Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 12/133/04. The contractual start date was in December 2014. The final report began editorial review in May 2018 and was accepted for publication in October 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Frank Kee was a member of the Public Health Research (PHR) Funding Board 2009–19 and the PHR Prioritisation Group 2016–19. Ruth F Hunter has received a National Institute for Health Research (NIHR) Career Development Fellowship. Ruth F Hunter and Wendy Hardeman have received funding from the NIHR Public Health Research programme separately from the current project grant. Wendy Hardeman has received funding from AbbVie Ltd (North Chicago, IL, USA) for consultancy outside the current project. The Northern Ireland Clinical Trials Unit received funds through the NIHR Public Health Research programme for its involvement in the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Tully et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Ageing and physical activity
Many countries, including the UK, are facing rapid growth in the proportion of the population aged ≥ 65 years. 1 Within the UK, Northern Ireland is projected to have the most rapid increase in the age of its population, with approximately 25% of the population projected to be aged ≥ 65 years by 2041. 2 Ageing is associated with functional decline, reduced quality of life and increased risk of morbidity, disability and mortality. 3 Payette et al. 3 have called for a renewed focus on the prevention of multimorbidity, which is set to double in the next 20 years. In addition, health problems emerge at a younger age in older adults from socioeconomically disadvantaged backgrounds, indicating the need for interventions targeting these individuals. 4
Physically active older adults are at a reduced risk of developing numerous chronic non-communicable diseases,5,6 all-cause mortality,7 poor self-rated health,8 falls9,10 and sarcopenia. 11 In addition to the physical health benefits, regular activity has been associated with improved cognitive function and reduced risk of dementia,12 and higher levels of health-related quality of life. 13 These associated physical and mental health benefits may lead to lower utilisation and cost of health-care services. 14 In addition, lower levels of physical activity are associated with poorer social health, such as increased social isolation (fewer number of interactions with others) and loneliness (feeling of being alone), in adults aged ≥ 65 years. 15,16
Physical activity levels of older adults in the UK
In the UK, it is recommended that older adults undertake at least 150 minutes of moderate-intensity physical activity per week. 17 Despite the possible benefits of being physically active, levels of inactivity increase with age. Two-thirds of adults aged ≥ 65 years are not meeting recommended levels, with significant inequalities in participation rates in people from socioeconomically disadvantaged areas. 18 Declining physical activity levels are a major public health concern in the UK due to the associated health-care costs, estimated to be £0.9B per year. 19 Coupled with the anticipated rise in the number of older adults in the UK and half of current lifetime spending on health care being incurred in old age,17 there is a need to develop effective interventions that promote active ageing.
Physical activity interventions for older adults
Systematic reviews of physical activity interventions for community-dwelling older adults20–23 have demonstrated that medium-term (up to 1 year) effects on physical activity are achievable with interventions that encourage older adults to perform some type of aerobic activity, of which walking is the predominant form. These reviews also highlight that many of the included interventions do not reach the people who would benefit the most. 21,22 Therefore, there is a need to develop interventions that specifically target groups who participate in low levels of physical activity, such as those from socioeconomically disadvantaged communities. These ‘hard-to-reach’ groups have their own unique needs that should be considered in designing an intervention.
The barriers to and motivators for physical activity reported by older adults are different from those in younger people. For older people, poor health and a lack of knowledge of, and belief in, the health benefits of physical activity are most frequently cited as the major barriers to regular participation. 24 Inactive older adults have identified their preference for individually tailored physical activity programmes, which take place outside intimidating settings such as gyms and which avoid the concern of slowing down others in group exercise. 25 Devereux-Fitzgerald et al. 26 recently reviewed the experience of older adults in previous physical activity interventions. Older adults’ doubts about their physical capability, or their need to engage in moderate-intensity physical activity in later life, were addressed through their experience of participating in the physical activity interventions. 26 Devereux-Fitzgerald et al. 26 also identified that older adults cited their enjoyment of social interaction with others in the intervention as a motivation to be physically active.
In addition to addressing individual and social determinants in physical activity interventions, research has demonstrated the influence of neighbourhood environments on supporting physical activity in older adults. Living in an area that is supportive of physical activity (i.e. more ‘walkable’) has been associated with higher levels of physical activity, especially in individuals who also have higher self-efficacy and social support. 27 Although it is not feasible to introduce wide-scale changes in the physical environment within behavioural interventions, previous research has shown the potential of physical activity interventions which seek to encourage the use of existing infrastructure for older adults. 28 Therefore, interventions designed on the basis of the socioecological model that seeks to address multiple levels of influence on physical activity behaviours (including individual, social and environmental factors) may have the potential to deliver sustained changes in physical activity. However, there are few interventions designed to address these multiple levels of influence in community-dwelling older adults.
Peer-led physical activity interventions
Peer-led interventions offer a model that may help older adults overcome many of the barriers to physical activity. Peer-led behaviour change interventions are a common and effective means of encouraging behaviour change, including in physical activity. 29,30 Peer mentors are trained, non-professional individuals, who are similar to the target population (e.g. in age and cultural background) and possess experiential knowledge of the target behaviour. 31,32 Peer mentors offer emotional support, motivation through positive reinforcement and relevant knowledge regarding problem-solving strategies. 33
In previous interventions, peer mentors have delivered skills training, provided advice and feedback, and offered social support. 32 The ‘motivational’ peer mentor is therefore an important source of social influence in interventions, addressing behavioural determinants such as self-efficacy, perceived competency to be active and self-determination. 32 However, most of the previous peer-led physical activity interventions did not employ a theoretical framework in their design phase, making it difficult to understand the potential mechanisms through which these interventions may have worked. 32
Aims of the ‘Walk with Me’ project
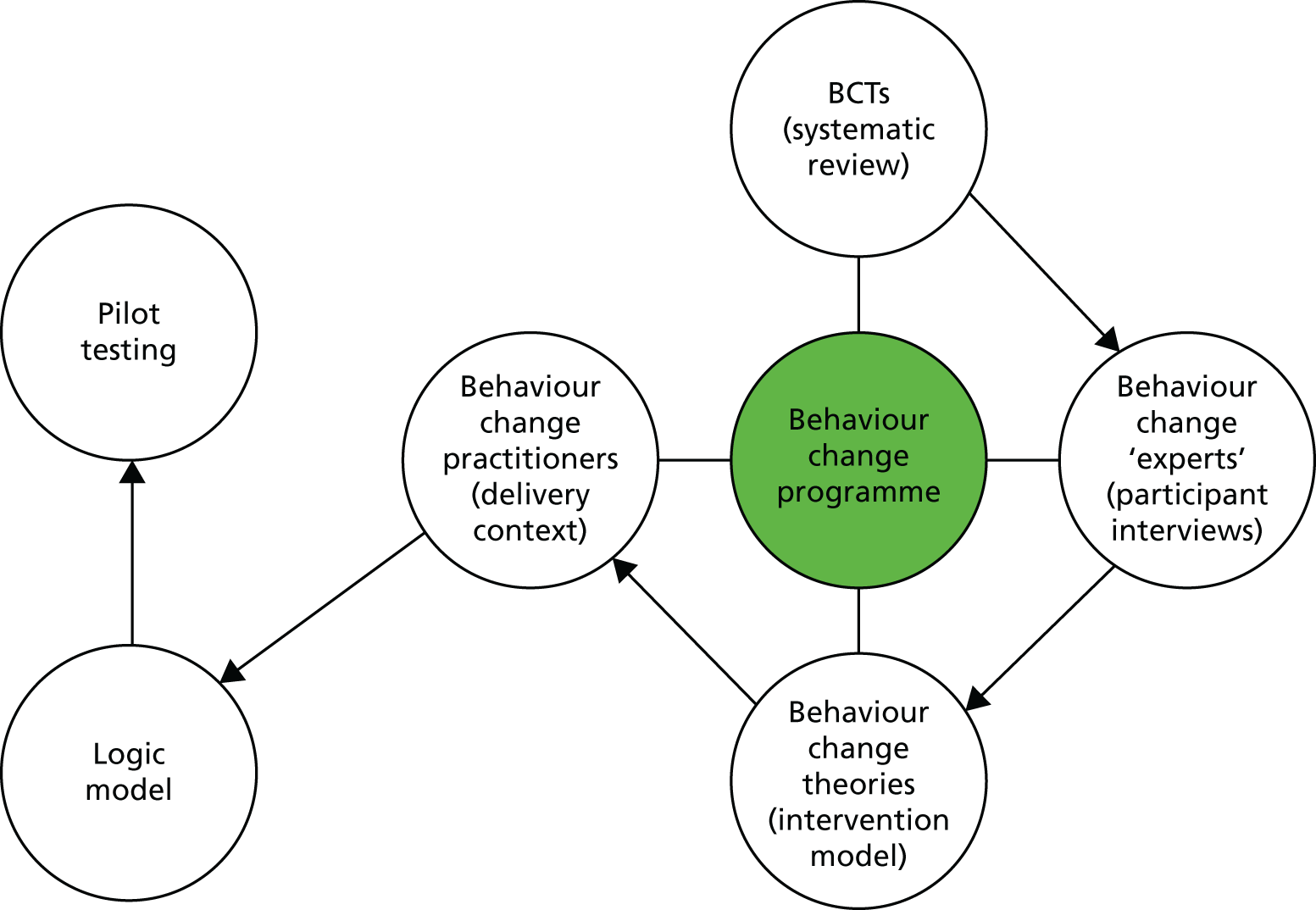
Using the Medical Research Council (MRC) framework for complex interventions,34 we designed and tested the feasibility of a multilevel peer-led physical activity intervention for older adults, tailored to meet the needs of the local community. The intervention package was developed after identifying appropriate behaviour change techniques (BCTs) through a rapid review of previous peer-led interventions. Following this, we conducted interviews with members of the target population to explore their preferences for, and their perceptions of, the feasibility of the BCTs identified in the rapid review. Using information from the first two stages, combined with behaviour change theory [social cognitive theory (SCT)] and input from practitioners regarding the context for the delivery of the proposed programme, we developed a peer-led physical activity intervention and logic model, and tested its feasibility in a pilot randomised controlled trial (RCT) (Figure 1). The aim of the pilot trial was to provide information on recruitment and attrition rates, intervention fidelity, data on the variability in objective physical activity measurements and the resources needed to support the development of a definitive trial. 35
FIGURE 1.
Integrated model to design intervention content.
Changes to the intervention delivery
It was originally planned that peer mentors would be managed under the existing walking group scheme in the Health and Social Care Trust. Owing to governance issues, it was not possible to arrange this in a timely manner, so the ‘Walk with Me’ study protocol needed to be amended. Therefore, some of the peer mentors were also insured and indemnified through Queen’s University Belfast.
Chapter 2 Rapid review to identify components used in previous peer-led interventions
Introduction
The first phase in the MRC complex intervention model is to gather relevant evidence and theory in order to develop a logic model for the implementation of the intervention, which includes the proposed causal pathways and relevant outcome measures. A rapid review approach36 was used to gather evidence and review the BCTs employed in previous peer-led physical activity interventions in adults aged > 18 years.
Peer-led interventions can be considered complex, as they involve multiple interacting BCTs. 34 This makes it difficult to identify the most effective techniques used within peer-led interventions to encourage physical activity behaviour change. 37 To standardise the extraction of components employed in previous interventions, Michie et al. 37 developed the BCT Taxonomy v1. This taxonomy provides standardised labels and definitions for 93 BCTs, hierarchically organised into 16 groupings. BCTs are ‘an observable, replicable, and irreducible component of an intervention, designed to alter or redirect causal processes that regulate behaviour’. 37 Previous evidence demonstrated an association between identification of BCTs and effective interventions for physical activity behaviour change. 38 Presently, there are no published studies identifying which BCTs are most widely used for physical activity behaviour change in peer-led interventions in older adults (aged > 60 years). The aim of our rapid review was to identify the BCTs employed in previous peer-led physical activity interventions.
Methods
Protocol registration
The review protocol was registered and published at PROSPERO (URL: www.crd.york.ac.uk/prospero/; registration number CRD42014009791).
Identification of studies
The following six databases were searched from inception until March 2015: MEDLINE, EMBASE, SPORTDiscus, The Cochrane Library, Physical Education Index and Web of Science. They were searched using a tailored and sensitive search strategy. Physical activity terms were based on those used in a previous Cochrane review of interventions to promote physical activity. 39 These were combined with peer-led intervention search terms derived from a previous review of peer-led interventions. 29 The search strategy was developed for MEDLINE and adapted for the other databases. A full list of terms is included in Appendix 1. In addition to searching electronic databases, the reference lists of included studies and relevant systematic reviews were searched for appropriate studies.
Inclusion criteria
The review was not restricted to interventions targeting only older adults, as we anticipated there would be very few peer-led interventions in this age group and this might limit the inclusion of potentially useful components. Therefore, studies involving community-dwelling adults aged > 18 years, interventions targeting changes in physical activity and interventions that reported a change in physical activity were included. Studies also needed to include a control or comparison group. No language restrictions were applied.
Study selection
All duplicate studies were removed with RefWorks 2.0 software (ProQuest, Ann Arbor, MI, USA). Two reviewers (ALW and MAT) independently screened the title and abstract of all remaining references to remove those that were obviously not relevant. The full text of remaining articles was obtained and screened for inclusion. When any discrepancies arose, consensus was reached through discussion with other authors.
Data extraction and management
The Cochrane Public Health Group data form was modified to meet the requirements of this review. The form was piloted by two authors (ALW) and (MAT) in a random sample of three studies to confirm that it captured relevant data. Data extracted included method of recruitment, type of peer who delivered the intervention, theoretical basis of intervention components, timing of intervention (frequency, intensity, duration) and method of delivery of outcome assessment.
Assessment of risk of bias in included studies
The risk of bias in the included studies was examined using the Cochrane risk of bias assessment tool (2014; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). This tool was extended to include risk of bias in specific assessments relating to physical activity interventions (e.g. use of objective measure of physical activity as an outcome measure). Two authors (ALW) and (MAT) independently assessed each study’s risk of bias. All discrepancies were resolved by the reviewers through discussion.
Identification of behaviour change techniques
Two trained reviewers (AW and CC) extracted information of the BCTs in included interventions. A detailed data extraction form was developed by three reviewers (MAT, CC and AW) (see Appendix 2). BCTs were extracted independently by two of the three reviewers (AW and CC), using the published BCT Taxonomy v1. 37 Discrepancies were resolved through discussion with a third reviewer (MAT).
Results
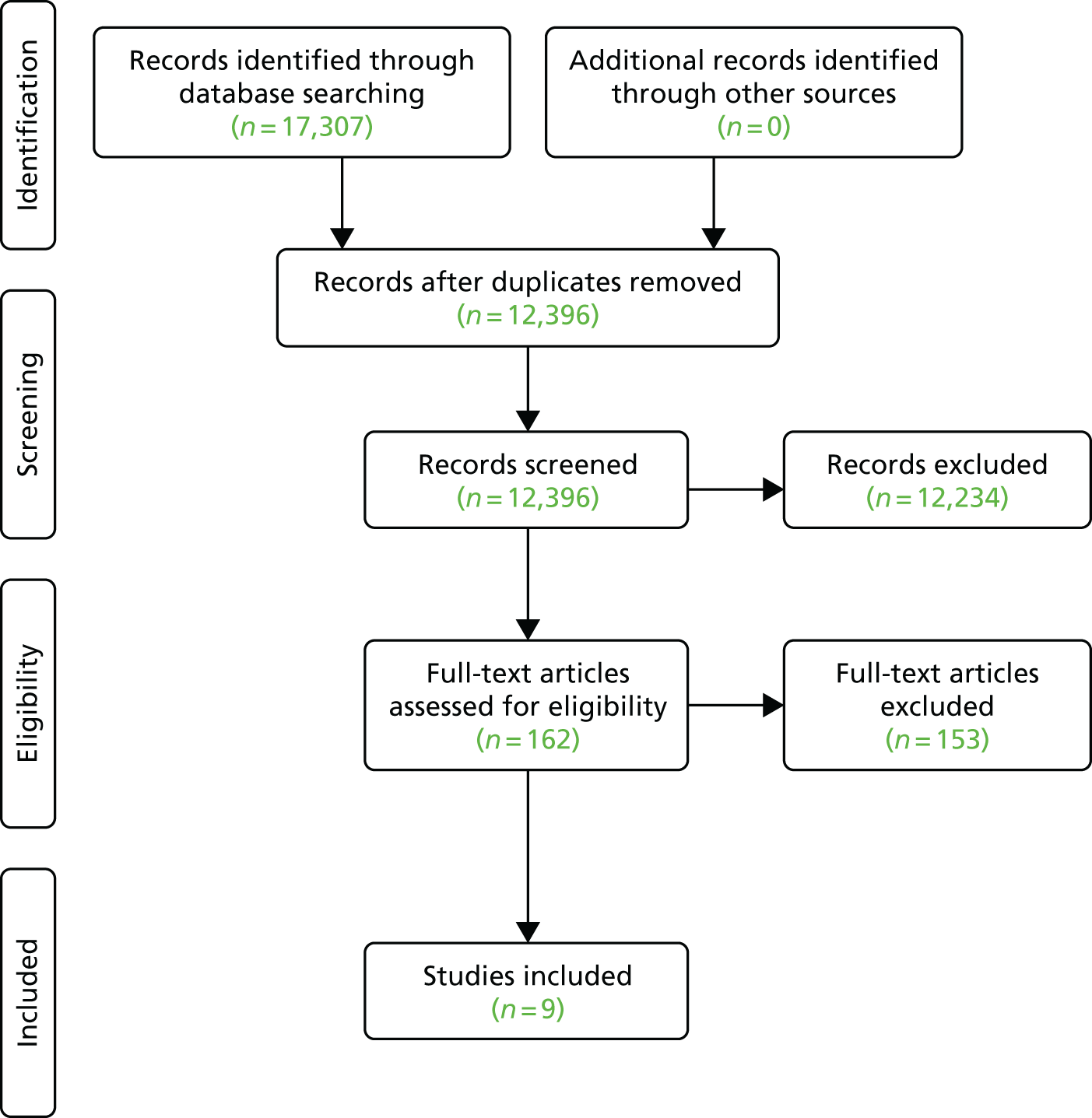
Overall, a total of 17,307 citations were identified from the database searches (Figure 2). After the removal of duplicates, 12,396 citations remained. After title and abstract screening, 162 full-text articles were assessed for inclusion. Most excluded studies did not measure free-living physical activity or were single-arm intervention studies with no control group (see Figure 2).
FIGURE 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for rapid review.
Characteristics of included studies
Nine studies (1780 participants with a mean age of 54.8 years) met the inclusion criteria and were included in this review. 40–48 Table 1 summarises in detail the key characteristics of included studies. Six of the nine studies were RCTs. 40–42,45,47,48 Two of these studies were conducted in specific groups of patients (male first-time cardiac patients44 and women with stage 0–3 breast cancer43). Most interventions were implemented in the USA (n = 5),40,41,43,44,48 with others in Canada (n = 2),45,46 the UK (n = 1)47 and Hong Kong (n = 1). 42 Overall, 69% of participants were female. Four of the nine studies involved ≥ 70% female participants. 40,41,45,46 One study involved exclusively female participants44 and one involved exclusively male participants. 45 In all studies, the authors reported that they had no conflict of interest to declare.
| Study | Sample population | Age (years), mean (SD) | Target population | Setting | Country | Sample size | Study design |
|---|---|---|---|---|---|---|---|
| Boyle et al.40 | Students (aged > 18 years) enrolled in a personal health class during the 2007–8 academic year |
Total sample: range not reported, 21.1 (4.47) Intervention: 21.2 (4.28) Control: 21.1 (4.67) |
Female: 74% White: 91% Full-time student: 96% |
University- and home-based programme | USA |
Total sample: n = 178 Intervention: n = 86 Control: n = 92 |
Quasi-experimental design |
| Buman et al.41 | Inactive/insufficiently active community-dwelling older adults living in a university community |
Total sample: range not reported, 63.42 (8.42) Active intervention: 63.49 (8.26) Standard community intervention: 63.35 (9.07) |
Female: 82% Married: 54% White: 91% Ethnicity: Hispanic |
Community-based programme | USA |
Total sample: n = 91 Active intervention: n = 44 Standard community intervention: n = 47 |
RCT |
| Castro et al.42 | Inactive (not active for > 60 minutes per week) older adults and living within San Francisco Bay area |
Total sample: range not reported, 59.1 (± 6.1) Peer mentors: 64.4 (± 5.8) |
Female: 65.8% Caucasian: 67.4% |
Community-based programme | USA |
Total sample: n = 181 Physical activity advice from staff arm: n = 61 Peer mentor arm: n = 61 Attention-matched control arm: n = 59 |
RCT |
| Lamb et al.43 | Inactive male and female middle-aged adults | Total sample: range 40–70 years, 50.8 (7.7) |
Taking < 120 minutes of MVPA Male: 47.7% |
Community-based programme | UK |
Total sample: n = 260 Advice group: n = 129 Health walks group: n = 131 |
RCT |
| Parent and Fortin44 | Male first-time cardiac surgery patients |
Total sample: range 40–69 years, 56.5 (7.7) Experimental: 57.6 (7.4) Control: 55.9 (7.8) |
Male: 100% | Home-based programme | Canada |
Total sample: n = 56 Experimental: n = 27 Control: n = 29 |
RCT |
| Pinto et al.45 | Inactive (< 30 minutes per week of vigorous exercise or 90 minutes per week of moderate intensity exercise for past 6 months). English-speaking women with stage 0–3 breast cancer (diagnosed in the past 5 years) and had completed surgery |
Total sample: range 55–65 years, 55.62 (9.55) Intervention: 55.64 (8.59) Control: 55.59 (10.59) |
White: 98.7% Hispanic: 6.6% Married: 82.9% Female: 100% |
Home-based programme | USA |
Total sample: n = 76 Intervention: n = 39 Controls: n = 37 |
Quasi-experimental design |
| Resnick et al.46 | Inactive urban community-dwelling older adults | Total sample: range 60–85 years, 73.3 (± 8.5) |
Female: 79% African American: 77% |
Community-based programme | USA | Total sample: n = 166 | Feasibility RCT |
| Thomas et al.47 | Inactive older adults (aged > 60 years) with no history of CVD of physical disabilities, from 24 community centres |
Buddy support group and pedometer: 71.7 (5.7) Control: 72.4 (5.7) |
Female: 67% Smoking: 53.7% |
Community-based programme | Hong Kong |
Total sample: n = 399 Buddy support group and pedometer group: n = 193 Control: n = 206 |
Cluster RCT |
| Tudor-Locke et al.48 | Inactive male and female participants with type 2 diabetes |
Total sample: range 38–71 years, 55.7 (7.3) Professional led: range 38–70 years, 54.8 (7.2) Peer led: range 42–71 years, 57.8 (7.4) |
Female: 82% Former smokers: 52.7% |
Community-based programme | Canada |
Total sample: n = 220 Professional led: n = 157 Peer led: n = 63 |
Quasi-experimental design |
Outcomes
Although total physical activity levels were reported in all studies, physical activity measures varied between them. However, all instruments were reported as being valid and reliable. Six studies reported assessing the impact of the intervention only on self-reported physical activity levels, including the National Health Interview Survey,40 the Jenkins Activity Checklist,44 the Stanford Five-City Project Physical Activity Questionnaire43 and the Yale Physical Activity Survey. 46 One study used an objective measure of walking (Yamax SW-200 pedometer; Yamax Corp., Tokyo, Japan). 48 Other studies used a combination of objective and self-report methods. No studies reported the cost-effectiveness of the intervention.
Methodological quality of included studies
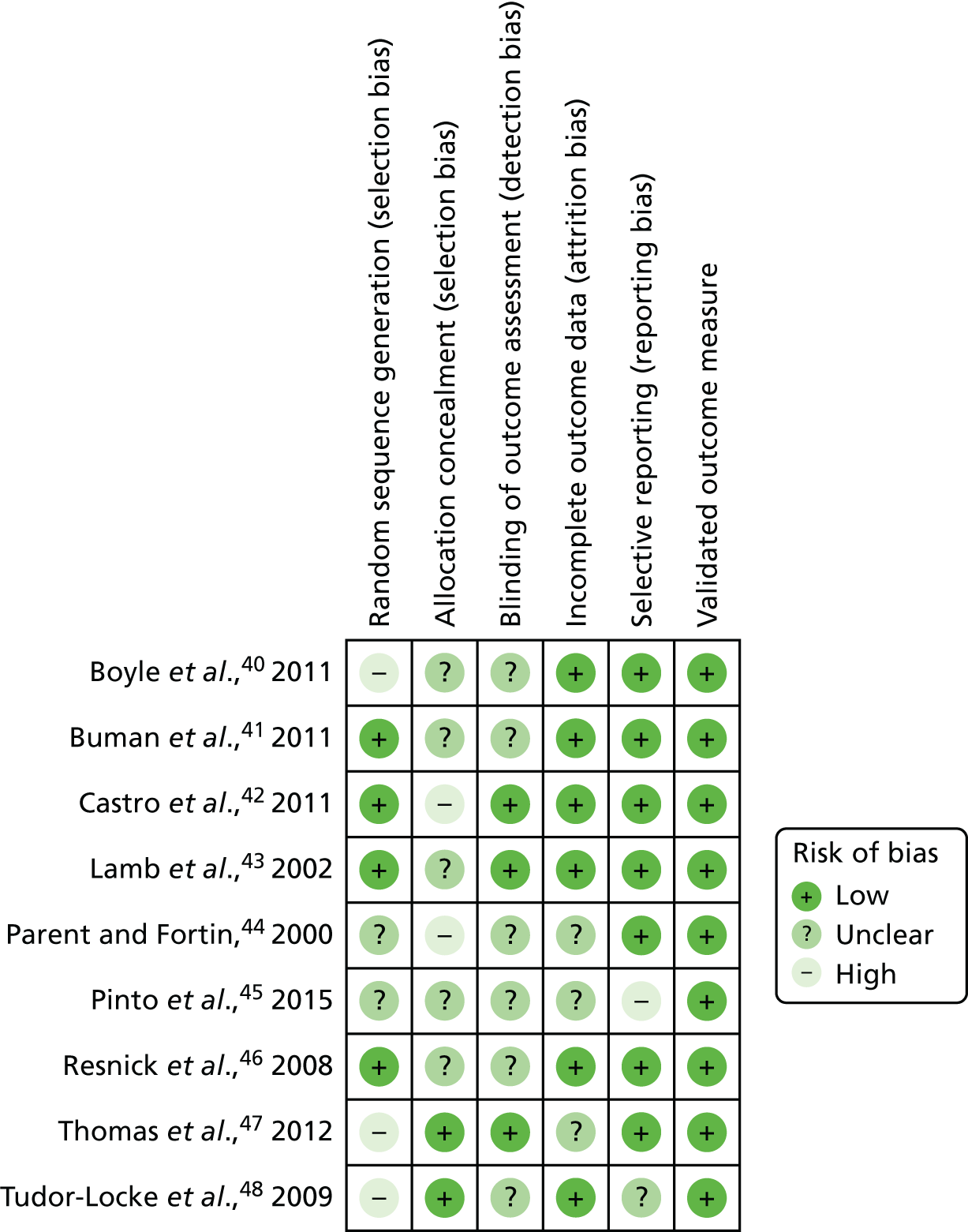
Six of the nine studies employed a randomised controlled design. 40–43,45,46 Allocation concealment was used in two studies. 47,48 A further four studies were rated as having a low risk of bias from random sequence generation. 41–43,46 Outcome assessors were blind to the allocation of participants in three of the nine studies. 42,43,47 It was unclear if outcome assessors were blind to allocation in the other six studies. Six of the nine studies were deemed to have a low risk of attrition bias. 40–43,46,48 Reporting bias was evident in only one study,45 which did not report all of the prespecified outcomes. Finally, all nine studies reported using a validated measure of physical activity (Figure 3).
FIGURE 3.
Risk of bias in included studies.
Intervention components
Several common approaches (models) were used to deliver the peer-led physical activity interventions. The first model identified was the group-based peer education. The role of the peers was to act as group leaders guiding participants to adopt a new behaviour that facilitated healthy outcomes. Five studies used a group-based approach to deliver the intervention, whereby peer mentors acted as group educators, social leaders or walking co-ordinators. 40,42,43,46,47 Another model used was the dyads model, whereby peer mentors offered one-to-one ‘buddy’-type support for participants. Three studies used this approach, with support offered either in person44,45 or by telephone. 41 Finally, one study offered a combination of group and one-to-one support. 48 In both models, peer mentors delivered skills training, goal-setting and feedback on progress, problem-solving activities and social support and acted as a role model for positive behaviour change. Interventions did not appear to include explicit strategies to encourage maintenance of physical activity. Five of the nine studies were based on a behavioural theory. Theories used were SCT,41,46 self-efficacy theory40,45,48 and social learning theory. 45
Characteristics of peer mentors
In four of the nine studies, peer mentors were recruited from the same source as the study participants, such as former patients,44,45 fellow university students40 or members of the same community centre (Table 2). 47 Peer mentors in other studies were former research participants,41,42 middle-aged lay instructors46 or trained peer leaders. 48 They were recruited from lists of participants in previous research studies41,42 or through existing organisations or groups such as university,40 patient groups or community centres,46,47 or peer leadership training courses. 48
| Study | Peer mentor characteristics | Recruitment of peer mentors | Eligibility | Additional training (type and hours of training) | Ongoing management of peer mentors | Incentives for peer mentors | Resources |
|---|---|---|---|---|---|---|---|
| Boyle et al.40 | Peer educator was a trainee exercise physiologist enrolled in an advanced undergraduate physiology class | Not reported | Not reported | Trained in physical fitness assessment and programming skills | Supervised by researchers | Not reported | Not reported |
| Buman et al.41 | Research participants from previous health promotion studies | Recruited from a registry of research participants from previous health promotion studies and through a local fair | Reported having a regular physical activity routine or had a basic background in health education | Not reported | Quality control checklists and scoring procedures were used to give the peer mentors feedback about ways to improve their efforts to facilitate group meetings. Programme staff met weekly with the mentor after each of the first five sessions to give feedback and coaching. Additional feedback was provided as needed throughout the intervention | Not reported | Volunteered their time without remuneration; however, in a few cases mentors were modestly reimbursed for their travel (approximately US$15 per session) |
| Castro et al.42 | Participants from previous research studies | Mailings to previous research participants and announcements to local active ageing community groups | Physically active (at least 150 minutes of MVPA per week) and willing to volunteer for 4–6 hours per week for a minimum of 1 year | Not reported | Peer mentors were assigned post-training practice sessions identical to professional staff, including assignments to rehearse advice and counselling components and practise completing forms to document the content and delivery of the interventions | Not reported | Peer mentors were provided with pre-paid telephone charge cards if they wished to make telephone calls to their contacts from home |
| Lamb et al.43 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Parent and Fortin44 | Previous patients who had recovered from cardiac surgery | Recruited by a research co-ordinator | Able to verbalise enthusiasm towards increased activity, stimulate motivation and share their successful rehabilitation after surgery | Given 6 hours’ training by the research co-ordinator on interaction principles (how to listen empathically and to reflect the patient’s feelings) and on cardiovascular disease and treatment | Not reported | Not reported | Not reported |
| Pinto et al.45 | Breast cancer survivors who provide information and emotional support for other breast cancer survivors | Recruited from an existing programme run by the American Cancer Society Reach to Recovery programme | Not reported | Trained by the American Cancer Society Reach to Recovery programme on how to deliver the exercise programme | Not reported | Not reported | Not reported |
| Resnick et al.46 | Middle-aged lay instructors | Not reported | Not reported | Full-day training session and a detailed procedure manual | Within an ongoing Senior Wellness Project | Not reported | Not reported |
| Thomas et al.47 | Members of community centre aged ≥ 60 years | Through older adults community centres | Aged ≥ 60 years, no history of myocardial infarction or stroke and physical disability | Not reported | Supervised by research assistants. Provided with an instruction manual on how to enlist a walking partner | Not reported | Cost of telephone calls were reimbursed |
| Tudor-Locke et al.48 | Nominated by professionals after the completion of a 16-week peer leadership training course | Recruited by professionals after the completion of 16-week peer leadership training course | Not reported | Additional half-day training on adult learning principles and facilitation skills | Not reported | Not reported | Travel costs. Peers were given the same resources as the professionals (overhead transparencies, checklists) |
Not all of the studies detailed the training offered to peer mentors. Those that did so reported training lasting from 6 hours to a full day. 44–46,48 Moderate-value resources were offered to support peer mentors, including reimbursement of expenses incurred such as travel costs and the cost of telephone calls (see Table 2). 41,42,47,48
Behaviour change techniques in peer-led physical activity interventions
The BCT Taxonomy v1 is clustered into groupings of BCTs that may be commonly used together in physical activity interventions. 37 Agreement between data extractors was fair (κ = 0.5). Therefore, all papers were reviewed a second time with a third reviewer (MT) to ensure accuracy in BCT data extraction.
The results from the assessment of BCTs identified that the most commonly used BCTs were goal-setting (behaviour) (n = 740–42,45–48); social support (emotional) (n = 740–46); instruction on how to perform the behaviour (n = 740,42–47); problem-solving (n = 641,42,45–48); adding objects to the environment (n = 641,42,45–48); demonstration of the behaviour (n = 440,43,46,47); behavioural practice/rehearsal (n = 440,43,46,47); self-monitoring of behaviour (n = 640–42,45,47,48); and social support (practical) (n = 640–42,45,47,48). The most commonly used groups of BCTs employed in peer-led physical activity interventions were (1) goals and planning [goal-setting (behaviour), n = 7;40–42,45–48 problem-solving, n = 6;41,42,45–48 action-planning, n = 2;41,47 and behavioural contract, n = 1];40 (2) feedback and monitoring [feedback on behaviour, n = 2;42,48 self-monitoring of behaviour, n = 6;40–42,45,47,48 self-monitoring of outcome(s) of behaviour, n = 1;45 and feedback on outcome of behaviour, n = 241,45]; (3) social support [social support (practical), n = 6;40–42,45,47,48 and social support (emotional), n = 740–46]; (4) shaping knowledge (instruction on how to perform the behaviour, n = 740,42–47); (5) comparison of behaviour (demonstration of the behaviour, n = 4;40,43,46,47 social comparison, n = 244,46); and (6) antecedents (adding objects to the environment, n = 641,42,45–48) (Table 3).
| BCT label | BCT group | Study | Frequency of BCT/nine studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boyle et al.40 | Buman et al.41 | Castro et al.42 | Lamb et al.43 | Parent and Fortin44 | Pinto et al.45 | Resnick et al.46 | Thomas et al.46 | Tudor-Locke et al.47 | |||
| 1. Goals and planning | 1.1 Goal-setting (behaviour) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||
| 1.2 Problem-solving | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| 1.4 Action-planning | ✓ | ✓ | 2 | ||||||||
| 1.8 Behavioural contract | ✓ | 1 | |||||||||
| 2. Feedback and monitoring | 2.2 Feedback on behaviour | ✓ | ✓ | 2 | |||||||
| 2.3 Self-monitoring of behaviour | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| 2.4 Self-monitoring of outcome(s) of behaviour | ✓ | 1 | |||||||||
| 2.7 Feedback on outcome of behaviour | ✓ | ✓ | 2 | ||||||||
| 3. Social support | 3.1 Social support (unspecified) | ✓ | 1 | ||||||||
| 3.2 Social support (practical) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| 3.3 Social support (emotional) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| 4. Shaping knowledge | 4.1 Instruction on how to perform the behaviour | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | ||
| 5. Natural consequences | 5.1 Information about health consequences | ✓ | ✓ | 2 | |||||||
| 5.6 Information about emotional consequences | ✓ | 1 | |||||||||
| 6. Comparison of behaviour | 6.1 Demonstration of the behaviour | ✓ | ✓ | ✓ | ✓ | 4 | |||||
| 6.2 Social comparison | ✓ | ✓ | 2 | ||||||||
| 8. Repetition and substitution | 8.1 Behavioural practice/rehearsal | ✓ | ✓ | ✓ | ✓ | 4 | |||||
| 9. Comparison of outcomes | 9.1 Credible source | ✓ | ✓ | 2 | |||||||
| 10. Reward and threat | 10.3 Non-specific reward | ✓ | 1 | ||||||||
| 10.9 Self-reward | ✓ | 1 | |||||||||
| 11. Regulation | 11.2 Reduce negative emotions | ✓ | 1 | ||||||||
| 12. Antecedents | 12.5 Adding objects to the environment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | |||
| 15. Self-belief | 15.1 Verbal persuasion about capability | ✓ | ✓ | 2 | |||||||
| 15.4 Self-talk | ✓ | 1 | |||||||||
Discussion
In this review of peer-led physical activity interventions, nine studies rated as having fair to good methodological quality were identified (i.e. low risk of bias). Interventions were designed around the social support that peer mentors could offer, either within groups or on a one-to-one basis. Intervention strategies were broadly developed to emphasise the peer mentor as a role model for positive behaviour change. Within the interventions, peer mentors delivered skills training, goal-setting and feedback, and problem-solving components. To equip them to do this, they were given short training sessions and offered ongoing support (see Table 2). Peer mentors were recruited either from groups of participants in previous interventions or from community centres. Physical activity was measured using validated instruments, but, as self-reported activity may be biased, there is a need for studies using objective measures of physical activity. A limitation of the study was that BCTs in the control groups were not coded, and thus we were unable to identify BCTs that were unique to the intervention. However, as we were not assessing effectiveness, this does not have an implication on the overall findings.
The BCTs employed in the interventions included in the review were used in the next stage of our study as the basis of interviews with older adults to determine their preferences for what could be included in an intervention.
Chapter 3 Feasibility and acceptability of proposed behaviour change and intervention strategies (qualitative interviews)
Introduction
The rapid review of existing literature (see Chapter 2) reporting peer-led physical activity interventions identified common groups of BCTs employed in previous interventions; these were goals and planning; feedback and monitoring; social support; shaping knowledge; comparison of behaviour; repetition and substitution; and antecedents. This qualitative study aimed to explore the feasibility of using some of the most commonly used BCTs in these groups [goal-setting, self-monitoring (behaviour), social support (practical and emotional), problem-solving, instruction on how to perform the behaviour, demonstration on the behaviour and adding objects to the environment] in a peer-led intervention for older adults. These BCTs aligned to SCT, which emerged from the rapid review as a promising theoretical framework for the design of the intervention. We sought to inform the development of our intervention content by eliciting, through semistructured interviews, the opinions and preferences of older adults living in socioeconomically disadvantaged communities regarding the use of these BCTs.
Methods
The Office for Research Ethics Committees Northern Ireland gave ethics approval for the study (reference number 14/NI/1330).
Participants
This phase of the study was carried out in the South Eastern Health and Social Care Trust (SEHSCT) area. All electoral wards within the SEHSCT area were ranked by quartiles of the Northern Ireland Multiple Deprivation Measure (NIMDM). The NIMDM score is constructed by combining population data relating to seven different domains (income; employment; health deprivation and disability; education, skills and training; proximity to services; living environment; crime and disorder). Community organisations [e.g. Colin Neighbourhood Partnership, The Resurgam Trust (community group), Hillhall Community Resource Centre and St Luke’s Family Centre] located within electoral wards with NIMDM scores in the top 25% (most disadvantaged quartile) were approached to facilitate identification and recruitment of potential participants. They were asked to identify individuals aged between 60 and 70 years living in the target areas (although we accepted some older individuals as they were available). The aim was to recruit a purposive sample of individuals (men and women of different ages and physical activity levels, living in urban and rural settings). None of the participants had experience of walking groups or peer-led health programmes.
Data collection
Semistructured one-to-one interviews were deemed the most appropriate method of gathering detailed information from participants regarding the feasibility and acceptability of BCTs. Interviews were conducted either in participants’ homes or in local community centres. Participants completed a brief questionnaire to provide demographic information and a self-assessment of their current health status (as poor, fair, good, very good). Each interview lasted approximately 1 hour and all were conducted by a female psychology graduate trained and experienced in qualitative methodology (IMcM).
At the beginning of each interview, participants were informed of the research topic and the aims of the study and asked to sign a consent form. All participants were informed that they could withdraw their support at any time during the process, and that their information would be held securely and used anonymously. A flexible interview schedule was developed for the interview (summarised in Box 1). This included questions about the role of physical activity in day-to-day life and participants’ views of setting physical activity goals (goal-setting); using a pedometer to monitor progress (self-monitoring, instruction on how to perform the behaviour, adding objects to the environment); problem-solving; working with a peer mentor (social support, practical and emotional); and going for a demonstration walk with a peer mentor (demonstration on the behaviour). Questions were supplemented with copies of self-monitoring diaries, pedometers and photos to elicit responses. A full copy of the interview schedule is included as Report Supplementary Material 1. After the interview, all participants were debriefed and provided with an opportunity to raise any questions or concerns.
Can you describe your typical day?
When do you feel you are physically active during the day?
Goal-setting and self-monitoringHave you ever heard of a pedometer before?
What do you think are the advantages of using something like a pedometer and diary?
What do you think are the disadvantages of using something like a pedometer and diary?
Problem-solvingCan you think of barriers or obstacles you have faced to increase your physical activity?
Did you find anything that helped you overcome these?
What do you like about this method?
What do you not like about this method?
Peer mentoringWhat do you think about the idea of using a peer mentor?
What would you want them to do with you?
Do you see any problems with using a peer mentor?
Demonstration walkWhat kind of information would people need to increase their walking?
Where would you like to go?
How often would you want to go?
Are there any problems with using a peer mentor?
Data analysis
Interviews were audio-recorded and transcribed anonymously. In line with current guidelines;49–51 a directed content analysis approach was adopted to understand the emotional responses and preferences expressed by the participants regarding the feasibility and acceptability of implementing the BCTs being examined. 49–51
Using the transcripts, the lead researcher (IMcM) generated initial codes which highlighted pertinent features of the data. This was achieved in a systematic manner by reading each line of the transcript and placing codes in the margins of the text. These initial codes were then collated into potential themes. The researcher then reviewed the themes in relation to the initially coded narratives and a thematic map was generated. Sufficient time was given to this coding process to ensure that coding was as robust as possible. The codes and themes were then given to another member of the research team (CC) who was familiar with the transcripts and who then confirmed the validity of the key themes. In addition, researchers (MEC and ES) experienced in qualitative analysis also reviewed the themes and subthemes. After 11 interviews, data saturation was achieved; another interview was completed to seek confirmation of the analysis. The findings were discussed with six participants in follow-up meetings during the intervention development phase for validation.
Results
The characteristics of each participant are summarised in Table 4.
| Participant ID | Sex | Age (years) | Employment status | Health status | Health problem limiting normal daily activities | Highest education level | Home owner | Living with partner or living alone | Number of people in household |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 61 | Retired | Fair | No | Secondary school | Own | Alone | 1 |
| 2 | Male | 92 | Retired | Good | Yes | Secondary school | Own | Alone | 1 |
| 3 | Female | 77 | Retired | Fair | No | Secondary school | Own | Alone | 1 |
| 4 | Female | 70 | Retired | Poor | Yes | Secondary school | Rent | Partner | 2 |
| 5 | Male | 80 | Retired | Fair | No | Secondary school | Own | Alone | 1 |
| 6 | Female | 67 | Retired | Good | Yes | Secondary school | Own | Alone | 1 |
| 7 | Female | 60 | Full-time work | Good | No | College | Own | Partner | 3 |
| 8 | Female | 62 | Retired | Excellent | No | Secondary school | Own | Partner | 2 |
| 9 | Female | 65 | Retired | Fair | Yes | Secondary school | Rent | Partner | 2 |
| 10 | Male | 63 | Retired | Fair | Yes | Secondary school | Own | Partner | 2 |
| 11 | Male | 72 | Retired | Fair | Yes | Secondary school | Rent | Partner | 2 |
| 12 | Male | 72 | Retired | Poor | Yes | Secondary school | Rent | Living alone | 1 |
Themes
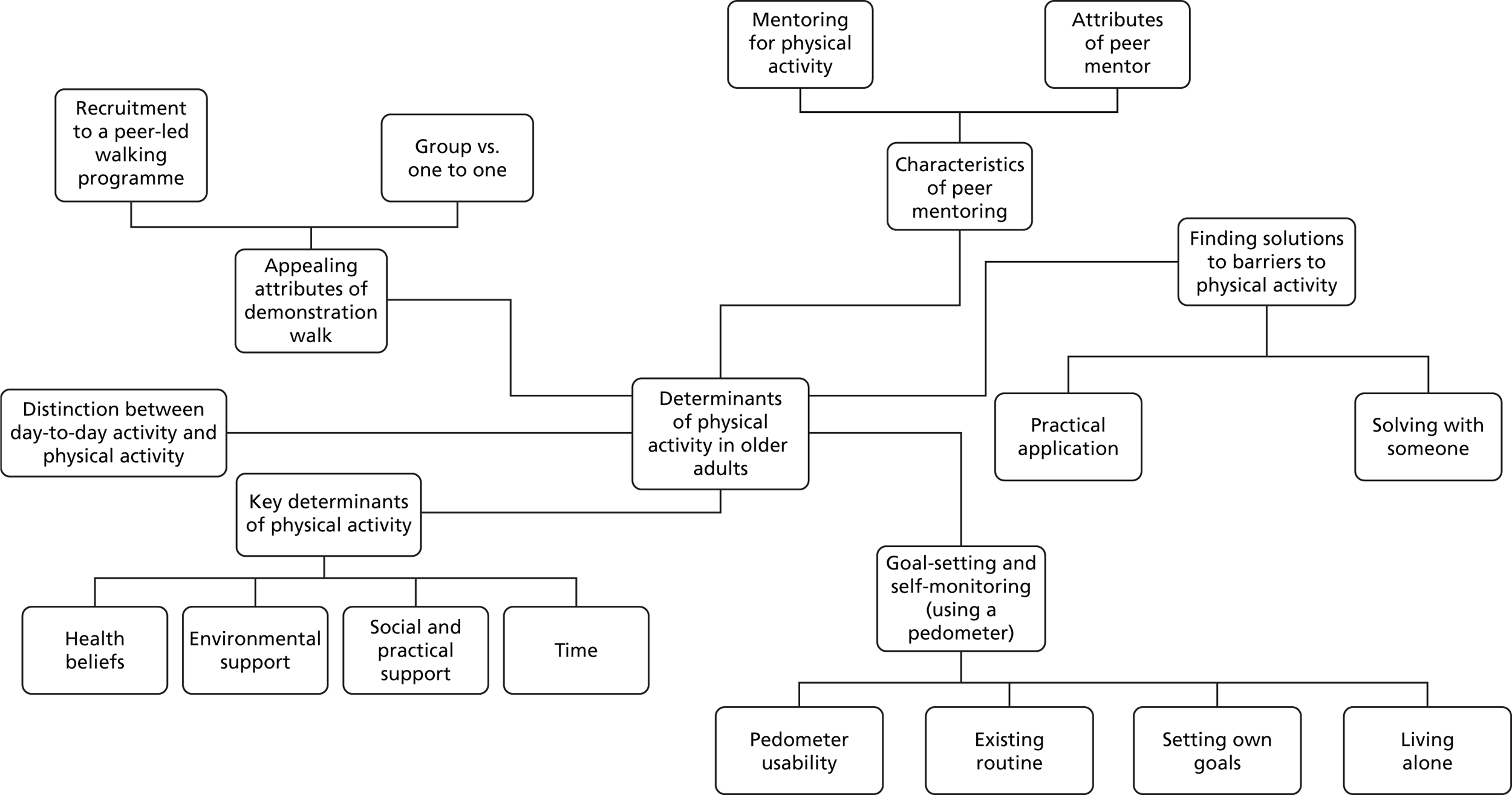
The key themes and subthemes are depicted in Figure 4. These included a distinction between day-to-day activity and physical activity; key determinants of physical activity; goal-setting and self-monitoring using a pedometer; characteristics of peer mentors; finding solutions to barriers to physical activity; and appealing attributes of demonstration walking. The themes are reported below, supported by relevant quotations, which are anonymised.
FIGURE 4.
Thematic map of ‘Walk with Me’ intervention development interview data.
Theme 1: distinction between day-to-day activity and physical activity
The interviewer clearly defined physical activity as activities in which people were ‘up and about’ and ensured that participants understood that physical activity is not limited to activities that are structured (e.g. going to the gym). In terms of the discussion, individuals echoed this understanding and described their typical day, which consisted of the activities of daily living, including housework (e.g. laundry, cooking and cleaning), as well as carer responsibilities for either grandchildren or partners:
. . . just have breakfast. And so I make my husband’s breakfast cause he’s not in good health . . . going to do the washing . . . go and get your washing upstairs and bring it down . . . I’d be up the stairs all the time . . . And would I never rest. I do knit . . .
Participant 9
The majority of individuals appeared to be busy simply carrying out day-to-day living activities, and physical activity was regarded as something that was for leisure, and not something that was ‘necessary’. It was also mainly limited to a number of physical activities, among which walking and gardening were the most frequently mentioned:
. . . the morning part of it was active enough, certainly, because erm my flower bed had got decidedly overgrown and um it needed a great deal of hoking and poking to get it into any sort of order at all.
Participant 3
My only activity at, at the moment is walking. Walking my dogs.
Participant 4
The findings suggest that older adults are potentially less physically active and may not be meeting the recommended guidelines for physical activity:
Really that’s all my activity because I’m not sport minded or anything [laughing].
Participant 2
Theme 2: key determinants of physical activity
Individual discussions suggested that there was a complex interplay between individuals’ beliefs about their health and how physical activity would affect it; the environment in which they lived and whether or not it allowed access to appropriate areas to walk; the level of support that they received, whether physical or social; and the amount of time that they could devote to physical activity. These various factors all appeared to have a potential influence on physical activity levels.
Health beliefs
Participants expressed feelings about the ‘inevitability’ that physical activity would decline over time as a result of changes in health and in social circumstances:
But unfortunately with erm the deterioration in my wife’s health I was gradually dropping, and the fact that I was getting older anyway (laughs).
Participant 3
Participants also suggested that existing health conditions prevented them from increasing their physical activity level:
Although I haven’t done it [physical activity] in about 2 months. Erm I think that one day I just done a bit too much and then fluid gathered in my knees. So, I need to be careful not to over do it.
Participant 11
However, participants also suggested that poor health could also be a motivator for increasing physical activity in the belief that it could alleviate the symptoms of existing conditions or even prevent new ones from developing:
I’m trying to use the hands. Trying to use the limbs by bending up and down . . . I still do it because I have to do it.
Participant 9
In addition, participants expressed a belief about the benefits of physical activity (e.g. for better mental health and weight management). Participants expressed different beliefs about their level of actual physical activity, with some believing that they should be taking more physical activity and others believing that their physical activity levels were adequate:
You’re out walking, you’re out active, you’re, you feeling better in yourself and your whole head lifts. My husband suffers badly with depression so, when we’re out walking, it lifts him. You’re out. Know what I mean?
Participant 9
I’m conscious that I need some sort of walking. Some sort of activity. I’ve retired now around 2 years. And erm I’ve noticed I’ve put on a bit of weight and I have myself, a certain weight I will not go over and I’m, you know, erm, normally watching my weight. And I am conscious that I need to be more active.
Participant 11
We are active, active enough. At the moment like . . . hopefully it stays that way.
Participant 10
However, the mixed belief regarding adequacy levels of physical activity may have been due to a misunderstanding about or a lack of awareness of the actual physical activity guidelines:
. . . but I don’t know what 300 steps would mean. Would that mean it’s good or bad?
Participant 9
Environmental support
Participants mentioned fear of traffic (cars and bicycles), dogs, antisocial behaviour and bad weather as key barriers to physical activity, even when appropriate places such as parks were available in their local areas:
Erm [coughs] I think that is becoming increasingly difficult as the roads, the roads have got so busy now. Aye. It’s not so pleasant. I can see myself now, um being reluctant enough to walk the roads with my dog because there’s so much traffic and so few now with . . . easy green edges to the roads where you can walk in comfort.
Participant 3
There’s green fields over there . . . But you wouldn’t walk. I mean I could walk about during the day, it’s fine. But at night, no, I wouldn’t.
Participant 9
Well I, I, don’t like walking round tow paths so I do not ‘cause it’s full of dogs’ doo doos and broken bottles. I don’t like that atmosphere along it. I like round [XXXX] bridge. I like places like that. You know, there’s good fresh air and you meet other people. You know what I mean? Somewhere like that you know. Or up the [XXXX] park. Now I would walk round [XXXX] park.’
Participant 11
I used to go to [XXXX] path but erm the amount of cyclists in that area. You’re just constantly stopping and starting and looking round you. And mostly cyclists. They don’t have a bell anymore and erm, . . . , they would probably frighten you sometimes. They’re right behind you before you realise they’re there.
Participant 12
However, having places to walk that were safe and accessible, or places that allowed them to get in touch with nature, could help to increase participants’ physical activity levels:
Well all the trees. There’s trees and nature. You see ducks and you see . . . So you go up there and you see ducks, and cows. Just trees. It’s not vandalised, not wrecked and ruined and destroyed. You know what I mean? So when you go up to the [XXXX] bridge, 10 minutes away in the car, sure it’s like a different world isn’t it?
Participant 13
Social and practical support for physical activity
Participants expressed a preference for combining social interaction with physical activity, thus making physical activity more enjoyable by focusing attention on socialising rather than on the activity itself:
I would like that one if you were going out with friends . . . you wouldn’t realise the distance . . . you don’t see time.
Participant 8
. . . you’re talking away and you’re. I would say, erm, and I would even think a couple of them there people would forget about their ailments when there’s a crowd cause they’re talking about different things.
Participant 12
Comments suggested that increased social support also increases confidence through learning from others or through the feeling of increased safety from the presence of others:
Safety in numbers. People feel safe out walking along with somebody else. Cause we’re afraid of the dogs and young ones and all messing about in the parks. Not so bad if . . . I’m on the toll path. But I’ve walked round [XXXX] park and I’ve felt a wee bit uneasy in it . . .
Participant 10
Physical support was also a motivator to increasing physical activity whereby providing physical aids to carry out gardening or a secure physical environment could increase physical activity levels:
Give me the tools. Instead of you bending down in pain, get the tools to dig but you standing up . . . so that would be. I would need to get out there and buy those tools that would be my solution. That’s my barrier.
Participant 9
However, the intensity of social support was seen as an important determinant of physical activity, and both lack of support and too much support were potential barriers to physical activity:
. . . if I need to go somewhere, to the hospital for an appointment to the clinic, my daughters are down straight away. Pick me up in the car, so, I don’t get to walk, and I would, and that, that would stop me from, you know.
Participant 9
Time
Participants suggested that their lack of physical activity was due to lack of time because of the many other activities that they took part in, as well as the carer responsibilities that they had for either partners or grandchildren:
I would like to have been gone out and having a walk. But that’s like a barrier. I have to stay in and mind three children.
Participant 7
However, even when time was available, participants preferred to take part in less intense physical activities (e.g. knitting and reading), and so appeared to lack motivation to increase their physical activity level:
I put her into the activity centre and then I’d sit and chat to my friends.
Participant 7
. . . drops me off . . . and will come back and pick me up for I wouldn’t walk all the way back.
Participant 7
Theme 3: goal-setting and self-monitoring (using a pedometer)
Self-monitoring and goal-setting were perceived as helpful approaches to increasing physical activity and, in particular, participants perceived that the pedometer would have a positive influence on their activity levels. Four subthemes were highlighted: pedometer usability, existing routine, setting own goals and living alone.
Pedometer usability
In general, participants expressed a positive attitude towards the pedometer:
Yes that would be a good thing. Yes . . . my son . . . uses that at work. He’d walk 5 mile a day.
Participant 11
There was a general awareness of the pedometer among participants, all of whom had either seen or heard of a pedometer before. In fact, one participant had actually used a pedometer for monitoring steps previously for a different study:
. . . and that would keep me active. That would keep me more active than erm because I do walk about a bit in the home . . .
Participant 9
Yes. I’ve seen them before. Our kids got them one time at McDonalds [Chicago, IL, USA] . . . wee wee yellow ones, and they were using them . . .
Participant 10
However, despite the overall positive view regarding pedometers, there were concerns about their actual use in practice:
Sure the last time . . . there wasn’t a step come up on it. So I wonder if this one’s working? That one wasn’t working sure it wasn’t?
Participant 10
In addition, participants also highlighted that pedometers should be simple to use:
I don’t want something like a magic phone . . . because I’ll not be able to use it [laughs].
Participant 4
. . . well that there’s handy . . . where erm you just, say clip it on to your jeans or something, and um, you just push a button and it starts, and pushes a button and it stops. Anything complicated, I wouldn’t be able to use, you know. But something like that there!
Participant 5
Participants also suggested that the device should be easy to wear and easy to see:
You have to be able to see it . . . the other one I used you were constantly putting your glasses on and off so that was a distraction too. But that has a nice clear face.
Participant 8
. . . the way I thought that if you could get like an arm band . . . it’s better than having something clipped on you.
Participant 8
Existing routine
Participants expressed an interest in using the pedometer because they felt that this would fit into their existing routines. For example, participants preferred to, or were more interested in, monitoring or counting current steps rather than trying any additional or new types of activities:
. . . so I really have a routine, most of the week . . . and then I’d be able to see what I’d done one day and what I’m doing on another day. Some days wouldn’t be as many as others. So it would give me a, a bit of a picture.
Participant 10
However, this does show that the provision of pedometer per se may not be sufficient to enact behaviour change and that participants need to be encouraged to use the pedometer to set step goals and monitor their progress:
But I wouldn’t want to be doing this diary all the time.
Participant 10
Setting own goals
Participants expressed a preference for being able to set their own goals, as opposed to having them set by someone else. This was mainly due to the feeling that self-set goals would be tailored to their own physical fitness or capability rather than general goals that might not be achievable:
Well the goal-setting, because it’s, as long as it’s, I set my own goals . . . you know . . . and doesn’t put too much pressure on individuals to achieve them.
Participant 12
It would really encourage you, I’d be ‘here, I gotta go out the night and all. I’ve got to fill this here in. see how many steps I’d done . . . It would encourage me just to be able to look and say ‘Look what I’ve done – 8000 today’, and then when it’s coming up to tea time I’d be going ‘come on let’s go and do another one . . . we’ll maybe try and get up to 10’ . . . I think that would be encouraging.
Participant 12
There was also a feeling that, even if goals were set, there should be flexibility to take account of ‘off’ days when existing conditions or tiredness might prevent them from achieving the targets:
I would get maybe, say for argument’s sake, about 50 yards before the pain, the pain gets gradually worse . . . you know. When would you want me to draw the line?
Participant 12
Finally, participants expressed concern about the added pressure that setting goals might put on them, despite the fact that doing this would motivate them to try to increase their physical activity levels:
I wouldn’t want the guilt on me . . . if I didn’t do the right amount. And I wouldn’t want to be feeling that I’d fallen behind . . .
Participant 12
Living alone
The use of a pedometer to set goals and monitor activity was viewed as a potentially useful solution for people living alone because it was something that could be carried out independently without any additional support:
. . . even in the house, you could do that on your own with the pedometer . . . that would interest me.
Participant 5
Participants expressed a view that people living alone could easily slip into levels of non-activity because there was no one else to motivate or encourage them to take part in physical activity. They saw the pedometer as a source of motivation and encouragement:
And in fact in a family environment possibly the, the advantages might be doubtful. But for anyone living alone. I think it would be a very useful in that it’s quite easy to lapse into a way of life which . . . would predominantly inactive . . .
Participant 3
Theme 4: characteristics of peer mentoring
Social support in the form of peer mentoring was viewed favourably by participants, and comments suggested that, for a peer-led intervention to be successful, the specifics of both the peer and the activity should be considered. In addition, participants suggested that they should have the opportunity to meet with the peer mentor at least once per week. Some participants said they would prefer to have peer contact by telephone, whereas others preferred face-to-face contact, and at a neutral location rather than at their home.
Mentoring for physical activity
Participants identified key components of the activity that needed to be addressed in order to motivate them to take part. First, the activity needed to be well planned, in terms of both timing and route of walking, but arrangements needed to be flexible:
When he phones and lets you know what’s happening in advance then you can pre-plan what’s going to happen or pre-plan to go to these different activities.
Participant 11
In addition, participants felt that the programme planned should create opportunities to try new activities (e.g. swimming), to visit places within their local area or outside their area that they had not been before, or to resume previous activities that they had stopped because their circumstances had changed (e.g. as a result of illness). Attention should also be given to including activities that the participants themselves identified as being pleasurable:
I do feel peer mentoring can introduce you to new ideas. Just because you’re elderly, it doesn’t mean to say you can’t have new ideas . . . you can show the peer something that you enjoy doing . . .
Participant 4
Activities needed to reflect the shared interests and the capabilities of both the peer and the participant:
I’d be interested in what they [the peer mentor?] would like to do too.
Participant 4
People might be reluctant to go out with other people on the basis that they might be . . . walking much further than they would.
Participant 3
In addition, the benefits of any planned activities should be stated at the outset of the programme so that there could be an understanding of the purpose in order to increase motivation to participate:
. . . she said it was good for your health. Good for your mental health, and once she mentioned mental health sort of thing, people listened. And this’ll relax you and relieve stress of the day.
Participant 9
In addition, although the aim of the physical activity intervention may be to increase levels of activity, its other potential beneficial effects were of significance. One interviewee commented on how motivation to continue with the programme long term was derived from their positive experience of it, specifically reporting how it helped promote relaxation:
So relaxing. And that’s what got me motivated.
Participant 9
Attributes of the peer mentor
The attributes of the peer were considered to be key to ensuring the success of a peer-led intervention. Participants expressed a desire for the peer mentor to be not only experienced and well trained but also medically aware to build confidence and trust:
. . . if they have got experience, that can restore your confidence, offering advice, discussing problems . . . Problem-solving with you . . .
Participant 4
. . . the peer would need to know, look this woman has high blood pressure, and this one has diabetes and she needs to carry something . . . Now my friend, she knows me well, and she has asthma and see before we go out like, [XXXX] knows to have all her things with her and she, she lets me know. So, that I’m aware when I’m out with her that this is a bag she has this particular coloured inhaler in . . .
Participant 10
In addition, although participants did not feel that they needed to have the same demographic characteristics as the peer mentors, such as age, they did consider that a peer mentor needs to be someone who is themselves physically active and physically capable to help and provide encouragement. However, it was also noted that other resources should be readily available if help were needed:
Even a young person. It wouldn’t matter what age they were. I think a young person anyway would get you up and get you out.
Participant 9
As long as they were a wee bit more active, . . . that they were able to . . . erm. It would be no good if they were worse than me probably . . . these days we all carry mobile phones.
Participant 5
The only other characteristic of the peer that was identified as important was sex: it was generally felt that the peer mentor should be of the same sex as the participant. However, it was suggested that participants with partners may not need a peer.
The most important component of a peer-led intervention for participants was the relationship that would develop. All participants felt that the relationship should be based on friendship, and a few key components that could secure the success of friendship development were dentified. First, participants identified the need for the peer and participant to have shared interests, and several participants suggested that, similar to dating, the peer’s interests and the participant’s interests should be ‘matched’ to ensure compatibility:
. . . before a peer was selected, that . . . there was a list that you could put down the sort of things that interest you.
Participant 4
Well I would like go out with somebody that’s not doom and gloom. A bit of jokiness . . . that’s what you need. What would be important to walk with is two or three people that you can have a wee bit of banter. You don’t want to hear about the price of tea. You don’t want to hear about their aches and pains. I don’t want to be conferring about aches and pains with anybody. I would avoid having a walk.
Participant 13
Participants suggested that the peer should be voluntarily motivated to spend time with them rather than being paid to do so:
. . . rather than . . . someone who is duty bound, to go ‘walkies’ on Wednesday afternoon, no matter whether it’s pouring or, or the roads are icy or whatever . . .
Participant 4
There was a feeling that friendship would lead to a stronger bond of loyalty, which would act as a physical activity motivator. In other words, friendship would ensure that people would be more reluctant to let others down and incentivise them to meet and carry out activities with the peer:
There’s always the, the feeling that ‘well I should turn up’ because if I don’t I’m letting, letting them down.
Participant 3
Theme 5: finding solutions to barriers to physical activity
Participants were not aware of what problem-solving might involve until it was explained to them. However, it was one of the most preferred options, perhaps because participants felt that it was something that they did on a day-to-day basis anyway. It was considered that problem-solving should be integrated into an overall programme rather than being used as a standalone activity:
I mean, if you’re, you’re getting to the point of deciding that we’re going to have regular walks together you would then come down to the point of where you were actually going to go.
Participant 3
However, participants identified many potential barriers relating to physical activity, including health-related issues; for example, arthritis may prevent walking or gardening, fear for personal safety may prevent walking in areas that were not busy or crowded, and child-minding responsibilities meant that it could be difficult to find time for physical activity. In general, participants felt that finding solutions to barriers was a useful exercise and that problem-solving with another person could help them see the broader picture and find solutions that they may not find by themselves:
It’s great if somebody helps me, because I just see the barrier. You know? And then if somebody like you was to talk about I would go ‘yeah that would be a good solution there’, you know.
Participant 9
However, there was a concern that solutions needed to be applied, progress monitored and feedback given on an ongoing basis to ensure success:
It’s just not practical. No.
Participant 2
Theme 6: appealing attributes of the demonstration walk
The inclusion of a ‘demonstration walk’ as part of the proposed intervention, whereby the peer mentor and participant would go for a walk together, in order to identify potential routes and locations in the local area, was not widely welcomed. The majority of participants suggested that they might consider taking part in a peer-led walk on a weekly basis if the areas outlined in the peer-led activity (above) were addressed. In addition, two areas that could increase motivation to taking part in the demonstration walk were identified.
Group versus one-to-one peer walk
Participants felt that a demonstration walk, if performed as a group activity, could be more enjoyable than walking only with a mentor, as it could provide an opportunity to meet other people with common interests and friendships could be developed within the group:
Where there are other people like yourself who would welcome you, welcome your company.
Participant 4
There was also a feeling that a group could avoid some of the difficulties that perhaps may arise in a one-to-one peer relationship. Thinking of performing the activity as a group was considered to be less daunting and to offer greater opportunity to meet someone with similar interests and capabilities:
. . . maybe even three or four people, . . . because maybe in a group, it’s easier.
Participant 5
However, participants also perceived that, although the group had advantages over a one-to-one peer relationship, it could be less attractive because it felt more like a structured activity and could be a less flexible arrangement:
A structured appointment is, is not a good idea, um, because older people have good days and bad days or don’t fancy putting on a, a waterproof and braving the storm.
Participant 4
I don’t really like the idea of a structured appointment.
Participant 4
Recruitment to a peer-led walking programme
In considering preferred approaches to recruitment, some participants reflected on their experience of joining various clubs. Although they had joined clubs in response to seeing information about them, they knew of other people who would have been eligible to join but did not:
There’s a lot of people in the same situation as what I am in the area but they don’t seem to be coming forward.
Participant 11
There was discussion within the group about the targeted selection of individuals for invitation to join a peer mentoring intervention. It was considered that such an approach from someone from the intervention would have been a more effective method of encouraging recruitment and one participant described how a personal approach had engendered enthusiasm for accepting an invitation to join another group. Such a personal approach to recruitment, rather than relying on posters and leaflets, was considered appropriate in order to ensure common interests or capability among prospective participants:
Well I was stopped one time when, when I was in shopping and this guy stopped me and erm he was telling me that he was trying to set this group up for a certain age, well not a certain age, but a certain gender of people and he was telling me the activities that they plan . . .
Participant 11
It was emphasised in the discussion that information relating to how to join a peer mentor walking group should be made very clear and that the process of recruitment should be as simple as possible for older individuals.
Well, . . . I’m not sure how they get in contact with people or do you have to get in contact with them? They [walking groups] might well make it easier for people to get in touch with them.
Participant 3
Discussion
Interviews revealed that older adults have busy lives, including housekeeping and carer responsibilities, and therefore engaging in other regular physical activity is not seen as a necessity. Walking appeared to be a common and preferred activity across all participants.
Similar to previous research,52 the findings suggest that increasing physical activity in older adults is a complex phenomenon because of the interplay between physical, psychological and environmental factors. For example, the current study identified that health-related beliefs, social support and the characteristics of the local neighbourhood environment are important determinants of physical activity that need to be considered in the development of any physical activity intervention development. However, the current study findings revealed that there were key areas within each BCT which should be explored with a target population, in order to address their barriers to physical activity.
Participants reported leading busy lives, but are not necessarily physically active. To increase physical activity, interventions should seek to increase awareness of the recommended physical activity guidelines and highlight the key health, emotional and social benefits of physical activity for older adults in order to persuade older adults to prioritise physical activity.
Tailoring interventions to personalised physical activity goals, such as step goals,20 can lead to sustained increases in physical activity. Our participants reported that they liked the idea of goal-setting and self-monitoring because they are easy to do and because they can be integrated into existing routines, leading to increased self-efficacy for physical activity. 53 However, the interviews revealed the importance of participants setting their own goals and of the ease of use of monitoring devices, so attention must be paid to selecting the right activity monitor (simple, easy to read, easy to carry and robust) and to delivering appropriate training on how to use it, and allowing participants to set their own goals.
Providing physical activity in combination with appropriate levels of support (whether physical support, such as gardening tools or walking aids, social support, such as peer mentoring or demonstration walking, or simply providing physical activity within a social setting), could decrease feelings of social isolation and loneliness, and increase feelings of motivation, confidence and safety, as well as make physical activity more enjoyable and ultimately increase overall levels of physical activity. Interventions that capitalise on social inclusion, such as peer mentoring or a demonstration walk, have great potential to succeed in increasing physical activity. 52 However, the relationship with the peer or the group is important to ensuring success, so a matching exercise to ensure shared interests between the peer/group and participant is key.
Discussion about the environment identified several defining characteristics which could increase physical activity. For example, individuals suggested that important features of the environment included a facilitated environment (well-maintained paths, aesthetically pleasing, scenic, close to nature, free of pollution), a safe environment (free from traffic, cyclists, dogs and crime), and one that was accessible (local or not). They also mentioned bad weather as a barrier to physical activity. Therefore, planned activities should take place away from traditional physical activity locations (e.g. gyms), but in a physically supportive environment (one that is ‘walkable’), with transport readily available or provided, and which also provides indoor or protected locations to facilitate walking in any weather. In addition, discussions around the demonstration walk identified a need to ensure robust recruitment that was proactive and personal to promote participation.
Interviews revealed conflicting beliefs relating to health, with individuals expressing the belief that physical activity can prevent and improve symptoms of current health conditions, such as arthritis, but can also create health issues through physical exhaustion or make existing symptoms worse, which could result in a lack of independence and the loss of the ability to get out and about. Thus, pain management is key to increasing physical activity, as is the tailoring of physical activity to individual needs. 54
In summary, there is merit in utilising each of the BCTs explored within the context of this study as each addresses barriers to physical activity. Interventions which include goal-setting and feedback, problem-solving, social support through peer mentors and behavioural practice with a peer mentor, as identified through the interview discussions, are perceived to have the greatest likelihood of succeeding in increasing physical activity in an older population living in an area of socioeconomic disadvantage.
Chapter 4 Development of the ‘Walk with Me’ peer-led walking intervention to increase physical activity in inactive older adults
The first phase in developing the complex intervention was to gather relevant evidence and theory to develop a logic model for the intervention, including the proposed causal pathways and relevant outcome measures. From the rapid review of peer-led physical activity interventions, the most common groups of BCTs used previously were related to goals and planning; feedback and monitoring; social support; shaping knowledge; comparison of behaviour; repetition and substitution; and antecedents. The intervention development interviews also identified other BCTs specifically relating to the health benefits of physical activity (information about health consequences). The behaviour change wheel55 was used to map promising BCTs, that is, those that had been successfully used in previous interventions included in the rapid review and that it was deemed could be feasibly delivered within the proposed context, on components of behaviour that reflect multiple levels [motivation (reflective and automatic), opportunities (physical and social environment) and capability (physical and psychological)]. The main output from this stage was a shortlist of proposed BCTs, mapped on to key intervention functions, for inclusion in the design of a pilot RCT.
In the next stage, we explored the perceived feasibility and preferences for strategies, which included particular BCTs, through interviews with older adults from our target communities. Taking account of the interview findings enabled us to avoid and overcome potential barriers to implementation within the intervention design, and to incorporate elements which were perceived to facilitate walking.
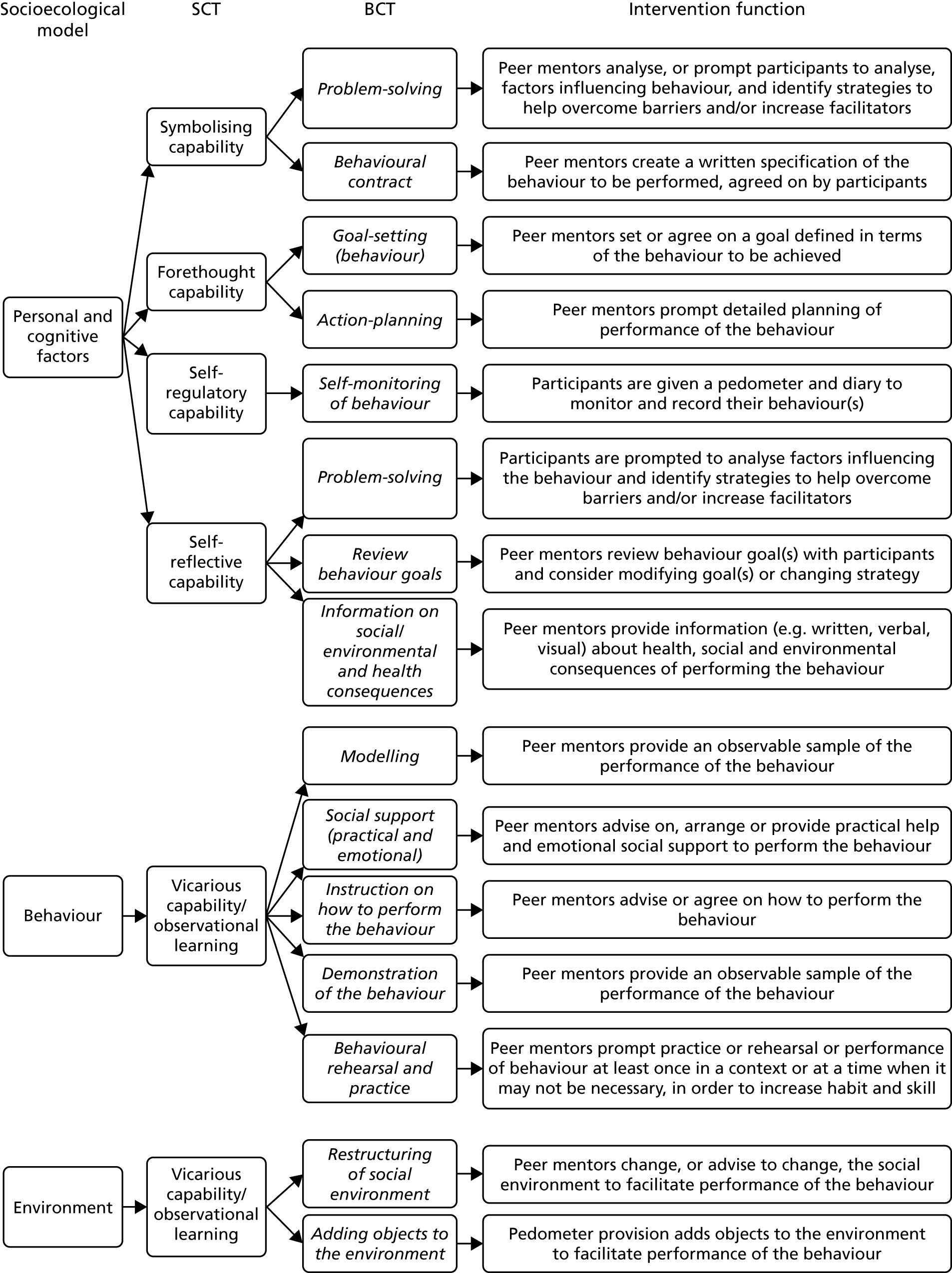
Social cognitive theory56 was used to provide a theoretical framework for designing the intervention as it maps well onto the socioecological model and the role that the physical, psychological and environmental factors identified in the interviews play in physical activity. SCT proposes that personal, environmental and behavioural factors reciprocally influence behaviours. BCTs identified through the rapid review were mapped onto the core set of psychosocial determinants and intervention functions of SCT (i.e. self-efficacy, outcome expectations, goals, and impediments and facilitators) (Figure 5). In addition, the socioecological model was used to provide a framework for a multilevel intervention design57 that addresses multiple levels of determinants, including individual, social and environmental factors. In addition to individual factors (such as feedback on current behaviour), we planned to address social factors, by providing peer mentors to act as a social support for change and environmental factors by matching the programme to local environmental opportunities.
FIGURE 5.
Behaviour change techniques mapped to intervention functions, SCT and socioecological model.
The logic model developed for this study (Figure 6) illustrates how the key intervention functions (BCT groupings) align with the basic capabilities of SCT,56 and how the process and behaviour outcomes of the intervention were measured.
FIGURE 6.
‘Walk with Me’ logic model. EQ-5D, EuroQol-5 Dimensions; GHQ-28, General Health Questionnaire-28 items; LSN, Lubben Social Network; WEMWBS, Warwick–Edinburgh Mental Well-being Scale.
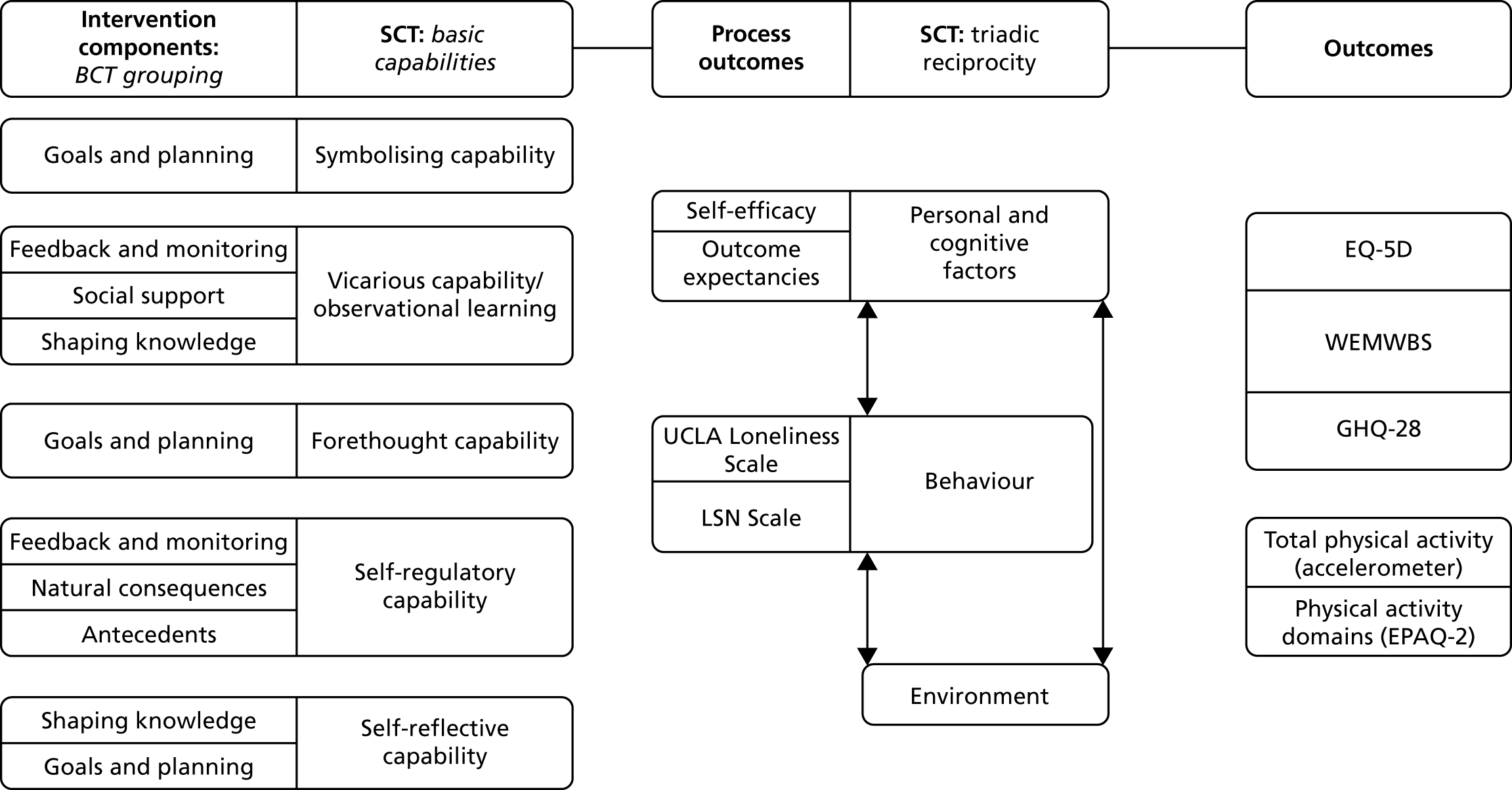
Chapter 5 Methods for a pilot randomised controlled trial of a peer-led walking programme to increase physical activity in inactive older adults
The aim of this phase of the project was to test the feasibility of delivering and evaluating a complex, peer-led, multicomponent, physical activity intervention in socioeconomically disadvantaged community-dwelling older adults.
The objectives of the pilot RCT were to:
-
determine the most efficient methods of recruitment to a peer-led physical activity intervention in older adults
-
assess the resources needed for the development of a future definitive trial
-
assess the feasibility of a RCT of a peer-led walking intervention in older adults in terms of rates of recruitment, retention of participants and data completeness, the administration of outcomes and the acceptability of the intervention
-
generate data to inform what sample size would be required in a definitive trial of a multilevel peer-led physical activity intervention, based on the variability in objective measurements of physical activity, and recruitment and attrition rates
-
measure the resource use associated with the intervention and estimate costs
-
pilot the use of a health and social care service use instrument and summarise the resource use and costs per group.
Participants
This study targeted community-dwelling older adults, aged 60–70 years and living in areas of socioeconomic disadvantage. For this study, socioeconomically disadvantaged communities were defined as those falling within the most disadvantaged quartile of electoral wards, based on the NIMDM (URL: www.nisra.gov.uk). For ease of administration, the pilot study was conducted in the SEHSCT and the Northern Health and Social Care Trust in Northern Ireland, which cover a large geographical area and a mix of urban and rural settings.
Recruitment
Previous research has identified difficulties in recruiting participants from socioeconomically disadvantaged communities. 58 We therefore employed a range of active and passive recruitment strategies to maximise the potential for efficient recruitment and to explore which methods appeared most effective. Active strategies involved the identification and referral of potential participants by community and voluntary organisations and in-person presentations to community groups based in target communities. Passive recruitment methods included the following: sending study information, along with a letter from their general practitioner (GP), to suitable patients from primary care practices in target communities; the distribution of leaflets and posters through general practices, community centres, libraries, health centres, faith-based groups and churches; and the use of e-mail lists and social media outlets of project partners. All practices with postcodes in the target electoral wards were identified from the Business Services Organisation records in Northern Ireland. Eleven practices were chosen for initial contact and selected with the aim of recruiting participants from a range of geographical locations and with varied characteristics. The practices were contacted by telephone and brief information was passed to the practice manager with an invitation to meet with the researcher to discuss the study in further detail. Each practice was offered an honorarium of £150 in recognition of the time involved and the administration costs of setting up the study, with a further £50 if two or more of the practice’s patients participated in the study.
Individuals who wished to participate were asked to contact the study team by telephone, in writing or by e-mail. Following initial contact, participants were screened for eligibility and invited to return written consent to participate. Those participants who were eventually recruited were asked how they learned of the study.
Eligibility criteria
The following inclusion criteria were employed to assess eligibility:
-
male or female aged 60–70 years
-
living in a socioeconomically disadvantaged community (defined as the lowest quartile of super output areas according to the NIMDM)
-
competent to give informed consent
-
not currently physically active (assessed using the General Practice Physical Activity Questionnaire)59
-
community dwelling (i.e. living in their own home)
-
planning to stay in the current residence during the next year
-
able to communicate in English
-
no self-reported recent history (within the last 6 months) of myocardial infarction or stroke, or physical limitations that would limit ability to participate in a walking programme (assessed using the Physical Activity Readiness Questionnaire). 60
Randomisation and allocation concealment
After baseline outcome measures were completed, participants were randomised to the intervention or the control group using block randomisation with randomly permuted block sizes. An independent statistician from the Northern Ireland Clinical Trials Unit generated the randomisation sequence using a computer program, and treatment allocations were concealed in sealed, sequentially numbered opaque envelopes. The envelope was not opened until after completion of baseline measures, at which point participants were informed of their group allocation.
‘Walk with Me’ intervention
The 12-week intervention comprised three stages: (1) activation (weeks 1–4), (2) goal-setting and problem-solving (weeks 5–8) and (3) signposting participants to other activity programmes in their community to encourage them to maintain their activity (weeks 9–12). Typically, meetings between mentors and participants began in an environment (community centre/coffee shop close to the planned walk location) where they could discuss the participants’ physical activity patterns in the previous week. During this time, participants and mentors also completed study records using weekly templates (see Appendix 3), discussed goal-setting and problem-solving, and set goals for the coming week. The structure for a typical meeting was outlined during the initial introductory session between a member of the research team, the participant and the mentor, and the participants and mentors were encouraged to continue this format to help establish a rapport with one another and to facilitate the delivery of the intervention content. Following a discussion of the planned duration and route, mentors and participants would then take part in a walk in the local environment/park. At the end of a typical session, plans were made to meet the following week, or 2 weeks later, to progress the programme. A full list of the BCTs to be delivered in the intervention is given in Table 5.
| Grouping and BCTs (expanded) | Intervention components (informed by the BCT Taxonomy v1) |
|---|---|
| Goals and planning | |
| Goal-setting (behaviour) | Peer mentors will set or agree on a goal defined in terms of the behaviour to be achieved |
| Action-planning | Peer mentors will prompt detailed planning of performance of the behaviour by including specific reference to (at least one of) context, frequency, duration and intensity of physical activity. Context may be environmental (physical or social) or internal (physical, emotional or cognitive) |
| Problem-solving | Peer mentors will analyse, or prompt the person to analyse, factors influencing the behaviour, and generate or select strategies that include overcoming barriers and/or increasing facilitators |
| Review behaviour goals | Peer mentors will review behaviour goal(s) jointly with the person and consider modifying goal(s) or behaviour change strategy in light of achievement |
| Behavioural contract | Peer mentors will create a written specification of the behaviour to be performed, agreed with the person and witnessed by another |
| Feedback and monitoring | |
| Self-monitoring of behaviour | Peer mentors will distribute (via the research team) pedometers and step diaries to the people whom they are mentoring so that they may monitor and record their physical activity behaviour(s) as part of the intervention |
| Social support | |
| Social support (practical) | Peer mentors will advise on, arrange or provide practical help for the performance of the behaviour |
| Social support (emotional) | Peer mentors will advise on, arrange or provide emotional social support for the performance of the behaviour |
| Shaping knowledge | |
| Instruction on how to perform the behaviour | Peer mentors will advise or agree on how to perform the behaviour |
| Natural consequences | |
| Information about health consequences | Peer mentors will provide information (e.g. written, verbal, visual) about health consequences of performing the behaviour |
| Information about social and environmental consequences | Peer mentors will provide information (e.g. written, verbal, visual) about social and environmental consequences of performing the behaviour |
| Comparison of behaviour | |
| Demonstration of the behaviour | Peer mentors will provide an observable sample of the performance of the behaviour |
| Repetition and substitution | |
| Behavioural rehearsal and practise | Peer mentors will prompt practice or rehearsal of the performance of the behaviour one or more times in a context or at a time when the performance may not be necessary, in order to increase habit and skill |
| Habit formation | Peer mentors will prompt rehearsal and repetition of the behaviour in the same context repeatedly so that the context elicits the behaviour |
| Graded tasks | Peer mentors will set easy-to-perform tasks, making them increasingly difficult, but achievable, until the behaviour is performed |
| Antecedents | |
| Adding objects to the environment | The provision of pedometers will add objects to the environment in order to facilitate performance of the behaviour |
| Restructuring of the social environment | Peer mentors will change, or advise the participant to change, the social environment in order to facilitate performance of the behaviour |
The intervention began with a face-to-face meeting between the peer mentor, the participant and a member of the research team. The role of the member of the research team was to facilitate initial discussions. At this introductory meeting, the discussion focused on building rapport, defining the role of the peer mentor and describing the main BCTs of the intervention (e.g. goal-setting, reviewing behavioural goals, problem-solving). At the end of this initial meeting, the participant was given a pedometer (Yamax SW-200) and a participant information and resource booklet which contained study contact details, weekly step diaries and a physical activity action-planning template. The participant and peer mentor undertook a short (5-minute) ‘familiarisation’ walk, during which the participant was shown how the pedometer worked and the accuracy of the device in recording steps was checked. The meeting concluded with the exchange of contact details and a plan to meet the following week.
The initial period of the intervention (activation stage, weeks 1–4) was designed to enable the participant and peer mentor to establish a rapport (e.g. by building a trusting relationship that is necessary for successful peer mentoring). During the first week, the participant recorded their baseline levels of physical activity using the pedometer. Following this, the participant, with the support of the mentor, set an initial step goal based on the average steps per day during the first week. 61,62 Participants and mentors discussed what a reasonable goal for the next week would be. The participant was encouraged to consider increasing their daily steps by 500 steps per day and continuing for the next week (approximately 5 minutes/day), and then the mentor and participant discussed how many more steps per day would be practical. Once a goal was decided on, the participant was asked to rate, on a 10-point Likert scale, how confident they were that they could meet this goal. If they rated their confidence as ≤ 7, the goal was revised downwards until they rated their confidence as ≥ 8. The participant and mentor then drew up an action plan to incorporate additional physical activity into the participant’s weekly schedule, and the participant agreed to meet to walk with their mentor at least once every 2 weeks. At these walks the peer mentor was able to advise the participant of the frequency, intensity, time and type of physical activity they should be taking part in (e.g. by discussing the physical activity guidelines for older adults, copies of which were included in the participant information and resource booklet).
The programme continued (weeks 5–8) with the participant and mentor meeting regularly to walk and discuss goals/barriers to increasing physical activity. These meetings enabled the mentor to demonstrate the appropriate walking pace to achieve moderate-intensity physical activity and enabled the participant to set individual physical activity goals by taking into consideration their capabilities. Weekly activity targets were reviewed and agreed. If the participant had difficulty increasing their physical activity, the mentor discussed strategies to overcome barriers to increasing physical activity (e.g. by discussing opportunities for physical activity in the local neighbourhood environment). During this period, the mentor and participant began to make plans to attend a local community- or leisure centre-based walking group or other local physical activity opportunities (to take place during weeks 10–12) that would help the participant maintain their activity level when the structured component of the intervention came to an end.
The final 4 weeks of the intervention emphasised behaviour rehearsal and practice by the participant walking regularly in a locally accessible physical activity environment (e.g. local park). In order to increase physical activity habit formation, the peer mentor prompted rehearsal and repetition of physical activity behaviour by meeting and discussing physical activity goals with the participant, via weekly/biweekly walks and contact over the telephone. The final weeks of the structured component of the intervention provided an opportunity for the participant and mentor to discuss other community-based physical activity opportunities and to attend a local community group to facilitate the maintenance of physical activity behaviours at the conclusion of the 12-week intervention.
Peer mentors
Peer mentor recruitment
To assist in delivering the programme, peer mentors were recruited, prior to and concurrently with participant recruitment, through partnerships with local community organisations, leisure centres and general practices and via referral from the physical activity co-coordinator based in the health and social care trust. Posters and leaflets were used to invite individuals who lived in the target areas, were aged 60–70 years and who were already physically active to consider participating in the study as peer mentors and to contact the research team. In addition, individuals who volunteered to take part in the intervention but were not eligible as they were already sufficiently physically active (i.e. meeting the current recommended level of 150 minutes per week) were invited to participate as a peer mentor.
Before being appointed as a peer mentor, these individuals attended a meeting with a member of the research team (typically at a local community venue), where they were provided with information on the study and the role of mentors within it. During this initial meeting, potential peer mentors were asked to (1) confirm their willingness to undergo the required training to deliver the programme, (2) describe their attitude and commitment to helping others increase their physical activity levels and (3) to complete baseline assessment measures of their health, well-being and physical activity. This session gave the prospective mentor an opportunity to discuss their personal interests, information which was used to assist in pairing the peer mentors with participants. Peer mentors completed AccessNI clearance prior to being matched with potential participants.
Matching and introducing peer mentors to participants
The information gained in the meeting between the researcher and potential peer mentor was used to build a mentor profile, identifying their activity likes and dislikes, and activity habits. This profile was used to facilitate matching mentors with study participants. Mentors and participants were also matched by sex and geographic location.
A member of the research team facilitated the initial introductory meeting between the mentor and the participant, and the structure for a typical meeting was outlined during this meeting (see ‘Walk with Me’ intervention). Participants and mentors were encouraged to continue this format in order to support the development of a rapport between them and to facilitate delivery of the intervention content.
Peer mentor training
Peer mentors received two individually delivered 1-hour face-to-face, one-to-one training sessions, 1 week apart, delivered by a member of the research team, and guided by the peer mentor training and support manual developed for this intervention. The aim of these sessions was to develop their skills, knowledge and confidence to promote physical activity among their peers. The training included information on the role and responsibilities of the peer mentor, including participant confidentiality; knowledge and education about physical activity; behavioural change techniques, including setting goals and monitoring performance; and problem-solving and practical approaches to overcome potential barriers to physical activity. During the training sessions mentors received information on the ‘Walk with Me’ programme, including the level of commitment required (biweekly meetings over a 12-week period); the main tasks and requirements; information about physical activity guidelines for older adults; education about BCTs and their role in the programme; how to model physical activity behaviours; helping their peer complete and record programme activities; and reporting on activities or providing feedback to the project team. Case studies were included in the training on each BCT, based around scenarios that the peer mentor may face (e.g. overcoming potential barriers to increasing physical activity). In addition, peer mentors engaged in role play to practise the use of BCTs, such as delivering instructions for using the pedometer and setting goals.
Mentors were trained in how to build and sustain an effective mentoring relationship with a peer, as well as skill-building in the areas of active listening, communication and providing social and emotional support. In addition, peer mentors received a training and support manual to promote intervention fidelity. The manual included information on the areas of the programme covered in the training sessions and copies of all of the materials they needed to deliver the intervention. They were also given a copy of the Public Health Agency information leaflet Ageing well by being active every day,63 which contains brief information on the physical activity guidelines for older adults and brief advice for older adults on how to become more active.
Additional follow-on support was delivered to mentors during the programme. A member of the research team met with the peer mentors three times (once a month), for 1 hour. This was to ensure that the mentor was still comfortable with the content of the intervention and involved a brief review of the original training, including the techniques of goal-setting and monitoring, a discussion of any issues which had arisen with participants (such as not turning up or not getting on), and the focus for the next phase of the intervention.
Ongoing support for peer mentors
During the pilot RCT, peer mentors were given open telephone access to a research team member for advice/support. They were also contacted by the project manager at least once per fortnight and asked to give an update on the programme, to identify proactively any problems with progress in the intervention delivery or with participant contact and engagement. During the course of the intervention, no issues were identified with participant/peer mentor contact and engagement.
Control group
After the collection of baseline data was complete, individuals allocated to the control group were given an information booklet on active ageing (the same booklet that was given to the intervention group). They did not receive any additional support to change their activity over the course of the research study. After the 6-month data collection point they were given the opportunity to discuss with a member of the research team the availability of local physical activity opportunities (e.g. local walking groups).
Outcome measures
Outcome measures were assessed at baseline, post intervention (12 weeks) and 6 months after baseline. The primary outcome measure was average daily minutes of moderate to vigorous physical activity (MVPA), measured over a 7-day period using a waist-worn ActiGraph GT3X+ accelerometer (ActiGraph, LLC, Pensacola, FL, USA). Non-wear time was defined as a run of zero counts lasting > 60 minutes. At least 5 valid days (including one weekend day) were required for inclusion in the analysis; a valid day was defined as a 24-hour period in which > 600 minutes of wear time were recorded. 64 Activity counts were recorded in 10-second epochs. Freedson cut-off points were applied to the data to calculate time spent daily sedentary (≤ 100 counts/minute) or undertaking light (101–1951 counts/minute), moderate (1952–5724 counts/minute) or vigorous (≥ 5725 counts/minute) physical activity. 65
To explore how participants adjusted their daily physical activity routines in response to the intervention, time spent in recreational-, occupational-, domestic- and travel-related physical activity was assessed using the validated EPIC Physical Activity Questionnaire (second version) self-reported physical activity questionnaire. 66
Secondary outcomes (see Figure 6) included physical and mental health, and mental well-being and were measured using the Short Form questionnaire-12 items (SF-12),67 the General Health Questionnaire-28 items (GHQ-28)68 and the Warwick–Edinburgh Mental Well-being Scale (WEMWBS). 69,70 Health-related quality of life was assessed using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), questionnaire. 71 Social engagement was measured with the UCLA Loneliness Scale72 and the Lubben Social Network (LSN) Scale. 73 Physical activity and social activity self-efficacy (10-point Likert scale rating confidence in ability to remain physically or socially active despite circumstances such as bad weather, boredom and pain),74 and physical activity and social activity outcome expectancies (five-point Likert scale rating likelihood of outcomes such as good health, improved appearance, reduced stress, companionship and motivation),75 were also measured. The internal consistency for the self-efficacy and outcome expectancy scales was high (Cronbach’s α = 0.91, 0.92, 0.88 and 0.91, respectively).
Process evaluation
Based on the MRC guidance on process evaluation,76 we used a mix of approaches. The fidelity of the delivery and receipt of the intervention was assessed through structured observation of intervention delivery by a member of the research team responsible for mentor training, semistructured interviews and focus groups with peer mentors and participants as part of the post-intervention follow-up (see Chapter 7), and review of intervention records and participant diaries. A member of the research team observed all of the first meetings between the peer mentor and the participant in person. The delivery was reviewed with the peer mentor as part of ongoing training. In addition, for each peer mentor, a randomly selected further meeting between them and a participant was audio-recorded to assess the content fidelity of delivery. The dose of intervention delivered was assessed by asking the peer mentors and a random sample of 12 trial participants to complete weekly checklists and record a diary of contacts. The peer mentor diary included information on the number of attempts to make contact with participants and the duration of each successful contact.
To assess if the intervention was working through the pathways proposed in the intervention logic model, changes in physical activity and social activity self-efficacy, and physical activity and social activity outcome expectancies, were measured. Post-intervention focus groups and semistructured interviews (see Chapter 7) were used to provide context to the research by examining how potential external factors may have influenced the delivery and functioning of the intervention.
Feasibility of conducting a definitive trial
The feasibility of conducting a definitive trial, defined as the ability to recruit participants within the time frame and retain a significant proportion of them within the trial, was assessed based on the recruitment and attrition rates and the qualitative feedback from participants and mentors. The recruitment rate was assessed by calculating the total number recruited as a proportion of the predefined target of 60 participants, within the time frame of the study. We predetermined that we would not proceed to a main RCT unless a recruitment rate of ≥ 60% was achieved. Attrition was measured as the proportion of participants who did not complete outcome measures at 6 months after baseline, either because they dropped out or because they failed to complete outcome measures. We predetermined that we would not proceed to a main RCT unless the attrition rate was < 30%, calculated as the number of participants who returned data at 6 months as a proportion of the number who started the study.
In addition, the decision to proceed to a definitive trial would be informed by the rates of unexplained adverse events in the intervention and the peer mentors’ views on feasibility of delivering the intervention, whether or not it could be delivered within the timeline and the sufficiency of the training and ongoing support.
Acceptability of the intervention
The acceptability of the intervention was assessed through a post-study exit questionnaire. The questionnaire, which was similar to that used in a previous physical activity intervention,77 required intervention group participants to rate their experience of the intervention and satisfaction with the information they received about this study.
In addition, all participants in the intervention group were invited to attend post-intervention focus groups or one-to-one semistructured interviews (see Process evaluation) with a researcher independent of the intervention delivery to discuss their views on the feasibility and acceptability of the intervention. Participants were asked to explore reasons for success in the intervention and challenges to increasing their physical activity. They were asked what they would change about the intervention if they were to take part again.
Peer mentors also were invited to attend separate post-intervention focus groups or one-to-one semistructured interviews (see Process evaluation) to provide feedback on their experiences of the intervention. Topics included challenges to intervention delivery, perceived success, barriers to implementation and suggestions on how to improve the delivery of the intervention. Primary questions related to the different BCTs employed, reviewing each in turn, considering what worked to increase engagement in walking for some individuals and what did not work for others. Control group participants were invited to attend semistructured interviews in which they were asked to give their feedback on their involvement in this arm of the study and their motivation to become involved in the research.
Transcripts from the focus groups and interviews were independently analysed using content and thematic analysis by two researchers. 50 These focus groups and interviews will further inform the development and design of a fully powered trial by enabling appropriate refinement of the intervention’s components and delivery for the subsequent RCT.
Assessment of harms
Although there is a low risk of harm from walk interventions, participants were encouraged to report adverse events resulting from activity (e.g. musculoskeletal problems, shortness of breath or falls). Adverse events reported by participants were recorded on a standard proforma. 78
Sample size
As this was a pilot study, no formal sample size calculation was conducted. However, it was expected that 60 participants would provide sufficient information to estimate variability in the primary outcome and inform the decision around a predicted effect size, to inform a sample size calculation for a fully powered future trial.
Measurement of the resource use associated with the intervention and associated costs
We measured costs from a public sector funder perspective. Resources were categorised according to the stage in the process at which they were incurred [planning and preparation for delivery (stage 1) or intervention delivery (stage 2)], in keeping with other trials of public health intervention. 79–82 Resources associated with the development of the intervention (stage 0) were not included in the overall costs, as they would not be incurred should the intervention be adopted into practice in the future. Details about the steps involved in developing the intervention are presented in Chapter 1 of this report. Stage 1 costs included recurring costs associated with the intervention materials and the delivery of peer mentor training by a trainer. Stage 2 costs covered trainer input associated with the initial intervention meeting between trainer, peer mentor and participant, ongoing meetings between trainer and peer mentors, and the provision of pedometers.
As the identification of the relevant resources occurred during study set-up, the research team was able to record the resource use prospectively over the course of the trial. The trainer in the trial was a post-doctoral research fellow employed by a university. However, we envisage that, if ‘Walk with Me’ were rolled out, then the role of the research fellow would be replaced by a health improvement officer (band 5). Training of mentors took place in community centres and coffee shops and no costs were associated with the training location. All mentors required criminal records checks by AccessNI and were paid expenses.
Piloting the health service use log
Although the trial was not designed to estimate cost-effectiveness, participants were asked to keep a record of their use of health and social care services using a study-specific log over the 6-month study period in order to pilot the use of the tool for a future definitive trial from a health service perspective (see Appendix 4). For each participant, the quantity of each service used was multiplied by the unit cost of that service to estimate the total cost of use. The costs for each service were then summed to calculate the total cost of all health service use for each participant. Unit costs were obtained from publicly available sources and set at 2017 prices (Table 6). 79 At the end of the health service use log, we asked participants to express how much they agreed/disagreed with particular statements about the log using a five-point Likert scale. As the time horizon for the analysis was < 1 year, discounting of costs was not necessary.
| Service use | Unit cost (£) | Source |
|---|---|---|
| GP visit | 38 | Unit Costs of Health and Social Care 2017, p. 16279 |
| GP telephone call | 15 | Unit Costs of Health and Social Care 2017, p. 16479 |
| GP out of hours | 94 | Unit Costs of Health and Social Care 2017, p. 162,79 11.4-minute consultation and 12-minute travel, £242 per hour |
| GP nurse | 11 | Unit Costs of Health and Social Care 2017, p. 160,79 based on 15.5 minutes and £42 per hour |
| Physiotherapist | 45 | Unit Costs of Health and Social Care 2017, p. 2079 |
| Podiatrist | 45 | Unit Costs of Health and Social Care 2017, p. 203,79 hospital-based professional staff |
| A&E visit | 246 | Unit Costs of Health and Social Care 2017, p. 110,79 see and Treat and Convey |
| Hospital clinic/outpatient | 137 | Unit Costs of Health and Social Care 2017, p. 110,79 weighted average of all outpatient attendances |
Statistical analysis
As this was a pilot study, statistical tests to determine the effectiveness of the intervention were not performed. Instead, the effects of the intervention were represented by point estimates. For the change in primary and secondary outcomes from baseline to each follow-up time point, 95% confidence intervals (CIs) were calculated. 83 Analysis was conducted by a researcher blind to group allocations. The Office for Research Ethics Committees Northern Ireland gave ethics approval for the study (reference number 14/NI/1330) and the trial was registered as ISRCTN23051918. Peer mentor recruitment commenced in July 2016. Baseline recruitment of participants commenced in December 2016, and post-intervention and 6-month follow-up data were collected between March 2017 and January 2018.
Chapter 6 Randomised controlled trial of a peer-led walking programme to increase physical activity in inactive older adults: results
To assess the feasibility of conducting a fully powered RCT of a peer-led walking programme in older adults, the objectives of the study were to (1) determine the most efficient methods of recruitment to a peer-led physical activity intervention in older adults; (2) assess the resources needed for the development of a future definitive trial; (3) assess the feasibility of a trial of a peer-led walking intervention in older adults in terms of rates of recruitment, retention of participants and data completeness, the administration of outcomes and the acceptability of the intervention; (4) generate data to inform what sample size would be required in a definitive trial of a multilevel peer-led physical activity intervention, based on the variability in objective measurements of physical activity and recruitment and attrition rates; (5) measure the resource use associated with the intervention and estimate costs; and (6) pilot the use of a health and social care service use instrument and summarise the resource use and costs per group.
Objective 1: participant and peer mentor recruitment and retention
General practices were selected to represent a range of geographical locations within the target socioeconomically disadvantaged areas. Of the 11 GP surgeries that were invited to participate, nine agreed to participate and to display a poster in their waiting areas. Seven of the 11 practices also agreed to send postal invitations (400 letters were sent to eligible patients), and in another practice the GPs decided that they would invite patients verbally at a weekend clinic at which influenza vaccinations were given (20 patients were invited). These five practices also agreed to opportunistically invite patients identified as eligible during surgeries (none was recruited).
Participant recruitment strategies also included presentations to community groups and older adults’ groups meeting in local libraries (n = 13), and display of flyers/posters in leisure centres (n = 4). In addition, five community-based older adults’ associations e-mailed their members to tell them about the study; an advert was placed in a free newspaper; and two organisations that telephone older and vulnerable adults on a daily basis informed their clients about the study. Challenges in and opportunities for recruiting individuals to physical activity research studies from general practice are discussed further in Chapter 8.
The peer mentors who delivered the intervention (n = 13) were recruited through a mixture of local community groups (n = 6), leisure centres (n = 3), general practices (n = 2) and referral from the physical activity co-coordinator based in the health and social care trust (n = 2).
To identify community groups to approach, we spoke to physical activity co-ordinators, searched online for groups and e-mailed 25 local elected representatives asking for a referral to groups in their community. In addition, we attempted to place adverts about the study in local newspapers, but this was not possible without incurring substantial cost.
Overall, from these various sources, 105 individuals (36 men and 69 women) contacted the study team and expressed an interest in participating. Of these, 56 individuals heard about the study through a letter from their GP and a further four individuals responded after seeing a poster or flyer in their health centre. Nineteen individuals responded to an e-mail from a leisure centre or an association for older adults and two individuals responded after seeing a flyer in their leisure centre. Fourteen individuals responded after attending a presentation at a community group run in a library, leisure centre or community centre. Four individuals heard about the study from a friend or family member and a further four individuals heard about the study through the health trust. Finally, one individual heard about the study through a poster in their local library and one individual through an advert in a community newspaper.
In total, 50 (48%) of the 105 individuals who expressed an interest in the study were deemed eligible and entered the study. The reasons for excluding individuals are summarised in Figure 7 (27 individuals were already physically active, 26 individuals were too busy to commit to the intervention or not interested in participating when they received further details and two individuals were excluded as they were too old and were not well enough to be eligible to participate). Therefore, 50 of the prespecified sample size of 60 participants were recruited (82%) over a 12-month period.
FIGURE 7.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the ‘Walk with Me’ pilot RCT.
Of the 50 individuals who participated, 26 (52%) received a letter from their GP inviting them to take part. A further 14 individuals (28%) took part after seeing a flyer or poster in their health centre. Five individuals (10%) were referred into the study by a friend, four individuals (8%) were recruited from groups in local libraries and one individual (2%) was recruited by a community organisation.
Before the end of the 12-week intervention, seven people had dropped out and did not complete the post-intervention measurements (four reported a change in their life circumstances, e.g. an increasing care commitment for an elderly relative such that they believed that they could not commit fully to the project; three reported a change in health condition/completion of a surgical procedure, the cause of which was unrelated to participation in the study), resulting in a retention rate at 12 weeks of 86% (43/50). All 43 participants (intervention group, n = 22; control group, n = 21) who were retained in the study returned data at 6 months. The percentage of participants who dropped was higher in the control group (19%) than in the intervention group (12.5%), indicating that the intervention did not discourage participation.
Participant characteristics
The characteristics of participants are summarised in Tables 7 and 8 and the flow of participants through the trial is described in Figure 7.
| Outcome (units or possible range) | Intervention group (N = 24), n (%) | Control group (N = 26), n (%) | Overall (N = 50), n (%) |
|---|---|---|---|
| Sex | |||
| Male | 5 (21) | 12 (46) | 17 (34) |
| Female | 19 (79) | 14 (54) | 33 (66) |
| Marital status | |||
| Married | 16 (67) | 21 (80) | 37 (74) |
| Single | 2 (8) | 3 (12) | 5 (10) |
| Widowed | 6 (25) | 1 (4) | 7 (14) |
| Separated | 0 (0) | 1 (4) | 1 (2) |
| Employment status | |||
| Retired | 16 (67) | 21 (80) | 37 (74) |
| Working full-time | 6 (25) | 3 (12) | 9 (18) |
| Working part-time | 2 (8) | 1 (4) | 3 (6) |
| Volunteer worker | 0 (0) | 1 (4) | 1 (2) |
| Car owner | |||
| Yes | 21 (88) | 25 (96) | 46 (92) |
| No | 3 (12) | 1 (4) | 4 (8) |
| Characteristic | Intervention group (n = 24) | Control group (n = 26) | Overall (n = 50) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| Age (years) | 64.04 (–3.47) | 62.58 to 65.51 | 64.92 (3.27) | 63.54 to 66.3 | 64.48 (3.36) | 63.5 to 65.46 |
| Sedentary time (minutes/day) | 705.5 (–196.01) | 620.74 to 790.27 | 695.22 (399.39) | 526.57 to 863.87 | 700.25 (313.3) | 608.26 to 792.24 |
| Light-intensity physical activity (minutes/day) | 172.21 (47.89) | 151.5 to 192.92 | 176.75 (55.86) | 153.16 to 200.34 | 174.53 (51.6) | 159.38 to 189.68 |
| Moderate-intensity physical activity (minutes/day) | 28.58 (13.56) | 22.71 to 34.44 | 34.38 (923.01) | 24.67 to 44.1 | 31.54 (19.01) | 25.96 to 37.12 |
| Vigorous-intensity physical activity (minutes/day) | 0.57 (1.39) | 0 to 1.17 | 0.57 (90.94) | 0.17 to 0.96 | 0.56 (1.17) | 0.22 to 0.91 |
| MVPA (minutes/day) | 29.15 (13.8) | 23.18 to 35.11 | 34.95 (23.44) | 25.05 to 44.84 | 32.11 (19.35) | 26.43 to 37.79 |
| Steps per daya | 5989 (1913) | 5162 to 6816 | 6693 (2587) | 5601 to 7786 | 6349 (2286) | 5678 to 7020 |
| Number of valid days | 6.61 (0.66) | 6.32 to 6.89 | 5.96 (1) | 5.54 to 6.38 | 6.28 (0.9) | 6.01 to 6.54 |
| Domestic physical activity (minutes/day) | 1167.76 (583) | 886.77 to 1448.76 | 1120.98 (846.49) | 735.67 to 1506.3 | 1143.2 (724.49) | 911.5 to 1374.91 |
| Occupational physical activity (minutes/day) | 15.44 (53.99) | –9.13 to 40.02 | 12.35 (54.41) | –13.12 to 37.82 | 13.93 (53.54) | 0 to 30.83 |
| Recreational physical activity (minutes/day) | 311.17 (339.99) | 122.89 to 499.45 | 364.62 (422.46) | 147.41 to 581.82 | 339.56 (380.85) | 202.25 to 476.88 |
| GHQ-28 (0–84) | 15.09 (9.24) | 11 to 19.19 | 18.75 (12.81) | 13.34 to 24.16 | 17 (11.27) | 13.65 to 20.35 |
| SF-12 | ||||||
| Total score (12–57) | 26.55 (2.52) | 25.37 to 27.73 | 26.54 (1.91) | 25.73 to 27.35 | 26.55 (2.18) | 25.88 to 27.21 |
| Physical health score (6–18) | 12.95 (1.23) | 12.37 to 13.53 | 12.67 (1.24) | 12.14 to 13.19 | 12.8 (1.23) | 12.42 to 13.17 |
| Mental health score (6–27) | 13.78 (2.04) | 12.9 to 14.67 | 13.88 (1.83) | 13.1 to 14.65 | 13.83 (1.91) | 13.27 to 14.39 |
| EQ-5D health score (/100) | 74.83 (21.78) | 65.41 to 84.24 | 72.5 (18.2) | 64.81 to 80.19 | 73.64 (19.85) | 67.81 to 79.47 |
| EQ-5D-5L index value (–0.59 to 1) | 0.81 (0.23) | 0.72 to 0.91 | 0.82 (0.14) | 0.77 to 0.88 | 0.82 (0.19) | 0.76 to 0.87 |
| Physical activity self-efficacy (1–10) | 7.07 (1.42) | 6.44 to 7.7 | 6.11 (2) | 5.22 to 7 | 6.59 (1.78) | 6.04 to 7.13 |
| Social activity self-efficacy (1–10) | 7.1 (1.39) | 6.48 to 7.71 | 5.93 (1.99) | 5.05 to 6.81 | 6.51 (1.8) | 5.97 to 7.06 |
| Physical activity outcome expectancy (1–10) | 4.18 (0.64) | 3.9 to 4.47 | 4.0 7 (0.57) | 3.81 to 4.32 | 4.12 (0.61) | 3.94 to 4.31 |
| Social activity outcome expectancy (1–10) | 4.45 (0.5) | 4.21 to 4.68 | 4.16 (0.71) | 3.84 to 4.47 | 4.3 (0.63) | 4.1 to 4.49 |
| LSN Scale | ||||||
| Total (0–90) | 46.15 (10.88) | 41.06 to 51.24 | 40.74 (11.49) | 35.77 to 45.71 | 43.26 (11.41) | 39.75 to 46.77 |
| Family (0–30) | 22.2 (4.63) | 20.03 to 24.37 | 18.83 (5.92) | 16.33 to 21.33 | 20.36 (5.57) | 18.67 to 22.06 |
| Neighbours (0–30) | 8.45 (6.39) | 5.46 to 11.44 | 9.35 (4.73) | 7.3 to 11.4 | 8.93 (5.51) | 7.23 to 10.63 |
| Friends (0–30) | 15.5 (5.72) | 12.82 to 18.18 | 12.7 (5.3) | 10.4 to 14.99 | 14 (5.61) | 12.27 to 15.73 |
| UCLA Loneliness Score (0–60) | 8.24 (11.75) | 2.89 to 13.59 | 12.87 (13.88) | 6.87 to 18.87 | 10.66 (12.97) | 6.72 to 14.6 |
| WEMWBS (14–70) | 52.74 (9.55) | 48.61 to 56.87 | 50.04 (10.29) | 45.7 to 54.39 | 51.36 (9.92) | 48.45 to 54.28 |
Of the 50 participants who gave written informed consent to participate, 24 were allocated to the intervention group and 26 were allocated to the control group. At baseline, the groups were balanced in terms of activity levels and health status. The overall mean age of participants was 64.5 years at baseline. Participants were predominantly female (66%). Individuals who did not complete the intervention were similar to those who completed in terms of age, health status, mental well-being, self-efficacy and outcome expectancy. Women accounted for a higher proportion of non-completers than of completers (non-completers, n = 6/7, 86%; completers, n = 27/43, 63%). In addition, non-completers had higher levels of loneliness according to their score on the UCLA Loneliness Scale (non-completers, 14.14 ± 17.97; completers, 10.00 ± 12.02) and lower levels of social engagement according to their score on the LSN Scale (non-completers, 32.00 ± 10.77; completers, 45.44 ± 10.30).
Peer mentors
Sixteen of the 23 individuals (70%) who contacted the study team expressing an interest in becoming a peer completed training. Thirteen individuals were matched to a participant and delivered the intervention (13/23, 57%), but three individuals were not matched to a participant as there were no participants needing a peer mentor in their community. Seven individuals who expressed an interest did not undertake training (citing lack of time because of family or other volunteering commitments).
Characteristics of the peer mentors are described in Table 9, and apart from their activity levels, they are similar to the participants.
| Characteristic | Mean (SD)a | 95% CIa |
|---|---|---|
| Sex, n (%) | ||
| Male | 3 | (23) |
| Female | 10 | (77) |
| Age (years) | 64.31 (5.23) | 61.14 to 67.47 |
| Sedentary (minutes/day) | 603.13 (48.45) | 572.35 to 633.91 |
| Light-intensity physical activity (minutes/day) | 197.1 (36.12) | 174.15 to 220.05 |
| Moderate-intensity physical activity (minutes/day) | 47.68 (25.09) | 31.74 to 63.63 |
| Vigorous-intensity physical activity (minutes/day) | 1.94 (2.82) | 0.14 to 3.73 |
| MVPA (minutes/day) | 49.62 (24.66) | 33.95 to 65.29 |
| Steps per day | 9157 (3445) | 6967 to 11,346 |
| Valid number of days wear time | 6.75 (0.75) | 6.27 to 7.22 |
| GHQ-28 (0–84) | 9.69 (4.99) | 6.68 to 12.71 |
| SF-12 | ||
| Total score (12–57) | 26.9 (2.13) | 25.38 to 28.42 |
| Physical health score (6–18) | 12.64 (1.36) | 11.72 to 13.55 |
| Mental health score (6–27) | 14.42 (1.24) | 13.63 to 15.2 |
| EQ-5D health score (/100) | 88.46 (7.47) | 83.95 to 92.97 |
| EQ-5D-5L index value (–0.59 to 1) | 0.91 (0.11) | 0.84 to 0.97 |
| Physical activity self-efficacy (1–10) | 7.7 (1.64) | 6.71 to 8.69 |
| Social activity self-efficacy (1–10) | 6.88 (1.93) | 5.71 to 8.05 |
| Physical activity outcome expectancy (1–10) | 4.48 (0.46) | 4.2 to 4.75 |
| Social activity outcome expectancy (1–10) | 4.49 (0.46) | 4.21 to 4.76 |
| LSN Scale | ||
| Total (0–90) | 50.08 (13.1) | 41.76 to 58.4 |
| Family (0–30) | 20.92 (4.44) | 18.24 to 23.61 |
| Neighbours (0–30) | 11.92 (5.75) | 8.45 to 15.4 |
| Friends (0–30) | 17.25 (5.93) | 13.49 to 21.01 |
| UCLA Loneliness Score (0–60) | 3.91 (3.65) | 1.46 to 6.36 |
| WEMWBS (14–70) | 62 (5.64) | 58.59 to 65.41 |
Objectives 2 and 3: the resources needed and feasibility of conducting a definitive trial
The results for recruitment and retention indicated that support from general practice, as well as from community organisations, is key to the development of a definitive trial. Further results regarding the level of completeness of valid data returned within the various outcome measures, including the extent of changes observed and process measures used in the pilot study, are reported below.
Data completeness
A summary of the completeness of data at each time point is included in Table 10. At baseline, all 50 participants agreed to wear the accelerometer, and 48 (96%) returned valid accelerometer data at baseline. At 12 weeks, of the 43 participants still in the study, two did not return valid accelerometer data, meaning that 95% (n/N = 41/43) of accelerometer data sets at 12 weeks were available for analysis. Finally, at 6 months, 40 (93%) of the 43 participants who wore an accelerometer returned valid data. Other outcomes were returned with a similar degree of completeness (see Table 10).
| Outcome | Baseline (N = 50), n (%) | 12 weeks (N = 43), n (%) | 6 months (N = 43), n (%) |
|---|---|---|---|
| Valid Actigraph data sets returned | 48 (98) | 41 (95) | 40 (93) |
| EPAQ-2 | 41 (82) | 35 (81) | 22 (51) |
| GHQ-28 | 46 (92) | 42 (98) | 43 (100) |
| SF-12 | |||
| Total score | 44 (88) | 40 (93) | 36 (84) |
| Physical health score | 44 (88) | 40 (93) | 36 (84) |
| Mental health score | 47 (94) | 41 (95) | 41 (95) |
| EQ-5D | 47 (94) | 43 (100) | 42 (98) |
| Physical activity self-efficacy | 44 (88) | 39 (91) | 41 (95) |
| Social activity self-efficacy | 44 (88) | 38 (88) | 40 (93) |
| Physical activity outcome expectancy | 44 (88) | 43 (100) | 41 (95) |
| Social activity outcome expectancy | 42 (84) | 42 (98) | 41 (95) |
| LSN Scale | |||
| Total | 43 (86) | 42 (98) | 43 (100) |
| Family | 44 (88) | 43 (100) | 43 (100) |
| Neighbours | 43 (86) | 42 (98) | 43 (100) |
| Friends | 43 (86) | 43 (100) | 43 (100) |
| UCLA Loneliness Score | 44 (82) | 39 (91) | 40 (93) |
| WEMWBS | 47 (94) | 43 (100) | 41 (95) |
Change in outcomes
Changes in outcome measures at 12 weeks and 6 months are reported in Table 11. The study was not powered to detect change, so only descriptive statistics are included. There did appear to be an increase in MVPA at 12 weeks and 6 months in the intervention group (7.42 ± 10.79 minutes/day and 6.30 ± 16.60 minutes/day, respectively), but the control group showed a decrease at 12 weeks (–8.02 ± 24.41 minutes/day) and a slight increase at 6 months (1.51 ± 29.54 minutes/day).
| Outcome | Change after 12 weeks | Change after 6 months | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention group (n = 22) | Control group (n = 21) | Intervention group (n = 22) | Control group (n = 21) | |||||
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| Sedentary time (minutes/day) | –82.19 (226.19) | –188.05 to 23.66 | 48.14 (149.64) | 118.17 to –21.9 | –26.26 (328.24) | 119.28 to –171.79 | 77.54 (265.9) | 209.77 to –54.69 |
| Light-intensity physical activity (minutes/day) | 3.83 (50.5) | –19.8 to 27.47 | –18.12 (49.26) | 4.93 to –41.18 | –6.38 (27.06) | 5.62 to –18.38 | –15.4 (49.81) | 9.37 to –40.17 |
| Moderate-intensity physical activity (minutes/day) | 6.3 (11.4) | 0.97 to 11.64 | –7.62 (24) | 3.18 to –19.68 | 5.6 (15.09) | 12.29 to –.09 | 1.74 (29.23) | 16.27 to –12.8 |
| Vigorous-intensity physical activity (minutes/day) | 1.11 (3.2) | –0.39 to 2.61 | –0.4 (0.99) | 0.06 to –0.87 | 0.71 (3.69) | 2.34 to –0.93 | –0.23 (0.83) | 0.18 to –0.65 |
| MVPAa | 7.42 (10.79) | 2.37 to 12.47 | –8.02 (24.41) | 3.4 to –19.45 | 6.31 (16.6) | 13.66 to –1.05 | 1.51 (29.54) | 16.19 to –13.18 |
| Steps per dayb | 720 (2032) | –231 to 1671 | –901 (3044) | 524 to –2325 | 543 (2271) | 1550 to –463 | 133 (3702) | 1974 to –1708 |
| Domestic physical activity (minutes/day) | 412.97 (721.31) | 108.39 to 717.56 | 285.41 (697.94) | 567.31 to 3.51 | 686.35 (517.67) | 904.94 to 467.76 | 620.37 (592) | 859.48 to 381.26 |
| Occupations physical activity (minutes/day) | 382.75 (489.19) | 176.18 to 589.32 | 59.26 (241.9) | 175.85 to –57.33 | 582.75 (503.11) | 795.19 to 370.31 | 622.63 (503.54) | 826.02 to 419.25 |
| Recreational physical activity (minutes/day) | 645.87 (517.55) | 427.33 to 864.41 | 408.08 (549.26) | 629.93 to 186.23 | 890.25 (443.76) | 1077.63 to 702.87 | 714.99 (443.15) | 893.98 to 536 |
| GHQ-28 | –2.1 (12.28) | –7.68 to 3.49 | –1.4 (10.7) | 118.17 to –21.9 | –3.05 (11.4) | 2.14 to –8.23 | –6.24 (13.51) | –0.09 to –12.39 |
| SF-12 | ||||||||
| Total score | –0.11 (2.23) | –1.18 to 0.97 | –0.05 (2.01) | 0.06 to –0.87 | 0.76 (2.95) | 2.28 to –0.75 | 0.22 (2.6) | 1.52 to –1.07 |
| Physical health score | 0.05 (1.47) | –0.66 to 0.76 | 0.001 (1.17) | 4.93 to –41.18 | 0.47 (1.33) | 1.15 to –0.21 | 0.06 (1.35) | 0.73 to –0.62 |
| Mental health score | –0.33 (2.03) | –1.26 to 0.59 | –0.05 (1.9) | 3.6 to –18.85 | 0.14 (2.61) | 1.33 to –1.05 | 0.05 (2.63) | 1.32 to –1.22 |
| EQ-5D health score | 6.91 (17.45) | –0.83 to 14.65 | 1.95 (19.66) | 3.4 to –19.45 | 7.71 (21.6) | 17.55 to –2.12 | 6.72 (22.71) | 18.02 to –4.57 |
| EQ-5D-5L index value | 0.06 (0.11) | 0.01 to 0.11 | 0.001 (0.22) | 524.29 to –2325.25 | 0.03 (0.1) | 0.08 to –0.02 | 0.04 (0.13) | 0.1 to –0.02 |
| Physical activity self-efficacy | –0.06 (1.36) | –0.74 to 0.62 | –0.1 (1.35) | 3.61 to –6.41 | 0.16 (1.56) | 0.91 to –0.59 | 0.23 (2.61) | 1.42 to –0.95 |
| Social activity self-efficacy | –0.33 (1.46) | –1.05 to 0.4 | –0.25 (1.32) | 0.55 to –0.55 | 1.35 (5.15) | 3.91 to –1.21 | –0.38 (1.49) | 0.3 to –1.06 |
| Physical activity outcome expectancy | 0.09 (0.44) | –0.1 to 0.29 | 0.0001 (0.0001) | 0.84 to –0.94 | 0.17 (0.36) | 0.36 to –0.02 | 0.06 (0.6) | 0.33 to –0.21 |
| Social activity outcome expectancy | –0.0002 (0.003) | –0.001 to 0.001 | 0.0005 (0.002) | 0.89 to –0.99 | 0.23 (0.72) | 0.57 to –0.12 | –0.06 (0.61) | 0.22 to –0.33 |
| LSN Scale | ||||||||
| Total | 2.05 (15.37) | –4.77 to 8.86 | 1.74 (6.91) | 5.07 to –1.59 | –2.5 (10.28) | 2.06 to –7.06 | –2.8 (9.51) | 1.65 to –7.25 |
| Family | 1.5 (12.67) | –4.12 to 7.12 | 1.14 (9.75) | 5.58 to –3.29 | –3.5 (4.94) | –1.31 to –5.69 | –3.24 (3.78) | –1.52 to –4.96 |
| Neighbours | –0.45 (3.61) | –2.06 to 1.15 | 1.16 (3.78) | 2.98 to –0.66 | 1.45 (4.16) | 3.3 to –0.39 | 0.9 (3.23) | 2.41 to –0.61 |
| Friends | 1 (3.39) | –0.51 to 2.51 | 1.25 (4.34) | 3.28 to –0.78 | –0.45 (6.15) | 2.27 to –3.18 | –0.65 (5.72) | 2.03 to –3.33 |
| UCLA Loneliness Score | –2.65 (9.16) | –6.93 to 1.63 | –1.11 (7.4) | 0.001 to –0.0006 | –0.1 (8.22) | 3.75 to –3.95 | –3.74 (9.62) | 0.9 to –8.37 |
| WEMWBS | 3.5 (9.99) | –0.93 to 7.93 | 5.57 (10.22) | –0.02 to –2.64 | 2.3 (9.13) | 6.57 to –1.97 | 4.71 (8.33) | 8.51 to 0.92 |
One individual in the control group returned to work as a postman between the end of the intervention and the 6-month follow-up, and increased his average MVPA per day from 18.8 minutes per day at baseline to 119.6 minutes per day at 6 months. This accounts for the large variance in the control group at 6 months. When this outlier was omitted from the analysis, the mean ± standard deviation (SD) in the control group at 6 months was –4.33 ± 16.55 minutes of MVPA per day, resulting in a difference in mean change between the groups of 10.64 minutes of MVPA per day.
Mixed findings were found for other outcomes, with a high degree of variability (see Table 11). The EQ-5D-5L health score and GHQ-28 score outcomes appeared to move in a positive direction in the intervention group, but these improvements were not sustained at 6 months. However, at 6 months there did appear to be improvements in physical activity and social activity outcome expectancies and self-efficacy for social activities in the intervention group. No changes in social isolation or loneliness were observed.
Process evaluation
All 12 participants who were selected at random agreed to allow us to audio-record a randomly selected session with their peer mentor, allowing us to assess the fidelity of delivery of intervention content. These conversations, which lasted 30–45 minutes, were recorded at the venue where the peer mentor met the participant, using a digital dictaphone. However, owing to a technical failure, all audio-recordings were accidentally deleted from the digital dictaphone before they were downloaded to a computer, and therefore it was not possible to analyse the content. All peer mentors and intervention participants who were asked also agreed to complete checklists (see Appendix 3) to assess the fidelity of delivery and receipt of the intervention. All mentors and participants completed weekly step diaries for all 12 weeks (Table 12). The fidelity checklists were not completed to the same extent. For the first 3 weeks, 9 of the 11 participants completed the fidelity checklists enabling us to identify which of the specified intervention components had been received (e.g. goal-setting, action-planning). From week 6 onwards, the rate of return of checklists was significantly lower (see Table 12). The return of fidelity checklists by mentors showed a similar pattern (see Table 12). The number of components reported as having been delivered by the mentor or as having been received by participants was high for the first 5 weeks (range 49–83%), but lower during the second half of the intervention. It was not clear from the checklists whether mentors and participants no longer kept accurate records or if the intended intervention content was no longer being delivered by mentors. Intervention fidelity is discussed further in the results of the post-intervention qualitative interviews (see Chapter 7).
| Week | Mentor (N = 9) | Intervention group (N = 11) | ||||
|---|---|---|---|---|---|---|
| Step diaries returned (n) | Checklists returned (n) | Proportion of components delivered (%) | Step diaries returned (n) | Checklists returned (n) | Proportion of components delivered (%) | |
| 1 | 9 | 6 | 74 | 11 | 9 | 84 |
| 2 | 9 | 7 | 83 | 11 | 9 | 82 |
| 3 | 9 | 8 | 72 | 11 | 9 | 75 |
| 4 | 9 | 5 | 69 | 11 | 7 | 84 |
| 5 | 9 | 5 | 52 | 11 | 7 | 54 |
| 6 | 9 | 4 | 46 | 11 | 4 | 51 |
| 7 | 9 | 4 | 41 | 11 | 4 | 48 |
| 8 | 9 | 4 | 35 | 11 | 4 | 51 |
| 9 | 9 | 4 | 40 | 11 | 3 | 43 |
| 10 | 9 | 4 | 31 | 11 | 3 | 43 |
| 11 | 9 | 4 | 31 | 11 | 2 | 36 |
| 12 | 9 | 4 | 40 | 11 | 2 | 36 |
| Average per week | 9 | 5 | 51 | 11 | 5 | 55 |
Acceptability of the intervention
Participants in the intervention group were invited to give feedback at the end of the study. Of the 22 participants who were still in the study, 17 returned exit questionnaires, and their responses are summarised in Table 13. Participants rated all aspects of the study positively (overall satisfaction, information received, peer mentor, pedometer). All 17 participants reported that they would take part again and would recommend the intervention programme to a friend. In the free-text comments, participants noted the benefits in terms of establishing and maintaining an active routine. Some participants noted the possible benefit of adding walking groups to the intervention and a dislike of having to complete so much paperwork.
| Question | Responses |
|---|---|
| 1. Overall were you satisfied with your involvement in the study? |
Very satisfied = 14 Somewhat satisfied = 3 Comments: |
| 2. Were you satisfied with the advice/information you received about this study (including the participant information sheet and other information)? |
Very satisfied = 17 Comments: |
| 3. How helpful do you think it was having a peer mentor to encourage you to undertake more physical activity? |
Great benefit = 15 Some benefit = 2 |
| 4. How helpful do you think the health promotion information in the leaflet was? |
Great benefit = 4 Some benefit = 13 |
| 5. How helpful do you think the behavioural change tools were (e.g. goal-setting, weekly planning schedule?) |
Great benefit = 14 Some benefit = 3 |
| 6. Do you think the pedometer helped to change the amount of physical activity you did? | Yes = 17 |
| 7. Would you recommend this scheme to a friend? | Yes = 17 |
| 8. Would you be happy to be involved in this type of scheme again? | Yes = 17 |
| 9. If we were to run this scheme again, what features did you like and would want us to keep the same? | Comments:Keep the same – enjoyed itKeep the sameJust the sameEnjoyed walking with [peer mentor] each weekEnjoyed it as was – helped me do additional activity on my ownLiked regular walksFlexible activity – used the leisure centre on rainy daysLike peer group approachLike using pedometer to track progressEnjoyed the regular meetings – helped me to become more active early in the day which I would never have done |
| 10. If we were to run this scheme again, what changes do you think could be made to improve it? | Comments:Disliked the paperwork – took enjoyment out of programme – less/no paperworkPerhaps some changes to the paperworkToo much paperwork to fill inTo meet with others who were also participating in the schemePerhaps organise occasional walking groupsGroup walkingWalk in groups?More people becoming involvedA little long for me – 8 weeks would be greatOK as is |
| 11. Do you have any other comments? | Comments:Was happy to be involvedVery enjoyable, and for me beneficial project |
Adverse events
There were no related or unexpected serious adverse events in this study. One participant reported a minor musculoskeletal injury (sprained ankle) during the intervention. This injury occurred while boarding a train and was therefore deemed to be unrelated to the intervention. After a short period of rest, the participant was able to resume and complete the study.
Objective 4: sample size calculation for definitive trial
Based on a mean difference in the change between groups of 4.8 minutes per day of MVPA (Cohen’s d = 0.29) at 6 months, and a SD of 16.6, 316 participants per group would be required at 90% power and a significance level of 0.05, allowing for 20% dropout as shown in the pilot RCT. We did not identify clustering of the results by peer mentor, with no obvious pattern in the data, suggesting that it was not the case that some peer mentors were more effective at delivering the intervention than others. We have therefore not adjusted the sample size to account for clustering.
If the individual in the control group who returned to work as a postman is excluded from the sample size calculation, then, based on a mean difference between groups of 10.6 minutes of MVPA per day (Cohen’s d = 0.64) and a SD of 16.6, 66 participants per group would be required at 90% power and a significance level of 0.05, allowing for 20% dropout. This effect size is similar to that found in a previous systematic review of pedometer interventions (Cohen’s d = 0.68). 84
A medium effect size (Cohen’s d) of 0.5 is a conservative estimate of the anticipated effect size (approximately mid-way between the values presented above). This is equivalent to a difference between groups of 8.5 minutes per day of MVPA at 6 months. Using this estimate, a sample size of 107 per group or a total sample size of 214 individuals would therefore be required for a definitive trial, at 90% power and a significance level of 0.05, allowing for 20% dropout.
Objectives 5 and 6: measure the resource use associated with the intervention and pilot the use of a health and social care service use instrument
The key resources identified for the planning and preparation stage (stage 1) are presented in Table 14, along with the associated costs. The costs are based on the training of 13 peer mentors and 24 participants. The total cost to deliver the intervention was £5055 and the mean cost per participant was £211. The main driver of costs was the time input required by the trainer, amounting to 5 hours of contact on a one-to-one basis with each mentor, and 1 hour of contact with every participant and their assigned peer mentor. These costs would be lower if peer mentors were paired with larger groups of participants, as this would mean fewer peer mentors would be required and therefore fewer training sessions delivered by the trainer. Similarly, if peer mentor training was delivered to larger groups of peer mentors instead of on a one-to-one basis, fewer training sessions would be required. However, the results of this study indicated that these alternative structures are not feasible. We also explored the impact of a band 4 health improvement officer delivering the training, as this would also be within the remit of their role, and the mean cost per participant reduced to £185.
| Resource use | Unit cost (£) | Number of units | Total cost (£) |
|---|---|---|---|
| Planning and preparation for delivery | |||
| Printing peer mentor manuals | 3.16 | 13 | 41.08 |
| Printing participant booklet | 2.12 | 24 | 50.88 |
| Physical activity information leafletsa | 0.08 | 24 | 0.00 |
| Yamax pedometers | 10.00 | 24 | 240.00 |
| Trainer input: peer mentor trainingb (one-to-one training sessions for peer mentor lasting 2 hours) | 36.00 | 26 | 936.00 |
| Travel costs for the trainer to deliver peer mentor training (based on 56p per milec) | 0.56 | 1180 | 660.58 |
| Criminal record check for mentors (based on cost of £26 per standard check for AccessNId) | 26.00 | 13 | 338.00 |
| Delivery of intervention | |||
| Trainer input: initial meeting (to facilitate initial 1-hour meeting between trainer, peer mentor and participant) | 36.00 | 24 | 864.00 |
| Trainer input: ongoing support (three 1-hour support sessions) | 36.00 | 39 | 1404.00 |
| Peer mentor input: travel and subsistence (to meet with participants) | 40.00 | 13 | 520.00 |
| Total cost of intervention | 5054.54 | ||
| Mean cost per participant | 210.61 | ||
| Sensitivity analysis: trainer rate per hourse | 184.65 | ||
Health service resource use
Table 15 shows the mean health service use per participant in the intervention and control groups. Three-quarters (n/N = 38/50; 76%) of participants returned their health service use log at the 6-month follow-up: 19 in each group. In general, use of health services was low for both groups. The log required participants to tick a numbered box each time they used a service; therefore, if no boxes were ticked, it was assumed that they had not used that service. There was no option to tick zero. Many patients recorded no service use at all (intervention, n = 14; control, n = 12; n/N = 26/50), as they returned blank logs.
| Health service | Intervention (n = 19) | Control (n = 19) | Difference between groups (95% CI) | ||
|---|---|---|---|---|---|
| Mean number of appointments | Mean (SD) cost (£) | Mean number of appointments | Mean (SD) cost (£) | ||
| GP visit | 0.26 | 10.00 (27.87) | 0.74 | 28.00 (54.98) | –18.00 (–46.68 to 10.68) |
| GP telephone call | 0.05 | 0.78 (3.40) | 0.53 | 7.79 (24.35) | –7.01 (–18.45 to 4.43) |
| GP out of hours | 0.05 | 4.97 (21.65) | 0.11 | 9.93 (29.76) | –4.97 (–22.09 to 12.16) |
| Practice nurse | 0.21 | 2.28 (6.84) | 0.32 | 3.43 (14.93) | –1.14 (–8.79 to 6.50) |
| Physiotherapist | 0.21 | 9.47 (41.29) | 0.58 | 26.05 (78.40) | –16.58 (–57.81 to 24.65) |
| Podiatrist | 0.05 | 2.37 (10.32) | 0 | 0 | 2.37 (–2.43 to 7.17) |
| A&E visit | 0.05 | 12.95 (56.44) | 0 | 0 | 12.95 (–13.31 to 39.21) |
| Hospital clinic/outpatient | 0.05 | 7.21 (31.43) | 0.32 | 43.26 (91.93) | –36.05 (–81.26 to 9.15) |
| Total cost | – | 50.03 (135.63) | – | 118.47 (197.52) | –68.44 (–179.92 to 43.05) |
Service use was overall slightly higher in the control group. For every other service type, the mean usage and corresponding costs were higher in the control group. The biggest difference in costs was due to more outpatient visits in the control group. Total costs were £68 lower for the intervention group.
The results from the feedback resource use log are presented in Table 16. In general, the feedback was positive. The majority of responders agreed or strongly agreed that they were willing to complete the log (18/20) and that it was easy to use (15/20). Just over half (10/19) of respondents agreed it was easy to remember to use the log, although six people remained neutral. Only two respondents agreed that the log was burdensome.
| Question | Response |
|---|---|
| Willing to complete log (n = 20) |
Strongly disagree, n = 2 (10%) Disagree, n = 0 Neither agree nor disagree, n = 0 Agree, n = 12 (60%) Strongly agree, n = 6 (30%) |
| Found log easy to use (n = 20) |
Strongly disagree, n = 2 (10%) Disagree, n = 0 Neither agree nor disagree, n = 3 (15%) Agree, n = 12 (60%) Strongly agree, n = 3 (15%) |
| Found easy to remember to use log (n = 19) |
Strongly disagree, n = 2 (10.5%) Disagree, n = 1 (5.3%) Neither agree nor disagree, n = 6 (31.6%) Agree, n = 8 (42.1%) Strongly agree, n = 2 (10.5%) |
| Found log burdensome (n = 19) |
Strongly disagree, n = 6 (31.6%) Disagree, n = 6 (31.6%) Neither agree nor disagree, n = 5 (26.3%) Agree, n = 2 (10.5%) Strongly agree, n = 0 |
Chapter 7 Acceptability of a peer-led walking programme to increase physical activity in inactive older adults: ‘Walk with Me’ study
Introduction
In keeping with the MRC framework for developing complex interventions,34 the ‘Walk with Me’ study was piloted to determine the acceptability of the programme and the feasibility of a definitive trial. The feasibility of the trial was discussed in Chapter 6; this chapter presents information on its acceptability based on the results of a qualitative evaluation undertaken with a subsample of those who delivered and received the ‘Walk with Me’ programme.
Aim and objectives
This phase of the project sought to explore the acceptability of the ‘Walk with Me’ study. More specifically, the objectives were to explore the acceptability of the intervention components from the perspective of those who received and delivered it; identify barriers to success and implementation; and identify possible improvements to the intervention that could be made.
Methods
Qualitative methodology involving interviews and focus groups was used to explore the acceptability of the ‘Walk with Me’ study, in order to identify possible changes to the intervention and proposed trial design, to improve their acceptability and the likelihood of successful delivery of a definitive RCT. Interviews and focus groups were conducted between March 2017 and January 2018.
Participants
Peer mentors and members of the intervention and control groups who completed the study were invited to take part in a post-intervention interview or focus group. Participant characteristics are presented in Table 17.
| ID | Sex | Age (years) | Employment status |
|---|---|---|---|
| Peer mentors | |||
| PM1 | Female | 67 | Retired |
| PM2 | Female | 67 | Retired |
| PM3 | Female | 62 | Part-time work |
| PM4 | Female | 65 | Retired |
| PM5 | Male | 67 | Retired |
| PM6 | Female | 64 | Retired |
| PM7 | Female | 50 | Retired |
| PM8 | Female | 70 | Retired |
| Intervention group participants | |||
| IP1 | Female | 66 | Retired |
| IP2 | Female | 62 | Retired |
| IP3 | Male | 60 | Retired |
| IP4 | Female | 61 | Retired |
| IP5 | Female | 68 | Retired |
| IP6 | Female | 68 | Retired |
| IP7 | Female | 61 | Part-time work |
| Control group participants | |||
| CP1 | Male | 64 | Retired |
| CP2 | Female | 63 | Voluntary work (part time) |
| CP3 | Female | 65 | Retired |
Data collection
Semistructured focus groups and interviews were considered the most appropriate methods for eliciting views and opinions on the ‘Walk with Me’ study. However, it proved difficult to find a convenient time and date to form focus group discussions. Consequently, only one focus group was conducted with four peer mentors. The remaining data were collected via one-to-one interviews (n = 14). The focus group and interviews lasted 30–45 minutes and were conducted in the local community or in participants’ homes. They were facilitated by a female independent researcher (JD) who has completed a MSc in health psychology and has experience in implementing qualitative research methods.
At the beginning of each interview and the focus group, participants were informed of the research topic, encouraged to share their views on the study and asked to keep in mind that both positive and negative views, and any recommendation on how the study could be improved, would be welcomed during the discussion. Three flexible schedules were developed to guide the discussions, one for intervention participants (Box 2), one for peer mentors (Box 3) and one for control participants (Box 4). These schedules contained questions that explored the study objectives but reflected the individual’s role in the intervention. However, as a semistructured approach was employed, participants were encouraged to discuss issues that arose but were not originally included in the schedule. Additional probing questions were also used to obtain more detailed information. All interviews and the focus group were recorded using a voice recorder. The audio files were transcribed verbatim and subjected to thematic analysis.
How did you hear about the study?
Why did you decide to take part?
InterventionTell me about your experiences as a participant in the ‘Walk with Me’ study.
How did you feel about completing the goal-setting sheet?
How did you identify and overcome barriers that prevented you from meeting your goals?
How did you feel about recording your steps?
How did you feel about using the pedometer?
Can you tell me about your meetings with your peer mentor?
Can you tell me about the walks you attended with your peer mentor?
What did you think about the amount of contact time you had with your peer mentor?
How did you feel about completing the paperwork involved?
What part of the study did you like the best?
What part of the study did you like the least?
What would you change about the study if you were to take part again?
Closing questionsWhat do you think were the benefits of taking part?
Would you take part in a similar programme again?
How did you hear about the study?
Why did you decide to take part?
TrainingWhat did you think about the training you received for becoming a peer mentor?
Could the training be improved?
InterventionIn your opinion . . .
How did your peers feel about setting goals?
Do you see any problems with using the goal-setting to increase physical activity?
Do you see any problems with using a weekly diary to record people’s steps?
How did your peers feel about developing an action plan?
What difference did using a pedometer make to their physical activity levels?
Can you tell me how your peers felt about the problem-solving strategies used?
How do you feel about peer mentors being used to support people’s physical activity?
What do you think about the amount of contact time you had with your mentee?
Can you explain how the paperwork in the training and support manual was used?
What aspects of the study worked best?
What aspects of the study did not work well?
Closing questionsHow could the study be improved to help peer mentors implement the programme?
If you had a chance to change the way this programme was implemented, what would you change?
How did you hear about the study?
Why did you decide to take part?
Group allocationHow did you feel about the group you were allocated to?
Can you tell me about the information leaflet you received?
What were the advantages of taking part?
What were the disadvantages of taking part?
PaperworkHow did you feel about completing the paperwork involved with this study?
Closing questionsWould you like to take part in a similar programme in the future, if offered to you?
Data analysis
The transcripts were analysed thematically in line with guidance provided by Braun and Clark. 50 To do so, the researcher (JD) familiarised herself with the data and generated initial codes. Relevant data were assigned to these codes, which were then allocated to themes that reflect the aims and objectives of this research. Themes and codes were reviewed to ensure they were clearly defined. After conducting 14 interviews and one focus group, data saturation was reached (i.e. no new information or themes were emerging from the data).
Results
This section presents our findings within two main themes: first, the acceptability of the ‘Walk with Me’ study from the perspective of peer mentors, members of the intervention group and members of the control groups; and, second, participants’ motivation to become involved in the study. Exemplar quotes are included to support these findings; however, all quotations have been anonymised. Therefore, the views of peer mentors (coded as PM1–8), intervention participants (coded as IP1–7) and control participants (coded as CP1–3) are labelled throughout this section.
Overall, peer mentors and members of the intervention and control groups spoke very positively about the ‘Walk with Me’ study. The majority described the intervention as an enjoyable experience and mentioned that they would be willing to take part in a further future study or to recommend the intervention to a friend:
I enjoyed the experience.
PM2
I thoroughly enjoyed it I have to say, I thought it was good.
IP5
Control participants believed that the ‘Walk with Me’ study could help older adults to increase their physical activity levels. In addition, the social support component of the intervention was viewed positively, suggesting that peer mentors might be a key feature influencing the acceptability of the programme:
I think especially when you reach that age group you’re either, before that you’re either into a mode of exercising and looking after yourself or you’re not and if you’re not in that mode then I think something that sort of heightens the idea that you should be I think is very good.
CP2
It would be worthwhile if it gets people motivated and gets people going . . . it’s good for the social aspect too.
CP2
Theme 1: acceptability of the ‘Walk with Me’ study
Peer mentors’ and intervention participants’ views of the acceptability of the ‘Walk with Me’ study were influenced by their experiences of benefits associated with participating and barriers to success/implementation of the intervention. They suggested several possible changes to enhance its acceptability. In relation to the control group, views of acceptability were influenced by perceived benefits from participation, willingness to be randomised and suggested changes to the treatment of a control group.
Peer mentors
Peer mentors were positive about their role and felt that both they and their peer benefited from participating, suggesting that benefits associated with the intervention promoted positive attitudes towards the study:
I feel that it was a two-way process and I really benefited from it as well as I was walking at times when I wouldn’t normally have walked and that was good for me as well as them because I was making that extra effort.
PM8
I would like to think that not only did I get her walking but just the conversations that we had when we were walking around I think was good for her mentally.
PM1
It certainly reinforced my own opinion that activity is really good and it has helped me walk more, you know, try and get out walking every day apart from all of the gym stuff that I do.
PM5
In general, it was agreed that the intervention was acceptable to peer mentors and that most of the components were relevant to increasing older adults’ physical activity levels. Peer mentors identified peer support and self-monitoring as the most beneficial components of the intervention, suggesting that these enhanced the acceptability of the study:
I know this is going to sound ridiculous but I think it’s the relationship they develop with you, friendships.
PM7
I think the pedometer played a lot and as I said before the very simple measurement, you know, and you can see it day-by-day and week-by-week you can see, you know, the improvement.
PM3
More specifically, peer mentors talked positively about walking with their peer, using the pedometer to monitor step counts and recording daily step counts. Peer mentors believed that these components were favoured by participants as they were enjoyable and directly contributed to their weekly physical activity and to meeting their step goals. They also found that seeing their participant increase their level of physical activity was rewarding:
I think probably just having someone to walk with.
PM6
When they see how many steps they’ve done that day sometimes they were actually surprised when they were wearing it and they say oh I can’t believe that today I’ve done . . . it makes a big difference, it motivates them, it encourages them most definitely.
PM7
It does give them a certain amount of self-satisfaction, you know, to see that they’re increasing their physical activity.
PM1
Goal-setting was mentioned, with more emphasis placed on step counts than longer-term goals. This may be because meeting a step goal is a more immediate process and longer-term goals were not specifically identified. Some expressed concern about implementing more formal components of the intervention, for instance the problem-solving process and setting longer-term goals, particularly as these involved completing paperwork:
The problem-solving, people don’t want it on paper, it becomes threatening, you know, do you understand, it makes it uncomfortable.
PM7
Based on the discussions, it is unclear the degree to which these components were implemented in their entirety, but evidence was present that they were utilised to some extent:
Our plans were quite loose if you want, our main objective was to just get out once a week and for them to do what they could the other days so it was a stepping stone and I was happy to do that . . . I don’t want to say cheating but there wasn’t the full package of having to go through the whole process.
PM5
Peer mentors spoke positively about helping people to increase their step count and felt that walking with participants was the best way to achieve this. Therefore, peer mentors were happy to arrange walks that encouraged their participant to become more active and directly contributed to their weekly step count:
What I would do if they said to me I want to do 10,000 steps today we went for a walk and we looked at the pedometer and if it said 9800 then I would walk with them and finish that with them and encourage them to complete that.
PM8
We just arranged it and decided we would walk for whatever distance, you know, and just carried on with that.
PM6
All peer mentors mentioned that they did not like the paperwork involved with their role. Most expressed concerns about the volume of paperwork and felt that participants also disliked this aspect of the intervention. Views were based on the essential paperwork, suggesting that additional support might be required to promote more positive attitudes towards completing necessary paperwork:
Again I just found them very repetitive I mean it was the same thing week after week so I mean there has to be a way to make it much more user-friendly.
PM2
There was consensus among peer mentors that they had received sufficient training to deliver the intervention activities required:
I think it was sufficient, I think, you know, when you have life experience you really know all of this anyhow.
PM6
However, most believed that the training and support manual was extensive and difficult to understand, suggesting that it may be more user-friendly if the information it contained was condensed:
It was explained very well but having said that if you go through the book it’s slightly confusing . . . could’ve been shortened down somewhat it just didn’t need to be as complicated really for somebody reading through it.
PM5
Peer mentors differed in the way they used the training and support manual throughout the study. Some indicated that they referred to the instructions to ensure they covered the necessary content each week, whereas others admitted that they referred to this information from time to time only:
Well, I did refer to it, yes, I read it all through initially and then just to keep on top of it I would, because it was broken up into different weeks and that and so you know as time went on I would’ve referred to those just to keep myself right.
PM6
Well, I did read through it but I wouldn’t say I used it every week, I looked at it now and again and sort of thought well I think we’re achieving what we were meant to be achieving.
PM1
Finding a time that was suitable to meet and the weather were identified as the main barriers to delivering the intervention. Despite this, there was a general agreement that the role of a peer mentor was easy to implement, but flexibility was considered important to sustaining an effective relationship with their peer:
We found it sometimes hard to co-ordinate getting ourselves out together, do you know, it may not have suited me whereas it suited the other girl, I found that bit hard.
PM4
Because of the bad weather and it was coming up to Christmas time which is the worst time ever, everybody is going mad, so it was quite difficult over that Christmas period.
PM8
In general, these barriers were overcome by rescheduling and identifying alternative forms of physical activity:
I suggested that they maybe go to aerobics so that if they weren’t able to walk at least you could do some activity inside that is similar to walking.
PM8
We just rearranged if the day didn’t suit we met at least once a week to walk.
PM2
Some peer mentors asked about the possibility of using a wrist-worn pedometer, suggesting that they would be more practical:
I definitely would have liked a wrist one cause I think it would’ve stayed on you all the time except when you’re in the shower.
PM3
In addition, it was suggested that the step diary should be produced as a monthly rather than weekly document, and that meeting other groups of peer mentors might be beneficial for peer mentors and participants:
If you look at it you go week 1, week 2, week 3, I can’t see why you can’t put week 1, week 2, week 3, week 4 on one sheet.
PM7
Maybe introduce us to another group even another couple or something . . . just to see how they felt and maybe get some ideas from them or whatever.
PM6
Intervention group participants
Most participants agreed that the intervention was enjoyable and beneficial. They spoke positively about the physical and psychological benefits they experienced, with some reporting a decline in cholesterol levels, a better quality of sleep, weight loss and feeling more relaxed after having participated in this study:
I enjoyed it because you’d have got a bit of a laugh and actually you found you were talking about things that you normally wouldn’t speak about when you’re in here, you know that way . . . I felt better and actually I think I was sleeping better too you know so but I think the weight loss was a big part of it.
IP5
For me, my cholesterol kind of came down.
IP3
The majority of participants felt that peer support was a key component and indicated that walking with their peer mentor was their favourite aspect of the intervention. It was clear that participants enjoyed the social support provided by having the company of a peer mentor and felt accountable to someone which enhanced their commitment to the programme:
Well, it’s easier to go walking when you have somebody else as to being on your own. I think the time goes in a lot quicker if you’re walking with somebody else and not being on your own.
IP1
Well, I think it started off making me feel accountable to somebody which is something for me personally that I like to be accountable to someone . . . every now again you need a wee nudge.
IP7
It made a big difference because you set aside that time, you knew you were going, you knew you were going to have a real good walk and it really was enjoyable so it definitely was great encouragement . . . you know and we just enjoyed each other’s company so that was a real bonus.
IP6
When asked how their peer supported them during the programme, participants rated walking with their peer mentor highly. The presence of the mentor encouraged them not only to meet their step count targets but also to achieve a higher level of intensity of walking activity:
I would say my walk out with the mentor is what I enjoyed most and really keeping up to my steps, making that effort to keep up to the steps.
IP6
We just said that we would walk together at least once a week which we did and kept in touch with each other . . . you found you were walking better and you were conscious of quickening your step and not just dragging yourself along, you know what I mean, you were conscious that right if you walk quicker you’ll feel better rather than just dawdling along.
IP5
Participants were also positive about goal-setting and self-monitoring. Most liked these components as it provided a sense of achievement and helped them to determine whether or not they were increasing their physical activity:
Because that was your goal and when you saw you hadn’t made it you thought right well I’m going to do that better the next time I’m out, I’m going to do better the next time around, I found it helpful.
IP5
‘Cause you can actually see what your progress is, see what you’ve done and realise you know with a little bit more effort you can up the number pretty quicker so it’s useful from that viewpoint.
IP3
Most participants liked keeping a step diary as they could visually see their progress. This enabled them to determine whether or not they were meeting their step goal or needed to walk more:
I found it good, I found it encouraging, the fact that you do write something down and you’re saying oh yesterday I did such and such, today I’ve done whatever . . . I did find that encouraging.
IP6
When you see it written down, especially when you go and take the weekly average because you’re thinking oh there’s a couple of days there where I didn’t meet my target but then when you juggle high days, so on average you do reach your target so you know so it’s a good enough wee point as well.
IP3
One participant mentioned that they liked it when their peer mentor reviewed their progress as it encouraged them to increase their step count for the following week.
She was checking them every week and I remember the other weeks, now I can’t exactly remember the figure, say I had set myself for 4000, I had that for a couple of weeks I think and she said to me right here it’s time you moved that up so it would’ve gone up to 5000 so she would’ve encouraged you to up it a wee bit and you finally made it eventually.
IP5
During the discussions, participants were asked about developing an action plan and the problem-solving process. In the context of the intervention, they identified action-planning in the form of setting step goals and making arrangements to walk with the peer mentor. Participants did not, however, describe problem-solving activity:
We chatted about what we currently did and exchanged phone numbers and we would phone or text each other and agree you know we would sort of agree well that’s OK we’ll meet same time next week same place and then if there was any change we would text so it wasn’t a formal action plan as such, you know, we talked it over and decided OK I want to walk solid for an hour and let’s see how many steps we get up.
IP3
In keeping with the views of peer mentors, participants disliked the paperwork, with the exception of the weekly step diary. They found it difficult to recall specific forms; most mentioned that they believed they tried to complete the paperwork but were unsure if they did it correctly, suggesting that more help might be required with this aspect of the intervention. It was also suggested that developing a smaller booklet containing the weekly step diaries would be useful:
Some of it was cumbersome paperwork . . . I get impatient when I’m doing paperwork.
IP3
Just filling in them forms . . . I think when people are really so busy in life filling in forms and that can be quite monotonous plus you don’t know if people really see . . .
IP7
. . . possibly more in a booklet form that you could sort of put in your handbag you know you were going around with this big sheet.
IP7
Some participants also disliked aspects of what was been asked in the pre- and post-intervention assessments:
Some of the things you hardly know what to answer, you know what I mean. Are you depressed, you know, all of those sort of questions, no I’m not depressed, you know what I mean, would people tell you if they were.
IP6
As for peer mentors, time constraints and the weather were reported as barriers. Motivation was also mentioned. Some participants believed that knowing their peer mentor helped them to overcome these barriers, as they felt comfortable with rescheduling their walks:
Well, the only thing is on one occasion I took a really bad cold or it could’ve been the flu but I just wouldn’t have the energy to go out so that was just on one occasion and I was on holiday one week as well . . . we just met the following week.
IP6
Well, the things we did was just mainly go walking, you know, if the weather was good . . . a few times when it was raining we went down to the mall down here and we went up and down the mall.
IP1
Laziness really, once I got out walking I was grand. It was just getting out of that habit of sitting on the sofa and once you got out it was good and when you came back you felt brilliant that you had got out and you had achieved what you were looking for.
IP5
The length of the intervention programme was acceptable to participants:
12 weeks was probably OK, an OK time to do things.
IP1
Some participants asked about the possibility of using smartphones or wrist-worn step counters instead of pedometers worn on the waist, suggesting that alternative methods of counting steps might enhance the acceptability of the intervention:
I must admit I’d prefer that [wrist-worn monitor] to the one on the back of my trousers . . . a Fitbit yes because it was just so easy to look at it and think you know it’s there.
IP6
It was also suggested that, if the intervention was to run again, including incentives such as a gym membership and providing an opportunity for all those involved in the study to meet might increase motivation, help increase physical activity levels and reassure participants that their progress and experiences of the intervention are similar to others:
I don’t know if it’s feasible, I don’t know what the budget is but for anybody who was interested in say going to a gym maybe provide a 3-month gym membership or something like that just so you can have a taster session if that’s something that you like and I think I would probably go for the taster session.
IP3
It would be nice sort of to do some sort of other activity every so many weeks, you know, as a group and do something slightly different than walking . . . I think you need something else put in there to keep people motivated cause that’s what I need at the minute, motivation.
IP5
I think to hear someone else’s experience and you know what they were getting from physical activity benefits of it and to see if I could learn something new.
IP3
Some participants mentioned they would like to continue walking with their peer mentor and felt that it might be useful for GPs to monitor whether or not people should be reintroduced to the intervention in the future:
. . . you can do something and be 100% behind it for a couple of weeks . . . just to come back and check and I think it gives you that wee thing right I’m still accountable here although you’re not meeting up the same whether that’s through the GP to check up and say right you’re doing great or maybe we should think about getting you back into the programme.
IP7
Control group
It was clear that those allocated to the control group believed that enrolling in the research study would help them to increase their activity levels and improve their health and provide an opportunity to meet people who live in their local area:
Well, I thought I was going to do something and I was going to increase my physical activities and contribute maybe to the overall health.
CP1
I’m retired and I don’t work, I’m cut off really from society in lots of ways and I thought that this was going to be a way of connecting with other people in the area, you know.
CP2
Consequently, participants allocated to this group mentioned that that they were disappointed to be allocated to this group:
I was in the control group so I was a bit disappointed that I wasn’t in the other group because I thought I’ve missed my chance to be really sort of taken by the hand and sort of helped to get into the mode of exercising or walking.
CP2
Although these individuals were disappointed with their role in the study, one participant mentioned that being involved had prompted then to join the gym, another had joined a local walking group and the third participant remained committed to increasing their physical activity in the future, but were unsure how they would achieve this:
Well, I don’t think the physical activity has increased . . . if I want to take part, you know, positively in the thing, I’ll have to find out what activities would be recommended for somebody like myself or I don’t know whether you decide yourself what you’ve to do or whether it’s just recommended to you.
CP1
Control participants’ views on the length of the study were mixed and influenced by their role in the study. However, overall, the length of the study was deemed as acceptable to increase activity levels:
I think that’s the right amount of time I think any shorter would not be sufficient and any longer would be just too long.
CP2
Given the above, when asked if there was anything they would like to change about this study, group allocation was mentioned: the control group participants would have preferred to have received some type of intervention. In addition, it was suggested that the diary used to record accelerometer wear time data and the questionnaires administered at the beginning and end of the study could be simplified:
I think the assessments were you had the tracker on and you had to put in when you took it off, I think that sheet to me was very difficult to understand . . . I think it’s important that it’s ultra-simple.
CP1
It was also suggested that those involved in the ‘Walk with Me’ study, irrespective of group allocation, would benefit from being introduced to others in their local area who participated, as a way of feeling more involved:
I would like a meeting of the people in the area who were involved in the research, just as a sort of as a rounding up of the whole research where they had people from the control group and the other group . . . maybe sort of mingle for half an hour or something where they could talk to whoever’s there to say well you know how did you find the group and did it really make a big impact on you . . . it’s so easy sort of to sign up for a group and sometimes you’re left in the air and I think when you meet up with a person involved in the research then it feels as if you were actually involved.
CP2
Theme 2: factors that influenced motivation to become involved
Recruiting from local community groups and GPs was a key feature in promoting the uptake to this study. There were common findings across the various categories of study participants, so that the views of peer mentors, intervention and control group participants are integrated in the report below.
Recruiting from communities
The findings suggest that recruiting participants and peer mentors who were familiar to one another within a local community motivated people to become involved, indicating that the recruitment strategies adopted contributed to the overall acceptability of the study:
[XXXX] and me are sort of just more friends like and she asked me would I do this with her.
IP1
I got the letter and said I was interested and my friend she agreed she wanted to do it, I didn’t want to be in charge of a big group of people.
PM2
When asked how more people could be recruited, it was suggested that people like to find out what would be involved and the benefits from others who have participated. Previous quotes also support this finding, suggesting that older adults might be motivated by learning from others:
The best way of advertising is word of mouth you know really if you’ve got people there that said to you right I really benefit from this . . . and says here that was really worthwhile.
CP1
It was suggested that if the study was to run again it might be useful to base its management within local community organisations with already established groups:
With the help of the library they can maybe extend it a wee bit more you know, different groups.
IP5
Recruiting from general practitioners
The majority of participants who took part in the discussions were motivated to become involved after receiving information about this study from their GP:
I got a letter from my doctor saying that there was going to be a study and would I be interested in taking part.
CP2
I got a letter from my general practice it was round about the time I turned 60 last year and asked me if I was interested in taking part . . . I had recently retired from work and had decided that I wanted to get myself fitter primarily by walking, not particularly interested in going to the gym but I do like getting out walking and it seemed perfect.
IP3
It was apparent that promoting this study through GPs increased its acceptability. Participants considered this approach as more personalised, with some mentioning that they were motivated to become involved because they had been personally selected by their GP as someone who would benefit from taking part:
. . . it felt like a personal invitation and so it was as if it was sort of, not targeted at me but you know just specifically for me because it had been highlighted that I was a person who could maybe be helped by this research.
CP2
Others indicated that receiving an invitation from their GP minimised any concerns they may have had about the legitimacy of this study:
You know, you just knew it was genuine.
CP3
Some peer mentors who were recruited following invitation from their GP but found to be ineligible to participate because of their level of physical activity said that they were motivated to take up the role of a peer mentor because they were interested in the opportunity to walk more and to help others:
I just found it interesting and although I go to the exercise classes I should probably walk a bit more and this was an opportunity that was going to force me to actually go out and walk more to be quite honest so that was probably the main reason plus I liked the idea of mentoring somebody who maybe wasn’t as active.
PM4
It gives you a bit of a feel good thing to think that you are actually helping people, that you’re encouraging them to do something that is for their benefit as well as for your own, you know.
PM3
Given this, it was apparent that GPs played a role in motivating participants to take part and aided the recruitment of peer mentors to this study. However, comments suggest that adopting this recruitment method might have an impact on participants’ expectations. For example, it was mentioned that, because the letter of invitation was sent from their GP, they thought there would be some general health checks:
I thought whenever the letter came through that they would do more . . . tell me how my cholesterol is and how my general health is . . . I thought there would be more tests that way but then there wasn’t, so I was kind of disappointed that way, that they didn’t do physical tests . . . you know nobody took your weight to see if you lost weight.
CP3
GPs . . . maybe should be checking well how did you get on with that and whether you’re still being active and maybe have taken records at the start of the programme and later on then, records of blood pressure, cholesterol all those wee things and mental state of a person and the GP taking that.
IP7
Discussion
Key findings
The results suggest that the ‘Walk with Me’ study was acceptable. Social support from the peer mentors, self-monitoring and the associated benefits of the intervention (e.g. increased walking; encouragement; enjoyment; physical and psychological) emerged as key features in whether or not participation in this programme was viewed favourably. In addition, a number of issues which have the potential to affect the success and implementation of this programme in the future were identified, namely the paperwork, formal aspects of the peer mentor role and control group treatment.
The paperwork involved had a negative impact on both peer mentors’ and participants’ experience of this study. It was not clear how peer mentors supported their participants in completing the paperwork; however, peer mentors’ and participants’ comments suggested that some additional support might be required. Participants themselves suggested that the information required should be reduced in amount, be more user-friendly and be presented in a smaller booklet format. It might also be useful to identify opportunities for peer mentors and participants to meet as a group, but the timing of this would need careful consideration. Such a meeting would provide an opportunity for those involved to meet others and discuss difficulties and reinforce the importance of completing the required paperwork.
Greater clarity needs to be given to participants, from the outset, on the importance of completing the self-reported outcome measures on health and psychological well-being, as it was evident from the findings that they did not see the relevance of this information. In addition, some participants recruited from general practices expressed an expectation that they would receive physical health check-ups as part of the intervention. This might be something to consider for future research (e.g. blood pressure checks or monitoring body mass index), and the inclusion of such components in baseline assessments may provide a tangible encouragement to control group participants’ continued involvement in the study.
Time and the weather emerged as potential barriers to participation in the study programme, but peer mentors and participants managed these well, implementing problem-solving strategies.
Conclusion
To conclude, these findings suggest that the ‘Walk with Me’ programme and proposed trial protocol were well received by those involved, the intervention has the potential to encourage older adults to walk more and suggested protocol changes have informed the design of a definitive trial.
Chapter 8 Recruiting participants from general practice
Introduction
Previous research has suffered from the under-recruitment of older adults into research studies. Reasons cited are that older adults may be less healthy and therefore may not meet narrow inclusion criteria or refuse to participate. 87 Physical activity studies often report low response rates to invitations to participate,88 though the need to improve understanding of effective methods to recruit trial participants has been recognised. 89
In the ‘Walk with Me’ study, recruitment via general practice accounted for approximately half of the individuals who expressed an interest in taking part in the study. However, previous research has shown that it is difficult to engage GPs in research. Nevertheless, engaging GPs in research can play a key role in extending knowledge and translating new information into practice. The most common reasons given by GPs for not participating in research are a lack of time, a preference for clinical care over research, lack of skills to conduct research and research regarded as less relevant in terms of clinical or professional value. 90,91
To further explore the feasibility of recruiting older adults and the barriers to and opportunities of recruiting participants to a physical activity research study from general practice, we conducted qualitative interviews with participants and staff from general practice.
Methods
A purposive sample of GPs engaged in recruiting participants for the ‘Walk with Me’ study were invited to take part in a short face-to-face semistructured interview. The aim of these semistructured interviews was to explore the barriers to GPs’ participation in recruitment of patients to physical activity research. In addition, practice managers were also invited for interview. Practice managers have direct knowledge of the running of a practice and workload of GPs and it was considered that their views would provide information about enablers of and barriers to recruitment from general practice. The interviews took place in each participant’s own practice. The interview schedule (see Report Supplementary Material 2) was developed after reviewing responses to a brief questionnaire returned from GPs in participating practices about their experience of recruiting to the ‘Walk with Me’ study and their views of how recruitment could be improved (responses were received from 24 GPs). Each interview was recorded and transcribed verbatim. Iterative analysis allowed further exploration of issues identified in earlier interviews. Interviews were conducted by a female trainee GP (DC) who was trained in qualitative methodology.
Further interviews were conducted with six intervention participants who had been recruited from general practices. A convenience sample of participants who had recently finished the intervention were invited to take part, and all agreed. The purpose of these interviews was to explore patients’ views on themes identified in GP interviews in relation to barriers to and facilitators of recruitment. The interviews took place at a location of the interviewee’s choice, including the local leisure centre, the interviewee’s home and the local park (as the participant wanted the interview to coincide with their morning walking schedule). Each interview was recorded and transcribed before the next interview took place to allow iterative analysis.
Data analysis
Interview transcripts were independently analysed by two people using a thematic analysis framework approach. 50 Initial codes were identified and themes collated (DC/MT). In discussion with a third researcher (MEC), these themes were reviewed and refined, ensuring clear definition. Data saturation was achieved.
Results
Practice demographics
Six practices agreed to participate in this aspect of study. The participating practices were multipartner with varying list sizes (Table 18), and located in both urban and rural settings.
| Practice | Location | Number of partners | Approximate list size (n) |
|---|---|---|---|
| A | Urban | 2 | 4200 |
| B | Urban | 6 | 9800 |
| C | Urban | 6 | 8640 |
| D | Rural | 2 | 3800 |
| E | Rural | 3 | 5300 |
| F | Urban | 5 | 8755 |
In total, four GPs, four practice managers and six participants agreed to participate in a semistructured interview. Three of the four GPs had returned questionnaires prior to the interview. Although the aim was to interview at least six GPs, data saturation was achieved with four GP and four practice manager interviews. Thematic analysis of the interviews identified themes and subthemes, based on the views expressed by GPs, practice managers and participants on recruitment to physical activity research. These themes – (1) barriers to recruitment, (2) facilitators of research recruitment and (3) suggested approaches to recruitment – are reported below, illustrated with supporting quotations which have been anonymised with the individual’s corresponding role [e.g. GP1 (GP), PM1 (practice manager), P1 (participant)].
Theme 1: barriers to recruitment
Expectations of the general practitioner–patient relationship
There was a consensus among GPs that their patients would not expect to receive advice or information about physical activity from them. Their comments suggested that GPs were reluctant to introduce the subject and invite patients to participate in physical activity research:
I find personally they’re [patients are] not that interested. They want a tablet or something. You mention it and they’re like ‘oh aye aye’. I think smoking cessation has become a bit more socially acceptable. You start on about the usual things like weight and you get varying responses. But I don’t think people expect it [mention of physical activity] really.
GP2
However, among the GPs’ comments, a deviant opinion was identified. One rural GP perceived that patients did expect to receive lifestyle advice from their GP and that those with cardiovascular disease relied on the GP’s approval to pursue physical activities. The majority of practice managers were also of the opinion that patients would expect physical activity advice/guidance from their GP:
They do not want to be doing anything without their GP saying it would be advisable to do that.
GP4
Every exercise programme says consult your GP before starting this programme, so I think there is an expectation there although I don’t know how well GPs are educated themselves to be able to provide that education to the patients.
PM4
In contrast to most GPs, participants’ comments tended to support the latter view. They welcomed advice about physical activity being given by their GP and responded positively to receiving information about the research project. Their responses appeared to be related to their views about what they hoped to achieve from consultation with their GP, in relation to improving their overall health:
I think they [GPs] could do with putting a line or two in there, get up and get moving.
P1
I think it’s good for GP practices to look at what you might call the holistic approach to people’s health, not just, here’s a prescription.
P3
Most GPs felt that patients had specific but variable expectations of what their GP could do to help and what they might accept as part of a management plan, particularly in relation to physical activity. It appeared that GPs were more prepared to discuss physical activity in detail, and to mention possible involvement in research, if patients themselves raised the issue. GPs’ comments also suggested that they/colleagues had particular habits in their consultation practice and that the extent to which they would usually give lifestyle advice was known by their patients: they considered it appropriate to fulfil their patients’ expectations. Interestingly, one participant had similar views to the GPs, in that there was a perception that older GPs were less ready to give lifestyle advice than those who were younger:
Some patients have unreachable expectations of their GP and some have no expectation of their GP. I think if someone came and asked about exercise yeah some would expect their GP to say you should be doing this that or the other.
GP1
I feel my parents’ generation just expect the doctor to give them a tablet for every ache or pain.
P5
One practice manager commented on the importance of the GP promoting physical activity research because of the high regard that they are held in by their patients. GPs reported a similar view. The perceived significance of the GPs’ endorsement of physical activity highlights the importance of GPs’ continued involvement in recruitment to physical activity research:
It encourages people to be physically active, I think people will take notice from the doctor.
PM2
If it comes from us it carries more likelihood of success.
GP4
One participant commented on the importance of the invitation to take part in physical activity research coming from the GP, but appeared to understand the constraints on their time in surgery consultations:
I think it makes more impact if the doctor says it, but they don’t have the time.
P6
However, several participants felt that the responsibility for maintaining health lay with themselves and that they should not be solely reliant on the GP’s influence, but should have a personal motivation to improve their health. They considered that displaying information about research participation in posters and leaflets was an appropriate method of recruitment, to which people should make autonomous decisions to respond:
You have to be your own advocate because you live with your body so you know the changes.
P4
I feel I should be looking after my own health, that’s my responsibility.
P5
Further comments illustrated how the GPs’ likelihood of inviting a patient to partake in research was also influenced by their perceptions of the patient’s response. The following quote illustrates one GP’s reluctance to invite those from whom a negative response was expected and how patients’ responses were often pre-judged:
The people who are most likely to benefit are the least likely to get involved.
GP2
Workload
Workload was identified as a significant issue in all interviews, with both GPs and practice managers citing this as a barrier to GPs recruiting participants. Most GPs found that it was difficult to complete the essential tasks of their clinical role, and that involvement in research, although it may be beneficial, was beyond their workload capabilities. Research was regarded as having less priority than issues which required immediate clinical management. In addition, the nature and extent of additional work required to recruit patients had an impact on their readiness to engage in the project:
It [research] feels like an ‘add on’ which is maybe not as important to clinicians.
GP1
. . . recruiting in addition to delivery of a clinical service probably feels more challenging in the current setting.
GP1
They’re just too busy, it’s their workload.
PM2
One GP commented that the increasing complexity of patients’ clinical conditions discouraged the introduction of research, as another issue, into the consultation:
With ever increasing workloads we are trying to fit more into consultations, patients are coming with not just one problem but several problems.
GP3
Interestingly, it was not just GPs’ workload which was perceived to have an impact on recruitment. Participants felt that one of the reasons that patients may decline to participate in physical activity research was their own workloads, in their busy day-to-day lives and their personal commitments:
It’s just other commitments, finding other things to do, even retired people.
P3
Time
Time was identified as a separate subtheme to workload. It encompasses limited consultation time and GPs’ difficulty in finding time to learn about a research project. All GPs who took part in the interviews felt that there was insufficient time during their 10-minute consultation with a patient to appropriately identify who may be suitable for the study and provide information about it. Practice managers held a similar view:
It’s going to take a little bit of time to explain a project to a patient so that is probably the greatest barrier that I would see.
GP3
The greatest barrier has got to be time just learning about these projects and following through with it.
GP3
The comments of GPs indicated that most practices would be happy to get involved with physical activity research as long as it did not interfere with other practice work. It appeared that the pressure of time needed to complete work to achieve good standards of clinical practice and governance within the practice far outweighed any monetary incentive that could be offered to incentivise recruitment to physical activity research:
It’s about the time, not the money.
GP2
Practices will do things they have time to do and that doesn’t conflict with other responsibilities.
GP1
Participants valued GPs’ involvement in recruitment but felt they did not have time for recruitment of patients to research:
They don’t have time [to involve patients on physical activity research].
P1
I think it makes more impact if the doctor says it but they don’t have the time.
P6
Weather
The weather emerged as a barrier to recruitment to physical activity research in interviews with all groups. One GP felt that the winter months may have had an impact on patients’ likelihood of engaging in physical activity and hence did not readily offer invitations or information about the study. This view was supported by practice managers, whose comments suggested that they would expect a higher rate of recruitment during more favourable weather conditions. Participants expressed varied views regarding the impact of poor weather on engagement in physical activity research that involved walking: some considered that weather was an important factor and others thought that people should be prepared to overcome its adverse influence:
. . . better time of the year, maybe winter not the best time, launch it in May.
GP2
I think a longer period of time to recruit and maybe during better weather, I think if you maybe started it in the spring time.
PM6
My mate says, ah sure go on pull the wellies on, the weather in Ireland does impact.
P4
Identification of potential participants
A further barrier to GPs’ recruitment was difficulties in identifying appropriate individuals. GPs recounted difficulty in remembering to invite potential participants during consultations mainly because of the time constraints and competing priorities, but also difficulty remembering the specific inclusion and exclusion criteria. Practice managers’ comments confirmed the view that GPs had difficulty in remembering to recruit and attributed this to the complex content of their consultations:
I think it’s the last thing in your head sometimes [referral to research] especially if there is paperwork involved.
GP2
Remembering who is eligible for recruitment might be another barrier.
GP3
I don’t think GPs remember to ask patients after they have dealt with everything else.
PM2
One practice manager cited limitations of software in their current electronic record system to identify patients who would fulfil the inclusion criteria for the current study and be eligible for postal invitation:
I think it’s hard to pick individuals, I don’t think doctors have the resources to do it, I think in terms of our search engines on EMIS PCS [operating system in practice] that we use I think it’s quite hard to do because these are sort of soft targets and you sort of need to know the patients, I think narrowing in on more specific criteria.
PM1
Theme 2: facilitators of research recruitment
Benefits to the practice
There was a general agreement among practice managers that research was of benefit to the practice. Their comments reflected a readiness to support physical activity research as they perceived that it has positive benefits in promoting health. Their comments also indicated how they perceived potential value for the health of their staff by supporting physical activity research. There were conflicting views among GPs about the benefits to the practice of monetary incentives to encourage research recruitment, with several reporting that a monetary payment would not make up for the necessary increased time investment. Only one GP considered that a monetary incentive to purchase equipment for the practice would be beneficial:
Obesity is obviously a huge problem, our diabetic clinics are increasing each month and I think anything we can to do improve their health and reduce pressure on us would help enormously.
PM1
I think it’s good for everyone to promote PA [physical activity]! Even for staff to encourage us to be physically active.
PM2
. . . if there is some sort of repayment that we could get equipment for our practice or some sort of reimbursement for the practice I think we would all sign up far quicker.
GP1
Participants equally had positive thoughts about research and so supported the practice being involved in it. They understood the potentially beneficial outcomes of physical activity research and expressed enthusiastic views that their practice could contribute to this process:
He told me it was research and I was delighted.
P1
Research is important, how else do we know if things work.
P5
Benefits to the general practitioner
Most GPs had positive perceptions about research: their difficulties in being actively engaged in their practice were attributed to the aforementioned barriers. They indicated a willingness to be involved in future research and reflected the need for researchers to highlight perceived benefits to the practice when seeking to engage them in research. They also highlighted how engagement in the research project had personal professional benefits, including reflection on the workings of their primary care team, extension of their knowledge about physical activity and positive feedback on their clinical activity in the area of health improvement:
. . . you know when you have research happening in a practice, like when you take part in clinical governance, when you take part in education, it just helps you to think slightly differently about what you’re doing, eh, and so I think it has a role of getting the wider GP team to think more broadly about what they are doing in terms of their daily work.
GP1
I do think there is a benefit to the practice which maybe isn’t immediately obvious. But in hindsight everyone will have learned something because they were involved.
GP1
I think from my own experience taking part in research I learned more about the clinical area and then in turn how effective I am as a clinician to patients.
GP1
. . . anything that has a demonstrated outcome, that if you know that you’re doing it its worthwhile and the patients or we benefit from it in terms of reduction of health need.
GP2
Benefits to the participant
Perceived benefits to their patients was a significantly positive factor in the GPs’ willingness to engage in physical activity research, cited in the majority of interviews. They regarded the research project as an opportunity to advise patients regarding healthy lifestyles and in the first steps of chronic disease management:
If it’s something that will benefit your patients you’re more likely to take it on board, I think relevance to general practice would be quite important.
GP3
One GP reported that patients involved in research usually receive additional time from health professionals and may learn more about their condition than they would in the course of ‘usual’ care. Involvement in research was also perceived to be of benefit to GPs’ development of their clinical skills, with potential benefit for patients’ care:
. . . individuals who take part get a bit more attention and maybe learn a bit more about their condition and I think it also has the added benefit that it educates the clinicians to some extent and improve their performance as clinicians and just through all of the training and feedback and taking part.
GP1
Similarly, participants cited benefits of their involvement in physical activity research and plans for continued physical activity efforts, corroborating GPs’ perceptions of health benefits for patients:
My cholesterol has come down and I’m contributing it to the programme, it’s a good result so it’s an incentive for me to keep going with the programme.
P3
For the participants, the benefit of companionship and the social contact that the study added to their lives was a significant benefit to them and they recognised a sense of interdependence with their peers involved in the study:
I think the programme is really good, especially buddying up, the peer programme, ’cause there is the thing about guilt.
P3
It was something to get me involved with other people again.
P4
For me the social element was as important as the walking the steps.
P4
Theme 3: suggested approaches to recruitment
Most interviewees suggested alternative strategies that they felt may boost recruitment. These included improved methods of self-referral and using the project team or practice staff to inform potential participants about the project. It was also suggested that GPs could target specific practice events or activities for recruitment, particularly of patients who would be infrequent attenders.
Self-referral to the research project
The study relied on posters displayed in waiting and reception areas to encourage self-referral, but there was limited uptake from this method. However, the interviewees approved of self-referral methods of recruitment and suggested that potential participants could be encouraged to engage by making relevant information more visible, including it on the practice’s website or using text messaging:
Well I suppose it’s always good if patients can self-refer, it’s becoming more widely used for example self-referral for antenatal, so if you can target people that perhaps you don’t need the step of the GP that would help.
GP3
. . . you could send out a text message and get them to self-refer, but you’d have to pay practices per message.
PM1
One participant also suggested that they would have liked to have been able to share their letter of invitation to the study with others who may have been interested and could have self-referred:
The letter looked like it was just a one person and a monitor but if it was extended to other people, like your partner or your friend then you could have got three people involved.
P4
‘In-person’ recruitment
Interestingly, ‘in-person’ recruitment strategies, where personal invitations were given directly face to face to potential participants, were approved by practice managers and echoed in comments from participants, but were not suggested by GPs. Although some participants felt that the GP should be opportunistically inviting patients when they attended the surgery, practice managers perceived that staff members other than the GP could contribute, at least in part, to the recruitment process. One approach suggested was that of asking patients when registering with the practice, for a consent to be contacted about future physical activity studies:
Talk to people like me when you’re [I’m] in for a visit.
P3
Maybe even like a promotion stall downstairs as people come in the front door, telling people about it. Passing out a few leaflets and trying to encourage people.
PM3
I think even while people are just sitting in the waiting areas, if you had someone there to approach them and tell them about the project, promote the project.
PM2
I think nurses are seeing patients with certain conditions like asthma and diabetes and they have more time to talk to them, whereas if a patient goes in to speak to the doctor, . . . it’s time, that takes up all the time.
PM2
Interviewees’ comments indicated that, although they perceived the involvement of GPs in issuing invitations as important, they also recognised that GPs’s time is limited. One participant suggested the possibility of the GP inviting potential participants by telephone, rather than trying to make time during surgery consultations or involving other members of the practice team:
Something like a telephone call, I think from the GP because that’s the initial contact with the surgery.
P2
Targeting events
Both GPs and practice managers raised the issue of targeting particular events or activities within the practice for concurrent efforts to promote recruitment to research, for example taking advantage of the large numbers of people who attend the practice for flu vaccination, who otherwise may not attend the practice on a regular basis:
Maybe during busy times like flu [vaccination] seasons.
GP2
Enhanced engagement with the practice
Enhancing promotion of the research project to all practice staff was favoured by several GP interviewees, with the aims of encouraging them to remind GPs about recruitment and of involving them directly in recruitment. The importance of personal contact between the researcher and the GPs/practice staff was highlighted as a means of heightening interest in supporting the project. In addition, to reduce the impact of time taken to learn about a project on a GP’s clinical workload, various options for informing GPs about the project outside their practice time were suggested:
I think again getting practice nurses involved and keeping at the GPs to constantly remind them.
GP2
It might be worth looking at those other health-care professionals who are involved in caring for the patient.
GP3
Targeting educational evenings for GPs in the building. You would get about 15–20 GPs at that across the practices.
GP3
Discussion
Summary of findings
The interviews have highlighted a number of challenges, principally the issues of workload and time constraints, to GPs’ recruitment of older people to physical activity research. The limited time that GPs have to dedicate to research recruitment might have had an impact on their willingness to engage. GPs also identified the difficulties they had in remembering to invite patients during the consultation and in identifying which patients may be appropriate to recruit to the study. The perceptions of patients’ expectations of GPs’ knowledge of physical activity and the perceptions of patients’ responses to invitations to participate in physical activity research influenced GPs’ engagement in recruitment. However, positive suggestions were made for improving recruitment from general practice in further studies.
A novel finding was the impact that weather had on the willingness of GPs to invite patients: they were less likely to offer an invitation if they felt patients would be likely to decline it because of poor seasonal weather conditions. We have also identified several potential facilitators that could enhance the recruitment process, including emphasis of the perceived health benefits that involvement in physical activity research could confer on the practice and its patients. There was also an overall positive perception among GPs of the enhanced personal professional knowledge that involvement in such a study could provide.
Several participants mentioned the potential for using media, such as text messaging and the use of the practice website, in promoting the study and aiding in the recruiting process. This is a potential area to improve the reach of a future definitive trial without unduly burdening the practice to publicise the study further.
There was an overall positive perception of physical activity research, but a consensus among GPs that they would find it difficult to undertake additional tasks to their current workload. From the outset, an aim of this study was to have minimal impact on the workload of GPs; however, for many, the thought of having any additional responsibility appeared to be a deterrent for involvement in research recruitment. This may have reflected insufficient education about the project requirements for the GPs, but it was very difficult to arrange meetings with them to discuss this because of their busy schedules. Many participants appeared enthusiastic about making a contribution to research, and one GP recognised that patients involved in research may receive additional time from health professionals.
Implications for a future definitive trial
These findings offer important insights to maximise the potential of recruiting participants from general practice. For GPs to even consider becoming involved in recruitment to physical activity research their concerns about additional workload need to be addressed by ensuring that added work is minimal. The majority of GPs felt that monetary incentives would not encourage their participation. However, some GPs did make the reference to how they are paid for other tasks through the Quality and Outcomes Framework. This system, which rewards GPs for the quality of care they provide, constitutes a significant portion of GP income and is supplemented by enhanced services payments. Perhaps if incentives were streamlined to encourage regular involvement in similar research projects, more GPs would be willing to become involved.
This study findings suggested that it may be beneficial to encourage other members of the practice team, such as nurses, to become involved in the recruitment of patients to physical activity research, alongside the use of letters of invitation. This may reduce the burden of responsibility and additional workload among GPs and maintain the use of the practice as a platform for recruitment. However, unless research recruitment is a recognised part of the role of other staff, it may be viewed by them as an ‘extra burden’, as it is by GPs, and will subsequently be challenging to implement.
One participant highlighted the potential to recruit more than one person per letter of invitation sent. Offering patients the opportunity to invite a friend or family member may enhance recruitment numbers and this approach may be developed in future work. Given the current expanding traction of social media and use of information technology, this area may be explored in greater detail in future studies to provide further community reach and greater engagement with GPs and patients.
Finally, the findings suggested that future trials should avail of the influx of patients to the GP surgery that occurs during the vaccination season and during specialty clinics. One practice did invite 20 patients verbally at a flu vaccination clinic, but this did not result in any contacts to the study. However, this may be reflective of the numbers required to be invited to obtain even a single study contact. For example, from the 400 letters sent by GPs in our study, the response rate was 14% (n/N = 56/400).
Chapter 9 Discussion
The ‘Walk with Me’ intervention was designed to engage socioeconomically disadvantaged older adults in regular physical activity. The theory- and evidence-based intervention was developed in accordance with the MRC framework for complex interventions,34 by using a mix of evidence from previous peer-led physical activity interventions and the input of socioeconomically disadvantaged older adults. The feasibility of delivering the intervention in order to evaluate its effect within a RCT was then assessed. The predetermined recruitment and attrition rates were reached: the intervention was delivered with a satisfactory level of fidelity in weeks 1–4, but delivery fidelity after that was less than optimal. Participants did report high levels of acceptability of the intervention within the pilot RCT. Retention and engagement in the study were high, with high levels of compliance in wearing the accelerometers to measure the primary outcome. Increases in physical activity behaviour in response to the intervention were evidenced in both the quantitative and the qualitative data, demonstrating the potential effectiveness of the ‘Walk with Me’ intervention.
Changes required for a main trial
The pilot trial has been a critical step in moving towards a definitive, fully powered RCT of a peer-led physical activity intervention for older adults. Several modifications are suggested to improve the implementation and evaluation of the intervention for a main trial.
We identified that it was possible to engage older adults aged 60–70 years to sign up to the trial. It should be noted that the individuals who agreed to participate were relatively healthy, and more tailoring of the recruitment process is needed in order to recruit less healthy individuals, given the focus of the intervention. Though the participants were classified as inactive when registering their interest in the study, accelerometer data revealed that they were reasonably active at baseline. Participants in this study were undertaking, on average, 32 minutes of MVPA per day at baseline. This is higher than the average MVPA in a sample of 1186 adults (aged 60–69 years) who participated in the National Health and Nutrition Examination Survey (NHANES) study in the USA (14.2 minutes of MVPA/day). 92 However, the levels of physical activity in our participants were lower than the levels of a similar cohort of 298 adults (aged 60–75 years) recruited to a recently reported walking intervention from general practice in England (43 minutes of MVPA/day). 93
In addition, two-thirds of participants were female. This is similar to the findings of a previous systematic review of recruitment to walking interventions, which identified that 70% of participants are female. 58 In addition, most (70%) of the male participants in our study were recruited through their GP, which suggests that this may be the most feasible way to recruit male participants to the study. Foster et al. 58 recommended monitoring participants’ responses to recruitment approaches and using different recruitment strategies, where necessary, to ensure balanced recruitment. Careful monitoring of recruitment by sex would be an important aspect of a definitive trial.
Given our finding that the most efficient way to recruit participants was through general practices, this is also likely to be the avenue to identify and recruit less healthy individuals. GPs supported the idea of recruiting patients to a physical activity trial, but the process needs to take place with minimal intrusion on the delivery of direct patient care. Using general practices to recruit participants is becoming increasingly complex and we have identified a variety of approaches that can be used (e.g. synchronising recruitment efforts with other activities in the practice, such as clinics).
After indicating their interest in participating, individuals were willing to accept randomisation to either an intervention or a control group; although those in the control group did express a desire for more than a waitlist condition. Future peer-led interventions could consider using an attention-matched control group, like that of Castro et al. ,42 who offered nutrition support instead of physical activity, although this may impact on the secondary outcomes. We therefore propose adding brief nutrition advice for the control group.
The ‘Walk with Me’ intervention included only individuals aged 60–70 years. Some community groups gave informal feedback that this may be restrictive in terms of implementation of the strategy in the real world, as their practice is to offer programmes to anyone who wishes to take them up (i.e. they would identify individuals outside this age band who would benefit from the programme and be capable of participating). There is therefore a case to be made to omitting an upper age limit from future inclusion criteria and using a measure of functional ability to identify eligible participants. We also propose removing the upper age limit for peer mentors, as participants indicated that the peer mentors’ ability to motivate and support was more important than their age for the successful delivery of the intervention.
The quality of data from the primary outcome was good, with at least 93% of participants returning a valid accelerometer data set at any time point. This demonstrates that the outcome measure was acceptable. Some participants reported that the self-reported outcomes were burdensome and took too long to complete. This may be the reason for the relatively lower rates of completeness of self-reported outcomes at baseline. These measures therefore need to be reduced in terms of their time requirement, and duplication of focus, such as avoiding the use of both GHQ-28 and SF-12 questionnaires. In addition, a measure of self-reported physical activity may not be needed. The purpose of including it was to capture the domains of physical activity where changes occurred, but it may not be sensitive enough to capture changes. As identified in Chapter 7, some participants expected to receive a health check as part of the intervention. We therefore propose adding measures of blood pressure and body mass index to a future study. In addition, greater efforts will be required to encourage the return of data from those who discontinue the intervention but do not withdraw from the study, including the offer of telephone interviews to collect outcome data.
In the post-intervention interviews, some participants reported that they would like to have had more support from the mentors in setting goals. Some participants felt they were left to set their own goals in the later parts of the programme, though this was not corroborated by the fidelity checklists. It will be important to emphasise the importance of following the approach to goal-setting set out in the programme manual with mentors in the ongoing support that is offered.
Assessment of fidelity and record-keeping proved challenging within the intervention. This may be because peer mentors and participants are not professionals and therefore are not used to the type of record-keeping which has worked well in previous walking programmes in clinical settings. 61 The importance of record-keeping will need to be emphasised with peer mentors and participants. Some modifications, with user input, to checklists to reduce the burden of record-keeping should be planned before undertaking a definitive trial. Other options will need to be explored, including mobile telephone apps or websites to make recording information less burdensome. In addition, a protocol for creating regular backups of digital audio-recordings will prevent the loss of data.
We propose amending the exclusion criteria to exclude those not in work at the start of the intervention, but planning on returning to work before the end of follow-up. This would avoid the situation that arose in our pilot study, in which a participant in the control group returned to work as a postman over the course of the study and increased the group’s average step count through work-related activity which could not be directly attributable to the intervention.
As described in our post-intervention interviews, the burden of paperwork was a barrier to the delivery of the programme and potentially to the development of the relationship between the peer mentor and participant. Reducing the volume of paperwork should help to foster good peer mentor–participant relationships.
During training, peer mentors were advised that if they encountered difficulties in their relationship with a participant then they should contact a member of the research team as soon as possible. In this scenario, the researcher would speak with both parties in an attempt to resolve issues. However, during the intervention we did not experience any difficulties in the relationship between peer mentors and participants.
Peer mentors reported that the main barriers to delivering the intervention were (1) finding a time that was mutually suitable for them and participants to meet and (2) the weather (see Chapter 7). Therefore, some training needs to be added to reinforce the importance of a flexible approach to working with participants and finding alternative venues (e.g. local shopping centres) to walk in when the weather is poor. This may be achieved through a top-up training session with peer mentor (delivered at the half-way point of the intervention), which may help to refresh training on the delivery of key intervention BCTs.
Assessment of intervention costs
The intervention cost £210.61 per participant. This included the cost of training the peer mentor, the pedometer and materials. The peer mentors volunteered their time to deliver the intervention, so the cost of their time has not been included.
Piloting health service use log
The main aim of including an economic component was to pilot the use of a health and social care services resource use instrument to capture health-care utilisation. Participants in both the intervention and the control groups were given the template log and asked to record their use of health and social services over the full 6-month period. Although we got a reasonable return of this at 6 months (76%), participants commented that it was burdensome to complete alongside the diaries that were used as part of the intervention. We therefore propose a modified and shortened version of this log for participants to use during the intervention, supplemented with a questionnaire at the end of the trial. We will also ensure that the questionnaire adequately records when a participant has not used a service. In our rapid review of literature (see Chapter 2), we did not identify any previous studies assessing the cost-effectiveness of a peer-led walking intervention. This emphasises the importance of including a health economic analysis in a definitive trial.
Strategic planning
Findings from the interviews conducted with peer mentors and participants following the pilot RCT suggested that recruiting peer mentors within target communities and matching them to participants within these communities motivated both the mentors and the participants to become involved in the study, indicating that the recruitment strategies that were adopted contributed to the overall acceptability of the study. There was consensus among peer mentors that they had received sufficient training to deliver the intervention activities required. However, most believed that the training and support manual was extensive and difficult to understand, suggesting that it may be more user-friendly if this information was condensed. Peer mentors reported using the training and support manual differently throughout the study: some indicated that they referred to the instructions to ensure they covered the necessary content each week, whereas others admitted that they referred to this information from time to time only. This suggests that some mentors may need more support than others.
In the interviews conducted during the development of the intervention, participants identified that having a peer mentor to try new activities with would help overcome barriers and motivate behaviour change. Likewise, matching to a peer mentor who is both physically active and someone with whom they could develop a friendship with was also considered an important factor. This was supported by the findings from the post-intervention interviews. Participants in the pilot RCT were very positive about the benefits of the support and friendship received from the mentors. They did express a desire to meet others in the programme for support during the intervention.
Although we originally planned to involve a smaller number of peer mentors, matched to groups of participants, this was not what happened in the trial. Instead, peer mentors were matched with just one or two participants. This was feasible as we were able to recruit mentors from individuals who volunteered to take part in the trial but were ineligible to do so as study participants as they were too active. The planning of peer mentor matching will need to be addressed in a full trial. Our findings are currently inconclusive regarding how quickly a peer mentor would be willing to engage with more than one participant, so it is not clear how many peer mentors would be willing to be paired with a second and subsequent participant within the time confines of a definitive trial. In addition, we did not identify clustering of the results by peer mentor, with no obvious pattern in the data suggesting that some peer mentors were more effective at delivering the intervention than others. We have therefore not included this in the proposed sample size for a fully powered trial.
Finally, it proved very difficult to integrate the management of peer mentors into existing volunteer structures in the health and social care trust within the scope of our pilot study. Some mentors were therefore managed through the university. This is manageable within the confines of a trial, though, for the longer-term implementation of the programme, their management through existing walk leader schemes would appear to be the most appropriate route.
Strengths and limitations
We have noted the lessons learnt from the current pilot study in Changes required for a main trial. However, there are a few additional points that should be noted.
Characteristics of the sample
The final sample were all living in socioeconomically disadvantaged areas. However, they were more active, healthier and more likely to be female than originally envisaged. We also under-recruited according to our planned sample size of 60 participants, but we still recruited enough participants to deem a definitive trial feasible according to our original criteria. This may limit the generalisability of the feasibility of the trial to these groups. In addition, we did not record information on comorbidities at baseline.
Recruiting more active individuals into studies is common in physical activity studies. More research is required to understand why less active individuals do not respond to invitations to participate in physical activity interventions. In addition, the sample was restricted to those aged 60–70 years. The advice of community groups was to remove the upper age limit. Along with the further engagement with GPs, removing the upper age limit may also lead to the inclusion of less healthy and less active participants.
Use of the pedometer to set goals and monitor progress
Participants reported that they found the pedometer a useful aid to setting goals and monitoring progress. Given that all participants returned weekly step diaries throughout the full 12 weeks of the intervention, we assume that there was very high compliance with wearing the devices. In the interviews after the pilot RCT, participants reiterated the need for pedometers to be simple to use and easy to see.
Measurement of outcomes
The use of accelerometers as an objective measure of physical activity is a key strength of this study. In the rapid review (see Chapter 2), only one previous trial used an objective measure. 48 In addition, compliance with the monitor is very high, suggesting high acceptability of the main outcome. As this was a pilot study, the sample size was small and, as the participants were relatively healthy, it was not unexpected that their self-reported health outcomes did not change considerably during the trial. Nonetheless, the use of both the GHQ-28 and the SF-12 questionnaires as measures of general health was overly burdensome on participants.
For pragmatic reasons, the final follow-up time point was 3 months after the end of the intervention (6 months after baseline). To ascertain if changes in physical activity are maintained over a longer period (> 12 months), an additional time point may need to be included in a fully powered definitive trial.
Process evaluation
The loss of audio-recordings to assess the fidelity of intervention delivery was unfortunate and limited the analyses that could be performed on fidelity to the checklists and the post-intervention interviews with mentors and participants. However, fidelity was measured in a number of other ways. Analysing data from the participant and peer mentor checklists and step diaries indicated that the intervention was delivered with acceptable fidelity, suggesting that the loss of the audio-recordings was not a significant limitation to the process evaluation.
Public involvement
Another strength of this study was the contribution of project partners and stakeholder representatives, who were proactive in providing guidance from their own public representatives. Through interviews, we sought the views of older adults in developing the intervention and the design was based on their views. Subsequently, study documentation, such as the peer mentor training and support manual and the participant information booklet, were read and revised by members of an older adults forum to ensure that the language and content were acceptable to the target population. This provided a valuable source of public involvement during the development phase of the intervention. Service users were also involved in delivering the intervention, in their role as peer mentors. Finally, two members of the public sat on the project steering committee and provided valuable advice on recruiting to, and maintaining the involvement of older adults in, the intervention.
Use of behaviour change theory and behaviour change techniques
A previous review of physical activity interventions in socioeconomically disadvantaged communities94 has shown that interventions based on behaviour change theory are more effective, though no single theory appeared to be more effective than others. Behaviour change theories provide hypothesised mechanism of intervention effects on desired outcomes. 95 Based on the findings from the systematic review (see Chapter 2) and intervention development interviews (see Chapter 3), we identified SCT as an appropriate theoretical framework for the design of the intervention. Interventions targeting constructs of SCT in physical activity interventions are effective in increasing motivation and ultimately increasing physical activity. 96 In a systematic review of interventions to increase motivation for physical activity, Knittle et al. 96 identified that motivation for behaviour change was a result of fostering of personal control over behaviour within interventions based on SCT, and this was shown to be achieved in previous interventions using BCTs such as goal-setting, action-planning, self-monitoring of behaviour, feedback on behaviour and problem-solving.
Conclusions
There is a paucity of evidence of the effects of peer-led walking programmes in older adults. The ‘Walk with Me’ intervention, developed from existing evidence, with input from community stakeholders, based on SCT and designed with the aim of promoting physical activity among older physically inactive adults in a socioeconomically disadvantaged population, was acceptable to participants. Our pilot study has informed approaches to recruitment and peer-mentoring planning for future work. Notably, participants reported that they valued recruitment via their GP, as this is someone they trust and would have confidence in their recommendation to participate. A need to reduce the burden of self-reported outcomes and address intervention fidelity in the later stages of the intervention was identified. This should be balanced against participants’ desire to have objective health measures, such as blood pressure and body mass index, included. Quantitative and qualitative information suggested that it would be feasible to conduct a definitive RCT to evaluate the intervention.
Acknowledgements
We are very grateful to the participants and mentors who willingly engaged in the ‘Walk with Me’ intervention. We would also like to thank Trudy Brown (SEHSCT), Nicola Arbuckle (Northern Health and Social Care Trust) and Danielle Sinclair (Public Health Agency) for their assistance with the study. Thanks also to Alan Ferret and Philip Reilly, the Public Health Agency representatives on the Project Steering Committee.
The study was undertaken within the UK Clinical Research Collaboration (UKCRC) Centre of Excellence for Public Health (Northern Ireland). The Centre of Excellence for Public Health (Northern Ireland) is a UKCRC Public Health Research Centre of Excellence, funded by the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, MRC, Research and Development Office for the Northern Ireland Health and Social Services, and the Wellcome Trust, under the auspices of the UKCRC.
Contributions of authors
Mark A Tully (Professor of Public Health) was the chief investigator of the project and took overall responsibility for the delivery of the project and writing of the report.
Conor Cunningham (Project Manager) project managed the study and co-drafted the report.
Ashlene Wright (PhD Student) contributed to the conduct and analysis of the rapid review.
Ilona McMullan (Research Assistant) completed the intervention development interviews, co-analysed the data and co-wrote the relevant chapter of the report.
Julie Doherty (Research Assistant) completed the post-intervention qualitative investigation, co-analysed the data and co-wrote the relevant chapter of the report.
Debbie Collins (GP trainee and Masters in Research Student) completed the interviews about recruitment, co-analysed the data and co-wrote the relevant chapter of the report.
Catrine Tudor-Locke (Professor of Kinesiology) contributed to the design of the peer-led intervention, the conduct of the trial and was a member of the project management group.
Joanne Morgan (Director of Community Development and Health Network) contributed to the development of recruitment methods, the conduct of the trial and was a member of the project management group.
Glenn Phair (Junior Health Economist) co-analysed the health economics data and was a member of the project management group.
Bob Laventure (Director of Later Life Training) advised on tailoring the intervention to older adults and was a member of the project management group.
Ellen EA Simpson (Senior Lecturer in Psychology) supervised the collection of qualitative data, co-analysed the data and co-authored the relevant chapters, contributed to the conduct of the trial and was a member of the project management group.
Suzanne M McDonough (Professor of Health and Rehabilitation) contributed to the design of the project, the assessment of fidelity of the intervention, the conduct of the trial and was a member of the project management group.
Evie Gardner (Head of Statistics) contributed to the design of the project, supervised the statistical component of the research and was a member of the project management group.
Frank Kee (Professor of Public Health) contributed to the design of the project, the conduct of the trial and was a member of the project management group.
Marie H Murphy (Professor of Exercise and Health) contributed to the design of the project, the conduct of the trial and was a member of the project management group.
Ashley Agus (Health Economist) led on the analysis of the health economics data and was a member of the project management group.
Ruth F Hunter (Lecturer in Physical Activity and Public Health) contributed to the design of the project, conduct of the trial and was a member of the project management group.
Wendy Hardeman (Senior Lecturer in Health Psychology at the School of Health Sciences) contributed to the design of the intervention, the conduct of the trial and was a member of the project management group.
Margaret E Cupples (Emeritus Professor of General Practice) contributed to the design and conduct of the project, co-analysed the qualitative data and was a member of the project management group.
All authors read, commented and agreed the final draft report.
Publication
Tully MA, Cunningham C, Cupples ME, Farrell D, Hardeman W, Hunter RF, et al. Walk with Me: a protocol for a pilot RCT of a peer-led walking programme to increase physical activity in inactive older adults. Pilot Feasibility Stud 2018;4:117.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- National Population Projections: 2016-Based Statistical Bulletin. Newport: Office for National Statistics; 2017.
- 2016-Based Population Projections for Northern Ireland. Belfast: Northern Ireland Statistics and Research Agency; 2017.
- Payette H, Gueye NR, Gaudreau P, Morais JA, Shatenstein B, Gray-Donald K. Trajectories of physical function decline and psychological functioning: the Quebec longitudinal study on nutrition and successful aging (NuAge). J Gerontol B Psychol Sci Soc Sci 2011;66:i82-90. https://doi.org/10.1093/geronb/gbq085.
- Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s physical activity guidelines. Int J Behav Nutr Phys Act 2010;7. https://doi.org/10.1186/1479-5868-7-38.
- Talbot LA, Morrell CH, Metter EJ, Fleg JL. Comparison of cardiorespiratory fitness versus leisure time physical activity as predictors of coronary events in men aged < or = 65 years and > 65 years. Am J Cardiol 2002;89:1187-92. https://doi.org/10.1016/S0002-9149(02)02302-0.
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care 1997;20:537-44. https://doi.org/10.2337/diacare.20.4.537.
- Mañas A, Del Pozo-Cruz B, García-García FJ, Guadalupe-Grau A, Ara I. Role of objectively measured sedentary behaviour in physical performance, frailty and mortality among older adults: a short systematic review. Eur J Sport Sci 2017;17:940-53. https://doi.org/10.1080/17461391.2017.1327983.
- Bamia C, Orfanos P, Juerges H, Schöttker B, Brenner H, Lorbeer R, et al. Self-rated health and all-cause and cause-specific mortality of older adults: individual data meta-analysis of prospective cohort studies in the CHANCES Consortium. Maturitas 2017;103:37-44. https://doi.org/10.1016/j.maturitas.2017.06.023.
- Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev 2011;11. https://doi.org/10.1002/14651858.CD004963.pub3.
- Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, et al. Exercise to prevent falls in older adults: an updated systematic review and meta-analysis. Br J Sports Med 2017;51:1750-8. https://doi.org/10.1136/bjsports-2016-096547.
- Steffl M, Bohannon RW, Sontakova L, Tufano JJ, Shiells K, Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging 2017;12:835-45. https://doi.org/10.2147/CIA.S132940.
- Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia?: a systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-510.
- Elavsky S, McAuley E, Motl RW, Konopack JF, Marquez DX, Hu L, et al. Physical activity enhances long-term quality of life in older adults: efficacy, esteem, and affective influences. Ann Behav Med 2005;30:138-45. https://doi.org/10.1207/s15324796abm3002_6.
- Sari N. Exercise, physical activity and healthcare utilization: a review of literature for older adults. Maturitas 2011;70:285-9. https://doi.org/10.1016/j.maturitas.2011.08.004.
- Shankar A, McMunn A, Banks J, Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol 2011;30:377-85. https://doi.org/10.1037/a0022826.
- Robins LM, Hill KD, Finch CF, Clemson L, Haines T. The association between physical activity and social isolation in community-dwelling older adults. Aging Ment Health 2018;22:175-82. https://doi.org/10.1080/13607863.2016.1242116.
- Start Active, Stay Active: A Report on Physical Activity From the Four Home Countries’ Chief Medical Officers. London: DHSC; 2011.
- Cruise SM, Hughes J, Bennett K, Kouvonen A, Kee F. The impact of risk factors for coronary heart disease on related disability in older Irish adults. J Aging Health 2019;31:165-84. https://doi.org/10.1177/0898264317726242.
- Scarborough P, Bhatnagar P, Wickramasinghe KK, Allender S, Foster C, Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006–07 NHS costs. J Public Health 2011;33:527-35. https://doi.org/10.1093/pubmed/fdr033.
- Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-75.
- Taylor AH, Cable NT, Faulkner G, Hillsdon M, Narici M, Van Der Bij AK. Physical activity and older adults: a review of health benefits and the effectiveness of interventions. J Sports Sci 2004;22:703-25. https://doi.org/10.1080/02640410410001712421.
- van der Bij AK, Laurant MG, Wensing M. Effectiveness of physical activity interventions for older adults: a review. Am J Prev Med 2002;22:120-33. https://doi.org/10.1016/S0749-3797(01)00413-5.
- King AC. Interventions to promote physical activity by older adults. J Gerontol A Biol Sci Med Sci 2001;56:36-4. https://doi.org/10.1093/gerona/56.suppl_2.36.
- Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med 2004;39:1056-61. https://doi.org/10.1016/j.ypmed.2004.04.003.
- Costello E, Kafchinski M, Vrazel J, Sullivan P. Motivators, barriers, and beliefs regarding physical activity in an older adult population. J Geriatr Phys Ther 2011;34:138-47. https://doi.org/10.1519/JPT.0b013e31820e0e71.
- Devereux-Fitzgerald A, Powell R, Dewhurst A, French DP. The acceptability of physical activity interventions to older adults: a systematic review and meta-synthesis. Soc Sci Med 2016;158:14-23. https://doi.org/10.1016/j.socscimed.2016.04.006.
- Carlson JA, Sallis JF, Conway TL, Saelens BE, Frank LD, Kerr J, et al. Interactions between psychosocial and built environment factors in explaining older adults’ physical activity. Prev Med 2012;54:68-73. https://doi.org/10.1016/j.ypmed.2011.10.004.
- Rosenberg DE. Outcomes of a multilevel walking intervention for older adults living in retirement communities. Diss Abstr Int 2011;71.
- Webel AR, Okonsky J, Trompeta J, Holzemer WL. A systematic review of the effectiveness of peer-based interventions on health-related behaviors in adults. Am J Public Health 2010;100:247-53. https://doi.org/10.2105/AJPH.2008.149419.
- Best KL, Miller WC, Eng JJ, Routhier F. Systematic review and meta-analysis of peer-led self-management programs for increasing physical activity. Int J Behav Med 2016;23:527-38. https://doi.org/10.1007/s12529-016-9540-4.
- Dorgo S, Robinson KM, Bader J. The effectiveness of a peer-mentored older adult fitness program on perceived physical, mental, and social function. J Am Acad Nurse Pract 2009;21:116-22. https://doi.org/10.1111/j.1745-7599.2008.00393.x.
- Ginis KA, Nigg CR, Smith AL. Peer-delivered physical activity interventions: an overlooked opportunity for physical activity promotion. Transl Behav Med 2013;3:434-43. https://doi.org/10.1007/s13142-013-0215-2.
- Dennis CL. Peer support within a health care context: a concept analysis. Int J Nurs Stud 2003;40:321-32. https://doi.org/10.1016/S0020-7489(02)00092-5.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Michie S, Abraham C, Eccles MP, Francis JJ, Hardeman W, Johnston M. Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-10.
- Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev 2012;1. https://doi.org/10.1186/2046-4053-1-10.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. https://doi.org/10.1037/a0016136.
- Baker PR, Francis DP, Soares J, Weightman AL, Foster C. Community wide interventions for increasing physical activity. Cochrane Database Syst Rev 2011;4. https://doi.org/10.1002/14651858.CD008366.pub2.
- Boyle J, Mattern CO, Lassiter JW, Ritzler JA. Peer 2 peer: efficacy of a course-based peer education intervention to increase physical activity among college students. J Am Coll Health 2011;59:519-29. https://doi.org/10.1080/07448481.2010.523854.
- Buman MP, Giacobbi PR, Dzierzewski JM, Aiken Morgan A, McCrae CS, Roberts BL, et al. Peer volunteers improve long-term maintenance of physical activity with older adults: a randomized controlled trial. J Phys Act Health 2011;8:257-66. https://doi.org/10.1123/jpah.8.s2.s257.
- Castro CM, Pruitt LA, Buman MP, King AC. Physical activity program delivery by professionals versus volunteers: the TEAM randomized trial. Health Psychol 2011;30:285-94. https://doi.org/10.1037/a0021980.
- Lamb SE, Bartlett HP, Ashley A, Bird W. Can lay-led walking programmes increase physical activity in middle aged adults? A randomised controlled trial. J Epidemiol Community Health 2002;56:246-52. https://doi.org/10.1136/jech.56.4.246.
- Parent N, Fortin F. A randomized, controlled trial of vicarious experience through peer support for male first-time cardiac surgery patients: impact on anxiety, self-efficacy expectation, and self-reported activity. Heart Lung 2000;29:389-400. https://doi.org/10.1067/mhl.2000.110626.
- Pinto BM, Stein K, Dunsiger S. Peers promoting physical activity among breast cancer survivors: a randomized controlled trial. Health Psychol 2015;34:463-72. https://doi.org/10.1037/hea0000120.
- Resnick B, Luisi D, Vogel A. Testing the senior exercise self-efficacy project (SESEP) for use with urban dwelling minority older adults. Public Health Nurs 2008;25:221-34. https://doi.org/10.1111/j.1525-1446.2008.00699.x.
- Thomas GN, Macfarlane DJ, Guo B, Cheung BM, McGhee SM, Chou KL, et al. Health promotion in older Chinese: a 12-month cluster randomized controlled trial of pedometry and ‘peer support’. Med Sci Sports Exerc 2012;44:1157-66. https://doi.org/10.1249/MSS.0b013e318244314a.
- Tudor-Locke C, Lauzon N, Myers AM, Bell RC, Chan CB, McCargar L, et al. Effectiveness of the First step Program delivered by professionals versus peers. J Phys Act Health 2009;6:456-62. https://doi.org/10.1123/jpah.6.4.456.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277-88. https://doi.org/10.1177/1049732305276687.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Finfgeld-Connett D. Use of content analysis to conduct knowledge-building and theory-generating qualitative systematic reviews. Qual Res 2014;14:341-52. https://doi.org/10.1177/1468794113481790.
- Hardcastle S, Taylor AH. Looking for more than weight loss and fitness gain: psychosocial dimensions among elder women in a primary-care exercise-referral program. J Phys Act Health 2001;9:313-28. https://doi.org/10.1123/japa.9.3.313.
- McAuley E, Lox C, Duncan TE. Long-term maintenance of exercise, self-efficacy, and physiological change in older adults. J Gerontol 1993;48:218-24. https://doi.org/10.1093/geronj/48.4.P218.
- Marley J, Tully MA, Porter-Armstrong A, Bunting B, O’Hanlon J, Atkins L, et al. The effectiveness of interventions aimed at increasing physical activity in adults with persistent musculoskeletal pain: a systematic review and meta-analysis. BMC Musculoskelet Disord 2017;18. https://doi.org/10.1186/s12891-017-1836-2.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986.
- Kerr J, Rosenberg DE, Nathan A, Millstein RA, Carlson JA, Crist K, et al. Applying the ecological model of behavior change to a physical activity trial in retirement communities: description of the study protocol. Contemp Clin Trials 2012;33:1180-8. https://doi.org/10.1016/j.cct.2012.08.005.
- Foster CE, Brennan G, Matthews A, McAdam C, Fitzsimons C, Mutrie N. Recruiting participants to walking intervention studies: a systematic review. Int J Behav Nutr Phys Act 2011;8. https://doi.org/10.1186/1479-5868-8-137.
- Ahmad S, Harris T, Limb E, Kerry S, Victor C, Ekelund U, et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60–74 year old primary care patients. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0324-8.
- Adams R. Revised physical activity readiness questionnaire. Can Fam Physician 1999;45.
- McDonough SM, Tully MA, O’Connor SR, Boyd A, Kerr DP, O’Neill SM, et al. The back 2 activity trial: education and advice versus education and advice plus a structured walking programme for chronic low back pain. BMC Musculoskelet Disord 2010;11. https://doi.org/10.1186/1471-2474-11-163.
- Heron N, Tully MA, McKinley MC, Cupples ME. Steps to a better Belfast: physical activity assessment and promotion in primary care. Br J Sports Med 2014;48:1558-63. https://doi.org/10.1136/bjsports-2012-091581.
- Public Health Agency . Ageing Well by Being Active Every Day. n.d. www.publichealth.hscni.net/publications/ageing-well-being-active-every-day (accessed 20 March 2019).
- Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nyström C, Mora-Gonzalez J, Löf M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med 2017;47:1821-45. https://doi.org/10.1007/s40279-017-0716-0.
- Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. accelerometer. Med Sci Sports Exerc 1998;30:777-81. https://doi.org/10.1097/00005768-199805000-00021.
- Wareham NJ, Jakes RW, Rennie KL, Mitchell J, Hennings S, Day NE. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol 2002;31:168-74. https://doi.org/10.1093/ije/31.1.168.
- Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, et al. The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med 1997;27:191-7. https://doi.org/10.1017/S0033291796004242.
- Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med 1979;9:139-45. https://doi.org/10.1017/S0033291700021644.
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-63.
- Lloyd K, Devine P. Psychometric properties of the Warwick-Edinburgh Mental Well-being Scale (WEMWBS) in Northern Ireland. J Ment Health 2012;21:257-63. https://doi.org/10.3109/09638237.2012.670883.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Russell DW. UCLA loneliness scale (version 3): reliability, validity, and factor structure. J Pers Assess 1996;66:20-4. https://doi.org/10.1207/s15327752jpa6601_2.
- Lubben JE. Assessing social networks among elderly populations. Fam Community Health 1998;11:42-5. https://doi.org/10.1097/00003727-198811000-00008.
- Resnick B, Palmer MH, Jenkins LS, Spellbring AM. Path analysis of efficacy expectations and exercise behaviour in older adults. J Adv Nurs 2000;31:1309-15. https://doi.org/10.1046/j.1365-2648.2000.01463.x.
- Steinhardt MA, Dishman RK. Reliability and validity of expected outcomes and barriers for habitual physical activity. J Occup Med 1989;31:536-46. https://doi.org/10.1097/00043764-198906000-00011.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Hunter RF, Tully MA, Davis M, Stevenson M, Kee F. Physical activity loyalty cards for behavior change: a quasi-experimental study. Am J Prev Med 2013;45:56-63. https://doi.org/10.1016/j.amepre.2013.02.022.
- Winter S, Collins D. Why do we do, what we do?. J Appl Sport Psychol 2015;27:35-51. https://doi.org/10.1080/10413200.2014.941511.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: University of Kent, Personal Social Services Research Unit; 2017.
- Sumnall H, Agus A, Cole J, Doherty P, Foxcroft D, Harvey S, et al. Steps Towards Alcohol Misuse Prevention Programme (STAMPP): a school- and community-based cluster randomised controlled trial. Public Health Res 2017;5.
- Lohan M, Aventin A, Maguire L, Curran R, McDowell C, Agus A, et al. Increasing boys’ and girls’ intentions to avoid teenage pregnancy: a cluster randomised controlled feasibility trial of an interactive video drama-based intervention in post-primary schools in Northern Ireland. Public Health Res 2017;5.
- Hollingworth W, Cohen D, Hawkins J, Hughes RA, Moore LA, Holliday JC, et al. Reducing smoking in adolescents: cost-effectiveness results from the cluster randomized ASSIST (A Stop Smoking In Schools Trial). Nicotine Tob Res 2012;14:161-8. https://doi.org/10.1093/ntr/ntr155.
- Lee EC, Whitehead AL, Jacques RM, Julious SA. The statistical interpretation of pilot trials: should significance thresholds be reconsidered?. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-41.
- Kang M, Marshall SJ, Barreira TV, Lee JO. Effect of pedometer-based physical activity interventions: a meta-analysis. Res Q Exerc Sport 2009;80:648-55. https://doi.org/10.1080/02701367.2009.10599604.
- The NHS Staff Council . NHS Terms and Conditions of Service Handbook n.d. www.nhsemployers.org/employershandbook/afc_tc_of_service_handbook_fb.pdf (accessed 24 April 2018).
- nidirect . AccessNI: Criminal Record Checks n.d. www.nidirect.gov.uk/articles/costs-and-turnaround-times (accessed 25 April 2018).
- Clegg A, Relton C, Young J, Witham M. Improving recruitment of older people to clinical trials: use of the cohort multiple randomised controlled trial design. Age Ageing 2015;44:547-50. https://doi.org/10.1093/ageing/afv044.
- Rogers A, Harris T, Victor C, Woodcock A, Limb E, Kerry S, et al. Which older people decline participation in a primary care trial of physical activity and why: insights from a mixed methods approach. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-46.
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-34.
- Sahin D, Yaffe MJ, Sussman T, McCusker J. A mixed studies literature review of family physicians’ participation in research. Fam Med 2014;46:503-14.
- Salmon P, Peters S, Rogers A, Gask L, Clifford R, Iredale W, et al. Peering through the barriers in GPs’ explanations for declining to participate in research: the role of professional autonomy and the economy of time. Fam Pract 2007;24:269-75. https://doi.org/10.1093/fampra/cmm015.
- Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chronic Dis 2012;9.
- Harris T, Kerry SM, Victor CR, Ekelund U, Woodcock A, Iliffe S, et al. A primary care nurse-delivered walking intervention in older adults: PACE (pedometer accelerometer consultation evaluation)-Lift cluster randomised controlled trial. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001783.
- Cleland CL, Tully MA, Kee F, Cupples ME. The effectiveness of physical activity interventions in socio-economically disadvantaged communities: a systematic review. Prev Med 2012;54:371-80. https://doi.org/10.1016/j.ypmed.2012.04.004.
- Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0605-9.
- Knittle K, Nurmi J, Crutzen R, Hankonen N, Beattie M, Dombrowski SU. How can interventions increase motivation for physical activity? A systematic review and meta-analysis. Health Psychol Rev 2018;12:211-30. https://doi.org/10.1080/17437199.2018.1435299.
Appendix 1 The MEDLINE search strategy for rapid review of peer-led physical activity interventions
| Search number | Search term |
|---|---|
| 1 | exp Exercise/ |
| 2 | exp Running/ |
| 3 | Walking/ |
| 4 | Physical Fitness/ |
| 5 | cardiovascular fitness.ti,ab. |
| 6 | Gardening/ |
| 7 | exp ‘Physical Education and Training’/ |
| 8 | Dancing/ |
| 9 | exp Sports/ |
| 10 | Fitness Centers/ |
| 11 | exp Recreation/ |
| 12 | exp ‘Play and Playthings’/ |
| 13 | Motor Activity/ |
| 14 | (fitness adj (class* or regime* or program*)).ti,ab. |
| 15 | cardiorespiratory fitness.ti,ab. |
| 16 | aerobic capacity.ti,ab. |
| 17 | ((led or health) adj walk*).ti,ab. |
| 18 | (physical adj5 (fit* or train* or activ* or endur* or exer*)).ti,ab. |
| 19 | ((moderate or vigorous*) adj activ*).ti,ab. |
| 20 | (exercise* adj5 (fit* or train* or activ* or endur* or aerobic)).ti,ab. |
| 21 | ((leisure or fitness) adj5 (centre* or center* or facilit*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] |
| 22 | ((promot* or uptak* or encourag* or increas* or start* or adher* or sustain* or maintain*) adj5 gym*).ti,ab. |
| 23 | ((promot* or uptak* or encourag* or increas* or start* or adher* or sustain* or maintain*) adj5 physical activ*).ti,ab. |
| 24 | ((promot* or uptak* or encourag* or increas* or start* or adher* or sustain* or maintain*) adj5 (exer* or keep fit or fitness class or yoga or aerobic*)).ti,ab. |
| 25 | ((decreas* or reduc* or discourage*) adj5 (sedentary or deskbound or ‘physical inactiv*’)).ti,ab. |
| 26 | sport*3.mp. or walk*3.ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] |
| 27 | (run* or jog*).mp. or yoga.ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] |
| 28 | (work or workplace or commut* or travel* or equipment or facility or park* or friendly or infrastructure).ti,ab. |
| 29 | bicycle*.ti,ab. |
| 30 | bike*1.mp. or biking.ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] |
| 31 | swim*1.mp. or swimming*.ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] |
| 32 | (exercis*3 adj5 aerobic*).ti,ab. |
| 33 | exertion*1.ti,ab. |
| 34 | strength training.ti,ab. |
| 35 | resilience training.ti,ab. |
| 36 | travel mode*1.ti,ab. |
| 37 | (active adj (travel*4 or transport* or commut*)).ti,ab. |
| 38 | (multimodal transport* or alternative transport* or alternative travel*).ti,ab. |
| 39 | recreation*1.ti,ab. |
| 40 | (‘use’ adj3 stair*).ti,ab. |
| 41 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 |
| 42 | peer group/ |
| 43 | peer based intervention*.ti,ab. |
| 44 | peer led intervention*.ti,ab. |
| 45 | peer education.ti,ab. |
| 46 | peer*.ti,ab. |
| 47 | peer support*.ti,ab. |
| 48 | peer counsel?ing*.ti,ab. |
| 49 | (group adj support*).ti,ab. |
| 50 | (group adj education*).ti,ab. |
| 51 | ((peer or opinion) adj leader*).ti,ab. |
| 52 | befriend*.ti,ab. |
| 53 | (home adj visit*).ti,ab. |
| 54 | (visit adj program*).ti,ab. |
| 55 | mentor*3.ti,ab. |
| 56 | Mentors/ |
| 57 | (associate* or rival* or companion* or compeer* or like* or match* or coequal*).mp. or co-equal*.ti,ab. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] |
| 58 | 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 |
| 59 | 41 and 58 |
| 60 | limit 59 to humans |
Appendix 2 Template for extracting behaviour change techniques
| BCT label | Excerpt | Page number and paragraph |
|---|---|---|
| 1. Goals and planning | ||
| 1.1. Goal-setting (behaviour) | ||
| 1.2. Problem-solving | ||
| 1.3. Goal-setting (outcome) | ||
| 1.4. Action-planning | ||
| 1.5. Review behaviour goal(s) | ||
| 1.6. Discrepancy between current behaviour and goal | ||
| 1.7. Review outcome goal(s) | ||
| 1.8. Behavioural contract | ||
| 1.9. Commitment | ||
| 2. Feedback and monitoring | ||
| 2.1. Monitoring of behaviour by others without feedback | ||
| 2.2. Feedback on behaviour | ||
| 2.3. Self-monitoring of behaviour | ||
| 2.4. Self-monitoring of outcome(s) of behaviour | ||
| 2.5. Monitoring of outcome(s) of behaviour without feedback | ||
| 2.6. Biofeedback | ||
| 2.7. Feedback on outcome(s) of behaviour | ||
| 3. Social support | ||
| 3.1. Social support (unspecified) | ||
| 3.2. Social support (practical) | ||
| 3.3. Social support (emotional) | ||
| 4. Shaping knowledge | ||
| 4.1. Instruction on how to perform the behaviour | ||
| 4.2. Information about antecedents | ||
| 4.3. Re-attribution | ||
| 4.4. Behavioural experiments | ||
| 5. Natural consequences | ||
| 5.1. Information about health consequences | ||
| 5.2. Salience of consequences | ||
| 5.3. Information about social and environmental consequences | ||
| 5.4. Monitoring of emotional consequences | ||
| 5.5. Anticipated regret | ||
| 5.6. Information about emotional consequences | ||
| 6. Comparison of behaviour | ||
| 6.1. Demonstration of the behaviour | ||
| 6.2. Social comparison | ||
| 6.3. Information about others’ approval | ||
| 7. Associations | ||
| 7.1. Prompts/cues | ||
| 7.2. Cue signalling reward | ||
| 7.3. Reduce prompts/cues | ||
| 7.4. Remove access to the reward | ||
| 7.5. Remove aversive stimulus | ||
| 7.6. Satiation | ||
| 7.7. Exposure | ||
| 7.8. Associative learning | ||
| 8. Repetition and substitution | ||
| 8.1. Behavioural practice/rehearsal | ||
| 8.2. Behaviour substitution | ||
| 8.3. Habit formation | ||
| 8.4. Habit reversal | ||
| 8.5. Overcorrection | ||
| 8.6. Generalisation of target behaviour | ||
| 8.7. Graded tasks | ||
| 9. Comparison of outcomes | ||
| 9.1. Credible source | ||
| 9.2. Pros and cons | ||
| 9.3. Comparative imagining of future outcomes | ||
| 10. Reward and threat | ||
| 10.1. Material incentive (behaviour) | ||
| 10.2. Material reward (behaviour) | ||
| 10.3. Non-specific reward | ||
| 10.4. Social reward | ||
| 10.5. Social incentive | ||
| 10.6. Non-specific incentive | ||
| 10.7. Self-incentive | ||
| 10.8. Incentive (outcome) | ||
| 10.9. Self-reward | ||
| 10.10. Reward (outcome) | ||
| 10.11. Future punishment | ||
| 11. Regulation | ||
| 11.1. Pharmacological support | ||
| 11.2. Reduce negative emotions | ||
| 11.3. Conserving mental resources | ||
| 11.4. Paradoxical instructions | ||
| 12. Antecedents | ||
| 12.1. Restructuring the physical environment | ||
| 12.2. Restructuring the social environment | ||
| 12.3. Avoidance/reducing exposure to cues for the behaviour | ||
| 12.4. Distraction | ||
| 12.5. Adding objects to the environment | ||
| 12.6. Body changes | ||
| 13. Identity | ||
| 13.1. Identification of self as role model | ||
| 13.2. Framing/reframing | ||
| 13.3. Incompatible beliefs | ||
| 13.4. Valued self-identify | ||
| 13.5. Identity associated with changed behaviour | ||
| 14. Scheduled consequences | ||
| 14.1. Behaviour cost | ||
| 14.2. Punishment | ||
| 14.3. Remove reward | ||
| 14.4. Reward approximation | ||
| 14.5. Rewarding completion | ||
| 14.6. Situation-specific reward | ||
| 14.7. Reward incompatible behaviour | ||
| 14.8. Reward alternative behaviour | ||
| 14.9. Reduce reward frequency | ||
| 14.10. Remove punishment | ||
| 15. Self-belief | ||
| 15.1. Verbal persuasion about capability | ||
| 15.2. Mental rehearsal of successful performance | ||
| 15.3. Focus on past success | ||
| 15.4. Self-talk | ||
| 16. Covert learning | ||
| 16.1. Imaginary punishment | ||
| 16.2. Imaginary reward | ||
| 16.3. Vicarious consequences | ||
Appendix 3 Example of the fidelity checklist completed by the peer mentor following the completion of weekly meeting with participant
Appendix 4 Health service use log
List of abbreviations
- BCT
- behaviour change technique
- CI
- confidence interval
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GHQ-28
- General Health Questionnaire-28 items
- GP
- general practitioner
- LSN
- Lubben Social Network
- MRC
- Medical Research Council
- MVPA
- moderate to vigorous physical activity
- NIMDM
- Northern Ireland Multiple Deprivation Measure
- RCT
- randomised controlled trial
- SCT
- social cognitive theory
- SD
- standard deviation
- SEHSCT
- South Eastern Health and Social Care Trust
- SF-12
- Short Form questionnaire-12 items
- UKCRC
- UK Clinical Research Collaboration
- WEMWBS
- Warwick–Edinburgh Mental Well-being Scale
