Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 13/10/14. The contractual start date was in February 2015. The final report began editorial review in May 2018 and was accepted for publication in January 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Gavin Perkins reports grants from the National Institute for Health Research (NIHR) during the conduct of the study and non-financial support from Intensive Care Foundation (Camberwell, VIC, Australia) outside the submitted work. Karen Rees reports grants from NIHR during the conduct of the study. Helen Parsons reports grants from NIHR during the conduct of the study. Zoe Fritz has received grant funding from the Wellcome Trust (London, UK) outside this study and is on the executive committee of the Resuscitation Council (UK) (London, UK); expenses are covered for meetings. Zoe Fritz is also chairperson of the Strategic Steering Group for ReSPECT (Recommended Summary Plan for Emergency Care and Treatment); expenses are covered for meetings. Sarah Symons is a member of the Bath Clinical Ethics Advisory Group and has received payment from the University of Warwick for time taken to comment on the study documents as patient and public involvement co-investigator. Anne Slowther is a member of the Board of Trustees of the UK Clinical Ethics Network (Newcastle upon Tyne, UK) and the Institute of Medical Ethics (St Helens, UK). She has received funding in grants from NIHR as a co-investigator outside this study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Bassford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Throughout this report we have used the term intensive care (rather than critical care) to describe both the physical location [intensive care unit (ICU)] and the health-care professional (intensive care doctor) making decisions about admission to the unit. We consider the terms intensive care and critical care to be synonymous.
Intensive care
An ICU is ‘a specifically staffed and equipped, separate and self-contained area of a hospital dedicated to the management and monitoring of patients with life-threatening conditions’ (reproduced with permission from Faculty of Intensive Care Medicine/Intensive Care Society). 1 Timely admission to an ICU is associated with more favourable outcomes. 2 It is thought that admission to intensive care gives a critically ill patient a 17–23% increase in their chances of surviving their acute illness. 3
Treatments delivered in intensive care include invasive monitoring of physiological parameters, renal replacement therapy, cardiovascular support and invasive and non-invasive ventilatory support. These treatments, although potentially life-saving, can place significant burdens on an individual patient. In the UK, the mortality rate for patients whose hospital stay involves admission to ICU is around 20%, and 10% of those who survive ICU die before leaving hospital. 4 Following ICU discharge, patients have an increased mortality rate that persists for at least 15 years. 5,6 In addition, ICU survivors have ongoing morbidity compared with their peers; among patients who require renal replacement therapy on ICU, only 28% survive for 1 year and 12% of survivors require ongoing renal dialysis. 7 Long-term psychological morbidity after ICU admission is also widely recognised. 8
Treatment in intensive care may not always be in the best interests of a critically ill patient. If intensive care does not return a critically ill patient to a life they value, then the intervention may have harmed rather than benefited that patient. For patients who do not survive ICU, an opportunity may have been missed for them to have a peaceful and dignified death.
Deciding whether to refer and/or admit a patient
When a patient is assessed as having a life-threatening illness, an initial decision must be made about whether or not they should be referred to ICU. This decision is made by the clinical team caring for the patient. The decision whether or not a patient should be admitted to ICU is usually made by the ICU doctor, although critical care outreach (CCOR) or emergency medical treatment teams may also contribute to the decision. Both the referral and the admission decision require clinical teams to consider the benefits and burdens of ICU treatment for the individual patient in question. Treatments delivered in an ICU may not be required or the burdens of ICU treatment, both short and long term, may outweigh any potential benefit. In some cases, palliative care may provide the best option for treatment of the patient’s condition. These decisions are not easy and require good clinical and ethical judgement.
Assessing the clinical situation
A patient’s acute clinical condition and the severity and nature of their past medical history are important considerations when making a decision about admitting him or her to ICU. Our previous scoping review found several studies confirming that these factors have an impact on the decision. 9–14 However, there is often uncertainty about prognostic indicators and the extent of chronic illness in an acute situation. Other, more value-laden factors also appear to influence the assessment of the clinical situation, including the age of the patient13,15–17 or the doctor’s assessment of the patient’s functional status or quality of life,14,18–20 whether a patient has a medical or a surgical diagnosis3,12,21 and whether they are assessed by a junior or a senior physician. 10,16,22–24
Evidence and prognostic indicators
Although prognostic information from observational studies is available to guide decision-making, the likelihood of death for a critically ill individual remains difficult to predict with an acceptable degree of certainty. Several prognostic tools for application to critically ill patients have been developed, the most widely used in the UK being the Acute Physiology and Chronic Health Evaluation (APACHE-II) score. 25 However, this is calculated after the patient has been admitted to ICU, so it is of limited value for referral or admission decisions. Most prognostic models predict either ICU or hospital mortality, rather than long-term survival (or quality of life). Therefore, prognostic models are primarily used to assess ICU quality and performance rather than as a framework for decision-making in individual cases. 26
Some individual patient-related factors have been found to be associated with increased mortality in ICU patients after hospital discharge, namely severity of illness, age and presence of comorbidities. 5 This information can inform the risk–benefit analysis when considering admission, but it does not provide a definitive prognosis for an individual patient.
There is evidence to suggest that, even if clinicians are provided with patient-specific prognostic information about end of life at the time of decision-making, this does not materially alter their clinical decision-making. 27
Patient’s values and wishes
The ethical principle of respect for patient autonomy is reflected in legal and professional regulation of clinical decision-making. 28,29 A person should be provided with relevant information and given an appropriate time to contribute to a decision about their treatment. However, when the decision is about whether to refer or admit a person to ICU, that person is often unable to take part in the discussion, and in most cases will lack the capacity to consent to, refuse or request treatment because of the severity of their illness. Guidance on how to use a shared decision-making model in the context of critical illness has been produced;30 however, it is still the clinician’s responsibility to operationalise the shared decision-making model within a specific context, including the urgency of the situation and the extent to which the patient and/or their family or surrogate decision-maker are able to participate. In the UK, if a patient lacks capacity, it is the responsibility of the clinician caring for them to first determine if there is any relevant advance statement or legal proxy to make decisions on the patient’s behalf, and then to make a decision that is in the patient’s best interests, consulting where possible with the patient’s family and those who know the patient well in order to understand what the patient’s wishes might be. 28
Critical illness often develops with little warning and the decision whether or not to admit a patient to ICU often needs to be made in an emergency situation or when a patient’s condition is deteriorating rapidly. Family members may not be present and, even if they are, there may be little time for extensive discussion with them. When families are consulted about the patient’s values and wishes, they are not always accurate in predicting what the patient might want in this situation. There is evidence that families acting as surrogate decision-makers poorly predict what the patient would wish for themselves, and may prioritise their own values and wishes, rather than those of the patient, when asked for their views on future therapy. 31
Resources and external influences
The safe delivery of intensive care treatments requires a high concentration of staff and resources. In an ICU, the nurse-to-patient ratio is 1 : 1 for a ‘level 3’ patient or 1 : 2 for a ‘level 2 patient’,1,32 compared with a standard ward where one nurse will look after nine patients. 33 In 2006 in Europe the cost of an intensive care bed was between €1168 and €2025 per day. 34 Delivering this level of nursing for all patients would quickly overwhelm the resources of any health-care system, and therefore access must be limited. 35 The UK has relatively few intensive care beds compared with many other countries,36 and pressure on the UK’s available ICU resources is a regular occurrence. A survey by the Faculty of Intensive Care Medicine in 201837 showed that 21% of ICUs regularly moved patients to other hospitals because of a lack of local intensive care resources. Surveys and cross-sectional studies10,38–43 of patients admitted to ICU have previously shown that the availability of ICU resources influences whether or not a patient is admitted.
Decision-making within intensive care is set in the context of wider organisational policies and priorities. Some hospitals have specific priority programmes, such as transplant surgery or major trauma, and institutional policy may prioritise these patient pathways for intensive care admission. 44,45 The behaviour of other clinicians (e.g. failure to specify end-of-life treatment plans or to secure an ICU bed before elective surgery) and family demands for life support can also create a perceived pressure to admit a patient to ICU. 46
These organisational and situational pressures add a further ethical dimension to the decision-making, in addition to the difficulty of balancing benefits and burdens of ICU treatment for a particular patient.
The views and values of the decision-maker
As evidence-based prognostic indicators are seen to be of limited value, and as the patients’ views may not be known, it is likely that other values and perceptions will have a bearing on the decision about whether or not to admit a patient to ICU. Previous experience of treating patients with similar conditions may influence the clinician’s perception of a patient’s potential to benefit from a particular treatment. A phenomenon known as ‘prognostic pessimism’ has been demonstrated in ICU clinicians assessing patients with chronic obstructive pulmonary disease (COPD). Patients with the most severe disease were found to benefit most from ICU treatment and valued the resultant extension to their life after hospital discharge, contrary to the expectations of clinicians who predicted that they would fare badly. 47 Similar findings have also been seen in patients with heart failure. 48,49 A large European study50 of decisions to admit to ICU found that, although older people were less likely to be admitted to ICU, the mortality benefit (mortality without ICU compared with that with ICU) was greater for older patients than for their younger counterparts. The authors concluded that ‘physicians should consider changing their intensive care triage practices for the elderly’. 50
Personal moral values and religious values have also been shown to influence clinicians’ decisions about withholding or withdrawing life-prolonging treatment, of which intensive care can be considered an example. 51
Fair access to intensive care
Given the diversity of values and external factors that influence decisions to admit to ICU, the lack of an effective prognostic tool and the relative lack of relevant guidance, it seems likely that decision-making will vary. Studies have shown variation in ICU admission decisions among individual ICU clinicians,47,52 between ICU staff and referring clinicians,9,53 between institutions in the same country18,50 and between physicians in different countries. 43 Although some variation in decision-making is inevitable, this could lead to inequity in the provision of ICU care. Currently in the UK, not all patients who would benefit from ICU receive it. The 2012 National Confidential Enquiry into Patient Outcomes and Death report54 Time to Intervene? noted that 37 out of the 392 patients studied who were admitted to a standard ward should have received ICU/high-dependency unit care. There is an ethical requirement to be fair to every patient when making decisions about their care, which means that there should be consistency in the reasons for making the decision and that these reasons should be explicit so that they can be justified if challenged.
Current guidance on decisions to admit patients to the intensive care unit
There is very little specific professional guidance on decision-making about admitting patients to intensive care. In 1996, the then UK Department of Health published guidelines55 on patients’ admissions to and discharge from ICU and high-dependency units. The main criteria for ICU admission in this guidance are whether or not the condition is reversible and the absence of a significant comorbidity, in addition to the need for ventilator or multiple organ support. The guidance does not define what constitutes reversibility or significant comorbidity. This document, although 22 years old, remains the only national UK guidance on admission to ICU. A Department of Health and Social Care report,56 published in 2000, on the organisation of critical care services in the UK did not further develop admission policy for intensive care but called for further guidance to be developed and implemented locally and nationally. Although some regional critical care networks have developed admission policies,57,58 the national guidance has not been updated to take into account new evidence or developments in professional guidance and legal frameworks such as the Mental Capacity Act 2005. 29
In 2016, the Society of Critical Care Medicine in the USA provided definitions of ‘inappropriate’ and ‘futile’ treatments, with the aim of resolving disagreement about these terms. They suggested that a treatment should generally be considered inappropriate:
when there is no reasonable expectation that the patient will improve sufficiently to survive outside the acute care setting, or when there is no reasonable expectation that the patient’s neurologic function will improve sufficiently to allow the patient to perceive the benefits of treatment.
Nates et al. 59
The Society of Critical Care Medicine also produced administrative guidance59 for developing services for best practice in ICU admissions. This guidance highlights the importance of the processes surrounding admission to ICU but offers little guidance for individual decision-making. Also in 2016, the World Federation of Societies of Intensive and Critical Care Medicine produced a summary60 of available evidence addressing four key questions relevant to decisions about intensive care admission: who will benefit from intensive care?; who makes the decision whether or not a patient will be admitted to intensive care?; what in-hospital factors limit patient access to intensive care; and what other factors should influence whether or not a patient is admitted to intensive care? Their conclusions did not provide specific guidance for decision-makers but gave more general points about the importance of fair allocation of ICU resources, the need to weigh the benefits and burdens of ICU for the individual patient, the importance of multidisciplinary input into decisions, and the limited usefulness of prognostic algorithms.
Decision-making for intensive care admissions: addressing the issue
Decisions about the provision of potentially life-saving but extremely burdensome treatment are clinically complex and ethically challenging. The clinicians who make these decisions are faced with clinical uncertainty, limited knowledge of the patient and external constraints which may preclude their preferred option, as well as being under pressure to make a decision quickly if the patient is deteriorating. They also have an ethical obligation to treat all patients fairly. Critically ill patients and their families may be unaware of how or why the decision has been made and have little opportunity to contribute to or challenge the process, yet the families have to live with the consequences of these decisions and may be very distressed if they feel that the decision was the wrong one, especially where the patient does not survive. There is a clear need for guidance and support for both clinicians and patients and their families when faced with these difficult decisions.
Therefore, this project focuses on understanding the decision-making process around referral and admission to the ICU in order to develop an intervention to support decision-makers, patients and their families, and to improve the decision-making process.
The project was designed to answer the research question:
-
What is required for an ethically justifiable, patient-centred decision-making process for unplanned and emergency admissions to adult intensive care?
The project aims were threefold:
-
to explore how decisions about whether or not to refer or admit a patient to adult intensive care are made in the acute and emergency situation
-
to identify and critically analyse the factors that should inform ICU referral and admission decisions from the perspective of patients and their families and the clinical decision-makers
-
to facilitate ethically justifiable, patient- and family-centred decision-making in these situations.
We sought to achieve these aims through a series of work packages (WPs) addressing specific objectives:
-
to describe current practice in decision-making for referral and admission to ICU (WP1)
-
to explore the experience of patients, families and clinicians involved in the decision-making process and their views on how these decisions should be made (WP1)
-
to determine the influence of different factors on decisions to admit a patient to ICU from the perspective of ICU clinicians and the general public (WP2)
-
to develop and test a decision-support framework (DSF), including education and support materials, that will facilitate ethically informed decision-making, including reasons and process (WP3)
-
to develop information for patients and families to help them understand and contribute to the decision-making process (WP3)
-
to develop and test a tool for assessing the impact of the DSF on ICU referral and admission decisions (WP4).
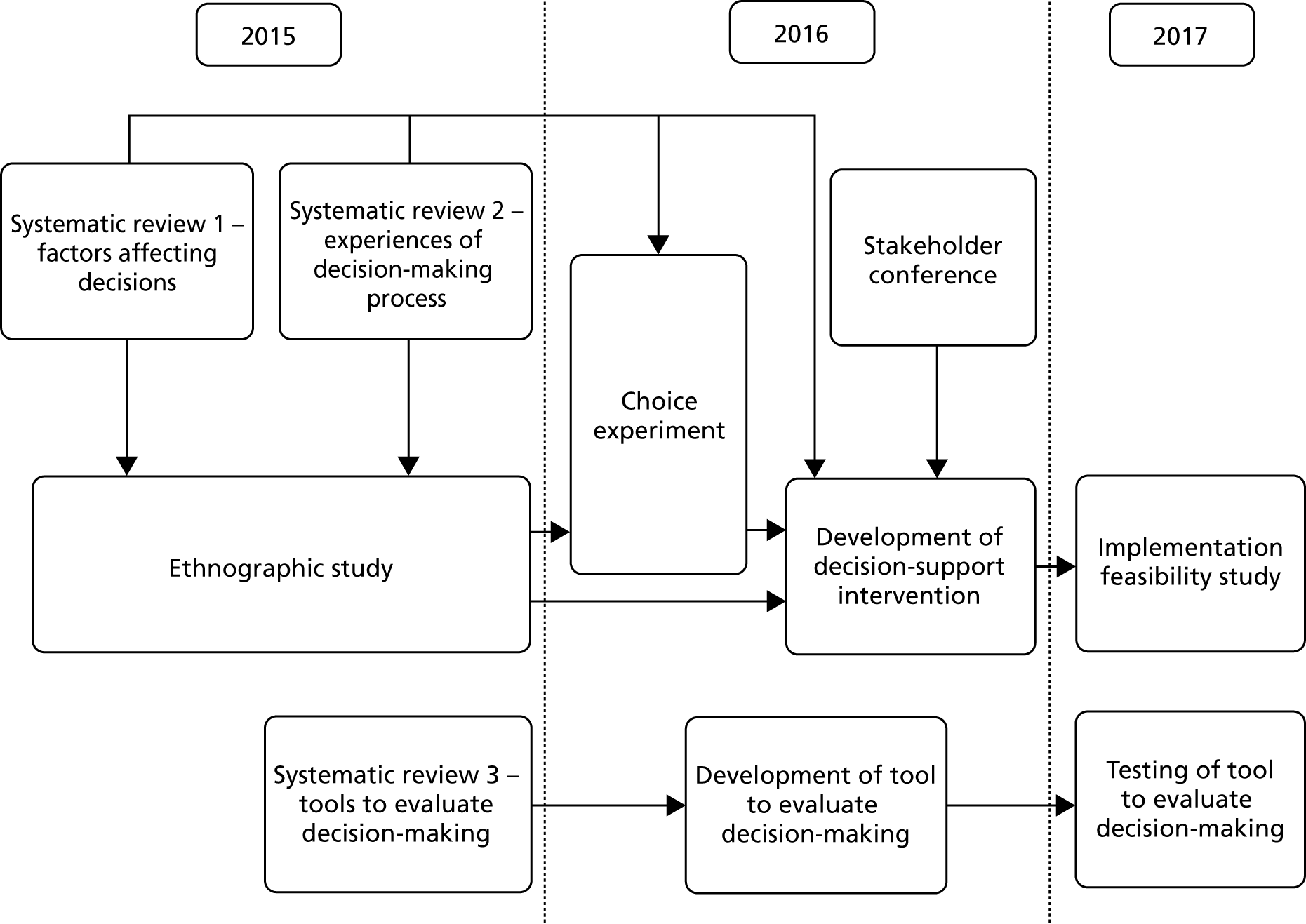
The project used a mixed-methods approach, including systematic reviews, a focused ethnographic study of current practice, a choice experiment questionnaire survey of intensive care consultants and CCOR nurses, a stakeholder conference and an implementation feasibility study. Figure 1 provides an overview of the study.
FIGURE 1.
Study diagram.
Ethics considerations
This project raised a number of ethical issues, particularly in terms of recruitment and consent to our ethnographic study. For the observation process, it was not possible to obtain consent from everyone who might be observed; therefore, information was provided in all clinical areas, consent was obtained from the ICU doctor being shadowed and specific agreement was obtained from the patient or family members for individual case observation. The approach to patients and/or family members at the time of decision-making was through the clinical team to minimise distress and protect privacy. A comprehensive system of tracking, recruitment and consent was developed to ensure that patients who regained capacity were informed of the study and gave appropriate consent for participation, and to approach family members for late-stage interviews (see Report Supplementary Material 1 and 2). Site-specific contacts for support for patients and families were identified. A protocol for responding to disclosures or observation of unsafe or unethical practice was developed and approved by the research ethics committee. In developing the evaluation tool, we accessed sections of anonymised patient records without explicit consent being obtained. We received approval for this from the Confidentiality Advisory Group of the Health Research Authority (15/CAG/0116). The whole project was approved by the Coventry and Warwickshire Research Ethics Committee (15/WM/0025) on 18 February 2015.
Chapter 2 Patient and public involvement
Introduction
Decisions about referral or admission to intensive care can have life-changing implications for patients and their families, and any project investigating these decisions must consider this impact in both the design and the conduct of the project. A project that involves critically ill patients and their families raises challenges for researchers around how to involve them while not adding to their existing distress. Given these two major considerations, the embedding of patient and public involvement (PPI) at every stage was identified as vital to the successful running and meaningful reporting of the project. We therefore included PPI in the design, development, analysis, reporting and oversight of the project.
Scope of patient and public involvement
Project design
Before submitting the application for funding, we held a patient and public engagement meeting. Attendees were recruited from a national ICU patient support charity, ICUsteps; patients from a NHS post-ICU clinic; and the University of Warwick’s Teaching and Research Action Partnership (UNTRAP). The purpose of this meeting was to seek input from the group on framing and prioritising the research questions and identifying appropriate methods for conducting the research that would be acceptable to patients and their families. During the meeting, the participants were presented with background information about referrals and admissions to intensive care and why there was a perceived need for a study. The presentation concluded with the proposed broad aim of the research. The group was asked to identify what they saw as important issues for the research to address, what the specific research questions might be and how to conduct the research in a way that was sensitive to patients and their families.
The group thought that this was an important area of research as it was crucial for patients and families in an extremely vulnerable situation to be able to trust both the professionals making these decisions and the decision-making process. Key questions identified by the group included how patients and families were involved in these decisions and communicated with; who was involved in making the decisions; how decisions about quality of life were made; and if ICU resources were important in the decision-making process. The group also offered suggestions on the timing and the number of interviews with family members to balance the needs of data collection with reducing family distress. These fed into the final project design.
Investigator team
Our investigator team included two PPI co-investigators (CW and SS), who were involved in the design and development of the project, provided guidance on the acceptability of the methods, commented on and amended all patient and family information materials (both study and intervention documents) and contributed to the writing and editing of reports from the different WPs.
Advisory group
We convened a patient and public involvement advisory group (PPIAG) of six members, some of whom had either been patients themselves or had experience of a relative being in an ICU. The group met 6-monthly throughout the project for updates from the project team and to provide advice on the conduct and findings of the project as it progressed. Individual members of the group also contributed more directly to specific WPs.
Project oversight
The project’s independent steering committee included two PPI members, one of whom had been a patient in an ICU. The committee met at 6-monthly intervals throughout the project to provide support to and oversight of the project.
Project development
The PPI co-investigators worked closely with the project team on developing information for patient and family participants and recruitment and consent processes in the ethnographic study. Two members of the PPIAG participated in the development of the draft decision-support intervention (DSI), attending project meetings and commenting on each stage of the process. One member led on initial drafting of patient and family information leaflets to be used as part of the intervention. Members of patient representative groups and the PPIAG attended the stakeholder conference and participated in focus groups to refine the content of the DSI. One of our PPI co-investigators (CW) chaired a session at the conference. Following the stakeholder conference, further extensive revision of patient and family information leaflets was overseen by the PPI co-investigators. Translated versions of these documents were checked for cultural acceptability among native speakers of the languages through our PPIAG contacts.
We were unable to obtain sufficient representation at the stakeholder conference for patients with mental health disorders and for elderly patients. We therefore attended local advocate group meetings to seek feedback on the DSI and its implementation.
Analysis
Two members of the PPIAG and one PPI co-investigator (CW) contributed to the analysis of the qualitative data in our ethnographic study. They attended data analysis meetings, read a selection of interview transcripts and contributed to the refinement of interview schedules and the development of an analysis framework.
Dissemination
Patient and public involvement co-investigators, members of the PPIAG and patient organisation representatives who attended the stakeholder conference were invited to the dissemination event at the end of the project. One PPI co-investigator (CW) gave a response to and reflection on the project findings from the patient and family perspective.
Summary
The importance of PPI was recognised at an early stage of development of the project and was integral to its development, conduct, delivery and successful completion. Involving PPI members in analysis raised issues of data protection, which were addressed using confidentiality agreements and standard operating procedures. PPI was occasionally challenging, as the involvement of individuals with different experiences and perspectives can create dissonance and disagreement. However, disagreement was always constructive and enriched the overall project development. The presence of PPI throughout the project also helped to ensure that the work retained its focus on the patients at the heart of the decision-making process, and that language and communication were consistently clear and accessible. Without PPI throughout the project, the outputs would have been less acceptable and credible as guides for patient-centred clinical decision-making. We were fortunate to have such engaged PPI co-investigators and advisory group members so that we were able to create an environment for meaningful PPI.
Chapter 3 Systematic literature reviews to explore existing evidence
Introduction
To identify what was already known about the subject, we carried out two systematic reviews to answer the following research questions:
-
What are the patient- and clinician-related factors that affect decisions around unplanned admissions to an ICU? (Factors review: PROSPERO CRD42015019711.)
-
What are the experiences of clinicians, patients and families of the process of referral and admission to an ICU? (Experiences review: PROSPERO CRD42015019714.)
Methods
Study identification
Because both reviews focused on the process of decision-making about referral and admission to ICU, we used the same search strategy and abstract screening process to avoid duplication between them. At the full-paper screening stage, we identified studies relevant to (1) the factors review, (2) the experiences review and (3) both reviews. The search strategy was informed by an initial scoping review of the literature, and had three broad areas using a combination of the following MeSH (medical subject heading) headings and keywords: (1) critical and intensive care, intensive care units and critical illness; (2) patient admission, transfer, triage and refusal to treat; and (3) professional decision-making and judgement, professional–family relations, choice behaviour and medical futility. Papers that referred to paediatrics or neonates were excluded. The following databases were searched: MEDLINE, EMBASE, Applied Social Sciences Index and Abstracts (ASSIA), all sections of The Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO and Web of Science. ‘Grey literature’ (Dissertation Abstracts Online, Index to Theses, OpenGrey) and references from key papers were also screened. We used forward and backward citation tracking from our full-text papers to identify further studies that our initial search had missed. Our full searches are presented in Appendix 1 (see Tables 25 and 26). We included papers published between 1980 and 2015 describing empirical research that focused on the process of decision-making about referral or admission of adult patients to ICU, and that investigated either factors affecting decision-making or the experiences of clinicians, patients or families. The search was run on 11 May 2015.
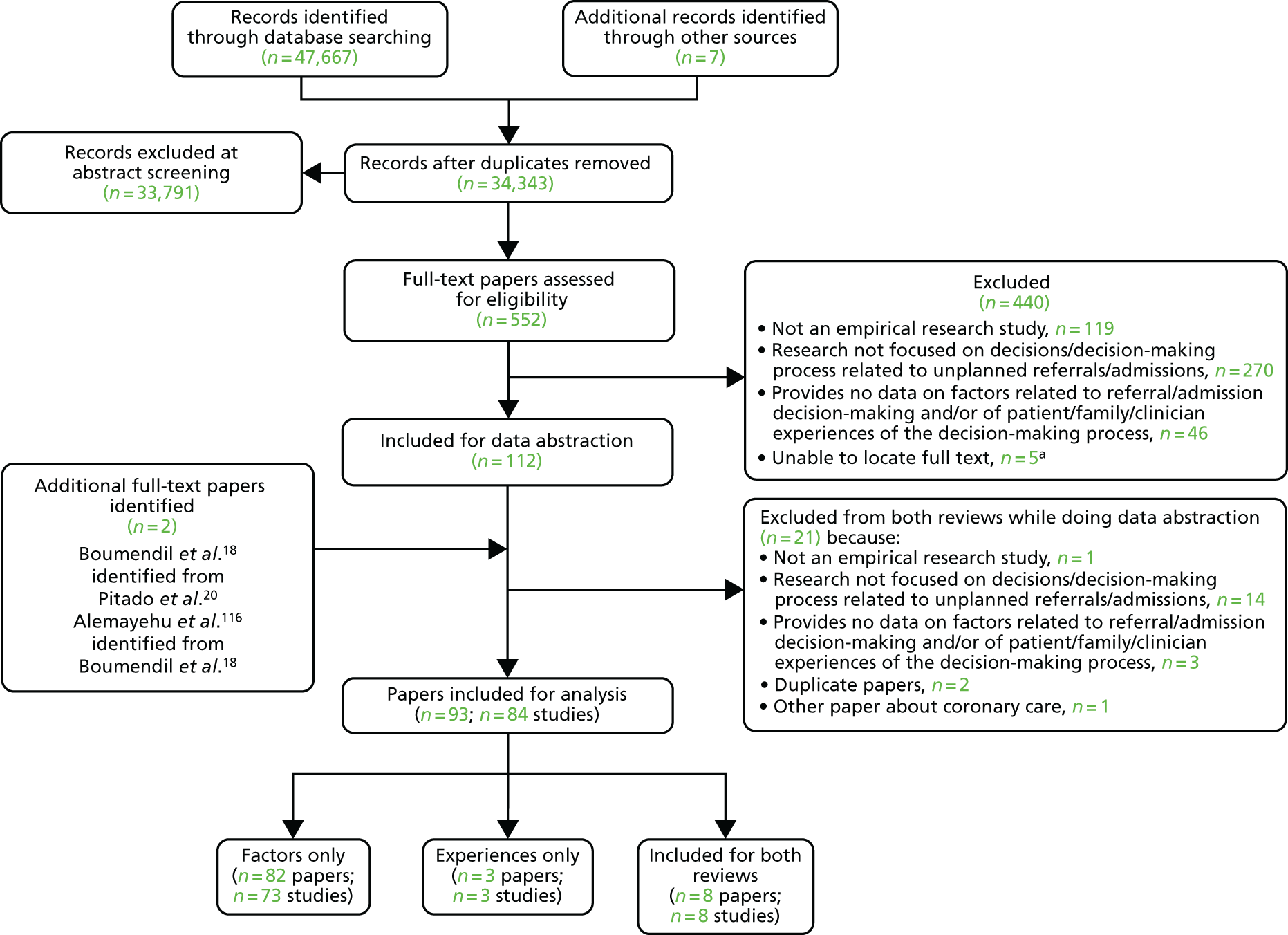
We identified 47,674 records, 34,343 after duplicates were removed. Abstracts were double-screened by a team of 13 reviewers [three members of the study team (CB, AS and HH) and 10 medical students trained in the process], and 552 records went forward for full-text retrieval and formal inclusion in/exclusion from the review (Figure 2). Full-text papers were also double screened by six members of the research team (CB, AS, ZF, HH, JT and MB).
FIGURE 2.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the main systematic review. a, We were unable to source these five full texts for a variety of reasons, including being unable to make contact with the author even after internet searching, e-mail-based invitation and telephoning, where possible; the author of two related conference abstracts also did not follow up in the end with further details on their unpublished full study findings (as promised earlier). Reproduced with permission from Rees et al. 61
In March 2018, we completed a brief update review to identify any relevant studies that had been published since our initial searches. A re-run of the original searches yielded over 10,000 returns. Given time constraints, we chose not to repeat the full review process. Instead, we adopted a pragmatic approach using the following method:
-
We searched PubMed for papers published between 1 May 2015 and 31 December 2017 using the search terms Critical care/CCU or intensive care/ICU AND decision-making AND admissions OR referrals.
-
We hand-searched the contents of the six journals that had provided more than one included paper in our original review. We searched all issues published from 1 May 2015 to 1 January 2018.
-
We searched the reference lists of any identified papers to check for further papers, as well as the reference list of a published review. 62
Ten papers were identified at abstract screening stage for full-text assessment by two reviewers (AS/KR or AS/CB). Three further papers were identified from the reference list of the published review. Eight studies (seven for factors and one for experiences) were added to the total number of studies included for analysis in the main systematic review.
Methodological quality assessment
Cohort studies were assessed using the Newcastle–Ottawa Scale. 63 The majority of studies were cross-sectional, and we used an adapted version of the Newcastle–Ottawa Scale to assess quality for this study design. 64 Clinical trials were assessed with the Cochrane Risk of Bias tool65 and qualitative studies were assessed using May and Pope’s qualitative evaluation criteria. 66 Each included study was scored for quality by two reviewers (AS/HH, CB/JT or ZF/MB) and any discrepancies were referred to a third reviewer for final decision (KR or FG) (see Appendix 2, Tables 27–29).
Data extraction
For studies relevant to the factors review, we grouped identified factors using the following process. We identified an initial list of factors based on our previous scoping review of the literature and categorised these into patient factors (medical/non-medical), clinician factors, organisational factors and others. These categories were further subdivided; for example, patient-related medical factors included type of acute illness, severity of acute illness and type of chronic illness. During data abstraction, we mapped each factor identified in a paper onto our predefined subcategories and collected any factors that did not map onto the category of ‘other’. Three members of the team (KR, CB and AS) then either categorised factors in the ‘other’ category into existing categories or created additional subcategories. For the experiences review, any relevant qualitative data were copied and pasted into a Microsoft Word (Microsoft Corporation, Redmond, WA, USA) document for analysis.
Analysis
The majority of data for the factors review were quantitative, but a small number of studies had descriptive qualitative data. When possible, we combined studies statistically using a meta-analysis. Owing to the potential confounding effects of each of the factors examined on the others, we focused on studies reporting multivariate analyses of independent factors affecting admission decisions. Where these were lacking, we explored descriptive associations but were cautious in our interpretation because of biases and confounding.
If there were sufficient studies, effect sizes from multivariate analyses for each factor were pooled using the generic inverse variance method using RevMan software (version 5.3; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). 67 The results from cohort studies were pooled together with the results from cross-sectional studies as we were concerned not with longitudinal associations but with decisions to admit and the factors affecting these that occur concurrently. The remaining studies were described narratively.
For the experiences review, a thematic analysis of any relevant qualitative data from the identified studies was conducted. Two research team members (SR and AS) read all of the data and developed initial codes from which themes were developed during discussion. The themes were tested in a further research meeting with a third member of the team (FG).
Results
From the initial review, 84 studies (93 papers) were included for analysis in both reviews, of which 81 studies (90 papers) were included in the factors review and 11 studies were included in the experiences review (see Figure 2). A further eight studies (seven for factors and one for experiences) were included from the brief update review. Overall, the quality of studies was moderate or poor, with 14 out of 19 cohort studies and 17 out of 61 cross-sectional studies being rated as high quality (see Appendix 2, Tables 27 and 28).
Factors review
The characteristics of the 88 included studies and the factors each study investigated are documented in Appendix 3 (see Table 31). The vast majority were observational.
The findings are reported under factor headings grouped as patient-related medical, patient-related non-medical, clinician-related and organisation-related (Table 1). For each of the factors analysed, the results are presented first for multivariate analyses. A summary of multivariate analysis results is presented in Appendix 4 (see Table 32). For factors for which there are no multivariate analyses, we report findings of descriptive studies. If multivariate analyses are present, we note the presence of descriptive studies.
| Factor | Studies with multivariate analyses (n) | Studies with univariate analyses (n) | Descriptive studies (n) |
|---|---|---|---|
| Patient related | |||
| Medical | |||
| Type of acute illness | 9 | 1 | 14 |
| Severity of acute illness | 8 | 1 | 36 |
| Presence of chronic illness | 6 | 1 | 21 |
| Severity of chronic illness | 5 | – | 14 |
| Functional status/quality of life measures | 14 | – | 25 |
| Nutritional status | 1 | – | 1 |
| Length of hospital stay | 1 | 1 | 5 |
| Trajectory of illness | 3 | – | 4 |
| Presence of DNACPR order | 3 | – | 6 |
| Non-medical | |||
| Age | 18 | – | 35 |
| Gender | 7 | 1 | 22 |
| Ethnicity | 4 | – | 5 |
| Patient preference | 3 | – | 15 |
| Family preference | 1 | – | 13 |
| Health insurance status | 2 | – | 4 |
| Clinician related | |||
| Seniority of ICU clinician | 2 | – | – |
| Seniority of referring clinician | – | – | – |
| Demography of ICU clinician | – | – | – |
| Physician’s attitude | – | – | – |
| Prognostic pessimism | 2 | – | – |
| Organisation related | |||
| ICU bed availability | 12 | – | 27 |
| Decision-maker present | 2 | ||
| Specialty of patient | 5 | – | 4 |
| Time of day | 2 | – | 5 |
| Experience/expertise of ward team | 1 | – | – |
| Hospital characteristics | 2 | – | 3 |
| Avoid conflict/litigation | – | – | 2 |
| Other | 3 | – | – |
Patient-related medical factors
Type of acute illness
Nine studies12,14,16,41,68–70,72,73 reported multivariate analyses of type of acute illness as a factor affecting decisions about patient admission to ICU. The types of acute illnesses considered between studies varied enormously and precluded meta-analyses. Distinct groupings were possible in the following categories: respiratory, cardiac/vascular, neurological, infections and emergency surgery. Three studies14,16,69 reported respiratory diagnoses as a factor associated with reduced likelihood of refusal or increased odds of admission, whereas one study70,71 found respiratory diagnoses to be associated with an increased odds of refusal. Cardiac or vascular diseases were reported in six studies,12,16,41,69,72,73 with increased odds of admission reported in four studies. 12,16,69,73 One study72 showed less likelihood of refusal to ICU with a diagnosis of cardiac failure than with a diagnosis of respiratory failure. In another study,41 cardiac disease was associated with refusal to admit. Four studies16,41,69–71 reported on neurological disease, with inconsistent results. Two of the studies16,69 showed that admission was more likely with a diagnosis of neurological disease, whereas the other two studies41,70,71 showed that the same diagnosis was associated with refusal to admit. Three studies68,69,72 reported that infections were an independent factor associated with ICU admission, and two studies12,72 reported independent effects of emergency surgery on admission decisions (see Appendix 4, Table 32).
Other diagnoses as independent predictors of admission decisions in multivariate models included shock and coma, which reduced the likelihood of refusal. 14 Haematologic etiology,68 injuries/poisonings/toxic effects of drugs,69 trauma and haematological malignancy12 and ‘worried’16 were all associated with increased likelihood of admission.
One study13 reported diagnosis to be a significant factor in multivariate analyses affecting refusal to admit to ICU, but it is unclear which individual diagnoses had the most impact. Another study20 reported univariate associations between categories of acute illness and odds of ICU refusal in an elderly cohort, but type of acute illness was not an independent predictor in the multivariate model. Fourteen further studies10,11,18,19,24,74–82 reported descriptive associations between types of acute illness and decisions about admission to ICU.
Severity of acute illness
Eight studies12,13,41,76,82–85 reported multivariate analyses of severity of acute illness as a factor. A number of different scales were used to assess severity of acute illness (see Appendix 4, Table 32). Results from individual studies were plotted, but the studies were not combined statistically because of considerable heterogeneity (I2 = 96%) and inconsistent direction of effect (Figure 3). There were no clear effects of severity of acute illness and decisions to admit patients to ICU (see Appendix 4, Table 32). It is possible that the heterogeneity and inconsistent direction of effect relates to questions around patients being too ill or too well to benefit from ICU care.
FIGURE 3.
Forest plot of studies reporting severity of acute illness scales in multivariate analyses. The pooled effect estimate is suppressed as heterogeneity is considerable (I2 = 97%) and the direction of effect is inconsistent between studies. a, APACHE II score; b, APS II score (Simplified Acute Physiology Score II without age, comorbidity and type of admission); c, MPM-0 (mortality predicted model at admission); d, All Patient Refined Diagnosis Related Group (APR DRG) grouper (measures chronic and acute disease severity); e, APACHE II (SE imputed); f, EWS; and g, severity of illness stage 2 (as defined by the authors): selective population – patients with AIDS with pneumonia. AIDS, acquired immunodeficiency syndrome; EWS, Early Warning Score; IV, instrumental variable; OR, odds ratio; SE, standard error.
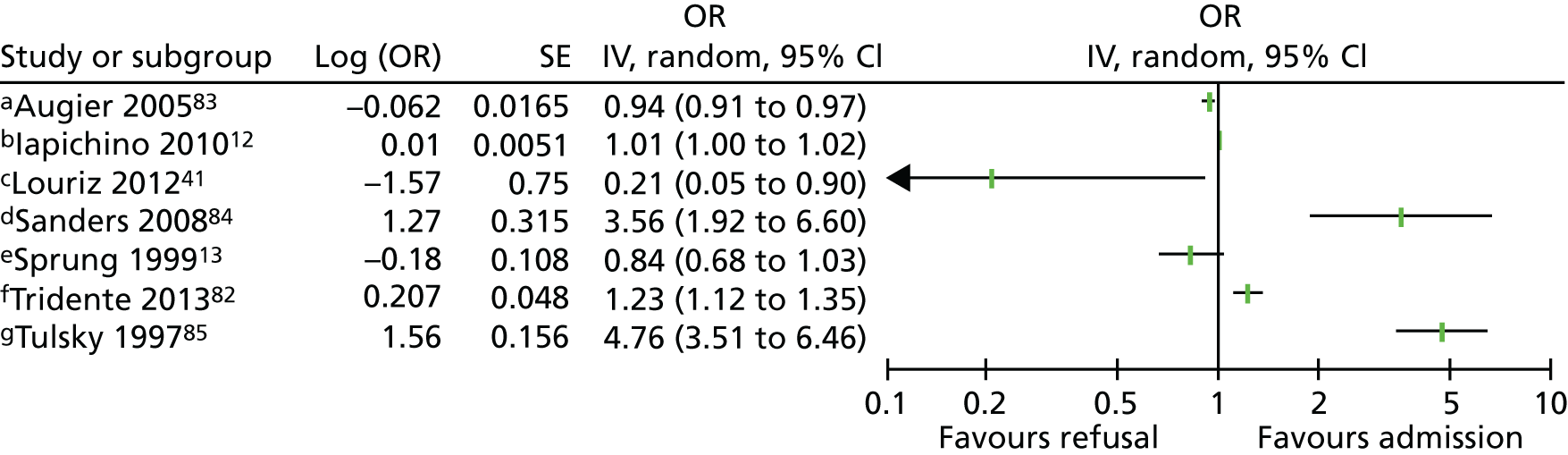
One further study20 reported a reduced odds of refusal of admission with increasing SOFA (Sequential Organ Failure Assessment) score [odds ratio (OR) 0.93, 95% confidence interval (CI) 0.87 to 1.0] in a univariate analysis, although this was not an independent predictor in the multivariate model. Thirty-six further studies10,19,21,22,24,42,44,72,73,75–77,80,81,86–107 reported descriptive associations between severity of acute illness and decisions about admission to the ICU.
Presence of chronic illness
Seven studies10,14,18,70,71,76,108 reported multivariate analyses of presence of chronic illness as a factor. Chronic illnesses investigated included dementia,108 metastatic cancer,10,18,76 mental disorder (psychotropic drug use),18 a combined category of chronic respiratory or heart failure or metastatic cancer without hope of remission,14 and a general category of ‘underlying chronic disease’70,71 (see Appendix 4, Table 32).
One study20 reported univariate associations between prior cognitive status and odds of ICU refusal, where refusal was associated with worse cognitive status, but this was not an independent predictor in the multivariate model. Twenty-one studies11,12,15,17,22,41,74,78,80,88–91,93,98,101,105,106,109–111 reported descriptive associations between the presence of chronic illness and decisions about admission to the ICU.
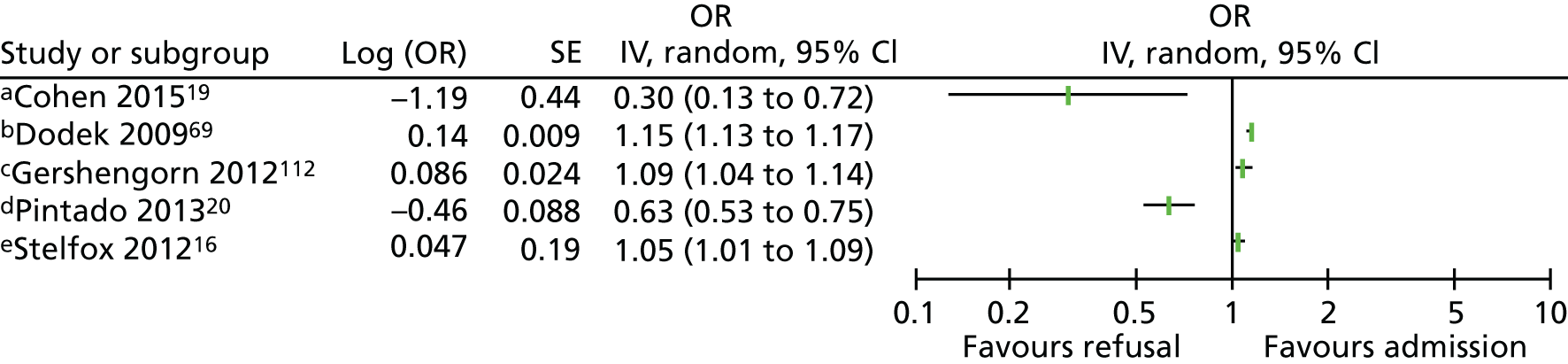
Severity of chronic illness
Five studies16,19,20,69,112 reported multivariate analyses for severity of chronic illness as a factor. Four studies16,20,69,112 assessed the severity of chronic illness using the Charlson Comorbidity Index. Results from individual studies have been plotted but not combined statistically because of considerable heterogeneity (I2 = 95%) and inconsistent direction of effect (Figure 4). Sensitivity analyses combining scales of the same type and removing selective populations had no effect on the very high level of heterogeneity. There are no clear effects from multivariate studies of severity of chronic illness and decisions to admit patients to the ICU. Fourteen further studies10,24,41,74,80,81,86,87,91,92,95,100,101,113 reported descriptive associations between the severity of chronic illness and decisions about admission to the ICU.
FIGURE 4.
Forest plot of studies reporting severity of chronic illness scales in multivariate analyses. The pooled effect estimate is suppressed as heterogeneity is considerable (I2 = 95%) and the direction of effect is inconsistent between studies. a, Elixhauser scale; b, Quan’s adaptation of Charlson Index; c, Charlson Comorbidity Index, selective population – diabetic ketoacidosis; d, Charlson Comorbidity Index, selective population – elderly cohort; and e, Charlson Comorbidity Index; IV, instrumental variable; OR, odds ratio; SE, standard error.
Functional status/quality of life
Fourteen studies10,12,14,15,17–21,68,70,76,82,114 reported multivariate analyses for functional status/quality of life as a factor using a number of different scales and measures, so we were unable to pool data statistically. Most studies reported on patient data from real-world settings but three reported simulation studies,17 surveys to health-care professionals using clinical vignettes114 or the theoretical future need of ICU in elderly patients admitted to emergency departments (EDs). 15
Measures of dependency/self-caring status related independently to admission decisions in the 11 studies10,12,14,18–21,68,70,76,82 in real-world settings; in most studies, increased dependency was associated with reduced odds of admission (see Appendix 4, Table 32).
Twenty-five further studies11,22,24,42,46,74,76,78,80,81,87-91,99–105,111,114,115 reported descriptive associations between functional status/quality of life and decisions about admission to the ICU.
Nutritional status
Only one study18 reported multivariate analyses of nutritional status on admission decisions. In this elderly cohort of patients aged > 80 years, nutritional status was independently associated with eligibility for ICU admission (see Appendix 4, Table 32). One descriptive study76 in patients aged > 80 years found no statistically significant differences in nutritional status between those referred and those not referred to ICU.
Pre-admission length of hospital stay
One study16 reported multivariate analyses for pre-admission length of hospital stay as a factor. One study84 reported univariate analyses where length of hospital stay of > 7 days increased the odds of ICU transfer compared with 1 or 2 days, but this was not an independent predictor in the multivariate model. Five additional descriptive studies12,18,50,68,110 reported previous length of hospital stay and admission decisions to the ICU with conflicting results.
Trajectory of illness
Three studies16,17,76 reported multivariate analyses of trajectory of illness as a factor. Previous hospitalisation in the past year was associated with reduced odds of admission to the ICU in one study,17 but hospitalisation in the past 6 months showed no difference in another. 76 Previous ICU admission during the hospitalisation was associated with increased odds of admission within 2 hours of medical emergency team (MET) activation16 (see Appendix 4, Table 32). Four further studies90,91,105,111 reported descriptive associations between trajectory of illness and ICU admission.
Presence of do-not-resuscitate order (variously described in studies as DNR/DNAR/DNACPR)
Three studies19,68,97 reported multivariate analyses of do not resuscitate orders and admission decisions to the ICU. All three studies showed that patients were less likely to be admitted with a do not resuscitate order.
Six descriptive studies78,89,92,98,116,117 reported associations between presence of do not resuscitate order and admission decisions. Three studies found that it resulted in significantly fewer ICU admissions. 89,116,117 In the others, do not attempt resuscitation status was seen as important98 or clinicians said that they would comply with a do not attempt resuscitation order. 78,92
Patient-related non-medical factors
Age
Eighteen studies12–18,20,21,69–73,76,84,97,106,112 examined age as a factor in multivariate analyses. Data on age were inconsistently reported. Six studies12,15,16,18,20,84 reported admission decisions per year increase in age, and data from these studies were pooled statistically (Figure 5). There was considerable heterogeneity (I2 = 99%), but magnitude and direction of effect between studies were similar, showing an increased odds of refusal to ICU with increasing age (OR 0.95, 95% CI 0.91 to 1.0; p = 0.05). Five studies12,16,18,20,84 were conducted in real-world settings and one15 was theoretical.
FIGURE 5.
Forest plot of studies reporting age in multivariate analyses, for each year increase in age and by age bands. IV, instrumental variable; OR, odds ratio; SE, standard error.
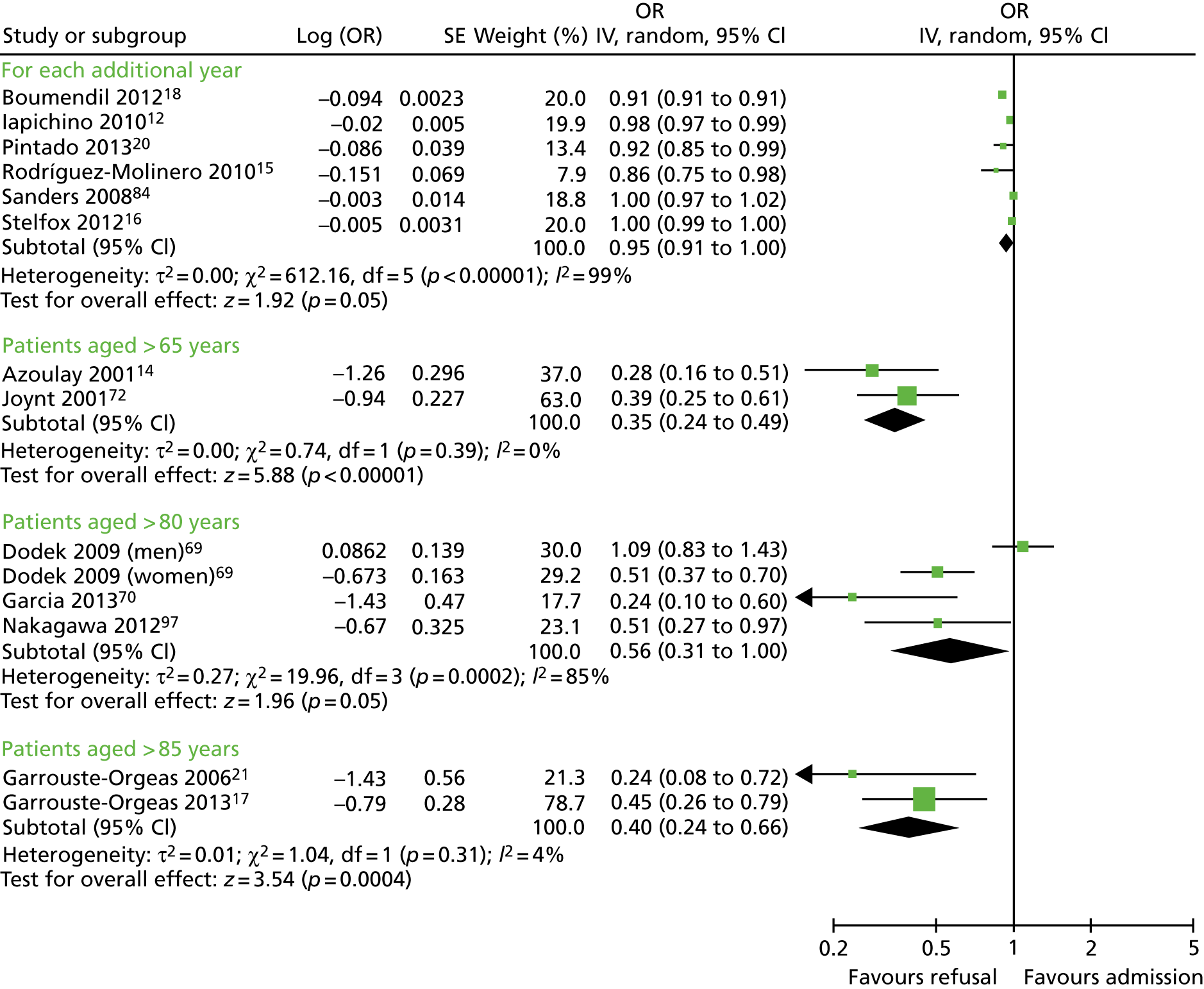
Other studies reported specifically on elderly cohorts or similar cut-off points and, where possible, we have pooled these statistically (see Figure 5). All multivariate analyses are summarised in Appendix 4, Table 32.
A further 35 studies (39 publications)10,11,19,22,24,41,68,74,75,77,78,80–83,87–91,93–95,99–101,105,107,109–111,114,118–124 looked at the association between age and admission to ICU in descriptive analyses.
Sex
Seven studies69,72,73,84,112,125,126 examined sex as a factor in multivariate analyses. Results were inconsistent between studies, with different age groups and ethnicity also playing a role.
Three studies73,84,112 reported no difference in admission decisions by sex when controlling for other covariates. One study72 found that being female reduced the odds of refusal to ICU compared with being male (the reference), but this did not reach statistical significance. Three studies69,125,126 found that men were more likely to be admitted than women (see Appendix 4, Table 32).
One study20 reported univariate associations between sex and ICU admission decisions and found no differences between men and women (OR 0.83, 95% CI 0.52 to 1.43). Twenty-two further studies10–13,18,19,21,24,41,50,68,70,74–76,79,81,89,94,106,110,127 reported descriptive associations between sex and decisions around admission to the ICU.
Ethnicity
Four studies73,85,112,125 reported multivariate analyses for ethnicity as a factor, with inconsistent findings. All studies were conducted in the USA.
Five additional studies11,68,75,109,110 reported the effects of ethnicity on admission decisions in descriptive analyses. Ethnic origin did not have an impact on admission decisions in these studies.
Patient preference
Three studies16,17,114 reported multivariate analyses of patient preferences and admission decisions to the ICU. Two studies16,17 of patient cohorts found increased odds of ICU admission when patient preferences for ICU care were considered (resuscitative vs. comfort care, and I accept vs. I refuse ICU care) (see Appendix 4, Table 32). A survey of health-care professionals,114 using a clinical vignette to determine decisions about admission to the last available ICU bed, found that the prior wishes of the patient were not an independent predictor for either physicians or nurses in predicting choice of patient for admission.
No further studies reported the effects of patient preference in univariate analyses, but 15 additional studies22,71,78,80,81,87,90,100,101,105,107,111,116,128,129 reported its effect on admission decisions in descriptive analyses, the majority being questionnaire studies of physician attitudes.
Family preference
One study114 reported multivariate analyses of family preferences and admission decisions to the ICU. This study was regarded as being of low methodological quality and did not show family considerations as an independent factor in affecting admission decisions in a clinical vignette study.
Thirteen additional studies38,46,71,78,80,81,91,96,100,101,105,130,131 reported the effects of family preference on admission decisions in descriptive analyses. The majority of these were questionnaire studies of physician attitudes and perceptions that variably reported the effect of family wishes on decision-making.
Health insurance
Two cohort studies73,112 reported multivariate analyses of health insurance and admission decisions to the ICU, one of which was in a population of patients with diabetic ketoacidosis. Both were US studies and explored differences between types of health insurance (see Appendix 4, Table 32).
Four additional studies22,68,91,98 reported the effects of health insurance on admission decisions in descriptive analyses.
Clinician-related factors
Seniority of intensive care unit clinician
Two studies16,68 reported multivariate analyses of clinician seniority and the effects on ICU admission decisions. One study16 found that attending physicians were more likely than more junior physicians to admit patients, whereas the other68 found that less experienced attending physicians (defined as those spending < 25% of their time in the ICU) were more likely to admit patients to ICU (see Appendix 4, Table 32).
Ten additional studies10,21,22,24,41,74,76,78,116,130 were found with descriptive analyses reporting variable results.
Seniority of referrer
No studies reported multivariate or univariate analyses of the effects of the seniority of the referrer on ICU admission decisions, but four descriptive studies11,120,132,133 were found reporting associations between referrer seniority and ICU admissions.
Personal characteristics/demography of intensive care unit clinician
No multivariate or univariate studies reported on the personal characteristics/demography of ICU clinicians and their association with ICU admission decisions, but two descriptive studies22,116 were found that were rated as being of low methodological quality. No consistent association was found with physician age, sex and whether or not they had children in responses to a number of clinical vignettes and importance of a number of criteria when considering admission. 22 Country of practice had an impact on decision-making, with Brazilian and US physicians choosing more aggressive treatment in response to clinical vignettes than Australian, Scottish and Welsh physicians. 116
Physician’s attitude
Three descriptive studies86,90,111 were found that were rated as being of low methodological quality. In one study,86 12% of physicians cited alcohol dependency as a ‘lifestyle decision’ when considering ICU admission. In the other studies,90,111 56% of Israeli physicians90 and 19% of US physicians111 thought that their personal attitude was important in deciding admission to the last ICU bed.
Prognostic pessimism
Two studies24,72 reported multivariate analyses of prognostic pessimism and admission decisions to the ICU. A high chance of mortality from the mortality prediction model was associated with increased odds of refusal to the ICU, as was physician-predicted risk of death of > 50% in the coming month. No further studies were found reporting on prognostic pessimism.
Organisational-related factors
Intensive care unit resource/bed availability
Twelve studies10,12,13,16,17,20,21,24,41,83,112,134,135 examined ICU bed availability as a factor. Results were reported variably by number of beds available and unit full/not full, so we were unable to pool data statistically. In eight studies,10,12,13,16,17,20,21,41 ICU bed availability was associated with likelihood of admission to ICU. Conversely, ICU occupancy levels had no significant effect or were only weakly predictive of likelihood of admission in three studies. 83,112,135,136 One study134 found that an increase in the use of mechanical ventilation in hospitalised nursing home residents with advanced dementia was associated with an increased number of ICU beds in a hospital (see Appendix 4, Table 32).
An additional 27 studies11,24,42,44,46,68,74,75,77,78,80,81,86–88,100,103,104,107,115,122,128–130,137–140 reported the effects of ICU bed availability on admission decisions in descriptive analyses.
Decision-maker present
Only two studies10,21 were found that reported circumstances in which an ICU clinician undertook triage, whether over the telephone or by examination. Both studies found that patients who were refused ICU admission were more likely to have been examined by an ICU clinician.
Specialty of patient
Five studies12,21,24,82,106 reported multivariate analyses of specialty of patient and admission decisions to the ICU. These found that patients referred from medical specialties were less likely to be admitted to ICU than those referred from surgical specialties.
Four further studies10,11,16,120 reporting descriptive analyses were found, reporting variable results.
Time of day
Two studies10,16,118,119 reported multivariate analyses of time of day and admission decisions to the ICU. These had conflicting results, with one10 reporting increased admissions during the day and the other16 reporting increased admissions at night (see Appendix 4, Table 32).
Five descriptive studies21,24,41,76,81 found no significant association between time of day and admission decisions, but one study120 found that patients referred out of hours were more likely to be admitted to the ICU (p = 0.005).
Experience and expertise of ward team
One study84 reporting multivariate analyses examined the effect of experience and expertise of the ward team on admission decisions. Registered nurse certification in a nursing specialty did not significantly predict ICU transfer after controlling for severity of illness (OR 0.43, 95% CI 0.1 to 1.73).
Hospital characteristics
Two studies85,112 reporting multivariate analyses examined the effect of hospital characteristics on admission decisions. The number of hospital beds, hospital percentage occupancy, ICU percentage occupancy, location (metropolitan, non-metropolitan) or teaching status of the hospital did not have an effect. 112 Among patients with AIDS (acquired immunodeficiency syndrome), admission was more likely in a Veterans Health Administration hospital than in a government hospital. 85
Three further descriptive studies73,113,131 found associations with characteristics of the hospital and decisions to admit to ICU.
Avoid conflict/litigation
Two descriptive studies38,78 were found that reported the effect of litigation on ICU admission decisions. In a survey38 of ICU clinicians in Milan exploring reasons for inappropriate admission, 5% cited threat of legal action as a reason. The other study78 found no association with admission to ICU.
Other
Three empirical studies12,97,112 reported multivariate analyses of other organisational factors not captured in the categories above (see Appendix 4, Table 32).
Experiences review
We identified 12 studies (14 publications), presented in Table 2. Eleven44,46,92,115,128,130,141–145 used qualitative and one100 used quantitative methods. Interviews were most commonly used for data collection. Two studies used questionnaires and four used multiple methods (including interviews, focus groups, observation, and analysis of documents). Five of the studies44,46,128,143,144 were carried out in North America and seven92,100,115,130,141,142,145 were carried out in Europe (including three92,141,142 in the UK). In general, the quality of the studies was mixed (see Appendix 2 for a review of quality assessment of studies). All but two of the qualitative studies were single-site or single-participant studies.
| Study (first author, year) | Objective of study | Data collection | Location | Participants | Number of participants | Limitations |
|---|---|---|---|---|---|---|
| Cullati, 2014130 | Importance of advanced care planning for seriously ill patients | Interviews | Switzerland | Health-care professionals | 24 | Single site; conference abstract and presentation |
| Hart, 2011128 | ICU clinicians’ reasons for bed rationing | Questionnaire (free text) | USA | Health-care professionals | 1086 | Abstract only; questionnaire |
| Santana Cabrera, 2008100 | Non-ICU doctors’ perceptions of ICU | Questionnaire | Spain | Health-care professionals | 116 | Single site; questionnaire |
| Hancock, 200792 | A CCOR nurse’s decision-making | Reflective piece | UK | Health-care professionals | 1 | One participant |
| Todres, 2000141 | Being a patient in ICU | Interview | UK | Patient (also an ICU nurse) | 1 | One participant |
| Oerlemans, 2015115 | Ethical dilemmas influencing admission and discharge | Interviews and focus groups | Netherlands | Health-care professionals | 44 | |
| Mielke, 200344 | Priority setting in ICU: evaluated in an ethical framework | Interviews, documents, observations | USA | Health-care professionals | 20 interviews | Single site |
| Fulbrook, 1999142 | Being a relative of a patient in ICU | Interview | UK | Relative (also an ICU nurse) | 1 | One participant |
| Cooper, 201346 | Scarcity in ICU context | Interviews | Canada | Health-care professionals | 22 | Single site |
| Martin, 2003143 | Neurosurgery patients’ access to ICU in an ethical framework | Interviews, documents, observations | Canada | Health-care professionals | 13 interviews | Single site |
| Danjoux Meth, 2009144 | Conflicts in the ICU | Interviews | Canada | Health-care professionals | 42 | |
| Charlesworth, 2017145 | ICU doctors’ decision-making practices in response to patient referrals | Ethnography (observations, interviews) | UK | Health-care professionals | 71 ICU reviews observed, 10 interviews | Single site |
Of the 12 studies, two looked at patients’ or relatives’ experiences (having only one participant each): Fulbrook et al. 142 and Todres et al. 141 The other 10 explored the experiences of health-care professionals, including ICU and general ward doctors and nurses, CCOR nurses, medical directors, respiratory technicians, hospital administrators, social workers and bioethicists. One study92 contained a detailed reflection of how a CCOR nurse made her clinical decisions based on one case, rather than a reflection on the wider process. We did not find any papers that focused solely on experiences of the process of referral and admission to ICU, but in these 12 we were able to identify data relevant to our research question.
Findings
Thematic analysis of the data from these studies identified three main themes: professional relationships, communication and working within external constraints. An overarching theme relevant to health-care professionals, patients and relatives was lack of agency or control.
Professional relationships
The existing and past relationships with clinical colleagues in the context of referring a patient to ICU had a substantial impact on how health-care professionals, specifically ICU doctors, referring doctors and ward nurses, experienced the decision-making process. Previous experience of having had a patient refused admission made some referrers less likely to refer, either because they assumed that the referral would be refused again or because the process had made them feel undervalued:100,115,130
And then you think, well as they are always aggressive, you are, you are a little afraid of calling them, yes. A dire consequence is you don’t dare ask for the consultation.
Medical doctor 6, Cullati et al. 130
One study115 reported that terms such as ‘arrogant’, ‘ivory tower’ and ‘island’ were used often to describe ICU consultants. Interprofessional relationships were often strained because of a lack of shared understanding about what ICU could achieve and what life was like caring for patients on a general ward. 44,115,144 This can lead to frustration and resentment. When professional relationships work well, the process runs smoothly. 44
Communication
Good communication between clinicians was seen as facilitating the referral and decision-making process, but poor communication was often described, leading to tensions between staff and harmful consequences for patients:
[You] go to see a patient and you don’t know what the therapeutic plan is, the patient is ill and we sometimes bring the patient down to the unit and then discover that actually the patient was for palliative care.
ICU team member, Cooper et al. 46
Concerns about communication were particularly noted in relation to patients and relatives. 44,46,115,141,142 ICU doctors commented that referring teams often avoided conversations about treatment goals and what transfer to ICU would mean for the patient, so the ICU team was put in the position of having to initiate those conversations.
However, ICU doctors also avoided conversations with relatives about the decision:
The ICU resident would have come down, done the consult and said to the ward team, ‘no.’ Or they may have said to the family, en passant, ‘Sorry, no,’ and then disappeared and then the family would have said, ‘Why?’
Nurse manager, Danjoux Meth et al. 144
By contrast, we found one study145 that reported a highly collaborative environment at their site, and this was seen as improving the experience of making decisions about ICU:
The other change that has come on in the last few years that is probably worthy of talking to you about is the collaborative way in which we make the decisions now . . . It is a supportive thing.
ICU consultant, Charlesworth et al. 145
Working within resource constraints
Several studies115,130,144,145 described the need for ICU and referring clinicians to negotiate limited availability of ICU beds, and the impact of this on both clinicians’ decision-making and patient care. External pressures included unrealistic expectations from the patient’s family,46,130 pressure from referring clinicians,46,143 and hospital policies on priority programme patients. 44 ICU doctors sometimes stretched ICU resources by reducing the nurse-to-patient ratio, creating conflict with nursing staff:144
We all knew that it [ICU treatment] wasn’t gonna make any difference . . . So it was hard for us to understand, given that our resources are very tight . . . why we were proceeding with the care of this patient.
Nurse manager, Danjoux Meth et al. 144
Lack of agency
An overarching theme of lack of agency was identified running through the studies. ICU doctors feel constrained by policies and pressure from other clinicians and families,44,46,143 referring teams feel that they are not listened to by ICU colleagues,100,115,130 and nurses are left caring for the critically ill, deteriorating patient with no power to challenge the doctor’s decision. 141,142,144 Patients and relatives are often excluded from the decision-making process. 44,141,142
The body of literature indicated a major gap in relation to patient and relative experiences, as these were included in only two studies, each of which contained the account of one participant. Furthermore, these participants were both ICU nurses by profession. The data in these two studies indicated that patients/relatives were not adequately involved in the process of referral and admission.
Summary
Our systematic reviews identified a large number of studies exploring a wide range of factors associated with decisions to admit a patient to ICU. There was marked heterogeneity among these studies, making it difficult to pool results, and many of the studies were of poor quality. The clearest associations identified were with age, sex, type of acute illness, presence of chronic illness, functional status, presence of do not attempt cardiopulmonary resuscitation (DNACPR) order, referring specialty, seniority of referrer and ICU bed availability. No clear association across studies was found with severity of acute or chronic illness. Few studies looked at the experience of stakeholders in the decision-making process and none had specifically explored this. Key themes identified in the data related to experience were communication, interprofessional relationships and perceived loss of control. One study we reviewed reported a positive, collaborative environment for ICU decision-making, in contrast to our overall findings. Very little is known about patients’ and families’ experience of this potentially life-changing decision.
Chapter 4 Understanding current practice
Introduction
This chapter reports a focused ethnographic study (observation and interviews) to explore current practice and inform subsequent WPs. We chose focused ethnography as this allows observation of what happens in a particular context, which for this study is hospitals with ICUs, and exploration of patterns of practice, ideas, beliefs, norms and values. 146,147
Our research questions were:
-
How are decisions about whether or not to admit a patient to an ICU made?
-
What are the experiences of patients, families and doctors involved in the decision-making process, and what are their views on how these decisions should be made?
-
What would constitute an ethically justifiable process for these decisions?
We first report the methods of data collection and analysis. We then report the analysis undertaken to inform the discrete choice experiment, followed by the analysis undertaken to inform the design of the DSF, accompanying training and professional support. We draw the analysis together at the end of the chapter to respond to our research questions.
Methods
In consultation with our PPI co-investigators and PPIAG, we designed detailed flow charts of participant recruitment and consent (see Report Supplementary Material 1 and 2).
We used a focused ethnography technique to observe and describe what happened before and after a decision was made whether or not to admit a patient to ICU. 148 We chose, as our main data collection, to observe decision-making events and interview doctors about these specific events as this keeps data collection grounded in what is actually happening and how doctors think at the time about decisions. The aim was to reduce social desirability bias of self-reported data from health professionals. 149 However, when asking about the ideal process, we wanted the doctors to think beyond the constraints of a specific case.
To gain understanding of the decision and what happened from the perspectives of those involved, we interviewed the observed intensive care doctors, patients or family members where possible, and referring doctors (see Report Supplementary Material 3–6 for interview schedules and observation template).
Six NHS hospitals across the East and West Midlands of England were sampled for diversity of type of hospital (university/district general) and ICU unit size (bed numbers: up to 20/21–40/> 40). The researcher, Mia Svantesson (a qualitative researcher with ethics training and ICU nursing experience in Sweden), undertook observation sessions over a 3-week period at each site. These were timetabled to include as many different ICU doctors as possible and to cover the whole 24-hour period and all days of the week. Mia Svantesson met the doctor to be shadowed at the start of the observation period and agreed a process that would not interfere with the clinician’s other work. Usually Mia Svantesson sat in or next to the ICU in a place where the doctor would remain aware of her, and when the doctor received a referral they signalled to Mia Svantesson to join them. The time spent waiting for referrals ranged from 8 to 16 hours. Mia Svantesson observed one to three decisions in each session, except for one session in which she observed five. The time spent with the doctor while the decision was made ranged from 10 minutes to 1.5 hours, except for one decision where the duration was 3.5 hours. Twelve of the 28 sessions were out of hours (between 17.00 and 24.00 or during a weekend).
Patients and family members seen by the doctor during observation were given the option of asking the researcher to leave the room. The limits we placed on observation minimised the burden on the clinical team but allowed sufficient time to include diverse participants and times of day/week, and to reach data saturation.
Interviews with the ICU and referring doctors occurred as soon as possible after the observed decision. Additionally, we interviewed specialist doctors who refer to ICU but did not refer during observation periods. For logistical reasons the interviews with these doctors were undertaken by Mia Svantesson and other team members, Frances Griffiths (a UK general practitioner and sociologist) and Anne Slowther (a UK general practitioner and ethicist). Patients with capacity at the time of their referral to ICU were given brief information about the study and asked if they agreed to a family member being interviewed. Before leaving hospital, surviving patients with capacity were asked for their consent to include data from any family interview already obtained, consent to approach a family member for an interview in 3 months and consent to be personally contacted 3 months later for an interview. Family members were approached by a member of the clinical team for interview within 48 hours of the observed decision. Early in the study, we realised that very few family members had been approached because of practical reasons, such as the family not being available, and because the clinical teams were concerned about causing distress. In consultation with our PPI advisors, and with ethics approval, the time window for approaching family members was extended to 72 hours in an attempt to increase recruitment. If the clinical team thought that an approach would cause distress, no approach was made. Patient and family member interviews were undertaken by Mia Svantesson. Patients and family members unable to engage in an interview in English were excluded.
Field notes were taken during observation about what was happening and Mia Svantesson’s impressions of whether or not a process was a good experience for patients and family members and for doctors, and what influenced this, including underlying values. We used a broad definition of values to include procedural values such as communication, time and mentoring, as well as ethical/moral values such as respect for persons, minimising harm, honesty and fairness. Mia Svantesson drew on ethical, professional and legal frameworks to inform her identification and exploration of ethical/moral values in the field. She checked her impressions through conversations with the observed doctors. The field notes were typed up and expanded immediately after each observation session. Doctors were asked during interview to describe their decision-making process related to an observed decision to admit or not to admit a patient to ICU (or their most recent decision if they had not been involved in an observed decision), including any communication with the patient/family, what they took into account in the process and how the process could be improved. The researcher explored what participants thought made a good decision-making process, and what hindered this. Patients/family members were asked during interview (initial interview and 3-month interview) to describe what they remembered of the situation they were in, the decision about admission to ICU, and their involvement, and whether or not they thought that the process could be improved. Interviews were audio-recorded, transcribed verbatim and anonymised. Observation and interview guides were refined based on analysis of initial data (see Report Supplementary Material 3–6). Data collection started in June 2015 and was complete by May 2016.
Data analysis was designed to inform subsequent WPs. Our project protocol specified analysis questions based on the needs of these WPs. We used these questions to guide our thematic analysis with constant comparison150,151 and adjusted the questions as the analysis proceeded. We present each analysis question as a section title in Results. The analysis was led by Mia Svantesson, with the rest of the WP research team (FG, AS, CB, JD, CW, AM and CB) and PPIAG members reading data and contributing to interpretation at a series of analysis meetings. All research team members and lay advisors read raw data from across the data set and provided an initial commentary on what in the data they thought was relevant to our analysis questions. Mia Svantesson then read each transcript line by line and labelled chunks of text that shared meaning with a code that was a short descriptive condensation. 152 Coding was facilitated by the software program NVivo 11 (QSR International, Warrington, UK). Codes were simultaneously grouped into content areas in order to manage the large number of data, and content areas were gradually abstracted into categories relevant to each analysis question. 152 Mia Svantesson undertook this process for all transcripts and Aimee McCreedy and Caroline Blake did so for samples of transcripts across the data set.
This process was reviewed by the wider research team during analysis meetings. Working analytical frameworks were created, responding to our analysis questions, that were shared during these analysis meetings. 153 As far as possible, data about a particular decision event were separated from data related to general reflections on ICU referral and decision-making. The latter were used only to understand what doctors considered the ideal process for ICU referral. We critically reviewed our emerging interpretation of the data by bringing our different disciplinary perspectives to the raw data and codes. Analysis was both descriptive, seeking to understand what was happening in relation to decisions to admit or not to admit patients to ICU, and analytical, understanding how and why the decision was experienced as it was, including the underlying values. In our thematic analysis, we use the judgements in the data as stated by the research participants about whether experiences were good or bad and impressions noted during fieldwork and checked with participants. However, we went further than this, using an empirical ethics approach. 154 The research team sought to identify from the data explicit and implicit ethical values and principles that were informing or framing the decision-making process, or participants’ views of what a good process should entail. We distinguished between ethical values (normative values or principles defining the obligations or duties inherent in both patient–doctor and interprofessional relationships) and instrumental values (what was important to facilitate enactment of the ethical values). Drawing on ethical theories and frameworks in relation to health-care decision-making and the results of our empirical analysis in an iterative process, we were able to provide a normative and procedural framework to inform the design of the DSF, accompanying training and patient and family support.
We present our results for each of our per-protocol analysis questions and provide illustrative data extracts labelled with the decision event to which the data relate and the role of the informant. When an extract is from field notes, [informant:] indicates that the text that follows is a verbatim quotation noted during observation. When no index event is included in the data extract label, the data are from an interview with a doctor who did not refer to ICU during the observation period at their hospital.
Results
Participants and decisions observed
In total, 55 decisions to admit or not to ICU were observed on wards or in EDs (Table 3) over 28 days, of which 6 were weekend days. In 18 of these decisions the referral was received after 17.00 and before 09.00 Monday to Friday, and in nine decisions the referral was received at the weekend (of which one was received after 17.00 and before 09.00). These decisions involved 46 patients (27 female; mean age 61 years; age range 19–94 years); nine of these patients had two decisions within 2 days. In these nine cases, the first decision was not to admit.
| Hospital size | Hospital department/unit | |||
|---|---|---|---|---|
| ED | Medical unita | Surgical unitb | Total N | |
| Small (n = 2) | 11 | 8 | 5 | 24 |
| Medium (n = 2) | 4 | 7 | 5 | 16 |
| Large (n = 2) | 5 | 5 | 5 | 15 |
| Total (N) | 20 | 20 | 15 | 55 |
In total, 42 different ICU doctors were observed, all of whom were interviewed. Thirty-one referring doctors were interviewed about referrals related to observed decision-making. Twenty-eight further specialist doctors who refer to ICU were interviewed (Table 4) (see Appendix 5, Table 33, for further information regarding interviews by site). The acute medical conditions of the patients, as initially reported, are summarised in Table 5.
| Specialty | Doctors involved in observed decision | Doctors not involved in observed decision | Total (n = 101) | ||
|---|---|---|---|---|---|
| Consultant | Registrar | Junior doctor | Consultant | ||
| Intensive care,a n | 23 | 19 | 0 | 1 | 42 |
| Medical specialties,b n | 7 | 8 | 6 | 15 | 36 |
| Surgical specialties,c n | 1 | 3 | 0 | 7 | 11 |
| Acute/emergency medicine,d n | 1 | 5 | 0 | 5 | 11 |
| Years’ experience in current specialty, mean (range) | 10.0 (0.5–22) | 4 (0.1–20) | 2 (0.2–4) | 10 (0.2–22) | 8 (0.1–22) |
| Years’ experience since graduation, mean (range) | 23 (10–36) | 10 (4–29) | 4 (1–12) | 22 (8–34) | 17 (1–41) |
| Acute medical condition reported initially | Number of patients | Admission/non-admission (n/n) |
|---|---|---|
| Respiratory failure | 17 | 7/10 |
| Cardiac arrest | 5 | 1/4 |
| Low blood pressure | 5 | 2/3 |
| Trauma | 3 | 1/2 |
| Bleeding | 3 | 2/1 |
| Pre–post-surgical problems | 3 | 1/2 |
| Pancreatitis | 3 | 0/3 |
| Renal failure | 2 | 1/1 |
| Intoxication | 2 | 1/1 |
| Seizures | 2 | 1/1 |
| Home ventilator, overnight admission | 1 | 1/0 |
From our observations, family members of 45 patients were identified as potentially eligible for interview. Seven were not asked for an interview as the clinical team thought that an approach would cause undue distress, and 13 were not available to ask or did not respond to the initial information. Fifteen declined to be interviewed. Therefore, 10 family members were interviewed soon after a decision had been made to admit or not to admit to ICU (two parents, four spouses/partners, five daughters or sons and one daughter-in-law).
We gained consent to approach 13 family members of patients for a late-stage interview approximately 3 months after the observed decision. Of these, four family members did not wish to be interviewed; contact was lost with one family; one patient was readmitted to ICU and the family were not re-contacted; and two patients, after regaining capacity, did not consent for their family to be contacted. One family member was not re-interviewed as their consent for further contact by the research team could not be verified from documentation. Four family members were interviewed about three patients. One family member was interviewed at the time of the decision and 3 months later.
Of the 45 patients for whom we have observation data, 11 agreed to interview 3 months after the decision, but only three were interviewed; the remainder declined or contact was lost. Of the 34 from whom we did not obtain consent for interview, 15 died in hospital, two did not regain capacity while in hospital so were not asked, four declined to participate and 13 were not invited to participate.
Analysis to inform the design of the choice experiment
When designing the choice experiment, we were specifically interested in factors that influenced the decision-making process. Our analysis questions were as follows.
What factors influence decision-making?
For many patients there were multiple factors at play, but when doctors mentioned several reasons both for and against admission, they rarely expressed them in terms of weighing them up. Sometimes there was a single factor that determined admission or non-admission. Each of the following was given as the single reason for admission:
-
severity of illness
-
patient being young and at risk of decompensating
-
uncertain diagnosis or lack of information
-
patient looking more ill than the physiological measures suggested
-
lack of patient safety on ward (e.g. insufficient staff to closely monitor patient).
Each of the following was given as the single reason for non-admission:
-
patient not being ill enough
-
patient having poor prior functional status
-
patient having severe premorbid illness
-
patient looking less ill than the physiological measures suggested
-
(in one case) the patient’s advance expressed wish not to be admitted to ICU.
Overall, the factors identified as influencing decisions about admission to ICU could be grouped into different types of information available to and interpreted by the ICU doctor. We explored these categories of information further in our analysis.
What types of information are used in ICU admission decisions and how are they valued by the decision-maker?
Clinical information
All doctors making a decision about ICU admission considered and valued clinical information about the patient: acute condition and its cause; comorbidities; duration of illness; current and previous treatment; previous health-care contacts, including hospital admissions; and physiological parameters. For some patients, particularly in the ED, information about their medical history was not available.
Overall look of the patient
Consistently throughout our observation data we found that ICU doctors placed particular value on the overall look of the patient:
I suspect he will be on a ventilator by the end of day, he looks very tired. If you look at him you’d put money on it being heart failure.
ICU registrar, field notes, index event 5, hospital 5
This was considered at least as important as physiological parameters:
I’d already formulated some thoughts in my head as to how she might look based on the numbers he [referring doctor] gave me. Actually she looked better than I thought she would.
ICU registrar, interview, index event 2, hospital 2
Functional status of patient
When assessing patients, ICU doctors almost always asked about functional status, usually in terms of how far the patient could walk:
What does he do for himself? How far can he walk?
Around the house.
Field notes, index event 6, hospital 1
One referring doctor referred to this assessment as the ICU doctor’s trademark, but referring doctors also took existing functional status into account:
. . . I mean there’s patients that I’ve seen that, that ordinarily could have benefitted if they were fitter and stronger to begin with but, I would normally work on identifying whether the patient is fit to go to intensive care or not before I would even think about whether they need it.
Referring doctor, hospital 2
Patient safety
Intensive care unit doctors took into careful account the safety on the wards of unwell patients or of those at risk of deteriorating:
There is not a huge number of staff, especially overnight and we were coming to the end of the day. Although it would be nice to say he’d be constantly encouraged to keep his oxygen on and constantly monitored overnight . . . on a busy ward overnight, with minimal nursing staff actually picking up any deterioration would be difficult.
ICU registrar, interview, index event 2, hospital 5
Number of available intensive care unit beds
Some ICU doctors recognised that the number of ICU beds available influenced their threshold for admission:
The number of beds and nurses that we’ve got on the unit . . . sets the tone for how you will view the next patient . . . (today’s patient) was borderline really so I felt that we should observe her on the ward first but when she deteriorated that tipped the balance, we took her . . . if we’d got more patients, more sicker patients I would have kept her on the ward a bit longer and tried a few other things.
ICU consultant, interview, index event 2, hospital 3
However, ICU doctors also talked about creating bed availability by discharging patients from ICU and using other beds for which there were high staff-to-patient ratios.
Views of patient or family members
Although ICU doctors discussed taking account of the views of the patient or family members as something they ideally would do, it was striking in the observation data how infrequently these views were sought. Patients were often in distress and family members were not always available. However, we also observed situations in which the patient/family was available and would have been able to give a view had they been asked.
When family members were consulted, they did not always know the details of the patient’s health and so had difficulty discussing treatment:
I think there’s quite a lot of grey areas with regards to the cancer . . . I think that’s partly down to mum not telling us the whole truth!
Daughter, interview, index event 3, hospital 1
Conversely, when the family knew the patient’s wishes, this helped the doctor to make a decision:
I was questioning [my decision] until the family said . . . he [patient] had specifically said, ‘Don’t ever let me go through this’ . . . It’s gold dust when people can actually tell you what they feel that the patient would want.
Referring medical registrar, interview, index event 1, hospital 1
Family members interviewed expressed frustration that they did not have an opportunity to speak to the clinical team. One family member who did speak to the clinical team commented that it was ‘just luck’ that she had happened to be there at the right time:
I don’t think he [doctor] planned to come during visiting hours, I think it was just luck.
Daughter, interview, index event 3, hospital 4
Age
All doctors, when presenting information about a patient to a colleague, would begin with the patient’s age. As this is the standard way to describe a patient, it was difficult to discern whether or not this was a factor influencing the decision. However, this referring doctor felt that he had to advocate hard for an older person to be admitted:
So if you had your 80 year old and you think ‘Oh they’re not going to take somebody whose 80 but they’re fighting fit apart from X’ then you ring up [ICU] and you say, ‘I’ve got this person they’re . . .’ and then you discuss it and that’s how you have to work with them. You put forward the case, they review them and then if the registrar says, ‘No’, then you ring the consultant and you put forward the case.
Referring doctor, hospital 6
Our analysis suggested that, in addition to clinical information, we should include the following in the choice experiment: the overall look of the patient, the patient’s functional status, patient safety, the number of available ICU beds, the views of the patient/family members, and the patient’s age. Analysis of data about the ideal pathway for ICU referral and decision-making (discussed later in this chapter) suggested that the length of experience as an ICU doctor influences a doctor’s approach to decision-making.
For the factors that influence decision-making, what is their range?
To undertake a choice experiment, each factor needs levels (or ranges) that are realistic and recognisable by choice experiment respondents. For most of the factors selected for the choice experiment (see Chapter 5), we drew on existing demographic and epidemiological data and clinical scoring systems. However, for two of the factors identified in our data, namely patient/family perspective and the gestalt or look of the patient, we used our analysis to provide levels for these factors in the choice experiment.
The range of patient/family perspectives
We have noted that information on patient wishes was often not available and that, even when patient/family views could have been elicited, the clinical team did not always do so.
When the decision was to admit a patient to ICU, our interviews with patients and family members suggest that they accepted the doctor’s judgement:
I was just happy to be guided by the professionals, I don’t think I was given any opportunity to make a decision myself . . . I was in and out of sleep and in pain.
Patient, interview, index event 3, hospital 4
I think he was making the decisions whether she needed to be on intensive care . . . or whether they could manage it in a ward that wasn’t quite so intensive . . . he just said she didn’t need to go on intensive care initially.
Daughter, interview, index event 3, hospital 1 (non-admission)
Patients or family members sometimes requested admission to ICU:
The ICU consultant kneels by her bed and talks slowly and repeats the information to the patient. He asks her whether she agrees with his decision, but she shakes her head and says she wants treatment.
Field notes, index event 2, hospital 6
Occasionally we observed family members being more demanding about ICU:
Son [interrupts]: ‘I am quite disappointed. There has been no X-ray, if I hadn’t been here . . . nothing at all would have been done, everything is going too slow. I want my mother to be admitted to ICU straight away’ . . . The referral surgeon explains reasons for conservative treatment.
Field notes, index case 5, hospital 4
Range of description of the look of the patient
Intensive care unit doctors described their subjective assessment of patients using a range of expressions, such as ‘holding their own’, tiring or deteriorating:
Just now she looks remarkably well.
He adds that she might need a HDU (high dependency unit) bed, but that she doesn’t need it tonight.
She is reasonably comfortable at the moment, but we don’t know which way it will go.
Field notes, index event 10, hospital 3
She looked unwell . . . she was breathing quickly and she was a bit sleepy which was concerning . . . her GCS [Glasgow Coma Scale] was normal . . . but she was clearly a bit tired . . . younger patients I find are more difficult to make decisions on because they are young, they have reserve and then they can suddenly fall off their perch.
ICU registrar, interview, index event 2, hospital 2
. . . deteriorating quite rapidly because of his problems with his spine and neurological problems . . . Then struggling with his breathing.
Referring senior house officer, interview, index event 3, hospital 6
Analysis to inform the design of the decision-support framework, accompanying training and patient and family support
Our analysis questions were designed to capture the whole process of referral and decision-making, including the difficulties encountered and the facilitators of a good decision-making process.
What is the variation in reasons for a referral to the intensive care unit team?
The design of the DSF and the accompanying training needed to take account of the different reasons for making referrals to the ICU. As expected, there were crisis situations in which a patient was suddenly deteriorating, and there were referrals when the ward or emergency teams recognised that a patient needed organ support or intensive monitoring. However, in many instances the ICU team was called for other reasons. These included:
-
help needed with a procedure – monitoring during endoscopy or ward team struggling to take blood gases
-
advice sought when doctors were unsure what was going on with the patient or wanted reassurance that they were doing the right thing:Surgical registrar, interview, index event 3, hospital 4
I asked the opinion from the ITU [intensive treatment unit] guy, the main issue was the breathing. I wanted to make sure that his respiratory function was well treated, that he didn’t need any more invasive treatment.
-
pre-emptive planning for a patient, or when the ward team wanted the ICU team to keep an eye on a patient when they knew this might not happen on the ward:Respiratory consultant, interview, index event 7, hospital 6
The reason for contacting ITU was to provide appropriate oversight . . . during the night . . . cover overnight is not great . . . one or two agency nurses possibly. There is no onsite kidney specialist overnight.
What is the variation in intensive care unit doctors’ approaches to the decision-making process?
We were concerned with understanding the consistency or not of admission decisions between ICU doctors. Both referring and ICU doctors noted that ICU doctors varied in their assessment of a patient’s need for ICU admission:
I accepted a patient for admission and at 5 o’clock I handed over this patient who was awaiting a scan . . . he [colleague] reviewed the patient and said admission was not needed . . . my junior registrar had to go and see the patient overnight . . . then admitted the patient . . . I think different people have different thresholds for admitting a patient . . .
ICU consultant (interview 1, non-admission), interview, index case 1, hospital 5
Intensive care unit doctors described differing approaches and attitudes to responding to referrals and requests for ICU admission among their colleagues. They often used the term ‘dove’ or ‘hawk’ to characterise themselves or other ICU consultants. Being a dove appeared to mean that, compared with hawks, they admitted patients more easily and were more responsive to needs other than admission:
So I was fairly clear that I didn’t think the patient would benefit from coming to intensive care but the patient still had needs and the patient in this case had end-of-life care needs that didn’t seem to be being addressed so I went down to the ward.
ICU consultant, interview, index event 3, hospital 5
Doctors who described themselves as doves viewed the decision to admit or not to ICU as subjective, and acknowledged that other ICU consultants might make a different decision for the same patient. Those who described themselves as hawks saw themselves as a gatekeeper to a limited resource. Both consultants and registrars were aware of the predisposition of their colleagues in this regard:
It sounds as though he needs to come to ICU.
We don’t take patients who might need ITU, we have only capacity for patients that do need a bed.
Field notes, index event 3, hospital 6
I thought his answer was heavily influenced by the fact that we were stuck for beds. But I think the particular consultant I was on with will say no very quickly.
ICU registrar, interview, index event 3, hospital 6
Previous experience influenced the approach taken by ICU doctors, as illustrated by the following example:
When I was a junior registrar . . . the [doctor] seeing the patient with me said to me, ‘This person is not for intensive care’, thinking that they were going to pass away. About 4 weeks later, the junior doctor who was on the medical ward where they were being treated bumped into me and said, ‘Oh remember that patient we saw a month ago? They’ve just been discharged home, absolutely fine!’ and I remember thinking . . . there is a lot more to it . . . I think I’ve become more willing to get patients into intensive care since then.
ICU consultant, interview, index event 4, hospital 1
These two approaches to decision-making may reflect the weight ICU doctors give to different ethical values. Being more inclined to admit a patient or to support those clinicians treating patients on the ward might suggest an emphasis on respect for patient autonomy, in addition to the professional value of collegiality, while being less inclined could be associated with giving more emphasis to being fair to all patients who may need ICU and feeling an obligation to use limited resources prudently. Both approaches may be underpinned by the duty to protect patients from harm. Inconsistency of decision-making based on the predisposition of the decision-maker or the relative importance (RI) given to implicit values is an ethical concern as it may lead to unfairness in patient access to ICU. To minimise this inconsistency, we would need to consider how our support intervention to improve ethical decision-making would encourage doctors to recognise the approach they take and to critically reflect on this.
What makes a good decision-making process, as experienced by doctors and patients/family members?
To understand what the decision-support, training package and patient/family support should aim to achieve, this analysis reports on what doctors and patients/family members experience as a good decision-making process within the current NHS context.
Experienced ICU doctors brought a sense of calm and reassurance to situations in which a patient was very unwell and the ward or ED team was struggling. This enabled everyone to work more effectively:
It’s a lot easier to do things in a calm environment and I think part of that comes from the intensivists themselves . . . they’re able to take charge of a situation with ease . . . even the most stressful situations.
Medical registrar, interview, index event 2, hospital 2
Intensive care unit doctors have more time for assessment than the doctors working on wards or in the ED. Referring and ICU doctors recognised this as important for a good decision-making process:
Resus was an exceptionally busy area . . . Their help with this, particularly to have more time to go through with the patient and their relative . . . the extra information allowed more . . . considered decision-making for this patient.
Referring emergency medicine registrar, interview, index event 3, hospital 1
Intensive care unit doctors valued comprehensive and trustworthy information about a patient. This came from the referring doctor’s knowledge of the patient and any knowledge from pre-alerts or from previous referrals or ICU admissions, as well as the ICU doctor’s own examination of the patient:
She [the patient] has been known to us for about 3 years with cancer. She’s been having chemotherapy and has been deteriorating slowly but is still completely fit and was going to work 2 or 3 weeks ago, and is keen to have treatment . . .
Oncologist consultant, interview, index event 2, hospital 6
Family members sometimes provided doctors with very helpful information, for example about how the patient had been a few days earlier, or information about a long-term condition. However, family members were often not approached or not available at the time an ICU doctor was making a decision.
Developing a holistic assessment of the patient was seen as important by some doctors:
So a global view for me isn’t just their acute medical problem. It’s finding out more about how the patient is in their usual life . . . So this man obviously has a family, he had a job . . . he’s got social obligations. He has a terminal illness but where is he in his journey towards the grave? The only question is the relative speed . . . Is this a new diagnosis of cancer and he hasn’t had time to adjust and tell people and make plans?
ICU consultant, interview, index event 3, hospital 5
Developing a holistic view involved building a relationship with the patient so that the patient felt empowered to share information and trust the ICU doctor to truly consider the best course of action for them. We observed examples of ICU doctors exploring patients’ concerns, providing reassurance, and seeking information from the patient and responding in turn to their questions.
Doctors talked about balancing benefits and burdens during decision-making:
It’s about balancing the burden that you’re going to impose on a patient for the benefit that they’re likely to get.
ICU consultant, interview, index case 2, hospital 2
However, the explicit balancing of factors for and against ICU admission was rarely observed.
Characteristics of ICU doctors that appeared to facilitate good decision-making were being approachable, being non-judgemental towards the referring team, being a good listener and mediator, having good analytical skills, being able to communicate and being prepared to justify their decisions:
He’s receptive to discussion and the concerns of referring clinician. He doesn’t accept every worry but he has a very receptive approach.
Respiratory consultant, interview, index event 7, hospital 6
Very helpful . . . he’s good at explaining why; his rationale . . . he’s also good at providing advice which is very helpful for us.
Junior medical referring doctor, interview, index event 4, hospital 1
Intensive care unit doctors also experienced good collaboration with the referring team:
They [referring team] were providing a good level of care . . . [They] help with patients stepping down which is not usually the case . . .
ICU consultant, interview, index event 7, hospital 3
Intensive care unit consultants often provided support to junior doctors. Throughout the hospitals, there seemed to be a tight web of close collaboration between the ICU registrar and the ICU consultants, with junior doctors feeling supported by their consultant colleagues:
I was very thankful for my consultant getting involved to a point where he was physically down in the emergency department. I certainly got the feeling from him that he was also of the opinion that if we don’t stop now the next few hours will be in vain.
ICU registrar, interview, index event 4, hospital 6
There seemed to be closer relationships between junior and senior ICU doctors than in other specialties. Many ICU consultants mentored referring junior doctors who felt abandoned by their consultants, particularly at night.
It was usually the ICU doctor who met with the family to explain a decision once it had been made. Family members appreciated receiving clear information and having the opportunity to ask questions:
He was brilliant, he was clear, very thorough, he repeated it and then he summarised it at the end. He was good because he gave all the information . . . He explained that my dad might have had a fall and that can cause some toxins in your body if you’ve been lying there for some time affecting his kidney. I was wondering why they were saying they were functioning so poorly . . . He explained that he didn’t think that he [patient] needed to go to intensive care and that he just needed lots and lots of fluids, that they would monitor him every 2 hours. It was nice to have some kind of time scale.
Daughter, interview, index event 6, hospital 3
This analysis includes good experiences that doctors and patients/family members have had of ICU admission decisions. These include experiences related to individual decision-making processes such as bringing calm to a difficult situation, taking a holistic approach and talking to the family. They also include experiences about how teams function and collaborate. The different aspects of a good admission decision-making process were not all present in any one decision.
Our proposed DSF, accompanying training and patient and family support needs to consider how to support clinicians to bring these good experiences to more decisions. For example, a decision-support could prompt doctors to explicitly balance reasons for and against admission and to ask families for their perspective. Family support could include explaining the decision-making process.
What contributes to the experience of a poor decision-making process?
Most of the observed decision-making processes were experienced as generally going well, although problems usually arose with some aspects.
Misunderstanding between the referring team and the ICU team about the reason for contacting the ICU team was observed. We give two examples in Table 6.
| Reason | |
|---|---|
|
The ICU doctor thinks referring doctor is demanding a specific treatment Referring doctor intends to alert ICU team that they might need help |
Index event 3, hospital 5The ICU registrar talks about a demand to give a general anaesthetic to a patient with a glioblastoma, having seizures, being aggressive and unmanageable on the ward [She frowns and does not seem content with the inquiry]. She says that this is not a good idea due to the cancerField notesHis exact words were ‘Can you come and give him a general anaesthetic?’ That was exactly the question I got . . . He’s probably on the ward with an aggressive patient. I think it’s sometimes difficult because it’s very easy to say well that’s a bit odd to admit, odd to requestICU registrarWe had to treat him. He was banging the doors, he was a risk to himself and he was a risk to other people. The nurses gave him some benzodiazepine. Then I thought, what will we do next [if] it doesn’t work for him? So rather than waiting 5 or 10 minutes and then asking the ITU to come – that might delay things – I thought just let them know that we might need helpMedical registrar |
|
The ICU doctor thinks referring doctor is seeking reassurance The referring doctor was keen on admission |
Index event 10, hospital 3They had a patient with pancreatitis, they hadn’t finished scoring her right at that point. They wanted us to review her because they were worried about her breathing potentially deteriorating . . . He was probably doing the right thing, referring the patient and he was probably reassured there was nothing too worrying going on, he wasn’t insisting on ITUICU registrarI wanted to have careful fluid management in this lady. I thought she would get more goal directed fluid therapy better in ICU than us giving fluids in the ward . . . I would prefer her to have been in ICU, but I understand the restrictions they have with beds. In ideal circumstances if there was an HDU bed I would have preferred for her to be more carefully monitored thereSurgical registrar |
Disagreements between intensive care unit doctors and referring team about whether or not a patient should be admitted
We observed instances of disagreement between doctors about whether a patient should receive treatment that would take them to ICU. In the ED these tensions were exacerbated by the urgency of the situation and the lack of information about the patient. In the following example, the ICU registrar was under pressure to intubate the patient but was unsure whether intubation was the right decision:
The ED team were pressing us to intubate her as well, it wasn’t something I wanted to do on my own . . . It’s very easy for someone to say, ‘Intubate, intubate!’ And I find often that it’s the other teams who are working with their decision, they seem to think intubation is the solution to everything and from experience it’s not!
ICU registrar, interview, index event 4, hospital 3
In the next example, also in the ED, it is again an ICU registrar feeling pressurised into providing support, this time for the cardiology team, while they undertake investigations. The ICU registrar was able to call his consultant for support:
And the fact that cardiology were coming to do an angio. I thought, we should be palliating this person . . . felt that I was being coerced into moving him there. They were escalating to cardiology and we were going to continue supporting and to admit him to ITU and I felt that that was inappropriate. So I was very thankful for my consultant getting involved to a point where he was physically down in the department.
ICU registrar, interview, index event 4, hospital 6
However, sometimes the referring doctor felt that their views about treatment were dismissed by the ICU doctors:
I thought the ICU registrar was a bit dismissive . . . they’d already made a decision that they were a cancer patient and that they weren’t going to be appropriate for critical care before they actually came and assessed her.
Referring oncologist consultant, interview, index event 2, hospital 6
The preceding examples support the view that consultant-to-consultant referrals might help to avoid misunderstanding and facilitate good decision-making. This is recommended in existing guidance for ICU referrals. The oncologist in the previous quotation expressed this view:
I should have just spoken to the consultant directly rather than speaking to one of the registrars. I quite often find when we speak to the juniors, as a consultant, they come along and they usually reject the patient. Whereas actually if you speak to the consultant you just get a different level of consultation.
Referring oncologist consultant, interview, index event 2, hospital 6
Chaotic environments make decision-making more difficult
Decision-making was difficult in chaotic situations, as the following quotations illustrate:
It was a bit frantic because she was wriggling around and thrashing, a lot of people around, everyone had different opinions, it was very difficult for everyone to get their opinion across rationally and it can seem sometimes the easy thing to do it can be to intubate but is it the right thing to do? . . . and I had lots of different people telling me . . .
ICU registrar, interview, index event 4, hospital 3
It’s very difficult to intubate a patient and ventilate and be thinking about the background at the same time.
ICU registrar, interview, index event 3, hospital 3
In one case, an ICU registrar said that his decision to admit had been influenced by his desire not to let the patient die in the environment of the resuscitation room.
Lack of information about the patient
Lack of information about the patient caused most frustration for ICU doctors trying to make a decision. In the resuscitation room of the ED, a lack of information about the patient was expected and accepted. For patients referred from the wards, ICU doctors often complained about a lack of up-to-date information, especially at night. Junior doctors might refer a patient without gathering all of the relevant information. ICU registrars often interpreted this as incompetence, which led them to mistrust any information that was provided. However, they also acknowledged that the referring junior doctors were under pressure with the volume of work:
They are overloaded with a lot of patients . . . they don’t have the time or they don’t have the resources . . . It was not clear who knew the patient best to give me a full handover.
ICU registrar, interview, index event 7, hospital 3
Lack of information about a patient sometimes influenced the decision whether or not to admit them:
Decisions are made later in the night by somebody who doesn’t know the patient and is put in the difficult position of taking this patient to ITU or not. Making the decision not to admit somebody to ITU at 11 o’clock at night, when you’ve never seen the patient before is much harder [than admitting].
ICU consultant, interview, index event 7, hospital 6
Poor communication with families
We observed poor communication with families, with doctors seeming impatient when asking them for information or their views.
Doctors found that family members had difficulty taking in how seriously ill their loved one was:
. . . she was called out of the blue to come into hospital quite quickly . . . she certainly did seem distracted, like all of a sudden she had to think about a lot more.
ICU registrar, interview, index event 3, hospital 6
Family members described difficulty taking in information, feeling blocked by being in shock, being tired or being upset:
I know he’s got a terminal illness . . . I just didn’t expect it . . . it was just what I heard [about do not resuscitate] . . . that’s the only bit I remember . . . I was tired . . . I was very upset. I probably didn’t grasp all of it, although it was explained well.
Partner, interview, index event 3, hospital 5
We identified problems throughout our observations, some of which are difficult to solve, for example a patient deteriorating rapidly in the ED with little information available about them and no family members able to provide it. Our proposed DSF, accompanying training and patient and family support is not going to contribute to solving this type of problem. However, these difficult situations are exacerbated by misunderstandings and poor communication between teams and with families, and lack of consultant support, especially at night. Our proposed training could therefore include training in communication including how to better articulate reasons for decisions. Appropriate consultant support for junior doctors is likely to reduce their anxiety and improve decision-making for patients.
What is considered an ideal process for decision-making?
In this section we report what doctors told us during interviews about what they thought would make an ideal process for decision-making. Data for this analysis come from both comments doctors made when discussing a referral and what they said when asked how the process of admitting patients to ICU could be improved. We mostly summarise what doctors said, limiting illustrative quotations to those relating to issues not already covered in this chapter.
Improved understanding of what is involved
Ideally, there would be understanding among the population generally about what ICU can achieve and what it cannot:
If the population understood that intensive care isn’t magic and that even if you survive intensive care you may not go back to your old self . . . there might not be so much of a push to refer for intensive care.
ICU consultant, interview, index event 2, hospital 6
Many ICU doctors said that they would like greater understanding among other clinical staff about what ICU can achieve and the burden of treatment:
Sometimes there is a misunderstanding about the rigours of intensive care, what it costs a patient to be put through a level 3 stay and the consequences of that for the patient and the family.
ICU consultant, interview, index event 2, hospital 2
A few referring doctors wanted more understanding among ICU doctors about which treatments can be delivered on wards:
In an ideal situation more could be offered outside ITU but we as physicians who don’t work in ITU are very well aware of the limitations of high-level care in any other part of the hospital.
Referring doctor, interview, index event 1, hospital 5
Several doctors said that decisions about care plans and treatment escalation should be made as soon as possible after admission.
Good communication between teams with relevant information
Both referring doctors and ICU doctors saw good communication between them as important:
As long as I can have a discussion with the intensivist on the whole I am pretty happy with the outcome.
Referring doctor, hospital 5
Consultant-to-consultant referral was considered ideal for cases that were not straightforward. However, doctors warned that insisting on this could delay referrals. They were also realistic about what was possible given the system in which they worked:
Very rarely do you have a consultant-to-consultant referral, particularly from the medical teams . . . it is impossible to achieve because of the way the physicians have their work organised.
ICU consultant, interview, index event 3, hospital 5
All doctors noted the importance of having information about the patient available as and when this was needed, but several acknowledged that good information recording did not always occur:
Sometimes they are supplying history that’s just not all the history. It’s not because they’re hiding facts, it’s only because they don’t have the time or they don’t have the resources.
ICU registrar, index event 7, hospital 3
Involvement of patients and their family
Most doctors thought that the ideal process would involve patients and their families and that the doctor would maintain an open mind about what the patient might want:
It’s a very interesting and when you start putting it to patients ‘Do you really want to come down to the intensive unit and have all these tubes and lines?’ And actually they generally don’t because if they’re dying, they know it. But you know sometimes they do [want admission] and sometimes I’ll be persuaded by a family [member]. You have to keep an open mind.
ICU consultant (second interview, admission), interview, index event 1, hospital 4
The patient’s own values regarding quality of life would be taken into account by the doctor making the decision, even when the doctor had assessed that patient as having poor functional status:
We can’t really say that [a patient’s] quality of life would be poor on the simple grounds that people put different value on what quality is. It’s very subjective . . . I had a patient a few months ago who said, ‘If I can walk from my bed to the end of the bed I would think I have a good quality of life’. And there’s another one who’d say, ‘If I can’t go swimming every day I don’t think there’s any reason [to live]’.
ICU registrar, interview, index event 1, hospital 1
Doctors wanted to provide their best possible prediction of outcome for patients and family members, yet they also recognised uncertainty:
What you’ve got to do is refine the probabilities to an extent that you feel you can make a decision but always acknowledge that there is a chance . . . that it’s wrong . . . I say to the family, ‘I think it is likely they’re going to die,’ . . . if they remarkably make a recovery . . . ’I’m very happy!’ That’s fine but I suspect I won’t be wrong.
ICU consultant, interview, index event 4, hospital 2
Several doctors commented on the importance of ensuring that the family receives a consistent message from the clinical team after the decision is made and that families should feel involved in the decision but not responsible for it:
We’ll know what the family’s wishes are but we won’t involve them in the decision-making until we’ve got a medical consensus. There’s no point saying to the family, ‘We would allow it but the neurologists don’t want us to, what do you want us to do?’ that doesn’t work. You’ve got to come to the point where you say, ‘We’re all of the opinion that we continue treatment for 24 hours longer, then review’ . . . it makes it more difficult if the family almost feel like they’ve got to decide.
ICU consultant, interview, index event 7, hospital 2
Holistic assessment of a patient’s needs and willingness to respond to a changing clicinal situation
Doctors commented that, ideally, each deteriorating patient would receive a joint assessment involving the ward and ICU teams before a crisis is reached, although they also reflected that cases are complex and a patient’s illness trajectory is uncertain. Doctors with more experience were more confident in their decision-making, considering the whole person and feeling more able to predict outcome while recognising the uncertainty:
The more experienced you get, the more you’ll tend to look at the whole picture so it’s not just a case of pneumonia . . . Greater experience allows you to predict with more confidence what the likely outcomes are likely to be but not always. So I often think the more I know the less I know because I don’t see the exceptions . . . Generally I think have a better ability to predict.
ICU consultant, interview, index event 2, hospital 1
The ideal process was seen as including a willingness and opportunity to review and change a decision, providing a ‘safety net’ for patients:
The most important thing with intensive care is to not go, ‘No we’re never going to take them’ because the situation changes . . . I’ve been wrong with my gut thinking that someone is on their way to getting better only to see them 24 hours later in a worse state.
ICU consultant, interview, index event 4, hospital 1
Doctors talked about ideally balancing the benefits and burdens of admission to ICU for each patient, including considering other treatment options such as palliative care, although, as we have noted, this explicit balancing was almost never observed during our field work.
Adequate resources, monitoring systems and support
All doctors recognised that ICU doctors described decision-making as easier when resources (staff, beds, equipment) are available and that this has an effect on patient safety. ICU doctors described that ideally ward teams would be willing and able to offer to help with step-down from ICU when the unit is full. Doctors recognised that the downside of the ideal situation of always having sufficient ICU beds is that some patients would be admitted who are borderline and may not need ICU treatment:
Patients are sometimes being picked up who may be wouldn’t have been brought up if the unit was full . . . people who are borderline, who could probably be OK on the ward.
ICU registrar, interview, index event 6, hospital 3
Some doctors suggested that a system to record referrals would improve patient care by facilitating appropriate review of patients who are not admitted:
There’s a ITU log book which works well . . . every patient we get called about goes into a log book so can refer back to that book . . . who’s been referred up and why . . . [What] I’ve offered them [on ward], I’ll review this patient tomorrow and Sunday if required.
ICU registrar, interview, index event 6, hospital 3
Doctors suggested that decisions should be made with another doctor, particularly when cases are borderline or more complex. They noted that collegiate decision-making reduced variation:
During the daytime there’s always two of us working together so we know how each other works and in the daytimes we discuss cases very freely. So I think there isn’t a huge amount of variation.
ICU consultant, interview, index event 2, hospital 1
However, they recognised the cost implications of this approach, particularly out of hours:
If we could have two people of appropriate seniority around at night it would be great but it’s far too expensive.
ICU consultant, interview, index event 2, hospital 6
Another suggestion for improving the consistency of decision-making was to hold regular consultant (or, indeed, wider ICU team) meetings to discuss cases and decisions:
All intensive care consultants meet once in a month . . . even though I have come from different hospitals my practice won’t be any different from the people here because we all sit every month and interact with each other, discuss cases . . . what’s right, what is wrong.
ICU consultant (interview 1, non-admission), interview, index event 1, hospital 5
Both consultants and registrars described the importance of consultants providing support for junior doctors during the decision-making process. This may include providing advice and support on the telephone, speaking directly to the other team’s consultant when there is a misunderstanding or disagreement, or stepping in to take over from their juniors:
If I don’t feel the junior doctor has got enough experience, or that they don’t quite grasp what it is that I am concerned about in the decision-making process, then I’ve got to come in and do it myself.
ICU consultant, interview, index event 4, hospital 5
Figure 6 illustrates the components of the ideal decision-making process as described in our data.
FIGURE 6.
The ideal decision-making process.
What is the range of values associated with the decision-making process?
The analysis reported so far describes the ICU decision-making process and identifies what makes a good, poor or ideal process. In this section we add to this analysis the explicit and implicit values informing and framing the decision-making process identified through our empirical ethics approach. Figure 7 summarises the current ICU admission decision-making process (central column), the values supporting a good decision-making process (left column) and the flaws contributing to a poor process (right column). We identify intrinsic moral values, such as respect, honesty and trust, and the instrumental values that enable the realisation of intrinsic moral values, such as time, analytical skills, communication and mentorship.
FIGURE 7.
The ICU admission decision-making process.
What would constitute an ethically justifiable process for intensive care unit admissions?
Our analysis so far shows that the decision-making process for referral and admission to ICU is complex, context dependent, and shaped by underpinning ethical/moral values. To develop a normative framework in which to conceptualise an ideal decision-making process, and to inform an intervention to support clinicians in achieving this, we considered two separate but interlinked processes. First, there is the decision about whether to refer or whether to admit an individual patient, and the cognitive process that a doctor should go through to make that decision. Second, there is the wider process from referral through decision-making to reviewing and communicating the decision as described in Figure 7. In identifying a normative framework for making the actual decision to refer or admit, we considered common medical ethical frameworks such as the Four Principles approach or the four quadrant approach. 155,156 The elements of these frameworks were identified in our thematic and ethical analysis of our data. However, we did not think that they captured the complexity of these decisions, the uncertainty of decision-making and the diversity of other values that fed into these decisions. We were also mindful that any framework we recommended should, as far as possible, fit easily within usual clinical reasoning frameworks to increase the likelihood that doctors would engage with it.
With regard to the wider process of referral and admission decision-making, we wanted to capture the key values of communication, shared understanding and ongoing review. The ethical framework of Accountability for Reasonableness157 appears to capture the key normative requirements identified from our analysis for a good decision-making process. This framework requires the following criteria to be met for an ethically justifiable decision-making process: decisions are based on reasons acceptable as relevant to all involved, should be transparent and open to review and should allow an opportunity for appeal. Our analysis identifies key elements of a good decision-making process that reflect these criteria, including an emphasis on holistic care (identifying and respecting patient and family views as well as those of other health-care professionals); the importance of communication and explicit ethical reasoning; and the need to reflect on personal biases, and allow opportunity for review in the light of changes in the situation. The criterion of review and opportunity for appeal is also noted to be important in our data. Below we set out the elements from our analysis that we consider necessary for an ethically justifiable decision-making process (Table 7), and the contextual and organisational requirements to support such a process. We describe how this normative framework informed the development of our intervention in Chapter 6.
| Elements of decision-making process | Values |
|---|---|
| Gathering information about the patient’s clinical condition and functional status from as many informants as possible | Holistic care |
| Identifying patient’s wishes, values and expectations as expressed by the patient themselves or interpreted by their family when possible | Person-centred care/respect for autonomy |
| Recording or signposting the gathered information that forms the basis for the decision to refer/admit or not to ICU so it is accessible to others providing care for the patient | Transparency |
Explicitly identifying the reasons for and against referral/admission to ICU, including:
|
Ethical judgement |
| Clear communication with colleagues, patients and family; actively listing to their contribution; expressing empathy for their situation; and communicating the decision, including any arrangements for review | Respect |
| Being aware of own biases based on personal values/previous experience | Honesty and integrity |
| Opportunity for respectful challenge to a decision | Fairness |
The context for such decisions should include:
-
understanding of what ICU can and cannot achieve both generally in society and specifically by all referring teams in the hospital
-
early decision-making about ceilings of care for patients
-
established referral pathway that recognises and accommodates the difference between referral during the working day and referral outside the working day and the working patterns of different clinical teams
-
working culture of honesty and respect between referring and ICU teams, taking account of their different pressures and limitations
-
working culture of mentorship for all junior staff so that they learn with support and test their decisions with more experienced staff
-
availability of colleagues to discuss decisions, particularly those that are borderline or complex
-
tracking of referrals and regular discussion of cases for ongoing learning.
The health-care provider organisation needs to recognise the tensions created for their clinical teams when:
-
resources are limited both for ward-based care for seriously ill patients and for ICU
-
the workload and work patterns of clinical teams reduce the availability of senior doctors for consultation by their junior staff.
Summary
Our analysis provides a structured account of what will be recognisable to ICU and referring doctors. Our account of the patient/family member experience may be familiar to those with this experience, although our low recruitment rate may mean that we have not captured the full diversity of their experience. This account of how ICU admission decisions are made provides a framework for developing an intervention to improve these decisions. The variation in how ICU doctors approach these decisions suggests that consistency is an issue that the intervention needs to both tackle and provide a means of evaluating.
Doctors and patients/family members had good experiences of the decision-making processes that we need to preserve. The data on good experiences provide insights into how and where an intervention could improve the process. When doctors obtained information about the patient’s wishes – whether from the patient or the family member – this was highly valued for informing the decision. However, family members, even when available, were not always asked for their perspective. Balancing the reasons for and against admission was considered a good thing to do but was rarely observed in practice.
Poor experiences of decision-making were often linked to the difficult situation in which the doctors were working, for example having little information available about a rapidly deteriorating patient. Poor communication exacerbated these problems, so any intervention would need to support communication between teams and with patients/family members.
From participating doctors we elicited a detailed description of the ideal decision-making process. An intervention targeted at doctors can tackle some aspects of the decision-making process where the experience falls short of the ideal, for example by encouraging balancing of reasons for and against admission, and talking to the family. Training and support could improve communication skills between teams and with patients and family and could improve understanding among clinicians of what can be achieved on ICU and on the wards. Other aspects of the process are beyond the reach of such an intervention, for example resource provision and public understanding of what ICU can achieve.
Through elucidating normative values during our analysis, we have been able to go beyond a description of the ICU admission decision to suggest what would constitute an ethically justifiable process for these decisions and the context that would support these decisions. This forms the foundation on which we can develop an intervention that aims to achieve a good process of ICU decision-making for as many patients as possible, while recognising the intrinsic difficulty of the decision itself.
Strengths and limitations
This project has several strengths. We had well-defined research questions and a field researcher familiar with the observation context, which can be an advantage for focused ethnography. 146 Coming from a different European country gave the researcher some degree of an outsider’s perspective considered important for more classical ethnography. 146 The field researcher’s expertise in ethics meant that she was able to draw on relevant frameworks while in the field to inform her conversations and interviews. Field work occurred in a range of sizes of NHS hospitals, and at different times of the day/week, providing a variety of contexts for the ICU admission decisions. Most NHS hospitals work within similar policy guidance and resource constraints, so our findings are likely to be transferable to most NHS hospitals that have an ICU. Most doctors involved in observed decisions were willing to be interviewed. Analysis was undertaken by a team with diverse academic, clinical and research backgrounds, who were able to challenge each other’s assumptions.
Although we achieved some patient/family interviews, we were disappointed not to achieve more, and this could be seen as a key limitation of the project. This was despite taking extensive advice from the PPIAG. Taking part in research in this context is extremely difficult for patients and families. The patient is usually too sick to engage in a discussion about research and their family is too worried about their loved one or too preoccupied with tasks associated with the crisis. More focus on training the clinical and nursing staff in how to support the families to participate in research at such a difficult time may have improved recruitment. 158 We attempted to recruit patients and families from post-ICU clinics to increase our number of participants, but this was also unsuccessful.
Doctors who did not refer during the observation period were interviewed by three different members of the team. This may mean that the data from these doctors were not collected as consistently as other data. However, the team members worked on the interview schedule development together and read each other’s interviews to minimise inconsistency. All members of the analysis team, and the researcher, had a clinical background, so we lacked the perspective that a non-clinical social scientist or ethicist would have brought to the data.
Chapter 5 Intensive care unit consultants’ and critical care outreach nurses’ preferences for intensive care unit admissions: a choice experiment
Introduction
Although our literature review and ethnographic study identified a range of factors that influence referral decisions, the extent of any influence and the priority given to different factors is not fully described. We used a choice experiment to investigate this issue. Our research questions were:
-
What is the influence of patients’ characteristics and ICU admission outcomes on ICU consultants’ and CCOR nurses’ decision-making?
-
What are the decision rules clinicians and nurses use to make their ICU decisions?
-
What is the variability of the decisions rules and preferences among clinicians and nurses?
-
How do patients’ concerns influence decisions about admissions to ICU?
The specific objectives of this WP were to:
-
examine consultants’ preferences for ICU admission (i.e. the importance that consultants attach to the patient-related factors when making an admission decision and how the probability of admitting a patient to ICU is influenced by changes in the patient-related factors) (addresses research questions 1 and 4)
-
examine the factors that influence CCOR nurses’ preferences for ICU admission (addresses research question 1)
-
explore heterogeneity in preferences among consultants and nurses (i.e. do consultants/nurses differ in their preferences for ICU admission and could eventual differences be explained by personal characteristics of the health professionals?) (addresses research questions 2 and 3)
-
compare preferences for ICU admission between CCOR nurses and consultants (i.e. do nurses give more or less importance to some patient-related factors than consultants?) (addresses research questions 2 and 3).
Methods
We obtained a quantitative assessment of both ICU consultants’ and CCOR nurses’ preferences for ICU admission using the choice experiment methodology. 159 This approach is frequently used in health economic and health services research to investigate patient, public and health professional preferences for a range of topics. 160,161 In designing and analysing our choice experiment, we used the International Society for Pharmacoeconomics and Outcomes Research guidelines. 162,163
A choice experiment is an attribute-based survey technique based on the assumption that the extent to which an individual values a health-care service (in our case ICU admission) depends on its features (in our case patient-related factors). The participants of a choice experiment are typically asked to choose from a limited number of choice options that systematically differ in their composition. Such choices require individuals to make trade-offs among the different features, and this information is then used to estimate the importance given to each feature.
Developing the choice experiment questionnaire can be summarised into three main steps:
-
identify relevant patients’ features for the ICU admission decision-making process
-
combine these patients’ features into hypothetical patients’ profiles and choice tasks
-
create the choice questionnaire.
Step 1: identifying patients’ features
A planned interim analysis of data from the initial systematic review (see Chapter 3) and data from two of the six hospitals in our ethnographic study (see Chapter 4) were used to identify relevant patient-related factors. The systematic review identified 88 studies that reported empirical research investigating the factors associated with decisions to admit or refuse admission to ICU. From these studies, we identified a list of factors to map to the list identified from the ethnographic study. We excluded factors that were not relevant to a UK NHS context (e.g. presence of health insurance). For the ethnographic study, we observed 15 ICU referrals and interviewed the consultants, as well as referring clinicians, about their decision-making. We interrogated the observation field notes and interview transcripts for data where we could discern an influence on the decision-making process. These data were coded descriptively and the codes were categorised into factors influencing decision-making. These factors were then mapped to the list of factors identified in the systematic review to check for congruence and any additional factors. The resulting factors were grouped into categories of similar meaning to achieve a workable list of potential choice experiment features. The qualitative data were checked to clarify meaning and to inform the decision to include or exclude an identified factor. For example, the data suggested that ‘communication with colleagues’ was mainly about process and did not influence the decision itself, so this factor was excluded. All of the factors in the final list included in the choice experiment were patient-related and covered the key factor categories from both the systematic review and the ethnographic data. Clinician- and organisation-related factors were reflected in the additional data collected in the choice questionnaire.
Family’s views about patient admission did not appear as an important factor in the systematic review. However, the ethnographic study showed that this was a relevant factor in understanding consultants’ admission decision-making. Moreover, one objective of this study was to determine whether or not patients’ preferences for ICU admission (as approximated by family’s views) significantly influence decision-making.
The next step was to identify levels for each attribute. We divided the attributes into those that related to established clinical guidance or scores such as the National Early Warning Score (NEWS), and those for which we needed to draw on the qualitative data for levels. For the latter, we reinterrogated the data to ensure that we covered the breadth of levels of each attribute. Attribute ‘levels’ were selected to reflect the range of observed clinical situations in our qualitative study. Levels of comorbidities were selected to reflect roughly comparable stages of disease: peridiagnosis, established disease and advanced disease with limited survival. The attributes and levels are presented in Table 8.
| Factor | Descriptor of level | Short forma |
|---|---|---|
| Age | 89 years | 89 |
| 79 years | 79 | |
| 66 years | 65 | |
| 39 years | 39 | |
| Type of main comorbidity | Prostate cancer | PCa |
| Heart failure | Heart failure | |
| COPD | COPD | |
| Dementia | Dementia | |
| Severity of main comorbidity | For ischaemic heart disease: echo shows severe LV impairment; numerous long hospital admissions; biventricular pacemaker and on spironolactone and furosemide twice per day | Severe |
| For chronic obstructive pulmonary disease: FEV1 of 28% predicted; two hospital admissions for exacerbations in the last year | ||
| For prostate cancer: a recent CT scan revealed bone metastases | ||
| For dementia: forgets many recent conversations and needs some help washing and dressing; family say they remain contented | ||
| For ischaemic heart disease: moderate heart failure on echo; on regular furosemide and ramipril | Moderate | |
| For chronic obstructive pulmonary disease: FEV1 of 45% predicted; three courses of steroids and antibiotics over the past 12 months | ||
| For prostate cancer: local spread on recent staging CT; on hormonal therapy with planned radiotherapy | ||
| For dementia: started on Aricept in the last month | ||
| For ischaemic heart disease: previous MI; recent echo shows LVH and a mildly decreased ejection fraction; on ramipril | Mild | |
| For chronic obstructive pulmonary disease: FEV1 of 65% predicted; one course of steroids and antibiotics in the past year | ||
| For prostate cancer: on hormonal therapy | ||
| For dementia: recently referred by GP to memory clinic for suspected diagnosis of dementia; otherwise well | ||
| Functional status | Mobilises around the ground floor of their home; cannot manage stairs; has carers twice a day | Bad |
| Mobile to shops with family; has to rest climbing stairs | Intermediate | |
| Mobilises independently; walks dog daily | Good | |
| Severity of acute illness | NEWS of 11 | 11 |
| NEWS of 8 | 8 | |
| NEWS of 5 | 5 | |
| ‘Look of patient’ as reported by registrar | Registrar saw the patient earlier and says that they look dreadful now | Bad |
| Registrar saw the patient earlier and tells you that they look like they are tiring | Intermediate | |
| Registrar has seen the patient and tells you that they are stable and ‘holding their own’ | Good | |
| Safety (capacity) on referring ward | Patient is on a busy acute ward with one trained nurse per eight patients; the ward sister is worried the ward cannot cope with looking after the patient | Bad |
| Patient is on a busy acute ward with one trained nurse per four patients; CCOR nurses are available to provide further support | Good | |
| Family’s views | The patient’s family say that they think the patient would not want to be admitted to ICU | No |
| The patient’s family say they have never discussed ICU admission or end-of-life care: they will leave all the decisions to the medical team | Unsure | |
| The patient’s family have already approached the ward doctors and said that they insist on the patient being admitted to ICU | Yes |
Step 2: designing patients’ profiles
The eight patients’ features were experimentally combined to form choice tasks following a multistage strategy. 162 First, we created a draft version of the choice experiment questionnaire based on a D-efficient design optimised for a multinomial logit (MNL) model with null priors (i.e. null a priori information about respondents’ preferences) and sent it to 30 ICU consultants. This quantitative piloting of the questionnaire fulfilled two objectives: to further improve the quality of the questionnaire by identifying wording issues and to obtain a set of preliminary results regarding consultants’ preferences for patients’ admission. We used this knowledge of consultants’ preferences to update the initial design of the experiment. This increased the statistical efficiency of the choice experiment, allowing for a more precise measurement of respondents’ preferences.
As we were interested in measuring the main effects of changes in the eight patients’ features (e.g. increase in patient’s age) on admission decisions, and also potential interaction effects between the type and severity of main comorbidity, it was necessary to include a minimum of 24 choice tasks in the questionnaire. 165 Each choice task includes two hypothetical patient profiles (Figure 8). Participants were asked to answer three choice questions: (1) would you admit patient A? (yes/no); (2) would you admit patient B? (yes/no); and (3) which patient should be admitted in priority? (A/B).
FIGURE 8.
Illustration of the choice task format. Reproduced from Bassford et al. 164 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
To reduce cognitive burden, the 24 choice tasks were divided into two blocks of 12 tasks, leading to two versions of the questionnaire. Participants were randomly allocated between these two versions. In addition to the 12 experimental tasks, we manually added a warm-up task (task #1) to familiarise the participants with the format of the choice tasks, and two data quality checks (a repeat of the warm-up task (#14) to test the stability of participants’ preferences for admission and a logical task (task #15) to check participants’ engagement). (More information about the data quality checks can be found in Appendix 6.) The order of the experimental tasks was randomised across participants to minimise the ordering effects that may result from learning/fatigue.
Step 3: creating the choice questionnaire
In addition to the choice tasks, the questionnaire included an information section that carefully explained the context of the study. We also collected information on respondents’ sociodemographic characteristics (e.g. age, sex) to ensure the generalisability of our results. We worked with a UK-based research company (Clinvivo Ltd, Tenterden, UK) to develop an electronic version of the questionnaire. The questionnaire was scripted such that participants could respond on either a standard or a tablet PC. The questionnaires can be found in Report Supplementary Material 7–10.
Sampling and recruitment strategy
Following a sample size computation for choice proportions,166 we needed to recruit a minimum of 130 ICU consultants and 130 CCOR nurses. For this sample size, it is possible to estimate a 33% choice probability with 90% accuracy and 95% confidence. However, we aimed to recruit more participants than the minimum needed to compensate for potential data quality issues (e.g. the exclusion of participants who do not fully engage with the experiment), and to explore preferences heterogeneity. Therefore, we aimed for 300 consultants (303 were obtained). Owing to the smaller overall number of CCOR nurses nationally, we could not increase the sample size to the same extent, so we aimed for 200 respondents (189 were obtained).
Intensive care units in NHS hospitals were invited to participate through regional clinical research networks (see Report Supplementary Material 11 for a list of participating hospitals). The local research nurses distributed an invitation to participate with a link to the choice experiment to ICU consultants and CCOR nurses in each of the 47 participating hospitals. In addition, an invitation to participate was e-mailed to all consultant members of the UK intensive care society and all members of the National Outreach Forum (for CCOR nurses).
Results: consultants’ preferences for intensive care unit admission
Sample of intensive care unit consultants
The personal characteristics of the ICU consultants are shown in Table 9. The sample mainly included male consultants (79.5%) who did not work for a university hospital (63.7%); 21.1% were aged < 40 years and 28.1% were aged > 50 years. The majority (76.9%) had been working in ICU for > 10 years.
| Characteristic | Consultants (%) (n = 303) | Nurses (%) (n = 189) |
|---|---|---|
| Sex | ||
| Male | 79.5 | 15.9 |
| Female | 20.5 | 84.1 |
| University hospital | ||
| Yes | 63.7 | 59.8 |
| No | 36.3 | 40.2 |
| Number of ICU beds | ||
| < 11 | 26.4 | 24.3 |
| 11–19 | 48.5 | 44.4 |
| > 19 | 25.1 | 31.2 |
| Years working in ICU | ||
| < 5 | 4.3 | 40.7 |
| 5–9 | 18.8 | 26.5 |
| 10–14 | 22.8 | 18.5 |
| 15–19 | 22.4 | 9.5 |
| > 19 | 28.1 | 27 |
| Age (years) | ||
| < 40 | 21.1 | 32.8 |
| 40–49 | 50.8 | 40.2 |
| > 49 | 28.1 | 27 |
Analysis of data quality
The results are reported in Table 10. The quality of responses was high, with 73.6% of participants meeting all four quality measures. No participants failed more than two tests; thus, none was removed from subsequent analyses. The criteria for assessing quality are reported in Appendix 6.
| Criterion | Consultants (%) (n = 303) | Nurses (%) (n = 189) |
|---|---|---|
| Logical consistency | ||
| Fail | 0.7 | 1.6 |
| Pass | 99.3 | 98.4 |
| Choice stability | ||
| Fail | 0 | 1 |
| Pass | 100 | 99 |
| Choice desirability | ||
| Fail | 0 | 1 |
| Pass | 100 | 99 |
| Response time | ||
| Fail | 14.2 | 9.5 |
| Pass | 85.8 | 90.5 |
| Summary: quality score (number of fail) | ||
| 0 | 73.6 | 71.4 |
| 1 | 26.1 | 24.9 |
| 2 | 0.3 | 3.7 |
| > 3 | 0 | 0 |
Preferences for intensive care unit admission
We modelled consultants’ preferences for ICU admission within the random utility maximisation framework. 167
From the responses, it is possible to derive a full rank ordering of the choice options (e.g. if patients A and B should be admitted but A is prioritised over B, then it is possible to infer that patient A > patient B > no admission). From this implicit ranking, it is possible to analyse consultants’ decisions as if they were made based on three options (i.e. which option do you prefer of admit patient A, admit patient B, and admit neither of them?). To take advantage of this data structure, we jointly analyse the probability of being first ranked (i.e. the MOST preferred choice option) and last ranked (i.e. the LEAST preferred choice option). This approach allows for a more accurate estimate of respondents’ preferences because (1) a choice from three options generated more information about preferences than a choice between two options, and (2) jointly modelling the MOST and LEAST preferred choices generated more observations about respondents’ choice behaviour than MOST preferred choices alone. This structure of the data is inspired by the best–worst scaling literature. 168
For each participant (n), at choice task (t), the probability of the option (j) to be first ranked is noted PntjMOST and its probability of being last ranked is noted PntjLEAST. These probabilities depend on the subjective values of the choice options (Untj), determined by a linear additive combination (Vntj) of patients’ features (Xntjk) with consultants’ preferences (βnk) and an error term (εntj) that is typically assumed to be identically and independently distributed as type I extreme value, leading to a MNL specification:
The estimated model parameters (βnk) capture the effect of a marginal change in patients’ features (e.g. ‘being 66 years old’ vs. ‘being 39 years old’). We report the simulated maximum likelihood estimates of these (βnk) preference parameters. To account for the panel nature of the data (i.e. multiple choices per participant), we include an individual error term (ωn) that is assumed to be Normal (0;σω). Because of this error term, the model log-likelihood needs to be simulated. We used 1000 Halton draws in addition to 200 draws as a ‘burn-in’ period and tried different sets of starting values to increase the chance of finding a global solution. To ease the interpretation of the results, we also computed ORs and scores of RI. 169
The results are reported in Table 11. All eight patient features have a significant effect on consultants’ decisions. All three age-related effects are significant and positive, meaning that younger patients are more likely to be admitted to ICU. Patients more likely to be admitted by consultants include those with prostate cancer, mild comorbidity severity, good functional status (mobility), a more severe acute condition (higher NEWS score), those who are not safe in their current (non-ICU) ward, those who receive a negative subjective assessment from the registrar and those for whom family insist on admission are more likely to be admitted by consultants.
| Factor | MLE (SE) | RI (%) | OR (95% CI) |
|---|---|---|---|
| Constant (no admission) | 3.671 (0.131)*** | – | – |
| Age (reference: 89 years) | |||
| 39 years | 2.488 (0.074)*** | 23.9 | 12.04 (10.42 to 13.91) |
| 66 years | 1.609 (0.063)*** | 5 (4.42 to 5.65) | |
| 79 years | 0.934 (0.066)*** | 2.55 (2.24 to 2.9) | |
| Comorbidity type (reference: prostate cancer) | |||
| COPD | –0.04 (0.06) | 3.8 | 0.96 (0.85 to 1.08) |
| Dementia | –0.391 (0.06)*** | 0.68 (0.6 to 0.76) | |
| Heart failure | –0.292 (0.069)*** | 0.75 (0.65 to 0.86) | |
| Comorbidity severity (reference: severe) | |||
| Mild | 1.859 (0.063)*** | 17.9 | 6.42 (5.67 to 7.26) |
| Moderate | 1.406 (0.062)*** | 4.08 (3.61 to 4.61) | |
| Functional status (reference: bad) | |||
| Good | 1.489 (0.054)*** | 14.3 | 4.43 (3.99 to 4.92) |
| Intermediate | 0.978 (0.056)*** | 2.66 (2.38 to 2.97) | |
| NEWS (reference: score = 5) | |||
| 11 | 0.784 (0.058)*** | 7.5 | 2.19 (1.96 to 2.45) |
| 8 | 0.12 (0.053)** | 1.13 (1.02 to 1.25) | |
| Look (reference: good) | |||
| Bad | 1.055 (0.056)*** | 10.2 | 2.87 (2.57 to 3.21) |
| Intermediate | 0.752 (0.06)*** | 2.12 (1.89 to 2.39) | |
| Safety (reference: good) | |||
| Bad | 0.26 (0.041)*** | 2.5 | 1.3 (1.2 to 1.41) |
| Family views (reference: unsure) | |||
| No | –1.791 (0.061)*** | 19.9 | 0.17 (0.15 to 0.19) |
| Yes | 0.277 (0.051)*** | 1.32 (1.19 to 1.46) | |
| SD individual errors | 0.962 (0.054)*** | – | – |
Patients’ age has the largest influence on consultants’ decisions (RI 23.9%), with 39-year-old patients 12 times more likely and 66-year-old patients five times more likely to be admitted than 89-year-old patients (the reference group). This is followed by family views (RI 19.9%). When the family is against admission (‘no’), the patient is six times less likely to be admitted than when the family is unsure about admission. The third most important effect is severity of comorbidity (RI 17.9%). Patients with mild comorbidity are 6.4 times more likely to be admitted than those with severe comorbidity. Least important are type of main comorbidity (RI 3.8%), patient’s safety in non-ICU ward (RI 2.5%) and the severity of acute condition/NEWS (RI 7.5%). Patients with COPD, heart failure and dementia are 1.04, 1.34 and 1.48 times less likely to be admitted, respectively, than patients with prostate cancer.
Preferences heterogeneity among intensive care unit consultants
We estimated a latent class logit (LCL) model to investigate preference heterogeneity. 170,171 This model allows the identification of different segments (or classes) of consultants who differ in their preferences for ICU admission. These classes can be thought of as different preference patterns adopted by consultants to decide whether or not patients should be admitted. The LCL model is a popular approach in the choice experiment literature to investigate preferences heterogeneity. Following the literature, we estimate several LCL models with an increasing number of preferences patterns (or latent classes) and retain the solution that optimises a measure of statistical performance known as Bayesian information criteria.
A solution with four preferences patterns provided the best model fit (see Appendix 8). The results for these patterns are presented in Figure 9. In all four patterns, consultants are more likely to admit younger patients, patients with a good functional status, patients whom the registrar reports as ‘tiring’, patients with a less severe comorbidity and patients whose family is not opposed to their admission. All four patterns appear to give little consideration to the level of resource in the non-ICU ward (safety factor) and to the type of comorbidity. However, the four patterns differ in the importance given to the different patient-related factors. A perfectly balanced decision-making process, giving equal weight to all eight patient factors, would be associated with RI scores of 12.5% for each factor (i.e. 100%/8 factors = 12.5%).
FIGURE 9.
Preference heterogeneity among ICU consultants. Reproduced from Bassford et al. 164 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
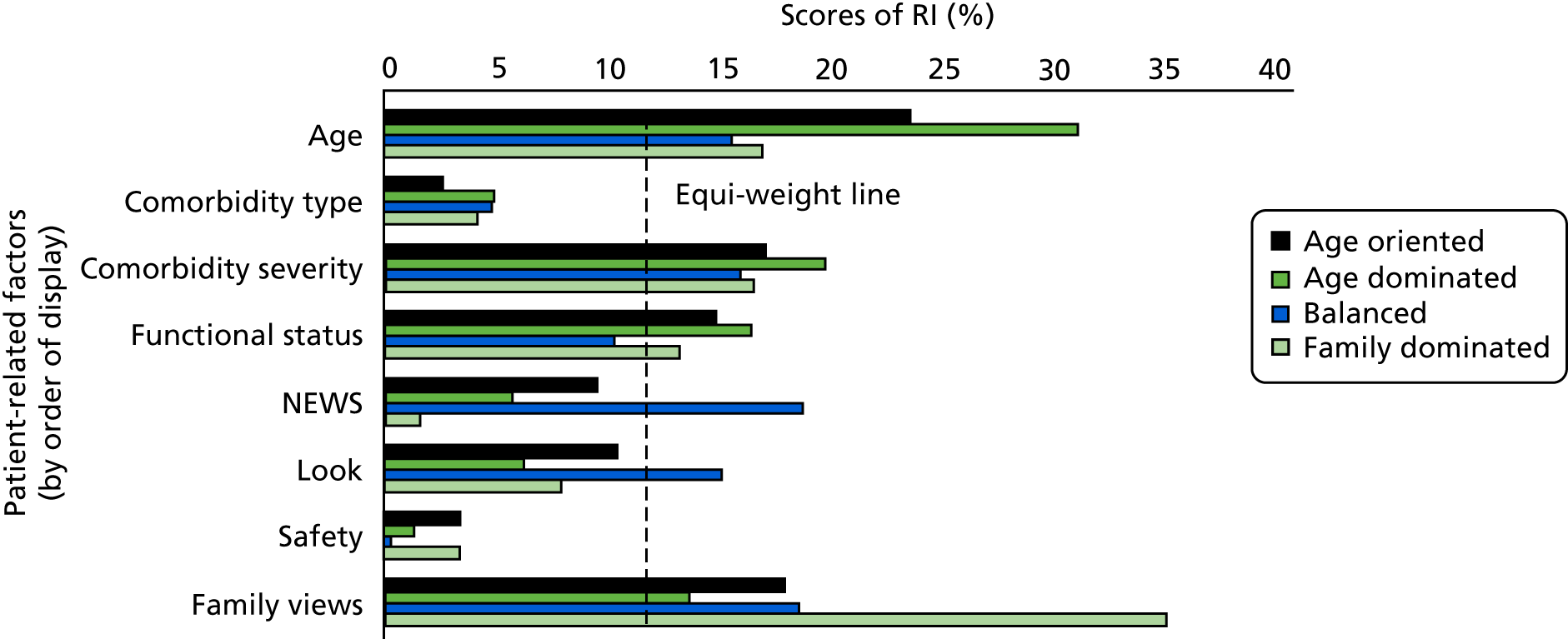
We define the first preference pattern as ‘age-oriented’ decision-making; although consultants in this group consider all patient-related factors (except type of comorbidity and patient safety), they give slightly more weight to the patient’s age (RI 24%). The second preference pattern describes ‘age-dominated’ decision-making because decisions are largely driven by the patient’s age (RI 31%). The third preferences pattern is described as ‘balanced’ decision-making, with consultants giving approximately similar importance to the eight patient-related factors. The last pattern indicates a ‘family-dominated’ decision-making (RI of family views 35%). These four patterns represent 31% (‘age-oriented’), 33.2% (‘age-dominated’), 17.4% (‘balanced’) and 18.4% (‘family-dominated’) of the ICU consultants.
Effects of consultants’ characteristics on preferences heterogeneity
We examined the effects of consultants’ characteristics (see Table 9) on the probability of their belonging to the different preference patterns by re-estimating the LCL model with characteristics as predictors of class membership probabilities. To maintain the structure of the LCL model, we constrained all preference parameters to remain the same, and then only the effects of the characteristics are freely estimated. For mathematical reasons, one class preference pattern has to be omitted, serving as a reference. We specified ‘family-dominated’ as the reference.
From a statistical perspective, the characteristics of the consultants explain very little of the variability in the preference patterns. Regression results are presented in Table 12. Only six effects (out of a possible 30) reach significance at the 5% level. The admission decision-making of consultants aged > 40 years is more likely to be ‘age-oriented’ and ‘balanced’ than ‘family-dominated’ than that of younger consultants. This is especially true for consultants aged > 50 years (as indicated by relatively large ORs). Consultants working in a medium-sized ICU (i.e. 11–19 beds) and those working at a university hospital are less likely to adopt ‘age-oriented’ and ‘balanced’ decision-making, respectively.
| Characteristic | Decision-making (reference: ‘family-dominated’), OR (95% CI) | ||
|---|---|---|---|
| ‘Age-oriented’ | ‘Age-dominated’ | ‘Balanced’ | |
| Constant | 2.11 (0.23 to 19.53) | 1.69 (0.21 to 13.54) | 0.79 (0.09 to 6.91) |
| Sex (reference: male) | |||
| Female | 0.77 (0.27 to 2.17) | 1.1 (0.43 to 2.85) | 1.68 (0.59 to 4.79) |
| Hospital (reference: not university) | |||
| University | 1.07 (0.44 to 2.6) | 0.84 (0.35 to 2.03) | 0.34 (0.12 to 0.96) |
| Age (reference: ≤ 40 years) | |||
| 41–50 years | 3.71 (1.15 to 12.01) | 2.43 (0.82 to 7.18) | 6.57 (1.47 to 29.4) |
| > 50 years | 16.36 (3.22 to 83.14) | 4.9 (0.97 to 24.66) | 12.72 (1.84 to 88.11) |
| Experience in ICU (reference: ≤ 4 years) | |||
| 5–9 years | 0.63 (0.06 to 6.29) | 1 (0.12 to 8.4) | 0.23 (0.02 to 2.24) |
| 10–14 years | 0.89 (0.09 to 8.76) | 1.53 (0.18 to 12.84) | 0.27 (0.03 to 2.63) |
| 15–19 years | 0.29 (0.03 to 3.19) | 0.44 (0.05 to 4.23) | 0.15 (0.01 to 1.6) |
| ≥ 20 years | 0.19 (0.02 to 2.28) | 0.24 (0.02 to 2.58) | 0.17 (0.02 to 1.99) |
| ICU size (reference: ≤ 10 beds) | |||
| 11–19 beds | 0.33 (0.12 to 0.91) | 0.61 (0.22 to 1.66) | 1.15 (0.37 to 3.63) |
| ≥ 20 beds | 0.62 (0.17 to 2.2) | 1.26 (0.36 to 4.42) | 2.32 (0.53 to 10.2) |
Influence of type of comorbidity on the effect of comorbidity severity
We re-estimated the model used for estimating consultants’ preferences, allowing for interaction effects between preferences for type and severity of main comorbidity. The results are presented in Figure 10. Increasing the severity of all comorbidities is associated with a decreased likelihood of admission to the ICU; however, differences are noted across the comorbidities. For mild severity, patients in all four comorbidity groups are more likely to be admitted than ‘patients with severe prostate cancer’. When the severity changes from mild to moderate, the probability of ICU admission falls only in patients with COPD. At the most severe level, dementia is the comorbidity most likely to result in the patient not being admitted to ICU, followed by severe heart failure and severe COPD. This pattern of results indicates that the effects of severity of main comorbidity on consultants’ decisions depend on the type of comorbidity.
FIGURE 10.
Interaction between the effects of type and severity of comorbidity in consultants’ decisions. The dashed line indicates a null effect on consultants’ admission decisions (i.e. OR 1). No CI is reported for severe prostate cancer because it corresponds to the reference category and then this effect was constrained to be null (OR 1). All other effects were estimated relative to this reference category. Reproduced from Bassford et al. 164 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
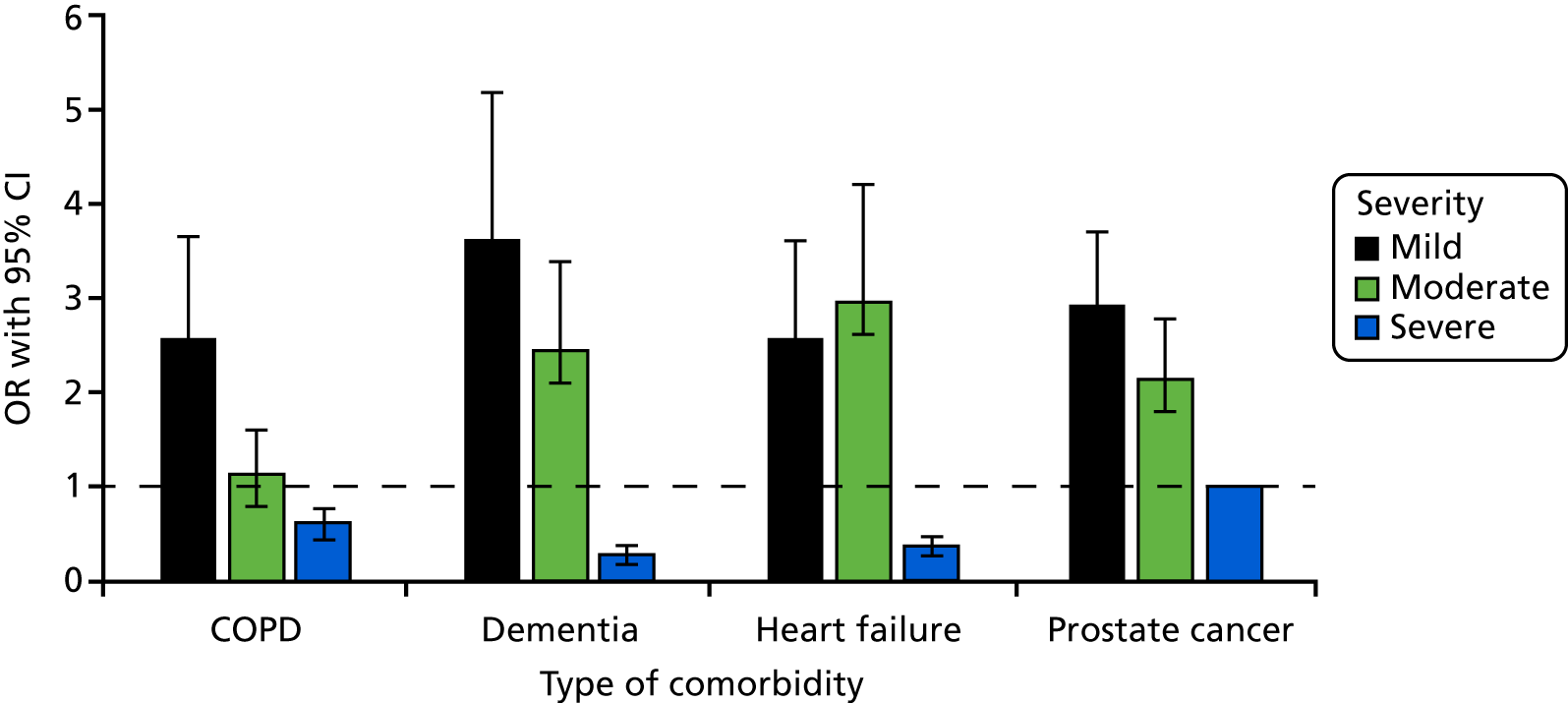
Results: clinical care outreach nurses’ preferences for intensive care unit admission
In this section we analyse CCOR nurses’ preferences, following the same structure and methods as for ICU consultants.
Sample of outreach nurses
The characteristics of the nurses are shown in Table 9. The sample mainly includes female nurses (84.1%) who do not work for a university hospital (59.8%) and are aged 40–49 years (40.2%).
Data quality
Overall, the quality of the choice data was good, with 71.4% of the CCOR nurses fully satisfying all four quality criteria and none of them failing more than two tests (see Table 10). Therefore, all of the observations were kept for the estimation of admission preferences.
Sample preferences of nurses
The results are reported in Table 13. All eight patient-related factors have a significant effect on nurses’ decisions. The patients’ age is the most influential feature, with a score of RI of 21.6%. This is followed by both severity of main comorbidity (RI 17.1%) and severity of acute condition (RI 17.4%). In comparison, two factors appear to have less influence on nurses’ admission decisions: the type of main comorbidity (RI 5.4%) and patient’s safety in ordinary (non-ICU) ward (RI 3.4%). The largest and smallest effects on nurses’ decision are related to age and type of main comorbidity, respectively. On average, a 39-year-old patient is 5.36 times more likely to be admitted than an 89-year-old patient, everything else being equal. On the other hand, a patient with prostate cancer is only 1.06 times more likely to be admitted than a patient with dementia. The second most influential effect is being critically ill: a patient with a NEWS of 11 is, on average, 3.86 times more likely to be admitted than a patient in a less severe condition (NEWS of 5).
| Factor | MLE (SE) | RI (%) | OR (95% CI) |
|---|---|---|---|
| Constant (no admission) | 2.944 (0.152)*** | – | – |
| Age (reference: 89 years) | |||
| 39 years | 1.679 (0.077)*** | 21.6 | 5.36 (4.61 to 6.23) |
| 66 years | 1.138 (0.072)*** | 3.12 (2.71 to 3.59) | |
| 79 years | 0.615 (0.071)*** | 1.85 (1.61 to 2.13) | |
| Comorbidity type (reference: prostate cancer) | |||
| COPD | –0.421 (0.069)*** | 5.4 | 0.66 (0.57 to 0.75) |
| Dementia | –0.061 (0.073) | 0.94 (0.82 to 1.09) | |
| Heart failure | –0.092 (0.076) | 0.91 (0.79 to 1.06) | |
| Comorbidity severity (reference: severe) | |||
| Mild | 1.328 (0.071)*** | 17.1 | 3.77 (3.28 to 4.34) |
| Moderate | 0.831 (0.07)*** | 2.3 (2 to 2.63) | |
| Functional status (reference: bad) | |||
| Good | 0.884 (0.06)*** | 11.4 | 2.42 (2.15 to 2.72) |
| Intermediate | 0.525 (0.062)*** | 1.69 (1.5 to 1.91) | |
| NEWS (reference: score = 5) | |||
| 11 | 1.352 (0.068)*** | 17.4 | 3.86 (3.38 to 4.41) |
| 8 | 0.295 (0.061)*** | 1.34 (1.19 to 1.51) | |
| Look (reference: good) | |||
| Bad | 0.946 (0.065)*** | 12.2 | 2.57 (2.27 to 2.92) |
| Intermediate | 0.65 (0.063)*** | 1.92 (1.69 to 2.17) | |
| Safety (reference: good) | |||
| Bad | 0.262 (0.046)*** | 3.4 | 1.3 (1.19 to 1.42) |
| Family views (reference: unsure) | |||
| No | –0.777 (0.064)*** | 11.5 | 0.46 (0.41 to 0.52) |
| Yes | 0.12 (0.059)** | 1.13 (1 to 1.27) | |
| SD individual errors | 1.104 (0.076)*** | – | – |
Preferences heterogeneity among clinical care outreach nurses
Five preference patterns were identified (see Report Supplementary Material 12). For all classes, nurses give similar importance to severity of comorbidity (RI ranges from 14% to 17%) and patient’s safety in a non-ICU ward (RI ranges from 1% to 5%) (Figure 11). The first preferences pattern gives relatively more importance to family views and functional status, and less importance to type of comorbidity and severity of acute condition (NEWS). Given the importance given to family views (RI 20%), we label this pattern ‘family-oriented’ decision-making. The second preferences pattern gives relatively more importance to patient’s age and registrar’s assessment, and less importance to acute condition (NEWS). Given the importance given to patient’s age (RI 27%), we label this pattern ‘age-oriented’ decision-making. The third pattern gives relatively more importance to type of comorbidity, functional status, severity of acute condition (NEWS) and family views, and relatively less importance to registrar’s assessment. Given that most factors show a similar level of importance, we label this pattern ‘balanced’ decision-making. The fourth pattern gives relatively more importance to severity of acute condition (NEWS) and less importance to functional status and family views. Given the importance given to NEWS (RI 27%), we label this pattern ‘NEWS-oriented’ decision-making. The last pattern gives relatively more importance to severity of acute condition (NEWS) and less importance to functional status, family views and type of comorbidity. This preferences pattern appears to be a more extreme version of the ‘NEWS-oriented’ decision-making, giving even more importance to NEWS (RI 39%), and is then labelled ‘NEWS-dominated’ decision-making.
FIGURE 11.
Preferences heterogeneity among CCOR nurses.
‘Family-oriented’ decision-making represents 66% (n = 125) of the sample; ‘age oriented’ represents 18% (n = 34); and ‘NEWS dominated’ represents 10% (n = 18). The two remaining preference patterns represent very few nurses: 5% (n = 10) and 1% (n = 2) for ‘balanced’ and ‘NEWS oriented’, respectively. Although nurses’ decisions reveal more types of decision-making than do those of ICU consultants, the relatively large market share for ‘family oriented’ suggests that CCOR nurses are less heterogeneous in their admission decisions than consultants.
Effect of nurses’ characteristics on preferences heterogeneity
The results are presented in Table 14. From a statistical perspective, allowing the characteristics of the nurses to predict decision-making styles leads to a minor improvement in performance. Although the log-likelihood of the model is improved (–3681.8 → –3662.9), this comes at the expense of 40 additional model parameters, leading to poorer Bayesian information criterion (8155 → 8454). Only four effects (out of a possible 40) reach significance at the 5% level, suggesting a limited contribution of characteristics to heterogeneity in preferences. Everything else being equal, CCOR nurses working in a larger ICU (i.e. number of beds ≥ 20) are more likely to adopt ‘NEWS-dominated’ decision-making than nurses working in a smaller ICU.
| Characteristic | Decision-making (reference: ‘NEWS-dominated’), OR (95% CI) | |||
|---|---|---|---|---|
| ‘Family-oriented’ | ‘Age-oriented’ | ‘Balanced’ | ‘NEWS-oriented’ | |
| Constant | 2.57 (0.51 to 13) | 4.22 (0.65 to 27.15) | 0.98 (0.12 to 8.35) | 1.94 (0.28 to 13.47) |
| Sex (reference: male) | ||||
| Female | 0.81 (0.23 to 2.77) | 0.58 (0.14 to 2.41) | 1.19 (0.23 to 6.18) | 1.11 (0.24 to 5.21) |
| Hospital (reference: not university) | ||||
| University | 1.51 (0.5 to 4.6) | 4.84 (1.29 to 18.19)** | 3.21 (0.8 to 12.88) | 3.14 (0.84 to 11.71)* |
| Age (reference: ≤ 40 years) | ||||
| 41–50 years | 0.81 (0.23 to 2.83) | 0.83 (0.2 to 3.5) | 2.18 (0.44 to 10.75) | 0.41 (0.08 to 1.98) |
| > 50 years | 1.19 (0.24 to 5.83) | 2.85 (0.47 to 17.43) | 5.8 (0.86 to 39.14)* | 1.3 (0.2 to 8.41) |
| Experience in ICU (reference: ≤ 4 years) | ||||
| 5–9 years | 1.37 (0.38 to 4.97) | 0.36 (0.07 to 1.73) | 0.81 (0.18 to 3.61) | 1.17 (0.25 to 5.53) |
| 10–14 years | 0.81 (0.18 to 3.72) | 0.51 (0.09 to 2.94) | 0.4 (0.07 to 2.37) | 1.02 (0.15 to 6.96) |
| 15–19 years | 1.15 (0.18 to 7.26) | 0.15 (0.01 to 1.97) | 0.15 (0.02 to 1.57) | 0.09 (0 to 11.05) |
| ≥ 20 years | 1.34 (0.16 to 11) | 0.16 (0.01 to 4.64) | < 0.01 | 1.42 (0.11 to 19.03) |
| ICU size (reference: ≤ 10 beds) | ||||
| 11–19 beds | 1 (0.27 to 3.7) | 0.42 (0.1 to 1.73) | 0.38 (0.09 to 1.66) | 0.5 (0.11 to 2.2) |
| ≥ 20 beds | 0.38 (0.09 to 1.65) | 0.06 (0.01 to 0.38)*** | 0.1 (0.02 to 0.59)** | 0.13 (0.02 to 0.72)** |
Influence of type of comorbidity on the effect of comorbidity severity
As with ICU consultants, there is evidence of an interaction between type and severity of comorbidity in CCOR nurses’ admission preferences (Figure 12). As anticipated, patients with mild comorbidity are more likely to be recommended for admission than those with severe prostate cancer. However, the magnitude of this effect depends on the type of comorbidity. Everything else being equal, patients with mild prostate cancer are more likely to be recommended for admission than patients with mild dementia, COPD or heart failure. This pattern tends to be stable across severity levels.
FIGURE 12.
Interaction between the effects of type and severity of comorbidity in nurses’ decisions. The dashed line indicates a null effect on nurses’ admission decisions (i.e. OR 1). No CI is reported for severe prostate cancer because it corresponds to the reference category and then this effect was constrained to be null (OR 1). All other effects were estimated relative to this reference category.
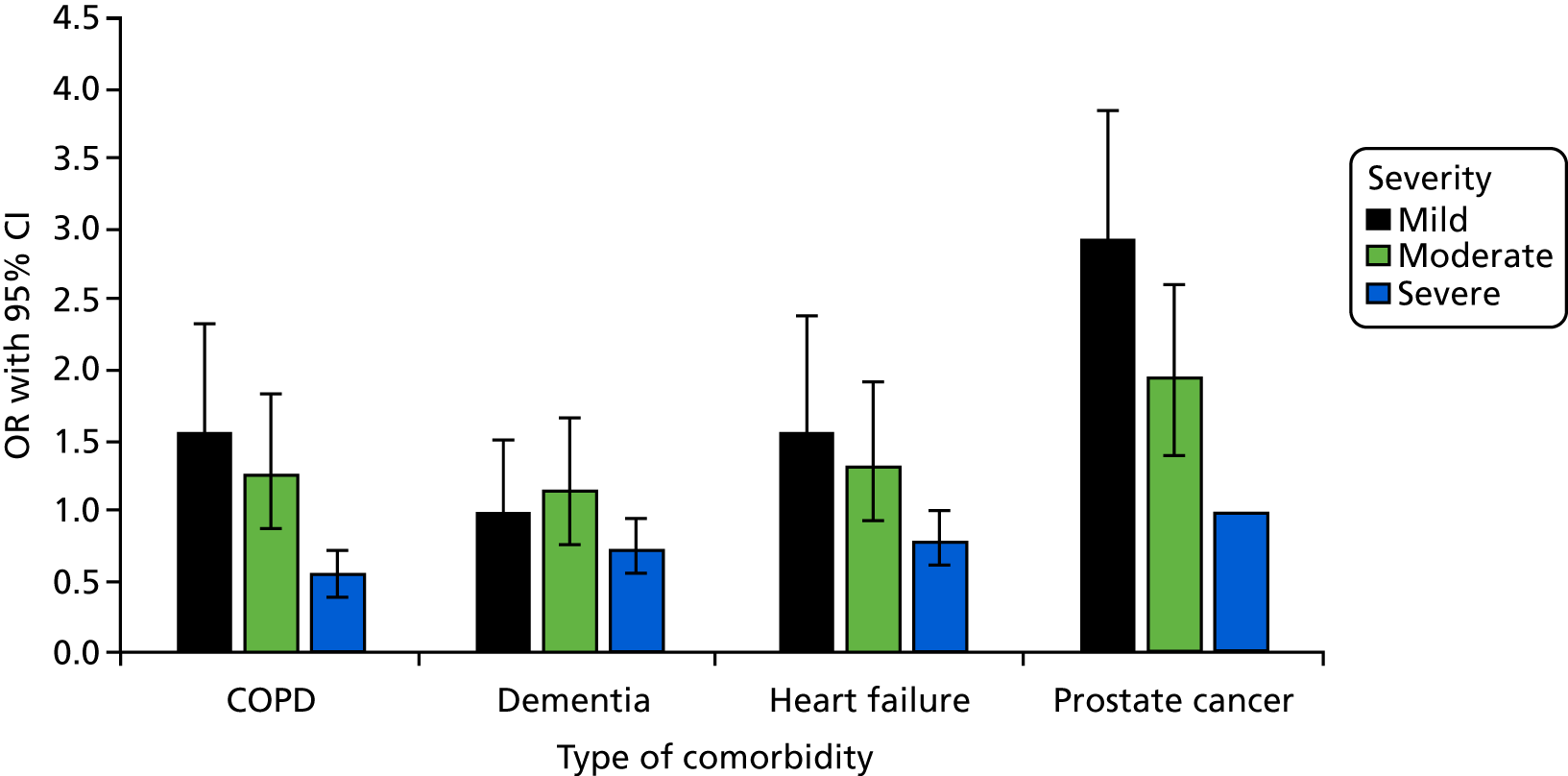
Comparison of consultants and outreach nurses’ preferences for intensive care unit admission
Comparing the results above suggests differences between ICU consultants and CCOR nurses. Notably we observe a smaller proportion of stable decisions for nurses (79%) than for consultants (88%); this ≈10-point difference reaches significance at the 5% level (χ2 = 6.82; df = 1; p = 0.009). Lower levels of stability may indicate that ICU admission decisions are more difficult for CCOR nurses.
The two groups also differ in the relative importance given to patient-related factors. Whereas severity of acute condition (NEWS) is ranked as one of the least important factors for consultants (RI 7.5%; rank 6/8), it appears to be one of the most important factors for nurses (RI 17.4%; rank 2/8). CCOR nurses give less importance to family’s views (RI 11.5%; rank 5/8) than do consultants (RI 19.9%; rank 2/8).
It is important to note that the difference in preference patterns (βnk) between nurses and consultants may be caused by differences in true preferences [e.g. consultants would give more (less) weight to family’s views than those of CCOR nurses] and/or differences in consistency of choices [e.g. nurses would be less (more) consistent than consultants in their admission decisions]. This is because the estimated parameter, βnk, is the product of a true preference and errors-related parameters. Therefore, when comparing preferences between ICU consultants and CCOR nurses, it becomes important to make a distinction between changes in true preferences and choices consistency (as approximated by variance in the model errors), as they would have different implications. For example, differences due to changes in the level of choices consistency suggest that CCOR nurses are less confident than ICU consultants in making admission decisions, which might reflect their limited participation in the decision-making process in real-life situations.
We thus compare consultants’ and nurses’ preferences for patients’ admission following a multisteps approach. We first test for the existence of a systematic change in choice consistency between nurses’ and consultants’ decisions, estimating a heteroscedastic MNL model. This allows the choice consistency to be a function of type of respondents (i.e. nurse vs. consultant). The results, shown in Figure 13, suggest a negative and significant effect, indicating that, on average, CCOR nurses are less consistent than ICU consultants in their admission decisions.
FIGURE 13.
Comparison of preferences between ICU consultants and CCOR nurses.
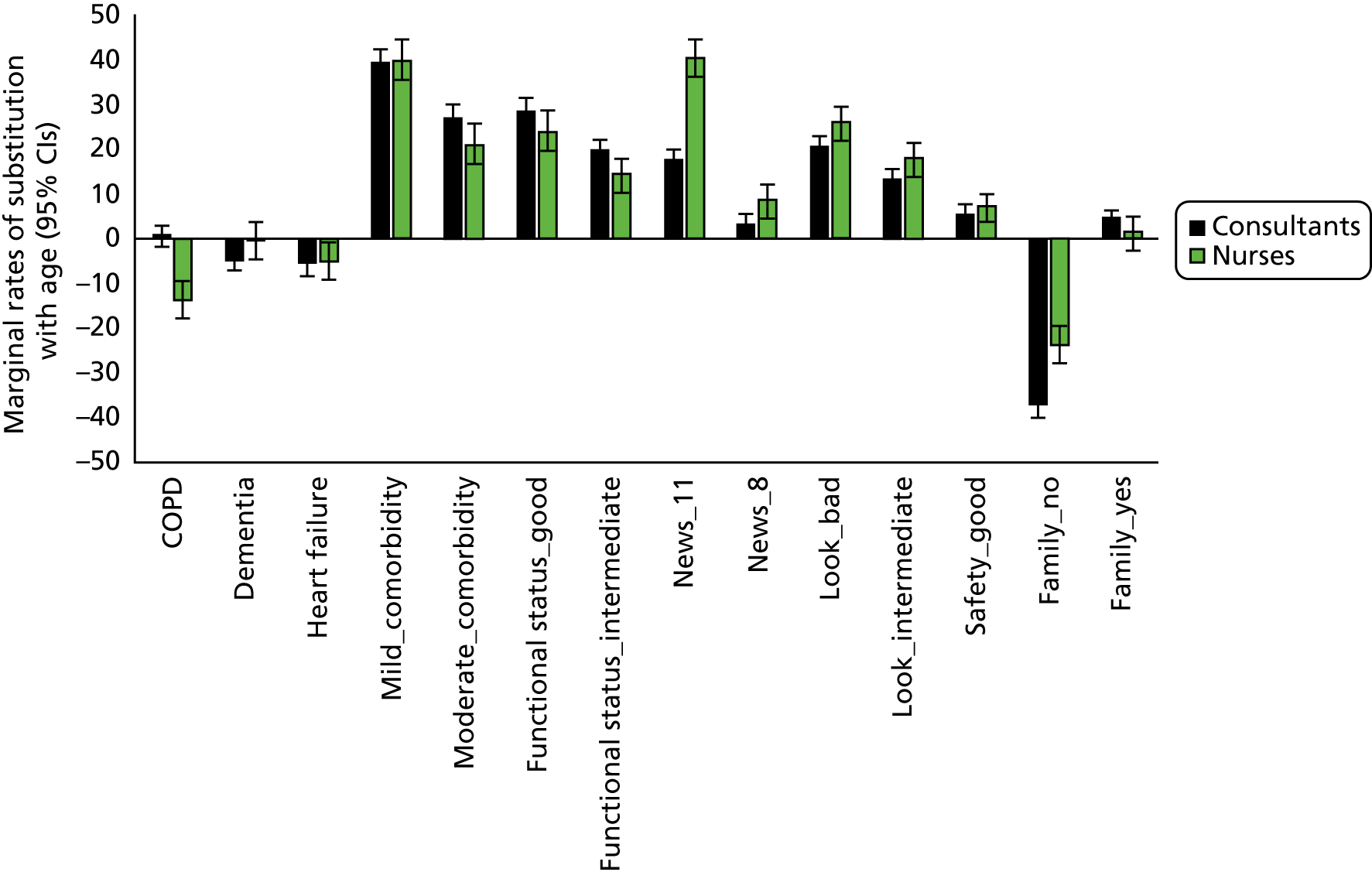
We then determine whether or not this difference in choice consistency would explain all of the differences in parameter estimates between the two samples. This is done by comparing the statistical performance (as measured by the log-likelihood value at convergence) of different MNL models. Under the assumption of similar choices consistency but dissimilar preferences (H1), the joint model log-likelihood for the two samples is –10,057.1 points. Under the assumption of similar choices consistency and preferences (H2), we obtain a log-likelihood of –10,295 points. Under the assumption of dissimilar choices consistency but similar preferences (H3), we obtain a log-likelihood of –10,206.7 points. The comparison of these three values indicates that allowing preferences for patients’ features to differ between the two samples would improve modelling performance more than allowing for changes in choices consistency (H2–H1 = –237.9 vs. H2–H3 = –88.3). This result suggests that CCOR nurses and ICU consultants are likely to differ in their true preferences for patients’ admission in addition to differing in the consistency of their choices.
Given these likely differences, we compare the preferences between the two samples by estimating a MNL model of the trade-offs between patients’ age and the other features. A similar approach has been used to directly estimate how much individuals would be willing to pay for changes in product composition. 172 In our case this approach is technically appealing because it rules out the influence of changes in choices consistency.
Overall, nurses and consultants appear to hold similar preferences regarding patients’ admission; for most patient features, the CIs overlap. However, some significant differences exist. Consultants give significantly more weight than nurses to families’ views. The trade-off between ‘family insisting for admission’ and patients’ age is 37.08 and 24.06 for consultants and nurses, respectively, indicating that ‘family insisting for admission’ is 1.5 times more important for consultants than for nurses (i.e. 37.08/24.06 = 1.54). Nurses also give significantly more weight to the severity of the acute condition (NEWS). A severe acute condition with ‘NEWS = 11 (temp: 37.7 °C; resp. rate: 23; SpO2: 90% on 60% FiO2; Glasgow Coma Score: 15; pulse: 90; systolic blood pressure: 85 mmHg after adequate iv fluids)’ is 2.3 times more influential in nurses’ decisions than in consultants’ (i.e. 40.17/17.61 = 2.28). Finally, nurses and consultants differ in the importance they give to COPD, with this having almost no impact on consultants’ decisions and a large effect on nurses’ decisions (ratio: 13.74/0.54 = 25.42) (see Appendix 7, Table 34).
Summary
This study is the first to use a validated economic method to elicit consultants and CCOR nurses’ preferences for patient admissions to ICU.
In terms of relative importance of the patient-related factors, our main study results indicate that (1) the patient’s age is by far the most important determinant of consultants’ admission decisions, (2) the severity of the main comorbidity has more influence on consultants’ decisions than the severity of the acute condition (as measured by the NEWS) and (3) patient assessment by the registrar has more influence than objective clinical assessment (NEWS).
Our results show considerable heterogeneity in consultants’ preferences. About one-third of the consultants give priority to functional status, severity of comorbidity and age when deciding whether or not to admit patients, 31% make admission decisions largely driven by the patient’s age, 17% appear to give approximately the same importance to different types of information, and the last group gives more importance to the family’s views.
Our results also indicate a complex relationship between the type and severity of the main comorbidity. As expected, patients presenting a more severe comorbidity are less likely to be admitted than patients with a less severe condition. Although a priori consultants do not appear to discriminate among the four types of comorbidity (COPD, prostate cancer, heart failure and dementia), this is no longer the case once severity is taken into account. For instance, patients with moderate COPD are less likely to be admitted than patients with mild severity of any other condition.
Finally, our results indicate that differences in admission decision-making exist between CCOR nurses and ICU consultants. Outreach nurses appear to be less consistent in their admission decisions than consultants, and give more importance to clinical features (e.g. NEWS).
Our study results can be used to inform ICU consultants about potential biases in their admission decision-making. In line with this educational purpose, and to best communicate our study results to consultants, we have developed two ‘ICU admission simulators’ (https://warwick.ac.uk/fac/med/research/hscience/sssh/research/intensive/; see Appendices 8 and 9). The first simulator can be used to see how the probability of ICU admission changes with a patient’s characteristics (as described in the choice experiment). The second simulator was developed to explore preferences heterogeneity. The user is asked to answer a limited number of choice questions as presented in the choice experiment. Based on their answers, and on the study results, the simulator predicts their probability of belonging to the four preference patterns identified in the study.
Strengths and weaknesses of the study
The study uses a validated and powerful preferences-elicitation method (namely choice experiment) to understand how consultants and CCOR nurses make admission decisions. We recruited a large representative sample of ICU consultants. We achieved a relatively high level of data quality, indicating that the preferences-elicitation instrument was well accepted by participants. We used a combination of ethnographic observations and systematic literature reviews to identify the most relevant features. A difficulty with all choice experiments, including this one, is that participants are asked to make hypothetical decisions, in this case regarding ICU admission. Although the choice tasks were created to mimic real-life situations, we cannot guarantee that ICU consultants and CCOR nurses would have made the same choices if the tasks had been real.
Chapter 6 Development of an intervention to support decision-making around referral and admission to intensive care
Introduction
Having investigated what is currently known about the decision-making process in our systematic reviews, and explored current practice and experience in our ethnographic study, we drew on our findings to develop an intervention that would support consistent, transparent and ethically justifiable patient-centred decision-making. In developing the DSI we were aware that it needed to be feasible to implement in daily practice.
Our specific objectives were to develop:
-
a DSF for decisions about referral and admission to ICU
-
support and training materials for clinicians using the framework
-
information for patients and families on decisions about referral and admission to ICU.
From our preceding work (systematic reviews, ethnography and choice experiment), we identified the key patient-related, situational and process-related factors that influenced (or were thought should influence) decisions about referral or admission to ICU. Using data from all three sources provided triangulation and strengthened our evaluation of these factors as being relevant to the decision-making process. These factors could then be emphasised, supported or mitigated within the intervention. From our ethnographic study, we also mapped the temporal and relational aspects of the decision-making process, the points of conflict, and the implicit and explicit values that informed it. This enabled us to map the DSI to current practice that would be recognisable to clinicians, and to address points of conflict and value positions in the associated educational support. Finally, the analysis in our ethnographic study specifically focused on what would constitute a good decision-making process and the normative framework to support this. This analysis fed directly into the development of the DSI and the implementation plan. For example, our analysis identified the importance of involving patients and their families in the decision-making process and of explicit balancing of harms and benefits, and the infrequency with which either of these occurred in practice. The choice experiment identified different preference patterns among ICU doctors, and this finding supported the need for explicit articulation of reasons when making a decision. Thus, in developing the DSI we needed to include prompts for both patient and family involvement and explicit balancing of reasons.
Development of the decision-support intervention
The development of the DSI (conceptual framework plus supporting resources) was in five phases:
-
initial development of a draft DSI (conceptual framework and supporting resources)
-
presentation of the DSI at a stakeholder conference including focus groups to identify areas for revision and refinement
-
post-conference refinement of the DSI based on this feedback
-
development of educational materials
-
refinement of the implementation strategy using normalisation process theory (NPT).
Initial development of the decision-support intervention
Developing a conceptual/cognitive framework
The first step was to develop a ‘best practice’ conceptual framework for decision-making, based on our previous work, that encapsulated what is important/essential to patients and clinicians in practice and what is ethically required.
Ethical reasoning is an integral part of clinical decision-making but is often implicit rather than explicit. Findings from our systematic review and ethnographic study show that clinicians’ values influence their decision-making, and that they do not explicitly balance different empirical and normative considerations when making a decision. There are some ethical frameworks and heuristics used in teaching medical ethics, most commonly a principlist approach. However, it seems unlikely from our observation work that providing a theoretical ethical framework for use in day-to-day practice would be successful. In seeking to develop a framework that could be embedded in daily practice, be congruent with clinical decision-making and prompt ethical reasoning, we identified the ‘accountability for reasonableness’ (AFR) framework157 as the most useful model on which to build our DSF. This is a commonly used ethical framework for considering the allocation of limited medical resources and focuses on the process of decision-making rather than relying on a specific moral theory. AFR has four requirements: decisions must be transparent, based on reasons that stakeholders can agree are relevant and revisable in the light of new evidence and arguments, and there should be an appeals process. Other authors have highlighted the requirement for priority setting decisions by clinicians (including ICU admissions) to be transparent (AFR requirement 1) and ethically justifiable (AFR requirements 2 and 3). 173,174 Therefore, any framework to support decision-making should improve the transparency and ethical justification of decisions about referral and admission to ICU. A further ethical requirement is one of equity, which can be interpreted as consistency of process (the factors taken into account and the decision-making process should be consistent across all patients and all decision-makers).
Process of developing the framework
A generic model of the steps in the decision-making process was created based on the findings from our ethnographic study (Figure 14).
FIGURE 14.
The generic model decision-making process. Reproduced from Rees et al. 175 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
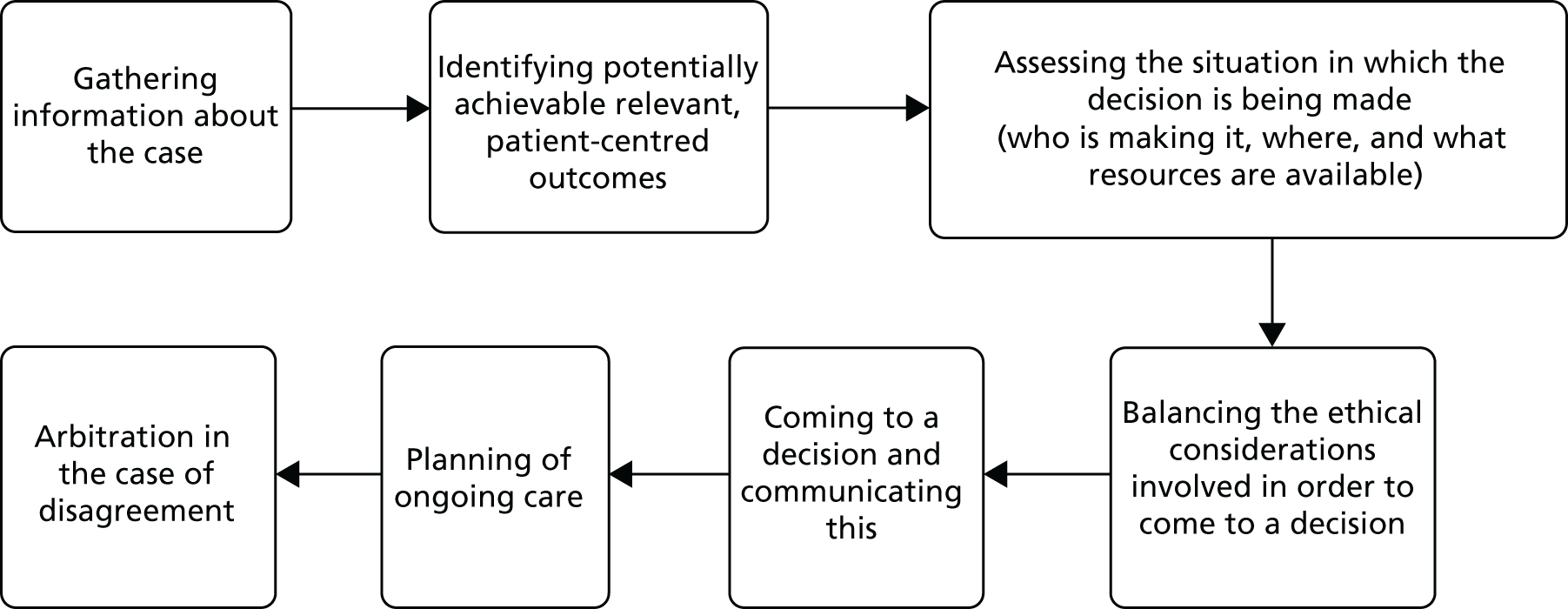
Clinical and non-clinical members of the research team were then asked to map the factors identified in the qualitative study and systematic reviews (tabulated in Appendix 10) to relevant stages of the process. From this, a draft framework was developed, specifying content at each stage in the decision-making process and ensuring that overall it was consistent with the AFR ethical requirements. The resulting framework was discussed and refined in a series of meetings involving members of the research team and two members of our PPIAG. The draft framework was then used as a basis for developing the supporting materials (Table 15).
| Stage in process | Factors to consider |
|---|---|
| Gathering relevant information |
Clinical information about current acute illness and relevant chronic illness Functional reserve of patient Patient values, wishes and relevant outcomes Need to recognise clinician biases in interpretation of information |
| Assessing and evaluating the situation |
Ward situation/resources available ICU resource availability (beds/nursing staff) Urgency of decision Experience of clinicians involved in decision |
| Balancing the ethical considerations in order to come to a decision | Explicit balancing of potential harms and benefits and reason for decision |
| Making and communicating the decision |
Specifically informing medical and nursing staff of decision and the reasons for it Communicating the decision and reasons for it to the patient and/or their family |
| Planning of ongoing care | Specific documentation of plan for review, including who, when and in what circumstances |
| Arbitration in cases of disagreement | System and process in place to support this |
Development of supporting resources for clinicians
The supporting resources of the draft DSI were:
-
documentation to prompt and record best practice in the referral and decision-making process
-
information for patients and families to help them participate in the decision-making process
-
educational resources to support the implementation of the intervention.
Development of the decision support documentation
We developed a referral form (for the referring team to use) and a decision-support form (for ICU doctors to use). The aim of the forms was to provide the clinician with a framework within which to structure their decision, and to prompt them to include the key steps identified as necessary to ensure an ethically justified decision-making process. A series of drafts were produced and revised at a number of meetings of the research team and members of the PPIAG. Feedback was also sought on their format and usability from clinicians at sites that had participated in the ethnographic study. The final versions, following revisions informed by the stakeholder conference, are presented in Appendices 11 and 12.
Electronic format
As part of the development work, we worked with the Health and Social Care Information Centre, now NHS Digital, to develop an electronic version of the referral and decision-support forms. Draft electronic copies of the referral and decision-support forms were created in the Lorenzo electronic health record system (DXC Technology, Tysons, VA, USA) by collaborators at the NHS Digital. This team also developed system agnostic criteria for electronic versions of the forms that IT developers in individual NHS trusts could use to incorporate the forms into local operating systems.
Development of patient and family information leaflets
The patient and family information leaflets were initially developed by a member of the study PPIAG. The research team and other members of the PPIAG then refined and expanded these drafts, drawing on the findings of the ethnographic study, particularly interviews with patients and families. These were presented at the stakeholder conference. The final versions, including revisions following the stakeholder conference, can be found in Appendices 13 and 14.
Consensus conference
A 1-day conference was held in July 2016 at the University of Warwick to gain the views of a wider stakeholder population regarding the content of the DSI and the facilitators of and barriers to its implementation.
Delegates were invited from the following stakeholder groups (see Appendix 15 for the list of delegate representatives):
-
Patients –
-
people who had survived a critical illness
-
patient groups who may be particularly likely to need intensive care treatment (e.g. those with heart disease or lung disease)
-
patient groups who may be disadvantaged in terms of access to health care (e.g. those with a learning disability)
-
-
ICU doctors (including professional body representation)
-
ICU nurses and CCOR nurses (including professional body representation)
-
referring specialty clinicians (e.g. cardiology, renal, elderly medicine, respiratory, surgery)
-
regulatory authority, legal and health-care management representation.
Method
Meeting sessions were chaired by a PPI co-investigator (CW) and by a senior member of the Faculty of Intensive Care Medicine. Before the event, delegates were sent copies of the draft DSI and a summary of the findings of the research to date. The event included presentations of the research on which the DSI was based and a demonstration of the electronic version of the referral and decision-support form. Delegates then participated in focus groups in which they were asked to consider specific elements of the intervention and answer a series of questions relating to these elements (see Report Supplementary Material 13 for topic guides). Each focus group included a cross-section of delegates to ensure a balance of disciplines and backgrounds within each group. The focus groups were facilitated by a member of the study team and the discussion was captured by a medical student acting as a scribe. The discussion was recorded with participants’ consent and recordings used to check accuracy when interpreting the written notes of the discussion. All focus groups were asked to consider the referral form and the decision-support form, and either patient or family information leaflet or learning outcomes for educational resources. Two key points from each group on each element considered were transcribed onto flip chart paper and placed around the main meeting room. In a subsequent plenary session all delegates were asked to read the flipcharts and write any further thoughts or suggestions on sticky notes and attach them to the flip charts.
Analysis
Following the conference, the scribes’ notes and points from the sticky notes were circulated to two members of the research team (AS and HH) for analysis. The data was categorised under the headings: referral form and process; DSF; patient and family information; education; and barriers and facilitators to implementation. A summary of this analysis was used by the research team to refine the documentation and develop a strategy for implementation of the DSI.
Post-consensus conference development of the decision-support intervention
A conference report, including the summary of key points and the changes made to the intervention in response, was sent to all delegates with an invitation to provide further comment (see Appendix 16). The DSI was then finalised for use in the implementation feasibility study. The changes made to the components of the DSI are outlined below.
Refining the components of the intervention
Development of the referral and decision-support documentation
The focus of all of the components of the DSI was broadened to include decision-making regarding all forms of critical care support. The term ‘family member’ was changed to ‘person closest to the patient’. The referral form was modified to be closer to the SBAR (Situation, Background, Assessment and Recommendation) format. Specific questions related to ICU bed availability and ward capacity were removed and reframed to ask first, what was the optimum treatment for the particular patient, and then to ask where this care could be safely delivered.
Development of the educational package
Key suggestions were incorporated into the development of training materials including the importance of communication, relevant knowledge of legal and regulatory frameworks, a clear ethical framework, and guidance for using the forms (see Report Supplementary Material 14 for copies of educational materials).
Development of the patient and family support material
New drafts of the patient and family information leaflets were written by members of the PPIAG and our PPI co-investigators. Notably the use of separate leaflets for patients and family/friends was developed rather than a single combined leaflet. The language was simplified and the layout improved. The finalised leaflets received a Crystal Mark from the Plain English Campaign. 176
Refining implementation of the intervention
To identify potential difficulties in the implementation of the DSI and to improve the chances of its successful implementation, eight intensive care consultants and CCOR nurses from sites that had been involved in the ethnographic study attended an implementation planning meeting. The meeting used an adapted NPT177 (see Report Supplementary Material 15) toolkit: a way of exploring changes to practice to understand how an innovation is implemented and integrated into the work of an organisation.
The DSI was presented, and how it was perceived was explored using adapted questions from the NPT toolkit. Responses were categorised into the four constructs of NPT: coherence (could they make sense of the intervention), cognitive participation (could they envisage their colleagues and other key stakeholders actively engaging in this process), collective action (could they envisage this working in practice and what might be the barriers and facilitators) and reflexive monitoring (how could they envisage the intervention changing practice and how would they evaluate this) (see Report Supplementary Material 15). This analysis revealed particular concerns regarding the active engagement of all stakeholders and uncertainty around organisational support for the intervention creating difficulties for implementation.
In response to this feedback, we adapted our implementation strategy to include approaching the medical directors of each NHS trust participating in the implementation study to secure organisational support for the intervention and research. We also adapted our educational resources and the train-the-trainer sessions to encourage and support stakeholder engagement.
The decision-support intervention
The final DSI is described briefly below.
Guidance for referral for intensive care support
We developed guidance to improve communication between clinical teams around referral. The guidance is summarised as follows:
-
Referral to intensive care should not be delegated to junior members of the clinical team.
-
Referring teams should clearly articulate what support they are seeking from the intensive care team.
-
The benefits and burdens of intensive care should be considered for each patient.
-
Ensure that the patient and/or their family has been consulted in the referral process.
-
The referral should follow recognised best practice using the SBAR format.
The referral form (see Appendix 11) provides a structured format for information gathering and communication with the intensive care clinicians to facilitate a collaborative decision-making process.
A decision-support framework to guide intensive care unit doctors when making decisions
The framework describes best practice for decision-making in three domains: (1) evidence – the collection of information relevant to the decision; (2) reasoning – using this evidence to identify relevant outcomes, balance the burdens and benefits of treatments and make recommendations for treatment; and (3) implementation – ensuring that decisions are acted on and communicated effectively. The framework aims to integrate ethical reasoning within the decision-making process in a practical and accessible manner. The decision-support form (see Appendix 12) enables the decision-making process to be recorded to facilitate transparency and consistency of decision-making.
A pocket-sized aide-mémoire (Figure 15), which has the referral guidance on one side and the framework for decision-making on the other, was provided to act as a reminder of the referral guidance and decision framework to referring teams and ICU doctors.
FIGURE 15.
A pocket-sized summary of the cognitive framework to act as an aide-mémoire.

Patient and family support information
Patient and family information leaflets (see Appendices 13 and 14) to be given to patients and/or their families/someone close to them when they are recognised as being critically unwell and a decision regarding ICU referral is being considered. The leaflets are intended to support discussion between clinicians and the patient/family and not to be given in isolation.
Educational resources
Educational resources to support the implementation of the DSI included (see Report Supplementary Material 14):
-
‘train-the-champion’ session for implementation champions (including an introductory presentation to the legal and ethical background)
-
a presentation of the DSI and its rationale at hospital Grand Rounds or other formal meetings
-
a shorter presentation for use at more informal or smaller departmental meetings, for example quality improvement meetings
-
a brief ‘key points’ presentation for opportunistic teaching of individuals or small groups
-
a table-top case-based simulation exercise to familiarise clinicians with the intervention.
The final version of all components of the DSI formed the basis of our implementation feasibility study (see Chapter 7).
Summary
This chapter has described the development of an intervention to support best practice in decision-making for escalation of treatment and admission to intensive care. The development was informed at each stage by the findings of our preceding WPs and input from our PPI co-investigators and advisory group. Accountability for Reasonableness provided the normative theoretical framework for the intervention. It was developed to fit with established clinical practice while offering support for improvement in that practice, and supported by resources to increase the likelihood of successful implementation.
Chapter 7 Feasibility study to explore implementation of the decision-support intervention
Introduction
In this chapter we report on an assessment of the feasibility of implementing the DSI in NHS daily practice.
The objectives of the feasibility study were:
-
to demonstrate the feasibility of implementing the DSI at an organisational level, including its associated materials and training, in a busy NHS trust
-
to explore intervention fidelity reviewing the actual use of the DSI and its impact on decision-making, and how this compares with its intended use
-
to explore the acceptability of the DSI, including the training and materials, to referring and ICU doctors.
Methods
Settings and recruitment
A description of the study was circulated to intensive care leads through the National Institute for Health Research Clinical Research Network. ICU leads were asked to indicate their interest in participating in the study and to state the number of ICU beds in their trust. We purposively sampled by size of ICU: one small (< 20 ICU beds), one medium (20–30 ICU beds) and one large (> 30 ICU beds).
We worked with the principal investigator at each selected trust to identify implementation champions, who were tasked with planning and running the implementation. The identified champions were, in site A, an ICU consultant and a CCOR nurse; in site B, two ICU senior trainees (registrars); and, in site C, an ICU consultant and two CCOR nurses sharing the role.
Pre implementation
Before the DSI was implemented at each site, we ran a 1-day training session for the implementation champions. The day included:
-
Presentations on the background to the study and findings from the early workstreams, and an introduction to the ethical and legal considerations in decision-making for the critically ill patient.
-
A detailed description and explanation of the intervention, with particular focus on the DSF and its documentation, the referral process, and patient and family information leaflets.
-
An introduction to the range of educational support materials provided for the implementation champions to use in engaging with staff in their trust about implementing the intervention.
-
An interactive discussion on the barriers to and facilitators of implementation in the specific context of the individual site. The implementation champions used this to develop a detailed implementation plan appropriate for their trust, detailing which referral wards were included and a timetable.
Each site was provided with copies of DSI documentation. Coloured and clearly labelled boxes that included referral forms, decision-support forms, and patient and family information leaflets were positioned on ICU and wards identified as likely referrers. In sites with CCOR nurses, the outreach team also had copies of the forms to take with them to wards when seeing patients. Patient and family information leaflets were translated into the two non-English languages most commonly spoken by patients in the relevant trust. The implementation champions were given a resource pack (hard copy and electronic) that included education materials and presentations for training.
Data collection
An initial 8-week period preceded the main data collection period for the implementation champions to implement the intervention. During that time, we conducted audio-recorded interviews (telephone or face to face) with the implementation champions at approximately fortnightly intervals (see Report Supplementary Material 16). We also observed two training sessions at each hospital (see Report Supplementary Material 17) and made field notes.
The subsequent 6 weeks was the main data collection period. To assess form use, including consistency, we aimed to identify, from ICU referral logs, the clinical records of all patients referred to ICU during the data collection period. One site already kept a detailed log, but the other two sites had to create a new system. We did not seek clinical records of logged referrals from wards not included in the study or for planned ICU admissions. From the clinical records of referred patients, we extracted the following data: the time and date of the ICU referral/review; the patient’s year of birth and sex; and the name and role of the person referring/reviewing. If a form was used, the presence or absence of information in each section was recorded. Information on daily ICU bed occupancy was obtained from the ICU manager.
Acceptability of the intervention was assessed in interviews with ICU doctors recieving a referral and doctors who had referred a patient during that period (see Report Supplementary Material 18 for the topic guide). We used the ICU referral logs and clinical records collected during the data collection period to identify potential participants, purposively sampling to include a mix of grades, specialties of (referring) doctors and those who had/had not used a form when referring/reviewing a patient. In one hospital it was not possible to reach the desired sample based on the referral log owing to the low number of logged referrals, and therefore the local principal investigator identified additional doctors to participate. We conducted a group interview with the CCOR nurses at one site. Interviews were semistructured. Interviewees were asked their thoughts on the DSI overall. A referral or decision form (either blank or completed by them if available), and details of a specific referral were used in the interview to prompt recollection of using/not using a form in specific cases. Interviews were audio-recorded with written consent from participants. We held a debriefing meeting at each site with the implementation champions, reflecting on the intervention implementation process. This was audio-recorded and field notes were taken.
Data analysis
Quantitative data analysis
Data extracted from the patient records were analysed as counts, percentages and, where there was a continuous variable, means and standard deviation. Informed by the results of our ethnographic study and systematic review, we looked for associations between patient, doctor or organisational factors and completion of the forms. Chi-squared tests were used to compare categorical variables, and Student’s t-test was used to compare continuous data. Analyses were carried out in R (The R Foundation for Statistical Computing, Vienna, Austria).
Qualitative data analysis
All field notes and interviews were thematically analysed with the aid of NVivo 11 software by Sophie Rees. Frances Griffiths reviewed coding for consistency. 150,178,179 The researcher (SR) listened to each implementation champion interview multiple times, took notes and transcribed key comments. These interviews were coded at the nodes: enablers, obstacles, disagreements, modifications and rationale for approach. The doctor and CCOR interviews were transcribed verbatim. Initially, data were coded at nodes identified from the protocol: acceptability, experience of using the forms and impact on decision-making. Other emergent nodes were added during coding. Then, within these broad codes, further coding was undertaken; for example, data in the ‘acceptability’ node were further coded to nodes ‘ease of use’ and ‘concerns about workload’. Analysis discussions were held with the whole research team.
Results: study population
All six implementation champions were interviewed to investigate implementation. To assess acceptability of the intervention, we approached 61 doctors (ICU doctors, n = 25; doctors who referred patients to ICU, n = 36), and three CCOR nurses across the three sites. Of these, 20 ICU doctors, 19 referring doctors from a range of specialties, and three CCOR nurses were interviewed (Table 16). A total of 333 referrals were logged across the three sites during the 6-week data collection phase (Table 17). We were unable to access 41 records during the study period, and 111 records were excluded because they were ineligible. Data were extracted from 181 patient records.
| Characteristic | Hospital (n) | Total (n) | ||
|---|---|---|---|---|
| A | B | C | ||
| Referring doctors | ||||
| Grade | ||||
| Consultant | 3 | 4 | 3 | 10 |
| Registrar | 3 | 2 | 2 | 7 |
| FY1/2 | 0 | 0 | 2 | 2 |
| Specialty | ||||
| Acute medicine | 2 | 0 | 1 | 3 |
| ED | 1 | 2 | 1 | 4 |
| Surgery | 1 | 0 | 1 | 2 |
| Haematology/oncology | 0 | 3 | 1 | 4 |
| Respiratory | 1 | 1 | 1 | 3 |
| Hepatology | 0 | 0 | 1 | 1 |
| Geriatrics | 0 | 0 | 1 | 1 |
| Medicine | 1 | 0 | 0 | 1 |
| Form usea | ||||
| Used | 5 | 2 | 4 | 11 |
| Never used | 1 | 4 | 3 | 8 |
| CCOR nurses | ||||
| 3 | 3 | |||
| ICU doctors | ||||
| Grade | ||||
| Consultant | 3 | 2 | 4 | 9 |
| Registrar | 3 | 4 | 4 | 11 |
| Form usea | ||||
| Used | 6 | 2 | 6 | 14 |
| Never used | 0 | 4 | 2 | 6 |
| Hospital | Total referrals logged (n) | Excluded (ineligiblea) (n) | Not assessed (unable to access notes) (n) | Final number of referrals examined (n) |
|---|---|---|---|---|
| A | 71 | 8 | 1 | 63 |
| B | 26 | 11 | 1 | 14 |
| C | 236 | 92 | 40 | 104 |
| Total | 333 | 111 | 42 | 181 |
Results: assessment of the implementation process, including fidelity, reach and reception of training
Implementation strategies
Each site took a different approach to implementation (see Appendix 17). Hospitals A and C opted to roll out the intervention to the whole site, whereas Hospital B identified three clinical departments: haematology/oncology, respiratory and ED (excluding out-of-hospital cardiac arrests). Each hospital placed boxes containing study forms in prominent positions on referring wards and on the ICU. Implementation champions at all three sites used the presentations provided by the research team at the Grand Round and departmental meetings, modifying them to make them trust-specific. The CCOR implementation champions did not use these resources, preferring to explain the study at team meetings, individually at shift changeovers and/or by utilising a group message service to remind colleagues about the study.
Challenges and enablers to implementation at the sites
Time and reach
The implementation champions had 8 weeks to implement the intervention at their site, a relatively short time to make such a change. Hospital C was the largest hospital and, despite optimistic expectations, the implementation champions felt daunted by the task of reaching all referrers in the available time:
It was unrealistic to think we could do it in 8 weeks in a hospital of our size.
Implementation champion 1, hospital C
Conversely, at hospital A, the implementation champions thought that the tight time frame provided an incentive to achieve maximum reach by the deadline. Throughout the implementation period, the implementation champions at hospital A felt confident that they could embed the intervention in the time provided:
It’s a small hospital so easy to meet people in corridors and chat to them.
Implementation champion 1, hospital A
The challenge was to raise enthusiasm among not only their own ICU teams, but also among potential referrers, who could be anywhere throughout the hospital. The implementation champions hoped to reach their target groups quickly, but they struggled to secure a place in the schedule for the referring department’s meetings and seminars, or for the grand round:
Most of these programmes are set up months in advance . . . it takes time to break into their timetable of events.
Implementation champion 1, hospital B
Another problem was the number of locums working at the sites, who were unlikely to have received the training or may have been less motivated to engage with the intervention.
At observed training sessions, the implementation champions spent time explaining the rationale behind the study and the reason why decision-making about referral/admission to ICU needed to be improved. However, we did not observe how well they explained this during the one-to-one opportunistic training that all sites carried out. Given the limited time for each formal session, the implementation champions’ focus was on informing rather than explaining the concept:
The background is really important but if the window you have to explain it isn’t very big then you just need to get to the nuts and bolts of what’s needed and the background takes a back seat.
Implementation champion 1, hospital B
We provided implementation champions with an exercise using a hypothetical case (see Report Supplementary Material 14) to use as part of their educational resources, but none of the sites chose to use this. The reasons given were a lack of time, a preference for discussion over individual exercises, and that the forms were intuitive enough that the exercise was not needed. However, our interview data indicate that some doctors misunderstood the underlying purpose of the DSF, and a more interactive training session may have made this clearer.
Site B decided to introduce the forms for referrals in only three clinical areas to overcome the challenges of embedding a change in a larger hospital. In practice, however, the involvement of only three clinical areas meant that ICU doctors forgot about the forms between referrals from these areas.
Selection of implementation champions
The status and credibility of implementation champions in a trust was important to the success of embedding the intervention. Whereas the consultants at hospitals A and C felt comfortable in the champion role, the registrars at hospital B expressed some unease about their credibility as change agents:
It’s difficult to disagree with people who are our consultants and are signing our feedback form; there’s only so much opposition or contrasting opinion I can vocalise.
Implementation champion 2, hospital B
The registrars felt confident encouraging other registrars to use the form, but less so doing this with their consultant colleagues. They assumed that in consultant-to-consultant referrals all elements of the decision-making framework would have been covered without using the form as a prompt. They also experienced resistance from senior colleagues who did not see the need for a decision-support form. This was reflected in the low use of the forms by ICU consultants at hospital B:
The forms are perceived to be superfluous for consultant-to-consultant referrals because all of the information that needs collecting will have been got and the bits of the referral process that the intervention requires will have been done.
Implementation champion 2, hospital B
I probably wouldn’t ask a consultant to fill in a form because I would assume that everything we needed to discuss we had done.
Implementation champion 1, hospital B
At hospitals A and C, the CCOR nurses, led by the CCOR implementation champion, were able to speak to referring doctors early in the process of considering if a patient should be referred to the ICU doctors. They also raised the profile of the intervention on the wards using a potential referral as an opportunity to let all doctors on the ward know about the referral form. Hospital B did not have CCOR, so it relied solely on ICU doctors prompting referrers about the form when a referral was being made.
At hospital A, the clinical director of acute medicine took active steps to embed the intervention in the unit’s working processes by uploading the referral form to the acute medics’ intranet and adding a prompt to their admission document. Doctors working in acute medicine were therefore regularly prompted to consider the form and knew how to access it easily. Some referrers in acute medicine at hospital A used the form without being prompted by CCOR or ICU. We did not observe or hear of any other examples of implementation champions emerging in referring teams. One senior referring doctor at hospital B reflected that this was something they should have done:
We didn’t do very well . . . I’d have seen myself as one of the people who encouraged people to do this and I’m afraid I’ve failed!
Referring doctor 4, hospital B
Our findings suggest that identifying champions in the referring teams facilitates embedding of the intervention in practice. A new practice is less likely to be viewed with hostility if it is supported by others in the same department. Even at hospital C, where CCOR nurses championed the intervention with referring teams while providing support for treatment of critically ill patients on the wards, there was sometimes a perception that the ICU specialty (including CCOR) was imposing the intervention and, thus, questioning the referring teams’ competence.
However, the implementation champions also sometimes struggled to motivate their own team:
[ICU] were less enthusiastic and optimistic than I expected . . . If I can’t get my own team on board what chance do I have with other teams?
Implementation champion 2, hospital B
I expected more resistance from [referrers] . . . it’s the ICU doctors I can’t understand not filling in the forms.
Implementation champion 3, hospital C
Having an implementation champion who frequently worked night shifts also helped at hospital A as they were able to encourage form completion overnight when compliance was likely to be worse because there were fewer staff.
Structure and process facilitators
Mutual support for the implementation champions and a system for reminding implementation champions, and, in turn, doctors, about the intervention appeared to be important facilitators of implementation. The CCOR team, research nurses and ICU doctors at hospital A shared an office and had regular conversations about the study. The research nurse checked the referral log daily to identify which clinical areas were not using referral forms so that these could be targeted to encourage uptake. Similarly, the CCOR implementation champions at hospital C monitored the ICU team’s form use, but only when they were on shift.
By contrast, no one in hospital B proactively monitored referral log and form use, and so there were fewer opportunities to remind both ICU and referring doctors to complete the forms. This may account for the recorded drop-off in referrals over time in hospital B, and also meant that the referral log was probably less accurate than at the other sites. The referral log appeared to be an important element of implementation by acting as a daily reminder to doctors and implementation champions.
Existing professional relationships and hierarchies
Although the main responsibility for driving implementation falls to the implementation champions, the success of implementation also depends on other professionals in the hospital engaging with, supporting and promoting the intervention. However, this can be challenging when an intervention crosses professional boundaries. At hospitals A and C, the CCOR team were all involved in prompting referring teams to use the form whenever they were asked to review a patient on the ward. At hospital A this appeared to work well, with only one example of a referring doctor refusing to complete a referral form when asked to do so by the CCOR nurse. However, at hospital C, the CCOR team experienced more resistance from referring teams where the suggestion of using the form appeared to trigger a more general expression of frustration and antagonism toward CCOR staff:
It’s awful the way we’re spoken to, when they’re not getting the answers they want or things like that.
Implementation champion 3 CCOR nurse, hospital C
Some referrers appeared to incorrectly regard the referral forms as a substitute for CCOR involvement or as somehow making the CCOR nurses redundant:
Questioning ‘Well what’s your ability then? I thought you were the Outreach person I thought you were supposed to liaise for us and now you’re asking us to fill a form in’ and creating quite difficult situations.
CCOR nurse 2, hospital C
I think when we’re asking them to fill a form in they’re like ‘Well we’ve referred to you’ . . . The ward see referring to us as a referral to ICU.
Implementation champion 3, CCOR nurse, hospital C
The experience of encountering stiff resistance from consultants was not evident from the interview data at hospital A, although we did not conduct a group interview with CCOR at this site. It may be that the smaller team at hospital A facilitated a more collaborative environment that was less threatened by the introduction of a new approach to established referral practices.
Consistency of form usage
The extent of form use varied between sites, with uptake at hospital A higher than at the other two hospitals (see Appendix 18, Figures 21–35 and Tables 38–46, for further analysis). Forms were used in 44.4% (28/63) of examined referrals in hospital A. Among examined referrals, hospital B achieved a form use rate of 21.4% (3/14), and hospital C achieved a rate of 19.2% (20/104).
Overall, 28.2% of referrals included a referral and/or decision form. Documentation of the ICU referral and/or review was frequently absent from the patient’s record and, if present, it often did not include the name/role of the referrer or ICU doctor. When these could be found in the notes, the details of the doctor (e.g. name and role) were frequently absent (see Appendix 19).
In total, 45 completed referral forms and 36 decision forms were identified across all three sites, with both forms being used in 30 cases. The referral forms were used more often (n = 45, 25%) than the decision forms (n = 36, 20%). If a referral form was present, there was a higher chance that a decision form would be present (16.6% vs. 3.3%; p < 0.001).
Factors associated with form use
Age was the only factor for which there was a statistically significant association with use of the referral and decision forms. The mean age of patients when a referral form was used was 70.4 years, compared with 60.4 years when it was not (p < 0.001 t-test). The mean age of patients when a decision form was used was 71.6 years, compared with 60.7 years when it was not (p < 0.001 t-test).
Both forms were more likely to be used during the day (08.00–20.00) than during the night (referral forms, p = 0.3289; decision forms, p = 0.1129) and referral forms were used more often during the week than at the weekend (p = 0.5018), although these findings were not statistically significant.
From the available data it appears that registrars used referral forms more often than consultants. When we could identify that a registrar referred, 66.7% (n = 26) used a form, whereas when a consultant made a referral, 38.5% (n = 5) used a form. By contrast, ICU consultants were more likely to use the decision form than ICU registrars [44.2% (n = 19) of consultant reviews compared with 28.9% (n = 11) of registrar reviews]. However, because of the large number of missing data, these findings are presented cautiously; in 66.9% and 54.7% of cases, respectively, no details about referrer or ICU reviewer role could be identified.
Patient sex and ICU bed availability did not have significant associations with form use. We were unable to assess whether or not patient condition had an impact on form use because we did not collect these data.
Form completion
We assessed the extent to which referral and decision-support forms were completed. In general, there were good completion rates for all sections of the forms, although some were completed more frequently than others (Table 18).
| Form section | Completed (n) | Completed (%) |
|---|---|---|
| Referral form sections | ||
| Situation (reason for referral) | 45 | 100 |
| Background | 45 | 100 |
| Patient’s values and wishes | 42 | 93.3 |
| Source of information regarding patient’s values and wishes | 4 | 8.9 |
| Discussed with referring team consultant | 31 | 68.9 |
| Recommendation | ||
| To obtain a review to consider admission | 33 | 73.3 |
| To obtain a review but not necessarily to admit | 7 | 15.6 |
| For assistance with a specific therapy | 0 | 0 |
| To obtain a review to plan care in the event of deterioration | 3 | 6.7 |
| Other | 0 | 0 |
| No box ticked | 2 | 4.4 |
| Decision-support form sections | ||
| Evidence: clinical | 35 | 97.2 |
| Evidence: ability to recover | 35 | 97.2 |
| Evidence: patient values and wishes | 34 | 94.4 |
| Source of information regarding patient’s values and wishes | 9 | 25 |
| Balancing: benefits | 31 | 86.1 |
| Balancing: burdens | 25 | 69.4 |
| Recommended treatment | 33 | 91.5 |
| Can care be delivered outside ICU? | ||
| Only in ICU | 14 | 38.9 |
| Outside ICU | 12 | 33.3 |
| Outside ICU but no resources | 0 | 0 |
| No box ticked | 10 | 27.8 |
| Ongoing care/review | ||
| Admit to ICU | 11 | 30.6 |
| Ongoing review | 4 | 11.1 |
| Review if anything changes | 5 | 13.9 |
| No box ticked | 16 | 44.4 |
| Patient contributed | 16 | 44.4 |
| Relative contributed | 10 | 27.8 |
| Nature of relative involvement | 8 | 22.2 |
Although the ‘patient values and wishes’ section was mostly completed, the ‘source of this information’ prompt was often not completed. Among the decision forms, 27.8% (n = 10/36) did not record where the recommended treatment could be delivered, and 44.4% (n = 16/36) did not record a decision about ongoing review. The section on the patient’s contribution to the decision was completed on 44.4% (n = 16/36) of forms, and the relative’s contribution on 27.7% (n = 10/36). A referring doctor’s name was documented on 30.5% (n = 11/36) of decision forms, but referral doctors rarely signed a decision form (8.33%, n = 3/36).
Acceptability of intervention to doctors
Ease of use
Those doctors who used the form generally felt that it was easy to use.
It wasn’t difficult . . . If we do this for all the patients we send in I don’t have an issue with that.
Referring doctor 6, hospital C, consultant, used form
The writing on my part is actually relatively straightforward.
ICU doctor 1, hospital A, registrar, used form
Some doctors described how use or knowledge of the framework and forms informed their decision-making even in cases where the form was not readily available.
Did it prompt you to talk to the patient?
No I already had because I knew what was on the form. So it had prompted me . . . I already knew what was on the form, what I needed to fill in.
Consultant, used form
Difficulties encountered in using the form were largely in relation to the ‘capacity to recover’ and ‘benefits and burdens’ sections. Identifying and articulating the benefits and, particularly, the burdens of care, was challenging for ICU doctors:
There are risks of developing all sorts of complications in critical care. Trying to predict what they’re going to be is quite difficult.
ICU doctor 3, hospital B, registrar, never used form
They’re quite hard to write how you would, how would you write this down? . . . The burdens are quite hard to articulate, although we know they’re there and we know they’re profound.
ICU doctor 2, hospital C, registrar, used form
The quantitative data also supported this finding. The benefits were documented more frequently (86.1%, n = 31) than the burdens (69.4%, n = 25).
Concerns about workload
A common concern about using the forms was the creation of extra work and duplication of effort in documenting information twice (in the patient record and on the form), and the effect on the doctor’s time for their other obligations both to the patient in question and to other patients:
It needs to not to be a replication of what we need to document in the notes.
Referring doctor 6, hospital A, acute medical consultant, used form
Just another form really . . . Feeling overwhelmed with paperwork.
Referring doctor 3, hospital B, haematology/oncology consultant, never used form
The intention was that the referral and decision-support forms would form part of the patient record and hence information would not need to be duplicated; however, some doctors recorded it in both because the form was not trust-approved documentation. Our implementation champions in post-study debrief meetings suggested that successful ongoing implementation would require the forms to be an integral part of the trust’s electronic patient record system to demonstrate trust approval and facilitate ease of use. The ICU doctors interviewed felt that it would be easier to direct referrers to an online form than to a box on a ward, and the electronic system could prompt the reviewer to use the decision form:
If you come, sort of come in as a critical care person taking the referral, [and] the form comes up then it would be easier to fill in.
ICU doctor 8, hospital C, consultant, used form
Linked to concerns over duplication was the view of many doctors that they were already doing what the forms were prompting them to do:
[It’s] pretty similar to what we already do just on a piece of paper.
Referring doctor 3, hospital B, consultant, never used form
I think it probably err is duplication of the full assessment that we write in the notes anyway.
ICU doctor 5, hospital B, registrar, never used form
Our sample may have been biased in favour of doctors who already document decisions well, given that those in our sample were identified through patient records. The quantitative data showed that doctors, particularly at hospitals B and C, often did not document their referral or ICU reviews in the patient records before the patient’s admission to ICU, suggesting that doctors had an inaccurate perception of how reliably they recorded referrals and reviews in the patient records.
A further reason why completing the forms was seen as extra work was that the forms, particularly referral forms, were introduced or used at an inappropriate point in the decision-making process. The referring team were usually prompted to use the form by ICU doctors or CCOR nurses following an initial referral of a patient to ICU, and thus the forms were introduced after a decision had been made to refer rather than being used as part of the process of clarifying the reasons for a referral:
I’d already had to ring up and . . . hand over everything on the phone and it was all documented in the notes. I just felt like I was kind of copying what I’d already done.
Referring doctor 5, hospital A, registrar, used form
I think if it was to be used long-term then it would be better on the wards because then they would be thinking to do it before calling us.
CCOR nurse 2, hospital C
Perceived threat to clinical authority and expertise
Some doctors saw the introduction of referral or decision-support forms as a question about their clinical judgement by either implying that they needed assistance to make a decision or requiring them to provide written evidence of their thought processes:
One could argue it’s intensive care saying: ‘You guys don’t know what you’re talking about, we’ll make the decision for you’.
Referring doctor 7, hospital C, consultant, used form
I don’t need it to help me make a decision because like otherwise what have I been doing for the last 10 years?
ICU doctor 1, hospital B, consultant, never used form
It’s almost saying y’know, ‘Here’s this thing that you’ve been doing for 20 years, just write down how you do it please?’
ICU doctor 2, hospital B, consultant, never used form
This sense of having their expertise questioned was described only by consultants, and, with one exception, by those who had never used the form.
The particular context of the emergency department
At all sites, uptake of the forms in ED was very low. Concerns about the lack of time to complete paperwork, expressed by many participants, were particularly evident among ED physicians:
We are too busy to complete forms, it’s as simple as that.
Referring doctor 4, hospital A, ED consultant, never used form
It’s basically regurgitating my notes that I’ve already done . . . So I’m not saying it’s not good but it’s not practical.
Referring doctor 4, Hospital C, Emergency medicine registrar, never used form
Another feature of the ED context described by participants is that a request for ICU support is one element in a complex web of multiple decisions being made in an emergency situation, something that the ICU referral form could not capture fully. This is in contrast to referrals for patients on a ward where the decision is usually more discrete:
This is only pertinent to referring them to ICU. There is a whole load of other stuff that needs to be in those notes because . . . our notes involve multiple focuses . . . it’s not just ICU we interact with . . . we’re such a junction box.
Referring doctor 5, Hospital B, ED consultant, used form
The complexity and urgency of a clinical situation in which doctors are dealing with a severely ill patient in the ED means that the decision-making process is much more fluid, with doctors taking on new information and revising their treatment plans often within minutes rather than hours. Communication is rapid and verbal rather than written, and formal documentation is likely to be done retrospectively once the patient is stable:
If they have anaphylaxis or GCS [Glasgow Coma Scale] of 3 with head injury and things how am I meant to know all of this background and things? They just collapsed in front of A&E [accident and emergency] and I have no medical history and think ‘I want you to be here to tube for me so that you look after the patient until I collate information and look at [electronic records system]’.
Referring doctor 4, hospital C, ED registrar, never used form
I’m constantly scoping for new information if I need to update and revise my decision and that’s, y’know that’s the whole modus operandi we have to be in.
Referring doctor 5, hospital B, ED consultant, used form
Intensive care unit doctors were also reluctant to prompt ED doctors to complete a form when making a referral:
What would’ve happened if you’d gone in and asked for their form?
[Laughs] They would’ve said ‘Fine you, you know you be the one to come and do this guy’s breathing for him and I’ll fill out a form’. 10 minutes later . . . and this guy arrests and dies in front of me then I’m, y’know he’s going to be like ‘Well . . .?’ So . . . it’s not appropriate at all.
ICU registrar, never used form
However, not all clinical situations in the ED are so time-critical that a more considered assessment prior to ICU referral is not possible, and, once the patient is stabilised in the ED, the decision-support form could be helpful in structuring a decision about whether or not admission to ICU is the best next step. As one ICU registrar commented:
[ED is] like a busy bazaar so it might be, sort of particularly adds a little bit of resting quiet normality to an otherwise slightly potentially mad referral with a lot of noise and a lot of things going on in the background.
ICU doctor 6, hospital B, registrar, used form
Results: impact on decision-making
Improving articulation and communication of referrals and decisions
Doctors who used the form often reported that it helped them to clearly set out the rationale for their decision and provided reassurance that their decision was sound:
It makes us as the referrers focus on exactly why we’re referring that patient.
Referring doctor 3, hospital A, acute medical consultant, used form
It just cemented and convinced me that actually I was doing the right thing . . . It gives you just a bit more err support in your decision-making so you can show . . . this is what I felt at the time.
ICU doctor 6, hospital B, registrar, used form
They also found that it helped with communication between doctors and teams, and promoted mutual understanding of decisions:
It helped me frame the conversation I was going to have with the ITU registrar in terms of this patient, OK she’s possibly borderline, she’s elderly, got some other comorbidities, not your kind of clear-cut ITU case but she’s functioning independently and her family say she’s got a good quality of life and I’ve got some evidence there to say that actually she should be, she’s probably got a reserve that could be managed in ITU.
Referring doctor 2, hospital A, registrar, used form
I guess it’s just to have better communication with the . . . patient’s primary team . . . to understand what our thought process is and why we sometimes would be a bit reluctant to admit a patient.
ICU doctor 4, hospital B, registrar, used form
Doctors thought that the forms were particularly useful for borderline cases where the arguments for and against admission to ICU were more nuanced and clear articulation of the reasoning was essential. For more clear-cut decisions, they saw the forms more as a checklist to record that everything had been considered:
I find this most useful for the kind of grey patients . . . the ones where the decision-making might be quite nuanced . . . The ones that it’s more obvious to me they should be going to ITU I’d see it as a . . . tick-box exercise.
Referring doctor 2, hospital A, registrar, used form
None of that’s relevant because they’re 21 and they’ve taken an overdose so y’know, but then for a 90-year-old from a nursing home that particular box [benefits and burdens] becomes much more crucial.
ICU doctor 6, hospital A, consultant, used form
Our quantitative data showed that older patient age was associated with higher use of both referral and decision forms and that decision forms were more likely to be used when patients had been in hospital longer than 2 days (p = 0.6994). Older patients are more likely to have complex comorbidities, and if patients deteriorate following admission then this may also suggest more complex situations that would support the interviewees’ views that they were more likely to use the forms here.
Using forms may facilitate the articulation and communication of relevant information and reasoning, but it does not guarantee that this will occur:
I’ve just looked at the e-records, this patient has metastatic cancer. He has got a colostomy, he’s got severe kyphoscoliosis, he had severe obstructive lung disease and [referrer] called him ‘fit and well’ . . . There are people who are putting the forms like that to me. And I’ve found myself seething in anger.
ICU doctor 2, hospital A, consultant, used form
The fact that this patient is err paranoid and refusing treatment isn’t even mentioned [on the completed referral form] . . . and that’s the main problem with this patient.
ICU doctor B, hospital C, consultant, used form
Failing to provide information or providing inaccurate information on a referral form might provoke more irritation than simply failing to mention it in a telephone call or on an entry in the medical record.
Considering patient wishes and values
Involving patients and/or their families in decisions about their care is both a legal and a moral requirement and a central tenet of professional guidance. 180 Data from our ethnographic study suggested that this was uncommon in the context of decisions regarding admission to ICU. Several referring doctor participants noted that the forms prompted them to specifically consider and document the views of the patient, something that they would not usually have done in this context:
I think the most important bit was actually speaking to the patient about their wishes. That I wouldn’t automatically think about.
Referring doctor 6, hospital B, registrar, used form
This form definitely made me write it in this case anyway . . . It’s a good prompt and something that you should think about documenting every time even if you’re not doing this form.
ICU doctor 7, hospital C, registrar, used form
Intensive care unit doctors often hoped or found that the form would encourage referring doctors to think about this earlier, and this was seen as a major benefit of the referral forms:
Trying to get the referring team in particular to, think more carefully and more sensibly about their patients and what they want for them and what’s in their best interests, and them to document that in advance of the patient falling in a heap would be very useful . . . I can think of a patient actually downstairs whereby the form prompted them to go and have that discussion. And in fact that patient didn’t come to ICU.
ICU doctor 3, hospital C, consultant, used form
However, a few participants expressed doubt about the usefulness of seeking the patient’s views about admission to ICU, given the time constraints in emergency situations and the difficulty for patients to have a sufficient understanding of both their condition and the nature of intensive care treatment:
Whether the patient can express coherently and with knowledge their own wishes given the complexity of their cases.
Referring doctor 2, hospital B, consultant, never used form
You [patient] don’t really understand . . . You don’t know what the pain and suffering of intensive care is.
ICU doctor 2, hospital C, registrar, used form
If someone is peri-arrest you can’t ask them.
Referring doctor 2, hospital C, registrar, never used form
The patient and family information leaflets were not implemented successfully at any of the sites, and, in general, participants were unaware of them. When asked to comment on them, participants thought that the concept was useful but noted practical difficulties around when to provide them to patients and their family:
Seeing it [ICU] can be quite scary and an information leaflet kind of warning them of what to expect is, is helpful.
Referring doctor 4, hospital A, registrar, used form
I don’t think he would have read it to be honest in that particular situation . . . I would probably prefer to use them in the less acute situation.
Referring doctor 6, hospital B, registrar, used form
I think it’s perfectly appropriate but I don’t think it’s going to work in the real world.
Referring doctor 6, hospital A, consultant, used form
The view that providing patient and family information leaflets would distress or overwhelm patients and family was reiterated in our debrief discussions with the implementation champions at all sites. A further practical problem, also noted with the referral forms, related to the timing of the use of the leaflets. We had envisaged these as supporting discussion with patients and/or their family about a decision to refer to ICU. However, as the prompt to use these usually occurred once a referral had been initiated, the usefulness of the leaflets was called into question.
These data indicate that our intervention prompted involvement of patients and family, and the documentation of these discussions, but revealed that doctors often struggle to make these conversations meaningful.
Improving documentation
Clear documentation of clinical decisions in a patient’s record is considered a key element of good practice,180 but evidence from our review of patient records shows that this does not always occur in the context of ICU referrals and reviews. Participants reflected that the structured documentation prompted by the forms improved transparency and accountability of decision-making:
Introducing an element of . . . accountability for that referral conversation is really, really useful.
Referring doctor 1, hospital B, registrar, never used form
It’s a good way of making sure that everyone documents the same . . . type of information.
ICU doctor 3, hospital A, registrar, used form
Although some doctors felt that they already documented their decision-making, most agreed that the form produced better documentation, particularly of the patient’s values and wishes, and of the benefits and burdens of ICU. They commented that documentation varied depending on whether or not a patient was to be admitted to ICU:
If I know I’m going to accept the patient I won’t justify the decision to accept in the notes because I’ve accepted them. It’s if I have to refuse the patient and then the documentation is different.
ICU doctor 2, hospital C, registrar, used form
If you go to a ward to see someone and decide to admit them sometimes there’s very little written in the notes on the basis that it’s all going to get repeated . . . [in] the clerking admission notes.
ICU doctor 6, hospital C, consultant, used form
However, our quantitative data do not support this suggestion. The majority of cases where the decision form was used were admitted (25/36, 69.4% of cases), which reflects the numbers of cases admitted overall (71.5%).
Even doctors who did not see a need for the form to aid their decision-making acknowledged that the improved documentation facilitated transparency, which could be beneficial for the doctor as well as the patient:
This is what I’ve done my whole life . . . it’s just a written-down version of what I do every day so . . . probably the most helpful thing for me is to get me to write it down rather than help me make the decision . . . [It] might save me from going to jail because I will have written things down in a more thorough format when anything goes wrong.
ICU doctor 1, hospital B, consultant, never used form
Summary
The uptake and use of the forms varied across sites, reflecting different approaches to and environments for implementation. Overall, the timescale was too short to establish sustained engagement, particularly in the larger hospitals, and uptake and engagement may have been greater if trusts had been committed to longer-term use of the forms. A key difficulty, related to the short implementation time, was the limited opportunity to educate doctors about the underlying conceptual framework and purpose of the forms before they were asked to use them. Facilitators to implementation included existing good relationships between staff within and between teams; enthusiasm and engagement from key people within those teams; opportunities for education and training; and the relative position of implementation champions within trusts.
Doctors who used the forms were generally supportive of them, with the majority of negative comments coming from doctors who had never used the form. It is not possible to say if these doctors would have had a more positive attitude to the forms had they used them, or whether their criticisms of the forms was a reason for their non-use. The key concerns expressed by doctors interviewed were duplication of documentation and increased workload. Having an electronic version of the form was identified as key to facilitating widespread embedding of the intervention. The referral forms were generally not considered acceptable in the context of ED as a result of lack of time and information. ICU doctors had particular difficulty explicitly articulating the benefits and burdens of intensive care. An important identified benefit was improving the understanding between referring teams and ICU doctors about each other’s reasoning in a particular case, and the prompt to consult with the patient and/or their family. The DSI was largely perceived as resulting in better documentation.
Strengths and limitations of the implementation feasibility study
Our implementation sites reflected the range of ICU settings in the NHS. The doctors interviewed were diverse in terms of specialty, grade, and use of the intervention documentation. Interviews focused on specific cases rather than hypothetical scenarios or generalisations. As the researcher was a non-clinician there was less risk of implicit assumptions about clinical practice being shared between researcher and interviewees. This would reduce the potential for lack of clarity of meaning in the data.
The 8-week implementation period was too short for full implementation. Retrieving clinical records during the time frame of the evaluation was challenging. Many referrers did not write their names clearly in the patient record so we were unable to identify them to request an interview. Although many challenges of its use were raised by participants, it is possible that those willing to take part in the interviews were more positive about the intervention. Poor documentation in patient records resulted in missing data so some of our results should be interpreted with caution. We were nevertheless able to identify differences in form use that were supported and complemented by the qualitative findings. The limited, and variable, uptake of the intervention across sites and the observed and reported challenges of implementing a system-wide change in practice make it difficult to come to a definite conclusion regarding implementation feasibility.
Although we observed formal training given by the implementation champions, we did not observe informal educational opportunities and nor did we observe referrals as they happened. We did not involve patients or their families in the feasibility study as participants, and so their voices are missing from the data. If the intervention is implemented more widely, future research will need to explore the process from the perspective of patients and families.
Chapter 8 Development and testing of a tool to evaluate ethical decision-making
Introduction
The aim of our DSI was to support ethically justified, person-centred, evidence-based decision-making about referral and admission to intensive care. Data from the intervention implementation feasibility study suggest that the intervention had an impact on ICU and referring doctors’ decision-making, particularly around the involvement of the patient and articulation of reasons for their decisions. Although this is encouraging, more rigorous evaluation is required of whether or not, and to what extent, this is reflected in improved quality of the decision-making process in practice. Such an evaluation is likely to be multifaceted. As our project has demonstrated, these decisions are often complex and context dependent, and involve a high degree of uncertainty. There will be reasonable differences of opinion about whether or not an individual decision was the right decision. The importance of process in ethical decision-making was identified in our ethnographic study and informed our choice of accountability for reasonableness as the ethical framework for our intervention. 181
Therefore, a key element of any future evaluation would need to focus on the decision-making process itself in addition to any specified secondary outcomes. Prior to this project, we were not aware of any standard instruments for evaluating the quality of ethical decision-making in clinical practice, and therefore we conducted a systematic literature review to identify any instrument that could be used to evaluate the effectiveness of our intervention with regard to ethical decision-making, or to inform he development of an appropriate instrument in the context of this study.
Systematic literature review
Our research question for this review was ‘What measures or models of evaluation have been used to assess the impact of interventions to improve ethical decision-making in acute medicine and emergency care?’ Early discussions within the investigator team clarified that limiting our review to the context of acute and emergency care was too restrictive, so our search strategy was widened to include evaluations of interventions to improve ethical decision-making in clinical care (PROSPERO 2016: CRD42016039054).
Method
We searched MEDLINE, EMBASE, PsycINFO and Web of Science (Science Citation Index and Social Sciences Citation Index) via Ovid using specific MeSH terms. We combined MeSH terms referring to clinical ethics, and included ethical decision-making; ethical decision; ethical value; moral deliberation; moral case deliberation; moral value; ethical deliberation; ethics consultation; ethics support; ethics education; ethics framework; ethical framework; evaluation; and outcome. We then combined the search results from all of the databases and removed duplicate articles. In addition, we searched grey literature using the same key terms. Our initial search was run on 21 March 2016. We repeated the search on 21 March 2018 to capture any studies published since that date (see Appendix 20, Tables 48–52, for search strategies).
We included empirical studies that evaluated interventions aimed at improving ethical decision-making in clinical care. Articles included also had to describe measures or tools that evaluated one or more components of the intervention(s). The combined searches yielded 4037 papers after deduplication (400 from the updated search in 2018) (Figure 16). Two primary reviewers (AI and AS) independently screened all included papers on title and abstract and identified 79 papers for full-text review. All full-text papers were read by both reviewers, and disagreement about final inclusion was resolved after discussion or by a third reviewer (KR). During the full-text review process, another three papers were identified from a bibliography search of included papers. Fourteen papers (12 studies)182–193–195 were included for data abstraction. All included studies were assessed for methodological quality of the evaluation tool development using an adapted version of the COSMIN (COnsensus-based Standards for the selection of health status Measurement INstruments) checklist (see Appendix 21, Table 53). 196
FIGURE 16.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for evaluation tool systematic review.
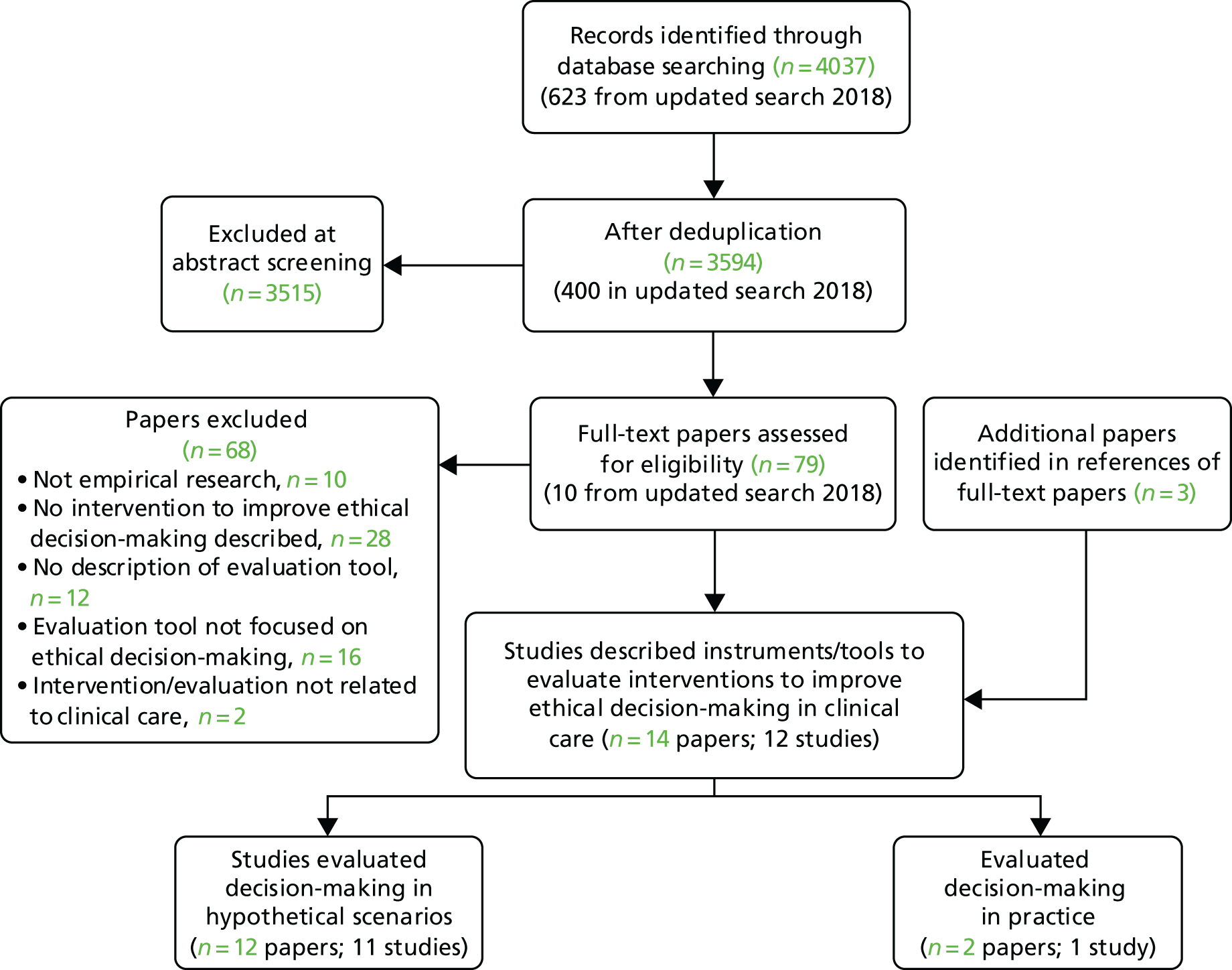
Results
Overall, the quality of the identified studies was variable, with some studies reporting detailed validity and reliability testing and others not describing any testing of the instrument. All but one of the studies described either the development of an evaluation tool or the use of an existing tool to assess educational interventions to improve ethical decision-making in medical or nursing students. The educational interventions were diverse and included a general medical curriculum with some lectures and discussion relating to ethics;182 an ethics course as part of a medical or nursing curriculum;183–186 an integrated ethics thread in a medical curriculum;187,188 a specific educational tool for teaching ethics in a nursing curriculum (guided design189); and a general medical or nursing undergraduate curriculum. 190–193,197 Of these 11 studies, seven182–189,192 described evaluation tools based on written assessments, two190,191 described an objective structured clinical examination (OSCE) station, and one193 described a combination of performance-based assessment and a standardised patient and written assessment of the same case.
Of the 10 studies describing written evaluation tools, two used the Judging About Nursing Decisions Test,198 which assesses nurses’ ability to judge which course of action in a series of scenarios most closely accords with the American Nurses Association’s code of ethics, and how likely the participant is to follow it. 189,197 One study182 modified the Defining Issues Test (DIT) and combined it with another instrument (the Problem Identification Test). Both of these tests were questionnaire surveys based on vignettes in which participants were asked to list the ethical issues in the vignette (Problem Identification Test) and choose the most suitable action from a list (DIT). The remaining seven studies described the development of new instruments. Six used case vignettes, although the number of cases varied. Three instruments183–185 required students to state what they would do in each case vignette and justify their reasons, while one instrument asked students to select an action from a prespecified list for each vignette and then to justify their chosen action. 187,188,192 The final instrument193 combined written assessment with a performance based assessment of ethical decision-making. One further instrument,186 the nursing ethical decision-making ability scale, was not clearly enough described in the study to establish whether or not case vignettes were used.
Among the performance-based assessments, the two studies190,191 evaluating OSCE stations found that inter-rater reliability was acceptable [intraclass correlation coefficient (ICC) of 0.3–0.89 across six stations in one study and 0.44–0.8 across four in the other], but internal consistency was low. The authors concluded that the OSCE assessment was not a feasible standalone method for the evaluation of clinical ethics. In the third performance-based assessment study,193 no report was given of the reliability of the assessment tool. A summary of the studies included in the review can be found in Appendix 21.
Only one study,194 which was identified in our 2018 updated search, described an instrument for evaluating ethical decision-making in actual clinical practice rather than using hypothetical scenarios. This instrument was developed to evaluate clinical ethics case consultation, which provides advice to clinicians making decisions about patient care rather than ethical decision-making by the clinician responsible for the patient’s care. However, in evaluating ethical reasoning and decision-making in actual clinical cases, it is the most relevant instrument for our project of all of the studies/tools identified. 194 The instrument [Ethics Consultation Quality Assessment Tool (ECQAT)] is used to evaluate written records of case consultations, which then form part of the patient clinical record. It is based on a holistic assessment model covering four key elements: identifying the ethics question, eliciting consultation-specific information, ethical analysis, and making practical recommendations. Inter-rater reliability was 43% for the individual key element scores and 74% for the overall holistic assessment score.
We found no instruments that were designed to evaluate interventions to improve ethical decision-making by clinicians that assessed the quality of their ethical decision-making in actual clinical practice. The ECQAT is a potential model for developing such an instrument, but this study had not been published prior to our initial search.
Development of the evaluation tool
Introduction
In the absence of an existing appropriate instrument to evaluate ethical decision-making in clinical practice, we developed an evaluation tool that was specific to the decision about admission to ICU and piloted it during the intervention implementation feasibility study. In a series of meetings, three members of the study team, Frances Griffiths (social scientist and general practitioner), Anne Slowther (ethicist and general practitioner) and Chris Bassford (intensive care consultant), agreed the broad framework of the evaluation tool. During these discussions we drew on a range of sources to inform our thinking:
-
an initial analysis of our ethnographic study data
-
anonymised extracts of 20 records of patients who had been admitted or referred to ICU from two of the hospitals participating in the ethnographic study
-
the findings of our systematic review of factors affecting decisions to admit to ICU
-
our concurrent development of a DSI based on our analysis of what would constitute a good decision-making process
-
existing ethical frameworks for decision-making.
It was agreed that the evaluation tool would be applied to the clinical record of a decision. This model of evaluation can be criticised because of the potential disparity between the actual process of decision-making and what is recorded in the patient notes. However, an evaluation tool that requires direct observation of the decision-making process would not be feasible to implement in a real-world situation. In addition, there is a more substantive ethical justification for this approach. If a requirement of ethical decision-making in this context is transparency, then it can be argued that having a clear record of the decision-making process – including the reasoning behind the decision – is itself an ethical criterion in any evaluation of the decision.
Key messages from our qualitative study included the importance of communication both between clinicians and with the patient and their family, clarity of process and responsibility for care of the patient, the absence of information about patients’ wishes and values, and a lack of explicit ethical reasoning in terms of weighing relevant factors in making a decision. Considering existing ethical frameworks for potential models, we selected AFR because of its focus on process, transparency, and review in the light of new evidence/information. 181 We considered that frameworks based only on ethical principles, for example ‘the four principles of biomedical ethics’, offered too narrow a model to capture the procedural elements. 155 However, the AFR framework itself does not specify the relevant reasons or factors that should be taken into account for specific decisions, and for this element of our evaluation tool we drew on our systematic review of factors, our ethnographic study data and our DSF.
Method
We first identified the dimensions of decision-making that we wanted to capture in an evaluation tool: ethics and values, transparency, consistency of process and opportunity for review. Consistency of process can be evaluated by looking at performance across decisions at any one time point and is not a substantive element of the evaluation tool itself. Within each dimension, we identified elements that should be included; for example, within the ethics and values dimension we would expect to see articulation of relevant factors (including clinical benefits and harms, and patient’s wishes), evidence to support these factors and a reasoned line of argument taking the factors into account.
We next interrogated anonymised patient records of decisions to admit (or not) a patient to ICU from two hospitals participating in the ethnographic study. Research nurses at each hospital selected 10 consecutive admissions to ICU, and one hospital, which had a referral logging system in place, also identified five consecutive cases in which a decision had been made not to admit the patient. For each record, the section relating to 24 hours before and 24 hours after a referral to ICU had been made was identified and photocopied. All patient and clinician identifiers were then redacted and the redacted copy was scanned and sent to the study team via encrypted nhs.net mail. The interrogation of the anonymised records included taking note of how our identified dimensions were recorded. This initial interrogation resulted in a draft evaluation tool with four sections:
-
factors that are required to inform ethical reasoning
-
evidence of ethical reasoning
-
communication and review of decision
-
factors that should not be present in decision-making without mitigation or explanation (e.g. age, DNACPR status).
Within these sections, further subdivisions were identified; for example:
-
factors that are required to inform ethical reasoning –
-
evidence of need for intensive care
-
evidence of capacity to recover
-
evidence of what is important to the patient.
-
The iterative process of interrogating the notes using the current version of the tool, refining the criteria and developing/refining scoring levels continued for a further four cycles. Concurrently, a non-clinical research fellow (SR) trialled the tool with a sample of records and noted areas that required clarification for a non-clinical scorer and developed a glossary of terms and a user guide of frequently asked questions (see Report Supplementary Materials 19 and 20). The penultimate version of the tool was given to four members of the research team from different clinical specialties (GP, intensive care; JD, general practice; ZF, acute medicine; and SQ, CCOR), who were each asked to score two sets of notes and provide comments on the scoring system and the tool in general. Each set of notes was scored by two members and the resulting scores were compared and discussed, along with the scorer’s feedback, at a meeting of all scorers and the core study team. This version was also sent to the principal investigators at the six hospitals that took part in our ethnographic study to seek their comments as a further check of face validity. Further changes were made to the tool to clarify the scoring criteria, and a finalised version of the tool was drafted (see Appendix 22). The key elements of the tool are presented in Table 19.
| Question number | Question | Possible score |
|---|---|---|
| Evidence of need (or not) for intensive care (descriptive evidence of system failure) | ||
| System | ||
| 1.1 | Cardiovascular | 0/1 |
| 1.2 | Respiratory | 0/1 |
| 1.3 | Renal | 0/1 |
| 1.4 | Neurology | 0/1 |
| Interpretation of evidence of system failure | ||
| 2.1 | Interpretation of acute clinical situation | 0/1 |
| 2.2 | Interpretation of acute clinical situation 2 | 0/1 |
| Evidence of capacity to recover | ||
| 3 | Description of factors that might affect capacity to recover | 0–2 |
| 4 | Interpretation of capacity to recover | 0–2 |
| Evidence of what is important to patient | ||
| 5 | Description of attempts to get data about patient wishes | 0–2 |
| 6 | Description of information about patient wishes | 0–2 or N/A |
| Ethical reasoning | ||
| 7 | Balancing of benefits and burdens of intensive care treatments | 0–2 |
| Reference to the factors in the balancing | ||
| 8 | Acute physiology/system failure (question 1) | 0/1 |
| 9 | Capacity to recover (question 2) | 0/1 |
| 10 | Patient wishes (question 3) | 0/1 |
| 11 | Link of balancing to specific treatment | 0/1 |
| Communication | ||
| 12 | Was the decision communicated to medical staff? | 0–2 |
| 13 | Was the decision communicated to nursing staff? | 0–2 |
| 14 | Was the decision communicated to family? | 0–2 or N/A |
| 15 | Was the decision communicated to patient? | 0–2 or N/A |
| Review | ||
| 16.1 | Need for review documented | 0/1 |
| 16.2 | Person or team needed to review specified | 0/1 |
| 16.3 | Circumstances for review specified | 0/1 |
| Red flag alert: factors that should not be present in decision-making without mitigation or other explanation | ||
| 17.1 | Advanced age | 0/1 |
| 17.2 | Quality of life | 0/1 |
| 17.3 | Functional status | 0/1 |
| 17.4 | Previous professional knowledge of patient | 0/1 |
| 17.5 | Presence of DNACPR order | 0/1 |
Testing of the tool
The tool was used to score a sample of patient records in each of the hospitals in the intervention implementation feasibility study. Each hospital kept a log of all referrals to ICU during the study. At each hospital, research nurses identified 20 consecutive records from eligible referrals (see Chapter 7 for eligibility criteria) at the beginning of the implementation phase and 20 consecutive referrals in the last 2 weeks of the data collection phase. Records were redacted, scanned and e-mailed to the study team in accordance with the protocol described for the development stage. One hospital preferred not to send redacted copies out of the trust, so the researchers scored the sample on site. Four reviewers independently assessed a total of 120 records from across the three sites. Two reviewers, Anne Slowther and Chris Bassford, both clinicians, reviewed all records; and two reviewers (SR and Julia Walsh, a researcher external to the study team), neither of whom was clinically trained, assessed a subset. JW checked 40 notes each from hospitals B and C, and Sophie Rees reviewed 40 notes from hospital A only. Three reviewers (CB, AS and SR) were members of the study team. Prior to scoring the records, each scored three sets of records from the original 25 records used in the tool development using the final version of the tool and compared their scorings, discussing any disagreements to reduce any differences in approach to use of the tool. JW was an external scorer and, prior to scoring the notes from the feasibility study hospitals, had had two training sessions in the use of the tool with Chris Bassford and Sophie Rees.
Analysis
A detailed description of the scoring system is set out in Appendix 23. Each section of the tool is scored separately and then scores for sections A–C are combined, at which point section B (capacity to recover) is weighted to maintain parity with section A (evidence of clinical need). The section D score (red flags) is reported separately.
Descriptive statistics were generated for the total number of decisions and for each set of decisions provided by a reviewer. For continuous variables, the interclass correlation coefficient was calculated with its 95% CI. To allow for generalisation beyond the study reviewers, the single random raters (ICC2)199 variant was used. For categorical data, the unweighted kappa statistic and its 95% CI were calculated for each pairwise comparison between reviewers. A decision was considered eligible to be entered into the reliability analyses if all reviewers had given it a valid score. Scores from the non-clinical reviewers (JW and SR) were combined as these reviewers never scored the same decision.
All analyses were computed in R using the ‘psych’ package. 200 Typically, an outcome measure would require a reliability of at least 0.7 to be considered a reliable instrument for use in a clinical setting. 201 However, to assist interpretation of the kappa statistic, Landis and Koch202 have suggested arbitrary boundaries to classify the value. These categories have been used for both the kappa and the ICC statistics throughout the results section and are shown in Table 20 for reference.
| Kappa statistic | Interpretation |
|---|---|
| < 0.00 | Poor |
| 0.00–0.20 | Slight |
| 0.21–0.40 | Fair |
| 0.41–0.60 | Moderate |
| 0.61–0.80 | Substantial |
| 0.81–1.00 | Almost perfect |
Presence of decision and review
Reviewers were asked to note any decision about admission to ICU and then to assess that decision using the scoring sheet. However, it was not always clear from the record whether or not a decision had been made. Decisions were categorised as having been identified and assessed. It was possible to identify that a decision had been made (e.g. the patient had clearly been admitted to ICU), but as there was no clear record of that decision in the notes it was not possible to assess it. This would be categorised as identified but not assessed. From the 120 case notes scrutinised (40 from each of the three study hospitals), 143 unique decisions were identified by at least one reviewer, resulting in 429 potential scores. However, only 234 actual scores were recorded (54.5%) as some but not all decisions were either identified or assessed by all three reviewers. Table 21 shows the categorisation each reviewer gave to each of the decisions identified by each study site.
| Hospital (number of decisions) | Decision category | Reviewer (number of decisions) | ||
|---|---|---|---|---|
| AS | CB | JW/SR | ||
| Aa (n = 46) | Not identified | 1 | 2 | 7 |
| Not assessed | 22 | 17 | 23 | |
| Assessed | 23 | 27 | 16 | |
| Bb (n = 44) | Not identified | 3 | 4 | 1 |
| Not assessed | 13 | 13 | 11 | |
| Assessed | 28 | 27 | 32 | |
| Cb (n = 53) | Not identified | 4 | 11 | 9 |
| Not assessed | 18 | 17 | 19 | |
| Assessed | 31 | 25 | 25 | |
Appendix 24, Figures 38–40, gives a more detailed analysis of the combinations of reviewers who gave each decision a score at each hospital.
Agreement varied between reviewers and between hospitals. All kappa values were significantly better than agreement due to random chance (i.e. the 95% CIs did not include zero); however, many values are only ‘fair’ (see Appendix 24, Table 54). Agreement was not necessarily dependent on the clinical knowledge of the cases, as sometimes kappa values were higher between non-clinical and clinical reviewers than between clinical reviewers at a hospital. Hospital B generally had higher agreement levels than hospitals A and C. This may indicate that some hospitals had clearer record-keeping processes than others.
The location of the decisions are given in Appendix 24, Table 56. The majority of identified decisions (54.5%) resulted in the patient being admitted to ICU; however, this was driven by hospital A only. Both B and C were more balanced, with slightly more decisions resulting in the patient not being admitted to ICU (see Appendix 24, Table 57).
Scores for each section of the tool
Section A: factors necessary to inform ethical reasoning (see Appendix 24, Table 58)
The median score across each hospital for this section remained constant, but the interquartile range (IQR) varied considerably. Figure 17 shows boxplots breaking down each hospital by reviewer. Distinct differences by both hospital and reviewer can be seen. However, these patterns do not appear to be systematic.
FIGURE 17.
Section A total score by hospital and reviewer.
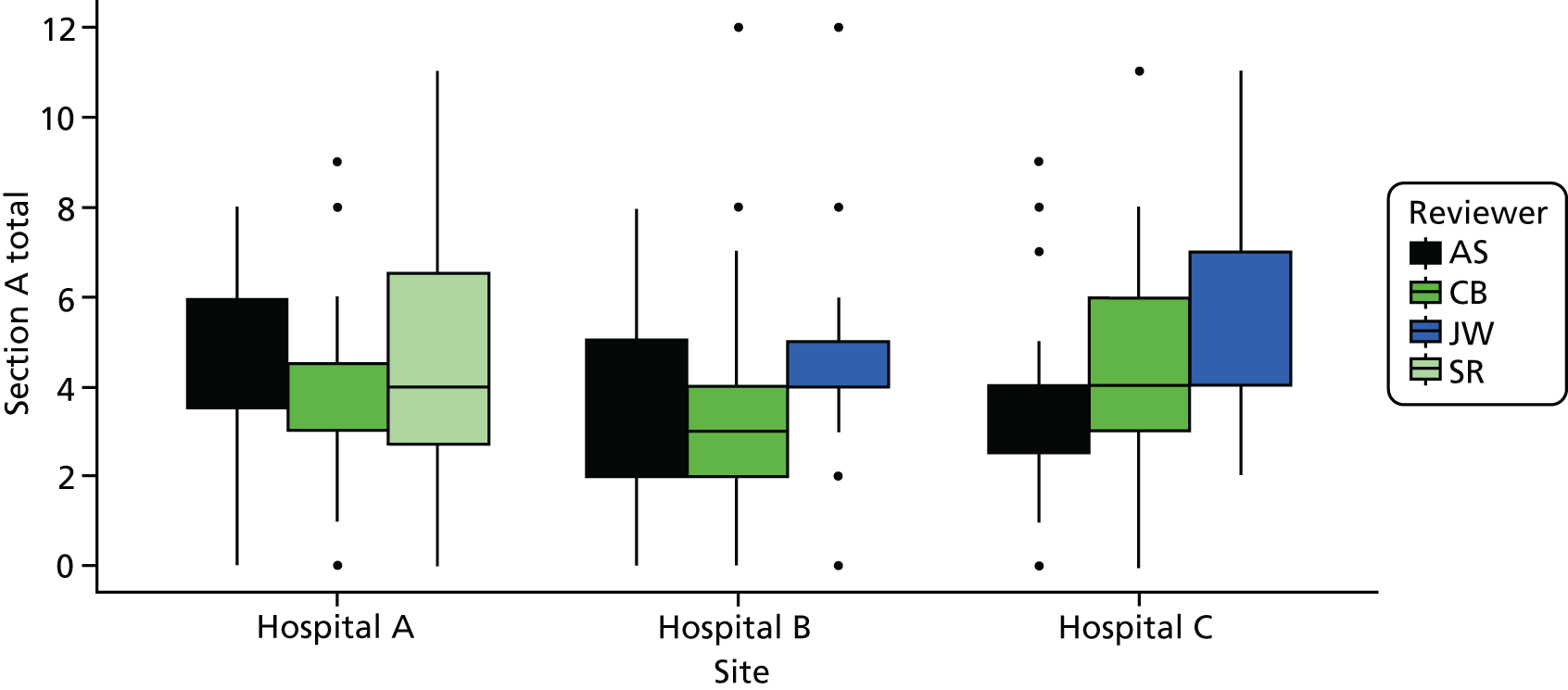
The overall reliability between the reviewers was ‘moderate’. However, at site level, there was a greater variability. The lowest agreement was between AS and JW at hospital B, which was not significantly different from random chance. All other ICC values observed were calculated to be better than random chance (Table 22).
| Hospital (N) | Comparison, ICC (95% CI); n decisions analysed) | |||
|---|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | All reviewers | |
| All (103) | 0.60 (0.42 to 0.74); 63 | 0.50 (0.28 to 0.66); 63 | 0.60 (0.37 to 0.75); 60 | 0.57 (0.41 to 0.70); 55 |
| Aa (31) | 0.52 (0.11 to 0.78); 19 | 0.63 (0.17 to 0.86); 15 | 0.65 (0.22 to 0.87); 14 | 0.61 (0.30 to 0.84); 13 |
| Bb (34) | 0.62 (0.30 to 0.82); 24 | 0.23 (–0.15 to 0.55); 26 | 0.41 (0.06 to 0.68); 27 | 0.42 (0.18 to 0.66); 24 |
| Cb (38) | 0.63 (0.28 to 0.83); 20 | 0.65 (0.15 to 0.86); 22 | 0.75 (0.40 to 0.90); 19 | 0.68 (0.41 to 0.85); 18 |
Looking at the inter-rater reliability of the individual items of section A, question 2 (‘interpretation of clinical situation’) had the lowest reliability. This item required interpretation of the clinical situation, so it is perhaps unsurprising that there was a marked difference between clinical and non-clinical reviewers for this question. For items 5 and 6 (patient wishes), reliability was generally higher than for the other items. This may be because specialist knowledge is not required to interpret the record with respect to patient wishes (see Appendix 24, Table 59).
Section B: ethical reasoning (see Appendix 24, Table 60)
The median scores across each hospital remained constant at zero, but the IQR varied considerably (Figure 18). At hospital A, the median score and IQR were zero, implying that the overwhelming majority of the 66 scored decisions were given a score of zero.
FIGURE 18.
Section B total score by hospital and reviewer. Owing to the large number of decisions given a score of zero, only the outlier scores are visible for reviewer CB.
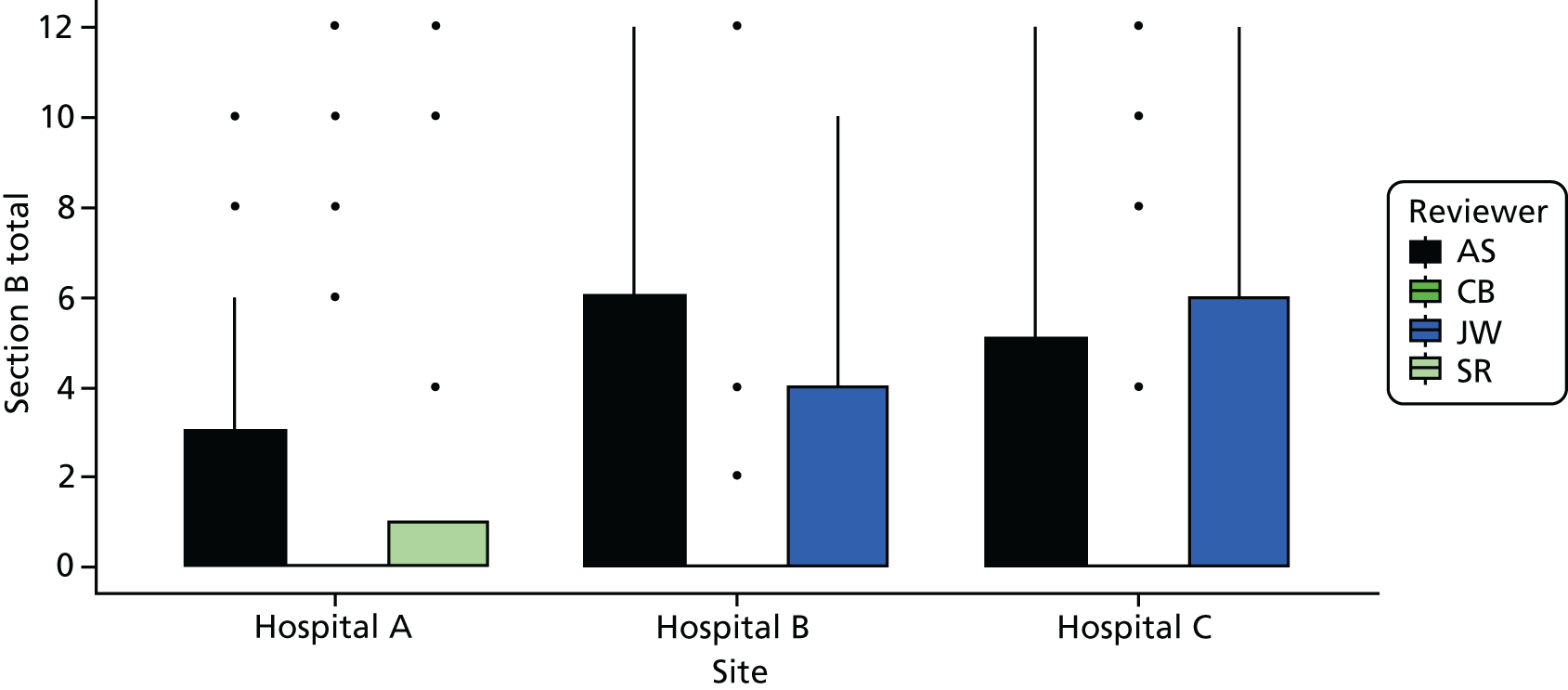
As for section A total score, at least one estimate of inter-rater reliability was not significantly better than random chance (hospital B comparing AS and CB). However, the reliability of AS and CB’s scores was otherwise at least moderate (Table 23). Looking at the inter-rater reliability of each question in section B, item 9 (‘Was capacity to recover included in the balancing?’) had the highest reliability between the reviewers, and item 8 (‘Was acute physiology/system failure included in the balancing?’) had the lowest (see Appendix 24, Table 61).
| Hospital (N) | Comparison, ICC (95% CI); n decisions analysed | |||
|---|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | All reviewers | |
| All hospitals (103) | 0.55 (0.35 to 0.70); 63 | 0.60 (0.42 to 0.74); 62 | 0.78 (0.62 to 0.87); 60 | 0.66 (0.52 to 0.77); 55 |
| Aa (31) | 0.63 (0.25 to 0.84); 19 | 0.55 (0.05 to 0.82); 15 | 0.94 (0.83 to 0.98); 14 | 0.79 (0.56 to 0.92); 13 |
| Bb (34) | 0.29 (–0.07 to 0.60); 24 | 0.48 (0.13 to 0.72); 26 | 0.42 (0.07 to 0.68); 27 | 0.37 (0.13 to 0.62); 24 |
| Cb (38) | 0.73 (0.44 to 0.88); 20 | 0.74 (0.47 to 0.88); 22 | 0.89 (0.74 to 0.96); 19 | 0.79 (0.60 to 0.91); 18 |
For section C (see Appendix 24, Table 62), the distribution of scores across each hospital remained reasonably consistent, with the IQR constant at 0–3. No hospitals achieved the maximum theoretical score of 10, and the maximum score of 8 was awarded once (Figure 19).
FIGURE 19.
Section C: total score by hospital and reviewer. Owing to the large number of decisions given a score of zero, only the outlier scores are visible for reviewer JW at hospital C.
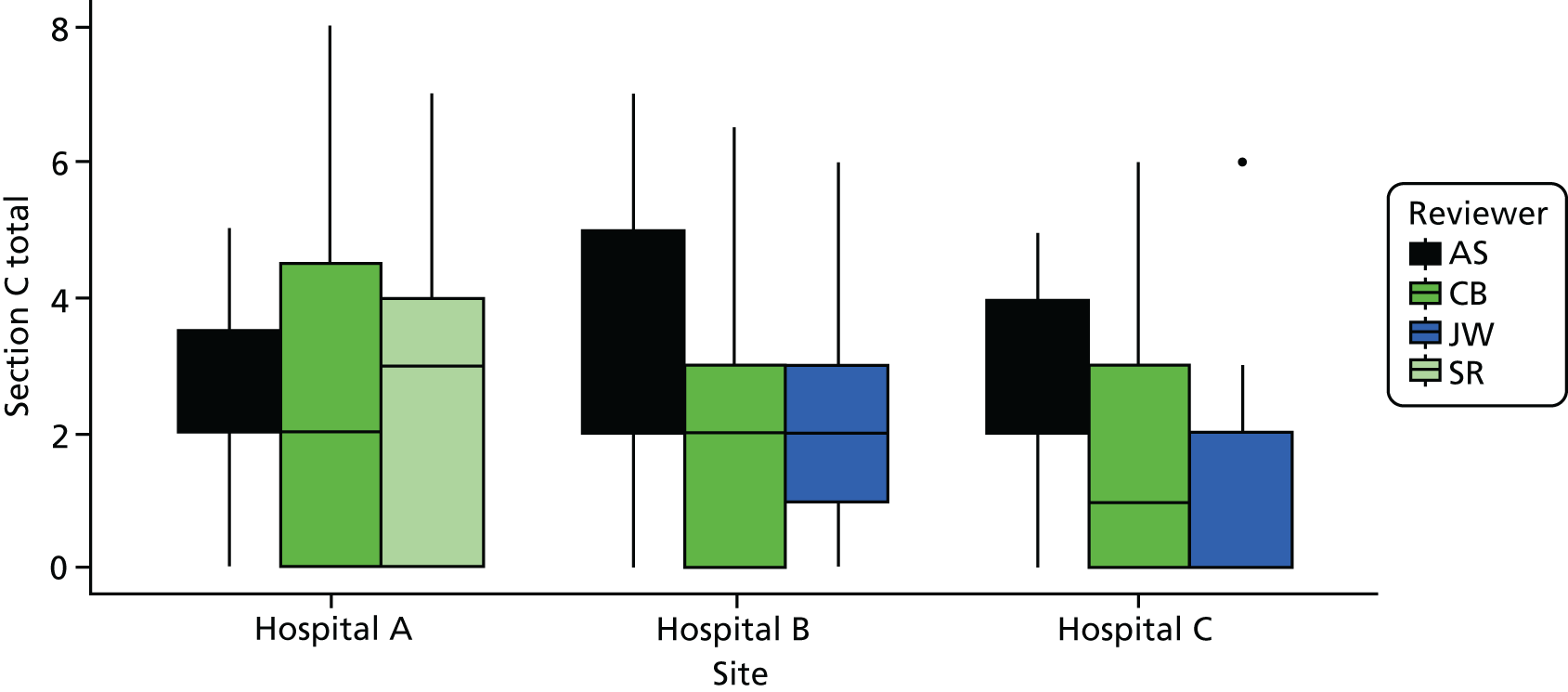
Table 24 shows that the inter-rater reliability of section C at hospital A is high between reviewers Anne Slowther and SR/JW, with reviewer Chris Bassford appearing to record very different scores. For individual items in section C, inter-rater reliability was generally moderate, except for decision communicated to family, which was fair (see Appendix 23, Table 63).
| Hospital (N) | Comparison, ICC (95% CI); n decisions analysed | |||
|---|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | All reviewers | |
| All (103) | 0.37 (0.13 to 0.57); 63 | 0.34 (0.06 to 0.56); 63 | 0.44 (0.21 to 0.62); 60 | 0.40 (0.23 to 0.56); 55 |
| Aa (31) | 0.41 (–0.06 to 0.72); 19 | 0.73 (0.35 to 0.90); 15 | 0.50 (–0.04 to 0.81); 14 | 0.56 (0.23 to 0.82); 13 |
| Bb (34) | 0.41 (0.0 to 0.70); 24 | 0.44 (0.07 to 0.70); 26 | 0.44 (0.01 to 0.70); 27 | 0.33 (0.09 to 0.59); 24 |
| Cb (38) | 0.34 (–0.05 to 0.66); 20 | 0.30 (–0.09 to 0.63); 22 | 0.32 (–0.11 to 0.66); 19 | 0.234 (0.07 to 0.63); 18 |
Section D: red flags
Only eight decisions were given any ‘red flags’ in section D. One decision was given two flags. Flags were given for the following: functional status (six decisions), previous knowledge of the patient (one decision) and presence of a DNACPR order (two decisions) (see Appendix 24, Table 64).
Total score (Figure 20 and see Appendix 24, Table 65). The maximum possible score is 36; however, the maximum score awarded to any decision was 30. Nine decisions (2.1% of all decisions) were marked as 0, and seven (1.6%) were awarded only 1 mark.
FIGURE 20.
Total score by hospital and reviewer.
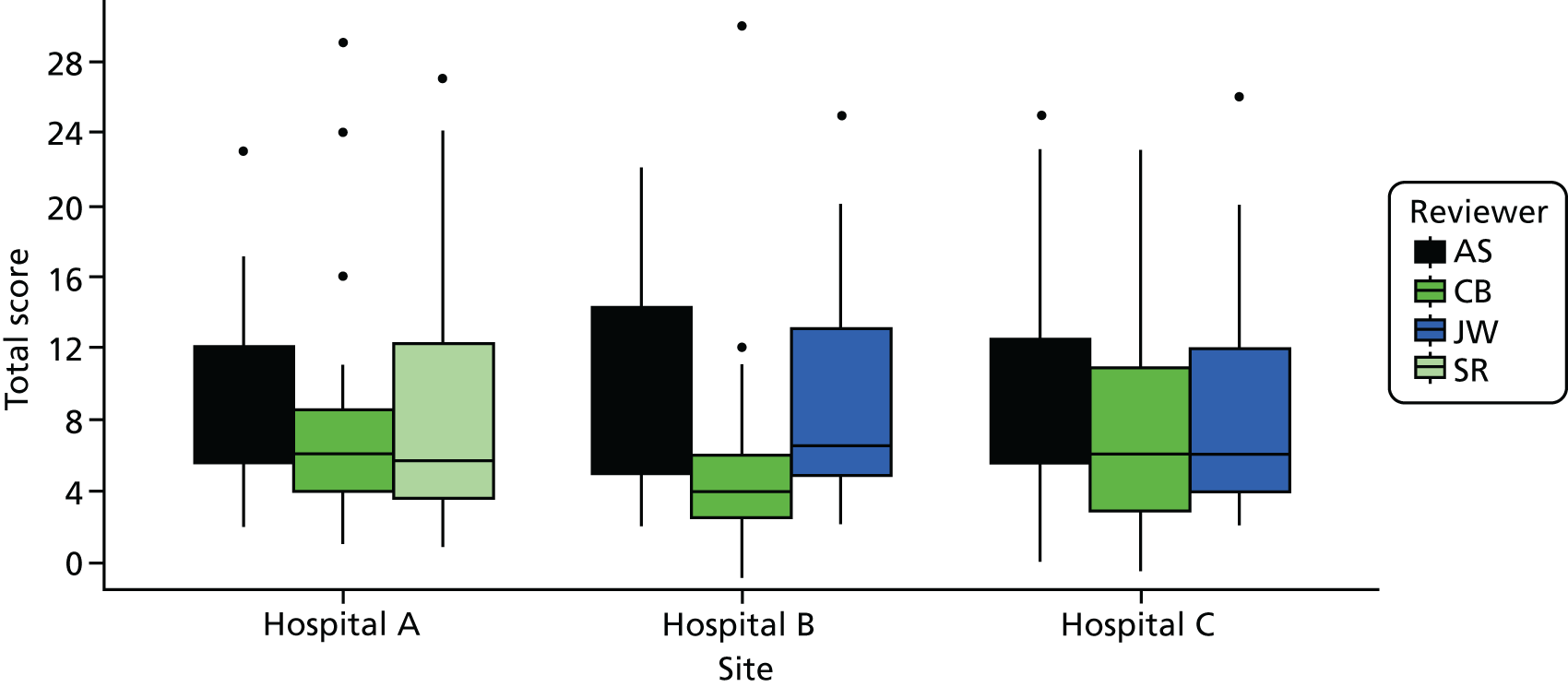
The overall reliability across all decisions was moderate to substantial. However, this varied considerably between hospitals (see Appendix 24, Table 66). The relationship between total score and outcome of decision and location of decision is shown in Appendix 24 (see Tables 67 and 68, and Figures 42 and 43).
We did not analyse for differences in score between pre-intervention and post-intervention records to look for responsiveness of the instrument. Given the low uptake of the intervention in the time available for implementation as described in Chapter 7 and confirmed by the small number of records with a referral or decision-support form present in our samples we considered it unlikely that any change could be demonstrated. Further work on reliability of the tool is required before this could be attempted (see below).
Summary
Our evaluation tool was grounded in our empirical data and stakeholder views about what was required for a transparent ethically justifiable decision around admission to ICU. Development work demonstrated face validity of the tool among ICU clinicians. However, applying the tool to clinical records proved challenging.
When asked to score the records of the decision to admit a patient into ICU, the initial step of identifying a decision is itself non-trivial and subject to disagreement. Out of a total of 429 potential decision scores, only 234 were recorded; 153 were identified as a decision but not scored (no data available to score); and 42 were unidentified decisions by one or more reviewers. Only 55 unique decisions were scored by all three reviewers at a hospital.
Of those decisions for which scores were given, reliability between both hospitals and reviewers varied considerably. In particular, hospital C had very poor reliability. This may indicate that sites have differing processes and standards for recording these decisions.
No clear trends were seen in reliability between reviewers. There were questions where agreement between the clinical reviewers was much higher than when compared with the non-clinical reviewers but often one clinical reviewer (AS) did not agree with the other reviewers. This trend was not continued at a total score level, where Anne Slowther and the non-clinical reviewers generally had greater reliability than with Chris Bassford. These results indicate that the current version of the tool is not sufficiently reliable to be used as a summative evaluation to assess decision-making in clinical practice. In Chapter 9 we reflect on the possible reasons for the lack of reliability, if and how the evaluation tool/process can be modified, and the situations in which the tool might be used.
Strengths and weaknesses
A major limitation of this study is the unanticipated problem of identification of referral decisions. As different reviewers extracted different numbers of decisions from a single set of notes, this made identification of each decision difficult. When possible, decisions were matched precisely, but it is likely that some decisions were incorrectly matched owing to missing data. This in turn reduced the effective sample size for the analysis, and is likely to have overestimated the errors when calculating the reliability. However, this finding is an important one for future evaluation studies using patient records.
Chapter 9 Discussion and conclusions
Discussion
This interdisciplinary mixed-methods project provides a unique insight into how decisions about whether or not to refer or admit a patient to ICU are made and how this decision-making process might be improved. The initial empirical investigation into current practice ensured that the development of any intervention would be grounded in, and relevant to, clinicians working in the NHS. A notable feature of all aspects of this project was the willingness of ICU doctors to participate, to have their practice observed, to complete a lengthy choice experiment and to engage with the intervention. This suggests that they recognise not only that these decisions are often difficult, but that how these decisions are made is important not just to patients and their families but also to society as a whole.
The current experience of decision-making around intensive care unit referral and admission
Complexity of factors influencing decisions
Previous work on ICU admission decision-making has predominantly focused on the factors associated with admission or refusal of admission, with less evidence on the actual process. Our systematic review found numerous studies that identified a wide range of factors influencing the decision. Factors included those that are likely to be seen as clinically and ethically justifiable, such as severity of comorbidity and type of acute illness, but also others that suggest that personal values and organisational constraints may affect the decisions made (age, functional status as perceived by clinician, time of day, seniority of referrer). The availability of ICU resources (beds or nursing staff) was also identified as an influential factor. Our ethnographic study confirmed that many of these factors had an effect on the decision whether or not to admit a patient to ICU. It also identified other factors not previously identified in the literature, most notably the subjective assessment of the patient by the ICU clinician (gestalt), and the safety of the ward environment in terms of the resources available to care for the patient.
Doctors in our qualitative study acknowledged that ICU bed availability influenced the type of decisions they made, and also the impact of the admission decision on other patients either already in ICU or potentially needing ICU treatment. They described deferring admission and trying ward-based treatment for longer if there was limited ICU capacity, and creating beds by transferring other patients out of ICU. This phenomenon has been noted in the literature. 16 ICU physicians in our experiences systematic review also referred to limited resources creating ethical difficulties in making these decisions. There is, however, a general reluctance to be explicit about the impact of limited ICU resource on decision-making and this was reflected in our stakeholder conference. A proposal to prompt ICU doctors to record this in the DSF was rejected by stakeholders.
Challenges related to ICU bed availability are not new and not limited to the UK NHS. The external constraint of limited resources creates an ethical dilemma for all doctors working in systems with limited resources: a tension between their duty to an individual patient and their duty to all patients in their care. However, this ethical tension is more acute in a situation where the individual patient in need of the resource is critically ill. Perhaps the ethical obligation is best articulated as a duty to provide the best possible treatment for the patient in the circumstances and explicitly state what actions are being taken to mitigate any effect of limited resources. This is the approach we took in our DSF.
Our choice experiment gave insight into the relative importance given to factors by ICU consultants and CCOR nurses, and the preference patterns that contribute to variability in decision-making. A key finding in our ethnographic study, which was mirrored in our choice experiment, was the greater importance given to the subjective assessment of the patient than to the measurement of physiological parameters or standardised physiological scoring systems such as NEWS. The concept of the clinical assessment of a critically ill patient as greater than the sum of individual physiological measurements (the gestalt) is recognised by clinicians but has had limited analysis in the literature. A study of consecutive patients assessed by a critical care consult team found weak correlation between the physician’s assessment of respiratory distress and a recognised physiological scoring system (APACHE II). 203 Gestalt has been investigated in the context of severe trauma, where it has been found to be unreliable in the prediction of massive transfusion204 and overall survival. 205 Similar findings have been described with gestalt in estimating probability of pulmonary embolism. 206 However, in all of these studies gestalt performed as well as other predictive scores or algorithms, highlighting the difficulty of prognostication in severely ill patients. One concern about gestalt in relation to ICU admission decisions is the phenomenon of prognostic pessimism. Studies have shown that ICU clinicians’ predictions of poor outcomes for specific patient groups, such as patients with COPD or elderly people, do not correlate with actual outcomes in these groups. 47,50 In the absence of sensitive prognostic tools, decisions about the treatment of critically ill patients will continue to include a gestalt assessment. Making the elements of this assessment more transparent will contribute to our understanding of this complex decision-making process and will enable the implicit value judgments inherent in the process to be challenged.
An important, although perhaps unsurprising, finding from the systematic review was that older age is independently associated with increased odds of a patient being refused admission to ICU. There was also evidence in the ethnographic study that ICU doctors considered young age a reason to support patient admission to ICU, and of referring doctors’ perception that ICU doctors had to be persuaded to take an elderly patient. The choice experiment supported this finding and also suggested that age has more influence on the admission decision than other factors such as functional status and presence or severity of comorbidity. The choice experiment also identified a distinct preference pattern, categorised as ‘age dominant’, suggesting that some consultants were influenced by the age of the patient more than others. It is not possible from the empirical data to determine why this is the case. It is possible that some ICU consultants, consciously or subconsciously, discriminate in favour of younger patients, or that they implicitly associate age with a reduced capacity to benefit from ICU (i.e. prognostic pessimism). 47,86 Another explanation could be that consultants use age as a proxy for capacity to recover when other information is unavailable, and that this heuristic is maintained even when specific information, such as physiological reserve or comorbidity, is known. The possibility that implicit assumptions affect decisions is one argument in favour of improving transparency of decision-making, making explicit the implicit in order to mitigate unfair discrimination. This is particularly important in the context of an ageing population and the legal obligations of UK doctors as set out in equality legislation. 207
Variability in decision-making about ICU admission was noted in our systematic review and evident in the ethnographic study and choice experiment. The choice experiment also showed differences between ICU consultants and CCOR nurses in the importance given to different factors when making these decisions. Although it is likely that the reasons for this variability are multifactorial, the choice experiment demonstrated that ICU consultants, and to a lesser extent CCOR nurses, have different preference patterns for factors influencing the decision. The reasons for these different preference patterns require further exploration, but their existence suggests that implicit value positions influence decisions. This is another argument for making the decision-making process transparent and explicitly articulating the reasons informing the decision.
Context, relationships and emotions
The wide range of patient-, clinician-, and organisational-related factors influencing decisions to refer or admit a patient to ICU illustrate the complexity of the decision. Our ethnographic study revealed that the contextual and relational features of the decision-making process contributed to the challenges faced by clinicians in these situations. These decisions are often time-critical and frequently made in the ED when relevant information may be limited or unavailable, increasing the uncertainty of predicting the consequences of any decision. The ED can be a chaotic environment, with multiple decisions being made about an individual patient and multiple patients requiring decisions. The ward environment may be less chaotic but it has less immediate senior clinical presence and junior doctors struggle to provide comprehensive assessments for referrals while managing day-to-day tasks for other patients in their care. In these situations the ICU doctor can bring a sense of reassurance and control, as was noted in our ethnographic study.
At the heart of these decisions is a web of relationships that need to be maintained for good decision-making to occur. We explore the specific issue of engaging with patients and family later in this chapter. The relationship between ICU doctor and referring team is also crucial. Mutual trust and respect between colleagues facilitates communication and shared understanding of how to achieve the best treatment for the patient. Difficult relationships between teams may lead to poor communication and delay in decision-making. Doctors in our implementation study commented that the requirement to clearly document reasons for their decisions had a positive impact on communication between referring and ICU teams, facilitating mutual understanding.
Managing relationships can be an additional challenge when high-stakes, complex decisions are being made and can place substantial emotional strain on clinicians. Moral distress is a well-recognised phenomenon in intensive care settings and has been noted to be associated with professional burnout, although it has not been specifically explored in the context of decisions about admission to ICU. 208 Our experiences systematic review identified some evidence of moral distress in both ICU and referring clinicians, and a recent study across nine ICUs in a publicly funded health system exploring the consequences of ICU capacity strain found that it contributed to moral distress and burnout among staff. 209 Our study noted the role that ICU consultants often took in providing support to junior staff in making these difficult decisions.
Ethical values and ethical reasoning
Our ethnographic study identified a range of ethical values, both implicit and explicit, shaping the decision-making process. Trust, respect and honesty were seen as important in supporting the interprofessional relationships necessary for good decision-making. Professional autonomy was valued by clinicians, and perceived lack of autonomy caused frustration. The importance of professional autonomy/agency was also identified in our experiences systematic review. Professional autonomy emerged as an important consideration when implementing our intervention, which some consultants perceived as questioning their ability to make decisions. Respect for patient autonomy and consideration of patients’ values when making decisions about their treatment and care is both an ethical and a legal requirement. 210 This was something that all clinicians in both our observational study and our intervention study identified as important, but there was little evidence that the patient’s views were routinely sought. We discuss this anomaly in more detail below. Most patients who are critically ill will lack the capacity to make a decision for themselves, and in the UK it is the duty of the doctor responsible for the patient’s care to make a decision in their best interests. Inherent in this duty is the obligation to identify and weigh the burdens and benefits of treatment for that particular patient. We found that although doctors talked about the benefits of ICU treatment and the potential harms in terms of likelihood of survival, they appeared to have great difficulty in explicitly weighing these benefits and burdens in individual cases. We noted only one example of this in our observation study, and, even when a framework prompting this process was provided in the intervention study, doctors still described difficulty in articulating the burdens and benefits of ICU care, and a reluctance to put these in writing. We have already noted the uncertainty associated with prognostic indicators in individual cases, and there is a recognised lack of ICU-specific patient-relevant outcomes that may explain some of this reluctance. 211 There is a substantial body of literature on the kinds of ethical issues facing ICU clinicians, but little has been published on how ICU clinicians make ethical decisions, or how they communicate their reasoning. A qualitative study specifically exploring priority setting decisions in a critical care unit found that both medical and non-medical reasons were used, but non-medical reasons were less well documented and understood. 44
Consultants’ concerns about clinical autonomy, coupled with their expressed difficulty in articulating their reasoning and the importance given to an intuitive feel for the ‘look of the patient’, may reflect the complex nature of clinical decision-making. There is an extensive body of literature on clinical decision-making that explores a range of elements in the process, including tacit knowledge,212 contextual experience213 and clinical heuristics, in addition to empirical evidence and formal reasoning. These implicit elements may be difficult to capture in a DSF or to articulate in a written record but may be essential to good patient care, particularly in a time pressured context of decision-making for a critically ill patient. However, there is a concern that reliance on an intuitive model of clinical reasoning (using clinical heuristics and personal experience) rather than a more analytical model can lead to cognitive biases that may adversely affect decision-making. 214,215 The empirical evidence on the relative importance or effectiveness of intuitive (type 1) or analytical (type 2) thinking in clinical decision-making is both mixed and limited, as are studies of interventions to improve clinical decision-making. 216 In a review of the causes of error in clinical reasoning, the authors found that interventions that encourage clinicians to mobilise and reorganise their knowledge and reflect on the content of the case appeared to have some benefit in reducing error in reasoning,217 particularly in complex cases. Presumably knowledge would include knowledge of the patient and their values as well as clinical knowledge.
It is not surprising that well-articulated ethical reasoning is absent in decision-making about ICU admission. As already noted, these decisions are often made in urgent situations when the priority is to act quickly to prevent further significant harm to the patient. As such, clinicians will tend to rely on clinical experience, algorithms and implicit values (type 1 thinking) rather than explicit formal reasoning (type 2 thinking). 214 In some cases this will be entirely reasonable, if not morally obligatory, in order to save the patient’s life. However, not all or even most decisions around admission to intensive care require this degree of urgency and often these decisions are complex, with uncertain outcomes. It is these decisions (the grey cases described by doctors in our ethnographic and implementation study) that we suggest require careful deliberation and consideration of the relevant evidence and values. It seems clear from our study that doctors making these decisions require support to do this type of thinking in these cases. We would also argue that explicit articulation of reasoning, including acknowledgment of the use of tacit knowledge and clinical experience, is necessary for transparency and consistency of decision-making. The challenge is to provide a framework that doctors find intuitive or familiar rather than simply adding ethics to their established clinical reasoning processes. Values, both desirable and undesirable, are not absent from their decision-making but are implicit. We are likely to be more successful in embedding improvement in ethical decision-making if we can facilitate a shift from implicit to explicit articulation of values using the language of clinical encounters.
Developing an intervention
At all stages of development, we sought to create a framework that was recognisable to clinicians, used non-academic language and could be embedded in established good clinical practice. However, it became clear during the stakeholder conference that education to support the implementation of a framework must include more specific engagement with the ethical concepts and principles underpinning it. This was confirmed in our implementation feasibility study when lack of time for champions to explain the underpinning ethical rationale behind the framework was identified as a challenge to successfully embedding the framework in practice.
The initial focus of the research was the decision whether or not to admit a patient to ICU. However, we observed that the ward teams had a wide range of reasons for seeking the help of the ICU doctor, and that intensive care input, when perceived to be working well, focused on the question of what was the best treatment for the critically ill patient rather than whether or not the patient should be admitted to the ICU. This prompted a paradigm shift in how the decision-making process (and, therefore, the support required) should be conceptualised, made concrete by a PPI participant in the stakeholder conference who suggested that the emphasis should be on not where the patient was treated, but how they were treated. This concept of intensive care as not constrained to the ICU has informed thinking on the development of CCOR teams,218 although the concept has been operationalised in a wide variety of ways, with inconclusive evidence of effectiveness. 219 If CCOR teams are to be used as an extension of intensive care beyond its boundaries, and CCOR nurses take on responsibility for decisions about admission to the ICU, it is worth noting the findings of our choice experiment, where differences were found between CCOR nurses and ICU consultants in the importance ascribed to factors such as NEWS when making the decision. Nevertheless, the broader message of patient-focused rather than location-focused decision-making from our empirical work led to a structuring of the DSF that first asked the question ‘What is the optimal treatment for this critically ill patient?’, and only then asked ‘Where can or should this treatment be delivered?’
A potential criticism of our intervention is that the process of making these decisions is a clinical skill that ICU doctors and referring teams exercise every day; there is no need for a framework to enable them to do this, a point made by some senior doctors in our implementation study. While it is clear that most health-care professionals make decisions and treat patients to a high professional standard, standards can be improved and patient care can benefit when checklists or prompts are used to support good practice in specific situations. There are numerous examples of checklists used in the ICU context, and their presence does not imply a lack of competence. 220,221 The recommendation for a checklist often arises from the observation that good practice can be compromised because of a combination of systemic, contextual and human factors. 222 This was identified in our ethnographic study. Reflecting the complex nature of decision-making for critically ill patients, the referral and DSFs developed in this study are less checklists but rather provide a structure for decision-making with prompts for decision-makers to consider. Our referral form was based on the SBAR tool, which is itself a checklist/framework that is widely used to support good communication between clinicians when escalating a problem or transferring care. 223
Is it feasible to implement the intervention in an acute NHS trust?
All feasibility sites were enthusiastic about implementing the intervention, which included support from the medical director. Despite the very short implementation period (8 weeks), we achieved an average of 28.2% usage of forms across sites. Following the study, two sites expressed an interest in taking the intervention or a variation of it forward as a service development. However, at all sites implementation was a challenge, and our feasibility study has provided useful data to facilitate future implementation initiatives. By using a mix of quantitative and qualitative methods, we were able to triangulate our data within the study and thus were able to understand how often, when and by whom the referral and decision-support forms were used, and also how and why clinicians used or did not use them. 224
The importance of context in successfully implementing interventions in health care is well documented. 225,226 The shared context of all of the sites was that of a busy NHS hospital, but the specific context of each site influenced implementation. The size of the organisation and the number of staff who had to be reached clearly had an impact, but so too did existing interprofessional relationships and structures (e.g. presence or absence of CCOR or existing systems for logging ICU referrals). Key difficulties common to all sites included having time for implementation, both longitudinally (for embedding the study into practice) and in individual champions’ time to devote to promoting the intervention. Linked to this is the challenge of institutional inertia. NHS trusts are large, complex organisations with well-established processes and protocols, and they cannot respond easily to rapid change (our implementation period was only 8 weeks). This represented a challenge for the champions, who had to break into existing training and information-giving opportunities within the trust to educate clinicians about the intervention. It was also a reason why it was impossible to integrate an electronic version of the forms into the trust electronic records system, something that all champions agreed would be essential for truly embedding the intervention into routine practice.
At all sites, to a greater or lesser extent, there was a mismatch between how we had envisaged the intervention working and how it actually worked in practice, particularly in the way the referral forms were used and the lack of use of patient and family information leaflets. Both of these documents were intended to be used when considering a decision to refer a patient to ICU; however, in most situations, use of the referral form was only prompted at the time a referral was being made, leading to frustration on the part of the referring team, who perceived the form as an additional burden rather than as the intended support. This emphasises the importance of understanding the context in which the intervention will be used and of being flexible in how it is implemented.
The nature of flexibility in implementation strategies has been highlighted by May et al.,225 who describe the importance of plasticity of intervention components and the elasticity of the normative and relational structure of the context in which the intervention is being implemented. Our intervention had some flexibility in the educational component, but the forms were less flexible. An electronic version would allow trusts to individualise the forms and process, which would increase flexibility and may improve uptake. However, the core structure and content of the referral and decision-support forms cannot become so flexible that key elements of the ethical decision-making process are absent. Negotiating the degree of flexibility necessary to maximise uptake while maintaining the integrity of the intervention will be key to successful implementation.
Interventions that involve normative changes in practice, such as introduction of shared decision-making or DSFs, can be experienced as a threat by the practitioners involved. 226 A few of the clinicians in the feasibility study expressed concerns that their clinical and ethical practice was being questioned by the requirement to complete a form. However, the main barriers to implementation were seen as more practical and related to concern about duplication of work, lack of time, and other organisational and situational pressures on decision-makers. These concrete barriers have been noted in other implementation studies of similar interventions, such as surgical checklists227 and advance care planning. 228
The challenge of involving patients and families in the decision-making process
Involving patients in decision-making about their medical care is both an ethical and a legal obligation for health professionals. Using a shared decision-making model is considered to be best practice, even in the ICU, where issues with a patient’s mental capacity pose challenges. 30 However, a striking finding throughout the project was the low level of involvement of patients and their families in the decision-making process. Clinician interviewees in the ethnographic study suggested that the patient’s views were highly valued when making a decision, and ICU consultants in the choice experiment considered the views of the family important. However, our observational data showed that, in practice, the views of patients or their families were seldom sought, and in the intervention implementation feasibility study the patient and family information leaflets, which were designed to support and promote their involvement in the decision-making process, were not used at any of the sites.
There are a number of reasons why active involvement of patients and their families may not occur when decisions are being made about a critically ill patient. The decision itself may need to be made quickly and often in an emergency situation when the patient is too sick to engage in discussion and their family is not available, and so it may not be possible to involve them. Clinicians may feel reluctant to engage in a discussion that involves the very real possibility that the patient will die, the uncertainty of outcomes, and the potential distress and harm that ICU interventions might cause. ICU doctors in the implementation study found it difficult to articulate the burdens of ICU on the decision-support form. Articulating these in a conversation with a critically ill patient or a distressed family member is likely to be even more difficult, and potentially distressing for the clinician. Feedback from site debrief meetings reinforced this concern during discussions of referring teams’ apparent reluctance to use the leaflets. This argument against the involvement of patients has previously been seen as a barrier to discussions about cardiopulmonary resuscitation229,230 or decisions to withdraw life-supporting treatment in ICU. 231,232
In other jurisdictions, consultation with family members or surrogates is legally mandated for important decisions about a patient who lacks capacity. Although surrogates often find this difficult, there is evidence that they prefer to take an active role in decision-making about end-of-life care in the ICU233,234 and experience little regret about the decisions they have made. 234,235 Patients wish to be involved in such discussions and do not experience undue distress from these. 236 In the context of DNACPR, UK law requires consultation with the patient or their family when this decision is being made. 237
Difficult conversations require good communication skills and there is a recognised need for training in this area among ICU clinicians. 238 Furthermore, negative attitudes and dysfunctional interdisciplinary relationships can be a barrier to patient and family involvement in decision-making. 239 Clarifying the responsibilities and processes involved in making these decisions may help. In end-of-life decisions, families can be confused about who is making the decision, which can have an impact on their ability to participate in the process. 240 When processes are clear, the family experience is better. 241
Challenges in evaluating ethical decision-making
A striking finding from our work in testing the evaluation tool was the difficulty reviewers had in identifying when a decision whether or not to admit a patient to ICU had been made from scrutinising the clinical record. This was true of the clinical as well as the non-clinical reviewers. There are several possible reasons for this finding. Lack of a detailed account of a decision does not in itself mean that a poor decision has been made. In urgent situations clinicians may feel that it is more important to make a decision and act on it than spend time recording in detail the reasons for the decision. Lack of clear documentation of this specific decision did not necessarily mean that general documentation in the patient record was poor. One of our sites had very clear, structured ICU records for patients who were admitted, which documented some of the information that would have informed the decision. However, this record was only for patients admitted and was not a retrospective documentation of the admission decision but the plan for ICU care.
The tool allowed only information and reasoning recorded by the person responsible for the decision to be scored, although explicit reference to previously documented evidence, such as physiological status, could be included. In some of our sample records, detailed notes by the critical care outreach team prior to ICU review of the patient included much of the documentation referred to in the score sheet. It is likely that an ICU doctor would have noted this when making their decision and therefore would not have recorded it again. However, it was not always clear how the doctor had used this information. The discrepancy between actual decision-making and the record of that decision is a weakness of any evaluation tool that is applied only to patient records. However, as we have stipulated a transparency requirement for ethical decision-making, and considering the potentially life-changing consequences of these decisions, it is reasonable to expect an adequate record of how the decision was made, and that this should be part of a standard of good decision-making. Our requirement for documentation of the decision-making process is in line with professional guidance for clinicians. 180
Although the overall total score reliability of the evaluation tool was moderate to substantial, there was considerable variability across sites, with reliability at some sites only fair. Variability was not linked to the clinical background of the reviewers, suggesting that clinical knowledge is not the main factor. A possible reason for the variability in scoring is the degree to which the scorers are required to interpret what is in the written record. It is not straightforward to assess what counts as evidence of capacity to recover or what demonstrates balancing of burdens and benefits and whether or not specific evidence has been used in that balancing. It may be that in our attempt to increase the granularity of our scoring system to increase its sensitivity, we reduced its reliability. Reliability could be potentially improved with better training of scorers, but this might limit its practical use.
The challenge of evaluating written records of complex decision-making processes using multiple criteria was also encountered by Pearlman et al. 194 when developing the ECQAT for assessing clinical ethics consultation records. Their approach was to adopt a holistic rather than an analytic scoring system. In this system, raters consider key elements and other factors that work together and give a score based on the total impression of the account rather than scoring individual items. Advantages include reduced likelihood of scorers struggling with interpretation of very specific criteria, and less time required for scoring. Possible disadvantages if assessors are clinicians is that they will overinterpret; that is, they will assume that the clinician has considered evidence or made a decision based on specific reasoning without clear evidence in the documentation. Critiques of holistic scoring models also recognise this concern, suggesting that they may mask deviances in how the descriptors for the holistic scores are applied. 242 However, it may be easier to give examples of what is considered a satisfactory or unsatisfactory decision-making process using this approach. Future development of the evaluation tool should consider both refining the individual criteria specifications in the current version and/or applying a holistic assessment approach to each section.
Although in its current version the tool cannot be recommended for summative evaluation of decision-making about admission to ICU, our experience in developing the tool suggests that it would be useful as a formative or an educational tool. Applying the tool to a sample of clinical records of patients who have recently been referred to a specific ICU during a quality improvement meeting would enable discussion of what counts as quality in decision-making and documentation, what counts as evidence, what reasons are relevant, and how reasoning should be articulated. Key points and lessons learned could feed back into future decision-making and recording, with the aim of improving overall quality of decision-making.
Strengths, weaknesses and methodological challenges
We have noted the strengths and weaknesses of the individual studies within this project in the relevant chapters. An overall strength of the project is its mixed-methods approach, which allowed the triangulation of findings between studies and enabled the development of an intervention firmly grounded in relevant empirical research evidence. We have been able to capture the process of decision-making around referral and admission to intensive care at nine NHS trusts in the UK (six in the ethnographic study and three in the implementation study) and also to capture decision preference patterns across a much wider cohort of intensive care consultants and CCOR nurses in our choice experiment. PPI and stakeholder involvement was strong throughout the project. The difficulty we had in involving patients and families in the project, specifically in the ethnographic study, was disappointing. We had anticipated that recruiting patients and families close to the decision-making event would be challenging and had worked with our PPI colleagues to develop processes to facilitate supportive approaches. Despite this, recruitment was difficult. We had not anticipated the difficulty in tracking patients following their discharge from the ICU to seek their consent for late-stage interviews, which further reduced potential recruitment. Thus, the patient and family perspective is not as strong as we had originally envisaged.
We also experienced challenges when conducting the two primary systematic reviews. The research questions required a broad search strategy, resulting in 34,000 abstracts and 83 included studies. The diverse methodologies of the studies and the heterogeneity of the results made it difficult to pool data and draw meaningful conclusions. In updating the reviews for this report, we adopted a narrative snowballing approach, using our existing knowledge of the literature and focusing on key journals that had the greatest number of relevant papers in our original review. The yield from this approach appears to be as productive as the formal systematic approach, although we cannot be certain that we have not missed a relevant paper. However, this approach was significantly more cost-effective, requiring only a maximum of three reviewers compared with a team of 16.
Conclusion
Summary of findings
This project has illuminated the complexity of decision-making around referral and admission to intensive care. Referring teams and ICU doctors making decisions about critically ill patients need to consider multiple sources of information with varying levels of uncertainty about prognosis or likely outcomes, often with little knowledge of the patient’s wishes or values. External constraints increase the pressure on the decision-making process. Our findings suggest that the focus should be, and in current practice often is, on the best treatment for the critically ill person, and how this can be delivered, rather than on whether or not they should be transferred to ICU. Good decision-making requires communication, both between colleagues and with patients and their families, strong interprofessional relationships built on mutual respect and trust, and careful consideration of the benefits and harms of intensive care for the individual patient. Although there are many examples of this process working well for patients, there is evidence that doctors find it difficult to articulate and explicitly balance the benefits and harms of treatment for patients, that implicit values can inform the decision, and that communication as part of the process of decision-making is often limited. Documentation of the decision to admit or not to intensive care is generally poor, making evaluation difficult and raising concerns about the transparency of the process. Support for junior doctors in making these difficult decisions is often patchy. The patient voice is often lacking in the decision-making process.
Implementing an intervention to support the decision-making process about treatment for a critically ill patient was challenging. Uptake and completion of the forms was low, at just under 30%. However, doctors who used the forms were generally positive about their use, observing that the forms helped them to articulate and communicate their thinking and prompted them to involve patients and their families. Concerns were raised about duplication of work and the time taken to complete the documentation distracting from patient care. Where the framework could be incorporated into existing systems, it appeared to work well. The implementation period in the feasibility study was, in retrospect, too short. Further work is needed, building on the lessons learned from the feasibility study.
Implications for practice
The concept of a structured intervention to support clinicians who are making decisions around referral or admission to ICU was generally welcomed by key stakeholders. NHS trusts wishing to implement such an intervention may need to consider how it will be incorporated into current working practices throughout the hospital; interventions aimed at improving decision-making are more likely to be effective if they engage with the whole process from the point at which the need for critical care support is first considered. Ensuring system-wide change needs senior institutional support and training for all clinical teams involved in the process. Incorporation into an electronic patient record and referral system will facilitate implementation. A quality improvement approach may be helpful when planning future intervention implementation work.
We identified a clear need for improved education of clinicians involved in decisions to refer or admit a patient to ICU, particularly around ethical reasoning and communication. Our project findings and resources may be helpful to professional organisations concerned with curriculum development and postgraduate training. National and international guidelines recommend consultant-to-consultant communication in these decisions, but our study suggests that this does not always occur, and junior doctors, particularly in referring teams, may feel unsupported. Given resource constraints and changes to team structures and working patterns within the NHS, trusts may wish to consider how best to support consultant-led decision-making and collaborative team-working, particularly out of hours.
Involving patients and their families in these difficult decisions appears to be extremely challenging for doctors, but the evidence from our study and others is that patients and families wish to be part of decision-making about their care, even in distressing and life-threatening situations. Further work needs to be undertaken to explore how information can be provided to patients and families to support their involvement. Examples from other areas of practice, such as the introduction of the national Recommended Summary Plan for Emergency Care and Treatment (ReSPECT), may be helpful in this regard.
Recommendations for future research
Our study has gone some way to suggesting how decision-making about referral and admission to intensive care can be improved. It has also identified areas in which further research is needed to inform future interventions and support evidence-based patient-centred decision-making. Priorities for research in this area suggested at our dissemination conference included:
-
Investigation into how to improve meaningful engagement of patients and families in the decision-making process. Difficulties in including patients and families in the decision-making process were evident in all parts of our research. Research to better understand the barriers to this involvement and to develop and test strategies to improve this aspect of the decision-making process is needed.
-
Development of patient-related outcome measures and investigation of the effect of interventions on patient-relevant outcomes. Short-term survival after a critical illness is a limited metric for judging the quality of care delivered by an ICU. Research to better understand the nature and range of specific outcomes that are important to critically ill patients is required to develop quantifiable metrics that can be used to improve patient care. This will allow more informed conversations with patients and families and inform evaluation and improvement of decision-making.
-
Development and evaluation of educational interventions to support ICU trainees with regard to decision-making around escalation of treatment decisions including admission to ICU.
-
Further development and implementation evaluation of DSI using a quality improvement approach.
-
Further work on validating the evaluation tool for ethical decision-making and to describe its role in service improvement and clinical research.
-
Evaluation of the importance of external influences on medical decision-making for the treatment of critically ill patients. We have shown that factors other than direct, patient-related, clinical information influences whether or not a patient is admitted to ICU. How these other, ‘external’, factors have an impact on the decision-making, and extent to which they have an impact, needs further investigation.
Achieving the best outcomes for an individual patient depends on everyone involved making the best possible decisions. Decisions about treatment for critically ill patients have life-changing consequences for the patients involved, and doctors making them will always be faced with difficult clinical and ethical challenges. This study has provided evidence and resources that will help clinicians and organisations aiming to improve decision-making for, and ultimately the care of, critically ill patients.
Acknowledgements
The study team would like to thank all the patients, family members and clinicians who participated in this research by being observed and/or interviewed in our ethnographic and implementation feasibility studies, or by completing the choice experiment questionnaire survey.
We would also like to thank the following:
The members of our Independent Study Steering Committee for their invaluable support and advice: chairperson Carl May; our PPI representatives Jenny Henderson and Ben Powis; and our specialist contributors Bobbie Farsides, Chris Danbury and Arne Risa-Hole.
From NHS Digital (formerly the Health and Social Care Information Centre), Chris Dickson, clinical informatics lead, and David Baker, business analyst, for their work on developing an electronic version of the referral and decision-support forms.
Our Patient and Public Advisory group, who contributed substantially to the project at all stages: Sue Tulip, Richard Grant, Elyas Khalifa, Jabeen Chaudhry and Sarah Broom.
The principal investigators at our study sites for their support and oversight of the project at their trusts.
The implementation champions at our three implementation sites for their enthusiasm and commitment in supporting the intervention in addition to their demanding clinical workload.
Dr Simon Baudouin for chairing the stakeholder conference.
Attendees of our stakeholder conference held in Warwick on July 2016. A list of participants can be found in Appendix 15.
Sam Johnson, academic support librarian at the University of Warwick, for assisting with developing and running the literature searches for the systematic reviews.
Kaja Heidenriech and Bronwyn Harris for contributing to coding and analysis of data from the ethnographic study.
The medical students who carried out abstract screening for our systematic reviews: Alasdair Anderson, Mark Ramsden, Stuart McClure, Stuart O’Connor, Josie Hough, Esther Illman, Martha Pearson and Lauren Sibley.
The research nurses at all of the NHS trusts who facilitated data collection and supported the research fellows.
Our project secretary, Louise Hutton, who provided support to the whole team throughout the project.
Contributions of authors
Chris Bassford (Honorary Associate Professor, ICU consultant) co-led the project with Anne Slowther, conceived and designed the study, supervised all WPs, data analysis and report writing, drafted study introduction and intervention development chapters, and editorially reviewed all chapters in the report.
Frances Griffiths (Professor of Medicine in Society) contributed to the study design, led WP1 (ethnographic study), supervised data collection and analysis in WP3 (implementation feasibility study), developed the evaluation tool with Chris Bassford and Anne Slowther, and drafted the ethnographic study chapter.
Mia Svantesson (Research Fellow) was the lead research fellow for the ethnographic study, responsible for data collection and analysis. She contributed to the development of the choice experiment and the DSI and provided early drafts of the ethnographic study chapter.
Mandy Ryan (Professor of Health Economics) led the choice experiment study, designed the choice experiment with Nicolas Krucien, supervised data analysis, contributed to the development of the DSI, and edited the choice experiment chapter.
Nicolas Krucien (Research Fellow) designed the choice experiment with Mandy Ryan, was responsible for choice experiment data analysis, contributed to the development of the DSI and drafted the choice experiment chapter.
Jeremy Dale (Professor of Primary Care) led the intervention development and implementation feasibility study, contributed to the analysis of data from the ethnographic study and testing of the evaluation tool, and provided editorial review of the feasibility study chapter.
Sophie Rees (Research Fellow) was the lead research fellow for the implementation feasibility study. She was responsible for data collection and analysis, contributed to development and testing of the evaluation tool, analysed qualitative data from the systematic reviews, drafted the implementation feasibility chapter and the experiences section of the systematic review chapter, and collated the report.
Karen Rees (Principal Research Fellow) was the lead for all systematic reviews, oversaw the protocol development and screening, analysed quantitative data for factors review and provided the initial draft of Chapter 3.
Agnieszka Ignatowicz (Research Fellow) was the principal researcher on the systematic review for evaluation tools. She drafted the protocol and contributed to screening of papers, quality assessment and analysis.
Helen Parsons (Senior Research Fellow) carried out the statistical analysis of quantitative data from the implementation feasibility study and evaluation tool testing, and contributed to the analysis section of the evaluation tool development chapter.
Nadine Flowers (Project Manager) oversaw recruitment and data collection for the choice experiment and contributed to data analysis of the factors systematic review.
Zoe Fritz (Wellcome Fellow in Society and Ethics) contributed to the development of the choice experiment and the DSI, screening and analysis of systematic reviews on factors and experiences, and development of the evaluation tool. She contributed to draft versions of the ethnographic study chapter and choice experiment chapter.
Gavin Perkins (Professor in Critical Care Medicine and ICU Consultant) contributed to the development of the choice experiment, the DSI and the evaluation tool. He contributed to draft versions of the ethnographic study chapter and choice experiment chapter.
Sarah Quinton (Honorary Associate Professor and Nurse Consultant) contributed to the development of the choice experiment and DSI, facilitated links with the CCOR nursing community, and advised on consent processes for the ethnographic study.
Sarah Symons (PPI Co-investigator) advised on the consent and recruitment processes for the ethnographic study, edited the patient and family information sheets, and contributed to the stakeholder conference and development of patient and family information leaflets for the intervention.
Catherine White (PPI Co-investigator) advised on the consent and recruitment processes for the ethnographic study. She contributed to data analysis, edited the patient and family information sheets, co-chaired the stakeholder conference and contributed to the development of the patient and family information leaflets for the intervention. She was a speaker at dissemination conference, commented on draft chapters and led the writing of a lay report.
Huayi Huang (Research Fellow) co-ordinated the abstract screening and data abstraction for the factors and experiences systematic reviews.
Jake Turner (Medical Student) contributed to the abstract screening and the analysis of systematic reviews.
Mike Brooke (Medical Student) contributed to the abstract screening and the analysis of systematic reviews.
Aimee McCreedy (Medical Student) contributed to the qualitative data coding and the analysis for the ethnographic study.
Caroline Blake (Medical Student) contributed to the qualitative data coding and the analysis for the ethnographic study.
Anne Slowther (Reader in Clinical Ethics, Clinical Ethicist) co-led the project with Chris Bassford, conceived and designed the study; supervised all of the WPs, data analysis and report writing; drafted the study discussion, systematic review and evaluation tool chapters; and editorially reviewed all of the chapters in the report.
Publications
Bassford CR, Krucien N, Ryan M, Griffiths FE, Svantesson M, Fritz Z, et al. UK intensivists’ preferences for patient admission to ICU: evidence from a choice experiment. Crit Care Med 2019;47:1522–30.
Rees S, Bassford C, Dale J, Fritz Z, Griffiths F, Parsons H, et al. Implementing an intervention to improve decision making around referral and admission to intensive care: results of feasibility testing in three NHS hospitals [published online ahead of print May 17 2019]. J Eval Clin Pract 2019.
Rees S, Griffiths F, Bassford C, Brooke M, Fritz Z, Huang H, et al. The experiences of health care professionals, patients, and families of the process of referral and admission to intensive care: a systematic literature review [published online ahead of print March 11 2019]. J Intensive Care Soc 2019.
Data-sharing statement
All qualitative data generated that can be shared are contained within the report. All data requests should be submitted to the corresponding author for consideration. Please note exclusive use will be retained until the publication of major outputs.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Faculty of Intensive Care Medicine . Core Standards for Intensive Care Units 2013.
- Cardoso LT, Grion CM, Matsuo T, Anami EH, Kauss IA, Seko L, et al. Impact of delayed admission to intensive care units on mortality of critically ill patients: a cohort study. Crit Care 2011;15. https://doi.org/10.1186/cc9975.
- Shmueli A, Sprung CL. Assessing the in-hospital survival benefits of intensive care. Int J Technol Assess Health Care 2005;21:66-72. https://doi.org/10.1017/S0266462305050087.
- Intensive Care National Audit and Research Centre (ICNARC) . Key Statistics from the Case Mix Programme – Adult, General Critical Care Units 1 April 2015 to 31 March 2016 2017.
- Brinkman S, de Jonge E, Abu-Hanna A, Arbous MS, de Lange DW, de Keizer NF. Mortality after hospital discharge in ICU patients. Crit Care Med 2013;41:1229-36. https://doi.org/10.1097/CCM.0b013e31827ca4e1.
- Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, et al. Determinants of long-term survival after intensive care. Crit Care Med 2008;36:1523-30. https://doi.org/10.1097/CCM.0b013e318170a405.
- Ng KP, Chanouzas D, Fallouh B, Baharani J. Short and long-term outcome of patients with severe acute kidney injury requiring renal replacement therapy. QJM 2012;105:33-9. https://doi.org/10.1093/qjmed/hcr133.
- Wade DM, Howell DC, Weinman JA, Hardy RJ, Mythen MG, Brewin CR, et al. Investigating risk factors for psychological morbidity three months after intensive care: a prospective cohort study. Crit Care 2012;16. https://doi.org/10.1186/cc11677.
- Patel A, Wisniewski M, Tulaimat A, Gonzalez-Carmago C, Khan J, Monti C, et al. Do physicians agree in their subjective assessment of patients evaluated for admission to the medical intensive care unit (MICU)?. Chest 2007;132. https://doi.org/10.1378/chest.132.4_MeetingAbstracts.446b.
- Garrouste-Orgeas M, Montuclard L, Timsit JF, Reignier J, Desmettre T, Karoubi P, et al. Predictors of intensive care unit refusal in French intensive care units: a multiple-center study. Crit Care Med 2005;33:750-5. https://doi.org/10.1097/01.CCM.0000157752.26180.F1.
- Howe DC. Observational study of admission and triage decisions for patients referred to a regional intensive care unit. Anaesth Intensive Care 2011;39:650-8. https://doi.org/10.1177/0310057X1103900419.
- Iapichino G, Corbella D, Minelli C, Mills GH, Artigas A, Edbooke DL, et al. Reasons for refusal of admission to intensive care and impact on mortality. Intensive Care Med 2010;36:1772-9. https://doi.org/10.1007/s00134-010-1933-2.
- Sprung CL, Geber D, Eidelman LA, Baras M, Pizov R, Nimrod A, et al. Evaluation of triage decisions for intensive care admission. Crit Care Med 1999;27:1073-9. https://doi.org/10.1097/00003246-199906000-00021.
- Azoulay E, Pochard F, Chevret S, Vinsonneau C, Garrouste M, Cohen Y, et al. Compliance with triage to intensive care recommendations. Crit Care Med 2001;29:2132-6. https://doi.org/10.1097/00003246-200111000-00014.
- Rodríguez-Molinero A, López-Diéguez M, Tabuenca AI, de la Cruz JJ, Banegas JR. Physicians’ impression on the elders’ functionality influences decision making for emergency care. Am J Emerg Med 2010;28:757-65. https://doi.org/10.1016/j.ajem.2009.03.016.
- Stelfox HT, Hemmelgarn BR, Bagshaw SM, Gao S, Doig CJ, Nijssen-Jordan C, et al. Intensive care unit bed availability and outcomes for hospitalized patients with sudden clinical deterioration. Arch Intern Med 2012;172:467-74. https://doi.org/10.1001/archinternmed.2011.2315.
- Garrouste-Orgeas M, Tabah A, Vesin A, Philippart F, Kpodji A, Bruel C, et al. The ETHICA study (part II): simulation study of determinants and variability of ICU physician decisions in patients aged 80 or over. Intensive Care Med 2013;39:1574-83. https://doi.org/10.1007/s00134-013-2977-x.
- Boumendil A, Angus DC, Guitonneau AL, Menn AM, Ginsburg C, Takun K, et al. Variability of intensive care admission decisions for the very elderly. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0034387.
- Cohen RI, Eichorn A, Motschwiller C, Laktikova V, La Torre G, Ginsberg N, et al. Medical intensive care unit consults occurring within 48 hours of admission: a prospective study. J Crit Care 2015;30:363-8. https://doi.org/10.1016/j.jcrc.2014.11.001.
- Pintado MC, Villa P, González-García N, Luján J, Molina R, Trascasa M, et al. Characteristics and outcomes of elderly patients refused to ICU. Scientific World Journal 2013;2013. https://doi.org/10.1155/2013/590837.
- Garrouste-Orgeas M, Timsit JF, Montuclard L, Colvez A, Gattolliat O, Philippart F, et al. Decision-making process, outcome, and 1-year quality of life of octogenarians referred for intensive care unit admission. Intensive Care Med 2006;32:1045-51. https://doi.org/10.1007/s00134-006-0169-7.
- Akpinar A, Ersoy N. Distributive justice and attitudes of intensive care physicians towards distribution of intensive care beds in Turkey. Konuralp Tip Dergisi 2013;5:4-11.
- Medow MA, Arkes HR, Shaffer VA. Are residents’ decisions influenced more by a decision aid or a specialist’s opinion? A randomized controlled trial. J Gen Intern Med 2010;25:316-20. https://doi.org/10.1007/s11606-010-1251-y.
- Shum HP, Chan KC, Lau CW, Leung AK, Chan KW, Yan WW. Triage decisions and outcomes for patients with Triage Priority 3 on the Society of Critical Care Medicine scale. Crit Care Resusc 2010;12:42-9.
- Afessa B, Gajic O, Keegan MT. Severity of illness and organ failure assessment in adult intensive care units. Crit Care Clin 2007;23:639-58. https://doi.org/10.1016/j.ccc.2007.05.004.
- Harrison DA, Parry GJ, Carpenter JR, Short A, Rowan K. A new risk prediction model for critical care: the Intensive Care National Audit. Research Centre (ICNARC) model. Crit Care Med 2007;35:1091-8. https://doi.org/10.1097/01.CCM.0000259468.24532.44.
- Connors AF, Dawson NV, Desbiens NA, Fulkerson WJ, Goldman L, Knaus WA, et al. A controlled trial to improve care for seriously iII hospitalized patients: the study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA 1995;274:1591-8. https://doi.org/10.1001/jama.1995.03530200027032.
- General Medical Council . Treatment and Care Towards the End of Life: Good Practice in Decision Making 2010.
- Great Britain . Mental Capacity Act 2005 2005.
- Kon AA, Davidson JE, Morrison W, Danis M, White DB. American College of Critical Care Medicine . Shared decision making in ICUs: an American College of Critical Care Medicine and American Thoracic Society Policy Statement. Crit Care Med 2016;44:188-201. https://doi.org/10.1097/CCM.0000000000001396.
- Shalowitz DI, Garrett-Mayer E, Wendler D. The accuracy of surrogate decision makers: a systematic review. Arch Intern Med 2006;166:493-7. https://doi.org/10.1001/archinte.166.5.493.
- Bray K, Wren I, Baldwin A, St Ledger U, Gibson V, Goodman S, et al. BACCN Standards for Nurse Staffing in Critical Care. Newcastle upon Tyne: British Association of Critical Care Nurses; 2009.
- Ball J, Pike G. Past Imperfect, Future Tense: Nurses’ Employment and Morale in 2009. London: Royal College of Nursing; 2009.
- Tan SS, Bakker J, Hoogendoorn ME, Kapila A, Martin J, Pezzi A, et al. Direct cost analysis of intensive care unit stay in four European countries: applying a standardized costing methodology. Value Health 2012;15:81-6. https://doi.org/10.1016/j.jval.2011.09.007.
- Truog RD, Brock DW, Cook DJ, Danis M, Luce JM, Rubenfeld GD, et al. Task Force on Values, Ethics, and Rationing in Critical Care (VERICC) . Rationing in the intensive care unit. Crit Care Med 2006;34:958-63. https://doi.org/10.1097/01.CCM.0000206116.10417.D9.
- Murthy S, Wunsch H. Clinical review: International comparisons in critical care – lessons learned. Crit Care 2012;16. https://doi.org/10.1186/cc11140.
- Faculty of Intensive Care Medicine . Critical Capacity: A Short Research Survey on Critical Care Bed Capacity 2018.
- Giannini A, Consonni D. Physicians’ perceptions and attitudes regarding inappropriate admissions and resource allocation in the intensive care setting. Br J Anaesth 2006;96:57-62. https://doi.org/10.1093/bja/aei276.
- Evans TW, Nava S, Mata GV, Guidet B, Estenssoro E, Fowler R, et al. Critical care rationing: international comparisons. Chest 2011;140:1618-24. https://doi.org/10.1378/chest.11-0957.
- Ward NS, Teno JM, Curtis JR, Rubenfeld GD, Levy MM. Perceptions of cost constraints, resource limitations, and rationing in United States intensive care units: results of a national survey. Crit Care Med 2008;36:471-6. https://doi.org/10.1097/CCM.0B013E3181629511.
- Louriz M, Abidi K, Akkaoui M, Madani N, Chater K, Belayachi J, et al. Determinants and outcomes associated with decisions to deny or to delay intensive care unit admission in Morocco. Intensive Care Med 2012;38:830-7. https://doi.org/10.1007/s00134-012-2517-0.
- Yap HY, Joynt GM, Gomersall CD. Ethical attitudes of intensive care physicians in Hong Kong: questionnaire survey. Hong Kong Med J 2004;10:244-50.
- Young PJ, Arnold R. Intensive care triage in Australia and New Zealand. N Z Med J 2010;123:33-46.
- Mielke J, Martin DK, Singer PA. Priority setting in a hospital critical care unit: qualitative case study. Crit Care Med 2003;31:2764-8. https://doi.org/10.1097/01.CCM.0000098440.74735.DE.
- Walter KL, Siegler M, Hall JB. How decisions are made to admit patients to medical intensive care units (MICUs): a survey of MICU directors at academic medical centers across the United States. Crit Care Med 2008;36:414-20. https://doi.org/10.1097/01.CCM.0000299738.26888.37.
- Cooper AB, Sibbald R, Scales DC, Rozmovits L, Sinuff T. Scarcity: the context of rationing in an Ontario ICU. Crit Care Med 2013;41:1476-82. https://doi.org/10.1097/CCM.0b013e31827cab6a.
- Wildman MJ, Sanderson C, Groves J, Reeves BC, Ayres J, Harrison D, et al. Implications of prognostic pessimism in patients with chronic obstructive pulmonary disease (COPD) or asthma admitted to intensive care in the UK within the COPD and asthma outcome study (CAOS): multicentre observational cohort study. BMJ 2007;335. https://doi.org/10.1136/bmj.39371.524271.55.
- Poses RM, Smith WR, McClish DK, Huber EC, Clemo FL, Schmitt BP, et al. Physicians’ survival predictions for patients with acute congestive heart failure. Arch Intern Med 1997;157:1001-7. https://doi.org/10.1001/archinte.1997.00440300111009.
- Smith WR, Poses RM, McClish DK, Huber EC, Clemo FL, Alexander D, et al. Prognostic judgments and triage decisions for patients with acute congestive heart failure. Chest 2002;121:1610-17. https://doi.org/10.1378/chest.121.5.1610.
- Sprung CL, Artigas A, Kesecioglu J, Pezzi A, Wiis J, Pirracchio R, et al. The Eldicus prospective, observational study of triage decision making in European intensive care units. Part II: intensive care benefit for the elderly. Crit Care Med 2012;40:132-8. https://doi.org/10.1097/CCM.0b013e318232d6b0.
- Bülow HH, Sprung CL, Baras M, Carmel S, Svantesson M, Benbenishty J, et al. Are religion and religiosity important to end-of-life decisions and patient autonomy in the ICU? The Ethicatt study. Intensive Care Med 2012;38:1126-33. https://doi.org/10.1007/s00134-012-2554-8.
- Dahine J, Mardini L, Jayaraman D. The perceived likelihood of outcome of critical care patients and its impact on triage decisions: a case-based survey of intensivists and internists in a Canadian, quaternary care hospital network. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0149196.
- Stawicki SP, Pryor JP, Hyams ES, Gupta R, Gracias VH, Schwab CW. The surgeon and the intensivist: reaching consensus in intensive care triage. J Surg Educ 2007;64:289-93. https://doi.org/10.1016/j.jsurg.2007.05.008.
- Findlay GP, Shotton H, Kelly K, Mason M. Time to Intervene? A Review of Patients who Underwent Cardiopulmonary Resuscitation as a Result of In-hospital Cardiac Arrest. London: National Confidential Enquiry into Patient Outcomes and Death; 2012.
- Executive N. Guidelines on Admission to and Discharge from Intensive Care and High Dependency Care Units. Wetherby: Department of Health and Social Care; 1996.
- Department of Health and Social Care . Comprehensive Critical Care: A Review of Adult Critical Care Services 2000.
- Mid Trent Critical Care Network . Admission and Operational Policy 2014.
- North West London Critical Care Network . Network Admissions Policy for Adult Critical Care Services 2017.
- Nates JL, Nunnally M, Kleinpell R, Blosser S, Goldner J, Birriel B, et al. ICU admission, discharge, and triage guidelines: a framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med 2016;44:1553-602. https://doi.org/10.1097/CCM.0000000000001856.
- Blanch L, Abillama FF, Amin P, Christian M, Joynt GM, Myburgh J, et al. Triage decisions for ICU admission: report from the Task Force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care 2016;36:301-5. https://doi.org/10.1016/j.jcrc.2016.06.014.
- Rees S, Griffiths F, Bassford C, Brooke M, Fritz Z, Huang H, et al. The experiences of health care professionals, patients, and families of the process of referral and admission to intensive care: a systematic literature review [published online ahead of print March 11 2019]. J Intensive Care Soc 2019. https://doi.org/10.1177/1751143719832185.
- James FR, Power N, Laha S. Decision-making in intensive care medicine – a review. JICS 2017. https://doi.org/10.1177/1751143717746566.
- Wells G, Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa, ON: The Ottawa Hospital; 2011.
- Patra J, Bhatia M, Suraweera W, Morris SK, Patra C, Gupta PC, et al. Exposure to second-hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta-analysis of 18 observational studies. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001835.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Cohen RI, Eichorn A, Silver A. Admission decisions to a medical intensive care unit are based on functional status rather than severity of illness. A single center experience. Minerva Anestesiol 2012;78:1226-33.
- Dodek P, Kozak JF, Norena M, Wong H. More men than women are admitted to 9 intensive care units in British Columbia. J Crit Care 2009;24:630.e1-8. https://doi.org/10.1016/j.jcrc.2009.02.010.
- Garcia E, Suarez R, Fuentes ME, Sanchez-Gonzalez MA, Campos JM, Sanchez M, et al. Predicted factors for ICU-admission refusal in an university tertiary care hospital. Intensive Care Med 2013;39:S330-S1.
- Garcia E, Suarez R, Fuentes ME, Sanchez-Gonzalez MA, Campos JM, Sanchez M, et al. ICU-admission refusal: a frequent type of limiting lifesustaining therapy in patients with co-morbidity and poor outcome. Intensive Care Med 2013;39.
- Joynt GM, Gomersall CD, Tan P, Lee A, Cheng CA, Wong EL. Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome. Intensive Care Med 2001;27:1459-65. https://doi.org/10.1007/s001340101041.
- Ohta BM. Determinants of Care for Medicare Recipients at the End of Life: Utilization and Decision Making in the Acute Care Hospital 2008.
- Borel M, Veber B, Hervé C, Rigaud JP, Moutel G, Rey N, et al. Conditions of decision making of admission or non-admission in surgical intensive care unit. Ann Fr Anesth Reanim 2012;31:203-7. https://doi.org/10.1016/j.annfar.2011.11.024.
- Chen LM, Render M, Sales A, Kennedy EH, Wiitala W, Hofer TP. Intensive care unit admitting patterns in the Veterans Affairs health care system. Arch Intern Med 2012;172:1220-6. https://doi.org/10.1001/archinternmed.2012.2606.
- Guidet B, Boumendil A, Garrouste-Orgeas M, Pateron D. Admitting elderly patients in intensive-care unit. An emergency-department perspective. Reanimation 2008;17:790-801. https://doi.org/10.1016/j.reaurg.2008.09.004.
- Mohammedi I, Martin O, Argaud L, St Denis M, Ferry T, Robert D. Patients refused in admission to an intensive care unit. Prospective evaluation of the causes and outcome. Presse Med 2003;32:1738-40.
- Naidoo K, Singh JA, Lalloo UG. Survey of ethical dilemmas facing intensivists in South Africa in the admission of patients with HIV infection requiring intensive care. South Afr J Crit Care 2013;29:28-32. https://doi.org/10.7196/sajcc.153.
- Raine R, Goldfrad C, Rowan K, Black N. Influence of patient gender on admission to intensive care. J Epidemiol Community Health 2002;56:418-23. https://doi.org/10.1136/jech.56.6.418.
- Tallgren M, Klepstad P, Petersson J, Skram U, Hynninen M. Ethical issues in intensive care – a survey among Scandinavian intensivists. Acta Anaesthesiol Scand 2005;49:1092-100. https://doi.org/10.1111/j.1399-6576.2005.00799.x.
- Toffart AC, Pizarro CA, Schwebel C, Sakhri L, Minet C, Duruisseaux M, et al. Selection criteria for intensive care unit referral of lung cancer patients: a pilot study. Eur Respir J 2015;45:491-500. https://doi.org/10.1183/09031936.00118114.
- Tridente A, Chick A, Keep S, Furmanova S, Webber S, Bryden D. Non medical factors influence likelihood of admission to critical care of acutely unwell patients. Intensive Care Med 2013;39:S401-S2.
- Augier R, Hambleton IR, Harding H. Triage decisions and outcome among the critically ill at the University Hospital of the West Indies. West Indian Med J 2005;54:181-6. https://doi.org/10.1590/S0043-31442005000300005.
- Sanders CL. Clinical Antecedents of a Medical Emergency Team Response As Predictors of ICU Transfer 2008.
- Tulsky JA, Cassileth BR, Bennett CL. The effect of ethnicity on ICU use and DNR orders in hospitalized AIDS patients. J Clin Ethics 1997;8:150-7.
- Berry P, Thomson SJ, Peck M, Standley T. A web-based survey to investigate physicians’ and intensivists’ attitudes to critical care admission for cirrhosis and multiple organ dysfunction. Gut 2014;63. https://doi.org/10.1136/gutjnl-2014-307263.202.
- Borel M, Veber B, Robillard F, Rigaud JP, Dureuil B, Hervé C. Admission of elderly in intensive care: does age affect access to care?. Ann Fr Anesth Reanim 2008;27:472-80. https://doi.org/10.1016/j.annfar.2008.03.015.
- Borel M, Veber B, Villette-Baron K, Hariri S, Dureuil B, Hervé C. Refusal of care in the intensive care: how makes decision?. Ann Fr Anesth Reanim 2009;28:954-61. https://doi.org/10.1016/j.annfar.2009.10.016.
- Cohen RI, Lisker GN, Eichorn A, Multz AS, Silver A. The impact of do-not-resuscitate order on triage decisions to a medical intensive care unit. J Crit Care 2009;24:311-15. https://doi.org/10.1016/j.jcrc.2008.01.007.
- Einav S, Soudry E, Levin PD, Grunfeld GB, Sprung CL. Intensive care physicians’ attitudes concerning distribution of intensive care resources. A comparison of Israeli, North American and European cohorts. Intensive Care Med 2004;30:1140-3. https://doi.org/10.1007/s00134-004-2273-x.
- Ersoy N, Akpinar A. Turkish nurses’ decision making in the distribution of intensive care beds. Nurs Ethics 2010;17:87-98. https://doi.org/10.1177/0969733009349992.
- Hancock HC, Durham L. Critical care outreach: the need for effective decision-making in clinical practice (part 2). Intensive Crit Care Nurs 2007;23:104-14. https://doi.org/10.1016/j.iccn.2006.06.002.
- Katz MH, Nicholson BW, Singer DE, Kelleher PA, Mulley AG, Thibault GE. The triage decision in pulmonary edema. J Gen Intern Med 1988;3:533-9. https://doi.org/10.1007/BF02596094.
- Kim SH, Chan CW, Olivares M, Escobar G. ICU admission control: an empirical study of capacity allocation and its implication for patient outcomes. Management Sci 2015;61:19-38. https://doi.org/10.1287/mnsc.2014.2057.
- Kostopoulou O, Wildman M. Sources of variability in uncertain medical decisions in the ICU: a process tracing study. Qual Saf Health Care 2004;13:272-80. https://doi.org/10.1136/qhc.13.4.272.
- McNarry AF, Goldhill DR. Intensive care admission decisions for a patient with limited survival prospects: a questionnaire and database analysis. Intensive Care Med 2004;30:325-30. https://doi.org/10.1007/s00134-003-2072-9.
- Nakagawa K, Vento MA, Seto TB, Asai SM, Koenig MA, Chang CW, et al. Clinical factors impacting triage decision of intracerebral hemorrhage patients in a region with limited neurocritical care capacity. Neurocrit Care 2012;17.
- Nuckton TJ, List ND. Age as a factor in critical care unit admissions. Arch Intern Med 1995;155:1087-92. https://doi.org/10.1001/archinte.1995.00430100123014.
- Santana Cabrera L, Gil Hernandez N, Mendez Santana A, Marrero Sosa I, Alayon Cabrera S, Martin Gonzalez JC, et al. Perception of ethical attitudes of intensive care nurses on treatment limitation. Enferm Intensiva 2010;21:142-9. https://doi.org/10.1016/j.enfi.2010.06.001.
- Santana Cabrera L, Sánchez-Palacios M, Rodríguez González F, Hernández Medina E, Casamitjana Ortega A, Fernández Arroyo M. Physicians’ attitudes and perceptions regarding the critical care and critical care specialty. Med Intensiva 2008;32:319-28. https://doi.org/10.1016/S0210-5691(08)76209-0.
- Schmidt M, Demoule A, Deslandes-Boutmy E, Chaize M, de Miranda S, Bèle N, et al. Intensive care unit admission in chronic obstructive pulmonary disease: patient information and the physician’s decision-making process. Crit Care 2014;18. https://doi.org/10.1186/cc13906.
- Thet MM, Chong WF, Tay SY, Seow E, Loh SS, Heng BHC. Factors associated with admission to medical intensive care unit high dependency unit from emergency department or death within 24 hours. Ann Acad Med Singapore 2013;1.
- Vincent JL. European attitudes towards ethical problems in intensive care medicine: results of an ethical questionnaire. Intensive Care Med 1990;16:256-64. https://doi.org/10.1007/BF01705162.
- Vincent JL. Forgoing life support in western European intensive care units: the results of an ethical questionnaire. Crit Care Med 1999;27:1626-33. https://doi.org/10.1097/00003246-199908000-00042.
- Oerlemans AJM, Wollersheim H, van Sluisveld N, van der Hoeven JG, Dekkers WJM, Zegers M. Rationing in the intensive care unit in case of full bed occupancy: a survey among intensive care unit physicians. BMC Anesthesiol 2016;16. https://doi.org/10.1186/s12871-016-0190-5.
- Caldeira VM, Silva Júnior JM, Oliveira AM, Rezende S, Araújo LA, Santana MR, et al. Criteria for patient admission to an intensive care unit and related mortality rates. Rev Assoc Med Bras 2010;56:528-34. https://doi.org/10.1590/S0104-42302010000500012.
- Orsini J, Butala A, Ahmad N, Llosa A, Prajapati R, Fishkin E. Factors influencing triage decisions in patients referred for ICU admission. J Clin Med Res 2013;5:343-9. https://doi.org/10.4021/jocmr1501w.
- Richardson SS, Sullivan G, Hill A, Yu W. Use of aggressive medical treatments near the end of life: differences between patients with and without dementia. Health Serv Res 2007;42:183-200. https://doi.org/10.1111/j.1475-6773.2006.00608.x.
- Barnato AE, Mohan D, Downs J, Bryce CL, Angus DC, Arnold RM. A randomized trial of the effect of patient race on physicians’ intensive care unit and life-sustaining treatment decisions for an acutely unstable elder with end-stage cancer. Crit Care Med 2011;39:1663-9. https://doi.org/10.1097/CCM.0b013e3182186e98.
- Tridente A, Chick A, Keep S, Furmanova S, Webber S, Bryden DC. Factors affecting critical care admission to a UK university hospital. Crit Care 2012;16:S180-S1.
- The Society of Critical Care Medicine Ethics Committee . Attitudes of critical care medicine professionals concerning distribution of intensive care resources. Crit Care Med 1994;22:358-62. https://doi.org/10.1097/00003246-199402000-00031.
- Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis*. Crit Care Med 2012;40:2009-15. https://doi.org/10.1097/CCM.0b013e31824e9eae.
- Corona A, Raimondi F. Critical care of HIV-infected patients: still a dilemma for Italian intensivists – results of a multicentre survey. Eur J Anaesthesiol 2010;27:377-82. https://doi.org/10.1097/EJA.0b013e3283333ac7.
- Levkoff S, Wetle T. Clinical decision making in the care of the aged. J Aging Health 1989;1:83-101. https://doi.org/10.1177/089826438900100106.
- Oerlemans AJ, van Sluisveld N, van Leeuwen ES, Wollersheim H, Dekkers WJ, Zegers M. Ethical problems in intensive care unit admission and discharge decisions: a qualitative study among physicians and nurses in the Netherlands. BMC Med Ethics 2015;16. https://doi.org/10.1186/s12910-015-0001-4.
- Alemayehu E, Molloy DW, Guyatt GH, Singer J, Penington G, Basile J, et al. Variability in physicians’ decisions on caring for chronically ill elderly patients: an international study. CMAJ 1991;144:1133-8.
- Beach MC, Morrison RS. The effect of do-not-resuscitate orders on physician decision-making. J Am Geriatr Soc 2002;50:2057-61. https://doi.org/10.1046/j.1532-5415.2002.50620.x.
- Garrouste-Orgeas M, Montesio LLM, Moreau DDM, Reignier JJR, Desmettre TTD, Boussat SSB, et al. Triaging patients to the ICU: a multicenter study of factors influencing admission decisions. Intensive Care Med 2003;29. https://doi.org/10.1007/s00134-003-1709-z.
- Garrouste-Orgeas M, Montuclard L, Timsit JF, Misset B, Christias M, Carlet J. Triaging patients to the ICU: a pilot study of factors influencing admission decisions and patient outcomes. Intensive Care Med 2003;29:774-81. https://doi.org/10.1007/s00134-003-1709-z.
- McCrossan L, Bickerstaffe W, Mostafa SM, Anderson L, Cheater L, Jayson D, et al. Referrals to intensive care: a region-wide audit. Crit Care 2007;11. https://doi.org/10.1186/cc5134.
- Piers RD, Benoit DD, Schrauwen WJ, Van Den Noortgate NJ. Factors influencing ICU referral at the end of life in the elderly. Z Gerontol Geriatr 2010;43:376-80. https://doi.org/10.1007/s00391-010-0151-4.
- Toffart AC, Lugosi M, Linda S, Pop O, Vesin A, Schwebel C, et al. Lung cancer patients with organ failures: determinant of ICU admission or palliative care, a hospital-wide prospective study. Intensive Care Med 2013;39.
- Tridente A, Chick A, Keep S, Furmanova S, Webber S, Bryden D. Functional status as a predictor of admission to critical care in acutely unwell patients. Intensive Care Med 2012;38:S119-20.
- Docherty AB, Anderson NH, Walsh TS, Lone NI. Equity of access to critical care among elderly patients in Scotland: a national cohort study. Crit Care Med 2016;44:3-13. https://doi.org/10.1097/CCM.0000000000001377.
- Barnato AE, Berhane Z, Weissfeld LA, Chang CC, Linde-Zwirble WT, Angus DC. Robert Wood Johnson Foundation ICU End-of-Life Peer Group . Racial variation in end-of-life intensive care use: a race or hospital effect?. Health Serv Res 2006;41:2219-37. https://doi.org/10.1111/j.1475-6773.2006.00598.x.
- Fowler RA, Sabur N, Li P, Juurlink DN, Pinto R, Hladunewich MA, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ 2007;177:1513-19. https://doi.org/10.1503/cmaj.071112.
- Just E, Casarett DJ, Asch DA, Dai D, Feudtner C. Sex-based differences in end-of-life care among hospitalized adults in the US. J Gen Intern Med 2013;28:S179-80.
- Hart JL, Kohn R, Halpern S. The final bed: a national, qualitative study of intensive care unit clinicians’ reasons for rationing. Am J Respir Crit Care Med 2011;183. https://doi.org/10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A6217.
- Zubek L, Elo G, Szabo L, Szucs O, Varga C, Gal J. Different point of view in therapy restriction. Comparison of the opinions of Hungarian intensive care nurses and physicians. Intensive Care Med 2012;38.
- Cullati S, Hudelson P, Ricou B, Nendaz M, Perneger TV, Dayer P, et al. The importance of advance care planning for seriously ill patients. A qualitative study of doctors’ experiences about admission to intensive care. Palliat Med 2014;28.
- Escher M, Perneger TV, Heidegger CP, Chevrolet JC. Admission of incompetent patients to intensive care: doctors’ responsiveness to family wishes. Crit Care Med 2009;37:528-32. https://doi.org/10.1097/CCM.0b013e3181958409.
- Dallison M, Jones H, Matthews P. Analysis of critical care referrals. Crit Care 2010;14. https://doi.org/10.1186/cc8701.
- Dunne E, Conrick-Martin I, Colreavy F, Marsh B. Prospective analysis of critical care referral patterns prior to the introduction of a national early warning score (EWS). Intensive Care Med 2012;38.
- Teno JM, Gozalo P, Khandelwal N, Curtis JR, Meltzer D, Engelberg R, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds. JAMA Intern Med 2016;176:1809-16. https://doi.org/10.1001/jamainternmed.2016.5964.
- Kelly SG, Hawley M, O’Brien J. Impact of bed availability on requesting and offering in-hospital intensive care unit transfers: a survey study of generalists and intensivists. J Crit Care 2013;28:461-8. https://doi.org/10.1016/j.jcrc.2012.10.070.
- Kelly SG, Hawley M, O’Brien JM. In-hospital intensive care unit transfers: impact of bed availability. Am J Respir Crit Care Med 2010;181. https://doi.org/10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A1657.
- Orsini J, Blaak C, Yeh A, Fonseca X, Helm T, Butala A, et al. Triage of patients consulted for ICU admission during times of ICU-bed shortage. J Clin Med Res 2014;6:463-8. https://doi.org/10.14740/jocmr1939w.
- Astles T, Cope T, Nagaraja S. Referrals made to critical care: a prospective service evaluation. Intensive Care Med 2013;39.
- Strauss MJ, LoGerfo JP, Yeltatzie JA, Temkin N, Hudson LD. Rationing of intensive care unit services. An everyday occurrence. JAMA 1986;255:1143-6. https://doi.org/10.1001/jama.1986.03370090065021.
- Kim SH, Chan CW, Olivares M, Escobar GJ. Association among ICU congestion, ICU admission decision, and patient outcomes. Crit Care Med 2016;44:1814-21. https://doi.org/10.1097/CCM.0000000000001850.
- Todres L, Fulbrook P, Albarran J. On the receiving end: a hermeneutic-phenomenological analysis of a patient’s struggle to cope while going through intensive care. Nurs Crit Care 2000;5:277-87.
- Fulbrook P, Allan D, Carroll S, Dawson D. On the receiving end: experiences of being a relative in critical care. Part 1. Nurs Crit Care 1999;4:138-45.
- Martin DK, Singer PA, Bernstein M. Access to intensive care unit beds for neurosurgery patients: a qualitative case study. J Neurol Neurosurg Psychiatry 2003;74:1299-303. https://doi.org/10.1136/jnnp.74.9.1299.
- Danjoux Meth N, Lawless B, Hawryluck L. Conflicts in the ICU: perspectives of administrators and clinicians. Intensive Care Med 2009;35:2068-77. https://doi.org/10.1007/s00134-009-1639-5.
- Charlesworth M, Mort M, Smith AF. An observational study of critical care physicians’ assessment and decision-making practices in response to patient referrals. Anaesthesia 2017;72:80-92. https://doi.org/10.1111/anae.13667.
- Higginbottom G, Pillay JJ, Boadu NY. Guidance on performing focused ethnographies with an emphasis on healthcare research. Qual Report 2013;18:1-6.
- Wall SS. Focused ethnography: a methodological adaptation for social research in emerging contexts. Forum Qual Sozialforsch 2014.
- Murchison J. Ethnography Essentials: Designing, Conducting and Presenting Your Research. New York, NY: Wiley; 2010.
- Larson KE, Bradshaw CP. Cultural competence and social desirability among practitioners: a systematic review of the literature. Children Youth Serv Rev 2017;76:100-11. https://doi.org/10.1016/j.childyouth.2017.02.034.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. London: SAGE Publications Ltd; 1998.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today 2004;24:105-12. https://doi.org/10.1016/j.nedt.2003.10.001.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Dunn M, Sheehan M, Hope T, Parker M. Toward methodological innovation in empirical ethics research. Camb Q Healthc Ethics 2012;21:466-80. https://doi.org/10.1017/S0963180112000242.
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. New York, NY: Oxford University Press; 2013.
- Sokol DK. The ‘four quadrants’ approach to clinical ethics case analysis; an application and review. J Med Ethics 2008;34:513-16. https://doi.org/10.1136/jme.2007.021212.
- Daniels N, Sabin J. Limits to health care: fair procedures, democratic deliberation, and the legitimacy problem for insurers. Philos Public Aff 1997;26:303-50. https://doi.org/10.1111/j.1088-4963.1997.tb00082.x.
- Dotolo D, Nielsen EL, Curtis JR, Engelberg RA. Strategies for enhancing family participation in research in the ICU: findings from a qualitative study. J Pain Symptom Manage 2017;54:226-30. https://doi.org/10.1016/j.jpainsymman.2017.03.004.
- Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. Dordrecht: Springer; 2008.
- de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145-72. https://doi.org/10.1002/hec.1697.
- Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 2014;32:883-902. https://doi.org/10.1007/s40273-014-0170-x.
- Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health 2013;16:3-13. https://doi.org/10.1016/j.jval.2012.08.2223.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health 2016;19:300-15. https://doi.org/10.1016/j.jval.2016.04.004.
- Bassford CR, Krucien N, Ryan M, Griffiths FE, Svantesson M, Fritz Z, et al. UK intensivists’ preferences for patient admission to ICU: evidence from a choice experiment. Crit Care Med 2019;47:1522-30. https://doi.org/10.1097/CCM.0000000000003903.
- Rose JM, Bliemer MCJ. Sample size requirements for stated choice experiments. Transportation 2013;40:1021-41. https://doi.org/10.1007/s11116-013-9451-z.
- Louviere JJ, Hensher DA, Swait JD, Adamowicz W. Stated Choice Methods: Analysis and Applications. Cambridge: Cambridge University Press; 2000.
- Train K. Discrete Choice Methods with Simulation. Cambridge: Cambridge University Press; 2009.
- Lancsar E, Louviere J, Donaldson C, Currie G, Burgess L. Best worst discrete choice experiments in health: methods and an application. Soc Sci Med 2013;76:74-82. https://doi.org/10.1016/j.socscimed.2012.10.007.
- Orme BK. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. Madison, WI: Research Publications LLC; 2010.
- Greene WH, Hensher DA. A latent class model for discrete choice analysis: contrasts with mixed logit. Transport Res-B Methodol 2003;37:681-98. https://doi.org/10.1016/S0191-2615(02)00046-2.
- Mentzakis E, Ryan M, McNamee P. Using discrete choice experiments to value informal care tasks: exploring preference heterogeneity. Health Econ 2011;20:930-44. https://doi.org/10.1002/hec.1656.
- Train K, Weeks M, Scarpa R, Alberini A. Applications of Simulation Methods in Environmental and Resource Economics. Berlin: Springer-Verlag; 2005.
- Hurst SA, Danis M. A framework for rationing by clinical judgment. Kennedy Inst Ethics J 2007;17:247-66. https://doi.org/10.1353/ken.2007.0021.
- Martin DK, Giacomini M, Singer PA. Fairness, accountability for reasonableness, and the views of priority setting decision-makers. Health Policy 2002;61:279-90. https://doi.org/10.1016/S0168-8510(01)00237-8.
- Rees S, Bassford C, Dale J, Fritz Z, Griffiths F, Parsons H, et al. Implementing an intervention to improve decision making around referral and admission to intensive care: results of feasibility testing in three NHS hospitals [published online ahead of print May 17 2019]. J Eval Clin Pract 2019. https://doi.org/10.1111/jep.13167.
- Plain English Campaign . Plain English Campaign 2018 n.d. www.plainenglish.co.uk/ (accessed 12 November 2018).
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- Guest G, MacQueen KM, Namey EE. Applied Thematic Analysis. London: SAGE Publications Ltd; 2011.
- Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods 2006;5:80-92. https://doi.org/10.1177/160940690600500107.
- General Medical Council . Good Medical Practice 2013.
- Daniels N, Sabin JE. Accountability for reasonableness: an update. BMJ 2008;337. https://doi.org/10.1136/bmj.a1850.
- Akabayashi A, Slingsby BT, Kai I, Nishimura T, Yamagishi A. The development of a brief and objective method for evaluating moral sensitivity and reasoning in medical students. BMC Med Ethics 2004;5. https://doi.org/10.1186/1472-6939-5-1.
- Siegler M, Rezler AG, Connell KJ. Using simulated case studies to evaluate a clinical ethics course for junior students. J Med Educ 1982;57:380-5. https://doi.org/10.1097/00001888-198205000-00006.
- McAlpine H, Kristjanson L, Poroch D. Development and testing of the ethical reasoning tool (ERT): an instrument to measure the ethical reasoning of nurses. J Adv Nurs 1997;25:1151-61. https://doi.org/10.1046/j.1365-2648.1997.19970251151.x.
- Savulescu J, Crisp R, Fulford KW, Hope T. Evaluating ethics competence in medical education. J Med Ethics 1999;25:367-74. https://doi.org/10.1136/jme.25.5.367.
- Chao SY, Chang YC, Yang SC, Clark MJ. Development, implementation, and effects of an integrated web-based teaching model in a nursing ethics course. Nurse Educ Today 2017;55:31-7. https://doi.org/10.1016/j.nedt.2017.04.011.
- Goldie J, Schwartz L, McConnachie A, Morrison J. The impact of three years’ ethics teaching, in an integrated medical curriculum, on students’ proposed behaviour on meeting ethical dilemmas. Med Educ 2002;36:489-97. https://doi.org/10.1046/j.1365-2923.2002.01176.x.
- Goldie J, Schwartz L, McConnachie A, Morrison J. The impact of a modern medical curriculum on students’ proposed behaviour on meeting ethical dilemmas. Med Educ 2004;38:942-9. https://doi.org/10.1111/j.1365-2929.2004.01915.x.
- Turner SL, Bechtel GA. The effectiveness of guided design on ethical decision making and moral reasoning among community nursing students. Nursing Connections 1998;11:69-74.
- Singer PA, Robb A, Cohen R, Norman G, Turnbull J. Evaluation of a multicenter ethics objective structured clinical examination. J Gen Intern Med 1994;9:690-2. https://doi.org/10.1007/BF02599011.
- Singer PA, Robb A, Cohen R, Norman G, Turnbull J. Performance-based assessment of clinical ethics using an objective structured clinical examination. Acad Med 1996;71:495-8. https://doi.org/10.1097/00001888-199605000-00021.
- Lohfeld L, Goldie J, Schwartz L, Eva K, Cotton P, Morrison J, et al. Testing the validity of a scenario-based questionnaire to assess the ethical sensitivity of undergraduate medical students. Med Teach 2012;34:635-42. https://doi.org/10.3109/0142159X.2012.687845.
- Smith SR, Balint JA, Krause KC, Moore-West M, Viles PH. Performance-based assessment of moral reasoning and ethical judgment among medical students. Acad Med 1994;69:381-6. https://doi.org/10.1097/00001888-199405000-00012.
- Pearlman RA, Foglia MB, Fox E, Cohen JH, Chanko BL, Berkowitz KA. Ethics Consultation Quality Assessment Tool: a novel method for assessing the quality of ethics case consultations based on written records. Am J Bioeth 2016;16:3-14. https://doi.org/10.1080/15265161.2015.1134704.
- Fins JJ, Kodish E, Cohn F, Danis M, Derse AR, Dubler NN, et al. A pilot evaluation of portfolios for quality attestation of clinical ethics consultants. Am J Bioeth 2016;16:15-24. https://doi.org/10.1080/15265161.2015.1134705.
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539-49. https://doi.org/10.1007/s11136-010-9606-8.
- Moe CS. Relationship of ethical knowledge to action in senior Baccalaureate nursing students. Nurs Educ Perspect 2018;39:363-5. https://doi.org/10.1097/01.NEP.0000000000000319.
- Ketefian S. Moral reasoning and ethical practice in nursing. Measurement issues. Nurs Clin North Am 1989;24:509-21.
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420-8. https://doi.org/10.1037/0033-2909.86.2.420.
- Revelle WR. psych: procedures for personality and psychological research. Evanston, IL, USA: Northwestern University; 2018.
- de Vet HCW, Terwee CB, Mokkink LB, Knol DL. Measurement in Medicine: A Practical Guide. Cambridge: Cambridge University Press; 2011.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Tulaimat A, Gueret RM, Wisniewski MF, Samuel J. Association between rating of respiratory distress and vital signs, severity of illness, intubation, and mortality in acutely ill subjects. Respir Care 2014;59:1338-44. https://doi.org/10.4187/respcare.02650.
- Pommerening MJ, Goodman MD, Holcomb JB, Wade CE, Fox EE, Del Junco DJ, et al. Clinical gestalt and the prediction of massive transfusion after trauma. Injury 2015;46:807-13. https://doi.org/10.1016/j.injury.2014.12.026.
- Goettler CE, Waibel BH, Goodwin J, Watkins F, Toschlog EA, Sagraves SG, et al. Trauma intensive care unit survival: how good is an educated guess?. J Trauma 2010;68:1279-87. https://doi.org/10.1097/TA.0b013e3181de3b99.
- Penaloza A, Verschuren F, Meyer G, Quentin-Georget S, Soulie C, Thys F, et al. Comparison of the unstructured clinician gestalt, the wells score, and the revised Geneva score to estimate pretest probability for suspected pulmonary embolism. Ann Emerg Med 2013;62:117-24.e2. https://doi.org/10.1016/j.annemergmed.2012.11.002.
- Great Britain . Equality Act 2010 2010.
- Fumis RRL, Junqueira Amarante GA, de Fátima Nascimento A, Vieira Junior JM. Moral distress and its contribution to the development of burnout syndrome among critical care providers. Ann Intensive Care 2017;7. https://doi.org/10.1186/s13613-017-0293-2.
- Bagshaw SM, Opgenorth D, Potestio M, Hastings SE, Hepp SL, Gilfoyle E, et al. Healthcare provider perceptions of causes and consequences of ICU capacity strain in a large publicly funded integrated health region: a qualitative study. Crit Care Med 2017;45:e347-e356. https://doi.org/10.1097/CCM.0000000000002093.
- General Medical Council (GMC) . Consent: Patients and Doctors Making Decisions Together 2008.
- Lim WC, Black N, Lamping D, Rowan K, Mays N. Conceptualizing and measuring health-related quality of life in critical care. J Crit Care 2016;31:183-93. https://doi.org/10.1016/j.jcrc.2015.10.020.
- Henry SG. Recognizing tacit knowledge in medical epistemology. Theor Med Bioeth 2006;27:187-213. https://doi.org/10.1007/s11017-006-9005-x.
- Salloch S, Otte I, Reinacher-Schick A, Vollmann J. What does physicians’ clinical expertise contribute to oncologic decision-making? A qualitative interview study. J Eval Clin Pract 2018;24:180-6. https://doi.org/10.1111/jep.12840.
- Kahneman D. Thinking, Fast and Slow. London: Penguin; 2012.
- Dawson NV, Arkes HR. Systematic errors in medical decision making: judgment limitations. J Gen Intern Med 1987;2:183-7. https://doi.org/10.1007/BF02596149.
- Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Making 2015;35:539-57. https://doi.org/10.1177/0272989X14547740.
- Norman GR, Monteiro SD, Sherbino J, Ilgen JS, Schmidt HG, Mamede S. The causes of errors in clinical reasoning: cognitive biases, knowledge deficits, and dual process thinking. Acad Med 2017;92:23-30. https://doi.org/10.1097/ACM.0000000000001421.
- Coombs M, Dillon A. Crossing boundaries, re-defining care: the role of the critical care outreach team. J Clin Nurs 2002;11:387-93. https://doi.org/10.1046/j.1365-2702.2002.00625.x.
- Marsh S, Pittard A. Outreach:’the past, present, and future’. Contin Educ Anaesthesia Crit Care Pain 2012;12:78-81. https://doi.org/10.1093/bjaceaccp/mkr062.
- Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis 2014;59:96-105. https://doi.org/10.1093/cid/ciu239.
- Walker IA, Reshamwalla S, Wilson IH. Surgical safety checklists: do they improve outcomes?. Br J Anaesth 2012;109:47-54. https://doi.org/10.1093/bja/aes175.
- Cook TM, Woodall N, Frerk C. Fourth National Audit Project . Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth 2011;106:617-31. https://doi.org/10.1093/bja/aer058.
- NHS Institute for Innovation and Improvement . Safer Care: SBAR n.d. www.england.nhs.uk/improvement-hub/wp-content/uploads/sites/44/2017/11/SBAR-Implementation-and-Training-Guide.pdf (accessed 30 September 2019).
- McNulty T, Ferlie E. Reengineering Health Care: The Complexities of Organizational Transformation. Oxford: Oxford University Press; 2002.
- May CR, Johnson M, Finch T. Implementation, context and complexity. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0506-3.
- May C. Agency and implementation: understanding the embedding of healthcare innovations in practice. Soc Sci Med 2013;78:26-33. https://doi.org/10.1016/j.socscimed.2012.11.021.
- Treadwell JR, Lucas S, Tsou AY. Surgical checklists: a systematic review of impacts and implementation. BMJ Qual Saf 2014;23:299-318. https://doi.org/10.1136/bmjqs-2012-001797.
- Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0116629.
- Perkins GD, Griffiths F, Slowther A-M, George R, Fritz Z, Satherley P, et al. Do-not-attempt-cardiopulmonary-resuscitation decisions: an evidence synthesis. Health Serv Deliv Res 2016;4. https://doi.org/10.3310/hsdr04110.
- Mentzelopoulos SD, Bossaert L, Raffay V, Askitopoulou H, Perkins GD, Greif R, et al. A survey of key opinion leaders on ethical resuscitation practices in 31 European Countries. Resuscitation 2016;100:11-7. https://doi.org/10.1016/j.resuscitation.2015.12.010.
- Kranidiotis G, Gerovasili V, Tasoulis A, Tripodaki E, Vasileiadis I, Magira E, et al. End-of-life decisions in Greek intensive care units: a multicenter cohort study. Crit Care 2010;14. https://doi.org/10.1186/cc9380.
- Coombs MA, Addington-Hall J, Long-Sutehall T. Challenges in transition from intervention to end of life care in intensive care: a qualitative study. Int J Nurs Stud 2012;49:519-27. https://doi.org/10.1016/j.ijnurstu.2011.10.019.
- Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care units. Am J Respir Crit Care Med 2011;183:915-21. https://doi.org/10.1164/rccm.201008-1214OC.
- Nunez ER, Schenker Y, Joel ID, Reynolds CF, Dew MA, Arnold RM, et al. Acutely bereaved surrogates’ stories about the decision to limit life support in the ICU. Crit Care Med 2015;43:2387-93. https://doi.org/10.1097/CCM.0000000000001270.
- Miller JJ, Morris P, Files DC, Gower E, Young M. Decision conflict and regret among surrogate decision makers in the medical intensive care unit. J Crit Care 2016;32:79-84. https://doi.org/10.1016/j.jcrc.2015.11.023.
- Sivakumar R, Knight J, Devlin C, Keir P, Ghosh P, Khan S. Communicating information on cardiopulmonary resuscitation to hospitalised patients. J Med Ethics 2004;30:311-12. https://doi.org/10.1136/jme.2002.002915.
- EWCA Civ. R (Tracey) V. Cambridge University Hospital NHS Foundation Trust [2014]. n.d.
- Nelson JE, Angus DC, Weissfeld LA, Puntillo KA, Danis M, Deal D, et al. End-of-life care for the critically ill: a national intensive care unit survey. Crit Care Med 2006;34:2547-53. https://doi.org/10.1097/01.CCM.0000239233.63425.1D.
- Visser M, Deliens L, Houttekier D. Physician-related barriers to communication and patient- and family-centred decision-making towards the end of life in intensive care: a systematic review. Crit Care 2014;18. https://doi.org/10.1186/s13054-014-0604-z.
- Baggs JG, Schmitt MH, Prendergast TJ, Norton SA, Sellers CR, Quinn JR, et al. Who is attending? End-of-life decision making in the intensive care unit. J Palliat Med 2012;15:56-62. https://doi.org/10.1089/jpm.2011.0307.
- Abbott KH, Sago JG, Breen CM, Abernethy AP, Tulsky JA. Families looking back: one year after discussion of withdrawal or withholding of life-sustaining support. Crit Care Med 2001;29:197-201. https://doi.org/10.1097/00003246-200101000-00040.
- Harsch C, Martin G. Comparing holistic and analytic scoring methods: issues of validity and reliability. Assess Educ 2013;20:281-307. https://doi.org/10.1080/0969594X.2012.742422.
- Beavan JR, Alshathar H. Physician’s attitudes towards admitting stroke patients to critical care units (CCU). Cerebrovasc Dis 2012;33:652-3.
- Schmidt M, Similowski T, Chaize M, De Miranda S, Belle N, Roche N, et al. How are patients with chronic obstructive pulmonary disease (COPD) prepared to the eventuality of an intensive care unit (ICU) admission?. Intensive Care Med 2012;38.
- Pastori MM, Sarti M, Pons M, Barazzoni F. Assessing the impact of bibliographical support on the quality of medical care in patients admitted to an internal medicine service: a prospective clinical, open, randomised two-arm parallel study. Evid Based Med 2014;19:163-8. https://doi.org/10.1136/ebmed-2014-110021.
Appendix 1 Search strategies for systematic reviews 1 and 2
| Search | Database (number of hits) | Total (number of hits) | ||||||
|---|---|---|---|---|---|---|---|---|
| MEDLINE | EMBASE | Web of Science | CINAHL | ASSIA | PsycINFO | The Cochrane Library | ||
| Initial | 8258 | 18,038 | 2166 | 1440 | 130 | 1804 | 1332 | 33,168 |
| After deduplication | 8405 | 23,926 | 6341 | 3298 | 494 | 2238 | 1699 | 46,401 |
| Database | Number of hits | |
|---|---|---|
| Initial | After deduplication | |
| Dissertations and Theses | 728 | 725 |
| Index to Theses | 498 | 438 |
| OpenGrey | 40 | 33 |
| Total | 1266 | 1196 |
MEDLINE
Date of search: 11 May 2015.
Search strategy
| 1 | intensive care.mp. or exp Critical Care/ or exp Intensive Care/ |
| 2 | critical care.mp. |
| 3 | exp Intensive Care Units/ or intensive care unit*.mp. |
| 4 | exp Critical Illness/ or critical illness*.mp. |
| 5 | icu.mp. |
| 6 | critically ill*.mp. |
| 7 | itu.mp. |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 |
| 9 | exp Triage/ |
| 10 | exp Patient Admission/ |
| 11 | exp Patient Transfer/ |
| 12 | exp Refusal to Treat/ |
| 13 | exp “Referral and Consultation”/ |
| 14 | exp Resource allocation/ |
| 15 | (triag* or admission* or admit* or refus* or deny or delay or refer* or limit* or transfer*).ti,ab. |
| 16 | 9 or 10 or 11 or 12 or 13 or 14 or 15 |
| 17 | professional practice.mp. or exp Professional Practice/ |
| 18 | exp Decision Making/ |
| 19 | exp Judgment/ |
| 20 | exp “Attitude of Health Personnel”/ |
| 21 | exp Medical Futility/ |
| 22 | exp Choice Behavior/ or choice behaviour.mp. |
| 23 | (futil* adj5 (care or treatment* or medical)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] |
| 24 | (judgement* or judgment* or decision* or choice* or prognostic pessimism).ti,ab. |
| 25 | (attitude* or experience*).ti,ab. |
| 26 | exp Professional-Family Relations/ |
| 27 | 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 |
| 28 | 8 and 16 and 27 |
| 29 | (neonat* or pediatric* or paediatric* or picu or nicu).ti,ab. |
| 30 | 28 not 29 |
EMBASE
Date of search: 11 May 2015.
Search strategy
| 1 | intensive care.mp. or exp intensive care/ |
| 2 | critical care.mp. |
| 3 | exp intensive care unit/ or intensive care unit*.mp. |
| 4 | exp critical illness/ or critical illness*.mp. |
| 5 | exp critically ill patient/ or critically ill patient*.mp. |
| 6 | critical care unit*.mp. |
| 7 | icu.mp. |
| 8 | itu.mp. |
| 9 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 |
| 10 | exp hospital admission/ |
| 11 | exp patient transport/ |
| 12 | exp patient abandonment/ |
| 13 | “refusal to treat”.mp. |
| 14 | exp patient referral/ |
| 15 | exp resource allocation/ |
| 16 | (triag* or admission* or admit* or refus* or deny or delay or refer* or limit* or transfer*).ti,ab. |
| 17 | 10 or 11 or 12 or 13 or 14 or 15 or 16 |
| 18 | professional practice.mp. or exp professional practice/ |
| 19 | exp decision making/ |
| 20 | exp health personnel attitude/ |
| 21 | (judgement* or judgment* or decision* or choice* or prognostic pessimism).ti,ab. |
| 22 | (futil* adj5 (care or treatment* or medical)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 23 | (attitude* or experience*).ti,ab. |
| 24 | (professional-family relation* or professional family relation*).mp. |
| 25 | 18 or 19 or 20 or 21 or 22 or 23 or 24 |
| 26 | 9 and 17 and 25 |
| 27 | (neonat* or pediatric* or paediatric* or picu or nicu).ti,ab. |
| 28 | 26 not 27 |
Web of Science
Date of search: 11 May 2015.
Search strategy
TOPIC: (“intensive care” or “critical care” or “intensive care unit*” or “critical illness*” or “critically ill*” or “critical care unit*” or icu or itu)
AND
TOPIC: (triag* or admission* or admit* or refus* or deny or delay or refer or referred or referral or limit or limited or limiting or transfer or transferred or “refusal to treat” or “referral and consultation” or “resource allocation”)
AND
TOPIC: (“professional practice” or “decision making” or judgement* or judgment* or “attitude of health personnel” or “medical futility” or choice* or decision* or “prognostic pessimism” or attitude* or experience* or “professional family relation*” or “professional-family relation*” or (futil* NEAR/5 (treatment* or care or medical)))
NOT
TOPIC: (neonat* or pediatric* or paediatric* or picu or nicu)
PsycInfo
Date of search: 11 May 2015.
Search strategy
((SU.EXACT.EXPLODE(“Intensive Care”) OR (“intensive care” OR “intensive care unit*” OR “critical care” OR “critical ill*” OR “critically ill*” OR “critical care unit*” OR itu OR icu))
AND
(SU.EXACT.EXPLODE(“Hospital Admission”) OR SU.EXACT.EXPLODE(“Resource Allocation”) OR (triag* OR admission* OR admit* OR refus* OR deny OR delay* OR refer* OR limit* OR transfer*))
AND
(SU.EXACT.EXPLODE(“Decision Making”) OR SU.EXACT.EXPLODE(“Judgment”) OR SU.EXACT.EXPLODE(“Health Personnel Attitudes”) OR SU.EXACT.EXPLODE(“Choice Behavior”) OR (“professional practice” OR “medical futility” OR futil* OR judgement* OR judgment* OR decision* OR choice* OR “prognostic pessimism” OR attitude* OR experience* OR “professional family relation*” OR “professional-family relation*”))) NOT
(neonat* OR pediatric* OR paediatric* OR picu OR nicu)
ASSIA
Date of search: 11 May 2015.
Search strategy
(((SU.EXACT.EXPLODE(“Intensive care units”) OR SU.EXACT.EXPLODE(“Intensive care”)) OR (“intensive care” OR “intensive care unit*” OR “critical care” OR “critical care unit*” OR “critical ill*” OR “critically ill*” OR itu OR icu))
AND
(SU.EXACT.EXPLODE(“Triage”) OR SU.EXACT.EXPLODE(“Admissions” OR “Emergency admission” OR “Readmission”) OR SU.EXACT.EXPLODE(“Refusal”) OR (SU.EXACT.EXPLODE(“Direct referrals”) OR SU.EXACT.EXPLODE(“Arrest referral schemes” OR “Direct referrals” OR “Extra contractual referrals” OR “Referrals” OR “Selfreferrals” OR “Weekend referrals”)) OR SU.EXACT.EXPLODE(“Resource allocation”) OR (triag* OR admission* OR admit* OR refus* OR deny OR delay OR refer* OR limit* OR transfer* OR “refusal to treat”))
AND
(SU.EXACT.EXPLODE(“Professional practices”) OR (SU.EXACT.EXPLODE(“Anticipatory decision” OR “Clinical decision making” OR “Collaborative decision making” OR “Decision making” OR “Dynamic decision making” OR “Groupthink” OR “Moral decision making”) OR SU.EXACT.EXPLODE(“Decisions” OR “End of life decisions”) OR SU.EXACT.EXPLODE(“Clinical decision making”)) OR SU.EXACT.EXPLODE(“Adjudicators” OR “Affective judgments” OR “Causal judgments” OR “Censure” OR “Clinical judgments” OR “Compliments” OR “Confidence judgments” OR “Frequency judgments” OR “Judgments” OR “Moral judgments” OR “Praise” OR “Probability judgments” OR “Professional judgments” OR “Reflective judgments” OR “Social censure” OR “Social judgments” OR “Subjective judgments” OR “Value judgments” OR “Visual judgments”) OR SU.EXACT.EXPLODE(“Career choice” OR “Choice” OR “Informed choice” OR “Life choice” OR “Object choice” OR “Parental choice” OR “Social choice”) OR SU.EXACT.EXPLODE(“Family-Health professional relationships”) OR (“professional practice*” OR judgement* OR judgment* OR decision* OR choice* OR “prognostic pessimism” OR attitude* OR experience* OR futil* OR “medical futility”)))
NOT
(neonat* OR pediatric* OR paediatric* OR picu OR nicu)
CINAHL
Date of search: 11 May 2015.
Search strategy
| Search option | Actions |
| S1 | (MH “Intensive Care Units+”) OR (MH “Critical Care+”) OR “intensive care” |
| S2 | “intensive care unit*” |
| S3 | “critical care” |
| S4 | (MH “Critical Illness”) OR (MH “Critically Ill Patients”) |
| S5 | “critical illness*” |
| S6 | “critically ill*” |
| S7 | “icu” |
| S8 | “itu” |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 |
| S10 | (MH “Triage”) |
| S11 | (MH “Patient Admission”) |
| S12 | (MH “Refusal to Treat”) |
| S13 | (MH “Referral and Consultation+”) |
| S14 | (MH “Resource Allocation+”) |
| S15 | (triag* or admission* or admit* or refus* or deny or delay or refer* or limit* or transfer* ) |
| S16 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 |
| S17 | (MH “Professional Practice+”) OR “professional practice” |
| S18 | (MH “Decision Making+”) |
| S19 | (MH “Judgment”) |
| S20 | (MH “Attitude of Health Personnel+”) |
| S21 | (MH “Medical Futility”) |
| S22 | judgment* or judgement* or decision* or choice* or “prognostic pessimism” |
| S23 | attitude* or experience* |
| S24 | (MH “Professional-Family Relations”) |
| S25 | S17 OR S18 OR S20 OR S21 OR S22 OR S23 OR S24 |
| S26 | S9 AND S16 AND S25 |
| S27 | neonat* or pediatric* or paediatric* or picu or nicu |
| S28 | s26 not s27 |
Dissertations and Theses and Index to Theses
Date of search: 23 June 2015.
Search strategy
all(“intensive care” OR “intensive care unit*” OR ICU OR ITU OR “critical care” OR “critical illness*” OR “critically ill*”)
AND
all(triage* OR admission* OR admit* OR refus* OR deny OR delay OR refer* OR limit* OR transfer*)
AND
all(judgement* OR JUDGMENT* OR decision* OR choice* OR “prognostic pessimism” OR attitude* OR experience* OR futil* OR “professional practice*” OR “professional family relation*”)
OpenGrey
Date of search: 22 September 2015.
Search strategy
(“intensive care” OR “intensive care unit*” OR ICU OR ITU OR “critical care” OR “critical illness*” OR “critically ill*”) AND (triage* OR admission* OR admit* OR refus* OR deny OR delay OR refer* OR limit* OR transfer*) AND (judgement* OR JUDGMENT* OR decision* OR choice* OR “prognostic pessimism” OR attitude* OR experience* OR futil* OR “professional practice*” OR “professional family relation*”)
Appendix 2 Methodological quality of included studies for systematic reviews 1 and 2
| Study (authors and year) | Selection (maximum 4 stars) | Comparability (maximum 2 stars) | Outcomes (maximum 3 stars) | Total number of stars (maximum 9) |
|---|---|---|---|---|
| Boumendil et al., 201218 | *** | ** | *** | 8 |
| Caldeira et al., 2010106 | **** | * | *** | 8 |
| Chen et al., 201275 | **** | ** | ** | 8 |
| Cohen et al., 201268 | **** | ** | *** | 9 |
| Cohen et al., 201519 | **** | ** | *** | 9 |
| Docherty et al., 2016124 | **** | ** | *** | 9 |
| Garrouste-Orgeas et al., 200621 | *** | ** | ** | 7 |
| Guidet et al., 200876 | **** | ** | ** | 8 |
| Joynt et al., 200172 | **** | – | ** | 6 |
| Katz et al., 198893 | *** | – | *** | 6 |
| Orsini et al., 2013107 | **** | * | ** | 7 |
| Pintado et al., 201320 | *** | ** | *** | 8 |
| Sanders et al., 200884 | **** | ** | *** | 9 |
| Shum et al., 201024 | **** | – | *** | 7 |
| Sprung et al., 199913 | **** | – | ** | 6 |
| Stelfox et al., 201216 | **** | ** | ** | 8 |
| The Eldicus Study (Sprung et al., 2012;50 Iapichino et al., 201012) | **** | * | *** | 8 |
| Teno et al., 2016134 | **** | ** | ** | 8 |
| Toffart et al., 201581 (this study also includes Toffart et al., 2013122) | **** | ** | *** | 9 |
| Study (authors and year) | Selection (maximum 3 stars) | Comparability (maximum 2 stars) | Outcomes (maximum 1 star) | Total number of stars (maximum 6) |
|---|---|---|---|---|
| Akpinar et al., 201322 | ** | – | – | 2 |
| Alemayehu et al., 1991116 | ** | ** | * | 5 |
| Astles et al., 2013138 | *** | – | * | 4 |
| Augier et al., 200583 | *** | ** | * | 6 |
| Azoulay et al., 200114 | *** | ** | * | 6 |
| Barnato et al., 2006125 | *** | ** | * | 6 |
| Barnato et al., 2011109 | ** | ** | * | 5 |
| Beach et al., 2002117 | ** | – | * | 3 |
| Beavan et al., 2012243 | * | – | – | 1 |
| Berry et al., 201486 | ** | – | – | 2 |
| Borel et al., 200887 | ** | – | * | 3 |
| Borel et al., 200988 | *** | – | * | 4 |
| Borel et al., 201274 | *** | – | * | 4 |
| Cohen et al., 200989 | *** | ** | * | 6 |
| Corona et al., 2010113 | * | – | – | 1 |
| Dallison et al., 2010132 | ** | – | – | 2 |
| Dodek et al., 200969 | *** | ** | * | 6 |
| Dunne et al., 2012133 | *** | – | * | 4 |
| Einav et al., 200490 | * | – | – | 1 |
| Ersoy et al., 201091 | ** | – | – | 2 |
| Escher et al., 2009131 | ** | – | * | 3 |
| Fowler et al., 2007126 | *** | – | * | 4 |
| Garcia et al., 201370,71 | *** | ** | * | 6 |
| Garrouste-Orgeas et al., 200510 | ** | ** | * | 5 |
| Garrouste-Orgeas et al., 201317 | *** | * | – | 4 |
| Gershengorn et al., 2012112 | *** | ** | * | 6 |
| Giannini et al., 200638 | ** | ** | – | 4 |
| Howe et al., 201111 | * | – | – | 1 |
| Just et al., 2013127 | *** | ** | * | 6 |
| Kelly et al., 2013135 | ** | – | – | 2 |
| Kim et al., 201594 | *** | – | * | 4 |
| Kim et al., 2016140 | *** | – | * | 4 |
| Levkoff et al., 1989114 | ** | * | – | 3 |
| Louriz et al., 201241 | ** | – | * | 3 |
| McCrossan et al., 2007120 | *** | – | * | 4 |
| McNarry et al., 200496 | *** | – | * | 4 |
| Mohammedi et al., 200377 | ** | – | * | 3 |
| Naidoo et al., 201378 | * | – | – | 1 |
| Nakagawa et al., 201297 | *** | – | * | 4 |
| Nuckton et al., 199598 | ** | – | – | 2 |
| Oerlemans et al., 2016105 | ** | * | * | 4 |
| Orsini et al., 2014137 | **** | * | * | 6 |
| Ohta et al., 200873 | *** | ** | * | 6 |
| Piers et al., 2010121 | ** | – | – | 2 |
| Raine et al., 200279 | *** | * | * | 5 |
| Richardson et al., 2007108 | *** | ** | * | 6 |
| Rodríguez-Molinero et al., 201015 | *** | ** | – | 5 |
| Santana Cabrera et al., 2008100 | ** | – | – | 2 |
| Santana Cabrera et al., 201099 | ** | – | – | 2 |
| Schmidt et al., 2014101 (this study includes Schmidt et al., 2012244) | ** | – | * | 3 |
| Society of Critical Care Medicine Ethics Committee Special Article, 1994111 | ** | – | – | 2 |
| Strauss et al., 1986139 | *** | – | * | 4 |
| Tallgren et al., 200580 | * | – | – | 1 |
| Thet et al., 2013102 | ** | – | * | 3 |
| Tridente et al., 201382 (this study includes Tridente et al., 2012,110 and Tridente et al., 2012123) | ** | ** | 5 | |
| Tulsky et al., 199785 | ** | ** | 5 | |
| Vincent et al., 1990103 | ** | – | – | 2 |
| Vincent et al., 1999104 | ** | – | – | 2 |
| Yap et al., 200442 | ** | – | – | 2 |
| Zubek et al., 2012129 | ** | – | – | 2 |
| Study | Selection bias | Performance bias (blinding of participants and personnel) (high risk/low risk/unclear) | Detection bias (blinding of outcome assessment) (high risk/low risk/unclear) | Attrition bias (incomplete outcome data) (high risk/low risk/unclear) | Selective outcome reporting (high risk/low risk/unclear) | Other sources of bias (high risk/low risk/unclear) | |
|---|---|---|---|---|---|---|---|
| Random sequence generation (high risk/low risk/unclear) | Allocation concealment (high risk/low risk/unclear) | ||||||
| Garrouste-Orgeas et al., 201317 | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk | Unclear |
| Pastori et al., 2014245 | Low risk | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear |
| Study (authors and year) | Worth or relevance (yes/no/unclear) | Clarity of research question (yes/no/unclear) | Appropriateness of design to the question (yes/no/unclear) | Context (yes/no/unclear) | Sampling (yes/no/unclear) | Data collection and analysis (yes/no/unclear) | Reflexivity of account (yes/no/unclear) |
|---|---|---|---|---|---|---|---|
| Charlesworth et al., 2017145 | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Cooper et al., 201346 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Cullati et al., 2014130 | Yes | Yes | Yes | Yes | Unclear | Unclear | No |
| Danjoux Meth et al., 2009144 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hancock et al., 200792 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Todres et al., 2000141 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ohta et al., 200873 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes |
| Mielke et al., 200344 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes |
| Kostopoulou et al., 200495 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Oerlemans et al., 2015115 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Fulbrook et al., 1999142 | Yes | Yes | Yes | Yes | Yes | Unclear | No |
| Martin et al., 2003143 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hart et al., 2011128 | Yes | Yes | Yes | Yes | Unclear | Yes | No |
Appendix 3 Table of study characteristics: systematic review 1
| Author and year | Study design | Participants and number | Study setting | Country | Patient factors | Clinician factors | Organisational factors | Other |
|---|---|---|---|---|---|---|---|---|
| Akpinar et al., 201322 | Cross-sectional | 228 intensive care physicians from university, state and private hospitals, as well as training hospitals connected to a university or the Ministry of Health | Two medical congresses | Turkey |
Presence of chronic illness Severity of acute illness Functional status/quality of life Age Patient preference Health insurance Other: whether or not the patient is under the care of a state institution; dependants; lifestyle factors contributing to the condition |
Seniority of clinician Religiosity Personal characteristics/demography of ICU clinician: age of clinician; sex of clinician; political views; whether or not clinician had children |
No |
Cost of treatment to society Social and economic effect on the family |
| Alemayehu et al., 1991116 | Cross-sectional | 897 physician respondents | Family practice, medical and geriatric rounds in academic medical centres (including teaching and community hospitals) | Australia, Brazil, Canada, Scotland, Sweden, USA and Wales |
Patient preference: presence of detailed treatment and resuscitative effort chart (documents wishes of family and patient) Presence of DNACPR order |
Seniority of clinician Personal characteristics/demography of ICU clinician: country of practice |
No | No |
| Astles et al., 2013138 | Cross-sectional | 1105 referrals captured over 203 days (40 days missing) | Single university hospital | UK | No | No | ICU bed availability | No |
| Augier et al., 200583 | Cohort |
Patients/health-care professionals Consecutive sampling of all patients referred to ICU service 356 requests for ICU admission studied in total (384: 28 exclusions) |
Single centre: 500-bed tertiary referral centre | Jamaica |
Age Severity of acute illness |
No | ICU bed availability | No |
| Azoulay et al., 200114 | Cross-sectional | All consecutive patients referred to ICU during the study period in 1292 patients included | 26 adult ICUs | France |
Presence of chronic illness Type of acute illness Functional status/quality of life Age |
No | No | No |
| Barnato et al., 2006125 | Cross-sectional | Patient data relating to 192,705 clinical cases | 674 acute care hospitals across Florida, Massachusetts, New Jersey, New York and Virginia | USA |
Ethnicity Sex |
No | No | No |
| Barnato et al., 2011109 | Randomised factorial simulation | 33 physicians (intensivists, acute physicians and emergency care physicians in one county) | Simulations took place in University of Pittsburgh Peter M Winter Institute for Simulation Education and Research | USA |
Ethnicity Presence of chronic illness: different type of cancer |
No | No | No |
| Beach et al., 2002117 | Cross-sectional | 241 health-care practitioners (attending physicians, medical residents) | Single department of medicine in a large urban academic medical centre | USA | Presence of DNACPR order | No | No | No |
| Beavan et al., 2012243 | Cross-sectional | 210 doctors (intensivists/anaesthetists, stroke, neurologists, geriatricians) | Web-based survey through e-mail and specialist society websites | UK | No | Other: referring clinician’s base specialty | No | No |
| Berry et al., 201486 | Cross-sectional | 144 clinicians (consultant physicians, consultant anaesthetists and specialist trainees) | NHS trusts in south of England | UK (England) |
Severity of chronic illness Severity of acute illness |
Physician’s attitude | ICU bed availability | No |
| Borel et al., 200887 | Cross-sectional (two studies: questionnaire survey and review of patient records) |
Questionnaire survey: 19 senior intensivists Response rate: 19 out of 34 (13 men and 6 women) Patient record study: 80 patients refused intensive care admission |
Questionnaire study: general critical care in five hospitals in one region Patient record study: one university hospital surgical ICU |
France |
Age Severity of chronic illness Severity of acute illness Functional status/quality of life Patient preference Other: non-compliance with treatment plan |
No | ICU bed availability | No |
| Borel et al., 200988 | Cross-sectional | Consecutive referrals to ICU who were denied admission to ICU (150 cases were analysed) | Surgical ICU in Rouen University hospital (16 beds) | France |
Presence of chronic illness Severity of acute illness: APACHE II score Age Functional status/quality of life: patient capacity/autonomy |
No | ICU bed availability | No |
| Borel et al., 201274 | Cross-sectional | 298 patients (149 admitted patients and 149 non-admitted patients) | Surgical ICU in Rouen University hospital (16 beds) | France |
Severity of chronic illness Age Presence of chronic illness Sex Type of acute illness Functional status/quality of life: limited patient autonomy |
Seniority of clinician Clinician communication skills: collegial decision |
ICU bed availability | No |
| Boumendil et al., 201218 | Cohort | 2646 patients in total (across both the admitted and the non-admitted cohorts) | 15 acute care hospitals in the Paris metropolitan region | France |
Presence of chronic illness: respiratory, cardiac, neuro, cancer Type of acute illness: medical condition associated with admission Functional status/quality of life Age Sex Nutritional status Pre-admission length of hospital stay (recent hospitalisation) |
No | No | No |
| Caldeira et al., 2010106 | Cohort | All patients aged > 18 years for whom an ICU bed was requested between 1 July and 30 September 2005. Total included 359 | Tertiary referral hospital | Brazil |
Severity of acute illness: APACHE II score; Multiple Organ Dysfunction Score Presence of chronic disease Age Sex |
No | Specialty of patient | No |
| Chen et al., 201275 | Cohort |
289,310 patients All adult nonsurgical admissions to any Veteran Affairs acute care hospital (on-site ICU admissions from the ED or the outpatient clinic) |
118 Veterans Affairs acute care hospitals | USA |
Type of acute illness Severity of acute illness: Veterans Affairs ICU severity score Age Sex Ethnicity |
No | ICU bed availability | No |
| Cohen et al., 200989 | Cross-sectional | 179 consecutive critical care consultations for possible admission to the MICU | University-affiliated teaching hospital in New York City with a 12-bed MICU | USA |
Presence of chronic illness Severity of acute illness: APACHE II score Functional status/quality of life: MRS Age Sex Presence of DNACPR order |
No | No | No |
| Cohen et al., 201268 | Cohort | Medical ICU consultations for 501 patients | A 12-bed medical ICU within a 500-bed academic adult hospital | USA |
Type of acute illness Severity of acute illness Functional status/quality of life Pre-admission length of hospital stay Age Sex Ethnicity: Caucasian; Hispanic; African; American; other Health insurance: Type of payer – commercial; managed care; self-pay; other Presence of DNACPR order |
Seniority of clinician: attending/spending < 25% of time in medical ICU | ICU bed availability | No |
| Cohen et al., 201519 | Cross-sectional | All patients admitted from the ED to a general medical ward for whom MICU consult was requested within 48 hours of admissions; 134 patients | 600-bed adult academic hospital | USA |
Severity of acute illness: APACHE II score Severity of chronic illness: Elixhauser score Type of acute illness Functional status/quality of life Age Sex Presence of DNACPR order |
No | No | No |
| Cooper et al., 201346 | Qualitative (interview study) | 22 ICU clinicians participated, out of 24 invited (12 ICU physicians, 4 ICU fellows, 2 ICU nursing team leaders, and 4 ICU resource nurses) | An ICU of a university-affiliated hospital | Canada |
Functional status/quality of life Family preference |
Clinician’s personal attitude |
ICU bed availability Avoid conflict/litigation Other: hospital prioritisation programme |
Pressure from referring clinicians Clinical uncertainty |
| Corona et al., 2010113 | Cross-sectional | 126 ICU physicians, from the 239 in the GiViTI professional/membership network | 440 ICUs, from all types of hospitals within the country | Italy | Severity of chronic illness | No | Hospital characteristics: presence of infectious disease ward | No |
| Cullati et al., 2014130 | Qualitative (individual, in-depth interviews, thematic analysis based) | 24 doctors (12 ICU doctors and 12 internal medicine doctors) | University hospitals across Geneva | Switzerland | Family preference | Seniority of clinician | ICU bed availability | Clinical uncertainty |
| Dallison et al., 2010132 | Cross-sectional | All referrals to intensive care service in a 6-week period | Single hospital | UK (Wales) | No | Seniority of referrer | No | No |
| Docherty et al., 2016124 | Cohort | All adult patients (aged > 16 years) admitted to ICU between 2005 and 2009. Total 40,142 | Scottish Intensive Care Society audit group database (all ICUs in Scotland) | Scotland | Age | No | No | No |
| Dodek et al., 200969 | Cross-sectional | Patients admitted to hospital between 1998 and 2008 | Nine tertiary, and community hospitals in one region | Canada |
Type of acute illness Severity of chronic illness: Quan’s adaptation of Charlson index Age Sex |
No | No | No |
| Dunne et al., 2012133 | Cross-sectional | 76 patients referred to ICU in a 1-month period | 500 bed hospital | Ireland | No | Seniority of referrer: referrer is consultant grade | No | No |
| Einav et al., 200490 | Cross-sectional | 43 intensive care physicians who were members of the Israel Society of Critical Care Medicine | Mail survey | Israel |
Presence of chronic illness Severity of acute illness Functional status/quality of life Age Patient preference Trajectory of illness |
Physician’s attitude | No |
Cost to society Social cost to family Social worth |
| Ersoy et al., 201091 | Cross-sectional | 136 intensive care nurses | Two ICU congresses in Turkey | Turkey |
Severity of acute illness Severity of chronic illness Presence of chronic illness Trajectory of illness: patient has not made progress during hospitalisation Functional status/quality of life Age Family preference Health insurance |
No | No |
Cost to society Patient social contribution Cost to family |
| Escher et al., 2009131 | Cross-sectional | 232, out of 402 certified professional society members (Swiss Society of Intensive Care Medicine) | Mail-survey | Switzerland | Family preference | No | Hospital characteristics: university hospital, or not | No |
| Fowler et al., 2007126 | Cross-sectional | 466,792 patients admitted consecutively to adult hospitals between 1 January 2001 and 31 December 2002 | 13 hospitals (four teaching and nine community) in one region (Ontario) | Canada | Sex | No | No | No |
| Garcia et al., 2013;71 Garcia et al., 201370 | Cross-sectional |
Garcia et al. , 2013:70 803 consecutive patients referred to intensive care service during 1 year Garcia et al. , 2013:71 281 consecutive patients with a decision to limit life-sustaining treatment by an ICU clinician over 1 year (using ICU refusal as a decision to limit life-sustaining treatment) |
University tertiary care hospital | Spain |
Age Sex Type of acute illness Presence of chronic illness Functional status/quality of life Patient preference Family preference |
No | No | No |
| Garrouste-Orgeas et al., 2005: consisting of three research papers: Garrouste-Orgeas et al., 2005;10 Garrouste-Orgeas et al., 2003;119 and Garrouste-Orgeas et al., 2003118 | Cross-sectional |
Garrouste-Orgeas et al. , 2005:10 all 574 adult patients referred to intensive care service (in June 2001) Garrouste-Orgeas et al. , 2003:119 all 334 patients referred to intensive care (over 8-month study period) Garrouste-Orgeas et al. , 2003:118 572 admissions decisions |
Garrouste-Orgeas et al. , 2005:10 11 ICUs (four medical and seven medical-surgical ICUs) Garrouste-Orgeas et al. , 2003:119 10-bed medical-surgical ICU in an acute-care 460-bed tertiary care hospital Garrouste-Orgeas et al. , 2003:118 11 ICUs |
France |
Type of acute illness Presence of chronic illness Severity of chronic illness: McCabe score Severity of acute illness Functional status/quality of life Age Sex |
Seniority of clinician |
ICU bed availability Decision-maker present Time of day Speciality of patient |
No |
| Garrouste-Orgeas et al., 200621 | Cohort | 180 patients aged ≥ 80 years who were triaged for admission to ICU | A 460-bed tertiary non-university hospital for adults | France |
Functional status/quality of life ADL Age Sex Severity of acute illness: Mortality prediction model |
Seniority of clinician: ICU experience of the triaging physician |
ICU bed availability Speciality of patient Time of day Decision-maker present Other: number of clinicians involved in triage |
No |
| Garrouste-Orgeas et al., 201317 | Cross-sectional | 220 physicians from the French Society for Critical Care stratified by geographic area (Paris vs. others) | Web-based survey | France |
Presence of chronic illness: cancer Functional status/quality of life: IADL and ADL scores; self-sufficiency Quality of life: WHOQOL-BREF Environment Age: ≥ 85 Patient preference Trajectory of illness: patient’s previous ICU admission; hospitalisation in the past year |
No |
ICU bed availability (three step) Other: geography – Paris area or not |
No |
| Gershengorn et al., 2012112 | Cross-sectional | 15,994 patient admissions identified from the Agency for Healthcare Research and Quality’s State Inpatient Database | 159 New York State acute care hospitals | USA |
Severity of chronic illness Age Sex Ethnicity Health insurance Other, non-medical: more affluent zip code |
No |
ICU bed availability Other Weekend/weekday Hospital characteristics: % utilisation non-diabetic ketoacidosis patients; location; volume diabetic ketoacidosis; number of hospital beds; % hospital beds designated ICU; teaching status; hospital occupancy |
No |
| Giannini et al., 200638 | Cross-sectional | 225 ICU physicians working in Milan | 20 ICUs in Milan | Italy | Family preference |
Other Assessment error; clinical doubt |
Avoid conflict/litigation Time to make a decision |
Pressure from seniors Pressure from referring team Pressure from management |
| Guidet et al., 200876 | Cohort |
Cohort study: 2646 patients aged > 80 years with a condition potentially requiring ICU admission across 15 centres (13 centres sampled across 12 months, two centres for less than this) 662 were referred; 330 were admitted Delphi: emergency care physicians across France; 30 physicians. No information about number invited |
Cohort study: emergency care departments in 15 hospitals across France | France |
Type of acute illness: severity of acute illness Presence of chronic illness Functional status/quality of life Nutritional status Age Sex Other: patient had a recent hospitalisation (i.e. within the last 6 months); number of medicines |
Seniority of clinician | Time of day | No |
| Hancock et al., 200792 | Qualitative (reflecting on practice relating to a single clinical case experienced by the study participant) | One critical care nurse-consultant | Single acute hospital | UK |
Severity of chronic illness (nature of renal disease relevant) Severity of acute illness (physiological parameters) Other: nurses’ perception of patient’s reserve (patient tiring); presence of DNACPR order |
No | No | Clinical uncertainty |
| Hart et al., 2011128 | Qualitative |
648 ICU physicians responded out of the 2206 invited (31%) 438 ICU nurses responded out of the 988 invited (44.3%) |
Care institutions across the country | USA |
Patient preference Other: perceived probability of benefit from ICU care |
No | ICU bed availability: administrative or legal influences | The need to use the last ICU bed to provide maximal social benefit |
| Howe et al., 201111 | Cross-sectional | Data on decision processes relating to 100 consecutive referrals to ICU from non-elective, acute medical inpatients | A closed, mixed medical-surgical and paediatric ICU; within a tertiary referral hospital serving all specialties except organ transplantation | Australia |
Type of acute illness Functional status/quality of life Presence of chronic illness Sex Age Ethnicity |
Seniority of referrer |
ICU bed availability Specialty of patient |
No |
| The ELDICUS study (consisting of Sprung et al., 2012,50 and Iapichino et al., 201012) | Cohort |
Sprung et al. , 2012:50 6796 patients referred/triaged for ICU admission; 5602 accepted, 1194 rejected Iapichino et al. , 2010:12 7994 patients eligible for the study (patients referred to ICU); 7877 patients were included in the analysis |
Sprung et al. , 2012:50 11 closed, general and specialty ICUs in seven European countries Iapichino et al. , 2010:12 11 closed ICUs in seven European countries |
Denmark; France; Italy; Israel; the Netherlands; Spain; the UK |
Functional status/quality of life Age Sex Type of acute illness Severity of acute illness Pre-admission length of hospital stay Presence of chronic illness: comorbidities |
No |
ICU bed availability Specialty of patient Other: ventilators in ward |
≥ 1 triage during admission |
| Joynt et al., 200172 | Cohort | 624 adult patients referred to ICU from December 1997 to June 1998 | A 22-bed multidisciplinary, adult and paediatric ICU, serving a 1400-bed university referral hospital | China (Hong Kong) |
Severity of acute illness: mortality probability model score Type of acute illness Age Sex |
Prognostic pessimism | No | No |
| Just et al., 2013127 | Cross-sectional | Detailed clinical administrative data on 98,314 hospitalised patients | 458 acute care hospitals | USA | Sex | No | No | No |
| Katz et al., 198893 | Cohort | 216 patients with cardiogenic pulmonary oedema entered into study; analysis on 108 patients who did not develop a complication in the emergency ward | Single emergency ward in general hospital | USA |
Presence of chronic illness Severity of acute illness: physiological parameters, pleural effusion Age |
No | No | No |
| Kelly et al., 2013135 (the other paper relating to this study is Kelly et al., 2010136) | Cross-sectional |
44 (out of 112 invited) residents and hospitalist attending physicians participated in the Generalist ICU Request study (response rate of 39%) 92 (out of 173 invited) intensivists participated in the second Intensivist ICU Offer study (response rate of 53%) |
For the Generalist ICU Request study, data were from an academic medical centre, and a community-based teaching hospital For the Intensivist ICU Offer study, data were from intensivists across 17 academic medical centres |
USA | No | No | ICU bed availability | No |
| Kim et al., 201594 | Cross-sectional | Total number of patient records included 70,133 patients admitted to a medical ward from the ED during a 12-month period | 15 hospitals with an ICU of 10 or more beds in an integrated health-care system | Not specified |
Severity of acute illness Age Sex |
No | No | No |
| Kim et al., 2016140 | Cohort | 70,133 patients admitted via ED to an inpatient unit. During 12-month period, 20% were eligible for study | 15 hospitals in integrated health system | USA | No | No | ICU bed availability | No |
| Kostopoulou et al., 200495 | Qualitative | 14 consultants in total (half respiratory medicine, half anaesthesia and intensive care) | Seven hospitals across the West Midlands region | UK |
Severity of acute illness: blood gases Severity of chronic illness: COPD severity suggested by frequent hospital admissions Functional status: exercise tolerance Estimated survival at 6 months |
Other: physician assessment of importance or relevance of clinical information; physician interpretation of clinical information | No | No |
| Levkoff et al., 1989114 | Cross-sectional | Telephone interviews with 251 health-care advisors (96 physicians, 121 nurses, 31 social workers and 3 psychologists) | Two large VA medical centre acute care hospitals and a combined VA acute care and long-term care facility | USA |
Functional status/quality of life Age Patient preference Family preference Other Cognitive status; degree of pain and suffering |
No | Other: rules and practices for admitting older people | No |
| Louriz et al., 201241 | Cross-sectional | 398 adult patients consecutively triaged for admission to ICU | A 12-bed medical ICU at a 1028-bed tertiary/university hospital | Morocco |
Presence of chronic illness: metastatic cancer Severity of acute illness: mortality predicted model at admission Severity of chronic illness: McCabe score Type of acute illness Age Sex |
Seniority of clinician |
ICU bed availability Time of day: 08.00–17.59 or 18.00–07.59 |
No |
| Martin et al., 2003143 | Qualitative | 13 key informants interviewed using theoretical sampling | Tertiary and quaternary centre teaching hospital | Canada | Family preference | Other: pressure applied by referring doctor |
Written guidelines Other: patients within the hospital rather than those being referred from external sources |
No |
| McCrossan et al., 2007120 | Cross-sectional | All patients referred to ICU (number not stated in abstraction form) | Six hospitals across one region (Merseyside) | UK | Age | Seniority of referrer |
Specialty of patient: surgical patients were more likely to be admitted than medical patients Time of day |
No |
| McNarry et al., 200496 | Cross-sectional |
Number invited not given 169 clinicians completed a questionnaire, 166 analysed |
Questionnaire distributed electronically through the Intensive Care Society | UK |
Severity of acute illness Family preference |
No | No | No |
| Mielke et al., 200344 | Qualitative (case-study based) | 20 health-care professionals | A combined medical-surgical ICU of a large urban university-affiliated hospital | Canada |
Severity of acute illness Other: transplant patient Family preference |
No |
ICU bed availability Other: availability of nursing staff in ICU |
No |
| Mohammedi et al., 200377 | Cross-sectional | Decisions relating to all patients triaged for admission to a medical ICU (251 patients), of whom 132 were refused admission | Critical care unit with 15 beds capacity, from a university hospital with 1100 beds | France |
Type of acute illness Severity of acute illness Age |
No | ICU bed availability | No |
| Naidoo et al., 201378 | Cross-sectional | Questionnaire distributed to conference delegates. 450 questionnaires to eligible participants out of 830 delegates; 90 questionnaires suitable for analysis | National medical conference | South Africa |
Age Type of acute illness Presence of chronic illness Functional status/quality of life Patient preference Family preference Presence of DNACPR order Other: ability to contribute to society |
Seniority of clinician: physician experience Other: threat/fear of litigation |
ICU bed availability Written guidelines Avoid conflict/litigation |
Human rights |
| Nakagawa et al., 201297 | Cross-sectional | 397 consecutive patients hospitalised for intracranial haemorrhage between 2006 and 2010 | A tertiary care centre (the only eight-bed neuroscience ICU for the state) | Not stated |
Severity of acute illness Age Presence of DNACPR order |
No | Other: transfer from another hospital | No |
| Nuckton et al., 199598 | Cross-sectional | 114 intensivists in the greater Chicago area | Form of survey (postal/online) not specified | USA |
Presence of chronic illness Severity of acute illness Age Health insurance: ability to pay Presence of DNACPR order Other: ability to contribute to society; patient attitude; family support; current health status (alcohol/smoking/exercise) |
No | No | No |
| Ohta et al., 200873 | Cross-sectional |
For the quantitative study, data on 3409 terminal patient admissions For the qualitative study, 13 clinicians |
For the quantitative study, 28 acute care hospitals in Maricopa County, Arizona For the qualitative study, intensive care and medical units of a 400-bed, private, non-profit, community acute care hospital |
USA |
Severity of acute illness: acuity on hospital admission (APR-DRG) level Type of acute illness Age Sex Ethnicity Health insurance |
No | Hospital characteristics: hospital bed capacity; for-profit organisation | No |
| Oerlemans et al., 2015115 | Qualitative |
19 interview participants (ICU physicians) Focus group participants: five ICU physicians; five general ward physicians; seven ICU nurses; eight general ward nurses |
General, teaching and academic hospitals | The Netherlands | Functional status/quality of life: clinician interpretation of quality of life | No |
ICU bed availability Written guidelines |
Other Clinical uncertainty |
| Oerlemans et al., 2016105 | Cross-sectional | Questionnaire survey sent to 751 members of Dutch Society of Intensive care; 166 respondents (21.8%) | Netherlands |
Severity of acute illness Presence of chronic illness Quality of life: perceived by patient; perceived by clinician Trajectory of illness Age Patient’s wishes Family wishes |
No | Presence of guideline |
Other Cost to society Social worth of patient Social and economic impact on family Pressure from patient or family Cost effectiveness Pressure from other physician |
|
| Orsini et al., 2013107 | Cohort |
All patients aged > 18 years referred for ICU admission between 1 August 2012 and 31 October 2012 Total 165 |
General community inner-city hospital | USA |
Severity of acute illness: APACHE II score Age Patient’s preferences: presence of advance directive |
No | ICU bed availability | No |
| Orsini et al., 2014137 | Cross-sectional | Patients aged ≥ 18 years referred to ICU at time of ICU overcrowding during April and May 2014. Total 92 patients | General community inner-city hospital | USA | No | No | ICU bed availability | No |
| Pastori et al., 2014245 | Intervention | 201 patients hospitalised on internal medicine wards that generated treatment questions (100 in the control group and 101 in the intervention group) | Internal medicine service of a non-university hospital | Switzerland | No | No | No | Provision of evidence-based medicine-related bibliographical support |
| Piers et al., 2010121 | Cross-sectional | 330 adult patients (aged > 16 years) who died in hospital during the study period (patients admitted to psychiatry, palliative day hospital and ED were excluded) | A single university hospital | Belgium | Age | No | No | No |
| Pintado et al., 201320 | Cohort | 338 patients evaluated for ICU admission. Four patients refused ICU care and were excluded from the analysis | A 14-bed medical and surgical ICU in a hospital | Spain |
Presence of chronic illness: comorbidity Type of acute illness Severity of chronic illness: Charlson Comorbidity Score; Cruz Roja Mental Scale Severity of acute illness: SOFA and APACHE II scores Functional status/quality of life: Barthel Index Age Sex |
No | ICU bed availability | No |
| Raine et al., 200279 | Cross-sectional | 11,074 patients admitted to ICU with a primary diagnosis in one of 10 prespecified categories | ICNARC database (includes 91 units, contributing units across tertiary and secondary hospitals) | UK (specifically in England, Wales and Northern Ireland) | Sex | No | No | No |
| Richardson et al., 2007108 | Cross-sectional | 169,036 patients aged > 65 years who died between October 1999 and September 2001 and used Veterans Health Administration health-care services in the last 2 years of their life | VA databases | USA |
Age Presence of chronic illness: dementia |
No | No | No |
| Rodríguez-Molinero et al., 201015 | Cross-sectional |
101 elderly patients. Randomly selected from those admitted to the ED from July to November 2003. All patients > 80 years, and patients between 65 and 79 years, provided that the latter had at least two comorbid chronic conditions Each patient’s physician and a family member also participated |
Four university teaching hospitals | Spain |
Functional status/quality of life: family assessment of (Katz and Barthel indices) Functional status/quality of life: physician’s assessment (Katz and Barthel indices) Presence of chronic illness: history of cancer; cognitive status Age |
No | No | No |
| Sanders et al., 200884 | Cohort | 140 adult inpatients, who were treated by a Medical Emergency Team (MET) for pre-defined clinical instability criteria | Academic medical centre 417-bed tertiary referral centre | USA |
Severity of acute illness Age Sex Pre-admission length of hospital stay |
Seniority of referrer: years of experience of the nurse initiating the medical emergency teams (MET) call Experience/expertise of ward team |
Other: nurse-to-bed ratio; frequency of vital signs measurements | No |
| Santana Cabrera et al., 2008100 | Cross-sectional | 116 non-intensivist doctors | A tertiary academic hospital | Spain |
Age Severity of chronic illness: baseline situation Severity of acute illness: short-term prognosis, divergent professional opinions about this Functional status/quality of life: sequela that might remain Patient preference Family preference |
No | ICU bed availability | No |
| Santana Cabrera et al., 201099 | Cross-sectional | 52 intensive care nurses (86.6% of all intensive care nursing staff at the hospital at the time) | A tertiary university hospital | Spain |
Severity of acute illness: length of life Functional status/quality of life |
No | No | No |
| Schmidt et al., 2014101 (this study also includes Schmidt et al., 2012244) | Cross-sectional |
200 pulmonologists randomly selected from the French language society of respiratory medicine (‘Société de Pneumologie de Langue Française’) database that comprises about 2000 names 173 responders (138 with no missing data) 175 ICU physicians derived from the ‘Famirea’ database (no explanation of this database) (135 responses, 119 with no missing data) |
The postal survey was country wide | France |
Age Presence of chronic illness: heart failure; depression (all patients had COPD) Severity of chronic illness: number of hospital admissions for ventilation in last year Functional status/quality of life: physician’s perception of Patient preference Family preference Other: smoking cessation; non-invasive home ventilation; patient has no family |
Other: respiratory nurse’s opinion; general practitioner’s opinion | No | No |
| Shum et al., 201024 | Cohort | 1346 (unique) patient referrals to ICU within the study period January to September 2007 | A ‘closed’, 20-bed, mixed medical-surgical ICU within a 2300-bed acute care tertiary hospital | China (Hong Kong) |
Functional status/quality of life Age Sex Severity of acute illness Type of acute illness Severity of chronic illness |
Seniority of clinician: ICU clinician seniority Prognostic pessimism |
ICU bed availability Time of day Specialty of patient |
No |
| Society of Critical Care Medicine Ethics Committee Special Article, 1994111 | Cross-sectional | 600 returned questionnaires, out of the 1148 registrants attending the symposium | The Annual Educational and Scientific Symposium of the Society of Critical Care Medicine | USA |
Presence of chronic illness Functional status/quality of life: self-reported by patient, or as viewed by physician Age Trajectory of illness Patient preference |
Physician’s attitude | No |
Cost to society Social cost to family Financial cost–benefit analysis Social worth |
| Sprung et al., 199913 | Cohort | All patients triaged for admission to ICU. Total 382 patients | (Eight-bed general critical care unit) in a tertiary medical centre with six critical care units | Israel |
Severity of acute illness: APACHE II score Age Sex Type of acute illness |
No | ICU bed availability | No |
| Stelfox et al., 201216 | Cohort | 3494 consecutive hospitalized adults with sudden clinical deterioration trigger a Medical Emergency Treatment (MET) activation | Alberta Health Services hospitals (regional clinical and administrative databases) | Canada |
Type of acute illness: reason for MET activation (cardiac, respiratory, neurological) Severity of chronic illness: Charlson index Age Trajectory of illness: previous ICU admission during hospital stay Pre-admission length of hospital stay Patient preference: baseline patient goals of care |
Seniority of clinician |
ICU bed availability Time of day: specialty of patient |
No |
| Strauss et al., 1986139 | Cross-sectional | 1151 ICU consecutive admissions (14% of which were readmissions of the same patients) | An 18-bed ICU, in a 286-bed acute-care university hospital | United States | No | No | ICU bed availability | No |
| Tallgren et al., 200580 | Cross-sectional | 51 ICU physicians (out of 83 physicians who received the questionnaire) | ICUs in Scandinavia (data from 51 ICUs) | Scandinavia (Denmark, Finland, Norway, Sweden) |
Age Type of acute illness Functional status/quality of life Presence of chronic illness Severity of chronic illness Severity of acute illness: patient preference Patient wishes Family wishes |
No | ICU bed availability | No |
| Teno et al., 2016134 | Cohort | Medicare beneficiaries hospitalised with advanced dementia between 1 January 2000 and 31 December 2013; 380,060 eligible patients | Minimum data set assessments linked with Medicare part A claims | USA | No | No | ICU bed availability | No |
| Thet et al., 2013102 | Cross-sectional | 3795 patients admitted to ED | ED of a tertiary hospital | Singapore |
Severity of acute illness Functional status/quality of life: ADL, or dependent on others for ADL |
No | No | No |
| Toffart et al., 201581 (this study also includes Toffart et al., 2013122) | Cohort | 140 patients with lung cancer presenting with at least one organ dysfunction | Teaching hospital in Grenoble, France; single site | France |
Severity of chronic illness: Charlson Index Severity of acute illness: LODS Type of acute illness Functional status/quality of life: ECOG-PS Patient preference Family preference Age Sex |
No |
ICU bed availability Time of day Specialty of patient |
No |
| Tridente et al., 201382 (this study also includes Tridente et al., 2012,110 and Tridente et al., 2012123) | Cross-sectional |
Tridente et al. , 2012:110 all patients referred to intensive care; total 201 patients (of whom 85 were declined) Tridente et al. , 2012:123 328 patients referred to intensive care (of whom 165 were declined) Tridente et al. , 2013:82 402 patients (of whom 186 were admitted) |
ICU in a large teaching hospital/university hospital | UK |
Age Sex Ethnicity Type of acute illness Presence of chronic illness Comorbidity Severity of acute illness: MEWS/reason for referral Functional status/quality of life Pre-admission length of hospital stay |
No | Specialty of patient | No |
| Tulsky et al., 199785 | Cross-sectional | Random sample of 1376 from potential sample of 1812 patients with Pneumocystis carinii pneumonia or HIV admitted to hospital | Hospitals in the cities of Chicago, Miami and Los Angeles | USA |
Ethnicity Severity of acute illness: stage 3 severity of Pneumocystis carinii pneumonia or HIV Other: intravenous-drug users |
No | Hospital characteristics: type of hospital – VA or government | No |
| Vincent et al., 1990103 | Cross-sectional | 242 members of the European Society of Intensive Care Medicine (590 questionnaires sent) | European Society of Intensive Care Medicine | Belgium; France; Germany; Italy; Netherlands; Portugal; Scandinavia; Spain; Switzerland; UK |
Severity of acute illness Functional status/quality of life |
No | ICU bed availability | No |
| Vincent et al., 1999104 | Cross-sectional | 1272 Western European members of the European Society of Intensive Care Medicine, of whom 504 responded | Postal questionnaire survey | Austria; Belgium; France; Germany; Greece; Italy; the Netherlands; Portugal; Scandinavia; Spain; Switzerland; UK |
Functional status/quality of life: including patient assessed quality of life Severity of acute illness: limited or poor prognosis |
No | ICU bed availability | No |
| Yap et al., 200442 | Cross-sectional |
95 questionnaires were sent to all intensive care doctors practising in 11 Hong Kong ICUs 65 ICU doctors completed the questionnaire |
11 ICUs in Hong Kong (in hospitals under the Hong Kong Hospital Authority) | China (Hong Kong) |
Severity of acute illness: no hope of survival Functional status/quality of life: physician and patient assessment |
No | ICU bed availability | No |
| Zubek et al., 2012129 | Cross-sectional | 302 completed questionnaires (from 191 ICU physicians and 102 ICU nurses) | Hungarian ICUs | Hungary | Patient preference | No | ICU bed availability | No |
Appendix 4 Factors associated with admission or refusal of admission in multivariate analysis (systematic review 1)
| Factor | Finding | OR (95% CI) | Study (first author and year) |
|---|---|---|---|
| Type of acute illness | |||
| Respiratory | Reduced odds of refusal | 0.36 (0.23 to 0.55) | Azoulay, 200114*** |
| Increased odds of admission | 4.23 (3.65 to 4.91) | Dodek, 200969*** | |
| Increased odds of refusal | 3.9 (3.17 to 4.9) | Stelfox, 201216*** | |
| 7.25 (2.96 to 17.1) | Garcia, 201370,71*** | ||
| Cardiovascular | Increased odds of admission | 1.9; p < 0.001 | Ohta, 200873*** |
| 2.7 (2.1 to 3.48) | Dodek, 200969*** | ||
| 1.68 (1.26 to 2.24) | Iapichino, 201012*** | ||
| 1.74 (1.38 to 2.19) | Stelfox, 201216*** | ||
| Reduced odds of refusal compared with reference respiratory failure | 0.53 (0.29 to 0.99) | Joynt, 200172** | |
| Increased odds of refusal | 14.26 (3.95 to 51.4) | Louriz, 201241** | |
| Infections | Increased odds of admission | 9.33 (7.81 to 11.14) | Dodek, 200969*** |
| 2.33 (1.5 to 3.63) | Cohen, 201268*** | ||
| Reduced odds of refusal compared with ref respiratory failure | 0.46 (0.23 to 0.91) | Joynt, 200172** | |
| Neurological | Increased odds of admission | 2.83 (2.33 to 3.43) | Dodek, 200969*** |
| 1.3 (1.07 to 1.53) | Stelfox, 201216*** | ||
| Increased odds of refusal | 6.17 (1.99 to 19.06) | Garcia, 201370,71*** | |
| 4.05 (1.33 to 12.28) | Louriz, 201241** | ||
| Emergency surgery | Increased odds of admission | 4.44 (3.49 to 5.64) | Iapichino, 201012*** |
| Reduced odds of refusal compared with reference respiratory failure | 0.12 (0.04 to 0.35) | Joynt, 200172** | |
| Severity of acute illness | |||
| APACHE II score | Increased odds of refusal | 0.94 (0.91 to 0.97) | Augier, 200583*** |
| Increased odds of refusal | 0.84 (0.68 to 1.03) | Sprung, 199913** | |
| APS II score | No difference | 1.01 (1.00 to 1.02) | Iapichino, 201012*** |
| MPM-0 | Increased odds of refusal | 0.20 (0.05 to 0.9) | Louriz, 201241** |
| Increased odds of refusal | 0.6 (0.53 to 0.68) | Guidet, 200876*** | |
| APR DRG grouper | Increased odds of admission | 3.56 (1.92 to 6.6) | Sanders, 200884*** |
| EWS | Increased odds of admission | 1.23 (1.12 to 1.35) | Tridente, 201382 |
| Severity of illness (as defined by authors) | Increased odds of admission | 4.76 (3.51 to 7.46) | Tulsky, 199785** |
| Presence of chronic illness | |||
| Metastatic cancer | Increased odds of refusal | 5.82 (2.22 to 15.28) | Garrouste-Orgeas, 200510*** |
| Reduced odds of admission | 1.61 (1.09 to 2.38) | Guidet, 200876*** | |
| 0.6 (0.33 to 1.05) | Boumendil, 201218*** | ||
| Dementia | Reduced likelihood of admission | 7.5 percentage points on probit model (6.9 to 8.1) | Richardson, 2007108*** |
| Mental disorder (use of psychotropic drugs) | Reduced odds of admission | 0.66 (0.45 to 0.95) | Boumendil, 201218*** |
| Chronic respiratory failure or heart failure or metastatic cancer without hope of remission | Increased odds of refusal | 2.24 (1.38 to 3.64) | Azoulay, 200114*** |
| ‘Underlying chronic disease’ | Increased odds of refusal | 8.9 (4.06 to 19.6) | Garcia, 201370,71*** |
| Severity of chronic illness | |||
| Charlson Comorbidity Index | Increased odds of admission | 1.09 (1.04 to 1.14) | Gershengorn, 2012112*** |
| 1.05 (1.10 to 1.09) | Stelfox, 201216*** | ||
| Increased odds of refusal | 0.63 (0.53 to 0.75) | Pintado, 201320*** | |
| Quans adaptation of Charlson | Increased odds of admission | 1.15 (1.13 to 1.17) | Dodek, 200969*** |
| Elixhauser scale | Increased odds of refusal | 0.3 (0.13 to 0.72) | Cohen, 201519*** |
| Functional status/quality of life measures | |||
| Dependence for daily activities | Increased odds of refusal | 8.03 (3.5 to 18.4) | Garcia, 201370*** |
| 14.2 (5.27 to 38.25) | Garrouste-Orgeas, 200510*** | ||
| Decreasing Barthel Index | Increased odds of refusal | 0.97 (0.96 to 0.98) | Pintado, 201320*** |
| Loss of functional status according to Katz ADL score | Increased odds of refusal | 0.93 (0.88 to 0.99) | Guidet, 200876*** |
| Patient living alone | Increased odds of refusal | 1.27 (0.99 to 1.62) | Guidet, 200876** |
| Karnovsky performance status > 40 | Increased odds of admission | 2.84 (2.23 to 3.62) | Iapichino, 201012*** |
| Self-caring status | Increased odds of admission | 2.16 (1.03 to 4.53) | Tridente, 201382 ** |
| 0.04; p < 0.001 | Garrouste-Orgeas, 200621** | ||
| WHOQOL-BREF environmental domain > 75 | Independent predictor of admission in patient aged > 80 years | 2.14 (1.25 to 3.65) | Garrouste-Orgeas, 201317** |
| Expected quality of life post treatment | Most important determinant of admission | None provided | Levkoff, 1989114* |
| Physician perception of functional status (Katz Index) | Independent factor affecting admission | 4.09 (1.81 to 9.25) | Rodríguez-Molinero, 201015** |
| Performance status Knaus Scale C or D | Increased odds of refusal | 3.098 (2.05 to 4.67) | Azoulay, 200114*** |
| Katz ADL per 1-point increase | Increased odds of admission | 1.32 (1.19 to 1.46) | Boumendil, 201218*** |
| MRS: more functional at baseline | Likelihood to admit to ICU | 0.31 (0.19 to 0.50) | Cohen, 201268*** |
| MRS: less functional at time of evaluation for ICU | Likelihood to admit to ICU | 2.38 (1.40 to 3.26) | Cohen, 201268*** |
| MRS: MRS high (more disability) pre hospital | Likelihood to admit to ICU | 0.132 (0.042 to 0.41) | Cohen, 201219*** |
| MRS: MRS high at ICU consultation | Likelihood to admit to ICU | 13.045 (4.74 to 35.95) | Cohen, 201219*** |
| Nutritional status | |||
| Normal vs. emaciated | Increased odds of admission | 0.42 (0.2 to 0.82) | Boumendil, 201218*** |
| Somewhat malnourished vs. emaciated | Increased odds of admission | 1.06 (0.68 to 1.6) | Boumendil, 201218*** |
| Trajectory of illness | |||
| Pre-admission length of hospital stay | Increased odds of admission for each additional day in hospital prior to MET activation | 0.99 (0.99 to 1.0) | Stelfox, 201216*** |
| Previous hospitalisation in last year | Reduced odds of admission | 0.51 (0.3 to 0.85) | Garrouste-Orgeas, 201317** |
| Recent hospitalisation in the last 6 months | No difference | 1.09 (0.84 to 1.4) | Guidet, 200876*** |
| Prior ICU admission during this hospital admission | Increased odds of admission | 1.88 (1.43 to 2.46) | Stelfox, 201216*** |
| Presence of do not attempt resuscitation order | Reduced odds of admission | 0.42 (0.2 to 0.89) | Cohen, 201268*** |
| 0.17 (0.03 to 0.98) | Cohen, 201519*** | ||
| 0.34 (0.16 to 0.72) | Nakagawa, 201297** | ||
| Age | |||
| Admission decisions per year increase in age | Reduced odds of admission | 0.91 (0.91 to 0.91) | Boumendil, 201218*** |
| 0.98 (0.97 to 0.99) | Iapichino, 201012*** | ||
| 0.92 (0.85 to 0.99) | Pintado, 201320*** | ||
| 1.00 (0.97 to 1.02) | Rodríguez-Molinero, 201015** | ||
| 0.86 (0.75 to 0.98) | Sanders, 200884*** | ||
| 1.00 (0.99 to 1.00) | Stelfox, 201216*** | ||
| Patient aged > 65 years | Reduced odds of admission | 0.28 (0.16 to 0.51) | Azoulay, 200114*** |
| 0.39 (0.25 to 0.61) | Joynt, 200172** | ||
| Patient aged > 80 years | Reduced odds of admission | 1.09 (0.83 to 1.43) | Dodek, 200969 (men)*** |
| 0.51 (0.37 to 0.7) | Dodek, 200969 (women)*** | ||
| 0.24 (0.10 to 0.60) | Garcia, 201370,71*** | ||
| 0.51 (0.27 to 0.97) | Nakagawa, 201297** | ||
| Patient aged > 85 years | Reduced odds of admission | 0.24 (0.08 to 0.72) | Garrouste-Orgeas, 200621** |
| 0.45 (0.26 to 0.79) | Garrouste-Orgeas, 201317** | ||
| Studies reporting age but not included in forest plot | |||
| 25–49 years | Reduced odds of admission | 0.85 (0.77 to 0.94) | Gershengorn, 2012112*** |
| ≥ 50 years | Reduced odds of admission | 0.83 (0.73 to 0.93) | Gershengorn, 2012112*** |
| 40–59 years | Increased odds of admission compared with reference of 15–39 years | 1.28 (1.03 to 1.58) | Dodek, 200969 (men)*** |
| Increased odds of admission compared with reference of 15–39 years | 1.26 (1.0 to 1.59) | Dodek, 200969 (women)*** | |
| 60–79 years | Increased odds of admission compared with reference of 15–39 years | 1.38 (1.12 to 1.71) | Dodek, 200969 (men)*** |
| Increased odds of admission compared with reference of 15–39 years | 1.35 (1.07 to 1.69) | Dodek, 200969*** (women) | |
| Age per decade | Increased odds of refusal | 0.858 (no variance reported) | Guidet, 200876*** |
| Unit of analysis not clear | Increased odds of refusal | 1.02 (no variance reported) | Ohta, 200873*** |
| 1.04 (1.02 to 1.07) | Sprung, 199913** | ||
| Unit of analysis not clear | No difference | 1.01 (0.99 to 1.03) | Caldeira, 2010106*** |
| Sex | |||
| Being female | Reduced odds of refusal | 0.7 (0.47 to 1.05) | Joynt, 200172** (white men compared with white women) |
| White men compared with white women | Increased odds of admission | 1.14 (1.12 to 1.17) | Barnato, 2006125*** |
| Older women compared with older men | Reduced odds of admission | 0.68 (0.66 to 0.71) | Fowler, 2007126** |
| Men aged > 80 years compared with women aged > 80 years | Increased odds of admission | 2.14 (1.55 to 2.94) | Dodek, 200969*** |
| Being female compared to male reference | No difference in admission decisions | 0.82 (0.42 to 1.6) | Sanders, 200884*** |
| 0.970 (no CI reported) | Ohta, 200873*** | ||
| Male vs. female patients admitted for diabetic ketoacidosis | No difference in admission decisions | p = 0.44 | Gershengorn, 2009112*** |
| Ethnicity | |||
| Black women or Hispanic women compared with white women | Increased odds of admission | Black women 1.07 (1.03 to 1.17); Hispanic women 1.18 (1.0 to 1.42) | Barnato, 2006125** |
| Black people and people from other ethnic groups compared with white people with diabetic ketoacidosis | Reduced odds of admission | Black 0.81 (0.73 to 0.90); other ethnic groups 0.86 (0.76 to 0.98) | Gershengorn, 2012112*** |
| Hispanic people compared with Caucasian people | Increased odds of admission (no difference for black, Asian, Native American people or people from other ethnic groups) | 1.66 (p = 0.05) | Ohta, 200873*** |
| African American and Latino/Latina people compared with white patients with AIDS | Increased odds of admission | African American people 1.59 (1.13 to 2.25); Latino/Latina people 1.47 (1.02 to 2.16) | Tulsky, 199785*** |
| Patient preference | |||
| Baseline patient goals for resuscitative vs. medical or comfort care | Increased odds of admission | 8.25 (5.93 to 11.46) | Stelfox, 201216*** |
| Accept vs. refuse ICU admission | Increased odds of admission | 10.6 (6.17 to 18.4) | Garrouste-Orgeas, 201317** |
| Health insurance status | |||
| Medicaid vs. Medicare | No difference | 0.97 (0.86 to 1.08) | Gersehengorn, 2012112 |
| Private pay vs. Medicare | No difference | 1.00 (0.90 to 1.12) | Gersehengorn, 2012112 |
| Self-pay vs. Medicare | No difference | 0.93 (0.80 to 1.07) | Gersehengorn, 2012112 |
| Medicare advantage vs. standard Medicare | Increased odds of admission | 1.363 | Ohta, 200873*** |
| Other (including those coded as no charge) vs. Medicare | Reduced odds of admission | 0.65 (0.5 to 0.85) | Gershengorn, 2012112*** |
| Clinician-related factors | |||
| Seniority of ICU clinician | |||
| Experience (spending < 30% of time in ICU) | Increased odds of admission | 2.44 (1.37 to 4.33) | Cohen, 201268*** |
| ICU physician extenders vs. ICU attending | Decreased odds of admission | 0.65 (0.44 to 0.98) | Stelfox, 201216*** |
| ICU residents vs. ICU attending | Decreased odds of admission | 0.69 (0.51 to 0.96) | Stelfox, 201216*** |
| ICU fellows vs. ICU attending | No difference | 1.23 (0.87 to 1.7) | Stelfox, 201216*** |
| Prognostic pessimism | |||
| Mortality prediction model: high chance of mortality | Increased odds of refusal | 2.4 (1.42 to 4.05) | Joynt, 200172** |
| Physician expected risk of death > 50% | Increased odds of refusal | 11.8 (4.6 to 30.5) | Shum, 201024** |
| Organisational-related factors | |||
| ICU bed availability | |||
| Bed availability | Increased odds of admission | 3.22 (0.36 to 3.75) | Iapichino, 201012*** |
| No ICU beds available/full unit | Increased odds of refusal | 0.26 (0.08 to 0.81) | Pintado, 201320*** |
| 3.16 (1.88 to 5.81) | Garrouste-Orgeas, 200510*** | ||
| 4.72 (1.37 to 16.2) | Garrouste-Orgeas, 200621** | ||
| 6.26 (4.14 to 9.46) | Louriz, 201241** | ||
| 3.2 (p = 0.01) | Sprung, 199913** | ||
| Decreased ICU bed availability | Reduced odds of admission within 2 hours of MET activation | p = 0.03 | Stelfox, 201216*** |
| ICU occupancy | Additional 10% capacity had no effect on admissions | 0.99 (0.94 to 1.04) | Gershengorn, 2012112*** |
| ICU bed occupancy level | Weakly predictive of likelihood of admission | 0.81 (0.66 to 1.00) | Augier, 200583*** |
| ICU bed availability |
1 available bed vs. 0 2 available beds vs. 0 > 2 available beds vs. 0 |
4.89 (1.99 to 12.0) 7.92 (3.09 to 20.30) 12.41 (4.49 to 34.26) |
Garrouste-Orgeas, 201317** |
| ICU bed availability | 1 bed available vs. 7 beds available | 62.5% probability of offering admission vs. 57.4%; p = 0.24 | Kelly, 2013135* |
| ICU bed availability | Increased odds of mechanical ventilation for nursing home residents with dementia per 10-bed increase in ICU availability | 1.06 (1.05 to 1.07) | Teno, 2016134*** |
| Decision-maker present | |||
| Examined by ICU clinician (patients aged > 80 years) | Increased odds of refusal | 5.75 (1.21 to 27.2) | Garrouste-Orgeas, 200621*** |
| Telephone assessment vs. examination by ICU clinician | Reduced odds of refusal | 0.23 (0.14 to 0.4) | Garrouste-Orgeas, 200510*** |
| Specialty of patient | |||
| Medical specialty | Increased odds of refusal | 5.96 (1.21 to 28.2) | Garrouste-Orgeas, 200621** |
| Less likely to be admitted than any other specialty | 0.46 (0.24 to 0.88) | Tridente, 201382*** | |
| Non-postoperative status less likely to be admitted | 26.3 (7.6 to 90.9) | Shum, 201024*** | |
| Surgical specialty | Increased odds of admission | 4.4 (3.49 to 5.64) | Iapichino, 201012*** |
| Elective surgical | Reduced odds of refusal | 0.43 (0.15 to 1.24) | Caldeira, 2010106*** |
| Emergency surgical | Reduced odds of refusal | 0.52 (0.17 to 2.32) | Caldeira, 2010106*** |
| Time of day | |||
| Daytime | Reduced odds of refusal | 0.52 (0.32 to 0.84) | Garrouste-Orgeas, 200310,118,119*** |
| Night-time (17.00–07.59) | Increased odds of admission | 1.52 (1.20 to 1.90) | Stelfox, 201216*** |
| Experience/expertise of ward team | |||
| Registered nurse certification | Not associated with ICU transfer | 0.43 (0.10 to 1.73) | Sanders, 200884*** |
| Hospital characteristics | |||
| Veterans Health Administration hospital (patients with HIV) | Increased odds of admission | 1.42; p = 0.04 | Tulsky, 199785*** |
| Government hospital (patients with HIV) | Reduced odds of admission | 0.57; p = 0.01 | Tulsky, 199785*** |
| Metropolitan vs. non-metropolitan | No difference | 0.46 (0.80 to 1.34) | Gershengorn, 2012112*** |
| Number of hospital beds (> 400 vs. < 100) | No difference | 0.8 (0.43 to 1.51) | Gershengorn, 2012112*** |
| Percentage of hospital beds designated as ICU beds (> 12.5% vs. < 5%) | No difference | 2.23 (0.97 to 5.16) | Gershengorn, 2012112*** |
| Teaching vs. non-teaching hospital | No difference | 0.78 (0.52 to 1.18) | Gershengorn, 2012112*** |
| Other | |||
| Weekend compared with weekday | Increased odds of admission | 1.14 (1.05 to 1.24) | Gershengorn, 2012112*** |
| Greater than one triage | Reduced odds of admission | 0.69 (0.58 to 0.82) | Iapichino, 201012*** |
| Transferred from another hospital (patients with intracerebral haemorrhage) | Increased odds of admission | 2.82 (1.51 to 5.28) | Nakagawa, 201297** |
Appendix 5 Summary of interviews by site
| Number of patient cases | Interview codes | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Total |
|---|---|---|---|---|---|---|---|---|
| 6 | 8 | 11 | 6 | 7 | 8 | 46 | ||
| Number of interviews linked to patient cases | 18 | 17 | 32 | 16 | 20 | 18 | 121 | |
| Consultant interviews | C | 4 | 7 | 6 | 6 | 6 | 6 | 35 |
| Registrar interviews | R | 6 | 4 | 11 | 3 | 4 | 3 | 31 |
| Outreach nurse interviews | O | 1 | 2 | 1 | 0 | 2 | 2 | 8 |
| Referring clinician interviews | D | 5 | 4 | 7 | 4 | 5 | 5 | 30 |
| Family (initial interview) | F | 2 | 0 | 4 | 1 | 3 | 0 | 10 |
| Family (2- to 3-month follow-up) | F Late | 0 | 0 | 2 | 1 | 0 | 1 | 4 |
| Patient (2- to 3-month follow-up) | P | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| Number of interviews not linked to patient cases | 3 | 6 | 3 | 6 | 6 | 4 | 28 | |
| Non-referring clinicians | nonR | 3 | 6 | 3 | 6 | 5 | 4 | 27 |
| Additional ‘general’ interview with clinician | General | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total number of interviews | 21 | 23 | 35 | 22 | 26 | 22 | 149 |
Appendix 6 Analysis of the data quality
We use four criteria to approximate the quality of the choices made by consultants and CCOR nurses:
-
Choice desirability: task #15 set all eight patient-related features to their best (i.e. most attractive in terms of ICU admission) level and worst level for patient A and B, respectively. The respondent was deemed to fail the desirability test when patient A was not prioritised over patient B.
-
Choice stability: task #14 was a repetition of task #1, used to test for consistency of responses. The rankings of the different choice options (i.e. patient A, patient B, neither of them) are compared between the two tasks. The respondent was deemed to fail the stability test when none of the top and last ranked options were not repeated.
-
Logical consistency: if A (B) should be admitted but not B (A), then B (A) should not be prioritised over A (B). This condition was verified for each task and respondent. Then we computed for each respondent the number of tasks in which the condition was satisfied. If this proportion was < 80%, then the respondent was deemed to fail the logical consistency test.
-
Response time: we recorded response times at the task level for each participant. We then identified ‘speedsters’ (i.e. participants who answered the choice questions ‘too quickly’ to make decisions that would accurately reflect their preferences for patient admission). For each choice task, we computed the first quintile of the response time distribution. Then, for each participant and each task we determined whether or not the observed response time was below the quintile value. If this proportion of very quick choices was > 50%, the respondent was deemed to fail the response time test.
The performance of the four tests is then summarised into a quality score ranging from 0 (when the participant passes the four tests) to 4 (when the participants fails the four tests). It was decided to exclude only participants who provide seemingly low-quality data (i.e. quality score of ≥ 3).
Appendix 7 Comparing preferences between intensive care unit consultants and critical care outreach nurses
| MLE | SE | p-value | |
|---|---|---|---|
| 1. Model parameters | |||
| A. Preference parameters | |||
| ASC | |||
| No admission | 4.667 | 0.140 | < 0.001 |
| Age (reference: 89 years) | |||
| 39 years | 2.342 | 0.067 | < 0.001 |
| 66 years | 1.514 | 0.059 | < 0.001 |
| 79 years | 0.883 | 0.056 | < 0.001 |
| Mobility (reference: bad) | |||
| Good | 1.264 | 0.047 | < 0.001 |
| Intermediate | 0.961 | 0.049 | < 0.001 |
| NEWS (reference: score = 5) | |||
| Score = 11 | 1.061 | 0.050 | < 0.001 |
| Score = 8 | 0.209 | 0.047 | < 0.001 |
| Look (reference: good) | |||
| Bad | 1.029 | 0.048 | < 0.001 |
| Intermediate | 0.698 | 0.050 | < 0.001 |
| Safety (reference: good) | |||
| Bad | 0.246 | 0.034 | < 0.001 |
| Family views (reference: no) | |||
| Unsure | 1.483 | 0.054 | < 0.001 |
| Yes | 1.724 | 0.054 | < 0.001 |
| Comorbidity (reference: severe prostate cancer) | |||
| Mild × COPD | 0.769 | 0.148 | < 0.001 |
| Moderate × COPD | –0.054 | 0.148 | 0.714 |
| Severe × COPD | –0.469 | 0.103 | < 0.001 |
| Mild × dementia | 0.981 | 0.150 | < 0.001 |
| Moderate × dementia | 0.613 | 0.138 | < 0.001 |
| Severe × dementia | –0.899 | 0.109 | < 0.001 |
| Mild × heart | 0.715 | 0.141 | < 0.001 |
| Moderate × heart | 0.729 | 0.148 | < 0.001 |
| Severe × heart | –0.741 | 0.101 | < 0.001 |
| Mild × prostate | 1.131 | 0.102 | < 0.001 |
| Moderate × prostate | 0.848 | 0.109 | < 0.001 |
| B. Heteroscedasticity parameters | |||
| Nurse (reference: consultant) | –0.490 | 0.039 | < 0.001 |
| 2. Model statistics | |||
| # Individuals | 492 | ||
| # Observations | 11,808 | ||
| # Parameters | 25 | ||
| Log-likelihood | –10,206.7 | ||
| BIC | 20,647.9 | ||
Appendix 8 Simulator choice experiment for intensive care unit admission decision-making: identifying preference patterns
This simulator has been developed based on the results from the choice experiment conducted as part of this NIHR-funded project. The choice experiment requires participants to make a series of choices based on hypothetical patient profiles. Each patient profile includes eight patient-related factors with a varying number of levels per factor. In each choice task, two hypothetical patient profiles were presented to the participants and they were asked three related questions: (1) would you admit patient A? (yes/no); (2) would you admit patient B? (yes/no); and (3) which patient should be given priority for admission? (patient A/B). We asked a sample of 303 ICU consultants to answer these choice questions.
Analysis of the consultants’ decisions identified four distinct patterns of preferences for ICU admission.
-
Pattern 1: ‘age-oriented decision-making’ – consultants belonging to this group tend to give relatively more weight to the patient’s age than to other factors.
-
Pattern 2: ‘age-dominated decision-making’ – consultants would base their admission decisions mainly on the patient’s age.
-
Pattern 3: ‘balanced decision-making’ – consultants would give approximately the same importance to all patient-related factors.
-
Pattern 4: ‘family-dominated decision-making’ – consultants’ admission decisions would be mainly driven by the family’s views regarding the patient’s admission (e.g. they insist on admission).
The objective of this simulator exercise is to determine which of these four patterns you are more likely to belong to. You are asked to complete three choice tasks (derived from the choice experiment). Based on your answers to the choice questions and results from the study, the simulator will return (1) the preferences pattern that is the most likely to explain your decisions; (2) the probably of belonging to this preferences pattern; and (3) finally the relative importance given to the different patient factors.
The simulator and instructions for its use can be found at the following URL: https://warwick.ac.uk/fac/sci/med/research/hscience/sssh/research/intensive/simulator_choice_experiment_1_for_icu_admission_decision.pdf (accessed 30 September 2019).
| Value_1 | Value_2 | Value_3 | Value_4 | |
|---|---|---|---|---|
| Age | 39 years | 66 years | 79 years | 89 years |
| Type of main comorbidity | COPD | Heart failure | Dementia | Prostate cancer |
| Severity of main comorbidity | Mild | Moderate | Severe | – |
| Functional status (mobility) | Good | Bad | Intermediate | – |
| Severity of acute condition (NEWS) | 5 | 8 | 11 | – |
| Family’s views | Yes | No | Unsure | – |
| Subjective assessment by the registrar | Good | Bad | Intermediate | – |
| Safety in ordinary ward | Good | Bad | – | – |
Appendix 9 Simulator choice experiment for intensive care unit admission decision-making: preference calculator
This simulator has been developed based on the results from the choice experiment conducted as part of this NIHR-funded project (HSDR 13/10/14). The choice experiment requires participants to make a series of choices based on hypothetical patient profiles. Each patient profile has eight patient-related factors with a varying number of levels per factor. In each choice task, two hypothetical patient profiles were presented to the participants and they were asked three related questions: (1) would you admit patient A? (Yes/No); (2) would you admit patient B? (Yes/No); and (3) which patient should be given priority for admission? (Patient A/B). We asked a sample of 303 ICU consultants to answer these choice questions.
Analysis of the consultants’ decisions identified four distinct patterns of preferences for ICU admission:
-
Pattern 1: ‘age-oriented decision-making’ – consultants belonging to this group tend to give relatively more weight to the patient’s age than to other factors.
-
Pattern 2: ‘age-dominated decision-making’ – consultants base their admission decisions mainly on the patient’s age.
-
Pattern 3: ‘balanced decision-making’ – consultants give approximately the same importance to all patient-related factors.
-
Pattern 4: ‘family-dominated decision-making’ – consultants’ admission decisions are mainly driven by the family’s views on the patient’s admission (e.g. they insist on admission).
The simulator and instructions on its use can be found at the following URL: https://warwick.ac.uk/fac/sci/med/research/hscience/sssh/research/intensive/simulator_choice_experiment_2_for_icu_admission_decision.pdf (accessed 30 September 2019).
Appendix 10 Table of factors informing decision-support intervention development
| Factor identified | WP1 | Systematic review | Influence on final DSI |
|---|---|---|---|
| Patient dignity | + | Addressed in educational resources included in information for family and patients | |
| Clinician authority/seniority in both ICU and referring team | + | + | Encourage senior clinician involvement by naming individuals on both referral and decision-making forms included in supporting educational material |
| Clinician personal views/beliefs/experiences | + | + | Use of a standardised process and cognitive framework to guide decision-making to enable clear, transparent and consistent decision-making process to reduce implicit biases |
| Communication between clinicians | + | + | Use of standardised referral and decision-making forms to clarify communication. Use of an SBAR model in the referral forms. Included in content of educational support material |
| Perceived competence of involved clinicians | + | Educational supporting materials to encourage good dialogue between clinicians and use of a consistent approach. Education on the potential harms of ICU. Development of decision-support and referral documents to clarify communication | |
| Family perspective | + | + | Family information sheets developed to help families participate in decision-making process |
| Functional status and reserve | + | + | Clarified as a potential source of information to guide judgement on the capacity to benefit from life-supporting treatment, and included on decision-support and referral forms |
| Lack of available information | + | Not directly addressed in this intervention. However, this intervention relies on best use of available information, and encourages information gathering from family and patient | |
| Kind of help requested not well communicated | + | Referral forms include section to document type of help requested as tick box with free-text option | |
| Likelihood of survival of patient | + | Included as part of balancing activity in cognitive framework and the decision-support and referral forms | |
| Likelihood of benefiting from ICU care | + | Included as part of cognitive framework and in the decision-support and referral forms | |
| Subjective ‘look’ of the patient | + | Emphasis on explicit reasoning and justification in the cognitive framework and in referral and decision-support forms | |
| Patient’s age | + | + | Implicit biases acknowledged within supporting educational material. Explicit reasoning and justification for reasons included in cognitive framework and referral and decision-support forms |
| Personal connection with patient | + | Use of a standardised process and cognitive framework to guide decision-making to enable clear, transparent and consistent decision-making process | |
| Premorbid state/comorbidities | + | + | Included as part of cognitive framework as contributing to judgement over capacity to recover from critical illness and included in the decision-support and referral forms |
| Acute medical condition | + | + | Included in the cognitive framework, decision-support and referral forms as contributing decision-making as the ‘need’ for intensive care treatment |
| Patients quality of life as perceived by clinician | + | + | Explicit reasoning and justification for reasons included in cognitive framework and referral and decision-support forms |
| Resource availability/ICU bed availability | + | + | The sequencing of decision regarding treatment required before considering resources to provide it to minimise the effect of resource availability on ICU admission decision |
| Safety concerns | + | Specific question about whether or not required care can be delivered safely on the ward | |
| Illness length and trajectory | + | Included as part of cognitive framework as contributing to judgement about capacity to recover from critical illness and included in the decision-support and referral forms | |
| Patient’s wishes | + | + | Specifically included in cognitive framework. Patient and family information sheets developed to aid participation in process. Specific requirement on referral and decision-support form to seek patient wishes. Included specifically in educational support material |
| Patient’s sex | + | Not directly addressed in this intervention | |
| Prognostic pessimism | + | Requirement for explicit reasoning and justification of decisions | |
| Time of day of referral | + | Use of a standardised process and cognitive framework to guide decision-making to enable clear, transparent and consistent decision-making process | |
| Presence of key decision-maker at bedside | + | Encouraging of consultant to consultant discussion and collegiate decision-making, included in educational supporting material and the documentation of senior involvement on referral and decision-support forms | |
| Ethnicity of patient | + | Patient and family information leaflets translated into minority languages | |
| Patient’s cognitive status | + |
Use of a standardised process and cognitive framework to guide decision-making to enable clear, transparent and consistent decision-making process Explicit reasoning and justification of decision required on referral and decision-support forms |
|
| Time available to make decision | + | Addressed in supporting educational material | |
| Clinical doubt | + | Addressed in supporting educational material | |
| Patients health insurance | + | Not relevant to UK practice, not addressed within this intervention | |
| Pressure from hospital hierarchy | + | Use of a standardised process and cognitive framework to guide decision-making to enable clear, transparent and consistent decision-making process | |
| Clinicians wish to avoid complaints and litigation | + | Use of a standardised process and cognitive framework to guide decision-making to enable clear, transparent and consistent decision-making process | |
| Presence of written guidelines | + | Not specifically addressed within this intervention | |
| Clinicians wish to avoid conflict | + | Use of a standardised process and cognitive framework to guide decision-making to enable a clear, transparent and consistent decision-making process | |
| Specialty discipline of patient’s acute illness | + | Use of a standardised process and cognitive framework to guide decision-making to enable a clear, transparent and consistent decision-making process. Included in educational supporting material | |
| Background discipline of intensive care decision-maker | + | Not addressed in this intervention | |
| Presence of a DNACPR order | + | Use of a ReSPECT form specifically included in decision-support and referral forms as a source of information. Included in educational material |
Appendix 11 Referral form
Appendix 12 Decision form
Appendix 13 Family information leaflet
Appendix 14 Patient information leaflet
Appendix 15 Stakeholder conference delegates
| Title | First name | Surname | Job role | Organisation name |
|---|---|---|---|---|
| Dr | Milind | Arolker | Palliative Care Consultant | Heart of England NHS Foundation Trust |
| Dr | Nicky | Ashby | University of Nottingham | |
| Mr | David | Baker | Clinical and Business Informatics Specialist | NHS Digital |
| Dr | Chris | Bassforda | Chief Investigator | UHCW |
| Dr | Simon | Baudouin | Project Chairperson | FICM Professional Standards Committee |
| Ms | Sarah | Broom | Lay Advisory Group | |
| Dr | Daniele | Bryden | Consultant intensive care physician | Faculty of Intensive Care Medicine |
| Ms | Sharon | Burton | Head of Standards and Ethics | General Medical Council |
| Ms | Elaine | Clarke | Nurse Manager | |
| Reverand | Alison | Coles | West Midlands Regional Representative for CHCC/Lead Chaplain for Walsall Healthcare | Walsall Healthcare NHS Trust |
| Professor | Jeremy | Dalea | Co-investigator | University of Warwick |
| Dr | Ron | Daniels | Chief Executive | Sepsis Trust |
| Mr | Chris | Dickson | Clinical and Business Informatics Specialist | NHS Digital |
| Mr | Roy | Dudley-Southern | NHS Service User and Commissioner | |
| Dr | Nadine | Flowersa | Project Manager | University of Warwick |
| Dr | Zoe | Fritza | Co-investigator | University of Warwick |
| Dr | Akif | Gani | Consultant Geriatrician | |
| Dr | Colin | Gelder | Consultant Physician | UHCW |
| Mr | Richard | Grant | Lay Advisory Group | |
| Ms | Sarah | Gray | Service Development Manager-Midlands | British Lung Foundation |
| Professor | Frances | Griffithsa | Co-investigator | University of Warwick |
| Ms | Bridget | Harper | Patient Representative | Age UK Coventry/Coventry Older Voices |
| Dr | Dan | Harvey | Consultant Intensive Care Physician | FICM Professional Standards Committee |
| Dr | Huayi | Huanga | Co-investigator | University of Warwick |
| Mrs | Louise | Hutton | Secretary | University of Warwick |
| Ms | Philippa | Jones | Associate Acute Oncology Nurse Advisor | Macmillan Cancer Support |
| Mr | Elyas | Khalifa | Lay Advisory Group | |
| Dr | Zahid | Khan | Consultant Intensive Care Physician | Intensive Care Society |
| Dr | Nadia | Khan | Consultant in Palliative Medicine | St Giles Hospice |
| Dr | Nicolas | Kruciena | Co-investigator | University of Aberdeen |
| Mr | Peter | McCullough | Consultant Surgeon | University Hospital Coventry |
| Mr | Sean | McGovern | HM Governor | Coventry |
| Ms | Julie | Midgley | NHS Solicitor | UHCW |
| Dr | Natalie | Pattison | Senior Clinical Nursing Research Fellow | BACCN |
| Dr | Nick | Scriven | Consultant in Acute Medicine | |
| Dr | Anne | Slowthera | Chief Investigator | University of Warwick |
| Mr | Peter | Spurgeon | HealthWatch Coventry Executive (Patients General) | HealthWatch |
| Dr | Andrew | Stewart | Lead Consultant Haematologist | University Hospitals of North Midlands/NHS England Acute Oncology |
| Dr | Ganesh | Suntharalingam | Consultant Intensive Care Physician | Intensive Care Society |
| Dr | Mia | Svantesson-Sandberga | Co-investigator | University of Warwick |
| Mrs | Sarah | Symonsa | Co-investigator | Royal United Hospitals Bath |
| Dr | Mark | Temple | Consultant Physician and Nephrologist RCP | Heart of England NHS Foundation Trust |
| Mr | Lloyd | Tingley | Neuromuscular Outreach Officer | Muscular Dystrophy UK |
| Ms | Emerald | Toogood | Legal Services Manager | |
| Mrs | Susan | Tulip | Lay Advisory Group | |
| Dr | Chris | Turner | Consultant in Emergency Medicine | University Hospital Coventry |
| Dr | Phil | Watt | ICU Consultant Anaesthetist | |
| Ms | Catherine | Whitea | Co-investigator | ICU steps |
Appendix 16 Report of the stakeholder conference
Appendix 17 Site implementation schedules
| Week | Hospital A | Hospital B | Hospital C |
|---|---|---|---|
| 1 |
E-mailed all consultants via medical director Spoke to Deteriorating Patients Group Informed CCOR nurses about the study E-mailed ICU colleagues |
Training at ICU meeting; ED consultants meeting |
Training to acute medicine CCOR implementation champions informed team at staff meeting Posters placed on referring wards |
| 2 | E-mailed ACCP and CCOR teams |
Training to anaesthetics and intensivists department meeting; thoracic surgery meeting Circulated materials to haematology; and to consultants at ED |
|
| 3 | Training to ACCPs, Grand Round, Anaesthetics and ICU meeting | Delivered training to haematology-oncology | Delivered training to acute medics, geriatrics, and haematology departments |
| 4 | Delivered training to junior doctors at ED | Delivered training to respiratory department and ED registrars | |
| 5 | Delivered training to respiratory department | E-mails sent out to clinical leads of referring specialties | |
| 6 | |||
| 7 | Delivered ‘Goody Bags’ to wards to raise awareness; started circulating ‘League Tables’ | Added study to ‘topic of the month’ (daily reminder to ITU medical staff) | |
| 8 | |||
| 9 | Delivered training at Grand Round | ||
| 10–14 | |||
| Continually over period | Opportunistic one-to-one training; raising awareness on referring wards | Mentioned daily at ICU consultant handover |
Emails to colleagues (ICU and CCOR) Mentioned every day at briefings on ICU Reminding CCOR every day |
Appendix 18 Form usage data analysis
Data cleaning notes
Bed occupancy
Bed occupancy was reported differently across the three sites. Therefore, to make fair comparisons, bed occupancy per day was calculated as the maximum occupancy of the site over one calendar day (midnight to midnight). Hence, at time of assessment more beds may have been available, and this represents a ‘worst case’ scenario. Details for each site are given in the section below. A summary graph illustrating the calculated bed capacity at each site over the 6 weeks of data is shown in Figure 21.
FIGURE 21.
Percentage of daily ICU bed occupancy.
Daily bed occupancy was then categorised as that the unit:
-
was full/over capacity
-
had one bed available
-
had two beds available
-
had three or more beds available.
Figure 22 shows the categorical breakdown of the bed occupancy data at each site. Note that hospital A did not report bed occupancy data on the first day the site was open (i.e. study day 1).
FIGURE 22.
Intensive care unit daily bed occupancy categories at each site.
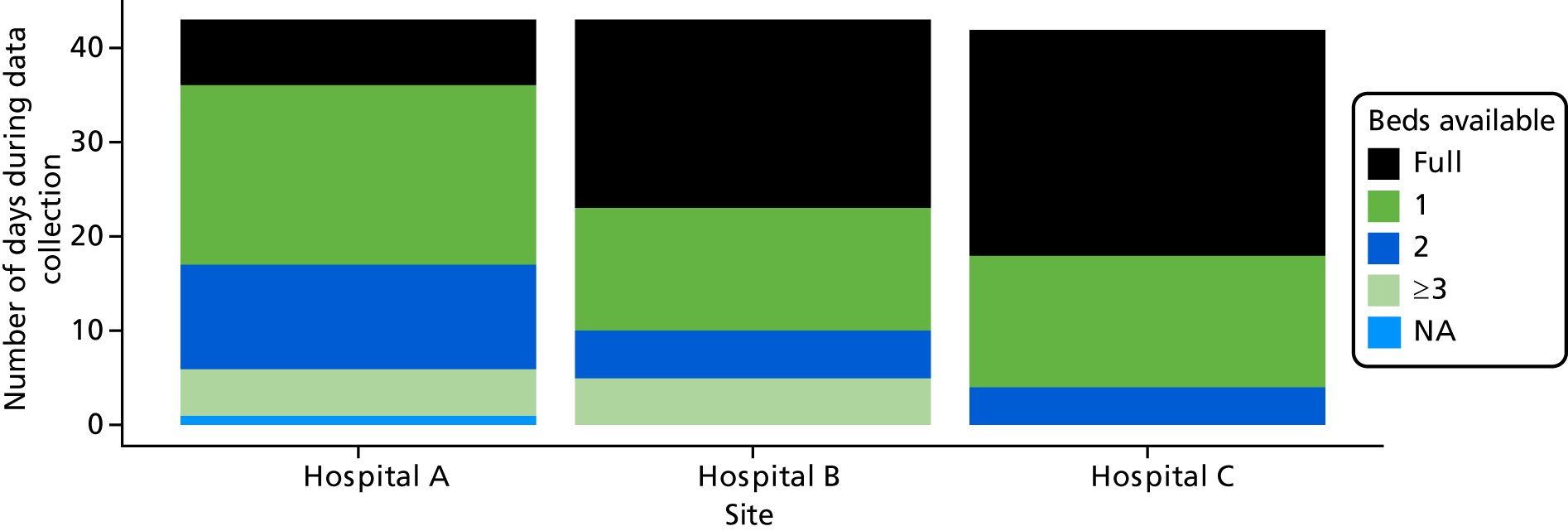
Hospital A
The number of beds available on each nurse shift (early, late and night) was reported. Maximum capacity was given as seven beds, with no details on level 2 and level 3. Bed occupancy per day was calculated as the maximum occupancy on any shift, and divided by seven to create a percentage.
Capacity for day 1 of the study at this site was not reported.
Hospital B
The number of level 3 and level 2 patients at both day and night were reported. Maximum capacity was reported as 20 beds or 16 level 3 patients. Figures were highlighted when reaching bed capacity.
Bed occupancy per day was calculated as the maximum number of level 3 equivalent patients (no. L3 + no. L2/2, rounded upwards) at any shift, and divided by 16 to create a percentage.
Hospital C
A total L3/L2 mix of 21 beds was reported as the maximum ICU capacity. The number of level 2 and level 3 beds available was reported at any point when the ward occupancy was changed. This was used to calculate the number and percentage of available beds.
Case descriptive statistics
| Case variable | Hospital A | Hospital B | Hospital C | All sites |
|---|---|---|---|---|
| Referrals, N (% of total) | 71 (21.3) | 26 (7.8) | 236 (70.9) | 333 |
| Eligible referral: yes, n (% of total referrals) | 63 (88.7) | 14 (53.8) | 104 (44.1) | 181 (54.4) |
| Reason ineligible, n (% of site ineligible) | ||||
| Duplicate referral/review | 4 (50.0) | 1 (8.3) | 1 (0.8) | 6 (3.9) |
| Unclear documentation | 2 (25.0) | 0 | 0 | 2 (1.3) |
| Prior to start date | 1 (12.5) | 0 | 0 | 1 (0.7) |
| Elective procedure | 1 (12.5) | 0 | 0 | 1 (0.7) |
| Unable to access records | 0 | 1 (8.3) | 40 (30.3) | 41 (27.0) |
| Did not meet site eligibility criteria | 0 | 10 (83.3) | 91 (68.9) | 101 (66.4) |
| Forms used | ||||
| Both | 18 (28.6) | 1 (7.1) | 11 (10.6) | 30 (16.8) |
| Decision form only | 1 (1.6) | 1 (7.1) | 4 (3.8) | 6 (3.3) |
| Referral form only | 9 (14.3) | 1 (7.1) | 5 (4.8) | 15 (8.3) |
| Neither | 35 (55.6) | 11 (78.6) | 84 (80.8) | 130 (71.8) |
| Admitted | ||||
| Missing | 0 | 6 (42.9) | 4 (3.8) | 10 (5.5) |
| No | 14 (22.2) | 4 (28.6) | 33 (31.7) | 51 (28.2) |
| Yes | 49 (77.8) | 4 (28.6) | 67 (64.4) | 120 (66.3) |
| Patient age (years) | ||||
| Mean (SD) | 67.4 (19.1) | 61.1 (17.0) | 60.6 (16.1) | 62.9 (17.4) |
| Missing, n (%) | 3 (4.8) | 0 | 0 | 3 (1.7) |
| Patient age (years) (category) | ||||
| < 40 | 6 (9.5) | 2 (14.3) | 12 (11.5) | 20 (11.0) |
| 40–59 | 14 (22.2) | 3 (21.4) | 34 (32.7) | 51 (28.2) |
| 60–79 | 18 (28.6) | 6 (42.9) | 49 (47.1) | 73 (40.3) |
| ≥ 80 | 22 (34.9) | 3 (21.4) | 9 (8.7) | 34 (18.8) |
| Missing | 3 (4.8) | 0 | 0 | 3 (1.7) |
| Patient sex | ||||
| Unknown/missing | 3 (4.8) | 0 | 0 | 3 (1.7) |
| Female | 28 (44.4) | 4 (28.6) | 43 (41.3) | 75 (41.4) |
| Male | 32 (50.8) | 10 (71.4) | 61 (58.7) | 103 (56.9) |
| Time of day, n (%) | ||||
| No data/no time given | 28 (44.4) | 8 (57.1) | 19 (18.3) | 55 (30.4) |
| Daytime (08.00–19.59) | 20 (31.7) | 4 (28.6) | 42 (40.4) | 66 (36.9) |
| Night-time (20.00–07.59) | 15 (23.8) | 2 (14.3) | 43 (41.3) | 59 (30.2) |
| Day of assessment | ||||
| Sunday | 6 (9.5) | 2 (14.3) | 8 (7.7) | 16 (8.8) |
| Monday | 6 (9.5) | 3 (21.4) | 12 (11.5) | 21 (11.6) |
| Tuesday | 12 (19.0) | 1 (7.1) | 18 (17.3) | 31 (17.1) |
| Wednesday | 9 (14.3) | 1 (7.1) | 13 (12.5) | 23 (12.7) |
| Thursday | 8 (12.7) | 4 (28.6) | 10 (9.6) | 22 (12.2) |
| Friday | 15 (23.8) | 1 (7.1) | 23 (22.1) | 39 (21.5) |
| Saturday | 7 (11.1) | 2 (14.3) | 20 (19.2) | 29 (16.0) |
| Week of data collection | ||||
| 1 | 7 (11.1) | 5 (35.7) | 29 (27.9) | 41 (22.7) |
| 2 | 12 (19.0) | 1 (7.1) | 17 (16.3) | 30 (16.6) |
| 3 | 11 (17.5) | 3 (21.4) | 9 (8.7) | 23 (12.7) |
| 4 | 6 (9.5) | 3 (21.4) | 19 (18.3) | 28 (15.5) |
| 5 | 12 (19.0) | 1 (7.1) | 9 (8.7) | 22 (12.2) |
| 6 | 15 (23.8) | 1 (7.1) | 21 (20.2) | 37 (20.4) |
| Days between admission and referral, n (%) | ||||
| Mean (SD) | 1.7 (3.6) | 2.5 (2.4) | 5.3 (7.0) | 3.1 (5.2) |
| Missing | 19 (30.2) | 4 (28.6) | 75 (72.1) | 98 (54.1) |
| 0–2 days | 37 (58.7) | 5 (35.7) | 15 (14.4) | 57 (31.5) |
| > 2 days | 7 (11.1) | 5 (35.7) | 14 (13.5) | 26 (14.4) |
| Bed capacity at referral | ||||
| No data | 2 (1.1) | 0 | 0 | 2 (1.1) |
| Full/over capacity | 13 (7.2) | 4 (2.2) | 54 (29.8) | 71 (39.2) |
| 1 bed available | 29 (16.0) | 5 (2.8) | 38 (21.0) | 72 (39.8) |
| 2 beds available | 16 (8.8) | 2 (1.1) | 12 (6.6) | 30 (16.6) |
| ≥ 3 beds available | 3 (1.7) | 3 (1.7) | 0 | 6 (3.3) |
Age at assessment
FIGURE 23.
Histogram of age at assessment by site. Only approximate patient age was calculated, as only year of birth was recorded for the purposes of patient confidentiality.
Time of day of assessment
FIGURE 24.
Time of day of assessment to referral to ICU.
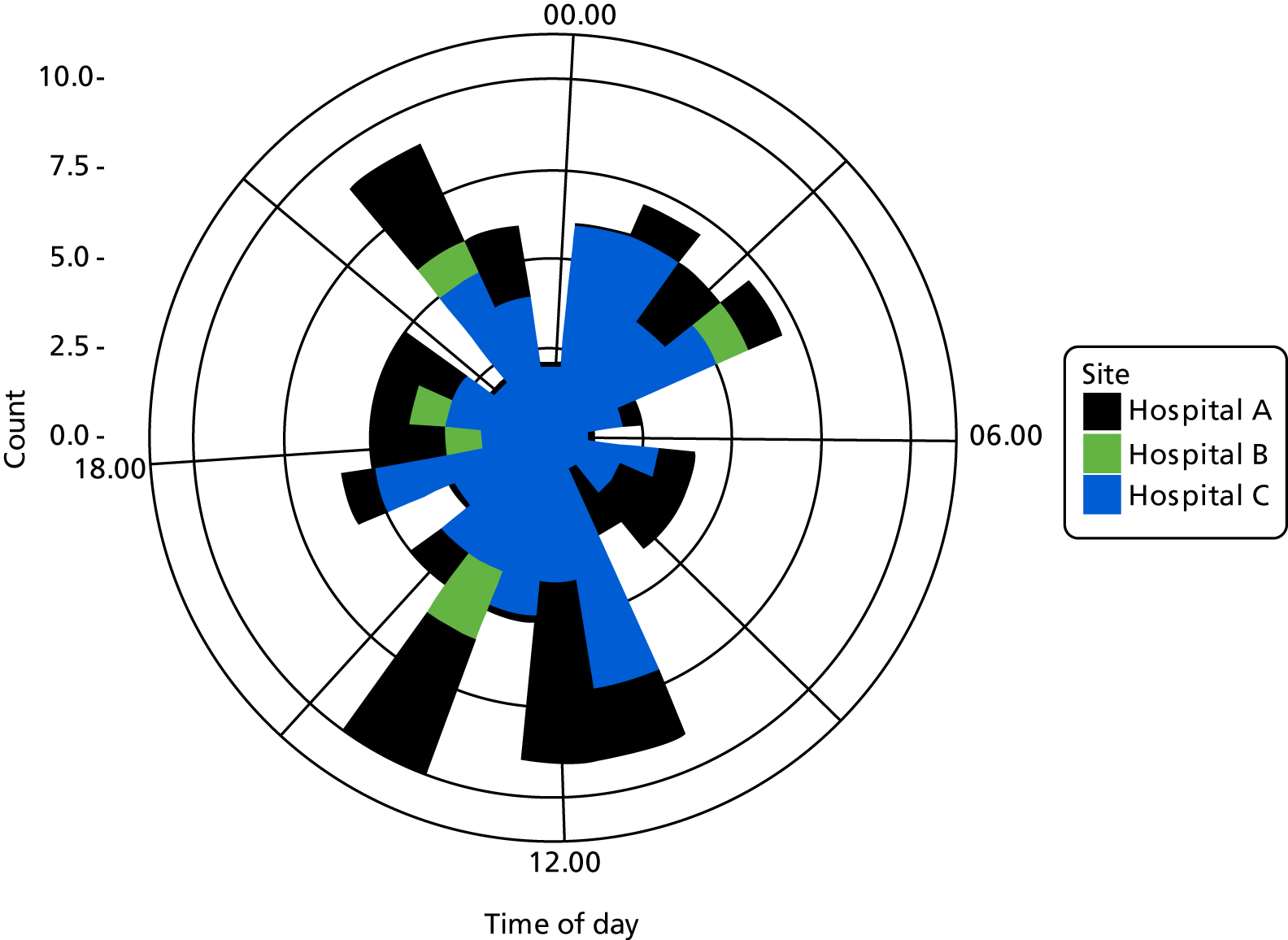
FIGURE 25.
Time of day of assessment of referral to ICU by site. (a) Hospital A; (b) hospital B; (c) hospital C.
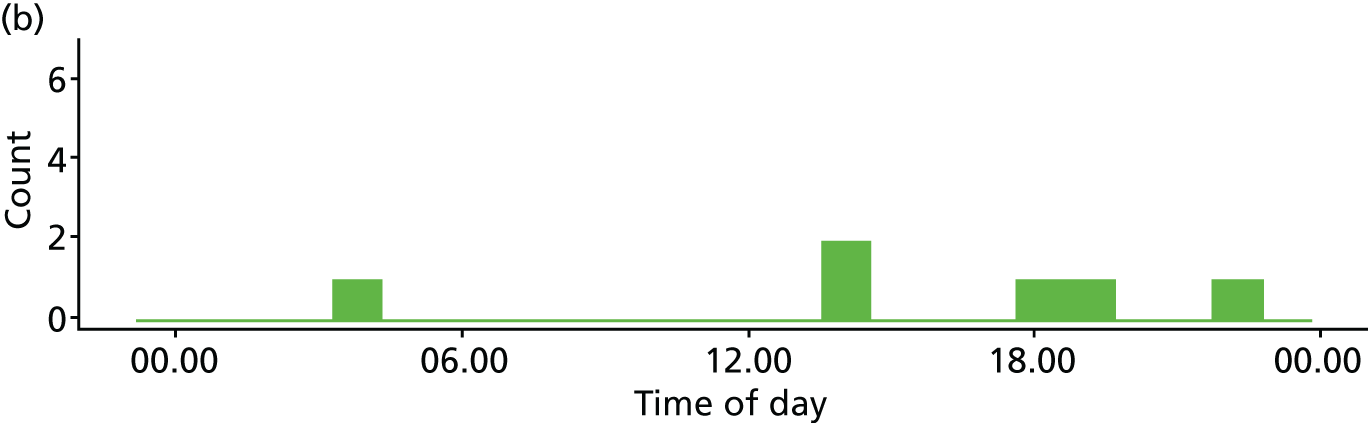
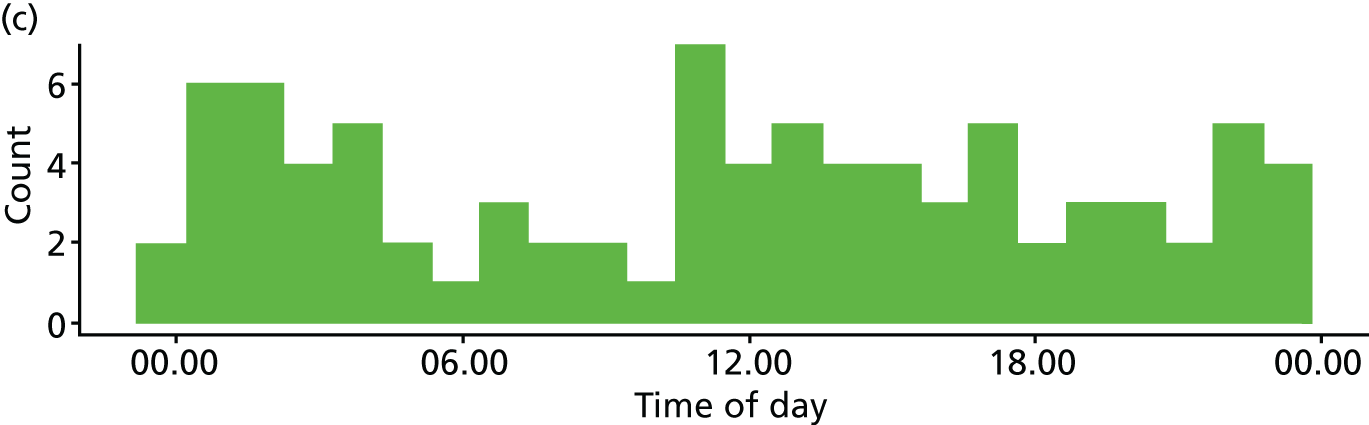
FIGURE 26.
Proportion of assessments of referral to ICU made during daytime.
Day of intensive care unit referral assessment
FIGURE 27.
Day of referral to ICU.
FIGURE 28.
Proportion of each day of the week when referral to ICU was made, by site.
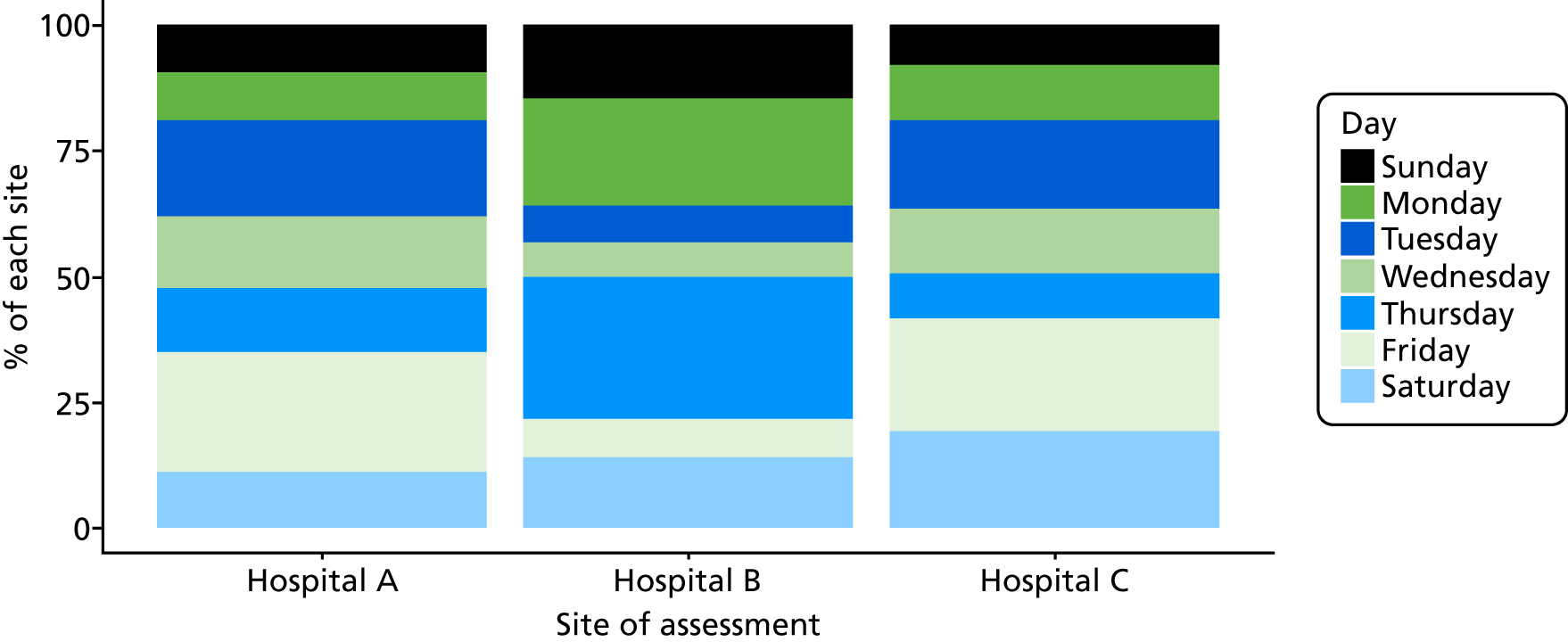
Week of data collection
FIGURE 29.
Week of data collection when referral to ICU was made.
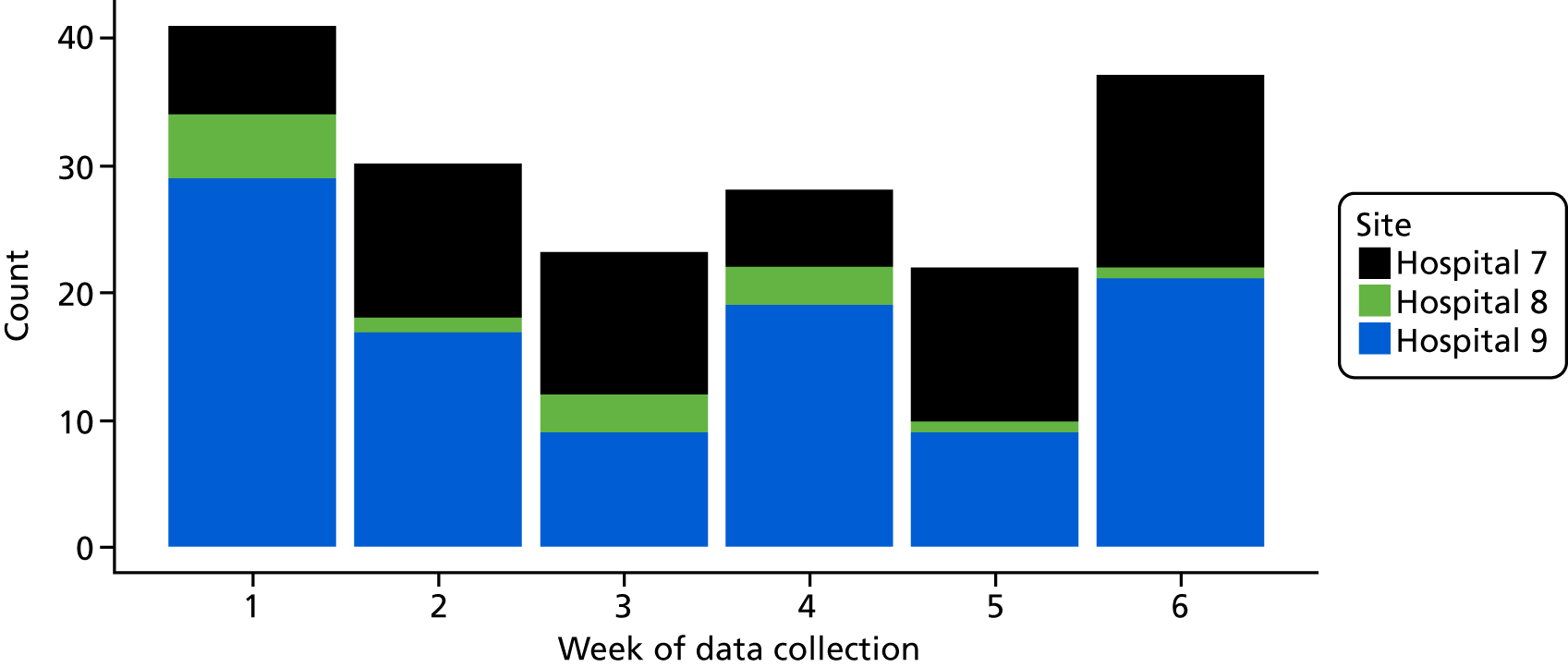
FIGURE 30.
Proportion of each day of week referral to ICU was made by site.
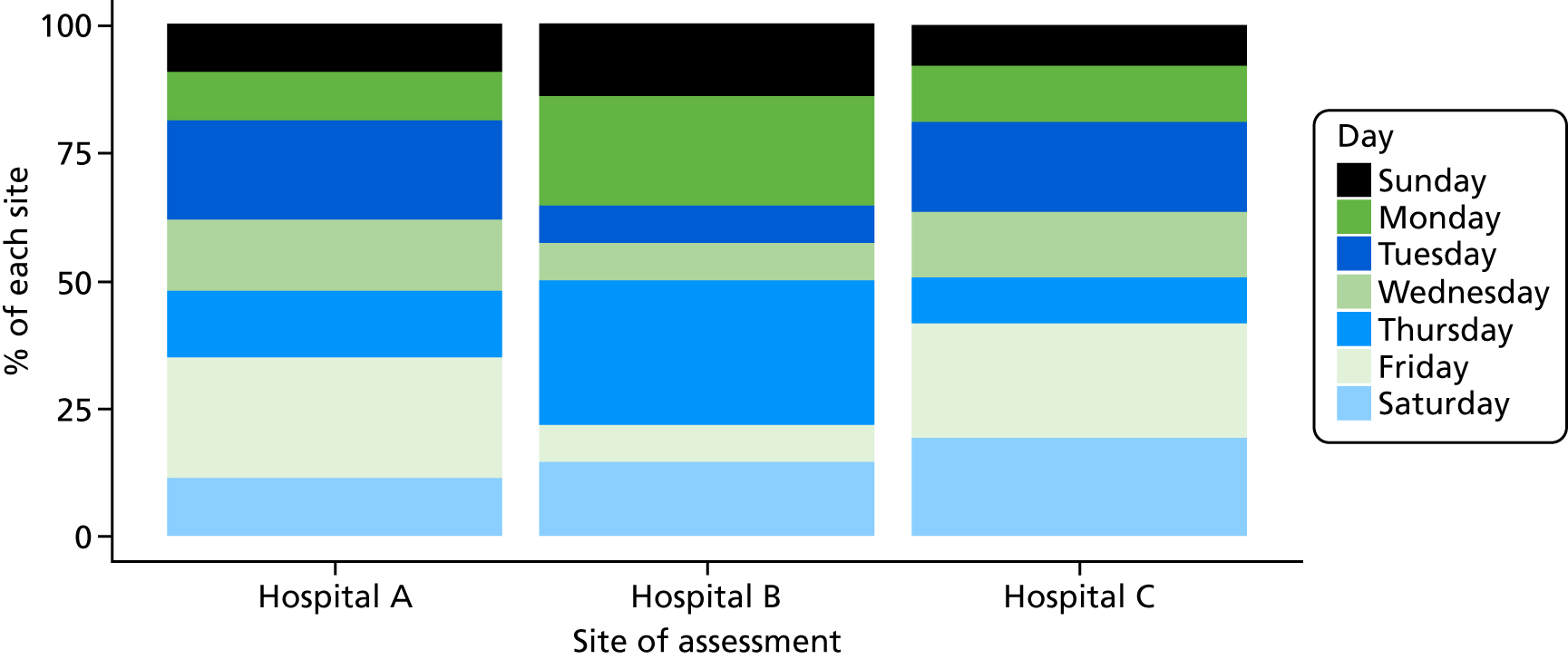
Days between admission and intensive care unit referral assessment
FIGURE 31.
Number of days between admission and when referral to ICU was made.
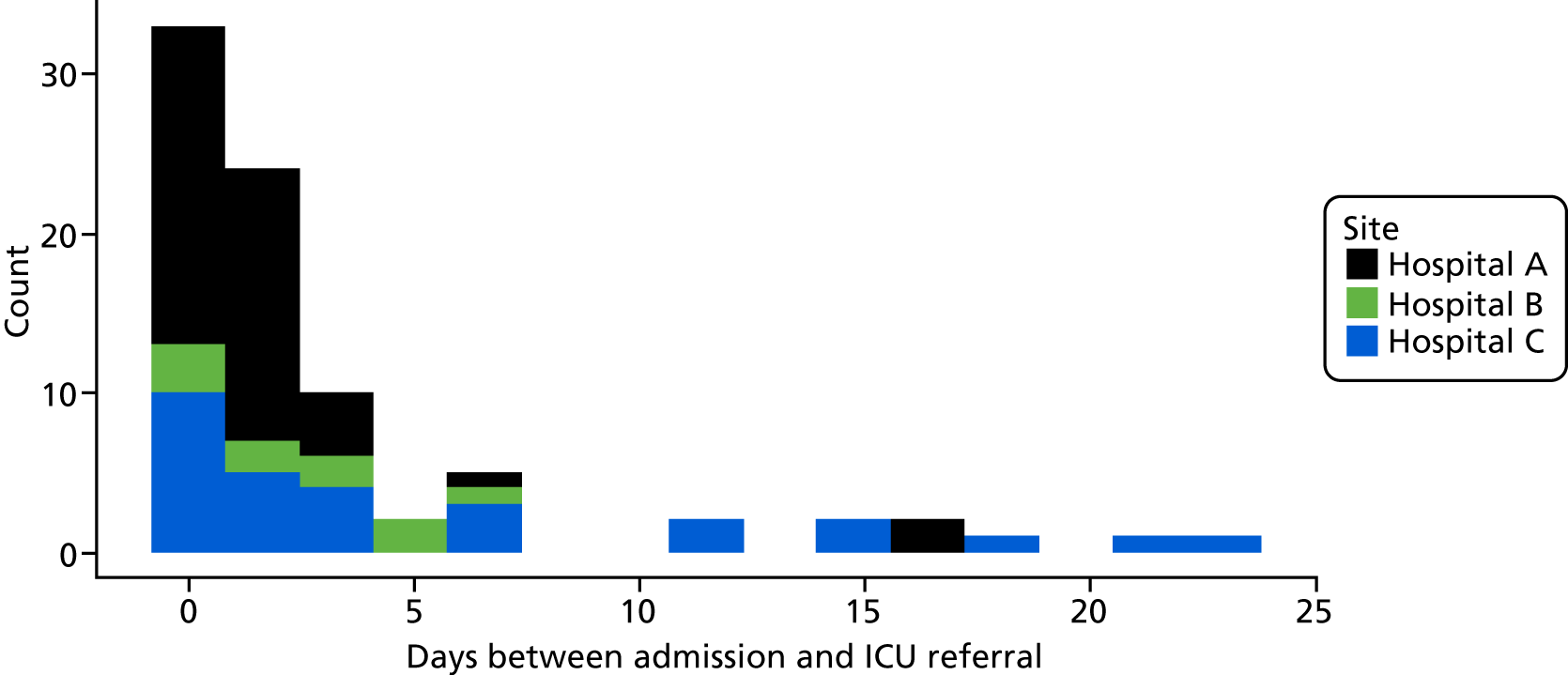
FIGURE 32.
Number of days between admission and when referral to ICU was made, by site.
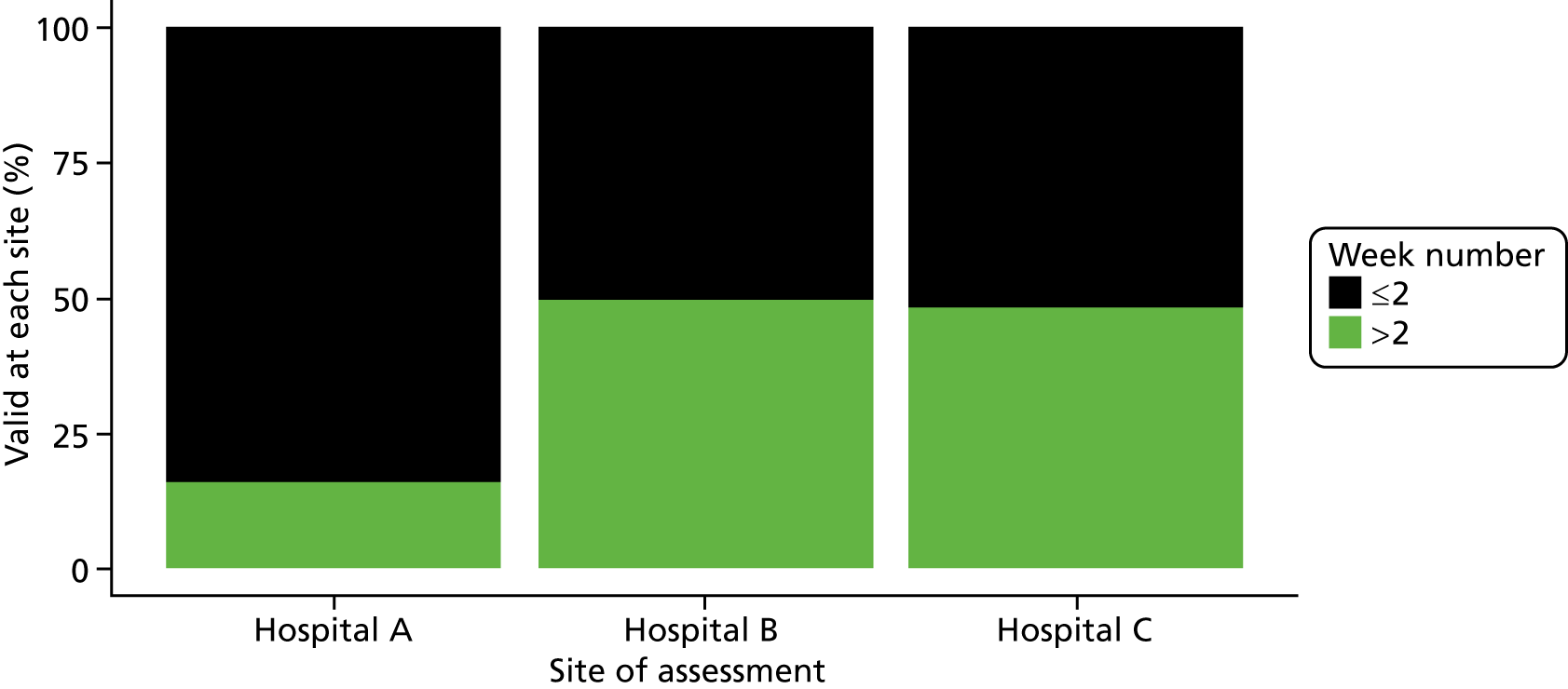
There is no evidence to suggest that there is any relationship between the time between admission and ICU referral, and the time that the referral is made. However, note that there is a large number of missing data for these two variables.
FIGURE 33.
Scatterplot of number of days between admission and when referral to ICU was made and time of referral.
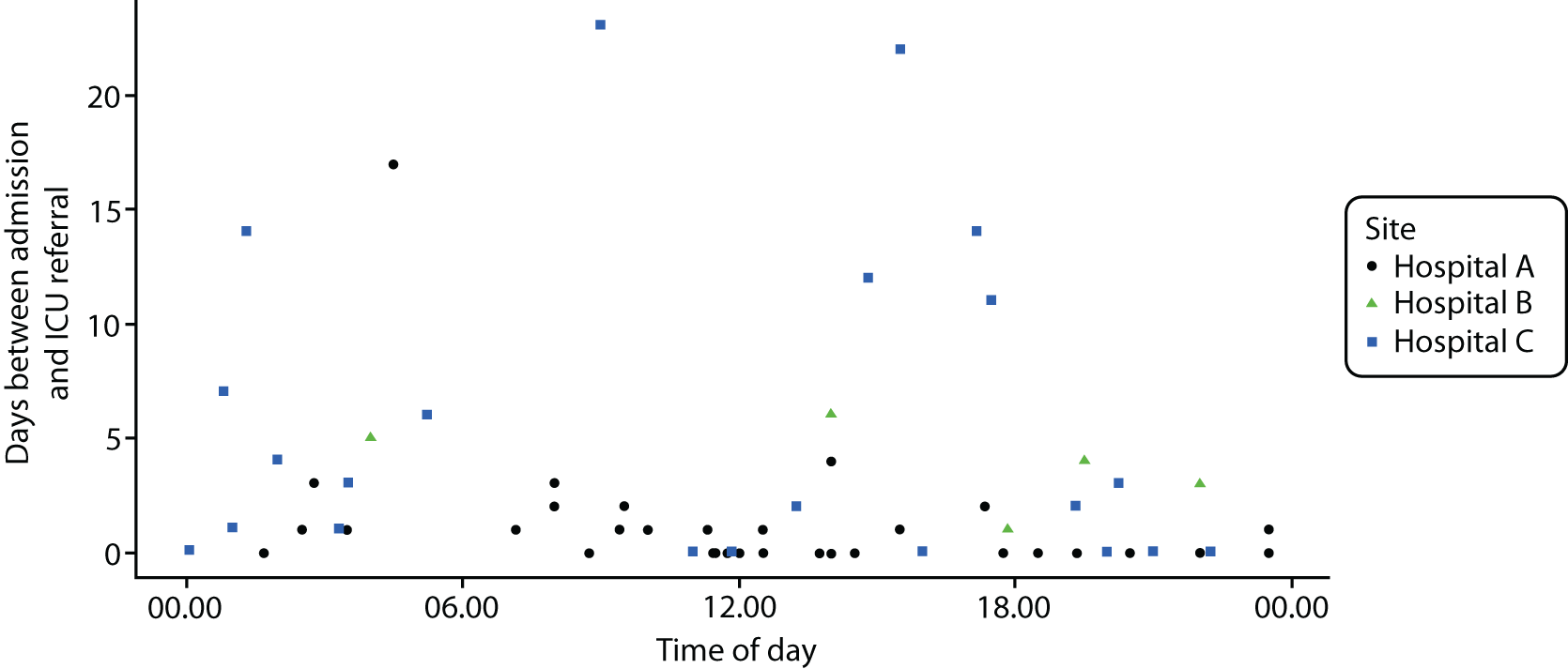
Critical care referral form
Referral form usage
A total of 45 of the 181 cases (25%) used the referral form. Table 39 shows how often each section of the form was completed.
| Form variable | Hospital A (N = 63) | Hospital B (N = 14) | Hospital C (N = 104) | All sites (N = 181) |
|---|---|---|---|---|
| Cases where referral form was used, n (%) | 27 (42.8) | 2 (14.3) | 16 (15.4) | 45 (24.9) |
| Situation/reason for referral, n (%) | ||||
| Details not given | 0 | 0 | 0 | 0 |
| Details given | 27 (100) | 2 (100) | 16 (100) | 45 (100) |
| Background, n (%) | ||||
| Details not given | 0 | 0 | 0 | 0 |
| Details given | 27 (100) | 2 (100) | 16 (100) | 45 (100) |
| Patient’s wishes, n (%) | ||||
| Details not given | 1 (3.7) | 0 | 2 (12.5) | 3 (6.7) |
| Details given | 26 (96.3) | 2 (100) | 14 (87.5) | 42 (93.3) |
| Source for patient’s wishes, n (%) | ||||
| Details not given | 24 (88.9) | 2 (100) | 15 (93.8) | 41 (91.1) |
| Details given | 3 (11.1) | 0 | 1 (6.2) | 4 (8.9) |
| Discussed with referring team consultant, n (%) | ||||
| Details not given | 11 (40.7) | 0 | 3 (18.8) | 14 (31.1) |
| Details given | 16 (59.3) | 2 (100) | 13 (81.2) | 31 (68.9) |
| Recommendation, n (%) | ||||
| Details not given | 2 (7.4) | 0 | 0 | 2 (4.4) |
| 1 – Consider admission | 17 (63.0) | 2 (100) | 14 (87.5) | 33 (73.3) |
| 2 – Review | 5 (18.5) | 0 | 2 (12.5) | 7 (15.6) |
| 3 – Therapy outside ICU | 0 | 0 | 0 | 0 |
| 4 – Plan in case of deterioration | 3 (11.1) | 0 | 0 | 3 (6.7) |
| 5 – Other | 0 | 0 | 0 | 0 |
| Discussed with ICU team: clinician rolea | ||||
| Details not given | 6 (22.2) | 0 | 6 (37.5) | 12 (26.7) |
| Outreach nurse/ACCP team | 3 (11.1) | 0 | 1 (6.2) | 4 (8.9) |
| Junior doctor | 2 (7.4) | 0 | 0 | 2 (4.4) |
| Registrar | 6 (22.2) | 2 (100) | 6 (37.5) | 14 (31.1) |
| Consultant | 10 (37.0) | 0 | 3 (18.8) | 13 (28.9) |
Referral details
For some referral details it was possible to extract this information, such as time of discussion with ICU team or clinician responsible for referral, from the hospital notes. Hence, data presented in Table 40 may have been collected from the referral forms or from patient notes.
| Referring clinician details | Hospital A (N = 63) | Hospital B (N = 14) | Hospital C (N = 104) | All sites (N = 181) |
|---|---|---|---|---|
| Cases where referral form was used, n (%) of cases | 27 (42.8) | 2 (14.3) | 16 (15.4) | 45 (24.9) |
| Discussed with ICU team: time | ||||
| Details not given/found | 41 (66.1) | 12 (85.7) | 99 (95.2) | 153 (84.5) |
| Daytime (08.00–19.59) | 15 (23.8) | 2 (14.3) | 1 (1.0) | 18 (9.9) |
| Night-time (20.00–07.59) | 6 (9.5) | 0 | 4 (3.8) | 10 (5.5) |
| Discussed with ICU team: day | ||||
| Details not given/found | 41 (65.1) | 12 (85.7) | 95 (91.3) | 148 (81.8) |
| Sunday | 1 (1.6) | 0 | 0 | 1 (0.6) |
| Monday | 4 (6.3) | 0 | 3 (2.9) | 7 (3.9) |
| Tuesday | 3 (4.8) | 0 | 0 | 3 (1.7) |
| Wednesday | 4 (6.3) | 0 | 1 (1.0) | 5 (2.8) |
| Thursday | 3 (4.8) | 1 (7.1) | 0 | 4 (2.2) |
| Friday | 4 (6.3) | 0 | 1 (1.0) | 5 (2.8) |
| Saturday | 3 (4.8) | 1 (7.1) | 4 (3.8) | 8 (4.4) |
| Clinician named in form/found in notes, n (%) of cases | 31 (49.2) | 6 (42.9) | 39 (37.5) | 76 (42.0) |
| Unique clinician ID named (n) | 24 | 6 | 33 | 63 |
| Cases per named clinician (n) | ||||
| 1 | 17 | 6 | 27 | 50 |
| 2 | 7 | 0 | 6 | 13 |
| 3 | 0 | 0 | 0 | 0 |
| Role, n (% of cases) | ||||
| Details not given/found | 35 (55.6) | 7 (50.0) | 79 (76.0) | 121 (66.9) |
| Outreach nurse/ACCP team | 1 (1.6) | 0 | 1 (1.0) | 2 (1.1) |
| Junior doctor | 4 (6.3) | 0 | 3 (2.9) | 7 (3.9) |
| Registrar | 18 (28.6) | 6 (42.9) | 15 (14.4) | 39 (21.5) |
| Consultant | 5 (7.9) | 1 (7.1) | 6 (5.8) | 12 (6.6) |
| Speciality,a n (% of cases) | ||||
| Details not given/found | 46 (73.0) | 3 (21.4) | 83 (79.8) | 132 (72.9) |
| Medical specialties | 8 (12.7) | 6 (42.9) | 8 (7.7) | 22 (12.2) |
| Surgical specialties | 2 (3.2) | 0 | 5 (4.8) | 7 (3.9) |
| Acute/emergency medicine | 7 (11.1) | 5 (35.7) | 8 (7.7) | 20 (11.0) |
Referral figures
FIGURE 34.
Time of day of discussion with ICU team member about possibility of admission.
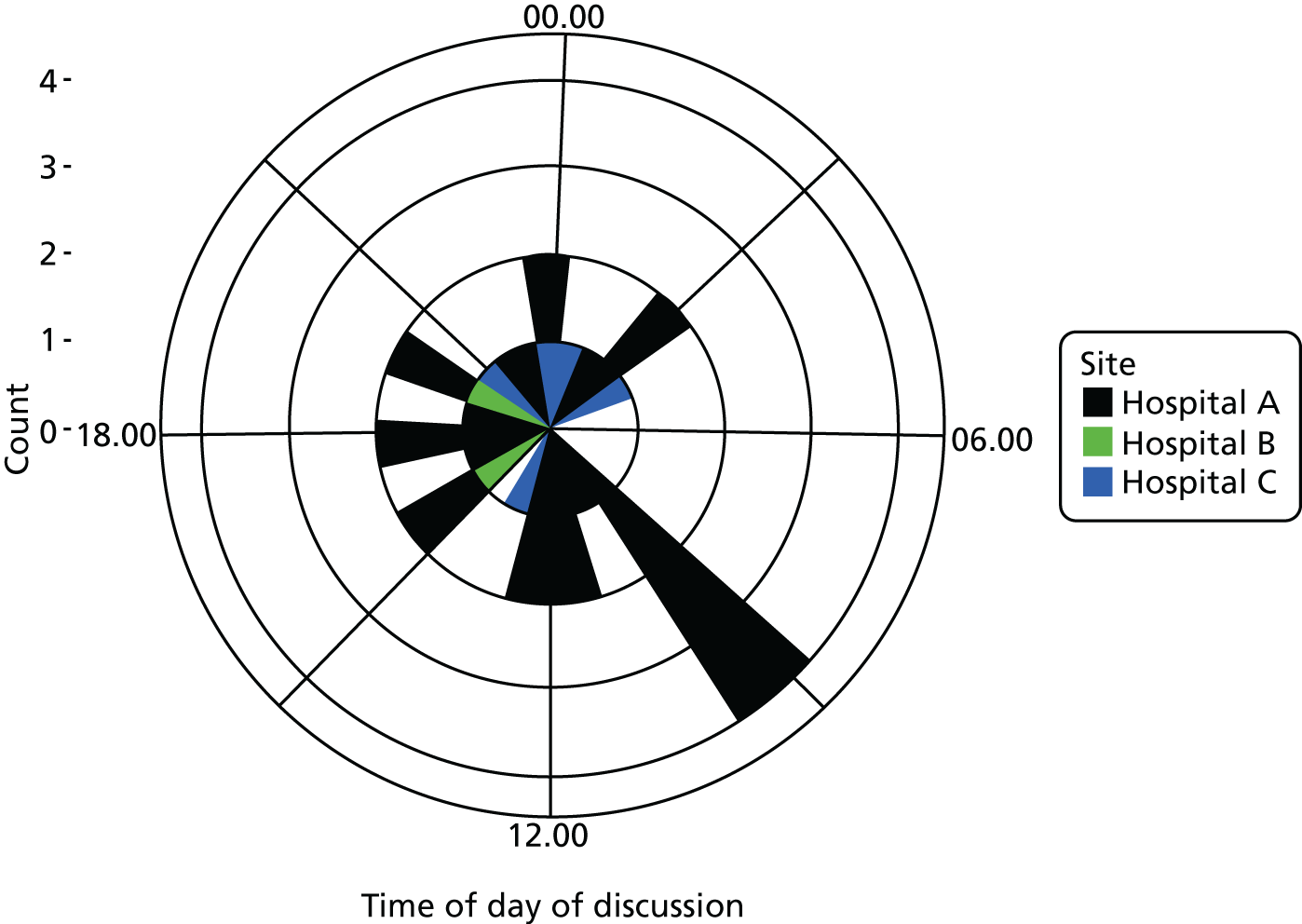
FIGURE 35.
Time of day of discussion with ICU team member about possibility of admission, by site. (a) Hospital A; (b) hospital B; (c) hospital C.
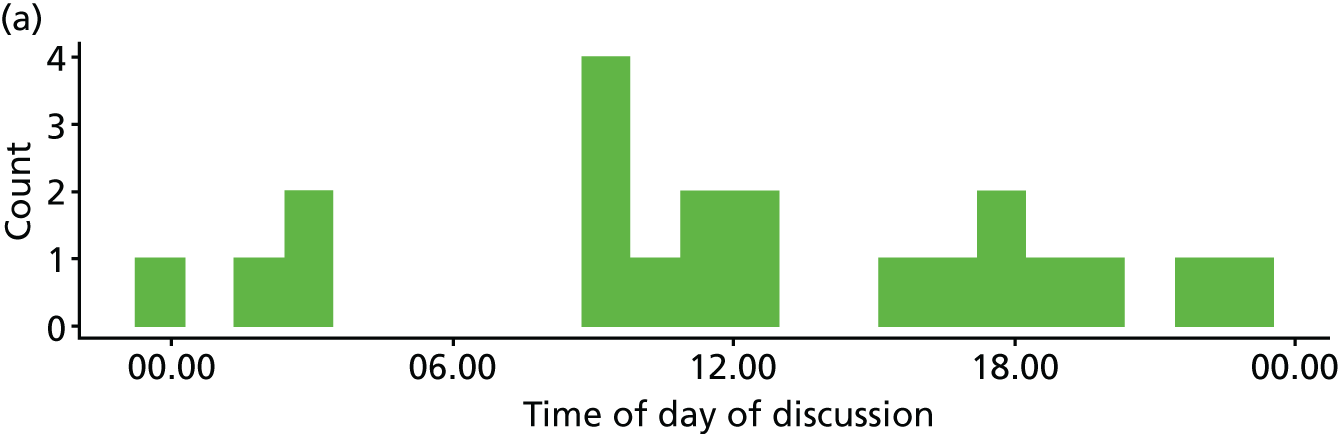
Decision form
Decision form use
In 36 of the 181 cases (20%) the referral form was used. Table 41 shows how often each section of the form was completed.
| Form variable | Hospital A (N = 63) | Hospital B (N = 14) | Hospital C (N = 104) | All sites (N = 181) |
|---|---|---|---|---|
| Cases where decision form was used, n (%) | 19 (30.2) | 2 (14.3) | 15 (14.4) | 36 (19.9) |
| Evidence: clinical, n (%) | ||||
| Not given | 1 (5.3) | 0 | 0 | 1 (2.8) |
| Details given | 18 (94.7) | 2 (100) | 15 (100) | 35 (97.2) |
| Evidence: ability to recover, n (%) | ||||
| Not given | 1 (5.3) | 0 | 0 | 1 (2.8) |
| Details given | 18 (94.7) | 2 (100) | 15 (100) | 35 (97.2) |
| Evidence: patient’s wishes, n (%) | ||||
| Not given | 2 (10.5) | 0 | 0 | 2 (5.6) |
| Details given | 17 (89.5) | 2 (100) | 15 (100) | 34 (94.4) |
| Source of patient wishes, n (%) | ||||
| Not given | 12 (63.2) | 2 (100) | 13 (86.7) | 27 (75.0) |
| Details given | 7 (36.8) | 0 | 2 (13.3) | 9 (25.0) |
| Balancing: benefits, n (%) | ||||
| Not given | 3 (15.8) | 0 | 2 (13.3) | 5 (13.9) |
| Details given | 16 (84.2) | 2 (100) | 13 (86.7) | 31 (86.1) |
| Balancing: burdens, n (%) | ||||
| Not given | 4 (21.1) | 1 (50.0) | 6 (40.0) | 11 (30.6) |
| Details given | 15 (78.9) | 1 (50.0) | 9 (60.0) | 25 (69.4) |
| Recommended treatment, n (%) | ||||
| Not given | 2 (10.5) | 0 | 1 (6.7) | 3 (8.3) |
| Details given | 17 (89.5) | 2 (100) | 14 (93.3) | 33 (91.5) |
| Can care be delivered outside ICU?, n (%) | ||||
| Not given | 4 (21.1) | 1 (50.0) | 5 (33.3) | 10 (27.8) |
| 1: only in ICU | 9 (47.4) | 0 | 5 (33.3) | 14 (38.9) |
| 2: outside ICU | 6 (31.6) | 1 (50.0) | 5 (33.3) | 12 (33.3) |
| 3: outside ICU, but no resource | 0 | 0 | 0 | 0 |
| Ongoing care/review, n (%) | ||||
| Not given | 11 (57.9) | 0 | 5 (33.3) | 16 (44.4) |
| 1: admit to ICU | 6 (31.6) | 1 (50.0) | 4 (26.7) | 11 (30.6) |
| 2: ongoing review | 2 (10.5) | 1 (50.0) | 1 (6.7) | 4 (11.1) |
| 3: review if changes | 0 | 0 | 5 (33.3) | 5 (13.9) |
| Patient contributed | ||||
| Not given | 8 (42.1) | 1 (50.0) | 11 (73.3) | 20 (55.6) |
| Details given | 11 (57.9) | 1 (50.0) | 4 (26.7) | 16 (44.4) |
| Relative contributed | ||||
| Not given | 11 (57.9) | 2 (100) | 13 (86.7) | 26 (72.2) |
| Details given | 8 (42.1) | 0 | 2 (13.3) | 10 (27.8) |
| Nature of relative involvement | ||||
| Not given | 12 (63.2) | 2 (100) | 14 (93.3) | 28 (77.8) |
| Details given | 7 (36.8) | 0 | 1 (6.7) | 8 (22.2) |
| Repeated assessment number, n (%) | ||||
| No data/not given | 8 (42.1) | 1 (50.0) | 11 (73.3) | 20 (55.6) |
| First | 11 (57.9) | 1 (50.0) | 4 (26.7) | 16 (44.4) |
| Referring team name, n (%) | ||||
| No data/not given | 12 (63.2) | 2 (100) | 11 (73.3) | 25 (69.4) |
| Details given | 7 (36.8) | 0 | 4 (26.7) | 11 (30.6) |
| Referring clinician signed decision form | ||||
| No data/not given | 18 (94.7) | 2 (100) | 13 (86.7) | 33 (91.7) |
| Details given | 1 (5.3) | 0 | 2 (13.3) | 3 (8.3) |
Decision details
Again, for some details of the decision process, it was possible to extract information from the hospital notes. Hence, data presented in Table 42 may have been collected from the referral forms or from patient notes.
| Form variable | Hospital A (N = 63) | Hospital B (N = 14) | Hospital C (N = 104) | All sites (N = 181) |
|---|---|---|---|---|
| Cases where decision form was used, n (%) | 19 (30.2) | 2 (14.3) | 15 (14.4) | 36 (19.9) |
| Day of ICU assessment, n (%) | ||||
| No data/not given | 38 (60.3) | 6 (42.9) | 84 (80.8) | 128 (70.7) |
| Sunday | 4 (6.3) | 2 (14.3) | 3 (2.9) | 9 (5.0) |
| Monday | 5 (7.9) | 0 | 3 (2.9) | 8 (4.4) |
| Tuesday | 3 (4.8) | 1 (7.1) | 5 (4.8) | 9 (5.0) |
| Wednesday | 2 (3.2) | 2 (14.3) | 2 (1.9) | 6 (3.3) |
| Thursday | 6 (9.5) | 1 (7.1) | 1 (1.0) | 8 (4.4) |
| Friday | 0 | 0 | 3 (2.9) | 3 (1.7) |
| Saturday | 5 (7.9) | 2 (14.3) | 3 (2.9) | 10 (5.5) |
| Time of ICU assessment, n (%) | ||||
| No data/not given | 39 (61.9) | 6 (42.9) | 84 (80.8) | 129 (71.3) |
| Daytime (08.00–19.59) | 18 (28.6) | 3 (21.4) | 13 (12.5) | 34 (18.8) |
| Night-time (20.00–07.59) | 6 (9.5) | 5 (35.7) | 7 (6.7) | 18 (9.9) |
| ICU team named in form/notes, n (%) of cases | 32 (50.8) | 9 (64.3) | 40 (38.5) | 81 (44.8) |
| Unique ICU teama named (n) | 16 | 8 | 21 | 45 |
| Cases per ICU team (n) | ||||
| 1 | 9 | 7 | 13 | 29 |
| 2 | 2 | 1 | 2 | 5 |
| 3 | 3 | 0 | 2 | 5 |
| 4 | 1 | 0 | 3 | 4 |
| 5 | 0 | 0 | 1 | 1 |
| 6 | 1 | 0 | 0 | 1 |
| ICU team role, n (%) of cases | ||||
| Details not given/found | 31 (49.2) | 5 (35.7) | 63 (60.6) | 99 (54.7) |
| Outreach nurse/ACCP team | 1 (1.6) | 0 | 0 | 1 (0.6) |
| Registrar | 13 (20.6) | 5 (35.7) | 20 (19.2) | 38 (21.0) |
| Consultant | 18 (28.6) | 4 (28.6) | 21 (20.2) | 43 (23.8) |
Decision form figures
FIGURE 36.
Date of ICU assessment on form or in notes.
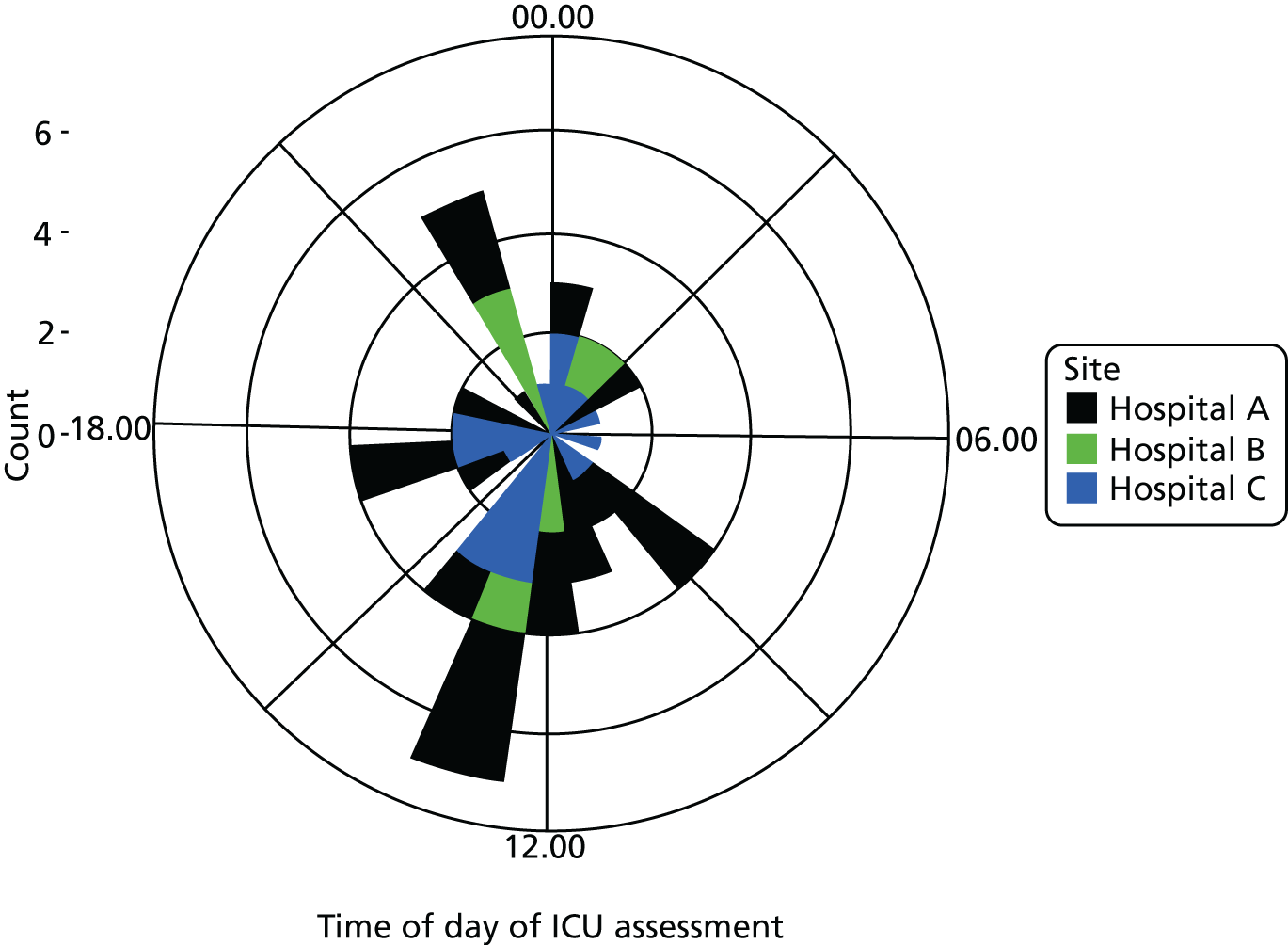
FIGURE 37.
Date of ICU assessment on form or in notes, by site. (a) Hospital A; (b) hospital B; (c) hospital C.
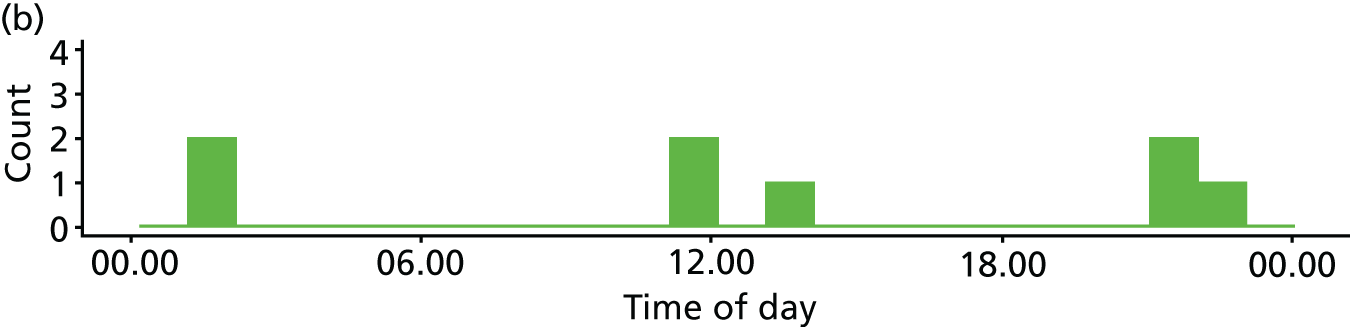
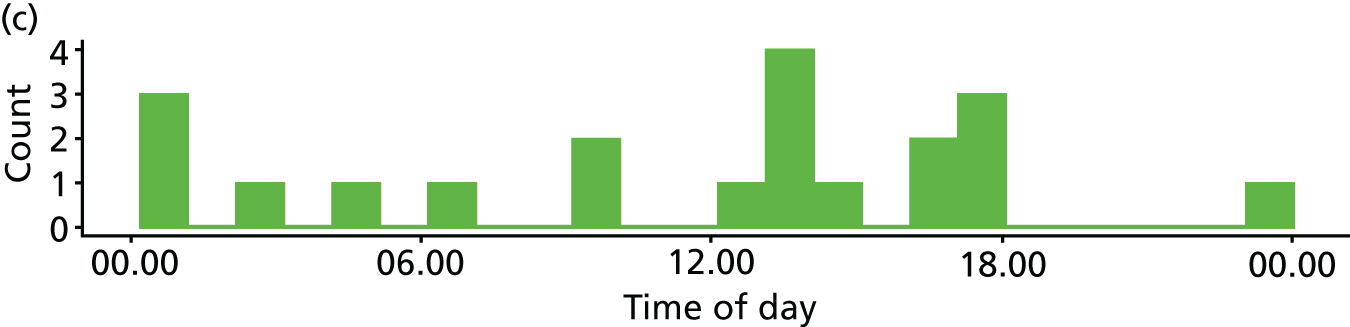
Factors associated with form use
Table 43 illustrates potential descriptive statistics of each referral by site. Unless indicated, the number of each category and the percentages of category of the row have been calculated. It should be noted that these analyses are exploratory only as no formal power calculation was employed a priori.
| Case variable | Referral form used, n (% of row) | p-value | |
|---|---|---|---|
| Yes | No | ||
| Eligible referral: yes, N (%) of known form use | 45 (24.9) | 136 (75.1) | – |
| Admitted | |||
| No | 13 (25.5) | 38 (74.5) | 1 |
| Yes | 31 (25.8) | 89 (74.2) | |
| Patient age (years), mean (SD) | 70.4 (16.3) | 60.4 (17.1) | 0.001a |
| Patient age category | |||
| < 40 | 3 (15.0) | 17 (85.0) | 0.001 |
| 40–59 | 5 (9.8) | 46 (90.2) | |
| 60–79 | 21 (28.8) | 52 (71.2) | |
| ≥ 80 | 16 (47.1) | 18 (52.9) | |
| Patient sex | |||
| Female | 21 (28.0) | 54 (72.0) | 0.5908 |
| Male | 24 (23.3) | 79 (76.7) | |
| Day of assessment | |||
| Weekend (Saturday or Sunday) | 9 (20.0) | 36 (80.0) | 0.5018 |
| Weekday (Monday–Friday) | 36 (26.5) | 100 (73.5) | |
| Time of assessment | |||
| Daytime (08.00–19.59) | 24 (36.4) | 42 (63.6) | 0.3289 |
| Night-time (20.00–07.59) | 16 (27.7) | 44 (73.3) | |
| Week of data collection | |||
| 1 | 8 (19.5) | 33 (80.5) | 0.8411 |
| 2 | 8 (26.7) | 22 (73.3) | |
| 3 | 7 (30.4) | 16 (69.6) | |
| 4 | 7 (25.0) | 21 (75.0) | |
| 5 | 4 (18.2) | 18 (81.8) | |
| 6 | 11 (29.7) | 26 (70.3) | |
| Days between admission and assessment | |||
| 0–2 | 31 (54.4) | 26 (45.6) | 0.8932 |
| > 2 | 13 (50.0) | 13 (50.0) | |
| ICU bed availability at assessment | |||
| Full/over capacity | 20 (28.2) | 51 (71.8) | 0.4240 |
| 1 bed available | 14 (19.4) | 58 (80.6) | |
| ≥ 2 beds available | 10 (27.8) | 26 (72.2) | |
| Referring clinician | |||
| ID given/found | 40 (52.6) | 36 (47.4) | 0.001 |
| ID not given/found | 5 (4.8) | 100 (85.2) | |
| Referring clinician: specialty | |||
| Medical specialty | 12 (54.5) | 10 (45.5) | b |
| Surgical specialties | 4 (57.1) | 3 (42.9) | |
| Acute medicine | 1 (33.3) | 2 (66.7) | |
| Emergency medicine | 5 (29.4) | 12 (70.6) | |
| Referring clinician: grade | |||
| Outreach nurse/ACCP team | 1 (50.0) | 1 (50.0) | b |
| Junior doctor | 5 (55.6) | 4 (44.4) | |
| Registrar | 26 (66.7) | 13 (33.3) | |
| Consultant | 5 (38.5) | 8 (61.5) | |
| Case variable | Decision form used, n (% of row) | p-value | |
|---|---|---|---|
| Yes | No | ||
| Eligible referral: yes, N (% of known form use) | 36 (19.9) | 145 (80.1) | – |
| Use referral form | |||
| Yes | 30 (16.6) | 15 (8.3) | < 0.001 |
| No | 6 (3.3) | 130 (71.8) | |
| Admitted | |||
| No | 11 (21.6) | 40 (78.4) | 1 |
| Yes | 25 (20.8) | 95 (79.2) | |
| Patient age (years), mean (SD) | 71.6 (13.9) | 60.7 (17.6) | < 0.001a |
| Age category (years) | |||
| < 40 | 1 (5.0) | 19 (95.0) | b |
| 40–59 | 5 (9.8) | 46 (90.2) | |
| 60–79 | 19 (26.0) | 54 (74.0) | |
| ≥ 80 | 11 (32.4) | 23 (67.6) | |
| Patient sex | |||
| Female | 14 (18.7) | 61 (81.3) | 0.8005 |
| Male | 22 (21.4) | 81 (78.6) | |
| Day of assessment | |||
| Weekend (Saturday or Sunday) | 9 (20.0) | 36 (80.0) | 1 |
| Weekday (Monday–Friday) | 27 (19.9) | 109 (80.1) | |
| Time of assessment | |||
| Daytime (08.00–19.59) | 20 (30.3) | 46 (69.7) | 0.1129 |
| Night-time (20.00–07.59) | 10 (16.7) | 50 (83.3) | |
| Week of data collection | |||
| 1 | 7 (17.1) | 34 (82.9) | b |
| 2 | 8 (26.7) | 22 (73.3) | |
| 3 | 5 (21.7) | 18 (78.3) | |
| 4 | 5 (17.9) | 23 (82.1) | |
| 5 | 2 (9.1) | 20 (90.9) | |
| 6 | 9 (24.3) | 28 (75.7) | |
| Days between admission and assessment | |||
| 0–2 | 20 (35.1) | 37 (64.9) | 0.6994 |
| > 2 | 11 (42.3) | 15 (57.7) | |
| ICU bed availability at assessment | |||
| Full/over capacity | 14 (19.7) | 57 (80.3) | 0.999 |
| 1 bed available | 14 (19.4) | 58 (80.6) | |
| ≥ 2 beds available | 7 (19.4) | 29 (80.6) | |
| Reviewing clinician | |||
| ID given/found | 31 (38.3) | 50 (61.7) | < 0.001 |
| ID not given/found | 5 (5.0) | 95 (95.0) | |
| Reviewing clinician: grade | |||
| Outreach nurse/ACCP team | 1 (100) | 0 | b |
| Registrar | 11 (28.9) | 27 (71.1) | |
| Consultant | 19 (44.2) | 24 (55.8) | |
| Decision form responsea | Not admitted | Admitted |
|---|---|---|
| Used decision form: yes | 11 (30.6) | 25 (69.4) |
| Ongoing care/review, n (%) | ||
| Not given | 2 (28.6) | 5 (71.4) |
| 1: admit to ICU | 0 | 14 (100) |
| 2: ongoing review | 6 (54.5) | 5 (45.5) |
| 3: review if changes | 3 (75.0) | 1 (25.0) |
| Balancing: benefits, n (%) | ||
| Not given | 1 (20.0) | 4 (80.0) |
| Details given | 10 (32.3) | 21 (67.7) |
| Balancing: burdens, n (%) | ||
| Not given | 1 (9.1) | 10 (90.9) |
| Details given | 10 (40.0) | 15 (60.0) |
The majority of patients for whom the decision form was used were admitted (25/36, 69.4% of patients). Among those patients for whom the decision form was used, most completed the need for ongoing care/review section (29/36, 80.6% of patients). All 14 patients who were marked as admitted to ICU were admitted, but only 5 of the 11 patients marked as ‘ongoing review’ were admitted (45.5%) and one of the four patients (25%) given the ‘review if condition changes’ was admitted. Of the seven patients who did not give a review response, five (71.4%) were admitted.
When considering the balancing of care, the benefits of care were given more frequently (n = 31, 86.1%) than the burdens (n = 25, 69.4%). Patients who were subsequently admitted tended to have benefits details (n = 21/31, 67.7%) more frequently given than the burdens (n = 15/25, 60.0%), but this may be an artefact of the small sample size.
| Case variable | Both forms used, n (% of row) | p-value | |
|---|---|---|---|
| Yes | No | ||
| Eligible referral: yes, N (% of known form use) | 30 (16.6) | 151 (83.4) | |
| Admitted | |||
| No | 9 (17.6) | 42 (82.4) | 1 |
| Yes | 21 (17.5) | 99 (82.5) | |
| Patient age (years), mean (SD) | 71.4 (14.4) | 61.2 (17.5) | 0.0013 |
| Age category (years) | |||
| < 40 | 1 (5.0) | 19 (95.0) | a |
| 40–59 | 4 (7.8) | 47 (92.2) | |
| 60–79 | 16 (21.9) | 57 (78.1) | |
| ≥ 80 | 9 (26.5) | 25 (73.5) | |
| Patient sex | |||
| Female | 12 (16.0) | 61 (84.0) | 0.9546 |
| Male | 18 (17.5) | 85 (82.5) | |
| Day of assessment | |||
| Weekend (Saturday or Sunday) | 6 (13.3) | 37 (86.7) | 0.6575 |
| Weekday (Monday–Friday) | 24 (17.6) | 112 (82.4) | |
| Time of assessment | |||
| Daytime (08.00–19.59) | 19 (28.8) | 47 (71.2) | 0.0314 |
| Night-time (20.00–07.59) | 7 (11.7) | 53 (88.3) | |
| Week of data collection | |||
| 1 | 6 (14.6) | 35 (85.4) | a |
| 2 | 5 (16.7) | 25 (83.3) | |
| 3 | 4 (17.4) | 19 (82.6) | |
| 4 | 4 (14.3) | 24 (85.7) | |
| 5 | 2 (9.1) | 20 (90.9) | |
| 6 | 9 (24.3) | 28 (75.7) | |
| Days between admission and assessment | |||
| 0–2 | 19 (33.3) | 38 (66.7) | 0.5871 |
| > 2 | 11 (42.3) | 15 (57.7) | |
| ICU bed availability at assessment | |||
| Full/over capacity | 12 (16.9) | 59 (83.1) | 0.9624 |
| 1 bed available | 11 (15.3) | 61 (84.7) | |
| ≥ 2 beds available | 6 (16.7) | 30 (83.3) | |
Appendix 19 Documentation of referrals and reviews by site
| Hospital, n (%) | ||||
|---|---|---|---|---|
| A | B | C | All | |
| No ICU referral documented | 4 (6.3) | 1 (7.1) | 28 (26.9) | 33 (18.2) |
| Referral identified but no doctor name documented | 26 (41.2) | 6 (42.9) | 33 (31.7) | 65 (35.3) |
| No ICU review documented | 4 (6.3) | 1 (7.1) | 45 (43.3) | 50 (27.6) |
| ICU review identified but no doctor name documented | 24 (38.1) | 2 (14.3) | 18 (17.3) | 44 (24.3) |
Appendix 20 Search strategies for systematic review 3
| Database | Retrieved (n) | After deduplication (n) |
|---|---|---|
| MEDLINE | 1170 | 1157 |
| EMBASE | 1375 | 681 |
| Web of Science | 545 | 166 |
| PsycInfo | 324 | 143 |
| Total | 3414 | 2147 |
| Results | Search type | Actions |
|---|---|---|
| 1 | Ethics, Clinical/ | 2902 |
| 2 | ethical decision making.mp. | 965 |
| 3 | ethical decision*.mp. | 1407 |
| 4 | ethical value*.mp. | 435 |
| 5 | moral deliberation*.mp. | 61 |
| 6 | moral case deliberation*.mp. | 19 |
| 7 | moral value*.mp. | 462 |
| 8 | ethical deliberation.mp. | 48 |
| 9 | Ethics Consultation/or ethics consultation*.mp. | 1154 |
| 10 | ethics support.mp. | 75 |
| 11 | ethics education.mp. | 504 |
| 12 | ethics framework*.mp. | 64 |
| 13 | ethical framework*.mp. | 616 |
| 14 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 7043 |
| 15 | exp Evaluation Studies as Topic/or evaluat*.mp. or outcome*.mp. | 4,054,850 |
| 16 | exp Program Evaluation/ | 60,694 |
| 17 | 15 or 16 | 4,061,053 |
| 18 | 14 and 17 | 1170 |
| Results | Search type | Actions |
|---|---|---|
| 1 | clinical ethics.mp. | 1216 |
| 2 | ethical decision making/or ethical decision making.mp. | 2144 |
| 3 | ethical decision*.mp. | 2645 |
| 4 | ethical value*.mp. | 576 |
| 5 | moral deliberation*.mp. | 70 |
| 6 | moral case deliberation*.mp. | 24 |
| 7 | moral value*.mp. | 553 |
| 8 | ethical deliberation.mp. | 56 |
| 9 | ethics consultation*.mp. | 629 |
| 10 | ethics support.mp. | 81 |
| 11 | ethics education.mp. | 591 |
| 12 | ethics framework*.mp. | 80 |
| 13 | ethical framework*.mp. | 829 |
| 14 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 6688 |
| 15 | exp evaluation/or evaluat*.mp. or outcome*.mp. | 5,115,815 |
| 16 | 14 and 15 | 1375 |
Web of Science: Core Collection (SCI and SSCI only) (ICU Ethics Final V2)
Date of search: 21 March 2016.
Search strategy
TOPIC: (“clinical ethics” or “ethical decision making” or “ethical decision*” or “ethical value*” or “moral deliberation*” or “moral case deliberation*” or “moral value*” or “ethical deliberation” or “ethical consultation*” or “ethics support” or “ethics education” or “ethics framework*” or “ethical framework*”)
AND
TOPIC: (evaluat* or outcome*)
AND
TOPIC: (medicine or medical or clinical or health)
PsycINFO (scholarly journals only)
Date of search: 21 March 2016.
Search strategy
(“clinical ethics” OR “ethical decision making” OR “ethical decision*” OR “ethical value*” OR “moral deliberation*” OR “moral case deliberation*” OR “moral value*” OR “ethical deliberation” OR “ethical consultation*” OR “ethics support” OR “ethics education” OR “ethics framework*” OR “ethical framework*”)
AND
(SU.EXACT.EXPLODE(“Program Evaluation”) OR SU.EXACT.EXPLODE(“Evaluation”) OR evaluat* OR outcome*)
AND
(medicine OR medical OR clinical OR health)
OpenGrey
Date of search: 3 March 2016.
146 references.
Search strategy
(ethic* OR moral) AND (evaluat* OR outcome*) AND (medic* OR clinic* OR health)
Dissertations and Theses
Date of search: 5 May 2016.
552 references.
Search strategy
all(“clinical ethics” OR “ethical decision making” OR “ethical decision*” OR “ethical value*” OR “moral deliberation*” OR “moral case deliberation*” OR “moral value*” OR “ethical deliberation*” OR “ethics consultation*” OR “ethics support” OR “ethics education” OR “ethics framework*” OR “ethical framework*” OR “medical ethics”) AND all(evaluat* OR outcome*) AND all(medic* OR health OR clinic* OR nurs* OR doctor* OR physician*)
Index to Theses
Date of search: 5 May 2016.
411 references.
Search strategy
(“clinical ethics” OR “ethical decision making” OR “ethical decision*” OR “ethical value*” OR “moral deliberation*” OR “moral case deliberation*” OR “moral value*” OR “ethical deliberation*” OR “ethics consultation*” OR “ethics support” OR “ethics education” OR “ethics framework*” OR “ethical framework*” OR “medical ethics”) AND (evaluat* or outcome*) AND (health or medic* or clinic* or nurs* or doctor* or physician*)
| Database | Number of hits | |
|---|---|---|
| Original | Deduplicated | |
| OpenGrey | 146 | 146 |
| Dissertations and Theses | 552 | 547 |
| Index to Theses | 411 | 353 |
| Total | 1109 | 1045 |
| Database | References retrieved | Date range | Deduplicated |
|---|---|---|---|
| MEDLINE | 182 | 21/03/16–03/18 | 182 |
| EMBASE | 238 | 21/03/16–03/18 | 115 |
| Web of Science | 122 | 2016–03/18 | 56 |
| PsycINFO | 55 | 2016–03/18 | 24 |
| Dissertations and Theses | 26 | 01/05/16–03/18 | 23 |
| Index to Theses | 0 | 01/05/16–03/18 | |
| OpenGrey | 0 | 2016–03/18 | |
| Total | 623 | 400 |
PsycINFO (via Ovid)
Date range searched: 1806 to March week 3 2018.
Search strategy
-
clinical ethics.mp. (337)
-
ethical decision making.mp. (1960)
-
ethical decision*.mp. (2404)
-
ethical value*.mp. (741)
-
moral deliberation*.mp. (102)
-
moral case deliberation*.mp. (25)
-
moral value*.mp. (1554)
-
ethical deliberation.mp. (53)
-
ethical consultation*.mp. (12)
-
ethics support.mp. (48)
-
ethics education.mp. (536)
-
ethics framework*.mp. (66)
-
ethical framework*.mp. (619)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 (6135)
-
exp Program Evaluation/ (19,270)
-
exp EVALUATION/ (101,036)
-
evaluat*.mp. (521,413)
-
outcome*.mp. (358,634)
-
15 or 16 or 17 or 18 (810,188)
-
(medicine or medical or clinical or health).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures] (1,099,046)
-
14 and 19 and 20 (408)
-
limit 21 to yr="2016 -Current" (55)
-
from 22 keep 1-55 (55)
Appendix 21 Included studies for systematic review of evaluation tools for interventions to improve ethical decision-making
| First author and year | Country | Name/brief description of evaluation tool | Target population | Validity testing | Reliability testing | Intervention evaluated | Language of tool | What was measured |
|---|---|---|---|---|---|---|---|---|
| Siegler, 1982183 | USA | A series of case vignettes with questions related to clinical and ethical dimension of the case; students were asked to give reasons for their answers to the individual question | Medical students | Scoring categorisation of reasons developed by several members of weighting of reasons reflected values of teaching staff | Two independent scorers each scored 10 students’ assessments and reached agreement on 88% of responses | Experimental teaching course in ethics | English | Ethical reflectiveness and reasoning |
| Smith, 1994193 | USA | Performance-based clinical skills assessment; students assessed on performance with standardised patient based on five behaviours and on written element of the assessment that asked students to list the moral conflicts identified and analyse two of them | Medical students | Not described | For written portion: the Spearman rank-correlation coefficients for pairs of readers who jointly rated more than 10 students | Medical curriculum | English | Moral reasoning and ethical judgement |
| Singer, 1994190 | Canada | OSCE; six ethics OSCE stations; stations based on actual cases described; scoring checklists developed using videotaped encounters between attending physicians and standardised patients | Medical students and residents | Performance of eight expert clinicians in response to the scenarios | Inter-rater reliability determined using intraclass correlation coefficient. Internal consistency reliability calculated using Cronbach’s alpha | Medical curriculum | English | Performance in the OSCE |
| Singer, 1996191 | USA | Four ethics stations on the OSCE; cases developed based on legal cases; scoring checklists developed by videotaping performances of 4–6 staff physicians on each of the stations, and then transcribed and reviewed by the physicians to identify comments most commonly mentioned and consistent with bioethical principles | Final-year medical students | Content validity tested by use of staff physicians in development of station | Inter-rater reliability scored using interclass correlation coefficients | Medical curriculum | English | Performance in the OSCE |
| McAlpine, 1997184 | Australia |
Ethical Reasoning Tool Case reflections are scored for each component of ethical reasoning against three professional response levels (traditional/traditional reflective/reflective). And eight components of ethical reasoning: (1) recognition of ethical issue; (2) use of ethical framework; (3) use of personal values; (4) use of professional values; (5) perception of the nurses role; (6) perception of therapeutic nurse–patient relationship; (7) communication patterns; (8) potential action |
Nursing students |
Content validity assessed by panel Construct validity Wilcoxon matched pairs signed-rank test used to test changes in scores from pre to post test. Confirmed by a content analysis of students reflections on completing the post test |
Philosopher not connected with the study used the tool to score a random sample of 25% of papers. At least 75% agreement on level of response was achieved for 11 out of 15 students | Ethics study unit in medical curriculum | English | Cognitive reasoning |
| Turner, 1998189 | USA | Ketefian’s JAND, six stories with ethical dilemmas in practice; respondents rank which behaviour is most professionally desirable (moral reasoning) and which is most likely to occur (ethical decision-making) | Community health nursing students | Content validity of JAND reported as being established with internal consistency measures giving alpha coefficients from 0.66 to 0.73 for ethical decision-making | Not described | Nursing curricula (nursing students enrolled in the study from three undergraduate programmes) | English | Ethical decision-making and moral reasoning |
| Savulescu, 1999185 | UK | Six vignettes constructed to reflect ethical issues arising in clinical practice; answers to vignettes evaluated by three markers with formal training in philosophy/bioethics and experience of teaching medical ethics, and using a set of principles/marking criteria developed for that purpose | Medical students | Content validity assessed by naive markers scores compared with marks by primary markers using the marking scheme | Test–retest reliability evaluated by the extent to which the same student answering the same script 2 months later was given the same mark, from the same rater | Medical ethics course in medical curriculum | English | Ethical awareness and core critical thinking skills |
| Goldie, 2002,187 and Goldie, 2004188 | UK |
Ethics and health care survey instrument (EQUAT) 12 case vignettes that include an ethical dimension; for nine there is consensus opinion regarding preferred answer and for three there is reasonable dissensus; participants asked to choose preferred answer and justify their decision |
Medical students | Not described | Not described | Integrated medical curriculum | English | Proposed behaviour in ethical situation |
| Akabayashi, 2004182 | Japan | Two-component survey: (1) Japanese version of the ethical sensitivity test (Problem Identification Test). Students are asked to list all the ethical issues related to each case in three vignettes; (2) two vignettes from the Japanese version of the DIT. In the DIT students are asked to choose the most suitable action, list reasons for that action and order the four most important reasons | Medical students and graduates (residents) | Referred to validity of the test in other papers | Not described | Medical curriculum with second year medical ethics lectures | Japanese | Moral sensitivity and reasoning |
| Lohfeld, 2012192 | UK | EHCQ-2 ethical dilemmas in 12 clinical vignettes; subjects are asked to choose the best option from several pre-set responses; rationale for the choice is also explored by asking subjects to write a short answer that explains their thinking. These explanations are then scored through a formal coding system | Medical students (final-year McMaster University students and final-year University of Glasgow students) | Content validity was ensured by having a team of experts review the cases and reach consensus on the final versions | Assessment of the performance of medical students on two occasions, separated by 2 weeks, using two or three trained raters at each site | Medical curriculum (McMaster, problem-based programme; Glasgow University, integrated, problem-based curriculum) | English | Ethical sensitivity |
| Pearlman, 2016;194 Fins, 2016195 | USA | A records-based assessment using the record of a clinical ethics case consultation. Scoring is based on four key elements of an ethics consultation (ethics question, consultation-specific information, ethical analysis, conclusions and recommendations). Each element is scored within two categories as acceptable/less than acceptable using four key descriptors: poor, less than adequate, adequate, and strong. Each element has a set of descriptors about what should be included in the record | Clinical ethics consultants | Verbal feedback from nine ASBH reviewers who were members of the SBH Quality Attestation Presidents Taskforce | Scoring of a sample of case consultation records as part of an ASBH quality attestation pilot. 43% inter-rater agreement between scores and 74% agreement regarding acceptable/not acceptable categories | Clinical ethics consultation | English | Identification of ethical issue, relevant information gathering, ethical analysis and ethical decision-making |
| Chao, 2017186 | Taiwan |
Nursing ethical decision-making ability scale Questionnaire comprising 30 questions reflecting four dimensions of ethical decision-making: recognising differences, comparing differences, self-dialogue and identifying implications. Self-assessment |
Nursing students | Not described. References validity testing in an unpublished paper | Not described | Web-based ethics course | Taiwanese | Self-assessment of ethical decision-making |
Appendix 22 Evaluation tool: final version
Appendix 23 Scoring system for evaluation tool
Data preparation
As part of the scoring process, reviewers were asked to note if there was a record of a decision to admit the patient to ICU in the notes, and then to score any decisions found based on the record given. Reviewers were asked to record the date, time and setting of the decision and if the patient was admitted to ICU after the decision had been made. If multiple decisions were recorded in the notes, reviewers were asked to score each review separately.
Reviewers and data extraction from referrals
Four reviewers independently assessed 120 patient referrals, 40 from each of the three study hospitals. Two reviewers (AS and CB), both clinically trained, reviewed all referrals; and two reviewers (JW and SR), neither clinically trained, assessed only a subset of referrals. JW assessed 80 referrals – those from hospitals B and C – while Sophie Rees reviewed 40 referrals from hospital A only.
All reviewers were trained by the study team prior to data extraction.
Presence of intensive care unit decision in notes
Reviewers were asked to mark each decision identified in the referrals as ‘assessed’ or ‘not assessed’. Decisions were considered present and assessable only if the documentation was made by the person responsible for decision-making. However, in some cases, reviewers did not mark the same number of decisions in each patient referral. In this case, the decision was considered to be ‘not identified’ by the reviewers who did not find the decision and the ID code was added to their scoring sheet. The date, time, admission and setting of the identified decisions for each referral was used to best match which decision had not been identified by the other reviewer(s). For instance, if reviewer 1 recorded one decision made for referral X on the ward (decision X-1), but reviewer 2 recorded two decisions for referral X, one in the ED (decision X-1) and one on the ward (decision X-2), the reviewer 1’s decision was recoded as X-2 and a new ‘not identified’ decision X-1 was added.
Furthermore, any decision was assumed to be ‘not present’ if no items were scored using the instrument. If at least one question was answered, a review decision was assumed to be present and the missing questions were scored as if marked as ‘0’.
Setting of review
Setting of decision has been coded as:
-
A&E/ED (including ‘CT Scan’)
-
cardiac arrest (regardless of other setting details; also includes ‘periarrest’ and ‘OOHCA’)
-
theatre (including ‘post op’ and ‘elective’)
-
ward (including ‘RCU’)
-
not given/unclear.
Cases labelled as ‘transfer’ were excluded from analysis, even if only one of the three reviewers had selected this location.
In cases where settings differed between reviewers, the setting given by the majority of reviewers was used, treating settings not given/unclear as missing. Only decisions for which all three reviewers gave the setting as ‘not given/unclear’ were labelled as such.
Date and time of decision
Where possible, the date and time of the decision were also recorded. However, this data item was not collected at the first hospital analysed. This was used for matching decisions only.
Admission to intensive care unit
Reviewers were asked to collect the outcome of each decision, that is, whether or not the patient was admitted to ICU. If anything other than a clear yes or no was recorded, this was coded as ‘not given/unclear’. However, this data item was not collected at the first hospital analysed (A) by the first reviewer (SR), as the instrument was amended to include this information after their review.
In the same manner as for setting data above, in cases where the outcome differed between reviewers, the outcome reported was the one given by the majority of reviewers. Outcomes were labelled as not given/unclear only when all three reviewers gave this option, and decisions for which no majority outcome was given were noted as such.
Scoring
The scoring questionnaire was broken into four separate sections as follows:
-
section A – clinical need (items 1–6)
-
section B – balancing of benefits and burdens (items 7–11)
-
section C – communication (items 12–16)
-
section D – red flags (item 17).
Each section is scored separately, as detailed below. Note that, unless explicitly stated in the answer scheme as a specific response, a ‘NA’ or blank item was scored as a 0 when a review was noted as assessed.
Section A
This section is marked out of a possible score of 12:
-
Item 1: description of evidence of system failure. For each of the four systems stated (cardiovascular, respiratory, renal and neurology), a mark is awarded if an adequate description is given in the notes up to a maximum score of 2. Zero marks are awarded if no system is described in sufficient detail. For example, if a judge marks that the cardiovascular, respiratory and renal systems were all adequately described (three systems), 2 marks are awarded.
-
Items 2–5: marked as scored (each maximum 2).
-
Item 6: information about patient wishes. Marked as scored (maximum 2). However, 2 marks were awarded if it was documented that this was not appropriate (NA) (i.e. the patient was unconscious).
Section B
This section is marked out of a possible 7 points:
-
Items 7–11: marked as scored (items 7 and 11, each maximum 2; items 8–10, each maximum 1).
Section C
This section is marked out of a possible 10 points:
-
Items 13 and 16: marked as scored (item 13, maximum 2; item 16, maximum 3).
-
Items 12, 14 and 15: Marked as scored (maximum 2). If marked as NA (i.e. not appropriate to communicate to patient, as patient unconscious), then 2 points were awarded.
Section D
This section is marked out of 5, and is a simple summation of each of the five Q17 subitems.
Total score
The total score was calculated as total score = section A total + 2 × section B total + section C total.
Section D is then reported separately.
This results in a maximum total score of 36 + 5.
Statistical methods
Descriptive statistics were generated for the total number of decisions and for each set of decisions provided by a reviewer. Scores given by each reviewer were calculated as stated in Chapter 8, Development of the evaluation tool, Analysis, with each section calculated separately, and then the total was computed.
For continuous variables, the interclass correlation coefficient was calculated along with its 95% CI. To allow for generalisation beyond the study reviewers, the single random raters (ICC2) variant was used. 199 For categorical data, the unweighted kappa statistic and its 95% CI were calculated for each pairwise comparison between reviewers. A decision was considered eligible to be entered into the reliability analyses if all reviewers had given the decision a valid score. At an individual item level, ‘not appropriate’ responses were considered missing to preserve the direct agreement in scoring. Scores from the non-clinical reviewers (JW and SR) were combined as these reviewers never scored the same decision.
All analyses were computed in R using the ‘psych’ package. 200
Interpretation of reliability
For the measures of reliability used here (ICC and kappa statistic), values typically lie between 0 and 1. A value of 1 can be thought of as perfect agreement: all reviewers produce the same score for the same decision. A value of 0 can be thought of as a random chance agreement: the reviewers have no more agreement than would happen if a random number was chosen as the score. Negative values may occur: small negative values for the ICC would represent larger variation occurring between the reviewers than between the decisions; small negative values for the kappa represents less agreement than random chance, but larger (near –1) may indicate reversed scoring from one rater. 201
Typically, an outcome measure would require a reliability of at least 0.7 to be considered a reliable instrument for use in a clinical setting. 201 However, to help aide interpretation of the kappa statistic, Landis and Koch202 suggested arbitrary boundaries to classify the value. These categories have been used for both the kappa and the ICC statistics throughout the results section and are shown in Table 54 for reference.
| Kappa statistic | Interpretation |
|---|---|
| < 0.00 | Poor |
| 0.00–0.20 | Slight |
| 0.21–0.40 | Fair |
| 0.41–0.60 | Moderate |
| 0.61–0.80 | Substantial |
| 0.81–1.00 | Almost perfect |
Appendix 24 Detailed analysis of scoring across reviewers and sites
At hospital A, 46 unique decisions were identified as eligible for review and scoring, 31 of which were assessed at least once. Figure 38 illustrates which combination of reviewers gave each decision a score (i.e. gave the category as assessed). Eight of these decision records (17%) were not identified by at least one reviewer, and no one reviewer identified all 46 decisions. Two records (4%) were identified by only one reviewer (one by AS and one by CB), but both of these decisions were indicated as ‘not assessed’ and therefore not scored by the identifying reviewer.
FIGURE 38.
Venn diagram of assessed decisions at hospital A.
As shown in Figure 39, at hospital B, 34 of the 44 unique decisions were assessed. Five decisions (11%) were missed by at least one reviewer, of which three (7%) were identified by only a single reviewer (two by JW and one by AS).
FIGURE 39.
Venn diagram of the number of assessed decisions by reviewer at hospital B.
At hospital C, 54 distinct decisions were initially identified. However, one reviewer identified that the patient subsequently died after the decision point; hence, this patient was removed from the analysis, leaving 53 eligible decisions, 38 of which were assessed at least once (Figure 40). Thirteen decisions (25%) were missed by at least one reviewer, of which 11 (21%) were identified by only one reviewer (i.e. not noted by the other two reviewers).
FIGURE 40.
Venn diagram of the number of assessed decisions by reviewer at hospital C.
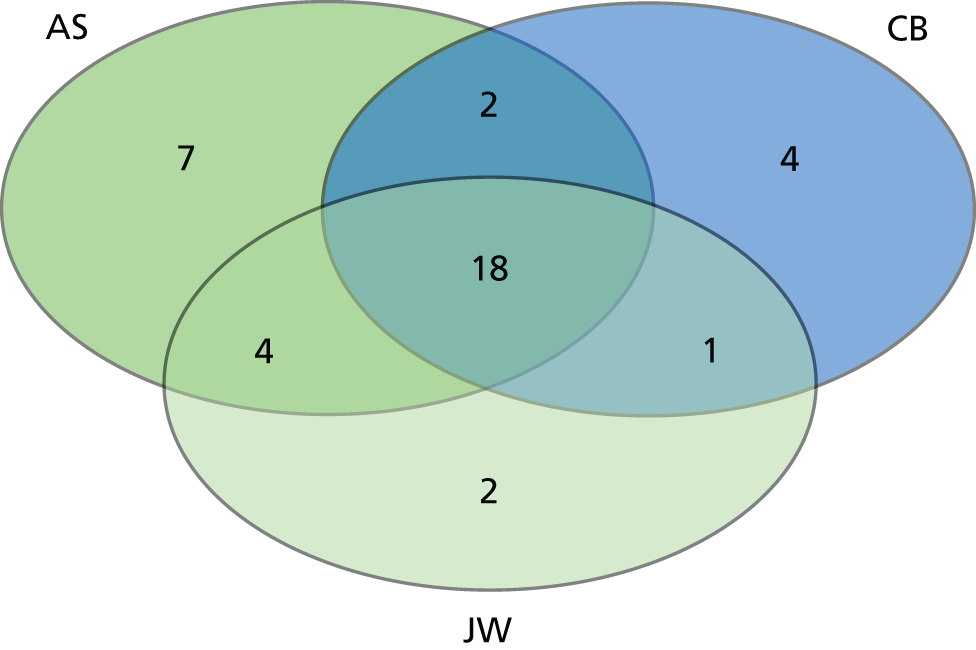
In Table 55, the agreement between reviewers on the given category of the decision is shown for each hospital (including not identified). Agreement varied between reviewers and between hospitals. For instance, Anne Slowther and Chris Bassford had a kappa agreement of 0.42 (‘moderate’; see Table 55) at hospital A, but 0.65 (‘substantial’) at hospital B and 0.38 (‘fair’) at hospital C. All kappa values were significantly better than agreement due to random chance (i.e. the 95% CIs did not include zero); however, many values are only ‘fair’.
| Hospital (n) | Comparison (kappa, 95% CI) | ||
|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | |
| Aa (46) | 0.42 (0.19 to 0.66) | 0.55 (0.36 to 0.75) | 0.32 (0.11 to 0.52) |
| Bb (44) | 0.65 (0.43 to 0.86) | 0.56 (0.32 to 0.79) | 0.76 (0.57 to 0.95) |
| Cb (53) | 0.38 (0.17 to 0.58) | 0.46 (0.28 to 0.65) | 0.49 (0.29 to 0.69) |
Agreement was not necessarily dependent on the clinical knowledge of the cases, as the non-clinical reviewers (JW and SR) sometimes had higher kappa values with one of the clinical reviewers (AS and CB) than between the clinical reviewers at that hospital. However, hospital B generally had higher agreement levels than hospitals A and C. This may indicate that some hospitals had more clear record-keeping processes than others, making extraction of the records easier.
Setting of review
As shown in Table 56, information concerning the number of settings given by each of the reviewing team is given. As noted, settings are given as the decisions given by the majority of reviewers. Decisions given as ‘disagreement’ were those cases with no majority (e.g. each reviewer gave a different response). These cases may be a result of unclear documentation in the patient notes, or of not giving clear guidance on the level of detail required before data extraction.
| Setting | Hospital, n (%) | |||
|---|---|---|---|---|
| A (N = 46) | B (N = 44) | C (N = 53) | All (N = 143) | |
| Unclear/not given | 1 (2.2) | 2 (4.5) | 0 | 3 (2.1) |
| A&E/ED | 4 (8.7) | 20 (45.5) | 9 (17.0) | 33 (23.1) |
| Cardiac arrest | 2 (4.3) | 1 (2.3) | 1 (1.9) | 4 (2.8) |
| Theatre | 17 (37.0) | 0 | 2 (3.8) | 19 (13.3) |
| Ward | 19 (41.3) | 16 (36.4) | 37 (69.8) | 72 (50.3) |
| Disagreement | 3 (6.5) | 5 (11.4) | 4 (7.5) | 12 (8.4) |
Outcome of decision
Table 57 shows the majority outcome of each of the unique decisions at each hospital. The majority of decisions (54.5%) resulted in the patient being admitted to ICU; however, this was driven by hospital A only. Both B and C were more balanced, with slightly more decisions resulting in not being admitted to ICU. For five decisions there were disagreements between reviewers, which may indicate that decisions have not been matched perfectly in all cases. This is of particular difficulty in hospital A, for which reviewer Sophie Rees did not record outcome data and other descriptive information was not collected.
| Admitted | Hospital, n (%) | |||
|---|---|---|---|---|
| A (N = 46) | B (N = 44) | C (N = 53) | All (N = 143) | |
| No | 9 (19.6) | 23 (52.3) | 28 (52.8) | 60 (42.0) |
| Yes | 35 (76.1) | 19 (43.2) | 24 (45.3) | 78 (54.5) |
| Disagreement | 2 (4.3) | 2 (4.5) | 1 (1.9) | 5 (3.5) |
Section A: clinical situation
Descriptive statistics of section A are shown in Table 58. Note that the maximum number of possible decisions represents the number of decisions if all unique decisions are assessed at least once were assessed by all reviewers. For example, at hospital A, 46 unique decisions were identified, 31 of which were assessed at least once. This results in a maximum total of 31 × 3 = 93 possible decisions scores for hospital A. Repeating this for all hospitals leads to a maximum total of 31 + 34 + 38 = 103 unique decisions that were assessed at least once.
| Decisions | Mean (SD) | Median (IQR) | Range (0–12) | Decision scores, n (% of maximum possible) | Maximum possible decision score |
|---|---|---|---|---|---|
| All decisions | 4.2 (2.4) | 4 (3–5) | 0–12 | 234 (75.7) | 309 |
| Hospital Aa | 4.3 (2.4) | 4 (3–6) | 0–11 | 66 (71.0) | 93 |
| Hospital Bb | 3.8 (2.4) | 4 (2–5) | 0–12 | 87 (85.3) | 102 |
| Hospital Cb | 4.5 (2.5) | 4 (3–6) | 0–11 | 81 (71.1) | 104 |
| Reviewer AS | 4.0 (2.2) | 4 (3–5) | 0–9 | 82 (79.6) | 103 |
| Reviewer CB | 3.8 (2.5) | 3 (2–4) | 0–12 | 79 (76.7) | 103 |
| Reviewer JW | 4.8 (2.3) | 4 (4–6) | 0–12 | 57 (79.2) | 72 |
| Reviewer SR | 4.6 (3.2) | 4 (2.75–6.5) | 0–11 | 16 (51.6) | 31 |
Here it can been seen that the median score across each hospital remained constant, but the IQR varied considerably. All hospitals and reviewers used the lowest possible score (0). Of the hospitals, only hospital B was given the maximum possible score (12); with both hospital A and hospital B receiving a maximum of 11. Among the reviewers, Chris Bassford and JW both gave the possible maximum, but the highest score given by Sophie Rees was 11 and by Anne Slowther was 9.
Table 59 shows the inter-rater reliability of the individual items of section A. Question 2 (‘Interpretation of clinical situation’) had the lowest reliability. Unsurprisingly, as this item required interpretation of the clinical situation, there was a marked difference between the clinical and non-clinical reviewers when reliability was no better than random chance. For items 5 and 6, reliability was generally higher than for the other items. This may be because no specialist knowledge is required to interpret patients’ wishes.
| Item (N = 103 possible decisions) | Comparison, ICC (95% CI); n decisions analysed | |||
|---|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | All reviewers | |
| 1: evidence of system failure | 0.63 (0.46 to 0.76); 63 | 0.23 (0.01 to 0.45); 63 | 0.33 (0.09 to 0.53); 60 | 0.47 (0.31 to 0.63); 55 |
| 2: interpretation of clinical situation | 0.36 (0.13 to 0.56); 63 | –0.08 (–0.32 to –0.17); 63 | 0.12 (–0.10 to –0.34); 60 | 0.15 (0.01 to 0.33); 55 |
| 3: description of capacity to recover | 0.34 (0.10 to 0.54); 63 | 0.41 (0.18 to 0.60); 63 | 0.55 (0.35 to 0.71); 60 | 0.44 (0.28 to 0.60); 55 |
| 4: interpretation of capacity to recover | 0.35 (0.11 to 0.55); 63 | 0.42 (0.20 to 0.60); 63 | 0.64 (0.47 to 0.77); 60 | 0.50 (0.34 to 0.65); 54 |
| 5: attempts to obtain patient wishes | 0.74 (0.60 to 0.83); 63 | 0.56 (0.37 to 0.71); 63 | 0.70 (0.54 to 0.81); 60 | 0.69 (0.57 to 0.79); 55 |
| 6: sources of patient wishes | 0.62 (0.44 to 0.75); 63 | 0.58 (0.39 to 0.72); 63 | 0.52 (0.31 to 0.69); 60 | 0.54 (0.39 to 0.68); 54 |
Scores: section B
Descriptive statistics of section B are shown in Table 60. Here, the scores are reported as out of 14 (i.e. the multiplication factor has been applied). All hospitals and reviewers used the lowest (0) and the same maximum (12 out of a possible 14) scores; however, the majority of reviewers gave a range of scores greater than zero, apart from Chris Bassford (who gave only 14 non-zero valid scores).
| Decisions | Mean (SD) | Median (IQR) | Range (0–14) | Decision scores, n (% of maximum possible) | Maximum possible decision scores |
|---|---|---|---|---|---|
| All decisions | 2.2 (3.7) | 0 (0–4) | 0–12 | 234 (75.7) | 309 |
| Hospital Aa | 1.9 (3.7) | 0 (0–0) | 0–12 | 66 (71.0) | 93 |
| Hospital Bb | 2.1 (3.2) | 0 (0–4) | 0–12 | 87 (85.3) | 102 |
| Hospital Cb | 2.7 (4.2) | 0 (0–4) | 0–12 | 81 (71.1) | 104 |
| Reviewer AS | 2.8 (3.8) | 0 (0–6) | 0–12 | 82 (79.6) | 103 |
| Reviewer CB | 1.4 (3.4) | 0 (0–0) | 0–12 | 79 (76.7) | 103 |
| Reviewer JW | 2.5 (3.7) | 0 (0–4) | 0–12 | 57 (79.2) | 72 |
| Reviewer SR | 2.2 (4.3) | 0 (0–1) | 0–12 | 16 (51.6) | 31 |
Table 61 shows the inter-rater reliability of each question in section B. For question 10 (‘balancing patient wishes’), reviewers Chris Bassford and JW differed only by the presence or absence of a decision. If both reviewers gave a score, these scores were identical. However, for this item, agreement with Anne Slowther was no better than random chance and so resulted in a ‘fair’ reliability overall. Otherwise, item 9 (‘was capacity to recover included in the balancing?’) had the highest reliability between the reviewers and item 8 (‘was acute physiology/system failure included in the balancing?’) had the lowest.
| Item (N = 103 eligible decisions) | Comparison, ICC (95% CI); n eligible decisions | |||
|---|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | All reviewers | |
| 7: balancing: benefits and burdens | 0.48 (0.27 to 0.65); 63 | 0.65 (0.49 to 0.77); 63 | 0.68 (0.51 to 0.79); 60 | 0.60 (0.46 to 0.73); 55 |
| 8: balancing: physiology | 0.20 (–0.03 to 0.41); 63 | 0.39 (0.17 to 0.58); 63 | 0.54 (0.31 to 0.70); 60 | 0.39 (0.23 to 0.55); 55 |
| 9: balancing: capacity to recover | 0.72 (0.57 to 0.82); 63 | 0.64 (0.46 to 0.76); 63 | 0.60 (0.41 to 0.74); 60 | 0.62 (0.48 to 0.74); 55 |
| 10: balancing: patient wishes | –0.03 (–0.28 to –0.22); 63 | –0.03 (–0.28 to –0.22); 63 | 1 (1 to 1); 60 | 0.24 (0.07 to 0.42); 55 |
| 11: link of balancing with treatment | 0.39 (0.16 to 0.57); 63 | 0.40 (0.18 to 0.59); 63 | 0.53 (0.32 to 0.69); 60 | 0.48 (0.32 to 0.63); 55 |
Scores: section C
Descriptive statistics of section C are shown in Table 62. Reviewer JW gave lower marks than the other reviewers, giving a score of 0 just under half of the time (n = 28, 49% of decisions assessed) and a maximum score of 4 (one at each hospital reviewed).
| Decisions | Mean (SD) | Median (IQR) | Range (0–10) | Decision scores, n (% of maximum possible) | Maximum possible decision scores |
|---|---|---|---|---|---|
| All decisions | 2.2 (1.9) | 2 (0–3) | 0–8 | 234 (75.7) | 309 |
| Hospital Aa | 2.5 (2.1) | 2.5 (1–8) | 0–8 | 66 (71.0) | 93 |
| Hospital Bb | 2.4 (1.9) | 2 (1–3) | 0–7 | 87 (85.3) | 102 |
| Hospital Cb | 2.0 (1.6) | 2 (0–3) | 0–6 | 81 (71.1) | 104 |
| Reviewer AS | 3.0 (1.7) | 3 (2–4) | 0–7 | 82 (79.6) | 103 |
| Reviewer CB | 2.0 (2.1) | 2 (0–3) | 0–8 | 79 (76.7) | 103 |
| Reviewer JW | 1.6 (1.4) | 2 (0–2) | 0–6 | 57 (79.2) | 72 |
| Reviewer SR | 2.5 (2.1) | 3 (0–4) | 0–7 | 16 (51.6) | 31 |
Table 63 illustrates the reliability for the individual items in section C. Reliability for questions 12, 15 and 16 was mostly moderate; however, Anne Slowther and Chris Bassford had only fair reliability for question 15 (‘communication with patient’). For question 14 (‘communication with family’), reliability was fair at best. In particular, agreement between Anne Slowther and the other reviewers was no better than random chance. For question 13 (‘communication to nursing staff’), no reviewer gave a score of 1 for any decision. Although this technically results in perfect agreement, the lack of other scores present means that the ICC cannot be calculated and that the reliability of the score is difficult to judge. Hence, the kappa statistic comparing the presence with the non-present has been reported; where it can been seen, the agreement was only slight. Anne Slowther and Chris Bassford agreed on 68 decisions (66% of decisions), Anne Slowther and Sophie Rees/JW agreed on 74 decisions (72%) and Chris Bassford and Sophie Rees/JW agreed on 71 decisions (69%).
| Item (n = 104) | Comparison, ICC (95% CI); n eligible decisions | |||
|---|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | All reviewers | |
| 12: communication to medical staff | 0.55 (0.35 to 0.70); 63 | 0.62 (0.44 to 0.75); 63 | 0.55 (0.35 to 0.71); 60 | 0.61 (0.46 to 0.73); 55 |
| 13: communication to nursing staff | –0.01a (–0.20 to 0.2) | 0.25a (0.05 to 0.45) | 0.20a (–0.01 to 0.40) | – |
| 14: communication to family | –0.15 (–0.35 to –0.07); 63 | 0.01 (–0.13 to –0.19); 63 | 0.31 (0.07 to 0.52); 60 | –0.02; (–0.11 to –0.12); 55 |
| 15: communication to patient | 0.28 (0.05 to 0.49); 63 | 0.75 (0.62 to 0.84); 63 | 0.37 (0.13 to 0.56); 60 | 0.54 (0.38 to 0.68); 55 |
| 16: review arrangements | 0.59 (0.40 to 0.73); 63 | 0.61 (0.43 to 0.74); 63 | 0.54 (0.33 to 0.69); 60 | 0.56 (0.41 to 0.69); 55 |
Scores: section D
As shown in Table 64, only eight decisions were given any ‘red flags’ in section D. The vast majority of decisions were given no warning ‘red flags’, and only one decision scored multiple flags (two flags, at hospital A by reviewer Anne Slowther; this decision was also flagged once by Sophie Rees).
| Decisions | Not flagged, n (% of maximum possible) | Red flagged, n (% of maximum possible) | Assessed decisions, n (% of maximum possible) | Maximum possible decisions |
|---|---|---|---|---|
| All decisions | 226 (73.1) | 8 (2.6) | 234 (75.7) | 309 |
| Hospital Aa | 60 (90.9) | 6 (6.5) | 66 (71.0) | 93 |
| Hospital Bb | 86 (98.9) | 1 (1.0) | 87 (85.3) | 102 |
| Hospital Cb | 80 (98.8) | 1 (0.9) | 81 (71.1) | 104 |
| Reviewer AS | 78 (75.7) | 4 (3.9) | 82 (79.6) | 103 |
| Reviewer CB | 79 (76.7) | 0 | 79 (76.7) | 103 |
| Reviewer JW | 56 (77.8) | 1 (1.4) | 57 (79.2) | 72 |
| Reviewer SR | 13 (41.9) | 3 (9.7) | 16 (51.6) | 31 |
Of the flagged decisions, six were flagged due to functional status, one was flagged due to previous knowledge of the patient and two were flagged due to the presence of a DNACPR order. The decision which was twice flagged was due to functional status and the presence of a DNACPR order. No decisions were flagged due to age or quality of life of the patient.
With so few ‘flagged’ decisions, calculation of the ICC is not possible. However, reliability between the three possible categories in section D is possible using kappa: if a decision was ‘red flagged’, ‘not flagged’ or ‘missed’. This is shown in Table 64 and pictorially in Figure 40. Here, reliability was generally low between all reviewers at all hospitals. Many estimates are negative, implying that agreement is no better than random chance.
Total score
Table 65 shows the descriptive statistics for the total score (sum of A + 2 * B + C). The maximum possible score is 36; however, the maximum score awarded to a decision was 30. Nine decisions (2.1% of all decisions) were given no marks at all, and seven (1.6%) were awarded only 1 mark.
| Decisions | Mean (SD) | Median (IQR) | Range (0–36) | Decision scores, n (% of maximum possible) | Maximum possible decision scores |
|---|---|---|---|---|---|
| All decisions | 7.9 (6.2) | 6 (4–11) | 0–30 | 234 (75.7) | 309 |
| Hospital Aa | 8.0 (6.5) | 6 (4–10.8) | 1–27 | 66 (71.0) | 93 |
| Hospital Bb | 7.5 (5.9) | 5 (4–11) | 0–30 | 87 (85.3) | 102 |
| Hospital Cb | 8.1 (6.5) | 6 (4–11) | 0–24 | 81 (71.1) | 104 |
| Reviewer AS | 8.4 (6.1) | 7 (4–12) | 0–23 | 82 (79.6) | 103 |
| Reviewer CB | 6.7 (6.2) | 5 (3–7) | 0–30 | 79 (76.7) | 103 |
| Reviewer JW | 8.4 (5.8) | 6 (4–12) | 2–25 | 57 (79.2) | 72 |
| Reviewer SR | 9.1 (8.1) | 5 (3.8–11.3) | 1–25 | 16 (51.6) | 31 |
No clear patterns of responses were evident from description. Both non-clinical reviewers (Sophie Rees and JW) did not award the minimum possible score and Chris Bassford gave the greatest range of marks.
Table 66 shows the inter-rater reliability of the total scores. Although the overall reliability across all decisions was moderate to substantial, it varied considerably between hospitals. Again, reliability at hospital C was generally worse than at the other hospitals, but hospital B also had one comparison that was no better than random chance. Reviewer Chris Bassford appears to have lower reliability than the other reviewers; however, at hospital B, the reliability of Chris Bassford against Anne Slowther and Sophie Rees/JW is higher than that for Anne Slowther against the non-clinical reviewers.
| Hospital (N) | Comparison, ICC (95% CI); n eligible decisions | |||
|---|---|---|---|---|
| AS vs. CB (clinical vs. clinical) | AS vs. SR/JW (clinical vs. non-clinical) | CB vs. SR/JW (clinical vs. non-clinical) | All reviewers | |
| All (103) | 0.66 (0.47 to 0.79); 63 | 0.65 (0.49 to 0.78); 63 | 0.76 (0.60 to 0.85); 60 | 0.69 (0.56 to 0.80); 55 |
| Aa (31) | 0.41 (–0.06 to 0.72); 19 | 0.73 (0.35 to 0.90); 15 | 0.50 (–0.04 to 0.81); 14 | 0.56 (0.23 to 0.82); 13 |
| Bb (34) | 0.41 (–0.00 to 0.70); 24 | 0.13 (–0.14 to 0.43); 26 | 0.44 (0.07 to 0.70); 27 | 0.33 (0.09 to 0.59); 24 |
| Cb (39) | 0.34 (–0.05 to 0.66); 20 | 0.30 (–0.09 to 0.63); 22 | 0.32 (–0.11 to 0.66); 19 | 0.34 (0.07 to 0.63); 18 |
Total score and outcome
Table 67 and Figure 41 illustrate the relationship between the outcome of the decision (admitted to ICU or outcome unknown) and the total score. Care must be given when interpreting these results, as the study was not formally powered to detect differences between scores for different categories; and at hospital A one reviewer did not record any admission data. However, the trend indicates that scores when the patient was not admitted to ICU were generally higher than when the patient was admitted.
| Decision resulted in admission to ICU | 7 (n = 66) | 8 (n = 87) | 9 (n = 81) | All hospitals (N = 234) |
|---|---|---|---|---|
| Unclear/not given | 9.4 (7.6) | 4.0 (–) | 11.0 (8.8) | 9.5 (7.6) |
| No | 11.3 (6.8) | 8.8 (4.7) | 11.0 (7.1) | 10.0 (6.1) |
| Yes | 5.8 (4.0) | 7.7 (7.4) | 6.0 (4.6) | 6.6 (5.8) |
FIGURE 41.
Venn diagram of decisions red flagged at least once.
Total score and setting
Table 68 and Figure 42 illustrate the relationship between the setting of the decision and the total score. As for the admission data, care must be given to interpreting these results. However, in this case, some clear trends are visible between the settings of the decisions.
| Setting (n decisions, %) | 7 (n = 66) | 8 (n = 87) | 9 (n = 81) | All hospitals (N = 143) |
|---|---|---|---|---|
| Unclear/not given | 9.1 (7.1) | 6.8 (4.1) | 6.0 (–) | 8.7 (6.7) |
| A&E/ED | 11.5 (7.7) | 7.7 (5.5) | 4.8 (3.0) | 7.2 (5.4) |
| Cardiac arrest | 6.0 (–) | 4.0 (–) | 14.0 (9.9) | 9.5 (7.8) |
| Theatre | 5.8 (5.8) | 9.5 (6.4) | NA | 6.8 (5.7) |
| Ward | 8.4 (6.2) | 9.2 (6.7) | 10.0 (7.0) | 9.4 (6.7) |
FIGURE 42.
Box plot of total score and outcome of decision by reviewer.
FIGURE 43.
Box plot of total score and location of decision by reviewer.
List of abbreviations
- A&E
- accident and emergency
- AFR
- accountability for reasonableness
- APACHE II
- Acute Physiology and Chronic Health Evaluation
- ASSIA
- Applied Social Science Index and Abstracts
- CCOR
- critical care outreach
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COPD
- chronic obstructive pulmonary disease
- DIT
- Defining Issues Test
- DNACPR
- do not attempt cardiopulmonary resuscitation
- DSF
- decision-support framework
- DSI
- decision-support intervention
- ECQAT
- Ethics Consultation Quality Assessment Tool
- ED
- emergency department
- HDU
- high dependency unit
- ICC
- intraclass correlation coefficient
- ICU
- intensive care unit
- IQR
- interquartile range
- ITU
- intensive treatment unit
- LCL
- latent class logit
- MeSH
- medical subject heading
- MET
- medical emergency team
- MNL
- multinomial logit
- NEWS
- National Early Warning Score
- NPT
- normalisation process theory
- OR
- odds ratio
- OSCE
- objective structured clinical examination
- PPI
- patient and public involvement
- PPIAG
- patient and public involvement advisory group
- ReSPECT
- Recommended Summary Plan for Emergency Care and Treatment
- RI
- relative importance
- SBAR
- Situation, Background, Assessment and Recommendation
- WP
- work package
Notes
-
Consent flow chart 1
-
Consent flow chart 2
-
Family interview schedule
-
Patient interview schedule
-
Clinician interview schedule
-
Observation template
-
Choice experiment 1 consultant questionnaire
-
Choice experiment 2 consultant questionnaire
-
Choice experiment 1 CCOR questionnaire
-
Choice experiment 2 CCOR questionnaire
-
List of participating hospitals
-
Search for optimal number of latent classes
-
Topic guides for stakeholder conference focus groups
-
Educational material for Implementation Champions
-
Normalisation process theory toolkit questions
-
Implementation champion telephone interview topic guide
-
Training session observation guide
-
Clinician interview topic guide
-
Glossary of terms
-
Evaluation tool user FAQs
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/hsdr/131014/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
