Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 03/14/03. The contractual start date was in December 2004. The draft report began editorial review in March 2010 and was accepted for publication in August 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2011. This work was produced by Glazener et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
In 2003, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme called for a randomised controlled trial (RCT) to determine the effectiveness and cost-effectiveness of pelvic floor muscle exercises, with and without biofeedback, for men with urinary incontinence at 6 weeks after prostate surgery. This report describes the research that was commissioned.
The Men After Prostate Surgery (MAPS) study was a major multicentre UK trial that aimed to establish whether a structured programme of conservative physical treatment, delivered personally by a trained health professional (therapist), resulted in better urinary and other outcomes compared with standard management with no professionally delivered pelvic floor muscle exercise regimen in men who were incontinent after prostate surgery. Men having (1) transurethral resection of the prostate (TURP) for benign prostatic hypertrophy and (2) radical prostatectomy for prostate cancer were randomised independently in separate trials but using common outcome measures.
Description of the underlying health problem
Urinary incontinence after prostate surgery and effect on well-being
Urinary incontinence is a debilitating condition that can be an iatrogenic consequence of prostate surgery. 1 The effect of urinary incontinence on quality of life can be profound. The economic costs can be personal (such as the need to use pads or devices and the deleterious effect on quality of life) and societal (use of health services and the need for residential or nursing home care). The effect on quality of life of urinary incontinence is greater than that of erectile dysfunction, another possible iatrogenic consequence of prostate surgery. 2
Continence mechanisms in men
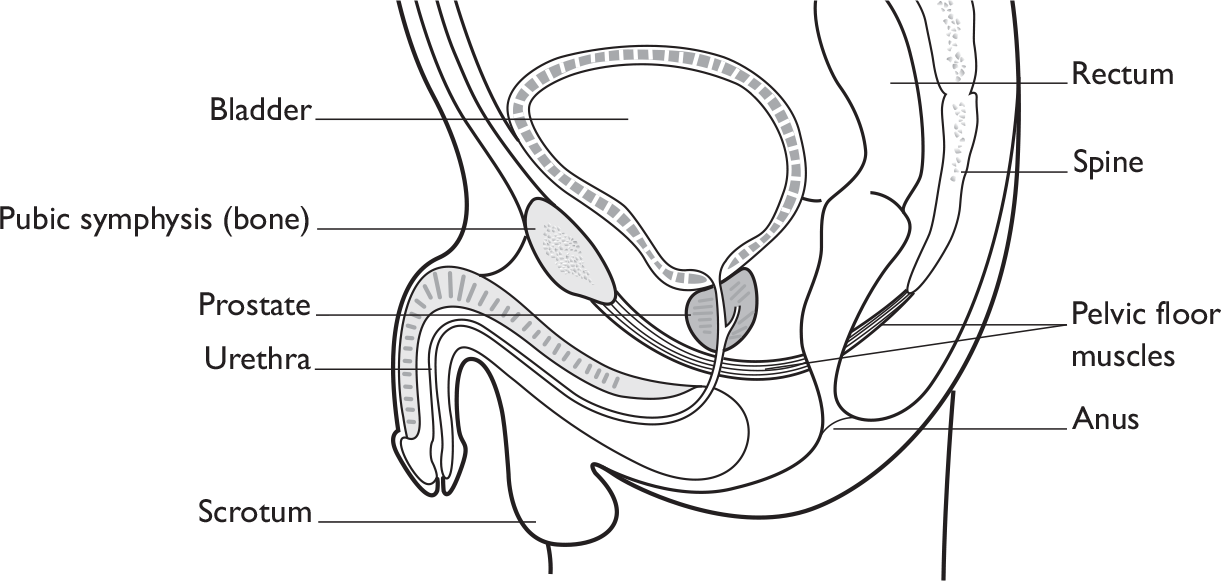
Urinary continence in men is achieved by the interaction of anatomical structures (bladder, urinary sphincter, urethra and the pelvic floor muscles; Figure 1) and neurological control. Continence is maintained by contraction of the sphincter and pelvic floor muscles and relaxation of the bladder muscle (detrusor muscle), while controlled, appropriate urination requires the relaxation of the sphincter and pelvic floor muscles at the same time as the bladder muscle contracts. This process is under neurological control. Failure in either muscle or neurological function or both will result in incontinence or urinary retention.
The male urinary sphincter may be divided into two functionally separate units, the proximal sphincter (nearest the bladder, consisting of the bladder neck, prostate and the portion of the urethra that passes through the prostate) and the distal sphincter (further away from the bladder, just below the prostate at the level of the pelvic floor muscles). The pelvic floor muscles contribute to the ability of the distal sphincter to keep the urethra closed.
Radical prostatectomy physically disrupts the integrity of both the muscles and the nerves, thus resulting in urinary incontinence. The proximal sphincter is removed during prostatectomy, and may also be damaged by radiation to the prostate. After prostatectomy, men achieve continence using the distal sphincter and the pelvic floor muscles that surround it. Thus, following prostatectomy, continence depends on the distal sphincter mechanism, which includes soft tissue supporting structures, the muscles of both the sphincter and the pelvic floor, and their intact innervation (both autonomic via the pelvic nerve and somatic via the pudendal nerve). In addition, the disruption of the nerve supply to the penis can interfere with normal erectile and hence sexual function.
Transurethral surgery for benign prostatic hypertrophy, while theoretically not disrupting the distal sphincter or the nerve supply to the pelvic floor muscles, does remove the proximal urethral sphincter. Such sphincter injury can result in incontinence.
Thus, urinary incontinence can be an iatrogenic outcome of prostate surgery. However, incontinence may also result from bladder dysfunction, which may persist from before surgery or be of new onset. Before surgery, men with benign prostatic hypertrophy have difficulty in emptying their bladders owing to bladder outlet obstruction. This may manifest as the lower urinary tract symptoms of frequency, urgency and urgency urinary incontinence. Detrusor overactivity may be demonstrated by urodynamic studies. While these symptoms are relieved by prostatectomy in over 75% of men,3,4 residual overactivity or incontinence may be accounted for by a variety of mechanisms, including persistent obstruction (38%), impaired contractility of the bladder detrusor muscle (25%) and sphincter deficiency (8%). 3
For a detailed description of the continence mechanisms and incontinence resulting from prostatic disease and its treatment see Koelbl et al.,5 pp. 299–308.
Definition of urinary incontinence
The MAPS study has used methods, definitions and units that conform to the standards jointly recommended by the International Continence Society and the International Urogynaecology Association, except where specifically noted. 6 These replace those formerly in use. 7
Urinary incontinence is defined as the ‘complaint of involuntary loss of urine’. 6 This can be subcategorised as follows:
Stress urinary incontinence (SUI) Complaint of involuntary loss of urine on effort or physical exertion (e.g. sporting activities) or on sneezing or coughing.
Urgency urinary incontinence (UUI) Complaint of involuntary loss of urine associated with urgency.
Mixed urinary incontinence (MUI) Complaint of involuntary loss of urine associated with urgency and also with effort or physical exertion or on sneezing or coughing.
Postmicturition leakage Complaint of a further involuntary passage of urine following the completion of micturition.
Urgency Complaint of a sudden compelling desire to pass urine that is difficult to defer.
In the MAPS study these were differentiated according to the men’s responses to questionnaires. Men could also categorise their incontinence as ‘other’ if they were incontinent under other circumstances, but we did not ask for clarification of the type of incontinence.
Incontinence can be further categorised according to the results of urodynamic studies (cystometry). These are:
Urodynamic stress incontinence (USI) Involuntary leakage of urine during filling cystometry, associated with increased abdominal pressure, in the absence of a detrusor contraction.
Detrusor overactivity (DO) Involuntary detrusor contractions occurring during filling cystometry. The symptoms of urgency and/or urgency incontinence may or may not occur.
However, we did not require the type of incontinence to be defined using urodynamics in MAPS.
Prevalence and natural history
The prevalence of urinary incontinence after radical prostatectomy is widely reported, ranging from 2% to 60%, albeit at varying times after operation. 8 The wide range in estimates of the incidence of postprostatectomy urinary incontinence may be explained by factors such as differences in populations, type of study, type of operation, definition of incontinence and time of assessment relative to surgery. Estimates of incontinence soon after radical operation are much higher (e.g. 82% in 1013 men9). The technique of radical prostatectomy also affects continence rates: the perineal approach,1 use of laser,10 preservation of the neurovascular bundle11 and bladder neck preservation12 have all been shown to be associated with lower urinary incontinence rates. The incidence also varies according to who measures it: doctors may underestimate urinary incontinence rates by as much as 75%. 13 The incidence of urinary incontinence decreases with time, and seems to plateau at 1 to 2 years after surgery,14 emphasising the need for long-term follow-up. 8 Other factors sometimes associated with postprostatectomy urinary incontinence include older age, previous TURP, preoperative lower urinary tract symptoms, obesity, clinical stage, race and ethnic differences. 8
In contrast, the prevalence of urinary incontinence after TURP is less widely reported. Based on a population audit of over 3000 men, an estimated 11% needed to use pads at 3 months after endoscopic resection of the prostate. 15
Significance in terms of ill health
Extent of problem in the UK
The number of men undergoing surgery for prostate disease is changing: in 2000–1, the number of TURPs in NHS hospitals in England was just under 30,000, while there were about 2000 open excisions of prostate (of which the majority would have been for prostate cancer). 16 By 2008–9, the number of TURPs had fallen to just over 25,000 (of which 2700 were with laser), while over 4000 open operations were performed. Thus, prostate surgery and its sequelae represent a considerable use of health resources and a health burden to men.
Description of standard management
Existing guidelines
For men who have undergone prostate cancer treatment, the current National Institute for Health and Clinical Excellence (NICE) guidelines acknowledge that urinary incontinence has been reported as a result, most especially stress incontinence (which is mentioned as either temporary or permanent). NICE highlights that incontinence may be a problem after brachytherapy and external beam radiotherapy, as well as in those men who have also had a TURP.
NICE guidelines highlight some of the treatments available to men, including physical (pelvic floor muscle re-education, bladder retraining), medical (drug therapy) or surgical (injection of bulking agents, artificial urinary sphincters or perineal sling) interventions, and they give the following recommendations for urinary incontinence management following prostate cancer treatment. 17
Current recommendations from NICE
-
Men experiencing troublesome urinary symptoms before treatment (of their prostate problem) should be offered a urological assessment.
-
Men undergoing treatment for prostate cancer should be warned of the likely effects of the treatment on their urinary function.
-
Health-care professionals should ensure that men with troublesome urinary symptoms after treatment should have access to specialist continence services for assessment, diagnosis and conservative treatment. This may include coping strategies, along with pelvic floor muscle re-education, bladder retraining and pharmacotherapy.
-
Health-care professionals should refer men with intractable stress incontinence to a specialist surgeon for consideration of an artificial urinary sphincter.
-
The injection of bulking agents into the distal urinary sphincter is not recommended to treat stress incontinence after prostate surgery in men.
No guidelines could be found for the treatment of urinary incontinence associated with either benign prostatic hypertrophy or TURP.
Treatment options
Treatment options for men with urinary incontinence after prostate surgery include:
-
containment using continence products, including absorbent products, sheaths, urine drainage bags, mechanical devices such as penile occlusive devices or clamps, and catheters (see Cottenden et al. 18 for a comprehensive review)
-
conservative options such as advice to modify lifestyle factors and pelvic floor muscle training (PFMT) (see Hay-Smith et al. 19 for a comprehensive review)
-
surgery using injectable urethral bulking agents, a male sling, an adjustable balloon device or an artificial urinary sphincter or, as a last resort, creation of a catheterisable continent stoma by bladder neck closure or urinary diversion into a rectal reservoir or ileocaecal pouch with a catheterisable stoma (see Herschorn et al. 20 for a comprehensive review).
However, few of these options are supported by reliable research evidence.
The decision to test conservative treatment
One of these options, PFMT, was the subject of a Cochrane review first published in 1999. 21 The review found that, although conservative treatment based on PFMT is offered to men with urinary incontinence after either type of prostate surgery, there was insufficient evidence to evaluate its effectiveness, cost-effectiveness and effect on quality of life. In the first version of that review, data from three small trials involving a total of 232 men provided estimates of the effects of PFMT on the chance of having incontinence after radical prostatectomy at 1 year: the relative risk of incontinence, comparing PFMT plus biofeedback versus control, was 0.55 [95% confidence interval (CI) 0.24 to 1.23]. 22–24 However, some of the men in two of these trials were not incontinent at baseline, and the trials were all small. Thus, the data did not provide conclusive evidence about whether conservative treatment might reduce incontinence at 1 year after operation.
As a consequence, the NIHR HTA programme commissioned primary research (the MAPS trial) to provide reliable evidence about the effectiveness of PFMT in this population.
In three subsequent updates of the Cochrane review (in 2001, 2004 and 2007), there was still insufficient evidence to guide the practice of providing men with PFMT after prostate surgery. The current (as yet unpublished) update (2011) will have an additional 16 included RCTs, but even after inclusion of data from these trials, no clear conclusions can be drawn.
The questions addressed by this study
The following questions were addressed, primarily in terms of regaining urinary continence at 12 months after recruitment:
-
For men with urinary incontinence 6 weeks after radical prostatectomy, what is the clinical effectiveness and cost-effectiveness of active conservative treatment delivered by a specialist continence physiotherapist or a specialist continence nurse compared with standard management?
-
For men with urinary incontinence 6 weeks after TURP, what is the clinical effectiveness and cost-effectiveness of active conservative treatment delivered by a specialist continence physiotherapist or a specialist continence nurse compared with standard management?
The hypothesis tested in each group of men (in two parallel but separate trials) was that active conservative management would result in a difference of 15% between the groups in the proportion of incontinent men at 1 year after recruitment. The two groups were considered independently because the underlying pathological mechanisms, the rates of incontinence and the chance of regaining continence were expected to be different in the two clinical populations. We recognised that standard management for the control arm in both trials was likely to include non-specialist advice about pelvic floor exercises, including leaflets. Men also had access to any normal care provided locally for men with urinary incontinence, such as pads and advice from continence nurse specialists on continence aids.
Chapter 2 Methods of study
This chapter will describe the methods used to identify and enrol the men in the two trials, and describe the methods of statistical and economic analysis.
Study design and populations
The MAPS study involved men who had urinary incontinence after prostate surgery. Two parallel but separate RCTs were conducted, amongst:
-
men having a radical prostatectomy, usually for prostate cancer
-
men having TURP, usually for benign prostatic hypertrophy.
Approval for this UK study was obtained from the Scottish Multicentre Research Ethics Committee (reference number MREC/04/10/01) and confirmed by each centre’s local research ethics committee and research and development department. The study was conducted according to the principles of good practice provided by research governance guidelines.
Local clinical centres
Centres willing to participate in MAPS were identified from a survey of members of the British Association of Urological Surgeons (BAUS), through personal communication [with urological surgeons and with staff from the Radiotherapy and Androgen Deprivation in Combination After Local Surgery (RADICALS) trial] and through the inclusion of the study on the National Cancer Research Network (NCRN). Each centre had a local principal investigator (lead urologist), who co-ordinated the activities of the local recruitment officer(s) and the local therapist(s). All men from all consultants providing prostate surgery in each centre were eligible, but there were some centres that agreed only to the recruitment of men having radical surgery, while others agreed only to the inclusion of those having TURP. Four centres recruited only to the radical prostatectomy trial: three of these sites recruited during the last 6 months of the recruitment period and included only men recruited to the radical prostatectomy trial at the request of the central office, in order to maximise the numbers in that trial. The fourth site had such a large throughput of men having radical prostatectomies that it did not have the capacity to recruit to the TURP trial as well. Seven centres recruited only men having TURP. This was due, in five of these seven, to existing local services for all men having radical surgery that included explicit teaching of PFMT: the staff were reluctant to ‘unpick’ this element of their service for fear of delivering lower-quality care than before (despite the service not being evidence based). Men were not recruited to the radical prostatectomy trial in the other two sites because of lack of capacity and low numbers of prostate procedures being undertaken locally.
Therapists and training
The therapists could be either specialist continence physiotherapists or nurses with specialist continence or urology training. All therapists received standardised bespoke instruction in the use of PFMT and bladder training for the conservative treatment of male urinary incontinence and PFMT for erectile dysfunction. Therapists used MAPS study instruction materials and documentation to further ensure standardisation of the intervention (see Chapter 3).
Participants
Men were approached at the time of admission for their prostate surgery or at pre-operative assessment clinics. They were initially asked for their consent to receive a screening survey questionnaire sent by post 3 weeks after their operation. Men who indicated in that questionnaire that they were incontinent were invited to participate in the appropriate RCT. Their eligibility was reviewed against the following criteria.
Inclusion criteria
-
Urinary incontinence at 6 weeks after prostate surgery (incontinence was defined as a response indicating a loss of urine to either of two questions in the screening questionnaire: ‘how often do you leak urine?’ and ‘how much urine do you leak?’.
-
Full informed consent.
-
Ability to comply with intervention.
Exclusion criteria
-
Formal referral for physiotherapy or teaching PFMT related to prostate surgery.
-
Radiotherapy planned or given during the first 3 months after surgery for men with prostate cancer.
-
Transurethral/endoscopic resection of prostate carried out as palliation for outflow obstruction in advanced prostate cancer (known as ‘channel TURP’).
-
Inability to complete study questionnaires.
Men with prostate cancer diagnosed at transurethral resection of the prostate
The literature suggested that approximately 15% of men have incidental prostate cancer when the prostatic chips removed at TURP are examined for pathology. 25 Within MAPS, men were still considered eligible for randomisation if the initial management plan did not include formal treatment (a wait and see policy). If the cancer was identified before randomisation, and either radiotherapy or radical prostatectomy was planned within the following 3 months, the man was not eligible for the TURP trial. However, men who were not randomised but subsequently readmitted for radical prostatectomy were eligible to be recruited as new participants to the radical prostatectomy group (after signing a new consent form and completing a new screening questionnaire after surgery). If cancer was diagnosed after randomisation, the men remained in the group to which they had been allocated even if radiotherapy or radical prostatectomy was carried out subsequently. These men could still have the MAPS intervention, if the timing of the new treatment allowed, and were followed up as per the MAPS protocol.
Thus, the MAPS study consisted of two stages: stage 1, the screening survey (used to identify eligible men), and stage 2, the two RCTs.
Screening for postoperative urinary incontinence (stage 1, the screening survey)
Potential MAPS participants were identified by recruitment officers in each clinical centre from amongst all men admitted to the urological ward(s) for prostate surgery. A log was kept of potentially eligible men, categorising reasons if they subsequently became ineligible or did not consent to receive a screening questionnaire. Each man was given a MAPS hospital information sheet (see Appendix 1.1) by the recruitment officer, and then, if interested in the study, each man was asked for his consent to be sent the screening questionnaire at 3 weeks after surgery. The hospital patient information sheet, the screening consent form (see Appendix 2.1) and the screening questionnaire (see Appendix 3.1) all included information about being contacted about further research.
The screening questionnaire was sent to men from the study office in Aberdeen at 3 weeks after the date of operation. A reminder letter with a second copy of the questionnaire was sent after 2 weeks to non-responders. If the returned questionnaire indicated that a man had urinary incontinence, he became eligible for stage 2 of MAPS.
Recruitment to the randomised controlled trial of conservative treatment (stage 2, the randomised controlled trials)
Each man who indicated on his screening questionnaire that he had urinary incontinence was sent an RCT patient information sheet (see Appendix 1.2), a baseline questionnaire (see Appendix 3.2), a urinary diary (see Appendix 3.5) and an RCT consent form (see Appendix 2.2) by the study office in Aberdeen. Men who were willing to be contacted by telephone were telephoned around a week later by a dedicated recruitment co-ordinator based at the MAPS study office in Aberdeen. The purpose of this call was to answer the men’s questions about the trial, to confirm eligibility and to obtain verbal consent to randomisation. Upon receipt of the signed RCT consent form, men were randomised to the intervention or standard care group. Men who did not respond within 14 days after the initial mailing-out were reminded by post and/or telephone.
Withdrawal
Men were free to withdraw from the study at any point without giving a reason. Verbal consent was obtained from men who initially agreed to enter the trial, but later decided to withdraw, to enable relevant data to be retained or collected through central NHS resources.
Randomisation and allocation to group
When the baseline questionnaire and the consent form were received, the Aberdeen MAPS study office randomised the man to the intervention or standard care group.
Randomisation was by computer allocation using the randomisation service of the Centre for Healthcare Randomised Trials (CHaRT, in the Health Services Research Unit, University of Aberdeen). Allocation was stratified by type of operation (radical prostatectomy or TURP) and minimised using centre, age and pre-existing urinary incontinence. The process was independent of all clinical collaborators.
The study office informed all men of their allocation by post. All groups received a lifestyle advice leaflet (see Appendix 4.2). For men allocated to the intervention group, the study office arranged for the local therapist (physiotherapist or continence nurse) to send out the necessary appointments. A letter and GP information sheet were sent to each participant’s GP. Copies of the GP’s letter and the consent form were sent to the hospital urological consultant for filing in the man’s hospital notes.
A flow chart summarising the trial recruitment processes and procedures is shown in Figure 2.
FIGURE 2.
Flow chart of the trial recruitment processes and procedures. Q, questionnaire.
Interventions
Intervention arm
The men in the intervention group attended for a MAPS therapist assessment of their symptoms after randomisation (see Chapters 6 and 11). The first appointment, for an hour, consisted of assessment and training, including customised goal setting for home practice of exercises. The men then attended a further three appointments, each lasting approximately 45 minutes, at around 2, 6 and 12 weeks after the first appointment. They were taught PFMT, with bladder training for men with urgency or urge incontinence. 26 This was supplemented by a booklet containing reminder instructions for PFMT and bladder training (see Pelvic Floor Exercises for Men Taking Part in the MAPS Study; Appendix 4.3). Men also received the lifestyle advice leaflet sent to the men in the standard care arm (see Appendix 4.2).
Biofeedback using digital anal examination was used to teach correct contraction technique and to monitor the strength of contractions. Although biofeedback used for diagnosis or training (repetitive exercising with machine-led feedback on the effectiveness of contractions) was not used routinely in the trial, therapists could use this at their discretion in individual cases. Further details of the intervention are given in Chapter 3.
Control arm
Men in the control group received standard care and a booklet containing supportive lifestyle advice only (without reference to PFMT) by post after randomisation (see Appendix 4.2). Men did not receive any formal assessment or treatment but were able to access usual care and routine NHS services if they felt they needed help with incontinence. This could include written advice if this was part of routine hospital care (such as leaflets containing instructions on PFMT).
Both arms
Use of NHS services, use of pads and practice of PFMT were documented in both groups using information from questionnaires (see Appendix 3.4) and 3-day urinary diaries (see Appendix 3.5) issued at baseline and 3, 6, 9 and 12 months after randomisation.
Data collection and processing
Men were recruited between January 2005 and September 2008. Follow-up continued with 3-monthly questionnaires and urinary diaries for 12 months from the date of the last randomisation, at which time the primary end point (incontinent or not) was measured using the International Consultation on Incontinence (ICI) Short Form questionnaire (ICI-SF). 27 Consent was sought to continue follow-up into the future. The men were also asked to consent to be contacted about other relevant research studies.
Questionnaires (see Appendices 3.1–3.4)
Men were sent postal questionnaires at baseline and 3, 6, 9 and 12 months. An additional health-care unit cost questionnaire was sent only at 6 months. The short questionnaires at 3 and 9 months contained only brief urinary incontinence, exercise and health-care utilisation questions.
Urinary diaries (see Appendix 3.5)
Men were asked to keep diaries at baseline and 3, 6, 9 and 12 months after randomisation, kept for 3 days at each time period.
Data processing
Data from the various sources outlined above were sent to the study office in Aberdeen for processing. Staff in the study office carried out extensive range and consistency checks to enhance the quality and accuracy of the data. Essential missing data were sought from the recruitment officers at the centres, or the men, by post, telephone or email as appropriate.
Outcomes
The primary clinical outcome was subjective report of urinary continence at 12 months. 27
Incontinence was defined as a response indicating a loss of urine to either of two questions in the screening questionnaire, ICI-SF: ‘how often do you leak urine?’ and ‘how much urine do you leak?’
The primary measure of cost-effectiveness was incremental cost per quality-adjusted life-year (QALY).
Secondary outcome measures were as follows.
Clinical
-
Subjective report of continence or improvement of urinary incontinence at 3, 6 and 9 months after randomisation, and improvement at 12 months.
-
Number of incontinent episodes in previous week (objective, from diary).
-
Use of absorbent pads, penile collecting sheath, bladder catheter or bed/chair pads.
-
Number and type of incontinence products used.
-
Coexistence, cure or development of urgency or urge incontinence.
-
Urinary frequency.
-
Nocturia.
-
Faecal incontinence (passive or urge).
-
Other bowel dysfunction (urgency, constipation, other bowel diseases).
-
Sexual function at 12 months (including information about erection, ejaculation, retrograde ejaculation, pain, change in sex life and reason for change).
Quality of life
-
Incontinence-specific quality of life outcome measure (10-point scale, ICI-Q). 27
-
General health measures (Short Form questionnaire-12 items, SF-12, and European Quality of Life-5 Dimensions, EQ-5D).
Use of health services for urinary incontinence
-
Need for alternative management for incontinence (e.g. surgery, drugs).
-
Use of GP, nurse, consultant urologist, physiotherapist.
-
Satisfaction with treatment of incontinence after prostate surgery.
Other use of health services
-
Visits to GP.
-
Visits to practice nurse.
Effects of interventions
-
Use of PFMT.
-
Lifestyle changes (weight, constipation, lifting, coughing, exercise).
Economic measures
-
Patient costs [e.g. self-care (e.g. pads, laundry), travel to health services, sick leave].
-
Cost of conservative trial treatment.
-
Cost of alternative or additional NHS treatments [e.g. pads, catheters, drugs (e.g. adrenergic agonists, anticholinergics, oral medication for erectile dysfunction), hospital admissions or further surgery].
-
Other measures of cost-effectiveness (e.g. incremental cost per additional man continent at 12 months).
Table 1 provides a summary of which study measures and outcomes were collected at each time point in the study.
| Study measure | Screening | Baseline | Month 3 | Month 6 | Month 9 | Month 12 |
|---|---|---|---|---|---|---|
| Consent/randomisation | ✓ | ✓ | ||||
| Sociodemographic characteristics | ✓ | |||||
| Operative details | ✓ | |||||
| Clinical characteristics | ✓ | ✓ | ||||
| Follow-up (outcome) questionnaires | ✓ | ✓ | ✓ | ✓ | ||
| Urinary diaries | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Urinary outcomes (primary) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Other urinary outcomes | ✓ | ✓ | ✓ | |||
| Health-care utilisation questions | ✓ | ✓ | ✓ | ✓ | ✓ | |
| SF-12, EQ-5D | ✓ | ✓ | ✓ | |||
| Exercise, including practice of PFMT | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Bowel outcomes | ✓ | ✓ | ✓ | |||
| Participant cost questionnaire | ✓ | |||||
| Sexual function outcomes | ✓ | |||||
| Lifestyle change outcomes | ✓ | |||||
| Satisfaction with treatment for incontinence | ✓ | |||||
| Further treatment for incontinence | ✓ |
Blinding
As the trial arm to which men were allocated could not be concealed after randomisation had occurred from either the man or the therapist, blinding of participants to intervention was not possible. However, outcome measures were assessed using questionnaires that were processed by MAPS study office staff who were not aware of the randomisation.
The statistician responsible for the final analyses was not the same as the one who performed the interim analyses for the Data Monitoring Committee. All statistical coding and results were agreed before the allocation was revealed.
Sample size
Based on the aim of detecting an absolute difference between intervention and control groups of 15% (30% to 15%) in the number of men who are still incontinent at 12 months, we calculated that we would need 174 men per arm of each trial to give 90% power to detect a significant difference at the 5% level. This would allow detection of a difference of 0.30 of a standard deviation (SD) at 80% power for continuous measures such as quality of life. Should the proportion of men who are incontinent be more than 30%, we would still have 80% power to detect a 15% change from 40% to 25%.
Allowing for a 13% dropout rate after enrolment in the RCT, we planned to recruit 200 men per arm. This would amount to 400 men in each of the two parallel trials, who would come from 615 incontinent men, assuming that 65% agree to join the trial. Based on conservative assumptions of 50% and 5% incontinent at 6 weeks after radical prostatectomy and endoscopic resection of prostate, respectively, and 80% response rates to the screening questionnaire, 1540 and 15,400 men would need to be approached. If a typical centre undertook 30 radical prostatectomies and 300 endoscopic resections of prostate each year, about 26 centres would be required for each trial recruiting over an average of 2 years.
Table 2 shows the number of men whom we estimated we would need to approach and hence the number of ‘typical sized’ clinical centres that would be required. In summary, we needed to screen around 17,000 men in stage 1 of the study, making conservative assumptions about likely response and participation rates. Based on these figures, a 2-year recruitment period in 26 centres would have been needed.
However, towards the end of planned recruitment (end September 2007), it became apparent that we would fall short of our minimum targets for men randomised. We therefore applied for a 9-month extension to recruitment, based on more accurate estimates of recruitment rates. In consultation with the Data Monitoring Committee and representatives of the HTA programme, recruitment was extended to July 2008 and, as a result, randomisation finished on 23 September 2008. There were no changes to the effective sample size sought (174 in each group at 12-month follow-up).
| Estimate | Radical prostatectomy | TURP |
|---|---|---|
| Men needed per arm (minimum) | 174 | 174 |
| Allowing for 13% dropout | 200 | 200 |
| Total men needed in two arms | 400 | 400 |
| Assuming 65% willing to enter RCT, no. of incontinent men needed | 615 | 615 |
| Percentage incontinent at 6 weeks (stage 2) | 50% | 5% |
| No. of men needed to reply to survey | 1230 | 12,300 |
| Assuming 80% response to survey, no. needed for survey (stage 1) | 1540 (approx.) | 15,400 (approx.) |
| No. of operations per typical centre | 30 | 300 |
| No. of typical centres needed in 2 years | 26 | 26 |
Statistical methods
Trial analyses
The principal comparisons in each trial were between men allocated to active therapy (up to four visits to a therapist plus the lifestyle advice leaflet) and men allocated to the control group (lifestyle advice leaflet only). The two populations of men (having radical prostatectomy or TURP) were analysed as separate trials. The primary outcome measure (urinary incontinence at 12 months) and secondary outcome measures were analysed using general linear models that adjusted for the minimisation covariates (age and pre-existing urinary incontinence) and, when possible, the baseline measure of the outcome. For the primary outcome only, unadjusted analyses were also reported. All analyses used 95% CIs. For the binary outcomes, a Poisson link function was used to estimate relative risks (instead of estimating odds ratios from a logistic model) and robust standard errors were used to estimate the CIs. 28 For illustrative purposes, the relative risk of the primary outcome was also transformed to a risk difference.
The primary statistical analysis was based on all men as randomised, irrespective of subsequent compliance with the treatment allocated (intention to treat). The intention-to-treat approach gives the least biased estimate of effectiveness of the two interventions. Given that it was likely that some of the participants would not attend the therapy sessions (e.g. because they were continent), a secondary comparison was conducted to estimate the efficacy of the treatment received (i.e. what is the effect if the participants actually received the treatment they were allocated to?). The so-called ‘per-protocol’ approach for estimating efficacy of treatment, in which compliers with treatment in each group are compared with each other, can have substantial selection bias. A more robust method is to use a latent variable approach. 29 We used the method of adjusted treatment received as described by Nagelkerke et al. 30 The method used a two-stage least squares approach, whereby treatment received and the residuals from that model were used as an independent variable in a second model together with the treatment received to estimate the effects on the primary outcome.
Missing items in the health-related outcome measures were treated as per the instructions for that particular measure. No further imputation for missing values was undertaken. The ways in which the data were analysed were prespecified in the statistical analysis plan, which was agreed in advance with the MAPS Trial Steering Committee.
Timing and frequency of analyses
A single principal analysis was carried out at 15 months after the last man was recruited. The Data Monitoring Committee considered confidential interim analyses of data on three occasions during the data collection period (January 2006 – 31 randomised to radical prostatectomy and 48 randomised to TURP; January 2007 – 180 randomised to radical prostatectomy and 200 randomised to TURP; January 2008 – 297 randomised to radical prostatectomy and 364 randomised to TURP). The Data Monitoring Committee did not recommend any amendments to the protocol on any occasion.
Planned secondary subgroup analyses
Subgroup analyses (separately for the two populations) explored the effect on urinary incontinence at 12 months after randomisation of:
-
pre-existing urinary incontinence (before prostate surgery)
-
age (up to 60 years, 61 years and over for radical prostatectomy; up to 70 years, 71 years and over for TURP)
-
body mass index (BMI) (up to 30 kg/m2, 30–34.9 kg/m2, 35 kg/m2 or greater)
-
type of incontinence at trial entry (SUI, UUI, MUI, postmicturition leakage)
-
other morbidity
-
type of therapist (physiotherapist or nurse)
-
centres with and without biofeedback machines.
Stricter levels of statistical significance (2p < 0.01) were sought, reflecting the exploratory nature of these analyses.
Ancillary analyses
Screening data
Descriptive statistics were tabulated to describe the derivation of the trial groups from the screening procedures, and included comparison of those who responded versus those who did not respond to the screening.
Therapist data
Descriptive data were tabulated to describe how the therapy intervention was implemented in each of the trials. This included a comparison across therapy visits (one to four) on incontinence, bowel and sexual problems and pelvic floor muscle performance.
Economics methods
Introduction
The economic evaluation was based on a within-trial analysis at 12 months after recruitment for men with urinary incontinence 6 weeks after radical prostatectomy or TURP. The question addressed was: what is the clinical effectiveness and cost-effectiveness of active conservative treatment delivered by a specialist continence physiotherapist or a specialist continence nurse compared with usual management? The perspective of the study was based on a societal viewpoint and included both the costs of the health service provider (the NHS) and those of the patients.
Measurement of resource use
The use of health services as a consequence of being incontinent was recorded prospectively for every participant in the study. Resource utilisation data were collected using questionnaires and urinary diaries. These data were collected using questionnaires sent to the participants at baseline, and 3, 6, 9 and 12 months. Resource utilisation data collected also included the intervention, i.e. the number of visits to the therapists, who were either specialist continence physiotherapists or continence nurse specialists. According to the protocol, PFMT intervention comprised four sessions. Details of the intervention are provided in Chapter 3. The first session of PFMT was 1 hour and the other three sessions were approximately 45 minutes each. Each session was conducted in a hospital department. The consumables required per session were gloves, K-Y Jelly, wipes and paper towels. No additional resources were required for the biofeedback as no equipment was used; verbal biofeedback was used to teach the men how to contract their muscles optimally and advise them on improvement from previous appointments.
Primary care and outpatient resource use included visits to the GP as well as to the outpatient department. The number of GP visits and the contact (doctor or nurse) were obtained from the 3-, 9- and 12-month follow-up questionnaires. Number of outpatient visits was obtained from the 3-, 6-, 9- and 12-month follow-up questionnaires. For the length of stay, the number of days the men were admitted was recorded. Other resource use included the number and type of drugs the patients were prescribed for their incontinence problems, the number of pads used and, finally, the number of bed and chair protectors used. The data reported by the patients were used to calculate the average and total resource use per patient.
The information generated from these questionnaires entailed manipulation of the data to perform the comparative analysis. Details of methods used to estimate resource use collected are included in Table 3.
| Resource | Relevant variables | Source | Reported outcome |
|---|---|---|---|
| Patient management | Physiotherapist 1st visit | DA | Number attending |
| Physiotherapist 2nd–4th visit | DA | Number attending | |
| GP visits | PQ | Number | |
| Primary care | Nurse visits | PQ | Number |
| Outpatient visits | PQ | Number | |
| Secondary care | Inpatients days | PQ | Number |
| Physiotherapy | PQ | Number | |
| Medications, e.g. tolterodine tartrate | PQ | Type and number | |
| Other | Pads | PQ | Type and number |
| Chair/bed protectors | PQ | Type and number | |
| Catheters | PQ | Type | |
| External sheaths | PQ | Type |
Identification of unit costs
As described above, costs focused on the direct health service costs associated with each treatment. Unit cost data were extracted from the literature or from relevant sources such as manufacturers’ price lists (British National Formulary, BNF)31 and NHS Reference Costs. 32 The year of the cost data is 2008 and the currency is pounds sterling (£).
The costs of the intervention included the cost of PFMT sessions. These comprised the costs of the staff involved, consumables and overheads. The costs of producing the leaflets for the trial were not included in the analysis as all the men in the trial received leaflets. Men in both groups received a booklet containing supportive lifestyle advice (without reference to PFMT) by post after randomisation (see Appendix 4.2). Men in the intervention group also received a MAPS pelvic floor exercise leaflet (see Appendix 4.3) from the therapist at the first visit. The booklet aimed both to support and to reinforce the anatomy teaching received during MAPS therapy appointments, as well as the exercise programme that had been set. It was therefore assumed that the costs would be the same for both groups. The cost of training that therapists received (1-day course) was included in the intervention costs because it was low and was not likely to impact on the overall costs.
The cost of the follow-up management comprised the cost per visit to both primary (GP and nurse appointments) and secondary (outpatient appointments and number of inpatient days) health-care providers. Unit costs for GP’s visits were obtained from the Personal Social Services Research Unit (PSSRU) unit costs of community care. 33 Unit costs for outpatient services were obtained from the Scottish Health Service Costs (SHCS) (Information and Statistics Division website34) for the primary analysis and the national reference costs in a sensitivity analysis (Department of Health website35). The inpatient costs were those of the wards to which the men were admitted.
Other costs considered included containment products. These comprised all the products that participants used, such as absorbent pads, penile collecting sheaths, bladder catheters and bed and chair protectors. The unit costs of these items were taken from the providers of these items or from the NHS suppliers, where available. The unit cost of sheaths is based on a weekly cost of sheaths, estimated assuming that one sheath is used each day, the reusable leg bag is used for 3 days and one night bag is used each night. The unit costs of the catheter were based on the assumption that the catheter was used over a 3-month period, and similar assumptions were made for the leg and night bags. The unit cost of the medications was taken from the BNF,31 and the cost per patient in terms of medication use was calculated by multiplying the unit cost by each number of units consumed for each patient. The costs considered were those of the drugs, not the prescription charges. Table 4 provides a summary of the unit costs for the resources used.
| Resource use | Unit cost (£) | Notes |
|---|---|---|
| Staff costs | 67 | Based on cost per hour of patient contact for Band 6 of the October–December 2007 NHS staff earnings estimates for qualified nurses33 |
| Cost of consumables | 0.90 | Based on cost of gloves, K-Y Jelly, couch roll, paper towels, wipes for four visits |
| Medications | Various | Cost based on recommended dosage |
| GP doctor visit | 36 | Per surgery consultation lasting 11.7 minutes33 |
| GP nurse visit | 11 | Based on cost per consultation33 |
| Physiotherapist visit | 31 | Based on cost of nurse-led clinic33 |
| Outpatient visit | 75 | Based on cost34 |
| Inpatient visit | 157 | Based on the average cost per day in a urology specialty ward34 |
| Pads | 0.17 | Cost per pad |
| Chair/bed protector | 0.15 | Cost per protector |
| Sheath | 8.46 | Weekly cost of sheath (condom) catheter, reusable leg bag and disposable night bag |
| Catheter | 2.73 | Weekly cost of catheter, reusable leg bag and disposable night bag |
The data describing the resource utilisation of participants were combined with estimates of unit costs for each of the areas of management considered. This allowed for estimation of total cost for each participant, as well as the average cost for each area of resource utilisation and average total cost. The results are reported in Chapters 8 and 13.
Participant costs of urinary incontinence
As the perspective of the study was the NHS and patient, those costs borne by participants and their families were also considered. Participants’ resource use was taken as time taken to access services (e.g. attend GP, physiotherapist, outpatient or inpatient appointments), travel costs and the time taken off usual activities owing to poor health. Similar costs were included for spouses, relatives or friends who accompanied them to their appointments. Travel costs to patients and their families were based on actual fares when public transport was used and published mileage rates in the case of those who used their own vehicles (HM Revenue and Customs website36). These data were collected through postal questionnaires administered at 12 months.
In the case of patients who would have been engaged in employed work, the value of their time was taken as the gross average full-time wage rate for men (Office for National Statistics website37). The value for those who were not in formal employment was based on 57% of the average national rate and 43% for those who may have been involved in leisure activities. 38 The costs of friends/relatives accompanying patients to hospital were estimated in the same way. These unit time costs, measured in terms of their natural and monetary terms, were combined with estimates of number of health-care contacts derived from the health-care utilisation questions. Self-purchased health care included items such as pads bought by the participant, prescription medicines and over-the-counter medications. Information about these was collected through the health-care utilisation questions. Patients’ time and travel costs were based on the information collected, and are described in Table 5.
| Resource | Resource use | Unit cost |
|---|---|---|
| Use of personal car to GP | Distance travelled | Cost per mile/km |
| Use of personal car to hospital | Distance travelled | Cost per mile/km |
| Use of public transport to GP | Ticket | Return cost of ticket |
| Use of public transport hospital | Ticket | Return cost of ticket |
| Medication purchased | Type and number | Cost of medicines |
| Loss of earnings | Number of days off work | Daily wage |
Quality of life
Effectiveness within the trial was measured in terms of QALYs and subjective continence at 12 months (assessed using data from the ICI-SF). Quality of life data were collected at baseline and 6 and 12 months. This was generated using generic health status measurement tools, the EQ-5D and SF-12, included in the questionnaires. The EQ-5D measure divides health status into five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Each of these dimensions has three levels, therefore 243 possible health states exist. 39 Responses of the patient EQ-5D questionnaires were transformed using a standard algorithm to produce a health state utility at each time point for each patient. The utility scores obtained at baseline and 6 and 12 months were used to estimate the mean QALY score for each group. The estimation of QALYs took account of the mortality of study participants. Participants who died within the study follow-up were assigned a zero utility weight from their death until the end of the study follow-up. QALYs before death were estimated using linear extrapolation between the QALY scores at baseline and all available EQ-5D scores up to death.
As described below in the section on the sensitivity analysis, the responses from the SF-12 questionnaire were also used as the basis of QALYs, and were mapped on to the existing Short Form questionnaire-6 Dimensions (SF-6D) measure using the algorithm by Brazier et al. 40 to allow utility values to be estimated for each time point. These utility scores were transformed into QALYs using the methods described above to provide an alternative measure of QALYs for each patient.
Incremental cost-effectiveness
Data collected on costs and effects of the interventions were combined to obtain an incremental cost-effectiveness ratio (ICER). This was performed by calculating the mean difference in costs between the interventions and control groups over the difference in effect between the interventions and control groups. This gives us the cost per additional QALY gained for the new interventions relative to standard practice.
The primary analysis was based on the 1-year follow-up of the trial and the outcome was the incremental cost per QALY. This outcome was chosen to reflect a societal decision-making perspective. The results are presented as point estimates of mean incremental costs, proportion of men continent, QALYs and cost per QALY. Measures of variance were based upon bootstrapped estimates of costs, QALYs and incremental cost per QALY. Incremental cost-effectiveness data are presented in terms of cost-effectiveness acceptability curves (CEACs).
Data analysis (economics)
As data were collected over a 1-year period, discounting was not carried out. The numbers of missing data for each variable used in the analyses of cost were quite low, and data that were missing were considered to be missing completely at random. Data reported as mean costs for both cases and controls were derived for each item of resource use and then compared using unpaired t-tests and linear regression adjusted for baseline values. As the data were not normally distributed, non-parametric bootstrapping was used to estimate confidence limits around the difference in cost for each area of resource use and total costs.
Sensitivity analysis
With all parameter estimates there are elements of uncertainty owing to the lack of available information. In order to explore the importance of such uncertainties and assumptions, various sensitivity analyses were conducted by varying some of the assumptions or estimates used in the analysis. Two types of sensitivity analyses were performed: one-way sensitivity analysis and threshold analysis.
The base-case analyses in terms of utilities were adjusted for patient outcomes at baseline to account for variability that might be present amongst the intervention groups. An unadjusted analysis was also performed to highlight the importance of this base-case assumption.
There is uncertainty around the QALY estimates as they were derived using one generic instrument, the EQ-5D. There is some debate over whether the dimensions in the EQ-5D are sensitive enough to capture the loss in quality of life for chronic health states of which the worst effects occur during acute episodes. Therefore, the responses from the SF-12 questionnaire were mapped on to the existing SF-6D measure using the algorithm by Brazier et al. 40 to allow utility values to be estimated for each time point. These utility scores were then transformed into QALYs using the same methods as used for the EQ-5D scores to provide an alternative measure of QALYs for each patient.
Modelling
Additional information for policy-makers was derived from a simple economic model that considers what difference in continence rates would result in a change in the conclusions about which treatment would be cost-effective. This analysis was performed from the perspective of the NHS.
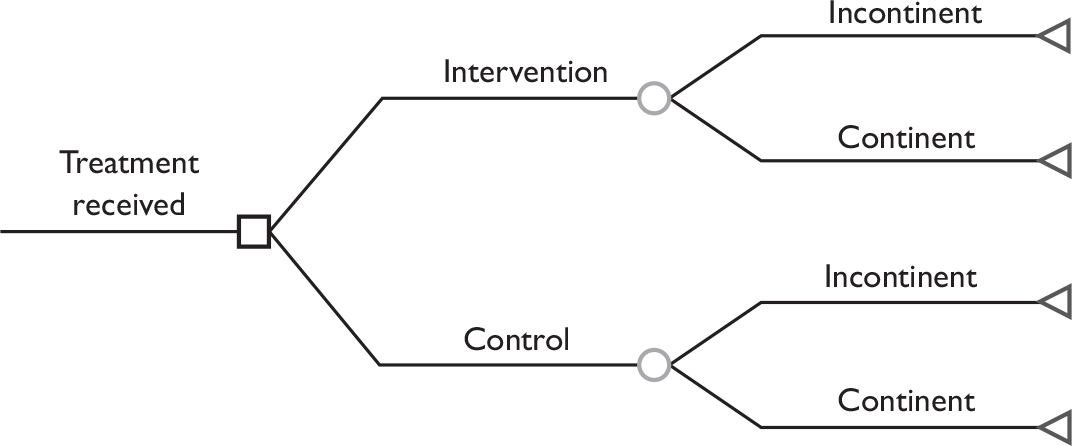
The data used to populate the model were based on the trial patient data, to inform on the probability of being incontinent at the end of 12 months, and the cost data. The data also included QALYs and costs derived for each participant, based on the group they were allocated to (intervention or control) The model is illustrated in Figure 3.
FIGURE 3.
Structure of the model used in economic analyses.
Management of the study
The MAPS study office, working in conjunction with our trials unit, and CHaRT in the Health Services Research Unit, University of Aberdeen, provided support for the clinical centres, randomisation, management of data collection, follow-up, data processing and analysis. The MAPS Project Management Group (grant holders and representatives from the study office) met formally at least monthly during the course of the study to discuss key trial issues.
The study was overseen by an independent Trial Steering Committee with an independent chairman and three other independent members. The remaining members were the grant holders. The Trial Steering Committee met annually on six occasions. An independent Data Monitoring Committee was also established, comprising an independent chairman and three other independent members, who met on three occasions. The trial statistician supplied, in strict confidence, interim analysis results for their consideration.
The University of Aberdeen assumed the role of sponsor for the study.
Table 6 shows the substantive changes to the MAPS study protocol since its first approval by the MREC: they were approved on the dates shown.
| Change to protocol | Date approved |
|---|---|
| Nomenclature: the operation types for the two groups of men are referred to as ‘radical’ and ‘TURP’ | 31 May 2005 |
| Nomenclature: intervention will be delivered by ‘therapists’ rather than ‘physiotherapists’ | 31 May 2005 |
| Formal referral to a therapist delivering PFMT before or after operation added as a specific exclusion criterion | 31 May 2005 |
| Multiple sclerosis or Parkinson’s disease no longer a specific exclusion criterion | 31 May 2005 |
| Trial Steering Committee concluded that the study should still aim to enrol 25–30 centres as this would allow for sites withdrawing and rates dropping off | 30 November 2005 |
| Amendment relating to the diagnosis of unsuspected prostate cancer in men undergoing TURP, and how this would be handled in the MAPS study | 30 November 2005 |
| New sponsor: University of Aberdeen | 30 November 2007 |
| Revised extension timings | 30 November 2007 |
Chapter 3 Intervention design, centres, therapists and therapy
In this chapter, the rationale for the intervention, the methods used to train the therapists in order to standardise the intervention and the types of therapists at each centre are described.
Introduction
The purpose of the MAPS trial was to compare return to continence with or without a structured PFMT programme, delivered by a trained therapist, in men after TURP or radical prostatectomy. Both groups received written information on recovery after surgery. The primary outcome was self-reported urinary incontinence at 12 months after randomisation.
Following radical prostatectomy, some degree of iatrogenic urinary incontinence is a recognised complication in up to 90% of men. 9 Following TURP for benign prostatic hypertrophy, the figure is around 10%. 15
Some physiotherapists already use PFMT and bladder training (BT) or urge suppression (US) techniques to treat men with urinary incontinence following prostate surgery, despite Cochrane reviews clearly showing that there is currently insufficient evidence to confirm whether or not these are effective. 26,41 Uncertainty also surrounds the most effective PFMT and BT/US protocols for specific clinical indications.
MAPS control protocol
All participants received a lifestyle advice leaflet (see Appendix 4.2) (control and intervention groups). Face and content validity were established by review of the literature,19 with a consumer representative of men who had urinary incontinence, and with health-care professionals. The final copy contained information about moderating fluid intake (avoiding too much or too little), and information on caffeine, cranberry juice, diet and obesity, constipation, general fitness, lifting, smoking, chest problems and urinary tract infections and was based on clinical practice recommendations. The control group had no further contact with the research team, apart from follow-up by questionnaires.
MAPS intervention protocol
In addition to the leaflet described above, all men in the intervention group received a structured PFMT intervention. The protocol was based on one used in a previous trial using PFMT to increase pelvic floor muscle strength for men with erectile dysfunction. 42 The BT/US techniques were based on those typically used in clinical practice for UUI and summarised in a Cochrane review. 43 The advice on fluid intake was based on standard clinical practice. 44
Constituent elements of the intervention protocol
Assessment of pelvic floor strength
During each therapy appointment, pelvic floor muscle contraction strength was evaluated by a digital anal examination using the Oxford score (graded 0–5). 45 An additional grade (6) was added to define a very strong anal squeeze. 46 The new grading system was used to assess separately the strength of both the external anal sphincter and the deeper puborectalis muscle (taken to represent the pelvic floor muscles). The external anal sphincter was assessed at 1–2 cm from the anal meatus, and the puborectalis at 3–4 cm from the anal meatus.
Verbal biofeedback from this examination was used to teach the men how to contract their muscles optimally, and advise them on improvement from previous appointments. At each assessment, the maximum duration of each contraction was timed by counting.
Pelvic floor muscle therapy regimen
PFMT was aimed at improving the strength of the pelvic floor muscles to allow effective contraction during exertion to prevent urinary leakage. PFMT consisted primarily of three maximum-strength contractions with a 10-second break between each one, practised in three positions (lying, sitting and standing) twice daily (see Appendix 4.3). Targets were set for the duration of each contraction, up to a maximum of 10 seconds, and revised in successive appointments if progress had been made. In addition, men were taught to carry out a sustained submaximal contraction of the pelvic floor muscles during walking and to perform a strong contraction before and during any event that might cause leakage, such as coughing or rising from sitting (‘the knack’). 47 Men were advised to eliminate urine remaining in the bulbar urethra by using a strong contraction after urination was finished, in order to prevent postmicturition dribble. 42 Contracting the pelvic floor muscles during sexual activity was also recommended to achieve, maintain or improve erectile strength.
Bladder training/urge suppression
Men with urgency or UUI were taught urge suppression techniques in order to avoid rushing to the toilet when the bladder was starting to contract (see Appendix 4.3). Fluid advice, including avoiding or reducing caffeine, was also offered.
Written supplementary guidance
The MAPS PFMT leaflet (see Appendix 4.3) aimed both to support and to reinforce the anatomy teaching received during appointments, as well as the exercise programme that had been set. To maximise understanding, careful consideration was given to the language and terminology used in this leaflet, taking into account the sensitive nature of incontinence and erectile dysfunction. The use of medical and anatomical terms was minimised in favour of a plain English approach (‘urine leakage’ for incontinence). 48
Drafts of the leaflets were reviewed for face and content validity by lay persons and health-care professionals with knowledge of men’s health and continence issues.
Understanding strategies selected for the MAPS intervention
In order to clarify key aspects of the rationale behind elements of the MAPS standardised intervention, the following areas were addressed.
Rationale for performing a digital anal examination
A digital anal examination was undertaken to assess the strength and endurance of, firstly, the external anal sphincter and, secondly, the puborectalis muscles. Wyndaele and Van Eetvelde49 demonstrated the reproducibility of assessing the puborectalis by anal assessment using grades 0–5. By assessing puborectalis muscle strength, the strength of the surrounding pelvic floor muscles would also be graded. The muscles were graded from 0 to 6, with 0 being ‘no flicker or contraction’ and 6 being ‘very strong, unable to withdraw finger. 42 Repeating the examination at subsequent visits enabled therapists to provide verbal feedback to men that their exercises were effective in building up muscle strength and to monitor progress.
Rationale for asking men to perform pelvic floor exercises in three positions
Pelvic floor muscles support the abdominal contents and prevent urinary leakage. The three positions provided a graded method of increasing the effect of gravity, in order to provide extra muscle work load. The pelvic floor muscles were recruited initially in a lying position, without the effect of gravity. As strengthening occurred, pelvic floor muscles were subject to a higher load by recruiting them in a sitting position, where the downwards descent of the pelvic floor would be partly prevented by the seat of the chair. A greater load would be placed on the pelvic floor during standing, when gravitational forces opposed the elevation of the pelvic floor during exercise. MAPS adopted this regimen for the intervention supported by evidence from four previously documented trials, which found it to be convenient, acceptable and comfortable for patients. 24,50–52 It was believed that men needed to be able to tighten their pelvic floor muscles in a number of positions, so that they could recruit them speedily during coughing and sneezing.
Rationale for performing three pelvic floor muscle contractions
The PFMT programme was aimed at increasing pelvic floor muscle strength in order to counteract increases of abdominal pressure during exertion. Based on clinical research of quadriceps strengthening using a progressive resistance machine, repeated computerised readings showed that the first contraction gave the patient the feel of the movement but failed to achieve maximum power. The second contraction attained maximum power, whilst the third failed to reach maximum power owing to fatigue. 53 Kegel54 stated that maximum power was a key element to gaining increased muscle strength. These principles informed the PFMT programme, considering that the maximum power of pelvic floor contraction would be attained using three muscle contractions in each position held for up to 10 seconds. The target was individually adjusted as performance improved.
Rationale for performing the regimen twice a day
Kegel54 recommended 300–400 pelvic floor muscle contractions a day to treat SUI in women.However, clinical practice has shown that patients find this level of commitment to be too arduous, resulting in attrition and demotivation. The principles of muscle building show that it is the quality of the contraction that is more important than the quantity. 53,55
The MAPS intervention was therefore designed to provide targets that were achievable in order to motivate men to maintain the regimen within the constraints of the protocol. In a previous trial,42 55 men were asked to perform their exercise sets only twice a day. After 3 months, all except one (who had severe back pain) showed a statistically significant increase in pelvic floor muscle strength. We therefore felt that this regimen had a proven ability to increase pelvic floor muscle strength.
Rationale for contracting the muscles as strongly as possible
The pelvic floor muscles consist of two-thirds slow-twitch continually tonic muscle fibres and one-third fast-twitch muscle fibres, which can be speedily recruited when extra support is needed during activities that increase intra-abdominal pressure. 56 Both fibre types are recruited during maximum contraction of the pelvic floor muscles. In order to achieve an increase in muscle bulk, the MAPS intervention used maximum voluntary effort, which was expected to result in the hypertrophy of muscles and an increase in local blood supply. 53,55
Rationale for functional use of muscles
Pelvic floor muscles need to be recruited to prevent leakage of urine during activities that increase intra-abdominal pressure. ‘The Knack’ is the technique, or learned skill, of tightening just before and during these activities. 47 Owing to its significant role in contributing to continence, teaching of ‘the Knack’ was therefore included as an element in the MAPS intervention.
Reasons for increasing pelvic floor muscle endurance
Slow-twitch muscle fibres fulfil a number of important functions: pelvic floor support, bladder and bowel control, sexual activity, posture and respiration. The upright posture stimulates the pelvic floor reflex, which results in contraction of the slow-twitch fibres in response to the weight of the abdominal contents. 57 In order to meet this demand, the pelvic floor muscles need to have sufficient muscle endurance to prevent urinary leakage. By encouraging the patient to tighten the pelvic floor muscles slightly during walking (as taught in the MAPS intervention), a functional method of potentially increasing the use of slow-twitch fibres and hence muscle endurance was achieved.
Rationale for tightening the pelvic floor muscles after urinating
One of the superficial pelvic floor muscles, the bulbocavernosus muscle, encircles the proximal 50% of the penis and tightens by reflex action at the end of micturition to facilitate emptying of the bulbar portion of the urethra. 58 Teaching men to contract their pelvic floor muscles strongly after they have completed micturition will result in the recruitment of the bulbocavernosus muscle along with the other pelvic floor muscles. 42 This muscle contraction will then facilitate the evacuation of residual urine from the bulbar urethra. This may restore or develop the reflex postvoid milking mechanism identified by Wille et al. 58 and termed the ‘urethrocavernosus reflex’ by Shafik and El-Sibai. 59 Thus, as an additional strategy to attain continence, participants were taught to perform consciously a pelvic floor muscle contraction immediately after micturition.
Rationale for tightening the pelvic floor muscles during sexual activity
The superficial bulbocavernosus and ischiocavernosus muscles are active during penile erection. 60 The bulb of the penis sits on the inferior aspect of the deeper layer of the pelvic floor muscles, which form a firm base for the erect penis. The bulbocavernosus muscle prevents blood from escaping through the deep dorsal vein during an erection. One study has shown that pelvic floor exercises can restore erectile function in 40% of men and improve it in a further 36%. 42 As this is another potential benefit of PFMT, it was decided that it would be appropriate to include erectile function in the MAPS intervention materials and be measured as a secondary outcome. However, it is not yet clear whether men will benefit after radical prostatectomy as the amount and degree of nerve damage caused by surgery is likely to be variable. Erectile function was a secondary outcome of the study.
Rationale for choice of urge suppression techniques
A detrusor contraction produces a desire (urge) to empty the bladder. If urgency sensations cannot be overcome, urinary incontinence may occur. The resulting fear of leakage can cause anxiety, breath-holding and descent of the diaphragm, which, coupled with abdominal muscle contraction, can produce early inappropriate micturition. A retrospective study in women has reported that effective urge suppression techniques include keeping calm, sitting down or standing still and waiting 1 minute until the initial urge sensation disappears. 46 PFMT can be used to strengthen the pelvic floor musculature and, together with urge suppression techniques, can help to restore bladder control.
Rationale for giving fluid, dietary and lifestyle advice
All men received fluid, dietary and relevant lifestyle advice as part of the therapy appointments, supplemented by written information (see Appendix 4.2). Advice included information that reducing fluid intake (underdrinking) to avoid leakage may lead to urinary tract infections, constipation and dehydration. 61 Conversely, drinking excessive amounts (in the belief that this is beneficial for health) may have adverse effects such as an increased risk of leakage. 62 However, men experiencing nocturia were advised that avoiding fluids 2 hours before bedtime may be helpful.
Drinks containing caffeine or alcohol may cause increased risk of urgency and men were advised to reduce or avoid them. 61 Anecdotal evidence has shown that certain foods (e.g. onions, spicy foods and curries) can cause increased gut peristalsis, which may also have an effect on the bladder, causing it to be overactive and contractile. Other risk factors for an overactive bladder were highlighted, including the effect of constipation, smoking and obesity. 63 Information on all these elements was included in the MAPS lifestyle advice leaflet (see Appendix 4.2).
Rationale for four appointments in 12 weeks
The value of psychological support for men following radical prostatectomy has been stressed in the literature,52 as has the intrinsic value of therapist contact in order to maintain patient motivation. 64 Within MAPS, therefore, a schedule of four appointments (at baseline, 2 weeks, 6 weeks and 12 weeks) was considered sufficient to monitor postsurgical muscle strength development and maintain motivation but not be too burdensome on patients or costly in terms of resources (chiefly therapist time).
Pelvic floor muscle strength can improve over a 3-month period of PFMT. 42 Men in the study received four appointments and were encouraged to continue their exercise regimen for life, with particular emphasis on functional work (e.g. contracting during activity or counteracting increases in intra-abdominal pressure by use of ‘the Knack’). A previous trial by van Kampen et al. 24 using pelvic floor exercises and functional use of these muscles showed significant reduction in urinary incontinence at 1, 6, and 12 months after radical prostatectomy, demonstrating that improvement was maintained while men continued to perform their exercises.
Summary of rationale for design of intervention
The MAPS intervention, combining PFMT, BT/US and fluid advice, was evidence based wherever possible. Where evidence was lacking, the intervention was based on expert clinical practice. The rationale underpinning the intervention was published in 2009. 65
The trial compared the structured PFMT intervention with standard care, in order to add to the current evidence base. This, in turn, should inform practice and treatment decisions for therapists, men with incontinence after prostate surgery, and providers of care.
Training for the therapists
Therapists were either specialist continence physiotherapists or specialist continence nurses. The intervention protocol was standardised by systematically training all the therapists during a bespoke training day programme, and by use of common trial forms for recording assessment and treatment data. Some therapists were trained on a one-to-one basis if they joined the study late. During the training day, an overview provided information on the anatomy and physiology of the lower urinary tract, the pelvic floor muscles and the abdominal muscles, together with information on how prostate surgery affects normal urine control. Therapists received instruction on the MAPS PFMT protocol including:
-
assessment and examination of men in a systematic manner
-
the diagnosis of SUI, UUI, postmicturition dribble and erectile dysfunction by history
-
grading the strength of the pelvic floor muscles during a digital anal examination by evaluating the anal sphincter and also the puborectalis sling at each visit
-
affirmation that all the pelvic floor muscles (including the transversus abdominis) should tighten during a maximum contraction and that, if the contraction was strong, they would see a scrotal lift and the penis moving slightly into the body
-
description of the MAPS-approved method of teaching PFMT
-
instruction in BT/US techniques
-
advice about fluid intake and other lifestyle advice corresponding to the leaflet
-
the role of PFMT in the treatment of erectile dysfunction
-
graded goal-setting for the men in terms of gradually increasing endurance of pelvic floor muscle contractions
-
documenting the treatment given at each visit.
Summary of the MAPS intervention
At the baseline assessment visit, the men were taught PFMT. BT/US techniques were included if men described urgency or UUI. The men had reinforcement sessions on three further occasions over 3 months – at around 2 weeks, 6 weeks and 12 weeks after the first appointment. Anal examination was repeated at each visit to document changes in pelvic floor muscle strength, and to provide feedback to the men on their progress.
Pelvic floor muscle training
Men in the intervention group were instructed:
-
to carry out three maximum pelvic floor contractions in three positions (lying supine with knees bent and feet on the couch, sitting with knees apart and standing with feet apart) twice per day
-
to ‘lift’ their pelvic floors slightly while walking
-
to tighten their pelvic muscles before activities that might cause them to leak, such as coughing
-
to tighten after urinating to eliminate the last few drops.
Biofeedback
Biofeedback involved monitoring the strength of a pelvic floor contraction (by digital anal assessment) and verbally relaying the information back to the men in order to confirm that they were performing contractions correctly (lifting up in a cranial direction) and to inform them when they were increasing the strength or duration of their contractions. Therapists were trained to consistently grade the pelvic floor muscle strength and endurance by digital anal assessment at each session. The findings were used to set progressive targets for the men. Treatment was therefore individualised and could be progressively increased for each man.
If it was felt clinically indicated, in addition to digital anal assessment, therapists used machine-mediated biofeedback with an anal biofeedback probe in centres where this was available (see Table 7), both for diagnosis and for teaching of correct muscle contraction.
| Centre | Physiotherapist | Specialist continence nurse | Biofeedback available in the centre | Biofeedback clinically indicateda | Number of men receiving biofeedback |
|---|---|---|---|---|---|
| Aberdeen | No | Yes | No | No | 0 |
| Ipswich | Yes | No | Yes | Yes (3R) | 2R |
| Dundee | No | Yes | No | No | 0 |
| Stockport | Yes | No | No | Yes (2R, 1T) | 0 |
| Tameside | Yes | No | Yes | Yes (3R, 8T) | 3R, 4T |
| Middlesbrough | Yes | No | No | Yes (8R) | 0 |
| Falkirk | Yes | No | No | No | 0 |
| Newcastle upon Tyne | Yes | No | No | No | 1R |
| Airedale | Yes | No | Yes | Yes (4R) | 4R |
| Reading | No | Yes | No | No | 0 |
| Wakefield | Yes | No | Yes | No | 0 |
| Ayr | Yes | No | Yes | Yes (1T) | 0 |
| Bristol | No | Yes | Yes | Yes (7R, 6T) | 7R, 6T |
| Stevenage | No | Yes | No | No | 0 |
| Inverness | No | Yes | No | No | 0 |
| Leeds | No | Yes | No | Yes (8R, 6T) | 0 |
| Inverclyde | Yes | No | No | No | 0 |
| Wolverhampton | Yes | No | No | No | 0 |
| Swansea | No | Yes | No | No | 0 |
| Sheffield | No | Yes | No | No | 0 |
| Ilford | No | Yes | Yes | No | 0 |
| Bolton | Yes | No | Yes | No | 0 |
| Taunton | No | Yes | No | No | 0 |
| Norwich | Yes | No | Yes | No | 0 |
| Yeovil | Yes | No | Yes | No | 0 |
| Edinburgh | Yes | No | No | Yes (5R, 1T) | 0 |
| Dunfermline | Yes | No | Yes | No | 0 |
| Cardiff | No | Yes | No | No | 0 |
| Macclesfield | No | Yes | No | No | 0 |
| Southmead | No | Yes | No | No | 0 |
| Crewe | Yes | No | Yes | No | 0 |
| Hillingdon | No | Yes | No | No | 0 |
| St Mary’s, London | No | Yes | Yes | No | 0 |
| Hope, Salford | No | Yes | No | No | 0 |
Bladder training/urge suppression
BT/US involved advice to gradually delay urination (by pelvic floor muscle contraction or calming/distracting activities) to teach the bladder to hold increasing volumes of urine. Men were instructed to relax for 1 minute when they first felt an ‘urge’, then walk calmly to the toilet or delay urination until the next ‘urge’.
Written information
All participants received the lifestyle advice booklet (see Appendix 4.2), and those in the intervention group also received the pelvic floor exercise booklet (see Appendix 4.3).
Ensuring standardisation of intervention
All staff delivering the intervention received exactly the same training in order to ensure consistency of their method of teaching and delivery of the PFMT, BT/US and biofeedback. Both specialist continence physiotherapists and specialist continence nurses were eligible to deliver the intervention, thus increasing the generalisability of the trial.
The therapists recorded their assessments and treatment programmes on standard study forms (see Appendix 4.1). Data were stored locally in case notes but collected and analysed centrally (see Chapters 6 and 11).
Centres, resources and therapists
Because physiotherapists were not available at every centre, and to increase the generalisability of the intervention to the NHS, we chose to train both specialist continence physiotherapists and specialist continence nurses (as above). There were 17 centres with therapists from a physiotherapy background, and 17 with a nursing background (Table 7).
Machine-led biofeedback was available in 13 centres (Table 7). Therapists were asked to declare whether they felt that biofeedback would be clinically indicated (whether or not a biofeedback machine was available) and also whether it was actually used. In five centres that had access to a biofeedback machine, and in four without such access, therapists reported that they would like to use one. A biofeedback machine was actually used in 5 of the 13 centres with access, for 17 men after radical surgery and 10 after TURP (Table 7).
Chapter 4 Centres and recruitment to the two trials
This chapter describes how the two trial groups were identified from the men admitted for radical prostatectomy and TURP at the recruiting hospitals. It reports the baseline comparability of the men in hospital, the response to the screening survey, and the characteristics of the study groups up to the point of entry to the RCTs.
Study recruitment
Men who were having prostate surgery were approached during their hospital stay by recruitment officers in each of the 34 centres. Men were asked to consent to being sent a screening questionnaire at 3 weeks after their surgery. If their response indicated that they were incontinent, they were invited to participate in the RCT of PFMT.
Centre screening and recruitment
Table 8 shows the number of men approached in each centre, and how many were eventually eligible for screening and randomisation. In total, 780 men having radical prostatectomy and 2836 men who had TURP were sent a screening questionnaire, 742 and 2590 responded, and 429 and 442 were eventually randomised. However, 18 of the ‘radical’ men from one centre were subsequently excluded after randomisation (postrandomisation exclusion) as therapy was not available during some of the period of screening in that centre, leaving 411 randomised to the radical prostatectomy trial.
| Centre | Radical prostatectomy | TURP | ||||
|---|---|---|---|---|---|---|
| n screened/N approached | n responded/N screened | n randomised/N responded to screening | n screened/N approached | n responded/N screened | n randomised/N responded to screening | |
| Aberdeen | 76/85 | 74/76 | 55/74 | 320/467 | 302/320 | 60/302 |
| Ipswich | 40/49 | 39/40 | 15/39 | 146/511 | 138/146 | 17/138 |
| Dundee | 1/1 | 1/1 | 1/1 | 27/37 | 27/27 | 4/27 |
| Stockport | 70/97 | 68/70 | 37/68 | 191/476 | 173/191 | 27/173 |
| Tameside | 30/32 | 26/30 | 18/26 | 145/203 | 125/145 | 19/125 |
| Glasgow Southern | 4/45 | 3/4 | 0/3 | |||
| Middlesbrough | 49/60 | 47/49 | 36/47 | |||
| Falkirk | 4/22 | 4/4 | 2/4 | 6/62 | 6/6 | 1/6 |
| Newcastle | 45/61 | 42/45 | 23/42 | 491/661 | 448/491 | 63/448 |
| Airedale | 5/15 | 5/5 | 2/5 | 19/109 | 18/19 | 3/18 |
| Reading | 69/98 | 68/69 | 32/68 | 144/408 | 131/144 | 18/131 |
| Wakefield | 15/23 | 15/15 | 6/15 | 59/150 | 57/59 | 11/57 |
| Ayr | 109/225 | 101/109 | 17/101 | |||
| Bristol | 26/51 | 26/26 | 16/26 | 110/244 | 104/110 | 24/104 |
| Stevenage | 0/3 | 27/108 | 26/27 | 8/26 | ||
| Inverness | 38/41 | 36/38 | 18/36 | 108/144 | 98/108 | 15/98 |
| Leeds | 118/168 | 113/118 | 77/113 | 205/623 | 180/205 | 24/180 |
| Inverclyde | 20/30 | 16/20 | 3/16 | |||
| Wolverhampton | 38/115 | 36/38 | 9/36 | |||
| Swansea | 31/36 | 30/31 | 15/30 | 43/63 | 38/43 | 6/38 |
| Sheffield | 63/67 | 61/63 | 27/61 | 112/143 | 102/112 | 22/102 |
| Ilford | 20/22 | 18/20 | 4/18 | |||
| Bolton | 8/12 | 6/8 | 3/6 | 51/155 | 43/51 | 8/43 |
| Taunton | 4/5 | 4/4 | 2/4 | 71/82 | 62/71 | 14/62 |
| Norwich | 12/24 | 12/12 | 5/12 | 18/72 | 17/18 | 1/17 |
| Yeovil | 1/2 | 1/1 | 0/1 | 40/63 | 39/40 | 10/39 |
| Edinburgh | 10/81 | 10/10 | 6/10 | 23/220 | 21/23 | 4/21 |
| Dunfermline | 5/6 | 5/5 | 2/5 | 91/108 | 82/91 | 14/82 |
| Cardiff | 107/194 | 97/107 | 19/97 | |||
| Macclesfield | 0/1 | 14/45 | 13/14 | 1/13 | ||
| Southmead | 29/86 | 24/29 | 15/24 | 44/150 | 38/44 | 8/38 |
| Crewe | 33/51 | 31/33 | 8/31 | |||
| Hillingdon | 1/1 | 1/1 | 1/1 | |||
| St Mary’s, London | 28/28 | 22/28 | 13/22 | |||
| Hope, Salford | 2/3 | 2/2 | 2/2 | |||
| Total (34) | 780/1158 | 742/780 | 429/742 | 2836/5986 | 2590/2836 | 442/2590 |
Reasons for not completing a screening questionnaire
Table 9a describes the reasons given for ineligibility for receiving a screening questionnaire. The most common reasons for ineligibility were that the men were missed (no contact with recruitment officer), they refused, they were unable to give informed consent or they were having radiotherapy or palliative surgery, or that the operation was not carried out or changed to another operation. Table 9b shows that the men having TURP who were ineligible for screening were older than those who consented to screening.
| Radical prostatectomy | TURP | |
|---|---|---|
| Approached | 1158 | 5986 |
| [Consented, N] | [804] | [2985] |
| Not consented | 354/1158 (31) | 3001/5986 (50) |
| Reasons for no consent | ||
| No reason given | 15 (4) | 65 (2) |
| Refused | 75 (21) | 601 (20) |
| Not approached/missed | 154 (44) | 1078 (36) |
| Unable to give informed consent | 20 (6) | 537 (18) |
| Referred for formal physiotherapy | 10 (3) | 3 (0) |
| Radiotherapy planned or palliative surgery | 6 (2) | 485 (16) |
| Surgery cancelled or changed (e.g. BNI) | 17 (5) | 198 (7) |
| On other trial (e.g. ProtecT) | 47 (13) | 3 (0) |
| Unclear/other reason | 10 (3) | 31 (1) |
| Radical prostatectomy | TURP | |||
|---|---|---|---|---|
| Eligible | Ineligible | Eligible | Ineligible | |
| Age, years [mean (SD) n] | 62.5 (5.9) 802 | 63.1 (6.5) 347 | 69.9 (8.3) 2972 | 73.4 (9.1) 2971 |
Table 10a describes the reasons why men who were eligible for screening (signed consent forms received at the study office in Aberdeen) were in the event not sent a screening questionnaire. The most common reason was that the planned operation was changed or cancelled after the men had signed a consent form. Only men who actually had a radical prostatectomy or TURP were eligible to be screened. Table 10b shows that there were no significant differences in age between those who were eligible and screened and those who were eligible and not screened.
| Radical prostatectomy | TURP | |
|---|---|---|
| Consented n/N (%) | 804/1158 (69.4) | 2985/5986 (49.9) |
| Consented and screened n/N (%) | 780/804 (97) | 2838/2985 (95) |
| Consented but not screened n/N (%) | 24/804 (3) | 147/2985 (5) |
| Reasons for not screening (n) | ||
| No reason given | 0 (0) | 3 (3) |
| Refused | 0 (0) | 11 (8) |
| Referred for formal physiotherapy | 1 (4) | 0 |
| Radiotherapy planned or palliative surgery | 1 (4) | 17 (12) |
| Surgery cancelled or changed (e.g. BNI) | 12 (50) | 106 (72) |
| On other trial (e.g. ProtecT) | 0 (0) | 1 (1) |
| Unclear/other reason | 10 (42) | 11 (6) |
| Radical prostatectomy | TURP | |||
|---|---|---|---|---|
| Screened | Not screened | Screened | Not screened | |
| Age, years [mean (SD) n] | 62.4 (6.0) 778 | 63.5 (5.2) 24 | 70.0 (8.2) 2825 | 69.3 (10.0) 147 |
Recruitment of men to the randomised controlled trial
Each man who indicated on his screening questionnaire that he had urinary incontinence was sent a baseline questionnaire and contacted around a week later by a dedicated recruitment officer based at the MAPS study office in Aberdeen. Eligibility was confirmed and, upon receipt of the signed RCT consent form, men were randomised to the intervention or standard care groups. Figures 4 and 5 show the flow of the number of patients who were approached to take part in the screening through to randomisation into the radical prostatectomy and TURP trials respectively.
FIGURE 4.
Flow chart of men from operation to recruitment to RCT: radical prostatectomy. a, Postrandomisation exclusion: therapy was not available during some of the period of screening in one centre (18 men).
FIGURE 5.
Flow chart of men from operation to recruitment to RCT: TURP.
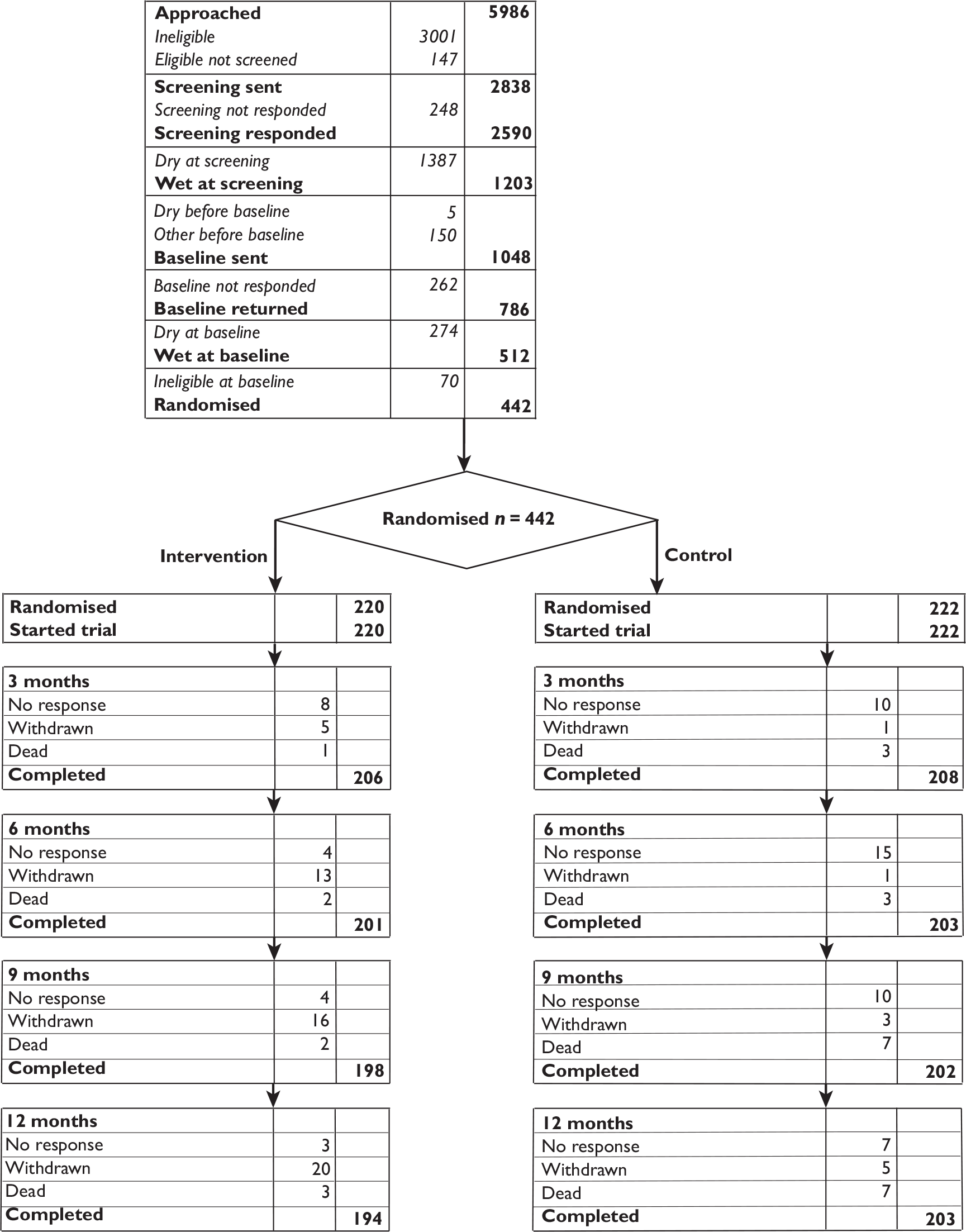
Reasons for screened patients not to be subsequently randomised
Table 11 describes the reasons why screened men were not randomised into the trials. The most common reason was that the men were continent at the screening questionnaire or subsequently continent by the time the baseline questionnaire or telephone recruitment call was conducted.
| Radical prostatectomy | TURP | |
|---|---|---|
| Number of men responding at screening but not sent a baseline questionnaire | ||
| Dry at screening | 51 | 1387 |
| Dry before baseline | 1 | 5 |
| Declined further contact | 26 | 122 |
| Referred for PFMT | 2 | 1 |
| Referred for radiotherapy | 1 | – |
| Moving away/unable to attend therapy | 1 | 1 |
| Permanently catheterised | – | 1 |
| No reason given | 2 | 25 |
| Number of men responding at baseline but not randomised | ||
| Dry before randomisation | 61 | 274 |
| Declined participation in RCT | 15 | 16 |
| Unable/unwilling to travel to therapy appointments | 11 | 32 |
| Dry after baseline | 8 | 18 |
| Attending PFMT training | 2 | – |
| Having radiotherapy | 5 | – |
| Medical problems | 1 | 4 |
| In another study | 1 | – |
| Postrandomisation exclusions | ||
| Therapy not available during some of the period of screening in one centre | 18 | – |
Recruitment rates
The original projections were based on assumptions from the literature that around 50% of men would be wet after a radical prostatectomy, and that 65% of these would be willing to enter an RCT of conservative treatment (see Table 2). For the men having TURP, we expected around 5% of men to be wet, and that a similar proportion (65%) would agree to randomisation. The sample size calculation had indicated that we would need to recruit 400 men per trial in order to achieve our prespecified difference, and that 26 centres would be sufficient to achieve this.
In the event, the numbers of men having operations at each centre varied widely (see Table 8) but the proportions of men responding, incontinent and willing to enter the RCT were much higher than our assumptions, especially in the TURP group. Despite this, a review of actual accrual rates led us to anticipate a shortfall in recruitment. We therefore prepared revised projections, based on more realistic assumptions and recruitment of extra centres, and were granted a 9-month extension in order to achieve our initial target sample size.
By the end of recruitment, 7144 men had been approached regarding the screening survey in 34 sites, and 853 men from these sites had been randomised. Figure 6 shows a graphical representation of recruitment against revised targets as agreed for the extension period. The jump in the projected recruitment line after December 2006 reflects the change after implementing the extension. The last men were approached in July 2008 and recruitment to the RCT ended on 23 September 2008.
FIGURE 6.
Recruitment to MAPS against revised targets agreed for the extension period.
Chapter 5 Radical randomised controlled trial: derivation and description of participants
This chapter describes the men derived from the screening survey in terms of their clinical characteristics and presents the baseline comparability between the randomised groups in the group having radical prostatectomy.
Comparison between those responding and not responding to screening survey
Table 12 shows the comparability at baseline of those responding and not responding to the screening survey in terms of their demographic and clinical characteristics. There were no clinically important differences between the responders and non-responders. The majority of men (around 80%) had a traditional abdominal retropubic prostatectomy, 2% had a perineal approach and just under 20% had a laparoscopic procedure. One or both nerve bundles were spared in just over 60% of operations amongst the responders.
| Radical prostatectomy | Responder | Non-responder |
|---|---|---|
| Number screened [n/N (%)] | 742/780 (95) | 38/780 (5) |
| Age [mean years (SD) n] | 62.5 (5.9) 740 | 61.1 (7.4) 38 |
| Weight [mean kg (SD) n] | 82.8 (12.2) 704 | 84.2 (12.7) 35 |
| Height (mean cm (SD) n] | 173.9 (13.1) 664 | 173.9 (8.4) 34 |
| Current smoker (yes) [n/N (%)] | 81/742 (11) | 7/38 (18) |
| Nights in hospital [mean (SD) n] | 6.2 (2.9) 687 | 5.7 (3.2) 33 |
| Type of operation | N = 740 | N = 38 |
| Abdominal retropubic prostatectomy [n/N (%)] | 585/740 (79) | 31/38 (82) |
| Perineal radical prostatectomy [n/N (%)] | 15/740 (2) | 0 |
| Laparoscopic radical prostatectomy [n/N (%)] | 140/740 (19) | 7/38 (18) |
| Nerve bundle sparing | N = 708 | N = 36 |
| One nerve bundle spared [n/N (%)] | 133/708 (19) | 10/36 (28) |
| Both nerve bundles spared [n/N (%)] | 302/708 (43) | 9/36 (25) |
| Neither nerve bundles spared [n/N (%)] | 90/708 (13) | 2/36 (6) |
| Unknown nerve bundle sparing [n/N (%)] | 183/708 (26) | 15/36 (42) |
Findings from screening survey
The average time of completion of the screening survey was at around 5 weeks after surgery (mean days since operation 38.1, SD 14.9).
Radical prostatectomy can be performed using three main routes: open abdominal, perineal, and laparoscopically. The last two are thought to be associated with less postoperative urinary incontinence and sexual dysfunction. 1,66 In addition, urinary incontinence is thought to be reduced in operations in which it is possible to spare one or both nerve bundles. 11,67,68 The majority of men had a traditional open abdominal prostatectomy, with only a few having a perineal approach (n = 15). Table 13 shows that, at screening, there was little difference in the chance of immediate incontinence according to the route of operation. Nor did the chance of incontinence differ according to the surgeon’s ability to spare one or both nerve bundles (Table 13). Long-term follow-up will be needed to confirm whether these findings persist. The most common type of incontinence was SUI (76%).
| Radical prostatectomy | All responders to screening questionnaire (n = 742)a | Abdominal route (n = 585) | Perineal route (n = 15) | Laparoscopic route (n = 140) |
|---|---|---|---|---|
|
Days since operation [mean (SD) n] |
38.1 (14.9) 732 | 33.0 (14.6) 578 | 30.0 (7.9) 15 | 34.5 (14.5) 139 |
| Number of men with any urine loss at screening questionnaire [n/N (%)] | 691/742 (93) | 543/585 (93) | 15/15 (100) | 131/140 (94) |
| Nerve bundle sparing: number of men with any urine loss [n/N (%)] | 658/708 (93) | 514/555 (93) | 15/15 (100) | 128/137 (93) |
| One nerve bundle spared [n/N (%)] | 126/133 (95) | 98/103 (95) | 5/5 (100) | 23/25 (92) |
| Both nerve bundles spared [n/N (%)] | 280/302 (93) | 212/230 (92) | 4/4 (100) | 64/68 (94) |
| Neither spared [n/N (%)] | 87/90 (97) | 71/74 (96) | 1/1 (100) | 15/14 (100) |
| Unknown sparing [n/N (%)] | 165/183 (90) | 133/148 (90) | 5/5 (100) | 26/29 (90) |
| ICI-QoL score owing to UIb [mean (SD) n] | 4.6 (5.6) 726 | 4.4 (3.2) 572 | 5.1 (3.3) 15 | 5.5 (3.3) 139 |
| ICI-Q scorec [mean (SD) n] | 10.8 (5.6) 740 | 10.4 (5.5) 585 | 11.9 (4.7) 15 | 12.3 (5.8) 140 |
| Number of men with urine loss before surgery [n/N (%)] | 47/740 (6) | 45/585 (8) | 0 | 2/140 (1) |
| Number of men with faecal incontinence after surgery [n/N (%)] | 18/742 (2) | 14/585 (2) | 1/15 (8) | 3/140 (2) |
| Type of incontinence | ||||
| SUI [n/N (%)] | 559/740 (76) | 427/585 (73) | 14/15 (93) | 118/140 (84) |
| UUI [n/N (%)] | 297/740 (40) | 227/585 (39) | 9/15 (60) | 61/140 (44) |
| MUI [n/N (%)] | 242/740 (33) | 175/585 (30) | 9/15 (60) | 58/140 (41) |
| Postmicturition leakage [n/N (%)] | 253/740 (34) | 185/585 (32) | 7/15 (47) | 61/140 (44) |
| Other incontinence [n/N (%)] | 334/740 (45) | 266/585 (45) | 6/15 (40) | 62/140 (44) |
Summary information for progress from screening questionnaire to randomisation
Of 691 men wet at screening, 33 were not eligible to be sent a baseline questionnaire (see Table 11 for reasons). Of the 658 men sent a baseline questionnaire, 533 (81%) responded, of whom 472 (89%) were still wet and 61 dry. A further 43 were excluded as they were ineligible for randomisation despite still being incontinent (see Table 11 for reasons). Finally, 429 men were randomised but 18 were excluded after randomisation as there was no therapy available during their recruitment period, leaving 411 men properly randomised, 205 in the intervention group and 206 in the control group (see Figure 4).
Men who recorded that they were wet at the screening survey were sent a further baseline questionnaire to confirm persistent leakage. Those who were still wet and consented were randomised to intervention or control. The average time to randomisation from the date of surgery was 8 weeks (mean 7.9, SD 2.7).
Comparability on baseline characteristics at trial entry
Table 14 shows that the men in the two randomised groups were comparable at baseline on the clinical and demographic characteristics recorded.
| Radical prostatectomy | Intervention (n = 205) | Control (n = 206) |
|---|---|---|
| Age in years [mean (SD) n, (min–max)] | 62.4 (5.8) 205, (47–76) | 62.3 (5.6) 206, (47–75) |
| BMI (kg/m2) [mean (SD) n, (min–max)] | 25.9 (2.9) 197, (19.4–39.5) | 26.3 (3.3) 202, (18.0–36.2) |
| Type of operation [n/N (%)]a | 204 | 205 |
| Abdominal | 157/204 (77) | 161/205 (79) |
| Perineal | 6/204 (3) | 4/205 (2) |
| Laparoscopic | 41/204 (20) | 40/205 (20) |
| TURP before surgery [n/N (%)] | 12/205 (6) | 4/201 (2) |
| Number of men not able to achieve erection before prostate surgery [n/N (%)] | 17/205 (8) | 18/202 (9) |
| Leakage of urine before operation [n/N (%)] | 14/205 (7) | 13/206 (6) |
| ICI-Q score at baseline [mean (SD) n]b | 11.2 (4.3) 205 | 11.5 (4.5) 206 |
| Number of men with severe incontinence at baseline [n/N (%)]c | 188/205 (92) | 189/206 (92) |
| Urinary frequency at baseline (per day) [mean (SD) n] | 7.4 (2.9) 187 | 7.9 (3.7) 192 |
| Nocturia at baseline (per night) [mean (SD) n] | 2.2 (1.2) 199 | 2.5 (1.6) 202 |
| Type of incontinence [n/N (%)] | 205 | 206 |
| SUI | 195/205 (95) | 195/206 (95) |
| UUI | 135/205 (66) | 156/206 (76) |
| MUI (both) | 132/205 (64) | 151/206 (73) |
| Postmicturition leakage | 166/205 (81) | 170/206 (83) |
| Other incontinence | 72/205 (35) | 91/206 (44) |
| Pad use | 180/205 (88) | 176/205 (86) |
| Other health problems | 89/204 (44) | 94/204 (46) |
| EQ-5D [mean (SD) n] | 0.8 (0.2) 200 | 0.8 (0.2) 206 |
| SF-12 mental [mean (SD) n] | 50.8 (10.5), 201 | 49.3 (10.7), 201 |
| SF-12 physical [mean (SD) n] | 42.7 (9.9), 201 | 41.8 (10.6), 201 |
Prior knowledge of pelvic floor exercises
Many men had been counselled before surgery about the possibility of urinary incontinence and sexual dysfunction after surgery. 69 Table 15 shows that almost all of the men (97% and 99% in the two groups) had prior knowledge of pelvic floor exercises for these problems. The most common sources of information were from nurses or continence advisors or from leaflets or books (Table 15).
| Source of information | Intervention | Control |
|---|---|---|
| From a doctor | 79/195 (41) | 72/191 (38) |
| From a nurse/continence advisor | 147/195 (75) | 136/191 (71) |
| From a physiotherapist | 21/195 (11) | 23/191 (12) |
| From leaflets or books | 127/195 (65) | 129/191 (68) |
| From the internet | 22/195 (11) | 34/191 (18) |
| From friends or family | 42/195 (22) | 43/191 (23) |
| From another source | 2/195 (1) | 2/191 (1) |
| At least one source of information | 190/195 (97) | 190/191 (99) |
Chapter 6 Radical randomised controlled trial: management received
This chapter describes how the intervention was implemented in the therapy arm of the radical RCT, and the progress of men through the intervention period (n = 205). The information in this chapter is derived from the therapy documentation (see Appendix 4.1), which was used primarily to guide the therapists while delivering the standardised intervention.
Compliance with therapy
Of the 205 men who were randomised to the intervention, 189 attended at least one visit (92%), and 85% attended every time (Table 16). The non-attenders were younger and lighter, but these differences were not clinically important (Table 17). Only 5 of the 16 men who did not attend were dry. The other main reason was that, after they were allocated to therapy, five men found it to be inconvenient or impossible to attend appointments, often owing to work commitments (Table 18).
| Radical prostatectomy | First visit | Second visit | Third visit | Fourth visit |
|---|---|---|---|---|
| Number of men attending [n (%)] | 189 (92) | 186 (91) | 177 (86) | 175 (85) |
| Radical prostatectomy | Attenders | Non-attenders |
|---|---|---|
| Age (years) | 62.6 (5.7) 189 | 59.8 (6.6) 16 |
| BMI (kg/m2) | 26.0 (3.0) 181 | 25.8 (1.5) 16 |
| Radical prostatectomy | Non-attenders (n) |
|---|---|
| Dry | 5 |
| Ill | 1 |
| Unable to attend | 5 |
| Declined | 0 |
| No reason given | 5 |
| Total | 16 |
Relationship between type of therapist and outcomes during therapy period
Half of the centres (17) used a physiotherapist to deliver the MAPS intervention, while in the other 17 the therapist had a nursing background (Table 19). There was no significant difference in the number of visits men made to physiotherapists or nurse therapists (Table 19). During the 3-month intervention period, there were no statistically significant differences in the mean ICI scores (a composite score reflecting urinary incontinence and its effect on quality of life) between therapists (Table 19).
| Radical prostatectomy | Physiotherapist | Continence nurse | Mean difference (95% CI), p-value |
|---|---|---|---|
| Number of attendances | 3.7 (1.0) 79, (3.5 to 3.9) | 3.4 (1.3) 126, (3.2 to 3.7) | 0.26 (–0.06 to 0.59), 0.113 |
| ICI-Q score | |||
| Visit 1 | 8.3 (4.7) 75, (7.2 to 9.4) | 7.6 (4.0) 114, (6.8 to 8.3) | 0.71 (–0.55 to 1.98), 0.267 |
| Visit 2 | 7.1 (4.1) 75, (6.2 to 8.1) | 6.6 (4.0) 111, (5.9 to 7.4) | 0.54 (–0.65 to 1.74), 0.372 |
| Visit 3 | 5.7 (4.1) 72, (4.8 to 6.7) | 5.5 (3.8) 105, (4.7 to 6.2) | 0.26 (–0.94 to 1.45), 0.674 |
| Visit 4 | 4.6 (3.8) 71, (3.7 to 5.5) | 4.1 (3.6) 104, (3.4 to 4.8) | 0.49 (–0.64 to 1.61), 0.396 |
Urinary incontinence during therapy period
Incidence of urinary incontinence
The therapists asked the men at each visit to rate their incontinence (in the previous week). This allowed the therapists to monitor the change in reported incontinence. They used the same form of question as the questionnaires, based on the ICI-SF instrument, which were also used to measure the primary outcome. The proportion of men with incontinence fell from 92% to 73%, while the mean ICI score decreased (improved) from around 8 at the start of treatment to around 4 afterwards (Table 20 and Figure 7).
| Radical prostatectomy | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Men incontinent [n/N (%)] | 172/187 (92) | 165/182 (91) | 143/171 (84) | 123/169 (73) |
| ICI-Q scorea [mean (SD) n] | 7.9 (4.3) 189 | 6.8 (4.1) 186 | 5.6 (3.9) 177 | 4.3 (3.7) 175 |
FIGURE 7.
Trend analysis of the proportion (%) of men incontinent at each visit: men having had radical prostatectomy. ICI-Q score: 0 = none, 21 = maximum (worst) score. Derived from questions 1–3 of the ICIQ-UI Short Form Questionnaire.
Type of urinary incontinence during therapy period
The distribution of type of incontinence reported by the men did not vary with time across the therapy visits (Table 21 and Figure 8) except that the proportion with stress incontinence alone decreased slightly (from 84% to 72%), and postmicturition leakage also decreased (from 63% to 25%).
| Radical prostatectomy | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Number of men | 189 | 186 | 177 | 175 |
| SUI | 152/181 (84) | 145/175 (83) | 118/162 (73) | 110/152 (72) |
| UUI | 35/173 (20) | 31/159 (19) | 33/154 (21) | 22/145 (15) |
| MUI (both SUI and UUI) | 28/182(15) | 26/174(15) | 24/162(15) | 17/155(11) |
| Postmicturition leakage | 113/179 (63) | 68/163 (42) | 49/157 (31) | 36/145 (25) |
| Other UI | 47/162 (29) | 35/148 (24) | 34/146 (23) | 27/135 (20) |
FIGURE 8.
Type of incontinence at each visit and change over time: men having had radical prostatectomy.
Incidence and type of bowel problems during therapy period
Therapists also enquired at each visit about whether the men experienced any bowel dysfunction in the previous week. The proportions of men with three different types of bowel dysfunction (faecal incontinence, faecal urgency and constipation) were low, and did not vary during the therapy period (Table 22 and Figure 9).
| Radical prostatectomy | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Faecal incontinence | 4/187 (2) | 3/183 (2) | 2/172 (1) | 2/171 (1) |
| Faecal urgency | 13/187 (7) | 11/183 (6) | 13/172 (8) | 6/171 (4) |
| Constipation | 23/187 (12) | 10/181 (5) | 11/171 (6) | 13/169 (8) |
FIGURE 9.
Type of bowel problems at each visit and change over time: men having had radical prostatectomy.
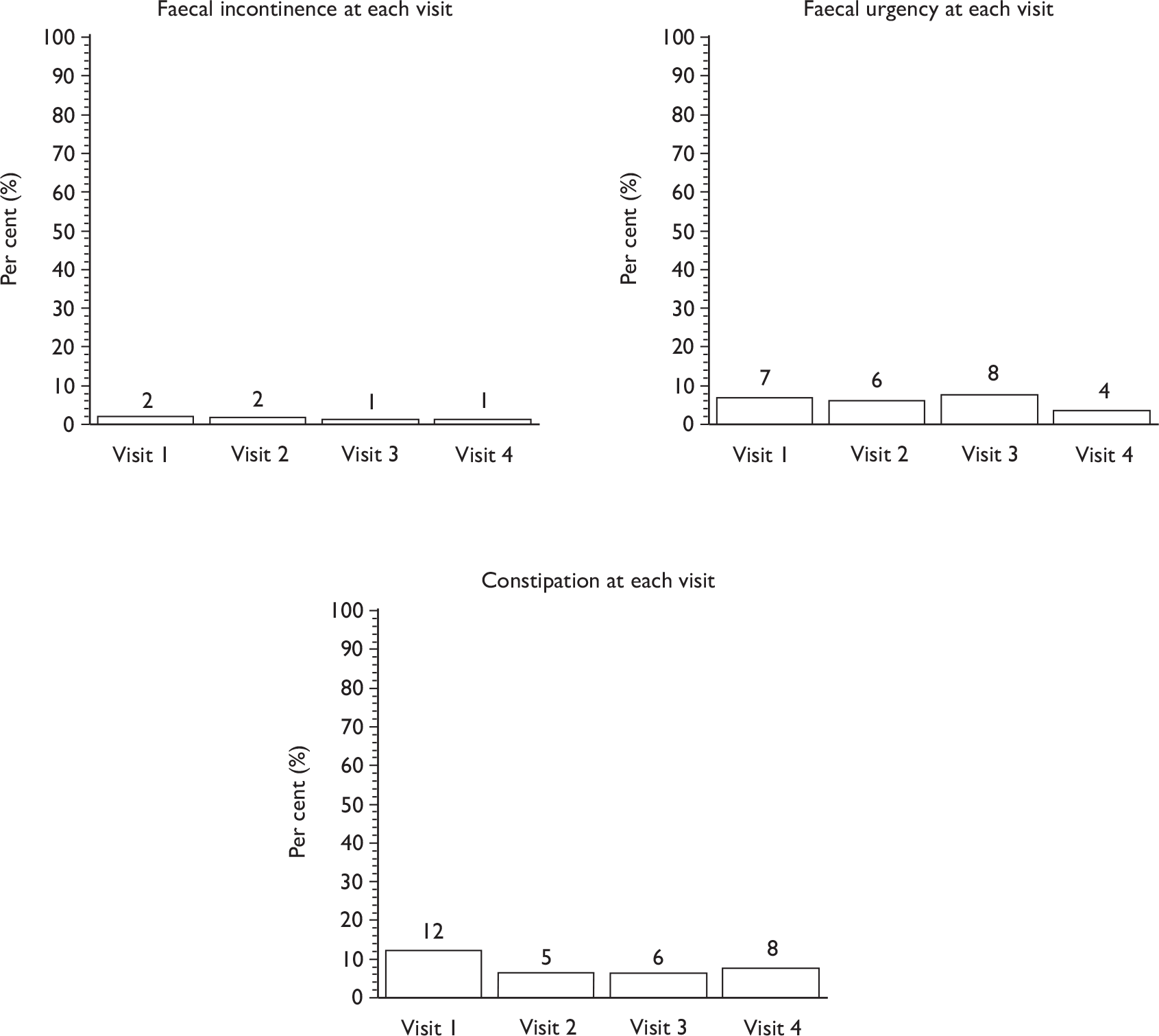
Incidence and type of sexual problems during therapy period
The questions relating to sexual problems were those used in routine clinical practice. They were not based on the questions men were asked at 12 months to assess their sexual function and activity 70 (see section G, 12-month questionnaire, Appendix 3.3).
The proportion of men with sexual dysfunction (‘difficulty gaining or maintaining an erection in the last week’) after radical surgery was high (85–90%) and this did not change during the therapy period. The corresponding proportion with premature ejaculation was very low and also did not vary with time (Table 23 and Figure 10).
| Radical prostatectomy | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Difficulty gaining erection | 156/176 (89) | 153/170 (90) | 142/159 (89) | 144/161 (89) |
| Difficulty maintaining erection | 148/169 (88) | 144/165 (87) | 128/148 (86) | 145/157 (92) |
| Premature ejaculation | 4/154 (3) | 4/150 (3) | 4/139 (3) | 2/143 (1) |
FIGURE 10.
Type of sexual problems at each visit and change over time: men having had radical prostatectomy.
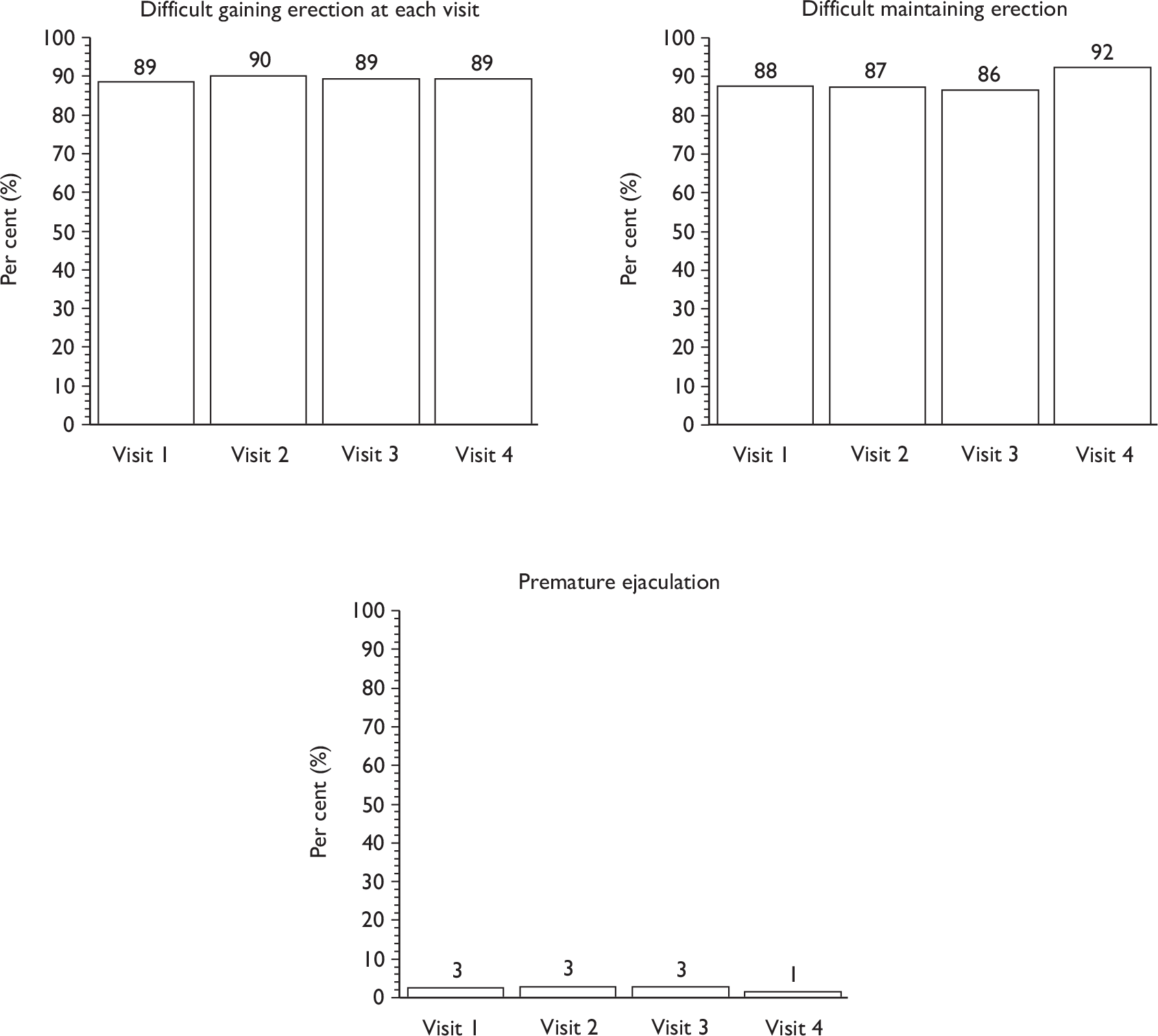
Examination of pelvic floor muscle performance during therapy visits
Therapists assessed the strength of the pelvic floor muscle contractions and their endurance (length of time men were able to hold a contraction) at each visit using digital anal assessment (see Chapter 3). The external anal sphincter and the internal puborectalis muscle were assessed separately. The internal puborectalis muscle strength was taken to be a measure of pelvic floor muscle strength.
For both the sphincter and the puborectalis, both strength and endurance improved during the therapy period (Table 24 and Figure 11). At baseline, only 15% of men had a strength of 5 or more, whereas by the fourth visit around 50% of men were able to contract strongly (5) or very strongly (6), and 85% had good muscle strength (4 or better). The therapists were trained to ask men to hold the pelvic floor muscle contraction for up to 10 seconds during the digital anal examination. This is in line with functional use of these muscles. However, some therapists assessed the maximum length of time for which men could hold a contraction. Of these men, some held the contraction for over 1 minute.
| Radical prostatectomy | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| A: External anal sphincter strength [mean (SD) n]a | 3.3 (1.0) 170 | 3.6 (0.9) 153 | 4.0 (1.0) 127 | 4.3 (1.0) 129 |
| 0 | 1/170 (0.5) | 0 | 0 | 0 |
| 1 | 1/170 (0.5) | 2/153 (1) | 1/127 (1) | 1/129 (1) |
| 2 | 29/170 (17) | 11/153 (7) | 9/127 (7) | 4/129 (3) |
| 3 | 71/170 (42) | 54/153 (35) | 20/127 (16) | 17/129 (13) |
| 4 | 48/170 (28) | 62/153 (41) | 57/127 (45) | 45/129 (35) |
| 5 | 19/170 (11) | 23/153 (15) | 38/127 (30) | 51/129 (39) |
| 6 | 1/170 (0.5) | 1/153 (1) | 2/127 (1) | 11/129 (8) |
| A: External anal sphincter endurance (seconds) [mean (SD) n]b | 6.1 (2.7) 170 | 7.6 (2.8) 153 | 9.3 (6.0) 127 | 10.6 (8.4) 129 |
| B: Puborectalis muscle strength [mean (SD) n]a | 3.4 (1.0) 169 | 3.7 (0.9) 153 | 4.1 (1.0) 126 | 4.4 (1.0) 128 |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 4/169 (2) | 3/153 (2) | 2/126 (2) | 1/128 (1) |
| 2 | 24/169 (14) | 8/153 (5) | 5/126 (4) | 3/128 (2) |
| 3 | 68/169 (40) | 43/153 (28) | 23/126 (18) | 19/128 (15) |
| 4 | 51/169 (30) | 71/153 (46) | 48/126 (38) | 39/128 (30) |
| 5 | 22/169 (13) | 28/153 (18) | 44/126 (35) | 52/128 (41) |
| 6 | 0 | 0 | 4/126 (3) | 14/128 (11) |
| B: Puborectalis muscle endurance (seconds) [mean (SD) n]b | 6.5 (3.0) 169 | 7.7 (2.7) 153 | 9.4 (6.1) 126 | 10.7 (8.5) 128 |
FIGURE 11.
Ability to contract anal sphincter and puborectalis muscle (pelvic floor) over time: men having had radical prostatectomy. (a) External anal sphincter strength/endurance. (b) Puborectalis muscle strength/endurance. 0 = No flicker; 1 = flicker; 2 = weak; 3= moderate movement; 4 = good resistance; 5 = strong resistance; 6 = very strong, unable to withdraw finger. Endurance of contraction in seconds: median, minimum, maximum and mean duration.
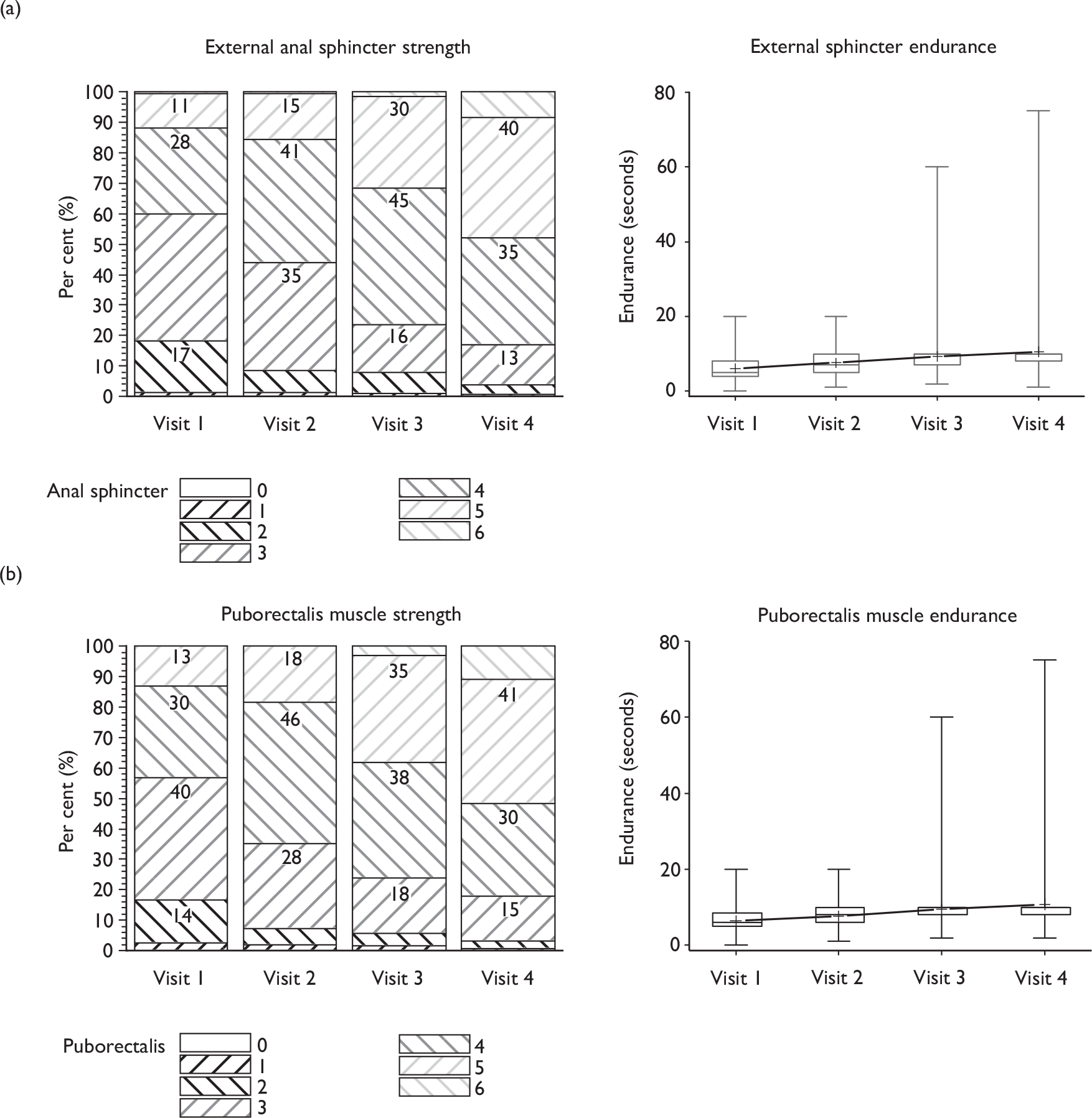
Examination and functional use of pelvic floor muscles
Therapists examined men at each visit to assess skin damage, skin infection, ability to tighten the anus, perform penile retraction and testicular lift, evidence of leakage on coughing and (for those who did leak) ability to prevent leakage on coughing. Very few men showed evidence of skin damage or infection (data not shown).
Four different aspects of functional use of pelvic floor muscles were assessed (ability to tighten anus; ability to perform penile retraction; leakage on coughing; ability to prevent leakage on coughing). While most men (around 95%) were able to contract well enough to tighten the anal sphincter at least a little from baseline onwards, the proportion able to demonstrate a testicular lift increased slightly with time (from 80% to 90%; Figure 12). The proportion who leaked when coughing decreased from about 18% to 9% during the therapy period. Around 80% of these men were able to contract their pelvic floor muscles sufficiently to prevent leakage when coughing at the first visit, and this improved only slightly to around 85% by the fourth visit.
FIGURE 12.
Aspects of ability to use pelvic floor muscles at each visit and change over time: men having had radical prostatectomy.
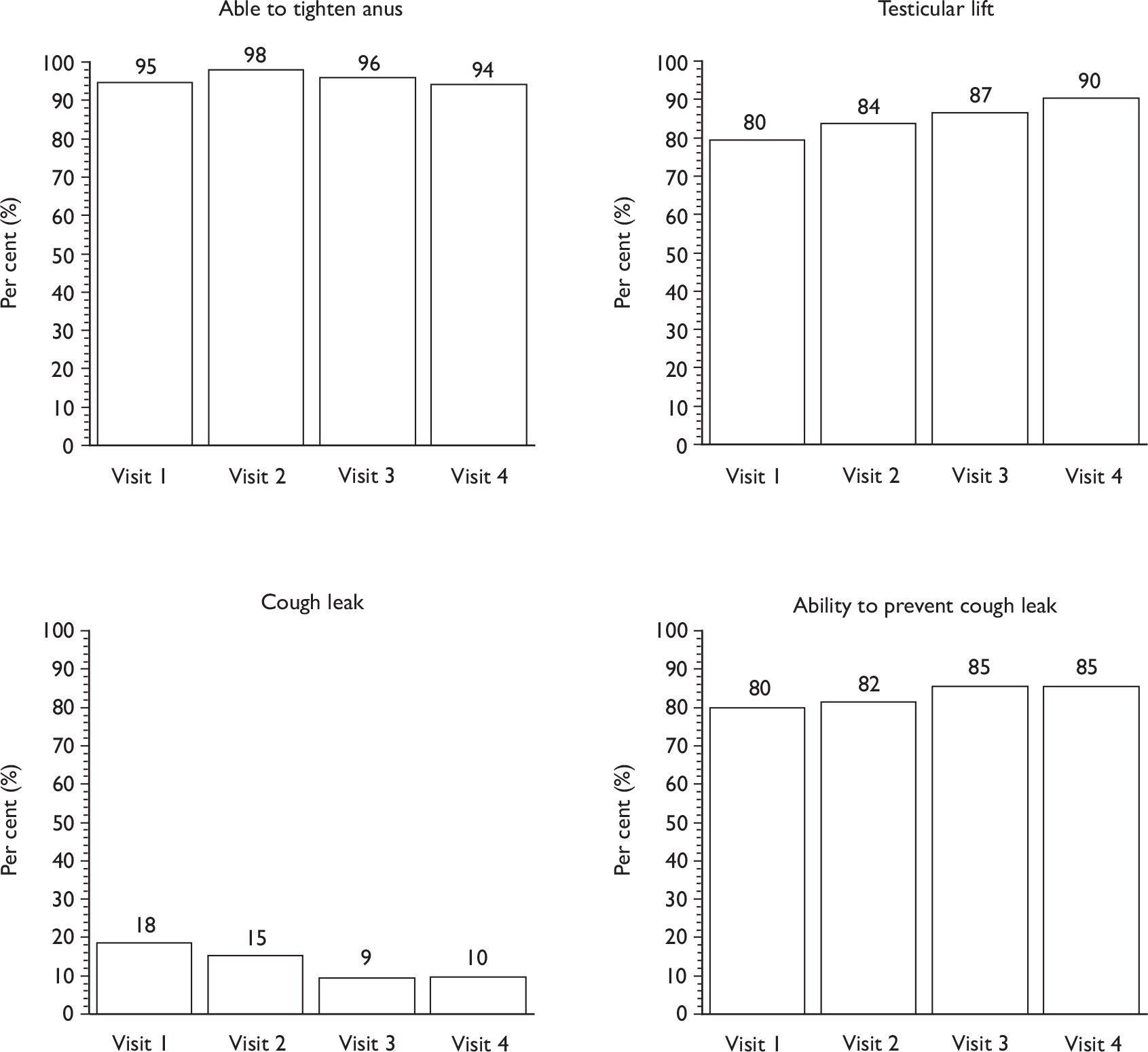
Use of machine-led biofeedback
Biofeedback was available in 13 of 34 MAPS centres, and was used clinically for MAPS men in five of them (see Table 7). Therapists would have liked access to this facility in four other centres where biofeedback was not available. Biofeedback can be used in two ways:
-
to feed back information to men that they are actually performing a correct pelvic floor contraction, and at what strength
-
as part of a repetitive training regimen when men are asked to use the machine to enable them to monitor their exercise function for a period of time (such as 20 minutes).
It was unclear which type of biofeedback was practised in the centres where this was available, but therapists from five centres recorded its use in 16 men (see Table 7) from the radical prostatectomy group (the number at each time point is shown in Table 25). In some cases men may have preferred anal examination using a machine rather than digital examination by the therapist for teaching of correct contractions. As it was most often used on the first visit, this suggests that suggests that biofeedback was used in a diagnostic rather than in a training capacity.
| Radical prostatectomy | Biofeedback indicated | Biofeedback actually implemented |
|---|---|---|
| Visit 1 | 12/123 (10) | 8/124 (6) |
| Visit 2 | 5/110 (4) | 5/110 (4) |
| Visit 3 | 5/94 (5) | 5/94 (5) |
| Visit 4 | 4/106 (4) | 4/106 (4) |
Chapter 7 Radical randomised controlled trial: outcomes and results
This chapter describes the results of the intervention amongst the men recruited to the radical prostatectomy RCT.
Patient flow
The derivation of the trial study groups and their progress through the trial is summarised in Figure 13. This is in the form of a CONSORT (Consolidated Standards of Reporting Trials) flow diagram. In total, 411 participants were recruited to the randomised trial: 205 randomly allocated to the intervention group and 206 to the control group. Nine men had withdrawn from follow-up by 12 months (although some information was available before their withdrawal in some cases). One of these nine, in the intervention group, subsequently died. His death was not attributed to the trial intervention. Sixteen participants (8%) in the intervention group did not attend any therapy sessions and were considered non-compliers with the intervention (see Chapter 6).
FIGURE 13.
Radical CONSORT diagram (men randomised n = 411). a, Postrandomisation exclusion: therapy was not available during some of the period of screening in one centre (18 men).
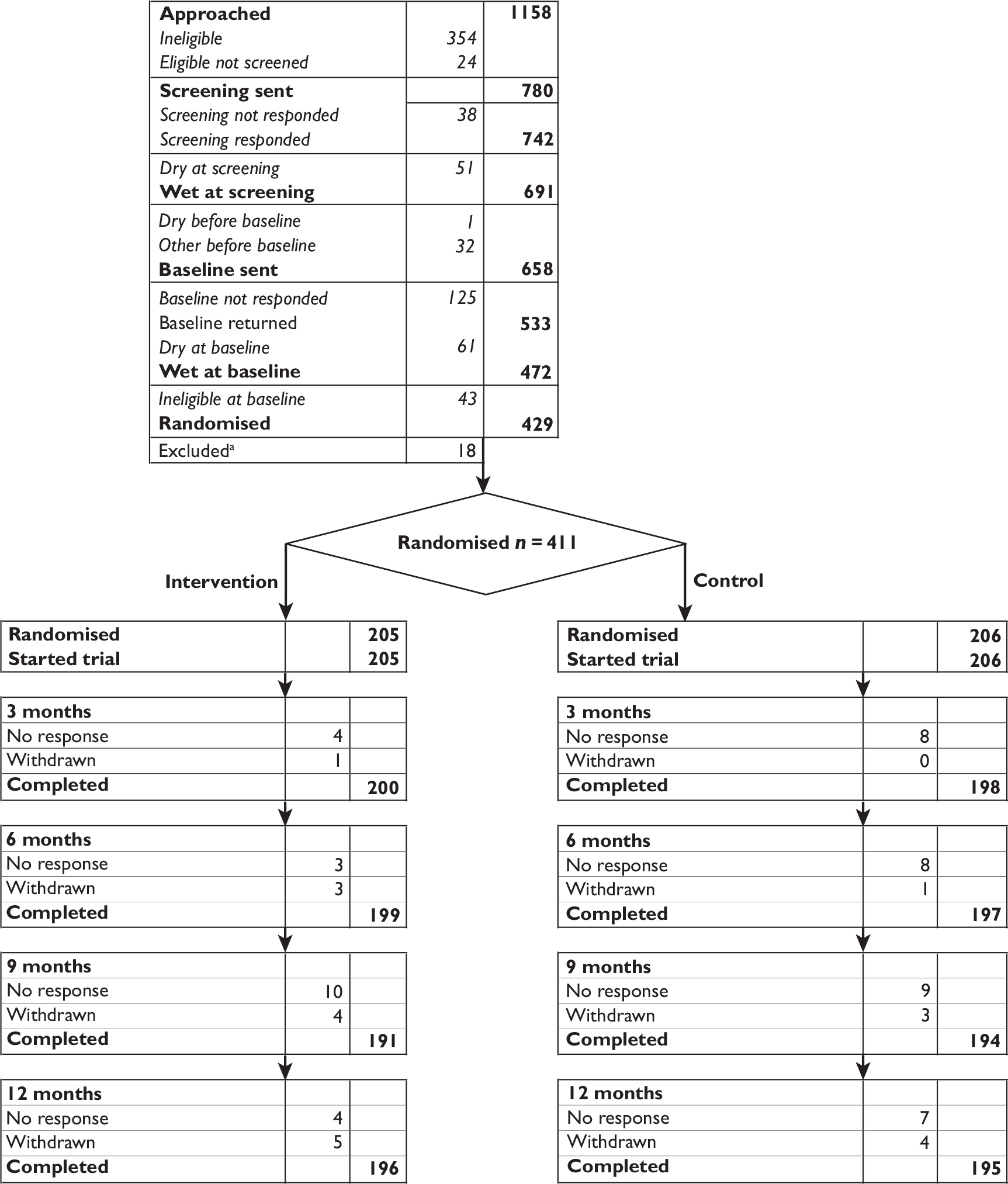
Response rates
Over 90% of all participants returned completed questionnaires. As shown in Figure 13, by the time of each follow-up some participants had formally withdrawn (five from the intervention group and four from the control group; Table 26), and so were not sent questionnaires. Of the participants for whom it was appropriate to send a follow-up questionnaire, over 95% returned it at each time point (Table 27a).
| Reason | Radical prostatectomy RCT | |
|---|---|---|
| Intervention | Control | |
| Ill | 1 | 1 |
| Dry | 1 | 1 |
| Catheterised permanently | 0 | 0 |
| No reason | 2 | 0 |
| Other | 1 | 2 |
| Total | 5 | 4 |
| Radical prostatectomy | Number sent | Number returned (%) | Percentage of all men |
|---|---|---|---|
| Baseline | |||
| Intervention | 205 | 205 (100) | 100 |
| Control | 206 | 206 (100) | 100 |
| 3 months | |||
| Intervention | 204 | 200 (98) | 98 |
| Control | 206 | 198 (96) | 96 |
| 6 months | |||
| Intervention | 202 | 199 (99) | 97 |
| Control | 205 | 197 (96) | 95 |
| 9 months | |||
| Intervention | 201 | 191 (95) | 93 |
| Control | 203 | 194 (95) | 94 |
| 12 months | |||
| Intervention | 200 | 196 (98) | 95 |
| Control | 202 | 195 (97) | 95 |
For return of urinary diaries, the response rate was slightly lower, but still approximately 90% at each time point (Table 27b).
| Radical prostatectomy | Number sent | Number returned (%) | Percentage of all men |
|---|---|---|---|
| Baseline | |||
| Intervention | 205 | 194 (95) | 95 |
| Control | 206 | 199 (97) | 97 |
| 3 months | |||
| Intervention | 204 | 188 (92) | 92 |
| Control | 206 | 187 (91) | 91 |
| 6 months | |||
| Intervention | 202 | 185 (92) | 90 |
| Control | 205 | 182 (89) | 88 |
| 9 months | |||
| Intervention | 201 | 178 (89) | 87 |
| Control | 203 | 181 (89) | 88 |
| 12 months | |||
| Intervention | 200 | 183 (92) | 89 |
| Control | 202 | 181 (90) | 88 |
Primary outcome: urinary incontinence at 12 months
The primary outcome was incontinence in men at 12 months after randomisation, measured by a positive response to one of two questions from the ICI-SF questionnaire (‘How often do you leak urine’ or ‘How much urine do you usually leak?’). Table 28 shows that the difference between the intervention and control groups in urinary incontinence at 12 months (75.5% vs 77.4%) was not statistically significant:
-
either when analysed by intention to treat (all men analysed in the groups to which they were randomised but results as given in the outcome questionnaires without adjustment for missing values)
-
or when analysed by ‘treatment received’, which adjusts the result by a factor related to the men who actually attended a therapist versus those who did not.
| Radical prostatectomy | Intervention | Control | RR (95% CI), p-value |
|---|---|---|---|
| Urinary incontinence at 12 months [n/N (%)] | 148/196 (75.5) | 151/195 (77.4) | Absolute risk difference (95% CI) –1.9% (–10% to 6%) |
| Intention to treat | |||
| Unadjusted analysis | 0.980 (0.879 to 1.094), 0.719 | ||
| Analysis adjusted for minimisation factors | 0.97 (0.87 to 1.09), 0.637 | ||
| Adjusted treatment received | |||
| Unadjusted analysis | 0.979 (0.877 to 1.093), 0.702 | ||
| Analysis adjusted for minimisation factors | 0.977 (0.876 to 1.090), 0.676 |
The above analyses were then repeated adjusting for the minimisation factors, but this did not alter the findings (Table 28). The corresponding risk difference for the unadjusted intention-to-treat analysis was –1.9% (95% CI –10% to 6%), thereby ruling out the likelihood that the trial prespecified difference of 15% in proportion incontinent between intervention and control group could have been missed.
Secondary outcomes
Urinary outcomes
Urinary incontinence was also measured at 3, 6 and 9 months after randomisation together with other urinary outcomes. Table 29a describes the various urinary outcomes at each follow-up and Table 29b shows the formal statistical testing of the differences at each time point. Figure 14 is a pictorial representation of the percentage of men incontinent at each follow-up and Figure 15 shows the change in mean ICI-score over time. The data show that there were no statistically significant differences between the intervention and control groups at any of the time points in terms of urinary incontinence and the other urinary outcomes measured.
| Radical prostatectomy | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Incontinence | ||||||||||
| Men with any incontinence [n/N (%)] | 205/205 (100) | 206/206 (100) | 172/200 (86) | 176/198 (89) | 158/197 (80) | 158/197 (80) | 144/191 (75) | 157/194 (81) | 148/196 (76) | 151/195 (77) |
| Men with severe incontinencea [n/N (%)] | 188/205 (92) | 189/206 (92) | 103/200 (52) | 107/198 (54) | 81/197 (41) | 79/197 (40) | 72/191 (38) | 84/194 (43) | 74/196 (38) | 78/195 (40) |
| ICI-Q scoreb | 11.2 (4.3) 205 | 11.5 (4.5) 206 | 6.3 (4.2) 198 | 7.2 (4.9) 198 | 5.4 (4.2) 197 | 5.6 (4.6) 197 | 5.1 (4.2) 186 | 5.6 (4.6) 194 | 4.9 (4.1) 196 | 5.4 (4.5) 195 |
| Frequency of daytime urinary incontinence from diaries | 6.9 (8.0) 181 | 6.9 (8.2) 180 | 3.3 (3.8) 139 | 3.9 (4.5) 139 | 3.3 (5.2) 117 | 3.5 (4.0) 110 | 3.1 (4.7) 107 | 3.3 (3.7) 110 | 3.0 (3.8) 105 | 2.9 (3.0) 106 |
| Effect of UI on QoL | 4.7 (3.1) 205 | 5.0 (3.1) 204 | 2.0 (2.3) 198 | 2.5 (2.8) 198 | 1.5 (2.1) 194 | 1.8 (2.5) 196 | 1.4 (1.9) 186 | 1.8 (2.5) 194 | 1.4 (2.0) 193 | 1.7 (2.3) 193 |
| Urinary frequency | ||||||||||
| Daytime urinary frequency | 7.4 (2.9) 187 | 7.9 (3.7) 192 | 7.1 (2.4) 187 | 7.1 (2.7) 188 | 7.0 (2.2) 183 | 7.0 (2.3) 189 | 6.8 (2.1) 187 | 7.4 (5.1) 185 | 6.8 (2.1) 184 | 7.0 (2.8) 183 |
| Daytime urinary frequency from diaries | 7.4 (2.6) 182 | 7.3 (2.6) 190 | 7.0 (2.0) 182 | 7.0 (2.5) 177 | 6.8 (2.1) 174 | 6.9 (2.9) 169 | 6.7 (1.9) 172 | 7.0 (2.1) 166 | 6.8 (2.6) 175 | 7.1 (4.1) 171 |
| Nocturia | 2.2 (1.2) 199 | 2.5 (1.6) 202 | 1.4 (1.1) 191 | 1.8 (1.2) 191 | 1.3 (1.0) 183 | 1.5 (1.0) 194 | 1.3 (1.4) 185 | 1.5 (1.3) 186 | 1.3 (1.0) 180 | 1.4 (1.0) 185 |
| Nocturia from diaries | 2.1 (1.2) 178 | 2.3 (2.0) 186 | 1.5 (0.9) 151 | 1.7 (1.0) 162 | 1.4 (0.9) 149 | 1.6 (1.0) 150 | 1.4 (0.9) 145 | 1.6 (1.0) 145 | 1.4 (0.9) 144 | 1.5 (0.9) 157 |
| Frequency of nocturnal incontinence from diaries | 1.4 (1.2) 90 | 1.6 (1.4) 90 | 1.1 (0.8) 35 | 1.6 (1.3) 45 | 1.1 (0.8) 24 | 1.1 (1.2) 37 | 1.1 (0.8) 25 | 1.2 (1.1) 36 | 1.4 (0.9) 26 | 1.6 (1.3) 27 |
| Radical prostatectomy | Effect size (95% CI), p-value | |||
|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | |
| Incontinence | ||||
| Men incontinent [RR (95% CI), p-value] | 0.97 (0.90 to 1.04), 0.366 | 1.00 (0.91 to 1.10), 0.990 | 0.93 (0.84 to 1.03), 0.174 | 0.97 (0.87 to 1.09), 0.637 |
| Men with severe incontinence [RR (95% CI), p-value]a | 0.95 (0.79 to 1.15), 0.595 | 1.02 (0.80 to 1.29), 0.875 | 0.86 (0.67 to 1.09), 0.208 | 0.93 (0.73 to 1.19), 0.582 |
| ICI-Q scoreb | –0.66 (–1.37 to 0.05), 0.068 | –0.15 (–0.86 to 0.55), 0.674 | –0.49 (–1.22 to 0.24), 0.188 | –0.34 (–1.05 to 0.38), 0.355 |
| Frequency of daytime urinary incontinence from diaries | –0.47 (–1.30 to 0.37), 0.274 | –0.09 (–1.03 to 0.85), 0.855 | –0.17 (–1.03 to 0.68), 0.690 | 0.04 (–0.65 to 0.12), 0.919 |
| Effect of UI on QoL | –0.37 (–0.79 to 0.05), 0.086 | –0.22 (–0.61 to 0.17), 0.266 | –0.24 (–0.63 to 0.15), 0.233 | 0.14 (–0.51 to 0.24), 0.476 |
| Urinary frequency | ||||
| Daytime urinary frequency | 0.01 (–0.49 to 0.52), 0.958 | 0.02 (–0.43 to 0.46), 0.944 | –0.61 (–1.44 to 0.22), 0.149 | –0.24 (–0.73 to 0.26), 0.346 |
| Daytime urinary frequency from diaries | 0.15 (–0.38 to 0.69), 0.577 | –0.02 (–0.66 to 0.62), 0.939 | –0.11 (–0.42 to 0.65), 0.679 | –0.07 (–0.89 to 0.73), 0.861 |
| Nocturia | –0.23 (–0.42 to –0.04), 0.020 | –0.09 (–0.25 to 0.07), 0.280 | –0.14 (–0.39 to 0.10), 0.248 | –0.04 (–0.21 to 0.14), 0.683 |
| Nocturia from diaries | –0.31 (–0.52 to –0.09), 0.005 | –0.18 (–0.41 to 0.04), 0.113 | –0.13 (–0.35 to 0.10), 0.277 | –0.15 (–0.35 to 0.06), 0.167 |
| Frequency of nocturnal incontinence from diaries | –0.18 (–0.44 to 0.07), 0.159 | –0.07 (–0.30 to 0.15), 0.516 | –0.07 (–0.31 to 0.18), 0.593 | –0.05 (–0.27 to 0.16), 0.617 |
FIGURE 14.
Per cent of men incontinent at each time point.
FIGURE 15.
Mean ICI-Q score at each time point. ICI-Q score: 0 = none, 21 = maximum (worst) score. Derived from questions 1–3 of the ICIQ-UI Short Form Questionnaire.
Type of incontinence
Table 30 and Figure 16 show the type of incontinence at baseline, and at 6 and 12 months after randomisation. Men could report more than one type of incontinence. At all time points the majority (70%) of men had (any) stress incontinence: this did not vary much after the first 6 months, and the proportions were not significantly different between the intervention and control groups (see Table 30 and Figure 16a). Around half of the men had urgency or mixed incontinence. The proportions of men with other types of urinary incontinence (urgency, Figure 16b; mixed, Figure 16c; postmicturition leakage, Figure 16d; and other types of incontinence) decreased over the first 6 months, but there was little further improvement, or difference between the groups.
| Radical prostatectomy | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | RR (95% CI), p-value | Intervention | Control | RR (95% CI), p-value | |
| SUI | 195/205 (95) | 195/206 (95) | 136/197 (69) | 135/197 (69) | 1.002 (0.88 to 1.14), 0.972 | 138/196 (70) | 128/195 (66) | 1.071 (0.94 to 1.22), 0.314 |
| UUI | 135/205 (66) | 156/206 (76) | 58/197 (29) | 87/197 (44) | 0.735 (0.57 to 0.94), 0.015 | 61/196 (31) | 83/195 (43) | 0.782 (0.61 to 1.00), 0.054 |
| Urgency | 131/205 (64) | 160/206 (78) | 76/197 (39) | 105/197 (53) | 0.827 (0.68 to 1.01), 0.066 | 80/196 (41) | 100/195 (51) | 0.879 (0.72 to 1.08), 0.218 |
| MUI (both SUI and UUI) | 132/205 (64) | 151/206 (73) | 55/197 (28) | 81/197 (41) | 0.752 (0.58 to 0.98), 0.033 | 59/196 (30) | 74/195 (38) | 0.843 (0.65 to 1.10), 0.208 |
| Postmicturition leakage | 166/205 (81) | 170/206 (83) | 87/197 (44) | 106/197 (54) | 0.831 (0.69 to 1.01), 0.059 | 102/196 (52) | 106/195 (54) | 0.924 (0.73 to 1.17), 0.512 |
| Other UI | 72/205 (35) | 91/206 (44) | 38/197 (19) | 52/197 (26) | 0.777 (0.54 to 1.12), 0.181 | 39/196 (20) | 39/195 (20) | 1.099 (0.74 to 1.63), 0.640 |
FIGURE 16.
Type of incontinence at baseline and at 6 and 12 months after randomisation. Men could report more than one type of incontinence. (a) Stress urinary incontinence. (b) Urgency urinary incontinence. (c) Mixed urinary incontinence (proportion of men reporting both stress and urgency urinary incontinence). (d) Postmicturition leakage (defined as ‘urine leaking when urination finished’).
Use of aids or protection for urinary incontinence
Table 31a shows the men’s use of aids to protect them from urinary leakage: this did not differ significantly according to the randomised groups at any of the follow-up time points. Table 31b presents the statistical analyses of these outcomes. About 40% of the men were still using pads at 12 months to protect themselves from leakage accidents, although in some cases this might have been more of a precaution than because they actually leaked.
| Radical prostatectomy | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Use of any protection | 183/205 (89) | 181/206 (88) | 103/205 (50) | 118/206 (57) | 76/205 (37) | 85/206 (41) | 72/205 (35) | 74/206 (36) | 65/205 (32) | 71/206 (34) |
| Use of body-worn pads (yes) | 180/205 (88) | 176/205 (86) | 101/177 (57) | 108/177 (61) | 74/161 (46) | 83/164 (51) | 67/154 (44) | 71/156 (46) | 63/159 (40) | 68/161 (42) |
| Number of body-worn pads in 24 hours [mean (SD) n] | 3.1 (2.3) 176 | 3.4 (2.8) 172 | 1.8 (1.4) 99 | 2.3 (1.8) 106 | 1.6 (1.4) 76 | 2.2 (1.8) 83 | 1.6 (0.9) 64 | 2.2 (1.8) 69 | 1.6 (1.1) 67 | 2.0 (1.8) 68 |
| Use of chair or bed pads (yes) | 47/201 (23) | 54/200 (27) | 14/174 (8) | 22/175 (13) | 11/153 (7) | 14/157 (9) | 10/154 (6) | 12/151 (8) | 5/149 (3) | 6/155 (4) |
| Number of chair or bed pads in 24 hours [mean (SD) n] | 1.4 (1.8) 47 | 1.4 (1.6) 54 | 1.1 (0.7) 12 | 1.1 (1.2) 20 | 1.0 (0.5) 10 | 1.5 (2.0) 10 | 1.1 (0.4) 8 | 1.1 (1.2) 10 | 1.0 (0.0) 5 | 1.4 (1.5) 5 |
| Use of external (sheath) catheter (yes) | 17/202 (8) | 18/205 (9) | 12/197 (6) | 18/195 (9) | 8/187 (4) | 11/189 (6) | 12/190 (6) | 7/189 (4) | 9/189 (5) | 5/188 (3) |
| Use of permanent catheter (yes) | 0/205 (0) | 0/203 (0) | 0/197 (0) | 0/196 (0) | 0/189 (0) | 0/192 (0) | 1/190 (1) | 0/189 (0) | 1/192 (1) | 1/191 (1) |
| Radical prostatectomy | Effect size (95% CI), p-value | |||
|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | |
| Use of any protection | 0.87 (0.73 to 1.05), 0.139 | 0.89 (0.70 to 1.13), 0.351 | 0.97 (0.75 to 1.25), 0.803 | 0.91 (0.69 to 1.19), 0.489 |
| Use of body-worn pads (yes) | 0.912 (0.78 to 1.07), 0.266 | 0.847 (0.68 to 1.05), 0.132 | 0.916 (0.72 to 1.16), 0.464 | 0.858 (0.67 to 1.10), 0.235 |
| Number of body-worn pads in 24 hours [mean difference (95% CI), p-value] | –0.40 (–0.77 to –0.02), 0.040 | –0.58 (–1.02 to –0.13), 0.012 | –0.54 (–0.99 to –0.10), 0.018 | –0.45 (–0.94 to 0.05), 0.075 |
| Use of chair or bed pads (yes) | 0.651 (0.34 to 1.23), 0.185 | 0.852 (0.39 to 1.87), 0.690 | 0.827 (0.37 to 1.83), 0.638 | 0.911 (0.25 to 3.28), 0.887 |
| Number of chair or bed pads in 24 hours [mean difference (95% CI), p-value] | 0.04 (–0.83 to 0.91), 0.919 | 0.01 (–1.60 to 1.63), 0.982 | ||
| Use of external (sheath) catheter (yes) | 0.741 (0.39 to 1.41), 0.360 | 0.690 (0.26 to 1.81), 0.450 | 1.720 (0.73 to 4.08), 0.218 | 1.948 (0.70 to 5.45), 0.204 |
| Use of permanent catheter (yes) | Not estimable | Not estimable | Not estimable | Not estimable |
Bowel function
In addition to urinary outcomes, men were also asked to describe some aspects of bowel function. Few men (< 10%) had faecal incontinence or constipation by the end of follow-up at 12 months, although rather more reported faecal urgency occasionally or more often. Table 32 and Figure 17 show that there were no differences in any aspect of bowel function between the men in the randomised groups.
| Radical prostatectomy | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Effect size (95% CI), p-value | Intervention | Control | Effect size (95% CI), p-value | |
| Faecal incontinencea | 6/205 (3) | 15/206 (7) | 11/190 (6) | 14/195 (7) | 0.97 (0.41 to 2.29), 0.968 | 16/193 (8) | 11/193 (6) | 1.56 (0.74 to 3.29), 0.241 |
| Faecal urgencya | 76/204 (37) | 92/205 (45) | 65/190 (34) | 80/195 (41) | 0.93 (0.73 to 1.18), 0.549 | 70/193 (36) | 86/193 (45) | 0.88 (0.70 to 1.10), 0.244 |
| Constipation | 31/205 (15) | 23/206 (11) | 12/189 (6) | 16/194 (8) | 0.60 (0.29 to 1.23), 0.165 | 11/193 (6) | 14/193 (7) | 0.69 (0.28 to 1.58), 0.359 |
| Any bowel dysfunctionb | 97/205 (47) | 110/206 (53) | 80/190 (42) | 94/195 (48) | 0.92 (0.75 to 1.13), 0.419 | 80/193 (42) | 98/193 (51) | 0.84 (0.69 to 1.03), 0.102 |
FIGURE 17.
Type of bowel problems at baseline and 6 and 12 months.
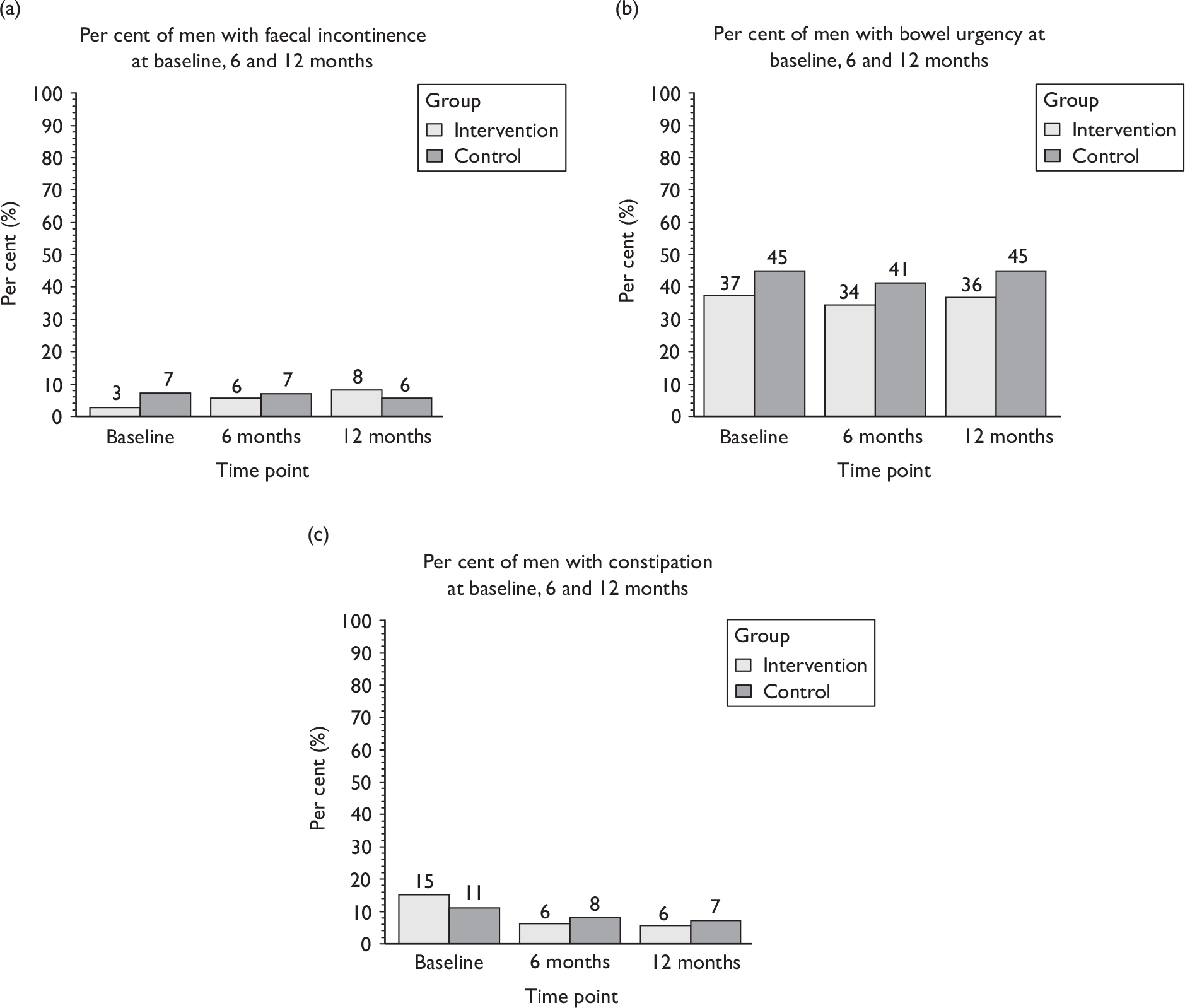
Sexual function
Table 33 compares the men in the randomised groups in terms of sexual function outcomes. Over 90% of men had normal erectile function before their operation. Although around one-third had an active sex life at 12 months, the majority said that this was less satisfactory than before their operation. There were, however, no differences at 12 months according to the randomised groups in terms of the proportion of men with an active sex life [RR 0.94 (95% CI 0.73 to 1.22); adjusted for age, urinary incontinence before surgery and baseline value, p = 0.661] or the proportion of men who rated their sex life as worse after the operation [RR 0.79 (95% CI 0.47 to 1.34); p = 0.391].
| Radical prostatectomy | Intervention | Control |
|---|---|---|
| Number of men not able to achieve erection before prostate surgerya | 17/205 (8) | 18/202 (9) |
| Number of men with active sex life at 12 months | 68/184 (37) | 73/184 (40) |
| Reasons for not having an active sex life | ||
| Because of urinary symptoms | 13/116 (11) | 10/113 (9) |
| Because of bowel symptoms | 0/116 | 0/111 |
| Because of prostate operation | 112/136 (82) | 106/132 (80) |
| Because of medical treatment | 1/116 (1) | 1/112 (1) |
| For another reason | 27/118 (23) | 30/112 (27) |
| Comparison of sex life with before prostate operation 12 months ago | ||
| Stayed the same | 22/163 (13) | 24/162 (15) |
| Better | 0/163 (0) | 3/162 (2) |
| Worse | 141/163 (87) | 135/162 (83) |
Table 34 compares the randomised groups in terms of problems with sexual function. There were no significant differences in sexual function outcomes between the intervention and control groups (Table 34 and Figure 18). Only about 20% of the men were able to achieve a normal erection or one with slightly reduced stiffness by 12 months after surgery, and almost all reported a lack of semen or no ejaculation. Of those men, few reported more than slight pain. Around 60% of men used drugs and about 20% used a vacuum device to help with sexual function. Only 20% reported urinary incontinence during intercourse.
| Radical prostatectomy | Intervention | Control |
|---|---|---|
| Difficulty with achieving erection | ||
| Normal stiffness | 6/189 (3) | 3/190 (2) |
| Reduced stiffness | 29/189 (15) | 38/190 (20) |
| Severely reduced stiffness | 49/189 (26) | 44/190 (23) |
| No erection possible | 105/189 (56) | 105/190 (55) |
| Bother with erection [mean (SD) n]a | 6.0 (3.3) 183 | 6.5 (3.1) 183 |
| Ejaculation | ||
| Normal quantity of semen | 0/187 (0) | 1/184 (1) |
| Reduced quantity of semen | 1/187 (1) | 2/184 (1) |
| Significantly reduced quantity of semen | 3/187 (2) | 3/184 (2) |
| Ejaculation but without semen | 65/187 (35) | 69/184 (38) |
| No ejaculation | 118/187 (63) | 109/184 (59) |
| Bother with ejaculation [mean (SD) n]a | 3.8 (3.7) 172 | 4.4 (3.7) 171 |
| Pain or discomfort with ejaculation | ||
| No pain | 94/117 (80) | 98/124 (79) |
| Slight pain | 14/117 (12) | 17/124 (14) |
| Moderate pain | 5/117 (4) | 5/124 (4) |
| Severe pain | 4/117 (3) | 4/124 (3) |
| Bother with pain or discomfort [mean (SD) n]a | 2.5 (3.4) 49 | 2.3 (3.1) 55 |
| Number of men using medication for sexual problems | 104/186 (56) | 110/189 (58) |
| Number of men using vacuum device for sexual problems | 47/185 (25) | 39/183 (21) |
| Number of men using either medication or a vacuum device for sexual problems | 116/188 (62) | 123/189 (65) |
| Number of men leaking urine during intercourse | 26/135 (19) | 30/139 (22) |
FIGURE 18.
Quality of erectile function at 12 months after prostate surgery: Radical prostatectomy.
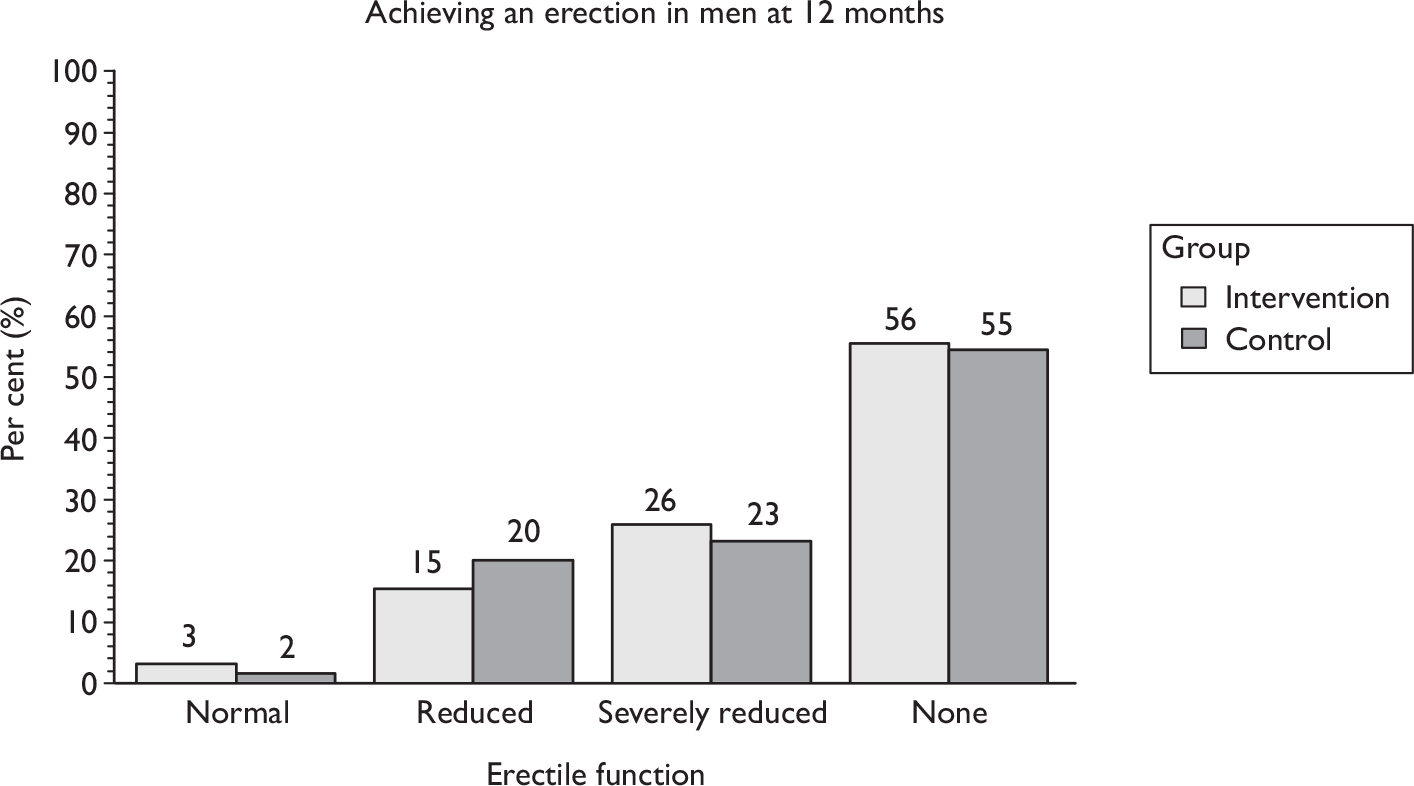
Quality of life
General health outcomes were measured using the EQ-5D and SF-12, the latter subdivided into role – mental (SF-12M) and role – physical (SF-12P) scores. The slight increase in the scores over time represents recovery from the operation but there were no differences between the randomised groups at any time point in EQ-5D or SF-12 scores (Table 35 and Figure 19).
| Radical prostatectomy | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Effect size (95% CI), p-value | Intervention | Control | Effect size (95% CI), p-value | |
| EQ-5D | 0.797 (0.216) 200 | 0.783 (0.225) 206 | 0.884 (0.205) 184 | 0.875 (0.189) 189 | 0.006 (–0.027 to 0.039) 0.725 | 0.879 (0.209) 187 | 0.887 (0.176) 189 | –0.013 (–0.047 to 0.021), 0.460 |
| SF-12M | 50.8 (10.5) 201 | 49.3 (10.7) 201 | 53.6 (8.3) 188 | 53.2 (8.1) 191 | 0.4 (–1.3 to 2.1) 0.615 | 52.9 (9.1) 190 | 53.6 (7.9) 191 | –0.9 (–2.6 to 0.9), 0.321 |
| SF-12P | 42.7 (9.9) 201 | 41.8 (10.6) 201 | 50.9 (9.4) 188 | 49.6 (9.9) 191 | 1.1 (–0.7 to 2.8) 0.246 | 51.4 (8.3) 190 | 51.2 (8.4) 191 | 0.0 (–1.6 to 1.6), 0.967 |
FIGURE 19.
Graphical representations of EQ-5D and SF-12 scores over time. SF-12M, SF-12 role – mental; SF-12P, SF-12 role – physical.
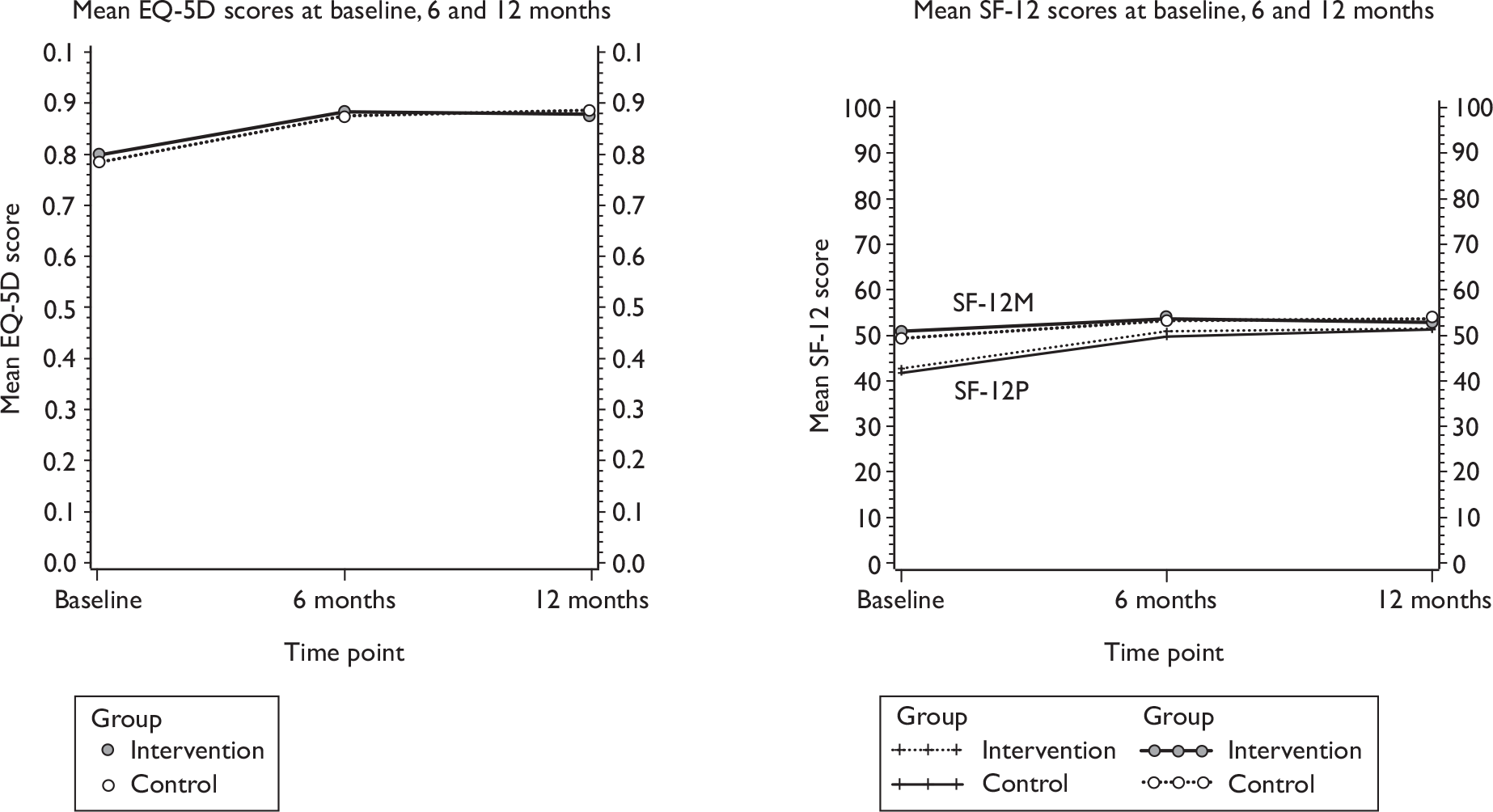
Pelvic floor muscle training
All men were asked to report on their practice of carrying out pelvic floor exercises at baseline and 6 and 12 months after randomisation. Initially a high proportion of men (over 80%) reported practising them, of whom around 80% carried them out every day. As this occurred before randomisation, it must reflect the high profile given to PFMT in the standard care of men after radical prostate surgery.
The prevalence of exercising in the control group had fallen by 6 months (to 62%), while men in the therapy group were more likely than controls still to be performing exercises (83%). This difference was maintained at 12 months: a significantly higher proportion of men from the intervention group were carrying out any PFMT at 12 months after randomisation (67%) compared with those in the control group (50%; see Table 36). Significantly more were practising for at least 3–4 days each week in the intervention group than in the control group (56% vs 36%). Although men in the intervention group were performing fewer daily contractions by 12 months than they had previously, the difference between randomised groups at this time point did not reach statistical significance (mean contractions 11.7 intervention vs 19.4 control) (see Table 36). This is likely to reflect the taught exercise regimen in the intervention group (aiming for 18 strong contractions every day) compared with recommendations from NICE,71,72 which are to perform eight contractions three times a day.
| Radical prostatectomy | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Effect size (95% CI), p-value | Intervention | Control | Effect size (95% CI), p-value | |
| Any PFMT in last week | ||||||||
| Yes | 176/205 (86) | 170/206 (83) | 156/188 (83) | 117/190 (62) | 1.329 (1.17 to 1.51), 0.001 | 128/191 (67) | 95/189 (50) | 1.296 (1.09 to 1.53), 0.003 |
| No | 22/205 (11) | 21/206 (10) | 32/188 (17) | 70/190 (36) | 63/191 (33) | 91/189 (48) | ||
| Don’t know | 7/205 (3) | 15/206 (7) | 0/188 (0) | 3/190 (2) | 0/191 (0) | 3/189 (2) | ||
| Days carrying out PFMT | ||||||||
| Every day | 145/205 (71) | 139/206 (67) | 96/188 (51) | 64/190 (34) | 67/192 (35) | 51/190 (27) | ||
| 5–6 days | 8/205 (4) | 8/206 (4) | 17/188 (9) | 10/190 (5) | 13/192 (7) | 5/190 (3) | ||
| 3–4 days | 9/205 (4) | 16/206 (8) | 19/188 (10) | 13/190 (7) | 26/192 (14) | 12/190 (6) | ||
| 1–2 days | 13/205 (6) | 6/206 (3) | 20/188 (11) | 28/190 (15) | 18/192 (9) | 28/190 (15) | ||
| None | 30/205 (15) | 37/206 (18) | 36/188 (19) | 75/190 (39) | 68/192 (35) | 94/190 (49) | ||
| Average contractions [mean (SD) n; mean difference] | 18.2 (29.4) 188 | 21.1 (45.0) 190 | –2.9 (–10.7 to 4.8), 0.457 | 11.7 (20.0) 192 | 19.4 (79.2) 189 | –7.8 (–19.4 to 3.9), 0.189 | ||
| Deliberate contractions whilst walkinga | 150/187 (80) | 118/184 (64) | 1.25 (1.10 to 1.42), 0.001 | 148/192 (77) | 121/186 (65) | 1.18 (1.04 to 1.35), 0.011 | ||
| Deliberate contractions before you do somethinga | 128/185 (69) | 111/184 (60) | 1.15 (0.98 to 1.33), 0.078 | 134/192 (70) | 113/187 (60) | 1.16 (1.00 to 1.34), 0.052 | ||
| Contracting reduces or stops leaking | 113/130 (87) | 105/125 (84) | 1.09 (0.96 to 1.24), 0.197 | 117/137 (85) | 97/126 (77) | 1.16 (1.00 to 1.34), 0.046 | ||
Men in the intervention group were also significantly more likely to perform contractions while walking. Differences between groups in performing a pelvic floor muscle contraction prior to increases in intra-abdominal pressure such as coughing or lifting (also known as ‘the Knack’) did not quite reach statistical significance (Table 36). There were no differences between the groups in terms of ‘the Knack’ reducing or stopping urinary leakage (although this analysis includes only the men using this technique).
Lifestyle outcomes
Men were also advised, in the lifestyle advice leaflet sent to both groups but reinforced by the therapists in the intervention group, about the benefits of general health strategies such as taking more exercise. There were few differences between the groups in terms of other types of exercise practised (Table 37) and no statistically significant difference in the proportion taking general exercise [RR 0.94 (95% CI 0.87 to 1.02); p = 0.151].
| Radical prostatectomy | 12 months [n/N (%)] | |
|---|---|---|
| Intervention | Control | |
| General exercise (yes)a | 160/189 (85) | 168/188 (89) |
| Exercise type | ||
| Walking | 150/189 (79) | 148/188 (78) |
| Swimming | 25/189 (13) | 19/188 (10) |
| Gardening | 101/189 (53) | 119/188 (63) |
| Running | 9/189 (5) | 14/188 (7) |
| Going to gym | 20/189 (11) | 20/188 (11) |
| Other | 36/189 (19) | 36/188 (19) |
| Changed exercise since prostate operation | ||
| No changes | 142/191 (74) | 121/191 (63) |
| I do less | 27/191 (14) | 39/191 (20) |
| I do more | 22/191 (12) | 31/191 (16) |
Finally, men in both groups were given (via the lifestyle advice leaflet) other general advice on lifestyle changes they could make that might help both with incontinence and with general health. Again, this advice was reinforced by the therapists for men in the intervention group. There were few differences between the groups in terms of changes made to lifestyle factors (Table 38).
Prespecified subgroup analyses
Preplanned subgroup analyses were carried out on the primary outcome (urinary incontinence at 12 months) according to factors that we thought would be prognostic. These factors were:
-
pre-existing urinary incontinence (before prostate surgery)
-
age (up to 60 years, 61 years and over)
-
BMI (up to 30 kg/m2, 30–34.9 kg/m2, 35 kg/m2 or greater)
-
type of incontinence at trial entry
-
SUI
-
UUI
-
MUI
-
postmicturition leakage
-
-
other morbidity
-
type of therapist (physiotherapist or nurse).
Whilst a subgroup analysis on the use of biofeedback machines was also prespecified, there were insufficient numbers of centres with biofeedback machines to do such an analysis (see Table 7).
Figure 20 shows the effect of subgroup analysis on the primary outcome (urinary incontinence at 12 months) according to the prespecified factors. The dotted line reflects the overall main effect of the intervention on incontinence rates. Stricter levels of statistical significance (2p < 0.01) were sought (99% CIs), reflecting the exploratory nature of these analyses. There were no apparent clinically relevant differences according to any subgroup and none of the formal tests for statistical interaction effects were significant.
FIGURE 20.
Forest plot of subgroup analyses: urinary incontinence at 12 months. PML, postmicturition leakage.

Satisfaction with treatment for urinary incontinence
Men were asked to score their satisfaction with the treatment they received for urinary incontinence (0 = ‘very unsatisfied’ to 10 = ‘very satisfied’) at 12 months after randomisation. Men in the intervention group were significantly more satisfied than those in the control group (see Appendix 5, Table 88). Thus, the therapy intervention did increase satisfaction rates despite the lack of difference in urinary outcomes.
| Radical prostatectomy | Intervention [n/N (%)] | Control [n/N (%)] |
|---|---|---|
| Weight | ||
| No need to lose weight | 75/190 (39) | 77/187 (41) |
| Haven’t tried to lose weight | 78/190 (41) | 73/187 (39) |
| Extra exercise to lose weight | 27/190 (14) | 29/187 (16) |
| Diet to lose weight | 12/190 (6) | 11/187 (6) |
| Other ways of losing weight | 15/190 (8) | 9/187 (5) |
| Fluid intake | ||
| Number of men making no changes to fluid intake | 90/191 (47) | 88/192 (46) |
| Drink more fluids | 55/191 (29) | 61/192 (32) |
| Drink more cranberry juice | 39/191 (20) | 36/192 (19) |
| Drink fewer caffeinated drinks | 49/191 (26) | 58/192 (30) |
| Drink less fluid in evenings | 49/191 (26) | 59/192 (31) |
| Other changes to fluid intake | 14/191 (7) | 18/192 (9) |
| Diet | ||
| Number of men making no changes to diet or food | 130/189 (69) | 133/191 (70) |
| More balanced diet | 36/189 (19) | 35/191 (18) |
| More fruit and vegetables | 49/189 (26) | 53/191 (28) |
| More fibre | 31/189 (16) | 35/191 (18) |
| Less fats or sugars | 45/189 (24) | 37/191 (19) |
| Other changes to food intake | 7/189 (4) | 7/191 (4) |
| Lifting | ||
| Number of men who reduce lifting | 81/193 (42) | 76/190 (40) |
| Smoking | ||
| Number of men who smoked | 16/193 (8) | 17/189 (9) |
| Number of men stopping smokinga | 1/16 (6) | 1/17 (6) |
| Number of men reducing smokinga | 8/16 (50) | 9/17 (53) |
| Chest or respiratory symptoms | ||
| Number of men who did have chest symptoms | 17/188 (9) | 19/184 (10) |
| Taking correct medicationa | 12/17 (71) | 6/19 (32) |
| Consulted GP about medicationa | 6/17 (35) | 8/19 (42) |
| Other changes to reduce respiratory symptomsa | 0/17 (0) | 1/19 (5) |
Chapter 8 Resource use and cost-effectiveness in the radical randomised controlled trial
This chapter describes the economic analyses for the radical prostatectomy RCT.
Description of the data available
Table 39 describes the number of men who contributed data for each of the areas of resource use and quality of life at each time point. Fewer data were available at the later data collection time points. For some areas of resource use (for example number of NHS pads at 12 months), only three-quarters of men indicated the quantity used. For other areas, for example hospital physiotherapist visits, nearly 90% of men provided data on the use of that resource even at 12 months. The difference between these two rates cannot be explained by the mode of data collection, as both were collected by participant-completed questionnaire. An alternative explanation might be the limited use of these services by 12 months, which meant that men did not answer the questions because they did not think they were relevant. Other explanations could be advanced, but there is no information to determine what the reasons are for men providing information for some areas of resource use but not for others.
| Baseline [n (%)] | 3 months [n (%)] | 6 months [n (%)] | 9 months [n (%)] | 12 months [n (%)] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| NHS-supplied pads | 201 (98) | 201(98) | 174 (85) | 175 (85) | 157 (77) | 162 (79) | 151 (74) | 154 (75) | 158 (77) | 157 (76) |
| Self-supplied pads | 197 (96) | 196 (95) | 170 (83) | 169 (82) | 152 (74) | 156 (76) | 148 (72) | 150 (73) | 153 (75) | 147 (71) |
| NHS-supplied bed/chair protector | 196 (96) | 194 (94) | 171 (83) | 173 (84) | 151 (74) | 153 (74) | 152 (74) | 149 (72) | 149 (73) | 154 (75) |
| Self-supplied bed/chair protector | 194 (95) | 194 (94) | 171 (83) | 167 (81) | 150 (73) | 153 (74) | 150 (73) | 149 (72) | 149 (73) | 154 (75) |
| Catheter | 205 (100) | 203 (99) | 197 (96) | 196 (95) | 189 (92) | 192 (93) | 190 (93) | 189 (92) | 192 (94) | 191(93) |
| Sheath | 202 (99) | 205 (99) | 197 (96) | 195 (95) | 187 (91) | 189 (92) | 190 (93) | 189 (92) | 189 (92) | 188 (91) |
| GP other visit | 188 (92) | 197 (96) | 188 (92) | 190 (92) | 182 (89) | 191 (93) | 182 (89) | 182 (88) | 190 (93) | 187 (91) |
| Nurse incontinent visit | 156 (76) | 166 (81) | 165 (80) | 154 (75) | 158 (77) | 158 (77) | 152 (74) | 147 (71) | 161 (79) | 156 (76) |
| Nurse other visit | 194 (95) | 183 (89) | 189 (92) | 188 (91) | 180 (88) | 184 (89) | 180 (88) | 186 (90) | 187 (91) | 188 (91) |
| Hospital doctor visit | 193 (94) | 184 (89) | 176 (86) | 184 (89) | 177 (86) | 191 (93) | 182 (89) | 174 (84) | 180 (88) | 183 (89) |
| Hospital nurse visit | 187 (91) | 191 (93) | 178 (87) | 174 (84) | 178 (87) | 183 (89) | 179 (87) | 172 (83) | 181 (88) | 179 (87) |
| Hospital physiotherapist visit | 191 (93) | 191 (93) | 184 (90) | 175 (85) | 172 (84) | 180 (87) | 179 (87) | 168 (82) | 177 (86) | 179 (87) |
| Private doctor visit | 204 (99) | 199 (97) | 192 (94) | 188 (91) | 183 (89) | 190 (92) | 183 (89) | 179 (87) | 187 (91) | 186 (90) |
| Private nurse visit | 191 (93) | 190 (92) | 188 (92) | 182 (88) | 178 (87) | 184 (89) | 180 (88) | 179 (87) | 183 (89) | 185 (90) |
| Private physiotherapist visit | 199 (97) | 195 (95) | 190 (93) | 185 (90) | 180 (88) | 184 (89) | 182 (89) | 180 (87) | 176 (86) | 178 (86) |
| Inpatient visit | 202 (99) | 205 (99) | 194 (95) | 193 (94) | 186 (91) | 193 (94) | 185 (90) | 186 (90) | 187 (91) | 191 (93) |
| Days off work | 194 (95) | 193 (94) | 179 (87) | 184 (89) | 170 (83) | 173 (84) | 178 (87) | 170 (83) | 177 (86) | 182 (88) |
Analysis of resource use and costs
Resource use
Table 40 details the average total resource use for the intervention and subsequent use of health services over the 12-month follow-up period after randomisation. The pattern of resource use was similar across both groups and there were few statistically significant differences between the two groups. In addition to differences in costs of the intervention (incurred only by those randomised to the intervention group), these costs included the use of pads paid for by the NHS, incontinence-related GP doctor and nurse visits, hospital physiotherapist visits and the number of days that participants were off work. The use of resources in each of these areas was higher for the control group, apart from the subsequent number of hospital physiotherapy visits, which was higher for the intervention group. A detailed description of the use of NHS and private provider health services is provided in Appendix 5.
| Area of resources use | Mean (SD) | Difference (95% CI)a | |
|---|---|---|---|
| Intervention | Control | ||
| Intervention | 3.55 (1.61) | 0 | 3.55 |
| Subsequent resource use | |||
| NHS-supplied pads usedb | 2.46 (3.86) | 3.53 (5.71) | –0.90 (–1.74 to –0.07) |
| NHS-supplied bed/chair protectors used | 0.18 (0.64) | 0.26 (1.41) | –0.08 (–0.29 to 0.12) |
| GP doctor incontinence-related visitb | 0.33 (1.17) | 0.63 (1.94) | –0.34 (–0.63 to –0.05) |
| GP doctor other visit | 4.17 (4.03) | 4.53 (4.03) | –0.24 (–1.00 to 0.52) |
| GP nurse incontinence-related visit | 0.16 (0.94) | 0.37 (1.50) | –0.17 (–0.40 to 0.06) |
| GP nurse other visit | 1.85 (2.21) | 2.16 (3.43) | –0.32 (–0.88 to 0.23) |
| Number of men using catheters | 1.17 (1.29) | 1.26 (1.29) | –0.09 (–0.34 to 0.16) |
| Number of men using sheaths | 0.20 (0.59) | 0.24 (0.61) | –0.03 (–0.14 to 0.08) |
| Hospital doctor visits | 0.44 (1.13) | 0.44 (1.14) | –0.01 (–0.23 to 0.21) |
| Hospital nurse visitsb | 0.62 (1.49) | 0.31 (0.88) | 0.32 (0.08 to 0.55) |
| Hospital physiotherapist visitsb | 0.79 (1.44) | 0.18 (1.17) | 0.62 (0.38 to 0.87) |
| Inpatient days | 0.04 (0.26) | 0.06 (0.29) | –0.02 (–0.07 to 0.04) |
| Number taking incontinence drugs | 0.29 (0.85) | 0.33 (0.86) | –0.05 (–0.21 to 0.11) |
| Self-purchased use of health care | |||
| Pads used | 1.61 (3.18) | 2.37 (4.60) | –0.62 (–1.34 to 0.09) |
| Bed/chair protector used | 0.25 (0.72) | 0.48 (2.18) | –0.17 (–0.47 to 0.14) |
| Private doctor visits | 0.13 (0.47) | 0.14 (0.53) | –0.02 (–0.12 to 0.07) |
| Private nurse visits | 0.15 (0.53) | 0.09 (0.35) | 0.07 (–0.02 to 0.15) |
| Private physiotherapist visits | 0.16 (0.60) | 0.08 (0.68) | 0.07 (–0.05 to 0.19) |
| Number of days off workb | 7.62 (24.71) | 15.20 (42.93) | –6.54 (–13.08 to –0.01) |
Costs
Participant time and travel costs
The average time and costs to participants and their companions (families or carers) of a contact with a GP, an outpatient consultation or an inpatient admission are reported in Table 41. These data were combined with the information on number of contacts (e.g. hospital doctor visits) that the trial participants had reported (Table 40) to estimate a monetary cost per patient for both intervention and control groups.
| Resource use | Time or monetary cost | Mean (SD) |
|---|---|---|
| Primary care consultation visit | Time spent going to and attending a primary care consultation (hours) | 0.67 (0.30) |
| Companion’s time off work (£) | 0.80 (2.40) | |
| Average cost to participant and companion of a primary care consultation (£) | 17.30 (36.75) | |
| Secondary care visit | Time spent attending a secondary care visit (hours) | 2.07 (1.51) |
| Companion’s time off work (£) | 9.05 (14.46) | |
| Average cost to participant and companion of travelling to a secondary care department (£) | 36.43 (49.17) | |
| Inpatient visit | Visits to participant during admission (number) | 4.04 (5.42) |
| Companion’s time off work (£) | 23.46 (73.12) | |
| Average cost to participant and companion of travelling to admission (£) | 82.78 (136.38) |
Estimation of societal costs
Table 42 details the mean cost per participant of the two interventions. The unit cost information in Tables 4 and 41 was combined with the resource use information reported in Table 40 to provide estimates of the total cost per participant. For the base-case analysis, based on societal costs, the mean total cost per participant in the intervention group was £1509 (SD £2802) and the mean cost in the control group was £2209 (SD £4835). The trend towards higher costs in the control group, –£588 (95% CI –£1330 to £153), was not statistically significant. The difference in mean societal cost was mainly due to the high number of days taken off work by the participants in the control arm of the trial.
| Area of resource use | Mean cost [£ (SD)] | Difference (95% CI)a | |
|---|---|---|---|
| Intervention | Control | ||
| NHS costs | |||
| Intervention | 198.30 (63.89) | 0 | 193.30 |
| Subsequent resource use | |||
| NHS-supplied padsb | 38.03 (59.71) | 54.67 (88.32) | –13.97 (–26.88 to –1.06) |
| NHS-supplied bed/chair protectors | 2.40 (8.74) | 3.58 (19.21) | –1.15 (–3.96 to 1.66) |
| GP doctor incontinence-related visitsb | 11.77 (42.11) | 22.54 (69.78) | –12.18 (–22.55 to –1.80) |
| GP doctor other visit | 150.15 (144.90) | 163.05 (145.08) | –8.72 (–36.09 to 18.66) |
| GP nurse incontinence-related visit | 1.72 (10.30) | 4.11 (16.55) | –1.85 (–4.38 to 0.68) |
| GP nurse other visit | 20.34 (24.29) | 23.76 (37.76) | –3.57 (–9.65 to 2.51) |
| Catheter | 0.46 (6.55) | 0.23 (3.27) | 0.24 (–0.77 to1.24) |
| Sheath | 22.00 (81.06) | 21.89 (74.81) | 0.73 (–12.33 to 13.80) |
| Hospital doctor visits | 32.93 (84.42) | 32.77 (85.22) | –0.72 (–17.19 to 15.75) |
| Hospital nurse visitsb | 19.20 (46.16) | 9.48 (27.19) | 9.83 (2.49 to 17.17) |
| Total hospital physiotherapy visitsb | 24.35 (44.72) | 5.57 (36.25) | 19.32 (11.79 to 26.85) |
| Inpatient days | 13.80 (46.65) | 20.07 (48.83) | –5.67 (–14.73 to 3.39) |
| Prescribed drugs | 28.96 (98.77) | 28.20 (86.21) | –2.99 (–19.96 to 13.98) |
| Total subsequent use cost | 358.42 (381.02) | 378.99 (399.45) | –17.31 (–89.81 to 55.19) |
| Total NHS cost | 556.72 (396.07) | 378.99 (399.45) | 181.02 (107.06 to 254.97) |
| Patient costs | |||
| Self-supplied pads | 35.17 (58.52) | 49.11 (82.18) | –12.88 (–26.58 to 0.81) |
| Self-supplied bed/chair protector | 0.87 (5.59) | 2.85 (21.74) | –1.14 (–4.03 to 1.76) |
| Private doctor visits | 4.02 (44.68) | 2.91 (29.49) | 0.03 (–7.01 to 7.07) |
| Private nurse visits | 0.00 (0) | 0.00 (0) | 0 |
| Private physiotherapist visits | 0.91 (9.66) | 0.30 (4.32) | 0.36 (–0.63 to 1.36) |
| Participant number of days off workb | 809.19 (2624.53) | 1614.14 (4558.83) | –694.77 (–1388.71 to –0.83) |
| Total patient costs | 832.72 (2628.03) | 1657.67 (4554.01) | –714.57(–1408.16 to –20.97) |
| Participant travel and companion travel and time off work costs | |||
| Intervention | 132.70 (43.43) | 0.00 (0) | |
| GP doctor incontinence-related visitsb | 5.66 (20.25) | 10.84 (33.55) | –5.85 (–10.84 to –0.87) |
| GP doctor other visit | 72.20 (69.67) | 78.40 (69.76) | –4.19 (–17.36 to 8.97) |
| GP nurse incontinence-related visit | 2.70 (16.21) | 6.47 (26.05) | –2.91 (–6.90 to 1.08) |
| GP nurse other visit | 32.00 (38.22) | 37.39 (59.42) | –5.62 (–15.19 to 3.95) |
| Total GP visits | 112.56 (97.54) | 133.10 (131.13) | –18.92 (–40.46 to 2.61) |
| Hospital doctor visits | 16.43 (42.12) | 16.35 (42.52) | –0.35 (–8.58 to 7.86) |
| Hospital nurse visitsb | 23.18 (55.72) | 11.44 (32.82) | 11.89 (3.01 to 20.73) |
| Hospital physiotherapy visitsb | 29.39 (53.98) | 6.72 (43.76) | 23.32 (14.23 to 32.41) |
| Total outpatient visitsb | 201.70 (118.93) | 34.51 (81.09) | 167.06 (147.36 to 188.77) |
| For inpatient visits | 3.23 (21.44) | 4.82 (24.05) | –1.30 (–5.61 to 3.02) |
| Total participant and companion travel and time off work costb | 450.19 (200.28) | 172.44 (180.86) | 272.74 (225.08 to 320.40) |
| Total participant and companion cost | 1150.21 (2671.60) | 1830.11 (4627.01) | –567.96 (–1274.10 to 138.15) |
| Total societal costs | 1508.63 (2802.37) | 2209.10 (4835.12) | –588.23 (–1329.83 to 153.37) |
Estimation of NHS costs
In terms of NHS costs incurred after the intervention was delivered, the mean total cost per patient in the intervention group was £358 (SD £381) and the mean cost in the control group was £379 (SD £399). There was, however, no evidence of a statistically significant difference in the cost of subsequent NHS services used. Intervention costs were, as would be expected, greater in the intervention group. Combining information on the cost of the interventions and the cost of subsequent NHS care resulted in a statistically significantly higher total cost per participant in the intervention group. This difference was driven almost entirely by the cost of the PFMT intervention itself.
Quality-adjusted life-years
Table 43 shows the EQ-5D scores for each arm of the trial at baseline and 6 and 12 months. Also reported is the mean difference between arms in EQ-5D score at 6 and 12 months. From these data it was estimated that the mean QALYs were 0.86 (SD 0.16, median 0.796) for the intervention arm and 0.86 (SD 0.19, median 0.796) for the control arm. The mean difference in QALYs after adjusting for minimisation and baseline EQ-5D scores was –0.002 (95% CI –0.027 to 0.023) higher for the intervention group, which was not statistically significant.
| Mean (SD) | Difference (95% CI) | ||
|---|---|---|---|
| Intervention | Control | ||
| Baseline EQ-5D |
0.80 (0.22) n = 200 |
0.78 (0.23) n = 206 |
|
| 6-month EQ-5D |
0.88 (0.21) n = 184 |
0.87 (0.19) n = 189 |
0.009 |
| 12-month EQ-5D |
0.88 (0.21) n = 187 |
0.89 (0.18) n = 189 |
–0.008 |
| QALYs |
0.86 (0.19) n = 170 |
0.86 (0.16) n = 179 |
–0.002 (–0.027 to 0.023)a |
Imputation was not performed on missing values in the base-case analysis. Simple plausible extreme value imputation on EQ-5D scores taking the 25th and 75th percentile values indicated that the mean difference in EQ-5D scores did not differ from that reported in Table 43.
Estimation of cost-effectiveness
Societal perspective
The cost-effectiveness of the intervention compared with control is dependent on whether the differences in QALYs are considered to be important to people with incontinence. Taking the mean difference in the total societal costs from Table 42 (–£588) and the mean difference in QALYs from Table 43 (–0.002) it can be seen that the intervention is, on average, more effective and less costly (Table 44).
| Difference in mean NHS costs [mean (95% CI)a] | –588.23 (–1329.83 to 153.37) |
| Difference in QALYs [mean (95% CI)] | –0.002 (–0.027 to 0.023) |
| ICER (£/QALY) | Organised PFMT is dominant |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 89.4% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 83.7% |
Uncertainty around the estimates of QALYs and costs was derived using 1000 bootstrap simulations. The bootstrap estimates in Figure 21 indicate that in most of the instances the intervention group had lower costs than the control; however, there was a relatively wide distribution in the difference in QALYs and costs.
FIGURE 21.
Representation of the uncertainty in differential mean costs and QALYs: societal perspective (radical prostatectomy).
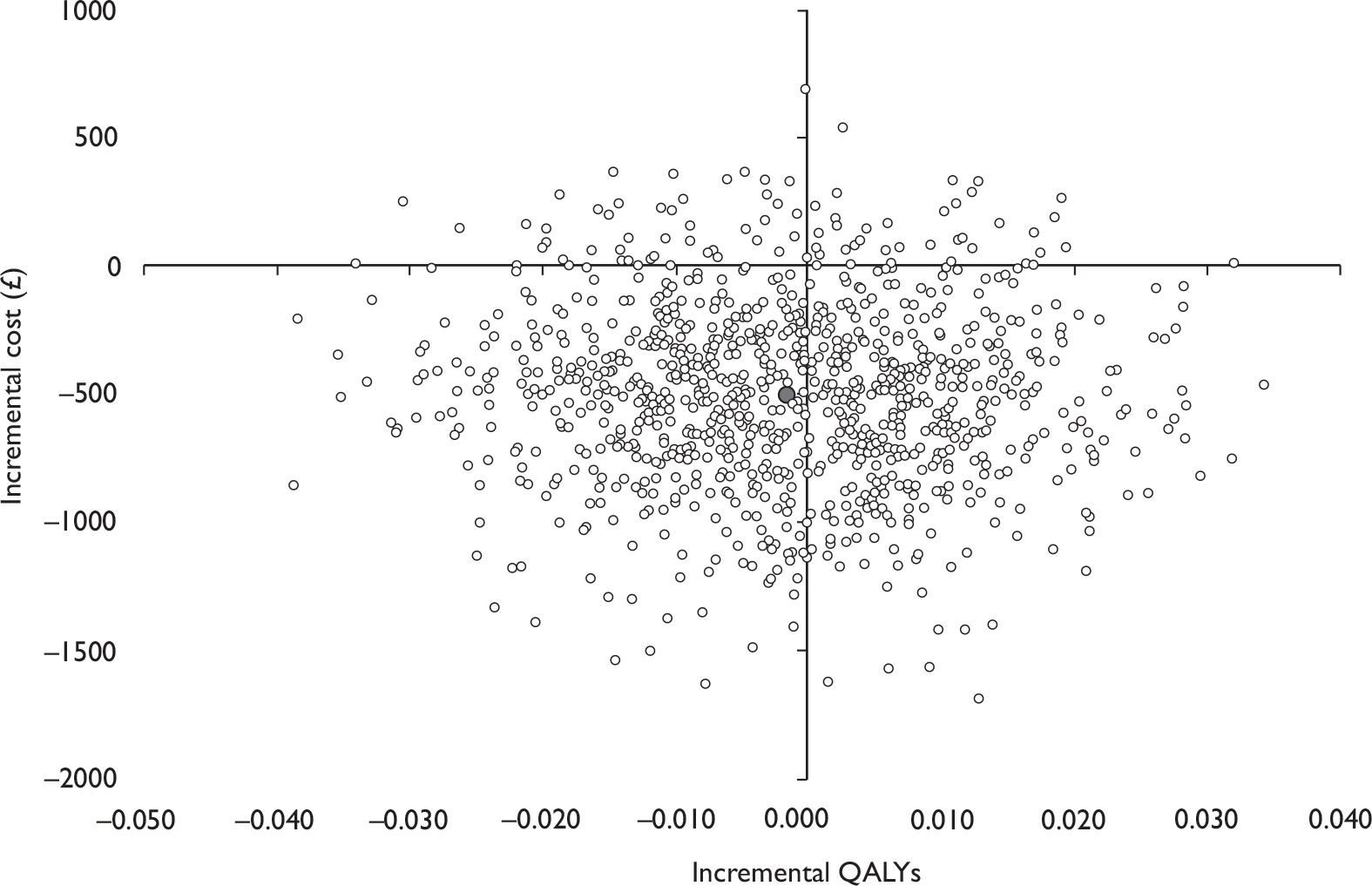
At a cost-effectiveness threshold of £20,000 per QALY, the intervention has a likelihood of 89% of being cost-effective, and at a threshold of £30,000 per QALY the intervention is 84% likely to be cost-effective (Figure 22). However, these results have to be interpreted cautiously as they are driven almost entirely by differences in time away from usual activities.
FIGURE 22.
Cost-effectiveness acceptability curve for intervention versus control: societal perspective (radical prostatectomy).
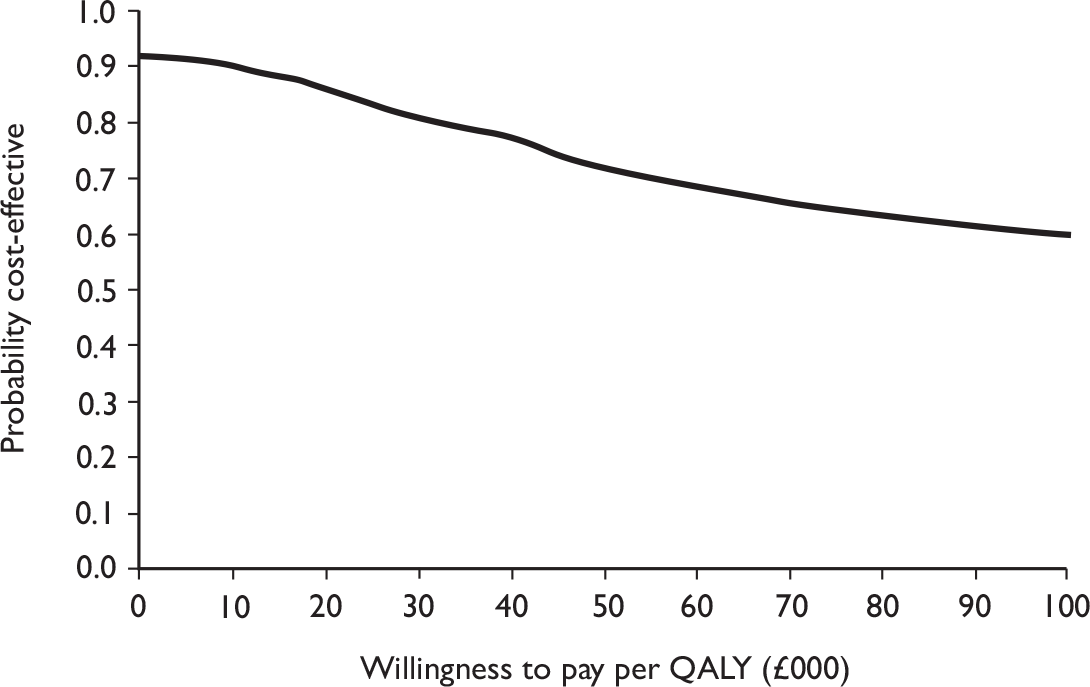
NHS perspective
Taking the mean difference in total NHS costs from Table 42 (£181) and the mean difference in the QALY estimate after adjusting for baseline EQ-5D scores from Table 43, the mean incremental cost per QALY is £90,510 (Table 45).
| Difference in mean NHS costs [mean (95% CI)] | 181.02 (107.06 to 254.97) |
| Difference in QALYs [mean (95% CI)] | –0.002 (–0.027 to 0.023) |
| ICER (£/QALY) | 90,510 |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 19.2% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 27.3% |
As with the societal perspective, bootstrap simulations were undertaken to estimate the uncertainty around the benefit and costs. The bootstrap estimates in Figure 23 indicate that the intervention group had higher costs than the control. However, there was a relatively wide distribution in the difference in QALYs.
FIGURE 23.
Representation of the uncertainty in differential mean costs and QALYs: NHS perspective (radical prostatectomy).
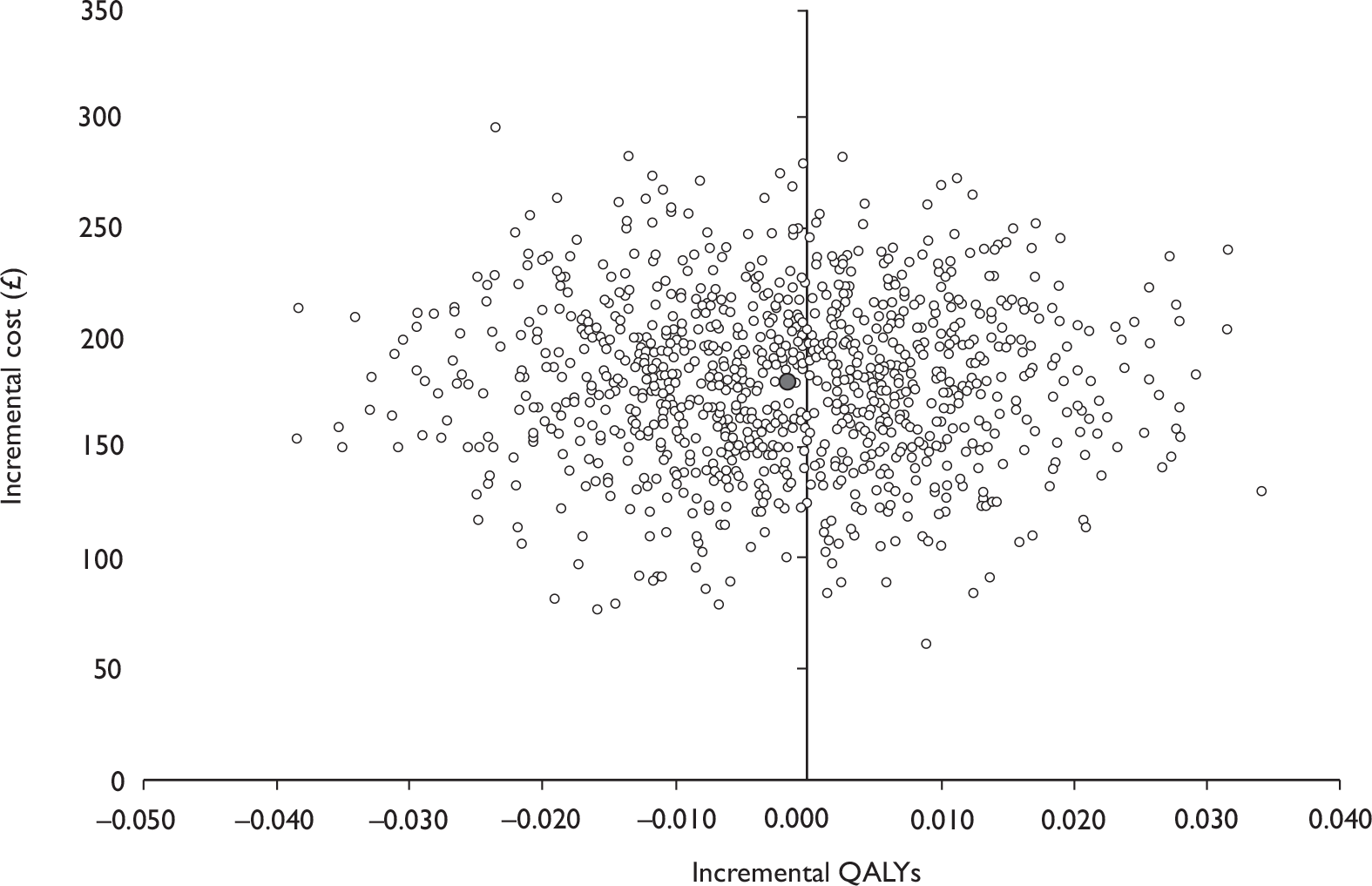
At a cost-effectiveness threshold of £20,000 per QALY, the intervention has a probability of 19% of being cost-effective, and at a threshold of £30,000 per QALY the intervention is 27% more likely to be cost-effective (Figure 24). At no point does the probability of being cost-effective reach 50%. This indicates that it is unlikely that PFMT is cost-effective.
FIGURE 24.
Cost-effectiveness acceptability curve for intervention versus control: NHS perspective (radical prostatectomy).
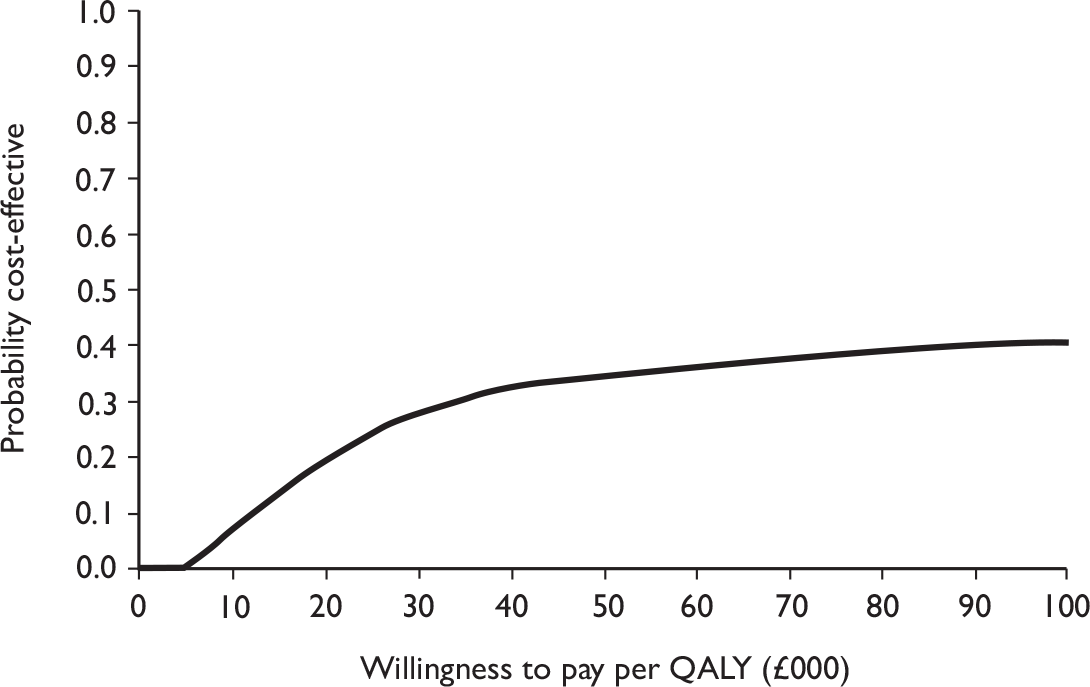
Sensitivity analysis
As mentioned in Chapter 2, sensitivity analysis is necessary to assess the robustness of the qualitative conclusion and identify areas where research is needed to more precisely estimate the values of those variables to which the result is sensitive. The variables that were considered uncertain in this study related to the costs and QALYs of the different services used.
Incremental cost per quality-adjusted life-year when differences are not adjusted for baseline differences
An unadjusted analysis was performed as a sensitivity analysis to highlight the importance of the assumption that the characteristics of the groups were not the same at baseline. The results of this analysis, from the perspective of the NHS, indicate that at a cost-effectiveness threshold of £20,000 per QALY the intervention has a probability of 45.3% of being cost-effective, and at a threshold of £30,000 per QALY the intervention is 50.2% more likely to be cost-effective (Figures 25 and 26).
FIGURE 25.
Representation of the uncertainty in differential mean costs and QALYs: using unadjusted data (radical prostatectomy).
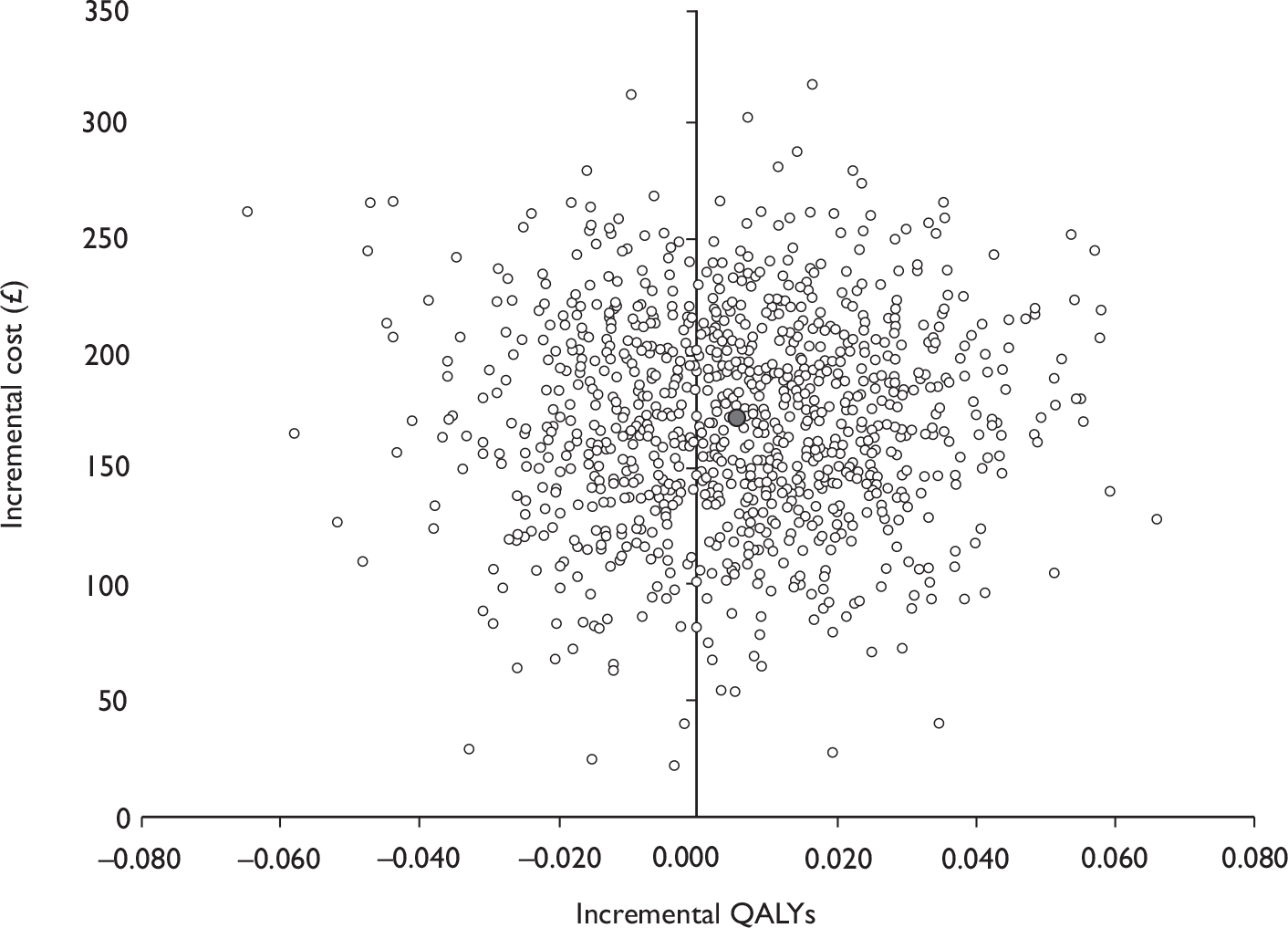
FIGURE 26.
Cost-effectiveness acceptability curve for intervention versus control: using unadjusted data (radical prostatectomy).
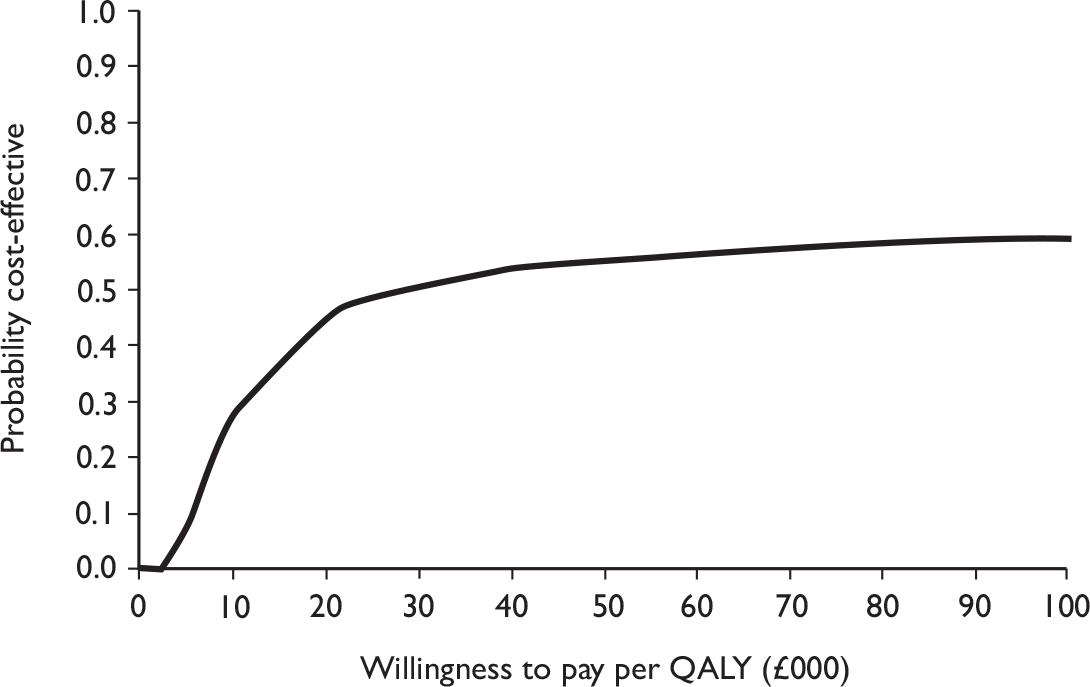
Basing quality-adjusted life-year estimates on SF-6D values
Table 46 reports the SF-6D scores for each arm of the trial at baseline and 6 and 12 months. These scores were slightly lower than those reported using the EQ-5D. From these data it was estimated that the mean QALYs were 0.80 (SD 0.11, median 0.806) for the intervention arm and 0.79 (SD 0.11, median 0.818) for the control arm. The mean difference in QALYs after adjusting for minimisation and baseline SF-6D scores was 0.005 (95% CI –0.022 to 0.012) higher for the intervention group, which was not statistically significant.
| Mean (SD) | Difference (95% CI)a | ||
|---|---|---|---|
| Intervention | Control | ||
| Baseline SF-6D |
0.71 (0.12) n = 201 (98%) |
0.69 (0.13) n = 200 (97%) |
|
| 6-month SF-6D |
0.82 (0.14) n = 188 (92%) |
0.81 (0.14) n = 189 (92%) |
0.012 |
| 12-month SF-6D |
0.82 (0.13) n = 189 (92%) |
0.84 (0.12) n = 189 (92%) |
–0.014 |
| QALYs |
0.80 (0.11) n = 172 (78%) |
0.79 (0.11) n = 166 (75%) |
–0.005 (–0.022 to 0.012) |
The results of the analysis using the SF-6D data when estimating incremental cost-effectiveness from the societal perspective were similar to those of the EQ-5D. Taking the mean difference in the total societal costs from Table 42 (–£588) and the mean difference in QALYs from Table 46 (–0.005); it can be seen that the intervention is, on average, more effective and less costly (Table 47).
| Difference in mean NHS costs [mean (95% CI)a] | –588.23 (–1329.83 to 153.37) |
| Differences in QALYs [mean (95% CI)a] | –0.005 (–0.022 to 0.012) |
| ICER (£/QALY) | Organised PFMT is dominant |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 85.0% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 79.8% |
At a cost-effectiveness threshold of £20,000 per QALY, the intervention has a likelihood of 85% of being cost-effective, and at a threshold of £30,000 per QALY the intervention is 79.8% likely to be cost-effective (Figures 27 and 28). Based on the societal perspective, these estimates indicate that the intervention is likely to be cost-effective. However, as with the base-case analysis, these results have to be interpreted cautiously as they are driven almost entirely by differences in time away from usual activities for which there is no obvious trial-related explanation.
FIGURE 27.
Representation of the uncertainty in differential mean costs and QALYs: societal perspective using SF-6D data (radical prostatectomy).
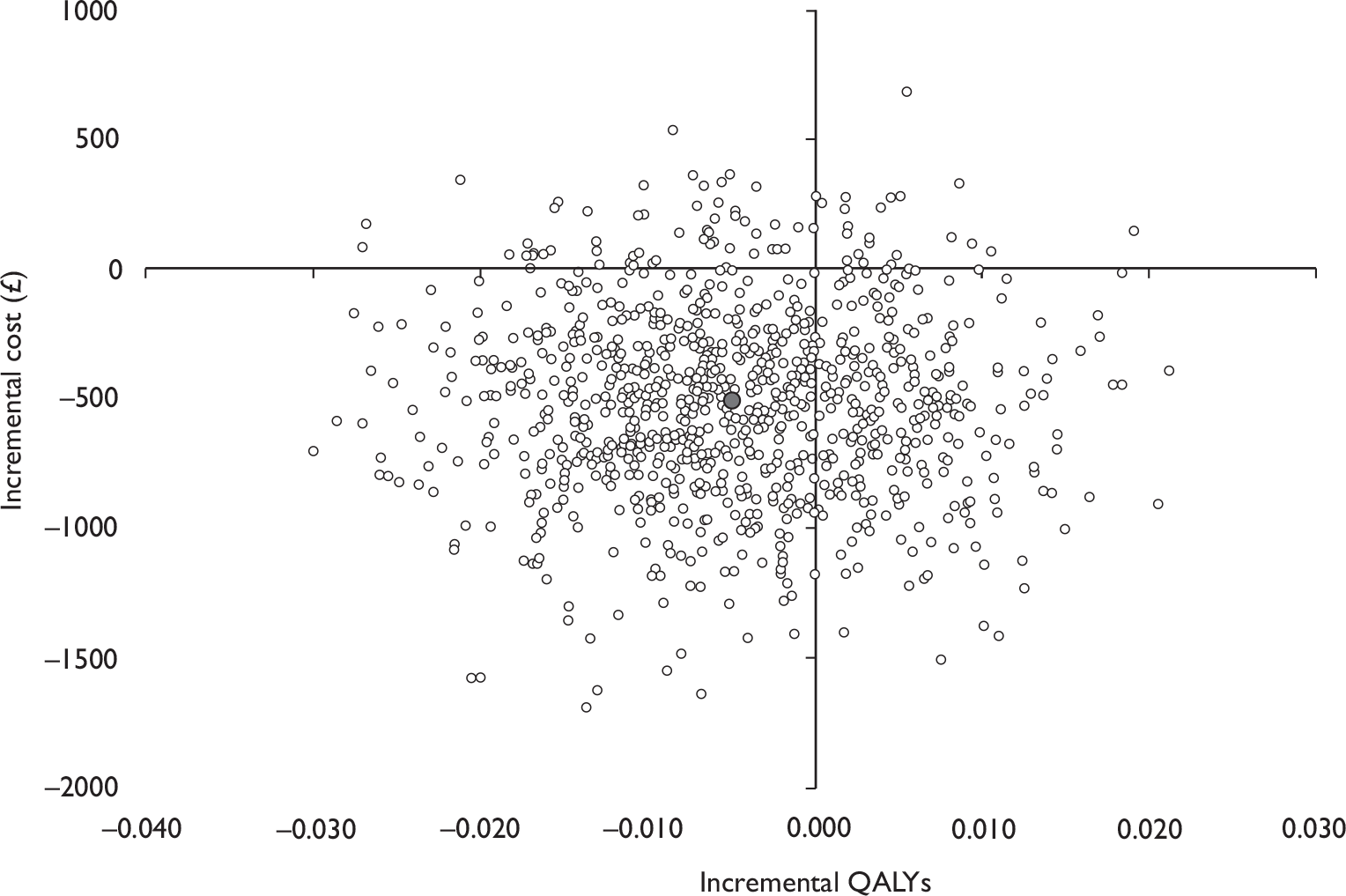
FIGURE 28.
Cost-effectiveness acceptability curve for intervention versus control: societal perspective using SF-6D data (radical prostatectomy).
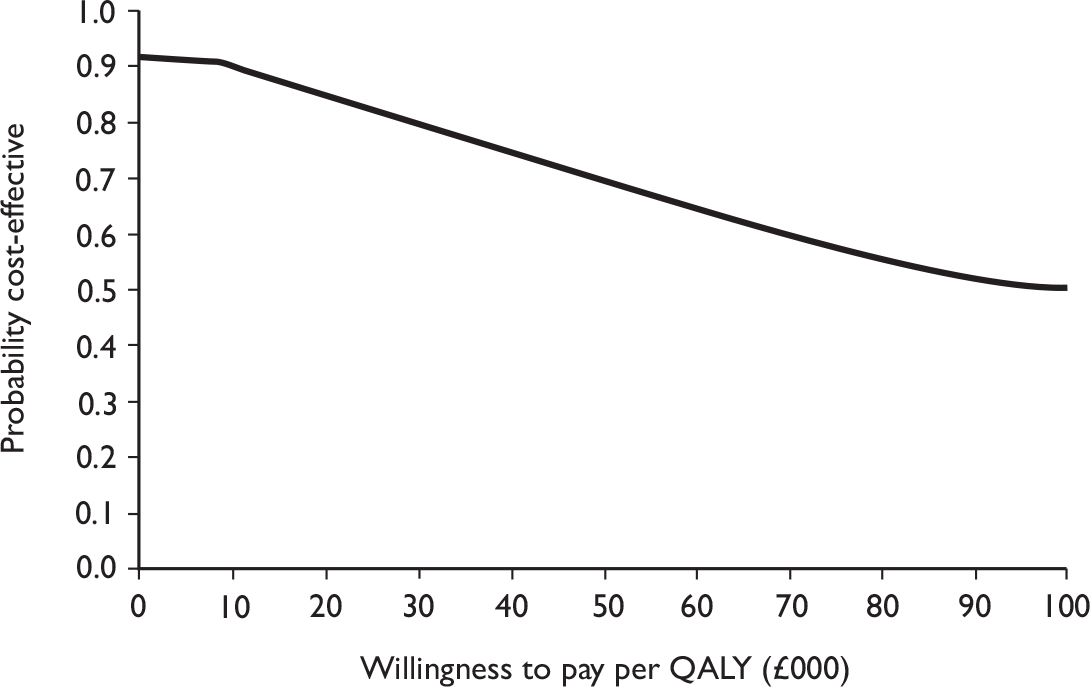
Taking the mean difference in total NHS costs from Table 42 and the mean difference in the QALY estimate after adjusting for baseline SF-6D scores from Table 46, the mean incremental cost per QALY from the NHS perspective is reduced to £36,204 (Table 48).
| Difference in mean NHS costs [mean (95% CI)a] | 181.02 (107.06 to 254.97) |
| Differences in QALYs [mean (95% CI)a] | –0.005 (–0.012 to 0.022) |
| ICER (£/QALY) | 36,204 |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 6.0% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 11.0% |
The probability of the intervention being cost-effective in this analysis was lower than that estimated when using the EQ-5D data. At a cost-effectiveness threshold of £20,000 per QALY the intervention has a probability of 6% of being cost-effective, and at a threshold of £30,000 per QALY the intervention is 11% likely to be cost-effective (Figures 29 and 30). At no point does the probability of being cost-effective reach 50%.
FIGURE 29.
Representation of the uncertainty in differential mean costs and QALYs: NHS perspective using SF-6D data (radical prostatectomy).
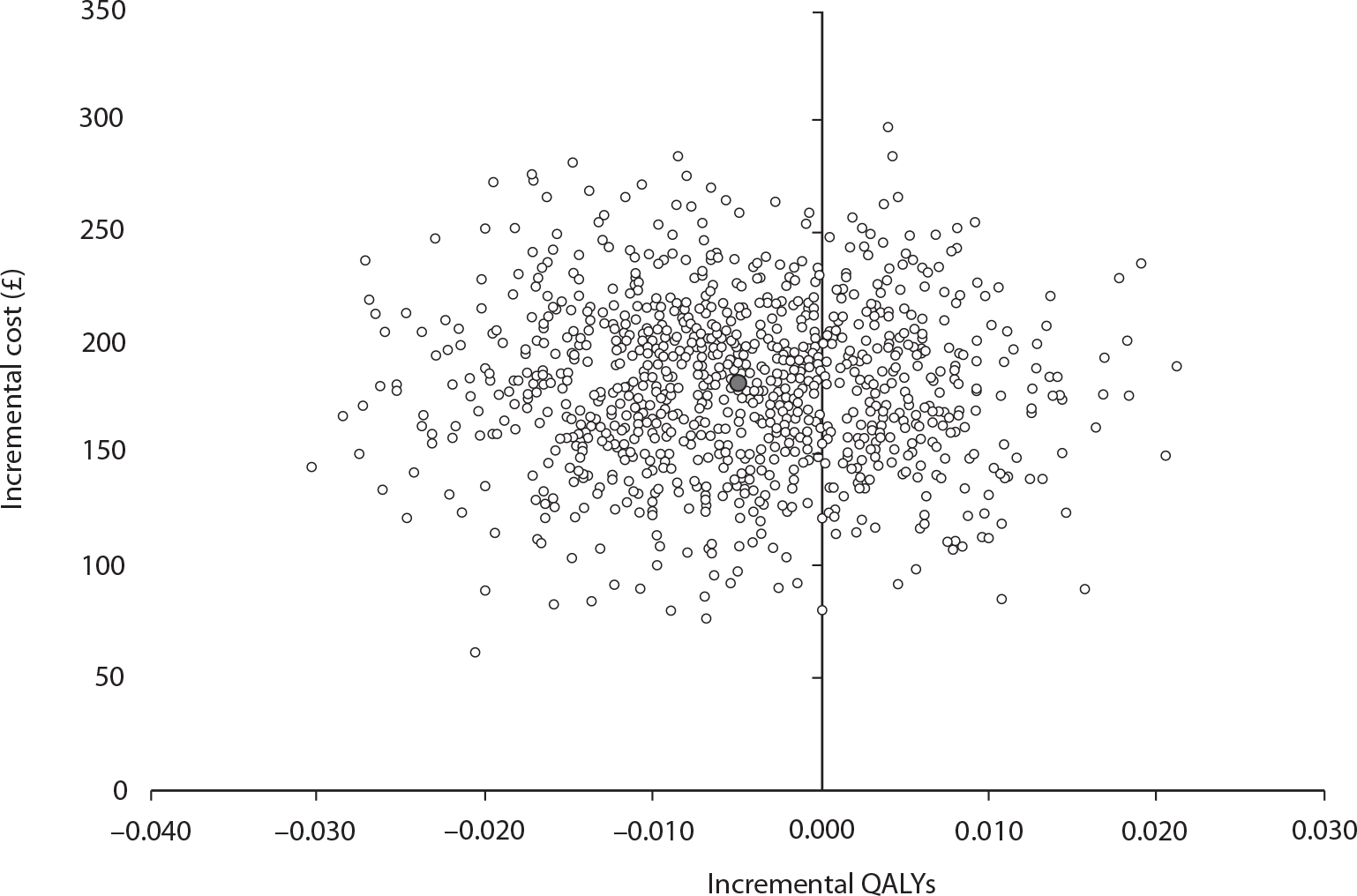
FIGURE 30.
Cost-effectiveness acceptability curve for intervention versus control: NHS perspective using SF-6D data (radical prostatectomy).
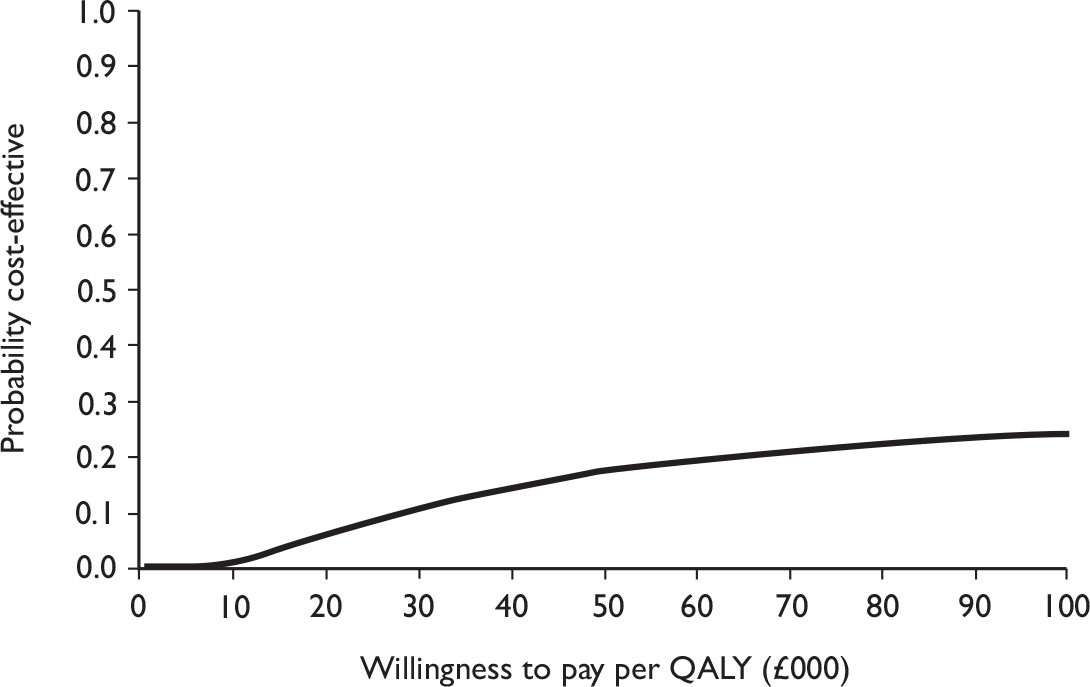
Threshold analysis around the cure rates
Further sensitivity analysis was performed by reanalysing the data by patient group for differences in costs and QALYs by continence status. A simple model was used to determine at what reduction in the rate of incontinence in the intervention group compared with the control the physical therapy would be cost-effective. Details of the parameters used in the model are given in Table 49.
| Parameter | Participants in intervention group who were continent | Participants in intervention group who were incontinent | Participants in control group who were continent | Participants in control group who were incontinent |
|---|---|---|---|---|
| Cost of intervention (£) | 196.34 (223.00) [66.82] | 204.56 (223.00) [56.00] | 0.00 (0.00) [0.00] | 0.00 (0.00) [0.00] |
| Total subsequent resource use costs (£) | 291.17 (239.19) [209.46] | 518.59 (365.39) [485.15] | 253.63 (214.21) [174.01] | 291.17 (239.19) [209.46] |
| Total NHS costs (£) | 487.51 (442.09) [212.57] |
723.16 (565.42) [495.26] |
253.63 (214.21) [174.01] | 291.17 (239.19) [209.46] |
| QALY | 0.92 (0.95) [0.08] | 0.84 (0.92) [0.21] | 0.91 (0.94) [0.10] | 0.84 (0.89) [0.18] |
| Probability of being continent/incontinent | 0.23 | 0.77 | ||
| Relative risk of being incontinent | 0.85–1 | 0.85–1 |
Figure 31 shows that when the rate of incontinence was reduced below 0.66 in the treatment group (while that of the control group was 0.77), the incremental cost per QALY would reduce to the level that society might be willing to pay. Reductions in the rates of incontinence are consistent with the reported CIs surrounding differences in continence rates. This suggests that, if smaller differences are clinically important, then, should these reductions be achieved, they would be potentially cost-effective.
FIGURE 31.
Probability that intervention is likely to be cost-effective: radical prostatectomy.
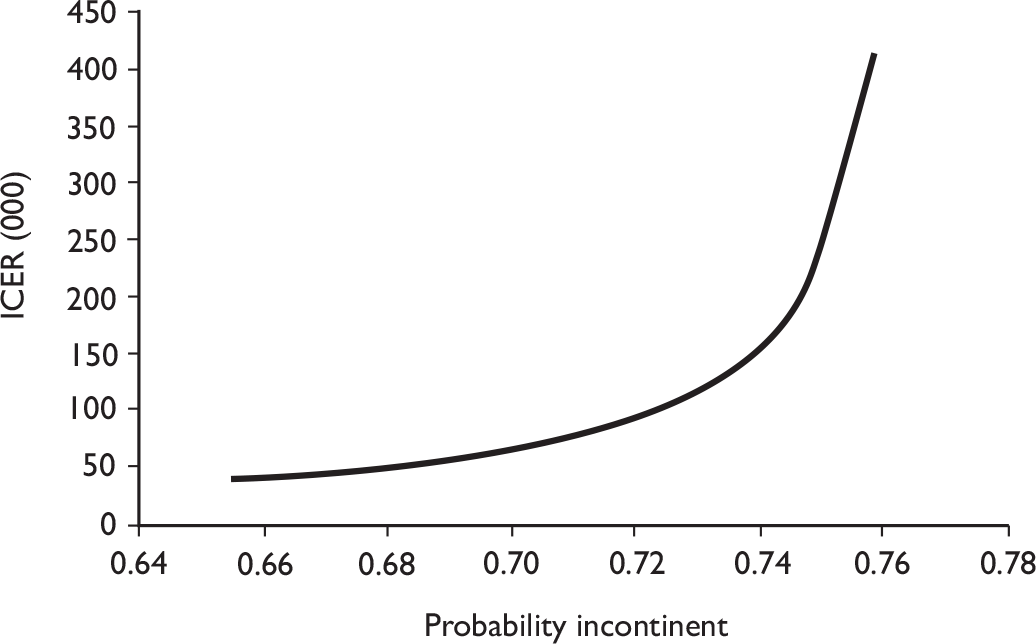
Conclusions
For men having radical prostatectomy, QALYs were similar in both groups, and the costs of those who received the intervention were higher, but not statistically significantly so, than the costs of those who did not. The costs of the intervention were less than those of the control when the analysis was performed from a societal perspective. However, these results need to be interpreted cautiously as they were largely influenced by the number of days that the participants said they were off work. The results of the analysis performed from the NHS perspective had lower costs for the control group, as anticipated. The cost-effectiveness results from a societal perspective favoured the intervention, and those from the NHS perspective favoured the control.
The model-based sensitivity analysis showed that a reduction in continence rates that is consistent with the CIs surrounding the relative risk of incontinence between treatment and control might be cost-effective. A judgement is required whether the smaller differences in incontinence are clinically significant. If they are, then a further judgement is required whether a larger trial would be worthwhile to identify these differences.
Chapter 9 Discussion of results of randomised controlled trial after radical prostatectomy
This chapter summarises the discussions relating to the radical prostatectomy RCT.
Summary of main findings
In the men who had a radical prostatectomy, there were no statistically significant differences in urinary, bowel or sexual function outcomes between the intervention and control groups, despite evidence of extra performance of PFMT and improvement in pelvic floor muscle strength over time in the intervention group. The estimated additional cost to the NHS was on average £181 (95% CI £107 to £255) higher in the intervention group than in the control group.
Recruitment and screening of men in hospital
We approached 1158 men having a radical prostatectomy in NHS hospitals and obtained consent to screen 804 of these men. Of those, 95% returned their screening survey, and over 90% of the responders were incontinent of urine at about 6 weeks after surgery (see Table 13). This prevalence was similar to that found by Kao et al. (82% in 1013 men). 9 The majority of the men (around 80%) had a traditional open retropubic radical prostatectomy, and around 55% had a procedure in which one or both nerve bundles were spared. Only 6% of the men had urinary incontinence before surgery, and 2% reported faecal incontinence (see Table 13). The average age of the men was 62 years.
Recruitment to randomised controlled trial and response rates
Of the 742 men who were incontinent at screening, 411 agreed to be randomised to a controlled trial of conservative treatment (PFMT and lifestyle advice) for urinary incontinence (205 in the intervention group and 206 in the control group). The groups were comparable at baseline on all the epidemiological and clinical characteristics measured (see Table 14). Almost all of the men had heard of pelvic floor exercises at some time prior to randomisation (see Table 15).
Conduct of the intervention
Compliance (attendance at therapy visits) with the intervention was high, with 92% of the men allocated to the intervention group attending at least one therapy visit and 85% attending all four of them. The most common reasons for not attending were becoming dry and finding it inconvenient to attend.
Association with type of therapist
Half of the centres used physiotherapists as the provider of the intervention, while the rest used nurse therapists (although all therapists received the same standardised training). About 40% of the men attended a physiotherapist while 60% attended a nurse therapist. However, there were no significant differences in the number of visits or the chance of urinary incontinence during the treatment period according to type of therapist (see Table 19).
At follow-up, no statistically significant association was demonstrated between the chance of incontinence at 12 months and type of therapist (see Figure 20).
Clinical symptoms during the therapy period
During the therapy period, the proportion of men with incontinence fell from 92% to 73% by the fourth visit (see Table 20 and Figure 7). Few men reported bowel problems, and these numbers did not vary much over time (see Table 22 and Figure 9). Around 90% of the men had problems with sexual function and these did not decrease with time (see Table 23 and Figure 10).
Clinical findings during the therapy period
Anal sphincter and pelvic floor muscle contraction strength increased over time in the intervention group: 40% of men rated their strength as good or better at the beginning of the therapy period, rising to 85% by the fourth visit (see Table 24 and Figure 11). However, around 15% still had only moderate or poor contraction strength at the end of the 3-month therapy period.
Machine-led biofeedback was available in only 13 of the 34 MAPS centres (see Table 7), and was used clinically in only five centres in 16 men (see Table 25). It was not clear whether this was for diagnosis or for repeated use to assist with training. However, almost all men had verbal biofeedback from their therapist following digital anal assessment of muscle contraction, to teach them to perform contractions correctly and to monitor improvement at each successive visit.
Practice of pelvic floor muscle training after end of therapy period
While over 80% of men in both the intervention and the control groups were practising PFMT at baseline (before they were randomised and before the intervention), this fell to 67% in the intervention group and 50% in the control group at the 12-month follow-up.
Findings of the randomised controlled trial
The primary outcome of the RCT was the proportion of men with urinary incontinence at 12 months after randomisation. This was measured using the ICI-SF questionnaire, and was also ascertained at 3, 6 and 9 months after randomisation. In addition, urinary outcomes were obtained from 3-day diaries completed by the men at each of these time points. The response rates were over 95% for the questionnaires and over 80% for the diaries (see Table 27).
Urinary outcomes
While the proportion of men with urinary incontinence fell from 100% at baseline to around 75% by 12 months, the majority of the decrease occurred in the first 3 months (to around 87%) with a further fall (to 80%) at 6 months. There was no statistically significant difference in the proportion of men with urinary incontinence between the intervention and control group at 12 months [75.5% vs 77.4%, absolute risk difference –1.9% (95% CI –10% to 6%) (see Table 28) or at any other time point (see Table 29 and Figure 14)].
These findings (of no statistically significant differences between the trial groups) were similar for all the urinary outcomes regardless of how (by questionnaire or diary) or when they were measured (see Table 29).
Severity of incontinence
If severe incontinence is defined as incontinence at least once a day and a moderate or large amount of leakage, around 40% of the men were still experiencing severe leakage at 12 months (see Table 29a) and 40% were also using pads (see Table 31a). Using the ICI score as a composite measure of severity and effect on quality of life, the same picture emerged: the majority of the improvement (decrease) in the score occurred in the first 3 months after randomisation with a further small improvement at 6 months (see Table 29 and Figure 15).
Types of incontinence
The most common type of incontinence was SUI (around 70%; see Table 30), while UUI and MUI affected around 40% of men. The prevalence of these and the other types of urinary symptoms was no different between the randomised groups (see Table 30 and Figure 16).
Subgroup analyses
Prespecified subgroup analyses were carried out on the primary outcome (urinary incontinence at 12 months). There were no significant differences between randomised groups in any of the subgroups (see Figure 20).
Other clinical outcomes
Men were also asked to report on bowel and sexual problems.
Bowel outcomes
Bowel problems that might be expected to be ameliorated by therapy or lifestyle advice included faecal incontinence, urgency and constipation. Men were also asked about bowel conditions such as ulcerative colitis, Crohn’s disease and irritable bowel syndrome. There were no differences at any time point in any aspect of bowel function or disease between the men in the randomised groups (see Table 32 and Figure 17).
Sexual function outcomes
Over 90% of men had normal erectile function before operation. Although around one-third had an active sex life at 12 months, the majority said that this was worse than before their operation (see Table 33). There were, however, no differences at 12 months according to the randomised groups in any of the aspects of sexual function measured (see Tables 33 and 34). Even at 12 months, 82% of intervention group men and 78% of control group men reported severely reduced or no erectile function (see Table 34 and Figure 18). Neither were there differences between the groups in the proportions of men with an active sex life [RR 0.94 (95% CI 0.73 to 1.22); p = 0.661] or the proportions of men whose sex life had become worse after the operation [RR 0.79 (0.47 to 1.34); p = 0.391; see Table 33].
Over half of the men had used a vacuum device or medication to improve sexual function (see Table 34). NICE guidelines17 suggest that phosphodiesterase type 5 (PDE5) inhibitors could be offered to men who experience loss of erectile function. If PDE5 inhibitors fail or are contraindicated, men could be offered vacuum devices, intraurethral inserts, penile injections or prostheses.
Quality of life outcomes
General health outcomes were measured using the EQ-5D and SF-12 (the latter subdivided into role – mental and role – physical scores). The slight increase in the scores over time can be assumed to represent recovery from operation, but there were no differences between the randomised groups at any time point in EQ-5D or SF-12 scores (see Table 35 and Figure 19).
Knowledge of pelvic floor muscle training in trial groups before intervention
Nearly all of the men in both groups (97% and 99% respectively) had received information about the use of pelvic floor exercises before starting the trial intervention (see Table 15). The most common sources were from nurses or continence advisors (over 70%), or from leaflets or books (around 65%; see Table 15).
Practice of pelvic floor muscle training in intervention and control groups
It is not surprising, given the prior knowledge of pelvic floor exercises, that over 80% of men in both groups reported carrying out some exercises before randomisation (see Table 36). However, by 6 months the men in the intervention group were more likely to be still carrying them out and this difference persisted at the 12-month follow-up (see Table 36).
Changes in lifestyle factors
Men in both groups were given written information about lifestyle changes that might improve aspects of both their general health and incontinence. In the intervention group, this advice was reinforced and individualised by the therapists. However, there were no significant differences in the uptake of any aspect of this advice at 12 months after randomisation (see Tables 37 and 38).
Economic outcomes
Costs to the NHS
Total costs to the NHS were on average £181 (95% CI £107 to £255) higher in the intervention group than in the control group. This difference was entirely due to the cost of providing the PFMT training in the intervention group. The use of other health services, and hence cost, was, on average, slightly lower in the control group, but this difference was not statistically significant.
Costs to the participants
Costs to the participants related to three broad elements: (i) the cost of private health care; (ii) the cost of accessing NHS care (in terms of time and out-of-pocket expenses); and (iii) the cost of time away from usual activities. On average, the costs of any private health care used were low and there was no evidence of any difference between groups. Similarly, the costs of accessing care other than the intervention were similar for the two groups. However, participants in the control arm appeared, on average, to have more days away from usual activities than participants in the intervention arm [mean difference –6.54 days (95% CI –13.08 days to –0.01 days)]. The mean cost of time away from usual activities was approximately £809 for the intervention group and £1614 for the control group. Given the assumptions made about valuing time away from usual activities, this difference was the key determinant of the differences in average total participant costs. It is not clear why there would be fewer days away from usual activities in the intervention group, given the lack of evidence for any treatment effect. Therefore, as these data are counterintuitive, they should be treated cautiously.
Overall costs to the NHS and participants
On average, the cost to the NHS and participants was £588 [–£1330 to £153] greater in the control group than in the intervention group. Although not statistically significant, the direction of effect was the result of differences in the mean time away from usual activities.
Quality-adjusted life-years
On average, QALYs were virtually identical in both the intervention and the control group [on average, QALYs were 0.002 higher in the intervention group (95% CI –0.027 to 0.023)].
Cost-effectiveness from the perspective of the NHS and participants
Based upon the point estimates of the mean difference in costs and QALYs the intervention is dominant. The point estimates are associated with considerable imprecision, so the probability that the intervention would be considered cost-effective at typical thresholds for society’s willingness to pay for a QALY were calculated (see Figure 22). This analysis suggested that there was over an 80% chance that organised PFMT training was cost-effective. As noted above, this result was almost entirely driven by the differences in days away from usual activities, and therefore should be treated with caution.
Cost-effectiveness from the perspective of the NHS
When the perspective of the economic evaluation was restricted to the NHS, the point estimate of the incremental cost per QALY was effectively dominated. Furthermore, there was less than a 20% chance that the intervention would be cost-effective should the threshold value for society’s willingness to pay for a QALY be £20,000.
Sensitivity analyses
The majority of the sensitivity analyses conducted did not greatly alter the conclusions of the economic evaluation. In one sensitivity analysis, conducted from the perspective of the NHS, the reduction in the rate of incontinence that would make the intervention cost-effective was estimated. The results of this analysis suggested that, should the intervention reduce the rate of incontinence by approximately 10%, then the provision of physical therapy could be cost-effective. This difference is similar to the lower end of the CIs surrounding the risk difference in incontinence rates. However, the trial was of sufficient size to rule out any but a small chance that this difference could exist.
Strengths and weaknesses (specific to the radical prostatectomy randomised controlled trial)
Recruitment
We approached 1158 men who were admitted to hospital for radical prostate surgery in order to identify and recruit our final population of 411 men who entered the RCT. Many of the men approached were ineligible or missed in hospital (354 men; see Table 9a) and 24 were subsequently found to be ineligible (see Table 10a). This scale of recruitment represented a large burden on the recruitment officers in the centres. However, we felt that this was the most efficient way of identifying our target population, which was men who had urinary incontinence after prostate surgery. Other methods, such as expecting local staff to identify incontinent men and recruit them to the RCT directly, might have been too burdensome and risked missing many men owing to pressure of routine work.
Generalisability of the trial population
Most of the men who agreed, when in hospital, to be screened 3 weeks later returned their screening questionnaire (742/780, 95%; Table 12). There were no significant differences in demographic or clinical characteristics when non-responders were compared with responders. Just over half (411/742, 55%) of the men who returned a screening questionnaire were eventually recruited into the RCT. Many of the remainder had become dry [53 (7%) at screening, 61 (8%) at baseline], and a further 125 (17%) were not eligible because they did not return their baseline questionnaire (see Figure 4 and Table 11). Of the 92 not accounted for, 26 (4%) declined further contact, 15 (2%) did not wish to be randomised, and the remainder had a variety of other reasons for not wishing to enter the trial (see Table 11). A further 18 (2%) were excluded after randomisation. Thus, our trial population represented 411/472 (87%) of the men who were incontinent and eligible to be randomised, but only 411/780 (53%) of men identified in hospital as having radical prostate surgery.
Response rates
Once randomised, participants were compliant in returning their questionnaires (over 90%) and urinary diaries (over 80%), while the withdrawal rates were very low. There was no evidence of differential dropout from the randomised groups, with outcome data available for 98% (intervention) and 97% (control) of the men continuing at 12 months (see Table 27a). This provides some reassurance that the outcome data are representative of the men in the RCT, and that bias from differential attrition was minimal.
Strengths and weaknesses of the economic analyses
The methods of the economic analysis were rigorous and reproducible, and efforts were made to assess the importance of uncertainty surrounding estimates of costs, effects and cost-effectiveness. As the study was not powered to detect differences in economic outcomes, it was anticipated that differences in costs and effects would not reach statistical significance. For this reason, conclusions from the economic evaluation were based upon the consideration of the balance of probabilities.
The conclusions from the economic analysis are sensitive to the perspective taken. When the perspective was the NHS, it was unlikely that the intervention would be cost-effective. When the perspective was widened to the NHS and patients, there was over an 80% chance that the intervention would be cost-effective. The main driver of this difference in the conclusions was the trend towards more time away from usual activities in the control group. It is not clear whether this trend was real or not, given the lack of any meaningful trends in either health or use of health services. Therefore, the conclusion drawn on the basis of the NHS and patient perspective should be treated with caution.
Chapter 10 Transurethral resection of the prostate: derivation and description of participants
This chapter describes the men derived from the screening survey in terms of their clinical characteristics and presents the baseline comparability between the randomised groups in the TURP group.
Comparison between those responding and not responding to the screening survey
Table 50 shows the comparability at baseline of those responding and not responding to the screening survey in terms of their demographic and clinical characteristics. There were no clinically important differences between responders and non-responders. The majority were standard TURPs, but 5% were laser TURPs, 15 were open abdominal procedures and two were transvesical. A further 16 men were all originally admitted for TURP but were subsequently found to need a different procedure. They were not randomised or followed up in MAPS.
| TURP | Responder | Non-responder |
|---|---|---|
| Number screened [n/N (%)] | 2590/2836 (91) | 246/2836 (9)) |
| Age [mean years (SD) n] | 69.9 (8.2) 2578 | 70.6 (9.0) 246 |
| Weight [mean kg (SD) n] | 81.9 (31.4) 2399 | 81.5 (15.0) 222 |
| Height [mean cm (SD) n] | 172.6 (14.0) 2226 | 171.6 (22.0) 213 |
| Current smoker [n/N (%)] | 285/2590 (11) | 44/246 (18) |
| Nights in hospital [mean (SD) n] | 3.8 (2.2) 2457 | 4.2 (2.6) 231 |
| Type of operationc | n = 2587 | n = 241 |
| Standard TURP [n/N (%)]a | 2431/2588 (94.0) | 224/246 (93.1) |
| Laser TURP [n/N (%)] | 140/2588 (5.4) | 12/246 (4.9) |
| No TURP (had other procedure) [n/N (%)]b | 16/2588 (0.6) | 5/246 (2.0) |
Findings from screening survey
The average time of completion of the screening survey was at around 5 weeks after surgery [mean days since operation 34.4 (SD 18.8)]. The majority of the men had a standard TURP, but laser was used for ablation in about 5%: this did not affect the chance of subsequent incontinence. Just under half of the men had urinary incontinence at the time of screening, and the most common type was UUI (Table 51).
| TURP | All responders to screening questionnaire (n = 2587) |
Standard TURP (n = 2431) |
Laser TURP (n = 140) |
No TURP (n = 16) |
|---|---|---|---|---|
| Days since operation [mean (SD) n] | 34.4 (18.8) 2518 | 34.1 (19.1) 2368 | 32.7 (12.5) 134 | 34.1 (9.8) 13 |
| Number of men with any urine loss at screening questionnaire | 1203/2585 (47) | 1129/2433 (46) | 66/140 (47) | 6/16 (38) |
| ICI-QoL score due to UI [mean (SD) n]a | 1.3 (2.4) 2414 | 1.3 (2.4) 2269 | 1.5 (2.7) 129 | 0.9 (2.0) 16 |
| ICI-Q score [mean (SD) n]b | 3.42 (4.7) 2568 | 3.4 (4.7) 2415 | 3.7 (4.9) 137 | 2.6 (3.9) 16 |
| Number of men with urine loss before surgery | 883/2588 (36) | 839/2432 (37) | 38/140 (29) | 6/16 (43) |
| Number of men with faecal incontinence after surgery | 112/2585 (4) | 105/2433 (4) | 7/140 (5) | 1/16 (6) |
| Type of incontinence | ||||
| SUI | 303/2585 (12) | 292/2433 (12) | 11/140 (8) | 1/16 (6) |
| UUI | 733/2585 (28) | 697/2433 (29) | 36/140 (26) | 1/16 (6) |
| MUI | 135/2585 (5) | 131/2433 (5) | 5/140 (4) | 0 |
| Postmicturition leakage | 446/2585 (17) | 413/2433 (17) | 29/140 (21) | 4/16 (25) |
| Other incontinence | 295/2585 (11) | 270/2433 (11) | 24/140 (17) | 1/16 (6) |
Summary information for progress from screening questionnaire to randomisation
Of 1203 men wet at screening, 155 were not eligible to be sent a baseline questionnaire (see Table 11 for reasons). Of the 1048 men sent a baseline questionnaire, 786 (75%) responded, of whom 512 (89%) were still wet and 274 dry. A further 70 were excluded as they were ineligible for randomisation despite still being incontinent (see Table 11 for reasons). Finally, 442 men were randomised, 220 in the intervention group and 222 in the control group (see Figure 5).
Men who recorded that they were wet at the screening survey were sent a further baseline questionnaire to confirm persistent leakage. Those who were still wet and consented were randomised to intervention or control. The average time to randomisation from the date of surgery was 8 weeks (mean 8.4, SD 3.4).
Comparability on baseline characteristics at trial entry
Table 52 shows that the men in the two randomised groups were comparable at baseline on the clinical and demographic characteristics recorded.
| TURP | Intervention (n = 220) | Control (n = 222) |
|---|---|---|
| Age in years [mean (SD) n, (min–max)] | 68.2 (7.7) 220, (47–90) | 67.9 (8.1) 222, (45–86) |
| BMI (kg/m2) [mean (SD) n, (min–max)] | 27.1 (4.1) 217, (15–48) | 27.1 (4.7) 215, (17–44) |
| Type of operation | 220 | 222 |
| Standard TURP | 199/220 (90) | 199/222 (90) |
| Laser TURP | 10/220 (5) | 15/222 (7) |
| TURP + other procedure | 11/220 (5) | 8/222 (4) |
| TURP before surgery | 23/217 (11) | 26/218 (12) |
| Number of men not able to achieve erection before prostate surgery | 67/214 (31) | 71/215 (33) |
| Leakage of urine before operation | 95/195 (49) | 102/205 (50) |
| ICI-Q scorea at baseline [mean (SD) n, (0–21 max)] | 8.6 (4.1) 219 | 8.7 (4.3) 222 |
| Number of men with severe incontinence at baselineb | 145/220 (66) | 144/222 (65) |
| Urinary frequency at baseline (per day) [mean (SD) n] | 8.6 (5.2) 205 | 7.9 (3.1) 199 |
| Nocturia at baseline (per night) [mean (SD) n] | 2.7 (1.6) 215 | 2.5 (1.5) 212 |
| Type of incontinence | 220 | 222 |
| SUI | 148/220 (67) | 136/222 (61) |
| UUI | 186/220 (85) | 183/222 (82) |
| MUI (both) | 129/220 (59) | 112/222 (50) |
| Postmicturition leakage | 151/220 (69) | 156/222 (70) |
| Other incontinence | 57/220 (26) | 44/222 (20) |
| Pad use | 71/220 (32) | 70/217 (32) |
| Other health problems | 138/220 (63) | 145/222 (65) |
| EQ-5D [mean (SD) n] | 0.8 (0.3) 213 | 0.8 (0.3) 208 |
| SF-12M [mean (SD) n] | 49.9 (10.4) 216 | 50.3 (10.4) 212 |
| SF-12P [mean (SD) n] | 42.7 (11.0) 216 | 43.2 (11.9) 212 |
Prior knowledge of pelvic floor exercises
Many men had been counselled before surgery about the possibility of urinary incontinence and sexual dysfunction after surgery, although this was less common in the men undergoing TURP than in those undergoing radical surgery. 69 Table 53 shows that the most common sources of information about pelvic floor exercises were leaflets or books or nurses or continence advisors: around 80% of men had some prior knowledge of pelvic floor exercises for these problems.
| Source of information | Intervention | Control |
|---|---|---|
| A doctor | 13/106 (12) | 13/119 (11) |
| A nurse/continence advisor | 34/106 (32) | 40/119 (34) |
| A physiotherapist | 6/106 (6) | 5/119 (4) |
| Leaflets or books | 46/106 (43) | 54/119 (45) |
| The internet | 2/106 (2) | 7/119 (6) |
| Friends or family | 18/106 (17) | 16/119 (13) |
| Another source | 10/106 (9) | 6/119 (5) |
| At least one source of information | 94/106 (89) | 89/119 (75) |
Chapter 11 Transurethral resection of the prostate: management received
This chapter describes how the intervention was implemented in the therapy arm of the TURP RCT, and the progress of men through the intervention period (n = 220). The information in this chapter is derived from the therapy documentation (see Appendix 4.1), which was used primarily to guide the therapists while delivering the standardised intervention.
Compliance with therapy
Of the 220 men who were randomised to the intervention, 189 attended at least one visit (86%), and 72% attended every time (Table 54). The non-attenders were slightly older and lighter, although these differences were not clinically important (Table 55). Only 7 of the 31 men who did not attend were dry. The other main reasons were that, after they were allocated to therapy, four men became ill and six men found it to be inconvenient or impossible to attend appointments, often because of work. The remainder simply declined or did not give a specific reason (Table 56).
| TURP | First visit | Second visit | Third visit | Fourth visit |
|---|---|---|---|---|
| Number of men attending | 189 (86%) | 173 (79%) | 163 (74%) | 158 (72%) |
| TURP | Attenders | Non-attenders |
|---|---|---|
| Age [mean years (SD) n] | 68.0 (7.9) 189 | 69.2 (6.9) 31 |
| BMI [mean kg/m2 (SD) n] | 27.2 (4.1) 187 | 26.3 (4.5) 30 |
| TURP | Non-attenders (n) |
|---|---|
| Dry | 7 |
| Ill | 4 |
| Unable to attend | 6 |
| Declined | 9 |
| No reason given | 5 |
| Total | 31 |
Relationship between type of therapist and outcomes during therapy period
Half of the centres (17) used a physiotherapist to deliver the MAPS intervention, while in the other 17 the therapist had a nursing background (Table 57). There was no significant difference in the number of visits men made to physiotherapist or nurse therapists (Table 57). During the 3-month intervention period, there were no statistically significant differences in the mean ICI scores (a composite score reflecting urinary incontinence and its effect on quality of life) between therapists (Table 57).
| TURP | Physiotherapist | Continence nurse | Significance [mean difference (95% CI), p-value |
|---|---|---|---|
| Number of attendances | 3.1 (1.5) 111, (2.8 to 3.4) | 3.1 (1.5) 109, (2.8 to 3.4) | 0.02 (–0.4 to 0.4), 0.901 |
| ICI-Q score | |||
| Visit 1 | 6.7 (4.7) 95, (5.7 to 7.7) | 6.4 (3.7) 94, (5.6 to 7.1) | 0.3 (–0.9 to 1.5), 0.602 |
| Visit 2 | 5.0 (3.8) 87, (4.2 to 5.8) | 4.5 (3.9) 86, (3.6 to 5.3) | 0.5 (–0.7 to 1.7), 0.387 |
| Visit 3 | 4.3 (3.6) 82, (2.7 to 4.3) | 3.8 (3.5) 81, (3.0 to 4.6) | –0.2 (–1.3 to 0.9), 0.691 |
| Visit 4 | 2.9 (3.9) 81, (2.0 to 3.8) | 3.1 (3.3) 77, (2.3 to 3.8) | –0.2 (–1.3 to 1.0), 0.775 |
Urinary incontinence during therapy period
Incidence of urinary incontinence
The therapists asked the men at each visit to rate their incontinence (in the previous week). This allowed the therapists to monitor the change in reported incontinence. They used the same form of question as the questionnaires, based on the ICI-SF instrument, which were also used to measure the primary outcome. During the 3-month intervention period, the proportion of men with incontinence fell from 82% to 52%, while the mean ICI score decreased (improved) from around 6.5 at the start of treatment to 3 afterwards (Table 58 and Figure 32).
| TURP | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Men incontinent [n/N (%)] | 154/188 (82) | 118/172 (69) | 97/162 (60) | 82/157 (52) |
| ICI-Q score [mean (SD) n] | 6.5 (4.23) 189 | 4.8 (3.87) 173 | 3.7 (3.53) 163 | 3.0 (3.58) 158 |
FIGURE 32.
Trend analysis of the proportion (%) men incontinent at each visit: TURP. ICI-Q score: 0 = none, 21 = maximum (worst) score. Derived from questions 1–3 of the ICIQ-UI Short Form Questionnaire.
Type of urinary incontinence during therapy period
The distribution of type of incontinence reported by the men did not vary with time across the therapy visits, except that the proportion with SUI decreased slightly (from 36% to 21%), the proportion with UUI decreased (from 57% to 20%) and the proportion with postmicturition leakage decreased (from 57% to 33%) (Figure 33 and Table 59).
| TURP | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Number of men | 189 | 173 | 163 | 158 |
| SUI | 59/162 (36) | 50/151 (33) | 42/146 (29) | 28/134 (21) |
| UUI | 95/166 (57) | 47/150 (31) | 38/144 (26) | 27/135 (20) |
| MUI (both SUI and UUI) | 34/170 (20) | 15/155(10) | 9/148 (6) | 5/138 (4) |
| Postmicturition leakage | 97/171 (57) | 67/154 (44) | 52/144 (36) | 45/136 (33) |
| Other UI | 28/146 (19) | 22/131 (17) | 24/123 (20) | 21/127 (17) |
FIGURE 33.
Type of incontinence at each visit and change over time: TURP.
Incidence and type of bowel problems during therapy period
Therapists also enquired at each visit about whether the men experienced any bowel dysfunction in the previous week. The proportions of men with three different types of bowel dysfunction (faecal incontinence, faecal urgency and constipation) were low and did not vary during the therapy period (Table 60 and Figure 34).
| TURP | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Faecal incontinence | 6/184 (3) | 3/170 (2) | 3/159 (2) | 2/156 (1) |
| Faecal urgency | 17/185 (9) | 9/170 (5) | 7/159 (4) | 11/156 (7) |
| Constipation | 30/184 (16) | 18/165 (11) | 21/156 (13) | 10/155 (6) |
FIGURE 34.
Type of bowel problems at each visit and change over time: TURP.
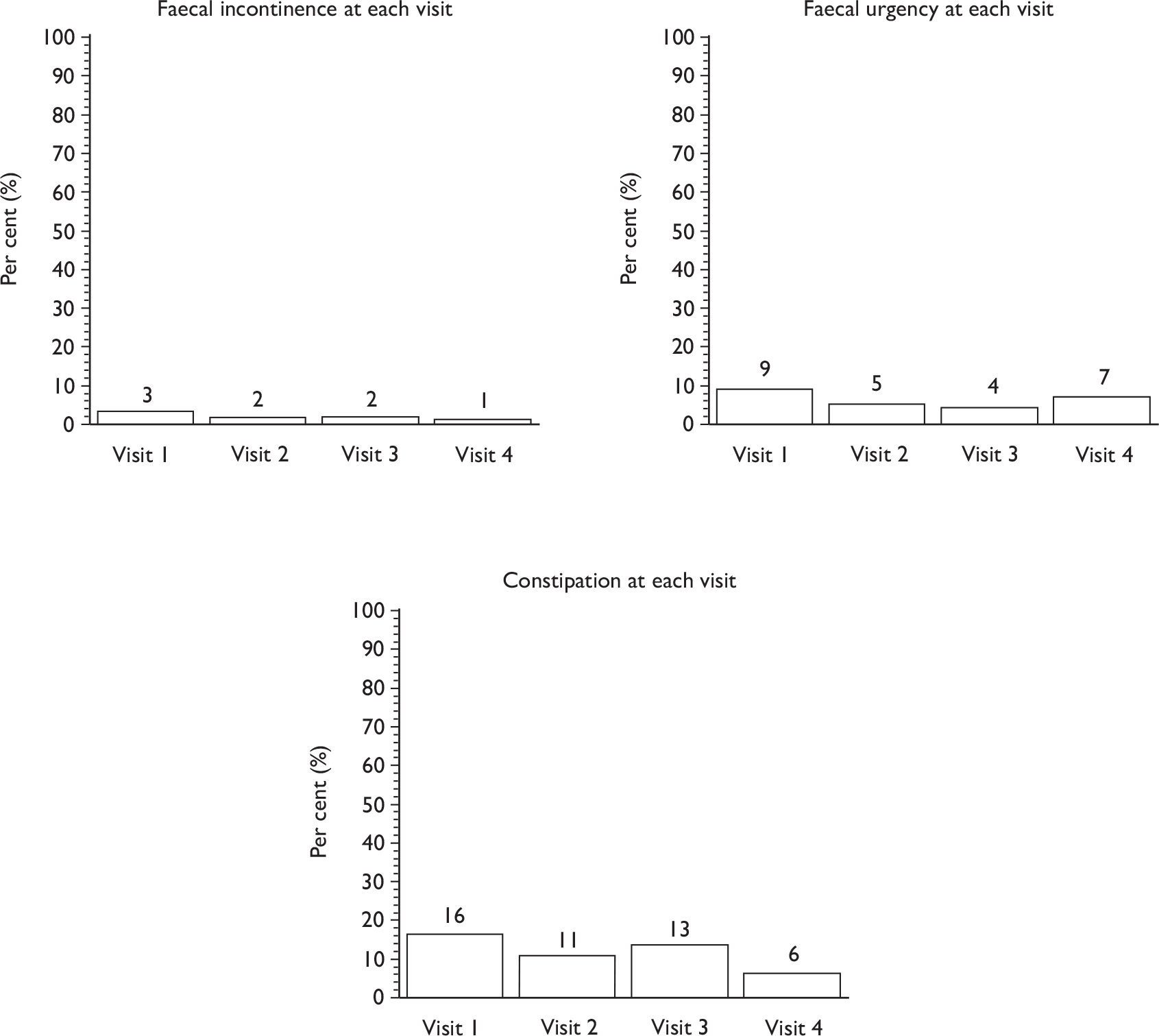
Incidence and type of sexual problems during therapy period
The questions relating to sexual problems were those used in routine clinical practice. They were not based on the questions men were asked at 12 months to assess their sexual function and activity70 (see section G, 12-month questionnaire, Appendix 3.3).
Just over half of the men reported sexual dysfunction (‘difficulty gaining or maintaining an erection in the last week’) (52–62%) and this did not change during the therapy period. The corresponding proportion with premature ejaculation was low (around 6%) and also did not vary with time (Table 61 and Figure 35).
| TURP | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| Difficulty gaining erection | 95/173 (55) | 77/149 (52) | 79/142 (56) | 71/137 (52) |
| Difficulty maintaining erection | 97/168 (58) | 86/146 (59) | 85/138 (62) | 80/134 (60) |
| Premature ejaculation | 8/152 (5) | 9/137 (7) | 8/130 (6) | 7/121 (6) |
FIGURE 35.
Type of sexual problems at each visit and change over time: TURP.
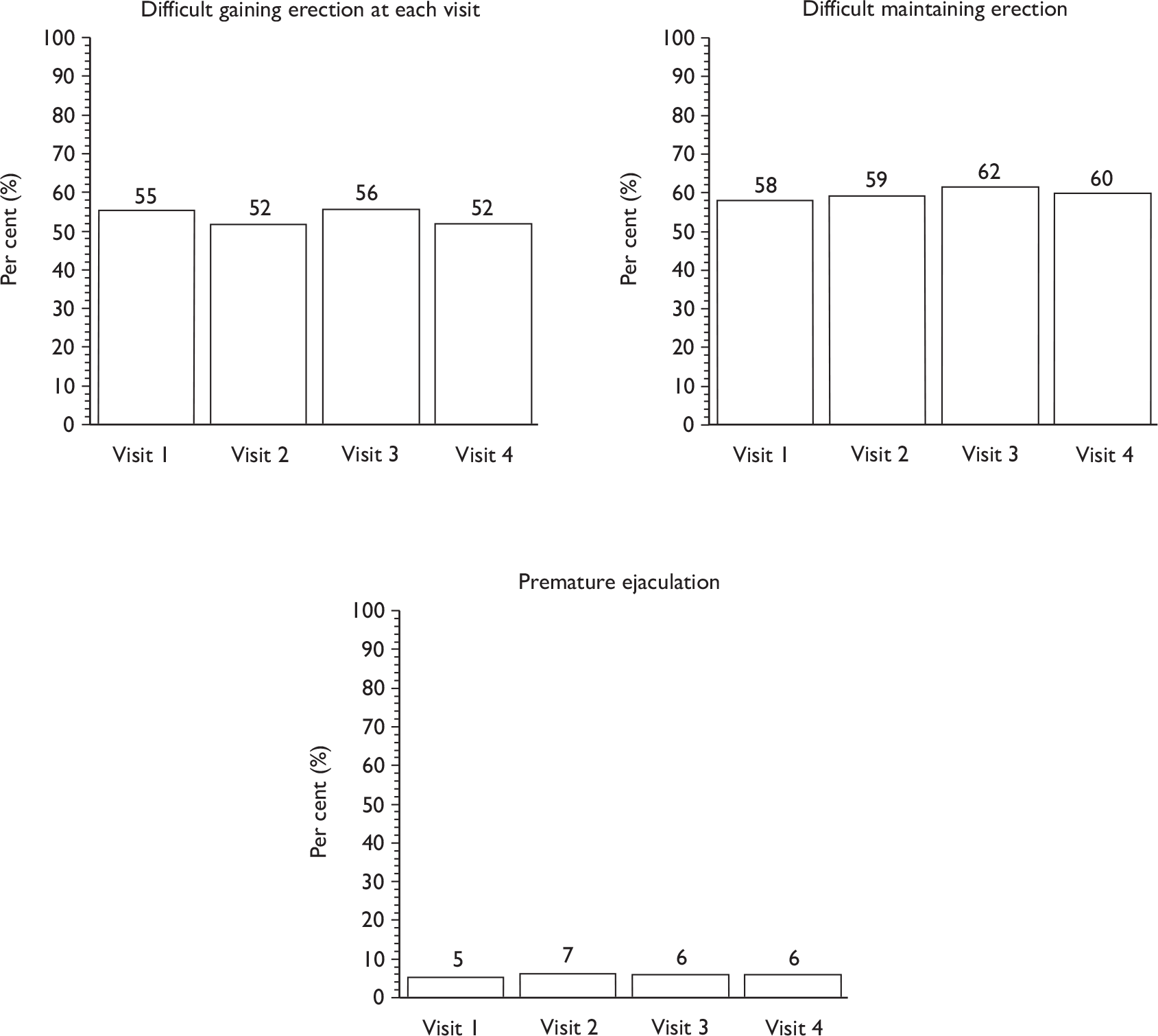
Examination of pelvic floor muscle performance during therapy visits
Therapists assessed the strength of the pelvic floor muscle contractions and their endurance (length of time men were able to hold a contraction) at each visit using digital anal assessment (see Chapter 3). The external anal sphincter and the internal puborectalis muscle were assessed separately. The internal puborectalis muscle strength was taken to be a measure of pelvic floor muscle strength.
For both the sphincter and the puborectalis, both strength and endurance improved during the therapy period (Table 62 and Figure 36). At baseline, only 4–6% of men had a strength of 5 or more, but by the fourth visit around 35–40% of men were able to contract strongly (5) or very strongly (6), while over 80% had good muscle strength (4 or better). The therapists were trained to ask men to hold the pelvic floor muscle contraction for up to 10 seconds during the digital anal examination. This is in line with functional use of these muscles. However, some therapists assessed the maximum length of time for which men could hold a contraction. Of these men, some held the contraction for over 1 minute.
| TURP | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|---|---|---|---|
| A: External anal sphincter strength [mean (SD) n]a | 3.1 (0.9), 153 | 3.5 (0.9), 125 | 3.9 (0.9), 119 | 4.2 (1.0), 108 |
| 0 | 1(1) | 0 | 0 | 0 |
| 1 | 6/153 (3) | 1/125 (1) | 1/119 (1) | 0 |
| 2 | 28/153 (18) | 17/125 (14) | 7/119 (6) | 5/108 (7) |
| 3 | 63/153 (41) | 34/125 (27) | 29/119 (24) | 16/108 (15) |
| 4 | 50/153 (33) | 59/125 (47) | 55/119 (46) | 48/108 (44) |
| 5 | 6/153 (4) | 14/125 (11) | 24/119 (20) | 29/108 (27) |
| 6 | 0 | 0 | 3/119 (2) | 10/108 (9) |
| A: External anal sphincter endurance (seconds) [mean (SD) n]b | 6.0 (2.9) 153 | 7.4 (3.0) 125 | 8.4 (3.3) 119 | 10.2 (5.3) 108 |
| B: Puborectalis muscle strength [mean (SD) n]a | 3.1 (1.0) 153 | 3.5 (0.9) 125 | 3.9 (0.9) 119 | 4.3 (1.0) 108 |
| 0 | 1/153 (1) | 0 | 0 | 0 |
| 1 | 8/153 (5) | 4/125 (3) | 1/119 (1) | 1/108 (1) |
| 2 | 23/153 (15) | 13/125 (10) | 7/119( 6) | 4/108 (4) |
| 3 | 67/153 (44) | 42/125 (34) | 30/119 (25) | 13/108 (12) |
| 4 | 44/153 (29) | 53/125 (42) | 52/119 (44) | 45/108 (42) |
| 5 | 8/153 (5) | 12/125 (10) | 25/119 (21) | 37/108 (34) |
| 6 | 2/153 (1) | 1/125 (1) | 4/119 (3) | 8/108 (7) |
| B: Puborectalis muscle endurance (seconds) [mean (SD) n]b | 6.0 (2.8) 153 | 7.6 (3.1) 125 | 8.5 (3.2) 119 | 10.2 (5.5) 108 |
FIGURE 36.
Ability to contract anal sphincter and puborectalis muscle (pelvic floor) over time:TURP. (a) External anal sphincter strength/endurance. (b) Puborectalis muscle strength/endurance.
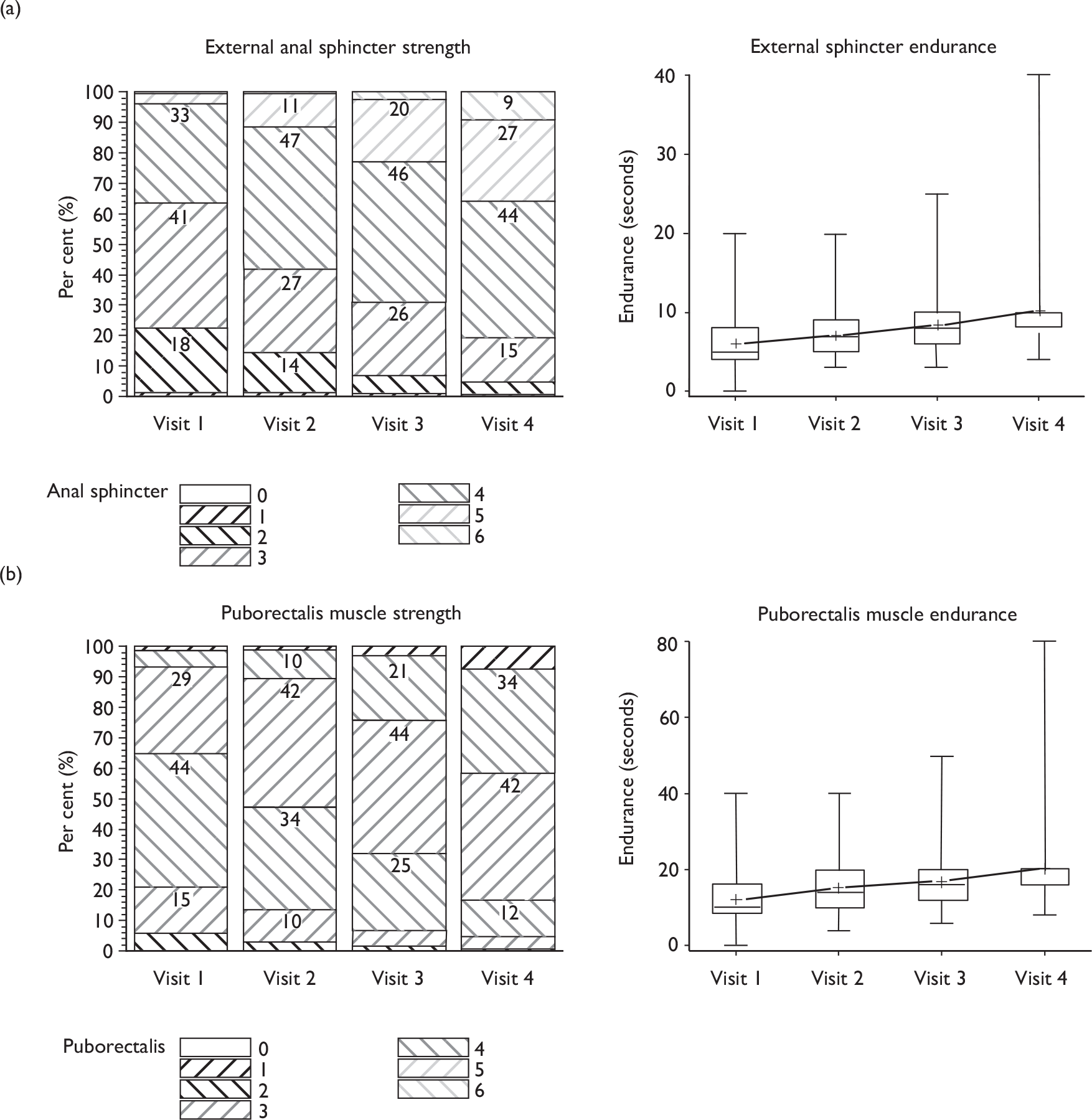
Examination and functional use of pelvic floor muscles
Therapists examined men at each visit to assess skin damage, skin infection, ability to tighten the anus and perform penile retraction and testicular lift, evidence of leakage on coughing and (for those who did leak) ability to prevent leakage on coughing. Very few men showed evidence of skin damage or infection (data not shown).
Four different aspects of functional use of pelvic floor muscles were assessed: ability to tighten anus; ability to perform penile retraction; leakage on coughing; ability to prevent leakage on coughing. While most men (over 95%) were able to contract well enough to tighten the anal sphincter at least a little from baseline onwards, the proportion able to demonstrate a testicular lift increased slightly with time (from 73% to 89%; Figure 37). The proportion who leaked when coughing was low (< 5%) and decreased very slightly during the therapy period. Around 77% of these men were able to contract their pelvic floor muscles sufficiently to prevent leakage when coughing at the first visit, and this improved to around 86% by the fourth visit.
FIGURE 37.
Aspects of ability to use pelvic floor muscles at each visit and change over time: TURP.
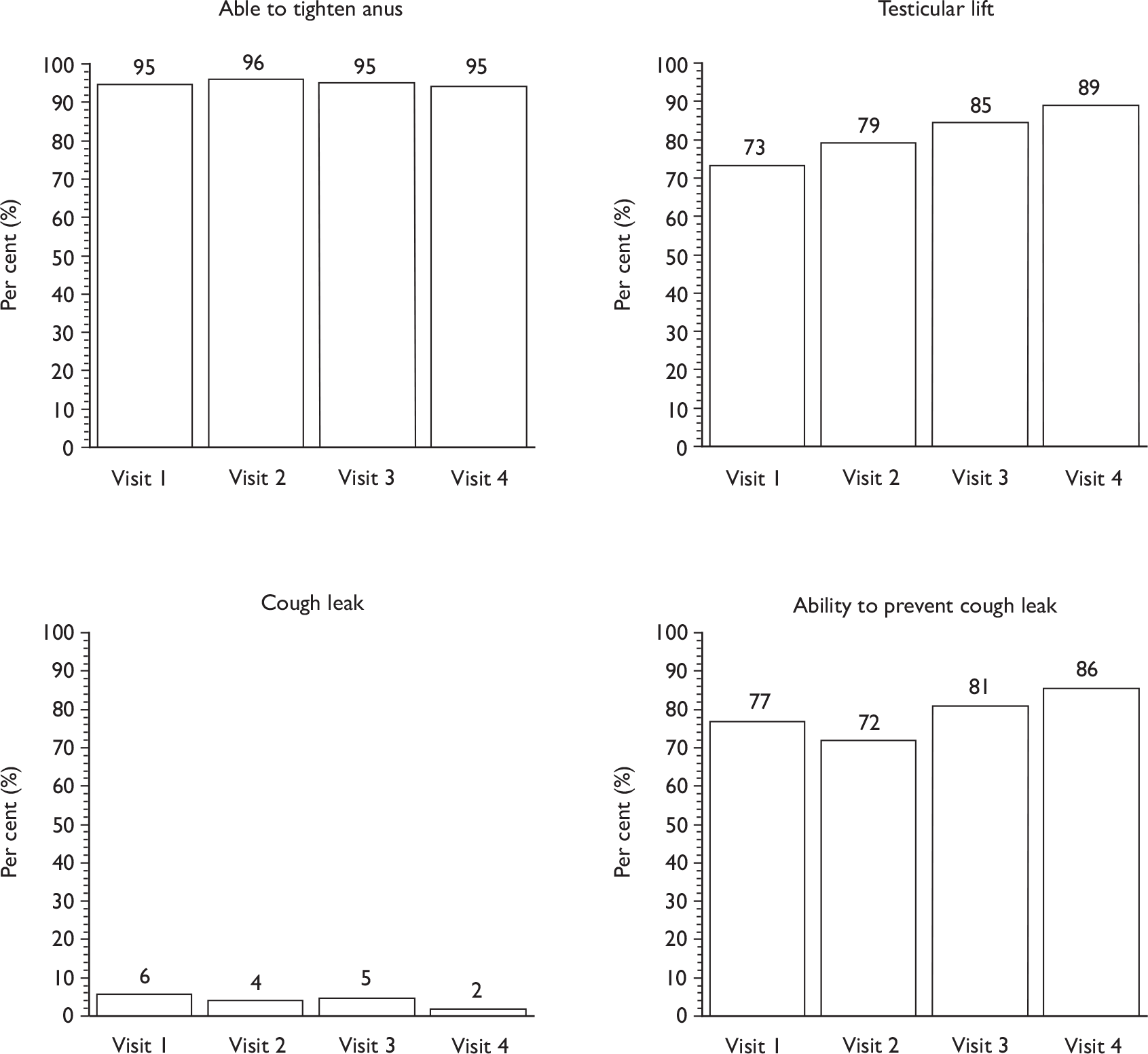
Use of machine-led biofeedback
Biofeedback was available in 13 of 34 MAPS centres, and was used clinically for men after TURP in two of them (see Table 7). Therapists would have liked access to this facility in four other centres where biofeedback was not available. Biofeedback can be used in two ways:
-
to feed back information to men that they are actually performing a correct pelvic floor contraction, and at what strength
-
as part of a repetitive training regimen when men are asked to use the machine to enable them to monitor their exercise function for a period of time (such as 20 minutes).
It was not clear which type of biofeedback was practised in the centres where it was available, but therapists from two centres recorded its use in 11 men (see Table 7) from the TURP group (Table 63). In some cases men may have preferred anal examination using a machine rather than digital examination by the therapist for teaching of correct contractions.
| TURP | Biofeedback indicated | Biofeedback actually implemented |
|---|---|---|
| Visit 1 | 10/92 (11) | 0/90 (0) |
| Visit 2 | 7/87 (8) | 5/85 (6) |
| Visit 3 | 3/81 (4) | 3/78 (4) |
| Visit 4 | 3/85 (3) | 3/85 (3) |
Chapter 12 Transurethral resection of the prostate: randomised controlled trial outcomes and results
This chapter describes the results of the intervention amongst the men recruited to the TURP RCT.
Patient flow
The derivation of the trial study groups and their progress through the trial is summarised in Figure 38. This is in the form of a CONSORT flow diagram. In total, 442 participants were recruited to the randomised trial: 220 randomly allocated to the intervention group and 222 to the control group. Twenty-five men had withdrawn from follow-up by 12 months (although some information was available prior to the time of their withdrawal in some cases). Ten men, three in the intervention group and seven in the control group, died before 12-month follow-up was reached. These deaths were not attributed to the trial intervention. Thirty-one men (14%) in the intervention group did not attend any therapy sessions and were considered non-compliers with the intervention (see Chapter 6) but were retained in their allocated group for the purpose of analysis.
FIGURE 38.
TURP CONSORT diagram (men randomised n = 442).
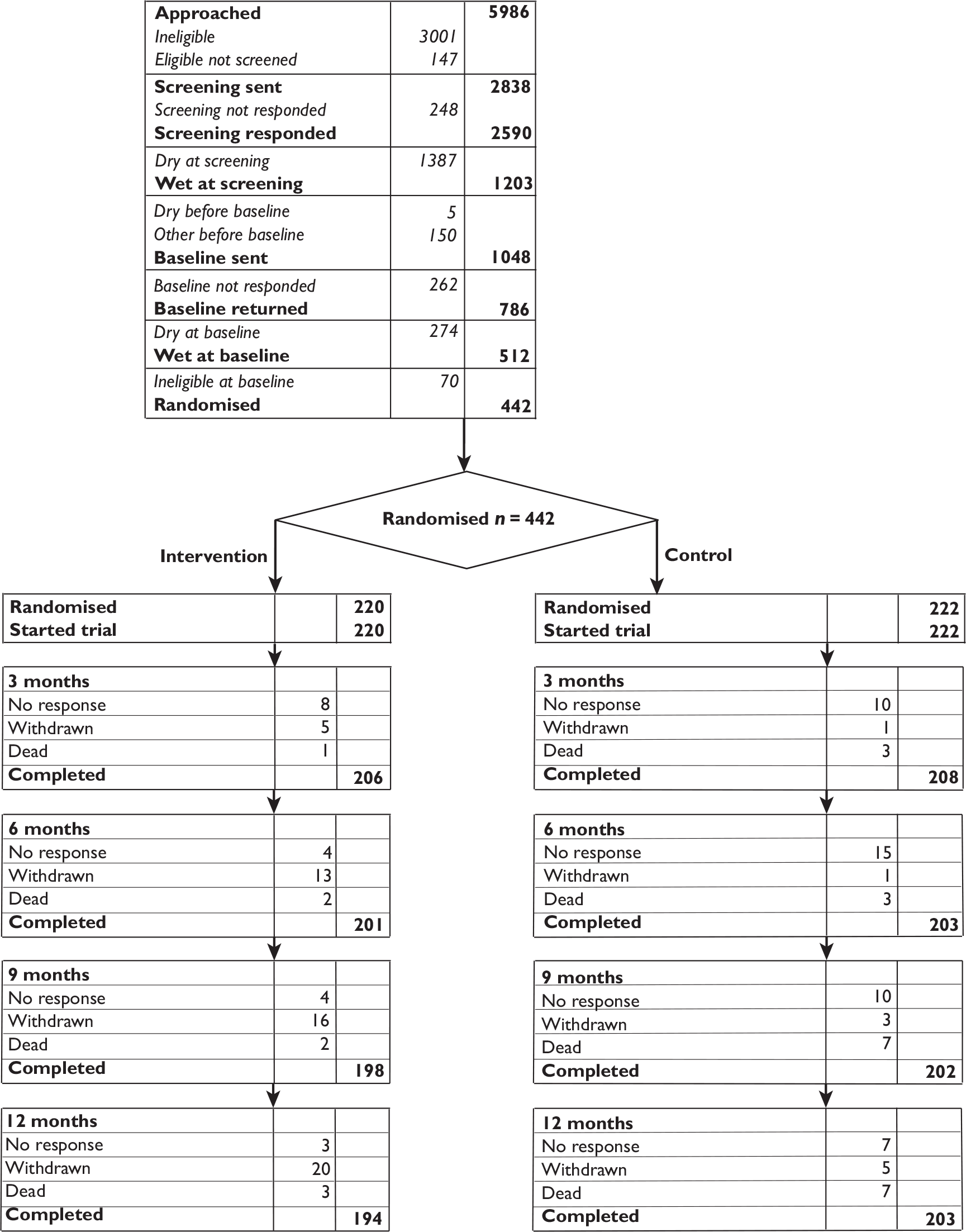
Response rates
Over 90% of all participants returned completed questionnaires. As shown in Figure 38, by the time of each follow-up some participants had formally withdrawn or died, and so were not sent questionnaires (Table 64). Of the participants for whom it was appropriate to send a follow-up questionnaire, over 90% returned it at each time point (Table 65a). For return of urinary diaries, the response rate was slightly less, but still approximately 90% at each time point (Table 65b).
| Reason | TURP | |
|---|---|---|
| Intervention | Control | |
| Ill | 6 | 2 |
| Dry | 6 | 1 |
| Catheterised permanently | 2 | 0 |
| No reason | 2 | 1 |
| Other | 4 | 1 |
| Total | 20 | 5 |
| TURP | Number sent | Number returned (%) | Percentage of all men |
|---|---|---|---|
| Baseline | |||
| Intervention | 220 | 220 (100) | 100 |
| Control | 222 | 222 (100) | 100 |
| 3 months | |||
| Intervention | 216 | 206 (95) | 94 |
| Control | 220 | 208 (95) | 94 |
| 6 months | |||
| Intervention | 208 | 201 (97) | 91 |
| Control | 219 | 203 (93) | 91 |
| 9 months | |||
| Intervention | 203 | 198 (98) | 90 |
| Control | 215 | 202 (93) | 91 |
| 12 months | |||
| Intervention | 199 | 194 (97) | 88 |
| Control | 211 | 203 (97) | 92 |
| TURP | Number sent | Number returned (%) | Percentage of all men |
|---|---|---|---|
| Baseline | |||
| Intervention | 220 | 207 (94) | 94 |
| Control | 222 | 203 (91) | 91 |
| 3 months | |||
| Intervention | 216 | 183 (85) | 83 |
| Control | 220 | 186 (85) | 84 |
| 6 months | |||
| Intervention | 208 | 184 (88) | 84 |
| Control | 219 | 183 (84) | 82 |
| 9 months | |||
| Intervention | 203 | 177 (87) | 80 |
| Control | 215 | 184 (86) | 83 |
| 12 months | |||
| Intervention | 199 | 176 (88) | 80 |
| Control | 211 | 181 (86) | 82 |
Primary outcome: urinary incontinence at 12 months
The primary outcome was incontinence in men at 12 months after randomisation, measured by a positive response to one of two questions from the ICI-SF questionnaire (‘How often do you leak urine’ or ‘How much urine do you usually leak?’). Table 66 shows that the difference between the intervention and control groups in urinary incontinence at 12 months (64.9% vs 61.6%) was not statistically significant:
| TURP | Intervention | Control | RR (95% CI), p-value |
|---|---|---|---|
| Urinary incontinence at 12 months [n/N (%)] | 126/194 (64.9) | 125/203 (61.6) | Absolute risk difference 3.4% (95% CI –6% to 13%) |
| Intention to treat | |||
| Unadjusted analysis | 1.055 (0.908 to 1.225), 0.486 | ||
| Analysis adjusted by minimisation factors | 1.057 (0.910 to 1.227), 0.471 | ||
| Adjusted treatment received | |||
| Unadjusted analysis | 1.048 (0.900 to 1.221), 0.546 | ||
| Analysis adjusted by minimisation factors | 1.049 (0.901 to 1.222), 0.538 |
-
either when analysed by intention to treat (all men analysed in the groups to which they were randomised but results as given in the outcome questionnaires without adjustment for missing values)
-
or when analysed by ‘treatment received’, which adjusts the result by a factor related to the men who actually attended a therapist versus those who did not.
The above analyses were then repeated adjusting for the minimisation factors, but this did not alter the findings (Table 66). The corresponding risk difference for the unadjusted intention-to-treat analysis was 3.4% (95% CI –6% to 13%), thereby ruling out the likelihood that the trial prespecified difference of 15% in the proportion incontinent between intervention and control group could have been missed.
Secondary outcomes
Urinary outcomes
Urinary incontinence was also measured at 3, 6 and 9 months after randomisation, together with other urinary outcomes. Table 67a describes the various urinary outcomes at each follow-up, and Table 67b shows the formal statistical testing of the differences at each time point. Figure 39 is a pictorial representation of the percentage of men incontinent at each follow-up, and Figure 40 shows the change in mean ICI-Q score over time. The data show that there were no statistically significant differences between the intervention and control groups at any of the time points in urinary incontinence and the other urinary outcomes measured.
| TURP | Baseline | 3 months | 6 months | 9 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||
| Incontinence | |||||||||||
| Men incontinent [n/N (%)] | 220/220 (100) | 222/222 (100) | 142/205 (69) | 132/208 (63) | 140/199 (70) | 129/201 (64) | 133/197 (68) | 131/202 (65) | 126/194 (65) | 125/203 (62) | |
| Men with severe incontinence [n/N (%)]a | 145/220 (66) | 144/222 (65) | 57/205 (28) | 57/208 (27) | 53/199 (27) | 49/201 (24) | 46/197 (23) | 45/202 (22) | 48/194 (25) | 49/203 (24) | |
| ICI-Q scoreb | 8.6 (4.1) 219 | 8.7 (4.3) 222 | 4.6 (4.0) 201 | 4.6 (4.8) 203 | 4.1 (3.7) 199 | 4.1 (4.3) 201 | 4.2 (4.0) 193 | 4.1 (4.3) 198 | 3.9 (3.7) 194 | 4.0 (4.3) 203 | |
| Frequency of daytime urinary incontinence from diaries | 3.01 (3.7) 204 | 2.7 (3.1) 201 | 1.31 (2.2) 182 | 1.4 (2.5) 184 | 1.1 (2.0) 184 | 1.4 (2.6) 181 | 1.2 (2.51) 177 | 1.31 (2.3) 182 | 1.4 (2.3) 175 | 1.2 (2.2) 179 | |
| Effect of urinary incontinence on quality of life | 3.4 (3.0) 215 | 3.5 (3.0) 221 | 1.5 (2.1) 201 | 1.6 (2.5) 203 | 1.2 (1.9) 194 | 1.4 (2.3) 198 | 1.3 (2.2) 193 | 1.4 (2.3) 198 | 1.2 (1.9) 190 | 1.3 (2.2) 199 | |
| Urinary frequency | |||||||||||
| Daytime urinary frequency | 8.6 (5.2) 205 | 7.9 (3.1) 199 | 6.9 (2.8) 186 | 6.6 (2.5) 177 | 6.6 (2.1) 186 | 6.7 (2.2) 181 | 6.6 (2.3) 182 | 6.7 (2.3) 183 | 7.0 (4.3) 177 | 6.5 (2.1) 178 | |
| Daytime urinary frequency from diaries | 7.6 (3.8) 204 | 7.7 (4.8) 201 | 6.6 (3.9) 182 | 6.3 (4.0) 184 | 6.2 (2.6) 184 | 6.0 (2.1) 181 | 6.4 (2.3) 177 | 6.2 (3.0) 182 | 6.1 (2.6) 175 | 6.1 (3.3) 179 | |
| Nocturia | 2.7 (1.6) 215 | 2.5 (1.5) 212 | 1.9 (1.5) 193 | 1.9 (1.4) 192 | 1.7 (1.1) 185 | 1.8 (1.2) 185 | 1.7 (1.2) 174 | 1.8 (1.5) 188 | 1.7 (1.4) 177 | 1.8 (1.6) 181 | |
| Nocturia from diaries | 2.2 (1.4) 204 | 2.2 (1.5) 201 | 1.6 (1.3) 182 | 1.6 (1.4) 184 | 1.5 (1.4) 184 | 1.5 (1.3) 181 | 1.6 (1.5) 177 | 1.5 (1.4) 182 | 1.5 (1.2) 175 | 1.6 (1.5) 179 | |
| Frequency of nocturnal incontinence from diaries | 0.8 (1.2) 204 | 0.7 (1.2) 201 | 0.3 (0.7) 182 | 0.4 (1.0) 184 | 0.3 (0.9) 184 | 0.3 (0.7) 181 | 0.3 (0.9) 177 | 0.4 (0.9) 182 | 0.4 (0.9) 175 | 0.4 (0.9) 179 | |
| TURP | Effect size (95% CI), p-value | |||
|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | |
| Incontinence | ||||
| Men incontinent [RR (95% CI), p-value] | 1.09 (0.95 to 1.25), 0.213 | 1.10 (0.96 to 1.26), 0.184 | 1.04 (0.91 to 1.20), 0.571 | 1.06 (0.91 to 1.23), 0.471 |
| Men with severe incontinence [RR (95% CI), p-value]a | 1.02 (0.74 to 1.39), 0.925 | 1.10 (0.78 to 1.53), 0.589 | 1.05 (0.73 to 1.50), 0.791 | 1.03 (0.73 to 1.45), 0.884 |
| ICI-Q scoreb | 0.03 (–0.74 to 0.81), 0.935 | 0.01 (–0.72 to 0.74), 0.973 | 0.09 (–0.68 to 0.85), 0.825 | –0.04 (–0.78 to 0.71), 0.925 |
| Frequency of daytime urinary incontinence from diaries | –0.21 (–0.74 to 0.32), 0.441 | –0.31 (–0.83 to 0.21), 0.248 | –0.06 (–0.64 to 0.52), 0.847 | –0.21 (–0.30 to 0.72), 0.421 |
| Effect of urinary incontinence on quality of life | –0.22 (–0.61 to 0.17), 0.273 | –0.13 (–0.50 to 0.25), 0.511 | –0.08 (–0.47 to 0.31), 0.697 | –0.13 (–0.50 to 0.24), 0.499 |
| Urinary frequency | ||||
| Daytime urinary frequency | 0.11 (–0.42 to 0.64), 0.692 | –0.14 (–0.57 to 0.30), 0.530 | 0.01 (–0.44 to 0.46), 0.960 | 0.35 (–0.40 to 1.09), 0.362 |
|
Daytime urinary frequency from diaries |
0.30 (–0.61 to 1.22), 0.518 | 0.20 (–0.40 to 0.80), 0.510 | 0.16 (–0.45 to 0.77), 0.608 | –0.19 (–0.87 to 0.50), 0.590 |
| Nocturia | –0.08 (–0.33 to 0.17), 0.537 | –0.16 (–0.35 to 0.04), 0.110 | –0.08 (–0.31 to 0.16), 0.527 | –0.05 (–0.30 to 0.20), 0.698 |
| Nocturia from diaries | 0.02 (–0.29 to 0.33), 0.911 | 0.10 (–0.20 to 0.39), 0.518 | 0.02 (–0.31 to 0.36), 0.886 | –0.12 (–0.43 to 0.19), 0.439 |
| Frequency of nocturnal incontinence from diaries | –0.10 (–0.38 to 0.18), 0.472 | 0.06 (–0.25 to 0.36), 0.717 | –0.15 (–0.46 to 0.16), 0.344 | 0.03 (–0.25 to 0.32), 0.807 |
FIGURE 39.
Per cent of men incontinent at each time point.
FIGURE 40.
Mean ICI-Q score at each time point. ICI-Q score: 0 = none, 21 = maximum (worst) score. Derived from questions 1–3 of the ICIQ-UI Short Form Questionnaire.
Type of incontinence
Table 68 and Figure 41 show the type of incontinence at baseline and at 6 and 12 months after randomisation. Men could report more than one type of incontinence. About two-thirds of men had SUI at baseline, reducing to about one-third over time. More men had urgency and UUI (than stress incontinence) at baseline. The proportions of men with the other types of urinary incontinence (mixed, Figure 41c; postmicturition leakage, Figure 41d; and other types of incontinence) decreased over time but there was little difference between the groups for any specific type of incontinence.
| TURP | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | RR (95% CI), p-value | Intervention | Control | RR (95% CI), p-value | |
| SUI | 148/220 (67) | 136/222 (61) | 71/199 (36) | 77/201 (38) | 0.87 (0.68 to 1.10), 0.246 | 71/194 (37) | 76/203 (37) | 0.91 (0.72 to 1.17), 0.477 |
| UUI | 186/220 (85) | 183/222 (82) | 77/199 (39) | 80/201 (40) | 0.97 (0.76 to 1.22), 0.772 | 72/194 (37) | 82/203 (40) | 0.92 (0.72 to 1.17), 0.483 |
| Urgency | 198/220 (90) | 193/222 (87) | 94/199 (47) | 97/201 (48) | 0.96 (0.79 to 1.18), 0.724 | 94/194 (48) | 94/203 (46) | 1.04 (0.85 to 1.27), 0.723 |
| MUI (both SUI and UUI) | 129/220 (59) | 112/222 (50) | 43/199 (22) | 59/201 (29) | 0.67 (0.48 to 0.92), 0.014 | 46/194 (24) | 58/203 (29) | 0.77 (0.56 to 1.06), 0.116 |
| Postmicturition leakage | 151/220 (69) | 156/222 (70) | 90/199 (45) | 90/201 (45) | 1.04 (0.85 to 1.28), 0.687 | 92/194 (47) | 87/203 (43) | 1.13 (0.92 to 1.39), 0.245 |
| Other UI | 57/220 (26) | 44/222 (20) | 22/199 (11) | 22/201 (11) | 0.93 (0.54 to 1.61), 0.800 | 18/194 (9) | 17/203 (8) | 1.04 (0.55 to 1.95), 0.911 |
FIGURE 41.
Type of incontinence at baseline, and at 6 and 12 months after randomisation: TURP. Men could report more than one type of incontinence. (a) Stress urinary incontinence. (b) Urgency urinary incontinence. (c) Mixed urinary incontinence (proportion of men reporting both stress and urgency urinary incontinence). (d) Postmicturition leakage (defined as ‘urine leaking when urination finished’).
Use of aids or protection for urinary incontinence
Table 69a shows the men’s use of aids to protect them from urinary leakage: this did not vary according to the randomised groups at any of the follow-up time points. Table 69b presents the statistical analyses of these outcomes. Just under 20% of the men were still using pads at 12 months to protect themselves from leakage accidents, although in some cases this might have been more of a precaution than because they actually leaked.
| TURP | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Use of any protection | 79/220 (36) | 77/222 (35) | 51/220 (23) | 40/222 (18) | 31/220 (14) | 26/222 (12) | 32/220 (15) | 26/222 (12) | 28/220 (13) | 31/222 (14) |
| Use of body-worn pads (yes) | 71/220 (32) | 70/217 (32) | 45/153 (29) | 30/147 (20) | 27/150 (18) | 21/145 (14) | 25/135 (19) | 23/137 (17) | 24/146 (16) | 24/136 (18) |
| Number of body-worn pads in 24 hours [mean (SD) n] | 2.5 (2.1) 69 | 2.3 (1.3) 67 | 2.2 (2.0) 41 | 1.9 (1.4) 29 | 1.7 (1.5) 31 | 1.6 (1.3) 24 | 2.3 (2.3) 24 | 1.6 (1.0) 22 | 1.5 (1.4) 28 | 1.6 (1.3) 26 |
| Use of chair or bed pads (yes) | 28/209 (13) | 22/214 (10) | 12/149 (8) | 12/142 (8) | 7/143 (5) | 8/128 (6) | 7/128 (5) | 7/132 (5) | 8/130 (6) | 9/122 (7) |
| Number of chair or bed pads in 24 hours [mean (SD) n] | 1.9 (1.5) 25 | 1.7 (1.2) 20 | 1.2 (0.8) 10 | 2.1 (2.3) 11 | 0.8 (1.0) 9 | 1.8 (1.6) 8 | 1.5 (0.6) 4 | 1.7 (1.6) 7 | 1.5 (1.0) 4 | 1.6 (1.3) 9 |
| Use of external (sheath) catheter (yes) | 6/219 (3) | 3/216 (1) | 10/201 (5) | 9/199 (5) | 6/187 (3) | 5/182 (3) | 7/186 (4) | 5/193 (3) | 4/185 (2) | 8/192 (4) |
| Use of permanent catheter (yes) | 2/220 (1) | 1/218 (0) | 2/204 (1) | 2/199 (1) | 1/187 (1) | 0/188 (0) | 2/186 (1) | 0/194 (0) | 1/188 (1) | 0/195 (0) |
| TURP | Effect size (95% CI), p-value | |||
|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | |
| Use of any protection | 1.27 (0.88 to 1.83), 0.197 | 1.19 (0.74 to 1.94), 0.475 | 1.24 (0.76 to 2.00), 0.388 | 0.91 (0.56 to 1.46), 0.683 |
| Use of body-worn pads (yes) | 1.32 (0.93 to 1.88), 0.114 | 1.03 (0.65 to 1.65), 0.886 | 1.03 (0.66 to 1.61), 0.903 | 0.96 (0.61 to 1.51), 0.852 |
| Number of body-worn pads in 24 hours [mean difference (95% CI), p-value] | –2.15 (–5.26 to 0.95), 0.144 | –0.38 (–1.14 to 0.38), 0.320 | –1.57 (–3.45 to 0.31), 0.081 | –0.14 (–0.94 to 0.66), 0.724 |
| Use of chair or bed pads (yes) | 0.75 (0.36 to 1.56), 0.446 | 0.70 (0.27 to 1.82), 0.467 | 0.97 (0.38 to 2.47), 0.943 | 0.68 (0.29 to 1.59), 0.373 |
| Number of chair or bed pads in 24 hours [mean difference (95% CI), p-value] | –2.09 (–5.38 to 1.19), 0.151 | 0.29 (–2.70 to 3.27), 0.780 | 0.76 (–6.47 to 7.99), 0.696 | 2.35 (–28.06 to 32.75), 0.506 |
| Use of external (sheath) catheter (yes) | 1.12 (0.46 to 2.74), 0.800 | 1.50 (0.37 to 6.04), 0.565 | 1.51 (0.47 to 4.88), 0.491 | 0.53 (0.17 to 1.68), 0.282 |
| Use of permanent catheter (yes) | 1.13 (0.13 to 9.57), 0.912 | Not estimable | Not estimable | Not estimable |
Bowel function
In addition to urinary outcomes, men were also asked to describe some aspects of bowel function. Few men (< 20%) had faecal incontinence or constipation occasionally or more often by the end of follow-up at 12 months, although rather more (around 50%) reported faecal urgency. Table 70 and Figure 42 show that there were no differences in any aspect of bowel function between the men in the randomised groups.
| TURP | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Effect size (95% CI), p-value | Intervention | Control | Effect size (95% CI), p-value | |
| Faecal incontinencea | 40/216 (19) | 29/218 (13) | 35/195 (18) | 29/194 (15) | 1.13 (0.74 to 1.73), 0.563 | 40/192 (21) | 36/199 (18) | 1.06 (0.74 to 1.52), 0.745 |
| Faecal urgencya | 130/216 (60) | 116/218 (53) | 96/195 (49) | 105/194 (54) | 0.86 (0.72 to 1.03), 0.094 | 107/192 (56) | 106/198 (54) | 1.00 (0.85 to 1.17), 0.966 |
| Constipation | 44/216 (21) | 35/218 (16) | 29/195 (15) | 24/191 (13) | 1.03 (0.66 to 1.59), 0.910 | 32/190 (17) | 28/196 (14) | 1.03 (0.69 to 1.53), 0.877 |
| Any bowel dysfunctionb | 146/216 (68) | 128/218 (59) | 113/195 (58) | 116/194 (60) | 0.91 (0.78 to 1.06), 0.226 | 121/191 (63) | 118/196 (60) | 0.97 (0.85 to 1.11), 0.640 |
FIGURE 42.
Type of bowel problems at baseline and 6 and 12 months: TURP.
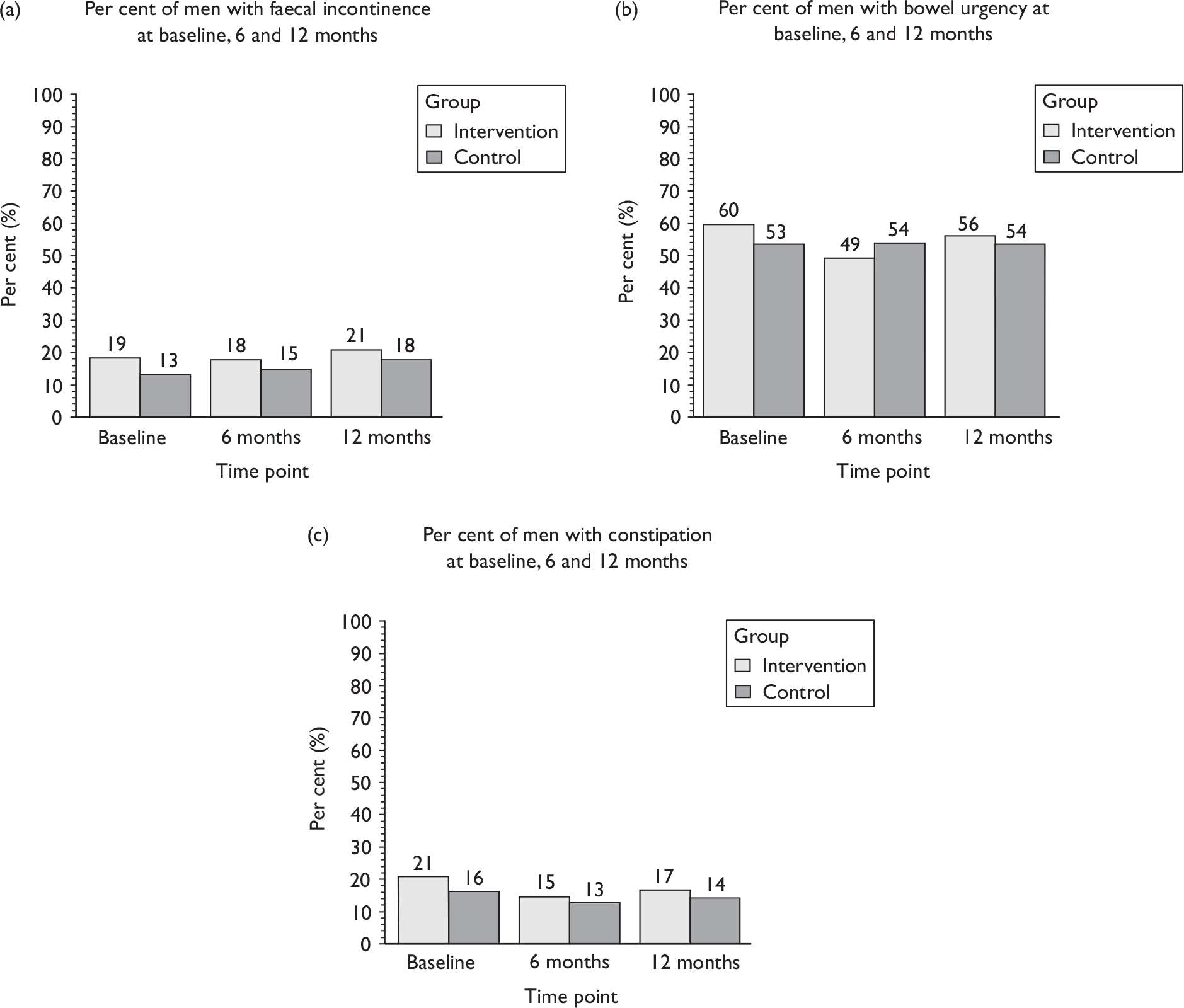
Sexual function
Table 71 compares the men in the randomised groups in terms of sexual function outcomes. One-third of men had difficulty with erection before surgery. Although just over one-third had an active sex life at 12 months, about half said that it was the same as before their operation. There were, however, no differences at 12 months according to the randomised groups in terms of the proportion of men with an active sex life [RR 1.04 (95% CI 0.80 to 1.36); adjusted for age, urinary incontinence before surgery and baseline value, p = 0.768] or the proportion of men who rated their sex life as worse after the operation [RR 0.99 (0.80 to 1.22), p = 0.912].
| TURP | Intervention | Control |
|---|---|---|
| Number of men not able to achieve erection before prostate surgerya | 67/214 (31) | 71/215 (33) |
| Number of men with active sex life at 12 months | 65/173 (38) | 66/184 (36) |
| Reasons for not having an active sex life at 12 months | ||
| Because of urinary symptoms | 7/108 (6) | 7/118 (6) |
| Because of bowel symptoms | 3/108 (3) | 1/118 (1) |
| Because of prostate operation | 42/113 (37) | 38/127 (30) |
| Because of medical treatment | 29/109 (27) | 25/119 (21) |
| For another reason | 51/110 (46) | 60/123 (49) |
| Sex life compared now with before prostate operation 12 months ago | ||
| Stayed the same | 76/145 (52) | 75/145 (52) |
| Better | 3/145 (2) | 5/145 (3) |
| Worse | 66/145 (46) | 65/145 (45) |
Table 72 compares the randomised groups in terms of problems with sexual function. There were no significant differences in sexual function outcomes between the intervention and control groups (Table 72, Figure 43). About half of the men were able to achieve a normal erection or one with slightly reduced stiffness by 12 months after surgery, and almost all reported a reduced quantity of semen or no ejaculation. Of those men, few reported more than slight pain. Around 15% of men used drugs, and about 1% used a vacuum device to help with sexual function. Only 2% reported urinary incontinence during intercourse.
| TURP | Intervention | Control |
|---|---|---|
| Difficulty with achieving erection | ||
| Normal stiffness | 37/177 (21) | 36/178 (20) |
| Reduced stiffness | 48/177 (27) | 48/178 (27) |
| Severely reduced stiffness | 40/177 (23) | 51/178 (29) |
| No erection possible | 52/177 (29) | 43/178 (24) |
| Bother with erection [mean (SD) n]a | 4.2 (3.7) 152 | 4.6 (3.9) 154 |
| Ejaculation | ||
| Normal quantity of semen | 7/174 (4) | 5/179 (3) |
| Reduced quantity of semen | 22/174 (13) | 27/179 (15) |
| Significantly reduced quantity of semen | 25/174 (14) | 27/179 (15) |
| Ejaculation but without semen | 62/174 (36) | 54/179 (30) |
| No ejaculation | 58/174 (33) | 66/179 (37) |
| Bother with ejaculation [mean (SD) n]a | 3.6 (3.7) 155 | 3.8 (3.7) 153 |
| Pain or discomfort with ejaculation | ||
| No pain | 121/142 (85) | 127/150 (85) |
| Slight pain | 15/142 (11) | 15/150 (10) |
| Moderate pain | 5/142 (4) | 7/150 (5) |
| Severe pain | 1/142 (1) | 1/150 (1) |
| Bother with pain or discomfort [mean (SD) n]a | 2.4 (3.6) 54 | 2.3 (3.4) 68 |
| Number of men using medication for sexual problems | 25/177 (14) | 20/186 (11) |
| Number of men using vacuum device for sexual problems | 2/175 (1) | 2/185 (1) |
| Number of men using either medication or a vacuum device for sexual problems | 26/177 (15) | 20/186 (11) |
| Number of men leaking urine during intercourse | 3/135 (2) | 3/133 (2) |
FIGURE 43.
Quality of erectile function at 12 months after prostate surgery: TURP.
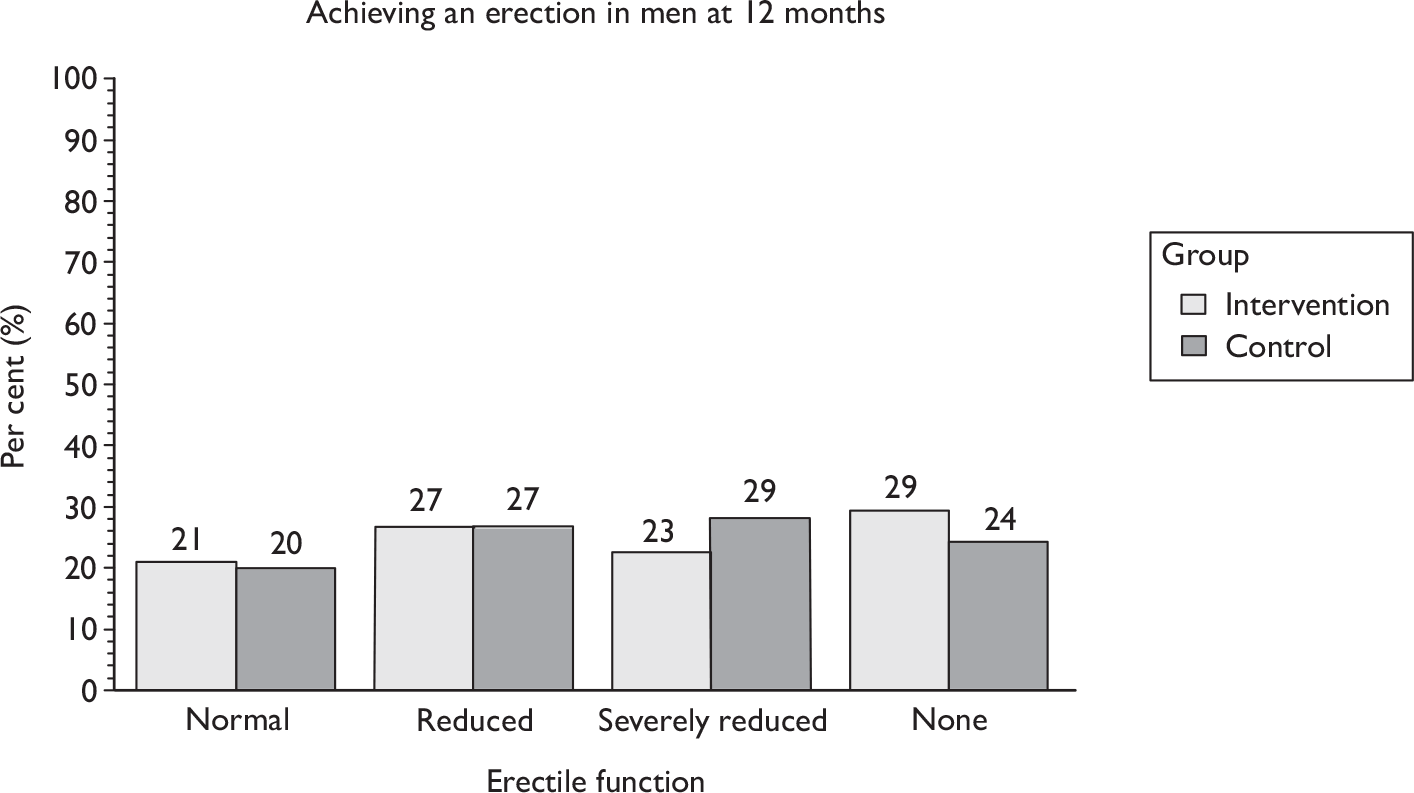
Quality of life
General health outcomes were measured using the EQ-5D and SF-12 (the latter subdivided into role – mental and role – physical scores). The slight increase in the scores over time probably represents recovery from the operation, but there were no differences between the randomised groups at any time point in EQ-5D or SF-12 scores (Table 73 and Figure 44).
| TURP | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Effect size (95% CI), p-value | Intervention | Control | Effect size (95% CI), p-value | |
| EQ-5D | 0.752 (0.270) 213 | 0.781 (0.251) 208 | 0.800 (0.257) 188 | 0.826 (0.235) 185 | –0.005 (–0.040 to 0.031), 0.789 | 0.784 (0.249) 177 | 0.791 (0.266) 189 | –0.005 (–0.040 to 0.031), 0.789 |
| SF-12M | 49.9 (10.4) 216 | 50.3 (10.4) 212 | 51.5 (9.5) 188 | 51.5 (10.5) 189 | –0.039 (–1.708 to 1.630), 0.964 | 52.6 (9.2) 188 | 51.7 (10.5) 193 | –0.039 (–1.708 to 1.630), 0.964 |
| SF-12P | 42.7 (11.0) 216 | 43.2 (11.9) 212 | 45.0 (11.6) 188 | 45.4 (12.6) 189 | 0.385 (–1.216 to 1.986), 0.636 | 44.5 (11.1) 188 | 44.0 (13.3) 193 | 0.385 (–1.216 to 1.986), 0.636 |
FIGURE 44.
Graphical representations of EQ-5D and SF-12 scores over time: TURP. SF-12M, SF-12 role – mental; SF-12P, SF12 role – physical.
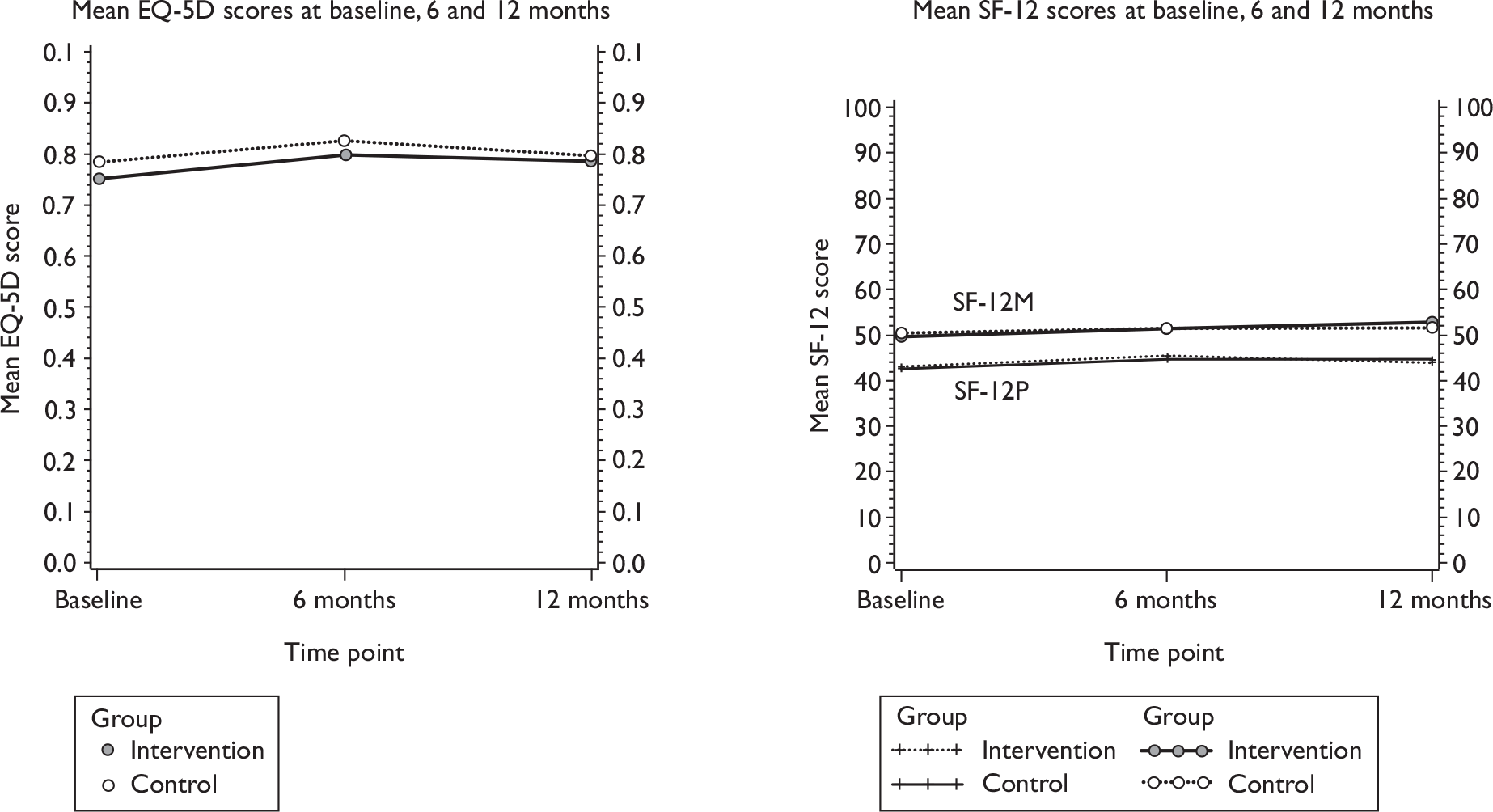
Pelvic floor muscle training
All men were asked to report on their practice of carrying out pelvic floor exercises at baseline and 6 and 12 months after randomisation. Initially around 20% of the men reported practising PFMT, of whom around 80% did it every day. The men in the control group continued with this frequency, while those in the intervention group were more likely to be carrying out PFMT at 6 and 12 months (75% and 65% respectively, Table 74). Significantly more, 64%, were practising for 3–4 days or more each week in the intervention group than in the control group at 6 months (14%). Men in the intervention group were also more likely to perform contractions while walking (81% vs 41%) or before a stress situation such as coughing or lifting by performing a pelvic floor muscle contraction before an increase in intra-abdominal pressure, also known as ‘the Knack’ (40% vs 28%). ‘The Knack’ was also more likely to reduce or stop urinary leakage in the intervention group (77% vs 62%).
| TURP | Baseline | 6 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Effect size (95% CI), p-value | Intervention | Control | Effect size (95% CI), p-value | |
| Yes | 47/213 (22) | 46/220 (21) | 146/194 (75) | 36/183 (20) | 3.93 (2.90 to 5.31), 0.001 | 122/188 (65) | 39/193 (20) | 3.20 (2.37 to 4.32), 0.001 |
| No | 104/213 (49) | 124/220 (56) | 44/194 (23) | 129/183 (70) | 65/188 (35) | 133/193 (69) | ||
| Don’t know | 62/213 (29) | 50/220 (23) | 4/194 (2) | 18/183 (10) | 1/188 (1) | 21/193 (11) | ||
| Days carrying out PFMT | ||||||||
| Every day | 26/213 (12) | 28/220 (13) | 75/194 (39) | 14/183 (8) | 51/188 (27) | 15/193 (8) | ||
| 5–6 days | 1/213 (0) | 1/220 (0) | 17/194 (9) | 0/183 (0) | 9/188 (5) | 4/193 (2) | ||
| 3–4 days | 12/213 (6) | 9/220 (4) | 32/194 (16) | 11/183 (6) | 38/188 (20) | 11/193 (6) | ||
| 1–2 days | 8/213 (4) | 8/220 (4) | 19/194 (10) | 10/183 (5) | 20/188 (11) | 9/193 (5) | ||
| None | 166/213 (78) | 174/220 (79) | 51/194 (26) | 149/183 (81) | 70/188 (37) | 155/194 (80) | ||
| Average contractions [mean (SD) n] | 12.4 (19.2) 194 | 2.7 (10.6) 183 | 9.6 (6.5 to 12.8), < 0.001 | 11.5 (22.8) 188 | 4.3 (16.4) 193 | 7.1 (3.1 to 11.1), 0.001 | ||
| Deliberate contractions whilst walkinga | 156/186 (84) | 67/163 (41) | 2.04 (1.68 to 2.48), <0.001 | 151/186 (81) | 71/175 (41) | 1.98 (1.64 to 2.40), < 0.001 | ||
| Deliberate contractions before doing somethinga | 74/179 (41) | 35/157 (22) | 1.85 (1.32 to 2.60), <0.001 | 74/183 (40) | 48/173 (28) | 1.47 (1.09 to 1.98), 0.012 | ||
| Contracting reduces or stops leaking | 77/92 (80) | 37/62 (60) | 1.32 (1.05 to 1.66), 0.016 | 79/103 (77) | 58/78 (62) | 1.24 (1.01 to 1.52), 0.043 | ||
Men in the therapy group were performing more contractions every day (11.5 vs 4.3) by 12 months (Table 74). This is likely to reflect the taught exercise regimen in the therapy group (aiming for 18 strong contractions every day). Recommendations from NICE72 suggest that men should perform eight contractions three times a day (24 per day). The low mean number of contractions reflects the high proportion of men in the control group (80%) not carrying out any exercises at all.
Lifestyle outcomes
Men were also advised, in the lifestyle advice leaflet sent to both groups and reinforced by the therapists in the intervention group, about the benefits of general health strategies such as taking more exercise. There were few differences between the groups in terms of other types of exercise practised (Table 75) except that men in the intervention group were more likely to practise walking [RR 1.18 (95% CI 1.02 to 1.35); p-value 0.024] or go swimming [RR 2.44 (95% CI 1.19 to 4.98); 0.014].
| TURP | Intervention | Control |
|---|---|---|
| General exercise (yes) | 148/184 (80) | 139/192 (72) |
| Exercise type | ||
| Walking | 134/184 (73) | 122/192 (62) |
| Swimming | 23/184 (13) | 10/192 (5) |
| Gardening | 98/184 (53) | 89/192 (45) |
| Running | 6/184 (3) | 5/192 (3) |
| Going to gym | 12/184 (7) | 10/192 (5) |
| Other | 23/184 (13) | 30/192 (16) |
| Changed exercise | ||
| No | 137/190 (72) | 142/197 (72) |
| Less | 17/190 (9) | 26/197 (13) |
| More | 36/190 (19) | 29/197 (15) |
Finally, men in both groups were given (via the lifestyle advice leaflet) other general advice on lifestyle changes they could make that might help both with incontinence and general health. Again, this advice was reinforced by the therapists for men in the intervention group. There were no significant differences between the groups in terms of changes made to lifestyle factors after TURP (Table 76).
| TURP | Intervention | Control |
|---|---|---|
| Weight | ||
| No need to lose weight | 68/184 (37) | 75/192 (39) |
| Haven’t tried to lose weight | 67/184 (36) | 83/192 (43) |
| Extra exercise to lose weight | 33/184 (18) | 19/192 (10) |
| Diet to lose weight | 23/184 (13) | 15/192 (8) |
| Other ways of losing weight | 16/184 (9) | 11/192 (6) |
| Fluid intake | ||
| Number of men making no changes to fluid intake | 66/189 (35) | 86/196 (44) |
| Drink more fluids | 84/189 (44) | 85/196 (43) |
| Drink more cranberry juice | 34/189 (18) | 36/196 (18) |
| Drink fewer caffeinated drinks | 52/189 (28) | 47/196 (24) |
| Drink less fluid in evenings | 67/189 (35) | 59/196 (30) |
| Other changes to fluid intake | 9/189 (5) | 6/196 (3) |
| Diet | ||
| Number of men making no changes to diet or food | 104/186 (56) | 109/193 (56) |
| More balanced diet | 47/186 (25) | 41/193 (21) |
| More fruit and vegetables | 67/186 (36) | 77/193 (40) |
| More fibre | 44/186 (24) | 42/193 (22) |
| Less fats or sugars | 50/186 (27) | 56/193 (29) |
| Other changes to food intake | 3/186 (2) | 6/193 (3) |
| Lifting | ||
| Number of men who reduce lifting | 90/191 (47) | 86/196 (44) |
| Smoking | ||
| Number of men who smoked | 25/186 (13) | 23/195 (12) |
| Number of men stopping smokinga | 1/25 (4) | 1/23 (4) |
| Number of men reducing smokinga | 14/25 (56) | 11/23 (48) |
| Chest or respiratory symptoms | ||
| Number of men who did have chest symptoms | 50/189 (26) | 41/189 (22) |
| Taking correct medicationa | 36/50 (72) | 24/41 (59) |
| Consulted GP about medicationa | 34/50 (68) | 23/41 (56) |
| Other changes to reduce respiratory symptomsa | 4/50 (8) | 3/41 (7) |
Prespecified subgroup analyses
Preplanned subgroup analyses were carried out on the primary outcome (urinary incontinence at 12 months) according to factors that we thought would be prognostic. These factors were:
-
pre-existing urinary incontinence (before prostate surgery)
-
age (up to 70 years, 71 years and over)
-
BMI (up to 30 kg/m2, 30–34.9 kg/m2, 35 kg/m2 or greater)
-
type of incontinence at trial entry
-
SUI
-
UUI
-
MUI
-
postmicturition leakage
-
-
other morbidity
-
type of therapist (physiotherapist or nurse).
Whilst a subgroup analysis on the use of biofeedback machines was also prespecified, there were insufficient numbers of centres with biofeedback machines to do so (see Table 7).
Figure 45 shows the effect of subgroup analysis on the primary outcome (urinary incontinence at 12 months) according to the prespecified factors. The dotted line reflects the overall main effect of the intervention on incontinence rates. Stricter levels of statistical significance (2p < 0.01) were sought (99% CIs), reflecting the exploratory nature of these analyses. There were no apparent clinically relevant differences according to any subgroup and none of the formal tests for statistical interaction effects was significant.
FIGURE 45.
Forest plot of subgroup analyses: urinary incontinence at 12 months.
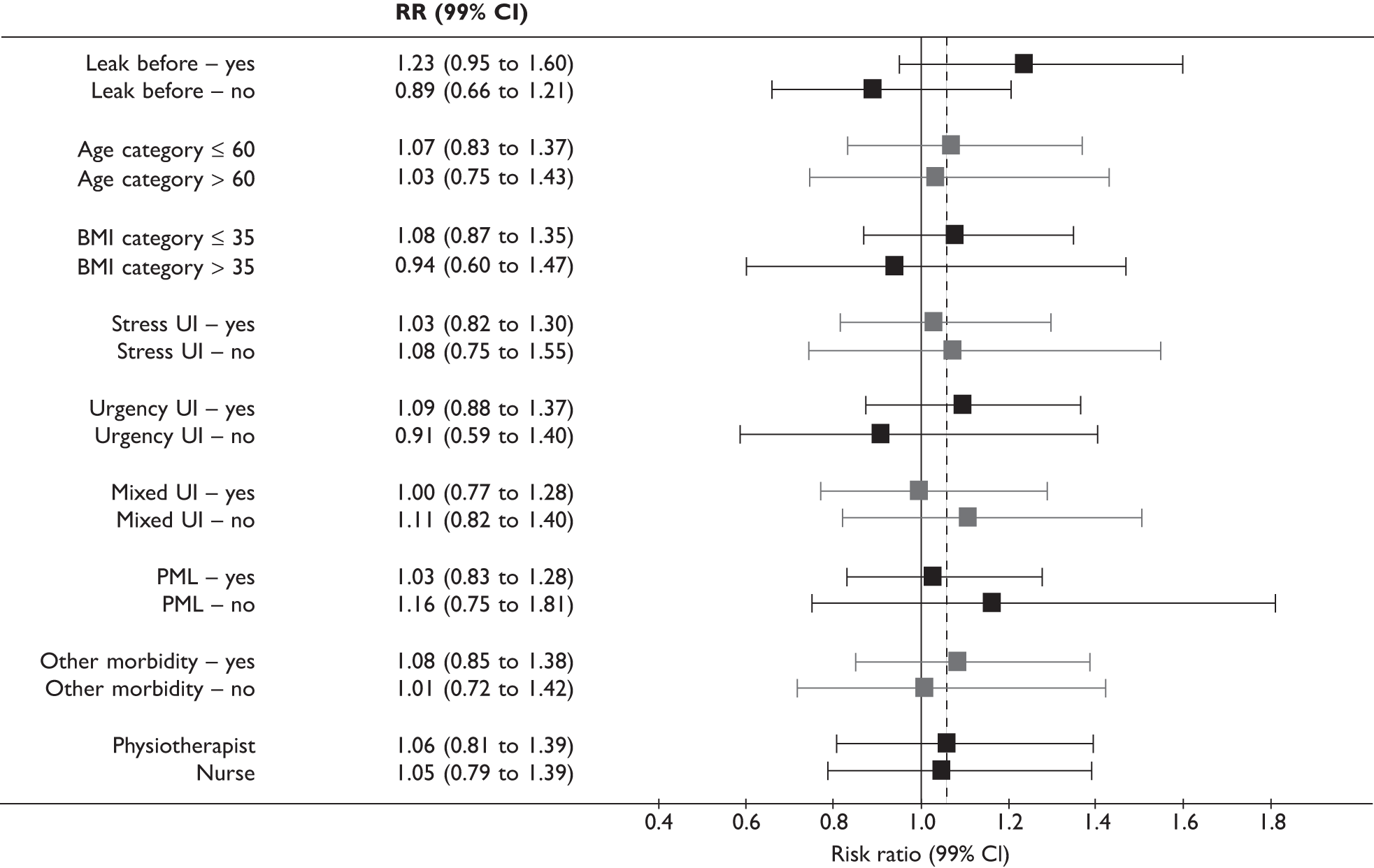
Satisfaction with treatment for urinary incontinence
Men were asked to score their satisfaction with the treatment they received for urinary incontinence (0 = ‘very unsatisfied’ to 10 = ‘very satisfied’) at 12 months after randomisation. Men in the intervention group were significantly more satisfied than those in the control group (see Appendix 5, Table 93). Thus, the therapy intervention did increase satisfaction rates despite the lack of difference in urinary outcomes.
Chapter 13 Resource use and cost-effectiveness in transurethral resection of the prostate randomised controlled trial
This chapter describes the economic analyses for the TURP RCT.
Description of the data available
Table 77 describes the number of men who contributed data for each of the areas of resource use and quality of life at each time point. Fewer data were available at the later data collection time points. For some areas of resource use (for example number of NHS pads at 12 months), only two-thirds of men indicated the quantity used. For other areas, for example inpatient admissions, 88% of men provided data on use of that resource even at 12 months. The difference between these two rates cannot be explained by the mode of data collection, as both were collected by participant-completed questionnaire. An alternative explanation might include the limited use of these services by 12 months, which meant that participants did not answer the questions because they did not think they were relevant. Other explanations could be advanced but there is no information to determine what the reasons are for men providing information for some areas of resource use but not others.
| Baseline [n (%)] | 3 months [n (%)] | 6 months [n (%)] | 9 months [n (%)] | 12 months [n (%)] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| NHS-supplied pads | 218 (99) | 214 (96) | 149 (68) | 146 (66) | 149 (68) | 144 (65) | 134 (61) | 136 (61) | 144 (65) | 135 (61) |
| Self-supplied pads | 216 (98) | 210 (95) | 144 (65) | 144 (65) | 148 (67) | 143 (64) | 133 (60) | 133 (60) | 142 (65) | 134 (60) |
| NHS-supplied bed/chair protector | 206 (94) | 212 (95) | 147 (67) | 141 (64) | 143 (65) | 128 (58) | 125 (57) | 132 (59) | 126 (57) | 122 (55) |
| Self-supplied bed/chair protector | 205 (93) | 212 (95) | 143 (65) | 140 (63) | 142 (65) | 128 (58) | 124 (56) | 132 (59) | 125 (57) | 121 (55) |
| Catheter | 220 (100) | 218 (98) | 204 (93) | 199 (90) | 187 (85) | 188 (85) | 186 (85) | 194 (87) | 188 (85) | 195 (88) |
| Sheath | 219 (100) | 216 (97) | 201 (91) | 199 (90) | 187 (85) | 182 (82) | 186 (85) | 193 (87) | 185 (84) | 192 (86) |
| GP incontinent visit | 151 (69) | 163 (73) | 141 (64) | 143 (64) | 121 (55) | 142 (64) | 118 (54) | 141 (64) | 120 (55) | 131 (59) |
| GP other visit | 200 (91) | 206 (93) | 195 (89) | 191 (86) | 186 (85) | 183 (82) | 186 (85) | 189 (85) | 182 (83) | 189 (85) |
| Nurse incontinent visit | 189 (86) | 195 (88) | 162 (74) | 157 (71) | 141 (64) | 145 (65) | 136 (62) | 148 (67) | 137 (62) | 149 (67) |
| Nurse other visit | 207 (94) | 211 (95) | 190 (86) | 191 (86) | 186 (85) | 176 (79) | 181 (82) | 186 (84) | 181 (82) | 191 (86) |
| Hospital doctor visit | 203 (92) | 204 (92) | 170 (77) | 190 (86) | 173 (79) | 183 (82) | 181 (82) | 187 (84) | 176 (80) | 178 (80) |
| Hospital nurse visit | 185 (84) | 189 (85) | 162 (74) | 172 (77) | 168 (76) | 172 (77) | 166 (75) | 181 (82) | 170 (77) | 174 (78) |
| Hospital physiotherapist visit | 205 (93) | 204 (92) | 182 (83) | 180 (81) | 171 (78) | 171 (77) | 172 (78) | 182 (82) | 168 (76) | 173 (78) |
| Private doctor visit | 215 (98) | 217 (98) | 187 (85) | 192 (86) | 184 (84) | 183 (82) | 184 (84) | 187 (84) | 180 (82) | 185 (83) |
| Private nurse visit | 191 (87) | 193 (87) | 181 (82) | 182 (82) | 179 (81) | 174 (78) | 178 (81) | 184 (83) | 176 (80) | 178 (80) |
| Private physiotherapist visit | 207 (94) | 206 (93) | 187 (85) | 190 (86) | 175 (80) | 171 (77) | 178 (81) | 186 (84) | 179 (81) | 175 (79) |
| Inpatient visit | 218 (99) | 220 (99) | 197 (90) | 198 (89) | 189 (86) | 188 (85) | 188 (85) | 193 (87) | 188 (85) | 195 (88) |
| Days off work | 206 (94) | 204 (92) | 185 (84) | 192 (86) | 183 (83) | 179 (81) | 176 (80) | 179 (81) | 183 (83) | 186 (84) |
Analysis of resource use and costs
Resource use
Table 78 details the average resource use for the intervention and subsequent use of health services over the 12-month follow-up period after randomisation. Resource utilisation was slightly higher across most areas in the intervention group than in the control group but the differences were very small and were not statistically significant, apart from nurse and hospital physiotherapy visits and the number of private physiotherapy visits. A detailed description of the use of NHS and private provider health services is provided in Appendix 5.
| Area of resource use | Intervention [mean (SD)] | Control [mean (SD)] | Difference (95% CI)a |
|---|---|---|---|
| Intervention visits | 3.10 (1.51) | 0 | –3.10 |
| Subsequent resource use | |||
| NHS-supplied pads used | 1.09 (3.28) | 0.77 (2.33) | 0.21 (–0.24 to 0.66) |
| NHS-supplied bed/chair protectors used | 0.14 (0.83) | 0.29 (1.54) | –0.18 (–0.40 to 0.04) |
| GP doctor incontinence-related visits | 0.38 (1.32) | 0.37 (0.98) | –0.02 (–0.23 to 0.20) |
| GP doctor other visit | 4.25 (4.32) | 4.00 (4.04) | 0.34 (–0.43 to 1.11) |
| GP nurse incontinence-related visit | 0.20 (1.00) | 0.20 (1.15) | 0.00 (–0.19 to 0.19) |
| GP nurse other visit | 2.47 (4.00) | 2.61 (4.56) | 0.01 (–0.75 to 0.76) |
| Number of men using catheters | 0.45 (0.92) | 0.34 (0.78) | 0.10 (–0.06 to 0.26) |
| Number of men using sheaths | 0.15 (0.52) | 0.15 (0.54) | –0.01 (–0.10 to 0.09) |
| Hospital doctor visits | 0.33 (1.48) | 0.37 (1.04) | –0.04 (–0.28 to 0.20) |
| Hospital nurse visitsb | 0.46 (1.33) | 0.15 (0.60) | 0.31 (0.12 to 0.51) |
| Hospital physiotherapy visitsb | 0.82 (1.68) | 0.06 (0.39) | 0.76 (0.53 to 0.99) |
| Inpatient days | 0.06 (0.44) | 0.05 (0.35) | 0.01 (–0.06 to 0.08) |
| Number taking incontinence drugs | 0.22 (0.66) | 0.36 (0.95) | –0.13 (–0.28 to 0.02) |
| Participant resource use | |||
| Self-supplied pads used | 1.10 (3.86) | 0.84 (3.58) | 0.26 (–0.42 to 0.94) |
| Self-supplied bed/chair protector used | 0.22 (1.51) | 0.36 (2.42) | –0.15 (–0.52 to 0.23) |
| Private doctor visits | 0.05 (0.28) | 0.10 (0.34) | –0.05 (–0.11 to 0.01) |
| Private nurse visits | 0.10 (0.62) | 0.05 (0.27) | 0.05 (–0.03 to 0.14) |
| Private physiotherapist visitsb | 0.26 (0.87) | 0.18 (2.29) | 0.22 (0.10 to 0.34) |
| Number of days off work | 4.14 (23.32) | 1.50 (11.48) | 2.63 (–0.58 to 5.84) |
Costs
Participant time and costs
The average time and costs to participants and their companions (families or carers) of a contact either with a primary care service provider or as an outpatient consultation or an inpatient admission are reported in Table 79. These data were combined with the information on number of contacts (e.g. hospital doctor visits) that the trial participants had reported (Table 78) to estimate a monetary cost per participant for both intervention and control.
| Resource use | Time or monetary cost | Mean (SD) |
|---|---|---|
| Primary care consultation visit | Time spent going to and attending a primary care consultation (hours) | 0.68 (0.29) |
| Companion’s time off work (£) | 1.10 (2.68) | |
| Average cost to participant and companion of a primary care consultation (£) | 11.29 (13.38) | |
| Secondary care visit | Time spent attending a secondary care visit (hours) | 1.73 (1.11) |
| Companion’s time off work (£) | 5.81 (9.69) | |
| Average cost to participant and companion of travelling to a secondary care department (£) | 25.25 (25.31) | |
| Inpatient visit | Number of visits to participant during admission | 2.10 (4.47) |
| Companion’s time off work (£) | 6.59 (31.69) | |
| Average cost to participant and companion of admission (£) | 30.21 (55.84) |
Estimation of societal cost
Table 80 details the mean costs per participant of the two interventions. The unit cost information in Tables 4 and 79 was combined with the resource use information reported in Table 78 to provide estimates of the total cost per participant. For the base-case analysis based on societal costs the mean total cost per participant in the intervention group was £984 (SD £2626) and the mean cost in the control group was £566 (SD £1285). There was evidence of higher costs in the intervention group: mean difference £420 (95% CI £55 to £785). The difference in mean societal cost was mainly due to the higher number of days taken off work by the participants in the intervention arm of the trial and the cost of the intervention itself.
| Area of resources use | Intervention [mean cost, £ (SD)] | Control [mean cost, £ (SD)] | Difference (95% CI)a |
|---|---|---|---|
| NHS costs | |||
| Intervention | 174.47 (82.89) | 0 | 174.47 |
| Subsequent resource use | |||
| NHS-supplied pads | 16.88 (50.74) | 11.85 (35.99) | 3.27 (–3.70 to 10.24) |
| NHS-supplied bed/chair protectors | 1.92 (11.32) | 3.94 (21.05) | –2.50 (–5.56 to 0.51) |
| GP doctor incontinence-related visits | 13.58 (47.53) | 13.46 (35.42) | –0.57 (–8.27 to 7.11) |
| GP doctor other visit | 153.16 (155.65) | 143.84 (145.32) | 12.13 (–15.53 to 39.79) |
| GP nurse incontinence-related visit | 2.20 (11.01) | 2.18 (12.63) | 0.12 (–2.12 to 2.14) |
| GP nurse other visit | 27.20 (44.04) | 28.74 (50.17) | 0.06 (–8.22 to 8.35) |
| Catheter | 1.28 (13.36) | 0.42 (4.44) | 0.87 (–1.030 to 2.73) |
| Sheath | 13.50 (62.93) | 13.38 (55.23) | 1.41 (–0.26 to 0.80) |
| Hospital doctor visits | 24.89 (111.12) | 27.70 (77.78) | –2.70 (–20.66 to 15.26) |
| Hospital nurse visits | 14.37 (41.19) | 4.75 (18.74) | 9.67 (3.68 to 15.67)b |
| Hospital physiotherapy visits | 25.36 (52.11) | 1.82 (12.20) | 23.52 (16.45 to 30.59)b |
| Inpatient days | 7.15 (47.89) | 6.89 (44.15) | 0.26 (–8.36 to 8.88) |
| Prescribed drugs | 14.51 (50.33) | 24.97 (76.77) | –7.24 (–18.84 to 4..36) |
| Total subsequent use cost | 318.13 (333.42) | 284.81 (315.07) | 34.43(–25.53 to 94.38) |
| Total NHS cost | 492.59 (355.95) | 284.81 (315.07) | 208.88 (146.69 to 271.07) b |
| Participant costs | |||
| Self-supplied pads | 19.41 (61.51) | 11.22 (36.97) | 8.22 (–1.17 to 17.60) |
| Self-supplied bed/chair protector | 0.31 (2.42) | 3.75 (29.81) | –3.45 (–7.38 to 0.48) |
| Private doctor visits | 0.00 (0.00) | 1.35 (15.90) | –1.35 (–3.46 to 0.76) |
| Private nurse visits | 0.00 (0.00) | 0.00 (0) | 0 |
| Private physiotherapist visits | 0.14 (2.09) | 4.75 (70.74) | –0.52 (–1.72 to 0.68) |
| Number of days off work | 439.76 (2476.70) | 158.82 (1219.55) | 279.32 (–61.62 to 620.28) |
| Total participant costs | 462.30 (2519.18) | 184.00 (1222.49) | 277.74 (–68.71 to 624.18) |
| Participant travel and companion travel and time off work costs | |||
| Intervention | 78.39 (38.18) | 0 | 78.39 |
| GP doctor incontinence-related visits | 4.26 (14.90) | 4.22 (11.11) | 0.18 (–2.59 to 2.23) |
| GP doctor other visit | 48.03 (48.81) | 45.11 (45.57) | 3.81 (–4.87 to 12.48) |
| GP nurse incontinence-related visit | 2.26 (11.30) | 2.24 (12.96) | 0.01 (–2.17 to 2.20) |
| GP nurse other visit | 27.92 (45.20) | 29.50 (51.49) | 0.06 (–8.44 to 8.57) |
| Total GP travel and time off work | 82.47 (85.60) | 81.06 (88.75) | 2.93 (–12.81 to 18.67) |
| Hospital doctor visits | 8.38 (37.41) | 9.33 (26.19) | –0.91 (–6.96 to 5.14) |
| Hospital nurse visits | 11.71 (33.55) | 3.87 (15.27) | 7.88 (3.00 to 12.76) |
| Hospital physiotherapy visits | 20.66 (42.44) | 1.48 (9.94) | 19.16 (13.40 to 24.92)b |
| Total outpatient travel and time off work costs | 197.52 (118.68) | 14.67 (41.33) | 13.65 (–4.99 to 32.29) |
| Total inpatient visits | 1.79 (13.27) | 1.50 (10.45) | 0.29 (–1.94 to 2.52) |
| Total participant travel and companion travel and time off work costs | 360.17 (187.89) | 97.23 (104.11) | 70.06 (39.73 to 100.39)b |
| Total participant and companion cost | 665.68 (2540.26) | 281.24 (1224.41) | 385.11 (35.21 to 35.01) b |
| Total societal costs | 983.81 (2626.28) | 566.05 (1284.97) | 419.50 (53.67 to 785.31) b |
Estimation of NHS costs
In terms of NHS costs incurred after the intervention was delivered, the mean total cost per patient in the intervention group was £493 (SD £356) and the mean cost in the control group was £285 (SD £315). There was, however, no evidence of a statistically significant difference in the cost of subsequent NHS services used. Intervention costs were, as would be expected, greater in the intervention group. Combining information on the cost of the interventions and the cost of subsequent NHS care resulted in a statistically significantly higher total cost per participant in the intervention group. The mean difference was £209 (95% CI £147 to £271). This difference was driven almost entirely by the cost of the PFMT intervention itself.
Quality-adjusted life-years
Table 81 reports the EQ-5D scores for each arm of the trial at baseline and 6 and 12 months. Also reported is the difference between arms in EQ-5D score at 6 and 12 months. From these data it was estimated that the mean QALYs were 0.78 (SD 0.24, median 0.85) for the intervention arm and 0.82 (SD 0.22, median 0.89) for the control arm. The mean difference in QALYs after adjusting for minimisation and baseline EQ-5D scores was –0.00003 (95% CI –0.026 to 0.026). The mean difference is equivalent to 0.011 days in full health over the 1-year time horizon.
| Intervention [mean (SD)] | Control [mean (SD)] | Difference (95% CI)a | |
|---|---|---|---|
| Baseline EQ-5D |
0.75 (0.27) n = 213 (97%) |
0.78 (0.25) n = 208 (94%) |
|
| 6-month EQ-5D |
0.80 (0.26) n = 188 (85%) |
0.83 (0.24) n = 185 (83%) |
–0.03 |
| 12-month EQ-5D |
0.78 (0.25) n = 177 (80%) |
0.79 (0.27) n = 189 (85%) |
–0.01 |
| QALYs |
0.78 (0.24) n = 162 (74%) |
0.82 (0.22) n = 163 (73%) |
–0.00003 (–0.026 to 0.026) |
Imputation was not performed on missing values in the base-case analysis. Simple plausible extreme value imputation on EQ-5D scores, taking the 25th and 75th percentile values, suggested that only if it is assumed that all missing values were equal to the 75th percentile range of EQ-5D scores would the mean difference in EQ-5D scores differ from that reported in Table 81. However, this mean difference still would not be significantly different.
Estimation of cost-effectiveness
Societal perspective
Taking the mean difference in costs from Table 80, and assuming that the mean difference in the QALYs from Table 81 is 0, then on average the intervention is both more costly and no more effective than the control and hence the intervention is dominated by the control (Table 82). Arithmetically, the ICER was 14 million because the difference in QALYs was just negative. However, this has been interpreted as being effectively 0 as the difference was so small as to be meaningless.
| Difference in mean costs [mean (95% CI)a] | 419.50 (53.67 to 785.31) |
| Difference in QALYs [mean (95% CI)a] | –0.00003 (–0.026 to 0.026) |
| ICER (£/QALY) | Dominated |
| Probability intervention is cost-effective when threshold is £20,000 | 11.2% |
| Probability intervention is cost-effective when threshold is £30,000 | 17.3% |
One thousand bootstrap simulations were undertaken to estimate the uncertainty around the benefits and costs. At a willingness to pay threshold of £20,000 per QALY the intervention has a probability of 11% of being cost-effective, and at a threshold of £30,000 per QALY the intervention is 17% likely to be cost-effective (Figures 46 and 47). At no point does the probability of being cost-effective reach 50%.
FIGURE 46.
Representation of the uncertainty in differential mean costs and QALYs: societal perspective (TURP).
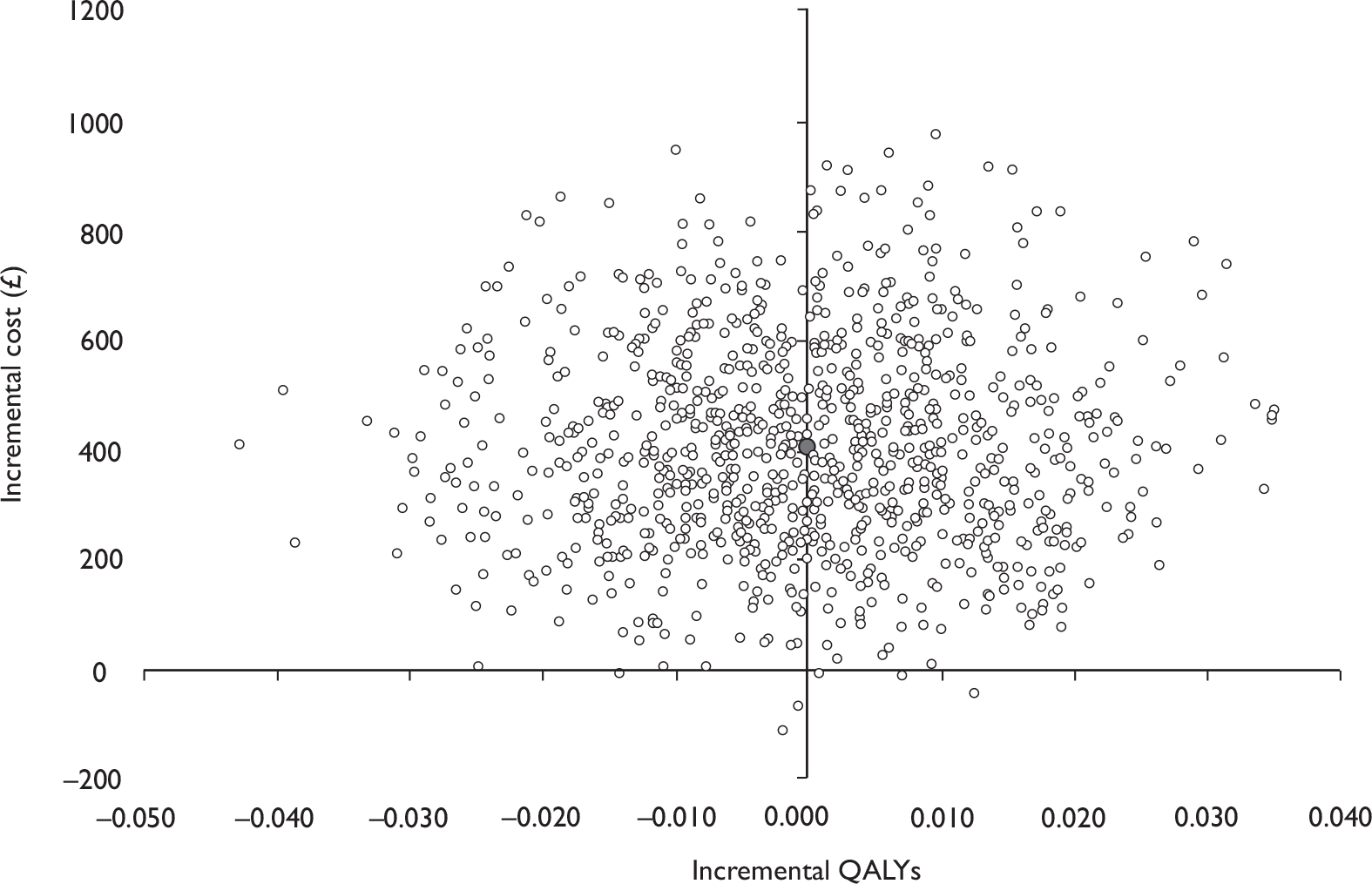
FIGURE 47.
Cost-effectiveness acceptability curve for intervention versus control: societal perspective (TURP).
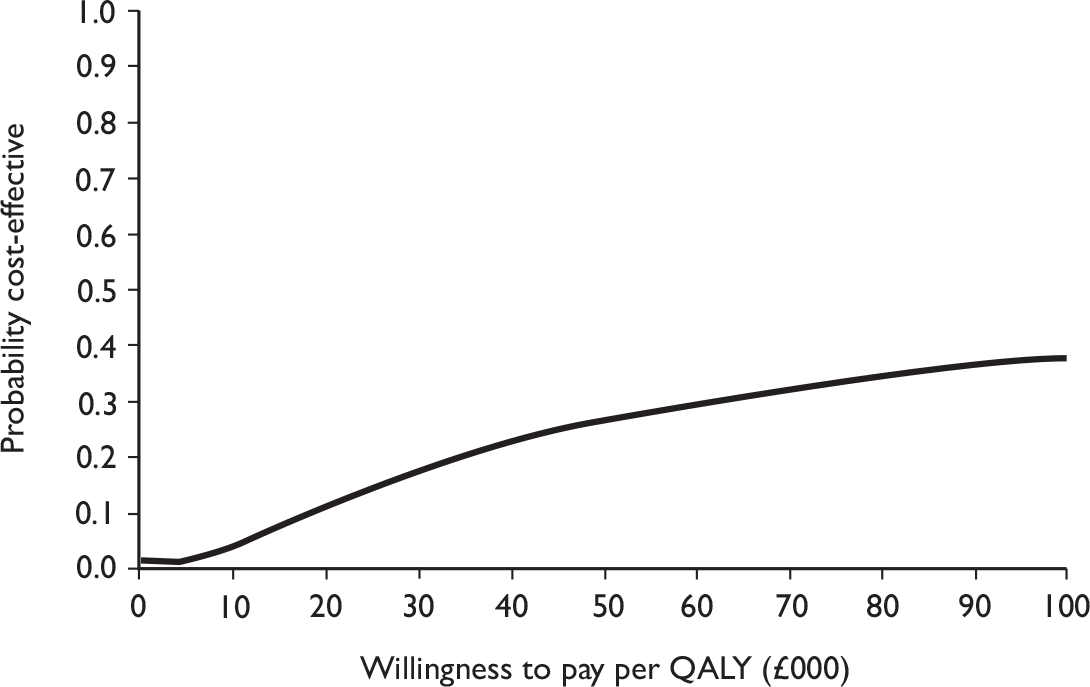
NHS perspective
Taking the mean difference in total NHS costs from Table 80 and assuming that the mean difference in QALYs is effectively zero means that the provision of PFMT is on average both more costly and no more effective than the control and hence dominated (Table 83). Arithmetically, the ICER was 6.9 million because the difference in QALYs was just negative. However, this has been interpreted as being effectively 0 as the difference was so small as to be meaningless.
| Difference in mean NHS costs [mean (95% CI)a] | 208.88 (146.69 to 271.07) |
| Difference in QALYs [mean (95% CI)a] | –0.00003 (–0.026 to 0.026) |
| ICER (£/QALY) | Dominated |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 20.0% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 29.4% |
As with the societal perspective, bootstrap simulations were undertaken to estimate the uncertainty around the benefit and costs. The bootstrap estimates in Figure 48 indicate that the intervention group had higher costs than the control; however, there was a relatively wide distribution in the difference in QALYs.
FIGURE 48.
Representation of the uncertainty in differential mean costs and QALYs: NHS perspective (TURP).
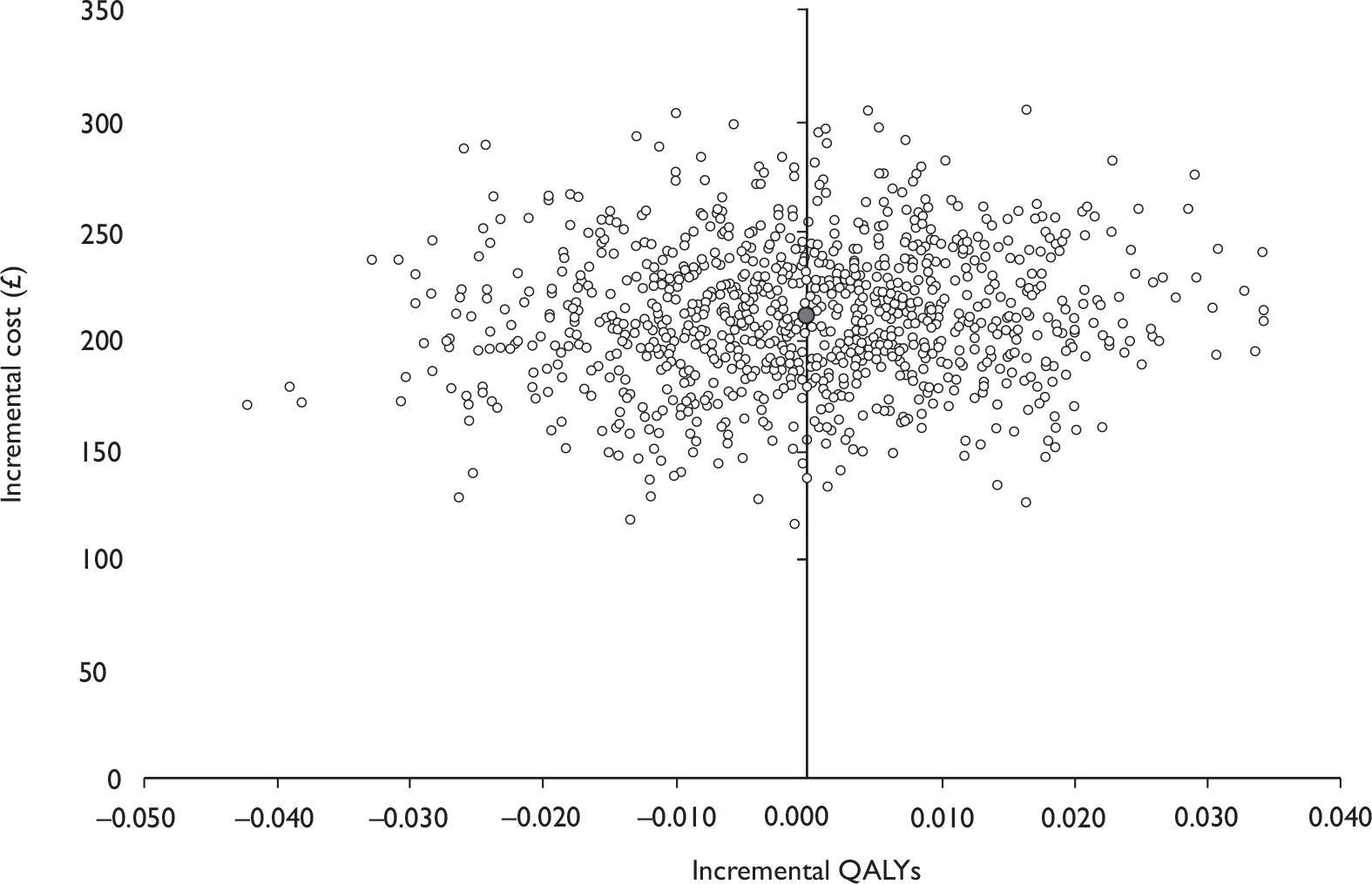
At a cost-effectiveness threshold of £20,000 per QALY, the intervention has a probability of 20% of being cost-effective and at a threshold of £30,000 per QALY the intervention is 29% likely to be cost-effective (Figure 49). At no point does the probability of being cost-effective reach 50%. This indicates that it is unlikely that PFMT is cost-effective.
FIGURE 49.
Cost-effectiveness acceptability curve for intervention versus control: NHS perspective (TURP).
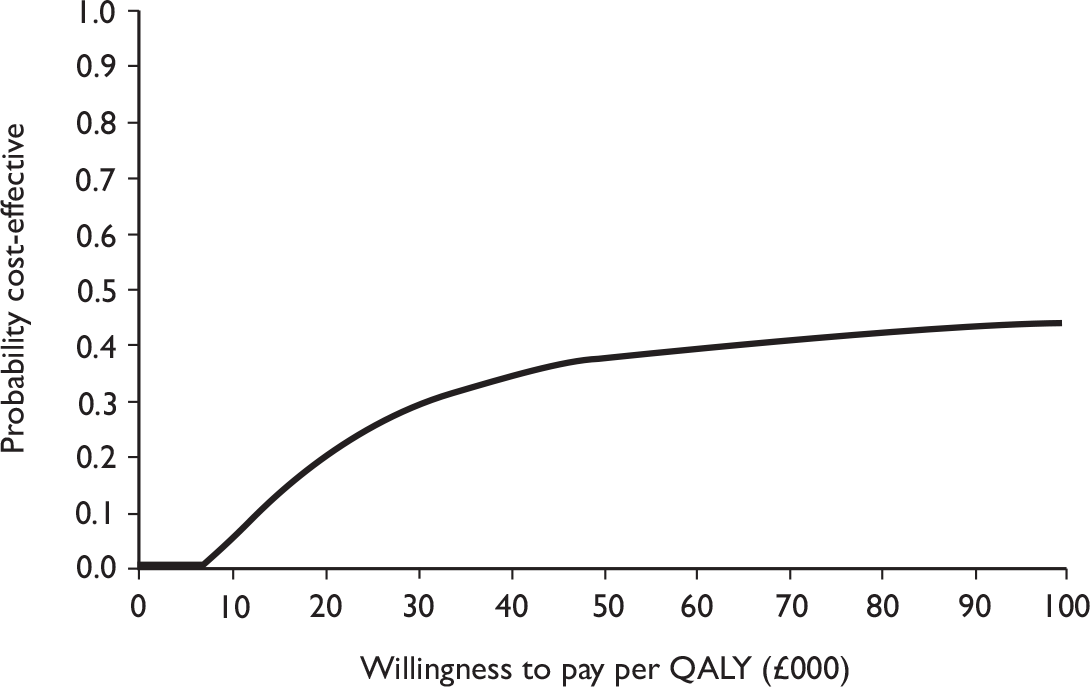
Sensitivity analysis
As mentioned in Chapter 2, sensitivity analysis is necessary to assess the robustness of the qualitative conclusion and identify areas where research is needed to more precisely estimate the values of those variables to which the result is sensitive. The variables that were considered uncertain in this study related to the cost of the different services used.
Incremental cost per quality-adjusted life-year when differences are not adjusted for baseline differences
An unadjusted analysis was performed as a sensitivity analysis to highlight the importance of the assumption that the characteristics of the groups were not the same at baseline. The results of this analysis, from the perspective of the NHS, indicate that at a cost-effectiveness threshold of £20,000 per QALY the intervention has a probability of 41.1% of being cost-effective, and at a threshold of £30,000 per QALY the intervention is 47.4% likely to be cost-effective (Figures 50 and 51).
FIGURE 50.
Representation of the uncertainty in differential mean costs and QALYs: using unadjusted data (TURP).
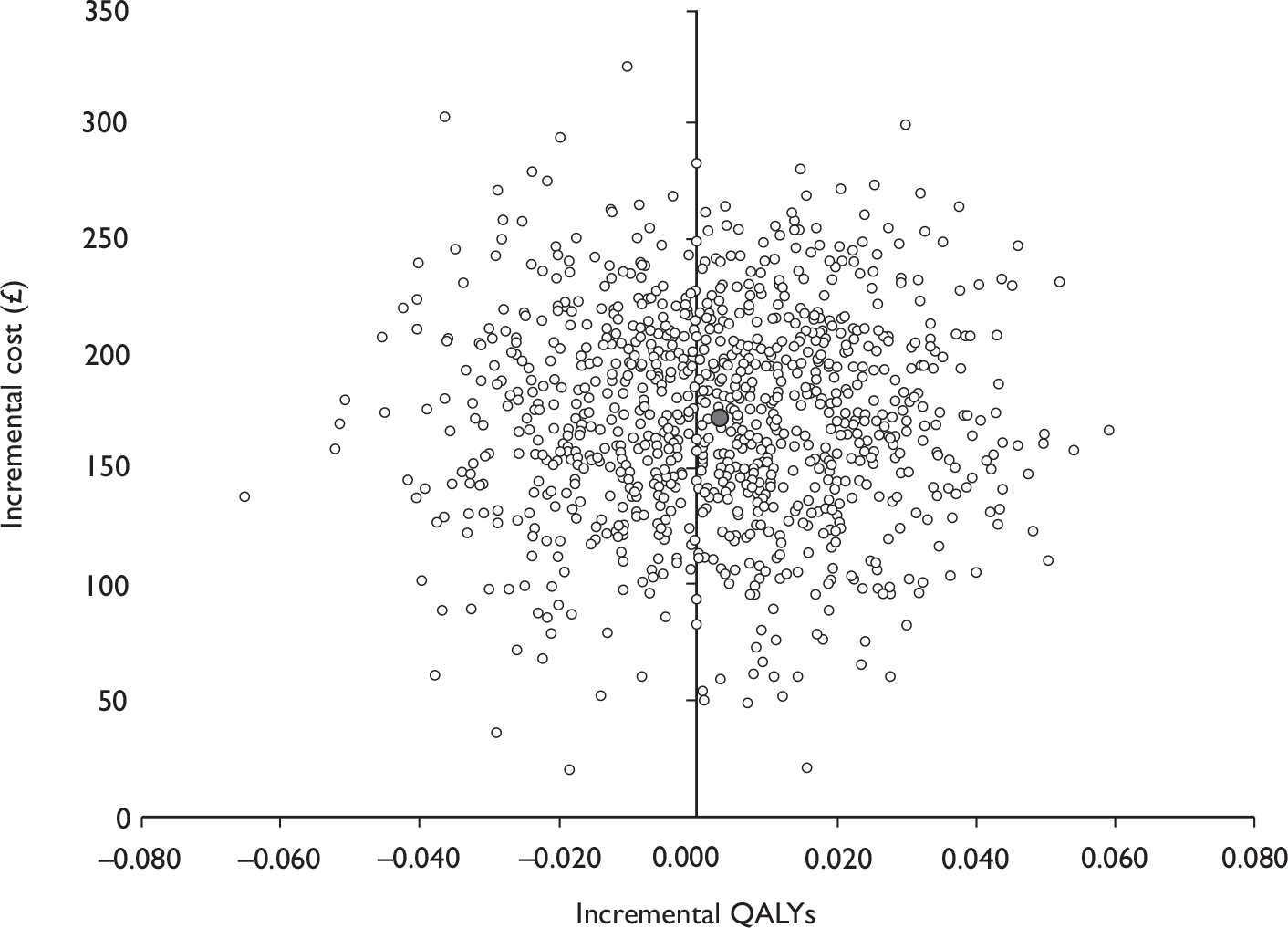
FIGURE 51.
Cost-effectiveness acceptability curve for intervention versus control: using unadjusted data (TURP).
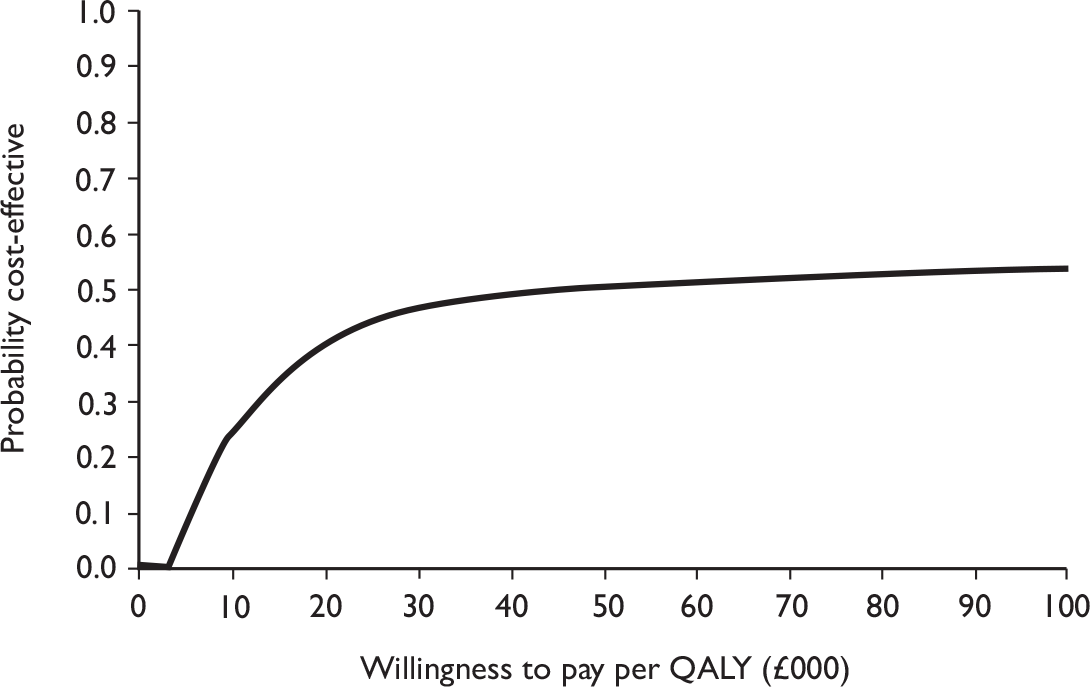
Basing quality-adjusted life-year estimates on SF-6D values
Table 84 reports the SF-6D scores for each arm of the trial at baseline and 6 and 12 months. These scores were slightly lower than those reported using the EQ-5D at the same time points. From these data it was estimated that the mean QALYs were 0.75 (SD 0.13, median 0.800) for the intervention arm and 0.77 (SD 0.15, median 0.783) for the control arm. The mean difference in QALYs, after adjusting for minimisation and baseline EQ-5D scores, was 0.004 (95% CI –0.012 to 0.022) higher for the control group, which was not statistically significant.
| Intervention [mean (SD)] | Control [mean (SD)] | Difference (95% CI)a | |
|---|---|---|---|
| Baseline SF-6D |
0.73 (0.14) n = 217 (99%) |
0.73 (0.15) n = 209 (94%) |
|
| 6-month SF-6D |
0.76 (0.15) n = 186 (85%) |
0.77 (0.16) n = 187 (84%) |
–0.01 |
| 12-month SF-6D |
0.77 (0.15) n = 186 (85%) |
0.76 (0.16) n = 191 (86%) |
0.01 |
| QALYs |
0.75 (0.13) n = 172 (78%) |
0.77 (0.15) n = 166 (75%) |
–0.004 (–0.020 to 0.012) |
The results of the analysis using the SF-6D data when estimating incremental cost-effectiveness from the societal perspective were similar to those of the EQ-5D. Taking the mean difference in the total societal costs from Table 80 (£420) and the mean QALY difference from Table 85 (–0.004) it can be seen that the intervention is on average less effective and more costly, indicating that it is dominated.
| Difference in mean NHS costs [mean (95% CI)a] | 419.50 (53.67 to 785.31) |
| Differences in QALYs [mean (95% CI)a] | –0.004 (–0.020 to 0.012) |
| ICER (£/QALY) | Dominated |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 1.9% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 3.9% |
At a cost-effectiveness threshold of £20,000 per QALY, the intervention has a likelihood of 2% of being cost-effective, and at a threshold of £30,000 per QALY the intervention has only a 4% likelihood of being cost-effective (Figures 52 and 53). Based on the societal perspective, these estimates indicate that the intervention is unlikely to be cost-effective.
FIGURE 52.
Representation of the uncertainty in differential mean costs and QALYs: societal perspective using SF-6D data (TURP).
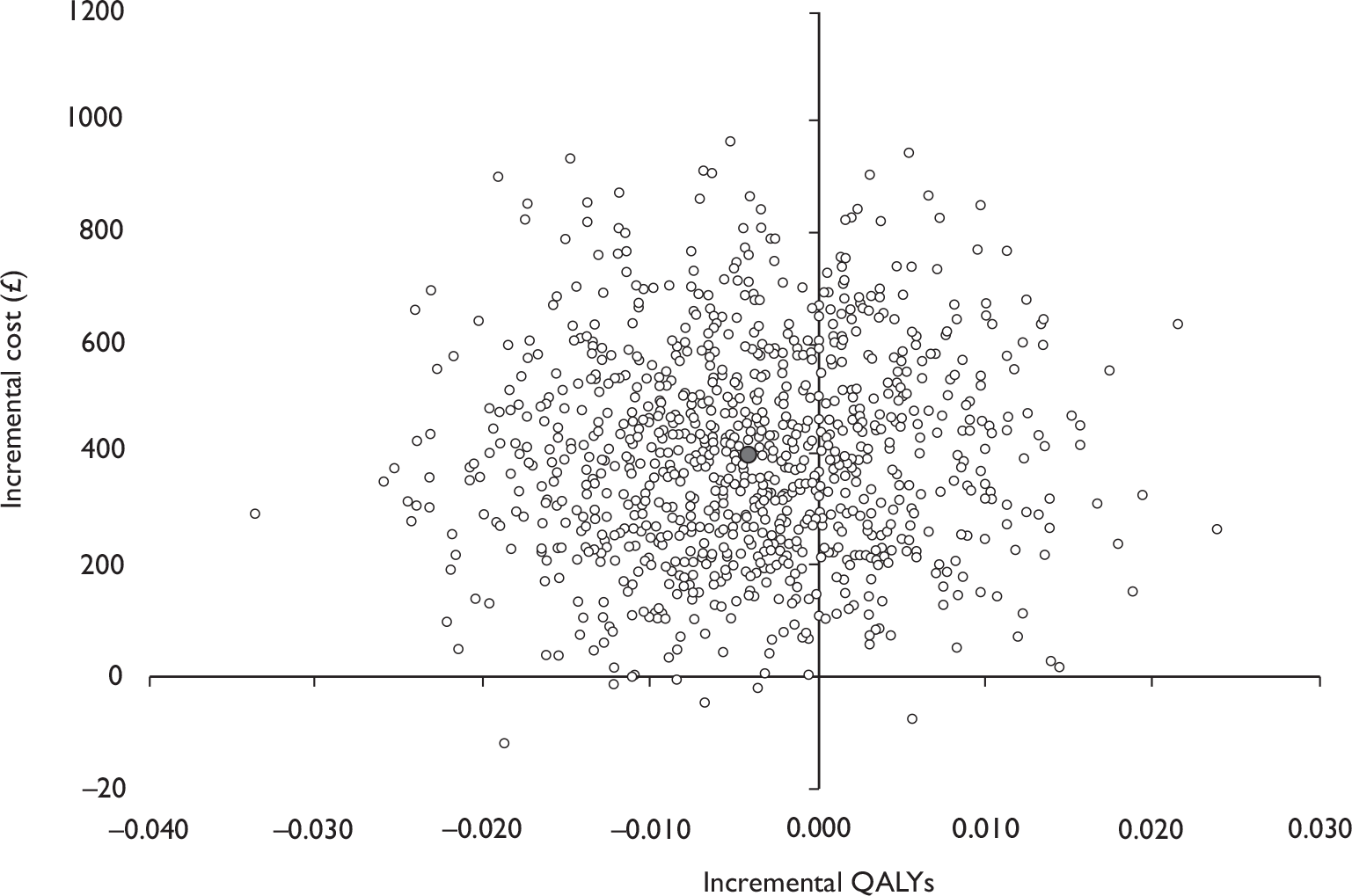
FIGURE 53.
Cost-effectiveness acceptability curve for intervention versus control: societal perspective using SF-6D data (TURP).
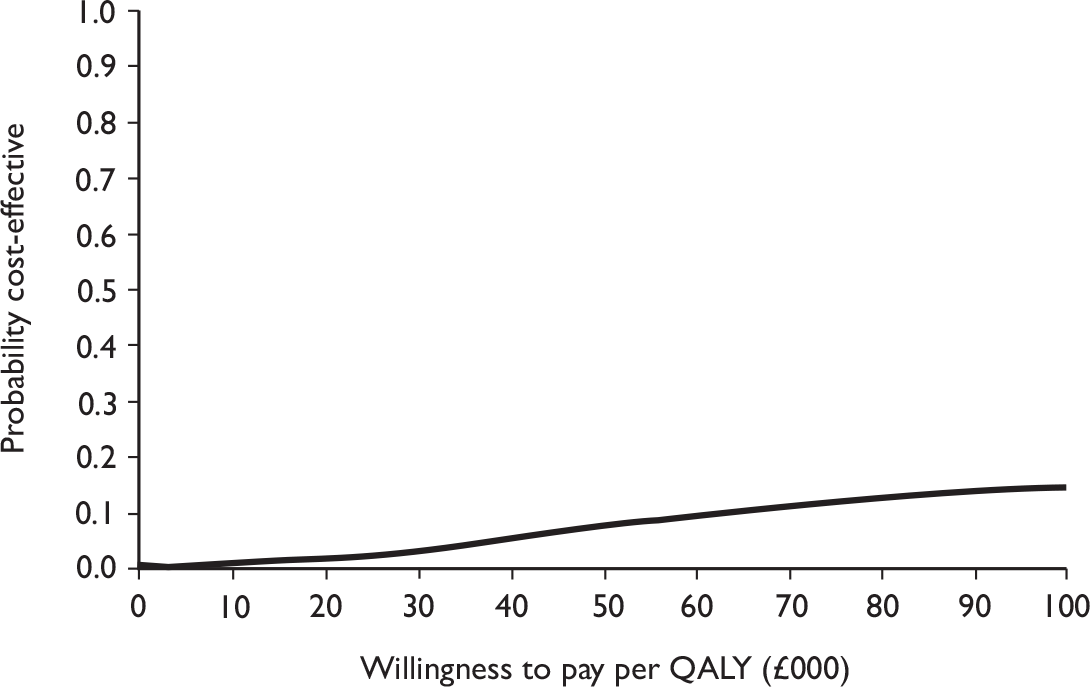
Taking the perspective of the NHS resulted in similar findings (Table 86). The probability of the interventions being cost-effective in this analysis was lower than that estimated when using the EQ-5D data. At a cost-effectiveness threshold of £20,000 per QALY the intervention has a probability of 4.2% of being cost-effective, and at a threshold of £30,000 per QALY the intervention has a 9% chance of being cost-effective (Figures 54 and 55). At no point does the probability of being cost-effective reach 50%, and it is unlikely that the intervention would be cost-effective.
| Difference in mean NHS costs [mean (95% CI)a] | 181.02 (107.06 to 254.97) |
| Differences in QALYs [mean (95% CI)a] | –0.004 (–0.020 to 0.012) |
| ICER (£/QALY) | Dominated |
| Probability intervention is cost-effective when threshold is £20,000 per QALY | 4.2% |
| Probability intervention is cost-effective when threshold is £30,000 per QALY | 8.9% |
FIGURE 54.
Representation of the uncertainty in differential mean costs and QALYs: NHS perspective using SF-6D data (TURP).
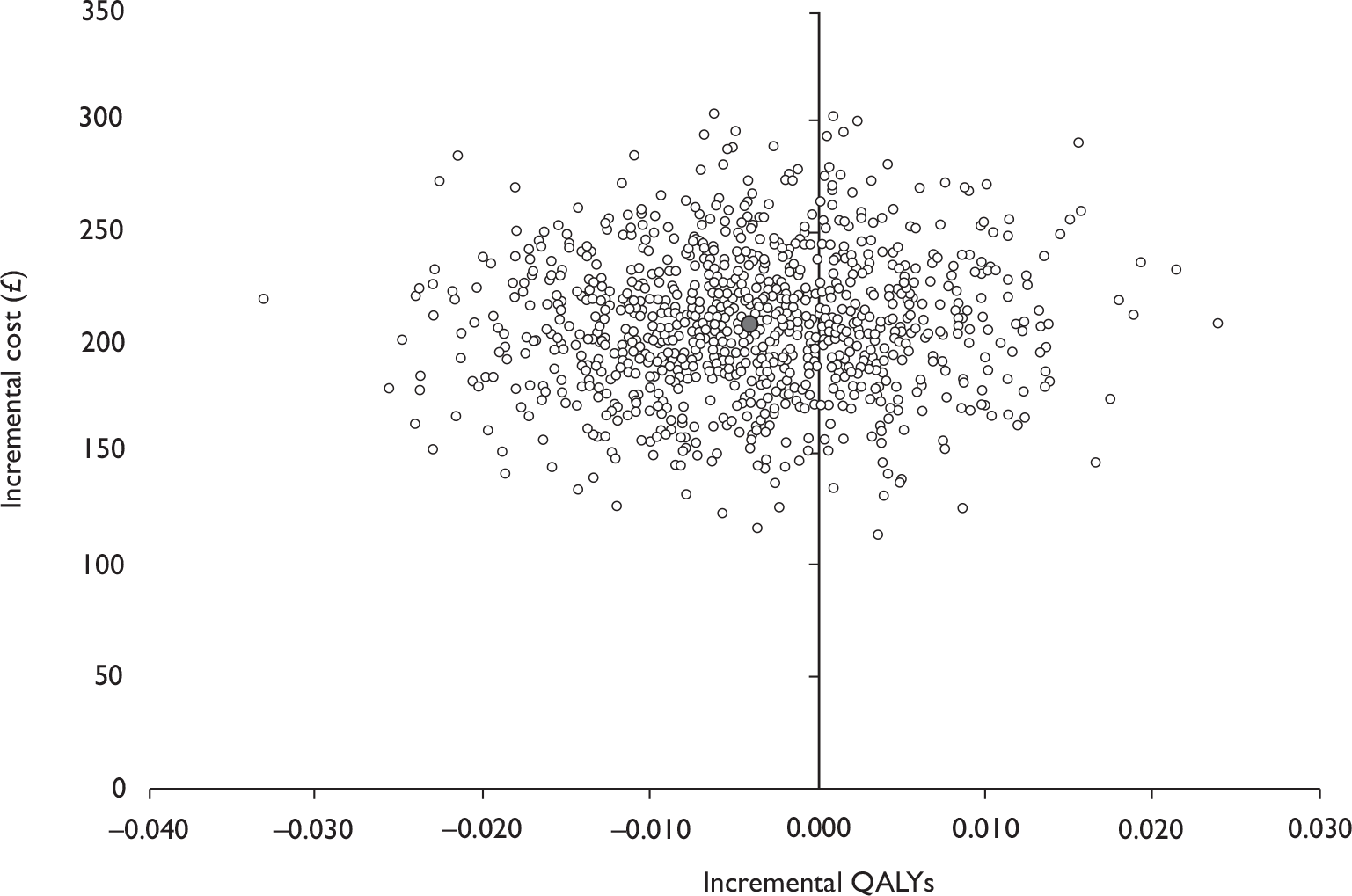
FIGURE 55.
Cost-effectiveness acceptability curve for intervention versus control: NHS perspective using SF-6D data (TURP).
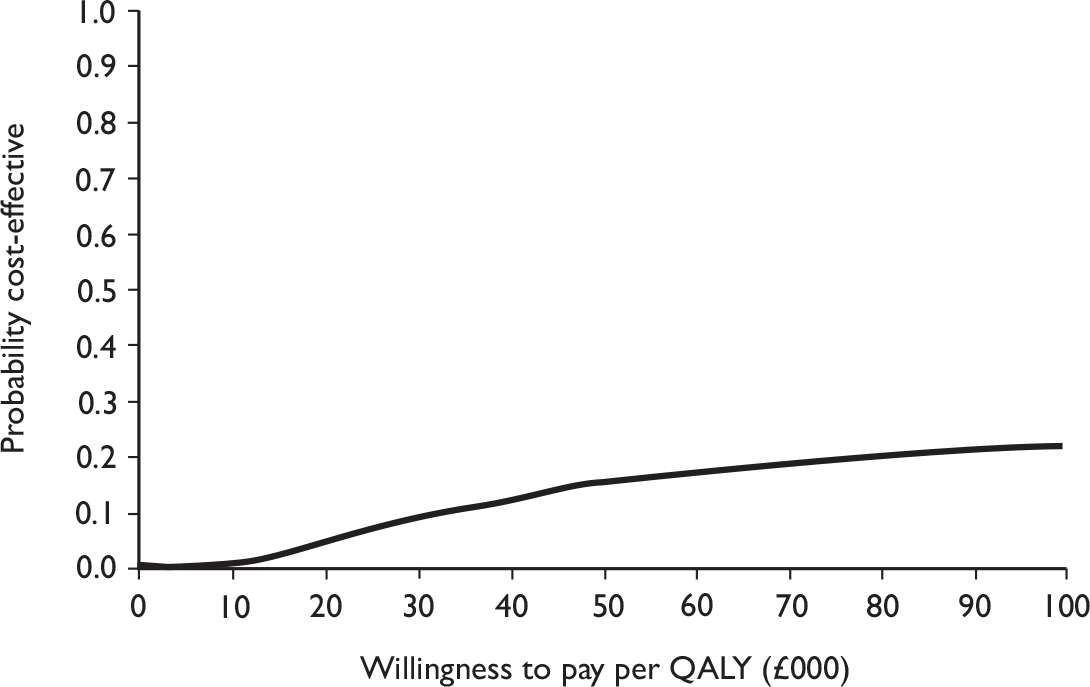
Threshold analysis around the cure rates
Further sensitivity analysis was performed by reanalysing the data by patient group for differences in costs and QALYs by continence status. A simple model was used to determine at what reduction in the rate of incontinence in the intervention group compared with the control group PFMT might be cost-effective. Details of the parameters used in the model are given in Table 87.
| Parameter | Participants in the intervention group who were continent | Participants in the intervention group who were incontinent | Participants in the control group who were continent | Participants in the control group who were incontinent |
|---|---|---|---|---|
| Cost of intervention (£) | 178.81 (223.00) [75.85] | 195.07 (223.00) [67.32] | 0.00 (0.00) [0.00] | 0.00 (0.00) [0.00] |
| Total subsequent resource use costs (£) | 304.69 (223.19) [308.33] | 425.02 (351.65) [351.14] | 225.67 (141.00) [241.53] | 433.08 (334.13) [368.07] |
| Total NHS costs (£) | 483.51 (417.41) [331.26] | 620.09 (572.00) [352.32] | 225.67 (141.00) [241.53] | 433.08 (334.13) [368.07] |
| QALYs | 0.82 (0.87) [0.20] | 0.77 (0.85) [0.26] | 0.87 (0.95) [0.19] | 0.79 (0.84) [0.23] |
| Probability of being continent/incontinent | 0.35 | 0.65 | 0.38 | 0.62 |
| Relative risk of being incontinent |
Varied between 0.85 and 1 |
Varied between 0.85 and 1 |
The results of the analysis indicate that the intervention was always dominated over the range of reductions in incontinence considered. Hence, it can be concluded that it is unlikely that the intervention could ever be considered cost-effective.
Conclusions
For men having TURP QALYs were similar in both groups, and costs for those who received the intervention were higher regardless of whether a societal or an NHS perspective was taken. Therefore, for both perspectives and over the range of sensitivity analyses conducted, it is unlikely that the provision of physical therapy for men who are incontinent after TURP would be cost-effective.
Chapter 14 Discussion of results of transurethral resection of the prostate randomised controlled trial
This chapter summarises the discussions relating to the TURP RCT.
Summary of main findings
In the men who had TURP, there were no statistically significant differences in urinary, bowel or sexual function outcomes between men in the intervention and control groups, despite evidence of extra performance of PFMT and improvement in pelvic floor muscle strength over time in the intervention group. The estimated extra cost to the NHS was on average £209 (95% CI £147 to £271) higher in the intervention group than in the controls.
Recruitment and screening of men in hospital
We approached 5986 men having TURP in NHS hospitals and obtained consent to screen 2832 of them. Of those, 91% returned their screening survey, and 46% were incontinent of urine at about 6 weeks after surgery (see Table 51). The majority of the men (around 94%) had a standard TURP, and a further 5% had a laser TURP. Around 36% of the men had urinary incontinence before surgery, and 4% reported faecal incontinence (see Table 51). The average age of the men was 70 years.
Recruitment to randomised controlled trial and response rates
Of the 1201 men who were incontinent at screening, 442 agreed to be randomised to a controlled trial of conservative treatment (including PFMT and lifestyle advice) for urinary incontinence (220 in the intervention group and 222 in the control group). The groups were comparable at baseline on all the epidemiological and clinical characteristics measured (see Table 52). Most men (around 80% in both groups) had heard of pelvic floor exercises at some point before starting the study (see Table 53).
Conduct of the intervention
Compliance with the intervention was high, with 86% of the men allocated to the intervention group attending at least one therapy visit and 72% attending all four of them. The most common reasons for not attending were becoming dry or ill, or finding it inconvenient to attend (see Table 56).
Association with type of therapist
Half of the centres used physiotherapists as the provider of the intervention, while the rest used nurse therapists (although all therapists received the same standardised training). About half of the men attended a physiotherapist while the other half attended a nurse therapist. However, there were no significant differences in the number of visits or the prevalence of urinary incontinence during the treatment period according to type of therapist (see Table 57).
At follow-up, no statistically significant association was demonstrated between the chance of incontinence at 12 months and type of therapist (Figure 57).
Clinical symptoms during the therapy period
During the therapy period, the proportion of men with incontinence fell from 82% to 52% by the fourth visit (see Table 58 and Figure 32). Few men reported bowel problems, and these numbers did not vary much over time (see Table 60 and Figure 34), although constipation was more common amongst the men who had a radical prostatectomy (who were around 10 years younger). Around half of the men had problems with sexual function and these did not improve with time (see Table 61 and Figure 35).
Clinical findings during the therapy period
Anal sphincter and pelvic floor muscle contraction strength increased over time in the intervention group: 37% of men had good or better strength at the beginning of the therapy period, rising to 80% by the fourth visit (see Table 62 and Figure 36). However, around 20% still had only moderate or poor contraction strength at the end of the 3-month therapy period.
Machine-led biofeedback was available in only 13 of the 34 MAPS centres, and was only actually used clinically in 10 men from two centres (see Table 63). When it was used it was not clear whether it was for diagnosis or for repeated use to assist with training. However, almost all men had verbal biofeedback from their therapist following digital anal assessment of muscle contraction, to teach them to perform contractions correctly and to monitor improvement at each successive visit.
Practice of pelvic floor muscle training after end of the therapy period
While around 20% of men in both the intervention and control groups were practising PFMT at baseline (before they were randomised and before the intervention), this increased to 65% in the intervention group at the 12-month follow-up, but remained at 20% in the control group.
Findings of the randomised controlled trial
The primary outcome of the RCT was the proportion of men with urinary incontinence at 12 months after randomisation. This was measured using the ICI-SF questionnaire, and was also ascertained at 3, 6 and 9 months after randomisation. In addition, urinary outcomes were obtained from 3-day diaries completed by the men at each of these time points. The response rates were almost all over 95% for the questionnaires and over 80% for the diaries (see Table 65).
Urinary outcomes
While the proportion of men with urinary incontinence fell from 100% at baseline to around 65% by 12 months, the majority of the decrease occurred in the first 3 months (to around 65%). There was no statistically significant difference between the intervention and control group in the proportion of men with urinary incontinence at 12 months [64.9% vs 61.6%, absolute risk difference 3.4% (95% CI –6% to 13%) at 12 months; see Table 66] or at any other time point (see Table 67 and Figure 39).
These findings (of no statistically significant differences between the trial groups) were similar for all the urinary outcomes regardless of how (by questionnaire or diary) or when they were measured (see Table 67).
Severity of incontinence
If severe incontinence is defined as leakage at least once a day and a moderate or large amount of leakage, around 25% of the men were still experiencing severe leakage at 12 months (see Table 67a) and around 17% were also using pads at 12 months (see Table 69a). Using the ICI score as a composite measure of severity and effect on quality of life, the same picture emerged: the majority of the improvement (decrease) in the score occurred in the first 3 months after randomisation with little further improvement (see Table 67 and Figure 40).
Types of incontinence
The most common type of incontinence was UUI (around 80%, see Table 68), while SUI and MUI affected around 60% of men. These and the other types of urinary symptoms were not different in the randomised groups at any time point, although they all decreased in frequency with time (see Table 68 and Figure 41).
Subgroup analyses
Prespecified subgroup analyses were carried out on the primary outcome (urinary incontinence at 12 months). There were no significant differences between randomised groups in any of the subgroups (see Figure 45).
Other clinical outcomes
Men were also asked to report on bowel and sexual problems.
Bowel outcomes
Bowel problems that might be expected to be ameliorated by therapy or lifestyle advice included faecal incontinence, urgency and constipation. Men were also asked about bowel conditions such as ulcerative colitis, Crohn’s disease and irritable bowel syndrome. There were no differences at any time point in any aspect of bowel function or disease between the men in the randomised groups (see Table 70 and Figure 42).
Sexual function outcomes
Around 70% of men had normal erectile function before operation. Although around one-third had an active sex life at 12 months, about half said that this was much the same as before their operation and just under half said that it was worse than before their operation (see Table 71). There were, however, no differences at 12 months, according to the randomised groups in any of the aspects of sexual function measured (see Tables 71 and 72). Around a half of the men had severely reduced or no erectile function (see Table 72). There were no differences at 12 months according to the randomised groups in terms of the proportion of men with an active sex life [RR 1.04 (95% CI 0.80 to 1.360), p = 0.768] or the proportion of men whose sex life had become worse after the operation [RR 0.99 (95% CI 0.80 to 1.22), p = 0.912] (see Table 71). Around 15% of men had used a vacuum device or medication to improve sexual function (see Table 72).
Quality of life outcomes
General health outcomes were measured using the EQ-5D and SF-12 (the latter subdivided into role – mental and role – physical scores). The slight increase in the scores over time can be assumed to represent recovery from operation, but there were no differences between the randomised groups at any time point in EQ-5D or SF-12 scores (see Table 73 and Figure 44).
Knowledge of pelvic floor muscle training in trial groups before intervention
Around four-fifths of the men in both groups had received information about the use of pelvic floor exercises before starting the trial intervention from at least one source (see Table 53). The most common sources were nurses or continence advisors (around 30%) or leaflets or books (over 40%, see Table 53).
Practice of pelvic floor muscle training in intervention and control groups
It is not surprising, given the level of prior knowledge of pelvic floor exercises, that 20% of men in both groups reported carrying out some exercises before randomisation (see Table 74). However, by 6 months the men in the intervention group were more likely to be still carrying them out and this difference persisted at the 12-month follow-up (see Table 74).
Changes in lifestyle factors
Men in both groups were given written information about lifestyle changes that might improve aspects of both their general health and incontinence. In the intervention group, this advice was reinforced and individualised by the therapists. However, there were no significant differences in the uptake of any aspect of this advice at 12 months after randomisation (see Tables 75 and 76).
Economic outcomes
Costs to the NHS
Total costs to the NHS were on average £209 (95% CI £147 to £271) higher in the intervention group than in the control group. This difference was primarily due to the cost of providing the PFMT training in the intervention group. The use of other health services, and hence cost, was similar between the groups.
Costs to the participants
On average, the costs of any private health care used were low and there was no evidence of any difference between groups. Similarly, the costs of accessing care other than the intervention were similar for the two groups. Participants in the intervention arm had on average more days away from usual activities than participants in the control arm and hence on average a higher cost. However, this difference was not statistically significant.
Overall costs to the NHS and participants
On average, the cost to the NHS and participants was greater in the intervention group than in the control group. This difference was not statistically significant.
Quality-adjusted life-years
On average, QALYs were virtually identical in both the intervention and the control group [mean difference –0.00003 (95% CI –0.026 to 0.026)].
Cost-effectiveness from the perspective of the NHS and participants
Based upon the point estimates of the mean difference in costs and QALYs, the intervention is dominated by the control intervention as it is on average more costly but associated with no more effectiveness. As there was considerable imprecision around the estimates of mean difference in costs and effects, the probability that the intervention would be cost-effective at the typical threshold for society’s willingness to pay for a QALY was calculated. This analysis suggested that there was less than an 18% chance that organised PFMT training was cost-effective.
Cost-effectiveness from the perspective of the NHS
When the perspective of the economic evaluation was restricted to the NHS, the intervention was dominated by the control intervention (it was more costly and no more effective). Furthermore, there was only a 20% chance that the intervention would be cost-effective if the threshold value for society’s willingness to pay for a QALY were £20,000.
Sensitivity analyses
The majority of the sensitivity analyses conducted did not greatly alter the conclusions of the economic evaluation. However, a sensitivity analysis conducted from the perspective of the NHS showed that, should the intervention reduce the rate of incontinence by approximately 15%, the provision of physical therapy might be cost-effective.
Strengths and weaknesses (specific to transurethral resection of the prostate randomised controlled trial)
Recruitment
We approached 5986 men who were admitted to hospital for TURP in order to identify and recruit our final population of 442 men who entered the RCT. Many of the men approached were ineligible or missed in hospital (3001 men, see Table 9a) and some were subsequently found to be ineligible (149 men; see Table 10a). This scale of recruitment represented a large burden on the recruitment officers in the centres. However, we felt that this was the most efficient way of identifying our target population, which was men who had urinary incontinence after prostate surgery. Other methods, such as expecting local staff to identify incontinent men and recruit them to the RCT directly, might have been too burdensome and risked missing many men owing to pressure of routine work.
Generalisability of the trial population
Most of the men who agreed, when in hospital, to be screened 3 weeks later, returned their screening questionnaire (2590/2838, 91%; Table 50): although the non-responders were older, more likely to smoke and spent more time in hospital, there were no clinically important differences in demographic or clinical characteristics when compared with responders. Just under one-fifth (442/2590, 17%) of the men who returned a screening questionnaire were eventually recruited into the RCT. Many of the remainder had become dry [1387 (54%) at screening, 274 (11%) at baseline], and a further 262 (10%) were not eligible because they did not return their baseline questionnaire (see Figure 5 and Table 11). Of the 227 (9%) not accounted for, 122 declined further contact, 16 did not wish to be randomised and the remainder had a variety of other reasons for not wishing to enter the trial (see Table 11). Thus, our trial population represents 442/512 (86%) of the men who were incontinent and eligible to be randomised, but only 442/2838 (15%) of men identified in hospital as having TURP.
Response rates
Once randomised, our participants were compliant in returning their questionnaires (over 90%) and urinary diaries (over 80%). While the withdrawal rates were slightly higher than from the radical prostatectomy RCT, there was no evidence of differential dropout from the randomised groups, with outcome data available for 97% (intervention) and 97% (control) of the men continuing at 12 months (see Table 65a). This provides some reassurance that the outcome data are representative of the men in the RCT, and that bias from differential attrition was minimal.
Strengths and weaknesses of economic analyses
The methods of the economic analysis were rigorous and reproducible, and efforts were made to assess the importance of uncertainty surrounding estimates of costs, effects and cost-effectiveness. As the study was not powered to detect differences in economic outcomes, it was anticipated that differences in costs and effects would not reach statistical significance. For this reason, conclusions from the economic evaluation were based upon the consideration of the balance of probabilities.
Regardless of the perspective taken for the analysis, there was little chance that the intervention would be cost-effective. Furthermore, none of the sensitivity analyses conducted changed the conclusions of this analysis.
Chapter 15 Overview of MAPS study
This chapter draws together the findings generated during the conduct of the MAPS study, discusses its strengths and weaknesses and sets the findings in context with those in the literature.
Summary of findings statement
Urinary incontinence
MAPS has shown that the provision of one-to-one conservative physical therapy for men with urinary incontinence after prostate surgery, either radical prostatectomy or TURP, does not result in better short- or long-term incontinence rates than standard management. The therapy intervention did, however, increase the number of men performing PFMT (compared with the control groups). From the perspective of the NHS, it is unlikely that, for either of the two patient groups considered, provision of one-to-one conservative physical therapy is cost-effective compared with standard care.
Pelvic floor muscle training
It seems that a great deal of information was available to men regarding PFMT in both the control and the intervention groups, for example from routine care in the NHS (from nurses, continence advisors, physiotherapists and doctors), books, leaflets, friends and the internet (Tables 15 and 53). It may be that this information was sufficient to allow men to manage their own incontinence. Therefore, this trial has not shown whether PFMT is effective or not; rather, it has shown that one-to-one sessions by a trained therapist are not necessary for instruction in PFMT and other aspects of conservative care such as bladder training and lifestyle advice. There was also no evidence to suggest that an intervention delivered by a trained continence nurse was more or less effective than that delivered by a trained physiotherapist.
Bowel function
Bowel dysfunction was uncommon. There was no evidence from MAPS that PFMT was effective in the treatment of faecal incontinence, constipation or bowel urgency.
Sexual function
There was no evidence from MAPS that PFMT was effective in the treatment of sexual dysfunction. Current NICE guidelines17 suggest that drugs or mechanical devices should be offered to men, and indeed around 60% of men had tried one or the other of these.
Quality of life
There was no difference between the intervention and control groups in either condition-specific or general measures of quality of life, in either of the clinical groups. The men did, however, show a gradual improvement in quality of life, consistent with a return to health after major surgery.
Prevention of risk of bias
Selection bias at trial entry (generation of allocation sequence, quality of concealment of randomisation process)
The randomisation programme was computer generated and used prespecified minimisation factors that varied according to each participant, which provided protection from selection bias.
Attrition bias (accounting for missing data, withdrawals and deaths)
We accounted for all deaths and men withdrawing from the trial. We did not impute data for them after their withdrawal but did use the information they supplied up to the point of loss of contact. However, the loss to follow-up was low and similar in the randomised groups of both trials.
Performance bias (blinding of participants and care deliverers to allocated group)
Neither the men nor the therapists could be blinded to the intervention, as this would not have been possible.
Detection bias (blinding of outcome assessments)
The majority of the outcomes were obtained using self-reported data from questionnaires posted from the MAPS study office to the men at their homes. The data were entered without the clerks being aware of the randomised allocation.
Strengths and weaknesses
Reliability of findings
One of the main strengths of the MAPS study is the consistency and robustness of the findings. No matter which way we compared the groups, all the different outcome measures concurred in failing to find clinically or statistically significant differences between the randomised groups in the clinical outcomes. Where statistically significant differences in costs were identified, these could be attributed to the cost of providing the intervention rather than the consequences of the intervention.
Trial management
We deliberately chose to minimise the trial processes that needed to occur at local centres, as most of the staff involved were engaged in routine delivery of care in the NHS. Recruitment officers at each site were tasked with approaching men who were admitted for prostate surgery. Rather than ask them to explain the RCT to all men, we chose to ask them only to obtain the men’s contact details and consent to receive a postoperative (screening) questionnaire. In this way, staff at the MAPS study office in Aberdeen were able to carry out the administration of the survey and subsequent contact only with men with incontinence in an efficient and standardised manner. This also facilitated follow-up by post and telephone.
If a man was randomised to the intervention group, the MAPS study office contacted the local therapist to ask that an appointment should be set up with the participant in accordance with local conditions.
In this way the burden of participating in research was reduced to a minimum in participating centres. We feel that this contributed to the success of recruitment to both the study and the RCT.
Design and content of pelvic floor muscle training regimen
The design of the PFMT regimen could not be based on evidence as there is no consensus on the most effective types of treatment and frequency of follow-up. The frequency of therapy visits (four in a 3-month period) was chosen to reflect current practice in the NHS. It would potentially have been feasible to roll out this pattern of visits to NHS practice if it had been effective.
The rationale underlying the choice of components is given in Chapter 3. The underlying assumption was that any treatment programme would have to be acceptable to men and practical to incorporate into their daily lives without becoming a burden. The emphasis was that men should continue to practise the exercises both during and after the end of the therapy period. The choice of nine strong contractions twice a day is not far removed from the NICE recommendations of eight contractions three times a day. 72
Standardisation of the therapy intervention
We tried to ensure standardisation in the delivery of the intervention by inviting all therapists to undertake a bespoke training programme. The programme and the intervention were formalised using standardised therapy documentation (see Appendix 4), which ensured a consistent content at each visit and between different therapists. The content of the documentation was based on similar documents used in a previous trial of PFMT for women with prolapse, and was also linked to that of the questionnaire (ICI-SF) that was used to obtain urinary outcome data from the men.
Prevalence of help and support services and information for men with urinary incontinence
Men in both groups had access to any standard care that would normally be provided locally for men with urinary incontinence. This would include the service provision of a continence nurse or a community nurse, who would provide continence aids, as well as general advice, which could include verbal instruction and leaflets on PFMT.
We recognised that this advice on PFMT might potentially dilute the measurable effect of our intensive PFMT intervention. Therefore, if a centre was to participate in MAPS, the staff had to agree that they would not provide specific instruction in pelvic floor anatomy, demonstrate PFMT or suggest a daily PFMT exercise regimen. However, they were permitted to provide a PFMT leaflet if this was part of their standard pattern of care. In addition, most men could and did access any care they needed, which included information on pelvic floor exercises from the literature, staff and elsewhere (see Tables 15 and 53). It might be that this would be sufficient for men to be able to perform adequate PFMT without the need for specialist advice from a trained therapist.
Effect of prior knowledge of pelvic floor exercises and provision of advice on practice of pelvic floor muscle training
It was clear that almost all of the men having a radical prostatectomy, and 80% of those having a TURP, were aware of the use of PFMT after operation, and they derived this information from a variety of sources (see Tables 15 and 53). Before randomisation to the intervention, in the radical RCT, 80% of the men in both the intervention and the control groups were initially performing at least some PFMT at baseline (see Table 36), compared with only 20% in each of the TURP groups (see Table 74). In the control groups, around 50% of the men in the radical trial were still performing some exercises at 12 months, while the proportion in the TURP trial remained the same as at baseline (20%). This could be regarded as an estimate of the background effect of the provision of PFMT advice outwith a specialist service.
In both trials the proportion of men continuing to do the exercises at 12 months was greater in the intervention group (radical prostatectomy 67%, TURP 65%) than in the control groups (50% and 20% respectively). Therefore, the therapists had succeeded in motivating the men to carry out more exercises. Nevertheless, this did not result in any difference in urinary or other clinical outcomes.
Choice of clinical effectiveness outcome measures
Urinary incontinence
We started from the premise that the outcomes of importance were those that mattered to the men. Clinicians’ assessments of patients’ outcomes often underestimate the degree of bother perceived by patients, and tend to focus on issues of lesser importance to patients. 73,74 The wide variety of different data collection methods and instruments limits the ability of researchers to compare similar clinical and research data. In the latest iteration of the ICI, Staskin et al. devote a chapter to the assessment of ‘patient-reported outcomes’ and provide grades of recommendation for a wide range of different instruments developed for this purpose. 73
As a result of an ICI initiative after the first meeting in 1998, an international advisory board was tasked to develop modular ICI questionnaires on each of the clinical issues in incontinence. This was quickly expanded to include wider urinary symptoms, bowel symptoms and vaginal symptoms. 18 The first fruit of this process was the ICI-SF urinary incontinence questionnaire, and we adopted this for our screening questionnaire. We felt that it was short and easy to complete and reliably assessed the aspects of urinary incontinence in which we were interested. This formed the basis for our assessment of male urinary incontinence at each time point.
The questionnaire has now been in use for about 5 years, but when MAPS was starting we felt that we needed another, more ‘objective’ (though still patient-reported), outcome measure. The most common method was to ask men to complete a urinary diary. In the past, researchers have experienced poor return rates for these diaries, which require participants to record fluid input and, worse, urine output for 3 or 5 days. We decided to reduce the burden of completion of diaries by simplifying it, and indeed obtained return rates of over 80%. There was good concordance between the men’s diary records and their questionnaire responses: for example, the two methods of recording daytime urinary frequency and nocturia agreed remarkably (see Table 29a).
Another aspect of the ICI-SF questions was the ability to generate a urinary incontinence score, as a composite of the amount of incontinence and the men’s assessment of its effect on quality of life. It was also possible to use the ‘quality of life’ question on its own as a measure of the effect of ‘leaking urine on everyday life’. Each separate method of measuring incontinence, however, gave the same general picture: of an improvement in the first 6 months and relatively little change thereafter.
Sexual function outcomes
We addressed this sensitive aspect of male function (symptoms of sexual dysfunction) by using the same outcome measures that were developed and piloted for the ProtecT trial,70 with permission from the study staff. The ICSmale and ICSsex questionnaires in ProtecT have already been used in related research. 75 Use of common and standardised outcome measures will enable direct comparisons to be made between the outcomes of MAPS and those of ProtecT.
Choice of economic effectiveness outcome measures
Both the EQ-5D and SF-12 are recognised measures for the measurement of health-related quality of life. Furthermore, both can be used to provide utility scores that can be used to estimate QALYs. The EQ-5D was taken as the basis of QALY estimates in the base-case analysis, as it is the preferred approach of NICE. 17 However, the SF-12 can be converted into similar population-based scores using an algorithm developed by Brazier et al. ,40 and this approach was used in a sensitivity analysis. The results of the cost-effectiveness analysis, based upon either the EQ-5D or SF-12 data, are, however, similar.
The perspective adopted for the economic analysis was that of both the NHS and the trial participant. This meant that the costs incurred by the NHS and by participants themselves were combined to produce an overall estimate of cost. While such an approach is not recommended by NICE,17 this methodological standpoint is contentious and it was felt that a more informed view about the desirability of the intervention would come from the consideration of a wider perspective.
For the TURP trial, the conclusions were sensitive to the choice of perspective (owing to a trend to more days away from usual activities in the control group). The trend towards fewer days away from usual activities following physical therapy was not consistent with the findings about quality of life or use of health services, so should be treated with caution. In the radical prostatectomy trial the conclusions were unaffected by the choice of perspective. A second analysis using an NHS-only perspective was also performed (i.e. only those costs that fell on the NHS were included and those that fell on the participants were excluded). For both trials combining information on the cost of the interventions and the cost of subsequent NHS care resulted in a statistically significantly higher total cost per participant in the intervention group. This difference was driven almost entirely by the cost of the PFMT intervention itself.
Long-term follow-up and potential for further research
When signing the original consent form in hospital, men also consented to being approached about other research in the future. This enabled us to apply to the ethics committee to commence long-term follow-up.
Two-year follow-up is ongoing. This will include information about persistent incontinence, the continuing need for treatment and whether the men have made use of any services (such as pads or surgery). The conclusions of the economic analysis will be reviewed in the light of longer-term follow-up data obtained.
We carried out a short survey to determine the level of provision of services at each site, both by asking the men about the services they used or needed and by asking the staff about what they provided.
Generalisability
Centres
The centres varied widely in size, with our largest centre approaching a total of 118 men having a radical prostatectomy and 661 having a TURP, while in the smallest, one man had a radical prostatectomy and 30 had a TURP.
Some centres, however, did not agree to recruit men from one of the clinical groups. In seven centres, we were able to recruit only men having TURP. This occurred most often because a service was already in place for men after radical surgery that contained specific instruction in PFMT. Centre staff were reluctant to unpick this aspect of their service (which formed part of a larger service addressing other continence needs). In one centre we did not recruit men having TURP because they treated such a large volume of men that recruitment would have been impractical. In a further three centres, which joined MAPS only for the final few months, we targeted recruitment for the radical prostatectomy group only as we needed to increase our sample size before the study finished.
Therapists
Because of the lack of physiotherapy availability in some centres, we allowed specialist continence or urology nurses to be trained as therapists: they delivered the intervention in about half of the centres and to just over half of the men. This would have increased the generalisability of the trial if the intervention were to be rolled out to the NHS. As specialist physiotherapists are in short supply, it would be possible to train other staff to deliver the PFMT intervention.
Men undergoing prostate surgery
Within each centre, we aimed to explain the research study to all the men admitted during the recruitment period. Owing to unavoidable local factors, such as holidays, some men were missed (154/1158 radical prostatectomies, 1078/5986 TURPs; see Table 9a), and a further 20% did not wish to participate in research. Many men also became ineligible when it became apparent that they did not meet the inclusion criteria (for example, almost 500 of the men admitted for TURP were in fact having a palliative ‘channel TURP’ for advanced local prostate cancer). The ‘ineligible’ men were older than those who were eligible for screening (see Table 9b). Some men were also not screened as, after consenting before operation, they became ineligible because in the event they did not undergo a prostatectomy (24/804 radical prostatectomies, 147/2985 TURPs; see Table 10a). Thus, apart from the men who declined participation in screening, we felt that we avoided systematic biases in recruiting our sample, and that we screened a representative sample of the population of eligible men undergoing prostate surgery.
Our trial was aimed at men who were still incontinent at 6 weeks after surgery. A considerable proportion of the men were known to be dry by the time they could have been randomised (113/742 radical prostatectomies, 1661/2590 TURPs) and a further number explicitly declined randomisation, did not return their baseline questionnaire or had another reason why they could not participate (218/742 and 489/2590 respectively). Thus, the men entering the two trials represented 411/742 (55%) of men undergoing radical surgery and 442/2590 (17%) undergoing TURP. These figures compare with our original estimates before the trial started that 50% of the men after radical prostatectomy and 5% after TURP would be randomised.
Timing of the intervention
Nearly all of the improvement in incontinence happened within the first 8 months after surgery for the radical prostatectomies (see Table 29a and Figures 14 and 15) and 5 months for the TURPs (see Table 67a and Figures 39 and 40). In retrospect, it might have been better to wait until at least 6 months after surgery before randomising men who were then still incontinent to specialist therapy. This would then have been targeted at men with a persistent problem (avoiding unnecessary treatment of men who recovered spontaneously). The MAPS findings cannot be extrapolated to this group of men, and we know of no evidence to suggest that a delayed intervention would have been more effective. However, it is clear that a substantial proportion of men remain wet with or without specific instruction.
Need for further treatment
At the end of follow-up at 12 months, around 14 months after surgery, a substantial proportion of men were still incontinent. Based on the original populations, 299/742 (40%) in the radical group and 251/2590 (10%) in the TURP group were still incontinent a year after surgery. For men with severe urinary incontinence (defined as incontinence at least once a day and a moderate or large amount of urine loss), the equivalent figures were radical prostatectomies, 152/742 (20%); and TURPs, 97/2590 (4%). Forty per cent of the men who had radical prostatectomy and 20% of those who had TURP were still using pads.
Although we are still carrying out follow-up at 2 years for these men, it seems likely that this provides a reasonable estimate of the men who are likely to have a continuing problem. We have now shown that four specialised sessions of conservative therapy have not benefited the men and are not likely to be cost-effective, but a considerable number of men are still severely affected by urinary incontinence. It is important to consider what further treatment can be offered to these men.
Need for further research
Surgical treatment for men with persistent urinary incontinence
For some men it may be appropriate to consider a surgical intervention. The options include an artificial sphincter that can be inflated and deflated to permit continence and micturition respectively. More recently, a mesh sling analogous to the minimally invasive slings used in women (such as tension-free vaginal tape and transobturator tape) has been introduced. A new RCT comparing these two options might be an appropriate way of evaluating their relative effectiveness and cost-effectiveness.
While NICE recommends surgery for men with intractable and bothersome SUI,17 there is no consensus on which surgery is most effective and cost-effective. The recommendation was based on case series evidence only. The MAPS study has shown that, based on the original number of men having prostate surgery, the proportion of men with severe urinary incontinence (defined as incontinence at least once a day and a moderate or large amount) amongst the radical prostatectomy group was 152/742 (20%) and amongst the TURP group 97/2590 (4%).
The type of incontinence in the radical group was mostly SUI, for which surgery might be appropriate. Current management advice from NICE17 is in favour of the artificial urinary sphincter. This operation has been available for SUI after prostatectomy for several years. Recently, transobturator ‘minimally invasive’ slings have been marketed for men,76 extrapolated from their use to treat SUI in women. Published evidence for these male slings is sparse and usually single-centre, uncontrolled case series. Men with SUI are increasingly aware of the sling option and often enquire about it in clinic.
NICE interventional procedure guidance suggests that current evidence (from case series only) on the safety and efficacy of suburethral synthetic sling insertion appears adequate to support the use of this procedure under normal arrangements for consent and audit. 76 However, this suggests that such male sling surgery ‘should only be undertaken by units that specialise in the investigation and treatment of postprostatectomy incontinence and that can offer alternative treatments, including the insertion of artificial urinary sphincters’. 76 Adverse effects of male slings were reported, and there was no evidence comparing them with other procedures.
Relative efficacy and adverse effects of the male sling are thus uncertain, and therefore a randomised trial comparing the artificial urinary sphincter with the male sling would be a high priority. Based on a population of 4000 men having radical prostatectomy annually in England, and 20% of those men having severe persistent SUI, up to 800 men per year might be available for a trial. Such a trial should include an economic evaluation.
As artificial urinary sphincter is normally performed only in tertiary centres, only a limited number of urological surgeons would be available to collaborate in such a trial but they would already be treating the majority of eligible men. A collaboration amongst these urologists might feasibly result in a rigorous RCT, which would in turn guide future management of men with persistent and severe urinary incontinence after prostate surgery.
Another possibility that could be tested is the addition of an anti-incontinence procedure such as a sling during the initial radical prostatectomy operation.
Validation of ICI-SF and other outcome measures
A large number of longitudinal data were collected on a variety of measures of urinary, bowel and sexual function outcomes, largely using the instruments developed, or being developed, by the ICI initiative. 27 Quantitative analysis, and work comparing the data with those from the urinary diaries, will allow further validation of these outcome measures for use in future research and clinical settings.
Long-term epidemiology after different types of prostate surgery
The men who agreed to be screened in MAPS also agreed to be contacted in the future about other research related to men’s health after prostate surgery. Some valuable epidemiological information was provided in the short term by their responses to the screening survey. We could capitalise on this by carrying out further epidemiological research in this cohort of men. We have detailed information on their clinical characteristics and baseline level of incontinence. This might inform the debate about different methods of prostatectomy and provide valuable prognostic information for counselling men in the future.
In addition, long-term follow-up of the men enrolled in the MAPS RCT would provide useful clinical and epidemiological information about the consequences of urinary incontinence, in particular the need for and use of NHS services, use of products such as pads and catheters, the need for admission to residential or nursing home care and the chance of receiving surgery.
Meaning of the study/relationship to other work in the field
The data from the MAPS study will be added to the Cochrane review in order to supplement the existing body of knowledge. The MAPS trial, however, will more than double the number of existing data on the treatment of men with incontinence after a radical prostatectomy, and provides unique data on men having a TURP.
Chapter 16 Implications for the NHS and further research
Implications for the NHS and patients
Incontinence is a major complication of prostate surgery. It does, however, resolve in a proportion of those initially affected. For those for whom incontinence does not quickly resolve, additional physical therapy for incontinence is unlikely to be effective or cost-effective compared with the current practice of provision of information about PFMT. This suggests that, in centres routinely offering specific PFMT therapy in one-to-one consultations with a trained physiotherapist or continence nurse for all men who are incontinent after prostate surgery, it may be possible to reallocate resources with potentially no loss of benefit.
Incontinence that persists into the longer term represents a considerable continuing burden to both the NHS and the men affected. Further management of these men is necessary.
Unanswered questions and further research
Treatment for men with urinary incontinence after prostate surgery
-
Physical therapy of the type used in this trial is not worthwhile, but the continuing burden of incontinence suggests that research into other treatments would be, for example research on the value of surgery in controlling symptoms. Specifically, an RCT comparing different surgical options for men with severe persistent urinary incontinence is needed (see Chapter 15).
-
While the MAPS study has demonstrated that specific instruction in PFMT from a therapist is not more effective than standard management, nevertheless many men are advised to carry out PFMT and indeed do so. However, MAPS did not test whether any other method of provision of advice about PFMT would be an effective and efficient way of reducing incontinence. If not, this would represent a waste of resources in teaching men or providing leaflets, and a waste of their own time in practising the exercises. Further research into the effectiveness of any other method of delivery of PFMT would be worthwhile.
Treatment for men with erectile dysfunction after prostate surgery
-
Of the men in the radical prostatectomy trial, 80% still had erectile dysfunction at the 12-month follow-up, and over 60% had tried various treatments. As PFMT was of no value to these men, research into effective and efficient treatment would be worthwhile. While men who did not also have urinary incontinence were not included in the RCT, it is possible that erectile dysfunction is equally prevalent in that group and might also merit further research and evaluation of treatment.
Validation of ICI-SF and other outcome measures
-
The MAPS data set can be used to improve the quality of further research and to improve other aspects of management. The data collected within MAPS can be used to further validate the ICI outcome measures for use in future research and clinical settings. These will support the work on standardised outcome measures being developed by the ICI initiative. 27 This would include quantitative analysis and work comparing the data with those from the urinary diaries.
Long-term consequences of different types of prostate surgery
-
Detailed epidemiological data gathered within MAPS can be analysed and will allow prospective follow-up of the men. This will inform the debate about different methods of prostatectomy and provide valuable prognostic information for counselling men in the future. Issues include the consequences of urinary incontinence, in particular the need for and use of NHS services, use of products such as pads and catheters, the need for admission to residential or nursing home care and the chance of receiving surgery.
Acknowledgements
This study would not have been possible without the help and involvement of all the men who so willingly completed their questionnaires and diaries and turned up for their therapy appointments. We are also very grateful to the staff at each of our centres for recruiting and motivating our participants. We thank the NIHR HTA programme for funding this study, and the staff of the HTA programme for their helpful administrative support.
The MAPS study office was based in CHaRT within the Health Services Research Unit, University of Aberdeen, and much of the success of the trial is due to the dedication of our study office staff: Claire Cochran, Louise Campbell, Lynne Swan, Diane Collins and Janice Cruden. Statistical support from Charles Boachie and Craig Ramsay was invaluable, as was the economics input from Mary Kilonzo and Luke Vale, and the information technology and database support from Gladys McPherson.
We also thank the members of the Trial Steering Committee [Professor Paul Abrams (Chair), Mrs Jane Dixon, Professor David Torgerson and Professor John Verrier-Jones] and the Data Monitoring Committee [Professor Peter Langhorne (Chair), Mrs Julia Brown, Mr Thomas McNicholas and Professor Christine Norton], whose voluntary support and advice were essential to the success of MAPS.
Sincere thanks are due to the staff of the MREC in Edinburgh, particularly Mrs Dorothy Garrow, for dealing with our 16 substantive amendments between 2004 and 2008. We are also grateful to the National Cancer Research Network (NCRN) and Professor Chris Parker of the RADICALS trial for access to men from extra centres, and to Professor Freddie Hamdy and Dr Athene Lane of the ProtecT trial, who provided access to outcome measures of male sexual dysfunction.
In addition, we are grateful to Kirsty Gordon, the first MAPS trial manager; John Norrie, the first director of CHaRT; and to the staff of the Health Services Research Unit, University of Aberdeen, including Marion Malcolm, Kathleen McIntosh, Karen McLeod and Julie Murdoch, for sterling support. We also thank the proofreaders: Susan Campbell, Clare Robertson, Seonaidh Cotton, Jonathan Cook and Jenni Hislop.
Particular thanks are due to Adrian Grant, without whose vision and drive this study would not have existed or been so successfully completed.
Contribution of authors
Professor Cathryn Glazener (Professor of Health Services Research, chief investigator) was the chief investigator of the study: she had complete involvement in and oversight of the study design, execution and data collection, and was responsible for the writing of the final report.
Mr Charles Boachie (Statistician, statistical analysis) contributed to the statistical analysis of the study and the writing of the results and discussion chapters.
Dr Brian Buckley (Chairman, Bladder and Bowel Foundation, consumer representative) contributed to the consumer aspect of the study and writing the final report.
Mrs Claire Cochran (Trial Manager), was responsible for the day-to-day management of the study and also contributed to writing the final report.
Professor Grace Dorey (Professor at the University of the West of England, intervention specialist) contributed to the design of the intervention component of the study and was also responsible for training the therapists recruited to provide the intervention to study participants.
Professor Adrian Grant (Director of Research, trialist) contributed to the overall study design and gave expert guidance on the writing of the final report.
Professor Suzanne Hagen (Programme Director, trial design) contributed to the design of the study and also to the choice and design of the outcomes measures.
Miss Mary Kilonzo (Research Fellow, health economics) contributed to the analysis of the health economics component of the study and also to the writing of the health economics chapters.
Mrs Alison McDonald (Senior Trial Manager, trialist) contributed to the design of the study, organised the authorisation of the study and contributed to the writing of the final report.
Mrs Gladys McPherson (Senior IT Manager, programming) designed the programming of the study database, carried out data analysis and contributed to the writing of the final report.
Professor Katherine Moore (Vice Dean, trialist and therapist perspective) contributed to the design of the therapy intervention and to the writing of the final report.
Professor James N’Dow (Professor of Urology, clinician) contributed his clinical expertise to the design of the study and to the final report writing.
Professor John Norrie (Professor of Biostatistics, Statistician) contributed to the design and conduct of the study, and the interpretation of the findings.
Dr Craig Ramsay (Healthcare Assessment Programme Director, Health Services Research Unit, statistical analysis) contributed to the statistical analysis of the study and also to the writing of the results chapters.
Professor Luke Vale (Professor of Health Technology Assessment, Health Economics Research Unit, health economics) contributed to the writing of the health economics chapters and to the interpretation of the health economics findings.
MAPS writing group
Professor Cathryn Glazener, Mr Charles Boachie, Dr Brian Buckley, Mrs Claire Cochran, Professor Grace Dorey, Professor Adrian Grant, Professor Suzanne Hagen, Miss Mary Kilonzo, Mrs Alison McDonald, Mrs Gladys McPherson, Professor Katherine Moore, Professor James N’Dow, Dr Craig Ramsay and Professor Luke Vale.
Project Management Group
Professor Cathryn Glazener, Professor Adrian Grant, Professor Grace Dorey, Professor James N’Dow, Professor Suzanne Hagen, Professor Katherine Moore, Dr Craig Ramsay, Professor Luke Vale, Professor John Norrie, Dr Brian Buckley, Mrs Alison McDonald, Mrs Gladys McPherson and Mrs Claire Cochran.
MAPS principal investigators in centres
Professor James N’Dow, Mr Peter Donaldson, Mr Derek Byrne, Mr Gerald Collins, Mr Muhammad Akhtar, Mr David Chadwick, Mr James Tweedle, Mr Tahseen Hasan, Mr Naeem Shaikh, Mr Peter Malone, Mr Subramanian Kanagasundaram, Mr Robert Meddings, Mr Rajendra Persad, Mr Gregory Boustead, Mr Sudhir Borgaonkar, Mr Owen Cole, Mr Ian Eardley, Mr Geoffrey Orr, Mr Nigel Philp, Mr Malcolm Lucas, Dr Derek Rosario, Mr Shiv Bhanot, Mr Mohammad Vandal, Ms Ling Lee, Mr Ruairidh MacDonagh, Mr Ralph Webb, Mr Tim Porter, Mr Prasad Bolina, Mr Roland Donat, Mr John MacFarlane, Professor Howard Kynaston, Mr David Gillatt, Mr Pradip Javle, Mr Alvan Pope, Mr Anup Patel and Mr Noel Clarke.
MAPS recruitment officers in centres
Mary Simpson, Linda Pennet, Paul Ridley, Chris Garlick, Charlotte Etheridge, Allison Robertson, Sarah McKenna, Jo Butler, Andrew Brown, Aelens Brauckman, Delyth Hague, Louise Taylor, Stephanie Ridgway, Julie Longworth, Jill Taylor, Sarah Mckenna, Kirsty Crozier, Patricia McClurey, Kathleen Riddle, Kathryn Procter, Sam Genner, Wendy Robson, Mark Davies, Nona Toothill, Gill Driver, Karen Wilmott, Navin Ravindranath, Christine Oxnard, Mary Hamilton, Jillian Connell, Katrina Hurley, Gill Larsen, Linda Fowler, Alison Finlay, Alison Steel, Lorraine Lamb, Liz Dalgaty, Brenda McCallum, Andy Smallwood, Jane Griffiths, Kez Richards, Louise Goodwin, Peter Holding, Joanne Howson, Louise Gray, Neale O’Brien, Angela Lee, Dorothy Sugden, Kate Pearce, Laura Jones, Valerie Powell, Margaret Austin, Ruth Broom, Clare Buckley, Debbie Gibbons, Louise Jones, Lisa Egan, Barbara Mayne, Sarah Thompson, Paul Allcoat, Irene Blythe, Samantha Holliday, Claire Jones, Loveness Chikopela, Claire Gaskell, Lisa Hardstaff, Barbara Townley, Marilyn McCurrie, Debbie Delgado, Andrew Harvey, Lisa Saville, Helen Corderoy, Suriya Kirkpatrick, Jenny Butler-Barnes, Vanessa Adamson, Carolyn Mansfield, Mariam Nasseri, Vikram Bohra, Gillian Hornzee and Jill Youd.
MAPS therapists in centres
Linda Pennet, Fiona Lennard, Allison Robertson, James Bolarin, Sue Hallam, Julie Lang, Philip Howard, Ann Gilchrist, Kath Moore, Madeline Frank, Karen Wilmott, Alison Coughlan, Annette Bell, Debbie Rigby, Gill Larsen, Alison Finlay, Elaine Cathcart, Lorraine Lamb, Ada Cardiff, Fiona Jarvis, Maureen Lumber, Louise Goodwin, Ann Evans, Kirsty Moore, Valerie Powell, Caroline Gill, Jill Branson, Kay Hildersley, Linda Haworth, Ann Yeats, Fiona Key, Debbie Delgardo, Margaret Atherton, Mariam Nasseri, Julia Muman and Jill Youd.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Gray M, Petroni GR, Theodorescu D. Urinary function after radical prostatectomy: a comparison of the retropubic and perineal approaches. Urology 1999;53:881-90.
- Mazur DJ, Merz JF. Older patients’ willingness to trade off urologic adverse outcomes for a better chance at five-year survival in the clinical setting of prostate cancer. J Am Geriatrics Soc 1995;43:979-84.
- Seaman EK, Jacobs BZ, Blaivas JG, Kaplan SA. Persistence or recurrence of symptoms after transurethral resection of the prostate: a urodynamic assessment. J Urol 1994;152:935-7.
- Warwick RT, Whiteside CG, Arnold EP, Bates CP, Worth PH, Milroy EG, et al. A urodynamic view of prostatic obstruction and the results of prostatectomy. Br J Urol 1973;45:631-45.
- Koelbl H, Nitti V, Baessler K, Salvatore S, Sultan A, Yamaguchi O. Incontinence: Fourth International Consultation on Incontinence. Paris: Health Publications Ltd; 2009.
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4-20.
- Abrams P, Cardozo LD, Fall M, Griffiths DJ, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn 2002;21:167-78.
- Milsom I, Altman D, Lapitan MC, Nelson R, Sillen U, Thom D. Incontinence: Fourth International Consultation on Incontinence. Paris: Health Publications Ltd; 2009.
- Kao TC, Cruess DF, Garner D, Foley J, Seay T, Friedrichs P, et al. Multicenter patient self-reporting questionnaire on impotence, incontinence and stricture after radical prostatectomy. J Urol 2000;163:858-64.
- Olsson LE, Salomon L, Nadu A, Hoznek A, Cicco A, Saint F, et al. Prospective patient-reported continence after laparoscopic radical prostatectomy. Urology 2001;58:570-2.
- Eastham JA, Kattan MW, Rogers E, Goad JR, Ohori M, Boone TB, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol 1996;156:1707-13.
- Lowe BA. Comparison of bladder neck preservation to bladder neck resection in maintaining postprostatectomy urinary continence. Urology 1996;48:889-93.
- McCammon KA, Kolm P, Main B, Schellhammer PF. Comparative quality-of-life analysis after radical prostatectomy or external beam radiation for localised prostate cancer. Urology 1999;54:509-16.
- Saranchuk JW, Kattan MW, Elkin E, Touijer K, Scardino PT, Eastham JA. Achieving optimal outcomes after radical prostatectomy. J Clin Oncol 2005;23:4146-51.
- Emberton M, Neal DE, Black N, Fordham M, Harrison M, Mcbrien MP, et al. The effect of prostatectomy on symptom severity and quality of life. Br J Urol 1996;77:233-47.
- National Health Service . Hospital Episode Statistics n.d. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937 (accessed January 2010).
- National Institute for Health and Clinical Excellence . Prostate Cancer: Diagnosis and Treatment 2008.
- Cottenden A, Bliss DZ, Buckley B, Fader M, Getliffe K, Paterson J, et al. Incontinence: Fourth International Consultation on Incontinence. Paris: Health Publications Ltd; 2009.
- Hay-Smith EJ, Berghamns LC, Burgio K, DuMoulin C, Moore KN, Nygaard I, et al. International Consultation on Urinary Incontinence. Plymouth, UK: Health Publications Ltd; 2009.
- Herschorn S, Bruschini H, Comiter C, Grise P, Hanus T, Kirschner-Hermanns R. Incontinence: Fourth International Consultation on Incontinence. Paris: Health Publications Ltd; 2009.
- Moore K, Cody DJ, Glazener C, Grant AM, Cody DJ, Glazener CMA, et al. The Cochrane Library, Issue 1. Oxford: Update Software; 1999.
- Bales GT, Gerber GS, Minor TX, Mhoon DA, McFarland JM, Kim HL, et al. Effect of preoperative biofeedback/pelvic floor training on continence in men undergoing radical prostatectomy. Urology Online 2000;56:627-30.
- Parekh AR, Feng MI, Kirages D, Bremner H, Kaswick J, Aboseif S. The role of pelvic floor exercises on post-prostatectomy incontinence. J Urol 2003;170:130-3.
- Van Kampen M, De Weerdt W, Van Poppel H, De Ridder D, Feys H, Baert L. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet 2000;355:98-102.
- Burford DC, Kirby M, Austoker J. Prostate cancer risk management programme: information for primary care; PSA testing in asymptomatic men. NHS Cancer Screening Programmes; 2009.
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev 2009;1.
- Bristol Urological Institute . International Consultation on Incontinence Modular Questionnaire (ICIQ) n.d. www.iciq.net.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6.
- White IR. Uses and limitations of randomization-based efficacy estimators. Statistical Methods Med Res 2005;14:327-47.
- Nagelkerke N, Fidler V, Bernsen R, Borgdorff M. Estimating treatment effects in randomized clinical trials in the presence of non-compliance. Stat Med 2000;19:1849-64.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary. 2009.
- Department of Health . NHS Reference Costs n.d. www.dh.gov.uk/en/publicationandstatistics/publication/publicationspolicyandguidance (accessed September 2009).
- Curtis L, Netten A. The costs of training a nurse practitioner in primary care: the importance of allowing for the cost of education and training when making decisions about changing the professional-mix. J Nurs Manag 2007;15:449-57.
- National Health Service (NHS) in Scotland Information and Statistics Division (ISD) . Scottish Health Services Costs. n.d. www.isdscotland.org/isd (accessed September 2009).
- Department of Health . Reference Costs. n.d. www.dh.gov.uk/en/publicationsandstatistics (accessed September 2009).
- HM Revenue and Customs . EIM31240 – Employees Using Own Vehicles for Work: Statutory Mileage Rates 2002 03 Onwards: Kinds of Vehicle n.d. www.hmrc.gov.uk (accessed September 2009).
- Office of National Statistics . Average Hourly Earning (without overtime). New Earning Survey Data Base. n.d. www.ons.gov.uk (accessed September 2009).
- Department of Transport . COBA 9 Manual 1989.
- EuroQol . EuroQol – a new facility for the measurment of health-related quality of life. Health Policy 1993;16:199-208.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF–36. J Health Econ 2002;21:271-92.
- Hunter KF, Moore KN, Glazener CMA. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev 2007;2.
- Dorey G, Speakman M, Feneley R, Swinkels A, Dunn C, Ewings P. Randomised controlled trial of pelvic floor muscle exercises and manometric biofeedback for erectile dysfunction. Br J Gen Pract 2004;54:819-25.
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev 2000;1.
- Stephens GR, Haslam J, Laycock J. Therapeutic management of incontinence and pelvic pain. London: Springer-Verlag; 2008.
- Beasley W. Quantitative muscle testing: principles and applications to research and clinical services. Arch Phys Med Rehabil 1961;42:398-425.
- Dorey G, Sultan H, Eckford S, Parker J, Lowe D. Pelvic floor muscle exercises and urge suppression technique in women with urge urinary incontinence: a retrospective study in a dedicated urogynaecology clinic. J Assoc Chart Physiother Womens Health 2006;99:43-8.
- Miller J, Ashton-Miller JA, DeLancey JOL. The Knack: use of precisely-timed pelvic muscle contraction can reduce leakage in SUI. Neurourol Urodyn 1996;15:1280-6.
- Buckley B. It’s the way you ask that matters: comparison of data relating to prevalence of incontinence aid use from two surveys of people with multiple sclerosis. J Wound Ostomy Continence Nurs 2006;33:26-9.
- Wyndaele JJ, Van Eetvelde B. Reproducibility of digital testing of the pelvic floor muscles in men. Arch Phys Med Rehabil 1996;77:1179-81.
- Burgio KL, Stutzman RE, Engel BT. Behavioral training for post-prostatectomy urinary incontinence. J Urol 1989;141:303-6.
- Paterson J, Pinnock CB, Marshall VR. Pelvic floor exercises as a treatment for post-micturition dribble. Br J Urol 1997;79:892-7.
- Moore KN, Valiquette L, Chetner MP, Byrniak S, Herbison GP. Return to continence after radical retropubic prostatectomy: a randomised trial of verbal and written instructions versus therapist-directed pelvic floor muscle therapy. Urology 2008;72:1280-6.
- DiNubile NA. Strength training. Clin Sports Med 1991;10:33-62.
- Kegel AH. Physiologic therapy for urinary incontinence. JAMA 1951;146:915-7.
- Guyton AC. Textbook of medical physiology. Philadelphia, PA: WB Saunders; 1986.
- Gosling JA, Dixon JS, Critchley HOD, Thompon SA. A comparative study of the human external sphincter and periurethral levator ani muscles. Br J Urol 1981;53:35-41.
- Gordon H, Logue M. Perineal muscle function after childbirth. Lancet 1985;2:123-5.
- Wille S, Mills RD, Studer UE. Absence of urethral post-void milking: an additional cause for incontinence after radical prostatectomy?. Eur Urol 2000;37:665-9.
- Shafik A, El-Sibai O. Mechanism of ejection during ejaculation: identification of a urethrocavernosus reflex. Archives Androl 2000;44:77-83.
- Claes H, Bijnens B, Baert L. The hemodynamic influence of the ischiocavernosus muscles on erectile function. J Urol 1996;156:986-90.
- Wilson PD, Berghmans B, Hagen S, Abrams P, Cardozo L, Khoury S. Incontinence management. Plymouth, UK: Health Publications; 2005.
- Valtin H. ‘Drink at least eight glasses of water a day.’ Really? Is there scientific evidence?. Am J Physiol Regulatory Integrative Comparative Physiol 2002;283:R993-R1004.
- Haidinger G, Temml C, Schatzl G, Brössner C, Roehlich M, Schmidbauer CP, et al. Risk factors for lower urinary tract symptoms in elderly men. Eur Urol 2000;37:413-20.
- Jackson J, Emerson L, Johnston B, Wilson J, Morales A. Biofeedback: a noninvasive treatment for incontinence after radical prostatectomy. Urol Nurs 1996;16:50-4.
- Dorey G, Glazener C, Buckley B, Cochran C, Moore K. Developing a pelvic floor muscle training regimen for use in a trial intervention. Physiotherapy 2009;95:199-20.
- Bishoff JT, Motley G, Optenberg SA, Stein CR, Moon KA, Browning SM, et al. Incidence of faecal and urinary incontinence following radical perineal and retropubic prostatectomy in a national population. J Urol 1998;160:454-8.
- Wei JT, Dunn RL, Marcovich ROBE, Montie JE, Sanda MG. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol 2000;164:744-8.
- Van Kampen M, De Weerdt W, Van Poppel H, Feys H, Campesino AC, Stragier J, et al. Prediction of urinary continence following radical prostatectomy. Urol Int 1998;60:80-4.
- Hagen S, Glazener C, Cochran C, Campbell L. Continence care for men having prostate surgery: advice and care before and after radical prostatectomy and TURP. Neurourol Urodyn 2009;28:690-1.
- Frankel SJ, Donovan JL, Peters TI, Abrams P, Dabhoiwala NF, Osawa D, et al. Sexual dysfunction in men with lower urinary tract symptoms. J Clin Epidemiol 1998;51:677-85.
- Scottish Intercollegiate Guidelines Network . Management of Urinary Incontinence in Primary Care 2004.
- National Institute for Health and Clinical Excellence . NICE Clinical Guideline 40. Urinary Incontinence: The Management of Urinary Incontinence in Women 2006.
- Staskin D, Kelleher C, Avery K, Bosch R, Cotterill N, Coyne K, et al. Incontinence: Fourth International Consultation on Incontinence. Paris: Health Publications Ltd; 2009.
- Spring S. Guidance for industry – patient reported outcome measures: use in medical product development to support labelling claims. Silver Spring, MD: FDA; 2006.
- Collin SM, Metcalfe C, Donovan JL, Lane JA, Davis M, Neal DE, et al. Associations of sexual dysfunction symptoms with PSA-detected localised and advanced prostate cancer: a case–control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. Eur J Cancer 2009;45:3254-61.
- National Institute for Health and Clinical Excellence . Suburethral Synthetic Sling Insertion for Stress Urinary Incontinence in Men 2008.
Appendix 1 Patient information sheets
Appendix 1.1 Hospital information sheet for Men After Prostate Surgery: research information leaflet for men having prostate surgery
Appendix 1.2 Randomised controlled trial patient information sheet: simple treatment for urinary incontinence in Men After Prostate Surgery. Invitation to help with research
Appendix 2 Consent forms
Appendix 2.1 Screening consent form: your health after prostate surgery
Appendix 2.2 Randomised controlled trial consent form: simple treatment for urinary incontinence in Men After Prostate Surgery
Appendix 3 Questionnaires
Appendix 3.1 MAPS screening questionnaire
Appendix 3.2 MAPS baseline questionnaire
Appendix 3.3 MAPS 12-month questionnaire
Appendix 3.4 MAPS participants’ cost questionnaire
Appendix 3.5 MAPS urinary diary
Appendix 4 Therapy documentation and participants’ advice leaflets
Appendix 4.1 Therapy documentation used during therapy appointments
Appendix 4.2 Lifestyle advice leaflet
Appendix 4.3 Pelvic floor exercises for men taking part in the MAPS study
Appendix 5 Health economics
Resource use tables: radical prostatectomy and transurethral resection of the prostate
| Intervention [mean (SD) n] | Control [mean (SD) n] | Mean difference (95% CI), p-value | |
|---|---|---|---|
| Satisfaction scorea | 7.5 (2.7) 175 | 6.6 (3.3) 158 | 0.83 (0.19 to 1.48), 0.012 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Seen GP [n/N (%)] | 118/205 (58) | 121/205 (59) | 133/200 (67) | 141/198 (71) | 124/199 (62) | 131/197 (66) | 103/191 (54) | 124/194 (64) | 104/196 (53) | 103/196 (53) |
| Number of times for UI | 0.61 (0.82) 85 | 0.56 (0.94) 79 | 0.13 (0.45) 135 | 0.36 (0.96) 145 | 0.17 (0.58) 125 | 0.21 (0.92) 135 | 0.17 (0.56) 106 | 0.20 (0.73) 129 | 0.10 (0.43) 109 | 0.28 (1.17) 108 |
| Number of times for other reason | 1.28 (0.93) 106 | 1.35 (0.91) 118 | 1.89 (1.26) 139 | 2.14 (1.78) 145 | 1.90 (1.51) 126 | 1.75 (1.06) 134 | 1.69 (1.23) 107 | 1.74 (1.44) 129 | 1.73 (1.19) 109 | 1.60 (1.04) 108 |
| Seen nurse [n/N (%)] | 135/205 (66) | 124/203 (61) | 77/200 (39) | 84/198 (42) | 73/199 (37) | 78/197 (40) | 74/191 (39) | 79/194 (41) | 70/196 (36) | 70/196 (36) |
| Number of times for UI | 1.17 (1.66) 90 | 1.54 (3.16) 97 | 0.23 (0.83) 77 | 0.60 (1.60) 84 | 0.15 (0.75) 75 | 0.22 (0.71) 81 | 0.04 (0.19) 82 | 0.07 (0.34) 83 | 0.03 (0.16) 73 | 0.04 (0.20) 73 |
| Number of times for other reason | 2.82 (3.32) 128 | 2.91 (4.10) 118 | 1.45 (1.53) 77 | 1.81 (2.45) 84 | 1.24 (0.65) 76 | 1.23 (0.95) 81 | 1.08 (1.08) 83 | 1.27 (1.16) 82 | 1.32 (0.92) 74 | 1.27 (1.38) 74 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Seen hospital doctor for UI [n/N (%)] | 25/200 (13) | 18/196 (9) | 19/200 (10) | 25/198 (13) | 19/199 (10) | 18/197 (9) | 14/191 (7) | 13/194 (7) | 21/196 (11) | 14/196 (7) |
| Number of times | 1.36 (0.64) 25 | 1.50 (1.69) 18 | 1.24 (0.62) 21 | 1.38 (0.85) 26 | 1.21 (0.79) 19 | 1.17 (0.51) 18 | 1.00 (0.73) 16 | 1.29 (0.61) 14 | 1.29 (0.56) 21 | 1.29 (0.73) 14 |
| Seen hospital nurse for UI [n/N (%)] | 32/187 (17) | 27/191 (14) | 32/178 (18) | 18/176 (10) | 19/178 (11) | 15/183 (8) | 8/179 (4) | 9/172 (5) | 12/181 (7) | 9/179 (5) |
| Number of times | 1.56 (1.05) 32 | 2.25 (4.33) 28 | 2.15 (0.97) 33 | 1.28 (0.75) 18 | 1.63 (0.76) 19 | 1.27 (0.46) 15 | 1.20 (0.92) 10 | 1.20 (0.42) 10 | 1.23 (0.73) 13 | 1.00 (0.47) 10 |
| Seen hospital physiotherapist for UI [n/N (%)] | 2/191 (1) | 3/191 (2) | 51/200 (26) | 5/198 (3) | 15/199 (8) | 5/197 (3) | 2/191 (1) | 3/194 (2) | 1/196 (1) | 5/196 (3) |
| Number of times | 0.50 (0.58) 4 | 0.75 (0.50) 4 | 2.54 (0.85) 52 | 2.60 (1.52) 5 | 1.73 (0.96) 15 | 3.00 (3.39) 5 | 1.20 (0.92) 10 | 1.20 (0.42) 10 | 0.50 (0.71) 2 | 1.00 (0.63) 6 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Seen private doctor for UI n/N (%) | 1/204 (0) | 0/199 (0) | 1/200 (1) | 1/198 (1) | 2/199 (1) | 1/197 (1) | 0/191 (0) | 1/194 (1) | 0/196 (0) | 2/196 (1) |
| Number of times | 2.00 (2.00) 1 | NR | 1.50 (2.12) 2 | 1.00 (0.0) 1 | 4.00 (5.66) 2 | 1.00 (0.0) 1 | 0.00 (0.0) 1 | 1.00 (0.0) 1 | NR | 1.67 (2.08) 3 |
| Seen private nurse for UI n/N (%) | 2/192 (1) | 2/190 (1) | 0/188 (0) | 0/182 (0) | 2/180 (1) | 2/186 (1) | 0/180 (0) | 0/179 (0) | 0/183 (0) | 0/185 (0) |
| Number of times | 1.00 (1.00) 1 | 1.33 (1.53) 3 | NR | 0.0 (0.0) 2 | 0.00 (0.00) 2 | 0.00 (0.00) 2 | 0.00 (0.0) 1 | NR | NR | 0.00 (0.0) 1 |
| Seen private physiotherapist for UI n/N (%) | 1/199 (1) | 2/196 (1) | 2/200 (1) | 2/198 (1) | 1/199 (1) | 0/197 (0) | 0/191 (0) | 0/194 (0) | 0/196 (0) | 0/196 (0) |
| Number of times | 2.00 (2.00) 1 | 0.50 (0.71) 2 | 2.00 (2.00) 3 | 2.00 (2.00) 1 | NR | NR | 0.00 (0.0) 1 | NR | NR | 0.00 (0.0) 1 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Admitted to hospital for UI | 4/202 (2) | 6/205 (3) | 1/200 (1) | 4/198 (2) | 4/199 (2) | 3/197 (2) | 2/191 (1) | 2/194 (1) | 1/196 (1) | 2/196 (1) |
| Operation for UI | 0/127 (0) | 1/118 (1) | 1/200 (1) | 5/198 (3) | 3/199 (2) | 1/197 (1) | 1/191 (1) | 1/194 (1) | 2/196 (1) | 2/196 (1) |
| Number of nights in hospital [mean (SD) n] | 2.50 (3.00) 4 | 2.67 (1.37) 6 | 1.00 (1.41) 2 | 1.00 (0.71) 5 | 0.50 (0.58) 4 | 1.33 (0.58) 3 | 0.67 (1.15) 3 | 1.00 (1.41) 2 | 1.00 (1.41) 2 | 0.33 (0.58) 3 |
| Medication or drugs for UI | 16/202 (8) | 13/203 (6) | 14/200 (7) | 18/198 (9) | 15/199 (8) | 18/197 (9) | 15/191 (8) | 17/194 (9) | 16/196 (8) | 15/196 (8) |
| Other treatment or advice for UI | 60/202 (30) | 57/200 (29) | 53/200 (27) | 17/198 (9) | 22/199 (11) | 14/197 (7) | 7/191 (4) | 11/194 (6) | 7/196 (4) | 8/196 (4) |
| Intervention [mean (SD) n] | Control [mean (SD) n] | Mean difference (95% CI), p-value | |
|---|---|---|---|
| Satisfaction scorea | 7.7 (3.0) 164 | 6.5 (3.4) 142 | 1.15 (0.43 to 1.88), 0.002 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Seen GP [n/N (%)] | 131/220 (60) | 127/218 (58) | 122/206 (59) | 119/208 (57) | 117/201 (58) | 101/203 (50) | 109/198 (55) | 108/202 (53) | 100/194 (52) | 108/204 (53) |
| Number of times for UI | 1.23 (1.25) 65 | 0.88 (1.07) 80 | 0.26 (0.68) 122 | 0.35 (0.79) 119 | 0.20 (0.83) 120 | 0.27 (0.74) 103 | 0.10 (0.60) 113 | 0.05 (0.27) 110 | 0.16 (0.58) 100 | 0.07 (0.32) 113 |
| Number of times for other reason | 1.47 (1.00) 115 | 1.67 (1.39) 125 | 2.11 (1.82) 123 | 2.07 (1.62) 121 | 2.07 (1.38) 121 | 2.00 (1.41) 104 | 1.97 (1.34) 116 | 2.02 (1.32) 113 | 2.18 (1.33) 101 | 1.99 (1.43) 119 |
| Seen nurse [n/N (%)] | 51/217 (24) | 50/219 (23) | 66/206 (32) | 73/208 (35) | 75/201 (37) | 68/203 (33) | 72/198 (36) | 77/202 (38) | 75/194 (39) | 73/204 (36) |
| Number of times for UI | 1.00 (0.94) 26 | 0.82 (1.03) 34 | 0.38 (1.63) 66 | 0.45 (1.86) 73 | 0.15 (0.54) 75 | 0.10 (0.53) 73 | 0.03 (0.16) 77 | 0.04 (0.19) 83 | 0.08 (0.36) 75 | 0.03 (0.16) 77 |
| Number of times for other reason | 1.11 (0.88) 46 | 1.60 (2.28) 50 | 1.94 (2.13) 66 | 1.66 (1.56) 73 | 1.89 (1.72) 81 | 1.92 (2.45) 74 | 1.71 (1.80) 78 | 2.11 (3.00) 84 | 1.82 (1.52) 77 | 2.22 (3.24) 78 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Seen hospital doctor for UI [n/N (%)] | 14/216 (6) | 11/215 (5) | 23/206 (11) | 29/208 (14) | 8/201 (4) | 18/203 (9) | 9/198 (5) | 13/202 (6) | 8/194 (4) | 12/204 (6) |
| Number of times | 1.14 (0.36) 14 | 2.00 (2.37) 11 | 1.58 (2.28) 24 | 1.23 (0.63) 30 | 1.22 (0.83) 9 | 1.05 (0.51) 20 | 1.44 (1.74) 9 | 1.00 (0.41) 13 | 1.30 (1.06) 10 | 0.86 (0.86) 14 |
| Seen hospital nurse for UI [n/N (%)] | 3/185 (2) | 8/189 (4) | 31/163 (19) | 12/173 (7) | 9/169 (5) | 9/173 (5) | 5/169 (3) | 3/181 (2) | 1/170 (1) | 1/174 (1) |
| Number of times | 1.50 (0.58) 4 | 2.25 (2.76) 8 | 2.41 (1.05) 34 | 1.42 (0.79) 12 | 2.30 (1.95) 10 | 1.00 (0.77) 11 | 0.57 (0.53) 7 | 1.00 (0.82) 4 | 0.33 (0.58) 3 | 0.50 (1.00) 4 |
| Seen hospital physiotherapist for UI [n/N (%)] | 1/205 (0) | 1/204 (0) | 43/206 (21) | 3/208 (1) | 27/201 (13) | 3/203 (1) | 4/198 (2) | 1/202 (0) | 4/194 (2) | 1/204 (0) |
| Number of times | 1.00 (0.00) 1 | 2.00 (0.00) 1 | 2.53 (0.69) 45 | 1.33 (0.58) 3 | 2.11 (1.45) 28 | 1.00 (1.00) 5 | 1.80 (2.39) 5 | 1.00 (1.41) 2 | 0.83 (0.75) 6 | 0.50 (1.00) 4 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Seen private doctor for UI [n/N (%)] | 0/215 (0) | 1/218 (0) | 0/206 (0) | 1/208 (0) | 0/201 (0) | 2/203 (1) | 0/198 (0) | 0/202 (0) | 0/194 (0) | 0/204 (0) |
| Number of times | NR | NR | NR | 1.00 (0.00) 1 | NR | NR | NR | NR | NR | NR |
| Seen private nurse for UI [n/N (%)] | 0/191 (0) | 1/194 (1) | 0/181 (0) | 0/182 (0) | 0/179 (0) | 1/175 (1) | 0/178 (0) | 0/184 (0) | 0/176 (0) | 0/178 (0) |
| Number of times | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Seen private physiotherapist for UI [n/N (%)] | 1/207 (0) | 1/206 (0) | 0/206 (0) | 1/208 (0) | 1/201 (0) | 0/203 (0) | 0/198 (0) | 1/202 (0) | 0/194 (0) | 1/204 (0) |
| Number of times | 1.00 (0.00) 1 | 8.00 (0.00) 1 | NR | NR | NR | 1.00 (0.00) 1 | NR | 10.00 (0.00) 1 | NR | 12.00 (0.00) 1 |
| Resource | Baseline | 3 months | 6 months | 9 months | 12 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Admitted to hospital for UI | 6/220 (3) | 4/221 (2) | 5/206 (2) | 5/208 (2) | 3/201 (1) | 4/203 (2) | 0/198 (0) | 1/202 (0) | 0/194 (0) | 1/204 (0) |
| Operation for UI | 2/128 (2) | 1/126 (1) | 2/206 (1) | 6/208 (3) | 0/201 (0) | 4/203 (2) | 0/198 (0) | 0/202 (0) | 0/194 (0) | 2/204 (1) |
| Number of nights in hospital [mean (SD) n] | 2.40 (1.52) 5 | 2.00 (2.38) 7 | 1.00 (1.73) 5 | 1.20 (1.30) 5 | 2.67 (1.53) 3 | 1.00 (1.22) 5 | NR | 0.00 (0.00) 4 | NR | 0.00 (0.00) 2 |
| Medication or drugs for UI | 24/217 (11) | 25/216 (12) | 16/206 (8) | 25/208 (12) | 14/201 (7) | 19/203 (9) | 10/198 (5) | 18/202 (9) | 9/194 (5) | 17/204 (8) |
| Other treatment or advice for UI | 10/216 (5) | 13/213 (6) | 44/206 (21) | 13/208 (6) | 21/201 (10) | 8/203 (4) | 3/198 (2) | 3/202 (1) | 4/194 (2) | 6/204 (3) |
Appendix 6 MAPS study protocol
List of abbreviations
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- BMI
- body mass index
- BNI
- bladder neck incision
- BT
- bladder training
- EQ-5D
- European Quality of Life-5 Dimensions
- ICER
- incremental cost-effectiveness ratio
- ICI-SF
- International Consultation on Incontinence Short Form questionnaire
- ICI-QoL
- International Consultation on Incontinence Quality of Life (score)
- ICI-UI
- International Consultation on Incontinence Urinary Incontinence (score)
- MAPS
- Men After Prostate Surgery (trial)
- MREC
- Multicentre Research Ethics Committee
- MUI
- mixed urinary incontinence
- NICE
- National Institute for Health and Clinical Excellence
- PFMT
- pelvic floor muscle training
- PML
- postmicturition leakage
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- risk ratio
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- SUI
- stress urinary incontinence
- TURP
- transurethral resection of the prostate
- UI
- urinary incontinence
- US
- urge suppression
- UUI
- urgency urinary incontinence
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Department of Public Health and Epidemiology, University of Birmingham
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Peter Brocklehurst, Professor of Women’s Health, Institute for Women’s Health, University College London
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor Irwin Nazareth, Professor of Primary Care and Head of Department, Department of Primary Care and Population Sciences, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norrie, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Professor of Primary Care Research, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Tom Foulks, Medical Research Council
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Diana Baralle, Consultant and Senior Lecturer in Clinical Genetics, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Public contributor
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Nicola Lennard, Senior Medical Officer, MHRA
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Public contributor
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Public contributor
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Matthew Thompson, Senior Clinical Scientist and GP, Department of Primary Health Care, University of Oxford
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Joanna Jenkinson, Board Secretary, Neurosciences and Mental Health Board (NMHB), Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Public contributor
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire County Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Mrs Jean Thurston, Public contributor
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Public contributor
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Public contributor
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Public contributor
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Public contributor
-
Mr Sankaran Chandra Sekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Public contributor
-
Mr Seumas Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Dr Matthew Wilson, Consultant Anaesthetist, Sheffield Teaching Hospitals NHS Foundation Trust
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan, Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Public contributor
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer, Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Public contributor
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Public contributor
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Public contributor
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington