Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/80/19. The contractual start date was in August 2017. The draft report began editorial review in May 2021 and was accepted for publication in May 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Heuvelman et al. This work was produced by Heuvelman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Heuvelman et al.
Chapter 1 Introduction
Depression is a common mental health condition and a leading cause of disability worldwide. 1 Prescribing of antidepressant medications for depression and other common mental health problems has markedly increased in recent decades. For example, within the National Health Service (NHS), the number of prescriptions of antidepressants almost doubled in one decade, from 36 million prescriptions in 2008 to 70.9 million prescriptions in 2018. 2 This increase in the number of prescriptions for antidepressants appears to be largely explained by a longer duration of prescriptions,3 which reflects the chronic course of depression.
Depression is particularly common in women of childbearing age, and antidepressants are commonly prescribed in this patient group. 4 Women receiving antidepressants who are planning pregnancy or those who discover they are pregnant while on antidepressants are, therefore, often faced with a decision about whether to continue or discontinue their antidepressant medication during the pregnancy. Furthermore, pregnancy itself may be a trigger for the onset or worsening of depression, and up to one in seven women suffer from depression during their pregnancy. 5–7 Untreated depression may have serious consequences, such as self-neglect and suicidal behaviour, in affected women and birth complications in their babies. 5,8 Therefore, initiation of antidepressant medications may be considered in the treatment of depression in pregnant women after considering the risks and benefits. 9
A study of a representative sample of UK primary care patients reported that 8.6% of women who had deliveries between 2004 and 2010 were prescribed antidepressants in the year before their pregnancy. 10 During pregnancy, 3.7% of these women were prescribed an antidepressant, but this number sharply increased to 12.9% in the year following the pregnancy. 10 These prescribing patterns reflect the advice to minimise fetal exposure to medications, but the rise in prescribing in the year following pregnancy may also suggest that many women are re-prescribed antidepressants after they have given birth, for either ongoing or worsened depressive features or because of a new onset of postnatal depression. 11
Weighing the potential benefits and harms of antidepressant use during pregnancy is challenging. A recent network meta-analysis of randomised controlled trials (RCTs) involving 21 commonly used antidepressants concluded that all antidepressants were more efficacious than placebo in the treatment of depression in adults. 12 However, pregnancy was an exclusion criterion in such RCTs; therefore, prescribing decisions in pregnancy have been reliant on observational data. Recent systematic reviews have highlighted the poverty of studies on the benefits of antidepressants during pregnancy or harms of discontinuing them. 13
The National Institute for Health and Care Excellence guidance on antenatal and postnatal mental health (CG192)9 advised that psychotropic use during pregnancy should be informed by the careful individualised weighing of benefits and risks, but acknowledged that data on long-term developmental outcomes are still scarce. In January 2016, the US Centers for Disease Control and Prevention (CDC) also called for further research on the safety of antidepressants during pregnancy, following the most recent data from a convenience sample of 5.8 million privately insured women in the USA of reproductive age showing over 15% filled claims of antidepressants. The CDC highlighted that such work would be important to provide accurate evidence and guidance for women of childbearing age given that many pregnancies are unplanned and first trimester exposure, therefore, is unavoidable. The US Preventative Services Task Force Recommendation Statement also published in 2016 recommended screening for depression in pregnant women but highlighted the lack of data on the benefits and harms of treatment during pregnancy. A comprehensive systematic review, carried out by the US Agency for Healthcare Research and Quality, concluded that ‘Evidence about the comparative benefits and harms of pharmacologic treatment of depression in pregnant and postpartum women was largely inadequate to allow informed decisions about treatment’. 14
For example, only a few previous studies have specifically investigated the outcomes of continuing or discontinuing antidepressants during pregnancy in relation to worsening or relapse of depression. These studies include a study of 201 women in the USA, which reported over a fivefold risk of relapse of major depression in those who discontinued antidepressants. 15 Another US study of 367 women with mild to moderate depression reported that, compared with non-users, women who discontinued antidepressants in pregnancy had a sixfold risk of a relapse of depression in the second half of pregnancy. 16 However, this latter study also reported a fivefold risk of relapse of depression in women who continued antidepressants without dosage modification [odds ratio (OR) 4.59, 95% confidence interval (CI) 1.44 to 14.64], although the findings for women who continued antidepressants with dosage modification were imprecise (OR 0.58, 95% CI 0.06 to 5.52). 16 Two large studies based on analysis of secondary data (n = 778 and n = 28,493) found little evidence of a risk of relapse of depression following discontinuation of antidepressants,17,18 although these studies acknowledged the limitations of using routinely collected data for effectiveness research. To date, only one RCT has been attempted to study this topic (the ‘Stop or Go’ trial in the Netherlands). 19 ‘Stop or Go’ was a pragmatic, multicentre, randomised non-inferiority trial that aimed to recruit 200 pregnant women with a gestational age of less than 16 weeks who were receiving selective serotonin reuptake inhibitor (SSRI) antidepressants without clinically relevant depressive symptoms. 19 The intervention group received preventative cognitive therapy-guided gradual discontinuation of antidepressants and the control group continued their antidepressant. A brief report of the results of this trial has been recently published,20 which highlighted that only 44 (of 200 planned) participants were recruited. Women in both groups had similar rates of a relapse of depression,20 although the trial was clearly underpowered to detect a meaningful difference.
Alongside the potential for benefits of antidepressants to pregnant women, there has been increasing discussion about the potential effects of antidepressants on fetal development. Most antidepressants do not appear to be associated with major congenital malformations,21,22 but there is evidence of an increased risk of persistent pulmonary hypertension of the newborn with some antidepressants, a rare but serious condition. 23 Apart from immediate birth outcomes, there has also been increasing interest in potential longer-term neurodevelopmental effects of antidepressant exposure during pregnancy.
All antidepressants cross the placental barrier and are available to the developing fetus,24 and their mechanism of action commonly involves an increase of the availability of serotonin in the synaptic cleft. 25 The serotonergic system is critical for fetal neurodevelopment and emerges early in embryogenesis. 26 Animal studies have reported that exposure to antidepressants in utero can lead to long-term impairments in cognitive, social and behavioural development that is attributed to disruptions in the serotonergic system. 26–31 It is, therefore, biologically plausible that similar effects on fetal neurodevelopment may occur in humans.
However, whether or not long-term development of the exposed offspring is affected as a result of in utero exposure to antidepressant medications is difficult to assess because maternal depression may independently affect offspring neurodevelopment. It is, therefore, difficult to determine whether antidepressants or depression in pregnancy are the cause of any observed adverse outcomes. This is known as confounding by indication32 and can be an obstacle to clinical guidance and decision-making. If antidepressant use during pregnancy was the cause of any adverse offspring outcomes, pregnant women would need to be made aware of this to make informed decisions; however, if these outcomes are a result of the underlying depression, the benefits of taking them and, therefore, treating the depression would outweigh the risks.
A number of studies have now been carried out to investigate potential long-term neurodevelopmental outcomes in offspring exposed to antidepressants in pregnancy – the majority studying autism spectrum disorder (henceforth autism),33–49 but also include attention deficit hyperactivity disorder (ADHD)49–53 and intellectual disability. 54 Many of the studies on autism reported unadjusted associations between antidepressant use during pregnancy and autism. However, all of these studies reported concern about confounding by indication and one major concern was the under ascertainment of depression owing to reliance on secondary-care records. The results of studies on risk of ADHD53 and intellectual disability54 have suggested that the association of antidepressant exposure with these conditions is unlikely to be causal.
Questions about medication effectiveness and safety are best answered using well-designed RCTs. However, RCTs in the area of medication use during pregnancy have not been carried out and pregnancy is one of the common exclusion criteria for controlled trials of investigational medicinal products owing to ethics concerns. 55 Furthermore, the feasibility of carrying out RCTs in pregnancy is a major issue because assessing potential long-terms risks to exposed offspring will require randomising very large numbers of pregnant women, and successful long-term follow-up may be unlikely. Clinical guidance on this issue is, therefore, likely to continue to rely on observational data. However, it is important that efforts are made to minimise the potential for confounding in results of such studies.
In the absence of RCTs, an efficient approach to studying outcomes related to antidepressants prescribed in pregnancy is to use routinely collected observational data to emulate the hypothetical RCT that would have been carried out and use methods that may minimise confounding bias and strengthen causal inference. 56 This approach also addresses constraints in time and cost given that routinely collected health-care data allow us to study large representative patient populations over long periods of time.
The aim of this research, funded by the Efficient study designs committee of the NIHR Health Technology Assessment Programme, was to address some of the gaps in the literature described above.
This research aimed to simulate two scenarios that could be tested among pregnant women with depression in a hypothetical target RCT asking the following research questions:
-
Does initiation of antidepressants for depression during pregnancy affect maternal service use outcomes and childhood neurodevelopmental outcomes?
-
Does continuation of antidepressant use during pregnancy for depression affect maternal service use outcomes and childhood neurodevelopmental outcomes?
To assess the robustness of the results, multiple methods for confounding control were used, including multivariable regression methods, propensity score matching to account for measured confounding factors, instrumental variable (IV) analysis using prescriber preference as an instrument to account for unmeasured confounding, negative control exposures for child outcomes (discontinuation of antidepressant before pregnancy where no gestational exposure occurred), comparison of risks of outcomes across indications for antidepressants other than depression, and analysis of exposure discordant pregnancies to account for confounders shared between pregnancies.
Chapter 2 Overview of the methods used in this project
This chapter provides an overview of the data and the methods used in this project. Further details on individual causal approaches are also provided in later chapters.
Design: observational cohorts emulating target randomised controlled trials
This study was an observational cohort study using data from the Clinical Practice Research Datalink (CPRD),57 and used multiple methods to strengthen causal inference. In discussion with our patient advisory group (PAG), and to inform decisions faced by pregnant women and clinicians, we identified two distinct clinical trial scenarios that would need to be emulated in our observational data. First, we examined the effects of initiating an antidepressant among women with depression not already prescribed antidepressant medications before they became pregnant. Second, we examined the effects of continuing antidepressants into pregnancy among those who were already prescribed antidepressant medications before they became pregnant. The protocol components of each of these hypothetical trials and our approach to emulating these in the observational data are described in Chapters 3 and 4, respectively. By making the target trial explicit in the selection of the study cohort and approach to statistical analysis, we can evaluate how well causal analysis of the observational data set emulates the target trial and, therefore, whether or not any observed associations are likely to represent the causal effects that would have been produced by an experimental study.
Study data: the Clinical Practice Research Datalink
This study used data from the CPRD, which is a large, ongoing database of anonymised primary care medical records for patients registered with a general practice in the UK. By 2015, the CPRD included data for over 11.3 million patients from 674 general practices in the UK, of whom 4.4 million patients were alive and registered, representing approximately 7% of the UK population. 57 Patients included in the CPRD are broadly representative of the UK population in terms of age, sex and ethnicity. 57
Identification of pregnancies and linkage of women to offspring
A validated set of algorithms that identify pregnancies within the CPRD is now integrated within the CPRD as a pregnancy register. 58 This register enables the identification of the dates, stages and outcomes of pregnancies within the CPRD. 58 The CPRD has also developed a probabilistic mother–baby link, which allows the patient identifier numbers of mothers and the patient identifier numbers of their live-born offspring to be linked, enabling the construction of an intergenerational cohort. 59
Linkage of Clinical Practice Research Datalink data with other resources
For consenting CPRD practices in England, which represent approximately 60% of patients in our data, it was possible to link the anonymised primary care records with other data sources. These sources include the Hospital Episode Statistics (HES), which has separate registers for inpatient admissions, outpatient care and accident and emergency (A&E) attendance in England. Linkage with the Office for National Statistics (ONS) mortality data and the Census small-area socioeconomic data was also available for this same subset.
The use of CPRD data for this project was approved by the CPRD’s Independent Scientific Advisory Committee (reference 17_225).
Study cohort selection
The CPRD data extract for this project covered dates between 1 January 1995 and 31 December 2017. Within this time frame, there were 344,720 pregnancies in the pregnancy register for which there was evidence of depressive symptoms or prescription of an antidepressant up to 1 year before or during pregnancy (see Report Supplementary Materials 1 and 2 for Read codes and product code lists). This was the eligible sample for our cohort construction, as described below.
We constructed two cohorts: (1) the pregnant women’s cohort contained all pregnancies for which women could be followed up for at least 2 years beyond their pregnancy end date, regardless of the pregnancy outcome or availability of linkage with the child in the CPRD mother–baby link; and (2) the mother and child cohort contained pregnancies that could be linked with the offspring patient records. A detailed description of the construction of each cohort is provided in the following sections.
Pregnant women’s cohort
Figure 1 shows the derivation of the pregnant women’s cohort used for the main analysis.
FIGURE 1.
Derivation of the pregnant women’s cohort.
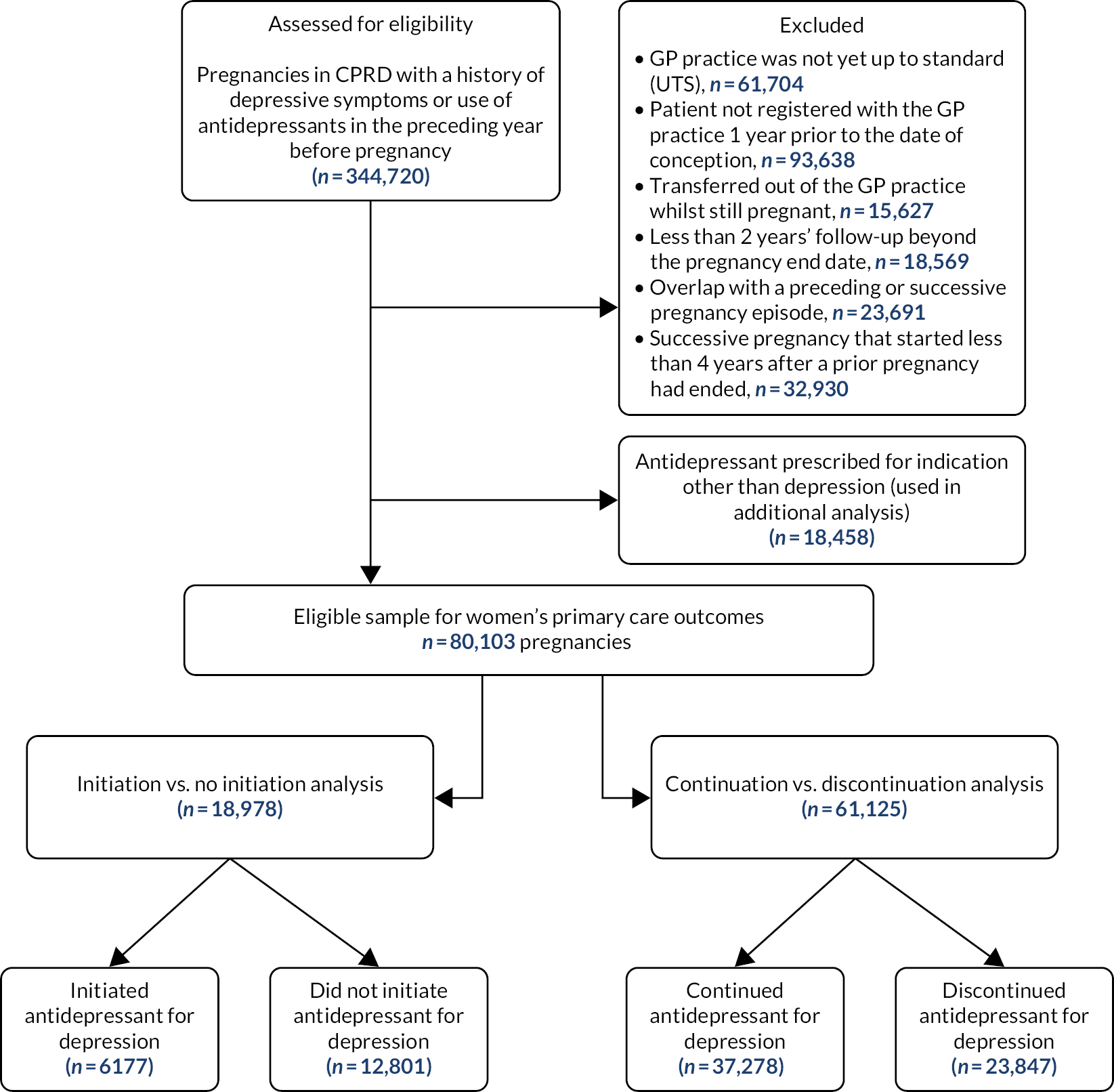
From the eligible sample of 344,720 pregnancies, we excluded:
-
records where the general practice was not yet up to standard (UTS) (n = 61,704). CPRD defines practices as being ‘up to standard’ if they have provided data on a consistent basis
-
records where the patient had not yet registered with her current general practice 1 year prior to the date of conception, as recorded in the pregnancy register (n = 93,638)
-
records suggesting that the woman had transferred out of the general practice while pregnant (n = 15,627)
-
records with less than 2 years’ follow-up beyond the pregnancy end date (n = 18,569)
-
records that showed overlap with a preceding or successive pregnancy episode (n = 23,691)
-
records of any successive pregnancy episode that started less than 4 years after a prior episode had ended to minimise biased results arising owing to the possibility of women being pregnant again or trying to conceive during follow-up (n = 32,930).
We set aside pregnancies for which antidepressants had been prescribed for indications other than depression (n = 18,458; these were used in additional analyses described in Variation by indication: depression compared with other indication for antidepressant prescribing); therefore, 80,103 pregnancies were included to study women’s primary care service use outcomes. Of these pregnancies, 45,358 were eligible for record linkage to study secondary care service outcomes. Among these, data on inpatient admission were available for pregnancies that had started on or after 1 April 1997 (n = 43,662); outpatient treatment data were available for pregnancies starting on or after 1 April 2003 (n = 35,674); and A&E attendance data were available for pregnancies starting on or after 1 April 2007 (n = 25,697).
Mother and child cohort
Figure 2 shows the derivation of the mother and child cohort.
FIGURE 2.
Derivation of the mother and child cohort.
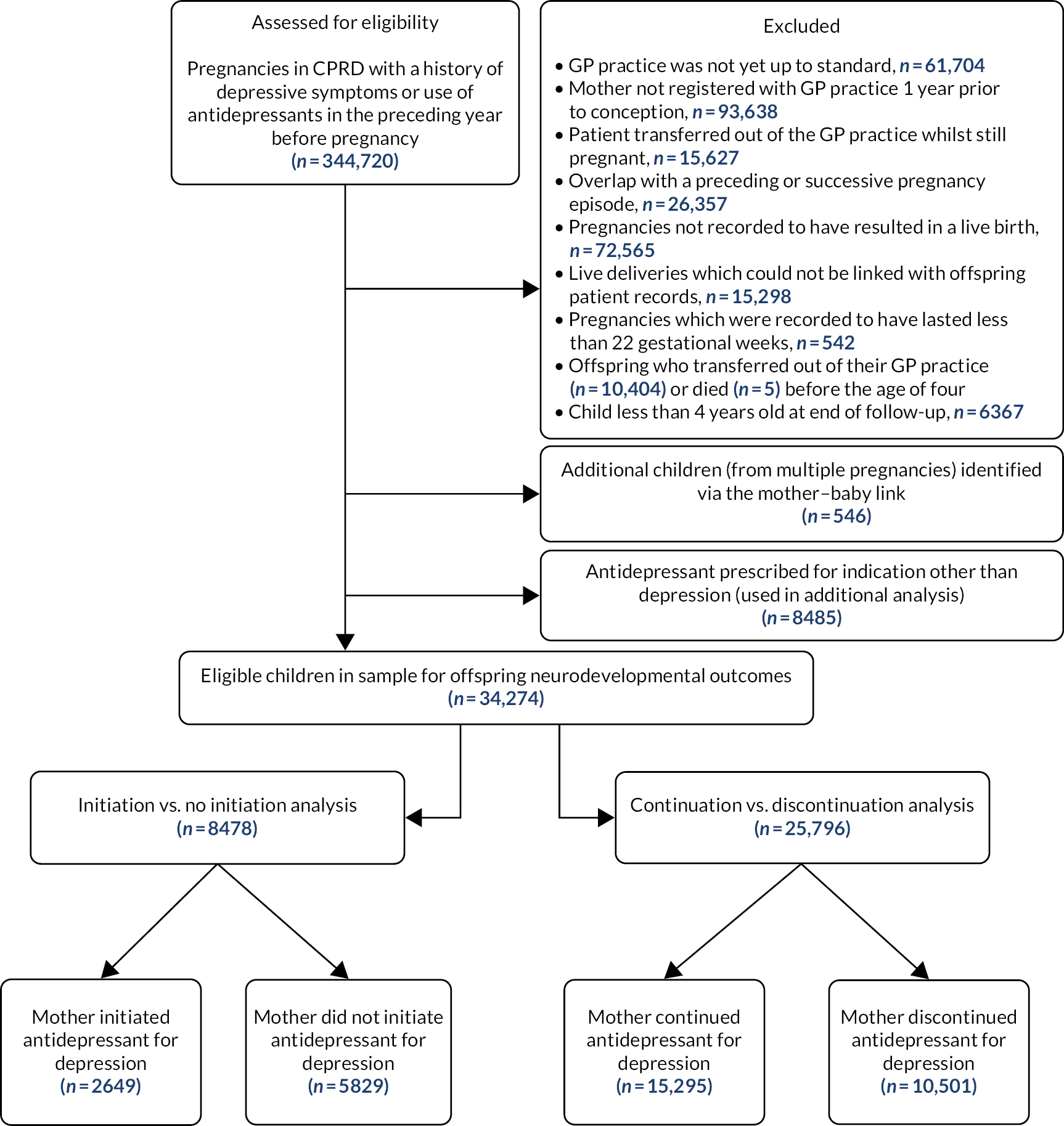
From the eligible sample of 344,720 pregnancies, we excluded:
-
records where the general practice was not yet UTS, as defined by CPRD (n = 61,704)
-
records where the patient had not yet registered with her current general practice 1 year prior to conception (n = 93,638)
-
records where the patient had transferred out of the general practice while still pregnant (n = 15,627)
-
records that showed overlap with a preceding or successive pregnancy (n = 26,357)
-
pregnancies not recorded to have resulted in a live birth (n = 72,565)
-
live deliveries that could not be linked with offspring patient records (n = 15,298)
-
pregnancies that were recorded to have lasted less than 22 gestational weeks (n = 542)
-
any offspring who transferred out of their general practice (n = 10,404) or died (n = 5) before the age of 4 years.
Given that the CPRD pregnancy register includes only the first child in cases of multiple deliveries, we identified an additional 546 children with at least 4 years’ follow-up in the mother–baby link data set, by matching the mother’s patient identification number and exact date of delivery. Setting aside mothers who were likely to have been prescribed antidepressants for indications other than depression (n = 8485) and children followed up for less than 4 years owing to being born after 2013 (n = 6367), we were able to include 34,274 children in the offspring cohort (mean age at end of follow-up 10.04 years, range 4–22 years).
Definition of treatment groups
As noted above, the treatment groups were based on two clinical scenarios that may be encountered by pregnant women with depression:
-
Women who have depression during pregnancy but were not receiving prior treatment may either be initiated with a prescription of antidepressants or not be prescribed antidepressant treatment.
-
Women currently prescribed antidepressants for the treatment of depressive symptoms may choose to continue taking these medications in pregnancy or to discontinue them before pregnancy.
To identify each pregnancy as belonging to one of four treatment groups, we extracted information on prescription start dates, daily recommended dose and number of doses prescribed from women’s medical records to identify periods of continuous prescribing before or during pregnancy (see Report Supplementary Material 3). Using these prescribing periods, we identified (1) women who initiated an antidepressant in pregnancy; (2) women who did not initiate an antidepressant in pregnancy; (3) women who continued an existing prescription into pregnancy; and (4) women who discontinued an antidepressant prescription prior to conceiving. We chose a 2-month grace period preceding the date of conception to take account of the longer pharmacological half-life of some antidepressants, which could still be active in pregnancy if taken shortly before conceiving. Therefore, women who discontinued or initiated antidepressants were required not to have been prescribed during this 2-month grace period. The rules used to define the treatment groups are detailed in Figure 3.
FIGURE 3.
Allocation of treatment groups.
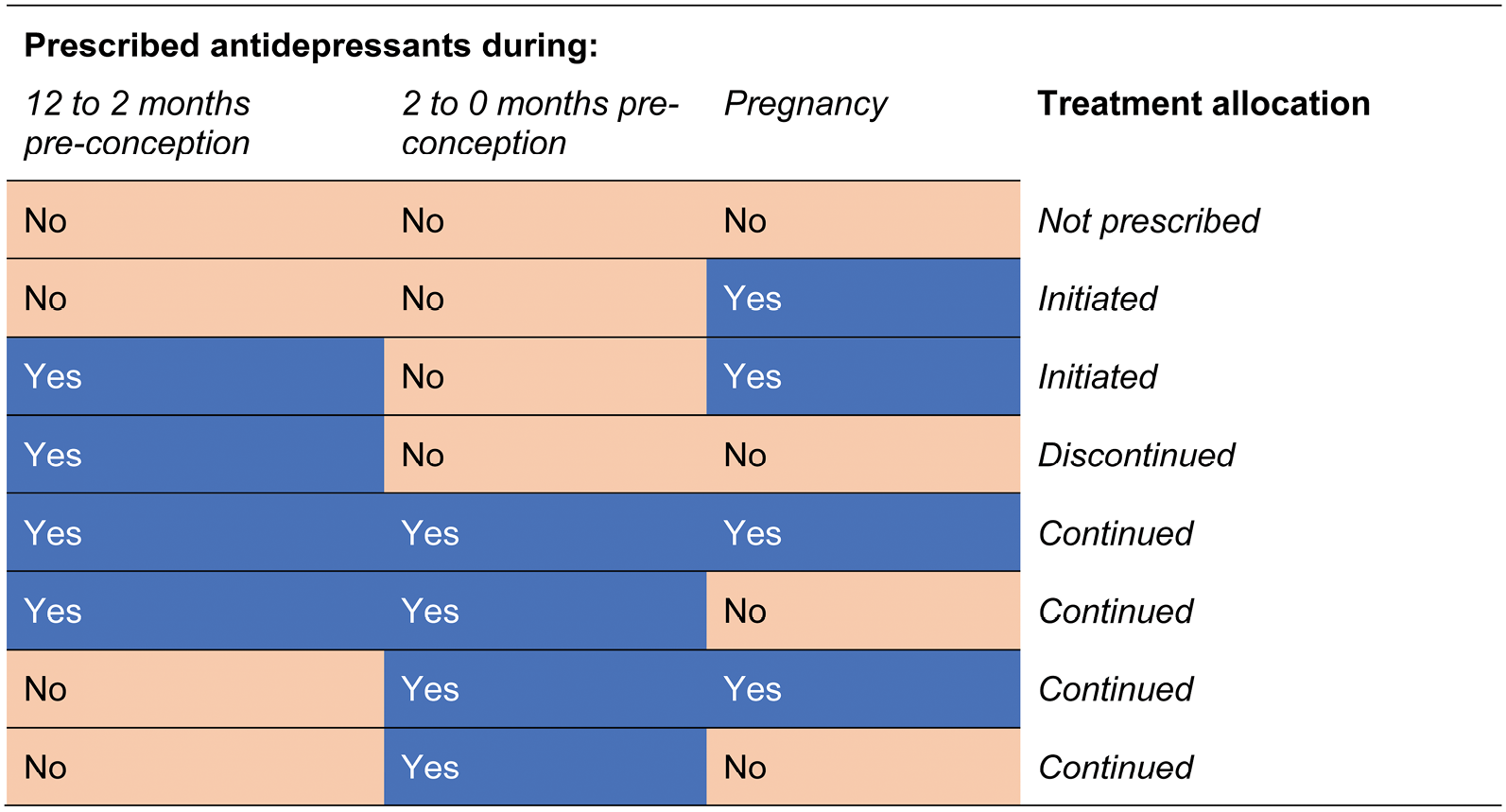
Women’s service use outcomes
We examined women’s use of health-care services during pregnancy and during each of the four consecutive 6-month follow-up periods after the pregnancy end date: 1–6 months, 7–12 months, 13–18 months and 19–24 months. The start of follow-up was defined as the day of estimated conception, as recorded in the CPRD pregnancy register, for women who received no treatment or discontinued or continued an existing prescription, and as the date of first prescription for women who initiated an antidepressant during pregnancy. Any resulting differential length of follow-up between treatment groups was adjusted for in our statistical models.
General practitioner consultations
During pregnancy and in each of the four consecutive 6-month follow-up periods, we counted the number of days on which women had consulted with their general practice. General practitioner (GP) consultations were required to have been face to face or by telephone and the staff member was required to be a doctor, nurse (including community psychiatric nurse) or psychologist. Report Supplementary Material 4 contains the operational definitions used in deriving this outcome. Following the same definitions, we counted the number of days on which women consulted with their general practice specifically for further episodes of depression or self-harm (including suicide attempts) during pregnancy and follow-up periods. Symptoms of depression and self-harm were identified in the consultation records using validated Read code lists (see Report Supplementary Materials 1 and 5) where the consultation type equalled ‘symptom’, ‘examination’, ‘diagnosis’, ‘administration’ or ‘presenting complaint’.
Referrals made by the general practitioner
We constructed a set of binary variables to indicate whether women had been referred by their GP to secondary services for depression or self-harm/suicide attempts during pregnancy or in each of the four consecutive 6-month follow-up periods. Referrals were defined as the presence of a referral record with a medical code for depression or self-harm/suicide attempt, where the NHS referral specialty classification equalled ‘mental handicap’, ‘mental illness’, ‘child and adolescent psychiatry’, ‘forensic psychiatry’, ‘psychotherapy’, ‘old age psychiatry’, ‘clinical psychology’, ‘learning disabilities’, ‘adult psychiatry’ or ‘community psychiatric nurse’, or where the Family Health Services Authority referral classification equalled ‘psychiatry’.
Inpatient admissions
Using linked HES data, we constructed a set of binary variables to indicate whether women had been admitted as an inpatient for a mental health problem [International Classification of Diseases, 10th Edition (ICD-10): F00–F99] or for intentional self-harm/suicide attempt or self-harm of undetermined intent (ICD-10: X60–X84 and Y10–Y34) during pregnancy or in each of four consecutive 6-month follow-up periods. These inpatient admissions were defined by a record where the main specialisation of the consultant equalled ‘accidents and emergency’, ‘learning disability’, ‘adult mental illness’, ‘forensic psychiatry’ or ‘psychotherapy’; where their treatment specialisation equalled ‘accidents and emergency’, ‘clinical psychology’, ‘learning disability’, ‘forensic psychiatry’, ‘psychotherapy’, ‘eating disorders’, ‘liaison psychiatry’, ‘perinatal psychiatry’, ‘mental health recovery and rehabilitation service’ or ‘mental health dual diagnosis service’; and where the method of admission equalled ‘A&E or dental casualty department’, ‘request for immediate admission by GP’, ‘consultant clinic’, ‘admission via mental health crisis resolution team’ or ‘other means’.
Outpatient treatment
Using linked HES data, we constructed a set of binary variables to indicate whether women had used outpatient services for a mental health problem during pregnancy or in each of four consecutive 6-month follow-up periods. Outpatient contacts were defined by outpatient records where the treatment specialty of the consultant equalled ‘adult mental illness’, ‘child and adolescent psychiatry’, ‘forensic psychiatry’ or ‘psychotherapy’.
Accident and emergency department attendance
Using linked HES data, we counted the number of instances women had presented to A&E services during pregnancy or in each of four consecutive 6-month follow-up periods. We considered only first A&E attendances (excluding any planned or unplanned follow-up for a prior attendance) and excluded attendances for assault, sports injuries or firework injuries, or where the patient had died on arrival to the A&E unit.
All-cause and cause-specific mortality
We used linked ONS mortality data to identify women who had died at any time after the end of the study pregnancy and specifically within the study window, that is within the 2-year period immediately following the pregnancy end date. In the pregnant women’s cohort, only 14 women had died within the 2-year period immediately following the pregnancy end date. We, therefore, did not carry out further analysis on mortality as an outcome.
Prescription of an antidepressant at 2-year follow-up
We determined whether mothers were still or again being prescribed antidepressants at the end of follow-up, that is 2 years after the pregnancy end date. Based on the assumption that antidepressants are generally prescribed where there is greater clinical need, we proxied the mother’s recovery from depression by not receiving antidepressants at the end of follow-up. We, therefore, examined all periods of continuous prescribing around this time and considered mothers to have recovered if (1) the end of follow-up did not coincide with a start or estimated end date of a prescription period; and (2) the end of follow-up did not fall within a period of continuous prescribing.
Offspring neurodevelopmental outcomes
Autism spectrum disorder
We examined the primary care clinical and referral records of linked offspring for the presence of autism spectrum disorder (referred to hereafter as autism) using a validated Read code list (see Report Supplementary Material 6). Offspring were considered positive on outcome if they had a primary care record that indicated autism, autism spectrum disorder, autistic disorder, Asperger syndrome, atypical autism, childhood autism, infantile autism, autistic psychopathy or pervasive developmental disorder, and if they had a record of autism when they were at least 4 years of age. A recent study60 validated the CPRD diagnosis, as recorded in the CPRD, against the clinical records for a subsample and reported a positive predictive value of 91.4%. Given that the HES data were available for only a subsample, and registered diagnoses are recorded in less than 5% of all outpatient attendances (during which most autism-related consultations would happen), we did not use linked data to supplement the autism diagnoses. 61
Intellectual disability
Following the same approach, we examined the primary care records of linked offspring for presence of intellectual disability that had been diagnosed when the child was at least 4 years of age. Children were considered positive on outcome if their primary care records indicated Read codes related to intellectual disability (see Report Supplementary Material 7 for the list of Read codes). These codes were similar to those used by previous studies of intellectual disability within CPRD,62,63 although we did not include codes for autism (i.e. someone with a code of autism would be counted as having an intellectual disability only if there were additional codes related to intellectual disability in their medical record). Similar to autism, linked data were not used to supplement these diagnoses because these data were available for only a subset and the HES outpatient registers had less than 5% recording of diagnostic data. 61
Attention deficit hyperactivity disorder
Primary care diagnoses of ADHD were identified by the presence of medical codes pertaining to ADHD or therapy records that indicated that the child had been prescribed ADHD medication when they were at least 4 years of age. Children were considered positive on outcome if they had a recorded Read code related to ADHD (see Report Supplementary Material 8) or if they had been prescribed any of the following ADHD medications: methylphenidate, dexamphetamine, atomoxetine, dextroamphetamine, amphetamine with dexamphetamine, or lisdexamphetamine (see Report Supplementary Material 9). Similar methods have been used to identify ADHD in previous CPRD studies. 64 As above, linked data were not used to supplement these diagnoses because these data were available for only a subset and the HES outpatient registers had incomplete recording of diagnostic data. 61
Covariates
To account for potential confounders of the treatment–outcome association, we included additional covariates in our statistical models or used them in matching procedures. Covariates extracted from primary care records were:
-
maternal age – defined as the age in years recorded on the pregnancy register
-
the number of days on which the woman consulted with her GP in the year prior to conception – a proxy for illness severity and health-care seeking behaviour
-
Charlson Comorbidity Index score – a continuous measure for presence of comorbid physical health conditions65 from a previously published code list66
-
psychiatric history of any of the following by the start of pregnancy – psychosis, anxiety, self-harm, bipolar affective disorder, eating disorders, personality disorders, sleep disorders and neuropathic pain (see Report Supplementary Materials 10–17 for Read code lists)
-
prescription of medications for physical health problems (any medications listed within BNF sections 1.1–1.9, 2.1–2.13, 3.1–3.11, 5.1–5.5, 6.1–6.7, 7.2–7.4, 8.1–8.3, 10.1–10.3, 13.5.3, 13.6.2 and 13.6.3)67
-
prescription of central nervous system agents (any medication listed within BNF sections 4.1, 4.2 and 4.4–4.10)
-
prescription of nutritional supplements in the year before or during pregnancy (defined as any supplements listed within BNF sections 9.1–9.12)
-
smoking status at the start of pregnancy – never smoked, current or ex-smoker or status unknown (details of Read codes and categorisation provided in Report Supplementary Material 18)
-
history of alcohol use by the start of pregnancy (see Report Supplementary Material 19)
-
administrative region of the general practice – The North or Yorkshire and the Humber, Midlands or East of England, the South excluding London, London, Northern Ireland, Scotland or Wales
-
calendar year – 1995–97, 1998–2000, 2001–03, 2004–06, 2007–09, 2010–12 or 2013–17
-
any recorded severity of prior depression – mild, severe or severity not recorded (see Report Supplementary Material 20; code lists were rated by two psychiatrists, DR and JE, to derive groups)
-
concurrent use of multiple antidepressants during the study period – a proxy for illness severity
-
switching from one antidepressant to another – a proxy for illness severity.
Using linked HES data, we extracted:
-
a variable indicating past inpatient admission where a mental health problem was mentioned as a primary or secondary diagnosis – a proxy for illness severity.
From linked Census data we extracted:
-
the ranked Index of Multiple Deprivation (IMD) quintile of the patient’s postcode area – a proxy for socioeconomic status.
The above two variables extracted from linked records were available for only the subsample of cases with linked data; therefore, they were included in within-multivariable regression and the generation of propensity scores in supplementary analyses only.
Methods to account for confounding
Multivariable regression
We used multivariable regression to estimate the maternal and child outcomes associated with initiating (described further in Chapter 3) or continuing (described further in Chapter 4) an antidepressant into pregnancy. For each outcome, we first estimated crude associations and then controlled statistically for the range of potential confounders described in Covariates. Further detail on the selection and specification of multivariable regression models is provided in Chapters 3 and 4.
Propensity score-matched regression
Alongside conventional multivariable regression, we carried out all analyses in subsets of the data for which we matched treatment groups on propensity scores for initiation and continuation of antidepressants during pregnancy. Propensity score matching (PSM) is a commonly used method in pharmaco-epidemiology that allows the identification of pairs of observations that are similar in all measured characteristics, except for treatment status. 68 It, therefore, aims to achieve balanced treatment groups, allowing for a like-with-like comparison that would be achieved by randomisation in RCTs. It has been argued that PSM may provide a more effective approach to minimising confounding bias than traditional multivariable regression methods because it can incorporate large numbers of potential covariates that may overwhelm traditional regression models. 69 However, the main constraint of PSM is that the groups can be matched only on characteristics that are measured, so confounding by unmeasured characteristics is still possible. 32 Furthermore, in analyses using PSM, individuals who cannot be matched for being too dissimilar are excluded from the analysis, which can affect statistical power because of reduced numbers.
In this study, we estimated propensity scores using classification and regression tree (CART) models69 separately for our two comparisons to match mothers who initiated antidepressants with mothers who received no treatment (see Chapter 3) and to match mothers who continued antidepressants into pregnancy with mothers who discontinued antidepressants before pregnancy (see Chapter 4). Further details are provided in the respective chapters.
Instrumental variable analysis
Instrumental variable regression is a statistical technique that can allow the estimation of causal effects in the presence of unmeasured confounding. 70 This is where unobserved characteristics of patients influence their likelihood of being prescribed antidepressants and at the same time influence risk of outcome, resulting in a confounded treatment effect. The rationale for IV analysis, in this particular context, is that the clinical decision to prescribe an antidepressant in pregnancy can be viewed as being influenced by three factors: first, whether or not the GP deems it safe to prescribe antidepressants to a pregnant patient given potential concerns about teratogenicity; second, the characteristics of the patients themselves, for instance their clinical characteristics, including severity of depression during pregnancy; and, third, the propensity of the physician to prescribe antidepressants. Using a well-specified IV, we can, therefore, distinguish between variability in treatment decisions owing patient characteristics (which may confound the treatment effect) and variability in treatment decisions as a result of whether or not GPs are willing to prescribe antidepressants in pregnancy (which is not determined by the characteristics of their current patient). For this reason, IV analysis can overcome unmeasured treatment-outcome confounding and, therefore, identify the causal effect of treatment on outcome. Broadly following methods proposed in earlier work,71 we aimed to capture as an IV the GPs previous prescribing practice of antidepressants in a pregnant patient given potential concerns around risks. Given that a GP’s views on medication safety cannot be directly observed, we proxied this by the number of times that they had issued an antidepressant in prior consultations with other pregnant patients. The validity of the result then depends on the extent to which the following assumptions are tenable: first, the instrument associates with the treatment (relevance assumption); second, the IV should influence only the outcome through the treatment variable (the exclusion restriction); and third, the IV does not share a common cause with the outcome (i.e. there are no confounders of the instrument outcome relationship) (the independence assumption). 70 If these IV assumptions are met, IV analysis can estimate the causal effect of treatment on an outcome.
We used IV analysis separately for women who initiated an antidepressant or continued with an existing prescription into pregnancy. Further details are provided in Chapter 5.
Matched treatment-discordance designs
Another approach to account for unmeasured confounding is the matched treatment-discordance design. This design is also commonly referred to as a sibling design when the matching is based on siblings to study outcomes in offspring of treatment or exposure discordant pregnancies. 72,73 We use the term treatment-discordance design because we have used this approach to study women’s outcomes across pregnancies, as well as outcomes for the offspring across pregnancies.
In this design, we consider consecutive pregnancies to the same woman that differed in terms of treatment status. For example, a woman may have not taken antidepressants in the first pregnancy but initiated an antidepressant in a second pregnancy, or she may have discontinued antidepressants in the first pregnancy but then continued antidepressants in a second pregnancy. These being pregnancies to the same women, any observed or unobserved characteristics that remain stable between pregnancies cannot confound the treatment–outcome association when they are analysed as matched pairs. For this reason, matched treatment-discordance designs are robust against both observed and unobserved confounders that are constant between pregnancies. Further detail on the selection and specification of statistical models used for these analyses is provided in Chapter 6.
Negative control analysis
We examined the risk of offspring neurodevelopmental outcomes where antidepressants were prescribed before but not during the pregnancy. 74 If prescription of antidepressants before the gestational period is associated with increased risk of an adverse outcome, it is unlikely that these associations are because of the effect of in utero exposure to the medication and would, therefore, suggest confounding by other characteristics. Further details of the method and results of these analyses are presented in Chapter 7.
Variation by indication: depression compared with other indication for antidepressant prescribing
To explore potential confounding by the indication, where the severity of depressive symptoms during pregnancy may influence both the likelihood of treatment and the risk of adverse outcome, we compared associations where antidepressants had been issued for depression with associations where antidepressants were likely to have been issued for other indications. A stronger association of antidepressants prescribed for depression is suggestive of confounding by the indication. Methods and results pertaining to these analyses are described in Chapter 8.
Additional analyses
In addition to analyses performed specifically to minimise confounding bias described above, we performed a range of additional analyses, as described in the following sections.
Association by timing of initiation in pregnancy
To identify potentially sensitive periods in fetal development, we compared risk of offspring neurodevelopmental problems where antidepressants were initiated in the first trimester with where they were issued in the second or third trimester. Further detail of the methods and the results of these analyses are presented in Chapter 9.
Dose response of associations
To assess dose–response relationships of antidepressant use with offspring neurodevelopmental disorders, we categorised the dose of antidepressants prescribed to each woman into low, moderate and high. It should be noted that, although such associations may highlight any dose–response relationships, they remain vulnerable to the possibility of confounding by the severity of the indication. Further detail of the methods and results for these analyses are presented in Chapter 9.
Associations for type of antidepressants
We examined associations with offspring neurodevelopmental outcomes where women had been prescribed SSRIs, tricyclic antidepressants (TCAs) or other types of antidepressants during pregnancy. Where women were issued different types of antidepressants during the same pregnancy, pregnancies counted independently to each risk estimate (e.g. women who were prescribed a SSRI and TCA were considered in the analysis of either drug type). Further detail and results for these analyses are presented in Chapter 9.
Associations by serotonin transporter receptor affinity
We examined the risk of offspring neurodevelopmental outcomes in relation to the serotonin transporter (SERT) affinity of antidepressant medications. 46,50 For these analyses, we compared women who were prescribed antidepressants in pregnancy with women who were not prescribed antidepressants in pregnancy. Further detail and results for these analyses are presented in Chapter 9.
Associations for specific antidepressant medications
Where we had sufficient numbers to enable statistical analyses, we report the associations of specific medications with neurodevelopmental outcomes. Where women were prescribed different medications within the same pregnancy, we counted them independently towards the risk estimates for all medications prescribed and then limited our analyses to women prescribed only a single medication within the same pregnancy as a sensitivity analysis. Further detail and results for these analyses are presented in Chapter 9.
Patient and public involvement
This project benefited from valuable patient and public involvement (PPI) from the very outset at the application for funding stage. We received important feedback on the study plan and design at the funding application stage from leaders of two perinatal mental health charities – Mothers for Mothers (Bristol, UK) (Mrs Maria Viner) and Bluebell Care (Bristol, UK) (Mrs Ruth Jackson). Following the project award, Mrs Maria Viner co-led the PPI strategy for this project along with Mrs Claire Storey who has significant experience of PPI in research. A bespoke PAG comprising women who have had lived experience of perinatal depression and had faced decision-making regarding medications during pregnancy was set up and three meetings were held where our PPI co-leads facilitated a discussion around important issues in relation to this project. Our co-leads purposefully recruited women known to the charity who were well and not currently in the decision-making process around medication use during pregnancy to ensure their well-being. The co-leads took particular care to ensure that the members of the PAG were supported during and after each group meeting in case any distressing issues arose.
At the start of the project, we discussed the research plan with the PAG and the challenges of decision-making regarding risks and benefits of medications during pregnancy, the portrayal of recent studies in the popular press and the media. In the next two meetings, we presented our progress and findings to the group and discussed their meaning and potential implications, as well as ideas for dissemination. The group will help support dissemination of the findings of this report upon publication.
Alongside the PAG, we also set up a Clinical Advisory Group (CAG) of multidisciplinary clinicians, which fed back on the aims of the project. The CAG meetings were later carried out within the meetings of the Health Integration Team for improving perinatal mental health, ‘IMPROVE’, based in Bristol. This unique local collaboration of service users, commissioners, service providers and researchers in the field funded by the Bristol Health Partners (www.bristolhealthpartners.org.uk/health-integration-teams/improving-perinatal-mental-health-hit/) (accessed 1 March 2021), where we received feedback on our methods and results.
These groups will continue to support the dissemination of our work to ensure that it reaches a wider audience.
Deviations from the protocol
The following deviations to the protocol were made:
-
we used the GP records within the CPRD to ascertain diagnoses of childhood neurodevelopmental conditions and did not supplement these diagnoses with HES records. This was because the HES outpatient register had less than 5% of diagnoses in outpatient appointments recorded
-
following feedback from the patient and CAGs and the discussions within the team in relation to a potential measure of ‘recovery’ from depression, we defined an additional outcome measure of women still being prescribed an antidepressant 2 years following the pregnancy as described in Prescription of an antidepressant at 2-year follow-up
-
we frequently encountered violations of non-proportionality of hazards; therefore, we did not use survival analysis in our traditional regression models and instead used logistic regression with cluster robust variance for the analysis of binary outcomes and negative binomial regression with cluster robust variance for count outcomes, while accounting for differential time at risk in all analyses by including the natural logarithm of a time-at-risk variable in our models, constraining its regression coefficient to one.
Chapter 3 Emulating the antidepressant initiation trial
This chapter describes our emulation of the target trial for initiation compared with no initiation of an antidepressant during pregnancy. Our aim was to examine the outcomes of initiating an antidepressant for depression during pregnancy compared with not initiating an antidepressant for depression during pregnancy. Figure 4 provides the specification of the target RCT and how we aimed to emulate it in observational CPRD data.
FIGURE 4.
Specification of the target initiation trial.

Methods
Study cohorts
Depending on the outcome under investigation, we used the pregnant women’s cohort or the mother and child cohort for analysis, as described Chapter 2, Study cohort selection, to use the largest available sample size relevant to each outcome.
Statistical analysis
First, we compared the characteristics of women in each arm of our target trial to assess differences in covariate distributions.
Logistic regression models with cluster-robust variances were used to estimate the relative odds associated with initiating an antidepressant in pregnancy for each of the following binary outcomes:
-
whether or not women consulted with their GP for depression or self-harm during pregnancy and in each of the four consecutive 6-month follow-up periods
-
whether or not women had been referred by their GPs to specialist services during pregnancy and each of the four consecutive 6-month follow-up periods
-
whether or not they had been admitted as an inpatient or outpatient to specialist mental health services during pregnancy and each of the four consecutive 6-month follow-up periods
-
whether or not they were still or again on antidepressants 2 years after the pregnancy end date
-
whether or not children resulting from the study pregnancies had been diagnosed with autism, ADHD or intellectual disability.
We used negative binomial regression models with cluster-robust variances to estimate incidence rate ratios for the following count outcomes:
-
the number of days on which the mother had consulted with her GP during pregnancy and further follow-up periods
-
the number of days on which the mother had consulted with her GP specifically for depression during pregnancy and further follow-up periods
-
the number of times the mother had attended A&E services during pregnancy and further follow-up periods.
To account for differential length of follow-up between treatment groups, for instance because of differences in time of initiation or length of pregnancy, we included the natural logarithm of a time-at-risk variable in our models, constraining its regression coefficient to one.
Multivariable regression
Using the models described above, we estimated crude associations between initiating an antidepressant during pregnancy and the range of outcomes described above. We then statistically adjusted our estimates for all potential confounders described in Chapter 2, Covariates. We did not, however, adjust for concurrent use of multiple antidepressants or switching between medications because these variables cannot be used to proxy illness severity for mothers who received no treatment during the study period. All analyses were conducted in Stata® 15.1/MP (Stata Corp LP, College Station, TX, USA).
Propensity score-matched regression
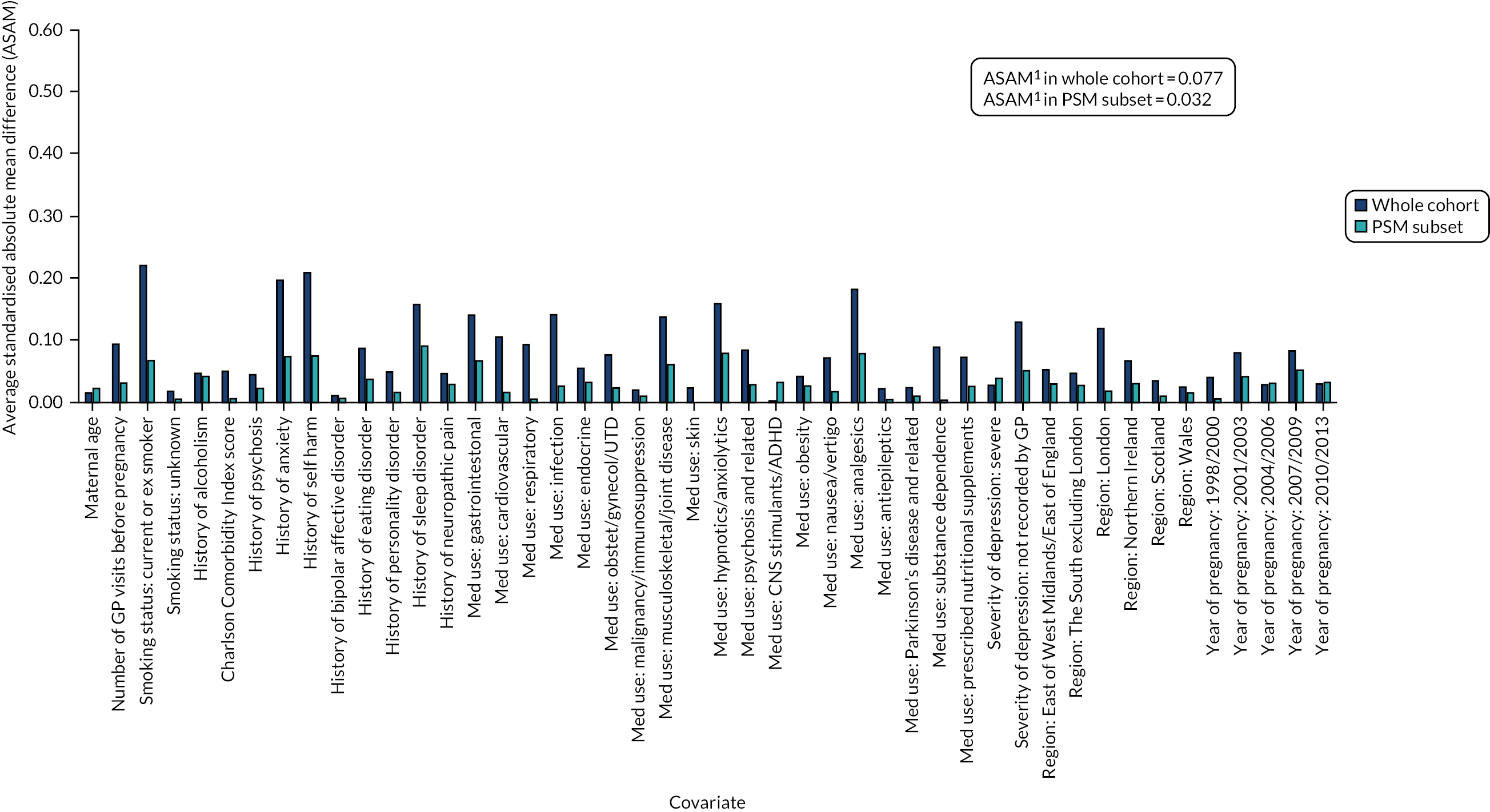
Using a CART model69 with 15,000 iterations in RStudio version 1.0.153 (The R Foundation for Statistical Computing, Vienna, Austria), we estimated a continuous score capturing women’s propensity to initiate an antidepressant in pregnancy based on their other measured characteristics. By contrast with propensity score estimation by parametric methods (where a single model is chosen to predict the data), a CART model uses a multitude of potential models, including interaction terms, and optimises its prediction across these. For this reason, CART models are well suited to predictive data modelling problems, such as propensity score estimation, because they do not depend on subjective decisions regarding the specification of the predictive model. Using the estimated propensity scores, we matched pregnancies during which women initiated antidepressants with pregnancies during which they received no treatment during the study period. Matches were carried out in a 1 : 1 ratio, without replacement and not allowing the propensity scores of matched pairs to differ by more than 0.2 standard deviations (SDs). We evaluated the quality of the matching algorithm by comparing standardised mean differences in covariate distributions before and after matching (Figures 5 and 6; see Report Supplementary Materials 21–23 for PSM analyses carried out in cohort subsets for which linked data were also included) and then exported the matched data sets to Stata 15.1/MP for statistical analysis. In these analyses, no further statistical adjustments for covariates were made because the groups were sufficiently balanced on the propensity score.
FIGURE 5.
Standardised absolute mean differences in covariate distributions between initiators and non-initiators of antidepressants in the women’s cohort before (blue) and after (orange) matching on propensity scores. Note: (1) ASAM = the Average Standardised Absolute Mean difference for all covariates.
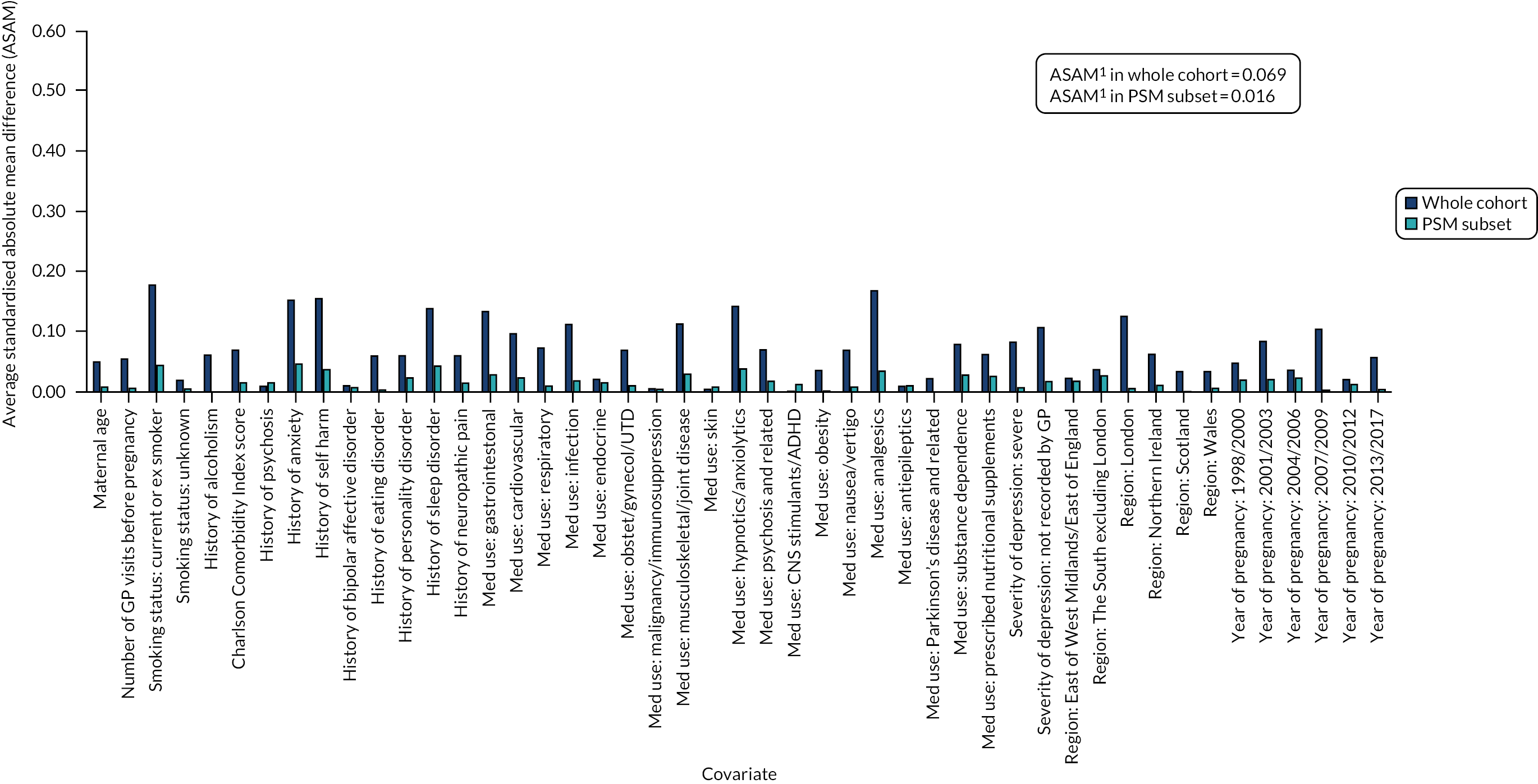
FIGURE 6.
Standardised absolute mean differences in covariate distributions between initiators and non-initiators of antidepressants in the offspring cohort, before (blue) and after (orange) matching on propensity scores. Note: (1) ASAM = the Average Standardised Absolute Mean difference for all covariates.
Results
Descriptive statistics of initiators versus non-initiators of antidepressants
Table 1 describes the characteristics of the study population of the women’s cohort by treatment status. There were 18,978 pregnancies in which women had evidence of depression during the pregnancy or within the preceding 12 months and, of these, antidepressants were initiated in 6177 pregnancies. Women who initiated an antidepressant during pregnancy were, on average, 0.3 years older and had seen the GP more frequently in the year prior to pregnancy than women who were not initiated on an antidepressant. At the start of pregnancy, women who were thereafter initiated on an antidepressant were more likely to have a history of physical comorbidities and, except for psychosis and bipolar affective disorder, were more likely to have a history of other psychiatric illness. They were also more likely to have been prescribed medications other than antidepressants in the year prior to or during pregnancy, to be current or ex-smokers, and to have a record of severe depression in their medical histories when they became pregnant. The treatment groups also differed in terms of area of residence within the UK and calendar year of the pregnancy.
| Variable | Not prescribed (N = 12,801) | Initiated (N = 6177) | p-value |
|---|---|---|---|
| Maternal age (years), mean (SD) | 28.00 (6.89) | 28.33 (6.66) | 0.002 |
| Number of GP visits in the year prior to pregnancy, mean (SD) | 5.89 (6.15) | 6.25 (6.69) | < 0.001 |
| Charlson Comorbidity Index score, n (%) | |||
| 0 | 9158 (71.54) | 4205 (68.08) | < 0.001 |
| 1 | 2877 (22.47) | 1530 (24.77) | |
| ≥ 2 | 766 (5.98) | 442 (7.16) | |
| Psychiatric history at the start of pregnancy, n (%) | |||
| Alcohol dependence | 86 (0.67) | 86 (1.39) | < 0.001 |
| Psychosis | 54 (0.42) | 30 (0.49) | 0.535 |
| Anxiety | 2912 (22.75) | 1836 (29.72) | < 0.001 |
| Self-harm | 1266 (9.89) | 958 (15.51) | < 0.001 |
| Bipolar affective disorder | 57 (0.45) | 32 (0.52) | 0.492 |
| Eating disorder | 258 (2.02) | 188 (3.04) | < 0.001 |
| Personality disorder | 79 (0.62) | 80 (1.30) | < 0.001 |
| Sleep disorder | 915 (7.15) | 716 (11.59) | < 0.001 |
| Neuropathic pain disorder | 469 (3.66) | 307 (4.97) | < 0.001 |
| Use of other medications, n (%) | |||
| Medications for physical health problems | 10,412 (81.34) | 5277 (85.43) | < 0.001 |
| Central nervous system agents | 4103 (32.05) | 2583 (41.82) | < 0.001 |
| Prescribed nutritional supplements | 1993 (15.57) | 1109 (17.95) | < 0.001 |
| Smoking status at the start of pregnancy, n (%) | |||
| Never smoked | 4887 (38.18) | 1860 (30.11) | < 0.001 |
| Current or ex-smoker | 7830 (61.17) | 4285 (69.37) | |
| Unknown | 84 (0.66) | 32 (0.52) | |
| Recorded severity of past depression, n (%) | |||
| Mild | 9777 (76.38) | 4348 (70.39) | < 0.001 |
| Severe | 387 (3.02) | 337 (5.46) | |
| Unknown | 2637 (20.60) | 1492 (24.15) | |
| Region of the general practice, n (%) | |||
| North East/North West/Yorkshire and the Humber | 2265 (17.69) | 1127 (18.25) | < 0.001 |
| East Midlands/West Midlands/East of England | 2696 (21.06) | 1359 (22.00) | |
| South West/South Central/South East | 3695 (28.86) | 1681 (27.21) | |
| London | 1271 (9.93) | 418 (6.77) | |
| Northern Ireland | 349 (2.73) | 244 (3.95) | |
| Scotland | 1155 (9.02) | 620 (10.04) | |
| Wales | 1370 (10.70) | 728 (11.79) | |
| Year of pregnancy, n (%) | |||
| 1995–97 | 663 (5.18) | 290 (4.69) | < 0.001 |
| 1998–2000 | 831 (6.49) | 480 (7.77) | |
| 2001–03 | 1487 (11.62) | 901 (14.59) | |
| 2004–06 | 2318 (18.11) | 1035 (16.76) | |
| 2007–09 | 2725 (21.29) | 1071 (17.34) | |
| 2010–12 | 2420 (18.90) | 1119 (18.12) | |
| 2013–17 | 2357 (18.41) | 1281 (20.74) | |
Table 2 describes the characteristics of the study population of the mother and child cohort by treatment status in terms of women with depression who were initiated on an antidepressant during pregnancy compared with women who were not initiated on an antidepressant and had the child’s record linked with a minimum follow-up period of 4 years. There were 8478 pregnancies in which women had evidence of depression and, of these pregnancies, antidepressants were initiated in 2649. The characteristics of women initiating in this cohort were largely similar to those described in Table 1 for the women’s cohort, barring that there was no age difference between women in the treatment groups observed.
| Not prescribed (N = 5829) | Initiated (N = 2649) | p-value | |
|---|---|---|---|
| Maternal age (years), mean (SD) | 28.62 (6.41) | 28.54 (6.12) | 0.555 |
| Number of GP visits in year prior to pregnancy, mean (SD) | 5.85 (5.85) | 6.49 (6.80) | < 0.001 |
| Charlson Comorbidity Index score, n (%) | |||
| 0 | 4131 (70.87) | 1803 (68.06) | 0.028 |
| 1 | 1345 (23.07) | 678 (25.59) | |
| ≥ 2 | 353 (6.06) | 168 (6.34) | |
| Psychiatric history at the start of pregnancy, n (%) | |||
| Alcohol dependence | 38 (0.65) | 30 (1.13) | 0.021 |
| Psychosis | 15 (0.26) | 16 (0.60) | 0.014 |
| Anxiety | 1368 (23.47) | 868 (32.77) | < 0.001 |
| Self-harm | 558 (9.57) | 463 (17.48) | < 0.001 |
| Bipolar affective disorder | 22 (0.38) | 13 (0.49) | 0.451 |
| Eating disorder | 125 (2.14) | 101 (3.81) | < 0.001 |
| Personality disorder | 39 (0.67) | 32 (1.21) | 0.012 |
| Sleep disorder | 431 (7.39) | 335 (12.65) | < 0.001 |
| Neuropathic pain disorder | 222 (3.81) | 126 (4.76) | 0.041 |
| Use of other medications, n (%) | |||
| Medications for physical health problems | 4776 (81.94) | 2289 (86.41) | < 0.001 |
| Central nervous system agents | 1826 (31.33) | 1113 (42.02) | < 0.001 |
| Prescribed nutritional supplements | 854 (14.65) | 457 (17.25) | 0.002 |
| Smoking status at the start of pregnancy, n (%) | |||
| Never smoked | 2331 (39.99) | 782 (29.52) | < 0.001 |
| Current or ex-smoker | 3468 (59.50) | 1850 (69.84) | |
| Unknown | 30 (0.51) | 17 (0.64) | |
| Recorded severity of past depression, n (%) | |||
| Mild | 4526 (77.65) | 2003 (75.61) | < 0.001 |
| Severe | 165 (2.83) | 156 (5.89) | |
| Unknown | 1138 (19.52) | 490 (18.50) | |
| Region of the general practice, n (%) | |||
| North East/North West/Yorkshire and the Humber | 1160 (19.90) | 510 (19.25) | < 0.001 |
| East Midlands/West Midlands/East of England | 1305 (22.39) | 654 (24.69) | |
| South West/South Central/South East | 1759 (30.18) | 743 (28.05) | |
| London | 443 (7.60) | 134 (5.06) | |
| Northern Ireland | 146 (2.50) | 99 (3.74) | |
| Scotland | 537 (9.21) | 273 (10.31) | |
| Wales | 479 (8.22) | 236 (8.91) | |
| Year of pregnancy, n (%) | |||
| 1995–97 | 114 (1.96) | 37 (1.40) | < 0.001 |
| 1998–2000 | 252 (4.32) | 137 (5.17) | |
| 2001–03 | 816 (14.00) | 452 (17.06) | |
| 2004–06 | 1351 (23.18) | 579 (21.86) | |
| 2007–09 | 1519 (26.06) | 604 (22.80) | |
| 2010–12 | 1777 (30.49) | 840 (31.71) | |
Table 3 provides descriptive statistics for the outcomes evaluated (number and percentages for categorical outcomes and the average number of events with SD for count outcomes) in the regression and propensity score analysis. We used the maximum data available for each outcome under investigation; given that linked data were available for only a subset of women, these analyses included a smaller number of women.
| Cohort used for multivariable regression analyses | Subset used for propensity score-matched regression analyses | |||
|---|---|---|---|---|
| Not prescribed | Initiated | Not prescribed | Initiated | |
| Women’s cohort | N = 12,801 | N = 6177 | N = 5679 | N = 5679 |
| Number of GP consultations, mean (SD) | ||||
| During pregnancy | 0.92 (0.93) | 1.29 (1.96) | 0.91 (0.93) | 1.27 (1.90) |
| 0–6 months after pregnancy | 0.60 (0.64) | 0.67 (0.71) | 0.60 (0.66) | 0.64 (0.68) |
| 6–12 months after pregnancy | 0.44 (0.57) | 0.51 (0.64) | 0.45 (0.59) | 0.49 (0.61) |
| 12–18 months after pregnancy | 0.43 (0.58) | 0.47 (0.62) | 0.43 (0.59) | 0.46 (0.59) |
| 18–24 months after pregnancy | 0.40 (0.55) | 0.45 (0.60) | 0.41 (0.57) | 0.43 (0.58) |
| Number of GP consultations for depression, mean (SD) | ||||
| During pregnancy | 0.05 (0.17) | 0.11 (0.59) | 0.05 (0.17) | 0.11 (0.61) |
| 0–6 months after pregnancy | 0.03 (0.11) | 0.06 (0.16) | 0.03 (0.11) | 0.06 (0.15) |
| 6–12 months after pregnancy | 0.02 (0.09) | 0.04 (0.12) | 0.02 (0.09) | 0.04 (0.12) |
| 12–18 months after pregnancy | 0.02 (0.09) | 0.03 (0.10) | 0.02 (0.08) | 0.03 (0.10) |
| 18–24 months after pregnancy | 0.02 (0.08) | 0.03 (0.10) | 0.02 (0.08) | 0.03 (0.10) |
| Consulted with GP for self-harm, n (%) | ||||
| During pregnancy | 9 (0.07) | 9 (0.15) | 5 (0.09) | 7 (0.12) |
| 0–6 months after pregnancy | 11 (0.09) | 11 (0.18) | 5 (0.09) | 9 (0.16) |
| 6–12 months after pregnancy | 9 (0.07) | 13 (0.21) | 3 (0.05) | 10 (0.18) |
| 12–18 months after pregnancy | 9 (0.07) | 6 (0.10) | 3 (0.05) | 5 (0.09) |
| 18–24 months after pregnancy | 10 (0.08) | 1 (0.02) | 5 (0.09) | 0 (0.00) |
| Referred by GP for depression, n (%) | ||||
| During pregnancy | 140 (1.09) | 36 (0.58) | 74 (1.30) | 33 (0.58) |
| 0–6 months after pregnancy | 59 (0.46) | 34 (0.55) | 24 (0.42) | 28 (0.49) |
| 6–12 months after pregnancy | 42 (0.33) | 25 (0.40) | 21 (0.37) | 24 (0.42) |
| 12–18 months after pregnancy | 29 (0.23) | 29 (0.47) | 12 (0.21) | 27 (0.48) |
| 18–24 months after pregnancy | 22 (0.17) | 15 (0.24) | 11 (0.19) | 13 (0.23) |
| Still or again on antidepressants at end of follow-up, n (%) | 985 (7.69) | 941 (15.23) | 459 (8.09) | 844 (14.86) |
| Women’s cohort with linked HES inpatient data | N = 7390 | N = 3482 | N = 3063 | N = 3063 |
| Admitted as an inpatient for a mental health issue, n (%) | ||||
| During pregnancy | 7 (0.09) | 7 (0.20) | 3 (0.10) | 3 (0.10) |
| 0–6 months after pregnancy | 12 (0.16) | 16 (0.46) | 7 (0.23) | 10 (0.33) |
| 6–12 months after pregnancy | 12 (0.16) | 19 (0.55) | 6 (0.20) | 14 (0.46) |
| 12–18 months after pregnancy | 7 (0.09) | 13 (0.37) | 3 (0.10) | 8 (0.26) |
| 18–24 months after pregnancy | 11 (0.15) | 9 (0.26) | 3 (0.10) | 6 (0.20) |
| Women’s cohort with linked HES outpatient data | N = 6173 | N = 2736 | N = 2378 | N = 2378 |
| Treated as outpatient for mental health issue, n (%) | ||||
| During pregnancy | 107 (1.73) | 75 (2.74) | 39 (1.64) | 60 (2.52) |
| 0–6 months after pregnancy | 101 (1.64) | 89 (3.25) | 40 (1.68) | 65 (2.73) |
| 6–12 months after pregnancy | 87 (1.41) | 70 (2.56) | 36 (1.51) | 47 (1.98) |
| 12–18 months after pregnancy | 63 (1.02) | 67 (2.45) | 25 (1.05) | 49 (2.06) |
| 18–24 months after pregnancy | 50 (0.81) | 46 (1.68) | 27 (1.14) | 33 (1.39) |
| Women’s cohort with linked HES A&E data | N = 4381 | N = 1883 | N = 1536 | N = 1536 |
| Number of A&E attendances, mean (SD) | ||||
| During pregnancy | 0.08 (0.30) | 0.10 (0.31) | 0.09 (0.23) | 0.09 (0.30) |
| 0–6 months after pregnancy | 0.04 (0.11) | 0.04 (0.12) | 0.04 (0.12) | 0.04 (0.10) |
| 6–12 months after pregnancy | 0.03 (0.10) | 0.04 (0.12) | 0.04 (0.11) | 0.04 (0.10) |
| 12–18 months after pregnancy | 0.03 (0.09) | 0.04 (0.11) | 0.03 (0.12) | 0.04 (0.10) |
| 18–24 months after pregnancy | 0.03 (0.09) | 0.04 (0.11) | 0.03 (0.11) | 0.04 (0.11) |
| Offspring cohort | N = 5829 | N = 2649 | N = 2245 | N = 2245 |
| Child diagnosed with autism, n (%) | 92 (1.58) | 49 (1.85) | 27 (1.20) | 44 (1.96) |
| Child diagnosed with ADHD, n (%) | 59 (1.01) | 45 (1.70) | 25 (1.11) | 36 (1.60) |
| Child diagnosed with intellectual disability, n (%) | 26 (0.45) | 15 (0.57) | 12 (0.53) | 9 (0.40) |
All neurodevelopmental conditions were relatively rare and were observed in less than 2% of the sample in either group. The prevalence of autism (1.85%) and ADHD (1.7%) was slightly greater in children of women who initiated antidepressants for depression in the main sample than in children of women who had depression but were not initiated on antidepressants (1.58% for autism and 1.01% for ADHD).
Results of multivariable regression and propensity score-matched analysis
To control for differences in measured characteristics between treatment groups, we examined associations between treatment status and the various outcomes while adjusting statistically for covariates and matching on the propensity to initiate antidepressants (Tables 4–6).
| Multivariable regression | Propensity score-matched regression | |||||
|---|---|---|---|---|---|---|
| Crudea | p-value | Fully adjustedb | p-value | IRR (95% CI) | p-value | |
| Number of GP consultationsc | ||||||
| During pregnancy | 1.26 (1.22 to 1.30) | < 0.001 | 1.23 (1.19 to 1.26) | < 0.001 | 1.26 (1.21 to 1.31) | < 0.001 |
| 0–6 months after pregnancy | 1.11 (1.08 to 1.15) | < 0.001 | 1.07 (1.04 to 1.11) | < 0.001 | 1.07 (1.03 to 1.12) | < 0.001 |
| 6–12 months after pregnancy | 1.15 (1.10 to 1.19) | < 0.001 | 1.10 (1.06 to 1.15) | < 0.001 | 1.09 (1.04 to 1.14) | < 0.001 |
| 12–18 months after pregnancy | 1.11 (1.06 to 1.15) | < 0.001 | 1.07 (1.03 to 1.12) | 0.001 | 1.06 (1.01 to 1.11) | 0.025 |
| 18–24 months after pregnancy | 1.12 (1.07 to 1.16) | < 0.001 | 1.09 (1.04 to 1.14) | < 0.001 | 1.07 (1.02 to 1.12) | 0.011 |
| Number of GP consultations for depressionc | ||||||
| During pregnancy | 1.65 (1.52 to 1.80) | < 0.001 | 1.67 (1.53 to 1.82) | < 0.001 | 1.66 (1.50 to 1.83) | < 0.001 |
| 0–6 months after pregnancy | 1.97 (1.80 to 2.15) | < 0.001 | 1.94 (1.76 to 2.13) | < 0.001 | 1.89 (1.68 to 2.11) | < 0.001 |
| 6–12 months after pregnancy | 1.65 (1.49 to 1.83) | < 0.001 | 1.57 (1.41 to 1.75) | < 0.001 | 1.59 (1.40 to 1.81) | < 0.001 |
| 12–18 months after pregnancy | 1.64 (1.47 to 1.84) | < 0.001 | 1.58 (1.41 to 1.77) | < 0.001 | 1.72 (1.49 to 1.97) | < 0.001 |
| 18–24 months after pregnancy | 1.54 (1.36 to 1.74) | < 0.001 | 1.47 (1.29 to 1.67) | < 0.001 | 1.48 (1.27 to 1.73) | < 0.001 |
| Consulted with GP for self-harmd | ||||||
| During pregnancy | 2.11 (0.81 to 5.53) | 0.127 | 1.92 (0.75 to 4.91) | 0.174 | 1.32 (0.40 to 4.36) | 0.651 |
| 0–6 months after pregnancy | 2.07 (0.90 to 4.79) | 0.087 | 1.64 (0.70 to 3.82) | 0.252 | 1.80 (0.60 to 5.38) | 0.292 |
| 6–12 months after pregnancy | 3.00 (1.28 to 7.02) | 0.011 | 2.81 (1.15 to 6.85) | 0.023 | 3.34 (0.92 to 12.13) | 0.067 |
| 12–18 months after pregnancy | 1.38 (0.49 to 3.88) | 0.540 | 1.36 (0.49 to 3.79) | 0.557 | 1.67 (0.40 to 6.98) | 0.484 |
| 18–24 months after pregnancy | 0.21 (0.03 to 1.62) | 0.133 | 0.11 (0.01 to 0.92) | 0.042 | N/A | N/A |
| Referred by GP to secondary services for depressiond | ||||||
| During pregnancy | 0.74 (0.51 to 1.08) | 0.124 | 0.72 (0.49 to 1.06) | 0.092 | 0.59 (0.38 to 0.90) | 0.015 |
| 0–6 months after pregnancy | 1.20 (0.78 to 1.82) | 0.409 | 1.10 (0.71 to 1.70) | 0.671 | 1.17 (0.68 to 2.02) | 0.579 |
| 6–12 months after pregnancy | 1.23 (0.75 to 2.03) | 0.405 | 1.15 (0.69 to 1.93) | 0.597 | 1.14 (0.64 to 2.06) | 0.655 |
| 12–18 months after pregnancy | 2.08 (1.24 to 3.48) | 0.005 | 2.04 (1.21 to 3.44) | 0.008 | 2.26 (1.14 to 4.46) | 0.019 |
| 18–24 months after pregnancy | 1.41 (0.73 to 2.73) | 0.301 | 1.24 (0.59 to 2.58) | 0.571 | 1.18 (0.53 to 2.64) | 0.684 |
| Prescription status at end of follow-upd | ||||||
| Prescribed an antidepressant | 2.29 (2.08 to 2.53) | < 0.001 | 2.16 (1.95 to 2.39) | < 0.001 | 2.06 (1.82 to 2.34) | < 0.001 |
| Multivariable regression | Propensity score-matched regression | |||||
|---|---|---|---|---|---|---|
| Crudea | p-value | Fully adjustedb | p-value | OR (95% CI) | p-value | |
| Admitted as inpatient for a mental health issuec | ||||||
| During pregnancy | 2.36 (0.76 to 7.39) | 0.139 | 1.66 (0.46 to 5.94) | 0.436 | 1.54 (0.36 to 6.55) | 0.562 |
| 0–6 months after pregnancy | 2.84 (1.34 to 6.01) | 0.006 | 2.22 (0.99 to 4.95) | 0.052 | 1.43 (0.54 to 3.76) | 0.470 |
| 6–12 months after pregnancy | 3.37 (1.64 to 6.96) | 0.001 | 2.81 (1.28 to 6.15) | 0.010 | 2.34 (0.90 to 6.09) | 0.082 |
| 12–18 months after pregnancy | 3.95 (1.58 to 9.92) | 0.003 | 3.43 (1.18 to 9.97) | 0.024 | 2.67 (0.71 to 10.07) | 0.147 |
| 18–24 months after pregnancy | 1.73 (0.72 to 4.20) | 0.219 | 1.28 (0.49 to 3.39) | 0.614 | 2.00 (0.50 to 8.01) | 0.327 |
| Treated as out-patient for a mental health issuec | ||||||
| During pregnancy | 1.94 (1.43 to 2.65) | < 0.001 | 1.91 (1.37 to 2.67) | < 0.001 | 1.97 (1.32 to 2.95) | 0.001 |
| 0–6 months after pregnancy | 2.02 (1.51 to 2.70) | < 0.001 | 1.92 (1.40 to 2.64) | < 0.001 | 1.64 (1.10 to 2.44) | 0.015 |
| 6–12 months after pregnancy | 1.84 (1.34 to 2.52) | < 0.001 | 1.63 (1.15 to 2.31) | 0.006 | 1.31 (0.85 to 2.03) | 0.225 |
| 12–18 months after pregnancy | 2.43 (1.72 to 3.44) | < 0.001 | 2.32 (1.59 to 3.38) | < 0.001 | 1.98 (1.22 to 3.22) | 0.006 |
| 18–24 months after pregnancy | 2.09 (1.40 to 3.13) | < 0.001 | 1.79 (1.13 to 2.85) | 0.014 | 1.22 (0.73 to 2.04) | 0.437 |
| Number of A&E attendancesd | ||||||
| During pregnancy | 1.26 (1.10 to 1.43) | 0.001 | 1.11 (0.98 to 1.27) | 0.113 | 0.98 (0.83 to 1.15) | 0.785 |
| 0–6 months after pregnancy | 1.18 (1.02 to 1.37) | 0.027 | 1.05 (0.91 to 1.21) | 0.511 | 1.02 (0.84 to 1.25) | 0.826 |
| 6–12 months after pregnancy | 1.23 (1.05 to 1.44) | 0.012 | 1.04 (0.90 to 1.22) | 0.575 | 0.95 (0.77 to 1.16) | 0.592 |
| 12–18 months after pregnancy | 1.30 (1.11 to 1.51) | 0.001 | 1.20 (1.03 to 1.41) | 0.022 | 1.05 (0.84 to 1.32) | 0.638 |
| 18–24 months after pregnancy | 1.44 (1.23 to 1.68) | < 0.001 | 1.34 (1.14 to 1.58) | < 0.001 | 1.09 (0.88 to 1.34) | 0.446 |
| Offspring neurodevelopmental outcome | Multivariable regression | Propensity score-matched regressionb | ||||
|---|---|---|---|---|---|---|
| Crudea,b | p-value | Fully adjustedb,c | p-value | |||
| Autism | 1.18 (0.83 to 1.67) | 0.366 | 1.23 (0.85 to 1.78) | 0.272 | 1.64 (1.01 to 2.66) | 0.044 |
| ADHD | 1.69 (1.14 to 2.50) | 0.008 | 1.48 (0.98 to 2.24) | 0.064 | 1.45 (0.87 to 2.42) | 0.158 |
| Intellectual disability | 1.27 (0.67 to 2.40) | 0.461 | 1.16 (0.63 to 2.14) | 0.634 | 0.75 (0.31 to 1.78) | 0.513 |
Table 4 presents results relating to women’s use of primary care services during pregnancy and within each of the four additional 6-month follow-up periods. Crude regression estimates suggested that women who had initiated an antidepressant consulted more frequently with their GPs, for any reason or specifically for depressive symptoms, during or up to 2 years after pregnancy than women who received no antidepressants. These women were also more likely to be prescribed an antidepressant medication at the end of the 2-year follow-up period. These associations remained after statistical adjustment for measured differences between treatment groups and in propensity score-matched analysis.
Differences between treatment groups in odds of consulting with the GP for episodes of self-harm were imprecise owing to a small number of observations (see Table 4). Where estimates were sufficiently powered, we observed greater odds of GP consultations for self-harm between 6 and 12 months after the pregnancy end date associated with initiating an antidepressant in multivariable regression analyses (OR 2.81, 95% CI 1.15 to 6.85). The apparently protective effect of initiating an antidepressant in terms of consulting for self-harm between 18 and 24 months after pregnancy (OR 0.11, 95% CI 0.01 to 0.92) was based on less than three treated individuals who experienced the outcome and is, therefore, likely to be unreliable.
With regard to GP referrals to secondary care services for depression during pregnancy, there was weak evidence that these were less likely among women who had initiated antidepressants compared with women who did not initiate antidepressants in pregnancy when the data were examined using multivariable regression analyses (OR 0.72, 95% CI 0.49 to 1.06) and comparably stronger evidence when using propensity score-matched regression analyses (OR 0.59, 95% CI 0.38 to 0.90). Conversely, we observed evidence for a twofold increased odds of referral to secondary services between 12 and 18 months after the pregnancy end date among women who had initiated antidepressants using both multivariable and propensity score-matched regression models.
Examining differences in continued need for antidepressants at the end of follow-up, we observed that women who had been initiated on an antidepressant in pregnancy were twofold more likely than women who had not been prescribed in the year before or during the study pregnancy to be prescribed an antidepressant 2 years after the pregnancy end date in regression (OR 2.16, 96% CI 1.95 to 2.39) and propensity score analyses.
The results of the analyses presented in Table 4 were very similar when repeated in the subset of the data with record linkages available to enable additional control for deciles of IMD as a covariate (see Report Supplementary Material 24).
The results pertaining to women’s use of secondary care services are presented in Table 5. These were broadly consistent with results for primary care outcomes in suggesting that odds of in-patient admission or out-patient treatment for a mental health problem were greater among women who had initiated antidepressants. However, low statistical power resulted in wide confidence intervals (CIs) around some estimates. While crude regression analyses suggested that A&E attendances were more common among women who had initiated antidepressants, these associations did not persist on statistical adjustment for potential confounders and/or matching on propensity scores.
Associations between initiation of an antidepressant and diagnoses related to neurodevelopmental problems in offspring are reported in Table 6. While we observed little evidence of associations in crude and multivariable regression analyses, there was some evidence for increased odds of offspring autism with initiation of an antidepressant in propensity score-matched analyses (OR 1.64, 95% CI 1.01, 2.66). There was evidence of increased odds of offspring ADHD with initiation of antidepressants in crude regression analyses, although this association attenuated on statistical adjustment for potential confounders and in propensity score-matched regression analyses, albeit with wide CIs. We observed little evidence for a difference in odds of offspring intellectual disability with initiation of an antidepressant during pregnancy.
Repeating the analysis presented in Table 6 on a smaller subset with availability of linked data to enable further adjustment for deciles of IMD led to similar point estimates in multivariable regression for all neurodevelopmental outcomes. The point estimates were attenuated for autism, and inflated for ADHD in the propensity score analysis, albeit with wide CIs due to smaller numbers (see Report Supplementary Material 25).
Chapter 4 Emulating the antidepressant continuation trial
This chapter describes our emulation of the target trial for examining the risks and potential benefits associated with continuing an antidepressant into pregnancy compared with discontinuing it prior to pregnancy. The specification of the target RCT for this question is provided in Figure 7.
FIGURE 7.
Specification of the target continuation trial.

Methods
Study cohorts
As described in the previous chapter, depending on the outcome under investigation we used the pregnant women’s cohort and the mother and child cohort for analysis. Each of these made optimal use of the available data, as described in Chapter 2, Study cohort selection.
Statistical analysis
We first compared the characteristics of women in each arm of our target trial, that is women who continued an antidepressant into pregnancy with women who discontinued prior to becoming pregnant, to assess differences in covariate distributions.
Logistic regression models with cluster-robust variance estimators were used to estimate the relative odds of the following outcomes: GP consultations for self-harm, GP referrals to specialist services, admission as an inpatient or outpatient to specialist mental health services, prescription status 2 years after the pregnancy end date, and diagnoses relating to autism, ADHD or intellectual disability in offspring from the age of 4 years.
We used negative binomial regression models with cluster-robust variance estimators to estimate incidence rate ratios for the number of days on which the mother had consulted with her GP, consulted with her GP specifically for depression and attended A&E services during pregnancy and further follow-up periods. To account differential length of follow-up between treatment groups, we included the natural logarithm of a time-at-risk variable in our models, constraining its regression coefficient to one.
Multivariable regression
We estimated crude associations between continuing an antidepressant into pregnancy and the various outcomes described earlier in this report, and then statistically adjusted our estimates for potential confounders (see Chapter 2, Covariates). All statistical analyses were conducted in Stata 15.1/MP.
Propensity score-matched regression
Using a CART model with 15,000 iterations,69 we estimated a continuous score capturing women’s propensity to continue an antidepressant in pregnancy based on their other measured characteristics. Using the estimated propensity scores, we then matched pregnancies where women continued antidepressants with pregnancies where women discontinued prior to conception. Matches were carried out in a 1 : 1 ratio, without replacement, with a calliper of 0.2 SDs. We evaluated covariate imbalance between treatment groups before and after matching (Figures 8 and 9; see Report Supplementary Materials 26–28 for PSM analyses carried out in cohort subsets in which linked data were also included) and then exported the matched data sets to Stata 15.1/MP for further analysis. No further statistical adjustment for covariates were made as the groups were sufficiently balanced on the propensity score.
FIGURE 8.
Standardised absolute mean differences in covariate distributions between continuers and discontinuers in the women’s cohort, before (blue) and after (orange) matching on propensity scores. Note: (1) ASAM = the Average Standardised Absolute Mean difference for all covariates.
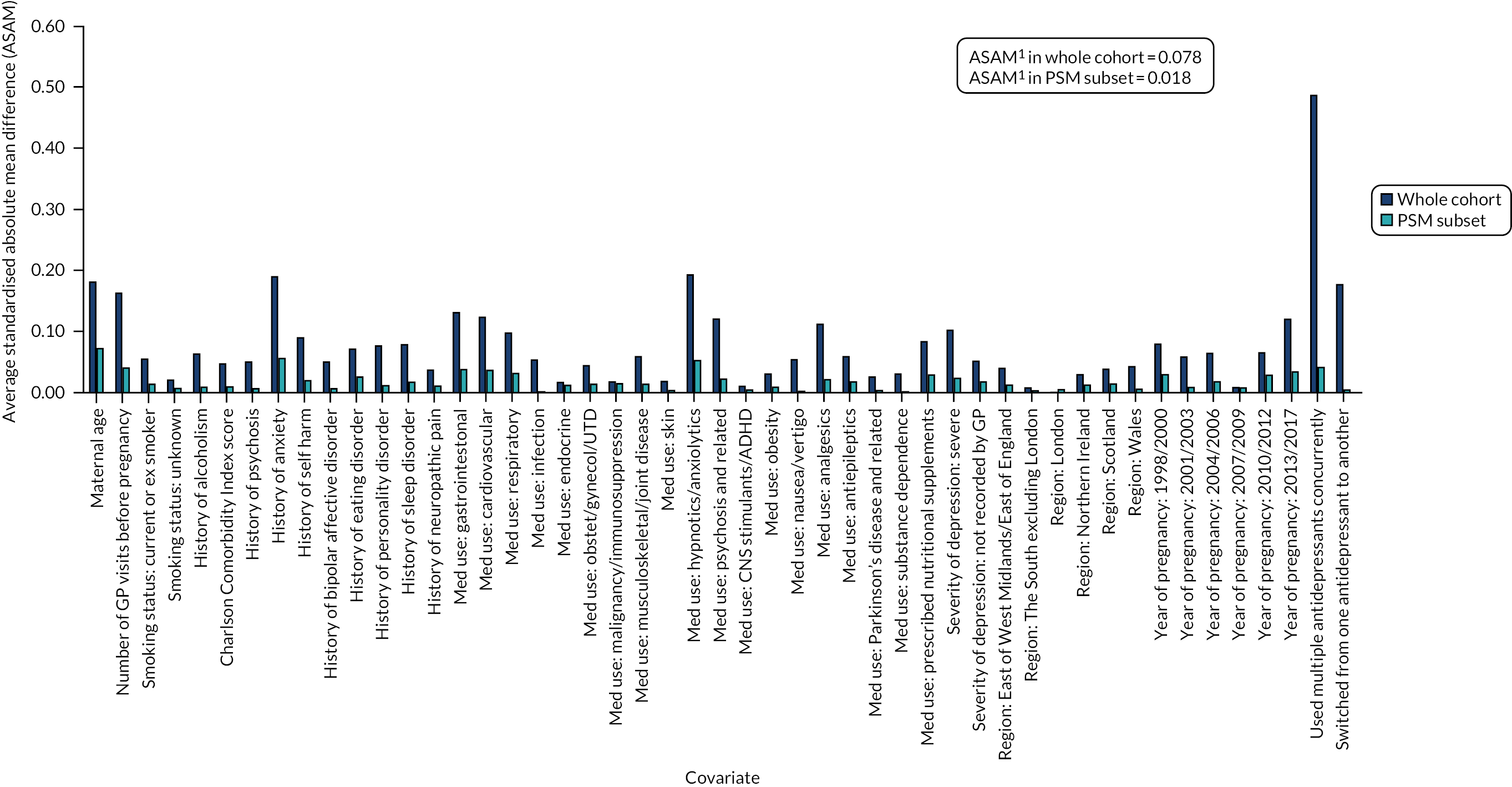
FIGURE 9.
Standardised absolute mean differences in covariate distributions between continuers and discontinuers in the mother and child cohort, before (blue) and after (orange) matching on propensity scores. Note: (1) ASAM = the Average Standardised Absolute Mean difference for all covariates.
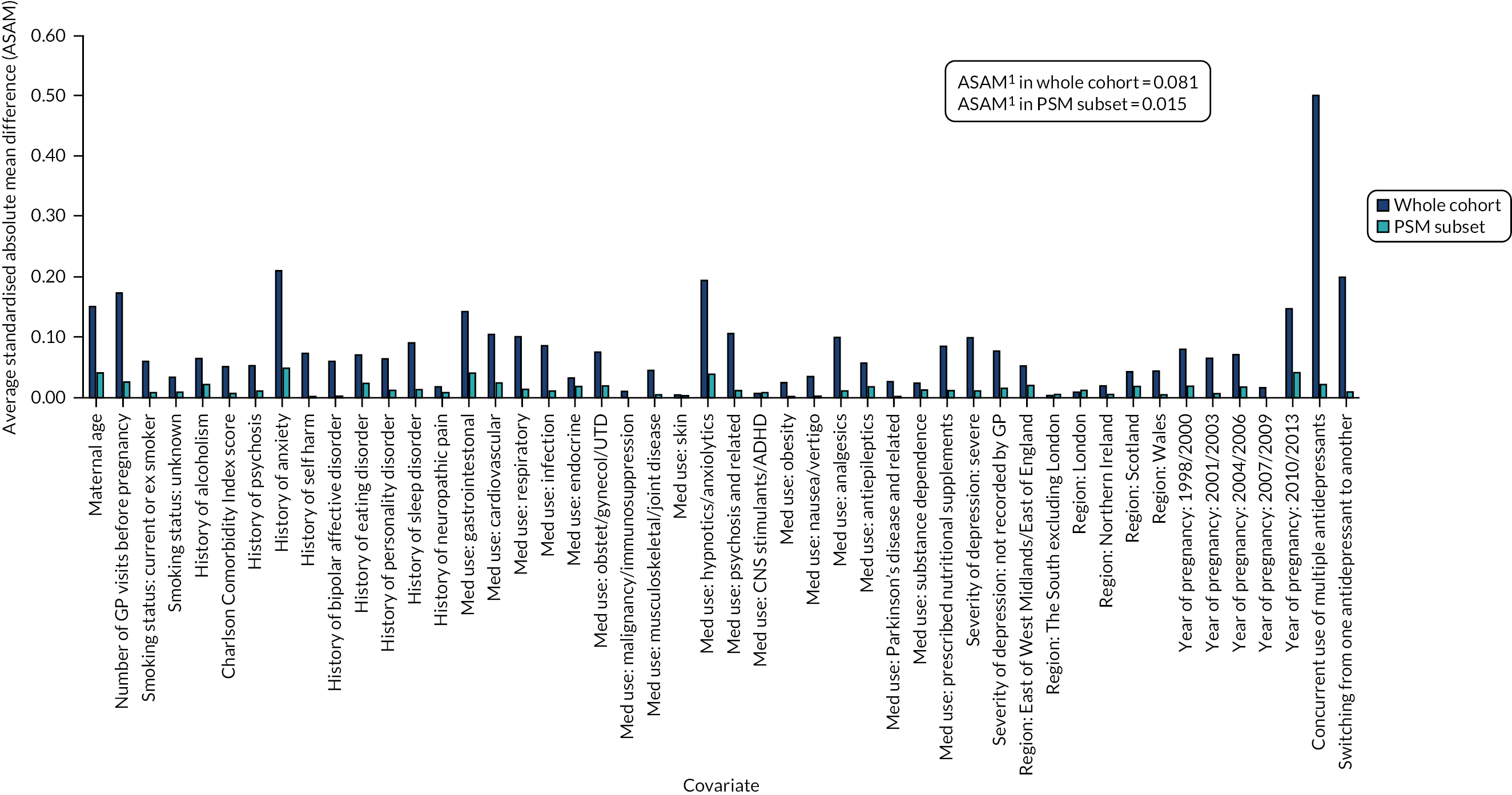
Results
Descriptive statistics of continuers versus discontinuers of antidepressants
Table 7 describes the characteristics of the study population of the women’s cohort by treatment status. There were 61,125 pregnancies in which women had a prior prescription of antidepressants for depression; of these 37,278 women continued the antidepressant into their pregnancy and 23,847 discontinued the antidepressant at least 2 months before the start of pregnancy. Women who continued antidepressants into pregnancy were, on average, 1.2 years older when they became pregnant and had consulted with their GPs on 1.2 more occasions in the year prior to pregnancy than women who had discontinued antidepressants prior to becoming pregnant. On becoming pregnant, women who had continued antidepressants were more likely to have a history of physical health comorbidities and a range of psychiatric comorbidities, to have been prescribed other medications during the study period and to be current or ex-smokers than women who discontinued antidepressants. They were also more likely to have records of both mild and severe depression in their medical histories, to have been prescribed multiple antidepressants at the same time or to have switched from one antidepressant to another during the study period than women who discontinued. There was evidence of a difference between treatment groups in the area of residence within the UK and calendar year of the pregnancy.
| Variable | Discontinued (N = 23,847) | Continued (N = 37,278) | p-value |
|---|---|---|---|
| Maternal age (years), mean (SD) | 29.05 (6.30) | 30.24 (6.60) | < 0.001 |
| Number of GP visits in year prior to pregnancy, mean (SD) | 6.57 (6.54) | 7.86 (7.90) | < 0.001 |
| Charlson Comorbidity Index score, n (%) | |||
| 0 | 16,651 (69.82) | 25,190 (67.57) | < 0.001 |
| 1 | 5640 (23.65) | 9427 (25.29) | |
| ≥ 2 | 1556 (6.52) | 2661 (7.14) | |
| Psychiatric history at the start of pregnancy, n (%) | |||
| Alcohol dependence | 311 (1.30) | 836 (2.24) | < 0.001 |
| Psychosis | 84 (0.35) | 298 (0.80) | < 0.001 |
| Anxiety | 8399 (35.22) | 16,651 (44.67) | < 0.001 |
| Self-harm | 3324 (13.94) | 6462 (17.33) | < 0.001 |
| Bipolar affective disorder | 111 (0.47) | 354 (0.95) | < 0.001 |
| Eating disorder | 698 (2.93) | 1634 (4.38) | < 0.001 |
| Personality disorder | 254 (1.07) | 812 (2.18) | < 0.001 |
| Sleep disorder | 2812 (11.79) | 5426 (14.56) | < 0.001 |
| Neuropathic pain disorder | 1401 (5.87) | 2538 (6.81) | < 0.001 |
| Use of other medications, n (%) | |||
| Medications for physical health problems | 20,446 (85.74) | 32,748 (87.85) | < 0.001 |
| Central nervous system agents | 10,162 (42.61) | 19,273 (51.70) | < 0.001 |
| Prescribed nutritional supplements | 2973 (12.47) | 5772 (15.48) | < 0.001 |
| Smoking status at the start of pregnancy, n (%) | |||
| Never smoked | 7867 (32.99) | 11,401 (30.58) | < 0.001 |
| Current or ex-smoker | 15,868 (66.54) | 25,747 (69.07) | |
| Unknown | 112 (0.47) | 130 (0.35) | |
| Recorded severity of past depression, n (%) | |||
| Mild | 15,927 (66.79) | 26,099 (70.01) | < 0.001 |
| Severe | 1025 (4.30) | 2039 (5.47) | |
| Unknown | 6895 (28.91) | 9140 (24.52) | |
| Region of the general practice, n (%) | |||
| North East/North West/Yorkshire and the Humber | 4416 (18.52) | 6387 (17.13) | < 0.001 |
| East Midlands/West Midlands/East of England | 4990 (20.93) | 7215 (19.35) | |
| South West/South Central/South East | 6610 (27.72) | 10,203 (27.37) | |
| London | 1535 (6.44) | 2401 (6.44) | |
| Northern Ireland | 1025 (4.30) | 1840 (4.94) | |
| Scotland | 2463 (10.33) | 4306 (11.55) | |
| Wales | 2808 (11.78) | 4926 (13.21) | |
| Year of pregnancy, n (%) | |||
| 1995–97 | 1190 (4.99) | 1276 (3.42) | |
| 1998–2000 | 1798 (7.54) | 2124 (5.70) | |
| 2001–03 | 3270 (13.71) | 4414 (11.84) | |
| 2004–06 | 4151 (17.41) | 5628 (15.10) | |
| 2007–09 | 4240 (17.78) | 6512 (17.47) | |
| 2010–12 | 4156 (17.43) | 7472 (20.04) | |
| 2013–17 | 5042 (21.14) | 9852 (26.43) | < 0.001 |
| Prescribed multiple antidepressants simultaneously, n (%) | 3662 (15.36) | 14,587 (39.13) | < 0.001 |
| Switched from one antidepressant medication to another, n (%) | 1346 (5.64) | 4183 (11.22) | < 0.001 |
Table 8 describes the characteristics of the study population of the mother and child cohort by treatment status used for these analyses (i.e. these refer to women whose pregnancies could be linked to the child’s records), with a minimum follow-up of 4 years. There were 25,796 pregnancies in which women had a prior prescription of antidepressants for depression; of these pregnancies, 15,295 women continued the antidepressant into their pregnancy and 10,501 discontinued the antidepressant by the start of pregnancy. The characteristics of women who continued antidepressants into pregnancy compared with those who discontinued in this cohort were similar to those described above for the women’s cohort.
| Variable | Discontinued (N = 10,501) | Continued (N = 15,295) | p-value |
|---|---|---|---|
| Maternal age (years), mean (SD) | 29.17 (5.78) | 30.06 (5.91) | < 0.001 |
| Number of GP visits in year prior to pregnancy, mean (SD) | 6.94 (6.54) | 8.30 (7.84) | < 0.001 |
| Charlson Comorbidity Index score, n (%) | |||
| 0 | 7257 (69.11) | 10,184 (66.58) | < 0.001 |
| 1 | 2577 (24.54) | 4029 (26.34) | |
| ≥ 2 | 667 (6.35) | 1082 (7.07) | |
| Psychiatric history at the start of pregnancy, n (%) | |||
| Alcohol dependence | 113 (1.08) | 306 (2.00) | < 0.001 |
| Psychosis | 23 (0.22) | 99 (0.65) | < 0.001 |
| Anxiety | 3709 (35.32) | 7015 (45.86) | < 0.001 |
| Self-harm | 1396 (13.29) | 2439 (15.95) | < 0.001 |
| Bipolar affective disorder | 34 (0.32) | 131 (0.86) | < 0.001 |
| Eating disorder | 317 (3.02) | 687 (4.49) | < 0.001 |
| Personality disorder | 103 (0.98) | 286 (1.87) | < 0.001 |
| Sleep disorder | 1274 (12.13) | 2371 (15.50) | < 0.001 |
| Neuropathic pain disorder | 635 (6.05) | 1001 (6.54) | 0.107 |
| Use of other medications, n (%) | |||
| Medications for physical health problems | 9077 (86.44) | 13,531 (88.47) | < 0.001 |
| Central nervous system agents | 4469 (42.56) | 7816 (51.10) | < 0.001 |
| Prescribed nutritional supplements | 1433 (13.65) | 2604 (17.03) | < 0.001 |
| Smoking status at the start of pregnancy, n (%) | |||
| Never smoked | 3585 (34.14) | 4829 (31.57) | < 0.001 |
| Current or ex-smoker | 6858 (65.31) | 10,412 (68.07) | |
| Unknown | 58 (0.55) | 54 (0.35) | |
| Recorded severity of past depression, n (%) | |||
| Mild | 7354 (70.03) | 11,048 (72.23) | < 0.001 |
| Severe | 434 (4.13) | 917 (6.00) | |
| Unknown | 2713 (25.84) | 3330 (21.77) | |
| Region of the general practice, n (%) | |||
| North East/North West/Yorkshire and the Humber | 2121 (20.20) | 2899 (18.95) | < 0.001 |
| East Midlands/West Midlands/East of England | 2506 (23.86) | 3324 (21.73) | |
| South West/South Central/South East | 3049 (29.04) | 4472 (29.24) | |
| London | 479 (4.56) | 722 (4.72) | |
| Northern Ireland | 480 (4.57) | 751 (4.91) | |
| Scotland | 1004 (9.56) | 1676 (10.96) | |
| Wales | 862 (8.21) | 1451 (9.49) | |
| Year of pregnancy, n (%) | |||
| 1995–97 | 235 (2.24) | 194 (1.27) | < 0.001 |
| 1998–2000 | 640 (6.09) | 680 (4.45) | |
| 2001–03 | 1792 (17.07) | 2231 (14.59) | |
| 2004–06 | 2428 (23.12) | 3083 (20.16) | |
| 2007–09 | 2345 (22.33) | 3541 (23.15) | |
| 2010–13 | 3061 (29.15) | 5566 (36.39) | |
| Prescribed multiple antidepressants simultaneously, n (%) | 1609 (15.32) | 6104 (39.91) | < 0.001 |
| Switched from one antidepressant medication to another, n (%) | 592 (5.64) | 1852 (12.11) | < 0.001 |
Table 9 provides descriptive statistics for the outcomes evaluated (number and percentages for categorical outcomes and the average number of events with SD for count outcomes) in the regression and propensity score analysis. We used the maximum data available for each outcome under investigation and given that linked data were available for only a subset of women those analyses included fewer women. All neurodevelopmental conditions were relatively rare and observed in less than 2% of either group. The prevalence of autism, ADHD and intellectual disability appeared similar in all groups irrespective of whether the women continued or discontinued antidepressants during pregnancy.
| Cohort used for multivariable regression analyses | Subset used for propensity score-matched regression analyses | |||
|---|---|---|---|---|
| Discontinued | Continued | Discontinued | Continued | |
| Women’s cohort | N = 23,847 | N = 37,278 | N = 22,650 | N = 22,650 |
| Number of GP consultations, mean (SD) | ||||
| During pregnancy | 0.89 (0.92) | 1.02 (1.04) | 0.89 (0.93) | 0.95 (0.96) |
| 0–6 months after pregnancy | 0.62 (0.66) | 0.68 (0.75) | 0.62 (0.67) | 0.63 (0.69) |
| 6–12 months after pregnancy | 0.47 (0.60) | 0.52 (0.68) | 0.47 (0.60) | 0.49 (0.61) |
| 12–18 months after pregnancy | 0.45 (0.59) | 0.48 (0.66) | 0.45 (0.60) | 0.46 (0.60) |
| 18–24 months after pregnancy | 0.42 (0.59) | 0.45 (0.66) | 0.42 (0.60) | 0.43 (0.60) |
| Number of GP consultations for depression, mean (SD) | ||||
| During pregnancy | 0.01 (0.06) | 0.05 (0.16) | 0.01 (0.06) | 0.04 (0.15) |
| 0–6 months after pregnancy | 0.03 (0.11) | 0.05 (0.13) | 0.03 (0.11) | 0.04 (0.12) |
| 6–12 months after pregnancy | 0.03 (0.10) | 0.03 (0.11) | 0.03 (0.10) | 0.03 (0.11) |
| 12–18 months after pregnancy | 0.02 (0.09) | 0.03 (0.10) | 0.02 (0.09) | 0.03 (0.10) |
| 18–24 months after pregnancy | 0.02 (0.09) | 0.03 (0.10) | 0.02 (0.09) | 0.02 (0.09) |
| Consulted with GP for self-harm, n (%) | ||||
| During pregnancy | 10 (0.04) | 37 (0.10) | 10 (0.04) | 18 (0.08) |
| 0–6 months after pregnancy | 15 (0.06) | 57 (0.15) | 15 (0.07) | 34 (0.15) |
| 6–12 months after pregnancy | 20 (0.08) | 42 (0.11) | 19 (0.08) | 19 (0.08) |
| 12–18 months after pregnancy | 19 (0.08) | 59 (0.16) | 19 (0.08) | 27 (0.12) |
| 18–24 months after pregnancy | 11 (0.05) | 51 (0.14) | 11 (0.05) | 21 (0.09) |
| Referred by GP for depression, n (%) | ||||
| During pregnancy | 29 (0.12) | 137 (0.37) | 27 (0.12) | 80 (0.35) |
| 0–6 months after pregnancy | 82 (0.34) | 159 (0.43) | 76 (0.34) | 96 (0.42) |
| 6–12 months after pregnancy | 52 (0.22) | 136 (0.36) | 46 (0.20) | 74 (0.33) |
| 12–18 months after pregnancy | 55 (0.23) | 109 (0.29) | 50 (0.22) | 63 (0.28) |
| 18–24 months after pregnancy | 40 (0.17) | 107 (0.29) | 39 (0.17) | 65 (0.29) |
| Still or again on antidepressants at end of follow-up, n (%) | 2158 (9.05) | 8080 (21.67) | 2079 (9.18) | 4359 (19.25) |
| Women’s cohort with linked HES data | N = 13,110 | N = 19,680 | N = 12,046 | N = 12,046 |
| Admitted as in-patient for mental health issue, n (%) | ||||
| During pregnancy | 6 (0.05) | 53 (0.27) | 5 (0.04) | 21 (0.17) |
| 0–6 months after pregnancy | 38 (0.29) | 101 (0.51) | 37 (0.31) | 44 (0.37) |
| 6–12 months after pregnancy | 28 (0.21) | 105 (0.53) | 25 (0.21) | 48 (0.40) |
| 12–18 months after pregnancy | 24 (0.18) | 77 (0.39) | 22 (0.18) | 25 (0.21) |
| 18–24 months after pregnancy | 24 (0.18) | 87 (0.44) | 22 (0.18) | 41 (0.34) |
| Women’s cohort with linked HES outpatient data | N = 10,388 | N = 16,377 | N = 9670 | N = 9670 |
| Treated as outpatient for mental health issue, n (%) | ||||
| During pregnancy | 100 (0.96) | 689 (4.21) | 98 (1.01) | 275 (2.85) |
| 0–6 months after pregnancy | 147 (1.42) | 736 (4.49) | 144 (1.49) | 285 (2.95) |
| 6–12 months after pregnancy | 141 (1.36) | 614 (3.75) | 136 (1.41) | 230 (2.38) |
| 12–18 months after pregnancy | 136 (1.31) | 558 (3.41) | 132 (1.37) | 223 (2.31) |
| 18–24 months after pregnancy | 138 (1.33) | 499 (3.05) | 134 (1.39) | 192 (1.99) |
| Women’s cohort with linked HES A&E data | N = 7203 | N = 12,230 | N = 6835 | N = 6835 |
| Number of A&E attendances, mean (SD) | ||||
| During pregnancy | 0.10 (0.32) | 0.10 (0.27) | 0.10 (0.32) | 0.09 (0.27) |
| 0–6 months after pregnancy | 0.04 (0.11) | 0.05 (0.13) | 0.04 (0.11) | 0.04 (0.11) |
| 6–12 months after pregnancy | 0.04 (0.11) | 0.04 (0.13) | 0.04 (0.11) | 0.04 (0.10) |
| 12–18 months after pregnancy | 0.04 (0.10) | 0.04 (0.12) | 0.04 (0.11) | 0.03 (0.09) |
| 18–24 months after pregnancy | 0.03 (0.10) | 0.04 (0.12) | 0.03 (0.10) | 0.03 (0.10) |
| Offspring cohort, n (%) | N = 10,501 | N = 15,295 | N = 9135 | N = 9135 |
| Child diagnosed with autism | 162 (1.54) | 250 (1.63) | 146 (1.60) | 154 (1.69) |
| Child diagnosed with ADHD | 135 (1.29) | 178 (1.16) | 119 (1.30) | 115 (1.26) |
| Child diagnosed with intellectual disability | 62 (0.59) | 65 (0.42) | 55 (0.60) | 49 (0.54) |
Results of multivariable and propensity score-matched regression analysis
To control for differences in measured characteristics between treatment groups, we examined associations between treatment status and the various outcomes while adjusting statistically for covariates, and matching on the propensity to initiate antidepressants, as shown in Tables 10–12.
| Multivariable regression | Propensity score-matched regression | |||||
|---|---|---|---|---|---|---|
| Crudea | p-value | Fully adjustedb | p-value | IRR/OR (95% CI) | p-value | |
| Number of GP consultationsc | ||||||
| During pregnancy | 1.15 (1.13 to 1.17) | < 0.001 | 1.00 (0.99 to 1.02) | 0.807 | 1.07 (1.05 to 1.09) | < 0.001 |
| 0–6 months after pregnancy | 1.10 (1.08 to 1.12) | < 0.001 | 0.96 (0.95 to 0.98) | < 0.001 | 1.02 (1.00 to 1.05) | 0.016 |
| 6–12 months after pregnancy | 1.11 (1.08 to 1.13) | < 0.001 | 0.97 (0.95 to 0.99) | 0.013 | 1.03 (1.00 to 1.05) | 0.027 |
| 12–18 months after pregnancy | 1.08 (1.06 to 1.10) | < 0.001 | 0.95 (0.93 to 0.97) | < 0.001 | 1.01 (0.99 to 1.04) | 0.272 |
| 18–24 months after pregnancy | 1.06 (1.03 to 1.08) | < 0.001 | 0.95 (0.93 to 0.97) | < 0.001 | 1.00 (0.98 to 1.03) | 0.815 |
| Number of GP consultations for depressionc | ||||||
| During pregnancy | 7.45 (6.77 to 8.21) | < 0.001 | 5.66 (5.13 to 6.25) | < 0.001 | 6.11 (5.51 to 6.79) | < 0.001 |
| 0–6 months after pregnancy | 1.43 (1.36 to 1.51) | < 0.001 | 1.24 (1.17 to 1.31) | < 0.001 | 1.32 (1.25 to 1.40) | < 0.001 |
| 6–12 months after pregnancy | 1.26 (1.20 to 1.34) | < 0.001 | 1.14 (1.07 to 1.21) | < 0.001 | 1.21 (1.13 to 1.29) | < 0.001 |
| 12–18 months after pregnancy | 1.30 (1.22 to 1.38) | < 0.001 | 1.17 (1.10 to 1.25) | < 0.001 | 1.24 (1.16 to 1.33) | < 0.001 |
| 18–24 months after pregnancy | 1.20 (1.13 to 1.28) | < 0.001 | 1.06 (0.99 to 1.14) | 0.078 | 1.13 (1.05 to 1.22) | 0.001 |
| Consulted with GP for self-harmd | ||||||
| During pregnancy | 2.36 (1.18 to 4.75) | 0.016 | 1.59 (0.76 to 3.29) | 0.215 | 1.79 (0.83 to 3.88) | 0.138 |
| 0–6 months after pregnancy | 2.43 (1.38 to 4.30) | 0.002 | 2.14 (1.19 to 3.85) | 0.011 | 2.27 (1.23 to 4.17) | 0.008 |
| 6–12 months after pregnancy | 1.34 (0.79 to 2.29) | 0.277 | 1.14 (0.66 to 1.95) | 0.645 | 1.00 (0.53 to 1.89) | 0.999 |
| 12–18 months after pregnancy | 1.99 (1.19 to 3.33) | 0.009 | 1.82 (1.05 to 3.18) | 0.034 | 1.42 (0.80 to 2.56) | 0.241 |
| 18–24 months after pregnancy | 2.97 (1.55 to 5.70) | 0.001 | 2.38 (1.25 to 4.53) | 0.008 | 1.91 (0.92 to 3.96) | 0.082 |
| Referred by GP to secondary services for depressiond | ||||||
| During pregnancy | 3.10 (2.08 to 4.64) | < 0.001 | 2.72 (1.79 to 4.13) | < 0.001 | 3.04 (1.96 to 4.70) | < 0.001 |
| 0–6 months after pregnancy | 1.24 (0.95 to 1.62) | 0.112 | 1.16 (0.87 to 1.54) | 0.321 | 1.26 (0.93 to 1.71) | 0.128 |
| 6–12 months after pregnancy | 1.68 (1.22 to 2.31) | 0.002 | 1.56 (1.12 to 2.18) | 0.009 | 1.61 (1.11 to 2.33) | 0.011 |
| 12–18 months after pregnancy | 1.27 (0.92 to 1.76) | 0.151 | 1.20 (0.84 to 1.72) | 0.322 | 1.26 (0.87 to 1.83) | 0.222 |
| 18–24 months after pregnancy | 1.71 (1.19 to 2.46) | 0.003 | 1.67 (1.13 to 2.47) | 0.010 | 1.67 (1.13 to 2.47) | 0.011 |
| Prescription status at end of follow-upd | ||||||
| Prescribed an antidepressant | 2.80 (2.66 to 2.94) | < 0.001 | 2.40 (2.27 to 2.53) | < 0.001 | 2.37 (2.24 to 2.51) | < 0.001 |
| Multivariable regression | Propensity score-matched regression | |||||
|---|---|---|---|---|---|---|
| Crudea | p-value | Fully adjustedb | p-value | OR/IRR (95% CI) | p-value | |
| Admitted as inpatient for a mental health issuec | ||||||
| During pregnancy | 5.94 (2.55 to 13.81) | < 0.001 | 3.52 (1.49 to 8.34) | 0.004 | 4.20 (1.59 to 11.14) | 0.004 |
| 0–6 months after pregnancy | 1.77 (1.22 to 2.58) | 0.003 | 1.31 (0.87 to 1.99) | 0.199 | 1.19 (0.77 to 1.84) | 0.437 |
| 6–12 months after pregnancy | 2.51 (1.65 to 3.80) | < 0.001 | 1.98 (1.29 to 3.03) | 0.002 | 1.92 (1.19 to 3.12) | 0.008 |
| 12–18 months after pregnancy | 2.14 (1.35 to 3.39) | 0.001 | 1.48 (0.91 to 2.40) | 0.111 | 1.14 (0.64 to 2.02) | 0.662 |
| 18–24 months after pregnancy | 2.42 (1.53 to 3.81) | < 0.001 | 1.83 (1.14 to 2.93) | 0.012 | 1.87 (1.11 to 3.13) | 0.018 |
| Treated as out-patient for a mental health issuec | ||||||
| During pregnancy | 4.60 (3.72 to 5.69) | < 0.001 | 2.92 (2.34 to 3.64) | < 0.001 | 2.90 (2.30 to 3.66) | < 0.001 |
| 0–6 months after pregnancy | 3.28 (2.74 to 3.92) | < 0.001 | 2.08 (1.72 to 2.52) | < 0.001 | 2.01 (1.64 to 2.46) | < 0.001 |
| 6–12 months after pregnancy | 2.83 (2.35 to 3.41) | < 0.001 | 1.84 (1.51 to 2.23) | < 0.001 | 1.71 (1.38 to 2.12) | < 0.001 |
| 12–18 months after pregnancy | 2.66 (2.20 to 3.22) | < 0.001 | 1.81 (1.48 to 2.22) | < 0.001 | 1.71 (1.37 to 2.12) | < 0.001 |
| 18–24 months after pregnancy | 2.33 (1.93 to 2.82) | < 0.001 | 1.59 (1.30 to 1.94) | < 0.001 | 1.44 (1.15 to 1.80) | 0.001 |
| Number of A&E attendancesd | ||||||
| During pregnancy | 1.07 (1.00 to 1.15) | 0.050 | 1.01 (0.94 to 1.08) | 0.752 | 0.97 (0.90 to 1.06) | 0.536 |
| 0–6 months after pregnancy | 1.12 (1.03 to 1.21) | 0.007 | 1.05 (0.97 to 1.13) | 0.213 | 1.02 (0.93 to 1.11) | 0.647 |
| 6–12 months after pregnancy | 1.09 (1.00 to 1.20) | 0.060 | 1.01 (0.93 to 1.10) | 0.777 | 1.00 (0.91 to 1.11) | 0.950 |
| 12–18 months after pregnancy | 1.05 (0.96 to 1.15) | 0.321 | 0.97 (0.90 to 1.06) | 0.554 | 0.92 (0.84 to 1.02) | 0.099 |
| 18–24 months after pregnancy | 1.10 (1.01 to 1.21) | 0.039 | 0.99 (0.91 to 1.08) | 0.873 | 0.96 (0.87 to 1.07) | 0.459 |
| Multivariable regression | Propensity score-matched regression | |||||
|---|---|---|---|---|---|---|
| Crudea | Fully adjustedb | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Autism | 1.06 (0.87 to 1.29) | 0.563 | 1.10 (0.90 to 1.35) | 0.354 | 1.06 (0.84 to 1.32) | 0.639 |
| ADHD | 0.90 (0.72 to 1.13) | 0.380 | 1.02 (0.80 to 1.29) | 0.889 | 0.97 (0.75 to 1.25) | 0.792 |
| Intellectual disability | 0.72 (0.51 to 1.02) | 0.063 | 0.81 (0.55 to 1.19) | 0.279 | 0.89 (0.61 to 1.31) | 0.555 |
For results relating to women’s use of primary care services during pregnancy and each of the four additional 6-month follow-up periods, see Table 10. Although crude associations suggested that women who had continued antidepressants had consulted with their GPs more frequently for any reason, statistical adjustment for covariates revealed a slight protective effect. However, this finding was not replicated in propensity score-matched regression analyses in which control for potential confounders would have been more efficient. In terms of GP consultations specifically for depression, women who had continued antidepressants consulted more frequently with their GPs than women who had discontinued antidepressants, with consistent associations observed for crude, multivariable and propensity score-matched regression analyses. In general, women who had continued an antidepressant were more likely to have consulted for self-harm or to have been referred to specialist mental health services for depression during pregnancy and in further follow-up periods. Women who had continued antidepressant in pregnancy also had over two-fold odds of still being prescribed antidepressants at 2-year follow-up than women who had discontinued. Repeating these analyses on a subsample with linked data for further adjustment with decile of IMD led to similar results (see Report Supplementary Material 29).
In term of outcomes related to secondary care (see Table 11), crude associations suggested that women who had continued an antidepressant were more likely than women who had discontinued an antidepressant to have received inpatient or outpatient treatment for a mental health problem during pregnancy or in further follow-up periods. Statistical adjustment for potential confounders and/or matching on propensity scores resulted in weaker associations, but odds generally remained elevated. Crude associations suggested that women who continued antidepressants had attended A&E services more frequently than those who discontinued antidepressants, although these associations attenuated to the null on statistical adjustment for potential confounders or after matching on the propensity to continue antidepressant treatment.
See Table 12 for a description of the associations between continuation of an antidepressants and diagnoses of neurodevelopmental disorders in offspring. We observed no differences in odds for autism or for intellectual disability for mothers who continued or discontinued antidepressants during pregnancy. Although we observed lower odds of offspring intellectual disability among women who had continued taking antidepressants, this association attenuated to the null on statistical adjustment for potential confounders and in propensity score-matched regression analyses. Repeating these analyses on the subset of the sample with linked data to further adjust for deciles of IMD led to broadly similar results with wider CIs (see Report Supplementary Material 30).
Chapter 5 Instrumental variable analysis
We carried IV analyses to further strengthen causal inference from associations reported in the emulated initiation and continuation trials. IV regression can allow the estimation of causal effects in the presence of unmeasured treatment–outcome confounding. A well-defined IV is associated with the outcome variable only because of the instrument’s effect on the treatment variable and has no confounders of the instrument–outcome association. We chose GPs’ prescribing behaviour with regard to their prior pregnant patients with depression as an IV, and then used this to instrument the decision to initiate or continue an antidepressant for their current pregnant patient with depression. Conceptually, the IV is intended to capture the willingness of GPs to prescribe antidepressants to pregnant patients given the potential concerns around teratogenicity. For this reason, we carried out these analyses only for the neurodevelopmental outcomes under study in relation to initiation or continuation of antidepressants in pregnancy.
Definition of potential instrumental variables
We defined 10 potential IVs based on the treatment decisions made in an increasing number of prior consultations with other pregnant patients with depression. For instance, the first instrument was defined as the treatment decision made in the last consultation with another pregnant patient, the second instrument as the number of times the GP had prescribed an antidepressant in the last two consultations with other pregnant patients, the third instrument as the number of times an antidepressant was prescribed among the last three pregnant patients, and so on. It is worth noting that instruments based on n prior treatment decisions will require GPs to have seen at least n prior pregnant patients with depression. IVs based on larger numbers of prior treatment decisions will, therefore, result in smaller numbers of observations being available for IV analysis. For each instrument, we calculated F-statistics for first-stage regressions and checked for associations between the instrument and the potential confounders of the treatment–outcome association using bias plots. We also examined the number of observations with complete IVs for each of the 10 IV definitions. Our aim was to select the instrument that was optimally associated with the treatment variable but minimally associated with potential confounders of the treatment–outcome association, while optimising the number of observations available for IV analysis.
Methods
Identification of the general practitioner
Many women in our sample will have been seen by different GPs during the course of their pregnancies. Therefore, we aimed to identify the GP who, by issuing or not issuing an antidepressant, had determined the woman’s the treatment status, and defined the IV on that GP’s prior prescribing behaviour. That is, for patients who initiated or continued an antidepressant into pregnancy, we identified the first GP to have prescribed an antidepressant in pregnancy, whereas for patients who received no treatment or discontinued an existing prescription, we identified the GP who had attended the first consultation after the estimated date of conception. Where antidepressants were issued as part of a repeat prescription, we did not consider prescriptions for which there had been no face-to-face or telephone consultation. We made these exclusions because it may be likely that, in these instances, the GPs would have been unaware of their patient being pregnant.
Treatment variables
We considered two treatment variables. The first treatment variable captured the initiation of an antidepressant during pregnancy compared with receiving no antidepressant treatment, and the second captured the continuation of an antidepressant into pregnancy compared with discontinuing at least 2 months prior to conception, as described in Chapter 2, Definition of treatment groups.
Outcome variables
Our outcome variables for these analyses included a diagnosis of (1) autism, (2) ADHD or (3) intellectual disability when the child was at least 4 years of age.
Statistical analysis
For all observations with a non-missing value on the IV, we estimated the associations using ordinary least squares (OLS) regression (using a linear probability model for the binary outcomes) to allow for comparison of these estimates with associations estimated using the IV method applying two-stage least squares regression. All associations were estimated using robust standard errors to account for clustering of patients treated by the same GP.
Results
Selection and evaluation of the instrumental variable
We found an IV based on eight prior consultations with other pregnant patients to be optimally associated with the treatment variables, while including an optimal number of observations in IV analyses. For the IV based on eight prior consultations, we checked associations of the instrument with potential confounders of the treatment–outcome association using bias component plots. 75 Results from these analyses suggested weak associations of the instrument with the following covariates: (1) number of GP visits in the year prior to pregnancy; (2) region of residence in the UK; (3) calendar year of the pregnancy; (4) concurrent use of multiple antidepressants; (5) maternal age; and (6) Charlson Comorbidity Index score. We, therefore, adjusted for these characteristics in the analytical models.
Results of standard and instrumental variable analysis
The results of the standard and IV analyses are presented in Table 13. Among observations with a non-missing value on the IV, there was no observed association between initiation or continuation of antidepressants and any of the neurodevelopmental outcomes in either the standard or the IV approach, although CIs were wide in all analyses. Using Wu–Hausman F-tests, there was little evidence for differences in effect size between OLS regression and IV estimates for these outcomes. This does not provide any evidence that residual confounding can explain our OLS results.
| OLSs regression coefficient | p-value | Two-stage least squares regression coefficient | p-value | Wu–Hausman F-test p-value | |
|---|---|---|---|---|---|
| Effect of initiating an antidepressant | |||||
| Offspring autism | 0.006 (–0.05 to 0.017) | 0.300 | 0.040 (–0.039 to 0.119) | 0.321 | 0.391 |
| Offspring ADHD | 0.002 (–0.006 to 0.010) | 0.648 | –0.004 (–0.059 to 0.050) | 0.873 | 0.820 |
| Offspring ID | 0.003 (–0.003 to 0.009) | 0.279 | 0.007 (–0.031 to 0.044) | 0.735 | 0.865 |
| Effect of continuing an antidepressant | |||||
| Offspring autism | 0.006 (–0.001 to 0.013) | 0.083 | –0.010 (–0.061 to 0.040) | 0.690 | 0.520 |
| Offspring ADHD | 0.003 (–0.002 to 0.008) | 0.211 | 0.000 (–0.039 to 0.040) | 0.982 | 0.890 |
| Offspring ID | –0.001 (–0.004 to 0.003) | 0.755 | –0.005 (–0.035 to 0.025) | 0.740 | 0.768 |
Chapter 6 Analysis of treatment-discordant pregnancies
The matched treatment-discordance design is another effective approach to strengthening causal inference when using observational data. Where the outcomes relate to siblings born to the same mother in treatment-discordant (or other exposure) pregnancies, this design is also known as a sibling or sibship design and is increasingly used in intergenerational observational studies strengthening causal inference of prenatal factors. 32,73,74 Given that we study both maternal and child outcomes in this study, and the unit for sampling was pregnant women with exposure discordant pregnancies, we refer to this analysis as a treatment-discordance design.
In this design, we consider consecutive pregnancies to the same woman that differed in terms of treatment status, for instance where they had received no antidepressants in the first pregnancy but initiated an antidepressant in the second pregnancy. By examining pregnancies to the same women as matched observations, all characteristics that are constant between pregnancies (e.g. time-stable socioeconomic factors or genetic risk for depression) cease to confound the treatment–outcome association. For this reason, the analysis of treatment-discordant pregnancies can help to reduce bias due to unmeasured time-stable confounders and, therefore, allow stronger causal inference from observational data. If associations observed in the emulated initiation and continuation trials (described in Chapters 3 and 4) are replicated in an analysis of treatment-discordant pregnancies, this would, therefore, suggest robustness against shared unmeasured confounding.
Methods
We first identified all women who had contributed more than one pregnancy to the study cohort and who differed in treatment status between pregnancies. Among these matched treatment-discordant pregnancies, only those that also differed in terms of outcome contributed to the analysis. Therefore, an inherent limitation of this approach is that the smaller number of treatment- and outcome-discordant observations can limit statistical power.
Definition of treatment discordance
Treatment discordance was defined as (1) having initiated an antidepressant in one pregnancy and having received no treatment in another pregnancy, or (2) having continued an antidepressant in one pregnancy and having discontinued antidepressants in another. All women contributing at least two pregnancies that were discordant in terms of treatment status were considered in the analysis.
Definition of outcome discordance
Women’s use of primary care
To maximise statistical power, we combined all follow-up beyond the pregnancy end date into a single 2-year window. For count outcomes, discordance was considered as the difference in count value between pregnancies. For example, having consulted with a GP on 2 more days in one pregnancy compared with another. Owing to small cell counts, it was only possible to examine primary care outcomes using the discordant-treatment method. We, therefore, report the following count outcomes in our analyses: (1) frequency of GP consultation during and after pregnancy; (2) frequency of GP consultation for depressive symptoms during or after pregnancy; and (3) the binary outcomes of women’s antidepressant prescription status 2 years after pregnancy.
Offspring neurodevelopmental outcomes
We examined discordance in matched offspring in terms of a diagnosis of (1) autism, (2) ADHD or (3) intellectual disability. These analyses were carried out for continuation compared with discontinuation of antidepressants only, as there were insufficient numbers to estimate the results for the initiation compared with no initiation of antidepressant analyses for these outcomes.
Statistical analysis
For binary outcomes, we first estimated associations using standard logistic regression models to assess whether or not the associations observed in Chapters 3 and 4 of this report were present in this subset of the data. Associations were estimated using robust standard errors, clustering on women’s patient identification numbers, as our estimates were based on comparisons of more than one pregnancy in the same women. We then used fixed-effects logistic regression models to estimate the matched treatment–outcome association. We followed a similar approach for count outcomes, first estimating associations using standard negative binomial regression and second using a fixed-effects negative binomial regression model to estimate the matched treatment–outcome association. We statistically adjusted all associations for calendar year and maternal age at delivery.
Results
Table 14 shows the results of the treatment-discordant design, as applied to women’s primary care outcomes. It is evident that owing to the smaller cell counts these estimates were less precise in both the standard regression analyses on this subset of data and the fixed-effects analyses, reflecting the matched design. In the service use outcomes relating to antidepressant initiation, women who initiated an antidepressant consulted with their GP more frequently for depressive symptoms than when they received no treatment in another pregnancy. In the service use outcomes relating to antidepressant continuation, women who continued antidepressants in one pregnancy compared with discontinuing in another consulted with their GPs more frequently for any reason as well as for depression specifically and were more likely to be prescribed antidepressants at 2 years of follow-up.
| Standard negative binomial regression | Fixed-effects negative binomial regression | |||
|---|---|---|---|---|
| IRR/OR (95% CI) | p-value | IRR/OR (95% CI) | p-value | |
| Matched pregnancies discordant for initiation | ||||
| GP consultationsa | ||||
| During pregnancy | 1.03 (0.86 to 1.23) | 0.768 | 1.14 (0.92 to 1.41) | 0.225 |
| In 2 years following pregnancy | 1.11 (0.88 to 1.39) | 0.386 | 0.97 (0.79 to 1.19) | 0.758 |
| GP consultations for depressiona | ||||
| During pregnancy | 1.24 (0.77 to 1.98) | 0.374 | 1.17 (0.52 to 2.65) | 0.702 |
| In 2 years following pregnancy | 1.77 (1.02 to 3.07) | 0.042 | 2.05 (1.13 to 3.72) | 0.018 |
| Standard logistic regression | Fixed-effects logistic regression | |||
| Prescribed at end of follow-upb | 1.31 (0.38 to 4.58) | 0.670 | 1.13 (0.31 to 4.07) | 0.858 |
| Matched pregnancies discordant for continuationc | ||||
| GP consultationsa | ||||
| During pregnancy | 1.12 (1.06 to 1.18) | < 0.001 | 1.06 (1.00 to 1.12) | 0.046 |
| In 2 years following pregnancy | 1.09 (1.03 to 1.15) | 0.004 | 1.07 (1.01 to 1.13) | 0.023 |
| GP consultations for depressiona | ||||
| During pregnancy | 3.20 (2.32 to 4.41) | < 0.001 | 6.23 (4.01 to 9.66) | < 0.001 |
| In 2 years following pregnancy | 1.27 (1.14 to 1.43) | < 0.001 | 1.24 (1.08 to 1.43) | 0.002 |
| Standard logistic regression | Fixed-effects logistic regression | |||
| Prescribed at end of follow-upb | 1.81 (1.41 to 2.32) | < 0.001 | 1.82 (1.41 to 2.36) | < 0.001 |
Table 15 shows the results of the treatment-discordant design, as applied to the neurodevelopmental outcomes in offspring of women who continued antidepressants in one pregnancy but not the other. Similar analyses were not possible for discordance in relation to initiation of antidepressants owing to zero cell counts. The results suggest little evidence of an association between continuation of antidepressants and any of the neurodevelopmental outcomes, although the CIs were wide.
| Offspring neurodevelopmental outcome | Standard logistic regression,a OR (95% CI) | p-value | Fixed-effects logistic regression,b OR (95% CI) | p-value |
|---|---|---|---|---|
| Offspring autism | 1.13 (0.57 to 2.23) | 0.721 | 1.65 (0.66 to 4.12) | 0.288 |
| Offspring ADHD | 0.93 (0.49 to 1.76) | 0.832 | 0.89 (0.33 to 2.40) | 0.824 |
| Offspring ID | 0.57 (0.15 to 2.16) | 0.409 | 0.64 (0.16 to 2.50) | 0.522 |
Chapter 7 Negative control analyses
When a particular exposure and outcome are being investigated, a negative control approach is one that utilises an additional exposure or outcome that would be liable to the same sources of confounding or bias as the ones under investigation, but for which causal associations cannot be plausibly ascribed. 74,76 For this study, we chose as a negative control the prescription of antidepressants before but not during pregnancy, that is where it is likely that no gestational exposure to antidepressants had occurred. These analyses were, therefore, relevant for outcomes only for which the timing of prescription within pregnancy could have potentially influenced risk (i.e. for offspring neurodevelopmental outcomes). If antidepressants prescribed before but not during the pregnancy period are associated with later risk of neurodevelopmental problems in offspring, these associations are unlikely to be attributable to the effects of the medications but would suggest confounding by other characteristics.
Methods
Definition of the treatment variable and its negative control
As a negative control, we identified all women who had discontinued an antidepressant at least 3 months prior to becoming pregnant, comparing them with a reference group that had not been prescribed antidepressants at all. To contrast the effect of the negative control with that of the actual treatment, we combined women who had initiated or continued an antidepressant in pregnancy and compared them with the same reference group. Any woman who had been prescribed antidepressants solely during the grace period, as described in Chapter 2, was not considered for these analyses.
Outcome variables
These analyses were relevant only for outcomes for which the timing of prescription in relation to the pregnancy period could have potentially influenced risk. We, therefore, carried them out only for neurodevelopmental outcomes in offspring where exposure to an antidepressant in utero may be a potentially causal mechanism of any observed association (i.e. autism, ADHD and intellectual disability).
Statistical analysis
We used logistic regression to compare associations with outcomes for the actual treatment variable (prescription during pregnancy) with its negative control (prescription before but not during pregnancy). Associations were estimated using cluster robust variance estimators to recognise the presence of consecutive pregnancies to the same women. We adjusted all associations for the potential confounders described in Chapter 2, Covariates.
Results
We observed similar ORs for neurodevelopmental outcomes in children of women who were treated with antidepressants during pregnancy and for the negative control (i.e. children of women who were treated with antidepressants before but not during pregnancy) with overlapping CIs, which all included the null (Table 16). In these analyses, there was little evidence of an association of a prescription of an antidepressant for depression before or during pregnancy compared with no prescriptions and any of the neurodevelopmental outcomes, although CIs were wide.
| Offspring neurodevelopmental outcome | Actual exposure: prescribed during pregnancy vs. not at all prescribed | Negative control: prescribed before pregnancy vs. not at all prescribed | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Autism | 1.15 (0.88 to 1.51) | 0.318 | 1.06 (0.81 to 1.39) | 0.665 |
| ADHD | 1.27 (0.91 to 1.78) | 0.163 | 1.26 (0.91 to 1.74) | 0.166 |
| Intellectual disability | 1.07 (0.65 to 1.75) | 0.796 | 1.21 (0.75 to 1.96) | 0.434 |
Chapter 8 Variation by indication for antidepressant prescription
To investigate potential confounding by the indication, we compared associations where antidepressants had been prescribed for depression with associations where they had been prescribed for other indications. If there were causal associations between antidepressant use during pregnancy and risk of an adverse outcome, we would expect these associations to be similar irrespective of the indication the antidepressant was issued for. A different risk associated with antidepressants prescribed for depression than for other conditions will be suggestive of confounding by indication.
Methods
Pregnancies where antidepressants had not been issued for depression
To identify pregnancies during which antidepressants had been issued for other indications than depression, we selected those where prescription had occurred during the study period, but no current or past depressive symptoms were present in women’s medical records. Readers should note that the pregnancies identified here are the same as those set aside as described in Chapter 2, Study cohort selection.
Treatment variables
As described in Chapter 2, Design: observational cohorts emulating target randomised controlled trials, and elsewhere in this report, we considered associations with the following treatment variables: (1) initiating an antidepressant during pregnancy compared with receiving no antidepressant treatment during the study period, and (2) continuing an antidepressant into pregnancy compared with discontinuing the antidepressant.
Outcome variables
Women’s use of primary and secondary health-care care services
We combined all follow-up beyond the pregnancy end date into a single 2-year window and examined the following outcomes: (1) the number of days on which women had consulted with their GPs; (2) inpatient admission to specialist mental health services; (3) outpatient treatment for a mental health problem; and (4) frequency of A&E attendance. We also examined women’s antidepressant prescription status 2 years after the study pregnancy end date. For the purpose of these analyses, we did not consider use of primary or secondary care services in relation to specific indications (e.g. the number of days consulted with the GP for depressive symptoms) as women prescribed antidepressants for other indications than depression would, by definition, not have consulted for depressive symptoms during the study period.
Offspring neurodevelopmental outcomes
Neurodevelopmental outcomes in offspring included a diagnosis of (1) autism, (2) ADHD or (3) intellectual disability.
Statistical analysis
We used logistic regression models to estimate the relative odds of binary outcomes where antidepressants had been prescribed for depression or for other indications. Negative binomial regression models were used to estimate the relative incidence of count outcomes. Associations were estimated using cluster-robust variance estimators to allow for clustering owing to consecutive pregnancies to the same women. We statistically adjusted the identified associations for the potential confounders described in Chapter 2, Covariates. Differential length of follow-up was accounted for in our statistical models.
Results
Table 17 shows the results of women and offspring outcomes by variation by indication for which the antidepressants were initiated compared with no treatment. Table 18 shows the results of the same analysis for the continuation of antidepressants compared with discontinuation.
| Antidepressants issued for depression | Antidepressants issued for other indication | |||
|---|---|---|---|---|
| IRR/OR (95% CI) | p-value | IRR/OR (95% CI) | p-value | |
| Primary care outcomes | ||||
| GP consultationsa | ||||
| During pregnancy | 1.22 (1.18 to 1.26) | < 0.001 | 1.05 (0.97 to 1.14) | 0.193 |
| After pregnancy | 1.08 (1.05 to 1.12) | < 0.001 | 1.15 (1.06 to 1.24) | 0.001 |
| Prescribed at end of follow-upb | 1.88 (1.71 to 2.08) | < 0.001 | 0.66 (0.49 to 0.89) | 0.007 |
| Secondary care outcomes | ||||
| In-patient admissionb | ||||
| During pregnancy | 1.86 (0.63 to 5.56) | 0.264 | 2.53 (0.36 to 17.89) | 0.353 |
| After pregnancy | 2.12 (1.36 to 3.31) | 0.001 | 2.18 (0.78 to 6.07) | 0.136 |
| Outpatient treatmentb | ||||
| During pregnancy | 1.88 (1.38 to 2.55) | < 0.001 | 1.14 (0.41 to 3.13) | 0.806 |
| After pregnancy | 1.90 (1.51 to 2.37) | < 0.001 | 1.88 (1.07 to 3.29) | 0.027 |
| A&E attendancea | ||||
| During pregnancy | 1.15 (1.02 to 1.31) | 0.027 | 1.06 (0.66 to 1.73) | 0.803 |
| After pregnancy | 1.17 (1.06 to 1.30) | 0.002 | 1.67 (1.27 to 2.21) | < 0.001 |
| Offspring neurodevelopmental outcomes | ||||
| Autismb | 1.27 (0.90 to 1.80) | 0.180 | 0.97 (0.53 to 1.80) | 0.933 |
| ADHDb | 1.45 (0.97 to 2.17) | 0.068 | 1.42 (0.68 to 2.95) | 0.355 |
| Intellectual disabilityb | 1.31 (0.74 to 2.31) | 0.357 | 2.13 (0.96 to 4.69) | 0.061 |
| Antidepressants issued for depression | Antidepressants issued for other indication | |||
|---|---|---|---|---|
| IRR/OR (95% CI) | p-value | IRR/OR (95% CI) | p-value | |
| Primary care outcomes | ||||
| GP consultationsa | ||||
| During pregnancy | 1.01 (0.99 to 1.02) | 0.363 | 0.91 (0.87 to 0.94) | < 0.001 |
| After pregnancy | 0.94 (0.93 to 0.96) | < 0.001 | 1.01 (0.97 to 1.06) | 0.609 |
| Prescribed at end of follow-upb | 2.54 (2.41 to 2.68) | < 0.001 | 0.82 (0.71 to 0.95) | 0.007 |
| Secondary care outcomes | ||||
| Inpatient admissionb | ||||
| During pregnancy | 3.29 (1.46 to 7.41) | 0.004 | 5.92 (1.83 to 19.11) | 0.003 |
| After pregnancy | 1.53 (1.21 to 1.93) | < 0.001 | 1.28 (0.70 to 2.34) | 0.417 |
| Outpatient treatmentb | ||||
| During pregnancy | 2.99 (2.41 to 3.72) | < 0.001 | 1.69 (0.98 to 2.91) | 0.057 |
| After pregnancy | 1.76 (1.55 to 2.01) | < 0.001 | 1.02 (0.70 to 1.49) | 0.920 |
| A&E attendancea | ||||
| During pregnancy | 0.97 (0.91 to 1.04) | 0.398 | 1.20 (1.00 to 1.44) | 0.047 |
| After pregnancy | 0.98 (0.93 to 1.03) | 0.447 | 1.06 (0.92 to 1.22) | 0.388 |
| Offspring neurodevelopmental outcomes | ||||
| Autismb | 1.16 (0.95 to 1.41) | 0.146 | 1.09 (0.77 to 1.53) | 0.633 |
| ADHDb | 1.00 (0.80 to 1.25) | 0.977 | 1.32 (0.93 to 1.88) | 0.123 |
| Intellectual disabilityb | 0.86 (0.59 to 1.25) | 0.420 | 0.82 (0.43 to 1.57) | 0.554 |
In both sets of analyses, a higher risk of being prescribed antidepressants 2 years after pregnancy was observed in women who initiated or continued antidepressants for depressive symptoms than in women who did not initiate or discontinue antidepressants. By contrast, a lower risk of being prescribed antidepressants 2 years after pregnancy was observed in women who initiated or continued antidepressants for indications other than depression than in women who did not initiate or discontinue antidepressants.
There was also evidence of a higher risk of inpatient admission for a mental health problem after pregnancy when antidepressants had been initiated or continued for depressive symptoms, but not when issued for other indications, compared with women who did not initiate or discontinue.
For other associations, there was no strong evidence for confounding by the indication due to overlap in 95% CIs and inconsistencies in the direction of risk differences for different outcomes and comparisons.
Chapter 9 Additional analyses: timing of initiation, dose response, antidepressant class, serotonin receptor affinity and individual antidepressant drugs
We carried out the following additional analyses to investigate associations between antidepressants prescribed during pregnancy and offspring neurodevelopmental outcomes: (1) associations by timing of initiation within pregnancy; (2) associations for low, moderate and high doses of antidepressants; (3) associations for SSRIs, TCAs or other types of antidepressants; (4) associations for antidepressants with low, moderate and high affinity for the SERT; and (5) associations for individual antidepressant medications. We confined these analyses to offspring neurodevelopmental outcomes where it was plausible that the timing of exposure, dose level, type of antidepressant, SERT affinity or specific medication prescribed may have influenced the size of the associated risks. Except for analyses pertaining to the timing of initiation, we combined groups who had initiated or continued antidepressants and compared these with groups who did not initiate or discontinued antidepressants to comprehensively capture all prescribing during pregnancy.
Associations by timing of initiation of antidepressants in pregnancy
Methods
We limited these analyses to the timing of initiation of antidepressants in pregnancy because we could clearly identify the point at which women were first exposed to antidepressants during pregnancy. We coded a time-specific exposure variable to indicate exposure in the first trimester compared with prescriptions issued in the second or third trimesters (these latter time points had to be combined because of small cell counts). We then used logistic regression models with cluster-robust variances to estimate the relative odds of offspring autism, ADHD and intellectual disability associated with initiating an antidepressant in the first trimester, or in the second or third trimesters, compared with offspring born to women who did not initiate an antidepressant. In addition, to providing crude estimates, we adjusted the ORs for potential confounding variables (see Chapter 2, Covariates).
Results
Table 19 shows the results of the analysis estimating relative odds of offspring neurodevelopmental disorders by timing of initiation of antidepressants in pregnancy. Overall, associations between antidepressants during pregnancy and offspring odds of autism, ADHD or intellectual disability did not appear to vary with timing of initiation. We observed weak evidence for an association between first trimester initiation of an antidepressant and offspring ADHD, although a similar association was observed for second or third trimester initiation of an antidepressant with wide CIs.
| Initiated in the first trimester | Initiated in the second or third trimester | |||||||
|---|---|---|---|---|---|---|---|---|
| Crudea,b | p-value | Adjustedb,c | p-value | Crudea,b | p-value | Adjustedb,c | p-value | |
| Autism | 1.29 (0.87 to 1.91) | 0.205 | 1.32 (0.86 to 2.01) | 0.202 | 0.96 (0.55 to 1.70) | 0.895 | 1.05 (0.59 to 1.87) | 0.861 |
| ADHD | 1.85 (1.20 to 2.85) | 0.006 | 1.51 (0.96 to 2.40) | 0.077 | 1.40 (0.77 to 2.56) | 0.275 | 1.42 (0.76 to 2.65) | 0.268 |
| Intellectual disability | 1.17 (0.55 to 2.50) | 0.688 | 1.11 (0.55 to 2.27) | 0.768 | 1.46 (0.60 to 3.57) | 0.402 | 1.47 (0.59 to 3.65) | 0.410 |
Associations by dose level
Methods
We identified the generic drug category and daily dose in milligrams for each individual prescription of an antidepressant. Using this information, we calculated tertiles of distributions of daily doses in milligrams separately for each of 34 generic drug categories, and then combined this information in a single dose level variable (first tertile defined as low doses, second tertile as moderate doses and third tertile as high doses). In case of pregnancies during which prescriptions had been issued at different dose levels, we used the highest daily dose prescribed. Using logistic regression models with cluster-robust variances, we estimated relative odds of offspring neurodevelopmental disorders associated with being prescribed a low, moderate or high dose of antidepressants during pregnancy, compared with not having been prescribed antidepressants while pregnant. We provide both crude and statistically adjusted estimates.
Results
There was some evidence for a dose–response association between antidepressants prescribed to the mother during pregnancy and offspring odds of autism, although the CIs around estimates for low, moderate and high doses overlapped (Table 20). There was no clear evidence for dose–response association with offspring ADHD or intellectual disability.
| Offspring neurodevelopmental outcomes | Crude | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Autism | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed a low dose | 1.05 (0.86 to 1.27) | 0.660 | 1.19 (0.96 to 1.46) | 0.106 |
| Prescribed a moderate dose | 1.24 (0.86 to 1.79) | 0.242 | 1.67 (1.09 to 2.55) | 0.018 |
| Prescribed a high dose | 1.42 (1.06 to 1.90) | 0.019 | 1.75 (1.27 to 2.40) | 0.001 |
| ADHD | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed a low dose | 1.00 (0.80 to 1.26) | 0.982 | 1.06 (0.83 to 1.36) | 0.630 |
| Prescribed a moderate dose | 1.18 (0.77 to 1.81) | 0.441 | 1.21 (0.73 to 1.99) | 0.455 |
| Prescribed a high dose | 1.03 (0.71 to 1.49) | 0.868 | 1.21 (0.78 to 1.89) | 0.399 |
| Intellectual disability | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed a low dose | 0.77 (0.53 to 1.11) | 0.157 | 0.75 (0.50 to 1.12) | 0.159 |
| Prescribed a moderate dose | 0.98 (0.49 to 1.94) | 0.943 | 1.11 (0.47 to 2.58) | 0.817 |
| Prescribed a high dose | 0.96 (0.55 to 1.69) | 0.899 | 1.18 (0.60 to 2.31) | 0.640 |
Associations by type of antidepressant
Method
We categorised individual antidepressants prescribed during pregnancy into the following groups: (1) SSRIs included prescriptions for citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine and sertraline; (2) TCAs included prescriptions for amitriptyline, clomipramine, dosulepin, doxepin, imipramine, lofepramine, mianserin, nortriptyline and trimipramine; and (3) ‘other’ antidepressants included agomelatine, duloxetine, isocarboxazid, mirtazapine, moclobemide, nefazodone, trazodone, phenelzine, reboxetine and venlafaxine. In cases where women had been prescribed different types of antidepressants during pregnancy, they counted independently towards analyses for each type (e.g. pregnancies where a SSRI and TCA had been prescribed were considered in analyses of SSRIs as well as in analyses of TCAs). We used logistic regression models with cluster-robust variances to estimate relative odds of offspring neurodevelopmental disorders associated with being prescribed a SSRI, a TCA, or an ‘other’ antidepressant during pregnancy, compared with not having been prescribed antidepressants while pregnant. We provide both crude and statistically adjusted estimates.
Results
The results of the analyses comparing no antidepressant prescription for depression in pregnancy with prescription of antidepressants grouped into SSRIs, TCAs and other antidepressants are provided in Table 21. We observed greater adjusted odds of autism among children whose mothers had been prescribed SSRIs (OR 1.26, 95% CI 1.04 to 1.53) or TCAs (OR 1.58, 95% CI 1.12 to 2.24) during pregnancy, although CIs for other antidepressants were wider, probably reflecting smaller numbers. There was little evidence for association between the type of antidepressant issued during pregnancy and later risk of ADHD or intellectual disability in resulting offspring.
| Neurodevelopmental outcome by prescription status | Crude | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI)1 | p-value | |
| Autism | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed SSRI | 1.15 (0.95 to 1.38) | 0.145 | 1.26 (1.04 to 1.53) | 0.018 |
| Prescribed TCA | 1.32 (0.96 to 1.83) | 0.089 | 1.58 (1.12 to 2.24) | 0.009 |
| Prescribed other antidepressant | 0.95 (0.55 to 1.63) | 0.852 | 1.25 (0.69 to 2.28) | 0.456 |
| ADHD | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed SSRI | 0.99 (0.79 to 1.23) | 0.916 | 1.09 (0.86 to 1.39) | 0.471 |
| Prescribed TCA | 1.38 (0.96 to 1.98) | 0.083 | 1.24 (0.83 to 1.85) | 0.302 |
| Prescribed other antidepressant | 1.07 (0.59 to 1.92) | 0.827 | 1.11 (0.57 to 2.16) | 0.768 |
| Intellectual disability | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed SSRI | 0.78 (0.55 to 1.10) | 0.160 | 0.82 (0.56 to 1.21) | 0.328 |
| Prescribed TCA | 1.04 (0.57 to 1.90) | 0.906 | 0.90 (0.46 to 1.77) | 0.763 |
| Prescribed other antidepressant | 1.18 (0.51 to 2.70) | 0.701 | 1.29 (0.48 to 3.46) | 0.616 |
Associations by serotonin transporter binding affinity of antidepressants
Methods
We identified prescriptions where antidepressants with low, moderate or high SERT affinity had been issued during pregnancy: (1) low-affinity medications included desipramine, nortriptyline, amoxapine, doxepin, trimipramine, trazodone, nefazodone and mirtazapine; (2) moderate-affinity medications included citalopram, imipramine, fluvoxamine, amitriptyline and venlafaxine; and (3) high-affinity medications included escitalopram, fluoxetine, paroxetine, sertraline, duloxetine and clomipramine. These groupings were based on previous work on this topic46,50 but it is important to note that the empirical evidence behind these remains limited and, therefore, results should be viewed with caution. Where women were prescribed antidepressants with different affinities, they were counted independently in each analysis. We used logistic regression models with cluster-robust variances to estimate relative odds associated with being prescribed a low, moderate or high SERT affinity medication compared with not having been prescribed antidepressants during pregnancy, providing both crude and statistically adjusted estimates.
Results
The point estimates of offspring odds of all neurodevelopmental outcomes were lower for higher-affinity antidepressants than those for lower-affinity antidepressants, although the CIs for all estimates overlapped (Table 22). There were increased odds of autism among children whose mothers had been prescribed moderate-affinity antidepressants (OR 1.50, 95% CI 1.16 to 1.94), and increased odds of ADHD among children whose mothers had been prescribed low-affinity antidepressants (OR 1.96, 95% CI 1.06 to 3.64), compared with children whose mothers had not been prescribed antidepressants during pregnancy. It should be noted that low- or moderate-affinity antidepressants are generally used for the treatment of more severe depression; therefore, these associations may be consistent with residual confounding by the severity of depression.
| Neurodevelopmental outcome by prescription status | Crude | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Autism | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed low-affinity antidepressant | 1.01 (0.56 to 1.80) | 0.984 | 1.46 (0.78 to 2.74) | 0.239 |
| Prescribed moderate-affinity antidepressant | 1.18 (0.95 to 1.48) | 0.140 | 1.50 (1.16 to 1.94) | 0.002 |
| Prescribed high-affinity antidepressant | 1.11 (0.90 to 1.37) | 0.323 | 1.18 (0.95 to 1.46) | 0.126 |
| ADHD | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed low-affinity antidepressant | 1.77 (1.06 to 2.97) | 0.030 | 1.96 (1.06 to 3.64) | 0.032 |
| Prescribed moderate-affinity antidepressant | 0.88 (0.67 to 1.17) | 0.384 | 1.05 (0.75 to 1.48) | 0.772 |
| Prescribed high-affinity antidepressant | 1.10 (0.87 to 1.40) | 0.422 | 1.14 (0.88 to 1.47) | 0.328 |
| Intellectual disability | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Prescribed low-affinity antidepressant | 1.21 (0.49 to 2.99) | 0.678 | 1.66 (0.54 to 5.15) | 0.376 |
| Prescribed moderate-affinity antidepressant | 0.61 (0.38 to 0.98) | 0.042 | 0.69 (0.39 to 1.22) | 0.203 |
| Prescribed high-affinity antidepressant | 0.91 (0.63 to 1.32) | 0.607 | 0.85 (0.57 to 1.28) | 0.443 |
Associations for individual antidepressant medications
Methods
Analyses of individual medications were limited by small cell counts. We will, therefore, report results only for which we had at least five observations within each cell of bivariate tables of exposure and outcome variables. For example, we required at least five instances in which women had been prescribed citalopram while pregnant with linked offspring who were later diagnosed with autism to report this result. Where women had been prescribed multiple medications during pregnancy, they counted independently towards analyses of each medication. We used logistic regression models with cluster robust variances to estimate associations with specific antidepressant medications, providing both crude and adjusted relative risk and odds estimates.
Results
The results of the associations of individual medications with neurodevelopmental outcomes are presented in Table 23. There were some variations in the outcomes in relation to individual medications. The adjusted odds of offspring autism were greater when mothers had been prescribed amitriptyline, citalopram, lofepramine or paroxetine during pregnancy. There was weak evidence that the odds of offspring ADHD were increased among mothers who had been prescribed amitriptyline or mirtazapine while pregnant, but little evidence for increased odds of intellectual disability with prescription of any individual antidepressant medications during pregnancy. The CIs of all these results are wider owing to smaller numbers contributing to the analyses.
| Neurodevelopmental outcome by prescription status | Crude | Adjustedb | ||
|---|---|---|---|---|
| OR (95% CI)a | p | OR (95% CI)a | p | |
| Autism | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Amitriptyline | 1.62 (1.08 to 2.44) | 0.020 | 2.02 (1.32 to 3.11) | 0.001 |
| Citalopram | 1.13 (0.87 to 1.46) | 0.354 | 1.57 (1.17 to 2.11) | 0.003 |
| Dosulepin | 1.14 (0.56 to 2.32) | 0.712 | 1.53 (0.73 to 3.20) | 0.262 |
| Escitalopram | 0.94 (0.46 to 1.91) | 0.867 | 0.93 (0.46 to 1.89) | 0.845 |
| Fluoxetine | 1.14 (0.88 to 1.46) | 0.318 | 1.16 (0.90 to 1.49) | 0.239 |
| Lofepramine | 1.98 (1.01 to 3.89) | 0.046 | 2.52 (1.23 to 5.17) | 0.012 |
| Mirtazapine | 0.93 (0.46 to 1.89) | 0.843 | 1.45 (0.67 to 3.14) | 0.346 |
| Paroxetine | 1.66 (1.09 to 2.50) | 0.017 | 2.04 (1.31 to 3.16) | 0.001 |
| Sertraline | 0.86 (0.58 to 1.27) | 0.453 | 1.37 (0.88 to 2.14) | 0.159 |
| Venlafaxine | 1.10 (0.64 to 1.90) | 0.726 | 1.46 (0.78 to 2.73) | 0.235 |
| ADHD | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Amitriptyline | 1.46 (0.90 to 2.38) | 0.125 | 1.74 (1.00 to 3.03) | 0.050 |
| Citalopram | 0.71 (0.50 to 1.00) | 0.050 | 0.93 (0.61 to 1.40) | 0.713 |
| Escitalopram | 1.24 (0.61 to 2.52) | 0.558 | 1.11 (0.52 to 2.38) | 0.783 |
| Fluoxetine | 1.13 (0.85 to 1.51) | 0.397 | 1.12 (0.82 to 1.51) | 0.480 |
| Lofepramine | 2.31 (1.13 to 4.73) | 0.022 | 1.74 (0.82 to 3.67) | 0.148 |
| Mirtazapine | 1.69 (0.92 to 3.13) | 0.094 | 2.03 (0.99 to 4.16) | 0.054 |
| Paroxetine | 1.29 (0.76 to 2.19) | 0.344 | 1.06 (0.61 to 1.84) | 0.847 |
| Sertraline | 0.80 (0.51 to 1.28) | 0.356 | 1.20 (0.71 to 2.04) | 0.497 |
| Venlafaxine | 1.13 (0.61 to 2.09) | 0.688 | 0.97 (0.48 to 1.96) | 0.927 |
| Intellectual disability | ||||
| Not prescribed | 1.00 | 1.00 | ||
| Amitriptyline | 0.89 (0.36 to 2.20) | 0.803 | 1.00 (0.39 to 2.58) | 0.999 |
| Citalopram | 0.48 (0.26 to 0.88) | 0.017 | 0.59 (0.29 to 1.19) | 0.140 |
| Fluoxetine | 1.01 (0.66 to 1.56) | 0.956 | 0.93 (0.58 to 1.47) | 0.741 |
| Lofepramine | 3.17 (1.28 to 7.85) | 0.013 | 1.88 (0.72 to 4.86) | 0.195 |
| Paroxetine | 1.14 (0.50 to 2.60) | 0.764 | 0.77 (0.32 to 1.85) | 0.554 |
| Sertraline | 0.44 (0.18 to 1.09) | 0.077 | 0.61 (0.23 to 1.58) | 0.304 |
| Venlafaxine | 1.14 (0.46 to 2.80) | 0.783 | 0.99 (0.33 to 2.93) | 0.984 |
Chapter 10 Triangulation of results and discussion
Maternal outcomes: triangulation of results for initiating or continuing an antidepressant during pregnancy
Table 24 summarises the evidence from the various analyses on maternal outcomes in relation to initiation compared with no initiation of antidepressants during pregnancy, and Table 25 provides the summary of evidence of these outcomes for the analyses for continuation compared with discontinuation of antidepressants for depression in pregnancy.
| Maternal outcome | Analytical approach | |||
|---|---|---|---|---|
| Multivariable regression | Propensity score-matched regression | Treatment-discordant pregnancies | Variation by indication for antidepressants | |
| GP consultations | Greater frequency among initiators | Greater frequency among initiators | Little evidence for greater frequency (null association) | More frequent when issued for depression |
| GP consultations for depression | Greater frequency among initiators | Greater frequency among initiators | Greater frequency among initiators | N/A |
| GP consultations for self-harm | Null association or greater risk among initiators | Null association or greater risk among initiators | N/A: insufficient numbers | N/A |
| GP referral for depression | Some evidence for fewer referrals in pregnancy but null or increased risk thereafter | Stronger evidence for fewer referrals during pregnancy but null or increased risk thereafter | N/A: insufficient numbers | N/A |
| Prescription status at end of follow-up | Initiators more likely prescribed at 2 years of follow-up | Initiators more likely prescribed at 2 years follow-up | Null associations | Initiators for depression more likely to be prescribed at 2 years of follow-up, less likely for ‘other’ indications |
| Inpatient admission for MH | Little evidence of association or greater risk among initiators | Little evidence of association | N/A: insufficient numbers | Little evidence for difference between prescribing for depression and other indications (overlapping 95% CIs) |
| Outpatient treatment for MH | Greater risk among initiators | Greater risk among initiators | N/A: insufficient numbers | Little evidence for difference between prescribing for depression and other indications (overlapping 95% CIs) |
| A&E attendance | Little evidence of association or greater frequency among initiators | Little evidence of association | N/A | Weak evidence for more frequent A&E attendance for ‘other’ indications |
| Maternal outcome | Analytical approach | |||
|---|---|---|---|---|
| Multivariable regression | Propensity score-matched regression | Treatment-discordant pregnancies | Variation by indication for antidepressants | |
| GP consultations | Lower frequency among those who continued | Little evidence of associations or increased frequency | Greater frequency with continuation in fixed-effects model | Lower frequency in pregnancy when prescribed for other indications, lower frequency after pregnancy when prescribed for depression |
| GP consultations for depression | Greater frequency among those who continued | Greater frequency among those who continued | Greater frequency with continuation in fixed-effects model | N/A: analysis not appropriate for outcome |
| GP consultations for self-harm | Null association or greater risk among those who continued | Null association or greater risk among those who continued | Insufficient numbers during pregnancy, greater risk thereafter | N/A: analysis not appropriate for outcome |
| GP referral for depression | Null association or greater risk among those who continued | Null association or greater risk among those who continued | Insufficient numbers during pregnancy, null association thereafter | N/A: analysis not appropriate for outcome |
| Prescription status at end of follow-up | Continuers more likely prescribed at 2 years of follow-up | Continuers more likely prescribed at 2 years of follow-up | Continuers more likely prescribed at 2 years of follow-up | Continuers for depression more likely to be prescribed at 2 years of follow-up if depression, less likely for other indications |
| In-patient admission for MH | Null association or greater risk among continuers | Null association or greater risk among continuers | Insufficient numbers during pregnancy, null association thereafter | Little evidence for confounding by indication (overlapping 95% CIs) |
| Out-patient treatment for MH | Greater risk among those who continued | Greater risk among those who continued | Weak evidence of greater risk in fixed-effects models | Weak evidence for greater risk if prescribed for depression |
| A&E attendance | Little evidence for association | Weak evidence for lower attendance at 1 year after pregnancy, otherwise little evidence for associations | Weak evidence of lower attendance during pregnancy in fixed-effects model | More frequent during pregnancy if prescribed for indications other than depression |
There was consistent evidence across the main (multivariable regression and propensity score regression) and additional analyses that women who initiated or continued antidepressants during pregnancy were more likely to have contact with health-care services at various times during and after pregnancy. These include the number of GP consultations (including consultations for depression, and self-harm where there were sufficient numbers available in analyses), GP referrals for depression and outpatient contacts and inpatient stays for mental health problems. Women who initiated or continued antidepressants in pregnancy were also more likely to continue to be prescribed an antidepressant 2 years following the end of pregnancy.
Child neurodevelopmental outcomes: triangulation of results for initiating or continuing an antidepressant during pregnancy
Table 26 summarises the evidence from the various analyses on child neurodevelopmental outcomes in relation to the mother’s initiation compared with no initiation of antidepressants during pregnancy, and Table 27 provides the summary of the evidence from analyses of these outcomes for continuation compared with discontinuation of antidepressants for depression in pregnancy.
| Child outcome | Analytical approach | |||||
|---|---|---|---|---|---|---|
| Multivariable regression | Propensity score-matched regression | Instrumental variable analysis | Treatment-discordant pregnancies | Negative control analysis | Variation by indication for antidepressants | |
| Offspring autism | Little evidence for greater risk | Evidence for greater risk among initiators | Little evidence for greater risk | N/A: insufficient numbers | Little evidence for unmeasured confounding (both associations null) | Little evidence for confounding by indication (both associations null) |
| Offspring ADHD | Weak evidence for greater risk among initiators | Little evidence for greater risk although point estimates consistent with multivariable regression | Little evidence for greater risk | N/A: insufficient numbers | Little evidence for unmeasured confounding (both associations null) | Little evidence for confounding by indication (overlapping 95% CIs) |
| Offspring intellectual disability | Little evidence for greater risk | Little evidence for greater risk | Little evidence for greater risk | N/A: insufficient numbers | Little evidence for unmeasured confounding (both associations null) | Little evidence for confounding by indication (overlapping 95% CIs) |
| Child outcome | Analytical approach | |||||
|---|---|---|---|---|---|---|
| Multivariable regression | Propensity score-matched regression | Instrumental variable analysis | Treatment-discordant pregnancies | Negative control analysis | Variation by indication for antidepressants | |
| Offspring autism | Little evidence for greater risk | Little evidence for greater risk | Little evidence for greater risk | Little evidence for greater risk | Little evidence of association with antidepressants prescribed during or before pregnancy | Little evidence for association with antidepressants prescribed for depression or other conditions |
| Offspring ADHD | Little evidence for greater risk | Little evidence for greater risk | Little evidence for greater risk | Little evidence for greater risk | Little evidence of association with antidepressants prescribed during or before pregnancy | Little evidence for association with antidepressants prescribed for depression or other conditions |
| Offspring intellectual disability | Little evidence for greater risk | Little evidence for greater risk | Little evidence for greater risk | Little evidence for greater risk | Little evidence of association with antidepressants prescribed during or before pregnancy | Little evidence for association with antidepressants prescribed for depression or other conditions |
There was consistent evidence that continuation of antidepressants into pregnancy was not associated with a higher risk of autism, ADHD or intellectual disability compared with discontinuing them before pregnancy in our main and supplementary analyses. The evidence was less consistent for the analyses on initiation compared with no initiation of antidepressants during pregnancy, and the lack of precision owing to smaller number and wider CIs was a disadvantage. For autism, propensity score-matched analyses showed some evidence of an association for women who initiated an antidepressant compared with those who did not initiate an antidepressant in pregnancy, and the CIs of other analyses were wide; therefore, we were unable to rule out an association with certainty. There was also evidence for stronger associations for higher doses of antidepressants prescribed, although this may reflect the severity of underlying depression. There was also some evidence of higher risk with antidepressants that have low and moderate SERT affinity than those with high SERT affinity antidepressants. High SERT affinity antidepressants are typically first-line antidepressants, which may be prescribed for milder forms of depression, although these groupings may not have strong empirical support so should be considered with caution. There was weak evidence in terms of higher point estimates for first trimester compared with later initiation, although the CIs were wide. Finally, there was variation by type of antidepressant, with higher risk estimates with tricyclics than SSRIs, and variation of risk estimates within individual antidepressants. All of these latter analyses have to be interpreted with caution because of the lack of statistical power and further work on larger samples will be able to address this limitation.
There was also weak evidence of an association between prescribing variation in the results for ADHD risks in relation to initiation or no initiation of antidepressants in pregnancy in the regression analysis with similarly raised point estimates in propensity score analysis but wide CIs crossing the null. There was also some variation for these in additional analyses although all suffered from low statistical power.
There was little evidence of any increase in risk of intellectual disability for either initiation or continuation of antidepressants in pregnancy consistently across the main and additional analyses, although CIs were wide in all cases.
Strengths
This study had a number of strengths. It was based on a large primary care sample in the UK that is broadly representative of the UK population. Prospectively recorded data were recorded from medical records minimising the possibility of recall bias.
The study benefited from valuable input from our experienced PPI co-leads and the PPI group comprising women with lived experience of perinatal depression. We received input on our plans and results throughout the life of the study, including important input on issues related to our research questions, the interpretation of results and how they might be perceived, and ongoing help in relation to meaningful dissemination of the findings.
To our knowledge, this is the first study on this topic to conceptualise the research question in terms of a clinical trial, with an attempt to emulate two distinct clinical scenarios, that is the decision to initiate or not initiate an antidepressant during pregnancy or the decision to continue or not continue and antidepressant during pregnancy; therefore, the results may support clinical decision-making for these distinct scenarios. Furthermore, our treatment groups comprised women with an underlying history of depression, that is women who would be potentially eligible for RCTs of initiation or continuation of antidepressants in pregnancy. Therefore, we attempted like-with-like comparisons as would be undertaken in randomised trials. This is particularly important where the risk of an outcome, for example offspring autism, is likely to be elevated in groups of women with the underlying indication of prescribing antidepressants. However, previous studies have included either general population comparison groups or less-specific comparison groups, such as women with a history of a mental illness but not specifically depression.
Another important strength of this study is the primary care setting. Although depression is overwhelmingly managed in primary care, most previous studies have had diagnostic data only for the underlying reason for antidepressant prescribing from secondary care samples. This would have underascertained depression in previous studies and thus compounded the potential problem of confounding by indication, a key issue raised in almost all previous studies.
The base sample we had was large for both initiation compared with no initiation and continuation compared with discontinuation of antidepressants. However, due to the offspring outcomes being relatively rare, there was still a problem with statistical power, most apparent in outcomes related to initiation compared with no initiation of antidepressants in pregnancy, particularly in causal inference approaches applied within smaller subsets. This can be ameliorated in future studies using CPRD, as the sample size with research quality data is continuously increasing. This issue of power also highlights why it is unlikely that it will be feasible to have RCT evidence to study such long-term offspring outcomes. Even if ethically and logistically permissible, such RCTs will require the recruitment of very large samples of pregnant women with several years of post-pregnancy follow-up.
A major strength of this study is the use of a range of causal inference methods, all of which have their own strengths and limitations. This project could be a template of how studies of medication use during pregnancy may make use of such methods to triangulate the results for better understanding of any potential causal mechanisms. However, a limitation for some of these approaches was the lack of statistical power, leading to wide CIs specially in the investigation of initiation of antidepressants and child neurodevelopmental outcomes.
Limitations
Several limitations of this study should be considered.
First, although we have noted the key strength of using primary care data to ascertain depression more completely, the possibility of measurement error in depression should be acknowledged. The terms we used to define depression included symptom codes such as ‘low mood’. This was because it is well established that UK GPs have been increasingly making use of symptom codes as opposed to diagnostic codes since the introduction of the Quality Outcomes Framework even when antidepressants are being prescribed. 77 Therefore, CPRD studies ascertaining depression solely through Read codes for a diagnosis of depression are likely to have low sensitivity.
Second, this study, like others on this topic, used prescription data and, therefore, it is not possible to comment on the adherence to the treatment prescribed.
Third, although we used a number of causal methods, several of the analyses which may have been more informative regarding causal estimates (e.g. the IV analysis), lacked precision because of small numbers.
Fourth, we could define outcomes only based on their presence in the medical records, and consultations typically record problems and diagnoses than measures of improvement. This was problematic in studying women’s outcomes for whom we were interested in studying potential benefits of antidepressant prescribing. We concluded that several of the outcomes that we studied, for example number of GP consultations following prescription of antidepressants, were intrinsically linked to the exposure (prescribing) as doctors would routinely follow-up patients who they prescribe medications to. For this reason, medical record data to study measures of effectiveness or improvement are limited in the absence of robust outcome measures routinely recorded in medical records.
Finally, we studied a range of maternal outcomes and long-term neurodevelopmental outcomes. However, there may be outcomes that do not fit into specific diagnostic categories we used. The decision regarding benefits or harms of medications is more complex and there may be other outcomes that are important to individuals who are considering these decisions.
Clinical implications
The most common clinical scenario in relation to antidepressant prescribing in pregnancy is of women who need to decide whether to continue or discontinue their antidepressants when planning pregnancy or after discovering they are pregnant. Women who continued antidepressants in pregnancy in this study had more severe depression and continued to need antidepressants for a longer period, received more frequent input from primary care and greater frequency of referrals to secondary mental health care. In this group of women, there was no increase in risk of offspring autism, ADHD or intellectual disability compared with women who discontinued antidepressant treatment.
Fewer women in the population needed to initiate antidepressants during pregnancy. Our study found that these women too had greater clinical need, were followed up more frequently in primary care, were more likely to be referred to secondary mental health care services and be prescribed antidepressants at 2-year follow-up. There was no strong evidence suggestive of risks of offspring ADHD or intellectual disability but a potential association with offspring autism would need further investigation, although there is a possibility that this is finding was observed due to chance or residual confounding.
The findings of this research may help clinicians and women make decisions; however, prescribing during pregnancy should always be a decision based on the clinical presentation and individual preferences after taking into account a broader range of factors than this study investigated.
Research implications
The CPRD is a powerful resource for perinatal pharmacoepidemiology. As the database is continually updated, a follow-up study on this topic would provide larger numbers and more precision in the results in relation to the outcomes of initiation of antidepressants during pregnancy, as well as longer period of follow-up.
Collection of standard outcome measures of depression in CPRD practices could allow for more robust assessment of effectiveness of antidepressants.
This study found variation of the relative risks for the neurodevelopmental outcomes by different antidepressants. As larger data sets become available, this information may be useful to understand outcomes of individual medications with more precision.
Our PPI work highlighted that there may be a wider set of outcomes (e.g. pregnancy loss or cardiac anomalies in offspring) that may be of interest to women in the decision-making process. These could be addressed in future work.
The methods used herein could be used as a template for pharmaco-epidemiological studies of other medications during pregnancy and provide an efficient approach towards clinical guidance in the absence of randomised trial evidence.
Acknowledgements
We thank our public contributors who attended the PPI groups facilitated by Maria Viner and Claire Storey for their valuable input on this project. We thank members of our clinical advisory group and members of the IMPROVE Perinatal Mental Health Integration team for their feedback. We thank Mrs Ruth Jackson, Chief Executive Officer of Bluebell Care Trust, for useful comments and feedback on our research plans during the funding application stage.
This research was also supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Contributions of authors
Hein Heuvelman (https://orcid.org/0000-0002-4662-7533) (Senior Research Associate, University of Bristol) conducted statistical analysis, interpretation of results and drafted the report.
Neil M Davies (https://orcid.org/0000-0002-2460-0508) (Senior Research Fellow, University of Bristol) supported design, supervised statistical analysis, interpretation of results and revision of the report.
Yoav Ben-Shlomo (https://orcid.org/0000-0001-6648-3007) (Professor of Epidemiology, University of Bristol) provided epidemiological expertise, interpretation of results and revision of the report.
Alan Emond (https://orcid.org/0000-0001-8029-2987) (Professor of Child Health, University of Bristol) supported design, provided child health expertise, interpretation of results and revision of the report.
Jonathan Evans (https://orcid.org/0000-0003-3171-640X) (Consultant Senior Lecturer in Psychiatry, University of Bristol) provided expertise in perinatal epidemiology and psychiatry, interpretation of results, revision of report.
David Gunnell (https://orcid.org/0000-0002-0829-6470) (Professor of Public Health, University of Bristol), provided expertise in epidemiology, interpretation of results, revision of report.
Rachel Liebling (https://orcid.org/0000-0003-3448-5918) (Consultant Obstetrician), provided expertise in obstetrics, interpretation of results, revision of report.
Richard Morris (https://orcid.org/0000-0001-7240-4563) (Professor in Medical Statistics, University of Bristol), provided statistical supervision, interpretation of results, revision of report.
Rupert Payne (https://orcid.org/0000-0002-5842-4645) (Consultant senior lecturer in primary care, University of Bristol), provided expertise in CPRD and primary care, interpretation of results, revision of report.
Claire Storey (https://orcid.org/0000-0002-5428-9909) (Chief Executive Officer, Mothers for Mothers), co-lead for patient and public involvement, interpretation of results, revision of report.
Maria Viner (https://orcid.org/0000-0001-8269-4824) (Mothers for Mothers), co-lead for patient and public involvement, interpretation of results, revision of report.
Dheeraj Rai (https://orcid.org/0000-0002-7239-3523) (Consultant senior lecturer in psychiatry, University of Bristol), supervised project, provided expertise in perinatal epidemiology, psychiatry and neurodevelopmental conditions, interpretation of results, drafted report.
Data-sharing statement
The analytic code for this study can be requested from the corresponding author. The CPRD data cannot be shared due to licencing agreements.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 2016;387:1672-85. https://doi.org/10.1016/S0140-6736(15)00390-6.
- Iacobucci G. NHS prescribed record number of antidepressants last year. BMJ 2019;364. https://doi.org/10.1136/bmj.l1508.
- Moore M, Yuen HM, Dunn N, Mullee MA, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ 2009;339. https://doi.org/10.1136/bmj.b3999.
- Mental health and wellbeing in England: Adult Psychiatric Morbidity Survey 2014. Leeds; 2016.
- Howard LM, Molyneaux E, Dennis CL, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet 2014;384:1775-88. https://doi.org/10.1016/S0140-6736(14)61276-9.
- Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med 2011;365:1605-11.
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 2005;106:1071-83.
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet 2014;384:1800-19. https://doi.org/10.1016/S0140-6736(14)61277-0.
- National Institute for Health and Care Excellence (NICE) . Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance. Clinical Guideline [CG192] 2014. www.nice.org.uk/guidance/cg192 (accessed 1 February 2022).
- Charlton RA, Jordan S, Pierini A, Garne E, Neville AJ, Hansen AV, et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. BJOG 2015;122:1010-20. https://doi.org/10.1111/1471-0528.13143.
- Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry 2020;19:313-27. https://doi.org/10.1002/wps.20769.
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357-66.
- McDonagh MS, Matthews A, Phillipi C, Romm J, Peterson K, Thakurta S, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol 2014;124:526-34. https://doi.org/10.1097/AOG.0000000000000410.
- Dawson AL, Ailes EC, Gilboa SM, Simeone RM, Lind JN, Farr SL, et al. Antidepressant prescription claims among reproductive-aged women with private employer-sponsored insurance – United States 2008-2013. MMWR Morb Mortal Wkly Rep 2016;65:41-6. https://doi.org/10.15585/mmwr.mm6503a1.
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295:499-507.
- Bérard A, Sheehy O, Zhao JP, Chambers C, Roth M, Bozzo P, et al. Impact of antidepressant use, discontinuation, and dosage modification on maternal depression during pregnancy. Eur Neuropsychopharmacol 2019;29:803-12.
- Swanson SA, Hernandez-Diaz S, Palmsten K, Mogun H, Olfson M, Huybrechts KF. Methodological considerations in assessing the effectiveness of antidepressant medication continuation during pregnancy using administrative data. Pharmacoepidemiol Drug Saf 2015;24:934-42. https://doi.org/10.1002/pds.3798.
- Yonkers KA, Gotman N, Smith MV, Forray A, Belanger K, Brunetto WL, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy?. Epidemiology 2011;22:848-54.
- Molenaar NM, Brouwer ME, Bockting CL, Bonsel GJ, van der Veere CN, Torij HW, et al. Stop or go? Preventive cognitive therapy with guided tapering of antidepressants during pregnancy: study protocol of a pragmatic multicentre non-inferiority randomized controlled trial. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-0752-6.
- Molenaar NM, Brouwer ME, Burger H, Kamperman AM, Bergink V, Hoogendijk WJG, et al. Preventive cognitive therapy with antidepressant discontinuation during pregnancy: results from a randomized controlled trial. J Clin Psychiatry 2020;81. https://doi.org/10.4088/JCP.19l13099.
- Wang S, Yang L, Wang L, Gao L, Xu B, Xiong Y. Selective Serotonin Reuptake Inhibitors (SSRIs) and the risk of congenital heart defects: a meta-analysis of prospective cohort studies. J Am Heart Assoc 2015;4. https://doi.org/10.1161/JAHA.114.001681.
- Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014;370:2397-407. https://doi.org/10.1056/NEJMoa1312828.
- Grigoriadis S, Vonderporten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. BMJ 2014;348. https://doi.org/10.1136/bmj.f6932.
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 2009;10:303-12. https://doi.org/10.1038/nrn2598.
- Taylor C, Fricker AD, Devi LA, Gomes I. Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal 2005;17:549-57.
- Lauder JM, Wallace JA, Krebs H. Roles for serotonin in neuroembryogenesis. Adv Exp Med Biol 1981;133:477-506. https://doi.org/10.1007/978-1-4684-3860-4_28.
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 2004;306:879-81.
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci 2007;10:588-97.
- Darling RD, Alzghoul L, Zhang J, Khatri N, Paul IA, Simpson KL, et al. Perinatal citalopram exposure selectively increases locus ceruleus circuit function in male rats. J Neurosci 2011;31:16709-15. https://doi.org/10.1523/JNEUROSCI.3736-11.2011.
- Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience 2016;321:24-41.
- Rodriguez-Porcel F, Green D, Khatri N, Harris SS, May WL, Lin RC, et al. Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anat Rec 2011;294:1726-35. https://doi.org/10.1002/ar.21402.
- Wood ME, Lapane KL, van Gelder MMHJ, Rai D, Nordeng HME. Making fair comparisons in pregnancy medication safety studies: an overview of advanced methods for confounding control. Pharmacoepidemiol Drug Saf 2018;27:140-7. https://doi.org/10.1002/pds.4336.
- Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr 2016;170:117-24. https://doi.org/10.1001/jamapediatrics.2015.3356.
- Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, Vigod SN. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA 2017;317:1544-52. https://doi.org/10.1001/jama.2017.3415.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011;68:1104-12. https://doi.org/10.1001/archgenpsychiatry.2011.73.
- El Marroun H, White TJ, van der Knaap NJ, Homberg JR, Fernández G, Schoemaker NK, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry 2014;205:95-102. https://doi.org/10.1192/bjp.bp.113.127746.
- Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord 2014;44:2558-67. https://doi.org/10.1007/s10803-014-2128-4.
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics 2014;133:e1241-8. https://doi.org/10.1542/peds.2013-3406.
- Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013;369:2406-15. https://doi.org/10.1056/NEJMoa1301449.
- Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol 2016;66:31-43.
- Liu X, Agerbo E, Ingstrup KG, Musliner K, Meltzer-Brody S, Bergink V, et al. Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. BMJ 2017;358. https://doi.org/10.1136/bmj.j3668.
- Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016;55:359-66. https://doi.org/10.1016/j.jaac.2016.02.013.
- Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev 2015;49:82-9. https://doi.org/10.1016/j.neubiorev.2014.11.020.
- Petersen I, Evans S, Nazareth I. Prenatal exposure to selective serotonin reuptake inhibitors and autistic symptoms in young children: another red herring?. Br J Psychiatry 2014;205:105-6.
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013;346. https://doi.org/10.1136/bmj.f2059.
- Rai D, Lee BK, Dalman C, Newschaffer C, Lewis G, Magnusson C. Antidepressants during pregnancy and autism in offspring: population based cohort study. BMJ 2017;358. https://doi.org/10.1136/bmj.j2811.
- Sørensen MJ, Grønborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013;5:449-59. https://doi.org/10.2147/CLEP.S53009.
- Viktorin A, Uher R, Reichenberg A, Levine SZ, Sandin S. Autism risk following antidepressant medication during pregnancy. Psychol Med 2017;47:2787-96. https://doi.org/10.1017/S0033291717001301.
- Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017;317:1553-62. https://doi.org/10.1001/jama.2017.3413.
- Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015;20:727-34. https://doi.org/10.1038/mp.2014.90.
- Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016;6. https://doi.org/10.1038/tp.2015.190.
- Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ 2017;357. https://doi.org/10.1136/bmj.j2350.
- Uguz F. Maternal antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in children: a systematic review of the current literature. J Clin Psychopharmacol 2018;38:254-9. https://doi.org/10.1097/JCP.0000000000000868.
- Viktorin A, Uher R, Kolevzon A, Reichenberg A, Levine SZ, Sandin S. Association of antidepressant medication use during pregnancy with intellectual disability in offspring. JAMA Psychiatry 2017;74:1031-8. https://doi.org/10.1001/jamapsychiatry.2017.1727.
- Brandon AR, Shivakumar G, Inrig SJ, Sadler JZ, Craddock Lee SJ. Ethical challenges in designing, conducting, and reporting research to improve the mental health of pregnant women: the voices of investigators and IRB members. AJOB Empirical Bioethics 2014;5:25-43. https://doi.org/10.1080/23294515.2013.851128.
- Hernan MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016;183:758-64.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Minassian C, Williams R, Meeraus WH, Smeeth L, Campbell OMR, Thomas SL. Methods to generate and validate a pregnancy register in the UK Clinical Practice Research Datalink primary care database. Pharmacoepidemiol Drug Saf 2019;28:923-33. https://doi.org/10.1002/pds.4811.
- Medicines & Healthcare Products Regulatory Agency . CPRD Mother Baby Link Documentation V1.2 2017. https://cprd.com/sites/default/files/2022-02/MotherBabyLink_Documentation_v1.2.pdf (accessed 1 February 2022).
- Hagberg KW, Jick SS. Validation of autism spectrum disorder diagnoses recorded in the Clinical Practice Research Datalink, 1990-2014. Clin Epidemiol 2017;9:475-82. https://doi.org/10.2147/CLEP.S139107.
- CPRD n.d. www.cprd.com/sites/default/files/Documentation_HES_OP_set18_v1.9.pdf (accessed 01 February 2022).
- Carey IM, Hosking FJ, Harris T, . An Evaluation of the Effectiveness of Annual Health Checks and Quality of Health Care for Adults with Intellectual Disability: An Observational Study Using a Primary Care Database. Southampton, UK; 2017.
- Sheehan R, Hassiotis A, Walters K, Osborn D, Strydom A, Horsfall L. Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study. BMJ 2015;351. https://doi.org/10.1136/bmj.h4326.
- Newlove-Delgado T, Hamilton W, Ford TJ, Stein K, Ukoumunne OC. Prescribing for young people with attention deficit hyperactivity disorder in UK primary care: analysis of data from the Clinical Practice Research Datalink. Atten Defic Hyperact Disord 2019;11:255-62. https://doi.org/10.1007/s12402-019-00288-6.
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. https://doi.org/10.1093/aje/kwq433.
- Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Family Practice 2010;11.
- Joint Formulary Committee . British National Formulary (BNF) 2019. https://bnf.nice.org.uk/ (accessed 9 April 2023).
- Rosenbaum PR, Rubin DB. Constructing a control-group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33-8.
- Lee BK, Lessler J, Stuart EA. Improving propensity score weighting using machine learning. Stat Med 2010;29:337-46. https://doi.org/10.1002/sim.3782.
- Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream?. Epidemiology 2006;17:360-72.
- Davies NM, Gunnell D, Thomas KH, Metcalfe C, Windmeijer F, Martin RM. Physicians’ prescribing preferences were a potential instrument for patients’ actual prescriptions of antidepressants. J Clin Epidemiol 2013;66:1386-96. https://doi.org/10.1016/j.jclinepi.2013.06.008.
- Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology 2012;23:713-20. https://doi.org/10.1097/EDE.0b013e31825fa230.
- Susser E, Eide MG, Begg M. Invited commentary: The use of sibship studies to detect familial confounding. Am J Epidemiol 2010;172:537-9. https://doi.org/10.1093/aje/kwq196.
- Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol 2016;45:1866-86.
- Jackson JW, Swanson SA. Toward a clearer portrayal of confounding bias in instrumental variable applications. Epidemiology 2015;26:498-504. https://doi.org/10.1097/EDE.0000000000000287.
- Smith GD. Negative control exposures in epidemiologic studies. Epidemiology 2012;23:350-1.
- Carreira H, Williams R, Strongman H, Bhaskaran K. Identification of mental health and quality of life outcomes in primary care databases in the UK: a systematic review. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029227.
List of abbreviations
- A&E
- accident and emergency
- ADHD
- attention deficit hyperactivity disorder
- CAG
- Clinical Advisory Group
- CART
- classification and regression tree
- CDC
- US Centers for Disease Control and Prevention
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- ICD-10
- International Classification of Diseases, 10th Edition
- IMD
- Index of Multiple Deprivation
- IRR
- incidence risk ratio
- IV
- instrumental variable
- N
- number of observations
- N/A
- not applicable
- NHS
- National Health Service
- NIHR
- National Institute for Health Research
- OLS
- ordinary least squares
- ONS
- Office for National Statistics
- OR
- odds ratio
- PAG
- patient advisory group
- PPI
- patient and public involvement
- PSM
- propensity score matching
- RCT
- randomised controlled trial
- SD
- standard deviation
- SERT
- serotonin transporter
- SSRI
- selective serotonin reuptake inhibitor
- TCA
- tricyclic antidepressant
- UK
- United Kingdom
- US
- United States of America
- UTS
- up to standard GP practice
Notes
-
Read terms and medical codes used to identify depressive symptoms
-
Read terms and medical codes used to identify symptoms related to self-harm
-
Read terms and medical codes used to identify intellectual disability
-
Read terms and medical codes used to identify symptoms related to self-harm
-
Read terms and medical codes used to identify bipolar affective disorder
-
Read terms and medical codes used to identify eating disorders
-
Read terms and medical codes used to identify personality disorders
-
Read terms and medical codes used to identify sleep disorders
-
Read terms and medical codes used to identify neuropathic pain disorders
-
Read codes and medical codes used to rate severity of depression
-
Standardised absolute mean differences in covariate distributions between initiators and non-initiators in the pregnant women’s cohort who could be linked with HES in-patient data, before (blue) and after (orange) matching on propensity scores
-
Standardised absolute mean differences in covariate distributions between initiators and non-initiators in the women’s cohort who could be linked with HES accidents and emergencies data, before (blue) and after (orange) matching on propensity scores
-
Standardised absolute mean differences in covariate distributions between initiators and non-initiators in the pregnant women’s cohort who could be linked with HES out-patient data, before (blue) and after (orange) matching on propensity scores
-
Results for mothers outcomes for antidepressant initiation versus no treatment. Sample restricted to pregnancies that could be linked with SES data, fully adjusted model includes variable measuring the IMD decile
-
Results for neurodevelopmental outcomes in relation to initiation versus no treatment with antidepressants in the mother and child cohort restricted to offspring with data linkage, fully adjusted model includes variable measuring the IMD decile
-
Standardised absolute mean differences in covariate distributions between continuers and discontinuers in the pregnant women’s cohort who could be linked with HES in-patient data, before (blue) and after (orange) matching on propensity scores
-
Standardised absolute mean differences in covariate distributions between continuers and discontinuers in the pregnant women’s cohort who could be linked with HES accidents and emergencies data, before (blue) and after (orange) matching on propensity scores
-
Standardised absolute mean differences in covariate distributions between continuers and discontinuers in the women’s cohort who could be linked with HES out-patient data, before (blue) and after (orange) matching on propensity scores
-
Results of maternal primary care outcomes in women who continued antidepressants versus those who discontinued. Sample restricted to pregnancies that could be linked with SES data, fully adjusted model includes variable measuring the IMD decile
-
Results for analyses investigating offspring neurodevelopmental outcomes in children of women who continued antidepressants in pregnancy versus those who discontinued. Sample restricted to those with linked data, fully adjusted model includes variable measuring the IMD decile
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/AQTF4490).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
