guardians of children at risk of clinical deterioration. We performed a clinical and economic evaluation of early co-amoxiclav treatment in at-risk children presenting with ILI in primary or ambulatory care settings, and explored the potential implications of early co-amoxiclav treatment on respiratory bacterial carriage and antibiotic resistance.
Our systematic review and meta-analysis included data from a total of 28 articles reporting data from 27 studies. We identified premature birth as a new risk factor for influenza-related complications in children. Other strong risk factors were neurological disorders, sickle cell disease, diabetes mellitus and an age of <2 years.
To help us gain a broader perspective on how clinicians assess risk, we also conducted a qualitative interview study to explore the factors that influence how GPs make decisions about whether to prescribe antibiotics to at-risk children presenting in primary care with ILI. Semi-structured interviews based on a case vignette devised by the research team were conducted with 41 GPs practising in the UK. The lack of relevant evidence-based clinical practice guidelines was reflected in the wide variation we observed in GPs’ opinions on the potential significance of underlying co-morbidities as well as in their approach to making clinical decisions based on complex global assessments. Additionally we conducted in depth qualitative interviews with parents and guardians of at-risk children. Through these interviews we learned that parents and guardians are not always prepared for their child’s deterioration during an influenza like illness and that parents and guardians on the whole hold positive attitudes to antibiotic use but are simultaneously aware of the need for responsible and targeted antibiotic use.
Some text in the following paragraph has been adapted with permission from Wang et al. 40 The text below includes minor additions and formatting changes to the original text.
To determine whether or not early co-amoxiclav treatment is effective at reducing the risk of reconsultation owing to clinical deterioration in at-risk children presenting with ILI in community-based health-care settings, we conducted a double-blind randomised placebo-controlled trial comparing a 5-day course of co-amoxiclav 400/57 with an appearance-matched placebo. Our trial recruited 271 children. Primary outcome data were available for 265 children. There was no evidence of a statistically significant difference in the proportions of children who reconsulted owing to clinical deterioration between the co-amoxiclav and placebo groups. There was also no evidence of a difference in the proportions of children in whom adverse events were reported between groups.
Our within-trial economic evaluation did not find evidence that early co-amoxiclav treatment improves quality of life or reduces health-care use and costs in at-risk children with ILI. Our nested microbiology evaluation also found that early co-amoxiclav treatment did not have any adverse impact on respiratory bacterial carriage over the 12-month period of observation, with no emergence of resistance. The most common bacterial isolate found was H. influenzae. We found no difference in antibiotic susceptibility in the H. influenzae isolates between the co-amoxiclav and placebo groups.
In the subgroup of children with laboratory-confirmed influenza, fewer children on the co-amoxiclav arm reconsulted with clinical deterioration and a statistically non-significant trend towards lower non-medication costs and total costs for participants randomised to co-amoxiclav than those randomised to placebo was observed. This subgroup should be regarded as exploratory only owing to the very small number of children with laboratory-confirmed influenza (37/271).
Strengths and limitations
To ensure that our definition of ‘at-risk’ groups was as comprehensive and evidence based as possible, our systematic review and meta-analysis analysed both published data and data provided by authors whose published study methods suggested that they had collected but not published the data that we required for our analysis. This strategy more than doubled the number of studies from which we were able to obtain data. Twelve studies published sufficient data for us to analyse in their full-text manuscripts. In addition, we managed to obtain data from a further 18 author groups whose studies had collected but not published relevant data.
For some potential risk factors examined in our systematic review and meta-analysis, we were unable to draw firm conclusions owing to either a high degree of heterogeneity between different studies or a low number of hospital admissions among children with particular conditions. Although we had access to individual participant data for some studies, which allowed us to adjust some analyses for age and the presence of multiple conditions, data were not available for us to adjust for other potential prognostic factors such as disease severity and vaccination status. Data were also mainly obtained from studies conducted in hospital ambulatory care and emergency department settings. This may limit the generalisability of our findings to primary care.
Our qualitative GP interview study recruited a diverse sample of GPs representing a range of experiences and practice characteristics. We also simulated the way in which GPs gather information during a consultation by making different parts of the case vignette available in stages and adapting the way information was given to the GP according to what they asked for. Although we did not interview practice nurses (who sometimes assess minor illness in children), the GPs who took part in our study felt that nurses would be unlikely to assess children with ILI if they were known to have underlying comorbidities. Our qualitative and focus group work with parents and guardians allowed this key stakeholder group to share their views and experiences, which were central to developing our online resource on ‘Flu or flu-like illness in chronically ill or disabled children’ on the healthtalk.org website. 38
The findings of our randomised controlled trial are highly generalisable to community-based health-care settings during seasonal influenza periods owing to our wide geographical coverage, recruitment from primary and other ambulatory care settings, pragmatic ILI case definition, and high retention rate for our primary outcome. Our main limitation was being able to recruit only 271 participants versus our original target sample size of 650 participants. This limited the statistical power of our trial and prevented us from being able to develop and validate our proposed risk scores. Nevertheless, the sample size we recruited was still sufficient to detect a reduction in the primary outcome from 21% (percentage observed in the placebo group) to 6.5% [absolute risk reduction (ARR) 14.5%] with 90% power or 21% to 8% (ARR 13%) with 80% power and 5% two-tailed alpha error. These ARRs are similar to the treatment effect we considered for our target sample size (40% to 26%, 14% ARR), albeit from a lower baseline. A larger sample would have allowed us to estimate our result with greater precision and detect a more conservative treatment effect. However, we would need to consider the clinical importance of a smaller effect size in the context of numbers needed to treat for benefit versus harm.
Our trial results may also have been influenced by the low proportion of participants with laboratory-confirmed influenza and bacterial isolates. Only 37/271 children had evidence of laboratory-confirmed influenza (14%). This meant that, although our subgroup analysis in children with laboratory-confirmed influenza found that a lower proportion of children in the co-amoxiclav group reconsulted owing to clinical deterioration than in the placebo group, this subgroup did not have sufficient statistical power to demonstrate whether or not this difference was statistically significant. Only 68/271 children had evidence of one or more bacterial isolates (25%). This had implications for our nested microbiology evaluation, which was also affected by around two-thirds of participants being lost to follow-up after 12 months.
We also observed considerable loss-to-follow-up rates for data collected from study diaries despite extensive consultation with patient representatives on how to maximise response rates. These consultations led to us to issue four 1-week study diaries (instead of a single study diary covering the whole 4-week period after study entry) and incorporate telephone calls to all parents and guardians after weeks 1 and 2 to remind them to return their children’s study diaries. However, even with these measures, EQ-5D-Y data were only available for around one-quarter of participants at day 28. These data were not sufficient to conduct a full cost-effectiveness analysis.
Implications for clinical practice
Our systematic review identified premature birth as a new risk factor for complications from influenza or ILI, particularly in children <2 years of age. This risk factor is not included in existing guidelines on risk factors from the UK Department of Health and Social Care,53 the US Advisory Committee on Immunisation Practices (ACIP)54 or the World Health Organization,55 which are not specific to paediatric populations. We also found no evidence that certain conditions identified as being risk factors in adults, including obesity and cardiac, respiratory and renal conditions, were also risk factors in children. This may have been because these conditions are associated with less advanced disease in children than in adults and highlights the importance of clinicians being aware of which risk factors are relevant to children specifically.
Although previously published trial findings have reported evidence of clinical benefit from antibiotic treatment in patients with ILI during an influenza epidemic, the findings of our randomised controlled trial do not support immediate antibiotic use in at-risk children presenting with ILI in primary or ambulatory care settings during seasonal influenza periods. Although our programme of research did not find evidence of early co-amoxiclav treatment being associated with any safety concerns or changes in respiratory bacterial carriage or antibiotic resistance, we did not find evidence of clinical or economic benefit in our study population either.
In the absence of further evidence to underpin quantitative risk assessments in at-risk children with ILI, the global assessments already implemented by GPs are still valuable and may also inform other aspects of clinical management such as follow-up and safety-netting. Clinicians may also consider using point-of-care testing for respiratory virus and bacterial infections in settings where this is available to guide antibiotic prescribing decisions, particularly in cases where at-risk children present with more than a simple cold but no evidence of an established bacterial infection that would require immediate antibiotics.
Implications for future research
Future research should aim to carry out the following:
-
Examine risk factors for complications of influenza or ILI in children presenting in primary care rather than in hospital-based ambulatory care settings.
-
Examine premature birth as a risk factor for complications of influenza or ILI in children in more detail, including quantifying risk in children of different ages and born after different gestational periods.
-
Develop more comprehensive risk assessment tools that allow health-care professionals to provide more personalised estimates of risk, including risks associated with different types of underlying condition, multiple comorbidities and other relevant prognostic factors.
-
Develop simple, clear risk assessment tools to inform parental decisions about whether or not to seek medical advice for a child with ILI when that child is known to have one or more underlying potential risk factors.
-
Determine whether or not strategies to identify children who are more likely to have laboratory-confirmed influenza might help support more clinically effective and cost-effective antibiotic use in at-risk children. Such strategies may include consideration of local surveillance data or use of point-of-care testing for influenza and other potentially pathogenic respiratory tract infections.
-
Identify efficient strategies for opportunistically recruiting a larger sample of patients with influenza that can be rapidly mobilised and implemented during a future influenza epidemic or pandemic.
Acknowledgements
We would like to thank our Programme Oversight Committee members, (Professor Willie Hamilton, Professor Judy Breuer, Professor Maureen Baker and Robert Newby) and ARCHIE Trial Data and Safety Monitoring Committee members (Professor Lorcan McGarvey, Mike Bradburn, Professor Surinder Birring and Professor Nick Francis) for their contribution and advice.
We would also like to thank Professor Sue Mallett and Professor Sue Ziebland for the oversight of the conduct, analysis and outcomes of work package A and work package B, respectively.
We would like to thank the ARCHIE study investigators for their support with the conduct of the clinical trial: Professor Michael Moore, Professor Alastair D Hay, Tricia Carver, Professor Ly-Mee Yu, Professor Paul Little, Professor Andrew Farmer and Professor Christopher Butler. In addition, we thank Professor Butler for acting as programme chief investigator during Professor Anthony Harnden’s 2014–5 sabbatical. We would also like to thank our regional trial co-ordinators, Lyn Liddiard, Tammy Thomas, Clare McIntyre, and staff at the Nuffield Department of Primary Care Health Sciences Clinical Trials Unit, David Judge (Programmer), Jenna Grabey (Data Manager), Sonya Beecher (Trial Co-ordinator), Dr Jill Mollison (Statistics) and Ushma Galal (Statistics).
The research team acknowledges the support of the National Institute for Health and Care Research (NIHR) Clinical Research Network (CRN). We thank Mandy Wan (Paediatric Research Pharmacist, NIHR CRN Children specialty) for her support and guidance regarding trial medication, from procurement to disposal, and Dan Doran-Richards for contributing to the development of our study website, communications strategy and other study-related publicity materials.
We also thank Dr Helen Ashdown for her contribution towards the systematic review of work package A and for conducting the GP interviews in work package B. We thank Dr Oliver van Hecke for his assistance with monitoring the reconsultation data extracted from medical records in work package C and for his contribution to work package D via his outputs on issues surrounding antibiotic resistance. We thank Dr Ines Rombach for her contribution to the analysis and reporting of work package E1. In addition, we would like to thank Dr Ulla Raisanen for kind permission to reproduce Figure 4.
Finally, we thank all the health-care professionals, general practice and hospital staff, children and parents/guardians who participated in the study across all work packages.
Contributions of authors
Dr Kay Wang (https://orcid.org/0000-0002-7195-1730) (NIHR Postdoctoral Fellow, Primary Care) acted as Oxford programme lead and lead for work package C (clinical trial); she contributed to all other work packages and aided in the preparation of the final report.
Dr Sharon Tonner (https://orcid.org/0000-0002-7775-9926) (Infectious Disease Clinical Trial Manager) acted as clinical trial manager from 2017 and as programme manager from 2018 until the end of the programme; she wrote all sections with the exception of Synopsis Work Package C, Synopsis Work Package D. Synopsis Work Package E1 and Synopsis Work Package E2 which were written by the respective work package lead.
Professor Malcolm G Semple (https://orcid.org/0000-0001-9700-0418) (Professor of Child Health and Outbreak Medicine) acted as work package D (microbiology) lead and oversaw the conduct, analysis and reporting of this work package for the final report.
Dr Jane Wolstenholme (https://orcid.org/0000-0001-7493-1850) (Associate Professor of Health Economics) acted as work package E2 (health economics) lead and oversaw the conduct, analysis and reporting of this work package for the final report.
Professor Rafael Perera (https://orcid.org/0000-0001-9700-0418) (Professor of Medical Statistics) acted as work package E1 (risk reduction scores) lead and oversaw the conduct, analysis and reporting of this work package for the final report.
Professor Anthony Harnden (https://orcid.org/0000-0003-0013-9611) (Professor of Primary Care) acted as programme chief investigator and oversaw the conduct and reporting for all work packages and preparation of the final report.
Publications
Journal articles
Gill PJ, Ashdown HF, Wang K, Heneghan C, Roberts NW, Harnden A, Mallett S. Identification of children at risk of influenza-related complications in primary and ambulatory care: a systematic review and meta-analysis. Lancet Respir Med 2015;3:139–49. https://doi.org/10.1016/S2213-2600(14)70252-8
Ashdown HF, Räisänen U, Wang K, Ziebland S, Harnden A, ARCHIE investigators. Prescribing antibiotics to at-risk children with influenza-like illness in primary care: qualitative study. BMJ Open 2016;6: e011497. http://doi.org/10.1136/bmjopen-2016-011497
van Hecke O, Wang K, Lee JJ, Roberts NW, Butler CC. Implications of antibiotic resistance for patient’s recovery from common infections in the community: a systematic review and meta-analysis. Clin Infect Dis 2017;65:371–82. https://doi.org/10.1093/cid/cix233
Wang K, Carver T, Tonner S, Semple MG, Hay AD, Moore M, et al. Early use of Antibiotics for at-risk children with InfluEnza (ARCHIE): protocol for a double-blind, randomised, placebo-controlled trial. BMJ Open 2018;8:e021144. http://doi.org/10.1136/bmjopen-2017-021144
Wolstenholme JL, Bargo D, Wang K, Harnden A, Räisänen U, Abel L. Preference based measures to obtain health state utility values for use in economic evaluations with child-based populations: a review and UK-based focus group assessment of patient and parent choices. Qual Life Res 2018;27:1769–80. https://doi.org/10.1007/s11136-018-1831-6
van Hecke O, Butler CC, Wang K, Tonkin-Crine S. Parents’ perceptions of antibiotic use and antibiotic resistance (PAUSE): a qualitative interview study. Antimicrob Chemother 2019;74:1741–7. https://doi.org/10.1093/jac/dkz091
van Hecke O, Fuller A, Bankhead C, Jenkins-Jones S, Francis N, Moore M, et al. Antibiotic exposure and ‘response failure’ for subsequent respiratory tract infections: an observational cohort study for UK preschool children in primary care. Br J Gen Pract 2019;69:e638–e646. https://doi.org/10.3399/bjgp19X705089
Wang K, Semple MG, Moore M, Hay AD, Tonner S, Galal U, et al. Early use of Antibiotics for at-risk children with InfluEnza (ARCHIE): a double-blind, randomised, placebo-controlled trial. Eur Respir J 2021;58:2002819. https://doi.org/10.1183/13993003.02819-2020
Rombach I, Wang K, Tonner S, Grabey J, Harnden A, Wolstenholme J, for the ARCHIE Collaborators Group. Quality of life, healthcare use and costs in at-risk children after early antibiotic treatment versus placebo for influenza-like illness: within-trial descriptive economic analyses of the ARCHIE randomised controlled trial. BMJ Open 2022 12:e049373. http://doi.org/10.1136/bmjopen-2021-049373
Other outputs
We interviewed the parents of children with a long-term health condition or disability who had previously become unwell owing to influenza/ILI. Parents’ beliefs regarding the relative necessity of antibiotics versus concerns that antibiotics may cause harm to their child was explored as a key theme. Interviews were compiled and publicly released, with consent, as a website segment: http://healthtalk.org/peoples-experiences/pregnancy-children/flu-or-flu-illness-chronically-ill-or-disabled-children/topics
van Hecke O, Wang K, Lee J, Roberts N, Butler C, Nuffield Department of Primary Care Health Sciences, University of Oxford. The Clinical Relevance of Antibiotic Resistance in Patients Presenting with Common Infections in Primary Care: A Systematic Review and Meta-analysis. Antimicrobial Resistance: Implications for the Future of Medicine, Richard Doll Society and Oxford Global Health Group meeting, Oxford, UK, 31 October 2015.
van Hecke O, Wang K, Lee J, Roberts N, Butler C, Nuffield Department of Primary Care Health Sciences, University of Oxford. The Clinical Relevance of Antibiotic Resistance in Patients Presenting with Common Infections in Primary Care: A Systematic Review and Meta-analysis. South West Meeting of the Society for the Academic Primary Care (SW SAPC) Cardiff, UK, 1–2 March 2016.
Ashdown H, Prescribing Antibiotics to “At-risk” Children with Influenza-like Illness in Primary Care: Qualitative Study. Paper presented at the North American Primary Care Research Group (NAPCRG) International Conference, Colorado Springs, CO, USA, November 2016.
van Hecke O, Association Between Antibiotic Exposure and Antibiotic “Response Failure” in Preschool Children with Acute Respiratory Tract Infections: An Observational Cohort Study. Paper presented at the General Practice Research on Infections Network Meeting, Oslo, Norway, 29–30 September 2016.
van Hecke O, The Implications of Antibiotic Resistance for Patients’ Recovery from Common Infections in the Community: A Systematic Review and Meta-analysis. Paper presented at the North American Primary Care Research Group (NAPCRG) International Conference, Colorado Springs, CO, USA, November 2016.
Van Hecke O, Butler C. It’s False to Believe That Antibiotic Resistance Is Only a Problem in Hospitals – GP Surgeries Are Seeing It Too. URL: https://theconversation.com/its-false-to-believe-that-antibiotic-resistance-is-only-a-problem-in-hospitals-gp-surgeries-are-seeing-it-too-73414 (accessed 24 October 2022).
Van Hecke O, Butler C. It’s false to believe that antibiotic resistance is only a problem in hospitals – GP surgeries are seeing it too. The Independent, 24 April 2017. URL: www.independent.co.uk/news/health/it-s-false-to-believe-that-antibiotic-resistance-is-only-a-problem-in-hospitals-gp-surgeries-are-a7695481.html (accessed 24 October 2022).
Data-sharing statement
The trial protocol, trial statistical analysis plan and microbiology statistical analysis plan, and de-identified participant-level data collected for the trial are available on request. Research data requests should be submitted to information.guardian@phc.ox.ac.uk for consideration by the research team.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
Appendix 1 Management arrangements
We conducted our research between January 2013 and October 2019 as part of a collaboration between the University of Oxford, University of Liverpool, University of Bristol and University of Southampton.
The University of Oxford led the research and Professor Anthony Harnden was the programme chief investigator.
Work package A led by Professor Susan Mallett, Professor of Diagnostic and Medical Statistics, University College London (formerly of the University of Oxford). This work package was written up by Dr Sharon Tonner and reviewed by Dr Kay Wang and Professor Harnden.
Work package B was led by Professor Sue Ziebland, Professor or Medical Sociology, University of Oxford. This work package was written up by Dr Sharon Tonner and reviewed by Dr Kay Wang and Professor Harnden.
Work package C was led by Dr Kay Wang, NIHR Postdoctoral Fellow, University of Oxford. The Nuffield Department of Primary Care Sciences Clinical Trial Unit (PC-CTU) managed the randomised controlled trial. The University of Liverpool (principal investigator Professor Malcolm G Semple), University of Bristol (principal investigator Professor Alastair D Hay) and University of Southampton (principal investigator Professor Mike Moore) acted as co-ordinating centres.
Work package D was led by Professor Malcolm G Semple, Professor in Child Health and Outbreak Medicine, University of Liverpool, with support from Alder Hey Children’s Hospital NHS Foundation Trust.
Work package E1 was led by Professor Rafael Perera, Professor of Medical Statistics, University of Oxford. Work package E2 was led by Dr Jane Wolstenholme, Senior Health Economist, University of Oxford.
A Programme Steering Committee led by an independent chair, Professor Willie Hamilton, University of Exeter, provided oversight on all work packages.
Mrs Tricia Carver acted as Programme Manager from 2013–8 and Dr Sharon Tonner acted as Programme Manager from 2018–20
Appendix 2 Work package D summary report
Introduction
Prescribing a beta-lactam antibiotic for acute RTIs in a general population of children is reported to transiently double the prevalence of antibiotic resistance in H. influenzae and S. pneumoniae. Prevalence of antibiotic resistance is observed to return to pre-treatment baseline levels within 12 weeks and 6 months for H. influenzae and S. pneumoniae, respectively. 56,57
The study population in ARCHIE was children considered at risk of severe influenza virus infection or complications of influenza virus infection. At-risk children receive more courses of antibiotics than otherwise healthy children, so may carry a greater proportion of resistant species in their respiratory tract flora. In addition, while developing the study proposal, our PPI work found that parents of at-risk children had concerns about whether or not commonly prescribed antibiotics would still be effective in treating future infections if their child was prescribed antibiotics early during an episode of influenza or ILI.
Antibiotic resistance is recognised as a threat to treating serious bacterial infections. The National Institute for Health and Care Excellence has issued guidance on antibiotic stewardship to reduce inappropriate prescribing. 58 Should antibiotics be found to be effective in reducing reattendance for at-risk children with ILI, then evidence is needed that describes the likelihood of acquisition of antibiotic resistance and its duration. This would inform the benefit versus harm discussion for future national guidance.
Work package D of the ARCHIE programme was a nested microbiology substudy, designed to investigate any potential impact of an additional course of antibiotics given early during an ILI episode on the carriage of important pathogenic bacteria and levels of antibiotic resistance over time.
Methods
Objective
We aimed to compare the group prevalence and antibiotic resistance of S. pneumoniae and H. influenzae at 12 months among children in the co-amoxiclav and placebo arms enrolled in ARCHIE. Data on swabs at 3 and 6 months was used to help impute information on children without a 12-month swab.
Design
The substudy was a nested longitudinal cohort study, where children had throat swabs collected at recruitment and then at 3, 6 and 12 months after recruitment. Swabs were examined for bacterial growth, and analysed by species identified and resistance to common anti-microbial agents.
Subjects gave consent for their primary care records to be reviewed and data pertinent to antimicrobial prescribing in the 3 months prior to enrolment and during the study were collated.
Sample Size
We estimated (a priori) that a sample of 210 children would be sufficient to detect a 20% increase in the proportion of ampicillin-resistant S. pneumoniae and Haemophilus spp. (i.e. from 30% of samples to 50% of samples) in the co-amoxiclav group versus the placebo group with 80% power and 5% alpha error. We planned to gain consent for 360 children to allow for a loss to follow-up at 12 months of approximately 40%.
Clinical sampling
Flocked throat (pharyngeal) swabs were collected by research nurses using a technique standardised by training and supplemented by a video of sampling from a real patient. 59 Swabs were immediately placed into a 1 ml Amies liquid transport medium and posted as first-class mail to a single NHS laboratory.
Microbiological method
Swabs were ‘plated’ by application of 1 μl and 10 μl loops of transport media on blood and chocolate agar bacterial growth media both with and without ampicillin (selective growth media). Colonies were counted by eye. Drug resistance was screened by radial diffusion inhibition using MAST™ (Mast Group Ltd. Mast House Derby Road Bootle Merseyside L20 1EA United Kingdom) disks and minimum inhibitory concentration quantified by MICE™ strips (Oxoid Altrincham, Cheshire, WA14 2DT) as per the British Society for Antimicrobial Chemotherapy Standards for Microbiological Investigations (ISO 9001:2008). 60
Statistical analysis
A statistical analysis plan for the microbiology data was agreed prior to the unblinding of the swab data. Analysis was largely descriptive, but data were available to apply a chi-squared test to H. influenzae results. 61
Swabs were reported by bacterial species cultured. Where more than one isolate of the same species was cultured, only the isolate that had evidence of resistance was included in the analysis. Where more than one bacterial species was cultured, these were analysed separately. This analysis is consistent with standard microbiological reporting by NHS microbiology laboratories.
Results
Throat swabs from 225 children were available at baseline, representing 83% of 271 children randomised in the ARCHIE trial. Throats swabs from 93 children were available at 12 months, representing 34% of children enrolled and reflecting a follow-up attrition rate of 66% over the year (see Table 4).
| Swabs obtained | Co-amoxiclav group (N = 136), n (%) | Placebo group (N = 135), n (%) |
|---|---|---|
| Swab taken at baseline | 114 (84) | 111 (82) |
| Consented to swabs at 3, 6 and 12 months | 103 (76) | 98 (73) |
| Swab taken at 3 months | 55 (40) | 45 (33) |
| Swab taken at 6 months | 47 (35) | 45 (33) |
| Swab taken at 12 months | 47 (35) | 46 (34) |
The baseline demographic characteristics of children in the two groups are provided in Report Supplementary Material 3. There were no significant differences in age or sex between groups.
The prevalence of bacterial species of potential pathogenic significance is described in Table 5. The pathogens of particular interest in this study with potential for selection for resistance were H. influenzae and S. pneumoniae.
| Bacterial isolates | Co-amoxiclav group (n) | Placebo group (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (N = 114) | 3 months (N = 55) | 6 months (N = 47) | 12 months (N = 47) | Baseline (N = 111) | 3 months (N = 45) | 6 months (N = 45) | 12 months (N = 46) | |
| H. influenzae | 20 | 11 | 7 | 6 | 32 | 10 | 12 | 6 |
| S. pneumoniae | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. aureus | 4 | 1 | 2 | 0 | 1 | 1 | 0 | 0 |
| Group A Streptococcus | 3 | 3 | 0 | 0 | 3 | 6 | 1 | 1 |
| Group C Streptococcus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group G Streptococcus | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| MRSA | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Mixed flora | 87 | 40 | 38 | 41 | 74 | 30 | 32 | 39 |
| No growth | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
The overall prevalence of H. influenzae at baseline was 23% (52/225). Despite randomisation, the prevalence of bacterial species cultured from the throats of children at baseline differed significantly between the co-amoxiclav and placebo groups. By chance, the proportion of H. influenzae isolates was greater in the placebo group than the co-amoxiclav group at baseline (29% vs. 18%, respectively, χ2 = 4.03; p = 0.0447). However, there were no statistically significant differences in prevalence of this species at 3, 6 or 12 months between the two randomised arms.
The prevalence of S. pneumoniae was very low, being cultured from only one participant at baseline and at 3 months. This may be indicative of the success of the childhood pneumococcal vaccine programme.
The prevalence of Streptococcus sp. remained low throughout the period of observation. There was no emergence of methicillin-resistant S. aureus at any time in either group.
There was no clinically significant difference in bacterial load for H. influenzae, Pneumoniae or Streptococcus sp. measured as colony-forming units per ml between groups. The prevalence of mixed flora was similar across the randomised groups.
The prevalence of resistant isolates is described in Table 6. Owing to small numbers of isolates, there was no statistical testing. There did not appear to be a clinically significant difference in the prevalence of ampicillin-resistant isolates of H. influenzae between groups over time. There was insufficient data to provide evidence for a change in prevalence of ampicillin resistance in Streptococcus sp. or cefoxitin resistance in S. aureus throughout the period of observation. The counterfactual observation is that no emergence of resistant isolates at any time in either group.
| Antibiotic resistant isolates | Co-amoxiclav group, n/N (%) | Placebo group, n/N (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | Baseline | 3 months | 6 months | 12 months | |
| Haemophilus-selective agar containing ampicillin | 6/20 (30) | 5/11 (46) | 1/7 (14) | 1/6 (17) | 7/32 (22) | 3/10 (30) | 4/12 (33) | 1/6 (17) |
| Streptococcus-selective agar containing ampicillin | 0/4 (0) | 0/4 (0) | 0/1 (0) | – | 0/6 (0) | 0/6 (0) | 0/1 (0) | 0/1 (0) |
| Methicillin-susceptible S. aureus Cefoxitin Sensitive | 4/4 (100) | 1/1 (100) | 2/2 (100) | – | 1/2 (50) | 1/1 (100) | – | – |
| MRSA Cefoxitin Resistant | 0/4 (0) | 0/1 (0) | 0/2 (0) | – | 1/2 (50) | 0/1 (0) | – | – |
The minimum inhibitory concentrations of H. influenzae isolates against ampicillin, co-amoxiclav and cefotaxime did not change significantly over time in either group (data available on request).
Antibiotic prescriptions in the 3 months prior to enrolment and during the follow-up period are described for each group in Report Supplementary Material 3. In the 3 months prior to enrolment in ARCHIE, 19% (45/232) of children were prescribed an antibiotic. In the 12-month follow-up period only 6% (13/232) children received any antibiotics (χ2 = 20.18; p < 0.00001). This large effect is both clinically important and statistically significant.
The number of prescriptions for any penicillin-based antibiotic, including those combined with beta-lactamase, were similar between the co-amoxiclav (18.6%, 22/118) and placebo (15.8%, 18/114) groups in the 3 months prior to enrolment. In both groups there were notably fewer antibiotics prescriptions issued in the 12-month follow-up period (co-amoxiclav 1.7%, 2/118 and placebo 7%, 8/114) than in the 3 months prior to enrolment. There was no significant difference between groups in antibiotic prescriptions issued by GPs in the follow-up period (see Report Supplementary Material 3; χ2 = 2.80 with Yates correction; p = 0.094, not significant).
Limitations
Recruitment into the main trial was lower than originally estimated, which had an impact on recruitment into the nested microbiology substudy. Retention rates between baseline and 12 months were also lower than anticipated. Data with which to conduct a detailed statistical analysis were therefore limited.
Discussion
Previous well-conducted longitudinal studies found short-term increases, doubling the risk of isolation of H. influenzae with resistant features within 2 weeks, but this level of prevalence was transient and returned to baseline at 12 weeks. 56,57
Obtaining baseline throat swabs from children was feasible, with 83% of children providing a swab at baseline; however, the retention of 34% of participants over the study period was lower than the 60% predicted. This, combined with the reduced recruitment target, meant there were only 47 subjects in the co-amoxiclav arm and 46 subjects in the placebo arm at 12 months.
There were sufficient participants and a sufficient proportion of swabs with H. influenzae isolated to provide evidence of no change in the prevalence of this species over time. An important counterfactual observation was that there was no observed increase in resistant isolates of H. influenzae, S. pneumoniae, Streptococcus sp. or MRSA over time. Transient suppression of normal flora did not lead to the selection and persistence of resistant species.
There was no evidence of increase in bacterial load of potential pathogens over time.
A surprising observation was the substantial reduction in number of antibiotics prescriptions issued to ARCHIE participants during the 12-month follow-up period, suggesting a strong placebo effect from trial participation. This may reflect the confidence parents had in the regular safety monitoring provided by study participation or an increased awareness about the risk of antibiotic resistance.
Conclusion
We found no evidence that a single 5-day course of co-amoxiclav given early to children at-risk of complications when presenting with ILI increased the long-term prevalence or antibiotic susceptibility of H. influenzae, the most commonly isolated potential pathogen. Neither did this additional course of antibiotics lead to the emergence of other resistant species such as Streptococcus sp. or MRSA.
Appendix 3 Work package E1 summary report
Introduction
Decision-making surrounding the treatment of children presenting with influenza and ILI is often complex with competing factors requiring consideration. We aimed to use data gathered as part of the ARCHIE study to identify which subsets of at-risk children may benefit most from treatment with co-amoxiclav to ensure that antibiotic stocks are managed responsibly and that children who are most likely to benefit do not have treatment delayed.
Methods
Objective
We originally sought to develop four multivariable risk scores. These were to be based on different outcomes and covariates.
Model 1
The outcome for model 1 was reconsultation owing to clinical deterioration.
The covariates comprised age, type of comorbidity, household smoking status, PCV and influenza vaccination status, influenza activity in the community, and early co-amoxiclav treatment.
Model 2
The outcome for model 2 was complications resulting in clinical intervention.
The covariates comprised age, type of comorbidity, household smoking status, PCV and influenza vaccination status, influenza activity in the community, and early co-amoxiclav treatment.
Model 3
The outcome for model 3 was reconsultation owing to clinical deterioration.
The covariates comprised variables related to the acute illness episode: duration of illness, heart rate and respiratory rate. Age was also included.
Model 4
The outcome for model 4 was complications resulting in clinical intervention.
The covariates comprised variables related to the acute illness episode: duration of illness, heart rate and respiratory rate. Age was also included.
Restriction
We stipulated at the outset of our research programme that if <100 children received a clinical intervention, we would only calculate risk scores based on reconsultation owing to clinical deterioration.
Analysis
A total of 74 children received a clinical intervention and 61 reconsulted owing to clinical deterioration. This meant that only the models for the outcome of consultation owing to clinical deterioration (i.e. models 1 and 3) were created. There was also the issue of total number of events, which meant that only a small number of covariates could be included. Based on a post hoc analysis following Riley’s62 recommendation for the total number of covariates (parameters) that can be included in a model, we identified that for a model with just two covariates, a sample of nearly 7000 children would have been required. This suggests that the models presented below should be treated as exploratory only. See Report Supplementary Material 4 for details on these calculations.
As the outcome was binary, we used logistic regression for our models.
For each risk score, our intention was to form three risk subgroups (low, medium and high risk). When estimating the sample size for our randomised controlled trial, we estimated that around 40% of children in the placebo group would reconsult owing to clinical deterioration. Based on this, we pre-specified that medium risk would be defined as a reconsultation rate of 30–40%, low risk as <30% and high risk as >40%.
Our selection of risk factors were as follows:
-
age
-
type of co-morbidity (as there were several of these, they have been treated separately)
-
household smoking status
-
administration of the PCV
-
early co-amoxiclav treatment (randomisation group).
We considered data from each participant at the time of initial presentation on the following factors:
-
duration of illness
-
heart rate
-
respiratory rate.
We also collected data on risk factors for laboratory-confirmed influenza. These included the following:
-
administration of the current season’s influenza vaccines
-
administration of previous season’s influenza vaccines.
A total of 37 participants had laboratory-confirmed influenza.
A total of 271 participants were randomised and reconsultation owing to clinical deterioration (yes/no), data are available for 265 participants. Of these 265, 61 children reconsulted owing to clinical deterioration.
The number of outcome events we observed was not sufficient to create a model that included all these characteristics simultaneously, based on Altman’s rule of n/10 and square root rule. 63 We therefore looked at univariable associations. Only risk factors that had an association with a p-value <0.1 were included in a multivariable model. Age was included in all evaluations, including ‘univariable’ ones.
Outcome: The outcome was reconsultation due to clinical deterioration (n = 265; events = 61).
Included covariates comprised age, type of comorbidity (respiratory, neurological, cardiac, renal and immune deficiency), household smoking status, PCV status, early co-amoxiclav treatment (intervention), duration of illness, heart rate, respiratory rate, laboratory-confirmed influenza, receipt of current season’s influenza vaccination and receipt of previous season’s influenza vaccination.
| Baseline covariate | ORa | 95% CI | p-value |
|---|---|---|---|
| Respiratory condition | 0.88 | 0.46 to 1.69 | 0.706 |
| Neurological condition | 1.40 | 0.42 to 4.67 | 0.579 |
| Cardiac condition | 2.27 | 0.77 to 6.69 | 0.138 |
| Renal condition | 7.05 | 0.63 to 79.44 | 0.114 |
| One or more smokers in household | 0.44 | 0.18 to 1.11 | 0.082 |
| Received current season’s influenza vaccination | 0.84 | 0.45 to 1.60 | 0.601 |
| Received previous season’s influenza vaccination | 1.30 | 0.67 to 2.55 | 0.435 |
| Received PCV | 1.30 | 0.36 to 4.75 | 0.690 |
| Duration of illness | 0.91 | 0.71 to 1.17 | 0.478 |
| Heart rate | 1.00 | 0.98 to 1.01 | 0.773 |
| Respiratory rate | 1.05 | 1.01 to 1.08 | 0.012 |
| Laboratory-confirmed influenza | 1.62 | 0.74 to 3.56 | 0.228 |
| Recruited from hospital ambulatory care setting | 1.01 | 0.54 to 1.92 | 0.966 |
Table 7 summarises the results of our univariable analyses. None of the types of comorbidities appeared to be associated with reconsultation. The only two variables that appeared to show a link were household smoking status (p = 0.082) and respiratory rate (p = 0.01). The only multivariable model t constructed was based on these two variables. Table 8 summarises the results of this multivariable model.
Multivariable model (logistic): reconsultation ≈ age + smoking + respiratory rate
| Baseline covariate | OR (adjusted)a | 95% CI | p-value |
|---|---|---|---|
| Intercept | 0.063 | 0.015 to 0.257 | <0.001 |
| Age | 1.054 | 0.945 to 1.177 | 0.345 |
| One or more smokers in household (yes) | 0.382 | 0.150 to 0.972 | 0.043 |
| Respiratory rate | 1.052 | 1.014 to 1.091 | 0.006 |
The model has moderate to low discrimination and calibration, as shown in Figures 6 and 7. The results are unlikely to be of significant clinical importance. As mentioned above, given the limited sample, these models should all be interpreted as exploratory.
FIGURE 6.
Area under receiver operating characteristic (based on lroc in Stata).
FIGURE 7.
Calibration plot (based on lowess in Stata).
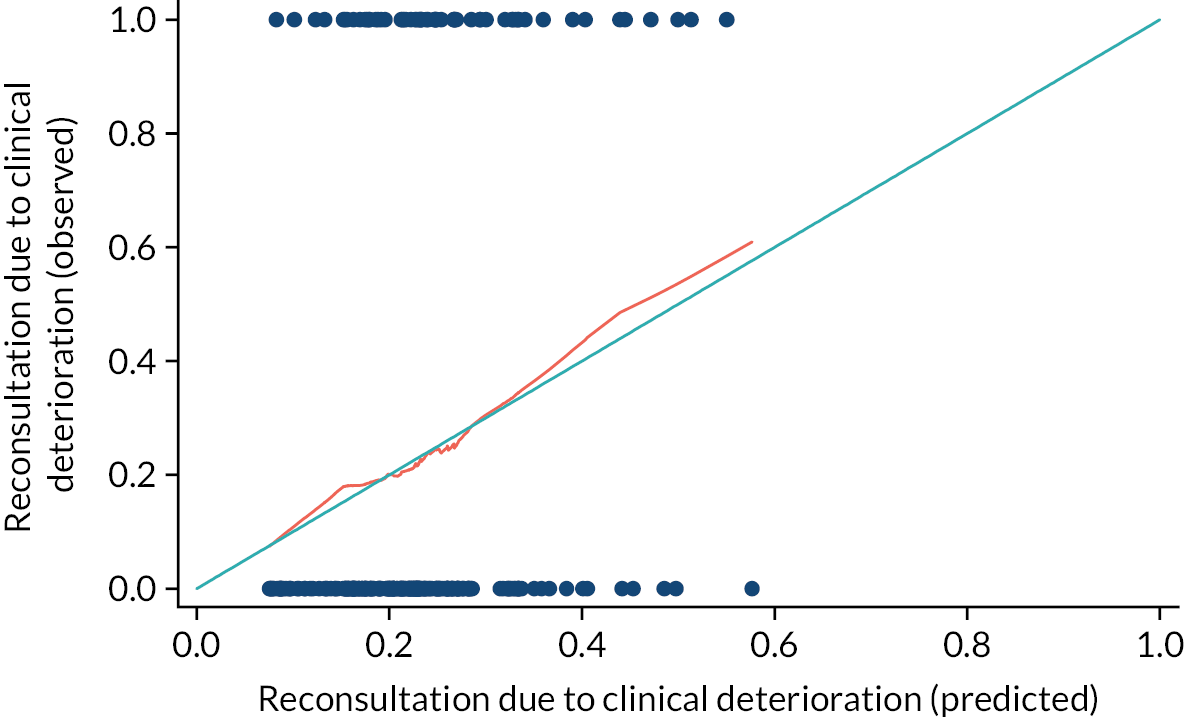
Limitations
The data available to construct multivariable models were severely limited by fewer recruits than originally projected (271 participants vs. the predicted 650). In addition, the event rate did not meet the threshold for enabling the calculation of all original proposed models and data were only available to calculate one limited multivariable model that should be considered exploratory only.
Conclusion
Based on the limited data available, no statistically significant associations with reconsultation owing to clinical deterioration were observed for the treatment arm (co-amoxiclav vs. placebo), a risk factor in the respiratory category (vs. other risk factors) or recruitment in secondary care versus primary care. The only multivariable model that could be constructed demonstrated an increase risk associated with higher respiratory rate and a decreased risk associated with a smoker in the household but these must be regarded as exploratory only. A far greater sample size would be required to produce accurate models.
- ARR
- absolute risk reduction
- CARIFS
- Canadian Acute Respiratory Infection and Flu Scale
- CHU9D
- Child Health Utility 9D
- CI
- confidence interval
- CRN
- Clinical Research Network
- EQ-5D-Y
- EuroQol-5 Dimensions, three-level youth version
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- ILI
- influenza-like illness
- IQR
- interquartile range
- LAIV
- live attenuated influenza vaccine
- MIC
- minimum inhibitory concentration
- MRSA
- methicillin-resistant Staphylococcus aureus
- NAI
- neuraminidase inhibitor
- NIHR
- National Institute for Health and Care Research
- OR
- odds ratio
- PCV
- pneumococcal conjugate vaccine
- PIL
- participant information leaflet
- PPI
- patient and public involvement
- RR
- risk ratio
- RTI
- respiratory tract infection
- SD
- standard deviation
- VE
- vaccine efficacy
Notes
-
Work package B interview schedules and GP regional demographics
-
Determining number of covariates (parameters) for prediction model in ARCHIE
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/WDFR7331
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
