Notes
Article history
The research reported in this issue of the journal was funded by the PHR programme as project number 15/49/32. The contractual start date was in January 2017. The final report began editorial review in March 2021 and was accepted for publication in September 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PHR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Wright et al. This work was produced by Wright et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Wright et al.
Chapter 1 Introduction
Background and rationale
Autism spectrum disorder (ASD) is a neurodevelopmental condition beginning in childhood characterised by atypical social communication, difficulties in creating and maintaining multiple social relationships, sensory reactivities or preferences, restrictive patterns of repetitive and stereotyped behaviour, and/or specific interests/preoccupations. 1 It is estimated to affect 1.1% of the UK population and up to 1.6% of children and young people (CYP) in the UK. 2–4 There are significant variations in the social, communicative and intellectual abilities of those with ASD, including increased rates of mental health problems such as anxiety, sleep problems, attention problems and behaviour problems. 5 Commonly experienced symptoms and problems can affect the individual into adulthood in areas such as social relationships, educational success and independent living. 6,7
Autistic CYP often experience difficulty with social and emotional skills and sometimes may not intuitively pick up on social rules or norms encountered in their daily lives. 8 Many autistic CYP attend mainstream school education environments, although this may expose them to difficulties in creating and maintaining relationships with their typically developing peers and, in turn, lead to increased feelings of isolation. 9,10 CYP learn and develop sociocognitive skills and gain language abilities through friendships and peer relationships, through which they also gain a sense of belonging to a peer group. 11 Autistic CYP often have smaller groups of friends than neurotypical peers and may face problems initiating/maintaining friendships,12,13 thereby potentially missing out on opportunities to develop their social and emotional competence, which can widen developmental differences between them and their typically developing peers.
Although it has been shown that many autistic CYP who struggle with social interactions find such situations anxiety-provoking,14 it has also been shown that they identify feelings of loneliness more frequently than their peers,13 suggesting an awareness of being excluded. Theories in this area suggest that autistic CYP may not be motivated by social interactions,15,16 but feelings of increased isolation perhaps indicate that they are simply differently motivated by such interactions. In addition, autistic CYP are at greater risk of being bullied by typically developing peers. 17 Evidence suggests that perceived exclusion from friendship groups can have negative effects on the quality of life, physical and mental health, emotional well-being and educational success of autistic and non-autistic CYP, creating concerns about appropriate education and health provisions. 18,19 Indeed, the National Institute for Health and Care Excellence (NICE) states in public health guidelines20 that the social and emotional well-being of CYP are significant factors in long-term outcomes, including educational attainment and physical and mental health.
Interventions to promote the development of social and emotional skills are a commonly used school-based approach both generally21 and for autistic CYP. 22–27 A Cochrane review28 showed some improvement in social competence following these interventions, although the published research was very limited, with only five studies meeting the inclusion criteria. 29–33 Different types of social skills interventions were assessed in these studies, including the following: a 20-week social adjustment enhancement curriculum for boys aged 8–12 years with high-functioning autism (HFA), Asperger syndrome and pervasive development disorders not otherwise specified;33 a manualised parent-assisted social skills intervention compared with a matched, delayed treatment control to improve friendship quality and social skills among autistic teenagers;31 12 weeks of a manualised intervention titled Children’s Friendship Training compared with a delayed treatment control group;29 a 16-week manualised social skills intervention designed to teach appropriate social behaviour compared with a waiting-list control group;30 and a manualised social intervention for CYP with HFA, including instruction and therapeutic activities, which focused on social skills, face–emotion recognition, interest expansion and interpretation of non-literal language. 32 A more recent review of school-based interventions that target social communication in autistic CYP found 22 studies, but only three of these reported significance or effect sizes,26 demonstrating a need for higher-quality future research methodologies. In this review26 many were adult-directed interventions.
Although social skills training interventions are commonly used and report moderate success with relatively low rates of attrition, studies to date have tended to use a skills deficit model, which potentially limits them. Common features include (1) being adult led and (2) focusing on skill building using various teaching methods. Participating CYP can also struggle to use learned skills outside the context of the interventions, a further limitation. 6,34
LEGO® (LEGO System A/S, Billund, Denmark) based therapy35 is a group-based social skills intervention specifically designed for autistic CYP that does not rely on adult-led teaching of skills. It has become popular in the UK, with many local authorities now recommending its use in schools. 36 The intervention focuses on collaborative building of LEGO sets in small groups of CYP, who are intrinsically motivated to play with this predictable and structured toy that has a wide range of alternative play sets appropriate for CYP of different chronological and developmental ages, genders, hobbies and interests. 37 The CYP’s own interests are used to motivate the learning and practising of social and emotional skills in a group context. Such naturalistic approaches used elsewhere have been shown to increase the likelihood that new skills will be used outside the context of the intervention, thus increasing overall effectiveness. 38
Although the use of LEGO® based therapy in schools across the UK is increasing, there is a lack of research evaluating the effectiveness of the intervention. A limited number of studies have been undertaken to assess LEGO® based therapy with autistic CYP, all of which had significant limitations in terms of research design, methodology and sample size. One of these was a small randomised controlled trial (RCT). 37
In 2004, Daniel LeGoff, the creator of LEGO® based therapy, used a repeated-measure waiting-list controlled trial to assess the impact of the intervention on the social competence of 47 autistic CYP aged 6 to 16 years. 39 He found that, compared with control participants, those receiving the intervention had greater motivation to initiate and greater ability to sustain social contact and decreased social impairment scores, as measured by the Gilliam Autism Rating Scale social interaction (GARS–SI) subscale. LeGoff and Sherman then undertook a retrospective cohort study to explore long-term outcomes associated with LEGO® based therapy. 40 Findings showed improvement on both the Vineland Adaptive Behaviour Scale socialisation domain (VABS–SD) and the GARS–SI in participants who had received LEGO® based therapy compared with control participants who had received similar, non-LEGO, therapies on a comparable timescale.
Owens et al. 37 undertook a small RCT of 31 CYP with Asperger syndrome and HFA aged 6–11 years, randomising between LEGO® based therapy and a Social Use of Language Programme (SULP). 41 A no-intervention control group was also followed up. The results showed some reduction in social difficulties in the LEGO® based therapy group compared with the SULP and control groups. The study had a number of limitations, including a lack of full randomisation: instead, one participant from each dyad matched on age, intelligence quotient (IQ), symptom severity and verbal IQ was allocated to either LEGO® based therapy or SULP. Intention-to-treat (ITT) analysis was not used and the sample size was small, with an attrition rate of 30% in each of the two groups. No measures were taken to assess the degree of delivery fidelity to the interventions.
The most recent UK study of LEGO® based therapy, and the first entirely school-based trial, used a single-case, non-concurrent, multiple-baseline-across-participants design to assess the impact of LEGO® based therapy on the social skills and the generalisability of those skills in autistic CYP. 42 This study had a very small number of participants (n = 6), who received a baseline phase consisting of between 2 and 13 sessions of free-play LEGO, each 15 minutes in length. Participants then received the intervention, which was delivered by trained school staff members 12 times (twice per week) for 45 minutes each. A maintenance phase followed the intervention in which observational data were collected from three 15-minute free-play LEGO sessions. The researchers reported significant improvements in social skills for five of the six participants and evidence that the improvements were maintained at follow-up.
Although the design of this study did allow for close examination of changes in behaviour of the participants, the sample size of six participants was very small, with only five receiving all 12 intervention sessions (one participant withdrew). The small sample size did not allow for varied participant demographics and characteristics, which may have affected the generalisability of, and conclusions to be drawn from, the study findings. There was no separate control arm for comparison or random allocation to treatment types. The authors also discuss their use of percentage of non-overlapping data analysis, which did not prove useful for effect size evaluation.
A scoping review of 15 studies investigating LEGO® based therapy was conducted in 2017. 43 Although the authors reported that LEGO® based therapy has the potential to improve various social skills, the consistently small sample sizes, together with the use of different study designs and outcome measures, makes drawing definite conclusions difficult. The review recommendations were for more rigorously designed trials of LEGO® based therapy using larger sample sizes. There is a need for a fully powered RCT to investigate the clinical effectiveness of LEGO® based therapy using a large sample size and standardised measures. Furthermore, as far as we are aware, no studies have examined the cost-effectiveness of LEGO® based therapy delivered in schools compared with other interventions or support services. This is a critical time to examine the effectiveness of LEGO® based therapy before it becomes a widely incorporated aspect of the school curriculum.
Research question, aims and objectives
The I-SOCIALISE (Investigating SOcial Competence and Isolation in children with Autism taking part in LEGO® based therapy clubs In School Environments) research team aimed to investigate the clinical effectiveness and cost-effectiveness of LEGO® based therapy on the social and emotional competence and perceived isolation of autistic CYP in mainstream school environments. The research design included cluster random allocation at the school level of CYP diagnosed with ASD to either LEGO® based therapy groups run in school environments in addition to usual support (i.e. the intervention arm) or usual support alone (i.e. the control arm).
Primary objective
-
To examine the clinical effectiveness of LEGO® based therapy groups on the social and emotional competence (specifically perceived social skills) of autistic CYP in schools, compared with usual support for autistic CYP, at 20 weeks after randomisation.
Secondary objectives
-
To examine the clinical effectiveness of LEGO® based therapy groups on the perceived social isolation of autistic CYP in schools, compared with usual support for autistic CYP, at 20 and 52 weeks.
-
To examine the clinical effectiveness of LEGO® based therapy groups on the academic competence of autistic CYP in schools, compared with usual support for autistic CYP, at 20 and 52 weeks.
-
To examine the clinical effectiveness of LEGO® based therapy groups on assertion, social control, externalising and internalising in autistic CYP in the school setting, compared with usual support for autistic CYP, at 20 and 52 weeks.
-
To examine the cost-effectiveness of LEGO® based therapy groups, compared with usual support for autistic CYP, at 52 weeks.
-
To examine the emotional and behavioural symptoms in those receiving LEGO® based therapy, compared with usual support for autistic CYP, at 20 and 52 weeks.
-
To determine if the impact of LEGO® based therapy is sustainable into the next academic year by comparing effectiveness on social and emotional competence (specifically perceived social skills) at 52 weeks after randomisation.
-
To examine the acceptability of the intervention at follow-up points using a purpose-designed questionnaire and telephone interviews at 20 weeks.
-
To examine treatment fidelity through independent observation of treatment sessions across schools and a self-report measure completed by the facilitator [i.e. a trained teacher or teaching assistant (TA)] after each session.
Chapter 2 Methods
This report is concordant with the Consolidated Standards of Reporting Trials (CONSORT) statement44 and with the CONSORT extension for cluster RCTs. 45
Main trial methods
Trial design
The I-SOCIALISE trial was a pragmatic, two-arm, cluster RCT at the school level with an internal pilot designed to examine the clinical effectiveness and cost-effectiveness of LEGO® based therapy groups for autistic CYP. The internal pilot study assessing recruitment feasibility was carried out over the first 10 months, for which a recruitment target of 120 CYP was set, one-third of whom (n = 40) were expected to have reached the primary end point of follow-up at 20 weeks after randomisation. Stop/go criteria were based on 75% of the recruitment target (n = 90) and 70% of the primary outcome target (n = 28) to assess the feasibility of continuing the trial. The trial also included an assessment of intervention fidelity and acceptability, a qualitative element and a nested economic evaluation.
Important changes to methods after trial commencement
August 2017
-
At this time the study protocol contained five objectives – the first five in the final list of objectives (see Chapter 1, Research question, aims and objectives). A sixth secondary objective was added to the existing five to enable the research team to examine the emotional and behavioural symptoms in CYP receiving the intervention compared with those in CYP in the control arm, using the Strengths and Difficulties Questionnaire (SDQ). The objectives were then re-ordered; this objective is now number five in the final list of objectives (see Chapter 1, Research question, aims and objectives). Two further secondary objectives were added later (see September 2018).
March 2018
-
The first follow-up time point was changed from 16 weeks post randomisation to 20 weeks post randomisation. This was implemented following concerns in the study team that the 12 sessions of LEGO® based therapy would not have been completed by all schools when approached for their 16-week follow-up and that resulting data would not be balanced, with schools at different stages of the intervention. This change was approved and implemented following discussion with the trial Data Monitoring and Ethics Committee (DMEC) and Trial Steering Committee (TSC). Prior to this change, one participant’s follow-up was collected at the 16-week time point. This participant’s school was allocated to the control arm of the study.
September 2018
-
The primary outcome measure used was clarified as being the social skills subscale of the Social Skills Improvement System (SSIS) (teacher), not the SSIS (teacher) total score.
-
The third secondary objective was amended to make it clear that the social skills subscale of the SSIS would be used at both follow-up time points to assess sustainability of the outcome.
-
Two additional secondary objectives were added, bringing the total to eight. These were (1) to examine the clinical effectiveness of the intervention on the academic competence of autistic CYP in the school setting, compared with usual support (measured using the academic competence scale of the SSIS), and (2) to examine the clinical effectiveness of the intervention on assertion, social control, externalising and internalising of autistic CYP in the school setting, compared with usual support (measured using the assertion, self-control and internalising subscales from the SSIS).
-
The per-protocol analysis was specified to include only those CYP who completed at least six sessions of the intervention.
-
The decision was made to offer training in LEGO® based therapy to schools in the trial allocated to the control arm directly following completion of the last participant follow-up (i.e. 52 weeks post randomisation) rather than at the end of the trial. This followed discussions in the study team and with the TSC around the ability of schools to access the training whether or not it was offered by the study team. It was hoped that this might also support continuing engagement, retention and compliance rates in control arm. This information was not known by the blinded research assistants (RAs) undertaking the research assessments or the families of autistic CYP; thus, there was no adverse impact on the trial and the blinding of RAs.
June 2019
January 2020
-
The study team implemented a prize draw for parents/guardians to increase participant retention and follow-up completion rates. This was approved by the University of York Research Ethics Committee (REC).
May 2020
-
An addendum to the SAP47 specified the addition of a sensitivity analysis to the impact of the COVID-19 pandemic, different teachers completing the primary outcome at baseline and 20 weeks and outcomes being provided outside the ideal survey window (i.e. 20–26 weeks and 52–60 weeks for the 20- and 52-week follow-up, respectively). The amendment was approved by the study’s DMEC and TSC and by the funder. The addendum to the SAP is available online. 47
July 2020
-
The health economics analysis method was amended based on detailed discussions with the Trial Management Group (TMG) about the most appropriate perspective to take in the main analysis. The amendment, approved by the study’s DMEC, TSC and REC and by the funder, altered the perspective from NHS and education to NHS and Personal Social Services (PSS), with the education perspective included in a sensitivity analysis. The rationale of the change was that the NHS/PSS and education perspective would be problematic in terms of making decisions, because there is no accepted threshold value by which to judge cost-effectiveness, and because there is also an issue with regard to who finances such programmes. On the other hand, the NHS and PSS perspective is the standard perspective recommended by NICE. Using such a perspective allows the cost-effectiveness of interventions to be assessed by comparing incremental cost-effectiveness ratio (ICER) results with the national willingness-to-pay (WTP) threshold.
October 2020
-
Additional exploratory analysis was requested by the TMG to explore the potential for observational analysis of the trial data in a subsequent substudy.
No other changes were made to the methods during the trial.
Participants and eligibility criteria
Participants were CYP meeting the inclusion criteria, their parents/guardians, associated teachers/TAs in the CYP’s schools who knew them well and facilitator teachers/TAs who could run the study intervention if the school was allocated to the intervention arm. CYP were asked to provide informed assent and parents/guardians provided informed consent for themselves and their CYP to participate. Optional consent items were included on the consent form regarding video-recording of some of the intervention sessions and future contact about this or other research. Additional CYP were also recruited to the study when schools had fewer than three eligible CYP (i.e. the number needed for a LEGO® based therapy group). These additional CYP did not need to meet the inclusion/exclusion criteria and a minimal amount of information was collected about these additional CYP, including chronological age, gender, any documented diagnoses and details of any special educational needs (SEN) support. A parent/guardian was asked to provide this information and to complete a consent form for their child or young person’s participation, which also included two optional consent items regarding video-recording of some of the intervention sessions and future contact about this or other research.
Inclusion criteria
A child or young person was included if they:
-
were aged 7–15 years at the time of randomisation of the school
-
attended a mainstream school in or between Year 2 and Year 10
-
had a sufficient understanding of English to be able to provide informed assent and read the LEGO® based therapy instructions and their parent/guardian had a sufficient understanding of English to be able to provide informed consent
-
had an ASD clinical diagnosis from a qualified assessing clinician or team [based on best-practice guidance leading to an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10)48 or Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V),1 diagnosis as reported by the child or young person’s parent/guardian and in the child or young person’s school records, which may include the school’s SEN register, an individual education plan (IEP), an individual healthcare plan, a my support plan (MSP), an education healthcare plan (EHCP) and an individual learning plan (ILP) or equivalent]
-
had the ability to follow and understand simple instructions (as determined by the associated teacher/TA or parent/guardian)
-
scored ≥ 15 points on the Social Communication Questionnaire (SCQ).
A school was included if it:
-
was a mainstream school (i.e. not a specialist or independent school) located in Leeds, York, Sheffield or surrounding areas in the north of England
-
had not used LEGO® based therapy with CYP in the current or preceding school term (where LEGO® based therapy was defined, for research purposes, as meeting all of the main fidelity checklist criteria)
-
had at least one child or young person diagnosed with ASD (in line with the CYP inclusion criteria).
Exclusion criterion
A child or young person was not eligible to take part in the study if they:
-
had physical impairments that would prevent them participating in the activities (as assessed by the associated teacher/TA).
Settings and locations where the data were collected
Data were collected from participants in mainstream primary and secondary schools in York, Leeds, Sheffield and surrounding areas in the north of England. Data acquired from CYP and their parent/guardian were collected either in their home or at the child or young person’s school, depending on the family’s preference. Data from associated teachers/TAs and facilitator teachers/TAs were collected in school, via post or through a secure link sent via e-mail. Follow-up data were collected from CYP, parents/guardians and teachers through face-to-face visits at home (families only) or school, via post or through a secure link sent via e-mail.
Intervention
LEGO® based therapy
LEGO® based therapy is a social skills intervention designed to support CYP with social communication difficulties such as ASD by offering scaffolded, playful opportunities to practise social skills and develop social competence. It was first developed by Daniel LeGoff, a paediatric neuropsychologist who observed that autistic CYP may be particularly drawn to LEGO bricks, perhaps because of their interests and strengths in systemising. 37,39,40 LEGO® based therapy is a group-based intervention in which CYP build LEGO models together under the guidance and facilitation of a trained adult. By building with others, CYP can practise social skills that may enable them to develop more successful social interactions and gain a sense of connection and group belonging.
Collaborative building
Key to the intervention is building LEGO sets co-operatively rather than individually.
When building a set with instructions, each child or young person takes turns to play the role of an ‘engineer’, who communicates the instructions, a ‘supplier’, who locates the appropriate brick and passes it to the builder, and a ‘builder’, who fits the model together. They follow the instructions together to finish the model as a team and take turns by CYP agreement to rotate the roles. The CYP then progress to collaborative ‘freestyle’ building in which they build models of their own design together. Sessions aim to be CYP led, following their interests as far as possible. The targets of the intervention are multiple and flexible but include core skills required for successful social interaction and peer relationships, including social communication, turn-taking, joint attention, problem-solving, emotion regulation and compromise.
Learning through play
The aim of LEGO® based therapy is to provide a setting with playful and fun activities in which CYP can learn through play that promotes skills development. Learning through play happens when activities are experienced as joyful, actively engaging, meaningful, iterative (i.e. involving experimentation and testing out ideas) and socially interactive. 49 In LEGO® based therapy, CYP develop skills in a naturalistic play setting, meaning that they are learning how to collaborate with others through doing rather than by learning in the abstract. As their social learning is connected to a here-and-now activity, with the meaningful goal of getting a LEGO model finished, the skills they are practising have immediate impact and relevance in other, everyday, contexts.
Adult facilitation
Playful learning is most effective when play is guided by an adult in a CYP-led, exploratory style rather than being directed and prescriptive. 50 Adult facilitation in LEGO® based therapy aims to guide CYP rather than directing them explicitly, in terms of both the LEGO building and the social interactions and challenges that arise from working together. Adults are encouraged whenever possible to follow the CYP’s lead and to allow CYP to solve their own challenges, stepping in to prompt through open questions only when needed (as outlined in the training and in the manual). When challenges arise, adults may work their way towards direct instructions if CYP are unable to solve issues themselves or through guidance and prompting. CYP are encouraged to practise and role-play new social strategies that they have discovered in the sessions. The adult’s role is also to praise and highlight the positive things happening in a session, socially and otherwise, using rewards as motivation.
Group rules and rewards
In LEGO® based therapy the CYP, supported by adult facilitation, develop their own group rules for the behaviour expected during the sessions. CYP can earn points for prosocial behaviour, skill in LEGO building and attempting to overcome challenges. They can also receive certificates to acknowledge progression of skills. The overarching aim is to motivate CYP to participate, collaborate and practise social interaction skills.
Fostering connection and social identity
Autistic CYP can find it hard to make friends and may feel lonely or socially isolated. LEGO® based therapy provides CYP with an opportunity to interact with peers through collaborative play that has clear social expectations to promote positive interaction experiences and develop a sense of social identity. By offering CYP the opportunity to be with others in a way that feels safe, secure and enjoyable, LEGO® based therapy provides an opportunity for CYP to experience social success. autistic CYP often have limited play experiences with others, leading to a vicious cycle of social isolation and peer rejection. 51 Therefore, LEGO® based therapy may play a preventative role, offering CYP the chance to be with others and have fun. Because LEGO is a popular toy, participating in LEGO® based therapy may open up new opportunities to interact with peers, who might show a natural interest in what the autistic CYP have been doing in their LEGO sessions. By showing others what they have made, autistic CYP can develop confidence, have the chance to talk to others and have further positive social interactions outside the LEGO® based therapy sessions, thus providing opportunities for the use of social skills in other settings.
Engagement and motivation
Many CYP find LEGO engaging,52 thus allowing an initial level of engagement with the materials that might not necessarily be experienced in other types of social skills interventions. Autistic CYP might typically be reluctant to participate in group activities or may struggle to initiate and maintain interactions with peers. With LEGO as the focus of the intervention, motivation to participate in the groups might be increased. LEGO is also familiar to most CYP, meaning that they may have a better understanding of what to expect in sessions and, therefore, potentially feel less anxious about attending.
Materials and procedures
LEGO® based therapy is a relatively new social skills intervention in the UK. School staff who had not previously attended a training course were therefore expected to have limited knowledge of this intervention. A LEGO® based therapy intervention manual and treatment protocol were designed by the study team, based on the work of Daniel LeGoff and co-applicant Gina Gomez de la Cuesta. Training sessions in LEGO® based therapy were conducted by Gina Gomez de la Cuesta and expert colleagues for local authority members with expertise in education settings and ASD in each recruiting area and for study team members. These sessions provided training in both LEGO® based therapy principles and how to train other professionals in its implementation. Local authority members and study team members were then able to run training sessions for school staff members put forward as facilitator teachers/TAs to run the intervention sessions in schools randomly allocated to the intervention arm.
Training materials used for these training sessions included:
-
a LEGO® based therapy manual developed by the study team, based on work by LeGoff et al. 35 and co-authored by Gomez de la Cuesta, a co-applicant on the study (see Report Supplementary Material 1)
-
a training PowerPoint® (Microsoft Corporation, Redmont, WA, USA) presentation created by co-applicant Gina Gomez de la Cuesta for use in training sessions for professionals who would go on to train school staff members
-
a training PowerPoint presentation created by co-applicant Gina Gomez de la Cuesta for use in training sessions with school staff members who would run the intervention sessions.
Each school staff member who had been put forward to run the intervention attended one training session that lasted for approximately 3 hours. Between 1 and 15 staff members attended each session, depending on how many schools had been randomised to the intervention arm in time to attend a particular training date. Facilitator teachers/TAs were also trained in how to complete the two forms to be completed after delivery of each intervention session: (1) a bespoke session attendance and resource use form, used to capture resource implications of the intervention and details of any adverse events (AEs) that occurred, and (2) a fidelity checklist, used to monitor the fidelity of intervention delivery to the intended delivery method.
Each LEGO® based therapy group was run in a participating school randomised to the intervention arm of the trial, with three CYP and one adult facilitator in each group. Schools were given all the materials needed to run their sessions, including LEGO sets with instructions and freestyle bricks. The CYP took turns playing one of the three roles. Groups were given LEGO sets containing picture-based instructions to build collaboratively, along with a tray on which to sort the LEGO bricks, and some free-play LEGO bricks. Facilitators were also given ‘brick club’ materials, including an example of some potential group rules, role cards (i.e. for the supplier, builder and engineer), free-play building ideas and a points system chart, all of which they were encouraged to use in the group. These materials were provided to facilitators in the abridged LEGO® based therapy manual (see Report Supplementary Material 1).
Schools with fewer than three CYP who were eligible for the study were asked to invite ‘additional CYP’ who may benefit from the groups. Informed assent/consent was gained from these CYP and their parents/guardians but there were no inclusion/exclusion criteria in place. Groups were able to go ahead with fewer than three CYP if any were absent owing to illness or other circumstances, though most groups included three CYP at all times.
Intervention provider
The facilitators delivering the LEGO® based therapy groups were school staff members, typically teachers or TAs, but sometimes the school’s special educational needs co-ordinator (SENCO), a learning mentor (LM) or another education professional. Each facilitator was required to have some prior knowledge of ASD.
Facilitators were trained by members of the study team in how to deliver the LEGO® based therapy intervention. Each training session lasted a total of 3 hours, during which facilitators would learn the theory of LEGO® based therapy and how to run the groups. They were given both a paper and an electronic copy of the LEGO® based therapy manual.
Mode and locations of delivery
All sessions of LEGO® based therapy were delivered face to face in school with the participating CYP and the group’s facilitator.
All sessions of LEGO® based therapy were delivered in schools randomised to the intervention arm of the trial. A permanent room in school was recommended to maintain consistency for the CYP and for ease of storage; however, materials could be moved between rooms if necessary. A minimum requirement was that the LEGO® based therapy took place in a quiet room with few to no interruptions and with enough table or carpet space to build LEGO sets.
Frequency
Schools were asked to run 12 sessions of LEGO® based therapy over a period of 12 weeks. It was recommended that sessions were run for 1 hour once per week for the 12-week period. However, multiple sessions in 1 week owing to illness and time constraints were acceptable. Sessions shorter than 1 hour in duration were also acceptable, but facilitators were encouraged to run groups for at least 45 minutes.
Tailoring and modifications
During the training of the LEGO® based therapy facilitators, the trainers emphasised that, although the LEGO® based therapy intervention could not be modified during the course of the study, some features of the delivery of the intervention could be adapted to meet the needs of each group of CYP. These features included, for example, decisions about the choice of rules and reward systems, acknowledging that not all CYP will need the same rules or be motivated by the same things. Each group was encouraged to agree their own group rules based on what they thought was important for them, for example ‘be kind and polite’ or ‘remember to share’. Schools were encouraged to use existing reward systems if present. Some alternative reward systems were also suggested, such as the use of stickers, LEGO brick collection pots or LEGO figures.
It was also acknowledged that the amount of time during each session spent building the sets collaboratively was likely to change over the 12-week period depending on the group’s ability to use skills learned in the collaborative build activities and then move towards freestyle building.
Fidelity
Intervention fidelity was assessed via two methods: (1) completion of the fidelity checklist after each session of LEGO® based therapy by all facilitators and (2) video-recording of a subset of sessions carried out. The self-report fidelity checklist is a 17-item questionnaire based on the main principles of LEGO® based therapy, which is designed to assess how closely the facilitator felt the session had followed the intended delivery method. These were completed after every session and were posted to the study team. Video-recordings were completed in schools where all participating CYP, their parents/guardians and the facilitator had consented to the recording. Recordings were then viewed by members of the study team and assessed for fidelity to the intended intervention delivery method and to the corresponding facilitator-completed fidelity checklist for each session.
Control arm: usual support
In addition to LEGO® based therapy, CYP in the intervention arm also received ‘usual support’, defined as the usual support they would receive from school, ASD specialist teachers, their general practitioners (GPs) and any other professionals. These data were collected using case report forms (CRFs) filled in independently by associated teachers and parents/guardians in both trial arms. Participating CYP in the control arm received usual support only.
Outcome measures
A range of outcome measures was used during the trial to assess the primary and secondary objectives.
Outcome measures were selected on the basis of (1) relevance for the study parameters and participant abilities, (2) brevity to reduce participant burden, (3) appropriate informant versions for those completing the measures and (4) acceptability to patient and public involvement (PPI) groups. The primary outcome measure, the SSIS, is a behaviour rating scale that can be completed by parents, teaching staff and appropriate students. It is widely used in national portfolio studies and has been shown to be sensitive to change resulting from interventions in autistic children. It has good levels of reliability and validity and is validated for use by teachers.
Measures were completed by CYP, parents/guardians, associated teachers/TAs who knew the CYP well and the facilitator teachers/TAs who delivered the intervention (if the child or young person’s school was allocated to the intervention arm). Data were collected face to face in family homes or at school, by post or online via a secure link sent via e-mail. All measures were completed by participants in both trial arms at baseline, 20 weeks after randomisation and 52 weeks after randomisation unless otherwise stated.
Associated teacher/teaching assistant questionnaires
-
The SSIS53 – three subscales:
-
Social skills subscale (primary outcome measure) – 46 items; higher scores indicate greater social competence. This measures social skills such as social communication, co-operation, social engagement, empathy, assertion, responsibility and self-control.
-
Problem behaviours subscale – 30 items; higher scores indicate fewer problem behaviours.
-
Academic competence subscale – seven items; higher scores indicate higher academic competence.
-
-
The SDQ54 – 25 items; higher scores indicate a higher chance of developing a mental health disorder.
-
Bespoke resource use questionnaires – used to capture the resource implications of usual support received by the CYP in both trial arms. Specific questions were included in the resource use form at 20 weeks to assess any AEs (at 20 weeks only).
Facilitator teacher/teaching assistant (intervention arm only)
-
A bespoke demographic information form collecting demographic information and information relating to training and experience of the facilitator teacher/TA (at baseline only).
-
A bespoke resource use questionnaire to capture the resource implications of running the LEGO® based therapy sessions at school (after each session):
-
Specific questions were included in the session resource use questionnaire to assess any AEs that might be attributable to the intervention (after each session).
-
-
A fidelity checklist based on the existing treatment manual35 (after each session) – 17 items; higher scores indicate higher treatment fidelity.
-
A bespoke questionnaire to assess acceptability of the intervention structured around the theoretical framework of acceptability (TFA)55 – 11 items; higher scores indicate greater acceptability (at 20 weeks only).
Children and young people questionnaires
-
The Multidimensional Scale of Perceived Social Support (MSPSS)56 – 12 items; higher scores indicate a higher degree of perceived social support.
-
The Asher Loneliness Scale (ALS)57 – 24 items; higher scores indicate lower levels of loneliness and social dissatisfaction.
-
Child Health Utility-9 Dimensions (CHU-9D)58 – nine items; higher scores indicate higher health utility.
Parent/guardian questionnaires (one parent/guardian only)
-
The SCQ59 – 40 items; higher scores indicate more social communication difficulties (at baseline only).
-
The SSIS53 – two subscales:
-
The social skills subscale (primary outcome measure) – 46 items; higher scores indicate greater social competence. This measures social skills such as social communication, co-operation, social engagement, empathy, assertion, responsibility and self-control.
-
The problem behaviours subscale – 33 items; higher scores indicate fewer problem behaviours.
-
-
The SDQ54 – 25 items; higher scores indicate a higher chance of developing a mental health disorder.
-
The EuroQol-5 Dimensions-Youth (EQ-5D-Y) [based on the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), proxy version]60 – five items; higher scores indicate higher health utility.
-
Bespoke resource use questionnaires to capture the health care and non-health resource implications attributable to the CYP’s ASD:
-
Specific questions were included in resource use form at 20 weeks to assess any AEs (at 20 weeks only).
-
-
Bespoke questionnaire to assess acceptability of the intervention structured around the TFA55 – 11 items; higher scores indicate greater acceptability (intervention arm only at 20 weeks only).
-
A bespoke demographic information form collecting demographic information pertaining to the CYP and the parent/guardian (at baseline only).
Adverse events and serious adverse events
Adverse events were reported at the 20-week follow-up point by parents/guardians and associated teachers in their CRFs and by facilitators in their session attendance and resource use form completed after each session of LEGO® based therapy. Reported AEs were assessed for seriousness and this information was reported to the trial manager, principal investigator and DMEC. An AE was classified as a serious adverse event (SAE) if it met the following criteria:
-
results in death
-
life-threatening
-
requires hospitalisation or prolongation of existing inpatient hospitalisation
-
results in persistent or significant disability or incapacity.
Three SAEs were reported during the trial: one in the intervention arm and two in the control arm. All were unrelated to the trial (see Table 21).
Changes to trial outcomes after the trial commenced (with reasons)
As discussed in Important changes to the methods after trial commencement, the study’s first follow-up time point was changed in March 2018 from 16 weeks post randomisation to 20 weeks post randomisation. This was implemented because of concerns in the study team that the 12 sessions of LEGO® based therapy would not have been completed by all schools when approached for their 16-week follow-up and that resulting data would not be balanced, with schools at different stages of the intervention. This change was approved and implemented following discussions with the trial DMEC and TSC. The primary outcome measure to be used in ITT analysis was clarified in September 2018 as the social skills subscale of the SSIS (as opposed to the entirety of the SSIS).
The fidelity checklist outcome measure completed by the facilitator teacher/TA after every session of LEGO® based therapy was altered in September 2018 after discussions around the ease of completion for participants. Question 14 originally included one question followed by a separate, second question. This was followed by an ‘or’ option leading to question 15. For clarity and ease of use by facilitators and study team members, the second question in question 14 was changed to become question 15a and question 15 became 15b.
During our study the country faced the consequences of the COVID-19 pandemic. UK schools closed multiple times over the course of the year (2020–1). However, for this study the impact was limited to the first ‘lockdown’ and school closures in March 2020. This coincided with the final months of data collection, meaning that for a small number of our 52-week follow-ups some CYP responses, for example in relation to levels of anxiety, may have been affected by the pandemic.
There was no impact on intervention delivery or the primary outcome measure because these had been completed by the summer of 2019.
Some additional analyses were conducted to assess the potential impact of the pandemic on the trial; these are discussed in Chapter 3, Recruitment and participant flow, Dates defining the periods of recruitment and follow-up.
Sample size calculations
The sample size used for the study was calculated based on a Cochrane review of five studies assessing the effects of social skills groups on the social competence of participants. 28 Four of the studies29–32 reported were RCTs that used standardised tools to measure social competence and could be synthesised via meta-analysis. When comparing group intervention receipt and usual support, the weighted mean standardised difference in social competence was found to be 0.47 [95% confidence interval (CI) 0.16 to 0.78] and the review stated that this represented a clinically significant change in social competence. When using this standardised effect size to calculate sample size to give 90% power and at the 5% two-sided significance, a total of 194 participants would be needed (i.e. 97 per trial arm). This number was increased to 232 participants (i.e. 116 per trial arm) to allow for an attrition rate of 16%, the highest of the reported rates in the Cochrane review of social skills interventions. 28 The sample size was further inflated to 240 participants to allow for trainer/school effects, assuming equal clusters of size four (i.e. two LEGO® based therapy intervention groups per school and two CYP participants with a diagnosis of ASD per LEGO® based therapy group). The intraclass correlation (ICC) was assumed to be 0.01 based on findings from the ASSSIST (Autism Spectrum Social Stories In Schools Trial) feasibility study. 65 This gave a total recruitment target of 240 participants (i.e. 120 participants per trial arm) or 12 participants per month over all recruiting areas.
Explanation of any interim analyses and stopping guidelines
The study included a 10-month internal pilot to assess the feasibility of conducting the trial. The expected recruitment target was 120 participants, based on a recruitment rate of 12 participants per month, one-third (n = 40) of whom were expected to have reached the primary end point of 20 weeks post randomisation. Stop/go criteria were used to assess whether or not the study should continue. This was based on 75% of expected recruitment at 10 months (n = 90) and 70% of the primary outcome target (n = 28). In reality we had only 8 months for recruitment because of school holidays and delays with study approvals leading to recruitment start delays, meaning that the recruitment rate target became 15 participants per month.
Method used to generate the random allocation sequence
The unit of randomisation was school, each of which was randomised using stratified randomisation lists based on two strata: stage of education (i.e. primary or secondary) and number of eligible and participating CYP (i.e. ≤ 6 or > 6 CYP). All consented CYP were included in the intervention clusters. A blinded statistician from the Clinical Trials Research Unit (CTRU) at the School of Health and Related Research (ScHARR), University of Sheffield (Sheffield, UK), generated four randomisation lists (i.e. one for each combination of the two strata) using random blocks in the statistical package R (The R Foundation for Statistical Computing, Vienna, Austria). Allocation to arms was undertaken remotely. The co-ordinating researcher telephoned the CTRU for the next entry on the appropriate list after establishing eligibility, obtaining consent and collecting baseline measures.
Type of randomisation and details of any restriction (such as blocking and block size)
Cluster randomisation was used at school level to avoid CYP in the same school receiving different interventions, thus limiting contamination. The allocation list was generated prior to recruitment using block randomisation, with each group of two or four recruited schools having a 1 : 1 balance between arms. Group membership was determined chronologically (by date of recruitment) in the four strata and the size of each subsequent group was selected at random.
Sequence generation, enrolment and assignation
Following random allocation, schools were informed of their allocation by the trial manager by post and by telephone or e-mail, and families were informed by post. Once schools allocated to the intervention had been informed of this, they were asked to select a member of staff to attend training in LEGO® based therapy and to run 12 sessions of the intervention before their 20-week follow-up. This method of enrolment was used successfully in a previous study of autistic CYP. 61
Blinding
All RAs in the study team were blind to treatment allocation to limit any potential bias at the two follow-up points (i.e. 20 and 52 weeks post randomisation). All forms were completed by participants themselves, and RAs had very little input, but, when instances of unblinding were reported, subsequent follow-ups were carried out by another blinded study team member, and these were recorded.
Trial statisticians were also blind to treatment allocation throughout the trial. The DMEC had access to unblinded data on request to enable it to investigate SAEs associated with the intervention.
Statistical methods
Intention-to-treat analysis was used to assess whether or not LEGO® based therapy had any effect on the social competence and perceived isolation of autistic CYP. This approach is appropriate for a pragmatic trial because of its focus on external validity despite dilution bias dangers when the regression slope may be biased towards zero. 62 Per-protocol analysis was also used based on a per-protocol definition of receiving six or more sessions of the LEGO® based therapy intervention. Comparability between baseline demographic and outcome measures was analysed and all data produced were assessed and reported based on RCT CONSORT guidelines44 and cluster RCT CONSORT guidelines. 45
The primary outcome measure was the social skills subscale of the SSIS completed by the associated teacher at 20 weeks post randomisation. This scale gives a summated score, treated as a continuous variable, with the significance level set at 5%.
As pre-specified in SAP version 2.0, the primary analysis of the primary outcome was performed using a multilevel mixed-effects model, with robust standard errors accounting for clustering within school. Covariates in the analysis were age, gender, baseline score on the social skills subscale of the SSIS, school (random effect), number of eligible and participating CYP (stratified by ≤ 6 or > 6 CYP), and school level (stratified by primary or secondary). The standardised mean difference, a two-sided 95% CI and p-values were presented. The primary analysis was performed on the ITT population.
Differences between arms were also summarised using unadjusted estimates with 95% CIs.
Secondary outcome variables were analysed using the same framework as the primary outcome. The sustainability analysis compared 20-week and 52-week estimates of differences between arms on the social skills subscale of the SSIS reported by the associated teacher.
Subgroup analysis of three levels of baseline symptom severity measured using the SCQ was undertaken in two ways. First, the interaction between subgroup and trial arm was added to the primary analysis model. Second, separate models were fitted for each of the three subgroups.
When data were missing from outcome measures because of withdrawals or attrition at follow-up points, multiple imputation (MI) methods were used in sensitivity analyses to reduce potential bias in the analyses resulting from the missing data.
All standardised effect sizes were calculated by rescaling the model estimated mean difference by the pooled standard deviation (SD) of the outcome at baseline, then multiplying by –1 for those outcomes in which a decrease in the score needs to be observed to find evidence in favour of the intervention arm.
Analysis populations
The following pre-planned analysis populations were studied for the primary outcome (analysis of secondary outcomes used the ITT population only):
-
ITT – those with data available for ≥ 42 of the 46 items in the primary outcome measure (social skills subscale at 20 weeks reported by associated teacher) and with recorded consent information
-
per protocol – the subset of the ITT population who received six or more of the 12 planned intervention sessions
-
complier average causal effect (CACE) – ITT analysis accounting for compliance (i.e. six or more sessions received)
-
MI population – imputation of missing social skills subscale scores using chained equations.
Additional analyses
Planned sensitivity analysis on the primary outcome
Sensitivity analyses were conducted for the primary outcome using the per-protocol, CACE and MI methods, and results were presented in a forest plot.
Additional sensitivity analyses accounted for the timeliness of follow-up data collection, associated teacher responses at the 20-week follow-up time point and the impact of UK lockdown measures resulting from the COVID-19 pandemic. The timelines analysis removed all responses submitted more than 26 weeks after randomisation (for the 20-week follow-up) and 60 weeks after randomisation (for the 52-week follow-up). The teacher analysis excluded all responses for which baseline and 20-week data had been provided by different teachers. The COVID-19 analysis removed all responses completed after 23 March 2020.
Unplanned sensitivity analysis on the primary outcome
Three unplanned analyses were undertaken to explore the potential for observational analysis of the trial data in a subsequent substudy.
First, two additional analysis populations were studied:
-
per protocol 2 – the subset of the ITT population for whom there was an average of two or more autistic CYP in therapy sessions
-
CACE 2 – ITT analysis accounting for compliance (i.e. CYP for whom there was an average of two or more autistic CYP in therapy sessions).
Second, subgroup analyses of the stratification variables used in the randomisation were undertaken using the same approach as the planned subgroup analysis.
Third, the three subgroup analyses undertaken for the primary outcome were repeated for six of the secondary outcomes: scores for the SDQ54 subscales peer problems and prosocial, ALS score,57 the MSPSS score and the parent-reported social score (all at 20 weeks) and the teacher-reported social score at 52 weeks. These were selected by clinical members of the team before they had knowledge of the results of the secondary outcome analysis.
Study oversight and management
The study was managed throughout by two trial managers, one at the ScHARR CTRU and one in the Leeds and York Partnership NHS Foundation Trust (LYPFT), with support from the Chief Investigator and CTRU Director. Oversight took the form of four groups: the TMG, the Operations Group, the TSC and the DMEC. TMG meetings, involving study team members and co-applicants, were held monthly for the majority of the study period until January 2019, when their frequency was changed to quarterly, with Operations Group meetings (involving core study team members) occurring monthly in their place. TSC and DMEC meetings occurred every 6 months for the duration of the study period and involved independent members and stakeholders with relevant expertise. In addition, a 6-monthly study report was submitted to the funder, the National Institute for Health and Care Research (NIHR) Public Health Research (PHR) programme, and regular communication occurred between all members of the study team.
Internal monitoring of the study was carried out in October 2018. At this time, each of the two sites [the Child Oriented Mental Health Intervention Centre (COMIC), University of York (York, UK), and the University of Sheffield CTRU] visited the other to check the study site files for completeness and accuracy. Findings were logged and any errors amended by the study team. In addition, in January 2019, the trial master file kept at the Sheffield CTRU was checked along with a randomly selected 10% sample of the completed CRFs at each site (COMIC, n = 9; Sheffield CRTU, n = 10). These were checked for accuracy of entry onto the bespoke clinical data management system. Data entry accuracy was relatively high.
External monitoring was then conducted at the COMIC research site in June 2019 by a member of the Harrogate and District NHS Foundation Trust’s research and development team. This included checking the site file for completeness and accuracy, assessing the trial’s Good Clinical Practice compliance, and source data verification (SDV) checking 10% of the completed CRFs at the site (n = 14). All errors noted in the monitoring report were amended by August 2019. This monitoring session confirmed the findings of the initial site monitoring in that there was a very small number of data entry errors. Therefore, the study team decided to carry out full SDV checks of all critical data (see Appendix 1, Table 29). Overall SDV error percentages were very low, with a total of eight forms out of 48 exceeding the 1% error rate. All identified errors were corrected on discovery.
Ethics arrangements and regulatory approvals
Positive ethics opinions for the study were obtained from the Health Research Authority (HRA) (18/HRA/0101) and from the University of York’s REC. Approvals for any changes to study documents or other required approvals were sought prior to implementation from the REC, the NIHR PHR programme, the sponsor and the HRA when necessary. The trial was not conducted in, and did not recruit from, the NHS, nor did it involve or identify participants based on their use of the NHS; thus, NHS ethics approval was not required. In addition, clinical trials authorisation was not required, as no medical devices or pharmaceutical elements were used.
Patient and public involvement
Study design
The study team worked closely with PPI members from different groups representing the target population to develop the original research proposal and in the early stages of study set-up. The PPI representatives included a parent/guardian of a child or young person with ASD and individuals from the Young Dynamos research advisory group (Bradford District Care NHS Foundation Trust, Shipley, UK) and the National Autistic Society (NAS) (London, UK). The Young Dynamos, a research advisory group from the Bradford District Care NHS Foundation Trust, is made up of young people of varying ages. The project was presented to the group, which comprised around 10 young people (the number differed at each meeting) and the two adult group leaders, at multiple time points throughout the study. Two members of the NAS gave valuable feedback regarding initial study design early in the project. A parent of a child with ASD helpfully agreed to be a member of our TSC and was able to provide input on discussion points throughout the study. We also gained input from a specialist teacher for autism from the York local authority (York, UK) during the study, which aided discussions. The recommendations provided by PPI representatives were implemented and contributed towards the smooth running and high recruitment rates of the trial.
Study oversight
The TSC included a parent/guardian of an autistic child. This representative was present at TSC meetings, which meant that the parent/guardian’s perspective was present when discussing the general running of the trial and any current problems faced by the study team. This type of PPI aided decision-making, and valuable insights were gained over the course of the trial. Ideally, a child or young person and a teacher would also have been involved, but this was not possible because TSC meetings often took place during school time.
The PPI representatives also provided helpful input for the plain English summary used in this report. In addition, a discussion was set up with PPI group members, clinicians and the TSC around the language to be used in this report to refer to the CYP participants with a diagnosis of ASD. It was noted that there are many terms that are used to refer to ASD, and some people with this diagnosis have a specific preference. The group concluded that it would be best to use the clinical terminology of ASD but to include an explanation of this along with acknowledgement of the many other terms preferred by this population.
The trial was presented in January 2020 at the NIHR Clinical Research Network Yorkshire and Humber’s Vision 2021: PPIE – Working Together in Research conference (York, UK, 28 January 2020) by the York trial co-ordinator and a young person from the Young Dynamos PPI group. The group’s involvement in the trial was described by the young person, and the involvement of other PPI members was also presented.
Health economic methods
Background
The trial design was a cluster RCT to investigate the clinical effectiveness and cost-effectiveness of the LEGO® based therapy intervention on the social and emotional skills of autistic CYP compared with usual support only. In this design, the health economics component was a cost–utility analysis, measuring the incremental cost-effectiveness ratio of the LEGO® based therapy intervention over the control arm.
Effectiveness
Effectiveness for the health economics analysis was measured using the EQ-5D-Y and the CHU-9D.
EuroQol-5 Dimensions-Youth
The EQ-5D-Y60 is a five-item, generic, preference-based measure of health-related quality of life (HRQoL) that can be completed by proxy person (i.e. parent/guardian) on behalf of the participant. The EQ-5D-Y comprises five dimensions: mobility, looking after oneself, doing usual activities, having any pain or discomfort and feeling worried. Each dimension presents three levels of problems: ‘no problems’ (level 1), ‘some problems’ (level 2) and ‘a lot of problems’ (level 3). There is also a visual scale of the child or young person’s overall health status from 0 (worst health imaginable) to 100 (best health imaginable). All questions refer to the child or young person’s health state ‘today’. The EQ-5D-Y has been shown to be a reliable and valid HRQoL instrument for use in CYP and adolescents,63 and it can be used for a cost–utility analysis.
Child Health Utility-9 Dimensions
The CHU-9D58 is a CYP-completed nine-item questionnaire that measures HRQoL. CYP are required to select one of five sentences for each question that describes how they feel in relation to the construct listed in that question. The constructs are ‘worried’, ‘sad’, ‘pain’, ‘tired’, ‘annoyed’, ‘schoolwork/homework’, ‘sleep’, ‘daily routine’ and ‘able to join in activities’. The CHU-9D also provides utility values, allowing the calculation of quality-adjusted life years (QALYs) for use in a cost–utility analysis.
Cost
Cost of the intervention
The cost of the intervention included the cost of training and the cost of delivering LEGO® based therapy. Training costs were calculated using the time spent by the trainer and included travel costs and the costs of materials and consumables (e.g. pens, paper, file folders, sticky notes, manuals used to deliver the intervention) used in the training. Costs associated with delivering the LEGO® based therapy intervention were calculated using the time spent by facilitator teachers/TAs in planning and conducting sessions and undertaking any additional work. A bespoke questionnaire developed by the research team was used for collecting data on the costs of training and delivering LEGO® based therapy.
Cost of the service use
Service use data were collected using bespoke questionnaires (completed by the parent/guardian and associated teacher of each CYP in the study) on the use of the following services:
-
community-based health services, including appointments with GPs, nurses, walk-in centre staff, social workers, family support workers, educational psychologists, educational welfare officers, and school and college nurses
-
mental-health-related services, including appointments with psychiatrists, psychotherapists, psychologists, Child and Adolescent Mental Health Services (CAMHS) therapists, mental health nurses, family therapists, GPs, school counsellors and privately paid mental health service staff
-
hospital-based services, including outpatient visits, inpatient admissions, accident and emergency department visits, and urgent care centre visits
-
school-based interventions/support provided by teachers
-
other services, including medication, privately paid services and productivity costs.
Parent-/guardian-completed resource use questionnaires informed individual-level use of the above-listed services, specifically primary and secondary healthcare and social care services. The tailored questionnaire was based on a previous questionnaire by Barrett et al. ,64 which has since been adapted for use in school-based trials. 65,66 Teacher-completed questionnaires captured any school-based interventions/support and the implications of a child or young person’s behaviour on school resources.
Service use was multiplied by unit costs to arrive at total cost in each arm. Unit costs of health and social service use were obtained from the UK national database of reference costs (2016–17)67 and the Personal Social Services Research Unit (PSSRU)’s Unit Costs of Health and Social Care 2015. 68 Medication costs were based on Prescription Cost Analysis, England 2017. 69 Data reported by the Department for Education70 were used to estimate the cost of teacher time, with any privately paid services being separately estimated using market prices. In cases in which funding sources for a member of staff were unclear, assumptions were made based on service location and published guidelines (such as PSSRU guidelines). 69
Economic analysis
The health economics analysis was conducted from a UK NHS and PSS perspective. This took the form of a within-trial cost–utility analysis to compare the LEGO® based therapy intervention with usual support for autistic CYP. Costs were measured using tailored service use questionnaires. Health outcomes were measured using QALYs based on the parent-/guardian-completed EQ-5D-Y as a health descriptor measure. QALYs were estimated using the area-under-the-curve approach between baseline and each follow-up.
Combining costs and QALYs, an ICER of cost per QALY gained was calculated and then compared with the national WTP threshold of £20,000–30,000 per QALY gained71 to assess the cost-effectiveness of the intervention.
Descriptive analysis
Total costs, including intervention and service utilisation costs and QALYs, were compared between intervention and control arms using appropriate descriptive analyses.
Handling missing data
Missing data existed in both service use and health outcome data. For service use, data were deemed missing when all questions under a particular section were left blank. If one of these questions was answered, the other answers were assumed to be 0. For the EQ-5D-Y, a section was considered missing if any of its five questions was not answered. Missing data were further imputed using Rubin’s MI method. 72
Regression analysis and bootstrapping
A regression model was used to compare mean costs and QALYs based on an ITT approach. The regression analyses were controlled for baseline differences in utility,73 costs and other baseline characteristics of autistic CYP, such as age, gender and SCQ score. The model specification followed the approach recommended by Glick et al. ,74 which considers the distribution of the dependent variable and any correlation found between cost and QALY outcomes. The ICER was then calculated based on the regression coefficients on intervention, because they represent the difference in mean cost and mean QALYs between the two groups. To take uncertainty into consideration, a non-parametric bootstrap resampling method was used to produce the CI for the ICER. This was carried out because the distribution of regression residuals was likely to be skewed. 75
The bootstrapped results were presented in the conventional form of a cost-effectiveness plane and a cost-effectiveness acceptability curve (CEAC). The uncertainty based on the outcomes of the 5000 bootstrap iterations was represented graphically on the cost-effectiveness plane, and the CEAC presented the probability of the intervention being cost-effective over a range of WTP thresholds per QALY gained. 76
Sensitivity analysis
A set of sensitivity analyses was conducted, as follows:
-
To assess the impact of the missing data, a sensitivity analysis using complete cases was conducted.
-
To account for the economic impact on the stakeholders, a sensitivity analysis adopting a NHS/PSS and education perspective was conducted.
-
To account for the economic impact outside the NHS/PSS perspective, a sensitivity analysis was conducted using a societal perspective to cover service costs from healthcare and education sectors, private expenses and costs of parental productivity loss.
-
A sensitivity analysis that used the CHU-9D instead of the EQ-5D-Y to estimate QALYs based on the UK population tariff58 was also conducted.
Qualitative methods
Qualitative methods
Interviews were conducted following the completion of the intervention with a subsample of facilitator teachers/TAs across school types (primary or secondary) who provided consent to be invited to participate in the qualitative substudy. The TFA55 and normalisation process theory77 were used to guide the design of the interview schedule and help frame the data analysis to aid our understanding of acceptability and implementation issues related to the intervention. Sekhon et al. 55 define acceptability as ‘a multi-faceted construct that reflects the extent to which people delivering or receiving a healthcare intervention consider it to be appropriate, based on anticipated or experienced cognitive and emotional responses to the intervention’. Sekhon et al. 55 outline seven component constructs of acceptability. Given that we have used the TFA to structure the measurement of intervention acceptability, the definitions of each construct have been included as a direct quotation for transparency as follows:
-
affective attitude – ‘How an individual feels about the intervention’55
-
burden – ‘The perceived amount of effort that is required to participate in the intervention’55
-
ethicality – ‘The extent to which the intervention has a good fit with an individual’s value system’55
-
intervention coherence – ‘The extent to which the participant understands the intervention and how it works’55
-
opportunity costs – ‘The extent to which benefits, profits or values must be given up to engage in the intervention’55
-
perceived effectiveness – ‘The extent to which the intervention is perceived as likely to achieve its purpose’55
-
self-efficacy – ‘The participant’s confidence that they can perform the behaviour(s) required to participate in the intervention’. 55
Normalisation process theory has been proposed as a way of identifying whether or not an intervention is likely to become part of routine practice. 78 It offers an explanatory model for the adoption and embedding of new practices into pre-existing routines based on the concept that changing such established routines is a complex process for individuals. The interview schedule was piloted with two teaching staff with experience of delivering LEGO® based therapy prior to commencing data collection as part of the trial.
Invitation e-mails were sent to all eligible facilitators who had given prior consent to participate in an interview, which included a short reminder of the interview purpose, and those who were still interested and available to participate responded accordingly.
All interviews were conducted by telephone by a member of the research team (AB, a postgraduate non-clinical RA at the time of study). Facilitators were asked to position themselves in a private location (e.g. work offices/private rooms on school premises) during the interview. All interviews were recorded and transcribed verbatim. Interview data were analysed using the framework analysis approach,79 supported by NVivo version 12 (QSR International, Warrington, UK) software.
Interview data were coded by two independently trained researchers in the research team. The coders met regularly to develop a coding manual and to ensure the grounding in original data of all developed codes. The framework used was amended to allow for new codes and the deletion of unnecessary codes, leading to a final framework representative of the whole data set. The final versions of the coding manuals were presented to the TMG and TSC to confirm validity, coherence and relevance.
Acceptability study
The acceptability of the intervention to CYP was assessed using the number of sessions attended and data collected from the facilitator and parent/guardian, because we did not want to overburden the CYP participants at each session. We designed a bespoke questionnaire based around the TFA55 to assess acceptability of the intervention to parents/guardians and facilitators at the 20-week time point. This questionnaire was piloted with two members of teaching staff with experience of delivering LEGO® based therapy and with the PPI members of the TMG prior to finalising content and design. All data were summarised descriptively. Results from the survey are presented using descriptive statistics.
Fidelity methods
Intervention fidelity was monitored and assessed using the following mechanisms throughout the study:
-
An abridged training manual created by the study team based on the LEGO® based therapy manual. 35
-
A training programme developed with the training manual and delivered by co-applicant and co-author of the LEGO® based therapy manual, Gina Gomez de la Cuesta.
-
Video-recording of a subsample of intervention sessions in schools; full written consent for this was obtained from the parents/guardians of all group members (and assent from group members themselves where possible) and the facilitator teacher/TA. Details are as follows:
-
A fidelity checklist developed from work by Gina Gomez de la Cuesta was used to monitor the content of the intervention sessions. This was also completed by facilitators after each session.
-
The aim was to record 10% (i.e. 72) of all intervention sessions in the study. These were sampled at school level (primary or secondary), with the aim of three session recordings per school, ideally one of the first four sessions, one of the second four sessions and one of the final four sessions.
-
Videos of the intervention sessions were reviewed by two independent observers to assess fidelity to the checklist, and inter-rater reliability was analysed.
-
Established criteria were used to classify the extent of observed fidelity of delivery:80 if ≤ 50% of intended content was delivered, this was classified as ‘low’ fidelity; if 51–79% of intended content was delivered, then this was classified as ‘moderate’ fidelity; and if 80–100% of intended content was delivered, then this was classified as ‘high’ fidelity. Descriptive statistics were produced on all completed fidelity checklists.
-
-
Global assessment of fidelity across all LEGO® based therapy sessions, including those that were not video-recorded and included in the observed fidelity assessment. Self-reported fidelity checklists were also administered to all intervention arm schools for completion by facilitators on a weekly basis, immediately at the end of each session.
-
The facilitator-completed checklist, which was compared with the recorded sessions when possible.
-
The proportion of intended components of LEGO® based therapy rated as delivered or not by facilitators. The same criteria were used to classify extent of fidelity as in the observed measurements: if ≤ 50% of intended content was delivered, then this was classified as ‘low’ fidelity; if 51–79% of intended content was delivered, then this was classified as ‘moderate’ fidelity; and if 80–100% of intended content was delivered, then this was classified as ‘high’ fidelity. Variations in proportion of fidelity of delivery were examined across session numbers and intervention groups. Descriptive statistics were produced on all completed fidelity checklists.
-
Chapter 3 Trial results
Recruitment and participant flow
A total of 564 schools in Leeds, York and Sheffield and their surrounding areas were contacted by telephone, e-mail or post to inform them of the I-SOCIALISE trial. Schools were contacted multiple times throughout the recruitment period, up to eight times in cases where schools did not respond. Among these schools, 238 confirmed their interest in the research; representatives from 131 schools met researchers to discuss participation in the trial and eligibility of CYP in their school; and 112 schools passed on study invitation packs to parents/guardians with any potentially eligible CYP, 284 of whom expressed an interest in participating. We did not record how many information packs were given out to all schools, only those that were given to parents/guardians who then expressed an interest in participating. From the 284 expressions of interest, 260 CYP participants from 103 schools provided parental consent/CYP assent to participate in the research; a total of 250 participants from 98 schools were randomised. The randomisation sequence allocated 50 schools (127 participants) to the intervention arm and 48 schools (123 participants) to the control arm. In total, 202 participants from 96 schools completed the study (Figure 1). Appendix 2, Figure 8 shows the as-treated CONSORT flow diagram. Related information is also detailed in Appendix 2, Tables 30 and 31.
FIGURE 1.
The CONSORT flow diagram: ITT. a, Following ITT principles, children (n = 3) who were allocated to the control arm but received intervention are included in the control arm.
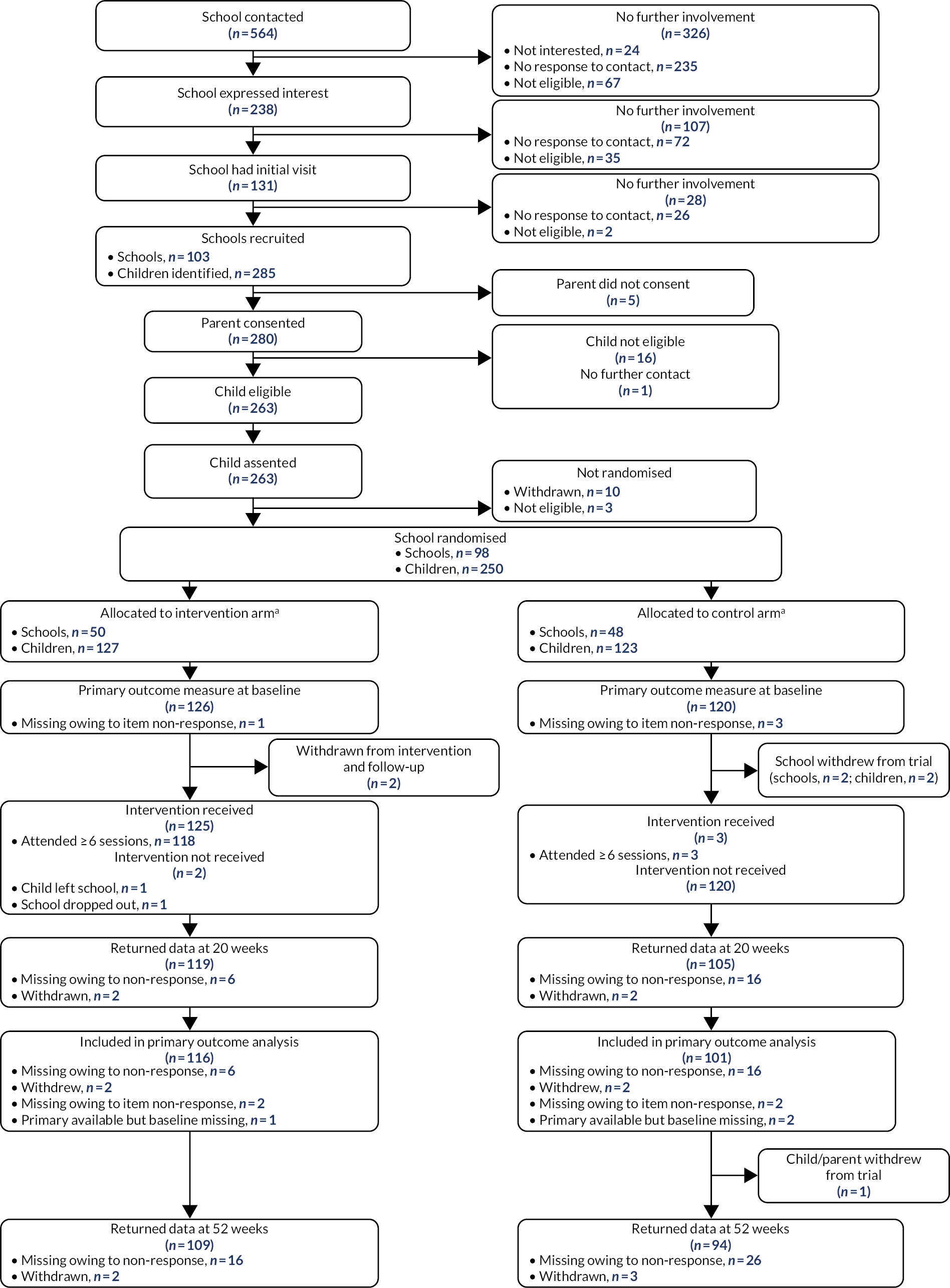
A school with three CYP participating in the trial was randomly allocated to the control arm but was informed that the CYP had been randomised to the intervention arm owing to human error. This constituted a protocol violation and was reported. The ITT analysis kept the misallocated school in the control arm. Therefore, for the analysis the ‘as-treated’ population included 51 schools (130 CYP) in the intervention arm and 47 schools (120 CYP) in the control arm. The pre-planned definition of ‘minimum dose’ for the per-protocol population was six sessions. The per-protocol population also excluded the three participants assigned to the control arm who received the intervention.
The primary outcome ITT analysis included 217 CYP (86.8%), 116 (91.3%) in the intervention arm and 101 (82.1%) in the control arm.
The protocol was clarified regarding CYP recruited to the study who were found to not have a clinical diagnosis of ASD and thus were ineligible for the trial. In line with the protocol the TMG decided that they would be withdrawn from the study and their data not included in the ITT analysis.
In a small number of cases in which RAs had become unblinded to participant treatment allocation, it was not possible for another RA to conduct the visit. In these cases, the study team made every effort to minimise the impact of the unblinding, including ensuring that the unblinded RA collected completed measures and did not provide direct assistance to participants completing self-report questionnaires. The number of follow-ups carried out by unblinded members of staff was recorded and such follow-ups were found to constitute only a small percentage of the total (Table 1).
| Follow-up time point | Participant type | Site | Mean | ||
|---|---|---|---|---|---|
| Leeds | York | Sheffield | |||
| 20 weeks | Parent | 85 | 77 | 81 | 81 |
| Child or young person | 92 | 96 | 88 | 92 | |
| Teacher | 84 | 82 | 100 | 89 | |
| 52 weeks | Parent | 84 | 74 | 88 | 82 |
| Child or young person | 89 | 84 | 88 | 87 | |
| Teacher | 89 | 84 | 100 | 91 | |
Losses and exclusions after randomisation
A small number of participants (n = 48) withdrew from the research following randomisation or were lost to follow-up and so did not complete the study. No participants were excluded. Table 2 presents withdrawal specifications. Withdrawal reasons can be found in Appendix 2, Table 32.
| Time point | Discontinuation category | Reason | Intervention arm (n) | Control arm (n) | Total (n) |
|---|---|---|---|---|---|
| Between randomisation and 20 weeks | Child or young person/parent withdrew | Parent unhappy with allocation | 0 | 2 | 2 |
| Lost to follow-up | N/A | 7 | 10 | 17 | |
| Decision taken to withdraw | Parent reported that child or young person was uncomfortable with sessions and surveys | 0 | 1 | 1 | |
| School no longer wanted to be in the study | 0 | 1 | 1 | ||
| Child or young person no longer attended the school | 1 | 0 | 1 | ||
| School withdrew | Not provided | 1 | 1 | 2 | |
| 20–52 weeks | Child or young person/parent withdrew | Personal/family issues | 0 | 1 | 1 |
| Parent unwilling to complete surveys | 0 | 2 | 2 | ||
| Lost to follow-up | N/A | 6 | 15 | 21 | |
| Total | 15 | 33 | 48 |
Dates defining the periods of recruitment and follow-up
Recruitment to the trial commenced in October 2017 and continued until March 2019. The 20-week post-randomisation follow-up collection period began in March 2018 and ended in October 2019. The 52-week post-randomisation follow-up collection period began in November 2018 and ended in June 2020. The COVID-19 pandemic had no impact on response rates for the primary end point (20 weeks after randomisation) and a small impact on data collection rates at 52 weeks for the second of the two school years in which we recruited. If the response rate had shown similar patterns in both years, we would have expected 222 responses at 52 weeks, rather than the 203 we received (Table 3).
| Follow-up time point | Recruitment phase, academic year | Participants (n) | No response (n) | Data provided (n) | Provided data (%) |
|---|---|---|---|---|---|
| 20 weeks | 2017/18 | 102 | 11 | 91 | 89 |
| 2018/19 | 148 | 15 | 133 | 90 | |
| 52 weeks | 2017/18 | 102 | 11 | 91 | 89 |
| 2018/19 | 148 | 36 | 112 | 76 |
The number of weeks between randomisation and outcome data collection is summarised in the left hand side of Table 4. Expected windows for the 20-week and 52-week follow-ups were 20–26 weeks and 52–60 weeks, respectively. The ITT analysis included all data irrespective of whether they were collected within or outside these expected follow-up windows. The impact on the analysis of data being collected outside these expected follow-up windows was explored by refitting the analysis models to the subset of data collected within the expected follow-up windows. This analysis is reported in the right hand side of Table 4. The only response returned after the start of the COVID-19 pandemic national lockdown was the 52-weeks follow-up booklet for one participant. Therefore, the sensitivity of the primary outcome to the COVID-19 pandemic lockdown is omitted.
| Follow-up time point | Weeks between randomisation and follow-up, n (median) [IQR] | Sensitivity analysis | |||
|---|---|---|---|---|---|
| Threshold | Returns before threshold (n) | Adjusted mean difference (95% CI) | p-value | ||
| 20 weeks | 220 (21.00) [19.86–23.14] | 26 weeks post randomisation | 196 | 2.96 (–1.18 to 7.09) | 0.16 |
| 52 weeks | 200 (53.93) [52.50–57.00] | 60 weeks post randomisation | 175 | 1.51 (–3.25 to 6.26) | 0.53 |
Baseline data
There were no major imbalances observed at baseline (Table 5). Trial participants tended to be male (78%) and the median age at recruitment was 9 years. Thirty percent of participants were attending secondary school and 27% of all participants were located in schools where there were at least six CYP with an ASD diagnosis (Table 6).
| Variable | Intervention arm (N = 127) | Control arm (N = 123) | All (N = 250) |
|---|---|---|---|
| Categorical | |||
| Gender, n (%) | |||
| Male | 102 (80) | 92 (75) | 194 (78) |
| Female | 25 (20) | 31 (25) | 56 (22) |
| Academic age (years),a n (%) | |||
| 6 | 9 (7) | 6 (5) | 15 (6) |
| 7 | 25 (20) | 21 (17) | 46 (18) |
| 8 | 23 (18) | 18 (15) | 41 (16) |
| 9 | 18 (14) | 24 (20) | 42 (17) |
| 10 | 14 (11) | 17 (14) | 31 (12) |
| 11 | 18 (14) | 12 (10) | 30 (12) |
| 12 | 7 (6) | 15 (12) | 22 (9) |
| 13 | 4 (3) | 5 (4) | 9 (4) |
| 14 | 9 (7) | 5 (4) | 14 (6) |
| Ethnicity, n (%) | |||
| English/Welsh/Scottish/Northern Irish/British | 102 (80) | 107 (87) | 209 (84) |
| Any other white background | 3 (2) | 1 (1) | 4 (2) |
| Pakistani | 5 (4) | 3 (2) | 8 (3) |
| Bangladeshi | 2 (2) | 0 (0) | 2 (1) |
| Any other Asian background | 2 (2) | 1 (1) | 3 (1) |
| White and Black Caribbean | 2 (2) | 1 (1) | 3 (1) |
| White and Asian | 1 (1) | 4 (3) | 5 (2) |
| Any other mixed/multiple ethnic background | 1 (1) | 2 (2) | 3 (1) |
| African | 3 (2) | 1 (1) | 4 (2) |
| Caribbean | 2 (2) | 0 (0) | 2 (1) |
| Arab | 1 (1) | 1 (1) | 2 (1) |
| Any other ethnic group | 2 (2) | 0 (0) | 2 (1) |
| Prefer not to say | 1 (1) | 2 (2) | 3 (1) |
| Continuous | |||
| Age (years) | |||
| n (%) | 127 (100) | 123 (100) | 250 (100) |
| Mean (SD) | 9.6 (2.3) | 9.7 (2.1) | 9.7 (2.2) |
| Median (IQR) | 9 (8, 11) | 9 (8, 11) | 9 (8, 11) |
| Minimum, maximum | 7, 15 | 7, 15 | 7, 15 |
| SCQ score, points | |||
| n (%) | 127 (100) | 123 (100) | 250 (100) |
| Mean (SD) | 25.1 (5.2) | 24.2 (5.2) | 24.7 (5.2) |
| Median (IQR) | 25 (22, 29) | 24 (21, 28) | 25 (21, 29) |
| Minimum, maximum | 15, 37 | 15, 36 | 15, 37 |
| SSIS social skills (teacher) score, points | |||
| n (%) | 126 (99) | 120 (98) | 246 (98) |
| Mean (SD) | 66.1 (19.3) | 64.9 (21.4) | 65.5 (20.4) |
| Median (IQR) | 64 (50, 79) | 66 (48, 77) | 65 (50, 79) |
| Minimum, maximum | 29, 117 | 26, 129 | 26, 129 |
| Variable | Intervention arm (N = 127), n (%) | Control arm (N = 123), n (%) | All (N = 250), n (%) |
|---|---|---|---|
| School level | |||
| Primary | 89 (70) | 86 (70) | 175 (70) |
| Secondary | 38 (30) | 37 (30) | 75 (30) |
| Number of children in school with ASD diagnosis | |||
| < 6 | 91 (72) | 92 (75) | 183 (73) |
| ≥ 6 | 36 (28) | 31 (25) | 67 (27) |
Summary data regarding CYP ASD diagnosis and baseline characteristics by arm are detailed in Appendix 2, Tables 33, 34 and 35. To explore baseline comorbidities, we conducted post hoc analyses looking at baseline scores for the five subscales of the SDQ and the five dimensions of the EQ-5D-Y (Tables 7 and 8, respectively). Using Goodman’s original descriptors,54 the SDQ showed that 74% of CYP were classed as borderline or abnormal on the prosocial scale, 55% were classed as borderline or abnormal on both hyperactive and peer problems scale, and 40% were classed as borderline or abnormal on both emotional and conduct scales (see Table 7). The EQ-5D-Y results showed that few CYP had issues with mobility or with pain or discomfort, but that problems with anxiety and engaging with usual activities were quite common (around 50% had some issue and 10% had many issues) and problems with looking after oneself were slightly more common (around 50% with some issues and 20% with many issues) (see Table 8). Medications used by CYP were captured by the parent/guardian questionnaires (see Appendix 2, Tables 39 and 40).
| Scale | Intervention arm (N = 127), n (%) | Control arm (N = 123), n (%) | All (N = 250), n (%) |
|---|---|---|---|
| Peer problem | |||
| Normal | 64 (50) | 51 (41) | 115 (46) |
| Borderline | 15 (12) | 26 (21) | 41 (16) |
| Abnormal | 48 (38) | 46 (37) | 94 (38) |
| Prosocial | |||
| Normal | 33 (26) | 31 (25) | 64 (26) |
| Borderline | 17 (13) | 14 (11) | 31 (12) |
| Abnormal | 77 (61) | 78 (63) | 155 (62) |
| Emotional | |||
| Normal | 83 (65) | 71 (58) | 154 (62) |
| Borderline | 13 (10) | 20 (16) | 33 (13) |
| Abnormal | 31 (24) | 32 (26) | 63 (25) |
| Conduct | |||
| Normal | 82 (65) | 74 (60) | 156 (62) |
| Borderline | 19 (15) | 15 (12) | 34 (14) |
| Abnormal | 26 (20) | 34 (28) | 60 (24) |
| Hyperactivity | |||
| Normal | 54 (43) | 57 (46) | 111 (44) |
| Borderline | 13 (10) | 17 (14) | 30 (12) |
| Abnormal | 60 (47) | 49 (40) | 109 (44) |
| Variable | Intervention arm (N = 127), n (%) | Control arm (N = 123), n (%) | All (N = 250), n (%) |
|---|---|---|---|
| Mobility (walking about) | |||
| No problems | 104 (82) | 103 (84) | 207 (83) |
| Some problems | 19 (15) | 17 (14) | 36 (14) |
| A lot of problems | 4 (3) | 2 (2) | 6 (2) |
| Missing data | 0 (0) | 1 (1) | 1 (0) |
| Looking after oneself | |||
| No problems | 37 (29) | 35 (28) | 72 (29) |
| Some problems | 63 (50) | 67 (54) | 130 (52) |
| A lot of problems | 27 (21) | 20 (16) | 47 (19) |
| Missing data | 0 (0) | 1 (1) | 1 (0) |
| Doing usual activities (e.g. school, hobbies, things with family or friends) | |||
| No problems | 51 (40) | 48 (39) | 99 (40) |
| Some problems | 67 (53) | 65 (53) | 132 (53) |
| A lot of problems | 9 (7) | 9 (7) | 18 (7) |
| Missing data | 0 (0) | 1 (1) | 1 (0) |
| Having pain or discomfort | |||
| No problems | 100 (79) | 87 (71) | 187 (75) |
| Some problems | 24 (19) | 33 (27) | 57 (23) |
| A lot of problems | 2 (2) | 2 (2) | 4 (2) |
| Missing data | 1 (1) | 1 (1) | 2 (1) |
| Feeling worried, sad or unhappy | |||
| No problems | 57 (45) | 30 (24) | 87 (35) |
| Some problems | 56 (44) | 74 (60) | 130 (52) |
| A lot of problems | 14 (11) | 18 (15) | 32 (13) |
| Missing data | 0 (0) | 1 (1) | 1 (0) |
Numbers analysed
Among the 250 CYP, scores from the social skills subscale of the SSIS (teacher) were available for 246 CYP at baseline (intervention arm, n = 126; control arm, n = 120) and 220 CYP at 20 weeks (intervention arm, n = 117; control arm, n = 103) (see Figure 1). However, three of those CYP for whom this score was available at 20 weeks did not have corresponding baseline data. Other baseline covariates (e.g. age, gender and school-level stratification variables) were available for all participants. Therefore, the ITT analysis population was 217 (intervention arm, n = 116; control arm, n = 101). This reflects the attrition rate of 12% for the primary outcome at 20 weeks. Social skills scores were missing because of loss to follow-up or item non-response, with four or more of the 46 items missing or invalid (see Figure 1).
Outcomes and estimation
Outcomes
The primary outcome was the social and emotional competence of the child or young person with an ASD diagnosis in a mainstream school setting measured 20 weeks post randomisation using the teacher-completed social skills subscale of the SSIS. The SSIS (teacher) completed at 52 weeks was a secondary outcome. Further secondary outcomes and measures are summarised in Table 9.
| Outcome | Measure | Completed by | Follow-up time point | |||
|---|---|---|---|---|---|---|
| Teacher | Parent | Child or young person | 20 weeks | 52 weeks | ||
| 1. Social and emotional competence | SSIS social skills subscale | ✓ | ✓ | ✓ | ||
| 2. Social isolation and loneliness | ALS | ✓ | ✓ | ✓ | ||
| MSPSS | ✓ | ✓ | ✓ | |||
| 3. Emotional and behavioural symptoms | SDQ | ✓ | ✓ | ✓ | ✓ | |
| SSIS problem behaviours subscale | ✓ | ✓ | ✓ | ✓ | ||
| 4. Academic competence | SSIS academic competence subscale | ✓ | ✓ | ✓ | ||
| 5. SSIS dimensions | Assertion subscale | ✓ | ✓ | ✓ | ✓ | |
| Self-control subscale | ✓ | ✓ | ✓ | ✓ | ||
| Internalising subscale | ✓ | ✓ | ✓ | ✓ | ||
| Externalising subscale | ✓ | ✓ | ✓ | ✓ | ||
Estimation
Method
The primary analysis of the primary outcome used a multilevel mixed-effects model with robust standard errors and accounting for clustering within school controlling for age, gender, baseline SSIS social skills subscale score, school (random effect) and the stratifying variables: number of autistic CYP in the school and school stage (primary or secondary). The primary analysis used complete cases in the ITT population. Statistical analyses used Stata® MP 16.0 (StataCorp LP, College Station, TX, USA)81 and all hypothesis tests were two-sided at the 5% level. Secondary outcome analysis used a similar approach. Secondary analyses adjusted for the baseline measure of the secondary outcome and the same additional covariates as the primary outcome. Subgroup analyses adjusted for covariates and baseline factors in a similar way, with the exception of adjusting by age if the subgroup was age related.
Summary of findings
The ITT analysis of the primary outcome is of borderline statistical significance (p = 0.06) at the pre-specified 5% level. However, the pre-planned sensitivity analysis of the primary outcome provides consistent evidence of LEGO® based therapy groups having a modest positive effect (standardised effect sizes between 0.15 and 0.21) on participant social and emotional skills measured using the social skills subscale of the SSIS at 20 weeks (see Table 12).
Only 3 out of the 31 pre-specified secondary outcomes were significant at the 5% level, and there is a high probability that these were observed by chance. However, the majority of the secondary outcome effect sizes favoured the intervention arm, which corroborates the modest impact observed for the primary outcome. Figures 2 and 3 present the outcomes observed at 20 and 52 weeks, respectively.
FIGURE 2.
Secondary outcomes at 20 weeks.
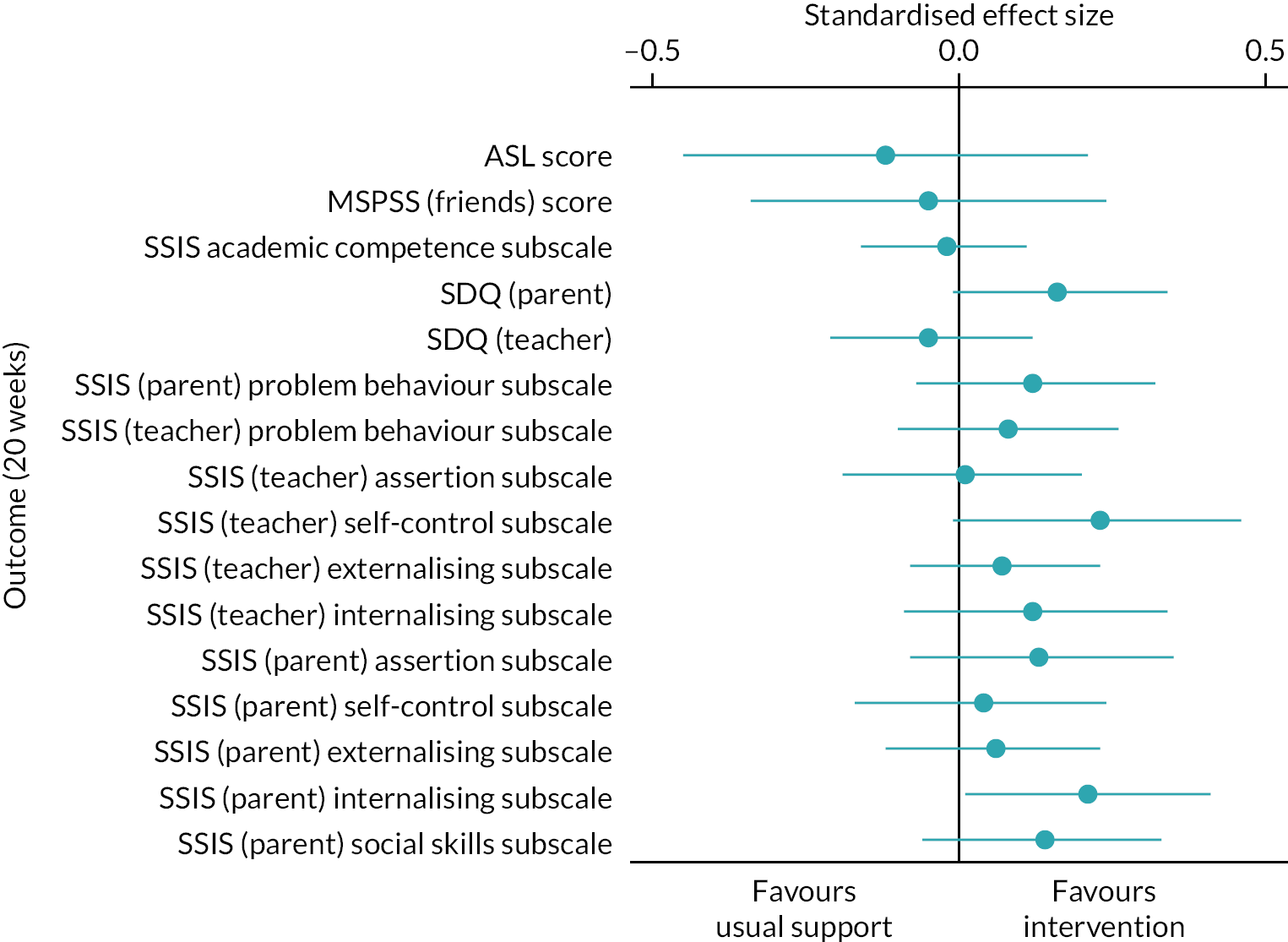
FIGURE 3.
Secondary outcomes at 52 weeks.
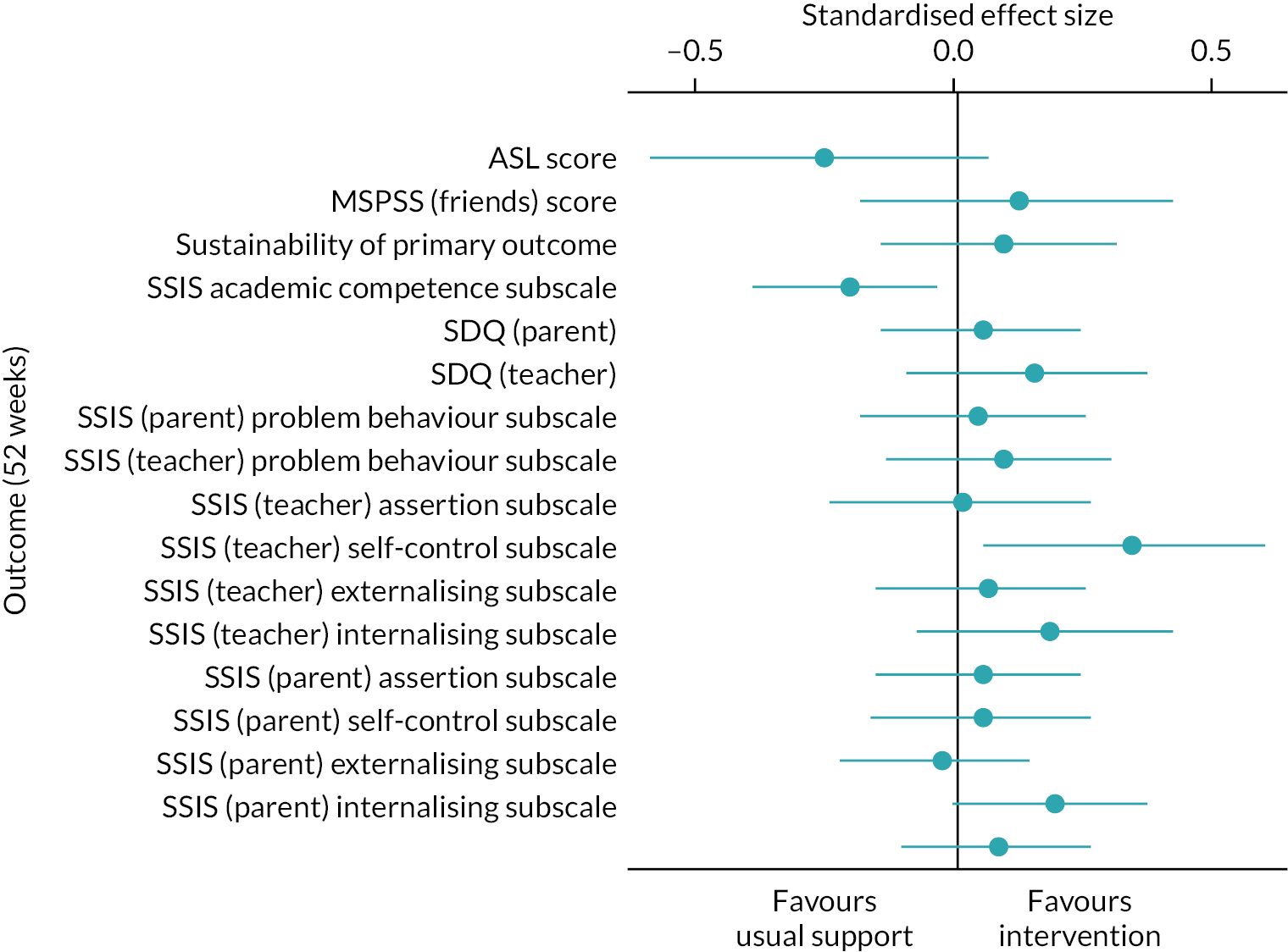
A large number of unplanned additional analyses were conducted to explore the potential for observational analysis of the trial data in a subsequent substudy. These analyses show that the effect size is potentially greater for certain subgroups and when there is a critical mass of autistic participants in therapy groups. These additional analyses are not intended to be confirmatory and are not used in drawing the overall conclusion of a modest borderline significant effect.
Primary outcome analysis
The primary analysis comparing LEGO® based therapy groups (i.e. the intervention arm) with usual support (i.e. the control arm) found a more modest, borderline statistically significant, increase in the social and emotional competence of CYP with an ASD diagnosis in the intervention arm than in the control arm. The difference between arms was 3.74 (95% CI –0.16 to 7.63; p = 0.06) (Table 10). In the original sample size calculation, a standardised effect size of 0.47 of a SD was the minimum clinically important difference (MCID). At baseline, the pooled SD for the primary outcome was 20.4 (Table 11). Hence, the rescaled MCID was 9.6 and the observed difference was smaller than this. Changes in primary outcome are detailed in Appendix 2, Table 37.
| Time point | Summary statistics | Model-based estimates | ICC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention arm (N = 127) | Control arm (N = 123) | Mean difference (95% CI) | |||||||||
| n (%) | Mean | SD | n (%) | Mean | SD | Adjusted mean difference (95% CI) | p-value | Controlling for covariates | Unconditional | ||
| Baseline | 126 (99) | 66.12 | 19.32 | 120 (98) | 64.90 | 21.45 | 1.22 (–3.88 to 6.32) | ||||
| 20 weeks | 117 (92) | 72.93 | 18.81 | 103 (84) | 69.78 | 20.21 | 3.15 (–2.01 to 8.31) | ||||
| Overall | 3.74 (–0.16 to 7.63) | 0.06 | 0.00 | 0.10 | |||||||
| Time point | Intervention arm (N = 127) | Control arm (N = 123) | All (N = 250) |
|---|---|---|---|
| Baseline | |||
| n (%) | 126 (99) | 120 (98) | 246 (98) |
| Mean (SD) | 66.1 (19.3) | 64.9 (21.4) | 65.5 (20.4) |
| Median (IQR) | 64 (50–79) | 66 (48–77) | 65 (50–79) |
| Minimum, maximum | 29, 117 | 26, 129 | 26, 129 |
| 20 weeks | |||
| n (%) | 117 (92) | 103 (84) | 220 (88) |
| Mean (SD) | 72.9 (18.8) | 69.8 (20.2) | 71.5 (19.5) |
| Median (IQR) | 74 (60–85) | 68 (57–79) | 71 (59–82) |
| Minimum, maximum | 35, 120 | 22, 133 | 22, 133 |
We had pre-specified a number of sensitivity analyses (Table 12), including an as-treated analysis to allow for the misallocated school. For the per-protocol analysis (six or more sessions attended) the p-value reduced to 0.036 and the difference between arms increased to 4.23 (95% CI 0.27 to 8.19). A second, post hoc per-protocol analysis (with two or more CYP with an ASD diagnosis in each session on average) also had a reduced p-value (p = 0.038) and an increase in the difference between arms to 4.56 (95% CI 0.24 to 8.87). For the as-treated analysis the p-value was further reduced to 0.026 and the adjusted mean difference again increased; however, the range of values included in the 95% CI remained below the MCID (difference between arms 4.37, 95% CI 0.53 to 8.20). Both per-protocol analysis models showed a statistically significant positive effect. The CACE analysis associated with the first per-protocol analysis reduced the difference between arms to 3.97, with a larger p-value than in the per-protocol analysis (95% CI –0.11 to 8.06; p = 0.056). The CACE analysis associated with the second, post hoc per-protocol analysis increased the difference between arms to 5.68, again with a larger p-value than in the per-protocol analysis but with a CI that includes the MCID (95% CI –0.18 to 11.54; p = 0.058). However, the analysis when using MI to allow for missing data at baseline and 20 weeks diminished the signal somewhat. The MI was undertaken in Stata MP 16.0 to test the robustness of findings for missing values of the primary outcome measure at baseline (n = 1 owing to missing survey items) and 20 weeks (n = 32 owing to withdrawal, non-response or missing survey items). There were no missing data for other baseline covariates. A total of 100 MI data sets were generated using chained equations, assuming missing at random and a multivariate normal distribution. Logistic modelling found no other covariates that were predictive of missing status. School random effects were included in the estimation stage of the MI procedure. Overall, there is consistent borderline statistically significant evidence that LEGO® based therapy groups (i.e. the intervention arm) had a modest positive effect on the social skills subscale of the SSIS at 20 weeks (see Table 12). The average causal response (ACR) analysis, as specified in the SAP, is omitted because the distribution of the number of sessions attended was negatively skewed and this violates the assumption required for an ACR analysis.
| Sensitivity analysis | n | Adjusted mean difference (95% CI) | p-value | Effect size |
|---|---|---|---|---|
| Pre-specified | ||||
| ITT | 217 | 3.74 (–0.16 to 7.63) | 0.060 | 0.18 |
| Sensitivity to misallocated school | 217 | 4.37 (0.53 to 8.20) | 0.026 | 0.21 |
| Sensitivity to teacher providing score | 184 | 3.92 (0.02 to 7.81) | 0.049 | 0.19 |
| Per protocol | 207 | 4.23 (0.27 to 8.19) | 0.036 | 0.21 |
| CACE | 217 | 3.97 (–0.11 to 8.06) | 0.056 | 0.19 |
| MI | 250 | 3.10 (–0.74 to 6.95) | 0.110 | 0.15 |
| Post hoc | ||||
| Per protocol 2 | 175 | 4.56 (0.24 to 8.87) | 0.038 | 0.22 |
| CACE 2 | 217 | 5.68 (–0.18 to 11.54) | 0.058 | 0.28 |
Secondary outcome analysis
Table 9 summarises the five secondary statistical objectives covered in the SAP; these include 33 associated end points (see Chapter 1, Research question, aims and objectives, and Chapter 2, Main trial methods, Important changes to methods after trial commencement). Tables 13 and 14 show full results and summary results, respectively. The health economic analysis and qualitative and fidelity results address the trial’s three additional secondary objectives. Secondary outcomes tended to have slightly higher rates of missing data than the primary outcome, so the analysis lacked some information. However, with respect to the secondary objectives, 2 of the 33 end points showed a significant difference between arms in favour of the intervention arm at the 5% level and four more showed a significant at the 10% level, although these small numbers of significant differences between arms may have occurred by chance. More generally, most of the 33 secondary end points showed a difference between arms in favour of the intervention arm and, although few were statistically significant, this consistent trend strengthens the evidence to support the hypothesis that LEGO® based therapy groups have some beneficial effects. A summary of secondary outcomes by arm is given in Appendix 2, Table 38. Overall data completeness is detailed in Appendix 2, Table 39.
| Secondary outcomes | Adjusted mean difference (95% CI) | p-value | Standardised effect size |
|---|---|---|---|
| Perceived social isolation and loneliness | |||
| ALS score, points | |||
| 20 weeks | –1.31 (–4.89 to 2.27) | 0.47 | –0.12 |
| 52 weeks | –2.83 (–6.39 to 0.74) | 0.12 | –0.26 |
| MSPSS (friends) subscale score, points | |||
| 20 weeks | –0.06 (–0.44 to 0.31) | 0.74 | –0.05 |
| 52 weeks | 0.16 (–0.23 to 0.56) | 0.41 | 0.12 |
| Sustainability of effect on social skills | |||
| SSIS (teacher) social skills subscale score, points | |||
| 52 weeks | 1.68 (–2.51 to 5.87) | 0.43 | 0.09 |
| Academic competence | |||
| SSIS academic competence subscale score, points | |||
| 20 weeks | –0.17 (–1.17 to 0.84) | 0.75 | –0.02 |
| 52 weeks | –1.57 (–2.91 to –0.24) | 0.02 | –0.21 |
| Emotional and behavioural symptoms | |||
| SDQ (parent) score, points | |||
| 20 weeks | –0.93 (–1.95 to 0.08) | 0.07 | –0.16 |
| 52 weeks | –0.30 (–1.43 to 0.84) | 0.61 | –0.05 |
| SDQ (teacher) score, points | |||
| 20 weeks | 0.30 (–0.72 to 1.32) | 0.57 | 0.05 |
| 52 weeks | –0.91 (–2.37 to 0.55) | 0.22 | –0.15 |
| SSIS (parent) problem behaviour subscale score, points | |||
| 20 weeks | –1.75 (–4.50 to 1.00) | 0.21 | –0.12 |
| 52 weeks | –0.59 (–3.72 to 2.55) | 0.71 | –0.04 |
| SSIS (teacher) problem behaviour subscale score, points | |||
| 20 weeks | –1.08 (–3.48 to 1.32) | 0.38 | –0.08 |
| 52 weeks | –1.19 (–4.09 to 1.71) | 0.42 | –0.09 |
| Assertion to self-control to externalising and internalising | |||
| SSIS (teacher) assertion subscale score, points | |||
| 20 weeks | 0.02 (–0.76 to 0.80) | 0.95 | 0.01 |
| 52 weeks | 0.05 (–0.96 to 1.05) | 0.93 | 0.01 |
| SSIS (teacher) self-control subscale score, points | |||
| 20 weeks | 0.94 (–0.04 to 1.92) | 0.06 | 0.23 |
| 52 weeks | 1.40 (0.25 to 2.54) | 0.02 | 0.34 |
| SSIS (teacher) externalising subscale score, points | |||
| 20 weeks | –0.50 (–1.54 to 0.54) | 0.35 | –0.07 |
| 52 weeks | –0.38 (–1.74 to 0.99) | 0.59 | –0.06 |
| SSIS (teacher) internalising subscale score, points | |||
| 20 weeks | –0.47 (–1.28 to 0.34) | 0.26 | –0.12 |
| 52 weeks | –0.68 (–1.64 to 0.28) | 0.16 | –0.18 |
| SSIS (parent) assertion subscale score, points | |||
| 20 weeks | 0.53 (–0.30 to 1.37) | 0.21 | 0.13 |
| 52 weeks | 0.19 (–0.61 to 0.99) | 0.64 | 0.05 |
| SSIS (parent) self-control subscale score, points | |||
| 20 weeks | 0.13 (–0.58 to 0.85) | 0.72 | 0.04 |
| 52 weeks | 0.19 (–0.57 to 0.94) | 0.63 | 0.05 |
| SSIS (parent) externalising subscale score, points | |||
| 20 weeks | –0.35 (–1.46 to 0.76) | 0.54 | –0.06 |
| 52 weeks | 0.21 (–0.97 to 1.39) | 0.73 | 0.03 |
| SSIS (parent) internalising subscale score, points | |||
| 20 weeks | –1.23 (–2.40 to –0.05) | 0.04 | –0.21 |
| 52 weeks | –1.11 (–2.23 to 0.02) | 0.05 | –0.19 |
| Additional outcome | |||
| SSIS (parent) social skills subscale score, points | |||
| 20 weeks | 2.54 (–1.03 to 6.11) | 0.16 | 0.14 |
| 52 weeks | 1.55 (–1.77 to 4.86) | 0.36 | 0.08 |
| Outcome | Measure | Respondent and follow-up time point | |||||
|---|---|---|---|---|---|---|---|
| Teacher | Parent | Child or young person | |||||
| 20weeks | 52 weeks | 20 weeks | 52 weeks | 20 weeks | 52 weeks | ||
| 1. Social and emotional competence | SSIS social skills subscale (higher scores are better) | ++ | + | ++ | + | ||
| 2. Social isolation and loneliness | ALS (higher scores are better) | – | –– | ||||
| MSPSS (higher scores are better) | – | + | |||||
| 3. Emotional and behavioural symptoms | SDQ (lower scores are better) | – | + | ++ | + | ||
| SSIS problem behaviours subscale (lower scores are better) | + | + | ++ | + | |||
| 4. Academic competence | SSIS academic competence subscale (higher scores are better) | – | –– | ||||
| 5. SSIS dimensions | Assertiona (higher scores are better) | + | + | + | + | ||
| Self-controla (higher scores are better) | + | ++ | + | + | |||
| Externalisingb (lower scores are better) | + | + | + | – | |||
| Internalisingb (lower scores are better) | + | + | + | + | |||
The first secondary objective (i.e. to examine the clinical effectiveness of LEGO® based therapy groups on the perceived social isolation of autistic CYP in schools, compared with usual support for autistic CYP, at 20 and 52 weeks) was assessed using the ALS and the MSPSS friends and significant other subscales. CYP in the intervention arm had slightly lower (i.e. worse) ALS scores than those in the control arm, and the gap widened at 52 weeks, although neither gap was significant: at 20 weeks, –1.31 points (95% CI –4.89 to 2.27 points; p = 0.47); at 52 weeks, –2.83 points (95% CI –6.39 to 0.74 points; p = 0.12). For the MSPSS, between-arm differences marginally favoured usual support at 20 weeks and the intervention at 52 weeks, but both differences were very small: at 20 weeks, –0.06 points (95% CI –0.44 to 0.31 points; p = 0.74); at 52 weeks, 0.16 points (95% CI –0.23 to 0.56 points; p = 0.41).
The sixth secondary objective [i.e. to determine if the impact of LEGO® based therapy is sustainable into the next academic year by comparing effectiveness on social and emotional competence (specifically perceived social skills) at 52 weeks after randomisation] was assessed using the SSIS social skills subscale as completed by the associated teacher at 20 weeks and 52 weeks after randomisation. At 20 weeks, the difference between arms was 3.74 points (95% CI –0.16 to 7.63 points; p = 0.06) (see Table 12) and at 52 weeks the difference was 1.68 points (95% CI –2.51 to 5.87 points; p = 0.43). Compared with the primary outcome analysis, which showed a borderline statistically significant effect in favour of the intervention, the effect at 52 weeks was diminished. There is no evidence that the modest effect of the intervention is sustained.
The second secondary objective (i.e. to examine the clinical effectiveness of LEGO® based therapy groups on the academic competence of autistic CYP in schools, compared with usual support for autistic CYP, at 20 and 52 weeks) was assessed using the SSIS academic competence scale as completed by the associated teacher at 20 weeks (–0.17 points, 95% CI –1.17 to 0.84 points; p = 0.75) and 52 weeks (–1.57 points, 95% CI –2.91 to –0.24 points; p = 0.02). Although the effect at 52 weeks was statistically significant and in favour of usual support, the effect at 20 weeks was very small and not statistically significant.
The fifth secondary objective (i.e. to examine the emotional and behavioural symptoms in those receiving LEGO® based therapy, compared with usual support for autistic CYP, at 20 and 52 weeks) was assessed using four completed components of two research instruments: first, the SDQ completed by the associated teacher at 20 and 52 weeks and by the parent/guardian at 20 and 52 weeks; second, the SSIS problem behaviours subscale completed by the associated teacher at 20 and 52 weeks and by the parent/guardian at 20 and 52 weeks. There is a borderline statistically significant effect seen for the SDQ completed by the parent/guardian at 20 weeks. Although this is reduced at 52 weeks and no longer borderline significant, the general trend favours the intervention arm. (For the medications being taken at baseline, see Appendix 2, Tables 40 and 41.)
The third secondary objective (i.e. to examine the clinical effectiveness of LEGO® based therapy groups on assertion, social control, externalising and internalising in autistic CYP in the school setting, compared with usual support for autistic CYP, at 20 and 52 weeks) was assessed using the SSIS assertion, self-control, internalising and externalising subscales completed by the associated teacher and the parent/guardian at 20 and 52 weeks. There are eight pairs of measurements assessed in this objective; for two of the pairs, the SSIS (teacher) self-control and SSIS (parent) internalising subscales, the effects, which are similar in magnitude, were borderline statistically significant. Again, considering all eight pairs, the general trend favours the intervention arm.
Additional analysis of primary outcome
Planned analysis
We undertook a pre-planned subgroup analysis to explore the moderating effects of symptom severity. This used the SCQ, a screening instrument administered at baseline only, as a proxy for severity, with higher scores representing more severe symptomatology. The estimated effect was greater for participants considered to have the less severe symptoms, that is, those in the bottom third of the SCQ distribution, and was lower for those in the middle third and lower again for those in the highest third (see subgroup analysis for primary outcome in Table 15). However, when an interaction term between subgroups and intervention was added to the primary outcome model for the ITT population, it was borderline statistically significant (p = 0.08).
| Subgroup analysis | n | Adjusted mean difference (95% CI) | p-value | Subgroup effect | Interpretation |
|---|---|---|---|---|---|
| Primary outcome | |||||
| SCQ score, points | |||||
| Low (15–22) | 78 | 8.32 (2.56 to 14.09) | 0.005 | Higher for less severe | |
| Medium (23–7) | 73 | 3.65 (–2.50 to 9.80) | 0.245 | ||
| High (28–37) | 66 | –2.64 (–9.71 to 4.43) | 0.465 | ||
| Subgroup interaction | 0.08 | ||||
| Selected secondary outcomes, points | |||||
| SDQ peer problems subscale score at 20 weeks (lower scores are better) | |||||
| Low | 82 | –0.34 (–1.01 to 0.33) | 0.317 | Lower for less severe | Supports subgroup analysis |
| Medium | 75 | 0.58 (–0.11 to 1.27) | 0.099 | ||
| High | 67 | 0.62 (–0.21 to 1.44) | 0.141 | ||
| SDQ prosocial subscale score at 20 weeks (higher scores are better) | |||||
| Low | 81 | 0.19 (–0.80 to 1.18) | 0.704 | Higher for less severe | Supports subgroup analysis |
| Medium | 75 | –0.25 (–1.12 to 0.62) | 0.571 | ||
| High | 67 | –0.31 (–1.33 to 0.71) | 0.557 | ||
| ALS score at 20 weeks (higher scores are better) | |||||
| Low | 56 | –3.12 (–6.80 to 0.57) | 0.098 | Lower for less severe | |
| Medium | 47 | –5.57 (–11.42 to 0.29) | 0.062 | ||
| High | 46 | 4.93 (–3.09 to 12.94) | 0.228 | ||
| MSPSS significant other subscale score at 20 weeks (higher scores are better) | |||||
| Low | 66 | 0.05 (–0.44 to 0.54) | 0.838 | No clear trend | |
| Medium | 59 | –0.22 (–1.00 to 0.55) | 0.572 | ||
| High | 51 | –0.08 (–0.74 to 0.58) | 0.817 | ||
| SSIS (parent) social subscale score at 20 weeks (higher scores are better) | |||||
| Low | 71 | 3.64 (–1.59 to 8.87) | 0.172 | No clear trend | |
| Medium | 63 | –0.20 (–5.92 to 5.53) | 0.946 | ||
| High | 57 | 5.11 (–2.15 to 12.37) | 0.168 | ||
| SSIS (teacher) social subscale score at 52 weeks (higher scores are better) | |||||
| Low | 68 | 2.24 (–4.73 to 9.20) | 0.529 | Higher for less severe | Supports subgroup analysis |
| Medium | 70 | 2.05 (–4.84 to 8.95) | 0.560 | ||
| High | 58 | –0.18 (–7.30 to 6.95) | 0.962 | ||
Post hoc analysis
To further explore the possible moderating effect of SCQ subgroups, we undertook an unplanned analysis, looking at the moderating effect of SCQ subgroups for six secondary outcomes. The six secondary outcomes were as follows: the SDQ peer problems and prosocial subscales, ALS score, MSPSS score and parent-reported social score (all at 20 weeks) as well as the teacher-reported social score at 52 weeks. These six secondary outcomes were selected by clinical members of the team before they had knowledge of the results of the secondary outcome analysis. This unplanned follow-up subgroup analysis found no clear pattern (see subgroup analysis for selected secondary outcomes in Table 15). Therefore, we conclude that the pattern found for the subgroup analysis using the primary end point may have occurred by chance and there is no convincing evidence of a differential effect for CYP with different levels of ASD symptomatology. This could be a subject for future research.
To investigate a tentative finding in the health economic analysis that the intervention led to less frequent use of CAMHS, we undertook an additional unplanned analysis looking at changes in the SDQ subscales and found no statistically significant difference between arms for any of the five subscales (Table 16).
| Time point | Intervention arm | Control arm | Mean difference (95% CI) | Adjusted mean difference (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean | SD | n (%) | Mean | SD | ||||
| Peer problem subscale (lower scores are better) | |||||||||
| Baseline | 127 (100) | 3.64 | 2.28 | 123 (100) | 3.98 | 2.18 | –0.35 (–0.90 to 0.20) | ||
| 20 weeks | 119 (94) | 3.66 | 2.29 | 105 (85) | 3.54 | 2.00 | 0.12 (–0.45 to 0.69) | 0.27 (–0.16 to 0.71) | 0.22 |
| 52 weeks | 109 (86) | 3.29 | 2.12 | 93 (76) | 3.70 | 2.20 | –0.41 (–1.01 to 0.19) | –0.27 (–0.74 to 0.19) | 0.25 |
| Prosocial subscale (higher scores are better) | |||||||||
| Baseline | 127 (100) | 4.11 | 2.36 | 123 (100) | 3.96 | 2.52 | 0.15 (–0.46 to 0.76) | ||
| 20 weeks | 118 (93) | 4.65 | 2.31 | 105 (85) | 4.66 | 2.52 | –0.00 (–0.63 to 0.63) | 0.00 (–0.48 to 0.48) | 1.00 |
| 52 weeks | 109 (86) | 5.10 | 2.37 | 93 (76) | 4.59 | 2.39 | 0.51 (–0.15 to 1.17) | 0.34 (–0.24 to 0.92) | 0.25 |
| Emotional subscale (lower scores are better) | |||||||||
| Baseline | 127 (100) | 3.86 | 2.52 | 123 (100) | 3.81 | 2.58 | 0.05 (–0.58 to 0.68) | ||
| 20 weeks | 119 (94) | 3.75 | 2.24 | 105 (85) | 3.76 | 2.39 | –0.01 (–0.62 to 0.60) | 0.03 (–0.44 to 0.51) | 0.89 |
| 52 weeks | 109 (86) | 3.54 | 2.63 | 93 (76) | 3.99 | 2.37 | –0.45 (–1.14 to 0.24) | –0.46 (–1.04 to 0.13) | 0.12 |
| Conduct subscale (lower scores are better) | |||||||||
| Baseline | 127 (100) | 1.98 | 2.18 | 123 (100) | 2.16 | 2.34 | –0.19 (–0.75 to 0.37) | ||
| 20 weeks | 119 (94) | 1.73 | 2.03 | 105 (85) | 1.89 | 1.92 | –0.15 (–0.67 to 0.37) | –0.11 (–0.42 to 0.20) | 0.49 |
| 52 weeks | 109 (86) | 1.73 | 2.21 | 93 (76) | 2.06 | 2.17 | –0.33 (–0.94 to 0.28) | –0.15 (–0.57 to 0.28) | 0.50 |
| Hyperactivity subscale (lower scores are better) | |||||||||
| Baseline | 127 (100) | 6.27 | 2.70 | 123 (100) | 5.80 | 2.89 | 0.46 (–0.23 to 1.15) | ||
| 20 weeks | 119 (94) | 5.77 | 2.51 | 105 (85) | 5.38 | 2.69 | 0.39 (–0.29 to 1.07) | 0.03 (–0.40 to 0.47) | 0.88 |
| 52 weeks | 109 (86) | 5.50 | 2.82 | 93 (76) | 5.48 | 2.81 | 0.02 (–0.76 to 0.80) | –0.08 (–0.66 to 0.50) | 0.79 |
Randomisation used stratification by stage of schooling (i.e. primary or secondary) and number of autistic CYP in the school. The additional unplanned exploratory analysis suggested an association between intervention and each of the stratifying variables (Tables 17 and 18). The effect was greater for secondary school CYP (7.45 points, 95 CI –0.93 to 15.83 points; p = 0.081) than for primary school CYP (1.86, 95% CI –2.08 to 5.80 points; p = 0.355) (see Table 17) and the effect was greater for schools with six or more autistic CYP (8.23 points, 95% CI 1.79 to 14.68 points; p = 0.012) than for schools with fewer than six autistic CYP (1.45 points, 95% CI –2.95 to 5.85 points; p = 0.519) (see Table 18). Follow-up subgroup analysis of these six selected secondary outcomes found no clear pattern in the association between the respective secondary outcomes and the number of autistic CYP in the school as the analysis of only three of the six secondary outcomes supported the hypothesis of greater effectiveness with higher numbers of autistic CYP (see Table 18). However, a similar analysis of these six selected secondary outcomes did find a pattern in the association between the respective secondary outcomes and level of schooling (secondary, primary) as five of the six secondary outcomes supported the hypothesis of greater effectiveness in secondary schools compared with primary schools (see Table 17). Therefore, we conclude that there is suggestive evidence that the intervention may be more effective in secondary schools, with potential for the impact to be clinically important.
| Subgroup analysis | n | Adjusted mean difference (95% CI) | p-value | Subgroup effect | Interpretation |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Primary school | 153 | 1.86 (–2.08 to 5.80) | 0.355 | Secondary schools higher | |
| Secondary school | 64 | 7.45 (–0.93 to 15.83) | 0.081 | ||
| Subgroup interaction | 0.20 | ||||
| Selected secondary outcomes, points | |||||
| SDQ peer problems subscale score at 20 weeks (lower scores are better) | |||||
| Primary school | 159 | 0.13 (–0.40 to 0.66) | 0.627 | Secondary schools higher | |
| Secondary school | 65 | 0.61 (–0.22 to 1.44) | 0.153 | ||
| SDQ prosocial subscale score at 20 weeks (higher scores are better) | |||||
| Primary school | 158 | –0.03 (–0.61 to 0.55) | 0.915 | Secondary schools higher | Supports subgroup analysis |
| Secondary school | 65 | 0.18 (–0.68 to 1.05) | 0.677 | ||
| ALS score at 20 weeks (higher scores are better) | |||||
| Primary school | 100 | –0.49 (–5.47 to 4.49) | 0.847 | Secondary schools higher | Supports subgroup analysis |
| Secondary school | 49 | 0.12 (–5.55 to 5.79) | 0.967 | ||
| MSPSS significant other subscale score at 20 weeks (higher scores are better) | |||||
| Primary school | 117 | –0.20 (–0.72 to 0.33) | 0.459 | Secondary schools higher | Supports subgroup analysis |
| Secondary school | 59 | 0.00 (–0.47 to 0.47) | 0.998 | ||
| SSIS (parent) social subscale score at 20 weeks (higher scores are better) | |||||
| Primary school | 135 | –0.08 (–4.11 to 3.95) | 0.970 | Secondary schools higher | Supports subgroup analysis |
| Secondary school | 56 | 10.46 (5.88 to 15.03) | 0.000 | ||
| SSIS (teacher) social subscale score at 52 weeks (higher scores are better) | |||||
| Primary school | 136 | 0.16 (–5.17 to 5.49) | 0.953 | Secondary schools higher | Supports subgroup analysis |
| Secondary school | 60 | 5.09 (–1.78 to 11.95) | 0.147 | ||
| Subgroup analysis | n | Adjusted mean difference (95% CI) | p-value | Subgroup effect | Interpretation |
|---|---|---|---|---|---|
| Primary outcome | |||||
| < 6 autistic pupils | 156 | 2.30 (–2.10 to 6.70) | 0.306 | Higher if ≥ 6 pupils | |
| ≥ 6 autistic pupils | 61 | 8.23 (1.79 to 14.68) | 0.012 | ||
| Subgroup interaction | 0.11 | ||||
| Subgroup analysis for selected secondary outcomes, points | |||||
| SDQ peer problems subscale score at 20 weeks (lower scores better) | |||||
| < 6 autistic pupils | 162 | 0.23 (–0.30 to 0.76) | 0.397 | Higher if ≥ 6 pupils | |
| ≥ 6 autistic pupils | 62 | 0.45 (–0.25 to 1.16) | 0.206 | ||
| SDQ prosocial subscale score at 20 weeks (higher scores better) | |||||
| < 6 autistic pupils | 161 | –0.10 (–0.67 to 0.48) | 0.740 | Higher if ≥ 6 pupils | Supports subgroup analysis |
| ≥ 6 autistic pupils | 62 | 0.33 (–0.77 to 1.42) | 0.558 | ||
| ALS score at 20 weeks (higher scores better) | |||||
| < 6 autistic pupils | 107 | –0.83 (–5.47 to 3.80) | 0.725 | Lower if < 6 pupils | |
| ≥ 6 autistic pupils | 42 | –3.29 (–6.10 to –0.47) | 0.022 | ||
| MSPSS significant other subscale score at 20 weeks (higher scores better) | |||||
| < 6 autistic pupils | 127 | –0.15 (–0.60 to 0.30) | 0.512 | Higher if ≥ 6 pupils | Supports subgroup analysis |
| ≥ 6 autistic pupils | 49 | 0.25 (–0.46 to 0.97) | 0.486 | ||
| SSIS (parent) social subscale score at 20 weeks (higher scores better) | |||||
| < 6 autistic pupils | 144 | 2.34 (–1.69 to 6.37) | 0.255 | Higher if ≥ 6 pupils | Supports subgroup analysis |
| ≥ 6 autistic pupils | 47 | 4.34 (–3.46 to 12.14) | 0.276 | ||
| SSIS (teacher) social subscale score at 52 weeks (higher scores better) | |||||
| < 6 autistic pupils | 140 | 3.65 (–1.30 to 8.61) | 0.148 | Lower if < 6 pupils | |
| ≥ 6 autistic pupils | 56 | –2.74 (–7.71 to 2.23) | 0.279 | ||
Intervention compliance
Planned analysis
Most participants met the per-protocol definition of compliance and attended six or more sessions (93%), and a large number attended ≥ 10 sessions (75%; Table 19).
| Level of schooling | Sessions attended per autistic child or young person, n (%) | |||
|---|---|---|---|---|
| 0–5 | 6–12 | 10–12 | 12 | |
| Primary | 2 (2) | 90 (98) | 74 (80) | 44 (48) |
| Secondary | 7 (18) | 31 (82) | 23 (61) | 12 (32) |
| All | 9 (7) | 121 (93) | 97 (75) | 56 (43) |
Post hoc analysis
Although not in the per-protocol definition for compliance, group make-up was recorded and it was found that 66% of secondary school sessions and 66% of primary school sessions included only one child or young person with an ASD diagnosis and other CYP joined the session to form a group of three (Table 20). In relation to group stability, the vast majority of CYP (89%) remained with the same group of CYP for all of their sessions (90%) (Table 21). We undertook an unplanned compliance analysis using participants in groups with an average of two or more autistic CYP in their sessions as the definition of compliance and this showed borderline significant evidence (p = 0.06) that larger numbers of autistic CYP in sessions were associated with a greater estimated effect (i.e. 5.7) (see Table 12). Indeed, the CI for effect for compliers had an upper bound above the MCID (i.e. 11.5). This suggests that LEGO® based therapy is more effective when more of the CYP in each session have a diagnosis of ASD, and the effectiveness in such settings has the potential to be clinically important. However, the sessions with a higher proportion of autistic CYP were mainly delivered in secondary schools (see Table 20) and the subgroup analysis in Table 17 suggests the potential for greater effectiveness in secondary schools in general. As such, it not possible to say whether the greater effect size observed in this compliance analysis is caused by the proportion of autistic CYP in the therapy groups or the secondary school settings where these higher proportions were most commonly observed.
| Variable | Primary school | Secondary school |
|---|---|---|
| Number of schools | 38 | 12 |
| Number of sessions | 574 | 166 |
| Number of CYP in session, n (%) | ||
| 1 | 2 (0) | 2 (1) |
| 2 | 66 (11) | 32 (19) |
| 3 | 506 (88) | 132 (80) |
| Number of autistic CYP in session, n (%) | ||
| 1 | 292 (51) | 30 (18) |
| 2 | 157 (27) | 96 (58) |
| 3 | 125 (22) | 40 (24) |
| CYP | n (%) |
|---|---|
| With ASD | 128 |
| Always grouped with same two children | 115 (90) |
| In sessions with three different children | 11 (9) |
| In sessions with four different children | 2 (2) |
All important harms or unintended effects in each arm
Small numbers of AEs were recorded during the trial. These were instances of emotional distress experienced by some CYP participating in the groups. All AEs recorded met the criteria detailed in the study’s protocol for possible expected AEs based on the trial population. These were reviewed by the study’s DMEC.
Two SAEs were reported in the intervention arm and one in the control arm. All SAEs were classified as isolated, mild in intensity and unrelated to the intervention (Table 22).
| Variable | Intervention arm (N = 127), n (%) | Control arm (N = 123), n (%) | All (N = 250), n (%) |
|---|---|---|---|
| Number of participants who experienced ≥ 1 AE | 1 (0.8) | 2 (1.6) | 3 (1.2) |
| Number of all SAEs (including repeated events) | 1 | 2 | 3 |
| SAEs related to intervention | |||
| Unrelated | 1 (100.0) | 2 (100.0) | 3 (100.0) |
| Frequency of SAE | |||
| Isolated | 1 (100.0) | 2 (100.0) | 3 (100.0) |
| Intensity of SAE | |||
| Mild | 1 (100.0) | 2 (100.0) | 3 (100.0) |
| Seriousness of SAE | |||
| Inpatient hospitalisation | 1 (100.0) | 2 (100.0) | 3 (100.0) |
| Outcome of SAE | |||
| Recovered | 1 (100.0) | 2 (100.0) | 3 (100.0) |
Chapter 4 Health economic results
Introduction
The primary aim of the economic evaluation was to assess the cost-effectiveness of LEGO® based therapy compared with usual support for autistic CYP from the NHS and PSS perspective. An economic evaluation from the societal perspective was included in the sensitivity analysis, which included costs from the educational system, private out-of-pocket expenses and parental productivity costs reported in the trial.
Missing data
Availability of quality-of-life and cost data
A total of 284 autistic CYP were recruited to the trial. After removing 34 ineligible CYP, 250 autistic CYP were available for analysis (LEGO® based therapy, n = 127; usual support, n = 123). The ‘complete case’ here refers to the parents/guardians of CYP who completed both EQ-5D-Y82 questionnaires and resource use questionnaires at any time point. Details of the quality-of-life and cost data available at each data collection time point are available in Appendix 3, Table 42. Quality-of-life data were fully available at any time point for 151 (62.1%) CYP, and cost data from the NHS and PSS perspective were available for 140 (57.6%) CYP. Overall, a total of 139 (55.6%) CYP had both EQ-5D-Y and resource use (from the NHS and PSS perspective) data at all three data collection time points. This sample constitutes the complete-case group, which is one of the two groups used for all the subsequent analyses. The second group (imputed-case or base-case group) is discussed in Chapter 2, Health economic methods.
Multiple imputation
To account for the data from the sample other than from the complete-case group (44.4%), missing utility scores and costs were further imputed with MI using chained equations (assuming missingness at random at each time point). The following variables were used in the imputation process to ensure best fit of the imputed results: trial arm, age, gender, site, utility scores and SCQ score at baseline. Because it would be speculative to impute missing utility at baseline, two CYP (0.8%) were excluded from the health economics study. The base-case (imputed) sample size was 248 CYP (intervention arm, n = 126; control arm, n = 123); this is the sample used for the primary cost-effectiveness analysis.
Baseline characteristics
Descriptive statistics of participating CYP’s characteristics and the additional predictors used in MI can be seen in Appendix 3, Table 43. These vary in relation to the main results, because the numbers of subjects included differed. This is based on the definitions of ‘complete case’ and ‘base case’ for the health economic analysis.
More than three-quarters of the CYP in the intervention arm and the control arm were male, and > 50% of the CYP in both arms were primary school age (7–11 years). Differences in SCQ scores at baseline were marginal across arms and samples. Overall, the baseline characteristics are consistent across samples (base case and complete case) and with the main statistical analysis.
Cost
Two types of costs were considered in this study: the cost of the intervention (obtained from the facilitators) and the cost of service use (self-reported by parents/guardians and teachers). All costs were expressed in 2018–9 Great British pounds (GBPs).
Unit costs
Individual-level resource use together with unit costs were used to calculate the total services cost for each autistic child or young person. Unit costs were obtained from published sources (i.e. NHS Reference Costs 2017–201883 and the PSSRU’s Unit Costs of Health and Social Care 2018),84 a national survey (i.e. the Childcare Survey 2018)85 and government departments (i.e. data reported by the Department for Education). 70 Appendix 3, Tables 44–52, present the summary of key unit costs used in the study.
Intervention costs
Intervention costs included both training and intervention session costs. The training cost information was obtained from the study team, and the estimated training costs were allocated to each session and each CYP who received the intervention. Information on intervention delivery costs was obtained directly from the facilitators using the self-reported questionnaires. Intervention sessions were costed on the basis of the salary of the professional involved. The total and average costs of each component that were used to deliver the LEGO® based therapy intervention can be seen in Appendix 3, Table 53. The main cost driver of training costs was trainer fees, whereas the main cost drivers of intervention delivery costs were the costs for preparation and delivery of the intervention and costs for LEGO bricks. On average, the estimated intervention cost per CYP was £96.96 (£25.29 for training and £71.67 for intervention delivery).
Service use and costs
The summarised service use per child or young person in each trial arm based on the complete cases only is presented in Appendix 3, Table 54. The difference in service use between the intervention and control arms was marginal at all three time points. At baseline, autistic CYP in the intervention arm seemed to have used slightly more NHS and education services (‘in the last 4 months’) than those in the control arm. After the intervention, such differences became smaller. In addition, CYP in the intervention arm were more likely to use fewer services at baseline. Caution is needed in interpreting these results, as these summarised service uses include various items with different unit costs.
The total costs broken down by perspective, type of service, trial arm and before and after imputation are presented in Table 23. Discounting was not applied owing to the short-term nature of the trial. The costs vary substantially from one participant to another, as illustrated by the wide 95% CIs.
| Costs | Base case, £ (95% CI) | Complete case, £ (95% CI) | ||
|---|---|---|---|---|
| Intervention arm (N = 126) | Control arm (N = 122) | Intervention arm (N = 80) | Control arm (N = 59) | |
| NHS and PSS | ||||
| Overall | 523.80 (372.40 to 675.10) | 677.60 (426.90 to 928.20) | 618.30 (428.30 to 808.20) | 751.50 (420.00 to 1083.00) |
| Community-based services | ||||
| CAMHS-related | 76.80 (39.50 to 114.20) | 232.60 (37.40 to 427.70) | 117.00 (49.80 to 184.20) | 267.40 (19.40 to 515.50) |
| Non-CAMHS-related | 115.00 (79.10 to 151.00) | 99.40 (68.80 to 129.90) | 119.70 (78.10 to 161.20) | 107.40 (66.80 to 148.10) |
| Hospital-based services | ||||
| Mental health-related | 19.60 (6.00 to 33.10) | 45.20 (11.40 to 78.90) | 18.50 (4.20 to 32.70) | 52.70 (–1.20 to 106.60) |
| Non-mental health-related | 60.00 (28.50 to 91.60) | 86.40 (37.30 to 135.60) | 78.90 (29.90 to 127.90) | 89.20 (30.90 to 147.40) |
| Medications | ||||
| Mental health-related | 195.20 (115.10 to 275.30) | 129.20 (73.80 to 184.60) | 210.80 (120.70 to 300.90) | 141.70 (75.20 to 208.10) |
| Non-mental health-related | 57.10 (17.70 to 96.50) | 84.80 (24.70 to 144.90) | 73.30 (22.40 to 124.30) | 93.10 (19.10 to 167.10) |
| Education system related | ||||
| Overall | 1203.60 (949.30 to 1457.90) | 1437.40 (1082.30 to 1792.40) | 1388.20 (989.10 to 1787.30) | 1632.80 (1041.30 to 2224.30) |
| School-based health | 164.30 (62.10 to 266.50) | 88.10 (20.00 to 156.10) | 181.90 (48.10 to 315.60) | 99.70 (13.20 to 186.20) |
| Intervention support | 711.60 (496.20 to 926.90) | 947.50 (645.20 to 1249.90) | 793.10 (458.10 to 128.10) | 1070.30 (614.60 to 1526.10) |
| General support | 327.20 (241.70 to 412.80) | 401.20 (261.90 to 540.50) | 368.40 (244.90 to 491.90) | 472.60 (236.50 to 708.80) |
| Private expenses | ||||
| Overall | 211.30 (129.20 to 293.30) | 317.20 (189.30 to 445.10) | 192.20 (104.90 to 279.50) | 328.90 (171.10 to 486.70) |
| Child care | 211.30 (129.20 to 293.30) | 317.20 (189.30 to 445.10) | 192.20 (104.90 to 279.50) | 328.90 (171.10 to 486.70) |
| Productivity | ||||
| Overall | 94.60 (57.40 to 131.80) | 113.90 (63.50 to 164.40) | 103.70 (61.80 to 145.60) | 111.40 (52.70 to 170.10) |
| Parental productivity loss | 94.60 (57.40 to 131.80) | 113.90 (63.50 to 164.40) | 103.70 (61.80 to 145.60) | 111.40 (52.70 to 170.10) |
| Total | ||||
| Overall | 2033.20 (1709.70 to 2356.70) | 2546.10 (2087.00 to 3005.30) | 2278.00 (1774.90 to 2781.10) | 2819.00 (2123.10 to 3514.90) |
In detail, the total mean service costs to the NHS providers (before imputation) were £618.30 (95% CI £428.30 to £808.20) in the intervention arm and £751.50 (95% CI £420.00 to £1083.00) in the control arm. Furthermore, the average total costs for the use of CAMHS-related community-based services, hospital-based services (both mental health and non-mental health-related) and non-mental-health-related medication were higher for the autistic CYP in the control arm than in the intervention arm. It is worth noting that some of the cost differences were likely to have been driven by the high-cost cases. For instance, the higher average total cost of CAMHS-related services in the control arm was driven by two high-cost cases, the first of which had 16 psychologist visits and the other 18 psychologist visits over the preceding 8 months. Because these high-cost cases were entirely plausible, we have decided to retain them in the data set without any adjustment.
The average total costs for the use of education services (before imputation) were £1388.20 (95% CI £989.10 to £1787.30) for the intervention arm and £1632.80 (95% CI £1041.30 to £2224.30) for the control arm. Apart from the school-based health-related services, CYP in the intervention arm used fewer education services than those in the control arm. As with the costs of CAMHS-related services, the higher average cost of school-based health-related services in the intervention arm was driven by one high-cost case, which had five education welfare officer visits per week for 36 weeks. Again, we decided to retain this in the analysis, as the scenario is plausible.
The CYP in the intervention arm also incurred fewer costs in private expenses and parental productivity losses than those in the control arm. Overall, CYP in the intervention arm incurred fewer costs across all the perspectives. This is observed in both the complete case and the base case. However, owing to the high-cost cases, the cost differences need to be interpreted with caution.
Quality of life
EuroQol-5 Dimensions-Youth
Table 24 shows the mean EQ-5D-Y utility scores between the two arms of the trial at each time point when scores were not imputed (complete case) and when scores were imputed (base case). In both arms, there was no significant change in EQ-5D-Y scores from baseline to 52 weeks. The fluctuations between baseline and 20 weeks, and between 20 weeks and 52 weeks, were small in both the base case and the complete case. After calculation using the area-under-the-curve approach, it was found that the LEGO® based therapy produced marginally higher mean QALYs (0.03 QALYs) than the usual support. Further details for the responses in each domain can be found in Appendix 3, Table 55.
| Variable | Base case, mean (95% CI) | Complete case, mean (95% CI) | ||
|---|---|---|---|---|
| Intervention arm (n = 126) | Control arm (n = 122) | Intervention arm (n = 126) | Control arm (n = 122) | |
| EQ-5D-Y | ||||
| Baseline | 0.79 (0.77 to 0.81) | 0.76 (0.74 to 0.79) | 0.79 (0.77 to 0.81) | 0.77 (0.74 to 0.79) |
| 20 weeks | 0.78 (0.76 to 0.81) | 0.76 (0.74 to 0.78) | 0.79 (0.76 to 0.81) | 0.75 (0.74 to 0.79) |
| 52 weeks | 0.79 (0.76 to 0.81) | 0.76 (0.74 to 0.79) | 0.80 (0.77 to 0.82) | 0.75 (0.73 to 0.80) |
| Total | 0.79 (0.77 to 0.80) | 0.76 (0.74 to 0.78) | 0.79 (0.77 to 0.81) | 0.76 (0.74 to 0.79) |
| CHU-9D | ||||
| Baseline | 0.83 (0.80 to 0.85) | 0.81 (0.79 to 0.84) | 0.84 (0.81 to 0.87) | 0.83 (0.80 to 0.86) |
| 20 weeks | 0.84 (0.82 to 0.86) | 0.80 (0.78 to 0.83) | 0.83 (0.80 to 0.86) | 0.78 (0.74 to 0.82) |
| 52 weeks | 0.83 (0.81 to 0.85) | 0.80 (0.77 to 0.83) | 0.81 (0.78 to 0.84) | 0.81 (0.77 to 0.85) |
| Total | 0.83 (0.82 to 0.85) | 0.80 (0.78 to 0.82) | 0.82 (0.80 to 0.85) | 0.80 (0.76 to 0.83) |
Child Health Utility-9 Dimensions
The change in mean CHU-9D utility scores between the two arms of the trial at any time point is presented in Table 24. CYP in the intervention arm had higher utility scores than those in the control arm at baseline. As in the EQ-5D-Y results, the observed changes at different time points in either arm were marginal. Such marginal differences were observed in both the complete case and the base case. Consistent with EQ-5D-Y results, LEGO® based therapy produced marginally higher (i.e. by 0.02) mean QALYs than did usual support. Further details for the responses in each domain can be found in Appendix 3, Table 56.
Economic analysis
Primary analysis
Table 25 shows the ICERs based on the base case for the primary analysis. On average, and before any adjustments, autistic CYP receiving LEGO® based therapy incurred £56.20 less in costs from the NHS and PSS perspective and gained 0.03 more QALYs (as measured by the EQ-5D-Y) than those receiving usual support.
| Trial arm | Costs (£), mean (95% CI) | QALYs, mean (95% CI) | Incremental cost, £ (95% CI) | Incremental QALY (95% CI) | ICER |
|---|---|---|---|---|---|
| Before bootstrap | |||||
| Intervention | 621.40 (471.10 to 771.70) | 0.78 (0.77 to 0.80) | –56.20 | 0.03 | Dominant |
| Control | 677.60 (426.90 to 928.20) | 0.76 (0.74 to 0.78) | |||
| After bootstrap | |||||
| Intervention | 536.10 (526.50 to 545.70) | 0.78 (0.78 to 0.78) | –250.90 (–272.70 to –228.90) | 0.009 (0.008 to 0.010) | Dominant |
| Control | 786.90 (772.90 to 801.00) | 0.77 (0.77 to 0.77) | – | – | – |
To account for the uncertainty and adjust for the imbalanced EQ-5D-Y utility scores at baseline, the estimates of incremental costs and the QALYs from regression were bootstrapped to simulate 5000 pairs of net cost and net outcomes, as recommended by NICE for health technology appraisals. 86 On average, after bootstrapping, autistic CYP receiving LEGO® based therapy incurred £250.90 (95% CI £272.70 to £228.90) less in costs and gained 0.009 (95% CI 0.008 to 0.010) more QALYs than those receiving usual support, which is equivalent to an extra 3.3 days of perfect health. The calculated ICER was below the NICE-recommended WTP threshold (i.e. £20,000–30,000 per QALY gained) for decision-making in England and Wales, indicating that LEGO® based therapy is likely to be cost-effective.
Figure 4 shows the cost-effectiveness plane for LEGO® based therapy compared with usual support based on 5000 bootstrapped estimates of incremental costs and incremental QALYs. The blue line represents the WTP threshold of £20,000 per QALY gained. The simulated estimates were largely below the threshold line and sat in the fourth quadrant, suggesting that LEGO® based therapy is likely to be cost-effective, although the incremental costs and QALYs were marginal.
FIGURE 4.
Base-case cost-effectiveness plane of LEGO® based therapy compared with usual support (outcome measure: QALY as measured by the EQ-5D-Y; NHS and PSS perspective).
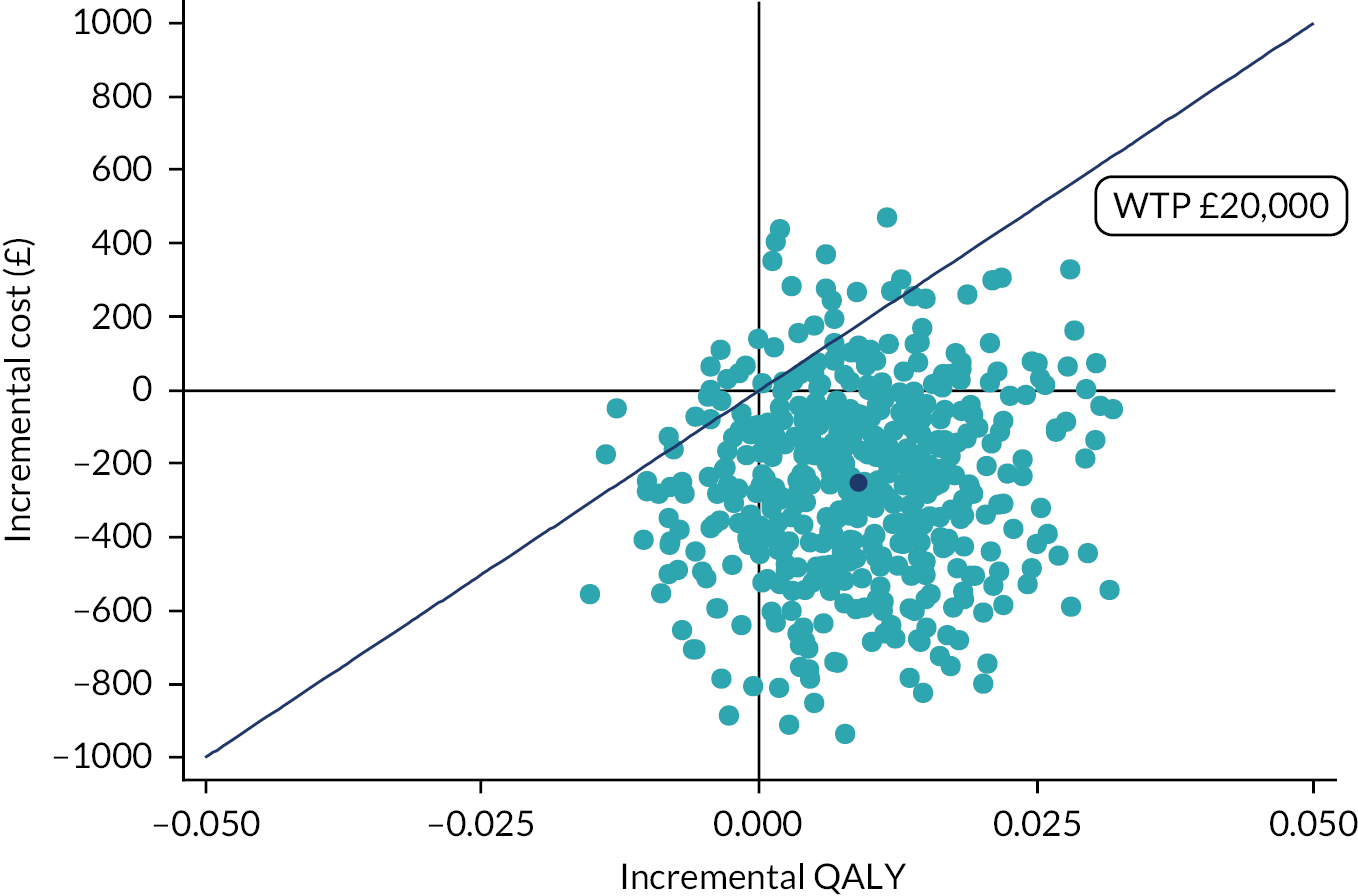
The CEAC of LEGO® based therapy compared with usual support is presented in Figure 5. The probability of LEGO® based therapy being cost-effective is 0.94 if decision-makers are willing to pay £20,000 for one QALY gained and 0.94 if they are willing to pay £30,000 per QALY gained.
FIGURE 5.
Base-case CEAC of LEGO® based therapy compared with usual support (outcome measure: QALY as measured by the EQ-5D-Y; NHS and PSS perspective).
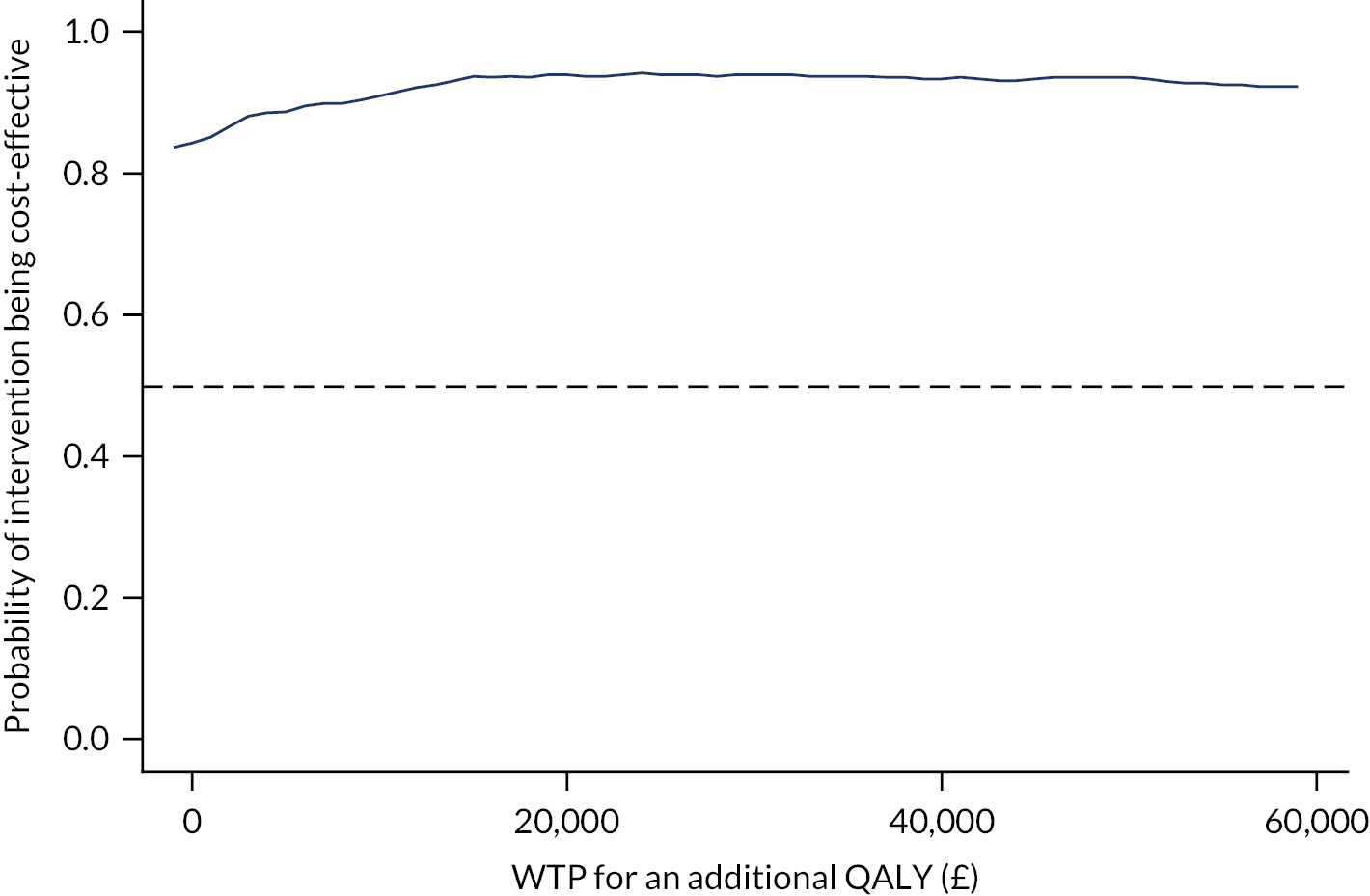
Sensitivity analyses
To account for uncertainty in the cost and QALY estimations, sensitivity analyses were conducted (Table 26). The mean incremental cost and QALY estimates from the complete case (scenario 1) were in line with the results from the best case (primary analysis), yielding a negative cost per QALY gained. Other sensitivity analyses using different cost perspectives and a different instrument (the CHU-9D) to measure QALYs were also conducted. In all of these sensitivity analyses, the ICER pairs lay below the NICE-recommended WTP threshold (£20,000-30,000 per QALY gained) (see Table 26) and the majority of the bootstrapped estimates sat in the fourth quadrant (Figure 6), suggesting that LEGO® based therapy is likely to be cost-effective.
| LEGO® based therapy vs. usual support | Incremental costs (£) (95% CI) | Incremental QALYs (95% CI) | ICER (£/QALY gained) |
|---|---|---|---|
| Scenario 1: complete-case analysis from the NHS and PSS perspective | –1279.70 (–1430.30 to –1129.10) | 0.011 (0.010 to 0.013) | Dominant |
| Scenario 2: assume outcomes were measured using the CHU-9D | –245.90 (–266.80 to –224.90) | 0.029 (0.028 to 0.030) | Dominant |
| Scenario 3: cost-effectiveness analysis from the NHS and education perspective | –510.90 (–551.80 to –470.00) | 0.009 (0.008 to 0.010) | Dominant |
| Scenario 4: cost-effectiveness analysis from the societal perspective | –376.00 (–420.60 to –331.30) | 0.009 (0.008 to 0.010) | Dominant |
FIGURE 6.
Sensitivity analysis: cost-effectiveness planes for LEGO® based therapy compared with usual support. (a) Base case, NHS and PSS perspective, EQ-5D-Y; (b) scenario 1, complete-case analysis; (c) scenario 2, NHS and PSS perspective, CHU-9D; (d) scenario 3, NHS and education perspective, EQ-5D-Y; and (e) scenario 4, societal perspective, EQ-5D-Y.
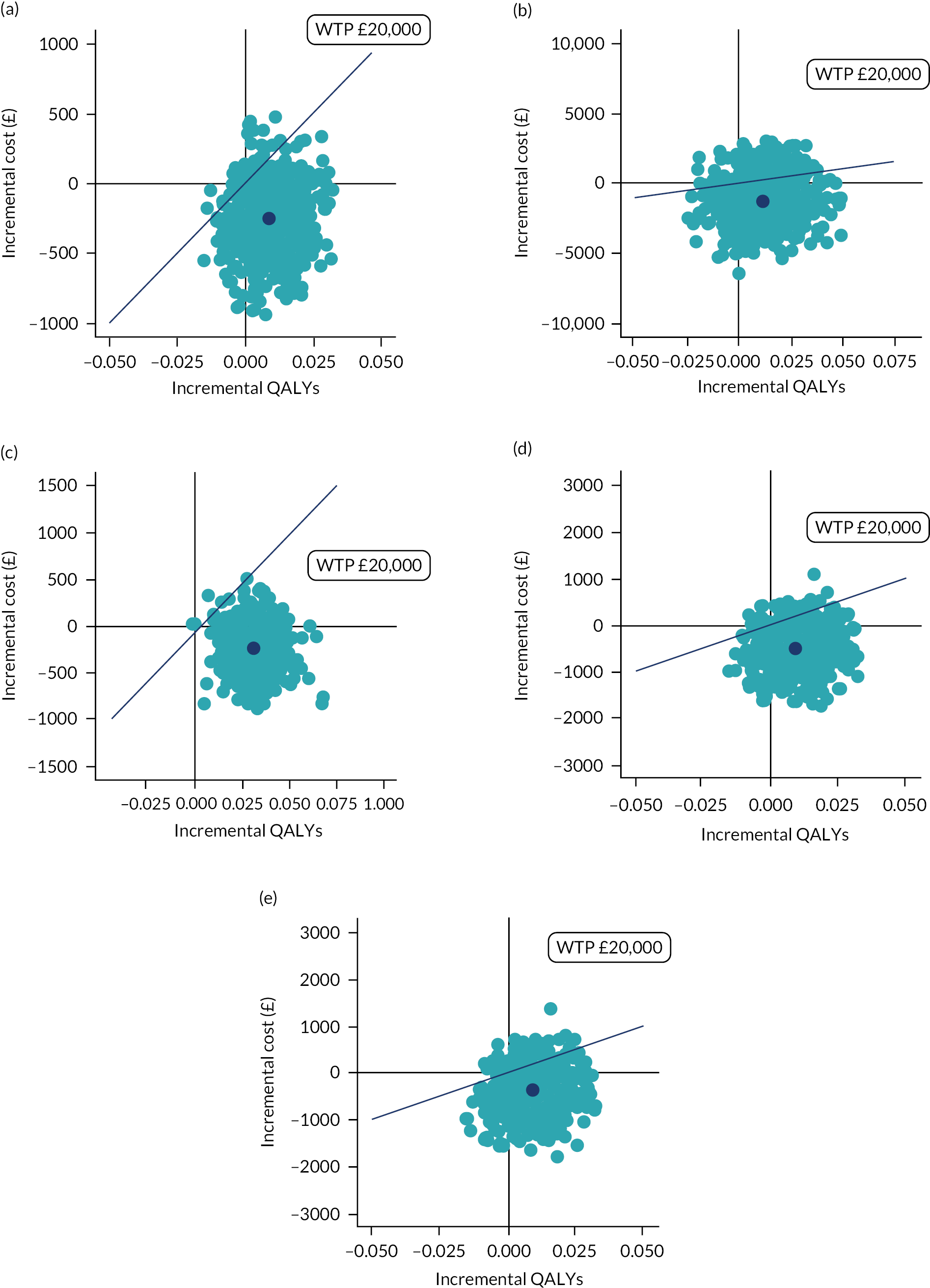
Summary
Compared with usual support, LEGO® based therapy marginally decreased service use costs and increased QALYs. This is evident in both primary and sensitivity analyses, which considered costs derived from various perspectives and QALYs measured by different instruments.
Chapter 5 Fidelity and acceptability results
Fidelity
Delivery of LEGO® based therapy sessions
A total of 69% of intervention groups received the required total of 12 LEGO® based therapy sessions and 93% received the per-protocol minimum of six sessions (see Figure 7). Overall, the median number of sessions delivered was 12 (range 3–12 sessions). A total of 749 LEGO® based therapy sessions were delivered as part of the trial. Appendix 4, Figure 10, shows the number of sessions delivered, grouped according to geographical location, and suggests that a similar dose of the intervention was delivered in Leeds, Sheffield and York.
Fidelity assessment of LEGO® based therapy: self-reported
Table 27 shows the proportion of components of LEGO® based therapy delivered by all facilitators during the trial, based on self-completion fidelity checklists and presented according to the ‘core content’ (items 1–4) and ‘all content’ (all 17 items) across all 12 sessions. The completion rate for the self-completed fidelity checklists was 99%, with only seven missing (from the total of 749). In line with guidance from Borrelli et al. ,80 self-reported fidelity was very high for both core content (99% of all sessions) and all content (91% of all sessions).
| Checklist item | Session number, n (%) | Total (N = 742),a % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 70) | 2 (N = 70) | 3 (N = 70) | 4 (N = 68) | 5 (N = 64) | 6 (N = 65) | 7 (N = 62) | 8 (N = 59) | 9 (N = 56) | 10 (N = 57) | 11 (N = 53) | 12 (N = 48) | ||
| Core content | |||||||||||||
| 1 | 69 (98.6) | 69 (98.6) | 70 (100) | 68 (100) | 64 (100) | 65 (100) | 61 (98.4) | 59 (100) | 56 (100) | 57 (100) | 53 (100) | 48 (100) | 99.6 |
| 2 | 64 (91.4) | 68 (97.1) | 69 (98.6) | 68 (100) | 62 (96.9) | 64 (98.5) | 58 (93.5) | 57 (96.6) | 54 (96.4) | 57 (100) | 52 (98.1) | 46 (95.8) | 96.9 |
| 3 | 69 (98.6) | 69 (98.6) | 70 (100) | 67 (98.5) | 64 (100) | 65 (100) | 62 (100) | 59 (100) | 56 (100) | 57 (100) | 53 (100) | 48 (100) | 99.6 |
| 4 | 65 (92.9) | 66 (94.3) | 69 (98.6) | 67 (98.5) | 64 (100) | 65 (100) | 61 (98.4) | 59 (100) | 55 (98.2) | 56 (98.2) | 52 (98.1) | 48 (100) | 98.1 |
| Mean total | 66.8 (95.4) | 68 (97.1) | 69.5 (99.3) | 67.5 (99.3) | 63.5 (99.2) | 64.8 (99.6) | 60.5 (97.6) | 58.5 (99.2) | 55.3 (98.7) | 56.8 (99.6) | 52.5 (99.1) | 47.5 (99) | 98.6 |
| Other items | |||||||||||||
| 5 | 66 (94.3) | 68 (97.1) | 68 (97.1) | 67 (98.5) | 62 (96.9) | 64 (98.5) | 62 (100) | 59 (100) | 56 (100) | 57 (100) | 52 (98.1) | 48 (100) | 98.4 |
| 6 | 66 (94.3) | 68 (97.1) | 69 (98.6) | 67 (98.5) | 59 (92.2) | 61 (93.8) | 60 (96.8) | 56 (94.9) | 54 (96.4) | 55 (96.5) | 53 (100) | 46 (95.8) | 96.3 |
| 7 | 68 (97.1) | 68 (97.1) | 70 (100) | 68 (100) | 62 (96.9) | 63 (96.9) | 61 (98.4) | 59 (100) | 56 (100) | 56 (98.2) | 53 (100) | 47 (97.9) | 98.6 |
| 8 | 69 (98.6) | 70 (100) | 68 (97.1) | 67 (98.5) | 63 (98.4) | 62 (95.4) | 62 (100) | 58 (98.3) | 54 (96.4) | 57 (100) | 52 (98.1) | 48 (100) | 98.4 |
| 9 | 69 (98.6) | 70 (100) | 70 (100) | 66 (97.1) | 63 (98.4) | 63 (96.9) | 61 (98.4) | 59 (100) | 55 (98.2) | 56 (98.2) | 52 (98.1) | 48 (100) | 98.7 |
| 10 | 60 (85.7) | 67 (95.7) | 66 (94.3) | 64 (133) | 62 (96.9) | 63 (96.9) | 60 (96.8) | 55 (93.2) | 52 (92.9) | 55 (96.5) | 49 (92.5) | 44 (91.7) | 97.2 |
| 11 | 70 (100) | 70 (100) | 66 (94.3) | 66 (97.1) | 60 (93.8) | 62 (95.4) | 59 (95.2) | 55 (93.2) | 54 (96.4) | 54 (94.7) | 52 (98.1) | 46 (95.8) | 96.2 |
| 12 | 56 (80) | 64 (91.4) | 65 (92.9) | 66 (97.1) | 57 (89.1) | 61 (93.8) | 59 (95.2) | 56 (94.9) | 53 (94.6) | 56 (98.2) | 49 (92.5) | 44 (91.7) | 92.6 |
| 13 | 70 (100) | 70 (100) | 69 (98.6) | 68 (100) | 61 (95.3) | 65 (100) | 62 (100) | 59 (100) | 55 (98.2) | 57 (100) | 53 (100) | 48 (100) | 99.3 |
| 14 | 44 (62.9) | 44 (62.9) | 47 (67.1) | 43 (63.2) | 40 (62.5) | 46 (70.8) | 43 (69.4) | 40 (67.8) | 38 (67.9) | 38 (66.7) | 40 (75.5) | 35 (72.9) | 67.5 |
| 15a | 45 (64.3) | 49 (70) | 51 (72.9) | 48 (70.6) | 40 (62.5) | 45 (69.2) | 43 (69.4) | 38 (64.4) | 38 (67.9) | 35 (61.4) | 35 (66) | 32 (66.7) | 67.1 |
| 15b | 50 (71.4) | 45 (64.3) | 45 (64.3) | 45 (66.2) | 45 (70.3) | 39 (60) | 40 (64.5) | 34 (57.6) | 36 (64.3) | 35 (61.4) | 36 (67.9) | 37 (77.1) | 65.8 |
| 16 | 55 (78.6) | 50 (71.4) | 55 (78.6) | 47 (69.1) | 50 (78.1) | 50 (76.9) | 48 (77.4) | 46 (78) | 44 (78.6) | 46 (80.7) | 44 (83) | 40 (83.3) | 77.8 |
| Mean total | 62.1 (88.7) | 63.2 (90.3) | 63.9 (91.3) | 61.9 (93.3) | 57.5 (89.9) | 59 (90.8) | 56.6 (91.3) | 53.4 (90.5) | 50.9 (91) | 52 (91.2) | 48.8 (92.1) | 44.3 (92.3) | 91.1 |
Delivery of core content was fairly stable over the 12 sessions (95–100%), with limited drift over time (i.e. at later sessions). Each school was required to deliver only one LEGO® based therapy group (with some schools delivering two groups). This pattern of high fidelity of delivery was stable for all content over the 12 sessions (89–93%), albeit with the caveat that only 69% of groups completed all 12 sessions.
The least commonly delivered components were as follows:
-
Was there an opportunity for each child or young person to show the other group members what they made, or talk about or play with what they made with others? (Yes, 67.5%.)
-
When a model was complete, was it appropriately displayed, or were photographs taken of the models? (Yes, 67%.)
-
If it was necessary to dismantle the models, was there warning and explanation given and were the children allowed to dismantle the models themselves (rather than the models being dismantled between sessions)? (Yes, 66%.)
-
Was there evidence of a reward system (e.g. LEGO points) to promote positive social behaviour, or LEGO certificates for attaining the different group levels (helper, builder, creator, etc.)? (Yes, 78%.)
The first three items are dependent on completion of the LEGO model (which does not necessarily happen in all sessions, for example when the model is complex and straddles sessions), whereas provision of the reward (item 16) should happen at any session. All other checklist items were delivered in > 90% of sessions.
The number of sessions delivered by facilitator can be seen in Appendix 4, Figure 9, and by location in Appendix 4, Figure 10. Appendix 4, Figure 11, shows the proportion of core and all content of the sessions according to individual therapists. A total of 63% of group therapists (n = 44) delivered 100% of core content and a further 24% of therapists (n = 17) delivered 98% of core content across all of the sessions completed. The remaining therapists (n = 9; 13%) delivered between 69% and 96% of core content. A total of 14% of therapists (n = 10) delivered 100% of all content; 63% of therapists delivered > 90% of all content and the remaining therapists (n = 16; 23%) delivered 73–89% of all content.
Fidelity assessment of LEGO® based therapy: independent rating
In addition to the self-assessed fidelity of intervention delivery, a subsample of LEGO® based therapy sessions were video-recorded to facilitate content analysis. The number of sessions recorded for each school that was fully consented into the fidelity assessment substudy is available in Appendix 4, Table 57. For a group to be included in the independent assessment of fidelity, consent to be video-recorded was required from all CYP and facilitator participants. In total, 63 sessions from 22 schools were recorded, which is equivalent to 9% of all LEGO® based therapy sessions in the trial. It was possible to organise recording of three sessions in 19 our of 22 schools, as planned, but logistical issues at other schools meant that it was possible to record only two sessions.
A total of 10 out of the 22 groups were from schools in Leeds, eight groups were from Sheffield schools and four were from York schools. The majority of groups (18/22) were from primary schools, and, accordingly, 51 our of 63 sessions were carried out in primary schools. The mean duration of sessions included in this substudy was 55 minutes (range 30–78 minutes). The recorded sessions comprised 20 early sessions (sessions 1–4), 22 middle sessions (sessions 5–8) and 21 later sessions (sessions 9–12) of LEGO® based therapy. No first sessions were recorded, with the number of all subsequent recorded sessions ranging from three to nine.
To provide an independent assessment of fidelity, all 63 sessions were independently reviewed and rated against the fidelity checklist by a trained researcher (AB). A subsample of 16 sessions was double rated by two senior researchers/co-applicants (BW and GG, who was also the senior trainer of the LEGO® based therapy) to ensure accuracy of rating. This double rating was completed at two time points (i.e. at the beginning and at the end of rating exercise). The level of inter-rater reliability was high at 84%,87 and all discrepancies between the two raters were discussed and a consensus reached with a third trained researcher (LC).
Table 28 presents the proportion of components of LEGO® based therapy delivered in those sessions included in the independent fidelity assessment and presented according to the core content (items 1–4) and all content (all 17 items) across sessions 2–12. In line with guidance from Borrelli et al. ,80 self-reported fidelity was high for core content (83% of all sessions) and moderate for all content (77% of all sessions).
| Session number, n (%) | Total (N = 742), % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 0) | 2 (N = 5) | 3 (N = 9) | 4 (N = 6) | 5 (N = 5) | 6 (N = 3) | 7 (N = 9) | 8 (N = 5) | 9 (N = 6) | 10 (N = 5) | 11 (N = 5) | 12 (N = 5) | ||
| Core content | |||||||||||||
| 1 | 0 (0) | 5 (100) | 8 (88.89) | 5 (83.33) | 5 (100) | 2 (66.67) | 9 (100) | 4 (80) | 4 (66.67) | 5 (100) | 4 (80) | 5 (100) | 80.46 |
| 2 | 0 (0) | 5 (100) | 9 (100) | 6 (100) | 5 (100) | 3 (100) | 9 (100) | 4 (80) | 6 (100) | 5 (100) | 4 (80) | 5 (100) | 88.33 |
| 3 | 0 (0) | 4 (80) | 5 (55.56) | 6 (100) | 3 (60) | 3 (100) | 9 (100) | 5 (100) | 5 (83.33) | 5 (100) | 3 (60) | 2 (40) | 73.24 |
| 4 | 0 (0) | 3 (60) | 7 (77.78) | 5 (83.33) | 3 (60) | 3 (100) | 7 (77.78) | 3 (60) | 4 (66.67) | 4 (80) | 2 (40) | 3 (60) | 63.80 |
| Mean total | 0 (0) | 4.25 (85) | 7.25 (80.56) | 5.5 (91.67) | 4 (80) | 2.75 (91.67) | 8.5 (94.44) | 4 (80) | 4.75 (79.17) | 4.75 (95) | 3.25 (65) | 3.75 (75) | 76.46 |
| Other items | |||||||||||||
| 5 | 0 (0) | 5 (100) | 9 (100) | 6 (100) | 5 (100) | 3 (100) | 9 (100) | 5 (100) | 6 (100) | 5 (100) | 5 (100) | 5 (100) | 91.67 |
| 6 | 0 (0) | 5 (100) | 6 (66.67) | 5 (83.33) | 5 (100) | 2 (66.67) | 9 (100) | 5 (100) | 5 (83.33) | 5 (100) | 5 (100) | 4 (80) | 81.67 |
| 7 | 0 (0) | 5 (100) | 6 (66.67) | 5 (83.33) | 4 (80) | 2 (66.67) | 7 (77.78) | 3 (60) | 5 (83.33) | 4 (80) | 3 (60) | 3 (60) | 68.15 |
| 8 | 0 (0) | 5 (100) | 9 (100) | 5 (83.33) | 5 (100) | 3 (100) | 9 (100) | 4 (80) | 6 (100) | 5 (100) | 5 (100) | 5 (100) | 88.61 |
| 9 | 0 (0) | 5 (100) | 9 (100) | 6 (100) | 4 (80) | 3 (100) | 9 (100) | 5 (100) | 6 (100) | 5 (100) | 4 (80) | 5 (100) | 88.33 |
| 10 | 0 (0) | 2 (40) | 3 (33.33) | 4 (66.67) | 3 (60) | 2 (66.67) | 4 (44.44) | 2 (40) | 1 (16.67) | 2 (40) | 1 (20) | 1 (20) | 37.31 |
| 11 | 0 (0) | 5 (100) | 8 (88.89) | 5 (83.33) | 3 (60) | 3 (100) | 7 (77.78) | 2 (40) | 4 (66.67) | 3 (60) | 3 (60) | 3 (60) | 66.39 |
| 12 | 0 (0) | 5 (100) | 7 (77.78) | 6 (100) | 4 (80) | 2 (66.67) | 7 (77.78) | 3 (60) | 3 (50.00) | 5 (100) | 2 (40) | 2 (40) | 66.02 |
| 13 | 0 (0) | 5 (100) | 9 (100) | 6 (100) | 5 (100) | 3 (100) | 9 (100) | 5 (100) | 6 (100) | 5 (100) | 4 (80) | 5 (100) | 90.00 |
| 14 | 0 (0) | 5 (100) | 7 (77.78) | 5 (83.33) | 3 (60) | 3 (100) | 7 (77.78) | 3 (60) | 4 (66.67) | 3 (60) | 4 (80) | 3 (60) | 68.80 |
| 15a | 0 (0) | 3 (60) | 7 (77.78) | 4 (66.67) | 4 (80) | 3 (100) | 6 (66.67) | 4 (80) | 2 (33.33) | 3 (60) | 3 (60) | 4 (80) | 63.70 |
| 15b | 0 (0) | 1 (20) | 2 (22.22) | 3 (50) | 1 (20) | 1 (33.33) | 3 (33.33) | 1 (20) | 4 (66.67) | 0 (0) | 2 (40) | 1 (20) | 27.13 |
| 16 | 0 (0) | 2 (40) | 7 (77.78) | 4 (66.67) | 2 (40) | 3 (100) | 7 (77.78) | 2 (40) | 3 (50) | 3 (60) | 1 (20) | 4 (80) | 54.35 |
| Mean total | 0.00 (0.00) | 4.12 (82.35) | 6.94 (77.12) | 5.06 (84.31) | 3.76 (75.29) | 2.59 (86.27) | 7.47 (83.01) | 3.53 (70.59) | 4.35 (72.55) | 3.94 (78.82) | 3.24 (64.71) | 3.53 (70.59) | 70.47 |
As with self-assessed fidelity, delivery of core content was stable over the 11 (out of 12) sessions (79–95%), with limited drift for later sessions (i.e. dropping to 65–75% in sessions 11 and 12). This pattern of fidelity of delivery was stable for all content over the sessions too, with a range of 65–86% throughout.
According to the independent rating, the least commonly delivered components were 15b and 15a (69%), both of which related to dismantling LEGO models; 10 (41%), which assessed if appropriate greetings took place and if these were prompted/encouraged; and 16 (59%), which related to clear use of rewards in the session. As with self-reported fidelity, it may be that some intervention components, such as displaying or dismantling models (items 15a and 15b), did not take place or, as in the case of the greetings (item 10) may have taken place outside the video-recording. However, evidence of the reward system was present in only 59% of the sessions. All other checklist items were delivered in > 70% of sessions.
Comparison of self-reported and independently rated fidelity assessment
Overall, self-reported fidelity of delivery was higher than independently rated fidelity of delivery, with fidelity for core content rated as 99% and 83%, respectively, and for all content rated as 91% and 77%, respectively. Despite these differences, both types of assessment can be classed as achieving high or high–moderate fidelity, according to Borrelli et al. 80
Appendix 4, Table 58, shows a comparison between the self-reported and independently rated delivery of individual components for all 63 sessions video-recorded as part of the independent assessment. Overall, there was 90% agreement between the self-reported and independent ratings of whether or not a component was delivered in the session. Where there was a conflict, this tended to be the result of facilitators reporting delivery of a component that was not observed independently (9%). For core content, the facilitators, for example, reported positive outcomes on items 3 and 4 (related to highlighting positive social interactions and problem solving by the CYP); for other items, such as group rules (item 7), greetings (item 10) or description of what will happen in the session (item 11), these were reported as present but were not perhaps visible or delivered on camera.
Acceptability: quantitative
All participants
Table 29 and Figure 7 show a summary of the assessment of acceptability of LEGO® based therapy to all facilitators and parents/guardians of autistic CYP in the trial (who evaluated this as proxy for their CYP). This was measured on a 5-point scale [from 1 (strongly disagree) to 5 (strongly agree)] across the different constructs of the TFA. This shows that acceptability of the intervention was high, with an overall median acceptability score for facilitators of 5 (range 4–5) and for parents/guardians of 4 (range 3–5). Acceptability to facilitators was generally higher than parents/guardians, and this was statistically significant (p = 0.000).
| Acceptability construct | Facilitators (N = 65), mean (range) | Parents/guardians (N = 98), mean (range) | Mann–Whitney U-test p-value | Facilitators (N = 65), %a | Parents (N = 98), %a |
|---|---|---|---|---|---|
| Affective attitude | 5 (4–5) | 5 (4–5) | 0.251 | 100 | 91.75 |
| Burden | 5 (4–5) | 3 (2.75–4) | 0.000 | 86.15 | 48.96 |
| Ethicality | 4 (4–5) | 4 (4–5) | 0.507 | 93.85 | 78.35 |
| Intervention coherence | 5 (5–5) | 3 (3–4) | 0.000 | 93.85 | 46.87 |
| Opportunity costs | 5 (4–5) | 4 (4–5) | 0.015 | 95.38 | 83.51 |
| Perceived effectiveness: general | 4 (4–5) | 4 (3–5) | 0.008 | 92.19 | 69.07 |
| Perceived effectiveness: social skills | 4 (4–5) | 4 (3–4) | 0.000 | 90.63 | 65.98 |
| Perceived effectiveness: academic confidence | 5 (4–5) | 4 (4–5) | 0.008 | 89.23 | 75.26 |
| Perceived effectiveness: communication skills | 5 (4–5) | 4 (4–5) | 0.002 | 95.38 | 84.54 |
| Perceived effectiveness: behaviour | 4 (4–5) | 4 (3–4) | 0.007 | 73.38 | 54.64 |
| Perceived effectiveness: overall | 5 (4–5) | 4 (3–5) | 0.000 | 87.72 | 69.90 |
| Self-efficacy | 5 (4–5) | 4 (4–5) | 0.000 | 100 | 79.59 |
| Overall acceptability | 5 (4–5) | 4 (3–5) | 0.000 | 91.64 | 70.77 |
FIGURE 7.
Acceptability of LEGO® based therapy: comparison of median scores for acceptability constructs for all participants. Higher scores indicate a greater level of acceptability.
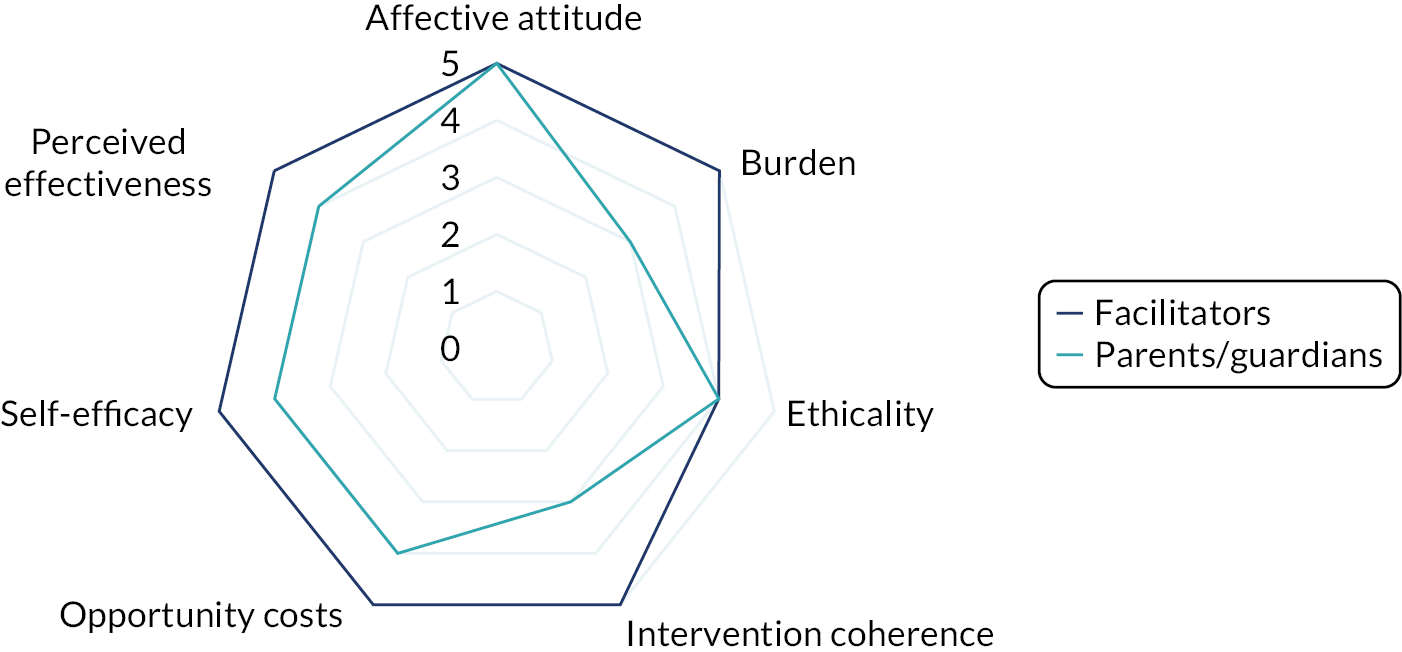
Looking at specific TFA constructs, facilitators rated all of these positively, with median scores of 4 or 5 on each. All facilitators positively rated ‘affective attitude’ (i.e. they liked the intervention) and ‘self-efficacy’ (i.e. they felt confident to deliver the intervention), with high scores on all other constructs, and, overall, 92% rated the acceptability of LEGO® based therapy positively. The lowest score for facilitators (73% for ‘perceived effectiveness on behaviour’) is still high.
Among parents/guardians, only ‘affective attitude’ received the maximum median score of 5, with 92% rating this positively. Overall, parents’/guardians’ acceptability score was slightly lower, at 71%, and this difference from the facilitators’ acceptability score was statistically significant (p = 0.000). With regards to specific TFA constructs, ‘burden’ (49%), ‘intervention coherence’ (47%) and ‘perceived effectiveness on behaviour’ (55%) were rated least positively by parents/guardians (although this is still fairly acceptable, with half of participants giving positive ratings). There were statistically significant differences between facilitator and parent/guardian ratings of acceptability for ‘burden’, ‘intervention coherence’, ‘self-efficacy’ (p = 0.000) and ‘perceived effectiveness’ (all dimensions) (p = 0.008–0.000).
Appendix 4, Tables 59 and 60, summarise the open-ended qualitative responses to the acceptability questionnaire given by facilitators and parents/guardians, respectively. Provision of the open-ended responses was optional, and the numbers reporting specific points are given for clarification only.
In general, the open-ended responses suggest that facilitators viewed LEGO® based therapy positively, and this was reportedly viewed positively by the CYP in their groups also. Despite some challenges, LEGO® based therapy was considered to be a rewarding and valuable experience. Facilitators were able to identify a number of perceived benefits from LEGO® based therapy during the sessions (e.g. communication, social skills and confidence), but some cautioned that these did not always translate into the classroom. A small number of facilitators highlighted problems with LEGO® based therapy delivery, including conflict, poor communication between two autistic CYP and CYP becoming fatigued at week 7 onwards. Five facilitators reported that their school had adopted LEGO® based therapy into practice after the end of the trial delivery, although sometimes adapted to six sessions. There were mixed views on the optimal duration for LEGO® based therapy, and one facilitator suggested that it was better suited to CYP of primary rather than secondary school age.
A majority of parents/guardians shared that their child had enjoyed taking part in LEGO® based therapy. Only a small number thought that their child had not enjoyed it, for example because they had lots of prior LEGO®-building experience or because of the gender composition of the group. Many parents/guardians highlighted the perceived benefits that their child had gained from LEGO® based therapy (e.g. improved communication, behaviour, patience or confidence), but some clarified that these improvements had not translated into behavioural changes at home. A small proportion of parents/guardians said that their child would have liked to continue with LEGO® based therapy in the longer-term, and that this might help develop their skills further, or lead to changes at home. A small proportion of parents/guardians highlighted that they lacked insight into their child’s experience of the intervention and had received no feedback from school regarding this.
Primary school participants
A summary of the assessment of acceptability of LEGO® based therapy to primary school facilitators and parents/guardians of primary school-age autistic CYP in the trial is available in Appendix 4, Table 61 and Figure 12. As with all participants, this shows that acceptability of the intervention was again high, with overall median acceptability scores of 5 (range 4–5) and 4 (range 3–5) for primary facilitators and parents/guardians, respectively. Similarly, acceptability to facilitators was generally higher than to parents/guardians, and this difference was statistically significant (p = 0.000).
In relation to specific TFA constructs, primary school facilitators rated all of these positively, with median scores of 4 or 5 on each. All facilitators positively rated ‘affective attitude’ and ‘self-efficacy’, with high scores on all other constructs, and, overall, 94% rated the acceptability of LEGO® based therapy positively. Again, the lowest rating from facilitators was for ‘perceived effectiveness on behaviour’, which at 71% is still positive.
For parents/guardians of primary school-age CYP, only ‘affective attitude’ and ‘opportunity costs’ received the maximum median score of 5, with 94% and 85% rating this positively, respectively. Overall, parents’ acceptability score was lower, at 76%, and the difference between parents/guardians and facilitators was statistically significant (p = 0.000). With regards to specific TFA constructs, ‘burden’ (42%), ‘intervention coherence’ (41%) and ‘perceived effectiveness on behaviour’ (56%) were rated least positively by parents. There were statistically significant differences between facilitator and parent/guardian ratings of acceptability on burden, intervention coherence, self-efficacy (p = 0.000), perceived effectiveness on behaviour (p = 0.05), academic confidence (p = 0.025), communication skills (p = 0.009), social skills (p = 0.001) and, correspondingly, overall perceived effectiveness (p = 0.000).
Secondary school participants
A summary of the assessment of acceptability of LEGO® based therapy to secondary school facilitators and parents/guardians of autistic CYP of secondary school age in the trial is available in Appendix 4, Table 62 and Figure 13. As with the whole group of participants, this shows that acceptability of the intervention was again high, with overall median acceptability scores of 5 (range 4–5) and 4 (range 3–5) for secondary facilitators and parents/guardians, respectively. Again, acceptability to facilitators was generally higher than to parents/guardians, and the difference was statistically significant (p = 0.000).
For specific TFA constructs, secondary school facilitators rated all of these positively, with median scores of 4 or 5 on each. Again, 100% of facilitators positively rated ‘affective attitude’ and ‘self-efficacy’, with high scores on all other constructs, and, overall, 94% rated the acceptability of LEGO® based therapy positively. Again, the lowest rating from facilitators was for ‘perceived effectiveness on academic confidence’, which is still high at 83%.
For parents/guardians of CYP of secondary school age, each of the TFA constructs was rated with a median score of 4. Overall, parents’ acceptability score was lower, at 68%, and the difference between parents/guardians and facilitators was statistically significant (p = 0.000). With regards to specific TFA constructs, ‘perceived effectiveness on social skills’ (60%) and ‘perceived effectiveness on behaviour’ (52%) were rated least positively by parents/guardians, with many other constructs rated positively by only 64% of participants. There were statistically significant differences between facilitator and parent/guardian ratings of acceptability on burden, intervention coherence (p = 0.005), self-efficacy (p = 0.006), perceived effectiveness on behaviour (p = 0.04), general perceived effectiveness (p = 0.03) and, correspondingly, overall perceived effectiveness (p = 0.000).
Comparison between primary and secondary facilitators
Although primary school facilitators generally rate the acceptability of LEGO® based therapy more positively, with median scores of 5 (range 4–5) compared with 4 (range 3–5) for secondary school facilitators, this difference was not statistically significant overall or on any of the individual constructs (see Appendix 4, Table 63 and Figure 14).
Comparison between parents
Parents/guardians of CYP of secondary school age generally rated LEGO® based therapy more positively than parents/guardians of CYP of primary school age, but this difference was not statistically significant overall: both groups had median scores of 4 (range 3–5) (see Appendix 4, Table 64 and Figure 15). There were statistically significant differences between the group ratings of ‘burden’ (p = 0.05) and ‘intervention coherence’ (p = 0.026).
Acceptability: qualitative
Participants
A total of 16 facilitators from the trial were consented to the qualitative acceptability substudy. The median duration of the interviews was 40 minutes (range 22–61 minutes). The majority of participants were working in primary schools (12/16). A total of 10 participants were working as TAs, four as SENCOs and two as teachers. All participants had previous experience of working with CYP with ASD and other SEN. None was actively delivering LEGO® based therapy (i.e. a study exclusion criterion) but nine indicated that they were familiar with it. Nine facilitators were from Leeds, two from Sheffield and five from York.
Analysis
On familiarisation with the data transcribed from the telephone interviews, we developed a list of preliminary codes. After several iterations, the final framework was developed: this included 26 codes, which could be distilled under eight categories (see Appendix 4, Table 65). Following this, indexing of the data set was undertaken and charts were produced to summarise the content of each category and code (and subcode). To help with interpretation of the data, these were then mapped to the TFA. 55 This mapping between categories and the seven TFA constructs is shown in Appendix 4, Table 66. The presentation of the themes is structured around the seven TFA constructs.
Affective attitude
Most of the facilitator participants explained that in their opinion the LEGO® based therapy was a positive experience for the CYP in their groups because these CYP were already familiar with and interested in LEGO. As LEGO® based therapy was generally seen as easy to explain to CYP and enjoyable to deliver, it was also viewed positively by facilitators:
I think what made it fairly easy to talk to them about it, is because they all had a knowledge and an interest in LEGO prior to the programme.
L/40/I2
Most facilitators said that their group had enjoyed participating in the intervention, and some CYP had asked to carry on with this when the 12 sessions had finished. Some participants found that the CYP in their groups were very excited and enthusiastic about taking part in LEGO® based therapy and would talk about the intervention in-between sessions in the classroom and the playground. Some participants also gave examples of other CYP who had not been directly involved in LEGO® based therapy wanting to take part:
I know mine have already come up to me and said ‘Are we doing it next year?’ They are so enthused by it.
S/28/I1
One of the secondary school facilitators highlighted reservations at their school, where it had initially been thought that LEGO would not be suitable for older CYP, but they found that the CYP enjoyed the sessions and found them to be ‘light relief’ from their usual timetable.
Overall, facilitators explained that they felt that LEGO® based therapy ran smoothly as an intervention and was successful; however, certain common challenges arose. For example, some CYP struggled to participate effectively in the groups if they were accomplished LEGO builders at home. This worsened when sets were perceived to be too small or simple, and the facilitators suggested that these CYP found it difficult to stick to their roles without rushing ahead with building:
Some of them had already made some of the models that were identified in the programme, so it was really guiding them because they had a tendency to run ahead.
L/40/I2
By contrast, other facilitators commented that, for groups of younger CYP, some of the LEGO sets were too complex, and the smaller pieces and intricate building could be difficult to manage for CYP with comparably poorer fine motor skills.
Burden
Most of the facilitators in this substudy did not view LEGO® based therapy as a major burden. Rather, the intervention groups were described as fitting relatively easily into their existing work schedule and compatible with current working practices in their schools. The study team remained in equipoise, but some schools made the decision to continue LEGO® based therapy after the trial. These were started only after our trial was competed. For example:
… and when we look at the broad spectrum of kids we’ve got here in this school, a lot of them are now requesting it as something that can go on pupil passbooks and things that this child has accessed this as an intervention. So you know, staff are coming to me and saying ‘do you think you could fit this child in?’ and I think it’s just seen now in school as something that we deliver.
L/35/I1
Overall, most facilitators explained that LEGO® based therapy had already been introduced more widely with other CYP in their schools (i.e. following completion of the intervention as part of the trial) or there were discussions about the possibility of expanding this. New groups had been set up at varying times (e.g. lunchtime or after school) to fit into the school schedule:
For the school, I think it’s certainly valued by me as I’m the SENCO, and for me it’s been really useful. It’s a skill now that I have, it’s a skill that I can now pass onto other people.
L/05/I1
. . . now we run LEGO club every lunchtime. So we have, I don’t remember the numbers, 50 or so children now engaged in LEGO therapy at some point during the term.
L/35/I1
In these schools, facilitators appeared hopeful that LEGO® based therapy could become another potential intervention available to help autistic CYP. On a practical level, in terms of delivery of the intervention, it was recognised that having timetabled protected time and the availability of a suitable space made integration into practice easier. The facilitators also noted that a private room or space in school to conduct LEGO® based therapy was important, as was using the same consistent, familiar space for every session. On occasion LEGO® based therapy sessions had to be held in a different location, and this change of venue had disrupted the sessions and CYP were ‘thrown’ when in an unfamiliar room. These challenges of ensuring the spatial continuity in schools, which are rapidly changing environments with a high demand for private space, were recognised.
Despite the positive comments about being able to fit the intervention into the school timetable, most facilitators said that the largest burden of delivering LEGO® based therapy in school was finding the time, and scheduling the sessions on a regular basis into timetables (i.e. accommodating the needs and preferences of CYP and teachers) relative to the facilitator’s timetable:
It’s time, because for me to have done this, I have had to take away an hour of my time every week, so yeah, it’s time.
S/28/I1
It’s primarily been the time, my time really and that’s always going to have a cost, but that’s the only thing really.
Y/21/I1
In some schools, it had been necessary to reorganise staff timetables to cover for those conducting LEGO® based therapy; however, most facilitators said that this change to staffing had a minimal impact and was not perceived as a major issue:
The class teacher is one member of staff down, so yes, it is, it is quite an impact and it’s hard to maintain an intervention every single week without a break in a school because schools are fluid places and they are flexible and things happen.
L/33/I1
The only difference really had been a re-jigging really of staffing on the playground.
L/35/I1
One facilitator described how they had run sessions after school and others had run the intervention groups during lunchtime. One facilitator felt that 12 sessions was a lot to fit in, and that fewer sessions may have been easier to accommodate into the school calendar.
Ethicality
All facilitators in this substudy explained that LEGO® based therapy fits well with their own professional values about what helps autistic CYP. Most agreed that the structure of the intervention is well tailored, in their opinion, to the needs of autistic CYP, helping them to communicate and socialise with others. Some facilitators also said that they appreciated gaining the new skill of delivering LEGO® based therapy, although we are aware that they were expressing these views before the study results were known:
Part of my job is to make these children feel successful and feel valued. And if I can use something like this, I know this will be successful, then why not? It’s gonna work, it’s gonna be a winner.
L/26/I1
The majority said that the intervention fitted well into their schools, as the main aim is to be as inclusive as possible for all CYP, including those with ASD and other CYP on the SEN register. Many suggested that their perceptions of benefits from LEGO® based therapy have helped to increase acceptance of the intervention in their school. For example, one secondary school participant explained that SEN can sometimes be seen as less of a priority, but the perceived benefits from LEGO® based therapy (e.g. helping to control anger and increase resilience) have been valued by the school, and it was seen as worthwhile:
So it fits with the goals, we are an inclusive school, we want everyone to achieve their best and we are, we often do a lot of different types of interventions in order to try and achieve those.
L/05/I1
I think [it fits in with school values] because we’re just striving to be a fully inclusive school and to give kids on the SEND [special educational needs and disabilities] register just the greatest opportunity they can.
L/35/I1
Intervention coherence
Teaching staff
All facilitators in this substudy stated that they had a clear understanding of LEGO® based therapy by the end of programme delivery. Most explained that, unless they had been directly involved, other teachers were aware only that LEGO® based therapy was happening in school and did not have a full understanding of the intervention and the purpose, thinking that the CYP were ‘just playing’ with LEGO:
It’s been more of a problem to explain to staff, they think it’s just an hour of playing with LEGO. And whereas, I had to explain how it works and whilst we’re playing with LEGO for the hour, there was very much a structure to it.
L/05/I1
Facilitators thought that it was important to give other teaching staffing a detailed explanation of the purpose and structure of LEGO® based therapy, or invite them to observe a session, to increase understanding and acceptance in their schools. In some cases, class teachers struggled to understand that LEGO® based therapy was not a reward but a tool to help develop the social skills of CYP:
We actually did make a LEGO therapy session in a staff meeting, just so we could show teachers what, how we were doing it, and I think . . . the easy thing was kind of doing a session with teachers, so that teachers could see the amount of communication that was required within the therapy sessions, and that was kind of the whole point of it really.
Y/21/I1
Facilitators identified a number of differences between LEGO® based therapy and other interventions for autistic CYP, principally the structured and repetitive nature of the intervention, which was felt to benefit autistic CYP. Furthermore, the design of LEGO® based therapy made planning and delivery easier for many facilitators; working with a toy product that most CYP already consider to be ‘fun’ and having a palpable end product that the CYP can visualise were also seen as positive features of the intervention:
I think as far as the intervention goes, this is, you see instant positive results. And you don’t get that in a lot of interventions. The very, very least it can do would make a child feel a bit better about themselves and if that’s the least it can do, then it’s a great intervention.
L/26/I1
Children and young people with autism spectrum disorder
Most facilitators thought that the CYP in their groups were enthusiastic about taking part but were not aware of the purpose of LEGO® based therapy. The facilitators also mentioned the importance of clearly explaining the structure of the sessions to their groups (i.e. roles and turn-taking) and the facilitators’ view that the group intervention was not simply about playing or about getting the models finished. They explained that some CYP struggled to see this perspective, especially those with lots of experience of LEGO play in everyday life:
. . . but it’s trying to talk to them about ‘it’s not just building, we’re not just playing, we’re going to do it in a structured way and you’ll each have a role to play’. So, once they get their head around that, then it’s pretty much plain sailing from there.
Y/06/I1
I think the main issue with the children was that their aim in their head, always went back to just finishing the model. But that’s not what it’s about, it was about different roles that we had to use. I think that was the main issue with understanding the LEGO therapy there.
L/43/I1
Parents
Facilitators were also asked about parental understanding of LEGO® based therapy. All secondary school facilitators reported that they had no feedback or contact from parents/guardians regarding the intervention. However, those working in primary settings felt that parents/guardians had a good understanding overall, but needed further explanation that it was ‘more than just building’. Facilitators also highlighted the importance of terminology and how the word ‘therapy’ may give the wrong initial impression to parents. In addition, some parents/guardians of the non-autistic CYP in groups often needed more clarification than parents/guardians of autistic CYP, to explain why their child had been chosen to be in the LEGO® based therapy groups:
I think certain parents might have the feeling that we’re just playing, we’re just literally building LEGO and having fun and it’s a social interaction thing, so discussing with them the fact that it runs deeper than that. That can be something that they find a little surprising maybe.
Y/06/I1
Opportunity costs
Most facilitators reflected that the benefits that they perceived from LEGO® based therapy far outweighed any resource implications or opportunity costs (e.g. staff time and planning) experienced in the delivery of the intervention. As already outlined, the main issue faced by the facilitators was timetabling and the juggling act required to deliver the intervention at the same time each week. Some facilitators identified a small cost to their own time, and a need to be more organised, but most felt that their workload was unaffected and that other staff helped them to fit the sessions in when there was a timetable clash (e.g. with school trips):
The teachers have never had a problem with them actually missing that lesson, as they know what those children come back to class like, and they’re very happy that it’s worth it and they’re not losing, they’re not missing anything.
L/43/I1
Related to this, most facilitators noted that, for LEGO® based therapy to continue in their schools, there would be an initial financial cost for the LEGO sets, which were perceived as expensive, and therefore the intervention must be seen as ‘worth it’ to justify this. Some of the schools had received donations and voluntary contributions from parents/guardians or planned to fundraise, but one school said that this large initial cost was a barrier to the continuation of LEGO® based therapy beyond the trial:
The other cost would be and that would be the real sticking point is, you know, having to buy the LEGO kits. That is, and that would have to be thought through carefully.
L/33/I1
Some facilitators noted that there was a key staff member (e.g. head teacher, inclusion manager or SENCO) who demonstrated clear enthusiasm for LEGO® based therapy and supported them doing this work during the trial:
I was allocated the time to do it, my school was behind me and they gave me the hours that I needed to it.
Y/06/I1
Perceived effectiveness
Children and young people with autism spectrum disorder
All facilitators said that they perceived LEGO® based therapy to be positive for their groups and that they had seen benefits during the sessions, the greatest of which were improved communication and socialising skills. The facilitators also stated that LEGO® based therapy had helped group members with team working, turn-taking, and being patient and calm with each other. Some also noticed that the intervention group members had become friends, which they felt would not have happened without the intervention:
Because I’d never heard him have a conversation before but he had the opportunity to do that in a quieter space.
Y/02/I1
One boy is autistic, and the other boy that shows lots of autistic traits, both on the playground would basically just play by themselves or walk around the perimeter of the playground and just play on their own. And they are now best friends.
L/41/I1
There was a definite increase in the amount of instructions this child could follow at one time.
L/35/I1
Other benefits noted included increased confidence in talking in class, improved language skills and greater resilience when tackling problems. However, some reported that, although they saw benefits for autistic CYP during the sessions, they were not sure that these benefits were visible beyond the group setting to other staff members or parents. They suggested that a longer-term intervention would perhaps help to embed longer-term benefits.
Children and young people without autism spectrum disorder
Most of those interviewed felt that LEGO® based therapy was beneficial to the non-autistic CYP who participated in the groups. They explained that these CYP would act in a supporting role in the group setting, which they then transferred to the classroom and playground. Facilitators suggested that similar benefits for non-autistic CYP as for autistic CYP had been observed, including language skills, confidence, patience and, in some CYP, fine motor skills.
Teaching staff
Most of the facilitators explained that they felt that they had gained beneficial new skills that they would continue to use both inside and outside the classroom. Indeed, some said that LEGO® based therapy had given them new approaches to communication to explore with autistic CYP, and some also suggested that participating in the trial reflected well on their school as a whole:
So I think that it has been positive and it is something that has created transferable knowledge and skills, not only for, I guess me, as an individual, but I guess it’s also from their [CYP’s] perspective as well.
L/40/I2
Self-efficacy
Most facilitators felt that the training for LEGO® based therapy provided as part of the trial adequately prepared them to deliver the manualised intervention in their schools. Most said that they enjoyed the training and felt well equipped to deliver the intervention afterwards. Some noted that receiving the group training format was helpful because they could ‘bounce ideas’ off one another:
It was a really, really good training session day. I really enjoyed it. I felt, after I came, I couldn’t wait to start.
L/07/I1
A couple of the facilitators stated that they would have preferred a little more training on conflict resolution during the sessions, and some, although they may have been nervous to start LEGO® based therapy after the training, discovered that this potential problem reduced after the first session:
I would say, you know, maybe in the training, go over a little bit more the issues you may encounter and how to deal with those.
L/33/I1
Nearly all the facilitators agreed that TAs should have the right set of skills to be able to deliver LEGO® based therapy in practice (i.e. outside the trial, if adopted by schools). Most suggested that patience is the most important skill needed, and it is important to give CYP enough time to solve their own problems before intervening. One facilitator explained that class teachers may not be as well equipped to deliver LEGO® based therapy as TAs and that facilitators, therefore, should be chosen carefully:
I think they need to be very patient and understanding. And I think they need to know their children and know what makes them tick.
L/46/I1
Related to this, the facilitators emphasised the importance of group dynamics to the success of the sessions. They explained that relationships between CYP were important to consider when assembling the group. Similarly, facilitators’ prior knowledge about, and positive relationships with, the CYP were helpful to ensure smooth and effective delivery of and participation in LEGO® based therapy:
You’ve got to really think about the group and the dynamics of your group.
L/26/I1
Most facilitators in this substudy did not and would not choose to make adaptations to the delivery of LEGO® based therapy; they felt that the intervention already worked well. Some made or suggested small changes, including running the sessions twice weekly (when there was a time constraint such as completion before half-term), giving out more certificates on a regular basis and using the LEGO® based therapy techniques in other settings (e.g. cooking lessons):
I don’t think I would adapt anything, because for me, it flowed really well.
L/07/I1
Twice a week did work really well. It might have felt a long time between sessions if we’d only done it once a week.
Y/21/I1
Summary
Overall, the evaluation of the fidelity and acceptability data shows a high level of intervention fidelity for many aspects of the delivery of LEGO® based therapy during the trial and that the facilitators felt able to deliver LEGO® based therapy as intended.
This finding is supported by data from the quantitative acceptability substudy, which showed that 100% of facilitators rated their ‘self-efficacy’ to deliver and ‘affective attitude’ towards LEGO® based therapy positively. Overall acceptability scores were positive for facilitators (median 5) and parents/guardians (median 4). Facilitator ratings of LEGO® based therapy were generally higher than those given by parents/guardians, and this was statistically significant for all participants and subgroups.
Overall, the qualitative substudy suggests that LEGO® based therapy was very acceptable to facilitators who had delivered this as part of the trial. The intervention was viewed positively by CYP and facilitators as well as by the wider school staff, so much so that in some schools it is being delivered post trial. LEGO® based therapy was not seen as a major burden on staff or wider school business, and, although some challenges with delivery were identified, they were generally perceived to be outweighed by the perceived benefits of the intervention. Facilitators felt that they had a clear understanding of LEGO® based therapy and that this was easy to communicate to others overall, but sometimes clarification of terminology and modelling the intervention were needed to increase understanding and acceptance. Facilitators also suggested that the LEGO® based therapy training prepared them well for delivery during the trial and that they did not identify any necessary adaptations, other than perhaps altering the frequency or total number of sessions.
Chapter 6 Discussion
Review of main findings
The I-SOCIALISE trial recruited 260 autistic CYP from 103 schools, 250 of whom (from 98 schools) were randomised to either LEGO® based therapy with usual support (i.e. the intervention arm) or usual support alone (i.e. the control arm). Using the main outcome measure of social skills (measured using the SSIS social skills subscale), a marginally significant modest positive difference of 3.74 points (95% CI –0.16 to 7.63 points; p = 0.06) was seen at 20 weeks post randomisation for CYP in the intervention arm using ITT analysis. The values contained in the 95% CIs for the ITT were below the pre-specified MCID of 9.6 points. However, the primary and pre-planned sensitivity analyses of the primary outcome provide consistent evidence of a modest positive difference, with standardised effect sizes between 0.15 and 0.21 of the SD of the points score at baseline. In particular, the estimated difference between arms increased slightly in the pre-planned per-protocol analysis to 4.23 points (95% CI 0.27 to 8.19 points; p = 0.036). Only 3 of the 31 pre-specified secondary outcomes were significant at the 5% level, but the majority of the effect sizes favoured the intervention arm, and this corroborates to some extent the modest impact observed for the primary outcome.
Compared with usual support alone, LEGO® based therapy with usual support was found to be likely to reduce overall costs (from both the NHS/PSS and the societal perspectives) and provide marginal improvement in QALYs, as evidenced by both primary and secondary analyses. Overall, the self-report and independent assessments of fidelity showed that the intervention was delivered with a high/moderately high degree of fidelity, and the intervention was reported as acceptable to both the teaching staff delivering the intervention and most parents/guardians (teachers > parents). Many of the schools in the trial considered LEGO® based therapy to be a helpful semistructured intervention and have continued to deliver the intervention after the end of the study. These modest and limited positive findings are considered in relation to the study as a whole, the strengths and weaknesses of the trial, and previous intervention evaluation research.
The pre-specified definition of a MCID (i.e. 9.6 points on the SSIS social skills subscale) was identified prospectively by clinicians on our research team based on a Cochrane review,28 which suggested that a MCID of 0.47 standard deviation would be appropriate. The modest positive findings in this trial can be contextualised by findings from other study outcomes. There is support from an improvement in internalising (emotion) scores from the parent-completed SSIS at the primary outcome time point (–1.23 points, 95% CI –2.40 to –0.05 points; p = 0.04) and also at 52 weeks (–1.23 points, 95% CI –2.40 to –0.05 points; p = 0.04). The total parent-/guardian-rated mental health score (using the SDQ) at the primary outcome time point also showed lower scores in the intervention arm than in the control arm, although this finding was not significant (–0.93 points, 95% CI –1.95 to 0.08 points; p = 0.07). Teacher-rated self-control approached statistical significance at 20 weeks (0.94 points, 95% CI –0.04 to 1.92 points; p = 0.06) and at 52 weeks (1.40 points, 95% CI 0.25 to 2.54 points; p = 0.02). The other outcome measures show no statistically significant differences. These individual results should be viewed with caution because of the number of secondary outcome measures; however, the forest plots indicate overall support for a modest improvement in outcomes.
This was a clinical effectiveness and cost-effectiveness RCT; therefore, we should consider the health economics outcomes carefully.
Cost-effectiveness analysis
Quality of life, using both the EQ-5D-Y and the CHU-9D, was rated higher by those in the intervention arm than those in the control arm. These differences are relatively small, but it is notable that both the parent-completed and the CYP-completed questionnaires showed the same positive differences. In addition, in the sensitivity analysis, when plotting the incremental cost of LEGO® based therapy against the incremental QALYs, the majority of bootstrapped incremental cost–effect pairs were in the fourth quadrant (i.e. the bottom right-hand quadrant). This indicates that, after taking uncertainty into consideration and adjusting for the imbalanced baseline utility, the differences in EQ-5D-Y and CHU-9D scores remain positive, and the costs of LEGO® based therapy remain lower than for usual support.
The results of the health economics analysis show a marginal increase in QALYs and reduction in school-based intervention costs [such as Social Stories™ (Carol Gray) and visual schedules] when comparing the intervention arm with the control arm. The results also show a reduction in overall costs (albeit with wide CIs), particularly through attendance at CAMHS. The lowered CAMHS costs found in the intervention therapy arm appear to be related more to a small number of CYP receiving high levels of high-tariff CAMHS support in the control arm than to a general drop across the whole group. This is a relatively positive finding in that many of the autistic CYP had evidence of co-occurring emotional and behavioural problems at baseline and CYP in both trial arms seemed to be receiving approximately similar support from CAMHS. It was also found that LEGO® based therapy resulted in a reduction in the mean frequency of CAMHS support received compared with baseline. Given the high threshold for receiving CAMHS support, it is likely that the CYP requiring support had more severe needs. Such findings should be treated with caution and need to be further investigated. The reduction in education costs may be related to the fact that CYP in a LEGO® based therapy intervention might be less likely to be put forward for other interventions (e.g. the SULP). 41 There was evidence, however, that schools reported a range of other interventions being received by autistic CYP in both arms of the trial, including Social Stories, visual schedules and one-to-one mentoring. The reduction in wider costs, including CAMHS costs, is worthy of comment. It could be that the clinical improvement or prevention of clinical deterioration lead to less likelihood of referral to CAMHS or a discharge from CAMHS if a child or young person is already in their care. It is important, however, to consider alternative explanations. For example, if the intervention improves comorbid problems, this might lead to a reduced likelihood of referral to CAMHS (e.g. for behavioural problems)88 or the child or young person may stop being seen by CAMHS. 89 We explored this by looking at changes in the subscales of the SDQ and found no statistically significant difference between arms for any of the five subscales. If a school-based intervention is improving a child or young person’s social skills and is viewed positively by teachers and parents/guardians, then this may reduce the likelihood for referral to CAMHS90 because of reduced levels of teacher and parent/guardian concern. However, research to date suggests that higher parental anxiety tends to reduce referral rates, not increase them, in many circumstances. 91 Other possible reasons for reduced costs might include a belief by a parent/guardian that an active intervention is happening and so for this reason taking part in another intervention at the same time is not necessary.
Qualitative analysis
Overall, there was a high level of intervention fidelity for the delivery of LEGO® based therapy.
The qualitative analysis suggested that LEGO® based therapy was very acceptable to facilitators and was also viewed positively by CYP and the wider school staff.
Facilitators believed that the LEGO® based therapy training prepared them well for delivery during the trial and did not identify any crucially necessary adaptations. Facilitators commonly reported observing improvements in both communication and social skills for CYP in intervention sessions. There were mixed findings about whether or not the CYP generalised these skills. Some described improvements being taken into other settings (e.g. into the playground), whereas others reported that some CYP appeared to struggle to generalise these skills. Previous authors have described problems with the generalisation of social skills learning in autistic CYP. 92 However, it is also clear from research that autistic CYP are not a homogeneous group and many autistic CYP can generalise learning in the right conditions, with some CYP finding this easier than others. 93,94 Identifying the components of effective interventions that create the conditions that both accommodate the differing needs of autistic CYP and promote skills development is an important area for future research.
For a new school-based intervention, training generally ran smoothly, with the intervention being delivered to a high level of fidelity and not too resource intensive for busy school environments in terms of training, supervision or delivery.
Subgroup and secondary analyses
Turning to the subgroup analysis to explore the moderating effects of ASD symptom severity (as measured using the SCQ), the results showed no clear pattern (see Table 15). There is no convincing evidence of a differential effect for CYP with different levels of ASD symptomatology. However, when an interaction term between subgroups and intervention was added to the primary outcome model for the ITT population it was borderline statistically significant (p = 0.08), and an additional unplanned sensitivity analysis looking at the subgroup effect for selected secondary outcomes was equivocal. How might we understand these findings? Taking an active role in LEGO® based therapy is reported to provide a structured, play-based opportunity for autistic CYP to develop socioemotional learning skills at a developmentally appropriate pace. 37
Post hoc exploratory analyses were conducted to explore the potential for observational analysis of the trial data in a subsequent substudy. These analyses show that the effect size is potentially greater for certain subgroups and where there is a critical mass of autistic participants in therapy groups. These additional analyses are not intended to be confirmatory and are not used in drawing the overall conclusion of a modest borderline significant effect. In this post hoc unplanned analysis we were able to look into the effect of school size and found some limited evidence that schools with six or more autistic CYP had a larger difference (8.23 points, 95% CI 1.79 to 14.68 points) than schools with fewer than six autistic CYP (1.45 points, 95% CI –2.95 to 5.85 points). Interestingly, in these schools the improvement approached the MCID. Our findings suggest a greater intervention effect when delivery adhered more closely to the protocol and there were two or more CYP with a diagnosis of ASD in each therapy session, as shown by the unplanned per-protocol analysis, which shows an effect of 4.56 points (95% CI 0.24 to 8.87 points; p = 0.038). These results should be read with caution but may provide direction for future research planning. It is instructive to consider in more detail the issue of numbers of autistic CYP in the school. There are a number of possible mechanisms. One of these is that schools with a large number of autistic CYP may have more staff training opportunities and larger numbers of skilled and trained teachers and TAs with knowledge and experience of ASD. This may mean that, when we were training teachers and TAs to deliver LEGO® based therapy, some may have already had considerable knowledge of ASD and practical experience of working with autistic CYP and that this aided their ability to deliver the intervention. Another possibility is that, because of the larger numbers of autistic CYP in the school, it was more likely that more than one autistic CYP would be in the group. There is some evidence in adult ASD research that peer-to-peer communication95 and more relaxed social interaction96,97 may be facilitated by autistic people being together.
The capacity to gain new social skills and integrate social and emotional learning in everyday social interactions are tasks integral to successful social functioning and relationships. 98,99 There is evidence that in autistic individuals these skills can continue to develop through childhood and into adulthood. 100 This skills development over time was perhaps also supported by our secondary post hoc analysis finding that the beneficial effect was higher for secondary school CYP, at 7.45 points (95% CI –0.93 to 15.83 points). However, we found no meaningful differences in loneliness scores between groups from baseline to either 20 or 52 weeks. These subgroup analyses were not pre-planned and the results should therefore be treated with caution. Although these findings are modest, they are interesting given that, prior to the study, during our development and consultation work, peer reviewers questioned whether or not secondary school-aged CYP would enjoy or engage in LEGO® based therapy. There are common misconceptions that LEGO is a toy for younger children. In fact, LEGO bricks appeal to a very wide developmental and chronological age range, including CYP and adults, as evidenced by very active adult fans of LEGO (AFOL) communities [e.g. there are AFOL groups with over 20,000 members on Facebook (Metaverse Platforms, Inc., Menlo Park, CA, USA) at the time of writing]. In fact, attrition rates and qualitative interviewing showed strong engagement and acceptability from secondary school participants. This is in line with the fact that the intervention was originally designed for CYP without learning disability, with a good level of language and in line with previous unpublished research exploring LEGO® based therapy that showed positive outcomes for children aged 9–17 years. 101
It is common practice in the UK for autistic CYP to be placed in mainstream schools, particularly if they do not have a learning disability. As a result, the population of autistic CYP is widely spread rather than concentrated in SEN schools. During recruitment we found that a number of primary schools had only one child or young person in the school who was eligible for the trial. This is reflected in the 51% of sessions in primary schools that were run with just one autistic child or young person. Simple infographic data collected from the additional non-autistic CYP showed that, although some were neurotypically developing CYP, others were being assessed for ASD or had other social communication difficulties. We were able to carry out an unplanned compliance analysis using participants in groups with an average of two or more autistic CYP in their sessions as the definition of compliance, and this provided borderline significant evidence (p = 0.06, 95% CI –0.2 to 11.5 points) that greater numbers of autistic CYP in sessions were associated with a higher estimated effect on the main outcome measure (i.e. 5.7 points). Indeed, the CI for effect for compliers had an upper bound above the MCID (at 11.5 points). These signals of possible positive effect may be interpreted in a number of ways. There is a suggestion that LEGO® based therapy is more effective where there are more autistic CYP in a group and in secondary schools in general. There is also a signal that this may also apply where there are more autistic pupils in the school. It may be that there is some confounding taking place, with, for example, larger schools (e.g. secondary schools with ASD units) contributing to all these factors. Larger schools may have larger numbers of staff trained in ASD or be better resourced to support autistic children. One approach to help disentangle this and get maximum use of the data collected is a subsequent in-depth causal impact analysis.
Long-term follow-up: main outcome measure at 52 weeks
Because the long-term outcome point was 52 weeks post randomisation, many of the CYP had moved into a new school year and therefore had a new teacher. Associated teachers were required to know the child or young person well, and this may be particularly relevant in this population owing to the importance of familiarity for autistic CYP; however, this was often difficult where CYP had moved schools and teachers were approached for follow-up after knowing the child or young person for only a short period. Having a more detailed knowledge about a child or young person could influence the rating of the outcome measures in a number of ways. On the one hand, it could be that teachers with longer-term knowledge of the child or young person are able to note subtle changes in the child or young person’s behaviours including, for example, social skills and interactions in the classroom setting. On the other hand, it could be that existing teachers were influenced by a bias in the knowledge that the child or young person had been in an intervention, introducing performance bias. However, it was also common for new teachers to be aware of the child or young person’s previous participation in the intervention, meaning that they may have been susceptible to the same bias. In addition, asking new teachers to complete the questionnaires about their associated child or young person, although necessary for long-term follow-up in a pragmatic school-based study, may have introduced some differences in perspective and styles of questionnaire completion to those of the initial teacher at previous time points. This raises the wider question of what might be the ‘best’ and/or most accurate way to record change in behaviours and skills over time in an education setting. For example, blinded outcome measures from RAs who observe social skills for short periods of time may be reliable in an efficacy evaluation research study but may not reflect overall everyday social competence, especially given the wide variety and variability of behaviours often seen in autistic CYP in different settings, on different days and in different social contexts. 102–104 Blinded observations usually take the form of 40-minute classroom or playground observations and thus may not reflect overall, everyday functioning. Teachers or parents/guardians who know the CYP well provide a different perspective; each may be able to rate CYP on complex social skills, but they are also likely to be influenced by a range of factors (e.g. a desire to see improvement, experience in home or education settings), which again may introduce bias. This is an ongoing concern for methodologists in all complex intervention evaluation efficacy and effectiveness studies of this nature.
It appears that no significant harm was caused by the intervention, based on the limited number of reports of AEs and SAEs throughout the trial. Minimal AEs were recorded and included instances of emotional distress experienced by some CYP participating in groups. Each instance fell under the category detailed in the study’s protocol as possible expected AEs based on the trial population and was reviewed by the study’s DMEC. It is important for those intending to train in and deliver LEGO® based therapy to be aware of and constructively manage these types of expected AEs. Three SAEs were reported but these were deemed unrelated to the intervention.
The trial in context
Despite the promise of school-based interventions and the results of several reviews exploring them,23–25,27,105,106 a recent systematic review and meta-analysis found that group work to develop social skills in autistic CYP shows no statistically significant improvement when using teacher report of outcomes. 22 This demonstrates the challenges of achieving positive outcomes and/or identifying appropriate intervention outcome measures in this vulnerable group. The modest but consistent positive findings in our study in this context are encouraging.
Establishing the delivery of effective school-based interventions has the potential to address inequities in healthcare outcomes. 107 CYP are in schools for long periods of time with both universal classroom support and targeted interventions devoted to their care. This creates the conditions for possible cost-effectiveness benefits owing to, for example, low implementation costs25 compared to specialist services, although in practice few school-based studies explore intervention cost-effectiveness. 25 Our study provides useful cost-effectiveness information.
To date, systematic reviews of interventions that improve social skills in autistic CYP and that can be delivered in schools22–27,105,106 include SULPs41 and other language support interventions, peer-mediated approaches,108 social skills training by allied professionals,109 play-based interventions,110 social skills interventions,23 behavioural interventions,111 communication skills interventions,112 emotion recognition support,113 school structural and cultural changes such as TEACCH®23 (Treatment and Education of Autistic and Related Communication Handicapped Children, University of North Carolina, Chapel Hill, NC, USA) and multicomponent interventions such as JASPER (Joint Attention, Symbolic Play, Engagement and Regulation)114 and SCERTS (Classroom Social, Communication, Emotional Regulation and Transactional Support). 115 These are delivered in a range of ways, including whole-school approaches,23 comprehensive treatment models,27,105 a range of targeted interventions,23,105 parent support or training,116 technological interventions111,117 and after-school clubs. 105 These often involve professionals from outside the classroom with specialist expertise, such as speech and language therapists, to deliver them. There are interventions that can be delivered by teachers or TAs in an individual group setting, including one-to-one mentoring, visual schedules or Social Stories. 61 However, few studies have been evaluated using RCT design,26 and to date the findings have been mixed.
Many interventions for improving social skills in autistic CYP are not naturalistic including, for example, adult-directed learning,26 modelling,118 video feedback,119 visual scripts120 or other techniques. The LEGO® based therapy intervention places CYP in a fun setting in which they are playing with toys. It makes use of intrinsic motivation121 by being enjoyable, unlike many other therapies for social skill development. Furthermore, this study has shown that the intervention can be delivered reliably in classrooms by school staff such as TAs and teachers with training and shows positive cost-effectiveness results.
Strengths and limitations of the trial
Strengths of the trial
To the best of our knowledge, the I-SOCIALISE trial is the first large-scale RCT evaluating the clinical effectiveness and cost-effectiveness of LEGO® based therapy in a UK education context. It was a pragmatic trial run in the real world, with teachers and TAs delivering the intervention. In our opinion, this study has successfully remedied a number of methodological limitations of previous trials of this intervention.
Recruitment to the trial was smooth and to time and target, with a very low attrition rate of 12% for the primary outcome at 20 weeks post randomisation (15% lost to follow-up across all outcome measures), which is in contrast to the drop-out rates of 28–75% reported in a meta-analytic review of child mental health studies. 122 Because strong predictors of drop-out rates found in the meta-analytic review122 were treatment and therapist factors, this was a positive finding.
The trial design incorporated a number of features to reduce bias. Cluster randomisation was used to reduce the possibility of contamination, with staff who were supporting either the intervention or control arm located in different schools to avoid them influencing each other. All trial participants were consented prior to informing the schools which arm the school had been allocated to in order to avoid selection bias. Frequent communication was maintained with both the intervention and control arm schools throughout the duration of the trial (i.e. the recruitment, intervention and follow-up phases) to reduce attrition bias. Control schools were offered the opportunity to receive training in LEGO® based therapy after the end of their involvement in the trial to reduce both demotivation in the control schools, which could impact on data completion, and any risk of contamination if school staff sought out intervention training in LEGO® based therapy from alternative sources during their involvement. Schools were made aware that the study had not yet concluded and results on the intervention’s efficacy and any potential harms were not yet available, but that this information would be shared once the study had completed and the findings had been approved.
Previous research undertaken in a school setting yielded less generalisable results owing to the low sample size. 42 The present study, however, was able to assess LEGO® based therapy with a relatively large sample (for studies of ASD) using well-recognised measures that assess social competence. The nested feasibility study included in this trial allowed us to first assess the feasibility of carrying out the trial and to discover any hinderances to implementation. The trial was specifically designed around the mainstream school environment with an awareness of the needs of education staff, timetabling and other logistical constraints and the needs of CYP (including those with and without ASD). PPI work aided the careful design of the study for this setting. Recruitment and intervention delivery were undertaken with school term times in mind, meaning that recruitment and follow-up periods were built around the long school summer holidays and a shorter amount of time for schools to deliver their 12 sessions of LEGO® based therapy when applicable. The trial team also anticipated that some mainstream schools (especially primary schools) were likely to have a relatively small number of eligible CYP and included in the trial protocol alternative arrangements for ‘additional CYP’ to join intervention groups where necessary.
The trial assessed both the clinical effectiveness and the cost-effectiveness of delivering LEGO® based therapy to autistic CYP in mainstream school settings and included longer-term follow-up. The findings will be of interest to NHS health and social care providers, local authorities, families and community professionals including school staff members.
The fidelity and acceptability work show good completion rates for the self-report measures (facilitators, 93%; parents, 77%) and the fidelity checklists (overall, 99%). The use of Sekhon et al. ’s55 TFA to structure the data collection and analysis in the different aspects of the acceptability substudy was helpful in unifying understanding of a broad concept of acceptability. As the use of the TFA increases in the future, this data set will provide valuable information and enable direct comparisons with the acceptability of other, similar, interventions.
A further strength of this research was the continuous involvement of PPI representatives and experts in LEGO® based therapy from the initial design of the research and the study set-up through to the end of the trial. The Young Dynamos and the NAS contributed to the initial design of the trial alongside a range of school and local authority staff, and provided the study team with invaluable feedback about implementation that allowed the trial to run smoothly throughout recruitment and follow-up stages, ultimately leading to over-recruitment and positive qualitative feedback from participants. Co-applicant Gina Gomez de la Cuesta, an expert in the field, was able to help create an intervention delivery manual (see Report Supplementary Material 1) and training package for all school staff members who were to run LEGO® based therapy sessions (i.e. the facilitators). She also delivered the training package to local authorities in each region and to the study team members who went on to successfully train the school staff, who in turn achieved high rates of treatment fidelity.
The training model used for the trial allowed us to train > 100 schools in the delivery of LEGO® based therapy. Our findings suggest that this model is appropriate for roll-out by local authorities. The study has also improved networking across local authorities, schools and parent groups, with the development of parent support groups and teacher-/school-based networks. This consolidated the expertise of the COMIC research team, which specialises in trialling CYP-friendly and CYP-oriented interventions for CYP with mental health difficulties.
Fidelity
Facilitators’ self-reported fidelity to the intervention was very high, at 99% for core content and 91% for all content. The core content addressed four areas: (1) whether or not the CYP were encouraged and supported to interact and co-operate, (2) if the CYP worked with other CYP to build a model using the three roles, (3) if the facilitator highlighted an aspect of positive social interaction and (4) if the facilitator highlighted an aspect of social interaction involving a challenge and prompted the CYP to problem-solve together. The self-reported fidelity percentages suggest that throughout the research the facilitators were able to deliver the intervention with reasonable adherence to the intervention training manual. For CYP to have received a per-protocol dose of the intervention, participants had to have attended six or more intervention sessions. Overall compliance levels were very good across both primary and secondary schools, with only 9 out of 130 participants attending fewer than six intervention sessions. This suggests good levels of acceptability among both facilitator and CYP participants.
Acceptability feedback
Facilitators did not report LEGO® based therapy feeling burdensome in the school context and reported that it was easy to incorporate into school life. Given the very intense school-life experiences of many education professionals, this outcome was most likely a result of the pragmatic approach to the training and intervention delivery. The qualitative findings from education staff, parents/guardians and CYP show that this is a well-liked intervention that can be delivered in the real world. Indeed, no CYP were excluded from the intervention groups, an important consideration when it is likely that the autistic CYP are also likely to have additional emotional and behavioural needs. 5 Facilitators had a good understanding of LEGO® based therapy and were generally confident in delivering it. There was also a widespread belief that LEGO® based therapy was fun but also that the intervention was delivering more than play for many CYP.
Limitations of the trial
Although the recruited sample is representative of the gender balance in ASD123 and representative of the regions from which we recruited in terms of participant ethnicities,124 it is not representative of the cultural diversity of the UK as a whole. Recruitment was slightly above target and, owing to missing primary outcome data at baseline or at 20 weeks, the overall effective attrition rate was 13%. This was lower than allowed for in the sample size calculations (which was 16%). However, at 20 weeks, the attrition rate was slightly higher in the control arm (at 18%) than in the intervention arm (at 9%). There was a tendency for the control arm to have slightly lower return rates for all measures, although overall return rates were high, at 85%.
Blinding of outcome assessors
Having unblinded outcome measurement is a weakness, although blinded assessments also come with weaknesses. Although some large meta-analyses of healthcare studies suggest that reported outcomes do not differ greatly depending on whether measurement is blinded or unblinded,125 other studies in the field of school-based research suggest that differences in effect sizes can be up to 36%. 126 However, even in high-stakes studies (e.g. in depression in CYP), blinding of outcome measurement is often not achieved because of the methodological challenges of obtaining accurate information about social, emotional or mental health status without knowing the child well. 127
Teacher-completed outcome measures
To assess the sustainability of the LEGO® based therapy intervention into the next academic year, a 52-week (i.e. 1-year) follow-up point was chosen. We worked closely with schools and implemented a number of strategies to support retention and the longer-term follow-up data collection, but despite this, and in common with other education intervention research, there are some limitations in the completeness of some measures, particularly in relation to the teacher-completed measures at 52 weeks.
CYP-completed outcome measures
The CYP-completed questionnaires (the MSPSS, ALS and CHU-9D) are all well validated for use in this population. However, although all the recruited CYP attended mainstream schools, RAs reported that some CYP struggled to complete these self-report questionnaires (some of which had up to 45 items) and/or had difficulties with the language and the format of the rating scales (especially the MSPSS and the ALS), even with the support available. Furthermore, the RAs observed that some CYP were better able to complete these questionnaires at school than at home. Some parents/guardians suggested that this was a result of the school context and that their child felt that work should be kept at school, not brought home. Obtaining the CYP perspective is important. Further research is needed on how best to support CYP, including those with ASD, to complete standardised outcome measures. This should include consideration of factors such as the form of the questionnaire as well as the setting in which the information is obtained (e.g. clinic/school/home, pen and paper/technology assisted).
Supervision and intervention training
For the consistent delivery of manualised interventions, regular supervision would usually be considered a marker of best practice. However, it was not possible for ongoing supervision of facilitators to be provided in this trial. The training team made an open offer for facilitators to make contact if necessary, but those who did make contact rarely asked for guidance on intervention delivery. Some schools reported to the trial team that they had deployed informal in-school supervision by senior staff.
Despite the planned training schedule agreed with the different local authorities, there were several occasions when the local authority’s staff were unable to do the training and study team members were required to deliver the intervention training to schools. Although the training requirement (and potentially the supervision component) is relatively modest for this community-based intervention, dedicated personnel time is an important resource constraint for local authorities, which would need to be addressed before a wider roll-out of this intervention could be successfully implemented into schools.
Complexity of LEGO
The study protocol included ensuring that the complexity of the LEGO sets was appropriate for the age and developmental stages of CYP in the LEGO® based therapy groups. However, despite this, a small number of primary school facilitator staff fed back that some of the LEGO sets were too complicated for the younger children and for some CYP with more severe ASD. Facilitators also reported that it is important that the LEGO set chosen is of the right complexity for each specific group. This aspect of the intervention was not assessed in the study but should be considered in any future study of LEGO® based therapy.
Health economic analysis
The economic analysis was subject to four limitations. First, funding sources for a few types of staff were not always clear. For example, speech and language therapists can be funded by the NHS, schools or local authorities. Such diversity causes difficulties when it comes to costing and reporting the results because detailed information about funders for each member of staff involved is unavailable. Several assumptions have been made based on service locations and published guidelines (i.e. Unit Costs of Health and Social Care 2018)84 for costing. Hence, the summarised cost results for different perspectives need to be treated with caution. However, the overall costs (from the societal perspective) should remain robust.
Second, a significant number of values required for the cost-effectiveness analysis were missing (see Appendix 3, Table 42), partly because of unintended causes and partly because of the attrition in response to questionnaires over time. Overall, around 55.6% of CYP had both EQ-5D-Y and resource use data (NHS/PSS) at all three time points. Among those who had missing values, around 95% had missing resource data and around 85% had missing EQ-5D-Y data. The sources of missing resource data across parents, teachers and facilitators. There is a concern that the number of missing values may introduce biased results as well as inaccurate conclusions. However, our results are unlikely to be affected because the results of complete case were consistent with the base case (i.e. imputed cases).
Third, the calculated intervention costs might have been underestimated. This is because several items associated with training and intervention sessions were not costed owing to data constraints. These included opportunity costs of trainee time, opportunity costs of school venue for delivering interventions, recruitment cost if intervention was rolled out and supervision costs. However, it is unlikely that the results of the dominance of the intervention over usual support would be affected, as these costs are considered to be small. The affects of these costs would be even smaller after allocating them to every CYP and for every session. Further research on the exploration and measurement of the costs with considerations is desirable.
Fourth, our economic analysis assessed the short-term cost-effectiveness of LEGO® based therapy between baseline and the 52-week follow-up only. The cost-effectiveness beyond the 1-year time frame remains unknown. Further model-based evaluation is required to assess longer-term cost-effectiveness (i.e. end of school age, early adulthood or lifetime) and allow for the measurement of CYP’s lost productivity in adulthood.
Qualitative methods
The acceptability substudy had three main limitations. First, this substudy included the perspectives of only facilitators (i.e. educational staff) and parents/guardians (albeit as a proxy for their child). Although both groups did reflect on the CYP’s experiences (in the qualitative interviews and the 20-week survey), it seems reasonable to suggest that greater insight into the acceptability of LEGO® based therapy to CYP could be gained by interviewing CYP directly. Second, a specific limitation of the qualitative interviews was the self-selecting sample of facilitators who provided informed consent to take part in this substudy. It is plausible that we have managed to capture the opinions of only those facilitators who viewed LEGO® based therapy most favourably. Third, it would have been useful to have recruited more facilitators from secondary schools to allow exploration of acceptability of LEGO® based therapy in that context in more depth. Despite these limitations, the findings from both the qualitative and quantitative components of the acceptability substudy are consistent, suggesting that these limitations may not have been too impactful.
Fidelity assessment methods
The limitations of the fidelity assessment include the reliance on video-recordings of only a subsample of LEGO® based therapy sessions. It is possible that these recordings did not provide an accurate representation of how LEGO® based therapy was delivered owing to the Hawthorne effect. 128 Having the resources to record all LEGO® based therapy sessions throughout the trial and in both primary and secondary schools (and then randomly sampling to identify the sessions for independent fidelity assessment) would have been a more robust methodology. However, the time-consuming, resource-intensive nature of that scale of work, including additional consents, particularly for busy staff in mainstream schools, outweighed the benefits of undertaking the work in that way. In addition, there was similarity between the independently and self-assessed record of fidelity, and, as highlighted in the acceptability substudy, facilitators reported that LEGO® based therapy was easy to deliver, as intended.
Implications for practice: meaning of the study and implications for clinicians and policy-makers
This trial addressed areas relevant to current NHS priorities and the NHS Long Term Plan,129 focusing on child mental health and child development,130,131 with particular focus on the delivery of interventions in education contexts. 132,133 On the basis of our findings in the round, although the level of clinical improvement did not meet our predetermined MCID, we believe that implementation of LEGO® based therapy in mainstream schools can provide cost-effective social skills development opportunities for autistic CYP. The intervention, which is both enjoyable for CYP and able to be integrated in everyday school provision, and which can potentially reduce the need for access to specialist NHS intervention and additional school-based interventions, suggests that LEGO® based therapy could be implemented without the need for additional resource in schools at a time when resources are very limited. We recommend that further research evidence on its use is obtained that, together with the findings from this study, can be considered by NICE.
In line with another small, school-based study of LEGO® based therapy42 we found that there were good levels of maintenance of fidelity to the intended intervention delivery. This is an important finding in relation to the training of education staff to deliver the intervention and of future trainers (e.g. in the local authorities). We were able to train school staff successfully in using a model that, with a relatively small amount of additional dedicated resource, could be rolled out on a larger scale. One small but notable gap in the largely positive fidelity ratings was this: facilitators appeared to use fewer rewards than in the original LEGO® based therapy design. 37,39 This could be related to cultural differences from the original work undertaken in the USA or to training and supervision of the education staff responsible for delivering the intervention (i.e. facilitators). These factors, such as the use of rewards, when delivering a community-based intervention in the UK context, provide an important reminder of the importance of ensuring that interventions are accessible across cultural groups and building this into both training and evaluation. Furthermore, it will be important to consider the specific skills that facilitators need to have. Levels of ASD knowledge and practical experience may well be important. Supervision and the development of LEGO facilitator networks may also provide a useful ongoing resource for schools and local authorities to support high levels of fidelity for this manualised intervention.
Recommendations for future research
Previous systematic reviews of social skills interventions in schools have suggested that there is relatively little research in this area and that more is needed. 106
LEGO® based therapy is a complex intervention,134 and we have examined whether or not it is clinically effective and cost-effective. How does LEGO® based therapy promote the learning of social skills? Is it in developing a child or young person’s social problem-solving skills?39,117 Is something else happening?135 For example, it may be that creating a regular opportunity for peer interaction using semistructured play creates more opportunities than usual for social mixing by reducing unoccupied time and/or increasing social interactions,51,136 or through behavioural activation137 or by promoting more opportunities for increased incidental learning of social skills. 138 Other theoretical mechanisms likely to be at play include structured experiential teaching of joint attention and learning of executive functioning skills, which are known to be delayed in some autistic CYP,139 and support for delayed learning of theory of mind skills140,141 and central coherence skills142 that emerge from joint play and discussions about the attitude and feelings of others in the context of that play. The self-esteem building that comes from successful co-operative play may also have beneficial social effects in itself. 135 Further research could explore the mechanisms important to the success of the intervention. One approach to help understand these complexities and get maximum use of the data collected would be to undertake a subsequent in-depth causal impact analysis.
Future research could be designed to examine whether or not subgroups can benefit in different ways at different times from LEGO® based therapy. For example, the suggestion from our work that older autistic CYP in mainstream secondary schools and/or those attending LEGO® based therapy groups with more than one autistic CYP show greater improvements in aspects of social skills competencies could be explored. Similarly, schools have been keen to use the intervention in wider groups of CYP. Future research could be carried out to discover which groups may benefit from it, such as CYP who do not have ASD but are at risk of other social communication problems or social and emotional development delays, such as deaf children who have had reduced exposure to language development,143 premature infants,144 those with language impairments145 or those with very distorted parenting experiences resulting in significant attachment problems. 146 Developmental psychology has long described a need to build on earlier skills before the introduction of later skills,147 with supportive facilitation148 and sequential positive interdependence. 149 Therapies related to social skills may well have a particular developmental place in the course of a autistic CYP’s life, and further research is needed to understand where such therapies are best placed. In this context, it may also be beneficial to investigate whether or not particular skills or tasks require some additional focus prior to or alongside the LEGO® based therapy groups.
The hypothesis that outcomes are better in schools with more autistic children could also be explored, particularly in regard to critical mass and school staff levels of knowledge both individually and collectively.
The early reports of LEGO® based therapy described an intervention group continuing for around 6 months, with some CYP participating for longer (up to 7 years) and/or receiving additional individual therapy sessions. 39 Our study has shown that it is possible to successfully undertake a RCT of LEGO® based therapy in mainstream school settings. However, further work would be needed to investigate the impact of changes of the ‘dose’ of the intervention for CYP with different levels of ASD severity and developmental and chronological age and a range of comorbidities. This is a complex issue in the developing child, and better ways of assessing and modelling cost-effectiveness over time may be helpful. Furthermore, to address inequalities in research, more work with families from more diverse backgrounds should be actively integrated into research calls and protocols, with strong PPI to support this.
In summary, important areas for future research include work to better understand mechanisms of effect, including different doses of intervention; further work on possible differential effects on subgroups, such as older children or schools with a critical mass of autistic students; and work on more sophisticated or long-term approaches to assessing and modelling cost-effectiveness.
Chapter 7 Conclusions
A new school-based intervention for CYP on the autism spectrum, LEGO® based therapy, delivered by school staff had a modest positive effect on social skills development, although this did not meet pre-set thresholds of clinical importance. This study suggests that the intervention can be cost-effective; is well received by participants, their families and by the school staff delivering the intervention; and has a modest positive effect on quality of life. Levels of fidelity to the intervention were good and the intervention was good value for money and has a relatively low level of intensity in terms of staff resources, supervision and delivery. It was largely well received by parents, staff and young people.
Acknowledgements
We gratefully acknowledge the hard work, support and advice from the following people.
Patient and public involvement representatives
The Young Dynamos research advisory group, the NAS, Karen Watson (PPI TSC member), the York Youth Council and several individuals, including Tina Hardman and Ann McLaren.
Participant screening and data collection
Sarah Jacob-Eshtan, Lisa Hackney, Rebecca Hargate, Sam Bennett, Emma Sellers, Holly Taylor, Alix Smith, Katie Sutherland, Richard Campbell, Rachel Hodkinson, Megan Garside, Jane Blackwell, Pavithra Kumar, Jennifer Lomas and Jules Beresford-Dent.
Administrative and clerical support
Sharon Bird, Rita Lynch, Daniel Gottschalk, Katy Harmston, Thasamia Akhtar, Louise Turner, Heather Dakin, Wendy Cattle and Catherine Arthurson.
Sponsor study monitoring
Tahir Idrees.
LEGO® based therapy expertise
Elinor Brett and Abigail Dodson.
Support on data management concerns
Emily Turton.
Support on ethics and governance issues
Sinead Audsley and Stephen Holland.
Others
The authors would also like to thank Daniel LeGoff for information about LEGO® based therapy in the USA, both the LYPFT and the University of York for supporting the COMIC, and the University of Sheffield CTRU for taking this research from an idea to a full trial.
We offer special thanks to the members of our two oversight committees:
-
TSC – David Cottrell (University of Leeds), Karen Watson (PPI), Sue Fletcher-Watson (University of Edinburgh), Fiona Warren (University of Exeter), Michael Morton (University of Glasgow) and Alison Thompson (LYPFT)
-
DMEC – Zoe Hoare (Bangor University), David Simms (Bradford District Care NHS Foundation Trust) and John O’Dwyer (University of Leeds).
Contributions of authors
Barry Wright (https://orcid.org/0000-0002-8692-6001) (Chair of Child Mental Health) conceived of/designed the work, was involved in the interpretation of data, produced the first draft of the report, revised the work critically for important intellectual content and was involved in the final approval of the version to be published.
Ellen Kingsley (https://orcid.org/0000-0002-0964-4588) (Research Fellow) was involved in the acquisition and interpretation of data, produced the first draft of the report, revised the work critically for important intellectual content and was involved in the final approval of the version to be published.
Cindy Cooper (https://orcid.org/0000-0002-2995-5447) (Director of CTRU) conceived of/designed the work, was involved in the interpretation of data, produced the first draft of the report, revised the work critically for important intellectual content and was involved in the final approval of the version to be published.
Katie Biggs (https://orcid.org/0000-0003-4468-7417) (Assistant Director of CTRU) conceived of/designed the work, was involved in the acquisition and interpretation of data, produced the first draft of the report, revised the work critically for important intellectual content and was involved in the final approval of the version to be published.
Matthew Bursnall (https://orcid.org/0000-0002-6519-3558) (Medical Statistician) was involved in the analysis and interpretation of data and revised the work critically for important intellectual content.
Han-I Wang (https://orcid.org/0000-0002-3521-993X) (Research Fellow) was involved in the analysis and interpretation of data and revised the work critically for important intellectual content.
Tim Chater (https://orcid.org/0000-0002-1138-0147) (Data and Information Systems Manager) conceived of/designed the work; was involved in the acquisition, analysis and interpretation of data; and revised the work critically for important intellectual content.
Elizabeth Coates (https://orcid.org/0000-0002-2388-6102) (Research Fellow) conceived of/designed the work, was involved in the analysis of data, conducted the qualitative research, was involved in the interpretation of data and revised the work critically for important intellectual content.
M Dawn Teare (https://orcid.org/0000-0003-3994-0051) (Professor of Biostatistics) was involved in the analysis and interpretation of data and revised the work critically for important intellectual content.
Kirsty McKendrick (https://orcid.org/0000-0003-0497-3582) (Trial Manager) was involved in the acquisition of data for the work and revised the work critically for important intellectual content.
Gina Gomez de la Cuesta (https://orcid.org/0000-0003-4152-1948) (Director) conceived of/designed the work, was involved in the interpretation of data and revised the work critically for important intellectual content.
Amy Barr (https://orcid.org/0000-0002-7990-7451) (RA) was involved in the acquisition of data for the work, conducted the qualitative research, was involved in the interpretation of data and revised the work critically for important intellectual content.
Kiera Solaiman (https://orcid.org/0000-0003-2244-5345) (RA) was involved in the acquisition of data for the work and revised the work critically for important intellectual content.
Anna Packham (https://orcid.org/0000-0003-2705-9339) (RA) was involved in the acquisition of data for the work and revised the work critically for important intellectual content.
David Marshall (https://orcid.org/0000-0001-5969-9539) (Research Fellow) conceived of/designed the work and revised the work critically for important intellectual content.
Danielle Varley (https://orcid.org/0000-0001-7208-8716) [Doctor of Philosophy (PhD) student] conceived of/designed the work, was involved in the acquisition of data for the work and revised the work critically for important intellectual content.
Roshanak Nekooi (https://orcid.org/0000-0002-2730-2207) (RA) was involved in the acquisition of data for the work and revised the work critically for important intellectual content.
Steve Parrott (https://orcid.org/0000-0002-0165-1150) (Reader in Health Economics) was involved in the analysis and interpretation of data and revised the work critically for important intellectual content.
Shehzad Ali (https://orcid.org/0000-0002-8042-3630) (Visiting Senior Research Fellow) conceived of/designed the work.
Simon Gilbody (https://orcid.org/0000-0002-8236-6983) (Director) conceived of/designed the work.
Ann Le Couteur (https://orcid.org/0000-0001-9991-3608) (Emerita Professor of Child and Adolescent Psychiatry) conceived of/designed the work, was involved in the interpretation of data, revised the work critically for important intellectual content and was involved in the final approval of the version to be published.
Data-sharing statement
Requests for patient-level data and statistical code should be made to the corresponding author or, if they are not available, the Director of the University of Sheffield CTRU and will be considered by members of the original TMG, who will release data on a case-by-case basis. The data will not contain any direct identifiers and we will minimise indirect identifiers and remove free-text data to minimise the risk of identification.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PHR programme or the Department of Health and Social Care.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 2013. https://doi.org/10.1176/appi.books.9780890425596.
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet 2006;368:210-5. https://doi.org/10.1016/S0140-6736(06)69041-7.
- Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry 2009;194:500-9. https://doi.org/10.1192/bjp.bp.108.059345.
- Office for National Statistics . 2011 Census Aggregate Data 2017.
- Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 2019;6:819-29. https://doi.org/10.1016/S2215-0366(19)30289-5.
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry Allied Discip 2004;45:212-29. https://doi.org/10.1111/j.1469-7610.2004.00215.x.
- Whitehouse AJ, Watt HJ, Line EA, Bishop DV. Adult psychosocial outcomes of children with specific language impairment, pragmatic language impairment and autism. Int J Lang Commun Disord 2009;44:511-28. https://doi.org/10.1080/13682820802708098.
- Travis L, Sigman M, Ruskin E. Links between social understanding and social behavior in verbally able children with autism. J Autism Dev Disord 2001;31:119-30. https://doi.org/10.1023/a:1010705912731.
- Department for Education and Skills . Autistic Spectrum Disorders: Good Practice Guidance 2002.
- Ochs E, Kremer-Sadlik T, Solomon O, Sirota KG. Inclusion as social practice: views of children with autism. Soc Dev 2001;10:399-41. https://doi.org/10.1111/1467-9507.00172.
- Bagwell CL, Newcomb AF, Bukowski WM. Preadolescent friendship and peer rejection as predictors of adult adjustment. Child Dev 1998;69:140-53. https://doi.org/10.1111/j.1467-8624.1998.tb06139.x.
- Bellini S, Peters JK, Benner L, Hopf A. A meta-analysis of school-based social skills interventions for children with autism spectrum disorders. Remedial Spec Educ 2007;28:153-62. https://doi.org/10.1177/07419325070280030401.
- Bauminger N, Kasari C. Loneliness and friendship in high-functioning children with autism. Child Dev 2000;71:447-56. https://doi.org/10.1111/1467-8624.00156.
- Carrington S, Graham L. Perceptions of school by two teenage boys with Asperger syndrome and their mothers: a qualitative study. Autism 2001;5:37-48. https://doi.org/10.1177/1362361301005001004.
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol 2004;40:271-83. https://doi.org/10.1037/0012-1649.40.2.271.
- Grandgeorge M, Bourreau Y, Alavi Z, Lemonnier E, Tordjman S, Deleau M, et al. Interest towards human, animal and object in children with autism spectrum disorders: an ethological approach at home. Eur Child Adolesc Psychiatry 2015;24:83-9. https://doi.org/10.1007/s00787-014-0528-9.
- Rowley E, Chandler S, Baird G, Simonoff E, Pickles A, Loucas T, et al. The experience of friendship, victimization and bullying in children with an autism spectrum disorder: associations with child characteristics and school placement. Res Autism Spectr Disord 2012;6:1126-34. https://doi.org/10.1016/j.rasd.2012.03.004.
- Killen M, Mulvey KL, Hitti A. Social exclusion in childhood: a developmental intergroup perspective. Child Dev 2013;84:772-90. https://doi.org/10.1111/cdev.12012.
- Sansoni J, Marosszeky N, Sansoni E, . Effective Assessment of Social Isolation. Wollongong, NSW: Centre for Health Service Development, Wollongong University; 2010.
- National Institute for Health and Care Excellence (NICE) . Autism in Under 19s: Support and Management 2013.
- Durlak JA, Weissberg RP, Dymnicki AB, Taylor RD, Schellinger KB. The impact of enhancing students’ social and emotional learning: a meta-analysis of school-based universal interventions. Child Dev 2011;82:405-32. https://doi.org/10.1111/j.1467-8624.2010.01564.x.
- Gates JA, Kang E, Lerner MD. Efficacy of group social skills interventions for youth with autism spectrum disorder: a systematic review and meta-analysis. Clin Psychol Rev 2017;52:164-81. https://doi.org/10.1016/j.cpr.2017.01.006.
- Hirvikoski T, Jonsson U, Halldner L, Lundequist A, de Schipper E, Nordin V, et al. A systematic review of social communication and interaction interventions for patients with autism spectrum disorder. Scand J Child Adolesc Psychiatry Psychol 2015;3:147-68. https://doi.org/10.21307/sjcapp-2015-016.
- Hughes C, Kaplan L, Bernstein R, Boykin M, Reilly C, Brigham N, et al. Increasing social interaction skills of secondary school students with autism and/or intellectual disability: a review of interventions. Res Pract Pers With Sev Disabil 2012;37:288-307. https://doi.org/10.2511/027494813805327214.
- Schmidt M, Werbrouck A, Verhaeghe N, Putman K, Simoens S, Annemans L. Universal mental health interventions for children and adolescents: a systematic review of health economic evaluations. Appl Health Econ Health Policy 2020;18:155-75. https://doi.org/10.1007/s40258-019-00524-0.
- Sutton BM, Webster AA, Westerveld MF. A systematic review of school-based interventions targeting social communication behaviors for students with autism. Autism 2019;23:274-86. https://doi.org/10.1177/1362361317753564.
- Wong C, Odom SL, Hume KA, Cox AW, Fettig A, Kucharczyk S, et al. Evidence-based practices for children, youth, and young adults with autism spectrum disorder: a comprehensive review. J Autism Dev Disord 2015;45:1951-66. https://doi.org/10.1007/s10803-014-2351-z.
- Reichow B, Steiner AM, Volkmar F. Social skills groups for people aged 6 to 21 with autism spectrum disorders (ASD). Cochrane Database Syst Rev 2012;7. https://doi.org/10.1002/14651858.CD008511.pub2.
- Frankel F, Myatt R, Sugar C, Whitham C, Gorospe CM, Laugeson E. A randomized controlled study of parent-assisted Children’s Friendship Training with children having autism spectrum disorders. J Autism Dev Disord 2010;40:827-42. https://doi.org/10.1007/s10803-009-0932-z.
- Koenig K, White SW, Pachler M, Lau M, Lewis M, Klin A, et al. Promoting social skill development in children with pervasive developmental disorders: a feasibility and efficacy study. J Autism Dev Disord 2010;40:1209-18. https://doi.org/10.1007/s10803-010-0979-x.
- Laugeson EA, Frankel F, Mogil C, Dillon AR. Parent-assisted social skills training to improve friendships in teens with autism spectrum disorders. J Autism Dev Disord 2009;39:596-60. https://doi.org/10.1007/s10803-008-0664-5.
- Lopata C, Thomeer ML, Volker MA, Toomey JA, Nida RE, Lee GK, et al. RCT of a manualized social treatment for high-functioning autism spectrum disorders. J Autism Dev Disord 2010;40:1297-310. https://doi.org/10.1007/s10803-010-0989-8.
- Solomon M, Goodlin-Jones BL, Anders TF. A social adjustment enhancement intervention for high functioning autism, Asperger’s syndrome, and pervasive developmental disorder NOS. J Autism Dev Disord 2004;34:649-68. https://doi.org/10.1007/s10803-004-5286-y.
- Licciardello CC, Harchik AE, Luiselli JK. Social skills intervention for children with autism during interactive play at a public elementary school. Educ Treat Child 2008;31:27-3. https://doi.org/10.1353/etc.0.0010.
- LeGoff D, Gomez De La Cuesta G, Krauss GW, Baron-Cohen S. LEGO®-Based Therapy: How to Build Social Competence Through Lego-Based Clubs for Children with Autism and Related Conditions. London: Jessica Kingsley Publishers; 2014.
- North Yorkshire County Council . North Yorkshire County Council Intervention Guidance 2019.
- Owens G, Granader Y, Humphrey A, Baron-Cohen S. LEGO therapy and the Social Use of Language Programme: an evaluation of two social skills interventions for children with high functioning autism and Asperger syndrome. J Autism Dev Disord 2008;38:1944-57. https://doi.org/10.1007/s10803-008-0590-6.
- Delprato DJ. Comparisons of discrete-trial and normalized behavioral language intervention for young children with autism. J Autism Dev Disord 2001;31:315-25. https://doi.org/10.1023/a:1010747303957.
- LeGoff DB. Use of LEGO© as a therapeutic medium for improving social competence. J Autism Dev Disord 2004;34:557-71. https://doi.org/10.1007/s10803-004-2550-0.
- LeGoff DB, Sherman M. Long-term outcome of social skills intervention based on interactive LEGO© play. Autism 2006;10:317-29. https://doi.org/10.1177/1362361306064403.
- Rinaldi W. Social Use of Language Programme. Infant and Primary School Teaching Pack. Cranleigh: Wendy Rinaldi; 2004.
- Levy J, Dunsmuir S. Lego therapy: building social skills for adolescents with an autism spectrum disorder. Educ Child Psychol 2020;37:58-83.
- Lindsay S, Hounsell KG, Cassiani C. A scoping review of the role of LEGO therapy for improving inclusion and social skills among children and youth with autism. Disabil Health J 2017;10:173-82. https://doi.org/10.1016/j.dhjo.2016.10.010.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-32.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345. https://doi.org/10.1136/bmj.e5661.
- Bursnall M. I-SOCIALSE Statistical Analysis Plan v 2.0. University of Sheffield, University of York, Leeds and York Partnership NHS Foundation Trust; n.d.
- Bursnall M. Addendum to the I-SOCIALSE Statistical Analysis Plan v 2.0. University of Sheffield, University of York, Leeds and York Partnership NHS Foundation Trust; n.d.
- World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research 1993.
- Zosh J, Hopkins EJ, Jensen H, Liu C, Neale D, Hirsh-Pasek K, et al. Learning Through Play: A Review of the Evidence. Billund: The LEGO Foundation; 2017.
- Zosh JM, Hirsh-Pasek K, Hopkins EJ, Jensen H, Liu C, Neale D, et al. Accessing the inaccessible: redefining play as a spectrum. Front Psychol 2018;9. https://doi.org/10.3389/fpsyg.2018.01124.
- Humphrey N, Symes W. Peer interaction patterns among adolescents with autistic spectrum disorders (ASDs) in mainstream school settings. Autism 2011;15:397-419. https://doi.org/10.1177/1362361310387804.
- Pimlott-Wilson H. Visualising children’s participation in research: Lego Duplo, rainbows and clouds and moodboards. Int J Soc Res Methodol 2012;15:135-48. https://doi.org/10.1080/13645579.2012.649410.
- Gresham AF, Elliott SN. Social Skills Improvement System (SSIS) Rating Scales. Bloomington, MN: Pearson Assessments; 2008.
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry 1997;38:581-6. https://doi.org/10.1111/j.1469-7610.1997.tb01545.x.
- Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2031-8.
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. J Pers Assess 1988;52:30-41. https://doi.org/10.1207/s15327752jpa5201_2.
- Asher SR, Hymel S, Renshaw PD. Loneliness in children. Child Dev 1984;55:1456-64. https://doi.org/10.2307/1130015.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. https://doi.org/10.2165/11587350-000000000-00000.
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire: Manual. Los Angeles, CA: West Psychol Serv; 2003.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Wright B, Marshall D, Adamson J, Ainsworth H, Ali S, Allgar V, et al. Social Stories™ to alleviate challenging behaviour and social difficulties exhibited by children with autism spectrum disorder in mainstream schools: design of a manualised training toolkit and feasibility study for a cluster randomised controlled trial. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20060.
- Torgerson DJ, Torgerson CJ. Designing Randomised Trials in Health, Education and the Social Sciences: An Introduction. New York, NY: Springer Publishers; 2008.
- Ravens-Sieberer U, Wille N, Badia X, Bonsel G, Burström K, Cavrini G, et al. Feasibility, reliability, and validity of the EQ-5D-Y: results from a multinational study. Qual Life Res 2010;19:887-97. https://doi.org/10.1007/s11136-010-9649-x.
- Barrett B, Byford S, Chitsabesan P, Kenning C. Mental health provision for young offenders: service use and cost. Br J Psychiatry 2006;188:541-6. https://doi.org/10.1192/bjp.bp.105.010108.
- Wright B, Marshall D, Collingridge Moore D, Ainsworth H, Hackney L, Adamson J, et al. Autism Spectrum Social Stories In Schools Trial (ASSSIST): study protocol for a feasibility randomised controlled trial analysing clinical and cost-effectiveness of Social Stories in mainstream schools. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005952.
- Marshall D, Wright B, Allgar V, Adamson J, Williams C, Ainsworth H, et al. Social Stories in mainstream schools for children with autism spectrum disorder: a feasibility randomised controlled trial. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011748.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2016–17 2017. https://improvement.nhs.uk/resources/reference-costs/ (accessed 11 March 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2016.
- Prescribing and Medicines Team . Prescription Cost Analysis, England 2017 2018.
- Department for Education . DfE Consolidated Annual Report and Accounts 2017 to 2018 2018.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- Rubin DB. Statistical matching using file concatenation with adjusted weights and multiple imputations. J Bus Econ Stat 1986;4:87-94. https://doi.org/10.1080/07350015.1986.10509497.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. https://doi.org/10.1002/hec.678.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Spencer L, Ritchie J, O’Connor W, Ritchie J, Lewis J, Nicholls CM, et al. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:52-63. https://doi.org/10.1111/j.1752-7325.2011.00233.x.
- Stata 16 Users Guide. TX, USA: Stata Press; 2019.
- The EuroQol Group . EQ-5D-Y User Guide. Basic Information on How to Use the EQ-5D-Y Instrument 2019. https://euroqol.org/publications/user-guides/ (accessed 11 March 2021).
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017–18 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2019.
- Family and Childcare Trust . Childcare Survey 2018 n.d. www.familyandchildcaretrust.org/childcare-survey-2018 (accessed 12 March 2021).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 11 March 2021).
- Hardeman W, Michie S, Fanshawe T, Prevost AT, Mcloughlin K, Kinmonth AL. Fidelity of delivery of a physical activity intervention: predictors and consequences. Psychol Health 2008;23:11-24.
- Costello EJ, Janiszewski S. Who gets treated? Factors associated with referral in children with psychiatric disorders. Acta Psychiatr Scand 1990;81:523-9. https://doi.org/10.1111/j.1600-0447.1990.tb05492.x.
- Miller LM, Southam-Gerow MA, Allin RB. Who stays in treatment? Child and family predictors of youth client retention in a public mental health agency. Child Youth Care Forum 2008;37:153-70. https://doi.org/10.1007/s10566-008-9058-2.
- Sayal K. Annotation: pathways to care for children with mental health problems. J Child Psychol Psychiatry 2006;47:649-59. https://doi.org/10.1111/j.1469-7610.2005.01543.x.
- Jongerden L, Simon E, Bodden DH, Dirksen CD, Bögels SM. Factors associated with the referral of anxious children to mental health care: the influence of family functioning, parenting, parental anxiety and child impairment. Int J Methods Psychiatr Res 2015;24:46-57. https://doi.org/10.1002/mpr.1457.
- Gunning C, Holloway J, Fee B, Breathnach Ó, Bergin CM, Greene I, et al. A systematic review of generalization and maintenance outcomes of social skills intervention for preschool children with autism spectrum disorder. Rev J Autism Dev Disord 2019;6:172-99. https://doi.org/10.1007/s40489-019-00162-1.
- Church BA, Rice CL, Dovgopoly A, Lopata CJ, Thomeer ML, Nelson A, et al. Learning, plasticity, and atypical generalization in children with autism. Psychon Bull Rev 2015;22:1342-8. https://doi.org/10.3758/s13423-014-0797-9.
- Schmidt C, Stichter JP. The use of peer-mediated interventions to promote the generalization of social competence for adolescents with high-functioning autism and Asperger’s syndrome. Exceptionality 2012;20:94-113. https://doi.org/10.1080/09362835.2012.669303.
- Crompton CJ, Ropar D, Evans-Williams CV, Flynn EG, Fletcher-Watson S. Autistic peer-to-peer information transfer is highly effective. Autism 2020;24:1704-12. https://doi.org/10.1177/1362361320919286.
- Davis R, Crompton CJ. What do new findings about social interaction in autistic adults mean for neurodevelopmental research?. Perspect Psychol Sci 2021;16:649-53. https://doi.org/10.1177/1745691620958010.
- Crompton CJ, Hallett S, Ropar D, Flynn E, Fletcher-Watson S. ‘I never realised everybody felt as happy as I do when I am around autistic people’: a thematic analysis of autistic adults’ relationships with autistic and neurotypical friends and family. Autism 2020;24:1438-48. https://doi.org/10.1177/1362361320908976.
- Arslan E, Durmuşoǧlu-Saltali N, Yilmaz H. Social skills and emotional and behavioral traits of preschool children. Soc Behav Pers 2011;39:1281-7. https://doi.org/10.2224/sbp.2011.39.9.1281.
- Glick GC, Rose AJ. Prospective associations between friendship adjustment and social strategies: friendship as a context for building social skills. Dev Psychol 2011;47:1117-32. https://doi.org/10.1037/a0023277.
- Laugeson EA, Gantman A, Kapp SK, Orenski K, Ellingsen R. A randomized controlled trial to improve social skills in young adults with autism spectrum disorder: the UCLA PEERS Program. J Autism Dev Disord 2015;45:3978-89. https://doi.org/10.1007/s10803-015-2504-8.
- Nguyen CTCH. Sociality in Autism: Building Social Bridges in Autism Spectrum Conditions through LEGO-Based Therapy 2016.
- Brown SM, Bebko JM. Generalization, overselectivity, and discrimination in the autism phenotype: a review. Res Autism Spectr Disord 2012;6:733-40. https://doi.org/10.1016/j.rasd.2011.10.012.
- Gresham FM, Elliott SN. The relationship between adaptive behavior and social skills: Issues in definition and assessment. J Spec Educ 1987;21:167-81. https://doi.org/10.1177/002246698702100115.
- Rao PA, Beidel DC, Murray MJ. Social skills interventions for children with Asperger’s syndrome or high-functioning autism: a review and recommendations. J Autism Dev Disord 2008;38:353-61. https://doi.org/10.1007/s10803-007-0402-4.
- Bond C, Symes W, Hebron J, Humphrey N, Morewood G, Woods K. Educational interventions for children with ASD: a systematic literature review 2008–13. Sch Psychol Int 2016;37:303-20. https://doi.org/10.1177/0143034316639638.
- Chang YC, Locke J. A systematic review of peer-mediated interventions for children with autism spectrum disorder. Res Autism Spectr Disord 2016;27:1-10. https://doi.org/10.1016/j.rasd.2016.03.010.
- Knopf JA, Finnie RK, Peng Y, Hahn RA, Truman BI, Vernon-Smiley M, et al. School-based health centers to advance health equity: a community guide systematic review. Am J Prev Med 2016;51:114-26. https://doi.org/10.1016/j.amepre.2016.01.009.
- Kasari C, Rotheram-Fuller E, Locke J, Gulsrud A. Making the connection: randomized controlled trial of social skills at school for children with autism spectrum disorders. J Child Psychol Psychiatry 2012;53:431-9. https://doi.org/10.1111/j.1469-7610.2011.02493.x.
- Mazurik-Charles R, Stefanou C. Using paraprofessionals to teach social skills to children with autism spectrum disorders in the general education classroom. J Instr Psychol 2010;37:161-9.
- Kossyvaki L, Papoudi D. A review of play interventions for children with autism at school. Int J Disabil Dev Educ 2016;63:45-63. https://doi.org/10.1080/1034912X.2015.1111303.
- Patterson SY, Smith V, Jelen M. Behavioural intervention practices for stereotypic and repetitive behaviour in individuals with autism spectrum disorder: a systematic review. Dev Med Child Neurol 2010;52:318-27. https://doi.org/10.1111/j.1469-8749.2009.03597.x.
- Hansen SG, Blakely AW, Dolata JK, Raulston T, Machalicek W. Children with autism in the inclusive preschool classroom: a systematic review of single-subject design interventions on social communication skills. Rev J Autism Dev Disord 2014;1:192-206. https://doi.org/10.1007/s40489-014-0020-y.
- Ramdoss S, Machalicek W, Rispoli M, Mulloy A, Lang R, O’Reilly M. Computer-based interventions to improve social and emotional skills in individuals with autism spectrum disorders: a systematic review. Dev Neurorehabil 2012;15:119-35. https://doi.org/10.3109/17518423.2011.651655.
- Goods KS, Ishijima E, Chang YC, Kasari C. Preschool based JASPER intervention in minimally verbal children with autism: pilot RCT. J Autism Dev Disord 2013;43:1050-6. https://doi.org/10.1007/s10803-012-1644-3.
- Morgan L, Hooker JL, Sparapani N, Reinhardt VP, Schatschneider C, Wetherby AM. Cluster randomized trial of the classroom SCERTS intervention for elementary students with autism spectrum disorder. J Consult Clin Psychol 2018;86:631-44. https://doi.org/10.1037/ccp0000314.
- Rispoli KM, Mathes NE, Malcolm AL. Characterizing the parent role in school-based interventions for autism: a systematic literature review. Sch Psychol 2019;34:444-57. https://doi.org/10.1037/spq0000283.
- Ke F, Whalon K, Yun J. Social skill interventions for youth and adults with autism spectrum disorder: a systematic review. Rev Educ Res 2018;88:3-42. https://doi.org/10.3102/0034654317740334.
- Elliot S, Roach A, Beddow P. Best Practices in School Psychology. Bethesda, ML: National Association of School Psychologists; 2008.
- Plavnick J, Sam A, Hume K, Odom S. Effects of video-based group instruction for adolescents with autism spectrum disorder. Except Child 2013;80:67-83. https://doi.org/10.1177/001440291308000103.
- Ganz JB, Heath AK, Lund EM, Camargo SP, Rispoli MJ, Boles M, et al. Effects of peer-mediated implementation of visual scripts in middle school. Behav Modif 2012;36:378-98. https://doi.org/10.1177/0145445512442214.
- Mastrangelo S. Play and the child with autism spectrum disorder: from possibilities to practice. Int J Play Ther 2009;18:13-30. https://doi.org/10.1037/a0013810.
- de Haan AM, Boon AE, de Jong JT, Hoeve M, Vermeiren RR. A meta-analytic review on treatment dropout in child and adolescent outpatient mental health care. Clin Psychol Rev 2013;33:698-711. https://doi.org/10.1016/j.cpr.2013.04.005.
- Lai MC, Baron-Cohen S, Buxbaum JD. Understanding autism in the light of sex/gender. Mol Autism 2015;6. https://doi.org/10.1186/s13229-015-0021-4.
- Office for National Statistics . Population and Demography for North Yorkshire 2018.
- Moustgaard H, Clayton GL, Jones HE, Boutron I, Jørgensen L, Laursen DRT, et al. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ 2020;368. https://doi.org/10.1136/bmj.l6802.
- Veloso A, Vicente SG, Filipe MG. Effectiveness of cognitive training for school-aged children and adolescents with attention deficit/hyperactivity disorder: a systematic review. Front Psychol 2019;10. https://doi.org/10.3389/fpsyg.2019.02983.
- Merry SN, Hetrick SE, Cox GR, Brudevold-Iversen T, Bir JJ, McDowell H. Psychological and educational interventions for preventing depression in children and adolescents. Cochrane Database Syst Rev 2011;12. https://doi.org/10.1002/14651858.CD003380.pub3.
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014;67:267-77. https://doi.org/10.1016/j.jclinepi.2013.08.015.
- NHS England . NHS Long Term Plan n.d. www.longtermplan.nhs.uk (accessed 25 February 2022).
- Department of Health and Department for Education . Transforming Children and Young People’s Mental Health Provision: A Green Paper 2017.
- Royal College of Paediatrics and Child Health . NHS Long Term Plan – A Summary of Child Health Proposals n.d. www.rcpch.ac.uk/resources/nhs-long-term-plan-summary-child-health-proposals (accessed 9 December 2020).
- Department for Health and Social Care and Department for Education . Government Response to the Consultation on Transforming Children and Young People’s Mental Health Provision: A Green Paper and Next Steps 2018.
- Wilson E. Where next for youth mental health? Reflections on current research and considerations for the future. J Ment Health 2020;29:371-5. https://doi.org/10.1080/09638237.2020.1766001.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Krasny L, Williams BJ, Provencal S, Ozonoff S. Social skills interventions for the autism spectrum: essential ingredients and a model curriculum. Child Adolesc Psychiatr Clin N Am 2003;12:107-22. https://doi.org/10.1016/S1056-4993(02)00051-2.
- Orsmond GI, Kuo HY. The daily lives of adolescents with an autism spectrum disorder: discretionary time use and activity partners. Autism 2011;15:579-99. https://doi.org/10.1177/1362361310386503.
- Tindall L, Mikocka-Walus A, McMillan D, Wright B, Hewitt C, Gascoyne S. Is behavioural activation effective in the treatment of depression in young people? A systematic review and meta-analysis. Psychol Psychother 2017;90:770-96. https://doi.org/10.1111/papt.12121.
- Brown W, McEvoy M, Bishop N. Incidental teaching of social behavior: a naturalistic approach for promoting young children’s peer interactions. Teach Except Child 1991;24:35-8. https://doi.org/10.1177/004005999102400109.
- Lai CLE, Lau Z, Lui SSY, Lok E, Tam V, Chan Q, et al. Meta-analysis of neuropsychological measures of executive functioning in children and adolescents with high-functioning autism spectrum disorder. Autism Res 2017;10:911-39. https://doi.org/10.1002/aur.1723.
- Andreou M, Skrimpa V. Theory of mind deficits and neurophysiological operations in autism spectrum disorders: a review. Brain Sci 2020;10. https://doi.org/10.3390/brainsci10060393.
- Baron-Cohen S. Theory of mind and autism: a review. Int Rev Res Ment Retard 2000;23:169-84. https://doi.org/10.1016/S0074-7750(00)80010-5.
- Happé F, Briskman J, Frith U. Exploring the cognitive phenotype of autism: weak ‘central coherence’ in parents and siblings of children with autism: I. Experimental tests. J Child Psychol Psychiatry 2001;42:299-307. https://doi.org/10.1111/1469-7610.00723.
- Wright B, Oakes P. Does socio-emotional developmental delay masquerade as autism in some deaf children?. Int J Ment Health Deaf 2012;2:45-51.
- Ritchie K, Bora S, Woodward LJ. Social development of children born very preterm: a systematic review. Dev Med Child Neurol 2015;57:899-918. https://doi.org/10.1111/dmcn.12783.
- Gerber S, Brice A, Capone N, Fujiki M, Timler G. Language use in social interactions of school-age children with language impairments: an evidence-based systematic review of treatment. Lang Speech Hear Serv Sch 2012;43:235-49. https://doi.org/10.1044/0161-1461(2011/10-0047).
- Luke N, Banerjee R. Differentiated associations between childhood maltreatment experiences and social understanding: a meta-analysis and systematic review. Dev Rev 2013;33:1-28. https://doi.org/10.1016/j.dr.2012.10.001.
- Cantor P, Osher D, Berg J, Steyer L, Rose T. Malleability, plasticity, and individuality: how children learn and develop in context. Appl Dev Sci 2019;23:307-37. https://doi.org/10.1080/10888691.2017.1398649.
- Wood D, Bruner JS, Ross G. The role of tutoring in problem solving. J Child Psychol Psychiatry 1976;17:89-100. https://doi.org/10.1111/j.1469-7610.1976.tb00381.x.
- Doolittle PE. Vygotsky’s Zone of Proximal Development as a theoretical foundation for cooperative Learning. J Excell Coll Teach 1997;8:83-103.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2018–2019 2018.
- Prescribing and Medicines Team . Prescription Cost Analysis, England 2018 2019.
- National Joint Council for Local Government Services . National Agreement on Pay and Conditions of Service 2018. www.unison.org.uk/content/uploads/2018/07/NJC-Green-Book-18.pdf (accessed 25 February 2022).
- Family and Childcare Trust . Childcare Survey 2019 n.d. www.familyandchildcaretrust.org/childcare-survey-2019 (accessed 12 March 2021).
- Office for National Statistics (ONS) . Average Weekly Earnings in Great Britain 2018 2018.
Appendix 1 Additional table to methods
| Time point | Participant | Form | Number of form items | Maximum items completed per participant | Minimum items completed per participant | Average items completed per participant | Total items completed | Total errors found | Percentage error rate |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Parent/guardian/child | Consent | 9 | 9 | 7 | 8.89 | 2312 | 10 | 0.43 |
| Parent/guardian | SCQ | 40 | 40 | 27 | 39.62 | 10,300 | 4 | 0.04 | |
| Parent/guardian | Eligibility and screening | 11 | 11 | 11 | 11.00 | 2860 | 5 | 0.17 | |
| Parent/guardian | Booklet completion | 3 | 3 | 2 | 2.34 | 607 | 3 | 0.49 | |
| Parent/guardian | Baseline information | 29 | 29 | 19 | 24.54 | 6357 | 26 | 0.41 | |
| Parent/guardian | Contact details | 7 | 7 | 1 | 6.14 | 1577 | 15 | 0.95 | |
| Parent/guardian | SSIS | 125 | 125 | 75 | 123.86 | 32,080 | 44 | 0.14 | |
| Parent/guardian | SDQ | 34 | 34 | 24 | 32.88 | 8516 | 31 | 0.36 | |
| Parent/guardian | RU | 78 | 78 | 0 | 23.12 | 6011 | 10 | 0.17 | |
| Parent/guardian | EQ-5D-Y | 6 | 6 | 5 | 5.99 | 1545 | 1 | 0.06 | |
| Child | Booklet completion | 3 | 3 | 1 | 2.25 | 578 | 2 | 0.35 | |
| Child | ALS | 24 | 24 | 4 | 23.72 | 5858 | 5 | 0.09 | |
| Child | MSPSS | 12 | 12 | 1 | 11.89 | 2914 | 9 | 0.31 | |
| Child | CHU-9D | 9 | 9 | 5 | 8.94 | 2199 | 10 | 0.45 | |
| Teacher | Booklet completion | 4 | 4 | 2 | 3.28 | 823 | 12 | 1.46 | |
| Teacher | SSIS | 129 | 129 | 75 | 127.24 | 31,938 | 40 | 0.13 | |
| Teacher | SDQ | 32 | 32 | 26 | 30.81 | 7734 | 14 | 0.18 | |
| Teacher | RU | 53 | 53 | 0 | 17.48 | 4546 | 54 | 1.19 | |
| 20-week follow-up | Parent/guardian | Booklet completion | 3 | 3 | 2 | 2.56 | 502 | 4 | 0.80 |
| Parent/guardian | SSIS | 125 | 125 | 67 | 122.32 | 23,975 | 83 | 0.35 | |
| Parent/guardian | SDQ | 34 | 34 | 25 | 33.01 | 6469 | 18 | 0.28 | |
| Parent/guardian | RU | 65 | 65 | 0 | 17.62 | 4582 | 65 | 1.42 | |
| Parent/guardian | ED-5D-Y | 6 | 6 | 5 | 5.99 | 1168 | 6 | 0.51 | |
| Parent/guardian | Acceptability form | 12 | 12 | 1 | 11.32 | 1109 | 10 | 0.90 | |
| Child | Booklet completion | 3 | 3 | 1 | 2.19 | 537 | 6 | 1.12 | |
| Child | ALS | 24 | 24 | 3 | 23.77 | 4374 | 22 | 0.50 | |
| Child | MSPSS | 12 | 12 | 4 | 11.94 | 2185 | 20 | 0.92 | |
| Child | CHU-9D | 9 | 9 | 8 | 8.98 | 1653 | 9 | 0.54 | |
| Teacher | Booklet completion | 4 | 4 | 2 | 3.60 | 806 | 15 | 1.86 | |
| Teacher | SSIS | 129 | 129 | 77 | 125.61 | 28,137 | 121 | 0.43 | |
| Teacher | SDQ | 32 | 32 | 25 | 30.68 | 6872 | 28 | 0.41 | |
| Teacher | RU | 57 | 57 | 0 | 16.28 | 4234 | 60 | 1.42 | |
| 52-week follow-up | Parent/guardian | Booklet completion | 3 | 3 | 2 | 2.49 | 451 | 3 | 0.67 |
| Parent/guardian | SSIS | 125 | 125 | 78 | 122.96 | 22,256 | 20 | 0.09 | |
| Parent/guardian | SDQ | 34 | 34 | 8 | 32.85 | 5945 | 14 | 0.24 | |
| Parent/guardian | RU | 66 | 66 | 0 | 17.91 | 4657 | 24 | 0.52 | |
| Parent/guardian | ED-5D-Y | 6 | 6 | 4 | 5.98 | 1082 | 3 | 0.28 | |
| Parent/guardian | Change in circumstances | 6 | 6 | 1 | 2.30 | 411 | 0 | 0.00 | |
| Child | Booklet completion | 3 | 3 | 1 | 2.12 | 512 | 3 | 0.59 | |
| Child | ALS | 24 | 24 | 14 | 23.79 | 4068 | 2 | 0.05 | |
| Child | MSPSS | 12 | 12 | 9 | 11.98 | 2024 | 1 | 0.05 | |
| Child | CHU-9D | 9 | 9 | 7 | 8.98 | 1527 | 1 | 0.07 | |
| Teacher | Booklet completion | 4 | 4 | 2 | 3.55 | 720 | 8 | 1.11 | |
| Teacher | SSIS | 129 | 129 | 78 | 125.36 | 25,574 | 36 | 0.14 | |
| Teacher | SDQ | 32 | 32 | 25 | 30.68 | 6197 | 13 | 0.21 | |
| Teacher | RU | 59 | 59 | 0 | 14.00 | 3639 | 42 | 1.15 | |
| All | Teacher | Consent | 3 | 3 | 0 | 2.93 | 911 | 7 | 0.77 |
| Teacher | Contact details | 10 | 10 | 1 | 7.36 | 2281 | 6 | 0.26 |
Appendix 2 Additional tables to statistical analyses
FIGURE 8.
The CONSORT flow diagram: as treated. a, Children (n = 3) who were allocated to the control arm but received the intervention are included in the intervention arm.
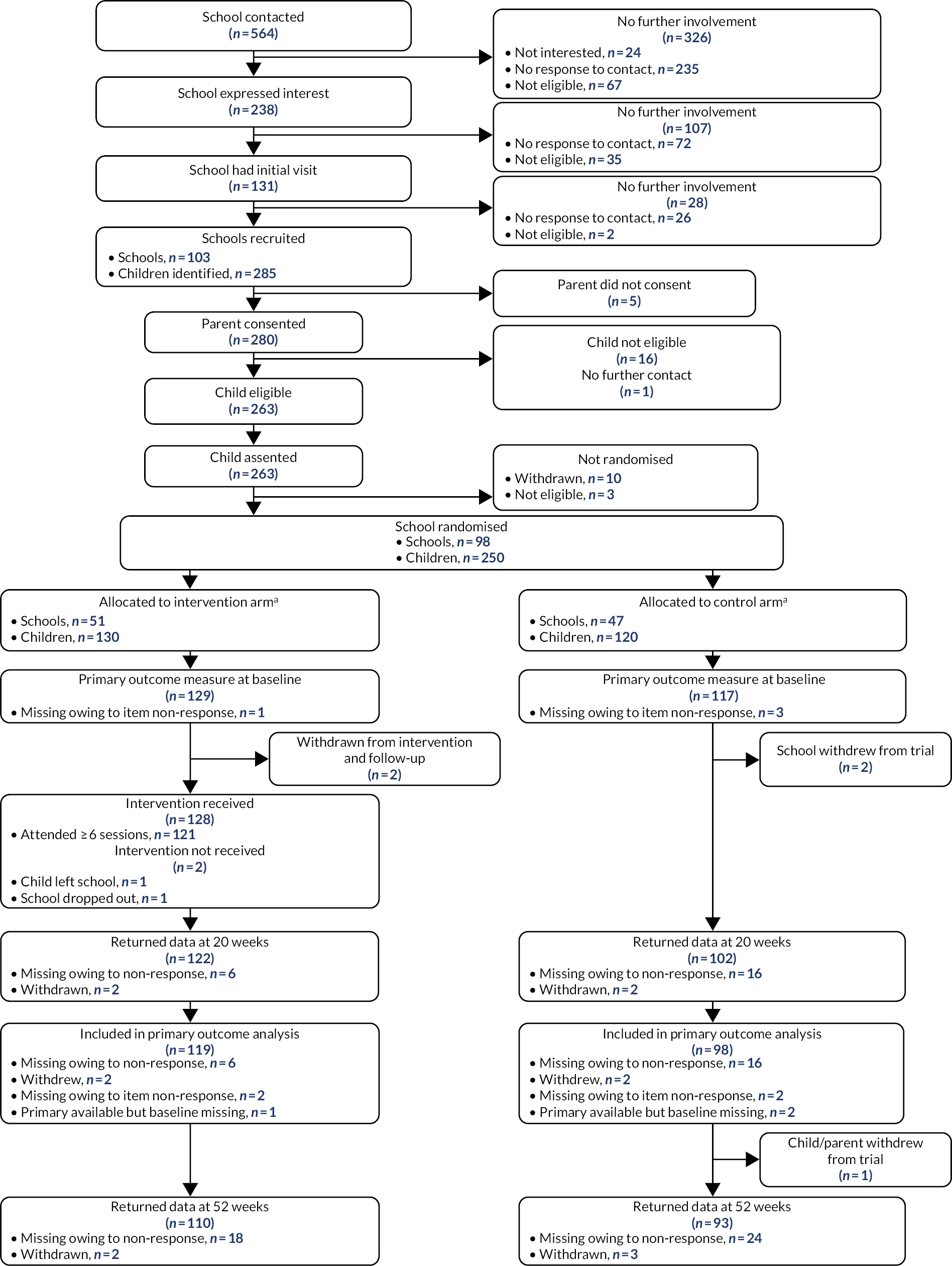
| Time point | Variable | Intervention arm (n) | Control arm (n) |
|---|---|---|---|
| Baseline | Primary collected | 126 | 120 |
| Missing owing to item non-response | 1 | 3 | |
| 20 weeks | Primary collected and participant included in primary analysisa | 116 | 98 |
| Primary collected; participant included in primary analysis; participant withdrew from intervention only between baseline and 20 weeksa | 0 | 3 | |
| Primary collected but baseline missing | 1 | 2 | |
| Missing owing to survey non-response | 6 | 16 | |
| Missing owing to item non-response | 2 | 2 | |
| Participant withdrew from intervention and evaluation between randomisation and 20 weeks | 2 | 2 | |
| 50 weeks | Primary collected | 107 | 88 |
| Missing owing to survey non-response | 16 | 26 | |
| Missing owing to item non-response | 2 | 1 | |
| Parent/guardian/CYP withdrew from evaluation between 20 and 52 weeks; teacher provided data | 0 | 5 | |
| Participant withdrew from intervention and evaluation between randomisation and 20 weeks | 2 | 2 | |
| Participant withdrew from intervention and evaluation between 20 and 52 weeks | 0 | 1 |
| Time point | Variable | Intervention arm (n) | Control arm (n) |
|---|---|---|---|
| Baseline | Primary collected | 129 | 117 |
| Missing owing to item non-response | 1 | 3 | |
| 20 weeks | Primary collected and participant included in the as-treated analysisa | 119 | 95 |
| Primary collected; participant included in the as-treated analysis; participant withdrew from intervention only between baseline and 20 weeksa | 0 | 3 | |
| Primary collected but baseline missing | 1 | 2 | |
| Missing owing to survey non-response | 6 | 16 | |
| Missing owing to item non-response | 2 | 2 | |
| Participant withdrew from intervention and evaluation between randomisation and 20 weeks | 2 | 2 | |
| 50 weeks | Primary collected | 108 | 87 |
| Missing owing to survey non-response | 18 | 24 | |
| Missing owing to item non-response | 2 | 1 | |
| Parent/guardian/CYP withdrew from evaluation between 20 and 52 weeks; teacher provided data | 0 | 5 | |
| Participant withdrew from intervention and evaluation between randomisation and 20 weeks | 2 | 2 | |
| Participant withdrew from intervention and evaluation between 20 and 52 weeks | 0 | 1 |
| Withdrawal | Intervention arm (N = 127), n (%) | Control arm (N = 123), n (%) | All (N = 250), n (%) |
|---|---|---|---|
| School withdrew between randomisation and 20 weeks | 1 (1) | 2 (2) | 3 (1) |
| CYP left school between randomisation and 20 weeks; unable to contact parent | 1 (1) | 0 (0) | 1 (0) |
| CYP/parent/guardian withdrew between randomisation and 20 weeks as unhappy with allocation; teacher provided all follow-up | 0 (0) | 2 (2) | 2 (1) |
| CYP/parent/guardian withdrew between randomisation and 20 weeks as unwilling to complete follow-up; teacher provided all follow-up | 0 (0) | 1 (1) | 1 (0) |
| CYP/parent/guardian withdrew between 20 and 52 weeks as unwilling to complete follow-up; teacher provided all follow-up | 0 (0) | 1 (1) | 1 (0) |
| CYP/parent/guardian withdrew between 20 and 52 weeks as unwilling to complete follow-up; teacher provided follow-up at 20 weeks | 0 (0) | 1 (1) | 1 (0) |
| CYP/parent/guardian withdrew between 20 and 52 weeks owing to personal/family reasons; teacher provided all follow-up | 0 (0) | 1 (1) | 1 (0) |
| Variable | Intervention arm (N = 127), n (%) | Control arm (N = 123), n (%) | All (N = 250), n (%) |
|---|---|---|---|
| ASD diagnosis | |||
| Yes | 127 (100) | 123 (100) | 250 (100) |
| Diagnosis conferred by | |||
| Psychiatrist | 7 (6) | 8 (7) | 15 (6) |
| Clinical psychologist | 27 (21) | 42 (34) | 69 (28) |
| Paediatrician | 20 (16) | 16 (13) | 36 (14) |
| Speech and language therapist | 1 (1) | 2 (2) | 3 (1) |
| Educational psychologist | 2 (2) | 3 (2) | 5 (2) |
| Ryegate Children’s Centre (Sheffield, UK) | 23 (18) | 16 (13) | 39 (16) |
| CAMHS | 5 (4) | 6 (5) | 11 (4) |
| Clinical nurse specialist | 1 (1) | 0 (0) | 1 (0) |
| Lime Trees Child and Adolescent Mental Health Service (York, UK) | 1 (1) | 0 (0) | 1 (0) |
| SENCO | 1 (1) | 0 (0) | 1 (0) |
| Panel of clinicians | 6 (5) | 4 (3) | 10 (4) |
| Unclear (illegible entry in free-text box) | 1 (1) | 1 (1) | 2 (1) |
| Not known/not sure | 2 (2) | 3 (2) | 5 (2) |
| Missing data | 30 (24) | 22 (18) | 52 (21) |
| Multidisciplinary diagnosis | |||
| No | 4 (3) | 8 (7) | 12 (5) |
| Yes | 113 (89) | 111 (90) | 224 (90) |
| Missing data | 10 (8) | 4 (3) | 14 (6) |
| Approximate number of years since ASD diagnosis | |||
| < 1 | 20 (16) | 20 (16) | 40 (16) |
| 1–2 | 44 (35) | 41 (33) | 85 (34) |
| 3–4 | 26 (20) | 20 (16) | 46 (18) |
| ≥ 5 | 31 (24) | 35 (28) | 66 (26) |
| Missing data | 6 (5) | 7 (6) | 13 (5) |
| Diagnosis letter received | |||
| No | 1 (1) | 4 (3) | 5 (2) |
| Yes | 124 (98) | 117 (95) | 241 (96) |
| Missing data | 2 (2) | 2 (2) | 4 (2) |
| CAMHS support | |||
| No | 115 (91) | 115 (93) | 230 (92) |
| Yes | 10 (8) | 8 (7) | 18 (7) |
| Missing data | 2 (2) | 0 (0) | 2 (1) |
| ASD diagnosis instrument | |||
| ADI-R | |||
| Yes | 14 (11) | 14 (11) | 28 (11) |
| Not ticked | 113 (89) | 109 (89) | 222 (89) |
| ADOS | |||
| Yes | 29 (23) | 36 (29) | 65 (26) |
| Not ticked | 98 (77) | 87 (71) | 185 (74) |
| CDI | |||
| Yes | 4 (3) | 5 (4) | 9 (4) |
| Not ticked | 123 (97) | 118 (96) | 241 (96) |
| DISCO | |||
| Yes | 12 (9) | 6 (5) | 18 (7) |
| Not ticked | 115 (91) | 117 (95) | 232 (93) |
| Clinical interview | |||
| Yes | 15 (12) | 21 (17) | 36 (14) |
| Not ticked | 112 (88) | 102 (83) | 214 (86) |
| Not sure/don’t know | |||
| Yes | 75 (59) | 74 (60) | 149 (60) |
| Not ticked | 52 (41) | 49 (40) | 101 (40) |
| Other | |||
| Yes | 5 (4) | 4 (3) | 9 (4) |
| Not ticked | 122 (96) | 119 (97) | 241 (96) |
| Characteristic | Primary available, n (%) | Primary missing, n (%) | ||||
|---|---|---|---|---|---|---|
| Intervention arm (N = 116) | Control arm (N = 101) | All (N = 217) | Intervention arm (N = 11) | Control arm (N = 22) | All (N = 33) | |
| Child’s gender | ||||||
| Male | 92 (79) | 74 (73) | 166 (76) | 10 (91) | 18 (82) | 28 (85) |
| Female | 24 (21) | 27 (27) | 51 (24) | 1 (9) | 4 (18) | 5 (15) |
| Child’s academic age (years) | ||||||
| 6 | 7 (6) | 6 (6) | 13 (6) | 2 (18) | 0 (0) | 2 (6) |
| 7 | 23 (20) | 16 (16) | 39 (18) | 2 (18) | 5 (23) | 7 (21) |
| 8 | 22 (19) | 16 (16) | 38 (18) | 1 (9) | 2 (9) | 3 (9) |
| 9 | 16 (14) | 19 (19) | 35 (16) | 2 (18) | 5 (23) | 7 (21) |
| 10 | 13 (11) | 15 (15) | 28 (13) | 1 (9) | 2 (9) | 3 (9) |
| 11 | 17 (15) | 7 (7) | 24 (11) | 1 (9) | 5 (23) | 6 (18) |
| 12 | 7 (6) | 12 (12) | 19 (9) | 0 (0) | 3 (14) | 3 (9) |
| 13 | 3 (3) | 5 (5) | 8 (4) | 1 (9) | 0 (0) | 1 (3) |
| 14 | 8 (7) | 5 (5) | 13 (6) | 1 (9) | 0 (0) | 1 (3) |
| Child’s ethnicity | ||||||
| English/Welsh/Scottish/Northern Irish/British | 94 (81) | 86 (85) | 180 (83) | 8 (73) | 21 (95) | 29 (88) |
| Any other white background | 2 (2) | 1 (1) | 3 (1) | 1 (9) | 0 (0) | 1 (3) |
| Pakistani | 5 (4) | 3 (3) | 8 (4) | 0 (0) | 0 (0) | 0 (0) |
| Bangladeshi | 1 (1) | 0 (0) | 1 (0) | 1 (9) | 0 (0) | 1 (3) |
| Any other Asian background | 2 (2) | 1 (1) | 3 (1) | 0 (0) | 0 (0) | 0 (0) |
| White and Black Caribbean | 1 (1) | 1 (1) | 2 (1) | 1 (9) | 0 (0) | 1 (3) |
| White and Asian | 1 (1) | 3 (3) | 4 (2) | 0 (0) | 1 (5) | 1 (3) |
| Any other mixed/multiple ethnic background | 1 (1) | 2 (2) | 3 (1) | 0 (0) | 0 (0) | 0 (0) |
| African | 3 (3) | 1 (1) | 4 (2) | 0 (0) | 0 (0) | 0 (0) |
| Caribbean | 2 (2) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) |
| Arab | 1 (1) | 1 (1) | 2 (1) | 0 (0) | 0 (0) | 0 (0) |
| Any other ethnic group | 2 (2) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) |
| Prefer not to say | 1 (1) | 2 (2) | 3 (1) | 0 (0) | 0 (0) | 0 (0) |
| ASD diagnosis | ||||||
| Yes | 116 (100) | 101 (100) | 217 (100) | 11 (100) | 22 (100) | 33 (100) |
| Diagnosis conferred by | ||||||
| Psychiatrist | 5 (4) | 5 (5) | 10 (5) | 2 (18) | 3 (14) | 5 (15) |
| Clinical psychologist | 25 (22) | 32 (32) | 57 (26) | 2 (18) | 10 (45) | 12 (36) |
| Paediatrician | 19 (16) | 14 (14) | 33 (15) | 1 (9) | 2 (9) | 3 (9) |
| Speech and language therapist | 1 (1) | 2 (2) | 3 (1) | 0 (0) | 0 (0) | 0 (0) |
| Educational psychologist | 2 (2) | 3 (3) | 5 (2) | 0 (0) | 0 (0) | 0 (0) |
| Ryegate Children’s Centre | 23 (20) | 13 (13) | 36 (17) | 0 (0) | 3 (14) | 3 (9) |
| CAMHS | 4 (3) | 5 (5) | 9 (4) | 1 (9) | 1 (5) | 2 (6) |
| Clinical nurse specialist | 1 (1) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lime Trees Child and Adolescent Mental Health Service | 1 (1) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
| SENCO | 1 (1) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
| Panel of clinicians | 5 (4) | 4 (4) | 9 (4) | 1 (9) | 0 (0) | 1 (3) |
| Free text not clear | 1 (1) | 0 (0) | 1 (0) | 0 (0) | 1 (5) | 1 (3) |
| Not known/not sure | 2 (2) | 3 (3) | 5 (2) | 0 (0) | 0 (0) | 0 (0) |
| Missing data | 26 (22) | 20 (20) | 46 (21) | 4 (36) | 2 (9) | 6 (18) |
| Multidisciplinary diagnosis | ||||||
| No | 3 (3) | 8 (8) | 11 (5) | 1 (9) | 0 (0) | 1 (3) |
| Yes | 104 (90) | 90 (89) | 194 (89) | 9 (82) | 21 (95) | 30 (91) |
| Missing data | 9 (8) | 3 (3) | 12 (6) | 1 (9) | 1 (5) | 2 (6) |
| Approximate number of years since ASD diagnosis | ||||||
| < 1 | 19 (16) | 16 (16) | 35 (16) | 1 (9) | 4 (18) | 5 (15) |
| 1–2 | 39 (34) | 34 (34) | 73 (34) | 5 (45) | 7 (32) | 12 (36) |
| 3–4 | 24 (21) | 16 (16) | 40 (18) | 2 (18) | 4 (18) | 6 (18) |
| ≥ 5 | 29 (25) | 29 (29) | 58 (27) | 2 (18) | 6 (27) | 8 (24) |
| Missing data | 5 (4) | 6 (6) | 11 (5) | 1 (9) | 1 (5) | 2 (6) |
| Diagnosis letter received | ||||||
| No | 1 (1) | 4 (4) | 5 (2) | 0 (0) | 0 (0) | 0 (0) |
| Yes | 113 (97) | 95 (94) | 208 (96) | 11 (100) | 22 (100) | 33 (100) |
| Missing data | 2 (2) | 2 (2) | 4 (2) | 0 (0) | 0 (0) | 0 (0) |
| CAMHS support | ||||||
| No | 104 (90) | 97 (96) | 201 (93) | 11 (100) | 18 (82) | 29 (88) |
| Yes | 10 (9) | 4 (4) | 14 (6) | 0 (0) | 4 (18) | 4 (12) |
| Missing data | 2 (2) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | 0 (0) |
| Characteristic | Primary available | Primary missing | ||||
|---|---|---|---|---|---|---|
| Intervention arm (N = 116) | Control arm (N = 101) | All (N = 217) | Intervention arm (N = 11) | Control arm (N = 22) | All (N = 33) | |
| Child’s age (years) | ||||||
| Mean (SD) | 9.6 (2.3) | 9.7 (2.1) | 9.6 (2.2) | 9.6 (2.5) | 9.8 (2.0) | 9.8 (2.1) |
| Median (IQR) | 9 (8–11) | 9 (8–11) | 9 (8–11) | 9 (7–12) | 10 (8–12) | 9 (8–12) |
| Baseline SCQ score (points) | ||||||
| Mean (SD) | 25.0 (5.3) | 24.2 (5.2) | 24.6 (5.3) | 26.5 (2.9) | 24.4 (5.1) | 25.1 (4.6) |
| Median (IQR) | 25 (21–29) | 24 (21–28) | 25 (21–29) | 27 (25–28) | 26 (21–28) | 26 (23–28) |
| Baseline social skills score (points) | ||||||
| Mean (SD) | 65.2 (18.9) | 65.0 (21.5) | 65.1 (20.1) | 76.8 (22.2) | 64.2 (21.9) | 68.5 (22.5) |
| Median (IQR) | 63 (50–79) | 65 (48–77) | 64 (50–78) | 78 (55–95) | 70 (41–81) | 72 (53–83) |
| Variable | Intervention arm (N = 127) | Control arm (N = 123) | All (N = 250) |
|---|---|---|---|
| 20 weeks | |||
| n (%) | 116 (91) | 101 (82) | 217 (87) |
| Mean (SD) | 7.7 (16.1) | 4.4 (16.5) | 6.1 (16.3) |
| Median (IQR) | 6 (–2 to 17) | 3 (–5 to 15) | 5 (–4 to 15) |
| Minimum, maximum | –34, 48 | –41, 51 | –41, 51 |
| 52 weeks | |||
| n (%) | 106 (83) | 90 (73) | 196 (78) |
| Mean (SD) | 7.0 (20.9) | 8.3 (20.9) | 7.6 (20.9) |
| Median (IQR) | 6 (–5 to 21) | 8 (–5 to 23) | 6 (–5 to 22) |
| Minimum, maximum | –64, 73 | –40, 62 | –64, 73 |
| Outcome | Time point | Summary measure | Intervention arm (N = 127) | Control arm (N = 123) | All (N = 250) |
|---|---|---|---|---|---|
| 1: Perceived social isolation and loneliness | |||||
| ALS score, points | Baseline | n (%) | 109 (86) | 103 (84) | 212 (85) |
| Mean (SD) | 37.6 (11.7) | 36.4 (11.7) | 37.0 (11.7) | ||
| Median (IQR) | 38 (28–45) | 35 (26–46) | 37 (28–45) | ||
| Minimum, maximum | 18, 74 | 16, 63 | 16, 74 | ||
| 20 weeks | n (%) | 87 (69) | 86 (70) | 173 (69) | |
| Mean (SD) | 37.3 (12.1) | 37.3 (11.5) | 37.3 (11.7) | ||
| Median (IQR) | 36 (27–44) | 37 (30–43) | 36 (29–44) | ||
| Minimum, maximum | 17, 70 | 16, 76 | 16, 76 | ||
| 52 weeks | n (%) | 82 (65) | 69 (56) | 151 (60) | |
| Mean (SD) | 36.0 (11.1) | 38.6 (10.6) | 37.2 (10.9) | ||
| Median (IQR) | 35 (26–45) | 37 (30–47) | 37 (29–46) | ||
| Minimum, maximum | 18, 66 | 18, 60 | 18, 66 | ||
| MSPSS significant other subscale score, points | Baseline | n (%) | 119 (94) | 116 (94) | 235 (94) |
| Mean (SD) | 5.8 (1.1) | 5.8 (1.2) | 5.8 (1.2) | ||
| Median (IQR) | 6 (5–7) | 6 (5–7) | 6 (5–7) | ||
| Minimum, maximum | 2, 7 | 1, 7 | 1, 7 | ||
| 20 weeks | n (%) | 90 (71) | 91 (74) | 181 (72) | |
| Mean (SD) | 5.8 (1.3) | 5.8 (1.2) | 5.8 (1.2) | ||
| Median (IQR) | 6 (5–7) | 6 (5–7) | 6 (5–7) | ||
| Minimum, maximum | 1, 7 | 3, 7 | 1, 7 | ||
| 52 weeks | n (%) | 90 (71) | 78 (63) | 168 (67) | |
| Mean (SD) | 5.8 (1.4) | 5.6 (1.2) | 5.7 (1.3) | ||
| Median (IQR) | 6 (5–7) | 6 (5–7) | 6 (5–7) | ||
| Minimum, maximum | 1, 7 | 2, 7 | 1, 7 | ||
| 2: Sustainability into next academic year | |||||
| SSI (teacher) social skills subscale score, points | Baseline | n (%) | 126 (99) | 120 (98) | 246 (98) |
| Mean (SD) | 66.1 (19.3) | 64.9 (21.4) | 65.5 (20.4) | ||
| Median (IQR) | 64 (50–79) | 66 (48–77) | 65 (50–79) | ||
| Minimum, maximum | 29, 117 | 26, 129 | 26, 129 | ||
| 52 weeks | n (%) | 107 (84) | 93 (76) | 200 (80) | |
| Mean (SD) | 74.8 (18.0) | 72.8 (18.5) | 73.8 (18.2) | ||
| Median (IQR) | 74 (62–86) | 73 (60–88) | 74 (61–87) | ||
| Minimum, maximum | 30, 121 | 29, 118 | 29, 121 | ||
| 3: Academic competence | |||||
| Academic competence points | Baseline | n (%) | 122 (96) | 121 (98) | 243 (97) |
| Mean (SD) | 9.6 (7.3) | 12.1 (7.6) | 10.8 (7.5) | ||
| Median (IQR) | 10 (2–15) | 13 (7–18) | 10 (4–16) | ||
| Minimum, maximum | 0, 28 | 0, 28 | 0, 28 | ||
| 20 weeks | n (%) | 112 (88) | 101 (82) | 213 (85) | |
| Mean (SD) | 10.1 (7.5) | 12.8 (8.2) | 11.4 (7.9) | ||
| Median (IQR) | 10 (4–15) | 13 (7–19) | 11 (4–17) | ||
| Minimum, maximum | 0, 28 | 0, 28 | 0, 28 | ||
| 52 weeks | n (%) | 100 (79) | 92 (75) | 192 (77) | |
| Mean (SD) | 9.7 (7.0) | 13.1 (7.8) | 11.3 (7.6) | ||
| Median (IQR) | 9 (4–15) | 14 (8–19) | 12 (5–17) | ||
| Minimum, maximum | 0, 28 | 0, 28 | 0, 28 | ||
| 4: Emotional and behavioural symptoms, points | |||||
| SDQ (teacher) score | Baseline | n (%) | 127 (100) | 123 (100) | 250 (100) |
| Mean (SD) | 15.7 (5.9) | 15.8 (5.8) | 15.8 (5.8) | ||
| Median (IQR) | 15 (12–20) | 15 (12–20) | 15 (12–20) | ||
| Minimum, maximum | 2, 31 | 1, 30 | 1, 31 | ||
| 20 weeks | n (%) | 119 (94) | 105 (85) | 224 (90) | |
| Mean (SD) | 14.9 (5.6) | 14.6 (5.6) | 14.8 (5.6) | ||
| Median (IQR) | 15 (11–19) | 15 (10–19) | 15 (11–19) | ||
| Minimum, maximum | 2, 27 | 3, 28 | 2, 28 | ||
| 52 weeks | n (%) | 109 (86) | 93 (76) | 202 (81) | |
| Mean (SD) | 14.1 (6.3) | 15.2 (6.1) | 14.6 (6.2) | ||
| Median (IQR) | 14 (10–18) | 15 (11–20) | 15 (10–19) | ||
| Minimum, maximum | 1, 29 | 2, 32 | 1, 32 | ||
| SDQ (parent) score | Baseline | n (%) | 127 (100) | 123 (100) | 250 (100) |
| Mean (SD) | 21.4 (5.4) | 22.2 (5.3) | 21.8 (5.3) | ||
| Median (IQR) | 22 (17–25) | 22 (18–26) | 22 (18–26) | ||
| Minimum, maximum | 10, 33 | 8, 34 | 8, 34 | ||
| 20 weeks | n (%) | 102 (80) | 94 (76) | 196 (78) | |
| Mean (SD) | 20.4 (5.6) | 21.8 (5.7) | 21.1 (5.7) | ||
| Median (IQR) | 21 (16–24) | 22 (18–26) | 21 (17–25) | ||
| Minimum, maximum | 7, 33 | 2, 35 | 2, 35 | ||
| 52 weeks | n (%) | 101 (80) | 79 (64) | 180 (72) | |
| Mean (SD) | 20.5 (5.7) | 20.6 (5.9) | 20.5 (5.8) | ||
| Median (IQR) | 20 (16–25) | 21 (17–24) | 20 (17–25) | ||
| Minimum, maximum | 7, 32 | 4, 37 | 4, 37 | ||
| SSIS (teacher) problem behaviours subscale score | Baseline | n (%) | 127 (100) | 119 (97) | 246 (98) |
| Mean (SD) | 30.7 (12.2) | 30.6 (13.9) | 30.7 (13.0) | ||
| Median (IQR) | 30 (20–41) | 29 (19–41) | 29 (20–41) | ||
| Minimum, maximum | 7, 65 | 2, 68 | 2, 68 | ||
| 20 weeks | n (%) | 119 (94) | 104 (85) | 223 (89) | |
| Mean (SD) | 27.8 (10.8) | 28.9 (13.4) | 28.3 (12.1) | ||
| Median (IQR) | 28 (20–36) | 28 (20–38) | 28 (20–36) | ||
| Minimum, maximum | 6, 57 | 3, 65 | 3, 65 | ||
| 52 weeks | n (%) | 109 (86) | 92 (75) | 201 (80) | |
| Mean (SD) | 27.8 (13.6) | 29.4 (13.0) | 28.5 (13.3) | ||
| Median (IQR) | 26 (18–38) | 29 (19–40) | 27 (19–39) | ||
| Minimum, maximum | 3, 64 | 4, 57 | 3, 64 | ||
| SSIS (parent) problem behaviours subscale score | Baseline | n (%) | 126 (99) | 121 (98) | 247 (99) |
| Mean (SD) | 48.7 (14.6) | 49.0 (13.5) | 48.8 (14.0) | ||
| Median (IQR) | 49 (38–58) | 50 (40–57) | 49 (39–58) | ||
| Minimum, maximum | 20, 84 | 7, 80 | 7, 84 | ||
| 20 weeks | n (%) | 100 (79) | 93 (76) | 193 (77) | |
| Mean (SD) | 45.6 (13.7) | 48.3 (12.4) | 46.9 (13.1) | ||
| Median (IQR) | 46 (35–55) | 48 (40–56) | 48 (38–56) | ||
| Minimum, maximum | 16, 85 | 11, 82 | 11, 85 | ||
| 52 weeks | n (%) | 101 (80) | 79 (64) | 180 (72) | |
| Mean (SD) | 46.6 (15.3) | 46.6 (12.4) | 46.6 (14.0) | ||
| Median (IQR) | 47 (36–57) | 46 (39–54) | 46 (38–55) | ||
| Minimum, maximum | 4, 83 | 19, 85 | 4, 85 | ||
| 5: Assertion, self-control, internalising and externalising | |||||
| SSIS (teacher) assertion subscale score, points | Baseline | n (%) | 126 (99) | 120 (98) | 246 (98) |
| Mean (SD) | 9.4 (4.2) | 9.7 (4.4) | 9.5 (4.3) | ||
| Median (IQR) | 9 (7–12) | 10 (7–13) | 10 (7–13) | ||
| Minimum, maximum | 0, 19 | 0, 18 | 0, 19 | ||
| 20 weeks | n (%) | 117 (92) | 103 (84) | 220 (88) | |
| Mean (SD) | 10.1 (3.7) | 10.4 (4.2) | 10.2 (3.9) | ||
| Median (IQR) | 10 (8–13) | 11 (7–13) | 11 (7–13) | ||
| Minimum, maximum | 0, 18 | 0, 20 | 0, 20 | ||
| 52 weeks | n (%) | 107 (84) | 93 (76) | 200 (80) | |
| Mean (SD) | 10.8 (3.6) | 10.9 (4.2) | 10.9 (3.9) | ||
| Median (IQR) | 11 (8–13) | 11 (9–14) | 11 (8–14) | ||
| Minimum, maximum | 1, 17 | 1, 20 | 1, 20 | ||
| SSIS (teacher) self-control subscale score, points | Baseline | n (%) | 126 (99) | 120 (98) | 246 (98) |
| Mean (SD) | 8.7 (4.3) | 8.2 (4.5) | 8.4 (4.4) | ||
| Median (IQR) | 9 (6–12) | 8 (5–11) | 8 (5–11) | ||
| Minimum, maximum | 0, 19 | 0, 20 | 0, 20 | ||
| 20 weeks | n (%) | 117 (92) | 103 (84) | 220 (88) | |
| Mean (SD) | 10.0 (4.3) | 9.0 (4.6) | 9.5 (4.5) | ||
| Median (IQR) | 11 (6–13) | 9 (6–12) | 10 (6–13) | ||
| Minimum, maximum | 0, 19 | 0, 21 | 0, 21 | ||
| 52 weeks | n (%) | 107 (84) | 93 (76) | 200 (80) | |
| Mean (SD) | 10.4 (4.2) | 8.9 (4.2) | 9.7 (4.2) | ||
| Median (IQR) | 11 (8–14) | 9 (6–12) | 10 (7–13) | ||
| Minimum, maximum | 0, 19 | 0, 18 | 0, 19 | ||
| SSIS (teacher) externalising subscale score, points | Baseline | n (%) | 127 (100) | 119 (97) | 246 (98) |
| Mean (SD) | 9.9 (6.7) | 10.5 (7.7) | 10.2 (7.2) | ||
| Median (IQR) | 9 (4–14) | 9 (4–17) | 9 (4–15) | ||
| Minimum, maximum | 0, 29 | 0, 31 | 0, 31 | ||
| 20 weeks | n (%) | 119 (94) | 104 (85) | 223 (89) | |
| Mean (SD) | 8.9 (5.8) | 9.6 (6.8) | 9.2 (6.3) | ||
| Median (IQR) | 8 (4–13) | 9 (4–13) | 9 (4–13) | ||
| Minimum, maximum | 0, 27 | 0, 32 | 0, 32 | ||
| 52 weeks | n (%) | 109 (86) | 92 (75) | 201 (80) | |
| Mean (SD) | 8.7 (6.7) | 9.7 (6.9) | 9.2 (6.8) | ||
| Median (IQR) | 6 (4–12) | 9 (4–15) | 7 (4–13) | ||
| Minimum, maximum | 0, 30 | 0, 26 | 0, 30 | ||
| SSIS (teacher) internalising subscale score, points | Baseline | n (%) | 127 (100) | 119 (97) | 246 (98) |
| Mean (SD) | 8.2 (3.4) | 8.1 (3.8) | 8.1 (3.6) | ||
| Median (IQR) | 8 (5–11) | 8 (6–11) | 8 (6–11) | ||
| Minimum, maximum | 1, 17 | 0, 19 | 0, 19 | ||
| 20 weeks | n (%) | 119 (94) | 104 (85) | 223 (89) | |
| Mean (SD) | 7.6 (3.4) | 8.2 (3.8) | 7.8 (3.6) | ||
| Median (IQR) | 7 (5–10) | 8 (5–11) | 8 (5–11) | ||
| Minimum, maximum | 1, 16 | 0, 18 | 0, 18 | ||
| 52 weeks | n (%) | 109 (86) | 92 (75) | 201 (80) | |
| Mean (SD) | 7.6 (3.9) | 8.1 (3.7) | 7.8 (3.8) | ||
| Median (IQR) | 7 (5–10) | 8 (6–10) | 8 (5–10) | ||
| Minimum, maximum | 0, 21 | 0, 17 | 0, 21 | ||
| SSIS (parent) assertion subscale score, points | Baseline | n (%) | 127 (100) | 121 (98) | 248 (99) |
| Mean (SD) | 10.8 (4.0) | 10.6 (4.1) | 10.7 (4.1) | ||
| Median (IQR) | 11 (8–14) | 11 (8–14) | 11 (8–14) | ||
| Minimum, maximum | 0, 19 | 1, 20 | 0, 20 | ||
| 20 weeks | n (%) | 100 (79) | 91 (74) | 191 (76) | |
| Mean (SD) | 11.3 (3.7) | 10.6 (4.1) | 11.0 (3.9) | ||
| Median (IQR) | 12 (8–14) | 10 (8–14) | 11 (8–14) | ||
| Minimum, maximum | 4, 20 | 0, 18 | 0, 20 | ||
| 52 weeks | n (%) | 101 (80) | 80 (65) | 181 (72) | |
| Mean (SD) | 11.1 (3.9) | 10.8 (4.0) | 11.0 (3.9) | ||
| Median (IQR) | 11 (9–14) | 11 (8–13) | 11 (8–14) | ||
| Minimum, maximum | 1, 20 | 2, 20 | 1, 20 | ||
| SSIS (parent) self-control subscale score, points | Baseline | n (%) | 127 (100) | 121 (98) | 248 (99) |
| Mean (SD) | 4.6 (3.5) | 4.1 (3.2) | 4.4 (3.4) | ||
| Median (IQR) | 4 (2–7) | 4 (2–6) | 4 (2–6) | ||
| Minimum, maximum | 0, 14 | 0, 17 | 0, 17 | ||
| 20 weeks | n (%) | 100 (79) | 91 (74) | 191 (76) | |
| Mean (SD) | 5.5 (3.5) | 5.0 (3.1) | 5.3 (3.3) | ||
| Median (IQR) | 6 (2–8) | 5 (3–7) | 5 (2–8) | ||
| Minimum, maximum | 0, 15 | 0, 16 | 0, 16 | ||
| 52 weeks | n (%) | 101 (80) | 80 (65) | 181 (72) | |
| Mean (SD) | 5.5 (3.8) | 4.9 (3.2) | 5.3 (3.5) | ||
| Median (IQR) | 6 (3–8) | 5 (2–7) | 5 (3–8) | ||
| Minimum, maximum | 0, 15 | 0, 16 | 0, 16 | ||
| SSIS (parent) externalising subscale score, points | Baseline | n (%) | 126 (99) | 121 (98) | 247 (99) |
| Mean (SD) | 16.8 (6.6) | 16.6 (6.4) | 16.7 (6.5) | ||
| Median (IQR) | 16 (13–21) | 17 (12–21) | 16 (12–21) | ||
| Minimum, maximum | 3, 34 | 3, 31 | 3, 34 | ||
| 20 weeks | n (%) | 100 (79) | 93 (76) | 193 (77) | |
| Mean (SD) | 15.5 (6.3) | 16.0 (5.7) | 15.7 (6.0) | ||
| Median (IQR) | 14 (11–21) | 16 (12–19) | 15 (11–20) | ||
| Minimum, maximum | 4, 32 | 2, 29 | 2, 32 | ||
| 52 weeks | n (%) | 101 (80) | 79 (64) | 180 (72) | |
| Mean (SD) | 15.8 (6.8) | 15.2 (5.5) | 15.6 (6.3) | ||
| Median (IQR) | 15 (11–20) | 16 (11–19) | 16 (11–19) | ||
| Minimum, maximum | 1, 35 | 3, 28 | 1, 35 | ||
| SSIS (parent) internalising subscale score, points | Baseline | n (%) | 126 (99) | 121 (98) | 247 (99) |
| Mean (SD) | 14.4 (6.0) | 15.4 (5.3) | 14.9 (5.7) | ||
| Median (IQR) | 14 (9–18) | 16 (11–19) | 16 (11–19) | ||
| Minimum, maximum | 0, 28 | 2, 27 | 0, 28 | ||
| 20 weeks | n (%) | 100 (79) | 93 (76) | 193 (77) | |
| Mean (SD) | 13.5 (6.0) | 16.1 (5.5) | 14.7 (5.9) | ||
| Median (IQR) | 13 (10–18) | 17 (12–20) | 15 (11–19) | ||
| Minimum, maximum | 2, 29 | 3, 30 | 2, 30 | ||
| 52 weeks | n (%) | 101 (80) | 79 (64) | 180 (72) | |
| Mean (SD) | 13.7 (6.1) | 15.3 (5.5) | 14.4 (5.9) | ||
| Median (IQR) | 13 (9–18) | 15 (12–19) | 14 (10–19) | ||
| Minimum, maximum | 0, 27 | 1, 29 | 0, 29 | ||
| Additional outcome | |||||
| SSIS (parent) social skills subscale score, points | Baseline | n (%) | 127 (100) | 121 (98) | 248 (99) |
| Mean (SD) | 58.5 (17.1) | 57.0 (16.9) | 57.8 (17.0) | ||
| Median (IQR) | 59 (45–69) | 58 (45–69) | 59 (45–69) | ||
| Minimum, maximum | 16–106 | 12–105 | 12–106 | ||
| 20 weeks | n (%) | 100 (79) | 91 (74) | 191 (76) | |
| Mean (SD) | 63.3 (18.0) | 59.3 (16.1) | 61.4 (17.2) | ||
| Median (IQR) | 61 (49–77) | 59 (50–68) | 60 (50–74) | ||
| Minimum, maximum | 25, 105 | 13, 100 | 13, 105 | ||
| 52 weeks | n (%) | 101 (80) | 80 (65) | 181 (72) | |
| Mean (SD) | 63.6 (19.7) | 61.5 (16.4) | 62.7 (18.3) | ||
| Median (IQR) | 64 (53–75) | 62 (52–72) | 64 (52–73) | ||
| Minimum, maximum | 23, 103 | 16, 110 | 16, 110 | ||
| Outcome | Time point | Intervention arm | Control arm | All | |||
|---|---|---|---|---|---|---|---|
| Questions completed, median (minimum, maximum) | Evaluable participants, % | Questions completed, median (minimum, maximum) | Evaluable participants, % | Questions completed, median (minimum, maximum) | Evaluable participants, % | ||
| SSI (teacher) social skills subscale score, percentage | Baseline | 100 (70, 100) | 99 | 100 (72, 100) | 98 | 100 (70, 100) | 98 |
| 20 weeks | 100 (67, 100) | 92 | 100 (63, 100) | 84 | 100 (63, 100) | 88 | |
| 52 weeks | 100 (76, 100) | 84 | 100 (65, 100) | 76 | 100 (65, 100) | 80 | |
| SSIS (teacher) problem behaviours subscale score, percentage | Baseline | 100 (90, 100) | 100 | 100 (70, 100) | 97 | 100 (70, 100) | 98 |
| 20 weeks | 100 (90, 100) | 94 | 100 (0, 100) | 85 | 100 (0, 100) | 89 | |
| 52 weeks | 100 (90, 100) | 86 | 100 (83, 100) | 75 | 100 (83, 100) | 80 | |
| Academic competence, percentage | Baseline | 100 (71, 100) | 96 | 100 (57, 100) | 98 | 100 (57, 100) | 97 |
| 20 weeks | 100 (0, 100) | 88 | 100 (0, 100) | 82 | 100 (0, 100) | 85 | |
| 52 weeks | 100 (0, 100) | 79 | 100 (71, 100) | 75 | 100 (0, 100) | 77 | |
| SSIS (parent) social skills subscale score, percentage | Baseline | 100 (93, 100) | 100 | 100 (72, 100) | 98 | 100 (72, 100) | 99 |
| 20 weeks | 100 (78, 100) | 79 | 100 (65, 100) | 74 | 100 (65, 100) | 76 | |
| 52 weeks | 100 (93, 100) | 80 | 100 (96, 100) | 65 | 100 (93, 100) | 72 | |
| SSIS (parent) problem behaviours subscale score, percentage | Baseline | 100 (79, 100) | 99 | 100 (70, 100) | 98 | 100 (70, 100) | 99 |
| 20 weeks | 100 (73, 100) | 79 | 100 (88, 100) | 76 | 100 (73, 100) | 77 | |
| 52 weeks | 100 (94, 100) | 80 | 100 (52, 100) | 64 | 100 (52, 100) | 72 | |
| ALS score, percentage | Baseline | 100 (0, 100) | 86 | 100 (88, 100) | 84 | 100 (0, 100) | 85 |
| 20 weeks | 100 (6, 100) | 69 | 100 (94, 100) | 70 | 100 (6, 100) | 69 | |
| 52 weeks | 100 (44, 100) | 65 | 100 (88, 100) | 56 | 100 (44, 100) | 60 | |
| MSPSS significant other subscale score, percentage | Baseline | 50 (0, 50) | 94 | 50 (38, 50) | 94 | 50 (0, 50) | 94 |
| 20 weeks | 50 (25, 50) | 71 | 50 (38, 50) | 74 | 50 (25, 50) | 72 | |
| 52 weeks | 50 (50, 50) | 71 | 50 (38, 50) | 63 | 50 (38, 50) | 67 | |
| SDQ (teacher) score, percentage | Baseline | 100 (88, 100) | 100 | 100 (92, 100) | 100 | 100 (88, 100) | 100 |
| 20 weeks | 100 (88, 100) | 94 | 100 (96, 100) | 85 | 100 (88, 100) | 90 | |
| 52 weeks | 100 (88, 100) | 86 | 100 (92, 100) | 76 | 100 (88, 100) | 81 | |
| SDQ (parent) score, percentage | Baseline | 100 (88, 100) | 100 | 100 (88, 100) | 100 | 100 (88, 100) | 100 |
| 20 weeks | 100 (92, 100) | 80 | 100 (96, 100) | 76 | 100 (92, 100) | 78 | |
| 52 weeks | 100 (92, 100) | 80 | 100 (0, 100) | 64 | 100 (0, 100) | 72 | |
| Variable | Intervention arm (N = 127), n (%) | Control arm (N = 123), n (%) | All (N = 250), n (%) |
|---|---|---|---|
| Mental health | |||
| Related medication(s) reported in free-text field | 2 (2) | 0 (0) | 2 (1) |
| No related medication(s) reported | 125 (98) | 123 (100) | 248 (99) |
| Physical health | |||
| Related medication(s) reported in free-text field | 14 (11) | 21 (17) | 35 (14) |
| No related medication(s) reported | 113 (89) | 102 (83) | 215 (86) |
| Neurodevelopment | |||
| Related medication(s) reported in free-text field | 23 (18) | 22 (18) | 45 (18) |
| No related medication(s) reported | 104 (82) | 101 (82) | 205 (82) |
| Variable | Intervention arm (N = 64), n (%) | Control arm (N = 69), n (%) | All (N = 133), n (%) |
|---|---|---|---|
| ADHD | 23 (36) | 15 (22) | 38 (29) |
| Antianxiety/antidepressant/antipsychotic | 2 (3) | 0 (0) | 2 (2) |
| Hay fever/allergy/asthma | 6 (9) | 15 (22) | 21 (16) |
| Skin problem(s) | 3 (5) | 3 (4) | 6 (5) |
| Neuromuscular problem(s) | 1 (2) | 0 (0) | 1 (1) |
| Nutrient deficiency | 3 (5) | 2 (3) | 5 (4) |
| Epilepsy | 3 (5) | 1 (1) | 4 (3) |
| Urinary (bedwetting) | 1 (2) | 2 (3) | 3 (2) |
| Gastrointestinal problem(s) | 8 (13) | 12 (17) | 20 (15) |
| Pain/inflammation | 2 (3) | 0 (0) | 2 (2) |
| Trouble with sleep | 10 (16) | 12 (17) | 22 (17) |
| Respiratory | 0 (0) | 2 (3) | 2 (2) |
| Other | 1 (2) | 2 (3) | 3 (2) |
| Infection | 0 (0) | 1 (1) | 1 (1) |
| Toothpastea | 1 (2) | 0 (0) | 1 (1) |
| Unknown | 0 (0) | 2 (3) | 2 (2) |
Appendix 3 Additional tables to health economic analyses
| Variable | Baseline, n (%) | Week 20, n (%) | Week 52, n (%) | Complete case, n (%) |
|---|---|---|---|---|
| Booklet (child or young person) completion | 241 (96.4) | 185 (74.0) | 171 (68.4) | 145 (58.0) |
| Booklet (parent) completion | 250 (100.0) | 196 (78.4) | 181 (72.4) | 160 (64.0) |
| Booklet (teacher) completion | 250 (100.0) | 224 (89.6) | 203 (81.2) | 192 (76.8) |
| Health economic data | ||||
| EQ-5D | 248 (99.2) | 194 (77.6) | 179 (71.6) | 155 (62.0) |
| CHU-9D | 231 (92.4) | 181 (72.4) | 168 (67.2) | 136 (54.3) |
| Costs from the NHS and PSS perspective | 233 (93.2) | 186 (74.4) | 173 (69.2) | 144 (57.6) |
| Costs from the societal perspective | 221 (88.4) | 158 (63.2) | 135 (54.0) | 100 (40.0) |
| EQ-5D and costs (NHS and PSS perspective) | 232 (92.8) | 184 (73.6) | 171 (68.4) | 139 (55.6) |
| EQ-5D and costs (societal perspective) | 220 (88.0) | 156 (62.4) | 133 (53.2) | 97 (38.8) |
| CHU-9D and costs (NHS and PSS perspective) | 215 (86.0) | 160 (64.0) | 135 (54.0) | 98 (39.2) |
| CHU-9D and costs (societal perspective) | 203 (81.2) | 134 (53.6) | 109 (43.6) | 69 (27.6) |
| Characteristic | Base case (N = 248) | Complete case (N = 139) | ||
|---|---|---|---|---|
| Intervention arm (n = 126) | Control arm (n = 122) | Intervention arm (n = 80) | Control arm (n = 59) | |
| Gender, n (%) | ||||
| Male | 101 (80.2) | 91 (74.6) | 68 (85.0) | 43 (72.9) |
| Age (years), n (%) | ||||
| 7-11 | 83 (65.9) | 79 (64.8) | 54 (67.5) | 43 (72.9) |
| 11-15 | 43 (34.1) | 43 (35.2) | 26 (32.5) | 16 (27.1) |
| Mean (SD) | 9.7 (2.3) | 9.8 (2.2) | 9.6 (2.2) | 9.6 (2.2) |
| Time (years) since ASD diagnosis | ||||
| Mean (SD) | 3.4 (2.7) | 3.6 (2.8) | 3.2 (2.4) | 3.6 (3.0) |
| SCQ score, points | ||||
| Mean (SD) | 25.1 (5.2) | 24.2 (5.2) | 24.9 (5.1) | 24.1 (5.0) |
| EQ-5D | ||||
| Mean (SD) | 0.79 (0.11) | 0.76 (0.11) | 0.79 (0.12) | 0.77 (0.11) |
| Site, n (%) | ||||
| Leeds | 37 (29.4) | 38 (31.2) | 31 (38.8) | 18 (30.5) |
| Sheffield | 70 (55.6) | 67 (54.9) | 34 (42.5) | 31 (52.5) |
| York | 19 (15.1) | 17 (13.9) | 15 (18.7) | 10 (17.0) |
| Number of intervention sessions | ||||
| Mean (SD) | 10.3 (2.3) | – | 10.5 (2.2) | – |
| Item | Unit cost (£) | Source | ||
|---|---|---|---|---|
| At homea | At clinic/surgery | Telephone/e-mail | ||
| GP | 44.12 | 37.40 | 37.70 | Unit Costs of Health and Social Care 2018 84 |
| Community nurseb | 33.72 | 27.00 | 27.00 | Unit Costs of Health and Social Care 2018 84 |
| Social care workerb | 28.72 | 22.00 | 22.00 | Unit Costs of Health and Social Care 2018 84 |
| Home care worker | 20.72 | 14.00 | 14.00 | Unit Costs of Health and Social Care 2018 84 |
| Family support workerb | 22.72 | 16.00 | 16.00 | Unit Costs of Health and Social Care 2018 84 |
| Helplinec | – | – | 6.00 | Unit Costs of Health and Social Care 2018 84 |
| Occupational therapist | 90.72 | 84.00 | 84.00 | Unit Costs of Health and Social Care 2018 84 |
| Physiotherapist | 46.72 | 40.00 | 40.00 | Unit Costs of Health and Social Care 2018 84 |
| Speech and language therapist | 94.72 | 88.00 | 88.00 | Unit Costs of Health and Social Care 2018 84 |
| Dentistb | 73.22 | 66.50 | 66.50 | Unit Costs of Health and Social Care 2018 84 |
| Item | Unit cost (£) | Source |
|---|---|---|
| Overnight stay in respite centre | 174.00 | Reference cost 2018/19150 (PX55Z Paediatric Respite Care) |
| Overnight stay with sharing care | 174.00 | Assuming the same as above |
| Day stay with another sharing care | 174.00 | Assuming the same as above |
| Item | Unit cost (£) | Source |
|---|---|---|
| Child psychiatrist | 227.00 | Reference cost 2018/19150 (service code 711: Child and Adolescent Psychiatry) |
| Child psychotherapist | 91.00 | Unit Costs of Health and Social Care 2018 84 |
| Child psychologist or clinical psychologista | 212.00 | Unit Costs of Health and Social Care 2018 84 |
| Mental health nurse or CAMHS therapistb | 59.00 | Unit Costs of Health and Social Care 2018 84 |
| Item | Unit cost (£) | Source |
|---|---|---|
| Accident and emergency | 133.00 | Reference cost 2018/19150 (service code: T01NA) |
| NHS walk-in centre | 53.00 | Reference cost 2018/19150 (service code: T04NA) |
| Urgent care centre | 53.00 | Reference cost 2018/19150 (service code: T04NA) |
| Outpatient visit | 148.00 | Reference cost 2018/19150 (national average) |
| Outpatient visit: mental health | 227.00 | Reference cost 2018/19150 (service code: 711) |
| Inpatient stay: knee fracture | 1604.00 | Reference cost 2018/19150 (HRG code: HE21G) |
| Inpatient stay: adenoidectomy | 1391.00 | Reference cost 2018/19150 (HRG code: CA62Z) |
| Inpatient stay: dental surgery | 1321.00 | Reference cost 2018/19150 (HRG code: CD01B) |
| Item | Chemical name | Dosage | Unit cost (£) | Source |
|---|---|---|---|---|
| Melatonin_Tab 2 mg | Melatonin | 2-mg tablet (once daily) | 1.69 per quantity | PCA 2018 (0401010ADAABKBK) |
| Equasym XL_Cap 20 mg | Methylphenidate hydrochloride | 20-mg capsule (once daily) | 1.00 per quantity | PCA 2018 (0404000M0BCAEAQ) |
| Movicol_Paed Pdr Sac | Macrogol 3350 | 6.9-g sachet (two sachets per day) | 0.15 per quantity | PCA 2018 (0106040M0BBAIAB) |
| Salbutamol_Inha 100 mcg | Salbutamol | Two puffs (as required) | 1.57 per quantity | PCA 2018 (0301011R0AAAAAA) |
| Cetirizine HCl_Tab 10 mg | Cetirizine hydrochloride | 10-mg tablet (once daily) | 0.03 per quantity | PCA 2018 (0304010I0AAAAAA) |
| Sod Picosulf_Oral Soln 5 mg | Sodium picosulfate | 5 mg (once daily) | 0.02 per quantity | PCA 2018 (0106020P0AAABAB) |
| Seretide 50 | Fluticasone propionate (inhalation) | 50 µg (two puffs daily) | 18.0 per quantity | PCA 2018 (0302000N0BCADBE) |
| Calceos_Tab | Colecalciferol | 500-mg tab (twice daily) | 0.06 per quantity | PCA 2018 (0906040G0BQAABW) |
| Strattera_Cap 40 mg | Atomoxetine hydrochloride | 40-mg capsule (once daily) | 1.90 per quantity | PCA 2018 (0404000S0BBABAB) |
| Clenil Modulite_Inha 50 mcg | Beclometasone dipropionate | 50 µg (two puffs daily) | 3.70 per quantity | PCA 2018 (0302000C0BPAABE) |
| Item | Unit cost (£) | Source |
|---|---|---|
| Educational psychologist | 23.20/hour | NJC ‘green book’152 (SCP 49) |
| Education welfare officer | 12.00/hour | NJC ‘green book’152 (SCP 25) |
| School or college nurse | 12.00/hour | NJC ‘green book’152 (SCP 25) |
| Item | Unit cost (£) | Source |
|---|---|---|
| Teacher | 16.30/hour | NJC ‘green book’152 (SCP 35) |
| TA | 9.70/hour | NJC ‘green book’152 (SCP 17) |
| High-level TA | 10.90/hour | NJC ‘green book’152 (SCP 22) |
| SENCO | 19.20/hour | NJC ‘green book’152 (SCP 41) |
| SEN assistant | 11.60/hour | NJC ‘green book’152 (SCP 24) |
| Learning mentor | 11.20/hour | NJC ‘green book’152 (SCP 23) |
| Literacy support assistant | 10.70/hour | NJC ‘green book’152 (SCP 21) |
| Form tutor | 21.70/hour | NJC ‘green book’152 (SCP 46) |
| Specialist teacher | 14.20/hour | NJC ‘green book’152 (SCP 30) |
| Item | Unit cost (£) | Source |
|---|---|---|
| Privately paid mental health services | 360.00/session | www.psychiatry-uk.com/fees/ (accessed 25 February 2022) |
| After-school club | 57.40/week | Childcare Survey 2019 153 |
| Holiday club | 133.40/week | Childcare Survey 2019 153 |
| Day care | 154.00/day | Unit Costs of Health and Social Care 2018 84 |
| Child care: home support | 25.00/hour | Unit Costs of Health and Social Care 2018 84 |
| Item | Unit cost (£) | Source |
|---|---|---|
| Average weekly earning of people in the UK | 522.00 | Office for National Statistics 2018154 |
| Item | Total cost | Time (minutes) per session per CYP (95% CI) | Cost (£) per session per CYP (95% CI) | Cost (£) per CYP (95% CI) |
|---|---|---|---|---|
| Training cost | ||||
| Trainer fee | 4262.00 | 2.01 | ||
| Refreshments | 10.00 | 0.00 | ||
| Materials and consumablesa | 178.00 | 0.08 | ||
| Trainer travel | 740.00 | 0.35 | ||
| Total | 5685.00 | 2.45 | 25.29 (24.30 to 26.29) | |
| Intervention cost | ||||
| LEGO bricks | 3903.00 | 1.84 | 18.97 (18.23 to 19.70) | |
| Materials and consumablesa | 580.50 | 0.27 | 2.78 (2.68 to 2.89) | |
| Intervention staff costs | ||||
| Planning | 3.0 (2.7 to 3.3) | 0.55 (0.50 to 0.60) | 5.46 (4.51 to 6.40) | |
| Set-up | 1.7 (1.6 to 1.7) | 0.31 (0.29 to 0.32) | 3.11 (2.76 to 3.46) | |
| Delivery | 18.4 (18.1 to 18.6) | 3.53 (3.45 to 3.61) | 35.50 (32.82 to 38.11) | |
| Clear-up | 1.8 (1.7 to 1.9) | 0.33 (0.31 to 0.34) | 3.35 (2.98 to 3.71) | |
| Additional work post sessionb | 1.3 (1.1 to 1.5) | 0.26 (0.22 to 0.29) | 2.11 (1.51 to 2.71) | |
| Help from other staff | ||||
| Set-up and clear-up | 0.1 (0.1 to 0.2) | 0.02 (0.01 to 0.03) | 0.25 (0.12 to 0.40) | |
| Additional work post sessionb | 0.1 (0.0 to 0.1) | 0.01 (0.01 to 0.02) | 0.14 (0.04 to 0.23) | |
| Total | 7.12 (6.99 to 7.26) | 71.67 (67.58 to 75.75) | ||
| Variable | Unit | Time point, mean (SD) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 20 weeks | 52 weeks | |||||
| Intervention arm (n = 80) | Control arm (n = 59) | Intervention arm (n = 80) | Control arm (n = 59) | Intervention (n = 80) | Usual support (n = 59) | ||
| NHS and PSS | |||||||
| Community-based services | |||||||
| CAMHS-related | Session | 0.70 (2.76) | 0.31 (0.88) | 0.23 (0.88) | 0.36 (1.06) | 0.63 (2.81) | 0.54 (2.46) |
| Non-CAMHS-related | |||||||
| GP | Appointment | 0.45 (0.97) | 0.24 (0.50) | 0.39 (1.02) | 0.22 (0.49) | 0.71 (1.41) | 0.66 (0.96) |
| Allied health professionals | Appointment | 0.44 (0.93) | 0.68 (2.20) | 0.43 (1.00) | 0.34 (0.71) | 0.78 (1.96) | 0.69 (1.56) |
| Social care service | Appointment | 0.49 (1.65) | 0.47 (1.34) | 0.26 (1.00) | 0.39 (2.11) | 0.99 (2.62) | 0.80 (2.06) |
| Hospital-based services/acute services | |||||||
| Emergency services | Visit | 0.19 (0.80) | 0.12 (0.46) | 0.13 (0.43) | 0.03 (0.26) | 0.20 (0.60) | 0.17 (0.62) |
| Inpatient stay | |||||||
| Mental health-related | Night | – | – | – | – | – | – |
| Non-mental health-related | Night | 0.01 (0.11) | – | 0.01 (0.11) | – | – | – |
| Outpatient visit/day case | |||||||
| Mental health-related | Visit | 0.06 (0.37) | 0.10 (0.44) | 0.08 (0.38) | 0.07 (0.31) | 0.05 (0.22) | 0.29 (1.37) |
| Non-mental health-related | Visit | 0.31 (1.71) | 0.17 (0.59) | 0.19 (0.75) | 0.24 (0.63) | 0.09 (0.40) | 0.24 (0.95) |
| Medication | |||||||
| Mental health-related | Type | 0.36 (0.80) | 0.20 (0.48) | 0.43 (0.81) | 0.27 (0.55) | 0.48 (0.87) | 0.32 (0.51) |
| Non-mental health-related | Type | 0.33 (0.62) | 0.41 (0.93) | 0.41 (0.90) | 0.53 (1.33) | 0.38 (0.79) | 0.59 (1.23) |
| Education-system-related | |||||||
| School-based health | Hour | 2.60 (10.00) | 2.17 (6.94) | 1.40 (5.80) | 3.12 (10.51) | 8.80 (32.99) | 2.17 (10.06) |
| General supporta | Hour | 8.54 (7.26) | 7.93 (12.68) | 7.48 (9.25) | 9.37 (17.34) | 18.94 (30.32) | 27.32 (45.13) |
| Intervention supporta | Hour | 5.50 (10.50) | 4.66 (6.74) | 6.78 (8.28) | 7.15 (11.03) | 8.37 (7.97) | 7.72 (10.56) |
| Private expenses: out of pocket | |||||||
| Child care | Session | 3.05 (7.35) | 4.10 (11.05) | 4.14 (9.30) | 5.98 (12.37) | 5.86 (13.58) | 12.29 (25.20) |
| Productivity | |||||||
| Parental productivity | Day | 0.45 (1.18) | 0.31 (0.93) | 0.45 (1.04) | 0.52 (1.51) | 0.58 (1.13) | 0.59 (1.40) |
| Variable | Time point, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 20 weeks | 52 weeks | |||||||
| Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | |
| Usual support (N = 59) | |||||||||
| Mobility | 52 (88.1) | 7 (11.9) | – | 46 (78.0) | 13 (22.0) | – | 46 (78.0) | 13 (22.0) | – |
| Self-care | 17 (28.8) | 32 (54.2) | 10 (17.0) | 16 (27.1) | 37 (62.7) | 6 (10.2) | 14 (23.7) | 35 (59.3) | 10 (17.0) |
| Usual activity | 25 (42.4) | 29 (49.1) | 5 (8.5) | 25 (42.4) | 27 (45.8) | 7 (11.8) | 22 (37.3) | 30 (50.8) | 7 (11.9) |
| Pain/discomfort | 45 (76.3) | 13 (22.0) | 1 (1.7) | 37 (62.7) | 22 (37.3) | – | 38 (64.4) | 21 (35.6) | – |
| Anxiety/depression | 15 (25.4) | 35 (59.3) | 9 (15.3) | 16 (27.1) | 34 (57.6) | 9 (15.3) | 18 (30.5) | 26 (44.1) | 15 (25.4) |
| LEGO® based therapy (N = 80) | |||||||||
| Mobility | 66 (82.5) | 11 (13.7) | 3 (3.8) | 61 (76.2) | 16 (20.0) | 3 (3.8) | 64 (80.0) | 13 (16.2) | 3 (3.8) |
| Self-care | 25 (31.3) | 36 (45.0) | 19 (23.7) | 26 (32.5) | 42 (52.5) | 12 (15.0) | 29 (36.3) | 36 (45.0) | 15 (18.7) |
| Usual activity | 33 (41.2) | 41 (51.2) | 6 (7.5) | 35 (43.7) | 36 (45.0) | 9 (11.3) | 43 (53.7) | 33 (41.3) | 4 (5.0) |
| Pain/discomfort | 64 (80.0) | 14 (17.5) | 2 (2.5) | 63 (78.7) | 13 (16.3) | 4 (5.0) | 59 (73.8) | 19 (23.7) | 2 (2.5) |
| Anxiety/depression | 40 (50.0) | 33 (41.3) | 7 (8.7) | 37 (46.2) | 34 (42.5) | 9 (11.3) | 39 (48.7) | 29 (36.3) | 12 (15.0) |
| Variable | Time point, n (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 20 weeks | 52 weeks | |||||||||||||
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | |
| Usual support (N = 45) | |||||||||||||||
| Worried | 20 (44.4) | 16 (35.6) | 7 (15.6) | – | 2 (4.4) | 22 (48.9) | 12 (26.7) | 5 (11.1) | 3 (6.7) | 3 (6.7) | 26 (57.8) | 13 (28.9) | 1 (2.2) | 3 (6.7) | 2 (4.4) |
| Sad | 32 (71.1) | 8 (17.8) | 2 (4.4) | 2 (4.4) | 1 (2.2) | 29 (64.4) | 6 (13.3) | 5 (11.1) | 4 (8.9) | 1 (2.2) | 34 (75.6) | 4 (8.9) | 2 (4.4) | 1 (2.2) | 4 (8.9) |
| Annoyed | 27 (60.0) | 13 (28.9) | 4 (8.9) | – | 1 (2.2) | 27 (60.0) | 10 (22.2) | 5 (11.1) | – | 3 (6.7) | 27 (60.0) | 10 (22.2) | 5 (11.1) | 2 (4.4) | 1 (2.2) |
| Tired | 9 (20.0) | 15 (33.3) | 8 (17.8) | 5 (11.1) | 8 (17.8) | 12 (26.7) | 13 (28.9) | 9 (20.0) | 5 (11.1) | 6 (13.3) | 10 (22.2) | 16 (35.6) | 6 (13.3) | 6 (13.3) | 7 (15.6) |
| Pain | 28 (62.2) | 6 (13.3) | 6 (13.3) | 3 (6.7) | 2 (4.4) | 21 (46.7) | 11 (24.4) | 4 (8.9) | 2 (4.4) | 7 (15.6) | 24 (53.3) | 12 (26.7) | 7 (15.6) | – | 2 (4.4) |
| Sleep | 21 (46.7) | 12 (26.7) | 5 (11.1) | 3 (6.7) | 4 (8.9) | 20 (44.4) | 3 (6.7) | 7 (15.6) | 9 (20.0) | 6 (13.3) | 17 (37.8) | 16 (35.6) | 4 (8.9) | 4 (8.9) | 4 (8.9) |
| Daily routine | 23 (51.1) | 12 (26.7) | 4 (8.9) | 3 (6.7) | 3 (6.7) | 19 (42.2) | 11 (24.4) | 7 (15.6) | 4 (8.9) | 4 (8.9) | 24 (53.3) | 10 (22.2) | 5 (11.1) | 3 (6.7) | 3 (6.7) |
| Work | 27 (60.0) | 15 (33.3) | 2 (4.4) | 1 (2.2) | – | 23 (51.1) | 11 (24.4) | 7 (15.6) | 1 (2.2) | 3 (6.7) | 28 (62.2) | 7 (15.6) | 7 (15.6) | 1 (2.2) | 2 (4.4) |
| Able to join activities | 23 (51.1) | 6 (13.3) | 10 (22.2) | 3 (6.7) | 3 (6.7) | 10 (22.2) | 14 (31.1) | 10 (22.2) | 9 (20.0) | 2 (4.4) | 20 (44.4) | 10 (22.2) | 10 (22.2) | 1 (2.2) | 4 (8.9) |
| LEGO® based therapy (N = 51) | |||||||||||||||
| Worried | 34 (66.7) | 8 (15.7) | 3 (5.9) | 2 (3.9) | 4 (7.8) | 33 (64.7) | 6 (11.8) | 7 (13.7) | 1 (2.0) | 4 (7.8) | 34 (66.7) | 7 (13.7) | 6 (11.8) | 3 (5.9) | 1 (2.0) |
| Sad | 40 (78.4) | 5 (9.8) | 2 (3.9) | 1 (2.0) | 3 (5.9) | 37 (72.5) | 5 (9.8) | 5 (9.8) | 1 (2.0) | 3 (5.9) | 36 (70.6) | 6 (11.8) | 6 (11.8) | 2 (3.9) | 1 (2.0) |
| Annoyed | 37 (72.5) | 7 (13.7) | 4 (7.8) | 2 (3.9) | 1 (2.0) | 34 (66.7) | 10 (19.6) | 5 (9.8) | – | 2 (3.9) | 37 (72.5) | 8 (15.7) | 3 (5.9) | 1 (2.0) | 2 (3.9) |
| Tired | 14 (27.5) | 19 (37.3) | 5 (9.8) | 3 (5.9) | 10 (19.6) | 17 (33.3) | 19 (37.3) | 4 (7.8) | 7 (13.7) | 4 (7.8) | 13 (25.5) | 19 (37.3) | 6 (11.8) | 4 (7.8) | 9 (17.6) |
| Pain | 33 (64.7) | 9 (17.6) | 3 (5.9) | 1 (2.0) | 5 (9.8) | 28 (54.9) | 9 (17.6) | 7 (13.7) | 3 (5.9) | 4 (7.8) | 27 (52.9) | 13 (25.5) | 6 (11.8) | 3 (5.9) | 2 (3.9) |
| Sleep | 26 (51.0) | 10 (19.6) | 6 (11.8) | 3 (5.9) | 6 (11.8) | 23 (45.1) | 8 (15.7) | 8 (15.7) | 4 (7.8) | 8 (15.7) | 20 (39.2) | 11 (21.6) | 11 (21.6) | 3 (5.9) | 6 (11.8) |
| Daily routine | 29 (56.9) | 8 (15.7) | 4 (7.8) | 4 (7.8) | 6 (11.8) | 24 (47.1) | 15 (29.4) | 7 (13.7) | 3 (5.9) | 2 (3.9) | 27 (52.9) | 12 (23.5) | 8 (15.7) | 3 (5.9) | 1 (2.0) |
| Work | 34 (66.7) | 7 (13.7) | 6 (11.8) | 1 (2.0) | 3 (5.9) | 33 (64.7) | 10 (19.6) | 5 (9.8) | 3 (5.9) | – | 32 (62.7) | 11 (21.6) | 4 (7.8) | 2 (3.9) | 2 (3.9) |
| Able to join activities | 30 (58.8) | 6 (11.8) | 5 (9.8) | 4 (7.8) | 6 (11.8) | 24 (47.1) | 8 (15.7) | 6 (11.8) | 8 (15.7) | 5 (9.8) | 20 (39.2) | 10 (19.6) | 7 (13.7) | 8 (15.7) | 6 (11.8) |
Appendix 4 Additional tables to qualitative analyses
| Site | Education level | Group ID | Session | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||
| Sheffield | Primary | S/04 | ✓ | ✓ | 2 | ||||||||||
| Sheffield | Primary | S/06 | ✓ | ✓ | ✓ | 3 | |||||||||
| Sheffield | Primary | S/07 | ✓ | ✓ | ✓ | 3 | |||||||||
| Sheffield | Primary | S/15 | ✓ | ✓ | ✓ | 3 | |||||||||
| Sheffield | Primary | S/17 | ✓ | ✓ | 2 | ||||||||||
| Sheffield | Primary | S/36 | ✓ | ✓ | ✓ | 3 | |||||||||
| Sheffield | Secondary | S/27 | ✓ | ✓ | ✓ | 3 | |||||||||
| Sheffield | Secondary | S/28 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/13 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/15 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/32 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/33 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/34 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/35 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/43 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Primary | L/46 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Secondary | L/40 | ✓ | ✓ | ✓ | 3 | |||||||||
| Leeds | Secondary | L/41 | ✓ | ✓ | ✓ | 3 | |||||||||
| York | Primary | Y/12 | ✓ | ✓ | ✓ | 3 | |||||||||
| York | Primary | Y/15 | ✓ | ✓ | ✓ | 3 | |||||||||
| York | Primary | Y/21 | ✓ | ✓ | ✓ | 3 | |||||||||
| York | Primary | Y/22 | ✓ | ✓ | 2 | ||||||||||
| Total | 0 | 5 | 9 | 6 | 5 | 3 | 9 | 5 | 6 | 5 | 5 | 5 | 63 | ||
FIGURE 9.
Number of sessions completed per facilitator (descending order).
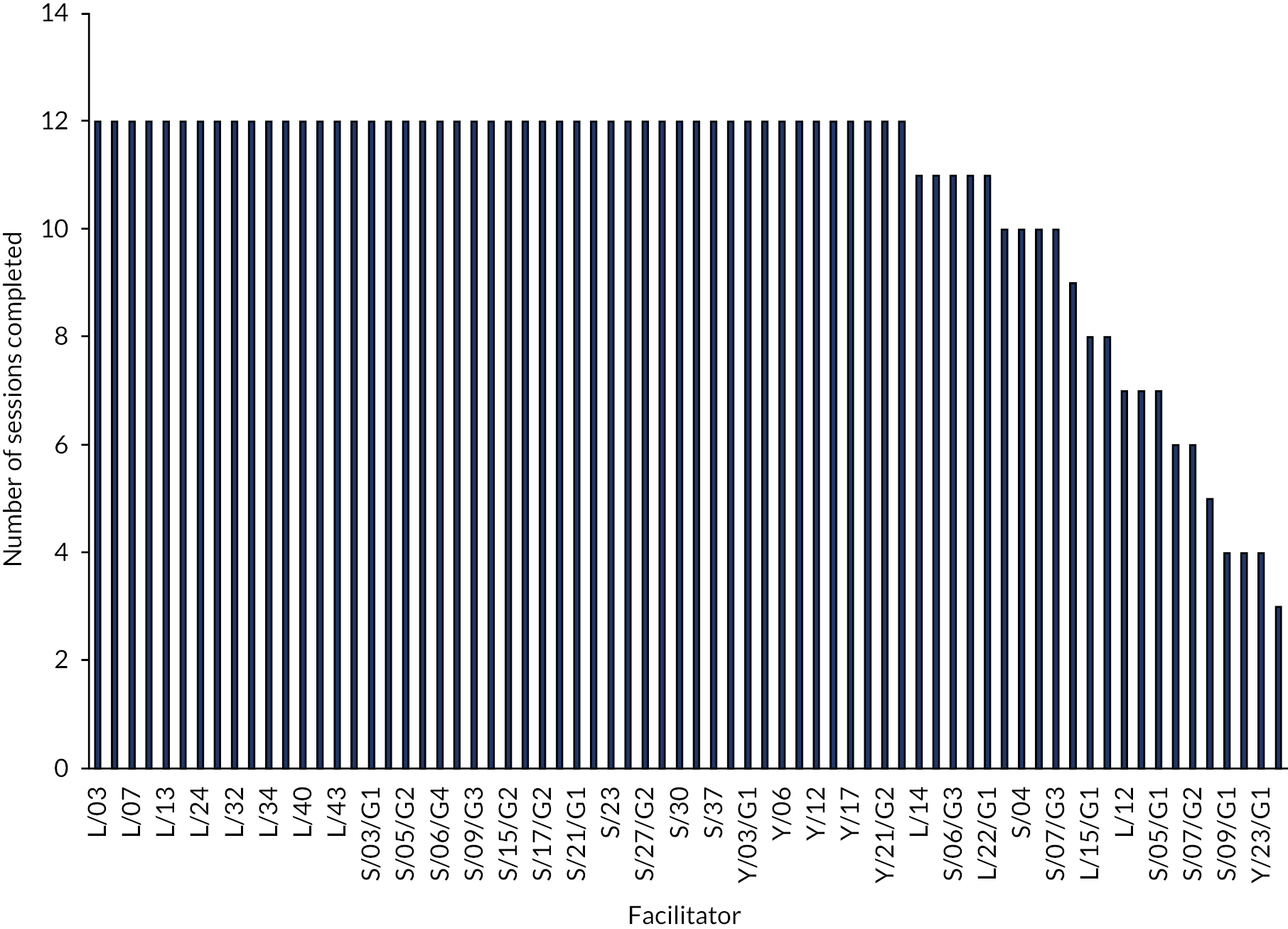
FIGURE 10.
Number of sessions completed per facilitator (grouped by location).
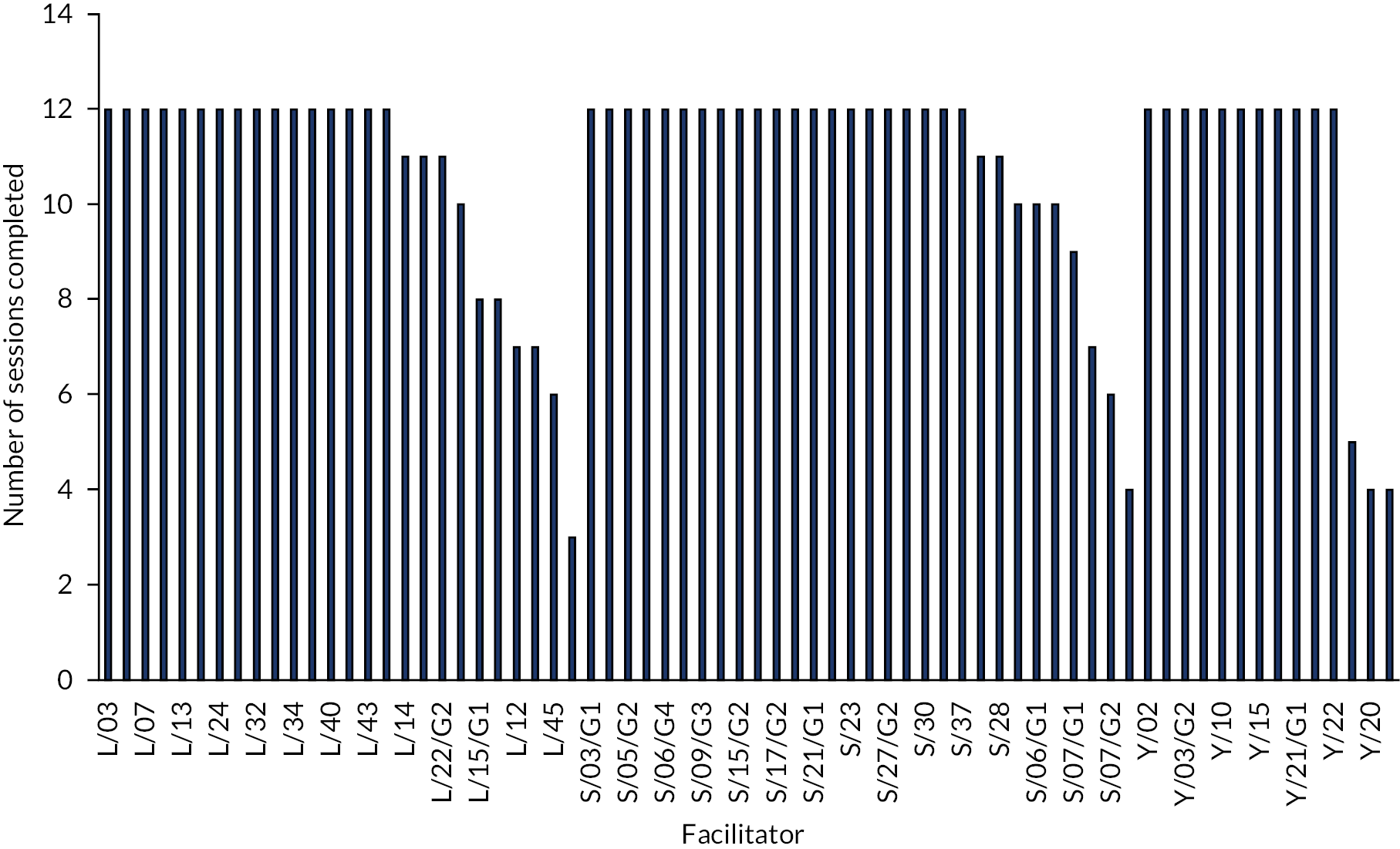
FIGURE 11.
Proportion of components of LEGO® based therapy delivered by facilitator (core and all items).
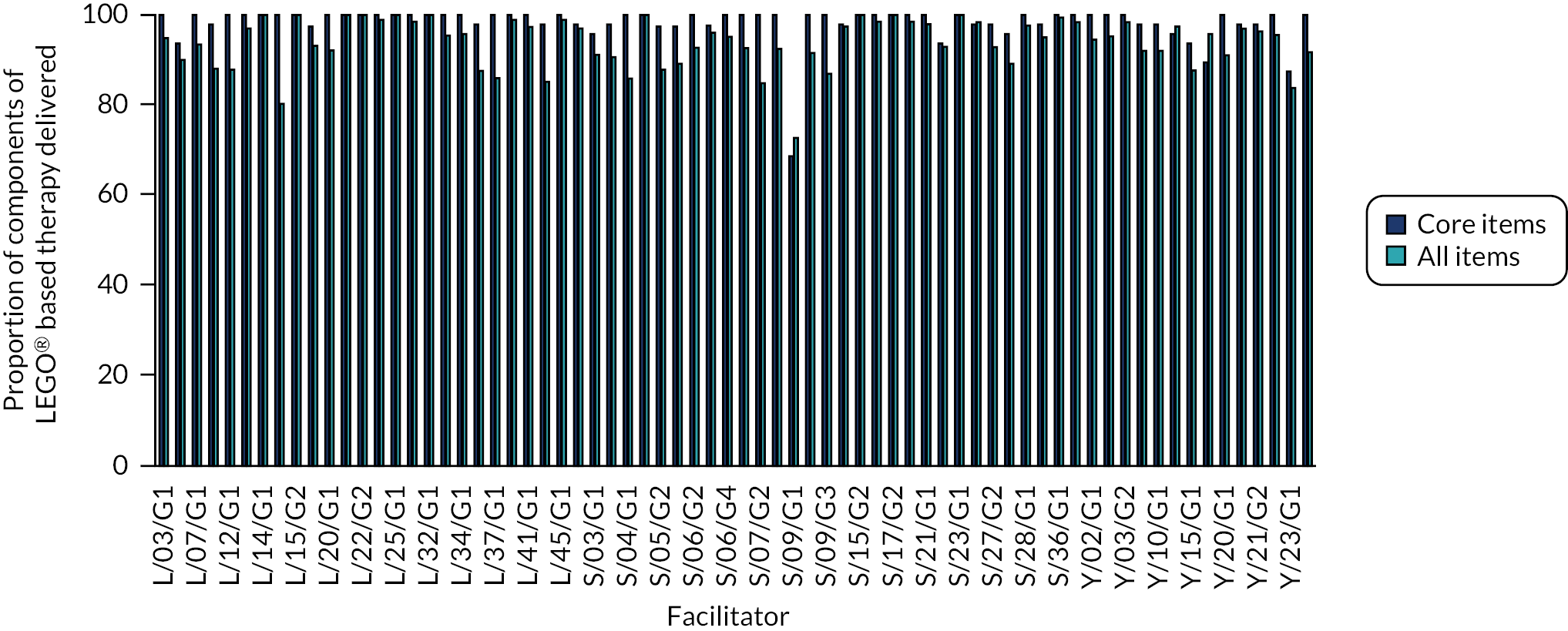
| Group ID | Session number | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12 | Item 13 | Item 14 | Item 15a | Item 15b | Item 16 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | SR | I | ||
| S/04 | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | N/A | N/A | ✗ | ✓ |
| S/04 | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | N/A | N/A | N/A | ✓ | N/A | N/A | M | ✗ |
| S/06 | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | M | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| S/06 | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| S/06 | 9 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | M | N/A | ✗ | N/A | N/A | N/A | ✓ | ✗ |
| S/07 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | ✓ |
| S/07 | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | ✗ | ✓ |
| S/07 | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✗ | ✗ |
| S/15 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| S/15 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| S/15 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ |
| S/17 | 6 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| S/17 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| S/27 | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| S/27 | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | N/A | N/A | ✓ | ✗ |
| S/27 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | N/A | ✗ | ✗ |
| S/28 | 4 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ |
| S/28 | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | N/A | ✓ | N/A | ✓ | ✓ | N/A | ✗ | ✗ |
| S/28 | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ |
| S/36 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| S/36 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | N/A | ✓ | ✓ |
| S/36 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | N/A | N/A | ✓ | ✓ |
| L/13 | 3 | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| L/13 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✗ | ✗ |
| L/13 | 11 | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| L/15 | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | N/A | N/A | ✗ | ✗ |
| L/15 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ |
| L/15 | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | M | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✗ | ✗ | ✗ | N/A | ✗ | ✗ | ✗ |
| L/32 | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| L/32 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| L/32 | 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| L/33 | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ |
| L/33 | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | M | ✓ | N/A | N/A | ✓ | ✓ | ✓ | ✓ |
| L/33 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ |
| L/34 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| L/34 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| L/34 | 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✗ |
| L/35 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| L/35 | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| L/35 | 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | N/A | N/A | N/A | N/A | N/A | ✓ | ✓ | ✓ |
| L/40 | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | M | ✗ |
| L/40 | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| L/40 | 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| L/41 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | N/A | N/A | N/A | N/A | ✓ | N/A | ✓ | ✗ |
| L/41 | 8 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| L/41 | 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| L/43 | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | N/A | N/A | ✗ | ✗ |
| L/43 | 8 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ |
| L/43 | 1 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | N/A | ✗ | N/A | N/A | N/A | ✗ | ✗ |
| L/46 | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| L/46 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| L/46 | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ |
| Y/12 | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | M | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | N/A | ✗ | ✓ |
| Y/12 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| Y/12 | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | N/A | ✓ | ✓ |
| Y/15 | 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Y/15 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | M | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | M | N/A | N/A | N/A | ✓ | ✓ |
| Y/15 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | M | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Y/21 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | ✓ | ✓ |
| Y/21 | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Y/21 | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | N/A | N/A | N/A | N/A | ✓ | ✓ |
| Y/22 | 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Y/22 | 9 | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Variable | Summary |
|---|---|
| 1. Enjoyed LEGO® based therapy | |
| 1.1. CYP | Fifteen facilitators said that the CYP involved in LEGO® based therapy enjoyed their sessions and looked forward to them every week |
| 1.2. Facilitator | Twenty-two facilitators said that, despite certain challenges, LEGO® based therapy was a rewarding and useful intervention to run and that they enjoyed delivering LEGO® based therapy sessions in their schools |
| 2. Benefits | |
| 2.1. During sessions | Eighteen facilitators said that LEGO® based therapy has clear benefits and that they have seen improvements in their CYP’s communication and social skills and confidence during the sessions. Three facilitators did say that, although they had seen the benefits during the sessions, these might not be reflected in the classroom |
| 2.2. Wider benefits | Eight facilitators said that their CYP had been more confident and had improved communication in the wider school setting. Two facilitators said that they had seen a positive effect in non-autistic CYP, including those with challenging behaviours |
| 3. Resources | Two facilitators said that they had trouble finding a suitable space for some of their sessions, as space in their schools was lacking. Two facilitators said that LEGO® based therapy was easy to set up and deliver |
| 4. Challenges | Five facilitators found LEGO® based therapy challenging to run at times. One facilitator said that having two autistic CYP in the group was tough, because they needed a lot of prompting to communicate, whereas others found that they struggled to find a suitable space to hold the sessions. One facilitator said that their CYP struggled with finding the necessary language to describe the pieces, and another said that their CYP lost motivation after seven or eight sessions, making the final sessions more difficult to run |
| 5. Implementation | Five facilitators said that their school has decided to continue with LEGO® based therapy and are trialling it with more groups or that they have rolled it out across their whole school. One school said that they will be implementing this over a half term (i.e. over 6 weeks), then revisiting it at a later date, rather than delivering it as a 12-week block |
| 6. Recommendations | One facilitator said that her group would have liked to have done more than 12 sessions; another said that 12 weeks was too long and that they would possibly split this up into two blocks in the future. Three secondary school facilitators said that LEGO® based therapy may be more suitable in a primary setting rather than in a secondary setting, and that secondary schools needed more complex sets for it to be effective. One facilitators said that they would include more freestyle play in future groups to allow CYP to be more creative, and another said that they would use CYP only in the same year group in the future |
| Variable | Summary |
|---|---|
| 1. Enjoyed LEGO® based therapy | Twenty-seven parents/guardians stated that their child enjoyed taking part in LEGO® based therapy. Eleven parents/guardians said that they had seen noticeable changes in their CYP’s behaviour and communication and social skills; however, seven parents/guardians said that, although their child enjoyed the sessions, they did not see any impact at home. One parent suggested that this may be because it is ‘too early’ to see any significant changes |
| 2. Benefits | |
| 2.1 Social skills | Seven parents/guardians felt that LEGO® based therapy has improved their child’s social skills, because it helped them with making new friends and interacting with other CYP in class |
| 2.2 Communication | Eight parents/guardians noticed that LEGO® based therapy had a positive impact on their CYP’s communication with their peers. Two parents/guardians said that their child has been more open and spoken about their feelings more at home |
| 2.3 Confidence | Two parents/guardians noticed that their child had grown in confidence after taking part in LEGO® based therapy sessions |
| 2.4 Calmness | One parent/guardian stated that their child/young person is more relaxed and calm since taking part in LEGO® based therapy |
| 3. CYP/parent/guardian would like to continue LEGO® based therapy | Three parents/guardians would have liked their child to continue with LEGO® based therapy because they had enjoyed it so much and to continue to develop their skills further and apply these at home |
| 4. Feedback from school | Four parents/guardians found that they were not sure how their child got on during LEGO® based therapy because they received little or no feedback from the school |
| 5. Did not enjoy LEGO® based therapy | Three parents/guardians stated that their child did not enjoy LEGO® based therapy owing to having lots of previous building experience or wanting more structure to the sessions |
| Acceptability construct | Facilitators (n = 48), median (range) | Parents/guardians (n = 72), median (range) | p-valuea | Facilitators (n = 48), %b | Parents/guardians (n = 72), %b |
|---|---|---|---|---|---|
| Affective attitude | 5 (4–5) | 5 (4–5) | 0.613 | 100 | 94.44 |
| Burden | 5 (4–5) | 3 (2–4) | 0.000 | 87.23 | 42.25 |
| Ethicality | 4 (4–5) | 4 (4–5) | 0.746 | 95.83 | 83.33 |
| Intervention coherence | 5 (5–5) | 3 (3–4) | 0.000 | 93.75 | 40.85 |
| Opportunity costs | 5 (4–5) | 5 (4–5) | 0.024 | 93.75 | 84.72 |
| Perceived effectiveness: general | 4 (4–5) | 4 (3–5) | 0.076 | 91.49 | 70.83 |
| Perceived effectiveness: social skills | 4 (4–5) | 4 (3–4) | 0.001 | 89.36 | 68.06 |
| Perceived effectiveness: academic confidence | 5 (4–5) | 4 (4–5) | 0.025 | 91.67 | 77.78 |
| Perceived effectiveness: communication skills | 5 (4–5) | 4.5 (4.5) | 0.009 | 95.83 | 84.72 |
| Perceived effectiveness: behaviour | 4 (3–5) | 4 (3–4) | 0.054 | 70.83 | 55.56 |
| Perceived effectiveness: overall | 5 (4–5) | 4 (3–5) | 0.000 | 91.49 | 70.83 |
| Self-efficacy | 5 (4–5) | 4 (4–5) | 0.000 | 100 | 76.39 |
| Overall acceptability | 5 (4–5) | 4 (3–5) | 0.000 | 93.75 | 76.38 |
FIGURE 12.
Acceptability of LEGO® based therapy: comparison of median scores for acceptability constructs for primary school participants. Higher scores indicate a greater level of acceptability.
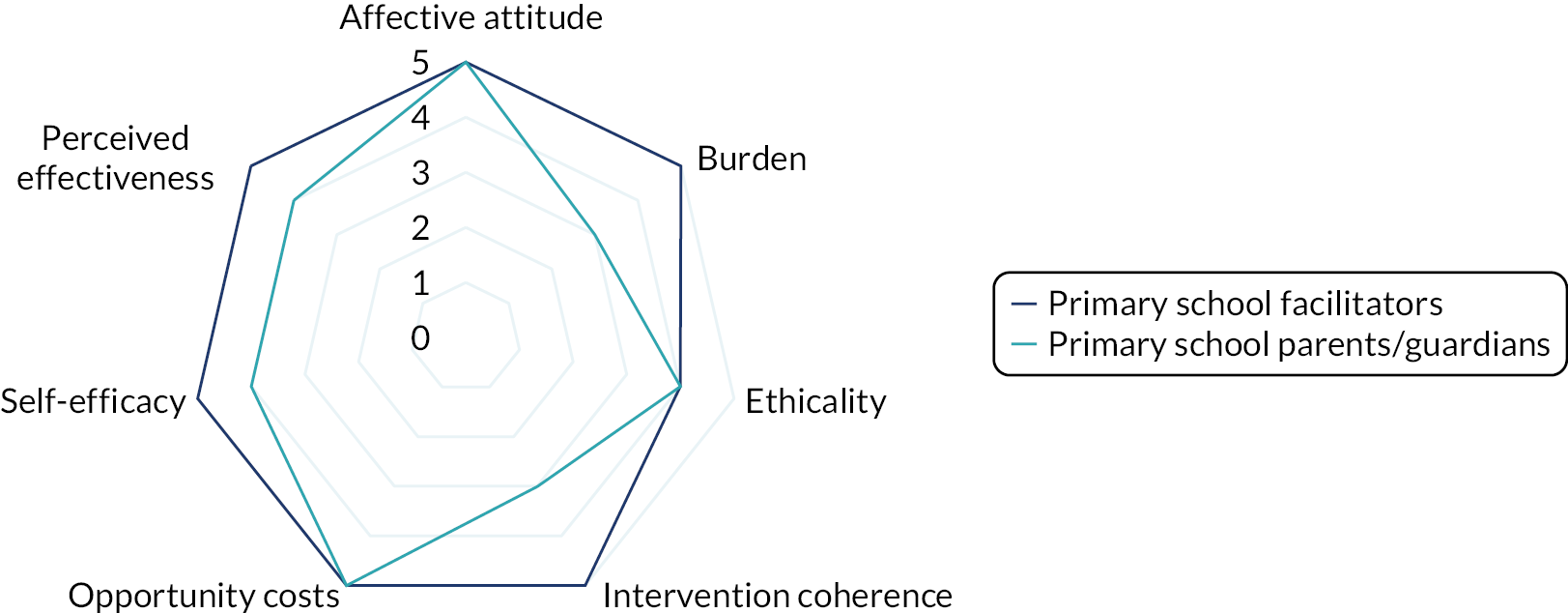
| Acceptability construct | Facilitators (n = 17), median (range) | Parents/guardians (n = 26), median (range) | p-valuea | Facilitators (n = 17), %b | Parents/guardians (n = 26), %b |
|---|---|---|---|---|---|
| Affective attitude | 5 (4–5) | 4 (4–5) | 0.171 | 100 | 84.00 |
| Burden | 4 (4–5) | 4 (3–5) | 0.084 | 88.24 | 68.00 |
| Ethicality | 5 (4–5) | 4 (3–5) | 0.107 | 88.24 | 64.00 |
| Intervention coherence | 5 (5–5) | 4 (3–5) | 0.005 | 94.12 | 64.00 |
| Opportunity costs | 5 (4–5) | 4 (4–5) | 0.304 | 94.12 | 84.00 |
| Perceived effectiveness: general | 5 (4–5) | 4 (3–5) | 0.029 | 94.12 | 64.00 |
| Perceived effectiveness: social skills | 4 (4–5) | 4 (3–4) | 0.088 | 88.24 | 60.00 |
| Perceived effectiveness: academic confidence | 5 (4–5) | 4 (3–5) | 0.152 | 82.35 | 68.00 |
| Perceived effectiveness: communication skills | 5 (4–5) | 4 (4–5) | 0.116 | 94.12 | 84.00 |
| Perceived effectiveness: behaviour | 4 (4–5) | 4 (3–4) | 0.038 | 88.24 | 52.00 |
| Perceived effectiveness: overall | 4 (4–5) | 4 (3–5) | 0.000 | 88.24 | 64.00 |
| Self-efficacy | 5 (5–5) | 4 (4–5) | 0.006 | 100 | 88.46 |
| Overall acceptability | 5 (4–5) | 4 (3–5) | 0.000 | 94.11 | 68.00 |
FIGURE 13.
Acceptability of LEGO® based therapy: comparison of median scores for acceptability constructs for secondary school participants. Higher scores indicate a greater level of acceptability.
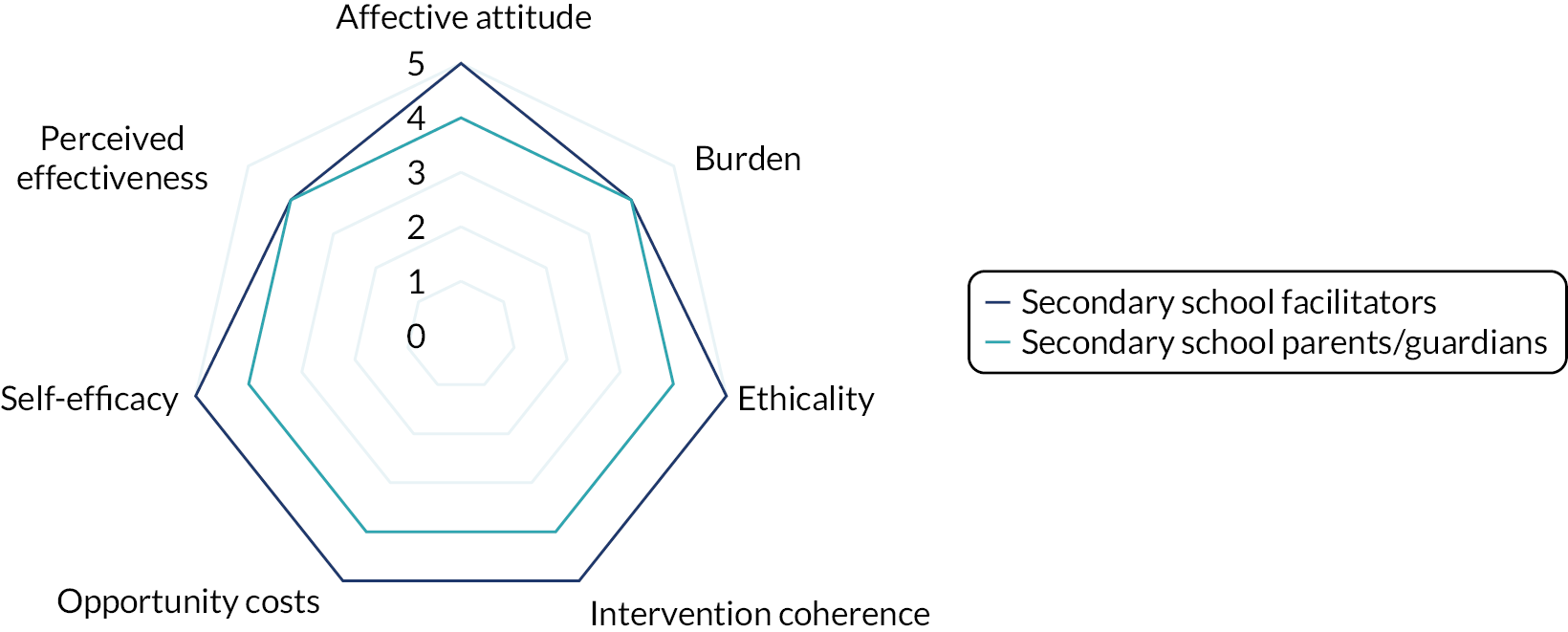
| Acceptability construct | Primary school facilitators (n = 48), median (range) | Secondary school facilitators (n = 17), median (range) | p-valuea | Primary school facilitators (n = 48), %b | Secondary school facilitators (n = 17), %b |
|---|---|---|---|---|---|
| Affective attitude | 5 (4–5) | 4 (4–5) | 0.641 | 100 | 100 |
| Burden | 5 (4–5) | 4 (3–5) | 0.781 | 87.23 | 88.24 |
| Ethicality | 4 (4–5) | 4 (3–5) | 0.228 | 95.83 | 88.24 |
| Intervention coherence | 5 (5–5) | 4 (3–5) | 0.708 | 93.75 | 94.12 |
| Opportunity costs | 5 (4–5) | 4 (4–5) | 0.172 | 93.75 | 94.12 |
| Perceived effectiveness: general | 4 (4–5) | 4 (3–5) | 0.456 | 91.49 | 94.12 |
| Perceived effectiveness: social skills | 4 (4–5) | 4 (3–4) | 0.234 | 89.36 | 88.24 |
| Perceived effectiveness: academic confidence | 5 (4–5) | 4 (3–5) | 0.337 | 91.67 | 82.35 |
| Perceived effectiveness: communication skills | 5 (4–5) | 4 (4–5) | 0.517 | 95.83 | 94.12 |
| Perceived effectiveness: behaviour | 4 (3–5) | 4 (3–4) | 0.643 | 70.83 | 88.24 |
| Perceived effectiveness: overall | 5 (4–5) | 4 (3–5) | 0.428 | 91.49 | 88.24 |
| Self-efficacy | 5 (4–5) | 4 (4–5) | 0.186 | 100 | 100 |
| Overall acceptability | 5 (4–5) | 4 (3–5) | 0.592 | 93.75 | 94.11 |
FIGURE 14.
Acceptability of LEGO® based therapy: comparison of median scores for acceptability constructs for primary and secondary school facilitators. Higher scores indicate a greater level of acceptability.
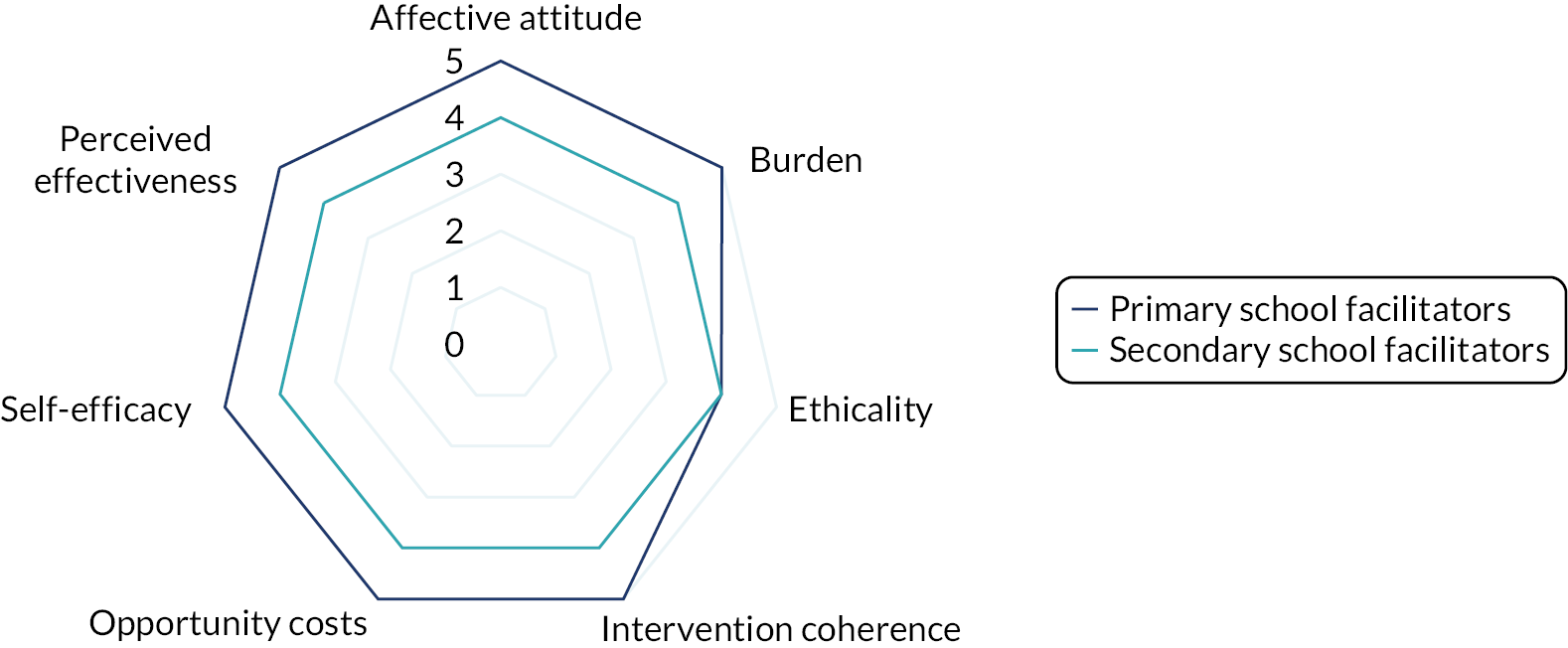
| Acceptability construct | Primary school parents/guardians (n = 72), median (range) | Secondary school parents/guardians (n = 26), median (range) | p-valuea | Primary school parents/guardians (n = 72), %b | Secondary school parents/guardians (n = 26), %b |
|---|---|---|---|---|---|
| Affective attitude | 5 (4–5) | 4 (4–5) | 0.054 | 94.44 | 84.00 |
| Burden | 3 (2–4) | 4 (3–5) | 0.052 | 42.25 | 68.00 |
| Ethicality | 4 (4–5) | 4 (3–5) | 0.172 | 83.33 | 64.00 |
| Intervention coherence | 3 (3–4) | 4 (3–5) | 0.026 | 40.85 | 64.00 |
| Opportunity costs | 5 (4–5) | 4 (4–5) | 0.392 | 84.72 | 84.00 |
| Perceived effectiveness: general | 4 (3–5) | 4 (3–5) | 0.474 | 70.83 | 64.00 |
| Perceived effectiveness: social skills | 4 (3–4) | 4 (3–4) | 0.469 | 68.06 | 60.00 |
| Perceived effectiveness: academic confidence | 4 (4–5) | 4 (3–5) | 0.205 | 77.78 | 68.00 |
| Perceived effectiveness: communication skills | 4.5 (4.5) | 4 (4–5) | 0.466 | 84.72 | 84.00 |
| Perceived effectiveness: behaviour | 4 (3–4) | 4 (3–4) | 0.564 | 55.56 | 52.00 |
| Perceived effectiveness: overall | 4 (3–5) | 4 (3–5) | 0.085 | 70.83 | 64.00 |
| Self-efficacy | 4 (4–5) | 4 (4–5) | 0.308 | 76.39 | 88.46 |
| Overall acceptability | 4 (3–5) | 4 (3–5) | 0.454 | 76.38 | 68.00 |
FIGURE 15.
Acceptability of LEGO® based therapy: comparison of median scores for acceptability constructs for primary and secondary school parents. Higher scores indicate a greater level of acceptability.
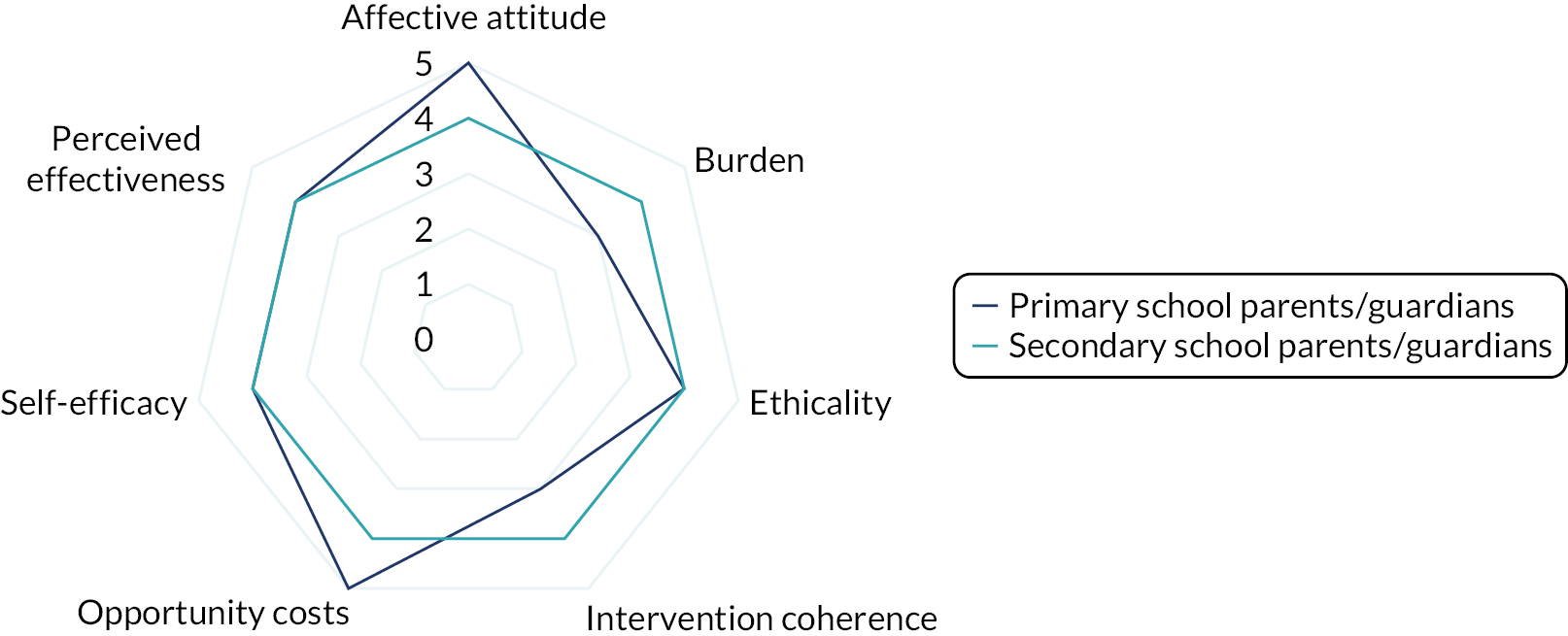
| Category | Code |
|---|---|
| 1. Understanding | 1.1 Parent |
| 1.1.1 Terminology | |
| 1.1.2 More than playing | |
| 1.1.3 Observation | |
| 1.1.4 Explaining | |
| 1.2 Teacher | |
| 1.2.1 Terminology | |
| 1.2.2 More than playing | |
| 1.2.3 Observation | |
| 1.2.4 Explaining | |
| 1.3 Parent | |
| 1.3.1 Terminology | |
| 1.3.2 More than playing | |
| 1.3.3 Observation | |
| 1.3.4 Explaining | |
| 1.4 Intervention differences | |
| 2. Benefits | 2.1 Autistic CYP |
| 2.1.1 Communication | |
| 2.1.2 Socialising | |
| 2.1.3 Confidence | |
| 2.1.4 Language skills | |
| 2.1.5 Concentration | |
| 2.1.6 Patience/calmness | |
| 2.1.7 Wider benefits | |
| 2.1.8 Resilience | |
| 2.1.9 Fine motor skills | |
| 2.2 Non-autistic CYP | |
| 2.2.1 Communication | |
| 2.2.2 Socialising | |
| 2.2.3 Confidence | |
| 2.2.4 Language Skills | |
| 2.2.5 Concentration | |
| 2.2.6 Patience/calmness | |
| 2.2.7 Wider benefits | |
| 2.2.8 Resilience | |
| 2.2.9 Fine motor skills | |
| 2.3 Professional | |
| 3. Implementation | 3.1 Continued therapy |
| 3.2 Expanded to other CYP | |
| 3.3 School/staff support | |
| 3.4 Commitment | |
| 3.5 Integration into practice | |
| 3.6 Cost of LEGO | |
| 4. Values | 4.1 School values |
| 4.2 Professional values | |
| 5. Resources | 5.1 Opportunity costs |
| 5.2 Staffing | |
| 5.3 Space | |
| 5.4 Time | |
| 5.5 Knowledge of ASD | |
| 6. Working practices | 6.1 Power |
| 6.2 Training | |
| 6.3 Adaptations | |
| 6.4 Facilitator skills | |
| 6.5 Recommendations | |
| 6.6 Importance of relationships | |
| 7. Acceptability | 7.1 Enjoyment of LEGO |
| 7.2 Examples | |
| 8. Challengesa |
| TFA construct | Category |
|---|---|
| Affective attitude | 1. Understanding |
| 7. Acceptability | |
| 8. Challenges | |
| Burden | 3. Implementation |
| 5. Resources | |
| Ethicality | 4. Values |
| Intervention coherence | 1. Understanding |
| Opportunity costs | 4. Values |
| Perceived effectiveness | 2. Benefits |
| 8. Challenges | |
| Self-efficacy | 6. Working practices |
| Category | TFA constructs | ||||||
|---|---|---|---|---|---|---|---|
| Affective attitude | Burden | Ethicality | Intervention coherence | Opportunity costs | Perceived effectiveness | Self-efficacy | |
| 1. Understanding | ✓ | ✓ | |||||
| 2. Benefits | ✓ | ||||||
| 3. Implementation | ✓ | ||||||
| 4. Values | ✓ | ✓ | |||||
| 5. Resources | ✓ | ||||||
| 6. Working practices | ✓ | ||||||
| 7. Acceptability | ✓ | ||||||
| 8. Challenges | ✓ | ✓ | |||||
Glossary
- Autism spectrum disorder
- This term is used throughout this report in line with the internationally recognised diagnostic definitions of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Washington, DC: American Psychiatric Publishing Inc.; 2013) and the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (World Health Organization. International Statistical Classification of Diseases and Related Health Problems Tenth Revision. 2019. URL: https://icd.who.int/browse10/2019/en; accessed 1 December 2020). The research team are aware, however, that there are a number of alternative terms used by members of the autistic community. Discussions with patient and public involvement representatives, experts in the field and other stakeholders associated with this research brought to our attention terms and phrases including autism, autism spectrum condition, on the autistic spectrum and child with/without autism. We appreciate that this is not an exhaustive list and that the rich diversity of this community cannot be captured by the terms mentioned here. For the purposes of this research, the term autism spectrum disorder should be understood to include these terms. The research team would like to thank those involved in the discussion of this terminology for their valued input.
- Brick builder
- The child who puts the pieces together.
- Brick engineer
- The child who describes the instructions.
- Brick supplier
- The child who finds and distributes the correct bricks.
- Facilitator
- A member of school staff who delivers the intervention.
- Gender
- This study sought to identify gender, not biological sex, as a participant characteristic; therefore, although the published protocol uses the term sex, the term gender is used throughout this report.
List of abbreviations
- ACR
- average causal response
- AE
- adverse event
- AFOL
- adult fans of LEGO
- ALS
- Asher Loneliness Scale
- ASD
- autism spectrum disorder
- CACE
- complier average causal effect
- CAMHS
- Child and Adolescent Mental Health Services
- CEAC
- cost-effectiveness acceptability curve
- CHU-9D
- Child Health Utility-9 Dimensions
- CI
- confidence interval
- COMIC
- Child Oriented Mental Health Intervention Centre
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTRU
- clinical trials research unit
- CYP
- children and young people
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-Y
- EuroQol-5 Dimensions-Youth
- GP
- general practitioner
- HFA
- high-functioning autism
- HRA
- Health Research Authority
- HRQoL
- health-related quality of life
- ICC
- intraclass correlation
- ICER
- incremental cost-effectiveness ratio
- I-SOCIALISE
- Investigating SOcial Competence and Isolation in children with Autism taking part in LEGO® based therapy clubs In School Environments
- ITT
- intention to treat
- LM
- learning mentor
- LYPFT
- Leeds and York Partnership NHS Foundation Trust
- MCID
- minimum clinically important difference
- MI
- multiple imputation
- MSPSS
- Multidimensional Scale of Perceived Social Support
- NAS
- National Autistic Society
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PHR
- Public Health Research
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RA
- research assistant
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SchARR
- School of Health and Related Research
- SCQ
- Social Communication Questionnaire
- SD
- standard deviation
- SDQ
- Strengths and Difficulties Questionnaire
- SDV
- source data verification
- SEN
- special educational needs
- SENCO
- special educational needs co-ordinator
- SSIS
- Social Skills Improvement System
- SULP
- Social Use of Language Programme
- TA
- teaching assistant
- TFA
- theoretical framework of acceptability
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WTP
- willingness to pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/VGTR7431).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
