Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 13/99/32. The contractual start date was in January 2015. The final report began editorial review in July 2016 and was accepted for publication in February 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Kate Hunt is deputy chairperson of the Research Funding Board for the National Institute for Health Research Public Health Research programme.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Gray et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The problem of obesity and sustained weight loss
Rising levels of obesity are a major challenge to public health. 1 In 2011, it was estimated that there would be 11 million more obese adults in the UK by 2030, resulting in up to 668,000 additional cases of diabetes, 461,000 additional cases of heart disease and stroke, 130,000 additional cases of cancer and up to 6.3 million quality-adjusted life-years (QALYs) lost, with associated medical costs set to increase by £1.9B–2B per year. 2
Although the behaviour change techniques (BCTs) and strategies that are effective in helping people achieve clinically significant short-term weight loss (by at least 5%3 of their initial body weight), by increasing physical activity (PA) and improving diet, are now well described,4–6 longer-term weight loss is less well researched and remains a challenge. Weight loss as a result of taking part in behavioural interventions typically peaks at around 6 months, followed by a plateau and then a gradual regain in weight at a rate of 1–2 kg per year (often with larger regains in the earlier years). 7–10 Participants in weight loss programmes typically regain 30–35% of lost weight in the first year post intervention, and most return to their baseline weight within 3–5 years. 11–14
As long-term weight maintenance is essential to maximise the health benefits associated with weight reduction,15 it is important to understand how to support people to sustain their weight loss following behavioural interventions. Systematic reviews indicate that a combination of energy and fat reduction, regular PA and behavioural strategies (such as regular weighing) is required for successful long-term weight loss. 16–18 Other reviews9,19 suggest that short-term weight loss, flexibility and variability in approach to diet and PA, and ongoing self-monitoring are important.
Some researchers have focused on the impact quality of motivation or locus of control on maintenance of behaviour change. Self-determination theory20,21 suggests that an understanding of motivation for behaviour change requires a consideration of innate psychological needs for autonomy (associated with the internalisation of regulation from external regulation, through introjected and identified regulation, to integrated regulation, in which a new behaviour becomes fully assimilated to the self), competence to perform behaviours, and relatedness to others. An empirical study22 of weight loss maintenance in relation to locus of control (the extent to which a person believes that they have control over events in their life) further demonstrated that an internal locus of control was associated with long-term weight loss maintenance.
Meanwhile, a recent systematic review23 of theoretical explanations for the maintenance of behaviour change suggested that people tend to maintain their behaviour if they are satisfied with the behavioural outcomes; if they enjoy engaging in the behaviour, or if the behaviour is congruent with their identity, beliefs and values; if they successfully monitor and regulate the newly adopted behaviour and have effective strategies to overcome barriers; if the new behaviours have become habitual; if their psychological and physical resources are plentiful (i.e. they are not subject to additional stressors such as life events); and if their environmental and social context supports the new behaviour.
Current evidence therefore suggests that fostering an internalised regulation of PA and dietary behaviours and an internal locus of control (which, in turn, are associated with habit formation and the routinisation of behaviours and their incorporation into values, self-identity and beliefs) are important for the long-term maintenance of weight loss. Self-monitoring, satisfaction with changes, and social and other environmental circumstances are also important. Nevertheless, the evidence on long-term weight loss remains limited, particularly in men. 16,24 More can be learned about the factors that are important for weight loss maintenance from follow-up studies of behavioural interventions that investigate who is successful in achieving long-term weight loss and how they do this. This will inform the development of future interventions to improve long-term outcomes.
Men and weight management
Since 1980, the prevalence of obesity in men worldwide has almost doubled. 1 In the UK, male obesity is among the highest in Europe and is forecast to increase at a faster rate than female obesity in the next 20 years. 2 Compared with women, men may be more vulnerable to the adverse health consequences of obesity. The tendency of men to carry excess fat abdominally puts them at greater cardiometabolic risk,25 and they are diagnosed with type 2 diabetes at a lower body mass index (BMI) than women. 26 In Scotland, obesity-related health risk is socially patterned, with men who are less affluent and less well educated at increased risk. 27 Nevertheless, men are under-represented in referrals to commercial weight management programmes (between 11%28 and 13%29 of referrals are men) and in NHS weight management services (23% of referrals are men). 30 A recent systematic review concluded ‘[t]hat men are under-represented suggests that methods to engage men in services, and the services themselves, are currently not optimal’. 31
Men’s reluctance to enrol in weight management programmes may in part reflect the way some men perceive weight management as a ‘diet’ or a ‘women’s’ issue. 32–34 It may also be influenced by the setting in which such programmes are delivered; for example, a Slimming World (Alfreton, UK) initiative35 to target men (by offering men-only groups) failed to increase their engagement by > 2% (i.e. from 3% to 5%). However, the potential of professional sporting organisations to attract men to health promotion activities is now widely recognised. 36–41 A National Institute for Health Research (NIHR) Public Health Research programme-funded randomised controlled trial (RCT) (09/3010/06)42 provided evidence of the success of professional football clubs in engaging men in a weight loss and healthy living programme [Football Fans in Training (FFIT)] and supporting them to lose weight42–44 and to make other positive changes to their health and behaviours up to 12 months after baseline measurement. The present study reports on the follow-up study (to 3.5 years post baseline measurement) of participants in the RCT of the FFIT programme at 13 of the top professional football clubs in Scotland (ISRCTN32677491). 45
Football Fans in Training: weight loss, physical activity and healthy eating for men
Here we provide a brief summary of the development of the FFIT programme, the key results from the previously funded evaluation and an update on the FFIT programme since the RCT results were published.
The FFIT programme was specifically designed to work with, rather than against, prevailing conceptions of masculinity, although it also takes account of best evidence in weight loss and behaviour change. 42,46 The FFIT programme is ‘gender-sensitised’ in relation to context (in the traditionally male environment of football clubs and men-only groups), content (for which information on the science of weight loss presented simply: ‘science but not rocket science’), discussion of alcohol, ‘branding’ (e.g. the use of football club insignia on programme materials) and style of delivery (using participative, peer-supported learning that encourages the men to interact for mutual learning and support, and positive male ‘banter’ to facilitate the discussion of sensitive subjects). The programme is delivered free of charge by community coaching staff at professional football clubs to groups of up to 30 men who are overweight or obese (participant-to-coach ratio 15 : 1) over 12 weekly sessions at football club stadia. At the start of the RCT, the coaches were trained over 2 days in the FFIT delivery protocol by the research team.
As Table 1 shows, each FFIT session combines advice on healthy eating and/or BCTs (‘classroom component’) with a coach-led group PA session using football club facilities. The BCTs are those known to be effective in PA and dietary interventions (self-monitoring, goal-setting, implementation intentions and feedback on behaviour)4 and social support, both from other participants and from their wider social networks,5 is also promoted (a full description of the use of BCTs within the FFIT programme is provided elsewhere46). Throughout the FFIT programme, men are encouraged to make behavioural changes that they can sustain long term, and to incorporate PA and healthy eating into their daily lives. The 12-week active phase is followed by a light-touch weight maintenance phase, including six e-mail prompts from coaches until 12 months after the start of the FFIT programme, and an invitation to a group reunion after 9 months. 46
| Classroom | PA | |
|---|---|---|
| Week 1 |
|
|
| Week 2 |
|
|
| Week 3 |
|
|
| Week 4 |
|
|
| Week 5 |
|
|
| Week 6 |
|
|
| Week 7 |
|
|
| Week 8 |
|
|
| Week 9 |
|
|
| Week 10 |
|
|
| Week 11 |
|
|
| Week 12 |
|
|
| Reunion |
|
|
Figure 1 provides an overview of the design of the FFIT RCT conducted in 2011/12; further details are provided elsewhere. 43,44 This was a pragmatic trial, with weight loss at 12 months as the primary outcome, in which 747 men (aged 35–65 years with an average BMI of ≥ 28 kg/m2) were randomly allocated either to the FFIT RCT intervention group (n = 374) or to a waiting list comparison group (n = 373) following baseline measurements at the 13 participating football clubs. The baseline measurements demonstrated that the programme successfully engaged men from across the socioeconomic spectrum43 whose excess body weight put them at a high risk of ill health. 47 At the baseline measurements, all men were given an information book on weight loss and feedback on their weight using a BMI wheel as a visual aid. As Figure 1 shows, men in the intervention group commenced the FFIT programme immediately (within 3 weeks of baseline measurement in August/September 2011) and men in the comparison group were offered a place on the FFIT programme at their football club 12 months later (in autumn 2012).
FIGURE 1.
The FFIT RCT waiting list design and timeline. LTMP, light touch maintenance phase.
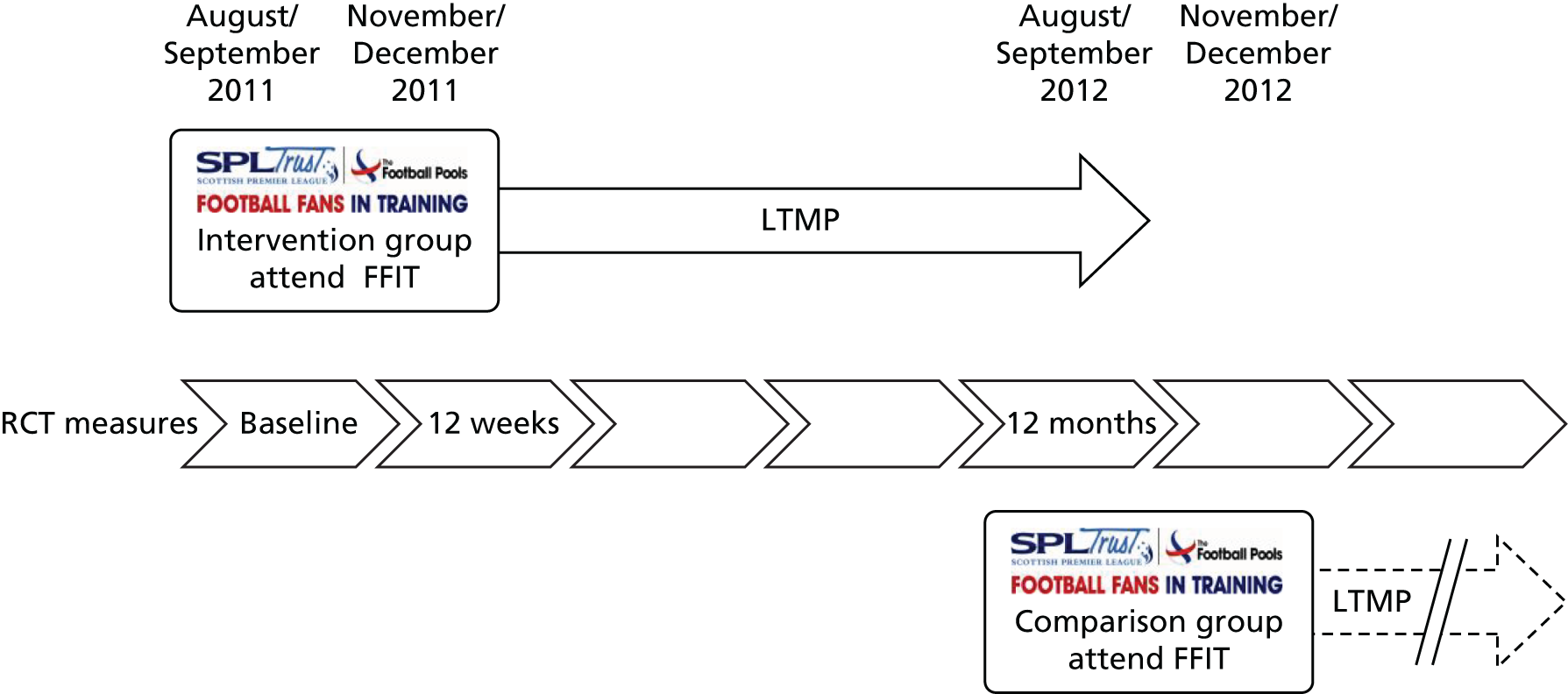
Baseline and follow-up measurements at 12 weeks and 12 months (with 92% retention overall at 12 months) were undertaken by a fieldwork team who were trained to standard measurement protocols. The waiting list design meant that all men in the RCT (i.e. the comparison group as well as the intervention group) were offered an opportunity to take part in the FFIT programme. However, because the comparison group took part in the FFIT programme after the end of the RCT [under ‘routine delivery’ (i.e. non-research) conditions], no post-intervention measurements were conducted with them.
During the RCT, the coaches delivering the FFIT programme reported weekly attendance at programme sessions to the research team; four out of five men in the intervention group attended at least 6 out of the 12 sessions during the active phase. 43 Observations of a sample (n = 26) of sessions across all football clubs suggested that the fidelity of delivery was good: coaches delivered 81 out of 93 (86%) key tasks. 43 At 12 months, the mean between-group weight loss difference was 4.94 kg [95% confidence interval (CI) 3.95 to 5.94 kg; p < 0.0001; adjusted for baseline weight and football club] in favour of the intervention group, and 39.0% of men in the intervention group (130/333), compared with 11.3% of men in the comparison group (40/355), had achieved a weight reduction from baseline of ≥ 5%. Significant between-group differences were also observed at 12 months in secondary outcomes including waist circumference, percentage body fat, resting blood pressure (BP), self-reported PA, dietary intake, alcohol consumption and psychological outcomes [self-esteem, affect and physical health-related quality of life (HRQoL)]. 43
The RCT demonstrated that the FFIT programme was cost-effective, with an incremental cost of £13,387 per QALY gained43 over the 12-month within-trial period. For a cost-effectiveness threshold of £20,000 per QALY, the probability that the FFIT programme was cost-effective, compared with no active intervention, was 0.72. This probability rose to 0.89 for a cost-effectiveness threshold of £30,000 per QALY. Longer-term modelling also showed favourable results. When the longer-term impact of the intervention was limited to 5 years, the cost-effectiveness of the FFIT programme was estimated to be between £1174 and £4475 per QALY gained depending on the assumptions made about the impact that this had on longer-term costs. 44
Focus groups comprising subsamples of men in the intervention group at each of the 13 RCT football clubs were conducted as part of the process evaluation at two time points: immediately post programme and at 12 months (when men were sampled to ensure that a range of experiences of weight loss maintenance were represented). These data suggested that men valued the way that the style of delivery of the FFIT programme and the materials themselves enabled them to build autonomy and take personal responsibility for their own behaviours, choosing which strategies best suited their own lives. 44 Men in the 12-month focus groups reported how focusing on a healthy, balanced diet with structured, organised eating patterns had helped them in their efforts to maintain their weight loss. Many also described how they had succeeded in incorporating new PA and healthy eating habits into their daily routine, and how they were continuing to self-monitor their weight and/or PA. There was evidence from the discussions that those who had successfully maintained their weight loss had, to at least some extent, developed internalised behavioural regulation. Many also spoke about the importance of ongoing social support, either from their partners and children or from fellow FFIT participants (in some football clubs, men had continued to meet up following the initial 12-week active phase of the programme). Some described how aspects of their identity had changed through participation in the FFIT programme, for example that they now saw themselves as an active, fit person. Nevertheless, men also reported a number of barriers to ongoing weight control after the programme, including injury, illness and stressful life events, such as bereavement, which hampered their ability to make long-lasting changes. 44
Similar to other RCTs,48,49 11.3% of men in the comparison group succeeded in losing ≥ 5% weight (as measured at 12 months) before starting the programme. This pre-intervention weight loss may reflect a number of potentially interacting factors. The act of signing up to take part in the FFIT RCT suggests that these men already had some degree of motivation to lose weight, and the experience of the baseline measurement sessions (which included feedback on their BMI status) may have heightened that motivation. Any man with elevated BP at the baseline measurements was advised to consult his general practitioner (GP), which may have acted as a further ‘wake-up call’. All men were also given a standard weight loss advice booklet50 and had the chance to speak briefly to the coach about the upcoming FFIT programmes. Finally, our recruitment activities within football clubs may also have changed men’s views about the acceptability of weight loss among men in general, or among their football-supporting peers in particular. Taken together, these factors suggest that the difference in weight loss between the groups is a conservative estimate of what the FFIT programme can deliver.
The FFIT programme continues to be delivered post RCT. In partnership with the Scottish Professional Football League (SPFL) Trust, the research team has developed a 2-day training package to train new coaches to deliver the FFIT programme, and has entered into an exclusive licence agreement with the SPFL Trust to allow it to oversee training, delivery, and ongoing quality assurance and monitoring of the FFIT programme worldwide. In Scotland, around 4500 men have now taken part in the FFIT programme, and the Scottish Government has funded further deliveries in 33 SPFL clubs. Seven football clubs are delivering the programme in England and 12 football clubs are delivering it in Germany. The Scottish Government has also commissioned an adapted version of the programme for women, which has been delivered in 25 Scottish football clubs.
Rationale for the current study
The FFIT RCT 12-month results compare favourably with those of RCTs of other men-only weight management programmes. 51,52 Nevertheless, longer-term follow-up is necessary to fully understand the potential public health benefit of the FFIT programme. The current study treats the FFIT RCT intervention and comparison groups as two longitudinal cohorts and reports their outcomes at 3.5 years after the RCT baseline measurements.
Aims and objectives
This longitudinal 3.5-year follow-up study had three main aims:
-
to investigate long-term weight trajectories from baseline to 3.5 years in men who were aged 35–65 years with a BMI of ≥ 28 kg/m2 at the start of the FFIT RCT
-
to establish the cost-effectiveness of the FFIT programme over the medium and longer term
-
to investigate the feasibility and utility of establishing low-cost, long-term, passive follow-up of the clinical health outcomes of current and future participants in the FFIT programme via routinely collected NHS records.
We aimed to address six underlying research objectives, as follows.
Objective 1: long-term weight outcomes
To investigate the extent to which:
-
participants in the intervention group achieved objectively measured long-term weight loss (at 3.5 years after baseline measurements and 3.5 years after commencing participation in the FFIT programme)
-
participants in the comparison group achieved objectively measured long-term weight loss (at 3.5 years after baseline measurements and 2.5 years after commencing participation in the FFIT programme)
-
weight trajectories and weight loss differed between the intervention group and the comparison group (at 3.5 years after baseline measurements).
Objective 2: randomised controlled trial secondary outcomes
To investigate the extent to which there were long-term changes in the intervention and comparison groups in the RCT secondary outcomes, and how these differed between the groups:
-
objective physical measurements – BMI, waist circumference, percentage body fat and resting BP
-
health behaviours – self-reported PA, sitting time, diet and alcohol intake
-
psychological outcomes – self-esteem, positive and negative affect, and physical and mental HRQoL.
Objective 3: predictors of long-term weight loss
To investigate:
-
the baseline predictors (age, BMI, education level, socioeconomic status, marital status, number of long-standing illnesses and orientation to masculine norms) of successful long-term weight loss in the two groups, and how these differed between the groups
-
how the following mediator variables predicted long-term weight loss after controlling for the baseline predictors in both groups, and how these differed between the groups –
-
change (from RCT baseline, 12 weeks and 12 months) in health behaviours (self-reported PA, sitting time, diet and alcohol intake)
-
change (from RCT baseline, 12 weeks and 12 months) in psychological status (self-esteem, positive and negative affect, and mental and physical HRQoL)
-
perceived autonomy, competence, relatedness and satisfaction with PA and dietary behaviours, as assessed at 3.5 years
-
the extent to which PA and healthy eating routines were established, the ongoing use of BCTs, ongoing contact with other FFIT participants, and major life events, as assessed by self-report at 3.5 years
-
end-of-intervention weight change (from objective RCT measurements in the intervention group and self-report in the comparison group) and pre-intervention weight change (from objective RCT measurements in the comparison group only)
-
self-reported injury and joint pain, as assessed at 12 months and 3.5 years.
-
Objective 4: men’s experiences
To describe men’s experiences (including their motivations, emotions and relations with others) of attempting to control their weight over the long term, their reasons for achieving or failing to achieve long-term weight loss and the strategies they continued to use or stopped using.
Objective 5: cost-effectiveness
To investigate the medium- and long-term cost-effectiveness of the FFIT programme by:
-
establishing the extent to which weight loss and positive behavioural changes were sustained beyond the first 12 months, and the subsequent impact that these had on the cost-effectiveness of the FFIT programme at 3.5 years
-
updating the modelling of the longer-term health outcomes and resource use of men who participate in the FFIT programme, and assessing the potential for longer-term cost-effectiveness
-
exploring heterogeneity of the cost-effectiveness of the FFIT programme.
Objective 6: long-term follow-up via medical records
To explore the potential of using linkage to routinely collected NHS data sets to allow long-term, low-cost, passive follow-up of future FFIT participants through investigation of the following:
-
utility – long-term clinical health outcomes (through data linkage on hospitalisations, mortality, prescribing, cancers, diabetes and, when possible, blood test results) of RCT participants, and the extent to which these were associated with long-term weight loss and behaviour change
-
feasibility – the extent to which men enrolling in routine implementation deliveries of the FFIT programme (in spring and autumn 2015) were prepared to give permission for transfer of their baseline, post-programme and 9-month weight measurements and BMI (as measured by coaches in participating football clubs) to the research team, and to agree to linkage to their NHS records.
In this report, we describe the methods and processes of data collection and analysis for the long-term weight outcomes, RCT secondary outcomes and predictors of long-term weight loss (see Chapter 2), the results of which are presented in Chapter 3 (objectives 1–3). Chapter 4 reports the qualitative analysis of men’s experiences, including methods and results (objective 4). Chapter 5 reports the economic analysis, including methods and results (objective 5). Chapter 6 reports the methods and results for the utility and feasibility of long-term follow-up via medical records (objective 6).
Chapter 2 General methods
Setting
The setting was the 13 SPFL clubs that took part in the FFIT RCT in 2011/12.
Overview of study design
We undertook a mixed-methods, longitudinal, follow-up study to investigate long-term weight trajectories in participants in both arms of the FFIT RCT. As both groups had taken part in the FFIT programme (the intervention group commenced in autumn 2011 and the comparison group were given the opportunity to do so in autumn 2012), the follow-up was designed as a cohort study.
The current chapter reports on the methods relating to data collection and analysis for long-term weight outcomes, RCT secondary outcomes and predictors of long-term weight loss. Methods relating to data collection and analysis for the qualitative interviews (see Chapter 4), economic evaluation (see Chapter 5) and utility and feasibility of data linkage (see Chapter 6) are presented in the relevant chapters.
The study protocol is available at www.nets.nihr.ac.uk/projects/phr/139932.
Ethics approval
Ethics approval for the study was obtained from the College of Social Sciences Ethics Committee at the University of Glasgow (CSS/400140075), which complies with the Economic and Social Research Council’s research ethics framework.
Retention strategies and contact with men at the 3.5-year follow-up
The personalised approach to recruitment and retention during the 2011/12 FFIT RCT resulted in 89% (333/374) of the intervention group and 95% (355/374) of the comparison group taking part in the 12-month measures. At this time, we asked participants if they would consent to being followed up in future. A total of 95% (316/333) of the intervention group and 98% (349/355) of the comparison group did so. We therefore had a potential FFIT follow-up cohort of 665 (89% of those who took part in the FFIT RCT).
Formal recruitment to the 3.5-year follow-up measures began in February 2015. To maximise attendance at the follow-up measures, we followed the retention protocols that had been successful in the RCT.
-
Measurement sessions were held at football club stadia at which FFIT community coaches were present.
-
At least two measurement sessions were held at each football club.
-
One month before the football club measurement sessions, men received a personalised letter and an information sheet about the follow-up study.
-
Three weeks before the football club measurement sessions, men were contacted individually by telephone to make an appointment, and appointments were confirmed by e-mail/post (in accordance with their preference).
-
Appointment reminder texts and e-mails were sent up to 48 hours in advance of the football club measurement sessions.
-
Men who did not attend their appointment were telephoned either during the football club measurement session to rearrange their appointment or after the session if they could not be reached during the session.
-
Men who were unable to attend football club measurement sessions were offered a fieldworker visit at their home (or at another convenient location if they preferred).
-
Men were offered £20 high street store vouchers (and travel expenses for stadia measurements) for taking part in the follow-up measurements.
In addition, in December 2014, prior to the study commencing, all men in the FFIT follow-up cohort were sent Christmas cards that included a request for any update of their contact details. A summary of the retention procedures and timeline is provided in the retention procedures for the FFIT follow-up study (see Report Supplementary Material 1).
Participants
The participants were men who participated in the FFIT RCT and who consented to being contacted for follow-up research at the RCT 12-month measurements (n = 665). These men were aged 35–65 years and had a BMI of ≤ 28 kg/m2 at RCT baseline.
Intervention
As this study was a longitudinal follow-up of participants in both arms of the FFIT RCT, no further interventions were conducted. A detailed description of the FFIT programme is provided in Chapter 1, Football Fans in Training: weight loss, physical activity and healthy eating for men, and is also available elsewhere. 46
Outcome assessment
The main outcome measures in the 3.5-year follow-up study are the same as those assessed at baseline, 12 weeks and 12 months during the FFIT RCT. They were set with reference to National Obesity Observatory guidance for the evaluation of weight management interventions. 53
Primary outcome
The primary outcome was objectively measured weight change from FFIT RCT baseline to 3.5 years expressed as a mean and as a percentage.
Secondary outcomes
The secondary outcomes were as follows:
-
change from baseline to 3.5 years in objectively measured BMI, waist circumference, percentage body fat and resting BP
-
PA – change from baseline to 3.5 years in self-reported frequency and duration of walking, moderate and vigorous activity and duration of sitting time over the last 7 days measured using the International Physical Activity Questionnaire-Short Form54
-
diet – change from baseline to 3.5 years in self-reported frequency of intake of key contributors to weight gain55 [e.g. fast foods, chocolate bars, chips, pies, sugary drinks and breakfast consumption, using questions adapted from the Dietary Instrument for Nutrition Education (DINE)56], and perceived changes in portion size using eight photographs representing different portions of foods that the research team considered to be important in weight gain (cheese, meat, pasta and chips)57
-
alcohol intake – change from baseline to 3.5 years in self-reported consumption over the last 7 days58
-
psychological outcomes –
-
change from baseline to 3.5 years in positive and negative affect as measured by the Positive and Negative Affect Schedule (PANAS)59
-
self-esteem as measured by the Rosenberg Self-Esteem Scale (RSE)60
-
physical and mental HRQoL as measured by the Short Form questionnaire-12 items (SF-12) Health Survey (version 2). 61
-
Baseline predictors
The baseline predictors were age, BMI, education level, socioeconomic status,71 marital status, orientation to masculine norms and number of long-standing illnesses, as measured at FFIT RCT baseline.
Mediators
The mediators measured included change from FFIT RCT baseline in health behaviours (self-reported PA, sitting time, diet and alcohol intake) and psychological outcomes (self-esteem, positive and negative affect, mental and physical HRQoL).
Other mediators were as follows:
-
self-determination theory constructs20 – reported self-regulation of PA and dietary behaviours as measured by the Treatment Self-Regulation Questionnaire (TSRQ),62 perceived autonomy in PA and dietary behaviours as measured by the Locus of Causality Scale (LCS),63 perceived competence in PA and dietary behaviours as measured by the Perceived Competence Scale (PCS)64 and perceived relatedness as measured by the Need for Relatedness Scale (NRS),65 all as reported at 3.5 years
-
perceived satisfaction with current PA and dietary behaviours,66 as reported at 3.5 years
-
self-reported use of behavioural techniques that are likely to be associated with long-term weight loss, including ongoing self-monitoring of weight/PA and the extent of establishment of dietary and PA daily routines, as reported at 3.5 years (see Q1a, Report Supplementary Material 2)
-
self-reported frequency of contact with other FFIT participants, coaches, football club-based initiatives and other health promotion/weight management initiatives since the end of the initial 12-week active phase of the FFIT programme, as reported at 3.5 years
-
self-reported major life events (e.g. bereavement, family illness, separation, divorce, redundancy) since the end of the initial 12-week active phase of the FFIT programme, as reported at 3.5 years
-
end-of-intervention weight change [from objective RCT baseline and 12-week measurements for the FFIT follow-up intervention (FFIT-FU-I) group and from self-reported (at 3.5 years) end-of-programme weight loss for the FFIT follow-up comparison (FFIT-FU-C) group] and pre-intervention weight change (from objective RCT baseline and 12-month measurements for the FFIT-FU-C group)
-
self-reported injury and joint pain, as reported at 12 months and at 3.5 years.
Health-care resource use, GP-prescribed medications and family history of coronary heart disease (CHD)/stroke67 were self-reported for the economic evaluation. Participants were also asked to report any long-standing illnesses (and the extent to which they limited activities) and smoking status at 3.5 years.
Procedures
Outcome and predictor assessment
Stadia measurements were conducted by a team of fieldworkers, trained to standardised protocols, during 29 sessions across the 13 football clubs between March and May 2015. Home visits took place between June and August 2015. Most home measurements were conducted at the participant’s home, but one participant attended at the University of Edinburgh and another requested a visit to his workplace. Percentage body fat was not recorded among these participants. In addition, some men who were contacted for home visits elected to provide only their weight (which was either objectively measured by a fieldworker visiting them at home or self-reported to a fieldworker over the telephone). Men who declined a home visit or giving self-reported weight by telephone and men whom the fieldworkers had been unable to reach by telephone or e-mail during the follow-up study were sent a weight-only self-report form and prepaid return envelope following the completion of home visits. All outcome data collection ended on 25 September 2015.
The fieldwork team
Data were collected by teams of fieldworkers. Each measurement session team had a designated fieldwork team leader and a fieldwork nurse responsible for BP measurement. Fieldworkers were trained to standard measurement protocols by experienced research and survey staff in the Medical Research Council (MRC)/Chief Scientist Office (CSO) Social and Public Health Sciences Unit. Training took place over 2 days for stadia measurement staff. This included a half-day of training for fieldwork team leaders only, and 1.5 days of training for fieldworkers, with training sessions led jointly by the fieldwork team leaders and members of the research team. A further day of training for home visit fieldworkers was provided to emphasise strict adherence to protocol during home visits to minimise detection bias. All fieldworkers wore T-shirts branded with FFIT research logos at in-stadia measurement sessions (Figure 2) and during home visits.
FIGURE 2.
Fieldwork team arriving at a FFIT follow-up 3.5 years in-stadia measurement session.

Measurement protocols
Objectively measured outcomes
The protocols for recording the objectively measured outcomes were identical to those used during the FFIT RCT. Weight (kg) was recorded using electronic scales (Tanita HD-352™, Milton Keynes, UK) and with participants wearing light clothing, not wearing shoes and having empty pockets. Height (cm) was measured without shoes using a portable stadiometer (Seca Leicester™, Chino, CA, USA). Waist circumference was measured twice (three times, if the first two measurements differed by ≥ 5 mm), and the mean was calculated. Resting BP was measured using a digital BP monitor (Omron HEM-705CP™, Milton Keynes, UK) by a fieldwork nurse. Body composition was measured using a Bodystat 1500MDD machine (Bodystat Ltd, Douglas, Isle of Man). All equipment was calibrated before fieldwork commenced.
Outcomes based on self-report
Participants completed two self-administered questionnaires. The first questionnaire included the self-report outcomes from the 12-month RCT measures (see schedule A, Report Supplementary Material 3). It also included additional questions on when participants attended the FFIT programme, their recollection of their weight loss at the end of the FFIT programme, interaction with other FFIT participants or coaches, changes in personal circumstances and family health history (see schedule B, Report Supplementary Material 3). The second questionnaire focused on additional mediator outcomes (see schedule B, Report Supplementary Material 3).
Fieldworkers were trained to assist any man who appeared to have literacy or other problems with questionnaire completion. All completed questionnaires were routinely checked before the participant left the measurement session (or the fieldworker left the home visit) to minimise missing data.
Copies of the two FFIT follow-up study questionnaires are provided (see Report Supplementary Material 2 and Report Supplementary Material 4).
Preparation of self-reported variables for analysis
Physical activity
Following standard procedures described in the International Physical Activity Questionnaire scoring protocol,54 we calculated and reported metabolic equivalent of task (MET) minutes per week for self-reported walking, vigorous, moderate and total PA, and time in minutes for daily sedentary time over the last 7 days. Activity times that exceed 180 minutes (3 hours) per day were truncated to 180 minutes to normalise the distribution of levels of activity, which are usually skewed in large population data sets. 68
Diet
We used the adapted DINE from the FFIT RCT43 to collect self-reported frequency of intake of various fatty and sugary food types, and fruit and vegetables over the previous 7 days (see questions 27–32, Report Supplementary Material 4). From these data, we calculated a fatty food score, a sugary food score, and a fruit and vegetables score (see Report Supplementary Material 5).
Fatty food types
Participants reported how many times, over the previous 7 days, they had eaten a serving of cheese, beef burgers or sausages, beef, pork or lamb, fried food, chips, bacon or processed meat, pies, quiches or pastries and crisps. They also reported the amount of milk, over the previous 7 days, that was used for drinking or in cereal, tea or coffee in a day, and what kind of milk they normally used.
Sugary food types
Participants reported how often, over the previous 7 days, they had eaten chocolate, sweets or biscuits and drunk sugary drinks (fizzy drinks, diluting juice or fruit juice).
Fruit and vegetables
Participants reported how many times a day, over the previous 7 days, they had eaten fruit and vegetables.
When there were missing values, the score was recoded to the smallest possible score (e.g. if number of times of eating cheese was missing, then the assumed frequency was ‘no times’) for all variables except milk. If milk (amount) was missing, then the entire milk score (milk amount × milk type) was assumed to be 1 (lowest possible value) regardless of milk type.
Portion size
We assigned numbers from 1 to 8 to the photographs of each food, with higher numbers representing larger portions.
Alcohol intake
Following Emslie et al. ,58 we converted responses to the 7-day recall diary for alcohol to standard units that are equivalent to 8 g of pure alcohol (e.g. half a pint of ordinary beer, lager or cider, a small glass of wine and one measure of spirit each contain 1 unit of alcohol). We calculated the total number of units reported in the past week.
Psychological outcomes
Scores for both the RSE and PANAS short form were normalised so that values could be calculated for participants who had missed one or two items contributing to each scale. The PANAS normalised scales scores range from 1 to 5, with higher scores indicating higher negative affect and higher positive affect. Higher scores on the RSE (normalised range 0–3) indicate better self-esteem. SF-12v2 scores were summarised separately for mental and physical HRQoL following standard algorithms. 61
Mediators
Treatment Self-Regulation Questionnaires: diet and physical activity
There are three subscales on each TSRQ: autonomous regulatory style, controlled regulatory style and amotivation. The responses to items on the three subscales were averaged (normalised) and reported separately for each behaviour. The scores for the items on the TSRQs range from 1 to 7, with higher scores indicating greater motivation.
Locus of Causality Scale: diet and physical activity
The scores for the three items on the LCS range from 1 to 7. Following the standard scoring protocol, scores were reversed on items 2 and 3, and the mean score for the three items was then calculated. Higher scores indicate greater self-determination or autonomy.
Perceived Competence Scale: diet and physical activity
The scores for the four items on the PCS range from 1 to 7. The mean score for the four items was calculated. Higher scores indicate greater perceived competence.
Need for Relatedness Scale
There are two subscales on the NRS: acceptance and intimacy. The scores for the items on the NRS range from 1 to 7. The responses to items on the subscales were averaged and reported separately for peers (specifically other men from the FFIT programme) and family. Higher scores indicate stronger relatedness.
Satisfaction with changes in physical activity, dietary and weight
The scores for the satisfaction items range from 1 to 9, with 1 being ‘very dissatisfied’ and 9 being ‘very satisfied’. There was also an option for participants to indicate if they had not made any changes (scored as ‘0’). For each item, participants who gave an answer of 0–6 were grouped together and compared with those who gave an answer of 7–9.
Use of behaviour change techniques
Items on the continued use of BCT scales were scored as follows: 1, ‘never’; 2, ‘rarely’; 3, ‘sometimes’; 4, ‘frequently’; and 5, ‘always’. For each item, participants who answered ‘sometimes’, ‘frequently’ or ‘always’ were grouped together and compared with those who answered ‘never’ or ‘rarely’. Strategies included using the pedometer (or self-monitoring of walking), self-monitoring weight, SMART (Specific, Measurable, Achievable, Realistic, Time-limited) goal-setting, tips on how to overcome setbacks, and social support for PA or diet.
Routinisation of physical activity and dietary behaviours
Items on the routinisation of behaviours scales were scored as follows: 1, ‘never’; 2, ‘rarely’; 3, ‘sometimes’; 4, ‘frequently’; and 5, ‘always’. For each item, participants who answered ‘frequently’ or ‘always’ were grouped together and compared with those who answered ‘sometimes’, ‘never’ or ‘rarely’. Items for routinisation of PA behaviours included daily walking, gym attendance, cycling, swimming or other forms of exercise, and attending a group programme. Items for routinisation of dietary behaviours included eating regular meals, watching portion sizes, limiting intake of certain types of food (e.g. fats, sugars), limiting overall calorie intake, limiting intake of sugary drinks, limiting intake of alcohol, consciously eating slowly and reading food labels.
Frequency of contact with other Football Fans in Training participants, coaches and health promotion/weight management initiatives since the end of the Football Fans in Training programme
Items on the continued contact scales were scored from 1, ‘very frequently’, to 5, ‘never’. For each item, participants who answered ‘very frequently’, ‘frequently’, ‘occasionally’ or ‘rarely’ were grouped together and compared with those who answered ‘never’.
Major life events since the end of the Football Fans in Training programme
A major life events scale was developed for the FFIT follow-up study, drawing on items from the Psychiatric Epidemiology Research Interview Life Events Scale. 69 Participants were asked to indicate if each of 17 life events had happened to them and, if so, the extent to which it had affected their day-to-day life both at the time, and currently. For the purposes of the mediator analyses, the total number of life events reported was calculated, as was the number of events in each of four categories (own health, personal circumstances, family health and work).
Prior weight change
Post-intervention weight change was estimated as follows: (1) the FFIT-FU-I group from objectively measured weight at 12 weeks minus baseline; and (2) the FFIT-FU-C group from self-reported post-intervention weight loss at 3.5 years. Post-intervention weight change was converted into the following categories for each group: 1, ‘did not lose weight’; 2, ‘lost up to 5%’; 3, ‘lost 5–10%’; and 4, ‘lost more than 10%’. Pre-intervention weight change was estimated in the FFIT-FU-C group only from objectively measured weight at 12 months minus baseline.
Short Form Conformity to Masculine Norms Inventory-22 items (measured at randomised controlled trial baseline)
The 22 items on the Short Form Conformity to Masculine Norms Inventory-22 items (CMNI-22)70 were scored as follows: 0, ‘strongly disagree’; 1, ‘disagree’; 2, ‘agree’; and 3, ‘strongly agree’. Following the scoring protocol, the scoring on nine of the items was reversed before scores on all items were summed to give an overall score. A higher CMNI-22 score indicates greater conformity to masculine norms.
Injury and joint pain as self-reported at 12 months and 3.5 years
For injuries, the number of lower limb injuries, the number of torso or upper body injuries and the total number and type of limitations due to injury were summed separately. In addition, participants were grouped and compared according to whether they had reported any injuries that limited walking, any injuries that limited using stairs and any injuries that limited PA. For joint pain, participants were assigned to groups (n = 7) according to reports of any:
-
upper joint pain (neck, back and upper limb) that persisted all of the time, compared with those who did not report upper joint pain all of the time
-
lower limb joint pain (hip, knee, ankle, foot/toes) all of the time, compared with those who did not report lower joint pain all of the time
-
upper joint pain that limited activities ‘to a moderate degree’ or more, compared with those who did not
-
lower limb joint pain that limited activities ‘to a moderate degree’ or more, compared with those who did not
-
upper joint pain, compared with those reporting ‘never’ or ‘don’t know’ for upper joint pain
-
lower limb joint pain, compared with those with those reporting ‘never’ or ‘don’t know’ for lower limb joint pain
-
joint pain, compared with those reporting ‘never’ or ‘don’t know’ for any joint pain.
Changes to outcomes
Diet
In our protocol we said that we would measure change from baseline to 3.5 years in self-reported frequency of intake of key contributors to weight gain (e.g. fast foods, chocolate bars, chips, pies and sugary drinks) and of breakfast using questions adapted from DINE. However, to be consistent with the reporting of the FFIT RCT,43 we summarised the 17 original variables into three scores (fatty food, sugary food, and fruit and vegetables; see Preparation of self-reported variables for analysis, Diet) indicative of healthy changes that men could have made to their diets.
Satisfaction with behaviour and weight changes
In our protocol we said that we would measure perceived satisfaction with current PA and dietary behaviours. However, given evidence from reviews that suggests that satisfaction with short-term outcomes (including weight) may predict long-term outcomes,19,23 we asked men how satisfied they were with what they had experienced as a result of the changes they had made to their diet, PA and weight at the end of the FFIT programme, as well as currently.
Sample size and power
All 665 men (intervention group, n = 316; comparison group, n = 349) who had consented to being contacted again for future follow-up were invited to participate in the FFIT follow-up study. Assuming an attrition rate of 20%, we estimated that we would have outcome measurements for 532 men (intervention group, n = 253; comparison group, n = 279). The standard deviation (SD) for the percentage change in weight loss during the RCT was approximately 10% in the intervention group. Assuming that this would be higher in the longer term (e.g. 15%) but similar in both groups, we estimated that we would have 80% power to detect a change in weight of 2.65% in the intervention group, 2.52% in the comparison group, and 1.83% overall.
Blinding
As this was not a RCT, and as both groups had had an opportunity to take part in the FFIT programme, blinding of participants was not relevant. Nevertheless, the fieldworkers conducting the measurements were not told to which group the men belonged.
Statistical methods
All participants with available data were included in the analysis. To investigate the long-term outcomes in relation to the primary (weight loss) and RCT secondary outcomes (BMI, waist circumference, percentage body fat, resting BP, and self-reported PA, diet, alcohol intake, positive and negative affect, self-esteem, and physical and mental HRQoL) (objectives 1 and 2), 12-month and 3.5-year outcomes were summarised separately by group (FFIT-FU-I or FFIT-FU-C) and overall. The Wilcoxon signed-rank test was used to test the change from baseline in 12-month and 3.5-year outcomes within groups, and the Mann–Whitney U-test was used to detect differences between groups. All outcomes were continuous. Each group was also analysed separately within mixed-effects (repeated measures) linear regression models, with adjustments for baseline value and visit (12 months and 3.5 years) as fixed effects, and participant and football club as random effects. The mean value (change from baseline), 95% CI and p-value were estimated from these models.
Differences in weight trajectories between the FFIT-FU-I group (who commenced the FFIT programme in September 2011) and the FFIT-FU-C group (who were offered places on the FFIT programme in September 2012) were investigated by considering both groups together and including additional fixed effect terms for group, and a group × visit interaction.
For each group, the predictors of change in weight (objective 3) were investigated by extending the repeated mixed-effects linear regression models described above to include each predictor separately to assess their individual impact. All predictors were then added to the model, and a backwards-selection method was applied to identify the independent predictors of change in weight. To determine whether or not there were different predictors for each group, the data from both groups were considered in a single model, with a predictor × group interaction term added.
When possible, we undertook additional analyses, including a backward-selection data-driven model, which included the following hypothesised predictors/mediators (measured at 12 months or 3.5 years) of long-term weight loss: end-of-intervention weight change; sustained PA and diet change; continued use of self-monitoring; major life events, injury and joint pain; social support; the extent to which behaviour had become routinised; satisfaction with behaviour change; self-regulation; perceived autonomy; perceived competence; and perceived relatedness.
Non-response bias was investigated by comparing the baseline characteristics of participants who agreed to take part in the 3.5-year measurements (total followed-up cohort) with those of participants who did not (not followed-up cohort) using appropriate statistical tests (t-test/Mann–Whitney U-test/chi-squared test/Fisher’s exact test).
The sensitivity of the overall results to a variety of assumptions about the primary outcome of the cohort not followed up was assessed by imputing missing outcome data for the cohort not followed up using the return to baseline and the last-value-carried-forward methods. The analyses described above were repeated for the imputed data.
In addition, sensitivity analyses of the change in primary outcome from baseline to 3.5 years were carried out using the weight measures from immediately before taking part in the FFIT programme as the baseline for both groups (i.e. for the FFIT-FU-C group, the RCT 12-month weight measurements were used as the ‘baseline’). Two sensitivity analyses were performed, the first using the total followed-up cohort, and the second excluding those in the FFIT-FU-C group who had lost ≥ 5% of their baseline weight by the RCT 12-month measures.
All analyses were conducted using SAS® software (version 5.1, SAS Institute Inc., Cary, NC, USA). (SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.)
Changes to protocol
There were no changes to protocol except those described in Changes to outcomes.
Public involvement
Extensive public engagement was undertaken throughout the development and evaluation of the FFIT programme. We worked closely with the SPFL Trust while developing the FFIT programme and during the conduct of the FFIT RCT in 2011/12. Subsequent discussions with the SPFL Trust about the value of long-term follow-up of RCT participants and of the potential for data linkage to routinely collected NHS records led to the development of the proposal for the FFIT follow-up study. Members of the SPFL Trust and other lay representatives were involved in the following ways.
Design of the Football Fans in Training follow-up study
The involvement of the SPFL Trust from the earliest stages of the development of the research questions and detailed project description ensured that the follow-up study had real-life application and would benefit the SPFL Trust and football clubs. A FFIT coach and a former FFIT participant also read and commented on the penultimate draft of the detailed project description to ensure that the research was relevant to a non-academic audience.
Management of the Football Fans in Training follow-up study
The SPFL Trust agreed to join the Study Steering Committee, and was represented at meetings by the operations manager, Derek Allison. The FFIT coach and the former participant who commented on the project description were also asked to join the Study Steering Committee. Their representation on this advisory group was important in ensuring that the findings were relevant and accessible to a non-academic audience.
Developing participant information resources
The SPFL Trust commented on the protocol that was developed as part of the data linkage feasibility work (described in Chapter 6, Feasibility of data linkage) for coaches to use to ask participants in the autumn 2015 and spring 2016 deliveries of the FFIT programme for permission to access their NHS records.
Undertaking and analysing the Football fans in Training follow-up study
The SPFL Trust attended all project team meetings (either the general manager, Nicky Reid, or the operations manager, Derek Allison), which allowed it to contribute to key decision-making across the project. It also worked closely with the project manager in facilitating links with, and training of, appropriate football club representatives to allow data collection in the data linkage feasibility study (see Chapter 6, Feasibility of data linkage).
Contributing to the reporting of the Football Fans in Training follow-up study
The SPFL Trust was given an opportunity to comment on all progress reports and this final report. The FFIT coach and the former FFIT participant were also given the opportunity to comment on this final report.
As part of the end-of-project dissemination activities (see Dissemination of research findings), we are working closely with the SPFL Trust FFIT Development Officer (Stevie Chalmers) in preparing a lay report presenting the results of the follow-up study. This collaboration will ensure that the lay report is a useful document for the SPFL Trust and football clubs to use to support their efforts to build partnerships with potential funders and organisations (e.g. the NHS) for future deliveries of the FFIT programme. The FFIT coach and former FFIT participant will also be asked to comment on the lay report.
Dissemination of research findings
We are working closely with the SPFL Trust in the organisation of end-of-project events to disseminate the findings of the FFIT follow-up study to non-academic audiences. FFIT coaches and participants are being invited to attend and play an active part in these events. This approach will ensure that these events have the maximum impact for the SPFL Trust and its member football clubs.
Chapter 3 Results: outcomes and predictors of long-term weight loss
Introduction
This chapter presents the results of the analysis in relation to the primary outcome (change in weight at 3.5 years), secondary outcomes from the FFIT RCT, and the predictors and mediators of weight loss at 3.5 years. Specifically, we aimed to investigate the:
-
extent to which participants in the RCT intervention group and comparison group achieved long-term weight loss, and how weight trajectories differed between the groups
-
extent to which there were long-term changes in the intervention and comparison groups in the RCT secondary outcomes, and how these differed between the groups
-
baseline predictors and mediators (after controlling for baseline predictors) of long-term weight loss, and how these differed between the groups.
Participant flow
As shown in Figure 3, of the 688 men who took part in the RCT 12-month measurements, 665 provided consent to be contacted again in future. When attempts were made to contact these 665 men in 2015, 87 (13%) withdrew consent [either completely (n = 43) or they did not want to take part in the current measurements but agreed to be contacted again in future (n = 44)]. Another 90 men (13%) could not be contacted despite multiple attempts. Hence, 488 men took part in the 3.5-year follow-up measurements (hereafter referred to as the total followed-up cohort). Of these, 333 attended measurement sessions in the stadia, 118 completed the full set of measurements and questionnaires in home visits and 37 provided weight-only data (three had their weight measured by a fieldworker during a home visit and 34 provided self-reported weight by telephone or post).
FIGURE 3.
Summary of flow of participants through the FFIT RCT and follow-up study. C, comparison group; I, intervention group.
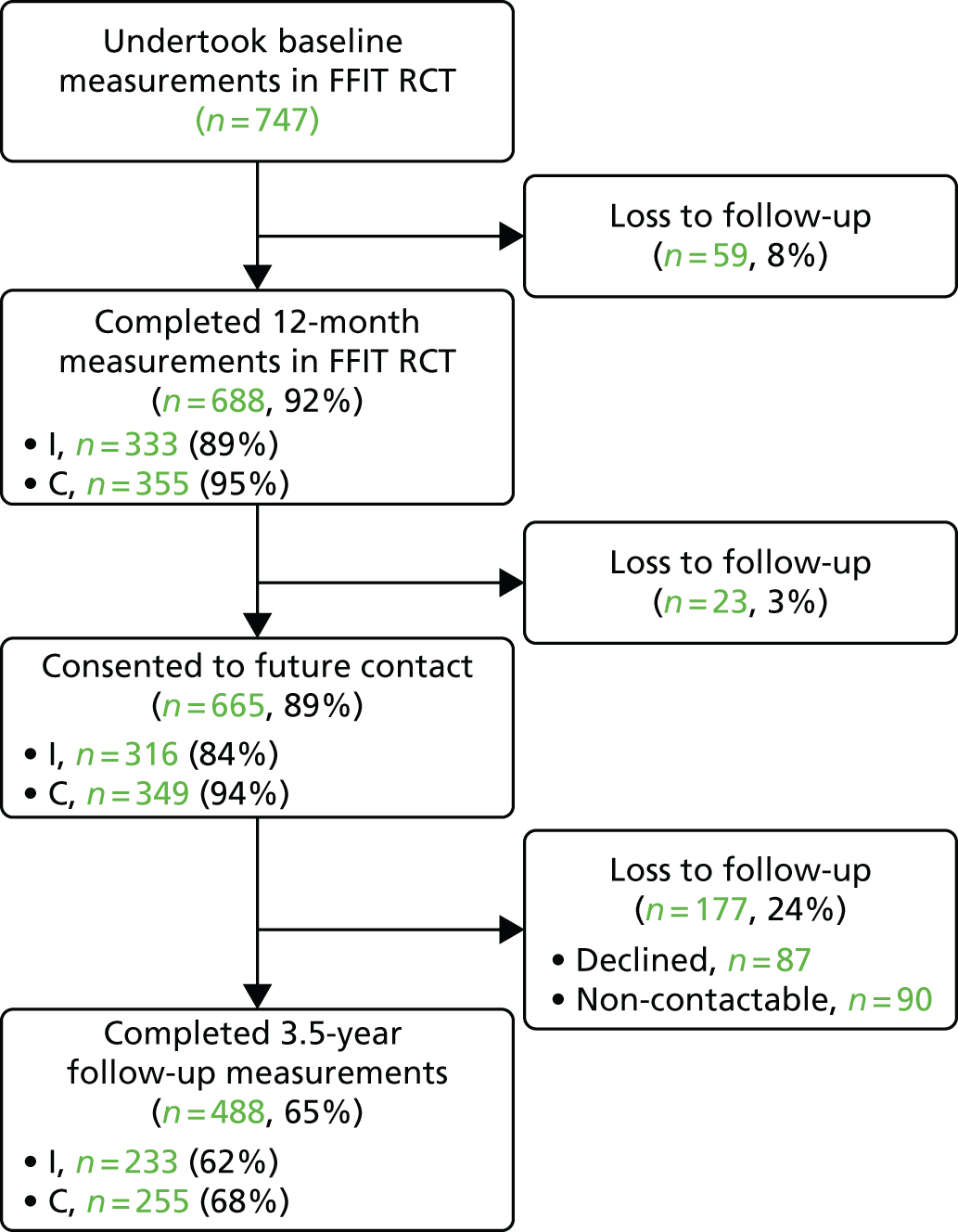
The FFIT-FU-I group comprised 62% (233/374) of men in the RCT intervention group and the FFIT-FU-C group comprised 68% (255/373) of men in the RCT comparison group. This equates to 65% (488/747) of men from the original RCT cohort. Although more participants in the RCT comparison group than in the RCT intervention group were followed up at 3.5 years, among those who consented to future contact at the 12-month measurements, follow-up was similar in both groups (intervention group, 74%; comparison group, 73%). Therefore, the differential follow-up at 3.5 years largely reflects the lower retention in the intervention group at the 12-month measurements (89% vs. 95% retention in the comparison group).
Baseline data
Table 2 shows the demographic characteristics of participants in the FFIT RCT (n = 747) and follow-up study (n = 488) cohorts. Small differences were seen in the age, employment status and housing tenure of those who took part in the 3.5-year measurements (total followed-up cohort) and those who did not (not followed-up cohort).
| Demographic characteristic | Cohort, n (%) | Group, n (%) | |||
|---|---|---|---|---|---|
| FFIT RCT (N = 747) | Not followed up (N = 259) | Total followed up (N = 488) | FFIT-FU-I (N = 233) | FFIT-FU-C (N = 255) | |
| Age (years), mean (SD) | 47.1 (8.0) | 46.2 (7.8) | 47.5 (8.0) | 47.3 (8.2) | 47.7 (7.9) |
| Ethnicity | |||||
| White (British/Scottish/Irish/other) | 735 (99.1) | 256 (99.2) | 479 (99.0) | 228 (98.7) | 251 (99.2) |
| Other | 7 (0.9) | 2 (0.8) | 5 (1.0) | 3 (1.3) | 2 (0.8) |
| Missing | 5 | 1 | 4 | 2 | 2 |
| SIMD71 (quintiles) | |||||
| 1 (most deprived) | 131 (17.8) | 45 (17.7) | 86 (17.8) | 40 (17.3) | 46 (18.3) |
| 2 | 131 (17.8) | 52 (20.5) | 79 (16.4) | 35 (15.2) | 44 (17.5) |
| 3 | 122 (16.6) | 42 (16.5) | 80 (16.6) | 43 (18.6) | 37 (14.7) |
| 4 | 165 (22.4) | 52 (20.5) | 113 (23.4) | 58 (25.1) | 55 (21.8) |
| 5 (least deprived) | 188 (25.5) | 63 (24.8) | 125 (25.9) | 55 (23.8) | 70 (27.8) |
| Missing | 10 | 5 | 5 | 2 | 3 |
| Employment status | |||||
| Paid work | 626 (84.0) | 210 (81.4) | 416 (85.4) | 201 (86.6) | 215 (84.3) |
| Education or training | 8 (1.1) | 8 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unemployed | 27 (3.6) | 13 (5.0) | 14 (2.9) | 3 (1.3) | 11 (4.3) |
| Not working (long-term sickness/disability) | 16 (2.1) | 3 (1.2) | 13 (2.7) | 8 (3.4) | 5 (2.0) |
| Retired | 32 (4.3) | 9 (3.5) | 23 (4.7) | 10 (4.3) | 13 (5.1) |
| Other | 36 (4.8) | 15 (5.8) | 21 (4.3) | 10 (4.3) | 11 (4.3) |
| Missing | 2 | 1 | 1 | 1 | 0 |
| Education level | |||||
| No qualifications | 71 (9.5) | 32 (12.4) | 39 (8.0) | 17 (7.3) | 22 (8.6) |
| Standard grades/Highers | 241 (32.3) | 83 (32.0) | 158 (32.4) | 73 (31.3) | 85 (33.3) |
| Vocational or HNC/HND | 240 (32.1) | 82 (31.7) | 158 (32.4) | 84 (36.1) | 74 (29.0) |
| University | 156 (20.9) | 53 (20.5) | 103 (21.1) | 48 (20.6) | 55 (21.6) |
| Other | 39 (5.2) | 9 (3.5) | 30 (6.1) | 11 (4.7) | 19 (7.5) |
| Housing tenure | |||||
| Owner occupied | 563 (75.4) | 179 (69.1) | 384 (78.7) | 182 (78.1) | 202 (79.2) |
| Other | 184 (24.6) | 80 (30.9) | 104 (21.3) | 51 (21.9) | 53 (20.8) |
| Marital status | |||||
| Married | 518 (69.3) | 181 (69.9) | 337 (69.1) | 149 (63.9) | 188 (73.7) |
| Living with partner | 95 (12.7) | 31 (12.0) | 64 (13.1) | 39 (16.7) | 25 (9.8) |
| Other | 134 (17.9) | 47 (18.1) | 87 (17.8) | 45 (19.3) | 42 (16.5) |
Table 3 shows that the not followed-up cohort had higher baseline weight, waist circumference, BMI, percentage body fat, and systolic and diastolic BP than the total followed-up cohort. Baseline PA, dietary, alcohol intake and psychological variables are provided in Report Supplementary Material 6. These show that men who took part in the 3.5-year measurements ate breakfast slightly more often than those did not; however, there were no other differences between the cohorts.
| Physical characteristic | Cohort, mean (SD) | Group, mean (SD) | |||
|---|---|---|---|---|---|
| FFIT RCT (N = 747) | Not followed up (N = 259) | Total followed up (N = 488) | FFIT-FU-I (N = 233) | FFIT-FU-C (N = 255) | |
| Weight (kg) | 109.5 (17.3) | 112.6 (17.2) | 107.8 (17.1) | 108.3 (17.9) | 107.4 (16.3) |
| Waist circumference (cm) | 118.4 (11.7) | 120.7 (11.7) | 117.1 (11.6) | 117.5 (12.3) | 116.8 (10.8) |
| BMI (kg/m2) | 35.4 (5.0) | 36.3 (5.0) | 34.9 (4.9) | 35.0 (5.1) | 34.8 (4.7) |
| Body fat (%) | 31.7 (5.5) | 32.5 (5.0) | 31.2 (5.6) | 31.3 (6.0) | 31.2 (5.3) |
| Missing | 10 | 3 | 7 | 4 | 3 |
| BP (mmHg) | |||||
| Systolic | 140.3 (16.3) | 142.5 (17.0) | 139.1 (15.8) | 137.5 (16.7) | 140.7 (14.9) |
| Diastolic | 88.8 (10.2) | 90.2 (10.7) | 88.1 (9.9) | 87.4 (10.0) | 88.8 (9.8) |
| Missing | 2 | 2 | 0 | 0 | 0 |
| Participants with a BMI of 28–30 kg/m2, n (%) | 72 (9.6) | 15 (5.8) | 57 (11.7) | 25 (10.7) | 32 (12.5) |
Outcomes
Long-term weight outcomes
Table 4 shows that, at 3.5 years, the mean weight loss from baseline in the FFIT-FU-I group was 2.90 kg (95% CI 1.78 to 4.02 kg; p < 0.0001); the equivalent figure for the FFIT-FU-C group was 2.71 kg (95% CI 1.65 to 3.77 kg; p < 0.0001). There were no between-group differences. Table 5 shows that similar proportions of men (≈32%) in both groups weighed ≥ 5% less than their baseline weight at 3.5 years.
| Change from baseline weight | Group | Difference between groups (FFIT-FU-C – FFIT-FU-I) | ||||
|---|---|---|---|---|---|---|
| FFIT-FU-I (N = 233) | FFIT-FU-C (N = 255) | |||||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Change to 12 months | ||||||
| Absolute (kg) | –5.49 (–6.51 to –4.47) | < 0.0001 | –0.68 (–1.32 to –0.03) | 0.1244 | 4.81 (3.61 to 6.02) | < 0.0001 |
| Percentage | –4.96 (–5.85 to –4.07) | < 0.0001 | –0.57 (–1.15 to 0.00) | 0.1425 | 4.39 (3.33 to 5.44) | < 0.0001 |
| Change to 3.5 years | ||||||
| Absolute (kg) | –2.90 (–4.02 to –1.78) | < 0.0001 | –2.71 (–3.77 to –1.65) | < 0.0001 | 0.19 (–1.35 to 1.73) | 0.7421 |
| Percentage | –2.52 (3.45 to –1.60) | < 0.0001 | –2.36 (–3.31 to –1.41) | < 0.0001 | 0.16 (–1.17 to 1.49) | 0.7266 |
| Proportion of men | Group, n (%) | |
|---|---|---|
| FFIT-FU-I (N = 233) | FFIT-FU-I (N = 255) | |
| Achieving at least 5% weight loss | 75 (32.2) | 81 (31.8) |
Table 4 also shows that, at the RCT 12-month measures, the FFIT-FU-I group had lost 5.49 kg (95% CI 4.47 to 6.51 kg) and the FFIT-FU-C group had lost 0.68 kg (95% CI 0.03 to 1.32 kg) from baseline. It is important and reassuring to note that the 12-month weight loss figures for the men who were followed to 3.5 years are very similar to those reported at the end of the trial, at which point the mean 12-month weight loss was 5.56 kg (95% CI 4.70 to 6.43 kg) in the intervention group and 0.58 kg (95% CI 0.04 to 1.12 kg) in the comparison group. 43 Thus, there was no selective loss to long-term follow-up owing to 12-month weight loss outcomes.
Long-term weight trajectories
Table 6 shows that men in the FFIT-FU-I group gained 2.59 kg (95% CI 1.61 to 3.58 kg; p < 0.001) between the 12-month and the 3.5-year measures (i.e. 2.44%, 95% CI 1.61% to 3.27%, of their baseline weight). This equates to a weight gain of 1.04 kg per year. Nevertheless, 3.5 years after baseline measurement, men in the FFIT-FU-I group still weighed 2.90 kg less on average, demonstrating a sustained weight benefit from taking part in the FFIT programme. Meanwhile, men in the FFIT-FU-C group (who had the opportunity to take part in the FFIT programme under ‘routine delivery’ conditions immediately after the 12-month measures) lost 2.03 kg (95% CI 1.08 to 2.98 kg; p < 0.001) or 1.79% (95% CI 0.92% to 2.65%) of their baseline weight during the same time period. The mean between-group difference in weight trajectories was 4.62 kg (95% CI 3.26 to 5.99 kg; p < 0.001) or 4.23% (95% CI 3.02% to 5.43%; p < 0.001).
| Change in weight | Group | Difference between groupsa (FFIT-FU-C – FFIT-FU-I) | ||||
|---|---|---|---|---|---|---|
| FFIT-FU-I (N = 233) | FFIT-FU-C (N = 255) | |||||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Absolute (kg) | 2.59 (1.61 to 3.58) | < 0.001 | –2.03 (–2.98 to –1.08) | < 0.001 | –4.62 (–5.99 to –3.26) | < 0.001 |
| Percentage (of baseline) | 2.44 (1.61 to 3.27) | < 0.001 | –1.79 (–2.65 to –0.92) | < 0.001 | –4.23 (–5.43 to –3.02) | < 0.001 |
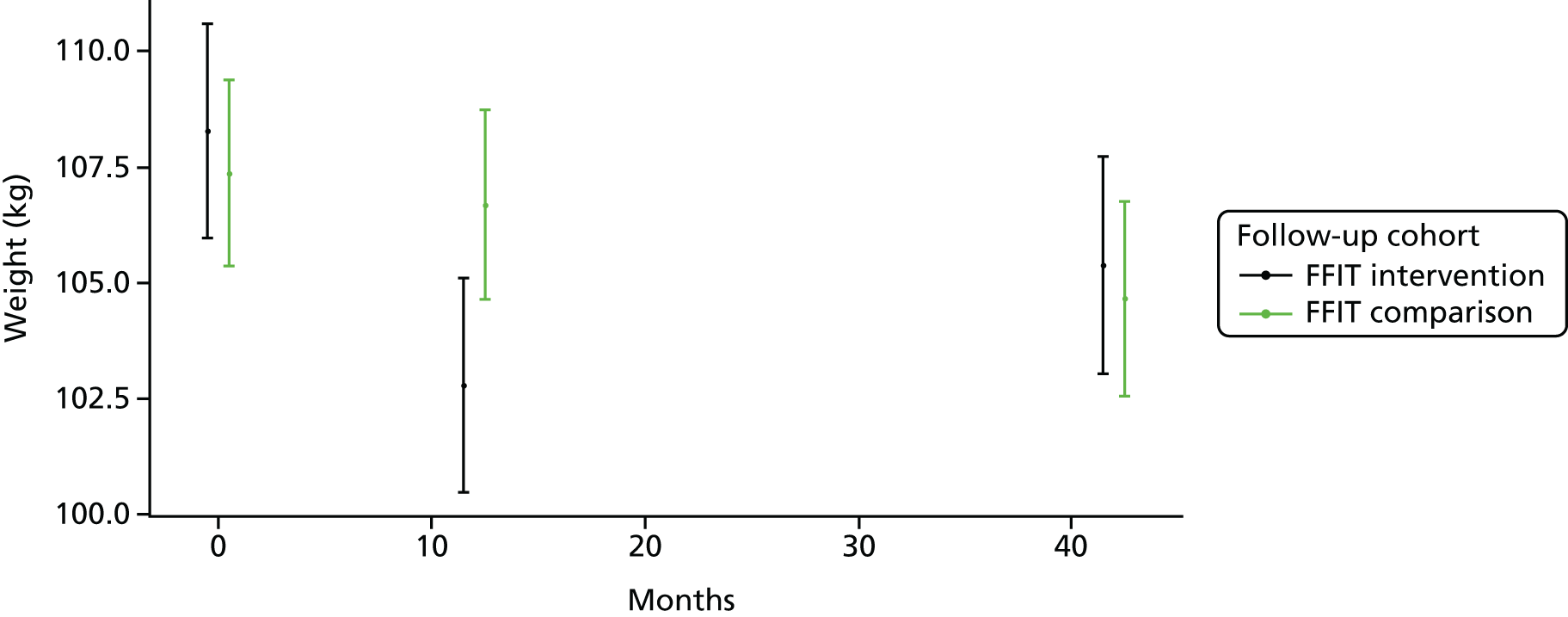
Figure 4 illustrates the data shown in Table 4, and clearly demonstrates that both groups weighed less at 3.5 years than at baseline.
FIGURE 4.
Mean weight (kg, 95% CI) of participants in the FFIT-FU-I and FFIT-FU-C groups at 12 months and 3.5 years after the FFIT RCT baseline measurements. Note that the y-axis [weight (kg)] does not start at zero.
Sensitivity analyses
Sensitivity analyses on the primary outcome (change in weight) were conducted to assess the sensitivity of the main analyses.
-
Loss to follow-up sensitivity analyses used a variety of assumptions about the long-term weight outcomes of men who had not taken part in follow-up measures at 3.5 years.
-
Baseline time points sensitivity analyses assessed the fact that both intervention and comparison groups had the opportunity to take part in the FFIT programme, but at different times.
In the loss to follow-up sensitivity analyses, the return-to-baseline and last-value-carried-forward methods were used to impute missing weight data at 12 months and 3.5 years for men who were not followed up. As men who were not followed up at 3.5 years (the not followed-up cohort) were, on average, heavier than the total followed-up cohort at baseline [112.8 kg (SD 17.2) vs. 107.8 kg (SD 17.1)] and 12 months [109.8 kg (SD 18.3) vs. 104.8 kg (SD 17.3)], the return-to-baseline sensitivity analysis is the most conservative and is reported here. The results of the last-value-carried-forward sensitivity analyses are shown in tables i and ii in Report Supplementary Material 7.
Table 7 shows that, in the return-to-baseline sensitivity analysis, the mean weight loss at 3.5 years was 1.81 kg (95% CI 1.09 to 2.52 kg) in the RCT intervention group (including imputed values) and 1.85 kg (1.12 to 2.58 kg) in the RCT comparison group (including imputed values). As in the main analyses, both figures were still significantly different from baseline, but there was no between-group difference.
| Change in weight | RCT group | Difference between groups (comparison – intervention) | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 374) | Comparison (N = 373) | |||||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Absolute (kg) | –1.81 (–2.52 to –1.09) | < 0.0001 | –1.85 (–2.58 to –1.12) | < 0.0001 | –0.05 (–1.07 to 0.98) | 0.7984 |
| Percentage | –1.57 (–2.16 to –0.98) | < 0.0001 | –1.61 (–2.27 to –0.96) | < 0.0001 | –0.04 (–0.92 to 0.84) | 0.7898 |
Table 8 shows that, between 12 months and 3.5 years, men in the RCT intervention group (including imputed values) gained 3.15 kg (95% CI 2.37 to 3.92 kg; p < 0.001) and those in the RCT comparison group (including imputed values) lost 1.30 kg (95% CI 0.59 to 2.01 kg; p < 0.001). Although, as expected, there were slight differences in these values from the main analyses, the change in weight over time in each group remained significant, as did the between-group difference in weight trajectories (4.45 kg, 95% CI 3.40 to 5.50 kg; p < 0.001).
| Change in weight | RCT group | Difference between groupsa (comparison – intervention) | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 374) | Comparison (N = 373) | |||||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Absolute (kg) | 3.15 (2.37 to 3.92) | < 0.001 | –1.30 (–2.01 to –0.59) | 0.0004 | –4.45 (–5.50 to –3.40) | < 0.001 |
| Percentage (of baseline) | 2.84 (2.18 to 3.50) | < 0.001 | –1.12 (–1.77 to –0.48) | 0.0007 | –3.96 (–4.88 to –3.04) | < 0.001 |
Full details of the baseline time points sensitivity analyses are provided in table iii in Report Supplementary Material 7 and confirm sustained weight loss in the FFIT-FU-I group at 3.5 years post intervention.
Randomised controlled trials secondary outcomes
Objectively measured physical outcomes
Table 9 shows that there were sustained improvements in waist circumference, BMI, percentage body fat, and systolic and diastolic BP at 3.5 years and 2.5 years after taking part in the programme (for the FFIT-FU-I group and FFIT-FU-C group, respectively). There were no between-group differences.
| Change in physical outcome | Group | Difference between groups (FFIT-FU-C – FFIT-FU-I) | ||||||
|---|---|---|---|---|---|---|---|---|
| FFIT-FU-I | FFIT-FU-C | |||||||
| n | Mean (95% CI) | p-value | n | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Waist (cm) | 214 | –2.90 (–3.89 to –1.91) | < 0.0001 | 237 | –2.64 (–3.64 to –1.65) | < 0.0001 | 0.25 (–1.15 to 1.66) | 0.7057 |
| BMI (kg/m2) | 233 | –0.96 (–1.31 to –0.60) | < 0.0001 | 255 | –0.88 (–1.22 to –0.54) | < 0.0001 | 0.08 (–0.42 to 0.57) | 0.7010 |
| Body fat (%) | 162 | –1.94 (–2.81 to –1.06) | < 0.0001 | 165 | –1.38 (–2.31 to –0.45) | < 0.0001 | 0.56 (–0.72 to 1.83) | 0.3085 |
| BP (mmHg) | ||||||||
| Systolic | 214 | –3.13 (–5.15 to –1.11) | 0.0080 | 235 | –4.58 (–6.42 to –2.74) | < 0.0001 | –1.45 (–4.17 to 1.27) | 0.1862 |
| Diastolic | 214 | –1.56 (–2.80 to –0.32) | 0.0308 | 235 | –2.95 (–4.24 to –1.67) | < 0.0001 | –1.39 (–3.18 to 0.39) | 0.0920 |
Self-reported physical activity
Table 10 shows that the total PA was significantly higher at 3.5 years than at baseline in both the FFIT-FU-I group [800.0 MET-minutes per week, interquartile range (IQR) –120 to 2514 MET-minutes per week] and the FFIT-FU-C group (919.0 MET-minutes per week, IQR –186 to 2909 MET-minutes per week), and that there were no significant between-group differences. A similar pattern of results was observed for vigorous and moderate PA, and for walking. Table 10 also demonstrates a sustained reduction in time spent sitting in both groups.
| Change in self-reported PA | Group | Difference between groups (FFIT-FU-C – FFIT-FU-I) | ||||||
|---|---|---|---|---|---|---|---|---|
| FFIT-FU-I | FFIT-FU-C | |||||||
| n | Median (IQR) | p-value | n | Median (IQR) | p-value | Mean (95% CI) | p-value | |
| Total PA (MET-minutes/week) | 213 | 800.0 (–120 to 2514) | < 0.0001 | 232 | 919.0 (–186 to 2909) | < 0.0001 | 148.7 (–427.5 to 724.9) | 0.6055 |
| Vigorous PA (MET-minutes/week) | 213 | 0 (0 to 1320) | < 0.0001 | 232 | 0 (0 to 1140) | < 0.0001 | 140.9 (–235.4 to 517.2) | 0.6872 |
| Moderate PA (MET-minutes/week) | 213 | 0 (0 to 700) | < 0.0001 | 232 | 0 (0 to 630) | < 0.0001 | 6.7 (–229.4 to 242.7) | 0.8298 |
| Walking (MET-minutes/week) | 213 | 297.0 (–66 to 1040) | < 0.0001 | 232 | 297.0 (–132 to 1287) | < 0.0001 | 1.1 (–237.6 to 239.8) | 0.8652 |
| Daily sitting (minutes) | 171 | –30 (–180 to 120) | 0.0389 | 189 | –30 (–180 to 60) | 0.0011 | –12 (–61 to 36) | 0.6121 |
Self-reported eating and alcohol intake
Table 11 shows that the consumption of fatty food and sugary food scores at 3.5 years were significantly reduced from baseline in both groups, and that there were no between-group differences. Fruit and vegetables consumption was significantly higher at 3.5 years in both groups, and, again, there were no between-group differences. Similar sustained improvements were also evident for portion sizes of cheese, meat, pasta and chips, and for alcohol consumption; all showed sustained reductions from baseline, and no between-group differences.
| Change in self-reported food and alcohol consumption | Group | Difference between groups (FFIT-FU-C – FFIT-FU-I) | ||||||
|---|---|---|---|---|---|---|---|---|
| FFIT-FU-I | FFIT-FU-C | |||||||
| n | Mean (95% CI) | p-value | n | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Score | ||||||||
| Fatty food | 214 | –3.86 (–4.83 to –2.89) | < 0.0001 | 236 | –3.16 (–3.99 to –2.33) | < 0.0001 | 0.70 (–0.57 to 1.97) | 0.3289 |
| Sugary food | 214 | –1.32 (–1.69 to –0.95) | < 0.0001 | 236 | –1.07 (–1.41 to –0.73) | < 0.0001 | 0.25 (–0.25 to 0.75) | 0.4264 |
| Fruit and vegetables | 214 | 0.50 (0.23 to 0.76) | < 0.0001 | 236 | 0.40 (0.14 to 0.65) | < 0.0001 | –0.10 (–0.47 to 0.27) | 0.5596 |
| Portion size | ||||||||
| Cheese | 198 | –1.12 (–1.41 to –0.83) | < 0.0001 | 213 | –1.12 (–1.41 to –0.83) | < 0.0001 | 0.00 (–0.41 to 0.41) | 0.9393 |
| Meat | 205 | –0.98 (–1.18 to –0.77) | < 0.0001 | 232 | –0.83 (–1.03 to –0.64) | < 0.0001 | 0.14 (–0.14 to 0.43) | 0.2017 |
| Pasta | 198 | –1.21 (–1.44 to –0.98) | < 0.0001 | 226 | –1.11 (–1.33 to –0.88) | < 0.0001 | 0.11 (–0.22 to 0.43) | 0.6339 |
| Chips | 183 | –1.08 (–1.32 to –0.84) | < 0.0001 | 217 | –0.84 (–1.07 to –0.61) | < 0.0001 | 0.24 (–0.09 to 0.58) | 0.0911 |
| Total alcohol (units/week) | 207 | –2.68 (–4.52 to –0.83) | 0.0070 | 233 | –4.28 (–6.06 to –2.50) | < 0.0001 | –1.61 (–4.16 to 0.95) | 0.2945 |
Self-reported psychological health and quality-of-life outcomes
Table 12 shows that improvements in self-esteem, positive and negative affect, and physical and mental HRQoL were sustained to 3.5 years in both groups, and that there were no between-group differences.
| Change in self-reported psychological outcomes | Group | Difference between groups (FFIT-FU-C – FFIT-FU-I) | ||||||
|---|---|---|---|---|---|---|---|---|
| FFIT-FU-I | FFIT-FU-C | |||||||
| n | Mean (95% CI) | p-value | n | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| RSE score | ||||||||
| Self-esteem | 214 | 0.23 (0.18 to 0.29) | < 0.0001 | 237 | 0.25 (0.20 to 0.30) | < 0.0001 | 0.01 (–0.06 to 0.09) | 0.5507 |
| PANAS score | ||||||||
| Positive affect | 214 | 0.27 (0.17 to 0.38) | < 0.0001 | 237 | 0.24 (0.16 to 0.32) | < 0.0001 | –0.04 (–0.17 to 0.09) | 0.8715 |
| Negative affect | 214 | –0.17 (–0.24 to –0.11) | < 0.0001 | 237 | –0.11 (–0.17 to –0.05) | < 0.0001 | 0.06 (–0.03 to 0.15) | 0.2429 |
| SF-12 score (HRQoL) | ||||||||
| Mental | 213 | 1.12 (–0.19 to 2.43) | 0.0145 | 235 | 2.63 (1.57 to 3.69) | < 0.0001 | 1.51 (–0.17 to 3.19) | 0.1619 |
| Physical | 213 | 1.98 (0.81 to 3.16) | < 0.0001 | 235 | 1.09 (–0.08 to 2.25)a | 0.0218a | –0.90 (–2.55 to 0.76) | 0.1008 |
Randomised controlled trial secondary outcome trajectories
Table 13 shows significant differences in the trajectories of many RCT secondary outcomes from 12 months to 3.5 years between the FFIT-FU-I and the FFIT-FU-C groups. These are likely to reflect the fact that the FFIT-FU-C group had the opportunity to take part in the FFIT programme during this time (from month 13). The results for the FFIT-FU-C group, therefore, reflect both the changes men made as a result of taking part in the FFIT programme and the maintenance, or lack of maintenance, of these changes over the 2.5 years following the completion of the programme.
| Change in secondary outcomes | Group | Difference between groupsa (FFIT-FU-C – FFIT-FU-I) | ||||
|---|---|---|---|---|---|---|
| FFIT-FU-I | FFIT-FU-C | |||||
| Mean (95% CI) | p-value | Mean (95% CI) | p-value | Mean (95% CI) | p-value | |
| Change in objectively measured physical outcomes | ||||||
| Waist (cm) | 4.41 (3.46 to 5.36) | < 0.0001 | –0.83 (–1.79 to 0.12) | 0.0865 | –5.26 (–6.61 to –3.91) | < 0.0001 |
| BMI (kg/m2) | 0.82 (0.50 to 1.13) | < 0.0001 | –0.66 (–0.96 to –0.35) | < 0.0001 | –1.47 (–1.91 to –1.04) | < 0.0001 |
| Body fat (%) | 0.40 (–0.44 to 1.24) | 0.3457 | –1.40 (–2.30 to –0.50) | 0.0025 | –1.84 (–3.09 to –0.59) | 0.0039 |
| BP (mmHg) | ||||||
| Systolic | 4.62 (2.98 to 6.27) | < 0.0001 | 1.38 (–0.25 to 3.01) | 0.0965 | –3.20 (–5.51 to –0.88) | 0.0068 |
| Diastolic | 3.05 (1.85 to 4.26) | < 0.0001 | 0.48 (–0.65 to 1.60) | 0.4058 | 2.55 (–4.19 to 0.90) | 0.0024 |
| Change in self-reported PA | ||||||
| Total PA (MET-minutes/week) | –439 (–871 to –8) | 0.0461 | 668 (292 to 1044) | 0.0005 | 1096 (526 to 1666) | 0.0002 |
| Vigorous PA (MET-minutes/week) | –542 (–824 to –261) | 0.0002 | 219 (–58 to 496) | 0.1203 | 760 (366 to 1155) | 0.0002 |
| Moderate PA (MET-minutes/week) | 45 (–118 to 208) | 0.5864 | 210 (46 to 374) | 0.0122 | 161 (–70 to 393) | 0.1722 |
| Walking (MET-minutes/week) | 55 (–115 to 226) | 0.5232 | 232 (67 to 398) | 0.0060 | 176 (–62 to 413) | 0.1468 |
| Daily time sitting (minutes) | 24 (–7 to 56) | 0.1328 | –16 (–43 to 12) | 0.2571 | –40 (–82 to 1) | 0.0572 |
| Change in self-reported diet and alcohol intake | ||||||
| Score | ||||||
| Fatty food | 0.71 (–0.10 to 1.53) | 0.0857 | –1.15 (–1.90 to –0.40) | 0.0026 | –1.88 (–2.98 to –0.77) | 0.0009 |
| Sugary food | –0.02 (–0.32 to 0.29) | 0.9168 | –0.54 (–0.86 to –0.22) | 0.0011 | –0.54 (–0.98 to 0.09) | 0.0185 |
| Fruit and vegetables | –0.42 (–0.67 to –0.17) | 0.0012 | 0.19 (–0.06 to 0.43) | 0.1305 | 0.60 (0.25 to 0.95) | 0.0007 |
| Portion size | ||||||
| Cheese | 0.22 (–0.04 to 0.48) | 0.0903 | –0.39 (–0.63 to –0.15) | 0.0017 | –0.61 (–0.96 to –0.26) | 0.0007 |
| Meat | 0.09 (–0.11 to 0.29) | 0.3736 | –0.31 (–0.51 to –0.12) | 0.0019 | –0.40 (–0.68 to –0.12) | 0.0048 |
| Pasta | 0.32 (0.12 to 0.52) | 0.0020 | –0.43 (–0.64 to –0.23) | < 0.0001 | –0.76 (–1.05 to –0.47) | < 0.0001 |
| Chips | 0.34 (0.16 to 0.53) | 0.0003 | –0.26 (–0.45 to –0.06) | 0.0093 | –0.61 (–0.88 to –0.34) | < 0.0001 |
| Total alcohol (units/week) | 0.69 (–0.95 to 2.33) | 0.4080 | –1.68 (–3.31 to –0.04) | 0.0449 | –2.42 (–4.74 to –0.10) | 0.0411 |
| Change in self-reported psychological health and quality-of-life outcomes | ||||||
| RSE score | ||||||
| Self-esteem | –0.01 (–0.06 to 0.03) | 0.5437 | 0.12 (0.07 to 0.16) | < 0.0001 | 0.13 (0.07 to 0.20) | < 0.0001 |
| PANAS score | ||||||
| Positive affect | –0.06 (–0.14 to 0.02) | 0.1482 | 0.17 (0.10 to 0.25) | < 0.0001 | –0.23 (–0.34 to –0.12) | < 0.0001 |
| Negative affect | 0.04 (–0.03 to 0.10) | 0.2735 | –0.02 (–0.07 to 0.03) | 0.4274 | –0.06 (–0.14 to 0.02) | 0.1494 |
| SF-12 score (HRQoL) | ||||||
| Mental | –1.20 (–2.36 to –0.04) | 0.0431 | 1.02 (0.06 to 1.99) | 0.0381 | 2.22 (0.73 to 3.72) | 0.0037 |
| Physical | –0.07 (–1.19 to 1.04) | 0.8971 | 0.81 (–0.23 to 1.85) | 0.1243 | 0.89 (–0.63 to 2.41) | 0.2485 |
Taking each group separately, the FFIT-FU-I group showed some regain in waist circumference and BMI, and increases in systolic and diastolic BP between 12 months and 3.5 years (although these were all significantly improved from baseline), whereas the FFIT-FU-C group showed tendencies towards improvement in these outcomes over this time period.
In relation to self-reported PA, although the FFIT-FU-I group showed some reduction in total and vigorous PA between 12 months and 3.5 years, levels of moderate PA and walking appeared to be sustained. The FFIT-FU-C group showed improvements in total and moderate PA, and in walking. Between-group differences in moderate PA and in walking were not significant.
The FFIT-FU-I group also demonstrated sustained reductions in intake of fatty and sugary foods, portion sizes of cheese and meat, and total alcohol consumption from 12 months to 3.5 years, but consumption of fruit and vegetables decreased. The FFIT-FU-C group showed improvements in all dietary outcomes (apart from fruit and vegetables consumption) between 12 months and 3.5 years.
Finally, improvements in all psychological outcomes, apart from mental HRQoL, were sustained between 12 months and 3.5 years in the FFIT-FU-I group. The FFIT-FU-C group demonstrated improvements in self-esteem, positive affect and mental HRQoL during this period.
Predictors of long-term weight loss
Baseline predictors of long-term weight loss
None of the prespecified baseline predictors (age, BMI, education level, socioeconomic status, marital status, orientation to masculine norms or number of long-standing illnesses) was significantly associated with weight loss at 3.5 years. For specific estimates and p-values, refer to tables 7.1–7.7 in Report Supplementary Material 8.
Mediators of long-term weight loss
Unlike the predictors, which are measured at baseline only, mediators of long-term weight loss were measured at multiple time points (12 weeks, 12 months and 3.5 years). Therefore, using these results to interpret the effect of each mediator on weight change, especially when the groups took part in the FFIT programme at different times, is not straightforward. Consequently, we performed multiple analyses at single time points. Mediators that were measured at 12 weeks and 12 months were analysed only in the FFIT-FU-I group, as the FFIT-FU-C group had yet to receive the intervention. The groups were combined for mediators measured at 3.5 years. The full results of the mediator analyses are presented in Report Supplementary Material 9. No 12-week variables (apart from objectively measured weight in the FFIT-FU-I group) showed any associations with long-term weight outcomes.
Table 14 shows that increases in self-reported total and vigorous PA, positive affect and physical HRQoL at 12 months all predict a lower weight at 3.5 years. In addition, increased total, vigorous and moderate PA, reduced intake of fatty and sugary foods, increased intake of fruit and vegetables, smaller portions of cheese, meat, pasta and chips, and increased positive affect, self-esteem and physical HRQoL at 3.5 years are associated with a lower weight at 3.5 years. Finally, increased time spent sitting at 3.5 years is associated with a higher weight at 3.5 years.
| Change in mediator | Length of time | |||
|---|---|---|---|---|
| 12 monthsb | 3.5 yearsc | |||
| Direction of effect | p-value | Direction of effect | p-value | |
| Change in self-reported PA (International Physical Activity Questionnaire) | ||||
| MET total | Negative | 0.014 | Negative | < 0.001 |
| MET vigorous | Negative | 0.024 | Negative | 0.011 |
| MET walking | NS | NS | Negative | < 0.001 |
| Sitting time | NS | NS | Positive | 0.043 |
| Change in self-reported diet (DINE score) | ||||
| Fatty food | NS | NS | Positive | < 0.001 |
| Sugary food | NS | NS | Positive | 0.003 |
| Fruit and vegetables | NS | NS | Negative | < 0.001 |
| Change in self-reported diet (portion size) | ||||
| Cheese | NS | NS | Positive | 0.038 |
| Meat | NS | NS | Positive | 0.005 |
| Pasta | NS | NS | Positive | < 0.001 |
| Chips | NS | NS | Positive | 0.003 |
| Change in self-reported psychological health outcomes | ||||
| Positive affect PANAS score | Negative | 0.009 | Negative | < 0.001 |
| Self-esteem | NS | NS | Negative | < 0.001 |
| Physical HRQoL | Negative | 0.013 | Negative | < 0.001 |
Table 15 shows that higher scores in relation to the self-determination theory constructs of autonomous regulation for diet and PA, perceived autonomy for diet and PA, perceived competence for diet and PA, and relatedness with peers (intimacy) and family members (acceptance), as well as higher satisfaction with current diet and PA, were associated with a lower weight at 3.5 years. In contrast, greater amotivation in relation to diet and PA was associated with a higher weight at 3.5 years.
| Mediator | Direction of effect | p-value |
|---|---|---|
| Quality of motivation (TSRQ) | ||
| Diet autonomous regulation | Negative | < 0.001 |
| Diet amotivation | Positive | 0.029 |
| PA autonomous regulation | Negative | < 0.001 |
| PA amotivation | Positive | 0.047 |
| Perceived autonomy (LCS) | ||
| Diet | Negative | < 0.001 |
| PA | Negative | < 0.001 |
| Perceived competence (PCS) | ||
| Diet | Negative | < 0.001 |
| PA | Negative | < 0.001 |
| Relatedness (NRS) | ||
| Peers intimacy | Negative | 0.049 |
| Family acceptance | Negative | 0.047 |
| Satisfaction with health behaviours | ||
| Current diet | Negative | < 0.001 |
| Current PA | Negative | < 0.001 |
Table 16 shows that self-reported routinisation of PA (walking, gym attendance and taking other forms of exercise), and healthy eating behaviours (eating regular meals, reducing portion sizes and reducing intake of fatty and sugary foods, sugary drinks and calories), self-monitoring of weight, reading the labels on food packaging, and ongoing contact with other participants and coaches from the FFIT programme were associated with a lower weight at 3.5 years.
| Mediator | Direction of effect | p-value |
|---|---|---|
| Routinisation of health behaviours | ||
| Walking | Negative | 0.001 |
| Attending gym | Negative | 0.021 |
| Cycling, swimming or other independent exercise | Negative | < 0.001 |
| Eating regular meals | Negative | 0.016 |
| Limiting portion size | Negative | < 0.001 |
| Limiting fatty/sugary food | Negative | < 0.001 |
| Limiting calories | Negative | < 0.001 |
| Limiting sugary drinks | Negative | 0.002 |
| Ongoing use of BCTs and practical strategies | ||
| Monitoring weight | Negative | < 0.001 |
| Reading food labels | Negative | 0.006 |
| Ongoing contact with people from the FFIT programme | ||
| Other FFIT participants | Negative | 0.043 |
| FFIT coaches | Negative | 0.041 |
| Number of major life events | ||
| None | NS | – |
Table 17 shows that greater weight loss at the end of the initial 12-week active phase of the FFIT programme (from objectively measured weight at 12-week RCT measurements in the FFIT-FU-I group, and self-reported retrospectively at 3.5 years by the FFIT-FU-C group) was associated with a lower weight at 3.5 years. Weight gain in the 12 months before starting the FFIT programme (from the RCT 12-month measurements in the FFIT-FU-C group only) was associated with a higher weight at 3.5 years.
| Mediator | Direction of effect | p-value |
|---|---|---|
| Objective post-intervention weight change categorya (FFIT-FU-I) | Negative | < 0.001 |
| Self-reported post-intervention weight change categoryb (FFIT-FU-C) | Negative | < 0.001 |
| Objective pre-intervention weight change (FFIT-FU-C) | Positive | < 0.001 |
Table 18 demonstrates that self-reported injuries that limit walking at 12 months predicted higher weight at 3.5 years, and that self-reported lower limb joint pain frequency, upper body and lower limb joint pain that limit activities, injuries that limit walking and using stairs, and total types of limitations due to injury were all positively associated with weight at 3.5 years.
| Mediator | Time assessed | |||
|---|---|---|---|---|
| 12 monthsb | 3.5 yearsc | |||
| Direction of effect | p-value | Direction of effect | p-value | |
| Lower limb joint pain frequency | NS | NS | Positive | 0.021 |
| Upper body joint pain that limits activities | NS | NS | Positive | 0.010 |
| Lower limb joint pain that limits activities | NS | NS | Positive | 0.023 |
| Any injuries that limit walking | Positive | 0.022 | Positive | 0.004 |
| Any injuries that limit using stairs | NS | NS | Positive | 0.010 |
| Total types of limitations due to injury | Positive | 0.036 | Positive | 0.009 |
Adverse events
Thirty new adverse events that were perceived to be related to participation in the FFIT programme were reported by men in the FFIT-FU-I group, and 16 were reported by men in the FFIT-FU-C group. In addition, linkage to routine NHS data sets (see Chapter 6, Long-term clinical health outcomes) revealed that one man in the FFIT RCT comparison group had died. A complete list of new adverse events reported at the 3.5-year measurements can be found in listing 6 A and B of Report Supplementary Material 8.
Summary and initial interpretation of results
The first aim of the FFIT follow-up study (see Chapter 1, Aims and objectives) was to investigate the long-term weight trajectories from baseline to 3.5 years (i.e. 3.5 years after the RCT intervention group commenced participation in the FFIT programme and 2.5 years after the comparison group did so) in men who were aged 35–65 years with a BMI of ≥ 28 kg/m2 at the start of the RCT. Here we summarise our results in relation to the three objectives that related to this aim.
Objective 1: long-term weight outcomes
Our first objective was to investigate the extent to which participants in the RCT intervention group and comparison group achieved objectively measured long-term weight loss, and how weight trajectories and weight differed between these groups (see Chapter 1, Aims and objectives). However, we start this section by describing the men who took part in the follow-up study and comparing them with those who did not take part.
Retention to the Football Fans in Training follow-up study
The use of the strategies that had been successful in achieving a retention rate of 92% at the 12-month RCT measurements44 resulted in the retention of 65% of the original RCT cohort at the 3.5-year follow-up study. There were some differences between men who took part in the 3.5-year measurements and those who did not. Men who were followed up were slightly older, more likely to be in paid work and more likely to own their home than those who were not followed up, perhaps reflecting a more stable lifestyle among those who were followed up. Men who took part in the 3.5-year measurements were also less overweight and had lower BP at baseline than those who did not take part in the follow-up study. Nevertheless, there were no differences in the RCT 12-month weight outcomes between men who were followed up and men who were not followed up, suggesting that success at maintaining weight loss to 12 months did not influence participation in the 3.5-year measures.
Long-term weight loss
The intervention group
Our results showed that men who took part in the FFIT programme during the RCT (the FFIT-FU-I group) succeeded in sustaining a weight loss from baseline of 2.90 kg (95% CI 1.78 to 4.02 kg; p < 0.0001) 3.5 years after the start of their participation in the FFIT programme. Around 32% (75/233) had retained a weight loss of ≥ 5% of their baseline weight at 3.5 years. This compares well with the 39% (130/355) of men who achieved this figure at the FFIT RCT 12-month measures. 44 These men had undergone the FFIT programme under research conditions, when coaches were supported by the research team, and researchers had observed two sessions at each football club and interviewed participants and coaches about their experiences. 44
The comparison group
At the 3.5-year follow-up, the FFIT-FU-C group showed a similar reduction in weight from baseline (as the FFIT-FU-I group) of 2.71 kg (95% CI 1.65 to 3.77 kg; p < 0.0001). This was 2.5 years after many (but not all) of this group started the FFIT programme. The men in the FFIT-FU-C group took part in the FFIT programme under ‘routine delivery’ conditions (after the research team transferred management of programme delivery to the SPFL Trust at the end of the RCT). Around 32% (81/255) of men had lost ≥ 5% of their weight at 3.5 years, compared with 11% (40/355) of men at the RCT 12-month measures. Thus, it appears that weight loss achieved at 3.5 years was similar regardless of whether men took part in the FFIT programme under ‘routine’ or ‘research’ conditions.
Long-term weight trajectories
Between 12 months and 3.5 years, as expected, there were marked differences in weight trajectories between the groups. Men in the FFIT-FU-I group regained almost half of the weight they had lost (2.59 kg, 95% CI 1.61 to 3.58 kg) compared with men in the FFIT-FU-C group who lost 2.03 kg (95% CI 1.08 kg to 2.98 kg) over this period. This difference probably reflects the fact that men in the comparison group had the opportunity to take part in the FFIT programme at the start of this period, whereas those in the intervention group had had this opportunity 12 months earlier. As a result, the 12-month to 3.5-year weight trajectories of men in the FFIT-FU-C group reflect both their initial weight loss during the FFIT programme and its subsequent maintenance or lack of maintenance, whereas the weight trajectories of the FFIT-FU-I group simply reflect maintenance or lack of maintenance post programme.
Objective 2: randomised controlled trial secondary outcomes
Our second objective was to investigate the extent to which there were long-term changes in the RCT secondary outcomes (objective physical measurements, self-reported health behaviours and psychological outcomes) and how these differed between the groups (see Chapter 1, Aims and objectives).
Long-term secondary outcomes in the intervention group
Men in the FFIT-FU-I group showed sustained improvements in physical, behavioural and psychological outcomes at 3.5 years. Improvements in physical outcomes (in addition to weight) included sustained reductions in BMI, waist circumference, percentage body fat, and systolic and diastolic BP. Self-reported PA was higher at 3.5 years than at baseline and, on average, men sat less, continued to eat fewer fatty and sugary foods, continued to eat more fruit and vegetables, had smaller portion sizes and showed a sustained reduction in the amount of alcohol that they reported drinking. This group also showed sustained improvements in self-esteem, positive and negative affect, and physical and mental HRQoL at 3.5 years.
Long-term secondary outcomes in the comparison group
Men in the FFIT-FU-C group showed improvements from baseline in all secondary outcomes, and to levels similar to those seen in the FFIT-FU-I group. Thus, at 3.5 years, BMI, waist circumference, and systolic and diastolic BP were all reduced in the FFIT-FU-C group. Men reported increased PA and decreased sitting time, and also showed improvements in consumption of fatty and sugary foods, fruit and vegetables, alcohol intake and portion sizes. Self-esteem, positive and negative affect, and physical and mental HRQoL were also improved at 3.5 years.
Long-term secondary outcome trajectories
As with weight, men in the FFIT-FU-I group showed some attenuation of the impact that the FFIT programme had across other physical, behavioural and psychological outcomes over the long term, although all were still significantly better at 3.5 years than they had been at baseline. There was some regain of waist circumference and BMI, and increases in systolic and diastolic BP, as well as reductions in self-reported total and vigorous PA, fruit and vegetable consumption and mental HRQoL between 12 months and 3.5 years. Nevertheless, there was no significant reduction during this period in levels of moderate PA and walking. In relation to diet, men were still managing to limit their intake of sugary foods and alcohol and their portion sizes of cheese and meat. Finally, improvements in all psychological outcomes, apart from mental HRQoL, were sustained from 12 months to 3.5 years.
Again, as with weight, the long-term secondary outcome trajectories of the FFIT-FU-C group reflect both the initial impact of the FFIT programme and the subsequent maintenance, or lack of maintenance, of the changes made. This group showed significant improvements from 12 months to 3.5 years in BMI but not in waist circumference or BP (the lack of improvement in BP may reflect our protocol at the RCT baseline measures, whereby any man with an elevated BP recording was advised to consult his GP). Men in this group also demonstrated increases in self-reported total and moderate PA, and in walking. Dietary improvements between 12 months and 3.5 years included reductions in the consumption of fatty and sugary foods (but no significant changes in fruit and vegetables intake), in portion sizes of all key foods and in alcohol intake. Finally, men also showed improvements in self-esteem, positive affect and mental HRQoL between 12 months and 3.5 years.
Significant between-group differences in the trajectories of most of these secondary outcomes reflect the fact that many FFIT-FU-C men took part in the FFIT programme immediately after the 12-month measures (i.e. in autumn 2012).
Objective 3: predictors of long-term weight loss
Our third objective was to investigate the baseline predictors of successful long-term weight loss in the two groups and how these differed as well as how mediator variables predicted long-term weight loss after controlling for the baseline predictors. These were investigated in both groups, and how these differed between the groups was also assessed.
Baseline predictors of successful long-term weight loss
None of the prespecified baseline predictors showed a significant relationship with weight loss at 3.5 years in either group.
Mediators of successful long-term weight loss
Physical activity and diet
Increased self-reported PA (walking at 3.5 years, and total and vigorous PA at 12 months and 3.5 years) and reduced sitting time (at 3.5 years) were associated with better long-term weight outcomes (i.e. lower weight at 3.5 years). In relation to diet, reduced consumption of fatty and sugary foods, smaller portions of cheese, meat, pasta and chips, and increased consumption of fruit and vegetables at 3.5 years were also associated with improved long-term weight outcomes.
Psychological status
Improvements in positive affect and physical HRQoL at 12 months and 3.5 years, and higher self-esteem at 3.5 years were associated with improved long-term weight outcomes.
Theoretical constructs
Autonomous regulation of PA and diet, an internal locus of control, perceived competence for PA and dietary behaviours, and relatedness to other men from the FFIT programme and family members were all associated with lower weight at 3.5 years. Amotivation in relation to PA and healthy eating was associated with poorer long-term weight outcomes. These findings are congruent with self-determination theory20,21 in that satisfaction of innate psychological needs is associated with more positive outcomes. Current satisfaction with the perceived results of being physically active and eating a healthier diet was also associated with improved long-term weight outcomes.
Behaviour change techniques, routinisation of physical activity and healthy eating, contact with Football Fans in Training participants and major life events
In relation to BCTs, only self-monitoring of weight was associated with better long-term weight outcomes. However, continued attention to food labels, regular PA (walking, gym attendance and other exercise), ongoing dietary restriction, regular mealtimes, and ongoing contact with other men and coaches from the FFIT programme were also associated with lower weight at 3.5 years. No associations were found between life events and long-term weight outcomes, in contrast to previous findings. 23
Prior weight change
Weight loss at the end of the FFIT programme was associated with lower weight at 3.5 years. Weight gain prior to taking part in the FFIT programme was associated with higher weight at 3.5 years.
Injury and joint pain
Self-reported injuries and joint pain at 3.5 years were both associated with poorer long-term weight outcomes, in particular injuries that limited walking and climbing stairs, and joint pain that limited activities. Injuries that limited walking at 12 months were also associated with increased weight at 3.5 years.
Chapter 4 Men’s experiences of trying to sustain weight loss long term
Introduction
The FFIT follow-up study provided an opportunity to investigate men’s experiences of attempts to sustain weight loss over several years after taking part in the FFIT programme (i.e. over 3.5 years in the FFIT-FU-I group and over 2.5 years in the FFIT-FU-C group). Specifically, we aimed to describe men’s experiences (including their motivations, emotions and relations with others) of attempting to control their weight over the long term, their reasons for achieving or failing to achieve long-term weight loss, and the strategies they continued to use or stopped using.
Methods
Sampling
Men who took part in the 3.5-year follow-up measurements were asked if they would be interested in being interviewed by telephone about their experiences of trying to manage their weight and lead a healthier lifestyle since taking part in the FFIT programme. As Table 19 shows, 88% (430/488) of men who took part in the measurements agreed to be interviewed. Seventy of these 430 men were purposively selected for interview, using a prespecified sampling strategy designed to capture a wide range of experiences of long-term weight loss, as follows.
-
Group 1 ‘long-term maintainers’: FFIT-FU-I men who achieved ≥ 5% weight loss at 12 months and at 3.5 years. This group were of interest because they succeeded in maintaining a clinically significant level of weight loss over the long term. In our original protocol we aimed to interview 15 men in this group.
-
Group 2 ‘long-term regainers’: FFIT-FU-I men who achieved ≥ 5% weight loss at 12 months but did not maintain this to 3.5 years. This group were of interest because, despite initial success, they had not maintained their weight loss over the long term. We aimed to interview 15 men in this group.
-
Group 3 ‘delayed responders’: FFIT-FU-I men who had not achieved ≥ 5% weight loss at 12 months but had achieved ≥ 5% weight loss at 3.5 years. This group were of interest because they were successful in achieving clinically significant weight loss at 3.5 years, even though they had not achieved it at 12 months. As we did not expect there to be many men in this group, we aimed to interview all of them.
-
Group 4 ‘long-term achievers’: FFIT-FU-C men who achieved ≥ 5% weight loss at 3.5 years. This group were of interest because they provided experience of succeeding in controlling their weight for 2.5 years after the FFIT study (and it included FFIT-FU-C men who had achieved ≥ 5% weight loss at 12 months and had maintained this to 3.5 years). We aimed to interview 15 men in this group.
-
Group 5 ‘pre-FFIT achievers’: FFIT-FU-C men who achieved ≥ 5% weight loss before taking part in the FFIT programme but did not maintain this to 3.5 years. This group were of interest because they had succeeded in losing weight using their own strategies before taking part in the FFIT programme, and we were interested to see how these experiences had had an impact on their attempts to sustain weight over the long term. It included one man who never took part in the FFIT programme. We aimed to interview 10 men in this group.
| Group | Total number of men in group | Agreed to interview, n (%) | Interviewed, n |
|---|---|---|---|
| Group 1: long-term maintainers | 53 | 46 (86.8) | 15 |
| Group 2: long-term regainers | 39 | 33 (84.6) | 15 |
| Group 3: delayed responders | 22 | 18 (81.8) | 15 |
| Group 4: long-term achievers | 81 | 73 (90.1) | 15 |
| Group 5: pre-FFIT achievers | 14 | 13 (92.9) | 10 |
| Men who did not fit the membership criteria of any group | |||
| FFIT-FU-I group | 119 | 106 (89.1) | n/a |
| FFIT-FU-C group | 160 | 141 (88.1) | n/a |
| Total | 488 | 430 (88.1) | 70 |
Attempts were also made to ensure that we sampled men from all football clubs, and included both men who had attended stadia measurements at 3.5 years and those who had received a home visit. As there were more men in the delayed responders group than we had expected (n = 22, of whom 18 agreed to be interviewed), we sampled 15 men to match the numbers interviewed in groups 1, 2 and 4.
Data collection
Six experienced qualitative researchers (Christopher Bunn, Craig Donnachie, Cindy M Gray, Graham Brennan, Kate Hunt and Lindsay Dalgarno), all but one of whom (Graham Brennan) had been involved in collection or analysis of earlier qualitative data on men’s experiences of the FFIT programme, conducted the telephone interviews between 30 June and 4 December 2015. We used a semistructured topic guide (see Report Supplementary Material 10) to address three subobjectives.
-
To identify the practices and techniques (e.g. incorporation of new PA and dietary behaviours into daily routine, self-monitoring and structured mealtimes) that men may have continued to use in attempting to control their weight over the long term, and those they adopted but had since stopped using.
-
To explore men’s experiences of motivation (including the extent to which regulation of PA and dietary behaviours are internalised or are part of a transformed everyday life), the role of emotions in changes made or not made, the extent to which men’s relations with others have supported their changed behaviours and/or the extent to which men’s relations with others have changed as a result of their new behaviours or practices.
-
To explore the extent to which (if at all) men viewed their identities differently in relation to performances of masculinity and health-related practices around diet, PA and other behaviours (e.g. health-care utilisation or sleep) following participation in the FFIT programme.
The interviews were audio-recorded, with participant consent, and transcribed verbatim. All were quality assured and anonymised before analysis. Men were given a £20 high street store voucher (in addition to the voucher they received for the 3.5-year measurements) to thank them for their participation in the interview.
Data analysis
We used a structured, thematic approach to analyse the anonymised interview transcripts. 72,73 A coding frame was developed by six members of the research team (Christopher Bunn, Craig Donnachie, Cindy M Gray, Graham Brennan, Kate Hunt and Sally Wyke) who each read a sample of transcripts from across the five interview groups to identify themes emerging from the data. The coding group then met face to face to agree seven ‘broad themes’: doing the FFIT programme; after the FFIT programme; weight; self-reflections; PA; diet; and social contexts and environments. The content of each broad theme is described in Report Supplementary Material 11. Subsequently, four researchers (Alice McLean, Christopher Bunn, Craig Donnachie and Graham Brennan) applied these themes to the data, reading and rereading the transcripts to ensure that all accounts and experiences (including deviant cases) were represented. NVivo 10 software (QSR International, Warrington, UK) was used to assist in the coding and organisation of data.
The broad themes were then read by six researchers (Alice McLean, Christopher Bunn, Craig Donnachie, Cindy M Gray, Graham Brennan and Sally Wyke), who noted all subthemes present, including expected subthemes that related to the main objectives (as described in Report Supplementary Material 11) and any unexpected topics. 74 Finally, the broad themes were used to develop matrices, in which each row represented a participant and each column represented a subtheme, to allow comparison of accounts across the different participant groups. A descriptive summary of each broad theme, highlighting similarities and differences between the groups, was then written and discussed by members of the qualitative subteam before being used to write this report.
In conducting our analysis, we remained mindful of theoretical accounts of behaviour change maintenance, including self-determination theory,20,21 and evidence from reviews suggesting that ongoing dietary changes, PA, self-monitoring (e.g. of weight16,17), and supportive social and physical environments are important in sustaining weight loss over the long term. 23 Results presents summary analyses. Each extract used is labelled to indicate the participant’s identifier (ID) number, the football club where he attended the FFIT programme and his interview group membership.
Results
Practices and techniques
As described in Chapter 1, Football Fans in Training: weight loss, physical activity and healthy eating for men, the FFIT programme is designed to provide men with a toolbox of strategies and techniques to support them in managing their weight, being physically active and eating healthily and to take this forward in their daily lives. 46 The 3.5-year interview data suggest that many men continued to use this toolbox to select strategies to help them sustain the changes they had made post programme.
Self-monitoring, goal-setting and review
Many men spoke about continuing to monitor their weight. For some, this involved weighing themselves on a regular (often weekly), or semiregular, basis. There were some differences between the groups. Although few pre-FFIT achievers specifically described weighing themselves, a number of long-term maintainers said that self-weighing was a satisfying or enjoyable experience:
I love it. I absolutely love it. I still weigh myself every Thursday morning.
Interview 50, football club 02, long-term maintainer
A number of men, across all groups apart from the pre-FFIT achievers, spoke about monitoring their weight in relation to weight targets, although sometimes these targets were rather loosely defined:
I don’t really make goals noo [now]. One o’ [of] my aims is tae [to] lose the stone again, an’ [and] that’ll happen.
Interview 21, football club 12, long-term achiever
Other men were more specific, both in relation to weight loss targets and in relation to an upper threshold of weight that they did not want to breach:
If I got to the 15-stone marker, I’ll quite likely start to panic. But it’s actually, it’s getting a . . . It’s getting a sorta [sort of] target, a maximum target and saying ‘No further’. But quite honestly, I would like to do it in terms o’ getting down to about 13 and a half stone.
Interview 7, football club 05, delayed responder
By contrast, a few men (long-term regainers and delayed responders) viewed weighing themselves as a negative experience. Some long-term regainers said that it could trigger low moods, with one describing how the thought of finding out his weight filled him with trepidation:
An’ I don’t weigh myself or anything like that because I’m very – I feel bad that, you know, that, you know, I’ve not kept it up an’, you know, I’ve not got down tae my ideal weight an’ all the rest of it. [. . .] What I am now, I haven’t got the faintest idea ‘cause, as I say, I’m actually frightened tae weigh myself. I think it would throw me intae [into] a spiral o’ depression, you know, if I actually knew what my weight was, you know?
Interview 55, football club 02, long-term regainer
Men also described more indirect methods of monitoring their weight, in particular in relation to how their clothes fitted and changes in the sizes of clothing they had to buy. Some men used both weighing themselves and the fit of their clothes to monitor their weight, but others had come to rely solely on how their clothes felt:
Do you monitor your weight?
No’ [Not] as such but, you know, it’s just how I feel, you know in my clothes and stuff like that.
Right, yeah. So whether or not you still fit those jeans and things like that? Yep.
Yep, definitely.
OK. Which rung of the belt you’re . . .?
Exactly. Stuff like that. Whatever the weight checking something like that, that’s mair o’ [more of] a sensible way o’ judging it for me.
Interview 22, football club 07, long-term achiever
Many men also continued to monitor their PA, some by tracking their step counts, others by being aware of distances walked or time spent walking. A couple of men described monitoring the intensity of their PA through time taken to cover specific distances. Although many men had embraced using the pedometer during and immediately after the FFIT programme, only a few had continued to use it long term. Some had stopped using their pedometer when they lost it or when the battery ran out, and others described how they simply found that they no longer needed it:
For the first . . . I can’t remember exact, say the first 18 months or so, we were given a pedometer and I wore that faithfully, all the time and I did keep a close track on my steps. As time went on I realised I didn’t need the pedometer, I knew by the amount of, length of time I was spending walking how many steps I was taking and, and what sort of exercise it was. So I know that if I walk for a hundred minutes, I do 10,000 steps, you know that kinda [kind of] thing? Just at the pace that I go and what it does.
Interview 12, football club 05, long-term maintainer
Some of the men who spoke about monitoring their PA set themselves daily or weekly targets to aim for in relation to steps or distance. However, the review of these targets was sometimes rather infrequent:
I just do keep a record of . . . well, it’s on the phone actually, but I keep a record on this app [application] that I’ve got on the phone and just look at it every 2 or 3 weeks, see how many steps I’ve done, see if I’ve reached my goals an’ all that sort o’ stuff.
Interview 34, football club 12, long-term regainer
It was notable that only one man in the Pre-FFIT achievers group mentioned continuing to monitor his PA, and then only on occasion:
Well, I’ve got an app on my phone, so I can occasionally just kinda jump onto that and hae [have] a look and see how far I’ve walked in a day, or how many steps I’ve done, or whatever.
Sure. And is that something you use quite often? Or not so often?
Och [Oh], just now and again.
Interview 23, football club 13, pre-FFIT achiever
Men in all groups mentioned self-monitoring less frequently in relation to diet than to PA, perhaps reflecting the lack of emphasis on self-monitoring of food intake in the FFIT programme (men were asked to keep a food diary only at the start and the end of the programme). Nevertheless, some men did describe continuing to keep food diaries, monitoring their calorie intake or weighing their food:
I used to get up in the morning and just pour a bowl of cereal and, you know, it would just be a case of – it would be overflowing, if you like. But the information that we were given about the portion size, it’s usually on the product, that really helped me in the fact that I then got a set of scales and I would say a full one portion equals 30 grams, and I would get a, you know, I’d put my plate on the scales and then pour the 30 grams in there, and that would do me, you know?
[. . .]
Do you still go to that extent or . . .?
I do, yes. Yes.
Interview 14, football club 06, long-term maintainer
Some men (especially delayed responders) had adopted new technologies to monitor their weight, PA and/or diet. In addition to pedometer apps, technologies mentioned included the Fitbit (Fitbit, San Francisco, CA, USA), Apple Watch (Apple Inc., Cupertino, CA, USA) and Garmin watches (Garmin Ltd, Olathe, KS, USA), and fitness and running/cycling apps, such as MyFitnessPal [MyFitnessPal, Inc., San Francisco, CA, USA]. Often technologies were used to try to achieve targets in relation to steps, diet or weight:
I use that MyFitnessPal . . . to record what calories I’m taking on a day-to-day basis. However, I give myself a 1400 calorie target Monday through Thursday. Weekends, I’m a bit more relaxed aboot [about] that, but if I sustain the 1400 Monday through Thursday, in conjunction with the kind o’ exercise programme, that seems tae work for me an’ I can still lose a pound or a couple o’ pounds, you know, every couple o’ weeks.
Interview 41, football club 04, delayed responder
One long-term achiever described how he and his wife used monitoring technologies to support each other in achieving their step targets:
I bought one [a Fitbit] and my wife bought one, so, we have healthy competition there. And it makes it fun with the app on the phone and whatnot, you know? And we can get a bit of fun banter going on there, you know? But, yeah, I’m not consciously checking the step count really, you know? When it buzzes on my wrist I know that that’s 10,000 steps for the day kind of thing, you know? And then, yeah [yes], I kind of – you know, at the end of the week we, kind of, compare our results and look at the trend in them and whatnot, you know?
Interview 9, football club 13, long-term achiever
Strategies used to remain physically active
The majority of men reported continuing to walk to stay more active after the FFIT programme, often describing how they were still managing to incorporate walking into their daily routine. Some talked about walking for leisure, including when listening to music or football commentaries on the radio, or with their wife, or simply because it gave them something to do:
I do much, much more walking, without a doubt. And think about exercise. I’m not saying I do exercise – I still do, evidently – but, yeah, there’s more thought in ‘Right, what am I doing? I’m sitting here doing nothing. Och, let’s go for a walk’.
Interview 31, football club 11, long-term achiever
Nevertheless, many spoke about purposeful walking, including walking to get the paper, walking instead of taking the car and walking their dogs (a couple had even bought dogs to encourage them to walk more):
. . . certainly, the exercise side o’ things, I mean, because I have a dog now, you know . . . well, I bought a dog when I retired because I always wanted a dog but we were working full-time it wasnae [wasn’t] very practical. But getting a dog was also part o’ the reason tae give me a good reason for going out an’ getting out every day.
Interview 64, football club 11, long-term achiever
Fewer pre-FFIT achievers mentioned walking compared with men in other groups, and although long-term regainers did report walking, their descriptions of what they did when walking often seemed vaguer and appeared to demonstrate less commitment to walking regularly than the groups that had been successful in losing weight long term (long-term maintainers, long-term achievers and delayed responders):
I try to go out walking every day with the dog for a good . . . for a decent hike, although I’ve been a bit slack recently.
Interview 65, football club 10, long-term regainer
Many men talked about how they had taken up other activities after the FFIT programme, sometimes instead of, but often in addition to, walking. Popular choices included cycling, running and going to the gym:
I never cycled during the programme really. I toyed with the idea of cycling tae work an’ got as far as applying for cycle tae work scheme but that was about the time o’ my redundancy, so that fell through. But the one treat I bought myself wi’ [with] my redundancy cheque was a cycle, you know? An’ I’ve actually got two now, so . . . an’ that’s my tool if I overeat now, I go out on the bike for half an hour tae get my calorie count back down.
Sure. And is that something you do quite regularly now then?
Yeah. Minimum of twice a week I’m out on the bike. Yeah.
Interview 4, football club 02, delayed responder
One long-term maintainer, a taxi driver, described how, for him, running was a more convenient way than walking of fitting PA around his work commitments:
So, whereas before you were going out walking, let’s be honest, walking’s quite time-consuming, and to do it . . . You know, for me anyway, because I wasn’t doing much in the taxi, it would maybe take an hour and a half each night to get up to that sort of level of exercise in terms of walking and that’s –
OK, because you’d be getting all your steps going in one go?
Exactly. Exactly. So now I’m maybe going running three times a week. You’ll still go for a walk now and again, but it’s not that sort of specific measuring it, whereas I go for a run three times a week.
Interview 13, football club 07, long-term maintainer
Another long-term maintainer described how, after a period of inactivity caused by stress at work, taking up one form of PA (going to the gym) had acted as a catalyst for him to do other things as well:
So, basically I’ve been stuck in the office, it was all, kind of, it was quite stressful working so long, and after the year, you know, you kind of get stuck in a sort of rut, if you like. And I just suddenly realised that the weight was slowly going back on, but not in a great extent, you know? I just thought ‘Right, I’m gonna [going to] have to do something else.’ And, so, we, kind of, joined a different gym and, yeah, that’s the way it’s, kind of, coming off again.
So, again you’re, sort of, going to the gym rather than walking. Is that . . .?
I am walking as well now.
Interview 14, football club 06, long-term maintainer
Some men also spoke about doing exercise programmes at home to help them maintain their PA levels. One long-term maintainer described how he turned to the exercises that he had been shown during the FFIT programme when the weather prevented him from going outside:
I think they used sort of bean bags, you know for stretching exercises that you can do like in the living room. You know in your own house. So sometimes, yeah, sometimes I do that. Like say if it’s inclement weather and I think, ‘Oh God, I’m no’ going out in that’. You know, so just throw a cushion on the floor and do some exercises inside.
Interview 29, football club 09, long-term maintainer
Another man (a delayed responder), who had struggled with his weight after the FFIT programme before joining a slimming club to help him overcome his addiction to food, explained in the interview how he had bought some gym equipment for home so he could exercise without feeling embarrassed in front of other people:
I also in my spare room got some . . . got an exercise . . . piece of exercise equipment. That meant I didn’t have the self-consciousness of going to the gym. I could exercise in private so to speak.
Interview 5, football club 01, delayed responder
Men from all groups described playing football. Sometimes (but not always) this was with other men who had taken part in the FFIT programme (in some football clubs ‘follow-on’ groups were formed at the end of the intervention so men could meet up to play football together). Nevertheless, some men were reluctant to play football, especially when it became too competitive. One long-term regainer described how, when play had become too serious, he had stopped attending the ‘follow-on’ group at his football club:
There were people coming intae [into] the group that didnae [didn’t] know what we were all about. They were just coming for the football. And that, we came off the cinder pitch which I loved, you know, and I couldnae [couldn’t] care if it was snowing, it didnae make any difference. If you’re playing on a cinder pitch nobody’s gonnae [going to] be throwing themselves intae challenges. [. . .] But when we got to the [professional sports facilities], the people were coming in, were coming flying off their feet and there was a lotta [lot of] people, there’s a lotta people admitted, if they sat down and admitted it, they got injuries. Injuries that they shouldn’t have had, and it wasnae caused by them, it was caused by others, you know? No, that’s a thing and then they started playing against other FFIT clubs, it got a bit too serious as well. But I suppose maybe I was looking for an excuse to end it, and I didnae get a choice [laughs], you know.
Interview 58, football club 13, long-term regainer
There was some evidence that long-term regainers may have been doing fewer forms of PA (instead of, or in addition to, walking) than men in the groups that had been more successful at achieving long-term weight loss (long-term maintainers, long-term achievers and delayed responders).
Finally, a number of men described taking part in events or challenges to give themselves a goal to train for. These included 5-km and 10-km runs (e.g. a weekly Park Run), cycling races, triathlons, charity walks and climbing mountains. In general, long-term maintainers appeared to push themselves further in the types of challenges they undertook than men in the other groups:
I like to have one event or two events on the calendar that I know I’ve got to step up to or I’ll fail it, and I don’t want to fail it, so . . .
So having targets helps. So having events and then I suppose working out the . . . I was going to say the steps, but yeah, the steps it takes to kind of be prepared for that?
Yeah, exactly, if you’ve got a 10-km run to do you’re gonnae make sure that you can do 10 km, do you know what I mean? You’re not gonnae turn up only being able to do 5 km, you’re gonnae do it all sort of thing.
Interview 13, football club 07, long-term maintainer
Strategies used for healthy eating and drinking
Almost all men across all groups described how they were continuing to make healthier choices in relation to food and drink. Strategies used included eating more fruit and vegetables; eating fewer processed foods and takeaways; reducing fat, sugar and salt consumption; switching from sugary, fizzy drinks to diet drinks, diluting juice or water; and taking food to work instead of buying from a canteen, vending machine or fast food outlet:
I have considerably cut down on a lot of things I shouldn’t have been eating and have been eating since I was a boy, notably fry-ups, very much reduced that at home. Significantly reduced takeaways. And eating a bit more fruit and veg [vegetables]. Maybe not quite as good at that over the last little period but yes, I have taken, I take, like, grapes and apples and oranges now that I just never bothered with before.
Interview 47, football club 08, long-term achiever
The changes I kinda [kind of] maist [most] stopped doing was I basically stopped eating . . . at my work, with . . . were using the facilities on site, basically because it was . . . You know, you’re having the roll and sausage for your breakfast, and having the macaroni cheese and chips at lunchtime and stuff. And I stopped that and . . . To this day I still go in there and have, you know, I have, like, fruit for breakfast and take in a . . . a salad that I make up for my lunch. So I’ve kind of got into that habit. So I’ve changed, definitely changed the, you know, at least a couple of meals when I’m in a working routine [. . .] And I kinda kept going wi’ that from . . . from day one of the course to still doing it now, you know, almost 4 years later.
Interview 54, football club 11, long-term regainer
The majority of the men (including most of the pre-FFIT achievers) still spoke about the importance of being aware of portion sizes. This mirrors the impact that the discussion of the Eatwell Plate and portion sizes in week 2 of the FFIT programme had on many men in the RCT 12-week and 12-month focus groups, who described it as powerful. 44 In the 3.5-year interviews, some men described strategies that they still used, such as weighing scales or smaller plates, to help them control portion size:
One of the changes, believe it or not, that I made was, I had bought these plates from Ikea, and they were actually huge plates, to the extent I had to actually cut a little bit out of the back of my cupboards so they’d fit in. When I met my partner, we had both talked about the portion size thing, and she’s obviously quite conscious of her weight and stuff, and we ended up giving the plates away, going to Ikea, and buying a smaller set [. . .] that was a big change, you know, it definitely made a difference because you have a smaller plate, you don’t put as much food on it, you know?
Interview 2, football club 12, pre-FFIT achiever
Across all groups, men talked about changing their pattern or intake of (unhealthy) snacks, reflecting another key message from the Eatwell Plate discussion in week 2 of the FFIT programme. Strategies used included eating fewer, or healthier, snacks between meals, and eating unhealthy snacks only from time to time and in smaller quantities. Some of the men, including long-term regainers and pre-FFIT achievers, described not allowing themselves to eat snacks after a certain time in the evening:
I don’t stop myself from eating what I want to eat but I do it more in a regulated way. If I was sitting and I say, ‘Right, I’m gonna have a packet o’ crisps,’ I have a packet of crisps, where instead of opening four packets an’ putting them in a bowl (because it doesn’t look like four packets when it’s in a bowl, it’s easier tae eat then) and then eat that whole bowl, I’ll get one packet o’ crisps and have that packet o’ crisps.
Interview 46, football club 12, delayed responder
There’s very few times now that my wife and I will eat after 9 o’clock tae half past 9 p.m. So, you know, I found that out in the programme and that’s been pretty easy, to be honest, because the odd time we would’ve been eating later, but not now. An’ even when we go away on holiday it’s sort of unwritten law that we just don’t eat or drink anything after half past nine.
Interview 34, football club 12, long-term regainer
Reflecting the food labelling activity in week 8 of the FFIT programme, a few men in each group spoke about continuing to read food labels, especially to monitor fat and sugar content:
I think obviously the eating part of it made us more aware of the fat content in food and stuff, so obviously that’s always a thing when I’m buying stuff now. You tend to look twice, whereas before you’d just be ‘Och!’, pick up any old sandwich and things, and maybe get a wee [little] bit too obsessive about it at times. But you’re obviously looking at the labels now and trying maybe get one that’s a wee bit less fattening, or [less] saturated fat or whatever.
Interview 53, football club 13, long-term regainer
Men also spoke about continuing to limit their alcohol intake, reflecting what they had discussed in week 5 of the FFIT programme. Some simply described cutting back on the amount of alcohol they consumed; others said that they had virtually eliminated alcoholic drinks. A couple of long-term achievers said that they had reduced the amount of alcohol they drank by going out less. However, the most commonly reported strategy was to limit drinking alcohol at home:
I used to have a drink every Saturday in life whether I was in the house or whether I was out. Now I don’t even drink in the house at all now so . . . I have to be going out now if I’m having a drink, but, as I say, I just don’t, I don’t touch alcohol at all now really . . . or I very seldom do. So that’s been a change for the good as well.
Interview 54, football club 11, long-term regainer
The 12-month focus groups had suggested that a flexible approach to healthy eating (e.g. men allowing themselves to eat unhealthy foods on occasion) was associated with successful weight loss. 44 In the 3.5-year interviews, men in all groups described continuing to adopt a flexible approach, in particular having a more relaxed approach to healthy eating at the weekend or when on holiday. Nevertheless, there was a notable difference in the way that this flexible approach was described between men who were successful in maintaining their weight loss (long-term maintainers, long-term achievers and some delayed responders) and those who were not (e.g. the long-term regainers). Those who were successful tended to use positive terminology (e.g. enjoying themselves while on holidays and at weekends) and often appeared to have clear strategies for ‘damage limitation’, as illustrated by this man’s account of his approach to holidays:
. . . even when I’m on holiday, I’m watching what I’m eating, you know? We went on our first ever all-inclusive holiday back in March, just for 7 days (and the things that are there to tempt you, you know, like the food and the alcohol and stuff like that). But we, we went walking every day and [. . .] went to the gym a couple of times when I was there. Yeah, and I did have a couple o’ things, you know, that maybe I shouldnae [shouldn’t] have eaten, but when I came back and went on the scales, I’d only put on a pound, so you know, whereas if I hadn’t, you know, been aware that I need to go walking, I need to go to the gym, I need to get a bit of exercise in here, and for lunch, let’s just have a little bit of seafood and salad and some fruit, you know? So . . . and that’s the way you think, you know?
Interview 49, football club 10, long-term achiever
By contrast, men who were less successful in maintaining weight loss spoke more negatively and often demonstrated a less tempered attitude to maintaining a healthy lifestyle while on holiday:
I’m just back from a sort of city break abroad an’, well, most o’ the diet went out the window then . . .
Interview 34, football club 12, long-term regainer
Barriers to and facilitators of sustained weight loss, physical activity and healthy eating
In the 12-month focus groups, common barriers to maintaining weight loss included injury and health problems, changes at work, holidays, festivities, poor weather, life events, and the costs associated with leading a healthy lifestyle. 44 The 3.5-year interviews revealed that many of these barriers continued to be issues over the longer term.
Injury and health problems
Men across all groups spoke about how injuries and ill health had been barriers to sustaining weight loss and being physically active. Problems described included long-standing health conditions, such as arthritis, depression and painful joints, as well as injuries of more limited duration, such as pulled muscles or broken bones:
I went back tae the gym and up until recently I was using that on a regular basis, an’ when I say recently I mean roughly up until last February, I would say, February this year. An’ the reason I stopped going is because I injured my shoulder, an’ I’m still waiting to get that fixed, if you like. I’ve been for X amount of analysis on it and . . . but still no further forward, an’ I go to the hospital I think next January to get . . . to see a specialist about it.
OK. So you’ve been injured and it has affected your physical activity quite significantly?
Absolutely. And not just the gym, many other things as well, gardening an’, well, social act-, my golf an’ stuff like that. All of that has been on hold since the injury.
Interview 34, football club 12, long-term regainer
I know a couple of them [FFIT participants] are still playing badminton together, and others are going to a sports club in town here for squash and things but as I say, my age and my tendonitis and things, my arthritis prevents me participating in that.
Interview 47, football club 08, long-term achiever
This man’s sentiments about age being a barrier to PA were echoed by some others in relation to sustaining weight loss, as illustrated by one delayed responder who complained that he was finding it harder to control his weight as he was getting older:
I am conscious I’m overweight and not as fit as I should be, so it’s just a gradual process of trying to get my weight down and get fitter than I was really. I know that at my age it’s not gonna happen overnight. It’s just a gradual process. At least if you don’t improve too much you won’t get worse.
Interview 44, football club 06, delayed responder
Physical activity and weight loss
A number of men in all groups described what they perceived to be a close relationship between PA and weight management. This was articulated in a number of ways. For example, some men spoke about how doing PA had contributed to their weight loss:
I’ve lost a significant amount of weight and I’m still losing weight because I’ve been more active. [. . .] The exercise (I knew that, as part of the course, itself, you know, burning the calories and watching what you’re eating, kind of thing, as well) was a common-sense type of part of the course, and stuff that I already knew, but you know, it puts it into effect when you’re actually, you know, you feel yourself losing weight and it’s because you’re exercising more.
Interview 6, football club 04, delayed responder
Men also spoke about the reverse situation, highlighting that not being physically active can lead to weight gain:
I did put weight back on again obviously [when he was depressed] because I wasn’t going to the gym and, if I was going to the gym, what I was eating – like I say, I’m not really too bad an eater, I don’t think now. If I was going to the gym I probably wouldn’t have put the weight on, you know, but, you know, keeping my appetite or, kind of, bad habit things where they were, and not going to the gym, you know, I wasn’t burning off that additional calories, so, you know, I did put a wee bit of weight on.
Interview 9, football club 13, long-term achiever
Some men suggested ways in which weight loss can lead to increased PA:
. . . certainly I was encouraged by that, you know, that as you lose – it’s amazing when you lose even a few pounds, you feel different when you’re walking, you know?
Interview 48, football club 01, long-term achiever
Finally, some men described how being more physically active had motivated them to eat more healthily:
I know for some people it’s losing the weight, giving them encouragement to really work hard in the gym. For me it was the opposite. I know I work hard in the gym and that encourages me to eat more sensibly and no’ undo the work that I was doing in the gym.
Interview 22, football club 07, long-term achiever
Men spoke about work as both a barrier to and a facilitator of being more physically active and eating a healthy diet. Some described how long, unsocial, unpredictable hours and shift patterns made it difficult to find time to do PA and to eat regular, well-balanced meals:
For me personally I’m a taxi driver, as I say, so in terms of my eating, my main challenge is, I’m up at 4 o’clock in the morning, about 4 o’clock in the morning, so my main challenge is watch what I’m eating and don’t take the easy option of the high-energy drinks and the chocolate bar to wake you up in the morning, you know, to get you that sort of energy boost. That’s my, now I’m good at that, and I’m strict at that. [. . .] And it’s difficult sometimes, like when you’ve got a job to the airport and it’s 4 o’clock in the morning. You’re yawning and you’re thinking, and I don’t drink coffee, so you know what I mean? I need a pick . . . And I’m going, ‘I’ve got to take this guy to the airport and I’m shattered. Oh, I’ll get a can of Red Bull’ you know what I mean? That’ll sort me oot [out]. And obviously you know that’s very bad, but it still happens.
Interview 13, football club 07, long-term maintainer
Some men also described their work as having a detrimental impact on their attempts to eat healthily. For example, a couple of long-term maintainers complained about the limited availability of healthy food options in the canteen, and the ‘cake culture’ in the office:
. . . the amount of cakes and biscuits in our office at the moment is just absolutely ridiculous. And I’m as bad as anybody else for buying them and eating them. So in some ways it’s better, but it’s . . . I’ve still got far too sweet a tooth, I’m afraid.
Interview 24, football club 08, long-term maintainer
The types of jobs men did and their workplace environment influenced their ability to fit PA into their daily routine. Some men reported having sedentary office jobs, whereas others had more active jobs or were able to walk to work and to find other ways of being physically active during their normal working day:
[W]ithin my work, you know, we’ve got a, I’ve got a building that’s got four floors, so what I do try and do is, I try and, you know, like use the actual stairs rather than the lift. You know, like wee things like, you know, at the car park, I’m in the car park, I try and not park that close and just to try and w–, you know, walk a little bit more.
Interview 19, football club 10, long-term regainer
Other men described how they were able to use flexitime to ensure that they could do the forms of PA that they enjoyed outside work:
‘Cause you know if you say, ‘Right, well I’m going to play badminton on a Tuesday and a Thursday, I’ll go to work early so that, you know, I’ll make up that hour’, and sort o’ stuff like that, so that helps. So I’m kind o’ fortunate that we’re on flexitime so . . . I would never have done that before, you know? I would just normally just go to work at the normal time, finish at the normal time but, you know, you’re making an effort to get up earlier so you’re into work earlier so you can have, like, an hour and a half for your lunch instead of 45 minutes. So you plan your, the whole thing makes you change so many different things about your life, you know, because you have to change to make things happen. So it’s all become part of a process that I’m now used to, I’m comfortable with it and, to be honest, I enjoy it, so it’s good.
Interview 49, football club 10, long-term achiever
Some men also described how changes in their employment had had an impact (either negatively or positively) on the amount of PA they were able to do. A number of men (disproportionately in the long-term regainers group) described how changing to more sedentary jobs, or jobs that required them to work longer hours, had reduced their PA. One long-term regainer said that uncertainty in his working life and a new role as a mortgage advisor had constrained the PA he was able to do:
I’ve just got this job recently, it’s a sort o’ self-employed job an’, you know, for instance, seeing people in the evenings an’ maybe getting home late at night. [. . .] So, you know, like, probably at the moment probably averaging about, you know, a couple o’ nights a week where I’m out, you know, seeing people, an’ I might not get back ‘til quite late, maybe 8, 9, 10 o’clock at night, that kind o’ thing. Again, so that kind o’ throws things out, you know? But I’m conscious that I do try tae go tae the gym quite regularly, an’ I’ve always tried to do that. But obviously those kind o’ demands, that’s kind o’ fallen by the wayside a bit.
Interview 55, football club 02, long-term regainer
In contrast, one delayed responder had made a conscious decision to seek a more active job after he was made redundant from his previous office job:
When I started the FFIT programme I was based in an office but I was . . . because it was [workplace name], they do redundancies every 2 years and they finally caught up wi’ me, but I made a conscious decision. [. . .] ‘Right, I’ve got redundancy, I’ll look for a more active job’, which I’ve got now. So that helps me keep the weight off as well. Yeah.
And in terms of your job now and in terms of I guess being more active, what kind of things does that involve, or how does it enable you to be more active?
Well, I’m a warehouse manager so I’m on my feet all the time now. I sit down for maybe half an hour at most in the day, and I work from 7 ‘til 5, whereas before I wasnae. An’ I’m on my feet for pretty much most o’ that, save for my lunch hour. Whereas before, 9 tae 5, I’d be sitting on my bum all day.
Interview 4, football club 02, delayed responder
A minority of men across all groups spoke about the negative impact that family commitments had on their ability to be physically active. Most of these men described how caring for their children reduced their time (and energy) to exercise:
As I say, I’m doing a compressed shift, so, I’m doing a 10-hour day. I’m in at 8 o’clock, and I’m home at, you know, half 6, so, by the time you get home and you’ve got the wee man [son] to deal with, get him his dinner, and get him to bed and stuff, it’s just a case of like ‘Oof, sit down,’ you know? Trying to motivate myself at that point to actually go out and do some more exercise is pretty difficult, to be honest.
Interview 2, football club 12, pre-FFIT achiever
Some of the men who had been successful in losing weight at 3.5 years described how they had overcome such challenges:
Some of the other guys meet up for a, kind of, a game of football thing, you know? But, yeah, again, the hectic lifestyle with the two children, you know, you’re either taking them on their football run yourself, or the dancing run, you know, so. But in between that I go to the gym, so, yeah.
Yeah. And is that in the evening that you carve out the time to do that, to go the gym? Or do you manage to fit that in during the day?
No. I do it on the way home from work. So I pass the door of the gym on the way home from work, so, no excuses, you know?
Interview 9, football club 13, long-term achiever
Very few men in the 3.5-year interviews reported major life events that had had an impact on their attempts to sustain weight loss long term.
Men in all groups described how the seasons had an impact on their PA and eating habits. Some cited winter conditions (e.g. darkness and slippery surfaces underfoot) and bad weather as making it more difficult, both physically and psychologically, for them to do PA:
I’m trying to lose a bit o’ weight again, so I joined the gym, as I said, just because it was a kinda easy thing to do and, och, during the winter, it’s kinda, during the winter it’s maybe harder to do ‘cause, you know, the temptation is to come in from work and it’s dark and, you know, you just settle down in front o’ the telly – so even going out to do anything involves a bit of an effort.
Interview 52, football club 04, long-term maintainer
In contrast, one long-term achiever said that he had found it easier to walk in winter, as there were too many other distractions in summer:
You know the funny thing about it is, the winter doesn’t bother me for going out walking. As long as it’s dry, that’s the main thing for me. If it’s cold, it doesnae [doesn’t] matter, you know? I’ll go out – I actually prefer walking in the dark rather than like summer nights. It sounds a bit strange, but you know, summer nights, you know, you’re more than likely maybe go and do some gardening, or something like that – go for a drive in the country, you know?
Interview 48, football club 01, long-term achiever
Festivities and celebrations were mentioned by a few men as being a challenge for healthy eating. Christmas, in particular, was associated with unhealthy foods. One pre-FFIT achiever described it as the time ‘when all the Celebrations [chocolates] boxes and [. . .] big tins of sweeties appear everywhere’ (interview 23, football club 13, pre-FFIT achiever). Times like these, when life deviates from the ‘normal routine’, were described as having the potential to lead to the re-emergence of unhealthy eating practices. One long-term maintainer felt that, although a bit of overindulgence on special occasions was inevitable, it was important have plans to get back on track as soon as possible:
There are times of the year, likes of Christmas, that you are gonnae come off the beaten track a wee bit and, kind of, overindulge, whether it be on chocolate or crisps or goodies, or people coming into your work at Christmas and baking cakes and leaving them out for the staff. That is going to happen for you, but as long as you can understand, ‘Yeah, I can do that now, but let’s get back to where I was before that!’ as well, and not just carry on the habit of having a packet of crisps at the same time every day and then two packets of crisps, and it builds up. Just knowing your limits and knowing that sometimes that, ‘Why should I need to starve myself? I’ve got a night out tonight, let’s go out and enjoy my night out’, you know what I mean? It’s not going to kill me in the overall grand scheme of things. Just don’t do it all the time.
Interview 68, football club 03, long-term maintainer
Finally, men described ways in which the outdoor physical environment could influence their PA. Most commonly, men from all groups, apart from the pre-FFIT achievers, spoke about ways in which the local physical environment facilitated PA. Some talked about how living near scenic areas encouraged them to be active:
I’ve never been into running, and what happened is on the walking part of it, I mean I live in a nice area, I’ve got miles and miles of woods at the back [of the house] now, I’m very fortunate. And I would walk and then I would walk a bit faster, and then I would do little bits where I’d do a little bit of trotting. And just led on from there and in the end I was sort of running, you know, 5, 6 miles every time, well every other night, something like that.
Interview 25, football club 13, long-term maintainer
However, a minority of men spoke about the outdoor physical environment as a barrier to PA. A couple (a delayed responder and a long-term regainer) said that they walked to work, but not back home, because the return journey was uphill. Another long-term regainer said that he had stopped cycling since having an accident and becoming more concerned about his safety:
I’ve stopped using that bike a wee bit, but that’s more through dangers on the roads than . . . end up killing myself than anything else.
Yeah. did you have a coupla [couple of] brushes with . . .?
Aye, there was a, one occasion wi’ a bus. [laughs] So . . .
Interview 16, football club 07, long-term regainer
Routinisation of physical activity and healthy eating
Throughout the FFIT programme, men were encouraged to make changes that suited their own eating and exercise preferences, and to try to incorporate them into everyday life. Men from all groups, apart from the pre-FFIT achievers, described how PA had become part of their daily routine. A number of long-term regainers, despite being less successful in long-term weight loss, still valued the fact that the FFIT programme had introduced regular PA into their lives:
So I’m sitting at a desk all day, you know? Eight, nine, ten hours. So that was the kind o’ scenario before I went on the course. After that, I’ve obviously I’m still doing the same kind o’ work, still working from home, but every lunch-time I now do a mile-round walk to go an’ buy my paper, as advertised on the television programme. Still doing that every day, primarily because it gets me out the house, get some fresh air, and forces me to take a longer break at lunch-time.
Interview 37, football club 02, long-term regainer
Men also spoke about how they continued to embrace the importance of eating regular meals (including breakfast), which was discussed in week 8 of the FFIT programme. Some described the importance of planning and organisation to help them achieve this:
It might sound a bit boring, but I now plan what I eat, what I will eat for the next day. You know, for example, I know exactly what I will eat tomorrow. I try and plan a menu. I find this helps me. I don’t know if it helps everybody, but I think it helps me. I try tae plan my eating for the week, so I’ll know what I’m gonna have Monday, Tuesday, Wednesday, Thursday, Friday. I have a bit of a blow out on a Saturday and a Sunday, but not to a great extent. But I do try tae, Monday tae Friday, I do try to plan what I’m gonna be eating, rather than just, you know, making decisions on the day. I feel for me personally it helps to plan the, to plan my programme for myself.
Interview 39, football club 13, delayed responder
Men across all groups spoke about the danger (and often the experience) of slipping back into old habits. Nevertheless, those who had been successful in long-term weight loss (long-term maintainers, long-term achievers and delayed responders) often appeared to recognise when old habits were re-emerging, and described the strategies they used to prevent these becoming established in their daily lives. For example, one long-term achiever described drawing on the knowledge and skills that he had learned during the FFIT programme to prevent him adopting old (unhealthy) eating habits when injury stopped him from following his normal PA routine:
I’ve slipped back into a couple of wee bad eating habits since I’ve been injured [. . .] but I know, I’ve identified them, you know, I know that ‘Right, that needs to get knocked on the head’. So, just a couple of bad habits [. . .] I’ve got a bad habit of eating a sandwich maybe about half 8, 9 o’clock [. . .] I used to have an 8 o’clock cut-off time for eating.
Interview 59, football club 04, long-term achiever
Men who were less successful at sustaining weight loss (long-term regainers and pre-FFIT achievers) seemed to take a more laissez-faire approach to the re-emergence of old habits:
I didn’t have any cheese whatsoever through the whole period [FFIT programme], you know? I mean, I’m not saying cheese is a big thing for me, it’s more chocolate and sweet things actually. So I hadn’t had them and I was sort of the belief that I’m OK as long as I don’t try . . . you know, it’s a bit like an addict with a drink or something like that, an alcoholic. Once I’ve tried some, that’s that, you know it’s . . . I can’t sort of rationalise that and say ‘Well, OK, that’s fine’. I say that, ‘Well, that’s it’. You know, I’ve . . . an’ then I’m a slippery slope again you see . . .
Interview 60, football club 11, long-term regainer
This section shows that men in all groups had retained some of the key messages around PA and healthy eating that they had learned during the FFIT programme over the long term. However, men in the more successful groups (in particular the long-term maintainers and long-term achievers) spoke in ways that suggested that they were more committed and disciplined about continuing to put these messages into practice than groups with less success in achieving long-term weight loss. For example, men in the pre-FFIT achievers group spoke less often about enacting the key healthy lifestyle advice from the FFIT programme, and men in the long-term regainers group were sometimes vague in their descriptions of how they put the tools and strategies from the FFIT programme into practice. Indeed, men in all groups spoke about facing continued challenges in maintaining their weight loss long term. The next section describes, in more detail, the reasons men gave for feeling motivated to continue to implement the changes they had made during the FFIT programme over the long term.
Participant experiences of motivation
Men described a wide range of factors that motivated them to try to maintain their weight loss, PA and dietary improvements over the long term, as well as what disrupted or undermined their motivation. Our analysis of men’s motivations was guided by self-determination theory,20,21 which suggests that autonomy (associated with the internalisation of regulation), competence and relatedness to other people are associated with long-term weight control. The following sections examine the men’s accounts in relation to the internalisation of behavioural control, from externalised regulation (i.e. doing things because of an external demand or reward), through introjected regulation (i.e. doing things because you feel you ‘should’) to identified regulation (i.e. doing things because they are personally important).
Externalised regulation (doing things because of an external demand or reward)
Men in all groups mentioned the role of external factors in influencing their efforts to lead a healthy lifestyle. Some told how family members reminded them of what they should and should not do, particularly in relation to healthy eating. One pre-FFIT achiever said that his partner had played a pivotal role in encouraging him to eat more healthily:
. . . being with my partner definitely helped me keep those changes up ’cause she’s there to keep me in check, you know? Whereas before I’d be, as I say, because you’re single and, when you work a long day, you come home, you just sometimes can’t be bothered cooking for yourself, and it’s certainly a lot easier when you’ve got somebody tae motivate you.
Interview 2, football club 12, pre-FFIT achiever
Other men described how discussions with health professionals about managing long-term health conditions, such as type 2 diabetes, hypertension and rheumatoid arthritis, had motivated them to try to keep their weight down:
I was certainly told by the doctor I really needed to lose about 5 stone. And, well, probably since I done the Football Fans in Training, it’s came down just over 2 stone, so . . . But they says ‘Don’t worry about how long it takes, just get there’, basically.
Interview 32, football club 08, delayed responder (diagnosed with type 2 diabetes since taking part in the FFIT programme)
Some men also described how compliments from other people acted as a reward that served to motivate them:
Oh, absolutely makes you feel a whole lot better about yourself and, like I say, if you’re getting compliments from people and stuff, you know, that’s gonnae help your self-esteem and make you feel better. There’s no doubt about that. Absolutely, you know, and it also keeps you motivated, you know, and . . . everybody’s the same, you know? If people say nice things about you, you’re gonna – it’s nice to have that, you know? But I think the challenge is to, rather than let that go over your head, is to keep it inside, and that keeps you motivated as well, you know? So it’s about learning to accept compliments. Before, you maybe werenae very good at that because it was about the way you looked and you knew that you were overweight and, you know, so it’s about keeping those inside you and using that as a continual motivation.
Interview 49, football club 10, long-term achiever
Introjected regulation (doing things because you feel you ‘should’)
Men from all groups described how they tried to manage their weight and lead a healthy lifestyle because they knew that they ‘should’ (e.g. they knew that it was good for their health), or to avoid negative emotions (e.g. embarrassment, guilt) or attain positive emotions (e.g. pride). Many said that they were aware of the health benefits of sustaining weight loss and leading a healthy lifestyle. Some reported that experiencing tangible benefits to their health and well-being, such as being able to reduce their medication, and improvements in back pain, quality of sleep and stamina, had spurred them on:
I suffer from high blood pressure, I had this at the start, and after the first 12 weeks of working there [on the FFIT programme], my – I went from hypertension just to high blood pressure. Now that’s fantastic as well, so my medication for that got reduced, and I really do believe that that’s been a direct impact or a combination of the exercise that we were doing there, and the eating habits that I had as well.
Interview 68, football club 03, long-term maintainer
Men described how the experience of negative emotions in relation to being overweight motivated them to keep the changes going. Some men recalled occasions, before commencing the FFIT programme, when they had felt embarrassed about their bodies, and they used these experiences to measure their progress:
[I]t’s lots of little things that make, you know, that you plan ahead, because you know you’re going to be embarrassed if such and such happens because you’re, you know, if, I always take my grandson to the [football] game or because if some other adult sat next to me [before he took part in the FFIT programme] we’d be struggling for space, ‘cause you know, my bum’s too big for the seat sort of thing, you know? And it’s these little things that . . . that make you want to . . . You know, lose weight.
Yeah, and do you find that that has changed for you?
Yes, aye, I can now, I can now sit on a seat myself [laugh].
Interview 59, football club 04, long-term achiever
Men also reported feeling disappointed and guilty when the new healthier PA and dietary routines that they had established were thwarted. For example, this long-term maintainer spoke about his negative emotional reaction to disruption to his normal PA routine:
I’ve kept it off, yeah, so, it’s a battle all the time just to keep it off, eh? It’s just – but that’s it, you just keep going. In fact, I feel – if I miss [an exercise] class now, I feel guilty.
Interview 51, football club 11, long-term maintainer
Other men were clear that negative emotions were unhelpful, and described how they sought to avoid them. One delayed responder told how the principles of the energy balance (discussed in week 11 of the FFIT programme) helped him keep a positive perspective on leading a healthy lifestyle:
I don’t treat the training as a chore and I don’t treat the eating as a sin. ‘Oh if I eat this, oh I’ve gottae [got to] do an extra mile, if I eat that.’ And I don’t hold back, if I want a packet o’ crisps, I’ll eat a packet o’ crisps, but I know that’s it. I don’t treat it where I feel to be guilty in doing any of it, which is good because I don’t feel guilty, I get on wi’ what I’m doing. I’ve been on these diets before, like Slimming World where you’ve gottae count this, and it’s a red day and a green day, and you cannae [can’t] eat this this day and you cannae eat that that day, and you can’t do this and you can’t do that, and you sit and you say tae yourself, ‘Oh I could really go this, I could really go that’. Where I just do it and work it off.
Interview 46, football club 12, delayed responder
In relation to positive emotions, pride in the changes they had succeeded in making was reported by men from all groups. One long-term maintainer described how losing weight helped him to feel good about the way he looked:
Well, I never used to bother aboot myself. I wouldnae [wouldn’t] get done up, if you know what I mean, to go oot. I wouldnae bother aboot shaving or anything, ken [you know]? Because you didnae think much o’ yourself before you started this course. And it makes you think a bit more about yourself when you’re . . . after you’ve done the course. And, sort of, didnae like going oot withoot [without] shaving or that, but as I say, I dinnae like looking as if I’m fat. That’s half the battle, if I dinnae look fat, I’ll feel better.
Interview 61, football club 08, long-term maintainer
Men across all groups described experiencing ups and downs in relation to sustaining their weight loss long term. For example, one delayed responder said ‘since the programme there’s been peaks an’ troughs’ (interview 41, football club 04, delayed responder). Weight regain was often accompanied by a range of negative emotions, including embarrassment, disappointment and guilt:
When I started to put weight back on, I felt guilty. I felt guilty that people had put me forward for this, or accepted me for it, and almost to a degree I’d let them down, I’d let myself down. And then you feel good when the weight starts to come back off. So . . .
Interview 17, football club 12, long-term regainer
Identified regulation (doing things because they are personally important)
Men from all groups spoke about how improvements in weight and lifestyle had enabled them to do things they valued and enjoyed, and that were personally important to them. These included playing or coaching football, doing more with their children, being able to do activities they enjoyed (e.g. hillwalking, deerstalking) more easily, or simply having more of an appetite for life:
I mentioned earlier about playing five-a-side, my son’s playing five-a-sides with me now, he – up until about 2 years ago he would be in company and, my friends would be saying aboot, ‘Oh your dad used to play football’. And he’s probably looking tae me, gaun [looking at me, going], ‘Nah [no] this wee dumpy guy here? Played football? At my level?’. And now we actually play five a-sides together. And I think he appreciates that actually I can kick a ball and play reasonable football. So that’s quite a good thing, it’s quite a good wee bonding thing as well.
Interview 62, football club 12, long-term maintainer
Although many men spoke about feelings of enjoyment in relation to PA, some also described enjoyment from eating a healthier diet and smaller portion sizes:
I enjoy cooking as it is, so the foods that I’m making for myself, it’s stuff that I enjoy eating but it’s also healthy stuff. Fish, pasta, rice, stuff like that that I like to cook. And obviously again portion control, I’m now enjoying what I’ve got, like I’m no’ wanting more, if you know what I mean. Or I’m no’ cooking too much that I’ve ate so much o’ it, an’ I cannae eat the rest. So I’m no’ wasting it, you know. So, but that’s a good thing that way.
Interview 40, football club 01, delayed responder
The preceding sections suggest that men in all groups (including those who had not sustained weight loss long term, i.e. long-term regainers and pre-FFIT achievers) had succeeded to some extent in internalising regulation for maintaining weight loss and positive behaviour changes. One long-term maintainer illustrated his shift from external regulation towards a more internalised form of regulation by describing how his weight loss was initially motivated by a desire not to be obese, a term that he saw as a ‘wake-up call’ when applied to him (at the RCT baseline measures). However, after successfully losing weight during the FFIT programme, he was now motivated to maintain the changes simply because he enjoyed his new lifestyle:
. . . now I hardly feel an incentive’s needed because I’m so much enjoying what we’re doing, it’s not a chore at all.
Interview 12, football club 05, long-term maintainer
According to self-determination theory, feelings of competence and relatedness to other people support this process of internalisation of regulation. 21 The following sections examine how competence and relatedness were portrayed by men in the 3.5-year interviews.
Competence
Men in all groups described feelings of competence and achievement in relation to weight control, PA, eating healthily and/or managing their health. Some men spoke confidently about their ability to use PA and diet to lose weight when they wanted to:
I feel as if I’m a lot more control over what I do, ’cause my wife was saying to me ‘Right we’ve got somebody coming up in a couple of weeks’. And I know I can, without being in some sort of like starvation, I know, if I think, I can just change my ways any time I like. And before I wouldnae really know what to do, to get that kind of weight loss or that fitness up, and just being really stupid and no’ eating for 2 days or something. So I now know that there’s nutritionist [nutritional] food oot there that I can make which isn’t bad for me. And I can cut oot a few things and do a wee bit of exercise, and I’m back on track again. So from that point of view it’s gave me, it’s gave me that wee bit of ownership of what I can do and what I cannae do.
Interview 62, football club 12, long-term maintainer
For most men, success in losing weight was accompanied by a real sense of achievement. Some illustrated this through accounts of being able to wear smaller-sized clothes:
Throwing oot the 40-plus waist – the 40-inch – sometimes it was almost a 42-inch waist, and throwing out those trousers and you know, gradually coming down. That’s the sort of thing that encouraged me, and also you know, when you go and buy the new trousers and everything, you don’t want to throw them out and go back to big stuff. So yeah, that’s encouraged me quite a lot. I’ve came down from like a two-XL to a large. And I’m still 36-inch waist, I’ve no managed to get below that, but it’s that . . . that’s encouraged me.
Interview 29, football club 09, long-term maintainer
Some men from all groups, apart from the pre-FFIT achievers, spoke about feeling competent and experiencing a sense of achievement in relation to PA, in particular when seeing improvements in their fitness and ability to run or play sports. One delayed responder described his satisfaction at now being able to play football with men who were much younger than him:
A minimum of twice a week I’m playing football with different groups. This is all through guys I’ve met through Football Fans in Training. I’m able to play football with guys that weren’t in Football Fans in Training because I’ve got healthier and I’m able tae keep up wi’ the younger kids, as it were.
Interview 4, football club 02, delayed responder
In relation to healthy eating, some men (again from all groups apart from the pre-FFIT achievers) described their skill in using PA to control their eating between meals, their ability to exert self-control over their eating habits or their competence in cooking healthy meals:
And we still do a lottae [lot of] the sortae [sort of] the cooking now, like I’m just standing here cooking my dinner for this evening which is made like bolognaise fae [from] scratch, ken. It’s no’ done wi’ like jars o’ sauces and things like that, ken; so a lot o’ our cooking techniques have totally changed.
Interview 38, football club 08, delayed responder
Finally, one delayed responder described how losing weight had allowed him to self-manage a long-standing back condition:
It turned out I had sciatica on the back o’ that, but because I’ve lost weight now it’s not bothering me any more. So it’s that kind o’ thing, you know? It’s made me think you can look after yourself without having tae go tae the doctor. I mean, the doctor tells you you’re overweight but you just think to yourself ‘Yeah, yeah, you’ve got tae say that’. But, no, it’s, the proof’s in the pudding.
Interview 4, football club 02, delayed responder
Relationships with other people
Family
In the RCT 12-month focus groups, men described how greater involvement and support from family members had helped them maintain the changes they had made. 44 In the 3.5-year interviews, men in all but the pre-FFIT achievers group continued to speak about family members in relation to their ongoing weight management efforts. One long-term achiever described how he had been a positive influence on his wife’s lifestyle:
She does a lot more walking. You know, she’ll come out wi’ me in the evenings sometimes when I go out wi’ the dog and things like that, so she’s, that has obviously wi’ the eating, as well, she’ll . . . ’cause, you know, we eat together obviously. But she’s changed her eating habits as well, so she’s kind o’ benefited from it through me.
Interview 64, football club 11, long-term achiever
Many men described taking up new activities with family members, or spending more time playing with their children and grandchildren. However, others described how, although they felt supported by their families, they pursued new health behaviours independently:
Well, I think my wife has been an excellent support. She’s kind o’ got on board herself an’ found things that work for her. And, again, it’s finding things that work for you. She won’t go out on a cycle with me ‘cause she can’t stand cycling, so I don’t force her tae do it. I go out for half an hour, put my earphones in an’ I listen tae an audiobook.
Interview 4, football club 02, delayed responder
Men reported that sometimes family members simply provided verbal encouragement rather than practical support. One long-term achiever described how feedback on the changes he had made from his adult children gave him a ‘buzz’ but also left him aware of the ongoing challenges he faced:
I mean my kids are always happy when they see me and I’m less heavy than I was before, ’cause they all say, you know, ‘Well . . . you want to live to see the next grandkid and you wanna . . . you know, you want to see these grandkids reaching 10 or 12 or 20’. So, you know . . .
Interview 59, football club 04, long-term achiever
Although most men spoke about the role of other family members in positive terms, a minority spoke about how making changes on their own, without family support or involvement, had been challenging for them. These men described how they found it difficult when others in their household were reluctant to change eating behaviours or continued to bring temptation into the house:
It is challenging [. . .] ’cause if my wife had come on board as well with it [. . .] if she’d participated in me, starting tae shop more in terms of like what’s healthy for us an’ what could we eat, you know, an’ how much should you be buying, it would undoubtedly be a lot easier, you know? Because from a convenience an’ a cost point of view, I’m just kind o’ going wi’ the flow, you know, in terms o’ the weekly shop [. . .] for me it’s a very solitary endeavour, you know, ‘cause, you know, my wife’s just . . . well, she’s not involved in it.
Interview 55, football club 02, long-term regainer
Other Football Fans in Training participants
Men in all groups attributed some influence over their long-term weight management to ongoing or lapsed contact with fellow FFIT participants. In many football clubs, men met up after the programme to do PA together. One group had formed a charity that fundraised for local causes. Another group had taken part in a weight loss challenge. Some men described the ‘follow-on group’ environment as an important contributor to their motivation to sustain the changes they had made during the FFIT programme:
If we hadn’t been this, as I say this, this little hard core of about six guys plus others who’ve come and gone, if we hadn’t had the group activity, we are all fairly sure we’d probably have not done anything. But we . . . even if . . . It’s a lovely night tonight, but even if it was pouring down with icy sleet on a November or February night, I would be more than happy to go to . . . Because you know that somebody you know is equally thinking ‘I’m quite happy to go because he’s going’. Or somebody else is going. So it’s all mo– . . ., you do motivate each other.
Interview 24, football club 08, long-term maintainer
One long-term regainer directly linked his weight regain to stopping attending the ‘follow-on group’ at his football club:
Because I fell away fae the group, the thing on a Monday night, I’ve crept back on – the weight’s crept back up to nearly what I was up tae when I first started.
Interview 56, football club 03, long-term regainer
Friends and work colleagues
Men, again from all groups, spoke about the role of wider networks of friends and work colleagues in helping them sustain the changes they had made on the FFIT programme. Inspired by his weight loss, one long-term maintainer had joined a triathlon club, and through this had overcome a fear of water:
I decided to take up triathlons, once I lost the weight . . . I’ve learned to swim, I had – if I was in the pool wi’ the kids, I didn’t even like putting my head under the water. I can now – I go to, I joined the triathlon club, they’ve taught me how to swim, I do front crawl, I do head under the water front crawl, I breathe wi’ every three – you know, it’s proper swimming. I’m no’ the fastest but it’s helped me get over that fear of water noo . . . there was a run we did on Boxing Day this year the Triathlon Club. And [wife] and the boys come along, and the Tri-Club really welcomed them to join in. So, they were having – they’re involved in it all as well, it’s no’ just me off doing my triathlon thing.
Interview 50, football club 02, long-term maintainer
For this man, weight loss provided a catalyst for engaging in new forms of PA, facilitated by engaging with a new social group, the ‘Tri-Club’, which also ‘welcomed’ his family’s involvement. Another man described how joining a cycling club had helped him rekindle his love of cycling. Similar to the long-term achiever who spoke about his participation in the FFIT programme having a positive influence on his wife (see Relationships with other people, Family), this man described how his reignited enthusiasm for cycling inspired one of his friends to also take up the hobby:
Again it’s just motivation, it’s motivation to do it. It’s, you know, sort of, especially wi’ cycling, sort of from bed to shed is the hardest, you know, bit that you’ll do, actually getting up in the morning, and going and grabbing your bike is the most difficult part, especially if the weather’s crap. Anyway, so it’s that, and I suppose again being part of a group, makes it easier because there’s almost an expectation that you’ll be out, or you’ll be out cycling or whatever. [. . .] And one of my other friends has got a bike and he’s taken up cycling, so I think I wouldn’t put that necessarily down to me, but maybe again the same as myself that they see me doing it, maybe think, ‘Well if he can do it then I can do it’.
Interview 45, football club 11, delayed responder
Some men described how they had encouraged work colleagues to change their behaviour, for example by setting up a self-weigh-in group or badminton club in their workplaces:
So that’s been a really positive that’s came out of that, and in a funny . . . fits in wi’ the thing we do at work wi’ the health at work thing, you know? The health at work profile that you get and . . . so we’ve had a lot of sort of plaudits from that, just because, at the start, there’s like three or four people say ‘Do you fancy a game of badminton,’ you know? We’ve got almost 20 people playing now so . . .
Interview 49, football club 10, long-term achiever
Nevertheless, other people were not always supportive. Some men described feeling distanced by friends making negative comments about their weight loss:
One of them actually told . . . one of my friends told me I looked gaunt [laughs], which I find quite amusing. [. . .] We’d, [other FFIT fan friend] and I had discussed that, and we reckoned that they were just, wished that they’d done it [laughs]. They were a bit jealous about it, so sticking a, putting the knife in a little bit [laughs]. I don’t know.
Interview 69, football club 03, long-term maintainer
The previous two sections have demonstrated how participation in the FFIT programme provided men from all groups with tools and strategies that allowed them to feel competent in being able to manage their weight, PA and diet over the long term (whether or not they were currently succeeding in doing so). Relationships with other people also featured in interviewees’ accounts of sustaining weight loss and behaviour change. Often these relationships were described as positive and supportive, but some men, often long-term weight regainers, spoke about the role of other people in undermining their attempts to manage their weight over the long term.
Changed identities: integrated regulation
When people feel autonomous in the choices they make, competent in the behaviours they practise to obtain the outcomes they desire, and supported by others in their efforts, self-determination theory suggests that they are more likely to be successful in maintaining changes long term. 21 Ultimately, some may achieve fully internalised (integrated) regulation, in which new behaviours become congruent with their sense of self, which may also be revised. Integration is the most stable form of regulation and, therefore, is most likely to support long-term maintenance of weight loss and related behaviours. 20
Some men in the 3.5-year interviews had clearly succeeded in achieving fully integrated regulation in relation to weight loss, PA and/or healthy eating. However, there was a clear difference between the groups who had succeeded in losing weight long term and those who had been less successful. Examples of integrated regulation featured far more clearly in the accounts of long-term maintainers, delayed responders and long-term achievers than in the accounts of long-term regainers and pre-FFIT achievers. One long-term maintainer told how participation in the FFIT programme had transformed his life:
I get such a buzz from the exercise, you see the weight is – it’s two-pronged this, you lose the weight and you feel good and you look good. But then because you’re exercising, you’ve got that extra buzz as well. And, you know, it’s just a double whammy o’ happiness.
Interview 50, football club 02, long-term maintainer
Some men described a clear distinction between the person they had become since taking part in the FFIT programme and their pre-FFIT self. They used terminology like having ‘a whole new way of thinking’ (interview 4, football club 02, delayed responder), enjoying ‘how I’ve become’ (interview 39, football club 13, delayed responder) and how doing the FFIT programme ‘really was a period that changed the way, my whole direction in many ways’ (interview 47, football club 08, long-term achiever). A couple of long-term maintainers even kept photographic reminders of who they had been (and did not want to become again):
[My wife] always does a family calendar every year as part of the Christmas present, and one of the women, and I’m glad she said it to me, she says ‘[Name], you look as if you’ve had a stroke in that photograph.’ I was sitting wi’ [wife] and the kids in this photograph on the couch, and my t-shirt’s tight, I look lethargic, my eyes half closed, my complexion . . . I look peely wally, I just look terrible. And she says ‘Honestly, [Name]. You look as if you’ve had a stroke in that photograph.’
Was that a trigger for you, do you think?
I keep that, that’s on the top of the biscuit tin in the kitchen. I keep it there.
Interview 50, football club 02, long-term maintainer
A number of men described how taking part in the FFIT programme had changed their views of their capacity to be physically active, in relation to either their fitness level or their age:
. . . actually just realising how much I enjoyed just exercising, you know, and sort of getting involved in that was quite good for me, I think. So again it’s back to that mind-set thing, of probably thinking, ‘Och, I’m too old, I can’t play football any more or I can’t go running or I can’t go cycling’ or whatever. And actually thinking, ‘Well, d’you know, I can do more than I think I can, so why not just try things and see what I enjoy doing’.
Interview 45, football club 11, delayed responder
Nevertheless, it was clear that even for men who were successful in long-term weight loss, the journey was not always smooth. One long-term maintainer described his constant battle to maintain his weight loss:
I’ve lost about 2 stone on the course, managed to keep that off. Well, I had actually lost 2 and a half stone but I’ve put about half a stone back on, but I’ve managed to try and keep it at that sortae level. I’m never gonnae be Skinny Malinky, so.
But, as you say, you lost quite a bit. You’ve kept it off actually, that’s –
I’ve kept it off, yeah, so, it’s a battle all the time just to keep it off, eh? It’s just – but that’s it, you just keep going.
Interview 51, football club 11, long-term maintainer
For this man, his view of himself as a person who would always be overweight was something that he still felt unable to change. Nevertheless, another man (a delayed responder) described how, although his journey had been difficult, by transforming more externalised motivations to lose weight (for his son’s wedding/health reasons) into more internalised motivations (going to the gym because he enjoyed it) this had allowed him to develop a new identity as a fit and active person:
When my son said he was getting married, we got the old wedding pictures out of that period in time [a previous wedding] when I was in a kilt, an’ I thought ‘Nah, I cannae wear a kilt to your wedding unless I lose some weight’. So he says ‘Well, you’d better lose some weight then’. So, again, that’s the kind o’ incentive for me tae pick up the thread again an’ get back on track.
Sure. So has that been quite an important prompt for you, would you say?
I’d say, yes, it has. Yes. I’d say that’s what prompted me into it. But having been now, what, 9 months, you know, losing weight and I’m going tae the gym probably three, four, times a week now, I don’t think I’ll ever slip back because I’m enjoying it. Before, I was doing it because I felt I needed to do it for the wrong reason, not for the wrong reasons but, you know, health reasons, my hip problem. Now, I’m doing it because I miss it, an’ I don’t want to go back intae the kind o’ lethargic day-to-day type of person I was.
Interview 41, football club 04, delayed responder
Summary and initial interpretation of results
The fourth objective of the FFIT follow-up study was to explore and describe men’s experiences (including their motivations, emotions and relations with others) of attempting to control their weight over the long term, their reasons for achieving or failing to achieve long-term weight loss, and the strategies they continued to use or stopped using (see Chapter 1, Aims and objectives). Below, we summarise our results in relation to the three detailed subobjectives.
Subobjectives
Subobjective 1: behaviour change techniques and practical strategies for controlling weight long term
The FFIT programme was designed to provide men with a toolbox of BCTs and practical strategies that they could choose from to help them incorporate PA and healthy eating (and drinking) into their normal daily routine. 46 Table 20 provides a summary of the BCTs and strategies that men across all 3.5-year interview groups said that they continued to use. However, there were some differences between groups, particularly in relation to PA. Men from the pre-FFIT achievers group were less likely to use self-monitoring, and did not appear to walk as much as men in other groups. Long-term regainers also seemed less committed to walking, and to engaging in fewer alternative forms of PA than some of the other groups.
| BCTs | Ways in which men described applying these BCTs |
|---|---|
| Self-monitoring | |
| Weight | Regular self-weighing |
| Feeling how clothes fit | |
| PA | Using a pedometer (although some men specifically said that they no longer did so) |
| Using distance covered or time spent walking | |
| Using new technology (e.g. more sophisticated pedometer/cycling/running apps or wearables) | |
| Diet | Using food diaries |
| Using new technology (e.g. calorie counting) | |
| Weighing food | |
| Goal-setting | |
| Weight | Having a weight loss target |
| Having an upper weight threshold to stay below | |
| PA | Steps or distance |
| PA challenges (e.g. a 5-km or 10-km run) | |
| Practical strategies | |
| For weight management | Eating less |
| Being more physically active | |
| Instigating regular weigh-ins at work | |
| For PA | Walking for leisure |
| Purposeful walking (including walking/getting a dog) | |
| Walking to work | |
| Using the stairs at work | |
| Parking the car further away | |
| Doing other PA (e.g. cycling, running or gym membership) | |
| Following a home-based exercise routine | |
| Playing football | |
| Attending FFIT ‘follow-on’ groups | |
| Membership of a sports club | |
| Involving family members | |
| Involving friends/work colleagues | |
| Using flexitime at work | |
| Changing jobs | |
| For healthy eating and drinking | Reducing portion sizes |
| Using smaller plates | |
| Eating fewer and/or healthier snacks | |
| Reading food labels | |
| Reducing fat, sugar and salt intake | |
| Eating more fruit and vegetables | |
| Eating fewer processed foods and takeaways | |
| Taking own food to work | |
| Switching from sugary drinks to healthier alternatives | |
| Not drinking alcohol at home | |
| Being more physically active | |
Similar to the accounts of behavioural maintenance in the RCT 12-month focus groups, 3.5-year interviewees described how PA (often walking) and eating regular meals had become part of their everyday routine, although the pre-FFIT achievers spoke about this less in relation to walking than those in the other groups. However, the men’s accounts suggested that work was sometimes required to maintain their new healthy habits. It also appeared that those who were succeeding in controlling their weight long term were better at avoiding slipping back into old (bad) habits and tended to take a more positive attitude in relation to facing up to (and often overcoming) challenges.
Again, as in the 12-month focus groups, some men described how health problems, injury, work and family commitments had prevented them from pursuing the new PA and dietary behaviours that they had adopted as a result of taking part in the FFIT programme. Similarly, changes in jobs or working practices were described, particularly by long-term regainers, as being disruptive to newly adopted PA and healthy eating routines. Seasonal factors, such as Christmas and holidays, were also described as disrupting normal routine, as well as providing temptation and a licence to consume unhealthy and/or more food and drink. Poor weather continued to be a challenge and a negative influence on men’s intentions to be physically active, and the outdoor physical environment (e.g. traffic on roads) was also cited by a few men as a reason for being unable to engage in some forms of PA.
Subobjective 2: participant experiences of motivation
According to self-determination theory,20,21 internalised regulation is associated with behavioural maintenance. External factors (e.g. health professionals, family members or friends) continued to be important influences on behaviour for some interviewees. However, men from all groups also described more internalised forms of regulation, including pursuing weight loss, PA and healthy eating behaviours because of the health benefits of doing so, or to avoid embarrassment, guilt or disappointment. Many men across the groups spoke about their pleasure in being able to do things they valued or enjoyed or that were personally important to them, including being able to do more things with their children or grandchildren. Some men also described moving from an external form of regulation (e.g. being told that they were obese at the RCT baseline measures) to now leading a healthier lifestyle because they were enjoying the changes that they had made.
Relationships with other people featured widely in the men’s accounts. Many described feeling supported by their family and doing more things together, but some (including those who were less successful in long-term weight loss) complained that lack of support and involvement from family members threatened to undermine their efforts. Friends (including FFIT participants) also played an important role in the men’s efforts to maintain the changes they had made. However, some men, often those who had been unsuccessful in controlling their weight in the long term, had been unable to attend ‘follow-on groups’ that were set up after the FFIT programme, for different reasons. A few men described how the changes they had made had allowed them to become a positive role model for others. This is consistent with practice theory, which describes how ‘novices’ learn experientially and assimilate new behaviours into their routinised practice to become ‘full practitioners’, and predicts that some people will become ‘carriers of practice’ and take pleasure in influencing the behaviour (e.g. PA) of their family and friends. 75
Subobjective 3: acquisition of changed identities
Self-determination theory20,21 suggests that people who achieve fully integrated regulation in relation to weight loss, PA and/or healthy eating will be more successful in maintaining change. This was borne out in the 3.5-year interviews, in which accounts of viewing themselves as different from the person they had been before the FFIT programme featured most clearly in the accounts of men who had succeeded in long-term weight loss. Some of these men appeared to have distanced themselves from the (unhealthy, unfit or overweight) person they had previously been, seeing themselves now by contrast as a ‘new’ man who had succeeded in fully embracing a new, healthy lifestyle.
Chapter 5 Economic evaluation: methods and results
Introduction
The FFIT RCT demonstrated that the FFIT programme was inexpensive to deliver and cost-effective over the 12-month within-trial period. The longer-term modelling also showed favourable results. 44 The aim of the current economic evaluation was to determine the medium- and long-term cost-effectiveness of the FFIT programme. Specifically, we aimed to:
-
establish the extent to which weight loss and positive behavioural changes are sustained beyond the first 12 months, and the subsequent impact that they have on the cost-effectiveness of the FFIT programme at 3.5 years
-
update the modelling of the longer-term health outcomes and resource use of men who participate in the FFIT programme, and assess the potential for longer-term cost-effectiveness
-
explore heterogeneity of the cost-effectiveness of the FFIT programme.
General methods
The cost-effectiveness analyses conducted in the FFIT follow-up study were based on self-reported health-care resource use, GP-prescribed medications and SF-12 outcomes that were collected during the stadia and home visit measurements described in Chapter 2, Procedures. The initial medium-term analysis estimated the cost-effectiveness of the FFIT programme compared with no active intervention at 3.5 years and required the assumption that there were no differences in costs or effects between the FFIT-FU-I group and a ‘no active intervention’ control group beyond the 3.5-year follow-up period. The longer-term (‘lifetime’) analysis employed a health economics model (see The Cardiovascular Disease Policy model) to estimate additional costs and benefits over the lifetime of the participant. Both analyses used the NHS and Personal Social Services perspective, which is favoured by the National Institute for Health and Care Excellence (NICE). 76
Hypothetical control scenarios
All cost-effectiveness analyses require a ‘no active intervention’ control. In the RCT cost-effectiveness analyses, the comparison group was used as the source of data for the ‘no active intervention’ control. 44 However, because the comparison group had the opportunity to take part in the FFIT programme after the RCT 12-month measures, their 3.5-year costs and outcomes data could not be used as the control for the 3.5-year cost-effectiveness analyses.
It was necessary, therefore, to construct hypothetical scenarios to operate as counterfactuals. We did this in two ways: first, by extrapolating RCT comparison group baseline data to take account of the fact that 11% of men had lost ≥ 5% of their body weight at 12 months (i.e. without the FFIT intervention) and, second, by extrapolating the 12-month data of the comparison group. We included this extrapolation because it used the last observed data for the comparison group, and we expected that it would provide the most conservative cost-effectiveness estimate. (Data from three men in the comparison group who died, two during the RCT and one during the follow-up period, were included up to the point of death.) Using the baseline and 12-month data, we modelled two possible weight trajectories: a population trajectory of 0.46 kg per year [the mean weight gain in men with no known intervention in the European Prospective Investigation into Cancer and Nutrition (EPIC) study77] and 1.04 kg per year (the mean weight gain of the FFIT-FU-I group from 12 months to 3.5 years, as reported in Chapter 3, Long-term weight trajectories). These weight gain trajectories were thought to be the most likely upper and lower boundaries. We produced the following six hypothetical ‘no active intervention’ control scenarios, as shown in Table 21.
-
Base case: the comparison group data are extrapolated from baseline. We assume that the controls put on weight from baseline (0 months to 3.5 years) in accordance with an average population trajectory in men of 0.46 kg per year.
-
Scenario 1: the comparison group data are extrapolated from baseline. We assume that the control group gained weight from baseline to 3.5 years at the same rate of 1.04 kg per year as the FFIT-FU-I group gained weight over the follow-up period (12 months to 3.5 years).
-
Scenario 2: the comparison group data are extrapolated from 12 months. We assume the control group gained weight after the RCT (12 months to 3.5 years) in accordance with an average population trajectory in men.
-
Scenario 3: the comparison group data are extrapolated from 12 months. We assume that the control group gained weight after the RCT at the same rate as the FFIT-FU-I group over the follow-up period.
-
Scenarios 4 and 5: these scenarios mirror scenario 1 and scenario 3, respectively, but exclude the 11% of men in the comparison group who achieved ≥ 5% weight loss at 12 months. We included them to reflect the sensitivity analyses for the primary outcome (see Using different baseline time points, baseline time points sensitivity analysis 2, Report Supplementary Material 7). We expect them to produce similar results to base case and scenario 1, respectively.
| RCT comparison group data | Weight gain trajectory | |
|---|---|---|
| Population (0.46 kg/year) | FFIT-FU-I (1.04 kg/year) | |
| Extrapolated from baseline to 3.5 years | Base case | Scenario 1 |
| Extrapolated from 12 months to 3.5 years | Scenario 2 | Scenario 3 |
| Extrapolated from 12 months to 3.5 years (excluding 11% who lost ≥ 5% weight at 12 months) | Scenario 4 | Scenario 5 |
Medium-term (3.5-year) analysis
Introduction
The aim of the medium-term analysis was to estimate the impact (in terms of costs and effects) associated with the FFIT programme in order to establish its cost-effectiveness over 3.5 years. The analysis compared the 3.5-year data from the FFIT-FU-I group with data extrapolated from the RCT comparison group baseline and 12-month measures in terms of (1) costs incurred over the 3.5-year period, (2) number of men achieving a ≥ 5% weight reduction at 3.5 years and (3) QALYs gained over the 3.5-year period as determined from SF-12 scores using the algorithm described by Brazier and Roberts. 78
Methods
Resource use and costs
The cost of providing the FFIT programme in the 13 SPFL clubs in the RCT was estimated to be £61,700, which is equivalent to £164 per FFIT participant. 44 In addition to the intervention costs, self-reported data on the number and type of any NHS resources used in the preceding 12-week period were collected at all time points (baseline, 12 weeks, 12 months and 3.5 years) from each participant. This included visits to the GP, practice nurse or physiotherapist, and any attendances at accident and emergency departments. Unit costs for each of these visits were taken from the Personal Social Services Research Unit, using 2011/12 costs in the RCT79 and 2014/15 costs in the follow-up study at 3.5 years80 (see table i, Report Supplementary Material 12). We used 12 weeks as the time frame over which participants were asked to recall their health resource use, as longer periods have been found to be potentially subject to greater recall bias. 81 Self-reported data on inpatient stays and outpatient appointments over the preceding 12 weeks were also collected at all time points. The unit costs, which were taken from Information Services Division Scotland tariffs82 for 2012 for RCT data and 2015 for 3.5-year follow-up data, and, when necessary, NHS Reference Costs for 2011–12 (RCT)83 and 2014–1584 (3.5-year follow-up), are shown in table iii in Report Supplementary Material 12.
In the RCT, the only between-group difference in costs at baseline was related to inpatient costs, with the comparison group having higher self-reported inpatient costs than the intervention group. 44 However, there was no pattern in the data [i.e. participants with higher baseline inpatient costs did not continue to have higher costs during the RCT or follow-up study periods (confirmed through data linkage; see Chapter 6, Long-term clinical health outcomes)].
Finally, self-reported data were collected at each time point on the numbers of GP prescriptions of antidepressants, painkillers, asthma, pain gels or creams, anti-inflammatories and sleeping tablets in the previous 12 weeks. These medications were identified as being most likely to be affected by the intervention, and were costed using unit costs for a typical prescription from the British National Formulary (BNF)85 (see table ii, Report Supplementary Material 12).
As we have no self-reported health resource use data between 12 months and 3.5 years, to estimate the total health resource costs associated with participation in the FFIT programme over the entire 3.5-year period, we imputed costs at £16 per year per BMI unit increase, as estimated in the UK Counterweight programme,86 between 1 year and 3 years 3 months (i.e. the period of time for which we have no self-reported follow-up resource use data), assuming that there was no inflation over the period. This value was added to (or, for overall weight lost, deducted from) the observed annual resource use costs at 12 months multiplied by 2.25 years. The costs based on 12-week recall at the 3.5-year follow-up (see table iii, Report Supplementary Material 12) were then added to this figure. For full details of the baseline and 12-month health resource use costs, see Wyke et al. 42
For the hypothetical control scenarios, costs were similarly imputed at £16 per year per unit of BMI increase between baseline or 12 months, and 3.5 years. The annual values were added to the observed health resource costs at baseline or 12 months, and multiplied by 3.5 or 2.5 years, respectively.
Outcome measurements
For the medium-term analysis, weight is assumed to be a modifiable risk factor that has an impact on costs and utilities. Our ‘imputations’ were informed by a review of the literature for weight and costs77,86 or by regressing self-reported SF-12 data collected at all time points (baseline, 12 weeks, 12 months and 3.5 years) against BMI in order to predict SF-12 utilities (HRQoL). How each variable was imputed for the six hypothetical control scenarios is described in detail in the following sections.
Weight and body mass index
In the FFIT-FU-I group, BMI was calculated using weight measurements taken at baseline, 12 weeks, 12 months and 3.5 years. A fixed height was assumed based on a best estimate that was modelled from the height measurements taken at baseline, 12 weeks and 12 months.
For the six hypothetical control scenarios, the weight was extrapolated from the RCT comparison group data by multiplying the average annual weight gain (0.46 kg or 1.04 kg) by 3.5 years or by 2.5 years. This was then added to each individual’s measured weight at baseline or at 12 months to give imputed weight at 3.5 years. The BMI was recalculated for each individual in the hypothetical control scenarios using their imputed weight at 3.5 years and fixed height (see table iv, Report Supplementary Material 12).
Utilities
The SF-12 scores from baseline, 12 weeks, 12 months and 3.5 years were converted into health utility weights using the Short Form questionnaire-6 Dimensions (SF-6D) algorithm. 78,87,88 These health utility scores were regressed against BMI and age in order to predict scores at 3.5 years in each of the hypothetical control scenarios. A cluster variable was included in the regression, given the multiple observations per participant. Age was dropped from the analysis, as it was found not to be associated with utilities. Values were fitted for each of the six hypothetical control scenarios by taking each individual’s BMI in each scenario as the predictor of their utility (see tables v and vi, Report Supplementary Material 12).
Analysis
The individual-level data on achieving a ≥ 5% weight reduction at 3.5 years were summed to provide the number of men attaining this outcome in each group (i.e. the FFIT-FU-I group and the six hypothetical control scenarios). The individual-level QALY data were averaged within each group. Differences in the average utility change between the FFIT-FU-I group and hypothetical control scenarios give an estimate of the QALYs gained from participation in the FFIT programme, assuming no differences beyond 3.5 years.
The cost associated with each individual is the sum of the per-participant intervention cost (for the FFIT-FU-I group) and the cost of NHS resource use and medications. Individual costs were averaged within each group to give an estimate of the average cost associated with the FFIT-FU-I group and the hypothetical control scenarios. The incremental cost associated with the FFIT programme is the difference between the average cost of the FFIT-FU-I group and the average cost of each hypothetical control scenario.
The incremental cost-effectiveness associated with the FFIT programme is presented as the incremental cost per additional individual who achieved ≥ 5% weight reduction over 3.5 years, and the incremental cost per QALY gained. Costs and utilities were discounted at 3.5% in accordance with NICE guidance. 76
Results
Utilities
Utility scores were fitted for each of the six hypothetical control scenarios using observed BMI at baseline, 12 weeks and 12 months, and the estimated BMI values at 3.5 years. The area under the curve method, using the trapezoid method,89 was used to determine the overall utilities. All available data were used at each time point (baseline, 12 weeks, 12 months and 3.5 years) to calculate the area under the curve (< 1% of utility scores were missing at baseline, and 8–11% of scores were missing at the various follow-up time points). Change from baseline was used to account for differences in the average baseline values between the FFIT-FU-I group and each of the hypothetical control scenarios in order to give an estimate of the utility change over the 3.5 years, as shown in Table 22.
| Group/scenario | SF-12 mean utility (time point) | QALYs | |||
|---|---|---|---|---|---|
| Baseline | 12 weeks | 12 months | 3.5 years | ||
| FFIT-FU-I group | 0.778 | 0.832 | 0.798 | 0.794 | 2.801 |
| Hypothetical control scenarios | |||||
| Base case | 0.768 | 0.790 | 0.782 | 0.782 | 2.739 |
| Scenario 1 | 0.768 | 0.790 | 0.782 | 0.780 | 2.736 |
| Scenario 2 | 0.768 | 0.790 | 0.782 | 0.783 | 2.741 |
| Scenario 3 | 0.768 | 0.790 | 0.782 | 0.782 | 2.738 |
| Scenario 4 | 0.768 | 0.790 | 0.782 | 0.781 | 2.738 |
| Scenario 5 | 0.768 | 0.790 | 0.782 | 0.779 | 2.736 |
Costs
The total cost associated with the FFIT programme was estimated to be £571,000 (95% CI £401,000 to £740,000), with an average cost of £2450 per participant. The minimum cost necessarily incurred in the FFIT-FU-I group is the cost of the FFIT programme itself, at £164. As shown in Table 23, the total cost associated with the hypothetical control scenarios was estimated to be between £521,000 (95% CI £410,000 to £632,000) and £697,000 (95% CI £480,000 to £914,000), with an average cost per participant of between £1640 and £1870 for the different hypothetical control scenarios.
| Group/scenario | Total costs (£) | 95% CI (£) | Average cost per person (£) |
|---|---|---|---|
| FFIT-FU-I group | 571,000 | 401,000 to 740,000 | 2450 |
| Hypothetical control scenarios | |||
| Base case | 684,000 | 468,000 to 901,000 | 1840 |
| Scenario 1 | 697,000 | 480,000 to 914,000 | 1870 |
| Scenario 2 | 617,000 | 469,000 to 764,000 | 1730 |
| Scenario 3 | 623,000 | 476,000 to 770,000 | 1740 |
| Scenario 4 | 521,000 | 410,000 to 632,000 | 1640 |
| Scenario 5 | 526,000 | 415,000 to 638,000 | 1660 |
At the 12-month follow-up, 130 men in the RCT intervention group had achieved and maintained ≥ 5% weight reduction, and 40 men in the comparison group had done so. At the 3.5-year follow-up, 53 men in the FFIT-FU-I group who had achieved ≥ 5% weight reduction at 12 months had maintained this to 3.5 years. An additional 22 men who had not achieved ≥ 5% weight reduction at 12 months had achieved this by 3.5 years. After modelling weight gain trajectories in the six hypothetical control scenarios, we examined how many of the 40 men in the comparison group who achieved ≥ 5% weight loss at 12 months were predicted to have maintained this to 3.5 years. In base case and scenario 1, as weight gain is modelled from baseline, no participant achieved ≥ 5% weight loss at 12 months. In the remaining scenarios (in which weight gain is modelled from 12 months) when a population trajectory is assumed, 34 men in the comparison group were predicted to have maintained ≥ 5% weight loss at 3.5 years, and when a FFIT-FU-I group trajectory is assumed, 24 men in the comparison group were predicted to have maintained ≥ 5% weight loss at 3.5 years.
Incremental cost-effectiveness ratios
As shown in Table 24, over 3.5 years the FFIT programme is more expensive than no active intervention, with an additional cost of £532–740 per individual. The results also indicate that the FFIT programme is more effective in terms of QALYs, with a gain of 0.046–0.051 QALYs over 3.5 years. As a result, the FFIT programme is associated with an incremental cost-effectiveness of £10,700–15,300 per QALY gained.
| Scenario | FFIT-FU-I | Hypothetical control scenarios | Incremental costs (£) | Incremental QALYs adjusting for baselinea | ICER cost per QALY (£) | ||
|---|---|---|---|---|---|---|---|
| Mean discounted costs (£) | Mean discounted QALYs | Mean discounted costs (£) | Mean discounted QALYs | ||||
| Base case | 2250 | 2.57 | 1680 | 2.51 | 563 | 0.0469 | 12,000 |
| 1 | 2250 | 2.57 | 1750 | 2.51 | 532 | 0.0499 | 10,700 |
| 2 | 2250 | 2.57 | 1590 | 2.51 | 663 | 0.0458 | 14,500 |
| 3 | 2250 | 2.57 | 1600 | 2.51 | 647 | 0.0478 | 13,500 |
| 4 | 2250 | 2.57 | 1500 | 2.51 | 740 | 0.0484 | 15,300 |
| 5 | 2250 | 2.57 | 1520 | 2.51 | 723 | 0.0505 | 14,300 |
Lifetime analysis
Introduction
The medium-term cost-effectiveness analysis assumes no differences in QALYs between the FFIT-FU-I group and hypothetical control scenarios beyond the 3.5-year follow-up period. The aim of the lifetime analysis was to use the data obtained at 3.5 years to update the modelling of the longer-term health outcomes and resource use of men who participate in the FFIT programme, that was undertaken in the RCT,44 to provide an estimate of the longer-term cost-effectiveness of the FFIT programme.
The Cardiovascular Disease (CVD) Policy model
The lifetime analysis involved linking the shorter-term impacts identified within the follow-up period to longer-term impacts through the use of a model. Following a review of published models, the Cardiovascular Disease (CVD) Policy model90,91 was selected for use in the lifetime cost-effective analysis during the FFIT RCT. 44 The model uses data from the Scottish Heart Health Extended Cohort (SHHEC),92,93 which measured ASsessing cardiovascular risk using Scottish Intercollegiate Guidelines Network guidelines (ASSIGN) risk factors [including age, sex, family history of CHD and stroke, socioeconomic status, diabetes, systolic BP, number of cigarettes smoked per day, and total and high-density lipoprotein (HDL) cholesterol] in random samples of the Scottish population recruited between 1984 and 1987. For a full description of the CVD Policy model, see Report Supplementary Material 13.
Methods
Employing the Cardiovascular Disease (CVD) Policy model in the Football Fans in Training follow-up study
Within the lifetime analysis in the FFIT follow-up study, the CVD Policy model was used simply as a means to extrapolate the medium-term results. The model uses individual sampling, which enabled us to generate estimates of long-term cost and outcomes for each individual within the FFIT-FU-I group with which to supplement the within-study data. This allowed us to estimate the longer-term costs and outcomes associated with the FFIT programme compared with no active intervention.
Weight and systolic BP were assumed to be the modifiable risk factors that have an impact on costs, life expectancy and QALYs. Weight and utilities were imputed for each of the six hypothetical control scenarios as described in Outcome measurements. Systolic BP was imputed for each hypothetical control scenario, informed by systematic review findings that 10% weight loss equates to a 6.1-mmHg drop in systolic BP on average7 (see table vii, Report Supplementary Material 12).
Updating the Cardiovascular Disease (CVD) Policy model to replace cholesterol with body mass index
We populated the CVD Policy model with data collected during the follow-up study on the risk factors for individuals in the FFIT-FU-I group. In the original longer-term modelling for the FFIT RCT, it was necessary to make a number of assumptions and adjustments in order to use the CVD Policy model. As shown in Table 25, three out of the nine ASSIGN risk factors (family history of CHD/stroke, and total and HDL cholesterol) were not collected during the RCT, and had to be imputed for each participant using information from other Scottish sources. 44
| ASSIGN risk factors | FFIT | |
|---|---|---|
| RCT | Follow-up study | |
| Age | ✓ | ✓ |
| Sex | ✓ | ✓ |
| Family history of CHD/stroke | ✗ | ✓ |
| Socioeconomic status | ✓ | ✓ |
| Diabetes | ✓ | ✓ |
| Systolic BP | ✓ | ✓ |
| Smoker | ✓ | ✓ |
| Total cholesterol | ✗ | ✓ BMI |
| HDL cholesterol | ✗ | ✓ BMI |
To avoid imputation in the current lifetime analysis, a question on family history of CHD/stroke67 was included in the follow-up study questionnaire (see Q41a, Report Supplementary Material 4), and the original model was updated using the original SHHEC data to replace the two cholesterol variables with a single BMI variable (see Table 25). Summary statistics showed that 50% of participants in the SHHEC data set had a BMI of > 25 kg/m2, and 10% had BMI of > 30 kg/m2. Even though the population prevalence of overweight and obesity may have increased since the SHHEC data were collected, we assume that the risk they pose has not and, thus, that the changing prevalence is not an issue. Table 26 describes the other variables used in the longer-term modelling, the data sources, and any assumptions made.
| Variable | Data source | Assumptions |
|---|---|---|
| Age | Age at RCT baseline + 3.5 years | None |
| Socioeconomic status | From RCT baseline (1 = most deprived) | Kept 2009 SIMD71 scores used in RCT for consistency rather than update to 2012 SIMD scores |
| Family history of CHD/stroke | From FFIT follow-up study | This question was not asked in the RCT. Instead, values were imputed for modelling. In the current lifetime analysis, the FFIT-FU-I group comprises only those men who provided follow-up data. All men in the original RCT comparison group are in the model, but not all took part in the follow-up study and, therefore, did not answer this question. We assume those who were ‘missing’ to have ‘no family history of CHD/stroke’. This provides a conservative estimate of family history of CHD/stroke in the six hypothetical control scenarios, thereby reducing the cost-effectiveness of the FFIT programme |
| Diabetic status | Taken as positive if men reported themselves to have diabetes at any data collection time point (baseline, 12 weeks, 12 months or 3.5 years) | As not all of the RCT comparison group took part in the follow-up study and, therefore, did not provide information of diabetes status at 3.5 years, potentially not all cases of diabetes are captured in the six hypothetical control scenarios. We assume those who were ‘missing’ to be ‘not diabetic’. As we would expect an increase in cases of diabetes over time with age and weight gain, this provides a conservative estimate of diabetes, which reduces the cost-effectiveness of the FFIT programme. In addition, as the comparison group was given the opportunity to take part in the FFIT programme after the 12-month measures, this may have had the effect of reducing the progression of diabetes among men who provided data at 3.5 years. This would again result in conservative estimates of diabetes in the six hypothetical control scenarios |
| Cigarettes per day | From RCT baseline or 12-month data | We assume that smoking status remains unchanged from the time point of extrapolation (baseline or 12 months) in the six hypothetical control scenarios. When men said that they did smoke, but did not provide a number of cigarettes per day, a mean of 15 cigarettes per day (based on the average daily number of cigarettes reported by men who smoked in the RCT) was imputed. When an estimation of cigarettes smoked (e.g. 5–10 per day) was provided, the mean was taken |
The FFIT follow-up economic evaluation supplementary tables provide the risk equations for each first event for men in the CVD Policy model with a BMI as a covariate (see tables viii–xi, Report Supplementary Material 12) and the original model that used cholesterol (see tables xii–xv, Report Supplementary Material 12). When BMI was included in the non-CVD death equation, it had a fairly strong (unintuitive) protective effect, as did cholesterol in the original model. In both models, it was assumed that this protective effect was an association, not causal, and therefore, it was disabled, and not included as part of the model. 90,91
Analysis
Comparing the Cardiovascular Disease (Body Mass Index) Policy model with the original Cardiovascular Disease (CVD) Policy model
The RCT 12-month data were rerun through the CVD (BMI) Policy model in order to compare the results with those of the original CVD Policy model. As in the RCT, men reporting a past CVD event were not excluded, imputed family history of CHD/stroke values were used, and some values for systolic BP were left as missing. In addition, in the RCT 12-month data set, BMI was missing for 11% of men in the intervention group and for 5% in the comparison group. In these cases, we used the average of BMI at baseline and 12 weeks or, when 12-week BMI was also missing, we used BMI at baseline alone.
These data were used to compute a post-intervention risk score from which the model generates post-intervention estimates of life expectancy and QALYs, and an estimate of the cumulative lifetime hospital costs for each participant. The impact of the FFIT programme is based on the differences between these estimates for the men in the FFIT-FU-I group and individuals in the hypothetical control scenarios.
Lifetime cost-effectiveness
The lifetime cost-effectiveness is presented in terms of QALYs and costs. These estimates were generated for each individual as the sum of the individual’s 3.5-year outcomes, and their estimate of life expectancy and the costs generated by the CVD (BMI) Policy model. The estimates of life expectancy, QALYs and costs were all discounted at a rate of 3.5%. 76 As this is a primary prevention model, those men reporting a past CVD event [FFIT-FU-I group, n = 8; RCT comparison group, n = 9 (as used to derive the hypothetical control scenarios)] were excluded from the lifetime analysis.
The costs and effects of the FFIT programme were calculated by averaging the costs and effects within the FFIT-FU-I group and within each of the hypothetical control scenarios. The incremental costs (£) and effects (QALYs) associated with the FFIT programme are the differences between the average costs and effects of the FFIT-FU-I group, and the average costs and effects of each hypothetical control scenario. The incremental cost-effectiveness associated with the FFIT programme is then presented in terms of incremental cost per QALY gained.
Cost-effectiveness acceptability curves and expected value of perfect information (EVPI) analyses
To estimate the uncertainty in the CVD (BMI) Policy model estimates, we undertook a probabilistic sensitivity analysis allowing for uncertainty in the estimation of all of the parameters within the model. Running this analysis required simulating 100–500 draws from the probability distributions for each of the parameters, and using each of these draws to estimate the life expectancy, QALYs and lifetime costs for each individual, generating an estimate of the average costs, life-years and QALYs for each group for each draw. The resulting uncertainty in the incremental costs and effects associated with the intervention were plotted on incremental cost-effectiveness planes. The cost-effectiveness acceptability curve presents the uncertainty surrounding the cost-effectiveness of the FFIT programme compared with no active intervention.
Finally, the uncertainty surrounding the decision to implement the FFIT programme was formally assessed, in terms of the associated costs and consequences, within a value-of-information (VOI) analysis. 94 In general, any decision made on the basis of cost-effectiveness may turn out to be wrong (i.e. the intervention is not actually cost-effective). When this is the case, resources used to provide the intervention are being wasted and QALYs are being lost. VOI analysis determines the potential value associated with undertaking additional research to generate more information that would reduce that uncertainty in future. VOI is assessed by the difference in the value of a decision taken with the current level of information and uncertainty, and the value of a decision taken with more information (and less uncertainty). When the cost of undertaking additional research is lower than the value associated with the decision made on the basis of more information, the research is worthwhile.
The expected value of perfect information (EVPI) is a specific type of VOI that estimates the value associated with eliminating all uncertainty surrounding a decision. If the cost of further research is greater than the EVPI at a given cost-effectiveness threshold, further research is not worthwhile. However, if the cost of further research is lower than the EVPI, the research is potentially worthwhile. Determining whether or not specific research is worthwhile requires a comparison of the value of the actual reduction in uncertainty achieved by that research, which can be assessed through the expected value of sample information, with the actual costs of the research. Here, the uncertainty surrounding the decision to implement the FFIT programme was formally assessed using the EVPI to determine the potential worth of undertaking further research. We used only the hypothetical base case for the EVPI analysis, as the results of all six scenarios were highly similar, and the base case was considered the most likely scenario. As in the RCT, the eligible population for this analysis was specified as Scottish men aged 35–65 years identified over the next 3 years with a BMI of ≥ 30 kg/m2: a population of 365,000 men. 44
Scenario analysis
Uncertainty about the long-term sustainability of behavioural change (structural uncertainty) was examined through a scenario analysis (again using the hypothetical base case scenario only) that limited the time frame for the risk reduction impact of the intervention (assuming that new behaviours and associated outcomes will not be sustained indefinitely). We restricted the effect of the FFIT programme to 2 years beyond the 3.5-year follow-up period (5.5 years in total since the FFIT-FU-I group started the FFIT programme) by returning to the individual’s baseline hazard predictions for BMI, systolic BP and smoking after 2 years in the model, with diabetic status, socioeconomic status and family history of CVD remaining unchanged. The FFIT-FU-I group and base case scenario were put through the same hazard predictions after 5.5 years.
Results
Comparing the Cardiovascular Disease (Body Mass Index) Policy model with the original Cardiovascular Disease (CVD) Policy model
The comparison of the CVD (BMI) Policy model with the original CVD Policy model indicated that the incremental costs and effects were approximately of the same order in both models. However, as shown in Table 27, costs were higher, and absolute QALYs were lower, in the CVD (BMI) Policy model.
| Study arm | Model | |||||
|---|---|---|---|---|---|---|
| CVD Policy | CVD (BMI) Policy | |||||
| Cost (£) | Life-years | QALYs | Cost (£) | Life-years | QALYs | |
| FFIT-FU-I | 19,500 | 66.9 | 65.6 | 27,700 | 66.1 | 62.2 |
| No active intervention | 18,400 | 66.5 | 65.2 | 26,300 | 65.7 | 61.8 |
| Incremental | 1070 | 0.430 | 0.380 | 1400 | 0.400 | 0.380 |
Lifetime cost-effectiveness
Table 28 shows that, over the lifetime modelling, the FFIT programme is more expensive than no active intervention, with an additional cost of around £1450–1680 per individual. The FFIT programme is also more effective, with an increase of 0.679–0.821 QALYs. As a result, the FFIT programme is associated with an incremental cost-effectiveness of £1790–2200 per QALY gained.
| Scenario | FFIT-FU-I groupa | Hypothetical control scenarios | ICER | ||||
|---|---|---|---|---|---|---|---|
| Discounted costsb (£) (95% CI) | Discounted QALYsb (95% CI) | Discounted costsb (£) (95% CI) | Discounted QALYsb (95% CI) | Incremental costs (£) | Incremental QALYs | ICER cost per QALY (£) | |
| Base case | 27,400 (27,200 to 27,500) | 65.8 (65.8 to 65.8) | 25,700 (25,600 to 25,800) | 65.0 (65.0 to 65.1) | 1680 | 0.781 | 2150 |
| 1 | 27,400 (27,200 to 27,500) | 65.8 (65.8 to 65.8) | 25,700 (25,600 to 25,800) | 65.0 (65.0 to 65.0) | 1640 | 0.782 | 2100 |
| 2 | 27,400 (27,300 to 27,500) | 65.8 (65.8 to 65.8) | 25,900 (25,800 to 26,000) | 65.1 (65.1 to 65.2) | 1500 | 0.679 | 2200 |
| 3 | 27,400 (27,200 to 27,500) | 65.8 (65.8 to 65.8) | 25,900 (25,800 to 26,000) | 65.1 (65.1 to 65.1) | 1510 | 0.691 | 2190 |
| 4 | 27,400 (27,200 to 27,500) | 65.8 (65.8 to 65.8) | 25,900 (25,800 to 26,000) | 65.0 (65.0 to 65.0) | 1450 | 0.809 | 1800 |
| 5 | 27,400 (27,200 to 27,500) | 65.8 (65.8 to 65.8) | 25,900 (25,800 to 26,000) | 65.0 (65.0 to 65.0) | 1470 | 0.821 | 1790 |
Cost-effectiveness acceptability curves and expected value of perfect information (EVPI) analyses
Figure 5 shows the uncertainty surrounding the estimates of the lifetime incremental costs and effects for the different hypothetical control scenarios. There is no uncertainty surrounding the existence of a cost difference; the FFIT programme is more expensive than no active intervention (i.e. all of the incremental costs are positive), although there is some uncertainty about the extent of the cost difference in each analysis. In addition, there is no uncertainty surrounding the existence of a difference in effect (i.e. all the incremental QALYs are positive), although again there is uncertainty about the extent of the effect difference. Furthermore, costs and health outcomes (effect) are highly correlated.
FIGURE 5.
Incremental cost-effectiveness planes for the six hypothetical control scenarios. (a) Base case and scenario 1; (b) scenarios 2 and 3; and (c) scenarios 4 and 5.
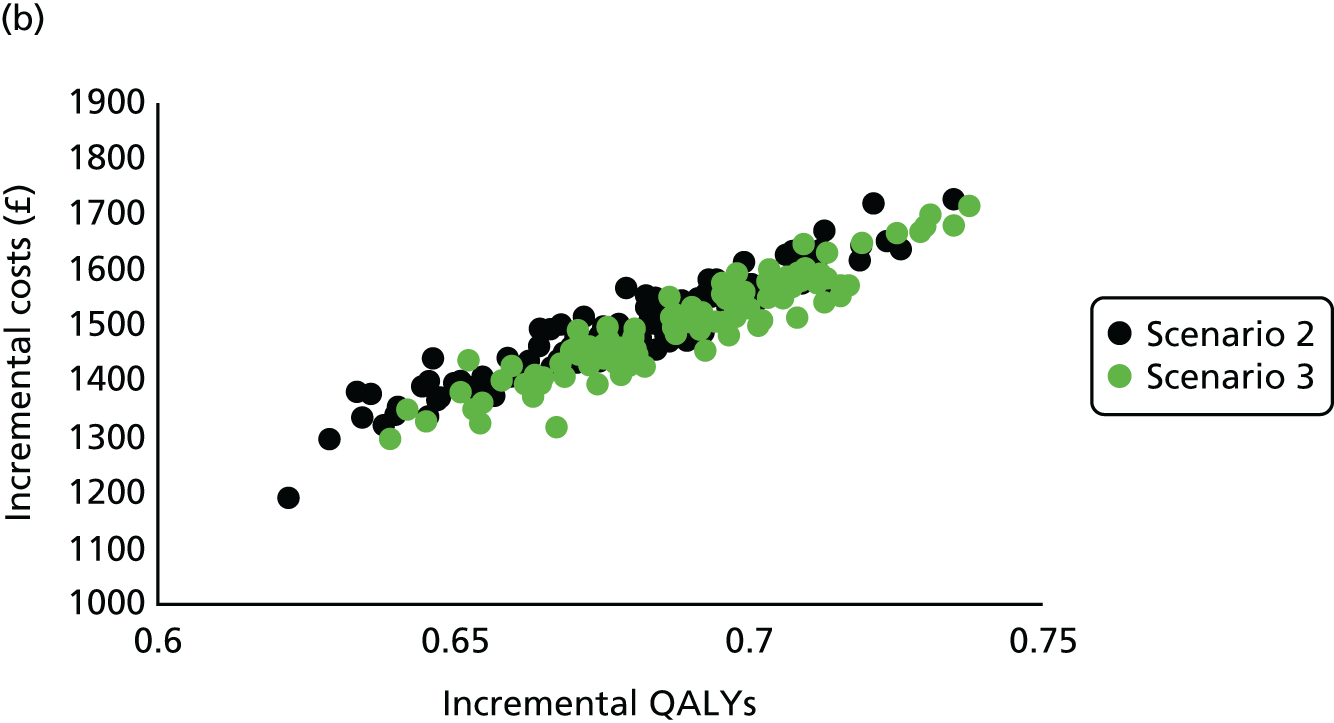
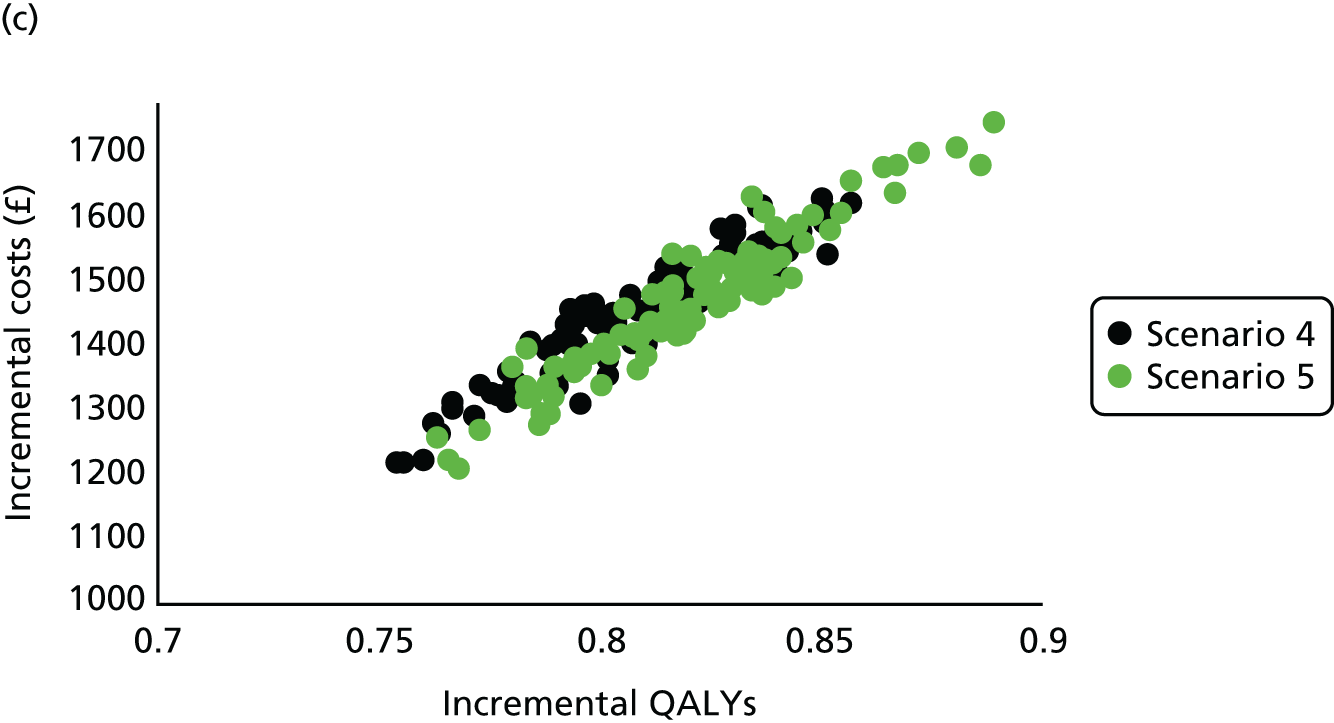
The cost-effectiveness acceptability curves for the lifetime analysis (Figure 6) illustrate the probability that the FFIT programme is cost-effective for any given value of the cost-effectiveness threshold. Figure 6 shows that in the base case scenario (which is also representative of scenarios 1–3) the FFIT programme becomes the cost-effective option at a willingness-to-pay threshold of just over £2000, and in scenario 4 (which is also representative of scenario 5) the FFIT programme becomes the cost-effective option at a willingness-to-pay threshold of just under £2000. This demonstrates that, when the decision-maker is willing to pay £20,000 or £30,000 per QALY (the willingness-to-pay threshold generally accepted by NICE76), there is no uncertainty that the FFIT programme is cost-effective compared with a no active intervention alternative. Indeed, if a decision-maker is willing to pay more than approximately £2500 per QALY, there is no uncertainty that the FFIT programme is cost-effective.
FIGURE 6.
Cost-effectiveness acceptability curves.
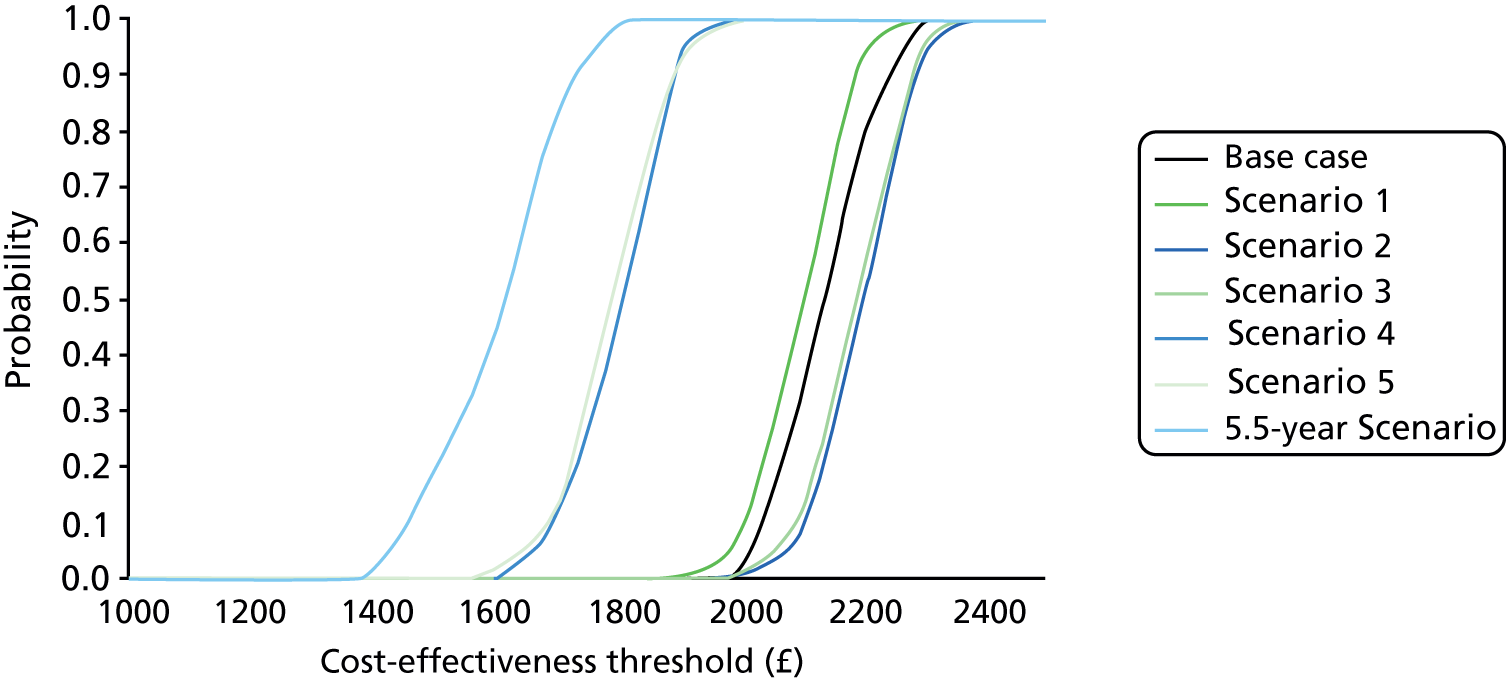
The EVPI analysis shown in Figure 7 demonstrates that there is very little value associated with further research if the decision-maker is willing to pay more than £2500 per QALY. For cost-effectiveness thresholds below this level, there is considerable value in undertaking further research. For the range of thresholds generally employed in the UK (£20,000–30,000 per QALY), there is no uncertainty about implementing the FFIT programme and, consequently, no potential value in undertaking further cost-effectiveness research.
FIGURE 7.
Expected value of perfect information (EVPI) for each eligible man: base case scenario.
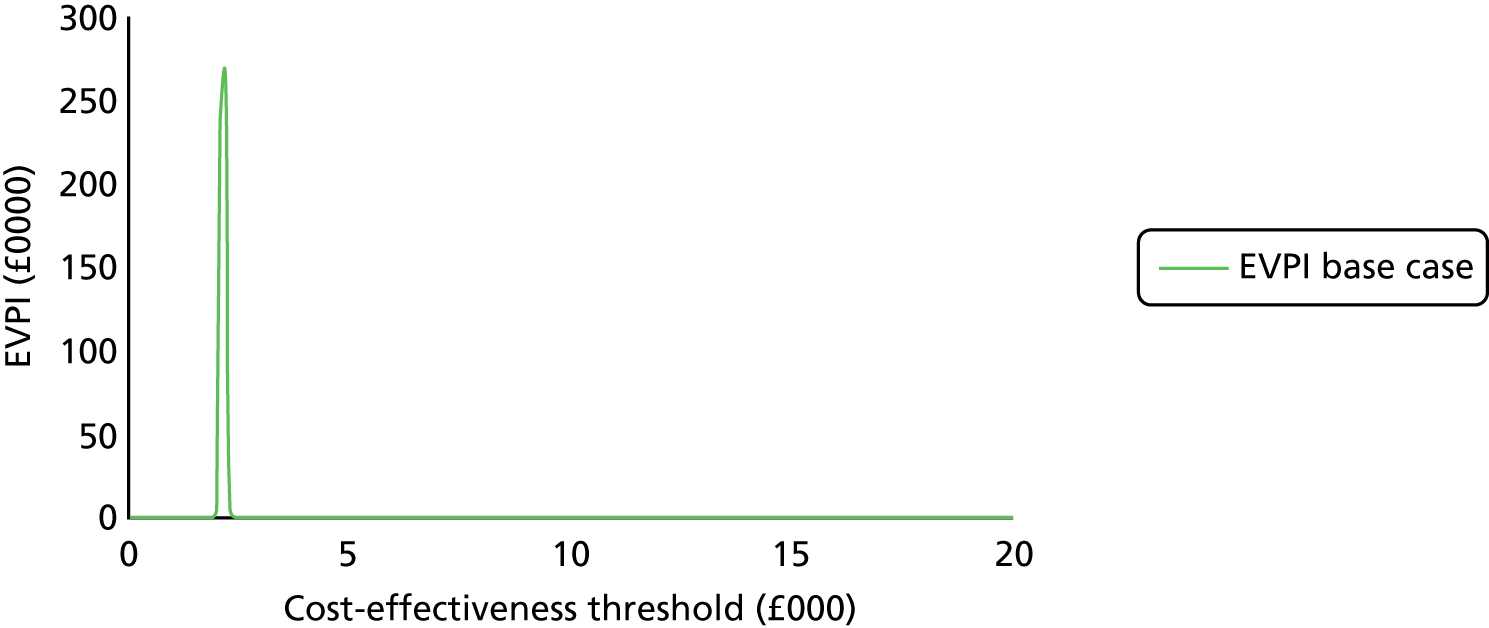
Scenario analysis
The scenario analysis (as shown in Table 29 and Figure 6) indicates that the FFIT programme remains cost-effective when the impact of the beneficial behaviour change on the intervention is limited to 5.5 years. Specifically, although the FFIT programme remains more expensive than no active intervention, the average additional cost is reduced to £1025 (95% CI –£85 to £1220) per individual (from £1680, 95% CI –£1480 to £1870, per individual in the lifetime base case analysis). The results also indicate that the FFIT programme remains more effective, but to a lesser extent, with an average increase of 0.639 (95% CI –0.595 to 0.693) QALYs (compared with 0.781, 95% CI –0.732 to 0.831 QALYs in the base case lifetime analysis). The overall result is to improve the cost-effectiveness of the FFIT programme, with an incremental cost-effectiveness ratio of £1600 per QALY gained (compared with £1790–2200 per QALY gained in the lifetime analysis).
| FFIT-FU-I group | Base case scenario | ICER | |||
|---|---|---|---|---|---|
| Cost (£) (95% CI) | QALYs (95% CI) | Cost (£) (95% CI) | QALYs (95% CI) | Incremental costs (£) (95% CI) | Incremental QALYs (95% CI) |
| 26,700 (26,600 to 26,900) | 65.7 (65.6 to 65.7) | 25,700 (25,600 to 25,800) | 65.0 (65.0 to 65.1) | 1025 (1480 to 1870) | 0.639 (0.595 to 0.693) |
Heterogeneity analysis
Introduction
We explored potential heterogeneity of the cost-effectiveness of the FFIT programme to see if there were any differences according to baseline BMI. BMI was selected for the heterogeneity analysis, as BMI is an important eligibility criterion for participation in the FFIT programme.
Methods
We undertook a bivariate analysis of the modelled lifetime QALYs and costs associated with the FFIT-FU-I group compared with the hypothetical base case scenario for differences in baseline BMI of > 30, > 35 and > 40 kg/m2.
Results
Table 30 shows that there is a difference in utility of 0.630 QALYs between the FFIT-FU-I group and the hypothetical base case scenario, but for men with a baseline BMI of > 35 kg/m2 there is a significantly greater effect in health outcomes. The difference in costs between the FFIT-FU-I group and the base case scenario is £1440, but in men with a BMI of > 40 kg/m2 the FFIT programme becomes cost saving [i.e. there is a cost-saving of £1290, 95% CI –£1440 to £2730, per individual].
| Risk factor | Coefficient | Standard error | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Discounted QALYs | |||||
| Intervention effect | –0.632 | 0.617 | –1.02 | 0.306 | –1.84 to 0.577 |
| BMI (kg/m2) | |||||
| > 30 | –0.571 | 1.01 | –0.560 | 0.574 | –2.56 to 1.42 |
| > 35 | –1.96 | 0.716 | –2.74 | 0.006 | –3.36 to –0.555 |
| > 40 | -0.672 | 0.957 | –0.700 | 0.483 | –2.55 to 1.20 |
| Constant | 71.0 | 2.22 | 32.1 | 0.000 | 66.7 to 75.3 |
| Discounted costs (£) | |||||
| Intervention effect | –1440 | 589 | –2.45 | 0.014 | –2600 to –287 |
| BMI (kg/m2) | |||||
| > 30 | –248 | 969 | –0.260 | 0.798 | –2150 to 1650 |
| > 35 | 719 | 684 | 1.05 | 0.293 | –622 to 2060 |
| > 40 | 2730 | 915 | 2.99 | 0.003 | 938 to 4520 |
| Constant | 25,200 | 2116 | 11.9 | 0.000 | 21,000 to 29,300 |
Summary and initial interpretation of results
The aim of the FFIT follow-up study economic evaluation (see Chapter 1, Aims and objectives) was to establish the cost-effectiveness of the FFIT programme over the medium and longer term. In this section, we discuss our results in relation to the three subobjectives that related to this aim.
Subobjectives
Subobjective 1: the impact that sustained changes have had as a result of taking part in Football Fans in Training on the cost-effectiveness of the programme at 3.5 years
The medium-term (3.5 year) analysis found similar cost-effective results to those of the FFIT RCT,44 and these results were well below the willingness-to-pay threshold range of £20,000–30,000 per QALY used by NICE. 76 In the follow-up study, the FFIT programme was associated an additional cost of £532–740 per individual compared with no active intervention. The results also indicate that the FFIT programme is more effective than no active intervention, with a gain of 0.046–0.051 QALYs over 3.5 years. As a result, the FFIT programme is associated with an incremental cost-effectiveness of between £10,700 and £15,300 per QALY gained.
In the scenarios that model a population weight gain trajectory (base case and scenarios 2 and 4; i.e. 0.46 kg per year), the participants in the hypothetical control scenarios gain as much weight as the participants in the scenarios that model the FFIT-FU-I group weight gain trajectory (scenarios 1, 3 and 5; i.e. 1.04 kg per year). The FFIT programme is therefore found to be slightly less cost-effective (but negligibly so in terms of decision-making) in the scenarios modelling the population trajectories.
Subobjective 2: updating the modelling of the longer-term health outcomes and resource use assessing the potential for cost-effectiveness
In the follow-up study lifetime analysis, the FFIT programme was associated with an incremental cost-effectiveness of around £2000 per QALY gained, with no uncertainty that the FFIT programme is cost-effective compared with a no active intervention for any value of the cost-effectiveness threshold beyond approximately £2500 per QALY. Therefore, if the decision-maker is willing to pay more than this value, there is no uncertainty that the FFIT programme is cost-effective.
Assumptions about the duration of the effect of the intervention are a key driver of cost-effectiveness, and we undertook a scenario analysis limiting the treatment effect of the FFIT programme to 5.5 years. The FFIT programme remained cost-effective, although there was a fall in the additional cost per QALY associated with the programme. This result reflects the fact that restricting the period over which behaviour change is sustained has a greater impact on the QALYs associated with the FFIT programme than on the QALYs associated with no active intervention (reducing the incremental outcomes associated with the FFIT programme). In addition, the reduction in QALYs has a greater impact on the lifetime costs associated with the FFIT programme than on the cost of no active intervention (reducing the incremental costs associated with the FFIT programme).
Subobjective 3: heterogeneity of the cost-effectiveness of the Football Fans in Training programme
As the predictor analyses conducted as part of the outcome analyses (see Chapter 3, Baseline predictors of long-term weight loss) did not show any baseline predictors of weight loss, we took the practical decision of basing the cost-effectiveness heterogeneity analyses on BMI, one of the main eligibility criteria for taking part in the FFIT programme. This analysis suggests that participation in the FFIT programme has a greater impact on health outcomes for men with a BMI of > 35 kg/m2, and that, although the FFIT programme is highly cost-effective overall, it becomes cost saving for men with a BMI of > 40 kg/m2.
Chapter 6 Data linkage utility and feasibility
Introduction
Routinely collected health records are increasingly used to support health research, both in undertaking observational studies and in supporting RCTs. 95–98 Scotland has created a national research platform for the provision of electronic patient records to approved research projects99 as part of an initiative from the Medical Research Council and other major funders in the UK to create a network of centres of excellence in research using e-health records. Previous work has suggested that routinely collected data are an accurate replacement for cardiovascular end points in clinical trials compared with rigorously collected clinical trial data. 100
The FFIT RCT showed that the FFIT programme helped men lose weight, become more active, improve their diet and maintain these changes to 12 months. 43,44 However, nothing was known about the impact that the programme had on clinical health outcomes. To address this gap, the FFIT follow-up study explored the utility and feasibility of using linkage to routine NHS data sets to assess participants’ clinical health outcomes. The specific aim of the ‘data linkage’ work was to investigate the potential for long-term, low-cost, passive follow-up through linkage to routinely collected NHS data.
First, in relation to the utility of data linkage (see Utility of data linkage), we aimed to investigate long-term clinical health outcomes (through linkage data on hospitalisations, mortality, prescribing, cancers, diabetes and, when possible, blood test results) of RCT participants, and the extent to which these were associated with long-term weight loss and behaviour change. Second, in relation to the feasibility of data linkage (see Feasibility of data linkage), we aimed to investigate the extent to which men enrolling in current routine deliveries of the FFIT programme were prepared to give permission for the transfer of their programme data (as collected by coaches) to the research team, and to agree to the linkage to their NHS records.
Utility of data linkage
Introduction
In August 2012, the 688 men who took part in the RCT 12-month measurements43,44 were asked if they would give consent for the research team to access their NHS records for future data linkage. Most men (648/688, 94%) gave consent; this equates to 87% (648/747) of the original FFIT RCT cohort. In the FFIT follow-up study, we aimed to apply to access these men’s NHS records to explore:
-
the proportion of consenting men who could be followed up through data linkage
-
the long-term clinical health outcomes from hospitalisations, mortality, prescribing, cancers, diabetes and blood test results
-
the extent to which these outcomes were associated with objectively measured change in weight (from baseline to 3.5 years) and self-reported long-standing illnesses at 3.5 years (to test the validity of our self-report data).
Methods
Definition of time periods for data linkage comparisons
In autumn 2015, we requested data to be extracted from the beginning of January 2009 to the most recent data available from each database. The period covered by the data linkage study was divided into different time periods to allow comparisons between the RCT intervention and comparison groups at various points in time, and across different periods of time for both groups combined. The four time periods (periods A–D) are shown in Figure 8 and defined as follows.
-
Period A: the 12 months before the intervention group started the FFIT programme (i.e. 12 months before the RCT baseline measurements). The start date of period A was 365 days prior to the randomisation date for each man.
-
Period B: the 12 months during which participants were involved in the RCT. For the intervention group, this included the period during which they took part in the FFIT programme; for the comparison group, this was the 12-month period prior to being offered the opportunity to take part in the FFIT programme. The start date of period B was the randomisation date for each participant, with the end date being 365 days later.
-
Period C: the 2.5-year period between the end of the RCT and the 3.5-year follow-up measures. At the beginning of this period, participants in the comparison group were all offered a place on the FFIT programme. The start date for period C for each participant corresponds to the end date of period B.
-
Period D: the 12 months during which participants in the comparison group were given the opportunity to take part in the FFIT programme (i.e. during the first 4 months of period C). Period D allowed comparison with period B in the intervention group. The start date of period D for each man was the autumn 2012 programme start date at the football club where he attended the FFIT programme.
FIGURE 8.
Data linkage time periods. a, FFIT programme delivery period.
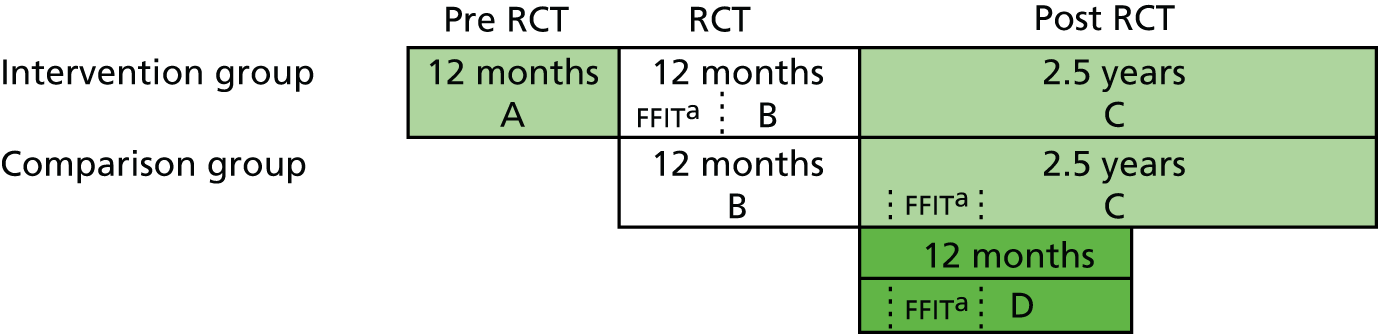
Frequencies of events and percentages of participants admitted to hospital and using medications were then reported according to the following comparisons.
-
Pre-FFIT programme delivery: compared the intervention group during period A with the comparison group during period B
-
During FFIT programme delivery: compared the intervention group during period B with the comparison group during period D
-
Long-term follow-up: compared the intervention group with the comparison group during period C
-
During FFIT RCT: compared the intervention group with the comparison group during period B
-
Pre-FFIT programme versus FFIT programme: the clinical health outcomes for the intervention group during period A and the comparison group during period B were combined and compared with the combined clinical health outcomes for the intervention group during period B and the comparison group during period D.
Data management
The RCT database was archived at the Robertson Centre for Biostatistics (RCB) at the University of Glasgow. RCB information security (IS) managers submitted participant names, gender, dates of birth, addresses and RCT ID numbers securely to a named contact at the electronic Data Research and Innovation Service (eDRIS: www.isdscotland.org/Products-and-Services/EDRIS) using a previously established secure file transfer process. The identifiable data were used at eDRIS to index each participant to their Community Health Index (CHI) number to allow the extraction of records on hospital episodes [Scottish Morbidity Records (SMR): SMR01 and SMR04], deaths and recorded cause of death (National Records of Scotland, Vital Events – Deaths),101 cancers (SMR06) and prescribing [Prescribing Information System (PIS)]. The extracted data had all identifiers replaced by ID numbers and were then transferred back to a named IS manager at RCB, as before. RCB IS managers linked the data to the RCT database using participants’ RCT ID numbers. The new linked data set was made available to the project statistician for analysis within a secured network.
NHS research and development (R&D) approval for the data linkage utility study was obtained from Greater Glasgow and Clyde Health Board (reference number GN15DI154). Further approval for linkage and use of routinely collected clinical data was obtained from the Public Benefit and Privacy Panel for Health and Social Care (reference number 1516-0215).
Preparation of linked data variables for analysis
Records from the SMR01, SMR04, SMR06 and recorded cause of death were classified using International Classification of Diseases, Tenth Edition (ICD-10) codes102 and Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures version 4 (OPCS-4) codes. 103 Prescribing data were classified using BNF categorisations,85 and other data sets used local coding schemes.
Analysis
Clinical outcomes were reported only for the participants who had consented to data linkage at the RCT 12-month measurements, with percentages of those who could be followed up through data linkage reported overall and by group (intervention and comparison groups) also summarised. Numbers of hospital admissions, deaths, cancer incidence and prescriptions were summarised for all participants who consented to data linkage, and also by group, as frequencies of events and percentages of participants. Continuous variables were summarised by the number of observations, number of missing values, mean and SD. Categorical variables were summarised by the number of observations, the number of missing values and the number and percentage of individuals in each category.
Formal group and time period comparisons were made using two-sample or paired t-tests or non-parametric equivalents, as appropriate, for:
-
number of hospital admissions
-
overall
-
by ICD-10 group (i.e. the first letter of the ICD-10 code) of primary diagnosis
-
-
number of prescriptions
-
overall
-
by BNF chapter (first-level classification) to reflect broad disease categories (e.g. CVD)
-
total number of BNF paragraphs (third-level classification) per participant to provide an indication of number of different conditions being treated
-
for specific medications, including antidepressants (BNF section 4.3), pain relief (BNF subsections 4.7.1, 4.7.2, 4.7.3), statins and other cholesterol-lowering drugs (BNF section 2.12), diabetes medications (BNF section 6.1), non-steroidal anti-inflammatory drugs (BNF subsection 4.7.1), aspirin (BNF approved name aspirin), hypertensives (BNF subsections 2.5.1, 2.5.2, 2.5.3, 2.5.4) and hypnotics and anxiolytics (BNF section 4.1).
-
The following outcomes were summarised overall and by RCT group, but no formal comparisons were performed owing to small numbers of:
-
deaths
-
cancer incidences
-
psychiatric hospital admissions
-
strokes or myocardial infarctions.
Mixed-effects regression models, with a random effect for football club and a fixed effect for RCT group, assessed the relationship between hospitalisations and prescriptions (yes vs. no) as the clinical outcome, and the following explanatory variables measured from the start date of time period B to the end date of time period C:
-
weight at RCT baseline
-
change in weight from baseline to 3.5 years
-
self-reported number of long-standing illnesses at 3.5 years.
All mixed-effects regression models were reported as relative risks or odds ratios, as appropriate, with corresponding 95% CIs and p-values.
Results
Proportion of consented men followed up through data linkage
As shown in Table 31, indexing was successfully completed for 645 men: 86.3% of the original RCT population and 99.5% of the data linkage consented group. Indexing was successful for more men in the comparison group (89.8%) than in the intervention group (82.9%), but this was mainly a result of the greater loss to follow-up at 12 months in the FFIT RCT intervention group.
| Population | Group, n (%) | ||
|---|---|---|---|
| FFIT RCT cohort | Intervention | Comparison | |
| RCT population | n = 747 | n = 374 | n = 373 |
| Data linkage consented population | 648 (86.7) | 312 (83.4) | 336 (90.1) |
| Data linkage analysis population | 645 (86.3) | 310 (82.9) | 335 (89.8) |
| Linkage not possible | 102 (13.7) | 64 (17.1) | 38 (10.2) |
| RCT 12-month measures population | n = 688 | n = 333 | n = 355 |
| Data linkage consented population | 648 (94.2) | 312 (93.7) | 336 (94.6) |
| Data linkage analysis population | 645 (93.8) | 310 (93.1) | 335 (94.4) |
| Linkage not possible | 43 (6.3) | 23 (6.9) | 20 (5.6) |
| Data linkage consented population | n = 648 | n = 312 | n = 336 |
| Data linkage analysis population | 645 (99.5) | 310 (99.4) | 335 (99.7) |
| Linkage not possible | 3 (0.5) | 2 (0.6) | 1 (0.3) |
Long-term clinical health outcomes
As shown in Table 32, linkage was performed for the 645 indexed men to hospital episode statistics, cancer registry, the PIS and National Records of Scotland death records. We had hoped to also link to diabetes care records and blood test laboratory results, but these were still unavailable at the time of the follow-up study. Owing to the small numbers of recorded deaths, cancer registrations and admissions to a psychiatric unit, statistical comparisons between the groups and across different time periods were restricted to general hospital admissions (SMR01) and prescribing (PIS) records.
| RCT DL cohort (N = 645) | DL intervention (N = 310) | DL comparison (N = 335) | ||||
|---|---|---|---|---|---|---|
| Number of linked participants (%) | Number of records | Number of linked participants (%) | Number of records | Number of linked participants (%) | Number of records | |
| Hospital admissions | ||||||
| SMR01 (general hospital admissions) | 211 (32.7) | 418 | 105 (33.9) | 228 | 106 (31.6) | 190 |
| SMR04 (psychiatric unit admissions) | 4 (0.6) | 6 | 2 (0.6) | 3 | 2 (0.6) | 3 |
| Cancer registry | 9 (1.4) | 9 | 5 (1.6) | 5 | 4 (1.2) | 4 |
| PIS prescribing | 610 (94.6) | 35,704 | 292 (94.2) | 17,768 | 318 (94.9) | 17,936 |
| National Records of Scotland death certificates | 1 | – | 0 | – | 1 | – |
General hospital admissions
General hospital admissions were categorised into broad subject groups using the ICD-10 code for the main reason for admission (e.g. codes starting with C are classed as neoplasms) (Table 33) and then split into different time periods (as described in Definition of time periods for data linkage comparisons). There were no differences between the intervention and the comparison group in general hospital admissions within any time period, or within the whole RCT cohort before and during the FFIT programme (see tables i–iv, Report Supplementary Material 14).
| RCT DL cohort (N = 645) | DL intervention (N = 310) | DL comparison (N = 335) | |
|---|---|---|---|
| Total number of admissions | 418 | 228 | 190 |
| Number of participants with ≤ 1 admission | 211 | 105 | 106 |
| Primary diagnosis, n (%) | |||
| Certain infectious and parasitic diseases | 8 (1.9) | 7 (3.1) | 1 (0.5) |
| Neoplasms | 31 (7.4) | 27 (11.8) | 4 (2.1) |
| Diseases of the blood/blood-forming organs, certain disorders involving the immune mechanism | 2 (0.5) | 0 (0.0) | 2 (1.1) |
| Endocrine, nutritional and metabolic diseases | 12 (2.9) | 1 (0.4) | 11 (5.8) |
| Mental, behavioural and neurodevelopmental disorders | 2 (0.5) | 0 (0.0) | 2 (1.1) |
| Diseases of the: | |||
| Nervous system | 25 (6.0) | 16 (7.0) | 9 (4.7) |
| Eye and adnexa | 12 (2.9) | 6 (2.6) | 6 (3.2) |
| Ear and mastoid process | 1 (0.2) | 0 (0.0) | 1 (0.5) |
| Circulatory system | 45 (10.8) | 26 (11.4) | 19 (10.0) |
| Respiratory system | 16 (3.8) | 8 (3.5) | 8 (4.2) |
| Digestive system | 61 (14.6) | 31 (13.6) | 30 (15.8) |
| Skin and subcutaneous tissue | 12 (2.9) | 7 (3.1) | 5 (2.6) |
| Musculoskeletal system and connective tissue | 52 (12.4) | 25 (11.0) | 27 (14.2) |
| Genitourinary system | 29 (6.9) | 15 (6.6) | 14 (7.4) |
| Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified | 74 (17.7) | 38 (16.7) | 36 (18.9) |
| Injury, poisoning and certain other consequences of external causes | 18 (4.3) | 12 (5.3) | 6 (3.2) |
| Factors influencing health status and contact with health services | 18 (4.3) | 9 (3.9) | 9 (4.7) |
Prescriptions
Prescriptions across all time periods were categorised using BNF chapters (Table 34) and split into the different time periods. Table 35 shows that, overall, men in the comparison group received more prescriptions in all time periods than those in the intervention group, but it suggests that this difference was mainly because individual men in the comparison group had more prescriptions. There were no differences in prescribing before and during the FFIT programme delivery period (see table v, Report Supplementary Material 14).
| RCT DL cohort (N = 645) | DL intervention (N = 310) | DL comparison (N = 335) | |
|---|---|---|---|
| Total prescriptions | 35,704 | 17,768 | 17,936 |
| Number of participants with at least one prescription | 610 | 292 | 318 |
| BNF chapter | |||
| 1. Gastrointestinal system | 2654 (7.4) | 1484 (8.4) | 1170 (6.5) |
| 2. Cardiovascular system | 12,174 (34.1) | 6216 (35.0) | 5958 (33.2) |
| 3. Respiratory system | 2542 (7.1) | 1182 (6.7) | 1360 (7.6) |
| 4. Central nervous system | 6120 (17.1) | 2763 (15.6) | 3357 (18.7) |
| 5. Infections | 1339 (3.8) | 635 (3.6) | 704 (3.9) |
| 6. Endocrine system | 3888 (10.9) | 1923 (10.8) | 1965 (11.0) |
| 7. Obstetrics, gynaecology and urinary tract disorders | 1184 (3.3) | 564 (3.2) | 620 (3.5) |
| 8. Malignant disease and immunosuppression | 103 (0.3) | 14 (0.1) | 89 (0.5) |
| 9. Nutrition and blood | 346 (1.0) | 253 (1.4) | 93 (0.5) |
| 10. Musculoskeletal and joint diseases | 2035 (5.7) | 1053 (5.9) | 982 (5.5) |
| 11. Eye | 338 (0.9) | 176 (1.0) | 162 (0.9) |
| 12. Ear, nose and oropharynx | 609 (1.7) | 237 (1.3) | 372 (2.1) |
| 13. Skin | 1446 (4.0) | 811 (4.6) | 635 (3.5) |
| 14. Immunological products and vaccines | 51 (0.1) | 32 (0.2) | 19 (0.1) |
| 15. Anaesthesia | 24 (0.1) | 6 (0.0) | 18 (0.1) |
| Others | 851 (2.4) | 419 (2.4) | 432 (2.4) |
| Variable | Data linkage analysis population | ||
|---|---|---|---|
| FFIT RCT cohort (N = 645) | Intervention (N = 310) | Comparison (N = 335) | |
| Total number of prescriptions | |||
| Pre-FFIT programme (IG period A vs. CG period B) | 8047 | 3379 | 4668 |
| During FFIT programme (IG period B vs. CG period D) | 8750 | 3692 | 5058 |
| Long-term follow-up (IG period C vs. CG period C) | 23,965 | 10,697 | 13,268 |
| During FFIT RCT (IG period B vs. CG period B) | 8360 | 3692 | 4668 |
| Number of participants with ≥ 1 prescription | |||
| Pre-FFIT programme (IG period A vs. CG period B) | 522 | 241 | 281 |
| During FFIT programme (IG period B vs. CG period D) | 513 | 241 | 272 |
| Long-term follow-up (IG period C vs. CG period C) | 574 | 269 | 305 |
| During FFIT RCT (IG period B vs. CG period B) | 522 | 241 | 281 |
Participants in the comparison group were more likely to have received at least one prescription than those in the intervention group in the 12 months prior to commencing the FFIT programme (83.9% vs. 77.7%; p = 0.0474), and during the RCT (83.9% vs. 77.7%; p = 0.0474). On average, the comparison group also received significantly more prescriptions than the intervention group in the 12 months prior to commencing the FFIT programme (comparison group, mean 13.9, SD 17.6; intervention group, mean 10.9, SD 14.6; p = 0.0200), and in the 12-month period during which they took part in the FFIT programme (comparison group, mean 15.1, SD 19.0; intervention group, mean 11.9, SD 15.9; p = 0.0313). Participants across both groups received more prescriptions in the 12 months during which they took part in the FFIT programme than in the 12 months prior to the FFIT programme (comparison group, mean 13.6, SD 17.6; intervention group, mean 12.5, SD 16.3; p = 0.0025). These differences seemed to be driven by cardiovascular system, urinary tract disorder, and musculoskeletal and joint diseases drugs (BNF chapters 2, 4, 7, and 10: see FFIT tables vi–vii, Report Supplementary Material 14, and tables 7.3.1–7.18.5, Report Supplementary Material 15).
Men in the comparison group were more likely than those in the intervention group to be taking antidepressants both before (17.9% vs. 11.6%; p = 0.0248) and during the FFIT RCT (17.9% vs. 12.3%; p = 0.0457). They were also more likely to be taking non-steroidal anti-inflammatory drugs in the 12 months prior to the RCT (17.9% vs. 12.3%; p = 0.0457). Across all participants who consented to data linkage, there was an increase in the average number of prescriptions per participant for statins or other cholesterol medications in the 12 months during which they took part in the FFIT programme compared with the 12 months before their participation in FFIT programme (prior to the FFIT programme, mean 1.0, SD 2.4; during the FFIT programme, mean 0.9, SD 2.1; p < 0.0001). Aspirin use showed the same pattern (prior to the FFIT programme, mean 0.4, SD 1.5; during the FFIT programme, mean 0.3, SD 1.3; p = 0.0338).
Associations between clinical health outcomes, weight and long-standing illnesses
The relationships between hospitalisations and prescriptions in the 3.5 years after the start of the FFIT RCT, and baseline weight, change in weight and number of long-standing illnesses at 3.5 years are shown in Table 36. Only the number of long-standing illnesses self-reported at 3.5 years was related to admittance to hospital and receiving a prescription.
| Explanatory variables | Odds ratio (95% CI) | p-value |
|---|---|---|
| Any admission to hospital | ||
| Baseline weight (kg) | 0.998 (0.989 to 1.008) | 0.753 |
| Change in weight from baseline to 3.5 years (kg) | 1.011 (0.988 to 1.035) | 0.340 |
| Number of long-standing Illnesses at 3.5 years | 1.615 (1.318 to 1.980) | < 0.001 |
| Any prescriptions | ||
| Baseline weight (kg) | 1.004 (0.990 to 1.023) | 0.677 |
| Change in weight from baseline to 3.5 years (kg) | 1.009 (0.970 to 1.049) | 0.661 |
| Number of long-standing illnesses at 3.5 years | 1.932 (1.117 to 3.343) | 0.019 |
Feasibility of data linkage
Introduction
The FFIT programme has now been delivered to almost 4500 men in Scotland, and the Scottish Government is currently funding further deliveries in 33 SPFL clubs. An opportunity therefore exists to build a cohort of men who will take part in future deliveries of the FFIT programme for long-term passive follow-up of health outcomes via linkage to routine NHS data sets. To test the feasibility of this, we aimed to investigate the extent to which men enrolling in routine implementation deliveries of the FFIT programme in 2015 were prepared to give permission for the transfer of their baseline and post-programme weight and BMI (as measured by coaches in participating football clubs) to the research team, and to agree to linkage to their NHS records.
Methods
Phase 1: spring 2015
The SPFL Trust identified 25 clubs as being due to start new deliveries of the FFIT programme between January and March 2015. Of these, 20 clubs were selected (on grounds of proximity to Central Scotland) for visits by members of the research team to ask participants for permission for data transfer and data linkage.
The research team aimed to visit the clubs during the early weeks (sessions 1–4) of the FFIT programme to maximise the number of participants in attendance. Once the session began, the researcher provided a brief overview of the rationale of the data linkage study and what this meant for the individual, and gave reassurances about anonymity in relation to men’s NHS records. All men were then asked to give written permission or refusal for future data linkage via the completion of a data linkage permission form. The protocol for phase 1 researcher data linkage visits to football clubs in spring 2015 is provided in Report Supplementary Material 16. Field notes, focusing on the men’s responses and questions asked, were written up electronically as soon as possible after each visit.
The number of men who were approached for data transfer and linkage permissions, as well as the number and percentage who gave permission, were reported overall and by club. A descriptive summary of the field notes was used to inform the development of a data linkage feasibility study coach protocol for coaches to pilot during routine deliveries of the FFIT programme in autumn 2015.
Phases 2a and 2b: autumn 2015
The aim of phase 2 was to test the feasibility of an implementation model (i.e. with the coaches who were delivering the FFIT programme requesting permissions for data transfer and linkage) that could be used in post-research deliveries of the FFIT programme. The SPFL Trust identified 22 clubs that were due to start new deliveries of the FFIT programme between September and December 2015.
Coaches delivering these FFIT programmes were trained by a member of the research team in the new data linkage feasibility study coach protocol (see Report Supplementary Material 16). The training was conducted either in person during the routine 2-day training of new coaches to deliver the FFIT programme (n = 4) or individually by telephone (n = 19). Coaches were asked to return all data (including the session during which permissions were collected and the total number of men registered on the current delivery of the FFIT programme) to the research team by Royal Mail tracked delivery, or courier.
Phase 2a
Delays in starting the FFIT programme at some clubs meant that only 11 out of 22 clubs delivered the FFIT programme in autumn 2015. The remaining clubs planned to deliver the FFIT programme in spring 2016. Researchers attended four of the clubs delivering the FFIT programme in autumn 2015 to observe the coaches delivering the data linkage protocol. Field notes were written up electronically as soon as possible after each session in accordance with an observation pro forma (see Report Supplementary Material 17).
The number of men who were approached for data linkage permissions, as well as the number and percentage who gave permission, were reported overall and by club. A descriptive summary of the observation field notes was used to refine the data linkage feasibility study coach protocol for phase 2b.
Phase 2b
Of the 11 clubs that were due to deliver the FFIT programme in spring 2016, 10 actually did so. Members of the research team attended five of these clubs to observe the delivery of the data linkage protocol. Field notes were written up, and data were summarised and reported as described for phase 2a.
Results
Phase 1
The research team visited 18 out of 20 clubs between January and May 2015 to ask participants in new deliveries of the FFIT programme if they would be willing to provide permission for data transfer and linkage. The remaining two clubs did not deliver the FFIT programme in spring 2015. The visits took place across programme sessions 1–4 as follows: two clubs were visited in session 1, six in session 2, five in session 3 and four in session 4, and one club was visited during the enrolment session (the coaches had informed the research team that this was when session 1 was scheduled). The total number of men in attendance was 297 (range 9–35 men at each club). Of these, 264 (88.9%) agreed to data transfer and linkage. The percentage of men giving permissions ranged from 70.0% to 100.0% across the clubs. (For detail, see table I, Report Supplementary Material 18. )
Common questions noted during early phase 1 visits (e.g. around anonymity and how the NHS records would be identified, what data would be made available to the research team and how the results of any data linkage would be reported) were incorporated into the explanation of the rationale for, and process of, data transfer and linkage for later phase 1 club visits. Within sessions 1–4 of the programme, session 2 appeared to be the optimal time for asking for data transfer and linkage permissions: the men had ‘settled’ into the programme and attendance was high.
Phase 2a
Coaches at 11 clubs asked participants for permission for data transfer and linkage between October and December 2015. Despite being fully trained to ask for permissions in session 2, fewer than half (5/11, 45.5%) did so. Others asked for permissions in session 1 (n = 1), session 3 (n = 1), session 4 (n = 1), session 5 (n = 2) and session 6 (n = 1). The total number of men who completed data linkage permission forms across the 11 clubs was 128 (range 5–19 men at each club), with 117 (91.4%, range 68.4–100.0% men at each club) agreeing to data transfer and linkage. (For detail, see table ii, Report Supplementary Material 18. )
Although coaches had been trained to report the total number of men who were registered on the current delivery of the FFIT programme, only 7 out of 11 (63.6%) did so. This meant that we were unable to gain an accurate picture of the proportion of men registered on the programme who agreed to data linkage and transfer. When coaches did provide information about the number of men registered on the programme, comparison with the number of data linkage permission forms returned suggested that attendance at later sessions was similar to attendance at the earlier sessions.
There was slightly more variation across the clubs in terms of the percentage of men returning completed data linkage permission forms who agreed to data transfer and linkage in phase 2a (range 68.4–100.0% men at each club) in comparison with phase 1 (range 70.0–100.0% men at each club). This variation may indicate some inconsistencies in the way the protocol was delivered. In the four clubs observed, it was clear that, despite having been trained in the data linkage delivery protocol, most of the coaches still lacked confidence in explaining the rationale and process of data linkage, and in answering questions. Often the process was rushed, with a lack of discussion, and it was not clear that the men fully understood what they were being asked to do.
Given the poor compliance with the delivery of the data linkage feasibility study coach protocol in session 2, and the apparently high attendance at some of the later sessions, the decision was taken to delay the introduction of information about data transfer and linkage until session 6 [when it was felt that the men’s relationships with the coach(es) should be established]. In addition, to encourage the men to fully consider the implications of data transfer and linkage before deciding whether or not to give their permission, and to reduce the time pressure on coaches having to deliver the whole process in a single session, men were not asked to complete a data linkage permissions form until session 7. Finally, instead of being a stand-alone document, the data linkage feasibility study coach protocol was incorporated into sessions 6 and 7 of the main FFIT coach delivery notes, to try to increase compliance with the timing of delivery.
Phase 2b
Coaches at 10 clubs asked participants for permission for data transfer and linkage between February and April 2016. Data on the timing of the delivery of the protocol were available for 6 out of 10 clubs, either coach-reported or from researcher observation field notes, and five out of six clubs delivered the information on data transfer and linkage during session 6, as per protocol. The total number of men who completed data linkage permission forms across the 10 clubs was 87 (range 5–17), and 78 of these (89.7%, range 76.9–100% men at each club) agreed to data transfer and linkage. (For detail, see table iii, Report Supplementary Material 18.)
The number of completed data linkage permission forms returned in phase 2b (range 4–17) was smaller than in phase 1 (range 9–35) and phase 2a (range 5–19), which may reflect the fact that the data linkage permission process was conducted over two sessions. This conclusion is supported by variation in the dates on the completed data linkage permission forms in some clubs, suggesting that coaches may have had some problems in getting men to complete their forms after session 6 (i.e. not all did it in session 7, as per protocol).
Observations were timed to coincide with the session in which the coaches informed the men about the data linkage process. In four out of five clubs, this activity took place in session 6. Coaches at the fifth club delivered it in session 3. In one club, session 6 was led by a coach who had not received training in the data linkage protocol, because the main coach (who had been trained) was unavailable. This led to confusion about the materials that were to be handed out, and the men being largely left to learn about the process by reading an information sheet. In three of the clubs, the activity was well delivered, with the coaches raising most (if not all) of the key points, including the fact that agreeing to data transfer and linkage was completely voluntary and would not affect men’s involvement in the FFIT programme. In the club where information on the data linkage process was delivered in session 3, the coach appeared rushed; nevertheless, the men did appear well informed, and the voluntary nature of the process was clearly emphasised. Therefore, it appears that with adequate training, and when the data linkage permissions activity is included in session 6, the coaches are able to deliver it confidently to allow men to be fully informed before deciding whether or not to agree to data linkage. However, asking men to complete permission forms the following week may have contributed to a lower rate of completion than in previous phases.
Summary and initial interpretation of results
The overarching aim of our data linkage work (see Chapter 1, Aims and Objectives) was to explore the utility and feasibility of using data linkage to routinely collected NHS data sets to allow long-term, low-cost, passive follow-up of FFIT participants. In the current section, we summarise our results in relation to our objectives.
Utility of data linkage
We aimed to investigate the long-term clinical health outcomes of RCT participants, and the extent to which these were associated with long-term weight loss and behaviour change. Specifically, we sought to explore:
-
the proportion of consenting men who could be followed up through data linkage
-
the long-term clinical health outcomes from hospitalisations, mortality, prescribing, cancers, diabetes and (when possible) blood test results
-
the extent to which these outcomes were associated with objectively measured change in weight and self-reported long-standing illnesses.
Following up consenting men through data linkage
The data linkage utility study demonstrated that it was highly feasible to follow up participants through data linkage within the Scottish system, with a 99.5% success rate in linking data to routine NHS records for men who provided permission to do so.
Long-term clinical health outcomes
The linkage data showed that high proportions of participants had been admitted to hospital and/or received prescribed medications from 2009 onwards. This finding suggests that the FFIT RCT cohort (or at least those who consented to data linkage) had a number of existing conditions before, during and after their participation in the RCT, confirming (as we reported previously47) that this was a high-risk group of men. We did not detect any significant differences in hospital admissions; however, it is important to note that the RCT power calculations were based on weight loss at 12 months (our primary outcome) and not on hospitalisations or prescriptions.
Nevertheless, there were some differences in prescriptions, with the RCT comparison group (or at least those who consented to data linkage) tending to receive more prescriptions than those in the intervention group. Differences were seen in both the number of men receiving at least one prescription and the number of prescriptions per man at various time points. Men across both groups also received more prescriptions in the 12-month period during which they took part in the FFIT programme than in the 12 months prior to the FFIT programme. Finally, there was some suggestion that, in the 12 months during which men took part in the FFIT programme, they received more preventative cardiovascular (e.g. statins and aspirin), urinary tract, and musculoskeletal and joint medication.
Associations between clinical health outcomes, change in weight and self-reported long-standing illnesses at 3.5 years
Small numbers of reported deaths, cancers, psychiatric hospital admissions, and strokes or myocardial infarctions meant that we were unable to explore associations between these outcomes and weight and long-standing illnesses. For hospital admissions and prescriptions, we did not find any associations with weight. Associations between hospital admissions and prescriptions and self-reported long-standing illnesses confirmed the validity of our self-reported long-standing illnesses data.
Feasibility of data linkage
We aimed to investigate the extent to which men who enrolled in routine deliveries of the FFIT programme in 2015 were prepared to give permission for passive long-term follow-up through transfer of data collected by coaches during the programme to the research team, and for data linkage to their NHS records.
This was trialled in two ways: first, members of the research team attended FFIT sessions to ask for permissions and, second, football club coaches asked participants for permissions as part of the routine delivery of the FFIT programme (which was a more sustainable process for future roll-out). In spring 2015, when members of the research team attended sessions, 89.2% (264/296) of men agreed. The proportions of men who agreed when coaches asked for permissions were similar (autumn 2015, 91.4%, 117/128; spring 2016, 89.7%, 87/78). Observations suggested that (with adequate training) coaches were able to deliver the data linkage permissions activity well in session 6 of the FFIT programme. We had asked the coaches to delay asking the men to complete permissions forms until session 7; however, the relatively small number of completed forms returned (compared with spring and autumn 2015) suggests that this protocol did not work well in practice and should be reviewed in any future roll-out.
Chapter 7 General discussion
Introduction
Rising levels of obesity and its associated health risks remain a major global health challenge. 1 Therefore, there is a clear need to develop interventions that can deliver sustained improvements in weight. One way of doing this is by targeting the health behaviours associated with weight gain, physical inactivity and poor diet at the individual level. Nevertheless, although the BCTs and practical strategies that are effective for short-term weight loss are well described,4–6 longer-term weight loss is less well understood, particularly in men.
In general, men are under-represented in weight loss intervention research: a recent review found that only 27% of participants in randomised trials were men. 104 The FFIT programme was designed to capitalise on the powerful social and psychological connections to the team that ‘being a fan’ creates105 to engage men in weight management through their football club. The 3.5-year follow-up of participants in a RCT designed to evaluate the outcomes of the FFIT programme up to 12 months presents an excellent opportunity to investigate long-term weight outcomes. The RCT comprised 747 men and reported mean weight losses of 5.80 kg (95% CI 5.27 to 6.33 kg) at 12 weeks (immediately post programme) and 5.56 kg (95% CI 4.70 to 6.43 kg) at 12 months in the intervention group, and (adjusted) between-group weight loss differences of 5.18 kg (95% CI 4.35 to 6.00 kg; p < 0.0001) at 12 weeks and 4.94 kg (95% CI 5.94 to 3.95 kg; p < 0.0001) at 12 months, both in favour of the intervention group. Retention at the 12-month measures was 92% (89% in the intervention group; 95% in the comparison group). All attendees at the 12-month measures were asked to consent to long-term follow-up; 97% did so.
In this report we present the findings of the 3.5-year FFIT follow-up study, a longitudinal cohort study that investigated the long-term weight, behavioural and psychological outcomes of RCT participants (both the RCT intervention group, who commenced FFIT in autumn 2011, and the RCT comparison group, who were given the opportunity to take part in FFIT in autumn 2012, although not all did so). We also present the results of a qualitative investigation of men’s experiences of trying to sustain weight loss long term, and a health economic evaluation of the medium-term and lifetime cost-effectiveness of the FFIT programme. Finally, we present the results of an investigation of the potential for and practicality of establishing a system of long-term, low-cost, passive follow-up of men taking part in future routine deliveries of the FFIT programme through linkage to NHS data sets.
Interpretation of results
Sustained improvements in weight, behavioural and psychological outcomes at 3.5 years
The follow-up study showed that men who took part in the FFIT programme during the RCT (the FFIT-FU-I group) retained a significant reduction in weight (2.9 kg from baseline) 3.5 years after the start of their participation in the FFIT programme. Almost one-third of men (32.1%, 75/233) demonstrated a clinically important weight loss (of ≥ 5%3) at 3.5 years in comparison with their baseline weight (although 67.8% did not). The FFIT-FU-I group also showed sustained improvements in waist circumference, percentage body fat, BMI, BP, self-reported PA, diet and alcohol consumption, and measures of psychological and physical well-being.
This level of long-term weight loss is better than reported by a previous weight loss management RCT106 in which, after losing on average 8.5 kg from baseline during a 6-month intervention, 1032 women and men were randomised to an interactive website, one-to-one monthly telephone contact or self-control weight management conditions. At 36 months from enrolment in the study (30 months from randomisation), those in the self-control group (the most similar condition to the FFIT programme) showed a mean (adjusted) reduction of 2.9 kg (SD 0.4) from their initial weight. The FFIT-FU-I group sustained this level of weight loss at 42 months.
Nevertheless, men in the FFIT-FU-I group did show some weight regain (2.59 kg) between the 12-month and 3.5-year measurements (equating to an average weight gain of 1.04 kg per year). Although this is more than estimates of annual weight gain in the general population (around 0.46 kg per year),77 it compares favourably with patterns of regain following participation in weight loss interventions, in which participants typically show a regain of 1–2 kg per year (often more, around 30–35% of weight lost, in the earlier years), with most participants returning to their baseline weight within 3–5 years. 7–14,18
Although there was a decrease in levels of self-reported total and vigorous PA between 12 months and 3.5 years, levels of walking remained stable. At 3.5 years, men were walking an extra 90 minutes (297 MET-minutes) per week more than at baseline. Our qualitative interviews revealed that many men were continuing to walk as part of their normal routine, as they had been encouraged to do during the FFIT programme. Long-term follow-ups of PA interventions are rare;107 therefore, the FFIT follow-up study provides important new evidence of how men’s initial enthusiasm for walking during the FFIT programme, as reported previously,108 has successfully translated into an ongoing behaviour that has become part of many men’s everyday lives.
Our findings also suggest that men were sitting less at 3.5 years than at baseline (by around 30 minutes per day), despite the fact that sedentary behaviour was not an explicit target of the programme. However, caution is required in interpreting this finding, as although the self-report question used to capture sitting time in the International Physical Activity Questionnaire-Short Form has good reliability and acceptable validity against accelerometers in epidemiological studies,109 to our knowledge its sensitivity to detect change in sitting has not been demonstrated.
In relation to diet, the FFIT-FU-I group appeared to be successful in sustaining improvements in intake of fatty and sugary foods and alcohol, and in reductions in portion sizes of cheese and meat from 12 months to 3.5 years. Our qualitative interviews showed that most men (regardless of success in long-term weight control) were continuing to use strategies that they had learned on the FFIT programme to improve their diet. The sugary food score in the modified DINE56 included consumption of biscuits, chocolates, sweets and sugary drinks, and many interviewees described continuing to eat fewer unhealthy snacks and to swap sugary drinks for diet drinks or water at 3.5 years. In the RCT 12-week focus groups, information on portion sizes emerged as a highly valued part of the programme,44 and the 3.5-year interviews indicated that men had adopted their own strategies (e.g. weighing their food or using smaller plates) to continue to limit portion size over the long term. Continued reductions in alcohol consumption appear to have been achieved mainly by limiting or completely cutting out alcoholic drinks when at home; very few men described cutting back on drinking when out socialising.
Finally, there was a decrease in fruit and vegetables consumption between 12 months and 3.5 years. Nevertheless, the FFIT-FU-I group was still eating more fruit and vegetables than at baseline. Fruit and vegetables consumption among Scottish men remains low (on average three portions per day);110 therefore, the modest long-term improvements in fruit and vegetables consumption that result from participation in the FFIT programme are an important finding that is associated with a reduction in risk of premature death (from any cause) of about 20%. 111
Long-term outcomes following ‘routine deliveries’ of Football Fans in Training
Men in the FFIT-FU-I group took part in the FFIT programme during the RCT when its delivery was overseen by the research team, who observed two sessions at each football club, interviewed the coaches and conducted focus groups with participants. Men in the FFIT-FU-C group were offered an opportunity to undertake the programme after the RCT 12-month measures. At this point, responsibility for the oversight, management and ongoing evaluation of the FFIT programme was passed from the research team to the SPFL Trust. The follow-up study has therefore presented an opportunity to investigate the long-term outcomes of men who took part in the first ‘routine deliveries’ of the FFIT programme, which potentially has more ecological validity than the more ‘research-intensive’ programme deliveries that took place during the RCT. At the 3.5-year measures (i.e. 2.5 years after having the chance to start participation in the FFIT programme), the long-term weight, PA, dietary and psychological outcomes of the FFIT-FU-C group were very similar to those of the FFIT-FU-I group. The weight outcomes were also comparable with those in the previous weight loss management RCT described in Sustained improvements in weight, behavioural and psychological outcomes at 3.5 years. 106
These findings demonstrate that there is no reason why the long-term outcomes from ongoing routine deliveries of the FFIT programme should differ from those obtained by men who took part in the programme under research conditions. Indeed, since the end of the RCT, the research team (with funding from the University of Glasgow) has worked closely with the SPFL Trust to develop a sustainable model to support the roll-out of the FFIT programme nationally and internationally. This ‘routine delivery’ model includes standardised 2-day training for new coaches to become accredited to deliver the FFIT programme, with a requirement for existing coaches to attend these sessions to update their skills and knowledge on a regular basis. In addition, the SPFL Trust has appointed a development officer to act as the main point of contact for all football clubs and to quality assure session delivery. Finally, as part of an exclusive licensing agreement between the University of Glasgow and SPFL Trust for the delivery of the FFIT programme, members of SPFL Trust and the core research team meet regularly to review audit data from all clubs.
This ‘routine delivery’ model has now been rolled out to 33 SPFL clubs, and the FFIT programme has already been delivered to almost 4500 men. To date, seven English football clubs have been trained by the SPFL Trust to deliver the FFIT programme and, following similar procedures, 12 football clubs in Germany (funded by German Cancer Aid, through a grant to IFT-Nord). The research team has also worked with the SPFL Trust to adapt the FFIT programme for delivery to women (currently being delivered in 25 SPFL clubs) to satisfy the demand for the programme from female fans and family members of men who have taken part in the FFIT programme.
Factors associated with long-term weight outcomes
The role of physical activity and diet
We did not find any subgroups of men who were more (or less) likely to achieve good long-term weight outcomes on the basis of our prespecified potential baseline predictors (age, BMI, education level, socioeconomic status, marital status, orientation to masculine norms and number of long-standing illnesses) Our mediator analyses suggested that regular PA (including walking and vigorous PA), and conscious dietary regulation (including reductions in fatty and sugar foods, portion sizes and regular mealtimes) and increased intake of fruit and vegetables were all associated with improved long-term weight outcomes. The findings are in line with existing evidence pointing to the role of both PA and dietary restriction in weight loss maintenance,16–18 and confirm the utility of many of the strategies that men were introduced to during the FFIT programme and still talked about using to control their weight in the 3.5-year qualitative interviews. Routinisation and the formation of new (PA and dietary) habits are important for behaviour maintenance;23 however, the men’s accounts suggested that work was often required to maintain new habits. They talked about the importance of planning and organisation to allow them to pursue PA and healthy eating in the face of competing work and family commitments.
Many men also spoke about the role of PA in weight loss and weight regain. For some, PA and weight appeared to be almost inextricably linked, whereas others spoke about a more indirect association (i.e. being more active encouraging them to eat better). Previous studies32,112,113 have highlighted that men are more likely to use PA as a strategy to manage their weight than women. Given the important role of PA in long-term weight control,16,17,19 the promotion of PA (e.g. encouraging the incorporation of walking into daily routine, as in the FFIT programme) may be a particularly useful strategy to improve weight loss maintenance in men.
The mediator analyses also revealed that increased self-reported sitting time was associated with poorer long-term weight outcomes, which is consistent with previous evidence of an association between increased sedentary time and risk of obesity. 114 The provision of information and advice around decreasing sedentary time, alongside increasing PA, may therefore be useful to improve the long-term outcomes of weight management programmes (see Further research).
Finally, given the finding of a positive association between the consumption of fruit and vegetables and long-term weight loss, and the fact that men in the FFIT-FU-I group had reduced their consumption of fruit and vegetables during the follow-up period, programmes aimed at men, who may be more reluctant than women to eat fruit and vegetables,115 should place more emphasis on the importance of finding sustainable ways to increase the intake of fruit and vegetables.
The role of behaviour change techniques
As theoretical accounts of behaviour change maintenance predict,23 regular self-monitoring of weight and a supportive social environment (e.g. through ongoing contact with other men and coaches from the FFIT programme) were associated with lower weight at 3.5 years. Most men had continued some form of monitoring of their weight (either directly, through weighing themselves, or indirectly, often through the fit of their clothes) and many also described continuing to monitor their PA (some, but not many, continuing to use pedometers or alternative strategies, which included being aware of the distance or time spent walking). Some men had adopted new technologies that allowed them to monitor steps taken and other forms of PA (e.g. running and cycling), and some also spoke about using these to monitor diet and calories consumed. A number of men continued to set themselves goals and targets in relation to weight and PA, although sometimes these goals were rather loosely defined. Nevertheless, some men found it useful to set an upper weight threshold that they did not want to cross to help them maintain their weight at a level that was comfortable for them.
The coaches who deliver the FFIT programme are trained to build on the men’s common interest in football and to encourage the formation of a positive group dynamic to allow the men to support each other in making changes to their PA and diet. 116 During the programme (in session 12) the coaches encourage the men to consider how they can meet up once the FFIT programme is finished. In the 3.5-year interviews, men described the importance of continuing to meet up with other FFIT participants in their ongoing efforts to control their weight. However, not all men were able to attend ‘follow-on group’ meetings. Some men complained that there was no such group at their football club, that the meeting times or locations were not convenient or that the activity that the group did (often playing football matches, which some men found too competitive or strenuous) did not suit them. Some of these men attributed their weight regain to their lack of ongoing contact with fellow FFIT participants, but others described finding sources of social support elsewhere, for example through sports club membership, other friends and work colleagues, and family members.
The role of innate psychological needs
Self-determination theory suggests that maintenance of behaviour change requires satisfaction of innate psychological needs for autonomy (associated with the internalisation of regulation), competence to perform behaviours and relatedness to others. 20,21 Our mediator analysis results were consistent with these hypothesised relationships: associations with improved long-term weight outcomes were found for autonomous regulation, internal locus of control, perceived competence for PA and dietary behaviours, and relatedness to other people. In the 3.5-year interviews, men described a range of factors that continued to regulate their behaviours. These included interactions with health professionals, family members and friends (external factors), and more internal factors, such as personal health benefits, avoidance of guilt or embarrassment, and doing things because they valued them (e.g. being able to do more things with their children or grandchildren) or simply because they found them to be enjoyable. A few men articulated their journey from initially wanting to make changes because of external factors, to continuing to manage their weight because they were enjoying the changes they had made. However, accounts of changed identities (or of viewing themselves differently) as a result of the changes that they had made were evident in interviews only with men who had been successful in long-term weight control. This is consistent with self-determination theory, which suggests that this fully integrated regulation is the most stable form of regulation and, therefore, most supportive of maintenance of change.
Our mediator analyses suggest that the type of relatedness with other FFIT participants that is supportive of positive long-term weight outcomes (intimacy) may be qualitatively different from that required from family members (acceptance). Consistent with this, the extent to which they were able to continue meeting up with other FFIT participants seemed important in many men’s accounts of their motivation to maintain their weight loss and lifestyle changes. However, men’s descriptions of maintenance of change and relationships with family members appeared more complex. This echoed findings from observations of non-RCT deliveries of the FFIT programme and post-programme participant focus groups, which suggested that although some men experienced wholehearted support from spouses/partners (characterised in our analysis as ‘facilitative allies’), for their efforts to improve their eating practices, others experienced more muted support (‘detached allies’) and in relatively rare cases felt that their efforts to change were undermined by spouses or partners. 117 Examples of supportive ‘facilitative or detached allies’ and family members ‘undermining change’ were also evident in the 3.5-year interviews, but these extended beyond spouses or partners and eating practices to include other family members (i.e. children) and PA.
Factors that hinder and support positive long-term weight outcomes
Many of the factors that hindered men’s attempts to control their weight long term were similar to those described in the RCT 12-month focus groups. 44 Associations between injury and joint pain and poorer long-term weight in the mediator analyses were further elucidated in the 3.5-year interviews in which men described how various injuries had restricted their PA. Other barriers identified by the men included poor weather, holidays and festivities, and time pressures. Nevertheless, contrary to theoretical accounts, which suggest that stressors that deplete psychological and physical resources undermine maintenance of behaviour change,23 there was no association between life events and long-term weight outcomes in the mediator analyses. One explanation may be that many life events can either disrupt or facilitate men’s efforts to lead a healthy lifestyle. For example, in the interviews, although some men spoke about how changing jobs had a negative impact (e.g. longer hours, moving to a desk job), others described it as an opportunity to improve their lifestyle (e.g. having a more regular working pattern, being on their feet all day). However, the way in which life events were measured in the follow-up study questionnaire was too crude to distinguish between such positive and negative impacts.
Throughout our analysis of the 3.5-year interviews, we systematically noted similarities and differences in accounts between men who appeared to have been successful in controlling their weight long term and those who had not. We found that almost all men had taken on board some of the main messages from the FFIT programme in relation to PA and healthy eating. Many were continuing to walk regularly and/or do other forms of PA. Nevertheless, there was evidence that men who were successful in long-term weight control may have been more ‘committed’ to being physically active than those who were not, with some men (e.g. long-term maintainers) describing setting themselves PA challenges to push themselves further. Likewise, in relation to diet, although men who had not succeeded in maintaining weight loss long term attempted to exercise dietary restriction, they may have experienced more frequent (or more severe) lapses than men who were successful in managing their weight long term. Men who were successful described clearer strategies to avoid slipping back into old (bad) habits, and generally spoke in more positive terms about facing up to (and overcoming) challenges.
It appeared that men who had lost weight before they began the FFIT programme but who had not maintained it (the pre-FFIT achievers) may have been less successful than other groups (including those who regained weight by 3.5 years) in putting into practice the BCTs, and practical PA and healthy eating strategies they had learned during the FFIT programme. One explanation may be that losing weight before FFIT had made them less receptive to some of the programme’s healthy lifestyle messages.
Finally, it was clear from the men’s accounts that even for those who were successful in controlling their weight at 3.5 years, the journey was not always smooth. The delayed responder group (men who had not achieved ≥ 5% weight loss at 12 months but went on to achieve it at 3.5 years) was particularly interesting in this respect. The accounts of some delayed responders suggest that participation in the FFIT programme provided them with tools for weight loss that they were able to put in practice when life circumstances (e.g. a health diagnosis, a change of job or a family wedding) subsequently provided an additional trigger for them to make changes that they were then able to maintain over the long term.
Medium-term and lifetime cost-effectiveness of the Football Fans in Training programme
The medium-term cost-effectiveness analysis demonstrated that the FFIT programme was cost-effective, with an incremental cost of £10,700–15,300 per QALY gained. In the lifetime analysis, the programme was associated with an incremental cost-effectiveness of around £2000 per QALY gained, with no uncertainty that the FFIT programme is cost-effective compared with a no active intervention for any value of the cost-effectiveness threshold beyond approximately £2500 per QALY. Therefore, if the decision-maker is willing to pay more than this value, there is no uncertainty at all that it is cost-effective. These results are comparable with the cost-effectiveness of community-based PA (US$14,000–69,000 per QALY) and behavioural (US$235–30,419 per QALY) interventions found in previous reviews. 118,119
In the medium-term analysis, the results of the hypothetical control scenarios in which the RCT comparison group 12-month data were extrapolated, after excluding the 11% of men who lost ≥ 5% weight at 12 months (scenarios 4 and 5), do not triangulate with the scenarios in which baseline data are extrapolated (base case and scenario 1), as we might have expected. (These scenarios all take into account the fact that the comparison group lost weight during the RCT. 44) This may reflect the fact that the 11% of men in the comparison group who lost weight at 12 months may have achieved this at a greater cost than men in the FFIT-FU-I group. Indeed, we know anecdotally that one man had bariatric surgery, which is associated with greater health-care costs. By removing these men from the hypothetical control scenarios, the incremental cost associated with the FFIT programme increases, making the FFIT programme appear less cost-effective in the medium term. Nevertheless, over the lifetime analysis, these scenarios do show that the FFIT programme has a greater effect in terms of QALYs gained than those scenarios in which 12-month data, including data for the 11% of men who lost weight before the FFIT programme, are extrapolated, as would be expected.
When modelling lifetime cost-effectiveness, cholesterol level is normally used as a variable in risk algorithms, with the impact of weight loss captured indirectly through its influence on cholesterol level and other risk factors (e.g. systolic BP). During the FFIT RCT, we did not measure cholesterol, and this variable (as well as family history of CHD/stroke) had to be imputed from external sources to calculate the ASSIGN score. This limited our ability to produce an accurate risk prediction for each individual. In the follow-up study, we included a question on family history of CHD/stroke, and modified the lifetime CVD Policy model (used in the RCT long-term modelling44) to replace cholesterol with a BMI using the original SHHEC data. However, the literature indicates that cholesterol level is a main predictor of CVD, and a recent article120 suggests that the excess risk of premature death associated with obesity has decreased over the past 40 years. We may, thus, expect to lose some predictive power using BMI as a proxy for cholesterol level. Nevertheless, the fact that we can use data collected from participants, rather than impute variables from different populations as we did in the RCT, strengthens our analyses.
Establishing low-cost, long-term follow-up through linkage to routine NHS data sets
Our data linkage work has demonstrated that it is feasible and worthwhile to establish long-term follow-up of the clinical health outcomes of FFIT participants via linkage to routine NHS data sets. With adequate training, coaches delivering the FFIT programme were able to fully inform participants about the rationale, process and implications of giving permission for data transfer and linkage. The procedure seemed acceptable to men in new deliveries of the FFIT programme in 2015 and 2016; the proportion providing permission was high.
Linkage to NHS data sets for previously consented men from the RCT revealed large numbers of hospital admissions and prescriptions since 2009, and linkage to General Register Office records revealed one death in the comparison group since the end of the RCT. These results confirm that the RCT cohort is a high-risk group of men, as we reported previously. 47 Information about psychiatric hospital admissions, stroke or myocardial infarction and cancer diagnoses was limited, reflecting the relatively low incidence of these events and the short follow-up period.
Strengths and limitations
The FFIT follow-up study has a number of strengths and some limitations. One of the major strengths is that our intensive retention strategies allowed us to complete follow-up at 3.5 years for 73% (488/665) of men who had consented to future contact at the RCT 12-month measurements. There were some differences between men who were followed up and those who were not, with the latter appearing to have less stability in their lives (i.e. being less likely to be in full-time employment and to be home owners) as well as having a higher baseline weight. Although we have no information about the long-term weight outcomes of these men, there was no difference in the 12-month weight outcomes between the men who were and the men who were not lost to follow-up, and sensitivity analyses conducted to account for loss to follow-up revealed a similar pattern of results as the main weight outcome analyses. In addition, a small number of men (37/488, 7.6%) provided weight-only data at 3.5 years (34 of whom provided self-reported weight). The removal of these 37 men from the 3.5-year weight outcome analysis did not significantly change the results [mean weight loss for FFIT-FU-I (weight-only) 3.02 kg, 95% CI 1.86 to 4.18 kg, compared with mean weight loss for FFIT-FU-I (all men) 2.90 kg, 95% CI 1.78 to 4.02 kg; and mean weight loss for FFIT-FU-C (weight-only) 2.80 kg, 95% CI 1.70 to 3.90 kg, compared with mean weight loss for FFIT-FU-C (all men) 2.71 kg, 95% CI 1.65 to 3.77 kg].
As in the FFIT RCT, almost all (99.0%) participants in the total followed-up cohort self-reported as white, reflecting the low ethnic diversity in many parts of Scotland. Ongoing research in four European countries [the European Fans in Training (EuroFIT) project;121 see Further research] will demonstrate whether or not the professional football club setting in other countries can attract more ethnic diversity to healthy lifestyle programmes for men.
As men in the RCT comparison group took part in the FFIT programme after the end of the RCT, we were unable to collect any data on their programme attendance, or short- (12-week) and medium-term (12-month) outcomes. This means that we lack important information about their long-term weight trajectories. Nevertheless, the fact that this group were involved in non-research deliveries means that we have valuable information on the long-term outcomes of men who take part in the FFIT programme under routine delivery conditions. The fact that their 3.5-year outcomes (i.e. 2.5 years after they had the opportunity to take part in the FFIT programme) were very similar to the 3.5-year outcomes of men who took part in FFIT at a time when the research team was heavily involved in FFIT programme deliveries provides ecological validity to our findings. The routine delivery model has been further enhanced in the period since the comparison group undertook the FFIT programme, and now includes standardised training, coach accreditation and quality assurance protocols. There is, therefore, no reason to believe that men taking part in future routine deliveries of the FFIT programme should not experience similar long-term outcomes to those described in this report.
Although the study clearly demonstrated that the FFIT programme helps men achieve long-term improvements in weight and other outcomes, around two out of three participants did not achieve ≥ 5% weight loss at 3.5 years. This suggests that improvements could be made. Our sampling strategy for the 3.5-year interviews allowed us to explore a range of experiences of trying to control weight long term across men who had taken part in the FFIT programme in research and routine deliveries. This work, and our mediator analysis, have allowed us to develop a clearer understanding of the factors that operate to either support or undermine long-term weight loss. This knowledge should be used to inform the development of future programmes aimed at improving long-term maintenance. We return to this in Further research.
The main limitation for the cost-effectiveness analyses was the lack of a ‘no active intervention’ arm at 3.5 years. Nevertheless, we undertook robust and multiple sensitivity analyses by modelling six hypothetical control scenarios with two different weight trajectories. We also acknowledge that NICE prefers the use of the EuroQol-5 dimensions questionnaire122 rather than the SF-1261 to generate health utilities. At the time the RCT was planned in 2010, there was uncertainty about whether or not the EuroQol-5 dimensions questionnaire would be responsive to change in such a ‘healthy’ population. Therefore, the decision was made to use the SF-12 during the RCT and to retain it for consistency during the follow-up study.
The medium-term cost-effectiveness analysis has a number of additional limitations. Not all men in the RCT were followed up. Therefore, we cannot establish the true cost per man who achieved clinically significant (≥ 5%) weight loss at 3.5 years, as the 3.5-year weight status of some men is unknown. However, in the hypothetical control scenarios, we modelled 3.5-year weight status for all men in the RCT comparison group, and were able to calculate the numbers in each scenario who maintained ≥ 5% weight loss.
Health resource use was self-reported in the FFIT follow-up study, as it had been in the RCT. This reflected the fact that data linkage to primary care, accident and emergency department, and hospital outpatient visit routine NHS data sets was not available at the time of the RCT or follow-up study. Medication use was also self-reported (again as it had been in the RCT); however, patterns of prescription medication use were confirmed using linked data. Furthermore, costing of medications was restricted to GP prescriptions of antidepressants, painkillers, asthma, pain gels/creams, anti-inflammatories and sleeping tablets. Other prescribed and over-the-counter medications were excluded. Nearly all of the medications included in the medium-term analysis are available without prescription. Therefore, the exclusion of over-the-counter medications is a limitation of using the NHS perspective preferred by NICE,76 as opposed to taking a wider societal perspective, which may be appropriate if the FFIT programme is considered to be a public health intervention. 123
There are some limitations associated with lifetime analysis. The studies informing the imputation of systolic BP contained predominantly female participants,7 whereas the participants in this study were male. The study informing the imputation of costs (Counterweight86) used a population that had prior contact with primary care,86 whereas this is not necessarily the case with men who took part in the RCT. The CVD (BMI) Policy model does not take into account other risk factors and outcomes from involvement in the FFIT programme, specifically changes in behaviour. The current study has demonstrated that, at 3.5 years, men still show improvements (over baseline) in their PA, diet and alcohol intake. A reason for not including PA and diet in our modelling is that they are self-reported, and therefore, may be subject to bias. Nevertheless, omitting these positive behavioural changes is likely to mean that our lifetime cost-effectiveness results are conservative. In addition, the CVD Policy model predicts hospitalisations; therefore, less serious events that could be treated in a primary care setting are not explicitly modelled. The result is that the projection of lifetime costs in the model is limited to hospital costs. However, primary care costs may be considerable following a non-fatal event, such as a stroke. Although this limitation has an impact on the estimate of an individual’s total cost, it should not have an impact on the incremental cost unless there is a large disparity in the number of events between the FFIT-FU-I group and the hypothetical control scenarios.
Further research
The FFIT follow-up study has shown that the FFIT programme helps men achieve long-term weight loss and improvements in other physical (e.g. waist circumference, BP) and PA, dietary and psychological outcomes, with almost one-third of the men still weighing ≥ 5% less than their baseline weight at 3.5 years. Nevertheless, this figure can clearly be improved. The mediator analyses and qualitative interviews have allowed us to understand the factors that are important for successful long-term outcomes. Therefore, further research is needed to use this knowledge to optimise the FFIT programme and other programmes to improve the long-term maintenance of weight and behavioural changes. We have used some of the lessons learned from the FFIT programme to develop a new programme, EuroFIT, for male football fans who are overweight or obese. The EuroFIT programme specifically targets sedentary behaviour as well as PA, dietary and weight outcomes and provides even greater support for the development of internalised regulation of behaviour,20,124 and for coping with setbacks and stressful situations. The EuroFIT programme has been evaluated in a RCT (2015–17) in 15 professional football clubs across four European countries (England, the Netherlands, Portugal and Norway), with outcomes, including objective PA, sedentary behaviour and weight, being measured to 12 months. 121
The high costs and participant burden of collecting bloods may compromise the accuracy of health economics models in community-based studies such as the FFIT programme. The development of alternative models that use less invasive variables (e.g. BMI instead of cholesterol level) is needed to improve estimates of the long-term cost-effectiveness of such interventions. Therefore, research is currently being undertaken by the health economics team comparing the time and resource implications involved in the collection of bloods with any (potential loss of) impact on predictive power of future events if cholesterol level is not included in the modelling.
Research recommendations
In terms of future research, further follow-up of the FFIT RCT cohort would enable investigation of whether or not the programme is able to support weight loss in the very long term. During the 3.5-year measurements, men were asked about the acceptability of completing an online questionnaire (instead of a paper questionnaire) in any future follow-up, and over 82% (357/434) said that they would be happy to do so. An online questionnaire could provide significant cost savings during measurements, as smaller fieldwork teams would be required to travel to football stadia to collect objective weight measurements.
In addition, very long-term follow-up of the clinical health outcomes of this cohort and of new men starting future deliveries of the FFIT programme is possible via data linkage to NHS data sets. The development of data linkage capabilities in Scotland over the next few years to include more complete general practice information, which could include BMI measurements, could present other novel and low-cost opportunities for assessing and modelling long-term outcomes. Nevertheless, this would require a suitable ‘synthetic’ comparator group to be identified (e.g. by identifying BMI-, age- and GP-matched control patients, or by using another suitable population group, e.g. from the Scottish Health Survey110). The potential to compare FFIT participants with matched control patients who have had no involvement in the programme, and to examine outcomes via routine data linkage could be very successful in determining the very long-term impact of the FFIT programme.
It must also be recognised that the FFIT programme operates mainly at the individual level, and does not address other (e.g. physical environmental) influences on weight control. Although it is clear that the physical environment does have an impact on levels of obesity,125 more research is needed to improve understanding of the mechanisms and potential differential effects on different population groups in order to develop interventions that can operate effectively to reduce obesity at an environmental level.
In the economic evaluation, the CVD Policy model, and the CVD (BMI) Policy model derived from it, relate to primary prevention only; therefore, all participants reporting a past CVD event were excluded from the cost-effectiveness lifetime analysis. Although it is possible to include men with established CVD through the secondary transition, currently only non-modifiable risk factors affect progression from the non-fatal CVD states to death in the model. This means that transition from the non-fatal CVD states to death will not be affected by the FFIT programme. Changing the secondary transition equations in the model to include modifiable risk factors (e.g. weight, systolic BP) to allow the model to assess secondary, as well as primary, prevention would be a useful further development of the CVD Policy model. Finally, different versions of the model’s predictions (i.e. with and without cholesterol as an input into the risk equations) could be compared with linked data from the FFIT programme as the linked data sets continue to grow in future.
Chapter 8 Conclusions
Rising levels of obesity and associated health risks demand innovative evidence-based interventions to help participants lose weight and maintain this over the long term. The evidence presented in this report shows that a 12-week weight management programme for men (FFIT) delivered by trained community coaches in professional football club stadia was effective in helping men achieve significant improvements in weight, PA, dietary and psychological outcomes 3.5 years after participation in the programme. The finding that similar improvements were achieved by men taking part in routine (non-research) programme deliveries is important for the ongoing implementation of FFIT; there is no reason why similar long-term outcomes should not be achieved by men taking part in future deliveries of the programme.
The report also provides evidence on factors that are associated with long-term weight loss in men, and how men put these into practice in their ongoing efforts to control their weight. They include regular PA, regular mealtimes and dietary restraint, self-monitoring of weight, reading food labels, and ongoing contact with other men and coaches from the FFIT programme. This new knowledge can help to improve the potential of future interventions to achieve lasting weight loss, and improved PA and dietary outcomes.
Our data linkage study suggests that the evidence base on long-term (and very long-term) outcomes following participation in the FFIT programme can be augmented through linkage to NHS records to provide passive, low-cost follow-up. Our results also show that the FFIT programme continues to be cost-effective at 3.5 years, with gains well below the willingness-to-pay threshold range of £20,000–30,000 per QALY used by NICE.
Finally, the continuing involvement of the SPFL Trust and FFIT representatives in the FFIT follow-up study has been essential to ensure that our research has maximum public health impact. Ongoing close collaboration between the research team and the SPFL Trust (which now manages and oversees all deliveries of the FFIT programme worldwide under an exclusive licence model) means that we are uniquely placed to translate our findings into practice, and to undertake future research to improve understanding of the essential components necessary to achieve positive long-term outcomes.
Acknowledgements
We are grateful to the FFIT participants who took part in the research. We warmly thank the football club community manager coaches in the football clubs (Aberdeen FC, Celtic FC, Dundee United FC, Dunfermline Athletic FC, Hamilton Academical FC, Heart of Midlothian FC, Hibernian FC, Inverness Caledonian Thistle FC, Kilmarnock FC, Motherwell FC, Rangers FC, St Johnstone FC and St Mirren FC) who provided facilities for, and attended, the 3.5-year follow-up stadia measurements. We also thank Albion Rovers FC, Alloa Athletic FC, Ayr United FC, Brechin City FC, Clyde FC, Dundee FC, Falkirk FC, Forfar Athletic FC, Greenock Morton FC, Montrose FC, Partick Thistle FC, Queen of the South FC, Queen’s Park FC, Raith Rovers FC, Ross County FC and Stenhousemuir FC, who took part in the data linkage feasibility study.
Our wholehearted thanks are due to members of the Population Health Research Facility at MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, and to the fieldwork team leaders and fieldworkers for their dedicated and careful work on the project. Particular thanks are due to Alan Pollock, who made all the initial contact calls to participants; Lindsay Dalgarno, who assisted with data collection for the follow-up interviews; Leanne Auckland and Greig Logan, who assisted with the data linkage utility observations; Elaine Hindle and Sally Stewart, who closely supported the project manager in the organisation and conduct of the fieldwork; and Doctor of Philosophy (PhD) students in the College of Social Sciences at the University of Glasgow, James Kaufman and Aisha Macgregor, who helped with the quality assurance of the interview transcripts. We also thank staff at the RCB for their help with data entry and data management, and Jenni Watson, Jackie Morris and Sweta Mathur for their support with the administration of the project over its lifetime.
We are grateful to colleagues at the SPFL Trust (Nicky Reid, Derek Allison, Stevie Chalmers and Alison Ballantyne) for their support and input throughout the study, and also thank Shaun Treweek (chairperson) and members of our Study Steering Committee [Martin White (a member of the NIHR Journals Library Board), Peter Craig, Alan Gray (FFIT Coach) and Graham Jamieson (former FFIT participant), Laura Stewart and Tony Rednall] for their valuable advice.
Finally, we thank Professor Hugh Tunstall-Pedoe and Professor Mark Woodward for allowing us access to the SHHEC data in order to adapt the original CVD Policy model for the lifetime analysis of the FFIT follow-up study. Any errors in undertaking analysis of the SHHEC data and modelling are our own.
No funding source had any role in the design or conduct of the study, in the collection, management, analysis or interpretation of the data, or in the preparation, review or approval of the manuscript.
Many people contributed to the development of the FFIT programme. We would particularly like to acknowledge Jim Leishman and colleagues at the Camelon Men’s Health Clinic.
Contributions of authors
Cindy M Gray (Senior Lecturer, Health and Wellbeing), in close collaboration with Kate Hunt and Sally Wyke particularly, and others, as specified below, wrote the original grant application, developed the FFIT programme, designed the RCT and follow-up study measurement schedules and qualitative topic guides, and interpreted the findings. She was chief investigator for the project reported here, project manager and co-applicant on the FFIT RCT, and co-chief investigator on the earlier feasibility pilot study with Sally Wyke and Kate Hunt. She drafted this report with input from other authors, as specified below.
Sally Wyke (Deputy Director, Institute of Health and Wellbeing, Health Social Sciences), in close collaboration with Kate Hunt and Cindy M Gray particularly, and others as specified below, wrote the original grant application, developed the FFIT programme, designed the RCT and follow-up study measurement schedules and qualitative topic guides, and interpreted the quantitative and qualitative findings. She provided detailed critical comments on the full report. She was a grant holder on this project, co-chief investigator with Kate Hunt on the FFIT RCT, and co-chief investigator on the earlier FFIT feasibility pilot study with Kate Hunt and Cindy M Gray.
Rachel Zhang (Statistician, Biostatistics) undertook the statistical analyses and first drafted parts of Chapter 3, Results: outcomes and predictors of long-term weight loss, and Chapter 6, Utility of data linkage. She provided critical comments on these in the full report.
Annie S Anderson (Professor, Nutrition) contributed to the design of the FFIT programme (in particular providing expertise on diet and dietary behaviour change) and the FFIT RCT. She contributed to the original grant application for the follow-up study and provided critical comments on the full report. She was a grant holder on this project, on the RCT and on the earlier FFIT feasibility pilot study.
Sarah Barry (Consultant Biostatistician, Biostatistics) oversaw the statistical input on this project from June 2015 to March 2015. She drafted the outcome and predictor, and data linkage analysis plans, and liaised closely with the chief investigator over the interpretation of the results.
Graham Brennan (Research Associate, Social Sciences) was the follow-up study project manager. He oversaw the day-to-day management of all outcome data collection, and co-led fieldworker training (with Cindy M Gray). He also oversaw the qualitative data collection, conducted interviews, contributed to the thematic analysis, led fieldwork and data collection for the data linkage feasibility study, and first drafted Chapter 2, General methods, and Chapter 6, Feasibility of data linkage.
Andrew Briggs (Professor, Health Economics) was a grant holder and contributed to the original grant application. He oversaw the economic evaluation.
Nicki Boyer (Research Assistant, Health Economics) undertook the economic analysis with Eleanor Grieve, Andrew Briggs and Ciaran Kohli-Lynch and provided critical comments on Chapter 5, Economic evaluation.
Christopher Bunn (Research Associate, Sociology) was a grant holder and contributed to the original grant application. He co-led (with Alice McLean) the qualitative evaluation with support from Cindy M Gray, and first drafted sections of Chapter 4, Men’s experiences of trying to sustain weight loss long term. He provided critical comments on the full report.
Craig Donnachie (Research Fellow, Gender and Health) conducted some of the qualitative interviews and played a lead role in the analysis of the qualitative data. He first drafted sections of Chapter 4, Men’s experiences of trying to sustain weight loss long term, and provided critical comments on the full qualitative chapter.
Eleanor Grieve (Research Fellow, Health Economics) was a grant holder and designed the economic evaluation in the grant application with support from Andrew Briggs. She led the economic analysis with support from Nicki Boyer, Ciaran Kohli-Lynch and Andrew Briggs, and first drafted Chapter 5, Economic evaluation.
Ciaran Kohli-Lynch (PhD Student, Health Economics) developed the CVD (BMI) Policy model and contributed to the economic analysis with Eleanor Grieve, Andrew Briggs and Nicki Boyer.
Suzanne Lloyd (Statistician, Biostatistics) was a grant holder and contributed to the study design and grant application. She led the statistical input on this project with support from Alex McConnachie until June 2015.
Alex McConnachie (Reader, Biostatistics) was a grant holder on this project and contributed to the study design and the original grant application. He oversaw and interpreted the statistical analysis, and provided critical comments on Chapter 2, General methods, and Chapter 3, Results: outcomes and predictors of long-term weight loss.
Colin McCowan (Professor, Health Informatics) was a grant holder on this project and contributed to the design of the data linkage study and the original grant application. He led the data linkage utility study, and oversaw and interpreted the data linkage analysis. He first drafted Chapter 6, Utility of data linkage, and provided critical comments on the full report.
Alice McLean (Investigator Scientist, Gender and Health) was a grant holder and contributed to the original grant application. She co-led the qualitative evaluation, led parts of the analysis (with support from Cindy M Gray), and first drafted sections of the qualitative chapter. She provided critical comments on Chapter 4, Men’s experiences of trying to sustain weight loss long term.
Nanette Mutrie (Professor, Sports Psychology) contributed to the design of the FFIT programme (in particular providing expertise on PA) and the FFIT RCT. She contributed to the original grant application and the interpretation of the statistical analysis, and provided critical comments on the full report, particularly in relation to PA. She was a grant holder on this project, on the FFIT RCT and on the earlier FFIT feasibility pilot study.
Kate Hunt (Professor, Behavioural Sciences and Health), in close collaboration Sally Wyke and Cindy M Gray particularly, and others as specified above, wrote the original grant application, developed the FFIT programme, designed the FFIT RCT and follow-up study measurement schedules and qualitative topic guides, and interpreted the quantitative and qualitative findings. She provided detailed critical comments on this report. She was a grant holder on this project, co-chief investigator with Sally Wyke on the FFIT RCT and co-chief investigator on the earlier FFIT feasibility pilot study with Sally Wyke and Cindy M Gray.
Publications
Bunn C, Wyke S, Gray CM, Maclean A, Hunt K. ‘Coz football is what we all have’: masculinities, practice, performance and effervescence in a gender-sensitised weight-loss and healthy living programme for men. Sociol Health Illn 2016;38:812–28.
Hunt K, on behalf of the FFIT Follow Up Project Team. Football Fans in Training: A Weight Loss and Health Living Programme Delivered to Men aged 35–65 by SPFL Clubs. Follow Up Study. SPFL-Trust All Club meeting, Hampden, Glasgow, UK, 16 March 2016.
Gray CM, Hunt K, Brennan G, Zhang R, Barry S, McConnachie A, et al. Life After FFIT: Do Men in a Weight Management Programme Delivered Through Elite Football Clubs Sustain Change Long Term? International Society of Behavioural Nutrition and Physical Activity, Cape Town, South Africa, 8–11 June 2016.
Gray CM, Hunt K, Wyke S, the FFIT Follow Up Project Team. Life After FFIT: Do Men in a Weight Management Programme Delivered through Professional Football Clubs Sustain Weight Loss, Physical Activity and Dietary Changes Long Term, and How? Scottish Physical Activity Research Connections, Edinburgh, UK, 26 October 2016.
Gray CM, Hunt K, Wyke S, the FFIT Follow Up Project Team. Life After FFIT: Do Men in a Weight Management Programme Delivered Through Professional Football Clubs Sustain Weight Loss, Physical Activity and Dietary Changes Long Term, and How? UK Society for Behavioural Medicine, Cardiff, UK, 1–2 December 2016.
Gray CM, Hunt K, Donnachie C, McLean A, Bunn C, Brennan G, Wyke S, the FFIT Follow Up Project Team. How do Men Sustain Long Term Weight Loss Following a Weight Management Programme Delivered Through Professional Football Clubs? International Society of Behavioural Nutrition and Physical Activity, Victoria, BC, Canada, 7–10 June 2017.
Gray CM, Wyke S, Zhang R, Anderson AS, Barry S, Boyer N, et al. Long-term weight loss trajectories following participation in a randomised controlled trial of a weight management programme for men delivered through professional football clubs: a longitudinal cohort study and economic evaluation. Int J Behav Nutr Phys Act 2018;15:60.
Data sharing statement
Data relating to the FFIT follow-up study may be obtained from the corresponding author on request.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557-67. https://doi.org/10.1016/S0140-6736(10)62037-5.
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815-25. https://doi.org/10.1016/S0140-6736(11)60814-3.
- Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: NICE; 2006.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. https://doi.org/10.1037/a0016136.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-119.
- Dombrowski SU, Sniehotta FF, Avenell A, Johnston M, MacLennan G, Araujo-Soares V. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev 2012;6:7-32. https://doi.org/10.1080/17437199.2010.513298.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8210.
- Ulen CG, Huizinga MM, Beech B, Elasy TA. Weight regain prevention. Clin Diabetes 2008;26:100-13. https://doi.org/10.2337/diaclin.26.3.100.
- MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity 2015;23:7-15. https://doi.org/10.1002/oby.20967.
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;129:S102-38. https://doi.org/10.1161/01.cir.0000437739.71477.ee.
- Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: current status. Health Psychol 2000;19:5-16. https://doi.org/10.1037/0278-6133.19.Suppl1.5.
- Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res 2004;12:151S-62S. https://doi.org/10.1038/oby.2004.282.
- Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 2001;74:579-84. https://doi.org/10.1093/ajcn/74.5.579.
- Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now?. Curr Opin Endocrinol Diabetes Obes 2009;16:347-52. https://doi.org/10.1097/MED.0b013e32832f5a79.
- Penn L, White M, Lindström J, den Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0057143.
- Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs 2009;24:58-80. https://doi.org/10.1097/01.JCN.0000317471.58048.32.
- Ramage S, Farmer A, Eccles KA, McCargar L. Healthy strategies for successful weight loss and weight maintenance: a systematic review. Appl Physiol Nutr Metab 2014;39:1-20. https://doi.org/10.1139/apnm-2013-0026.
- Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ 2014;348. https://doi.org/10.1136/bmj.g2646.
- Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005;6:67-85. https://doi.org/10.1111/j.1467-789X.2005.00170.x.
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000;55:68-7. https://doi.org/10.1037/0003-066X.55.1.68.
- Deci EL, Ryan RM. The ‘what’ and ‘why’ of goal pursuits: human needs and the self-determination of behavior. Psychol Inquiry 2000;11:227-68. https://doi.org/10.1207/S15327965PLI1104_01.
- Anastasiou CA, Fappa E, Karfopoulou E, Gkza A, Yannakoulia M. Weight loss maintenance in relation to locus of control: the MedWeight study. Behav Res Ther 2015;71:40-4. https://doi.org/10.1016/j.brat.2015.05.010.
- Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev 2016;10:277-96. https://doi.org/10.1080/17437199.2016.1151372.
- Patrick K, Calfas KJ, Norman GJ, Rosenberg D, Zabinski MF, Sallis JF, et al. Outcomes of a 12-month web-based intervention for overweight and obese men. Ann Behav Med 2011;42:391-40. https://doi.org/10.1007/s12160-011-9296-7.
- Young MD, Morgan PJ, Plotnikoff RC, Callister R, Collins CE. Effectiveness of male-only weight loss and weight loss maintenance interventions: a systematic review with meta-analysis. Obes Rev 2012;13:393-408. https://doi.org/10.1111/j.1467-789X.2011.00967.x.
- Lovejoy JC, Sainsbury A. Stock Conference 2008 Working Group . Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 2009;10:154-67. https://doi.org/10.1111/j.1467-789X.2008.00529.x.
- Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, et al. Do men develop type 2 diabetes at lower body mass indices than women?. Diabetologia 2011;54:3003-6. https://doi.org/10.1007/s00125-011-2313-3.
- The Scottish Health Survey 2011. Edinburgh: National Statistics Publication for Scotland; 2012.
- Stubbs RJ, Pallister C, Whybrow S, Avery A, Lavin J. Weight outcomes audit for 34,271 adults referred to a primary care/commercial weight management partnership scheme. Obes Facts 2011;4:113-20. https://doi.org/10.1159/000327249.
- Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485-92. https://doi.org/10.1016/S0140-6736(11)61344-5.
- Ross HM, Laws R, Reckless J, Lean M, Counterweight Project T. Evaluation of the Counterweight Programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008;58:548-54. https://doi.org/10.3399/bjgp08X319710.
- Robertson C, Archibald D, Avenell A, Douglas F, Hoddinott P, Boyers D, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18350.
- Kiefer I, Rathmanner T, Kunze M. Eating and dieting differences in men and women. J Mens Health Gender 2005;2:194-201. https://doi.org/10.1016/j.jmhg.2005.04.010.
- Gough B. ‘Real men don’t diet’: an analysis of contemporary newspaper representations of men, food and health. Soc Sci Med 2007;64:326-37. https://doi.org/10.1016/j.socscimed.2006.09.011.
- Gough B, Conner MT. Barriers to healthy eating amongst men: a qualitative analysis. Soc Sci Med 2006;62:387-95. https://doi.org/10.1016/j.socscimed.2005.05.032.
- Bye C, Avery A, Lavin J. Tackling obesity in men – preliminary evaluation of men-only groups within a commercial slimming organization. J Hum Nutr Diet 2005;18:391-4. https://doi.org/10.1111/j.1365-277X.2005.00642.x.
- Pringle A. The growing role of football as a vehicle for interventions in mental health care. J Psychiatr Ment Health Nurs 2009;16:553-7. https://doi.org/10.1111/j.1365-2850.2009.01417.x.
- Duffin C. Match of the day. Nurs Stand 2006;20:22-3. https://doi.org/10.7748/ns.20.36.22.s30.
- Snow T. Footie fans kicked into fitness action. Nurs Stand 2004;19. https://doi.org/10.7748/ns.19.3.9.s24.
- Witty K, White A. Tackling men’s health: implementation of a male health service in a rugby stadium setting. Commun Pract 2011;84:29-32.
- Brady AJ, Perry C, Murdoch DL, McKay G. Sustained benefits of a health project for middle-aged football supporters, at Glasgow Celtic and Glasgow Rangers Football Clubs. Eur Heart J 2010;31:2966-8.
- Wyke S, Hunt K, Gray CM, Fenwick E, Bunn C, Donnan PT, et al. Football Fans in Training (FFIT): a randomised controlled trial of a gender-sensitised weight loss and healthy living programme for men – end of study report. Public Health Res 2015;3.
- Zwolinsky S, McKenna J, Pringle A, Daly-Smith A, Robertson S, White A. Optimizing lifestyles for men regarded as ‘hard-to-reach’ through top-flight football/soccer clubs. Health Educ Res 2013;28:405-13. https://doi.org/10.1093/her/cys108.
- Hunt K, Wyke S, Gray CM, Anderson AS, Brady A, Bunn C, et al. A gender-sensitised weight loss and healthy living programme for overweight and obese men delivered by Scottish Premier League football clubs (FFIT): a pragmatic randomised controlled trial. Lancet 2014;383:1211-21. https://doi.org/10.1016/S0140-6736(13)62420-4.
- Hunt K, Wyke S, Gray C, Bunn C, Singh B, Conrad D, et al. Sports-Based Health Interventions: Case Studies from Around the World. New York, NY: Springer; 2016.
- Wyke S, Hunt K, Gray CM, Team FP. Football Fans in Training (FFIT): a randomized controlled trial of a gender-sensitive weight loss and healthy living programme delivered to men aged 35-65 by Scottish Premier League (SPL) football clubs. Public Health Res 2015;3.
- Gray CM, Hunt K, Mutrie N, Anderson A, Leishman J, Dalgarno L, et al. Football Fans in Training: the development and optimization of an intervention delivered through professional sports clubs to help men lose weight, become more active and adopt healthier eating habits. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-232.
- Hunt K, Gray CM, Maclean A, Smillie S, Bunn C, Wyke S. Do weight management programmes delivered at professional football clubs attract and engage high risk men? A mixed-methods study. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-50.
- Anderson AS, Craigie AM, Caswell S, Treweek S, Stead M, Macleod M, et al. The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ 2014;348. https://doi.org/10.1136/bmj.g1823.
- Waters L, George AS, Chey T, Bauman A. Weight change in control group participants in behavioural weight loss interventions: a systematic review and meta-regression study. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-120.
- So You Want to Lose Weight?. London: British Heart Foundation; 2009.
- Morgan PJ, Lubans DR, Collins CE, Warren JM, Callister R. 12-month outcomes and process evaluation of the SHED-IT RCT: an internet-based weight loss program targeting men. Obesity 2011;19:142-51. https://doi.org/10.1038/oby.2010.119.
- Standard Evaluation Framework for Weight Management Interventions. London: NHS; 2009.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: World Cancer Research Fund/American Institute of Cancer Research; 2007.
- Roe L, Strong C, Whiteside C, Neil A, Mant D. Dietary intervention in primary care: validity of the DINE method for diet assessment. Fam Pract 1994;11:375-81. https://doi.org/10.1093/fampra/11.4.375.
- Food, Portion Sizes. London: Her Majesty’s Stationery Office; 1993.
- Emslie C, Lewars H, Batty GD, Hunt K. Are there gender differences in levels of heavy, binge and problem drinking? Evidence from three generations in the west of Scotland. Public Health 2009;123:12-4. https://doi.org/10.1016/j.puhe.2008.06.001.
- Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS). J Cross-Cultural Psychol 2007;38:227-42. https://doi.org/10.1177/0022022106297301.
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171-8. https://doi.org/10.1016/S0895-4356(98)00109-7.
- Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ Res 2007;22:691-702. https://doi.org/10.1093/her/cyl148.
- Markland D, Hardy L. On the factorial and construct validity of the Intrinsic Motivation Inventory: conceptual and operational concerns. Res Q Exerc Sport 1997;68:20-32. https://doi.org/10.1080/02701367.1997.10608863.
- Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care 1998;21:1644-51. https://doi.org/10.2337/diacare.21.10.1644.
- Richer SF, Vallerand RJ. Construction and validation of the perceived relatedness scale. Rev Eur Psychol App 1998;48:129-38.
- Baldwin AS, Rothman AJ, Hertel AW, Linde JA, Jeffery RW, Finch EA, et al. Specifying the determinants of the initiation and maintenance of behavior change: an examination of self-efficacy, satisfaction, and smoking cessation. Health Psychol 2006;25:626-34. https://doi.org/10.1037/0278-6133.25.5.626.
- University of Dundee . Assign Family History Questionnaire 2008. http://assign-score.com/estimate-the-risk/family-history (accessed 17 November 2016).
- International Physical Activity Questionnaire . IPAQ Scoring Protocol n.d. https://sites.google.com/site/theipaq/scoring-protocol (accessed 17 November 2016).
- Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: the Peri Life Events Scale. J Health Soc Behav 1978;19:205-29. https://doi.org/10.2307/2136536.
- Hamilton CJ, Mahalik JR. Minority stress, masculinity, and social norms predicting gay men’s health risk behaviors. J Counsel Psychol 2009;56:132-41. https://doi.org/10.1037/a0014440.
- Scottish Government . Scottish Index of Multiple Deprivation 2009. www.scotland.gov.uk/Topics/Statistics/SIMD/ (accessed 17 November 2016).
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Corbin J, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Soc 1990;13:3-21. https://doi.org/10.1007/BF00988593.
- Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ 2000;320:114-16. https://doi.org/10.1136/bmj.320.7227.114.
- Shove E, Pantzar M, Watson M. The Dynamics of Social Practice: Everyday Life and How It Changes. London: Sage Publications; 2012.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Freisling H, Pisa PT, Ferrari P, Byrnes G, Moskal A, Dahm CC, et al. Main nutrient patterns are associated with prospective weight change in adults from 10 European countries. Eur J Nutr 2016;55:2093-104. https://doi.org/10.1007/s00394-015-1023-x.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Curtis L. Unit Cost of Health and Social Care 2011 2011. www.pssru.ac.uk/project-pages/unit-costs/2011/index.php (accessed 16 November 2016).
- Curtis L. Unit Cost of Health and Social Care 2015 2015. www.pssru.ac.uk/project-pages/unit-costs/2015/index.php (accessed 16 November 2016).
- Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev 2006;63:217-35. https://doi.org/10.1177/1077558705285298.
- Information Services Division . Information Services Division Scotland n.d. www.isdscotland.org/ (accessed 16 November 2016).
- Reference Costs 2011–12. London: Department of Health and Social Care; 2012.
- Reference Costs 2014–15. London: Department of Health and Social Care; 2012.
- British National Formulary (online). London: BMJ Group and Pharmaceutical Press; n.d.
- Tigbe WW, Briggs AH, Lean ME. A patient-centred approach to estimate total annual healthcare cost by body mass index in the UK Counterweight programme. Int J Obes 2013;37:1135-9. https://doi.org/10.1038/ijo.2012.186.
- McCabe C, Brazier J, Gilks P, Tsuchiya A, Roberts J, O’Hagan A, et al. Using rank data to estimate health state utility models. J Health Econ 2006;25:418-31. https://doi.org/10.1016/j.jhealeco.2005.07.008.
- Kharroubi SA, Brazier JE, Roberts J, O’Hagan A. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ 2007;26:597-612. https://doi.org/10.1016/j.jhealeco.2006.09.002.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Lawson KD, Lewsey JD, Ford I, Fox K, Ritchie LD, Tunstall-Pedoe H, et al. A cardiovascular disease policy model: part 2-preparing for economic evaluation and to assess health inequalities. Open Heart 2016;3. https://doi.org/10.1136/openhrt-2014-000140.
- Lewsey JD, Lawson KD, Ford I, Fox KA, Ritchie LD, Tunstall-Pedoe H, et al. A cardiovascular disease policy model that predicts life expectancy taking into account socioeconomic deprivation. Heart 2015;101:201-8. https://doi.org/10.1136/heartjnl-2014-305637.
- Tunstall-Pedoe H, Woodward M, Tavendale R, A’Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ 1997;315:722-9. https://doi.org/10.1136/bmj.315.7110.722.
- Woodward M, Brindle P, Tunstall-Pedoe H. SIGN group on risk estimation . Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2007;93:172-6. https://doi.org/10.1136/hrt.2006.108167.
- Claxton KP, Sculpher MJ. Using value of information analysis to prioritise health research: some lessons from recent UK experience. PharmacoEconomics 2006;24:1055-68. https://doi.org/10.2165/00019053-200624110-00003.
- Boyle PJ, Feijten P, Feng Z, Hattersley L, Huang Z, Nolan J, et al. Cohort Profile: the Scottish Longitudinal Study (SLS). Int J Epidemiol 2009;38:385-92. https://doi.org/10.1093/ije/dyn087.
- Gray L, Batty GD, Craig P, Stewart C, Whyte B, Finlayson A, et al. Cohort profile: the Scottish health surveys cohort: linkage of study participants to routinely collected records for mortality, hospital discharge, cancer and offspring birth characteristics in three nationwide studies. Int J Epidemiol 2010;39:345-50. https://doi.org/10.1093/ije/dyp155.
- Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM. West of Scotland Coronary Prevention Study Group . Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med 2007;357:1477-86. https://doi.org/10.1056/NEJMoa065994.
- West of Scotland Coronary Prevention Study Group . Computerised record linkage: compared with traditional patient follow-up methods in clinical trials and illustrated in a prospective epidemiological study. J Clin Epidemiol 1995;12:1441-52.
- Pavis S, Morris AD. Unleashing the power of administrative health data: the Scottish model. Public Health Res Pract 2015;25. https://doi.org/10.17061/phrp2541541.
- Barry SJ, Dinnett E, Kean S, Gaw A, Ford I. Are routinely collected NHS administrative records suitable for endpoint identification in clinical trials? Evidence from the West of Scotland Coronary Prevention Study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0075379.
- National Records of Scotland . Vital Events – Deaths n.d. www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/deaths (accessed 18 May 2018).
- International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Geneva: World Health Organization; 1992.
- Classification of Surgical Operations. London: Royal College of General Practitioners; 1992.
- Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity 2012;20:1234-9. https://doi.org/10.1038/oby.2011.140.
- Hirt ER, Clarkson JJ, Kahle LR, Close A. Consumer Behavior Knowledge for Effective Sports and Event Marketing. New York, NY: Routledge; 2011.
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139-48. https://doi.org/10.1001/jama.299.10.1139.
- Denison E, Vist GE, Underland V, Berg RC. Interventions aimed at increasing the level of physical activity by including organised follow-up: a systematic review of effect. BMC Fam Pract 2014;15. https://doi.org/10.1186/1471-2296-15-120.
- Hunt K, McCann C, Gray CM, Mutrie N, Wyke S. ‘You’ve got to walk before you run’. Positive evaluations of a walking programme as part of a gender sensitised weight management programme delivered to men through professional football clubs. Health Psychol 2013;32:57-65. https://doi.org/10.1037/a0029537.
- Rosenberg DE, Bull FC, Marshall AL, Sallis JF, Bauman AE. Assessment of sedentary behavior with the International Physical Activity Questionnaire. J Phys Act Health 2008;5:30-44. https://doi.org/10.1123/jpah.5.s1.s30.
- The Scottish Health Survey 2014. Edinburgh: A National Statistics Publication for Scotland; 2015.
- Khaw KT, Bingham S, Welch A, Luben R, Wareham N, Oakes S, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European prospective investigation into cancer and nutrition. Lancet 2001;357:657-63. https://doi.org/10.1016/S0140-6736(00)04128-3.
- Lewis S, Thomas SL, Hyde J, Castle DJ, Komesaroff PA. A qualitative investigation of obese men’s experiences with their weight. Am J Health Behav 2011;35:458-69. https://doi.org/10.5993/AJHB.35.4.8.
- Tsai SA, Lv N, Xiao L, Ma J. Gender differences in weight-related attitudes and behaviors among overweight and obese adults in the United States. Am J Mens Health 2016;10:389-98. https://doi.org/10.1177/1557988314567223.
- Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. Am J Prev Med 2011;41:207-15. https://doi.org/10.1016/j.amepre.2011.05.004.
- Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Prev Chronic Dis 2008;5.
- Bunn C, Wyke S, Gray CM, Maclean A, Hunt K. ‘Coz football is what we all have’: masculinities, practice, performance and effervescence in a gender-sensitised weight-loss and healthy living programme for men. Sociol Health Illn 2016;38:812-28. https://doi.org/10.1111/1467-9566.12402.
- MacLean A, Hunt K, Gray C, Smillie S, Wyke S. How do men’s female relatives feature in their accounts of changing eating practices during a weight-management programme delivered through professional football clubs?. Int J Mens Health 2014;13:121-38.
- Roux L, Pratt M, Tengs TO, Yore MM, Yanagawa TL, Van Den Bos J, et al. Cost effectiveness of community-based physical activity interventions. Am J Prev Med 2008;35:578-88. https://doi.org/10.1016/j.amepre.2008.06.040.
- Griffiths UK, Anigbogu B, Nanchahal K. Economic evaluations of adult weight management interventions: a systematic literature review focusing on methods used for determining health impacts. Appl Health Econ Health Policy 2012;10:145-62. https://doi.org/10.2165/11599250-000000000-00000.
- Afzal S, Tybjærg-Hansen A, Jensen GB, Nordestgaard BG. Change in body mass index associated with lowest mortality in Denmark, 1976–2013. JAMA 2016;315:1989-96. https://doi.org/10.1001/jama.2016.4666.
- van Nassau F, van der Ploeg HP, Abrahamsen F, Andersen E, Anderson AS, Bosmans JE, et al. Study protocol of European Fans in Training (EuroFIT): a four-country randomised controlled trial of a lifestyle program for men delivered in elite football clubs. BMC Public Health 2016;16. https://doi.org/10.1186/s12889-016-3255-y.
- Brooks R, Rabin R, de Charro F. The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective. Dordrecht: Kluwer; 2003.
- Incorporating Health Economics. Methods for the Development of NICE Public Health Guidance. London: NICE; 2012.
- Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act 2012;9. https://doi.org/10.1186/1479-5868-9-78.
- Government Office for Science . Foresight. Tackling Obesities: Future Choices – Obesogenic Environments – Evidence Review 2007. www.gov.uk/government/uploads/system/uploads/attachment_data/file/295681/07-735-obesogenic-environments-review.pdf (accessed 17 November 2016).
List of abbreviations
- app
- application
- ASSIGN
- ASsessing cardiovascular risk using Scottish Intercollegiate Guidelines Network guidelines
- BCT
- behaviour change technique
- BMI
- body mass index
- BNF
- British National Formulary
- BP
- blood pressure
- CHD
- coronary heart disease
- CHI
- Community Health Index
- CI
- confidence interval
- CMNI-22
- Short Form Conformity to Masculine Norms Inventory-22 items
- CSO
- Chief Scientist Office
- CVD
- cardiovascular disease
- DINE
- Dietary Instrument for Nutrition Education
- eDRIS
- electronic Data Research and Innovation Service
- EuroFIT
- European Fans in Training
- EVPI
- expected value of perfect information
- FFIT
- Football Fans in Training
- FFIT-FU-C
- Football Fans in Training follow-up comparison
- FFIT-FU-I
- Football Fans in Training follow-up intervention
- GP
- general practitioner
- HDL
- high-density lipoprotein
- HRQoL
- health-related quality of life
- ICD-10
- International Classification of Diseases, Tenth Edition
- ID
- identifier
- IQR
- interquartile range
- IS
- information security
- LCS
- Locus of Causality Scale
- MET
- metabolic equivalent of task
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRS
- Need for Relatedness Scale
- PA
- physical activity
- PANAS
- Positive and Negative Affect Schedule
- PCS
- Perceived Competence Scale
- PhD
- Doctor of Philosophy
- PIS
- Prescribing Information System
- QALY
- quality-adjusted life-year
- RCB
- Robertson Centre for Biostatistics
- RCT
- randomised controlled trial
- RSE
- Rosenberg Self-Esteem Scale
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SHHEC
- Scottish Heart Health Extended Cohort
- SMART
- Specific, Measurable, Achievable, Realistic, Time-limited
- SMR
- Scottish Morbidity Records
- SPFL
- Scottish Professional Football League
- TSRQ
- Treatment Self-Regulation Questionnaire
- VOI
- value of information
