Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-1209-10071. The contractual start date was in July 2012. The final report began editorial review in February 2021 and was accepted for publication in February 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Wyld et al. This work was produced by Wyld et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Wyld et al.
SYNOPSIS
Research summary
The original proposal has largely been adhered to in the programme grant, with a few minor variations.
Prospective cohort study
The study recruited 3450 (with a target of 3500) women aged ≥ 70 years, but recruitment was slower than expected and an extension was required from the original 5 years to 6 years. We also increased the number of centres from 22 to 56 to enhance recruitment. A further delay was caused by the UK Cancer Registry, where we planned to get our survival data updated before final analysis. Legal changes caused by the new General Data Protection Regulations1 meant that, although our study was legally compliant with the old legislation, it no longer complied with consent requirements in the new system. A lengthy negotiation process was required to permit access, which added 9 months to our timelines. In total, the study ran for 7 years, rather than the original estimate of 5 years.
Decision support intervention development
The development proceeded to time and target, resulting in the production of decision support interventions (DESIs) suitable to trial in the cluster-randomised controlled trial.
The cluster-randomised controlled trial
The trial started later than expected owing to a delay in receiving National Institute for Health and Care Research (NIHR) approval for phase 2 of the programme, and compounded by slower than anticipated recruitment. Therefore, an extension and an increase in centre numbers were required. However, we did ultimately recruit to target: 1330 out of the target 1328 women.
Health economic analysis
This was completed, but delayed by the registry data access issue noted in Prospective cohort study.
Variation study
This is completed and published.
Overall
The programme has met all of its targets, but was delayed by 24 months. All expected outputs have been published in scientific journals. The online tool has been Medicines and Healthcare products Regulatory Agency (MHRA) approved, is available on the web and is in regular clinical use.
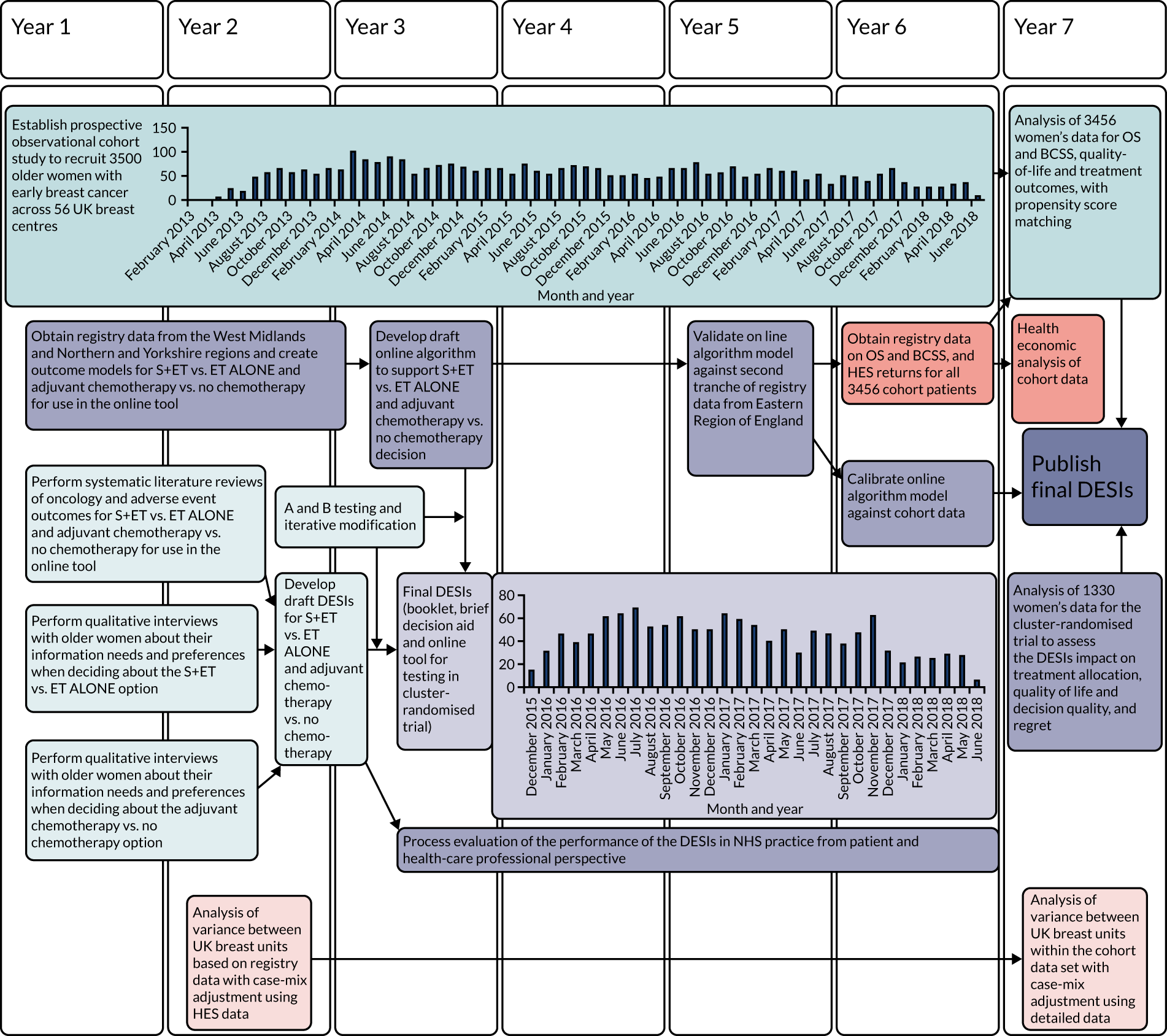
Research pathway diagram
The research pathway diagram is presented in Figure 1.
FIGURE 1.
Research pathway diagram: summary of the relational timelines for the Age Gap programme grant. BCSS, breast-cancer-specific survival; ET ALONE, primary endocrine therapy; HES, Hospital Episode Statistics; OS, overall survival; S+ET, surgery plus adjuvant endocrine therapy. Light-blue shading indicates development of the DESI. Mid-blue shading represents the cohort study. Light-purple shading indicates the cluster RCT. Mid-purple shading indicates development of the models. Light-orange shading indicates analysis of variance. Mid-orange shading indicates health economic analysis.
General background introduction
Breast cancer in older women
Breast cancer is the most common cancer to affect women, with over 55,000 women diagnosed annually,2 and one-third of cases being in women aged ≥ 70 years. The clinical significance of breast cancer is proportionately less in older women, as breast-cancer-specific mortality is overtaken by other-cause mortality once a woman is in her eighties. However, women aged > 80 years still have a higher risk of dying from breast cancer than women in their seventies, which may be because of suboptimal treatment. 3 It is equally important to avoid overtreatment and causing unnecessary harms in women who are very frail. 4 There are currently no tools available that specifically support decision-making in older women with early-stage breast cancer who are faced with a choice of primary endocrine therapy (ET ALONE) or surgery plus adjuvant endocrine therapy (S+ET). The Adjuvant! Online tool5 and the NHS PREDICT tool6 provide survival estimates only for women who have had surgery. This programme of research has developed, validated and trialled an online tool specifically for supporting decision-making in older women.
Surgical treatment of breast cancer in older women
Surgery may be unnecessary for some frailer older women, as disease control may be achieved using antioestrogens alone (ET ALONE). Up to 90% of breast cancers in older women are oestrogen sensitive7,8 and therefore respond well to antioestrogens, potentially allowing women to avoid surgery.
A systematic review of randomised controlled trials (RCTs) evaluating the role of S+ET versus ET ALONE demonstrated no survival difference between the two treatments, but inferior local control for ET ALONE compared with S+ET. 9–12 Longer-term follow-up and a patient-level meta-analysis have recently suggested that survival is superior with S+ET than with ET ALONE (Richard Gray, University of Oxford, 2019, personal communication). These trials were methodologically flawed, and it is therefore not justified to suggest that ET ALONE is not appropriate for any older women. Overtreatment with surgery for very frail older women may be harmful. A recent study of 6000 nursing home residents with breast cancer, all of whom were treated with surgery, reported high morbidity and mortality rates and high levels of long-term functional impairment, although the authors acknowledge that some of the deterioration and deaths may have occurred regardless of their breast cancer treatment. 4
Since these trials were performed in the 1980s, the practice of ET ALONE has changed and is now usually offered only to women with oestrogen-receptor-positive (ER+) cancers, and aromatase inhibitors have largely replaced tamoxifen. 13–16 ET ALONE may, therefore, be more efficacious if potential candidates are selected appropriately, based on health status and tumour biology. From a patient’s perspective, older women express high levels of satisfaction with both S+ET and ET ALONE. 17 Factors cited in favour of ET ALONE are avoiding hospitalisation and surgery, and a desire to retain independence. 17
The purpose of the Age Gap study was to gather data to guide treatment selection for both patients and clinicians to avoid women being overtreated or undertreated.
Adjuvant chemotherapy in older women
Adjuvant chemotherapy is usually advised for high-recurrence-risk breast cancer; however, in women aged ≥ 70 years, there is less certainty about the survival benefits of adjuvant chemotherapy owing to the lack of trial data and to toxicity concerns. 18 The Oxford overview of adjuvant chemotherapy demonstrated reduced mortality for women up to age 69 years,19,20 but there were insufficient data regarding women aged ≥ 70 years to draw firm conclusions. 20
As a result of this uncertainty, in the UK, the National Audit of Breast Cancer in Older Patients (NABCOP) found a two-thirds reduction in chemotherapy use in women aged ≥ 70 years with high-risk disease. 21 Avoidance of chemotherapy in some women may be justified by concerns about poor treatment tolerance; for others, treatment may be well tolerated and improve survival. Rates of mortality and morbidity associated with chemotherapy are higher in women aged ≥ 70 years, with higher rates of haematological toxicity, febrile neutropenia22 and early discontinuation. 23 There is wide variation in chemotherapy rates across the UK,24 which suggests that age- and health-stratified guidelines are required. Generating evidence to support guideline development was one of the aims of this study.
Clinician practice variation in the UK
There is wide variation in rates of non-surgical treatment of breast cancer in the UK, ranging from 5% to 50%,21 suggesting that some women may be overtreated and others undertreated, and highlighting the need for age- and health-stratified guidelines. A similar picture is also seen in the use of adjuvant chemotherapy, with wide variation in rates of use between UK breast units. 24
One of the aims of the Age Gap study was to assess the extent and causes of these variations.
Shared decision-making and decision support
Shared decision-making is joint decision-making by a patient and health-care professional(s) in which they are both experts: the patient on what matters most to them and the health-care professional on the clinical evidence. It can be particularly beneficial in the context of treatment decisions that are preference sensitive, such as those described above for older women with breast cancer. DESIs aim to support shared decision-making and improve the quality of treatment decisions. A systematic review of DESIs showed that they lead to several benefits, such as increased knowledge, greater participation in decision-making, greater comfort with decisions and more accurate risk perceptions. 25
One of the aims of the Age Gap study was to develop DESIs to support older women and their clinicians when engaging in shared decision-making regarding breast cancer treatment.
Aims and objectives
Aims and objectives
-
To provide guidance for the treatment of frailer older women with ER+ breast cancer to ensure optimal treatment with either S+ET or ET ALONE.
-
To provide guidance on the treatment of fitter older women with high-recurrence-risk breast cancer to ensure optimal use of adjuvant chemotherapy with or without trastuzumab.
-
To design, validate and evaluate (in a RCT) a DESI for older women faced with either of the above choices.
-
To determine the health economics of S+ET compared with ET ALONE in older women with ER+ early-stage breast cancer.
-
To determine the extent and causes of variation in UK practice relating to older women with breast cancer.
Work packages
-
Summation of evidence of current UK practice for older women with early-stage breast cancer.
-
Determination of the information needs and preferences of older women when deciding on their preferred breast cancer treatment using a mixed qualitative and quantitative methodology.
-
Development of DESIs, including an online tool (using the above models), to be evaluated in a randomised trial.
-
Development of outcome models in older women with breast cancer, comparing S+ET with ET ALONE, or adjuvant chemotherapy with no chemotherapy, using UK registry data.
-
Acquisition of data from a cohort study of older women with breast cancer to validate the treatment thresholds identified above.
-
Cluster-randomised trial of the DESIs’ impact on treatment allocation and outcomes for older women with breast cancer.
-
Health economic analysis comparing the cost efficacy of ET ALONE with that of S+ET in older women, based on a mixture of published data, registry data and cohort study data.
-
Study of the degree and causes of variation in treatment allocation between UK breast units using registry data, cohort study data and mixed-methods research.
Evidence summary of current UK practice in older women with early-stage breast cancer
Three systematic reviews were undertaken of the evidence relating to the oncological efficacy of and adverse events related to ET ALONE compared with S+ET, and adjuvant chemotherapy compared with no chemotherapy in older women. The three reviews are:
-
a systematic review and meta-analysis of randomised trials comparing ET ALONE and S+ET11
-
a systematic review of cohort studies comparing ET ALONE with S+ET10
-
a systematic review of the efficacy and side effects of chemotherapy for breast cancer in older women (not published, see Appendix 1).
The data from these reviews were used to develop an evidence summary on which the factual content of the decision support tools was based.
Defining the information needs and preferences of older women with breast cancer
The next stage in the development of the DESIs was to determine the information needs and preferences of older women. These DESIs focused on two key decisions:
-
whether to have S+ET or ET ALONE
-
following surgery, whether or not to have adjuvant chemotherapy.
A multicomponent mixed-methods study was undertaken to acquire this information, and design and refine the DESIs. The components of the study were:
-
a systematic review of literature on intervention efficacy and adverse events (see Evidence summary of current UK practice in older women with early-stage breast cancer)
-
qualitative interviews with patients regarding information needs and preferences (see Patient interview study to determine the preferred format and content for the surgery plus adjuvant endocrine therapy versus primary endocrine therapy decision support intervention and Patient interview study to determine preferred format and content for the adjuvant chemotherapy versus no chemotherapy decision)
-
a quantitative survey of patient information needs and preferences (see Questionnaire study to determine the preferred content for the surgery plus adjuvant endocrine therapy versus primary endocrine therapy decision support intervention)
-
draft decision support tools (see Development and testing of the decision support interventions)
-
DESI modification based on interviews and focus groups with healthy, age-appropriate volunteers and health-care professionals (see Development and testing of the decision support interventions)
-
DESI modification based on interviews with patients (see Development and testing of the decision support interventions)
-
DESI modification based on field testing with patients (see Development and testing of the decision support interventions)
-
language review by the Plain English Campaign (Stockport, UK; see Development and testing of the decision support interventions)
-
graphic and web design input
-
evaluation of final DESIs in a cluster-randomised controlled trial (see Cluster-randomised controlled trial).
These components are described below.
Patient interview study to determine the preferred format and content for the surgery plus adjuvant endocrine therapy versus primary endocrine therapy decision support intervention
Introduction
The aim of this study was to explore information needs using in-depth qualitative interviews and then to quantify preferences using a bespoke questionnaire. This work has been published. 26
Methods
Women aged > 75 years who were under follow-up after treatment for early-stage breast cancer and had been offered a choice of S+ET or ET ALONE at diagnosis were recruited for interview.
A topic guide was developed by the expert reference group and patient and public involvement (PPI) panel. Topics included sources of information used; information they were given or would prefer to aid their decision-making; their preferences about the process of decision-making; and their preferred format for information, that is booklets, verbal, digital versatile disc (DVD), audio, internet or mobile applications (apps). Interviews were digitally recorded and transcribed verbatim. A framework approach was used for analysis.
Results
Thirty-three older women were interviewed (median age 82 years, range 75–95 years) at a median of 20 months after diagnosis. Twenty-two women underwent ET ALONE and 11 underwent S+ET. The median interview duration was 50 minutes (range 23–85 minutes). There were three interview themes:
-
the decision-making process
-
satisfaction with the decision-making process
-
the impact of breast cancer.
The majority of women were pleased with their decision and the decision-making process. The majority preferred a shared decision-making model in which they had significant involvement, although a minority preferred a passive role. Women wanted the information to be jargon free, concise, personalised and in everyday language. For information delivery, they prefered face-to-face discussion, booklets and a question-and-answer format; they wished to avoid online material, ‘apps’ and DVDs. For information graphics, they preferred verbal descriptors, and to avoid percentages and frequencies.
Conclusion
The information needs of the majority of older women were focused on the practical implications of treatment and its impact on physical function and independence. They wanted information presented in a simple, jargon-free manner, ideally face to face. These preferences were taken into account when developing the DESIs, which would comprise a booklet; brief decision aid; and a personalised printout from an online tool, which would be used by the clinician, inserted in the booklet and given to the patient.
Questionnaire study to determine the preferred content for the surgery plus adjuvant endocrine therapy versus endocrine therapy alone decision support intervention
The above qualitative findings were quantified using a bespoke patient questionnaire used with a larger cohort. This study has been published. 27,28
Methods
A cohort of older women (aged > 75 years) were recruited across 10 UK sites. Eligibility criteria were identical to those in Patient interview study to determine the preferred format and content for the surgery plus adjuvant endocrine therapy versus primary endocrine therapy decision support intervention. Questionnaire face and content validity were based on the interviews (see Patient interview study to determine the preferred format and content for the surgery plus adjuvant endocrine therapy versus primary endocrine therapy decision support intervention), and included 30 items relating to preferred information content and nine items relating to preferred media and presentation. An integrated, previously validated, decision-making-preference questionnaire assessed whether older women wanted a passive, active or shared role. 29
Results
A total of 247 women were invited to take part, of whom 101 returned questionnaires (41% response rate). The median age was 82 years (range 75–99 years).
Desired treatment information included the safety and side-effects of surgery, post-operative pain and duration of hospitalisation, fitness for surgery ‘at their age’, impact on independence, availability of support and oncological efficacy.
The majority of women preferred face-to-face informaton provision from doctors (81%), with fewer preferring face-to-face provision from nurses (37%) or the use of a booklet or leaflet (33%). There was little interest in information online or via an app, DVD or video. Very few women had access to the internet (29%). In terms of preferred display of data, verbal descriptors were strongly preferred (e.g. ‘breast cancer is common’), followed by use of proportions (e.g. ‘1 in 8’). Pictograms, bar and pie charts were not highly rated, with < 10% preferring information in this format.
In terms of preferred decision-making style, 35 out of 93 (38%) women preferred the health-care professional to choose the treatment, 36 (39%) preferred to decide themselves and 22 (24%) preferred shared decision-making. 28
Conclusion
Older women expressed their preferences regarding the information they desired to support decision-making about the S+ET versus ET ALONE decision, and their preferred format of information. These data were used to develop a draft DESI, which was then field tested, as described in Development and testing of the decision support interventions.
Patient interview study to determine preferred format and content for the adjuvant chemotherapy versus no chemotherapy decision
Introduction
A similar method was used to derive the information needs and preferences of older women facing a decision about adjuvant chemotherapy after surgery. A series of qualitative interviews, not part of this programme, regarding the factors influencing older women’s choices had been performed30 and these transcripts were reanalysed to extract specific information relating to information needs and preferences.
Methods
The study was part of a larger multicentre observational study [Adjuvant chemotherapy in elderly women with breast cancer (ACHEW)] across 24 UK breast units. 30 Patients who had received adjuvant chemotherapy were recruited for interview. Interviews were based on a topic guide, including provision of information and participation in decision-making. Interview transcripts were re-read for the Age Gap study to focus on informational needs and preferences, which were then used in DESI development.
Results
A total of 58 out of 95 (61%) eligible women participated. The median age was 73 years (range 70–83 years). The majority (58.5%) of women preferred to make the decision in collaboration with a clinician, 22.6% preferred to delegate responsibility to a clinician and 18.9% wanted to make their own decision. Shared decision-making was the preferred choice in women accepting adjuvant chemotherapy (63.9%). The main reasons for accepting adjuvant chemotherapy were prevention of recurrence and clinician recommendation. Women were interested in the side effects of treatment, duration of treatment, route of administration, impact on quality of life (QoL), impact due to their age and comorbidities. Low survival benefits and clinician recommendations influenced decisions to decline adjuvant chemotherapy. Information sources were usually written, and those available were generally viewed positively (56%) and well read (96%). Many wished for personalised information, which was often lacking. There was little interest in information from the internet (17%).
These items were used to develop the DESIs.
Conclusion
The derived information was used to develop the Age Gap decision tool. The focus was on personalised, age-specific information, available in a printed format (a booklet and personalised printout from an online tool) and giving detailed practical information about adjuvant chemotherapy, its benefits and side effects.
Development and testing of the decision support interventions
Introduction
Data from the literature reviews, expert reference group, patient interviews and questionnaires were used to draft decision support tools for both the S+ET versus ET ALONE decision and the adjuvant chemotherapy or no chemotherapy decision. These were then tested with patients and clinicians for content, comprehensibility and accuracy, before being field tested with patients and clinicians. The resulting DESI were then finalised by English language review and graphic design input prior to being trialled in the cluster-randomised controlled trial.
This process has been published. 31
Usability testing
Following initial development, prototype DESIs (brief decision aid, booklets) were tested for usability, acceptability and utility using semistructured interviews. Preliminary testing was first conducted among healthy volunteers aged ≥ 70 years. This was followed by testing with patients who had made a decision regarding breast cancer treatment in the last 12 months, before finally testing the DESI with those currently facing the treatment decision. Iterative modifications were made between each phase.
Sample recruitment
Volunteers
Female volunteers were recruited from breast cancer charities and local community groups.
Patients
Patients were recruited via five UK breast units: Cardiff, Brighton, Doncaster, Sheffield and Southampton. Recruitment continued until data saturation.
Interviews and analysis
Semistructured interviews based around an interview topic guide regarding the DESIs were audio-recorded and transcribed verbatim. Interview topics included DESI content, layout, usefulness, comprehension and potential improvements. Transcripts were analysed using a framework approach.
Results
Sample characteristics
Primary endocrine therapy versus surgery plus adjuvant endocrine therapy decision support intervention
Twenty-two women were interviewed who were aged between 75 and 94 years (median 82.5 years).
Adjuvant chemotherapy decision support intervention
Interviews were completed with 14 women aged between 70 and 87 years (median 74 years).
Surgery plus adjuvant endocrine therapy versus primary endocrine therapy, and adjuvant chemotherapy decision support intervention content feedback
The content of the DESIs was viewed positively, with the right amount of clear, relevant information, which was easily understood. There were some alterations to the text and images to make it more easily understood, and other text items were emphasised further to reflect their importance based on feedback.
Decision support intervention use/implementation
The DESIs were generally thought to be helpful. The importance of discussion with health-care professionals was highlighted.
Discussion
Feedback from participants about the DESIs included many positive comments, but areas of confusion were noted and amendments were made.
Conclusion
Using an iterative process of feedback and improvements, the DESIs were found to be acceptable to and usable for patients. These DESIs were then trialled in the cluster-randomised trial (see Cluster-randomised controlled trial).
Developing outcome models for older patients with early-stage breast cancer
The final component of the decision support package was an online, interactive algorithm, allowing cancer and non-cancer survival prediction, tailored to the age, tumour characteristics and health of the patient. The resulting online tool would be part of the DESI package tested in the cluster-randomised trial. The initial models were developed using retrospective UK registry data from two of the largest UK registry regions, Northern and Yorkshire [Northern and Yorkshire Cancer Registry and Information Service (NYCRIS)], and the West Midlands [West Midlands Cancer Intelligence Unit (WMCIU)], validated using data from a third region [East Anglia; Eastern Cancer Registry and Intelligence Centre (ECRIC)]. Development had three components:
-
development of the first draft of the S+ET versus ET ALONE model using WMCIU and NYCRIS data on 23,849 women aged ≥ 70 years between 2002 and 2010 (see Development of a survival outcome model for surgery plus adjuvant endocrine therapy versus primary endocrine therapy for breast cancer in older women using UK registry data)
-
internal model validation using a more recent tranche of WMCIU/NYCRIS data up to January 2017 using Flexsurv (R Foundation for Statistical Computing, Vienna, Austria) (see Validation of the statistical predictive model)
-
external model validation using registry data from ECRIC (see Adjuvant chemotherapy model development).
These models have been published,32–34 and the process and outcomes are described in more detail in the subsequent sections. The models performed well in terms of survival prediction and were used to populate the algorithm for the Age Gap decision tool, which was used in the cluster-randomised controlled trial.
Development of a survival outcome model for surgery plus adjuvant endocrine therapy versus endocrine therapy alone for breast cancer in older women using UK registry data
Introduction
A retrospective cohort analysis was conducted using UK Cancer Registry data from two English cancer registration regions that are demographically representative of the wider UK population (West Midlands, and Northern and Yorkshire). The effect of ET ALONE versus S+ET on survival outcomes was assessed and a model developed to provide data for an online algorithm to be tested in the cluster-randomised controlled trial. This work has been published. 32,35
Methods
Data on all invasive breast cancer in women aged ≥ 70 years between 2002 and 2010 were acquired from two UK cancer registries (West Midlands, and Northern and Yorkshire). Variables in the analysis included age, tumour stage and oestrogen receptor status, comorbidity index and whether treated with surgery or not. Survival data were derived from death certifications. Cause of death was classified as either ‘breast cancer related’ or ‘not breast cancer related’. Only women with ER+ disease were included. Comorbidity was derived from linked Hospital Episode Statistics (HES) and aggregated using the Charlson Comorbidity Index (CCI)36 by counting diagnostic codes recorded in the 18 months prior to diagnosis. 37
A full prognostic survival model was constructed by combining the hazards predicted by two submodels. The breast-cancer-specific model was a Royston–Parmar, restricted, cubic spline model. 38 This model had eight covariables (treatment, age, comorbidity, mode of detection, deprivation, lymph node status, tumour grade and size). 32 The other-cause-mortality model had three covariates: age, comorbidity and frailty; these were modelled with the proportional hazards assumption. Frailty was approximated using a version of the activities of daily living (ADL) score. 39 This variable was not recorded in the registry data; therefore, a Markov chain Monte Carlo approach was adapted from Koissi and Högnäs40 to infer frailties.
Results
The 5-year breast-cancer-specific survival (BCSS) in the ET ALONE and S+ET arms was 69% and 90%, respectively, which was in favour of S+ET. Analysis of subgroups according to age or comorbidity stratification demonstrated that, as age or comorbidity increased, the degree of separation between the survival curves lessened, and, in the oldest and least fit groups, there was less benefit from undergoing surgery, regardless of cancer stage at diagnosis.
Conclusion
This analysis suggests that, for older breast cancer patients with ER+ disease, the hazard of breast cancer death was greater with ET ALONE than with S+ET. Findings from this analysis were used to develop a web-based clinical algorithm to aid decision-making (see Design of an online tool to support decision-making for older women with early-stage breast cancer). The final model used in the online tool differed slightly from the above, as, during validation, a technical problem was identified, which was rectified in the final version used in the online tool. The validation process of this model is described in the next section.
Validation of the statistical predictive model
Background
The statistical models for the Age Gap online tool were developed to predict risk of breast cancer and non-breast cancer deaths over time for an individual woman given her age, breast cancer status and comorbidity. Phase 1 of the validation involved internal validation and phase 2 involved external validation using cancer registry data. This work has been published. 34
Phase 1: validation using registry data (internal)
Internal validation was undertaken using the updated registries data set. In the case of breast-cancer-specific mortality results, comparison of observed and predicted 2- and 5-year survival outcomes were well matched, with discrimination of between 0.73 and 0.77. The model was also well calibrated. There was a slight overestimate of breast cancer mortality, with an overestimation of 0.2 and 0.9 percentage points at 2 and 5 years, respectively. All-cause mortality was underestimated by 1 percentage point at 2 years and overestimated by 0.7 percentage points at 5 years.
Phase 2: external validation
External validation was undertaken using a data set from the ECRIC of breast cancer in women aged ≥ 70 years registered from 2002 to 2012.
The model performed well, with discrimination results ranging from 0.75 to 0.80. Overall calibration of the model in the external data set was also good. At 5 years, predicted breast cancer mortality exceeded that observed for the training data by 4%, with the absolute breast cancer mortality rate difference being 0.2%.
Conclusion
The developed model, which was both internally and externally validated using registry data, performed well, with a high degree of prognostic accuracy. The model permits outcome stratification for age and comorbidity according to whether a woman has S+ET or ET ALONE. This model was used to develop the age- and fitness-stratified online outcome tool for the cluster-randomised controlled trial.
Adjuvant chemotherapy model development
Introduction
Adjuvant chemotherapy is recommended for high-recurrence-risk, early-stage breast cancer patients. Meta-analysis41 reported that adjuvant chemotherapy was associated with a reduced risk of recurrence (12%) and death (13%) in patients aged ≥ 70 years, compared with no chemotherapy.
A survival outcome model was, therefore, developed using retrospective analysis of data for breast cancer patients aged ≥ 70 years, from two English cancer registry regions, diagnosed between 2002 and 2012. This compared the effects of adjuvant chemotherapy or no chemotherapy in women aged ≥ 70 years. The outcomes were stratified by age, cancer stage and biology, and health status. The resultant outcome model was used in the Age Gap online algorithm. The work has been published. 33
Methods
Cancer registration records were obtained for all breast cancer diagnoses in women aged ≥ 70 years between 2002 and 2012 from two English registries. Comorbidity was derived from linked HES data and aggregated into a proxy CCI. English indices of deprivation42 (2010) were derived from postcodes.
Analyses were restricted to women diagnosed with stage I–III disease, treated with surgery and adjuvant chemotherapy or no chemotherapy. Some analyses were restricted to patients at high risk of recurrence.
Associations between patients, treatment characteristics and survival were investigated using multivariate proportional hazard regression, using Royston–Parmar, restricted, cubic spline parametric models. 38 Missing data were handled using multiple imputation with chained equations.
Results
Between 2002 and 2012, a total of 29,728 women were diagnosed with breast cancer, of whom 11,735 were included in analysis (stage I–III disease, treated with surgery). Very few patients aged ≥ 80 years received adjuvant chemotherapy, precluding meaningful analysis (n = 122; < 1%), so analyses were restricted to patients aged 70–79 years. Women who had adjuvant chemotherapy were generally younger, were in better health, and had worse disease stage and adverse biology [oestrogen receptor negative (ER–), human epidermal growth factor receptor-2 positive (HER-2+)].
The logistic regression confirmed that a younger age at diagnosis, increased nodal involvement, tumour size and grade, and having ER– or HER-2+ disease were all associated with increased probability of receiving adjuvant chemotherapy. For example, the odds ratio (OR) of adjuvant chemotherapy receipt for each year that the woman was aged ≥ 70 years was 0.763 [95% confidence interval (CI) 0.741 to 0.787; p < 0.001]. Having an ER+ cancer was associated with an OR of adjuvant chemotherapy receipt of 0.305 (95% CI 0.261 to 0.357; p < 0.001); conversely, for HER-2+ disease, the OR was 2.904 (95% CI 2.476 to 3.406; p < 0.001). Similarly, node positivity (1–3 nodes positive: OR 3.739, 95% CI 3.120 to 4.483; p < 0.001) and increased tumour size (per mm increase in tumour size: OR 1.015, 95% CI 1.011 to 1.019; p < 0.001) were also associated with increase adjuvant chemotherapy use.
Naive comparison of survival outcomes between the two treatment groups showed that BCSS and overall survival (OS) were worse for patients who received adjuvant chemotherapy than for those who did not. However, this arises from the difference in risk profiles between the two groups. When all high-risk patients were considered, adjuvant chemotherapy was associated with reduced BCSS compared with no chemotherapy. However, when the analysis was repeated with higher-risk-scoring patients only, the positive effect of adjuvant chemotherapy becomes clear.
The Royston–Parmar model results showed that adjuvant chemotherapy was associated with a significant reduction in the hazard of breast-cancer-specific mortality compared with no chemotherapy, after adjustments were made for patient-level characteristics [hazard ratio (HR) 0.74].
Discussion
This study found that adjuvant chemotherapy was associated with a reduction in breast-cancer-specific mortality for patients aged 70–79 years with high-recurrence-risk cancer, after adjustment for patient characteristics.
High-recurrence-risk disease characteristics were associated with adjuvant chemotherapy use. However, many high-risk patients did not receive chemotherapy, in particular those patients aged > 80 years. This may be justifiable given that older patients are more likely to die of other causes than of breast cancer, so the absolute gains in survival may be small.
This age-, comorbidity- and tumour-characteristic-stratified outcome model was, therefore, suitable to use in the online Age Gap tool that would be tested in the cluster-randomised controlled trial.
Design of an online tool to support decision-making for older women with early-stage breast cancer
Introduction
An integral component of the DESI was an online tool to permit personalised survival prediction, stratified according to tumour stage, biology, fitness and frailty. The models described previously were used to ‘power’ the online tool, with stratification for patient age and comorbidity based on the CCI score. The tools were developed to be useable for both health-care professionals and patients. The development process has been summarised in a publication. 31
Methods
The design of the online tool was based on data derived from the developed models for the ET ALONE versus S+ET, and adjuvant chemotherapy models (see Developing outcome models for older patients with early-stage breast cancer and Adjuvant chemotherapy model development), supplemented with published frailty data39 and evidence from patient interviews about preferred formats for data presentation (see Defining the information needs and preferences of older women with breast cancer). The output from the tool presented data in text, pictogram or bar chart formats. In addition, in response to patient feedback, stratified outputs could be printed off in an easy-to-read format that could be inserted in the booklets developed for the tool, allowing outcomes to be personalised. A draft version was user tested with both health-care professionals and patients, and modified according to feedback.
Three focus groups were undertaken, including six surgeons, seven clinical nurse specialists and nine oncologists.
Results
Feedback from clinicians
The tool was welcomed. All clinicians liked the layout and found it intuitive, quick and easy to use. The printout of the personalised risk sheet was also welcomed. Some clinicians felt that their current pathway of care would make using the online tool difficult, with most stating they would work around the problems.
Items of concern and the responses made by the team are shown in Appendix 2, Table 1.
Feedback from patients
Eight patients were interviewed, with varied feedback regarding data display formats, which were amended. Items of concern and the response made by the team are shown in Appendix 3, Table 2.
Figures 2 and 3 are screenshots of the final online tool for the S+ET versus ET ALONE tool used during interviews. Figures 4 and 5 are screenshots of the adjuvant chemotherapy tool. Screenshots of the booklets are shown in Appendix 6, Figure 19.
FIGURE 2.
Data collection sheet: comparing S+ET and ET ALONE. Reproduced with permission from the University of Sheffield.
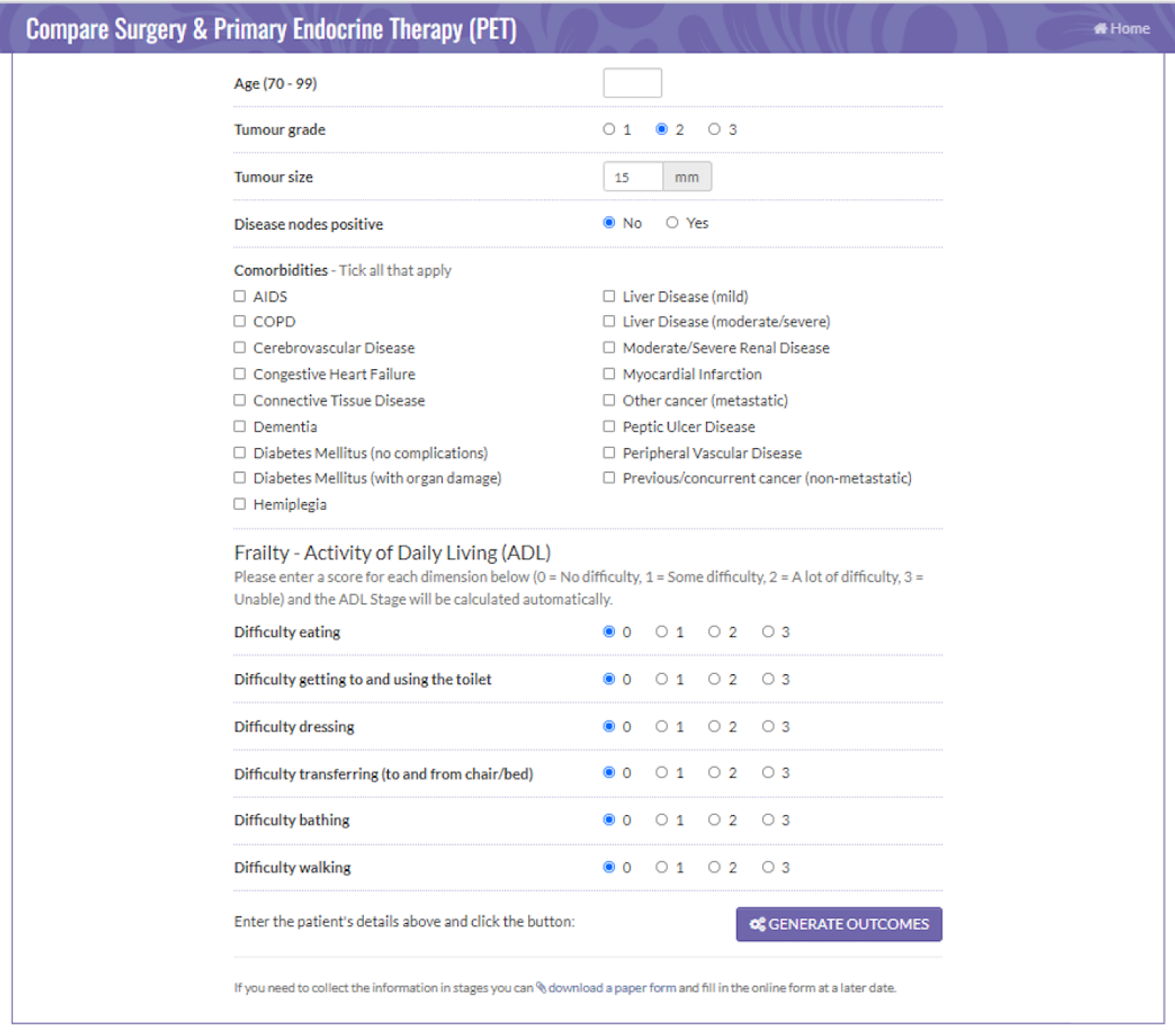
FIGURE 3.
Pictogram showing the survival estimates at 2 years, comparing S+ET and ET ALONE. Reproduced with permission from the University of Sheffield.
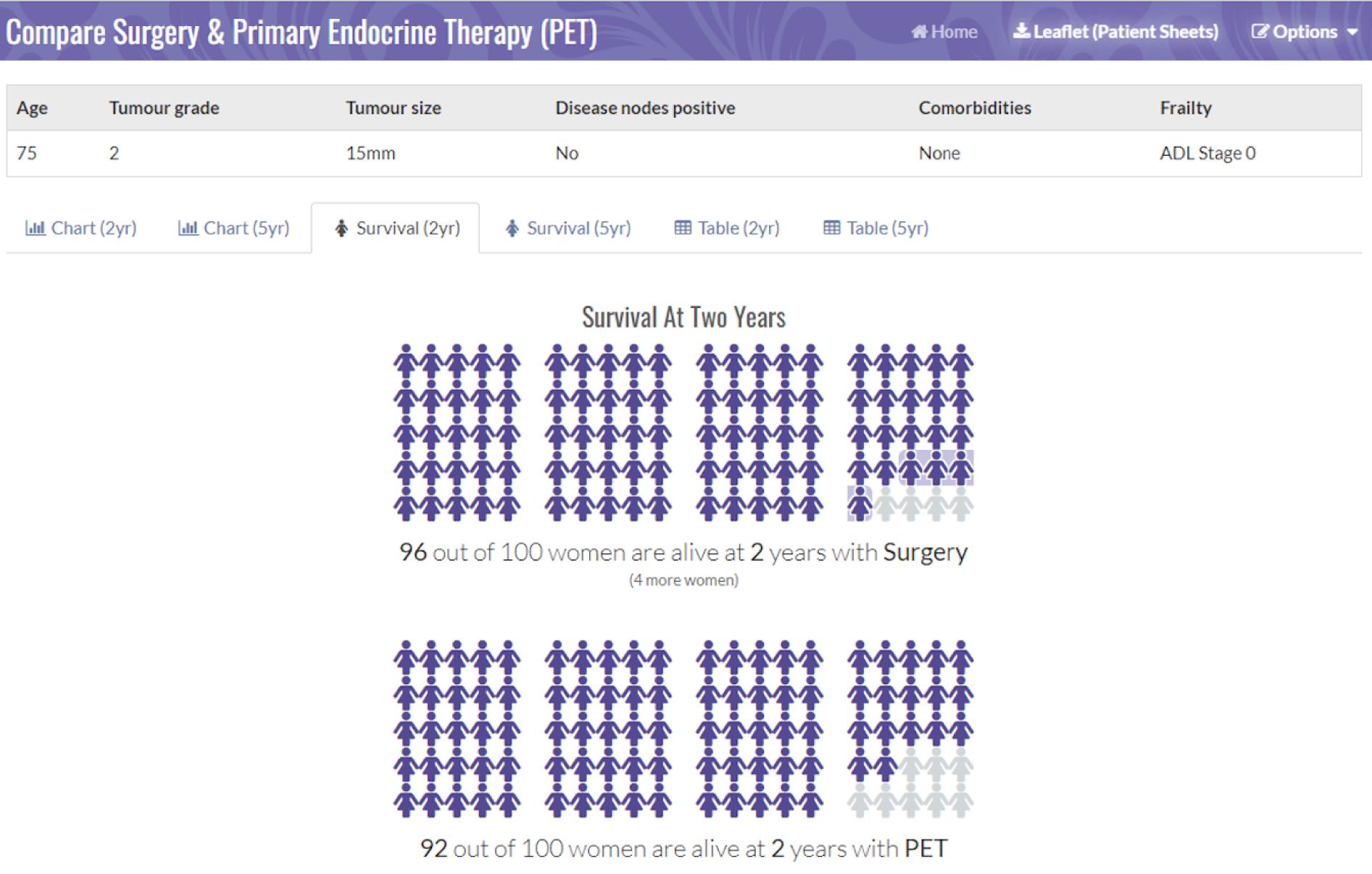
FIGURE 4.
Data collection sheet: comparing adjuvant chemotherapy and no chemotherapy. Reproduced with permission from the University of Sheffield.
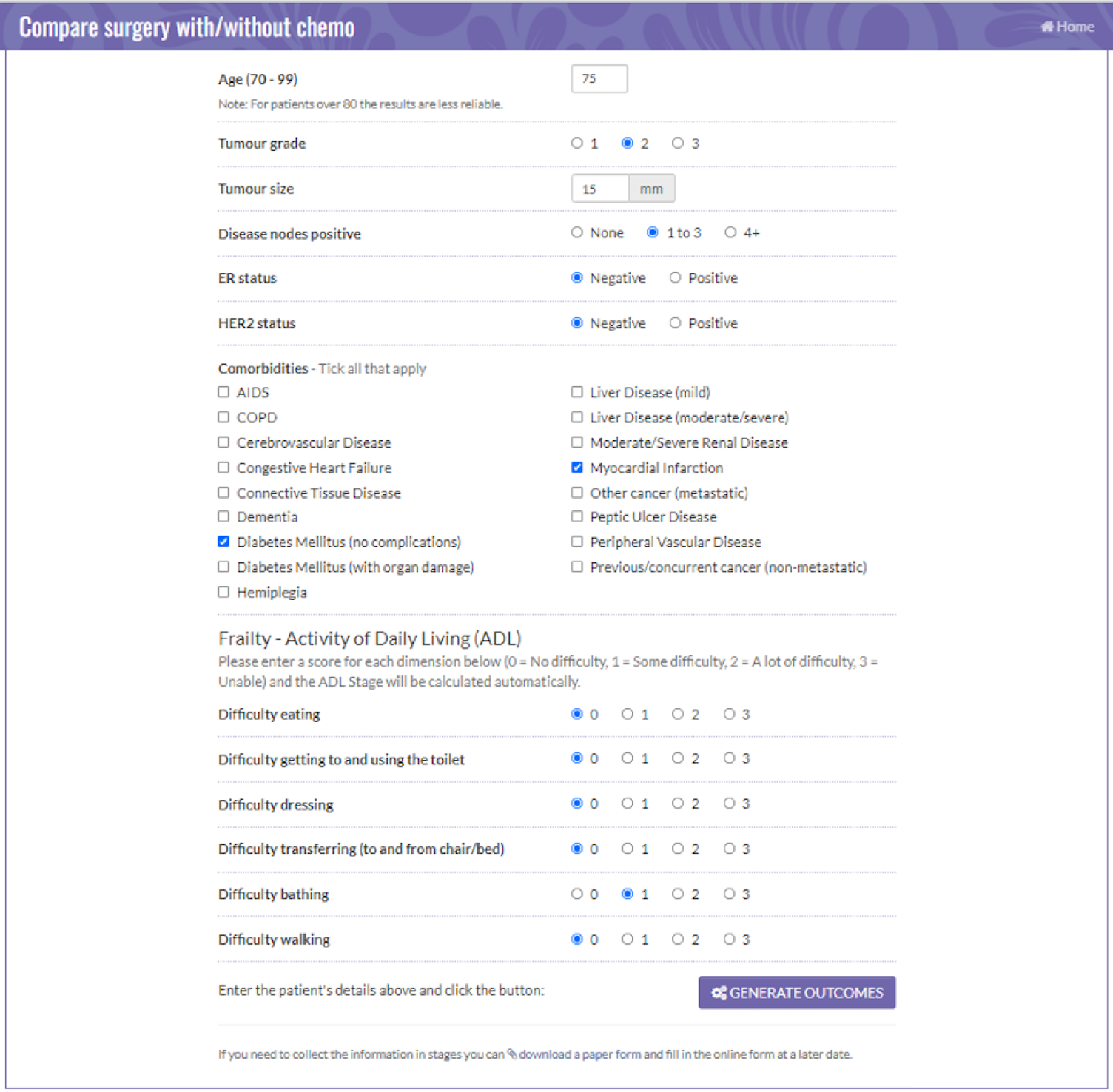
FIGURE 5.
Pictogram showing the survival estimates at 2 years, comparing adjuvant chemotherapy and no chemotherapy. Reproduced with permission from the University of Sheffield.
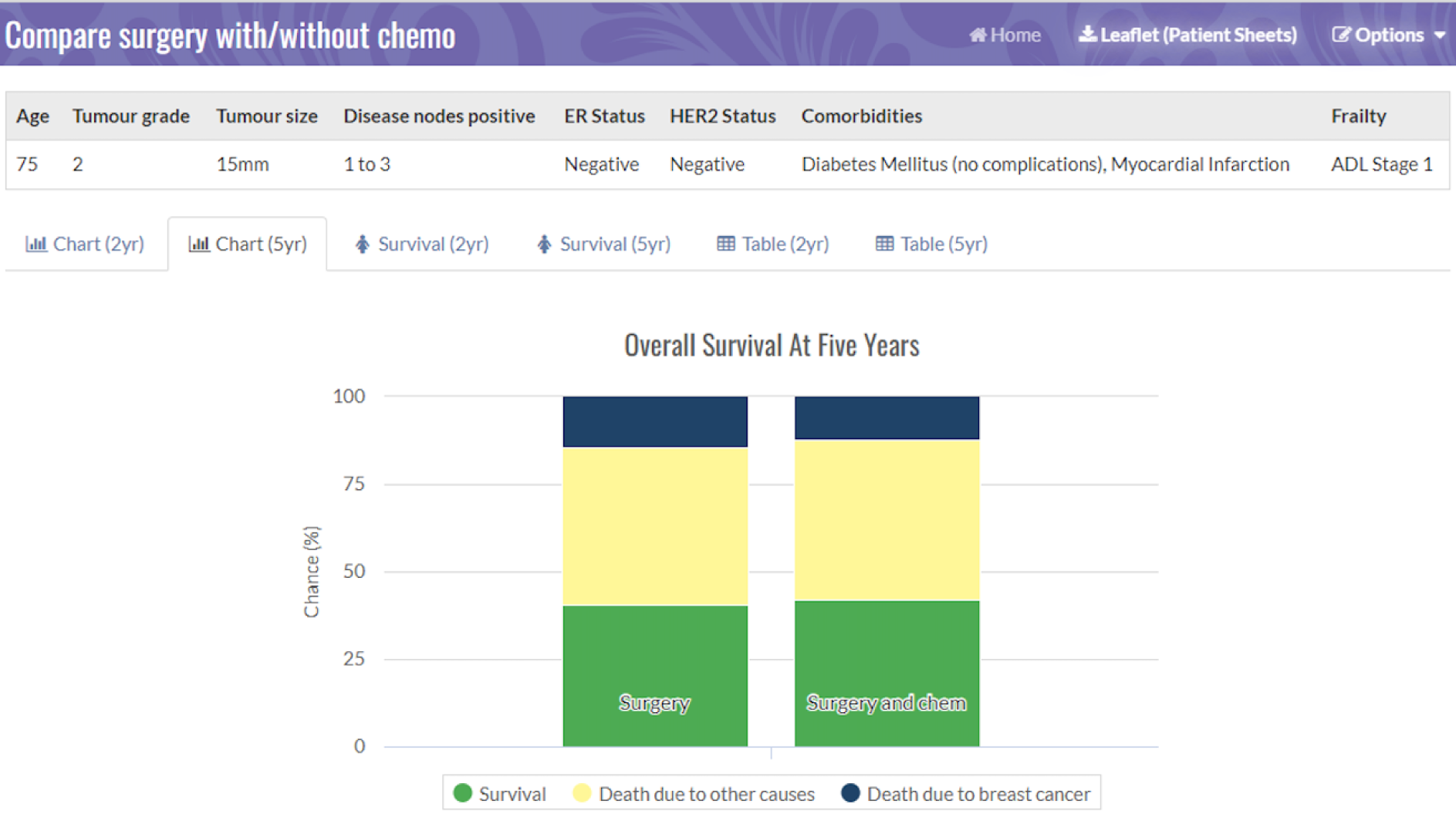
The tool now has MHRA approval and can be accessed online. 43
Examples of the brief decision aid for ET ALONE versus S+ET, and for the adjuvant chemotherapy or no chemotherapy decision are shown in Appendix 4, Table 3, and Appendix 5, Table 4, respectively. Images relating to the booklets are shown Appendix 6, Figure 19, and images relating to the outputs from the online tool are shown in Figure 2 and Appendix 7, Figure 20.
The Age Gap prospective observational multicentre cohort study general methods and results
Introduction
The purpose of this study was to recruit a large prospective cohort of older women with early-stage breast cancer with baseline information regarding health, cancer stage, biology and treatment. These data were analysed using propensity score matching to adjust for baseline variation in health and fitness to determine outcomes (survival and QoL) between women treated with S+ET versus ET ALONE, or adjuvant chemotherapy versus no chemotherapy. This would allow us to determine optimal age- and health-stratified thresholds for treatment allocation. Several specific, focused analyses were performed, which are summarised in subsequent sections, including:
-
general cohort methods and results
-
surgery outcomes (see Outcomes from surgery for older women with early-stage breast cancer)
-
overall and BCSS outcomes for women having S+ET versus ET ALONE (see Endocrine therapy alone versus surgery plus adjuvant endocrine therapy: survival analysis)
-
QoL outcomes for women having S+ET versus ET ALONE (see Quality-of-life variation between primary endocrine therapy and surgery plus adjuvant endocrine therapy)
-
overall and BCSS outcomes for women following surgery who did or did not have adjuvant chemotherapy (see Adjuvant chemotherapy versus no chemotherapy in women with high-recurrence-risk breast cancer using both unmatched and propensity-score-matched analyses)
-
QoL outcomes for women following surgery who either did or did not have adjuvant chemotherapy (see Quality-of-life impacts of chemotherapy)
-
treatment allocation and survival outcomes in women with or without cognitive impairment (see Analysis of the impact of cognitive impairment on outcomes for older women with early-stage breast cancer)
-
health economic outcomes for women who had S+ET versus ET ALONE (see Cost-effectiveness analysis comparing surgery plus adjuvant endocrine therapy with primary endocrine therapy).
General methods
Study design
This was a prospective, pragmatic, multicentre, observational UK cohort study.
The cohort study has been published in various component parts, detailed in each section as listed above. A more detailed overview of methods is given in Appendix 8, and described in a published paper that summarises both the cohort and cluster methodologies. 44
Aims
To determine overall and propensity-score-matched outcomes for older women (aged ≥ 70 years) with early-stage breast cancer according to whether they receive ET ALONE or S+ET, and, for women with high-recurrence-risk breast cancer, whether or not they received adjuvant chemotherapy.
Objective
To undertake propensity-score-matched analysis of prospectively collected cohort data to allow risk-stratified comparison of outcomes between women receiving ET ALONE and those receiving S+ET, and those receiving adjuvant chemotherapy and no chemotherapy.
Outcomes
Primary outcome
The primary outcome was OS.
Secondary outcomes
Secondary outcomes were BCSS, failure-free survival (FFS), time to local recurrence (progression in ET ALONE), time to metastatic recurrence, QoL and adverse events [Common Terminology Criteria for Adverse Events (CTCAE) classification]. 45
Propensity-score-matched analysis was performed to adjust for baseline variation in patient and disease characteristics between treatment groups.
Levels of participation
Women were invited to participate after diagnosis but before treatment commencement.
Patients with full cognitive capacity could select one of two levels of participation: full participation (including QoL forms) or partial participation (excluding QoL). Women with significant cognitive incapacity did not complete QoL questionnaires.
Baseline data collection
At baseline, women underwent assessment using a series of validated tools, including the:
-
CCI36
-
abridged Patient-Generated Subjective Global Assessment (aPG-SGA)46,47
-
ADL48
-
Instrumental Activities of Daily Living (IADL)49
-
Mini-Mental State Examination (MMSE)50
-
Eastern Cooperative Oncology Group Performance Status (ECOG-PS). 51
In addition, QoL was assessed using the:
-
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC-QLQ-C30),52 a generic cancer QoL tool
-
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-BR23 (EORTC-QLQ-BR23),53 a breast-cancer-specific QoL module
-
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-ELD14 (EORTC-QLQ-ELD14),54 an older-person-specific module
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L)55 to permit calculation of quality-adjusted life-years (QALYs) for health economics assessment.
Baseline data were collected regarding primary tumour stage and biotype.
Follow-up assessments
Patients were followed up at 6 weeks, and at 6, 12, 18 and 24 months.
Patients could also consent to accessing cancer registry data for long-term follow-up to 10 years.
Participating UK breast units
The study recruited from 56 breast units in England and Wales (see Appendix 8, Table 5).
Eligibility criteria
Inclusion criteria
Women aged ≥ 70 years with operable breast cancer were eligible.
Study treatments
This was a pragmatic cohort study with no change to normal treatment.
Statistical analysis
Propensity-score-matched analysis
Two analyses were performed:
-
In the case of women with ER+ breast cancer, we compared the survival and QoL outcomes in those who received S+ET with the outcomes in those who received ET ALONE.
-
In the case of women with high-recurrence-risk cancers, following surgery [i.e. patients who met any of the following criteria: (1) HER-2+; (2) ER–; (3) ER+ and a histological grade of 3; (4) nodes positive or (5) a high Oncotype DX™ recurrence score], we compared the survival and QoL outcomes for those treated with adjuvant chemotherapy and those treated without chemotherapy.
Overall survival and BCSS were calculated according to treatment allocation. Kaplan–Meier curves were derived for each treatment type by age, disease characteristics, comorbidity and frailty subgroups. OS was compared between treatment groups using a Cox proportional hazards model, adjusting for important prognostic factors.
General cohort results
Recruitment
The study recruited 3416 women between January 2013 and June 2018. The median age was 77 years (range 69–102 years). The median follow-up was 52 months. The age distribution of the cohort was slightly skewed relative to that of the wider population of older UK women with breast cancer, with a slight preponderance of women in the 70–80 years age group and a slight deficit of women aged > 90 years. This was due to selective recruitment and retention. These results have been published. 59
Conclusion
The Age Gap cohort study recruited 3414 women aged ≥ 70 years, demonstrating that recruitment in this age group is feasible. Recruitment was slightly skewed towards younger, fitter women, which must be considered when interpreting the generalisability of the results.
Various specific analyses of the cohort study are presented in the subsequent sections.
Outcomes from surgery for older women with early-stage breast cancer
Introduction
This analysis was performed to describe the allocation of surgery (mastectomy vs. wide excision, and sentinel node biopsy vs. axillary clearance) and the safety and adverse events associated with it. This may enhance understanding of the impacts of surgery. These data have been published. 60
Methods
For this analysis, surgery was classified as major (i.e. mastectomy and/or ALND) or minor (i.e. BCS with or without SLNB). In the case of patients who had a bilateral procedure for invasive breast cancer, surgery was assessed as two unilateral procedures.
Outcomes
Mortality related to surgery was defined as death within 30 days of surgery or surgery being documented as contributing to cause of death. Death from breast cancer was assessed by death certification and expert review of all causes of death. Causes of death were categorised as breast cancer-related or other causes. Complications, obtained by follow-up to 2 years, were categorised using the CTCAE28 and as systemic (i.e. atelectasis, stroke, infarction, deep vein thrombosis/embolism, arrhythmia, allergic reaction or somnolence) or local (i.e. lymphoedema, neuropathy, functional difference, wound pain, wound, necrosis, infection, haematoma, seroma or haemorrhage).
Results
This analysis examined the data from 2816 women who underwent surgery. Of these, 62 had bilateral surgery; therefore, 2858 surgical events are reported. The median age of the surgical cohort was 76 years (range 70–95 years). The majority underwent breast conservation surgery (n = 1716) and 1138 underwent a mastectomy. For axillary surgery, 575 women underwent axillary clearance, 2203 underwent sentinel node biopsy and 76 underwent no axillary surgery.
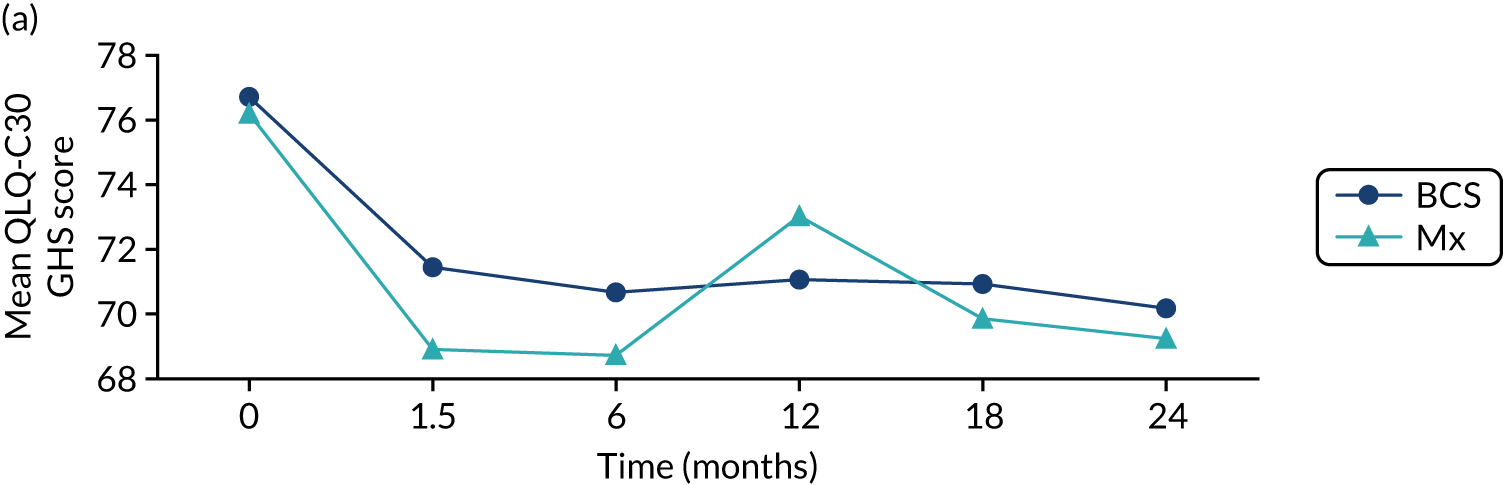
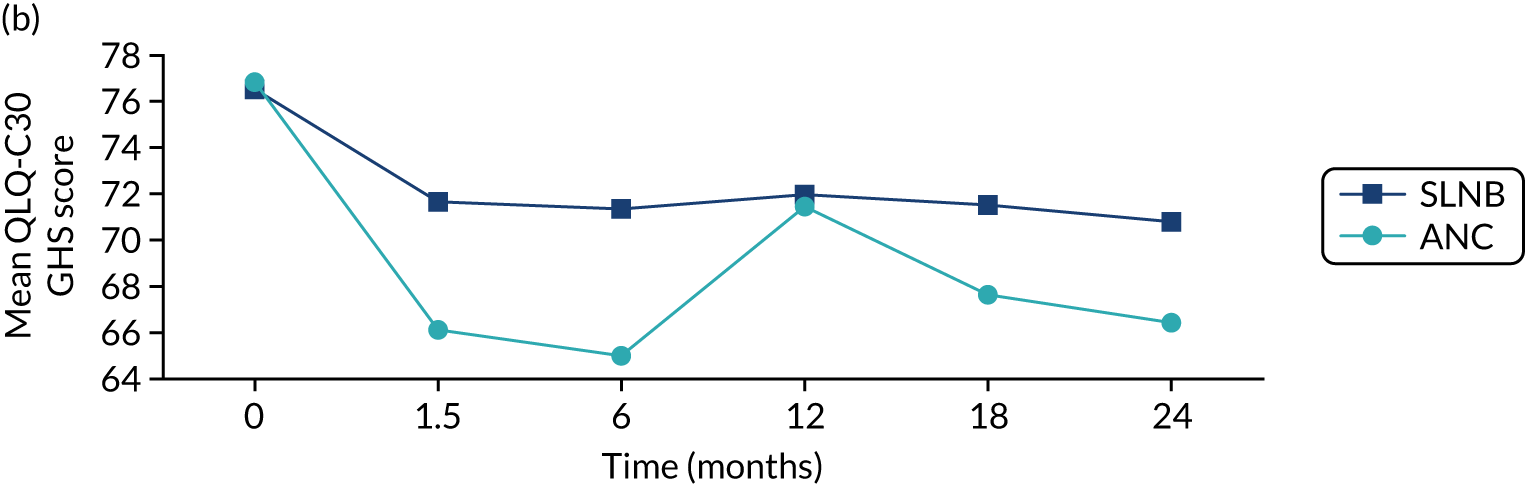
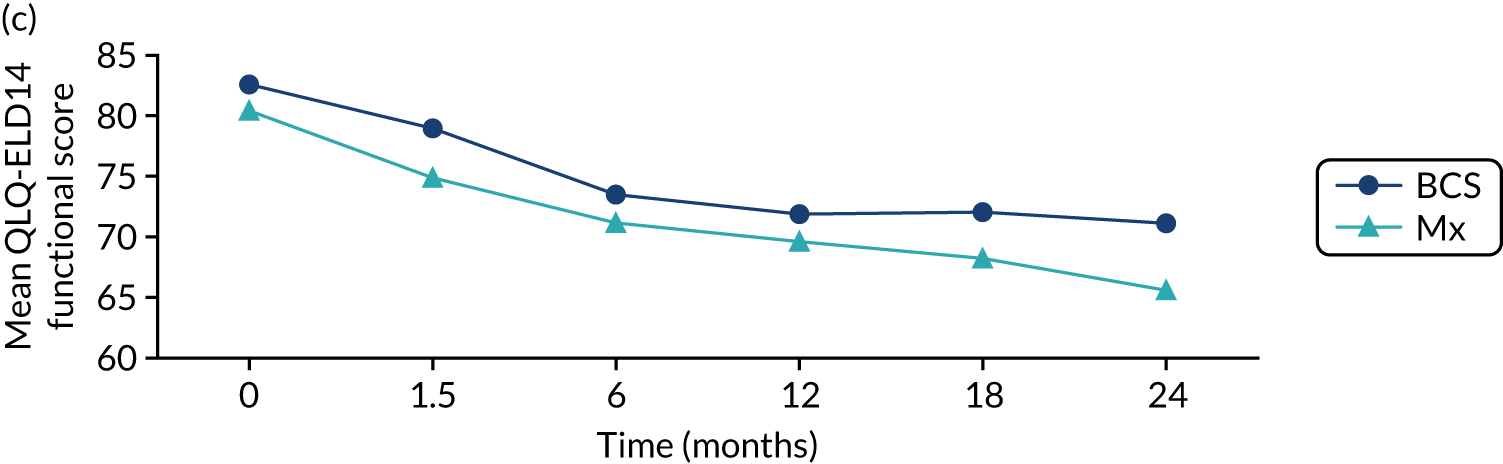
There were no deaths directly attributable to surgery, although there were three deaths within 30 days of surgery (to which surgery theoretically may have contributed). There were 551 adverse events recorded from these 2858 procedures (19.3%), although the majority of these were simple wound complications. Only 2.1% of women had more serious systemic adverse events, such as cardiac, respiratory or cerebrovascular events. QoL was adversely affected by surgery, with impacts clearly seen in the post-operative period (the 6-week time point; Figure 6). These were usually transient, but some residual negative impact was seen in some domains up to 2 years. Negative QoL impacts were more likely with major surgery (i.e. mastectomy, axillary clearance). There was also a long-term negative impact on physical function, implying a lack of resilience in this age group.
FIGURE 6.
Selected mean QoL domain scores following different types of surgery. (a) Axillary clearance vs. sentinel node biopsy: global health status; (b) mastectomy vs. breast conservation: global health status; (c) axillary clearance vs. sentinel node biopsy: functional QoL score; (d) mastectomy vs. breast conservation: breast symptoms; (e) axillary clearance vs. sentinel node biopsy: arm symptoms; and (f) mastectomy vs. breast conservation: body image. ANC, axillary node clearance; BCS, breast conservation surgery; Mx, mastectomy; SLNB, sentinel node biopsy. Surgery usually occurred between 0 and 1.5 months.
Conclusions
In this cohort, breast surgery is generally safe and well tolerated, with few serious adverse events and no deaths. However, there is a negative impact on QoL that is more significant in women who have more major surgery. These risks need to be communicated to women before surgery as part of shared decision-making. We are in the process of developing the online tool to include risk-stratified QoL and adverse event outcomes to be displayed alongside survival outcomes.
Endocrine therapy alone versus surgery plus adjuvant endocrine therapy: survival analysis
Introduction
This study has used real-world data reflecting current UK practice to determine whether or not there is a group of older women who may be safely offered ET ALONE with little negative impact on their risk of death from breast cancer. The analysis corrected for treatment selection bias using propensity score matching and included only women with ER+ cancers. This analysis has been published. 61
Methods
The cohort recruited women aged ≥ 70 years with operable breast cancer and collected detailed baseline data regarding health, frailty, tumour stage and biology. Normal treatment allocation was permitted to derive a real-world cohort. Analysis of unadjusted outcomes is reported; in addition, to correct for baseline variation in patient allocation to these two treatment arms, propensity score matching was used to create a matched cohort of women with similar baseline characteristics. 61
Results
Recruitment
This analysis was confined to women with ER+ cancer. Of the 2854 patients with ER+ cancer, 2354 (82%) received S+ET and 500 (18%) received ET ALONE. The median follow-up was 52 months. The two treatment cohorts differed significantly in their characteristics. The median age of the ET ALONE group was 84 years (range 70–102 years), whereas the median age of the S+ET group was 76 years (range 69–94 years). In addition, the burden of comorbidity was higher in the ET ALONE group, with a median CCI score of 6 [interquartile range (IQR) 4–7] for ET ALONE, compared with 4 (IQR 3–5) for S+ET. Tumour stage and biology were broadly similar.
Because of this variation in cohort characteristics, baseline adjustment was achieved using propensity score matching. Logistic regression was used to calculate propensity scores for treatment allocation using the ADL, the IADL, the MMSE, the ECOG-PS, the aPG-SGA, the CCI, number of medications and age as covariates.
In the final matched data set, a suitable match was found for 240 (48%) ET ALONE patients: 184 were matched with two S+ET patients and 56 were matched with only one. The resulting matched cohort characteristics were excellent.
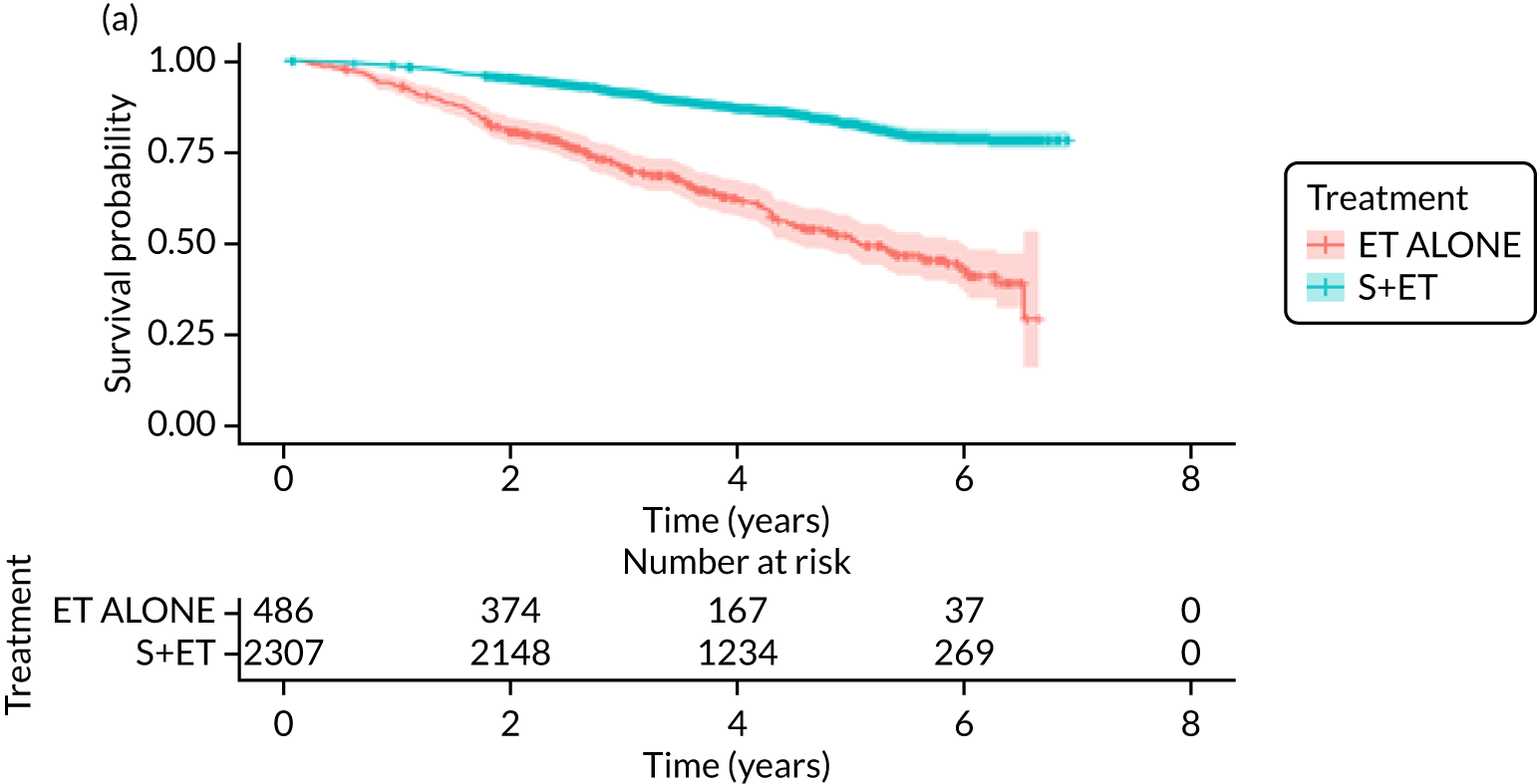
Overall survival (unmatched analyses)
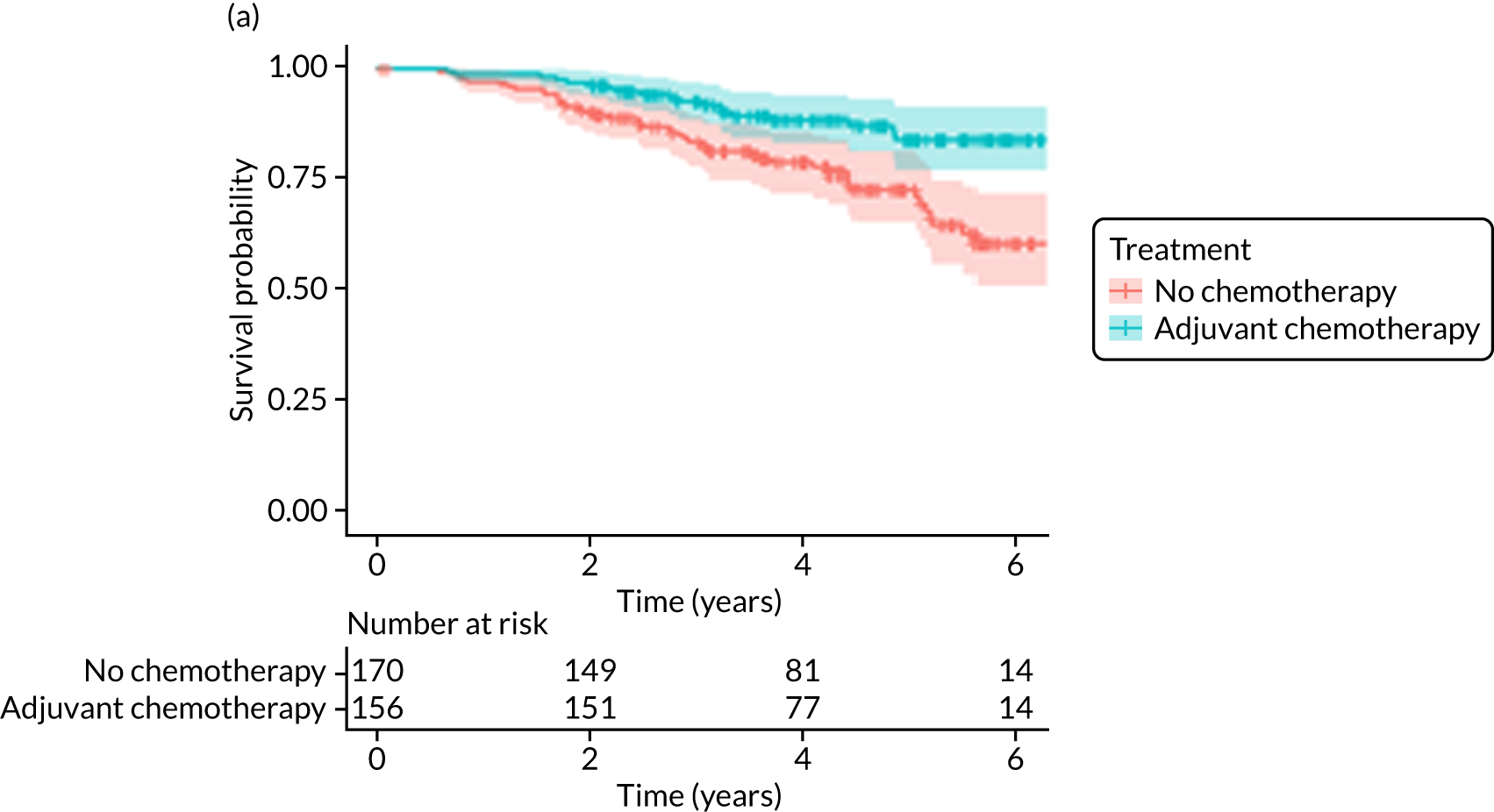
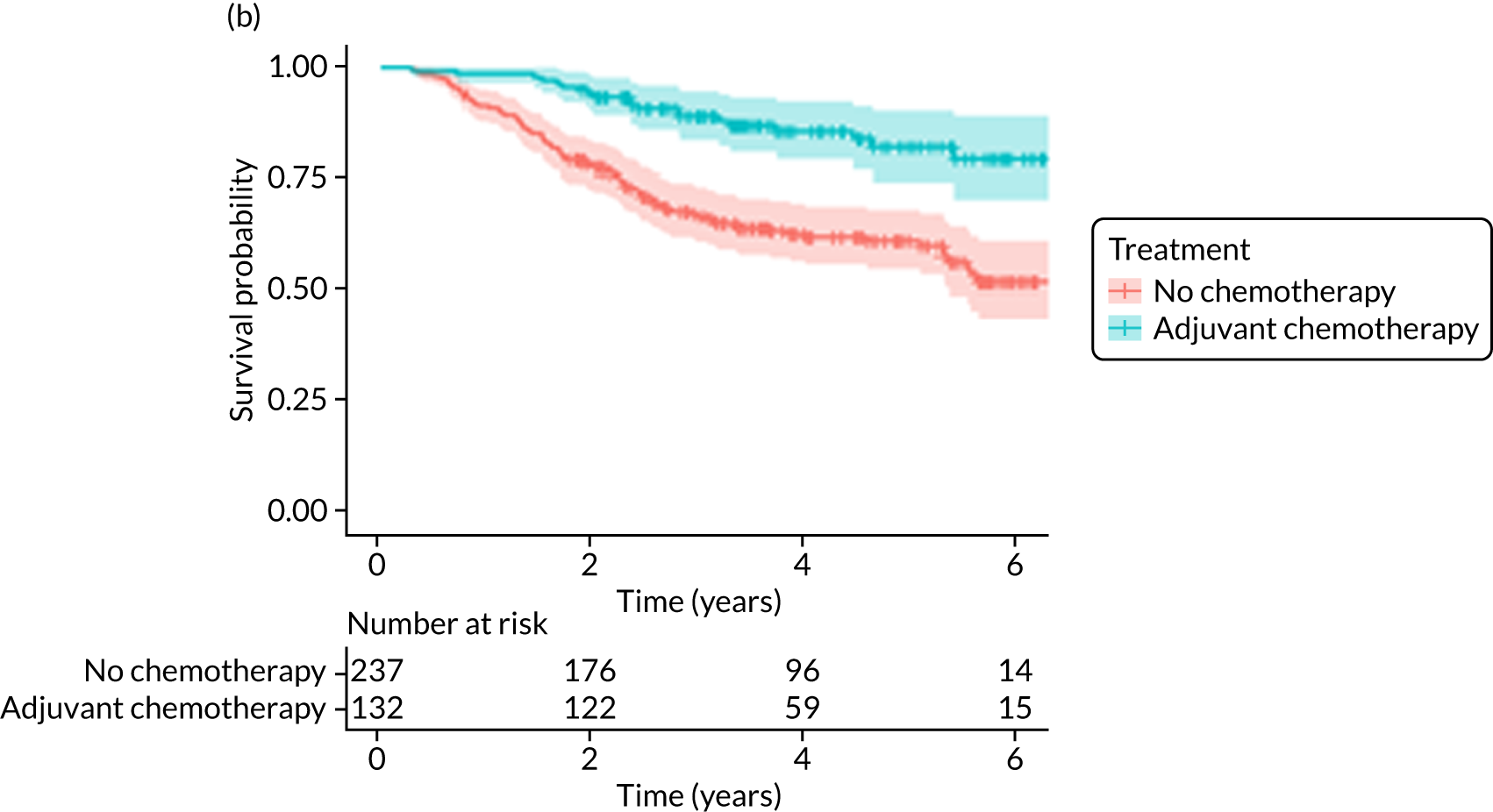
Of the 486 patients who received ET ALONE, 203 (41.8%) died during follow-up, compared with 336 out of 2307 (14.6%) of the S+ET patients. Patients treated with ET ALONE had inferior OS compared with those treated with S+ET (unadjusted HR 0.27, 95% CI 0.23 to 0.33; p < 0.001), but adjusting for case mix via multivariable Cox regression reduced this difference (adjusted HR 0.83, 95% CI 0.63 to 1.09; p = 0.18). The survival curves are shown in Figure 7.
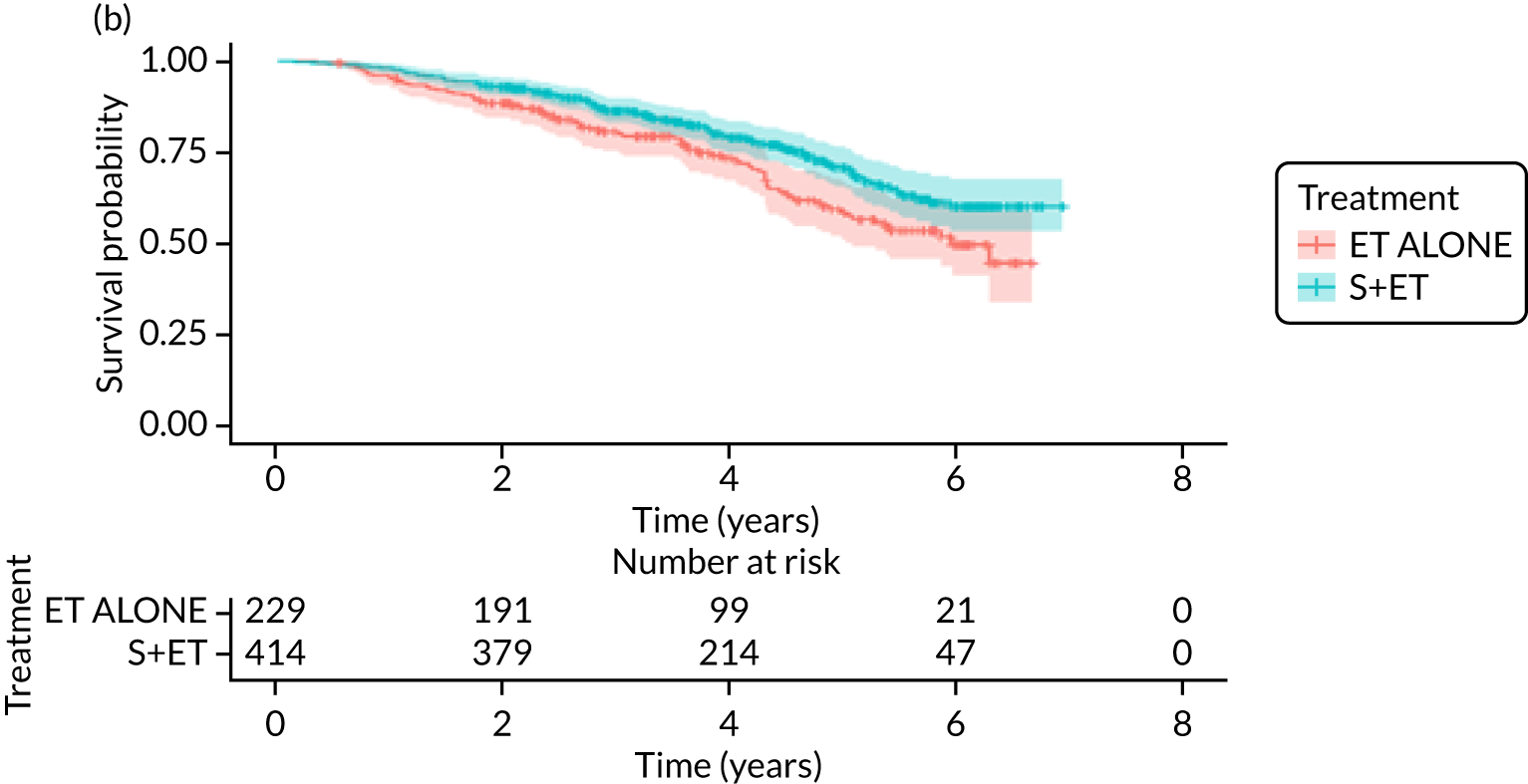
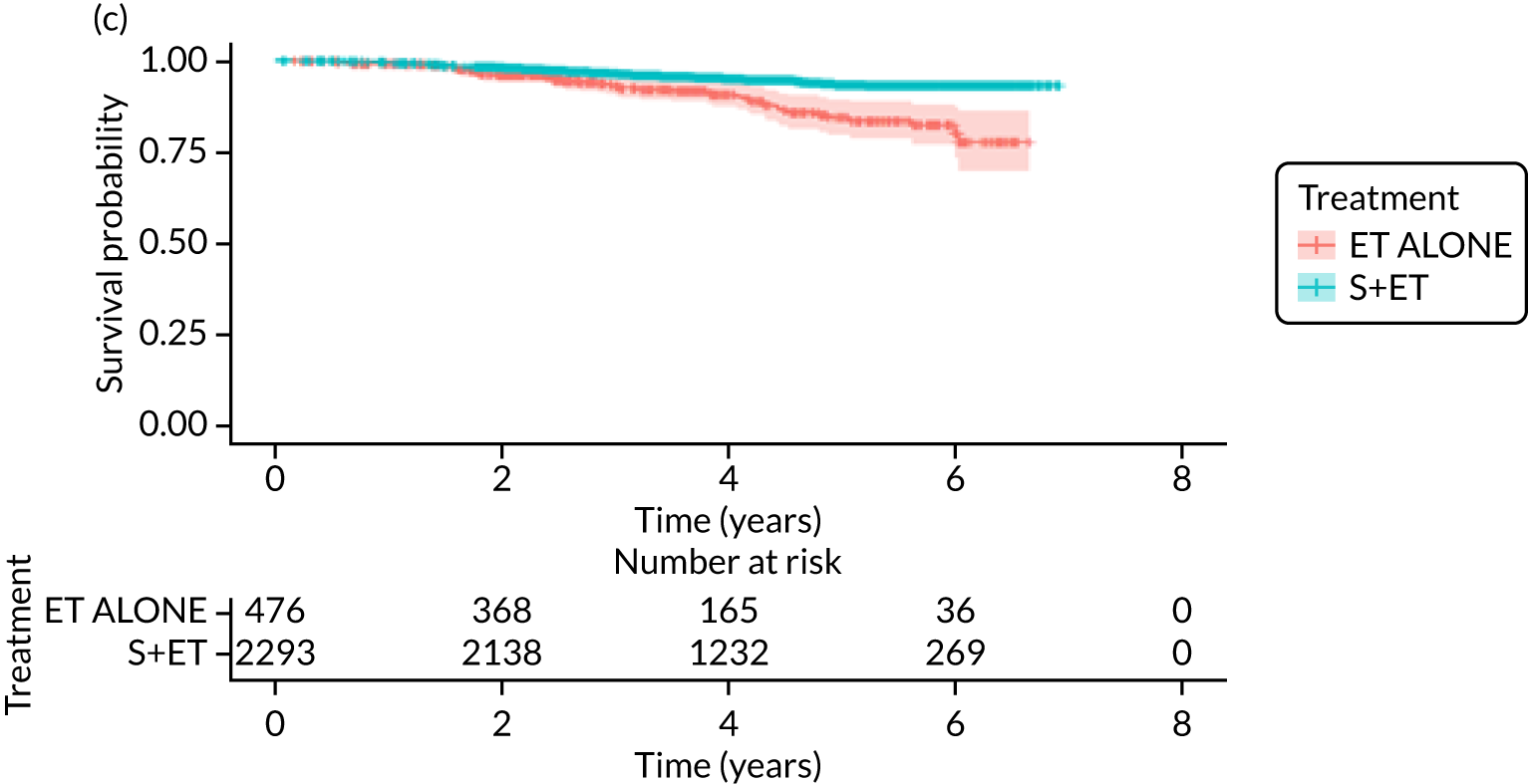
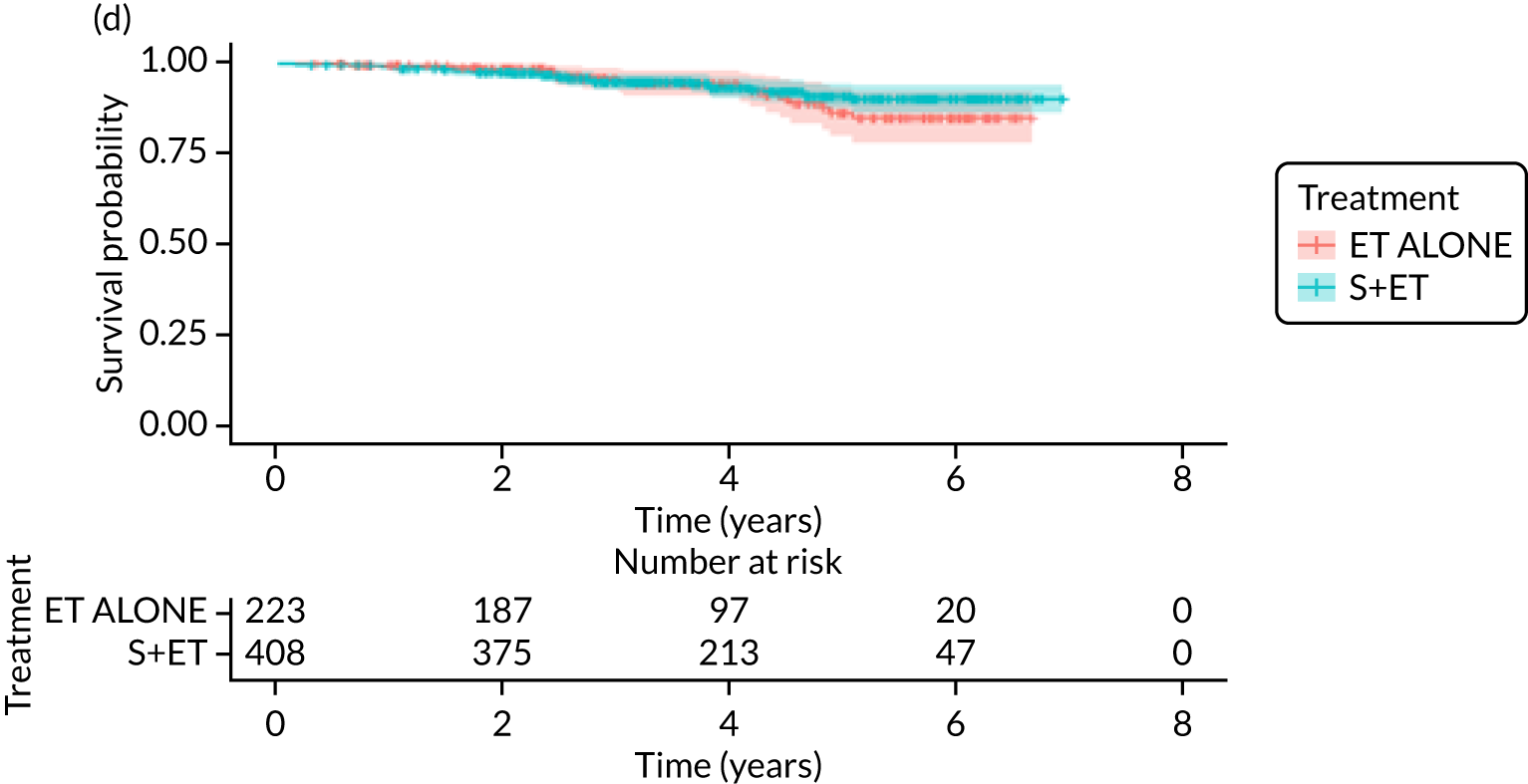
FIGURE 7.
Kaplan–Meier survival curves for women with ER+ breast cancer treated with either S+ET or ET ALONE in matched and unmatched cohorts. (a) OS: unmatched; (b) OS: matched; (c) BCSS: unmatched; and (d) BCSS: matched.
Overall survival (matched analyses)
Of the 229 patients who received ET ALONE, 79 (34.5%) died from all causes during follow-up, compared with 106 out of 414 (25.6%) S+ET patients (matched, unadjusted HR 0.66, 95% CI 0.49 to 0.90; p = 0.008). Matched patients treated with ET ALONE had inferior OS compared with those treated with S+ET. Further adjustment for case mix reduced, but did not remove, this difference (matched, adjusted HR 0.72, 95% CI 0.53 to 0.98; p = 0.037).
Breast-cancer-specific survival (unmatched analyses)
A total of 45 out of 476 (9.5%) patients died from breast cancer in the ET ALONE group, compared with 113 out of 2293 (4.9%) in the S+ET group (unadjusted HR 0.41, 95% CI 0.29 to 0.58; p < 0.001). The BCSS of patients treated with ET ALONE was inferior to that of those treated with S+ET, but adjusting for case mix reduced this difference (HR 0.89, 95% CI 0.52 to 1.53; p = 0.68).
Breast-cancer-specific survival (matched analyses)
In the matched analysis, of the 223 women, there were 17 (7.6%) breast cancer deaths for ET ALONE, compared with 27 (6.6%) deaths among 408 women for S+ET (HR 0.80, 95% CI 0.43 to 1.47; p = 0.46). Adjusting for residual imbalance via multivariable regression provided similar findings (HR 0.74, 95% CI 0.40 to 1.37; p = 0.34).
Recurrence and progression
Unmatched analyses
Rates of overall (locoregional and metastatic) recurrence in the unmatched cohort were higher in patients in the ET ALONE group (33/451; 7.3%) than the S+ET group (113/2325; 4.9%). Locoregional recurrences (progression in ET ALONE) were experienced by 6 out of 451 (1.33%) women for ET ALONE and 25 out of 2325 (1.07%) women for S+ET. After adjusting for age, baseline health status, physical function and Nottingham Prognostic Index (NPI), S+ET had no effect on the rate of recurrence (HR 1.01, 95% CI 0.52 to 1.95; p = 0.981).
Matched analysis
On matching, the differences in rates of recurrence were not significant and the HR for recurrence between the two treatments was 1.11 (95% CI 0.55 to 2.26; p = 0.775).
Discussion
Patient characteristics differed significantly between groups, with ET ALONE generally allocated to older, frailer women than those allocated S+ET. Propensity matching allowed creation of well-matched cohorts of women, although some degree of bias persisted, as demonstrated by the residual variation in OS, where the impact of non-breast-cancer causes of death was still slightly higher in the ET ALONE group than the S+ET group. As a result, although S+ET was still associated with an OS benefit, this difference disappeared in both BCSS and recurrence-/progression-free survival at 52 months’ follow-up.
Surgery plus adjuvant endocrine therapy was the primary treatment modality in 82% and ET ALONE in 18% of women, which is slightly lower than a recent national audit,62 which reported a ET ALONE rate of 24%. This variation may reflect selective recruitment of the less frail into the trial and the technical challenge of recruiting women receiving ET ALONE, in whom treatment often starts on the day of diagnosis, leaving a very narrow window for recruitment.
This study suggests that older women with a short life expectancy of ≤ 5 years derive little survival benefit from S+ET, but women likely to survive > 5 years may start to see endocrine resistance develop on ET ALONE. This accords with previous RCTs63–65 in which survival outcomes at 5 years were equivalent between S+ET and ET ALONE, but outcomes diverged over the longer term.
One of the key aims of this work was to identify a subgroup of women who may safely be offered ET ALONE. It would appear that women with a life expectancy of ≤ 5 years (aged ≥ 85 years with significant comorbidities, or aged > 90 years) should be offered an informed choice, highlighting the potential differences in survival, adverse events and QoL.
Quality-of-life outcomes between treatment groups are presented in Quality-of-life variation between primary endocrine therapy and surgery plus adjuvant endocrine therapy.
Quality-of-life variation between endocrine therapy alone and surgery plus adjuvant endocrine therapy
This analysis has been published. 66
Background
There are very few published data regarding QoL outcomes in older women comparing S+ET and ET ALONE. One previous randomised trial comparing ET ALONE and S+ET measured psychiatric morbidity using the General Health Questionnaire-28 (GHQ-28)67 score up to 2 years after diagnosis. 68 The study found that 9 out of 49 (18%) surgical patients and 2 out of 49 (4%) ET ALONE patients had scores indicative of psychiatric morbidity, whereas, by 2 years, there were 6 out of 49 (12%) patients with scores indicative of psychiatric morbidity in both arms. The GHQ-28 has no domains that would reflect the specific impacts of breast cancer. The European Organisation for Research and the Treatment of Cancer (EORTC) QoL instruments are much more specific to the impacts of breast cancer treatment (i.e. the EORTC-QLQ-BR2353) and the QoL concerns relevant to older people (i.e. the EORTC-QLQ-ELD1454). The purpose of this study was to explore the QoL differences between S+ET and ET ALONE in older women with early-stage breast cancer using highly specific QoL tools.
Methods
Quality-of-life assessment was performed on the matched and unmatched cohorts from the ET ALONE versus S+ET analysis using the validated European Organisation for Research and the Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ) instruments described in The Age Gap prospective observational multicentre cohort study general methods and results.
Owing to significant baseline variation between the unmatched groups in all QoL domains, analyses are largely descriptive and map the changes caused by treatment in each group. In the smaller matched cohort (matched as described in Endocrine therapy alone versus surgery plus adjuvant endocrine therapy: survival analysis), comparative analyses are presented.
Results
EORTC-QLQ-C30: unmatched analysis
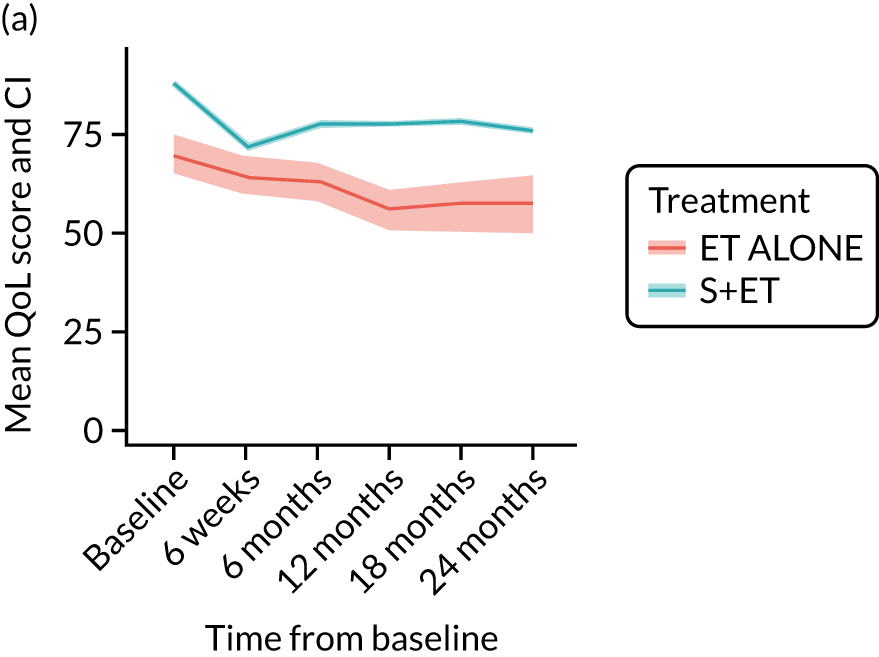
The number of patients completing the EORTC-QLQ-C30 global health status at baseline was 1902 out of 2854 (67%): 1644 received S+ET (70% of the total of 2354 patients) and 258 received ET ALONE (52% of the total of 500 patients). Patients treated with ET ALONE had worse baseline scores than those treated with S+ET across all domains of the EORTC-QLQ-C30. In the S+ET group, a steeper change in mean scores between baseline and 6 weeks is apparent in the role functioning, social functioning and fatigue domains (Figure 8).
FIGURE 8.
Summary of key changes in QoL during treatment for breast cancer using the EORTC instruments. (a) Role functioning (EORTC-QLQ-C30 domain); (b) social functioning (EORTC-QLQ-C30 domain); (c) fatigue (EORTC-QLQ-C30 domain); (d) arm symptoms (EORTC-QLQ-BR23 domain); (e) breast symptoms (EORTC-QLQ-BR23 domain); (f) systemic therapy side effects (EORTC-QLQ-BR23 domain); (g) burden of illness (EORTC-QLQ-ELD14 domain); (h) joint stiffness (EORTC-QLQ-ELD14 domain); and (i) family support (EORTC-QLQ-ELD14 domain). Surgery took place between baseline and 6 weeks.
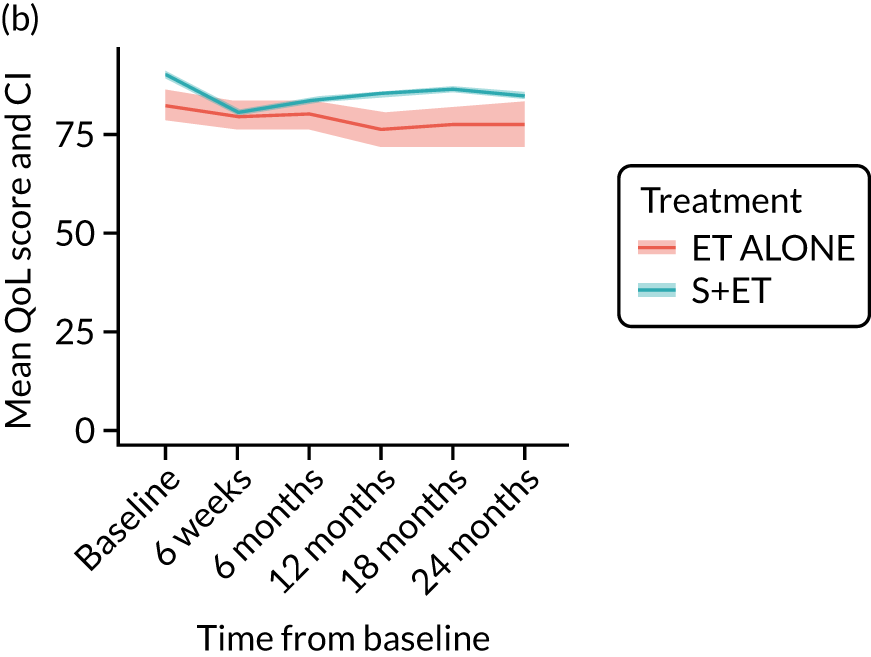
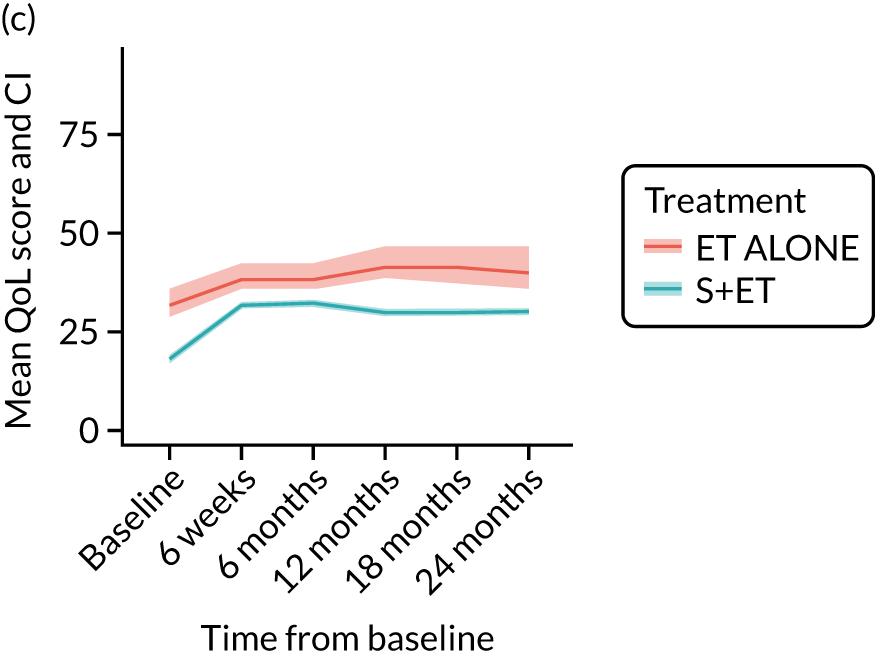
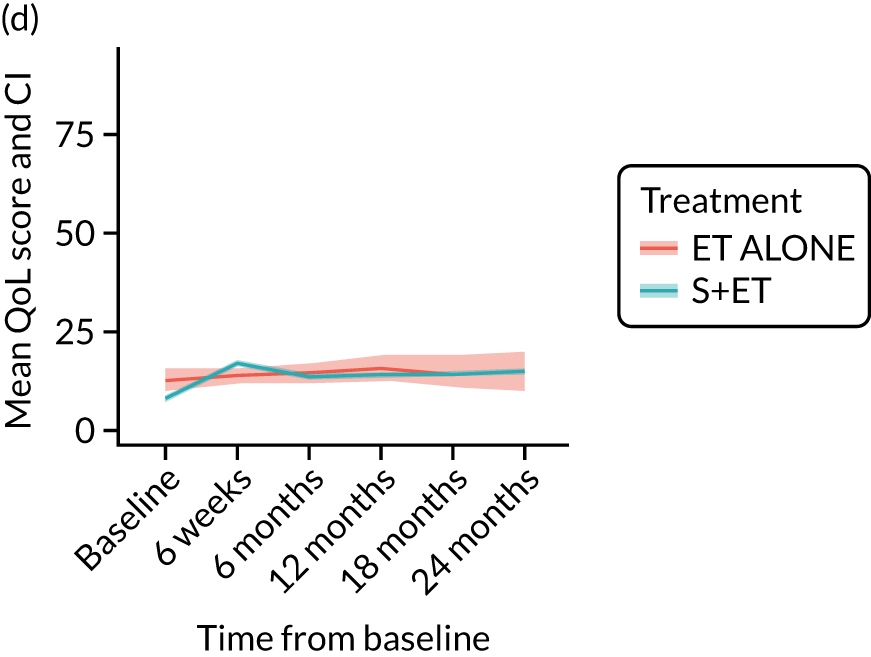
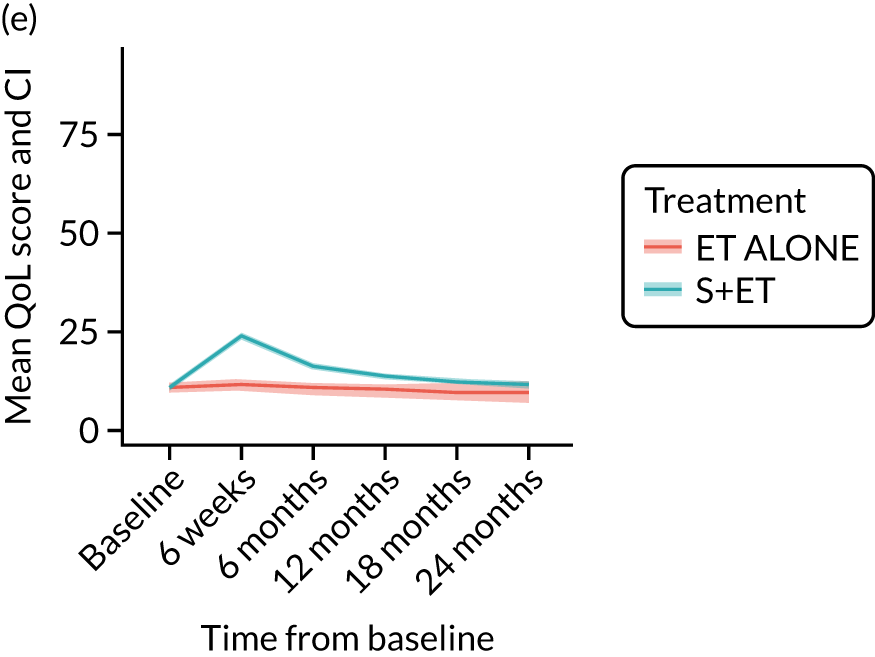
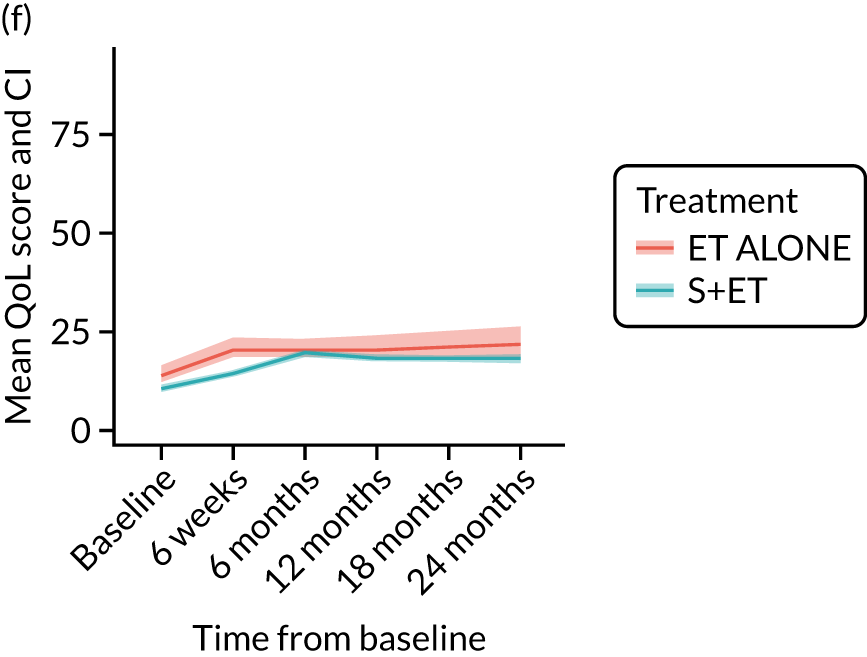
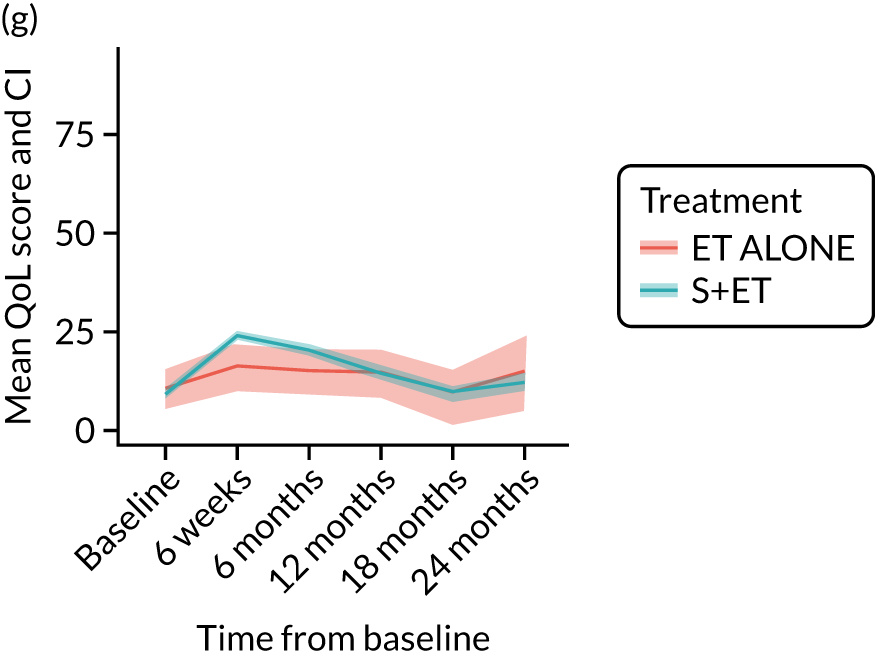
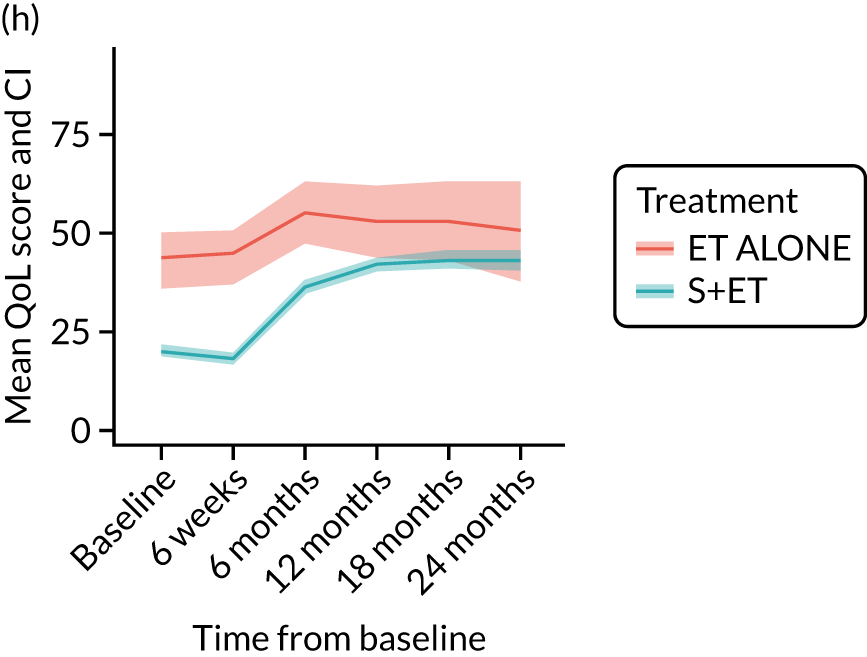
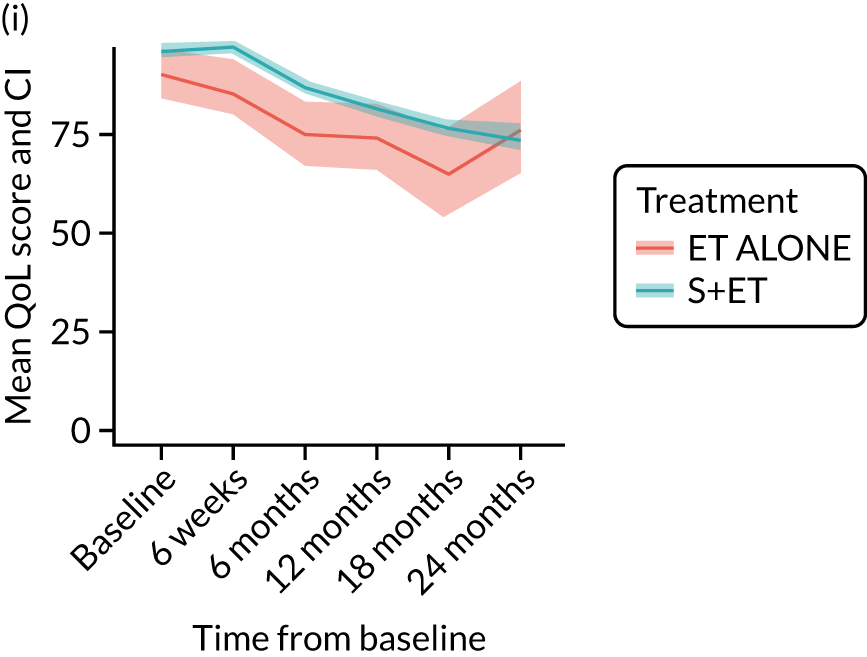
EORTC-QLQ-C30: major or minor surgery (plus adjuvant endocrine therapy) versus endocrine therapy alone analysis (matched)
Analysis according to whether the surgery was major (mastectomy and/or axillary clearance) or minor (wide local excision and/or sentinel node biopsy) or ET ALONE highlighted the impact of major surgery compared with ET ALONE on key domains of the EORTC-QLQ-C30 instrument. Major surgery has a more notable negative impact than ET ALONE on global health status and role function at 6 weeks (HR –9.59, 95% CI –16.96 to –2.21).
EORTC-QLQ-BR23 outcomes: breast-cancer-specific quality-of-life outcomes
EORTC-QLQ-BR-23: unmatched analysis
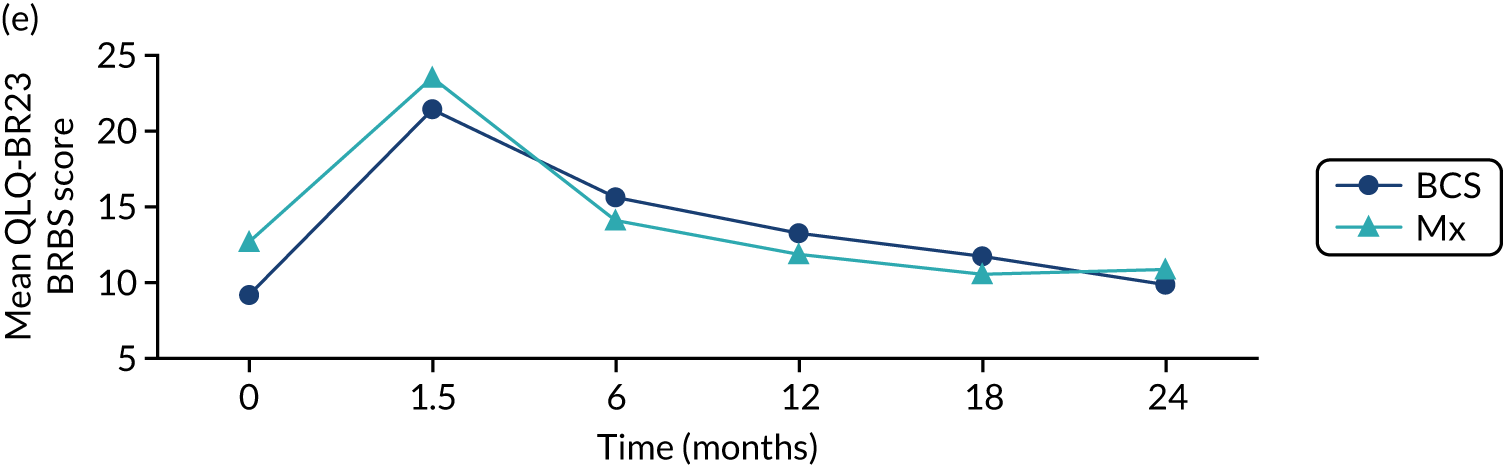
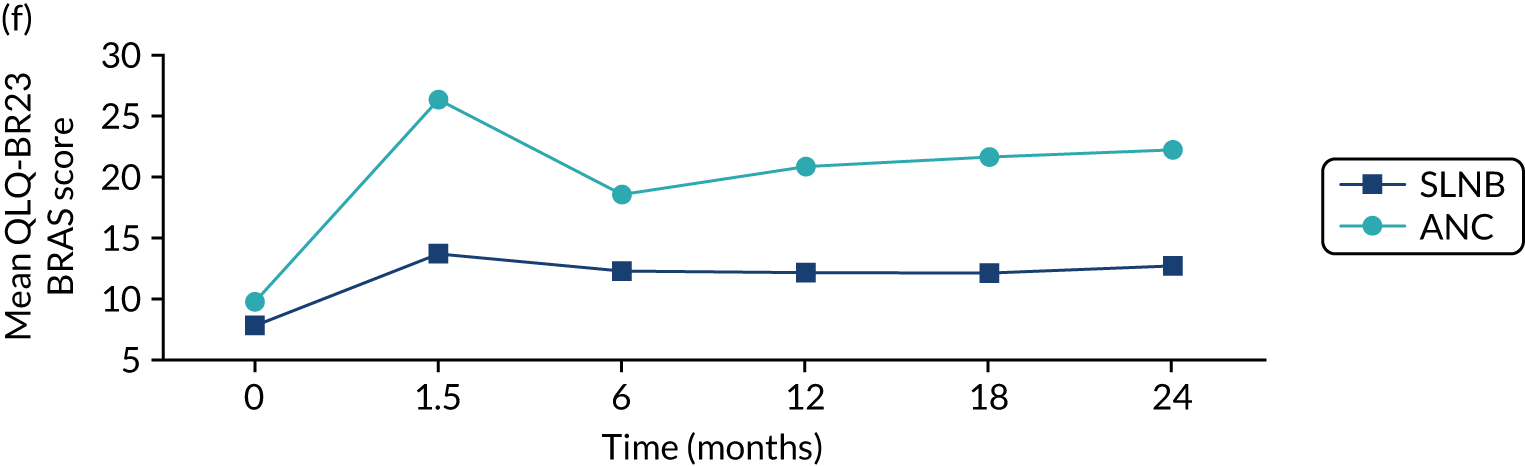
The mean scores for each domain of the EORTC-QLQ-BR23 at each time point (see Figure 8) show that there were several domains in which surgery had an impact (6-week time point). Mean breast symptom scores increased between baseline and 6 weeks for both groups: S+ET patient scores increased from 10.3 [standard deviation (SD) 13.2] to 23.0 (SD 18.8) and ET ALONE patient scores increased from 10.1 (SD 15.7) to 10.8 (SD 15.7). The score for the S+ET group returned to normal by 24 months.
The arm symptom scores increased between baseline and 6 weeks: S+ET patient scores increased from 8.4 (SD 14.1) to 16.6 (SD 18.3) and ET ALONE patient scores increased from 12.5 (SD 18.1) to 14 (SD 18.9). S+ET scores did not return to baseline levels even at 24 months, suggesting a long-term impact of axillary surgery.
For other domains, scores were similar.
EORTC-QLQ-BR23: major versus minor surgery analysis – matched cohort
In the matched cohort, women having major surgery had significantly worse arm symptoms (HR 8.85, 95% CI 3.63 to 14.07) than ET ALONE patients, and the symptoms did not return to baseline even at 2 years. Minor surgery had a lesser effect that, again, persisted to 2 years.
EORTC-QLQ-ELD14 outcomes: older-age-specific quality-of-life domains
EORTC-QLQ-ELD14: unmatched data
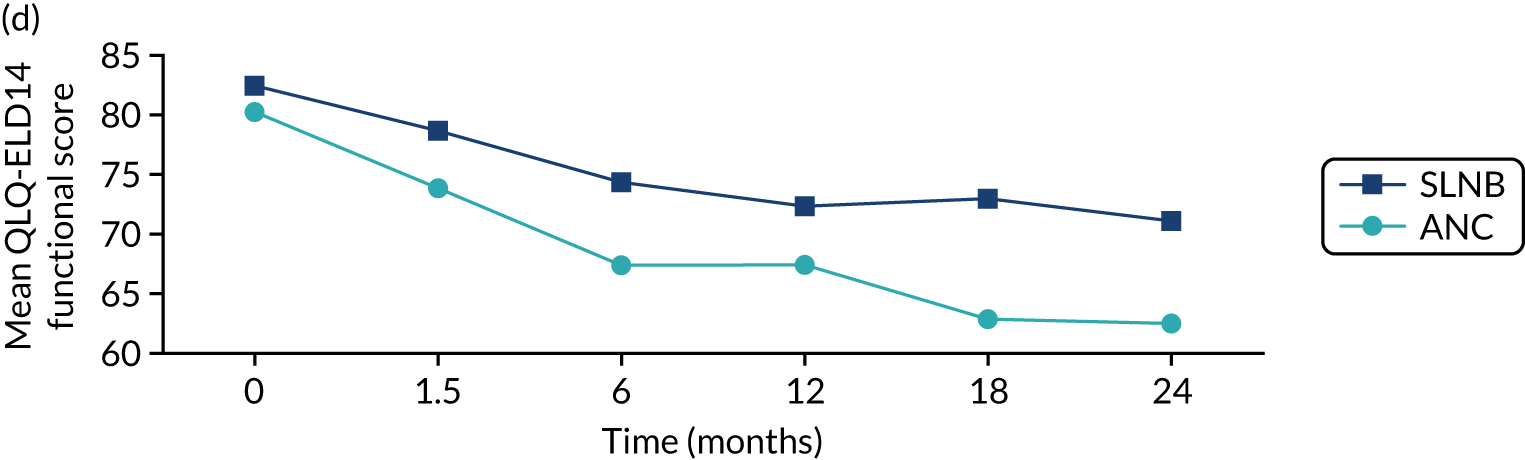
In the unmatched data, differences in baseline scores were apparent for the majority of domains. Following surgery, at the 6-week time point, burden of illness scores increased from 21.1 (SD 23.8) to 25.2 (SD 27.5) in the ET ALONE group, compared with a change from 20.3 (SD 23.5) to 30.4 (SD 24.7) for the surgery group. The burden of illness scores returned to baseline levels in the surgery group by 18 months.
EORTC-QLQ-ELD14: major versus minor surgery analysis – matched cohort
In the matched cohort, comparison of major surgery and ET ALONE caused a significant increase in the burden of illness (HR 7.57, 95% CI 0.58 to 14.56) at 6 weeks that, although it improved with time, did not fully return to normal even at 24 months. By contrast, comparison of minor surgery and ET ALONE had a less marked impact (HR 5.17, 95% CI –1.99 to 12.32), which did return to normal.
EQ-5D-5L and visual analogue scale outcomes
EQ-5D-5L: unmatched data
Analysis of unmatched data by domain shows the impact of surgery on several domains, notably ability to perform usual activities, pain and discomfort, and anxiety and depression. All these scores become more adverse between baseline and 6 weeks, which is when surgery usually occurrs. It is noteworthy that, for all these domains, the scores do not return to baseline levels after this treatment-induced deterioration. In ET ALONE patients, the pattern is a slow decline over the 2-year period.
Discussion
Surgery has a clinically relevant impact on a range of QoL domains. Most of these impacts return to baseline levels by 24 months, but there may be some permanent impairment. In ET ALONE patients, the pattern of changes are those of a slow decline across all domains over the 2-year period. These impacts need to be considered and discussed with older women when deciding treatment options. Considering the survival benefit seen in fitter, older women with breast cancer, for most women, despite the largely transient negative impacts of surgery, surgery will be the better option.
However, for older, frailer women with significant comorbidities, for whom any survival benefit may be small, these QoL impairments become more significant. For some of these women, surgery will have no benefit and impair the quality of their remaining lifespan. Older women need to be made aware of this, and we plan to develop the online decision tool to include QoL data so that this may be factored into the decision-making process.
Adjuvant chemotherapy versus no chemotherapy in women with high-recurrence-risk breast cancer using both unmatched and propensity-score-matched analyses
Introduction
The available evidence relating to adjuvant chemotherapy in older women suggests that there is a reduced level of benefit for older women compared with younger women. Nevertheless, benefit is present in some women aged between 70 and 80 years, although there are very limited data for those aged > 80 years. 69 The objectives of this analysis were to determine health-status-stratified outcomes for older women (aged ≥ 70 years) with breast cancer according to whether or not they received adjuvant chemotherapy. This analysis has been published. 70
Methods
General methods
See The Age Gap prospective observational multicentre cohort study general methods and results.
Statistical analyses for the adjuvant chemotherapy survival analysis
The relationships between systemic therapy use and tumour and patient characteristics were evaluated using univariable and multivariable logistic regression. High-risk breast cancers were defined as nodal involvement, ER–, HER-2+, grade 3, or an Oncotype DX score of > 25.
For both OS and BCSS, a Cox proportional hazards model was fitted using regression-based adjustment based on covariates of treatment; age; categories of the aPG-SGA, ADL, IADL, CCI, MMSE and ECOG-PS; number of medications; and NPI71 and human epidermal growth factor receptor-2 (HER-2) status for all high-risk patients.
In addition, a propensity-score-matched analysis of high-risk patients was performed, with exact matching on NPI and HER-2 status, and logistic regression was used to calculate propensity scores for treatment in relation to age, aPG-SGA category, ADL category, IADL category, MMSE category, CCI category, ECOG-PS category and number of medications.
Results
Characteristics of patients
A total of 1520 out of the 2811 patients who underwent surgery had high-recurrence-risk cancer. Of those, 381 (25%) patients subsequently underwent adjuvant chemotherapy within 6 months. As expected, patients undergoing adjuvant chemotherapy were typically younger and fitter and had cancer with a worse prognosis. The median age was 73 years (IQR 71–76 years) for the adjuvant chemotherapy group and 76 years (IQR 73–80 years) for the no chemotherapy group. The median CCI score was 0 (IQR 0–2) for the adjuvant chemotherapy group and 1 (IQR 0–2) for the no chemotherapy group. Tumour stage was generally higher and tumour biology more adverse in women having adjuvant chemotherapy than woman not having chemotherapy.
Only 150 out of 332 (45.1%) women with HER-2+ cancers received adjuvant chemotherapy plus trastuzumab. Only women with high-recurrence-risk tumours (1520/2811; 54%) were analysed. Of these, 376 (25%) received adjuvant chemotherapy.
Propensity matching
In a propensity-score-matched analysis, 200 patients who received adjuvant chemotherapy were matched to 350 who did not.
Overall survival
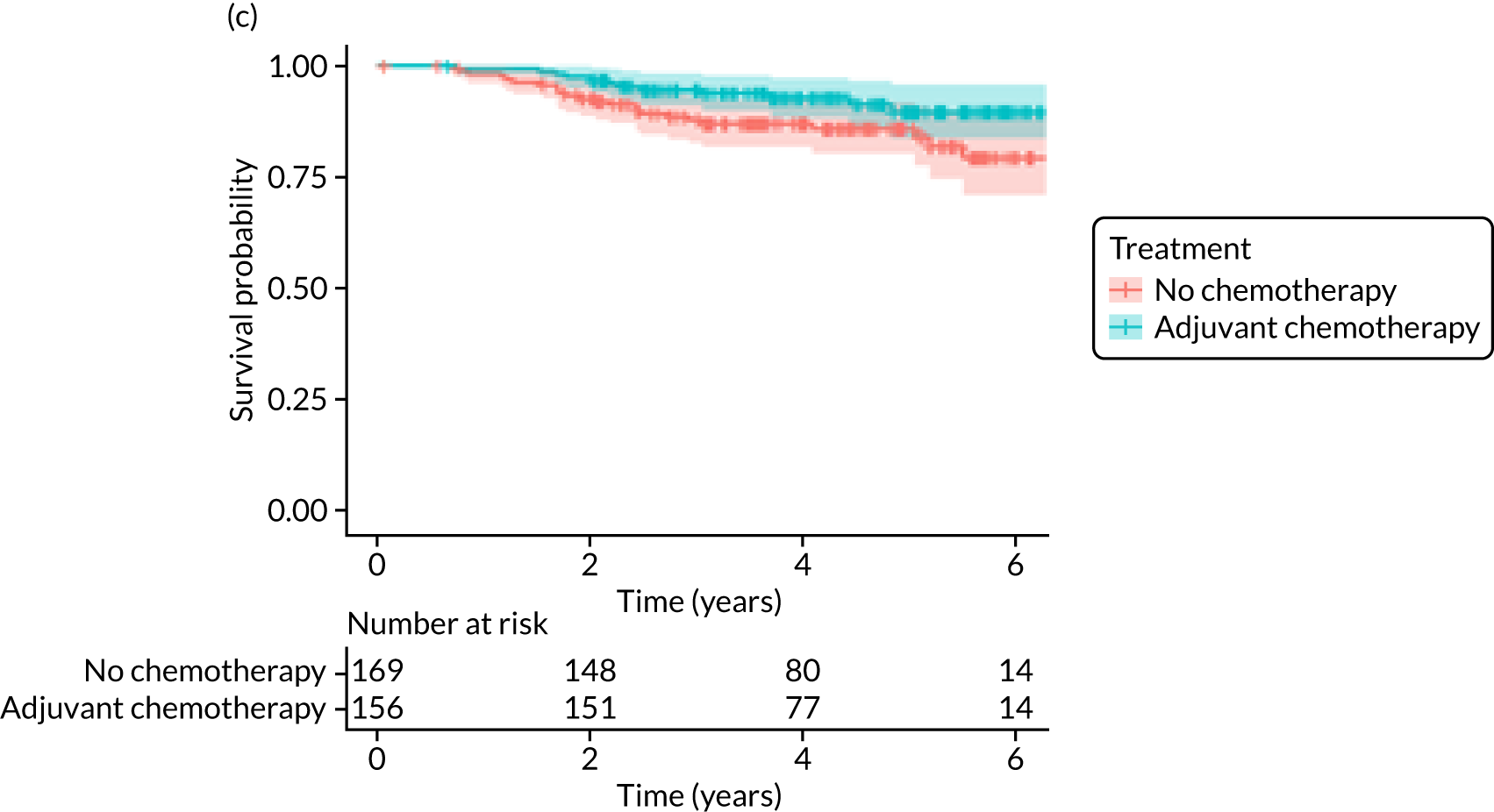
The median follow-up of 52 months is reported for 1495 out of 1520 high-risk patients (adjuvant chemotherapy, n = 371; no chemotherapy, n = 1124). Receiving adjuvant chemotherapy was associated with a longer OS than not receiving chemotherapy, but the difference was not statistically significant when adjusted for other covariates: unadjusted HR 0.545 (95% CI 0.40 to 0.73; p < 0.001) and adjusted HR 0.87 (95% CI 0.58 to 1.28; p = 0.47; Figure 9).
FIGURE 9.
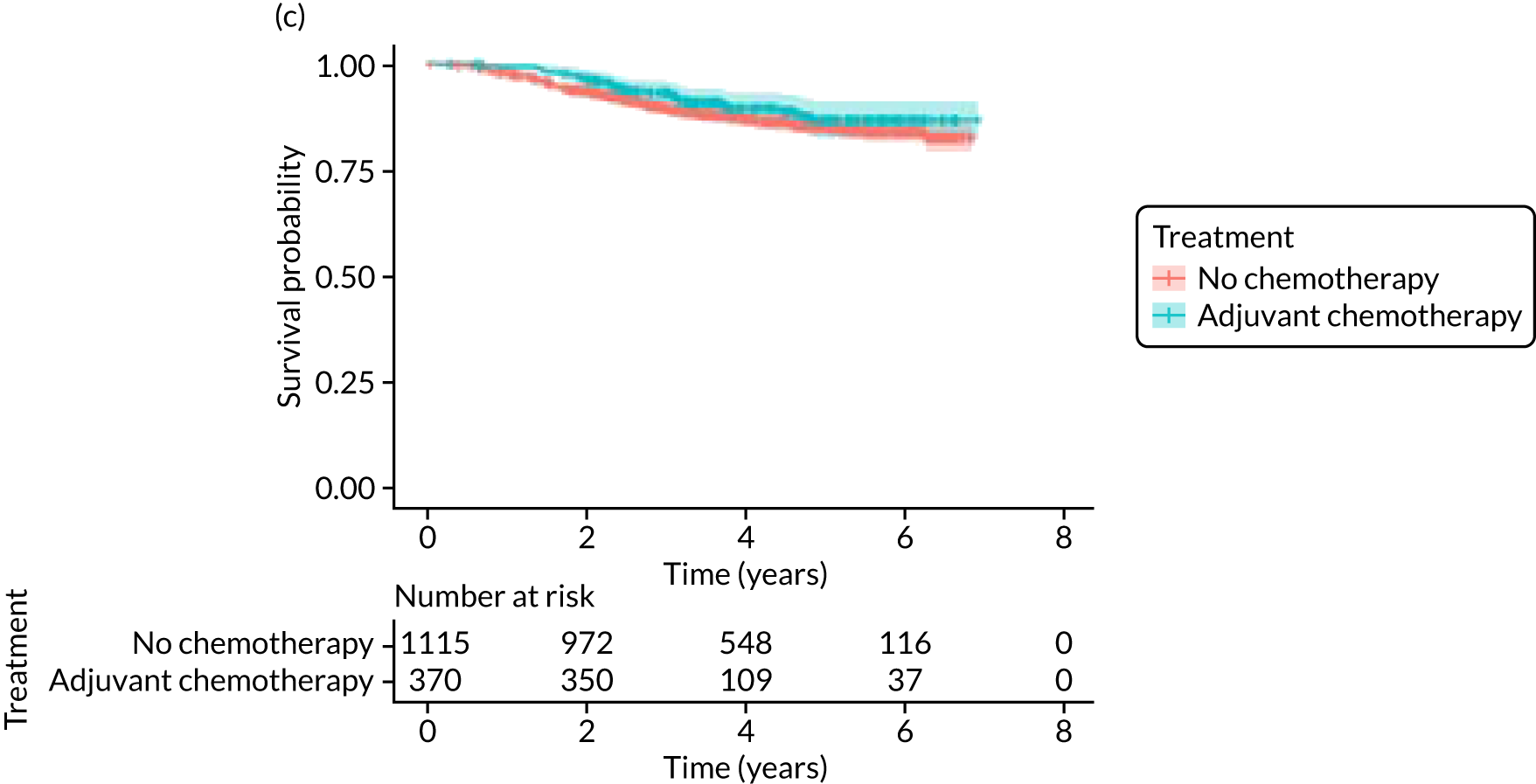
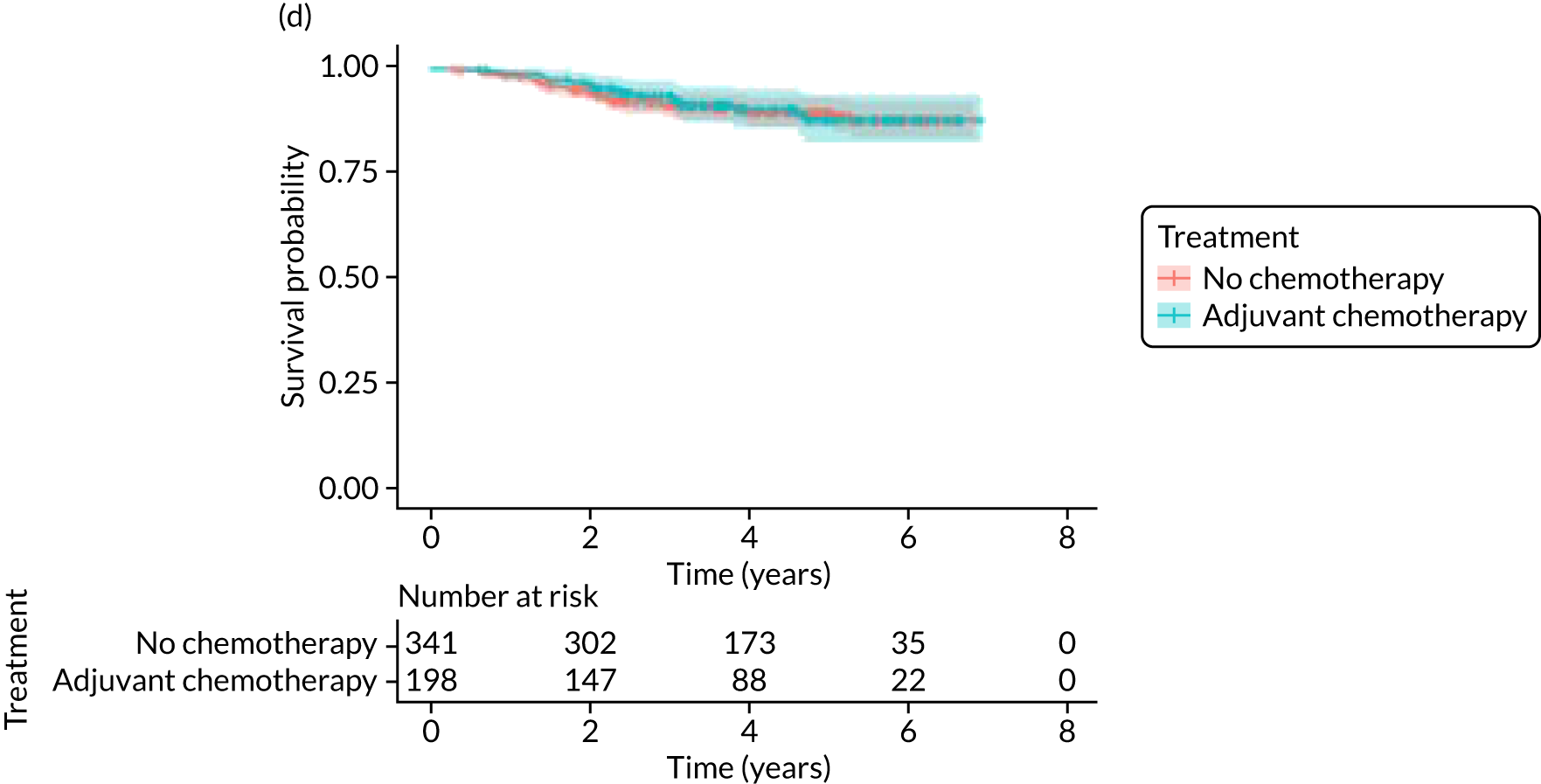
Kaplan–Meier survival curves for adjuvant chemotherapy or no chemotherapy in matched and unmatched groups. (a) OS: unmatched 1; (b) OS: matched 1; (c) BCSS: unmatched 2; (d) BCSS: matched 2; (e) metastatic recurrence-free survival: unmatched 3; and (f) metastatic recurrence-free survival: matched 3.
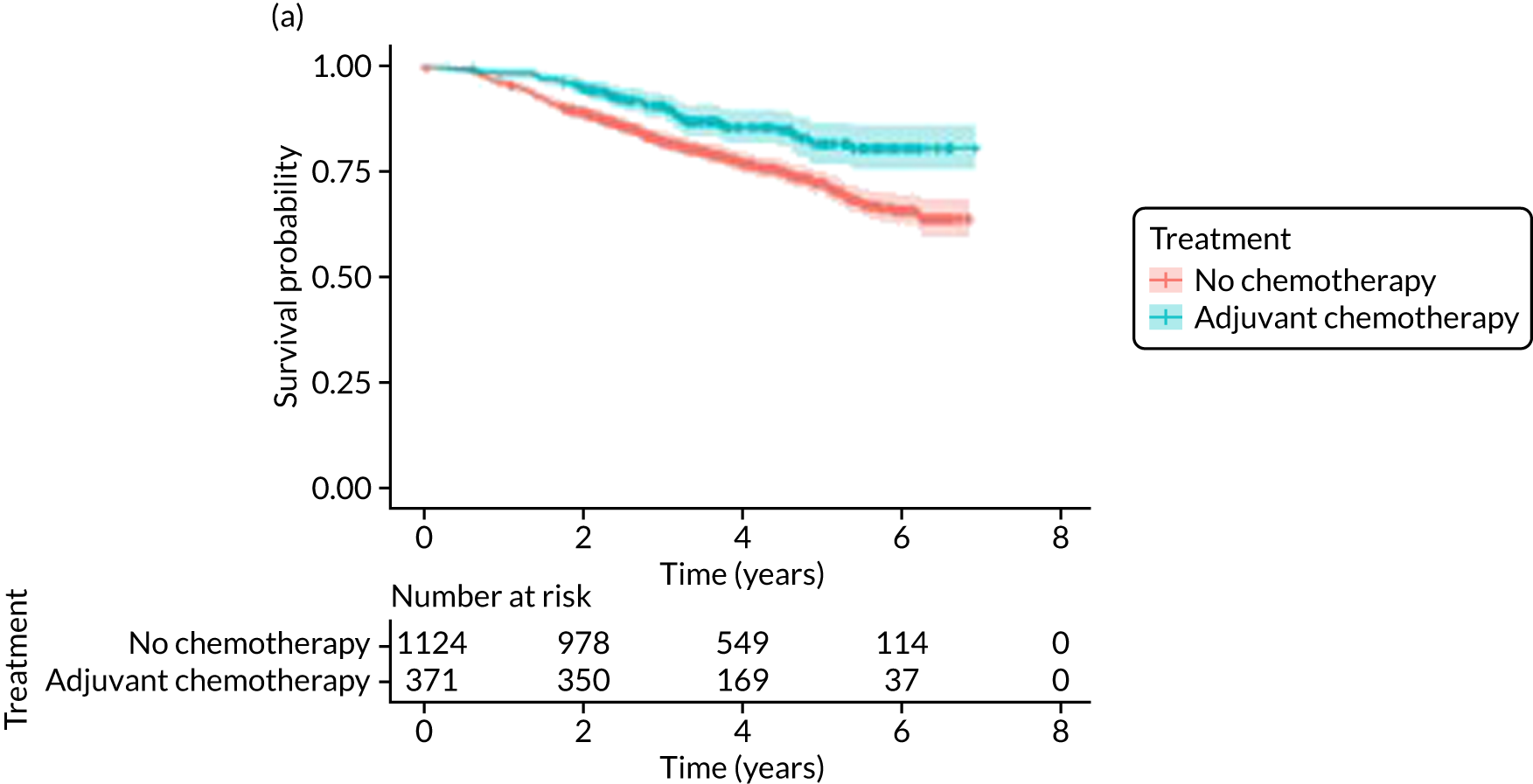
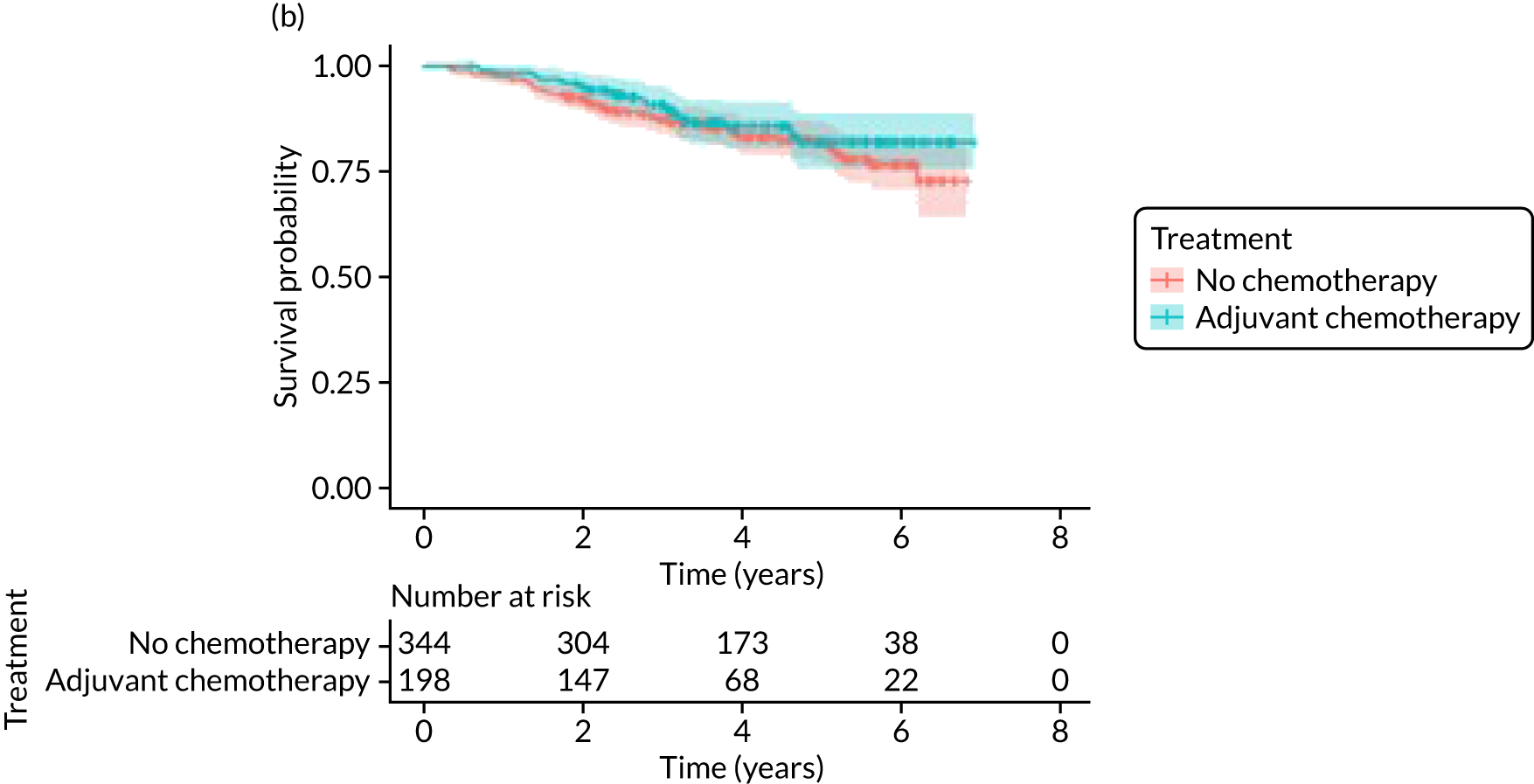
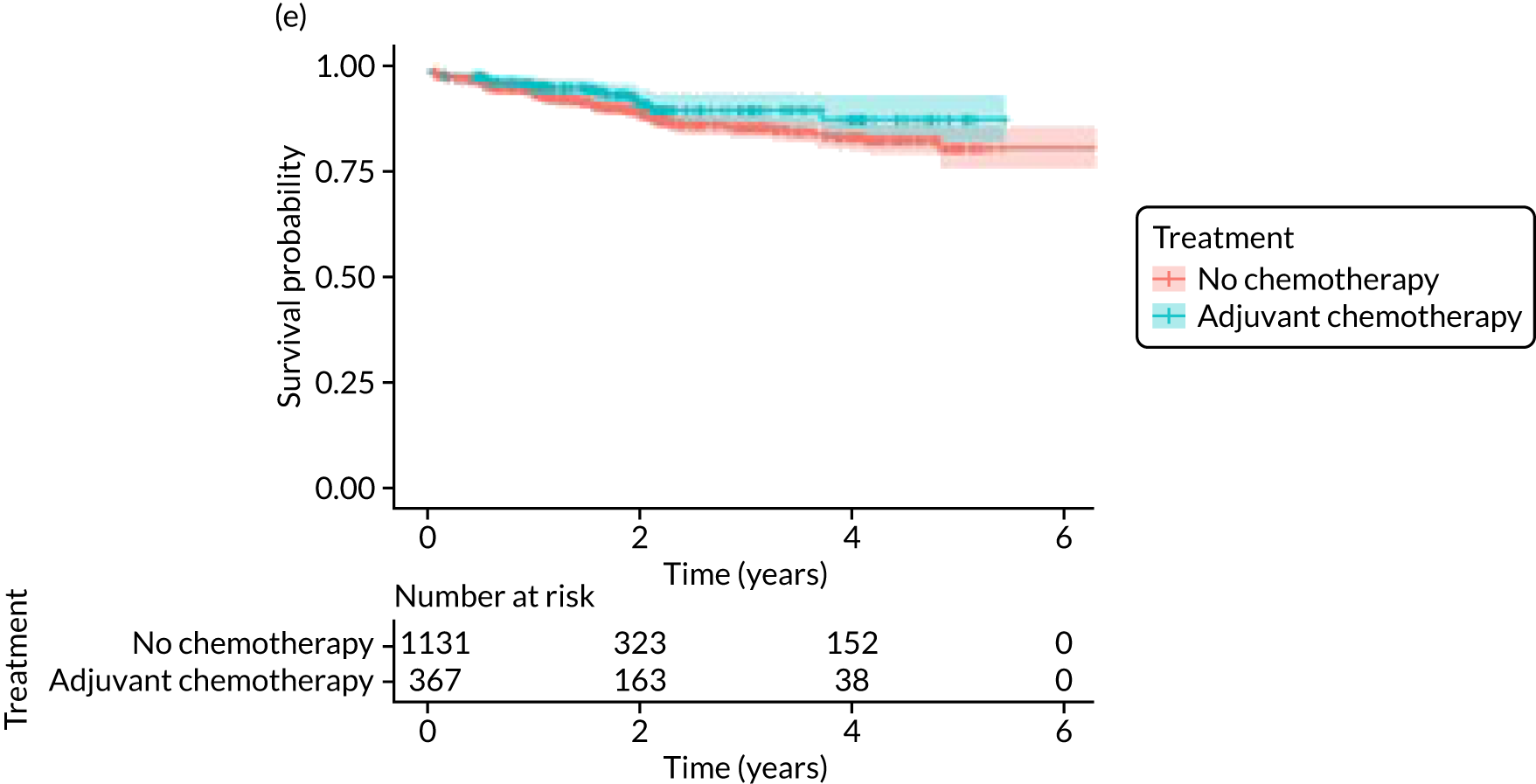
In the matched population, adjuvant chemotherapy was associated with a longer OS than no chemotherapy, although this was not statistically significant: unadjusted HR 0.79 (95% CI 0.50 to 1.3; p = 0.32) and adjusted HR 0.81 (95% CI 0.50 to 1.31; p = 0.38; see Figure 9).
Breast-cancer-specific survival
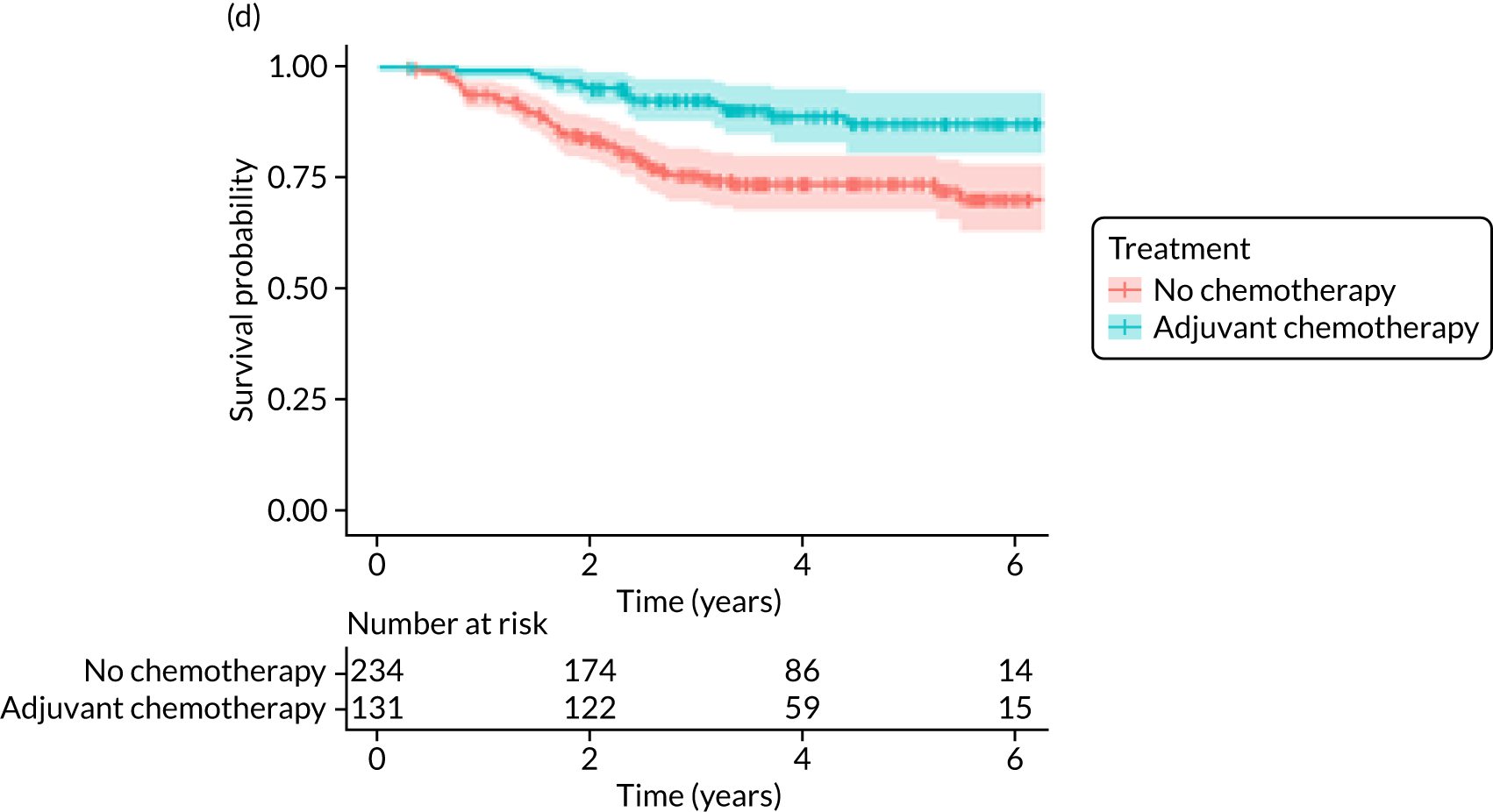
Adjuvant chemotherapy did not improve BCSS in the non-matched (unadjusted HR 0.76, 95% CI 0.53 to 1.1; p = 0.15; adjusted HR 0.92, 95% CI 0.56 to 1.53; p = 0.76) or matched populations (unadjusted HR 0.93, 95% CI 0.52 to 1.66; p = 0.80; adjusted HR 0.92, 95% CI 0.50 to 1.69; p = 0.77; see Figure 9), compared with no chemotherapy.
Metastatic-recurrence-free survival
Adjuvant chemotherapy was associated with a lower risk of metastatic recurrence than no chemotherapy in the unmatched population (unadjusted HR 0.67, 95% CI 0.43 to 1.04; p = 0.08; adjusted HR 0.36, 95% CI 0.19 to 0.68; p = 0.002). In 541 matched patients, adjuvant chemotherapy was associated with a lower risk of metastatic recurrence than no chemotherapy (unadjusted HR 0.53, 95% CI 0.26 to 1.07; p = 0.08; adjusted HR 0.43, 95% CI 0.20 to 0.92; p = 0.03; see Figure 9).
Breast-cancer-subtype-specific analysis of survival
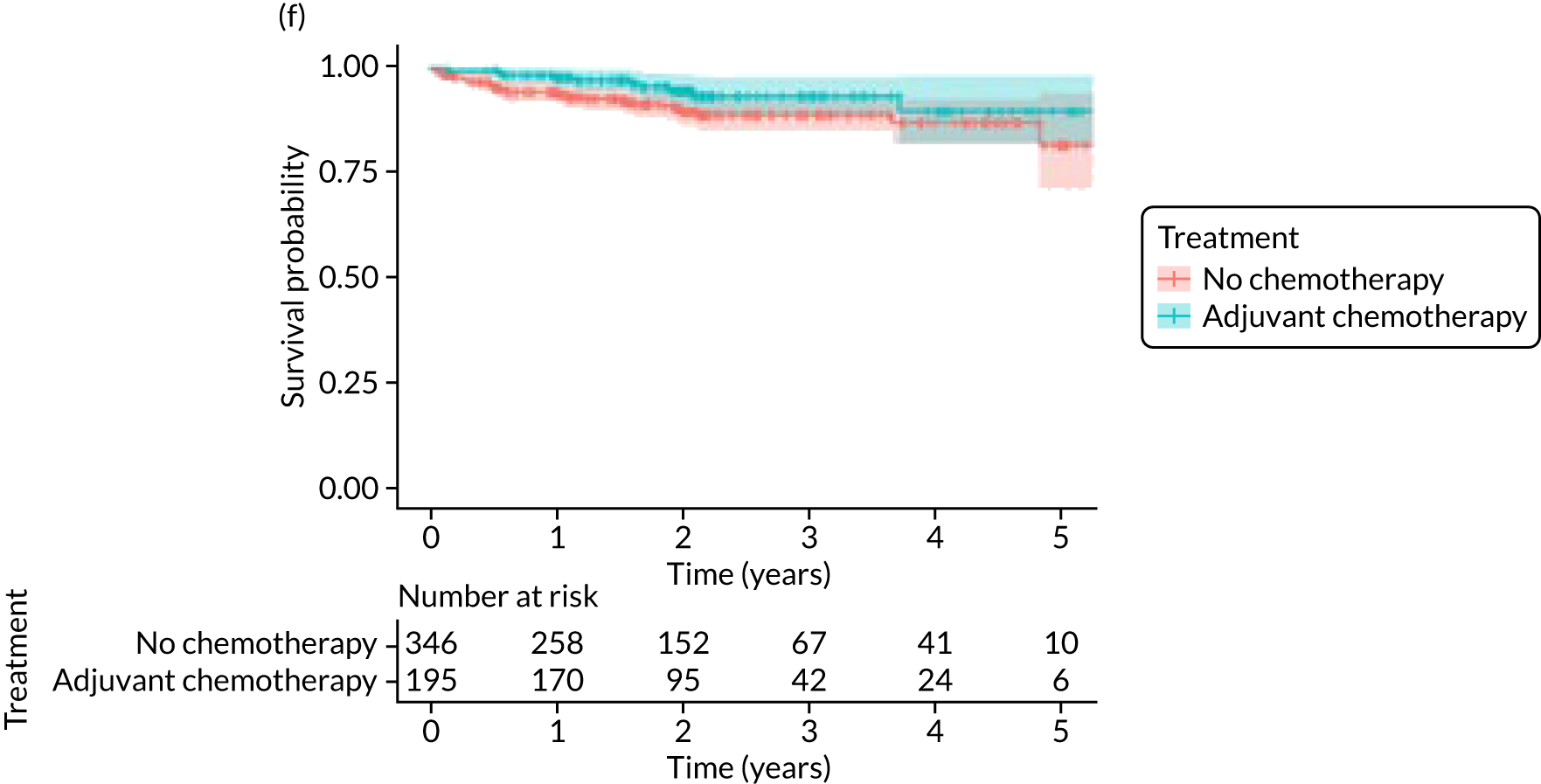
Breast cancers that are ER– and/or HER-2+ are particularly chemosensitive. Therefore, additional exploratory analyses were performed in these subgroups (Figure 10).
FIGURE 10.
Kaplan–Meier survival curves for OS and BCSS in patients treated with adjuvant chemotherapy or no chemotherapy in subgroups with ER– or HER-2+ cancers. (a) OS: HER-2+; (b) OS: ER–; (c) BCSS: HER-2+; and (d) BCSS: ER–.
There were 369 patients with ER– breast cancer with known mortality status, of whom 132 (35.8%) received adjuvant chemotherapy. In a propensity-score-matched analysis of 136 patients, adjuvant chemotherapy was associated with improvements in OS (HR 0.20, 95% CI 0.08 to 0.49) and BCSS (HR 0.12, 95% CI 0.03 to 0.44).
There were 326 patients with HER-2+ breast cancer and known mortality status, of whom 156 (47.9%) received adjuvant chemotherapy with or without trastuzumab. There were fewer deaths from breast cancer and other causes in those who received adjuvant chemotherapy with or without trastuzumab, but, in a matched analysis of 137 patients, the differences were not statistically significant for OS (HR 0.63, 95% CI 0.27 to 1.48) or BCSS (HR 0.50, 95% CI 0.16 to 1.63).
Adjuvant chemotherapy toxicity
Among the 397 patients receiving adjuvant chemotherapy, there was one death (0.25%) due to adjuvant chemotherapy (due to congestive heart failure) and 117 (29.5%) patients had an episode of infection. Among the 144 patients who received trastuzumab, four (2.8%) experienced cardiac failure within the first 6 months and 10 (7%) experienced cardiac failure within the first 12 months.
Discussion
Older women treated with adjuvant chemotherapy are younger and fitter and have higher-risk breast cancer (i.e. higher stage, more adverse biology) than those not treated with chemotherapy. When matching for these baseline characteristics is performed, the OS and BCSS benefits disappear. However, in metastatic-recurrence-free survival, a small difference persists in favour of adjuvant chemotherapy in the population of all women, unselected for tumour biology. This apparent benefit did not translate to a survival benefit at 52 months’ follow-up, but longer-term follow-up will be needed to clarify this. 72
It is recognised that the benefits of adjuvant chemotherapy are small for patients with ER+, human epidermal growth factor receptor-2 negative (HER-2–) breast cancer. This study found a benefit from adjuvant chemotherapy in women with ER– breast cancer, with a reduction in the number of breast cancer deaths. These data are consistent with an analysis of US Surveillance, Epidemiology, and End Results (SEER) data, which suggested that the benefits of adjuvant chemotherapy in older women were restricted to those with ER– breast cancer. 73
These data relate to women aged between 70 and 79 years. No comment can be made about women aged > 80 years, as there were insufficient patients aged > 80 years receiving adjuvant chemotherapy to permit analysis.
It is clear that the risks of adjuvant chemotherapy must be considered and balanced against these survival benefits for certain subgroups of women. The present study found that mortality rates from adjuvant chemotherapy were very low and side effects were consistent with previous analyses in this setting. 18 QoL outcomes are reviewed in Quality-of-life impacts of adjuvant chemotherapy.
Quality-of-life impacts of adjuvant chemotherapy
Introduction
There are few data about QoL outcomes during adjuvant chemotherapy in older women. 74 This study has evaluated QoL in the Age Gap cohort, comparing women who did and did not receive adjuvant chemotherapy. Considering the high priority that older women give to maintaining their QoL, these data are of great clinical value. This article has been published. 75
Methods
General methods
See The Age Gap prospective observational multicentre cohort study general methods and results.
The Age Gap cohort was analysed, comparing women who did or did not have adjuvant chemotherapy after surgery who had a high risk of recurrence. Both matched and unmatched analyses were performed.
Propensity score matching
The matching process is as described in Adjuvant chemotherapy versus no chemotherapy in women with high-recurrence-risk breast cancer using both unmatched and propensity-score-matched analyses.
Quality-of-life assessment
This is as described in Quality-of-life variation between primary endocrine therapy and surgery plus adjuvant endocrine therapy.
Statistical analyses
The analysis was conducted for patients with high-recurrence-risk cancer for whom QoL questionnaires were available. The mean difference (MD) and 95% CI in the domain scores at each time point, adjusted for baseline scores, were calculated using linear regression models.
The effect of adjuvant chemotherapy on global health scores over time for participants considered as being at high risk was estimated using a mixed-effects linear model that allowed for time, treatment, treatment–time interaction and baseline global health status score. The model was fitted to all high-risk patients and to the propensity-score-matched patients only. For the unmatched analysis, the model also adjusted for age and baseline functionality scores.
Results
Cohort characteristics
Women receiving adjuvant chemotherapy were younger and fitter and had higher stage disease and more adverse biology. Consequently, matching was used to correct for this baseline variation. Most adjuvant chemotherapy was administered between baseline and 6 months (n = 393), with only four women having adjuvant chemotherapy between 6 and 12 months. The impact of adjuvant chemotherapy is, therefore, most apparent at the 6-month time point.
Impact of adjuvant chemotherapy on quality-of-life according to adjuvant chemotherapy use (unmatched): EORTC-QLQ-C30
The impact of adjuvant chemotherapy on each domain of the EORTC-QLQ-C30 is shown in Figure 11. As shown in the figure, significant differences in baseline scores between groups were seen and we adjusted for these. The impact of adjuvant chemotherapy was significant on most domains at 6 months, including global health status (MD –9.20, 95% CI –11.95 to –6.44; p < 0.001), physical functioning (MD –8.05, 95% CI –10.21 to –5.89; p < 0.001), role functioning (MD –17.59, 95% CI –21.24 to –13.95; p < 0.001), cognitive functioning (MD –5.55, 95% CI –7.97 to –3.13; p < 0.001), social functioning (MD –18.72, 95% CI –22.17 to –15.27; p < 0.001), and financial problems (MD 3.28, 95% CI 1.16 to 5.39; p = 0.002), all of which largely persisted to 12 months, but had returned to near baseline levels by 18 months.
FIGURE 11.
EORTC-QLQ-C30, EORTC-QLQ-BR23 and EORTC-QLQ-ELD14 selected mean scores and CIs in women treated with adjuvant chemotherapy or no chemotherapy. (a) Global health status/QoL; (b) social functioning; (c) appetite loss; (d) fatigue; (e) body image; (f) systemic therapy side effects; (g) future perspective; (h) upset by hair loss; (i) family support; (j) burden of illness; (k) worries about others; and (l) worries.
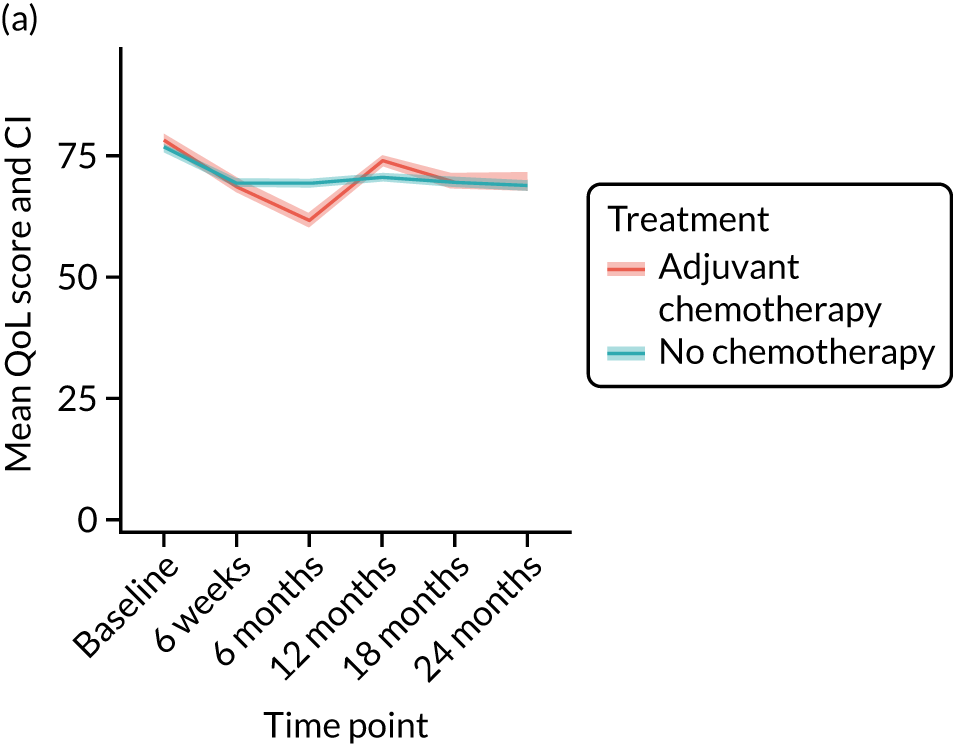
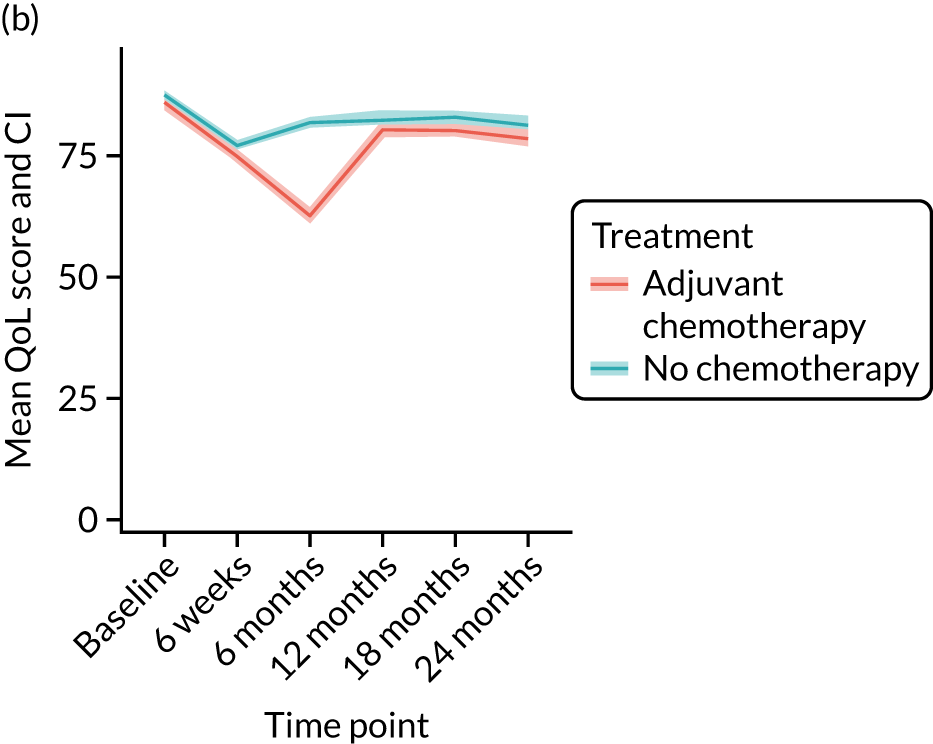
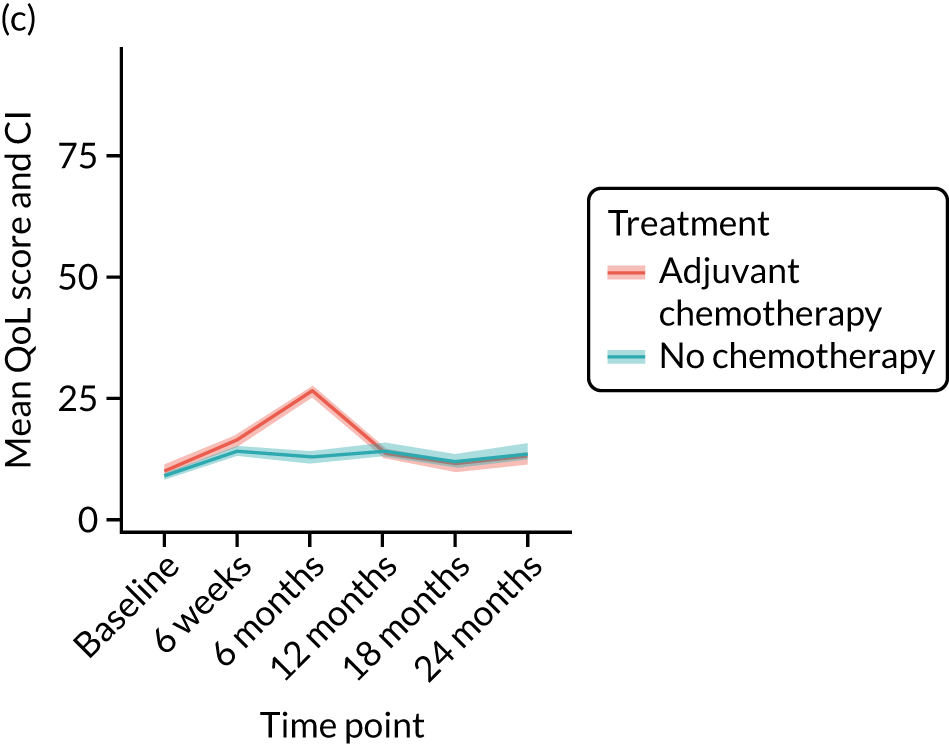
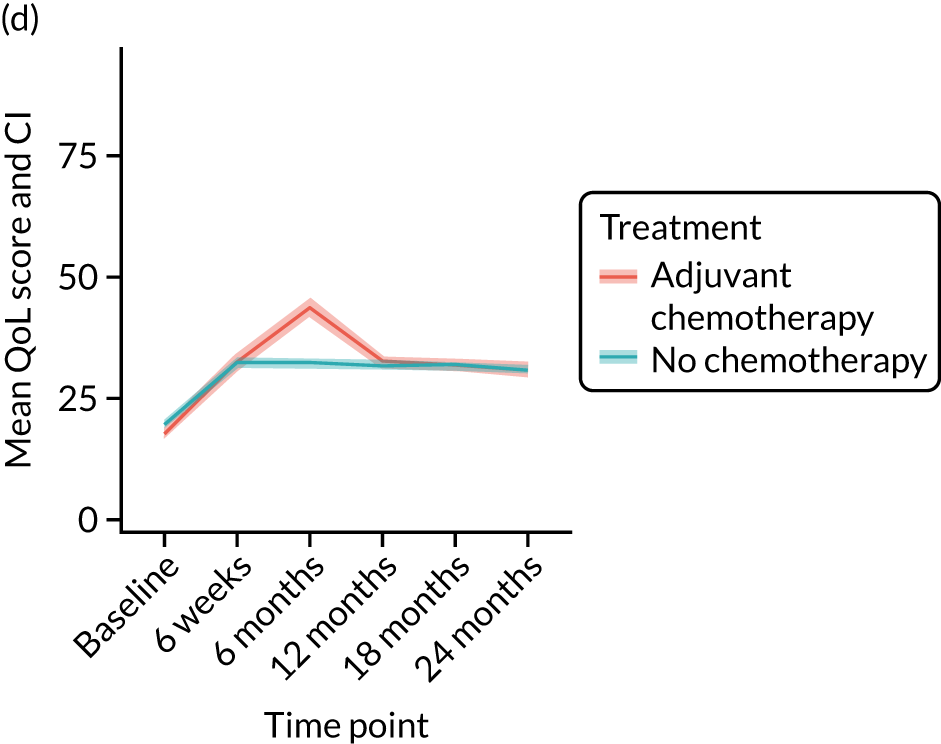
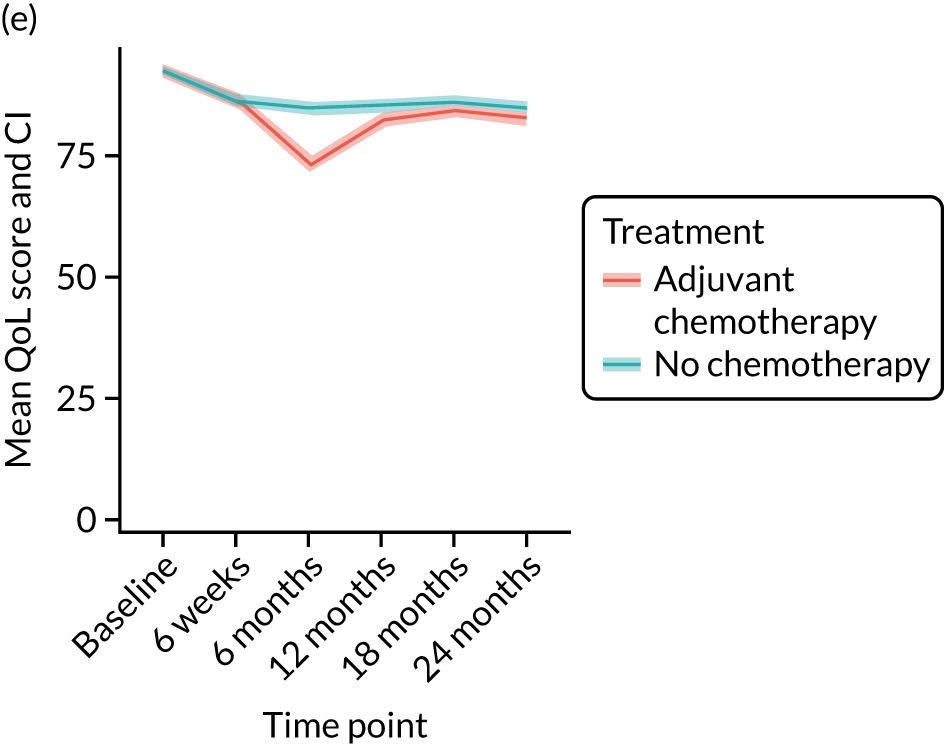
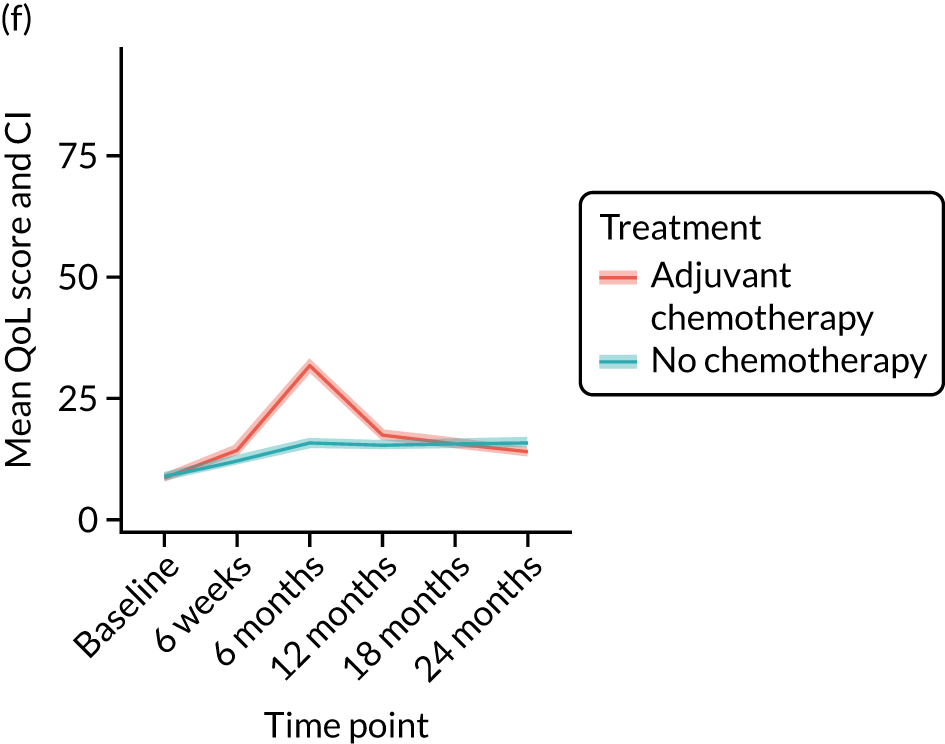
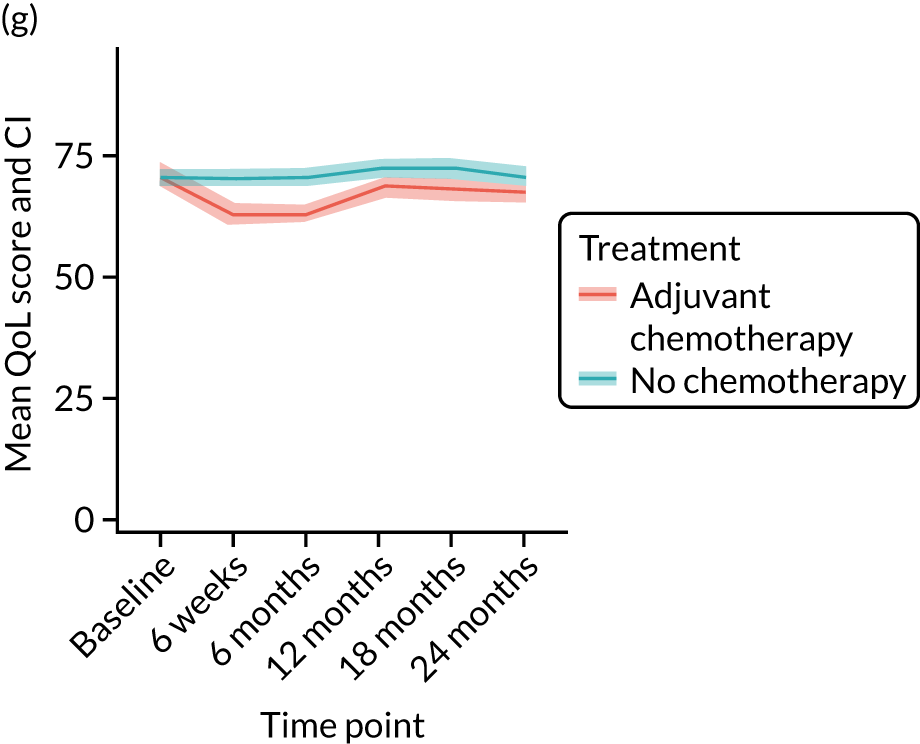
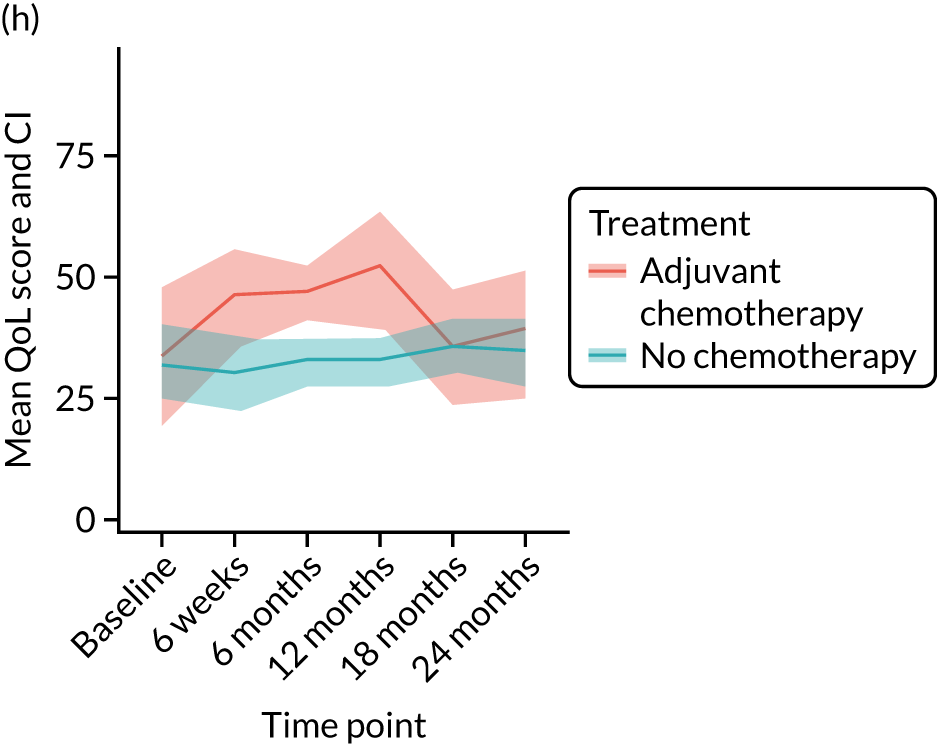
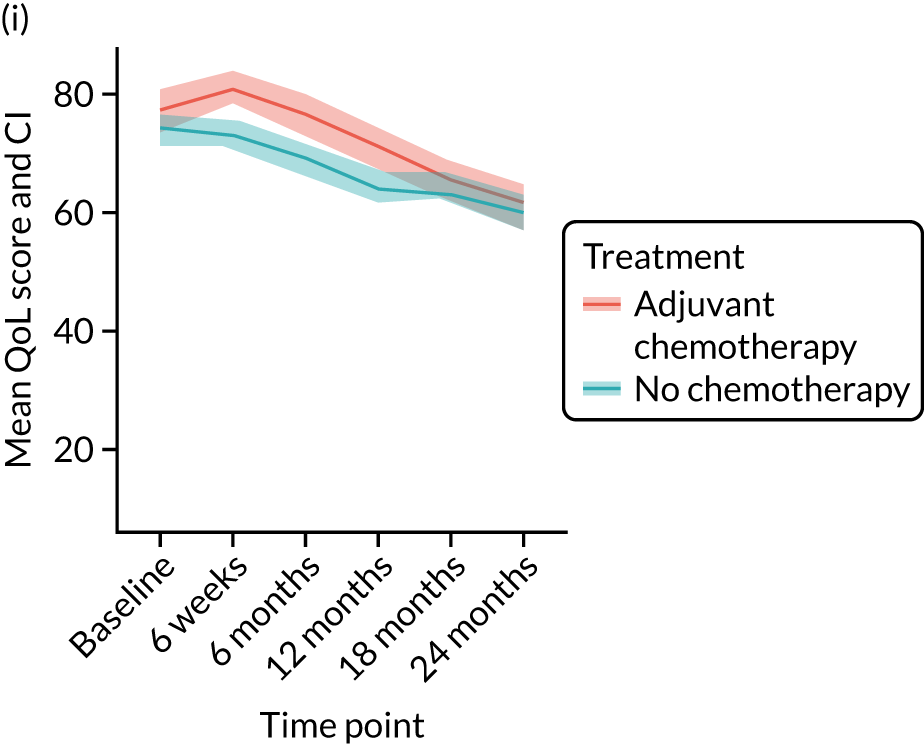
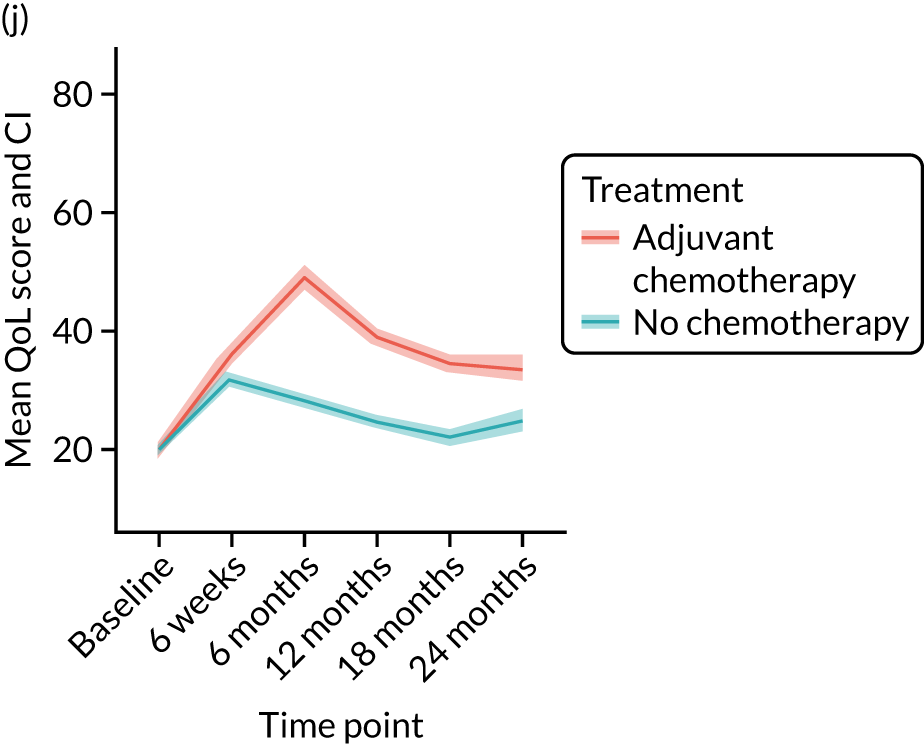
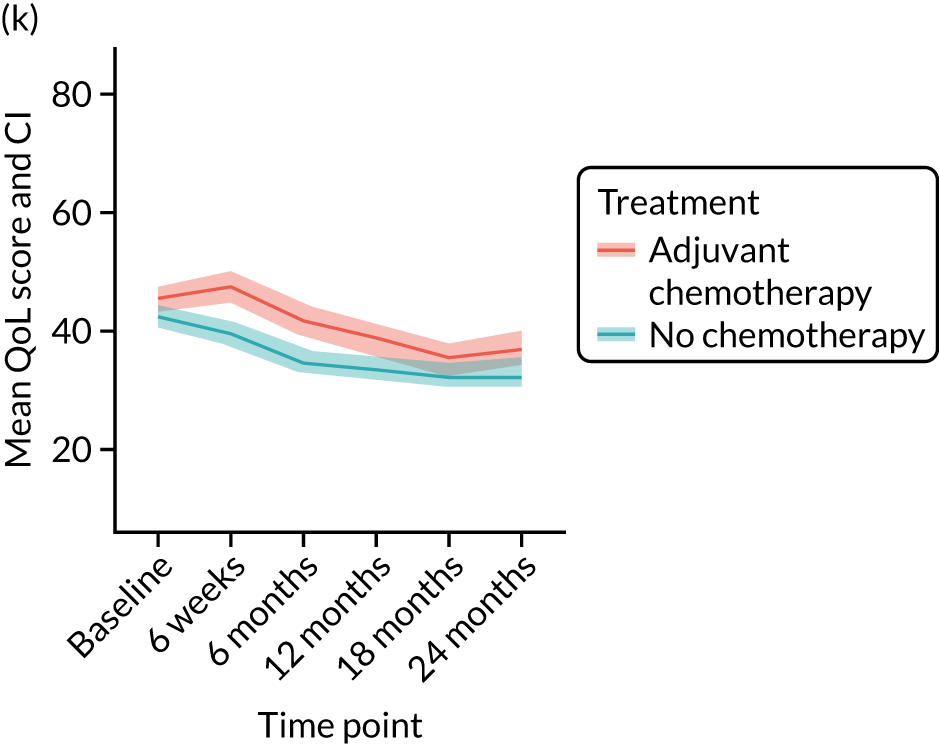
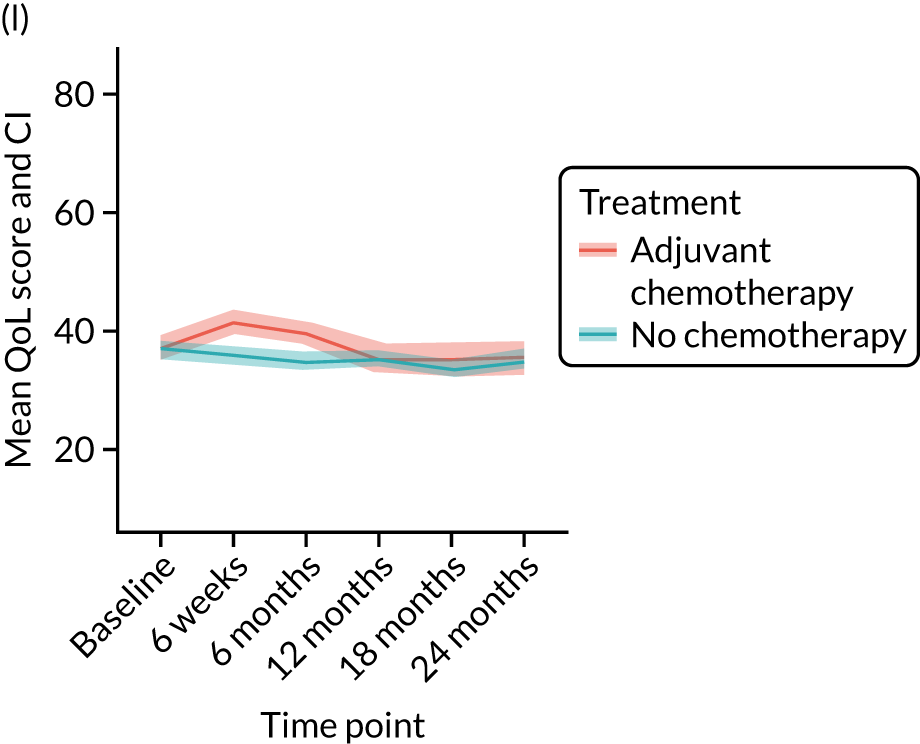
Impact of adjuvant chemotherapy on breast cancer-specific quality-of-life (unmatched): EORTC-QLQ-BR23
After adjustment for baseline measurements, patients receiving adjuvant chemotherapy experienced a significant decline in several EORTC-QLQ-BR23 domain mean scores at 6 months, including future perspectives (MD –7.54, 95% CI –11.28 to –3.80; p < 0.001) and systemic therapy side effects (MD 16.97, 95% CI 15.00 to 18.94; p < 0.001). By 18 months, the differences remained significant for future perspective (MD –4.97, 95% CI –9.37 to –0.57; p = 0.027) and arm symptoms (MD 3.27, 95% CI 0.01 to 6.54; p = 0.049), and at 24 months the difference remained significant for arm symptoms only (MD 4.02, 95% CI 0.13 to 7.9; p = 0.043; see Figure 11).
Impact of adjuvant chemotherapy on older adult-specific quality-of-life (unmatched): EORTC-QLQ-ELD14
Scores were significantly different between patients receiving and patients not receiving adjuvant chemotherapy in most domains at 6 months (worries about others: MD 6.19, 95% CI 2.44 to 9.95; p = 0.001; worries: MD 4.18, 95% CI 0.89 to 7.46; p = 0.013; burden of illness: MD 21.60, 95% CI 17.82 to 25.39; p < 0.001), and the impact on mobility also became significant (MD 9.82, 95% CI 6.87 to 12.78; p < 0.001). Burden of illness was the only domain significantly influenced to 18 months (MD 12.99, 95% CI 8.81 to 17.17; p < 0.001) and 24 months (MD 8.80, 95% CI 3.93 to 13.66; p < 0.001; see Figure 11).
Impact of adjuvant chemotherapy on global health domains (unmatched): EQ-5D-5L
The mean scores on the visual analogue scale were significantly worse in patients receiving adjuvant chemotherapy than in those not receiving chemotherapy at 6 months only (adjusted MD –6.57, 95% CI –8.74 to –4.40; p < 0.001); changes were no longer significant at the remaining time points.
Conclusions
Adjuvant chemotherapy has a clinically and statistically significant, largely transient, negative effect on many domains of QoL and this must be taken into consideration when advising patients about whether or not to undergo adjuvant chemotherapy. As seen in the adjuvant chemotherapy survival analysis, survival benefit is largely seen in women with adverse biology (ER– disease), aged between 70 and 79 years. For women outside this age range and with ER+/HER-2– disease, the risk-to-benefit ratio must be carefully considered. It is clear that, for women with adverse tumour biology, in whom a survival benefit may be obtained, these ‘costs’ may be justified. Overall, the impacts of adjuvant chemotherapy are transient, but there is still some residual negative impact at 24 months that may never fully resolve. Older women considering adjuvant chemotherapy must be made aware of the potential survival benefits of adjuvant chemotherapy, and also the potential risks and negative QoL impacts.
Analysis of the impact of cognitive impairment on outcomes for older women with early-stage breast cancer
Background
One of the key subgroups of women considered for non-standard care are those with cognitive impairment. Rates of dementia are increasing as population ageing occurs, and severe dementia is a significant cause of death. The management and outcomes for women with dementia and breast cancer were of interest. This work has been published. 76
Methods
General methods
See The Age Gap prospective observational multicentre cohort study general methods and results.
Cognition categorisation
Cognition status was identified in the following ways:
-
Proxy consent (the patient was assented to the study by a consultee). These women were assumed to have severe cognitive impairment.
-
Self-consenting patients –
-
Cognition status indicated by completion of the CCI, which has a field for dementia (yes/no). The majority of these women also completed a MMSE, but, if this was absent, they were categorised as having mild cognitive impairment.
-
Cognition status indicated by participant completion of the MMSE, which has the categories cognition as normal (score of 27–30), mild cognitive impairment (score of 21–26), moderate cognitive impairment (score of 11–20) and severe cognitive impairment (score of 0–10).
-
Data analysis
Overall survival and BCSS were compared for patients with and without cognitive impairment by matching. Two matching approaches were used:
-
matching with up to three non-impaired patients who had the same category of NPI (risk categories ≤ 3.4, > 3.4 to ≤ 5.4, > 5.4), oestrogen receptivity and treatment (surgery and ER+, surgery and ER–, or ET ALONE and ER+), and age to within a calliper width of 1 year (approximately one-sixth of a SD)
-
matching on NPI category, oestrogen receptor (ER) category and treatment, and a more detailed propensity score, including functionality (the ADL, IADL and ECOG-PS), nutrition (the aPG-SGA) and comorbidity (CCI score, excluding dementia and age), to a calliper of 0.015 propensity score units (≈ 0.2 SD), with up to two matches allowed.
The comparisons were quantified using Kaplan–Meier curves and Cox regression, presented for unadjusted and matched analyses; matching was accounted for by use of shared frailty terms.
Results
Patient characteristics
Cognitive impairment was associated with greater age, reduced levels of physical functionality (lower median ADL and IADL score) and higher rates of comorbidity than no cognitive impairment. Tumour size was also slightly larger in women with cognitive impairment than in those without.
Treatment allocation
Treatment allocation showed a larger percentage of women with cognitive impairment were treated in a non-guideline concordant manner than women without cognitive impairment. Treatment with S+ET decreased from 84% for those with normal cognition to 39.8% for those with severe cognitive impairment (p < 0.001). Rates of adjuvant chemotherapy and radiotherapy halved for those with severe cognitive impairment compared with those with normal cognition.
Survival outcomes
Overall survival at the median 52 months’ follow-up reduced from 81% for women with normal cognition to 36% for those with severe dementia. BCSS also decreased from 40.6% for women with normal cognition to 14% for those with severe dementia. However, because of the heterogeneity of patient characteristics (age, treatment, health status, physical function, tumour size), these differences cannot be ascribed to dementia. On matching, when adjusted for age, treatment type and physical function, the significance of the variation in OS was reduced, but not fully eliminated. The effect of these adjustments on BCSS and progression-free survival (PFS) were no longer significant. This suggests that dementia is a significant predictor of all-cause mortality, but not disease-specific mortality (Figure 12).
FIGURE 12.
Kaplan–Meier survival curves for women with or without dementia, with both unmatched and matched cohorts. (a) OS: survival proportion; (b) OS: matched on age, treatment and NPI; (c) OS: matched on age, treatment, NPI and functionality; (d) BCSS: unmatched; (e) BCSS: matched on age, treatment and NPI; and (f) BCSS: matched on age, treatment, NPI and functionality.
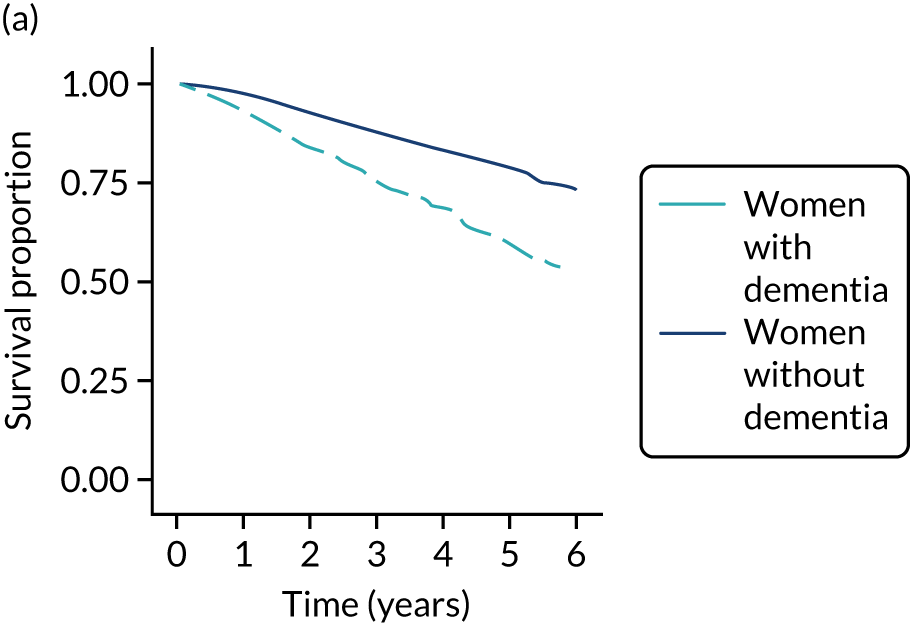
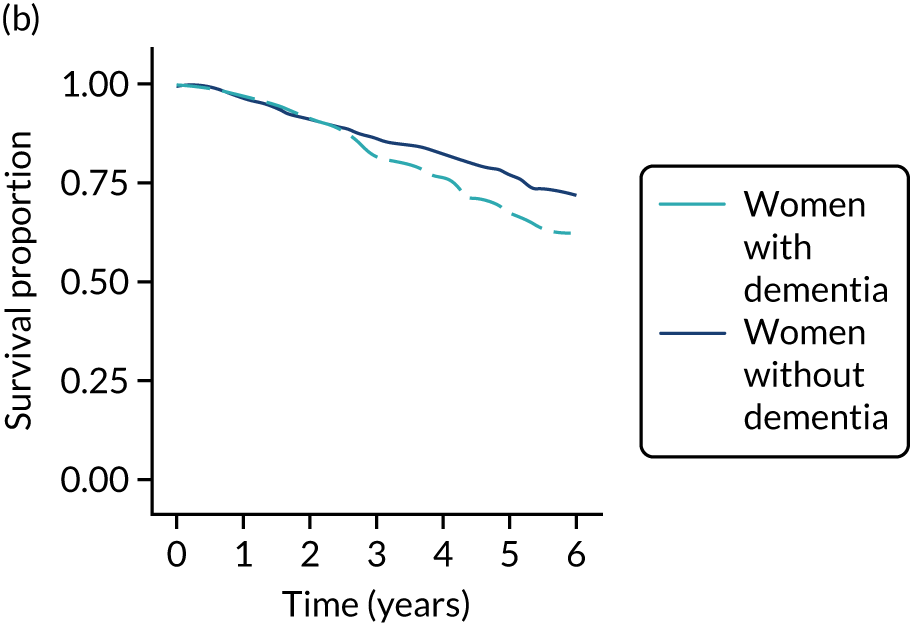
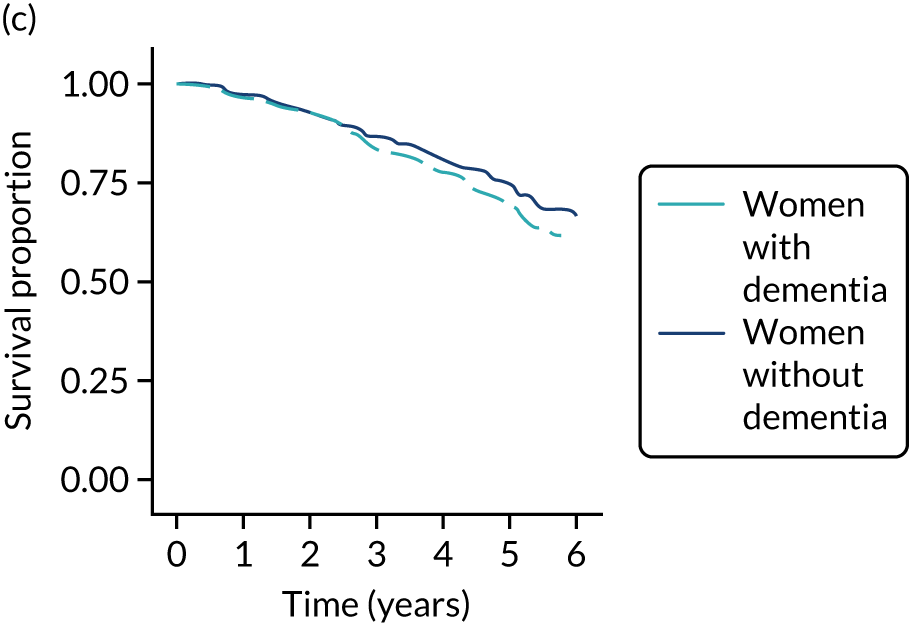
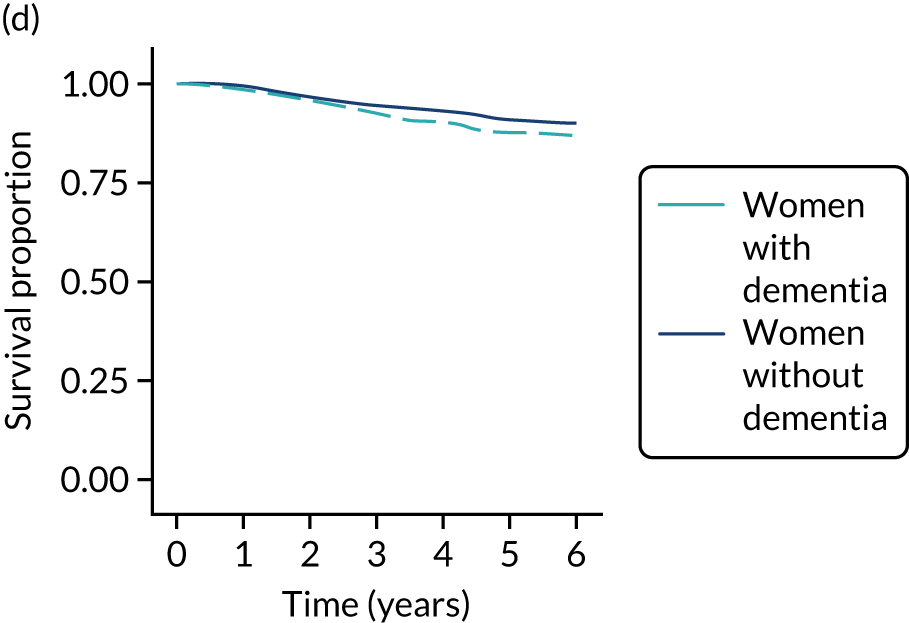
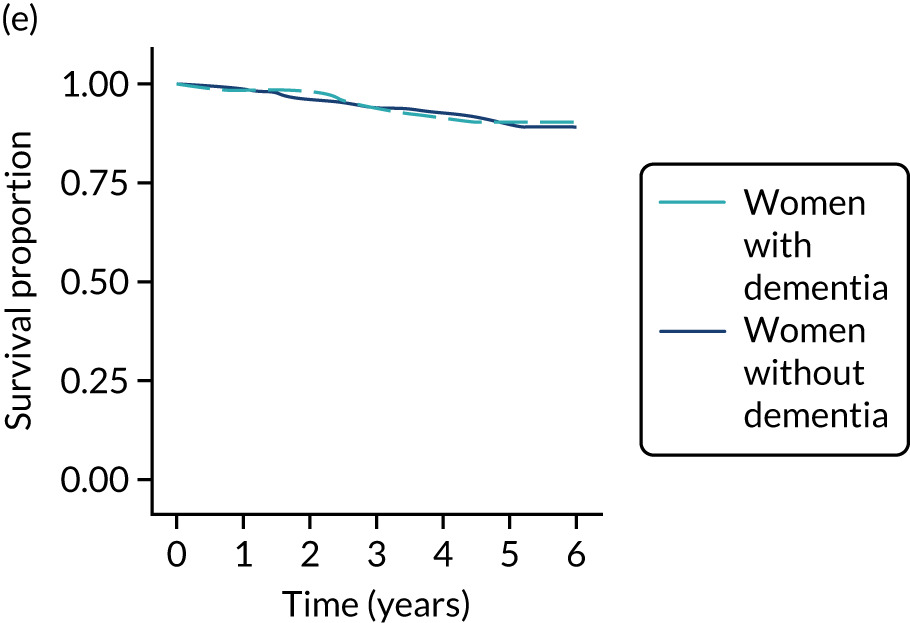
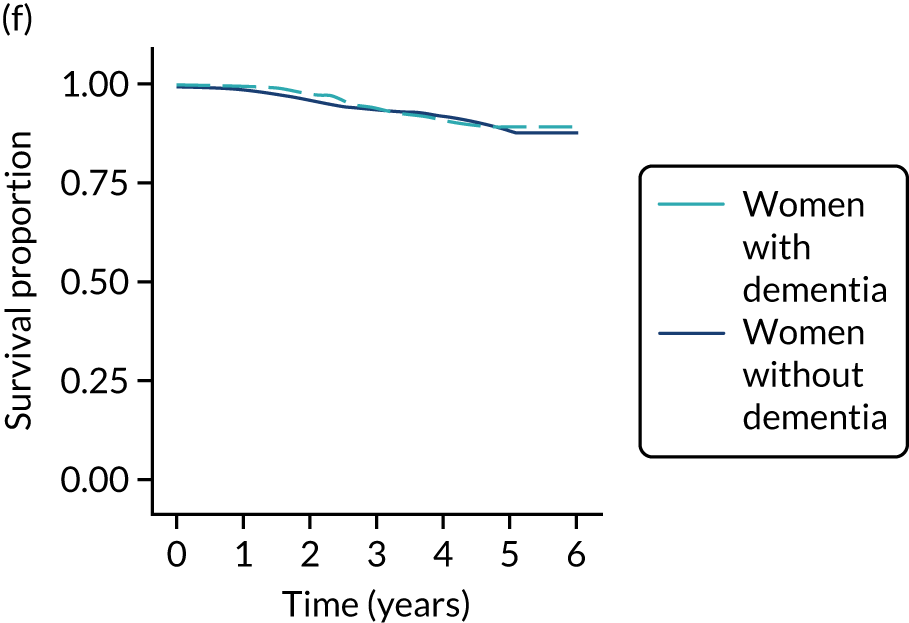
Conclusions
This analysis has demonstrated variation in the treatments that older women with cognitive impairment receive compared with those that women with normal cognitive function receive. The association is confounded by the link between older age and a higher rate of comorbidity in women with cognitive impairment. The use of ET ALONE is higher in women with cognitive impairment, particularly those with severe impairment.
Reasons for the lower rates of surgery in the cognitively impaired groups compared with the non-cognitively impaired groups may include clinicians offering ET ALONE because of a perceived risk, patient and carer preferences for low-risk treatment options, and the patients’ carers’ wish to optimise QoL. Other factors may be clinician preference, and the complexities of surgical consent in patients with cognitive impairment.
There is no difference in the rates of progression-free survival between the groups at 52 months’ follow-up. These data suggest that non-standard treatment may be appropriate for some older women with severe dementia, whose life expectancy is likely to be short, and that women with mild and moderate dementia are still likely to benefit from standard care. Dementia does not appear to be an independent predictor of inferior cancer outcomes.
Cost-effectiveness analysis comparing surgery plus adjuvant endocrine therapy with primary endocrine therapy
Introduction
The objective of this study was to use the Age Gap data, combined with other evidence, in a de novo cost-effectiveness model, using individual-level simulation in a partitioned survival model framework, to compare S+ET and ET ALONE. This analysis has been published. 77
Methods
Model structure
A partitioned survival model was developed in the open-source software package R, version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria). 78 The primary analysis was a cost–utility analysis, which estimated the lifetime health outcomes achieved, measured in QALYs and the health service costs incurred for patients receiving the two treatment options (S+ET vs. ET ALONE). The incremental cost-effectiveness ratio (ICER) for S+ET compared with ET ALONE for different patient age, health and disease subgroups was calculated.
The OS curves and causes of death for the ET ALONE and S+ET arms were modelled using a model previously developed for prediction of survival in the online Age Gap Decision Tool for the ET ALONE versus S+ET decision. 32 To power the patient-subgroup-specific cost-effectiveness analysis, separate survival curves were produced for patients aged 70, 80 and 90 years; for comorbidity scores of 0, 1, 2 and ≥ 3; for both positive and negative lymph node status; and for each treatment (i.e. 48 survival curves). A weighted average was calculated over the other patient, cancer and treatment characteristics included in the prognostic model, for which the weights were determined using the appropriate proportions of the characteristics in the cohort study data44 or published sources. Treatment costs were taken from published sources. Discounting was applied at 3.5% per annum for both costs and QALYs.
Health utility measurement
Health utility was based on the EQ-5D-5L. Utilities were drawn from the literature. Utilities were adjusted for age,79 comorbidity,80 and lymphoedema. 81 Short-term decrements after surgery (3 months)82 and for patients receiving adjuvant chemotherapy (12 months) were also applied.
Results
Surgery plus adjuvant endocrine therapy delivered more QALYs than ET ALONE, and was also cheaper, for a fit, 70-year-old, node-negative patient, that is, S+ET dominates ET ALONE for this subgroup. The ICER remained below £10,000 for most patient subgroups, and increased for the oldest, least fit patients only. S+ET was also cost-effective compared with ET ALONE at a threshold of £20,000 for all patient groups, except for patients aged ≥ 90 years, with a comorbidity score of 2 who were node negative, and for all patients aged ≥ 90 years with a comorbidity score of ≥ 3. The cost-effective benefit of S+ET reduced significantly with increasing age and rates of comorbidity.
Conclusions
This study shows that the cost-effective benefit of S+ET reduces with age and comorbidities, and, ultimately, becomes non-cost-effective in an unfit population aged ≥ 90 years. This correlates with the Age Gap survival analyses (see Endocrine therapy alone versus surgery plus adjuvant endocrine therapy: survival analysis).
Cluster-randomised controlled trial
The developed DESIs were tested in a cluster-randomised controlled trial nested within the cohort study. 44 The primary outcome was QoL of all patients, whether or not the tools were used, on the basis that the quality of decision-making would alter, potentially altering the wider allocation of therapies. Treatment allocation of all patients was compared to assess the impact of the DESIs on rates of surgery and adjuvant chemotherapy. The methods for this study have been published. 44 The results of the trial have also been published. 83
Introduction
There are no information resources specifically tailored to older women or the breast cancer treatment choices they may face. We have developed and piloted a series of DESIs specifically to support decision-making in older women with breast cancer,27,31 and have used a cluster-randomised controlled trial methodology to determine their impact on shared decision-making and in improving treatment allocation and QoL in older women.
Methods
Study design
This was a prospective, parallel-group, cluster-randomised controlled trial to compare the use of a DESI with usual care for older women with breast cancer. The trial was nested within the cohort study (see The Age Gap prospective observational multicentre cohort study general methods and results). Randomisation (stratified by high or low rates of ET ALONE or adjuvant chemotherapy) was between:
-
usual care – usual decision-making practice for older women (aged ≥ 70 years) with breast cancer
-
intervention – optional use of the DESIs (option grid, information booklet and online algorithm, plus training in their use) to support decision-making.
Trial interventions
The DESIs comprised:
-
two brief decision aids84 (see Appendices 4 and 5)
-
two booklets providing information about both S+ET versus ET ALONE and adjuvant chemotherapy versus no chemotherapy (see Appendix 6)
-
an online tool from which a patient-facing personalised section could be printed off to give specific estimates of the risks and benefits of each treatment based on personal and cancer characteristics (see Appendix 7)
-
a clinician training package.
Aims
-
To enhance the level of patient participation and decision quality for older women with breast cancer. The DESIs focused on two specific choices: (1) use of S+ET versus ET ALONE for older women with ER+ cancers and (2) use of adjuvant chemotherapy or no chemotherapy for older women with high risk cancers.
-
To improve and standardise clinician management of breast cancer in older women by using an online algorithm.
Outcomes
Primary outcome
The primary outcome was global QoL (questions 29 and 30 of the EORTC-QLQ-C30) at 6 weeks and 6 months after diagnosis.
Secondary outcomes
The secondary outcomes were decision quality and regret, anxiety, and knowledge.
Other outcomes
Other outcomes were:
-
OS and BCSS
-
treatment allocation between usual-care and intervention sites
-
a detailed process evaluation (see Process evaluation).
Clinical trials registration number
This trial is registered as ISRCTN46099296.
Baseline data collection
In addition to the data items collected for the cohort study (see The Age Gap prospective observational multicentre cohort study general methods and results), the following were also collected:
-
decision regret scale at 6 weeks and 6 months85
-
CollaboRATE score at baseline86
-
Spielberger State-Trait Anxiety Inventory score at 6 weeks and 6 months87
-
knowledge and treatment preference, measured using a bespoke questionnaire, at baseline
-
brief illness perception questionnaire at 6 weeks and 6 months88
-
coping [using the brief Coping Orientation to Problems Experienced inventory] at baseline, 6 weeks and 6 months. 89
Follow-up assessments
Patients were followed up at 6 weeks, and at 6, 12, 18 and 24 months. Decision-quality measures were recorded for patients who had relevant treatment decisions offered to them. Data were collected regarding whether or not a choice was offered and whether or not the DESIs were used. If a treatment choice was offered, decision quality and regret were measured.
Participating UK breast units
The study recruited from 46 breast units (see Appendix 8, Table 5).
Inclusion and exclusion criteria
These were identical to the cohort study (see The Age Gap prospective observational multicentre cohort study general methods and results).
Training package on use of the decision support interventions
All participating units were offered training for the study. In addition, training materials were available on the study website, including an educational video.
Sample size calculation
The sample size calculation was based on the primary end point: global health status QoL score (questions 29 and 30 of the EORTC-QLQ-C30) at 6 months. It was assumed that 50 breast units would be randomised and recruit a set number of women per cluster,90 based on a mean QoL score of 58.2, with a SD of 21, and a clinically significant difference set at 7 points (of medium clinical relevance). 91
Statistical analysis
Cluster-randomised trial analysis
A marginal generalised linear model, with coefficients estimated using generalised estimating equations, with robust standard errors and an exchangeable autocorrelation matrix, was used to analyse the outcomes and allow for the clustered nature of the data.
Other outcomes
The following summary statistics were reported overall and by intervention group (DESI intervention or usual care):
-
number and cause of deaths
-
number receiving ET ALONE, S+ET, adjuvant chemotherapy or no chemotherapy
-
OS at 6 months.
Survival analysis
Kaplan–Meier curves were derived for each intervention group (DESI or usual care) and compared using multivariate modelling (Cox’s proportional hazards model).
Results
Recruitment and patient characteristics
Patient flows are shown in Figure 13. There were 46 sites recruiting to the RCT (intervention, n = 21; usual care, n = 25) between December 2015 and June 2018.
FIGURE 13.
Cluster-randomisation and patient recruitment summary.
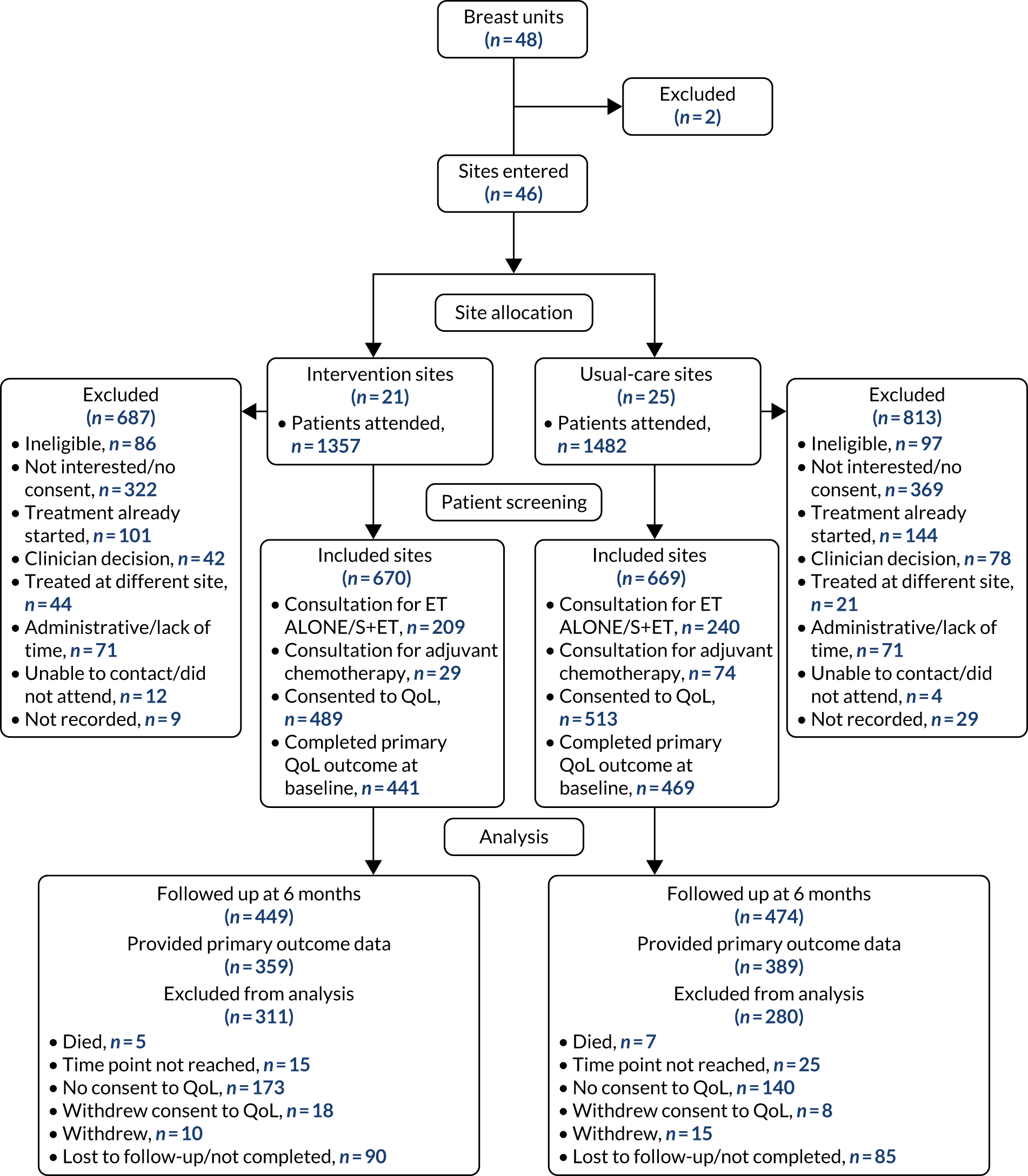
The median follow-up duration was 36 months. The baseline characteristics of the two arms were well matched.
Training in and use of the decision support interventions in intervention sites
A total of 229 health-care professionals at intervention sites received training in using the DESIs.
Usage data from the online algorithm recorded 494 individual log-ins.
A total of 326 women were offered a choice of ET ALONE or S+ET.
Primary outcome: quality-of-life at baseline, and at 6 weeks and 6 months after diagnosis
Quality-of-life scores
There were no differences in baseline global health status; any domain of the EORTC-QLQ-C30, EORTC-QLQ-BR23 or EORTC-QLQ-ELD14 at baseline; or scores at 6 weeks or 6 months in the intention-to-treat (ITT) analysis.
In the per-protocol analysis (including women offered the relevant treatment choice only), a small but significant difference was seen at the 6-month time point, with a mean global health status score (intervention: mean score 70.7, SD 17.4; usual care: mean score 66.8, SD 20.1; p < 0.04).
Secondary outcomes
Treatment allocation
Overall, 21% (123/591) of patients with an ER+ tumour underwent ET ALONE at intervention sites, compared with 15% (88/570) at usual-care centres (difference 5.5%, 95% CI 1.1% to 10.0%; p = 0.02). Similarly, uptake of adjuvant chemotherapy was lower in intervention sites than usual-care sites [10% (69/670) vs. 15% (99/669), respectively; difference –4.5%, 95% CI –8.0% to 0.0%; p = 0.013]. Comparison with historical practice in each intervention centre confirmed that this change in practice was genuine using locally weighted scatterplot smoothing (LOWESS) curves.
Kaplan–Meier survival outcomes
Survival
The median follow-up was 36 months. Survival was similar in the two arms, with an estimated 2-year survival of > 90% in both arms and a HR of 1.07 (95% CI 0.80 to 1.43; p = 0.633) in favour of the usual-care arm (Figure 14). Of the 184 deaths (intervention, n = 94; usual care, n = 90), two-thirds were unrelated to breast cancer, giving a HR for BCSS of 0.88 (95% CI 0.54 to 1.44; p = 0.609) in favour of the intervention arm (see Figure 14). Sixty patients (usual care, n = 35; intervention n = 25) experienced recurrence (HR 0.86, 95% CI 0.51 to 1.43; p = 0.556; see Figure 14).
FIGURE 14.
The OS, BCCS and time to recurrence for ITT and per-protocol analyses. (a) ITT analysis: OS; (b) ITT analysis: BCCS; (c) ITT analysis: time to recurrence; (d) per-protocol analysis: OS; (e) per-protocol analysis: BCCS; and (f) per-protocol analysis: time to recurrence.
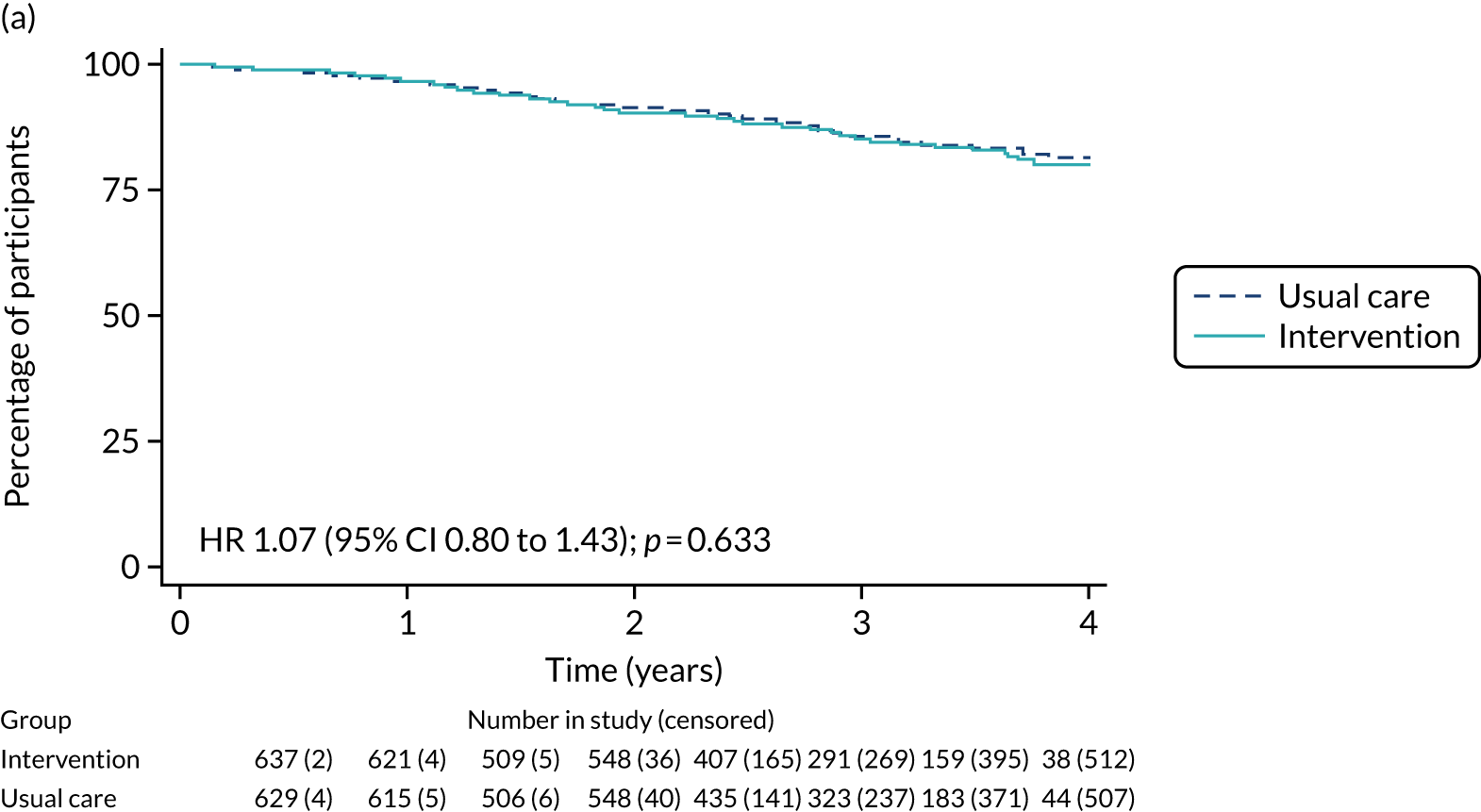
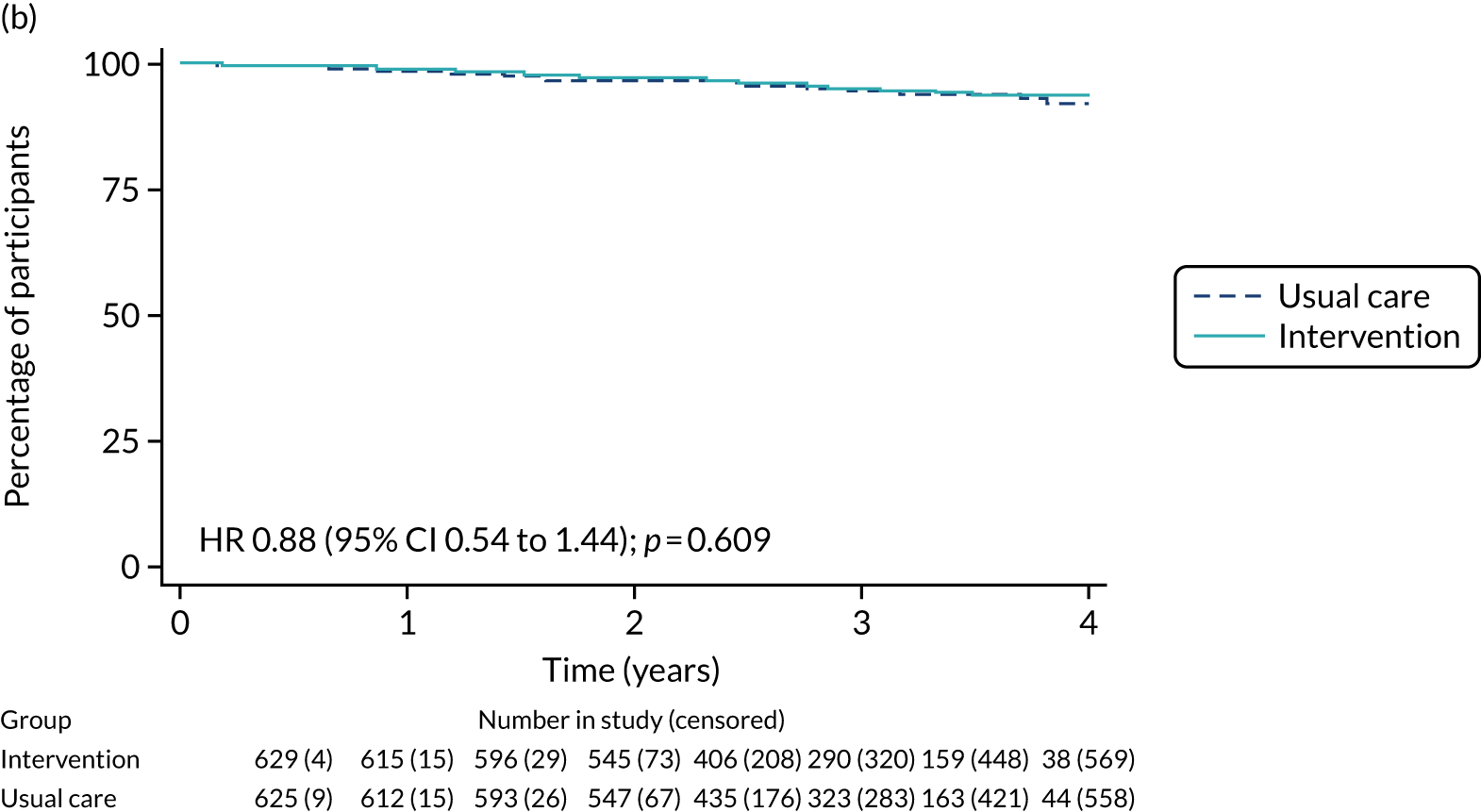
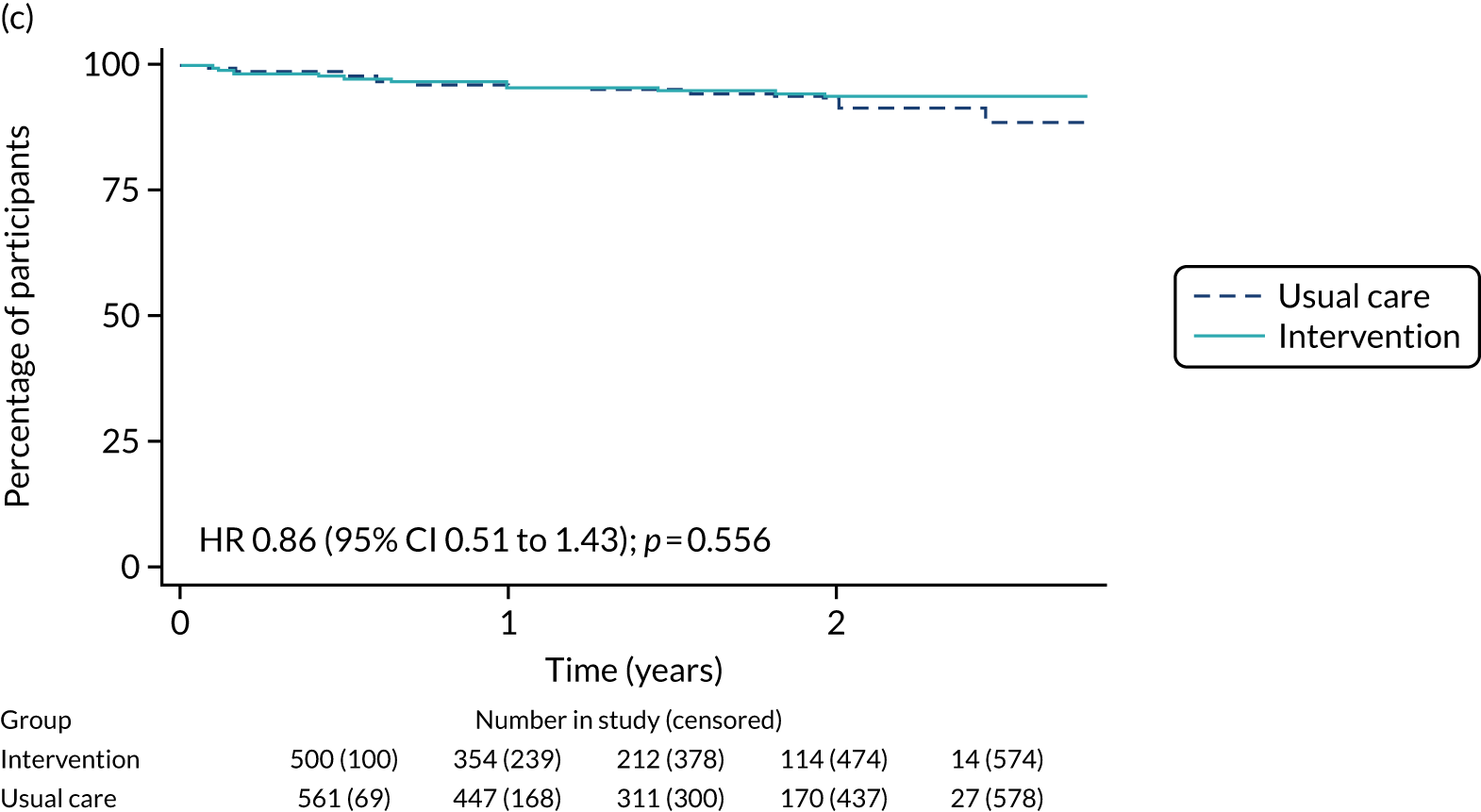
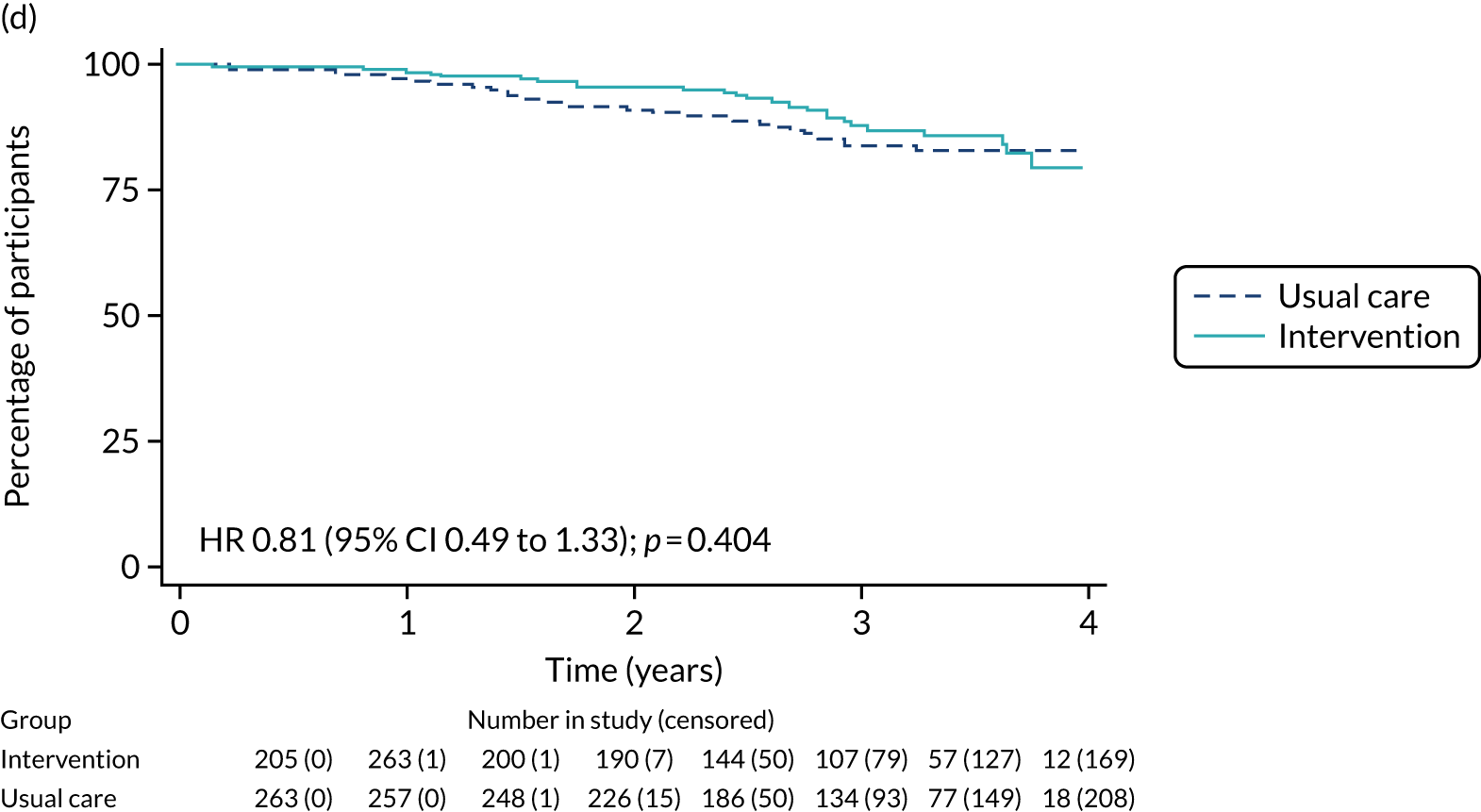
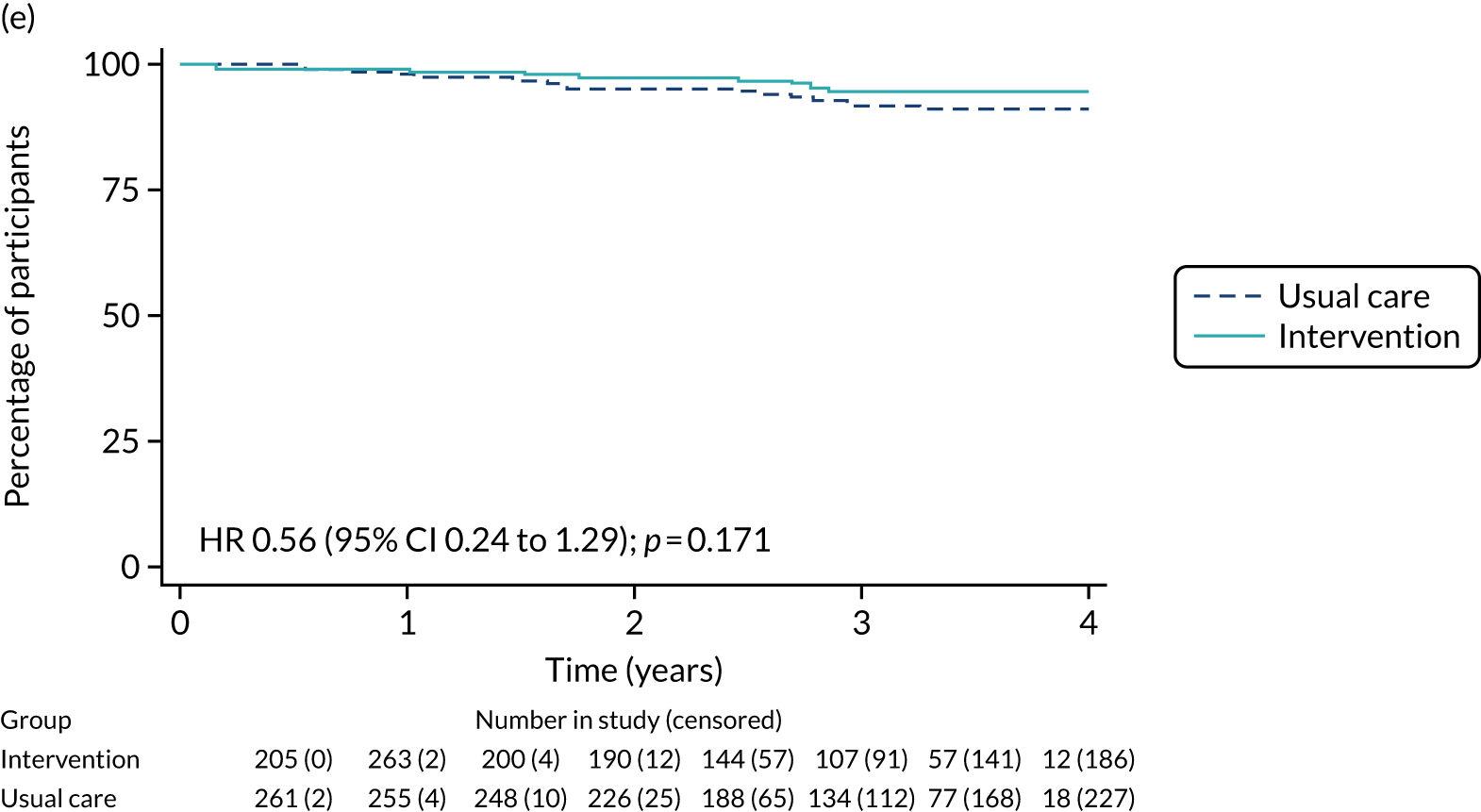
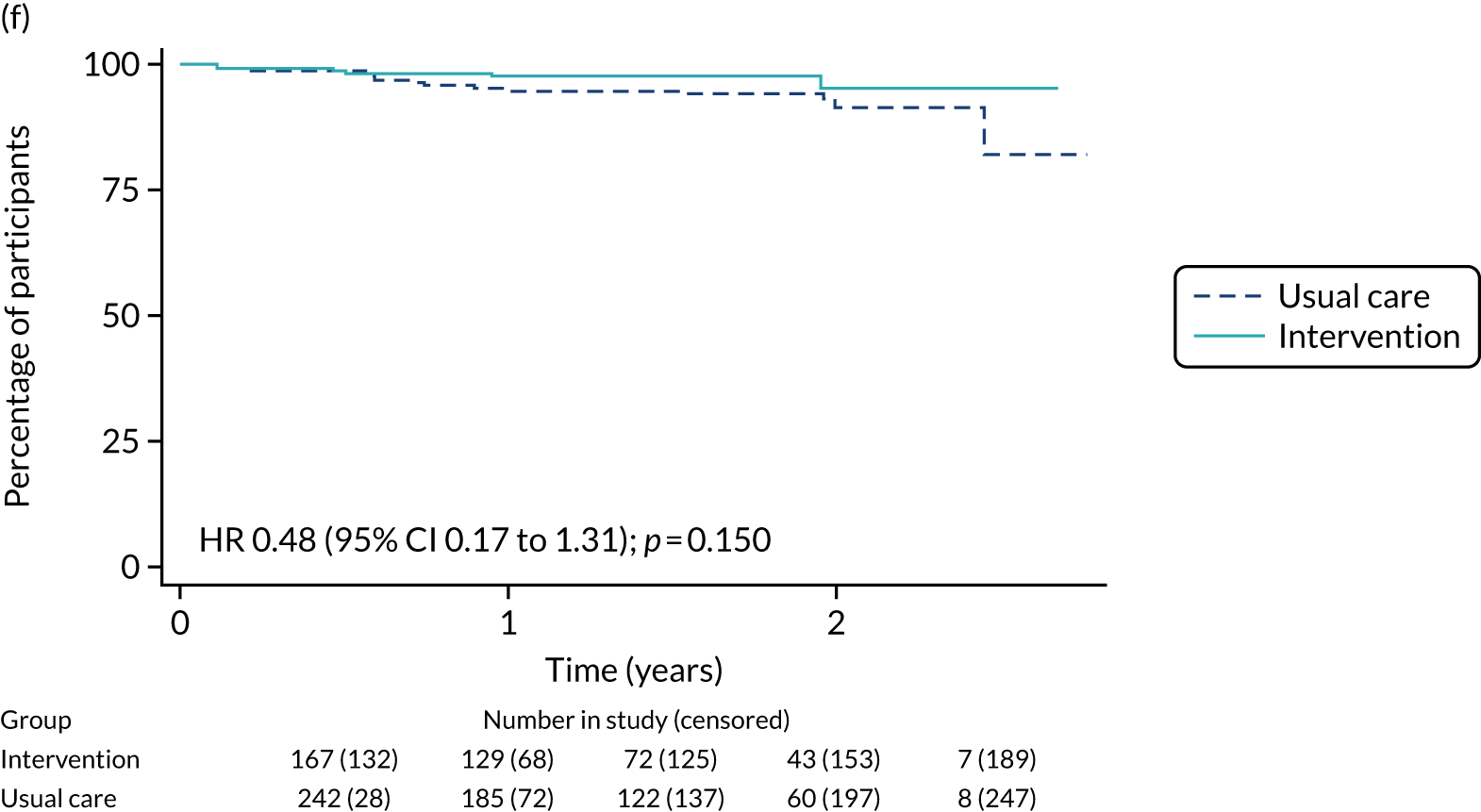
Decision-quality measures
There were no differences in any of the tools to evaluate decision quality (CollaboRATE, decision regret); however, knowledge scores were significantly higher in the intervention arm than the usual-care arm for those offered a choice of S+ET or ET ALONE. For adjuvant chemotherapy knowledge questions, the number of respondents was too low for meaningful analysis.
Discussion
This large, multicentre, cluster-randomised controlled trial is the first to evaluate the effects of two DESIs on clinical decision-making in older women with operable breast cancer. In the ITT analysis, use of the tools did not affect global QoL at 6 months, but the tools were intended for only those women who had been offered either of the relevant choices. Preplanned per-protocol analysis of women offered either choice showed a small improvement in QoL. Treatment choices were altered, with lower rates of S+ET and adjuvant chemotherapy at intervention sites than at usual-care sites. Patient knowledge was enhanced by using the DESIs, suggesting that the DESIs improve informed decision-making.
Analysis of survival outcomes at a median of 36 months’ follow-up showed that, in the ITT analysis, there was minimal survival difference; however, the per-protocol analysis did show some separation of the survival curves, indicating a small potential survival benefit among participants who underwent treatment consultations, as intended by the intervention. Nevertheless, longer follow-up is required.
The DESIs are now available online,43 following granting of MHRA approval. We plan to develop the online tool to incorporate QoL and adverse event outcomes, stratified by patient characteristics and treatment choice, considering the differences in these outcomes demonstrated in the cohort study QoL analyses and the patient feedback about the importance of these outcomes for their decision-making.
Process evaluation
An integral component of the cluster-randomised controlled trial was to assess the use of the DESIs, and their dose, reach, practicality, acceptability and usefulness to both health-care professionals and patients. The process evaluation has been published. 92
Introduction
Medical Research Council guidance on the evaluation of complex interventions were used to investigate the context, implementation and mechanisms of impact of the intervention to explore how the intervention led to its effect. 93 Shared decision-making was a short-term outcome by which the intervention was expected to improve QoL through greater confidence in making the best decision, and with less regret about the choice made. 44 Levels of shared decision-making between the two trial arms were also, therefore, measured.
Methods
In brief, the process evaluation interviewed 73 women and 10 health-care professionals from recruitment sites. It developed and used a range of questionnaire tools to evaluate the dose, reach, fidelity, adaptations, and barriers to and facilitators of the use of the Age Gap tool. Site use of the DESIs was also recorded.
Results
The DESIs were generally highly regarded by both patients and clinicians, and helped facilitate shared decision-making. However, engagement with the DESIs in clinical practice was suboptimal for a number of reasons, and this limits our understanding of their value and likely adoption in routine practice.
Conclusion
The process evaluation sought feedback about the dose, reach, practicality, acceptability and usefulness of the DESIs. The results were favourable, but engagement was limited in some centres.
Variation in rates of surgery (plus adjuvant endocrine therapy) or endocrine therapy alone between UK breast units: case-mix-adjusted variation in rates of non-surgical treatment between breast units
The final work package was to assess the variation in practice in case-mix-adjusted rates of surgery between UK units and the underlying reasons for this variation.
This work package comprised two sections:
-
registry analysis of case-mix-adjusted rates of ET ALONE or S+ET
-
case-mix-adjusted analysis of rates of S+ET or ET ALONE within the Age Gap cohort study.
These analyses have been published. 28,94–96
Introduction
In the UK, there is considerable variation in rates of ET ALONE in women aged ≥ 70 years,21 with rates of between 12% and 40%. 97 Previous authors have used UK registry data to identify factors affecting whether or not surgery is performed in older breast cancer patients, but, to the best of our knowledge, to date, none have studied the variation in treatment allocation based on individual hospitals and clinicians. 98–100 Our research analysed UK practice relating to women aged ≥ 70 years with ER+ breast cancer to determine whether or not the variation observed at hospital- and clinician-levels persists following adjustment for patient characteristics, and tumour stage and biological subtype.
Methods
Records on operable ER+ breast cancers diagnosed in women aged ≥ 70 years between 2002 and 2010 were acquired from two UK cancer registries (WMCIU and NYCRIS). Data on patient and tumour characteristics were included, which were derived from the registries, English Indices of Multiple Deprivation 2010 and HES. A proxy CCI score36 (excluding cancer) was calculated for each patient. 37 The proportion of patients undergoing surgery was calculated for each clinician and hospital. Multivariable logistic regression was used to estimate the probability of a woman undergoing surgery based on age, proxy CCI score, level of socioeconomic deprivation, tumour detection method, tumour size, tumour grade, tumour node metastasis (TNM) stage and nodal status. Missing data were handled using multiple imputation101 to produce 25 imputed data sets, and combining the results. 102
Expected rates of surgery were calculated for each health-care professional and breast unit by summing individual patient probabilities, determined from the logistic regression model. Rates of surgery were risk adjusted by dividing the observed rate by the expected rate for each health-care professional and hospital, and multiplying by the UK national rate. 103 Data are displayed as funnel plots with 95% (2 SD) and 99% (3 SD) SD lines. Logistic regressions were performed in IBM SPSS Statistics, version 21 (IBM SPSS Statistics, Armonk, NY, USA), and multiple imputations were performed using R (version 3.0.1). The analysis was also repeated on the Age Gap cohort data using similar methodology.
Results
Cancer registry analysis
Cancer registration records were obtained for 23,960 patients. After applying the exclusion criteria, 17,129 records remained for analysis. In total, 9955 out of 17,129 (58.1%) patients were treated surgically. The median age was 79 years (range 70–103 years).
The unadjusted rates of surgery varied between hospitals and clinicians, with 39 out of 68 (57.4%) hospitals and 73 out of 167 (43.7%) clinicians falling outside the 95% limits on the funnel plots (Figure 15).
FIGURE 15.
Funnel plots showing unadjusted and adjusted rates of surgery at hospital and clinician levels using registry data, or at hospital level using Age Gap cohort data. (a) Hospital level (registry data): unadjusted; (b) hospital level (registry data): adjusted; (c) clinical level (registry data): unadjusted; (d) clinician level (registry data): adjusted; (e) hospital level (Age Gap data): unadjusted; and (f) hospital level (Age Gap data): adjusted.
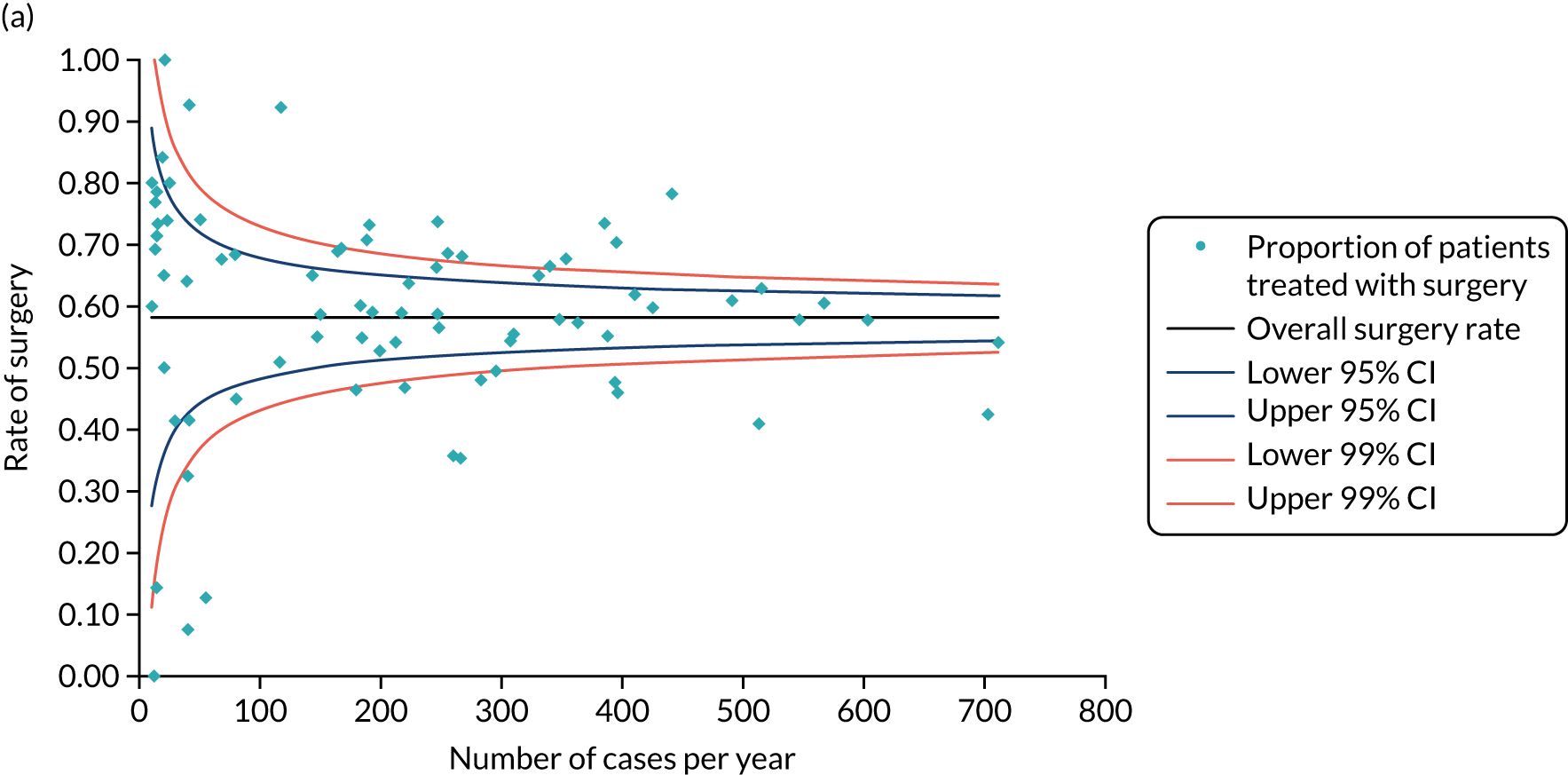
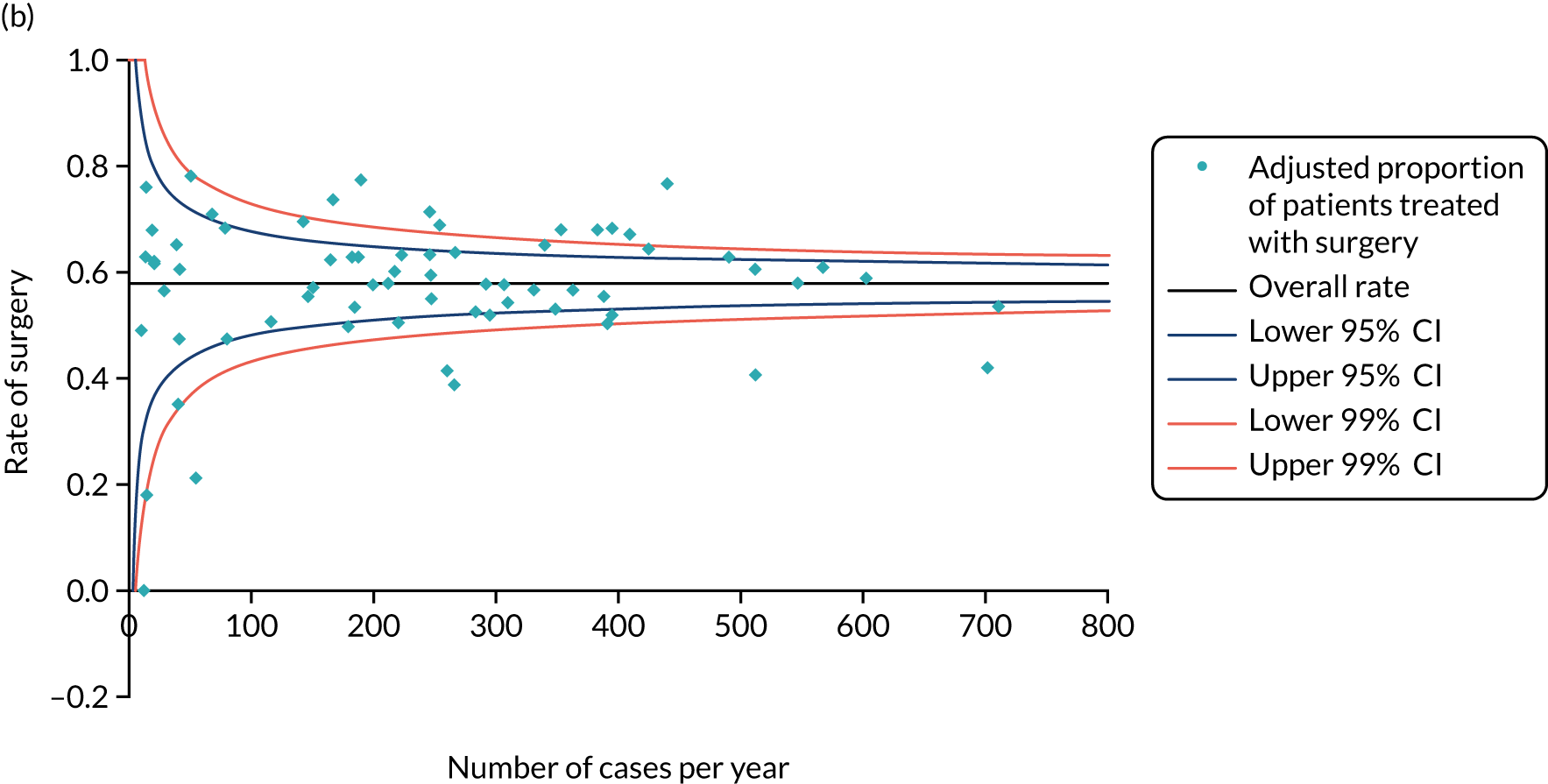
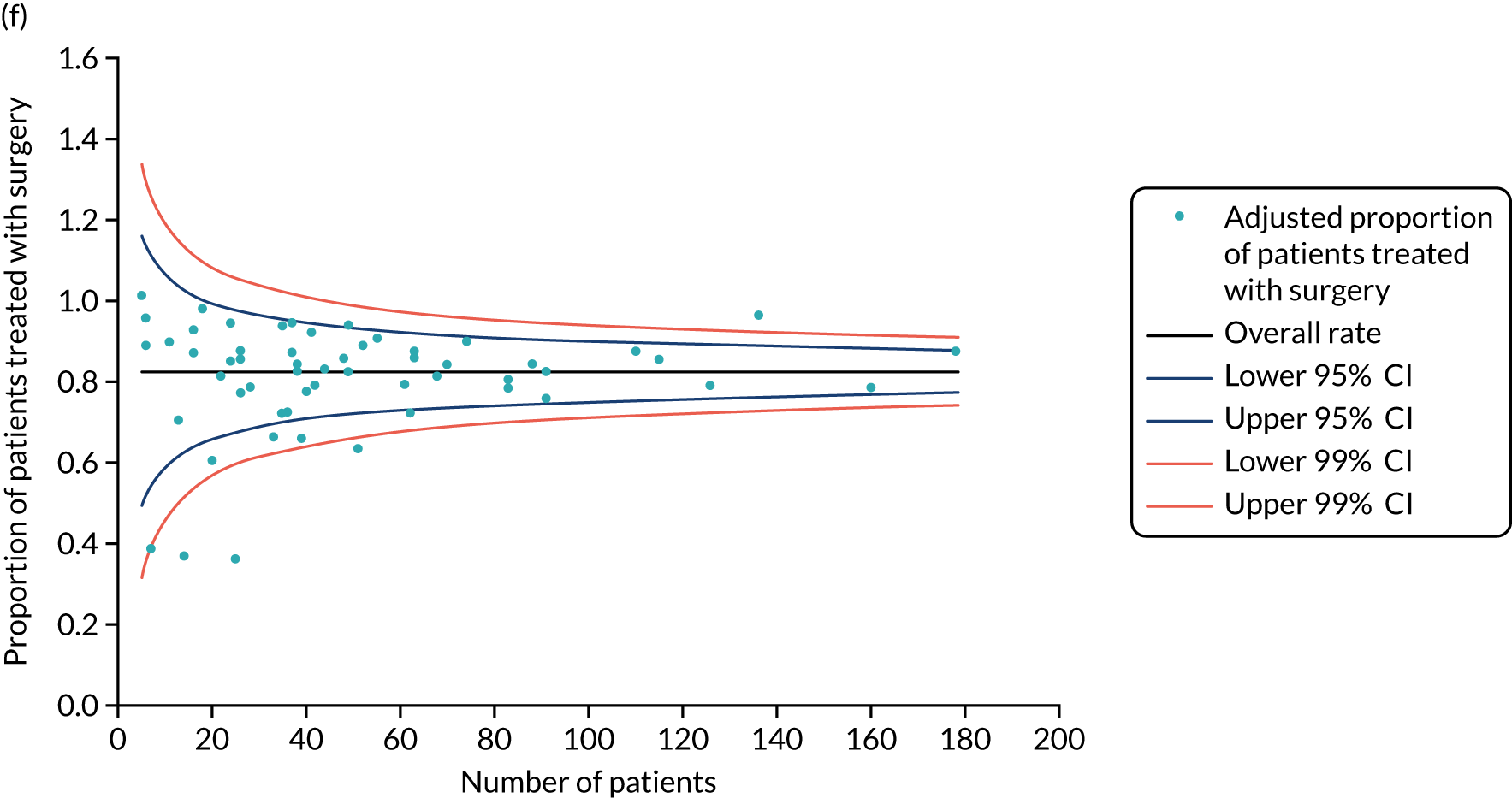
Adjusting for case mix did not significantly reduce the variation in surgery rates, with 30 out of 68 (44.1%) hospitals falling outside the 95% limits (see Figure 15). However, at clinician level, adjusting for case mix did reduce the variation in surgery rates, with 17 out of 167 (10.2%) clinicians falling outside the 95% limits on the funnel plot (see Figure 15).
Replication of variation analysis on the Age Gap cohort
The same methodology as for the cancer registry variation analysis was used to examine the variation between breast units recruiting to the 56 Age Gap study sites. Only variation by unit was analysed (see Figure 15).
Discussion
This study found considerable variation in the rates of surgery despite case mix adjustment in both the registry and Age Gap data analyses. Analysis of the reasons for this variation confirmed that thresholds for comorbidity, tumour characteristics, dementia and physical function vary widely. Age, comorbidity, frailty, dementia, tumour biology and stage all have an impact on treatment allocation. This underlines the need for fitness-stratified guidelines. The reasons for this variation are explored in Health-care professional interviews and questionnaire study to determine the reasons for treatment variation in older women with early-stage breast cancer.
Health-care professional interviews and questionnaire study to determine the reasons for treatment variation in older women with early-stage breast cancer
This mixed-methods study examined the views and opinions of a range of health-care professionals towards S+ET or ET ALONE in older women, and explored the factors they take into consideration. This work has been published. 95,96
Methods
A mixed-methods study design was used, comprising initial interviews with a range of health-care professionals, including breast surgeons, specialist nurses and geriatricians. 97 Interviews were recorded, transcribed and analysed using the framework approach. 104
A postal questionnaire was designed using the themes identified in the qualitative interviews to ensure content validity. Face validity and acceptability were assessed during piloting with five health-care professionals. The survey was posted to clinician and nurse members of the UK Association of Breast Surgeons (ABS). In addition, this study attempted to determine the most significant variables influencing this choice in UK health-care professionals using a discrete choice experiment (DCE)105 that used a series of hypothetical scenarios. Key scenario variables included patient age, comorbidity, cognitive status, functional status, and tumour stage and biology. A geriatrician then estimated the probable life expectancy for each scenario and categorised it as < 2 years, 2–5 years or > 5 years, although this information was not shown to participants. For each scenario, individuals were asked to state whether they would choose S+ET, ET ALONE or had no preference. The final DCE had 17 scenarios. 95 Analysis was by multinomial regression using Stata®, version 13 (StataCorp LP, College Station, TX, USA).
An example of a scenario is shown in Figure 16.
FIGURE 16.
Example of a discrete choice scenario.
Results
Semistructured qualitative interviews
Thirty-four interviews were conducted, with interviewees comprising 20 surgeons, 13 breast care nurses and one geriatrician. The mean duration of interviews was 33 minutes (16–55 minutes). Twenty-one (62%) were from units with a high rate of non-surgical treatment, with the remaining 14 from units with a low rate of non-surgical treatment.
Quantitative postal questionnaire survey
The response rate was 251 out of 641 (39%) questionnaires returned.
Results
Attitudes of health-care professionals to treating older women with operable breast cancer
Critical issues were fitness for surgery (comorbidity, frailty), biology and stage of cancer. Age was not noted as important, but did have some weight in the decision, with 205 out of 245 (83.7%) rating age as having ‘some importance’ in decision-making. Comorbidities were the most important factor determining fitness for surgery, with all respondents (248/248; 100%) rating this as important. The presence of significant dementia divided health-care professional opinion, with 102 out of 248 (41.1%) agreeing with the statement ‘all women aged ≥ 70 years with operable ER+ breast cancer, who have significant dementia should be treated with ET ALONE’.
Experience of surgical treatment in older women with breast cancer
Surgery was considered the superior option for older women with breast cancer, with 79.9% (199/249) of respondents agreeing with the statement ‘all women aged ≥ 70 years with operable breast cancer should be offered an operation regardless of age’. Tumour stage was a significant factor, with the fact that larger node-positive tumours require more extensive surgery having an impact on choice. Oestrogen receptor status was regarded as important to most surgeons (216/248; 87.1%) when deciding to use ET ALONE.
Experience of endocrine therapy alone as a treatment in older women with breast cancer
The majority of health-care professionals felt that ET ALONE was inferior to surgery and should not be routinely offered to any woman aged ≥ 70 years without considering that woman’s health status. The duration of ET ALONE disease control was not well understood, with responses regarding the median duration of control ranging from < 18 months to > 5 years. The consensus duration from the literature is ≈ 24 months.
Results: questionnaire study
The response rate was 40.2% (258/641). Most respondents (75%) were breast surgeons; 21% were breast care nurses. The results (Figures 17 and 18) show wide-ranging opinions regarding optimal patient management, with some scenarios having a three-way split between surgery, ET ALONE and undecided. It was clear that, in women with > 5 years predicted life expectancy, surgery was preferred, but, in women in poorer health, variability was greater. All five variables, patient age, comorbidity, cognitive status, functional status, and tumour stage and biology, influenced the decision, but comorbidity, cognition and tumour size were most clearly associated with a preference for ET ALONE.
FIGURE 17.
Patient and tumour characteristics, and their importance in shaping the advice health-care professionals would give to an older patient for whom a choice of ET ALONE or S+ET may be considered (n = 258).
FIGURE 18.
Treatment preferences according to estimated life expectancy of each scenario.
Conclusion
This study found wide variation of opinion among health-care professionals regarding the use of ET ALONE for older women, which may be one of the factors underlying wide UK variation. The lack of guidelines on the characteristics of women for whom ET ALONE may be appropriate means that such guidelines are urgently needed. It is hoped that the Age Gap online tool will aid clinical decision-making and harmonise selection of treatment.
Overall summary and study limitations
The trial has confirmed the findings of other RCTs and cohort studies, namely that the OS is worse for unmatched women on ET ALONE than those on S+ET; when matched, in a frailer, older, less fit cohort, the difference reduces but does not disappear, reflecting imperfect matching. BCSS is increased with S+ET in unmatched cohorts. However, on matching to create an older, less fit cohort, the disease-specific survival benefit is lost and QoL is needlessly impaired. Although surgery is generally physically well tolerated in the older, less fit woman, disease-specific survival benefit is lost and QoL is impaired in patients undergoing surgery. Although QoL is impaired in the short term, these effects have largely, but not completely, resolved by 2 years.
This study is, to the best of our knowledge, the first attempt to stratify outcomes for age and fitness, allowing us to suggest thresholds for offering ET ALONE. This should ideally be offered for a woman with an ER+ positive cancer with an estimated life expectancy of < 4 years, which would typically equate to a fit woman aged ≥ 90 years, or a less fit woman in her mid-eighties.
For adjuvant chemotherapy, this study has shown relatively low rates of use in this age group, with minimal use for those aged > 80 years. There appears to be little survival benefit from the use of adjuvant chemotherapy in women with ER+ cancers, but some survival benefit in women with ER– disease. Adjuvant chemotherapy has significant adverse effects and impacts on QoL, although these effects are largely short term and fully resolve by 2 years.
The information needs of older women were studied in detail and permitted the development of two bespoke DESIs. Testing within a RCT provided good qualitative feedback about the tools, which were well received by clinicians and patients. The tool enhanced shared decision-making and resulted in significantly altered treatment choices and patient knowledge levels. Importantly, the intervention centres demonstrated significant increases in the use of ET ALONE and reductions in the use of adjuvant chemotherapy associated with enhanced information provision and shared decision-making. This is in keeping with studies demonstrating that older patients value QoL and maintaining independence.
The Age Gap tool is MHRA approved and is available online for open-access use. 43
It is hoped that use of the online tool will increase over time. As of the start of 2021, the tool had been accessed over 10,000 times in the first year since it was launched, with access from 70 countries. The tool has been mentioned in the National Audit of Breast Cancer in Older Patients: 2021 Annual Report,106 wherein it is recommended as a useful tool for supporting decision-making in older women. The trial and the tool were showcased at the UK Association of Breast Surgery conference in January 2021 in a session devoted to improving outcomes for women aged ≥ 70 years. It is hoped that, with time, the tool will become part of standard of care for decision-making in older women, which will be further assisted by our plans for further tool development and validation.
Limitations
The Age Gap study has several limitations to consider when interpreting the findings. The most significant is the duration of follow-up. In the cohort analysis comparing S+ET and ET ALONE, all women had ER+ cancers, for which the natural history of the disease is slow. At present, median follow-up is 52 months and, ideally, analysis should be repeated at a median follow-up of 10 years. Previous comparisons of S+ET and ET ALONE, although showing a trend in favour of S+ET at 5 years’ follow-up, show a benefit to S+ET when analysed at longer follow-up. Future research plans to revise the analysis with 10-year data will be made. As a result, the online tool demonstrates outcome at 2 and 5 years only, and, again, this will be updated when modelling has been extended to 10 years.
Another limitation is that, although the Age Gap cohort study is very large, with > 3400 women recruited, when propensity score matching is applied, the sample size inevitably falls, meaning that the matched analysis was smaller. This reduces the chance of statistical significance, although the matched population is still of a reasonable size and the findings are robust. With any matching process there is always a trade-off between the degree of matching and the size of the matched sample. It is felt that the present analysis has optimally matched, while maintaining an adequate sample size.
One limitation of the cluster trial is the fact that median follow-up is only 36 months, which is too short for truly meaningful interpretation, for the same reasons outlined for the cohort study.
Another problem with the cluster trial was the reduction in power caused by the fact that completion of QoL questionnaires was optional. This was chosen to reduce trial burden in this group of often frail women, but, consequently, although our headline recruitment was adequate, the number completing questionnaires was not. Most women who did not elect to complete QoL forms were older and less fit, and therefore more likely to be the type of women for whom ET ALONE is preferable. If we had mandated 100% form completion, we would have selected a population of women for whom S+ET would be preferable, which would have skewed the survival analysis. There was, therefore, no optimal way to design the trial to meet both needs.
Recruitment was slightly skewed in favour of younger and fitter women, as we would expect in this challenging research demographic. However, the fact that the trial managed to recruit > 3400 women, with the oldest woman aged 102 years, is a testament to the support of older patients and also the study design being as accessible as possible for this group.
The models used to populate the online tool require further validation work, particularly the adjuvant chemotherapy model, which we have yet to validate against an external data set. It was also not possible to undertake the health economic modelling relating to adjuvant chemotherapy outcomes owing to lack of time (due to significant delays in recruitment and in gaining access to registry data). Again, this work is planned for the future. The models themselves, although using the best available evidence, did not have a direct frailty measure included, instead making use of imputation of external frailty impacts. Similarly, data held by the UK Cancer Registry during the period studied had missing data for ER status and use of endocrine therapy, although the model inferred ER status from whether or not endocrine therapy was used to ensure the data set was as complete as possible.
Suggestions for future work
The Age Gap team are currently using the QoL data from the cohort study to develop an enhanced online tool that not only provides survival outcomes, but also shows the personalised impact of treatment on QoL and adverse events. This enhanced online tool will then be prospectively evaluated on a further cohort of older women. It is also planned to revise the Age Gap models of survival within the online tool with survival outcomes up to 10 years.
It is also hoped that the adjuvant chemotherapy cost-effectiveness analysis will be completed.
A major theme that emerged from the qualitative data was the desire of older women to maintain their QoL and independence, and they are often prepared to trade this against length of life at any cost. Pilot data have been acquired that show that this is the case, especially for the older women in this age group, and this work will be published separately. It is planned to undertake further mixed-methods analysis of this issue, which will be important for understanding the reasons why many women make treatment choices that may have potentially adverse cancer outcomes.
Another area of interest that merits further study is the role of clinicians in directing decision-making, and how their conscious or unconscious bias may have an impact on treatment choices. We have already explored this with the variation-in-practice interviews and questionnaires, and Malcolm WR Reed and Jenna Morgan are currently undertaking a more detailed investigation of the role of unconscious age bias in treatment selection, which will be published separately.
Acknowledgements
The Age Gap Trial Steering Group and Data Monitoring and Ethics Committee (DMEC) wish to express their sincere gratitude to the following:
-
all of the patients and carers who have taken part in the study
-
all local principal investigators, clinical team members, research nurses and local research and development staff for all their efforts in recruiting to the study
-
the members of the PPI group, some of whom are Trial Steering Committee (TSC) members and others of whom have helped with various trial functions during the study
-
the ABS for supporting the study, raising the profile of the work and encouraging its members to become involved
-
NHS Digital and the National Cancer Registration and Analysis Service (NCRAS) for allowing us access to registry data, and their help in navigating the complex and changing legislation
-
the study sponsor, Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust (Doncaster, UK), whose research staff have been very supportive of the study, in particular Amy Lee and Emma Adams
-
university research finance officers for assistance with complex budget management, in particular Chris Day and Mark Sayers
-
NIHR as the funder of the study.
Patient and public involvement
The Age Gap programme has benefited from a high level and quality of PPI support and engagement across all aspects of the work. We had three experienced and active PPI representatives on the TSC who attended every TSC (twice yearly) since the study began. We had PPI representatives provide input into the original grant application to assist in developing the concept of the project and ensuring it was patient focused and acceptable to patients. The PPI representatives have been involved in discussions about study development and design at each stage, making many valuable suggestions about feasibility, acceptability, patient focus and ethics. They have also been involved in the drafting of all study protocols for each of the many stages of the project and editing all patient facing documents such as consent forms and patient information sheets. They have advised on the conduct of the trial and helped with raising the profile of the study by presenting at academic meetings. They have been involved in the interpretation of qualitative interviews; their perspective has been slightly different from that of the medical researchers and has added depth to our interpretation. They were particularly helpful in the development of the decision support tools, with excellent feedback about design and content. In addition, they have provided detailed comments and editing on all publications arising from this work and are credited with full authorship on all key papers. They have also provided valuable feedback on the final report and have assisted in our plans for follow-on funding applications.
Contributions of authors
Lynda Wyld (https://orcid.org/0000-0002-4046-5940) (Professor of Surgical Oncology) was the chief investigator. She contributed to study development, funding acquisition, trial design, recruitment, governance, management, budget and staff management, writing reports, data analysis and interpretation, and drafting of publications.
Malcolm WR Reed (https://orcid.org/0000-0001-7442-2132) (Professor of Surgical Oncology) was the co-chief investigator. He contributed to study development, funding acquisition, trial design, recruitment, governance, writing reports, analysis and drafting of publications.
Karen Collins (https://orcid.org/0000-0002-4317-142X) (Emeritus Professor of Health Services Research) was the lead for the qualitative study elements. She contributed to study development, funding acquisition, trial design, recruitment, governance, writing reports and drafting of publications.
Sue Ward (https://orcid.org/0000-0002-3344-0913) (Senior Research Fellow in Health Economics and Decision Science) contributed to the development of registry and cohort study models, health economic analysis, data analysis, and drafting of publications.
Geoff Holmes (https://orcid.org/0000-0002-8861-9638) (Senior Research Fellow in Health Economics and Decision Science) contributed to development of registry and cohort study models, health economic analysis, data analysis and drafting of publications.
Jenna Morgan (https://orcid.org/0000-0002-5337-5581) (NIHR Clinical Lecturer in Surgery) was the lead for the variation elements of analysis. She contributed to study development, design, governance, data analysis and drafting of publications.
Mike Bradburn (https://orcid.org/0000-0002-3783-9761) (Senior Medical Statistician) was the lead statistician for the project. He contributed to development of the statistical analysis plan, data analysis, data interpretation, writing reports and drafting of publications.
Stephen Walters (https://orcid.org/0000-0001-9000-8126) (Professor of Medical Statistics) was the senior statistician for the project. He contributed to study development, funding acquisition, trial design, oversight of statistical analysis and drafting of publications.
Maria Burton (https://orcid.org/0000-0002-5411-8181) (Senior Research Fellow in Health and Social Care Research) undertook the process evaluation, and was the lead for the qualitative work during the Age Gap tool development, qualitative interviews, trial design, statistical analysis and interpretation and drafting of publications.
Kate Lifford (https://orcid.org/0000-0002-9782-2080) (Research Associate in Health Psychology) was the co-lead for the process evaluation and qualitative analyses, development and evaluation of the online tool, qualitative interviews, data analysis and interpretation, and drafting of publications.
Adrian Edwards (https://orcid.org/0000-0002-6228-4446) (Professor of General Practice) was the co-lead for development of the decision support tool and its evaluation, and lead for the process evaluation and its analysis and interpretation, methodological expertise on decision support tools, data interpretation and drafting of publications.
Kate Brain (https://orcid.org/0000-0001-9296-9748) (Professor of Health Psychology) was an advisor on development and evaluation of the decision support tool, funding acquisition and editing of publications.
Alistair Ring (https://orcid.org/0000-0003-1230-2973) (Consultant Medical Oncologist and Honorary Reader in Breast Cancer Clinical Trials) was the lead for adjuvant chemotherapy and trastuzumab analyses, including study design, conduct, analysis, interpretation and write-up. He was the study advisor for all systemic therapy elements.
Esther Herbert (https://orcid.org/0000-0002-1224-5457) (Medical Statistician) contributed to statistical design, analysis and interpretation, and writing reports and publications.
Thompson G Robinson (https://orcid.org/0000-0003-2144-2468) (Professor of Stroke Medicine) was an advisor on geriatric medicine and assessment, funding acquisition, study design and management, data interpretation, and drafting and editing of publications.
Charlene Martin (https://orcid.org/0000-0002-0988-0353) (Post-Doctoral Research Associate) was the study manager, study monitor, and lead for analyses relating to cognitive impairment, statistical analysis and interpretation, data quality assurance, trial governance and ethics, and drafting and editing of publications.
Tim Chater (https://orcid.org/0000-0002-1138-0147) (Senior Data Manager) contributed to the set-up, management and monitoring of the Age Gap database, data security and quality assurance. He also contributed to the editing of publications.
Kirsty Pemberton (https://orcid.org/0000-0002-3666-2386) (Data Manager) contributed to the set-up and monitoring of the Age Gap database, data security and quality assurance. She also contributed to the editing of publications.
Anne Shrestha (https://orcid.org/0000-0002-5879-5534) (Research Fellow in Surgery) contributed to the design and analysis of QoL data, data collection, trial initiation visits, trial management and drafting and editing of publications.
Anthony Nettleship (https://orcid.org/0000-0003-0658-3668) (Information Technology Specialist) contributed to the design, development and set up of the Age Gap decision tool, tool monitoring and troubleshooting, and editing of publications.
Paul Richards (https://orcid.org/0000-0002-0320-9083) (Research Fellow in Health Economic Modelling) contributed to the development of initial model of outcomes for ET ALONE versus surgery and adjuvant chemotherapy versus no chemotherapy, and editing of publications.
Alan Brennan (https://orcid.org/0000-0002-1025-312X) (Professor of Health Economics and Decision Sciences) contributed to trial development, funding acquisition, study design, trial management, health economic and outcome model development oversight and validation, drafting and editing of publications.
Kwok Leung Cheung (https://orcid.org/0000-0003-2973-0755) (Professor of Breast Surgery and Medical Education) was an expert on geriatric oncology, primary endocrine therapy, trial steering group member and editing of publications.
Annaliza Todd (https://orcid.org/0000-0002-5888-8263) (Age Gap Trial Monitor) contributed to study monitoring, data quality assurance, data source verification, database management, research governance, and drafting and editing of publications.
Helena Harder (https://orcid.org/0000-0002-7296-8227) (Research Fellow) contributed to qualitative interviews and analysis of adjuvant chemotherapy interviews for the Age Gap study, and editing of publications.
Riccardo Audisio (https://orcid.org/0000-0002-7538-4809) (Professor of Surgical Oncology) contributed to the trial design and development; was an expert on geriatric oncology, surgery in the older patient and geriatric assessment; was a Trial Steering Group member; and contributed to editing of manuscripts.
Nicolo Matteo Luca Battisti (https://orcid.org/0000-0002-1063-1717) (Medical Oncologist) was an expert in chemotherapy in older patients, statistical analysis and interpretation of adjuvant chemotherapy, trastuzumab and radiotherapy data, and drafting and editing of publications.
Juliet Wright (https://orcid.org/0000-0002-8482-081X) (Professor of Elderly Medicine) was an expert on frailty assessment and geriatric medicine, was a member of the Trial Steering Group, and contributed to editing of manuscripts.
Richard Simcock (https://orcid.org/0000-0002-9828-4114) (Consultant in Clinical Oncology) was an expert advisor on radiotherapy in older women, was a member of the TSC, and contributed to editing of manuscripts.
Christopher Murray (https://orcid.org/0000-0001-6939-4384) (Managing Director) contributed software and IT expertise, and to funding acquisition, study design and development; was a co-developer of the Age Gap online tool, and was a member of the TSC.
Alastair M Thompson (https://orcid.org/0000-0003-0604-7267) (Professor of Surgery) was chairperson of the DMEC, and contributed to trial oversight and safety monitoring, progress monitoring, trial design and development, and editing of publications.
Margot Gosney (https://orcid.org/0000-0001-9004-7571) (Professor of Elderly Care Medicine) was a member of the DMEC, and contributed to trial oversight and safety monitoring, progress monitoring, trial design and development and editing of publications.
Matthew Hatton (https://orcid.org/0000-0003-2778-7926) (Consultant Clinical Oncologist and Honorary Professor of Clinical Oncology) was a member of the DMEC, contributed to trial oversight and safety monitoring, progress monitoring, and trial design and development, was an advisor on radiotherapy issues, and contributed to drafting and editing of publications.
Fiona Armitage (https://orcid.org/0000-0003-4311-0093) (Clinical Nurse Specialist) was an advisor on nursing issues, and contributed to trial set-up and trial initiation visits, development and evaluation of the online tool, and data interpretation and analysis.
Julietta Patnick (https://orcid.org/0000-0002-5500-3335) (Visiting Professor of Cancer Screening) contributed to funding acquisition, study development and editing of publications.
Tracy Green (https://orcid.org/0000-0002-7198-2828) (PPI representative) was a member of the TSC, and contributed to trial design, trial development, review of trial materials, ethical review and editing of publications.
Deirdre Revill (https://orcid.org/0000-0002-5074-4177) (PPI representative) was a member of the TSC, and contributed to trial design, trial development, review of trial materials, ethical review and editing of publications.
Jacqui Gath (https://orcid.org/0000-0001-8875-5894) (PPI representative) was a member of the TSC, and contributed to trial design, trial development, review of trial materials, ethical review and editing of publications.
Kieran Horgan (https://orcid.org/0000-0003-4173-1777) (Consultant Breast Surgeon and Honorary Professor of Surgery) was the recruitment lead at Leeds Teaching Hospitals NHS Trust; contributed to patient recruitment, consenting, follow-up, data collection and editing of publications; was chairperson of the NABCOP; and contributed expertise in the management of breast cancer in older women.
Chris Holcombe (https://orcid.org/0000-0002-6014-8053) (Professor of Surgery and Consultant Breast Surgeon) was recruitment lead at Liverpool; contributed to patient recruitment, consenting, follow up, data collection and editing of publications, and contributed expertise in the management of breast cancer in older women.
Matt Winter (https://orcid.org/0000-0001-6192-9874) (Consultant Medical Oncologist and Honorary Reader) was recruitment lead at Sheffield, and contributed to patient recruitment, consenting, follow-up, data collection and editing of publications.
Jay Naik (https://orcid.org/0000-0002-4793-8878) (Consultant Medical Oncologist) was recruitment lead at Harrogate, and contributed to patient recruitment, consenting, follow-up, data collection and editing of publications.
Rishi Parmeshwar (https://orcid.org/0000-0002-8162-2746) (Consultant Breast Surgeon) was recruitment lead at Morecambe, and contributed to patient recruitment, consenting, follow-up, data collection and editing of publications.
Publications
Ring A, Battisti NML, Reed MWR, Herbert E, Morgan JL, Bradburn M, et al. Bridging the Age Gap: observational cohort study of effects of chemotherapy and trastuzumab on recurrence, survival and quality of life in older women with early breast cancer. Br J Cancer 2021;125:209–19. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC8292504/pdf/41416_2021_Article_1388.pdf (accessed September 2021).
Martin C, Shrestha A, Morgan J, Bradburn M, Herbert E, Burton M, et al. Treatment choices for older women with primary operable breast cancer and cognitive impairment: results from a prospective, multicentre cohort study. J Geriatr Oncol 2021;12:705–13. URL: www.geriatriconcology.net/action/showPdf?pii=S1879-4068%2820%2930528-2 (accessed September 2021).
Battisti NML, Hatton MQ, Reed MWR, Herbert E, Morgan JL, Bradburn M, et al. Observational cohort study in older women with early breast cancer: use of radiation therapy and impact on health-related quality of life and mortality. Radiother Oncol 2021;161:166–76. URL: https://orca.cardiff.ac.uk/142497/ (accessed September 2021).
Morgan JL, Shrestha A, Reed MWR, Herbert E, Bradburn M, Walters SJ, et al. Bridging the age gap in breast cancer: impact of omission of breast cancer surgery in older women with oestrogen receptor-positive early breast cancer on quality-of-life outcomes. Br J Surg 2021;108:315–25. URL: https://academic.oup.com/bjs/article/108/3/315/6184678 (accessed September 2021).
Dharmarajan KV, Presley CJ, Wyld L. Care Disparities Across the Health Care Continuum for Older Adults: Lessons from Multidisciplinary Perspectives. American Society of Clinical Oncology Educational Book 41. Alexandria, VA: American Society of Clinical Oncology; 2021. URL: https://eprints.whiterose.ac.uk/174377/ (accessed September 2021).
Wyld L, Reed MWR, Collins K, Burton M, Lifford K, Edwards A, et al. Bridging the age gap in breast cancer: cluster randomised trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg 2021;108:499–510. URL: https://academic.oup.com/bjs/article/108/5/499/6182547 (accessed September 2021).
Holmes GR, Ward SE, Brennan A, Bradburn M, Morgan JL, Reed MWR, et al. Cost-effectiveness modelling of surgery plus adjuvant endocrine therapy versus primary endocrine therapy alone in UK women aged 70 and over with early breast cancer. Value Health 2021;24:770–79. URL: https://eprints.whiterose.ac.uk/cgi/request_doc?docid=2230501 (accessed September 2021).
Burton M, Lifford KJ, Wyld L, Armitage F, Ring A, Nettleship A, et al. Process evaluation of the Bridging the Age Gap in Breast Cancer decision support intervention cluster randomised trial. Trials 2021;22:447. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC8278730/pdf/13063_2021_Article_5360.pdf (accessed September 2021).
Wyld L, Reed MWR, Morgan J, Collins K, Ward S, Holmes GR, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer 2021;142:48–62. URL: www.ejcancer.com/action/showPdf?pii=S0959-8049%2820%2931274-0 (accessed September 2021).
Todd A, Martin C, Morgan J, Herbert E, Bradburn M, Burton M, et al. Age specific recruitment and retention to a large multicentre observational breast cancer trial in older women: the Age Gap trial. J Geriatr Oncol 2020;12:714–23. URL: www.geriatriconcology.net/action/showPdf?pii=S1879-4068%2820%2930487-2 (accessed September 2021).
Battisti NML, Reed MWR, Herbert E, Morgan JL, Collins KA, Ward SE, et al. Bridging the Age Gap in breast cancer: impact of chemotherapy on quality of life in older women with early breast cancer. Eur J Cancer 2021;144:269–80. URL: www.ejcancer.com/action/showPdf?pii=S0959-8049%2820%2931359-9 (accessed September 2021).
Ward SE, Holmes GR, Morgan JL, Broggio JW, Collins K, Richards PD, et al. Bridging the Age Gap: a prognostic model that predicts survival and aids in primary treatment decisions for older women with oestrogen receptor-positive early breast cancer. Br J Surg 2020;107:1625–32. URL: https://bjssjournals.onlinelibrary.wiley.com/doi/full/10.1002/bjs.11748 (accessed September 2021).
Lifford KJ, Edwards A, Burton M, Harder H, Armitage F, Morgan JL, et al. Efficient development and usability testing of decision support interventions for older women with breast cancer. Patient Prefer Adherence 2019;13:131–43. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC6338238/pdf/ppa-13-131.pdf (accessed September 2021).
Ward SE, Holmes GR, Ring A, Richards PD, Morgan JL, Broggio JW, et al. Adjuvant chemotherapy for breast cancer in older women: an analysis of retrospective English cancer registration data. Clin Oncol 2019;31:444–52. URL: https://eprints.whiterose.ac.uk/146368/4/Clin%20Oncology%202019_chemo_%20authors%20accepted%20%20version_with%20tables%20and%20figs.pdf (accessed September 2021).
Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psycho-Oncology 2019;28:1367–80. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC6619389/pdf/PON-28-1367.pdf
Martin C, Shrestha A, Burton M, Collins K, Wyld L. How are caregivers involved in treatment decision-making for older people with dementia and a new diagnosis of cancer? Psycho-Oncology 2019;28:1197–206. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC6563536/pdf/PON-28-1197.pdf (accessed September 2021).
Ward SE, Richards PD, Morgan J, Holmes GR, Broggio J, Collins K, et al. Omission of surgery in older women with early breast cancer has an adverse impact on breast cancer-specific survival. Br J Surg 2018;105:1454–63. URL: https://eprints.whiterose.ac.uk/132928/4/ETALONE%20SURVIVAL%20paper_BJS_accepted%20_with%20tables%20%2526%20figures_changes%20accepted.pdf (accessed September 2021).
Collins K, Reed M, Lifford K, Burton M, Edwards A, Ring A, et al. Bridging the age gap in breast cancer: evaluation of decision support interventions for older women with operable breast cancer: protocol for a cluster randomised controlled trial. BMJ Open 2017;7:e015133. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC5642653/pdf/bmjopen-2016-015133.pdf (accessed September 2021).
Morgan JL, Walters SJ, Collins K, Robinson TG, Cheung KL, Audisio R, et al. What influences healthcare professionals’ treatment preferences for older women with operable breast cancer? An application of the discrete choice experiment. Eur J Surg Oncol 2017;43:1282–7. URL: https://eprints.whiterose.ac.uk/113923/1/Morgan%20et%20al%20FINAL%20word%20version%20of%20accepted%20paper%20EJSO%202017.pdf (accessed September 2021).
Burton M, Kilner K, Wyld L, Lifford KJ, Gordon F, Allison A, et al. Information needs and decision-making preferences of older women offered a choice between surgery and primary endocrine therapy for early breast cancer. Psycho-Oncology 2017;26:2094–100. URL: https://eprints.whiterose.ac.uk/115105/3/Information%20needs%20and%20DM%20of%20older%20women%20with%20breast%20cancer%20good%20final%20draft.pdf (accessed September 2021).
Richards P, Ward S, Morgan J, Lagord C, Reed M, Collins K, Wyld L. The use of surgery in the treatment of ER+ early stage breast cancer in England: variation by time, age and patient characteristics. Eur J Surg Oncol 2016;42:489–96. URL: https://eprints.whiterose.ac.uk/96118/3/WRRO_96118.pdf (accessed September 2021).
Morgan JL, Richards P, Zaman O, Ward S, Collins K, Robinson T, et al. The decision-making process for senior cancer patients: treatment allocation of older women with operable breast cancer in the UK. Cancer Biol Med 2015;12:308–15. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC4706524/pdf/cbm-12-04-308.pdf (accessed September 2021).
Morgan JL, Collins K, Robinson TG, Cheung KL, Audisio R, Reed MW, Wyld L. Healthcare professionals’ preferences for surgery or primary endocrine therapy to treat older women with operable breast cancer. Eur J Surg Oncol 2015;41:1234–42. URL: https://shura.shu.ac.uk/10090/1/Collins_Healthcare_professionals%E2%80%99_preferences_for_surgery_.pdf (accessed September 2021).
Morgan J, Richards P, Ward S, Francis M, Lawrence G, Collins K, et al. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br J Surg 2015;102:1056–63. URL: https://eprints.whiterose.ac.uk/87495/12/registry_variation_in_surgery_rates_paper_submitted_version.pdf (accessed September 2021).
Morgan JL, Burton M, Collins K, Lifford KJ, Robinson TG, Cheung KL, et al. The balance of clinician and patient input into treatment decision-making in older women with operable breast cancer. Psycho-Oncology 2015;24:1761–6. URL: https://eprints.whiterose.ac.uk/169849/3/PON%20Manuscript%20balance%20of%20clinician%20and%20patient%20input%20psychoonoclogy%20accepted%20version.pdf (accessed September 2021).
Lifford KJ, Witt J, Burton M, Collins K, Caldon L, Edwards A, et al. Understanding older women’s decision making and coping in the context of breast cancer treatment. BMC Med Inform Decis Mak 2015;15:45. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC4461993/pdf/12911_2015_Article_167.pdf (accessed September 2021).
Morgan J, Wyld L, Collins KA, Reed MW. Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus). Cochrane Database Syst Rev 2014;5:CD004272. URL: www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004272.pub3/epdf/full (accessed September 2021).
Morgan JL, Reed MW, Wyld L. Primary endocrine therapy as a treatment for older women with operable breast cancer – a comparison of randomised controlled trial and cohort study findings. Eur J Surg Oncol 2014;40:676–84. URL: https://eprints.whiterose.ac.uk/169876/3/Manuscript%20for%20submission%20to%20EJSO%20%28Morgan%29%20plus%20tables%20submitted%20version.pdf (accessed September 2021).
Burton M, Collins K, Caldon L, Wyld L, Reed M. Information needs of older women faced with a choice of primary endocrine therapy or surgery for early-stage breast cancer: a literature review. Curr Breast Cancer Rep 2014;6:235–44. URL: https://shura.shu.ac.uk/8484/1/Burton_Information_needs_of_older_Women.pdf (accessed September 2021).
Burton M, Collins KA, Lifford KJ, Brain K, Wyld L, Caldon L, et al. The information and decision support needs of older women (> 75 years) facing treatment choices for breast cancer: a qualitative study. Psycho-Oncology 2015;24:878–84. URL: https://eprints.whiterose.ac.uk/115105/3/Information%20needs%20and%20DM%20of%20older%20women%20with%20breast%20cancer%20good%20final%20draft.pdf (accessed September 2021).
Trial sponsor
This trial was sponsored by Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust, Clinical Research Office, Doncaster Royal Infirmary (Doncaster, UK).
Data-sharing statement
The Age Gap data set will be used in 2025/26 for 10-year survival outcome analysis, when we plan to make contact with the UK Cancer Registry again to gain access to 10-year survival outcomes for both the ET ALONE versus surgery cohort, and the adjuvant chemotherapy versus no chemotherapy cohort. Once these key final papers have been published, we will make the data set (of fully anonymised data) available for open access in 2027. All data requests should be submitted to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- European Parliament, Council of the European Union . Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation). OJ L 119 2016.
- Cancer Research UK . Breast Cancer Statistics 2022. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer (accessed May 2022).
- Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol 2010;28:2038-45. https://doi.org/10.1200/JCO.2009.25.9796.
- Tang V, Zhao S, Boscardin J, Sudore R, Covinsky K, Walter LC, et al. Functional status and survival after breast cancer surgery in nursing home residents. JAMA Surg 2018;153:1090-6. https://doi.org/10.1001/jamasurg.2018.2736.
- Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 2005;23:2716-25. https://doi.org/10.1200/JCO.2005.06.178.
- Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G, et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 2010;12. https://doi.org/10.1186/bcr2464.
- Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst 2000;92:550-6. https://doi.org/10.1093/jnci/92.7.550.
- McCarty KS, Silva JS, Cox EB, Leight GS, Wells SA, McCarty KS. Relationship of age and menopausal status to estrogen receptor content in primary carcinoma of the breast. Ann Surg 1983;197:123-7. https://doi.org/10.1097/00000658-198302000-00001.
- Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L. Hormonal therapies for early breast cancer: systematic review and economic evaluation. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11260.
- Morgan JL, Reed MW, Wyld L. Primary endocrine therapy as a treatment for older women with operable breast cancer – a comparison of randomised controlled trial and cohort study findings. Eur J Surg Oncol 2014;40:676-84. https://doi.org/10.1016/j.ejso.2014.02.224.
- Morgan J, Wyld L, Collins KA, Reed MW. Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus). Cochrane Database Syst Rev 2014;5. https://doi.org/10.1002/14651858.CD004272.pub3.
- Johnston SJ, Kenny FS, Syed BM, Robertson JFR, Pinder SE, Winterbottom L, et al. A randomised trial of primary tamoxifen versus mastectomy plus adjuvant tamoxifen in fit elderly women with invasive breast carcinoma of high oestrogen receptor content: long-term results at 20 years of follow-up. Ann Oncol 2012;23:2296-300. https://doi.org/10.1093/annonc/mdr630.
- Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527-32. https://doi.org/10.1023/a:1013128213451.
- Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Jänicke F, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 2001;19:3808-16. https://doi.org/10.1200/JCO.2001.19.18.3808.
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 2003;21:2101-9. https://doi.org/10.1200/JCO.2003.04.194.
- Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365:60-2. https://doi.org/10.1016/S0140-6736(04)17666-6.
- Husain LS, Collins K, Reed M, Wyld L. Choices in cancer treatment: a qualitative study of the older women’s (> 70 years) perspective. Psycho-oncology 2008;17:410-6. https://doi.org/10.1002/pon.1242.
- Adjogatse D, Thanopoulou E, Okines A, Thillai K, Tasker F, Johnston SR, et al. Febrile neutropaenia and chemotherapy discontinuation in women aged 70 years or older receiving adjuvant chemotherapy for early breast cancer. Clin Oncol 2014;26:692-6. https://doi.org/10.1016/j.clon.2014.05.002.
- Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, Godwin J, et al. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet 2008;371:29-40. https://doi.org/10.1016/S0140-6736(08)60069-0.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) . Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432-44. https://doi.org/10.1016/S0140-6736(11)61625-5.
- Jahuri Y, Gannon M, Medina J, Cromwell D, Horgan K, Dodwell D. National Audit of Breast Cancer in Older Patients: 2019 Annual Report 2019.
- Karavasilis V, Papadimitriou C, Gogas H, Kouvatseas G, Pentheroudakis G, Koutras A, et al. Safety and tolerability of anthracycline-containing adjuvant chemotherapy in elderly high-risk breast cancer patients. Clin Breast Cancer 2016;16:291-8.e3. https://doi.org/10.1016/j.clbc.2015.12.001.
- Ngamphaiboon N, O’Connor TL, Advani PP, Levine EG, Kossoff EB. Febrile neutropenia in adjuvant docetaxel and cyclophosphamide (TC) with prophylactic pegfilgrastim in breast cancer patients: a retrospective analysis. Med Oncol 2012;29:1495-501. https://doi.org/10.1007/s12032-011-0035-5.
- Ring A, Harder H, Langridge C, Ballinger RS, Fallowfield LJ. Adjuvant chemotherapy in elderly women with breast cancer (AChEW): an observational study identifying MDT perceptions and barriers to decision making. Ann Oncol 2013;24:1211-19. https://doi.org/10.1093/annonc/mds642.
- Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4. https://doi.org/10.1002/14651858.CD001431.pub5.
- Lifford KJ, Witt J, Burton M, Collins K, Caldon L, Edwards A, et al. Understanding older women’s decision making and coping in the context of breast cancer treatment. BMC Med Inform Decis Mak 2015;15. https://doi.org/10.1186/s12911-015-0167-1.
- Burton M, Kilner K, Wyld L, Lifford KJ, Gordon F, Allison A, et al. Information needs and decision-making preferences of older women offered a choice between surgery and primary endocrine therapy for early breast cancer. Psycho-oncology 2017;26:2094-100. https://doi.org/10.1002/pon.4429.
- Morgan JL, Burton M, Collins K, Lifford KJ, Robinson TG, Cheung KL, et al. The balance of clinician and patient input into treatment decision-making in older women with operable breast cancer. Psycho-oncology 2015;24:1761-6. https://doi.org/10.1002/pon.3853.
- Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, O’Neil J, et al. Information needs and decisional preferences in women with breast cancer. JAMA 1997;277:1485-92. https://doi.org/10.1001/jama.1997.03540420081039.
- Harder H, Ballinger R, Langridge C, Ring A, Fallowfield LJ. Adjuvant chemotherapy in elderly women with breast cancer: patients’ perspectives on information giving and decision making. Psycho-oncology 2013;22:2729-35. https://doi.org/10.1002/pon.3338.
- Lifford KJ, Edwards A, Burton M, Harder H, Armitage F, Morgan JL, et al. Efficient development and usability testing of decision support interventions for older women with breast cancer. Patient Prefer Adherence 2019;13:131-43. https://doi.org/10.2147/PPA.S178347.
- Ward SE, Richards PD, Morgan JL, Holmes GR, Broggio JW, Collins K, et al. Omission of surgery in older women with early breast cancer has an adverse impact on breast cancer-specific survival. Br J Surg 2018;105:1454-63. https://doi.org/10.1002/bjs.10885.
- Ward SE, Holmes GR, Ring A, Richards PD, Morgan JL, Broggio JW, et al. Adjuvant chemotherapy for breast cancer in older women: an analysis of retrospective English cancer registration data. Clin Oncol 2019;31:444-52. https://doi.org/10.1016/j.clon.2019.03.005.
- Ward SE, Holmes GR, Morgan JL, Broggio JW, Collins K, Richards PD, et al. Bridging the Age Gap: a prognostic model that predicts survival and aids in primary treatment decisions for older women with oestrogen receptor-positive early breast cancer. Br J Surg 2020;107:1625-32. https://doi.org/10.1002/bjs.11748.
- Richards P, Ward S, Morgan J, Lagord C, Reed M, Collins K, et al. The use of surgery in the treatment of ER+ early stage breast cancer in England: variation by time, age and patient characteristics. Eur J Surg Oncol 2016;42:489-96. https://doi.org/10.1016/j.ejso.2015.12.012.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- Morris EJ, Taylor EF, Thomas JD, Quirke P, Finan PJ, Coleman MP, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011;60:806-13. https://doi.org/10.1136/gut.2010.232181.
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175-97. https://doi.org/10.1002/sim.1203.
- Stineman MG, Xie D, Pan Q, Kurichi JE, Zhang Z, Saliba D, et al. All-cause 1-, 5-, and 10-year mortality in elderly people according to activities of daily living stage. J Am Geriatr Soc 2012;60:485-92. https://doi.org/10.1111/j.1532-5415.2011.03867.x.
- Koissi M-C, Högnäs G. Using WinBUGS to study family frailty in child mortality, with an application to child survival in Ivory Coast. Afr Pop Stud 2005;20. https://doi.org/10.11564/20-1-384.
- Early Breast Cancer Trialists’ Collaborative Group . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. https://doi.org/10.1016/S0140-6736(05)66544-0.
- Noble S, McLennan D, Noble M, Plunkett E, Gutacker N, Silk M, et al. English Indices of Deprivation 2019 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833947/IoD2019_Research_Report.pdf (accessed May 2022).
- University of Sheffield . Age Gap Decision Tool n.d. https://agegap.shef.ac.uk/.
- Collins K, Reed M, Lifford K, Burton M, Edwards A, Ring A, et al. Bridging the age gap in breast cancer: evaluation of decision support interventions for older women with operable breast cancer: protocol for a cluster randomised controlled trial. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015133.
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010.
- Read JA, Crockett N, Volker DH, MacLennan P, Choy ST, Beale P, et al. Nutritional assessment in cancer: comparing the Mini-Nutritional Assessment (MNA) with the scored Patient-Generated Subjective Global Assessment (PGSGA). Nutr Cancer 2005;53:51-6. https://doi.org/10.1207/s15327914nc5301_6.
- Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12:15-9. https://doi.org/10.1016/0899-9007(95)00067-4.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965;14:61-5. https://doi.org/10.1037/t02366-000.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179-86. https://doi.org/10.1093/geront/9.3_Part_1.179.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. https://doi.org/10.1097/00000421-198212000-00014.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996;14:2756-68. https://doi.org/10.1200/JCO.1996.14.10.2756.
- Johnson C, Fitzsimmons D, Gilbert J, Arrarras JI, Hammerlid E, Bredart A, et al. Development of the European Organisation for Research and Treatment of Cancer quality of life questionnaire module for older people with cancer: the EORTC QLQ-ELD15. Eur J Cancer 2010;46:2242-52. https://doi.org/10.1016/j.ejca.2010.04.014.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 Scoring Manual. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
- Wheelwright S, Darlington AS, Fitzsimmons D, Fayers P, Arraras JI, Bonnetain F, et al. International validation of the EORTC QLQ-ELD14 questionnaire for assessment of health-related quality of life elderly patients with cancer. Br J Cancer 2013;109:852-8. https://doi.org/10.1038/bjc.2013.407.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Todd A, Martin C, Morgan J, Herbert E, Bradburn M, Burton M, et al. Age specific recruitment and retention to a large multicentre observational breast cancer trial in older women: the Age Gap Trial. J Geriatr Oncol 2021;12:714-23. https://doi.org/10.1016/j.jgo.2020.10.015.
- Morgan JL, George J, Holmes G, Martin C, Reed MWR, Ward S, et al. Breast cancer surgery in older women: outcomes of the Bridging Age Gap in Breast Cancer study. Br J Surg 2020;107:1468-79. https://doi.org/10.1002/bjs.11617.
- Wyld L, Reed MWR, Morgan J, Collins K, Ward S, Holmes GR, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer 2021;142:48-62. https://doi.org/10.1016/j.ejca.2020.10.015.
- Horgan KDD, Jauhari Y, Gannon M, Medina J, Cromwell D. National Audit of Breast Cancer in Older People (NABCOP) Annual Report 2018. www.nabcop.org.uk/reports/nabcop-2018-annual-report/ (accessed December 2018).
- Fennessey M, Bates T, McRae K, Riley D, Houghton J, Baum M. Randomised trial of surgery plus tamoxifen vs. tamoxifen-alone in women over age 70 with operable breast cancer. Br J Surg 2004;91:699-704. https://doi.org/10.1002/bjs.4603.
- Mustacchi G, Ceccherini R, Milani S, Pluchinotta A, De Matteis A, Maiorino L, et al. Tamoxifen alone vs adjuvant tamoxifen for operable breast cancer of the elderly: long-term results of the phase III randomized controlled multicenter GRETA trial. Ann Oncol 2003;14:414-20. https://doi.org/10.1093/annonc/mdg117.
- Kenny FS, Robertson JFR, Ellis IO, Elston CW, Blamey RW. Long-term follow-up of elderly patients randomized to primary tamoxifen or wedge mastectomy as initial therapy for operable breast cancer. Breast 1998;7:335-9. https://doi.org/10.1016/S0960-9776(98)90077-7.
- Morgan JL, Shrestha A, Reed MWR, Herbert E, Bradburn M, Walters SJ, et al. Bridging the age gap in breast cancer: impact of omission of breast cancer surgery in older women with oestrogen receptor-positive early breast cancer on quality-of-life outcomes. Br J Surg 2021;108:315-25. https://doi.org/10.1093/bjs/znaa125.
- Goldberg DP, Cooper B, Eastwood MR, Kedward HB, Shepherd M. A standardized psychiatric interview for use in community surveys. Br J Prev Soc Med 1970;24:18-23. https://doi.org/10.1136/jech.24.1.18.
- Fallowfield L. Quality of life in elderly women with breast cancer treated with tamoxifen and surgery or tamoxifen alone. J Womens Health 1994;3:17-20. https://doi.org/10.1089/jwh.1994.3.17.
- Ring A, Reed M, Leonard R, Kunkler I, Muss H, Wildiers H, et al. The treatment of early breast cancer in women over the age of 70. Br J Cancer 2011;105:189-93. https://doi.org/10.1038/bjc.2011.234.
- Ring A, Battisti NML, Reed MWR, Herbert E, Morgan JL, Bradburn M, et al. Bridging The Age Gap: observational cohort study of effects of chemotherapy and trastuzumab on recurrence, survival and quality of life in older women with early breast cancer. Br J Cancer 2021;125:209-19. https://doi.org/10.1038/s41416-021-01388-9.
- Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992;22:207-19. https://doi.org/10.1007/BF01840834.
- Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019;381:307-16. https://doi.org/10.1056/NEJMoa1903765.
- Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol 2006;24:2750-6. https://doi.org/10.1200/JCO.2005.02.3028.
- Kornblith AB, Lan L, Archer L, Partridge A, Kimmick G, Hudis C, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol 2011;29:1022-8. https://doi.org/10.1200/JCO.2010.29.9859.
- Battisti NML, Reed MWR, Herbert E, Morgan JL, Collins KA, Ward SE, et al. Bridging the Age Gap in breast cancer: impact of chemotherapy on quality of life in older women with early breast cancer. Eur J Cancer 2021;144:269-80. https://doi.org/10.1016/j.ejca.2020.11.022.
- Martin C, Shrestha A, Morgan J, Bradburn M, Herbert E, Burton M, et al. Treatment choices for older women with primary operable breast cancer and cognitive impairment: results from a prospective, multicentre cohort study. J Geriatr Oncol 2021;12:705-13. https://doi.org/10.1016/j.jgo.2020.12.006.
- Holmes GR, Ward SE, Brennan A, Bradburn M, Morgan JL, Reed MWR, et al. Cost-effectiveness modeling of surgery plus adjuvant endocrine therapy versus primary endocrine therapy alone in UK women aged 70 and over with early breast cancer. Value Health 2021;24:770-9. https://doi.org/10.1016/j.jval.2020.12.016.
- Narita S, Tsuchiya N, Kumazawa T, Maita S, Numakura K, Obara T, et al. Comparison of surgical stress in patients undergoing open versus laparoscopic radical prostatectomy by measuring perioperative serum cytokine levels. J Laparoendosc Adv Surg Tech A 2013;23:33-7. https://doi.org/10.1089/lap.2012.0348.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA. Health-related quality of life and healthcare utilization in multimorbidity: results of a cross-sectional survey. Qual Life Res 2013;22:791-9. https://doi.org/10.1007/s11136-012-0214-7.
- Meng Y, Ward S, Cooper K, Harnan S, Wyld L. Cost-effectiveness of MRI and PET imaging for the evaluation of axillary lymph node metastases in early stage breast cancer. Eur J Surg Oncol 2011;37:40-6. https://doi.org/10.1016/j.ejso.2010.10.001.
- Lovrics PJ, Cornacchi SD, Barnabi F, Whelan T, Goldsmith CH. The feasibility and responsiveness of the health utilities index in patients with early-stage breast cancer: a prospective longitudinal study. Qual Life Res 2008;17:333-45. https://doi.org/10.1007/s11136-007-9305-2.
- Wyld L, Reed MWR, Collins K, Burton M, Lifford K, Edwards A, et al. Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg 2021;108:499-510. https://doi.org/10.1093/bjs/znab005.
- Marrin K, Brain K, Durand M-A, Edwards A, Lloyd A, Thomas V, et al. Fast and frugal tools for shared decision making: how to develop option grids. Eur J Pers Cent Healthc 2013;1:240-5. https://doi.org/10.5750/ejpch.v1i1.657.
- Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Med Decis Making 2003;23:281-92. https://doi.org/10.1177/0272989X03256005.
- Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.3085.
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301-6. https://doi.org/10.1111/j.2044-8260.1992.tb00997.x.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- Fillion L, Kovacs AH, Gagnon P, Endler NS. Validation of the shortened COPE for use with breast cancer patients undergoing radiotherapy. Curr Psychol 2002;21:17-34. https://doi.org/10.1007/BF02903157.
- Campbell MJ, Walters SJ. How to Design, Analyse and Report Cluster Randomised Trials in Medicine and Health Related Research 2014. https://doi.org/10.1002/9781118763452.
- Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 2011;29:89-96. https://doi.org/10.1200/JCO.2010.28.0107.
- Burton M, Lifford KJ, Wyld L, Armitage F, Ring A, Nettleship A, et al. Process evaluation of the Bridging the Age Gap in Breast Cancer decision support intervention cluster randomised trial. Trials 2021;22. https://doi.org/10.1186/s13063-021-05360-z.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Morgan J, Richards P, Ward S, Francis M, Lawrence G, Collins K, et al. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br J Surg 2015;102:1056-63. https://doi.org/10.1002/bjs.9842.
- Morgan JL, Walters SJ, Collins K, Robinson TG, Cheung KL, Audisio R, et al. What influences healthcare professionals’ treatment preferences for older women with operable breast cancer? An application of the discrete choice experiment. Eur J Surg Oncol 2017;43:1282-7. https://doi.org/10.1016/j.ejso.2017.01.012.
- Morgan JL, Collins K, Robinson TG, Cheung KL, Audisio R, Reed MW, et al. Healthcare professionals’ preferences for surgery or primary endocrine therapy to treat older women with operable breast cancer. Eur J Surg Oncol 2015;41:1234-42. https://doi.org/10.1016/j.ejso.2015.05.022.
- Breast Cancer Clinical Outcome Measures . Breast Cancer Clinical Outcome Measures (BCCOM) Project: Analysis of the Management of Symptomatic Breast Cancers Diagnosed in 2004. 3rd Year Report December 2007 2007.
- Wishart GC, Greenberg DC, Chou P, Brown CH, Duffy S, Purushotham AD. Treatment and survival in breast cancer in the Eastern Region of England. Ann Oncol 2010;21:291-6. https://doi.org/10.1093/annonc/mdp301.
- Ali AM, Greenberg D, Wishart GC, Pharoah P. Patient and tumour characteristics, management, and age-specific survival in women with breast cancer in the East of England. Br J Cancer 2011;104:564-70. https://doi.org/10.1038/bjc.2011.14.
- Lavelle K, Downing A, Thomas J, Lawrence G, Forman D, Oliver S. Are lower rates of surgery amongst older women with breast cancer in the UK explained by comorbidity?. Br J Cancer 2012;170:1175-80. https://doi.org/10.1038/bjc.2012.192.
- Su Y-S, Yajima M, Gelman AE, Hill J. Multiple imputation with diagnostics (mi) in R: opening windows into the black box. J Stat Soft 2011;45:1-31. https://doi.org/10.18637/jss.v045.i02.
- Rubin D. Multiple Imputation from Non-Response in Surveys. New York, NY: John Wiley & Sons; 1987.
- Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med 2005;24:1185-202. https://doi.org/10.1002/sim.1970.
- Ritchie J, Spencer L, Huberman AM, Miles MB. The Qualitative Researcher’s Companion. London: SAGE Publications Ltd; 2002.
- Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ 2000;320:1530-3. https://doi.org/10.1136/bmj.320.7248.1530.
- Gannon M, Miller K, Medina J, Cromwell D, Horgan K, Dodwell D. National Audit of Breast Cancer in Older Patients: 2021 Annual Report 2021.
- Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 2012;13:e148-60. https://doi.org/10.1016/S1470-2045(11)70383-7.
- Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol 2007;25:1858-69. https://doi.org/10.1200/JCO.2006.10.4208.
- Cameron C, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195-205. https://doi.org/10.1016/S0140-6736(16)32616-2.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. NEJM 2005;353:1673-84. https://doi.org/10.1056/NEJMoa052122.
- Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859-65. https://doi.org/10.1200/JCO.2006.09.1611.
- Autier P, Boniol M, La Vecchia C, LaVecchia C, Vatten L, Gavin A, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ 2010;341. https://doi.org/10.1136/bmj.c3620.
- Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. JCO 2007;25:3699-704. https://doi.org/10.1200/JCO.2007.10.9710.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205-16. https://doi.org/10.1093/jnci/92.3.205.
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805-35. https://doi.org/10.1097/EDE.0b013e3181577511.
Appendix 1 Systematic review of adjuvant chemotherapy outcomes in women aged ≥ 70 years with breast cancer
Introduction
The Early Breast Cancer Triallists’ Collaborative Group analysis of adjuvant chemotherapy in women aged ≥ 70 years found that adjuvant chemotherapy reduced the risk of recurrence by 12% and death by 13%. 41 The results were not statistically significant owing to the small number of women aged ≥ 70 years; nonetheless, international guidelines recommend that adjuvant chemotherapy should be considered in fitter older women with high-risk breast cancer. 107 Despite this, numerous studies report low rates of adjuvant chemotherapy and trastuzumab in older women. 21,24,108 Adjuvant chemotherapy omission may result from concerns about toxicity, but age-specific data on this are sparse and are reviewed below. This review was used to populate the adjuvant chemotherapy DESI with age-specific outcome data.
Methods
Studies were identified from MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and the Cochrane Library. The following medical subject heading (MeSH) terms were used to search for adjuvant chemotherapy toxicities in elderly patients with early-stage breast cancer: ‘Breast’, ‘Cancer’, ‘Neoplasm’, ‘Malignancy’, ‘Older’, ‘Aged’, ‘Elderly’, ‘Frail’, ‘adjuvant chemotherapy’, ‘toxicity’, ‘side effects’, ‘adverse effects’, ‘adverse events’ and ‘tolerability’. We searched for relevant studies of trastuzumab toxicity in older patients with early-stage breast cancer using the same MeSH terms, but ‘adjuvant chemotherapy’ was omitted and ‘adjuvant’, ‘Trastuzumab’, ‘Herceptin’ and ‘HER-2’ were used instead.
Results
In total, 627 studies relevant to toxicities of adjuvant chemotherapy and 313 relevant to adjuvant trastuzumab in older people were identified. Of these, 20 were selected that reported toxicity from adjuvant chemotherapy. These comprised five RCTs, three pooled analyses of RCTs, four cohort studies and eight retrospective studies.
In total, data from four studies relevant to adjuvant trastuzumab toxicity in older patients were reviewed; this included one cohort study and three retrospective studies.
Rates of toxicity from adjuvant chemotherapy in older women were as follows:
-
The febrile neutropenia/neutropenic sepsis rate was between 0% and 29%.
-
Anthracycline-based regimens had a reported febrile neutropenia rate of 0–29%, whereas non-anthracycline regimens had reported rates of between 0% and 16.7%.
-
Adjuvant chemotherapy toxicity-related death rates ranged from 0% to 1.5%.
-
Trastuzumab-related cardiac morbidity in older patients ranged from 0.2% to 9.2%, with arrhythmias occurring in 0.2–1% of patients (grade 1 severity).
Only 16% of the patients entering the HERA (herceptin adjuvant) and NSABP (national surgical adjuvant breast and bowel project) B31/N9831 adjuvant trastuzumab trials109,110 were aged ≥ 60 years, but the benefits of trastuzumab in this subgroup were the same as for the whole-study populations. In the HERA trial, the incidence rates of cardiac adverse events for those aged ≥ 60 years compared with those aged < 60 years were not significantly different (3.75% vs. 3.61%). 111 However, there were too few patients aged ≥ 65 years to analyse this older group and the clinical trial population selected in the HERA trial may not be representative of the general breast cancer population. The data reviewed suggest that the majority of older patients who are treated with adjuvant trastuzumab complete the course of treatment with low risk of short-term cardiac complications, although there are few data regarding longer-term risks.
Discussion
The median reduction in breast cancer-associated mortality between 1989 and 2006 was 37% for women aged < 50 years, compared with a 2% reduction for women aged > 69 years. 112 The reasons for this are multifactorial, but undertreatment is likely to have contributed. Undertreatment is partly due to understandable concerns over life-threatening toxicities of adjuvant chemotherapy. However, given that the median survival of a 70-year-old woman in the UK is 15.9 years, it becomes apparent that conservatism could leave a cohort of fit, older women inadequately treated who are likely to live long enough to experience a recurrence. Adverse event rates in this older age group are acceptable in published series. 113
Appendix 2 Summary of clinician feedback and responses during development of the online tool component of the decision support interventions
| Clinician feedback | Response |
|---|---|
| Both tools | |
| Where do the data come from? | Addressed by the introduction of a FAQs sheet that explained the status of the data |
| Has the tool been validated? | Addressed by the introduction of a FAQs sheet that explained the status of the data |
| Why is performance status not included? | Addressed by the introduction of a FAQs sheet that explained the status of the data |
| Comments about the definitions assigned to the CCI | Addressed by the introduction of a FAQs sheet that explained the status of the data |
| It is important to be able to calculate relapse | It was explained during training sessions that the current data do not allow for this calculation in older women |
| Predicting survival to 10 years is too long | The option of a 10-year survival prediction was removed |
| Would be useful to print out the data collection page for use in MDTs | A function was added to allow this |
| Helpful to have navigation button to return to data collection page | A navigation button was added |
| Difficulty in accessing the tool due to internet browser and firewall issues | Contact made with site IT to address this |
| Personalised sheet | |
| Would be advantageous to be able to choose which visual displays to print out | The option to choose was added |
| Colour printing is not always available, and this would affect the visual displays | Shading was added to the graphs |
| The use of the word ‘death’ in the personalised sheet would limit the number of patients whom clinicians felt they could give the information to | The statements were reversed to show the number of women who would be alive |
| Adjuvant chemotherapy tool only | |
| Would like to be able to combine ET ALONE and/or S+ET into the adjuvant chemotherapy tool | It was explained during training sessions that this may be a future development |
| A HER-2 status is required – adjuvant chemotherapy tool | HER-2 status was inserted |
| Would be better to include the level of the ER+ status | It was explained that not all sites calculated this |
| Preference for nodal status to be entered as ‘0’, ‘1–3’ or ‘≥ 4’ | Data collection page altered to this format |
| Highlight items that are based on particularly limited data | The following statement was added to address the data related to older women: ‘Note: For patients over 80 the results are less reliable’. Other items were highlighted in the FAQs and during training sessions |
Appendix 3 Summary of patient feedback and responses during development of the on line tool
| Patient feedback | Response |
|---|---|
| Some of the words were said to be off-putting [e.g. ‘statistics at two years’, and ‘chance (%)’ ‘survival’ used on the personalised sheet] |
‘Statistics at two years’ replaced by ‘ What will have happened to women like you 2 years after they start their breast cancer treatment?’ Axis on graph relabelled ‘number of women’ rather than ‘chance (%)’ Columns on graphs relabelled ‘alive’, ‘died of breast cancer at 2 years’ and ‘died of other causes at 2 years’ |
| Some women were unsure how to interpret the pictograms |
A legend was added to enhance understanding Key: alive, this shows the extra women alive compared to the other treatment option, died from breast cancer or other causes. |
| 10 years is too long to estimate survival | This was removed |
Appendix 4 Endocrine therapy alone versus surgery: brief decision aid
| Question | Hormone-blocking pills | Surgery and hormone-blocking pills |
|---|---|---|
| What does the treatment involve? | Taking a pill every day as long as the pills keep working. The hormone blocking pill is not a type of chemotherapy | An operation to remove the cancer is usually done under general anaesthetic (while you sleep). Some or all the underarm glands may be removed and tested at the same time. Some women go home the same day. A few stay in hospital for up to 4 days. You will also be given pills to take for at least 5 years |
| How does the treatment work? | The pills block a hormone called oestrogen to shrink or stop the cancer growing | By removing the part of the breast that contains the cancer (often called a lumpectomy) or all of the breast (called a mastectomy) |
| Is there a difference between the treatments in how long I will live or if the cancer will spread to other parts of the body? | There is no difference between the treatments in terms of how long you will live or if the cancer will spread | There is no difference between the treatments in terms of how long you will live or if the cancer will spread |
| What are the chances of the breast cancer coming back? | There is a risk that the cancer will start to grow again in about 30–50 in 100 women (30–50%) in 3–5 years. If this happens you may be given different pills or have an operation. This will not change how long you will live compared with having surgery and pills | There is a risk that the cancer will come back again in the breast or mastectomy scar in up to 10 in 100 women (10%) in 3–5 years. If this happens you may be given different pills or have an operation. This will not change how long you will live compared with having pills only |
| Will anything else happen at the start of treatment? | You will have check-ups every 3–6 months to make sure the cancer is not growing | If part of your breast is removed (lumpectomy), you may also have radiotherapy to the remainder of the breast (X-ray treatment) at hospital visits for 3–6 weeks. If the whole of your breast is removed you will not usually have radiotherapy |
| Can I carry on with my normal activities? | Yes | Most women take 2 weeks to get back to normal activities. Some take up to 5 weeks |
| Will I have to go for hospital check-ups? | When the cancer has started to shrink, you will have check-ups every 3–6 months. Your GP may do the check-ups | Typically, you will have a check-up after 1, 3 and 5 years. You may be offered a mammogram every year for 5 years |
| What are the risks or side effects of the treatment? | Common side effects include tiredness, hot flushes and aching joints, often in the hands and feet | The surgery is relatively safe and not usually very painful. A few women have problems with bleeding or infection. Arm swelling (lymphoedema) happens in roughly 15 in 100 women (15%) if all the underarm glands are removed. The pills also have side effects |
Appendix 5 Adjuvant chemotherapy versus no chemotherapy: brief decision aid
Options at a glance
Here are questions women with breast cancer like yours often ask about the treatment options, along with the answer to each question.
| Question | Chemotherapy | No chemotherapy |
|---|---|---|
| What does the treatment involve? | You will attend hospital usually every few weeks for chemotherapy over a 12- to 24-week period. You will have blood tests before each treatment to check all is well. If you have a Herceptin-sensitive cancer you may also have three weekly Herceptin injections for 1 year. You may also be offered other treatments such as radiotherapy or hormone-blocking pills | Following your surgery you may be offered treatments such as radiotherapy or hormone-blocking pills but will otherwise just be followed up |
| How does the treatment work? | Chemotherapy and Herceptin both work by targeting cancer cells and killing them | N/A |
| Is there a difference between the treatments in how long I will live? | Chemotherapy increases the chances of long-term cure for breast cancer in most women by between 2% and 30%. The advantage varies according to cancer type. Your doctor will be able to advise about the precise increase chance of cure | For women with high-risk cancers the risk of the cancer coming back is higher without chemotherapy. This risk varies depending on the type of cancer, your age and fitness |
| What are the chances of the breast cancer coming back? | Chemotherapy ± Herceptin reduce the chances of the cancer coming back both in the breast area and also in other parts of the body (secondary breast cancer) | This varies with the type of cancer you had. Your doctor will be able to advise you about the precise risk and how this would be affected by chemotherapy |
| Can I carry on with my normal activities? | Yes. However, you may feel tired and need more rest. You should avoid contact with people with flu | Yes |
| Will I have to go for hospital check-ups? | During treatment, you will have regular visits for treatments, usually every 3 weeks and blood tests in between. Once treatment is complete you will have normal follow-up as described opposite | You will usually have a check-up after 1, 3 and 5 years. You may be offered a mammogram every year for 5 years |
| What are the risks or side effects of the treatment? | The severity and type vary with treatment type. Some women are more affected than others. Side effects commonly include tiredness, hair thinning or hair loss, nausea and an increased risk of infection | If the cancer comes back, you may need other treatments to help with this |
Appendix 6 Cover illustrations for the two decision support tool booklets
FIGURE 19.
Illustration of the front covers of the two booklet components of the decision support tool. (a) Chemotherapy or no chemotherapy; and (b) surgery and hormone-blocking pulls or hormone-blocking pills only. Reproduced with permission from the University of Sheffield.


Appendix 7 Examples of printouts for the endocrine therapy alone versus surgery online algorithm
FIGURE 20.
Screenshots of the outputs from the online decision support tool, showing an example of a woman in her eighties demonstrating a small survival advantage from surgery. Reproduced with permission from the University of Sheffield.
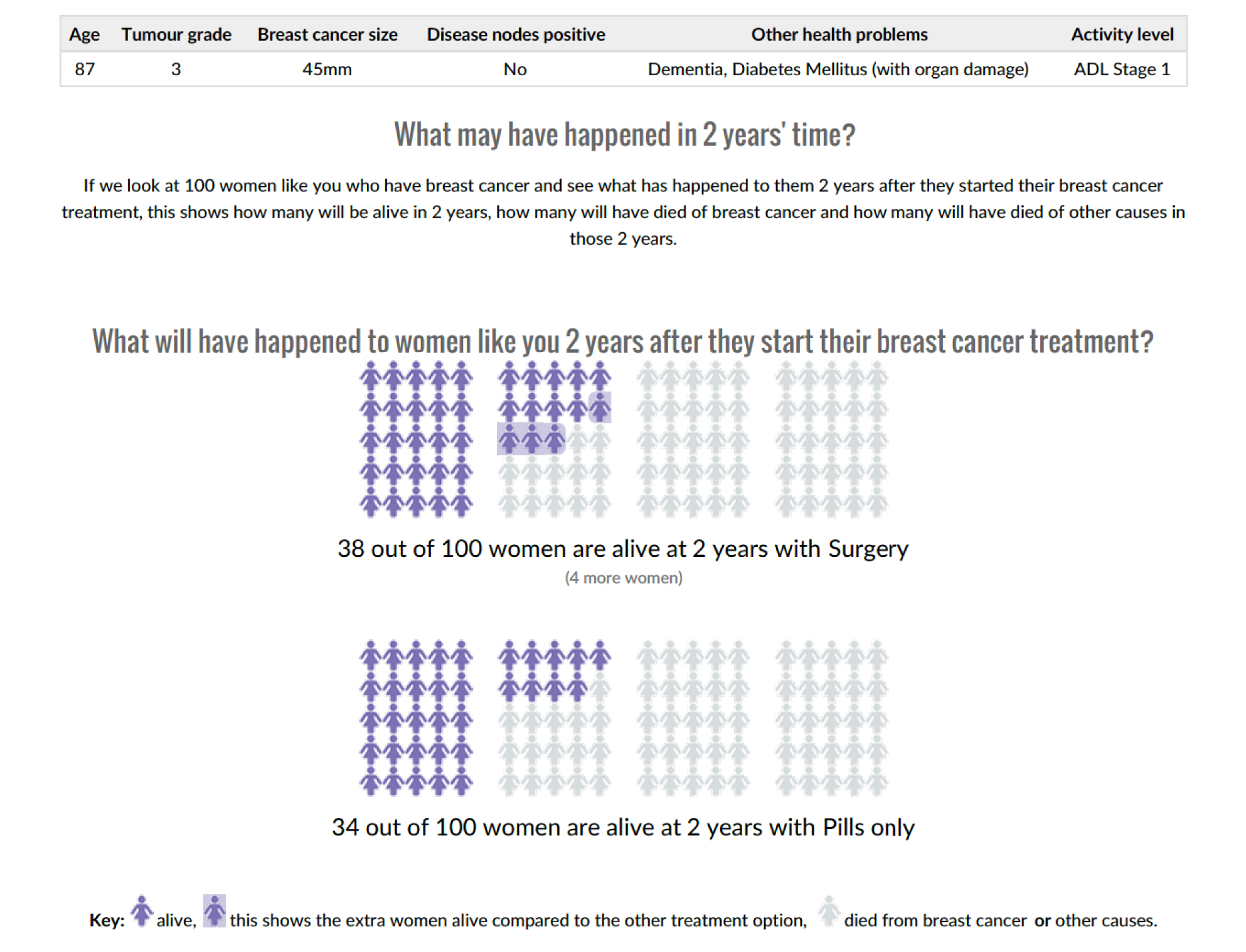
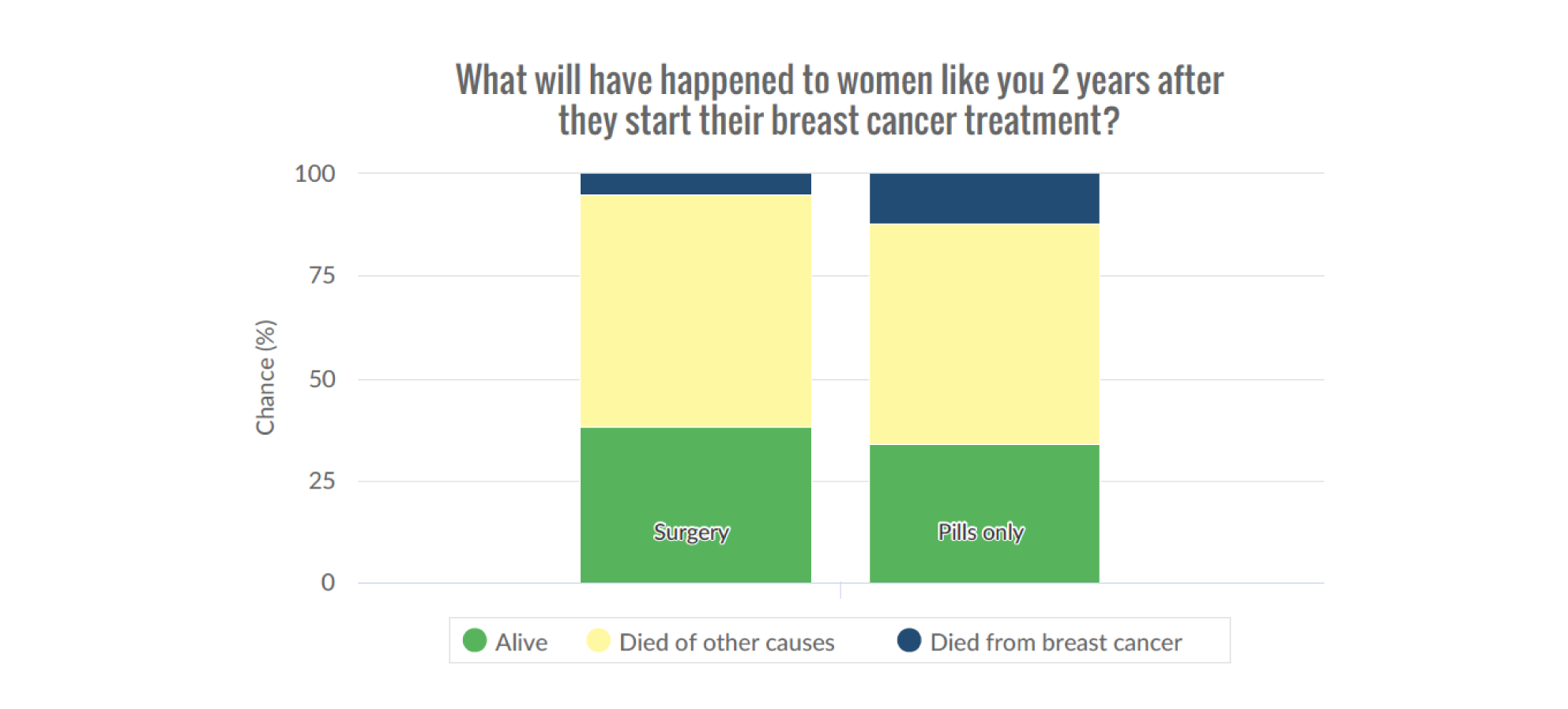
Appendix 8 The Age Gap cohort study: detailed general methods and results
Introduction
This section gives an overview of the detailed methods and general results of the Age Gap cohort study. These are relevant to various sections in the main report.
General methods
Study design
This was a prospective, pragmatic, multicentre, observational UK cohort study.
Aims
To determine health-status-stratified outcomes for older women (aged ≥ 70 years) with early-stage breast cancer according to whether they receive ET ALONE or S+ET, or, for women with high-recurrence-risk breast cancer, whether or not they received adjuvant chemotherapy.
Objectives
To undertake propensity-score-matched analysis of prospectively collected cohort data to allow risk-stratified comparison of outcomes between women receiving ET ALONE versus S+ET, or adjuvant chemotherapy versus no chemotherapy.
Outcomes
Primary outcome
The primary outcome was OS.
Secondary outcomes
Secondary outcomes were BCSS, FFS, time to local recurrence (progression in ET ALONE), time to metastatic recurrence, QoL and adverse events (CTCAE45).
Propensity-score-matched analysis was performed to adjust for baseline variation in patient and disease characteristics between treatment groups.
Ethics and research governance
Ethics approval was obtained in 2012 [Integrated Research Application System (IRAS) 12 LO 1808].
Study sponsor
The study was sponsored by Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust.
Levels of participation
Women were invited to participate after diagnosis, but before treatment commencement.
Patients with full cognitive capacity could select one of two levels of participation: full participation (including QoL forms) or partial participation (excluding QoL). Women with significant cognitive incapacity did not complete QoL questionnaires.
Baseline data collection
At baseline, women underwent assessment using a series of validated tools, including the:
In addition, QoL was assessed using the:
-
EORTC-QLQ-C30,52 a generic cancer QoL tool
-
EORTC-QLQ-BR23,53 a breast-cancer-specific QoL module
-
EORTC-QLQ-ELD14,54 an older-person-specific module
-
EQ-5D-5L55 to permit calculation of QALYs for health economics assessment.
Baseline data were collected regarding the primary tumour stage and biotype. Staging for metastatic disease was performed if clinically indicated, but otherwise was presumed to be stage M0.
Follow-up assessments
Patients were followed up at 6 weeks, and at 6, 12, 18 and 24 months. All patients were assessed for evidence of local, regional or systemic recurrence at each visit. Treatment details and adverse events were recorded using the CTCAE. 45 In addition, patients receiving ET ALONE underwent clinical (or ultrasound) examination to assess disease response at each visit. Response was assessed based on the Response Evaluation Criteria In Solid Tumors (RECIST) criteria. 114
The study also requested optional consent for long-term access to cancer registry data for long-term follow-up purposes up to 10 years.
Participating UK breast units
The study recruited from 46 breast units in England and Wales.
| Site number | Site name | Local principal investigator | RCT site type |
|---|---|---|---|
| 1 | Sheffield | Lynda Wyld and Matt Winter | Intervention |
| 2 | Barnsley | Julia Dicks | Usual care |
| 3 | Doncaster | Clare Rogers | Usual care |
| 4 | Milton Keynes | Amanda Taylor | Intervention |
| 5 | Scunthorpe and Grimsby | Rajesh Vijh (Scunthorpe) and Jenny Smith (Grimsby) | Usual care |
| 6 | Leicester | Monika Kaushik | Usual care |
| 7 | Derby | Kwok Leung Cheung | Usual care |
| 8 | East Lancashire | Julie Iddon | Intervention |
| 9 | Harrogate | Matthew Adelekan | Intervention |
| 10 | St Helens and Knowsley | Riccardo Audisio | Usual care |
| 11 | York | Rana Nasr (York and Scarborough) | Intervention |
| 12 | Liverpool | Chris Holcombe | Intervention |
| 13 | Airedale | Claire Murphy | Not in RCT |
| 14 | Leeds | Kieran Horgan | Intervention |
| 15 | Bradford | Rick Linforth | Usual care |
| 16 | Cardiff | Helen Sweetland | Usual care |
| 17 | Aneurin Bevan University Health Board | Simon Waters (Royal Gwent Hospital), Theresa Howe (Nevill Hall Hospital) | Usual care |
| 18 | Lancaster | Rishi Parmeshwar | Intervention |
| 19 | Coventry | Abigail Tomlins | Intervention |
| 20 | Grantham | Anzors Gvaramadze | Not in RCT |
| 21 | Lincoln | Anzors Gvaramadze | Not in RCT |
| 22 | Pilgrim | Anzors Gvaramadze | Not in RCT |
| 23 | Hull | Peter Kneeshaw | Intervention |
| 24 | Nottingham | Lisa Whisker | Usual care |
| 25 | Southport | Anwar Haq | Not in RCT |
| 26 | Leighton | Vanessa Pope | Usual care |
| 27 | Royal Marsden | Jenny Rusby | Usual care |
| 28 | Cheltenham General | Sarah Vestey | Usual care |
| 29 | Guy’s and St Thomas’ | Michael Douek | Usual care |
| 30 | Dorset County | Caroline Osborne | Usual care |
| 31 | Mid Essex | Sascha Miles-Dua | Not in RCT |
| 32 | Mid Yorkshire | Jay Naik | Usual care |
| 33 | Bristol | Zoe Winters | Not in RCT |
| 34 | Chesterfield | Iman Azmy | Intervention |
| 35 | Rotherham | Inder Kumar | Intervention |
| 36 | Darent Valley, Dartford | Seema Seetharam | Intervention |
| 37 | Kingston | Karyn Shenton | Usual care |
| 38 | Colchester | Mukesh Mukesh | Usual care |
| 39 | Yeovil | Caroline Osborne | Usual care |
| 40 | Croydon | Sanjay Joshi | Intervention |
| 41 | North Tees | Colm Hennessy | Intervention |
| 42 | South Tees (James Cook University Hospital) | Imtiaz Cheema | Intervention |
| 43 | Luton and Dunstable | Mei-Lin Ah-See | Intervention |
| 44 | Weston General | Rachel Ainsworth | Intervention |
| 45 | Tameside | Stephanie Ridgway | Usual care |
| 46 | Macclesfield | Lisa Barraclough | Not in RCT |
| 47 | Wrightington, Wigan and Leigh Teaching Hospitals (Royal Albert Edward Infirmary) | Angela Power | Usual care |
| 48 | Birmingham | Fiona Hoar | Intervention |
| 49 | Kings Mill | Rebecca Boulton | Usual care |
| 50 | Wythenshawe | Nigel Bundred | Intervention |
| 51 | Aintree | Peter Robson | Intervention |
| 52 | Brighton | Gargi Patel | Usual care |
| 53 | St Margaret’s | Ashraf Patel | Intervention |
| 54 | St Mary’s | Steve Parker | Usual care |
| 55 | Oxford | Asha Adwani | Usual care |
| 56 | Frimley and Wexham | Ruth Davis (Wexham), Raouf Daoud (Frimley) | Intervention |
Eligibility criteria
Inclusion criteria
-
Female.
-
Aged ≥ 70 years at diagnosis.
-
Primary, operable (TNM categories: T1, T2, T3, N0, N1 and M0), invasive breast cancer.
-
Ability to give informed consent or have proxy consent if cognitively impaired.
Exclusion criteria
-
Disease unsuitable for surgery.
-
Previous invasive breast cancer within 5 years.
Women with reduced cognitive capacity
The study recruited older women with cognitive impairment by asking for consent from a personal consultee. These patients did not complete QoL questionnaires.
Study treatments
The study was a pragmatic cohort study with no change to normal treatment.
Statistical analysis
Data are reported and presented according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for pragmatic trials. 115
Propensity-score-matched analysis
Two analyses were performed:
-
For women with ER+ breast cancer, we compared the survival and QoL outcomes for those treated with S+ET versus those treated with ET ALONE
-
For women with high-recurrence-risk cancers [i.e. patients who meet any of the following criteria: (1) HER-2+; (2) ER–; (3) ER+ and a histological grade of 3; (4) nodes positive or (5) a high Oncotype DX recurrence score], we compared the survival and QoL outcomes for those treated with adjuvant chemotherapy and those treated without chemotherapy.
Survival analysis
Overall survival and BCSS were calculated according to treatment allocation. Kaplan–Meier curves were derived for each treatment type by age, disease characteristics, comorbidity and frailty subgroups. OS was compared between treatment groups using Cox’s proportional hazards model, adjusting for important prognostic factors.
List of abbreviations
- ABS
- Association of Breast Surgeons
- ADL
- activities of daily living
- aPG-SGA
- abridged Patient-Generated Subjective Global Assessment
- app
- mobile application
- BCSS
- breast-cancer-specific survival
- CCI
- Charlson Comorbidity Index
- CI
- confidence interval
- CTCAE
- Common Terminology Criteria for Adverse Events
- DESI
- decision support intervention
- DMEC
- Data Monitoring and Ethics Committee
- DVD
- digital versatile disc
- ECOG-PS
- Eastern Cooperative Oncology Group Performance Status
- ECRIC
- Eastern Cancer Registry and Intelligence Centre
- EORTC
- European Organisation for Research and the Treatment of Cancer
- EORTC-QLQ
- European Organisation for Research and the Treatment of Cancer Quality of Life Questionnaire
- EORTC-QLQ-C30
- European Organisation for Research and the Treatment of Cancer Quality of Life Questionnaire-C30
- EORTC-QLQ-BR23
- European Organisation for Research and the Treatment of Cancer Quality of Life Questionnaire-BR23
- EORTC-QLQ-ELD14
- European Organisation for Research and the Treatment of Cancer Quality of Life Questionnaire-ELD14
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ER
- oestrogen receptor
- ER+
- oestrogen receptor positive
- ER–
- oestrogen receptor negative
- ET ALONE
- primary endocrine therapy
- FFS
- failure-free survival
- HER-2
- human epidermal growth factor receptor-2
- HER-2+
- human epidermal growth factor receptor-2 positive
- HER-2–
- human epidermal growth factor receptor-2 negative
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- IADL
- Instrumental Activities of Daily Living
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MD
- mean difference
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MMSE
- Mini-Mental State Examination
- NABCOP
- National Audit of Breast Cancer in Older Patients
- NIHR
- National Institute for Health and Care Research
- NPI
- Nottingham Prognostic Index
- NYCRIS
- Northern and Yorkshire Cancer Registration and Information Service
- OR
- odds ratio
- OS
- overall survival
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- S+ET
- surgery plus adjuvant endocrine therapy
- SD
- standard deviation
- TNM
- tumour node metastasis
- TSC
- Trial Steering Committee
- WMCIU
- West Midlands Cancer Intelligence Unit
