Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/90/03. The contractual start date was in November 2017. The draft report began editorial review in August 2021 and was accepted for publication in February 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Worthington et al. This work was produced by Worthington et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Worthington et al.
Chapter 1 Introduction
Parts of this chapter are reproduced from Frost et al. 1 © The Author(s) 2019. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Scientific background and review of current literature
Lower urinary tract symptoms (LUTS) in men substantially affect quality of life, work and other activities; such problematic LUTS are described as ‘bothersome’ according to the impact on the patient. 2 LUTS can relate to storage (increased urinary frequency, nocturia, urgency, incontinence), voiding (slow stream, hesitancy, straining) or post-voiding (post-voiding dribble, sensation of incomplete emptying) symptoms.
Men usually present with a range of LUTS. Particularly high-impact LUTS for men are urgency/urgency incontinence, post-micturition dribble, nocturia and increased urinary frequency. 3–5 In population-based studies, measures of disease-specific, health-related quality of life are substantially worse among men with higher symptom severity and bother ratings than among men with low symptom severity and bother ratings. 6 LUTS can be caused by prostate enlargement, leading to obstruction and/or bladder dysfunction, but are also influenced by men’s lifestyle and habits, such as overall fluid and caffeine intake.
National Institute for Health and Care Excellence (NICE) Clinical Guideline 97 recommends that, on initial presentation with LUTS, men should undergo key assessments to exclude serious medical conditions [malignancy and urinary tract infection (UTI)] and should be asked to complete a bladder diary, to assess the impact of their LUTS and to aid diagnosis. 7 Conservative treatment measures (fluid advice, bladder training, urethral compression and release, and pelvic floor muscle exercises) are then recommended as initial interventions. 7 On the basis of a systematic review of assessment and therapy of male LUTS, the European Association of Urology (EAU) guidelines on male LUTS for secondary care state that categorising precise symptoms (storage/voiding/post voiding) is an expectation of urological practice, and EAU also recommends conservative treatment measures. 8
Proper symptom assessment and explanation of conservative measures are more time-consuming and complex than they may at first appear, owing to differences in individual symptoms and potential causative factors, and the need for conservative care to be tailored. As the average general practitioner (GP) consultation lasts 12 minutes and covers 2.5 problems,9 and given a lack of resources to support the guidelines and conservative approach, the proportion of men receiving the recommended standard of care in primary care is potentially small.
In terms of symptom assessment, a 2016 Royal College of Physicians audit of continence care in primary care showed that < 50% of patients had completed a bladder diary and < 30% had a validated symptom score. 10 Thus, men may undergo limited assessment mainly to exclude serious underlying conditions, potentially resulting in treatment for LUTS being sidelined.
The frequency of delivery of conservative treatment measures was also low, with the Royal College of Physicians’ audit demonstrating that < 65% of men had used lifestyle modification, < 50% used behavioural modification and < 35% used bladder training (worse among men aged > 65 years). 10 Ineffective delivery of conservative measures in primary care can mean that men simply receive a prescription of medication to treat the prostate, are inappropriately referred to secondary care or endure persistent symptoms.
The evidence to support conservative interventions is also limited. The Cochrane review on lifestyle interventions for the treatment of urinary incontinence in adults11 suggested that there is insufficient evidence to justify fluid advice for treatment of urgency incontinence. However, an NHS evidence update indicated that self-management may have a role in the treatment of LUTS,12 citing a post hoc analysis13 of a single-centre randomised controlled trial (RCT)14 of 140 men with LUTS assigned to standard care plus a self-management programme or standard care alone. Men assigned to the self-management programme reported better voided volumes, and reduced daytime frequency and nocturia. The trial had a relatively small patient population and was conducted in a single tertiary treatment centre. According to NICE Clinical Guideline 97, a multicentre RCT is needed to determine if such results could be replicated in everyday clinical practice. 15
Rationale for the trial
The risk of LUTS increases with age, with a prevalence of up to 30% in men aged > 65 years;7 therefore, the number of patients affected is likely to increase as the population ages. As most men with LUTS are managed within primary care, the burden on primary care is likely to grow, and, consequently, so will the need for effective provision of LUTS care.
As only a small proportion of men with LUTS receive the recommended assessments, or recommended standard of conservative care as initial treatment within primary care, there is a clear need for an effective, evidence-based intervention that can be feasibly implemented in routine primary care to support delivery of NICE guidelines. The TRIUMPH (TReatIng Urinary symptoms in Men in Primary Health care using non-pharmacological and non-surgical interventions) trial aimed to address this need within primary care. In addition, with evidence lacking on the effectiveness of self-management programmes for LUTS, the trial aimed to provide the evidence needed to justify this approach and the current guidelines.
The TRIUMPH trial intervention sought to provide detailed conservative care advice for male LUTS. The trial was primarily designed to establish whether or not this approach improved symptomatic outcomes compared with usual care, and whether or not the result was sustained over a longer duration beyond the last follow-up contact with the healthcare professional (HCP). If the TRIUMPH approach proved to be effective, it would provide the necessary resource for general practices to effectively deliver conservative treatment through a pathway led by a practice nurse/healthcare assistant (HCA), reducing GP reconsultation for symptoms, prescribing and unnecessary secondary care referrals.
Trial aims and objectives
The key aim of the TRIUMPH trial was to determine whether or not a standardised and manualised care intervention achieves superior symptomatic outcome, compared with usual care, for male LUTS, with a primary outcome of overall International Prostate Symptom Score (IPSS) measured 12 months after consent, in a primary care setting.
The secondary objectives were to compare the two trial arms with regard to:
-
disease-specific quality of life
-
symptomatic outcomes
-
cost effectiveness
-
relative harms
-
use of NHS resources
-
overall quality of life and general health
-
acceptability of assessment and provision of care
-
patients’ perception of their LUTS condition.
Chapter 2 Methods
Parts of this chapter are reproduced from Frost et al. 1 © The Author(s) 2019. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter are also reproduced from the published statistical analysis plan by MacNeill and Drake. 16 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Trial design
The TRIUMPH trial was a multicentre, pragmatic, two-arm cluster RCT of a care pathway based on a standardised and manualised care intervention (TRIUMPH intervention arm) and usual care (comparator arm) for men with LUTS. The trial was conducted in 30 general practice sites within nine Clinical Commissioning Groups (CCGs) across the West of England and Wessex Clinical Research Network (CRN) regions in the UK, recruiting patients from June 2018 to August 2019. Sites were randomised to the intervention or comparator arm, with men with bothersome LUTS consented from these sites. The main aim of the trial was to determine whether or not the TRIUMPH intervention arm achieves superior symptomatic outcome, compared with the usual-care arm, for male LUTS, with a primary outcome of overall IPSS measured 12 months after consent.
The trial design included an internal pilot recruitment phase of 4 months’ duration, primarily to verify that recruitment was possible before progression to the main phase of the trial. Targets for progression to the main phase of the trial were as follows:
-
The number of practices agreeing to take part was at least 18 (75%) by the end of month 6.
-
The number of patients recruited was at least 120 by the end of month 4 of the recruitment phase.
Ethics approval and research governance
Approval from the National Research Ethics Service Committee North West – Preston (reference number 18/NW/0135) was received on 11 April 2018 and applied to all NHS sites that took part in the trial.
The trial is registered at the International Standard Randomised Controlled Trial Number registry with the reference ISRCTN11669964.
All participants provided their written, informed consent to participation before entering the trial. Participants consented by post, following GP screening of their medical notes and a screening call by CRN nurses/clinical practitioners.
All serious adverse events (SAEs) were recorded; the sponsor and Research Ethics Committee were notified within 15 days of any SAEs categorised as unexpected and related. General practices were responsible for reporting SAEs for their trial participants during the course of the trial; however, participants were also asked to self-report any inpatient stays in their follow-up questionnaires, which prompted GP review. All other adverse events not deemed serious were collected from participant electronic medical records (EMRs) at the end of participants’ 12-month involvement in the trial, as part of the secondary outcomes.
A number of protocol changes were made during the course of the trial, as listed below:
-
The Self-Assessment Goal Achievement questionnaire that was originally planned was replaced with the Brief Illness Perception Questionnaire (B-IPQ). The B-IPQ was more relevant to the trial, as the Self-Assessment Goal Achievement questionnaire mentions goals set with the healthcare providers, which was not applicable to the TRIUMPH intervention.
-
Qualitative interviews with a small number of men who decided not to take part in the TRIUMPH trial (decliner interviews) were added.
-
As well as practice nurses, HCAs were specified as able to deliver the intervention.
-
Hypercalcaemia was removed from the exclusion criteria.
-
A criterion about a competing primary care LUTS study was added to general practice eligibility requirements.
-
‘Date of LUTS diagnosis’ was removed from the baseline data to be collected, as this date is arbitrary and depends on when the patient chose to seek help regarding his symptoms, and therefore was not considered relevant.
-
Specification of an exact figure for the number of eligible patients required by general practices to take part in the trial was removed, as determined by a pre-randomisation practice database search.
The protocol was also updated during the trial to make minor clarifications to trial procedures.
Participants remained in the trial unless they chose to withdraw, or if they were unable to continue. Participants could withdraw fully from the trial or from specific elements without giving a reason. Any data collected up until the point of withdrawal were retained. Participants were informed of this in the patient information leaflet prior to consent.
General practice recruitment and selection
The trial recruited from two centres: West of England and Wessex CRNs. The CRNs invited general practices to express an interest in taking part in the trial. The general practice inclusion and exclusion criteria were as follows:
-
inclusion criterion – adequate number of eligible patients, determined by pre-randomisation practice database search (to achieve site target recruitment of 35 patients)
-
exclusion criterion – unable to provide adequate treatment room space and availability for trial or practice nurse/HCA to complete HCP training and baseline visits.
However, to achieve a balanced range of practices, the following factors were also considered in practice selection:
-
number of potentially eligible patients, on conduct of a preliminary database search
-
patient list size
-
deprivation score (calculated using the general practice postcode)
-
preference for intervention delivery (practice staff or trial research nurses).
The sites selected to take part in the trial underwent site initiation training. An internal pilot phase was conducted with eight initial sites over a period of 4 months before the main phase of the trial, for which a further 22 sites were recruited. In total, 32 general practices were recruited within nine CCGs across the West of England and Wessex CRN regions in the UK. These constituted 30 sites, owing to a collaboration of three practices operating a hub-and-spoke model, sharing nurse resource, and therefore randomised as a single site for the trial.
Participants
Participant population
The TRIUMPH trial was a pragmatic trial, comprising adult men who considered themselves to have bothersome LUTS and who presented to primary care within the preceding 5 years with at least one symptom. Only men already known to have LUTS (prevalent cases) were screened for inclusion in the trial. Screening was undertaken once by each site before randomisation, so men newly presenting with LUTS (incident cases) after site randomisation were not included. Detailed inclusion and exclusion criteria are provided in Table 1.
| Inclusion criterion |
| Adult men above the age of 18 years old who have bothersome LUTS |
| Exclusion criteria |
|
Patient screening and invitation
The process of patient invitation and screening is detailed below:
-
General practices conducted a single database search to identify potentially eligible patients. The database search was developed specifically for the trial, based on the trial inclusion and exclusion criteria (see Appendix 1, Table 38), for both EMIS Web (EMIS Health, Leeds, UK) and SystmOne (The Phoenix Partnership, Leeds, UK) patient administration systems used in primary care sites in both CRN regions.
-
The patient list identified by the search was manually screened by GPs at the site against patients’ EMRs using the eligibility criteria listed (see Appendix 1, Table 38), including criteria that could not be fully included in the search, such as lack of capacity to consent, and referral to or review by secondary care.
-
A de-identified screening log populated with eligibility codes for all patients identified from the search was sent to the central trial team.
-
Invitation letters, with patient information leaflets and expression of interest (EOI) forms, were mailed out to eligible patients by practices using an approved third party [Docmail® (Docmail Ltd, Radstock, UK), a secure service that automates sending invitation letters to potential participants]. A single mail-out was conducted by each site, before notification of their randomisation, to avoid any bias in patient selection.
-
Interested patients completed their EOI forms online or returned paper copies by post. Participants could also decline to take part on their returned EOI form, and indicate whether or not they would be willing to take part in a decliner interview for the qualitative element of the trial.
-
Clinical Research Network nurses or clinical practitioners trained by the trial team telephoned interested patients. These calls were conducted while masked to the allocation of the practice, and therefore the patient, to avoid any bias. Calls were conducted to confirm eligibility, particularly the subjective criterion of whether or not the patient’s LUTS were bothersome to him; to ensure patient understanding of the trial; to answer any questions; and to confirm willingness to participate.
Following a review of the pilot phase figures, the key changes that were made to the conduct of the trial for the main phase were an increase in the maximum number of patients invited by a single site from 150 to 220, and confirmation that 30 sites would be required to maintain the trial power calculation in the light of variation in the number of patients recruited per site. The decision was also taken to conduct only a single invitation mail-out from each site, rather than inviting additional patients in a second mail-out to achieve site targets as necessary, to avoid introducing any bias post randomisation. Although screening of all patients for both mail-outs would have been conducted before randomisation, rescreening would have been required to check patient status before a later second mail-out, when sites would have been aware of their allocation. Details of the processes for the two phases were as follows:
-
Pilot phase. During the pilot phase of the trial, sites manually screened up to 325 potentially eligible patients identified from their database search, in order of NHS number. The maximum number of patients for an individual site to include in its invitation mail-out was 150; however, the screening allowed for the potential of a second mail-out. For sites with > 150 eligible patients, the central trial team randomly identified 150 patients for invitation.
-
Main phase. During the main phase of the trial, sites with > 220 eligible patients screened up to this number, in order of NHS number. The maximum number of patients invited to participate in the trial from a single site was 220.
Patient consent
Patients deemed willing and eligible to participate in the trial following their telephone call with the CRN nurse or practitioner were posted a consent form and a questionnaire containing baseline measures specific to trial arm for completion (Table 2). All patients received the same consent forms and questionnaires, but those in the intervention arm also received a bladder diary to be completed before their face-to-face visit.
| Schedule | Screening (pre consent) | Trial period | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient enrolment | Post enrolment | |||||||
| Baseline | Visit 1 | 1 week | 4 weeks | 12 weeks | 6 months | 12 months | ||
| Practice eligibility screen | ✗ | |||||||
| Practice allocation | ✗ | |||||||
| CRN eligibility screen | ✗ | |||||||
| Informed consent | ✗ | |||||||
| Interventions | ||||||||
| Usual care | •—————————————————————————————————————• | |||||||
| HCP-delivered booklet | ✗a | |||||||
| Follow-up nurse contacts | ✗a | ✗a | ✗a | |||||
| Assessments | ||||||||
| Case report form | ✗a | ✗a | ✗a | ✗a | ✗ | |||
| ICIQ bladder diary | ✗a | |||||||
| IPSS | ✗ | ✗ | ✗ | |||||
| ICIQ-UI-SF | ✗ | ✗ | ✗ | |||||
| B-IPQ | ✗ | ✗ | ✗ | |||||
| EQ-5D-5L | ✗ | ✗ | ✗ | |||||
| Interviews | •—————————————————————————————————————• | |||||||
| Resource use via GP EMRs | ✗ | |||||||
For patients in both arms, the return of the completed consent form by post demonstrated explicit consent to participate in the trial. Alongside providing explicit consent to take part in the trial, the men were also asked on the consent form if they were willing to consent to (1) being contacted by a qualitative researcher to undertake an interview and (2) being contacted about other research. Declining to consent to these did not disqualify a man from participating in the main trial.
All men who entered the trial were logged with the central trial office at the University of Bristol and given a unique study number. Each patient’s GP was informed by the central trial team by letter about the patient’s participation in the trial. Sites were encouraged to update their GP EMRs to record participation.
Interventions
The TRIUMPH trial intervention arm
Principle of the TRIUMPH trial intervention
Presentations of LUTS are typically a composite of different symptom combinations. The TRIUMPH trial intervention targeted component symptoms with specific educational information and active management. This was provided in a standardised way in the form of a booklet that patients could read in their own time to encourage take-up of the information. However, the intervention also provided manualised care, with a HCP using basic assessments and discussion with the patient to direct them to the most applicable information in the booklet. The discussion considered their personal circumstances, symptom needs, bothersomeness of these symptoms and impact on quality of life. The TRIUMPH trial intervention arm therefore offered standardised and manualised care according to the symptomatic presentation of the individual participants.
The TRIUMPH trial intervention aimed to address key limitations in the current provision of care to men with LUTS in primary care through the use of symptom scores and bladder diaries to effectively diagnose specific LUTS, the provision of effective written materials, the training of nurses/HCAs in the interpretation of symptom scores and the use of the theory of planned behaviour to support self-management. 17
The TRIUMPH trial intervention booklet
The TRIUMPH trial intervention booklet was developed for the trial from the British Association of Urological Surgeons’ patient information sheets, and underwent sequential development in multiple iterations and consultations involving patients, HCPs and health psychologists. The booklet comprises written information and illustrations, and is titled Helping You to Take Control of Your Waterworks [the booklet is available on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169003/#/ (accessed March 2022)]. The sections included are as follows:
-
advice on drinks and liquid intake
-
advice on controlling an urgent need to urinate
-
exercising the muscles between the legs (pelvic floor) to help stop bladder leakage
-
advice on emptying the bladder as completely as possible
-
advice on getting rid of the last drops
-
reducing sleep disturbance caused by needing to urinate.
The booklet is water-resistant and able to lie flat when open, which is useful when potentially used in the bathroom. Pictorial representations were used for clarity and avoid the use of potentially embarrassing images. Sections are tabbed and colour-coded for specific symptoms and advice.
The TRIUMPH trial intervention procedure
The details of the TRIUMPH trial intervention procedure are as follows:
-
The intervention was delivered by a trained HCP, either a general practice clinical nurse, a research nurse or a HCA, or a dedicated trial research nurse, depending on site preference.
-
The HCP reviewed the patient’s baseline urinary symptoms using their completed IPSS, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form (ICIQ-UI-SF) and International Consultation on Incontinence Questionnaire (ICIQ) bladder diary. Any missing elements of questionnaires/bladder diaries were discussed with the patient to obtain approximate measures for the symptom assessment. Updated responses were used only for these purposes and baseline data remained as originally completed.
-
The participant attended for a single intervention visit, during which the HCP discussed their individual symptoms and level of bother. The HCPs were provided with decision tools (see Report Supplementary Material 1) to assist them in tailoring the treatment for each participant at their intervention visit, based on their symptoms.
-
The HCP provided the participant with the TRIUMPH trial booklet.
-
The delivery of the booklet was individualised by the HCP, who directed the participant to the relevant sections of the booklet, and therefore steps to take personally. A maximum of three sections were recommended to each participant and tabbed with discreet stickers. If more than three sections were identified as relevant to a participant, the three most bothersome symptoms were chosen. The choice of a maximum of three sections was guided by patient and public involvement (PPI) consultation on what they considered to be a manageable level of advice to follow.
-
To encourage and gauge adherence to the intervention, regular participant contact was provided following the initial face-to-face appointment. Follow-up contacts were conducted by telephone at 1 week, and then by telephone, e-mail or text at 4 and 12 weeks, according to participant preference. Participants retained the intervention booklet at the end of this period.
Participants in the intervention arm continued to receive usual care from their GP for their LUTS when necessary; details of this were collected at 12 months.
The TRIUMPH trial intervention training
The chief investigator developed the training and delivered this to the TRIUMPH trial research nurses. Materials were developed to support the training, as well as the intervention visits. The TRIUMPH trial nurses attended general practices randomised to the intervention arm to deliver training to practice nurses or HCAs for those sites opting to use their own staff. Training included examples of bladder diaries and how primary care staff should interpret them for the purpose of the trial. Nurses were trained on the responses to the questionnaires and which sections of the booklet they should direct the participant to. This was supported by a checklist developed specifically for the trial (see Report Supplementary Material 1). Ongoing support was available for practice nurses/HCAs in the form of regular teleconferences held by the trial team and trial research nurses.
Usual-care arm
Usual care (the comparator arm for the TRIUMPH trial) in this trial requested that sites continue to follow their standard local practice for trial participants. Usual care was chosen as the comparator for the trial to reflect the actual care provided by control arm general practices, rather than the NICE guidance, which may be variably implemented. The qualitative aspect of this trial explored what usual care looked like for a sample of comparator and intervention practices (see Chapter 5).
Post-trial care
Following the end of the trial, participants in the intervention arm retained the booklet provided, and participants in the control arm were provided with the booklet, alongside a summary of the results of the trial. Their LUTS care was the responsibility of their GP throughout the trial and after they had completed the TRIUMPH trial at 12 months.
Outcome measures
Primary outcome
The primary outcome measure was the participant-reported IPSS at 12 months after consent. The IPSS is validated, extensively tested in LUTS research and widely employed in urology services. 18 It produces a score from 0 to 35, with higher scores indicating more severe symptoms. The end point of 12 months was chosen to measure whether or not any effect of the TRIUMPH trial intervention on LUTS was sustained after the initial 12-week intervention delivery period.
Secondary outcomes
The following secondary outcomes were collected by questionnaire at 6 and/or 12 months.
-
International Prostate Symptom Score-Quality of Life Index (IPSS-QoL) – LUTS quality-of-life score measured at 6 and 12 months.
-
IPSS – overall urinary symptom score at 6 months.
-
ICIQ-UI-SF19 – measured at 6 and 12 months. This questionnaire supplements the IPSS with the measurement of incontinence.
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L)20 – measure of health status.
-
B-IPQ21 – patient perception of their LUTS. It was modified slightly, with permission, to ask participants about ‘urinary symptoms’ rather than ‘illness’.
The number of adverse events (specified as UTIs, catheterisations, urinary retention, prostatitis or death) and the number of referrals to secondary care (urology) were extracted from primary care EMRs at 12 months.
Sample size
This trial was powered to detect a mean between-group difference of 2 points on the primary outcome of IPSS at 12 months post consent. This target difference was chosen because, although the recognised minimum important difference in IPSS is 3.0 points,22 men may be bothered by just one symptom (e.g. nocturia). A change of 2 points might mean a small improvement in two symptoms or a bigger improvement in one symptom.
To inform the sample size calculation, a scoping search was conducted with local general practices within the NHS Bristol, North Somerset and South Gloucestershire CCG to gain a sense of the likely number of patients available on their lists based on our inclusion and exclusion criteria. This search suggested that an average-sized general practice might identify 100 patients. We originally assumed that 50% of these patients would be eligible and, of these, 70% would consent, so each practice would consent ≈35 eligible patients. Our estimates of eligibility rates, consent and loss to follow-up were conservative and based on our experience running pragmatic trials in primary care settings.
Based on this, we estimated that 840 patients were needed from at least 24 practices to detect a difference in IPSS of 2 [common standard deviation (SD) of 5: in line with the assumptions made in UPSTREAM (Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods3)] with 90% power and a significance level of 5%. Our estimate incorporated a design effect to account for clustering of effects in practices, which assumed that practices would be able to recruit 35 patients each (i.e. mean cluster size of 35) and that the intracluster correlation coefficient (ICC) between practices would be 0.05, an estimate in line with results from other primary care studies. 23 We allowed for up to 30% of men being lost to follow-up.
During the early stages of recruitment, however, it became apparent that practices were not consistently consenting 35 patients and that the variability in recruitment would necessitate more practices being recruited to achieve our objective of 90% power to detect a mean difference in IPSS of 2 points. Using information from the numbers and proportions of patients consenting from practices recruited early in the trial, and projections regarding practices soon to start recruiting patients, we revised our assumptions and assumed that the mean number of patients consented at each practice would be 26 and that the coefficient of variation for the mean cluster size between practices would be 0.26. Based on these revised assumptions, this increased the number of practices required for the study by six; the Trial Management Group agreed to recruit additional practices. This amendment was agreed with both the Trial Steering Committee and the funder.
Randomisation and implementation
General practices were the unit of allocation to the two trial arms. Practices were randomised on a 1 : 1 basis to either receive the intervention or continue care as usual [control group (CG)] by a statistician from the Bristol Trials Centre, who was outside the trial team and was masked to the identity of practices. This was done after the practice list searches had been conducted, lists were screened by GPs and the mail-out was uploaded (see Patient screening and invitation). As there were a relatively small number of general practices in the trial, minimisation was used to allocate practices to treatment arms to ensure balance. Allocation was minimised by centre (West of England and Wessex), practice size (numeric variable) and area-level deprivation [Index of multiple deprivation (IMD), a numeric variable] of the practice. A random element was incorporated in the minimisation procedure such that there was a 40% probability that allocation was random. When allocation was random, there was a 50 : 50 chance of practices being allocated to either arm.
Although it is common to use lower-layer super output area (LSOA) deprivation scores to estimate deprivation for individuals (using home postcodes to identify the LSOA), it has been shown that middle-layer super output area (MSOA) data better reflect the area-level deprivation of general practices. 24 Therefore, general practice postcodes were mapped onto LSOAs first and then onto MSOAs. Population-averaged IMD scores (from 2015) were then calculated based on the scores of LSOAs within each MSOA.
Masking
Two statisticians and two health economists supported this trial. The senior statistician and senior health economist were masked throughout the trial. A junior statistician performed all disaggregated analyses according to the statistical analysis plan16 and the junior health economist conducted the economic analyses according to the health economics analysis plan. The junior statistician attended closed meetings of the independent Data Monitoring Committee as required. The CRN support team was masked to minimise selection and recruitment bias. The remaining members of the trial team were masked to aggregate data only. General practice staff, research nurses involved in delivering the intervention and participants were not masked.
The protocol was written before recruitment end and published in Trials. 1 The statistical analysis plan16 was written and agreed by the trial team in July 2020, prior to completion of participant follow-up. The health economics analysis plan was written and agreed by the trial team in February 2021, prior to database lock.
Data collection
Participant-reported outcomes
The components and timing of follow-up measures are shown in Table 2. All participants were asked to complete self-reported outcome measures in the form of questionnaires (IPSS, ICIQ-UI-SF, EQ-5D-5L and B-IPQ) at baseline (postal), and 6 and 12 months (postal, online or telephone) post enrolment. Participants were sent one reminder to return their baseline materials, and up to three reminders to return their 6- and 12-month questionnaires.
All participants were provided with trial progress updates at 3 and 9 months via a newsletter to maintain engagement with the trial and encourage responses to follow-up questionnaires. The newsletter did not provide any detail on the TRIUMPH trial intervention.
Intervention delivery
Trial-designed case report forms were completed for the intervention arm only, at the intervention visit and during the 12-week treatment phase, to collect details of the booklet sections advised to the participant and feedback on the booklet.
Electronic medical record data extraction
Data extraction from GP EMRs was conducted by sites a minimum of 1 month after their final participant had completed follow-up. Database searches were developed specifically for the trial, for both EMIS Web and SystmOne patient administration systems used in primary care sites in both CRN regions. The EMR data extracted comprised baseline measures, clinical outcome measures and health economic outcome measures. The baseline and clinical data extracted are detailed in the following list; the health economic data are described in Chapter 4.
-
Baseline comorbidities: any comorbidity coded on a participant’s record according to the Quality and Outcomes Framework.
-
Baseline urinalysis: the most recent urinalysis results (normal/abnormal) in the 6 months pre consent.
-
Baseline renal function: the most recent estimated glomerular filtration rate (eGFR) in the 6 months pre consent.
-
Baseline medication: relevant prescriptions issued to patients in the 3 months pre consent.
-
Baseline GP consultations: the number of consultations in the 12 months pre consent.
-
Baseline referrals: referrals to urology in the 12 months pre consent.
-
Adverse events: UTI, catheterisation, urinary retention, prostatitis, deaths in the 12 months post consent.
-
Secondary care referrals: urology referrals in the 12 months post consent.
Patient and public involvement
We involved PPI representatives at all stages of the project. We had a patient representative on our Trial Management Group (also a co-applicant involved at the grant application stage) and a patient representative on our Trial Steering Committee. Both contributed to the management of the trial, providing an invaluable patient perspective to all aspects of trial conduct. In addition, we have held wider patient advisory group meetings throughout the trial; our two PPI representatives had a substantial role in planning, as well as chairing, these meetings.
One of the key roles of the PPI input was in the development of the TRIUMPH trial intervention booklet, which resulted in key changes to aid clarity and usability. We also undertook PPI review of the patient-facing trial materials, including the patient questionnaires, newsletters and website. Further PPI has included discussion of some of the initial qualitative findings relating to men’s experiences of the patient pathways for LUTS within the NHS, as well as routes for implementation and dissemination. Overall, PPI has played an invaluable part in the trial from conception to dissemination.
Statistical methods
Baseline data analyses
The baseline characteristics of patients and practice characteristics were compared between the two arms by reporting relevant summary statistics to determine whether or not any potentially influential imbalance occurred by chance. Baseline characteristics were summarised using means, SDs, medians [interquartile ranges (IQRs)] or number (%) depending on the nature of the data and their respective distributions.
Primary analysis
Analysis and reporting were in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines,25,26 with the primary analyses being conducted on an intention-to-treat (ITT) basis. The primary outcome was the IPSS collected at 12 months post consent. It was described in each treatment group using means and SDs. Comparisons between treatment arms were made using a mixed-effect multilevel linear model [individual patients (level 1) nested within general practices (level 2)] and adjusting for patient-level baseline IPSS and practice-level variables used in the randomisation. Results for a model adjusting only for baseline scores have also been presented. The results have been presented as the mean between-group difference with 95% confidence interval (CI), p-value and model ICC with corresponding 95% CI.
Secondary outcomes
The approach for the analysis of the secondary outcomes was on an ITT basis, defined as analysing all participants according to the group to which their practice was randomised. IPSS at 6 months were analysed in the same manner as the primary outcome using a linear mixed model [individual patients (level 1) nested within general practices (level 2)], adjusting for baseline scores and minimisation variables. A separate repeated measures analysis using a linear mixed model [6-monthly observations of IPSS (level 1), nested within participants (level 2) and nested within general practices (level 3)] was also conducted to incorporate all time points. The ICIQ-UI-SF, IPSS-QoL and B-IPQ at 6 and 12 months were studied in the same manner.
Whether or not a patient had a referral to secondary care was studied using a logistic mixed model with individual patients nested within general practices. The model adjusted for minimisation variables and whether or not the patient had a referral to urology pre baseline.
Sensitivity analyses
Predefined sensitivity analyses were conducted to assess the sensitivity of the primary analysis to various assumptions, and are described in this section. As these are exploratory in nature, 95% CIs and p-values are presented, but are interpreted with due caution.
-
Imbalance of baseline characteristics. We planned to explore the sensitivity of the primary analysis to imbalance of baseline characteristics by running models adjusting for these variables. Imbalance is defined as a difference of 10%, or half a SD, between treatment groups.
-
Clustering by HCA/nurse. To allow for clustering of outcomes within nurses/HCAs delivering the intervention, a model was run grouping the patient-level data into clusters based on the combination of the practice where the patient is registered and the nurse/HCA delivering the intervention (first visit). The primary analysis of the IPSS at 12 months was repeated using a single random effect for this level of clustering and the results were compared with those of the primary analysis.
-
Per-protocol analyses. Five per-protocol analyses were conducted using different definitions of protocol compliance:
-
excluding those in the intervention arm who received no intervention booklet by the time of the primary outcome measure at 12 months and had none of the follow-up contacts at 1, 4 and 12 weeks
-
excluding those in the intervention arm who received the intervention booklet by the time of the primary outcome measure at 12 months, but had only two of the three follow-up contact visits (regardless of whether these were early or late)
-
excluding those in the intervention arm who received the intervention booklet by the time of the primary outcome measure at 12 months and had only one follow-up contact (regardless of whether this was early or late)
-
excluding those in the intervention arm who received the intervention booklet by the time of the primary outcome measure at 12 months but had no follow-up contact
-
excluding those in the intervention arm who did not receive the follow-up contact in the protocolised format (i.e. by telephone, post or e-mail).
-
-
Complier-average causal effect (CACE) analysis. Recognising the inherent bias in estimates derived from per-protocol analyses, we planned a CACE analysis of the primary outcome. Compliers were defined as those who received the intervention booklet by the time of the primary outcome follow-up. Non-compliers were defined as those who had not received the booklet at all or received it after the primary outcome time point. The CACE estimates were to be obtained using instrumental variable regression including the same variables used in the primary analysis, with randomised group as the instrumental variable and the indicator variable for compliance.
-
Impact of COVID-19. By the time the COVID-19 outbreak was declared a pandemic by the World Health Organization on 11 March 2020, the trial had already reached its target recruitment and was no longer recruiting. Recognising that trial participation and symptom reporting may have been affected by the outbreak and subsequent lockdown, we used descriptive statistics to explore whether or not there were differences in the proportions of missing data for the primary outcome (IPSS at 12 months) before and after 11 March 2020. We also assessed whether or not IPSS at 12 months differed depending on whether data were collected before or during the COVID-19 pandemic. Scores before and after 11 March 2020 were described using descriptive statistics and the primary analysis model was refitted including a binary term to indicate whether the outcome was measured before 11 March 2020 (0) or from 11 March 2020 onwards (1).
-
Excluding patients later found to be ineligible. A small number of patients participated in the trial who, after consenting and providing data, were found to be ineligible, but were included in the primary ITT analysis. A sensitivity analysis of the primary outcome excluding these individuals was conducted.
Subgroup analyses
Four prespecified subgroup analyses were performed to explore whether or not baseline characteristics modified the effectiveness of the intervention:
-
Nature of LUTS at baseline. The ratio of the IPSS voiding subscore to the storage subscore has been used to describe the relative dominance of voiding to storage LUTS. This measure was used to explore whether or not the treatment effect differed by the nature of LUTS at baseline, as quantified using this ratio.
-
Intervention delivery. We explored whether or not the effect of the intervention differed according to whether a practice nurse/HCA or TRIUMPH trial nurse delivered the intervention to the participant.
-
Method of contact. Participants in the intervention arm specified how they preferred to be contacted by the research team for their intervention follow-up contacts. This might be by telephone or text, for example. The frequency of each preferred mode of contact, the model of contact used and how often this differed were reported. We explored whether or not the effect of the intervention differed by model of contact by including a ‘preferred method of contact’ and treatment group interaction term in the model.
-
Intervention ‘dose’. To explore the possibility that the number of nurse/HCA contacts modified the effect of the intervention, we created a ‘dose’ variable equal to the number of nurse/HCA contacts that participants at the intervention practices received (0–3). The primary analysis was repeated using the ‘dose’ variable as the treatment variable.
In all cases, effect modification was assessed including an interaction term in the regression model, and formal tests of interaction were performed to test whether or not the treatment effect differed between these groups. As with all other analyses, a significance level of 5% was used and, as these analyses were not statistically powered, they are interpreted with due caution as exploratory. A post hoc subgroup analysis was also performed according to whether or not the primary outcome was collected before the start of the COVID-19 pandemic.
Multiple imputation
For our analysis of the primary outcome, we investigated the influence of missing data using sensitivity analyses that made different assumptions regarding missingness: ‘best’- and ‘worst’-case scenarios and multiple imputation by chained equations (MICE) to impute missing data.
Chapter 3 Results
Parts of this chapter are reproduced from Frost et al. 1 © The Author(s) 2019. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
Recruitment
Recruitment of general practices
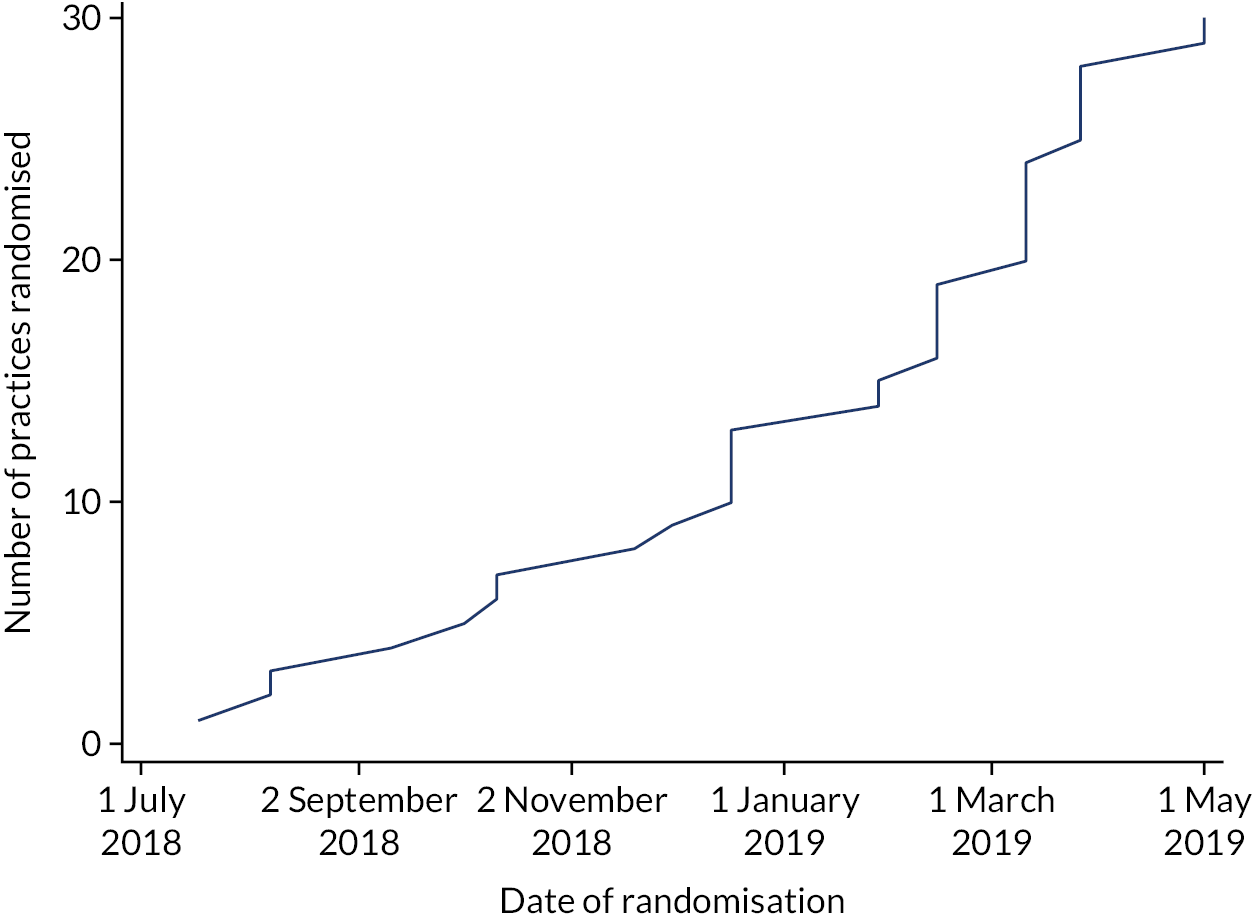
As outlined in General practice recruitment and selection, the West of England and Wessex CRNs invited general practices to express an interest in taking part in the trial. A total of 83 practices expressed an interest, of which 64 performed an initial search (Figure 1). On performing the search, 18 practices were excluded because they did not have sufficient numbers of patients and 16 other practices did not progress further. Practices were recruited between July 2018 and May 2019 (Figure 2). After having conducted contractual agreements and consent processes with practices, we randomised 30 general practice sites. These comprised 32 general practices, owing to one collaboration of three practices operating a hub-and-spoke model and sharing nurse resource, and therefore randomised as a single site for the trial.
FIGURE 1.
The CONSORT flow diagram.
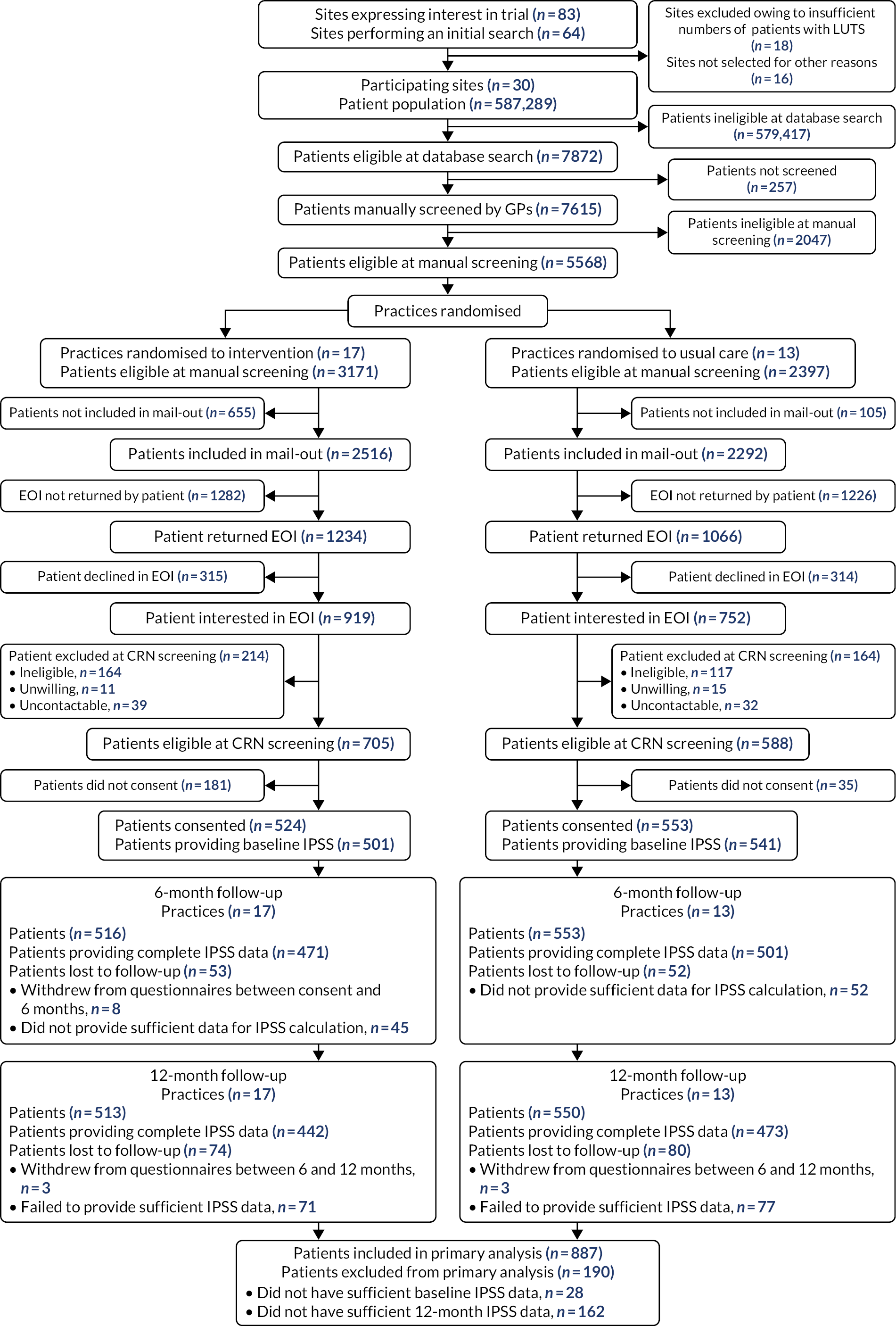
FIGURE 2.
Cumulative number of practices randomised over time.
Characteristics of participating general practices
At the time of recruitment, practices provided estimated list sizes; these ranged from 7600 to 48,623 patients (mean 19,576) (see Appendix 1, Table 39). Although routinely available data on general practices in this region do not allow us to identify and merge smaller practices operating as a hub-and-spoke model as participating practices have, participating practices reflect some of the larger practices in the region [regional median practice size in June 2019: 9440 patients (IQR 6429 to 13,239)]. Intervention practices, on average, recruited 31 patients each and control practices recruited 43 patients each, on average.
Patient-level socioeconomic data were not available for all registered practice patients. General practices were therefore characterised using area-level deprivation, which was estimated for the hypothesised catchment area for the practice (estimated as the MSOA). Deprivation scores ranged from 4.22 to 33.62, and scores were slightly higher in the usual-care arm [mean 16 (SD 8.39)] than in the intervention arm [mean 11 (SD 5.00)], indicating higher levels of socioeconomic deprivation.
Patient screening and randomisation of sites
When participating practices conducted a detailed database search, as outlined in Patient screening and invitation, they identified 7872 potentially eligible patients. A random selection of 160 patients from two practices were not screened as their practices were very large and they had already committed to screening a set number of patients, and 97 patients were not screened owing to practice capacity. Manual screening by GPs of the remaining 7615 patients used the eligibility criteria outlined (see Appendix 1, Table 38). Reasons for ineligibility are outlined in Table 3.
| Reason for exclusion | n (%) |
|---|---|
| GP screening/mail-out | |
| Patients not manually screened by GP (unable to confirm eligibility) | 257 (4) |
| Patients ineligible at GP manual screening | 2047 (30) |
| Reasons for ineligibility (inclusion/exclusion criteria) | |
| Currently being treated for prostate or bladder cancer | 50 |
| Lack of capacity | 85 |
| Does not have LUTS | 871 |
| Is aged < 18 years | 4 |
| Has poorly controlled diabetes mellitus | 18 |
| Previous prostate surgery | 71 |
| Recently referred for, or currently under, urological review | 441 |
| Relevant neurological disease or referral | 56 |
| Unable to complete assessments in English | 9 |
| Unable to pass urine without a catheter | 6 |
| Undergoing neurological testing for LUTS | 38 |
| Visible haematuria | 42 |
| Othera | 299 |
| Patients not included in mail-out | 760 (11) |
| Patient EOI | |
| EOI not returned by patient | 2508 (37) |
| Patients declined in EOI | 629 (9) |
| CRN telephone screening | |
| Patients excluded at CRN screening | 378 (6) |
| Ineligible | 281 (4) |
| Unwilling | 26 (0) |
| Uncontactable | 71 (1) |
| Consent | |
| Patients did not consent | 216 (3) |
Once general practices completed their manual screening of patient lists, sites were randomised. The randomisation process, which was minimised by centre, practice size and area-level deprivation of the practice, resulted in 17 sites being randomised to deliver the intervention (3171 eligible patients) and 13 sites being randomised to deliver usual care (2397 eligible patients). Practices themselves, however, were not informed of their allocation until patient screening had been completed and the invitation letter mail-out uploaded (see Patient screening and invitation).
Patient recruitment
Letters of invitation were sent to 4808 of the 5568 eligible patients (intervention practices, 2516; usual-care practices, 2292). Randomly selected eligible patients were not included in this mail-out (intervention practices, n = 655; usual-care practices, n = 105), as a maximum number was specified for mail-out from individual sites to avoid disproportionate recruitment at larger practices (see Patient screening and invitation). Expressions of interest were returned by 2300 patients [intervention practices, n = 1234 (49%); usual-care practices, n = 1066 (47%)], of whom 1671 [intervention practices, n = 919 (74%); usual-care practices, n = 752 (71%)] confirmed their interest in participating. Using available age data on those who were sent letters of invitation (Table 4), we observed that those who expressed an interest in participating in the trial were similar in age to those who said that they were not interested (mean age 68.7 and 71.0 years, respectively). Those who did not get in touch to confirm their interest were much younger (mean age 62.7 years).
| EOI status | N | n a | Mean age (years) | SD |
|---|---|---|---|---|
| Returned EOI and interested | 1671 | 1671 | 68.68 | 10.13 |
| Returned EOI and not interested | 629 | 629 | 70.99 | 11.05 |
| Did not return EOI | 2508 | 2508 | 62.72 | 14.10 |
| All groups combined | 4808 | 4808 | 65.87 | 12.91 |
Those who expressed an interest in participating were approached for screening by the CRN team. A total of 71 patients were not contactable at this stage; of those whom the CRN teams were able to reach, 281 were deemed ineligible, 26 were unwilling to proceed further and 1293 were found to be eligible. Men who were eligible at CRN screening and were willing to participate in the trial tended to be slightly younger than those eligible but unwilling to participate, although comparable to those ineligible at screening (mean age 68.3 years vs. 74.1 years vs. 70.7 years) (Table 5).
| Eligibility and willingness to participate | N | n a | Mean age (years) | SD |
|---|---|---|---|---|
| Eligible at CRN screening and willing to participate in the trial | 1293 | 1293 | 68.32 | 9.72 |
| Eligible at CRN screening and not willing to participate in the trial | 26 | 26 | 74.12 | 8.05 |
| Ineligible at CRN screening | 281 | 281 | 70.68 | 10.62 |
| Not contactable by the CRN | 71 | 71 | 66.45 | 13.70 |
| All groups combined | 1671 | 1671 | 68.68 | 10.13 |
Patients who were found to be eligible at the CRN screening stage were invited to participate in the trial and 1077 consented [intervention practices: n = 524 (74%); usual-care practices: n = 553 (94%)]. The men who were eligible and consented to participate tended to be slightly older than those who were eligible and did not consent (68.7 years vs. 66.5 years, respectively) (Table 6).
| Eligible men | All practices | Intervention practices | Usual-care practices | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n a | Mean | SD | Minimum, maximum | N | n a | Mean | SD | Minimum, maximum | N | n a | Mean | SD | Minimum, maximum | |
| Eligible men who consented to participate | 1077 | 1077 | 68.68 | 9.26 | 30, 95 | 524 | 524 | 68.95 | 9.27 | 32, 94 | 553 | 553 | 68.44 | 9.25 | 30, 95 |
| Eligible men who did not consent to participate | 216 | 216 | 66.51 | 11.61 | 26, 90 | 181 | 181 | 66.22 | 11.89 | 26, 90 | 35 | 35 | 68.03 | 10.10 | 38, 89 |
| Both groups combined | 1293 | 1293 | 68.32 | 9.72 | 26, 95 | 705 | 705 | 68.25 | 10.07 | 26, 94 | 588 | 588 | 68.41 | 9.29 | 30, 95 |
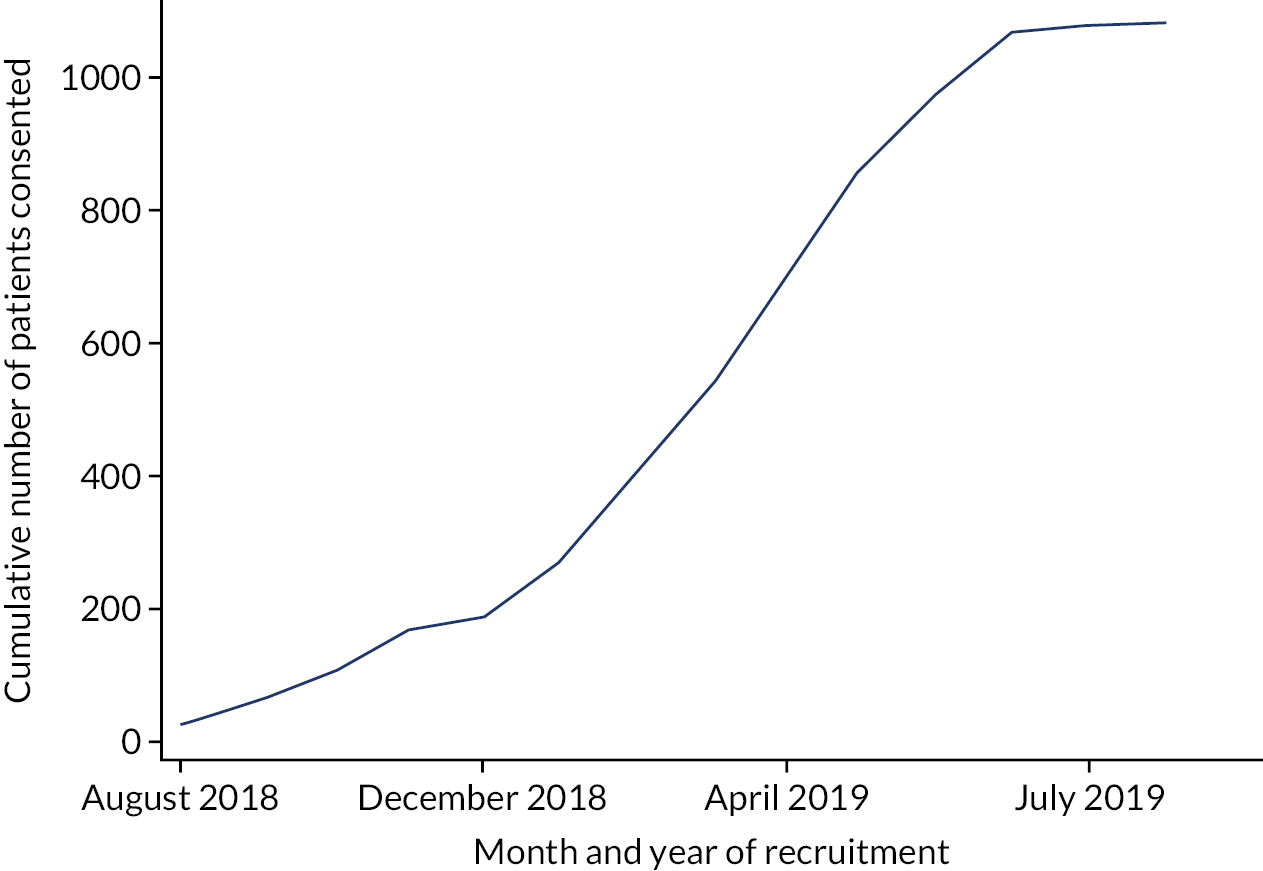
Patient recruitment over time is illustrated in Figure 3 and recruitment by practice is presented in Appendix 1, Table 40.
FIGURE 3.
Cumulative patient recruitment over time.
The CONSORT flow diagram (see Figure 1) describes how and when participants were lost to follow-up between consent and 12 months. Ultimately, 154 participants were lost to follow-up and 915 of the recruited 1077 participants (85.0%) provided primary outcome data at 12 months.
Pilot phase
The 4-month internal pilot phase of the trial was successfully completed in November 2018. A total of 142 participants were recruited, exceeding the target of 120 participants. Sixteen practices had formally agreed to take part in the trial during the pilot phase, which, although slightly short of the target of 18, was sufficient for progression to the main phase of the trial. The key changes that were made to the conduct of the trial between the pilot phase and the main phase of the trial were an increase in the maximum number of patients invited by a single site from 150 to 220, and confirmation that 30 sites would be required to maintain the trial power calculation in the light of variation in the number of patients recruited per site. The decision was also taken to conduct only a single invitation mail-out from each site, rather than inviting additional patients in a second mail-out to achieve site targets as necessary, to avoid introducing any bias post randomisation. Although screening of all patients for both mail-outs would have been conducted before randomisation, rescreening would have been required to check patient status before a later, second, mail-out, when sites would have been aware of their allocation.
Baseline data
The baseline characteristics of the participants of the TRIUMPH trial are presented in Table 7. The mean age was 69 years in the intervention arm and 68 years in the usual-care arm, although the trial included some younger men who were in their thirties. In both arms, participants were overwhelmingly white, with only 13 participants overall describing themselves as being from another ethnic group. In both arms, most men were married or in civil partnerships. Socioeconomic status was determined using area-level IMD based on the home postcode; participants were from all five quintiles of deprivation, although the proportion from the least deprived quintile was in both arms higher than the 20% we would expect if participants were equally distributed by quintile (intervention arm, 49.0%; usual-care arm, 42.9%). Participants self-reported height and weight; mean values of both were comparable between treatment arms.
| Characteristics | Intervention | Usual care | ||
|---|---|---|---|---|
| n a | Value | n a | Value | |
| Total number of participants (N) | 524 | 553 | ||
| Demographic characteristics | ||||
| Age (years), mean (SD) [minimum, maximum] | 524 | 68.95 (9.27) [32, 94] | 553 | 68.44 (9.25) [30, 95] |
| Ethnicity, n (%) | 522 | 550 | ||
| White | 513 (98.28) | 542 (98.55) | ||
| Black/African/Caribbean/Black British | 1 (0.19) | 1 (0.18) | ||
| Mixed/multiple ethnic groups | 2 (0.38) | 2 (0.36) | ||
| Asian/Asian British | 3 (0.57) | 2 (0.36) | ||
| Other ethnic group | 2 (0.38) | 0 (0.00) | ||
| Disclosure declined | 1 (0.19) | 3 (0.55) | ||
| Marital status, n (%) | 517 | 543 | ||
| Single | 21 (4.06) | 25 (4.60) | ||
| Married | 429 (82.98) | 440 (81.03) | ||
| Civil partnered | 7 (1.35) | 15 (2.76) | ||
| Divorced | 31 (6.00) | 32 (5.89) | ||
| Widowed | 27 (5.22) | 28 (5.16) | ||
| Disclosure declined | 2 (0.39) | 3 (0.55) | ||
| IMD score, median (IQR) [minimum, maximum] | 506 | 8.80 (5.75–13.71) [1.18, 60.30] | 525 | 9.89 (6.21–15.45) [1.64, 55.13] |
| IMD quintile, n (%) | 506 | 525 | ||
| 1 (most deprived) | 17 (3.36) | 21 (4.00) | ||
| 2 | 33 (6.52) | 37 (7.05) | ||
| 3 | 67 (13.24) | 106 (20.19) | ||
| 4 | 141 (27.87) | 136 (25.90) | ||
| 5 (least deprived) | 248 (49.01) | 225 (42.86) | ||
| Clinical characteristics | ||||
| Height (cm), mean (SD) [minimum, maximum] | 518 | 176.72 (6.77) [152.40, 198.12] | 550 | 176.93 (7.41) [157.48, 208.28] |
| Weight (kg), mean (SD) [minimum, maximum] | 510 | 83.35 (14.45) [55.02, 152.41] | 549 | 83.89 (14.29) [53.98, 136.98] |
| EMRs search data | ||||
| Number of comorbidities, n (%) | 478 | 544 | ||
| 0 | 151 (31.59) | 171 (31.43) | ||
| 1 | 160 (33.47) | 197 (36.21) | ||
| > 1 | 167 (34.94) | 176 (32.35) | ||
| Most recent urine analysis results in the 6 months pre baseline: abnormal, n (%) | 79 | 1 (1.27) | 52 | 2 (3.85) |
| Kidney function: most recent eGFR (ml/minute/1.73m2) measure in the 6 months pre baseline | 170 | 215 | ||
| Number of patients with an eGFR measure | 170 | 215 | ||
| eGFR: mean (SD) | 73.46 (15.72) | 74.56 (13.17) | ||
| eGFR: median (IQR) | 76.5 (65–87) | 75 (66–87) | ||
| eGFR: minimum, maximum | 28, 98 | 36, 100 | ||
| CKD stages based on most recent eGFR in the 6 months pre baseline, n (%) | 170 | 215 | ||
| ≥ 90 ml/minute/1.73 m2 (normal) | 28 (16.47) | 33 (15.35) | ||
| 60–90 ml/minute/1.73 m2 (CKD stages G1 and G2) | 114 (67.06) | 154 (71.63) | ||
| 30–59 ml/minute/1.73 m2 (CKD stage G3) | 27 (15.88) | 28 (13.02) | ||
| < 30 ml/minute/1.73 m2 (CKD stages G4 and G5) | 1 (0.59) | 0 (0.00) | ||
| Number of GP consultations in the 12 months before baseline | 478 | 544 | ||
| Mean (SD) | 4.4 (3.7) | 4.8 (5.0) | ||
| Median (IQR) | 4 (2-6) | 4 (2-6) | ||
| Minimum, maximum | 0, 23 | 0, 58 | ||
| Referrals to urology in the 12 months pre baseline, n (%) | 478 | 544 | ||
| None | 464 (97.07) | 525 (96.51) | ||
| One | 14 (2.93) | 19 (3.49) | ||
| More than one | 0 (0.00) | 0 (0.00) | ||
| Patient-reported symptoms and quality of life | ||||
| IPSS symptoms, mean (SD) [minimum, maximum] | ||||
| Incomplete emptying | 512 | 1.66 (1.46) [0, 5] | 549 | 1.85 (1.49) [0, 5] |
| Frequency | 514 | 2.68 (1.33) [0, 5] | 551 | 2.92 (1.37) [0, 5] |
| Intermittency | 514 | 1.87 (1.64) [0, 5] | 549 | 1.96 (1.69) [0, 5] |
| Urgency | 513 | 2.14 (1.61) [0, 5] | 549 | 2.35 (1.66) [0, 5] |
| Weak stream | 510 | 1.93 (1.53) [0, 5] | 549 | 2.02 (1.66) [0, 5] |
| Straining | 513 | 0.85 (1.19) [0, 5] | 548 | 0.99 (1.31) [0, 5] |
| Nocturia | 516 | 2.59 (1.36) [0, 5] | 551 | 2.43 (1.25) [0, 5] |
| Total IPSS score, mean (SD) [minimum, maximum] | 501 | 13.62 (5.83) [1, 33] | 541 | 14.59 (6.58) [2, 34] |
| IPSS-QoL score, mean (SD) [minimum, maximum] | 516 | 3.47 (1.19) [0, 6] | 551 | 3.55 (1.13) [0, 6] |
| ICIQ-UI-SF total score, mean (SD) [minimum, maximum] | 513 | 3.57 (3.57) [0, 14] | 542 | 3.93 (3.66) [0, 15] |
| ICIQ-UI-SF: when does urine leak?, n (%) | 523 | 553 | ||
| Never | 185 (35.37) | 162 (29.29) | ||
| Leaks before you can get to the toilet | 205 (39.20) | 237 (42.86) | ||
| Leaks when you cough/sneeze | 24 (4.59) | 24 (4.34) | ||
| Leaks when you are asleep | 12 (2.29) | 15 (2.71) | ||
| Leaks when you are physically active | 23 (4.40) | 27 (4.88) | ||
| Leaks when you have finished urinating/are dressed | 175 (33.46) | 205 (37.07) | ||
| Leaks for no obvious reason | 36 (6.88) | 42 (7.59) | ||
| Leaks all of the time | 1 (0.19) | 1 (0.18) | ||
| EQ-5D-5L utility score, median (IQR) [minimum, maximum]b | 522 | 0.84 (0.77–1) [–0.118, 1] | 547 | 0.84 (0.75–1) [–0.06, 1] |
| EQ VAS score, median (IQR) [minimum, maximum] | 522 | 80 (70–90) [15, 100] | 551 | 80 (70–90) [15, 100] |
| B-IPQ total score, mean (SD) [minimum, maximum] | 440 | 38.74 (11.02) [1, 75] | 478 | 39.40 (10.36) [6, 72] |
| Bladder diary, n (%) | ||||
| Incontinence | 502 | 100 (19.92) | N/A | N/A |
| Urgency | 507 | 364 (71.79) | ||
| Nocturiac | 261 | 222 (85.06) | ||
Electronic medical records were used to gather further information on the clinical characteristics at baseline. As not all practices were able to provide such data, more data were missing in the intervention arm. Approximately one-third of participants had no record of comorbidities, one-third had one comorbidity and one-third had more than one comorbidity. The distribution was comparable between treatment arms. The median number of GP consultations in the 12 months before baseline was the same in both arms (4 consultations), but the proportion with a referral to urological services in that period was slightly lower in the intervention arm than in the usual-care arm (intervention arm, 2.93%; usual-care arm, 3.49%).
We also used primary care EMRs to assess recent urine and kidney function tests. Few men in either arm reported having undergone urine analyses in the 6 months before baseline (intervention arm, n = 79; usual-care arm, n = 52), and few of these had abnormal test results (intervention arm, 1.27%; usual-care arm, 3.85%). Kidney function testing was more common (intervention arm, n = 170; usual-care arm, n = 215), and the most recent eGFR measure was similar in both arms. The majority of men in both arms who had such testing had chronic kidney disease stage 1 or 2 (intervention arm, 67.1%; usual-care arm, 71.63%).
International Prostate Symptom Score, the primary outcome measure, was measured at baseline; the total score was slightly lower in the intervention arm (mean total score 13.62 points) than in the usual-care arm (mean total score 14.59 points). When examining individual symptoms, the symptoms with the highest mean scores were frequency, nocturia and urgency. The IPSS-QoL scores were very similar in both arms (intervention: 3.47 points; usual care: 3.55 points).
The ICIQ-UI-SF total symptom scores were similar in both groups (intervention arm, mean of 3.6, usual care arm, mean of 3.9). When asked when men leaked urine, the most common responses were before getting to the toilet (intervention, 39.2%; usual care, 42.86%), and when finished urinating and dressed (intervention arm, 33.46%; usual-care arm, 37.07%). Approximately one-third of men in each arm said they never leaked (intervention arm, 35.37%; usual-care arm, 29.29%). Men in the intervention arm completed a bladder diary at baseline to help inform intervention delivery; they most frequently reported nocturia (85.06%) and urgency (71.79%). The EQ-5D-5L data were comparable in both arms, as were the B-IPQ data.
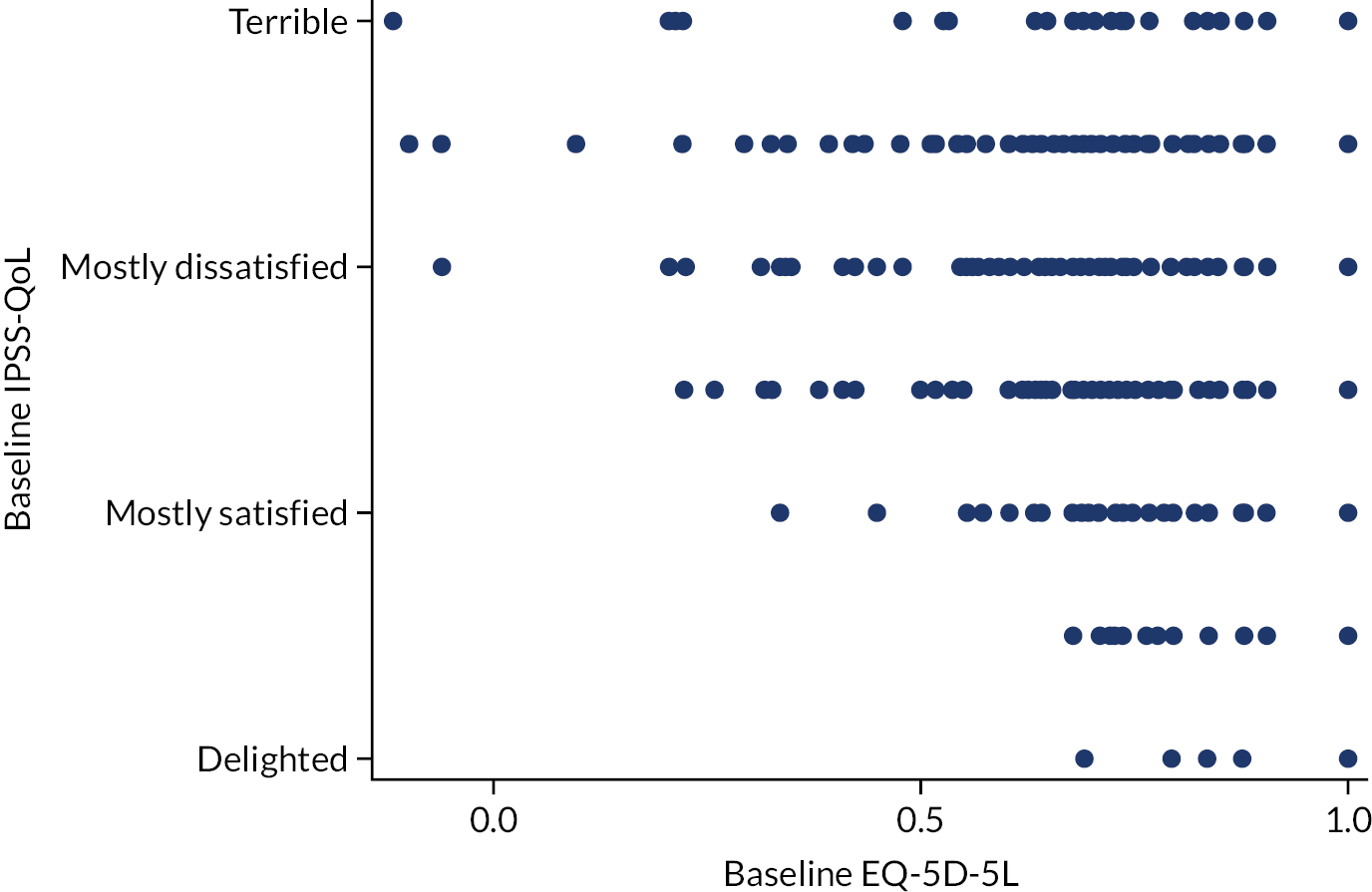
To explore whether or not the disease-specific IPSS-QoL score yields information different from the EQ-5D-5L, we plotted the baseline values of one versus the other (Figure 4). The correlation coefficient of –0.22 suggests a weak to no linear association between the two measures, that the IPSS-QoL score yields information different from that of the EQ-5D-5L and that the use of both is not redundant.
FIGURE 4.
Relationship between the EQ-5D-5L and IPSS-QoL scores at baseline.
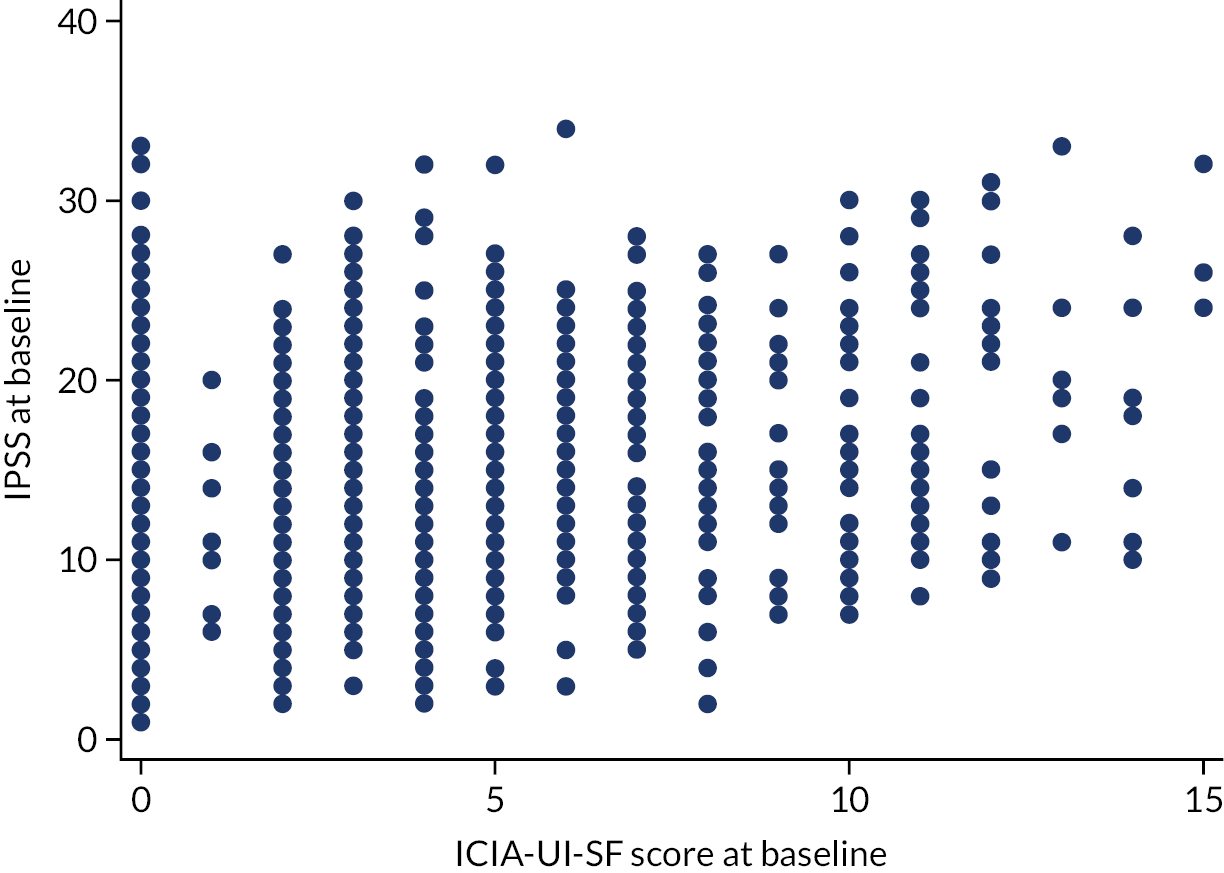
The IPSS does not capture all urinary symptoms; leakage is captured only in the ICIQ-UI-SF. Therefore, we explored how IPSS at baseline correlated with ICIQ-UI-SF scores at baseline. A scatterplot of the two variables is presented in Figure 5. There was little evidence that the two measures were strongly correlated (p = 0.28), suggesting that those with a higher IPSS do not necessarily have higher ICIQ-UI-SF scores, and vice versa, thus confirming the value of both measures.
FIGURE 5.
Baseline IPSS and baseline ICIQ-UI-SF scores.
As well as using EMRs to identify GP consultations and clinical test results, we also explored the prescriptions that patients were issued in the 3 months pre baseline with a potential indication for treating LUTS (Table 8). Relevant medications for LUTS are alpha-adrenergic antagonists (alfuzosin, doxazosin, tamsulosin) and phosphodiesterase-5 inhibitors (tadalafil) to treat bladder outlet obstruction, and antimuscarinics (oxybutynin, solifenacin, tolterodine, trospium) and beta-3 agonist (mirabegron) to treat overactive bladder syndrome. Approximately 40% of men had at least one prescription of this type in this period. The most common prescription by far was tamsulosin, prescribed to 30.5% of men in the intervention arm and 34.01% of men in the usual-care arm. Oxybutynin and doxazosin were the next most commonly prescribled treatments; both were more common in the usual-care arm.
| EMR search | Trial group | |
|---|---|---|
| Intervention | Usual care | |
| Total number of patients | 524 | 553 |
| Number of patients for whom EMR data were available | 478 | 544 |
| Patients having at least one relevant prescription, n (%) | 188 (39.33) | 227 (41.72) |
| List of medications as observed, n (%) | ||
| Tamsulosin | 146 (30.54) | 185 (34.01) |
| Oxybutynin | 8 (1.67) | 24 (4.41) |
| Doxazosin | 2 (0.42) | 19 (3.49) |
| Solifenacin | 17 (3.56) | 14 (2.57) |
| Tolterodine | 9 (1.88) | 12 (2.21) |
| Mirabegron | 11 (2.30) | 9 (1.65) |
| Alfuzosin | 6 (1.26) | 3 (0.55) |
| Tadalafil | 1 (0.21) | 2 (0.37) |
| Trospium | 0 (0) | 1 (0.18) |
| Solifenacin/tamsulosin fixed-dose combination | 4 (0.84) | 0 (0) |
Intervention delivery
Seven out of 16 West of England sites and 10 out of 14 Wessex sites were randomised to the intervention arm. The intervention was delivered by the TRIUMPH trial research nurses at nine sites (three West of England, six Wessex), by practice nurses/HCAs at six sites (three West of England, three Wessex) and by both at two sites (one West of England, one Wessex) (Table 9). Overall, compliance with the intervention visit and follow-up contacts was very high (see Table 9), as was adherence to patient preferences in the format of their follow-up visits (Table 10).
| Site ID | Centre | Delivered by practice nurse/HCA or TRIUMPH nurse | Number of patients consented | Intervention visit, n (%) | 1-week follow-up, n (%) | 4-week follow-up, n (%) | 12-week follow-up, n (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients receiving this stage of the intervention | Loss to follow-up | Patients receiving this stage of the intervention | Loss to follow-up | Patients receiving this stage of the intervention | Loss to follow-up | Patients receiving this stage of the intervention | Loss to follow-up | ||||
| B10 | WoE | TRIUMPH | 32 | 32 (100) | 0 (0) | 32 (100) | 0 (0) | 31 (96.9) | 1 (3.1) | 32 (100) | 0 (0) |
| B11 | WoE | TRIUMPH | 35 | 34 (97.1) | 1 (2.9) | 34 (97.1) | 1 (2.9) | 34 (97.1) | 1 (2.9) | 34 (97.1) | 1 (2.9) |
| B12 | WoE | Practice nurse/HCA | 40 | 38 (95.0) | 2 (5.0) | 38 (95.0) | 2 (5.0) | 38 (95.0) | 2 (5.0) | 36 (90.0) | 4 (10.0) |
| B13 | WoE | Practice nurse/HCA | 20 | 20 (100) | 0 (0) | 20 (100) | 0 (0) | 20 (100) | 0 (0) | 20 (100) | 0 (0) |
| B22 | WoE | Practice nurse/HCA | 40 | 38 (95.0) | 2 (5.0) | 37 (92.5) | 3 (7.5) | 35 (87.5) | 5 (12.5) | 31 (77.5) | 9 (22.5) |
| B32–B34 | WoE | Mixed | 63 | 63 (100) | 0 (0) | 59 (93.7) | 4 (6.3) | 63 (100) | 0 (0) | 62 (98.4) | 1 (1.6) |
| B36 | WoE | TRIUMPH | 32 | 32 (100) | 0 (0) | 31 (96.9) | 1 (3.1) | 32 (100) | 0 (0) | 32 (100) | 0 (0) |
| S15 | Wessex | Practice nurse/HCA | 24 | 22 (91.7) | 2 (8.3) | 20 (83.3) | 4 (16.7) | 21 (87.5) | 3 (12.5) | 21 (87.5) | 3 (12.5) |
| S18 | Wessex | Practice nurse/HCA | 25 | 25 (100) | 0 (0) | 25 (100) | 0 (0) | 25 (100) | 0 (0) | 25 (100) | 0 (0) |
| S19 | Wessex | TRIUMPH | 13 | 13 (100) | 0 (0) | 13 (100) | 0 (0) | 13 (100) | 0 (0) | 12 (92.3) | 1 (7.7) |
| S21 | Wessex | TRIUMPH | 29 | 28 (96.6) | 1 (3.4) | 26 (89.7) | 3 (10.3) | 27 (93.1) | 2 (6.9) | 28 (96.6) | 1 (3.4) |
| S26 | Wessex | TRIUMPH | 41 | 41 (100) | 0 (0) | 39 (95.1) | 2 (4.9) | 40 (97.6) | 1 (2.4) | 40 (97.6) | 1 (2.4) |
| S27 | Wessex | TRIUMPH | 36 | 36 (100) | 0 (0) | 35 (97.2) | 1 (2.8) | 35 (97.2) | 1 (2.8) | 35 (97.2) | 1 (2.8) |
| S28 | Wessex | Practice nurse/HCA | 15 | 15 (100) | 0 (0) | 7 (46.7) | 8 (53.3) | 13 (86.7) | 2 (13.3) | 13 (86.7) | 2 (13.3) |
| S30 | Wessex | Mixed | 29 | 28 (96.6) | 1 (3.4) | 25 (86.2) | 4 (13.8) | 27 (93.1) | 2 (6.9) | 28 (96.6) | 1 (3.4) |
| S40 | Wessex | TRIUMPH | 33 | 33 (100) | 0 (0) | 33 (100) | 0 (0) | 33 (100) | 0 (0) | 33 (100) | 0 (0) |
| S41 | Wessex | TRIUMPH | 17 | 17 (100) | 0 (0) | 15 (88.2) | 2 (11.8) | 17 (100) | 0 (0) | 17 (100) | 0 (0) |
| Across WoE practices | 262 | 257 (98.1) | 5 (1.9) | 251 (95.8) | 11 (4.2) | 253 (96.6) | 9 (3.4) | 247 (94.3) | 15 (5.7) | ||
| Across Wessex practices | 262 | 258 (98.5) | 4 (1.5) | 238 (90.8) | 24 (9.2) | 251 (95.8) | 11 (4.2) | 252 (96.2) | 10 (3.8) | ||
| Formats and preferences | N or n (%) |
|---|---|
| Number of participants in the intervention arm | 524 |
| Participants who received the booklet | 516 (98) |
| Participants who had their telephone call at week 1 | 489 (93) |
| Participants who had all three contacts | 470 (90) |
| Week 4 | |
| Preference | |
| N | 508 |
| Phone | 364 |
| Text | 18 |
| 126 | |
| Method of contact | |
| N | 501 |
| Phone | 356 |
| Text | 24 |
| 121 | |
| Week 12 | |
| Preference | |
| N | 508 |
| Phone | 348 |
| Text | 17 |
| 143 | |
| Method of contact | |
| N | 498 |
| Phone | 323 |
| Text | 26 |
| 149 | |
| Participants who had their contact in the preferred method at week 4 | 477 (91) |
| Participants who had their contact in the preferred method at week 12 | 475 (91) |
| Participants who had the week 1 contact by telephone and both the 4- and 12-week visits in their preferred method | 414 (79) |
Participants were directed to different sections of the intervention booklet based on their baseline data, including the bladder diary. We recorded the sections of the booklet that each participant was referred to in the trial case report forms, and compared the baseline IPSS and ICIQ-UI-SF scores between groups (see Appendix 1, Table 41). The most commonly referred booklet sections related to ‘drinks and water intake’, ‘controlling an urgent need to pass urine’ and ‘reducing sleep disturbance caused by needing to pass urine’.
Protocol deviations
No serious protocol deviations were experienced during the trial. Protocol deviations are summarised in Table 11 and detailed in Appendix 1, Table 42.
| Protocol deviation category | Trial group (n) | Overall (N) | |
|---|---|---|---|
| Intervention | Control | ||
| Consent | 3 | 0 | 3 |
| Intervention visit/booklet delivery | 4 | 0 | 4 |
| Intervention follow-up contacts | 88 | 0 | 88 |
| Follow-up | 16 | 1 | 17 |
| COVID-19 | 74 | 93 | 167 |
| Total | 185 | 94 | 279 |
Follow-up rates and International Prostate Symptom Score data completeness
Follow-up rates were monitored by the practice over the trial period and are described in Table 12. All had at least 80% follow-up for the 12-month visit. Completeness of the primary outcome (IPSS at 12 months) ranged from 75.8% to a high of 95.5%. Overall completeness of the primary outcome was > 80% in both arms.
| Centre | Arm | Practice ID | Total number of patients consented | Follow-up,a n (%) | Complete IPSS, n (%) | ||
|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 6 months | 12 months | ||||
| WoE | Intervention | B10 | 32 | 30 (93.8) | 29 (90.6) | 28 (87.5) | 29 (90.6) |
| WoE | Intervention | B11 | 35 | 32 (91.4) | 30 (85.7) | 32 (91.4) | 30 (85.7) |
| WoE | Intervention | B12 | 40 | 37 (92.5) | 38 (95.0) | 36 (90.0) | 37 (92.5) |
| WoE | Intervention | B13 | 20 | 17 (85.0) | 16 (80.0) | 17 (85.0) | 16 (80.0) |
| WoE | Intervention | B22 | 40 | 36 (90.0) | 34 (85.0) | 33 (82.5) | 32 (80.0) |
| WoE | Intervention | B32-B34 | 63 | 57 (90.5) | 55 (87.3) | 55 (87.3) | 52 (82.5) |
| WoE | Intervention | B36 | 32 | 30 (93.8) | 26 (81.3) | 28 (87.5) | 25 (78.1) |
| Wessex | Intervention | S15 | 24 | 22 (91.7) | 22 (91.7) | 22 (91.7) | 21 (87.5) |
| Wessex | Intervention | S18 | 25 | 25 (100) | 24 (96.0) | 24 (96.0) | 23 (92.0) |
| Wessex | Intervention | S19 | 13 | 11 (84.6) | 11 (84.6) | 10 (76.9) | 10 (76.9) |
| Wessex | Intervention | S21 | 29 | 27 (93.1) | 27 (93.1) | 24 (82.8) | 25 (86.2) |
| Wessex | Intervention | S26 | 41 | 38 (92.7) | 38 (92.7) | 36 (87.8) | 36 (87.8) |
| Wessex | Intervention | S27 | 36 | 35 (97.2) | 33 (91.7) | 35 (97.2) | 32 (88.9) |
| Wessex | Intervention | S28 | 15 | 15 (100) | 13 (86.7) | 15 (100) | 12 (80.0) |
| Wessex | Intervention | S30 | 29 | 28 (96.6) | 25 (86.2) | 27 (93.1) | 22 (75.9) |
| Wessex | Intervention | S40 | 33 | 32 (97.0) | 28 (84.9) | 32 (97.0) | 25 (75.8) |
| Wessex | Intervention | S41 | 17 | 17 (100) | 15 (88.2) | 17 (100) | 15 (88.2) |
| Across intervention practices | 524 | 489 (93.3) | 464 (88.6) | 471 (89.9) | 442 (84.4) | ||
| WoE | Control | B16 | 22 | 21 (95.5) | 21 (95.5) | 21 (95.5) | 21 (95.5) |
| WoE | Control | B20 | 55 | 50 (90.9) | 50 (90.9) | 49 (89.1) | 49 (89.1) |
| WoE | Control | B24 | 37 | 33 (89.2) | 32 (86.5) | 33 (89.2) | 31 (83.8) |
| WoE | Control | B25 | 46 | 44 (95.7) | 41 (89.1) | 42 (91.3) | 41 (89.1) |
| WoE | Control | B31 | 50 | 43 (86.0) | 42 (84.0) | 40 (80.0) | 40 (80.0) |
| WoE | Control | B35 | 52 | 48 (92.3) | 42 (80.8) | 46 (88.5) | 40 (76.9) |
| WoE | Control | B37 | 36 | 32 (88.9) | 31 (86.1) | 32 (88.9) | 30 (83.3) |
| WoE | Control | B38 | 55 | 54 (98.2) | 48 (87.3) | 53 (96.4) | 47 (85.5) |
| WoE | Control | B39 | 58 | 54 (93.1) | 50 (86.2) | 53 (91.4) | 48 (82.8) |
| Wessex | Control | S14 | 22 | 22 (100) | 21 (95.5) | 21 (95.5) | 21 (95.5) |
| Wessex | Control | S17 | 32 | 31 (96.9) | 30 (93.8) | 30 (93.8) | 30 (93.8) |
| Wessex | Control | S23 | 54 | 50 (92.6) | 47 (87.0) | 48 (88.9) | 45 (83.3) |
| Wessex | Control | S29 | 34 | 33 (97.1) | 31 (91.2) | 33 (97.1) | 30 (88.2) |
| Across usual-care practices | 553 | 515 (93.1) | 486 (88.0) | 501 (90.6) | 473 (85.5) | ||
Twenty-one participants withdrew from the trial, 17 from the intervention arm (Table 13). The most common reasons for withdrawal were ill health and not having sufficient time/finding the trial too inconvenient/not wanting to continue. The nature and level of individual withdrawals from the trial are outlined in Appendix 1, Table 43.
| Reason for withdrawal | Overall (N) | Trial group (n) | |
|---|---|---|---|
| Intervention | Control | ||
| Change in symptoms | 3 | 2 | 1 |
| Died | 3 | 2 | 1 |
| Ill health | 6 | 4 | 2 |
| No reason given | 3 | 3 | 0 |
| No time/too inconvenient/did not want to continue | 5 | 5 | 0 |
| Consented to another LUTS study | 1 | 1 | 0 |
| Total | 21 | 17 | 4 |
Data quality
Every effort was made to ensure that outcome data were collected at the appropriate month from baseline. In both arms, 12-month questionnaires were completed near the follow-up time point as intended (mean number of months since baseline: intervention arm, 12.30; usual-care arm, 12.18).
The pattern of missing data was explored by identifying variables recorded at baseline that were associated with ‘missingness’ of IPSS at 6 and 12 months (primary outcome). Appendix 1, Table 44, presents the frequency and proportion of missing IPSS data at 6 months by baseline characteristics. We observed that missingness seems to be associated with marital status, as those with complete data were more likely than those with missing data to be married. There was no evidence of a difference in missingness by baseline IPSS, but those with missing IPSS at 6 months tended to have slightly higher B-IPQ and ICIQ-UI-SF scores. These differences, however, were not observed when looking at missingness of IPSS at 12 months (see Appendix 1, Table 45).
Statistical outcomes and estimation
Primary outcome
The mean IPSS at follow-up was lower than at baseline in both treatment groups. When comparing groups, scores at follow-up were slightly lower in the intervention arm [mean 11.60 (SD 6.21)] than in the usual-care arm [mean 13.88 (SD 6.84)], suggesting that symptoms were worse in the usual-care arm (Table 14). After adjusting for baseline IPSS and minimisation variables, the between-arm difference was –1.81 (95% CI –2.66 to –0.95; p < 0.001), strongly suggesting a treatment effect. The treatment effect was similar when adjusting only for baseline values and not variables used in the randomisation.
| Trial arm | IPSS at baseline | IPSS at 12 months | Crude difference in mean IPSS between baseline and 12 months | Difference in meansa (95% CI) | p-value | Difference in meansb (95% CI) | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum, maximum | n | Mean | SD | Minimum, maximum | ||||||
| Intervention | 501 | 13.62 | 5.83 | 1, 33 | 442 | 11.60 | 6.21 | 1, 35 | –2.13 | –1.81 (–2.66 to –0.95) | < 0.001 | –1.79 (–2.56 to –1.01) | < 0.001 |
| Usual care | 511 | 14.59 | 6.58 | 2, 34 | 473 | 13.88 | 6.84 | 0, 32 | –0.58 | ||||
| Total | 915 | 12.78 | 6.64 | 0, 35 | |||||||||
| ICC (95% CI) | 0.011 (0.001 to 0.086) | 0.014 (0.002 to 0.080) | |||||||||||
A summary of symptom-specific scores for the IPSS and ICIQ-UI-SF at each time point is presented in Appendix 1, Table 46.
Secondary outcomes
Urinary symptoms over time
International Prostate Symptom Scores were studied at 6 months as a secondary outcome and, as with the primary outcome analysis, there was evidence that there was a greater improvement in symptoms in the intervention arm than in the usual-care arm (Table 15). The treatment effect, however, was slightly smaller than for the primary outcome (–1.68, 95% CI –2.34 to –1.02; p < 0.001).
| Trial arm | n | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value | Difference in meansb (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Intervention | 471 | 11.51 | 6.14 | 1, 35 | –1.68 (–2.34 to –1.02) | < 0.001 | –1.83 (–2.50 to –1.17) | < 0.001 |
| Usual care | 501 | 13.76 | 6.58 | 1, 32 | ||||
| Total | 972 | 12.67 | 6.47 | 1, 35 | ||||
| ICC (95% CI) | 0.000 (0.000 to 0.000) | 0.009 (0.001 to 0.093) | ||||||
Similar results were observed when a repeated-measures approach was used to study IPSS using both 6- and 12-month follow-up data (Table 16).
| Trial arm | Number of repeated measures | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value | Difference in meansb (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Intervention | 913 | 11.56 | 6.17 | 1, 35 | –1.70 (–2.35 to –1.05) | < 0.001 | –1.80 (–2.44 to –1.16) | < 0.001 |
| Usual care | 974 | 13.82 | 6.71 | 0, 32 | ||||
| Total | 1887 | 12.73 | 6.55 | 0, 35 | ||||
| ICC (95% CI) | 0.005 (0.0002 to 0.115) | 0.011 (0.002 to 0.056) | ||||||
Trajectories in IPSSs over time were also described by tabulating the number and percentage of men who move between IPSS categories between baseline and 6 months or between 6 and 12 months (see Appendix 1, Table 47). Nearly half of all men (47.5%) stayed in the same category and 37.1% improved over the 12 months. Improvement was slightly more common in the intervention arm.
Incontinence
Patient-reported incontinence symptoms were assessed using the ICIQ-UI-SF at 6 and 12 months. At both time points, scores were higher, suggestive of worse symptoms, in the usual-care arm (Table 17). After adjusting for baseline ICIQ-UI-SF scores and variables used in the minimisation, scores were slightly lower in the intervention arm than in the usual-care arm at both time points (difference in means at 6 months: –0.53, 95% CI –1.02 to –0.04; p = 0.033; difference in means at 12 months: –0.74, 95% CI –1.15 to –0.33; p < 0.001).
| Trial arm | n | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value | Difference in meansb (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| 6 months | ||||||||
| Intervention | 476 | 3.58 | 3.47 | 0, 15 | –0.53 (–1.02 to –0.04) | 0.04 | –0.74 (–1.22 to –0.27) | 0.004 |
| Usual care | 504 | 4.48 | 4.13 | 0, 20 | ||||
| Total | 980 | 4.04 | 3.85 | 0, 20 | ||||
| ICC (95% CI) | 0.022 (0.007 to 0.072) | 0.033 (0.013 to 0.084) | ||||||
| 12 months | ||||||||
| Intervention | 453 | 3.66 | 3.61 | 0, 18 | –0.74 (–1.15 to –0.33) | < 0.001 | –0.66 (–1.04 to –0.29) | 0.001 |
| Usual care | 480 | 4.53 | 4.14 | 0, 18 | ||||
| Total | 933 | 4.11 | 3.91 | 0, 18 | ||||
| ICC (95% CI) | 0.000 (0.000 to 0.000) | 0.004 (< 0.001 to 0.375) | ||||||
Quality of life
Lower urinary tract symptom-specific quality of life was assessed using the IPSS-QoL measure at 6 and 12 months. Scores range from 0 to 6, with higher scores reflecting worse quality of life. At both time points, scores were slightly higher in the usual-care arm; this was also observed in models adjusting for baseline scores and variables used in the randomisation (Table 18).
| Trial arm | n | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value | Difference in meansb (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| 6 months | ||||||||
| Intervention | 483 | 3.02 | 1.19 | 0, 6 | –0.29 (–0.43 to –0.15) | < 0.001 | –0.28 (–0.41 to –0.14) | < 0.001 |
| Usual care | 511 | 3.35 | 1.25 | 0, 6 | ||||
| Total | 994 | 3.19 | 1.23 | 0, 6 | ||||
| ICC (95% CI) | Could not be estimated | 0.006 (< 0.001 to 0.166) | ||||||
| 12 months | ||||||||
| Intervention | 463 | 2.90 | 1.31 | 0, 6 | –0.34 (–0.50 to –0.18) | < 0.001 | –0.31 (–0.46 to –0.17) | < 0.001 |
| Usual care | 483 | 3.27 | 1.25 | 0, 6 | ||||
| Total | 946 | 3.09 | 1.29 | 0, 6 | ||||
| ICC (95% CI) | 0.004 (< 0.001 to 0.274) | 0.004 (< 0.001 to 0.251) | ||||||
Lower urinary tract symptom perception
Patient perception of LUTS was assessed at 6 and 12 months using the B-IPQ, where scores range from 0 to 90, with higher scores reflecting a more threatening view of their symptoms. The mean scores at baseline were 38.7 in the intervention arm and 39.4 in the usual-care arm. A greater decrease was seen in the intervention arm at 6 and 12 months (Table 19). At 6 months, the between-group difference after adjusting for baseline values and minimisation variables was –5.34 (95% CI –6.69 to –3.99); at 12 months the difference was slightly smaller (–4.78, 95% CI –6.31 to 3.25).
| Trial arm | n | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value | Difference in meansb (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| 6 months | ||||||||
| Intervention | 450 | 33.42 | 11.87 | 2, 73 | –5.34 (–6.69 to –3.99) | < 0.001 | –5.11 (–6.31 to –3.90) | < 0.001 |
| Usual care | 430 | 38.30 | 11.53 | 1, 76 | ||||
| Total | 880 | 35.80 | 11.95 | 1, 76 | ||||
| ICC (95% CI) | < 0.001 (< 0.001 to < 0.001) | < 0.001 (< 0.001 to < 0.001) | ||||||
| 12 months | ||||||||
| Intervention | 419 | 33.82 | 12.01 | 0, 69 | –4.78 (–6.31 to –3.25) | < 0.001 | –4.57 (–5.92 to –3.21) | < 0.001 |
| Usual care | 427 | 38.44 | 12.18 | 0, 71 | ||||
| Total | 846 | 36.15 | 12.31 | 0, 71 | ||||
| ICC (95% CI) | < Could not be estimated | < 0.001 (< 0.001 to < 0.001) | ||||||
Secondary care referrals
Overall, 7.6% of men received a referral to secondary care (urology) during the 12-month follow-up period. There was little difference between treatment arms after adjusting for minimisation variables and whether or not a referral had been made pre baseline (odds ratio 0.91, 95% CI 0.51 to 1.62) (Table 20).
| Trial arm | Participants in treatment arm (N) | Participants with referral, n (%) | ORa (95% CI) | p-value | ORb (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Intervention | 478 | 35 (7.3) | 0.91 (0.51 to 1.62) | 0.757 | 0.89 (0.52 to 1.54) | 0.685 |
| Usual care | 544 | 43 (7.9) | ||||
| Total | 1022 | 78 (7.6) | ||||
| ICC (95% CI) | 0.02 (0.00 to 0.41) | 0.04 (0.01 to 0.26) | ||||
Statistical ancillary analyses
Subgroup analyses
We characterised the nature of LUTS at baseline for participants by taking the ratio of the IPSS voiding to storage scores. Including this as a continuous interaction term in our model of IPSS at 12 months provided no evidence that the treatment effect differed according to this ratio (p = 0.971). Similarly, when we distinguished between those who received the intervention via a TRIUMPH nurse (n = 249) and those who received it via a practice nurse (n = 190), we found no strong evidence that the treatment effect differed (p = 0.387). There was, however, very weak evidence (p = 0.094) that the treatment effect differed by how intervention participants preferred follow-up by the clinical team (telephone, n = 310; text or e-mail, n = 210).
Subgroup analysis by the number of contact visits received was complicated by the fact that there was little variability in the data (Table 21). Only five intervention participants had only one follow-up contact, 25 had two follow-up contacts and the rest had three. Unsurprisingly, the results for those who received three follow-up contacts reflects the primary analysis results.
| Follow-up contacts (n) | Participants in each level of subgroup who provided IPSS at 12 months (n) | Difference in means (95% CI) | p-value |
|---|---|---|---|
| 1 | 5 | –0.68 (–5.07 to 3.70) | 0.760 |
| 2 | 25 | –1.27 (–3.41 to 0.86) | 0.242 |
| 3 | 407 | –1.84 (–2.70 to –0.99) | < 0.001 |
A post hoc subgroup analysis was conducted to assess whether or not treatment effects for the primary outcome (IPSS at 12 months) and IPSS-QoL (12 months) differed depending on whether the data were collected before or during the COVID-19 pandemic. The results are summarised in Tables 22 and 23. We observed no strong evidence that the treatment effect on IPSS-QoL differed by COVID-19 period (p = 0.383), although there was some difference in the primary outcome (p = 0.010), in that the treatment effect was slightly greater in the pre-COVID period. These results must be interpreted with caution, however, as these are exploratory and the study was not powered to detect such effects.
| Interaction with treatment arm | Participants in each level of subgroup (n) | p-value of LRT |
|---|---|---|
| Primary outcome received before or after COVID-19 (11 March 2020) |
|
0.010 |
| IPSS-QoL subgroup analysis based on treatment effect for those with outcome received before or after COVID-19 |
|
0.383 |
| Subgroup | Control (n) | Intervention (n) | Adjusted difference in means (95% CI) | p-value |
|---|---|---|---|---|
| Before COVID-19 (11 March 2020) | 174 | 227 | –2.82 (–3.84 to –1.79) | < 0.001 |
| After COVID-19 (11 March 2020) | 299 | 215 | –1.01 (–2.00 to –0.01) | 0.047 |
Sensitivity analyses
Imbalances between treatment groups at baseline
There was no evidence of imbalance between the groups in terms of baseline characteristics. As a result, the planned sensitivity analysis additionally adjusting for imbalance was not conducted.
Clustering by nurse/healthcare assistant
The primary analysis was repeated using a practice + nurse cluster to allow for clustering by nurse/HCA at a given practice. The results for this model are presented alongside the original primary outcome analysis in Table 24. Accounting for this clustering slightly reduces the treatment effect from an adjusted mean difference of –1.81 to –1.79, but there remains strong evidence that this differs from the null.
| Trial arm | n | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value | Difference in meansb (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Intervention | 442 | 11.60 | 6.21 | 1, 35 | –1.81 (–2.66 to –0.95) | < 0.001 | –1.79 (–2.53 to –1.06) | 0.004 |
| Usual care | 473 | 13.88 | 6.84 | 0, 32 | ||||
| Total | 915 | 12.78 | 6.64 | 0, 35 | ||||
| ICC (95% CI) | 0.01 (0.00 to 0.09) | < 0.001 (< 0.001 to < 0.001) | ||||||
Per-protocol analyses
Five per-protocol analyses were prespecified based on varying ‘amounts’ of the intervention delivered to the participant. The vast majority of participants in the intervention arm received the intervention as intended (Table 25).
| Intervention arm participants (N = 524) who received | n (%) |
|---|---|
| The intervention booklet | 516 (98.47) |
| All three follow-up contacts (1, 4 and 12 weeks) [n (%) of those who received the booklet] | 473 (91.67) |
| Two follow-up contacts [n (%) of those who received the booklet] | 33 (6.40) |
| One follow-up contact [n (%) of those who received the booklet] | 7 (1.36) |
| Zero follow-up contacts [n (%) of those who received the booklet] | 3 (0.58) |
| Follow-up contacts in the protocolised format (received all three follow-ups, week 1 by telephone call and weeks 4 and 12 however the patient preferred, but not face to face) | 414 (79.01) |
As the vast majority of participants received the full (or near full) intervention, some of the analyses were restricted to a very small number of participants, and thus were extremely underpowered. The fifth per-protocol analysis was restricted to those who received the full intervention as intended and included 344 intervention participants (Table 26). The treatment effect in this analysis was slightly larger than that observed in the ITT analysis.
| Analysis | Intervention (n)a | Usual care (n)a | Total | Mean | SD | Minimum, maximum | Difference in meansb (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| ITT | 424 | 463 | 887 | 12.79 | 6.64 | 0, 35 | –1.81 (–2.66 to –0.95) | < 0.001 |
| Per-protocol 1: including all in the control arm and those in the intervention arm who received the intervention booklet and had three follow-up contacts | 391 | 463 | 854 | 12.84 | 6.67 | 0, 35 | –1.84 (–2.70 to –0.99) | < 0.001 |
| Per-protocol 2: including all in the control arm and those in the intervention arm who received the intervention booklet and had two follow-up contacts | 24 | 463 | 487 | 13.76 | 6.80 | 0, 32 | –0.83 (–3.16 to 1.50) | 0.484 |
| Per-protocol 3: including all in the control arm and those in the intervention arm who received the intervention booklet and had one follow-up contact | 5 | 463 | 468 | 13.88 | 6.85 | 0, 32 | –0.57 (–5.12 to 3.99) | 0.807 |
| Per-protocol 4: including all in the control arm and those in the intervention arm who received the intervention booklet and had no follow-up contacts | 2 | 463 | 465 | 13.90 | 6.83 | 0, 32 | –4.25 (–11.38 to 2.89) | 0.243 |
| Per-protocol 5: including all in the control arm and those in the intervention arm who received the intervention in the protocolised format [all three follow-ups, week 1 was delivered by telephone and weeks 4 and 12 were delivered in the participant’s preferred follow-up method (but not face to face)] | 344 | 463 | 807 | 12.83 | 6.63 | 0, 35 | –2.06 (–2.92 to –1.20) | < 0.001 |
A CACE analysis was planned, but as only nine participants in the intervention arm were deemed non-adherent according to our prespecified definition, the analysis was not conducted.
Excluding participants who were later found to be ineligible
Three participants were recruited to the trial and began follow-up before it was clear that they were, in fact, ineligible for the trial. We repeated the primary outcome analysis excluding these three participants and found that this made no difference to the primary analysis, as shown in Appendix 1, Table 48.
Impact of COVID-19
Half (50.5%) of primary outcome data for the intervention arm were collected pre COVID, compared with only 36.2% of the data from usual-care practices. When comparing the mean IPSS at 12 months pre COVID with the data collected during the pandemic, we see that scores in the usual-care arm were higher pre COVID (Table 27). There was also some evidence that the rate of missing primary outcome data was slightly higher during the COVID-19 pandemic (17.5%) than before it (9.4%).
| IPSS at 12 months | n; mean (SD) | ||
|---|---|---|---|
| Usual care | Intervention | Overall | |
| Collected before 11 March 2020 | 174; 15.02 (6.98) | 227; 11.34 (6.17) | 401; 12.94 (6.78) |
| Collected after 11 March 2020 | 299; 13.22 (6.68) | 215; 11.88 (6.25) | 514; 12.66 (6.53) |
A sensitivity analysis adjusting for whether the primary outcome was collected pre COVID or post COVID showed no difference from the primary analysis (Table 28).
| Analysis | n | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Primary analysis of IPSS at 12 months | 887 | 12.79 | 6.64 | 0, 35 | –1.81 (–2.66 to –0.95) | < 0.001 |
| Analysis additionally adjusting for COVID-19 pandemic | 887 | 12.79 | 6.64 | 0, 35 | –1.89 (–2.69 to –1.09) | < 0.001 |
Sensitivity analyses to examine the impact of missing data
Table 29 presents the results of the complete-case ITT analysis using 887 participants alongside analyses for which missing data were imputed using three different methods. In the first method it was assumed that those for whom data were missing had the lowest possible symptom scores (best-case scenario); this analysis yielded very similar results to the primary complete-case analysis. In the worst-case scenario, it was assumed that those in whom data were missing had high symptom scores, equal to the mean plus 2 SDs [12.78 + (2 × 6.84)]. The difference in means was smaller than that observed in the ITT and best-case scenario, but remained greater than the null (p = 0.017). In the multiple imputation analysis (MICE), the treatment effect was slightly smaller than in the complete-case analysis, yet still indicated a greater improvement in the intervention arm compared with the usual-care arm.
| Analysis | Imputed (n) | Used in analysis (n) | Mean (overall) | SD | Difference in meansa (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| Intervention | Usual care | ||||||
| Complete-case ITT | 0 | 0 | 887b | 12.79 | 6.64 | –1.81 (–2.66 to –0.95) | < 0.001 |
| Best-case scenario | 77 | 78 | 1042b | 10.89 | 7.63 | –1.89 (–2.89 to –0.88) | < 0.001 |
| Worst-case scenario | 77 | 78 | 1042b | 14.82 | 7.82 | –1.14 (–2.08 to –0.20) | 0.017 |
| MICEc | 82 | 80 | 1077 | –1.61 (–2.57 to –0.66) | 0.001 | ||
Post hoc sensitivity analysis allowing for clustering within general practice groups
In the case of the general practices that formed a single collaboration, we performed a sensitivity analysis allowing for clustering at the collaboration level (see Appendix 1, Table 49). The results, however, were very similar to the primary analysis, although the estimated ICC was slightly smaller.
Adverse events
Using EMRs, we sought to identify whether patients had LUTS-related adverse events or other urinary ‘expected’ adverse events. We found that a low number of patients reported such events, and reporting was similar in both arms (Table 30).
| Expected adverse event | Received intervention (n) | Received usual care (n) |
|---|---|---|
| Prostatitis | 2 (one patient had one occurrence and the other had four occurrences) | 2 (one patient had one occurrence and the other had six occurrences) |
| LUTS-related UTI | 2 (one patient had three occurrences and the other had two occurrences) | 3 (each patient had one occurrence) |
| Urinary retention | 1 (one patient had one occurrence) | 2 (one patient had two occurrences and the other had four occurrences) |
| Catheterisations | 0 | 0 |
When considering all SAEs identified through the trial SAE reporting procedures (regardless of whether they were related to LUTS), we found that there were 94 SAEs in total (47 in each arm) and there were three deaths (two in the intervention arm and one in the usual-care arm). All but two SAEs that did not result in death were resolved (Table 31). Individual SAEs are outlined in Appendix 1, Table 50. All were unrelated to the intervention, with the exception of five that were deemed unlikely to be related to the intervention.
| Received intervention | Received usual care | Overall | |
|---|---|---|---|
| Adverse events | |||
| Total number of SAEs (n) | 47 | 47 | 94 |
| Total number of related AEs (n) | 0 | 0 | 0 |
| Total number of deaths (n) | 2 | 1 | 3 |
| Status of SAEs, n (%) a | |||
| Resolved | 45 (95.74) | 44 (93.62) | 89 |
| Ongoing | 0 | 2 (4.26) | 2 |
| Died | 2 (4.26) | 1 (2.13) | 3 |
Statistical results summary
The trial was successful in meeting general practice site recruitment targets, and patient recruitment exceeded the updated sample calculations. The treatment groups were broadly balanced on baseline characteristics, but the primary outcome measure (IPSS) was slightly lower in the intervention arm than in the usual-care arm (intervention arm, 13.62; usual-care arm, 14.59).
The intervention was successfully delivered: 98% of men received their intervention booklet and 90% had all three contacts forming the intervention.
The primary outcome analysis found that there was a reduction in IPSS, suggesting improved symptoms, between baseline and 12 months in both arms, but the reduction was greater in the intervention arm (–1.81, 95% CI –2.66 to –0.95). The difference was smaller than the difference of 2 points in overall IPSS that we sought to detect in the sample size calculation, and smaller than the minimal clinically important difference of 3 points. The improvement in IPSS, however, was mirrored by improvements in the ICIQ-UI-SF, the IPSS-QoL measures and the B-IPQ. The primary analysis estimates were robust to ways of accounting for clustering and multiple imputation of missing data. High levels of adherence to intervention delivery meant that per-protocol analyses largely reflected the primary analysis.
Although there was no strong evidence that the treatment effect was modified by the nature of LUTS at baseline, by whom it was delivered or by how contact was made in the intervention, a post hoc analysis suggests that the treatment effect may have been stronger in the pre-COVID period.
Chapter 4 Economic evaluation
Introduction
The purpose of this chapter is to report the methods and results of the within-trial economic evaluation of the TRIUMPH trial. Details on site and participant recruitment are described in Chapter 2 in General practice recruitment and selection and Participants, respectively. A cost–utility analysis comparing a manualised and standardised non-pharmacological intervention with usual care for men experiencing bothersome LUTS was conducted from a UK NHS perspective. The analysis compared costs and quality-adjusted life-years (QALYs) from randomisation to 12 months’ follow-up, the time horizon of the trial. A secondary analysis compared mean differences in change in IPSS with mean differences in costs.
Methods
Measurement and valuation of relevant resource use
Resource use was captured for each individual patient’s 12-month follow-up period, through four primary sources: trial management records, case report forms, primary care EMRs and self-report questionnaires. Data extraction from GP EMRs is described in Chapter 2, Electronic medical record data extraction.
The resources required for training were obtained through primary sources including trial management records and expert opinion from the lead nurses. These resources included staff time, and travel and teleconference expenses. Staff delivering the intervention prospectively recorded on case report forms their time spent in the delivery of the intervention, both at the initial clinic visit and during subsequent follow-up contacts.
Primary care EMRs were used to obtain resource use in relation to (1) primary care consultations for any reason with a GP, nurse, HCA or pharmacist; (2) LUTS-related prescribed medication; and (3) for some of the participants, LUTS-related secondary care activity [outpatient, day case, inpatient and accident and emergency (A&E) visits].
Secondary care LUTS-related healthcare use (outpatient, day case, inpatient and A&E visits) was obtained from self-completed questionnaires 6 and 12 months post intervention. The questionnaires were either completed online or sent and returned by post. This information was supplemented by a review of secondary care letters (received by primary care practices) of participants who had a urology referral recorded in their primary care EMR data but had not recorded this in the follow-up questionnaires.
Details of all resource use were measured; the unit cost sources used to value them are reported in Table 32. Resource use was valued using 2018/19 costs. When a unit cost was not available for the year of analysis, it was inflated to current prices using the NHS cost inflation index as published in Curtis and Burns’ Unit Costs of Health and Social Care 2019. 27
| Resource | Unit cost (£) | Source of unit cost |
|---|---|---|
| GP surgery visit | 34a,b | Curtis and Burns, 201927 |
| GP telephone call | 26.27a,b,c | Curtis and Burns, 201927 |
| GP home visit | 87.28a,b,d | Curtis and Burns, 201927 |
| Practice nurse surgery visit | 10.85b,e | Curtis and Burns, 201927 |
| Practice nurse telephone call | 8.25b,f | Curtis and Burns, 201927 |
| HCA surgery visit | 6.67e | Curtis and Burns, 201927 |
| HCA telephone call | 5.07f | Curtis and Burns, 201927 |
| Pharmacist surgery visit | 11.63e | Curtis and Burns, 201927 |
| Pharmacist surgery telephone call | 8.84f | Curtis and Burns, 201927 |
| NHS 111 call | 12.26 | Pope et al.28 2017 |
| Trainers (professor, lead nurses) | Varies | gUniversity of Bristol pay scales, 2018/1929 |
| hCurtis and Burns, 201927 | ||
| Intervention booklet | 6.15 | University of Bristol printing service charge30 |
| Intervention visit time per minute | Variesi | Curtis and Burns, 201927 |
| Follow-up contact time per minute | Variesj | Curtis and Burns, 201927 |
| Teleconference call per minute | 0.15 | BT conference call service charge31 |
| Car mileage | 0.45k | HM Revenue and Customs32 |
| Outpatient procedures | Varies | NHS National Cost Collection, 2018/1933 |
| Outpatient visits | 110 | NHS National Cost Collection, 2018/1933 |
| Day cases | Varies | NHS National Cost Collection, 2018/1933 |
| Inpatient admissions | Varies | NHS National Cost Collection, 2018/1933 |
| A&E attendances | 159 | NHS National Cost Collection, 2018/1933 |
| Medications | Varies | Prescription Cost Analysis, 201934 |
| NHS Drug Tariff, 201935 |
The printing of the intervention booklets, which were given to participants at their initial intervention visit, was valued using the actual price charged by the University of Bristol’s printing services for printing all booklets. From this aggregate price, it was possible to calculate the unit cost per booklet. As all booklets had to be prepared in advance, it was assumed that all participants randomised to the intervention arm incurred the booklet cost.
The NHS staff time used for training and for the intervention was valued using Curtis and Burns’ Unit Costs of Health and Social Care 2019. 27 The University of Bristol staff time used for training was valued using the university’s 2018/19 pay scales29 and included basic salary, National Insurance and superannuation. All primary healthcare resource use was valued using Curtis and Burns’ Unit Costs of Health and Social Care 2019. 27 Medications were assigned a unit cost based on the prescription cost analysis for 2019. 34 If a unit cost was not available in the prescription cost analysis, which was rare, the unit costs reported in the 2019 NHS Drug Tariff were used. 35
Secondary healthcare resource use was valued using NHS costs published in the 2018/19 National Cost Collection. 33 For outpatient visits a weighted average of the unit costs for consultant- and non-consultant-led visits was used.
Outcome data collection and valuation
The primary outcome for the economic evaluation was the QALY, as recommended in NICE’s reference case for interventions funded by the NHS. 37 Participants completed the EQ-5D-5L at baseline and at the 6- and 12-month follow-ups. At baseline the questionnaire was administered via post; at the 6- and 12-month follow-ups it could be completed online or via post. Participants’ EQ-5D-5L scores were mapped to the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), valuation set using a validated mapping function by van Hout et al. 38 This approach is currently recommended by NICE. The EQ-5D-3L valuation set enables a utility score to be calculated for each patient based on published UK population utility values. Utility scores were then used to calculate the QALYs for each patient using an area-under-the-curve approach. The approach accounted for any deaths that occurred during the 12-month trial period.
The primary outcome of the effectiveness analysis (i.e. change in IPSS) was also evaluated in a secondary cost-effectiveness analysis.
Data cleaning and missing costs and outcomes
The EMR data were derived from two different information technology (IT) systems; therefore, a standardised cleaning process was implemented to ensure that the final analysis data set was comparable across the two systems (detailed in Appendix 2).
Clinician opinion was used to ascertain whether or not a secondary care visit was related to LUTS. In terms of missing data within questionnaires, if other sections of the questionnaire had been completed but the resource use questions had been left blank, then it was assumed that zero resource was incurred. If any part of a resource use question was unanswered or any items of the EQ-5D-5L were left blank, the participants were excluded from the complete-case analysis.
Simple mean imputation was used to impute data in the case of the few participants whose case report forms did not include duration of the follow-up visit at week 1 (n = 1) or week 4 (n = 7). If a participant did not attend the follow-up visit, then the duration of the follow-up visit was coded as zero.
Analysis
All economic analyses were based on ITT, whereby all participants were analysed in accordance with the allocation group they were assigned to at the point of randomisation. As costs and benefits were not assessed beyond the 12-month follow-up period, discounting was not required. Similarly, as this trial was a definitive trial, it was not within the scope of the work package to extrapolate the costs and outcomes beyond the 12-month trial period.
Resource use quantities were multiplied by their relevant unit costs to calculate the total mean resource use and total mean cost by resource category and allocation group, as well as total mean NHS costs. Similarly, mean EQ-5D-5L domain scores for each time point were calculated and reported by allocation group.
A multilevel model (MLM) was used to account for the clustered data and to adjust for the minimisation variables (practice size, IMD and centre) and for the general practice IT system and baseline utility for costs and QALYs, respectively. The MLM approach used has been recommended by El Alili et al. ,39 who found MLMs to perform better than ordinary least squares regression for trial-based economic evaluations that are based on clustered data. The MLM was combined with bias-corrected and accelerated bootstrapping to calculate the adjusted mean cost and QALYs, and the adjusted mean differences in costs and QALYs (and their associated 95% CIs) between groups. This type of bootstrapping was used to account for the skewness in the cost and QALY data.
Cost-effectiveness analysis
Outputs from the MLM were used to estimate the incremental net monetary benefit (INMB) statistic and the associated CIs at NICE’s recommended willingness-to-pay thresholds of £20,000 and £30,000 per QALY. A cost-effectiveness plane was created to graphically show the differences in costs and health outcomes between the trial arms. A cost-effectiveness acceptability curve was constructed for a range of willingness-to-pay thresholds using the parametric p-value approach, a method employed by El Alili et al. 39 and Hoch et al. 40
A secondary analysis examined mean differences in IPSS (adjusted for baseline IPSS and minimisation variables) with the adjusted mean differences in costs (adjusted for minimisation variables and general practice IT system).
Sensitivity analyses
Several sensitivity analyses were conducted to explore the uncertainty in the methodological choices made in the base-case analysis.
A seemingly unrelated regression (SUR) model was used as an alternative approach to analysing the data.
Alternative approaches to handling missing data were performed; these included the following:
-
Multiple imputation by chained equations using predictive mean matching was used in combination with the SUR model. The covariates in the multiple imputation model were site identification, baseline utility, centre, practice size, IMD and general practice system. The model was run by allocation group and a randomisation seed was set to ensure reproducible imputations. A total of 25 imputations were performed and combined with Rubin’s rule in Stata 16.1. 41
-
Participants whose secondary care data were based on letters rather than self-report were excluded from the analysis.
-
Participants who had missing questionnaire data, first, had their costs imputed to zero and their QALYS imputed to a value 10% higher than the mean QALY value for their respective trial arms. Second, they had their costs imputed to the highest participant cost for secondary care per arm, and their QALYs to a value 10% lower for their respective trial arms. These analyses were conducted to examine the impact of missing questionnaire data being potentially missing not at random.
-
The most frequently reported quantity of tablets per medication item in data from practices using EMIS Web were used to impute the missing quantities in data from practices using SystmOne. It had not been possible to extract information in relation to quantity of medications from the SystmOne practices. In the base-case analysis the mean quantity of tablets per medication item from EMIS Web practices were used to impute these missing data.
Uncertainty in the way HCPs code the mode of delivery for their consultations was explored by assuming that all contacts with the same type of HCP have the same unit cost (face-to-face unit cost) regardless of mode of delivery. In terms of intervention costs, training costs were excluded to test the assumption that these costs would be captured in the published unit costs. A further analysis excluded all intervention costs. Finally, the model was adjusted for GP consultation costs for the 12-month pre-consent period.
Subgroup analysis
An analysis was performed in which participants who completed follow-up from 11 March 2020 (11 March 2020 is the date when the World Health Organization declared the COVID-19 outbreak a pandemic) were compared with those who completed follow-up before this date.
Results
Complete NHS cost data were available for 902 (83.8%) of the 1077 consented trial participants, and complete QALY data were available for 904 (83.9%) participants. The complete-case analysis was conducted on 866 (80.4%) participants, 413 (78.8%) in the intervention arm and 453 (81.9%) in the usual-care arm.
Unadjusted mean resource use and costs are present in Table 33. Table 33 also reports the adjusted mean costs for primary care consultations, medications and secondary care visits, which were similar between the arms. Disaggregated data for unadjusted EQ-5D-5L domain scores, utility scores and total QALYs are reported in Table 34. Overall, mean unadjusted domain scores, utility scores and QALYs were similar across both groups and all time points. Intervention costs for the intervention arm equated to £39 per patient, excluding intervention training costs reduced this to £31 per patient.
| Resource category | Measurement | Intervention arm (N = 524) | Usual-care arm (N = 553) | ||||
|---|---|---|---|---|---|---|---|
| n | Resource use, mean (SD) | Cost (£), mean (SD) | n | Resource use, mean (SD) | Cost (£), mean (SD) | ||
| GP consultations, all types | Number of consultations | 478 | 4.60 (4.67) | 145.86 (162.62) | 544 | 5.00 (5.68) | 158.17 (180.05) |
| Practice nurse consultations, all types | Number of consultations | 478 | 2.78 (3.64) | 28.77 (38.58) | 544 | 3.61 (5.75) | 38.41 (58.48) |
| HCA consultations, all types | Number of consultations | 478 | 1.16 (2.05) | 7.70 (13.67) | 544 | 0.59 (2.07) | 3.91 (13.82) |
| Pharmacist consultations, all types | Number of consultations | 478 | 0.15 (0.54) | 1.60 (5.59) | 544 | 0.15 (0.90) | 1.36 (7.18) |
| Total primary care consultations | Number of consultations | 478 | 8.69 (7.57) | 183.93 (182.04) | 544 | 9.35 (10.73) | 201.84 (219.49) |
| Tamsulosin | Items prescribed | 478 | 2.29 (4.02) | 15.37 (25.04) | 544 | 3.06 (4.59) | 18.61 (28.09) |
| Solifenacin | Items prescribed | 478 | 0.35 (1.68) | 12.66 (60.50) | 544 | 0.24 (1.47) | 7.65 (47.75) |
| Mirabegron | Items prescribed | 478 | 0.21 (1.35) | 9.39 (61.77) | 544 | 0.18 (1.31) | 7.95 (60.75) |
| Other LUTS medications | Items prescribed | 478 | 0.42 (1.74) | 8.35 (48.55) | 544 | 0.81 (2.96) | 8.00 (40.38) |
| Total medications | Items prescribed | 478 | 3.27 (4.74) | 45.77 (100.50) | 544 | 4.29 (5.75) | 42.22 (98.97) |
| NHS 111 encounters | Number of calls | 459 | 0 | 0.00 | 479 | 0.01 (0.14) | 0.08 (1.72) |
| Outpatient visits | Number of visits | 459 | 0.07 (0.34) | 8.04 (36.75) | 479 | 0.08 (0.37) | 8.96 (40.82) |
| Outpatient procedures | Number of procedures | 459 | 0.03 (0.20) | 4.00 (27.78) | 479 | 0.05 (0.31) | 6.43 (39.65) |
| Day case | Number of cases | 459 | 0 | 0.00 | 479 | 0 | 0.00 |
| Inpatient stay | Number of stays | 459 | 0 | 0.00 | 479 | 0.00 (0.09) | 7.10 (155.49) |
| A&E visits | Number of visits | 459 | 0.00 (0.09) | 0.69 (14.84) | 479 | 0.01 (0.08) | 0.99 (12.56) |
| Total secondary care visits | Number of visits/encounters | 459 | 0.11 (0.46) | 12.73 (54.71) | 479 | 0.15 (0.66) | 23.56 (171.33) |
| Total intervention delivery costs | 524 | 31.48 (10.16) | – | 0.00 | |||
| Total intervention training costs | 524 | 7.66 | – | 0.00 | |||
| Total intervention costs | 524 | 39.14 (10.16) | – | 0.00 | |||
| Total NHS costs (unadjusted) | 429 | 272.37 (189.06) | 473 | 275.63 (321.36) | |||
| Total adjusted primary care consultation costsa | 478 | 174.48 (£142.80 to £206.16) | 544 | 205.61 (£166.05 to £245.18) | |||
| Total adjusted medication costsa | 478 | 40.49 (£28.28 to £52.71) | 544 | 46.39 (£34.90 to £57.88) | |||
| Total adjusted secondary care consultation costa,b | 459 | 13.78 (£0.37 to £27.19) | 479 | 25.83 (£0.06 to £51.59) | |||
| Domain | Baseline | 6 months | 12 months | Total QALYs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention arm | Usual-care arm | Intervention arm | Usual-care arm | Intervention arm | Usual-care arm | Intervention arm | Usual-care arm | |||||||||
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | |
| Mobilitya | 1.42 (0.77) | 523 | 1.38 (0.74) | 551 | 1.37 (0.72) | 486 | 1.38 (0.73) | 513 | 1.38 (0.74) | 461 | 1.41 (0.76) | 485 | ||||
| Self-carea | 1.09 (0.37) | 523 | 1.09 (0.38) | 551 | 1.09 (0.37) | 486 | 1.10 (0.41) | 512 | 1.08 (0.36) | 460 | 1.08 (0.39) | 484 | ||||
| Usual activitya | 1.42 (0.73) | 523 | 1.39 (0.70) | 550 | 1.38 (0.70) | 550 | 1.35 (0.70) | 511 | 1.42 (0.77) | 460 | 1.41 (0.72) | 484 | ||||
| Pain/discomforta | 1.69 (0.82) | 523 | 1.71 (0.80) | 549 | 1.65 (0.78) | 486 | 1.66 (0.80) | 513 | 1.70 (0.80) | 459 | 1.65 (0.78) | 484 | ||||
| Anxiety/depressiona | 1.40 (0.65) | 522 | 1.41 (0.67) | 549 | 1.38 (0.66) | 484 | 1.40 (0.65) | 512 | 1.37 (0.63) | 459 | 1.46 (0.70) | 485 | ||||
| Utility scoreb | 0.83 (0.17) | 522 | 0.83 (0.16) | 547 | 0.84 (0.17) | 483 | 0.84 (0.16) | 509 | 0.83 (0.17) | 457 | 0.83 (0.17) | 482 | ||||
| Total QALYs | 0.84 (0.16) | 444 | 0.84 (0.15) | 460 | ||||||||||||
The adjusted mean incremental differences in costs and QALYs were similar between the two arms. Compared with the usual-care arm, the intervention arm had slightly lower mean costs (adjusted mean difference of –£29.99, 95% CI –£109.84 to £22.63) and a small gain in QALYs (adjusted mean difference of 0.001, 95% CI –0.011 to 0.014). At a threshold of £20,000 per QALY the INMB was £48.01 (95% CI –£225.83 to £321.85), suggesting that the intervention is cost-effective, but there is uncertainty around this estimate, which is also reflected in the intervention having only a 63% probability of being cost-effective at this threshold (Figures 6 and 7).
FIGURE 6.
Cost-effectiveness plane from an NHS perspective.

FIGURE 7.
Cost-effectiveness acceptability curve from an NHS perspective.
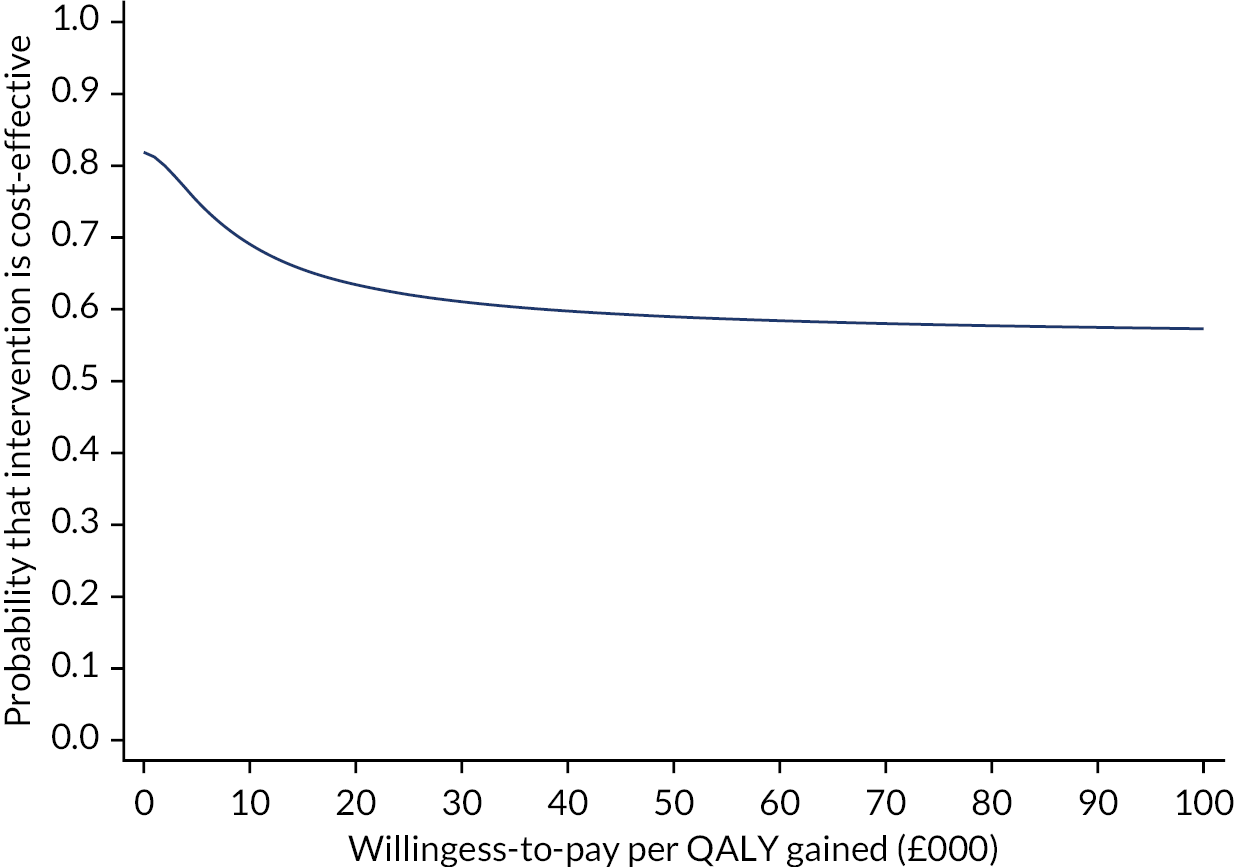
The sensitivity analysis (Table 35) applied to the complete-case population shows similar results, with a small positive INMB and wide CIs. Two of the sensitivity analyses that addressed missing data (multiple imputation, and zero costs and higher QALY imputation for missing questionnaire data) led to small negative INMBs and wide CIs. The sensitivity analysis considering the impact of using the highest costs and a lower QALY value for missing questionnaire data led to a positive INMB and wide CIs. It is of note that removing the intervention costs from the analysis led to a significant reduction in cost (–£70, 95% CI –£154 to –£18).
| Trial arm | n | Adjusted,a mean (95% CIb) | Incremental adjusted, mean (95% CIb) | ICER (£ per QALY) | INMB (£) at £20,000 per QALY (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | ||||
| Base case: complete-case analysis with MLM | |||||||
| Intervention | 413 | 253.53 (215.85 to 291.22) | 0.84 (0.83 to 0.84) | –29.99 (–109.84 to 22.63) | 0.001 (–0.011 to 0.014) | Intervention dominates usual care | 48.01 (–225.83 to 321.85) |
| Usual care | 453 | 283.52 (235.40 to 331.64) | 0.84 (0.83 to 0.84) | ||||
| Alternative analysis: complete-case analysis with SUR | |||||||
| Intervention | 413 | 258.05 (229.90 to 286.21) | 0.84 (0.83 to 0.84) | –20.00 (–62.50 to 22.50) | 0.001 (–0.010 to 0.011) | Intervention dominates usual care | 35.54 (–182.20 to 253.28) |
| Usual care | 453 | 278.05 (251.42 to 304.68) | 0.84 (0.83 to 0.84) | ||||
| Multiple imputation with SUR | |||||||
| Intervention | 524 | 275.03 (248.63 to 301.43) | 0.83 (0.83 to 0.84) | 1.42 (–38.61 to 41.46) | –0.002 (–0.012 to 0.009) | Usual care dominates intervention | –35.42 (–248.71 to 177.87) |
| Usual care | 553 | 273.61 (248.26 to 298.96) | 0.84 (0.83 to 0.84) | ||||
| Complete-case analysis with MLM: applying unit cost for face-to-face contacts used for telephone contacts | |||||||
| Intervention | 413 | 262.52 (222.73 to 302.32) | 0.84 (0.83 to 0.84) | –34.81 (–114.27 to 19.65) | 0.001 (–0.011 to 0.014) | Intervention dominates usual care | 52.83 (–222.33 to 327.99) |
| Usual care | 453 | 297.33 (248.37 to 346.29) | 0.84 (0.83 to 0.84) | ||||
| Complete-case analysis with MLM: excluding participants whose secondary care data are based on letters | |||||||
| Intervention | 399 | 249.83 (212.76 to 286.90) | 0.84 (0.83 to 0.84) | –26.68 (–96.34 to 25.11) | 0.001 (–0.011 to 0.014) | Intervention dominates usual care | 30.59 (–234.61 to 295.79) |
| Usual care | 434 | 276.52 (229.62 to 323.42) | 0.84 (0.83 to 0.84) | ||||
| Complete-case analysis with MLM: medication quantities for SystmOne based on most frequently reported, rather than average | |||||||
| Intervention | 413 | 259.39 (219.57 to 299.21) | 0.84 (0.83 to 0.84) | –29.89 (–118.22 to 24.25) | 0.001 (–0.011 to 0.014) | Intervention dominates usual care | 47.92 (–226.82 to 322.66) |
| Usual care | 453 | 289.28 (239.48 to 339.08) | 0.84 (0.83 to 0.84) | ||||
| Complete-case analysis with MLM: removal of training costs | |||||||
| Intervention | 413 | 245.87 (208.19 to 283.56) | 0.84 (0.83 to 0.84) | –37.65 (–117.50 to 14.97) | 0.001 (–0.011 to 0.014) | Intervention dominates usual care | 55.67 (–218.17 to 329.51) |
| Usual care | 453 | 283.52 (235.40 to 331.64) | 0.84 (0.83 to 0.84) | ||||
| Complete-case analysis with MLM: removal of all intervention costs | |||||||
| Intervention | 413 | 213.55 (176.34 to 250.77) | 0.84 (0.83 to 0.84) | –70.28 (–153.84 to –18.45) | 0.001 (–0.011 to 0.014) | Intervention dominates usual care | 88.31 (–185.50 to 362.12) |
| Usual care | 453 | 283.84 (235.59 to 332.09) | 0.84 (0.83 to 0.84) | ||||
| Complete-case analysis with MLM: adjusting for 12-month pre-consent GP consultation visits | |||||||
| Intervention | 413 | 277.53 (245.93 to 309.14) | 0.84 (0.83 to 0.84) | –3.02 (–49.52 to 44.02) | 0.001 (–0.011 to 0.014) | Intervention dominates usual care | 21.50 (–246.93 to 289.03) |
| Usual care | 453 | 280.56 (244.16 to 316.96) | 0.84 (0.83 to 0.84) | ||||
| MLM: simple imputation of £0 for secondary care per arm and a QALY value 10% higher than the mean QALY value per arm | |||||||
| Intervention | 456 | 254.12 (216.02 to 292.21) | 0.84 (0.83 to 0.85) | –20.19 (–91.44 to 30.56) | –0.006 (–0.020 to 0.008) | Intervention less costly, but fewer QALYs | –96.06 (–394.16 to 202.04) |
| Usual care | 516 | 274.31 (231.20 to 317.42) | 0.85 (0.84 to 0.86) | ||||
| MLM: simple imputation of the highest participant cost for secondary care per arm and a QALY value 10% lower than the mean QALY value per arm | |||||||
| Intervention | 456 | 330.66 (273.59 to 387.73) | 0.82 (0.82 to 0.83) | –322.19 (–435.12 to –232.31) | –0.004 (–0.015 to 0.010) | Intervention less costly, but fewer QALYs | 243.74 (–39.00 to 526.48) |
| Usual care | 516 | 652.85 (582.44 to 723.25) | 0.83 (0.82 to 0.84) | ||||
| Complete-case analysis with MLM: subgroup analysis for participants completing 12 months’ follow-up before 11 March 2020 | |||||||
| Intervention | 236 | 255.12 (133.88 to 376.35) | 0.83 (0.81 to 0.84) | –33.04 (–209.94 to 438.39) | –0.001 (–0.027 to 0.010) | Intervention less costly, but fewer QALYs | –155.66 (–730.36 to 419.03) |
| Usual care | 169 | 288.16 (23.20 to 553.11) | 0.84 (0.82 to 0.85) | ||||
| Complete-case analysis with MLM: subgroup analysis for participants completing 12 months’ follow-up from 11 March 2020 | |||||||
| Intervention | 177 | 229.31 (119.77 to 338.85) | 0.86 (0.81 to 0.90) | –67.77 (–219.86 to 74.92) | 0.029 (0.002 to 0.133) | Intervention dominates usual care | 640.30 (–1445.07 to 2726.67) |
| Usual care | 284 | 297.07 (204.54 to 389.61) | 0.83 (0.76 to 0.90) | ||||
A secondary analysis comparing difference in mean IPSS (see Table 14) and mean costs (see Table 35) found that costs were lower and improvement in IPSS was greater in the intervention arm, indicating that the intervention was dominant; therefore, no incremental cost-effectiveness ratios were created.
Just over half (57.1%, n = 236) of participants from the intervention arm and just over one-third (37.3%, n = 169) of usual-care participants completed 12 months’ follow-up before 11 March 2020 (see Table 35). Costs in each arm were similar for this subgroup compared with the base-case analysis, whereas QALYs were slightly lower in the intervention arm than in the usual care when compared with the base-case analysis, resulting in an INMB of –£156 (95% CI –£730 to £419). Among those participants who completed 12 months’ follow-up on or after 11 March 2020, costs were slightly lower in the intervention arm, and slightly higher in the the usual-care arm, than in the base-case analysis. The intervention arm also had a higher number of QALYs, which result in an overall higher INMB of £640 (95% CI –£1445 to £2727).
Discussion
From an NHS perspective, the mean costs and QALYS were similar across both trial arms. A slightly lower mean cost in the intervention arm (a £30 cost difference) led to a small positive INMB at the NICE willingness-to-pay threshold of £20,000 per QALY, and a 63% probability of the intervention being the cost-effective option. There is therefore some uncertainty in the finding of the intervention being cost-effective at the NICE £20,000 per QALY threshold. With the exception of two sensitivity analyses, both alternative methods for dealing with missing data, the initial results were robust.
This is the first economic evaluation carried out on a definitive trial in which the value for money of a manualised and standardised non-pharmacological intervention, compared with usual care, for men experiencing bothersome LUTS has been evaluated.
A key strength of this trial was the use of routine data, which minimised the number of missing data and the burden on participants. Participants typically struggle to recall and name the type of HCPs they have engaged with. 42 At each practice, EMR data were available from one of England’s two main general practice IT systems (EMIS Web or SystmOne). There were, however, differences between the IT systems: SystmOne was unable to extract data on the reason for a consultation and the quantities of medication prescribed. Consequently, a standardised approach was applied to reduce the potential for bias that may arise from using a different IT system. First, all consultations were included in the analysis and, second, medication quantities were based on the average quantities prescribed for the EMIS Web cohort. The IT system was also adjusted for in the regression analysis.
It was not possible to retrieve EMR data for any participants from one of the sites allocated to the intervention arm, which provided 5.5% (n = 29) of the intervention participants. The site attributed this to time constraints. Similarly, owing to time constraints at the general practices, it was not possible to request secondary care letters for all participants for whom data were missing. Prioritising participants with a urology referral in their EMR data was deemed a pragmatic approach. It was assumed that no secondary care resources were consumed if (1) no urology referral was recorded in the patient’s EMR data set and (2) the patient had answered other questions in the follow-up questionnaire booklet.
Future cluster randomised studies using EMR data to capture healthcare resource use should consider stratifying for the general practice IT system within the randomisation process, as well as controlling for this variable, as seen in this trial. Differences within the general practice IT systems, if arms are imbalanced, could lead to bias if there are differences in the number of resource use data available within each system. In this trial, a standardised approach was applied to reduce the impact of the IT systems; this meant that it was not possible to mask the second health economist, as the cleaning of the EMR data required the removal of intervention-related consultations which had already been captured in the case report form. Nevertheless, the lead health economist was masked to the randomisation allocation until all data had been cleaned and valued.
The primary analysis was a complete-case analysis that included 80.4% (n = 866) of all participants. Data were likely to be missing completely at random for the 3.5% (n = 38) of participants for whom EMR data or secondary care letters were not returned by sites or who had changed practice within the trial time frame. In the case of the remaining participants who were excluded from the complete-case analysis (16.1%, n = 173), all or some questionnaire data were missing. It is therefore possible that the complete-case analysis excluded participants who had better or worse outcomes or used more or fewer resources than the participants for whom we have observed data. Although the mechanism of missingness was assessed and the explanatory variables indicated that missingness was at random, it is not possible to rule out the mechanism ‘missing not at random’.
It may be the case that participants whose 12-month follow-up was after 11 March 2020 used less healthcare resource because of the impact of the COVID-19 pandemic on primary and secondary care services in the UK. This is important to consider given that the proportion of participants reaching their 12-month follow-up during the pandemic was one-fifth (19.8%) higher in the usual-care arm than in the intervention arm. That is to say, it could be possible that resource use was underestimated in the usual-care arm; however, the costs in this arm were higher for those participants whose 12-month follow-up occurred during the pandemic, and it is unclear why this is the case.
A further potential limitation is that costs and outcomes have been evaluated over only a short time horizon. It was beyond the scope of this work to compare the arms over a longer time horizon.
The cost-effectiveness analysis showed that costs and outcomes were similar in both arms. The removal of the intervention costs from the analysis (mean cost per person of £39) led to a significant difference in costs of £70 in favour of the intervention arm, indicating that, if the costs of implementing the intervention could be reduced, then the roll-out of this intervention could potentially lead to cost savings for the NHS.
Chapter 5 Qualitative study
Introduction
Qualitative methods are an increasingly important component of RCTs in health care,43 as they are able to bring ‘in situ’ insight into the processes and dynamics of an intervention and its delivery by capturing the views and experiences of patients and clinicians over the course of a trial. The findings from qualitative studies can help make sense of quantitatively measured outcomes;44 can ascertain otherwise unexplored, but important, dimensions of impact; and can support clinical recommendations and implementation plans. 45 The qualitative component embedded within the TRIUMPH trial set out to explore patients’ views and experiences of:
-
LUTS (symptoms, treatment and management) prior to the TRIUMPH trial
-
the intervention and take-up of the structured self-help guidance
-
outcomes from the intervention/usual care.
In addition, the qualitative study incorporated the views and experiences of clinicians, including nurses who presented the trial self-help material to intervention arm participants, and GPs from whose populations the TRIUMPH trial participants were recruited, and in whose hands ‘usual care’ continued.
The qualitative study set out to contextualise the quantitative findings; provide greater understanding of the experiences of those who were and those who were not offered self-help guidance; offer reflections on the intervention and its delivery from the perspective of clinicians as well as patients; and report on the current experiences of men with LUTS in relation to primary care and self-management (the context within which any recommendations for the improved care of men with LUTS must be situated). Alongside being of relevance to our understanding of the trial outcomes, this work provides insights into NHS primary care and treatment of men with LUTS, and the status, salience and use of self-help advice and guidance in routine practice.
Methods
Recruitment and sampling
All trial participants who had consented at baseline to being approached by a qualitative researcher were identified. Interviewees were purposively sampled to include:
-
men in both the intervention and the control arms of the trial (those who did and those who did not receive TRIUMPH trial self-help guidance, anticipating n = 30 in the intervention arm and n = 15 in the control arm) and included within this sample:
-
men from across the spectrum of participating general practice sites (including a maximally diverse range of practice sites)
-
men with a diversity of LUTS experiences (including men with mild, men with moderate and men with severe IPSS, and those with various kinds of LUTS, including ‘post-micturition dribble’, ‘frequency’, ‘urgency’, ‘nocturia’ and ‘leakage’ issues)
-
men of different ages (including older and younger men with LUTS).
-
In addition, over the course of the trial, purposive sampling attended to the lack of diversity in a majority ‘white’ and ‘married’ sample by seeking to include, in addition to the primary sampling framework, men who were:
-
ethnic minorities, as well as white men
-
single, as well as men who were married, civil partnered, divorced, separated or widowed.
Selected potential participants were sent an information leaflet describing the qualitative study by post/e-mail (according to their preference) and this was followed up with a telephone call, providing an opportunity to discuss the qualitative study. Telephone contact was attempted three times, and answer machine messages were left when possible. Those who were reached by telephone but declined the invitation to an interview gave the following reasons: recent bereavement, ill health, work abroad and holidays; those remaining did not respond to answer machine messages and could not be reached at the times they were called. For willing participants, a convenient time was arranged for the interview to take place, either in person or by telephone, with telephone interviews offered as per the research interviewer’s preference. Full consent to participate was recorded at the outset of each interview.
The majority of qualitative study men were interviewed twice, first at baseline (in the weeks following receipt of the intervention guidance for those in the intervention arm, and in the weeks following their consent to participation for those in the control arm) then later during follow-up phase of the trial (before the end of the 12-month follow-up period, and between 3 and 9 months after baseline interviews). In addition, a number of lone ‘feasibility’ interviews were conducted with men in the intervention arm only, to explore in greater depth participants’ views and experiences of the intervention delivery and the structured self-help guidance. Clinicians were also interviewed, following invitations sent by e-mail to relevant GPs, nurses and HCAs at all participating general practices (‘relevant’ included TRIUMPH trial site principal investigators, clinicians with dedicated research roles within the practice, those with special interests in LUTS/urology who were also part of wider TRIUMPH trial research team and those engaged in delivering the intervention). Clinician interviews included nurses/HCAs who delivered the intervention to men at different general practices, and GPs from participating sites (both intervention and usual-care sites). Furthermore, at the outset of the study, a small sample of men who declined to take part in the trial, but who consented to a brief telephone interview to discuss their reasons for declining, were interviewed to better understand some of the reasons why men chose not to take part.
Interviews
Comprehensive interview guides (see Report Supplementary Material 2–6) were developed for the baseline, follow-up, feasibility, clinician and decliner interviews, to explore the key qualitative areas of interest. Questions included in the topic guide were open-ended, and included follow-up prompts, to support a thorough exploration of relevant issues. Topic guides were used fluidly: interviews typically covered all the areas of interest indicated on the guide, but emphasis on aspects of topics shifted according to the experiences of the participant, and over the course of the study as interviews progressed, to ensure that emerging themes were explored adequately. Participants were encouraged to describe and reflect freely on their experiences in relevant areas, and specific views, comments and experiences of interest were interrogated more deeply. All but one participant (who chose to have a face-to-face interview) were interviewed by telephone (men were offered face-to-face interviews if preferred; for the most part, men expressed ease with the convenience of a telephone interview, and for some the relative anonymity of a telephone interview was also a factor). Interviews typically lasted 60–90 minutes at baseline and 20–60 minutes at follow-up; clinician interviews typically lasted 45–90 minutes and brief ‘decliner’ interviews lasted around 10–15 minutes. Interviews were conducted between December 2018 and February 2020. Participants were recruited to the point of data saturation at which no further substantive themes were emerging in relation to the aims and objectives of the qualitative study.
Analysis of the interviews
All of the interviews were audio-recorded and transcribed verbatim. Transcripts were prepared concurrently, as interviews continued, with analyses conducted in parallel, so that emerging themes could inform the focus of ongoing interviews. Transcripts were read and re-read for familiarisation, codes devised and themes developed. Transcript data were imported into the software package NVivo 12 (QSR International, Warrington, UK) to support formal electronic coding and data management. Data were coded according to coding frames, with emerging themes and patterns of relevance to the study aims drawn out and described as the data built up. Data were continuously coded until few, if any, new codes, themes or subthemes of relevance were found.
Results
Participants
We carried out a total of 115 interviews: 91 with participants, 14 with clinicians and 10 brief interviews with men who had declined to take part in the trial. Early in the trial we interviewed 45 men at baseline (30 men in intervention arm, 15 men in the control arm) and carried out a further 13 one-off feasibility interviews. Later we carried out 33 follow-up interviews with 22 previously interviewed men in the intervention arm and 10 previously interviewed men in the control arm. Participants interviewed in the early stage of their participation in the trial were typically interviewed 0–3 months after joining the trial (within the first weeks of receiving the TRIUMPH trial guide for those in the intervention arm, but allowing for practice variation in the time between initial recruitment and intervention delivery, as well as time to make telephone contact and arrange qualitative interviews). Follow-up interviews typically took place between 3 and 9 months after the initial early-stage baseline interview (as participants neared the completion of their 12-month involvement in the trial, while allowing for inclusion in the qualitative component of the study of those who joined the main trial in later waves of recruitment, to capture patients from a maximally diverse range of geographic regions and practices). Purposive sampling, to ensure balance, provided a sample made up of men who:
-
had IPSS ranging from 3 to 31, with a mean of 14.84 according to baseline measures
-
had a range of LUTS experiences, including men with more severe problems (higher scores, of ≥ 3 out of 5) relating specifically to an urgent need to urinate (23 men), urinary leakage (12 men), a frequent need to urinate (31 men) and nocturia (25 men), and men experiencing post-micturition dribble (22 men)
-
ranged in age from 40 to 84 years, with a mean age of 64.95 years
-
were from a diverse range of general practice sites, including 27 general practices (with 17 intervention arm and 10 usual-care arm general practice sites covered by the sample).
The cohort comprised four men who identified as black, Asian or mixed race and 54 who identified as white. Seven men were single, four widowed, three divorced, four civil partnered and 38 married. Tables 36 and 37 set out participant characteristics for the men who took part in the TRIUMPH qualitative study (including men who declined to take part in the trial) and clinicians, respectively. Personal information, including biographic and LUTS related, was not collected or stored for ‘declining’ men, 10 of whom were interviewed.
| Characteristic | Interview sample | ||
|---|---|---|---|
| Intervention arm (n = 30) | Usual-care arm (n = 15) | Feasibility group (n = 13) | |
| Age (years), mean (range) | 67.69 (44–84) | 59.47 (40–81) | 65.14 (51–80) |
| IPSS, mean (range) | 13.97 (3–30) | 15.2 (4–31) | 16.29 (8–24) |
| LUTS indicateda (n) | |||
| Urgency | 20 | 11 | 11 |
| Leakage | 15 | 14 | 9 |
| Frequency | 30 | 15 | 13 |
| Nocturia | 30 | 13 | 13 |
| Post-voiding dribble | 8 | 10 | 4 |
| Ethnicity ( n ) | |||
| Black/African/Caribbean | 1 | – | – |
| Asian | 1 | – | – |
| Mixed race | – | 1 | 1 |
| White | 28 (1) | 14 | 12 |
| Marital status ( n ) | |||
| Single | 6 | – | 1 |
| Married | 18 | 12 | 8 |
| Civil partnered | 1 | 1 | 2 |
| Divorced | 1 | – | 2 |
| Widowed | 4 | – | – |
| Not stated | – | – | 2 |
| Characteristic | Interview sample | |
|---|---|---|
| GPs (n = 10) | Nurse practitioners (n = 4) | |
| Female (n) | 5 | 4 |
| Male (n) | 5 | 0 |
| Age (years), mean (range) | 49 (42–59) | 45 (31–58) |
| Years qualified, mean (range) | 23 (3–36) | 13 (2.5–25) |
| Years in current role, mean (range) | 15 (3–21) | 7 (2.5–10) |
| Delivering intervention | 0 | 4 |
| Control site | 5 | 0 |
| Intervention site | 5 | 4 |
Conceptual framework/themes
Major themes and a conceptual framework (Figure 8) were developed through a process of recursive data analysis. The qualitative team were unmasked (men in the intervention/usual-care arms knew that they had/had not received the intervention; however, men in the usual-care arm did not know and were not informed about the content of the intervention/booklet). Men’s views, experiences and outcomes were linked to their intervention/usual-care arm status throughout the interview and analytic process.
FIGURE 8.
Conceptual framework/themes.

At its broadest level, the conceptual framework (see Figure 8) for those in the intervention arm who were receptive to, and engaged with, the guidance on offer (which was a striking majority of those interviewed) is a heartening journey. There was a shift from pre-trial/usual-care experiences of dispirited coping or resigned tolerance (living in the shadow of disruptive, annoying, anxiety-provoking, embarrassing, stigmatising, age- and decline-associated symptoms) to post-intervention enthused self-management, increased understanding, therapeutic empowerment, renewed efforts and symptom improvement.
For men in the intervention arm, whether the specific changes reported were minimal or wide-ranging, the overwhelming sentiment was upbeat; men described relief and gratitude at being better-informed, enthusiastically reported symptom-related benefits and said that ‘all men should have this guidance’.
Receptiveness to, and appetite for, the intervention was linked to prior LUTS experiences and tolerance of symptoms, prior satisfaction with the outcomes of primary care, personal factors (e.g. character, lifestyle) and experiences of the intervention (particularly men’s experiences of the relevance, novelty, practicality and acceptability of the booklet content). Understandably, for these reasons, a minority of men were not receptive to the guidance, reporting no change in their behaviours.
For men in both the intervention and the usual-care arms, the degree of prior dispiritedness, coping, resignation and tolerance was linked to (1) LUTS experiences (kinds and experiences of LUTS), (2) experiences of primary care (the decision to visit a GP, GP responses and levels of satisfaction with the outcomes of primary care support, the drivers and especially inhibitors of further GP visits) and (3) personal factors.
Control group men continued to live in the shadow of dispiriting symptoms, eager for support and guidance, but commonly inhibited from returning to their GP. These themes and components of the conceptual framework are explored in more detail below.
Theme 1a: kinds of lower urinary tract symptoms
Common to all three groups of study men (those in the intervention arm, feasibility group (FG) and usual-care arm) was a familiar constellation of kinds (and experiences, see below) of LUTS, broadly in line with current clinical depictions of LUTS among men7,46 (see Figure 8, ‘1a. Kinds of LUTS’, and Appendix 3 for further accounts).
Nocturia
Many men opened their account of LUTS onset with a description of the emergence of increasingly regular (most nights or every night) and multiple (more than once or many times) night-waking to urinate (clinically termed nocturia). Many stated that this precipitated their first LUTS-related GP visit, or, more accurately for some, their first ‘in passing’ LUTS-related question to a GP:
… the main problem I have encountered is having to get up in and being disturbed repeatedly in the night to pee and it was sort of at least three times a night.
Intervention group (IG) man 2, age 69 years
Beyond nocturia, men’s symptoms fell into clinically recognised clusters (nocturia was experienced within both symptom clusters; urgency and frequency were also prevalent across clusters, but associated distinctly with either difficulty voiding or difficulty holding/risk of leakage).
‘Voiding’ issues
These included a frequent, frustrating and, for some, painful urge to urinate accompanied by slow or weak flow, difficulty initiating micturition, a sense of incomplete emptying of the bladder and (more rarely) signs of and concerns about, or experience of, acute retention:
… what I found as it progressed it’s not possible to urinate to order, in effect, and you can be going into theatre and you know that you need to [urinate] … and you’re going to be sitting in a chair for 2 and half hours, but you can’t do anything and so you end up sitting uncomfortably. You want to, but you can’t do anything …
IG1, age 63 years
Yeah, I had to go down the [hospital name] in the middle of the night and they kept me in and they emptied it [by catheterisation for a week], ’cause I just could not get any out. But that’s only happened once.
IG15, age 75 years
‘Storage’ issues
This included a frequent (daytime and/or night-time) need to urinate, coupled with urgency, loss of control and leakage, and daytime and/or (much more rarely) night-time incontinence, and was sometimes diagnosed as ‘overactive bladder syndrome’ or a very frequent urge to urinate through the day and night (but without slow- or intermittent-flow issues):
One thing I would say it has made a difference to my life is the fact that I’ve been getting leakage and so where in the past I would have said I’m very keen to go the loo but I will put it off until it’s convenient, but then [now] I get leakage before urinating if I do that.
Control group (CG) man 5, age 66 years
Alongside these clusters, some men described additional, familiar post-micturition issues, which are discussed in the following sections.
Post-voiding dribble
Some men gave accounts of a small wet patch, often once dressed, just after dressing following urinating:
Don’t know, sort of late 40s, I suppose, I started noticing this is not right, I started getting worried about it and the symptoms was go to the toilet and then give it a shake and let it all drip out, do myself up, get back to my seat and en route back to my seat or sit on the settee some more would come out.
CG11, age 48 years
Feeling of incomplete emptying
Some men reported feeling that the bladder retains some urine or cannot be successfully emptied, leaving the unsettling feeling of still needing to urinate; indeed, large residual urine volumes can often be found in the bladder in urological assessments of urinary function:
What I do get is frustration that I haven’t done it, I haven’t emptied, because I want to get on.
CG1, age 58 years
… so what happens is when I’ve gone then there’s always some left really.
Feasibility group (FG) man 8, age 69 years
Recurrent urinary tract infection
Aetiologically, and in terms of treatment options, UTIs are a separate concern, and not the focus of this study, but, for some men, recurrent UTIs were nonetheless described as part of, or interacting with, their broader LUTS experience, or sense of LUTS onset. Because these experiences are relevant to self-help guidance responses, they are included:
Well when I empty my bladder it doesn’t fully empty, so I’m getting water retention and I think it’s because of that which has been causing a lot of the infections …
CG2, age 62 years
Diffuse/multiple symptoms
Some men experience a diffuse range of symptoms that stretch across the spectrum of clusters and symptoms and may also include UTIs:
I was aware that passing urine wasn’t as speedy as it might be, or as full a force as it might be. Also … routinely I get up twice in the night to go to the loo. That has been the case for a long time … urine wasn’t getting discharged as quickly as it might, which was leading to the likelihood of infection [UTI] being more prevalent … [and] More recently … a greater urgency, I suppose, when I feel I need to pass water. I can hold on, but I need to get to relieve myself as quick as possible. It hasn’t caused any particular problems, but it’s something I’m aware of … I’ve always got to be aware of whether there’s any cover locally that I can secrete myself into if I need to … It’s not something I can put off for several hours thinking, ‘When I get home, I’ll go to the loo’.
CG6, age 70 years
Conclusion: theme 1a – kinds of lower urinary tract symptoms
Interviews gave insight into the range and kinds of LUTS evident within a community cohort of men (see Figure 8, 1a). The cohort appeared entirely apt, which suggests that GP identification processes were effective; all men displayed LUTS, of varying degrees of severity and with a full range of symptoms. Kinds of LUTS were in line with clinical descriptions of LUTS and, although men did not self-categorise in terms of ‘voiding’ or ‘storage’ issues, these categories were appropriate delineators and clearly apparent within the interview sample. Some men reported UTIs, which interacted with their LUTS; for these men, the boundaries between LUTS and UTIs are less experientially meaningful than those that might be clinically imposed. In addition, a number of men reported diffuse symptoms that straddled categories. The prevalence of nocturia across categories was notable. It is widely reported that a large proportion of men with LUTS do not ever reach primary care;47 given that this sample was necessarily made up of men with LUTS who were known to primary care, it is perhaps unsurprising that many men had experienced symptoms for many years and had moderate or severe symptoms, both descriptively and in terms of their IPSS. This symptom longevity and severity of symptom experience, of relevance to men’s interest in the trial and willingness to take part, belies the burden of LUTS within the community, which falls within, and, for many more men, is likely to fall beyond, the remit of primary care.
Theme 1b: experiences of lower urinary tract symptoms
Men’s descriptions of their day-to-day lived experience share much with long-standing qualitative reports of men’s LUTS experiences. 48–52 Men describe a range of daily, and nightly, disturbances, frustrations, irritations, embarrassments, anxieties and fears, normalised conceptually as ‘old men’s problems’, despite a number of men in the qualitative study being aged < 60 years (see Figure 8, theme 1b, and see Appendix 4 for further accounts).
Tiredness and distress from sleep disturbance
Men with nocturia described tiredness and distress, because of constant nightly sleep disturbance and concomitant negative impacts on levels of tiredness, ability to function/work and mood:
[I]t was bothering me a lot because you are going into work and you are absolutely shattered … Tiredness and irritable and being miserable all the time.
IG4, age 69 years
Stress, anxiety and fear
Men described diverse anxieties relating to various aspects of LUTS. These included logistical anxieties (the need to plan journeys and trips, to know the location of toilets and to plan toilet breaks), anxieties around the potential for leakages during social events, anxieties that symptoms will inevitably worsen and that LUTS is an indication of relentless age-related decline, anxieties around inaction and not knowing what action to take, vicious cycles of anxiety whereby constant surveillance to manage symptoms increased tension in ways that perversely worsened symptoms and anxieties that symptoms might indicate prostate cancer:
Well, it was having to check yourself all the time, I’ve got to go to the loo before I do this. I couldn’t go to a meeting or go out because I’d have to check where the loos were. The whole cycle was making me more and more anxious. It would get to the point where I would say, ‘I’m not doing that. I can’t be bothered doing that. It’s too much stress’ …
CG1, age 58 years
Worrying that I wasn’t going to be, you know – have an accident and that sort of thing, I wasn’t able to get to a loo in time, that sort of thing. That was really worrying.
IG30, age 73 years
I suppose I never articulated this at the time but feeling concerned about a loss of control over my life … ‘Loss of control over my life’ sounds rather pretentious but I can’t think of a better way of phrasing it.
IG6, age 78 years
… that anxiety, if you see what I mean, of not doing anything.
IG26, age 72 years
… I suppose mentally because you know you’ve got a problem you make it worse for yourself thinking, ‘When can I get to the loo next?’ … Just a general urgency really of wanting to go, and anxiety I guess.
FG4, age 70 years
I sometimes worry that it might be something like sort of cancer, which thankfully it’s proved not to be. That’s when it preys on my mind.
CG2, age 62 years
Embarrassment, awkwardness and shame
Men described feeling embarrassed, awkward and a sense of stigma-related shame, associated with LUTS:
It’s quite soul destroying. It makes you feel like when you were a child and you used to wet the bed. The stigma and stuff like that. It’s going back to that stage and thinking that I’m a grown man, I shouldn’t be doing this.
CG2, age 62 years
If I was driving up a motorway and I just passed a service station, I’d be in agony by the time I got to the next one and sometimes obviously I dribbled. It was getting embarrassing … It’s just got to a stage where it was getting too embarrassing to go out.
FG7, age 78 years
I don’t think I would have discussed the urgency problem with [my wife], actually, thinking about it. No, it would have been around getting up in the night … [Interviewer: What stops you …?] I suppose embarrassment. It’s to do with … self-esteem. You feel that you are diminished, in your own view, that I lack control … Therefore, I would feel embarrassed …
IG6, age 78 years
Frustration, annoyance, disturbance, disruption and feeling down
Many men described feeling annoyed and frustrated by their symptoms, whether because of frequent night-waking, slow flow, a frequent need to urinate or wet patches; for some, the level of disruption was more a source of frustration and irritation than anxiety, whereas for others it was frustrating, distressing and concerning. The word ‘bothersome’, widely used in the clinical symptom assessments,53 was occasionally used unprompted by men in the sample (although it is perhaps a somewhat outmoded term), and remains apt in relation to this constellation of responses to the experience of LUTS (although insufficient in relation to the full range of distressing experiences described by men):
I’m not banging my head or gonna commit suicide about it, but it does bother me a little bit, sometimes I have to change underwear a good two, three times a day, possibly … it can be a bit annoying at times.
CG11, age 48 years
Normalisation and discounting as ‘part of getting old’
Many men describe their symptoms as something inevitably associated with ageing or ‘old men’s problems’. 48–52,54 This age-related normalisation is, in one sense, reassuring, in that ‘normal’ also means that there is not a more concerning explanation for, or condition underlying, LUTS. Normalisation is, however, also potentially dismissive, in the sense that it suggests inevitability. Inevitability is thoroughly undermining of potential efforts to ameliorate the condition through enhanced understanding and self-help, implicitly carrying a sense instead that there is no need to attend to anything but the most severe symptoms (at which point pharmaceutical/surgical treatment options might be more palatable):
Probably I just get on with it, yeah. A good description I heard from a doctor on YouTube [YouTube, LLC, San Bruno, CA, USA], ‘Getting old is not for sissies’, and I do consider this urinary problem along with all the other aches and pains is part of getting old.
CG13, age 70 years
Conclusions: theme 1b – experiences of lower urinary tract symptoms
Men describe a range of distressing symptom experiences, which ‘bothersome’ perhaps overneatly subsumes (see Figure 8, theme 1b). These range from tiredness and low or bad mood associated with frequent sleep disturbance, through a fleet of anxieties and stresses associated with concern about being caught short (leakage, whether post-voiding dribble or incontinence and wet clothing/visible damp patches), to being unable to initiate micturition after long periods holding. The associated logistical planning and surveillance required to ensure ready access to toilets during public/social activities, alongside embarrassment, shame, dispiritedness, frustration and annoyance, mean that LUTS disrupt everyday life. Many men said that they did not allow these symptoms to interrupt their daily activities, but some said that, over time, as symptoms worsened, they had withdrawn from certain or all social events and activities. The sense that these symptoms are an inevitable part of ageing was a predominant feature of accounts and was something that had, for many, been reinforced by the common tendency among men to shrug off symptoms as ‘part of getting old’.
Theme 1c: personal and social factors
Men’s contextual personal and social circumstances (see Figure 8, theme 1c, ‘Personal/social factors’) varied and shaped the interview cohort in ways that are likely to be relevant to experiences of LUTS, engagement with health services, involvement in the TRIUMPH trial and take-up of self-help guidance (see Figure 8, 1c; see also Appendix 5 for further accounts).
Men had varying degrees of additional contextual health concerns at the time of their involvement in the trial. The degree and centrality of other health issues factored in men’s focus on LUTS and willingness and ability to engage with the intervention; many men could be characterised as ‘well or very well’:
Generally very fit and healthy really, physically active and no health problems.
IG3, age 53 years
A number of men had considerable additional health issues alongside LUTS, but these were well-managed/in remission and did not stop LUTS from being a considerable additional concern/focus. Some men had conditions that interacted with their LUTS, such as diabetes, UTIs, prostate infections, or irritable bowel syndrome, whereby symptoms related to the respective conditions were, at times, indistinguishable or interacted (e.g. needing to urinate frequently, pain or sensitivity associated with excretion/voiding):
I also suffer from irritable bowel syndrome which concatenates with the urinary problems.
CG5, age 66 years
Some men had more active current and/or overwhelming health issues, which meant that LUTS were a peripheral concern at the time of their initial involvement in the trial:
Well, I’m in and out for tests on the operation … It is a bit concerning really … I had to go into the hospital and have tests and he said there’s blood being lost somewhere … Shortness of breath … I find it difficult walking. I get pains in the stomach whenever I try to walk … I think time’s run out now anyway…
IG10, age 71 years
Life circumstances/lifestyle
Men’s lifestyles varied: some were working, many were retired, some were carers for their partners and some were younger men with children at home, alongside other commitments. Most men were married, but some were civil partnered, widowed, divorced or single. The men often appeared relatively educated (describing careers in engineering, research, medicine, teaching, civil service, accountancy and other professional roles), affluent (homeowners, with gardens, a car, taking flights, holidays abroad) and active (describing regular activities including cycling, walking, swimming, golf, cruises, scuba diving, theatre trips, football/cricket matches, going to the cinema and shopping) and unperturbed by the paperwork and other tasks necessary to participate in the TRIUMPH trial. However, the sample was not uniform: among those interviewed were also a number of skilled tradesmen (e.g. working/previously working in manual engineering, electrical engineering, carpentry, gardening, firefighting, flight-attending), some had officer-/captain-rank military backgrounds, others were unable to work because of disabilities or ill health, or were in recovery from serious health conditions (e.g. cancer, heart surgery, depression, accidental injury), and some described more sedentary, less health-conscious/active lifestyles.
History of urinary tract infections/lower urinary tract symptoms/bed-wetting
Some men described long histories of urinary issues; for some these related to long-standing issues with LUTS and/or UTIs (for some these histories extended over 20–40 years). For others, there were traumatic childhood experiences of bed-wetting into late childhood/early adulthood. For some of those involved in the trial, urinary issues were a traumatic and/or debilitating lifelong experience. There is a growing literature relating to the life-course experience of urinary issues. 55 Some of the study men who had long histories of urinary issues (such as bed-wetting in later childhood) or long histories of UTIs or LUTS also described considerable sensitivity, embarrassment and shame in relation to their urinary experiences. In some of these cases, alongside dispiritedness and shame in relation to LUTS, men reported long-term mental health issues, in particular depression in adulthood.
Men with long-term recurrent UTIs:
… I’ve always had a problem with urinary tract infections for most of my life … I’m getting about two or three infections a year … [Interviewer: When did you first start to experience that?] I think it must have been about when I was about 22, 23, something like that. Yeah, so about 40 years you could say.
CG2, age 62 years
Men with childhood/adolescent urinary issues:
Well, um … I’m not sure [when LUTS started] because when I was – I had a problem with bed-wetting until I was almost 20 and so I’m wondering whether I have always had a problem of some description … It was horrible, truly horrible. I used to go to school, and often I used to go to school smelling and it was just horrible, completely from beginning to end. It just made me feel really bad.
FG14, age 58 years
Men with a family history of prostate cancer
Some men had fathers or brothers who had been diagnosed with prostate cancer. One man had two brothers diagnosed with prostate cancer in the previous 12 months (this man was one of the few men of black, mixed-race or Asian heritage, perhaps underscoring varying disease prevalence and experience among diverse communities and the kinds of issues that may warrant more focused attention and be underdocumented in a predominantly white sample). These men were understandably particularly alert to the possibility of prostate cancer:
My eldest brother he’s had his prostate removed but he’s doing alright now. My youngest brother who’s got prostate cancer, he hasn’t started treatment yet because they found it at a very, very early stage.
IG27, age 68 years
Gender
Issues of gender, or ‘being a man’, were apparent, especially when it came to men sharing their experiences, and in their help-seeking behaviours. Men said they did not readily talk about LUTS with friends, family members or clinicians. 56,57 Although some men described comfortably talking (at least with some others) about their symptoms, others identified their own and/or other men’s reluctance or embarrassment to do so, in anything other than a jocular and dismissive way, as an aspect of gender. This tendency can be seen to increase the chances that symptoms will be downplayed, and reduce everyday opportunities for sharing LUTS experiences, information and guidance among men:
… probably a bit typical of men, I think, not just in lower urinary tract symptoms, but all kinds of other things, men just don’t talk to each other about those sorts of things. There’s a couple of close friends, who I’ve talked to about it, but mainly in a fairly light-hearted way, almost, as part of banter, really.
IG25, age 68 years
Character/personality and health-seeking behaviours
Men also varied in their health-seeking behaviours.
Proactive/not complacent
Some men said that they mostly felt comfortable independently exploring or researching health conditions, in print or online, and readily seeking clinical guidance:
I look up everything … I’ve always done it no matter what, even if I’m buying something, I’m researching it to be honest … I think that forewarned is forearmed if you know what I mean? So you can plan for things.
IG22, age 45 years
As soon as there’s a twitch I go and get sorted out.
FG9, age 65 years
Reticent/avoidant
Some men were much more anxious about exploring health concerns, especially without clinical guidance or oversight, and mostly avoided doing so:
… I didn’t want to know. I thought if I ignore it, it’ll go away …
IG9, age 75 years
Stoic
Others were more stoic in their tolerance of various symptoms, and consequently unlikely to seek support unless symptoms became in some way alarming. This ‘stoic’ approach went hand in hand with processes of normalisation and discounting (see Normalisation and discounting as ‘part of getting old’), and is a common barrier to seeking support in relation to LUTS:
I’m quite stoic in my outlook and I tend to think, you know, you’re not gonna get through life without putting up with something or other and as things go, it’s not all that bad.
CG8, age 65 years
Conclusions: theme 1c – personal and social factors
Men’s LUTS-related experiences and health-seeking behaviours were shaped by a number of personal and social dimensions (see Figure 8, 1c), in particular (1) health (whether relatively well or unwell); (2) lifestyle/life circumstances, including marriage and working status (being married/civil partnered/single, being retired/in work/unemployed, having caring responsibilities), and having a relatively ‘healthy’ lifestyle (being active/fit, taking care with diet/alcohol, attending to mental health); (3) having a life course/long history of urinary issues; (4) having a family history of prostate cancer; (5) gender dynamics related to discussing symptoms/help-seeking; and (6) character/personality in relation to seeking health-related information/health checks (being proactive, avoidant or stoic). The qualitative sample suggested a predominance of apparently relatively affluent, married, retired men with largely healthy lifestyles, who were also fairly proactive in their health-seeking behaviours. Nevertheless, there were also busy working men, skilled tradesmen, unemployed men, men with young children, men caring full-time for partners, men caring regularly for grandchildren (to support working adult children), men with severe additional health issues, many men who did not discuss their symptoms with others and men who felt anxious about seeking health-related information/guidance, including (or especially) online via internet search engines. Despite apparent educational and lifestyle advantages, and relative health-related proactivity, many men described very lengthy periods with disruptive symptoms, without seeking clinical or other support.
Overall conclusions: theme 1 – lower urinary tract symptoms experiences
The TRIUMPH qualitative study cohort were men with a full range of LUTS, including long-term and severe symptoms, which impeded men’s lives, causing distress, tiredness, anxiety, embarrassment, frustration and annoyance, and, at worst, affected work and/or stopped men taking part in valued social activities. Some men described considerable and traumatic life-course urinary issues (relating to chronic recurrent UTIs and, more rarely, bed-wetting into early adulthood).
Many of the men were retired and married with grown-up children and appeared to be relatively affluent retired professionals, in reasonably good health, with relatively active lifestyles. The qualitative sample was also overwhelmingly white, including only four men of black, mixed-race or Asian heritage. This issue of bias (relating not to the kinds or severity of LUTS experiences, but to the culture, race, lifestyles and life circumstances of the men who volunteered to take part) will be returned to (see Discussion and conclusions). Nevertheless, the qualitative study includes accounts of men who were black, mixed race or Asian (also Polish), as well as men who had considerable additional health issues; men who had less active lifestyles; men who were younger and working, including skilled tradesmen; men with military backgrounds; and men who were unemployed and/or unable to work following bouts of ill-health.
Other useful population dynamics were evident, of relevance to men’s willingness, engagement and uptake of health-related information and guidance, in particular gender dynamics, associated with men’s discomfort in relation to, and dissuasion from, raising the issue of LUTS (and other non-life-threatening health conditions), and levels of proactivity, anxiety-related avoidance and stoicism in relation to health-related information, advice and help-seeking.
Theme 2: visiting the general practitioner/entering primary care
Men were asked to describe their LUTS-related contact with primary care. This gave useful insight into the drivers of GP visits, and the ways in which men with LUTS have been, and are currently, supported within primary care (and, if referrals had taken place, within secondary or specialist/consultant-led urological care) from the perspectives of male primary care patients with LUTS in (south-west) England. An exploration of men’s primary care experiences and post-primary care knowledge and awareness of their LUTS condition, and of self-help guidance received via primary care, offered insight into the status of, and need for, enhanced information provision and self-help guidance within primary care for men with LUTS (see Figure 8, theme 2).
Theme 2a: drivers of the general practitioner visit
The men in the TRIUMPH trial were identified by their general practices as men with some LUTS-related GP contact. For some this GP contact had been fairly recent, for instance within the preceding 1 or 2 years, but many men recalled initial discussions with their GP about LUTS taking place very many years previously (for some, ≥ 20 years prior to engagement in the TRIUMPH trial); some did not recall a designated LUTS consultation, but rather felt that they had probably mentioned their LUTS ‘in passing’ at some stage, and some recalled prostate cancer concerns and prostate-specific antigen (PSA) tests, rather than LUTS-focused discussions. This meant that recollections varied from distant, vague, summary or non-existent, to more recent, detailed and/or relatable. Nevertheless, there were similarities across time in the accounts of what drove men to visit or raise LUTS with their GP (see Figure 8, 2a): namely symptom severity, and, beyond symptom severity, concerns about prostate cancer were prevalent (and often it was a combination of these factors; see Appendix 6 for additional accounts).
Symptom severity (and gradual onset/high thresholds of severity)
Many men described a gradual worsening of symptoms, often over lengthy time periods, before ultimately making an appointment with their GP. Any kind of LUTS could reach a threshold to precipitate a GP appointment: for very many it was initially sleep disturbance; for others worsening frequency, urgency and/or hesitancy or slow-flow issues; a particularly painful or debilitating UTI (alongside LUTS); diffuse or multiple LUTS; or an incident of acute urinary retention. For a few men the threshold seemed low (e.g. waking once a night over a period of months) and this ‘low’ threshold was often accompanied by concerns about the possibility of prostate cancer (see Fear of prostate cancer). However, for many more men, the threshold was very high (e.g. waking six or seven times a night for 20 years before seeking help or advice from primary care), with a tendency to discount even relatively severe symptoms for prolonged periods, often years, before any action was taken:
It had become more often over a period of time, but whereas you start from not having to go at all and just wake up in the morning and go to the toilet, you then find once during the night I’d get up and then a year or 2 later you’d notice it’s twice a night I’m getting up now, and then a year or 2 later I got up three times last night … It developed over probably 5 or 10 years and it’s when it reaches the point where it becomes three and maybe even four times a night that then you really feel ‘isn’t there something we can do about this?’ and that’s when I went off to see the GP.
IG19, age 75 years
Fear of prostate cancer
As has been reported elsewhere, for many men, concerns about prostate cancer were a major factor in the decision to consult a GP. 50,52,54 Often the precursor to seeking clinical input was the experience of an acquaintance, friend or family member, or indeed a well-known public figure, who had been diagnosed with prostate cancer and described LUTS as a precursor or an associated symptom. For others, persistent/worsening LUTS increased tacit cancer concerns, triggering the decision to seek clinical support. Some reported concerned spouses/partners encouraging a check-up as symptoms continued or deteriorated. Several had read/heard about the PSA test and were keen to access this, for reassurance that their LUTS were not related to prostate cancer; some felt that these kinds of tests were, or, if not, ought to be, included in a general health ‘MOT’ for men in older-age brackets, and sought it out on that basis. Among TRIUMPH trial men, prostate cancer concerns were a common precursor to, and component of, primary care LUTS-related consultations (see Appendix 6 for additional accounts):
It was basically the fact I started to get reduced flow and difficulty urinating and a sense of urgency as well all the time, and I was living with this feeling of always needing to go to the toilet, but then going to the toilet and not being able to pass water. [Interviewer: What got you to the GP? What prompted you to take action?] Basically, because a friend got prostate cancer and had similar symptoms, so I decided I’d best go and have investigation done.
FG2, age 52 years
Notably, men seemed more inclined or determined to visit their GP because of prostate cancer concerns than because of LUTS, which may mean that men with mild/moderate LUTS are more likely to present to their GPs seeking PSA tests and reassurance in relation to prostate cancer, rather than LUTS remedy, despite enduring LUTS.
Recognising that the main drivers of GP visits in relation to LUTS are ‘severity of symptoms’ and ‘life-threatening cancer concerns’ raises a number of issues:
-
Men are liable to downplay their LUTS, including with their GPs.
By implicitly characterising LUTS as a mundane, trivial, inevitable facet of old age, men disregarded even severe and debilitating long-term LUTS experiences. This way of downplaying (at times even severe, distressing and disruptive) LUTS for many months or years affects men’s chances of seeking timely primary care, when chronic symptoms are less acutely severe.
-
There is a major disparity between men’s propensity to take action on prostate cancer concern and their propensity to take action on LUTS.
For some men, regular health checks, including PSA tests, and taking a proactive stance in relation to health and concerning symptoms were an important part of looking after themselves. 58,59 Paradoxically, men felt that it was important not to ignore health concerns in relation to (the relatively low risk of) prostate cancer, and simultaneously downplayed and discounted the need to visit or revisit their GP in relation to (present and enduring) LUTS. 60 The difference between the characterisation of clinically pressing (‘life-threatening’) prostate cancer concern and, by contrast, clinically unworthy (‘mundane/trivial’) LUTS was stark.
-
Men are liable to navigate their GP consultations away from LUTS and towards PSA testing.
It is evident that patient-led GP consultations involving mild to moderate LUTS may be overly oriented towards attending to prostate cancer concerns, and the pros and cons of PSA testing. Given the exigencies of the GP consultation, this is likely to reduce both the focus on, and the opportunity to discuss, LUTS alleviation, and self-management guidance in any depth: FG11, age 73 years
… with hindsight this thing about the high PSA and the testing for prostate, took over the symptoms that I’d really gone to the doctor about, not that I’m criticising that because, you know, it’s the sensible thing to do, but once they came back negative it was: ‘oh well, have these tablets’ and I think at that stage it would have been very useful to have a booklet. OK, it’s all come back negative on the prostate, have a read of this booklet and then we’ll go from there in terms of treatment options and exercises and so on …
Just ‘mentioned in passing’
A few men could not recall a dedicated LUTS-related GP consultation, but felt that they may have mentioned their LUTS at some stage ‘in passing’. Therefore, ongoing LUTS for some men in the trial had not yet reached a threshold that they felt might justify a dedicated GP consultation. This perhaps reflects the predominant position among men with LUTS in the population, who, for the most part, do not reach the attention of primary care. 47 Unfortunately, this may be as close as some men get to discussing their LUTS with a GP or with anyone:
I think I did go to the doctor for something else and then I brought that up while I was with him. So, I didn’t make a special appointment to go and see him, from memory.
IG15, age 75 years
Conclusions: drivers of the general practitioner visit
To justify a visit to the GP, men described the need for symptoms to become sufficiently alarming, that is very disruptive, distressing or potentially life-threatening (see Figure 8, 2a). This might be the result of severely disruptive symptoms, the onset of a very painful UTI, incessant/frank incontinence, waking frequently at night, needing to urinate very frequently in the day and/or night, acute retention, blood in urine and/or prostate cancer concerns. This tendency can be seen as a barrier to the uptake of timely information and guidance on LUTS and LUTS self-management among men. The ‘severity’/’life-threatening’ barrier (the counterpart of the ‘normalisation/discounting’ barrier; see Normalisation and discounting as ‘part of getting old’) to accessing timely self-help guidance appears to operate within the GP consultation, whereby acute severity of LUTS and/or prostate cancer concern skews the discussion, reducing the likelihood that the day-to-day self-management of less acute symptoms will be discussed in any detail, as opposed to reassurance, PSA testing and pharmacological intervention options.
Theme 2b: the consultation – general practitioner responses
Patterns were evident in GP consultation descriptions (see Figure 8, 2b), which could be grouped according to those that offered the following.
Reassurance alone: ‘That’s what happens with old men’
Clinical guidelines recommend that GPs offer reassurance to men with LUTS who are concerned about their symptoms. 7,46 Because many of the trial men arrived at their GPs to talk about their LUTS with concerns about prostate cancer, it is perhaps unsurprising that many trial men described their GP offering reassurance that LUTS was ‘nothing to worry about’ and a common symptom among ageing men. The guidance is clinically appropriate, given that LUTS is not a reliable indicator of prostate cancer and the majority of men with LUTS will not have prostate cancer. 60 However, given men’s tendency to discount LUTS as an inevitable part of ageing, there are some key problems with this approach. First, reassurance alone fails to address LUTS; following their GP’s reassurance, men described a sense that enduring LUTS was something they just had to deal with by themselves as part of ageing:
My recollection of the exercise [GP consultation] is ‘That’s what happens with old men’, and so I didn’t feel that really anything came out of going to see my GP.
IG19, age 75 years
Most … I’ve been told [by the GP], ‘Oh that’s your age. Learn to live with it.’ But that’s as far as it went really.
IG11, age 76 years
Second, men described taking months, or years, to present LUTS issues to their GP, which means we might best view ‘raising LUTS with a GP’ as a relatively rare moment in a potentially long trajectory of LUTS. From this vantage, providing cursory reassurance that LUTS is a normal part of ageing can be seen as a failure to offer robust sensitive support (be it information, conservative care guidance or other assessment or treatment),7,46 at a rare opportunity to do so.
Physical examinations, routine tests, referrals and diagnoses
For men who received more than simply ‘reassurance’, there was a familiar pattern of assessment and diagnosis and a familiar pattern of limited or absent self-management guidance. Most men recalled describing their LUTS and/or being assessed by their GP, mostly by digital rectal examination, alongside various blood and urine tests (men rarely knew what kinds of blood tests they had received, unless this was a PSA test). This corresponds with GPs’ accounts (see Interviews with clinicians). In the absence of indicators of a UTI, diabetes, blood in urine and physical signs of prostate tumour, most men were diagnosed with suspected ‘enlarged prostate’, as well as being reassured that this was age-related, most likely benign and nothing to worry about:
I have had several times with my GP … a digital examination, just because I’m aware I’ve got an enlarged prostate. Whether that was because of the urinary infection, they’re checking because of that, or simply because the other related symptoms might have led to thinking there was an issue … But each time, they have said, ‘Yes. It is enlarged, but there’s no indication of it being untoward’. It was an age thing, they thought, rather than anything more sinister.
CG6, age 70 years (also referred for urological assessment, had more than one PSA test and prescribed medication to ease flow, but no recall of being given self-management guidance/advice)
Treatments (pharmacological intervention)
Many men were offered pharmacological treatment by their GPs (often tamsulosin and sometimes finasteride, but other drugs were mentioned, including sildenafil, mirabegron and solifenacin). Some turned this down, while others tried medication either successfully or unsuccessfully, with or without unpleasant side effects or symptom alleviation. Others reported continuing with medications for years, and concern that symptoms might worsen if they stopped. Some men preferred not to take medication. Rarely were these men offered detailed self-management alongside or prior to pharmacological intervention:
… I felt sick, headaches, I just felt unwell most of the time. But within about 2 days of stopping taking them, I felt absolutely fine, except of course I had to get up in the night again.
IG5, age 66 years
… he checked my prostate and that needed … well, I’m on tamsulosin. I can’t remember why … if it was too hard or too soft, I can’t remember now … That was a few years ago.
CG1, age 58 years
… She said, ‘I can give you something to stop you getting up so many times during the night, but the problem with it is that it dries out your digestive tract’, and so I chose not to take anything …
FG14, age 58 years
The common features of pharmacological intervention accounts were as follows: (1) men did not regularly revisit LUTS-related medication use with their GP; (2) men remained unsure of the appropriate threshold for revisiting symptoms within primary care; (3) some men felt that surgery was something they might favour in the future, if/when things became really intolerable; and (4) most men taking medication for LUTS were unfamiliar with recommended self-management approaches (beyond, typically, fluid and caffeine reduction in the evening) prior to the TRIUMPH trial (for CG men, this continued to be the case throughout the trial).
Referral to urology
Men with more complicated symptoms often described referral to urology and a range of further assessments (including flow-rate tests, flexible cystoscopy, computerised tomography, residual bladder volume assessment, magnetic resonance imaging, biopsies and ultrasonography). This led to further diagnosis and pharmacological treatment (with outcomes as outlined above in relation to treatment experiences). A few of these men were able to describe the physiological issues underlying their LUTS in more detail and the proposed mechanisms of pharmacological interventions more precisely, but diagnosis/treatment often continued to be oriented around inconclusive assessment outcomes or vaguely around ‘enlarged prostate’ or ‘overactive bladder’. One study participant reported receiving the kind of guidance offered in the TRIUMPH trial booklet through his prior contact with urology; however, he was a notable anomaly: most men had very limited prior knowledge of recommended self-help techniques, even following referral to urology. Men rarely recalled GPs performing extensive (or any) urological assessments beyond questions/discussion of symptoms and very occasionally bladder diaries, and this chimed with clinicians’ accounts (see Interviews with clinicians, and Milosevic et al. 54).
In summary, a considerable number of participants had been referred to urology specialists, where a wider range of assessments had taken place. Often this resulted in the prescription of additional/adapted LUTS medication, but did not routinely lead to extensive self-management discussion/or necessarily to more detailed symptom explanation/understanding.
Symptom explanations, self-management advice/guidance
It was evident in men’s understanding of their condition, and from descriptions of the explanations, treatment and guidance they had been offered through primary care, that there was a lack of explanation of the physiological underpinnings of their LUTS [beyond ‘enlarged’ prostate, ‘benign prostatic hyperplasia’ (BPH) or ‘overactive bladder’], and of detailed self-management options:
Interviewer: Did the doctor offer you any advice, or support … or anything in relation to the urgency? Any guidance?
CG7, age 61 years: Not that I can remember.
Interviewer: From that point when you went, have you done anything to change your lifestyle in any way?
CG7: No, not really. I just carry on as normal … I was just – possibly for want of a better word – just left to get on with it, I suppose.
Only a very few men described receiving more detailed physiological explanation (usually following specialist urological assessment). Few men reported extensive prior self-management guidance (either via their GP or through urology referral). One man was taken through bladder drill guidance and pelvic floor exercises as a result of urology referral and assessment, and another was asked by his GP to complete a bladder, food and symptom diary in the context of a combined suspected irritable bowel syndrome and LUTS presentation. This chimed with GP accounts: according both to interviews with TRIUMPH trial men and clinicians, bladder diaries, and especially pelvic floor exercises for men and bladder drills, are not routine parts of GPs’ LUTS consultations with men (apart from, perhaps, when GPs have a specialist urology interest, or a special interest in self-help; see Interviews with clinicians).
On the other hand, many men said their GP had asked about and advised on fluid and caffeine intake, often recommending, for instance, a reduction of fluid intake and caffeine in the evenings to alleviate night-waking to urinate:
I think the general advice was not to overdrink liquids before going to bed. She didn’t mention anything about diet or anything like that, just reducing the liquid intake.
FG12, age 59 years
The prostate-specific antigen test loop
Interviews with study men revealed a cycle of missed opportunities to address LUTS by offering appropriate self-management guidance that we have called the ‘PSA test loop’. Cyclically, men with LUTS, with mild to moderate/severe symptoms, described attending their GP primarily concerned that their symptoms could indicate prostate cancer. These men described primary prostate cancer concerns, and many actively sought a PSA test, discussed the pros and cons of PSA testing, and were given advisory leaflets, before deciding to go ahead with the PSA test. While some of these men went on to discuss LUTS treatment options (typically pharmacological intervention options) with their GPs following their PSA test results, many men phoned in for their PSA test results and on receipt of a ‘low’ PSA result, treated the consultation as complete: their prostate cancer concerns were alleviated. For many of these men LUTS physiology, self-management and symptom alleviation were not discussed further.
I was concerned to have my prostate checked … I think that’s the first thing that took me to my GP and on the whole I haven’t actually consulted him about the difficulties that I have passing water … you just realise you have to learn to live with it to some extent, until I suppose it gets absolutely desperate, then you say to the doctor ‘I’ve got to have something done, even if it’s surgery’.
FG10, age 77 years
We have called this the PSA test loop because many men described their relief following a ‘low’ PSA test result, and concurrent failure to attend further to their ongoing, gradually worsening LUTS presentation, eventually triggering further prostate cancer concerns. This results in repetitions of the PSA test cycle, with repeated failure to discuss conservative symptom management, rather than cancer concerns/PSA tests (see Appendix 6 for a long extract with a very clear account of this cycle).
No memory, no treatment
A few men described having no memory of their LUTS-related GP consultation, and similarly no recall of an offer of any LUTS-related treatment:
I have no memory of going to the GP, I obviously must have done, I’ve never had any treatment in the sense of any medication offered or anything like that so, I’ve just been putting up with it basically.
IG29, age 85 years
I spoke to the doctor and then, I don’t know, you guys got involved and then, yeah, that’s it, yeah. I’ve been to my doctor, he doesn’t really do anything for me, per se, on that …
CG11, age 48 years
These men may have forgotten the detail of a distant encounter. Indeed, some men openly said that they did not feel that they could rely on their memory of the consultation. Nevertheless, this gives the impression that some form of more lasting self-help guidance material may well be helpful.
Conclusions: theme 2 – general practitioner responses
Consultation accounts (see Figure 8, 2b) can be grouped into those that (1) offered cursory reassurance (that LUTS are nothing to worry about, and a common issue in older age); (2) involved a fuller assessment, including physical digital rectal examination and various diagnostic tests to rule out other issues (e.g. diabetes/UTI), basic lifestyle discussions/guidance around liquid intake and caffeine, and diagnosis (typically) of BPH/enlargement; (3) offered pharmacological medication; (4) involved referral for fuller urological investigation prior to diagnosis (typically BPH, enlarged prostate or overactive bladder syndrome); (5) were inadequate in relation to explanation and self-management approaches; and (6) resulted in PSA testing (and often the ‘PSA test loop’ cycle). Some men described GP encounters (and referrals to urology) involving all of these components; for others, cursory reassurance summed up the entire encounter, and reinforced the notion that LUTS were an inevitable part of ageing and not a clinical concern (an issue that has been reported elsewhere). 50,54 What was missing from most accounts of GP responses was detailed explanation of the urinary system/probable physiology of a man’s particular LUTS (beyond the more generic ‘enlarged prostate’ explanation), and thorough discussion of self-management techniques. For the most part, men remained ill-informed after primary care engagement.
Theme 2c: outcomes and satisfaction
As has been reported elsewhere,54 men described positive relationships (see Figure 8, 2c) with their GPs and satisfaction with the outcomes of their LUTS-related primary care consultation. Despite displaying weak knowledge of their conditions (e.g. the mechanisms at play), often experiencing enduring symptoms with/without ongoing pharmacological treatment and having limited self-management guidance, men mostly did not report dissatisfaction with their GP encounters. Indeed, a few men had found their treatment (typically pharmacological intervention) helpful and continued to take medication, in some cases for years, typically without regular review, but happy that this helped alleviate symptoms. Some men reported acceptable side effects (including complete loss of libido for one man using finasteride, but which was reported as something that no longer felt concerning). 61 Other men were more disappointed either with being offered ‘reassurance alone’ or as a result of unacceptable side effects that led to the cessation of medication (e.g. ejaculation problems, dry mouth, unpleasant side effects that were more severely distressing than LUTS). This begs the question: why do men who often continue to have untreated, ongoing, worsening symptoms report satisfaction with GP encounters that did not result in better understanding or symptom alleviation? The answer seems to be that men’s expectations in relation to LUTS care and support are very low; the belief that LUTS is inevitable and unworthy of clinical attention runs deep – men often felt they ought not really to be bringing their LUTS concerns to their GP, and were often genuinely relieved to be told that LUTS was not likely to be related to concerning underlying factors (e.g. prostate cancer).
General practitioners rely on patients to come forward if or when symptoms become sufficiently problematic or worrying. Unfortunately, in the case of LUTS, as a consequence of men’s reluctance to bother their GP with this ‘unworthy’ condition, many men do not return to their GPs for review or to discuss gradually worsening ongoing LUTS (inhibitors of GP attendance are discussed further in Theme 3: resigned tolerance and inhibitors of seeking further clinical support). Other notable outcomes of the GP encounter, of relevance to the study, and/or highlighted through interviews are discussed in the following sections.
Poor knowledge, awareness and understanding of recommended self-help guidance
In line with findings from other research in this area, on the whole, men seemed poorly informed about their LUTS condition. 48,50–52 This was the case even despite a vested interest in LUTS alleviation, and often taking a proactive stance in relation to general health (e.g. attending to diet, weight and staying active). Prior to involvement in the TRIUMPH trial (and for CG men throughout the trial), most men lacked all but the most basic understanding and awareness of clinically supported self-help guidance for LUTS (see Appendix 6):
The night-time waking I just accept and I don’t know if maybe having to rush to the toilet, maybe I just think I hope it will go away some time. I wouldn’t know whether medically anything can be done about that so I’ve just accepted it as it is.
CG4, age 81 years
[Interviewer: What do you think of as being the cause?] I’ve no idea. Not being a medical person I’ve no idea what the cause may be so I just take it as something that’s happening and sort of get on with it.
CG4, age 81 years
Intervention arm men who received the TRIUMPH trial booklet were, for the most part, unfamiliar with much of the self-management guidance it contained. Many men with signs of post-voiding dribble had not come across this terminology, nor the urethral bulb massaging technique (prior to their involvement in the TRIUMPH trial). Men in the usual-care arm with symptoms of post-voiding dribble remained unfamiliar with the term and unaware of any technique that might alleviate it:
… also when I’ve finished urinating and then I quite often have a leakage afterwards. It’s as if what I’ve released from the bladder has gone down as far as the prostate and what is distal to the prostate has gone out but there is a dead leg between the bladder, sphincter and prostate, which occasionally will leak after urinating.
CG5, age 66 years (despite clearly giving it some careful thought, this usual-care arm man remained unaware that ‘post-voiding dribble’ is a common LUTS experience that might be alleviated through the urethral bulb massaging technique)
For the most part, self-management guidance was either absent or had not been a memorable or extensive component of LUTS-related primary care, despite many men having trialled, or still taking, medication to alleviate their LUTS. Even men who had reached specialist consultant-led urology services did not routinely report having received or implemented in-depth self-management guidance.
Follow-up
Men often reported a singular LUTS consultation, or sometimes an initial series of consultations whereby LUTS assessment led to urology referral, diagnosis and medication trial, or resulted in PSA testing (and cycles of the PSA test loop). After initial contact, interviews indicated long periods of time (years) in which men did not seek additional follow-up in relation to ongoing symptoms or for medication review:
… [the doctor] prescribed me these tamsulosin and sent me on my way and that was it really. I suppose, maybe, I should have gone back to her after a while, but that’s been it.
IG24, age 72 years
Overall conclusions: theme 2 – lower urinary tract symptoms-related primary care
The tendency among TRIUMPH trial men was to discount LUTS, and to reach primary care only if/when concerned that their symptoms may be indicative of a life-threatening condition (prostate cancer), or only after substantial, severe and lengthy periods enduring symptoms with little if any support, information or guidance. The explanation, given men’s accounts of their understanding and experiences of LUTS, is that men treat LUTS as an unfortunate but inevitable aspect of ageing, and have little hope of remedy, beyond a last resort of surgery. Some, but not all, men are keen to try pharmacological interventions, and some find these helpful; others experience unpleasant side effects, or minimal alleviation of symptoms, and are not keen to continue taking medication indefinitely. The dominant narrative that LUTS are ‘just part of ageing’ unfortunately appears to obliterate the seeking of a more constructive explanation and/or self-management guidance. TRIUMPH trial interviews suggest that not enough is currently done to counter this narrative within GP consultations; indeed, the standard reassurance offered to many men may serve to reinforce despondency, and needs an overhaul.
Much is covered in GP consultations (not just reassurance, but very often also tests and examinations to rule out other conditions, symptom discussion and referrals to urology to gauge aetiology, pharmacological interventions to reduce the size of the prostate and/or to relax the urethral sphincters, and so on). Indeed, men report being satisfied with their LUTS-related GP consultations, despite their symptoms persisting. It is important to situate men’s satisfaction in the context of their positive regard for their GP, their very low expectations in relation to LUTS remedy and their belief that they ought not really to bother their GP about LUTS.
Men do not miss what they do not know to expect. TRIUMPH trial interviews give the very strong impression that in-depth self-management is not a routine part of most GP LUTS consultations, easily slipping out of primary, and even secondary, care, perhaps because men are focused on prostate cancer concerns (and cycle through PSA tests loops without ever attending to their LUTS), or have, by the time they reach the GP, rather severe symptoms. The gap in men’s own understanding of the physiological mechanisms, and in their awareness that clinically recommended self-management approaches exist, has not, by most accounts, been sufficiently targeted or addressed, despite self-help guidance or ‘conservative care’ being recommended best practice in the first stages of LUTS care and treatment. 7,46
As things stand, it appears that, by the time many men (at least those not focused on PSA tests) reach a threshold of symptom severity that leads them to seek treatment for LUTS from their GP, they are liable to move straight into a pharmacological pathway, bypassing earlier gradual stages of LUTS onset, when LUTS self-management might have been appropriately offered:
Well, I think again, it would have been useful to have a booklet like that [the TRIUMPH trial booklet] at a much earlier stage of my symptoms. In other words, I don’t think I ever had a detailed discussion with the GP or, you know, I don’t think anybody has ever sat down to me or given me anything to read giving an explanation to the symptoms … I mean I read the booklet and yes, it made sense, but I think because I was taking these tablets, that reduced the symptoms, I didn’t feel there was any particular need to, you know, do anything, if you like.
FG11, age 73 years
Theme 3: resigned tolerance and inhibitors of seeking further clinical support
Resigned tolerance
As set out previously (see Theme 1a: kinds of lower urinary tract symptoms and Theme 1b: experiences of lower urinary tract symptoms), TRIUMPH trial interviews found that many trial men were living with compromising, embarrassing and difficult symptoms for long periods of time without seeking support and without accessing recommended clinical self-management guidance (see Figure 8, 3b). As outlined previously (see Theme 1c: personal and social factors), men tended to tolerate frustrating, distressing and depressing symptoms with stoicism, as an inevitable facet of old age, believing that only the most severe disturbance, or prostate cancer concern, might warrant further assessment and intervention.
In place of clinically recommended self-management guidance, men described making do with a range of their own unsatisfactory techniques to attempt to mask or overcome their LUTS. Men described some caffeine reduction, and lessening fluids before bedtime or before long journeys; using long jumpers or shirts to hide potential leakages; squeezing and shaking and using a tissue to wipe in an attempt to avoid post-voiding dribble; planning journeys around multiple toilet breaks; anxiously locating/remembering the location of toilets; and, at worst, staying home and avoiding social situations made awkward, uncomfortable and/or distressing by LUTS. A few men had begun to use pads occasionally, for instance on long journeys or trips away, to avoid the anxiety of potential bed-wetting in hotels/friends’ houses and to ease the stress of long journeys, but the stigma around pad use was strong; men more often viewed pad use unfavourably as an unwelcome sign of irreversible age-related decline, rather than a straightforward symptom support. Men’s unguided self-management responses were idiosyncratic and unstructured, and were most often attempts to conceal, rather than address, the underlying physiological causes of symptoms:
I use a weird technique, which I’ve invented, which is to sort of try and syphon it out with loo paper so that it sort of pulls out the wee … Except that it hasn’t been very effective.
FG8, age 69 years
Men’s resigned tolerance in relation to chronic LUTS was often underpinned by a weak understanding of the physiology of their LUTS, thoroughly inadequate knowledge of recommended self-management strategies, and limited knowledge of pharmacological interventions available and how these might operate in relation to their symptoms.
Inhibitors of seeking further clinical support
Men repeatedly discounted their LUTS as a symbol of old age, rather than a physiological condition that might be alleviated (see Figure 8, 3a). This was especially the case when symptoms were mild or moderate, but even with severe symptoms men were often uncomfortable, uncertain and considered it to be inappropriate to raise LUTS with their GP, frequently stating that they did not want to waste or take up their GPs time with commonplace, old-men’s problems, for which they anticipated no remedy. Men who might welcome further advice were unsure about the threshold of symptoms that might appropriately cause them to arrange to see their GP. Men understood the outcome of primary care involvement to be assessment and diagnosis (ideally ruling out pernicious health concerns such as cancer); pharmacological treatment; or referral for urological assessment and, in the most serious cases, surgery. For men who were not keen to take medication and who did not consider their LUTS severe enough to embrace the risks of surgery, there perhaps seemed little point in seeking a GP’s input:
I would probably fight shy of bothering the GP with these symptoms, because I would, and probably mistakenly, think it’s not worth bothering them with. Although it’s tiresome to deal with, it’s never seemed to be a medical something you’d go to the doctor about.
FG8, age 69 years
My gut feeling is it’s just going to stay like it or get slightly worse and I have to put up with it, really … GPs are very busy with people with more serious things … whereas the urinary symptoms, I thought was just a nuisance.
CG5, age 66 years
I thought I’d go for a bit longer to see if it recurs, within a month or two of that time. If not, then put it down to some aberration of some kind, rather than go and bother the GP about it again …
IG6, age 78 years (after two incidents of frank incontinence while sleeping at night)
This again belies a general lack of awareness of the range or efficacy of available self-management techniques (and targeted pharmacological interventions), revealing weaknesses in primary care, in everyday social discourse (in terms of sharing LUTS experiences, explanations and remedies), and in public health messaging.
Conclusions: theme 3 – resigned tolerance and inhibitors of revisiting primary care
Beyond the GP consultation, in the population at large, the shrouding of men’s LUTS’ experiences [diminished, stigmatised and hidden under the normalising and discounting notion of ‘old men’s problems; suppressed by gendered tendencies to avoid open discussion of these kinds of experiences (see Gender); and masked by ‘stoic’ tendencies to put up with ‘mundane’ symptoms] operates such that LUTS-related information and guidance for men are not proactively or commonly colloquially discussed or disseminated (see Figure 8, 3a and 3b). As a consequence, it seems that, despite the availability of best-practice guidelines supporting conservative self-management care for LUTS,7,46 men remain broadly unfamiliar with much of this material (see Theme 2c: outcomes and satisfaction) and unlikely to seek it out either through NHS online resources or through their local GP. Low levels of LUTS self-management dissemination reinforces a vicious cycle that further inhibits men from seeking out, being offered and passing on self-management guidance. In the grip of this lacuna, a great many men get on as best they can, masking, rather than addressing, their LUTS, with a sense of resigned tolerance and rarely considering it appropriate to seek help.
Theme 4: experiences of the intervention
How men responded to the intervention (see Figure 8, 4a and 4b) depended on a number of inter-related factors. Foremost was receptiveness, which related to the nature of men’s LUTS and readiness to take action. In addition, men’s character, lifestyle, life circumstances and concurrent health issues were all important factors. From a trial perspective, TRIUMPH trial communications and men’s experience of meeting with the trial nurse or HCA who delivered the intervention, along with their experience of the booklet itself, were critical (see Appendix 7 for additional accounts).
Receptiveness
Receptive men: men who received the intervention positively
Given the nature of men’s prior concealment and discounting of, at times distressing, symptoms, and apparent weaknesses in the dissemination of clinically recommended LUTS self-management guidance, both within and beyond primary care, it is perhaps unsurprising that, more often than not, the men welcomed the TRIUMPH trial intervention. Men often described being pleased to find themselves in the intervention arm and a little less pleased to find themselves in the usual-care arm. Alongside taking part for various altruistic reasons (to support the NHS, to contribute to health research, to support fellow patients), men often hoped that the trial might offer some help for their own neglected, but disruptive, ongoing symptoms:
I was starting to have a few accidents. In certain cases, embarrassing accidents, and it was a concern of mine. Then this letter comes through, and I thought that’s what I want to sort out.
FG9, age 65 years
When I got the stuff through the door, I thought ‘good, maybe I’ll learn something that’s going to help’ because obviously my symptoms were getting worse at this point …
FG2, age 52 years
Men who were especially receptive to the intervention spoke of:
-
mild to moderate/severe symptoms, which were untreated/unsuccessfully treated, disruptive, distressing and frustrating, and which they wished to remedy
-
long-term tolerance of disruptive symptoms, and reluctance to revisit/bother their GP with LUTS issues
-
a perception that the TRIUMPH trial booklet content was novel and illuminating
-
a belief that self-management techniques might alleviate symptoms, and a belief in self-management/lifestyle changes as an important part of good health (reflected in lifestyle)
-
confidence in making changes (sometimes because of engagement in self-help programmes related to other health issues, e.g. diabetes prevention programmes, or other efforts to get fit and improve mental and physical health).
For the most part, the TRIUMPH trial intervention was warmly received by men in the intervention arm, and at times rather sadly wished for by men in the usual-care arm (see Appendix 7 for further accounts):
… it would be nice to have been able to have somebody to talk to about it and to explain or to try and help me with other ways ’cause I’m sure there’s got to be ways to help deal with the way that I have to do, what I have to do. The way, what my diet is etc., etc. The way I live my life. But it’s finding that person. There doesn’t seem to be anybody interested.
CG10, age 54 years
… a little pamphlet, little tiny book or, yeah, to me, I would have been happy to have something like that, like a little bible thing, you know, you can refer to it, ‘OK, I’ve tried that technique, cutting down my tea, it’s still not working, what else can I do?’, you know?
CG11, age 48 years (a control group man, describing the kind of help he would like)
Unreceptive men: men who were ambivalent about and/or disinterested in the intervention
A few men were more sceptical or dismissive from the outset. One man felt that a large RCT of this nature, based around printed self-management guidance, might constitute a waste of health resources. A minority of men were more ambivalent, disengaged or uninterested in the guidance offered; this kind of response often related to:
-
minor symptoms – milder, less disruptive or distressing symptoms and/or taking medication with beneficial effect and so feeling less compelled to take on self-help guidance
-
very severe symptoms (being ‘too far down the line’) – frequent incontinence, regular pad use and/or contemplating surgery
-
a feeling that the guidance does not offer anything much new or worth trying
-
considering the strategies awkward, difficult, unpalatable or disruptive to implement
-
not believing sufficiently that the strategies could be helpful and/or sufficient to alter symptoms. IG20, age 74 years IG24, age 73 years (IG24 said he would be unlikely to keep hold of the booklet)
There was perhaps a slight relevance to me but not a huge amount. And the bit that I was directed to [urethral bulb technique], I find is moderately effective at night but I don’t really find it practicable when I’ve got clothes on … It’s not practicable most of the time.
I did read those. All the stuff that’s been sent to me, I’ve read it through once. But nothing really was earth-shattering …
Character, lifestyle, life circumstances and concurrent health issues
Men’s character, lifestyle, life circumstances and concurrent health issues (see Theme 1c: personal and social factors) were relevant to receptiveness, in particular to men’s ease of engagement with the trial. For instance, some men described spouses/partners reading the booklet and making fluid/caffeine intake and dietary changes alongside them, offering daily support to the implementation of these kinds of lifestyle changes. Conversely, a man undergoing multiple unrelated hospital procedures felt unable to take on LUTS self-management advice, as did a man involved in the full-time care of his chronically ill partner. Retired men often described an ease of implementation related to having the time and space at home to focus on various aspects of the guidance. Conversely, working men were more susceptible to the food and drinks present in their environments (such as the tea/coffee offered frequently to tradespeople in people’s homes), and/or had to implement self-management lifestyle changes despite the tiredness of working shifts/night shifts. Some men embraced the guidance wholeheartedly, as it arrived at a time in their lives when they were already making substantial lifestyle changes (to diet and exercise) as a result of other health issues (e.g. post cancer treatment, following alcohol cessation as a result of alcohol dependency or to tackle diabetes and/or obesity).
Despite often being relatively health conscious and proactive in attending to their health, most men were unfamiliar with (at least some aspects of) the self-management guidance in the booklet. This unfamiliarity contributed to receptiveness, in that men often considered the material to be novel and illuminating (discussed further in The booklet).
TRIUMPH trial communication (administration/paperwork)
Most men were positive about, and untroubled by, their experience of the trial’s administration (e.g. letters introducing the trial, consent sheets, regular forms to complete, occasional telephone calls) and the paperwork involved in participation:
… it’s all relevant. It’s been good to do [the paperwork].
CG1, age 58 years
No problem at all.
CG6, age 70 years
I was quite happy; the initial letter that I had was quite instructive. The contacts that I’ve had with different people have been very positive …
CG6, age 71 years
This differed from the small sample of ‘decliner’ men who agreed to a short telephone interview subsequent to declining to take part (see Interviews with ‘declining’ men): some felt that the paperwork would be overly burdensome. The positivity of the trial men in relation to the paperwork involved in participation may reflect, in part, a sample of men who appeared to be relatively affluent, well-educated and retired, with some spare time to offer (however, this was not always the case, as is reflected in the interview study data, which include working men and men who described reading difficulties, but did not find the paperwork overly burdensome).
Issues with bladder diaries
One caveat relates to the bladder diaries that the intervention arm men were asked to complete. Many men made no complaint about bladder diaries, with some actually described completing the bladder diary as an interesting and informative experience in relation to their LUTS (and their desire to better understand any patterns or aggravating factors):
The bladder diary was useful. It’s something I’ll probably do again at some point just to see if there has been a good change. It’s a good way to monitor.
FG5, age 56 years
However, some men said that they found completion of the bladder diary somewhat irksome, especially men who were working (and needed to carry a measuring pot with them to work, for instance):
I think the one tricky thing was doing the bladder diary at the start; my lifestyle doesn’t actually lend itself that easily, being able to find somewhere to go collecting urine, to measure urine really.
IG3, age 53 years
A few men ultimately took part without completing their bladder diaries:
… the bottom line is when you need to go for a pee you need to go for a pee and it’s not necessarily always easy to find somewhere appropriate where you can go where you can measure the amount that you’re actually getting rid of.
IG16, age 57 years
Apart from issues with the bladder diary, men seemed positive about, and at ease with, the trial administration and communication, including, for the most part, the paperwork involved in participation.
Meeting the nurse/healthcare assistant and follow-up communication
The TRIUMPH trial intervention arm men met with a specially trained nurse or HCA at their usual surgery; some surgeries chose to use the TRIUMPH trial research nurse, others involved their own dedicated research nurse/HCA. Often men spoke favourably about this meeting, describing the clinician contact (the face-to-face meeting and the ensuing telephone calls) as very helpful:
I had quite a long conversation. She was very helpful, very understanding and I can’t think of any questions that I asked but she was very supportive in talking to me.
FG1, age 71 years
The fact that you get rung up to ask how you’re getting on, that, I think, is important. I did start making a serious effort to do the exercises once I started on the course, but they ring up to find out how I was getting on which, if I had been backsliding, I think would provide a bit of stimulus and get back and do them.
IG6, age 78 years
Men referred to the highlighting of relevant sections as useful:
Yes, when I initially was given the booklet, the nurse who gave it to me went through and marked out the pages that were most relevant, which I thought was helpful, but it needed concentrating on. Other than that it’s been a chat on the phone.
FG12, age 59 years
She was very good; they did say read the whole of the book, but there were parts of it more suited to me which she pointed out.
FG6, age 60 years
Some men mentioned specific personal concerns about undertaking aspects of the guidance and/or getting help or advice from the TRIUMPH trial research nurse/HCA with interpreting diagrams or descriptions of exercises and appreciating this personalised, in-person introduction to the booklet:
I talked to the research nurse at [surgery name, and nurse name] … we talked through my symptoms and she put little stickers on the sections that [she] thought were relevant to me and she was right … Well [named research nurse] assured me that the pelvic floor exercises wouldn’t create any problems with my inguinal hernia.
IG2, age 69 years
Well I started the exercises that the book gave me and the nurse told me how to do it.
FG7, age 78 years
For some men, the research nurse/HCA involvement at the outset, and oversight of their progress through telephone contact, was integral to the success of the guidance; they found this contact supportive and motivating and felt that they would not have made the changes they made with a booklet/guidance alone:
Personally, I got more out of the meeting than I did out of the book. But, on the other hand, you could easily say – I could easily say – it just endorsed what was in the book.
IG9, age 75 years
However, others felt that the meeting with the nurse was less necessary and that the material in the booklet was accessible, self-evident and sufficient to enable them to take action:
She outlined some areas in the book that I needed to read, but the booklet’s so simple you just read the lot anyway, it’s quite easy to follow and easy to read.
IG22, age 45 years
It seems the need among trial men for personal qualified clinical contact to support the guidance, whether in person and/or by telephone, was mixed; some really appreciated this contact, the personalisation of advice and the opportunity for discussion, and felt that they would not have made sense of, or taken up, the guidance, without it. Others described the booklet in and of itself as an appropriate means of disseminating the guidance, and felt comfortable taking what they needed from it, with or without additional clinical input (although most seemed to appreciate the focus on the identified relevant sections).
The booklet
For the most part, the men were strongly positive about the booklet, in terms of its (1) style (accessible language use, diagrams and images, material quality) and content (offering explanation as well as clear self-management guidance) and (2) structure (focused sections, which meant that the guidance could be tailored to their particular LUTS experiences). Overall, many men responded positively to the booklet:
I think the book’s been perfect. I mean … I give it a 10 out of 10 for everything practically.
FG9, age 65 years
I think overall, it’s tremendously reassuring to have this sort of booklet, and to see that all these things are very well recognised and demonstrated and that there are techniques for helping with them.
FG8, age 69 years
I think basically the sooner this gets out to everybody with symptoms the better.
FG2, age 52 years
I just really appreciate the fact that being given the booklet, and being explained and chatted through it with the nurse and everything, it then gave me the motivation to actually start trying things properly and to try it all together and the fact that then it’s made a big improvement.
FG2, age 52 years
There were a number of ways in which men responded positively to the booklet.
Booklet style and content
The men spoke appreciatively about the length and extensiveness of the booklet, the diagrams and pictures, the readability of the language, the durability of the material and the tone of the booklet. The men also described the content favourably as offering an explanation of their symptoms and information about their physiology, as well as clear, usable, self-management guidance, in an accessible format:
I think it’s the right length. I’m glad you didn’t make it any longer. I think it’s a read that you can do, and not be overwhelmed, in fact, which is always a good thing, you read it thinking ‘well I need to go into more depth on a couple of bits’, which I would have thought is the purpose of it … the diagrams are to the point but done in a way that is practical … it’s informative. I don’t think it patronises … I think it’s something that it’s good to go back to … Not only that … you can’t destroy this thing, it’s going to last forever.
FG5, age 56 years
[A] very clear book. I’ve followed a lot of the advice …
FG8, age 69 years
I thought it was good, good in the information and techniques and that. The one where you engage your core, it really works.
IG7, age 54 years
Mostly the men praised the style and layout, and especially the aspects of the content they found useful, but the men responded extremely heterogeneously to the subsections and were sometimes ambivalent about sections that had not been selected for their focus (while recognising others may have found the section more useful), or reported skipping over sections that were not novel to them, or which they found difficult to follow or irrelevant to their own LUTS, and therefore unhelpful.
Booklet structure
The men also spoke positively about their experience in making use of the booklet and commented on the structure that allowed them to select relevant parts, rather than feel it necessary to read everything at once, so they could visit or revisit parts that were relevant or needed refreshing, or which had been selected for their focus, without feeling they needed to read the whole booklet:
The other thing I thought that was good about the book was initially I came back and I just read the two or three pages that were applicable to me, this is no real criticism, but the bigger the booklet, the less you read it. I put this in my office, picked it up more than once – two or three times at least – and just reread the bits that were applicable to me. Then since then, I’ve gone through the rest of the book and found it quite good. Very good.
IG9, age 75 years
I think it wouldn’t hurt for more people to read this because, as I say, I’ve read it more than once, it’s not just a case of sitting here and read it once and forget it. I’ve gone back to things, not all of it in one go, but I’ve read sections that I think, ‘Oh hang on a minute!’ So, I’ve done that, you know, the bit about getting rid of the last bit, when you have to do things to yourself to do that, you might not get it quite right first time, your urethral bulb business. You think, ‘well OK we’ll have another go’, and eventually you get it right, which I’ve now managed to do.
IG5, age 66 years
The men specifically spoke positively about (1) ease of use; (2) the informative, explanatory and engaging content and tone; (3) the empowering nature of supported self-management; (4) novelty; and (5) the usefulness of specific sections.
Ease of use
The men seemed to respond very favourably to elements of the specific guidance suggested to them, often reporting finding it easy to implement the suggested lifestyle changes/techniques:
… it said what you had to do and it was quite straightforward and clear. Yes, the first or second time I tried doing those exercises I could feel the effect … It was good because I knew I could go somewhere now and if I got an urgency, I could stop the urgency.
IG7, age 54 years
I found it very easy to make sense of … I mean the jargon is explained in there.
FG5, age 56 years
I thought it was really good actually. Yeah, it’s good, it covers everything. Well, as much as, you know, all in layman’s terms, tells you what to do.
IG22, age 45 years
Being informative, explanatory and engaging
The men were happy to understand more about the underlying nature and physiology of their LUTS and appreciated the non-prescriptive tone of the book, and the information on offer, which supported engagement with the guidance:
[B]y reading the booklet it described the symptoms that I had and it gave a medical term for it so that is or was useful; I’ve certainly kept the booklet and if the issues came back one of the first things I would do would be to read the booklet again, to remind myself of, you know, the options and the things that could be done.
FG11, age 73 years (with ongoing mild symptoms and for whom medication has been relatively successful)
Because by reading the booklet and reading the whole thing, it really explained the physiological side of the problems as well as then going into a bit more depth with the diet side and then also the pelvic floor exercises and then it was just a bit more explanation; it doesn’t say ‘don’t do these things’. It doesn’t say ‘avoid caffeine’. It says keep it down …
FG2, age 52 years
Well, I think again, it would have been useful to have a booklet like that at a much earlier stage of my symptoms. In other words, I don’t think I ever had a detailed discussion with the GP or, you know, I don’t think anybody has ever sat down to me or given me anything to read giving an explanation to the symptoms.
FG1, age 73 years
The empowering nature of self-management
Men also described the process of trialling approaches to see what worked for them as an empowering and positive process, a virtuous cycle, of guided and supported, but self-governed, experimentation, outcome monitoring and adjustment:
… You can actually put together a direct link if you like … it’s actually proved their advice is true.
FG11, age 73 years
… actually it was also quite empowering to be told that it is OK to make that value judgement that, if you want to go out and have a few drinks and enjoy your night out, and you know there’s going to be a price to pay for it, that’s OK.
IG16, age 57 years
It’s not going to go away so I’m going to have to do something, and by doing these things I’ve got a certain amount of control over it.
FG2, age 52 years
Successful interventions motivated men to continue with adaptations, and to trial other aspects of the guidance. Less helpful interventions led men to drop elements that seemed unduly troublesome to implement and unhelpful in relation to symptoms, maintaining a sense of choice, control and sensitive responsivity over the changes implemented in relation to the symptoms endured. Men enthusiastically reported positive outcomes and a sense of being better informed about, and more adept in response to, their symptoms.
Novelty
I thought it was useful that I received this booklet though … That it was pointing out things that I’d never even considered doing …
FG12, age 59 years
Men’s positivity often related to the novelty of the material, as well as its clarity and usefulness. In these responses men were heterogeneous: one man had never heard that salt might interfere with his symptoms and found salt reduction an easy and effective means to alleviate symptoms; very many men had never heard of pelvic floor exercises for men; some men with post-voiding dribble found the urethral bulb technique ‘life-changing’ in relation to that symptom; many men reported dramatic changes to caffeine use and fluid intake that dramatically affected symptoms; and others found great confidence through application of the bladder drill and pelvic floor exercises. Beyond the section on pelvic floor, which was almost ubiquitously novel to the men in the trial, for each section there would be at least one man who felt that the contents were already familiar and not worth trying and another who found changes in this area transformational. This heterogeneity in relation to the utility of sections and responses among men meant that the comprehensive nature of the booklet, having multiple approaches contained in one well-structured place, was particularly beneficial.
Feedback on specific sections
As discussed, men’s responses to the sections were as heterogeneous as their individual LUTS profiles. However, there were some notable responses to each section.
Men had often been asked by their GPs about fluid or caffeine intake prior to their involvement in the TRIUMPH trial. Nevertheless, many still found the section on fluid intake informative. Men reported prior misunderstandings about the amount of fluid they needed to drink each day, often quoting the figure ‘2 l’ as the recommended amount, which they had previously been attempting to achieve. Some men had previously cut down on fluid and caffeine intake in the evening or late evening, but not more generally reduced fluid intake or eliminated caffeine. The detail of the guidance, including the reminder that fruit and vegetables, and foods such as pasta and rice that are cooked in water, contain water was helpful to alleviate competing health concerns around reducing fluid intake. Some who reduced/eliminated certain drinks/foods/salt experienced dramatically alleviated symptoms and were encouraged to continue with these changes to their diet/fluid intake:
I have basically reduced my fluid intake a bit. Previously I was under the impression, before I started the study and before I read the booklet, there was this always magic figure of 2 l a day. If you read the book it’s, ‘Well, no, actually. Less than that’. It is less than what I have been drinking.
IG6, age 78 years
I don’t have any caffeine any more. I’ve gone caffeine free, and I try to avoid tomato pastas, because I found really rich tomato-based pasta sauces were causing me problems, and then also I reduced my alcohol intake and also changed what I drink … The change in diet definitely made a big difference in how active my bladder is, how sensitive you could say … I know now what can aggravate my bladder.
FG2, age 52 years (diagnosed with, and medicated for, overactive bladder)
… basically, you’ve found that once you’ve started to avoid caffeine you’ve got some relief …
FG2, age 52 years
Notably, men who had long histories of UTIs found the fluid intake guidance harder to take on board, having been told for many years to drink more water to reduce the chances of UTI, and wishing to avoid triggering any further unpleasant and disruptive UTIs. These men may need additional support and guidance to benefit from fluid intake advice to alleviate LUTS, without fear of aggravating UTIs.
Most men described pelvic floor exercises for men as an entirely novel technique; those who had heard of pelvic floor exercises associated these with women’s gynaecological issues (clinician interviews revealed a similar bias of association among GPs). Men who trialled pelvic floor exercises described a difference in their levels of control, and in the frequency/urgency of needing to urinate. Men often described the pelvic floor exercises as easy to grasp from the descriptions used in the booklet, or with extra input from the TRIUMPH nurse/HCA, and said they felt that the exercises had helped. Some men were extremely enthusiastic to discover that this technique was applicable to men, surprised that it was not more widely known about and keen for this to be better disseminated among men:
I didn’t realise until I got this booklet that men had a pelvic floor, to be quite honest! But, learning how to squeeze that muscle, I find it doesn’t seem natural at all, but whenever you start doing something new, often it takes a while before you think, ‘Yes, that’s it. That’s what I’m trying to achieve’. By just squeezing that muscle, that has also helped with – that has helped me stop having to run to the loo.
FG14, age 58 years
Well I started the exercises that the book gave me. … hell of a difference … I wasn’t dribbling … I was only waking up twice a night … I’ve still got the urge to go when I’ve gotta go … but there was definitely a relief, it helped.
FG7, age 78 years
Nevertheless, some men who were recommended to trial pelvic floor exercises as part of the trial did not attempt to do so; some seemed daunted and maybe needed additional input and encouragement. Fluid and dietary changes seemed to be more readily implemented:
It’s just the pelvic floor exercises and other technique which I haven’t got to grips with yet, but I will try again.
FG8, age 69 years
Maintaining the recommended pelvic floor exercise programme may also be challenging: men reported remembering to do the exercises only on certain occasions (e.g. when driving or in bed), or performing the exercises only once or twice a day. One man said that, following his engagement with the trial, he had started researching LUTS self-help for men independently; as a consequence, he discovered some applications that support pelvic floor exercising for men (by sending regular reminders during the day to encourage regular pelvic floor exercising). These kinds of tools might be a useful addition to the guidance:
I’ve been trying to do it two to three times a day, but sometimes because there’s so much been going on at the moment, it sometimes is only about once a day.
FG1, age 71 years
A few men said they had skipped over the pelvic floor section because they felt that their exercise classes and/or gym routines already covered their ‘core’. However, this subsuming of ‘pelvic floor’ within ‘core’ could be a misunderstanding; it would be useful if (as with many women’s exercise classes) the pelvic floor was isolated within men’s standardised exercise regimes.
Similarly to pelvic floor exercises, bladder drill was mostly unknown to trial men prior to their engagement in the TRIUMPH trial (and, along with pelvic floor exercises, was not spontaneously mentioned by CG men, and so appeared not to have been recommended to them). As with pelvic floor exercises alone, fewer men appeared to take up this guidance than fluid intake/diet changes; however, those who did said they experienced benefits:
[It’s] better because if I can avoid going to the toilet the minute it tells me I’ve got to go, and I can get through it doing that a couple of times, end up going the third time, I’ve then actually got more in my bladder to actually try and pass so the whole thing then of going to the toilet less means then I’m not getting the – how can I explain it? Going to the toilet lots, you end up almost with, your urethra ends up being sensitive as well, but by going to the toilet less your whole system is calmer.
FG2, age 52 years
Men singled out the advice to ‘take your time’ as particularly helpful. Talking through tendencies to rush, inhibitions and apprehensions with a TRIUMPH trial nurse/HCA appeared, alongside the booklet guidance, to support a shift from inhibited rushing to taking the time needed:
… it was always a bit of a rush and then you think oh, you know, and then you stop and in a couple of minutes you want to go again, you know what I mean, you’ve not emptied the bladder properly. But I didn’t realise that was what was happening, I needed to take more time to empty.
IG30, age 73 years
… and then the thing about emptying your bladder as completely as possible … I’ll actually stay at the toilet and I find that I can basically urinate say 250 ml and I can wait and if I stay waiting, at about 5 minutes later I can urinate the same amount again … If I stand and wait for long enough, eventually it will empty again.
FG2, age 52 years
Spending longer time at the toilet. They said people don’t care how long you’re there … I thought it [the booklet and nurse meeting] was absolutely brilliant.
IG11, age 76 years
A number of trial men experienced post-voiding dribble; many said they had not mentioned this symptom to their GP and very few men had been offered the urethral bulb massaging technique as part of their primary care. Mostly the urethral bulb massaging technique was new to trial men. Some men were daunted by the description/images in the booklet or found the technique tricky to master (some said the descriptions and images were helpful and adequate; one that a 3D model might have helped):
Yeah. The bulb thing, I found difficult to do … I’ve just not really been able to kind of follow it terribly well and I feel so stupid.
FG8, age 69 years
Those men who got the hang of it were often delighted to find a relatively simple and effective fix to address this previously embarrassing and frustrating symptom:
Learning how to do that, clear the bulb, has been brilliant … It did take some time, and sometimes it seems to work better than others, but just by pressing in the right place, I was able to get the last, or a little bit more urine out and I didn’t feel as though I was going to end up dripping. Yes, it’s awkward. You have to get – you have to press in the right place, but anyway, I’ve found a degree of success with that … One of the best things in the booklet.
FG14, age 58 years
Men in the CG with unreported and unsupported post-voiding dribble described their continued struggle with this symptom, without realising it had a name or a technique that might reduce it; the dispiritedness of CG men, in contrast to intervention arm men’s relief and delight at discovering a fix, was especially stark in relation to post-voiding dribble and having/not having access to the urethral bulb massaging technique:
[I]t’s just, I’m 48 now, I don’t really wanna be a 60-year-old man walking down the road with a nappy around me, that’s just me, personally … it gets me a little bit low at times thinking, ‘oh God, I’m gonna be wetting myself’ …
CG11, age 48 years (with untreated symptoms of post-voiding dribble, but without awareness that this is a common symptom; he visited his GP and had a PSA test following blood in urine, but ongoing LUTS were not addressed. He had no plan to revisit his GP and was unaware that self-management guidance might be available, but wished there was some guidance to better support symptoms)
This aspect of the booklet was the most familiar to men, as a result of discussions with their GP (about fluid and caffeine intake and sleep preparation), and/or through other sources of guidance on sleep improvement (by reducing light and noise disturbance, relaxation techniques). GPs had often mentioned afternoon/evening fluid and caffeine intake, and men were mostly familiar with the idea that caffeine could interfere with sleep and/or was a diuretic and many had trialled reduced caffeine and fluid intake in the evening prior to the TRIUMPH trial. This is perhaps in part because the symptom men most commonly said they brought to the attention of their GPs was nocturia (rather than other often undisclosed additional symptoms), but may also reflect general practices (see Interviews with clinicians). Trial men who reported improved sleeping with less night-waking to urinate often associated this with other aspects of the booklet, for instance pelvic floor exercises, bladder training, taking their time to urinate (including at night), and general fluid reduction and caffeine elimination. Men were not negative about this section, and some found it supportive, but the content was less remarked on. It may be the case that waking up in the night to urinate and the resultant sleep disturbance is less stigmatised and more readily discussed, so men were better informed in this area.
In general, men were poorly informed about the nature and causes of LUTS, the self-management options and pharmacological or surgical treatments available, and when it might be appropriate to seek support from their GP. Men appreciated the clear description of symptoms that required clinical attention and welcomed this aspect of the booklet. This underlined the extent to which men were lacking, and in need of, clear guidance around their symptoms, including when to seek help.
Overall outcomes: theme 4
Improved symptoms, better informed, increased self-efficacy
Men described feeling much better about their LUTS, and in some cases about themselves (see Figure 8, 4c). Having a better explanation and understanding of, and better information and guidance around, self-management appeared to offer an increased sense of control and empowerment in relation to their symptoms. As much as supporting LUTS, this renewed sense of control challenged stigmatised notions of inevitable decline with age, and provided instead a sense of attainable restoration of control and function through achievable efforts that preserved a sense of self-efficacy in the face of ageing. Indeed, for many men, LUTS alleviation seemed slight, perhaps one fewer instance of night-waking and/or a reduced urgency with a bit more time to reach a toilet before leakage, yet the sense of increased control, of viable action and of better understanding brought greater benefits than those measured by symptom alleviation alone. This enhanced sense of self-efficacy and preservation of physical form through self-management techniques is something that needs to be assessed in relation to men’s sense of themselves as active and able, and in relation to their well-being, or as one man described it, to his cheerfulness and his feeling less old:
I felt quite embarrassed about it, but now that’s not happening; it’s obviously made me feel quite a bit more cheerful … You’re getting old and you think it’s one of the signs of getting old, but then you think ‘well, actually if I can do something about it, it’s gonna make me feel a lot better’ and that’s what I found is it made me feel less old … As we get older obviously things don’t work as well as they did and if you can do something about it, it’s quite good for your mental health aspect as well.
FG1, age 71 years
I’ve got mixed feelings because I still get up at least twice during the night, but on a more positive front, I feel more in control and that’s just made a difference … I’m able to do something about [post-voiding dribble], so that has made things a lot better … Well, I think the information is good … I think it – the information that I’ve had, the techniques I’ve been advised to do – have been good for me on an individual basis, so I’m very happy about that.
FG14, age 58 years
Benefits in the context of wider health concerns
The broader benefits, relating to increased self-efficacy and well-being, should not be underestimated and need to be situated in the context of wider health concerns. Feeling enabled and empowered to take action on ill health, and to challenge stigmatising notions of ageing decline, might have a benefit in relation to other health concerns and outcomes. This model of treatment and support situates the patient in a position of positive, informed and proactive self-management of health. This is in line with and supports best-practice treatment recommendations62 in relation to a whole range of health conditions, especially those most associated with age (such as heart disease, diabetes, back pain, arthritic pain). Being well-informed, maintaining an active lifestyle, having a good diet and having a positive disposition in relation to self-efficacy and potential symptom amelioration are the flagbearers of better outcomes in relation to many conditions that are increasingly prevalent with age, and for which social, psychological, environmental and physiological aspects play interwoven parts. Indeed, for some trial men, the TRIUMPH trial recommendations were all the more readily attempted in the context of recent health management lifestyle interventions, such as diabetes prevention programmes, which had already underscored the benefits of self-management and lifestyle adaptations.
Benefits related to dissemination: increased knowledge, openness and discussion of symptoms, and availability and sharing of supportive information
One of the most distressing aspects of LUTS evidenced in the TRIUMPH trial, as elsewhere,48,50,52,63 is the extent to which men are inhibited from discussing or seeking help in relation to declining and troubling symptoms, often over the course of many years and sometimes despite severe symptoms that negatively affect mental health and participation in valued activities.
Notably, although men were asked not to share the contents of the booklet over the course of the trial, a number of trial men described opening conversations about LUTS with friends and family as a result of taking part in the trial. This suggests that a publicised roll-out of self-management guidance could have the welcome potential to dramatically shift the level of public discourse in relation to everyday LUTS experiences, which might increase men’s awareness of and readiness to seek guidance, and to share useful information.
This would be a hugely welcome cultural shift in relation to men’s LUTS and a tremendously positive outcome in relation to overcoming the shrouding and masking of LUTS, supporting the everyday dissemination of self-help strategies and the destigmatising of these symptoms:
… of course, when we’re talking about blokes don’t talk about it to each other … I basically opened up about the issue about waterworks and of course the topic of the conversation was the urethral bulb and trying to find it. You know what I mean? … The fact we were talking about it … I have to say that that was probably one of the best things about the book …
I know a lot of people of my age and a good few of them we’ve all, believe it or not we’ve actually discussed this, which is unusual for blokes to do that. We all went, ‘Well this is weird, isn’t it, because blokes don’t do this sort of thing?’ I said, ‘Yes we do now …!’
IG5, age 66 years
Less positive outcomes and ambivalent or negative responses
As discussed, some men made no or few changes and found little, if any, benefit (see Figure 8, 4b):
I sort of read the booklet through and thought ‘yes, it all makes sense’, but there was no matter of, well, you know, that’s something I’ve really got to do; there was nothing in it which made me think, ‘wow, this is the answer to everything’.
FG11, age 73 years
Some found partial benefit, for instance with daytime, but not night-time, symptoms:
I still had those symptoms, but since I’ve had that booklet, quite a few of the techniques and things in there has improved the daytime things, but I still have a lot of disrupted sleep.
IG7, age 54 years
As with all self-help, men needed to be sufficiently motivated to make changes. For some men, involvement in the trial was transformational in relation to symptom alleviation and quality of life. Men who made changes and found benefit were inspired to try other aspects of the booklet and became active investigators in relation to the impact of their lifestyle on their symptoms, ready to trial different approaches and to take on or drop aspects of guidance as they found them helpful. Conversely, men who felt minimal interest and/or found little benefit in their attempts at modification were discouraged from making or trialling further aspects.
It is unlikely that this guidance will inevitably benefit all men with LUTS. There is likely to be a ‘sweet spot’ for self-management intervention. Men with most concern for their symptoms and most distress in relation to their symptoms and most readiness to address their symptoms, but whose symptoms are not so severe as to feel overwhelming and in need of urgent pharmacological or surgical attention, are perhaps the most likely to welcome and diligently trial these approaches.
Men with less concern about their symptoms and less interest in taking up the guidance offered were nevertheless mostly ambivalent or despondent, rather than plainly negative, critical or antagonised. Disengaged men often commented that they thought the information might well be useful and beneficial to others with more distressing symptoms. These men did not report negative consequences; they reported making little effort to make changes and little outcome, and, unlike men who found the intervention useful, said they were unlikely to keep hold of the booklet. These men were in the minority. Most intervention arm men were positive about the information they had been given and said they would keep hold of the booklet for future reference in anticipation of new or changing symptoms and to refresh their memory over time if they found their efforts lapsed and their symptoms worsened. Some men were emphatically positive: many said they would definitely not throw it away:
… you know, it’s my bible.
IG11, age 76 years
The perceived gain in terms of symptom control and self-efficacy among those who took up the guidance was marked; exceptionally so in some men. Likewise, there was an upbeat sense of new knowledgeability and understanding among intervention arm men who had, prior to the study, been considerably distressed, ill-informed and uncertain of what, if any, action to take (as continued to be the case for usual-care men).
Overall, the TRIUMPH qualitative study offers strong support for the widest possible dissemination of the trial guidance, with a target of men with distressing symptoms and a willingness to make lifestyle changes, but in recognition also of the very many men living in the shadows of enduring LUTS who are unlikely to seek support until the availability and potential benefits of self-help options are more widely known and more openly discussed. The more men who take action and find benefits, the more men might eventually come to recognise, discuss and share self-management approaches, with the result that men might begin to see self-management as an option and as a viable remedy. Successful application, even among a somewhat biased sample of proactive motivated men, is not just of (sometimes tremendous) benefit to these men, but holds the potential to shift the wider narrative on LUTS care among men in ways that promote self-management as an option for many more men.
Interviews with ‘declining’ men
Ten participants who had declined the invitation to take part in the main trial, but had indicated that they were happy to be contacted to discuss their reasons for doing so, were interviewed (five from Wessex and five from West of England general practices). The ‘declining’ men’s interviews were brief (approximately 10–15 minutes) telephone conversations that sought to gain an understanding of these men’s reasons for non-participation and to provide a small window of insight into sample bias in relation to participating men.
The most common reason ‘declining’ men gave for choosing not to take part was that the trial appeared to be a time-consuming and effortful undertaking, including questionnaires, a bladder diary and visits to the general practice to meet a clinician. Men variously said that they felt too old for this additional burden, they were too busy with full-time work, they were already overloaded because of a house move or ill health, or they had reading and/or memory difficulties that made paperwork arduous. As noted, TRIUMPH trial men, by contrast, were mostly at ease with the paperwork and other trial demands, although some were also working full time; were dealing with additional ill health and/or caring responsibilities; and, in some cases, also had reading difficulties. Nevertheless, a relatively affluent, retired and educated trial sample bias is evident.
The second main reason that ‘declining’ men gave for not taking part was interesting in that, rather than being different from the accounts of trial men, it resonated strongly with them, in particular with the accounts of those who remained somewhat disengaged over the course of the trial and who were less likely to take up the guidance or find benefit. ‘Declining’ men said that the trial did not seem relevant because they had visited their GP about LUTS years previously, their symptoms were no longer concerning following effective medication or PSA test results that had addressed their fear of cancer, or they had additional overwhelming health issues that took precedence over LUTS concerns. As noted, although similarly despondent men were also present in the TRIUMPH trial sample, trial men tended to have a motivational bias, volunteering partly in the hope that they might find some benefit at a time when symptoms warranted attention, and with a keenness to take action.
Notably, as with trial men, despite struggling unsupported with ongoing LUTS, ‘declining’ men considered their LUTS to be an inevitable part of ageing (which is common among similarly aged men), and did not have much hope of remedy:
Yeah, waking up in the night and if we go away anywhere and I want the toilet if I’m driving, I’ve got to stop and go, so you know anyways I have a mishap then. And if I go too long when I do want to go, I can’t go … If they could help me, I’d do something else like … but when you talk to different people they’re doing the same thing. The older you get, the more things you get go wrong …
Declining interviewee (DI) 10
Although only very briefly questioned on the subject, ‘declining’ men reported little if any prior self-management guidance beyond fluid intake guidance and general recommendations to take exercise. If they were able to describe their underlying physiological conditions, they tended to mention ‘enlarged prostate’. None of the men mentioned pelvic floor exercises, and, when prompted directly, were unfamiliar with the term.
In summary, this small group of men who were disinclined to take part in the TRIUMPH trial primarily because of the time and effort involved did not seem so different from trial men in their LUTS experiences, in their lack of awareness of self-management techniques or in their resigned tolerance of ongoing symptoms that were considered an inevitable and irredeemable facet of age.
Some of these men spoke favourably about the trial objectives, reaffirming the sense that, although men may not expect to find remedy, or for others to care about their LUTS, such care and interest is in fact very welcome:
… I do totally agree with what the project is about … is nice to think that somebody’s out there who actually cares and is interested …
DI 19
Interviews with clinicians
Clinician interviews (n = 14) included GPs at usual care (n = 5) and intervention (n = 5) practice sites and interviews with research nurses (n = 4) who were trained to deliver the TRIUMPH trial intervention at intervention sites and interacted directly with intervention arm TRIUMPH trial men within their practices. GPs were not involved in the delivery of the TRIUMPH booklet and any contact they may have had with TRIUMPH trial men over the course of the trial was incidental; they were mostly unaware of which of their male patients had chosen to take part. GPs described an average of 2–6 weekly contacts with men presenting with LUTS (in the context of seeing 80–100 patients a week on the basis of full-time working hours) and agreed that, given the choice, men with LUTS were more likely to see a male GP; as a consequence, male GPs had somewhat higher rates of contact with men presenting with LUTS than their female counterparts.
Clinician interviews are very briefly summarised to provide insight into (1) the population of men with LUTS as experienced by GPs in primary care; (2) the nature and process of men’s primary care LUTS-related consultations, with a focus on the position of self-management guidance within primary care, from the perspective of GPs; (3) the receptiveness, engagement and outcomes of self-help guidance among TRIUMPH trial men (nurses involved in intervention delivery only); and (4) reflections on the future implementation of LUTS guidance for men. What is noteworthy about the clinician interviews is the extent to which they corroborate the men’s accounts in the qualitative study, albeit from a variety of different perspectives.
The population of men with lower urinary tract symptoms who reach primary care
General practitioners readily described men with LUTS presenting within their practices as falling into one of two categories: the first (and some felt predominantly) was men who were concerned about prostate cancer, and the second was men who were primarily concerned to alleviate (more severe) LUTS. This resonates strongly with men’s accounts of their decision to consult their GP and the pattern of primary care consultations that emerges from men’s descriptions.
However, GPs often felt that ‘men with concern about prostate cancer’ had minimal or no LUTS issues:
Sometimes they sound really mild and their only worry is about prostate cancer, and actually they really don’t mind the symptoms. You realise the IPSS score will be low, and you realise that they haven’t really come for any treatment. And you realise that the last question on the IPSS was likely to be, ‘If you spend the rest of your life like this, how would you feel?’ And they’re satisfied, and really it’s all about getting prostate cancer ruled out.
HCP5
Yet TRIUMPH trial men who had reached their GP with concern about prostate cancer had often endured LUTS for lengthy periods. Men also described repeated cycles of PSA testing over the course of years that had failed to attend to their LUTS, which we have termed the ‘PSA test loop’. Men’s accounts suggest that men are liable to downplay ‘trivial’ LUTS concerns when their primary focus is to alleviate prostate cancer concern/undertake a PSA test. GPs appeared, in this sense, insensitive to the burden of untreated, concealed and downplayed LUTS in the adult male population, and the extent to which men consider it inappropriate to raise LUTS with their GP unless symptoms become intolerable. Whereas we might see men with very mild symptoms presenting to primary care as a golden opportunity to impart self-management guidance, GPs described offering reassurance (in relation to prostate cancer concerns, alongside PSA testing) and downplayed unconcerning symptoms, on the assumption that men would come back if/when symptoms became more problematic. This fits with men’s descriptions of their consultation experiences and our concerns that GPs’ reassurance feeds into men’s already strong tendency to discount and normalise, rather than proactively attend to and seek support in relation to, their LUTS.
The nature and process of general practitioner consultations for men with lower urinary tract symptoms, with a focus on self-management
Similarly to trial men, GPs’ accounts described the consultation process in terms of LUTS assessment/discussion (GPs referred to the IPSS as a framework for asking patients about their LUTS), urine and blood tests to rule out other conditions (including PSA testing; there was considerable disparity between clinicians in relation to thresholds for offering a PSA test, but agreement on the need to discuss the pros and cons/risks with patients) and a tendency to move fairly rapidly to medication if symptoms warranted it.
When it came to self-management, a few GPs with specialist urology interests and workloads spoke of utilising a full raft of approaches, including bladder diary assessments, pelvic floor exercise regimes, bladder drill, urethral bulb massaging, and fluid intake and dietary advice. Some GPs also said they often gave out printed sheets that covered all these aspects and suggested men read them. However, as with trial men, several GPs (both men and women) had not really considered the pelvic floor as part of self-management guidance for men (as opposed to for women), and one felt that this was also not routinely advised in letters for men following referral to urology specialists (a source of specialist treatment guidance and learning for GPs). As experienced by trial men, a number of GPs focused on self-management conversations that covered fluid intake and caffeine, especially in the evening, or in relation to diet and the aggravation of overactive bladder sensitivities:
For the majority then is we start saying, ‘OK, tell me about how much tea you drink, tell me about how much coffee you drink, whether you drink any energy drinks’ …
HCP4
Some GPs saw their role in strongly physiological terms and felt that pharmacological intervention to reduce prostate size was the right focus for a clinician in relation to men with signs of enlarged prostate and LUTS. However, in contrast, and perhaps a sign of the shifting sands of (and individuality among) GPs’ professional stances, one GP described much more strongly foregrounding self-management approaches during all her consultations, including those for men with LUTS, and linking symptoms first to self-management, before offering other treatments:
I’m very aware of frailty and anticholinergic indexes and the burden of taking drugs and I think if you can sort something out without having to take a drug, I think that’s a very strongly held belief of my own. I often do say to patients, this is where I stand, so you have to say if you feel that you would much rather take a drug than do all this other stuff, then that is a choice you’ve got and you have to bear in mind that you’ve chosen a doctor who is on this end of the spectrum about that … I probably am on one end of the scale about wanting people to try lifestyle things first, rather than just go for medications that often have side effects and high numbers needed to treat to actually make a big difference.
HCP12
Receptiveness, engagement and outcomes of the intervention among TRIUMPH trial men (nurses involved in intervention delivery only)
The TRIUMPH trial nurses reported a similar pattern to that found through interviews with trial men. For those men who were receptive to the guidance, it was enthusiastically received and, at times, transformational. Nurses were positive about their experiences delivering the booklet guidance and hopeful about its wider dissemination, in view of the positive impact it had on some of the men with whom they had contact. Men for whom LUTS were a substantial problem, severely affecting life choices, were able to vastly improve, and at times completely remedy, their symptoms, through determined application of the intervention guidance. Other men were less responsive, did not see the value in the guidance and were less concerned about addressing their symptoms.
Reflections on the future implementation of lower urinary tract symptoms guidance for men
Clinician interviews reinforced the impression that, although GPs are receptive to self-management approaches, their consultations do not routinely foreground self-management, and the depth and range of self-management approaches offered by different GPs varies tremendously. GPs may be unaware of the extent to which men might be prone to concealing and downplaying LUTS, and the extent to which LUTS might need to be seen as highly stigmatised symptoms of old age that men treat as unworthy of both disclosure and clinical attention. GPs may also insufficiently apprehend the paucity of men’s awareness of self-management guidance, and the lack of access to, and community circulation of, this material. Better understanding, and, in some cases, education in relation to the range of self-management approaches, in relation to LUTS might encourage GPs to offer a greater depth and diversity of self-management, and to better orient consultations around this material.
There remain, however, a number of barriers to the dissemination of LUTS self-management guidance in primary care. GPs’ consultations cover a lot of ground and there are competing interests: necessary tests to rule out other conditions, and requests for PSA tests that require discussion. It is easy for extensive self-management discussion to slip out of a 10-minute consultation. The availability of alternative mechanisms for delivery of this material within the practice, whether nurse or HCA led, through men’s groups or primary care urology clinics, might make it possible to refer men in need of more thorough self-management guidance to settings where these can be more adequately addressed.
Discussion and conclusions
The qualitative study offers insight into men’s LUTS experiences and their outcomes following their contact with primary care, and engagement with the TRIUMPH trial.
Men do not seek what they do not know exists, and so do not attend GPs seeking self-management remedies as they are unfamiliar, and, unless they are willing to trial medication or seek surgery, men do not imagine any useful outcome to seeking clinical care. Men were also often uncomfortable and unwilling to bother their GP about symptoms that were not ‘serious’ or life-threatening. The mundane distress and disruption of LUTS, and association with stigmatised notions of ageing, further discouraged men from seeking clinical care. Instead, men seek GP input because of concerns about prostate cancer, or only after lengthy periods enduring increasingly severe LUTS, or because of blood in urine or a debilitating UTI; consequentially, the consultation tends to orient around a routine series of tests and pharmacological interventions to alleviate acute symptoms. We found that structured, in-depth self-management is not sufficiently embedded within GP consultations and easily slips out of consultations; instead, PSA test discussions, tests to rule out other conditions, medication options and rudimentary caffeine and fluid intake advice predominate. As a consequence, men leave primary care with little but reassurance, or with medication, but rarely with fulsome information, explanation and guidance, and often continue to resignedly tolerate difficult disruptive symptoms.
Men spoke with great enthusiasm about their experiences of the intervention, perhaps because it brought information, explanation and self-management approaches to men who had missed out on these things and were often struggling with neglected symptoms. Those men who were receptive to the guidance (because of their life circumstances, character and LUTS experiences), as well as it alleviating symptoms, were delighted to be better informed, and more in control, able to independently research symptoms and investigate self-management approaches; better able to decide if or when to see their GP; felt less anxious about their symptoms; no longer felt that LUTS indicated inevitable ageing decline; and were encouraged to take action in relation to their health.
There was a willingness to engage with self-help and a sense that this was a good option (compared with surgery or long-term medication). The format of the guidance was appreciated, although some parts were perceived as tricky and many men also found the nurse/HCA support helpful.
Men in the usual-care arm continued to struggle with poorly explained LUTS, without the guidance that might have supported them and without considering it appropriate to return to their GP.
Unexpectedly, some men spontaneously began sharing the content of the booklet with friends, family and acquaintances, offering some additional hope that the wider dissemination of this kind of guidance might begin to overcome men’s stigmatising perception that LUTS is an inevitable part of ageing and unworthy of care, and support a cultural shift in men’s willingness to talk about their LUTS and to share useful information and guidance. Once men know that techniques are available, they might also be more likely to seek these from primary care, instead of PSA tests or urology referral.
The qualitative study indicates that primary care recommendations for the care and treatment of LUTS may need to be reconsidered and refreshed, particularly the misapplication of ‘reassurance alone’, in view of men’s strong tendency to tolerate, downplay and dismiss their LUTS. The diverse range of supportive self-management options needs to be emphasised and disseminated, so these are more widely available to both GPs and their male LUTS patients.
Strengths and limitations of the qualitative study
The qualitative study included a relatively large sample of primary care men and was able to capture diverse and enduring LUTS experiences. It included single, married, divorced and civil partnered men, and the views of men with multiple additional health concerns. The sample was self-selecting within GP cohorts; this appeared to favour (1) men who wanted help in relation to their neglected symptoms and so were receptive, (2) men who had time and capacity to be at ease with completing paperwork and (3) older retired men with relatively comfortable lifestyles. Conversely, the trial included fewer men with the most complex health needs, disabilities and/or caring responsibilities; working men; and unemployed men. In particular, the trial was taken up almost exclusively by men who were white, and the qualitative sample as a result included the views and experiences of only four men who were black, Asian or mixed race. This lack of cultural diversity suggests that focused work targeting diverse UK cultural communities is necessary to address LUTS self-management and support LUTS primary care across diverse UK communities.
The study allowed men to reflect on experiences in-depth and in their own words, taking us beyond symptom measurement and revealing strong support for better guidance and support around LUTS self-care and a willingness to trial self-management approaches.
Interviews with clinicians cohered with men’s experiences, strengthening these findings.
Men provided sensitive and deeply thoughtful accounts of distressing, intimate, embarrassing and stigmatised symptoms, and reflected on the nature of stigma and age in relation to LUTS. Men also responded to the trial guidance, and to the possibility that men might have better access to this material in the future, with tremendous and spontaneous enthusiasm; this was supported by the experiences of HCPs delivering the intervention to trial men. It became an important component of the qualitative study to convey the strength of men’s appetite for this material. Nevertheless, some interviewed men were neither interested nor especially impressed, and it is probable that other men with less positive responses would also have been less willing to speak about their experiences and less likely to engage with the qualitative study as a consequence; the strong social desire to serve a trial well likewise favours more positive and less negative accounts. To counter this concern, the accounts of usual-care men, who did not receive the intervention, reinforced the need for better access to self-help guidance.
Summary of the findings and implications
The study provided insight into knowledge of, beliefs about and experiences of LUTS; GP care and guidance; and men’s awareness of self-management techniques. It revealed insufficient prior focus on self-management, and commensurate inadequacies in physiological and conservative care knowledge and understanding. Interviews with both primary care men with LUTS and with GPs indicated that in-depth, rigorous, structured and targeted LUTS self-management is not yet sufficiently embedded in the primary care of LUTS in men.
Men who received the intervention were willing to trial lifestyle changes and reported positive outcomes that reinforced their willingness to maintain changes and take further self-help action; the guidance appeared to increase men’s self-efficacy and empowerment in relation to their health. Men welcomed the guidance heartily because it addressed neglected symptoms and the sense of despondency attached to LUTS as an inevitable and somewhat depressing symbol of ageing decline that they were otherwise prone to resignedly tolerating, unless symptoms became sufficiently alarming, often in the light of prostate cancer concerns.
Both men’s accounts and those of GPs suggested that men often reach primary care because of prostate cancer concerns, and that self-management is often a more cursory element of GP care, with reference to fluid intake and caffeine, which is far more likely to be discussed than pelvic floor exercises (which both men and GPs associated with women’s rather than men’s LUTS self-management). GP care instead tends to focus on discussions of prostate cancer testing, on testing to rule out other underlying conditions and on medication. Interviews with men and clinicians showed that the full range of in-depth self-management guidance is not yet a cornerstone of primary care consultation, despite that it is supported by both men and their GPs. This suggests that current NICE and EAU clinical guidelines and recommendations are unlikely to be met without cultural change, a change that this intervention demonstrates some power to support.
The trial also found that the clinical guidance around offering reassurance to men with LUTS needs to be clarified; the way this reassurance is offered, and what is offered alongside this in terms of support, guidance and information, need to be more sensitively considered and better practices reinforced. Reassuring men that LUTS is ‘just a normal part of getting old’, without offering further information, support or in-depth guidance on self-management, may serve the aim of alleviating prostate cancer concerns, but strongly risks reinforcing men’s tendency to discount distressing LUTS experiences, and simultaneously strengthens, rather than addresses, the pernicious stigma associated with LUTS, and entangles concepts of age, inevitable decline and urinary dysfunction, thereby reducing the chances that men will seek, share and discover symptom support and alleviation of distress.
In short, the qualitative study strongly supports embedding the trial booklet, guidance and HCP training firmly within primary care, and developing men’s LUTS primary care services within practices, such as nurse- or HCA-facilitated men’s groups or primary care urology clinics to support more thorough and widespread dissemination of self-management guidance, and which are able to support LUTS self-management when this is not feasible within GP consultations. Interviews with men further supported a targeted public health campaign to promote LUTS awareness, discussion and self-management approaches more broadly, in ways that might reach the very large number of men aged > 40 years who, interviews suggest, are prone to tolerating distressing enduring LUTS, believing that LUTS are an inevitable and irredeemable part of ageing that are unworthy of clinical time, and who are not aware of, and so do not seek out, recommended self-management guidance.
Chapter 6 Discussion and conclusions
Summary and interpretation of main findings
Among a population of men with moderate-severity LUTS allocated to the TRIUMPH intervention (mean IPSS at baseline of 13.6) or usual care (mean IPSS at baseline of 14.6), the difference between the two arms in mean IPSS score at 12 months (primary outcome) was –1.81 in favour of the intervention arm (p < 0.001) after adjustment for baseline IPSS and minimisation variables (see Table 14). Secondary outcomes, notably the incontinence measure (ICIQ-UI-SF) (difference in means –0.74 at 1 year; see Table 17) and IPSS-QoL (–0.31; see Table 18), likewise showed evidence of improvement, compared with the change in symptoms seen with usual care. A greater decrease was also seen in the B-IPQ (see Table 19). The outcomes consistently showed advantage for the intervention. The results suggest strong evidence of a treatment effect.
The trial population was recruited by inviting men who were already known to have LUTS, that is men who were already recorded in GP EMRs as having previously presented with one or more of these symptoms (‘prevalent LUTS’). Hence, the changes achieved by the intervention are in addition to the prior care put in place by practices, potentially including medication. There was no selection based on the type(s) of LUTS present in men joining the trial. Hence this is a pragmatic trial, reflecting the real-world spectrum and the recognised co-existence of LUTS types in many affected people.
The assessment of participant LUTS was based on an understanding of the range of LUTS people can experience, categorised as storage, voiding and post voiding (also known as post micturition). The IPSS was used for the primary outcome, because of its extensive use in research and the detailed understanding of its performance. However, it was previously identified that the IPSS does not measure two symptoms that can be highly bothersome for men, specifically incontinence and post-micturition dribble. 64 Hence, IPSS alone was not considered a sufficient diagnostic assessment in determining which symptoms to prioritise in selecting three sections of the intervention booklet to recommend for individual participants, hence the inclusion of ICIQ-UI-SF, which is an incontinence symptom patient-reported outcome measure and includes questions that can elicit the presence of post-micturition dribble.
The bladder diary used in LUTS assessment was the ICIQ bladder diary,65 which is the only validated diary. This is considered a vital tool for understanding the mechanism of increased voiding frequency, and nocturia in particular. 10,66 However, bladder diaries do seem to represent a barrier for many patients, in terms of the inconvenience of measuring voided volumes over 3 full days, and also reliable provision of complete information. Varied willingness to complete a bladder diary at baseline may have contributed to the lower consent rate in the intervention arm. Only participants in the intervention arm were asked to complete one for symptom assessment to inform intervention delivery. Comparisons with the usual-care arm, therefore, cannot be made. Baseline measures of other symptom outcomes remained largely balanced between the two arms despite the disparity in consent rate. It is, however, noteworthy that the mean IPSS in the intervention arm was approximately 1 unit less than that of the usual-care arm.
The intervention was developed with extensive PPI contribution to achieve clear use of language and to convey the concepts effectively. The intention was to ensure that fundamental understanding would underpin effectiveness and long-term benefit. Overall, the sections did achieve clarity, as indicated in Chapter 5, Theme 4: experiences of the intervention. In the qualitative interviews, men spoke positively about ease of use, and the informative, explanatory and engaging content and tone. Two sections in particular took considerable development: ‘Getting rid of the last drops’ and ‘Reducing sleep disturbance caused by needing to pass urine’. These aim to treat highly bothersome symptoms (post-micturition dribble and nocturia, respectively), and, in both cases, materials previously available for patients failed to achieve insight and benefit. We note that the unadjusted mean nocturia scores were lower in the intervention arm than in the CG at 12 months.
Appendix 1, Table 41 shows that approximately two-thirds of the patients were referred to the sections on ‘Drinks and fluid intake’, ‘Controlling an urgent need to pass urine’ and ‘Reducing sleep disturbance caused by needing to pass urine’. For the other booklet sections, approximately one-third of participants were referred to each. These recommendations were made by the HCP, based on the evaluation of IPSS, ICIQ-UI-SF and bladder diary findings. Hence, choices were individualised according to the severity of individual symptoms and the bother (which does not always correspond with severity). Of course, the booklet was a standardised printing, so that participants were able to go through the entire booklet out of personal choice.
The type of HCP (nurse or HCA) undertaking the assessment and intervention did not appear to affect outcomes. Accordingly, the intervention appears well suited to delivery in clinical practice by either of these professionals. Site initiation training covered examples of bladder diaries and how primary care staff should interpret them for the purposes of the trial, including how this would direct the recommendation for relevant sections of the intervention booklet, using a checklist developed for the trial (see Report Supplementary Material 1). Equivalent training would be needed for introduction into routine clinical practice. A key part of the training relates to the interpretation of a bladder diary, as completion of a diary varies considerably according to willingness of patients to complete it; hence, extracting the meaningful information is not always straightforward.
The sustained response to the intervention over time is particularly noteworthy. The intervention was delivered with one individual session and three follow-up contacts using a contact modality based on the patient’s preference. The last follow-up was scheduled for 12 weeks, and hence the primary outcome capture at 12 months indicates that response is sustained for the medium term. Indeed, Tables 15 and 16 show there is minimal change in IPSS between 6 and 12 months.
The trial aimed to recruit a diverse population of participants, but the ethnicity data given in Table 7 show that white participants overwhelmingly outnumbered all other ethnic representation. The reasons for this are not clear, and the observation does have implications for generalisability. The deprivation index also showed a marked predominance of men from the two least deprived quintiles. Likewise, this will affect the generalisability of results, given the nature of the intervention. The COVID-19 pandemic took hold during the follow-up phase of the trial. A shift towards electronic questionnaire completion when possible, which was already an option for trial participants, enabled follow-up to continue. There did not appear to be any clear difference in the baseline characteristics of patients participating at that time.
There was no difference in secondary care referral rates between the two arms. We were unable to capture reasons for referral, although prostate cancer screening was commonly mentioned during qualitative interviews. Adverse event rates did not substantially differ between the two arms, except that life-threatening adverse events were more commonly seen in the intervention arm, as reported in Table 30. This did not appear to be a causative relationship.
The primary analysis of IPSS at 12 months was robust to ways of accounting for clustering and multiple imputation of missing data. High levels of adherence to the intervention delivery meant that per-protocol analyses largely reflected the primary analysis.
Economic evaluation
This is the first economic evaluation of a manualised and standardised non-pharmacological intervention for male LUTS. From an NHS perspective, mean costs and QALYs were similar across both trial arms, with a slightly lower mean cost (£30 difference) in the intervention arm. Hence, there was a small positive INMB at the NICE willingness-to-pay threshold of £20,000 per QALY, and a 63% probability of the intervention being the cost-effective option.
Electronic medical record data were available from one of England’s two main GP IT systems (EMIS Web or SystmOne) for each site; this minimised the number of missing data and burden on the participants. However, SystmOne was unable to extract data related to the reason any given consultation was undertaken, and aspects of resulting prescriptions. Accordingly, steps were taken to minimise the potential for bias from using the EMR systems, and adjustments were made in the regression analysis. This made it difficult to maintain masking to randomisation allocation for the health economic analysis. Completeness of data was affected by time constraints in sites, notably absence of EMR data from one site in the intervention arm (covering 5.5% of the intervention participants), and lack of secondary care letters for participants with missing data. Accordingly, it was assumed that no secondary care resources were consumed if the participant had no urology referral recorded in their EMR data set and had completed other questions in the questionnaire booklet. Sensitivity analyses tested these assumptions.
The analysis included 80.4% (n = 866) of the trial participants. For 16.1% of participants, questionnaire data were incomplete, and ‘missing not at random’ could not be ruled out. Some participants had their 12-month follow-up after 11 March 2020, and hence may have reduced healthcare use as a result of the impact of the COVID-19 pandemic in the UK. This may have affected the usual-care arm more than the intervention arm.
Overall, the results of the economic evaluation indicate that the costs should not be seen as a limiting factor for the roll-out of the TRIUMPH trial intervention.
Qualitative study
From qualitative interviews, it is apparent that the intervention was well received, and had a positive effect on men’s symptoms and attitude to their LUTS. Men in the intervention arm described relief and gratitude at being better informed, and reported symptom-related benefits. A minority of men were not receptive to the guidance. This was mainly linked to experiences of LUTS and of primary care.
Participants were men with a full range of LUTS, including long-term and severe symptoms, which caused distress, tiredness, anxiety, embarrassment, frustration and annoyance, impinging on and worsened by the pressures of occupational activities and, at worse, leading to complete withdrawal from valued social activities. For many men, justifying a visit to the GP reflected that symptoms had become very disruptive or distressing. Severely disruptive symptoms were particularly important, such as a severe UTI, incontinence, urinating very frequently in the day and/or night, acute retention, blood in urine and/or prostate cancer concerns. These were also factors influential to the willingness, engagement and uptake of health-related information and guidance.
The interviews also highlighted that structured, in-depth self-management is not sufficiently embedded within GP consultations. Consultation accounts ranged widely, covering simple reassurance, assessment, medication, explanation and self-management, PSA testing and referral inconsistency. Detailed explanations and self-management guidance were particularly lacking, and it is clear that men often remained ill-informed after their primary care contact. Men were broadly unfamiliar with self-help information to support LUTS, and were unlikely to seek it out. Hence, the TRIUMPH qualitative study supports dissemination of trial guidance, particularly when distressing symptoms are present and men are willing to make lifestyle changes, but also more broadly to support wider population-level awareness of the availability of tailored self-management options to support LUTS among men.
Non-participation was often due to the perception that the trial could be time-consuming and effortful, with some men saying that they felt too old and others saying that they were too busy for this additional burden. Of course, attitudes varied substantially, and have to be considered in the light of the demographic make-up of the trial population. Some ‘declining’ men said that the trial did not seem relevant to their current circumstances, in particular their symptom experiences; this strongly indicates that personal relevance is crucial for engagement with the intervention.
Barriers to the dissemination of LUTS self-management guidance in primary care are hugely important. GPs’ consultations have to cover multiple issues in a constrained time. Discussions around the implications of PSA testing can be time-consuming and take precedence, with implications for the inclusion of self-management information and guidance. Achieving delivery of this material without relying on GP availability is potentially very beneficial.
Men do not attend GPs seeking self-management remedies, and are much more likely to go the GP with concerns about prostate cancer or after lengthy periods enduring severe LUTS. The resulting consultation may well focus on diagnostic tests and medication, and overlook assessment to guide self-management. It is vital that GPs identify that a presentation with LUTS (even when symptoms are dismissively described) should not conclude solely with prostate cancer screening; once excluded, it is vital to return to the actual presenting complaint, and to structured self-management guidance. A further key point is to review professional treatment recommendations, notably the use of basic reassurance, because we identified that men have a strong tendency to tolerate and downplay their LUTS.
In this population with prevalent LUTS, the intervention was enthusiastically received because it brought information, explanation and self-management where they had not previously been provided. There was a willingness to engage with self-help and a sense that this was a good option, contrasting with the situation for men in the usual-care arm who continued to tolerate distressing symptoms without access to potentially helpful support and guidance. Indeed, men demonstrated an appetite for guidance and some intervention arm men spontaneously sometimes shared content with others. In some ways this could serve an advocacy role to help bring acceptability to discussion in this area.
Strengths and limitations
Strengths of the trial include recruitment to target and excellent follow-up rates, resulting in informative and robust conclusions. The primary outcome was also intentionally timed to allow the longer-term benefit of the TRIUMPH trial intervention to be captured, at 12 months post consent, following a 12-week intervention delivery. This is key, as continuing motivation is a concern in the longer-term effectiveness of lifestyle and conservative interventions.
Limitations of the trial include the need for trial participants to be unmasked, as a patient-reported primary outcome was used. In addition, the trial population was self-selected for trial inclusion, and included only those who had previously sought help from their GP for their LUTS. The small number of men of black, Asian or mixed-race ethnicity was also a limitation, particularly in view of potential ethnic differences in health beliefs regarding LUTS. 67 A potential limitation is that sites recruiting to the usual-care arm would potentially have developed renewed awareness of the condition and the current approaches to management as a result of participating in the trial. Potentially this would decrease the observed differences between randomised groups, given that there was some improvement in the usual-care arm in the primary outcome.
Overall evidence and generalisability
The trial findings show a significant improvement in prevalent LUTS, based on an assessment by trained staff, individualised recommendations for priority sections in a standardised booklet and HCP follow-up. HCPs used their best estimates to make up for missing data in the assessments; some staff may not feel able to undertake this step.
A study of men undergoing drug therapy supported the practice of powering studies to detect group mean differences in IPSS of at least 3 points. 22 This study had a recruitment pool that was rather different from that of the TRIUMPH trial: it enrolled participants from secondary care, and participants had to be aged ≥ 45 years, have a flow rate of at least 4 ml/second and have an IPSS of between 8 and 24 on two screening visits. The finding of a group mean difference of –1.8 is less than the accepted minimal clinically important difference of 3 points. In developing the trial, we considered that a lower difference in mean IPSS of 2 points could be meaningful if affecting particularly bothersome symptoms, such as nocturia or post-micturition dribble. Because the manualisation of the intervention meant that participants were specifically directed to advice relating to their more bothersome symptoms, the selective reduction in IPSS would be more likely in the clinically important areas. Considerations on the clinical importance of this difference need to factor in that the trial (1) used a non-drug intervention, (2) was based in primary care and (3) was unselective in terms of type or severity of LUTS. Indeed, many men were already established on medications used to treat LUTS, as indicated in Table 8.
It is important to recognise that the trial population was predominantly white. Furthermore, based on practice postcode, there was less representation from low-affluence areas. Hence, generalisability will be affected by the nature of the population served by the practice, willingness of staff to undertake training and facilities for follow-up.
Recommendations for research
In order of priority, the trial results suggest the following recommendations for further research:
-
Establishing the role of manualised and standardised care earlier in the clinical pathway. The trial population was drawn from the prevalent LUTS patients identified through diagnosis codes at practices. It will be important to establish whether or not equivalent, or better, changes in IPSS are achieved at the time of first presentation, that is incident LUTS. Intuitively, manualised and standardised care based on self-help measures would precede prescription of LUTS medications, to enhance compliance and potentially reduce medication use. Looking at incident LUTS patients would determine whether or not manualised and standardised care reduces prescriptions issued to treat LUTS.
-
Generalising the findings to a more diverse population. The non-white participants were a small part of the trial, so conclusions and recommendations from the TRIUMPH trial have to be considered accordingly. To explore the potential for this approach in a more diverse population, research is needed to understand the barriers to participation, to determine what adaptations to study design are needed to enable participation in a future study.
-
Rationalising the assessment and combining it with the intervention. Development of a unified assessment and outcome tool is highly desirable; potentially, this could include a bladder diary adapted for use in primary care, with fewer data collection requirements (notably volume measurement throughout the entire 3 days, bladder sensation reporting and fluid intake) and indicators of key information (e.g. ‘sleep’ and ‘wake’ times) to maximise completion and facilitate interpretation. This would then require further evaluation when used in conjunction with the intervention booklet.
-
Adapting the design of the health economic research. Future health economic studies should consider stratifying for practice EMR systems within the randomisation process and controlling for this variable.
-
Evaluating the need for repeated follow-up. Three follows-up contacts were made. It is possible that fewer would be sufficient. The trial had very high follow-up rates, such that there are very few data relating to men who had fewer follow-ups.
Implications for health care
Nurses and HCAs are suitable for the assessment and delivery of conservative treatment of LUTS using the TRIUMPH trial booklet.
Training in the approach to assessment and delivery of the intervention can be given in approximately 1 hour, which is likely to be a crucial requirement for clinical practice, and could potentially be delivered online.
In a prevalent LUTS population, we found a mean difference in IPSS of –1.8 points between the intervention and usual-care arms, associated with congruent improvements in quality of life and other secondary outcomes. The improvement was not accompanied by an increase in adverse events, and was sustained for many months after conclusion of the intervention.
Qualitative findings indicate that rigorous self-management guidance is insufficiently embedded within primary care for men with LUTS. Men had little awareness that LUTS is a condition that can be alleviated through a range of clinically recommended self-management techniques. Men who were receptive were willing and often eager to trial lifestyle approaches, found these helpful, and enthusiastically welcomed the guidance and intervention, saying it should be available to all men.
Stigmatised representations of LUTS as an inevitable and irreparable aspect of age-related decline were prevalent. Men often neglected enduring and distressing symptoms as ‘just a part of ageing’ and visited their GP only when symptoms became severe or alarming, often with concern about the possibility of prostate cancer. It appears that GP consultations tend, as a consequence, to orient around PSA testing and other tests to rule out possible underlying conditions, and move quickly into medication discussions, meaning that, ultimately, in-depth self-management easily slips out of primary care for men with LUTS, despite cycles of PSA testing (which we have termed ‘the PSA test loop’).
Inadequate self-management guidance within primary care is potentially a missed opportunity. The qualitative findings support the development of practice-based nurse- or HCA-facilitated primary care LUTS services for men that can better support self-management guidance beyond the constraints of the GP consultation, and might also help to foster and embed a culture of supported self-management guidance for men with LUTS within primary care. GPs and men with LUTS were supportive of self-management as an appropriate treatment option (alone or alongside medication, dependent on symptoms and aetiology).
More broadly, there is potentially a need to reach men in other settings, to challenge misconceptions and address the paucity of knowledge, awareness and understanding of LUTS and LUTS treatment options, with a focus on self-management. A broader, targeted public health campaign for men with LUTS could be extremely helpful to increase men’s awareness of the availability of self-help (and other clinically recommended) guidance to support LUTS. Without this, the information men receive about LUTS is often related to campaigns focused on prostate cancer, which can be alarming and unhelpful with respect to LUTS care.
Finally, the reassurance offered to men with LUTS by clinicians needs to be adjusted in recognition of denigrating and stigmatised beliefs about LUTS. Telling men that LUTS are ‘nothing to worry about’ and ‘just a normal part of ageing’ is inadequate and insensitive to men’s tendency to downplay and disregard their LUTS; dismissive reassurance feeds despondency, inaction and a sense of helpless decline. Conversely, self-management guidance promoted increased self-efficacy and better symptom management and outcomes.
Conclusion
The trial evaluated an intervention using a standardised information booklet, with manualisation by a nurse or HCA guiding the participants to the most appropriate sections of the booklet based on key individual assessments, after which three follow-up contacts were undertaken. This intervention consistently showed benefit for men’s LUTS and quality of life across a range of outcome measures, when compared against usual practice in a UK primary care setting. Improvements were sustained for a full year. Effect sizes were relatively small, but had the potential to achieve valuable clinical impact, with minimal risk of adverse effects. Qualitative data showed that men valued the intervention highly, and intervention costs were marginally lower than usual care.
Acknowledgements
This trial was designed and delivered in collaboration with the BTC, a UK Clinical Research Collaboration-registered Clinical Trials Unit in receipt of NIHR Clinical Trials Unit support funding. The University of Bristol acted as the sponsor for this trial and the trial was hosted by the NHS Bristol, North Somerset and South Gloucestershire CCG. The TRIUMPH trial research team acknowledges the support of the NIHR CRN.
Trial data were collected and managed using REDCap electronic data capture tools hosted at the University of Bristol. 68,69 REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external sources.
The authors would like to thank all participants, principal investigators and their teams at each of the TRIUMPH trial sites for their involvement, and the West of England and Wessex CRNs for their role in the trial, in particular the CRN staff who conducted the TRIUMPH trial patient screening calls. The authors would also like to thank the members of the Trial Steering Committee and the Data Monitoring Committee.
TRIUMPH study group
-
Chief investigator: Marcus Drake.
-
Trial managers: Jo Worthington and Jessica Frost.
-
BRTC co-director (Bristol Trials Centre): Athene Lane.
-
Senior trial manager: Jodi Taylor.
-
Trial statisticians: Emily Sanderson and Stephanie MacNeill.
-
Trial research nurses: Samantha Clarke, Joanne Thompson, Margaret Macaulay, Miriam Avery, Jackie Broadbridge and Pauline Rachman.
-
Qualitative researchers: Nikki Cotterill and Jessica Wheeler.
-
Health economists: Sian Noble, Madeleine Cochrane and Caoimhe Rice.
-
Administrative support: Charlotte McDonald, Nicholas Christoforou and Aneta Taylor.
-
Data management: David Carmichael.
Trial sites and principal investigators
| General practice | Principal investigator |
|---|---|
| Portishead Medical Group | Dr Matthew J Ridd |
| West Walk Surgery | Dr Sam Davies |
| Tyntesfield Medical Group | Dr Jonathan Rees |
| Nightingale Valley Practice | Dr Katharine Alsop |
| Kingswood Health Centre | Dr Helen Stoddart/Dr Alex Hickson |
| Mendip Vale Medical Practice | Dr Richard Reed |
| Clevedon Medical Centre | Dr Matthew Hoghton |
| Pioneer Medical Group | Dr Susie Cary |
| Lansdowne Surgery | Dr Nicholas Swale |
| Rowden Surgery | Dr Richard Gaunt |
| The Family Practice | Dr James Baker |
| Pembroke Road Surgery | Dr Rohan Perera |
| Whiteladies Medical Group | Dr Steve Granier |
| Heart of Bath Medical Partnership | Dr Tim Johnson |
| Portland Practice | Dr Shane Hegingbotham |
| Hathaway Medical Centre | Dr Philip Grimmer |
| Phoenix Health Group | Dr Naomi Vernon |
| Trowbridge Health Centre | Dr Tobias Cookson |
| Wareham Surgery | Dr Nathan Francis |
| The Adam Practice | Dr Patrick Moore |
| Swan Medical Group | Dr Rajdeep Laly |
| Abbeywell Surgery | Dr Tracey Ryan |
| Andover Health Centre | Dr Lucy Allen |
| The Friarsgate Practice | Dr Stephen Fowler |
| Highcliffe Medical Centre | Dr Zelda Cheng |
| Three Chequers Medical Practice | Dr Craig Kyte |
| Gosport Medical Centre | Dr Bozena Gorecka |
| Cowplain Family Practice | Dr Nicola Millen |
| Gillingham Medical Practice | Dr William Fenton |
| Homewell Curlew Practice | Dr Helen Whiting |
| The Wilson Practice | Dr Oliver Kemp |
| Salisbury Medical Practice | Dr Polly Jacobs |
Trial oversight committees
We thank all the members of the trial oversight committees for their valued contributions.
TRIUMPH Trial Steering Committee
Professor Peter Bower (chairperson), Professor Siobhan Creanor, Mr Malcom Pick and Mr Mark Speakman.
TRIUMPH Data Monitoring and Ethics Committee
Mr Chris Harding (chairperson), Professor Suzanne Richards and Dr Jennifer Nicholas.
Trial Management Group
Marcus Drake, Jo Worthington, Jessica Frost, Stephanie MacNeill, Sian Noble, Margaret Macaulay, Emily Sanderson, Luke Robles, Athene Lane, Hashim Hashim, Gordon Taylor, Nikki Cotterill, Jodi Taylor, Matthew J Ridd, Madeleine Cochrane, Lucy McGeagh, Jon Rees, Mandy Fader, Jess Wheeler, Miriam Avery, Jo Thompson and Sam Clarke.
Contributions of authors
Jo Worthington (https://orcid.org/0000-0002-2860-3511) (Trial Manager) managed the co-ordination of the trial throughout, and contributed to aspects of trial design, development of the trial protocol and procedures, and to writing the report.
Jessica Frost (https://orcid.org/0000-0002-2454-311X) (Trial Manager) managed the co-ordination of the trial throughout, and contributed to aspects of trial design, development of the trial protocol and procedures, and to writing the report.
Emily Sanderson (https://orcid.org/0000-0003-2268-4194) (Research Associate, medical statistics) conducted the statistical analysis and contributed to writing the report.
Madeleine Cochrane (https://orcid.org/0000-0003-1856-3293) (Research Associate, health economics) conducted the analysis of the economic evaluation and contributed to writing the report.
Jessica Wheeler (https://orcid.org/0000-0003-0802-7286) (Senior Research Associate in qualitative research) conducted the qualitative study and contributed to writing the report.
Nikki Cotterill (https://orcid.org/0000-0001-6921-2712) (Associate Professor in continence care) was a co-applicant and the lead qualitative researcher on the trial, was involved in designing and managing the qualitative analysis and contributed to writing the report.
Stephanie J MacNeill (https://orcid.org/0000-0001-6553-1433) (Senior Lecturer in medical statistics) was a co-applicant and the lead statistician for the trial, was involved in trial design and in managing the statistical analysis and contributed to writing the report.
Sian Noble (https://orcid.org/0000-0002-8011-0722) (Senior Lecturer, health economics) was a co-applicant and the lead health economist for the trial; was involved in designing, managing and conducting the health economic evaluation; and contributed to writing the report.
Miriam Avery (https://orcid.org/0000-0001-7772-9412) (Research Nurse) delivered the TRIUMPH intervention in the Wessex hub and was a member of the Trial Management Group.
Samantha Clarke (https://orcid.org/0000-0002-3100-3111) (Lead Urology Research Nurse) was lead research nurse in the West of England for the trial, and was a member of the Trial Management Group.
Mandy Fader (https://orcid.org/0000-0002-0801-543X) (Professor of Continence Technology) was a co-applicant, and contributed to the trial design and oversight of the trial in the Wessex hub.
Hashim Hashim (https://orcid.org/0000-0003-2467-407X) (Professor, Consultant Urological Surgeon) was a co-applicant, and contributed to the trial design and oversight of the trial.
Lucy McGeagh (https://orcid.org/0000-0002-9226-5115) (Senior Research Associate in Cancer, Chartered Psychologist) was a co-applicant providing expertise on the behavioural aspects of the trial, and contributed to the trial design and oversight of the trial.
Margaret Macaulay (https://orcid.org/0000-0003-1737-4589) (Senior Research Nurse) supported the set-up and recruitment of primary care sites, managed and delivered the TRIUMPH intervention in the Wessex hub, and contributed to oversight of the trial.
Jonathan Rees (https://orcid.org/0000-0001-8014-4407) (general practitioner partner) was a co-applicant providing primary care expertise, contributed to the trial design and oversight of the trial, and was a principal investigator for his trial site.
Luke Robles (https://orcid.org/0000-0003-2882-9868) (Senior Research Associate in Cancer) was a co-applicant providing expertise on the behavioural aspects of the trial, and contributed to the trial design and oversight of the trial.
Gordon Taylor (https://orcid.org/0000-0002-8029-2503) (PPI representative) was a co-applicant providing a patient perspective, contributed to the trial design and oversight of the trial and took a leading role in wider patient advisory group meetings.
Jodi Taylor (https://orcid.org/0000-0001-7171-8923) (Head of Trial Management and Operations) contributed to trial co-ordination and oversight of the trial and to writing the report.
Joanne Thompson (https://orcid.org/0000-0001-6598-3085) (Research Nurse) delivered the TRIUMPH intervention in the West of England hub and was a member of the Trial Management Group.
J Athene Lane (https://orcid.org/0000-0002-7578-4925) (Professor of Trials Research) was a co-applicant, contributed to the trial design, was involved in management of the trial and contributed to writing the report.
Matthew J Ridd (https://orcid.org/0000-0002-7954-8823) (Reader in Primary Health Care, general practitioner) was a co-applicant providing primary care expertise, contributed to the trial design and oversight of the trial, and was a Principal Investigator for his study site.
Marcus J Drake (https://orcid.org/0000-0002-6230-2552) (Professor of Physiological Urology, Associate Director of Research for the Bristol NHS Hospital Trusts) was chief investigator and clinical lead for the trial, and, as such, conceived the trial, participated in its design and co-ordination, and contributed to writing the report.
All authors read and approved and/or commented on the final report.
Publications
Frost J, Lane JA, Cotterill N, Fader M, Hackshaw-McGeagh L, Hashim H, et al. TReatIng Urinary symptoms in Men in Primary Healthcare using nonpharmacological and non-surgical interventions (TRIUMPH) compared with usual care: study protocol for a cluster randomised controlled trial. Trials 2019;20:546.
Worthington J, Frost J, Lane JA, Robles L, Rees J, Taylor G, et al. Effective management of male lower urinary tract symptoms in primary care. Br J Gen Pract 2021;71:388–9.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Frost J, Lane JA, Cotterill N, Fader M, Hackshaw-McGeagh L, Hashim H, et al. TReatIng Urinary symptoms in Men in Primary Healthcare using non-pharmacological and non-surgical interventions (TRIUMPH) compared with usual care: study protocol for a cluster randomised controlled trial. Trials 2019;20. https://doi.org/10.1186/s13063-019-3648-1.
- Hunter DJ, McKee M, Black NA, Sanderson CF. Health status and quality of life of British men with lower urinary tract symptoms: results from the SF-36. Urology 1995;45:962-71. https://doi.org/10.1016/S0090-4295(99)80116-2.
- Bailey K, Abrams P, Blair PS, Chapple C, Glazener C, Horwood J, et al. Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods (UPSTREAM) for diagnosis and management of bladder outlet obstruction in men: study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-1087-1.
- Selman LE, Ochieng CA, Lewis AL, Drake MJ, Horwood J. Recommendations for conducting invasive urodynamics for men with lower urinary tract symptoms: qualitative interview findings from a large randomized controlled trial (UPSTREAM). Neurourol Urodyn 2019;38:320-9. https://doi.org/10.1002/nau.23855.
- Lewis AL, Young GJ, Abrams P, Blair PS, Chapple C, Glazener CMA, et al. Clinical and patient-reported outcome measures in men referred for consideration of surgery to treat lower urinary tract symptoms: baseline results and diagnostic findings of the Urodynamics for Prostate Surgery Trial; Randomised Evaluation of Assessment Methods (UPSTREAM). Eur Urol Focus 2019;5:340-50. https://doi.org/10.1016/j.euf.2019.04.006.
- Girman CJ, Jacobsen SJ, Rhodes T, Guess HA, Roberts RO, Lieber MM. Association of health-related quality of life and benign prostatic enlargement. Eur Urol 1999;35:277-84. https://doi.org/10.1159/000019861.
- The Management of Lower Urinary Tract Symptoms in Men. London: NICE; 2010.
- Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher S, Mamoulakis C, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2015;67:1099-109. https://doi.org/10.1016/j.eururo.2014.12.038.
- Salisbury C, Procter S, Stewart K, Bowen L, Purdy S, Ridd M, et al. The content of general practice consultations: cross-sectional study based on video recordings. Br J Gen Pract 2013;63:e751-9. https://doi.org/10.3399/bjgp13X674431.
- Gibson W, Harari D, Husk J, Lowe D, Wagg A. A national benchmark for the initial assessment of men with LUTS: data from the 2010 Royal College of Physicians National Audit of Continence Care. World J Urol 2016;34:969-77. https://doi.org/10.1007/s00345-015-1702-5.
- Imamura M, Williams K, Wells M, McGrother C. Lifestyle interventions for the treatment of urinary incontinence in adults. Cochrane Database Syst Rev 2015;12. https://doi.org/10.1002/14651858.CD003505.pub5.
- National Institute for Health and Care Excellence (NICE) . Lower Urinary Tract Symptoms: Evidence Update March 2012 2012.
- Yap TL, Brown C, Cromwell DA, van der Meulen J, Emberton M. The impact of self-management of lower urinary tract symptoms on frequency-volume chart measures. BJU Int 2009;104:1104-8. https://doi.org/10.1111/j.1464-410X.2009.08497.x.
- Brown CT, Yap T, Cromwell DA, Rixon L, Steed L, Mulligan K, et al. Self management for men with lower urinary tract symptoms: randomised controlled trial. BMJ 2007;334. https://doi.org/10.1136/bmj.39010.551319.AE.
- National Institute for Health and Care Excellence . Lower Urinary Tract Symptoms in Men: Management 2015. www.nice.org.uk/guidance/cg97 (accessed 29 December 2021).
- MacNeill SJ, Drake MJ. TRIUMPH: TReatIng Urinary Symptoms in Men in Primary Healthcare Using Non-Pharmacological and Non-Surgical Intervention n.d. https://research-information.bris.ac.uk/ws/portalfiles/portal/248729952/TRIUMPH_SAP_v1_2_45_1_.pdf (accessed 31 July 2020).
- Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179-211. https://doi.org/10.1016/0749-5978(91)90020-T.
- Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549-57. https://doi.org/10.1016/S0022-5347(17)36966-5.
- Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30. https://doi.org/10.1002/nau.20041.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- Barry MJ, Cantor A, Roehrborn CG. CAMUS Study Group . Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. J Urol 2013;189:987-92. https://doi.org/10.1016/j.juro.2012.08.257.
- Campbell MK, Fayers PM, Grimshaw JM. Determinants of the intracluster correlation coefficient in cluster randomized trials: the case of implementation research. Clin Trials 2005;2:99-107. https://doi.org/10.1191/1740774505cn071oa.
- Griffin T, Peters TJ, Sharp D, Salisbury C, Purdy S. Validation of an improved area-based method of calculating general practice-level deprivation. J Clin Epidemiol 2010;63:746-51. https://doi.org/10.1016/j.jclinepi.2009.07.019.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345. https://doi.org/10.1136/bmj.e5661.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Pope C, Turnbull J, Jones J, Prichard J, Rowsell A, Halford S. Has the NHS 111 urgent care telephone service been a success? Case study and secondary data analysis in England. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014815.
- University of Bristol . Professorial Salary Ranges History 2018–2019 n.d. www.bristol.ac.uk/hr/salaries/gradem-prof-history.html#a1819 (accessed 2 March 2021).
- University of Bristol . Bespoke Printing 2018 n.d. www.bristol.ac.uk/print-services/bespoke-print-promotional-products-and-signage/ (accessed 2 March 2021).
- BT . BT Public Sector: Higher Education IT Solutions 2018 19 n.d. https://business.bt.com/public-sector/universities/ (accessed 2 March 2021).
- HM Revenue and Customs . Travel – Mileage and Fuel Rates and Allowances 2019 n.d. www.gov.uk/government/publications/rates-and-allowances-travel-mileage-and-fuel-allowances/travel-mileage-and-fuel-rates-and-allowances (accessed 2 March 2021).
- NHS . 2018/19/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (accessed 29 March 2022).
- NHS Business Services Authority . Prescription Cost Analysis – England 2019 n.d. www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis/prescription-cost-analysis-england-2019 (accessed 17 August 2020).
- NHS Business Services Authority . NHS Drug Tariff 2019 n.d. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff (accessed 2 March 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- National Institute for Health and Care Excellence . Developing NICE Guidelines: The Manual: 7 Incorporating Economic Evaluation n.d. www.nice.org.uk/process/pmg20/chapter/incorporating-economic-evaluation (accessed 2 March 2021).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- El Alili M, van Dongen JM, Goldfeld KS, Heymans MW, van Tulder MW, Bosmans JE. Taking the analysis of trial-based economic evaluations to the next level: the importance of accounting for clustering. PharmacoEconomics 2020;38:1247-61. https://doi.org/10.1007/s40273-020-00946-y.
- Hoch JS, Hay A, Isaranuwatchai W, Thavorn K, Leighl NB, Tu D, et al. Advantages of the net benefit regression framework for trial-based economic evaluations of cancer treatments: an example from the Canadian Cancer Trials Group CO.17 trial. BMC Cancer 2019;19. https://doi.org/10.1186/s12885-019-5779-x.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Thorn JC, Brookes ST, Ridyard C, Riley R, Hughes DA, Wordsworth S, et al. Core items for a standardized resource use measure: expert Delphi consensus survey. Value Health 2018;21:640-9. https://doi.org/10.1016/j.jval.2017.06.011.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. https://doi.org/10.1136/bmj.b3496.
- Hudak PL, McKeever PD, Wright JG. Understanding the meaning of satisfaction with treatment outcome. Med Care 2004;42:718-25. https://doi.org/10.1097/01.mlr.0000132398.11342.a8.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- Gravas S, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, Mamoulakis C, et al. EAU Guidelines on Non-Neurogenic Male LUTS Including Benign Prostatic Obstruction n.d. https://d56bochluxqnz.cloudfront.net/documents/EAU-Pocket-on-Non-neurogenic-Male-LUTS-2020.pdf (accessed 29 March 2022).
- Speakman M, Kirby R, Doyle S, Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) – focus on the UK. BJU Int 2015;115:508-19. https://doi.org/10.1111/bju.12745.
- Gannon K, Glover L, O’Neill M, Emberton M. Men and chronic illness: a qualitative study of LUTS. J Health Psychol 2004;9:411-20. https://doi.org/10.1177/1359105304042350.
- Wareing M. Lower urinary tract symptoms: a hermeneutic phenomenological study into men’s lived experience. J Clin Nurs 2005;14:239-46. https://doi.org/10.1111/j.1365-2702.2004.01003.x.
- Lammers HA, van Wijnhoven R, Teunissen TA, Harmsen S, Lagro-Janssen AL. Why do men suffering from LUTS seek primary medical care? A qualitative study. J Eval Clin Pract 2015;21:931-6. https://doi.org/10.1111/jep.12407.
- Coyne KS, Sexton CC, Kopp Z, Chapple CR, Kaplan SA, Aiyer LP, et al. Assessing patients’ descriptions of lower urinary tract symptoms (LUTS) and perspectives on treatment outcomes: results of qualitative research. Int J Clin Pract 2010;64:1260-78. https://doi.org/10.1111/j.1742-1241.2010.02450.x.
- Ikenwilo D, Heidenreich S, Ryan M, Mankowski C, Nazir J, Watson V. The best of both worlds: an example mixed methods approach to understand men’s preferences for the treatment of lower urinary tract symptoms. Patient 2018;11:55-67. https://doi.org/10.1007/s40271-017-0263-7.
- Agarwal A, Eryuzlu LN, Cartwright R, Thorlund K, Tammela TL, Guyatt GH, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur Urol 2014;65:1211-7. https://doi.org/10.1016/j.eururo.2014.01.019.
- Milosevic S, Joseph-Williams N, Pell B, Cain E, Hackett R, Murdoch F, et al. Managing lower urinary tract symptoms in primary care: qualitative study of GPs’ and patients’ experiences. Br J Gen Pract 2021;71:e685-92. https://doi.org/10.3399/BJGP.2020.1043.
- Ellsworth P, Marschall-Kehrel D, King S, Lukacz E. Bladder health across the life course. Int J Clin Pract 2013;67:397-406. https://doi.org/10.1111/ijcp.12127.
- Robertson S. Understanding Men and Health: Masculinities, Identity and Well-being. Maidenhead: Open University Press; 2007.
- Yousaf O, Grunfeld EA, Hunter MS. A systematic review of the factors associated with delays in medical and psychological help-seeking among men. Health Psychol Rev 2015;9:264-76. https://doi.org/10.1080/17437199.2013.840954.
- O’Brien R, Hunt K, Hart G. ‘It’s caveman stuff, but that is to a certain extent how guys still operate’: men’s accounts of masculinity and help seeking. Soc Sci Med 2005;61:503-16. https://doi.org/10.1016/j.socscimed.2004.12.008.
- Tannenbaum C, Frank B. Masculinity and health in late life men. Am J Mens Health 2011;5:243-54. https://doi.org/10.1177/1557988310384609.
- Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Lower urinary tract symptoms and risk of prostate cancer: the HUNT 2 Cohort, Norway. Int J Cancer 2008;123:1924-8. https://doi.org/10.1002/ijc.23713.
- Malde S, Umbach R, Wheeler JR, Lytvyn L, Cornu JN, Gacci M, et al. A systematic review of patients’ values, preferences, and expectations for the diagnosis and treatment of male lower urinary tract symptoms. Eur Urol 2021;79:796-809. https://doi.org/10.1016/j.eururo.2020.12.019.
- NHS . The NHS Long Term Plan n.d. www.longtermplan.nhs.uk/ (accessed 29 December 2021).
- Solvang M, Elnegaard S, Jarbøl DE. Urological symptoms among 23,240 men in the general Danish population – concerns about symptoms, their persistence and influence on primary care contacts. Scand J Prim Health Care 2018;36:227-36. https://doi.org/10.1080/02813432.2018.1487377.
- Ito H, Young GJ, Lewis AL, Blair PS, Cotterill N, Lane JA, et al. Grading severity and bother using the International Prostate Symptom Score and International Consultation on Incontinence Questionnaire Male Lower Urinary Tract Symptoms Score in men seeking lower urinary tract symptoms therapy. J Urol 2020;204:1003-11. https://doi.org/10.1097/JU.0000000000001149.
- Bright E, Cotterill N, Drake M, Abrams P. Developing and validating the International Consultation on Incontinence Questionnaire bladder diary. Eur Urol 2014;66:294-300. https://doi.org/10.1016/j.eururo.2014.02.057.
- Cornu JN, Abrams P, Chapple CR, Dmochowski RR, Lemack GE, Michel MC, et al. A contemporary assessment of nocturia: definition, epidemiology, pathophysiology, and management – a systematic review and meta-analysis. Eur Urol 2012;62:877-90. https://doi.org/10.1016/j.eururo.2012.07.004.
- Welch LC, Botelho EM, Tennstedt SL. Race and ethnic differences in health beliefs about lower urinary tract symptoms. Nurs Res 2011;60:165-72. https://doi.org/10.1097/NNR.0b013e3182159cac.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform 2019;95. https://doi.org/10.1016/j.jbi.2019.103208.
Appendix 1 Supplementary tables
Parts of this appendix are reproduced from Frost et al. 1 © The Author(s) 2019. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The text below includes minor additions and formatting changes to the original text.
| Criteria | Database search |
|---|---|
| Inclusion criteria | |
| Adult men (aged ≥ 18 years) | Males > 18 year old |
| Bothersome LUTS | LUTS; BPH; Nocturia; Urinary Symptoms; Prostatism; Overactive Bladder [within preceding 5 years] |
| Exclusion criteria | |
| Lack of capacity to consent | Dementia; Learning Disability; Psychosis; Schizophrenia [ever] – additional check in manual screen for any other indication of lack of capacity |
| Unable to pass urine without a catheter (indwelling or intermittent catheterisation) | Catheter code [in preceding 3 months] |
| Relevant neurological disease or referral | Dementia; Parkinson’s; MS; Previous stroke [ever] Additional check in manual screen for any other neurological disease/referral that may affect LUTS |
| Undergoing urological testing for LUTS | N/A – manual screen only |
| Currently being treated for prostate or bladder cancer | Prostate or bladder cancer [ever] |
| Previous prostate surgery | TURP; Prostatectomy; BNI [ever] |
| Poorly controlled diabetes mellitus | Latest HbA1c > 65 |
| Recently referred or currently under urology review | N/A – manual screen only |
| Visible haematuria | Visible haematuria [in preceding 6 months] |
| Unable to complete assessments in English | N/A – manual screen only |
| Centre | Arm | Practice ID | Type of EMR system used | Practice size (n patients) | Patients consented (n) | Area-level deprivation of the practice based on practice postcodea |
|---|---|---|---|---|---|---|
| WoE | Intervention | B10 | EMIS Web | 18,590 | 32 | 9.17 |
| WoE | Intervention | B11 | EMIS Web | 13,400 | 35 | 7.47 |
| WoE | Intervention | B12 | EMIS Web | 33,000 | 40 | 4.22 |
| WoE | Intervention | B13 | EMIS Web | 15,744 | 20 | 20.04 |
| WoE | Intervention | B22 | EMIS Web | 16,111 | 40 | 6.01 |
| WoE | Intervention | B32–B34 | EMIS Web | 48,632 | 63 | 10.66 |
| WoE | Intervention | B36 | SystmOne | 14,000 | 32 | 4.64 |
| Wessex | Intervention | S15 | SystmOne | 32,000 | 24 | 12.56 |
| Wessex | Intervention | S18 | EMIS Web | 18,615 | 25 | 5.21 |
| Wessex | Intervention | S19 | EMIS Web | 14,000 | 13 | 14.23 |
| Wessex | Intervention | S21 | EMIS Web | 24,500 | 29 | 6.64 |
| Wessex | Intervention | S26 | SystmOne | 25,500 | 41 | 15.07 |
| Wessex | Intervention | S27 | EMIS Web | 9818 | 36 | 13.82 |
| Wessex | Intervention | S28 | EMIS Web | 15,849 | 15 | 7.05 |
| Wessex | Intervention | S30 | EMIS Web | 15,138 | 29 | 14.11 |
| Wessex | Intervention | S40 | EMIS Web | 14,404 | 33 | 19.826 |
| Wessex | Intervention | S41 | SystmOne | 22,500 | 17 | 12.22 |
| Mean (SD) across intervention practices | 20,694 (9714) | 31 (12.00) | 11 (5.00) | |||
| WoE | Control | B16 | EMIS Web | 12,498 | 22 | 23.27 |
| WoE | Control | B20 | EMIS Web | 26,229 | 55 | 7.1 |
| WoE | Control | B24 | EMIS Web | 20,000 | 37 | 33.62 |
| WoE | Control | B25 | SystmOne | 7600 | 46 | 20.07 |
| WoE | Control | B31 | SystmOne | 16,200 | 50 | 22.19 |
| WoE | Control | B35 | SystmOne | 22,360 | 52 | 5.62 |
| WoE | Control | B37 | SystmOne | 16,000 | 36 | 10.89 |
| WoE | Control | B38 | EMIS Web | 32,000 | 55 | 14.87 |
| WoE | Control | B39 | SystmOne | 30,571 | 58 | 27.21 |
| Wessex | Control | S14 | SystmOne | 8030 | 22 | 14.21 |
| Wessex | Control | S17 | EMIS Web | 20,000 | 32 | 11.69 |
| Wessex | Control | S23 | SystmOne | 11,000 | 54 | 9.21 |
| Wessex | Control | S29 | SystmOne | 13,000 | 34 | 11.53 |
| Mean (SD) across usual-care practices | 18,114 (7998) | 43 (12.71) | 16 (8.39) | |||
| Mean (SD) across all practices | 19,576 (8956.98) | 35.90 (13.48) | 13.15 (7.12) | |||
| Centre | Arm | Practice ID | Patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Included in initial mail-out (n) | Returning EOI form | Interested in further screening | Eligible at CRN screening | Consented | |||||||
| n | %a | n | %b | n | %c | n | %d | ||||
| WoE | Intervention | B10 | 150 | 76 | 50.67 | 58 | 76.32 | 47 | 81.03 | 32 | 68.09 |
| WoE | Intervention | B11 | 150 | 86 | 57.33 | 57 | 66.28 | 47 | 82.46 | 35 | 74.47 |
| WoE | Intervention | B12 | 150 | 90 | 60 | 71 | 78.89 | 55 | 77.46 | 40 | 72.73 |
| WoE | Intervention | B13 | 108 | 50 | 46.3 | 34 | 68 | 26 | 76.47 | 20 | 76.92 |
| WoE | Intervention | B22 | 165 | 79 | 47.88 | 62 | 78.48 | 53 | 85.48 | 40 | 75.47 |
| WoE | Intervention | B32–B34 | 220 | 118 | 53.64 | 104 | 88.14 | 86 | 82.69 | 63 | 73.26 |
| WoE | Intervention | B36 | 220 | 80 | 36.36 | 53 | 66.25 | 43 | 81.13 | 32 | 74.42 |
| Wessex | Intervention | S15 | 150 | 62 | 41.33 | 42 | 67.74 | 29 | 69.05 | 24 | 82.76 |
| Wessex | Intervention | S18 | 127 | 63 | 49.61 | 49 | 77.78 | 37 | 75.51 | 25 | 67.57 |
| Wessex | Intervention | S19 | 99 | 43 | 43.43 | 27 | 62.79 | 20 | 74.07 | 13 | 65 |
| Wessex | Intervention | S21 | 118 | 68 | 57.63 | 51 | 75 | 39 | 76.47 | 29 | 74.36 |
| Wessex | Intervention | S26 | 181 | 81 | 44.75 | 60 | 74.07 | 47 | 78.33 | 41 | 87.23 |
| Wessex | Intervention | S27 | 146 | 74 | 50.68 | 57 | 77.03 | 50 | 87.72 | 36 | 72 |
| Wessex | Intervention | S28 | 108 | 53 | 49.07 | 36 | 67.92 | 23 | 63.89 | 15 | 65.22 |
| Wessex | Intervention | S30 | 118 | 58 | 49.15 | 47 | 81.03 | 37 | 78.72 | 29 | 78.38 |
| Wessex | Intervention | S40 | 174 | 97 | 55.75 | 75 | 77.32 | 43 | 57.33 | 33 | 76.74 |
| Wessex | Intervention | S41 | 132 | 56 | 42.42 | 36 | 64.29 | 23 | 63.89 | 17 | 73.91 |
| Across intervention practices | 2516 | 1234 | 49.05 | 919 | 74.47 | 705 | 76.71 | 524 | 74.33 | ||
| WoE | Control | B16 | 103 | 40 | 38.83 | 31 | 77.5 | 25 | 80.65 | 22 | 88 |
| WoE | Control | B20 | 220 | 82 | 37.27 | 63 | 76.83 | 58 | 92.06 | 55 | 94.83 |
| WoE | Control | B24 | 194 | 88 | 45.36 | 58 | 65.91 | 40 | 68.97 | 37 | 92.5 |
| WoE | Control | B25 | 204 | 98 | 48.04 | 55 | 56.12 | 48 | 87.27 | 46 | 95.83 |
| WoE | Control | B31 | 205 | 95 | 46.34 | 67 | 70.53 | 54 | 80.6 | 50 | 92.59 |
| WoE | Control | B35 | 220 | 99 | 45 | 73 | 73.74 | 57 | 78.08 | 52 | 91.23 |
| WoE | Control | B37 | 159 | 67 | 42.14 | 51 | 76.12 | 41 | 80.39 | 36 | 87.8 |
| WoE | Control | B38 | 181 | 89 | 49.17 | 73 | 82.02 | 59 | 80.82 | 55 | 93.22 |
| WoE | Control | B39 | 210 | 105 | 50 | 72 | 68.57 | 59 | 81.94 | 58 | 98.31 |
| Wessex | Control | S14 | 106 | 57 | 53.77 | 39 | 68.42 | 24 | 61.54 | 22 | 91.67 |
| Wessex | Control | S17 | 186 | 73 | 39.25 | 53 | 72.6 | 32 | 60.38 | 32 | 100 |
| Wessex | Control | S23 | 222 | 127 | 57.21 | 81 | 63.78 | 56 | 69.14 | 54 | 96.43 |
| Wessex | Control | S29 | 82 | 46 | 56.1 | 36 | 78.26 | 35 | 97.22 | 34 | 97.14 |
| Across control practices | 2292 | 1066 | 46.51 | 752 | 70.54 | 588 | 78.19 | 553 | 94.05 | ||
| Across WoE practices | 2859 | 1342 | 46.94 | 982 | 73.17 | 798 | 81.26 | 673 | 84.34 | ||
| Across Wessex practices | 1949 | 958 | 49.15 | 689 | 71.92 | 495 | 71.84 | 404 | 81.62 | ||
| IPSS and ICIQ-UI-SF sections | Booklet sections | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drinks and water intake | Controlling bladder leakage | Controlling an urgent need to pass urine | Emptying bladder completely | Getting rid of the last drops | Reducing sleep disturbance | |||||||
| Referred (n = 345) | Not referred (n = 170) | Referred (n = 160) | Not referred (n = 355) | Referred (n = 347) | Not referred (n = 168) | Referred (n = 142) | Not referred (n = 373) | Referred (n = 132) | Not referred (n = 383) | Referred (n = 348) | Not referred (n = 167) | |
| IPSS, mean (SD) | ||||||||||||
| Incomplete emptying | 1.44 (1.41) | 2.12 (1.48) | 1.40 (1.35) | 1.78 (1.50) | 1.55 (1.44) | 1.89 (1.49) | 2.99 (1.31) | 1.15 (1.17) | 1.93 (1.39) | 1.57 (1.48) | 1.62 (1.45) | 1.74 (1.50) |
| Frequency | 2.61 (1.38) | 2.84 (1.23) | 2.72 (1.21) | 2.67 (1.39) | 2.89 (1.28) | 2.27 (1.35) | 3.07 (1.26) | 2.54 (1.33) | 2.68 (1.39) | 2.69 (1.32) | 2.56 (1.33) | 2.95 (1.31) |
| Intermittency | 1.77 (1.66) | 2.04 (1.58) | 1.62 (1.59) | 1.97 (1.65) | 1.86 (1.63) | 1.87 (1.66) | 2.58 (1.55) | 1.58 (1.59) | 2.12 (1.64) | 1.77 (1.63) | 1.78 (1.61) | 2.03 (1.68) |
| Urgency | 1.90 (1.61) | 2.63 (1.49) | 2.55 (1.59) | 1.95 (1.58) | 2.69 (1.51) | 1.03 (1.16) | 1.95 (1.56) | 2.21 (1.62) | 2.11 (1.58) | 2.15 (1.61) | 1.95 (1.57) | 2.54 (1.61) |
| Weak stream | 1.83 (1.54) | 2.13 (1.50) | 1.81 (1.48) | 1.98 (1.55) | 1.93 (1.53) | 1.92 (1.52) | 2.47 (1.59) | 1.71 (1.45) | 2.22 (1.56) | 1.82 (1.50) | 1.78 (1.45) | 2.22 (1.65) |
| Straining | 0.81 (1.20) | 0.89 (1.14) | 0.52 (0.88) | 0.99 (1.27) | 0.84 (1.17) | 0.84 (1.20) | 1.39 (1.42) | 0.63 (1.00) | 0.83 (1.15) | 0.84 (1.19) | 0.83 (1.16) | 0.87 (1.22) |
| Nocturia | 2.57 (1.40) | 2.59 (1.28) | 2.43 (1.36) | 2.64 (1.35) | 2.65 (1.34) | 2.42 (1.39) | 2.45 (1.34) | 2.62 (1.36) | 2.18 (1.22) | 2.71 (1.38) | 2.84 (1.31) | 2.04 (1.30) |
| ICIQ-UI-SF | ||||||||||||
| Q1: how often leak, mean (SD) | 1.03 (1.24) | 1.58 (1.19) | 1.85 (1.11) | 0.92 (1.20) | 1.35 (1.25) | 0.91 (1.20) | 1.05 (1.24) | 1.27 (1.25) | 1.76 (1.24) | 1.02 (1.20) | 0.98 (1.17) | 1.68 (1.27) |
| ICIQ, n (%) | ||||||||||||
| Q4: leak before toilet | 103 (29.9) | 99 (58.2) | 113 (70.2) | 89 (25.1) | 189 (54.3) | 13 (7.8) | 34 (23.9) | 168 (45.0) | 38 (29.0) | 164 (42.7) | 118 (34.0) | 84 (50.0) |
| Q4: leak cough/sneeze | 12 (3.5) | 12 (7.1) | 15 (9.3) | 9 (2.5) | 15 (4.3) | 9 (5.4) | 10 (7.0) | 14 (3.8) | 9 (6.9) | 15 (3.9) | 10 (2.9) | 14 (8.3) |
| Q4: asleep | 9 (2.6) | 3 (1.8) | 7 (4.4) | 5 (1.4) | 10 (2.9) | 2 (1.2) | 2 (1.4) | 10 (2.7) | 2 (1.5) | 10 (2.6) | 6 (1.7) | 6 (3.6) |
| Q4: active | 14 (4.1) | 9 (5.3) | 16 (9.9) | 7 (2.0) | 17 (4.9) | 6 (3.6) | 6 (4.2) | 17 (4.6) | 5 (3.8) | 18 (4.7) | 10 (2.9) | 13 (7.7) |
| Q4: after urinating | 99 (28.7) | 74 (43.5) | 58 (36.0) | 115 (32.5) | 106 (30.5) | 67 (40.1) | 52 (36.6) | 121 (32.4) | 108 (82.4) | 65 (16.9) | 90 (25.9) | 83 (49.4) |
| Q4: no reason | 8 (5.2) | 17 (10.0) | 21 (13.0) | 14 (4.0) | 27 (7.8) | 8 (4.8) | 6 (4.2) | 29 (7.8) | 10 (7.6) | 25 (6.5) | 23 (6.6) | 12 (7.1) |
| Q4: all the time | 1 (0.3) | 0 | 0 | 1 (0.3) | 1 (0.3) | 0 | 0 | 1 (0.3) | 0 | 1 (0.3) | 1 (0.3) | 0 |
| Protocol deviation | Number | Arm |
|---|---|---|
| Consent | ||
| Three patients retrospectively identified as ineligible for trial. Patients retained in the trial as per the statistical analysis plan | 3 | Intervention |
| Intervention visit/booklet delivery | ||
| Unable to contact patient for their intervention visit | 2 | Intervention |
| Patient history of urinary retention at intervention visit, so booklet sections revised after discussion with chief investigator | 1 | Intervention |
| Patient reviewed by GP prior to starting intervention because of symptoms identified | 1 | Intervention |
| Intervention follow-up contacts | ||
| Missed 1-week intervention follow-up contact | 29 | Intervention |
| Missed 4-week intervention follow-up contact | 8 | Intervention |
| Missed 12-week intervention follow-up contact | 12 | Intervention |
| Follow-up not in the protocol-dictated format | 28 | Intervention |
| Intervention follow-up outside protocol-dictated time frame | 10 | Intervention |
| Site was unable to offer patients e-mail follow-up contacts owing to practice policy | 1 | Intervention |
| Follow-up | ||
| Patient answered questions regarding feedback on the intervention booklet in both 6- and 12-month questionnaires, having never received the booklet | 1 | Intervention |
| Patients left their trial general practice during their time in the trial | 13 | Intervention |
| Patient moved general practice and reported potential SAE in the 6-month questionnaire. SAE data were obtained from the patient’s new general practice | 1 | Intervention |
| Large-print documents provided to patient because of eyesight difficulties | 1 | Intervention |
| Partially redacted patient-identifiable data for two SAEs reported to UHBW as sponsor’s representative | 1 | Control |
| COVID-19 | ||
| 177 patients were changed from postal questionnaires to electronic questionnaires from 19 March 2020 during the coronavirus outbreak. All patients were notified by e-mail and given the opportunity to continue with postal questionnaires if needed | 167 | 74 intervention; 93 control |
| Total | 279 | |
| Practice ID | Arm | Date | Months since consent | Reason | Withdrawal from Q/EMR/int |
|---|---|---|---|---|---|
| B12 | Intervention | 23 November 2018 | 1.48 | Consented to another LUTS trial | Q/EMR/int |
| B12 | Intervention | 20 December 2018 | 2.04 | Ill health (subsequently died) | Q |
| B11 | Intervention | 9 January 2019 | 3.25 | Too inconvenient | Q/EMR/int |
| S15 | Intervention | 21 January 2019 | 1.97 | Did not want to attend face-to-face appointment | Int |
| S19 | Intervention | 29 March 2019 | 2.37 | Ill health | Q/EMR/int |
| S21 | Intervention | 29 April 2019 | 1.08 | No reason given | Q/int |
| B22 | Intervention | 23 May 2019 | 4.24 | No reason given | Q/int |
| S27 | Intervention | 23 July 2019 | 1.51 | Wife objected to participation in the trial | Q/EMR/int |
| S26 | Intervention | 23 August 2019 | 3.48 | Ill health (lacks capacity) | Q |
| S30 | Intervention | 3 October 2019 | 5.45 | Did not want to attend face-to-face appointment | Int |
| B32 | Intervention | 7 October 2019 | 2.92 | Patient felt that the trial was not relevant to him | Int |
| S23 | Control | 21 October 2019 | 6.34 | Ill health | Q/EMR |
| B33 | Intervention | 4 December 2019 | 7.66 | Died | Q/EMR/int |
| S26 | Intervention | 11 December 2019 | 6.44 | Change in symptoms | Q |
| B20 | Control | 15 January 2020 | 12.12 | Ill health and change of circumstance | Q |
| B13 | Intervention | 28 January 2020 | 13.4 | Too busy to take part in the trial | Q/int |
| B20 | Control | 3 February 2020 | 11.76 | Died | Q/EMR |
| B24 | Control | 10 February 2020 | 9.86 | Patient felt that the trial was not relevant to him | Q |
| B36 | Intervention | 21 April 2020 | 12.48 | Ill health | Q/int |
| B34 | Intervention | 12 May 2020 | 11.86 | Died | Q/EMR/int |
| S30 | Intervention | 22June 2020 | 14.55 | No reason given | Q/EMR/int |
| Characteristic | Missing | Present | p-value | ||
|---|---|---|---|---|---|
| n a | Value | n a | Value | ||
| Total number of participants, n (%) | 105 (9.83) | 972 (90.17) | |||
| Treatment arm, n (%) | |||||
| Intervention | 53 (50.48) | 471 (48.46) | 0.694 | ||
| Usual care | 52 (49.52) | 501 (51.54) | |||
| Demographic characteristics | |||||
| Age (years), mean (SD) [minimum, maximum] | 105 | 68.56 (11.05) [32, 94] | 972 | 68.70 (9.05) [30, 95] | 0.887 |
| Ethnicity, n (%) | |||||
| White | 103 | 101 (98.06) | 969 | 954 (98.45) | 0.304 |
| Black/African/Caribbean/Black British | 1 (0.97) | 1 (0.10) | |||
| Mixed/multiple ethnic groups | 1 (0.97) | 3 (0.31) | |||
| Asian/Asian British | 0 (0.00) | 5 (0.52) | |||
| Other ethnic group | 0 (0.00) | 2 (0.21) | |||
| Disclosure declined | 0 (0.00) | 4 (0.41) | |||
| Marital status, n (%) | |||||
| Single | 102 | 10 (9.80) | 958 | 36 (3.76) | < 0.001 |
| Married | 68 (66.67) | 801 (83.61) | |||
| Civil partnered | 3 (2.94) | 19 (1.98) | |||
| Divorced | 7 (6.86) | 56 (5.85) | |||
| Widowed | 12 (11.75) | 43 (4.49) | |||
| Disclosure declined | 2 (1.96) | 3 (0.31) | |||
| IMD score, median (IQR) [minimum, maximum] | 100 | 10.20 (6.17–17.45) [2.41, 54.06] | 931 | 9.08 (5.91–14.76) [1.18, 60.30] | 0.120 |
| IMD quintile, n (%) | |||||
| Quintile 1 (most deprived) | 100 | 4 (4.00) | 931 | 43 (3.65) | 0.534 |
| Quintile 2 | 9 (9.00) | 61 (6.55) | |||
| Quintile 3 | 21 (21.00) | 152 (16.33) | |||
| Quintile 4 | 27 (27.00) | 250 (26.85) | |||
| Quintile 5 (least deprived) | 39 (39.00) | 434 (46.62) | |||
| Clinical characteristics | |||||
| Height (cm), mean (SD) [minimum, maximum] | 104 | 175.75 (7.60) [157.48, 198.12] | 960 | 176.95 (7.05) [152.40, 208.28] | 0.102 |
| Weight (kg), mean (SD) [minimum, maximum] | 100 | 84.26 (14.54) [56.25, 136.98] | 942 | 83.50 (14.38) [53.98, 152.41] | 0.618 |
| EMR search data | |||||
| Number of comorbidities, n (%) | |||||
| None | 90 | 28 (31.11) | 932 | 294 (31.55) | 0.814 |
| One | 34 (37.78) | 323 (34.66) | |||
| More than one | 28 (31.11) | 315 (33.80) | |||
| Most recent urine analysis results in the 6 months pre baseline: abnormal, n (%) | 10 | 0 (0.00) | 121 | 3 (2.48) | 0.639 |
| Kidney function: most recent eGFR (ml/minute/1.73 m2) measure in the 6 months pre baseline | |||||
| Number of patients with an eGFR measure | 27 | 27 | 358 | 358 | 0.989 |
| eGFR: mean (SD) | 74.11 (16.16) | 74.07 (14.22) | |||
| eGFR: median (IQR) | 75 (61, 89) | 76 (66, 86) | |||
| eGFR: minimum, maximum | 31, 98 | 28, 100 | |||
| CKD stages based on most recent eGFR (ml/minute/1.73 m2) in the 6 months pre baseline, n (%) | |||||
| ≥ 90 (normal) | 27 | 6 (22.22) | 358 | 55 (15.36) | 0.799 |
| 90–60 (CKD stages G1 and G2) | 17 (62.96) | 251 (70.11) | |||
| 30–59 (CKD stage G3) | 4 (14.81) | 51 (14.25) | |||
| < 30 (CKD stages G4 and G5) | 0 (0.00) | 1 (0.28) | |||
| Number of GP consultations in the 12 months before baseline | |||||
| Mean (SD) | 90 | 4.48 (3.64) | 932 | 4.62 (4.49) | 0.766 |
| Median (IQR) | 3 (2-7) | 4 (2-6) | |||
| Minimum, maximum | 0, 21 | 0, 58 | |||
| Referrals to urology in the 12 months pre baseline, n (%) | |||||
| None | 90 | 85 (94.44) | 932 | 904 (97.00) | 0.191 |
| One | 5 (5.56) | 28 (3.00) | |||
| More than one | 0 (0.00) | 0 (0.00) | |||
| Medications at baseline, n (%) | |||||
| At least one prescription | 90 | 32 (35.56) | 932 | 393 (41.09) | 0.307 |
| At least one prescription of tamsulosin | 26 (28.89) | 305 (32.73) | 0.458 | ||
| At least one prescription of oxybutynin | 2 (2.22) | 30 (3.22) | 0.604 | ||
| At least one prescription of doxazosin | 1 (1.11) | 20 (2.15) | 0.509 | ||
| At least one prescription of solifenacin | 2 (2.22) | 29 (3.11) | 0.638 | ||
| At least one prescription of tolterodine | 1 (1.11) | 20 (2.15) | 0.509 | ||
| At least one prescription of mirabegron | 3 (3.33) | 17 (1.82) | 0.324 | ||
| At least one prescription of alfuzosin | 1 (1.11) | 8 (0.86) | 0.806 | ||
| At least one prescription of tadalafil | 0 (0.00) | 3 (0.32) | 0.59 | ||
| At least one prescription of trospium chloride | 0 (0.00) | 1 (0.11) | 0.756 | ||
| At least one prescription of solifenacin and tamsulosin | 0 (0.00) | 4 (0.43) | 0.533 | ||
| Patient-reported symptoms and quality of life | |||||
| IPSS symptoms, mean (SD) [minimum, maximum] | |||||
| Incomplete emptying | 104 | 1.82 (1.53) [0, 5] | 957 | 1.75 (1.48) [0, 5] | 0.671 |
| Frequency | 104 | 3.13 (1.33) [0, 5] | 961 | 2.77 (1.35) [0, 5] | 0.011 |
| Intermittency | 104 | 2.00 (1.72) [0, 5] | 959 | 1.91 (1.66) [0, 5] | 0.585 |
| Urgency | 102 | 2.49 (1.69) [0, 5] | 960 | 2.22 (1.63) [0, 5] | 0.113 |
| Weak stream | 102 | 2.08 (1.71) [0, 5] | 957 | 1.97 (1.59) [0, 5] | 0.498 |
| Straining | 104 | 0.92 (1.27) [0, 5] | 957 | 0.92 (1.25) [0, 5] | 0.972 |
| Nocturia | 105 | 2.59 (1.33) [0, 5] | 962 | 2.50 (1.30) [0, 5] | 0.5 |
| Total IPSS, mean (SD) [minimum, maximum] | 100 | 14.95 (6.36) [4, 34] | 942 | 14.04 (6.23) [1, 33] | 0.164 |
| IPSS-QoL score; mean (SD) [minimum, maximum] | 105 | 3.71 (1.11) [0, 6] | 962 | 3.49 (1.16) [0, 6] | 0.059 |
| ICIQ-UI-SF total score; mean (SD) [minimum, maximum] | 103 | 4.83 (3.99) [0, 15] | 952 | 3.64 (3.56) [0,15] | 0.001 |
| ICIQ-UI-SF: when does urine leak?, n (%) | |||||
| Never | 105 | 25 (23.81) | 970 | 321 (33.09) | 0.053 |
| Leaks before you can get to the toilet | 51 (48.57) | 391 (40.31) | 0.102 | ||
| Leaks when you cough/sneeze | 7 (6.67) | 41 (4.23) | 0.25 | ||
| Leaks when you are asleep | 3 (2.86) | 970 (2.47) | 0.812 | ||
| Leaks when you are physically active | 6 (5.71) | 44 (4.54) | 0.586 | ||
| Leaks when you have finished urinating/are dressed | 42 (40.00) | 338 (34.85) | 0.294 | ||
| Leaks for no obvious reason | 11 (10.48) | 67 (6.91) | 0.181 | ||
| Leaks all of the time | 0 (0.00) | 2 (0.21) | 0.641 | ||
| EQ-5D-5L utility score, mean (SD) [minimum, maximum] | 105 | 0.82 (0.17) [–0.06, 1] | 964 | 0.83 (0.17) [–0.12, 1] | 0.327 |
| EQ VAS, mean (SD) [minimum, maximum] | 104 | 75.23 (17.32) [20, 100] | 969 | 78.22 (15.37) [15, 100] | 0.060 |
| B-IPQ total score, mean (SD) [minimum, maximum] | 92 | 42.07 (10.47) [17, 62] | 826 | 38.75 (10.68) [1, 75] | 0.005 |
| Bladder diary (intervention arm only), n (%) | |||||
| Incontinence | 45 | 7 (15.56) | 457 | 93 (20.35) | 0.445 |
| Urgency | 46 | 35 (76.09) | 461 | 329 (71.37) | 0.498 |
| Nocturia | 25 | 20 (80.00) | 236 | 202 (85.60) | 0.456 |
| Characteristic | Missing | Present | p-value | ||
|---|---|---|---|---|---|
| n a | Value | n a | Value | ||
| Total number of participants | 163 | 915 | |||
| Treatment arm, n (%) | |||||
| Intervention | 82 (50.62) | 442 (48.31) | 0.638 | ||
| Usual care | 80 (49.38) | 473 (51.69) | |||
| Demographic characteristics | |||||
| Age (years), mean (SD) [minimum, maximum] | 162 | 68.33 (10.63) [35, 93] | 915 | 68.75 (9.00) [30, 95] | 0.601 |
| Ethnicity, n (%) | |||||
| White | 160 | 156 (97.50) | 912 | 899 (98.57) | 0.194 |
| Black/African/Caribbean/Black British | 1 (0.63) | 1 (0.11) | |||
| Mixed/multiple ethnic groups | 2 (1.25) | 2 (0.22) | |||
| Asian/Asian British | 0 (0.00) | 5 (0.55) | |||
| Other ethnic group | 0 (0.00) | 2 (0.22) | |||
| Disclosure declined | 1 (0.63) | 3 (0.33) | |||
| Marital status, n (%) | |||||
| Single | 158 | 6 (3.80) | 902 | 40 (4.43) | 0.126 |
| Married | 121 (76.58) | 748 (82.93) | |||
| Civil partnered | 7 (4.43) | 15 (1.66) | |||
| Divorced | 14 (8.86) | 49 (5.43) | |||
| Widowed | 9 (5.70) | 46 (5.10) | |||
| Disclosure declined | 1 (0.63) | 4 (0.44) | |||
| IMD score, median (IQR) [minimum, maximum] | 154 | 10.02 (5.80–15.77) [1.68, 54.06] | 877 | 9.08 (6.04–14.76) [1.18, 60.30] | 0.240 |
| IMD quintile, n (%) | |||||
| Quintile 1 (most deprived) | 154 | 7 (4.55) | 877 | 31 (3.53) | 0.510 |
| Quintile 2 | 15 (9.74) | 55 (6.27) | |||
| Quintile 3 | 27 (17.53) | 146 (16.65) | |||
| Quintile 4 | 38 (24.68) | 239 (27.25) | |||
| Quintile 5 (least deprived) | 67 (43.51) | 406 (46.29) | |||
| Clinical characteristics | |||||
| Height (cm), mean (SD) [minimum, maximum] | 160 | 176.91 (7.52) [157.48, 198.12] | 904 | 176.82 (7.04) [152.4, 208.28] | 0.884 |
| Weight (kg), mean (SD) [minimum, maximum] | 152 | 83.63 (15.15) [55.02, 136.98] | 890 | 83.57 (14.28) [53.98, 152.41] | 0.959 |
| EMR search data | |||||
| Number of comorbidities, n (%) | |||||
| None | 143 | 52 (36.36) | 879 | 270 (30.72) | 0.351 |
| One | 44 (30.77) | 313 (35.61) | |||
| More than one | 47 (32.87) | 296 (33.67) | |||
| Most recent urine analysis results in the 6 months pre-baseline: abnormal, n (%) | 14 | 1 (7.14) | 117 | 2 (1.17) | 0.294 |
| Kidney function: most recent eGFR (ml/minute/1.73m2) measure in the 6 months pre baseline | |||||
| Number of patients with an eGFR measure | 48 | 48 | 337 | 337 | 0.768 |
| eGFR: mean (SD) | 74.65 (15.93) | 73.99 (14.13) | |||
| eGFR: median (IQR) | 77 (64–88.5) | 76 (66–86) | |||
| eGFR: minimum, maximum | 32, 100 | 28, 98 | |||
| CKD stages based on most recent eGFR (ml/minute/1.73m2) in the 6 months pre baseline, n (%) | |||||
| ≥ 90 (normal) | 48 | 8 (16.67) | 337 | 53 (15.73) | 0.982 |
| 90–60 (CKD stages G1 and G2) | 33 (68.75) | 235 (69.73) | |||
| 30–59 (CKD stage G3) | 7 (14.58) | 48 (14.24) | |||
| < 30 (CKD stages G4 and G5) | 0 (0.00) | 1 (0.30) | |||
| Number of GP consultations in the 12 months before baseline | |||||
| Mean (SD) | 143 | 4.22 (3.60) | 879 | 4.67 (4.54) | 0.251 |
| Median (IQR) | 3 (2-6) | 4 (2-6) | |||
| Minimum, maximum | 0, 21 | 0, 58 | |||
| Referrals to urology in the 12 months pre baseline, n (%) | |||||
| None | 143 | 139 (97.20) | 879 | 820 (96.70) | 0.753 |
| One | 4 (2.80) | 29 (3.30) | |||
| More than one | 0 (0.00) | 0 (0.00) | |||
| Medications at baseline, n (%) | |||||
| At least one prescription | 143 | 50 (34.97) | 879 | 365 (41.52) | 0.139 |
| At least one prescription of tamsulosin | 44 (30.77) | 287 (32.65) | 0.656 | ||
| At least one prescription of oxybutynin | 3 (2.10) | 29 (3.30) | 0.444 | ||
| At least one prescription of doxazosin | 2 (1.40) | 19 (2.16) | 0.551 | ||
| At least one prescription of solifenacin | 5 (3.50) | 26 (2.96) | 0.728 | ||
| At least one prescription of tolterodine | 0 (0.00) | 21 (2.39) | 0.062 | ||
| At least one prescription of mirabegron | 2 (1.40) | 18 (2.05) | 0.603 | ||
| At least one prescription of alfuzosin | 0 (0.00) | 9 (1.02) | 0.224 | ||
| At least one prescription of tadalafil | 0 (0.00) | 3 (0.34) | 0.484 | ||
| At least one prescription of trospium chloride | 0 (0.00) | 1 (0.11) | 0.687 | ||
| At least one prescription of solifenacin and tamsulosin | 0 (0.00) | 4 (0.46) | 0.419 | ||
| Patient-reported symptoms and quality of life | |||||
| IPSS symptoms, mean (SD) [minimum, maximum] | |||||
| Incomplete emptying | 160 | 1.75 (1.48) [0, 5] | 901 | 1.76 (1.48) [0, 5] | 0.936 |
| Frequency | 160 | 2.43 (1.39) [0, 5] | 905 | 2.80 (1.35) [0, 5] | 0.700 |
| Intermittency | 158 | 1.98 (1.72) [0, 5] | 905 | 1.90 (1.65) [0, 5] | 0.591 |
| Urgency | 159 | 2.28 (1.62) [0, 5] | 903 | 2.24 (1.64) [0, 5] | 0.796 |
| Weak stream | 158 | 1.90 (1.60) [0, 5] | 901 | 1.99 (1.60) [0, 5] | 0.509 |
| Straining | 159 | 0.87 (1.18) [0, 5] | 902 | 0.93 (1.27) [0, 5] | 0.578 |
| Nocturia | 161 | 2.58 (1.34) [0, 5] | 906 | 2.50 (1.30) [0, 5] | 0.429 |
| Total IPSS score, mean (SD) [minimum, maximum] | 155 | 14.19 (6.23) [3, 34] | 887 | 14.11 (6.25) [1, 33] | 0.880 |
| IPSS-QoL score, mean (SD) [minimum, maximum] | 161 | 3.50 (1.12) [0, 6] | 906 | 3.51 (1.17) [0, 6] | 0.919 |
| ICIQ-UI-SF total score, mean (SD) [minimum, maximum] | 158 | 4.01 (4.03) [0, 15] | 897 | 3.71 (3.55) [0, 15] | 0.333 |
| ICIQ-UI-SF: when does urine leak?, n (%) | |||||
| Never | 162 | 54 (33.33) | 913 | 292 (31.98) | 0.734 |
| Leaks before you can get to the toilet | 70 (43.21) | 372 (40.74) | 0.557 | ||
| Leaks when you cough/sneeze | 9 (5.56) | 39 (4.27) | 0.466 | ||
| Leaks when you are asleep | 5 (3.09) | 22 (2.41) | 0.612 | ||
| Leaks when you are physically active | 8 (4.94) | 42 (4.60) | 0.851 | ||
| Leaks when you have finished urinating/are dressed | 53 (32.72) | 327 (35.82) | 0.447 | ||
| Leaks for no obvious reason | 16 (9.88) | 62 (6.79) | 0.163 | ||
| Leaks all of the time | 0 (0.00) | 2 (0.22) | 0.551 | ||
| EQ-5D-5L utility score, mean (SD) [minimum, maximum] | 162 | 0.84 (0.17) [–0.06, 1] | 907 | 0.83 (0.16) [–0.118, 1] | 0.427 |
| EQ VAS, mean (SD) [minimum, maximum] | 161 | 77.52 (16.38) [20, 100] | 912 | 78.00 (15.37) [15, 100] | 0.717 |
| B-IPQ total score, mean (SD) [minimum, maximum] | 139 | 38.55 (10.27) [16, 62] | 779 | 39.18 (10.76) [1, 75] | 0.520 |
| Bladder diary (intervention arm only), n (%) | |||||
| Incontinence | 71 | 13 (18.31%) | 431 | 87 (20.19%) | 0.714 |
| Urgency | 73 | 49 (67.12%) | 434 | 315 (72.58%) | 0.338 |
| Nocturia | 37 | 27 (73.00%) | 224 | 195 (87.10%) | 0.026 |
| Time point | Items | Intervention | Usual care | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Baseline | IPSS symptoms | ||||
| Incomplete emptying | 512 | 1.66 (1.46) | 549 | 1.85 (1.49) | |
| Frequency | 514 | 2.68 (1.33) | 551 | 2.92 (1.37) | |
| Intermittency | 514 | 1.87 (1.64) | 549 | 1.96 (1.69) | |
| Urgency | 513 | 2.14 (1.61) | 549 | 2.35 (1.66) | |
| Weak stream | 510 | 1.93 (1.53) | 549 | 2.02 (1.66) | |
| Straining | 513 | 0.85 (1.19) | 548 | 0.99 (1.31) | |
| Nocturia | 516 | 2.59 (1.36) | 551 | 2.43 (1.25) | |
| 6 months | IPSS symptoms | ||||
| Incomplete emptying | 483 | 1.45 (1.41) | 510 | 1.79 (1.46) | |
| Frequency | 483 | 2.25 (1.41) | 510 | 2.66 (1.38) | |
| Intermittency | 482 | 1.58 (1.53) | 510 | 1.93 (1.65) | |
| Urgency | 482 | 1.81 (1.52) | 510 | 2.31 (1.60) | |
| Weak stream | 479 | 1.53 (1.44) | 508 | 1.83 (1.58) | |
| Straining | 482 | 0.71 (1.12) | 509 | 0.91 (1.28) | |
| Nocturia | 483 | 2.23 (1.23) | 509 | 2.38 (1.18) | |
| 12 months | IPSS symptoms | ||||
| Incomplete emptying | 461 | 1.46 (1.42) | 484 | 1.88 (1.55) | |
| Frequency | 461 | 2.33 (1.40) | 484 | 2.68 (1.41) | |
| Intermittency | 460 | 1.56 (1.54) | 482 | 1.87 (1.65) | |
| Urgency | 455 | 1.80 (1.52) | 482 | 2.24 (1.64) | |
| Weak stream | 459 | 1.53 (1.42) | 482 | 1.91 (1.61) | |
| Straining | 457 | 0.60 (1.00) | 484 | 0.89 (1.24) | |
| Nocturia | 461 | 2.21 (1.27) | 483 | 2.34 (1.17) | |
| N | n (%) | N | n (%) | ||
| Baseline | ICIQ-UI-SF: when does urine leak?, n (%) | 523 | 553 | ||
| Never | 185 (35.37) | 162 (29.29) | |||
| Leaks before you can get to the toilet | 205 (39.20) | 237 (42.86) | |||
| Leaks when you cough/sneeze | 24 (4.59) | 24 (4.34) | |||
| Leaks when you are asleep | 12 (2.29) | 15 (2.71) | |||
| Leaks when you are physically active | 23 (4.40) | 27 (4.88) | |||
| Leaks when you have finished urinating/are dressed | 175 (33.46) | 205 (37.07) | |||
| Leaks for no obvious reason | 36 (6.88) | 42 (7.59) | |||
| Leaks all of the time | 1 (0.19) | 1 (0.18) | |||
| 6 months | ICIQ-UI-SF: when does urine leak?, n (%) | 524 | 553 | ||
| Never | 183 (34.92) | 172 (31.10) | |||
| Leaks before you can get to the toilet | 186 (35.50) | 212 (38.34) | |||
| Leaks when you cough/sneeze | 12 (2.29) | 10 (1.81) | |||
| Leaks when you are asleep | 9 (1.72) | 16 (2.89) | |||
| Leaks when you are physically active | 19 (3.63) | 21 (3.80) | |||
| Leaks when you have finished urinating/are dressed | 120 (22.90) | 168 (30.38) | |||
| Leaks for no obvious reason | 34 (6.49) | 34 (6.15) | |||
| Leaks all of the time | 1 (0.19) | 1 (0.18) | |||
| 12 months | ICIQ-UI-SF: when does urine leak?, n (%) | 524 | 553 | ||
| Never | 174 (34.92) | 160 (28.93) | |||
| Leaks before you can get to the toilet | 177 (35.50) | 203 (36.71) | |||
| Leaks when you cough/sneeze | 15 (2.29) | 15 (2.71) | |||
| Leaks when you are asleep | 16 (1.72) | 25 (4.52) | |||
| Leaks when you are physically active | 21 (3.63) | 20 (3.62) | |||
| Leaks when you have finished urinating/are dressed | 111 (22.90) | 153 (27.67) | |||
| Leaks for no obvious reason | 21 (6.49) | 31 (5.61) | |||
| Leaks all of the time | 1 (0.19) | 0 (0.00) | |||
| Change in IPSS from baseline | Intervention (N = 442) | Usual care (N = 470) | Overall (N = 912) |
|---|---|---|---|
| Improved | 167 (37.78) | 171 (36.38) | 338 (37.06) |
| Stayed in the same category | 202 (45.70) | 231 (49.15) | 433 (47.48) |
| Got worse | 53 (11.99) | 50 (10.64) | 103 (11.29) |
| Fluctuated | 18 (3.83) | 38 (4.17) |
| Primary analysis of IPSS | n | Mean | SD | Minimum, maximum | Difference in meansa (95% CI) | p-value |
|---|---|---|---|---|---|---|
| At 12 months | 887 | 12.79 | 6.64 | 0, 35 | –1.81 (–2.66 to –0.95) | < 0.001 |
| At 12 months excluding patients later found to be ineligible | 884 | 12.78 | 6.64 | 0, 35 | –1.81 (–2.65 to –0.96) | < 0.001 |
| Trial group | n | Mean | SD | Minimum, maximum | Difference in meansb (95% CI) | p-value | Difference in meansc (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Intervention | 442 | 11.60 | 6.21 | 1, 35 | –1.81 (–2.66 to –0.95) | < 0.001 | –1.80 (–2.61 to –0.99) | < 0.001 |
| Usual care | 473 | 13.88 | 6.84 | 0, 32 | ||||
| Total | 915 | 12.78 | 6.64 | 0, 35 | ||||
| ICC (95% CI) | 0.01 (0.00 to 0.09) | 0.006 (< 0.001 to 0.123) | ||||||
| Centre | Received intervention or usual care | Months since baseline | Description | Related to intervention? | Outcome |
|---|---|---|---|---|---|
| WoE | Intervention | 11.08 | Septal hypertrophic cardiomyopathy | Not related | Resolved |
| WoE | Intervention | 7.99 | Elective admission for left-hip replacement | Not related | Resolved |
| WoE | Intervention | 12.72 | Iron-deficiency anaemia | Not related | Resolved |
| WoE | Intervention | 12.72 | Suspected MI admission to A&E with presyncope and slow AF | Not related | Resolved |
| WoE | Intervention | 7.30 | NSTEMI | Not related | Resolved |
| WoE | Intervention | 10.98 | Encephalopathy, hepatocellular carcinoma, acute kidney injury, pneumonia | Not related | Death |
| Wessex | Intervention | 4.80 | Fast AF | Unlikely to be related | Resolved |
| Wessex | Intervention | 4.01 | Left-knee replacement | Not related | Resolved |
| Wessex | Intervention | 6.81 | Stroke | Not related | Resolved |
| Wessex | Intervention | 8.78 | Facial trauma peritonsillar swelling following an accidental fall | Not related | Resolved |
| WoE | Intervention | 11.05 | Flare of diverticular disease | Not related | Resolved |
| WoE | Intervention | 5.56 | Metastatic carcinoma of pancreas | Not related | Death |
| WoE | Intervention | 9.83 | Short of breath, chest tightness, admitted to A&E. Diagnosed with acute NSTEMI | Not related | Resolved |
| Wessex | Intervention | 5.13 | Junctional failure of posterior construction, revision surgery | Not related | Resolved |
| Wessex | Intervention | 4.01 | Complete heart block | Not related | Resolved |
| Wessex | Intervention | 3.62 | AF | Unlikely to be related | Resolved |
| Wessex | Intervention | 0.69 | Admitted for cellulitis; while in hospital, diagnosed with a heart block | Not related | Resolved |
| WoE | Intervention | 8.15 | Elective right-knee replacement | Not related | Resolved |
| WoE | Intervention | 5.72 | Transient ischaemic attack | Unlikely to be related | Resolved |
| WoE | Intervention | 2.93 | Fall, likely secondary to vasovagal syncope | Not related | Resolved |
| WoE | Intervention | 10.78 | Acute NSTEMI | Not related | Resolved |
| Wessex | Intervention | 8.91 | Progressive-onset chest pain: acute coronary syndrome | Not related | Resolved |
| Wessex | Intervention | 1.64 | Acute onset of confusion | Not related | Resolved |
| WoE | Intervention | 11.97 | Bilateral pneumonia | Not related | Resolved |
| Wessex | Intervention | 3.58 | Left inguinal hernia, requiring repair | Not related | Resolved |
| WoE | Intervention | 2.27 | Onset of chest pain | Not related | Resolved |
| WoE | Intervention | 8.35 | Multiresistant pseudomonas respiratory infection | Not related | Resolved |
| Wessex | Intervention | 3.52 | Abdominal pain and bloating | Not related | Resolved |
| WoE | Intervention | 9.17 | Infective exacerbation of bronchiectasis | Not related | Resolved |
| WoE | Intervention | 1.71 | Release of third and fourth toes | Not related | Resolved |
| Wessex | Intervention | 7.56 | Bilateral epistaxis | Not related | Resolved |
| WoE | Intervention | 2.01 | Ventricular ectopic beats | Not related | Resolved |
| Wessex | Intervention | 3.75 | Elective hospital admission for laparoscopic excision of GIST lesion of the stomach | Not related | Resolved |
| WoE | Intervention | 10.45 | Pulmonary embolism, hepatocellular carcinoma with metastatic local spread, cirrhosis of the liver with portal hypertension | Not related | Resolved |
| Wessex | Intervention | 2.53 | Hip replacement | Not related | Resolved |
| WoE | Intervention | 0.39 | Shortness of breath, productive cough | Not related | Resolved |
| Wessex | Intervention | 9.73 | Haematoma overlaying permanent pacemaker box | Not related | Resolved |
| WoE | Intervention | 7.17 | Acute uncomplicated diverticulitis | Not related | Resolved |
| WoE | Intervention | 7.73 | Severe aortic stenosis, coronary artery disease | Not related | Resolved |
| Wessex | Intervention | 5.13 | Further junctional failure of posterior construction and fracture | Not related | Resolved |
| Wessex | Intervention | 3.68 | Acute coronary syndrome, anterior NSTEMI | Not related | Resolved |
| WoE | Intervention | 13.22 | Inguinal hernia repair | Not related | Resolved |
| Wessex | Intervention | 4.96 | Postural hypertension secondary to antihypertensive medication | Not related | Resolved |
| WoE | Intervention | 4.11 | Heart block | Not related | Resolved |
| Wessex | Intervention | 5.13 | Elective posterior fusion of lumbar spine | Not related | Resolved |
| Wessex | Intervention | 1.15 | NSTEMI, myocarditis, treated as a NSTEMI: echocardiogram showed mild left ventricular systolic dysfunction | Not related | Resolved |
| Wessex | Intervention | 0.46 | Main symptom was fever and fall on level ground | Not related | Resolved |
| WoE | Control | 6.41 | Arthroscopic decompression of left subacromial joint (procedure) | Not related | Resolved |
| WoE | Control | 12.03 | Shoulder pain and carpal tunnel syndrome | Not related | Resolved |
| Wessex | Control | 1.87 | Transient ischaemic attack | Not related | Resolved |
| Wessex | Control | 6.84 | UTI, unplanned admission | Not related | Resolved |
| Wessex | Control | 22.13 | Delirium, upper respiratory infection and urinary retention | Not related | Resolved |
| WoE | Control | 2.53 | Angina | Not related | Resolved |
| WoE | Control | 5.49 | Left total hip replacement | Not related | Resolved |
| WoE | Control | 0.10 | Acute retention urine | Unlikely to be related | Resolved |
| Wessex | Control | 5.95 | NSTEMI | Not related | Ongoing |
| WoE | Control | 8.84 | Right inguinal hernia | Not related | Resolved |
| WoE | Control | 7.23 | Elective total knee replacement | Not related | Resolved |
| Wessex | Control | 8.42 | Exacerbation of congestive cardiac failure: shortness of breath and paroxysmal nocturnal dyspnoea | Not related | Resolved |
| WoE | Control | 9.21 | Chest pain | Not related | Resolved |
| Wessex | Control | 7.20 | Unstable angina, mild coronary artery disease | Not related | Resolved |
| WoE | Control | 9.47 | Syncope | Not related | Resolved |
| Wessex | Control | 0.49 | Right-knee arthritis, elective admission for right total knee replacement | Not related | Resolved |
| WoE | Control | 1.05 | Road traffic accident, contusion of cervical cord | Not related | Resolved |
| WoE | Control | 2.27 | Ischaemic heart disease | Not related | Resolved |
| WoE | Control | 9.86 | COPD, CVA, type 2 diabetes | Not related | Resolved |
| WoE | Control | 4.67 | Osteoarthritis of the hip, admission for elective left-hip replacement | Not related | Resolved |
| WoE | Control | 9.53 | COPD, CVA, type 2 diabetes | Not related | Resolved |
| Wessex | Control | 6.31 | Planned admission for right robotic partial nephrectomy for right lower pole renal tumour, postoperative lower respiratory tract infection | Not related | Resolved |
| WoE | Control | 2.47 | Infected toe | Not related | Resolved |
| Wessex | Control | 5.95 | NSTEMI | Not related | Resolved |
| WoE | Control | 5.79 | Left axillary dissection, metastatic malignant melanoma left axilla | Not related | Resolved |
| WoE | Control | 8.94 | Double lumbar decompression | Not related | Resolved |
| WoE | Control | 7.27 | Bowel obstruction due to bowel cancer | Not related | Resolved |
| Wessex | Control | 6.48 | Right-sided inguinal hernia | Not related | Resolved |
| WoE | Control | 6.84 | Sepsis | Not related | Resolved |
| WoE | Control | 6.84 | Exacerbation of COPD necessitating hospital admission | Not related | Resolved |
| WoE | Control | 8.71 | Grade I L4/L5 spondylolisthesis with canal stenosis. L4/L5 decompression and instrumental costolateral fusion with cemented screws | Unlikely to be related | Resolved |
| WoE | Control | 0.39 | AF, heart failure, non-ischaemic cardiomyopathy | Not related | Resolved |
| WoE | Control | 1.84 | Right laparoscopic nephrectomy for right kidney tumour | Not related | Resolved |
| Wessex | Control | 7.23 | Prostate cancer | Not related | Resolved |
| Wessex | Control | 4.96 | Bladder outflow obstruction, transitional cell carcinoma of bladder | Not related | Resolved |
| WoE | Control | 8.91 | Bladder stone | Not related | Resolved |
| WoE | Control | 3.39 | Collapse? Cardiac cause | Not related | Resolved |
| WoE | Control | 4.24 | Fall – minor head injury | Not related | Resolved |
| WoE | Control | 5.75 | Anterior cervical discectomy and fusion C3/C4 spinal cord | Not related | Resolved |
| WoE | Control | 5.56 | Wound dehiscence of left-hip replacement, post hip replacement | Not related | Resolved |
| WoE | Control | 1.22 | Haematuria/kidney pain | Not related | Resolved |
| Wessex | Control | 1.81 | Haematuria | Not related | Resolved |
| WoE | Control | 4.90 | NSTEMI | Not related | Resolved |
| WoE | Control | 7.40 | Carcinoma of sigmoid colon diagnosed, hospitalisation for surgery | Not related | Ongoing |
| WoE | Control | 3.75 | Hip fracture: right periprosthetic hip fracture | Not related | Resolved |
| WoE | Control | 12.00 | Acute urinary retention and AF | Not related | Resolved |
| WoE | Control | 11.61 | Community-acquired pneumonia | Not related | Death |
Appendix 2 Electronic medical records: data cleaning for economic evaluation
Multiple staff terms (n = 79) were first grouped into key staff categories of interest for the analysis (GP, nurse, HCA and pharmacist). When there was uncertainty in the staff term used, this was clarified with a GP. A GP consultation was assumed (because the majority of consultations were with a GP) when information on the staff member who provided the primary care consultation was missing (0.08% of consultation data). When information on the consultation type was missing (0.58% of consultation data), a weighted average of the unit costs for GP surgery, telephone and home visits was used for each relevant HCP. Finally, to avoid double-counting the intervention and follow-up visits, all practices were asked to check and confirm whether or not their nurses and/or HCAs had recorded any intervention activity in the patient’s EMR data. If they had, then a single consultation was removed if it took place 2 weeks prior to or after the date of the intervention or follow-up visit, as recorded in the case report form. This criteria was informed by pilot work carried out for two practices that used the EMIS Web IT system and could provide the reasons for the consultation. The average quantity of tablets prescribed per medication item for participants from general practices that used the EMIS Web IT system were used to inform the quantity of tablets prescribed per medication item for the practices that used the SystmOne IT system. It was not possible to extract this information from the SystmOne practices.
Appendix 3 Theme 1a: kinds of lower urinary tract symptoms – further accounts
Nocturia
I was having to get up more frequently during the night, regularly three times, and if I had coffee or a small glass of alcohol, it could easily be five.
IG6, age 78 years
The main one was having to get up three or four times during the night to go to the toilet.
IG7, age 54 years
Because I was kept awake at night … it went to four or five times …
FG3, age 81 years
… the main problem is … I go to the toilet probably three times in a night, which affects sleep. That’s the main thing really, that’s why I went.
FG6, age 60 years
‘Voiding’ issues
I get frustrated when I haven’t weed enough. I stop and then I have to come back a few minutes later and go through the process again … It’s incredibly painful in the morning, if I don’t get up and empty my bladder. It’s extremely painful. What I do get is frustration that I haven’t done it, I haven’t emptied, because I want to get on … I want to get on and do other stuff and I keep having to come back and … so it’s the time really.
CG1, age 58 years
… part of the problem, apart from the experience at night-time, there is also slow flow and once I get it started, it’s very slow and it takes an awful long time to finish … You spend a long time standing there.
IG2, age 69 years
I did a flow test which did indeed show I have a very poor stream. I had drunk a lot of water and my bladder was quite overfull and it took me quite a long time to empty my bladder and I had a residual volume of around 250 ml, so clearly there is a bit of an issue …
IG5, age 53 years
It was basically the fact I started to get reduced flow and difficulty urinating and a sense of urgency as well all the time, and I was living with this feeling of always needing to go to the toilet, but then going to the toilet and not being able to pass water …
FG2, age 52 years
There is an urgency when I do need to go to the loo. When I get there I can hardly go … Prior to having that examination they’d also sent me to another urologist who did a flow thing and examined by ultrasound my bladder and that’s when it became apparent that I wasn’t emptying my bladder.
CG2, age 62 years
So, I’ll wee and then I’ll know that there’s more to come, but it’s not easy to make it happen. So, I’ll stick around. I’ll try different positions, standing up, sitting down, and seeing if either encourages it. Then usually I can get another dribble out, and I think ‘ah that’s it’, but I never quite feel completely sort of liberated from … There is always a slight sense that there’s something still there.
FG8, age 69 years
‘Storage’ issues
That’s right yeah. I mean, you know, it comes to a point where the length of walk you take with your dog depends on how your bladder can hold up … I think the worst bit is bed-wetting … Also there was one incident when I actually couldn’t control my bladder and when I got back to my car after a walk and sat down and it just flooded out.
CG2, age 62 years
… it was a case of having to rush to the loo quite quickly and occasionally I had a little bit of leakage …
FG1, age 71 years
I couldn’t hold my bladder basically and I was urinating upwards of 20 times a day and just as many during the night as well … When it was at its worse last year, which is when I went back to the doctors, I would be crouched down and I would pass a little basically.
CG3, age 42 years (diagnosed with bladder sensitivity)
Well basically I noticed I was getting up more and more at night and I was sort of – as time went on I also realised I actually have to plan my journeys to make sure how things go. It just crept up and if I – in my job I used to be in meetings a lot, I found I had to excuse myself quite a lot and it got worse and worse and worse … And I went through a phase where I have gone from just it being an annoyance, to when it was at its very worst I counted a couple of nights when I was waking up within an hour of the last time.
IG27, age 68 years
‘Post-voiding dribble’: accounts of a small wet patch, often once dressed, just after urinating
… I got to a point where, after shaking, no matter how much I shook, you know, I suddenly realised there’s a slight dampness there and I realised I was leaking …
IG27, age 68 years
It’s just a slight leakage after I’ve peed. It’s not at any random time, it doesn’t happen when I’m at work or you know … not consistently anyway, it’s mainly at night when I’ve peed in the night and gone back to bed and they’ll be a slight leakage afterwards.
CG14, age 62 years
After I went to have a wee I was having leakage afterwards …
IG11, age 76 years
… when I’ve finished urinating and then I quite often have a leakage afterwards … It’s as if what I’ve released from the bladder has gone down as far as the prostate and what is distal to the prostate has gone out, but there is a dead leg between the bladder sphincter and prostate, which occasionally will leak after urinating.
CG5, age 66 years
Appendix 4 Theme 1b: experiences of lower urinary tract symptoms – further accounts
Tiredness and distress from sleep disturbance
Well, sleep is the one, because it’s every night. I rarely get more than sort of 4 hours’ sleep generally. When I do go to bed I’m usually awake, or up within 4, 4 and a half hours, sometimes less, but that’s the general. Then I’m up and need to urinate … so it does have effects on my patterns of sleep, and my amount to sleep. Luckily, I’m not working now. I mean I can work around it. It would have been more of a problem when I was in full-time work … because of the tiredness.
FG5, age 56 years
I was angry, because I wasn’t getting any sleep … I say ‘angry’, that’s not fair, but I was … oh talk about someone being in a bad mood … permanently, because of lack of sleep.
CG9, age 53 years
I went through a phase where I have gone from just it being an annoyance, to when it was at its very worst … I was waking up within an hour of the last time. I just wasn’t getting any sleep because I kept getting up to wee … I wee and then I go back to bed and I want to wee again.
IG27, age 68 years
Up to four times a night … Generally, throughout the day at work, I’m either yawning or stretching and I just – it’s as simple as that. I’m not falling asleep or anything. I’m just yawning or I’m stretching most of the time!
FG14, age 58 years
… Then I was unbearable at work. I was just getting no sleep at all …
CG9, age 53 years
Stress, anxiety and fear
Logistical anxieties (the need for careful planning around journeys/trips/activities away from home, which must be planned around regular stops, or which can make travelling in groups particularly difficult, and can also make trips to new places, where toilet locations are not known/may not be readily available, somewhat stressful):
In a way it does control the way I live and same as if I, I recently went on a long train journey from [city] to [city]. Fortunately, I was able to control my bladder long enough, I didn’t drink very much because to get to the loo on the train was not easy but it’s what I have to do. I don’t see any other way of doing it and I think it’s something that I worry about, I think about all the time. It’s the first thing I think about when I go anywhere, where’s the nearest toilet, always where’s the nearest toilet and how long it’s going to take me to get there.
CG10, age 54 years
Interviewer: What did it [the closure of public toilets in city centres, which the interviewee raised as an issue] mean to you personally?
CG13, age 70 years: Well that I couldn’t go out. The last time I went into [city shopping centre] I had to plan it mentally in advance, because I knew I was going to have to … I can tell you the story if you like [laughs].
Interviewer: Please do.
CG13: [City shopping centre] is a nightmare, isn’t it? So, they’ve been shutting all the loos, this is going back a couple of years ago when they started it. I had to go to see the [brand of bank] bank, which is right bang in the middle of [city shopping centre], and I knew I was going to have a problem. I managed to park my car in the multistorey opposite [high street shop], and there’s no loos in there. I ended up finding a dark corner and going, because by the time I’d left home and drove into town, which is 20 minutes, I need to go. So, I ended up going in the corner in this car park.
Anxieties around seated social activities/exacerbation of retention (when there may be a long wait between opportunities to use a toilet, or when using the toilet frequently may be awkward, be noticeable and involve disturbing others, e.g. the cinema, theatre, long journeys):
Yeah, I go to football matches and there, ’cause it’s 90 minutes, you get up at half-time, you can, but I don’t want to be getting up in the middle of the match. So I avoid drinking to any great amount before I go to a match, for that reason, ’cause that is awkward, if you’ve got to get past people and they’re watching a match …
IG15, age 75 years
… it got to a point where if I was travelling and particularly doing some work in [another country, in remote locations, with limited medical facilities, there was a] slight concern that actually if your bladder gets too full, it can actually get very difficult to get started, so my concern particularly was around the risk of going into acute retention when I was travelling. I had just been to [another country] and had a bit of a difficult trip driving back [from a remote place] where we stopped in a service station and I was absolutely desperate to go and it took me about 5 minutes before I could start going, so I think that was probably the trigger that caused me concern, because I thought ‘heck, could I be going into acute retention here?’
IG3, age 53 years (a man with clinical training in urological function/general practice medicine)
Anxieties around social or public engagements (where leakages could occur and be evident, when toilets cannot be reached in time or post-voiding dribble is an issue):
… a total nightmare with the wet patches, bursting to go and having to run to the loo.
IG11, age 76 years
Anxieties that symptoms will inevitably worsen and that LUTS is an indication of relentless age-related decline/loss of control:
It’s just I’m 48 now, I don’t really wanna be a 60-year-old man walking down the road with a nappy around me, that’s just me, personally … it won’t get me suicidal, but yeah, it gets me a little bit low at times thinking, ‘oh God, I’m gonna be wetting myself’ … It’s in my head is that what I’m gonna be like within the next 5, 10 years? Am I gonna have to take an extra pair of jeans with me or some extra boxer shorts with me in a little bag somewhere, a rucksack or something? Which is doable, but yeah, it’s a little bit annoying and causes stress.
CG11, age 48 years
Vicious cycles of anxiety:
… if I’ve had a drink then it starts going through my head … thinking, ‘Will I want to go to the toilet? Will I need the toilet? Is there somewhere I can go?’ Rather than putting it to the back of your mind and try and relax myself and ignore it completely so that you don’t think about that. But I think of it straight away, and that’s when it tends to play up.
CG7, age 61 years
… the actual overall feeling when you’re anxious, makes you want to go to the loo anyway. It’s the fight or flight, when you’re trying to dump as much weight as possible, isn’t it? I’m aware of all that.
CG1, age 58 years
… the thing which I’ve come to the view, but this is only my personal view, that it’s very related to how relaxed you are and all of these things where you can’t go, and you want to, are times when you’re not relaxed for some other reason, you know if I’m flying, crossing the Atlantic or something, just the whole thing about this is not relaxing, you know, you’ve worried about what it’s going to be like when you get there and etc. etc. But the, you know, going to the theatre, or a show, or something like that, that’s not relaxing because you’re worried you’re not going to disturb people and so on. And so, the view which I’ve come to, is that it’s enormously related, it’s more related to whether I’m relaxed, or not, than it is to whether I’ve had particular things to drink or not, or at least that’s what it seems to me.
IG1, age 63 years
Underlying-cause anxieties (concern that something serious might be wrong, whereby experiences of daily LUTS trigger fears, in particular of prostate or bladder cancer; notably, these kinds of underlying concerns were also a driver of GP visits among interviewed men):
My brother had similar symptoms and he had an infection, which caused the total blockage. That’s what prompted me to start thinking about it and worrying about prostate cancer and the like …
IG2, age 69 years
… it had been going on for a couple of years, but this time last year it was at its peak and it was getting obviously frustrating, so I was thinking it’s been going on for that long, I haven’t really got a bladder infection, although that is at the back of my mind, or there was something underlying, like bladder cancer basically. Something like that. You don’t like to say it, do you? So that was possibly in the back of my mind thinking it might be something like that because it has been going on for so long basically.
CG3, age 42 years
It was more the concern it was prostate cancer I was more bothered about then.
CG5, age 66 years
Embarrassment, awkwardness and shame
Stigma associated with a sense of age-related decline:
You’re getting old and you think it’s one of the signs of getting old …
FG1, age 71 years
Feeling socially uncomfortable and embarrassed (about having to use the toilet frequently, or about the appearance of a wet patch, or feeling unclean/needing to wash frequently):
It gets embarrassing when you are avoiding a drink and it starts to have a knock-on effect on me because I start to become dehydrated. Simply because I can’t control the exit of urine like I’d like to be able to do.
CG10, age 54 years
… because I basically had got to the point where I didn’t go out anymore, to the pub or anything like that, because it’s an embarrassment.
FG2, age 52 years
It was embarrassing going out and going to the loo. When you’re out and you got the wet patch.
IG11, age 76 years
… the other aspect is slight leakage … so obviously that’s, you know, can be a little bit embarrassing and mean extra showers and so on.
CG14, age 62 years
… the frequent urgency and leakage symptoms … have been restricting my lifestyle somewhat … I drive a minibus [for a local group] and it’s embarrassing when I go to pick somebody up at their house to have to ask them if I might use their loo before driving to their appointment …
CG5, age 66 years
… we have a changing room area which all the chaps get changed in and if I go to the loo and a bit conscious of it [dribbling after urinating], so I am constantly checking for that and I will wait in the toilet just to make sure that I don’t have that, because it’s something that’s obviously quite visible and you don’t really wanna be ridiculed as such in a factory situation …
CG15, age 41 years
Frustration, annoyance, disturbance, disruption and feeling down
I just get worn down with it all. I lose all kind of sort of vim and vigour and I can’t do … well it stops me from doing the things that I love to do, like going out walking … or whatever. You know, that’s a downer as well because I feel I’m sort of hemmed in. I just get fed up. Not quite suicidal but you can get a bit fed up with things. Why is it happening to me all the time?
CG2, age 62 years
Well, it’s frustrating now. That is the big thing, it’s frustrating because I want to get on and do something else. It seems to be that I have to take more time, take more care about it. Like before, you just go to wee and get on with your life, but now you have to be more conscious of it. If that’s clear to you.
CG1, age 58 years
Normalisation and discounting
I’ve been working in [another country] the last couple of years … a colleague over there said that his mother always used to say, you know, if you wake up in the morning and nothing’s hurting, you’ve died and gone to heaven, and it’s a bit like you know, with old age … you know, I’m not in a bad way.
IG1, age 63 years
I suppose I’m just conscious – whether it’s reality or more of a myth – but the idea that as you’re older, the likelihood is that you’ll need to nip out from a meeting or whatever to use the loo more so than if you were in younger years … I go to a lot of [charitable organisation] meetings and there are several men of my age, or possibly even a bit older, who it’s not at all uncommon for them to nip out of the room while the meeting is going on – not making any issue of it – but it’s pretty obvious to me. They may not have any of the similar background as me, but they’re similarly old …
CG6, age 70 years
I’ve never really consulted my GP specifically to say I’m getting up too often in the night and I’m having difficulty passing water. It’s something that I’ve noticed, obviously I’ve heard about the development of this problem in older men and that obviously creates an expectation, which means that you just realise you have to learn to live with it to some extent until I suppose it gets absolutely desperate, then you say to the doctor I’ve got to have something done, even if it’s surgery.
FG10, age 77 years
Appendix 5 Theme 1c: personal and social factors – further accounts
Contextual health issues
Many men could be characterised as ‘well or very well’:
Absolutely spot on other than high cholesterol and a reflux problem, that’s all.
IG11, age 76 years
… A few sort of niggles with the age, but I think on the whole it’s [general health] very good and I keep myself very active.
IG9, age 75 years
Health is good, the only – my only – I’m not sure how to describe it – anyway, I take an antidepressant. I take a small dosage of an antidepressant and I am doing for as long as I can remember but that’s – apart from that, yes in good health …
FG14, age 58 years
A number of men had considerable additional health issues alongside LUTS, but these were well-managed/in remission and did not stop LUTS from being a considerable additional concern/focus:
I have a long-term condition which I suppose might well affect … I don’t know whether it would affect the answers to the questions, or not. But nearly 20 years ago I was diagnosed as HIV [human immunodeficiency virus] positive, but I’ve been very successfully on treatment ever since then and have had no ill effects at all. I’ve been very fortunate …
CG6, age 70 years
Reasonable, I think, for my age. I had angina and an operation for that in 2012. It was an angioplasty and stent, which was probably the most major … I know it’s not exactly a major op[eration], but it’s probably the most major thing I’ve had in recent years.
IG6, age 78 years
I suffer sarcoidosis … I’ve got lesions in my lungs. My body thinks I’ve got a disease there and it fights against it when I’ve really not got anything wrong except these lesions … It gives me hot sweats and sometimes extreme tiredness … I was diagnosed about a year ago … I’ve got a small blockage in one of the arteries to the heart, but they’re not worried about that at all. That’s when they found I had this other issue.
IG7, age 54 years
I have a lot of arthritis issues, this is osteoarthritis not rheumatoid arthritis, my hands are basically worn out because after 50 years of working with them I’ve destroyed them!
IG5, age 66 years
I’m just recovering from non-Hodgkin lymphoma in 2016 and I had chemotherapy and radiotherapy, and during my treatment, due to muscle degradation, I ended up with a spinal compression, so I had to have a microscopic discectomy done to relieve pressure on the nerves in my back.
FG2, age 52 years
If I had to prioritise with the health issues I’ve had over the last, say, 10 years, the cancer would come top, then the knees would come next, then probably the gall bladder which I had out, then probably the urinating which comes down the list.
CG13, age 70 years
Some men had conditions that interacted with their LUTS, such as diabetes, UTIs, prostate infections, or irritable bowel syndrome, where symptoms related to these conditions were at times indistinguishable or overlapped (e.g. needing to urinate frequently, pain or sensitivity associated with excretory organs/voiding):
I’m type 1 diabetic … I guess they overlap anyway, if just slightly, you get high blood sugar then you tend to – do try and wee out the sugar, if you like, so that may lead to extra visits to get rid of that, so that’s always been something that’s happened.
CG14, age 62 years
The only thing sort of I hinted earlier, it’s not in my case, I don’t think, an isolated urine thing. I think it’s a whole package. In fact until today talking to you, it never really actually joined the dots. So, I think that’ll be useful when I go and see the doctor, I can just say that I’m having a bit of trouble with both aspects, and that might inform what they want to do.
FG8, age 69 years
Some men had more active current and/or overwhelming health issues that meant that LUTS were a peripheral concern at the time of the initial interview:
Has its ups and downs, I have just gone through a period where I have been poorly with my chest and cough, cough, cough, bringing up lots of sputum, coughing up blood at times. I have had X-rays … Had blood tests, rheumatoid arthritis, all relating to the problems I have got in my chest, bronchial, whatever it’s called, aspergillosis something like that.
IG4, age 64 years
At the moment it seems to be going downhill, generally speaking. I’ve got problems with the kidneys, the bladder. I’ve also got asthma and I’m trying to get over a recent infection … a chest infection. I’ve also had an infection in the prostate … It’s been a bit debilitating at times.
CG2, age 62 years
Life circumstances/lifestyle
Many men were married and retired, living at home with their partners; a few were similarly retired and living with their civil partners:
I’m in a civil partnership … just the two [of us at home] …. I’ve been retired for 10 years …
CG6, age 70 years
Some men were younger and working, some living with partners and with children at home, a few without children, or single, or living alone (with partners living elsewhere):
Well, I’m 46 years of age, I live on my own. I work, I run my own business, I’m [a skilled trade] … I’m single …
IG14, age 46 years
I’m single … I don’t have a family of my own, children and such. I live in [town] and I have two lodgers. I’m a [trade], self-employed.
IG13, age 52 years
There is me and my partner [name], so just the two of us … I work full time and I’m [profession].
FG14, age 58 years
Some were unable to work because of ill health/had been made redundant:
I’m 48, I live by myself … I’m not working and I’m unemployed. I suffer with mental health problems, as well … I’m not too bad at the minute, but I’ve just been having a bad time with life, so I’m on meds [medications] a lot …
CG11, age 48 years
Single … [living] … on my own. Technically speaking, I got made redundant, but that was a few years ago, and I’ve sort of been retired since most of it.
IG17, age 65 years
Many men (other than those in the midst of more severe illness) described relatively, or in some cases exceptionally, healthy, or health-conscious, lifestyles, and/or taking an active interest in exercise, diet and mental health:
We’ve gone onto the protein diet, both me and my wife, which is aiding us both in losing weight. I’ve been off alcohol now for about, I suppose, 16, 17 months, something like that, which is a change of lifestyle considering I’m ex-army … for about the last 15 years, I used to run 3 miles Monday, Wednesday and Friday until my legs gave up a bit. Now, I still do the 3 miles, but I’m walking at a fast pace. I meet up with my [nationality] lady friend halfway round and we’re both soundboards of each other’s problems and that sort of thing. It’s something I look forward to sort of clear the mind.
FG9, age 65 years
I haven’t been ill once this year, since I’ve been keeping myself fit. I’m in tip-top form, yeah. Near the start of the year I had some, I wouldn’t say mental issues, but it was just a case of fazing through giving up drinking and trying to keep fit, but I’m more determined than ever, now … as part of my training … there’s now cycling involved … there’s a lot of cardio training as well … I’m burning between 1000 and 1400 calories a day, at the moment … my diet’s changed, as well.
IG14, age 46 years
… I’m a marathon runner. I’ve half a dozen marathons under my belt. What I do is I swim, I outdoor swim, I lake swim. I’m generally fit and healthy … during the week I do watch what I eat with Spartan discipline and then at the weekend I just let myself go when I’m not working … I have 5 days’ good food and 2 days of absolute garbage. Mental health, I have ups and downs, some of the things over the years that are coming back to haunt me a little bit. I wasn’t always in the fire service, I was in the military before I joined the fire service … But I’m not scared to go to a counsellor and lie on the couch for half an hour and have a chat.
CG9, age 53 years
I’ve got weights and everything, don’t do it every single day, but yeah, I haven’t done much for a few months ’cause I’ve been low. But no, I’m not saying I’m Mr fit man but I’m quite – just try and keep in trim and that, I’m not overweight, if anything I’m skinny.
CG11, age 48 years
As well as the walking, cycling, I am also a diver, scuba diving … And I also, you know, in terms of activities and things like that, I’m still going skiing every year. So yes, I’d generally – so I’m in reasonably good shape for a 68-year-old.
IG25, age 68 years
A few men described more sedentary/less health-concerned/healthy lifestyles:
As soon as I try to do anything, I’m breathless. I find it very difficult to do anything really … I’m, overweight as well … I just stay at home.
IG10, age 71 years
History of urinary tract infections/lower urinary tract symptoms/bed-wetting
Some of the men described long histories of urinary issues; for some, these related to long-standing issues with LUTS and/or UTIs (for some these histories extended over 20–40 years). For others, there were traumatic childhood experiences of bed-wetting into late childhood/early adulthood. For some of those involved in the trial, urinary issues were a traumatic and/or debilitating lifelong experience. There is a growing literature relating to the life-course experience of urinary issues, although it is currently largely absent from the qualitative literature on LUTS experiences in adulthood: this may be a gap. The TRIUMPH qualitative study findings indicate that a history of urinary issues is prone to shaping the depth of a man’s sensitivity, embarrassment, dispiritedness and shame/stigma associated with LUTS in ways that may be linked to mental ill health in adulthood and may hamper LUTS-related help-seeking.
Men with long-term recurrent UTIs:
… I did have several bouts of a urinary infection. In fact, the very first time I had it, I didn’t think it was a urinary tract infection, actually, and it was the doctor who said, ‘No. You’ve got a urinary problem’. But that was a long, long time ago. It did seem to crop up fairly regularly …
CG6, age 70 years
Men with childhood/adolescent urinary issues:
As far as I can remember I’ve always not been very good at holding it in, if you know what I mean. Certainly, even when I was at school, I seem to remember having to leave the classroom to go. What prompted me to seek medical attention was for about 20 years I’d not had a continual night’s sleep; a normal night would be six or seven times to get up and urinate.
CG13, age 70 years
… I’d say since mid-40s and I knew I was coming of an age, so I’m trying to do what I can … ’cause I used to wet the bed as a child … I remember being about 12, 13 years old and my brother and myself, because he did it as well, basically, used to wet the bed, because my dad used to wet the bed up until he was 20 years old. We had all the alarms and all sorts of things … never understood why, all I can put it down to was muscle was a bit weak in that area.
CG11, age 48 years
Gender
Issues of gender, or ‘being a man’, were apparent, in particular when it came to men sharing their experiences, help-seeking behaviours (such as talking about LUTS with friends, family members or a GP) and the kinds of support they found helpful (e.g. structured printed material, diagrams, discussed further in relation to men’s responses to the intervention; see Chapter 5, The booklet, Booklet style and content and Booklet structure). Although some men described comfortably talking (at least with some others) about their symptoms, others identified their own and/or other men’s reluctance or embarrassment to do so as an aspect of gender:
I know a lot of men might be embarrassed to admit it [LUTS], but unfortunately that’s something that probably happens a lot and I guess a lot of men won’t talk about it.
CG15, age 41 years
I don’t think men tend to talk about that sort of thing [LUTS].
IG2, age 69 years
… well you know us blokes, we don’t like to talk about … well medically, I know I’m rabbiting on about it, we don’t normally like to talk about our medical conditions, especially when it involves our lower regions.
CG13, age 70 years
Character/personality and health-seeking behaviours
Men also varied in their health-seeking behaviours; some men described themselves as relatively proactive, independently exploring or researching their health conditions, and/or readily seeking clinical guidance; for others, thinking about or exploring health concerns, especially without clinical guidance or oversight, was seen as anxiety-inducing, and to be avoided.
Proactive/not complacent, health-information-seeking, via online/printed publications, or primary care:
… I don’t actually trawl the internet for information, but I sort of pick it up from various sources and also the rest of the media, typically newspapers. I tend to glance at least at reports on this topic and my wife might spot something and refer it and say you ought to read this if I haven’t already read it. It’s that sort of thing really. I just maintain a curiosity of that topic because I have a vested interest, simple as that really … at the back of my mind is the thought that this is something which needs to be, I need to keep under review and not become complacent about it …
FG10, age 77 years
… I don’t get advice regularly enough, but I do have annual check-ups, blood tests … I would want to keep up the regular link with the GP and their advice …
IG21, age 76 years
… they [a popular tabloid newspaper] do, 1 day a week there’s about six pages on health and I just skip read it and if it hits anything that I’m interested in, sometimes I keep it. I’ve got a file on, I call health issues, so I put it in there … you go to the doctors, there’s a notice board full of stuff, I don’t start reading all that, so there’s nothing else … but when I was prescribed these medications, I went online and looked to see what they said online about them. I read the little leaflet in the book and if you read that, sometimes that can be frightening.
CG15, age 75 years
Again, I’m not a hypochondriac, but if there’s something there, I want it checked out.
CG9, age 53 years
Reticent: anxious avoidance of seeking health-related information, either online or through primary care:
CG1, age 58 years: Yeah, I suppose in the last few years I’ve only wanted to go to a doctor if I had to … I only go if I have to …
Interviewer: Would you visit a website for that kind of information?
CG1: It’s difficult, really. Yeah.
Interviewer: Is that anxiety-related?
CG1: Yeah, oh definitely anxiety … It’s being very protective of myself. I wouldn’t put myself under any kind of stress unless I have to, so the actual thought of ‘I’ve got something else wrong with me’ and having to deal with that, it’s a trigger for stress and I don’t want that, you know?
Appendix 6 Themes 2a, 2b and 2c: visiting the general practitioner, drivers, the consultation, outcomes – further accounts
Drivers of the general practitioner visit
Symptom severity (and gradual onset/high thresholds of severity):
What prompted me to seek medical attention was [that] for about 20 years I’d not had a continual night’s sleep; a normal night would be six or seven times to get up and urinate … I would guess the reason why that happened was, because you could go back to when I very first had to get up to urinate, I don’t know how long ago that would have been, 40 years ago, and I might have had it once. Then maybe the following year I had to do it twice, and gradually it built up into I was going roughly every hour … if I suddenly went from not doing it to having to keep going every hour, I would have gone to the doctors then.
CG13, age 70 years
IG6: I was having to get up more frequently during the night, regularly three times, and if I had coffee or a small glass of alcohol, it could easily be five. I was also concerned about urgency, even having small leaks associated with urgency. Only ever small ones but, nevertheless. I did also have a problem with small leaks while I was asleep. All of those persuaded me to go and see my GP.
Interviewer: How long had those symptoms been going on for, or worsening for, before you went to see the GP?
IG6: I would guess it was probably a couple of years. It’s one of those things that gradually get worse, so at what point do you notice them, and then at what point do you decide to do something about them? I think it may even have been longer than that, come to think of it.
IG6, age 78 years
Basically I was having a few issues where at night I was going to bed and would always go to the loo before I went to bed and then I would get into bed and feel like I needed to go again, so that was one of my initial problems that I noticed and I was getting occasions where I was getting a feeling of needing to go to the loo, have a wee and basically nothing, getting nothing at all, so that was happening pretty regularly and then I was noticing after that, starting to notice that once I had been to the loo I was getting a bit of, sort of after I had finished, having a little bit of dribble, I guess you would call it, afterwards … so after all these things came up I went and saw a doctor … by the time I had plucked up the courage to go and see doctors and speak to someone, it was kind of a little bit of everything …
CG15, age 41 years
I couldn’t hold my bladder basically and I was urinating upwards of 20 times a day and just as many during the night as well … At least I would say six times normally in the night.
CG3, age 42 years
Fear of prostate cancer
CG5: … probably 20 years ago … probably in the 1990s … I had a PSA test which was normal, and I’ve had several PSA since then …
Interviewer: [What were] your symptoms … in the 1990s when you first went to the GP?
CG5: Well difficulty in passing urine and weak flow.
Interviewer: Any night-time waking?
CG5: Not then. That has become more frequent … It was more the concern it was prostate cancer I was more bothered about then. It has only recently become more of a practical problem.
CG5, age 66 years
What triggered that slightly was my brother-in-law having prostate cancer.
CG12, age 71 years
… I was a little bit concerned about it [prostate cancer] because I have some friends who have had prostate problems and it’s been very much in the news in the last few years. [Interviewer asks if this concern was mentioned to a GP] … I told him my symptoms and, you know, I’m not sure I’ve actually mentioned this, but the fact that he sent me off for a PSA test showed that he picked up on my concern.
CG14, age 62 years
After my first appointment, I was in work and when we clock in to go in work, someone had left some prostate cancer leaflets, so I just picked one up and it just happened, it was almost a bit of a coincidence, so I picked it up and had a little flick through that and that sort of brought my attention to it then, so that was after I had the initial problems.
CG15, age 41 years
I sometimes worry that it might be something like sort of cancer, which thankfully it’s proved not to be. That’s when it preys on my mind.
CG2, age 62 years
I was thinking that I’ve either got a bladder infection, although I’ve still got it and it had been going on for a couple of years, but this time last year it was at its peak and it was getting obviously frustrating, so I was thinking it’s been going on for that long, I haven’t really got a bladder infection, although that is at the back of my mind, or there was something underlying, like bladder cancer basically. Something like that. You don’t like to say it, do you? So that was possibly in the back of my mind, thinking it might be something like that because it has been going on for so long basically … [A little later in the interview] Also, my father had had prostate cancer as well. He had that about 10 years ago …
CG3, age 42 years
I’m on a register on our surgery, because on my dad’s side of the family they all died of cancer-related illnesses under the age of 65 … Yeah. Where I’m going with that is, is because my dad had prostate cancer … I keep an eye on it … Once a year I get my bloods done, and because of my general health and everything else that’s going along, they do tests, they have a little look at my bloods for everything else. They don’t just check my kidney function, they do the whole range. So, unless anything happens, if I notice blood then I would go and get it checked.
CG9, age 53 years
Basically, because a friend got prostate cancer and had similar symptoms, so I decided I’d best go and have investigation done.
FG2, age 52 years
I had a close colleague who had prostate cancer for some years and he was always worried, it’s the PSA levels, isn’t it? And I’ve had that done a couple of times …
IG1, age 63 years
… it may have been 3 years ago. My brother had similar symptoms and he had an infection, which caused the total blockage. That’s what prompted me to start thinking about it and worrying about prostate cancer and the like, but the GP has set my mind at rest. I think it’s quite unlikely that I will get prostate cancer and so I’m feeling a lot more comfortable about it nowadays.
IG2, age 69 years
Well, my dad’s got prostate cancer, yeah … I talked to him about this and he said, ‘oh, well actually, I might as well tell you now, I’ve got prostate cancer but the doctors aren’t going to do anything about it, they said I’ll outlive it, it’s very slow-growing’. So I thought yeah, and I went and got checked …
IG13, age 52 years
I’ve got five brothers, you know, there’s six of us and within the last year three of them have been diagnosed with some form of cancer … One’s in [UK country] and he’s got prostate cancer. One is in [UK city] and he’s got prostate cancer … It was a concern for me and that’s why when the first brother was diagnosed and the doctors told him, ‘if you’ve got brothers’, you know, ‘they should get checked out’. I had recently been checked in any case, but when I told the doctors about my brother and then my second brother, they’ve checked me as well ….
IG27, age 68 years
The consultation: general practitioner responses
Reassurance alone: ‘That’s what happens with old men’
It’s something I’ve more or less learnt to live with, ’cause it doesn’t seriously disrupt or interfere with my quality of life I would say. I mean, it would be nice if I didn’t have these symptoms, but it’s not something that I think I would want any kind of invasive treatment for … as far as he could tell from the rectal examination, there was nothing sinister and that my prostate was enlarged but that was to be expected for someone of my age.
FG10, age 77 years
Well, the first time I mentioned it was some years ago as adjunct to something else I was at the doctor’s for. I can’t even remember what. He must have said, you know, ‘Everything else alright?’ and I said ‘Well I’ve got this thing’ and he did a prostate exam and said it wasn’t massively enlarged and wait to see what happened, and frankly I kind of put it down after that to a sort of – well you grow old and this is gonna happen.
CG8, age 65 years
I don’t remember it [the first LUTS-focused GP consultation] specifically, but it would have been along the lines of ‘I keep having to go to the toilet and I wonder why; do I have anything wrong with my prostate?’ It would have been along those lines. And then, as a result of that, I think the first time I had a prostate examination and a PSA test and then when that was reviewed it was, ‘There doesn’t seem to be a problem’. So that would have been roughly how the first one went.
IG28, age 76 years
They [the GP] did some blood tests on me and they said ‘Yeah, you’re coming to the age where … it’s something quite common for the men of your age’ so that was probably the end of the conversation.
FG13, age 59 years
Physical examinations, routine tests, referrals, diagnoses, treatments
FG11, age 73 years: … eventually I went to the doctor, the GP, and that set off a test for prostate cancer, so I went through all the regime for that. Fortunately, all came back negative.
Interviewer: Were you concerned about prostate cancer before, was that in your mind already or did the GP sort of bring that up in the context of your symptoms?
FG11: The GP sort of felt an enlarged prostate and that, in turn, triggered the specialist and so on … If I remember right, there was somebody else in the golf club who was having the same symptoms as me, he said something like ‘Oh I’ve been given some tablets by the doctor, it’s made all the difference’ so that sort of … Based on that, the GP came up with the tablets … and they miraculously have made, you know, complete difference to these situations.
Interviewer: Could you give me the drug name again?
FG11: Yes, I’ll spell it: s-o-l-i-f-e-n-a-c-i-n succinate.
(This patient later explained that, alongside his digital rectal examination, his GP had undertaken a PSA test and the result was ‘high’, possibly due to an untreated recent UTI, hence his referral to urology. This man was satisfied with improvements he related to medication.)
CG1, age 58 years: About 3 years ago, I would say …
Interviewer: The doctor gave you a physical check and also blood tests? Do you remember what kind of check-ups you had at that time?
CG1: Physical check yes, but I … I may have had blood tests, I’m not quite certain …
Interviewer: Yeah. OK. You were then put straight on tamsulosin, or was that …
CG1: Yes. Straight on tamsulosin.
Interviewer: And you’ve stayed on that since then?
CG1: Yes. Yes.
Interviewer: OK. Have there been any further follow-up with your GP? Have you had any additional …?
CG1: No, not really. They have started medicine reviews [related to two other unrelated conditions], but no discussion about that at all.
Interviewer: In the med[ication]s review, has the medication in relation to your urinary tract, has it stayed the same or gone down?
CG1: It’s stayed the same.
(This patient originally visited his GP because of blood in his urine. He describes enduring mild LUTS, including night-waking and frequency/sensation of not emptying. This man continued to take medication without review of LUTS, and despite ongoing symptoms.)
Symptom explanations, self-management advice/guidance
Interviewer: Before you came into the study had anyone talked about self-help guidance?
FG13, age 59 years: … No. No.
Interviewer: Not fluid intake or pelvic floor or anything like that?
FG13: Nothing.
Interviewer: That first consultation – did the GP talk to you about any other kinds of approaches beyond medication that you might use?
FG14, age 58 years: The other thing that she mentioned is that she suggested that I drink quite a lot of tea and I think I told her that. She said that if you start drinking decaffeinated tea, that will probably reduce the amount of times that you have to go to the loo.
FG2: … He did the old blood test and that was all normal and then I went to urology and I had the usual examinations and also they did a thing where they filled my bladder with tubes and everything and video X-ray to see if my bladder was filling and emptying properly.
Interviewer: What did they tell you?
FG2: … they said maybe I had overactive bladder syndrome and with that I was sent away to say you’ve got overactive bladder syndrome and here’s a pamphlet.
Interviewer: OK, and what information were you given then about overactive bladder syndrome and how to deal with it?
FG2: Basically, it was just a leaflet that explained what overactive bladder syndrome was and you should start looking at your diet and fluid intake and just managing it that way and it was very vague really.
FG2, age 52 years
I hadn’t actually really talked to the GP about symptoms beforehand … Well, that’s not quite true. When I went to the GP it was more concerned about the prostate and thinking that was what was causing the problem. But I suspect that’s why I then started on the finasteride, but as far as I can remember the doctor didn’t say anything about trying to do pelvic floor exercises at that time.
FG1, age 71 years
The ‘PSA test loop’
The following lengthy extract is from an interview with a 75-year-old man with an IPSS of 24 (symptom severity grade 3, ‘most severe’). The passage opens with a description of his symptoms, from the early part of the interview, and then moves on to a later section of the interview, focused on his historical experience of GP consultations and treatment. It provides a clear depiction of what we have called the ‘PSA test loop’:
Interviewer: … moving on to your lower urinary tract symptoms, when did those first start to appear and what kinds of symptoms have you experienced?
Interviewee: About maybe as long as 5 years ago the frequency and volume of urine started – the volume started to decrease, the frequency started to increase. Over that period it has progressively got worse and as about 6 months to a year ago, it was inconvenient at night. I would be going to the toilet maybe two or three times in the night and just small volumes and during the day as well, not quite as frequent during the day but reasonably frequent with great urgency … because of the frequency and all that, I’ve had a number of PSA tests, probably four in the last 5 or 6 years and a couple of discussions and then the prostate examinations up the back passage probably, maybe three or four times.
[Later in the interview.]
Interviewer: … I just want to go back to your first visit to the GP about these symptoms, if you can remember that, because it’s a while back now. Because you mentioned that you’d been several – you’ve had several PSA tests and several prostate examinations, manual examinations. And I just want to get a little picture of that process because you’ve summarised it, but I’m interested in a bit more of the detail. So, when you first went what was that – do you remember that - the first time you spoke to a GP about your lower urinary tract symptoms?
Interviewee: No, but it would have been – I don’t remember it specifically, but it would have been along the lines of ‘I keep having to go to the toilet and I wonder why; do I have anything wrong with my prostate?’, it would have been along those lines. And then, as a result of that, I think the first time I had a prostate examination and a PSA test and then when that was reviewed it was, there doesn’t seem to be a problem. So that would have been roughly how the first one went.
Interviewer: And that was roughly how long ago?
Interviewee: I reckon 5 years ago.
Interviewer: Five years ago, OK. And what took you to the GP that first time, was it because the symptoms were bothering you or was it more because you were worried that they might be a sign of prostate cancer, what was the ….
Interviewee: Both.
Interviewer: Both, OK.
Interviewee: Both, the first thing I noticed is the frequency of going and then that leads to the realisation that it could be your prostate is now enlarged, pressing on your bladder, etc. So one leads to the other, if you see my point.
Interviewee: Yes, so your GP took that concern seriously it sounds like and gave you a PSA test and gave you a physical examination. What about dealing with the symptoms themselves once you’d established that the PSA was in a normal range and there was no sign as far as the manual investigation …?
Interviewee: No further action at all.
Interviewer: So you didn’t start medication?
Interviewee: No, none at all.
Interviewer: You didn’t talk about lifestyle changes?
Interviewee: None at all.
Interviewer: No discussion of pelvic floor exercises … or fluid intake?
Interviewee: No none at all.
Interviewer: OK. And then …
Interviewee: Didn’t mention it at all.
Interviewer: Nothing like that OK.
Interviewee: And subsequently it’s not really been mentioned at all in other PSA tests and examinations.
Interviewer: OK, so now just describe that process to me, that was 5 years ago and you said in the last 6 to 12 months things worsened, is that correct, about 12 months ago?
Interviewee: Probably started worsening 12 months ago, yes 6 to 12 months ago.
Interviewer: And before that, so between 5 years and 12 months if that makes sense, what was the – how often did you go back to the GP with these symptoms?
Interviewee: At least twice and probably three times, it always resulted in a PSA test and at least twice another prostate examination.
Interviewer: OK, so every time you went round this loop of PSA testing and prostate examination. But in none of those times was there a discussion of how you might alleviate your symptoms through lifestyle changes?
Interviewee: No none at all. And one of the reasons is because you were so, well you’re not anxious when you’ve had a PSA test, I know the PSA test is not 100% but when you’ve had it and it shows a low reading and a prostate examination says it’s OK, there’s a feeling of relief, ‘oh that’s alright I’ll move on’. And the symptoms themselves tend to be put into the background. I don’t know if you follow what I’m trying to say?
Outcomes and satisfaction (including knowledge, awareness and understanding of recommended self-help guidance)
There follows a long extract (edited down in places) from an interview with a 48-year-old man with indications of enduring undiagnosed post-micturition dribble, which seems to have slipped through both GP and urological assessment, without appropriate self-management guidance (e.g. the urethral bulb massaging technique) being offered:
Interviewer: So tell me what, have you been to the GP about these symptoms? Did you ever go and see someone about them?
Interviewee: Yeah, that’s why they got in contact with – I spoke to the doctor and then, I don’t know, you guys got involved and then, yeah, that’s it, yeah. I’ve been to my doctor; he doesn’t really do anything for me, per se …
Interviewer: Let me just understand what your GP, when you first went to the GP to talk about this, what did you say? How did you describe it to the GP?
Interviewee: I just said I keep peeing a lot and do the dribble, wait, do myself up and then, moving a few feet of leaving the toilet or a minute and a half after finishing, stuff comes out.
Interviewer: What did he say to you, or she say to you?
Interviewee: They said they’ll do some tests on me … the doctor gave me a … test … and found blood in my urine.
Interviewer: So you did a urine sample and there was blood in your urine, is that right?
Interviewee: Yeah, yeah, there was, yeah. That was ages ago, this was when they first got in contact with me. But that’s all been sorted out, I’m fine on that. But I still have these, I still have, you know, when I go to … toilet I can guarantee, within 3 seconds after doing myself up … some more is gonna come out.
Interviewer: Yeah, so those symptoms have not changed, at all, that’s not been addressed, at all?
Interviewee: No, no, not really … I haven’t really got worse and haven’t got any better, it’s the same …
Interviewer: Just tell me a bit more about the [time] you had blood in your urine, what was the treatment for that? What happened?
Interviewee: Nothing, ’cause it didn’t come to anything, I can’t remember, it was a long time ago now.
Interviewer: When you say a long time ago, years ago?
Interviewee: Oh, they sent me to [local hospital] and I’ve had a thing put down me, a camera down my … down my penis …
Interviewer: Yeah and so they did some investigation, they used actually a camera?
Interviewee: Yeah, did some investigation … couldn’t find anything, I’m all clear.
Interviewer: Did they do a digital rectal examination where … that’s a finger in your bum, is probably the straightforward way of describing it?
Interviewee: I think they did, I think they did.
Interviewer: Did they do a PSA test? Does that sound familiar …?
Interviewee: Yeah, yeah, yeah, they were concerned that, ’cause of the blood, they were concerned I might have prostate cancer, but I didn’t.
Interviewer: That was all ruled out as fine?
Interviewee: Yeah, everything was all ruled out.
Interviewer: OK, so, at that time, did anyone talk to you about self-help or lifestyle approaches?
Interviewee: No, no, no …
[A little later in the interview.]
Interviewee: I’m still having – I’m not banging my head or gonna commit suicide about it, but it does bother me a little bit, sometimes I have to change underwear a good two, three times a day, possibly. I don’t need nappies per se, as such, yet, but no, it can be a bit annoying at times.
Interviewer: Yeah, no, I understand that and has anyone ever tried to describe any techniques for dealing with that?
Interviewee: No, you’re the first person, we’ve had the longest conversation, other than when – like with the urine in my blood and stuff, this is the longest conversation so far.
Interviewer: Did anyone talk with you about water, the amount of caffeine that you’re drinking, the amount of drinks you’re drinking?
Interviewee: No, no, I mentioned in one of the forms, I said I do drink a lot of tea, I put my hand up to that. So I know tea can make you go to, caffeine and that can make you go to the loo. So no, no one, you’re the first person, no one talks about any symptoms, any tricks, nothing.
Interviewer: OK, so you went to the GP, you got no advice about self-help or guidance or lifestyle guidance.
Interviewee: No, not really.
Interviewer: But you did get referred on because of blood in your urine and you did have a lot of tests to rule out prostate cancer and anything that might be more concerning. But, as part of that, you didn’t have a conversation about lifestyle stuff?
Interviewee: No.
Interviewer: Like drinks or exercise or anything like that, that might be – tea or coffee?
Interviewee: No, no, no, I don’t do exercise these days myself.
Interviewer: Alcohol?
Interviewee: But no, no one gave me any information on that.
[Later in the interview.]
Interviewer: What kind of support would you like in relation to these symptoms?
Interviewee: I don’t know, I don’t know if there is support out there, so I don’t know. I talk to my Mum about it from time to time, but yeah, she don’t wanna – she’s older than me, she don’t wanna be listening to blokes talking about urine and stuff. So yeah, I’ve got no help, really, as such, of what to do, just get on with it.
Interviewer: But what would be helpful …?
Interviewee: Yeah, maybe, well, I can’t really see, I don’t know, talking, if they can get some other techniques might help. But I don’t know, I can’t – I don’t really know what help for the symptoms I can get … Yeah, but no, like someone talking to me about lifestyle changes, yeah, so even if it was just on the phone …
Interviewer: In terms of receiving a booklet, like written information?
Interviewee: Yeah, something like – I know I’ve said I suffer dyslexia, but I’m not like a total dingbat. So yeah, a little pamphlet, little tiny book or, yeah, to me, I would have been happy to have something like that, like a little bible thing, you know, you can refer to it, OK, I’ve tried that technique, cutting down my tea, it’s still not working.
Appendix 7 Theme 4: experiences of the intervention
Receptive men
I thought that taking part in it, I might find out something from it which might help me.
FG10, age 77 years
The main reason was I wanted to try and do something to help myself, but also it was explained to me that if the advice in the booklet worked, then potentially it could help a lot of other men as well. I thought not only am I going to help myself, but I may be able to help other people as well.
FG14, age 58 years
I thought it was a good thing to do frankly, I’m fairly public-spirited and I don’t mind taking part in trials, I thought well, you know, I do have a problem, maybe they can show me something I can control it with.
IG29, age 85 years
Unreceptive men
I did read those. All the stuff that’s been sent to me, I’ve read it through once. But nothing really was earth-shattering. I didn’t think, ‘Oh my gosh …’.
IG24, age 73 years (IG24 said he would be unlikely to keep hold of the booklet)
The booklet
I just really appreciate the fact that being given the booklet and being explained and chatted through it with the nurse and everything, it then gave me the motivation to actually start trying things properly and to try it all together and the fact that then it’s made a big improvement.
FG2, age 52 years
Very good, very positive. They gave me a booklet to read through which I’ve read … been trying to practise …
FG1, age 71 years
I think the book was very helpful. When I tried before [pelvic floor exercises], I was relying on the GP’s description, and I don’t know whether I was doing it right or not. By reading the book, I was able to have confidence that I was doing it right. That really is one difference.
IG6, age 78 years
… it’s all very well explained, I quite liked the way it’s put together.
IG5, age 66 years
… I’m a practical person and so if – when I had an interview at the GP surgery to go through the booklet and the sections of the booklet that might be useful to me, and so basically, I tried those to see whether anything would work for me. I think some of the ideas in the book did work for me.
FG14, age 58 years
I found it a real eye-opener to be honest, reading that booklet … I thought it was absolutely brilliant … it was absolutely brilliant. Perhaps they ought to put them on display in the doctors, you know; a booklet to help yourself.
IG11, age 76 years
List of abbreviations
- A&E
- accident and emergency
- B-IPQ
- Brief Illness Perception Questionnaire
- BPH
- benign prostatic hyperplasia
- BTC
- Bristol Trials Centre
- CACE
- complier-average causal effect
- CCG
- Clinical Commissioning Group
- CG
- control group
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRN
- Clinical Research Network
- DI
- declining interviewee
- EAU
- European Association of Urology
- eGFR
- estimated glomerular filtration rate
- EMR
- electronic medical record
- EOI
- expression of interest
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FG
- feasibility group
- GP
- general practitioner
- HCA
- healthcare assistant
- HCP
- healthcare professional
- ICC
- intracluster correlation coefficient
- ICIQ
- International Consultation on Incontinence Questionnaire
- ICIQ-UI-SF
- International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form
- IG
- intervention group
- IMD
- Index of Multiple Deprivation
- INMB
- incremental net monetary benefit
- IPSS
- International Prostate Symptom Score
- IPSS-QoL
- International Prostate Symptom Score-Quality of Life Index
- IQR
- interquartile range
- IT
- information technology
- ITT
- intention to treat
- LSOA
- lower-layer super output area
- LUTS
- lower urinary tract symptoms
- MICE
- multiple imputation by chained equations
- MLM
- multilevel model
- MSOA
- middle-layer super output area
- NICE
- National Institute for Health and Care Excellence
- PPI
- patient and public involvement
- PSA
- prostate-specific antigen
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SUR
- seemingly unrelated regression
- TRIUMPH
- TReatIng Urinary symptoms in Men in Primary Health care using non-pharmacological and non-surgical interventions
- UTI
- urinary tract infection
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/GVBC3182).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
