Notes
Article history paragraph text
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 11/45/01. The protocol was agreed in October 2011. The assessment report began editorial review in March 2012 and was accepted for publication in May 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Executive summary
Background
Ultrasound (US) scanning and other imaging technologies [e.g. computed tomography (CT) and magnetic resonance imaging (MRI)] are important in the management of many patients with liver disease. Imaging sometimes identifies focal abnormalities in the liver that cannot be characterised initially and may need further investigation, the main aim of which is to distinguish between liver cancers and benign abnormalities not likely to require further treatment. One important factor in selecting an imaging test is the ability to provide a rapid diagnosis, both to facilitate prompt treatment in patients who do have cancer and to minimise anxiety in the majority who do not. Most liver lesions are found at an initial unenhanced US scan. If the liver abnormality is not characterised by this test, the patient is usually referred for additional imaging (MRI and/or CT) and may require biopsy when additional imaging remains uncertain. CT and MRI can require additional waiting time, CT uses ionising radiation and the intravenous contrast agent can, on rare occasions, cause kidney damage, and some patients cannot undergo MRI (e.g. because of pacemakers or claustrophobia). The use of contrast agents may improve the ability of US to distinguish between liver cancer and benign abnormalities and, because it can be performed at the same appointment as unenhanced US, more rapid diagnoses may be possible and some CT and MRI examinations may be avoided.
Objectives
To compare the clinical effectiveness and cost-effectiveness of contrast-enhanced ultrasound (CEUS) using SonoVue® (Bracco UK Ltd, High Wycombe, UK) with that of contrast-enhanced CT (CECT) and contrast-enhanced MRI (CEMRI) for the assessment of adults with focal liver lesions (FLLs) in whom previous liver imaging is inconclusive.
Methods
A systematic review was conducted to summarise the evidence on the clinical effectiveness of CEUS using the contrast agent SonoVue compared with the clinical effectiveness of CECT and CEMRI for the assessment of adults with FLLs in whom previous liver imaging has been inconclusive. Search strategies were based on the target condition (primary or secondary liver cancer) and intervention (SonoVue CEUS), as recommended in current methodological guidance (www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm). Eight bibliographic databases including MEDLINE, EMBASE, Cochrane Database of Systematic Reviews and Database of Abstracts of Reviews of Effects were searched from 2000 to September/October 2011. Research registers and conference proceedings were also searched. Systematic review methods followed published guidance (www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm). The risk of bias in diagnostic test accuracy (DTA) studies was assessed using a modified version of the QUADAS-2 tool, and in the single controlled clinical trial was assessed using an adaptation of The Cochrane Collaboration's risk of bias tool. Accuracy results were summarised in tables and the text, stratified by clinical indication for imaging [characterisation of FLLs detected on US surveillance of cirrhosis patients, detection of liver metastases, characterisation of incidentally (US) detected FLLs, assessment of response to treatment of liver malignancy] and further stratified by target condition [primary hepatocellular carcinoma (HCC), liver metastases or ‘any liver malignancy’] and/or comparator test(s) (CECT, CEMRI, both), as appropriate. The review included only one group of four similar studies (comparable clinical indication, index test and comparator, target condition and diagnostic criteria). Pooled estimates of sensitivity and specificity, with 95% confidence intervals (CIs), were calculated using a random-effects model and a sensitivity analysis was undertaken to assess the effect of excluding one large study that used a suboptimal reference standard. Between-study clinical heterogeneity was assessed qualitatively.
The health economic analysis focused on populations in whom clinical opinion indicated that there was most likely to be a benefit from the use of CEUS. These were also the populations with most data on test performance. Specifically, most data on the detection of metastases were available from patients with colorectal cancer (CRC). In addition, clinical opinion confirmed that liver metastases from CRC were the main focus of testing. Therefore, the health economic analysis used three models to assess the value of CEUS in the following three populations:
-
characterisation of FLLs detected on routine surveillance of patients with cirrhosis
-
detection of liver metastases in patients with CRC
-
characterisation of incidentally detected FFLs.
In each model, CEUS was compared with CECT, CEMRI using gadolinium contrast agent (Gd-CEMRI) and/ or CEMRI using superparamagnetic iron oxide contrast agent (SPIO-CEMRI). The averagecosts, expected life-years and expected quality-adjusted life-years (QALYs) per patient were calculated for each comparator, if accuracy data were available.
The cirrhosis surveillance model was a modified version of a model produced by the Health Economics Group, Peninsula Technology Assessment Group (PenTAG), Institute of Health Service Research, Peninsula Medical School (the PenTAG cirrhosis surveillance model) [Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess 2007; 11(34)]. The population of interest was those with a diagnosis of compensated cirrhosis deemed eligible to enter a surveillance programme. It was a probabilistic state transition (Markov) cohort model constructed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The model used a lifetime time horizon and the cycle duration was 1 month. Patients in the model can develop HCC. In the base-case analysis surveillance is every 6 months and stops at age 70 years. During this surveillance (US, combined with CEUS, CECT or CEMRI when inconclusive), the probability of identifying a small (< 2 cm) or medium (2–5 cm) HCC depends on test accuracy. In the base case, accuracy was taken from Leoni et al. (Leoni S, Piscaglia F, Golferi R, Camaggi V, Vidili G, Pini P, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol 2010; 105:599–609). Large (> 5 cm) tumours are always identified at surveillance. If the tumour is not identified (false-negatives), it grows and may be identified at the next surveillance or when symptomatic. Patients without HCC who are incorrectly diagnosed (false-positives) were assumed to be rapidly discovered before treatment.
The liver metastases from CRC model is a modified version of the metastatic model developed by Brush et al. , adapted to assess the cost-effectiveness of CEUS compared with CECT and CEMRI in detecting metastases from CRC after inconclusive US [Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, et al. The value of FDG positron emission tomography/computed tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess 2011; 15(35)]. The population of interest was patients who had previously had surgical treatment for primary CRC and who, during routine follow-up, were identified as potentially having a metastatic recurrence. A decision tree combined with a probabilistic state transition (Markov) cohort model, constructed using Microsoft Excel, was used. The model used a lifetime time horizon and the cycle duration was 1 year. The probability of correctly detecting metastases depends on test accuracy. In the base case, accuracy was taken from Mainenti et al. (Mainenti PP, Mancini M, Mainolf C, Camera L, Maurea S, Manchia A, et al. Detection of colorectal liver metastases: prospective comparison of contrast enhanced US, multidetector C T, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging 2010; 35:511–21). It was assumed that patients with undetected metastases (false-negatives) would be identified within a year if they were still alive. These patients are expected to have a lower quality of life and prognosis, but only in the first year. In the base-case analysis, patients who are inaccurately diagnosed as having metastases (false-positives) are identified, because it is considered likely that clinicians will require confirmatory biopsy before initiating treatment. They are therefore not unnecessarily treated.
Patients with incidentally detected FLLs can have a variety of underlying diseases, for example HCC, metastases and various benign lesions. The prognosis and costs for patients with HCC were modelled using the cirrhosis model, and the prognosis and costs for patients with liver metastases were modelled using the liver metastases model. The FLL model used was a decision-analytic model with a lifetime time horizon. Test accuracy data were taken from the findings of the systematic review. The sensitivity and specificity of CEUS and CECT in identifying any malignancy were based on the results of a meta-analysis of four studies. CEUS and CEMRI could be compared using only one study. For different reasons it was assumed that patients with an incorrect test result (i.e. false-positive and false-negative results) would be correctly identified within 1 year. This was a conservative assumption biased against CEUS.
The impact of uncertainty about the various input parameters on the outcomes was explored through sensitivity analyses.
Results
Of the 854 references identified, 19 (describing 18 studies) were included in the review. Hand searching of conference proceedings identified a further three studies. Twenty of the 21 studies included in the systematic review were DTA studies. The majority of these were judged to be at low or unclear risk of bias with respect to the ‘index test’, ‘comparator test’ and ‘reference standard’ domains. Reporting quality was poor and a number of studies were reported only as conference abstracts. High risk of bias ratings for the ‘patient selection’ domain arose from retrospective study design or inappropriate exclusions (e.g. patients with a low probability of malignancy). High risk of bias ratings for the ‘flow and timing’ domain most frequently arose from exclusion of > 10% of patients from analyses. Test accuracy studies varied in terms of target condition, definitions of a positive imaging test and lesion size assessed. Overall, there was no clear indication that any of the imaging modalities considered (CEUS, CECT or CEMRI) offered superior performance for any of the populations or clinical applications considered.
Studies conducted in cirrhosis patients undergoing routine surveillance all concerned the differentiation of HCC from other lesion types. The definition of a positive test varied across studies and estimates of sensitivity and specificity were inconsistent, even when studies used similar definitions. There was no consistent evidence for any significant difference in performance between the three imaging modalities and three MRI contrast media assessed. It is unclear whether or not CEUS alone is adequate to rule out HCC for FLLs of < 30 mm in this population; one study indicated that CEUS may be better at ruling out HCC for FLLs of 11–30 mm, with very small FLLs (< 10 mm) not considered.
Studies of the diagnosis of liver metastases using contrast-enhanced imaging with vascular contrast media (CEUS, CECT and Gd-CEMRI) gave similar definitions of a positive test when reported. Two studies reported data for SPIO-CEMRI. There was no consistent evidence for any difference in test performance between the three imaging modalities and different contrast media assessed. The limited data available indicate that CEUS alone may be adequate to rule out liver metastases in patients with CRC.
Studies of patients with incidentally detected FLLs mainly reported data on diagnosis of ‘any malignancy’. Studies were consistent in their definitions of the criteria for HCC, which were similar to those reported in published guidelines. Studies reported per-patient or equivalent data. All studies reported no significant difference in the accuracy of CEUS and CECT or CEMRI for the characterisation of focal FLLs. The pooled estimates of sensitivity for the identification of ‘any liver malignancy’ using CEUS and CECT were 95.1% (95% CI 93.3% to 96.6%) and 94.6% (95% CI 92.7% to 96.1%), respectively, and the corresponding specificity estimates were 93.8% (95% CI 90.4% to 96.3%) and 93.1% (95% CI 89.6% to 95.8%), based on data from four studies. The single study comparing CEUS with CEMRI reported similar sensitivity and lower specificity for both modalities. High estimates of sensitivity indicate that CEUS alone may be adequate to rule out liver malignancy in this population.
In the surveillance of cirrhosis, CEUS was found to be as effective as but £379 (95% CI £324 to £1060) less costly than CECT. This indicates that CEUS dominates CECT. Gd-CEMRI was found to be £1063 (95% CI £449 to £1492) more costly than CEUS and gained 0.022 (95% CI −0.002 to 0.050) more QALYs. This resulted in an incremental cost-effectiveness ratio (ICER) of £48,545 per QALY gained. This ICER would be deemed unacceptable given a willingness-to-pay threshold of £20,000 per additional QALY. CEUS can therefore be considered the most cost-effective option when used after inconclusive US. Changing the source of accuracy data corroborated the dominance of CEUS over CECT. CEUS was cost-effective compared with Gd-CEMRI in most sensitivity analyses.
In the diagnosis of liver metastases from CRC, CEUS was found to cost £1 (95% CI −£1.26 to £1.28) more than CECT and at a lifetime time horizon they yielded equal QALYs per patient. Both Gd-CEMRI and SPIO-CEMRI were dominated by CECT because they were more costly and equally as effective. When increasing the proportion of patients with metastases or changing the source of accuracy data, CEUS was found to dominate CECT. In these additional analyses, Gd-CEMRI was not cost-effective compared with CEUS, or dominated by CEUS. If it is not assumed that patients incorrectly diagnosed with metastases are identified by biopsy before any unnecessary treatment, the lower specificity of CEUS has greater consequences. CEUS is then the most costly and the least effective option, and Gd-CEMRI dominates. However, it is questionable whether or not this would happen in practice.
In the characterisation of incidentally detected FLLs, CEUS was found to be very slightly more effective (0.0002 QALYs; 95% CI −0.00110 to 0.00140) than CECT and £52 (95% CI −£81 to −£22) less costly. Compared with CEMRI, CEUS was also slightly more effective (0.0026 QALYs; 95% CI −0.0058 to 0.0135 QALYs) and less costly (−£131; 95% CI −£194 to −£69). An increased prior probability of malignant lesions increased the QALYs gained by CEUS compared with both CECT and CEMRI, thereby confirming its dominance. When the consequences of an incorrect diagnosis of HCC and metastases were made more or less severe, CEUS dominated CECT and CEMRI. When the data source for the performance of CEUS and CECT was switched from the meta-analysis to one of the four studies used in the meta-analysis, the cost-effectiveness results changed only slightly, and did not alter the dominance of CEUS over CECT.
Conclusions
The results of our systematic review suggest that SonoVue CEUS could provide similar diagnostic performance to other imaging modalities (CECT and CEMRI) for the three main clinical applications considered: characterisation of FLLs detected on US surveillance of cirrhosis patients, detection of liver metastases in patients with CRC and characterisation of incidentally detected FLLs. However, some caution is required in the interpretation of these findings as studies were generally small and heterogeneous with respect to the target condition (HCC, liver metastases or ‘any malignancy’), definitions of a positive imaging test and lesion size assessed.
The cost-effectiveness analysis indicated that the use of CEUS instead of CEMRI was cost-effective. The use of CEUS instead of CECT was considered cost-effective in the surveillance of cirrhosis and the characterisation of incidentally detected FLLs, with similar costs and effects for the detection of liver metastases from CRC. Although conclusions can be very dependent on the management of incorrectly diagnosed lesions, it is expected that CEUS can reduce costs without reducing quality of life and survival. It should be noted that, although no data were available on this issue, experience with CEUS could have an important impact on diagnostic accuracy; availability of experienced operators and training requirements are likely to be important considerations for the implementation of this technology.
If the main use of liver imaging is considered to be the rapid rule-out of malignancy, equivalent diagnostic performance may be sufficient for SonoVue CEUS to be preferred over other imaging modalities. A potential advantage of using SonoVue CEUS would be the option of completing the assessment at the same time as the initial unenhanced US. Although this would be unlikely to reduce waiting times (compared with other imaging modalities) sufficiently to change clinical outcome, the potential to provide more rapid diagnosis without repeat hospital visits is likely to be preferred by patients and may also reduce costs.
Suggested research priorities
The ideal study to address questions of clinical effectiveness would be a large multicentre RCT in which patients are randomised to receive further testing/monitoring, therapeutic planning and/or treatment based on different imaging strategies (SonoVue CEUS, CECT, CEMRI). Long-term observational studies assessing the clinical consequences of incorrect initial diagnoses may also be informative for future cost-effectiveness analyses. Standardisation of the definition of a positive imaging test for each target condition (HCC, liver metastases) followed by further, high-quality DTA studies is needed to confirm our findings on test accuracy. Future DTA studies should ideally compare the performance of all three imaging modalities (SonoVue CEUS, CECT and CEMRI) in the same patient group and report numbers of non-diagnostic images and imaging-related adverse events. Studies comparing all three imaging modalities could provide a useful vehicle for the collection of information on patients' preferences. Further investigation of the potential role of CEMRI, using newer ‘combined’ vascular and hepatocyte-specific contrast agents, may also be warranted. The practicality and effectiveness of SonoVue CEUS in the assessment of multiple lesions in both lobes of the liver should also be considered.
Study registration
This study is registered as PROSPERO: CRD42011001694.
Funding
Funding for this study was provided by the Health Technology Assessment programme of the National Institute for Health Research.
Chapter 1 Objective
To compare the clinical effectiveness and cost-effectiveness of contrast-enhanced ultrasound (CEUS) using the contrast agent SonoVue® (Bracco UK Ltd, High Wycombe, UK) with that of contrast-enhanced computed tomography (CECT) and contrast-enhanced magnetic resonance imaging (CEMRI) for the assessment of adults with focal liver lesions (FLLs) in whom previous liver imaging has been inconclusive.
Chapter 2 Background and definition of the decision problem
Conditions and aetiologies
The indication for this assessment is the characterisation of FLLs and detection of liver metastases in adults and the target conditions are malignancies of the liver [primary hepatocellular carcinoma (HCC) or liver metastases].
In the context of this assessment, the term ‘focal lesion in the liver’ refers to any focal area of perceived difference seen on an imaging study and occurring in one specific area of the liver. FLLs can be broadly classified as benign (e.g. haemangioma, focal nodular hyperplasia, focal fatty infiltration or sparing and adenoma) or malignant [e.g. primary HCC, cholangiocarcinoma (CCC) or liver metastases], with the identification or exclusion of malignancy being the primary aim of diagnostic imaging. The distinction between benign and malignant determines the individual's prognosis and the subsequent treatment strategy. Benign, asymptomatic FLLs usually do not require any treatment. Depending on the specific type of lesion, the individual may be monitored and the lesion rescanned in 6–12 months. Once a malignant lesion is identified it is important to distinguish between primary and secondary cancers as this is likely to impact on how the individual is managed. Malignant lesions may be treated by a range of interventions including chemotherapy, liver resection (surgery) and local ablative therapy. The treatment of primary HCC has been addressed in published guidelines1,2 and the National Institute for Health and Care Excellence (NICE) has issued guidance on a number of individual interventions for primary HCC and liver metastases (see Appendix 6). However, expert opinion suggests that practice within the NHS may vary significantly across regions based on clinician preference.
Although liver cancer is rare in the UK (age-standardised rates are 4.7 per 100,000 men and 2.9 per 100,000 women),3 it is the second most rapidly increasing cancer in men and the third in women (increases of 38% and 28%, respectively, in the last decade). 4 However, as 70–75% of FLLs assessed in the NHS may be benign, one possible benefit of CEUS may be the rapid rule-out of malignancy with an associated reduction in anxiety for patients and families. The current practice of referring patients with inconclusive unenhanced ultrasound (US) for CEMRI and/or CECT may result in a wait of up to several months.
Because SonoVue CEUS should be used only when unenhanced US is inconclusive, we consider its primary application to be for the characterisation of lesions (benign or malignant) in patients with known FLLs; most patients who have already undergone unenhanced US and who have proceeded to CEUS are likely to have FLLs (seen at unenhanced US), the nature of which remains uncertain. Detection of FLLs at unenhanced US may be ‘incidental’ (FLLs detected in patients undergoing abdominal US for symptoms and/or biochemistry suggestive of possible liver disease or for other reasons unrelated to possible liver disease) or the result of routine surveillance of patients with cirrhosis. CEUS may also identify additional FLLs over and above those detected on unenhanced US. Other relevant applications include the detection of specific types of malignant FLLs [e.g. liver metastases from colorectal carcinoma (CRC), recurrent or residual disease following treatment of a known malignancy]. A recent systematic review reported ranges for the sensitivity and specificity of SonoVue CEUS for the detection of liver metastases from CRC of 79–100% and 95–100%, respectively,5 but this review did not provide any comparison with the accuracy of other imaging techniques.
Description of technologies under assessment (SonoVue)
SonoVue is a second-generation contrast agent that uses sulphur hexafluoride microbubbles for CEUS imaging in adults. It is used to enhance the echogenicity of the blood and can thus improve the signal-to-noise ratio in US. SonoVue should be used only in patients for whom unenhanced US is inconclusive. 6 Low solubility gas contrast agents such as SonoVue allow imaging at low mechanical index, which in turn leads to effective tissue signal suppression. 6 First-generation agents have now been superseded by second-generation agents and are no longer available in Europe.
The SonoVue product information lists its applications as:
-
echocardiography – provision of opacification of cardiac chambers and enhancement of left ventricular echocardial border delineation in patients with suspected or known cardiovascular disease
-
Doppler US of the macrovasculature – detection or exclusion of abnormalities in the cerebral arteries, extracranial carotid arteries or peripheral arteries
-
Doppler US of the microvasculature – visualising the vascularity of liver and breast lesions for lesion characterisation.
The focus of this assessment was CEUS of the liver.
SonoVue consists of a kit containing a vial of sulphur hexafluoride gas and phospholipid powder, a prefilled syringe of solvent (sodium chloride solution) and a transfer and a separate delivery system. The saline is introduced into the vial by the delivery system and, once reconstituted, microbubbles are formed. These microbubbles are the contrast agent, which is injected into a peripheral vein at the antecubital fossa. When the US probe is placed on the abdomen, US waves cause the microbubbles to resonate so that a signal is picked up by a transducer and an image is formed on a screen.
As this contrast agent is a pure blood pool agent it remains within the patient's blood vessels and, depending on the type of lesion, it shows a pattern of uptake similar to that of computed tomography (CT) or magnetic resonance imaging (MRI) vascular contrast agents. The contrast agent is broken down by the body after a few minutes and the sulphur hexafluoride gas is exhaled through the lungs and the phospholipid component of the microbubble shell is metabolised (re-entering the endogenous phospholipid metabolic pathway). The adverse event rate associated with the use of SonoVue for liver imaging is likely to be similar to or lower than that associated with other imaging modalities (CECT or CEMRI). A post-marketing study, published in 2006, included 23,188 abdominal investigations and reported adverse events in 29 cases, of which only two were graded as serious. 7
The dual blood supply of liver tissue from the hepatic artery (25–30%) and the portal vein (70–75%) means that three vascular phases can be visualised using CEUS: the hepatic arterial phase (starting approximately 10–20 seconds after injection of the contrast agent into a peripheral vein and lasting for approximately 10–15 seconds); the portal venous phase (following the hepatic arterial phase and lasting until approximately 2 minutes after the initial injection); and the late phase (following the portal venous phase and lasting until clearance of the contrast agent from the hepatic parenchyma, up to 4–6 minutes after the initial injection). The arterial phase provides information on the extent and pattern of vascularity in the lesion, and the portal venous and late phases provide information on the washout of the contrast agent from the lesion compared with that of normal liver tissue. 6
The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) produced guidelines and good clinical practice recommendations for CEUS in 2004. The latest version of the guidelines was published in 2008 and is currently being updated. 6 The 2008 EFSUMB guidelines recommend the use of CEUS for the characterisation of FLLs in the following indications:
-
in patients with incidental findings on routine US
-
for the investigation of lesions or suspected lesions in chronic hepatitis or liver cirrhosis
-
for the investigation of lesions or suspected lesions in patients with a history of malignancy
-
in patients with inconclusive MRI/CT or cytology/histology results
-
for the characterisation of portal vein thrombosis.
The guidelines recommend the use of CEUS for the detection of FLLs in the following indications:
-
to rule out liver metastases
-
in selected cases, when clinically relevant for treatment planning and as a complement to CECT and/or CEMRI, to assess the number and location of liver metastases
-
for the surveillance of patients with known malignancy
-
in suspected CCC, when other imaging is inconclusive
-
in suspected liver trauma (in some situations).
The EFSUMB guidelines provide information on the typical enhancement patterns associated with various types of benign and malignant liver lesions;6Table 1 shows the typical enhancement patterns described for the malignant lesions considered in this assessment.
| Arterial phase | Portal venous phase | Late phase | |
|---|---|---|---|
| HCC in cirrhosis | Hyper-enhancing, complete | Iso-enhancing | Hypo-/iso-enhancing |
| Non-enhancing areas | Non-enhancing areas | ||
| HCC in non-cirrhotic liver | Hyper-enhancing | Hypo-/non-enhancing | Hypo-/non-enhancing |
| Liver metastases (hypovascular) | Rim enhancement | Hypo-enhancing | Hypo-/non-enhancing |
| Liver metastases (hypervascular) | Hyper-enhancing, complete | Hypo-enhancing | Hypo-/non-enhancing |
When considering the post-treatment assessment of patients who have undergone percutaneous ablation therapies, CEUS can potentially provide useful information when unenhanced US cannot. This is because assessment of vascularisation and tissue perfusion is essential to enable differentiation of tissue necrosis from residual tumour. 6
Other similar US contrast agents (e.g. Luminity®, Lantheus Medical Imaging, and Optison®, GE Healthcare) are indicated for use in echocardiography only. Therefore, no equivalent alternative technologies were considered in this assessment.
Comparators
Patients with inconclusive unenhanced US are currently referred for CECT and/or CEMRI. The comparators for this assessment are therefore CECT and CEMRI. Contrast-enhanced MRI generally uses gadolinium-based vascular contrast agents, which can differentiate between benign and malignant FLLs based on vascular enhancement patterns in a similar way to CECT and CEUS. However, CEMRI of the liver can also use hepatocyte-specific contrast agents such as superparamagnetic iron oxide (SPIO). Hepatocyte-specific contrast agents are taken up by Kupffer cells in the normal liver and benign lesions and may therefore aid the identification of malignant lesions, which are generally deficient in Kupffer cells, particularly when such lesions are hypervascular. 8,9 ‘Combined’ vascular and hepatocyte-specific contrast agents such as gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) can also be used. 10 A recent systematic review compared the accuracy of SonoVue CEUS, CECT and CEMRI for the differentiation of malignant and benign liver lesions. The reported sensitivities were 88% (95% CI 87% to 90%), 90% (95% CI 88% to 92%) and 86% (95% CI 83% to 88%), respectively, and corresponding specificities were 81% (95% CI 79% to 84%), 77% (95% CI 71% to 82%) and 81% (95% CI 76% to 85%). 11 However, these data were based on indirect comparisons, and estimates for CEMRI combined studies using vascular contrast agent with studies using hepatocyte-specific contrast agent.
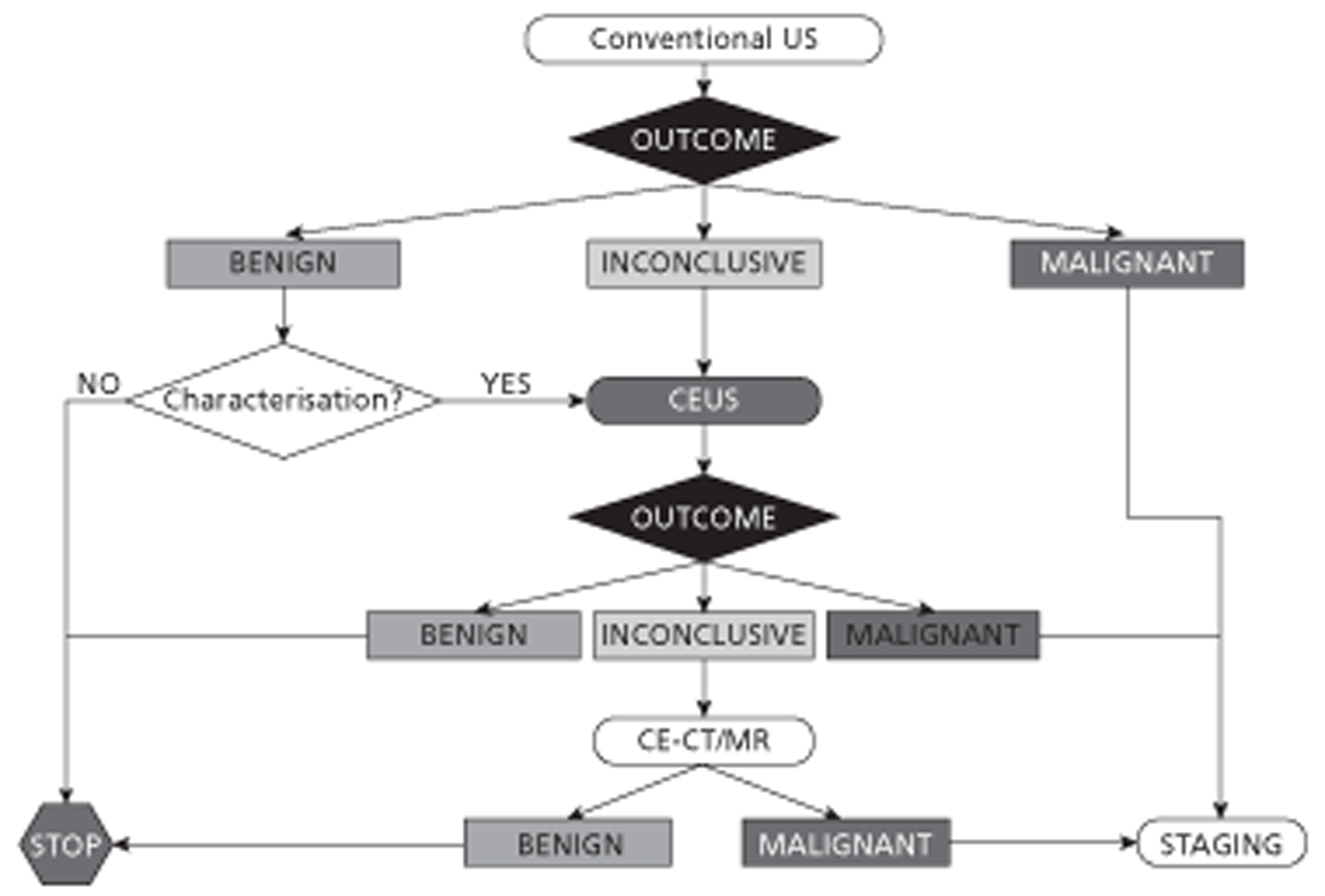
Contrast-enhanced ultrasound could be included in the diagnostic pathway as a replacement for CECT/ CEMRI (Figure 1) or as a triage step to reduce the use of CECT/CEMRI (Figure 2).
FIGURE 1.
Diagnostic algorithm for liver imaging: CEUS as a replacement test for CECT/CEMRI.
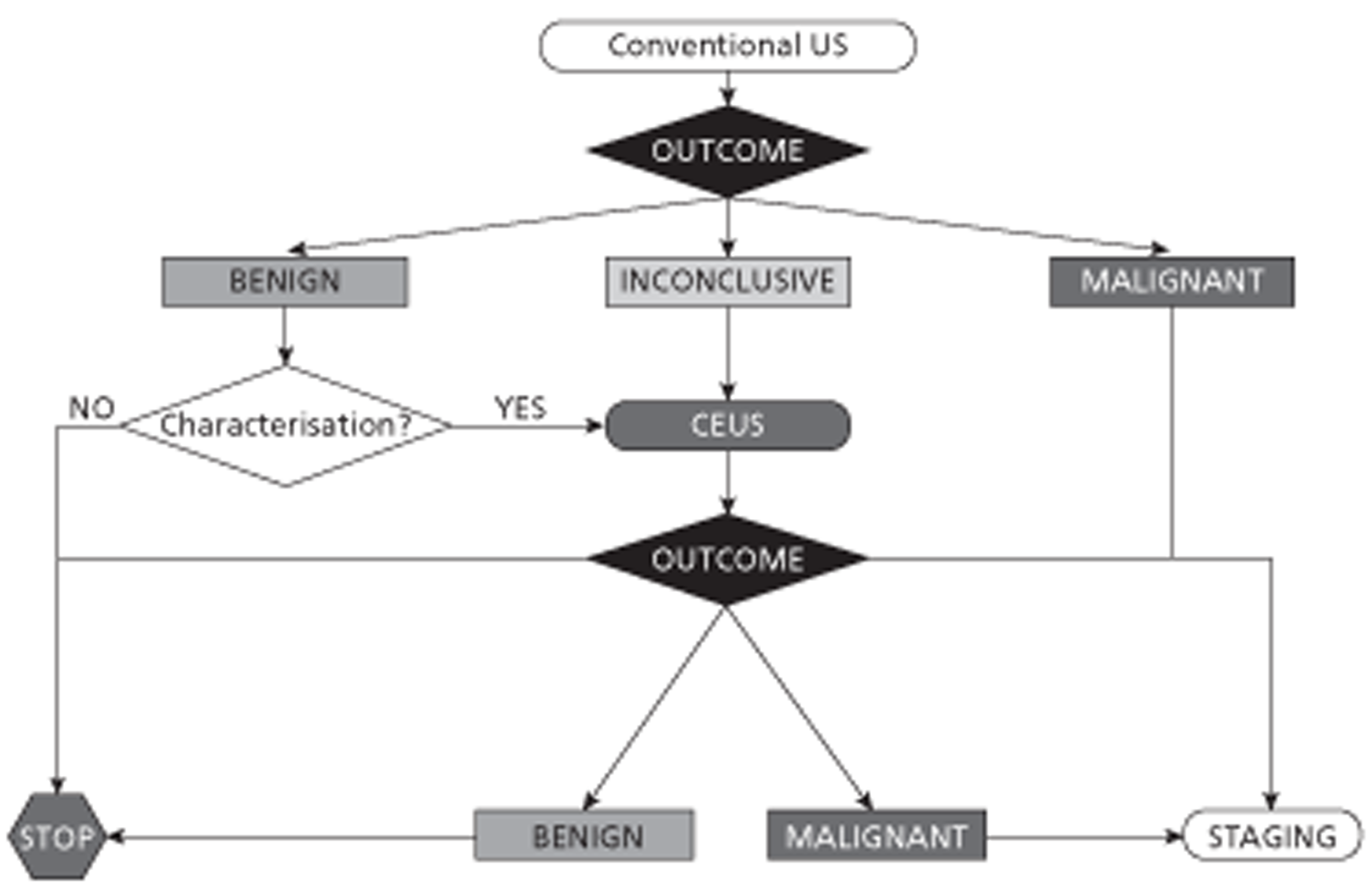
FIGURE 2.
Diagnostic algorithm for liver imaging: CEUS as a triage test to reduce the use of CECT/CEMRI.
Expert opinion indicated that biopsy would not be performed on the basis of unenhanced US examination alone; therefore, biopsy was not considered a relevant comparator for CEUS.
Care pathways/current practice
Focal liver lesions found on unenhanced US may be ‘incidental’ (FLLs detected in patients undergoing abdominal US for symptoms and/or biochemistry suggestive of possible liver disease or for other reasons unrelated to possible liver disease) or appear as the result of routine surveillance of patients with cirrhosis. In both cases investigation is focused upon characterisation of lesions, primarily to determine whether they are benign or malignant. Other relevant applications include the detection of specific types of malignant FLL such as liver metastases from CRC. The care pathways for each of these applications are described below.
In general, care pathways for patients with liver malignancy are guided by prognosis. Prognosis depends on both the stage of the tumour and underlying liver function. For any care pathway, survival time of the patient is the key variable of interest. Improvements in survival by any therapeutic option are largely dependent on the disease stage at diagnosis. The earlier the diagnosis, the greater the chance of a successful treatment.
Incidentally detected focal liver lesions
A focal lesion in the liver refers to any tissue abnormality occurring in one specific area of the liver. FLLs can be classified into two main categories, namely benign or malignant. Benign FLLs include haemangioma, focal nodular hyperplasia, focal fatty sparing and adenoma. Malignant FLLs include primary cancer of the liver, known as HCC, and secondary cancers of the liver (metastases) resulting from primary cancers occurring elsewhere in the body (e.g. CRC, breast cancer, lung cancer and pancreatic cancer).
Once a lesion has been incidentally detected in an individual the foremost concern is to differentiate between benign and malignant lesions. This distinction determines the individual's prognosis and the subsequent treatment strategy. Benign liver lesions, because of their asymptomatic nature, often require no treatment. In such cases it is common for the individual to be monitored and the lesion rescanned in 6–12 months. Once a malignant lesion is identified it is important to distinguish between primary and secondary cancers, as this is likely to impact how the individual is managed. Malignant lesions may be treated by a range of interventions including chemotherapy, liver resection (surgery), radiofrequency ablation (RFA) and transarterial therapies such as selective internal radiation therapy for metastatic lesions secondary to CRC. A fine needle aspiration biopsy to assist in the diagnosis is not always needed and involves the risk of bleeding and the seeding of neoplastic cells (along the needle tract). It has been argued that the biopsy provides little additional information beyond what can be established from a patient history, medical examination, laboratory testing and imaging. 12
Cirrhosis surveillance
Guidelines from the UK Hepatocellular Group advise that, for all patients with cirrhosis who might be suitable candidates for treatment for HCC, surveillance using abdominal US and alpha-fetoprotein (AFP) estimation should be considered. 2 If surveillance is offered it should involve abdominal US assessments in combination with serum AFP estimation at 6-month intervals. US is used for surveillance because it is low risk, non-invasive and has good acceptance by patients. However, fibrous septa and regenerative nodules characteristic of cirrhosis produce a coarse US pattern that can inhibit detection of small HCCs. 13 If the US is inconclusive, confirmatory testing will take place using CECT or CEMRI. The decision about whether to use CEMRI or CECT as the next imaging modality following the initial US scan is highly dependent on clinician preferences and local availability. Although CEMRI in general has a better sensitivity and specificity than CECT for the detection and characterisation of FLLs, the main disadvantage of MRI is the often long waiting times; it can sometimes take up to 6 months for the presence or absence of a FLL to be confirmed. A focal lesion in the liver of a patient with cirrhosis is highly likely to be HCC. 2 Biopsy is rarely required for diagnosis as this can usually be established radiologically, and seeding of tumour in the needle tract occurs in 1–3% of cases. Therefore, it is advised to avoid biopsy of potentially operable lesions when possible. Clinical practice guidelines from the European Association for the Study of the Liver (EASL) state that non-invasive diagnostic criteria for HCC (hypervascular in the arterial phase with washout in the portal venous or delayed phases) can be applied in cirrhotic patients; one imaging technique is needed for lesions of > 1 cm diameter while two techniques are recommended in suboptimal settings and biopsy is recommended only when a diagnosis cannot be reached using non-invasive criteria. 13 HCC can be curatively treated with surgery, either hepatic resection or liver transplantation. 2 Palliative treatments include percutaneous ethanol injection (PEI), RFA and transarterial chemoembolisation (TACE).
Surgical resection is the treatment of choice for HCC in non-cirrhotic patients. Cirrhotic patients need to be carefully selected for resection because they are especially prone to postoperative liver failure and increased risk of death. Survival after resection improves if the disease is diagnosed during the very early stages when liver function is preserved, the patient is asymptomatic and the nodule size is small (single, < 2 cm); it can then exceed 50% at 5 years. Taking liver function into account can help to identify patients in whom the resection could lead to decompensation of the liver and death, when resection might not be the treatment of choice. In contrast, more advanced liver tumours preclude resection. Commonly, the indication for resection is limited to patients with single tumours in the liver, without signs of vascular invasion and dissemination by the tumour. Benefits from other treatment options, such as adjuvant chemotherapy, are uncertain. Recurrence of HCC is very frequent and exceeds 70% at 5 years. Repeated resection is possible if intrahepatic dissemination of the tumour has not occurred. Liver transplantation is an option for early-stage HCC (< 5 cm or with up to three nodules < 3 cm) but is not recommended for more advanced stages. If resection or transplantation is not appropriate, percutaneous ablation (local tumour cell destruction by chemicals or temperature) can be applied to patients with early-stage HCC.
Non-curative (palliative) treatment options may be considered when disease has progressed to medium or more advanced stages and surgery or percutaneous ablation is not considered appropriate. During tumour growth the tumour becomes highly arterialised, meaning that most blood that supplies the tumour is from the hepatic artery. During transarterial embolisation (TAE), acute arterial obstruction is provoked, which causes ischaemic tumour necrosis. If TAE is combined with a chemotherapeutic agent, which is injected into the hepatic artery prior to the procedure, the procedure is called TACE. TACE is indicated if the tumour has multiple nodules, without affecting blood vessels or dissemination outside the liver. Complete necrosis of the tumour is rarely achieved after one treatment, thus treatment needs to be repeated several times. Response to treatment improves survival, which varies from 20% to 60% at 2 years depending on tumour stage, liver function and general health status. Systemic chemotherapy in treating HCC is sometimes used although it is not recommended by the American Association for the Study of Liver Diseases (AASLD). 1 EASL clinical practice guidelines recommend sorafenib (Nexavar®, Bayer Schering) as the standard systemic therapy in patients with well-preserved liver function (Child-Pugh class A), advanced HCC or tumours progressing after locoregional therapies. 13
Patients at an advanced stage of the disease, characterised by failure of liver function, tumour growth and dissemination or physical impairment, will not benefit from the above treatments and might therefore be enrolled in trials of new agents. In the terminal stage symptomatic treatment is appropriate. 1
Liver metastases for colorectal cancer
For cancers of both the colon and the rectum, surgical resection is the mainstay of definitive treatment. 14After surgical resection, patients may present with metastases. Metastases often first occur in the liver and this may be the only site of spread in 30–40% of patients with advanced disease. 15 For a patient discovered to have isolated liver metastases, CT of the chest, abdomen and pelvis should be performed to determine whether or not metastases at multiple sites are present. Isolated liver metastases of colorectal origin are commonly resected, with or without preoperative chemotherapy. In cases of small liver metastases, colon and liver resection might be combined in one surgery. Metastases at multiple sites may also be resected, with or without chemotherapy, or will be palliatively treated. If resection is not appropriate, systemic treatments such as chemotherapy in combination with other medication may be used; however, response to treatment is generally poor. Ablative therapy may also be considered; however, this is recommended only in the context of randomised controlled trials. As with HCC, recurrence of metastases after liver resection occurs in up to 60% of patients. 15
Patients without metastases are advised to undergo regular surveillance with a minimum of two CTs of the chest, abdomen and pelvis in the first 3 years and regular serum carcinoembryonic antigen (CEA) tests (at least every 6 months in the first 3 years). 14 Follow-up after liver resection is very dependent on local protocols but may include CT of the chest and liver and CEA testing for 5 years.
Chapter 3 Assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical-effectiveness of SonoVue CEUS for the assessment of FLLs in adults with previously inconclusive liver imaging. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care,16 the NICE Diagnostic Assessment Programme interim methods statement17 and the Cochrane Handbook for DTA Reviews. 18
Inclusion and exclusion criteria
Participants
Study populations eligible for inclusion were adults (≥ 18 years) in whom previous liver imaging has been inconclusive, including patients being assessed for:
-
suspected primary HCC
-
suspected secondary malignancy (liver metastases)
-
response to treatment/recurrence of known liver malignancy.
Setting
Relevant settings were secondary or tertiary care.
Interventions
The intervention (index test) was SonoVue CEUS.
Comparators
Comparator tests eligible for inclusion were:
-
CECT
-
CEMRI.
Reference standard
Studies reporting the diagnostic accuracy of SonoVue CEUS for the characterisation of FLLs (identification of liver malignancy) or the detection of liver metastases were required to use histology, following biopsy or surgical excision, to confirm the diagnosis in patients with positive index test results. Patients who test negative on the index test will generally not undergo biopsy or surgical treatment; clinical/radiological follow-up for a minimum of 6 months was therefore considered an acceptable reference standard in these patients.
Protocol modification
The reference standard criteria were extended for studies on the characterisation of FLLs only (suspected HCC) to include studies that use EASL/AASLD non-invasive diagnostic criteria (two concordant imaging test results) as the reference standard. 1,13 This modification does not apply to test accuracy studies on the detection of liver metastases. This extension of the inclusion criteria was made because clinical opinion indicated that biopsy of small, test-positive lesions may be considered unethical in this population and that the original criterion (biopsy for imaging test-positive patients/lesions and 6-month follow-up for imaging test-negative patients/lesions) may result in important studies being excluded.
Outcomes
Studies reporting the following outcomes were considered relevant:
-
effect of testing on treatment plan (e.g. surgical or medical management, or palliative care), when information on the appropriateness of the final treatment plan is also reported
-
effect of pretreatment testing on clinical outcome (e.g. overall survival, progression-free survival)
-
prognosis – the ability of the test result to predict clinical outcome (e.g. overall survival, progression-free survival, response to treatment)
-
test accuracy and number of patients/lesions classified as non-diagnostic by SonoVue CEUS.
For included studies reporting any of the above outcome measures, the following outcomes were considered, if reported:
-
the acceptability of tests to patients or surrogate measures of acceptability (e.g. waiting time and associated anxiety)
-
adverse events associated with testing (e.g. claustrophobia, reaction to contrast media)
-
additional FLLs detected by CEUS, over and above those seen on unenhanced US.
Radiation exposure was not considered a relevant outcome as the population is mostly older adults in whom additional incident cancers due to imaging-related radiation are likely to be minimal. In addition, a previous technology assessment (new-generation CT for cardiac imaging) showed that including radiation exposure in modelling did not influence the results of cost-effectiveness analyses. 19
Study design
The following study designs were eligible for inclusion:
-
Randomised or non-randomised controlled trials in which participants are assigned to the intervention or comparator test, for treatment planning, and outcomes are compared at follow-up.
-
Observational studies that report the results of multivariable regression modelling, with clinical outcome (e.g. survival, response to treatment) as the dependent variable and the index test result as an independent variable. Included studies should control adequately for potential confounders (e.g. age, tumour stage, previous treatment, results of other imaging).
-
Test accuracy studies in which the index test is compared with one or more of the comparators and the reference standard. Test accuracy studies of the index test alone were included when these were conducted in patients who had previously undergone one or more of the comparator tests (e.g. a study of the accuracy of SonoVue for the diagnosis of HCC in patients with inconclusive findings on CECT).
Included test accuracy studies were required to report the absolute numbers of true-positive, false-negative, false-positive and true-negative index test results or sufficient information to allow their calculation.
The following study/publication types were excluded:
-
preclinical and animal studies
-
reviews, editorials and opinion pieces
-
case reports
-
studies reporting only technical aspects of the test or image quality
-
studies with < 10 participants.
Search strategy
Search strategies were based on target condition and intervention, as recommended in the CRD's Guidance for Undertaking Reviews in Health Care and the Cochrane Handbook for DTA Reviews. 16,18,20
The following databases were searched for relevant studies from 2000 to September/October 2011:
-
MEDLINE (2000–September 2011 Week 4) (OvidSP)
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (2000–5 October 2011) (OvidSP)
-
EMBASE (2000–2011 Week 39) (OvidSP)
-
Cochrane Database of Systematic Reviews (CDSR) (The Cochrane Library Issue 10, 2011) (Wiley)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2011) (Wiley)
-
Database of Abstracts of Reviews of Effects (DARE) (2000–6 October 2011) (via The Cochrane Library)
-
Health Technology Assessment (HTA) database (2000–6 October 2011) (via The Cochrane Library)
-
DARE (1 January 2011–6 October 2011) (CRD website)
-
HTA database (1 January 2011–6 October 2011) (CRD website)
-
Science Citation Index (SCI) (2000–6 October 2011) (Web of Science)
-
National Institute for Health Research (NIHR) HTA database (2000–2011) (internet).
Supplementary searches were undertaken on the following resources to identify grey literature and completed and ongoing trials:
-
National Institutes of Health ClinicalTrials.gov (2000–7 October 2011) (www.clinicaltrials.gov/)
-
Current Controlled Trials (2000–7 October 2011) (www.controlled-trials.com/)
-
World Health Organization International Clinical Trials Registry Platform (ICTRP) (2000–7 October 2011) (www.who.int/ictrp/en/)
-
EU Clinical Trials Register (EU CTR) (2000–8 October 2011) (www.clinicaltrialsregister.eu/).
Searches were undertaken to identify studies of SonoVue/sulphur hexafluoride CEUS in the diagnosis of liver cancer (primary and metastases). The main EMBASE strategy for each set of searches was independently peer reviewed by a second information specialist using the PRESS EBC (Peer Review of Electronic Search Strategies Evidence-Based Checklist). 21 Search strategies were developed specifically for each database and the keywords associated with liver cancer (primary and metastases) were adapted according to the configuration of each database. Searches took into account generic and other product names for the intervention. No restrictions on language or publication status were applied. Limits were applied to remove animal studies. Full search strategies are reported in Appendix 1.
Electronic searches were undertaken on the following conference abstracts:
-
EFSUMB (EUROSON):
-
Radiological Society of North America (RSNA)
-
European Congress of Radiology (ESR)
-
2011: www.myesr.org/cms/website.php?id=/en/past_congresses/ecr_2011/ecr_2011_book_of_abstracts.htm
-
2010: www.myesr.org/cms/website.php?id=/en/ecr_2010/book_of_abstracts.htm
-
2009: www.myesr.org/cms/website.php?id=/en/ecr_2009/ecr_2009_book_of_abstracts.htm
-
2008: www.abstractsonline.com/viewer/?mkey={9AF35541-5128-444B-9D15-447022358A3F}
-
2007: www.abstractsonline.com/viewer/?mkey={9A26688A-5BBE-4366-AE14-5AC99DF8F8E4}
-
2006: www.abstractsonline.com/viewer/?mkey={6748FA35-D7A5-44B0-B8D4-4E2E51850B06}.
-
We planned to search the British Medical Ultrasound Society (BMUS) conference abstracts (2006–11) but these were not available online.
Identified references were downloaded into EndNote X4 software (Thomson Reuters, CA, USA) for further assessment and handling.
References in retrieved articles were checked for additional studies.
Inclusion screening and data extraction
Two reviewers (MW and VG) independently screened the titles and abstracts of all reports identified by searches and any discrepancies were discussed and resolved by consensus. Full copies of all studies deemed potentially relevant were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of studies excluded at the full-paper screening stage are presented in Appendix 5.
Studies listed in submissions from the manufacturer of SonoVue, Bracco UK Ltd, were first checked against the project reference database in EndNote X4; any studies not already identified by our searches were screened for inclusion following the process described above. Studies referenced by the manufacturer and excluded at the full-paper screening stage are noted in Appendix 5. Appendix 5 also includes a list of studies referenced by the manufacturer that were excluded at title and abstract screening.
When there was insufficient information for full inclusion assessment, study authors were contacted for clarification.
Data were extracted on study details (study design, participant recruitment, setting, funding, stated objective and clinical indication for testing relevant to this assessment for which data were reported), study participants (total number of participants and total number of FLLs, study inclusion criteria, study exclusion criteria, participant age and gender distribution, participant characteristics relevant to liver cancer risk, lesion size and final diagnoses), details of the index test, comparator(s) and reference standard (technical details of the test, details of who interpreted tests and how, threshold used to define a positive test) and study results. All but one of the studies included in the review were diagnostic test accuracy (DTA) studies and the results extracted from these studies were unit of analysis (patient or lesion); numbers of true-positive, false-negative, false-positive and true-negative test results; numbers of patients or lesions classified as non-diagnostic by SonoVue CEUS and/or comparator(s). The remaining study was a controlled trial that compared assessment with conventional imaging (CECT or CEMRI) plus unenhanced US with assessment with conventional imaging (CECT or CEMRI) plus SonoVue CEUS prior to RFA; data were extracted from this study to calculate odds ratios (ORs) and mean differences for dichotomous and continuous patient-relevant outcomes respectively. Data were extracted by one reviewer using a piloted, standard data extraction form and checked by a second (MW and VG); any disagreements were resolved by consensus. Chinese-language studies were extracted by one reviewer (MW) working with a native speaker (KL) and the only German language study was extracted by one reviewer and checked by a second (VG and HR) Full data extraction tables are provided in Appendix 4.
Quality assessment
The evidence-based QUADAS tool22–24 is recommended for assessing the methodological quality of test accuracy studies. 16,18 A revised version of QUADAS (QUADAS-2) has recently been published25 (www.QUADAS.org). QUADAS-2 more closely resembles the approach and structure of the Cochrane risk of bias tool. It is divided into four key domains covering participant selection, index test, reference standard and the flow of patients through the study (including the timing of tests). Each domain is rated for risk of bias (low, high or unclear) and the tool provides signalling questions in each domain to aid reviewers in reaching a judgement. The participant selection, index test and reference standard domains are also separately rated for concerns regarding the applicability of the study to the review question (low, high or unclear). Thus, QUADAS-2 separates bias from external validity (applicability) and does not include any items that assess only reporting quality. The QUADAS-2 tool does not currently include domains specific to the assessment of studies comparing multiple index tests, such as those included in this assessment. Further development of QUADAS-2 in this area is planned. A modified version of the QUADAS-2 tool, which includes an additional domain for the comparator test and additional signalling questions in the flow and timing domain, has been used in this assessment. Review-specific guidance was produced for the use of the modified version of QUADAS-2 and is reported in Appendix 2.
The results of the quality assessment are summarised and presented in tables and graphs in the results section of the systematic review and are presented in full, by study, in Appendix 3. No diagnostic accuracy data set included in this assessment was of sufficient size to allow statistical exploration of between-study heterogeneity based on aspects of risk of bias. The findings of the quality assessment were used to inform recommendations for future research.
The risk of bias in the controlled clinical trial was assessed using a table based on The Cochrane Collaboration's tool for assessing risk of bias. 26
Methods of analysis/synthesis
The results of the DTA studies included in this review were summarised by clinical indication for imaging (characterisation of FLLs detected on routine surveillance of cirrhosis patients using unenhanced US, detection of liver metastases in patients with known primary malignancy, characterisation of incidentally detected FLLs visualised on unenhanced US, assessment of response to treatment in known liver malignancy) and further stratified by target condition (HCC, liver metastases or ‘any liver malignancy’) and/ or comparator test(s) (CECT, CEMRI, both), as appropriate. For all included studies the absolute numbers of true-positive, false-negative, false-positive and true-negative test results, as well as sensitivity and specificity values, with 95% confidence intervals (CIs) were presented in results tables for index test, comparator and target condition reported. When multiple data sets were reported (e.g. for per-patient and per-lesion data, different diagnostic criteria, different lesion sizes) these were extracted in full. Data on the number of non-diagnostic tests were also included in the results tables and described in text summaries. No study reported data on patient preferences and one study reported absence of index test-associated adverse events; the latter was recorded in the relevant results table.
When groups of similar studies (comparable clinical indication, index test and comparator, target condition and diagnostic criteria) included four or more data sets, we planned to construct summary receiver operating characteristic (SROC) curves and calculate summary estimates of sensitivity and specificity with 95% CIs using the bivariate modelling approach;27–29 four data sets are the minimum requirement to ft models of this type. However, the review included only one group of four similar studies and this group included one study that used a suboptimal reference standard (as described in the protocol modification noted in Inclusion and exclusion criteria). Pooled estimates of sensitivity and specificity with 95% CIs were therefore calculated using a random-effects model and forest plots were constructed showing the sensitivity and specificity estimates from each study together with pooled estimates. A sensitivity analysis was undertaken to assess the effect of excluding the large study that used a suboptimal reference standard; these analyses were conducted using MetaDiSc 1.4 (www.hrc.es/investigacion/metadisc_en.htm). 30
Between-study clinical heterogeneity was assessed qualitatively. Statistical heterogeneity was assessed for the one meta-analysis undertaken using the chi-squared test and inconsistency was quantified using the I2 statistic,31 although these measures are of limited value given the small number of studies involved. There were no data sets of sufficient size (minimum 10) to allow statistical exploration of sources of heterogeneity by including additional covariables in the SROC model.
Where meta-analysis was considered unsuitable for the data identified (e.g. because of heterogeneity and/or small number of studies), studies were summarised using a narrative synthesis. Text and tables were stratified by clinical indication and target condition, as described above. Where appropriate, the results of individual studies were plotted in the receiver operating characteristic (ROC) plane.
Results of the assessment of clinical effectiveness
The literature searches of bibliographic databases identified 854 references. After initial screening of titles and abstracts, 175 were considered to be potentially relevant and ordered for full-paper screening. No additional papers were ordered based on screening of the industry submission; all studies submitted had already been identified by the bibliographic database searches. No additional studies were identified from searches of clinical trials registries. Of the 175 publications considered potentially relevant, three32–34 could not be obtained within the time scale of this assessment; these were held in British Library stacks that are currently closed for asbestos removal or were not held by the British Library. Four studies, reported as conference abstracts, did not contain sufficient information to complete inclusion assessment and authors were contacted for additional information;35–38 one response was received and all four studies were finally excluded. Figure 3 shows the flow of studies through the review process and Appendix 5 provides details of all publications excluded at the full-paper screening stage with reasons for exclusion.
FIGURE 3.
Flow of studies through the review process.
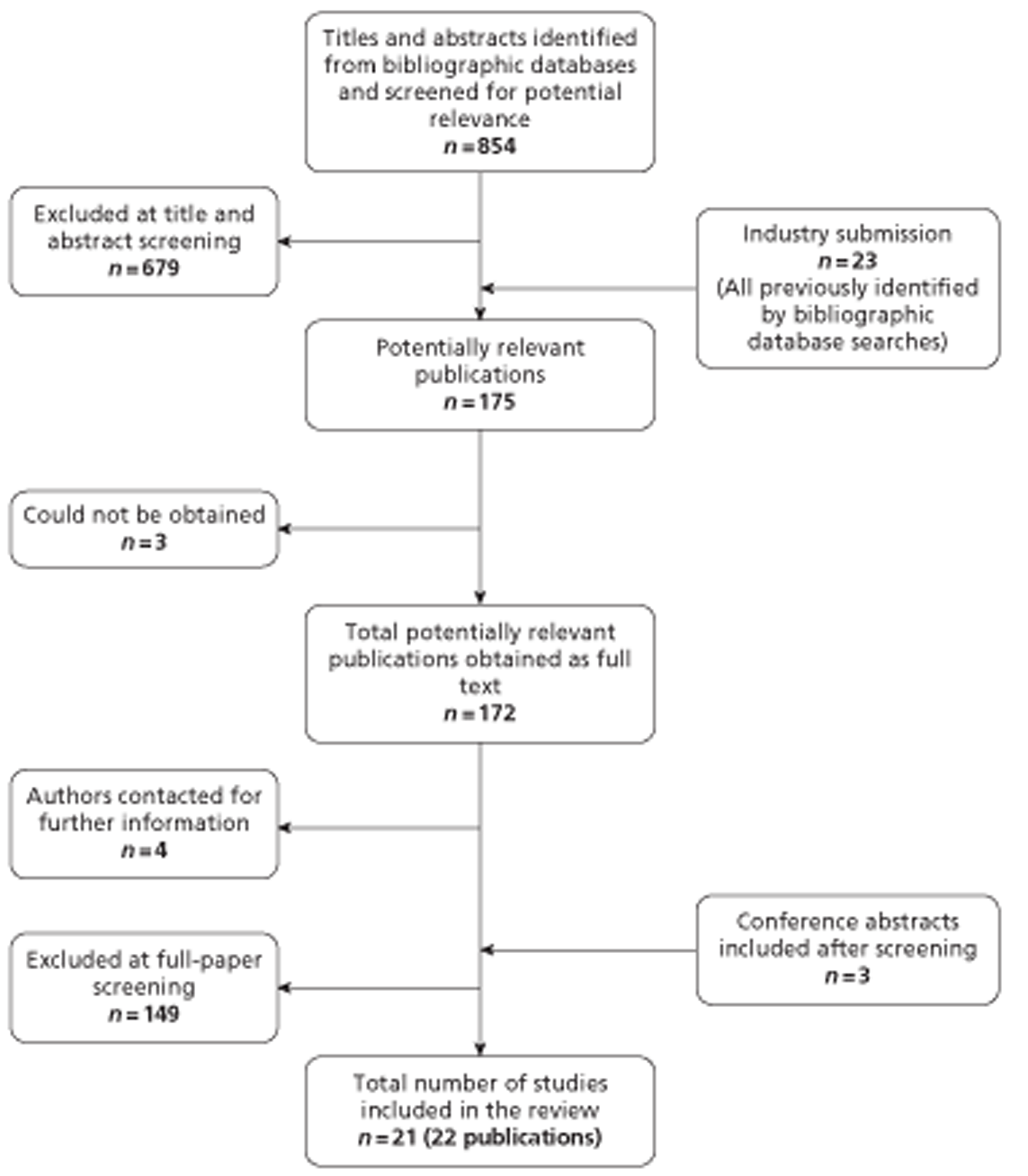
Based on the searches and inclusion screening described above, 19 publications of 18 studies were included in the review. Hand searching of conference proceedings resulted in the inclusion of a further three studies, which were published in abstract form only. 39–41 A total of 21 studies in 22 publications were therefore included in the review.
All but one of the included studies were test accuracy studies; of the 20 test accuracy studies, seven concerned the use of SonoVue CEUS for the characterisation of FLLs detected at routine surveillance of patients with cirrhosis,42–48 four assessed the performance of SonoVue CEUS for the detection of liver metastases in patients with known primary cancers (CRC),39,49–51 six concerned the use of SonoVue CEUS for the characterisation of incidentally detected FLLs41,52–56 and three considered the use of SonoVue CEUS to assess response to treatment in patients with liver cancer. 40,57,58 The remaining study was a controlled trial that compared assessment with conventional imaging (CECT or CEMRI) plus unenhanced US with assessment with conventional imaging (CECT or CEMRI) plus SonoVue CEUS prior to RFA. 59 This study reported the following patient-relevant outcomes: successful ablation, tumour progression, incidence of new HCC, incidence of repeat RFA, local progression-free survival, new tumour-free survival and post-therapy complications.
All included studies were published in 2006 or later. Sixteen of the 21 included studies were conducted in Europe (the majority in Italy or Spain) and the remaining five studies were conducted in China (including two Chinese-language publications). Two studies reported funding from the manufacturer of SonoVue55,56 and 13 studies did not report any information on funding sources.
Table 2 shows the details of the included studies, the clinical indication for imaging for which they reported data and the target conditions (primary HCC, liver metastases, ‘any liver malignancy’ or response to treatment) and comparator tests assessed. Further details of the characteristics of study participants and the technical details of the conduct of the index test (SonoVue CEUS), comparator test(s) and reference standard (where applicable) and their interpretation are reported in the data extraction tables presented in Appendix 4.
| Study ID | Study design | Objective | US (CCTs and RCTs only) | Combined imaging | Comparator CECT | Comparator CEMRI | Any liver malignancy | Primary HCC | Metastases | Treatment success | Study design and outcome extracted |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blondin 201148 | Retrospective analysis based on a search of the radiological information system between January 2007 and March 2009 | To compare the diagnostic accuracy of CEUS and hepatobiliary contrast-enhanced MRI of the liver in evaluating FLLs in patients with liver cirrhosis | ✓ | ✓ | DTA Accuracy data (characterisation of FLLs detected at cirrhosis surveillance): HCC vs benign |
||||||
| Catala 200752 | Prospective cohort of adult patients (≥ 18 years) with FLLs detected on US December 2002–August 2003 Single centre, Spain One author supported in part by a grant from the Carolina Foundation |
To compare the diagnostic accuracy of real-time evaluation by CEUS using SonoVue vs SCT in the characterisation of FLLs and to determine the degree of correlation between the two techniques | ✓ | ✓ | ✓ | ✓ | ✓ | DTA Accuracy data (characterisation of incidentally detected FLLs): separate data for HCC, liver metastases and any liver malignancy |
|||
| Chen 200759 Related publication60 excluded as duplicate |
Prospective CCT of patients with HCC who were being assessed before RFA treatment July 2002–March 2005 Single centre, China Funding NR |
To evaluate the use of CEUS in assessing patients for RFA and to compare the efficacy of RFA after CEUS with the efficacy of RFA after US | ✓ | ✓ | ✓ | CCT | |||||
| Clevert 200951 | Prospective cohort of consecutive patients with suspected liver malignancya Recruitment dates NR Two centre, Germany Funding NR |
To assess the diagnostic performance of CHI with SonoVue compared with biphasic multislice CECT for the detection of malignant liver lesions | ✓ | ✓a | DTA Accuracy data (detection of liver metastases) |
||||||
| Dai 200843 | Prospective cohort of consecutive patients with confirmed cirrhosis, without extrahepatic malignancy, who had indeterminate liver nodules on surveillance US March 2004–March 2005 Single centre, China Funding NR |
To investigate the diagnostic value for indeterminate small (1–2 cm) hepatic nodules detected by surveillance US in patients with cirrhosis using CEUS compared with helical CECT | ✓ | ✓ | DTA Accuracy data (characterisation of FLLs detected at cirrhosis surveillance): HCC vs benign |
||||||
| Feng 200757 (Chinese language) | Prospective cohort of patients with known liver malignancy (21 HCC, 3 metastases), undergoing cryosurgery November 2004–February 2006 Single centre, China Funding NR |
To evaluate the role of CEUS in assessing the short-term therapeutic response of hepatic carcinoma with cryosurgery | ✓ | DTA Accuracy data (detection of treatment success) |
|||||||
| Flor 200939 (abstract only) | Prospective cohort of patients with known primary cancer and indeterminate liver lesions on MDCT Recruitment dates NR Single centre, Italy Funding NR |
To evaluate the role of plain US and CEUS in characterising small indeterminate MDCT-detected FFLs in patients with known primary cancer | ✓ | DTA Accuracy data (detection of liver metastases) |
|||||||
| Forner 200844 | Prospective cohort of asymptomatic patients with Child–Pugh A/B cirrhosis and no history of HCC, with a new liver nodule detected on surveillance US November 2003–August 2006 Two centre, Spain and USA Supported by grants from Instituto de Salud Carlos III, Spain; BBVA Foundation; Fundación Cientifica de la Asociación Española de Ayuda contra el Cáncer, Spain, grant nos PI 05/150, 06/132 and 05/645; NIH-NIDDK grant no. 1R01DK076986–0 |
To evaluate the accuracy of CEUS and dynamic MRI for the diagnosis of nodules of ≤ 20 mm detected during US surveillance | ✓ | ✓ | DTA Accuracy data (characterisation of FLLs detected at cirrhosis surveillance): HCC vs benign |
||||||
| Gierbliński 200853 | Prospective cohort of patients with incidentally detected solid liver lesions, referred for biopsy June 2005–March 2006 Single centre, Poland Funding NR |
To determine whether or not CEUS is an accurate method to differentiate FLLs and reduce the need for fine-needle biopsy | ✓ | DTA Accuracy data (characterisation of incidentally detected FLLs): any malignancy vs benign |
|||||||
| Giorgio 200745 | Prospective study of consecutive patients with cirrhosis and a single liver nodule of ≤ 30 mm identified on surveillance US September 2003–June 2004 Single centre, Italy Funding NR |
To evaluate the role of low mechanical index CEUS for the characterisation of small HCC in cirrhotic patients compared with ultrafast gadolinium-enhanced MRI | ✓ | ✓ | DTA Accuracy data (characterisation of FLLs detected at cirrhosis surveillance): HCC vs benign |
||||||
| Jonas 201150 (abstract only) | Prospective study of consecutive patients with CRC metastases who were considered candidates for curative surgery and who underwent complete preoperative workup 2005–7 Single centre, Sweden Funding NR |
To assess the sensitivity and specificity of four imaging modalities (CEUS, CECT, CEMRI and FDG-PET) in detecting liver metastases in patients with CRC | ✓ | ✓ | ✓ | DTA Accuracy data (detection of liver metastases) |
|||||
| Leoni 201042 | Retrospective analysis of a study of consecutive patients with cirrhosis with one to three liver nodules (1–3 cm) detected at surveillance US September 2003–November 2005 Single centre, Italy No financial support |
To assess the diagnostic contribution of vascular contrast-enhanced techniques and the possible additional contribution of SPIO MRI for the diagnosis of HCC in cirrhosis | ✓ | ✓ | ✓ | DTA Accuracy data (characterisation of FLLs detected at cirrhosis surveillance): HCC vs benign |
|||||
| Li 200754 | Prospective study of patients with FLLs detected at US and unenhanced CT Recruitment dates NR Single centre, China Supported by the Clinical New Technology Foundation of Southwest Hospital (SWH2005A004) |
To compare the efficacy of contrast-enhanced pulse-inversion harmonic sonography for the characterisation of FLLs with that of contrast-enhanced helical CT | ✓ | ✓ | DTA Accuracy data (characterisation of incidentally detected FLLs): any malignancy vs benign |
||||||
| Lüttich 200640 (abstract only) | Cohort of patients with HCC undergoing RFA treatment Recruitment dates NR Single centre, Spain Funding NR |
✓ | ✓ | DTA Accuracy data (detection of treatment success) |
|||||||
| Mainenti 201049 | Prospective study of consecutive patients with histologically proven CRC who were scheduled for surgery July 2005–March 2007 Single centre, Italy Funding NR |
To compare CEUS, MDCT, MRI with extracellular contrast agent (Gd-CEMRI), MRI with intracellular contrast agent (SPIO-CEMRI) and PET/CT in the detection of hepatic metastases from CRC | ✓ | ✓ | ✓ | DTA Accuracy data (detection of liver metastases) |
|||||
| Quaia 200946 | Prospective study of patients with cirrhosis who had at least one hepatocellular nodule detected on surveillance US Recruitment dates NR Two centre, Italy Funding NR |
To assess the added diagnostic value of CEUS combined with 64-row MDCT in the assessment of hepatocellular nodule vascularity in patients with liver cirrhosis | ✓ | ✓ | ✓ | DTA Accuracy data (characterisation of FLLs detected at cirrhosis surveillance): HCC vs benign |
|||||
| Sangiovanni 201047,61 | Prospective study of patients with cirrhosis who had at least one hepatocellular nodule detected on surveillance US April 2006 to NR Single centre, Italy Funded by grant no. PUR 2008, University of Milan and a personal donation (Dr Aldo Antognozzi) |
To assess the sensitivity, specificity and economic impact of all possible sequential combinations of contrast imaging techniques in patients with cirrhosis with 1- to 2-cm liver nodules undergoing US surveillance | ✓ | ✓ | ✓ | ✓ | DTA Accuracy data (characterisation of FLLs detected at cirrhosis surveillance): HCC vs benign |
||||
| Seitz 200955 (linked to Seitz 201056) | Cohort of 267 patients who underwent SCT from a prospective study of 1349 consecutive patients with newly detected solid liver mass visible during routine US. Data extracted for the subgroup of patients (158) in whom diagnosis was histologically confirmed (2 × 2 data could not be extracted for the remaining patients) May 2004–December 2006 Multicentre, Germany, Austria and Switzerland Funded by Bracco Research (Konstanz, Germany) for the online data forms, quality control, calculations and statistical analyses |
To evaluate the diagnostic value of CEUS for the characterisation of FLLs in a prospective multicentre study in clinical practice. For this purpose CEUS was compared with SCT, the standard radiological method | ✓ | ✓ | ✓ | DTA Accuracy data (characterisation of incidentally detected FLLs): separate data for HCC, liver metastases and any liver malignancy |
|||||
| Seitz 201056 (linked to Seitz 200955) | Cohort of 269 patients who underwent MRI from a prospective study of 1349 consecutive patients with newly detected FLLs identified on US. Data extracted for the subgroup of patients (84) in whom diagnosis was histologically confirmed (2 × 2 data could not be extracted for the remaining patients) May 2004–December 2006 Multicentre, Germany Funding by Bracco Research (Konstanz, Germany) for the online data forms, quality control, calculations and statistical analyses |
To assess the diagnostic performance of CEUS (compared with MRI) in a large patient cohort with FLLs recently discovered by US but not yet characterised | ✓ | ✓ | ✓ | DTA Accuracy data (characterisation of incidentally detected FLLs): separate data for HCC, liver metastases and any liver malignancy |
|||||
| Solbiati 200641 (abstract only) | Retrospective analysis of data from patients with incidentally detected FLLs 5-year experience, dates not specified Single centre, Italy Funding NR |
To assess the diagnostic performance and cost-effectiveness of CEUS in the characterisation of FLLs | ✓ | ✓ | DTA Accuracy data (characterisation of incidentally detected FLLs): any malignancy vs benign |
||||||
| Zhou 200758 (Chinese language) | Retrospective analysis of data from patients undergoing non-surgical treatment for HCC June 2005–June 2006 Single centre, China Funding NR |
To investigate the value of CEUS for non-surgical treatment response in HCC | ✓ | ✓ | DTA Accuracy data (detection of treatment success) |
Accuracy of SonoVue contrast-enhanced ultrasound for the characterisation of focal liver lesions detected on surveillance of patients with cirrhosis
Seven studies reported comparisons of SonoVue CEUS with other imaging techniques for the characterisation of FLLs detected on unenhanced US surveillance of patients with known cirrhosis. 42–48 One study, by Sangiovanni et al. , was reported as both a full paper47 and a conference abstract. 61 All of the studies in this section reported accuracy data for the differentiation of HCC from other liver lesions only and one study45 reported that there were no imaging-related adverse events. In total, the seven studies in this section reported 369 diagnoses of malignant liver lesions, of which 366 were HCC; the remaining lesions comprised two CCC and one liver metastasis. All studies in this section reported per-lesion data; three studies reported data for one lesion per patient, equivalent to per-patient test performance. 44,45,47 Studies generally focused on the characterisation of small to medium FLLs. Four studies prespecified the size of FLLs considered: < 30 mm42,45,46 or < 20 mm. 47 In two studies the mean size was 15 ± 3 mm43 and 14 mm (range 7-20 mm). 44 The remaining study did not specify lesion size as an inclusion criterion or report mean lesion size. 48 Two studies explicitly excluded lesions of < 10 mm42,47 and one study reported stratified data for different lesion sizes (< 10 mm and 11–30 mm). 45 Two studies compared SonoVue CEUS with CECT,43,46 three studies compared SonoVue CEUS with CEMRI45,48,62 and the remaining two studies compared SonoVue CEUS with both CECT and CEMRI. 42,47 One study included in this section explicitly reported that patients had an uncertain diagnosis following unenhanced US. 43 Five studies had previous unenhanced US examination as an inclusion criterion, and the ‘concern regarding applicability’ criterion for quality assessment was rated ‘unclear’ for these studies (see Table 3). 42,44–47 The remaining study was a retrospective analysis of information derived from a radiology database; inclusion criteria specified only that patients should have received both CEUS and CEMRI and histological confirmation of diagnosis (examinations prior to contrast-enhanced imaging were not specified), and the ‘concern regarding applicability’ criterion was therefore rated ‘high’ risk of bias for this study48 Comparators and imaging criteria used to define a positive test for HCC varied across studies and no meta-analyses were therefore undertaken. All but one42 of the studies in this section used histological confirmation in all patients or histological confirmation of imaging-positive patients and follow-up of imaging-negative patients as the reference standard.
| Study ID | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index test | Comparator test | Reference standard | Flow and timing | Patient selection | |
| Blondin 201148 | ☹ | ? | ? | ☺ | ☺ | ☹ |
| Dai 200843 | ☺ | ☺ | ☺ | ? | ☺ | ☺ |
| Forner 200844 | ? | ? | ☺ | ? | ? | ? |
| Giorgio 200745 | ☺ | ☺ | ☺ | ☺ | ☺ | ? |
| Leoni 201042 | ☹ | ☺ | ☺ | ☹ | ☹ | ? |
| Quaia 200946 | ☹ | ☺ | ☺ | ? | ☹ | ? |
| Sangiovanni47,61 | ☹ | ? | ? | ☺ | ☺ | ? |
All studies in this section were rated as ‘low’ or ‘unclear’ risk of bias for the ‘index test’ and ‘comparator test’ domains of the quality assessment tool. Two studies recruited consecutive samples of patients without inappropriate exclusions and were rated as ‘low’ risk of bias for ‘patient selection’. 43,45 Four studies were rated as ‘high’ risk of bias for the ‘patient selection’ domain because of the retrospective study design48 or inappropriate exclusions. 42,46,47 Two studies excluded very small lesions (< 10 mm);42,47 as these lesions may be more difficult to characterise, their exclusion may result in overestimations of test performance. One study excluded lesions with peripheral enhancement on CECT, which was considered to be indicative of a high probability of haemangioma. 46 Two of the three studies were also rated as ‘high’ risk of bias for the ‘flow and timing domain’ of the assessment, in one case because the reference standard used was not independent of the imaging test results42 and in the other because a high proportion of lesions (approximately 40%) were excluded because a histopathological reference standard was not performed. 46 One study was also rated as ‘high’ risk of bias for the ‘reference standard’ domain because a suboptimal reference standard (concordance between at least two imaging test results) was used in the majority of cases. 42
The two studies that compared CEUS and CECT had slightly differing definitions of a positive imaging test (hyperenhancement in the arterial phase followed by portal venous washout43 and hyperenhancement in the arterial phase with or without portal venous washout). 46 Neither study reported a significant difference in performance between imaging modalities for the differentiation of HCC from other liver lesions and neither study specified exclusion of very small FLLs. However, no data for very small FLLs were reported; in one study 46% of lesions were 10-15 mm and 54% were 16–20 mm43 and in the other study all lesions were in the range 10–30 mm. 46 The study by Dai et al. 43 reported slightly higher estimates of test performance, particularly for CECT specificity (see Table 4). The sensitivity estimates for CEUS and CECT were 91.1% (95% CI 80.4% to 97.0%) and 80.4% (95% CI 67.6% to 89.8%), respectively, and the corresponding specificities were 87.2% (95% CI 74.3% to 95.2%) and 97.9 (95% CI 88.7% to 99.9%). 43 The definition of HCC used by this study corresponded most closely with that reported in the EFSUMB guidelines on the use of CEUS. 6Table 1 summarises the typical enhancement patterns seen in various malignant FLLs. Quaia et al. 46 reported sufficient data to allow calculation of sensitivity and specificity for the combination of CEUS and CECT, with a positive finding on either imaging technique treated as ‘test positive’; they reported an increase in sensitivity for combined imaging compared with either CEUS or CECT alone with no change in specificity.
| Study ID | Patient or lesion data | Index test or comparator | Reference standard | TP | FN | FP | TN | Sensitivity (95% CI) (%)a | Specificity (95% CI) (%)a | Non-diagnostic | Adverse events | Acceptability to patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC | ||||||||||||
| SonoVue CEUS compared with CECT | ||||||||||||
| Dai 200843 | n = 103 FLLs in 72 patients (per-lesion data) | CEUS SonoVue | Histopathology following biopsy, with negative biopsy confirmed by a minimum of 6 months' follow-up | 51 | 5 | 6 | 41 | 91.1 (80.4 to 97.0) | 87.2 (74.3 to 95.2) | None | NR | NR |
| HCC = positiveb | ||||||||||||
| CECT with Somatom Plus 4 (Siemens Medical Systems, Erlangen, Germany) | 45 | 11 | 1 | 46 | 80.4 (67.6 to 89.8) | 97.9 (88.7 to 99.9) | ||||||
| HCC = positiveb | ||||||||||||
| Quaia 200946SonoVue CEUS compared with CEMRI | n = 121 FLLs (≤ 30 mm) in 106 patients (per-lesion data) | CEUS sulphur hexafluoride-filled microbubbles | FNB in all lesions | 64 | 8 | 15 | 34 | 88.9 (79.3 to 95.1) | 69.4 (54.6 to 81.7) | n = 4 inadequate CEUS examinations excluded from study | NR | NR |
| HCC = positivec (readers 1 and 2) | 63 | 9 | 18 | 31 | 87.5 (77.6 to 94.1) | 63.3 (48.3 to 76.6) | ||||||
| CECT with Aquilion (Toshiba Medical Systems, Tochigi-ken, Japan) or Brilliance (Philips, Cleveland, OH, USA) | 53 | 19 | 14 | 35 | 73.6 (61.9 to 83.3) | 71.4 (56.7 to 83.4) | n = 10 inadequate CECT examinations excluded from study | |||||
| 51 | 21 | 14 | 35 | 70.8 (58.9 to 81.0) | 71.4 (56.7 to 83.4) | |||||||
| HCC = positivec (readers 1&2) | ||||||||||||
| CEUS + CECT | 70 | 2 | 14 | 35 | 97.2 (90.3 to 99.7) | 71.4 (56.7 to 83.4) | See above | |||||
| HCC = either test positive (readers 1 and 2) | 70 | 2 | 15 | 34 | 97.2 (90.3 to 99.7) | 69.4 (54.6 to 81.7) | ||||||
| SonoVue CEUS compared with CEMRI | ||||||||||||
| Blondin 201148 | n = 47 FLLs in 33 patients | CEUS SonoVue | Histology (surgery or biopsy) in all lesions | 38 | 3 | 3 | 3 | 93 (80 to 98) | 50 (42 to 88) | None | NR | NR |
| HCC = positiveb | ||||||||||||
| Gd-EOB-DTPA CEMRI with MAGNETOM Avanto (Siemens) | 37 | 4 | 1 | 5 | 90 (77 to 97) | 83 (36 to 100) | ||||||
| HCC = positiveb | ||||||||||||
| Forner 200844 | n = 89 patients (one lesion per patient) | CEUS SonoVue | FNB for test positive, imaging follow-up for test negative | 47 | 13 | 4 | 25 | 78.3 (65.8 to 87.9) | 86.2 (68.3 to 96.1) | |||
| HCC suspiciousd or conclusiveb = positive | ||||||||||||
| CEUS SonoVue | 31 | 29 | 2 | 27 | 51.7 (38.4 to 64.8) | 93.1 (77.2 to 99.2) | ||||||
| HCC conclusiveb = positive | ||||||||||||
| Gd-CEMRI with Symphony system (Siemens) | 51 | 9 | 3 | 26 | 85.0 (73.4 to 92.9) | 89.7 (72.6 to 97.8) | ||||||
| HCC suspiciousd or conclusiveb = positive | ||||||||||||
| Gd-CEMRI | 37 | 23 | 1 | 28 | 61.7 (48.2 to 73.9) | 96.6 (82.2 to 99.9) | ||||||
| HCC conclusiveb = positive | ||||||||||||
| Giorgio 200745 | n = 73 FLLs (one lesion per patient) | CEUS SonoVue | US-guided FNB in all patients | 37 | 11 | 1 | 24 | 77.1 (62.7 to 88.0) | 96.0 (79.6 to 99.9) | None | No side effects observed in any patients | NR |
| HCC = positivee | ||||||||||||
| n = 21 FLLs (≤ 10 mm) | 3 | 8 | 0 | 10 | 27.3 (6.0 to 61.0) | 100 (69.2 to 100) | ||||||
| n = 52 FLLs (11–30 mm) | 34 | 3 | 1 | 14 | 100 (69.2 to 100) | 93.3 (68.1 to 99.8) | ||||||
| n = 73 FLLs (one lesion per patient) | Gd-CEMRI with Symphony system | 43 | 5 | 3 | 22 | 89.6 (77.3 to 96.5) | 88.0 (68.8 to 97.5) | NR | ||||
| HCC = positivef | ||||||||||||
| n = 21 FLLs (≤ 10 mm) | 8 | 3 | 1 | 9 | 72.7 (39.0 to 94.0) | 90.0 (55.5 to 99.7) | ||||||
| n = 52 FLLs (11–30 mm) | 35 | 2 | 2 | 13 | 94.6 (81.8 to 99.3) | 86.7 (59.5 to 98.3) | ||||||
| SonoVue CEUS compared with CECT and CEMRI | ||||||||||||
| Leoni 201042 | n = 75 FLLs in 60 patients (10–30 mm) | CEUS SonoVue | Two or more concordant imaging results (n = 44), FNB (n = 14) or follow-up at 3-month intervals (n = 1) for positive test | 37 | 18 | 2 | 18 | 67.3 (53.3 to 79.3) | 90.0 (68.3 to 98.8) | None | NR | NR |
| HCC = positiveg | ||||||||||||
| CECT with Emotion 6 (Siemens) | 37 | 18 | 2 | 18 | 67.3 (53.3 to 79.3) | 90.0 (68.3 to 98.8) | ||||||
| HCC = positiveg | ||||||||||||
| Gd-CEMRI with Signa (GE Medical Systems, WI, USA) | FNB (n = 7) or follow-up at 3-month intervals (n = 9) for test negative | 45 | 10 | 1 | 19 | 81.8 (69.1 to 90.9) | 95.0 (75.1 to 99.9) | |||||
| HCC = positiveg | ||||||||||||
| n = 68 FLLs (10–30 mm) | SPIO-CEMRI with Signa | 35 | 15 | 1 | 17 | 70.0 (55.4 to 82.1) | 94.4 (72.7 to 99.9) | Seven FLLs not assessed with SPIO-MRI | ||||
| HCC = positiveg | ||||||||||||
| n = 75 FLLs (10–30 mm) | CEUS + CECT + CEMRI | 54 | 1 | 5 | 15 | 70.0 (55.4 to 82.1) | 75.0 (50.9 to 91.3) | None | ||||
| HCC = any test positive | ||||||||||||
| Sangiovanni 201047,61 | n = 55 FLLs selected from 67 FLLs in 64 patients (10–20 mm) | CEUS SonoVue | FNB in all lesions | 9 | 25 | 0 | 21 | 26.5 (12.9 to 44.4) | 100 (83.9 to 100) | None | NR | NR |
| HCC = positiveb | ||||||||||||
| CEUS SonoVue | 23 | 11 | 5 | 16 | 67.6 (49.5 to 82.6) | 76.2 (52.8 to 91.8) | ||||||
| HCC = positiveh | ||||||||||||
| CEUS SonoVue | 13 | 21 | 1 | 20 | 38.2 (22.2 to 56.4) | 95.2 (76.2 to 99.9) | ||||||
| HCC = positivei | ||||||||||||
| CECT with Definition system (Siemens) | 16 | 18 | 0 | 21 | 47.1 (29.8 to 64.9) | 100 (83.9 to 100) | ||||||
| HCC = positiveb | ||||||||||||
| CECT | 22 | 12 | 4 | 17 | 64.7 (46.5 to 80.3) | 81.0 (58.1 to 94.6) | ||||||
| HCC = positiveh | ||||||||||||
| CECT | 18 | 16 | 0 | 21 | 52.9 (35.1 to 70.2) | 100 (83.9 to 100) | ||||||
| HCC = positivei | ||||||||||||
| n = 53j FLLs (10–20 mm) | Gd-CEMRI with Avanto system (Siemens) | 14 | 18 | 0 | 21 | 43.8 (26.4 to 62.3) | 100 (83.9 to 100) | |||||
| HCC = positiveb | ||||||||||||
| Gd-CEMRI | 21 | 11 | 8 | 13 | 65.6 (46.8 to 81.4) | 61.9 (38.4 to 81.9) | ||||||
| HCC = positiveh | ||||||||||||
| Gd-CEMRI | 19 | 13 | 1 | 20 | 59.4 (40.6 to 76.3) | 95.2 (76.2 to 99.9) | ||||||
| HCC = positivei | ||||||||||||
| CEUS + CECT + CEMRI | 22 | 12 | 0 | 21 | 64.7 (46.5 to 80.3) | 100 (83.9 to 100) | ||||||
| HCC = at least one test positiveb | ||||||||||||
| Liver metastases | ||||||||||||
| No studies identified | ||||||||||||
| Any malignancy | ||||||||||||
| No studies identified | ||||||||||||
Three studies compared CEUS and CEMRI; two used gadolinium contrast-enhanced magnetic resonance imaging (Gd-CEMRI)44,45 and one used Gd-EOB-DTPA-CEMRI,48 a ‘combined’ vascular and hepatocyte-specific contrast agent. The two studies that compared CEUS and Gd-CEMRI used different definitions of a positive imaging test result and only Forner et al. 44 reported data for a definition of HCC, which corresponded with that given in the EFSUMB guidelines,6 which they described as ‘conclusive’ HCC. Forner et al. 44 also reported data for a definition of ‘suspicious’ HCC (hyperenhancement in the arterial phase without portal venous washout). Sensitivity and specificity were similar for CEUS and Gd-CEMRI using either criterion. Specificity tended to increase and sensitivity to decrease for both imaging modalities when the stricter ‘conclusive’ definition of HCC was used. This study did not stratify data by lesion size; however, very small lesions (≤ 10 mm) were included (15% of lesions were < 10 mm, 49% were 10–15 mm and 36% were 16–20 mm). The authors also stated that use of the AASLD criteria (concordant, ‘conclusive’ findings on CEUS and CEMRI) resulted in 100% specificity but low sensitivity (33%) (data not reported). Giorgio et al. 45 used (arterial phase) hypervascularity as the definition of a positive test and stratified data by lesion size. There was no significant difference in the performance of CEUS and Gd-CEMRI for the differentiation of HCC from benign lesions, for FLLs between 11 and 30 mm, and both techniques had sensitivity and specificity values > 85% (see Table 4). For very small FLLs (≤ 10 mm), the sensitivity of CEUS was lower than that of CEMRI (27% vs 73%); for both imaging techniques, sensitivity was poor when the analysis was restricted to very small FLLs. 45 Imaging test performance estimates were similar for the ‘all lesion’ data set from Georgio et al. 45 and the ‘suspicious’ diagnostic criteria data set from Forner et al. ;44 these data sets were similar in terms of diagnostic criteria and distribution of lesion size. The study that used Gd-EOB-DTPA-CEMRI did not report any information on lesion size. 48 The criteria used to define a positive imaging test result matched the definition of HCC given in the EFSUMB guidelines. 6 Sensitivity estimates were similar and high (> 90%) for both CEUS and Gd-EOB-DTPA-CEMRI (see Table 4). Specificity appeared lower for CEUS than for Gd-EOB-DTPA-CEMRI; however, the small number of patients with benign lesions in this study resulted in high imprecision in specificity estimates: 50% (95% CI 42% to 88%) for CEUS and 83% (95% CI 36% to 100%) for Gd-EOB-DTPA-CEMRI.
The two studies that assessed all three imaging modalities42,47 both reported data using a definition of HCC that broadly corresponded to that given in the EFSUMB guidelines,6 although Leoni et al. 42 stated ‘typical enhancement pattern’ without specifying portal venous/late phase washout and Sangiovanni et al. 47 also reported data using arterial hyperenhancement and portal venous washout separately as the definitions of HCC. Both studies assessed Gd-CEMRI and one study also assessed CEMRI using SPIO, a contrast agent that is selectively taken up by Kupffer cells in the normal liver and benign lesions and can therefore be used to identify HCCs, which are generally deficient in Kupffer cells. 47 When the EFSUMB-consistent definition of HCC was used, the two studies reported similar specificity estimates for all imaging modalities and for both MRI contrast agents; however, Leoni et al. 42 tended to report higher estimates of sensitivity. Sensitivity estimates from these studies were generally lower than those from studies with an EFSUMB-consistent definition of HCC that compared only CECT with CEUS43 or CEMRI with CEUS. 44,48 Leoni et al. 42 reported that Gd-CEMRI had the highest sensitivity of the imaging modalities assessed [81.8% (95% CI 69.1% to 90.9%)]. Both studies reported sufficient data to allow calculation of sensitivity and specificity estimates, with a positive result on any of the three imaging modalities treated as index test positive. Data from Leoni et al. 42 indicated that combining the three imaging modalities in this way could increase sensitivity [98.2% (95% CI 90.3% to 100%)] and decrease specificity [75.0% (95% CI 50.9% to 91.3%)] relative to any of the three imaging modalities alone. By contrast, combined imaging modality data from Sangiovanni et al. 47 did not appear to indicate significant improvements in sensitivity.
Table 3 provides a summary of the QUADAS-2 assessments for studies in this section and Table 4 summarises individual study results.
Accuracy of SonoVue contrast-enhanced ultrasound for the detection of liver metastases in patients with known primary malignancy
Two studies compared SonoVue CEUS with both CECT and CEMRI (SPIO-CEMRI in one study and both SPIO-CEMRI and Gd-CEMRI in the other study) for the detection of liver metastases in patients with known CRC. 49,50 Both studies reported per-lesion accuracy data and one study49 also reported per-patient data. These two studies reported a total of 46 diagnoses of metastatic liver lesions. One of these studies included only patients with known liver metastases who were being considered for curative surgery and was therefore rated as having ‘high’ concerns regarding applicability. 50 One study, which compared CEUS and CECT and reported data on the detection of any liver malignancy, was included in this section because the diagnostic status of participants at baseline was unclear and 52 of the 59 positive final diagnoses were liver metastases (primary tumours: colon 43, breast 5, neuroendocrine 2, renal 2); this study was rated ‘unclear’ for concerns regarding applicability. 51 One further study, which did not include a comparator test, was included in this section. 39 This study was included in the review because it reported an inclusion criterion of ‘indeterminate MDCT [multidetector computed tomography]-detected FLLs in patients with known primary cancers’ (various locations) and could therefore provide information on how SonoVue CEUS performs in patients who have had previous imaging other than US and in whom the diagnosis remains uncertain. All studies in this section used histological confirmation in all patients or histological confirmation of imaging-positive patients and follow-up of imaging-negative patients as the reference standard.
Two of the four studies included in this section were reported only as conference abstracts,39,50 resulting in a frequent judgement of ‘unclear’ risk of bias on quality assessment domains (see Table 5). Of the two full papers in this section,49,51 Clevert et al. 51 was rated ‘high’ risk of bias for the ‘flow and timing’ domain of QUADAS-2 because 21% of participants were excluded from the CECT analysis; both studies were judged to be at ‘low’ or ‘unclear’ risk of bias for all other domains. The study by Jonas et al. 50 was rated as ‘high’ risk of bias for the ‘patient selection’ domain because it aimed to assess the ability of imaging modalities to detect liver metastases while including only patients with known liver metastases.
| Study ID | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index test | Comparator test | Reference standard | Flow and timing | Patient selection | |
| Clevert 200951 | ☺ | ☺ | ☺ | ? | ☹ | ? |
| Flor 200939 (abstract only) | ? | ? | NA | ? | ? | ☺ |
| Jonas 201150 (abstract only) | ☹ | ? | ? | ? | ? | ☹ |
| Mainenti 201049 | ☺ | ☺ | ☺ | ? | ☺ | ? |
When definitions of a positive imaging test were reported, studies that assessed imaging tests using vascular contrast media (CEUS, CECT and Gd-CEMRI) gave various descriptions of peripheral rim enhancement as the criteria for liver metastases. In addition, two studies reported data for CEMRI using the hepatocyte-specific contrast agent SPIO. 49,50 Jonas et al. 50 reported 100% specificity and, similarly, high (83–97%) estimates of sensitivity for all three imaging modalities (CEUS, CECT and SPIO-CEMRI). Mainenti et al. 49 also reported high (83–100%) specificity values for all imaging modalities and for both per-lesion and per-patient data. Per-patient sensitivity estimates were also consistent across all imaging modalities (83% in all cases);49 however, for both CEUS and CECT, the sensitivity estimates appeared lower for per-lesion data (50% and 69% respectively) than for per-patient data. 49 For both CEMRI methods, the per-lesion estimate of sensitivity (81%) was similar to the per-patient estimate. 49 By contrast, Clevert et al. 51 reported per-patient data and found similarly high (> 95%) estimates of sensitivity for both CEUS and CECT; however, specificity appeared lower for CECT than for CEUS [71.4% (95% CI 47.8% to 88.7%) and 97.6% (95% CI 87.1% to 99.9%) respectively] and images were non-diagnostic in approximately 15% of CT examinations.
Table 5 provides a summary of the QUADAS-2 assessments for studies in this section and Table 6 summarises individual study results.
| Study ID | Patient or lesion data | Index test or comparator | Reference standard | TP | FN | FP | TN | Sensitivity (95% CI) (%)a | Specificity (95% CI) (%)a | Non-diagnostic (n patients/lesions) | Adverse events | Acceptability to patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRC | ||||||||||||
| SonoVue CEUS compared with CECT and CEMRI | ||||||||||||
| Jonas 201150 (abstract only) | n = 48 FLLs in 20 patients (by lesion data) | CEUS SonoVue M = positive |
Histology in all resected test-positive lesions | 26 | 4 | 0 | 18 | 86.7 (69.3 to 96.2) | 100 (81.5 to 100) | NR | NR | NR |
| SPIO-CEMRI (no further details reported) M = positive |
29 | 1 | 0 | 18 | 96.7 (82.8 to 99.9) | 100 (81.5 to 100) | ||||||
| All patients followed up for at least 36 months | ||||||||||||
| CECT (no further details reported) M = positive |
25 | 5 | 0 | 18 | 83.3 (65.3 to 94.4) | 100 (81.5 to 100) | ||||||
| Mainenti 201049 | n = 34 patients | CEUS SonoVue M = positiveb |
FNB for imaging test positive | 5 | 1 | 4 | 24 | 83.3 (35.9 to 99.6) | 85.7 (67.3 to 96.0) | None | NR | NR |
| n = 57 FLLs | 8 | 8 | 5 | 36 | 50.0 (24.7 to 75.3) | 87.8 (73.8 to 95.9) | ||||||
| n = 34 patients | CECT with Aquilion 4 (Toshiba) M = positivec |
12 months' follow-up for imaging test negative | 5 | 1 | 1 | 27 | 83.3 (35.9 to 99.6) | 96.4 (81.7 to 99.9) | ||||
| n = 57 FLLs | 11 | 5 | 7 | 34 | 68.8 (41.3 to 89.0) | 82.9 (67.9 to 92.8) | ||||||
| n = 34 patients | Gd-CEMRI (Gyroscan Intera 1.5T, Philips Medical Systems, Best, the Netherlands) M = positived |
5 | 1 | 0 | 28 | 83.3 (35.9 to 99.6) | 100 (87.7 to 100) | |||||
| n = 57 FLLs | 13 | 3 | 0 | 41 | 81.3 (54.4 to 96.0) | 100 (91.4 to 100) | ||||||
| n = 34 patients | SPIO-CEMRI M = positivee |
5 | 1 | 1 | 27 | 83.3 (35.9 to 99.6) | 96.4 (81.7 to 99.9) | |||||
| n = 57 FLLs | 13 | 3 | 1 | 40 | 81.3 (54.4 to 96.0) | 97.6 (87.1 to 99.9) | ||||||
| Other primary tumours | ||||||||||||
| SonoVue CEUS compared with CECT | ||||||||||||
| Clevert 200951 | n = 100 patients (maximum five lesions per patient) | CHI SonoVue Any liver malignancy = positivef |
Histology for all FLLs | 58 | 1 | 1 | 40 | 98.3 (90.9 to 100) | 97.6 (87.1 to 99.9) | None | NR | NR |
| n = 92g patients (maximum five lesions per patient) | CECT, Somatom Sensation 16 or 64 (Siemens) Any liver malignancy = positivef |
56 | 2 | 6 | 15 | 96.6 (88.1 to 99.6) | 71.4 (47.8 to 88.7) | 13 (excluded from analysis) | NR | NR | ||
| SonoVue CEUS following inconclusive CECT/CEMRI | ||||||||||||
| Flor 200939 (abstract only) | n = 26 FLLs | CEUS SonoVue M = positive |
FNB or 3- to 6-month follow-up | 4 | 1 | 0 | 21 | 80.0 (28.4 to 99.5) | 100 (83.9 to 100) | None | NR | NR |
Accuracy of SonoVue contrast-enhanced ultrasound for the characterisation of incidentally detected focal liver lesions
Five studies reported comparisons of SonoVue CEUS with other imaging techniques for the characterisation of incidentally detected liver lesions identified by unenhanced US. 41,52,54–56 All of these studies reported accuracy data for the differentiation of malignant from benign liver lesions and three studies also provided stratified data for the identification of HCC and the identification of liver metastases. 52,55,56 All but one of the studies in this section reported data on one lesion per patient and the remaining study41 reported per-lesion data for 694 lesions in 686 patients. Therefore, although data are reported per lesion, all results reported in this section can be considered equivalent to per-patient test performance. Four studies compared SonoVue CEUS with CECT41,52,54,55 and one of these52 also reported data on the combined performance of SonoVue CEUS and CECT, with a positive result on either test treated as positive. One study compared SonoVue CEUS with CEMRI. 55 No study reported comparative accuracy data for all three imaging modalities. None of the comparative accuracy studies described in this section explicitly stated that patients had an uncertain diagnosis following unenhanced US, although all patients had a prior unenhanced US examination and therefore the applicability criterion for the quality assessment was rated ‘unclear’ in all cases.
One further study, which did not include a comparator test, was included in this section. 53 This study was included in the review because it reported an inclusion criterion of ‘previous US and/or CT that had suggested the possibility of malignant liver lesions (not sufficiently proven benignancy)’ and could therefore provide information on how SonoVue CEUS performs in patients who have had previous imaging other than US and in whom the diagnosis remains uncertain. Altogether, the six studies included in this section reported 805 diagnoses of malignant liver lesions; these included 459 HCC, 333 liver metastases and 13 CCC. It should be noted that overlap between the study populations of Seitz et al. 55 and Seitz et al. 56 is highly likely as these two publications by the same group reported a very similar study design and identical recruitment periods; Seitz et al. 55 reported a comparison of SonoVue CEUS with CECT and Seitz et al. 56 reported a comparison of SonoVue CEUS with CEMRI in a smaller group of patients. All but one41 of the studies in this section used histological confirmation in all patients or histological confirmation of imaging-positive patients and follow-up of imaging-negative patients as the reference standard.
Studies were generally poorly reported, resulting in a judgement of ‘unclear’ risk of bias for many of the QUADAS-2 domain assessments. No study in this section reported recruiting a consecutive or random sample of participants and the ‘patient selection’ domain of QUADAS-2 was consequently rated ‘high’ or ‘unclear’ risk of bias in all cases. In addition, one study53 excluded patients who were unable to undergo biopsy and both Seitz et al. studies55,56 divided participants into two subgroups based on probable diagnoses after unenhanced US (‘suspected benign’ and ‘suspected malignant’). For the Seitz et al. studies, accuracy data could be extracted only for the ‘suspected malignant’ subgroup; this may have resulted in a higher than usual prevalence of malignancy and possible overestimation of test performance. Two studies were also rated as ‘high’ risk of bias for the ‘flow and timing’ domain, in one case52 because more than half of the participants initially recruited were excluded from the analyses (either because more than 1 month had elapsed between SonoVue CEUS and CECT or because positive lesions could not be confirmed by pathology) and in the second case41 because the reference standard used was not independent of the index test results. This study was also rated ‘high’ risk of bias for the ‘reference standard’ domain because a suboptimal reference standard (concordance between at least two imaging modalities) was used in the majority of cases.
All of the comparative accuracy studies in this section reported no significant difference in the accuracy of CEUS and CECT or CEMRI for the characterisation of focal FLLs. 41,52,54–56 The primary analysis in all studies was for the differentiation of malignant from benign lesions. Studies used similar criteria to define HCC (hyperenhancement in the arterial phase followed by portal venous/late phase washout) and liver metastases (peripheral rim enhancement in the arterial phase, decreasing in the portal venous and late phases). These criteria are consistent with the typical enhancement patterns described in the EFSUMB guideline on the use of CEUS6 (see Table 1). Pooled estimates of test performance for distinguishing malignant from benign FLLs, derived from the four studies that compared CEUS with CECT,41,52,54–56 indicated that sensitivity and specificity were similar for the two imaging modalities. The pooled estimates for the sensitivity of CEUS and CECT were 95.1% (95% CI 93.3% to 96.6%) and 94.6% (95% CI 92.7% to 96.1%) respectively. The pooled estimates for the specificity of CEUS and CECT were 93.8% (95% CI 90.4% to 96.3%) and 93.1% (95% CI 89.6% to 95.8%) respectively. I2 values were moderate (50–75%) for CEUS and high (> 75%) for CECT. Figures 4 and 5 illustrate the sensitivity and specificity values for each study comparing CEUS and CT, with pooled estimates. Sensitivity analyses excluding the study that used a suboptimal reference standard41 showed a trend towards lower estimates of test performance and reduced heterogeneity (I2 values were low, < 50%, in all cases). The new pooled estimates for the sensitivity of CEUS and CECT were 92.3% (95% CI 88.2% to 95.3%) and 87.4% (95% CI 82.7% to 91.3%), respectively, and the new pooled estimates for specificity were 88.2% (95% CI 79.8% to 93.9%) and 82.8% (95% CI 73.6% to 89.8%) respectively. It should be noted that exclusion of the study by Solbiati41 resulted in a large reduction in sample size (694 FLLs from a total sample size of 1038 FLLs) and hence greater imprecision (wider CIs) in the estimates of sensitivity and specificity.
FIGURE 4.
Forest plot of sensitivity and specificity of CEUS for the identification of any liver malignancy in patients with incidentally detected FLLs.
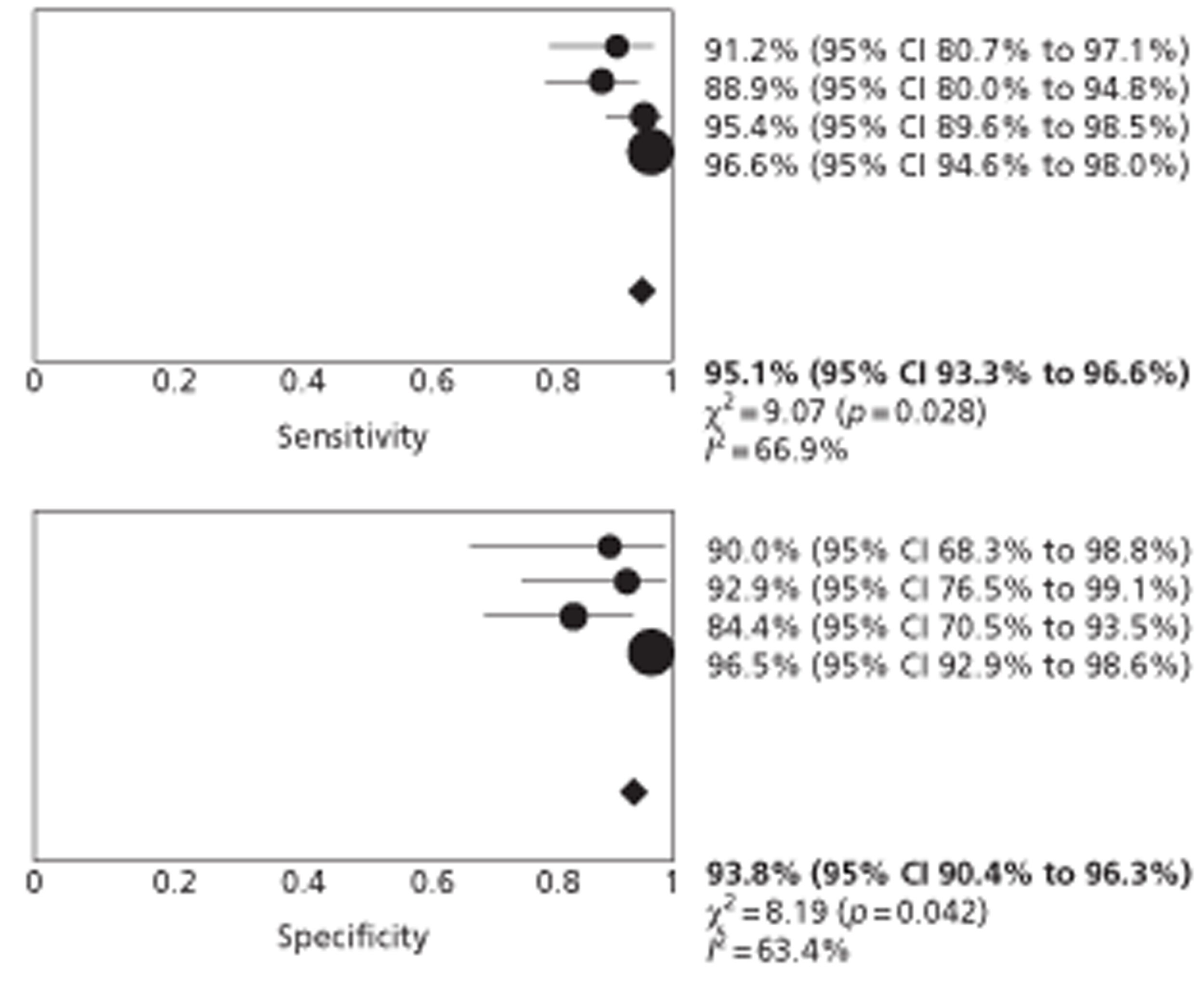
FIGURE 5.
Forest plot of sensitivity and specificity of CECT for the identification of any liver malignancy in patients with incidentally detected FLLs.
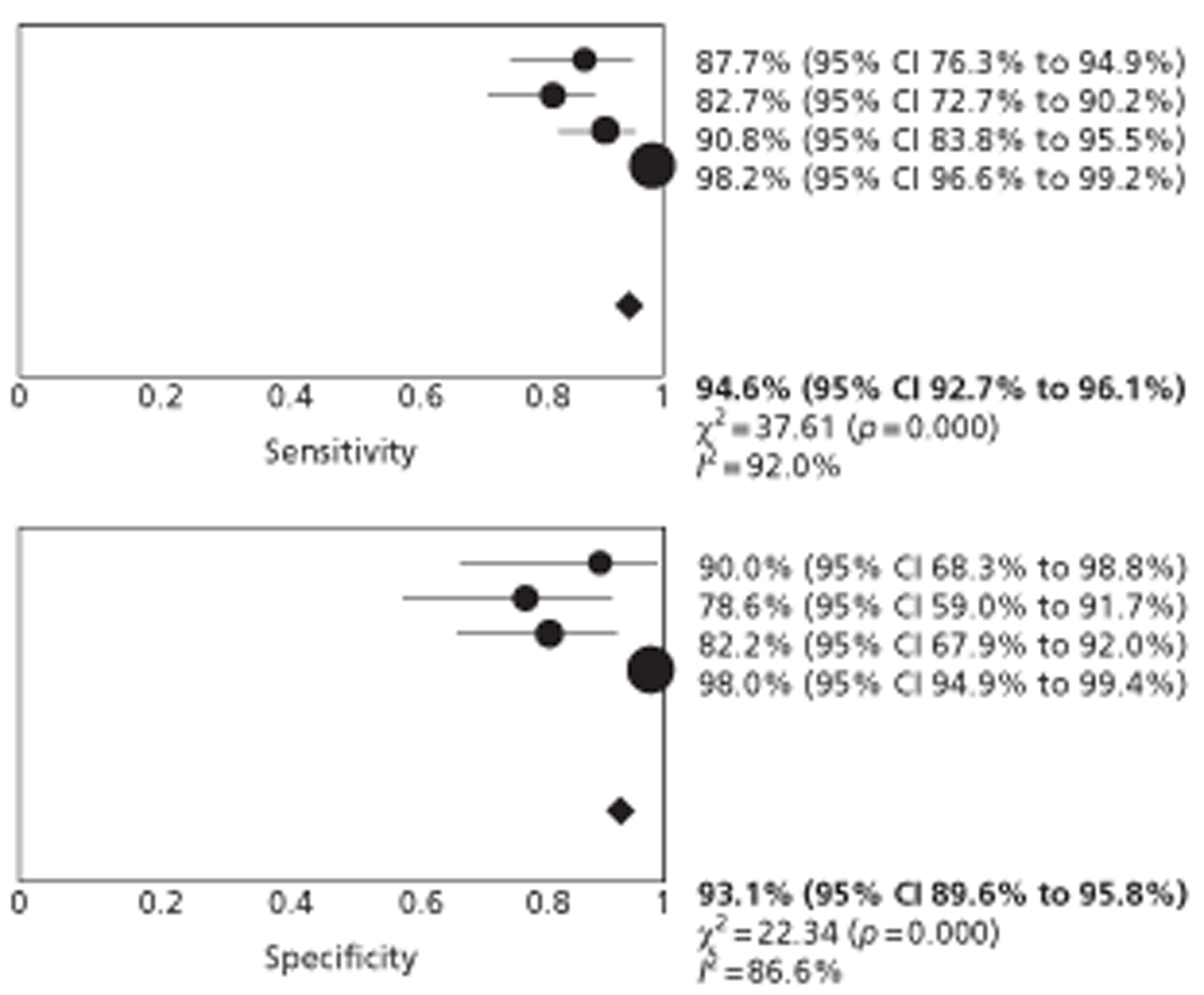
The single study that compared CEUS with CEMRI found no significant difference between the performance of the two imaging modalities for the differentiation of malignant from benign FLLs. The reported sensitivities were 90.0% (95% CI 80.0% to 97.0%) and 81.8% (95% CI 69.1% to 90.9%), respectively, and the reported specificities were 66.7% (95% CI 46.3% to 83.5%) and 63.0% (95% CI 42.4% to 80.6%) respectively. This study used gadolinium-enhanced MRI in all patients, with the addition of SPIO-MRI in an unspecified number of patients.
One study reported sufficient data to allow calculation of sensitivity and specificity for the combination of CEUS and CECT, with a positive finding on either imaging technique treated as ‘test positive’. 52 These data indicated that the addition of CECT to the imaging workup would not increase the accuracy of diagnosis over that obtained by CEUS alone; the sensitivity and specificity of CEUS for differentiating malignant from benign lesions were 91.1% (95% CI 78.8% to 97.5%) and 93.8% (95% CI 79.2% to 99.2%), respectively, and for CEUS and CECT combined were 93.3% (95% CI 81.7% to 98.6%) and 93.8% (95% CI 79.2% to 99.2%) respectively. Three studies reported sufficient data to derive estimates of test performance by lesion type (HCC and liver metastases), two comparing CEUS and CECT52,55 and one comparing CEUS and CEMRI. 56 The sensitivity and specificity of CEUS and CECT were similar for the characterisation of HCC; however, one study indicated that CEUS may be more sensitive than CECT for the characterisation of metastases [92.9% (95% CI 82.7% to 98.0%) compared with 67.9% (95% CI 54.0% to 79.7%)]. 55 The sensitivity and specificity of CEUS and CEMRI were similar for both HCC and liver metastases. 56
Table 7 provides a summary of the QUADAS-2 assessments for studies in this section and Table 8 summarises individual study results. Figure 6 shows the results for differentiation of malignant from benign FLLs for all studies in this section, plotted in the ROC plane.
| Study ID | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index test | Comparator test | Reference standard | Flow and timing | Patient selection | |
| Catala 200752 | ☹ | ☺ | ☺ | ? | ☹ | ? |
| Gierbliński 200853 | ☹ | ? | NA | ☺ | ? | ? |
| Li 200754 | ? | ☺ | ☺ | ? | ☺ | ? |
| Seitz 200955 | ☹ | ? | ? | ? | ? | ? |
| Seitz 201056 | ☹ | ? | ? | ? | ? | ? |
| Solbiati 200641 (abstract only) | ☹ | ? | ? | ☹ | ☹ | ? |
| Study ID | Patient or lesion data | Index test or comparator | Reference standard | TP | FN | FP | TN | Sensitivity (95% CI) (%)a | Specificity (95% CI) (%)a | Non-diagnostic | Adverse events | Acceptability to patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC | ||||||||||||
| SonoVue CEUS compared with CECT | ||||||||||||
| Catala 200752 | n = 77 patients (one lesion per patient) | CEUS SonoVue HCC = positiveb CECT with Somatom Plus 4 (Siemens) HCC = positiveb CEUS + CECT HCC=either test positive |
Histology following biopsy or surgery for test positive, MRI and follow-up ≥ 12 months for test negative | 41 | 4 | 2 | 30 | 91.1 (78.8 to 97.5) | 93.8 (79.2 to 99.2) | None | NR | NR |
| 39 | 6 | 2 | 30 | 86.7 (73.2 to 94.9) | 93.8 (79.2 to 99.2) | |||||||
| 42 | 3 | 2 | 30 | 93.3 (81.7 to 98.6) | 93.8 (79.2 to 99.2) | |||||||
| Seitz 200955 (related publication Seitz 201056) | Subgroup B (suspected malignant lesion)c | CEUS SonoVue HCC = positive |
FNB n = 154 (remaining four lesions excluded) | 34 | 6 | 4 | 110 | 85.0 (70.2 to 94.3) | 96.5 (91.3 to 99.0) | None | NR | NR |
| n = 158 FLLs (one lesion per patient) | CECT (device not specified) HCC = positive |
28 | 12 | 6 | 108 | 70.0 (53.5 to 83.4) | 94.7 (88.9 to 98.0) | |||||
| SonoVue CEUS compared with CEMRI | ||||||||||||
| Seitz 201056 (related publication Seitz 200955) | Subgroup B (suspected malignant lesion)c | CEUS SonoVue HCC = positive |
FNB n = 82 (two lesions excluded) | 23 | 6 | 11 | 42 | 79.3 (60.3 to 92.0) | 79.2 (65.9 to 89.2) | NR | NR | NR |
| n = 84 FLLs (one lesion per patient) | Gd-CEMRI and SPIO-CEMRI in some cases (number unspecified) (device not specified) HCC = positive |
24 | 5 | 13 | 13 | 82.8 (64.2 to 94.2) | 75.5 (61.7 to 86.2) | |||||
| SonoVue CEUS following inconclusive CECT/CEMRI | ||||||||||||
| Gierbliński 200853 | n = 100 patients (one lesion per patient) | CEUS SonoVue HCC = positiveb |
FNB with clinical and imaging follow-up for biopsy-negative patients | 7 | 2 | 1 | 90 | 77.8 (40.0 to 97.2) | 98.9 (94.0 to 100) | None | NR | NR |
| Liver metastases | ||||||||||||
| SonoVue CEUS compared with CECT | ||||||||||||
| Catala 200752 | n = 77 patients (one lesion per patient) | CEUS SonoVue M = positived |
Histology following biopsy or surgery for test positive, MRI and follow-up ≥ 12 months for test negative | 11 | 1 | 0 | 65 | 91.7 (61.5 to 99.8) | 100 (94.5 to 100) | None | NR | NR |
| CECT with Somatom Plus 4 (Siemens) M = positived pattern |
11 | 1 | 0 | 65 | 91.7 (61.5 to 99.8) | 100 (94.5 to 100) | ||||||
| CEUS + CECT M = either test positive |
11 | 1 | 0 | 65 | 91.7 (61.5 to 99.8) | 100 (94.5 to 100) | ||||||
| Seitz 200955 (related publication Seitz 201056) | Subgroup B (suspected malignant lesion)c | CEUS SonoVue M = positive |
FNB n = 154 (four lesions excluded) | 52 | 4 | 17 | 81 | 92.9 (82.7 to 98.0) | 82.7 (73.7 to 89.6) | None | NR | NR |
| n = 158 FLLs (one lesion per patient) | CECT (device not specified) M = positive |
38 | 18 | 23 | 75 | 67.9 (54.0 to 79.7) | 76.5 (66.9 to 84.5) | |||||
| SonoVue CEUS compared with CEMRI | ||||||||||||
| Seitz 201056 (related publication Seitz 200955) | Subgroup B (suspected malignant lesion)c | CEUS SonoVue M = positive |
FNB n = 82 (two lesions excluded) | 17 | 5 | 15 | 45 | 77.3 (54.6 to 92.2) | 75.0 (62.1 to 85.3) | NR | NR | NR |
| n = 84 FLLs (one lesion per patient) | Gd-CEMRI and SPIO-CEMRI in some cases (number unspecified) (device not specified) HCC = positive |
14 | 8 | 14 | 46 | 63.6 (40.7 to 82.8) | 76.7 (64.0 to 86.6) | |||||
| SonoVue CEUS following inconclusive CECT/CEMRI | ||||||||||||
| Gierbliński 200853 | n = 100 patients (one lesion per patient) | CEUS SonoVue M = positivee |
FNB with clinical and imaging follow-up for biopsy-negative patients | 13 | 1 | 2 | 84 | 92.9 (66.1 to 99.8) | 97.7 (91.9 to 99.7) | None | NR | NR |
| Any malignancy | ||||||||||||
| SonoVue CEUS compared with CECT | ||||||||||||
| Catala 200752 | n = 77 patients (one lesion per patient) | CEUS SonoVue Any malignancy (HCCb or Md) = positive CECT with Somatom Plus 4 (Siemens) Any malignancy (HCCb or Md) = positive CEUS + CECT Either test positive = positive |
Histology following biopsy or surgery for index test positive, MRI and follow-up ≥ 12 months for index test negative | 52 | 5 | 2 | 18 | 91.2 (80.7 to 97.1) | 90.0 (68.3 to 98.8) | None | NR | NR |
| 50 | 7 | 2 | 18 | 87.7 (76.3 to 94.9) | 90.0 (68.3 to 98.8) | |||||||
| 53 | 4 | 2 | 18 | 93.0 (83.0 to 98.1) | 90.0 (68.3 to 98.8) | |||||||
| Li 200754 | n = 109 patients (one lesion per patient) | CEUS SonoVue Any malignancy (HCC,f CCC,g Mh) = positive |
Histopathology following surgical resection or FNB | 72 | 9 | 2 | 26 | 88.9 (80.0 to 94.8) | 92.9 (76.5 to 99.1) | Three lesions not visualised. All were malignant and are classified as FN | NR | NR |
| CECT with Somatom Sensation (Siemens) Any malignancy (HCC, CCC, M) = positive |
67 | 14 | 6 | 22 | 82.7 (72.7 to 90.2) | 78.6 (59.0 to 91.7) | Seven lesions not visualised. Five were malignant and are classified as FN; two were benign and are classified as TN | |||||
| Seitz 200955 (related publication Seitz 201056) | Subgroup B (suspected malignant lesion)c n = 158 FLLs (one lesion per patient) |
CEUS SonoVue Any malignancy = positivei CECT (device not specified) Any malignancy = positive |
FNB n = 154 (four lesions excluded) | 104 | 5 | 7 | 38 | 95.4 (89.6 to 98.5) | 84.4 (70.5 to 93.5) | None | NR | NR |
| 99 | 10 | 8 | 37 | 90.8 (83.8 to 95.5) | 82.2 (67.9 to 92.0) | |||||||
| Solbiati 200641 (abstract only) | n = 694 FLLs in 686 patients, one lesion missing from analysis (per-lesion data) | CEUS SonoVue Any malignancy (HCC, M, CCC) = positive CECT (device not specified) Any malignancy (HCC, M, CCC) = positive |
Concordant CEUS and CT result (n = 656) or FNB when results were discordant (n = 38) | 478 | 17 | 7 | 191 | 96.6 (94.6 to 98.0) | 96.5 (92.9 to 98.6) | One (results missing for one lesion) | NR | NR |
| 486 | 9 | 4 | 194 | 98.2 (96.6 to 99.2) | 98.0 (94.9 to 99.4) | |||||||
| SonoVue CEUS compared with CEMRI | ||||||||||||
| Seitz 201056 (related publication Seitz 200955) | Subgroup B (suspected malignant lesion)c n = 84 FLLs (one lesion per patient) |
CEUS SonoVue Any malignancy = positivee |
FNB n = 82 (two lesions excluded) | 50 | 5 | 9 | 18 | 90.9 (80.0 to 97.0) | 66.7 (46.3 to 83.5) | Nine lesions (six benign and three malignant); these were classified as FP and FN respectively | NR | NR |
| Gd-CEMRI and SPIO-CEMRI in some cases (number unspecified) (device not specified) HCC = positive |
45 | 10 | 10 | 17 | 81.8 (69.1 to 90.9) | 63.0 (42.4 to 80.6) | Nine lesions (three benign and six malignant); these were classified as FP and FN respectively | |||||
| SonoVue CEUS following inconclusive CECT/CEMRI | ||||||||||||
| Gierbliński 200853 | n = 100 patients (one lesion per patient) | CEUS SonoVue Any malignancy (HCCb or Me) = positive |
FNB with clinical and imaging follow-up for biopsy-negative patients | 21 | 2 | 3 | 74 | 91.3 (72.0 to 98.9) | 96.1 (89.0 to 99.2) | None | NR | NR |
FIGURE 6.
Receiver operating characteristic plane plot comparing performance of imaging tests for the differentiation of malignant from benign lesions in patients with incidentally detected FLLs.
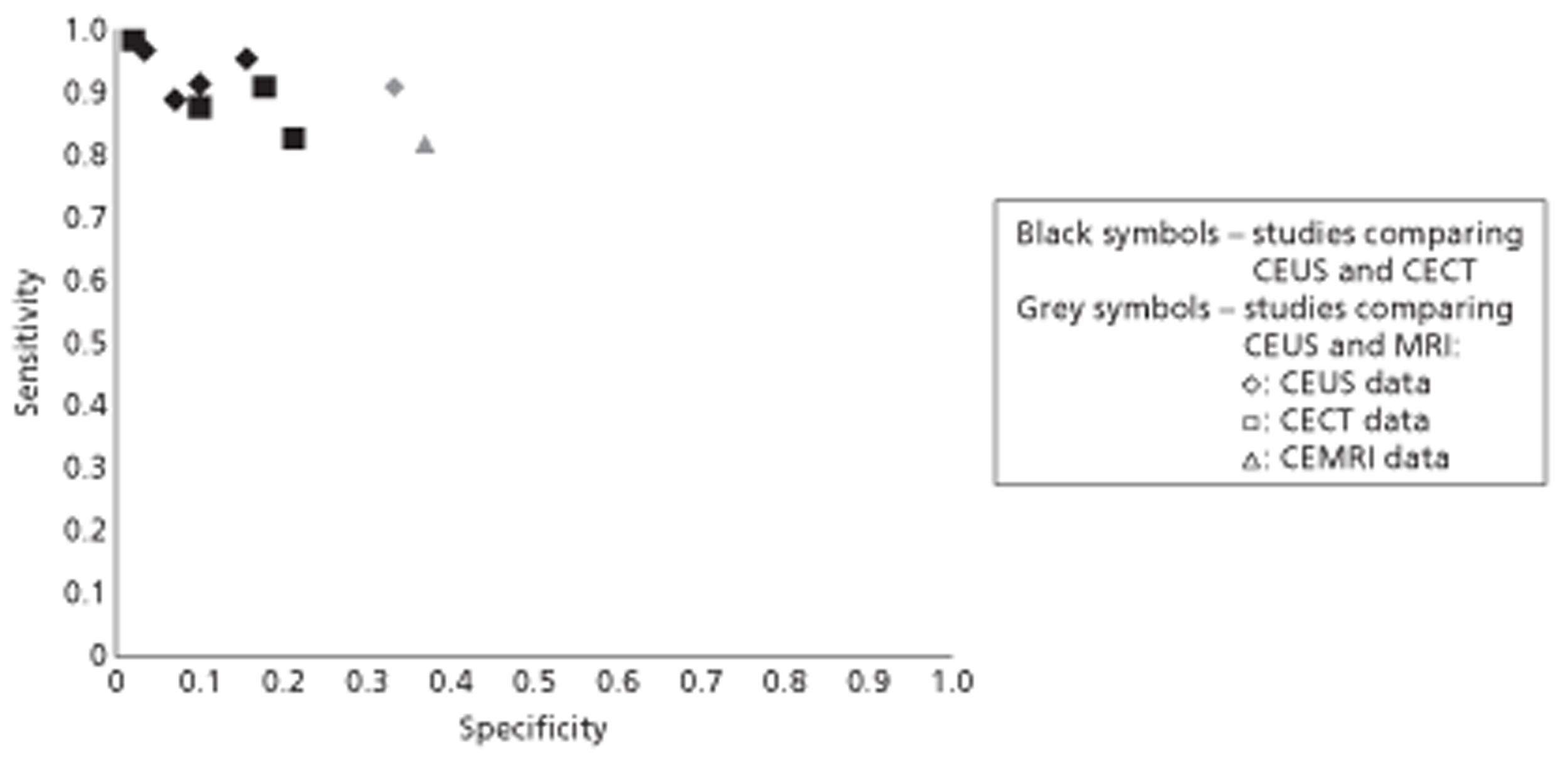
Accuracy of SonoVue contrast-enhanced ultrasound for the determination of treatment success in patients with known liver malignancy
Three studies reported comparisons of SonoVue CEUS with other imaging modalities for the assessment of treatment success (complete response) in patients with malignant liver lesions (mainly HCC). 40,57,58 Two were Chinese-language publications57,58 and the other was published only as a conference abstract. 40 The two Chinese studies reported per-lesion data, with one57 reporting only one lesion per patient, and the remaining study reported only per-patient data. 40 The studies assessed patients following cryosurgery,57 RFA40 and ‘non-surgical treatment’. 58 Sample sizes were small: in total, studies reported data for 105 lesions (102 HCC and three liver metastases) in 97 patients. All three studies included only patients who were undergoing treatment for known liver malignancies and all studies were therefore rated as having ‘low’ concerns regarding applicability.
Studies were generally poorly reported and all QUADAS-2 risk of bias domains were rated ‘unclear’.
One of the two Chinese studies compared CEUS with CECT or CEMRI (numbers of patients receiving CECT and CEMRI, respectively, were not specified)57 and the other compared CEUS with CECT. 58 Both studies reported similar, high sensitivity (95.5–100%) and specificity (83.3–100%) for all imaging modalities, although small sample sizes resulted in wide CIs. One study reported sufficient data to allow the calculation of sensitivity and specificity for the combination of CEUS and CECT, with a negative finding on either imaging technique treated as ‘test negative’ for complete response. 58 These data indicated that the addition of CECT would not increase the accuracy of the assessment of response to treatment over that obtainable by CEUS alone; the sensitivity and specificity of CEUS for detecting complete response were 97.8% (95% CI 88.5% to 99.9%) and 94.4% (95% CI 72.7% to 99.9%), respectively, and for CEUS and CECT combined were 97.8% (95% CI 88.5% to 99.9%) and 100% (95% CI 81.5% to 100%) respectively. The remaining study compared CEUS with Gd-CEMRI and included only 15 patients undergoing RFA, with five final diagnoses of ‘complete ablation’. 40 The results of the two techniques were identical; sensitivity for the detection of complete ablation was 80% (95% CI 28.4% to 99.5%) and there were nine false-positives, resulting in a very low estimate of specificity [10.0% (95% CI 3.0% to 44.5%)].
Table 9 provides a summary of the QUADAS-2 assessments for studies in this section and Table 10 summarises individual study results.
| Study ID | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index test | Comparator test | Reference standard | Flow and timing | Patient selection | |
| Feng 200757 | ? | ? | ? | ? | ? | ☺ |
| Lüttich 200640 (abstract only) | ? | ? | ? | ? | ? | ☺ |
| Zhou 200758 | ? | ? | ? | ? | ? | ☺ |
| Study ID | Patient or lesion data | Index test or comparator | Reference standard | TP | FN | FP | TN | Sensitivity (95% CI) (%)a | Specificity (95% CI) (%)a | Non-diagnostic | Adverse events | Acceptability to patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feng 200757 (Chinese language) | n = 26 malignant (23 HCC, 3 M) lesions in 23 patients treated with cryosurgery | CEUS SonoVuecomparatTest positive = complete response to treatment CECT or CEMRI (no details reported) Test positive = complete response to treatment |
Histopathological diagnosis | 20 | 1 | 0 | 5 | 100 (83.2 to 100) | 83.3 (35.9 to 99.6) | None | NR | NR |
| Lüttich 200640 (abstract only) | n = 15 patients treated with RFA | Sulphur hexafluoride CEUS Test positive = complete ablation Gd-CEMRI (no details reported) Test positive = complete ablation |
Biopsy | 4 | 1 | 9 | 1 | 80.0 (28.4 to 99.5) | 10.0 (3.0 to 44.5) | None | NR | NR |
| 4 | 1 | 9 | 1 | 80.0 (28.4 to 99.5) | 10.0 (3.0 to 44.5) | |||||||
| Zhou 200758 (Chinese language) | n = 64 HCC lesions in 56 patients who had undergone non-surgical treatment | CEUS SonoVue Test positive = complete response to treatment (no enhancement) CECT, Somatom Balance, (Siemens) Test positive = response to treatment (no enhancement) |
Positive imaging test (no enhancement) confirmed by imaging follow-up at 3 months Negative imaging test (partial enhancement) confirmed by FNB |
45 | 1 | 1 | 17 | 97.8 (88.5 to 99.9) | 94.4 (72.7 to 99.9) | None | NR | NR |
| 45 | 1 | 3 | 15 | 97.8 (88.5 to 99.9) | 83.3 (58.6 to 96.4) | |||||||
| CEUS + CECT Either test negative (partial enhancement) = negative |
45 | 1 | 0 | 18 | 97.8 (88.5 to 99.9) | 100 (81.5 to 100) |
Effectiveness of SonoVue contrast-enhanced ultrasound for treatment planning in patients with known liver malignancy
One controlled clinical trial compared SonoVue CEUS with unenhanced US (control) when added to routine imaging (CECT or CEMRI) for pretreatment assessment of patients undergoing RFA for HCC. 59 This study assessed the effect of CEUS on treatment effectiveness (successful ablation) as the primary outcome measure. Secondary outcomes were incidence of tumour progression, new HCC, repeat RFA and post-therapy complications, and duration of local progression-free survival and new tumour-free survival. The CEUS and control groups were similar at baseline in terms of age, gender distribution, numbers who had CECT and numbers who had CEMRI, TNM (tumour, lymph node, metastases) stage, tumour size and number, and numbers who had Child–Pugh class A cirrhosis.
This non-randomised study was considered to have ‘risk of bias’ in a number of areas. Alternate allocation of patients to the CEUS and control groups means that clinicians could predict patient allocation before recruitment. The nature of the study precluded the blinding of patients, and the blinding of assessors and/ or clinicians planning RFA protocols was not clear. Finally, 14 patients who were considered unsuitable for RFA after imaging assessment (nine in the CEUS group and five in the control group) were excluded from the analyses.
There were no significant differences in the rates of successful ablation (primary outcome) or post-therapy complications between the CEUS group and the control group. Use of CEUS in the pretreatment imaging protocol was found to significantly reduce incidence of tumour progression, new HCC and repeat RFA over a 2-year follow-up period; ORs were 0.35 (95% CI 0.13 to 0.95), 0.34 (95% CI 0.16 to 0.72) and 0.33 (95% CI 0.17 to 0.66) respectively. The use of CEUS also increased local progression-free survival [mean difference 7.2 months (95% CI 6.6 months to 7.8 months)] and new tumour-free survival [mean difference 11.7 months (95% CI 11.1 months to 12.3 months)].
Table 11 provides a summary of the risk of bias assessment for this study and Table 12 summarises the results.
| Item | Judgement | Description |
|---|---|---|
| Adequate sequence generation? | Yes | Alternate allocation |
| Allocation concealment? | No | Alternate allocation means that assignment of an individual patient to a test group can be easily predicted |
| Blinding? | No | Patients could not be blinded to the tests being undertaken and it was not clear whether or not those assessing the efficacy of treatment were aware of test allocations. It was not clear if those who designed the RFA protocol knew the results of CEUS and US or of only one of the tests |
| Were patient characteristics comparable at baseline? | Yes | |
| Incomplete outcome data addressed? | Yes | All outcomes assessed appear to be reported for all patients |
| Free of selective reporting? | Yes | All outcomes assessed appear to be reported for all patients |
| Free of other bias? | No | Patients in both groups who were judged to be unsuitable for RFA were excluded from the analyses |
| Study ID | Population | Intervention | Comparator | Outcomea | n with outcome (I) | n with outcome (C) | OR (95% CI)b | Mean ± SD(I) | Mean ± SD(C) | Mean difference (95% CI)a |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen 200759 (related publication60) | Patients with HCC, undergoing RFA treatment | CEUS SonoVue and CECT or CEMRI before treatment (83 patients, 114 tumours) | US and CECT or CEMRI before treatment (82 patients, 107 tumours) | Primary treatment effectiveness (successful ablation) | 77 | 71 | 1.99 (95% CI 0.70 to 5.66) | NA | NA | NA |
| Tumour progression | 6 | 15 | 0.35 (95% CI 0.13 to 0.95) | NA | NA | NA | ||||
| New HCC | 13 | 29 | 0.34 (95% CI 0.16 to 0.72) | NA | NA | NA | ||||
| Repeat RFA | 17 | 36 | 0.33 (95% CI 0.17 to 0.66) | NA | NA | NA | ||||
| Local progression-free survival | NA | NA | NA | 40.5 ± 1.9 months | 33.3 ± 2.2 months | 7.2 (95% CI 6.6 to 7.8) | ||||
| New tumour-free survival | NA | NA | NA | 38.1 ± 2.0 months | 26.4 ± 2.0 months | 11.7 (95% CI 11.1 to 12.3) | ||||
| Post-therapy complications | 1 | 3 | 0.32 (95% CI 0.03 to 3.15) | NA | NA | NA |
Summary of clinical effectiveness results
Twenty of the 21 studies included in the systematic review were DTA studies: seven compared the performance of imaging modalities for the characterisation of FLLs detected on surveillance of cirrhosis patients using unenhanced US; four compared the performance of imaging modalities for the detection of liver metastases in patients with known primary cancer (CRC); six compared the performance of imaging modalities for the characterisation of incidentally detected FLLs identified by unenhanced US; and three compared the performance of imaging modalities for the determination of treatment response in patients with liver cancer.
The majority of included test accuracy studies were judged to be at ‘low’ or ‘unclear’ risk of bias with respect to the ‘index test’, ‘comparator test’ and ‘reference standard’ domains. ‘Unclear’ ratings for these domains most frequently arose from insufficient detail in the reporting of how tests were interpreted, particularly blinding of interpreters to other test results. Reporting quality was generally poor and a number of studies were reported only as conference abstracts, resulting in a high proportion of ‘unclear’ risk of bias ratings across domains (Figure 7). ‘High’ risk of bias ratings for the ‘patient selection’ domain arose from the use of a retrospective study design or from inappropriate exclusions of particular patient groups (e.g. exclusion of patients with a low probability of malignancy). ‘High’ risk of bias ratings for the ‘flow and timing’ domain arose from exclusion of > 10% of patients from analyses or, in two cases, from incorporation of index test results in the reference standard. The last two studies were also rated as ‘high’ risk of bias for the ‘reference standard’ domain.
FIGURE 7.
Summary of QUADAS-2 assessments.
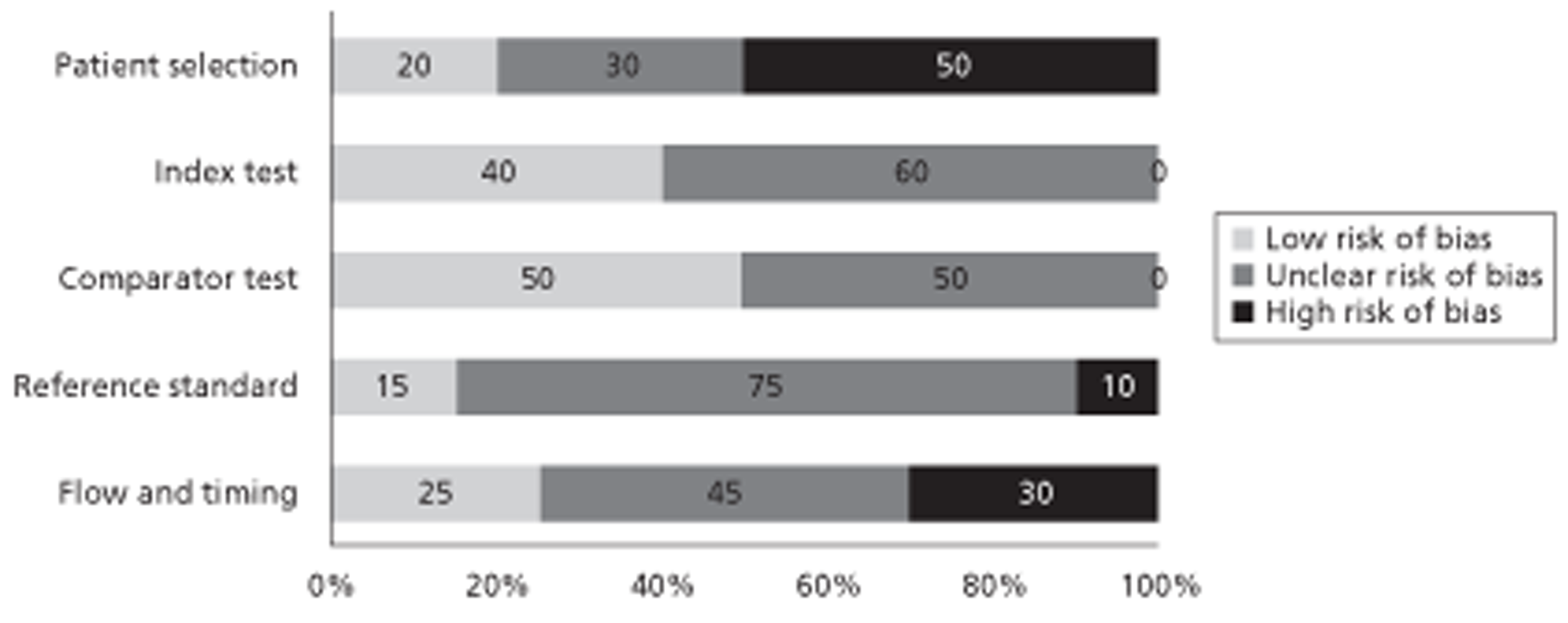
Studies varied in terms of target condition (HCC, liver metastases or ‘any malignancy’), definitions of a positive imaging test in studies of the same target condition, and lesion size assessed. Overall, there was no clear indication that any of the imaging modalities (CEUS, CECT or CEMRI) or contrast media considered offered superior performance for any of the clinical applications assessed.
Studies conducted in cirrhosis patients undergoing routine surveillance all concerned the differentiation of HCC from other lesion types in small to medium (< 30 mm) FLLs. The definition of a positive test for HCC varied, including arterial enhancement followed by portal venous washout, arterial enhancement alone and portal venous washout alone. There was no consistent evidence for any significant difference in test performance between the three imaging modalities and three MRI contrast media assessed. Results were inconsistent for the studies that reported an EFSUMB-consistent definition of HCC (arterial phase enhancement followed by portal venous/late phase washout). One study comparing CEUS and CECT reported high per-lesion sensitivity (91% and 80% respectively) and specificity (87% and 98% respectively) estimates; all lesions in this study were between 10 and 20 mm. Two studies comparing CEUS and Gd-CEMRI reported inconsistent sensitivity estimates for CEUS (93% and 52%), with the lower sensitivity estimate arising from a study that included very small (≤ 10 mm) FLLs. Two studies comparing all three imaging modalities reported similar, high specificity estimates (> 90% in most cases) for all imaging modalities; however, sensitivity estimates were inconsistent between the two studies. Sensitivity estimates were 67% and 27% for CEUS; 67% and 47% for CECT; and 82% and 44% for Gd-CEMRI. Sensitivity estimates from these two studies were generally lower than those in studies that compared only two imaging modalities using a similar definition of HCC and similar lesion size. There was some evidence from one study comparing CEUS and Gd-CEMRI that these techniques may be better at ruling out HCC in FLLs between 11 and 30 mm (sensitivities for CEUS and CEMRI were 92% and 95% respectively) than in small FLLs of ≤ 10 mm (sensitivities 27% and 73% respectively); however, this study did not use an EFSUMB-consistent definition of HCC. There was also some evidence from two studies that combined imaging using CEUS and CECT or all three imaging modalities, in which any positive imaging result was treated as ‘test positive’, that combined imaging may increase sensitivity. Overall, inconsistent estimates of sensitivity mean that it is unclear whether or not CEUS alone is adequate to rule out HCC for FLLs < 30 mm in this population; CEUS alone may be adequate to rule out HCC for FLLs 11–30 mm, with very small FLLs (< 10 mm) not considered.
Studies of the diagnosis of liver metastases using imaging with vascular contrast media (CEUS, CECT and Gd-CEMRI) in which definitions of a positive imaging test were reported gave various descriptions of peripheral rim enhancement as the criterion for liver metastases. Two studies reported data for SPIO-CEMRI. There was no consistent evidence for any significant difference in test performance between the three imaging modalities assessed and different MRI contrast media assessed. Both per-patient and per-lesion sensitivity estimates were generally high in all studies [> 83% for all imaging modalities and both MRI contrast agents in two studies of patients with CRC and > 95% for both CEUS and CECT in a third study of patients with various primary cancers (majority CRC)]. The limited data available indicate that CEUS alone may be adequate to rule out liver metastases in patients with known primary malignancies.
The primary outcome measure reported by studies conducted in patients with incidentally detected FLLs was test accuracy for the differentiation of malignant from benign liver lesions. Studies used arterial enhancement followed by portal venous washout to define a positive test for primary liver cancer (HCC) and peripheral rim enhancement to define a positive test for liver metastases; these criteria are consistent with those defined in the EFSUMB guidelines on the use of CEUS6 (see Table 1). All studies reported no significant difference in the accuracy of CEUS and CECT or CEMRI for the characterisation of focal FLLs. All but one study reported data for one lesion per patient and the remaining study reported data for 694 lesions in 686 patients; data were therefore treated as per patient. The pooled estimates of sensitivity for the detection of ‘any liver malignancy’ were approximately 95% for both CEUS and CECT and the pooled estimates of specificity were 94% and 93%, respectively, based on data from four studies. The single study comparing CEUS with CEMRI used Gd-CEMRI in all patients, with the addition of SPIO-CEMRI in an unspecified number of cases, and reported sensitivity estimates of 91% and 82%, respectively, and specificity estimates of 67% and 63% respectively. Data from one study indicated that combined imaging using both CEUS and CECT, in which a positive result on either modality was treated as ‘test positive’, did not increase sensitivity. High estimates of sensitivity indicate that CEUS alone may be adequate to rule out liver malignancy in this population.
Two Chinese-language studies compared imaging modalities for the assessment of response to treatment (cryosurgery and non-surgical treatment) in patients with HCC. One study compared CEUS and CECT in the same patients and the other compared CEUS and CECT or CEMRI. All sensitivity estimates were > 95% and all specificity estimates were > 80%. These very limited data indicate that CEUS may provide information on response in patients treated for HCC. However, these data are very limited and may not be directly applicable to UK clinical practice; further studies, ideally conducted in a UK setting, are required to confirm findings.
One controlled clinical trial indicated that the inclusion of CEUS in pretreatment imaging protocols for patients undergoing RFA for HCC may result in reduced incidence of disease progression, new HCC and repeat RFA, and increased local progression- and new tumour-free survival compared with unenhanced US. However, no difference was found in the primary outcome, successful ablation. High-quality RCTs are needed to determine the relative effectiveness of different imaging strategies for treatment planning.
Chapter 4 Assessment of cost-effectiveness
Search strategy
Searches were undertaken to identify cost-effectiveness studies of US, MRI and CT in the diagnosis of liver cancer. As with the clinical effectiveness searching, the main EMBASE strategy for each set of searches was independently peer reviewed by a second information specialist using the PRESS EBC checklist. 21 Search strategies were developed specifically for each database and searches took into account generic and other product names for the intervention. No restrictions on language or publication status were applied. Limits were applied to remove animal studies. Full search strategies are reported in Appendix 1.
The following databases were searched for relevant studies from 2000 to October/November 2011:
-
MEDLINE (2000–September 2011 Week 4) (OvidSP)
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (2000–10 October 2011) (OvidSP)
-
EMBASE (2000–11 Week 40) (OvidSP)
-
NHS Economic Evaluation Database (NHS EED) (2000–11) (via The Cochrane Library)
-
NHS EED (1 January 2011–12 October 2011) (CRD website)
-
Health Economic Evaluations Database (HEED) (2000–12 October 2011) (Wiley) (http://onlinelibrary.wiley.com/book/10.1002/9780470510933)
-
SCI (2000–7 October 2011) (Web of Science).
Supplementary searches on FLLs and liver cancers were undertaken on the following resources to identify guidelines and guidance:
-
National Guidelines Clearinghouse (NGC) (2005–10 November 2011) (www.guideline.gov/)
-
Guidelines International Network (GIN) (2005–10 November 2011) (www.g-i-n.net)
-
NICE guidance (up to 10 November 2011) (http://guidance.nice.org.uk/)
-
TRIP database (limited to guidelines) (2005–10 November 2011) (www.tripdatabase.com/)
-
HTA database (2005–10 November 2011) (CRD website).
Identified references were downloaded in EndNote X4 software for further assessment and handling.
References in retrieved articles were checked for additional studies.
Review of economic analyses of SonoVue
A total of 1194 titles and abstracts were screened from which 40 papers were selected. After full-paper screening 36 studies were excluded and four that met the inclusion criteria were included. A summary of each of these studies is provided in Table 13, with a quality checklist based on Drummond and Jefferson63 provided in Table 14. Faccioli et al. 64 developed a decision model to assess the costs of testing for benign FLLs after the introduction of CEUS. In total, 398 benign FLL patients (angiomas, focal nodular hyperplasias and pseudolesions) with suspicious lesions at baseline US from the radiology department of a hospital in Italy between 2002 and 2005 were reviewed and entered into the model. All lesions underwent CEUS and 98 also underwent CT. The average follow-up was 22 months and none of the CEUS diagnoses changed during the follow-up.
| Study details | Faccioli 200764 | Romanini 200766 | Sangiovanni 201047 | Sirli 201067 | Zaim 201168 |
|---|---|---|---|---|---|
| Time horizon | NA | NA | NA | NA | 24 months |
| Objective | To perform a cost analysis of CEUS in the study of benign FLLs with intermediate appearance on US | To evaluate the clinical and economic consequences of the introduction of CEUS into the diagnostic clinical algorithm for the characterisation of incidental FLLs | To assess the sensitivity, specificity, diagnostic accuracy and economic impact of all possible sequential combinations of contrast imaging techniques in patients with cirrhosis with 1- to 2-cm liver nodules undergoing US surveillance | To evaluate if CEUS is a cost-efficient method for the first-line examination for FLL characterisation | To evaluate the cost-effectiveness of the application of CEUS as a diagnostic imaging technique in the first-line characterisation of FLLs in the Netherlands |
| Source of effectiveness information/testing accuracy data | 398 benign FLL patients between 2002 and 2005 | Consecutive patients with FLLs presenting from January 2002 to October 2005 (575 FLLs) | 64 patients with 67 de novo liver nodules | 316 FLLs were included in the CEUS evaluation performed during a 6-month period | 170 prospectively enrolled patients > 18 years were included in a single-centre study at EMC |
|
|
|
AASLD approach:
|
|
|
| Reference standard | NA | CECT/CEMRI | Histology following FNB | NA | Imaging (MRI/CT), biopsy/surgical specimens and clinical judgement |
| Unit costs | Source: Hospital administrative office, SIRM publication,65 resource management service of this hospital | Source: NHS Italy and hospitals | Italian NHS | Source: Mean costs practised in Timisoara | Source: CVZ, Dutch tariffs and EMC69 [all costs reported in 2009 were inflated to 2010 values using the Centraal Bureau voor de Statistiek (CBS) website: www.cbs.nl] |
| Measure of benefit | Measured by the amount of money saved | Measured by the amount of money saved | Measured by the amount of money saved | Measured by the amount of money saved | Life-year gained |
| Study type | Cost analysis | Cost analysis | Cost analysis | Cost comparison study (prospective study) | Cost-effectiveness study |
| Model assumptions | NA | NA | NA | NA | The costs and effectiveness calculation for metastatic patients does not include secondary sites |
| Patients belonging to the benign group assumed to have the same life expectancy | |||||
| It was assumed that the time horizon does not capture patients undergoing liver transplantation; only screening for liver transplantation was included in the analysis | |||||
| All treatment, follow-up and non-follow-up costs and probabilities were assumed based on the reference standard | |||||
| Perspective | Radiology department of this hospital | NHS and hospitals in Italy | Italian NHS | NA | Hospital in the Netherlands |
| Discount rate | NA | NA | NA | NA | Costs 4%, effects 1.5% |
| Uncertainty around cost-effectiveness ratio expressed | NA | NA | NA | NA | CEUS strategy was cost-effective at a threshold of 20,000 euros per life-year in 90% of the simulation; MRI/CT strategy was cost-effective at a threshold of 20,000 euros per life-year in 10% of the simulation |
| Sensitivity analysis | NA | NA | NA | NA | Resource use and unit cost data were tested by varying the costs by ± 30% of the mean |
| Outcome (cost and life-years per QALY) per comparator | Total cost saving from 2002 to 2005: 47,055.33 euros | NHS:
|
AASLD approach:
|
|
|
| Summary of incremental analysis | Equivalent to 118.23 euros saving per person | Total saving for the NHS: 78,902 euros (162.70 euros per patient) Total saving for hospitals: 85,065.96 euros (175.39 euros per patient) |
Incremental per-patient cost for CEUS was estimated to be –452 euros (–160 euros for the diagnostic phase and –292 euros for the treatment phase) |
| Faccioli 200764 | Romanini 200766 | Sangiovanni 201147 | Sirli 201067 | Zaim 201168 | |
|---|---|---|---|---|---|
| Study design | |||||
| The research question is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The economic importance of the research question is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The viewpoint(s) of the analysis are clearly stated and justified | ✗ | ✓ | ✓ | ✗ | ✓ |
| The rationale for choosing alternative programmes or interventions compared is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The alternatives being compared are clearly described | ✓ | ✓ | ✓ | ✓ | ✓ |
| The form of economic evaluation used is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | ✓ | ✓ | ✓ | ✓ |
| Data collection | |||||
| The source(s) of effectiveness estimates used are stated | ✓ | ✓ | ✓ | ✓ | ✗ |
| Details of the design and results of the effectiveness study are given (if based on a single study) | ✓ | ✓ | ✓ | ✓ | ✗ |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | NA | NA | NA | NA | NA |
| The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methods-to-value benefits are stated | ✓ | ✓ | ✓ | ✓ | ✗ |
| Details of the subjects from whom valuations were obtained are given | ✓ | ✓ | ✓ | ✓ | ✓ |
| Productivity changes (if included) are reported separately | NA | NA | NA | NA | NA |
| The relevance of productivity changes to the study question is discussed | ✗ | ✗ | ✗ | ✗ | ✗ |
| Quantities of resource use are reported separately from their unit costs | ✓ | ✗ | ✓ | ✓ | ✗ |
| Methods for the estimation of quantities and unit costs are described | ✓ | ✗ | ✓ | ✗ | ✗ |
| Currency and price data are recorded | ✓ | ✓ | ✓ | ✓ | ✓ |
| Details of currency of price adjustments for inflation or currency conversion are given | ✗ | ✗ | ✗ | ✗ | ✗ |
| Details of any model used are given | NA | NA | NA | NA | NA |
| The choice of model used and the key parameters on which it is based are justified | ✓ | ✓ | ✓ | ✓ | ✓ |
| Analysis and interpretation of results | |||||
| Time horizon of costs and benefits is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The discount rate(s) is stated | ✗ | ✗ | ✗ | ✗ | ✓ |
| The choice of discount rate(s) is justified | ✗ | ✗ | ✗ | ✗ | ✓ |
| An explanation is given if costs and benefits are not discounted | ✗ | ✗ | ✗ | ✗ | NA |
| Details of statistical tests and CIs are given for stochastic data | ✗ | ✗ | ✓ | ✗ | ✗ |
| The approach to sensitivity analysis is given | NA | NA | NA | NA | ✓ |
| The choice of variables for sensitivity analysis is justified | NA | NA | NA | NA | ✗ |
| The ranges over which the variables are varied are justified | NA | NA | NA | NA | ✗ |
| Relevant alternatives are compared | ✓ | ✓ | ✓ | ✓ | ✓ |
| Incremental analysis is reported | ✓ | ✓ | ✗ | ✓ | ✓ |
| Major outcomes are presented in a disaggregated as well as aggregated form | ✓ | ✓ | ✓ | ✓ | ✓ |
| The answer to the study question is given | ✓ | ✓ | ✓ | ✓ | ✓ |
| Conclusions follow from the data reported | ✓ | ✓ | ✓ | ✓ | ✓ |
| Conclusions are accompanied by the appropriate caveats | ✓ | ✓ | ✓ | ✓ | ✓ |
Equipment costs (purchase and service contract costs), agents and related costs (contrast agents, saline solution, medical supplies and films) and human resource costs (radiologists, technicians, nurses and administrative staff) were evaluated within the model. The calculation of equipment costs was based on utilisation time per examination, considering both purchase price and depreciation; these were all obtained from the hospital administrative office with a constant annual depreciation rate. The costs of all medical staff and administrators (per minute) were derived from the Società Italiana di Radiologia Medica (SIRM) publication. 65 The formula for the ‘total saving’ calculation was CT × n – [(CEUS-US) × n], with n representing the number of examinations. The cost year was 2006.
For each US examination, the total cost was 46.36 euros and disaggregated costs were 8.43 euros for equipment, 5.96 euros for agents and related costs and 31.97 euros for human resource costs. In each CEUS examination, equipment costs were 8.43 euros, agents and related costs were 43.04 euros and human resources were 50.04 euros, giving a total cost of 101.51 euros. For each CECT examination, the aggregate cost was 211.48 euros, calculated by summing 68.27 euros for equipment costs, 62.96 euros for agents and related costs and 80.25 euros for human resource costs. The total saving from replacing CEUS as the second-line diagnostic procedure for the 398 patients modelled was 47,055.33 euros
Romanini et al. 66 conducted a multicentre prospective study to evaluate the economic and clinical outcomes after the introduction of CEUS in diagnostic procedures for incidentally detected FLLs. A total of 485 patients presenting with uncharacterised FLLs, without liver cirrhosis, were recruited into the study from January 2002 to October 2005. All patients underwent two diagnostic strategies, that is, patients were their own control group:
-
US → CEUS → (if inconclusive) CECT/CEMRI
-
US → CECT/CEMRI → (if inconclusive) CEMRI.
Cost items included diagnostic examinations, health-care professional time, pharmaceuticals, laboratory tests, medical devices and material for imaging. Reimbursement for baseline US was 51.13 euros, for CEUS was 76.13 euros, for CT with or without contrast agent was 164.75 euros and for MRI with or without contrast agent was 259.70 euros, according to a regional reimbursement price list. Other variable hospital costs were obtained from hospitals joining the study. From the Italian NHS perspective, the conventional diagnostic pathway with CECT and CEMRI cost a total of 134,576.60 euros. A total saving of 78,902 euros could be made by adopting the CEUS strategy, that is, 162.70 euros per patient. From the hospitals' perspective, the total expenditure incurred by the conventional approach was 147,045 euros, compared with 61,979 euros using the CEUS strategy. The reimbursement to the hospital per person for the conventional strategy was 277 euros, 26 euros less than the original spending by the hospital; for the CEUS strategy the reimbursement agency paid only 114.79 euros to the hospital, 13 euros less than the original spending by the hospital.
Sirli et al. 67 conducted a prospective study in the Department of Gastroenterology and Hepatology in a hospital in Romania to evaluate the cost differences when CEUS replaced CECT/CEMRI as the first-line examination for FLL characterisation. All of the CEUS liver evaluations performed from September 2009 to March 2010 were included in the study. The cost of a CEUS positive diagnosis was compared with the cost of a CECT and/or CEMRI positive diagnosis. The cost of a CECT/CEUS examination was added when the CEUS result was inconclusive:
-
CEUS→ (when inconclusive) CECT
-
CEUS→ (when inconclusive) CEMRI
-
CECT
-
CEMRI.
Contrast-enhanced ultrasound provided a conclusive diagnosis for 250 of 316 FLLs; the remaining 66 required further imaging (CECT or CEMRI). Therefore, the total examination cost for CEUS followed by CECT when necessary was 75,690 Romanian new leu (RON) [180 RON (cost for single CEUS examination) × 316 + 285 RON (cost for single CECT examination) × 66]. The total cost following the second strategy was 99,780 RON [180 RON (cost for single CEUS examination) × 316 + 650 RON (cost for single CEMRI examination) × 66]. When using CECT only the total cost was 90,060 RON and when using CEMRI only the total cost was 205,400 RON. To sum up, by adopting CEUS for first-line FLL characterisation, the cost saving per person was 45.5 RON compared with CT as first line and 334.2 RON compared with MRI as first line.
Sangiovanni et al. 47 conducted a study to assess the diagnostic accuracy and also the economic impact of all possible diagnostic strategy combinations in characterising FLLs (including only 1- to 2-cm lesions) in Italy. Compensated cirrhosis patients diagnosed with liver nodules under US surveillance were included in this study. All possible examinations [CT, MRI, CEUS and US-guided fine-needle biopsy (FNB)] were performed until a final diagnosis was obtained. The study assessed costs using two approaches. The first was in accordance with AASLD guidelines, with the final diagnosis of HCC needing concordant results from at least two imaging techniques; a third examination was recommended only when the previous two were discordant. FNB was performed only when the vascular pattern observed was different in the first two diagnostic procedures. The second approach was to perform a single scan and then perform subsequent scans if the result was inconclusive; although not stated, it appeared that FNB was performed only if all three scans were inconclusive.
The AASLD approach implied three possible permutations, that is:
-
CEUS and CT → (when inconclusive) MRI → (if required) FNB
-
CEUS and MRI → (when inconclusive) CT → (if required) FNB
-
CT and MRI → (when inconclusive) CEUS → (if required) FNB
The study criteria approach implied six possible permutations, that is:
-
CEUS → (when inconclusive) CT → (when inconclusive) MRI (if required) FNB
-
CEUS → (when inconclusive) MRI → (when inconclusive) CT (if required) FNB
-
CT → (when inconclusive) CEUS → (when inconclusive) MRI (if required) FNB
-
CT → (when inconclusive) MRI → (when inconclusive) CEUS (if required) FNB
-
MRI → (when inconclusive) CEUS → (when inconclusive) CT (if required) FNB
-
MRI → (when inconclusive) CT → (when inconclusive) CEUS (if required) FNB.
Following the AASLD guideline approach, CEUS + CT with MRI and FNB when required was considered the cheapest combination, with a total aggregate cost of 26,440 euros, equivalent to 479 euros per person. This strategy was 79 euros cheaper per person than CEUS + MRI → CT → FNB and 144 euros cheaper per person than CT + MRI → CEUS → FNB. The most inexpensive strategy using the study criteria approach was CEUS → CT → MRI → FNB: 535 euros per person, within the range of 9–45 euros cheaper than the rest of the strategies.
The study conducted by Zaim et al. 68 assessed cost-effectiveness when CEUS was applied as the second-line imaging technique in FLL characterisation. Patients with a FLL diagnosis were recruited between January 2009 and June 2010 in a medical centre in the Netherlands. All participants had at least one baseline US and received both the conventional imaging strategy, which was US, followed by MRI or CT, and CEUS. Those diagnosed with benign lesions underwent a minimum of 6 months of follow-up. Those with malignant lesions underwent curative or palliative treatments. Costs included costs of diagnostic techniques (US, CEUS, CT, MRI, laboratory tests and liver biopsy), surgical resection, intensive care stays, hospitalisation, outpatient visits and various treatment strategies (RFA, TACE, chemotherapy, palliative care and liver transplantation). All unit prices were based on Farmacotherapeutisch Kompas (CVZ) and Dutch tariffs and Erasmus Medical Centre (EMC) data at the 2010 rate. 69 The time horizon was 24 months with a 1.5% discount rate for health outcomes and 4% for costs. Deterministic and probabilistic sensitivity analyses were performed in the study. The discounted cost per patient undergoing CEUS was 8309 euros; this was less than that for patients following the conventional strategy, which was 8761 euros per person. The aggregate cost saving was 452 euros per person, of which 160 euros constituted the diagnostic phase and 292 euros the treatment phase. Total discounted life-years gained per patient were 1.538 for the CEUS strategy and 1.536 for the conventional strategy. The results of the probabilistic sensitivity analyses indicated that, when the cost-effectiveness threshold was 20,000 euros per life-year, the CEUS strategy was cost-effective in 90% of the simulation and the MRI/CT strategy was cost-effective in only 10% of the simulation.
Although all of the studies were of reasonably good quality, they did not fully address our research question. Limitations included restricted information about disease management and progression, choice of equipment and administrative procedures in different settings, inclusion of costing elements in the calculation and health outcomes. Zaim et al. 68 was the only paper that modelled disease management and reported relevant health outcomes; however, the follow-up lasted only 24 months.
Model structure and methodology
The aim of the health economic analysis was to investigate the cost-effectiveness of CEUS using the contrast agent SonoVue for the assessment of adults with FFLs in whom unenhanced US or other liver imaging is inconclusive. In the analysis we focused on the clinical applications for which the most data on test performance were available (see previous chapters) and for which we are most likely to see a clinical benefit from the use of CEUS. Therefore, the health economic analysis assessed the value of CEUS in the following three populations:
-
characterisation of FLLs detected on routine surveillance of patients with cirrhosis
-
detection of liver metastases in patients with CRC
-
characterisation of incidentally detected FLLs.
The comparators included the following liver imaging techniques:
-
CECT
-
Gd-CEMRI
-
SPIO-CEMRI.
Three separate models were used to assess the cost-effectiveness of CEUS using the contrast agent SonoVue in the populations specified above:
-
a cirrhosis surveillance model
-
a liver metastases of CRC model
-
an incidentally detected FLL model.
In all models the mean costs and life-years and quality-adjusted life-years (QALYs) gained per patient were calculated for each comparator. Costs and benefits were discounted at 3.5%. The three models are described in detail in the following sections.
Cirrhosis surveillance model
The cirrhosis surveillance model is a modified version of a model produced by the Health Economics Group, Peninsula Technology Assessment Group (PenTAG), Institute of Health Service Research, Peninsula Medical School (the PenTAG cirrhosis surveillance model). 70 This model was developed to assess the cost-effectiveness of several surveillance strategies in cirrhotic patients to identify HCC, using periodic serum AFP testing and/or liver US examination with CT as a confirmatory imaging technique, followed by treatment with liver transplantation or resection when appropriate. One of the research recommendations made by the authors was to assess the value of CEUS in surveillance strategies for cirrhotic patients. For the assessment of the value of CEUS in cirrhosis surveillance, this model required adaptation, because it did not allow for a confirmatory test with less than perfect accuracy. Also, the original model did not allow the comparison of different confirmatory tests.
The population of interest in the cirrhosis surveillance model in this assessment consisted of those with a diagnosis of compensated cirrhosis deemed eligible to enter a surveillance programme [aged ≤ 70 years with no pre-existing medical conditions that would preclude treatment with a liver transplant or hepatic resection (including current alcohol or intravenous drug abuse)]. The model allowed separate analysis of each of three cirrhosis aetiologies: alcoholic liver disease (ALD), hepatitis B virus (HBV) and hepatitis C virus (HCV). In the base-case analysis, results were produced for a mixed cohort weighted according to the following proportions: 57.6% ALD, 7.3% HBV and 35.1% HCV (expert opinion; as in the PenTAG model70). A probabilistic state transition (Markov) cohort model, constructed using Excel (Microsoft Corporation, Redmond, WA, USA), was used. The time horizon was lifetime and the cycle duration was 1 month.
The model diagram is shown in Figure 8. States are shown as boxes and allowable state transitions are shown as arrows. The basis of the model was the disease process or ‘natural history’ of cirrhosis. Within the natural history model, a distinction was made between those with compensated and those with decompensated cirrhosis. Those with compensated cirrhosis can progress to decompensated cirrhosis, which is irreversible and associated with excess mortality, costs and quality of life decrements. The rate of incidence of HCC is the same in those with compensated and decompensated cirrhosis. HCC can be either diagnosed or occult. Three classes of tumours were distinguished: small tumours (< 2 cm), medium tumours (2–5 cm) and large tumours (> 5 cm). Tumour size was used as a surrogate measure of all characteristics of tumour progression. Hence, tumour progression was modelled by a tumour growth rate. Both test performance in identifying tumours and treatability of the tumour are dependent on the tumour size. For example, for larger tumours there is a greater likelihood of identification. Incidental/symptomatic presentation of HCC is possible for those with both compensated and decompensated cirrhosis, for all tumour sizes, although with significantly lower probabilities for small and medium-sized tumours.
FIGURE 8.
Model diagram for cirrhosis surveillance, based on Thomson Coon et al. 70 Each cycle all patients who are alive can stay in the same health state, die from non-HCC related causes, or move according to the shown transitions. Patients with compensated cirrhosis can decompensate, by moving to the corresponding decompensated cirrhosis health state. Patients who are palliative or untreatable can die from HCC. Light grey states represent occult HCC. Dotted lines represent detection of HCC after screening or showing symptoms; stacked lines represent tumour growth; solid lines represent transplant.
The surveillance programme and treatment components are superimposed onto the disease process. The technical performance of each testing strategy was modelled using decision trees. The testing strategies consisted of unenhanced US followed by CEUS, CECT, Gd-CEMRI or SPIO-CEMRI as a confirmatory imaging test. In the base-case analysis surveillance was every 6 months and stopped for people who reached the age of 70 years. It was also assumed that compliance was 100%. The decisions trees are shown in Figure 9.
FIGURE 9.
Decision tree structure for the cirrhosis model. Note: The confirmatory tests are the comparators in this analysis: CEUS, CECT, Gd-CEMRI and SPIO-CEMRI.
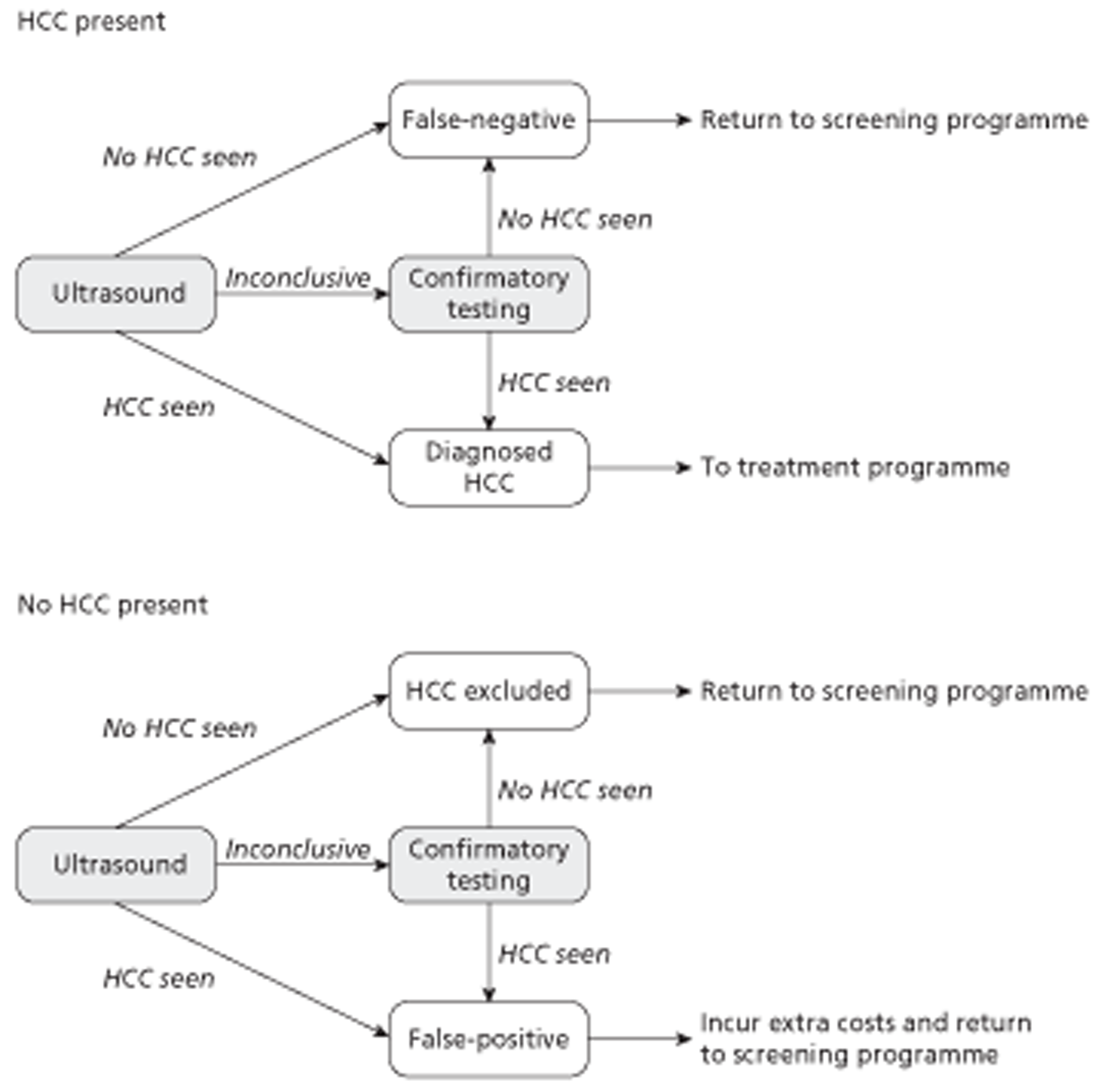
The treatments considered in the model are liver transplantation and liver resection. People can enter the transplant waiting list following diagnosis of either surgically treatable HCC or decompensated cirrhosis. There is no prioritisation of people waiting for a transplant. During the time on the waiting list people are subject to the same natural history process as during prelisting. There is no waiting list for liver resection for HCC. Some people are deemed unsuitable for surgical treatment, including those whose tumours are large or whose tumours become large while on the transplant waiting list. Small tumours are deemed more amenable to surgical treatment than medium-sized tumours. People who undergo successful liver transplant or resection enter a simplified disease process in which post-transplant or post-resection mortality, costs and utilities are taken into account. People with small and medium-sized tumours that are deemed to be surgically untreatable enter a series of states to model palliative care. Palliative care includes PEI, RFA and TACE and supportive care. Once people progress to untreatable large HCC, an excess mortality and associated costs and utilities are applied to reflect the palliation provided by TACE for a proportion of these people. An overview of the key structural assumptions is provided in the following section. A more detailed description of the model structure can be found in Thompson Coon et al. 70
Summary of structural assumptions (adapted from Thompson Coon et al.70)
-
All tumours are uninodular, with diameter used as a surrogate index of all characteristics of tumour progression.
-
Progression from compensated to decompensated cirrhosis is irreversible.
-
The rate of incidence of HCC is the same in compensated and decompensated livers.
-
The presence of a HCC tumour has no direct effect on mortality until it becomes ‘large’, at which point it becomes symptomatic and is associated with an additional mortality rate.
-
Incidental/symptomatic diagnosis is possible alongside all interventions, including ‘no surveillance’.
-
The ceiling age for surveillance is 70 years.
-
In the base case there is 100% compliance with the surveillance programme.
-
There is a small rate of false-positive diagnoses as a result of surveillance, all of which are assumed to be rapidly discovered before treatment, as both resection and transplant involve further diagnostic workup.
-
There is no waiting list for liver resection.
-
There is no prioritisation of people on the transplant waiting list.
-
No ablative therapies are applied to patients on the transplant waiting list.
-
Some people are deemed to have surgically untreatable tumours at the time of diagnosis of HCC.
Liver metastases of colorectal cancer model
The CRC metastases model is a modified version of the metastatic model developed by Brush et al. 71 This model was developed to assess the cost-effectiveness of fluorodeoxyglucose positron emission tomography (FDG-PET)/CT as an add-on device in detecting metastatic cancer compared with conventional imaging (CT). The model was adapted to assess the cost-effectiveness of CEUS compared with CECT, Gd-CEMRI and SPIO-CEMRI in detecting metastases from CRC after an inconclusive unenhanced US scan. In addition to changing the comparators in the model, we added the cost of a whole-body CT scan for all patients with a positive test to detect whether or not metastases at extra sites are present. We also changed the way that false-positives were handled, and changed the watch and wait strategy to correspond with latest guidance. The watch and wait strategy was given not only to patients without metastases but also to those patients treated and still alive. A final addition was that we assigned false-negatives poorer survival in the first year because they are not treated immediately. These adaptations are described in more detail below. A decision tree combined with a probabilistic state transition (Markov) cohort model, constructed using Excel, was used. The time horizon was lifetime and the cycle duration was 1 year.
Figure 10 depicts the decision tree structure used for the metastases model. Patients who had previously had surgical treatment for primary CRC and in a routine follow-up assessment (involving a clinical examination and CEA testing) were found to have rising CEA levels and were identified as potentially having a metastatic recurrence received an unenhanced abdominal US scan. When this US scan was deemed inconclusive, the patient entered the decision tree and could receive CEUS, CECT, Gd-CEMRI or SPIO-CEMRI. Similar to the Brush et al. model,71 the decision tree splits the patient population according to true disease status (metastatic recurrence or no metastatic recurrence) before applying the DTA estimates, so that accurate and inaccurate diagnoses can be identified.
FIGURE 10.
Decision tree structure for the liver metastases from CRC model.
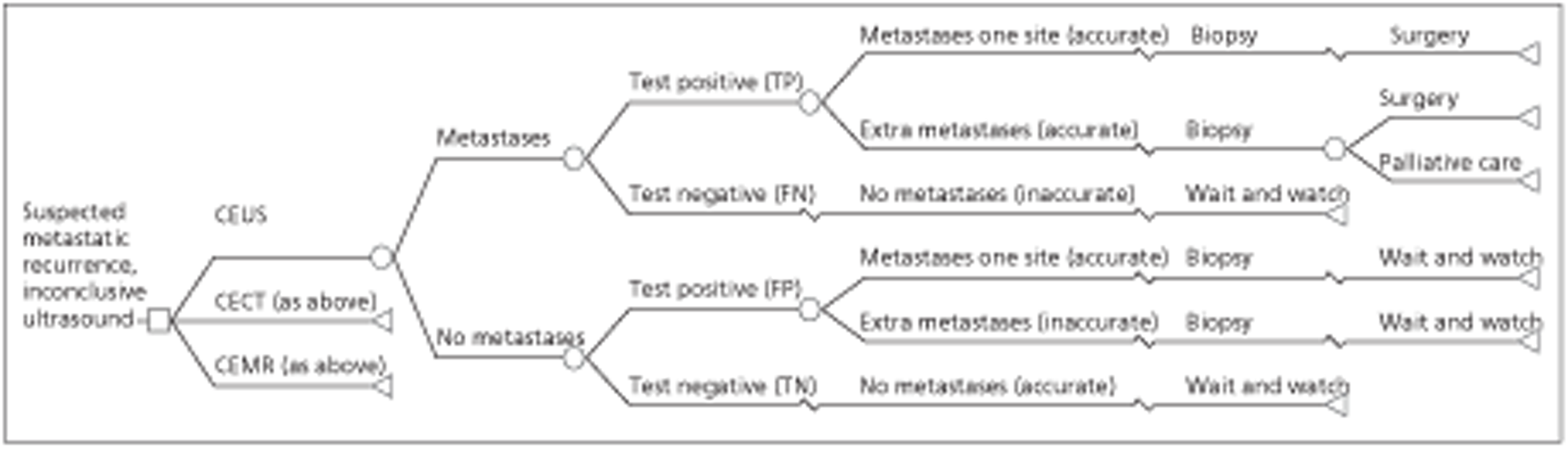
In this model, imaging (CEUS, CECT, Gd-CEMRI or SPIO-CEMR) will identify either metastases (test positive) or no metastases (test negative). After a positive test, patients receive a whole-body CECT scan to identify whether there are metastases at one site or at multiple sites. In the base case it was assumed that all patients in the model receive a biopsy to confirm the metastases before treatment, and it was assumed that biopsy is 100% accurate. Thus, in contrast to the Brush et al. model,71 patients with a false-positive test result will not receive treatment. Patients with a positive biopsy (true-positives) receive treatment. In line with Brush et al. 71 it was assumed that all patients with metastases at a single site will receive preoperative chemotherapy and surgery for metastases, and that patients with metastases at multiple sites are assumed to be non-curable and will receive either preoperative chemotherapy followed by surgery and palliative care, or chemotherapy and palliative care. In line with the Brush et al. model, patients with a negative test result are followed up in a watch and wait strategy for 3 years. Also in line with the Brush et al. model, for patients who are inaccurately diagnosed as having no metastases (false-negatives), the true diagnosis is assumed to be identified within a year if the patient is still alive. These metastases can be detected during scans in the watch and wait strategy or because the patient becomes symptomatic. This delayed detection involves a second scan (CEUS, CECT, Gd-CEMRI or SPIO-CEMRI, depending on the comparator), a whole-body CT and a biopsy.
After the decision tree phase, a state transition (Markov) model was used to follow up the patients (Figure 11). After the second year, when every patient is correctly diagnosed, patients can either stay in their health state or die. In the first 3 years, patients without metastases and those who were treated were assumed to be followed up using the watch and wait strategy.
FIGURE 11.
Simplified schematic diagram of the Markov model for follow-up of patients in the CRC metastases model.
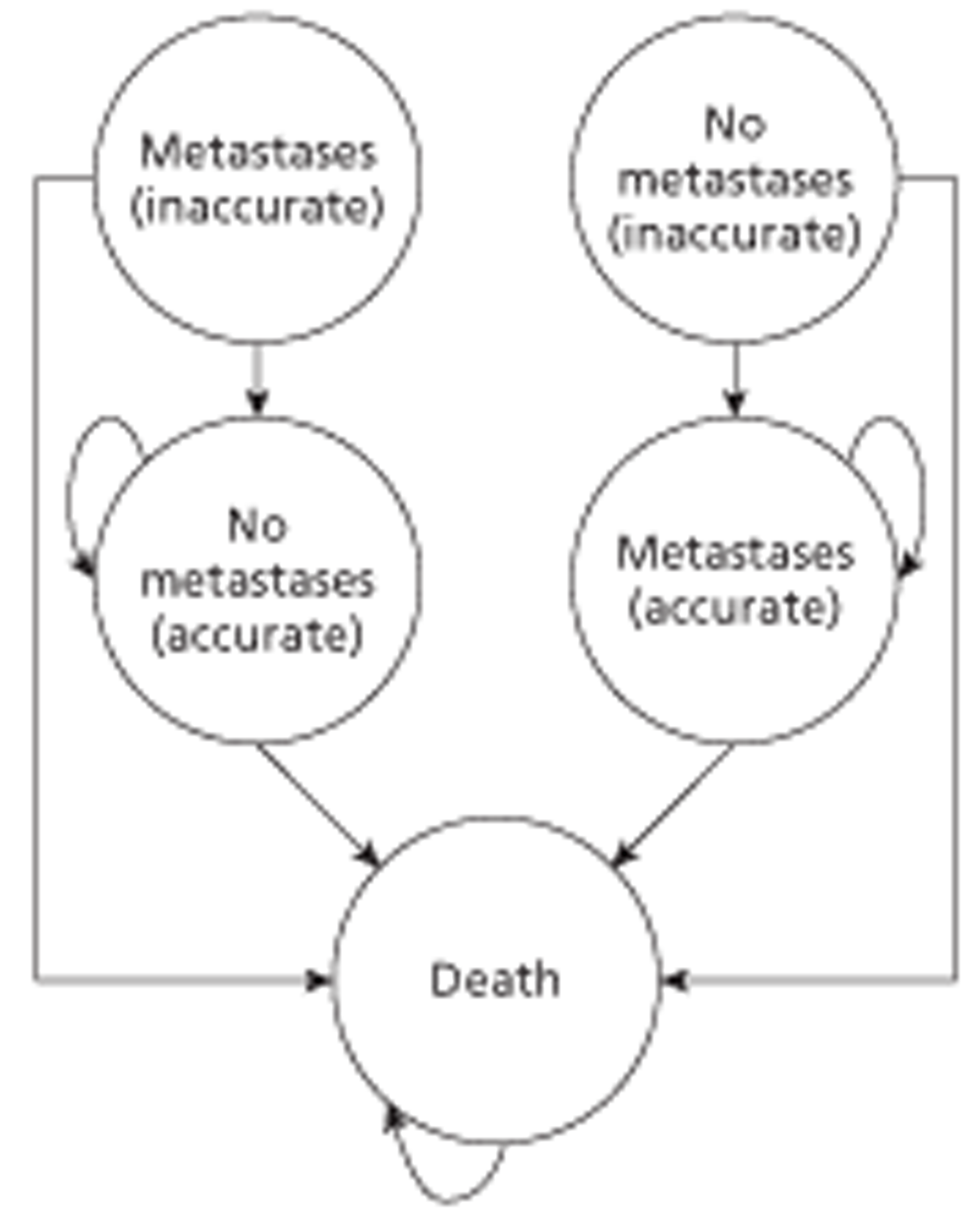
Summary of structural assumptions
-
For patients who are inaccurately diagnosed as having no metastases, the true diagnosis is identified within a year if the patient is still alive, either through regular tests in the watch and wait strategy or because the metastases become symptomatic.
-
All patients with a positive test result receive a whole-body CT scan to identify whether or not metastases are present at multiple sites. This scan does not detect inaccuracies of the previous (positive) test.
-
Patients who are inaccurately diagnosed as having metastases receive a biopsy and are therefore not treated for their metastases.
-
All patients with metastases at a single site will receive preoperative chemotherapy and metastatic surgery.
-
Patients with both hepatic and extrahepatic metastases are assumed to be non-curable and will receive one of two treatment options: preoperative chemotherapy followed by metastatic surgery and palliative care or chemotherapy and palliative care.
-
All patients identified as having no metastatic recurrence, as well as patients who have been treated for their metastases, would be treated with a watch and wait strategy in which they would be followed up annually for 3 years.
-
If there are no metastases at baseline, metastases will not occur. The watch and wait strategy is used to detect local recurrences and these are not incorporated in the model.
Incidentally detected focal liver lesion model
Patients with incidentally detected FLLs can have a variety of diseases, ranging from malignant lesions such as HCC and metastases to different types of benign lesions. Figure 12 illustrates the different combinations of test results and lesion types. The choice of lesion categories was based on similarities and differences in treatments, costs and prognosis.
FIGURE 12.
Description of patient categories and their treatments used in the incidentally detected FLL model.
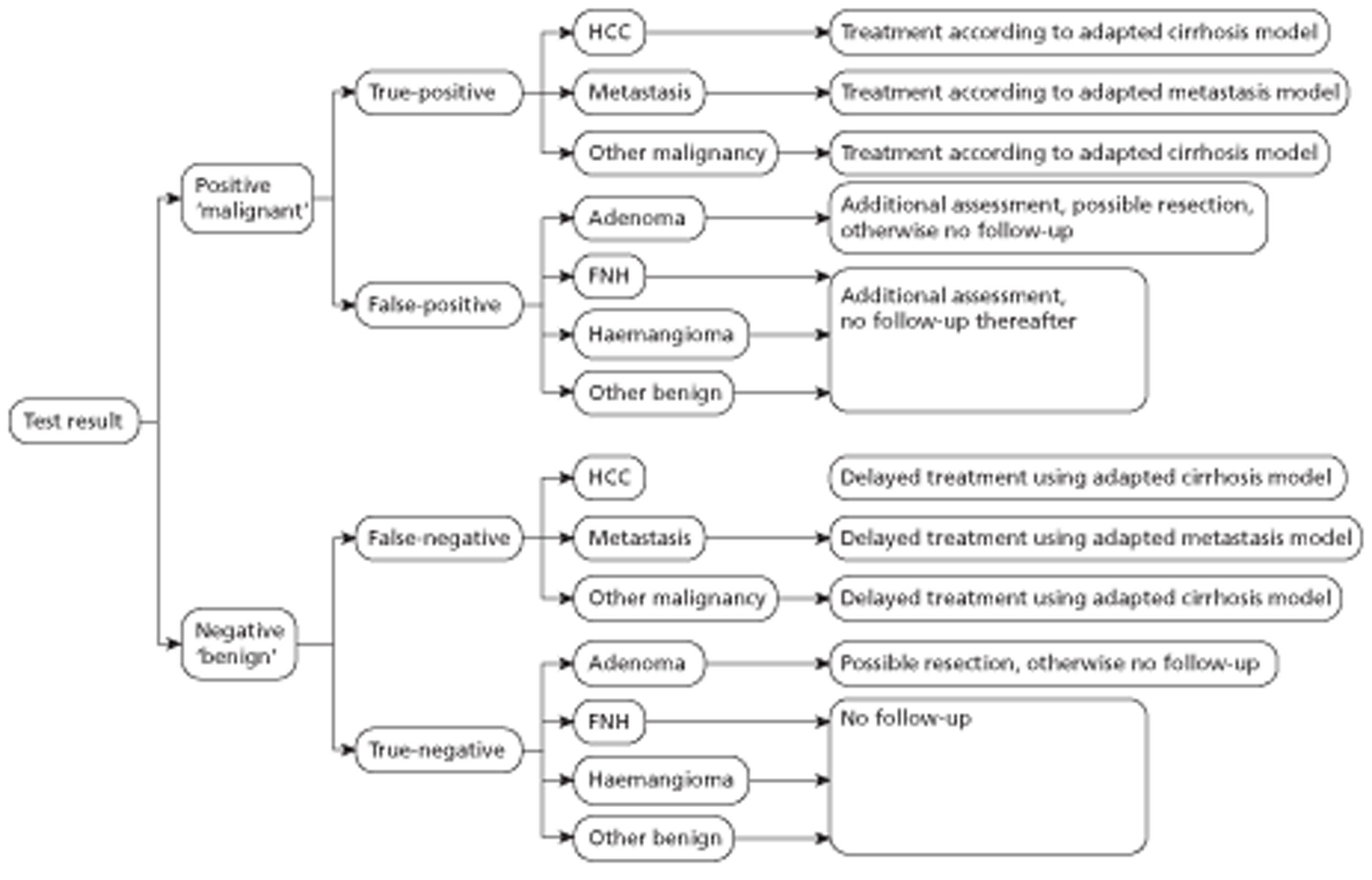
The prognosis, costs and QALYs seen among patients diagnosed with HCC were modelled using the cirrhosis model, whereas the prognosis, costs and QALYs among patients with liver metastases were modelled using the liver metastases model. The incidentally detected FLL model therefore incorporated elements of the cirrhosis model and elements of the liver metastases model as well as some new elements. The cirrhosis model required adjustments before it could be incorporated into these analyses. One important issue related to when HCC is diagnosed. In particular, although none of the patients in the cirrhosis surveillance model has HCC at the start of the simulation, all HCC patients in the incidentally detected FLL model will have HCC at the start of the simulation.
The economic and health consequences of false-positive and false-negative results were modelled in the following ways. First, it was assumed that patients with HCC who were not correctly identified at baseline would be correctly diagnosed within several months, as essentially all of these patients will have important risk factors (e.g. alcohol misuse, newly diagnosed cirrhosis or hepatitis) that are identified at baseline. Patients with a false-positive diagnosis (in particular, patients with a benign tumour that was misclassified as a malignant tumour) were assumed to undergo one additional follow-up consult as a result of this misclassification. This was viewed as a conservative assumption that would bias the assessment against CEUS and in favour of the comparators (CECT, CEMRI), as a false-positive result might lead to even greater costs than the cost of simply one extra visit and as CEUS was found to have a lower rate of false-positives in the DTA studies.
The costs, life-years and QALYs for patients having a malignancy other than HCC or metastases were assumed to be equal to those for HCC patients (see Figure 12). These other types of malignant lesions (e.g. lymphoma) were infrequently seen among patients with an incidentally detected FLL and the studies comparing CEUS with CECT or CEMRI provided little information about these lesions. Given the heterogeneity in costs and QALYs within this group (and even among patients with the same malignancy), we chose to set the base-case values to the costs and QALYs seen with HCC patients and emphasise that this was an assumption. However, it was known in advance that the costs and QALYs of these patients would have a limited effect on the cost-effectiveness of CEUS compared with the comparators for two reasons: the values for the sensitivity of CEUS and the comparators were very similar and the prior probability of other malignancies was small. In fact, the only possible way in which the values for costs and QALYs of other malignancies could have any effect on the overall cost-effectiveness was if the costs and QALYs changed dramatically if the malignancy were to be incorrectly classified as a benign lesion (i.e. a false-negative test result). The impact of this false-negative effect was therefore examined using sensitivity analysis.
Summary of structural assumptions made in the incidentally detected focal liver lesion model
-
Patients with HCC have a small HCC lesion and compensated cirrhosis at the time of assessment. The cirrhosis surveillance model made it possible to explore the impact of assuming that these patients have a medium lesion and compensated cirrhosis at the time of assessment, and the costs and QALYs associated with this alternative were used in a sensitivity analysis.
-
Patients with HCC who are incorrectly diagnosed at baseline will be correctly diagnosed later (within several months). This is assumed because these patients will be followed up because of the presence of some of the risk factors known to result in HCC (e.g. history of alcohol misuse, hepatitis B or C).
-
Patients diagnosed with an apparently benign lesion do not undergo treatment unless they have a (hepatic) adenoma, in which case they may undergo a resection.
-
The mean costs and health outcomes of patients with incidentally detected FLLs that are metastatic can be estimated using the model for liver metastases from CRC, because the highest proportion of liver metastases will originate from CRC. For example, Catala et al. 52 reported that 7 of the 12 patients with metastases in their study had CRC, and this corresponds with findings elsewhere in the literature as well as frequencies reported by one of the clinicians queried during this study.
Model parameters
Cirrhosis surveillance model
Test performance
It was assumed that the surveillance strategy started with unenhanced US. The test performance of US used in the model was based on the study by Bennett et al. ,72 as used in the HTA report by Thompson Coon et al. 70 (Table 15). This study was preferred over other studies because it distinguished between small, medium and large tumours and had a relatively large sample size (n = 200).
Additional imaging takes place following an inconclusive unenhanced US scan. The percentage of unenhanced US examinations that are inconclusive was estimated to be 43%, based on information provided by the manufacturer of SonoVue during the scoping phase of this assessment.
| Parameter | Value | Distributiona | TP | FN | FP | TN | |
|---|---|---|---|---|---|---|---|
| Sensitivity for identifying tumours | Small | 0.11 | Dirichlet | 3 | 25 | 6 | 118 |
| Medium | 0.29 | Dirichlet | 2 | 5 | 0 | 2 | |
| Large | 0.75 | Dirichlet | 3 | 1 | 0 | 0 | |
| False-positive rate | US | 0.04 | Dirichlet | See data used to model sensitivity | |||
In the systematic review seven studies42,43–46,48,61 that compared CEUS with at least one of the comparators (CECT, Gd-CEMRI or SPIO-CEMR) for the characterisation of FLLs detected during routine surveillance of cirrhotic patients were identified. In the base-case analysis the probability of identifying a HCC, as well as the proportion of people with a false-positive test result, was taken from the study by Leoni et al. 42 (Table 16). The main reason for using this study was that it used diagnostic criteria matching the EFSUMB guidance on the use of CEUS,6 and reported data on the performance of CEUS, CECT and Gd-CEMRI in the same population, whereas most other studies compared CEUS with either CECT or CEMRI. A potential disadvantage of the Leoni et al. study was that it used a suboptimal reference standard (concordance between at least two imaging test results) for the majority of patients. Leoni et al. also reported accuracy data for SPIO-CEMRI, which were not incorporated in the base-case analysis. The study included patients with liver lesions between 1 and 3 cm; therefore, in the base case we used these results to model the diagnostic accuracy for both small (< 2 cm) and medium-sized (2–5 cm) tumours. The sensitivity for the identification of large HCCs was assumed to be 100% for all confirmatory imaging tests and this assumption was agreed by the clinical experts.
| Parameter | Value | Distributiona | TP | FN | FP | TN | |
|---|---|---|---|---|---|---|---|
| Sensitivity for identifying small and medium tumours | CEUS | 0.67 | Dirichlet | 37 | 18 | 2 | 18 |
| CECT | 0.67 | Dirichlet | 37 | 18 | 2 | 18 | |
| Gd CEMRI | 0.82 | Dirichlet | 45 | 10 | 1 | 19 | |
| False-positive rate | CEUS | 0.03 | Dirichlet | See data used to model sensitivity | |||
| CECT | 0.03 | Dirichlet | See data used to model sensitivity | ||||
| Gd CEMRI | 0.01 | Dirichlet | See data used to model sensitivity | ||||
Transition probabilities
The transition probabilities were all taken from the cirrhosis surveillance model reported in Thompson Coon et al. 70 A detailed description of the estimates of the transition probabilities can be found in this HTA report. An overview of the parameters used in the model that affect transition probabilities is provided in Table 17.
| Parameter | Value | Source | Distribution | Range of values used in sensitivity analysis | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Mean age at start (years) | ALD | 53.3 | Roberts et al.73 | Normal | SE = 0.1789 | 43.3 | 63.3 |
| HBV | 44.0 | Fattovich et al.74 | Normal | SE = 0.1789 | 34.0 | 54.0 | |
| HCV | 54.0 | Fattovich et al.75 | Normal | SE = 0.1789 | 44.0 | 64.0 | |
| % male | ALD | 67.1 | ONS76 | Beta | α = 211, β = 90 | 50.0 | 90.2 |
| HBV | 86.5 | Fattovich et al.74 | Beta | α = 302, β = 47 | 82.6 | 89.7 | |
| HCV | 58.1 | Fattovich et al.75 | Beta | α = 223, β = 161 | 53.1 | 62.9 | |
| Upper age limit for surveillance (years) | 70 | AAa | Log-normal | Mean of logs = 4.249, σ = 0.006 | 60 | 80 | |
| Composition of mixed aetiology cohort (%) | ALD | 57.6 | EOb | Fixed | – | – | |
| HBV | 7.3 | Fixed | – | – | |||
| HCV | 35.1 | Fixed | – | – | |||
| Annual incidence of cirrhosis decomposition (%) | ALD | 3.3 | Assumed same as HBV | Beta | α = 5, β = 156 | – | – |
| HBV | 3.3 | Fattovich et al.77 | Beta | α = 5, β = 156 | – | – | |
| HCV | 5.3 | Beta | α = 7, β = 129 | – | – | ||
| Annual incidence of HCC (%) | ALD | 1.7 | Fattovich et al.78 | Beta | α = 10, β = 574 | – | – |
| HBV | 2.2 | Beta | α = 9, β = 392 | – | – | ||
| HCV | 3.7 | Beta | α = 47, β = 1237 | – | – | ||
| Probability that tumour increases in size | Small to medium tumours | 0.056 | Taouli et al.79 | Beta PERTc | λ = 4 | 0.036 | 0.089 |
| Medium to large tumours | 0.036 | Beta PERTc | λ = 4 | 0.023 | 0.056 | ||
| Annual symptomatic/incidental presentation rate for HCC (%) | Small tumours | 1.6 | Rates calibrated to be in line with Trevisani et al.80 | Beta | α = 160, β = 9840 | 0 | 16.2 |
| Medium tumours | 12.1 | Beta | α = 121, β = 879 | 0% | 30.3 | ||
| Large tumours | 50 | Beta | α = 500, β = 500 | 0 | 100 | ||
| Proportion with decompensated cirrhosis who are listed for OLT (%) | 90 | AAa | Beta PERTc | λ = 4 | 80 | 100 | |
| Proportion with HCC who receive resection (%) | Small tumours | 20 | AAa | Beta PERTc | λ = 4 | 10 | 30 |
| Medium tumours | 5 | Beta PERTc | λ = 4 | 2 | 10 | ||
| Proportion with HCC who are listed for OLT (%) | Small tumours | 75 | AAa | Beta PERTc | λ = 4 | 65 | 85 |
| Medium tumours | 85 | Beta PERTc | λ = 4 | 80 | 88 | ||
| Proportion with HCC who are deemed surgically untreatable (%) | Small tumours | 5 | AAa | Fixed | – | – | |
| Medium tumours | 10 | Fixed | – | – | |||
| Monthly probability of receiving OLT once on waiting list | 0.2541 | UK Transplant81 | Beta | α = 577, β = 1694 | – | – | |
| Annual mortality rate due to compensated cirrhosis (%) | 0 | AAa | Fixed | – | – | ||
| Annual mortality rate due to decompensated cirrhosis (%) | ALD | 17.7 | Average HBV and HCV | Beta | α = 17, β = 81 | 12.7 | 32.5 |
| HBV | 22.5 | Fattovich et al.77 | Beta | α = 7, β = 26 | 18.9 | 32.5 | |
| HCV | 12.9 | Fattovich et al.75 | Beta | α = 8, β = 57 | 12.7 | 14.0 | |
| 90-day mortality rate for patients undergoing OLT (%) | ALD | 6.0 | UK Transplant81 | Beta PERTc | λ = 4 | 0.0 | 12.6 |
| HBV | 15.0 | Beta PERTc | λ = 4 | 4.7 | 25.3 | ||
| HCV | 7.4 | Beta PERTc | λ = 4 | 3.0 | 11.8 | ||
| Proportion of patients surviving 1 year following OLT (%) | ALD | 92.0 | UK Transplant81 | Beta PERTc | λ = 4 | 84.5 | 99.5 |
| HBV | 78.0 | Beta PERTc | λ = 4 | 65.9 | 90.1 | ||
| HCV | 87.6 | Beta PERTc | λ = 4 | 81.9 | 93.3 | ||
| Proportion of patients surviving 5 years following OLT (%) | ALD | 54.7 | UK Transplant81 | Beta PERTc | λ = 4 | 38.2 | 71.3 |
| HBV | 68.5 | Beta PERTc | λ = 4 | 54.3 | 82.8 | ||
| HCV | 55.8 | Beta PERTc | λ = 4 | 41.0 | 70.6 | ||
| 90-day mortality rate for patients undergoing resection (%) | 3.9 | Llovet et al.82 | Beta PERTc | λ = 4 | 1.3 | 10.8 | |
| Proportion of patients surviving 1 year following resection (%) | 85.0 | Llovet et al.82 | Beta PERTc | λ = 4 | 79.0 | 88.0 | |
| Proportion of patients surviving 3 years following resection (%) | 62.0 | Llovet et al.82 | Beta PERTc | λ = 4 | 54.0 | 76.0 | |
| Proportion of patients surviving 5 years following resection (%) | 51.0 | Llovet et al.82 | Beta PERTc | λ = 4 | 36.0 | 58.0 | |
| Annual mortality rate associated with occult large HCC (%) | 72.9 | Greten et al.83 | Beta PERTc | λ = 4 | 34.6 | 97.3 | |
| Annual mortality rate associated with known large HCC (%) | 64.4 | Greten et al.83 | Beta PERTc | λ = 4 | 33.6 | 84.8 | |
Costs
The cost of CEUS (in addition to unenhanced US) was based on expert opinion, both from clinicians and the manufacturer. The cost of the contrast was assumed to be £48.70 (estimate supplied by the manufacturer and agreed by clinicians). This cost includes the cost of cannulation. In addition, we expected CEUS to take more time than the unenhanced US scan. Therefore, we used the difference between the reference cost of an US scan of < 20 minutes (£55) and the reference cost of an US scan of > 20 minutes (£71) as the additional time cost of CEUS. 84 The total additional costs of CEUS were therefore estimated to be £65. This implies that CEUS is performed in the same appointment as the unenhanced US scan. The costs of the other diagnostic tests, outpatient appointments, orthotopic liver transplantation (OLT) and resection were based on NHS reference costs (NHSRC). 84
All other cost inputs were based on Thompson Coon et al. ,70 recalculated to the 2011 price level. 85 A detailed description of these costs can be found in this HTA report. 70 The parameters used in the model affecting costs are listed in Table 18.
| Parameter | Cost (£) | Source | Distribution | Range of values used in sensitivity analysis | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Unit costs | ||||||
| US scan | 55 | Per scan | NHSRC84 | Beta PERTa | 40 | 65 |
| SonoVue contrast agent | 49 | Per scan | Expert opinion | Beta PERTa | 40 | 60 |
| Additional time for CEUS | 16 | Per scan | NHSRC84 | Beta PERTa | 0 | 39 |
| CECT (one area) | 116 | Per scan | Beta PERTa | 88 | 126 | |
| Gd-CEMRI (one area) | 189 | Per scan | Beta PERTa | 137 | 226 | |
| SPIO-CEMRI (one area) | 189 | Per scan | Beta PERTa | 137 | 226 | |
| Outpatient appointment | 150 | Per appointment | Beta PERTa | 72 | 228 | |
| OLT | 26,329 | Per operation | Beta PERTa | 20,169 | 38,406 | |
| Resection | 6521 | Per operation | Beta PERTa | 1812 | 7246 | |
| State costs | ||||||
| All compensated cirrhosis states | 1394 | Per year | Thompson Coon et al.,70 updated to 2011 | Beta PERTa | 867 | 1961 |
| All decompensated cirrhosis states | 11,335 | Per year | Beta PERTa | 7738 | 14,931 | |
| All known HCC states | 1486 | Per yearb | Beta PERTa | 743 | 2971 | |
| Post OLT (year 1) | 11,923 | Per patient per year | Beta PERTa | 5835 | 18,021 | |
| Post OLT (year 2 onwards) | 1889 | Per patient per year | Beta PERTa | 992 | 2796 | |
| Post resection | 4266 | Per patient per year | Beta PERTa | 2824 | 5752 | |
| Palliative care (small and medium tumours) | 1955 | Per yearb | Beta PERTa | 977 | 3909 | |
| Palliative care (large tumours) | 214 | Beta PERTa | 106 | 428 | ||
| Event costs | ||||||
| False-positive diagnosis | 618 | Per false-positive diagnosis | Thompson Coon et al.,70updated to 2011 | Beta PERTa | 419 | 961 |
| Symptomatic/incidental diagnosis | 198 | Per diagnosis | Beta PERTa | 94 | 287 | |
Utilities
Utilities were taken from the HTA report by Thompson Coon et al. 70 (Table 19)
| Parameter | Value | Source | Distribution | Range of values used in sensitivity analysis | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Compensated cirrhosis | 0.75 | Chong et al.86 | Beta PERTb | 0.66 | 0.83 |
| Decompensated cirrhosis | 0.66 | Chong et al.86 | Beta PERTb | 0.46 | 0.86 |
| Untreatable HCC | 0.64 | Chong et al.86 | Beta PERTb | 0.44 | 0.86 |
| Month of OLT | 0.50 | AAa | Beta PERTb | 0.30 | 0.60 |
| Post OLT (year 1) | 0.69 | Ratcliffe et al.87 | Beta PERTb | 0.64 | 0.74 |
| Post OLT (year 2 onwards) | 0.73 | Ratcliffe et al.87 | Beta PERTb | 0.67 | 0.78 |
| Month of resection | 0.50 | AAa | Beta PERTb | 0.30 | 0.60 |
Liver metastases of colorectal cancer model
Test performance
Chapter 3 reports the results of two studies identified that assessed the accuracy of CEUS compared with CECT and/or Gd-CEMRI and/or SPIO-CEMRI in detecting liver metastases in CRC patients after inconclusive unenhanced US. 49,50 The test performance found in the Mainenti et al. study49 was used in the base case as this study compared all three alternative tests (CECT, Gd-CEMRI, SPIO-CEMRI) with CEUS. In this study, based on a total of 34 patients, sensitivity was 83% for all comparators. Specificity was lowest for CEUS (86%), followed by CECT (96%), SPIO-CEMRI (96%) and Gd-CEMRI (100%). An overview of the test performance is presented in Table 20. A Dirichlet distribution based on the observed counts was used to assess the uncertainty surrounding these results.
| Parameter | Value | Distributiona | Observed counts (n = 34) | ||||
|---|---|---|---|---|---|---|---|
| TP | FN | FP | TN | ||||
| Sensitivity | CEUS | 0.83 | Dirichlet | 5 | 1 | ||
| CECT | 0.83 | Dirichlet | 5 | 1 | |||
| Gd-CEMRI | 0.83 | Dirichlet | 5 | 1 | |||
| SPIO-CEMRI | 0.83 | Dirichlet | 5 | 1 | |||
| Specificity | CEUS | 0.86 | Dirichlet | 4 | 24 | ||
| CECT | 0.96 | Dirichlet | 1 | 27 | |||
| Gd-CEMRI | 0.96 | Dirichlet | 0 | 28 | |||
| SPIO-CEMRI | 1.00 | Dirichlet | 1 | 27 | |||
Transition probabilities
All transition probabilities used in the model are listed in Table 21 and are in line with the probabilities used in the Brush et al. model. 71 The probability of having metastases after CRC is expected to be 40%. 88 Even though the population modelled in the present analysis has already had an inconclusive US scan and may therefore be a slightly different population, we expected this figure to also apply to our population. Of those patients with metastases, approximately 30% have them at one site. 89
| Parameter | Value | Source | Distribution | |
|---|---|---|---|---|
| Cancer prevalence | ||||
| Probability of having metastases | 0.40 | Saunders et al.88 | Beta | SE = 0.1 |
| Probability of having metastases at one site | 0.30 | Lejeune et al.89 | Beta | SE = 0.1 |
| Treatments | ||||
| Metastases: preoperative chemotherapy and metastatic surgery | 1.00 | Assumption based on Brush et al.71 | Fixed | |
| Extra metastases: preoperative chemotherapy and metastatic surgery | 0.20 | MSAC90 | Beta | SE = 0.04 |
| Watch and wait | 1.00 | Assumption based on Brush et al.71 | Fixed | |
| 5-year overall survival | ||||
| No metastases | 0.85 | American Cancer Society91 | Beta | SE = 0.01 |
| Metastases: surgery for cure | 0.24 | AJCC92 | Beta | SE = 0.03 |
| Extra metastases: metastatic surgery and palliative care | 0.12 | AJCC92 | Beta | SE = 0.04 |
| Extra metastases: palliative care | 0.06 | AJCC92 | Beta | SE = 0.04 |
In line with Brush et al. 71 we assumed that all patients with metastases at a single site receive preoperative chemotherapy and metastatic surgery. Patients with extra metastases receive either preoperative chemotherapy followed by metastatic surgery and palliative care (20%) or chemotherapy and palliative care. All patients without a metastatic recurrence are followed up using a watch and wait strategy.
Five-year overall survival rates were extracted from Brush et al. 71 Patients who were inaccurately classified as having no metastases and who therefore failed to receive treatment in the first year were expected to have a higher probability of dying in this first year than those who were immediately treated for their metastases. Therefore, in the first year patients who had undetected metastases at one site had the probability of dying of those who were treated for extra metastases with surgery. Similarly, patients who had undetected metastases at multiple sites who could have been treated with surgery were assumed to have the probability of dying of those patients who received palliative care. Patients with undetected metastases at multiple sites who would have received palliative care were assumed not to experience increased mortality. After 1 year, all patients were assigned the mortality rate that belonged to their type of metastases and treatment. The survival rates were converted to yearly probabilities and extrapolated to 10 years, after which patients were assumed to have survived their disease and returned to the average mortality rate for their age. 93 To inform this mortality rate, the model assumed a starting age of 50 years and a male-to-female ratio of 55 : 45. 94
Costs
Both the costs of the imaging techniques and the costs of subsequent treatment were taken into account (Table 22). The costs of CEUS were similar to those in the cirrhosis surveillance model. Because all patients already received an unenhanced US scan, the costs of CEUS consisted of the cost of the extra time used for CEUS as opposed to an unenhanced US scan (£16) and the cost of the contrast (£48.70). CECT was assumed to scan three areas (chest, abdomen, pelvis) whereas CEMRI was assumed to scan two to three areas. The costs of biopsy, whole-body CT and the watch and wait strategy were based on NHSRC reference costs. 84 The watch and wait strategy consisted of two CECT scans over 3 years and a serum CEA test twice a year for 3 years. 14 Costs of treatment were based on the costs used by Brush et al. 71
| Parameter | Cost (£) | Source | Distribution | Range of values used in sensitivity analysis | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| SonoVue contrast agent | 49 | Expert opinion | Beta PERTa | λ = 4 | 40 | 60 |
| Additional time for CEUS | 16 | NHSRC84 | Beta PERTa | λ = 4 | 0 | 39 |
| CECT (three areas) | 162 | NHSRC84 | Beta PERTa | λ = 4 | 120 | 192 |
| Gd-CEMRI (two to three areas) | 366 | NHSRC84 | Beta PERTa | λ = 4 | 175 | 374 |
| SPIO-CEMRI (two to three areas) | 366 | NHSRC84 | Beta PERTa | λ = 4 | 175 | 374 |
| Biopsy | 1437 | NHSRC84 | Beta PERTa | λ = 4 | 989 | 1798 |
| Whole-body CT | 162 | NHSRC84 | Beta PERTa | λ = 4 | 120 | 192 |
| Chemotherapy | 11,532 | BNF 58,95 ISD,96, Cancer Research UK97 | Fixed | |||
| Surgery | 9134 | SD96 | Normal | SD = 1827 | ||
| Palliative care | 2468 | Guest et al.98 | Normal | SD = 494 | ||
| Watch and wait | 110 | NHSRC,84 NICE14 | Beta PERTa | 82 | 130 | |
Utilities
All utility scores used in the model were based on Brush et al. 71 and are presented in Table 23. Patients who were inaccurately diagnosed as having no metastatic recurrence and who therefore failed to receive treatment in the first year were assigned a disutility for that year to account for the negative impact on their quality of life. Likewise, patients without metastases who unnecessarily received treatment (in a sensitivity analysis) were assigned a lower utility score to account for the negative impact of this unnecessary treatment on their quality of life.
| Parameter | Value | Source | Distribution | |
|---|---|---|---|---|
| No metastases | 0.91 | Ramsey et al.99 | Beta | SE = 0.11 |
| Metastases at one site | 0.84 | Ramsey et al.99 | Beta | SE = 0.12 |
| Extra metastases: surgery for cure | 0.74 | Langenhoff et al.100 | Beta | SE = 0.21 |
| Extra metastases: palliative care | 0.52 | Tengs and Wallace101 | Beta | SE = 0.08 |
| Patients receiving unnecessary metastatic surgery | 0.74 | Langenhoff et al.100 | Beta | SE = 0.14 |
| Patients receiving unnecessary palliative care | 0.61 | Tengs and Wallace101 | Beta | SE = 0.20 |
| Disutility for patients who fail to receive surgery | 0.30 | Assumption based on Tengs and Wallace101 | Gamma | SE = 0.08 |
| Disutility for patients who fail to receive palliative care | 0.20 | Assumption based on Tengs and Wallace101 | Gamma | SE = 0.08 |
It was assumed that the average utility experienced by patients in a particular stage was constant for 5 years post diagnosis. Patients who were still alive 5 years post diagnosis were assigned age-specific utility weights based on UK population norms. 102
Incidentally detected focal liver lesion model
Test performance
As noted earlier, different studies have compared CEUS with CECT and CEMRI in its ability to characterise incidentally FLLs. Three different types of diagnostic outcome have been studied: diagnosis of any malignancy, diagnosis of HCC and diagnosis of metastases. Of these three, the most common outcome has been any malignancy. In addition, most studies have compared CEUS with CECT; only one has compared CEUS with CEMRI. These two factors made it impossible to combine all results into one analysis without important assumptions (listed in Incidentally detected FLL model). This issue was resolved by utilising the test performance results in various ways.
The approach used in the base-case analysis was to take the results from the meta-analysis of four studies that compared CEUS with CECT in their ability to differentiate between malignant and benign lesions (described in Chapter 3, Accuracy of SonoVue CEUS for the characterisation of incidentally detected FLLs). The following results illustrate how similar the performance of CEUS and CECT are (Table 24). The CIs shown were calculated using the exact method.
| Estimate (%) | 95% CI (exact method) (%) | |
|---|---|---|
| Sensitivity of CEUS | 95.1 | 93.3 to 96.6 |
| Sensitivity of CECT | 94.6 | 92.7 to 96.1 |
| Specifcity of CEUS | 93.8 | 90.4 to 96.3 |
| Specifcity of CECT | 93.1 | 89.6 to 95.8 |
In addition to using the sensitivity and specificity values from the meta-analysis, we also used the results from the individual studies (see Chapter 3, Accuracy of SonoVue CEUS for the characterisation of incidentally detected FLLs for details). Dirichlet distributions were applied when the results from these individual studies were used. Use of these distributions had no influence on the prior probability of the different diagnoses as test performance and prior probability were combined to calculate the post-test probability using Bayes' theorem.
In the past, only one study has compared the test accuracy of CEUS with MRI. 56 As noted in Chapter 3 (see Accuracy of SonoVue CEUS for the characterisation of incidentally detected FLLs), this study reported that all patients in subgroup B underwent Gd-CEMRI, and that a subset of these patients also underwent SPIO-CEMRI. It is therefore difficult to refer to the accuracy of Gd-CEMRI or SPIO-CEMRI in the characterisation of incidentally detected FLL. For this reason, in the sections relating to the use of MRI in the characterisation of incidentally detected FLL, we refer to CEMRI.
As noted above, some studies examined the ability of imaging tests to correctly identify HCC and metastases. While modelling, we made it possible to use these results instead of the results based on malignancy compared with no malignancy. With regard to the outcome of malignancy compared with no malignancy, we assumed that any mistakes in diagnosis were made at random and were not associated with any particular lesion type. For example, if a malignant lesion was incorrectly classified by CEUS as a benign lesion, the type of benign lesion in that instance was determined according to the relative frequencies of the different benign lesion types.
Nevertheless, a number of different probabilities were used in this model. The first set of probabilities related to the prior probabilities (or prevalence) of the different types of lesions at the time of assessment (Table 25). The prevalence of malignant lesions varied substantially between the diagnostic accuracy studies included in the systematic review. In one study the probability of any malignancy was 23%,53 whereas in another it was 74%. 52 In the final protocol for this study it was stated that expert opinion had suggested that as many as 70–75% of FLLs assessed in the NHS may be benign. This percentage might be higher if the population in question were to be limited to incidentally detected FLLs. The clinicians surveyed during the present study were of the opinion that the chance of malignancy was rather low in this population. As a consequence, we used a low probability of malignancy in the base-case scenario. The values shown in Table 25 were based on the results of Bartolotta et al. ,103 who reported a low probability of malignancy of 4.3%. As Bartolotta et al. reported no patients with HCC in their study, we increased this to 0.05 to introduce a small chance that a patient with HCC would appear on occasion in the analysis.
| Type of lesion | Prior probability | Distribution | Alpha | Beta |
|---|---|---|---|---|
| Metastases | 0.0211 | Beta | 3 | 139 |
| HCC | 0.0004 | Beta | 0.05 | 141.95 |
| CCC | 0.0070 | Beta | 1 | 141 |
| Other malignancy | 0.0004 | Beta | 0.05 | 141.95 |
| Haemangioma | 0.4993 | Beta | 70.9 | 71.1 |
| Focal nodular hyperplasia | 0.3169 | Beta | 45 | 97 |
| Hepatocellular adenoma | 0.0141 | Beta | 2 | 140 |
| Focal fatty sparing | 0.0704 | Beta | 10 | 132 |
| Other benign lesion | 0.0704 | Beta | 10 | 132 |
| Probability of malignant lesion | 0.0289 | Beta | NA | NA |
| Probability of benign lesion | 0.9711 | Beta | NA | NA |
As noted above, care was taken to ensure that the estimates of test performance were kept separate from the prior probabilities of the different malignancies by combining prior probability, sensitivity and specificity using Bayes' theorem. This enabled us to vary the prior probability of malignancy in sensitivity analyses.
The incidentally detected FLL model was a decision-analytic model and not a Markov model and therefore did not directly involve the modelling of health states. The prognosis of patients following the initial diagnostic assessment was estimated using existing disease models and background mortality data (national vital statistics). The prognosis associated with the two most important types of malignant lesions (HCC and metastases) was estimated using the two other models applied in this HTA (i.e. the cirrhosis model and the liver metastases model). The following assumptions were made regarding the prognosis of patients with incidentally detected FLLs.
Summary of assumptions made in the incidentally detected focal liver lesion model regarding probabilities
-
Patients with HCC have a small HCC lesion and compensated cirrhosis at the time of assessment. The cirrhosis surveillance model made it possible to explore the impact of assuming that these patients have a medium-sized lesion and compensated cirrhosis at the time of assessment, and the costs and QALYs associated with this alternative were used in a sensitivity analysis.
-
Patients with HCC who are incorrectly diagnosed at baseline will be correctly diagnosed later (within several months). This is assumed because these patients will be followed up because of the presence of one or more risk factors for HCC, such as newly diagnosed cirrhosis and hepatitis. The impact of delayed treatment is 1 less life-year, 1 less QALY and 5% extra costs. The impact of delayed treatment was varied in sensitivity analyses.
-
Patients diagnoses with an apparent benign lesion do not undergo treatment unless they have a (hepatic) adenoma, in which case they may undergo a resection [base-case chance of resection: [base-case chance of resection 50% (but varied in sensitivity analyses)].
Costs
The costs of diagnostic tests, outpatient appointments, biopsy, OLT and resection were taken from the NHSRC. 84 Many of the values used in the incidentally detected FLL analyses were similar to those used in the cirrhosis analyses (Table 26). All other cost inputs were based on Thompson Coon et al. ,70 recalculated to the 2011 price level using the Personal and Social Services Research Unit (PSSRU) unit costs. 85
| Cost (£) | Lower (£) | Upper (£) | Distribution | |
|---|---|---|---|---|
| Imaging | ||||
| US | 16 | 0 | 39 | Beta PERT |
| Contrast | 48.70 | 40 | 60 | Beta PERT |
| CEUS | 65 | |||
| CECT | 116 | 88.21 | 126.33 | Beta PERT |
| CEMRIa | 189 | 137.27 | 225.89 | Beta PERT |
| Mean cost (£) | Standard error (£) | Distribution | ||
| HCC (correctly diagnosed) | 24,645 | 3980 | Normal | |
| HCC (incorrectly diagnosed) | 25,877 | 3980 | Normal | |
| Metastasis (correctly diagnosed) | 7518 | 1808 | Normal | |
| Metastasis (incorrectly diagnosed) | 7894 | 1808 | Normal | |
| Follow-up (total) | 150 | (min.-max.) 144-156 | Beta PERT (when varied) | |
| Resection | 6521 | (min.-max.) 1812-7246 | Beta PERT | |
The costs of treating HCC and metastases were based on the calculations in the cirrhosis surveillance and liver metastases models (see Table 26). However, adaptations of the cirrhosis model were needed before the results could be used for these analyses. In particular, it was assumed that a small tumour was found at diagnosis. Therefore, the total costs shown here cannot be compared with the total costs reported for cirrhosis surveillance. In contrast, the estimated costs of liver metastases treatment were based directly on the base-case results for liver metastases reported later in this chapter. Although it could be argued that some cost components (such as the costs of the initial diagnostic assessment) should be removed as they are not relevant for the incidentally detected FLL model, we chose to leave the total costs unchanged to allow the reader to trace the origin of these cost estimates. Moreover, these costs are greatly overshadowed by the other treatment-related costs and the standard error.
Utilities
Patients with an incidentally detected lesion that is benign are expected to lead a normal life in the future. For this reason it was assumed that their life expectancy and quality of life would not be different from those of the general population. In contrast, patients with a malignant lesion can have a poorer quality of life. The impact of disease on health utilities was based on the results of the cirrhosis and liver metastases models, as HCC and liver metastases are two important types of malignant lesion that may be identified. More information about the impact that these have on utilities is provided in the other sections of this chapter.
One factor not included in the analysis was the extent of disutility resulting from the anxiety caused by an incorrect diagnosis. Another type of disutility not explicitly included in the analysis related to the possible disutility from any delay before undergoing the test. Differences in waiting time between CEUS, CECT and CEMRI are expected, as CEUS can be performed right after the unenhanced US, as part of the same examination. However, it is uncertain how much disutility may be caused by differences in waiting time.
Summary of assumptions made in the incidentally detected focal liver lesion model regarding utilities
-
Patients with HCC who are incorrectly diagnosed at baseline will be correctly diagnosed later (within several months). This is assumed because these patients will be followed up because of other risk factors, such as newly diagnosed cirrhosis and hepatitis. The impact of delayed treatment is 1 fewer QALY.
-
Patients diagnosed with an apparent benign lesion will have a life expectancy and quality of life equal to those seen among people in the general population of the same age and sex.
Additional analyses
First, one-way sensitivity analyses were performed for all key parameters, especially for parameters in the models that were based on expert opinion. Next, probabilistic sensitivity analyses were performed using parameter distributions instead of fxed values. The chosen distributions are presented for each input parameter in Tables 25 and 26. Decision uncertainty regarding mutually exclusive alternatives is reflected using cost-effectiveness planes and cost-effectiveness acceptability curves. Specific additional analyses (including one-way sensitivity analyses) are listed in the following sections for each model.
Cirrhosis surveillance model
The proportion of patients receiving confirmatory imaging (the proportion of patients with an inconclusive unenhanced US scan: 43%) was an uncertain parameter in the model. Therefore, we performed a sensitivity analysis in which CEUS, CECT and Gd-CEMRI were used for a proportion of patients equal to the proportion of patients with a positive unenhanced US scan (as a minimum estimate of the patients requiring confirmatory imaging). Second, we reduced the proportion of inconclusive unenhanced US scans considerably (20% instead of 43%). Next, we conducted sensitivity analyses on the age limit of surveillance (90 years instead of 70 years), the frequency of screening (every year instead of every 6 months) and the tumour sizes for which the accuracy data were applied (small only instead of small and medium-sized).
Finally, scenario analyses were conducted using other sources for the accuracy of the tests. As alternative sources we used the articles by Dai et al. ,43 Quaia et al. ,46 Blondin et al. 48 and Giorgio et al. 45 (using data for 11- to 30-mm lesions). Dai et al. and Blondin et al. were included as other examples of studies that used a standard (EFSUMB guidelines6) definition of HCC, and Giorgio et al. and Quaia et al. were included to explore the effects of using other definitions of HCC. The study by Forner et al. 44 was not used because it included a significant proportion of patients with very small (< 10 mm) FLLs and the study by Sangiovanni et al. 47 was not used because it was considered to be an ‘outlier’ (accuracy results differed substantially from those of other, apparently similar studies).
Liver metastases from colorectal cancer model
First, we analysed the impact of not having a biopsy before treatment on the expected costs and effects. This would imply that patients who were inaccurately detected as having metastases would receive treatment, as was assumed in the Brush et al. model. 71 Second, we examined the impact of a 80% instead of a 40% probability of having metastases. We did this because our population of patients who have already received an unenhanced US scan may be slightly different from the population in Brush et al. 71 and may consist of more patients with metastases.
Next, we performed scenario analyses using other sources as input for the accuracy of the tests. Although the results refer to lesions instead of patients, we used the sensitivity and specificity reported in Jonas et al. 50 to assess the impact on the expected costs and effects. We also used the sensitivity and specificity reported in Clevert et al. ;51 this study included some patients with primary cancers other than CRC, but the majority (> 80%) of metastases diagnosed were from CRC.
Incidentally detected focal liver lesion model
A number of different parameters were varied to investigate their impact on the cost-effectiveness of CEUS. First, we increased the probability of a malignant lesion. We also examined the impact of basing the values for the sensitivity and specificity of CEUS and CECT on individual studies rather than on the meta-analysis. We then examined whether or not assuming that all patients with HCC had medium-sized lesions instead of small lesions would have an effect on the results. Lastly, we analysed the impact of changing the costs and health loss from an incorrect diagnosis of HCC or metastasis.
Results
Cirrhosis surveillance model
Effectiveness of surveillance
In the base case we compared CEUS, CECT and Gd-CEMRI (Table 27). Based on the accuracy data, as found by Leoni et al. ,42 we found that the proportion of patients dying from HCC was slightly higher for CEUS (17%) and CECT (17%) than for Gd-CEMRI (16%). This resulted in a slightly higher number of expected discounted life-years (13.76) and QALYs (10.18) gained by Gd-CEMRI than by CEUS and CECT (13.73 and 10.15 respectively).
| CEUS | CECT | Gd-CEMRI | |
|---|---|---|---|
| Proportion dying from HCC (%) | 17 | 17 | 16 |
| Proportion dead by age 75 (%) | 54 | 54 | 53 |
| Number of total life-years | 13.730 | 13.730 | 13.764 |
| Number of total QALYs | 10.153 | 10.153 | 10.175 |
Costs of surveillance
The total discounted costs were lowest for CEUS (£35,744), followed by CECT (£36,124) and Gd-CEMRI (£36,807) (Table 28). The main cost difference was in the imaging costs. Because Gd-CEMRI had a higher sensitivity than CEUS and CECT, HCC was identified at an earlier stage, improving the options for treatment. This also resulted in higher maintenance and treatment costs for CEMRI compared with CEUS and CECT.
| CEUS | CECT | Gd-CEMRI | |
|---|---|---|---|
| Surveillance | 1559 | 1939 | 2420 |
| Imaging | 1436 | 1816 | 2359 |
| False-positive | 123 | 123 | 61 |
| Maintenance | 23,631 | 23,631 | 23,687 |
| Symptomatic detection | 12 | 12 | 11 |
| Compensated cirrhosis | 13,043 | 13,043 | 13,014 |
| Decompensated cirrhosis | 2119 | 2119 | 2092 |
| Known HCC | 380 | 380 | 379 |
| Post transplant | 7822 | 7822 | 7931 |
| Post resection | 3 | 3 | 56 |
| Palliative | 57 | 57 | 59 |
| Transplant waiting list | 195 | 195 | 198 |
| Treatment | 10,554 | 10,554 | 10,700 |
| Transplantation | 10,504 | 10,504 | 10,644 |
| Resection | 50 | 50 | 56 |
| Total | 35,744 | 36,124 | 36,807 |
Cost-effectiveness of surveillance
Contrast-enhanced ultrasound was found to have the lowest discounted lifetime cost per patient (£35,744), followed by CECT (£36,124) and Gd-CEMRI (£36,807) (Table 29). Compared with CEUS, CECT was as effective and more costly and was thus considered to be dominated by CEUS. Gd-CEMRI was £1063 (95% CI £449 to £1492) more expensive than CEUS per patient, but also yielded 0.022 (95% CI −0.002 to 0.050) more QALYs, resulting in an incremental cost-effectiveness ratio (ICER) of £48,454 per QALY gained. As this is above the typical willingness-to-pay threshold of £30,000 per QALY gained, Gd-CEMRI was not deemed cost-effective compared with CEUS.
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 35,744 | 10.153 | |||||||
| CECT | 36,124 | 10.153 | 379 | 0.000 | Dominated | CEUS | 379 | 0.000 | Dominated |
| Gd-CEMRI | 36,807 | 10.175 | 1063 | 0.022 | 48,454 | CEUS | 1063 | 0.022 | 48,454 |
Additional analyses for surveillance
Sensitivity analyses
First, we analysed the impact of using CEUS, CECT and CEMRI as confirmatory imaging for a proportion of patients equal to the proportion of patients with a positive unenhanced US scan (Table 30). In line with the base-case analysis, CEUS was as effective and less costly than CECT. Gd-CEMRI was also more costly (£321) and more effective (0.025 QALYs) than CEUS, resulting in an ICER of £12,806 per QALY gained. Based on a willingness-to-pay threshold of £20,000–30,000, this indicated that Gd-CEMRI was cost-effective compared with CEUS in this analysis.
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 35,828 | 10.220 | |||||||
| CECT | 35,867 | 10.220 | 39 | 0.000 | Dominated | CEUS | 39 | 0.000 | Dominated |
| Gd-CEMRI | 36,148 | 10.245 | 321 | 0.025 | 12,806 | CEUS | 321 | 0.025 | 12,806 |
Second, we changed the proportion of inconclusive US scans from 43% to 20% (Table 31), the age limit of surveillance to 90 years instead of 70 years (Table 32), the frequency of screening to every year instead of every 6 months (Table 33) and the accuracy data to use only those that applied to small tumours instead of small and medium-sized tumours (Table 34). Only when changing the proportion of inconclusive US scans was Gd-CEMRI cost-effective compared with CEUS, with an ICER of £16,121 per QALY gained. In all other sensitivity analyses CEUS dominated CECT and was cost-effective compared with Gd-CEMRI.
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 35,784 | 10.192 | |||||||
| CECT | 35,959 | 10.192 | 176 | 0.000 | Dominated | CEUS | 176 | 0.000 | Dominated |
| Gd-CEMRI | 36,408 | 10.216 | 624 | 0.024 | 16,121 | CEUS | 624 | 0.024 | 16,121 |
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY(£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 36,163 | 10.164 | |||||||
| CECT | 36,593 | 10.164 | 430 | 0.000 | Dominated | CEUS | 430 | 0.00 | Dominated |
| Gd-CEMRI | 37,367 | 10.188 | 1204 | 0.023 | 51,619 | CEUS | 1204 | 0.023 | 51,619 |
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 34,431 | 10.093 | |||||||
| CECT | 34,629 | 10.093 | 198 | 0.000 | Dominated | CEUS | 198 | 0.000 | Dominated |
| Gd-CEMRI | 35,025 | 10.109 | 594 | 0.016 | 37,619 | CEUS | 594 | 0.016 | 37,619 |
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 36,054 | 10.191 | |||||||
| CECT | 36,432 | 10.191 | 378 | 0.000 | Dominated | CEUS | 378 | 0.000 | Dominated |
| Gd-CEMRI | 36,966 | 10.195 | 913 | 0.004 | 244,840 | CEUS | 913 | 0.004 | 244,840 |
Scenario analyses
Scenario analyses were conducted using other sources for data on the accuracy of the tests. As alternative sources we first used the articles by Dai et al. 43 and Quaia et al. 46 These studies both compared CEUS and CECT. Dai et al. used a definition of a positive test for HCC which was comparable with that used in the EFSUMB guidelines,6 whereas Quaia et al. did not. Using data from either study, CEUS was found to be less costly and more effective than CECT (Tables 35 and 36).
| Strategy | Cost (£) | QALYs | Compared with CEUS | ||
|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 36,203 | 10.188 | |||
| CECT | 36,332 | 10.184 | 129 | −0.004 | Dominated |
| Strategy | Cost (£) | QALYs | Compared with CEUS | ||
|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 36,479 | 10.185 | |||
| CECT | 36,767 | 10.180 | 288 | −0.005 | Dominated |
Next, we used Blondin et al. 48 and Giorgio et al. 45 as sources for input for the accuracy of CEUS and Gd-CEMRI (Tables 37 and 38 respectively). Blondin et al. used a definition of a positive test for HCC which was comparable with that used in the EFSUMB guidelines,6 whereas Giorgio et al. did not. Based on Blondin et al. , Gd-CEMRI was found to be more costly and less effective than CEUS. Based on Giorgio et al, using only data for lesions between 11 and 30 mm, Gd-CEMRI was found to be more costly, but also more effective than CEUS. However, the resulting ICER of £297,695 was very high.
| Strategy | Cost (£) | QALYs | Compared with CEUS | ||
|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 36,248 | 10.190 | |||
| Gd-CEMRI | 36,948 | 10.187 | 700 | −0.003 | Dominated |
| Strategy | Cost (£) | QALYs | Compared with CEUS | ||
|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 36,034 | 10.189 | |||
| Gd-CEMRI | 37,078 | 10.192 | 1044 | 0.004 | 297,695 |
Probabilistic sensitivity analysis
Over 5000 replications, CEUS has the highest probability of being cost-effective for thresholds < £55,000 (Figure 13). Above this threshold, Gd-CEMRI has the highest probability of being cost-effective. At a threshold of £20,000–30,000, the probability that CEUS, CECT or Gd-CEMRI is cost-effective is 99%, 0% and 1% respectively.
FIGURE 13.
Cost-effectiveness acceptability curves: cirrhosis surveillance (effects are QALYs; both costs and effects are discounted).
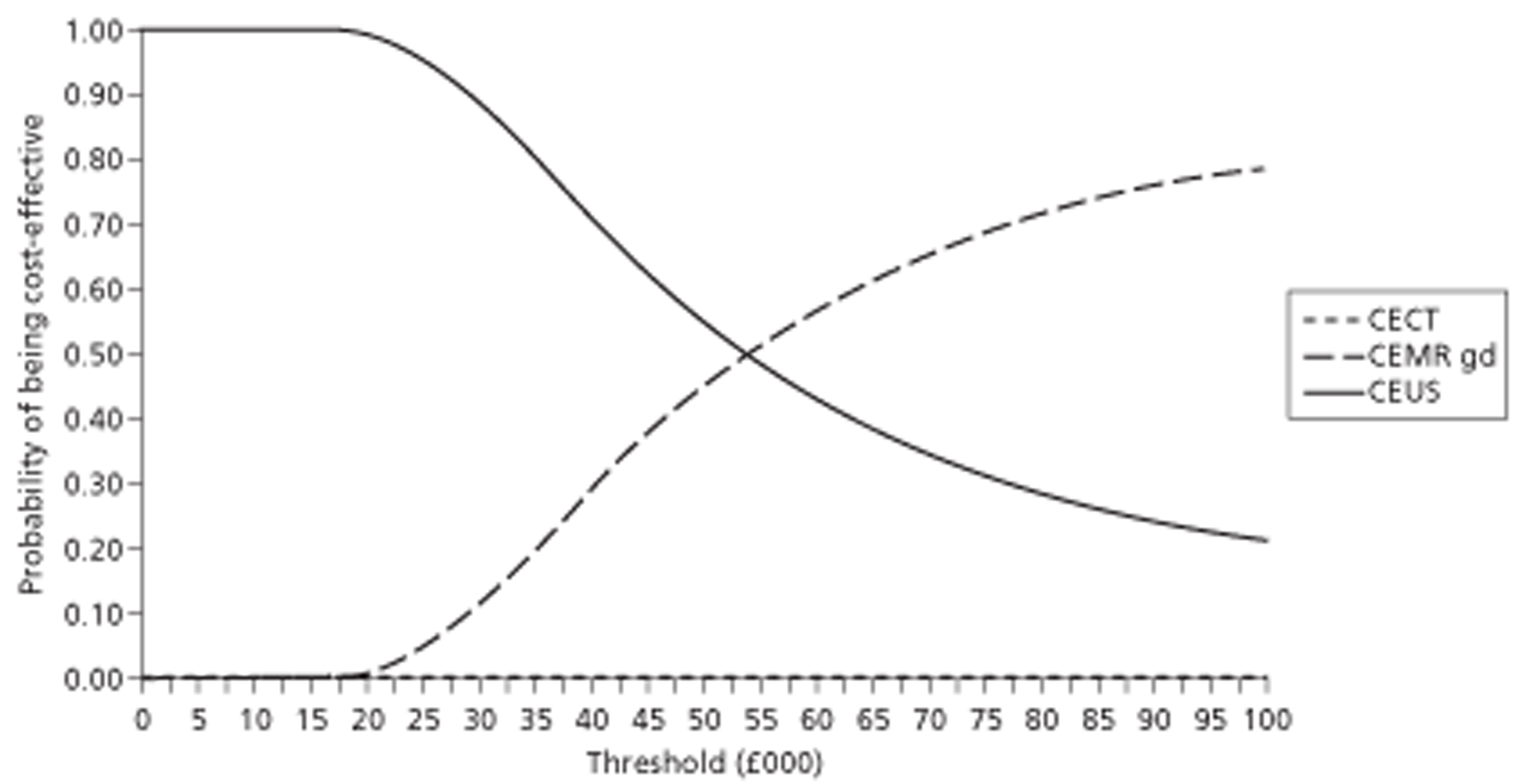
Table 39 provides an overview of the results of all of the sensitivity and scenario analyses.
| Analysis | Comparator | Compared with CEUS | ||
|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | ||
| Base-case analysis | ||||
| CECT | 379 | 0.000 | Dominated | |
| Gd-CEMRI | 1063 | 0.022 | 48,454 | |
| Sensitivity analyses | ||||
| Imaging used as confirmatory after all positive non-enhanced US examinations | CECT | 39 | 0.000 | Dominated |
| Gd-CEMRI | 321 | 0.025 | 12,806 | |
| Proportion of inconclusive US scans 20% instead of 43% | CECT | 176 | 0.000 | Dominated |
| Gd-CEMRI | 624 | 0.024 | 16,121 | |
| Age limit for screening 90 years instead of 70 years | CECT | 430 | 0.000 | Dominated |
| Gd-CEMRI | 1204 | 0.023 | 51,619 | |
| Annual screening instead of every 6 months | CECT | 198 | 0.000 | Dominated |
| Gd-CEMRI | 594 | 0.016 | 37,619 | |
| Accuracy data for small tumours only instead of for small and medium-sized tumours | CECT | 378 | 0.000 | Dominated |
| Gd-CEMRI | 913 | 0.004 | 244,840 | |
| Scenario analyses | ||||
| Dai et al.43 used as source for accuracy data | CECT | 129 | −0.004 | Dominated |
| Quaia et al.46 used as source for accuracy data | CECT | 288 | −0.005 | Dominated |
| Blondin et al.48 used as source for accuracy data | Gd-CEMRI | 700 | −0.003 | Dominated |
| Giorgio et al.45 used as source for accuracy data | Gd-CEMRI | 1044 | 0.004 | 297,695 |
Liver metastases of colorectal cancer model
Effectiveness of diagnosing liver metastases
As indicated previously, Mainenti et al. 49 found that the sensitivities of CEUS, CECT, Gd-CEMRI and SPIO-CEMRI were equal. This resulted in an equal number of cases incorrectly diagnosed without metastases (false-negatives) in the base-case analysis. Because of a lower specificity, the number of cases incorrectly diagnosed with metastases (false-positives) was highest for CEUS, followed by CECT, SPIO-CEMRI and Gd-CEMRI. Because false-positive results were assumed to be detected with a biopsy before treatment, differences in specificity did not affect the expected life-years and QALYs (Table 40).
| CEUS | CECT | Gd-CEMR | SPIO-CEMRI | |
|---|---|---|---|---|
| Number of discounted total life-years | 10.40 | 10.40 | 10.40 | 10.40 |
| Number of discounted total QALYs | 8.36 | 8.36 | 8.36 | 8.36 |
Costs of diagnosing liver metastases
An overview of the total discounted costs in the different cost categories per test strategy is given in Table 41. Although CEUS is less costly than CECT, the total diagnostic cost in the CEUS strategy is higher than that in the CECT strategy. This is because all patients with a positive test result receive a whole-body CT and biopsy, and in the CEUS strategy more patients have a positive test result. This implies that in the CEUS strategy, unnecessary additional diagnostic tests are performed. Because patients without metastases are not treated, and all metastases are eventually detected, costs of treatment are similar. Because of the higher total diagnostic cost, the average total discounted cost of CEUS (£7547) per patient is slightly higher than that for CECT (£7545). The average total discounted costs per patient for both Gd-CEMRI (£7724) and SPIO-CEMRI (£7758) are higher than those for CEUS and CECT, with SPIO-CEMRI having the highest cost because of unnecessary whole-body scans and biopsies.
| CEUS | CECT | Gd-CEMRI | SPIO-CEMRI | |
|---|---|---|---|---|
| Diagnostics | 795 | 793 | 971 | 1006 |
| Initial imaging | 67 | 169 | 381 | 381 |
| Whole-body scan | 75 | 64 | 61 | 64 |
| Biopsy | 653 | 560 | 529 | 560 |
| Treatment | 6716 | 6716 | 6716 | 6716 |
| Surgery/chemotherapy | 3583 | 3583 | 3583 | 3583 |
| Palliative care | 2901 | 2901 | 2901 | 2901 |
| Watch and wait | 232 | 232 | 232 | 232 |
| Total | 7511 | 7510 | 7688 | 7722 |
Cost-effectiveness of diagnosing liver metastases
In the base-case analysis, the different imaging techniques to detect liver metastases from CRC resulted in equal expected lifetime QALYs (8.364). CECT was found to be the least costly test, with expected costs of £7510 per patient. The expected lifetime cost per patient of CEUS was only slightly more than that for CECT (£7511). Gd-CEMRI (£7688) and SPIO-CEMRI (£7722) were both more costly than, and thus dominated by, CECT and CEUS. Although technically speaking CECT dominates CEUS, their effectiveness is equal and their expected costs are extremely close (Table 42).
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 7511 | 8.364 | |||||||
| CECT | 7510 | 8.364 | −1 | 0.000 | Dominant | CEUS | −1 | 0.000 | Dominant |
| Gd-CEMRI | 7688 | 8.364 | 177 | 0.000 | Dominated | CECT | 178 | 0.000 | Dominated |
| SPIO-CEMRI | 7722 | 8.364 | 211 | 0.000 | Dominated | CECT | 212 | 0.000 | Dominated |
Additional analyses for diagnosing liver metastases
Sensitivity analyses
When it is assumed that patients with a positive test do not undergo biopsy but are treated for their disease, implying that patients without metastases can receive unnecessary treatment, the lower specificity of CEUS leads to a loss in QALYs (Table 43). CEUS now yields the lowest number of QALYs (8.343) and is most expensive (£8335), while Gd-CEMRI, which is the most accurate, yields the highest number of QALYs (8.364) and is the least expensive (£7158). In this sensitivity analysis, Gd-CEMRI dominates the other tests because of its better accuracy.
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 8335 | 8.343 | |||||||
| CECT | 7321 | 8.359 | −1015 | 0.016 | Dominant | CEUS | −1015 | 0.016 | Dominant |
| Gd-CEMRI | 7158 | 8.364 | −1177 | 0.021 | Dominant | CECT | −162 | 0.005 | Dominant |
| SPIO-CEMRI | 7537 | 8.359 | −798 | 0.016 | Dominant | Gd-CEMRI | −379 | 0.005 | Dominated |
If CEUS is combined with biopsy (see Table 42), and CECT, Gd-CEMRI and SPIO-CEMRI are not followed by biopsy (see Table 43), then CEUS and Gd-CEMRI are most effective, both yielding 8.364 QALYS. However, CEUS is more costly than, and is thus dominated by, Gd-CEMRI. CECT and SPIO-CEMRI are now dominated by Gd-CEMRI.
If it is assumed that, instead of 40%, 80% of the initial population has metastases, the expected number of QALYs is 4.078 for all tests (Table 44). CEUS is now the least costly strategy, being £71 less costly than CECT. Because there is no difference between the tests in QALYs, the least costly test, CEUS, dominates all other tests.
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 14,419 | 4.078 | |||||||
| CECT | 14,490 | 4.078 | 71 | 0.000 | Dominated | CEUS | 71 | 0.000 | Dominated |
| Gd-CEMRI | 14,700 | 4.078 | 281 | 0.000 | Dominated | CEUS | 281 | 0.000 | Dominated |
| SPIO-CEMRI | 14,711 | 4.078 | 292 | 0.000 | Dominated | CEUS | 292 | 0.000 | Dominated |
Scenario analyses
We examined the expected costs and effects using different sources for the accuracy of the tests. First, we incorporated the accuracy data of Jonas et al. 50 (Table 45). This study compared CEUS, CECT and SPIO-CEMRI and found perfect specificity for all tests, with sensitivities of 87%, 83% and 97% respectively. CECT was slightly more costly (£7) and slightly less effective (0.005 QALYs) than CEUS and thus was dominated by CEUS. SPIO-CEMRI was more costly and more effective than CEUS, resulting in an ICER of £43,318 per QALY gained.
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 7468 | 8.369 | |||||||
| CECT | 7475 | 8.364 | 7 | −0.005 | Dominated | CEUS | Dominated | ||
| SPIO-CEMRI | 8055 | 8.382 | 587 | 0.014 | 43,318 | CEUS | 587 | 0.014 | 43,318 |
The slightly lower sensitivity and specificity of CECT compared with CEUS found by Clevert et al. 51 resulted in CEUS being £300 less costly and yielding 0.002 more QALYs than CECT (Table 46).
| Strategy | Cost (£) | QALYs | Compared with CEUS | Compared with next most cost-effective strategy | |||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | Comparator | Incremental cost (£) | Incremental QALYs | Incremental cost per QALY (£) | |||
| CEUS | 7821 | 8.384 | |||||||
| CECT | 8121 | 8.382 | 300 | −0.002 | Dominated | CEUS | 300 | −0.002 | Dominated |
Probabilistic sensitivity analyses
Based on the probabilistic sensitivity analysis with 5000 replications, we found that CEUS and CECT have a similar probability of being cost-effective across all willingness-to-pay thresholds (Figure 14). CEUS has a slightly higher probability of being cost-effective up to a threshold of £20,000, after which CECT has a somewhat higher probability of being cost-effective. At a threshold of £20,000 per QALY, CECT has the highest probability of being cost-effective (48%), followed by CEUS (47%), Gd-CEMRI (3%) and SPIO-CEMR (2%).
FIGURE 14.
Cost-effectiveness acceptability curves: liver metastases from CRC (effects are QALYs; both costs and effects are discounted).
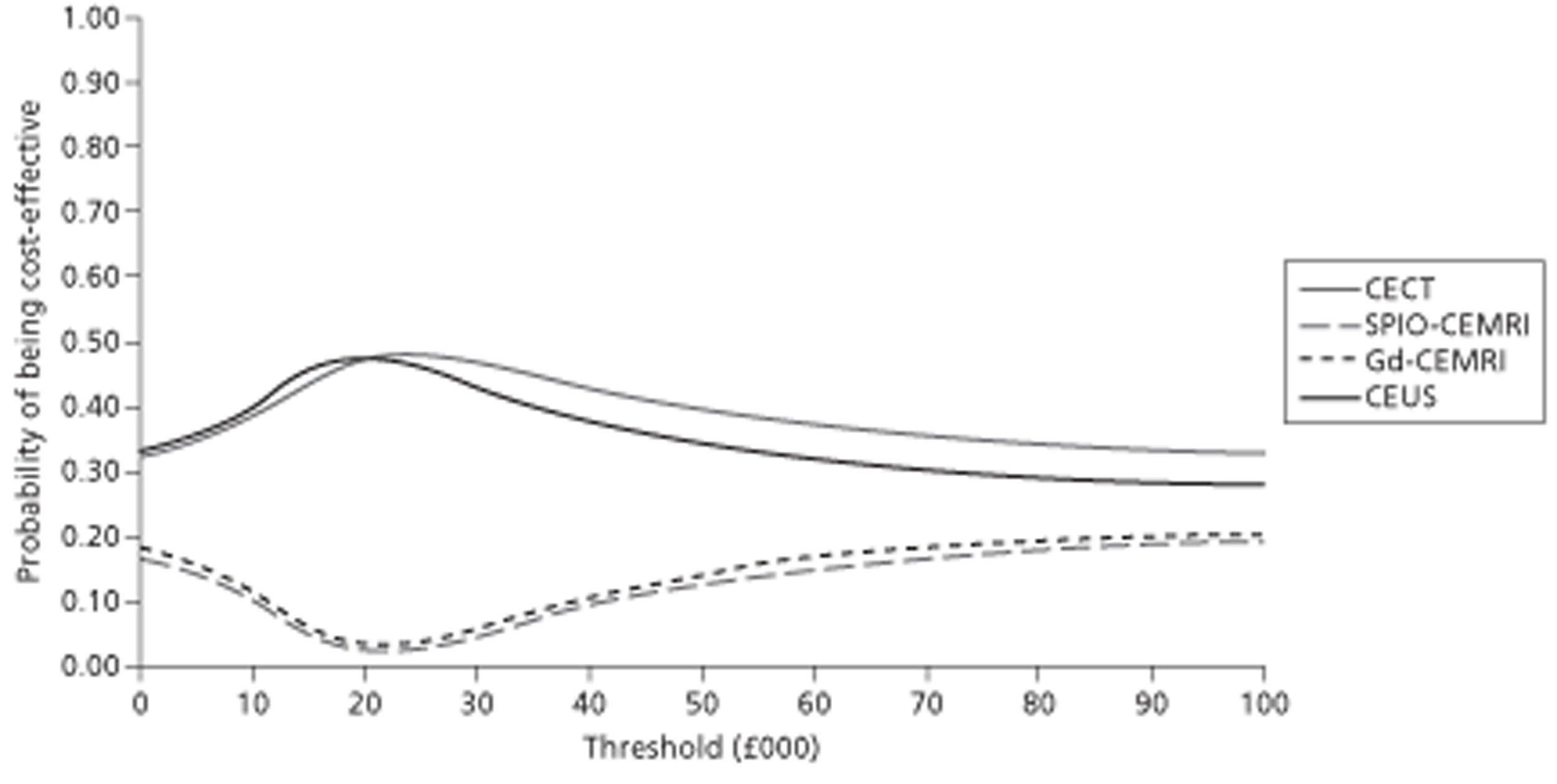
Incidentally detected focal liver lesion model
Effectiveness
Table 47 shows the effectiveness results from the base-case analysis. Two pairs of results are shown here: the first pair shows the results of CEUS compared with CECT, and the second pair shows the results of CEUS compared with CEMRI. The two sets are kept separate because four studies compared CEUS with CECT whereas one study compared CEUS with CEMRI. Very small differences in effectiveness (life-years and QALYs) were seen between CEUS and the two comparators. This was to be expected as the test performance results of the tests were not very different.
| Comparisons | Life-years | QALYs |
|---|---|---|
| CEUS (vs CECT) | 17.205 | 13.330 |
| CECT | 17.205 | 13.330 |
| CEUS (vs CEMRI) | 17.204 | 13.329 |
| CEMRI | 17.201 | 13.327 |
Costs
As with the effectiveness results, the small differences in test performance results resulted in small differences in overall costs (Table 48). The critical factor for any difference in costs is simply the cost of the initial test.
| CEUS (vs CECT) | CECT | CEUS (vs CEMRI) | CEMRI | |
|---|---|---|---|---|
| Initial assessment | 73.5 | 125 | 112.6 | 242 |
| Initial imaging | 64.7 | 116 | 64.7 | 189 |
| False-positive costs | 8.9 | 9.9 | 47.9 | 53 |
| Treatment | 397 | 397 | 398 | 400 |
| Metastases | 159 | 159 | 160 | 160 |
| HCC | 9 | 9 | 9 | 9 |
| Other malignancies | 183 | 183 | 183 | 184 |
| Adenoma | 46 | 46 | 47 | 47 |
| Total | 471 | 522 | 511 | 642 |
Cost-effectiveness
The following results were seen in the base-case analysis (Table 49). As expected, the lower costs of CEUS combined with the slightly better test performance meant that CEUS dominated both CECT and CEMRI. The main factor in these calculations was the cost of the tests.
| Comparison | Incremental costs (£) | Incremental QALYs | Incremental cost per QALY (£) |
|---|---|---|---|
| CEUS vs CECT | −52 | 0.0002 | Dominant |
| CEUS vs CEMRI | −131 | 0.0026 | Dominant |
Additional analyses
Additional analyses changed the absolute costs and effectiveness of the different strategies; however, this did not lead to any dramatic changes in the incremental costs and effectiveness of CEUS compared with CECT or CEMRI. The most critical factor in the analyses related to the costs of the tests. The impact of any other elements (e.g. prior probabilities of a particular diagnosis, costs of treatment) was minimal because the accuracies of the tests were so similar.
Sensitivity analyses
The first sensitivity analysis involved varying the prior probability of malignancy to a value much higher than that used in the base-case scenario. In this analysis, the prior probability was raised from the base-case value of 2.89% to 94% [based on the highest percentages for HCC and metastasis reported in the individual studies (58% of patients with HCC52 and 36% of patients with metastases)]. 55 Although this exceptionally high probability of malignancy was not viewed as realistic in daily practice, it was seen as a way to explore the degree of robustness of the results. As expected, the higher probability of malignancy reduced the absolute number of QALYs and increased the costs (Table 50). However, it increased the incremental QALYs only slightly and had no effect on incremental costs and therefore essentially had no effect on the cost-effectiveness of CEUS compared with CECT or CEMRI.
| Comparison | QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | Incremental cost per QALY (£) |
|---|---|---|---|---|---|
| CEUS (vs CECT) | 6.654 | 17,121 | 0.005 | −56 | Dominant |
| CECT | 6.649 | 17,177 | |||
| CEUS (vs CEMRI) | 6.614 | 17,160 | 0.086 | −202 | Dominant |
| CEMRI | 6.529 | 17,362 |
When the data source for the performance of CEUS and CECT was switched from the meta-analysis to one of the four studies used in the meta-analysis, the cost-effectiveness results changed only slightly.
We also examined the effect on the results of assuming that all patients with HCC had medium-sized lesions instead of small lesions. When we applied this in the model and also increased the risk of HCC to the highest value seen in the DTA studies (58% of patients with HCC52), there was no effect on the cost-effectiveness of CEUS compared with CECT or CEMRI.
When the consequences of an incorrectly diagnosed malignant lesion were made more severe (i.e. by reducing QALYs or increasing costs), this improved the cost-effectiveness of CEUS compared with CECT or CEMRI. For example, if an incorrect diagnosis of HCC and metastases led to a doubling of the costs (compared with the costs following a correct diagnosis) and the QALYs were set to zero, CEUS remained the dominant strategy. Table 51 shows the results of this analysis.
| Comparison | QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | Incremental cost per QALY (£) |
|---|---|---|---|---|---|
| CEUS (vs CECT) | 13.321 | 486 | 0.001 | −54 | Dominant |
| CECT | 13.320 | 540 | |||
| CEUS (vs CEMRI) | 13.312 | 541 | 0.020 | −162 | Dominant |
| CEMRI | 13.293 | 702 |
As expected, when an incorrect diagnosis of HCC or metastases did not result in any health or economic consequences, there was no difference in effectiveness between CEUS, CECT and CEMRI. However, because a difference in costs was still observed, this could be viewed as a situation of extended dominance in both comparisons (Table 52).
| Comparison | QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | Incremental cost per QALY (£) |
|---|---|---|---|---|---|
| CEUS (vs CECT) | 13.332 | 469 | 0.000 | −52 | Extended dominance |
| CECT | 13.332 | 521 | |||
| CEUS (vs CEMRI) | 13.332 | 509 | 0.000 | −130 | Extended dominance |
| CEMRI | 13.332 | 639 |
Probabilistic sensitivity analyses
Probabilistic sensitivity analyses revealed that there was no uncertainty about the cost savings of CEUS compared with CECT (mean difference −£52, 95% CI −£81 to −£22) but some uncertainty about their differences in effectiveness (mean difference 0.00014, 95% CI −0.00100 to 0.00130). Note that these CIs were based on symmetrical beta PERT distributions for the cost parameters. When the original beta PERT distributions were used, a mean difference of −£46 (95% CI −£71 to −£21) was found.
Figure 15 shows the cost-effectiveness acceptability curve comparing CEUS with CECT. This curve shows that the probability of CEUS being cost-effective compared with CECT is > 95% at willingness-to-pay thresholds of up to £20,000.
FIGURE 15.
Cost-effectiveness acceptability curve comparing CEUS with CECT: incidentally detected FLL model (effects are QALYs; both costs and effects are discounted).
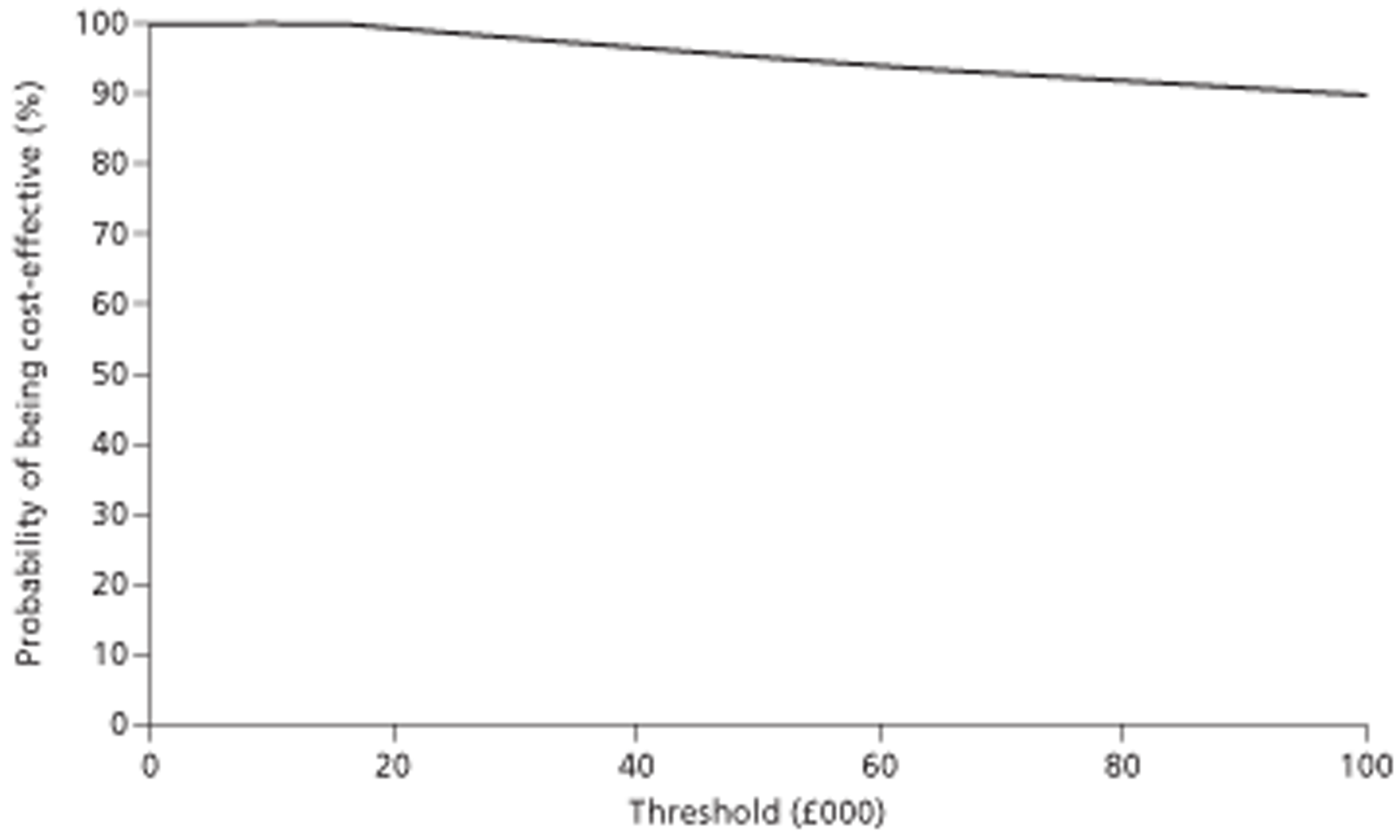
When the differences in costs and effects between CEUS and CEMRI are visualised on the cost-effectiveness plane, it is clear that there is little doubt about the cost savings of CEUS compared with CEMRI, but some uncertainty about their differences in effectiveness.
The results of probabilistic sensitivity analyses comparing CEUS with CEMRI were similar to those shown above for CEUS compared with CECT. There was less certainty about the expected amount of cost savings of CEUS compared with CEMRI (mean difference −£131, 95% CI −£194 to −£69) and some uncertainty about their differences in effectiveness (mean difference 0.0039, 95% CI −0.0058 to 0.0135). Once again, these calculations were made using symmetrical beta PERT distributions for cost parameters to ensure that the point estimate for the cost difference would correspond with the point estimate based on the deterministic analysis. When the original beta PERT distributions were used, a mean difference of −£125 (95% CI −£183 to −£67) was found.
Figure 16 shows the cost-effectiveness acceptability curve comparing CEUS with CEMRI. Here we see that the probability of CEUS being cost-effective compared with CEMRI is > 95% at all willingness-to-pay thresholds between £0 and £20,000.
FIGURE 16.
Cost-effectiveness acceptability curve comparing CEUS with CEMRI: incidentally detected FLL model (effects are QALYs; both costs and effects are discounted).
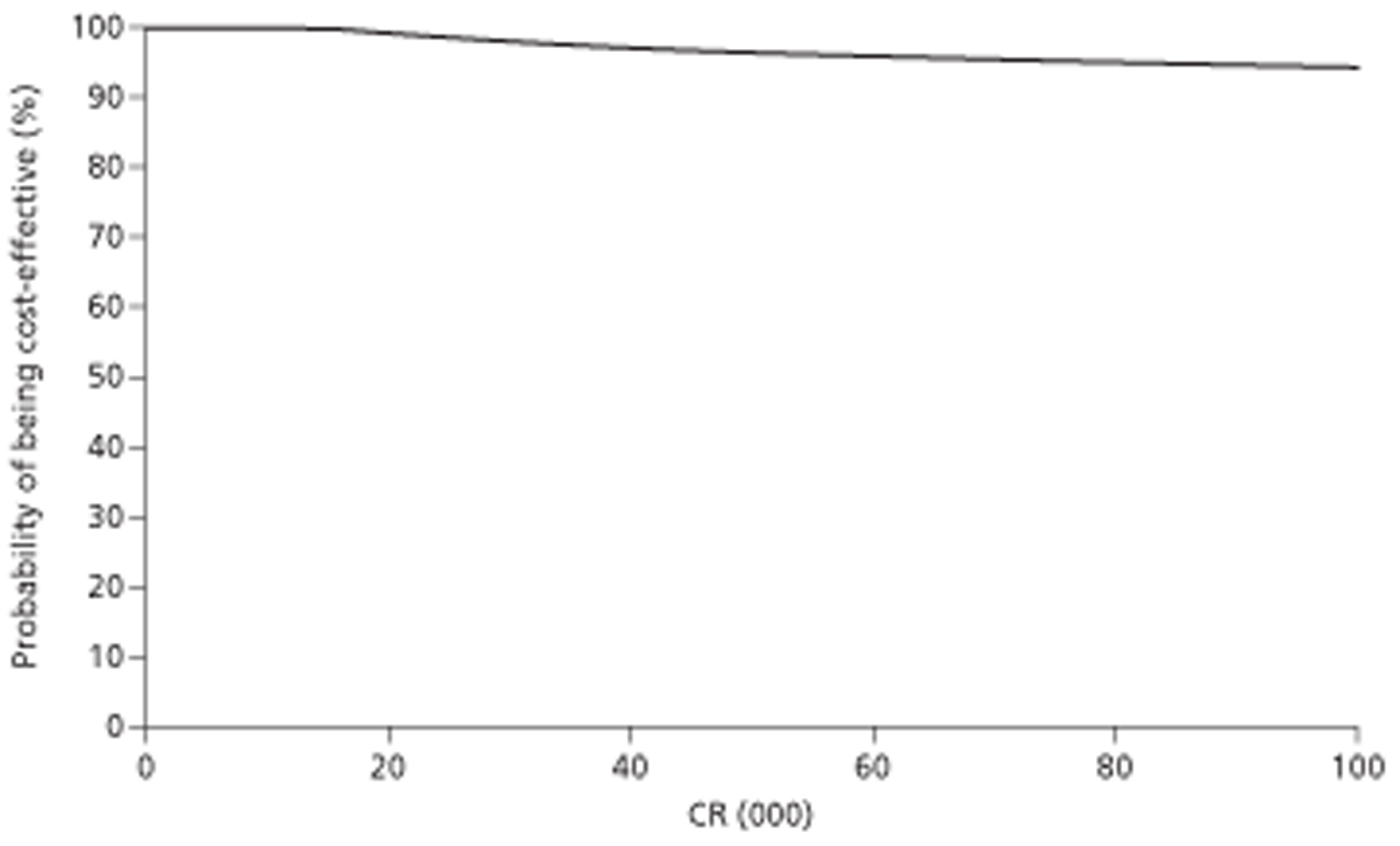
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
Twenty of the 21 studies included in the systematic review were DTA studies: seven compared the performance of imaging modalities for the characterisation of FLLs detected on surveillance of cirrhosis patients using unenhanced US; four compared the performance of imaging modalities for the detection of liver metastases in patients with known primary cancers; six compared the performance of imaging modalities for the characterisation of incidentally detected FLLs identified by unenhanced US; and three compared the performance of imaging modalities for the determination of treatment response in patients with liver cancers.
The only controlled clinical trial identified indicated that the inclusion of CEUS in pretreatment imaging protocols for patients undergoing RFA for HCC may result in a reduced incidence of disease progression, new HCC and repeat RFA, and increased local progression-free and new tumour-free survival, compared with unenhanced US. However, this was a small non-randomised study that had a number of methodological weaknesses and no difference was found in the primary outcome of successful ablation. High-quality RCTs are needed to determine the relative effectiveness of different imaging strategies for treatment planning.
Test accuracy studies varied in terms of target condition (HCC, liver metastases or ‘any malignancy’), definitions of a positive imaging test used by studies of the same target condition, and lesion size assessed. Overall, there was no clear indication that any of the imaging modalities considered (CEUS, CECT or CEMRI) offered superior performance for any of the clinical indications assessed. This is consistent with two other recently published systematic reviews, which found no significant difference in the performance of CEUS, CECT and CEMRI for the characterisation of FLLs. 11,104 Neither of these two reviews reported details of the clinical application of imaging in the included studies (i.e. were FLLs incidentally detected, detected on surveillance or detected during the assessment for liver metastases in patients with known primary cancers) or of the target conditions (e.g. HCC, liver metastases or ‘any liver malignancy’), and one review104 did not specify the use of SonoVue as the contrast agent for CEUS.
The majority of included test accuracy studies were judged to be at ‘low’ or ‘unclear’ risk of bias with respect to the ‘index test’, ‘comparator test’ and ‘reference standard’ domains. ‘Unclear’ ratings for these domains most frequently arose from insufficient detail in the reporting of how tests were interpreted, particularly blinding of interpreters to other test results. Reporting quality was generally poor and a number of studies were reported only as conference abstracts, resulting in a high proportion of ‘unclear’ risk of bias ratings across QUADAS-2 domains (see Figure 7). ‘High’ risk of bias ratings for the ‘patient selection’ domain arose from the use of a retrospective study design or from inappropriate exclusions of particular patient groups (e.g. exclusion of patients with a low probability of malignancy); exclusion of patients with a low probability of disease might result in underestimations of test accuracy, although this was not apparent from the results observed. ‘High’ risk of bias ratings for the ‘flow and timing’ domain arose from exclusion of > 10% of patients from analyses or, in two cases, from incorporation of index test results in the reference standard. The last two studies were also rated as ‘high’ risk of bias for the ‘reference standard’ domain.
Test accuracy studies included in this review were grouped by clinical application: characterisation of FLLs detected on routine unenhanced US surveillance of patients with known cirrhosis, detection of liver metastases in patients with known primary tumours (CRC), characterisation of FLLs in patients with incidentally detected lesions and assessment of response in patients treated for liver malignancy.
Studies conducted in cirrhosis patients undergoing routine surveillance all concerned the differentiation of HCC from other lesion types in small to medium-sized (< 30 mm) FLLs. The definition of a positive test for HCC varied across studies. Studies assessing CEMRI used three contrast agents: gadolinium, a vascular contrast agent; SPIO, a hepatocyte-specific contrast agent which is taken up by Kupffer cells in the normal liver and benign lesions and may therefore aid identification of HCC, which are generally deficient in Kupffer cells, particularly when such lesions are hypervascular;8,9 and Gd-EOB-DTPA-CEMRI, a ‘combined’ vascular and hepatocyte-specific contrast agent. 10 There was no consistent evidence for any significant difference in test performance between the three imaging modalities and three MRI contrast media assessed. When a definition of HCC consistent with that given in the EFSUMB guidelines6 (arterial phase enhancement followed by portal venous/late phase washout) was used, estimates of the sensitivity and specificity of each of the imaging modalities assessed varied across studies. There was some evidence from one study that compared CEUS and Gd-CEMRI that these imaging techniques may be better at ruling out HCC in FLLs of between 11 and 30 mm (sensitivities for CEUS and Gd-CEMRI were 92% and 95% respectively) than in small FLLs of ≤ 10 mm (sensitivities of 27% and 73% respectively), although this study did not use an EFSUMB-consistent definition of HCC. It is therefore possible that some of the variation in sensitivity estimates seen across studies of FLLs of < 30 mm may be due to differences in the size distribution of FLLs included. There was also some evidence from two studies that combined imaging using CEUS and CECT or all three imaging modalities, in which any positive imaging result was treated as ‘test positive’, that combined imaging may increase sensitivity. Inconsistent estimates of sensitivity mean that it is unclear whether or not CEUS alone is adequate to rule out HCC for FLLs of < 30 mm in this population; CEUS alone may be adequate to rule out HCC for FLLs of 11–30 mm, with very small FLLs (< 10 mm) not considered.
Studies of the diagnosis of liver metastases using imaging with vascular contrast media (CEUS, CECT and Gd-CEMRI) in which definitions of a positive imaging test were reported gave various descriptions of peripheral rim enhancement as the criteria for liver metastases. Two studies also reported data for SPIO-CEMRI. There was no consistent evidence for any difference in test performance between the three imaging modalities and the different contrast media assessed. Per-patient sensitivity estimates from two studies were generally high [83% for all imaging modalities and both MRI contrast agents in one study of patients with CRC and > 95% for both CEUS and CECT in a second study of patients with various primary cancers (majority CRC)]. The only previous systematic review identified, which assessed SonoVue CEUS for the diagnosis of liver metastases, did not include any comparator tests and reported sensitivities for CEUS ranging from 79% to 100%. The limited data available indicate that CEUS alone may be adequate to rule out liver metastases in patients with known primary malignancies.
The primary outcome measure reported by studies conducted in patients with incidentally detected FLLs was test accuracy for the differentiation of malignant from benign liver lesions. Studies consistently used definitions of the imaging criteria for HCC and liver metastases that were similar to those reported in the EFSUMB guidelines on the use of CEUS. 6 All studies reported no significant difference in the accuracy of CEUS and CECT or CEMRI for the characterisation of FLLs. All but one study reported data for one lesion per patient and the remaining study reported data for 694 lesions in 686 patients; data were therefore treated as per patient. The pooled estimates of sensitivity for the identification of ‘any liver malignancy’ were approximately 95% for both CEUS and CECT and the pooled estimates of specificity were 94% and 93%, respectively, based on data from four studies. The single study comparing CEUS with CEMRI used Gd-CEMRI in all patients, with the addition of SPIO-CEMRI in an unspecified number of cases, and reported sensitivity estimates of 91% and 82%, respectively, and corresponding specificity estimates of 67% and 63%. Data from one study indicated that combined imaging using both CEUS and CECT, in which a positive result on either modality was treated as ‘test positive’, did not increase sensitivity. This, combined with the high estimates of sensitivity, indicates that CEUS alone may be adequate to rule out liver malignancy in this population.
Two Chinese-language studies comparing imaging modalities for the assessment of response to treatment (cryosurgery and non-surgical treatment) in patients with HCC reported per-lesion sensitivity estimates of > 95% and specificity estimates of > 80% for complete response, using CEUS, CECT and CECT or Gd-CEMRI.
These data indicate that CEUS may provide information on response in patients treated for HCC. However, these data are very limited and may not be directly applicable to UK clinical practice; further studies, ideally conducted in a UK setting, are required to confirm findings. The possibility of the rapid detection of residual tumour tissue using CEUS has the potential to allow the immediate extension of interstitial therapy; however, no data were identified on any therapeutic consequences of using CEUS to assess response to initial treatment.
Cost-effectiveness
The cost-effectiveness analysis of the use of CEUS in patients with an inconclusive unenhanced US test indicated that the use of CEUS instead of CEMRI was considered cost-effective. The use of CEUS instead of CECT was considered cost-effective in the surveillance of cirrhosis and the characterisation of incidentally detected FLLs, whereas the two techniques were similar in terms of costs and effects in the detection of liver metastases from CRC.
In the surveillance of cirrhosis, CEUS was found to be as effective as but £379 less costly than CECT. This indicates that CEUS dominates CECT. Gd-CEMRI was found to be £1063 more costly than CEUS and gained 0.022 more QALYs. This resulted in an ICER of £48,545 per QALY gained. This ICER is deemed unacceptable given the currently used willingness-to-pay thresholds of £20,000 and £30,000 per additional QALY. CEUS can therefore be considered the most cost-effective option after inconclusive unenhanced US. These base-case results were based on one source for accuracy, the study by Leoni et al. 42 Using the two other studies that compared CEUS and CECT corroborated the dominance of CEUS over CECT, showing even lower effectiveness for CECT. Compared with Gd-CEMRI, CEUS was cost-effective in most sensitivity analyses, except when all positive unenhanced US examinations were subject to confirmatory testing instead of the inconclusive US examinations, and when the proportion of patients having an inconclusive US scan was considerably lower (20% instead of 43%). These two analyses resulted in acceptable ICERs for Gd-CEMRI compared with CEUS of £12,806 and £16,121 respectively.
In the diagnosis of liver metastases from CRC, CEUS was found to have similar costs and effects to those of CECT. Using a lifetime time horizon the two techniques yielded equal QALYs per patient, with CEUS costing £1 more than CECT. Both Gd-CEMRI and SPIO-CEMRI were dominated by CECT in this population because they were more costly and equally as effective. However, in the base-case analysis it was assumed that patients who were incorrectly diagnosed with liver metastases would receive a biopsy before they were treated and that this mistake would be discovered. If this is not assumed and patients could receive unnecessary treatment, the lower specificity of CEUS had larger consequences. Under this assumption, CEUS is both the most costly and the least effective option, and Gd-CEMRI dominates all other tests. However, it is questionable whether or not this would occur in practice. If the proportion of patients having metastases were higher, CEUS would dominate the other tests. Based on the two other studies that reported accuracy data in this population,50,51 CEUS was found to dominate CECT. Gd-CEMRI yielded 0.014 more QALYs but was also £587 more costly than CEUS, resulting in an ICER of £43,318 per QALY gained. As this is above the willingness-to-pay threshold of £30,000 per QALY, Gd-CEMRI is deemed not cost-effective compared with CEUS.
The final evaluation involved the comparison of CEUS with CECT and CEMRI in the characterisation of incidentally detected FLLs. In the base-case analysis, no large differences in effectiveness were found between the three imaging strategies (incremental QALYs: CEUS vs CECT: 0.00016; CEUS vs CEMRI: 0.0026). However, a difference in costs was found (CEUS vs CECT: −£52; CEUS vs CEMRI: −£131) and this resulted in a situation of dominance. Probabilistic sensitivity analysis revealed that there was little uncertainty about the cost-effectiveness of CEUS compared with the other two tests. Additional analyses changed the absolute costs and effectiveness of the different strategies but did not lead to dramatic changes in the incremental costs and effectiveness of CEUS compared with CECT or CEMRI. One critical factor in the analyses related to the costs of the tests. This could mean that local conditions may play a role in deciding which test is preferable, assuming that the costs of these tests can be influenced by local conditions.
Strengths and limitations of the assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms,20 search strategies were developed to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened, many of which did not meet the inclusion criteria of the review.
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment, for example a significant difference between the treatment and control groups that favours treatment. This is not the case for test accuracy studies, which measure agreement between index test and reference standard. It would seem likely that studies finding greater agreement (high estimates of sensitivity and specificity) will be published more often. In addition, test accuracy data are often collected as part of routine clinical practice, or by retrospective review of records; test accuracy studies are not subject to the formal registration procedures applied to RCTs and are therefore more easily discarded when results appear unfavourable. The extent to which publication bias occurs in studies of test accuracy remains unclear; however, simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 105 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic and reliability is limited. 105 We did not undertake a statistical assessment of publication bias in this review; however, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts.
Clear inclusion criteria were specified in the protocol for this review and the one protocol modification that occurred during the assessment has been documented in Chapter 3 of this report (see Inclusion and exclusion criteria). The eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for excluding all of the studies considered potentially relevant at initial citation screening (see Appendix 5). The review process followed recommended methods to minimise the potential for error and/or bias. 16 Studies were independently screened for inclusion by two reviewers and data extraction and quality assessment were carried out by one reviewer and checked by a second (MW and VG). Any disagreements were resolved by consensus. Chinese-language studies were extracted by one reviewer (MW) working with a native speaker (KL) and the only German-language study was extracted by one reviewer (VG).
With one exception, all studies included in the review were test accuracy studies. The methodological quality of these studies was assessed using a modification of the QUADAS-2 tool. 25 The QUADAS tool has been recommended for assessing the methodological quality of test accuracy studies16,18 and has been widely adopted by researchers and key organisations such as The Cochrane Collaboration, NICE and the Institut für Qualität and Wirtschaftlichkeit im Gesundheitswesen (IQWiG) in Germany. It has been mentioned in more than 200 abstracts in the DARE database and has been cited more than 500 times. The revised version of QUADAS (QUADAS-2) has recently been published. 25 QUADAS-2 more closely resembles the approach and structure of the Cochrane risk of bias tool. It is structured into four key domains covering participant selection, index test, reference standard and the flow of patients through the study (including the timing of tests). Each domain is rated for risk of bias (low, high or unclear) and the tool provides signalling questions in each domain to help reviewers in reaching a judgement. The participant selection, index test and reference standard domains are also separately rated for concerns regarding the applicability of the study to the review question (low, high or unclear). However, the QUADAS-2 tool does not currently include domains specific to the assessment of studies comparing multiple index tests; further development of QUADAS-2 in this area is planned. This assessment used a modified version of the QUADAS-2 tool that includes an additional domain for the comparator test and additional signalling questions in the flow and timing domain. It should be noted, however, that these components of the tool were not developed using the same rigorous evidence-based approach as for the core QUADAS-2 tool. The inclusion criteria for this review were considered to largely match the review question and questions of applicability were therefore relevant only to the patient selection domain. The review-specific guidance used in our QUADAS-2 assessment is reported in Appendix 2. The results of the risk of bias assessment are reported in full for all included studies in Appendix 3 and in summary in Chapter 3 (see Results of the assessment of clinical effectiveness). However, the usefulness of this assessment was limited by poor reporting of primary study methods, particularly with respect to how the index and comparator tests and the reference standard were applied. This issue was exacerbated because four of the 20 test accuracy studies (20%) were reported only as conference abstracts.
The systematic review conducted for this assessment represents an improvement on previously published systematic reviews11,104,106 in that it focuses on studies that directly compared the performance of SonoVue CEUS with at least one other imaging modality, as well as clearly distinguishing between both the clinical application and the target condition of imaging.
Hierarchical or bivariate models are considered the optimal methods for estimating SROC curves and pooled estimates of sensitivity and specificity. 16,27 The bivariate model analyses sensitivity and specificity jointly, retaining the paired nature of the original data, and has been shown to produce equivalent results to the hierarchical SROC model in the absence of other study-level covariates. 28 However, the fitting of this model requires a minimum of four data sets. There was only one group of four studies in this assessment for which meta-analytic pooling was considered potentially appropriate (similar clinical application, target condition and comparator test). One of these studies used a suboptimal reference standard and a sensitivity analysis was used to investigate the influence of this study upon the overall estimate of test performance, reducing the data set to three studies; for this reason, a random-effects model was used to generate pooled estimates of sensitivity and specificity, with 95% CIs.
In addition to the limited potential for meta-analysis and the general methodological quality issues outlined above, there were a number of reporting/methodological problems specific to this review. Of particular concern for this assessment was the way in which data were reported in terms of the unit of analysis. The main reason for undertaking liver imaging in the populations considered is likely to be to rule out primary liver cancer or liver metastases. Therefore, patient-level analyses of test performance are of particular interest. Some of the studies included in this review reported per-patient analyses; however, no study clearly stated how per-patient test results were defined (e.g. was the presence of any positive lesion regarded as a positive test for the whole patient). Some of the included studies reported per-lesion data (multiple lesions per patient). These type of within-patient ‘clustered’ data are a common feature of test accuracy studies and are likely to result in a correlation between results within each patient, which should be accounted for in any statistical analysis. 107 Uncorrected estimates of sensitivity and specificity derived from such data are likely to be accurate, but imprecision will be underestimated. 107 Of greater concern are those studies that reported data for one lesion per patient (treated as per-patient data in this assessment) but in which multiple lesions per patient were present, as was the case for the majority of studies evaluating SonoVue CEUS for the characterisation of incidentally detected FLLs. 52,54–56 These studies generally selected the largest lesion or the lesion ‘most suspicious for malignancy’ for inclusion in analyses, with the result that estimates of test performance may have been exaggerated. It might be argued that, when considering the ability of a test to rule out malignancy, performance for the characterisation of smaller ambiguous lesions is an important consideration.
All assessments of diagnostic accuracy are underpinned by the assumption that the reference standard, against which the index and comparator tests are evaluated, is 100% sensitive and 100% specific. The inclusion criteria specified by the protocol for this assessment allowed the use of different reference standards for test-positive and test-negative patients (histology and clinical follow-up respectively). This approach was used because it may be considered unethical to perform biopsy of test-negative patients or lesions. However, delayed verification, as represented by clinical follow-up, is inherently flawed in that follow-up must be of sufficient duration for any false-positive or false-negative test results to become apparent, but prolonged follow-up may also result in changes in disease state and hence misclassification of test results. In addition, a protocol modification allowed the inclusion of studies on the characterisation of FLLs (suspected HCC) that used EASL/AASLD non-invasive diagnostic criteria (two concordant imaging test results) as the reference standard. Two additional studies were included in the review as a result of this protocol modification. 42,41 Studies using this type of reference standard may be subject to incorporation bias. However, the implications of this are unclear; the review of sources of variation and bias in test accuracy studies, conducted as part of the development of QUADAS, found no evidence on the effects of incorporation bias,23 and the update of this review, conducted during the development of QUADAS-2, found two contradictory studies, one reporting no effect of incorporation bias on accuracy and one reporting increased sensitivity and reduced specificity in the presence of incorporation bias (unpublished data).
The clinical applicability of accuracy data included in this review may have some limitations. The inclusion criteria for this assessment specified that SonoVue CEUS should be used for the characterisation of FLLs when unenhanced US examination was considered inconclusive. Although all study participants had imaging-detected FLLs before undergoing SonoVue CEUS, only one study43 explicitly stated that unenhanced US was inconclusive. Perhaps more importantly, the prevalence of malignancy appeared high in studies assessing the accuracy of CEUS and other imaging modalities for the characterisation of incidentally detected FLLs; these study populations may not be representative of the population with incidental FLLs seen in clinical practice. When any information on the interpretation of CEUS was reported (see Appendix 4), the majority of DTA studies included in this review reported consensus interpretation by multiple experienced operators (experience ranging from > 2 years to 20 years). It should be noted that operator training and experience may have important effects on the diagnostic performance of CEUS, but insufficient data are currently available to explore these potential effects.
The majority of included studies reported no information on funding; two studies reported funding from the manufacturer of SonoVue. 55,56
Cost-effectiveness
In this study we built three separate models for the three different potential uses of CEUS: surveillance of cirrhosis, detection of liver metastases from CRC and characterisation of incidentally detected FLLs. All three models were based on existing models that had previously informed NICE guidance. 70,71 When necessary we updated and improved these models. The model for incidentally detected FLLs was a combination of the two updated and improved models.
In each of the three analyses we used evidence to inform parameters that was relevant for the UK and as up-to-date and of as high a quality as possible. When evidence was not available from published studies or databases, we used the most likely and plausible ranges based on expert opinion.
As expected, the main driver of the models was the accuracy of the different tests. There was only one group of four studies in this assessment for which meta-analytic pooling was considered potentially appropriate (similar clinical application, target condition and comparator test): the use of CEUS to characterise incidentally detected FLLs. As a consequence, the estimated cost-effectiveness of CEUS for the surveillance of cirrhosis and the diagnosis of liver metastases from CRC had to be based on single studies. Scenario analyses were performed using other available studies and these analyses showed that in general the source for accuracy influences the costs and effects of the different tests. However, the use of different sources resulted in similar conclusions. CEUS was found to be the most cost-effective test for the surveillance of cirrhosis, and the two alternative sources for the liver metastases model produced favourable results for CEUS.
In general, the studies used to estimate test accuracy appeared to involve different types of patient populations. The studies used for the incidentally detected FLL model, for example, defined incidentally detected FLLs in different ways. Interestingly, regardless of the variation in composition of the patient populations, there was never an instance when the test accuracy results of CEUS and CECT were very different. All studies concluded that the two tests were comparable in performance.
Another main driver was the clinical pathway of incorrectly diagnosed patients. Although the pathway may be straightforward for false-negative patients, as their disease may be correctly diagnosed at a later stage of the initial workup, it is not as clear for false-positive patients. In the liver metastases from CRC model we assumed that patients who are inaccurately diagnosed as having metastases would receive biopsy before treatment. This implies that patients were not unnecessarily treated. However, it is unclear what happens to these patients in practice. Therefore, we performed a sensitivity analysis in which patients without metastases were treated if they were incorrectly diagnosed. In this sensitivity analysis CEUS was found to be the least effective and most costly option. Although we do not expect it to be realistic that patients without metastases will actually receive treatment, it is important to note this factor.
Besides being less costly, CEUS has the advantage compared with CECT and especially CEMRI that it is highly accessible. All patients already receive an unenhanced US examination and can be immediately diagnosed using CEUS as part of the same examination. A potential benefit of CEUS is therefore the reduction in anxiety in patients because a malignant lesion is ruled out sooner as a result of not having to wait too long for another test. This benefit was not taken into account in the analysis as little evidence is available on the effect of anxiety on quality of life. It might be expected that the effects of using CEUS are therefore underestimated. Although the length of wait associated with other imaging modalities is uncertain, the consideration of this anxiety factor would only further support the use of CEUS over CECT or CEMRI.
Uncertainties
Clinical effectiveness
None of the clinical applications of liver imaging considered in this review was evaluated by a large number of studies; the maximum was seven studies on the performance of imaging modalities for the characterisation of FLLs detected on surveillance of cirrhosis patients using unenhanced US. Although this review benefits from focusing on studies that directly compared the performance of SonoVue CEUS with that of other imaging modalities, as noted above in the strengths and limitations section, only two studies on the characterisation of FLLs detected on surveillance of cirrhosis patients42,47 and two studies on the detection of liver metastases in patients with known primary cancers49,50 compared all three imaging modalities under assessment (CEUS, CECT and CEMRI). Most studies that assessed CEMRI used gadolinium-based vascular contrast agent, which has a comparable mode of operation with that of CEUS and CECT. However, CEMRI of the liver can also be conducted using hepatocyte-specific contrast agents such as SPIO or ‘combined’ vascular and hepatocyte-specific agents such as Gd-EOB-DTPA; only four of the studies included in our systematic review reported data for these types of contrast agent. 42,49,48,50 Studies were generally small (15 of the 20 DTA studies included fewer than 100 participants) and, within clinical applications, studies varied in terms of target condition (HCC, liver metastases or ‘any malignancy’), definitions of a positive imaging test used by studies of the same target condition, comparator imaging technologies and lesion size assessed. In addition, four of the 20 test accuracy studies were reported only as conference abstracts,39–41,50 which further limited the available data. These factors meant that, as detailed above in the statement of principal findings for the clinical effectiveness assessment, only one meta-analysis was undertaken (studies comparing CEUS with CECT for the characterisation of incidentally detected FLLs). Based on the available data, SonoVue CEUS appeared to offer similar diagnostic performance to that of other imaging modalities (CECT and CEMRI) for all clinical applications considered, but data were generally insufficient to support firm conclusions.
SonoVue CEUS is generally used for the characterisation or detection of liver lesions in patients for whom unenhanced US examination has proved inconclusive. In addition to test accuracy, it is therefore particularly important to assess the proportion of patients in whom US examination remains inconclusive even after contrast-enhancement compared with the proportion in whom comparator imaging technologies are inconclusive. Four of the 20 DTA studies included in this review explicitly reported the numbers of participants in whom imaging was inconclusive; three studies indicated that SonoVue CEUS was inconclusive in slightly fewer patients than CECT (0%,51 3%46 and 3%54 for SonoVue CEUS compared with 14%,51 8%46 and 6%54 for CECT). One study reported 11% inconclusive imaging results for both SonoVue CEUS and CEMRI. 56 Although not explicitly stated, all other included studies appeared to report complete data sets and hence may be inferred to have had no inconclusive imaging examinations.
When diagnostic accuracy is comparable across imaging modalities, comparison of adverse event rates associated with the different imaging options, as well as consideration of patients' preferences, are also of particular importance. Only one of the DTA studies included in this review reported any information on adverse events related to testing; the authors of this study stated that there were no adverse events associated with SonoVue CEUS but did not report any information about the comparator technology Gd-CEMRI. 45 A large, retrospective safety study of SonoVue CEUS in abdominal applications, which did not meet the inclusion criteria for this review, reported data from 23,188 investigations in 29 centres in Italy. 7 This study found 29 cases of adverse events, of which two were graded as serious, one as severe, three as moderate and 23 as mild. There were no fatal adverse events. One of the serious adverse events occurred in a patient with prostate cancer who was being investigated to characterise a liver lesion suspected of metastases; this patient complained of dyspnoea with signs of bronchoplasm, slight hypotension and bradycardia within 1 minute after injection of SonoVue. The majority of non-serious adverse events resolved without intervention and included itching, mild dizziness, moderate hypotension, headache, sensation of warmth and nausea and vomiting. None of the studies identified reported any information on patient preferences.
Acceptability to patients and the potential for reduced anxiety provided by the ability to conduct CEUS at the same appointment as the initial US examination are also likely to be important factors in the choice of imaging modality; however, no studies were identified that reported these outcome measures.
It should be further noted that, although this review provides some evidence on the accuracy of SonoVue CEUS for the characterisation of FLLs and the detection of liver metastases and response to treatment of liver cancers, only one study59 was identified that reported the effects of imaging with SonoVue on patient outcomes; the ultimate aim of any research on clinical tests should be to determine impact on patient management and clinical outcomes. As described earlier in the statement of principal findings for the clinical effectiveness assessment, this study indicated that the inclusion of CEUS in pretreatment imaging protocols for patients undergoing RFA for HCC may result in some improved outcomes compared with unenhanced US. Overall, the effects, if any, of imaging with SonoVue CEUS on management and outcome of patients with FLLs remain uncertain.
Cost-effectiveness
Many studies emphasised that the participating clinicians had years of experience in the use of CEUS. It is possible that the diagnostic accuracy of CEUS may be poorer if the user has little experience. However, widespread implementation of CEUS might also improve the experience with CEUS and ultimately improve accuracy.
The main uncertainty surrounding the cost-effectiveness of CEUS is how patients who are incorrectly diagnosed are managed. Arguably, this is very different across locations. In the cirrhosis surveillance model, patients are screened twice a year and it is expected that a lesion, although it may have grown and therefore be potentially less treatable, will be detected eventually. In the liver metastases from CRC model, patients with metastases will have associated symptoms and it is therefore justifiable to assume that metastases will be detected within a year. Patients with incidentally detected lesions also often have associated risk factors or evidence of liver disease, which may have been the indication for initial testing with unenhanced US or which may have been identified at this examination; hence, it is expected that their complaints will worsen and that their lesions will be detected within several months. How patients with a false-positive test result are managed might be more complex. We assumed that, in all models, these patients would receive additional costs of unnecessary additional diagnostic tests but would not undergo inappropriate treatment as the correct diagnosis would be determined after additional diagnostic workup. In the liver metastases of CRC model we examined the extreme situation in which all patients who were incorrectly diagnosed with metastases would receive treatment for these metastases. As this involves the costs of the treatment as well as reduced quality of life, this has a considerable impact on the results.
In the cirrhosis surveillance model, the actual use of CEUS impacted the results. If CEUS were used after all positive instead of after all inconclusive unenhanced US examinations, or if the proportion of inconclusive unenhanced US tests were lower, Gd-CEMRI would be cost-effective compared with CEUS.
Chapter 6 Conclusions
Implications for service provision
The results of our systematic review suggest that SonoVue CEUS could provide similar diagnostic performance to other imaging modalities (CECT and CEMRI) for the three main clinical applications considered: characterisation of FLLs detected on surveillance of cirrhosis patients using unenhanced US, detection of liver metastases in patients with known primary cancers (CRC) and characterisation of incidentally detected FLLs identified by unenhanced US. However, some caution is required in the interpretation of these findings as studies were generally small and heterogeneous with respect to the target condition (HCC, liver metastases or ‘any malignancy’), definitions of a positive imaging test used by studies of the same target condition, comparator imaging technologies and lesion size assessed. Available data were insufficient to draw firm conclusions of the effectiveness of CEUS in treatment planning and the determination of treatment response.
The cost-effectiveness analysis of the use of CEUS in patients with an inconclusive unenhanced US test indicated that the use of CEUS instead of CEMRI was considered cost-effective. The use of CEUS instead of CECT was considered cost-effective in the surveillance of cirrhosis and the characterisation of incidentally detected FLLs, whereas the two techniques were similar in terms of costs and effects in the detection of liver metastases from CRC. Although these conclusions can be very dependent on the actual management of incorrectly diagnosed lesions, it is expected that the use of CEUS can reduce costs without reducing quality of life and survival. It should be noted that experience with using CEUS can have an important impact on diagnostic accuracy.
If the main use of liver imaging in these populations is considered to be the rapid rule-out of malignancy, equivalent diagnostic performance may be sufficient for SonoVue CEUS to be preferred over other imaging modalities when unenhanced US is inconclusive. A potential advantage of using SonoVue CEUS would be the option of completing the assessment at the same time as the initial unenhanced US examination. Although this would be unlikely to reduce waiting times (compared with other imaging modalities) sufficiently to change clinical outcome, the potential to provide more rapid diagnosis without repeat hospital visits is likely to be preferred by patients and may also reduce costs (e.g. by avoiding the administration costs of scheduling new appointments).
Suggested research priorities
All but one of the studies included in our systematic review were DTA studies of liver imaging for the clinical applications specified in our protocol: characterisation of FLLs detected on surveillance of cirrhosis patients using unenhanced US, detection of liver metastases in patients with known primary cancers (CRC), characterisation of incidentally detected FLLs identified by unenhanced US and determination of treatment response in patients with liver cancers. The test accuracy study design compares the results of a new test (index test) with those of the reference standard (which are assumed always to be correct); it is therefore inherently not capable of comparing tests in terms of their ultimate impact on patient outcome. The only study included in this review that reported data on patient outcomes considered the impact on clinical outcomes following treatment of using SonoVue CEUS for pretreatment assessment. This study had a number of methodological limitations and found significant effects of SonoVue CEUS only on secondary outcomes. The ideal study to address questions of clinical effectiveness would be a large multicentre RCT in which patients are randomised to receive further testing/monitoring, therapeutic planning and/or treatment based on different imaging strategies (SonoVue CEUS, CECT, CEMRI); evaluation in more than one centre is preferred to minimise performance bias. Long-term observational studies assessing the clinical consequences of incorrect initial diagnoses may also provide an important source of information for future cost-effectiveness analyses. Other potentially important factors that may affect decision-making in the NHS and for which information is currently lacking include patient preferences and the acceptability of different imaging modalities, the potential effects of reduced anxiety arising from a more rapid diagnosis and the potential effects of operator experience on the diagnostic performance of CEUS.
Test accuracy data identified in this assessment were relatively sparse and studies were heterogeneous with respect to the target condition (HCC, liver metastases or ‘any malignancy’), definitions of a positive imaging test used by studies of the same target condition, comparator imaging technologies and lesion size assessed. Standardisation of the definition of a positive imaging test for each target condition followed by further high-quality DTA studies are therefore needed to confirm our findings. Future DTA studies should ideally compare the performance of all three imaging modalities (SonoVue CEUS, CECT and CEMRI) in the same patient group and should also report the numbers of patients in whom imaging with each modality is non-diagnostic as well as any imaging-related adverse events; studies comparing all three imaging modalities could provide a useful vehicle for the collection of information on patients' preferences. Further investigation of the potential role of CEMRI using newer ‘combined’ vascular and hepatocyte-specific contrast agents may also be warranted. The QUADAS-2 assessment highlighted limitations in the reporting of many studies included in our review; future studies should follow the STARD (STAndards for the Reporting of Diagnostic accuracy studies) guidelines for reporting test accuracy studies. 108,109
Acknowledgements
The authors acknowledge the clinical advice and expert opinion provided by Jane Smith (née Bates) MPhil, DMU, DCR, Consultant Ultrasound Practitioner, St James's University Hospital, Leeds Teaching Hospitals NHS Trust; Gail Coster MBA, DMU, BSc, DCR(R), Trainee Consultant Radiographer, Mid Yorkshire Hospitals NHS Trust; and Dr Tim Hoare, Consultant Radiologist, Newcastle Upon Tyne Hospitals NHS Foundation Trust. The authors would also like to thank Rob Anderson, Paul Hewson, Gabriel Rogers and Ken Stein, Health Economics Group, PenTAG, Institute of Health Service Research, Peninsula Medical School, for making the PenTAG cirrhosis surveillance model available.
The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA programme. Any errors are the responsibility of the authors.
Contribution of authors
Marie Westwood, Viktoria Gloy and Heike Raatz planned and performed the systematic review and interpretation of evidence. Manuela Joore, Janneke Grutters and Ken Redekop planned and performed the cost-effectiveness analyses and interpreted the results. Nigel Armstrong and Kelly Lee contributed to the planning and interpretation of the cost-effectiveness analyses and acquisition of input data for modelling. Kate Misso devised and performed the literature searches and provided information support to the project. Jos Kleijnen and Johan Severens provided senior advice and support to the systematic review and cost-effectiveness analyses respectively. All parties were involved in drafting and/or commenting on the report.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36.
- Ryder S. UK Guidelines for the management of suspected hepatocellular carcinoma (HCC) in adults (revised edition). Nottingham: Nottingham University Hospitals NHS Trust; 2009.
- Cancer statistics registrations: registrations of cancer diagnosed in 1999, England (Series MB1 no. 30). London: ONS; 2002.
- Cancer incidence for common cancers – UK statistics. London: Cancer Research UK; 2011.
- Cabassa P, Bipat S, Longaretti L, Morone M, Maroldi R. Liver metastases: sulphur hexafluoride-enhanced ultrasonography for lesion detection: a systematic review. Ultrasound Med Biol 2010;36:1561-7.
- Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS): update 2008. Ultraschall Med 2008;29:28-44.
- Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006;32:1369-75.
- Ward J, Guthrie JA, Scott DJ, Atchley J, Wilson D, Davies MH, et al. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology 2000;216:154-62.
- Lutz AM, Willmann JK, Goepfert K, Marincek B, Weishaupt D. Hepatocellular carcinoma in cirrhosis: enhancement patterns at dynamic gadolinium- and superparamagnetic iron oxide-enhanced T1-weighted MR imaging. Radiology 2005;237:520-8.
- Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics 2009;29:1707-24.
- Guang Y, Xie L, Ding H, Cai A, Huang Y. Diagnosis value of focal liver lesions with SonoVue-enhanced ultrasound compared with contrast-enhanced computed tomography and contrast-enhanced MRI: a meta-analysis. J Cancer Res Clin Oncol 2011;137:1595-605.
- Schwartz J, Kruskal J, Basow D. UpToDate. Waltham, MA: UpToDate; 2011.
- Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, . European Association for the Study Of The Liver; European Organisation for Research and Treatment of Cancer . EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43.
- Colorectal cancer: the diagnosis and management of colorectal cancer. Full guideline. London: NICE; 2011.
- Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut 2006;55:iii1-8.
- Systematic reviews: CRD's guidance for undertaking reviews in health care. York: University of York; 2009.
- Diagnostics Assessment Programme: interim methods statement (version 2). London: NICE; 2010.
- Handbook for DTA reviews. 2011.
- Westwood M, Al M, Burgers L, Redekop W, Lhachimi S, Armstrong N, et al. Computed tomography (CT) scanners for cardiac imaging – Somatom Definition Flash, Aquilion One, Brilliance iCT and Discovery CT7. York: Kleijnen Systematic Reviews Ltd; 2011.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7.
- McGowan J, Sampson M, Lefebvre C. An evidence based checklist for the peer review of electronic search strategies (PRESS EBC). Evid Based Libr Inf Pract 2010;5:1-6.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. A systematic review of sources of variation and bias in studies of diagnostic accuracy. Ann Intern Med 2004;140:189-202.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. A systematic review finds that diagnostic reviews fail to incorporate quality despite available tools. J Clin Epidemiol 2005;58:1-12.
- Whiting P, Rutjes AW, Westwood M, Mallet S, Deeks JJ, Reitsma JB, et al. The development of QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36.
- Higgins JPT, Green S. The Cochrane Collaboration. 2011.
- Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PMM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90.
- Harbord RM, Deek JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51.
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 2008;61:1095-103.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
- Bohata S, Pavlik T, Chlumska D, Valek V. Contribution of the contrast enhanced ultrasound in the differential diagnosis of the focal lesions. Ceska Radiol 2010;64:11-9.
- Chen M-H, Wu W, Yang W, Gao W, Dai Y, Yin S-S, et al. Application of contrast-enhanced ultrasonography in selecting indication of radiofrequency ablation among hepatocellular carcinoma patients. Zhonghua Yi Xue Za Zhi 2005;85:3491-4.
- Passamonti M, Vercelli A, Azzaretti A, Rodolico G, Calliada F. Characterization of focal liver lesions with a new ultrasound contrast agent using continuous low acoustic power imaging: comparison with contrast enhanced spiral CT. Radiol Med 2005;109:358-69.
- Banghui P, Chiche L, Alkofer B, Salame E, Bouvard N, Lepennec V. Imaging modalities before liver resection for colorectal metastases: which, when and how many? Paper presented at the 9th World Congress of the International Hepato-Pancreato-Biliary Association, Buenos Aires, Argentina, 18–22 April 2010. HPB 2010;12.
- Ercolani G, Zanello M, Rojas L, Ravaioli M, Cescon M, Gaudio MD, et al. A prospective comparative evaluation of pre- and intraoperative imaging techniques in chemo-pretreated or not pretreated patients with colorectal liver metastases. Paper presented at the 9th Congress of the European-African HPBA (E-AHPBA), Cape Town, South Africa, 12–16 April 2011. HPB 2011;13:25-6.
- Loria F, Loria G, Frosina L, Crea G, Basile S, Cantoni S. Metastatic disease of the liver: comparison of CEUS vs. US and CT in the evaluation of diagnostic performance and confidence. Paper presented at the 9th World Congress of the International Hepato-Pancreato-Biliary Association, Buenos Aires, Argentina, 18–22 April 2010. HPB 2010;12.
- Kono Y, Alton K, Rose SC, Hassanein TI, Mattrey RF. The ability of contrast-enhanced ultrasound (CEUS) to predict final outcome within 1 week after transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC). Hepatology 2004;40:706-7A.
- Flor N, Serantoni S, Barbosa Da Silva F, Impellizzeri E, Danaro M, Cornalba G, et al. Characterization of Small Indeterminate Focal Liver Lesions at Multiphasic MDCT in Patients With Known Primary Cancer: US and Contrast-Enhanced Ultrasound As Problem-Solving Approach? n.d.
- Lüttich A, Puig J, Martín J, Darnell A, Malet A, Veintemillas M. Can the Response to Radiofrequency Ablation of Hepatocarcinoma Be Adequately Measured by Means of Contrast-Enhanced US and Dynamic MRI? n.d.
- Solbiati L. D-55: Diagnostic Performance and Cost-Effectiveness of Contrast-Enhanced Ultrasound in the Characterization of Focal Liver Lesions 2006.
- Leoni S, Piscaglia F, Golfieri R, Camaggi V, Vidili G, Pini P, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol 2010;105:599-60.
- Dai Y, Chen MH, Fan ZH, Yan K, Yin SS, Zhang XP. Diagnosis of small hepatic nodules detected by surveillance ultrasound in patients with cirrhosis: comparison between contrast-enhanced ultrasound and contrast-enhanced helical computed tomography. Hepatol Res 2008;38:281-90.
- Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104.
- Giorgio A, De Stefano G, Coppola C, Ferraioli G, Esposito V, Di Sarno A, et al. Contrast-enhanced sonography in the characterization of small hepatocellular carcinomas in cirrhotic patients: comparison with contrast-enhanced ultrafast magnetic resonance imaging. Anticancer Res 2007;27:4263-9.
- Quaia E, Alaimo V, Baratella E, Medeot A, Midiri M, Cova MA. The added diagnostic value of 64-row multidetector CT combined with contrast-enhanced US in the evaluation of hepatocellular nodule vascularity: implications in the diagnosis of malignancy in patients with liver cirrhosis. Eur Radiol 2009;19:651-63.
- Sangiovanni A, Manini MA, Lavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59:638-44.
- Blondin D, Erhardt A, Crynen K, Sagir A, Scherer A, Kropil P, et al. Diagnosis of focal liver lesions in cirrhotic patients: comparison of contrast-enhanced ultrasound using sulphur hexafluoride (SF6) microbubbles and MRI using Gd-EOB-DTPA. Z Gastroenterol 2011;49:23-9.
- Mainenti PP, Mancini M, Mainolfi C, Camera L, Maurea S, Manchia A, et al. Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging 2010;35:511-21.
- Jonas E, Nilsson H, Larsson J, Freedman J. Detection of colorectal cancer liver metastases: an assessment of four imaging modalities. Paper presented at the 9th Congress of the European- African HPBA (E-AHPBA), Cape Town, South Africa, 12–16 April 2011. HPB 2011;13.
- Clevert DA, Jung EM, Stock KF, Weckbach S, Feuerbach S, Reiser M, et al. Evaluation of malignant liver tumors: biphasic MS-CT versus quantitative contrast harmonic imaging ultrasound. Z Gastroenterol 2009;47:1195-202.
- Catala V, Nicolau C, Vilana R, Pages M, Bianchi L, Sanchez M, et al. Characterization of focal liver lesions: comparative study of contrast-enhanced ultrasound versus spiral computed tomography. Eur Radiol 2007;17:1066-73.
- Gierbliński IW, Wocial T, Jarosz D. In which cases can contrast-enhanced ultrasound replace fine needle aspiration biopsy? Clinical feasibility study. Nowotwory 2008;58:238-45.
- Li R, Guo Y, Hua X, He Y, Ding J, Guo A, et al. Characterization of focal liver lesions: comparison of pulse-inversion harmonic contrast-enhanced sonography with contrast-enhanced CT. J Clin Ultrasound 2007;35:109-17.
- Seitz K, Strobel D, Bernatik T, Blank W, Friedrich-Rust M, Herbay AV, et al. Parts of this manuscript were presented at the Ultrasound Dreilaändertreffen. Davos; 2008.
- Seitz K, Bernatik T, Strobel D, Blank W, Friedrich-Rust M, Strunk H, et al. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM multicenter trial): CEUS vs. MRI – a prospective comparison in 269 patients. Ultraschall Med 2010;31:492-9.
- Feng XY, Sun XY, Tan BB, Qian GJ, Wu MC. Evaluation of enhanced ultrasonography for hepatic carcinoma with cryosurgery. J Interv Radiol 2007;16:246-8.
- Zhou P, Liu X-Y, Li R-Z, Nie W-P, Liu S. Comparison of treatment response in primary hepatocellular carcinomas with contrast-enhanced ultrasonography and contrast-enhanced helical CT. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2007;32:690-4.
- Chen MH, Wu W, Yang W, Dai Y, Gao W, Yin SS, et al. The use of contrast-enhanced ultrasonography in the selection of patients with hepatocellular carcinoma for radio frequency ablation therapy. J Ultrasound Med 2007;26:1055-63.
- Chen MH, Yang W, Yan K, Dai Y, Wu W, Fan ZH, et al. The role of contrast-enhanced ultrasound in planning treatment protocols for hepatocellular carcinoma before radiofrequency ablation. Clin Radiol 2007;62:752-60.
- Sangiovanni A, Manini MA, Romeo R, Iavarone M, Fraquelli M, Forzenigo LV, et al. Prospective validation of AASLD guidelines for the early diagnosis of hepatocellular carcinoma in cirrhotic patients. Hepatology 2007;46:241-2A.
- Cokkinos DD, Blomley MJ, Harvey CJ, Lim A, Cunningham C, Cosgrove DO. Can contrast-enhanced ultrasonography characterize focal liver lesions and differentiate between benign and malignant, thus providing a one-stop imaging service for patients?. J Ultrasound 2007;10:186-93.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83.
- Faccioli N, D’Onofrio M, Comai A, Cugini C. Contrast-enhanced ultrasonography in the characterization of benign focal liver lesions: activity-based cost analysis. Radiol Med 2007;112:810-20.
- Nomenclature SIRM-SNR delle prestazioni radiologiche. Tav III; 2006.
- Romanini L, Passamonti M, Aiani L, Cabassa P, Raieli G, Montermini I, et al. Economic assessment of contrast-enhanced ultrasonography for evaluation of focal liver lesions: a multicentre Italian experience. Eur Radiol 2007;17:F99-106.
- Sirli R, Sporea I, Martie A, Popescu A, Danila M. Contrast enhanced ultrasound in focal liver lesions: a cost efficiency study. Med Ultrason 2010;12:280-5.
- Zaim R, Taimr P, Redekop W, Uyl-de Groot C. Economic evaluation of contrast-enhanced ultrasound (CEUS) in the characterization of focal liver lesions (FLL) in the Netherlands. Rotterdam, Netherlands: Institute for Medical Technology Assessment, Department of Health Policy and Management, Erasmus University; Department of Gastroenterology and Hepatology, Erasmus MC University Hospital; 2011.
- Dutch Health Care Insurance Board . Farmacotherapeutisch Kompas n.d. www.fk.cvz.nl/ (accessed October 2012).
- Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess 2007;11.
- Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, et al. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess 2011;15.
- Bennett GL, Krinsky GA, Abitbol RJ, Kim SY, Theise ND, Teperman LW. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol 2002;179:75-80.
- Roberts SE, Goldacre MJ, Yeates D. Trends in mortality after hospital admission for liver cirrhosis in an English population from 1968 to 1999. Gut 2005;54:1615-21.
- Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology 1995;21:77-82.
- Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463-72.
- Mortality statistics: deaths registered in England and Wales (Series DR), 2010. Newport: ONS; 2011.
- Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002;97:2886-95.
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50.
- Taouli B, Goh JS, Lu Y, Qayyum A, Yeh BM, Merriman RB, et al. Growth rate of hepatocellular carcinoma: evaluation with serial computed tomography or magnetic resonance imaging. J Comput Assist Tomogr 2005;29:425-9.
- Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 2001;34:570-5.
- Liver transplant services: donor organ use – protocols and guidelines for adults undergoing cadaveric liver transplantation. Bristol: UK Transplant; 2005.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434-40.
- Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 2005;92:1862-8.
- NHS reference costs 2009–2010. London: Department of Health; 2011.
- Unit costs of health and social care. Canterbury: University of Kent; 2011.
- Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003;98:630-8.
- Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl 2002;8:263-70.
- Saunders TH, Mendes Ribeiro HK, Gleeson FV. New techniques for imaging colorectal cancer: the use of MRI, PET and radioimmunoscintigraphy for primary staging and follow-up. Br Med Bull 2002;64:81-99.
- Lejeune C, Bismuth MJ, Conroy T, Zanni C, Bey P, Bedenne L, et al. Use of a decision analysis model to assess the cost-effectiveness of 18F-FDG PET in the management of metachronous liver metastases of colorectal cancer. J Nucl Med 2005;46:2020-8.
- Assessment report. Canberra, Australia: MSAC; 2007.
- Colorectal cancer: facts and figures. Special edition. Atlanta, GA: American Cancer Society; 2005.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. New York, NY: Springer; 2009.
- UK interim life tables, 1980–82 to 2008–10. Newport: ONS; 2011.
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9.
- British national formulary. London: BMA and RPS; 2009.
- Costs book 2009. Edinburgh: NHS National Services Scotland; 2009.
- Cancer Research UK . Treating Bowel Cancer n.d. http://cancerhelp.cancerresearchuk.org/type/bowel-cancer/treatment/ (accessed 5 January 2012).
- Guest JF, Ruiz FJ, Greener MJ, Trotman IF. Palliative care treatment patterns and associated costs of healthcare resource use for specific advanced cancer patients in the UK. Eur J Cancer Care 2006;15:65-73.
- Ramsey SD, Andersen MR, Etzioni R, Moinpour C, Peacock S, Potosky A, et al. Quality of life in survivors of colorectal carcinoma. Cancer 2000;88:1294-303.
- Langenhoff BS, Krabbe PF, Peerenboom L, Wobbes T, Ruers TJ. Quality of life after surgical treatment of colorectal liver metastases. Br J Surg 2006;93:1007-14.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Bartolotta TV, Taibbi A, Midiri M, Matranga D, Solbiati L, Lagalla R. Indeterminate focal liver lesions incidentally discovered at gray-scale US: role of contrast-enhanced sonography. Invest Radiol 2011;46:106-15.
- Xie L, Guang Y, Ding H, Cai A, Huang Y. Diagnostic value of contrast-enhanced ultrasound, computed tomography and magnetic resonance imaging for focal liver lesions: a meta-analysis. Ultrasound Med Biol 2011;37:854-61.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93.
- Cantisani V, Ricci P, Erturk M, Pagliara E, Drudi F, Calliada F, et al. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med 2010;31:500-5.
- Zhou X, McClish D, Obuchowski N. Statistical methods in diagnostic medicine. New York, NY: Wiley Interscience; 2002.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41-4.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003;138:W1-12.
- International Working Party . Terminology of nodular hepatocellular lesions. Hepatology 1995;22:983-93.
Appendix 1 Literature search strategies
Appendix 2 Study-specific guide to completion of QUADAS-2
Study-specific guide to completion of QUADAS-2 (PDF download)
Appendix 3 Quality assessment: QUADAS-2 results
Appendix 4 Data extraction tables
Appendix 5 Table of excluded studies with rationale
Appendix 6 National Institute for Health and Care Excellence guidance relevant to the treatment of liver malignancies
National Institute for Health and Care Excellence guidance relevant to the treatment of liver malignancies (PDF download)
Appendix 7 PRISMA check list
Appendix 8 Protocol
Glossary
- Cholangiocarcinoma
- Cancer of the bile ducts, which drain bile from the liver into the small intestine.
- Cirrhosis
- A consequence of liver disease, most commonly alcoholism, hepatitis B and C, or fatty liver disease. It is characterised by replacement of liver tissue with fbrosis and scar tissue, leading to loss of liver function.
- Computed tomography
- A medical imaging technique using tomography created by computer processing to generate a three-dimensional internal image from a series of two-dimensional radiographic images.
- Contrast-enhanced ultrasound
- The application of a contrast agent to conventional ultrasonography. Ultrasound contrast agents rely on the different ways that sound waves are reflected from interfaces between substances, for example microbubbles and human tissue. The difference in echogenicity (ability to reflect ultrasound waves) between microbubbles and surrounding tissues is very high and intravenous contrast injection can be used to visualise blood perfusion and to distinguish between benign and malignant tissue.
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modeling
- A theoretical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False-negative
- Incorrect negative test result – number of diseased persons with a negative test result.
- False-positive
- Incorrect positive test result – number of non-diseased persons with a positive test result.
- Focal nodular hyperplasia
- A benign, usually asymptomatic tumour of the liver, which rarely grows or bleeds and has no malignant potential. It is often characterised by a central stellate scar.
- Haemangioma
- The most common benign tumour of the liver, usually of mesenchymal origin and comprising masses of atypical blood vessels.
- Hepatocellular carcinoma
- The most common type of liver cancer, usually secondary to scarring of the liver (cirrhosis) or hepatitide viral infection (hepatitis B or C).
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test whose performance is being evaluated.
- Magnetic resonance imaging
- A medical imaging technique that uses nuclear magnetic resonance to image the nuclei of atoms inside the body. It provides good contrast between the different tissues of the body and can be useful in distinguishing malignant from benign tumours.
- Markov model
- An analytical method particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- Metastasis
- The spread of a disease from one organ or part to another non-adjacent organ or part.
- Opportunity cost
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality of life
- An individual's emotional, social and physical well-being and his or her ability to perform the ordinary tasks of living.
- Quality-adjusted life-year
- A measure of health gain used in economic evaluations in which survival duration is weighted or adjusted by the patient's quality of life during the survival period.
- Radiofrequency ablation
- A medical procedure in which tumour tissue is ablated using the heat generated from the high-frequency alternating current.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity that result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test against which the index test is compared.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- Transarterial chemoembolisation
- A minimally invasive medical procedure to restrict blood flow to the tumour; frequently used to treat hepatocellular carcinoma.
- True-negative
- Correct negative test result – number of non-diseased persons with a negative test result.
- True-positive
- Correct positive test result – number of diseased persons with a positive test result.
List of abbreviations
- AASLD
- American Association for the Study of Liver Diseases
- AFP
- alpha-fetoprotein
- ALD
- alcoholic liver disease
- CCC
- cholangiocarcinoma
- CEA
- carcinoembryonic antigen
- CECT
- contrast-enhanced computed tomography
- CEMRI
- contrast-enhanced magnetic resonance imaging
- CEUS
- contrast-enhanced ultrasound
- CI
- confdence interval
- CRC
- colorectal carcinoma
- CRD
- Centre for Reviews and Dissemination
- CT
- computed tomography
- DARE
- Database of Abstracts of Reviews of Effects
- DTA
- diagnostic test accuracy
- EASL
- European Association for the Study of the Liver
- EFSUMB
- European Federation of Societies for Ultrasound in Medicine and Biology
- FDG-PET
- fuorodeoxyglucose positron emission tomography
- FLL
- focal liver lesion
- FNB
- fne-needle biopsy
- Gd-CEMRI
- gadolinium contrast-enhanced magnetic resonance imaging
- Gd-EOB-DTPA
- gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid
- HBV
- hepatitis B virus
- HCC
- hepatocellular carcinoma
- HCV
- hepatitis C virus
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- MDCT
- multidetector computed tomography
- MRI
- magnetic resonance imaging
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PEI
- percutaneous ethanol injection
- PenTAG
- Peninsula Technology Assessment Group
- PRESS EBC
- Peer Review of Electronic Search Strategies Evidence-Based Checklist
- QALY
- quality-adjusted life-year
- RFA
- radiofrequency ablation
- ROC
- receiver operating characteristic
- RON
- Romanian new leu
- SCI
- Science Citation Index
- SPIO-CEMRI
- superparamagnetic iron oxide contrast-enhanced magnetic resonance imaging
- SROC
- summary receiver operating characteristic
- TAE
- transarterial embolisation
- TACE
- transarterial chemoembolisation
- TNM
- tumour, lymph node, metastases
- US
- ultrasound
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.