Notes
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed commercial-in-confidence and academic-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of commercial-in-confidence and academic-in-confidence data removed and replaced by the statement ‘commercial-in-confidence and/or academic-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 08/236/01. The contractual start date was in April 2011. The draft report began editorial review in January 2012 and was accepted for publication in September 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rob Jones has received hospitality (value ≤£20) from Amgen Inc. on one occasion, not related to denosumab. He has received clinical trials support, speaker fees and consultancy honoraria from Novartis Pharmaceuticals UK (all unrelated to zoledronic acid). Clive Mulatero has acted in a consultant advisory role for AstraZeneca, Boehringer Ingelheim, GE Healthcare Systems and Roche, received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Pierre Fabre and Roche, and received research funding from Boehringer Ingelheim. The other authors have no competing interests
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Ford et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Brief statement describing health problem
Cancer is the leading cause of death in women and the second commonest cause of death in men; almost 30% of all deaths in England and Wales are caused by cancer. 1 Breast, prostate, lung and colorectal cancers are the commonest causes of cancer death in the UK. 2 In most cases, death is caused not by the primary tumour but by metastases or their complications. Almost any cancer can metastasise to bone, but cancers of the breast, prostate, lung, bladder, thyroid and kidney spread to bone most often. Cancer disrupts the architecture of bone, causing structural weakness. Subsequently, patients may suffer severe bone pain, pathological fractures or spinal cord compression (SCC), further reducing quality of life and adding to the burden of disease. Treatments that alleviate, prevent or delay these events offer the possibility of improving a patient's quality of life.
Overview of types of cancer commonly spreading to bone
Breast cancer
Bone metastases and their consequences depend on the type of primary tumour. Breast cancer is the commonest cancer in women. In the UK, approximately 124 women per 100,000 are diagnosed with breast cancer each year. 2 Approximately 0.5% of women have bone metastases at diagnosis, with 4.7% developing bone metastases in 5 years. 3 Bone metastases are associated with reduced median survival of approximately 24 months and 5-year survival of 20%. 4 However, survival is more heavily dependent on the presence of visceral organ metastases. Breast cancer commonly spreads to bone, liver, lung and brain. It has been estimated that breast cancer patients with metastatic disease only to bone survive 6 months longer than those with bone metastases and metastases outside a bone (1.6 years compared with 2.1 years). 5
Breast cancer most commonly originates from cells lining ducts or lobules (namely ductal carcinoma or lobular carcinoma). The natural history of the tumour is dependent on a range of different variables which, in turn, contribute to classification. Tumour–node–metastasis (TNM) is the most important prognostic classification and refers to the size of the tumour (T), spread to lymph nodes (N) and presence of metastases (M). Low-grade or precancerous cells are referred to as in situ carcinoma and do not cause metastases, unless the tumour progresses to an invasive carcinoma. Tumour aggressiveness can be predicted by the degree to which tumour cells are differentiated; poorly differentiated cells tend to be more aggressive, whereas well-differentiated cells are less so. Treatment and prognosis depend on receptors expressed by tumour cells. The three most important are oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2). Generally tumours that are receptor negative are less responsive to treatment and have a worse prognosis.
Prostate cancer
In men the most common cancer is prostate cancer. Approximately 98 men per 100,000 are diagnosed with prostate cancer in the UK each year. Almost 24 men per 100,000 each year die because of prostate cancer. 2 Prostate cancer often progresses to involve bone. At diagnosis 22% of patients have stage IV disease and a further 25% will develop clinically detectable metastases over the course of the disease. 6 One study found that 90% of patients with prostate cancer had some evidence of bone involvement at death. 7 Survival is reduced considerably in the presence of bone metastases, and 5-year survival drops from 56% in patients without bone metastases to 3% in patients with bone metastases. 8 However, this does not imply that bone metastases cause death per se, but rather, they occur in more aggressive cancers.
Prostate cancer originates in glandular cells and is therefore categorised as an adenocarcinoma. Similar to breast cancer, the TNM classification is the most important prognostic indicator. A worse prognosis is associated with the presence of disease in lymph nodes, or beyond. The grade of tumour cells is measured using the Gleason score. A high Gleason score suggests a poorly differentiated tumour and therefore poorer prognosis. Prostate-specific antigen (PSA) is a protein released by the prostate and can be a marker for cancer. However, there has been much debate around PSA testing. High levels of PSA can be found in patients without cancer and normal levels can be found in patients with cancer. 9 Prostate tumours are dependent on androgens to progress. Therefore, antiandrogen treatment can delay progression by either chemical or surgical castration. When tumours respond to castration therapy they are classified as castration-sensitive prostate cancer (CSPC), and when tumours no longer respond to castration treatment they are classified as castration-resistant prostate cancer (CRPC). Hormone-sensitive and hormone-refractory nomenclature has been used. However, some tumours remain dependent on androgens (and amenable to further androgen deprivation)10 to progress irrespective of castration therapy; here, the term castration resistant is more accurate.
Lung cancer
Lung cancer is the second commonest cancer, after breast (in women) and prostate (in men), and has an incidence of 48 per 100,000 per year. Lung cancer prognosis is very poor. More people die from lung cancer each year than from any other cancer (40 patients per 100,000). 2 One-year survival is 25% (in men) and 26% (in women). Five-year survival is only 7.8% (in men) and 8.7% (in women) and reflects cancers that are detected early, at a surgically resectable stage. 11 Spread of tumour to bone is common in lung cancer. Up to 36% of patients with lung cancer have evidence of bone metastases at death. 12 Other organs to which lung cancer often metastasises include the adrenal glands and the brain.
Classification of lung cancer is histological. Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) constitute more than 95% of all lung cancers. NSCLC includes squamous cell carcinoma, adenocarcinoma and large cell carcinoma. SCLC carries a worse prognosis and metastases are usually present at diagnosis. Both SCLC and NSCLC are staged using the TNM classification, or categorised as stage IA (better prognosis) to IV (worse prognosis).
Other solid tumours
Almost any cancer can metastasise to bone. At autopsy, 35–42% of thyroid, renal and bladder tumours have evidence of bone metastases. 13 Colorectal cancer mainly spreads to the liver, but in 6–10% of cases metastasises to bone. 14,15 Since colorectal cancer is the third commonest cancer, after breast (in women), prostate (in men) and lung, the actual number of patients with bone involvement is considerable. Each cancer has different subclassifications, each with its own pathophysiology, treatment and prognosis. For example, papillary thyroid cancer has a very good prognosis compared with anaplastic thyroid cancer. Bladder tumours may be superficial, requiring only local ablation therapy, or may be muscle invasive, requiring surgical resection or radical radiotherapy to the bladder. Therefore, the pathway to bone metastases in each cancer type varies according to primary site, cell type, classification and antineoplastic treatment.
Pathophysiology of bone metastasis
Bone provides an ideal environment for adhesive tumour cells, illustrated by the ‘seed and soil hypothesis’. 16 Blood flow through bone marrow provides ample opportunity for transportation of ‘seeds’ (tumour cells). A range of growth factors provides suitable ‘soil’. Once tumour cells have been established in bone marrow, the normal physiology of bone remodelling is disrupted.
Normal bone remodelling is dependent on the balance between osteoblasts and osteoclasts on the trabecular surfaces. Osteoblasts arise from mesenchymal stem cells and are responsible for bone formation. A cascade of bone proteins and growth factors drive and halt the bone formation process.
Osteoclasts resorb bone. They derive from the monocyte–macrophage lineage and rely on various cytokines and osteoblastic products to develop. One such cytokine is a tumour necrosis factor called receptor activator of nuclear factor κ-B ligand (RANKL). Through increased expression of RANKL, osteoclasts are induced and therefore bone resorption increases. Bone resorption results in calcium release. When combined with increased calcium reabsorption in the kidneys, this can lead to hypercalcaemia of malignancy (HCM).
Bone metastases result in an imbalance of osteoclast and osteoblast activity. If osteoclasts are primarily activated, bone resorption increases and metastases are more lytic in nature. Osteolytic lesions are thin lesions owing to the active resorption of bone and can be detected on plain radiograph. Appearance can be from a single well-defined lesion to multiple ill-defined lesions.
If osteoblasts are activated, bone formation increases and bone metastases are more sclerotic in nature. Sclerotic lesions are caused by increased bone formation so these lesions tend to be denser. The fact that these lesions are denser results not in normal/increased bone strength, but rather in weakness because of disruption of the bone matrix. Therefore, any imbalance of osteoblasts or osteoclasts causes disruption of the essential bone architecture and results in bone weakness.
Traditionally it was thought that bone metastases could be osteolytic, osteoblastic or mixed. Prostate cancer generally results in predominantly osteoblastic lesions and breast cancer predominantly osteolytic lesions. 17 However, current opinion is that a spectrum exists, with no metastasis being purely osteolytic or osteoblastic. 18
Clinical sequelae of bone metastases
The impact of bone metastases on patients is considerable. Bone metastases are associated with a worse prognosis, reduced quality of life and increased risk of complications. Quality of life is decreased by bone pain, reduced mobility and complications such as pathological fracture, SCC and HCM. Metastatic bone pain can be of a constant or intermittent nature, and it is not unusual for strong opioid analgesics to provide little relief. Alternatives to first-line analgesics include radiotherapy, bisphosphonates (BPs), corticosteroids or radionucleotides. Mobility may be reduced because of bone pain and other complications. Immobility places individuals at risk of other complications such as thromboembolism and lower respiratory tract infection, further increasing morbidity.
Complications are caused by weakness in the bone or disrupted calcium homoeostasis. Either osteoblastic or osteolytic lesions can cause pathological fractures, defined as pathological because minimal or no force is required. The commonest sites for fractures are the axial skeleton and long bones. Vertebral body collapse is common and can cause deformity of the spine. Saad and colleagues19 demonstrated that pathological fractures were correlated with reduced survival. Surgical fixation or radiotherapy can be used to prevent or treat pathological fractures.
The most serious complication of bone metastasis is SCC. Impingement of the spinal cord (i.e. SCC) is caused by either vertebral body collapse or direct tumour growth into the spinal canal. Even with emergency treatment, SCC can cause irreversible neurological damage, paraplegia and death. Neurological damage can range from mild sensory loss to complete paraplegia with loss of bowel and bladder function.
A further serious complication of bone metastases is hypercalcaemia (i.e. HCM). High circulating levels of calcium are caused by release of calcium from metastases and dysregulation in the kidney. HCM causes a typical pattern of unpleasant, non-specific symptoms. Untreated it can lead to coma, cardiac arrhythmias and death.
The term ‘skeletal-related event’ (SRE) is used to group the following complications together for research purposes: pathological fracture, SCC, and radiotherapy or surgery to bone. Some definitions include hypercalcaemia or change in antineoplastic therapies. The marketing authorisation for denosumab defines the term SRE as pathological fracture, SCC, and radiation to bone or surgery to bone. SREs should be considered as a spectrum of conditions, from unnoticed asymptomatic fractures to SCC resulting in paralysis.
Brown and colleagues,20 using randomised controlled trial (RCT) data, investigated baseline prognostic factors for patients experiencing a SRE. They found that significant factors included age, pain score, prior history of SRE, lesion type (osteolytic, osteoblastic or mixed) and elevated bone-specific alkaline phosphatase (BSAP) or lactate dehydrogenase (LDH). Bone pain at diagnosis has also been associated with increased SRE risk. 21 The incidence of SREs in patients with bone metastases without previous BP treatment was 3.5 events per year. 22 Sathiakumar and colleagues,23 using Medicar-linked data, found increased risk of death in patients with bone metastases from prostate cancer plus a SRE compared with patients with bone metastases plus no SRE. Yong and colleagues24 found a similar result in breast cancer. However, the majority of trials of bone-modifying agents aimed at delaying SREs in patients with bone metastases have not been shown to affect overall survival.
In addition, bone metastases have wider implications for patients. Aside from the symptoms and complications, the diagnosis of bone metastases substantially increases health-care contact. Patients may require a change in antineoplastic medications, careful titration of analgesics, radiotherapy, intravenous BPs, radiological imaging or frequent blood tests. More frequent health-care appointments can be especially difficult for patients who live in rural locations or do not have ready access to transport. Bone pain, decreased mobility and SREs undoubtedly have a further impact on patients and their families. Bone pain is characteristically severe and can be difficult to control. SREs can result in lengthy hospital stays and reduced mobility, especially in the case of communicated pathological fractures or SCC. The combination of increased contact with health care, reduced mobility and increased pain inevitably restricts daily activities and results in patients requiring a higher level of care. Increased care has a subsequent impact on carers and social services.
Measurement of disease
Investigations for bone metastases and skeletal-related events
Bone metastases and SREs can be measured in several different ways. 25 At the time of cancer diagnosis clinicians may screen for metastases. The decision to screen depends on stage of tumour and patients' symptoms. Skeletal scintigraphy (bone scan) uses injected radioactive material, which is then scanned with a gamma camera. Areas of increased bone metabolism are shown. This test shows the whole skeleton and is advantageous for a broad examination of the skeleton in asymptomatic patients. Plain radiographs (X-rays) are used for investigation of specific bones where metastases are suspected. Other investigations can then be used to investigate bone lesions, such as computerised tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) and single-photon emission CT (SPECT).
Bone markers, measured in blood or urine, have been used to monitor bone turnover in clinical trials. Patients with bone metastases and elevated bone markers are at increased risk of SREs. 26 It has been suggested that bone markers could be used to stratify risk of SRE in individuals with bone metastases, assisting in the choice of bone-modifying agents and monitoring treatment response. 27,28 There are several different bone markers, including BSAP, osteocalcin and N-terminal type 1 procollagen peptides (PINPs) markers for monitoring bone formation, and urinary or serum collagen type 1 cross-linked C telopeptide (CTX) and urinary collagen type 1 cross-linked N-telopeptide (NTX) for monitoring bone resorption. Denosumab trials have included measures of NTX and BSAP as secondary outcomes. 29–31 NTX increases in response to osteoclast-mediated bone resorption and can be measured in the blood or urine. During BP treatment, normalised levels of NTX appear to be associated with a reduced risk of SREs. 32,33 BSAP reflects osteoblastic activity by measuring bone formation. BP and denosumab treatment have been found to reduce BSAP. Conversely, persistent elevation of BSAP despite BP treatment is associated with increased SREs. 32 American Society of Clinical Oncology (ASCO) guidelines do not recommend the use of bone markers outside the trial setting. 34
In routine clinical practice acute uncomplicated pathological fractures are generally investigated by plain radiography. In the trial setting, regular skeletal surveys have been used to screen and diagnose pathological fractures. A skeletal survey is performed by taking plain radiographs of the skull, chest, spine, pelvis and long bones of the arms and legs. Therefore, both asymptomatic (lesions demonstrated radiologically but the patient does not complain of any symptoms) and symptomatic fractures will be observed. For pathological fractures of the spine, plain radiographs may not be sufficient. There may be uncertainty about the presence of a fracture and plain radiographs do not assess the integrity of the spinal canal. In this scenario, imaging with a MRI or CT scan may be necessary. In the case of suspected SCC, MRI is the investigation of choice.
Hypercalcaemia often presents with non-specific symptoms and is easily diagnosed on blood test. Signs and symptoms worsen as serum calcium increases. A serum calcium of more than 2.6 mmol/l is suggestive of hypercalcaemia.
Measuring skeletal-related events
There are several ways of recording SRE data in clinical trials:
-
time to first SRE
-
time to first and subsequent SREs (multiple event analysis)
-
SRE incidence
-
proportion of patients with at least one on-study SRE
-
skeletal morbidity rate (SMR) – number of events per year
-
skeletal morbidity period rate (SMPR) – the number of 12-week periods with new SREs divided by the total observational time.
It is important to note that SRE as a composite end point includes both complications of bone metastases (pathological fracture and SCC) and therapeutic or preventative measures (radiotherapy and surgery). Caution is needed because radiotherapy and surgery would be considered best supportive care (BSC). 35,36 Therefore, measures of radiotherapy and surgery contribute to both the treatment and the outcome measure.
Trinkaus and colleagues37 compared observational SRE frequency in ‘real life’ with SRE frequency in the intravenous BP trials. They found that the rate of SREs was higher in the trial setting than in ‘real life’. This may reflect the fact that bone scans are undertaken fequently in trials.
The various methods of assessing SRE data have evolved to overcome specific problems.
Some outcomes, such as proportion of patients with at least one on-study SRE or SMR, fail to consider time delays in SREs. For example, an individual who suffers SCC on day 1 of a trial is considered equivalent to an individual who suffers SCC after a year. To overcome this issue, time to first SRE can be measured. This outcome does not distinguish the number or timing of subsequent SREs. Consequently, the multiple-event analysis was developed. 38 The Andersen–Gill system is the commonest method used for multiple-event analysis. It includes a measure of both time and number of events. This method has been criticised because it fails to differentiate between individuals who have died and individuals who have left the trial for another reason. 39 Other methods have been described that also attempt to take mortality into account. 40,41
The choice of SRE measure depends on what is considered the most important outcome. To measure SRE prevention, the proportion of patients experiencing a SRE would be more suitable. To measure a reduction in rate, SMR/SMPR would be most appropriate. However, to measure delay, time to first or time to first and subsequent SRE would be more appropriate.
The situation is made more complex because more than one SRE may occur in relation to a single event and therefore the second SRE is dependent on the first. For example, an individual may suffer a pathological fracture, which is treated by radiotherapy or surgery (two SREs). In the pivotal denosumab and BP trials, a subsequent SRE is counted only after a 21-day period. This is not the case for SMR, which assumes independence for each event and can therefore lead to multiple counting of events. In an attempt to address this issue the SMPR outcome has been used.
The incidence of SREs is generally not considered appropriate because of underestimation of time variability within the data (similar criticism could be made of SMR). 42 A patient who suffers several SREs within the first 6 months is considered equivalent to a patient who suffers the same number of events over several years. The former patient is likely to have a reduced quality of life compared with the latter.
Trials have consistently used SRE as a composite outcome. This undoubtedly increases efficiency and power, but some caution is needed. However, the impact on health-care resources and a patient's quality of life is vastly different for SCC compared with an asymptomatic rib fracture. Nor does this SRE composite outcome directly measure factors that are important to patients such as mobility or pain (these are measured indirectly through need for radiotherapy or surgery). 43
Burden of bone metastases and skeletal-related events on health care and society
Undoubtedly, bone metastases and SREs require considerable health-care resources. In 2010, Pockett and colleagues44 reported the hospital burden associated with bone metastases and SREs from breast, prostate and lung cancer in Spain. They collected data on over 28,000 patients over 1 year. The incidence of hospital admission was greatly increased when a SRE occurred. Among patients with breast cancer, the hospital admission incidence rate was 95 per 1000 patients over 3 years for non-SRE-related metastatic bone disease and 211 per 1000 for SRE-related admissions. Among those with lung and prostate cancer, the incidence was 156 (lung) and 163 per 1000 patients (prostate) over 3 years for non-SRE-related metastatic bone disease and 260 and 150 for a SRE-related admission, respectively.
Current service provision
Current management of bone metastases and skeletal-related events
There are four National Institute for Health and Care Excellence (NICE) clinical guidelines (CGs) relevant to this appraisal:
-
Breast cancer – CG81. 45
-
Prostate cancer – CG58. 46
-
Metastatic SCC – CG75. 47
-
Lung cancer – CG121. 48
These guidelines recommend the use of BPs in:
-
all patients with advanced breast cancer and newly diagnosed bone metastases45
-
patients with ‘hormone-resistant’ prostate cancer and painful bone metastases when other treatments (including analgesics and palliative radiotherapy) have failed46
-
patients with breast cancer or multiple myeloma, plus vertebral involvement to reduce pain and prevent complications. 47
Bisphosphonates are not currently recommended to prevent skeletal complications in prostate cancer46 or tumours with vertebral involvement, excluding breast and multiple myeloma. 47 The lung cancer guideline48 states ‘methods of treating bone metastases include radiotherapy, BPs and nerve blocks’49 and ‘the effect of BPs . . . needs more research’. 50
ASCO has recently published guidelines concerning the use of bone-modifying agents in metastatic breast cancer. 34 Based on clinical efficacy, not cost-effectiveness, ASCO has recommended the use of zoledronic acid, disodium pamidronate or denosumab in patients with bone metastases from breast cancer.
The Scottish Intercollegiate Guidelines Network (SIGN) suggests that there is insufficient evidence to recommend BPs for first-line treatment of cancer-related pain, but it does recommend that BPs should be considered. 51 The SIGN breast cancer guideline52 recommends BPs in patients with metastatic breast cancer and symptomatic bone metastases.
An expert panel of European clinical oncologists has published recommendations. 53 Based on clinical effectiveness, but without economic evaluation, they recommended that all patients with bone metastases from lung cancer should be prescribed a BP.
Bisphosphonates
Bisphosphonates reduce bone resorption by inhibiting osteoclasts. 54 Clinical effectiveness starts after 6–12 months of treatment. 55 There are first-, second- and third-generation BPs. Early non-aminobisphosphonates include clodronate and etidronate. The addition of a nitrogen group to the BP structure was found to increase potency by inhibition of the 5-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway. These aminobisphosphonates include ibandronic acid, disodium pamidronate and zoledronic acid.
During the early studies of oral nitrogen-containing BPs, an association with oesophagitis was frequently reported. 56 Therefore, zoledronic acid and disodium pamidronate are available only as intravenous preparations. Ibandronic acid is available as an oral or intravenous preparation. Intravenous BPs are excreted rapidly from the kidneys and are typically associated with a higher incidence of hypocalcaemia and renal impairment than oral BPs. 57 Administration time varies from 15 minutes for zoledronic acid to 120 minutes for disodium pamidronate.
Oral BPs are absorbed by passive diffusion in the gastrointestinal tract. As a result, less than 6% of the active compound is absorbed, and this is further reduced with the presence of food. In addition, oral BPs increase the risk of oesophageal erosions, inflammation and neoplasm. 58 It is therefore recommended that patients remain upright for 30–60 minutes after ingestion. Consequently, oral BPs become burdensome for patients. 59 Location of treatment is important to patients. One study found that patients prefer administration at home, but this is not often possible with intravenous treatments. 60
Bisphosphonates are considered to be relatively safe drugs. Possible adverse reactions include renal failure, osteonecrosis of the jaw (ONJ), hypocalcaemia and acute-phase reaction. To avoid renal impairment, renal function is checked before administration, dose is adjusted if necessary and the intravenous infusion is given slowly. McDermott and colleagues61 assessed predictors of renal impairment in patients given zoledronic acid. The following predictive factors were found on multivariate analysis: age, myeloma or renal cell cancer, number of doses, concomitant non-steroidal anti-inflammatory drug therapy and current or prior treatment with cisplatin. ONJ has only recently been associated with BPs;62 ONJ leads to oral or periodontal lesions, which are usually associated with previous dental procedures. Hypocalcaemia can be rectified with oral calcium. Acute-phase reaction usually presents with transient pyrexia following first administration.
Four BPs are currently licensed in the UK for bone metastases:
-
Zoledronic acid (Zometa™, Novartis, Basel, Switzerland) is licensed for the reduction of bone damage in advanced malignancies involving bone. It is administered by intravenous infusion over at least 15 minutes at a dose of 4 mg every 3–4 weeks.
-
Disodium pamidronate (Aredia®, Novartis) is licensed for osteolytic lesions and bone pain in bone metastases associated with breast cancer or multiple myeloma. It is administered by slow intravenous infusion (over at least 2 hours) at a dose of 90 mg every 4 weeks.
-
Sodium clodronate (Bonefos™, Bayer Schering, Berlin, Germany; Clasteon™, Beacon, Tunbridge Wells, UK; Loron 520™, Roche, Basel, Switzerland) is licensed for osteolytic lesions, hypercalcaemia and bone pain associated with skeletal metastases in patients with breast cancer or multiple myeloma. It is administered by mouth at a dose of 1.6–3.2 g daily.
-
Ibandronic acid (Bondronate™, Roche) is licensed for the reduction of bone damage in bone metastases in breast cancer. It is administered either by mouth (50 mg daily) or by intravenous infusion (6 mg every 3–4 weeks).
Therefore, zoledronic acid is the only drug licensed for cancer involving bone, other than breast or multiple myeloma. Zoledronic acid has been the most studied BP and, according to expert opinion, is the most widely used BP. The patent for zoledronic acid is expected to expire in 2013. There are currently no firm criteria to advise when BPs should be stopped.
Best supportive care
Best supportive care varies between each primary cancer type.
In patients with breast cancer and bone metastases, BSC encompasses the use of BPs to prevent SRE and reduce pain. However, for the purpose of this report, the definition of BSC does not include BPs. Pain is also managed by the use of both simple and opioid analgesics, corticosteroids and non-steroidal anti-inflammatory agents. External beam radiotherapy is used to control pain at specific sites and, less commonly now, systemic radiopharmaceuticals may be used to alleviate widespread pain at multiple sites not controlled by other means. All patients with metastases in a long bone should be assessed for the risk of pathological fracture and referred to an orthopaedic surgeon for consideration of prophylactic fixation. Not all patients will require treatment with all modalities discussed above. The NICE guidelines currently recommend that all patients with bone metastases receive a BP, while ASCO guidelines recommend the use of a bone-modifying agent in patients with bone metastases and evidence of bone destruction. There is variation in the use of the other interventions mentioned, dependent on local practice and patient factors.
In patients with bone metastases, current BSC encompasses the use of systemic anticancer therapies including chemotherapy and further hormone therapies. Palliative external beam radiotherapy and systemic radionucleotides, such as strontium-89, are widely used and may be used on multiple occasions to treat metastatic bone pain. Despite these measures, pain may continue to be burdensome, and analgesics, often requiring specialist pain services, are frequently required. Attitudes to systemic anticancer therapies used in this context vary across the UK; in particular, there remains widespread controversy about the optimal timing of docetaxel-based chemotherapy, some clinicians opting to use it to prevent symptoms such as bone pain, whereas others save it until symptoms become burdensome. Two new drugs, cabazitaxel and abiraterone acetate, which are licensed for this indication, may change BSC patterns in this population, but neither drug has been the subject of published NICE review and access outside of clinical trials remains limited in the UK. The treatment of SRE is similar to that of other solid tumours (OSTs). Pathological fractures can be treated or prevented with surgery, radiotherapy or analgesics. Current practice is that BPs are not given to prevent complications of bone metastases, such as pathological fractures and SCC. However, BPs are used to treat pain when first-line analgesics have not alleviated pain.
In patients with lung cancer with bone metastases, BSC may include chemotherapy, palliative radiotherapy, antibiotics, steroids, surgery, analgesics and antiemetics. 63 Certain treatments are aimed at slowing disease progress (chemotherapy), while others are aimed at alleviating (analgesics and antiemetics) or preventing (surgery to prevent pathological fracture) symptoms. BSC may vary according to the location or primary tumour and presence of distal metastases. BPs are generally not used to prevent SREs. However, clinicians may consider BPs as a second-line analgesic option for painful bone metastases. BSC for pathological fracture and SCC in lung cancer is similar to that for OSTs.
Current treatments of skeletal-related events
Treatment of pathological fractures depends on the severity of injury, the bones involved and the degree of destruction. Management options include analgesics, immobilisation, surgical fixation, radiotherapy or a combination of the above. The impact of pathological fractures varies widely; some may be unnoticed and asymptomatic while more severe fractures may be associated with SCC and paraplegia.
Management of metastatic SCC has been described. 47 The guidelines highlight the need for early diagnosis and imaging with MRI. Acute treatment recommendations include good nursing care, corticosteroids and appropriate case selection for surgery or radiotherapy. Moreover, the guidelines make recommendations for long-term care, including management of pressure ulcers, bladder or bowel incontinence, postural hypotension and lung secretions, prevention of thromboprophylaxis and planning for rehabilitation or long-term care.
Hypercalcaemia of malignancy can present with various different signs and symptoms. If untreated, HCM can lead to confusion, drowsiness or coma. Rehydration and BP treatment are the cornerstone of management. Loop diuretics and steroids can also be used. Older agents such as plicamycin, calcitonin and gallium nitrate are not commonly administered.
Variation in service
There is variation among oncologists in the choice of BPs and more so in breast cancer, for which four BPs are licensed. With no clear guidelines about which BP to use, the decision is often made by the individual clinician. Based on expert opinion, zoledronic acid is the most widely used BP.
Bisphosphonates are used consistently in breast cancer; however, the use of BPs in other cancers varies. Among patients with metastatic tumours other than breast cancer, some clinicians use BPs routinely, wherease others reserve BPs only for uncontrolled pain and still others rarely use BPs. With the imminent patent expiry of zoledronic acid and the anticipated reduction in price, patterns of use may change significantly in the near future.
Fallowfield and colleagues64 conducted a UK survey to evaluate BP prescribing habits among oncologists. They found that 53% of oncologists gave intravenous and oral drugs, 40% gave only intravenous drugs and 7% gave only oral drugs. Zoledronic acid (56–85%) and disodium pamidronate (23–42%) were the commonest intravenous drugs, and ibandronic acid (66%) was the commonest oral BP used. Reasons reported for using oral preparations included ‘health authority/primary care trust only funds oral preparation’, ‘local guidelines dictate which patients receive oral/intravenous’ and ‘intravenous preparations are not listed on the local formulary’.
Variation in BSC exists between treatment centres. Local policy, available resources and clinician prescribing habits all affect the likelihood of patients being offered certain BPs, analgesics or antineoplastic medications.
Current service cost
Bisphosphonates are an adjuvant to BSC. British National Formulary (BNF) 62 gives a list price for zoledronic acid of £174.17, which can be administered as a 15-minute intravenous infusion. Disodium pamidronate is given a list cost of £165.00 in BNF62 and is administered as a slow intravenous injection over at least 2 hours every 4 weeks. Additional costs include staff time to administer BPs, monitoring costs, in particular monitoring of renal function, and capital costs.
The technology
Summary of intervention and important subgroups
Denosumab is a fully human monoclonal antibody. It has been designed to reduce osteoclast-mediated bone destruction through the inhibition of the RANKL. Its mechanism of action therefore varies from that of current BPs.
Tumour cells appear to increase the release of RANKL through activation of osteoblasts. RANKL, in turn, promotes osteoclast activity. Therefore, inhibition of RANKL reduces bone destruction. Denosumab is the first monoclonal antibody developed with this mode of activity.
Denosumab (Prolia®, Amgen, Thousand Oaks, CA, USA) is currently licensed for treatment of osteoporosis and bone loss caused by hormone ablation treatment in prostate cancer. Prolia is given in a dose of 60 mg every 6 months. Denosumab (Xgeva®, Amgen) for the prevention of SREs in bone metastases from solid tumours was granted marketing authorisation in July 2011. Multiple myeloma was not included within the marketing authorisation and therefore has been removed from the decision problem chapter of this report. Denosumab is administered as a 120 mg subcutaneous injection every 4 weeks. Xgeva is administered in a higher dose and more frequently than Prolia.
The Food and Drug Administration in the USA, on 18 November 2010, granted approval for a new indication for denosumab, to include the prevention of SREs in patients with bone metastases from solid tumours, to be marketed under a new proprietary name, Xgeva.
Current usage in the National Health Service
Denosumab has only recently been granted licensing authorisation in the UK. The assessment group (AG) is unaware of any current use in clinical practice.
Anticipated costs associated with intervention
Denosumab is admistered by 4-weekly subcutaneous injection in hospital while patients receive other therapy such as chemotherapy, at an outpatient appointment or potentially in primary care or through a dedicated health visitor domestic visit. The direct drug cost is £309.86 per dose. (Commercial-in-confidence information has been removed.)
Chapter 2 Definition of the decision problem
This section specifies the decision problem, outlines the key issues and provides an explanation of changes made between the scope and protocol or subsequent to the protocol.
Decision problem
The purpose of this report is to assess the clinical effectiveness and cost-effectiveness of denosumab within its licensed indication for the prevention of SREs in patients with bone metastases from solid tumours. Denosumab offers an alternative treatment to BPs, or an addition to BSC, for the prevention of SREs.
Scope: Denosumab
Protocol: Denosumab
The intervention is denosumab (Xgeva), administered every 4 weeks at a dose of 120 mg as a subcutaneous injection.
Scope Adults with bone metastases from solid tumours and adults with multiple myeloma
Protocol Adults with bone metastases from solid tumours and bone disease in multiple myeloma
The population assessed is adults with bone metastases from solid tumours. The scope requested that each tumour type be presented separately. Breast, prostate and NSCLC are the tumours that most commonly metastasise to bone. This grouping is reflected in the published literature. Therefore, the population is divided into those with breast cancer, prostate cancer, NSCLC and OSTs.
As far as the evidence allows, a subgroup based on prior history of SRE is considered.
Multiple myeloma is not included in the marketing authorisation for denosumab and has therefore been withdrawn from the decision problem.
Relevant comparators
Bisphosphonates such as sodium clodronate, disodium pamidronate, ibandronic acid and zoledronic acid
-
Best supportive care
-
Breast cancer – BPs
-
Prostate cancer, lung cancer and OSTs – BPs and BSC
Denosumab is compared with BPs and BSC.
The comparator of BSC is not mutually exclusive with denosumab or BP treatment. Both on-study and in ‘real life’ patients receive BSC, irrespective of denosumab or BP treatment. Therefore, a more accurate description of the comparators would be denosumab plus BSC compared with BPs plus BSC or BSC alone. However, for the purpose of this report the terms denosumab, BPs and BSC are used.
In breast cancer, denosumab is compared with BPs. Denosumab is compared with zoledronic acid, disodium pamidronate, ibandronic acid and sodium clodronate, depending on available literature.
In prostate cancer the NICE guideline46 recommends the use of BPs when conventional analgesics fail. Zoledronic acid is the only BP licensed and is the most commonly used. Therefore, denosumab is compared with BSC and zoledronic acid.
In NSCLC the NICE guideline48 states that ‘methods of treating bone metastases include radiotherapy, BPs and nerve blocks’. No clear guidance exists about when BPs should be administered. Zoledronic acid is the only BP licensed. Therefore, in NSCLC denosumab is compared with BSC and zoledronic acid.
In OSTs, excluding breast, prostate and NSCLC, no clear guidance exists about the circumstances under which BPs should be administered. Zoledronic acid is the only BP licensed. Therefore, denosumab is compared with BSC and zoledronic acid.
In patients with bone metastases from solid tumours who are eligible for a BP but are contraindicated (e.g. due to renal impairment), denosumab is compared with BSC.
The metastatic SCC NICE guideline47 recommends the use of BPs in (1) breast cancer to reduce pain and the risk of vertebral fracture/collapse and (2) prostate cancer to reduce pain if conventional analgesics fail to control pain. The guideline recommends that BPs are not used to treat pain, or with the intention of preventing metastatic SCC, in patients with vertebral involvement from solid tumour types other than breast and prostate cancer.
There is wide variation in the use of BPs for the management of patients with bone metastases in the UK. Patterns of use depend on local and national guidelines, and physician and patient preferences. Expert opinion is used to assess the use of unlicensed BPs in solid tumours other than breast cancer.
Outcomes
The outcome measures to be considered include:
-
Time to first SRE (pathological fracture, SCC, radiation or surgery to the bone)
-
Time to first and subsequent SRE
-
Incidence of SREs
-
SMR
-
Hypercalcaemia
-
Survival
-
Pain
-
Health-related quality of life (HRQoL)
-
Adverse effects of treatment
-
As per scope
The above outcomes are assessed according to available literature and suitability for network meta-analysis (NMA). In addition, the proportion of patients experiencing an on-study SRE is included. This outcome is synonymous with crude incidence of patients experiencing an on-study SRE.
Where the evidence allows, each type of SRE is presented separately. SRE is defined as pathological fracture, radiotherapy to bone, surgery to bone or SCC.
The use of SRE as a composite end point is discussed in Chapter 1 and Chapter 11. The term SRE is used in trials but not in clinical practice. The main criticism is that SRE encompasses a wide spectrum of possible health states, from asymptomatic fractures to SCC resulting in paraplegia, and does not directly measure pain or mobility. Including treatments (radiotherapy and surgery) in addition to complications (fracture and SCC) can make results difficult to interpret.
According to clinical advisors, the minimal clinically significant change in time to first SRE would be a 20% reduction in hazard ratio (HR) (R Jones). Mathias and colleagues65 correlated Brief Pain Inventory (BPI) scores and quality-of-life scores [European Quality of Life-5 dimensions (EQ-5D) and Function Assessment of Cancer Therapy (FACT)] using data from the trial by Stopeck and colleagues31 comparing denosumab and zoledronic acid in breast cancer with bone metastases. The authors concluded that a two-point change, or more, in BPI score should be considered as clinically meaningful.
Key issues
The place of denosumab within the treatment pathway is a crucial issue. The following possible places in the treatment pathway are considered:
-
Bone metastases from breast cancer.
-
An alternative to BPs as a first-line treatment in the prevention of SREs.
-
Second-line treatment for patients who have a SRE on a BP.
-
-
Bone metastases from prostate, NSCLC and OSTs, excluding breast cancer.
-
An alternative to BSC as a first-line treatment in the prevention of SREs.
-
As a first-line therapy for the secondary prevention of SREs in patients who have already suffered a SRE.
-
An alternative to BPs as a second-line therapy for prevention of SREs in patients for whom BSC has not proved adequate.
-
-
Bone metastases from breast cancer, prostate cancer, NSCLC and OSTs.
-
As a second-line treatment in patients unable to tolerate intravenous BPs, or for whom they are contraindicated.
-
The three main challenges with this appraisal are (1) a population that includes all solid tumours, (2) widespread variation in the use of comparators and (3) limited evidence suitable for inclusion in a NMA.
Three Phase III clinical trials have evaluated denosumab compared with zoledronic acid in breast cancer,31 prostate cancer,29 and OSTs (excluding breast and prostate) and multiple myeloma. 30 Breast, prostate and lung cancer are the tumours that most commonly metastasise to bone, although almost any tumour has the potential to do so. Treatment effect could be influenced if tumour types are combined or considered separately. In this appraisal, breast cancer, prostate cancer and NSCLC are considered separately; all OSTs are combined. Furthermore, at diagnosis of bone metastases patients may have been exposed to a variety of therapies. These include chemotherapy, hormonal therapy, radiotherapy or surgery. Therefore, the evidence of a treatment, which is given in addition to these therapies, and in a variety of tumour types, requires careful interpretation.
Comparators include BPs and BSC. There has been no NICE technology appraisal for the use of BPs in bone metastases. Four NICE guidelines give recommendations on the use of BPs in advanced breast cancer,45 prostate cancer,46 lung cancer48 and metastatic SCC. 47 Variation in practice exists in the use of BPs between tumour types and the choice of BP. Although zoledronic acid is the only licensed BP for solid tumours other than breast cancer, other BPs may be used off licence. Not only does BP use vary, but also BSC varies between geographical region and tumour type. Therefore, BSC is defined by clinical experts. There is no direct evidence comparing denosumab with current BSC. Placebo or no active treatment is used as a proxy for BSC. To compare denosumab with BSC several network meta-analyses are required. Only data that are sufficiently homogeneous, in terms of population, intervention, comparators, outcomes assessed, SRE definition and timeframe, can be included.
Other treatment-effect and cost-effect modifiers include:
-
symptomatic versus asymptomatic fractures (pivotal denosumab studies report combined symptomatic and asymptomatic fractures; the inclusion of asymptomatic fractures may overestimate treatment effects)
-
overall survival (tumours with extended survival may benefit more from denosumab)
-
place of administration of denosumab (community versus hospital).
Overall aims and objectives of assessment
To appraise the clinical effectiveness and cost-effectiveness of denosumab within its licensed indication for the treatment of bone metastases from solid tumours and multiple myeloma
ProtocolTo appraise the clinical effectiveness and cost-effectiveness of denosumab within its licensed indication for the treatment of bone metastases from solid tumours and bone disease in multiple myeloma
The purpose of this review is to appraise the clinical effectiveness and cost-effectiveness of denosumab, within its licensed indication, for the treatment of bone metastases from solid tumours. Multiple myeloma is not included in the marketing authorisation for denosumab and has therefore been withdrawn from the decision problem. As stated above, results are presented separately based on the type of primary cancer: (1) breast cancer, (2) prostate cancer, (3) NSCLC and (4) OSTs excluding breast, prostate or NSCLC. Where evidence allows, data for each type of SRE (pathological fracture, requirement for radiation therapy to bone, surgery to bone, or SCC) are presented separately. In addition, where evidence allows, data on patients with a history of SREs are presented separately.
The following aspects are not included in the aim of this report:
-
denosumab for the prevention of bone metastases
-
the clinical effectiveness and cost-effectiveness of BPs relative to BSC.
Chapter 3 Methods for reviewing effectiveness
Identification of studies
Studies were identified by searching electronic databases and relevant websites, contact with clinical experts and the scrutiny of bibliographies of retrieved papers.
The databases searched were MEDLINE (1948 to April 2011), EMBASE (1980 to March 2011), The Cochrane Library (all sections; Issue 1, 2011) and Web of Science with Conference Proceedings (1970 to May 2011). Auto-alerts were set-up in MEDLINE and EMBASE to identify any studies indexed after the above searches were done. Other sources, including the 2010 and 2011 meeting abstracts of ASCO and the American Urological Association, and the San Antonio Breast Cancer symposium were also searched. Searches were limited to English-language studies only.
Full details of all searches are shown, see Appendix 1.
Inclusion and exclusion criteria
Types of studies
The following studies were considered for inclusion:
-
Systematic reviews and RCTs.
There was no restriction on the number of patients in trials, because those with inadequate numbers, and hence power, would have been useful when combined in a meta-analysis.
If there were any high-quality existing systematic reviews that met the inclusion criteria, we would have considered updating them; however, no relevant systematic reviews were identified.
-
Observational studies were used, in addition to RCTs, for data on quality of life and safety.
Only studies published in full and published abstracts that reported additional outcomes or analyses from studies already published in full were included.
Meeting abstracts were tabulated for use in the discussion to indicate ongoing research (for recent abstracts), or possible sources of publication bias (for older abstracts not subsequently published in full).
Types of participants
The population considered were adults with confirmed carcinoma of the following:
-
breast
-
prostate
-
NSCLC or
-
OSTs
plus, evidence of at least one bone metastasis.
We considered separately patient groups based on location or type of primary cancer, where data permitted.
Types of interventions
Denosumab (trade name Xgeva), manufactured by Amgen, was given as a subcutaneous injection at dose of 120 mg every 4 weeks. The approved indication for denosumab is the prevention of SREs (pathological fracture, radiation to bone, SCC or surgery to bone) in adults with bone metastases resulting from solid tumours.
We excluded studies (such as pharmacokinetic or drug tolerability studies) in which patients were given only a single dose of a drug and where studies compared different routes of administration of the same BP. In studies that have arms with more than one dose of a licensed comparator drug, only arms of studies that used the UK-licensed doses of the drug were included.
Types of comparators
The relevant comparators are (1) BPs and (2) BSC.
Bisphosphonates
Bisphosphonates considered as a comparator included:
-
sodium clodronate
-
disodium pamidronate
-
ibandronic acid
-
zoledronic acid.
Etidronate was initially considered as an unlicensed (for this purpose) comparator, because of its much lower cost. However, clinical advice suggests that it should be used infrequently because it may cause gastrointestinal toxicity.
Currently, zoledronic acid has UK marketing authorisation for the reduction of bone damage in all advanced malignancies involving bone. Disodium pamidronate and sodium clodronate are licensed for breast cancer and multiple myeloma, and ibandronic acid is licensed only for breast cancer. However, we also considered inclusion of trials of these BPs when used outside their licensed indications.
Clinical experts and NICE guidelines were consulted to determine the place of BPs in the care pathway. For patient groups in which BPs are considered the current standard of care, denosumab was compared with BPs only.
A BP class effect was not assumed. As data allowed, all BPs would be included within a NMA.
Best supportive care (excluding bisphosphonates)
Best supportive care was considered a comparator where BPs were not recommended. This varied depending on the type of cancer. The relevant NICE CGs are CG81 for advanced breast cancer,45 CG58 for prostate cancer,46 CG121 for lung cancer48 and CG75 for metastatic SCC. 47 All of these guidelines recommend radiotherapy and analgesics within BSC. Other recommended supportive care for bone metastasis includes surgical fixation in breast cancer and multiple myeloma, strontium-89 in prostate cancer and nerve blocks in lung cancer.
Breast cancer
NICE CG81 on breast cancer recommends offering BPs to patients with newly diagnosed bone metastases to prevent SREs and to reduce pain. 45 Therefore, BSC was not used as a comparator in patients with advanced breast cancer and bone metastases. The planned NMA is shown in Figure 1.
FIGURE 1.
Network meta-analysis for those with bone metastases from breast cancer.
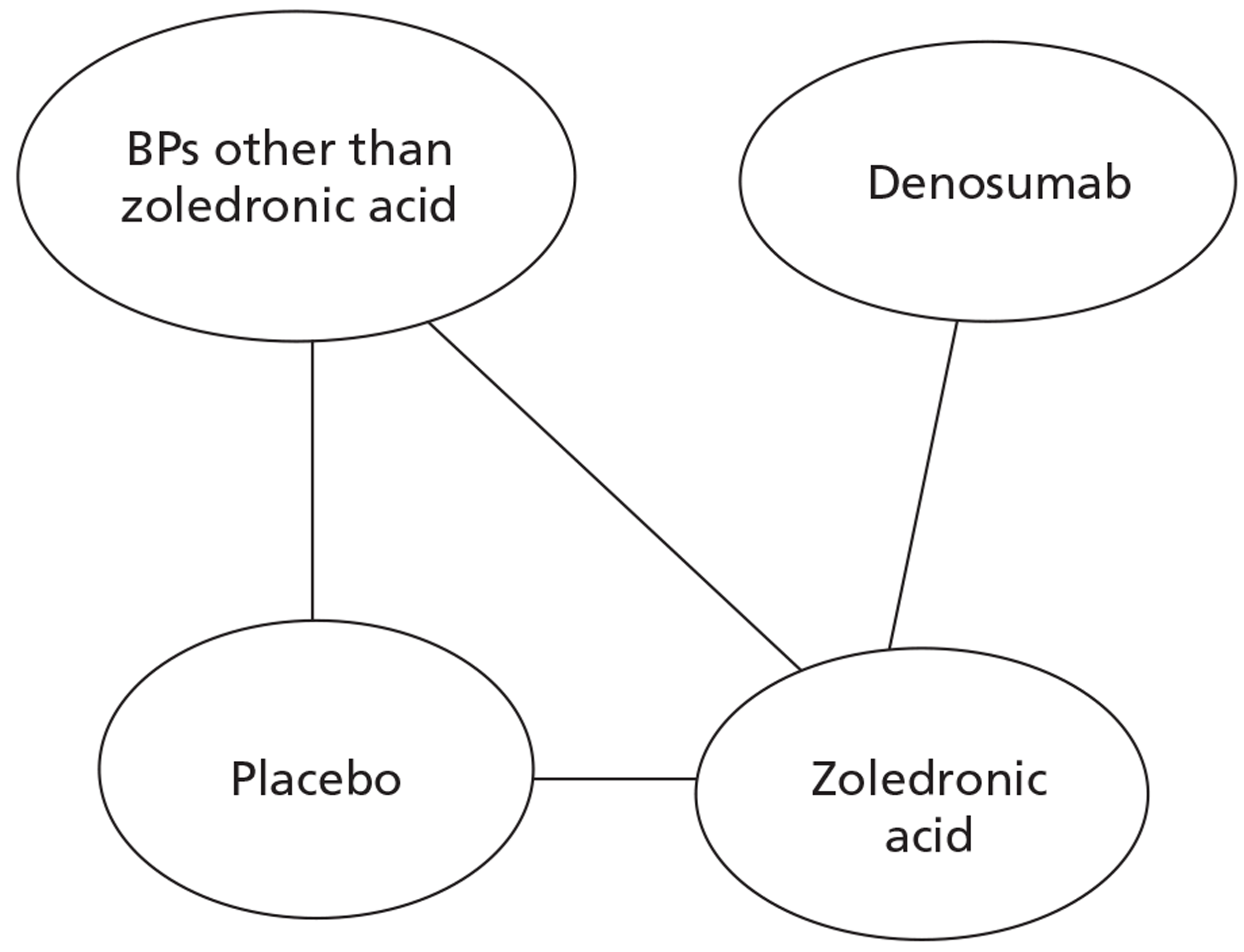
Prostate cancer
The NICE guidance, CG58, on prostate cancer recommends that ‘the use of BPs to prevent or reduce the complications of bone metastases in men with hormone-refractory prostate cancer is not recommended. Bisphosphonates for pain relief may be considered for men with hormone-refractory prostate cancer when other treatments (including analgesics and palliative radiotherapy) have failed’. 46 Therefore, in prostate cancer denosumab is compared with both BPs and BSC.
Lung cancer
No guideline recommendation for the use of BPs exists for bone metastases from lung cancer. NICE CG121 suggested that there was insufficient evidence to recommend BPs as a first-line treatment in bone metastases from lung cancer. 66 However, the standard treatments such as analgesics, or single-fraction radiotherapy, are recommended for the relief of symptoms from bone metastasis.
As the NICE guidelines for prostate and lung cancer recommend BSC before giving a BP, for these patient groups we plan to include BSC as a comparator, where data exist. The planned NMA for prostate cancer, lung cancer and OSTs is shown in Figure 2.
FIGURE 2.
Network meta-analysis for those with bone metastases from prostate cancer, lung cancer or OST.
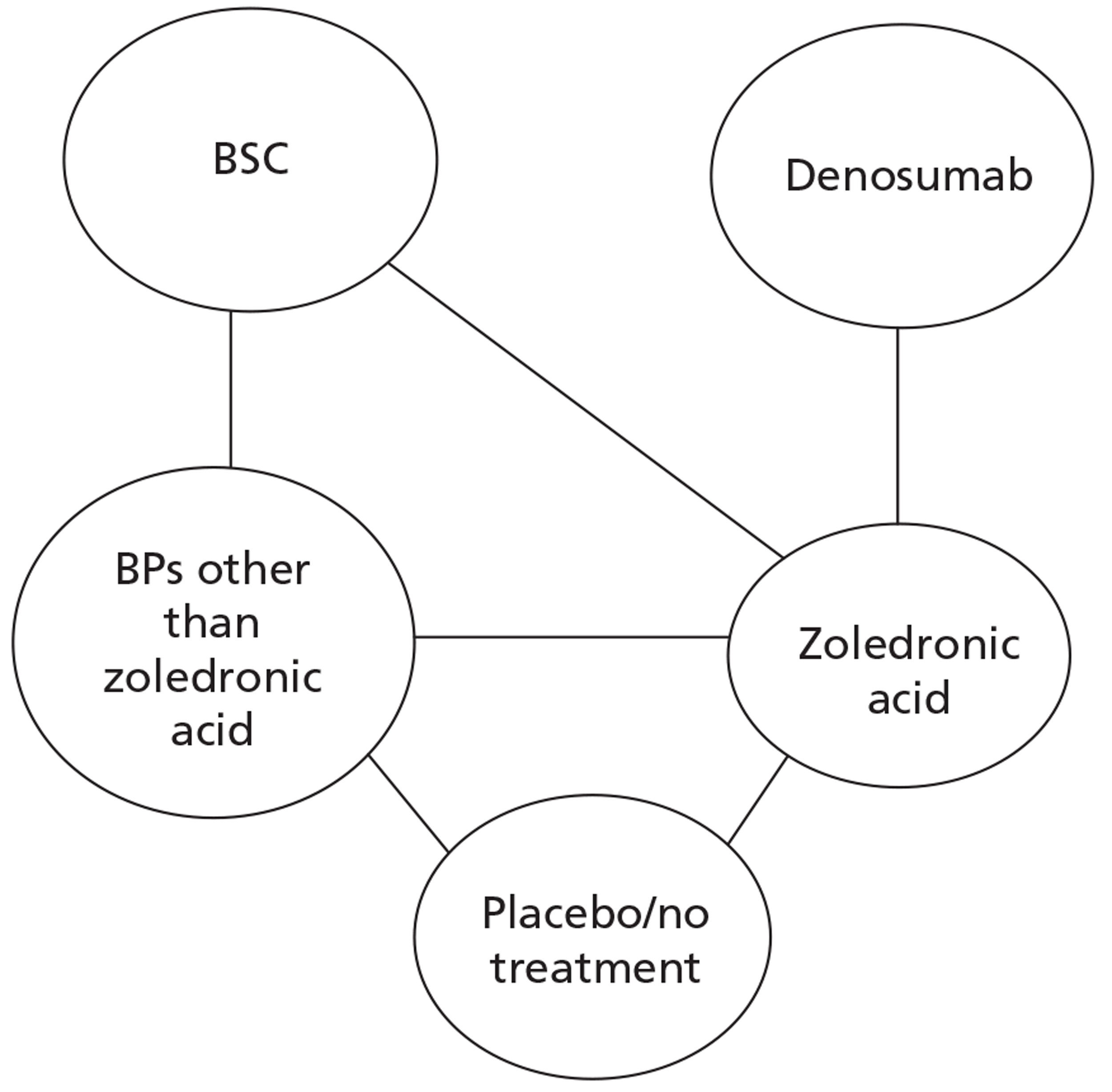
Other solid tumours
In the protocol we stated that if we obtained enough data on OSTs for which no relevant NICE guidelines existed, we would seek expert opinion as to the place of BPs in the clinical pathway.
Expert opinion suggested that BPs, mainly zoledronic acid, were used in OSTs. Therefore, the network diagram will be as in Figure 2 and denosumab is compared with both BPs and BSC.
Types of outcomes
These included:
-
time to first on-study SREs (SRE defined as pathological fracture, requirement for radiation therapy to bone, surgery to bone, or SCC)
-
time to first and subsequent on-study SRE
-
SMR
-
incidence of SREs
-
prevention of hypercalcaemia
-
overall survival rate
-
pain
-
HRQoL
-
adverse events related to treatment (including hypocalcaemia, ONJ, renal toxicity, acute-phase reactions).
Data extraction strategy
Selection of studies
Study selection was made independently by two reviewers (PR, JF) by screening titles, abstracts and full-text papers. Discrepancies were resolved by discussion. There was no requirement for a third reviewer.
Data extraction and management
Data were extracted from the included studies by one reviewer, using a standardised data extraction form (see Appendix 2), and checked by a second. Discrepancies were resolved by discussion. There was no need for a third reviewer. Any study data received from the manufacturer's submission (MS) that met the inclusion criteria were extracted and quality was assessed in accordance with the procedures outlined in the protocol for the assessment.
Critical appraisal strategy
The quality of the individual studies was assessed by one reviewer, and independently checked for agreement by a second reviewer.
The quality of the RCTs was assessed using the Cochrane risk-of-bias tool67 (see Appendix 3), which includes the following components:
-
adequate sequence generation
-
allocation concealment
-
blinding
-
incomplete outcome data addressed
-
free of selective reporting.
Any sponsorship or conflict of interests mentioned was recorded.
Methods of data synthesis
Initially we looked for head-to-head trials of denosumab versus BPs or BSC. Our initial scoping searches indicated that at present there were only three published Phase III trials of denosumab that included our relevant population. All three use zoledronic acid as a comparator. The three patient groups included in the three trials are (1) patients with advanced breast cancer, (2) patients with CRPC and (3) patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Therefore, to be able to compare denosumab with BPs other than zoledronic acid, or with BSC, the search was widened to allow for NMA. This included head-to-head BP trials, placebo-controlled BP trials or BSC-controlled trials.
Assessment of heterogeneity
Trials meeting the inclusion criteria were assessed for heterogeneity. The studies were examined for similarity with respect to population, intervention, comparators, outcomes, SRE definition and time frame. If trials were sufficiently homogeneous, a NMA of denosumab versus BP and BSC was carried out to pool direct and indirect evidence from randomised trials in a single analysis.
Patient groups were analysed separately based on location or type of primary cancer. When sufficient data were available, subgroup analyses were performed to examine the effect of treatment depending on the type of SRE, history of SREs, prior use of BP, prior type of BSC, different adjuvant therapies, different routes of administration of the BPs, and the location of the metastases.
An indirect comparison/NMA was performed as shown in Figure 1 and Figure 2.
Statistical technique of network meta-analysis
The NMAs were carried out using methods for mixed treatment comparisons described by Lu and Ades. 68 The Bayesian software package WinBUGS (MRC Biostatistics Unit, Cambridge, UK), which employs Markov chain Monte Carlo methods, was used for the analyses.
Network meta-analyses were conducted for all the cancer types included in this appraisal. Outcomes analysed were time to first SRE (HRs), time to first and subsequent SRE (rate ratios from Anderson–Gill38 multiple event analyses reported in primary studies), SMR ratios (for breast and prostate cancer only) and the proportion of patients with at least one on-study SRE. The proportions of patients with a SRE were also analysed by SRE type for breast and prostate cancer and by SRE history (SRE naive/experienced) for breast cancer.
Fixed effects models were used for time to first SRE, adopting an approach recommended by the NICE Decision Support Unit69 for modelling trial-based summary measures, which can be applied to modelling HRs on the log hazard scale. The trial-level data included in the models comprised log HRs and their standard error. Where HRs were not reported or derivable in the primary study, Kaplan–Meier estimates and numbers at risk (if available) were used, applying the methods of Tierney and colleagues70 to estimate the HR. Pairwise HRs were estimated from the median of the posterior distribution with credible intervals taken from the 2.5% and 97.5% percentiles. Two chains were used in the Markov chain Monte Carlo analyses, each with 10,000 simulations following a burn-in of 10,000. The same approach was taken for modelling rate ratios in the analysis of time to first and subsequent SREs.
For SMR and proportions of patients with a SRE, random effects models were adopted using arm-based data. The data included in the SMR models were mean SMR and standard deviation (SD) along with the number of patients. Where SDs were not reported, values were imputed by taking the mean of reported SDs from other studies but for the same treatment. The robustness of the imputation was tested by comparing results with those obtained by treating missing data as an uncertain parameter. For the proportions with a SRE, the numbers of patients and the numbers with a SRE were used. Posterior distributions for relative treatment effects were estimated from the absolute risks of outcome from the relevant individual treatments. Median estimates and credible intervals were taken from 10,000 Markov chain Monte Carlo simulations after a burn-in of 10,000.
To estimate the absolute risk of outcome in the analyses of arm-based data, it was necessary to include an estimate of the baseline risk of the control treatment in the models. Zoledronic acid was treated as the reference treatment in each analysis as it is the treatment common to the largest number of trials and is present in multiple included studies for each NMA. Single-arm meta-analyses of zoledronic acid were conducted to estimate baseline risk, from studies included in the NMA that had zoledronic acid as one of its comparators. The data in the time-to-event analyses, however, were trial-based and baseline risk could not be estimated, and so the absolute effect of the reference treatment was set to zero in these models.
The quality of the models was examined by inspecting convergence using Gelman–Rubin–Brooks plots, assessing autocorrelation between iterations of the Markov chain and checking whether or not the Monte Carlo error was less than 5% of the posterior SD.
Methods for estimating quality of life
Quality-of-life data for patients who had experienced bone metastases and SREs were obtained from the studies identified from the clinical effectiveness searches, the MS, and the denosumab clinical study reports (CSRs). A further systematic review of the effects on quality of life of SREs arising from metastatic bone disease and from myeloma bone disease was undertaken (see Chapter 9, Systematic reviews of cost-effectiveness studies and quality-of-life studies).
Chapter 4 Results: breast cancer
The clinical effectiveness chapters (see Chapter 4 on breast cancer; Chapter 5 on prostate cancer; Chapter 6 on NSCLC; Chapter 7 on OST excluding NSCLC; and Chapter 8 on OST including NSCLC) follow the same structure. Information is provided on the quantity of research available, followed by the results and then a summary of the chapter. For the outcomes of time to first on-study SRE, risk of first and subsequent on-study SRE, SMR and incidence of SREs, information is also reported, where available, for SRE by type, and history of SRE. Towards the end of each chapter there is a separate section reporting the results of the NMA. Chapter 6 (on NSCLC) and Chapter 7 (on OST excluding NSCLC) are subgroups of one trial. Therefore, Chapter 8 (on OST including NSCLC) has been included to present the outcomes for which the trial was powered and outcomes which are not presented within the aforementioned subgroups.
Quantity of research available: overall review of clinical effectiveness
As a single search strategy was designed to identify all potentially relevant studies for the clinical effectiveness review, information on the overall numbers of studies is given in the first three sections, as well as information specifically relating to breast cancer. The remaining sections focus on breast cancer.
Number and type of studies included
Overall
A flow diagram outlining the screening process for the overall review of clinical effectiveness is shown in Figure 3.
FIGURE 3.
Flow diagram of the searches and screening process.
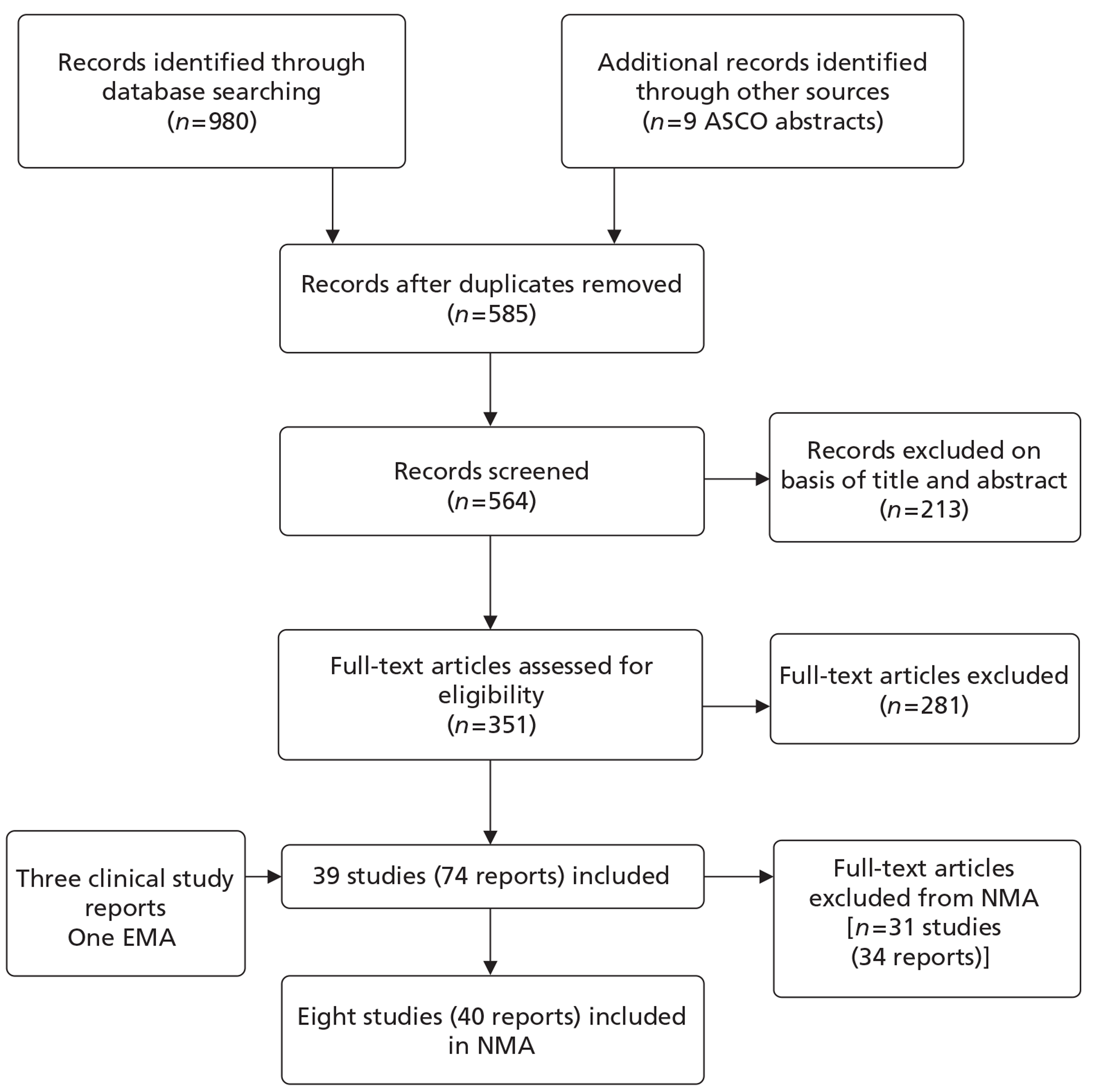
The searches identified 989 records, of which 585 were unique studies (after removing duplicates). Following screening of titles and abstracts, the full text of 352 articles was obtained for further assessment. With the addition of four reports received from the manufacturer, this resulted in 39 studies (74 reports) meeting the inclusion criteria for the review of clinical effectiveness (see Appendix 4). However, of these 39 studies, 31 were not able to contribute data to the AG's NMA and none reported denosumab, and therefore these studies were not reported further in the results chapters. The reasons why they were not able to contribute data to the NMA included:
-
studies did not report uniform definition of SREs
-
studies did not report standardised SRE rates
-
studies did not report outcomes separately for different cancer types
-
studies included patient groups where some patients were not diagnosed with bone metastases.
Of these 31 studies, 6 reported on bone metastases from breast cancer,71–76 13 reported on bone metastases from prostate cancer77–89 and 12 reported on bone metastases from OSTs. 90–101
Of the remaining eight studies that did contribute data to the network meta-analyses, four reported breast cancer31,102–104 [18 reports22,31,102–116; and Amgen Ltd. Multiple Technology Appraisal: Denosumab for the treatment of bone metastases from solid tumours (unpublished report). London: National Institute for Health and Care Evidence; 2011], two reported prostate cancer29,117 (15 reports19,29,117–129) and two reported OSTs, excluding breast and prostate cancer30,130 (seven reports30,130–135). Therefore, across the review of clinical effectiveness, eight studies (40 reports) contributed data to the NMAs.
All of the included studies were RCTs. No systematic reviews were identified that exactly met our inclusion criteria. The ASCO clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer was the most relevant systematic review identified. This review included denosumab, disodium pamidronate and zoledronic acid but did not include ibandronate or clodronate (because they are not licensed for this indication in the USA), which, therefore, were not considered further. 34
A search of safety-related articles identified 28 additional studies. 61,62,136–161
Breast cancer
The primary comparator for denosumab was considered to be BPs (zoledronic acid, disodium pamidronate, ibandronic acid or sodium clodronate) as recommended in NICE guideline CG81 for all patients with advanced breast cancer and newly diagnosed bone metastases. 45
One RCT (10 reports,31,105,106,110–114,116 including CSR 20050136) was identified comparing denosumab with zoledronic acid, with the primary published report considered to be that by Stopeck and colleagues. 31 An additional three studies contributed data to the NMA. One study, by Kohno and colleagues,102 compared zoledronic acid with placebo. One study (four reports22,103,107,115) compared disodium pamidronate with placebo, with the primary published report considered to be that by Lipton and colleagues. 103 One study (three reports104,108,109) compared zoledronic acid with disodium pamidronate, with the primary published report considered to be the 2003 paper by Rosen and colleagues. 104
Number and type of studies excluded
A list of the 281 potentially relevant studies identified by the search strategy for which full-text papers were obtained but which subsequently failed to meet the inclusion criteria is given in Appendix 5. These studies were excluded because they failed to meet one or more of the inclusion criteria in terms of the type of study, participants, intervention or outcomes reported. Three trials of denosumab, one in patients with breast cancer,162 one in patients with prostate cancer163 and one in patients with OSTs,164 were excluded because they used mixtures of BPs as a comparator and did not report the outcomes separately for each type of BP. Table 1 shows the numbers of studies excluded along with the reasons for their exclusion.
| Reasons for exclusion | Number of studies |
|---|---|
| Not a RCT | 93 |
| Reviews | 69 |
| Other study design | 24 |
| Comparing doses of radiotherapy | 23 |
| Not a relevant patient group | 26 |
| Dose-ranging study | 21 |
| Not a required dose used | 17 |
| No relevant outcomes | 30 |
| Economic study | 10 |
| Adjuvant use of drug | 20 |
| No relevant comparators | 7 |
| No relevant interventions | 18 |
| Multiple myeloma patient group | 14 |
| Treatment of hypercalcaemia | 2 |
| Total | 281 |
Characteristics of the included studies
Overall
All 31 studies that were excluded from the NMA included comparisons of BPs with placebo or another BP, and some compared BSC with placebo or another BSC. Table 2 provides a summary of the interventions and comparators included in the trials and a list of studies included or excluded from the NMA. Studies meeting the inclusion criteria but not contributing data to the NMA were not reported on in the chapters on clinical effectiveness because none provided direct evidence on denosumab compared with BPs, placebo or BSC. However, the results from these studies have been presented in appendices; see Appendix 6 for the characteristics of the participants and description of the interventions/comparators with the reasons for exclusions from the NMA and Appendix 7 for the results of these studies. Appendix 8 shows the characteristics of the included studies.
| Comparison | No. of studies | Primary tumour | Intervention | Comparator | Study ID |
|---|---|---|---|---|---|
| Included in NMA (n = 8) | |||||
| Denosumab vs zoledronic acid | 3 | Breast | Denosumab (s.c.) | Zoledronic acid (i.v.) | Stopeck 201031 |
| Prostate | Denosumab (s.c.) | Zoledronic acid (i.v.) | Fizazi 201129 | ||
| NSCLC (subgroup) | Denosumab (s.c.) | Zoledronic acid (i.v.) | Henry 201130 | ||
| OST | Denosumab (s.c.) | Zoledronic acid (i.v.) | Henry 201130 | ||
| BPs vs placebo/another BP | 5 | Breast | Zoledronic acid (i.v.) | Placebo | Kohno 2005102 |
| Breast | Zoledronic acid (i.v.) | Disodium pamidronate (i.v.) | Rosen 2003a104 | ||
| Breast | Disodium pamidronate (i.v.) | Placebo | Lipton 2000103 | ||
| Prostate | Zoledronic acid (i.v.) | Placebo | Saad 2002117 | ||
| NSCLC (subgroup) | Zoledronic acid (i.v.) | Placebo | Rosen 2003b130 | ||
| OST | Zoledronic acid (i.v.) | Placebo | Rosen 2003b130 | ||
| Excluded from NMA (n = 31) | |||||
| BP vs placebo/another BP | 27 | Breast | Ibandronate (oral) | Placebo | Body 200472 |
| bandronate (i.v.) | Placebo | Body 200371 | |||
| bandronate (i.v.) | Placebo | Heras 200974 | |||
| Clodronate (oral) | Placebo | Elomaa 198873 | |||
| Clodronate (oral) | Placebo | Paterson 199376 | |||
| Clodronate (oral) | Open | Kristensen 199975 | |||
| Clodronate (oral) | Placebo | Dearnaley 200379 | |||
| Prostate | Clodronate (i.v.) | Placebo | Elomaa 199280 | ||
| Clodronate (i.v.) | Open | Kylmala 199382 | |||
| Clodronate (i.v.) | Placebo | Ernst 200381 | |||
| Clodronate (i.v. + i.m. + oral) | Placebo | Adami 198977 | |||
| Clodronate (i.v. + oral) | Placebo | Kylmala 199783 | |||
| Clodronate (i.v.) | Placebo | Strang 199789 | |||
| Disodium pamidronate (i.v.) | Placebo | Small 200387 | |||
| Etidronate (i.v. + oral) | Placebo | Smith 198988 | |||
| Clodronate (oral) | Placebo | Arican 199990 | |||
| OST | Clodronate (oral) | Placebo | Brown 200792 | ||
| Clodronate (oral) | Placebo | O’Rourke 199596 | |||
| Clodronate (oral) | Placebo | Piga 199897 | |||
| Clodronate (oral) | Placebo | Robertson 199598 | |||
| Clodronate (oral) | Disodium pamidronate (i.v.) | Jagdev 200194 | |||
| Ibandronate (oral) | bandronate (i.v.) | Mystakidou 200895 | |||
| Ibandronate (i.v.) | Placebo | Heras 200793 | |||
| Zoledronic acid (i.v.) | Placebo | Lipton 2003101 | |||
| Zoledronic acid (i.v.) | Disodium pamidronate (i.v.) | Berenson 200191 | |||
| Zoledronic acid (i.v.) | Placebo | Zaghloul 201099 | |||
| Zoledronic acid (i.v.) | Open | Zhao 2011100 | |||
| BSC vs placebo/another BSC | 4 | Prostate | Strontium chloride (i.v.) | Placebo | Buchali 198878 |
| Strontium chloride (i.v.) | FEM | Nilsson 200584 | |||
| Strontium chloride (i.v.) | Placebo | Porter 199385 | |||
| Strontium chloride (i.v.) | Radiotherapy | Quilty 199486 | |||
Breast cancer
Table 3 shows summary information for the four studies that provided direct evidence for denosumab or were included in the NMA. The study by Kohno and colleagues102 was undertaken between May 2000 and May 2003 and enrolled adults with at least one osteolytic bone metastasis from breast cancer from 51 centres in Japan. Patients received 4 mg zoledronic acid or placebo every 4 weeks for 12 months. The primary outcome was the ratio of the SRE rate (defined as the total number of SREs divided by the total years on study) for patients treated with zoledronic acid divided by the SRE rate for the placebo group. Follow-up was 52 weeks. The study was funded by Novartis.
| Study criteria | aKohno 2005102 | Lipton 2000103 | Rosen 2003a104 | Stopeck 201031 | ||||
|---|---|---|---|---|---|---|---|---|
| Zoledronic acid | Placebo | Disodium pamidronate | Placebo | Zoledronic acid | Disodium pamidronate | Denosumab | Zoledronic acid | |
| Randomised | 114 | 113 | 367 | 387 | 378 | 388 | 1026 | 1020 |
| Age (years)b | 54.3 | 53.5 | See notes | 58 | 56 | 57 | 56 | |
| ECOG status 0–1 | 101 (89%) | 101 (89%) | 265 (72%) | 267 (69%) | (87%) | (81%) | 955 (93%) | 932 (92%) |
| Time from diagnosis (months)c | ||||||||
| Of breast cancer | 41.3 | 44.0 | NR | NR | 78±67 | 71±62 | NR | NR |
| Of bone metastases | 3.9 | 3.9 | See notes | See notes | 17.5 ± 33.85 | 12.6 ± 21.68 | 2.1 | 2.0 |
| Previous SREs | 39 (34%) | 47 (42%) | NR | NR | 232 (62%) | 244 (63%) | 378 (37%) | 373 (37%) |
The study by Lipton and colleagues103 reports results of two similarly conducted RCTs. 22,115 The studies were undertaken between 1990 and 1996 and enrolled women with stage IV breast cancer and at least one predominantly lytic metastatic bone lesion measuring ≥ 1 cm from 106 centres in the USA, Canada, Australia and New Zealand. Patients received 90 mg disodium pamidronate every 3–4 weeks or placebo every 4 weeks for 24 cycles. The primary outcome was the SMR, defined as the ratio of the number of skeletal complications experienced by a patient divided by the time on the trial for that patient (expressed as the number of events per year). Follow-up was 24 months. The study was funded by Novartis.
The study by Rosen and colleagues104 was undertaken between October 1998 and January 2000 and enrolled women with at least one bone metastasis (osteolytic, osteoblastic, or mixed) secondary to stage IV breast cancer. The primary analysis of this study included advanced multiple myeloma, but a subgroup of those patients with breast cancer is presented separately. 110 The study was described as multicentre and international. Patients received 4 mg zoledronic acid or 90 mg disodium pamidronate every 3–4 weeks for 24 months. Zoledronic acid was initially infused over 5 minutes in 50 ml of hydration solution. However, because of concerns over renal safety a protocol amendment in June 1999 changed the infusion time to 15 minutes and increased the volume of the infusion to 100 ml. The primary outcome was the proportion of patients who experienced at least one SRE during the study period. Follow-up was 25 months. The study was funded by Novartis.
The study by Stopeck and colleagues31 was undertaken between April 2006 and December 2007 and enrolled women with confirmed breast cancer and at least one bone metastasis from 322 centres in Europe, North America, South America, Japan, Australia, India and South Africa. However, few (academic-in-confidence information has been removed) of patients were from the UK (MS). Patients with creatinine clearance < 30 ml/minute, prior intravenous BP treatment, current or prior oral BPs for the treatment of bone metastases, non-healed dental/oral surgery and prior malignancy within 3 years before random assignment were excluded. Patients received a subcutaneous injection of 120 mg denosumab and an intravenous infusion of placebo or an intravenous infusion of 4 mg zoledronic acid and a subcutaneous injection of placebo every 4 weeks. The study was powered to detect both non-inferiority and superiority with respect to time to first on-study SRE (primary outcome), and risk of first and subsequent on-study SREs. Follow-up was around 34 months. The study was funded by Amgen and Daiichi Sankyo.
Quality of the included studies
Table 4 shows the results of the risk of bias assessment for the four studies that were included in the NMA. See Appendix 9 for risk of bias assessment for other included studies.
| Risk of bias criteria | Kohno 2005102 | Lipton 2000103 | Rosen 2003a104 | Stopeck 2010a31 |
|---|---|---|---|---|
| Adequate sequence generation | Yes | Yes | Yes | Unclear |
| Adequate allocation concealment | Yes | Yes | Yes | Unclear |
| Blinding | Yes | Yes | Yes | Yes |
| Incomplete outcome data addressed | No | Unclear | Yes | Yes |
| Free of selective reporting | Yes | Unclear | Yes | Yes |
The study by Lipton and colleagues103 used computer-generated randomisation, whereas the study by Rosen and colleagues104 reported an automated system and the study by Kohno and colleagues102 employed a dynamic balancing method. Although the study by Stopeck and colleagues31 was described as randomised, no further details were given of the sequence generation or allocation concealment. In the study by Lipton and colleagues103 patients, investigators and other study personnel were blinded, the study by Kohno and colleagues102 involved blinded radiographic assessment and the studies by Stopeck and colleagues31 and Rosen and colleagues104 were described as double blind. The study by Kohno and colleagues102 did not provide an explanation as to the reasons why around 33% of patients in the zoledronic acid group and 36% in the placebo group did not complete the study. It was unclear in the study by Lipton and colleagues103 whether or not the issue of incomplete outcome data had been addressed (reasons for discontinuation stated but number discontinued not given for one trial; Hortobagyi and colleagues 199622) or whether or not the study was free of selective reporting of outcomes (the stated primary end point and end point for power calculation were different for one trial; Theriault and colleagues 1999115).
Assessment of effectiveness
This section reports the clinical effectiveness and safety of denosumab for the treatment of bone metastases from breast cancer compared with BPs or placebo for those comparative studies included in the NMA. See Appendix 7 for the results for the following outcomes reported by those studies comparing BPs with placebo that were not included in the NMA.
Time to first on-study skeletal-related event
Table 5 shows the results for time to first on-study SRE as reported in the studies by Lipton and colleagues,103 Kohno and colleagues,102 Stopeck and colleagues31 and Rosen and colleagues. 104
| Study ID | Outcomes | Measures | Values | p-value | |
|---|---|---|---|---|---|
| Intervention | Comparator | ||||
| Denosumab (n = 1026) | Zoledronic acid (n = 1020) | ||||
| aStopeck 201031 | Time to first SRE (∼ 34 months' study duration) | Median months | Not reached | 26.4 | NA |
| Time to first SRE (from 4 months' extended treatment phase) | Median months | 32.4 | 27.4 | NA | |
| Delay to first on-study SRE | HR (95% CI) | 0.82 (0.71 to 0.95) | p < 0.01 (superiority analysis) | ||
| Zoledronic acid (n = 114) | Placebo (n = 113) | ||||
| aKohno 2005102 | Time to first SRE (excluding HCM) | Median days | Not reached | 364 (∼12.1 months) | 0.007 |
| Time to first SRE (including HCM) | Median days | Not reached | 360 (∼12 months) | 0.004 | |
| Disodium pamidronate (n = 367) | Placebo (n = 387) | ||||
| Lipton 2000103 | Time to any first SRE | Median months (95% CI) | 12.7 (9.6 to 17.2) | 7.0 (6.2 to 8.5) | < 0.001 |
| Time to first pathological fracture | Median months | 25.2 | 12.8 | 0.003 | |
| Time before requiring bone radiation | Median months | Not reached | 16.0 | < 0.001 | |
| Zoledronic acid (n = 378) | Disodium pamidronate (n = 388) | ||||
| Rosen 2003a104 | Time to first SRE (chemotherapy treated) | Median days | 349 (∼ 11.6 months) | 366 (∼ 12.2 months) | 0.826 |
| Time to first SRE (hormone therapy treated) | Median days | 415 (∼ 13.8 months) | 370 (∼ 12.3 months) | 0.047 | |
| Time to first SRE (lytic) | Median days | 310 (∼ 10.3 months) | 174 (∼ 5.8 months) | 0.013 | |
| Time to first SRE (non-lytic) | Median days | NR | NR | NR | |
In the study by Stopeck and colleagues,31 median time to first on-study SRE was not reached in the denosumab group compared with a median of 26.4 months in the zoledronic acid group during approximately 34 months of follow-up [HR 0.82; 95% confidence interval (95% CI) 0.71 to 0.95; p ≤ 0.0001]. Figure 4 shows the Kaplan–Meier estimates of the time to first on-study SRE. The MS reported that denosumab reduced the risk of a symptomatic SRE (academic-in-confidence information has been removed) and reduced the proportion of patients with symptomatic SREs (academic-in-confidence information has been removed). After an extended 4 months of blinded follow-up, Stopeck and colleagues reported that the median time to first on-study SRE was longer in the denosumab group compared with the zoledronic acid group by 5 months (32.4 vs 27.4 months).
FIGURE 4.
Kaplan–Meier (KM) estimates of time to first on-study SRE. Source: MS. Reproduced with permission from Stopeck et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132–39. 30
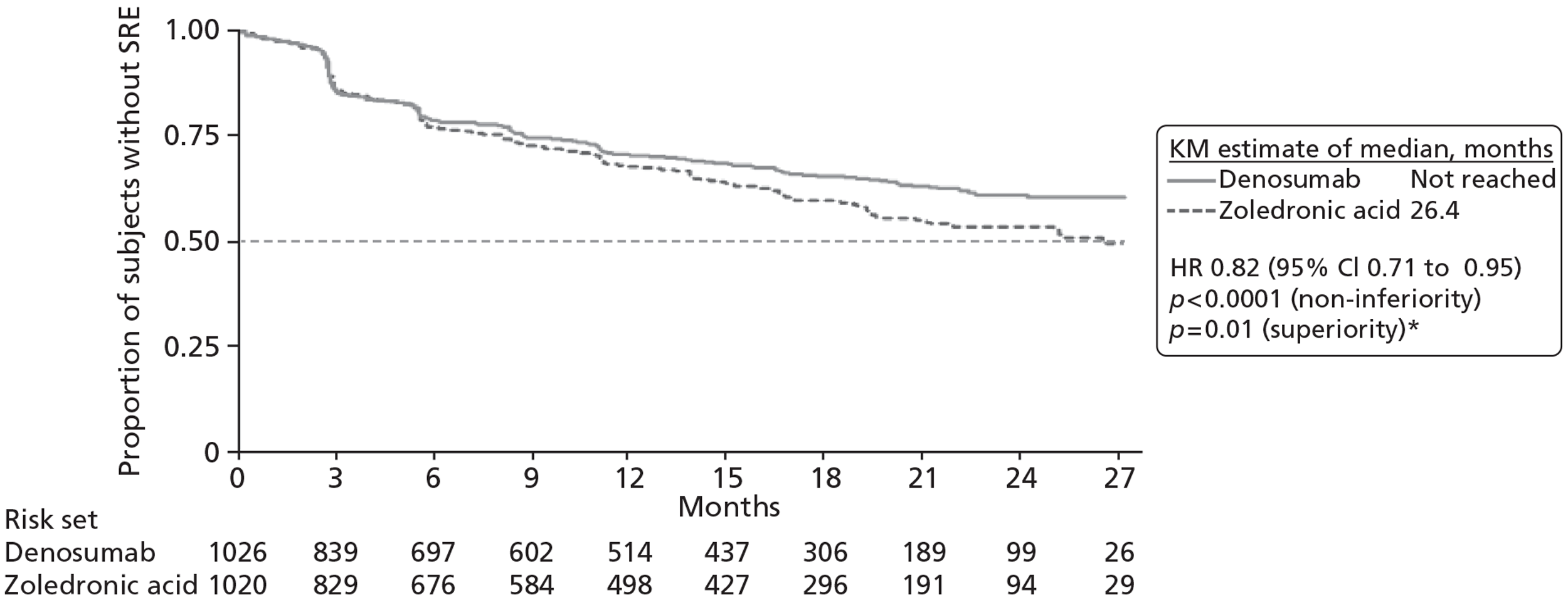
The median time to first on-study SRE was significantly longer in the BPs group compared with the placebo group in the study by Kohno and colleagues102 (not reached vs approximately 12 months; p = 0.007) and Lipton and colleagues103 [12.7 (95% CI 9.6 to 17.2) vs 7.0 (95% CI 6.2 to 8.5) months; p < 0.001]. The median time to first SRE was similar in the BPs groups as reported in trials by Lipton and colleagues103 (12.7 months) and Rosen and colleagues104 (∼ 11.6 to 13.8 months). There was no difference in the time to first SRE including or excluding hypercalcaemia as reported in the trial by Kohno and colleagues. 102
Skeletal-related events by type
In the denosumab RCT Stopeck and colleagues31 did not report SRE by type. The MS reported that denosumab reduced the risk for time to radiation in bone by (academic-in-confidence information has been removed) compared with zoledronic acid. Table 6 shows the distribution of first on-study SRE by type of SRE in the denosumab study. The distribution of type of SRE was similar across the treatment groups, with radiation to bone and pathological fracture being the most commonly occurring.
In the study by Lipton and colleagues,103 the median time to first pathological fracture was significantly longer in the disodium pamidronate group compared with the placebo group (by almost 12 months). The time before requiring bone radiation was not reached in the disodium pamidronate group compared with a median of 16 months in the placebo group (p < 0.001). 103
| SRE | Denosumab (n = 1026 randomised) | Zoledronic acid (n = 1020 randomised) |
|---|---|---|
| Number of events (%) | Number of events (%) | |
| Overall | 315 (100%) | 372 (100%) |
| Radiation to bone | AiC information has been removed | AiC information has been removed |
| Pathological fracture | AiC information has been removed | AiC information has been removed |
| SCC | AiC information has been removed | AiC information has been removed |
| Surgery to bone | AiC information has been removed | AiC information has been removed |
History of skeletal-related events
The MS reported time to first on-study SRE by history of SRE for the denosumab study 136 (Table 7). This showed that for those without a prior SRE (academic-in-confidence information has been removed). Covariate analysis showed that patients with a prior SRE history had an increased risk (academic-in-confidence information has been removed) compared with those without a SRE history.
| SRE history | Denosumab | Zoledronic acid |
|---|---|---|
| Overall | ||
| Number | 1026 | 1020 |
| HRa (95% CI) | 0.82 (0.71 to 0.95) | |
| p-value | 0.0101 | |
| No prior SRE | ||
| Number | 648 | 647 |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Prior SRE | ||
| Number | 378 | 373 |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Covariate effect | ||
| Point estimate (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
The study by Rosen and colleagues,104 comparing zoledronic acid with disodium pamidronate, reported time to first on-study SRE by lytic and non-lytic subgroup. There was no significant difference between the non-lytic treatment groups. For those lytic cases, the time to first SRE was much longer in the zoledronic acid (∼ 10.3 months) group compared with the disodium pamidronate group (∼ 5.8 months).
Risk of first and subsequent on-study skeletal-related events
Table 8 shows the results for risk of first and subsequent on-study SREs.
| Study ID | Treatment duration | Outcomes | Measures | Values, variance | p-value | |
|---|---|---|---|---|---|---|
| Intervention | Comparator | |||||
| Denosumab (n = 1026) | Zoledronic acid (n = 1020) | |||||
| Stopeck 201031 | ∼ 34 months | Risk of developing multiple SREs | Rate ratio (95% CI) | 0.77 (0.66 to 0.89) | 0.001 | |
| From 4 months' extended treatment phase | Risk of first and subsequent on-study SRE | Rate ratio (95% CI) | 0.78 (0.68 to 0.90) | 0.002 | ||
| Zoledronic acid (n = 114) | Placebo (n = 113) | |||||
| Kohno 2005102 | 12 months | Risk for developing SREs (multiple-event analysis) | Risk ratio (95% CI) | 0.59 (0.375 to 0.914) | 0.019a | |
| Excluding HCM | ||||||
| Risk for developing SREs (multiple-event analysis) | Risk ratio (95% CI) | 0.56 (0.363 to 0.867) | 0.009a | |||
| Including HCM | ||||||
| Zoledronic acid (n = 378) | Disodium pamidronate (n = 388) | |||||
| Rosen 2003a104 | 25 months | Risk of developing any SRE (multiple-event analysis) | Risk ratio (95% CI) | 0.799 (0.657 to 0.972) | 0.025 | |
| Including HCM | ||||||
| 25 months | Risk of developing a SRE | Risk ratio (95% CI) | 0.693 (0.527 to 0.911) | 0.009 | ||
| Including HCM; hormone therapy treated | ||||||
| 13 months | Risk for multiple skeletal events (total) | HR (95% CI) | 0.801 (Not reported) | 0.037 | ||
| Excluding HCM | ||||||
| 13 months | Risk for multiple skeletal events (lytic) excluding HCM | HR (95% CI) | 0.704 (Not reported) | 0.010 | ||
| 13 months | Risk for multiple skeletal events (non-lytic) excluding HCM | Not reported | Not reported | 0.760 | ||
Stopeck and colleagues31 reported a risk reduction of 23% [relative risk (RR) 0.77; 95% CI 0.66 to 0.89; p = 0.001] for the denosumab group compared with the zoledronic acid group over 34 months, with the risk remaining similar when the duration of treatment was extended by another 4 months (RR 0.78; 95% CI 0.68 to 0.90; p = 0.002). Figure 5 shows the cumulative mean number of SREs (multiple-event analysis).
FIGURE 5.
Cumulative mean number of SREs (multiple-event analysis). (Academic-in-confidence information has been removed.) Source: MS.
Kohno and colleagues102 and Rosen and colleagues104 reported the risk for developing multiple SREs for zoledronic acid compared with placebo and disodium pamidronate, respectively. In both studies, zoledronic acid significantly reduced the risk of developing multiple SREs when HCM was included in the SRE analysis (44% reduction compared with placebo102 and approximately 20% reduction compared with disodium pamidronate). 104 Similar results were reported when HCM was excluded from the SRE analysis (the risk of developing multiple SREs was 41% lower in the zoledronic group compared with the placebo group and 20% lower compared with the disodium pamidronate group).
Skeletal-related events by type
None of the studies reported risk of first and subsequent SREs by individual SRE type.
The MS reported the distribution of first and subsequent on-study SRE by type of SRE in the denosumab RCT (study 136) (Table 9). As for first on-study SRE by type, the distribution of type of SRE was similar across the treatment groups, with radiation to bone and pathological fracture again the most commonly occurring.
| SRE | Denosumab (n = 1026 randomised) | Zoledronic acid (n = 1020 randomised) |
|---|---|---|
| Number of events (%) | Number of events (%) | |
| Total confirmed events | AiC information has been removed | AiC information has been removed |
| Radiation to bone | AiC information has been removed | AiC information has been removed |
| Pathological fracture | AiC information has been removed | AiC information has been removed |
| SCC | AiC information has been removed | AiC information has been removed |
| Surgery to bone | AiC information has been removed | AiC information has been removed |
Prior history of skeletal-related events
The MS reported risk of first and subsequent on-study SRE by history of SRE for study 136 (Table 10). (Academic-in confidence information has been removed.) Covariate analysis as presented in the manufacturer's table showed that patients with a history of SRE had an increased risk (academic-in confidence information has been removed) compared with those without a SRE history.
| SRE history | Denosumab | Zoledronic acid |
|---|---|---|
| Overall | ||
| Number | 1026 | 1020 |
| Rate ratio (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| No prior SRE | ||
| Number | 648 | 647 |
| Rate ratio (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Prior SRE | ||
| Number | 378 | 373 |
| Rate ratio (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Covariate effect | ||
| Point estimate (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
Skeletal morbidity rate
Table 11 shows the results for SMR. The SMR is defined as the ratio of the number of SREs per patient divided by the patient's time at risk. The MS stated that for the SMR calculations a 21-day event window was used for counting on-study SREs, so that any event occurring within 21 days of a previous event was not counted as a separate on-study SRE.
| Study ID | Treatment duration | Outcomes | Measures | Values, variance | Difference between groups | p-value | |
|---|---|---|---|---|---|---|---|
| Intervention | Comparator | ||||||
| Denosumab (n = 1026) | Zoledronic acid (n = 1020) | ||||||
| Stopeck 20103 | ∼ 34 months | SMR (defined as the ratio of the number of SREs per patient/the patient's time at risk) | Mean events per patient per year | 0.45 | 0.58 | Denosumab reduced risk by 22% | 0.004 |
| Zoledronic acid (n = 114) | Placebo (n = 113) | ||||||
| Kohno 2005102 | 12 months | SRE rate (defined as the total number of SREs divided by the total years on study) | No. of events per patient-years | 0.63 | 1.1 | SRE rate ratio 0.57 (unadjusted) | 0.016 |
| All patients | |||||||
| SRE rate | No. of events per patient-years | 1.55 | 1.91 | SRE rate ratio 0.81 (unadjusted), 0.61 (adjusted)a | 0.027 | ||
| Patients with prior fracture | |||||||
| SRE rate | No. of events per patient-years | 0.33 | 0.78 | SRE rate ratio 0.43 (unadjusted) | |||
| Patients without prior fracture | |||||||
| Disodium pamidronate (n = 367) | Placebo (n = 387) | ||||||
| Lipton 2000103 | 24 months | SMR (any skeletal complication excluding HCM) | No. of events per year (mean, SD) | 2.4 (5.5) | 3.7 (5.5) | Not reported | < 0.001 |
| SMR (any skeletal complication including HCM) | No. of events per year (mean, SD) | 2.5 (5.6) | 4.0 (6.1) | Not reported | < 0.001 | ||
| Radiation to bone | No. of events per year (mean, SD) | 0.7 (1.9) | 1.2 (2.4) | Not reported | < 0.001 | ||
| Radiation to bone for pain relief | 0.5 (1.6) | 1.0 (2.2) | Not reported | < 0.001 | |||
| Pathologic fracture | 1.6 (4.1) | 2.2 (4.5) | Not reported | 0.002 | |||
| Surgery to bone | 0.10 (0.58) | 0.15 (0.53) | Not reported | 0.009 | |||
| SCC | 0.04 (0.30) | 0.07 (0.60) | Not reported | 0.772 | |||
| Hypercalcaemia | 0.07 (0.36) | 0.37 (1.75) | Not reported | < 0.001 | |||
| Zoledronic acid (n = 378) | Disodium pamidronate (n = 388) | ||||||
| Rosen 2003a104 | 25 months | SMR excluding HCM | Events per year | 0.9 | 1.49 | Not reported | 0.125 |
| 25 months | SMR including HCM | Events per year | 0.91 | 1.57 | Not reported | 0.102 | |
| 25 months | SMR (hormonal treated) | Events per year | 0.83 | 1.37 | Not reported | 0.39 | |
| 13 months | SMR excluding HCM | Events per year, mean (SD) | 0.98 (2.04) | 1.55 (5.03) | Not reported | 0.073 | |
| 13 months | SMR lytic | Events per year, mean (SD) | 1.16 (2.32) | 2.36 (7.16) | Not reported | 0.008 | |
| 13 months | SMR non-lytic | Events per year, mean (SD) | 0.81 (1.69) | 0.97 (2.47) | Not reported | 0.904 | |
| 13 months | SMR hormonal therapy treated | Events per year | 0.33 | 0.58 | Not reported | 0.015 | |
Stopeck and colleagues31 reported that the mean SMR (ratio of the number of SREs per patient divided by the patient's time at risk) was significantly lower in the denosumab group (0.45 events per patient per year) compared with the zoledronic acid group (0.58 events per patient per year) (p = 0.004). The studies by Kohno and colleagues102 and Lipton and colleagues103 comparing BPs with placebo reported that SRE events occurred less frequently in the BPs group (0.63 to 2.4 events per year) than in the placebo group (1.1 to 3.7 events per year). In the study by Rosen and colleagues104 the SMR rate was lower for zoledronic acid compared with disodium pamidronate (0.9 events per year vs 1.49 events per year), although the difference was not statistically significant (p = 0.125). In the study by Kohno and colleagues102 the rate of SREs was reduced by 39% (0.61; p = 0.027) in the zoledronic acid group compared with the placebo group when adjusted for whether or not patients had experienced prior pathological fracture before study entry. A similar SMR was reported when HCM was included or excluded from the analysis in the studies by Lipton and colleagues103 and Rosen and colleagues. 104
Skeletal-related events by type
The MS did not report SMR by type of SRE.
The study by Lipton and colleagues103 comparing disodium pamidronate with placebo reported SMR for different types of SREs including radiation to bone, radiation to bone for pain relief, pathological fracture, surgery to bone, SCC and hypercalcaemia. A statistically significant difference was reported between disodium pamidronate and placebo for all types of SRE other than SCC. Among all the SREs, the highest rate (events per year) was reported for pathological fracture (1.6 vs 2.2) and the lowest rate was reported for SCC (0.07 vs 0.37).
Prior history of skeletal-related events
The MS did not report SMR by prior history of SREs.
In the study by Kohno and colleagues102 the SRE rate reduction for zoledronic acid was more than 30% higher in patients without a prior fracture (unadjusted SRE rate ratio 0.43) than in patients with a prior fracture (unadjusted SRE rate ratio 0.81).
In the subgroup analysis of patients with lytic lesions, Rosen and colleagues104 reported SRE rates in the zoledronic acid arm (1.16 events per year) that were almost half of those in the disodium pamidronate arm (2.36 events per year; p = 0.008). In those with non-lytic lesions, the difference between the treatment groups for SRE rate was reported to be non-significant (0.81 vs 0.97; p = 0.904).
Incidence of skeletal-related events
Table 12 shows the results for the crude incidence of SREs.
| Study ID | Outcomes | Measures | Values, variance | p-value | |
|---|---|---|---|---|---|
| Intervention | Comparator | ||||
| Denosumab (n = 1026) | Zoledronic acid (n = 1020) | ||||
| Stopeck 201031 | Proportion of patients who experienced any on-study SRE | At 34 months | 30.7% | 36.5% | NR |
| Zoledronic acid (n = 114) | Placebo (n = 113) | ||||
| Kohno 2005102 | Proportion of patients with at least one SRE (excluding HCM) | At 1 year | 29.8% | 49.6% | 0.003 |
| Proportion of patients with at least one SRE (including HCM) | 30.7% | 52.2% | 0.001 | ||
| Proportion with fractures | At 1 year | 25.4% | 38.9% | NR | |
| Proportion with radiation to bone | At 1 year | 8.8% | 17.7% | NR | |
| Proportion with surgery to bone | At 1 year | 0.0% | 0.9% | NR | |
| Proportion with SCC | At 1 year | 3.5% | 11.5% | NR | |
| Proportion with hypercalcaemia | At 1 year | 2.6% | 8.8% | NR | |
| Disodium pamidronate (n = 367) | Placebo (n = 387) | ||||
| Lipton 2000103 | Proportion with any SRE (excluding HCM) | At 2 years | 51% | 64% | < 0.001 |
| Proportion with any SRE (including HCM) | At 2 years | 53% | 68% | < 0.001 | |
| Proportion with radiation to bone | At 2 years | 29% | 43% | < 0.001 | |
| Proportion with radiation to bone for pain relief | At 2 years | 25% | 37% | < 0.001 | |
| Proportion with pathological fracture | At 2 years | 40% | 52% | 0.002 | |
| Proportion with surgery to bone | At 2 years | 6% | 11% | 0.008 | |
| Proportion with SCC | At 2 years | 3% | 3% | 0.762 | |
| Proportion with hypercalcaemia | At 2 years | 6% | 13% | 0.001 | |
| Zoledronic acid (n = 378) | Disodium pamidronate (n = 388) | ||||
| Rosen 2003a104 | Proportion with any SRE (excluding HCM) | At 25 months | 46% | 49% | NR |
| At 13 months | 43% | 45% | NS | ||
| Proportion with any SRE: lytic subgroup | At 13 months | 48% | 58% | 0.58 | |
| Proportion with any SRE: non-lytic subgroup | At 13 months | 38% | 36% | NR | |
Stopeck and colleagues31 reported that at approximately 34 months of treatment, 30.7% of those receiving denosumab compared with 36.5% receiving zoledronic acid experienced any on-study SRE. The MS reported an annualised SRE rate based on the number of SREs observed in each treatment arm divided by the number of patient-years for each treatment arm and reported this outcome both with and without a 21-day event window.
Table 13 shows the annualised SRE rate both with and without the 21-day window for study 136. The MS reported that the primary analysis of annualised SRE rates was based on all SREs reported in each arm of the study (calculated without a 21-day window). Subsequently, a post-hoc analysis of the annualised SRE rate applying the trial-defined 21-day window for SREs was conducted. Both analyses show that the annualised SRE rate was lower in patients receiving denosumab compared with those receiving zoledronic acid.
| Annualised SRE rate per patient | Denosumab (n = 1026) | Zoledronic acid (n = 1020) |
|---|---|---|
| Subject years | AiC information has been removed | AiC information has been removed |
| Without 21-day window | ||
| Number of events | AiC information has been removed | AiC information has been removed |
| Annualised rate | AiC information has been removed | AiC information has been removed |
| With 21-day window | ||
| Number of events | AiC information has been removed | AiC information has been removed |
| Annualised rate | AiC information has been removed | AiC information has been removed |
A statistically significant difference in favour of BPs compared with placebo for patients experiencing an on-study SRE was reported in the studies by Kohno and colleagues102 and Lipton and colleagues. 103 The proportion of patients experiencing at least one on-study SRE at 1 year was significantly lower by 20% in the zoledronic acid group compared with the placebo group (29.8% vs 49.6%) in the study by Kohno and colleagues. 102 In the study by Lipton and colleagues,103 at 2 years, the disodium pamidronate group experienced a lower rate of SREs compared with the placebo group (51% vs 64%). Rosen and colleagues,104 comparing zoledronic acid with disodium pamidronate, reported a non-significant difference between the groups for the crude incidence of SREs at 13 or 25 months. Rosen and colleagues104 further reported non-significant difference in the crude incidence of SREs between zoledronic acid and disodium pamidronate for those with lytic lesion. For those with non-lytic lesion a similar crude incidence was reported between the groups.
Skeletal-related events by type
The MS did not report this outcome.
The studies by Kohno and colleagues102 and Lipton and colleagues103 reported the proportions of patients experiencing types of SRE at 1 year and 2 years, respectively. For each type of SRE reported (other than for SCC in the study by Lipton and colleagues103), the BP group experienced lower rates compared with placebo. In the study by Lipton and colleagues, 103 the difference between the treatment groups for each type of SRE was statistically significant other than for SCC. In both studies the most frequently occurring type of SRE was fractures (25.4% vs 39.8% at 1 year in the study by Kohno and colleagues102 and 40% vs 52% at 2 years in the study by Lipton and colleagues103), followed by radiation to the bone.
In a subgroup analysis comparing patients with lytic and non-lytic lesions, Rosen and colleagues104 reported a non-significant difference for the proportion experiencing a SRE between zoledronic acid and disodium pamidronate in each subgroup at 13 months.
Prior history of skeletal-related events
None of the studies reported incidence of SRE by prior history of SREs.
Prevention of hypercalcaemia
In study 136, (academic-in-confidence information has been removed) (CSR 136).
Kohno and colleagues102 reported that 2.6% (3/114) of the zoledronic acid group and 8.8% (10/113) of the placebo group experienced hypercalcaemia.
Overall survival
A non-significant difference in overall survival was reported for denosumab compared with zoledronic acid in the study by Stopeck and colleagues31 (HR 0.95; 95% CI 0.81 to 1.11; p = 0.49). The MS reported this (academic-in-confidence information has been removed) for denosumab versus (academic-in-confidence information has been removed) for zoledronic acid (MS). In the study by Lipton and colleagues103 overall median survival was slightly longer in the disodium pamidronate group (19.8 months) compared with the placebo group (17.8 months) although the difference was not statistically significant (p = 0.976). In a subgroup analysis of women < 50 years Lipton and colleagues103 reported a significantly longer median overall survival in the disodium pamidronate group compared with the placebo group (24.6 vs 15.7 months; p = 0.009).
Prior history of skeletal-related events
None of the studies reported overall survival by prior history of SREs.
Pain
Stopeck and colleagues31 reported the proportion of patients with no/mild pain at baseline (n = 1042) developing moderate/severe pain at study visits for up to 73 weeks. The severity of pain and interference with daily functioning were assessed using the Brief Pain Inventory-Short Form (BPI-SF) instrument, completed by patients at baseline, day 8 and before each monthly visit through to the end of the study. In each study visit week, the proportion of patients with no/mild pain at baseline, reporting moderate/severe pain was lower in the denosumab group (range 14.8% at 73 weeks to 19.9% at 25 weeks) compared with the zoledronic acid group (range 22.1% at 13 weeks to 27.4% at 37 weeks). The median time to developing moderate/severe pain in patients with no/mild pain at baseline was reported to be significantly longer in the denosumab group compared with the zoledronic acid group (295 vs 176 days; HR 0.78; 95% CI 0.67 to 0.92; p = 0.0024).
The median time to worsening pain (≥ 2-point increase from baseline in BPI-SF worst pain score) non-significantly favoured denosumab compared with zoledronic acid (8.5 vs 7.4 months, p = 0.822) and was similar between groups for time to pain improvement (median 82 days vs 85 days; HR 1.02; 95% CI 0.91 to 1.15; p = 0.7245).
(Academic-in-confidence information has been removed) (MS).
There was no statistical difference at study end point in the use of strong analgesics in breast cancer (academic-in-confidence information has been removed) (MS).
(Academic-in-confidence information has been removed) (CSR 136).
Lipton and colleagues103 reported, for disodium pamidronate compared with placebo, mean change in pain scores and analgesic scores from baseline to 24 months. Bone pain was evaluated using a scoring system that quantified both severity and frequency of bone pain. 103 The bone pain score was determined by multiplying the bone pain severity score by the bone pain frequency score. The mean pain score decreased significantly in the disodium pamidronate group (−0.07; SD 3.07) compared with the placebo group (1.14; SD 3.42) over the 24 months (p = 0.015). Similarly, the mean analgesic score decreased significantly in the disodium pamidronate group (−0.06; SD 3.28) compared with the placebo group (1.84; SD 3.73). At the last visit mean pain score and analgesic score were increased in both groups, but was significantly lower in the disodium pamidronate group compared with the placebo group (p < 0.001).
Health-related quality of life
Functional Assessment of Cancer Therapy
The Functional Assessment of Cancer Therapy – Breast (FACT-B) questionnaire consists of the Functional Assessment of Cancer Therapy -General (FACT-G) questionnaire plus additional questions specific to breast cancer. For each component of the FACT-B [FACT-G total score, FACT-B total score, physical well-being domain, functional well-being domain and trial outcome index (TOI): a composite of the functional well-being domain, physical well-being domain, and the prostate cancer subscale], a higher score indicates better HRQoL.
Stopeck and colleagues31 reported quality of life using the FACT-G questionnaire completed by patients at baseline, day 8, and before each monthly visit through to the end of the study (73 weeks). At 73 weeks 30% of patients had discontinued the study (academic-in-confidence information has been removed) (CSR 136).
Patients were divided into two subgroups at baseline: no/mild pain or moderate/severe pain, based on BPI. For those with no/mild pain at baseline, an average of 4.1% more patients (range −0.6% to 9.3%) treated with denosumab had a ≥ 5-point increase in the FACT-G score and an average of 2.4% fewer patients (range −4.4% to 6.3%) had a ≥ 5-point decrease in the FACT-G score at 18 months compared with those patients treated with zoledronic acid. For those with moderate/severe pain at baseline, a similar proportion of patients treated with denosumab had either a ≥ 5-point increase (average 3% more; range −1.7% to 7.9%) or a ≥ 5-point decrease (average 3.5% fewer; range −1.1% to 11.5%) in the FACT-G score at 18 months compared with those treated with zoledronic acid. 106 An average of 3.2% (range 1% to 7%) more patients in the denosumab group experienced a clinically meaningful improvement in quality of life (≥ 5-point increase in FACT-G total score) from week 5 through to week 73. 105
European Quality of Life-5 Dimensions
For both components of EQ-5D [the health index and the visual analogue scale (VAS)], a higher score indicates a more preferred health status. For the health index questions of the EQ-5D, a three-level response was used to assess quality of life (academic-in-confidence information has been removed) (CSR 136).
(Academic-in-confidence information has been removed) (CSR 136).
Lipton and colleagues,103 comparing disodium pamidronate with placebo, reported mean change in the quality-of-life scores from baseline to 24 months and to the last visit. Quality of life was evaluated using the Spitzer quality-of-life index. From baseline to the last visit quality of life worsened in both the disodium pamidronate group (−1.80; SD 2.81) and the placebo group (−2.13; SD 2.63) (p = 0.088).
Adverse events related to treatment
Hypocalcaemia
The MS reported that hypocalcaemia events were mainly non-serious and transient and resolved either spontaneously or with calcium supplementation (MS). More hypocalcaemia adverse events occurred in the denosumab group than in the zoledronic acid group [5.5% (56/1020) vs 3.4% (34/1013) respectively].
Kohno and colleagues102 reported that 39% of the zoledronic acid group and 7% of the placebo group experienced grade 1 hypocalcaemia. There were no grade 2 or 3 hypocalcaemia events in the zoledronic acid group, while one patient in each group experienced grade 4 hypocalcaemia. 102 Lipton and colleagues,103 comparing disodium pamidronate with placebo, reported that one patient (1/367) discontinued disodium pamidronate after a symptomatic hypocalcaemia episode. Rosen and colleagues104 did not report this outcome in their study comparing zoledronic acid with disodium pamidronate.
An observational study165 reported on 177 patients receiving BPs over 13 months. They found the incidence of hypocalcaemia to be 15.8% in patients treated with zoledronic acid over this period. However, this study included all grades of hypocalcaemia.
Osteonecrosis of the jaw
The rates of ONJ in the denosumab RCT were low and similar between the denosumab group and the zoledronic acid group [2.0% (20/1020) vs 1.4% (14/1013); p = 0.39]. 31 The cumulative incidence of ONJ in the denosumab and zoledronic acid groups, respectively, was 0.8% and 0.5% at 1 year, 1.9% and 1.2% at 2 years, and 2.0% and 1.4% at 3 years. 31 Stopeck and colleagues31 reported that, as of February 2010, 10 (50%) denosumab-treated patients and six (43%) zoledronic acid-treated patients had resolution of the ONJ event; 10 (50%) denosumab-treated patients and nine (64%) zoledronic acid-treated patients reported local infection; and seven patients in each group (35%, denosumab; 50%, zoledronic acid) reported undergoing limited surgical procedures such as debridement and sequestrectomy.
None of the other RCTs or observational studies reported ONJ.
Renal toxicity
In the denosumab RCT, a statistically significant lower rate of adverse events potentially associated with renal impairment occurred in the denosumab group compared with the zoledronic acid group [4.9% (50/1020) vs 8.5% (86/1013), respectively; p = 0.001]. 31 Stopeck and colleagues31 also reported that the rates of severe and serious adverse events (SAEs) associated with renal impairment were also lower for denosumab than for zoledronic acid (0.4% vs 2.2%, and 0.2% vs 1.5%, respectively). The incidence of renal adverse events among patients with baseline renal clearance ≤ 60 ml/minute was also lower in the denosumab group (5.9%) than in the zoledronic acid group (20.0%), and a greater proportion of patients had decreases in their baseline creatinine clearance from ≥ 60 ml/minute to < 60 ml/minute with zoledronic acid (16.1%) compared with denosumab (12.7%). 31
It should be noted that, as zoledronic acid is contraindicated in patients with poor renal function, such patients were excluded from the denosumab study. The manufacturer stated that the incidence of renal toxicity observed in the denosumab group represented a background rate for patients with advanced cancer, as such patients were predisposed to renal dysfunction, for example through the use of nephrotoxic drugs (MS).
Rosen and colleagues130 reported that there was no significant difference in renal safety profiles between the 4 mg zoledronic acid group and the 90 mg disodium pamidronate group. After 25 months, a change in the creatinine level of more than 0.5 mg/dl from baseline had occurred in 7.7% of patients in the zoledronic acid group and 6.0% of patients in the disodium pamidronate group. 130
Kohno and colleagues102 stated that there was no evidence of decreased renal function among patients in either group. In the zoledronic acid group, mean serum creatinine was 0.79 mg/dl at baseline and 0.78 mg/dl at the end of study while in the placebo group it was 0.79 mg/dl at baseline and 0.85 mg/dl at the end. In one patient in the zoledronic acid group, serum creatinine increased notably from a baseline of 1.3 mg/dl to 2.0 mg/dl, compared with seven patients in the placebo group. No patient in the zoledronic acid group developed a Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or 4 serum creatinine increase, while one patient in the placebo experienced such an event. 102
Acute-phase reactions
Acute-phase reactions encompass flu-like symptoms including pyrexia, chills, flushing, bone pain, arthralgias and myalgias. 31 Stopeck and colleagues31 reported that acute-phase reactions in the first 3 days after treatment were 2.7 times more common in the zoledronic acid group than in the denosumab group [27.3% (277/1013) vs 10.4% (106/1020), respectively]. In the MS, SAEs of acute-phase reactions within 3 days of first dose were reported. (Academic-in-confidence information has been removed.)
Other adverse events
Table 14 shows, for the denosumab RCT, rates of a number of selected other adverse events, including those leading to treatment discontinuation, CTCAE grade 3 or 4 events, serious and fatal adverse events. The rates for both groups were broadly similar.
| Adverse event | Denosumab (n = 1020) | Zoledronic acid (n = 1013) |
|---|---|---|
| AE leading to treatment discontinuation | 98 (10%) | 125 (12%) |
| CTCAE ≥ grade 3 AE | 609 (60%) | 635 (63%) |
| Serious AE | 453 (44%) | 471 (47%) |
For details of all other adverse events extracted from the RCTs meeting the review's inclusion criteria and also adverse events extracted from a number of observational studies identified, see Appendix 10.
Network meta-analysis
A NMA was undertaken by the AG. A NMA was also presented within the MS. The AG included four studies30,31,103,104 and the MS's NMA included 11 studies. Table 15 shows the comparisons and outcomes reported by the AG's and MS's NMAs.
| Comparisons | Time to first SRE | Time to first and subsequent SRE | SMR/SMPR | Proportion of patients with on-study SRE |
|---|---|---|---|---|
| Denosumab vs zoledronic acid | AG + MS | AG + MS | AG + MS | AG |
| Denosumab vs placebo | AG + MS | AG + MS | AG + MS | AG |
| Denosumab vs disodium pamidronate | AG + MS | AG + MS | AG + MS | Neither |
| Zoledronic acid vs placebo | AG + MS | AG + MS | AG + MS | AG |
| Denosumab vs ibandronic acid | MS | MS | Neither | Neither |
To convert time to event analysis, the statistical technique outlined by Tierney and colleagues70 was used. Although this is an accepted method of converting to HRs, assumptions are made, and this adds a further layer to the uncertainties of the NMA. This was performed for time to first SRE for Kohno and colleagues102 (zoledronic acid vs placebo HR 0.56; 95% CI 0.36 to 0.85) and Rosen and colleagues104 (zoledronic acid vs disodium pamidronate: HR 0.97; 95% CI 0.78 to 1.20). Conversion of Kohno and colleagues102 was straightforward using the number of observed events and p-value between groups. Conversion of Rosen and colleagues104 involved combining the lytic and non-lytic Kaplan–Meier curves. 109 The number of patients without a SRE at each time point and number at risk were then used to produce a HR. The HRs calculated by the AG and manufacturer were the same for Kohno and colleagues,102 but different for Rosen and colleagues. 104 It is unclear what the precise method was that was used by the manufacturer to calculate the HR for the Rosen study.
The manufacturer included 11 studies in the NMA. Five studies were considered too heterogeneous by the AG for the reasons outlined in Table 16. One study was not included in the AG's NMA because it was non-English language (French). The AG used pooled results of two studies,103 while the MS used unpooled studies. 107,115
| Study | Reason that AG considered study too heterogeneous |
|---|---|
| Heras 200974 | Different definition of SRE (includes change in antineoplastic medications) |
| Body 200371 | Different definition of SRE (excludes SCC) |
| Paterson 199376 | Different definition of SRE (excludes surgery and SCC) |
| Kristensen 199975 | Different definition of SRE (includes HCM, excludes need for surgery and SCC) |
| Body 200472 (Tripathy 2003166) | Different definition of SRE (excludes SCC) |
Time to first on-study skeletal-related event
The results from the AG's and MS's NMAs are shown below in Table 17.
| Comparison | AG's NMA HR (95% CI) | MS's NMA HR (95% CI) |
|---|---|---|
| Denosumab vs zoledronic acid | 0.82 (0.71 to 0.95) | AiC information has been removed |
| Denosumab vs disodium pamidronate | 0.79 (0.61 to 1.03) | AiC information has been removed |
| Denosumab vs placebo | 0.46 (0.29 to 0.72) | AiC information has been removed |
| Zoledronic acid vs placebo | 0.56 (0.36 to 0.86) | AiC information has been removed |
| Denosumab vs ibandronic acid | Not performed | AiC information has been removed |
In both the AG's NMA and the MS's NMA, time to first SRE favoured denosumab compared with zoledronic acid, disodium pamidronate and placebo. In the AG's NMA, the difference was statistically significant for denosumab versus zoledronic acid and denosumab versus placebo (academic-in-confidence information has been removed). The AG did not compare denosumab with ibandronic acid because they considered the studies too heterogeneous to provide meaningful results. (Academic-in-confidence information has been removed.) Risk of first and subsequent on-study SREs (academic-in-confidence information has been removed).
The results for risk of developing first and subsequent on-study SREs are provided below in Table 18.
| Comparison | AG's NMA RR (95% CI) | MS's NMA RR (95% CI) |
|---|---|---|
| Denosumab vs zoledronic acid | 0.77 (0.66 to 0.89) | AiC information has been removed |
| Denosumab vs disodium pamidronate | 0.62 (0.48 to 0.80) | AiC information has been removed |
| Denosumab vs placebo | 0.45 (0.28 to 0.72) | AiC information has been removed |
| Zoledronic acid vs placebo | 0.59 (0.37 to 0.91) | AiC information has been removed |
| Denosumab vs ibandronic acid | Not performed | AiC information has been removed |
Risk of first and subsequent SREs favoured denosumab compared with zoledronic acid, disodium pamidronate or placebo in both the AG's NMA and the MS's NMA. In the AG's NMA the difference was statistically significant. (Academic-in-confidence information has been removed.) SMR and SMPR (academic-in-confidence information has been removed).
The AG did not have access to SMPR for denosumab compared with zoledronic acid and were therefore unable to perform this comparison (Table 19).
| Comparison | SMR | SMPR | |
|---|---|---|---|
| AG's NMA rate ratio (95% CI) | MS's NMA rate ratio (95% CrI) | MS's NMA rate ratio (95% CrI) | |
| Denosumab vs zoledronic acid | 0.90 (0.67 to 1.09) | AiC information has been removed | AiC information has been removed |
| Denosumab vs disodium pamidronate | 0.73 (0.41 to 1.06) | AiC information has been removed | AiC information has been removed |
| Denosumab vs placebo | 0.47 (0.25 to 0.67) | AiC information has been removed | AiC information has been removed |
| Zoledronic acid vs placebo | 0.52 (0.32 to 0.70) | AiC information has been removed | AiC information has been removed |
| Denosumab vs ibandronic acid | Not performed | AiC information has been removed | AiC information has been removed |
The SMRs in both the AG's NMA and the MS's NMA favour denosumab. There was a statistically significant difference for denosumab compared with placebo (AG's NMA), zoledronic acid compared with placebo (AG's NMA). (Academic-in-confidence information has been removed.) Proportion of patients with on-study SRE (academic-in-confidence information has been removed).
The AG undertook a NMA comparing the proportion of patients with an on-study SRE (Table 20). This is a less informative outcome as it does not differentiate between lengths of study. However, the AG judged the study lengths to be similar enough to be included within the NMA. It also provided an opportunity to compare interventions by individual SRE.
| Comparison | Any SRE OR (95% CI) | Pathological fracture OR (95% CI) | Radiation to bone OR (95% CI) | Surgery to bone OR (95% CI) | SCC OR (95% CI) |
|---|---|---|---|---|---|
| Denosumab vs zoledronic acid | 0.77 (0.11 to 4.86) | 0.80 (0.06 to 10.11) | 0.72 (0.06 to 8.62) | 1.03 (0.08 to 13.15) | 1.30 (0.10 to 17.94) |
| Denosumab vs placebo | 0.36 (0.03 to 3.96) | 0.42 (0.01 to 15.96) | 0.31 (0.01 to 12.48) | 0.38 (0.00 to 30.47) | 0.34 (0.01 to 14.73 |
| Zoledronic acid vs placebo | 0.47 (0.09 to 2.23) | 0.53 (0.04 to 6.89) | 0.43 (0.03 to 6.28) | 0.37 (0.01 to 12.97) | 0.26 (0.02 to 3.89) |
Compared with zoledronic acid denosumab non-significantly reduced the risk of any SRE, pathological fracture and radiation to bone. There was a non-significant increase in SCC compared with zoledronic acid. Compared with placebo both denosumab and zoledronic acid non-significantly reduced the risk of each individual SRE. It should be noted that none of the above results was statistically significant and the NMA is not sufficiently powered to detect differences. Individual SREs should not be compared with each other, for example comparing the effectiveness of an intervention to prevent pathological fractures compared with SCC, because of the low numbers of events.
Summary
Only one study, by Stopeck and colleagues,31 was identified comparing denosumab with the primary comparator zoledronic acid. Three other studies contributed data to the indirect comparisons of denosumab versus BSC undertaken by the AG (these three studies were also included in the MS's NMA) and are therefore also reported in this chapter. Kohno and colleagues102 compared zoledronic acid with placebo, Rosen and colleagues104 compared zoledronic acid with disodium pamidronate, and Lipton and colleagues103 compared disodium pamidronate with placebo. All studies were generally of good quality. In terms of generalisability, all studies were multicentre and the first two were international. In the Kohno and colleagues study102 the patients were all Japanese and all had osteolytic lesions. The Stopeck and colleagues study31 was the largest, randomising 2046 patients, although few (academic-in-confidence information has been removed) were from the UK. All participants in this study had advanced breast cancer with one or more bone metastases, Eastern Cooperative Oncology Group (ECOG) status ≤ 2 and a life expectancy of ≥ 6 months. Patients with severe renal impairment, current or prior BP treatment, non-healed dental/oral surgery or prior malignancy within 3 years before randomisation were excluded. The study was powered to detect both non-inferiority and superiority with respect to time to first and risk of first and subsequent on-study SREs.
The study by Stopeck and colleagues31 reported a statistically significant difference in favour of denosumab compared with zoledronic acid in both the median time to first on-study SRE (not yet reached vs 26.4 months), most of which were radiation to bone or pathological fractures, and the risk of developing first and subsequent on-study SREs.
In the study by Kohno and colleagues,102 the median time to first on-study SRE was significantly longer in the zoledronic acid group than in the placebo group (not reached vs around 12 months), whereas the risk of developing multiple SREs was 41% lower in the zoledronic acid group. Likewise, in the study by Lipton and colleagues,103 the time to first on-study SRE was significantly longer in the disodium pamidronate group than in the placebo group (12.7 vs 7 months). In the study by Rosen and colleagues,104 comparing zoledronic acid with disodium pamidronate, the median time to first on-study SRE was broadly similar (around 11.6 vs 12.2 months) while the risk of developing multiple SREs was 20% lower in the zoledronic acid group.
Kohno and colleagues, in the denosumab RCT (academic-in-confidence information has been removed), reported that 2.6% of the zoledronic acid group and 8.8% of the placebo group experienced hypercalcaemia.
Stopeck and colleagues reported no difference in overall survival between denosumab and zoledronic acid (HR 0.95; 95% CI 0.81 to 1.11). Lipton and colleagues103 reported that median overall survival was slightly longer in the disodium pamidronate group than in the placebo group (19.8 vs 17.8 months).
Denosumab delayed the time to development of moderate or severe pain by more than 4 months compared with zoledronic acid (around 10.5 vs 6.3 months). Lipton and colleagues103 reported that the mean pain score decreased significantly in the disodium pamidronate group (−0.07) compared with the placebo group (1.14). The FACT quality-of-life scores were similar in the denosumab and zoledronic acid groups, and likewise there were no notable differences between the groups in terms of EQ-5D. Lipton and colleagues,103 using the Spitzer quality-of-life index, noted that from baseline to the last visit quality of life worsened in both the disodium pamidronate group (−1.80) and the placebo group (−2.13).
In terms of adverse events, slightly more hypocalcaemia events occurred in the denosumab group than in the zoledronic acid group (5.5% vs 3.4%), likewise for ONJ (2.0% vs 1.4%). There was a statistically significant lower rate of adverse events potentially associated with renal impairment (4.9% vs 8.5%), while fewer patients in the denosumab group experienced acute-phase reactions (10.4% vs 27.3%). The rates for adverse events leading to treatment discontinuation, CTCAE grade 3 or 4, or SAEs were broadly similar between the denosumab and zoledronic acid groups.
In the study by Kohno and colleagues,102 39% of the zoledronic acid group and 7% of the placebo group experienced grade 1 hypocalcaemia. Rosen and colleagues104 reported that there was no significant difference in renal safety profiles between the zoledronic acid and disodium pamidronate groups, whereas in the study by Kohno and colleagues102 there was no evidence of decreased renal function in either the zoledronic acid or placebo groups.
The AG's NMA included fewer trials than the MS's NMA, improving homogeneity; however, this reduced the number of outcomes and available comparisons. The MS's NMA included six more studies. It is the opinion of the AG that inclusion of these six additional studies introduced significant methodological heterogeneity to the NMA. All treatment effects were in the same direction in both AG's NMA and MS's NMA. The results from the AG's NMA show that denosumab, compared with zoledronic acid or placebo, significantly delayed the time to first SRE. For these comparisons and denosumab versus disodium pamidronate, denosumab significantly reduced the risk of first and subsequent SRE, and denosumab compared with placebo significantly reduced the SMR. (Academic-in-confidence information has been removed.) The proportion of SREs was non-significantly reduced in all SRE types, except for SCC. However, these results are subject to considerable uncertainty and should be interpreted with caution.
Chapter 5 Results: prostate cancer
Quantity of research available
Number and type of studies included
The flow diagram outlining the screening process for the overall review is shown in Figure 3 (see Chapter 4).
The primary comparator for denosumab was considered to be BSC, as in the NICE guideline on the diagnosis and treatment of prostate cancer the use of BPs to prevent or reduce the complications of bone metastases in men with hormone-refractory prostate cancer is not recommended. 46 BSC was defined as including palliative radiotherapy and analgesics. As the guideline states that BPs for pain relief may be considered when other treatments (including analgesics and palliative radiotherapy) have failed, BPs were considered as a secondary comparator in relation to this group of patients.
No RCTs were identified comparing denosumab with BSC. One RCT (six reports29,122,124,125,127,129) was identified comparing denosumab with the BP zoledronic acid. The primary published report for this study was considered to be that by Fizazi and colleagues. 29 One study (nine reports19,117–121,123,126,128) comparing zoledronic acid with placebo was identified and this study also contributed data to the indirect comparison of denosumab versus BSC. The primary report for this study was considered to be the 2002 paper by Saad and colleagues. 117
Number and type of studies excluded
For information on studies that were excluded from the review see Chapter 4, Number and type of studies excluded, and see Appendix 5 for a list of these studies along with the reasons for their exclusion. These studies were excluded because they failed to meet one or more of the inclusion criteria in terms of types of study, participants, intervention or outcomes reported.
Characteristics of the included studies
Appendix 8 shows the characteristics of the included studies. Table 21 shows summary information for the two studies that provided direct evidence for denosumab or were included in the NMA.
| Criteria | Fizazi 201129 | Saad 2002117 | ||
|---|---|---|---|---|
| Denosumab | Zoledronic acid | Zoledronic acid | Placebo | |
| Randomised | 950 | 951 | 214 | 208 |
| Age (years)a | 71 (64–77) | 71 (66–77) | 71.8 (7.9) | 72.2 (8.0) |
| Ethnicity | ||||
| White | 829 (87%) | 810 (85%) | 178 (83%) | 173 (83%) |
| Other | 121 (13%) | 141 (15%) | 36 (17%) | 35 (17%) |
| ECOG status 0–1 | 882 (93%) | 886 (93%) | 197 (92%) | 190 (91%) |
| Time from diagnosis (months)b | ||||
| Of prostate cancer | 37.5 (18.1–75.4) | 41.2 (18.3–82.0) | 62.2 ± 43.5 | 66.6 ± 46.9 |
| Of bone metastases | 3.94 (1.22–15.67) | 5.19 (1.31–16.10) | 23.8 ± 26.1 | 28.4 ± 30.7 |
| Previous SREs | 232 (24%) | 231 (24%) | 66 (31%) | 78 (38%) |
The study by Fizazi and colleagues29 was undertaken between May 2006 and October 2009 and enrolled men aged ≥ 18 years with confirmed prostate cancer and at least one bone metastasis, from 342 centres in 39 countries. However, (academic-in-confidence information has been removed) few patients were from the UK (MS). Exclusion criteria included creatinine clearance < 0.5 ml/second, current or previous treatment with intravenous BP or oral BP for bone metastases, planned radiation therapy or surgery to bone, life expectancy < 6 months, current or previous osteonecrosis or osteomyelitis of the jaw or any planned invasive dental procedure during the study. Patients received a subcutaneous injection of 120 mg denosumab and an intravenous infusion of placebo or an intravenous infusion of 4 mg zoledronic acid and a subcutaneous injection of placebo every 4 weeks. The study was powered to detect both non-inferiority and superiority with respect to time to first on-study SRE (primary outcome), and time to first and subsequent SRE. Follow-up was 41 months for the blinded treatment phase. The study was funded by Amgen.
The study by Saad and colleagues117 was undertaken between June 1998 and January 2001 and enrolled prostate cancer patients with a documented history of bone metastases, from more than 136 centres in the USA, Europe, South America and Australasia. Patients received 4 mg zoledronic acid or placebo every 3 weeks (a third arm in which 221 patients were assigned to an initial dose of 8 mg per week was not considered to meet our inclusion criteria). All patients also received a 500 mg calcium supplement and 400–500 IU of vitamin D daily. Pain management, including analgesics, radiation therapy, or other treatment, was at the discretion of the treating physician. The primary outcome was the proportion of patients having at least one SRE. Follow-up was 15 months (with an extension phase to 24 months). The study was funded by Novartis.
Quality of the included studies
Table 22 shows the results of the risk of bias assessment for the studies by Fizazi and colleagues29 and Saad and colleagues. 117
| Criteria | Fizazi 201129 | Saad 2002117 |
|---|---|---|
| Adequate sequence generation | Yes | Yes |
| Adequate allocation concealment | Yes | Yes |
| Blinding | Yes | Yes |
| Incomplete outcome data addressed | Yes | Yes |
| Free of selective reporting | Yes | Yes |
Both studies were good-quality studies with low risk of bias as assessed against the criteria in Table 22. The study by Fizazi and colleagues29 employed computer-generated randomisation, with an interactive voice response system used to assign patients (1 : 1 ratio) to treatment. Patients, study staff and investigators were masked to treatment assignment throughout the primary analysis period. Both primary and secondary efficacy end points included all randomised patients, irrespective of administration of study treatments (intention to treat), while the safety data set included all patients from the full analysis set who received at least one dose of study treatment. There was adequate description of withdrawals and losses to follow-up, and all of the prespecified outcomes were reported.
The study by Saad and colleagues117 employed a computer-generated list of randomisation numbers to assign patients. Treatment assignments were revealed to study personnel and any other persons involved in study conduct or monitoring only after the last patient had completed the last study visit. The study was double blind, patients lost to follow-up were described and all of the prespecified outcomes were reported.
Assessment of effectiveness
Time to first on-study skeletal-related event
The study by Fizazi and colleagues29 reported a statistically significant difference in favour of denosumab compared with zoledronic acid in the median time to first on-study SRE (20.7 vs 17.1 months; HR 0.82; 95% CI 0.71 to 0.95; p = 0.0002), reducing the risk of this event by 18% compared with zoledronic acid. Figure 6 shows the Kaplan–Meier estimates of the time to the first on-study SRE. The MS reported that denosumab reduced the risk of a symptomatic SRE (academic-in-confidence information has been removed) and reduced the proportion of patients with symptomatic SREs [to 25% (academic-in-confidence information has been removed)].
FIGURE 6.
Kaplan–Meier (KM) estimates of time to first on-study SRE. Reproduced with permission from Fizazi et al. Denosumab vs zoledronic acid for treatment of bone metastases in men with castration resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813–22. 29
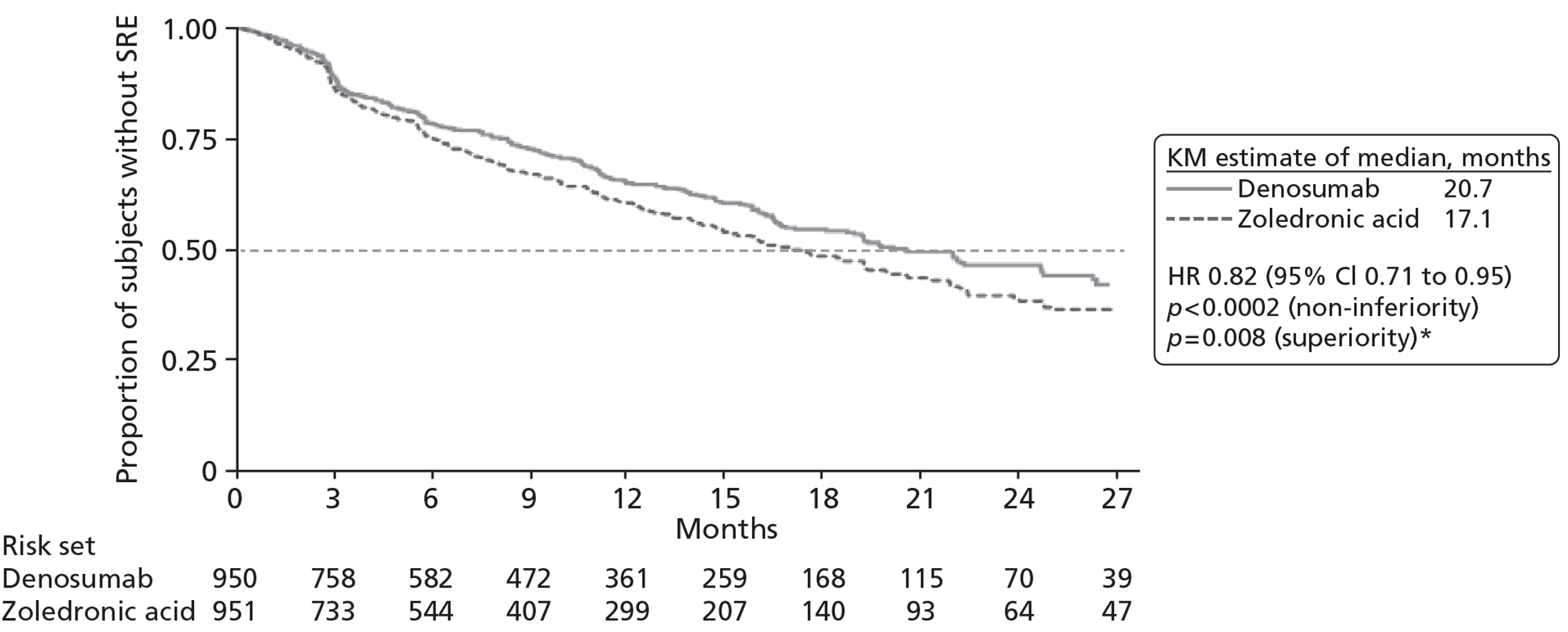
The study by Saad and colleagues118 reported a statistically significant difference in favour of zoledronic acid compared with placebo in the median time to first on-study SRE (488 vs 321 days; HR 0.68; 95% CI 0.51 to 0.91; p = 0.009), reducing the risk of this event by 32% compared with placebo.
Skeletal-related event by type
Neither study reported the time to first SRE for individual SREs.
Table 23 shows the distribution of first on-study SRE by type of SRE in the study by Fizazi and colleagues. 29 The distribution of type of SRE was similar across the treatment groups, with radiation to bone and pathological fracture being the most commonly occurring.
| SRE | Number of events (%) | |
|---|---|---|
| Denosumab (n = 950 randomised) | Zoledronic acid (n = 951 randomised) | |
| Overall | 341 (100%) | 386 (100%) |
| Radiation to bone | 177 (51.9%) | 203 (52.6%) |
| Pathological fracture | 137 (40.2%) | 143 (37.1%) |
| SCC | 26 (7.6%) | 36 (9.3%) |
| Surgery to bone | 1 (0.3%) | 4 (1.0%) |
Saad and colleagues117 did not report this outcome.
Prior history of skeletal-related events
The MS reported time to first on-study SRE by prior history of SREs for study 103 (Table 24). This showed a statistically significant difference in favour of denosumab for those patients with no prior SRE (academic-in-confidence information has been removed). Covariate analysis showed that patients with a prior SRE history had an increased risk (academic-in-confidence information has been removed) compared with those without a SRE history.
| SRE history | Denosumab | Zoledronic acid |
|---|---|---|
| Overall | ||
| Number | 950 | 951 |
| HR (95% CI) | 0.82 (0.71 to 0.95) | |
| p-value | 0.008 | |
| No prior SRE | ||
| Number | 718 | 720 |
| HR (95% CI) | 0.80 (0.67 to 0.95) | |
| p-value | 0.011 | |
| Prior SRE | ||
| Number | 232 | 231 |
| HR (95% CI) | 0.88 (0.67 to 1.16) | |
| p-value | 0.3675 | |
| Covariate effect | ||
| Point estimate (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
Saad and colleagues117 reported that the median time to first on-study SRE for those with a previous SRE (n = 144) was 361 days for the zoledronic acid group compared with 258 days for the placebo group (p = 0.066), whereas for those with no previous SRE (n = 277) it was 499 days for the zoledronic acid group and 337 days for the placebo group (p = 0.065). 119
Risk of first and subsequent on-study skeletal-related events
The study by Fizazi and colleagues29 reported a statistically significant difference in favour of denosumab compared with zoledronic acid in the risk of developing first and subsequent on-study SREs (RR 0.82; 95% CI 0.71 to 0.94; p = 0.004, adjusted for multiplicity p = 0.008). Figure 7 shows the cumulative mean number of SREs (multiple-event analysis).
FIGURE 7.
Cumulative mean number of SREs (multiple-event analysis). Reproduced with permission from Fizazi et al. Denosumab vs zoledronic acid for treatment of bone metastases in men with castration resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813–22. 29
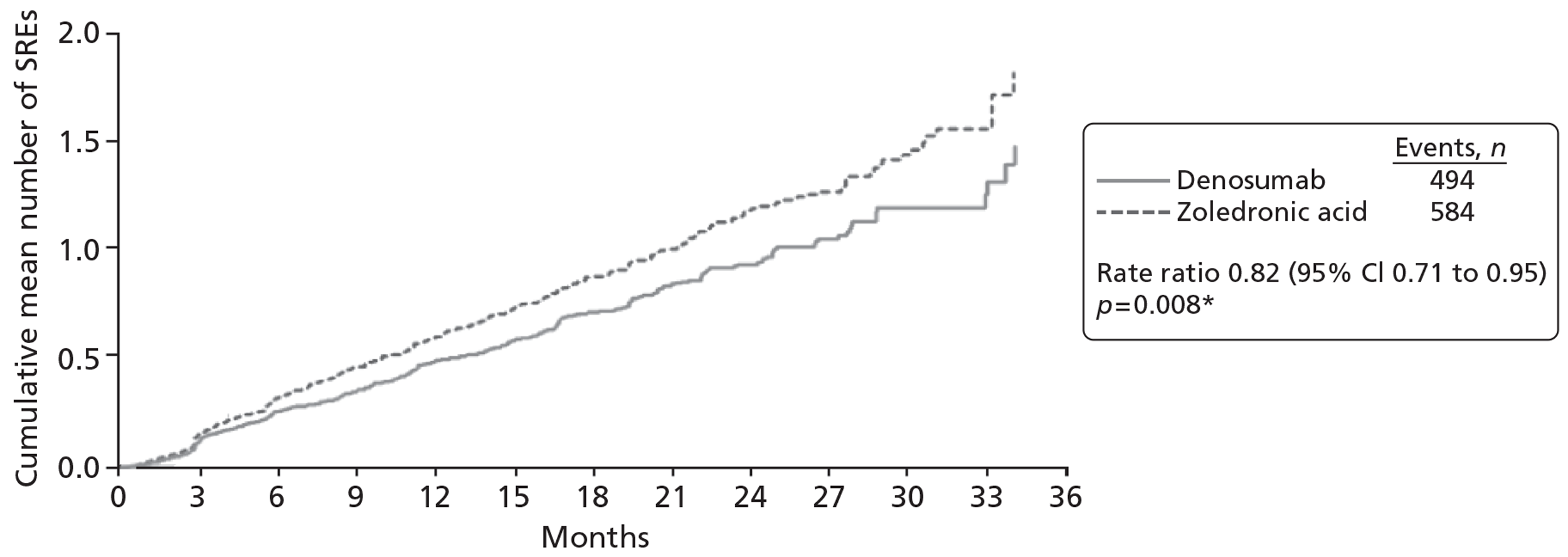
Saad and colleagues117 reported a statistically significant difference in favour of zoledronic acid compared with placebo in the risk of developing first and subsequent on-study SREs (RR 0.64; 95% CI not reported; p = 0.002).
Skeletal-related event by type
Neither study reported risk of first and subsequent on-study SRE by type of SRE.
The MS reported the distribution of first and subsequent on-study SREs by type of SRE in the denosumab RCT (study 103) (Table 25). As for first on-study SRE by type, the distribution of type of SRE was similar across the treatment groups, with radiation to bone and pathological fracture again the most commonly occurring.
| SRE | Denosumab (n = 950 randomised) | Zoledronic acid (n = 951 randomised) |
|---|---|---|
| Number of events (%) | Number of events (%) | |
| Total confirmed events | 494 (100%) | 584 (100%) |
| Radiation to bone | AiC information has been removed | AiC information has been removed |
| Pathological fracture | AiC information has been removed | AiC information has been removed |
| SCC | AiC information has been removed | AiC information has been removed |
| Surgery to bone | AiC information has been removed | AiC information has been removed |
Prior history of skeletal-related events
The MS reported risk of developing first and subsequent on-study SREs by history of SRE for study 103 (Table 26). (Academic-in-confidence information has been removed.) Covariate analysis as presented in the manufacturer's table showed that patients with a history of SRE had an increased risk (academic-in confidence information has been removed) compared with those without a SRE history [although in the text the manufacturer reported the covariate effect (academic-in confidence information has been removed) and increased risk (academic-in confidence information has been removed)].
| SRE history | Denosumab | Zoledronic acid |
|---|---|---|
| Overall | ||
| Number | 950 | 951 |
| Rate ratio (95% CI) | 0.82 (0.71 to 0.94) | |
| p-value | 0.0044 | |
| No prior SRE | ||
| Number | 718 | 720 |
| Rate ratio (95% CI) | 0.79 (0.67 to 0.94) | |
| p-value | 0.0067 | |
| Prior SRE | ||
| Number | 232 | 231 |
| Rate ratio (95% CI) | 0.88 (0.68 to 1.13) | |
| p-value | 0.3081 | |
| Covariate effect | ||
| Point estimate (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
Saad and colleagues reported that among the 144 patients with a SRE before study entry, zoledronic acid significantly reduced the risk of SREs by 40% compared with placebo (RR 0.60; p = 0.028), and among the 277 patients without a SRE before study entry, zoledronic acid significantly reduced the overall risk of SREs by 33% compared with placebo (RR 0.67; p = 0.027). 119
Skeletal morbidity rate
The SMR is defined as the ratio of the number of SREs per patient divided by the patient's time at risk. Information on this outcome for the denosumab RCT was reported in the MS, which stated that for the SMR calculations a 21-day event window was used for counting on-study SREs, so that any event occurring within 21 days of a previous event was not counted as a separate on-study SRE.
The MS for study 103 compared the annual SMR with denosumab (academic-in-confidence information has been removed) with zoledronic acid [0.79 vs 0.83 (academic-in-confidence information has been removed)]. Saad and colleagues117 reported that the mean SMR for all SREs combined and for each individual type of SRE was lower for patients who received zoledronic acid than for those who received placebo.
Skeletal-related event by type
The MS did not report SMR by type of SRE.
Table 27 shows the SMR by type of SRE for the study by Saad and colleagues. 117
| SRE | Zoledronic acid (n = 214) | Placebo (n = 208) | p-value |
|---|---|---|---|
| All SREs | 0.80 (0.57 to 1.03) | 1.49 (1.03 to 1.94) | 0.006 |
| Pathological fractures | 0.21 (0.11 to 0.31) | 0.45 (0.27 to 0.63) | 0.009 |
| Radiation therapy to bone | 0.44 (0.27 to 0.60) | 0.88 (0.48 to 1.28) | 0.084 |
| Surgery to bone | 0.03 (0.00 to 0.07) | 0.06 (0.01 to 0.11) | 0.509 |
| SCC | 0.14 (0.00 to 0.28) | 0.23 (0.04 to 0.42) | 0.247 |
Prior history of skeletal-related event
SMR by history of SRE was not reported for the denosumab RCT.
Saad and colleagues reported that the mean on-study SRE per year, for those patients with a previous SRE (n = 144) was 0.8 for zoledronic acid compared with 2.3 for placebo (p = 0.036), whereas for those with no previous SRE it was 0.77 for zoledronic acid and 0.98 for placebo (p = 0.06). 119
Incidence of skeletal-related events
In the denosumab RCT (study 103) 780 SREs occurred in 1045 patient-years in the denosumab arm and 943 occurred in 996 patient-years in the zoledronic acid arm, with the number-needed-to-treat analysis showing that compared with zoledronic acid, treatment of five patients with denosumab would prevent an additional SRE (first or subsequent) per year. 124
The MS reported an annualised SRE rate based on the number of SREs observed in each treatment arm divided by the number of patient-years for each treatment arm and reported this outcome both with and without a 21-day event window.
Table 28 shows the annualised SRE rate both with and without the 21-day window for study 103. The MS reported that the primary analysis of annualised SRE rates was based on all SREs reported in each arm of the study (calculated without a 21-day window). Subsequently, a post-hoc analysis of the annualised SRE rate applying the trial-defined 21-day window for SREs was conducted. Both analyses show that the annualised SRE rate was lower in patients receiving denosumab compared with those receiving zoledronic acid.
| Annualised SRE rate per patient | Denosumab (n = 950) | Zoledronic acid (n = 951) |
|---|---|---|
| Subject years | AiC information has been removed | AiC information has been removed |
| Without 21-day window | ||
| Number of events | AiC information has been removed | AiC information has been removed |
| Annualised rate | AiC information has been removed | AiC information has been removed |
| With 21-day window | ||
| Number of events | AiC information has been removed | AiC information has been removed |
| Annualised rate | AiC information has been removed | AiC information has been removed |
In the study by Saad and colleagues117 statistically significantly fewer patients in the zoledronic acid group compared with the placebo group experienced at least one SRE [33.2% (71/214) vs 44.2% (92/208), respectively; p = 0.021].
Skeletal-related event by type
Incidence of SREs by type of SRE was not reported for the denosumab RCT.
Table 29 shows the proportions of patients with different types of SRE for the study by Saad and colleagues. 117 More SREs occurred in the placebo group overall. The most frequently occurring SRE in both groups was radiation therapy to bone, followed by pathological fractures.
| SRE | Zoledronic acid (n = 214) | Placebo (n = 208) | p-value |
|---|---|---|---|
| All SREs | 71 (33.2) | 92 (44.2) | 0.021 |
| Pathological fractures | 28 (13.1) | 46 (22.1) | 0.015 |
| Radiation therapy to bone | 49 (22.9) | 61 (29.3) | 0.136 |
| Surgery to bone | 5 (2.3) | 7 (3.4) | 0.514 |
| SCC | 9 (4.2) | 14 (6.7) | 0.256 |
Prior history of skeletal-related events
Neither study reported incidence of SRE by history of SREs. However, Saad and colleagues117 reported that for those with a previous SRE (n = 144), the proportion of patients with one or more SRE while on study was 41% (27/66) for zoledronic acid compared with 51% (40/78) for placebo (p = 0.215), whereas for those with no previous SRE (n = 277) this was 37% (54/147) for zoledronic acid compared with 47% (61/130) for placebo (p = 0.087). 119
Prevention of hypercalcaemia
This was discussed in study 103, (academic-in-confidence information has been removed) (CSR 103).
Saad and colleagues117 did not report hypercalcaemia.
Overall survival
In the denosumab RCT, median overall survival was similar between the groups, with a median overall survival of 19.4 months (95% CI 18.1 to 21.7 months) for the denosumab group compared with 19.8 months (95% CI 18.1 to 20.9 months) for the zoledronic acid group (HR 1.03; 95% CI 0.91 to 1.17; p = 0.65). 29
In the study by Saad and colleagues,117 median survival was 546 days (around 18.2 months) for the zoledronic acid group and 464 days (around 15.5 months) for the placebo group (p = 0.091).
Prior history of skeletal-related events
Neither study reported overall survival by history of SREs.
Pain
The MS stated that the denosumab RCT used the BPI-SF, which captures information on the intensity of pain (pain severity) and the degree to which pain interferes with function (pain interference) in patients with cancer. The BPI-SF scores range from 0 to 10, with a higher score indicating more severe pain (0 = no pain, 1–4 = mild pain, 5–6 = moderate pain and 7–10 = severe pain). Pain analyses included evaluation of changes from baseline in BPI-SF worst pain score; evaluations of time to pain worsening, time to moderate or severe pain, or time to pain improvement; and the proportions of patients meeting these criteria.
The MS reported that denosumab delayed the time to development of moderate or severe pain in patients with no or mild pain at baseline by around 1 month compared with zoledronic acid (median 5.8 months vs 4.9 months) although the difference was not statistically significant (HR 0.89; 95% CI 0.77 to 1.04; p = 0.1416) (MS). Denosumab also significantly decreased the proportion of patients with no/mild pain at base who progressed to moderate or severe pain [relative decrease (academic-in-confidence information has been removed) over 73 weeks]. The median time to worsening pain (≥ 2-point increase from baseline in BPI-SF worst pain score) was similar in the denosumab and zoledronic acid groups. (Academic-in-confidence information has been removed.) There was no significant difference in time to pain improvement (≥ 2-point decrease from baseline) between denosumab and zoledronic acid (academic-in-confidence information has been removed) (MS).
There was no statistically significant difference at study end point or any study time point (19 study time points) in the use of strong analgesics.
The study by Saad and colleagues117 also used the BPI instrument, with the pain score a composite of four pain scores (worst pain, least pain, average pain of the last 7 days, and pain right now), and was the primary efficacy variable for the quality-of-life assessments. Saad and colleagues117 reported that the mean pain scores increased from baseline in each group at every 3-month interval, except at 3 months, when the zoledronic acid group exhibited a slight decrease from baseline. The mean increase from baseline in pain score at 15 months was 0.58 (95% CI 0.29 to 0.87) in the zoledronic acid group compared with 0.88 (95% CI 0.61 to 1.15) in the placebo group (p = 0.134). Saad and colleagues117 also reported that fewer patients in the zoledronic acid group experienced bone pain than in the placebo group [51% (108/214) vs 61% (127/208), respectively].
Health-related quality of life
Functional Assessment of Cancer Therapy
The Functional Assessment of Cancer Therapy – Prostate (FACT-P) questionnaire consists of the FACT-G questionnaire plus additional questions specific to prostate cancer. For each component of the FACT-P (FACT-G total score, FACT-P total score, physical well-being domain, functional well-being domain, and TOI: a composite of the functional well-being domain, physical well-being domain, and the prostate cancer subscale), a higher score indicates better HRQoL.
Table 30 shows the change in FACT scores from baseline to week 73. (Academic-in-confidence information has been removed) (CSR 103).
| Scale | Denosumab (n = 950) | Zoledronic acid (n = 951) | ||
|---|---|---|---|---|
| Baseline mean (SD) | Change from baseline to week 73 | Baseline mean (SD) | Change from baseline to week 73 | |
| FACT-B/-P total score | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Physical well-being | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Functional well-being | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| TOI | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| FACT-G total score | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Saad and colleagues117 reported that the total FACT-G score decreased from baseline to the last measurement, with no statistically significant differences between the zoledronic acid and placebo groups.
European Quality of Life-5 Dimensions
For both components of EQ-5D (the health index and the VAS), a higher score indicates a more preferred health status. For the health index questions of the EQ-5D, a three-level response was used to assess quality of life. (Academic-in-confidence information has been removed) (CSR 103).
Saad and colleagues117 reported that the EQ-5D scores decreased from baseline to the last measurement, with no statistically significant differences between the zoledronic acid and placebo groups.
Adverse events related to treatment
Data relating to adverse events were collected primarily from the included RCTs and supplementary data were included from observational studies where available.
Hypocalcaemia
The MS reported that hypocalcaemia events were mainly non-serious and transient and either resolved spontaneously or with calcium supplementation (MS). More hypocalcaemia adverse events occurred in the denosumab group than in the zoledronic acid group [13% (121/943) vs 6% (55/945), respectively], a statistically significant difference (p < 0.0001). 29 Calcium decreases of grade 3 or higher occurred in 48 patients (5%) receiving denosumab and 13 patients (1%) receiving zoledronic acid.
In the study by Saad and colleagues,117 1.9% (4/214) of patients in the zoledronic acid group experienced grades 3 or 4 hypocalcaemia compared with none in the placebo group.
Osteonecrosis of the jaw
In the denosumab RCT, more patients in the denosumab group than in the zoledronic acid group experienced ONJ events [2% (22/943) vs 1% (12/945)], although the difference was not statistically significant (p < 0.09). 29 Of those, 17 (77%) on denosumab and 10 (83%) on zoledronic acid had a history of tooth extraction, poor oral hygiene, or use of a dental appliance. Fizazi and colleagues29 reported that, by April 2010, 10 patients (45%) on denosumab had received limited surgical treatment for ONJ (debridement, sequestrectomy, or curettage) and two (9%) had undergone bone resection, whereas three patients (25%) on zoledronic acid had received limited surgery and one (8%) had undergone bone resection. They also reported that, overall, resolution of ONJ, as defined by mucosal coverage, was recorded in four patients (18%) on denosumab and one patient (8%) on zoledronic acid.
Saad and colleagues117 did not report ONJ.
The proportion of patients experiencing ONJ was slightly lower than in observational studies (see Appendix 11). Walter and colleagues160 retrospectively studied patients prescribed BPs and found that 18.6% of patients experienced ONJ (time at risk not reported). However, three other observational studies reported a cumulative incidence of 2.2–6.5% over 12–15 months. 62,137,144
Renal toxicity
In the denosumab RCT, a similar rate of adverse events potentially associated with renal impairment occurred in the denosumab and zoledronic acid groups [15% (139/943) vs 16% (153/945), respectively]. 29 The rates of SAEs associated with renal impairment were also similar [5.9% (56/943) vs 5.6% (53/945) respectively] (MS). It should be noted that, as zoledronic acid is contraindicated in patients with poor renal function, such patients were excluded from the trial. The manufacturer stated that the incidence of renal toxicity observed in the denosumab group represented a background rate for patients with advanced cancer, as such patients were predisposed to renal dysfunction, for example owing to the use of nephrotoxic drugs (MS).
Saad and colleagues117 reported that renal function deterioration occurred in 15.2% of patients who received zoledronic acid and 11.5% of those receiving placebo. They stated that Kaplan–Meier estimates of time to first renal function deterioration indicated a comparable RR between the groups, so that compared with the placebo group the zoledronic acid group had a RR of 1.07 (95% CI 0.46 to 2.47; p = 0.882). 117
Observational studies of zoledronic acid reported a higher incidence of renal toxicity. Oh and colleagues152 found that 23.8% of patients experienced renal toxicity over 10 months while Bonomi and colleagues137 reported a figure of 6.5%. However, these studies had a broader definition of renal toxicity than the RCTs.
Acute-phase reactions
In the denosumab RCT, during the first 3 days of treatment, fewer patients in the denosumab group than in the zoledronic acid group experienced symptoms associated with acute-phase reactions [8% (79/943) vs 18% (168/945), respectively]. 29
Saad and colleagues117 did not report this outcome.
Other adverse events
Table 31 shows, for the denosumab RCT, rates of a number of selected other adverse events, including those leading to treatment discontinuation, CTCAE grade 3 or 4 events, serious and fatal adverse events. The rates for both groups were broadly similar.
| Adverse event | Denosumab (n = 943) | Zoledronic acid (n = 945) | p-value |
|---|---|---|---|
| AE leading to treatment discontinuation | 164 (17%) | 138 (15%) | 0.10 |
| CTCAE grade 3 or 4 AE | 678 (72%) | 628 (66%) | 0.01 |
| Serious AE | 594 (63%) | 568 (60%) | 0.20 |
| Fatal AE | 283 (30%) | 276 (29%) | 0.72 |
Saad and colleagues117 reported that similar proportions of patients who received zoledronic acid (9.8%) and placebo (10.1%) discontinued the study drug because of a SAE.
In the denosumab group, 337 patients (36%) developed anaemia compared with 341 (36%) in the zoledronic acid arm. In the study by Saad and colleagues, a higher proportion of patients in the zoledronic acid group than in the placebo group experienced anaemia (26.6% vs 17.8%). 117 The clinical significance of this is unclear.
For details of all other adverse events extracted from the RCTs meeting the review's inclusion criteria and also adverse events extracted from a number of observational studies identified, see Appendix 11.
Network meta-analysis
The AG and manufacturer performed a NMA for prostate cancer. Both NMAs included only two studies. 29,117 The definition of SRE differed between the studies. Saad and colleagues117 included change in antineoplastic medications. Therefore, the results should be interpreted with caution. Table 32 shows the differences between the AG's NMA and MS's NMA.
| Comparison | Time to first SRE | Risk of first and subsequent SRE | SMR | Proportion of patients with on-study SRE | |
|---|---|---|---|---|---|
| All patients | Subgroup of patients with SRE at baseline | ||||
| Denosumab vs zoledronic acid | AG + MS | AG + MS | AG + MS | AG | AG |
| Denosumab vs placebo | AG + MS | AG + MS | AG + MS | AG | AG |
| Zoledronic acid vs placebo | AG + MS | AG + MS | AG + MS | AG | AG |
Time to first skeletal-related event
Results from the NMAs for time to first on-study SRE are shown in Table 33.
| Comparison | AG's NMA HR (95% CI) | MS's NMAHR (95% CI) |
|---|---|---|
| Denosumab vs zoledronic acid | 0.82 (0.71 to 0.95) | AiC information has been removed |
| Denosumab vs placebo | 0.56 (0.40 to 0.77) | AiC information has been removed |
| Zoledronic acid vs placebo | 0.68 (0.50 to 0.91) | AiC information has been removed |
The NMA results from both the AG and the MS show that time to first SRE favoured denosumab compared with zoledronic acid or placebo. The AG's NMA found these differences to be statistically significant in favour of denosumab, (academic-in-confidence information has been removed).
Risk of first and subsequent skeletal-related events
The NMA results for risk of developing first and subsequent on-study SREs are shown in Table 34.
| Comparison | AG's NMA RR (95% CI) | MS's NMARR (95% CI) |
|---|---|---|
| Denosumab vs zoledronic acid | 0.82 (0.71 to 0.94) | AiC information has been removed |
| Denosumab vs placebo | 0.53 (0.39 to 0.72) | AiC information has been removed |
| Zoledronic acid vs placebo | 0.64 (0.48 to 0.85) | AiC information has been removed |
The NMA results show the risk of developing first and subsequent SREs favoured denosumab compared with zoledronic acid or placebo. The AG's NMA found these differences to be statistically significant in favour of denosumab, (academic-in-confidence information has been removed).
Skeletal morbidity rate
The NMA results for SMR are shown in Table 35.
| Comparison | AG's NMA RR (95% CI) | MS's NMARR (95% CI) |
|---|---|---|
| Denosumab vs zoledronic acid | 0.95 (0.46 to 1.47) | AiC information has been removed |
| Denosumab vs placebo | 0.52 (0.07 to 0.82) | AiC information has been removed |
| Zoledronic acid vs placebo | 0.54 (0.11 to 0.83) | AiC information has been removed |
| Comparison | Any SRE | Pathological fracture | Radiation to bone | Surgery to bone | SCC | No prior SRE | Prior SRE |
|---|---|---|---|---|---|---|---|
| Denosumab vs zoledronic acid | 0.81 (0.07 to 10.40) | 0.91 (0.07 to 12.06) | 0.79 (0.06 to 10.16) | 0.58 (0.04 to 7.34) | 0.73 (0.06 to 9.65) | 0.82 (0.06 to 10.01) | 0.81 (0.07 to 10.27) |
| Denosumab vs placebo | 0.53 (0.01 to 18.80) | 0.48 (0.01 to 18.46) | 0.57 (0.02 to 19.20) | 0.39 (0.01 to 15.95) | 0.44 (0.01 to 16.32) | 0.53 (0.01 to 19.50) | 0.53 (0.01 to 19.57) |
| Zoledronic acid vs placebo | 0.64 (0.05 to 7.51) | 0.53 (0.04 to 7.06) | 0.72 (0.06 to 8.87) | 0.68 (0.05 to 10.20) | 0.60 (0.05 to 7.80) | 0.65 (0.05 to 8.72) | 0.65 (0.05 to 8.29) |
The AG's NMA found a non-significant difference in favour of denosumab compared with zoledronic acid and a significant difference in favour of denosumab compared with placebo, whereas there was a statistically significant difference in favour of zoledronic acid compared with placebo. (Academic-in-confidence information has been removed.)
Proportion of patients with on-study skeletal-related events
The AG compared the proportion of patients with an on-study SRE for individual SREs and with a subgroup with a SRE history. This outcome does not differentiate between time on study and, therefore, the results should be interpreted with caution. However, it provides an opportunity to indirectly compare SRE types and SRE history.
Denosumab non-significantly favoured zoledronic acid and placebo throughout. Owing to the small numbers, however, these results should not be used to compare the relative effectiveness of denosumab for preventing individual SRE types.
Summary
No studies were identified comparing denosumab with the primary comparator BSC. One study29 compared denosumab with zoledronic acid. Another study,117 comparing zoledronic acid with placebo, contributed data to the indirect comparisons of denosumab versus BSC undertaken by both the AG and the MS and therefore was also reported in this chapter. In terms of generalisability, both studies were multicentre, international good quality RCTs. The Fizazi study29 was the larger, randomising 1901 patients compared with 422 for the Saad study. 117 However, in the Fizazi study29 few (academic-in-confidence information has been removed) patients were from the UK. All participants in this study were men aged ≥ 18 years with life expectancy ≥ 6 months, confirmed prostate cancer and at least one bone metastasis. The exclusion criteria included patients with severe renal impairment or current or previous BP treatment for bone metastases, or current or previous ONJ. The study was powered to detect both non-inferiority and superiority with respect to time to first, and time to first and subsequent, on-study SRE.
The study by Fizazi and colleagues29 reported a statistically significant difference in favour of denosumab compared with zoledronic acid in both the median time to first on-study SRE (20.7 months vs 17.1 months), most of which were radiation to bone or pathological fractures, and the risk of developing first and subsequent on-study SREs. The annual SMR was also significantly lower in the denosumab group, as was the annualised SRE rate.
In the study by Saad and colleagues117 there was a statistically significant difference in time to first on-study SRE in favour of zoledronic acid compared with placebo (488 days vs 321 days), a lower SMR for the zoledronic acid group and a statistically significant lower incidence in the numbers of patients who experienced at least one SRE in the zoledronic acid group (33.2%) compared with the placebo group (44.2%).
The denosumab RCT reported on hypercalcaemia. (Academic-in-confidence information has been removed.) Saad and colleagues117 did not report this outcome.
In the denosumab study overall survival was similar between the groups (19.4 months for the denosumab group compared with 19.8 months for the zoledronic acid group). Saad and colleagues118 reported a median survival of 546 days (around 18.2 months) for the zoledronic acid group and 464 days (around 15.5 months) for the placebo group.
Denosumab delayed the time to development of moderate or severe pain by around 1 month compared with zoledronic acid (median 5.8 vs 4.9 months). Saad and colleagues117 reported that the mean increase from baseline in pain score at 15 months was 0.58 (95% CI 0.29 to 0.87) for the zoledronic acid group compared with 0.88 (95% CI 0.61 to 1.15) for the placebo group.
In terms of quality of life, for FACT-G, (academic-in-confidence information has been removed); Saad and colleagues117 reported that the total FACT-G score and the EQ-5D scores decreased from baseline to the last measurement, with no statistically significant differences between the zoledronic acid and placebo groups.
In terms of adverse events, there were statistically significantly more hypocalcaemia events in the denosumab group compared with the zoledronic acid group (13% vs 6%), slightly more ONJ events (2% vs 1%) and slightly fewer adverse events potentially associated with renal impairment (15% vs 16%), while fewer patients in the denosumab group experienced acute-phase reactions (8% vs 18%). The rates for adverse events leading to treatment discontinuation, CTCAE grade 3 or 4, or serious or fatal adverse events were broadly similar between the denosumab and zoledronic acid groups.
In the study by Saad and colleagues,117 2% of patients in the zoledronic acid group experienced grade 3 or 4 hypocalcaemia compared with none in the placebo group, and renal function deterioration occurred in 15.2% of patients who received zoledronic acid compared with 11.5% of those receiving placebo (ONJ and acute-phase reactions were not reported). Similar proportions of patients who received zoledronic acid (9.8%) and placebo (10.1%) discontinued the study drug because of a SAE.
The AG's NMA reported statistically significant differences in favour of denosumab compared with placebo for time to first on-study SRE, risk of developing first and subsequent SREs and SMR (academic-in-confidence information has been removed).
Chapter 6 Results: non-small cell lung cancer
This chapter reports NSCLC alone. As NSCLC alone, OSTs excluding NSCLC and OSTs including NSCLC were reported by the same two studies,30,130 information on the characteristics of the included studies and quality of the included studies is reported here and not repeated in Chapter 7 (on OSTs excluding NSCLC) or Chapter 8 (on OSTs including NSCLC).
Quantity of research available
See Chapter 4, Quantity of research available.
Number and type of studies included
The flow diagram outlining the screening process for the overall review is shown in Figure 3.
Number and type of studies excluded
See Chapter 4 for information on studies that were excluded from the review and Appendix 5 for a list of these studies along with the reasons for their exclusion. These studies were excluded because they failed to meet one or more of the inclusion criteria in terms of types of study, participants, intervention or outcomes reported.
Characteristics of the included studies
Two trials reported on bone metastases secondary to OSTs (excluding breast cancer and prostate cancer) and were included for the indirect comparison. 30,130 Both trials included a subgroup of patients with bone metastases secondary to NSCLC and reported outcomes for that group of patients. Appendix 8 shows the characteristics of the included studies. Table 37 shows summary information for the two studies that provided direct evidence for denosumab or were included in the NMA.
| Baseline characteristic | Henry 201130 | Rosen 2003b130 | ||
|---|---|---|---|---|
| Zoledronic acid | Denosumab | Zoledronic acid | Placebo | |
| Randomised | 890 | 886 | 257 | 250 |
| Age (years), median (range) | 61 (22–87) | 60 (18–89) | 64 | 64 |
| Sex (% male) | 552 (62%) | 588 (66%) | 158 (61%) | 159 (64%) |
| ECOG status 1 or below | 728 (82%) | 748 (84%) | 211 (83%) | 215 (87%) |
| Primary tumour type | ||||
| NSCLC | 352 (40%) | 350 (39%) | 124 (49%) | 120 (49%) |
| Multiple myeloma | 93 (10%) | 87 (10%) | NR | NR |
| Other | 455 (50%) | 449 (51%) | 130 (51%) | 130 (51%) |
| Time from diagnosis of bone metastasis (months), median (range) | 2 (0–130) | 2 (0–152) | 3.8 | 2.5 |
| Previous SREs | 446 (50%) | 440 (50%) | 166 (65%) | 179 (73%) |
Data from the trial comparing denosumab with zoledronic acid were derived from three sources: (1) the peer-reviewed publication by Henry and colleagues,30 which included multiple myeloma, but also presented certain outcomes for subgroups; (2) the MS, which included a post-hoc analysis excluding 179 patients with multiple myeloma (n = 800 denosumab, n = 797 zoledronic acid included for analysis); and (3) CSR 244 including multiple myeloma, which was included with the MS.
The study by Henry and colleagues30 was undertaken between June 2006 and May 2008 and enrolled patients aged ≥ 18 years with confirmed solid tumours (except breast and prostate) or multiple myeloma and at least one bone metastasis or osteolytic lesion (in the case of multiple myeloma), from 321 centres worldwide. However, few (academic-in-confidence information has been removed) patients were from the UK (MS). Exclusion criteria included creatinine clearance < 30 ml/minute, prior treatment with intravenous BPs, planned radiation or surgery to bone, and unhealed dental/oral surgery. Patients received 120 mg denosumab subcutaneously (plus intravenous placebo) or 4 mg zoledronic acid intravenously (adjusted for renal impairment plus subcutaneous placebo) every 4 weeks. Before the randomisation process, patients were stratified by tumour type that included NSCLC, myeloma, or other, previous SRE and systemic anticancer therapy at enrolment. The overall study was powered to detect non-inferiority and superiority for time to first on-study SRE (primary outcome) and risk of first and subsequent on-study SRE. Study duration was median 7 months and length of follow-up was 34 months. The study was funded by Amgen.
The study by Rosen and colleagues130 enrolled patients aged ≥ 18 years with osteolytic, osteoblastic, or mixed bone metastases from solid tumours (excluding breast and prostate cancer). Patients received 4 mg or 8 mg zoledronic acid intravenously or placebo every 3 weeks for 9 months. Before the randomisation process, patients were stratified by tumour type that included NSCLC or OST. The duration of the study was 9 months. The primary outcome was the proportion of patients with at least one SRE. During the trial there was a study protocol amendment. Patients randomised to the 8 mg zoledronic acid arm were changed to 4 mg because of renal toxicity concerns.
The study by Henry and colleagues30 included 40% of patients with NSCLC, 10% with multiple myeloma and 50% with other tumours where half of the included participants belonged to ECOG status 1. Similarly, the study by Rosen and colleagues130 included 49% of patients with NSCLC and the rest with OSTs including SCLC (7–8%), renal cell carcinoma (8–11%), unknown primary (7%), head and neck (2%), thyroid (1–2%) and other (24%) where more than 80% of patients had ECOG status 1 or below.
In the study by Henry and colleagues30 reporting on denosumab, 87% to 96% received antineoplastic or anticancer treatment. However, none of the patients had received previous intravenous BP treatment. Fifty per cent of the included participants had had a previous SRE at baseline while 40% and 46% had received radiotherapy and surgery, respectively. More than 80% had received chemotherapy in the trial by Rosen and colleagues130 reporting zoledronic acid and 3% had previously received BP treatment, while 68% had had a previous SRE at baseline (65% in zoledronic acid and 73% in placebo).
The definition of SRE in both trials included pathological fracture, radiation or surgery to bone, and SCC. In addition, Rosen and colleagues130 included hypercalcaemia in the definition of SRE for secondary efficacy analysis. A subsequent SRE was defined as an event occurring more than 21 days after the previous SRE in both trials by Henry and colleagues30 and Rosen and colleagues. 130
The characteristics of the subgroup of patients with bone metastases from NSCLC was reported in the manufacturer's CSR 244 of the denosumab RCT and are shown in Table 38.
| Baseline characteristic | Denosumab (n= 350) | Zoledronic acid (n= 352) |
|---|---|---|
| Mean age (SD) | AiC information has been removed | AiC information has been removed |
| Proportion female | AiC information has been removed | AiC information has been removed |
| Time from diagnosis to randomisation, median months (range) | ||
| Of lung cancer | AiC information has been removed | AiC information has been removed |
| Of bone metastases | AiC information has been removed | AiC information has been removed |
| Visceral metastases | AiC information has been removed | AiC information has been removed |
| ECOG status | ||
| 0 | AiC information has been removed | AiC information has been removed |
| 1 | AiC information has been removed | AiC information has been removed |
| 2 | AiC information has been removed | AiC information has been removed |
Quality of the included studies
Table 39 shows the results of the risk of bias assessment for the studies by Henry and colleagues30 and Rosen and colleagues. 130
| Criteria | Henry 201130 | Rosen 2003b131 |
|---|---|---|
| Adequate sequence generation | Yes | Unclear |
| Adequate allocation concealment | Yes | Unclear |
| Blinding | Yes | Yes |
| Incomplete outcome data addressed | Yes | No |
| Free of selective reporting | Yes | Yes |
The study by Henry and colleagues30 was of good quality with low risk of bias as assessed against the criteria in Table 39. In the study by Rosen and colleagues130 it was unclear whether or not sequence generation and allocation concealment were adequate. The study by Henry and colleagues30 used an interactive voice response system to randomly assign patients (1 : 1 ratio) to treatment groups. An individual independent of the study team prepared the random assignment schedule. The study was double blind and study dose and outcomes were blinded throughout the primary analysis. There was adequate description of withdrawals and losses to follow-up and all of the prespecified outcomes were reported. Both primary and secondary efficacy end points included all randomised patients (intention-to-treat analysis).
The study by Rosen and colleagues130 did not state the randomisation process and mentioned only that the participants were stratified by tumour type before randomisation. The study was double blind, patients lost to follow-up were described and all of the prespecified outcomes were reported; however, not all secondary outcomes were fully reported.
Assessment of effectiveness
Time to first on-study skeletal-related event
Henry and colleagues30 reported a HR of 0.84 (95% CI 0.64 to 1.10; p = 0.20) for denosumab compared with zoledronic acid for time to first on-study SRE for NSCLC, indicating a non-significant risk reduction for denosumab compared with zoledronic acid. (Academic-in-confidence information has been removed) (CSR 244). The study by Rosen and colleagues130 reported longer median time to first on-study SRE in the zoledronic acid group compared with the placebo group (171 vs 151 days); however, the difference was not significant (p = 0.188).
Neither study reported SRE by type or prior history of SRE for this outcome.
Risk of first and subsequent on-study skeletal-related event
The study by Henry and colleagues30 did not report the risk of developing multiple SREs (first and subsequent on-study SREs) for the NSCLC subgroup. (Academic-in-confidence information has been removed) (CSR 244). In the study by Rosen and colleagues,130 a 27% risk reduction of multiple SREs by the use of zoledronic acid was reported relative to placebo (HR 0.73; p = 0.061). A similar risk reduction was reported when HCM was included in the analysis (HR 0.71; p = 0.036).
Neither study reported SRE by type or prior history of SRE for this outcome.
Skeletal morbidity rate
Neither study reported SMR for the NSCLC subgroup.
Incidence of skeletal-related events
(Academic-in-confidence information has been removed) (CSR 244). The study by Rosen and colleagues130 reported that in the NSCLC group of patients, a similar proportion of patients experienced SREs in the zoledronic acid group and in the placebo group (42% vs 45%; p = 0.007).
Neither study reported SRE by type or prior history of SRE for this outcome.
Prevention of hypercalcaemia
Neither study reported hypercalcaemia for the NSCLC subgroup.
Overall survival
An ad hoc analysis for overall survival in a trial by Henry and colleagues30 reported that denosumab significantly improved overall survival relative to zoledronic acid by 21% (HR 0.79; 95% CI 0.65 to 0.95).
The study by Rosen and colleagues130 did not report this outcome.
Prior history of skeletal-related events
Neither study reported overall survival by prior history of SRE for those with NSCLC.
Pain
Neither study reported this outcome for those with NSCLC.
Health-related quality of life
Neither study reported this outcome for those with NSCLC.
Adverse events related to treatment
There were no published or unpublished data on adverse events including hypocalcaemia, ONJ, renal toxicity, acute-phase reactions or other adverse events reported separately for those with NSCLC. See Chapter 8, Adverse events related to treatment for adverse events reported for all OSTs including NSCLC.
Network meta-analysis
The AG group performed a NMA of NSCLC alone, using subgroups from the Henry and Rosen studies. 30,130 The manufacturer did not perform this analysis. Three outcomes were included: time to first on-study SRE (Table 40), risk of first and subsequent SREs (Table 41) and the proportion of patients with an on-study SRE (Table 42).
| Comparison | AG's NMA, HR (95% CI) |
|---|---|
| Denosumab vs zoledronic acid | 0.84 (0.64 to 1.10) |
| Denosumab vs placebo | 0.68 (0.45 to 1.03) |
| Zoledronic acid vs placebo | 0.81 (0.59 to 1.11) |
| Comparison | AG's NMA, RR (95% CI) |
|---|---|
| Denosumab vs zoledronic acid | 0.87 (0.68 to 1.12) |
| Denosumab vs placebo | 0.63 (0.42 to 0.97) |
| Zoledronic acid vs placebo | 0.73 (0.52 to 1.02) |
| Comparison | AG's NMA, OR (95% CI) |
|---|---|
| Denosumab vs zoledronic acid | 0.96 (0.08 to 11.7) |
| Denosumab vs placebo | 0.83 (0.02 to 30.6) |
| Zoledronic acid vs placebo | 0.87 (0.07 to 11.2) |
Time to first on-study skeletal-related event
The results for time to first on-study SRE are shown in Table 40. The NMA results favoured denosumab compared with zoledronic acid or placebo for time to first on-study SRE but were not statistically significant.
Risk of first and subsequent on-study skeletal-related events
The results for the risk of developing first and subsequent on-study SREs are presented below in Table 41. The NMA results favoured denosumab compared with zoledronic acid or placebo for risk of developing first and subsequent SREs, although only the result compared with placebo was statistically significant.
Proportion of patients with on-study skeletal-related events
Results for the proportion of patients with an on-study SRE are shown below in Table 42. The NMA results favoured denosumab compared with zoledronic acid or placebo for the proportion of patients with an on-study SRE but were not statistically significant. These results should be interpreted with additional caution because this outcome does not differentiate between lengths of study, thereby adding to the uncertainty.
Summary
Only one study, by Henry and colleagues,30 was identified that compared denosumab with zoledronic acid. Another study comparing zoledronic acid with placebo, by Rosen and colleagues,130 met the inclusion criteria for the NMA and so is reported in this chapter. The study by Henry and colleagues30 was a good-quality RCT with low risk of bias, whereas the study by Rosen and colleagues130 did not report sufficient information on randomisation. In terms of generalisability, the Henry study30 was multicentre and international while the Rosen study130 was multicentre. However, in these studies patients with NSCLC did not form the whole patient population but rather were a subgroup of a population that included patients with bone metastases from a range of OSTs, excluding breast and prostate cancer. The studies reported outcomes for all OSTs grouped together, and separately for NSCLC – approximately 40% (n = 702) of patients in the Henry study30 and 50% (n = 244) in the Rosen study130 – and OSTs excluding NSCLC. The proportion of NSCLC patients from the UK was not reported. In both studies the exclusion criteria included severe renal impairment or prior treatment with BPs. Study duration was longer in the Henry trial30 (primary analysis at 34 months) compared with the Rosen trial130 (9 months). The Henry study30 was not powered to detect either non-inferiority or superiority for time to first on-study SRE or risk of first and subsequent on-study SREs for the NSCLC subgroup alone.
For those with bone metastases from NSCLC, a non-significant difference favouring denosumab over zoledronic acid in time to first on-study SRE was reported in the study by Henry and colleagues. 30 (Academic-in-confidence information has been removed) (CSR 244). No data were reported on SMR, incidence of SRE, hypercalcaemia, pain or quality of life. The study by Henry and colleagues30 reported a statistically significant difference in favour of denosumab for overall survival (21% risk reduction with denosumab) for patients with NSCLC.
The study by Rosen and colleagues130 reported a non-significant difference favouring zoledronic acid over placebo in time to first SRE and time to first and subsequent SREs. A similar proportion of SREs were reported in the two groups. No data were reported for SMR, hypercalcaemia, overall survival, pain or quality of life. Adverse events were not reported separately for the subgroup of patients with NSCLC.
In the AG's NMA, there was a statistically significant difference in favour of denosumab compared with placebo for risk of developing first and subsequent SREs, while the direction of effect for SMR favoured denosumab but was not statistically significant.
Chapter 7 Results: other solid tumours (excluding non-small cell lung cancer)
This chapter reports outcomes for OSTs excluding NSCLC, breast cancer, prostate cancer or multiple myeloma.
Quantity of research available
See Chapter 4, Quantity of research available.
Number and type of studies included
The flow diagram outlining the screening process for the overall review is given in Figure 3.
Number and type of studies excluded
For information on studies that were excluded from the review see Chapter 4, Number and type of studies excluded, and see Appendix 5 for a list of these studies along with the reasons for their exclusion. These studies were excluded because they failed to meet one or more of the inclusion criteria in terms of types of study, participants, interventions or outcomes reported.
Characteristics of the included studies
As these were the same trials that reported the subgroup of patients with lung cancer separately (Henry and collagues30 and Rosen and colleagues130), see Chapter 6, Characteristics of the included studies for details of the characteristics of the included studies for the overall studies.
Quality of the included studies
As these were the same trials that reported the subgroup of patients with lung cancer separately, see Chapter 6, Quality of the included studies for the quality of the included studies for the overall studies.
Assessment of effectiveness
Time to first on-study skeletal-related event
Henry and colleagues30 reported that denosumab reduced the risk of having a first on-study SRE relative to zoledronic acid by 21% (HR 0.79; 95% CI 0.62 to 0.99; p = 0.04) for OSTs excluding NSCLC. The CSR 244 reported median time to first on-study SRE (academic-in-confidence information has been removed) for zoledronic acid and (academic-in-confidence information has been removed) for the denosumab group.
The study by Rosen and colleagues130 reported that the median time to developing a first SRE was significantly longer in the zoledronic acid group (314 days) than in the placebo group (168 days) (p = 0.051).
Neither study reported SRE by type or prior history of SRE for this outcome for the subgroup with OSTs excluding NSCLC.
Risk of first and subsequent on-study skeletal-related events
The published paper by Henry and colleagues30 did not report risk of developing first and subsequent on-study SREs. (Academic-in-confidence information has been removed) (CSR 244). The study by Rosen and colleagues130 reported a 26% reduction in the risk of developing multiple SREs for the zoledronic acid group compared with the placebo group (HR 0.74, CI not reported); however, the difference was non-significant (p = 0.136).
Neither study reported SRE by type or prior history of SRE for this outcome for the subgroup of patients with OSTs excluding NSCLC.
Skeletal morbidity rate
Neither study reported SMR for those with OSTs excluding NSCLC.
Incidence of skeletal-related events
The published study by Henry and colleagues30 did not report incidence of SREs for the subgroup of patients with OSTs excluding NSCLC. (Academic-in-confidence information has been removed) (CSR 244).
In the study by Rosen and colleagues,130 the proportion of patients with a SRE was significantly lower in the zoledronic acid group (33%) compared with the placebo group (43%) (p = 0.11) for those with OSTs (excluding NSCLC).
Neither study reported SRE by type or prior history of SRE for this outcome for the subgroup of patients with OSTs excluding NSCLC.
Prevention of hypercalcaemia
Neither study reported prevention of hypercalcaemia for those with OSTs excluding NSCLC.
Overall survival
All patients
An ad hoc analysis by Henry and colleagues30 reported a non-significant difference in overall survival between the denosumab and zoledronic acid groups (HR 1.08; 95% CI 0.90 to 1.30).
The study by Rosen and colleagues130 did not report overall survival for those with OSTs excluding NSCLC.
Prior history of skeletal-related events
Neither study reported overall survival by history of SREs for those with OSTs excluding NSCLC.
Pain
Neither study reported the outcome of pain for those with OSTs excluding NSCLC.
Health-related quality of life
Neither study reported quality of life for those with OSTs excluding NSCLC.
Adverse events related to treatment
Adverse events including hypocalcaemia, ONJ, renal toxicity, acute-phase reactions or other adverse events were not reported separately for those with OSTs excluding NSCLC. See Chapter 8, Adverse events related to treatment, for information on adverse events reported for patients with OSTs including NSCLC.
Network meta-analysis
The AG performed a NMA of OSTs, excluding breast cancer, prostate cancer, multiple myeloma and NSCLC, using subgroups from the Henry and Rosen studies. 30,130 The manufacturer did not perform this analysis. Three outcomes were included: time to first on-study SRE (Table 43), risk of first and subsequent on-study SRE (Table 44) and the proportion of patients with an on-study SRE (Table 45).
| Comparison | AG's NMA, HR (95% CI) |
|---|---|
| Denosumab vs zoledronic acid | 0.79 (0.62 to 0.99) |
| Denosumab vs placebo | 0.30 (0.11 to 0.82) |
| Zoledronic acid vs placebo | 0.37 (0.14 to 1.01) |
| Comparison | AG's NMA, RR (95% CI) |
|---|---|
| Denosumab vs zoledronic acid | 0.83 (0.67 to 1.03) |
| Denosumab vs placebo | 0.61 (0.39 to 0.97) |
| Zoledronic acid vs placebo | 0.74 (0.49 to 1.10) |
| Comparison | AG's NMA, OR (95% CI) |
|---|---|
| Denosumab vs zoledronic acid | 0.68 (0.05 to 8.81) |
| Denosumab vs placebo | 0.44 (0.01 to 17.13) |
| Zoledronic acid vs placebo | 0.65 (0.05 to 8.19) |
Time to first on-study skeletal-related event
The results for time to first on-study SRE are shown in Table 43. There was a statistically significant difference in favour of denosumab compared with zoledronic acid or placebo for this outcome.
Risk of first and subsequent on-study skeletal-related events
The NMA results for risk of developing first and subsequent SREs are presented in Table 44. There was a statistically significant difference in favour of denosumab compared with placebo for this outcome.
Proportion of patients with an on-study skeletal-related event
The results for the proportion of patients with an on-study SRE are shown in Table 45. The results for denosumab compared with zoledronic acid or placebo were not statistically significant although the direction of effect favoured denosumab. These results should be interpreted with additional caution because this outcome does not differentiate between lengths of study, thereby adding to the uncertainty.
Summary
As these two studies were the same studies that contained the subgroups of NSCLC patients, see also Chapter 6, Summary for information on the characteristics, quality and generalisability of the overall studies. One further point to note in terms of generalisability is that data from patients with a range of different types of solid tumour (excluding breast, prostate or NSCLC) were pooled to provide an overall estimate for OSTs. The Henry study30 was not powered to detect non-inferiority or superiority for OSTs excluding NSCLC.
For those with bone metastases from OSTs excluding NSCLC, there was a significant risk reduction for denosumab compared with zoledronic acid in time to first on-study SRE (21% reduction with denosumab in the study by Henry and colleagues30) (academic-in-confidence information has been removed) (CSR 244). (Academic-in-confidence information has been removed.) In the study by Henry and colleagues,30 no statistically significant difference was reported for overall survival. No data were reported for SMR, hypercalcaemia, pain or quality of life.
The study by Rosen and colleagues130 reported a statistically significant difference between zoledronic acid and placebo in time to first on-study SRE (314 days vs 168 days); however, a non-significant difference in risk of first and subsequent on-study SREs was reported. Significantly lower incidence of SREs was reported for zoledronic acid (33%) compared with placebo (43%) but the difference was not statistically significant (p = 0.11). No data were reported for hypercalcaemia, overall survival, pain or quality of life. Adverse events were not reported separately for OSTs excluding NSCLC.
In the AG's NMA, there was a statistically significant difference in favour of denosumab compared with zoledronic acid or placebo for time to first on-study SRE and compared with placebo for risk of developing first and subsequent SREs, while for the proportion of patients with an on-study SRE there was no statistically significant difference, although the direction of effect favoured denosumab.
Chapter 8 Results: other solid tumours (including non-small cell lung cancer)
This chapter reports outcomes for OSTs including NSCLC (but excluding breast cancer or prostate cancer). Data taken from the CSR may include multiple myeloma and this has been highlighted where applicable.
Quantity of research available
See Chapter 4, Quantity of research available
Number and type of studies included
The flow diagram outlining the screening process for the overall review is shown in Figure 3.
Number and type of studies excluded
For information on studies that were excluded from the review, see Chapter 4, Number and types of studies excluded, and for a list of these studies along with the reasons for their exclusion, see Appendix 5. These studies were excluded because they failed to meet one or more of the inclusion criteria in terms of types of study, participants, intervention or outcomes reported.
Characteristics of the included studies
As these were the same trials (Henry and colleagues30 and Rosen and colleagues130) that reported the subgroup of patients with lung cancer separately, see Chapter 6, Characteristics of the included studies for details of the characteristics of the included studies.
Quality of the included studies
As these were the same trials that reported the subgroup of patients with lung cancer separately, see Chapter 6, Qualities of the included studies for details of the quality of the included studies.
Assessment of effectiveness
Time to first on-study skeletal-related event
Results for time to first on-study SRE are shown in Table 46. In the MS post-hoc analysis of study 244 of OSTs (excluding myeloma), the median time to first on-study SRE was longer for denosumab (academic-in-confidence information has been removed) compared with zoledronic acid (academic-in-confidence information has been removed) with a risk reduction of 19% [HR 0.81; 95% CI 0.68 to 0.96; p = 0.03 (superiority)]. Some patients in the zoledronic acid group (academic-in-confidence information has been removed) and the denosumab group (academic-in-confidence information has been removed) were reported to experience a first on-study SRE. The MS (excluding multiple myeloma) further reported that the median time to first symptomatic SRE was significantly shorter for denosumab compared with zoledronic acid (HR 0.81; 95% CI 0.66 to 0.99; p = 0.0383). The study by Henry and colleagues30 (including multiple myeloma) reported a statistically significant difference in favour of denosumab compared with zoledronic acid in delaying time to first on-study SRE by 16% (HR 0.84; 95% CI 0.71 to 0.98; p = 0.0007). The median time to first on-study SRE was significantly longer for denosumab (20.6 months) than for zoledronic acid (16.3 months) (p = 0.03). However, when adjusted for multiple comparisons (using the Hochberg procedure) to test for superiority for time to first SRE, the difference was not significant (p = 0.06).
| Study ID | Measures | Denosumab | Zoledronic acid | p-value |
|---|---|---|---|---|
| Henry 201130(including multiple myeloma) | Number randomised | 886 | 890 | NA |
| Median months | 20.6 | 16.3 | 0.03 | |
| HR (95% CI) | 0.84 (0.71 to 0.98) | 0.0007 | ||
| Post-hoc analysis CSR 244 (excluding multiple myeloma) | Number randomised | 800 | 797 | NA |
| Median months | 21.4 | 15.4 | NA | |
| HR (95% CI) | 0.81 (0.68 to 0.96) | 0.03 (superiority) | ||
| 0.001 (inferiority) |
The study by Rosen and colleagues130 reported significantly longer median time to first SRE for zoledronic acid (230 days) compared with placebo (163 days) (p = 0.023). Analysis of median time to first event excluding HCM and including death was longer for zoledronic acid (136 days) compared with placebo (93 days) (p = 0.039).
Skeletal-related events by type
The time to radiation to the bone was reported in the post-hoc analysis of study 244 (excluding multiple myeloma). The median time to radiation to the bone in the zoledronic group and in the denosumab group (academic-in-confidence information has been removed), and the risk reduction for denosumab (academic-in-confidence information has been removed) (MS) were reported.
In the study by Henry and colleagues30 (including multiple myeloma), denosumab reduced the risk of having radiation to bone by 22% compared with zoledronic acid (HR 0.78; 95% CI 0.63 to 0.97; p = 0.03). 134
(Academic-in-confidence information has been removed) (CSR 244).
Table 47 shows the distribution of first on-study SRE by type of SRE as reported in the MS (post-hoc analysis of CSR 244, excluding multiple myeloma). The distribution of type of SRE was similar across the treatment groups, with radiation to bone and pathological fracture being the most commonly occurring.
| SRE | Number of events (%) | |
|---|---|---|
| Denosumab (n = 800 randomised) | Zoledronic acid (n = 797 randomised) | |
| Overall | AiC information has been removed | AiC information has been removed |
| Radiation to bone | AiC information has been removed | AiC information has been removed |
| Pathological fracture | AiC information has been removed | AiC information has been removed |
| SCC | AiC information has been removed | AiC information has been removed |
| Surgery to bone | AiC information has been removed | AiC information has been removed |
The study by Rosen and colleagues130 reported that the median time was not reached for individual SRE except for median time to first pathological fracture, which was longer in the zoledronic acid group (238 days) compared with the placebo group (161 days) (p = 0.031). Rosen and colleagues131 further reported that the time to first vertebral fracture and time to first radiation therapy were significantly longer in the zoledronic acid group (p = 0.05).
Prior history of skeletal-related events
The MS reported time to first on-study SRE by prior history of SREs for post hoc study 244 (excluding myeloma) (Table 48). (Academic-in-confidence information has been removed.)
| SRE history | Denosumab | Zoledronic acid |
|---|---|---|
| Overall | ||
| Number | 800 | 797 |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| No prior SRE | ||
| Number | AiC information has been removed | AiC information has been removed |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Prior SRE | ||
| Number | AiC information has been removed | AiC information has been removed |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Covariate effect | ||
| Point estimate (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
The published study by Henry and colleagues30 did not report time to first on-study SRE by previous history of SRE. (Academic-in-confidence information has been removed) (CSR 244).
Rosen and colleagues130 did not report time to first on-study SRE by previous history of SRE.
Risk of first and subsequent on-study skeletal-related events
The MS (post-hoc analysis of study 244 excluding multiple myeloma) reported that denosumab reduced the risk of developing first and subsequent SREs compared with zoledronic acid. Using Anderson–Gill multiple event analysis (any events occurring at least 21 days apart), the result demonstrated borderline statistical significance (RR 0.85; 95% CI 0.72 to 1.00) (Table 49). The cumulative number of on-study SREs was lower for denosumab (328) than for zoledronic acid (374) (MS).
| Study ID | Measures | Denosumab (n = 890) | Zoledronic acid (n = 886) | p-value |
|---|---|---|---|---|
| Henry 201130 (including multiple myeloma) | Number randomised | 886 | 890 | NA |
| Number of events | 392 | 436 | NA | |
| Rate ratio (95% CI) | 0.90 (0.77 to 1.04) | 0.14 | ||
| Post-hoc analysis CSR 244 (excluding multiple myeloma) | Number analysed | 800 | 797 | NA |
| Number of events | 328 | 374 | NA | |
| Rate ratio (95% CI) | 0.85 (0.72 to 1.00) | 0.048 |
Henry and colleagues30 (when including multiple myeloma) reported a non-significant risk reduction for first and subsequent on-study SREs (without the 21-day window) for denosumab compared with zoledronic acid (RR 0.90; 95% CI 0.77 to 1.04; p = 0.14).
Rosen and colleagues130 reported that zoledronic acid reduced the risk of multiple SREs by 27% compared with placebo (HR 0.732; p = 0.017).
Skeletal-related events by type
Neither study reported multiple event analysis for SRE by type.
In the MS (post-hoc analysis CSR 244), there was no difference reported between denosumab and zoledronic acid for the proportion of patients with each type of SRE. The distribution of each type of SRE is shown in Table 50. Radiation to bone and pathological fracture were the most commonly occurring SREs, whereas surgery to bone and SCC were reported for only a small proportion of patients.
| SRE | Number of events (%) | |
|---|---|---|
| Denosumab (n = 800 randomised) | Zoledronic acid (n = 797 randomised) | |
| Total number of events | AiC information has been removed | AiC information has been removed |
| Radiation to bone | AiC information has been removed | AiC information has been removed |
| Pathological fracture | AiC information has been removed | AiC information has been removed |
| SCC | AiC information has been removed | AiC information has been removed |
| Surgery to bone | AiC information has been removed | AiC information has been removed |
The published studies by Henry and colleagues30 and Rosen and colleagues130 did not report on risk of first and subsequent on-study SREs by type of SRE.
Prior history of skeletal-related events
The MS reported risk of first and subsequent on-study SREs by history of SRE for post hoc study 244 (excluding multiple myeloma) (Table 51). (Academic-in-confidence information has been removed) (MS).
| SRE history | Denosumab | Zoledronic acid |
|---|---|---|
| Overall | ||
| Number | 800 | 797 |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| No prior SRE | ||
| Number | AiC information has been removed | AiC information has been removed |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Prior SRE | ||
| Number | AiC information has been removed | AiC information has been removed |
| HR (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
| Covariate effect | ||
| Point estimate (95% CI) | AiC information has been removed | |
| p-value | AiC information has been removed | |
(Academic-in-confidence information has been removed) (CSR 244).
The studies by Henry and colleagues30 and Rosen and colleagues130 did not report risk of first and subsequent on-study SREs by prior history of SRE.
Skeletal morbidity rate
The published study by Henry and colleagues30 did not report data on SMR. (Academic-in-confidence information has been removed) (Table 52).
| Annualised SRE rate per patient | Denosumab (n = 800) | Zoledronic acid (n = 797) |
|---|---|---|
| Subject years | AiC information has been removed | AiC information has been removed |
| Without 21-day window | ||
| Number of events | AiC information has been removed | AiC information has been removed |
| Annualised rate | AiC information has been removed | AiC information has been removed |
| With 21-day window | ||
| Number of events | AiC information has been removed | AiC information has been removed |
| Annualised rate | AiC information has been removed | AiC information has been removed |
| Mean annual SMR | ||
| Rate | AiC information has been removed | AiC information has been removed |
| p-value | AiC information has been removed | |
(Academic-in-confidence information has been removed) (CSR 244). Rosen and colleagues130 reported a slightly lower SMR (the number of events per year) for zoledronic acid (2.24; SD 9.12) than for placebo (2.52; SD 5.11); however, the difference was non-significant (p = 0.069). When hypercalcaemia was included in the analysis, the SMR was statistically significantly lower for zoledronic acid than for placebo [2.24 (SD 9.12) vs 2.73 (SD 5.29)].
Skeletal-related events by type
The SMR by type of SRE was not reported for the denosumab RCT. 30
Rosen and colleagues130 reported that the SMR for each type of SRE was lower in the zoledronic acid treatment groups than in the placebo group except for surgery to bone and SCC; however, no data were reported.
Prior history of skeletal-related events
Neither study reported SMR by history of SREs.
Incidence of skeletal-related events
The study by Henry and colleagues30 did not report incidence of SREs. In the MS (post-hoc analysis of CSR 244 excluding multiple myeloma), the annualised SRE rate (number of events per subject years) (academic-in-confidence information has been removed). The results are shown in Table 52.
(Academic-in-confidence information has been removed) (CSR 244).
The study by Rosen and colleagues130 reported a non-significant difference between zoledronic acid and placebo in the proportion of SREs experienced (38% vs 44%; p = 0.127).
Skeletal-related events by type
Incidence of SREs by SRE type was not reported for the denosumab RCT.
Rosen and colleagues130 reported the distribution of SRE type in zoledronic acid compared with placebo as shown in Table 53. For each individual SRE, a lower proportion of patients receiving zoledronic acid experienced a SRE than those receiving placebo. Radiation to bone and pathological fracture were the most frequently occurring SREs while SCC occurred least.
| SRE | Number of events (%) | p-value | |
|---|---|---|---|
| Zoledronic acid (n = 257 randomised) | Placebo (n = 250 randomised) | ||
| All SRE (excluding HCM) | 38% | 44% | 0.127 |
| Radiation to bone | 69 (27%) | 81 (32%) | NR |
| Pathological fracture | 40 (16%) | 53 (21%) | NR |
| Vertebral | 20 (8%) | 30 (12%) | |
| Non-vertebral | 26 (10%) | 29 (12%) | |
| Surgery to bone | 11 (4%) | 9 (4%) | NR |
| SCC | 7 (3%) | 10 (4%) | NR |
| HCM | 0 | 8 (3%) | 0.004 |
| Any SRE (including HCM) | 97 (38%) | 117 (47%) | 0.039 |
Prior history of skeletal-related events
Neither study reported incidence of SRE by history of SREs.
Prevention of hypercalcaemia
(Academic-in-confidence information has been removed.) (CSR 244).
In the study by Rosen and colleagues130 there was no HCM in the zoledronic group, whereas in the placebo group 3% of patients experienced HCM.
Overall survival
Henry and colleagues30 reported no difference between denosumab and zoledronic acid for overall survival (HR 0.95; 95% CI 0.83 to 1.08; p = 0.43). In the MS median overall survival was balanced between the groups, with median time for survival 10.7 months in the denosumab group and 10.0 months in the zoledronic acid group. The risk reduction for overall survival (excluding multiple myeloma) was not statistically significant (0.92; 95% CI 0.81 to 1.05; p = 0.2149).
Rosen and colleagues130 reported time to median death, which was similar in the zoledronic acid group (203 days) and the placebo group (183 days) (p = 0.623).
Prior history of skeletal-related events
Neither study reported overall survival by history of SREs.
Pain
The MS reported pain outcomes assessed using BPI-SF. The median time to developing moderate or severe worst pain was evaluated in a subgroup of patients with no/mild pain (n = 323 for denosumab; n = 273 for zoledronic acid). The median time to developing moderate or severe worst pain (worst pain score > 4) in this group was longer in the denosumab group (3.7 months) than in the zoledronic acid group (2.8 months, HR 0.81; 95% CI 0.66 to 0.99; p = 0.038). The MS further reported that denosumab delayed the time to worsening pain (≥ 2-point increase from baseline in BPI-SF worst pain score) compared with zoledronic acid (4.7 months vs 3.9 months; p = 0.040). (Academic-in-confidence information has been removed.) The study by Henry and colleagues134 reported similar results in those with OSTs and including multiple myeloma (169 days vs 143 days; HR 0.85; 95% CI 0.73 to 0.98; p = 0.02).
(Academic-in-confidence information has been removed) (CSR 244).
There was no statistically significant difference at the study end point in the use of strong analgesics in OSTs (post-hoc analysis excluding multiple myeloma).
(Academic-in-confidence information has been removed) (CSR 244).
The study by Rosen and colleagues130 comparing zoledronic acid with placebo reported an increase in pain score from baseline to month 9 for mean BPI composite pain score and mean analgesic score in both groups, suggesting increased pain and use of analgesics. This study further reported that the mean composite pain score was decreased from baseline to month 9 for zoledronic acid for those who had pain at baseline; however, no data were reported.
Health-related quality of life
Functional Assessment of Cancer Therapy – General
(Academic-in-confidence information has been removed) (Table 54).
| Scale | Denosumab 120 mg (n = 800) | Zoledronic acid 4 mg (n = 797) | ||
|---|---|---|---|---|
| Baseline, mean (SD) | Change from baseline to week 45, mean (SD) | Baseline, mean (SD) | Change from baseline to week 45, mean (SD) | |
| Physical well-being | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Functional well-being | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| FACT-G total score | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
(Academic-in-confidence information has been removed) (CSR 244).
The study by Rosen and colleagues130 stated that there were no statistically significant differences between zoledronic acid and placebo with respect to any of these global quality-of-life outcomes and that changes in FACT-G scores were also comparable between treatment groups; however, no data were reported.
European Quality of Life-5 Dimensions
(Academic-in-confidence information has been removed) (CSR 244).
Adverse events related to treatment
Hypocalcaemia
Henry and colleagues30 reported that 10.8% of denosumab-treated patients had hypocalcaemia compared with 5.8% of zoledronic acid-treated patients. The statistical difference between the groups was not reported. Grade 3 or 4 decreases in albumin-adjusted calcium values were reported in nine patients (1.0%) receiving zoledronic acid and 20 patients (2.3%) receiving denosumab. Although the number of patients reporting hypocalcaemia is small the total number of events is higher for denosumab compared with zoledronic acid (academic-in-confidence information has been removed) (CSR 244).
The study by Rosen and colleagues130 did not report hypocalcaemia.
Observational studies reported a higher incidence of hypocalcaemia compared with the RCTs. However, the observational studies are likely to have broader criteria for hypocalcaemia. Chennuru and colleagues138 reported an incidence of 8.3% over 2 years in patients prescribed zoledronic acid. Zuradelli and colleagues161 reported an incidence of 4.6% in patients prescribed zoledronic acid (time at risk not reported).
Osteonecrosis of the jaw
Henry and colleagues30 reported that rates of ONJ were similar in the denosumab (1.3%) and zoledronic acid (1.1%) groups (p = 1.00). The cumulative incidence rates of ONJ at years 1 and 3 was reported to be slightly higher in the zoledronic acid group compared with the denosumab group, which was 0.6% versus 0.5% at year 1 and 1.3% versus 1.1% at year 3 (p = 1.0). At year 2, ONJ events were slightly higher in the denosumab group (1.1%) compared with the zoledronic acid group (0.9%).
The study by Rosen and colleagues130 did not report ONJ.
Two large observational studies were found. Hoff and colleagues147 reported an incidence of 0.7% (29/3994) over 21.2 months in patients taking zoledronic acid or disodium pamidronate. Vahtsevanos and colleagues159 reported an incidence of 4.9% (80/1621) over 20.4 months in patients taking any BP.
Renal toxicity
Henry and colleagues30 reported that renal adverse events occurred more often in the zoledronic acid group (10.9%) than in the denosumab group (8.3%). In both treatment groups, renal failure was reported to be similar. The MS reported a higher number of patients in the zoledronic acid group compared with the denosumab group with serious renal adverse events (34 patients compared with 24 patients). (Academic-in-confidence information has been removed) (CSR 244). The small discrepancy in these results is unclear.
Rosen and colleagues130 reported that the proportion of patients with decreased renal function was higher in the zoledronic acid group than in the placebo group. When zoledronic acid was given as a 5-minute infusion, the proportion of patients with decreased renal function was much higher in the zoledronic acid group (16.4%) than in the placebo group (5.6%). After the implementation of a 15-minute infusion of the given dose, 10.9% in the zoledronic acid group and 6.7% in the placebo group experienced decreased renal function.
The largest observational study155 (n = 966) evaluated renal impairment in patients taking any BP and found an incidence of 2.9% over 9.6 months.
Acute-phase reactions
Henry and colleagues30 reported that acute-phase reactions occurred more often in the zoledronic acid group (14.5%) than in the denosumab group (6.9%). In the MS, SAEs of acute-phase reaction occurred within 3 days of first dose. (Academic-in-confidence information has been removed.)
Rosen and colleagues130 did not report this outcome.
Other adverse events
In the study by Henry and colleagues,30 SAEs were reported in 66% of those treated with zoledronic acid and in 63% of those treated with denosumab (p = 0.16). Pyrexia and anaemia were reported to be significantly higher in the zoledronic acid group than in the denosumab group. Other adverse events were similar in both groups.
In the study by Rosen and colleagues,130 a higher proportion in the zoledronic acid group than in the placebo group was reported to have nausea (46% vs 34%), vomiting (36% vs 29%) and dyspnoea (33% vs 26%). The incidence of bone pain was reported to be higher in the placebo group (59%) than in the zoledronic acid group (51%).
There were no other adverse events of note from the observational studies assessed. Anaemia was similar between all groups.
For details of all other adverse events extracted from the RCTs meeting the review's inclusion criteria and also adverse events extracted from a number of observational studies identified, see Appendix 12.
Network meta-analysis
The AG and manufacturer performed a NMA of OSTs excluding breast cancer and prostate cancer but including NSCLC. Two studies were included in each NMA (Henry and colleagues30 and Rosen and colleagues130). Including a mixture of cancers within a NMA increases heterogeneity significantly. Therefore, these results should be interpreted with caution. The AG also performed a NMA of the proportion of patients with an on-study SRE.
Time to first on-study skeletal-related event
The results for time to first on-study SRE are shown in Table 55. The AG's NMA results were statistically significant in favour of denosumab compared with zoledronic acid or placebo. (Academic-in-confidence information has been removed.)
| Comparison | AG's NMA, HR (95% CI) | MS's NMA, HR (95% CI) |
|---|---|---|
| Denosumab vs zoledronic acid | 0.81 (0.68 to 0.96) | AiC information has been removed |
| Denosumab vs placebo | 0.49 (0.30 to 0.78) | AiC information has been removed |
| Zoledronic acid vs placebo | 0.60 (0.38 to 0.93) | AiC information has been removed |
Risk of first and subsequent on-study skeletal-related events
The results for risk of developing first and subsequent on-study SREs are presented in Table 56. The AG's NMA results were statistically significant in favour of denosumab compared with placebo, whereas the result for the comparison with zoledronic acid was not statistically significant, although the direction of effect favoured denosumab. (Academic-in-confidence information has been removed.)
| Comparison | AG's NMA, RR (95% CI) | MS's NMA, HR (95% CI) |
|---|---|---|
| Denosumab vs zoledronic acid | 0.85 (0.72 to 1.00) | AiC information has been removed |
| Denosumab vs placebo | 0.62 (0.46 to 0.85) | AiC information has been removed |
| Zoledronic acid vs placebo | 0.73 (0.56 to 0.95) | AiC information has been removed |
Proportion of patients with on-study skeletal-related event
The results for the proportion of patients with an on-study SRE are shown in Table 57.
| Comparison | AG's NMA, OR (95% CI) |
|---|---|
| Denosumab vs zoledronic acid | 0.79 (0.07 to 9.45) |
| Denosumab vs placebo | 0.58 (0.02 to 19.48) |
| Zoledronic acid vs placebo | 0.74 (0.06 to 8.83) |
In the AG's NMA, the differences between denosumab and zoledronic acid or placebo were not statistically significant, although the direction of effect favoured denosumab. This outcome does not account for differences in length of study, thereby adding to the uncertainty, and thus these results should be interpreted with caution.
Summary
See also Chapter 6, Summary, first paragraph, for information on the characteristics, quality and generalisability of the studies. In terms of generalisability, data from patients with a range of different types of solid tumour (excluding breast or prostate) were pooled to provide an overall estimate for OSTs. The Henry study30 was powered to detect non-inferiority or superiority for OSTs including NSCLC and multiple myeloma.
For those with bone metastases from OSTs, the study by Henry and colleagues30 reported a statistically significant difference in favour of denosumab compared with zoledronic acid in delaying time to first on-study SRE (20.6 months vs 16.3 months with 16% risk reduction by denosumab). However, a non-significant difference was reported in the risk of developing first and subsequent on-study SREs. The SMR and annualised SRE rate were also significantly lower in the denosumab group in the study by Henry and colleagues. 30
The MS reported (academic-in-confidence information has been removed) on risk reduction for first and subsequent on-study SRE (15% reduction for denosumab). (Academic-in-confidence information has been removed) (MS). Overall survival was similar for both groups.
In the study by Rosen and colleagues,130 a statistically significant difference in favour of zoledronic acid compared with placebo was reported in time to first SRE (230 days vs 163 days) and risk of developing first and subsequent SREs (risk reduction by 27% with zoledronic acid). No significant difference between the groups was reported for SMR and for incidence of SRE.
The MS reported on hypercalcaemia. (Academic-in-confidence information has been removed.) In the study by Henry and colleagues30 no significant difference between denosumab and zoledronic acid in overall survival was reported. Delay in worsening clinically significant pain at 45 weeks was reported, which favoured denosumab (169 days) compared with zoledronic acid (143 days). The MS reported (academic-in-confidence information has been removed).
In the study by Rosen and colleagues,130 no hypercalcaemia events were reported in the zoledronic acid group whereas these occurred in 3% of patients in the placebo group. No significant differences in overall survival and quality of life (changes in FACT-G scores) were reported. No data were reported for pain outcomes.
In the study by Henry and colleagues30 there were more hypocalcaemia events in the denosumab group (10.8%) compared with the zoledronic acid group (5.8%), fewer renal adverse events (8.3% vs 10.9%) and acute-phase reactions (6.9% vs 14.5%), whereas similar events of ONJ (1.3% vs 1.1%) were experienced by patients. The incidence of SAEs was similar in both groups (63% vs 66%; p = 0.16).
Rosen and colleagues130 reported that, compared with the placebo group, more patients in the zoledronic acid group experienced decreased renal function (10.9% vs 6.7%) and less bone pain (51% vs 59%). No data were reported on hypocalcaemia, ONJ or acute-phase reaction.
The AG's NMA reported a statistically significant difference in favour of denosumab compared with placebo for time to first on-study SRE and risk of developing first and subsequent on-study SREs. (Academic-in-confidence information has been removed.)
Chapter 9 Assessment design and results: cost-effectiveness
This chapter consists of the following main sections: Systematic reviews of cost-effectiveness studies and quality-of-life studies; Critique of the manufacturer's submission; and Independent economic assessment.
All costs and prices in this report are in 2010 pounds sterling. Costs in foreign currency amounts are converted to pounds sterling at the 5 April exchange rate of the relevant year. Where no year is stated for prices, it is assumed to be the year of the publication. Indexation to 2010 prices applies the Hospital and Community Health Services (HCHS) index as drawn from the Personal Social Services Research Unit Costs of Health and Social Care. 167 Original amounts are given in square brackets.
Systematic reviews of cost-effectiveness studies and quality-of-life studies
Search strategy and quantity of research available
Two separate literature searches were conducted to identify studies considering cost-effectiveness and quality of life. First, studies focusing on cost-effectiveness or quality of life in relation to bone metastases and SREs were sought; this search identified 468 papers. After having screened the titles and abstracts, 131 full-text papers were retrieved.
A second search was conducted to identify studies considering cost-effectiveness or quality of life in relation to denosumab and BPs. This search identified 2600 papers. After having screened the titles and abstracts, 139 full-text papers were retrieved.
The databases searched were MEDLINE (1948 to May Week 3 2011), EMBASE (1980 to 2011 Week 21), MEDLINE In-Process & Other Non-Indexed Citations (2 June 2011), NHS Economic Evaluation Database (June 2011), Science Citation Index (1970 to June 2011), Social Science Citation Index (1970 to June 2011), Conference Proceedings Citation Index – Science (1990 to June 2011) and Conference Proceedings Citation Index – Social Science & Humanities (1990 to June 2011). Conference proceedings from the 2010 and 2011 meetings of ASCO were hand-searched. The searches had no date restrictions, but were limited to English-language papers.
Full details of the search strategies used and websites consulted are documented in Appendix 1.
Results: cost-effectiveness studies
Full papers
Dranitsaris and Hsu168 estimate the cost-effectiveness of disodium pamidronate compared with BSC over a 12-month trial among breast cancer patients with bone metastases. This drew on the findings of Hortobagyi and colleagues,22 who report the clinical effectiveness of the then only relevant disodium pamidronate trial. Over a mean duration of therapy of 10 months, disodium pamidronate and BSC were associated, respectively, with the following events:
-
non-vertebral fractures: 20% vs 30%
-
radiation to the bone: 19% vs 33%
-
surgery to the bone: 4 % vs 10%
-
any SRE: 46% vs 62%
-
any SRE excluding hypercalcaemia: 43% vs 56%.
Costs per health state were estimated by chart review, with unit costs being drawn from the Princess Margaret Hospital and the Centenary Hospital of Ontario, Canada.
The main aspects of the paper that are of interest are the utility data, which are drawn from a time trade-off (TTO) exercise among 25 women from the Canadian general public and 25 female health workers. There is a lack of detail within the paper, and it seems likely that the health state descriptors include elements of both the treatment aspects and the clinical effectiveness for each arm. With this noted, the TTO exercise yields the following estimates (Table 58).
| Health state | Average public | % | Average health workers | % |
|---|---|---|---|---|
| SRE with disodium pamidronate | 5.46 months | 46 | 4.80 months | 40 |
| No SRE with disodium pamidronate | 7.73 months | 64 | 9.92 months | 83 |
| SRE with placebo | 3.68 months | 31 | 4.13 months | 34 |
| No SRE with placebo | 6.76 months | 56 | 7.89 months | 66 |
The source of the anticipated benefit from disodium pamidronate over placebo when no SRE is experienced is unclear and is not specified within the paper. Health worker responses are reasonably consistent, with a consistent reduction in quality of life from a SRE of around 50% for both the disodium pamidronate and the placebo health states. Results are more mixed within the public responses, with SREs causing a similar, approximate 50%, reduction in quality of life in the placebo group, but only a 30% reduction in the disodium pamidronate group.
Dranitsaris and Hsu168 estimate that disodium pamidronate results in an additional cost of £1758 (C$2800). Based on the SRE rates including hypercalcaemia of 46% and 62%, this results in an estimated gain from disodium pamidronate of 0.15 quality-adjusted life-years (QALYs), with an associated cost-effectiveness of £11,740 (C$18,700) per QALY based on public preferences and £10,359 (C$16,500) per QALY based on health-care worker preferences. Results are sensitive to the costs of surgery to the bone.
Hillner and colleagues169 estimate the cost-effectiveness of disodium pamidronate compared with BSC for breast cancer patients over a 2-year time horizon in the USA. The utility values are taken from expert opinion, with fractures at 0.8, radiation at 0.6, surgery at 0.4 and both hypercalcaemia and SCC at 0.2. The duration applied to these is not clear from the paper, but it may be 1 month. Disodium pamidronate is estimated to result in an additional 1.13 months SRE free with a net cost increase of £3593 (US$3968) for chemotherapy patients, resulting in a cost-effectiveness of £97,973 (US$108,200) per QALY. For hormone-treated patients the correspoding amounts are 0.82 additional months free of SRE at a cost of £6958 (US$7685) to yield a cost-effectiveness of £276,444 (US$305,300) per QALY.
Ross and colleagues,55 in the 2004 Health Technology Assessment (HTA) monograph reviewing the role of BPs in metastatic disease, model the cost per SRE avoided for breast cancer patients with bone metastases. This uses a cost-effectiveness Markov model with a monthly cycle. This simulates rates of SREs, with the health states also including hypercalcaemia and pain reduction, this latter being distinct from palliative radiotherapy. Note that SCC is not considered. The RRs for SREs and hypercalcaemia in the model for BPs compared with BSC are not differentiated by BP, but are differentiated by event type:
-
0.90 for vertebral fracture
-
0.79 for non-vertebral fracture
-
0.71 for palliative radiotherapy
-
0.59 for surgery to the bone
-
0.51 for hypercalcaemia.
Direct drug and administration costs are based on the cost of disodium pamidronate plus an oncology outpatient appointment. The cost per fracture is taken as the average of the relevant inpatient health-care resource groups (HRGs) within NHS reference costs £2786 (£2017), with surgery to the bone being costed at £2813 (£2036), while radiotherapy is based on three radiotherapy sessions in an outpatient setting to yield a cost of £978 (£708). Ross and colleagues55 undertook their own bottom-up costing for hypercalcaemia to estimate an average cost of £4840 (£3503). Note that this study was undertaken when discount rates were differentiated between costs at 6% and benefits at 1%.
The model estimates a 4-year survival of 16%, with patients being treated monthly with disodium pamidronate until death or to the end of the fourth year. This results in an average 1.45 SREs being averted compared with BSC: 0.54 non-vertebral fractures, 0.16 vertebral fractures, 0.64 courses of palliative radiotherapy and 0.12 episodes of surgery to the bone. An additional 0.34 episodes of hypercalcaemia are modelled as being prevented together with an average 3.2 months bone pain reduction. The total cost of therapy is estimated to be £7235 (£5237), but cost offsets reduce this to £613 (£444). Excluding hypercalcaemia, this results in a cost per SRE avoided of £423 (£306). With the application of a 0.33 QALY loss per SRE drawn from Dranitsaris and Hsu169 as reviewed above but adjusted for an increased SRE duration of 22 months, this translates into a cost-effectiveness estimate of £1851 (£1340) per QALY gained.
Reed and colleagues170 (supported by Novartis) compare the cost-effectiveness of zoledronic acid with BSC for prostate cancer patients with bone metastases, mainly within the context of the USA and Medicare. This analyses within-trial SRE rates and resource utilisation data over 15 months to estimate the cost per SRE avoided. An additional cost–utility analysis is conducted based on the EQ-5D VAS scores. The average number of SREs within the zoledronic acid group is 0.78 compared with 1.24 in the BSC group, resulting in incremental cost-effectiveness ratios (ICERs) of £11,137 ($12,300) per SRE avoided and £105,976 (US$159,200) per QALY.
De Cock and colleagues171 model the cost-effectiveness of oral ibandronate compared with zoledronic acid and disodium pamidronate among UK breast cancer patients receiving hormonal therapy. Treatment with oral ibandronate is estimated to result in a direct utility gain of 0.02 compared with intravenous administration. Discontinuation rates are also assumed to be lower, it being estimated that 96.9% of ibandronate patients are treated for an average of 7.2 months out of a total survival of 14.3 months. This compares with 71% for zoledronic acid and 73% for disodium pamidronate, although 12% of these patients switch to oral ibandronate. Oral ibandronate is estimated to be as effective as zoledronic acid for those on therapy in preventing SREs, and both are slightly superior to disodium pamidronate. Given this, ibandronate is estimated to yield an additional 0.02 QALYs over both zoledronic acid and disodium pamidronate, while saving £390 (£307) and £201 (£158), respectively.
In a parallel paper, De Cock and colleagues172 model the cost-effectiveness of oral ibandronate compared with zoledronic acid and disodium pamidronate among UK breast cancer patients receiving chemotherapy. This applies the same SRE rates and RRs for those on therapy as those applied in De Cock and colleagues,172 with the same discontinuation rates and percentages switching to oral ibandronate. There is also the same anticipated average survival of 14.3 months and the same quality-of-life values. There is the same average gain from ibandronate of 0.02 QALYs compared with zoledronic acid and disodium pamidronate, but the costs savings differ marginally: £490 (£386) compared with zoledronic acid and £285 (£224) compared with disodium pamidronate.
Guest and colleagues173 (supported by Mayne Pharma) undertake a cost minimisation analysis of disodium pamidronate compared with zoledronic acid for breast cancer patients in the UK, with a 1-year time horizon. This draws clinical effectiveness estimates from the literature, distinguishing between those on chemotherapy and those on hormonal therapy. Disodium pamidronate is estimated to be marginally superior in preventing any SRE among the chemotherapy group, and slightly inferior to zoledronic acid in preventing any SRE among the hormonal therapy group. These rates are then qualified by rates of individual SREs, with disodium pamidronate typically resulting in slightly more of all SREs among those experiencing a SRE, with the exception of fractures among those receiving hormonal therapy. Disodium pamidronate has a higher discontinuation rate, particularly among those being treated with hormonal therapy. For chemotherapy treated patients, this results in an average 3.77 SREs for disodium pamidronate compared with 2.79 for zoledronic acid. For hormone-treated patients, this resulted in an average 3.44 SREs for disodium pamidronate compared with 2.93 for zoledronic acid. The authors conclude that there is little clinical difference, and that as a consequence cost minimisation is appropriate.
Drug administration times for the base case are estimated as 184 to 214 minutes for disodium pamidronate compared with 204 to 232 minutes for zoledronic acid, though this latter includes patients waiting 90 minutes for test results. It is unclear quite how this has been costed. Expert opinion supplies much of the resource-use estimates (Table 59).
| Resource use | Hypercalcaemia | Vertebral fracture | Non-vertebral fracture | SCC |
|---|---|---|---|---|
| Inpatient | 31% for 3 days | 45% for 10 days | 20% for 7 days | 31% for 20 days |
| • 33% oncology | • 17% oncology | • 70% oncology | • 83% oncology | |
| • 67% general ward | • 17% orthopaedic | • 15% orthopaedic | • 17% general ward | |
| • 66% general ward | • 15% general ward | |||
| Outpatient | 2 oncology OP appt. | 2 oncology OP appt. | 2 oncology OP appt. | 2 oncology OP appt. |
| Radiotherapy | 12% of patients | 79% of patients | 85% of patients | 75% of patients |
| Surgery | 1% of patients | 42% of patients | 7% of patients | 19% of patients |
In the light of the above, disodium pamidronate is estimated to be cost-saving compared with zoledronic acid: £1130 (£936) for chemotherapy patients and £776 (£643) for hormone-treated patients.
Reed and colleagues170 (supported by Novartis) compare the costs and consequences of zoledronic acid with disodium pamidronate among breast cancer patients with bone metastases, again mainly within the context of the USA and Medicare. This analyses within-trial SRE rates and resource utilisation data, with a mean patient follow-up of 10 months. Zoledronic acid is estimated to have a RR of a SRE of 0.80 compared with disodium pamidronate. Costs in the zoledronic acid group are estimated to be marginally higher: £14,218 (US$15,703) compared with £14,198 (US$15,680) for disodium pamidronate. This was not taken through to a cost-effectiveness estimate specific to breast cancer patients.
Botteman and colleagues174 (authorship includes an employee of Novartis) compare the cost-effectiveness of zoledronic acid, oral ibandronate, intravenous ibandronate, disodium pamidronate, oral clodronate and BSC for breast cancer patients with bone metastases. This uses a cost–utility model from a UK NHS perspective, with a monthly cycle over a 10-year time horizon. Patients can discontinue active therapy due to non-compliance, which might be because of an adverse event. Fifty per cent of those discontinuing move on to another active therapy: oral if previously on intravenous and intravenous if previously on oral. Disease progression is also assumed to lead to therapy being stopped.
A baseline annual rate of 3.05 SREs is assumed for BSC, with this being multiplied by the relevant HR to arrive at the treatment-specific SRE rates: 0.56 for zoledronic acid, 0.62 for oral ibandronate, 0.71 for intravenous ibandronate and 0.70 for disodium pamidronate.
Quality-of-life values for without a SRE and with a SRE are drawn from Dranitsaris and Hsu168 on the grounds that it was the only published source available. There is some arbitrariness in the estimation of benefits, with the oral ibandronate being assumed to be postponed to the 12th week, while oral clodronate was assumed to have half the benefits of the other therapies. Survival was unaffected by treatment, with a mean survival of 20 months.
Zoledronic acid is estimated to require 11 minutes of physician time, 11 minutes of pharmacy technician time and 44 minutes of nurse time, in contrast to 8, 12 and 152 minutes for disodium pamidronate and 10, 11 and 98 minutes for intravenous ibandronic acid. This results in staff administration costs of £42.17 (£37.42) for zoledronic acid, £88.23 (£78.29) for disodium pamidronate and £65.20 (£57.85) for intravenous ibandronic acid.
The SRE costs are averaged across the SREs, with an average inpatient cost of £2272 (£2016) plus an additional average of £1826 (£1620) outpatient and care in the community costs. These are stated as being based on the Ross and colleagues55 BPs review HTA monograph.
The base-case results are an average 6.11 SREs for BSC, with this being reduced to 3.71 SREs for zoledronic acid; 4.41 SREs for disodium pamidronate; 4.46 for intravenous ibandronate; and 4.06 for oral ibandronate with this last SRE possibly being the result of the high discontinuation rate and second-line intravenous therapy. Given the figure for BSC and the average survival of 2 years, it is not obvious how progression was included in the modelling.
Average QALY estimates are surprisingly similar between the BPs – 1.18 QALYs to 1.20 QALYs – and BSC – 0.99 QALYs. Total costs are £21,032 (£18,662) for BSC, with disodium pamidronate and intravenous ibandronate exceeding this by £127 (£113) and £516 (£458), respectively, to yield cost-effectiveness estimates relative to BSC of £658 (£584) per QALY and £2671 (£2370) per QALY. Zoledronic acid and oral ibandronate are estimated to save £2554 (£2267) and £2382 (£2114) compared with BSC, and so dominate it, with zoledronic acid further dominating oral ibandronate. Across the therapies, zoledronic acid is estimated to be the preferred treatment at all values of willingness to pay.
Joshi and colleagues (who include Botteman and a Novartis employee) estimate the cost-effectiveness of zoledronic acid compared with BSC for NSCLC patients across five European countries in what appears to be an update of the Botteman 2009 abstracts, as summarised below. 175–181 This is based on the NSCLC subset of the Phase III trial populations, within which the median survivals were not statistically different between zoledronic acid, 201 days, and BSC, 157 days. As a consequence, a Weibull distribution is fitted to the zoledronic acid arm to yield an estimated average survival of 272 days. This is then multiplied by each arm's SRE-specific SMR to derive the number of SREs: 1.38 for zoledronic acid and 2.17 for BSC, though the latter includes some episodes of hypercalcaemia.
The SREs are assumed to be associated with only 1 month loss of quality of life, the baseline NSCLC HRQoL of 0.63 being reduced by 6.8% by vertebral fracture, 20% by non-vertebral fracture, 40% by radiation therapy, 60% by surgery to the bone and 80% by both SCC and hypercalcaemia, as drawn from Hillner and colleagues. 169 This results in zoledronic acid being estimated to yield 0.44 QALYs compared with 0.42 QALYs for BSC.
For the UK, in common with the approach of the 2004 Ross HTA monograph,55 the costs per SRE were derived mainly from averaging a range of HRG costs. This yields costs of £138 (€187) for vertebral fracture; £4520 (€6105) for non-vertebral fracture; £745 (€1007) for radiation to the bone; £2456 (€3318) for surgery to the bone; £3714 (€5017) for SCC; and £3822 (€5163) for hypercalcaemia. Administration costs and supplies for zoledronic acid are based on the micro-costing of DesHarnais and colleagues182 with 11-minute physician time, 11-minute pharmacist time and 44-minute nurse time to yield a total administration cost of £38.82 (€52.43). Total UK costs are reported as £3062 (€4136) for zoledronic acid compared with £3086 (€4168), which suggests a small net saving from zoledronic acid of £22 (€32), though the paper reports this as a saving of £155 (€209). The 0.79 fewer SREs are estimated to provide cost offsets of £1217 (€787), and zoledronic acid is estimated to dominate BSC for NSCLC patients with bone metastases.
Carter and colleagues183 (a similar authorship list to Joshi and colleagues' 2011 NSCLC paper,181 and with the support of Novartis) model the cost-effectiveness of zoledronic acid versus BSC for prostate cancer patients in France, Germany, Portugal and the Netherlands. 183 Quality-of-life data are drawn from the Reed and colleagues184 paper through a back calculation using the ICER and the estimated additional costs. This suggests an average gain from zoledronic acid over placebo of 0.034 QALYs. Rates of individual SREs are estimated solely to inform the drug and SRE costing exercise attached to this estimate of QALY gains. The base-case results are that 0.759 SREs are avoided on average, generating savings of between £2094 (€2396) and £3162 (€3617) per patient. The direct drug and administration costs of zoledronic acid are less geographically variable at between £3012 (€3446) and £3269 (€3704), with the resulting increase in costs leading to cost-effectiveness estimates ranging from a low of £2124 (€2430) in the Netherlands, to a high of £31,476 (€36,007) in France.
Xie and colleagues185 (supported by Novartis) estimate the cost-effectiveness of denosumab compared with zoledronic acid for patients with hormone refractory prostate cancer with bone metastases. This uses a 1-year Markov model with a 13-week cycle. The justification for using a 1-year time horizon rather than a 3-year time horizon is the anticipation of zoledronic acid being available in generic form from March 2013. But the analysis is from a US perspective, and the costs are not particularly relevant. The paper is of interest in part because in addition to modelling rates of SREs, the probability of a SRE is dependent on whether the patient is progression free or with progression. The likelihood of progression is not differentiated by treatment arm, but progression increases the rate of SREs by 2.14 compared with the without-progression SRE rate, as drawn from Tchekmedyian and colleagues. 186 Among those without progression denosumab was estimated to have a RR of first on-study SRE of 0.83 and a HR of 0.82 for subsequent SREs, with these estimates probably being carried over to the with-progression patients (Table 60).
| Time horizon | Zoledronic acid | Denosumab | Net | |||
|---|---|---|---|---|---|---|
| 1-year time horizon | ||||||
| Drug and administration | £6734 | $10,960 | £11,815 | $19,230 | £5081 | $8270 |
| Total cost | £16,914 | $27,528 | £21,714 | $35,341 | £4800 | $7813 |
| SREs | 0.60 | 0.49 | −0.11 | |||
| ICER | £43,641 | $71,027 | ||||
| 3-year time horizon | ||||||
| Drug and administration | £12,271 | $19,972 | £21,532 | $35,044 | £9261 | $15,072 |
| Total cost | £34,169 | $55,612 | £42,683 | $69,468 | £8513 | $13,856 |
| SREs | 1.46 | 1.18 | −0.28 | |||
| ICER | £31,532 | $51,319 | ||||
These cost-effectiveness results are summarised below (Table 61), within which unless otherwise stated the cost-effectiveness estimates are the cost per QALY for the more effective treatment over the less effective treatment.
| Main author | Year | Cancer | Country | Horizon | SREs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Denosumab | Zoledronic acid | Disodium pamidronate | Oral ibandronic acid | BSC | Cost per QALY or other c/e | |||||
| aDranitsaris168 | 1999 | Breast | Canada | 12 months | n.a | n.a | £11,740 (CND$18,700) public TTO | |||
| £10,359 (CND$16,500) expert TTO | ||||||||||
| bHillner169 | 2000 | Breast | USA | 2 year | 2.09 | 3.23 | £97,973 (US$108,200) chemotherapy patients | |||
| 2.60 | 3.43 | £276,444 (US$305,300) hormone therapy patients | ||||||||
| cRoss55 | 2004 | Breast | UK | 4 year | 5.68 | 7.47 | £1851 (£1340) | |||
| aReed184 | 2004 | Prostate | USA | 15 months | 0.78 | 1.24 | £11,137 (US$12,300) per SRE | |||
| dDe Cock171 | 2005 | Breast | UK | Lifetime | 2.00 | 2.49 | 2.00 | Oral ibandronic acid dominant in chemotherapy patients | ||
| • Saving £390 (£307) vs zoledronic acid | ||||||||||
| • Saving £201 (£158) vs disodium pamidronate | ||||||||||
| dDe Cock172 | 2005 | Breast | UK | Lifetime | 2.00 | 2.10 | 2.00 | Oral ibandronic acid dominant in hormone therapy patients | ||
| • Saving £490 (£386) vs zoledronic acid | ||||||||||
| • Saving £285 (£224) vs disodium pamidronate | ||||||||||
| eGuest173 | 2005 | Breast | UK | 1 year | Cost minimisation: disodium pamidronate cost saving | |||||
| 2.79 | 3.77 | • Saving £1130 (£936) chemotherapy patients | ||||||||
| 2.93 | 3.44 | • Saving £776 (£643) hormone therapy patients | ||||||||
| aBotteman174 | 2006 | Breast | UK | 10 years | 3.71 | 6.11 | Dominant £2554 (£2267) cost saving | |||
| aJoshi181 | 2011 | Lung | UK + 4 EU | 0.75 year average OS | 1.44 | 2.01 | Dominant £155 (€209) UK cost saving | |||
| aCarter183 | 2011 | Prostate | Netherlands | 0.83 | 1.59 | £2124 (€2430) | ||||
| Portugal | £7565 (€8655) | |||||||||
| Germany | £20,614 (€23,582) | |||||||||
| France | £31,476 (€36,007) | |||||||||
| aXie185 | 2011 | Prostate | USA | 1 year | 0.49 | 0.60 | £43,641 (US$71,027) per SRE avoided | |||
| 3 year | 1.18 | 1.46 | £31,532 (US$51,319) per SRE avoided | |||||||
Available only as abstracts
A number of other papers available only as abstracts were identified by the literature review. Few details are provided within the abstracts and the results for zoledronic acid compared with BSC, or for denosumab versus zoledronic acid, are summarised below for completeness. Note that all these studies are supported by Novartis. The AG has also been in contact with John Carter of Pharmerit with a view to accessing the full texts of the two cost–utility studies of denosumab versus zoledronic acid. Apparently these are ready for full publication and will be made available, but are yet to be received by the AG.
Note that some of the abstracts that were identified, for example Stephens for lung,187 simply report the results available in other abstracts, in this case Botteman188 for lung, and are therefore not repeated in Table 62.
| Lead author | Year | Cancer | Country | Horizon | SREs | Cost per QALY or other c/e | ||
|---|---|---|---|---|---|---|---|---|
| Denosumab | Zoledronic acid | BSC | ||||||
| Botteman189 | 2005 | Breast | Germany | Lifetime | 3.95 | 5.62 | £21,424 (€26,795) | |
| Botteman176 | 2009 | Lung | UK + 4 EU | Lifetime | 1.32 | 2.07 | Dominant £209 (€219) UK saving | |
| Botteman188 | 2009 | Lung | UK, France, Germany | Lifetime | 1.32 | 2.07 | Dominant £386 (€417) UK saving | |
| Botteman180 | 2009 | Renal | UK, France, Germany | Lifetime | 0.66 | 1.74 | Dominant £711 (£699) UK saving | |
| Botteman190 | 2010 | Prostate | Netherlands | 15 months | 0.83 | 1.66 | Dominant | |
| Portugal | 15 months | 0.83 | 1.66 | Dominant | ||||
| France | 15 months | 0.83 | 1.66 | £25,281 (€28,648) | ||||
| Germany | 15 months | 0.83 | 1.66 | £13,916 (€15,770) | ||||
| aEl Ouagari191 | 2005 | Breast | Canada | Lifetime | 3.44b | Dominant over other BPs | ||
| aMeijboom192 | 2009 | Prostate | France | 15 months | 0.83 | 1.66 | £26,541 (€28,648) | |
| Germany | 15 months | 0.83 | 1.66 | £8572 (€9252) | ||||
| aCarter183 | 2011 | Breast | USA | 28 months | 0.69 | 1.01 | £395,459 (US$643,626) | |
| aSnedecor193 | 2011 | Prostate | USA | 27 months | 1.04 | 1.29 | £766,831 (US$1,248,051) | |
| Yu194 | 2011 | Prostate | USA | 1 year | 0.56 | 0.67 | £43,756 (US$66,864) per SRE | |
Results: quality-of-life studies
Clohisy and colleagues195 use the SF-36 to estimate the quality-of-life impacts of surgery for skeletal metastases among 52 US patients, of whom 39 completed the preoperative questionnaire and 23 completed the questionnaire 6 weeks subsequent to surgery, this rate falling to 10 questionnaire completions at the 1-year point. The SF-36 scores over time across a range of dimensions are shown in Table 63.
| Dimension | Preoperative | 6 weeks postoperative | 3 months postoperative | 6 months postoperative | 1 year postoperative |
|---|---|---|---|---|---|
| Physical functioning | 21.7 | 22.8 | 25.1 | 36.9 | 38.5 |
| Role–physical | 2.9 | 4.7 | 4.5 | 9.4 | 16.3 |
| Bodily pain | 20.4 | 36.4 | 45.2 | 47.8 | 50.6 |
| General health | 45.0 | 44.3 | 39.7 | 42.3 | 50.3 |
| Vitality | 27.1 | 33.0 | 37.0 | 42.3 | 50.0 |
| Social functioning | 39.1 | 48.4 | 47.7 | 62.5 | 68.8 |
| Role–emotional | 24.8 | 29.0 | 17.4 | 33.3 | 16.7 |
| Mental health | 54.3 | 55.7 | 61.7 | 62.0 | 50.4 |
These values are not readily translatable into quality-of-life values. The high rate of attrition in the rate questionnaire completion rate may also call into question the reliability of extrapolation from the preoperative through to the postoperative.
Falicov and colleagues35 also investigate the quality-of-life impacts of surgery for skeletal metastases at the same time points as Clohisy and colleagues195 but using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30, the Health Utilities Index–3 and the EQ-5D among 85 Canadian patients with an average age of 58.6 years. Median survival was a little less than 1 year. EQ-5D data are available from 77 of these patients and are valued using the UK social tariff to provide a histogram of the number of patients in the first postoperative year in 0.1 QALY ranges, from −0.2 to −0.1 QALYs (one patient) through to near full health 0.9 to 1.0 QALY (two patients).
The resulting distribution is strongly bimodal with peaks at 0.0 to 0.2 QALYs and 0.6 to 0.7 QALYs, with an implied global average of 0.26 QALYs. It appears that the lower peak and the implied average first-year QALY may be in large part determined by survival. The results are not easily amended for this, though the second peak at 0.6 to 0.7 QALYs cannot be entirely discounted. Possibly because of patient numbers these results are not further analysed by cancer type.
As summarised in the Matza and colleagues ASCO abstract,196 judging from the authorship list it appears that Amgen has commissioned a TTO study among 126 members of the UK general public to estimate the disutilities arising from a number of SREs: SCC without paralysis, SCC with paralysis, pathological fracture of the rib, pathological fracture of the arm and pathological fracture of the leg, radiation to the bone over 2 weeks with 10 administrations, radiation to the bone with only two administrations, and surgery to the bone (Table 64). This involves assessing a 2-year lifespan with cancer and bone metastases, with subsequent assessment of this health state with the various SREs added to it. The base health state utility has a mean estimate of 0.47. The abstract reports the SRE disutilities as QALYs, whereas the electronic copy of the model submitted by the manufacturer reported these as utility decrements and reconstructs the QALY decrement on the assumption that they apply for 11 months. Note that the Amgen model when applying the TTO values also assumes that vertebral fracture has the same disutility as the average across pathological fractures to the rib, arm and leg.
| SRE | Abstract | Modela |
|---|---|---|
| SCC no paralysis | 0.68 | 0.269 |
| SCC with paralysis | 0.44 | |
| Vertebral fracture | n.a. | 0.036 |
| Non-vertebral fracture | 0.07 | 0.036 |
| 2 weeks' radiation | 0.10 | 0.038 |
| 2 radiation administration | 0.05 | |
| Surgery to the bone | 0.14 | 0.071 |
Professor John Brazier was involved in the study and has been approached by the AG with a view to accessing the full paper. Professor Brazier passed this request to Amgen in mid-September 2011. There is little detail on the TTO exercise within the published abstract. It appears that the Amgen modelling may have taken the 2-year QALY loss and broadly have converted it pro rata to an 11-month QALY loss. Whether or not this is correct within the context of the TTO exercise is impossible to tell from the published abstract.
Miksad and colleagues197 (with some indeterminate support from Pfizer and Merck, possibly institutional) estimate the quality-of-life impact from the various stages of ONJ: stage 0 with no evidence of necrotic bone, stage 1 with exposed or necrotic bone but no infection, stage 2 with infection, pain and erythema and stage 3 with pathological fracture, extra oral fistula or osteolysis (Table 65). Of the 54 cancer patients with ONJ contacted by telephone, 34 agreed to undertake questionnaires to assess quality of life by the VAS, TTO with a horizon of 48 weeks and EQ-5D over the telephone.
| Method | Stage 0 | ONJ decrements | ||
|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | ||
| VAS | 0.76 | −0.10 | −0.33 | −0.51 |
| TTO | 0.86 | −0.05 | −0.22 | −0.29 |
| EQ-5D | 0.82 | −0.05 | −0.33 | −0.61 |
Within a cost–utility analysis of palliative radiotherapy, van den Hout and colleagues198 estimate the quality of life among 1157 patients with bone metastases from the primary cancers: 39% breast cancer patients, 25% lung cancer patients, 23% prostate cancer patients and 13% patients with other cancers. This applies the EQ-5D valued using the UK social tariff. Limited quality-of-life differences are found between different methods of delivering radiotherapy, which is the focus of the paper. But for current purposes the evolution of the average quality of life may be of more immediate interest (Table 66). Van den Hout and colleagues198 provide a graph of the evolution of quality of life before death, with the value being relatively constant at around 0.60 in the penultimate year, but declining in a concave fashion over the year before death. This is admittedly average across a range of cancers and van den Hout198 does not report the number of questionnaires available for each time point, but it may be an important qualifier to any modelling.
| Months to death | Utility | Multiplier |
|---|---|---|
| 1 | 0.20 | 34% |
| 2 | 0.25 | 43% |
| 3 | 0.30 | 52% |
| 4 | 0.33 | 57% |
| 5 | 0.37 | 63% |
| 6 | 0.40 | 69% |
| 7 | 0.40 | 69% |
| 8 | 0.43 | 74% |
| 9 | 0.45 | 78% |
| 10 | 0.48 | 83% |
| 11 | 0.53 | 91% |
| 12 | 0.58 | 100% |
Weinfurt and colleagues128 (a named author being employed by Novartis with an additional grant for the study being given by Novartis) estimate the quality-of-life impact of the first on-study SRE among 248 prostate cancer patients who experienced at least one SRE during a zoledronic acid RCT: radiation to the bone, pathological fracture and other first on-study SREs (Table 67). Pooling of the SREs other than radiation and pathological fracture may have been necessary because of the small sample size. For each SRE only patients who experience it as their first on-study SRE are included. The EQ-5D data are valued using the UK social tariff. The analysis apparently controls for other patient characteristics, with the pre-SRE and post-SRE levels being characterised by assessments up to 100 days before the SRE and 100 days after. Before any on-study SRE the baseline average quality of life is 0.70. The first on-study SREs are associated with the following decrements at the first HRQoL measurement within 100 days of SRE diagnosis:
| Main Author | Year | Method | Estimate | SRE type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V fracture | NV fracture | Radiation | Surgery | SCC | Othera | Any | ||||
| Darnitsaris168 | 1999 | TTO general public | HRQoL loss while on disodium pamidronate | −0.19 | ||||||
| TTO experts | HRQoL loss while on disodium pamidronate | −0.43 | ||||||||
| TTO general public | HRQoL loss while on BSC | −0.26 | ||||||||
| TTO experts | HRQoL loss while on BSC | −0.31 | ||||||||
| Hillner169 | 2000 | Expert opinion | HRQoL loss (assumed 1 month duration) | −0.20 | −0.20 | −0.40 | −0.60 | −0.80 | ||
| Reed184 | 2004 | Patient EQ-5D VAS | HRQoL loss within ± 30 days of SRE | 0.07 | ||||||
| HRQoL loss within ± 60 days of SRE | 0.06 | |||||||||
| HRQoL loss within ± 90 days of SRE | 0.05 | |||||||||
| Falicov35 | 2006 | EQ-5D UK tariff | QALY for remaining lifetime | 0.26 | ||||||
| Weinfurt128 | 2006 | EQ-5D UK tariff | HRQoL loss: measurement ≤ 100 days of SRE | −0.13 | −0.07 | −0.02 | ||||
| Matza196 | 2011 | TTO UK public | 2-year QALY loss | −0.07 | −0.10 to −0.05 | −0.14 | −0.44 to −0.68 | |||
-
radiation to the bone −0.07
-
pathological fracture −0.13
-
other SREs pooled −0.02.
Results: resource-use studies
Full papers
Resource use: drug and administration costs
DesHarnais Castel and colleagues182 (supported by Novartis) provide a USA-based micro-costing study of zoledronic acid and disodium pamidronate among patients with metastatic bone disease. This draws data from three outpatient chemotherapy infusion sites, which were also participating in a concurrent zoledronic acid trial. For zoledronic acid average staff times for preinfusion, preparation and set up, administration and follow-up are estimated as 16 minutes, 6 minutes, 40 minutes and 4 minutes, respectively, to give a total of 66 minutes. For disodium pamidronate the times are 16 minutes, 5 minutes, 148 minutes and 4 minutes, respectively, to give a total time of 173 minutes.
Barrett-Lee and colleagues199 (supported by Roche) provide a UK-based study of the costs of administering intravenous BPs among breast cancer patients with bone metastases. This is across three cancer centres, with the first 50 administrations from the start of study being analysed through audit forms. Only 71% of the completed forms relate to breast cancer patients, and results are only reported for these patients. Zoledronic acid provided 67% of administrations, with the vast majority of the remainder being disodium pamidronate. Zoledronic acid is reported as taking an average 4-minute preparation time coupled with 18-minute administration time, though it is not clear whether this is patient time or staff time. Disodium pamidronate is reported as requiring 4 minutes and 93 minutes, respectively. Perhaps the most relevant statistic is that 77% of the breast cancer patients receiving a BP infusion were making a hospital visit solely for this purpose.
Oglesby and colleagues200 (supported by Amgen) undertook a time and motion study of the time and costs of administering zoledronic acid among 42 breast cancer patients and 26 prostate cancer patients in the USA. This concludes that among patients not receiving chemotherapy the overall mean time per administration was 1 hour 9 minutes, whereas among patients receiving chemotherapy it was 3 hours 1 minute, though this latter includes 1 hour 15 minutes specific to the chemotherapy infusion. The average across patients was a little under 2 hours.
Houston and colleagues148 (supported by Roche) within a UK-based study of renal function changes and NHS resource use among 189 patients, estimate an average staff time per zoledronic acid administration of 28 minutes, compared with 6 minutes for oral ibandronate. 148
Resource use: skeletal-related events and adverse events
Malmberg and colleagues201 in a Sweden-based cost-effectiveness study of adding strontium 89 to external radiotherapy among prostate cancer patients estimate the average cost per radiotherapy episode as £5382 (SEK31,011) for those in county, and £8433 (SEK48,585) for those out of county, this latter figure being higher due to the higher rate of inpatient admissions.
Groot and colleagues202 estimate the resource use associated with SREs among 28 prostate cancer patients in the Netherlands over a 2-year period, during which 61 SREs are experienced (Table 68). The majority of SREs are radiotherapy to the bone, most of which are treated as outpatient procedures.
| Outpatient | SREs | Treatment cost | Total cost | |||||
|---|---|---|---|---|---|---|---|---|
| External-beam RT | 25 | £1033 | €1187 | £1033 | €1187 | |||
| Strontium-89 | 21 | £1579 | €1815 | £1579 | €1815 | |||
| Inpatient | LoS | Inpatient cost | Treatment Cost | Total Cost | ||||
| External-beam RT | 3 | 12 | £3091 | €3553 | £1033 | €1187 | £4124 | €4740 |
| Pain management and RT | 1 | 22 | £5667 | €6514 | £1033 | €1187 | £6700 | €7701 |
| SCC and RT | 4 | 29 | £7534 | €8660 | £1033 | €1187 | £8567 | €9847 |
| Hip operation | 2 | 14 | £3477 | €3997 | £1074 | €1234 | £4551 | €5231 |
| Hip operation with CC | 1 | 129 | £33,231 | €38,196 | £2394 | €2752 | £35,625 | €40,948 |
| Fixation of femur fracture | 1 | 16 | £4121 | €4737 | £965 | €1109 | £5086 | €5846 |
| Pain management and RT | 3 | 10 | £2576 | €2961 | £2576 | €2961 | ||
Delea and colleagues203 (supported by Novartis) estimate the costs associated with SREs among 534 US lung cancer patients using data from an insurance claims database. The average SRE-related cost over a 3-year time horizon is estimated as £7974 (US$11,979) with 90% of this occurring within 2 months of the first claim.
Delea and colleagues204 (supported by Novartis) in a similar analysis estimate the costs associated with SREs among 617 US breast cancer patients with bone metastases through a matched pairs analysis of an insurance claims database, of whom 52% experienced at least one SRE. The average lifetime treatment cost of SREs is £8981 (US$13,940). Other costs are also higher in the SRE patient group, by £22,055 (US$34,233) with the average increase among SRE patients being £31,036 (US$48,173).
Lage and colleagues205 (supported by Amgen) undertake a retrospective analysis of a US insurance claims database to estimate the costs of SREs among prostate cancer patients. The average annual costs per individual SRE are radiotherapy: £3143 (US$5930); fracture: £1685 (US$3179); surgery to the bone: £1176 (US$2218); and SCC: £244 (US$460). The annual average per patient is calculated as £6609 (US$12,469).
Barlev and colleagues206 (supported by Amgen) estimate the direct inpatient costs arising from pathological fracture, surgery to the bone and SCC among multiple myeloma, prostate cancer patients with bone metastases and breast cancer patients with bone metastases through a USA Medicare-related database. For prostate cancer patients the average inpatient costs for pathological fracture, surgery to the bone and SCC are £14,652 (US$22,390), £27,546 (US$42,094) and £39,125 (US$59,788) respectively, while for breast cancer patients they are £17,627 (US$26,936), £22,735 (US$34,742) and £39,194 (US$59,894).
Critique of the manufacturer's submission
Patient groups, indications and comparator treatments
The comparators for each cancer are chosen by the manufacturer partly in the light of NICE's CGs, but current prescribing patterns as identified through a manufacturer-commissioned patient chart review coupled with drug use data sourced from the IMS Oncology AnalyzerTM (IMS Health®, PA, USA; URL: www.imshealth.com/deployedfiles/imshealth/Global/Content/StaticFile/IMS_Oncology_Analyzer_Fact_Sheet.pdf) also help to determine these (it appears that the prescribing and treatment data of tables 13 and 14 of the MS) (Table 69).
| Patient group | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| Bisphosphonate tolerant | |||
| All patients | Zoledronic acid | Not presented | Not presented |
| SRE naive | Not presented | BSC | BSC |
| SRE experienced | Not presented | Zoledronic acida | Zoledronic acidb |
| Bisphosphonate contraindicated | |||
| All patients | Not presented | Not presented | Not presented |
| SRE naive | Not presented | BSCc | BSC |
| SRE experienced | Not presented | Zoledronic acid | Zoledronic acid |
For breast cancer, the NICE guideline45 recommends consideration of BPs for patients diagnosed with bone metastases. This is reflected in the manufacturer's prescription data, within which zoledronic acid is the most frequently used BP. In the light of this, zoledronic acid is chosen as the primary comparator for breast cancer.
But note that this does not preclude consideration of patient subgroups: the cost-effectiveness of denosumab among patients who are SRE naive at baseline may differ from that for those who are SRE experienced at baseline. It may also be appropriate to consider BSC as a comparator for those contraindicated to BPs. The manufacturer's case review concluded that 8% of breast cancer patients with bone metastases will probably never be treated with BPs.
For prostate cancer, the NICE guideline46 recommends consideration of BPs for pain relief only when other conventional analgesics and palliative radiotherapy have failed. The manufacturer's case review suggests that 49% of prostate cancer patients have received BPs. It is not clear from the submission to what extent this BP use is a short course, and to what extent it is ongoing continuous use of BPs. The case review also suggests that an additional 19% of patients are likely to receive BPs in the future. Within this, zoledronic acid is the main drug, with over 90% market share. The manufacturer uses this to split the analysis into SRE-naive patients, for whom the comparator is BSC, and SRE-experienced patients which is used as a proxy for uncontrolled pain, for whom the primary comparator is zoledronic acid.
For lung cancer, the NICE guideline48 does not recommend the use of BPs. The metastatic SCC guideline provides similar recommendations for breast cancer and for prostate cancer to the cancer-specific guidelines summarised above. But it adds to this that BPs should not be used in other cancers to treat spinal pain with the intention of preventing metastatic SCC except as part of a RCT. Despite this, the manufacturer's case review suggests that 37% of patients with OSTs have been treated with BPs, with another 13% likely to receive them in the future. Again, it is not clear from the submission to what extent this BP use is a short course, and to what extent it is ongoing continuous use of BPs. Zoledronic acid is the main BP used, with an 80% market share. The manufacturer uses this to split the analysis for OST patients into SRE-naive patients, for whom the comparator is BSC, and SRE-experienced patients, for whom the primary comparator is zoledronic acid.
Within the manufacturer's modelling there appears to be no specific consideration of uncontrolled pain from bone metastases despite use of conventional analgesics and palliative radiation therapy to the bone. This subgroup does not appear to have been defined or analysed within the manufacturer's analyses, but the manufacturer notes that among prostate patients who were SRE experienced at baseline, 80% also had painful bone metastases at baseline. The corresponding figure for OST patients is 86%. In the light of this, the manufacturer has taken the subgroup of patients who were SRE experienced at baseline as a proxy for the likelihood of having uncontrolled pain from bone metastases.
Given data availability, the additional comparators of disodium pamidronate and ibandronic acid are also considered for breast cancer. Similarly, for OSTs, data availability permits the consideration of disodium pamidronate as an additional comparator for SRE-experienced patients.
Manufacturer's model structure summary
The manufacturer separately models three cancer groups: breast cancer, prostate cancer and all OSTs including lung cancer. While the parameter inputs to the modelling of the three cancers differ, the model structure is essentially the same across the three cancers: a cost–utility Markov model; a 4-week cycle to reflect dosing frequency; and a 10-year time horizon for the base case. The AG judges the manufacturer's model to be of good quality and structure, and rebuilds it with some structural additions for its own economic analysis. As a consequence, the manufacturer's model is summarised in detail below.
For a given cancer, all patients within the manufacturer's model are assumed to have the same survival risk. This is derived from a survival analysis (Weibull for breast cancer, gamma for prostate cancer and log-logistic for OSTs based on the Akaike information criterion: tables 53 and 54 of the MS) of the denosumab trial data, pooled across the denosumab and zoledronic acid arms. This is augmented by age-specific non-cancer deaths drawn from general population data. The reason for augmenting the survival curve estimated from the trial data with age-specific non-cancer deaths is not immediately obvious. It may be to help prevent the possible overextrapolation of survival given the survival curves for breast cancer, prostate cancer and OSTs in the MS, or it may be to enable sensitivity analyses around the baseline age to be examined. (The probabilistic modelling treats the baseline age as being deterministic.)
The key assumption, supported by the clinical trials, is that there is no overall survival difference between denosumab and zoledronic acid, with this assumption of no survival differences also being carried over to the other comparators where applicable. In other words, survival is not affected by rates of SREs.
The manufacturer's model divides patients into those who are SRE naive at start of treatment and those who are SRE experienced at start of treatment. The baseline rates of SREs are drawn from the zoledronic acid arm of the relevant denosumab trial.
-
For the SRE-naive, another time-to-event analysis is undertaken using the time to first on-study SRE data from SRE-naive patients in the zoledronic acid arm. The HRs for the other comparators are applied to this to estimate the evolution of first SREs among SRE-naive patients for the comparator arms.
-
For the SRE-experienced, a constant rate of SREs is assumed. This rate is drawn from all on-study SREs among the SRE-experienced at baseline. Note that the manufacturer does not include subsequent SREs among those who were initially SRE naive at baseline. The manufacturer justifies this on the basis that it would break randomisation. It is not clear to the AG why this applies, and including these SREs as a sensitivity analysis may be desirable. RRs are applied to this rate to estimate the rates for the comparator arms.
The balance between the different types of SREs is taken from the denosumab trials, pooled across the arms.
Individual SREs are associated with a HRQoL loss estimated using EQ-5D data from the denosumab trials. These estimates are cancer specific, and are summarised in greater detail in Chapter 9, Clinical data and effectiveness. It is assumed that the HRQoL loss associated with a SRE can extend up to 5 months before the month of its identification, and up to 5 months subsequent to the month of its identification. This yields an overall absolute QALY decrement for each SRE. A utility level is also estimated for SRE-naive patients, and for SRE-experienced patients. SRE-naive patients experiencing a SRE have the SRE experienced utility applied thereafter.
Individual SREs are also associated with a cost. The base case estimates these from a manufacturer-commissioned observational study as summarised in greater detail in Chapter 9, Resource use. The manufacturer's expert opinion suggested that vertebral fracture would be asymptomatic to the degree that treatment would be unlikely, and the base case applies no cost to vertebral fractures. (Between 40% and 45% of fractures in breast cancer, 50% and 70% of fractures in prostate cancer and 40% and 50% of fractures in OSTs including lung were vertebral fractures.)
Rates of the SAEs of ONJ, renal toxicity, hypercalcaemia, hypocalcaemia and skin infections are estimated from the clinical trials separately for denosumab and for zoledronic acid. These are also associated with discontinuation rates as drawn from the clinical trials. Additional non-SAE-specific discontinuations are included in the model, with these being the main source of patients discontinuing active treatment for both denosumab and zoledronic acid. The risk of a SRE among those discontinuing is assumed to be equal to that for BSC.
The HRQoL impact of an adverse event draws on the same EQ-5D data as those used for estimating the HRQoL impact of SREs. Note that a unified overall model is not presented, and the data are analysed separately for SREs and for adverse events. The assumed duration of HRQoL impacts is lifetime for ONJ and renal toxicity, whereas the duration of HRQoL impacts from hypercalcaemia, hypocalcaemia and skin infections is as apparently recorded within the individual patient level data.
Clinical data and effectiveness
Patient characteristics
Baseline patient characteristics are drawn from the relevant denosumab trials (Table 70).
| Characteristic | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| Age (years) | 57 | 71 | 60 |
| Female | 99% | 0% | 36% |
| SRE naive | 59% | 74% | 49% |
Survival data
On the basis of the Akaike information criterion, the survival analysis of the data pooled across the arms of the denosumab trials suggests modelling breast cancer survival using a Weibull, prostate cancer using a gamma and OSTs using a log-logistic functional form (Table 71).
| Parameter | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| Distribution | Weibull | Gamma | Log-logistic |
| Intercept | 7.2206 | 6.5823 | 5.7772 |
| Scale | 0.7775 | 0.9240 | 0.7154 |
| Shape | 0.6243 |
The key assumption in the above is that there is no overall survival difference between denosumab and zoledronic acid, with this assumption of no survival differences also being carried over to the other comparators where applicable. In other words, survival is not affected by rates of SREs. Any frailty distribution around multiple SREs in the same patient similarly is assumed to not affect survival. The survival curves are, for reasons that are not entirely clear, augmented with the age-specific non-solid tumour mortality rates as drawn from UK life tables. This results in the following survival percentages within the modelling (Table 72).
| Year | Breast cancer | Prostate cancer | OSTs | |||
|---|---|---|---|---|---|---|
| Fitted curve | + general mortality | Fitted curve | + general mortality | Fitted curve | + general mortality | |
| 1 | 83% | 83% | 68% | 66% | 46% | 46% |
| 2 | 64% | 64% | 41% | 39% | 24% | 24% |
| 3 | 47% | 47% | 25% | 23% | 15% | 15% |
| 4 | 34% | 33% | 15% | 14% | 11% | 11% |
| 5 | 24% | 23% | 9% | 8% | 8% | 8% |
| 6 | 16% | 16% | 6% | 5% | 6% | 6% |
| 6 | 16% | 10% | 6% | 3% | 6% | 5% |
| 7 | 11% | 7% | 4% | 2% | 5% | 4% |
| 8 | 7% | 4% | 2% | 1% | 4% | 3% |
| 9 | 5% | 3% | 2% | 1% | 4% | 3% |
| 10 | 3% | 3% | 1% | 1% | 3% | 3% |
Balance between types of skeletal-related events
The balance between the different SREs is taken from the denosumab trials, with the data being pooled between the arms (Table 73). The balance between the SRE types is time invariant, with the exception that once a SRE-naive patient has experienced a first SRE the balance between SREs is that for subsequent SREs as applied to SRE-experienced patients.
| SRE | Breast cancer | Prostate cancer | OSTs | |||
|---|---|---|---|---|---|---|
| SRE naive | SRE exp. | SRE naive | SRE experienced | SRE naive | SRE experienced | |
| Vertebral fracture | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-vertebral fracture | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Radiation to the bone | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Surgery to the bone | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| SCC | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Rates of skeletal-related events for zoledronic acid
Zoledronic acid is taken as the numéraire against which the other treatments' HRs and RRs are measured. The rates of first SREs and subsequent SREs for the comparator treatments are derived through the application of the relevant HRs and RRs. The rates of SREs for zoledronic acid are split into:
-
the time to first on-study SRE for SRE-naive patients
-
the SRE rate per cycle for patients who are SRE experienced at baseline.
Times to first skeletal-related event among skeletal-related event-naive patients
A reasonably standard set of time to event functional forms are fitted to the time to first on-study SRE among SRE-naive patients for the zoledronic acid arm of the denosumab trials. This results in the log-normal form being assessed as best by the Akaike information criterion for prostate cancer and OSTs.
But the gamma function is estimated as being superior for breast cancer patients with an Akaike information criterion of 3327 compared with 3330 for the log-normal, which is the next best fit. The manufacturer justifies the adoption of a common log-normal form on the basis of the probabilistic model often simulating a shape parameter for the gamma distribution of less than 0.08, which is apparently problematic. But even if this is the case, it would seem desirable to have applied the fitted gamma function within the deterministic modelling to test any sensitivity to this assumption. Unfortunately, the submission does not outline the parameterised form of the gamma distribution for breast cancer. If the central estimate for this postpones the first SRE beyond that suggested by the fitted log-normal distribution this may have tended to bias the analysis in favour of denosumab. The parameter estimates are shown in Table 74.
| Parameter | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| Intercept | 6.8849 | 6.3098 | 6.1074 |
| Scale | 1.6315 | 1.4547 | 1.5229 |
Rates of subsequent skeletal-related events among skeletal-related event experienced patients
The SRE cycle rate is calculated as the total number of SREs divided by the patient-years of exposure, and adjusted to the 28-day cycle length. The base case applies the 21-day window definition of a SRE, which results in the following cycle rates. The manufacturer assumes a cycle lasts 4/52nds of 1 year within this calculation. This is marginally longer than the true 28/365ths and serves to slightly increase the rate of SREs within the zoledronic arm, but this is unlikely to have much, if any, material effect on results (Table 75).
| Quantity | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| Patient-years of exposure | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| SREs | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Cycle rate based no 4/52 | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Cycle rate based no 28/365 | CiC information has been removed | CiC information has been removed | CiC information has been removed |
Note that the SRE rate per cycle for SRE-experienced patients excludes the data on SREs subsequent to the first on-study SRE among the SRE naive at baseline patients. The manufacturer justifies this on the grounds that it would break randomisation. This justification is not understood by the AG. It could be argued that applying the SRE rate estimated from patients who were SRE experienced at baseline to the patients who were SRE naive at baseline but have experienced an on-study SRE is a more serious violation of randomisation or stratification within the trials. Note also that the proportions of patients who were SRE naive at baseline were 59% for breast cancer, 74% for prostate cancer and 52% for OSTs.
Hazard ratios and relative risks for skeletal-related events for comparator treatments
The MS applies the hazard ratios for time to first on-study SRE and RRs for time to first and subsequent SRE as estimated from the denosumab trial data for denosumab versus zoledronic acid (table 24 of the MS), and from the NMA for the other comparators (tables 50, 51 and 52 of the MS) with zoledronic acid being the numéraire as outlined above. These are summarised in Table 76.
| Submission tables 29, 50, 51 and 52a | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| TTF HR vs zoledronic acid | |||
| Pooled across all patients | |||
| BSC/placebo | AiC information has been removed | 1.493 | 1.370 |
| Ibandronic acid | AiC information has been removed | ||
| Disodium pamidronate | AiC information has been removed | AiC information has been removed | |
| Denosumab | 0.820 | 0.820 | AiC information has been removed |
| Denosumab SRE naive | AiC information has been removed | 0.800 | AiC information has been removed |
| Denosumab SRE experienced | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| RR TTF&Subs vs zoledronic acid | |||
| Pooled across all patients | |||
| BSC/placebo | AiC information has been removed | 1.563 | 1.366 |
| Ibandronic acid | AiC information has been removed | ||
| Disodium pamidronate | AiC information has been removed | AiC information has been removed | |
| Denosumab | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Denosumab SRE naive | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Denosumab SRE experienced | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Note that while the submission suggests that the subgroups of SRE-naive and -experienced patients are analysed separately, the subgroup-specific HRs and RRs for denosumab versus zoledronic acid are not applied. Only pooled results are presented for comparator drugs because owing to a lack of published data neither the AG nor the manufacturer was able to undertake a NMA for SRE-experienced or SRE-naive patients. The modelling submitted by the manufacturer applies the HRs and RRs pooled across all patients, whether modelling SRE-naive patients or SRE-experienced patients. This is likely to have mainly affected the cost-effectiveness results presented for prostate cancer and for the OSTs group.
It would seem sensible to apply the SRE-naive- and -experienced-specific HRs and RRs for denosumab versus zoledronic acid when analysing these subgroups. The SRE-experienced-subgroup-specific central estimates suggest a smaller effect from denosumab compared with the pooled estimates for these patients.
Adverse events and discontinuations
The model includes the following SAEs:
-
ONJ
-
renal toxicity
-
hypercalcaemia
-
hypocalcaemia
-
skin infections.
For the main comparators of denosumab and zoledronic acid the rates of these are drawn from the denosumab trials. Each of these SAEs is also associated with a treatment-specific discontinuation rate, again drawn from the denosumab trials (Table 77). A further treatment-specific general discontinuation rate is drawn from the denosumab trials, though it is not clear whether or not the definition of this excluded the discontinuations due to SAEs. The key assumption within the handling of adverse events and discontinuations is that their rates are constant over the period of the modelling.
| SAE | Breast cancer | Prostate cancer | OSTs | |||
|---|---|---|---|---|---|---|
| Per cycle | Discontinuations | Per cycle | Discontinuations | Per cycle | Discontinuations | |
| Zoledronic acid | ||||||
| ONJ | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Renal toxicity | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Hypercalcaemia | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Hypocalcaemia | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Skin infection | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Other discontinuation | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Total per cycle | AiC information has been removed | CiC information has been removed | AiC information has been removed | CiC information has been removed | AiC information has been removed | CiC information has been removed |
| Denosumab | ||||||
| ONJ | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Renal toxicity | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Hypercalcaemia | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Hypocalcaemia | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Skin infection | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Other discontinuation | CiC information has been removed | CiC information has been removed | CiC information has been removed | |||
| Total per cycle | AiC information has been removed | CiC information has been removed | AiC information has been removed | CiC information has been removed | AiC information has been removed | CiC information has been removed |
The rates of adverse events for the other BPs are drawn from the literature, and are assumed to apply equally across the three cancer groups being modelled. Discontinuation rates due to SAEs for the other BPs are assumed to be the average across the rates observed for denosumab and zoledronic acid.
Discontinuation rates for the other BPs not due to SAEs are drawn from another three papers within the literature.
Rates of adverse events for BSC are assumed to be zero. This may be unrealistic and may tend to worsen the cost-effectiveness estimates for the active treatments relative to BSC. In the main, adverse event rates do not appear to be key model drivers as there is sufficient differentiation between active treatments and BSC in terms of reducing SRE rates. Sensitivity analyses that compare active treatments with BSC and assume minimal differences between them in terms of SRE rates may not be reliable, as the assumption of zero adverse events in the BSC arm may have come to the fore of the analysis. But given the cost-effectiveness estimates for active treatments versus BSC as outlined below this may not be a particular concern (it is also, at least in part, addressed in the AG modelling through sensitivity analyses that assume zero adverse events for all treatments). Note that those discontinuing denosumab or BP therapy are assumed to immediately assume the BSC RR for SREs. There is no waning protective effect from having received denosumab or BP therapy.
Discontinuations also introduce what may appear to be a perversity within the model structure. The model estimates both denosumab and zoledronic acid to have a very poor cost-effectiveness when compared with BSC. Because of this, a treatment that has a high discontinuation rate sees patients rapidly move off active treatment and on to the more cost-effective BSC. As a consequence, a high discontinuation rate for an active treatment improves the cost-effectiveness estimate for that treatment. This requires some qualification, in that the situation is more complicated if the main sources of discontinuations are SAEs, with their associated HRQoL and cost impacts. But as can be seen from the above, for both denosumab and zoledronic acid the vast majority of discontinuations are not related to SAEs.
Resource use
The manufacturer undertook a systematic literature review to try to identify the costs associated with SREs and adverse events as outlined in the MS. Out of the 150 papers identified by the search, six were found to have data relevant to the modelling. From these six papers, only the cost of treating hypercalcaemia £4579 (£3791 in 2004) as drawn from the Ross HTA journal publication55 is used.
Drug and administration costs
The list price of denosumab is £309.86 per vial. The manufacturer cites the BNF as the source of the direct drug costs of the comparators. The BNF used by the manufacturer may predate the current BNF62, which differs slightly from table 72 of the submission, giving the list prices as:
-
£174.17 for a 4-mg vial of Zometa®zoledronic acid
-
£165.00 for a 90-mg vial of generic disodium pamidronate.
This compares with the costs applied by the manufacturer of £183.30 and £167.73 respectively. This mainly affects the comparison with zoledronic acid, the manufacturer cost for it being 5% higher than BNF62.
To estimate the administration costs associated with the different administration routes the manufacturer commissioned a micro-costing study, as summarised in the MS. This study was undertaken in the UK among 80 oncology nurses and 20 oncology pharmacists. It is unclear to what extent any of the nursing staff would have had actual experience of denosumab, but they would obviously be fully familiar with subcutaneous injections. The micro-costing study provided estimates of the staff times involved in administering denosumab and BPs, and costed these from a NHS perspective using standard Personal Social Services Research Unit staff costs.
Note that the micro-costing study prompted respondents about the administration times associated with different infusion durations: ‘Question: It is assumed that an infusion of IV X would typically occur over a minimum of X minutes according to the SPC. Is this correct for your centre? If not, please specify the infusion time.’ This wording may have framed responses to the question. It also does not appear to ask whether or not the duration of the intravenous infusion involved any additional nursing time: 15 minutes for zoledronic acid, 15 minutes of intravenous ibandronic acid and 90 minutes for disodium pamidronate. These timings were included in the costing.
For the comparison between denosumab and zoledronic acid the main differences in terms of minutes of staff time reported by the oncology nurses and as outlined in the MS to the nearest minute are given (Table 78).
| Administration element | Denosumab | Zoledronic acid | Disodium pamidronate | Intravenous ibandronic acid | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | Mean | Median | Mean | Median | |
| Preadministration | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Drug preparation | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Drug administration | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Of which drug infusion | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Postadministration | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Total (minutes) | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Total (hours) | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Staff cost | AiC information has been removed | £33.24 | AiC information has been removed | £66.28 | AiC information has been removed | £138.49 | AiC information has been removed | AiC information has been removed |
Owing to the apparently highly skewed nature of replies, the manufacturer has chosen to use the medians rather than the means for costing purposes. The requirement to make this adjustment may suggest that the micro-costing study is not entirely reliable. (Academic-in-confidence information has been removed.)
The manufacturer estimates that denosumab will result in staff time savings compared with zoledronic acid (academic-in-confidence information has been removed) per administration. These arise in part from the preadministration savings (academic-in-confidence information has been removed), but more from drug administration savings (academic-in-confidence information has been removed) within which avoiding the need for infusion saves (academic-in-confidence information has been removed) staff time.
Taking these elements together with the consumables and fixed costs estimated within the micro-costing study yields the total annual direct drug and administration costs (Table 79).
| Administration element | Denosumab | Zoledronic acid | Disodium pamidronate | Intravenous ibandronic acid | Oral ibandronic acid |
|---|---|---|---|---|---|
| Direct drug costs per administration | |||||
| Manufacturer BNF | £183.30 | £167.73 | £183.69 | £183.69 | |
| BNF62 | £174.17 | £165.00 | |||
| Without PAS | £309.86 | ||||
| With PAS | CiC information has been removed | ||||
| Administration | |||||
| Staff time | £33.24 | £66.28 | £138.49 | £66.28 | £4.50 |
| Monitoring cost | £0.00 | £1.41 | £1.41 | £1.41 | £1.41 |
| Consumables | £0.44 | £7.31 | £7.24 | £7.31 | £0.00 |
| Capital costs | £0.06 | £0.52 | £1.84 | £0.52 | £0.00 |
| Annual totals as per manufacturer | |||||
| Without PAS | £4466.80 | £3364.66 | £4117.23 | £3369.73 | £2464.80 |
| With PAS | CiC information has been removed | ||||
| Annual totals BNF62 | |||||
| Without PAS | £4466.80 | £3245.97 | £4081.74 | £3369.73 | £2464.80 |
| With PAS | CiC information has been removed | ||||
Without the patient access scheme (PAS) the annual denosumab cost of £4467 is around £1102 more expensive than zoledronic acid.
The PAS proposed by the manufacturer has recently been approved. (Commercial-in-confidence information has been removed.)
(Commercial-in-confidence information has been removed.)
The base case assumes 4-weekly dosing for both denosumab and the BPs. The manufacturer also supplies a sensitivity analysis that retains 4-weekly dosing for denosumab, but assumes that a percentage of BP patients receive 3-weekly dosing in line with their chemotherapy regimen.
Within the denosumab trials intravenous therapy could be withheld because of elevated creatine. This affects the average dose received within the zoledronic acid arm. The CSRs provide the subject incidence of intravenous dose withholding, though it is not clear to the AG whether this corresponds to the number of patients having their dose withheld or the number of doses withheld. It appears possible that since exposure to zoledronic acid could be resumed only once creatine levels had returned to acceptable levels, some of these incident patients may have had more than one dose withheld. But on the conservative assumption that the incident patient dose withheld data is equivalent to only one dose being withheld the figures in Table 80 are implied.
| Numbers and percentages withheld | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| n | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| n intravenous zoledronic acid withheld | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| % intravenous zoledronic acid withheld | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Average zoledronic acid doses | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Total zoledronic acid dose exposure | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| % zoledronic acid withheld | AiC information has been removed | AiC information has been removed | AiC information has been removed |
The impact of this has not been included within the direct drug and administration costs calculated by the manufacturer.
Skeletal-related event costs
The STARs costing study
The STARs costing study is a manufacturer-commissioned observational study across the USA, Canada, the UK, Germany, Italy and Spain. This recruited patients with bone metastases secondary to breast cancer, prostate cancer, lung cancer or multiple myeloma who had had a SRE during the previous 90 days. Subjects were followed up for an average of around 18 months.
Health-care resource use across a number of different categories was collected: inpatient data, outpatient visits, procedures, emergency room visits, nursing home use and home health visits. The attribution of this resource use to a SRE was apparently at investigator discretion, with no details of the methods for this being reported in the submission.
The health-care resource use drawn from the STARs study for the submission is specific to the (academic-in-confidence information has been removed) UK patients within the study. The STARs study included multiple myeloma patients but, from the data presented in the electronic copy of the manufacturer's model, it appears that the (academic-in-confidence information has been removed) multiple myeloma SREs have been excluded from the total (academic-in-confidence information has been removed) observed to leave (academic-in-confidence information has been removed) SREs split into (academic-in-confidence information has been removed) SREs among breast cancer patients, (academic-in-confidence information has been removed), lung cancer patients and (academic-in-confidence information has been removed) prostate cancer patients.
Trim points and manufacturer's costings
For the derivation of the average inpatient cost per event the manufacturer's costings include an allowance for the excess bed-days within the NHS reference costs. The manufacturer calculates a weighted average length of stay across elective inpatients, non-elective long-stay inpatients and non-elective short-stay inpatients for the identified HRGs. This average HRG length of stay is taken as the trim point. If the average length of stay observed within the STARs study exceeds this, the manufacturer costs this excess at the excess bed day rate for the identified HRGs, averaged across elective inpatients and non-elective long-stay inpatients (Table 81).
| Inpatient days | Vertebral fracture | Non-vertebral fracture | Radiation to the bone | Surgery to the bone | SCC |
|---|---|---|---|---|---|
| Average inpatient stays per patient | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Average duration per stay | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Of which assumed within trim point | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Of which assumed excess bed-days | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
For instance, the average length of stay across the three HRGs identified for non-vertebral fractures treated as an inpatient is calculated as 7.93 days. Among those treated as inpatients for non-vertebral fracture, the STARs study average length of stay is given (academic-in-confidence information has been removed). The manufacturer calculates the excess bed-days (academic-in-confidence information has been removed) minus 7.93 days: (academic-in-confidence information has been removed) days are costed at £217 per day to yield an excess bed-day cost (academic-in-confidence information has been removed). This is added to the weighted average inpatient cost across the three HRGs (academic-in-confidence information has been removed) to yield an overall total cost for non-vertebral fractures treated on an inpatient basis (academic-in-confidence information has been removed).
But the 2010–11 episode trim points for the three identified HRGs (HD39A, HD39B and HD39C) are 45 days, 21 days and 19 days, respectively. Whereas the average treatment duration within the STARs study will encompass a spread of values, it is questionable whether or not any allowance for excess bed-day costs should have been made by the manufacturer.
These considerations around excess bed-day trim points apply throughout the manufacturer's costings of inpatient stays for the other SREs and AEs.
Radiotherapy to the bone costing
For reasons that are not clear, to cost radiotherapy planning and administration the manufacturer uses 2008–9 reference costs and indexes these for inflation, rather than using the 2009–10 reference costs which are employed for all the other SREs.
For the planning of radiotherapy the manufacturer includes the HRG codes SC01Z through to SC03Z, which seems reasonable. It may be more questionable to have included SC04Z relating to planning multiple phases of complex radiotherapy and SC010Z relating to planning ‘other’ radiotherapy. The weighted average cost across inpatients, day cases, outpatients and ‘other’ settings is applied to all those receiving radiotherapy.
Similarly, for the delivery of radiotherapy the manufacturer includes the HRG codes SC21Z through to SC24Z, all of which relate to delivering a single fraction of radiotherapy. Again, it may be more questionable to have included SC29Z relating to the delivery of ‘other’ radiotherapy, the unit costs of this typically being somewhat higher than that of the HRGs specifically relating to delivering a single fraction of radiotherapy. The weighted average cost across inpatients, day cases, outpatients and ‘other’ settings is multiplied by the average number of fractions (academic-in-confidence information has been removed) drawn from the STARs study.
Base-case skeletal-related event costs
The STARs-based costing results in the following cost estimates (Table 82).
| Cost element | Vertebral fracture | Non-vertebral fracture | Radiation to the bone | Surgery to the bone | SCC |
|---|---|---|---|---|---|
| n | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Inpatient cost | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Outpatient cost | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Emergency care cost | AiC information has been removed | AiC information has been removed | |||
| Home health visits | AiC information has been removed | AiC information has been removed | AiC information has been removed | ||
| Procedures | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Total STARs cost | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Base-case cost applied | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
Vertebral fracture is something of an outlier within these costings, with quite significant costs being associated with outpatient visits and outpatient procedures. Possibly because of the questionable reliability of the resource use around vertebral fractures and the numbers observed (academic-in-confidence information has been removed), coupled with expert opinion that vertebral fractures are typically asymptomatic to the extent of not being treated, the manufacturer applies no cost for vertebral fractures in the base case.
Adverse event costs
As already noted, the cost of treating hypercalcaemia, £4579 (£379; 2004), as drawn from the Ross HTA monograph is used for the base case.
For hypocalcaemia the manufacturer assumes that this will require one haematology consultant-led outpatient appointment, one intravenous calcium injection, and two follow-up visits. Each visit is associated with a blood test, to yield a total cost per event of £443.
For the other adverse events the manufacturer assumes that all will be treated as inpatients and simply averages the inpatient cost over a range of HRGs:
-
ONJ HRGs: CZ16 minor maxillofacial procedures, CZ17 intermediate maxillofacial procedures, CZ18 major maxillofacial procedures, and CZ19 complex maxillofacial procedures, to arrive at an average cost of £2465
-
renal toxicity HRGs: all LA07 acute kidney injury and all LA08 chronic kidney disease but not LA09 general renal disorders, to arrive at an average cost of £1681
-
skin infections HRG: only JD04B minor skin disorders category 3 with Intermediate CC at £1440.
Quality of life
The EQ-5D data were administered during the denosumab trials, and this data set is probably the best source of HRQoL data for estimating the impact of SREs on patient quality of life for the purposes of economic modelling. For the health index questions of the EQ-5D, a three-level response was used to assess quality of life. As explored in greater detail below, the manufacturer has undertaken an involved analysis of these data. Prior to exploring the analysis presented by the manufacturer two quite large caveats are in order:
-
At the stakeholder-briefing meeting, the manufacturer undertook to supply the full EQ-5D data analysis report as an appendix to the NICE submission. This report has not been supplied.
-
The submission and its appendices provide no detail of the functional forms that were tested during the EQ-5D data analysis. No statistical justification for the functional form chosen by the manufacturer over other candidate functional forms is presented.
The key assumption underlying the functional form chosen by the manufacturer is that only SREs and adverse events related to metastatic bone disease and its treatment affect deviations from the baseline HRQoL. In the context of the underlying condition(s) being cancer with the possibility of progression, the development of metastatic disease in areas other than the bone and the relatively short anticipated average survival this appears to be a very strong assumption. Other covariates not included within the manufacturer's model might be anticipated to be significant, and it might also be anticipated that there could be a general cancer-specific time trend to the patient HRQoL, such as that within the van den Hout and colleagues198 reference summarised in the quality-of-life review above. Not considering progression within the modelling of utility is surprising.
The other key assumption is that the most appropriate functional form is to estimate the HRQoL impact of a SRE from 5 months before its diagnosis, through diagnosis, and on through to 5 months subsequent to its diagnosis: 11 months in total. For fractures, it is not obvious why the extended period of time before the fracture being identified is required.
Note that the MS makes the assumption that utility 6 months before the diagnosis of a SRE is at the relevant baseline value, whether SRE naive or SRE experienced, and that 6 months subsequent to the diagnosis of the SRE it returns to the baseline SRE-experienced level. Given this, the overall QALY impact of a SRE is in effect calculated as the area between the curves. To illustrate this within the graphs of the calculation of disutility for SRE-naive and -experienced patients in the submission, the manufacturer anticipated impacts of radiation to the bone for a breast cancer patient. Figure 8 replicates this for the 11 months centred on radiation to the bone at T0 for a SRE-experienced breast cancer patient, where the vertical axis measures the HRQoL and the horizontal axis is in time in months.
FIGURE 8.
HRQoL evolution due to radiation to the bone for a breast cancer patient (academic-in-confidence information has been removed).
This is perhaps the neatest evolution of HRQoL due to a SRE within the manufacturer's analysis. It can be taken as an argument in favour of estimating the QALY impact of radiation to the bone as the area between the SRE-experienced straight line for those not experiencing a SRE and the curve for the evolution of HRQoL associated with radiation to the bone of a SRE-experienced patient.
But not all the curves are quite so tidy, as shown in full in Appendix 13. Cherry-picking to a similar degree but in the opposite direction to the manufacturer, the evolution of HRQoL due to vertebral fracture within the OSTs group of cancer patients for a SRE-experienced patient is shown in Figure 9.
FIGURE 9.
HRQoL evolution due to vertebral fracture for a OST cancer patient (academic-in-confidence information has been removed).
It is not obvious that the HRQoL impact of the vertebral fracture should be taken as far back as 5 months before its diagnosis. The dip at 4 months before diagnosis of vertebral fracture is not maintained and might be better discarded as an effect. It is also possibly questionable to include the estimated effects for the full 5 months subsequent to the diagnosis of the vertebral fracture. From the above, the argument could be made that the HRQoL impact of vertebral fractures is limited to the 2 months subsequent to T0.
These considerations outlined may apply in the opposite direction for the evolution of HRQoL because of SCC. While the picture varies across the cancers there is some similarity in terms of a possibly permanent effect, as would be anticipated given that a proportion of patients will have some degree of paralysis (Figure 10).
FIGURE 10.
HRQoL evolution due to SCC for a prostate cancer patient (academic-in-confidence information has been removed).
In this instance it can be argued that evaluating the QALY impact of SCC for only the 5 months subsequent to diagnosis of SCC may have underestimated the HRQoL impact of SCC. The HRQoL decrements estimated for the months subsequent to SCC for the SRE-experienced patient are presented in Table 83, together with the baseline HRQoL value for SRE-naive and -experienced patients.
| SRE experienced | Breast cancer | Prostate cancer | OSTs |
|---|---|---|---|
| SRE-naive baseline HRQoL | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| SRE-experienced baseline HRQoL | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Permanent loss from first SRE | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| SCC HRQoL decrements | |||
| First month post diagnosis | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Second month post diagnosis | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Third month post diagnosis | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Fourth month post diagnosis | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| Mean decrement post diagnosis | AiC information has been removed | AiC information has been removed | AiC information has been removed |
The total QALY decrements associated with SREs as presented by the manufacturer are summarised in Table 84. For the SRE-naive patient experiencing a SRE there is a permanent loss from the first SRE that is experienced. This accounts for much of the difference in the SRE QALY impacts between SRE-naive and -experienced patients. It is not clear that the full discounted impact of this is within the numbers below.
| SRE | Breast cancer | Prostate cancer | OSTs | |||
|---|---|---|---|---|---|---|
| SRE naive | SRE experienced | SRE naive | SRE experienced | SRE naive | SRE experienced | |
| Vertebral fracture | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Non-vertebral fracture | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Radiation to the bone | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Surgery to the bone | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| SCC | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
In the main, however, based on a fairly crude assessment of the central values derived and the graphs of the evolution of HRQoL over time as in Appendix 13, the manufacturer's analysis of the EQ-5D data does not appear to have arrived at unreasonable estimates for the impacts of SREs. But this retains the caveat that no detail of the EQ-5D study in terms of the alternative functional forms that were tested has been provided by the manufacturer. There is also no provision for other elements of the cancers, such as progression, to affect patient quality of life, which may have led to bias.
The manufacturer's model corrects the SRE utility decrements to avoid projecting any effect priors to the start of treatment, i.e. during the first five cycles of the model; for instance, for the third 28-day cycle to exclude the impacts of the fifth and fourth months before a SRE.
The manufacturer's model appears to correctly adjust the post-SRE HRQoL decrements for those dying in the 5 months subsequent to an event in order not to project SRE HRQoL impacts beyond death (Table 85).
| Adverse event | Breast cancer | Prostate cancer | OSTs | |||
|---|---|---|---|---|---|---|
| Average days | Decrement | Average days | Decrement | Average days | Decrement | |
| ONJ | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Renal toxicity | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Hypercalcaemia | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Hypocalcaemia | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Skin infection | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
The HRQoL impact of an adverse event draws on the same EQ-5D data as those used for estimating the HRQoL impact of SREs. A unified overall model is not presented and the data are analysed separately for SREs and for AEs.
The assumed duration of HRQoL impacts is lifetime for ONJ and renal toxicity, whereas the duration of HRQoL impacts from hypercalcaemia, hypocalcaemia and skin infections is as recorded within the individual patient level data.
Manufacturer's modelling conformity to National Institute for Health and Care Excellence reference case
The manufacturer's model broadly conforms to the NICE reference case as summarised in Table 86.
| Attribute | Reference case and TA methods guidance | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Comparator(s) | Therapies routinely used in the NHS, including technologies regarded as current best practice | Partial |
| Given the NICE breast cancer guideline, assessing denosumab only compared with BPs for the main analysis is reasonable. But this ignores the patient group contraindicated to BPs, for whom BSC would have been the appropriate comparator | ||
| For both prostate and lung cancer the manufacturer splits the patient groups into SRE naive and SRE experienced at baseline. For SRE-naive patients denosumab is assessed against BSC, which is appropriate | ||
| SRE experience is taken to be a close proxy for uncontrolled pain despite use of conventional analgesics. This enables the manufacturer to model denosumab against BPs for these patients. The evidence presented by the manufacturer that these patients are on ongoing BP use in the UK is not clear-cut. There is also no consideration of those contraindicated to BP use | ||
| Patient group | As per NICE scope | Yes |
| Perspective costs | NHS and Personal Social Services | Yes |
| Perspective benefits | All health effects | Yes |
| Form of evaluation | Cost-effectiveness analysis | Yes. Cost–utility analyses |
| Time horizon | Sufficient to capture differences in costs/outcomes | Yes. 10 years, which is in effect lifetime |
| Synthesis of evidence on outcomes | Systematic review | Yes. A NMA is undertaken. But note that this differs from the AG's NMA in part due to the studies that are included |
| Outcome measure | QALYs | Yes |
| Health states for QALY | Using a standardised validated instrument | Yes. Drawn from trial-based EQ-5D data |
| Benefit valuation | TTO or standard gamble | Yes. EQ-5D converted to utilities using the UK social tariff |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the public | Yes. The UK social tariff |
| Discount rate | An annual rate of 3.5% on both costs and health effects | Yes |
| Equity | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes |
| Probabilistic modelling | Probabilistic modelling | Yes. With the exception of the direct drug costs and the unit costs of drug administration, the model was fully probabilistic |
| Sensitivity analysis | A range of univariate sensitivity analyses are presented |
Manufacturer's base-case results
What follows are the manufacturer-reported estimates for the cost-effectiveness of denosumab compared with the primary comparator, plus additional pairwise comparisons where the NMA provides effectiveness estimates for other BPs.
Unfortunately, the manufacturer has not reported results relative to BSC for those contraindicated to BPs.
Breast cancer: all patients
The base-case results (Table 87) are that denosumab prevents on average around 0.21 SREs compared with zoledronic acid. Among those contraindicated to BPs, denosumab is anticipated to prevent on average around 0.91 SREs compared with BSC. These yield a gain from denosumab of 0.007 QALYs compared with zoledronic acid. Excluding the PAS, the net overall cost increase from denosumab is £1483 compared with zoledronic acid. Including the PAS, denosumab is estimated to yield cost savings of £483 compared with zoledronic acid. This results in the following cost-effectiveness estimates for denosumab within the pairwise comparisons (Table 88).
| Quantity | Breast cancer: all patients | |||
|---|---|---|---|---|
| Denosumab | Zoledronic acid | Disodium pamidronate | Ibandronic acid | |
| Life-years (undiscounted) | 3.45 | 3.45 | 3.45 | 3.45 |
| Life-years (discounted) | 3.16 | 3.16 | 3.16 | 3.16 |
| SREs | 2.13 | 2.34 | 2.47 | 2.30 |
| Net SREs vs denosumab | 0.00 | + 0.21 | + 0.34 | + 0.17 |
| QALYs | 1.912 | 1.904 | 1.898 | 1.907 |
| Net QALYs vs denosumab | 0.000 | −0.007 | −0.013 | −0.005 |
| Costs | ||||
| Treatment | ||||
| Excluding PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Including PAS | CiC information has been removed | |||
| SREs | £2932 | £3241 | £3435 | £3199 |
| AEs | £93 | £137 | £317 | £37 |
| Death | £4356 | £4356 | £4356 | £4356 |
| Total costs | ||||
| Excluding PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Including PAS | CiC information has been removed | |||
| Net excluding PAS vs denosumab | £0 | −£1483 | £1487 | −£72 |
| Net including PAS vs denosumab | £0 | £483 | £3453 | £1895 |
| Quantity | Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ICER |
|---|---|---|---|---|---|
| Denosumab | CiC information has been removed | 1.912 | |||
| With PAS | CiC information has been removed | ||||
| Zoledronic acid | CiC information has been removed | 1.904 | £1484 | 0.007 | £203,387 |
| With PAS | −£483 | Denosumab dominant | |||
| Disodium pamidronate | CiC information has been removed | 1.898 | −£1486 | 0.013 | Denosumab dominant |
| With PAS | −£3453 | Denosumab dominant | |||
| Ibandronic acid | CiC information has been removed | 1.907 | £72 | 0.005 | £13,835 |
| With PAS | −£1895 | Denosumab dominant |
Without the PAS, the cost-effectiveness of denosumab compared with zoledronic acid is estimated as £203,387 per QALY. The additional benefit of 0.007 QALYs does not warrant the additional cost of £1483. Probabilistic modelling undertaken by the manufacturer results in an identical central estimate of a 0.007 QALY gain over zoledronic acid for a similar average additional cost of £1490.
With the PAS, denosumab is estimated to be cost saving relative to zoledronic acid. Given the small additional QALY gain, this results in denosumab dominating zoledronic acid. Probabilistic modelling undertaken by the manufacturer results in the same central estimate of QALYs gained with a similar average cost saving of £481 from denosumab compared with zoledronic acid.
Prostate cancer: skeletal-related event experienced
The QALY gains anticipated from denosumab over zoledronic acid are slightly smaller than but similar to those within breast cancer at 0.006 QALYs with the lower survival limiting the potential for patients' gains (Table 89). Excluding the PAS the incremental cost of denosumab is estimated as £922 versus zoledronic acid, but with the PAS denosumab results in cost savings of £281 compared with zoledronic acid. This results in the following cost-effectiveness estimates (Table 90).
| Quantity | SRE-experienced patients (26%) | |
|---|---|---|
| Denosumab | Zoledronic acid | |
| Life-years (undiscounted) | 2.17 | 2.17 |
| Life-years (discounted) | 2.04 | 2.04 |
| SREs | 1.98 | 2.12 |
| Net SREs vs denosumab | 0.00 | +0.14 |
| QALYs | 1.089 | 1.083 |
| Net QALYs vs denosumab | −0.006 | |
| Costs | ||
| Treatment | ||
| Excluding PAS | CiC information has been removed | CiC information has been removed |
| Including PAS | CiC information has been removed | |
| SREs | £2810 | £3010 |
| AEs | £165 | £125 |
| Death | £4625 | £4625 |
| Total costs | ||
| Excluding PAS | CiC information has been removed | CiC information has been removed |
| Including PAS | CiC information has been removed | |
| Net excluding PAS vs denosumab | −£922 | |
| Net including PAS vs denosumab | £281 | |
| Comparator | Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ICER |
|---|---|---|---|---|---|
| Denosumab | CiC information has been removed | 1.089 | |||
| With PAS | CiC information has been removed | ||||
| Zoledronic acid | CiC information has been removed | 1.083 | £922 | 0.006 | £157,276 |
| With PAS | −£281 | Denosumab dominant |
Without the PAS, the cost-effectiveness of denosumab versus zoledronic acid is estimated as £157,276 per QALY. Probabilistic modelling undertaken by the manufacturer suggests the same average gain of 0.006 QALYs from denosumab over zoledronic acid for a similar average cost of £918. With the PAS, denosumab is estimated to result in a cost saving of £281 compared with zoledronic acid and as a consequence, given the small gain of 0.006 QALYs, is estimated to dominate zoledronic acid. Probabilistic modelling undertaken by the manufacturer indicates the same average gain from denosumab over zoledronic acid of 0.006 QALYs with an additional average cost saving of £286.
Prostate cancer: skeletal-related event naive
For the SRE-naive patients, who made up 74% of the denosumab trial population, the base-case cost-effectiveness results are summarised in Table 91.
| Comparator | Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ICER |
|---|---|---|---|---|---|
| Denosumab | CiC information has been removed | 1.189 | |||
| With PAS | CiC information has been removed | ||||
| BSC | CiC information has been removed | 1.150 | £3993 | 0.039 | £102,067 |
| With PAS | £2790 | £71,320 |
Without the PAS, denosumab is estimated to have a cost-effectiveness compared with BSC of £102,067 per QALY. With the PAS, the cost-effectiveness estimate falls but only to £71,320 per QALY, which is also well above normal cost-effectiveness thresholds. Probabilistic modelling by the manufacturer is in line with this, with denosumab yielding a central estimate of 0.039 QALYs over BSC but at an average net cost of £2776.
Other solid tumours: skeletal-related event experienced
The QALY gains anticipated from denosumab are smaller than those estimated for the previous analyses: 0.004 QALYs compared with zoledronic acid (Table 92). Excluding the PAS the incremental cost of denosumab is estimated as £757 versus zoledronic acid but sees cost savings of £2118 versus disodium pamidronate. With the PAS, denosumab results in cost savings of £43 compared with zoledronic acid and the net saving relative to disodium pamidronate increases to £2918. This results in the following cost-effectiveness estimates (Table 93).
| Quantity | SRE-experienced patients (48%) | ||
|---|---|---|---|
| Denosumab | Zoledronic acid | Disodium pamidronate | |
| Life-years (undiscounted) | 1.76 | 1.76 | 1.76 |
| Life-years (discounted) | 1.64 | 1.64 | 1.64 |
| SREs | 1.37 | 1.46 | 1.47 |
| Net SREs vs denosumab | 0.00 | +0.08 | +0.10 |
| QALYs | 0.765 | 0.761 | 0.759 |
| Net vs denosumab | −0.004 | −0.006 | |
| Costs | |||
| Treatment | |||
| Excluding PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Including PAS | CiC information has been removed | ||
| SREs | £2556 | £2714 | £2754 |
| AEs | £57 | £57 | £183 |
| Death | £4612 | £4612 | £4612 |
| Total costs | |||
| Excluding PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Including PAS | CiC information has been removed | ||
| Net excluding PAS vs denosumab | −£757 | £2118 | |
| Net including PAS vs denosumab | £43 | £2918 | |
| Comparator | Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ICER |
|---|---|---|---|---|---|
| Denosumab | CiC information has been removed | 0.765 | |||
| With PAS | CiC information has been removed | ||||
| Zoledronic acid | CiC information has been removed | 0.761 | £757 | 0.004 | £205,580 |
| With PAS | −£43 | Denosumab dominant | |||
| Disodium pamidronate | CiC information has been removed | 0.759 | −£2118 | 0.006 | Denosumab dominant |
| With PAS | −£2918 | Denosumab dominant |
Without the PAS, the cost-effectiveness of denosumab versus zoledronic acid is estimated as £205,580 per QALY. Probabilistic modelling undertaken by the manufacturer paints a similar picture at central estimates, with an average gain from denosumab over zoledronic acid of 0.004 QALYs at an average net cost of £749.
With the PAS, denosumab is estimated to result in a cost saving of £43 compared with zoledronic acid and, given the small gain of 0.004 QALYs, to dominate zoledronic acid. Probabilistic modelling undertaken by the manufacturer again paints a similar picture to the deterministic modelling, with an average gain from denosumab over zoledronic acid of 0.004 QALYs with a small cost saving of £45.
Other solid tumours: skeletal-related event naive
For the SRE-naive patients, who made up 52% of the denosumab trial population, the base-case cost-effectiveness results are summarised in Table 94.
| Comparator | Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ICER |
|---|---|---|---|---|---|
| Denosumab | CiC information has been removed | 0.803 | |||
| With PAS | CiC information has been removed | ||||
| BSC | CiC information has been removed | 0.782 | £2530 | 0.021 | £122,499 |
| With PAS | £1730 | £83,763 |
For the primary comparator of BSC, even with the PAS the resulting cost-effectiveness estimate for denosumab of £83,763 per QALY is again well above normal cost-effectiveness thresholds. Probabilistic modelling is in line with this, with denosumab yielding an average 0.021 QALYs over BSC but at an average net cost of £1724.
Manufacturer's structural and sensitivity analyses
The manufacturer undertakes a range sensitivity analyses that apply:
-
time horizons of 2 and 5 years
-
no 21-day window for the definition of SREs
-
costs to vertebral fracture as estimated from the STARs costing exercise
-
the SRE costs as estimated from NHS reference cost admission rates
-
the manufacturer commissioned TTO utilities and the Weinfurt utilities129
-
starting ages of 50 and 65 years
-
a balance between 3-weekly and 4-weekly dosing for intravenous BP administrations
-
oral administration for ibandronic acid
-
community administration for denosumab
-
no discontinuations and a constant 0.025 discontinuation rate per cycle for all treatments
-
sensitivity analyses around the discount rates.
Many of these sensitivity analyses have relatively little impact on the outcomes of the modelling. The full sensitivity analyses presented by the manufacturer for the with-PAS scenario are included in Appendix 14 of this report.
For the breast cancer modelling across all patients, without the PAS results are reasonably sensitive to:
-
the time horizon adopted, which if only 2 years worsens the ICER for denosumab compared with zoledronic acid from £203,000 per QALY to £254,000 per QALY, which provides some of the rationale for undertaking the modelling and extrapolation of effects
-
the source of utilities, with the TTO values increasing the net gain from denosumab by around 20% with parallel effects on the ICERs, while the Weinfurt utilities decrease the net gain from denosumab by a slightly smaller percentage
-
ibandronic acid being administered orally, which worsens the ICER for denosumab compared with it to £387,000 per QALY
-
the frequency of dosing for the intravenous BPs, as would be anticipated, reducing the net cost of denosumab over zoledronic acid by around 20% and causing the ICER to fall to £161,000 per QALY. For the other comparisons, including some 3-weekly, intravenous dosing is sufficient for denosumab to be cost saving and so dominant
-
the discontinuation rates assumed, with a zero discontinuation rate increasing the net lifetime costs from denosumab use. This mainly affects the comparison with ibandronic acid where the ICER worsens per QALY. (Commercial-in-confidence information has been removed.)
With the PAS, similar effects are observed among breast cancer patients in terms of the changes to the net QALYs and net costs but the sensitivity analyses still result in denosumab being estimated to be cost saving and to confer small QALY gains, and so dominate the other treatments. Only oral ibandronic acid stands out with a small net cost from denosumab use (commercial-in-confidence information has been removed), resulting in a cost-effectiveness estimate of £387 per QALY.
For SRE-experienced prostate cancer patients, without the PAS, results are reasonably sensitive to:
-
excluding the 21-day window from the identification of SREs, with this improving the ICER for denosumab compared with zoledronic acid from £157,000 per QALY to £89,000 per QALY
-
basing the utility estimates on the Weinfurt reference, which worsens the ICER to £384,000 per QALY
-
the frequency of dosing for the intravenous BPs, reducing the net cost of denosumab over zoledronic acid and causing the ICER to fall to £125,000 per QALY
-
community administration of denosumab, causing the ICER to fall per QALY. (Commercial-in-confidence information has been removed.)
With the PAS, as for the breast cancer modelling, similar effects are observed in terms of the changes to the net QALYs and net costs but the sensitivity analyses still result in denosumab being estimated to be cost saving and to confer small QALY gains, and so dominate zoledronic acid.
For SRE-naive prostate cancer patients, even with the PAS, the sensitivity analyses result in ICERs in the range of £50,000 per QALY to £355,000 per QALY, which are outside the range usually considered to be cost-effective.
For SRE-experienced patients with OSTs, for the comparison with zoledronic acid the cost-effectiveness of denosumab without the PAS is reasonably sensitive to:
-
excluding the 21-day window from the identification of SREs, with this improving the ICER for denosumab compared with zoledronic acid from £206,000 per QALY to £144,000 per QALY
-
basing the utility estimates on the Weinfurt reference, which worsens the ICER to £420,000 per QALY
-
the frequency of dosing for the intravenous BPs, reducing the net cost of denosumab over zoledronic acid and causing the ICER to fall to £176,000 per QALY
-
community administration of denosumab, causing the ICER to fall per QALY (commercial-in-confidence information has been removed)
-
zero discontinuations across treatments which improves the ICER per QALY. (Commercial-in-confidence information has been removed.)
With the PAS, as for the modelling of prostate cancer and breast cancer, similar effects are observed in terms of the changes to the net QALYs and net costs but the sensitivity analyses still result in denosumab being estimated to be cost saving and to confer small QALY gains, and so dominate zoledronic acid.
For SRE-naive OST patients, even with the PAS, the sensitivity analyses result in ICERs in the range £70,000 per QALY to £320,000 per QALY and would not typically be considered cost-effective.
The assessment group's critique of the manufacturer's model and results
The manufacturer's case is broadly that the average patient benefits from the reduced number of SREs are not large. (Commercial-In-confidence information has been removed.)
But for patients for whom zoledronic acid is not indicated, the manufacturer accepts that even with the PAS the relatively small patient gains do not justify the additional cost of denosumab. The manufacturer's cost-effectiveness estimates for denosumab compared with BSC are typically closer to £100,000 per QALY than £50,000 per QALY, even with the PAS.
There are some concerns around the reasonableness of the manufacturer's argument that case review indicates the majority of patients have had or are likely to have treatment with BPs. These may be short courses rather than continuous ongoing treatment, the latter seeming to be the manufacturer's intention in terms of denosumab use.
The estimation of utility decrements from the trials' EQ-5D data is at first pass impressive, but the complete lack of detail about the alternative functional forms that have been tested raises concerns. It also seems surprising that other aspects of the underlying cancers were not included as covariates. With this caveat and as there is no consideration of progression within the utility data, the general model structure employed by the manufacturer appears reasonable. It is also in line with the NICE reference case.
The manufacturer's implementation of the utility data within the model may have two errors within it. If so, these are likely to pull in opposite directions. The model appears to attempt to correct so as not to project benefits before the start of therapy. But it appears that this may cut off the patient benefits in the 5 months following a SRE occurring in the first cycle of the model, in the 4 months following a SRE occurring in the second cycle of the model, etc. Pulling in the opposite direction, it also appears that the SRE decrement among SRE-naive patients is measured from the SRE-naive baseline HRQoL for the 5 months subsequent to a SRE, but the patient is modelled as also stepping down to the SRE-experienced HRQoL for this period and beyond. This may double-count the impact of first SREs in the 5 months subsequent to their incidence.
Independent economic assessment
Methods
Before any cost-effectiveness modelling, some basic considerations should be borne in mind. Within the literature there are two broad strands of cost-effectiveness assessments: the straightforward assessments of within-trial costs and benefits and the more complicated modelling of costs and benefits with extrapolation to death, this latter also permitting other comparators to be included than just those studied within the trial. The more complicated modelling, including that of the Amgen submission [Amgen Ltd. Multiple Technology Appraisal: Denosumab for the treatment of bone metastases from solid tumours (unpublished report). London: National Institute for Health and Care Evidence; 2011], typically treats metastatic bone disease as a chronic condition. This gives rise to a SRE rate in one arm under consideration, with comparator treatments affecting this rate. There are additional considerations around distinguishing between the time to first SRE for SRE-naive patients compared with the rate of subsequent SREs for SRE-experienced patients. Almost by definition, extrapolation beyond the trial is likely to alter the patient balance towards SRE-experienced patients as SRE-naive patients experience SREs. Cost-effectiveness may differ between SRE-naive patients and SRE-experienced patients.
But even in the light of this, given that the condition is typically modelled as being chronic and stable through to death with discontinuations immediately leading to the BSC risk of an event, there is an argument for a simple economic assessment of the within-trial outcomes before any more sophisticated cost–utility economic modelling and extrapolation.
The more simple-minded within-trial assessment trial considers the economic implications of:
-
the average number of treatments in each arm of the trials
-
the average number of SREs per patient in each arm of the trials
-
the average number of SAEs per patient in each arm of the trials
-
the average months on study within each arm and how this may condition the above.
Unfortunately, the AG does not have access to sufficiently disaggregate data to present this analysis for the SRE-naive and -experienced subgroups.
Other than the paper by Xie and colleagues,185 the cost-effectiveness literature has not explicitly modelled progression or considered any explicit stopping rule. There are three main reasons why disease progression may affect cost-effectiveness:
-
The rate of SREs may change at progression.
-
A proportion of patients discontinue therapy at progression, which may differ between treatments.
-
The general patient quality of life and the quality-of-life impacts from SREs may change at progression.
Modelling the above would require the progression-free survival curves for each cancer, which are available from the denosumab CSRs. But it would also require the time to first SRE and the rate or time to subsequent SREs within the zoledronic acid arm to be split by those without disease progression and those with progression. These data are not readily available. There would also be the question of whether or not the relative effect for the other comparators would remain constant at progression. The additional concern about how to model the quality-of-life impacts of SREs among progression-free patients and patients with progression is also not readily addressable given the quality-of-life estimates within the literature and the Amgen submission.
The AG views the structure of the manufacturer's model as a reasonable basis for the estimation of cost-effectiveness. There is no suggestion that treatments affect the rate of progression or overall survival. If progression changes the rate of SREs, this can be explored by sensitivity analyses that change the rate of SREs from a given cycle in the model onwards. Quality of life declining towards the end of life can be explored through a structural sensitivity analysis that applies the EQ-5D utilities of van den Hout and colleagues. 198
In the light of this, the AG has rebuilt the model using the same overall structure as the manufacturer's model, the main adjustments within this being to the treatment of utilities to adjust for not projecting benefits to before the start of treatment, and to measure any utility decrements subsequent to a SRE from the SRE-experienced baseline utility. In the absence of other data, the average utility decrement for SREs within lung cancer has been assumed to be the same as within the OSTs including lung cancer trial.
The base case of the modelling applies the results of the AG's NM. Additional structural elements added to the model are the facility for SCC to have a sustained HRQoL impact beyond 5 months from diagnosis, and a decay in quality of life in the final year, as estimated by van den Hout and colleagues. 198 These are applied as sensitivity analyses only to the base case.
Given the AG's NMA results, cost-effectiveness results are presented for four cancer groups:
-
breast cancer
-
prostate cancer
-
OSTs including lung cancer
-
lung cancer.
These are further subdivided into;
-
all patients
-
SRE-naive patients
-
SRE-experienced patients.
The model structure can be presented diagrammatically (Figure 11).
FIGURE 11.
Cost–utility model structure.
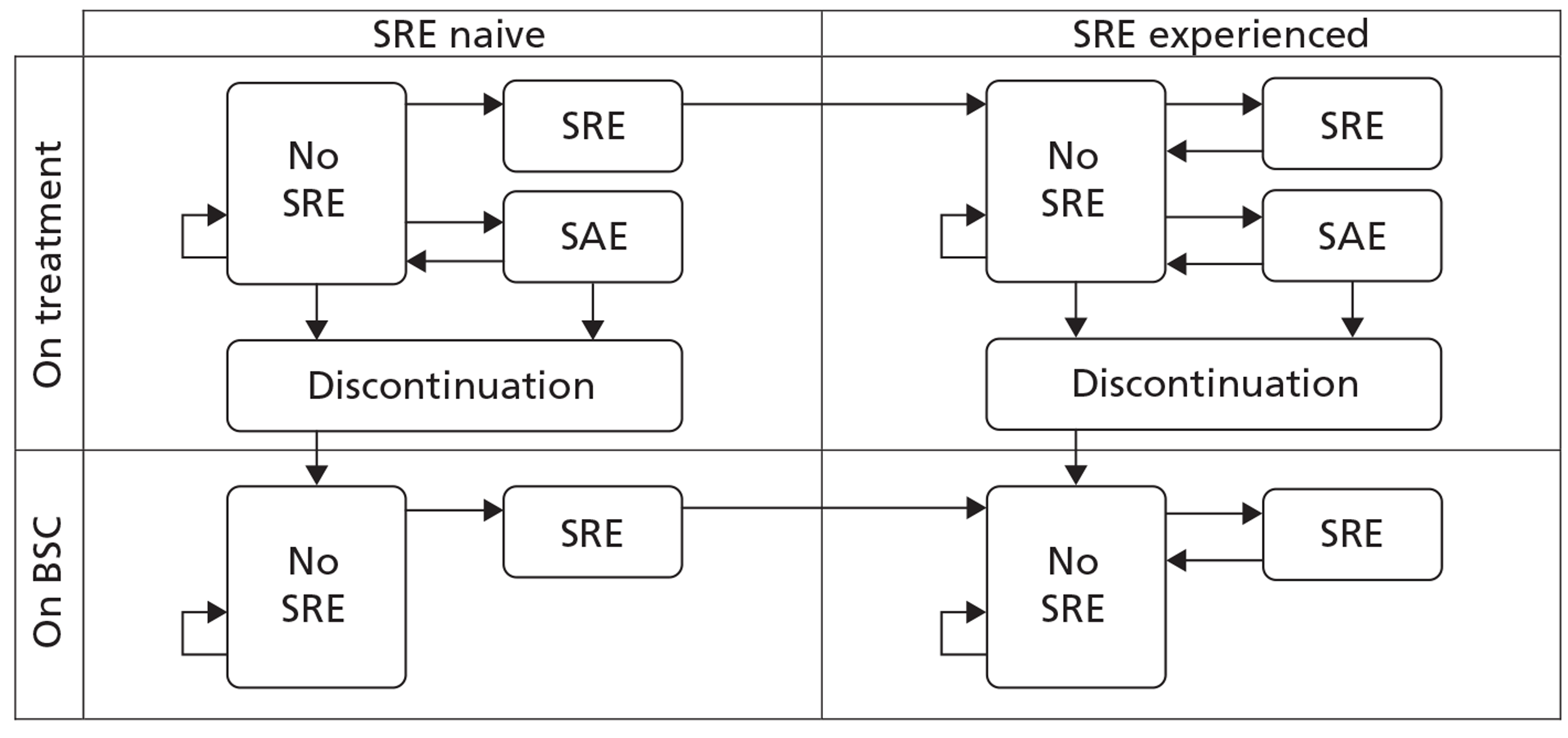
The following analyses are presented (Table 95), and compared with those of the manufacturer.
| SRE RR and HR | Breast cancer | Prostate cancer | OST + lung cancer | Lung cancer | ||||
|---|---|---|---|---|---|---|---|---|
| Pooled | Specific | Pooled | Specific | Pooled | Specific | Pooled | Specific | |
| Manufacturer | ||||||||
| All patients | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| SRE naive | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ |
| SRE experienced | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ |
| AG | ||||||||
| All patients | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| SRE naive | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| SRE experienced | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
For the above, the cost–utility analyses that employ the pooled HRs and RRs are presented as the base case. A range of univariate sensitivity analyses around these estimates are then presented in summary format.
The AG views the structural sensitivity analyses that employ the SRE-naive- and -experienced-specific HRs and RRs as sufficiently important for the full results of their impacts on the base case to be reported. This is complicated by the results of the AG's NMA being pooled across all patients, i.e. not being specific to SRE-naive or SRE-experienced patients. In the light of this and the manufacturer's summary of subgroup by SRE history for time to first and time to first and subsequent on-study SRE, the structural sensitivity analyses apply the SRE-specific head-to-head clinical effectiveness estimates for the effectiveness of denosumab compared with zoledronic acid, while retaining the results of the AG's NMA for the other comparator(s). This distinction is not available for the modelling of lung cancer.
Clinical parameters and effectiveness data for the modelling
The simplistic analysis of CSR data draws the rates of SREs and SAEs from the CSRs, the manufacturer's model and the MS, with cross checks between the two sources.
The cost–utility modelling draws heavily on the manufacturer's model.
Hazard ratios and relative risks of skeletal-related events
The base cases apply the results of the AG's NMA. The results of the manufacturer's NMA are applied as sensitivity analyses. The structural sensitivity analyses applying the SRE-naive and -experienced HRs and RRs apply to those summarised in Table 76.
Survival
Overall survival is mainly drawn from the manufacturer's model and as summarised in Table 71. Overall survival for lung cancer is drawn from the estimate for zoledronic acid presented within Joshi and colleagues182 using a Weibull extrapolation with survival at a given day being determined by:
Note that Joshi and colleagues181 do not report any standard errors or significance testing for these Weibull parameters, and that as a consequence, in contrast to the other probabilistic modelling, the probabilistic modelling of lung cancer treats the overall survival curve deterministically.
Time to first skeletal-related event and rate of subsequent skeletal-related events
Owing to the manufacturer having access to individual patient-level data restricted to the SRE-naive patient subgroup, the base cases for breast cancer, prostate cancer and OSTs including lung cancer apply the time to first SRE curves presented within the MS and summarised in Table 74. These are not available for lung cancer, and the base cases apply the AG estimate for this as summarised in Table 96 and Table 97. The additional AG estimates for the time to first SRE for zoledronic acid are applied as sensitivity analyses within the modelling.
| Functional form | SRE naive | All patients | ||||
|---|---|---|---|---|---|---|
| Breast | Prostate | Breast | Prostate | OST + lung | Lung | |
| Weibull | 0.000249 | 0.000115 | 0.000351 | 0.000148 | 0.000335 | 0.000128 |
| Log-logistic | 0.000225 | 0.000106 | 0.000272 | 0.000081 | 0.000380 | 0.000092 |
| Log-normal | 0.000205 | 0.000114 | 0.000213 | 0.000074 | 0.000383 | 0.000088 |
| Gamma | 0.000242 | 0.000105 | 0.000294 | 0.000083 | 0.000325 | 0.000111 |
| Patient type | Distribution | Intercept | Scale | Shape |
|---|---|---|---|---|
| SRE naive | ||||
| Breast | Log-normal | 3.62 | 1.84 | |
| Prostate | Gamma | 3.51 | 1.28 | 0.8 |
| All patients | ||||
| Breast | Log-normal | 3.33 | 1.97 | |
| Prostate | Log-normal | 2.85 | 1.48 | |
| OST + lung | Gamma | 3.55 | 1.54 | 0.82 |
| Lung | Log-normal | 2.62 | 2.73 | |
For similar arguments, the base cases for breast cancer, prostate cancer, and OSTs including lung cancer apply a cycle rate of SREs within the zoledronic acid arm as estimated by the manufacturer from trial data specific to the SRE-experienced subgroup. For lung cancer the AG has, in the absence of other data, estimated cycle rates based on the pooled data across all patients; i.e. not specific to the SRE-experienced subgroup (Table 98).
| Zoledronic arms (with 21-day window) | Prior SRE | All patients | ||
|---|---|---|---|---|
| Breast | Prostate | OST + lung | Lung | |
| Sample size | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Length of study (months) | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Length of study (years) | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Overall survival hazard rate (estimate) | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Patient-years of exposure | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Cumulative mean rate at end of study | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| SREs (estimate) | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| SMR | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Cycle length (days) | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Cycle rate | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
Discontinuation rates and serious adverse events
The base case applies those of the manufacturer's model, as summarised in Table 77. In the absence of any other data, the rates for modelling of lung cancer are assumed to be the same as those for the OSTs including lung cancer modelling.
Quality-of-life values
Despite the lack of detail around their estimation, the AG views the manufacturer's estimates for the quality-of-life impacts from SREs and SAEs as the best that are available. The balance between the SREs results in average QALY decrement per SRE as outlined in Table 99.
| Breast cancer | Prostate cancer | OSTs | |||
|---|---|---|---|---|---|
| SRE naive | SRE experienced | SRE naive | SRE experienced | SRE naive | SRE experienced |
| AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
The lower average SRE QALY decrement in breast cancer patients compared with patients with other cancers arises mainly from the lower proportion of SREs, which are either radiation to the bone or SCC. The average SRE QALY decrement among SRE-experienced breast cancer patients is further affected by non-vertebral fractures being estimated to have a particularly small impact on HRQoL in this group. Note that the QALY decrements reported above for the SRE-naive patients do not take into account the step change in utility when moving from being SRE naive to SRE experienced and continuing through to death, as outlined in Table 83.
Modelling a sustained quality-of-life impact from SCC beyond the 5 months subsequent to the compression is implemented by calculating the discounted expected cycles of survival from 5 months subsequent to the compression through to the model horizon. This is then multiplied by the per cycle QALY decrement associated with SCC. The QALY decrement can be either the average or the maximum decrement estimated during the 5 months subsequent to the compression, as outlined in Table 83.
Modelling decay in quality of life in the final year adjusts the total within-cycle QALY by the proportionate decline in utility as outlined in Table 66, taking the modelled survival into account. The proportion of patients anticipated to survive to 12 months beyond the cycle requires no adjustment to be made to their QALY. Working back from this, the proportion anticipated to survive to 11 months beyond the cycle has the percentage reduction in utility for being 11 months to death, as drawn from Table 66, applied. This is worked back through to the proportion anticipated to survive only 1 month beyond the cycle being modelled, which has the proportionate decline in utility for being 1 month to death applied. Summing these gives a total overall QALY multiplier to apply to the total within-cycle QALY. For instance, within the first cycle of the breast cancer model this gives rise to a multiplier of 0.96, which by the 12th cycle has fallen to 0.93.
Health-related quality-of-life values for SAEs are as per Table 85. The manufacturer's assumption of a permanent decrement from ONJ and renal toxicity has been adopted for the base case, with a sensitivity analysis limiting this to the average duration observed within the trials.
Resource use
The direct drug and administration costs for the base cases are as per the MS, correcting only the zoledronic acid price and the disodium pamidronate price for BNF62. Note that these costings do not attempt to correct for doses of zoledronic acid being withheld because of renal toxicity. Given the uncertainty around the future price of zoledronic acid as a result of imminent patent expiry, a common set of sensitivity analyses are presented that incrementally reduce this price by 5%.
Note that removing the 15-minute nursing time for zoledronic acid infusion that the manufacturer adds post hoc to the time and motion survey is equivalent to a reduction in the price of zoledronic acid of around 7%. In the light of this, sensitivity analyses around zoledronic acid administration costs have not been separately presented.
In common with the Ross HTA report55 and the MS, the AG's costings of events rely to a large extent on averaging reference costs, coupled with some expert opinion on the balance between the proportion of patients admitted as a result of the event and the proportion treated as either day cases or outpatients. As already noted, the manufacturer's costings include excess bed-days on the basis of the trim point being the average length of stay. These have been excluded from the AG costings, with the exception of the SCC costing. For SCC, NICE CG75 suggests an average £892 (£844) for patient rehabilitation drawn from CG75. Even this may underestimate the full cost of SCC, given that a proportion of patients will be paralysed to a greater or lesser extent and require ongoing care.
Costs for SAEs are less in line with those of the manufacturer, mainly because the manufacturer typically assumes that all would be treated on an inpatient basis, though this does include a proportion of day cases (Table 100). AG expert opinion suggests that an elective or non-elective inpatient admission is unlikely for ONJ, skin infections or renal toxicity caused by BP use. In the light of this, ONJ has been costed on the basis of 90% being treated as day cases with the remainder being admitted; skin infections on the basis of 90% being treated as outpatients with one initial and two follow-up appointments; and renal toxicity on the basis of 90% being treated as outpatients with one initial and two follow-up appointments, with the remainder being treated as day cases. Sensitivity analyses find these distinctions to have relatively little impact.
| Event | AG | Manufacturer |
|---|---|---|
| SREs | ||
| Vertebral fracture | £294 | AiC information has been removed |
| Non-vertebral fracture | £1581 | AiC information has been removed |
| Radiation to the bone | £662 | AiC information has been removed |
| Surgery to the bone | £7269 | AiC information has been removed |
| SCC | £7311 | AiC information has been removed |
| SAEs | ||
| ONJ | £1220 | £2465 |
| Renal | £496 | £1681 |
| Hypercalcaemia | £4579 | £4579 |
| Hypocalcaemia | £443 | £443 |
| Skin | £370 | £1440 |
As in the manufacturer's base case, the cost of vertebral fractures is set to zero on the basis that most are sufficiently asymptomatic to not require treatment. Within the probabilistic modelling the rates of SREs are treated probabilistically, but the unit costs are treated deterministically. (In the light of referee comment, treating the NHS reference costs underlying the SRE and SAE average costs as being deterministic may have slightly understated the degree of uncertainty around the overall resource use associated with SREs and SAEs. Distributions could and perhaps should have been placed on the underlying NHS reference costs, based on the interquartile ranges reported. But it seems likely that any resulting distributions would have to be treated as being independent, which would tend to reduce the overall uncertainty associated with the distributions of average costs per SRE and per SAE compared with that of the underlying NHS reference costs. The opinion of the AG is that, in the light of the final results, this amendment to the modelling would not be expected to have any significant impact on the central estimates of the probabilistic modelling. But it is conceded that this omission will have caused some underestimation of the degrees of uncertainty around the central estimates within the probabilistic modelling.)
Univariate sensitivity analyses
A range of univariate sensitivity analyses are presented for the lifetime cost–utility modelling (Table 101).
| Description | Abbreviated |
|---|---|
| Base case | Base case |
| Amgen STARs costing | Amgen STARs |
| Amgen NMA results | Amgen NMA |
| Amgen STARs costings and NMA results | Amgen STARs+NMA |
| No HRQoL step change for naive to experienced | No naive util step |
| SCC permanent utility effect of the average P1–P5 decrement | SCC ongoing mean |
| SCC permanent utility effect of the maximum P1–P5 decrement | SCC ongoing max. |
| No general mortality | No gen. mortality |
| 5-year horizon | 5-year horizon |
| 2-year horizon | 2-year horizon |
| van den Hout utility multipliers for last year of life | vd Hout utility |
| ONJ and renal toxicity utility impact beyond trial average | SAE P1+ |
| Excluding SAEs | No SAE |
| General discontinuations at the end of the average treatment then constant | Gen. discs. EoT |
| No general discontinuations | No gen. discs. |
| No discontinuations | No discs. |
| AG TTF functional form from naive for breast and prostate | TTF form AG naive |
| AG TTF functional form all patients for breast, prostate and OSTL | TTF form AG all |
The results of these are presented in full for all patients, for SRE-naive patients and for SRE-experienced patients for the comparison of denosumab with zoledronic acid and for the comparison of denosumab with BSC. But given the results of the analyses for the comparisons with BSC result in cost-effectiveness estimates typically in excess of £100,000 per QALY, even with the PAS, these are generally not reported in the main body of the text. For the sake of space, the body of the report presents only the summary of these for all patients for breast cancer, and all patients and SRE-experienced patients for the remaining analyses. Where the sensitivity analysis results in a cost-effectiveness estimate for denosumab versus BSC of less than £50,000 per QALY this is individually reported in the text, and whether this applies to all patients, SRE-naive patients or SRE-experienced patients.
In addition to these, as zoledronic acid is shortly coming off patent, the approximate changes in the price of zoledronic acid that would be required for the cost-effectiveness of denosumab relative to zoledronic acid to be £20,000 per QALY and £30,000 per QALY are reported.
Presentation of results
For the lifetime cost–utility modelling a common format has been adopted for each of the four cancer groups being modelled. The results of the base-case deterministic modelling that apply the AG's NMA results are presented in detail, coupled with the associated cost-effectiveness acceptability frontiers (CEAFs) from the probabilistic modelling. The range of univariate sensitive analyses are then tabulated, followed by a summary of the main points arising from them and of the impact of reductions in the price of zoledronic acid. This is then followed by a detailed presentation of results from the application of the SRE-naive- and -experienced-specific HRs and RRs. This latter is as per the base case, only with the SRE-naive- and -experienced-specific HRs and RRs for denosumab versus zoledronic acid being applied, as summarised in Table 76.
Results
Within-trial data analysis
Using data from the CSRs and the submission permits the average number of doses administered and the numbers of SREs to be presented, together with the numbers of SAEs, for each arm. The following presents these on the basis of net number of events per patient-year together with their costs, coupled with the average number of drug administrations per patient-year and the costs of this.
To cost the SREs and SAEs, and to assess their QALY impact, the individual events can be assessed separately. But this may result in the analysis being driven by a very small net difference in costly events between the arms. The same average distribution between SREs has been assumed for each arm as has been applied within the more involved cost–utility modelling and as reported in Table 73 above. The resulting average SRE unit cost and average SRE QALY impact can then be applied to the net difference between the arms. This latter will be referred to as average event based, the former as individual event based. The average total QALY decrements per event are drawn from the MS as summarised above.
Breast cancer
The direct on-trial drug and administration costs are as shown (Table 102).
| Event | Zoledronic acid | Denosumab | Net p.a. | Unit cost | Net p.a. | QALY decrement | Net p.a. |
|---|---|---|---|---|---|---|---|
| Patient-years | AiC information has been removed | AiC information has been removed | |||||
| n (full analysis set) | AiC information has been removed | AiC information has been removed | |||||
| SREs average event | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1222 | CiC information has been removed | AiC information has been removed | 0.003 |
| SCC | CiC information has been removed | CiC information has been removed | CiC information has been removed | £7311 | AiC information has been removed | ||
| Surgery to bone | CiC information has been removed | CiC information has been removed | CiC information has been removed | £7269 | AiC information has been removed | ||
| Fracture | CiC information has been removed | CiC information has been removed | CiC information has been removed | £895 | AiC information has been removed | ||
| Radiation to bone | CiC information has been removed | CiC information has been removed | CiC information has been removed | £662 | AiC information has been removed | ||
| SREs individual event | CiC information has been removed | 0.005 | |||||
| n (safety set) | AiC information has been removed | AiC information has been removed | |||||
| SAEs average event | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1396 | CiC information has been removed | AiC information has been removed | 0.000 |
| ONJ | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1220 | AiC information has been removed | ||
| Renal toxicity | CiC information has been removed | CiC information has been removed | CiC information has been removed | £496 | AiC information has been removed | ||
| Hypercalcaemia | CiC information has been removed | CiC information has been removed | CiC information has been removed | £4579 | AiC information has been removed | ||
| Hypocalcaemia | CiC information has been removed | CiC information has been removed | CiC information has been removed | £443 | AiC information has been removed | ||
| Skin infection | CiC information has been removed | CiC information has been removed | CiC information has been removed | £370 | AiC information has been removed | ||
| SAEs individual event | CiC information has been removed | 0.001 | |||||
| Mean administrations | AiC information has been removed | AiC information has been removed | |||||
| Per patient-year | AiC information has been removed | AiC information has been removed | |||||
| Drug and admin excluding PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||||
| Drug and admin including PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed |
This can be further summarised as shown (Table 103).
| Results | Average event assessment | Individual event assessment | ||||
|---|---|---|---|---|---|---|
| Costs | QALYs | ICER | Costs | QALYs | ICER | |
| Results excluding PAS | £1098 | 0.003 | £378,487 | £1147 | 0.006 | £190,841 |
| Results including PAS | −£26 | 0.003 | Dominant | £23 | 0.006 | £3783 |
This analysis is relatively straightforward and sees denosumab increase total costs by between £1101 and £1149 compared with zoledronic acid. This suggests crude estimates of the on-trial cost-effectiveness excluding the PAS of between £191,000 and £378,000 per QALY compared with zoledronic acid. However, with the PAS, denosumab is estimated to be broadly cost neutral, with this ranging between a cost saving of £26 and a small additional cost of £23 depending on how the costs of SREs and SAEs are summed. This results in denosumab being estimated to range from dominating zoledronic acid to having a very acceptable cost-effectiveness ratio of £3783 per QALY. Because of the small QALY gains estimated in the above, relatively small changes in the price of zoledronic acid cause quite large changes in the cost-effectiveness estimates. (CiC information has been removed.)
Prostate cancer
The direct on-trial drug and administration costs are as shown (Table 104).
| Event | Zoledronic acid | Denosumab | Net p.a. | Unit cost | Net p.a. | QALY decrement | Net p.a. |
|---|---|---|---|---|---|---|---|
| Patient-years | AiC information has been removed | AiC information has been removed | |||||
| n (full analysis set) | 951 | 950 | |||||
| SREs average event | 584 | 494 | CiC information has been removed | £1247 | CiC information has been removed | AiC information has been removed | 0.008 |
| SCC | CiC information has been removed | CiC information has been removed | CiC information has been removed | £7311 | AiC information has been removed | ||
| Surgery to bone | CiC information has been removed | CiC information has been removed | CiC information has been removed | £7269 | AiC information has been removed | ||
| Fracture | CiC information has been removed | CiC information has been removed | CiC information has been removed | £694 | AiC information has been removed | ||
| Radiation to bone | CiC information has been removed | CiC information has been removed | CiC information has been removed | £662 | AiC information has been removed | ||
| SREs individual event | CiC information has been removed | 0.016 | |||||
| n (safety set) | AiC information has been removed | AiC information has been removed | |||||
| SAEs average event | CiC information has been removed | CiC information has been removed | CiC information has been removed | £857 | CiC information has been removed | AiC information has been removed | −0.001 |
| ONJ | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1220 | AiC information has been removed | ||
| Renal toxicity | CiC information has been removed | CiC information has been removed | CiC information has been removed | £496 | AiC information has been removed | ||
| Hypercalcaemia | CiC information has been removed | CiC information has been removed | CiC information has been removed | £4579 | AiC information has been removed | ||
| Hypocalcaemia | CiC information has been removed | CiC information has been removed | CiC information has been removed | £443 | AiC information has been removed | ||
| Skin infection | CiC information has been removed | CiC information has been removed | 0.000 | £370 | AiC information has been removed | ||
| SAE individual event | CiC information has been removed | 0.000 | |||||
| Mean administrations | AiC information has been removew | AiC information has been removed | |||||
| Per patient-year | AiC information has been removed | AiC information has been removed | |||||
| Drug and admin excluding PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed | ||||
| Drug and admin including PAS | CiC information has been removed | CiC information has been removed | CiC information has been removed |
This can be further summarised (Table 105).
| Results | Average event assessment | Individual event assessment | ||||
|---|---|---|---|---|---|---|
| Costs | QALYs | ICER | Costs | QALYs | ICER | |
| Results excluding PAS | £1214 | 0.007 | £165,881 | £1228 | 0.016 | £77,129 |
| Results including PAS | £111 | 0.007 | £15,190 | £126 | 0.016 | £7904 |
Again, the principal immediate uncertainty may relate to the cost of zoledronic acid.
As for breast cancer, this analysis for prostate cancer is relatively straightforward and sees denosumab increase total costs by between £1214 and £1228 compared with zoledronic acid. This suggests crude estimates of the on-trial cost-effectiveness excluding the PAS of between £77,000 and £166,000 per QALY compared with zoledronic acid. Within this analysis there is a greater absolute QALY discrepancy between the average event-based analysis and the individual event-based analysis. This arises in large part from the crude estimate of the impact on the annual incidence of SCC. Whether or not this is an argument for assessing the SREs on an individual event basis is a moot point, but it seems conceivable that there may be different effects in osteolytic cancers compared with osteoblastic cancers.
With the PAS, denosumab is estimated to result in an average cost increase of between £111 and £126 per annum. Given the differences in the QALY estimates, this results in cost-effectiveness estimates ranging between £7904 per QALY and £15,190 per QALY. Because of the small QALY gains estimated using the average event-based method, as for breast cancer, relatively small changes in the price of zoledronic acid cause large changes in the cost-effectiveness. With the PAS, a fall in the price of zoledronic acid between that in the average event analysis (commercial-in-confidence information has been removed) and that in the individual event analysis (commercial-in-confidence information has been removed) would be sufficient to make the additional cost of denosumab not justify the relatively small average QALY gains.
Other solid tumours excluding multiple myeloma
Unfortunately, the CSR, the manufacturer's model and the submission do not provide sufficient detail to be able to present this analysis for the patient group of OSTs excluding multiple myeloma.
Cost–utility modelling
Breast cancer base case
The modelling applies the AG's NMA results in Table 106.
| Comparator | SREs | Net | QALYs | Net | Tx costs | Net | All costs | Net | ICER |
|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||
| BSC | 3.159 | −0.988 | 1.821 | 0.027 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £6242 | £229,547 |
| Including PAS | CiC information has been removed | £4292 | £157,829 | ||||||
| Zoledronic acid | 2.383 | −0.211 | 1.841 | 0.007 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1707 | £245,264 |
| Including PAS | CiC information has been removed | −£243 | Dominant | ||||||
| Denosumab | 2.171 | 1.848 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| Disodium pamidronate | 2.445 | −0.274 | 1.839 | 0.010 | CiC information has been removed | CiC information has been removed | CiC information has been removed | −£1303 | Dominant |
| Including PAS | CiC information has been removed | −£3253 | Dominant | ||||||
| SRE naive | |||||||||
| BSC | 2.807 | −0.962 | 1.850 | 0.035 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £6308 | £181,092 |
| Including PAS | CiC information has been removed | £4358 | £125,109 | ||||||
| Zoledronic acid | 2.031 | −0.186 | 1.876 | 0.008 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1747 | £209,345 |
| Including PAS | CiC information has been removed | −£203 | Dominant | ||||||
| Denosumab | 1.845 | 1.884 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| Disodium pamidronate | 2.022 | −0.177 | 1.875 | 0.009 | CiC information has been removed | CiC information has been removed | CiC information has been removed | −£1168 | Dominant |
| Including PAS | CiC information has been removed | −£3118 | Dominant | ||||||
| SRE experienced | |||||||||
| BSC | 3.667 | −1.025 | 1.780 | 0.016 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £6146 | £379,539 |
| Including PAS | CiC information has been removed | £4196 | £259,113 | ||||||
| Zoledronic acid | 2.888 | −0.247 | 1.791 | 0.005 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1649 | £332,185 |
| Including PAS | CiC information has been removed | −£301 | Dominant | ||||||
| Denosumab | 2.641 | 1.796 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| Disodium pamidronate | 3.055 | −0.414 | 1.786 | 0.010 | CiC information has been removed | CiC information has been removed | CiC information has been removed | −£1498 | Dominant |
| Including PAS | CiC information has been removed | −£3448 | Dominant | ||||||
The net gain from denosumab over zoledronic acid of 0.007 QALYs is in line with that estimated by the manufacturer. But this remains a relatively small gain, which without the PAS requires an additional £1707, resulting in a cost-effectiveness of £245,264 per QALY.
Among those in whom BPs are contraindicated, the cost-effectiveness of denosumab compared with BSC is broadly similar. Patient gains are larger at 0.027 QALYs but the net cost rises by a similar amount to £6242, resulting in a cost-effectiveness estimate of £229,547 per QALY.
With the PAS, the anticipated cost savings are less than anticipated by the manufacturer, but this appears to be broadly in line with the assumed costs of SREs and SAEs. Given the cost saving and the anticipated patient gains, denosumab is estimated to dominate zoledronic acid. Probabilistic modelling over 2000 iterations is broadly in line with this, estimating the same 0.007 QALYs, but a slightly smaller average cost saving of £243. The likelihood of denosumab being cost-effective compared with the BPs is estimated as 98% for a willingness to pay of £20,000 per QALY and as 100% for a willingness to pay of £30,000 per QALY.
For those in whom BPs are contraindicated, the cost-effectiveness of denosumab compared with BSC is again considerably worse, with a central estimate across all these patients of £157,829 per QALY. Across all patients the probabilistic modelling suggests similar central estimates of 0.028 QALYs and a net cost of £4269 to yield a cost-effectiveness estimate of £154,944 per QALY. The likelihood of denosumab being cost-effective compared with the BPs and BSC is estimated as 0% for a willingness to pay of £20,000 per QALY and as 0% for a willingness to pay of £30,000 per QALY (Figure 12).
FIGURE 12.
Breast cancer cost-effectiveness acceptability curves (CEACs) and CEAFs including the PAS. (The CEAF has been overlaid on top of the CEACs for reasons of space, but they are presented separately in Appendix 15.) (a) CEAF excluding BSC: all patients; (b) CEAF including BSC: all patients; (c) CEAF excluding BSC: SRE-naive patients; (d) CEAF including BSC: SRE-naive patients; (e) CEAF excluding BSC: SRE-experienced patients; (f) CEAF including BSC: SRE-experienced patients.
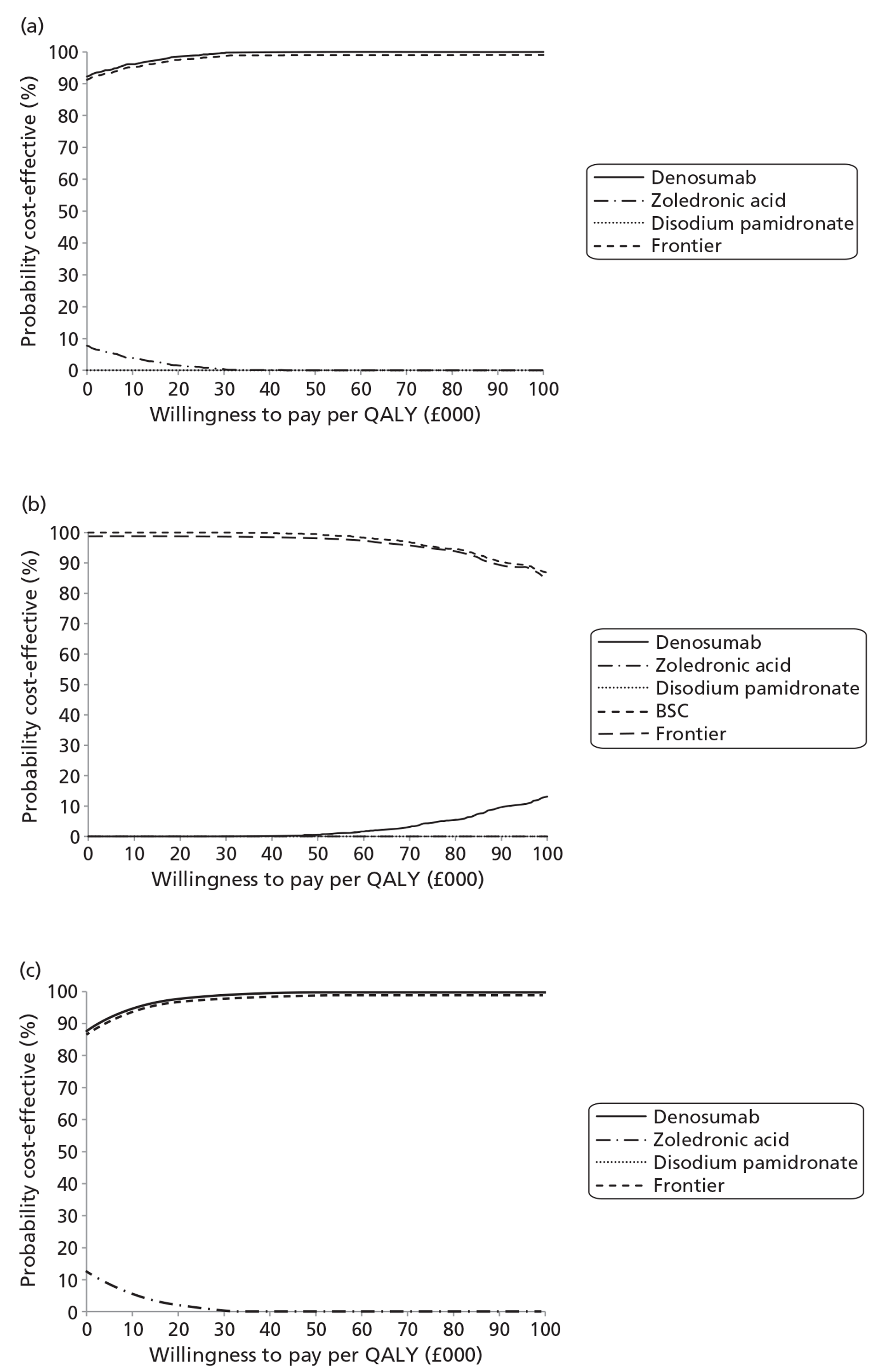
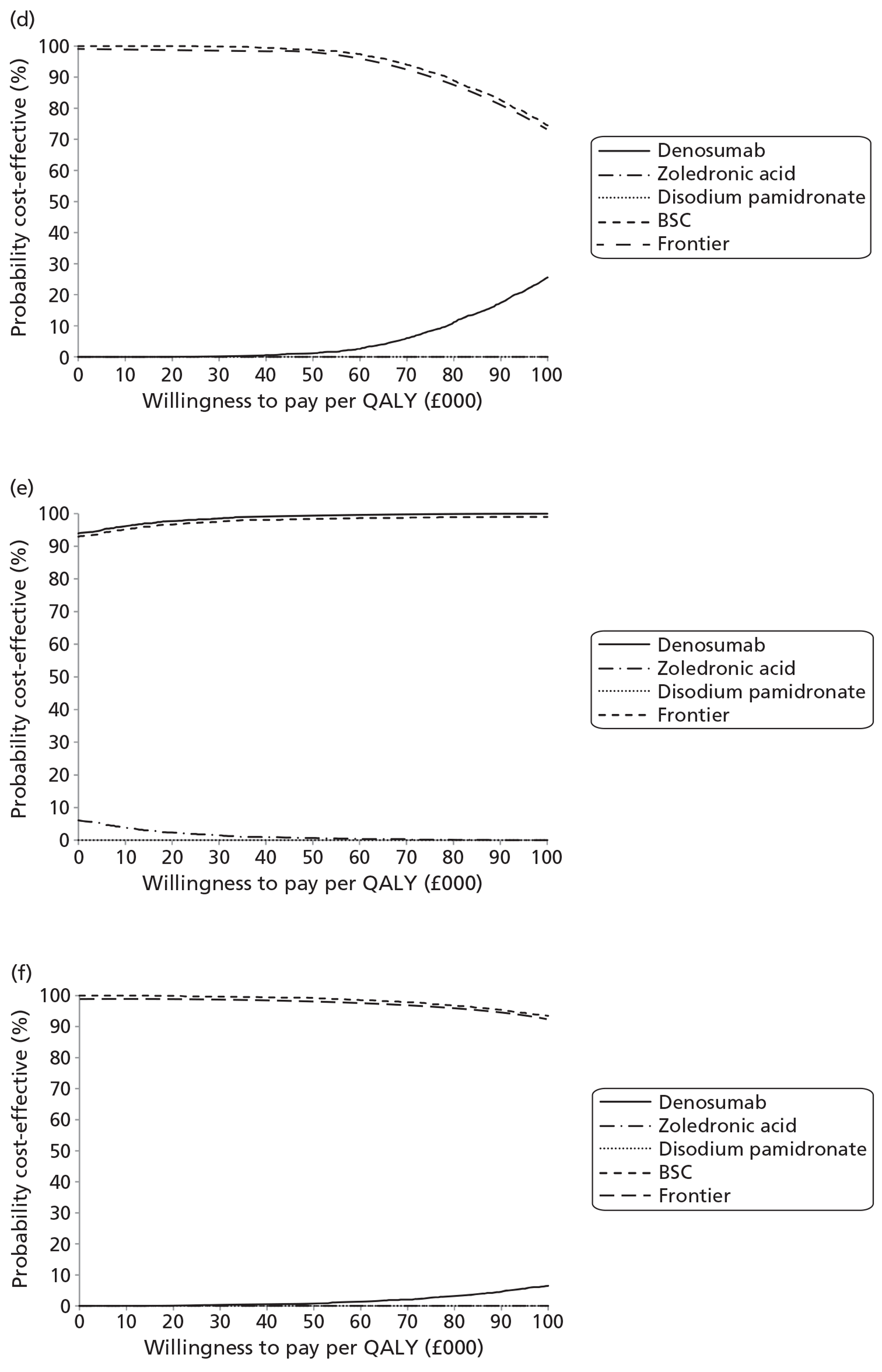
Breast cancer sensitivity analyses
The univariate sensitivity analyses for the all-patient modelling for the cost-effectiveness of denosumab compared with zoledronic acid are presented in Table 107.
| Sensitivity analyses | All patients vs BSC | All patients vs zoledronic acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excluding PAS | Including PAS | SREs | QALYs | ICER | ICER including | Excluding PAS | Including PAS | SREs | QALYs | ICER excluding | ICER including | |
| Base case | £6242 | £4292 | −0.988 | 0.027 | £229,547 | £157,829 | £1707 | −£243 | −0.211 | 0.007 | £245,264 | Dominant |
| Amgen STARs | £6623 | £4673 | −0.988 | 0.027 | £243,559 | £171,841 | £1782 | −£168 | −0.211 | 0.007 | £255,996 | Dominant |
| Amgen NMA | £6324 | £4374 | −0.922 | 0.025 | £257,431 | £178,053 | £1705 | −£245 | −0.213 | 0.007 | £242,776 | Dominant |
| Amgen STARs+NMA | £6683 | £4733 | −0.922 | 0.025 | £272,032 | £192,655 | £1781 | −£170 | −0.213 | 0.007 | £253,470 | Dominant |
| No naive util step | £6242 | £4292 | −0.988 | 0.017 | £366,760 | £252,172 | £1707 | −£243 | −0.211 | 0.005 | £362,999 | Dominant |
| SCC ongoing mean | £6242 | £4292 | −0.988 | 0.033 | £189,204 | £130,090 | £1707 | −£243 | −0.211 | 0.008 | £208,302 | Dominant |
| SCC ongoing max. | £6242 | £4292 | −0.988 | 0.035 | £179,091 | £123,137 | £1707 | −£243 | −0.211 | 0.009 | £198,682 | Dominant |
| No gen. mortality | £6277 | £4316 | −0.996 | 0.027 | £228,819 | £157,307 | £1717 | −£245 | −0.213 | 0.007 | £244,512 | Dominant |
| 5-year horizon | £6102 | £4204 | −0.935 | 0.025 | £239,758 | £165,176 | £1670 | −£229 | −0.199 | 0.007 | £256,441 | Dominant |
| 2-year horizon | £4781 | £3319 | −0.653 | 0.016 | £291,409 | £202,319 | £1309 | −£153 | −0.139 | 0.004 | £308,247 | Dominant |
| vd Hout utility | £6242 | £4292 | −0.988 | 0.025 | £249,169 | £171,320 | £1707 | −£243 | −0.211 | 0.006 | £266,094 | Dominant |
| SAE P1+ | £6242 | £4292 | −0.988 | 0.026 | £242,970 | £167,058 | £1707 | −£243 | −0.211 | 0.013 | £134,378 | Dominant |
| No SAE | £6276 | £4300 | −1.001 | 0.028 | £224,711 | £153,953 | £1773 | −£203 | −0.214 | 0.006 | £291,955 | Dominant |
| Gen. discs. EoT | £7077 | £4864 | −1.125 | 0.031 | £226,401 | £155,602 | £1947 | −£266 | −0.243 | 0.008 | £242,500 | Dominant |
| No gen. discs. | £11,493 | £7912 | −1.841 | 0.046 | £251,628 | £173,216 | £3167 | −£414 | −0.400 | 0.012 | £259,902 | Dominant |
| No discs. | £11,744 | £8085 | −1.883 | 0.047 | £252,493 | £173,819 | £3237 | −£422 | −0.409 | 0.012 | £260,510 | Dominant |
| TTF form AG naive | £6235 | £4285 | −0.994 | 0.028 | £225,904 | £155,252 | £1707 | −£243 | −0.211 | 0.007 | £244,209 | Dominant |
| TTF form AG all | £6147 | £4197 | −1.060 | 0.030 | £205,611 | £140,382 | £1687 | −£263 | −0.227 | 0.008 | £222,101 | Dominant |
The sensitivity analyses suggest that the AG's and manufacturer's estimates are broadly in line. Applying the manufacturer's estimates for costs and effectiveness has little impact, whereas applying the AG's estimates for the functional form for the time to first SRE again has very little impact.
The main sensitivity of results is around the SAEs and the discontinuation rates, given the higher rate of renal failure within the zoledronic acid arm, and the assumption that this lasts for longer than that measured in the trials affects results. If SAE ONJ and renal failure durations are the average remaining cohort survival, the anticipated benefits from denosumab over zoledronic acid increase by up to half, with a parallel impact on the cost-effectiveness estimate. Excluding discontinuations also has quite a large impact when compared with BSC, although the increase in the net patient gains is broadly mirrored by an increase in the net cost, resulting in a relatively static ICER.
A reduction in the price of zoledronic acid (commercial-in-confidence information has been removed) results in the cost-effectiveness of denosumab compared with zoledronic acid across all breast cancer patients including the PAS worsening to levels that might not be considered cost-effective. Applying the head-to-head SRE-naive- and -experienced-specific clinical effectiveness results for denosumab versus zoledronic acid, while retaining the remainder of the AG's NMA, gives the results in Table 108.
| Comparator | SREs | Net | QALYs | Net | Treatment costs | Net | All costs | Net | ICER |
|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||
| BSC | 3.159 | −0.997 | 1.821 | 0.027 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £6227 | £232,756 |
| Including PAS | CiC information has been removed | £4277 | £159,866 | ||||||
| Zoledronic acid | 2.383 | −0.221 | 1.841 | 0.007 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1693 | £259,484 |
| Including PAS | CiC information has been removed | −£258 | Dominant | ||||||
| Denosumab | 2.162 | 1.848 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| Disodium pamidronate | 2.445 | −0.283 | 1.839 | 0.009 | CiC information has been removed | CiC information has been removed | CiC information has been removed | −£1317 | Dominant |
| Including PAS | CiC information has been removed | −£3268 | Dominant | ||||||
| SRE naive | |||||||||
| BSC | 2.807 | −0.948 | 1.850 | 0.034 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £6323 | £188,162 |
| Including PAS | CiC information has been removed | £4373 | £130,133 | ||||||
| Zoledronic acid | 2.031 | −0.173 | 1.876 | 0.007 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1763 | £247,591 |
| Including PAS | CiC information has been removed | −£187 | Dominant | ||||||
| Denosumab | 1.859 | 1.883 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| Disodium pamidronate | 2.022 | −0.163 | 1.875 | 0.008 | CiC information has been removed | CiC information has been removed | CiC information has been removed | −£1152 | Dominant |
| Including PAS | CiC information has been removed | −£3102 | Dominant | ||||||
| SRE experienced | |||||||||
| BSC | 3.667 | −1.069 | 1.780 | 0.017 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £6089 | £360,413 |
| Including PAS | CiC information has been removed | £4139 | £244,979 | ||||||
| Zoledronic acid | 2.888 | −0.290 | 1.791 | 0.006 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1592 | £280,994 |
| Including PAS | CiC information has been removed | −£359 | Dominant | ||||||
| Denosumab | 2.598 | 1.797 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| Disodium pamidronate | 3.055 | −0.457 | 1.786 | 0.011 | CiC information has been removed | CiC information has been removed | CiC information has been removed | −£1555 | Dominant |
| Including PAS | CiC information has been removed | −£3505 | Dominant | ||||||
For breast cancer, as the subgroup-specific HRs and RRs for denosumab compared with zoledronic acid are broadly similar to the estimates pooled across all patients, applying the subgroup-specific HRs and RRs has relatively limited impact on results.
Prostate cancer base case
The modelling that applies the AG's NMA gives the results in Table 109.
| Comparator | SREs | Net | QALYs | Net | Tx costs | Net | All costs | Net | ICER |
|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||
| BSC | 2.185 | −0.543 | 1.065 | 0.035 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £3951 | £112,415 |
| Including PAS | CiC information has been removed | £2766 | £78,713 | ||||||
| Zoledronic acid | 1.772 | −0.130 | 1.090 | 0.009 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1059 | £111,603 |
| Including PAS | CiC information has been removed | −£125 | Dominant | ||||||
| Denosumab | 1.642 | 1.100 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE naive | |||||||||
| BSC | 2.049 | −0.528 | 1.088 | 0.039 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £3969 | £103,003 |
| Including PAS | CiC information has been removed | £2785 | £72,269 | ||||||
| Zoledronic acid | 1.650 | −0.129 | 1.116 | 0.011 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1061 | £99,561 |
| Including PAS | CiC information has been removed | −£123 | Dominant | ||||||
| Denosumab | 1.521 | 1.127 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE experienced | |||||||||
| BSC | 2.574 | −0.587 | 0.997 | 0.025 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £3897 | £152,916 |
| Including PAS | CiC information has been removed | £2713 | £106,446 | ||||||
| Zoledronic acid | 2.122 | −0.135 | 1.016 | 0.006 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1053 | £170,854 |
| Including PAS | CiC information has been removed | −£131 | Dominant | ||||||
| Denosumab | 1.987 | 1.023 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
Larger patient gains are anticipated for prostate cancer patients. This is partly because of a higher proportion of SCC within the overall incidence of SREs. But the analysis is broadly similar to that for breast cancer. Without the PAS, the relatively small patient gain of 0.009 QALYs at an additional cost of £1059 results in a cost-effectiveness compared with zoledronic acid of £111,603 per QALY. However, with the PAS, cost savings and dominance over zoledronic acid are anticipated.
The cost-effectiveness is estimated to be slightly worse among the SRE experienced than across the patient group as a whole, though this may be partly the result of the step change in HRQoL that is applied when SRE-naive patients experience their first SRE. But with the PAS, cost savings are again anticipated, which again results in dominance over zoledronic acid. The probabilistic modelling suggests central estimates of a gain of 0.009 QALYs and a cost saving of £123 across all patients. The likelihood of denosumab being cost-effective compared with the BPs across all patients is estimated as 99% for a willingness to pay of £20,000 per QALY and as 100% for a willingness to pay of £30,000 per QALY (Figure 13).
FIGURE 13.
Prostate cancer CEAFs including the PAS. (a) CEAF excluding BSC: all patients; (b) CEAF including BSC: all patients; (c) CEAF excluding BSC: SRE-naive patients; (d) CEAF including BSC: SRE-naive patients; (e) CEAF excluding BSC: SRE-experienced patients; (f) CEAF including BSC: SRE-experienced patients.
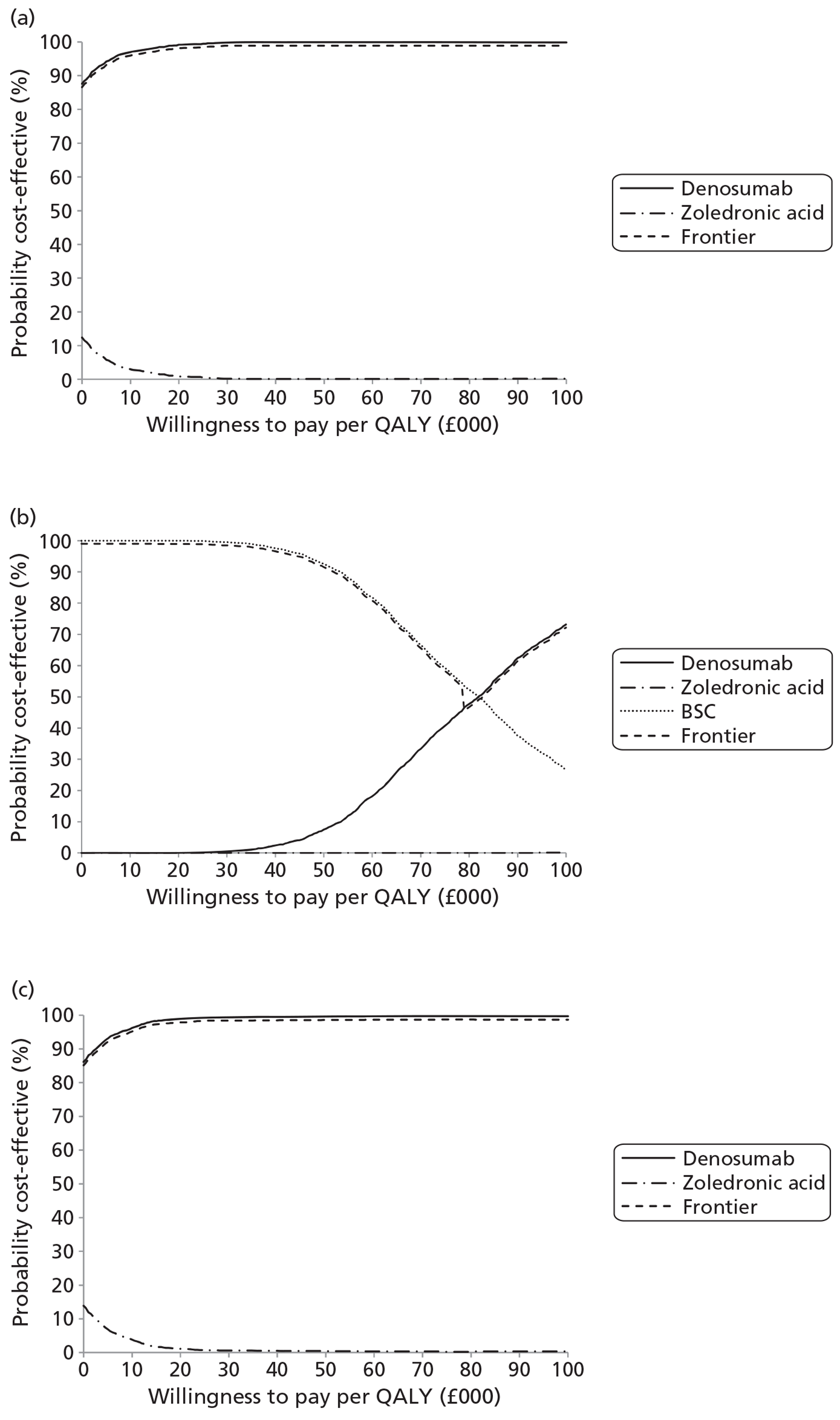
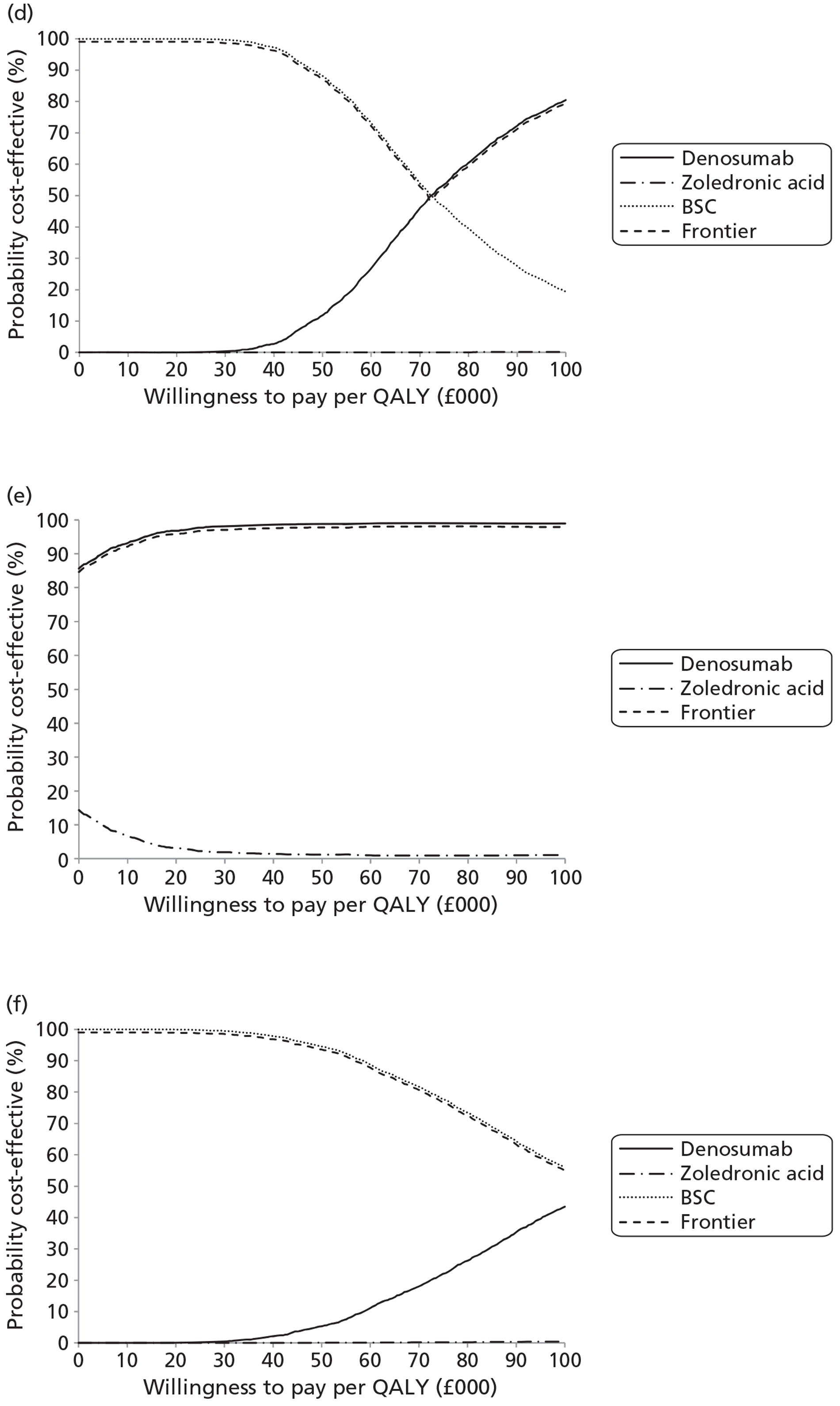
For those in whom BPs are contraindicated, even with the PAS, the cost-effectiveness of denosumab compared with BSC is poor, at between £70,000 per QALY and £240,000 per QALY. Across all patients the probabilistic modelling suggests similar central estimates of 0.035 QALYs and a net cost of £2764 to yield a cost-effectiveness estimate of £78,756 per QALY. The likelihood of denosumab being cost-effective compared with the BPs and BSC across all patients is estimated as 0% for a willingness to pay of £20,000 per QALY and as 0% for a willingness to pay of £30,000 per QALY.
Prostate cancer sensitivity analyses
The univariate sensitivity analyses for the SRE-experienced patient modelling for the cost-effectiveness of denosumab are presented in Table 110.
| Sensitivity analyses | SRE-naive patients vs BSC | SRE-experienced patients vs zoledronic acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excluding PAS | Including PAS | SREs | QALYs | ICER excluding | ICER including | Excluding PAS | Including PAS | SREs | QALYs | ICER excluding | ICER including | |
| Base case | £3969 | £2785 | −0.528 | 0.039 | £103,003 | £72,269 | £1053 | −£131 | −0.135 | 0.006 | £170,854 | Dominant |
| Amgen STARs | £4195 | £3010 | −0.528 | 0.039 | £108,848 | £78,114 | £1100 | −£84 | −0.135 | 0.006 | £178,502 | Dominant |
| Amgen NMA | £3965 | £2780 | −0.532 | 0.039 | £101,900 | £71,460 | £1054 | −£130 | −0.134 | 0.006 | £172,124 | Dominant |
| Amgen STARs+NMA | £4191 | £3007 | −0.532 | 0.039 | £107,716 | £77,276 | £1101 | −£83 | −0.134 | 0.006 | £179,785 | Dominant |
| No naive util step | £3969 | £2785 | −0.528 | 0.026 | £153,733 | £107,862 | – | – | – | – | – | – |
| SCC ongoing mean | £3969 | £2785 | −0.528 | 0.049 | £80,415 | £56,420 | £1053 | −£131 | −0.135 | 0.009 | £116,820 | Dominant |
| SCC ongoing max. | £3969 | £2785 | −0.528 | 0.057 | £69,884 | £49,032 | £1053 | −£131 | −0.135 | 0.011 | £95,965 | Dominant |
| No gen. mortality | £4054 | £2843 | −0.546 | 0.040 | £101,176 | £70,945 | £1076 | −£135 | −0.138 | 0.006 | £170,261 | Dominant |
| 5-year horizon | £3961 | £2781 | −0.520 | 0.038 | £104,689 | £73,497 | £1050 | −£130 | −0.135 | 0.006 | £170,852 | Dominant |
| 2-year horizon | £3620 | £2553 | −0.429 | 0.030 | £120,521 | £85,018 | £959 | −£108 | −0.122 | 0.006 | £171,394 | Dominant |
| vd Hout utility | £3969 | £2785 | −0.528 | 0.034 | £118,235 | £82,955 | £1053 | −£131 | −0.135 | 0.005 | £195,155 | Dominant |
| SAE P1+ | £3969 | £2785 | −0.528 | 0.024 | £162,306 | £113,877 | £1053 | −£131 | −0.135 | 0.007 | £158,518 | Dominant |
| No SAE | £3983 | £2773 | −0.540 | 0.042 | £95,819 | £66,716 | £1074 | −£135 | −0.143 | 0.007 | £159,100 | Dominant |
| Gen. discs. EoT | £4789 | £3358 | −0.644 | 0.047 | £101,327 | £71,045 | £1348 | −£83 | −0.177 | 0.008 | £165,677 | Dominant |
| No gen. discs. | £7571 | £5312 | −1.037 | 0.068 | £111,073 | £77,935 | £1987 | −£272 | −0.267 | 0.012 | £163,163 | Dominant |
| No discs. | £7875 | £5526 | −1.081 | 0.071 | £111,674 | £78,358 | £2169 | −£180 | −0.298 | 0.013 | £161,126 | Dominant |
| TTF form AG naive | £3993 | £2809 | −0.507 | 0.037 | £107,860 | £75,867 | – | – | – | – | – | – |
| TTF form AG all | £3953 | £2769 | −0.541 | 0.040 | £100,060 | £70,085 | – | – | – | – | – | – |
Prostate cancer patient benefits are more sensitive to the assumed duration of the quality-of-life impact from SCC than those of breast cancer patients. The anticipated net QALY gain from denosumab compared with zoledronic acid increases by up to around 40% depending on whether the mean decrement post diagnosis or the maximum decrement post diagnosis is carried forward.
If the average (or maximum) SCC utility decrement is carried forward in the modelling for SRE-naive prostate cancer patients, this yields a cost-effectiveness estimate for denosumab with the PAS compared with BSC of £56,420 per QALY (or £49,023 per QALY). There are limited data on the rates of paralysis from SCC and the cost estimates from averaging reference costs may be too low. CG75 suggests an average therapy cost of £14,173 (£13,705). Adding this to the average rehabilitation costs and applying the average SCC decrement through to death results in a cost-effectiveness estimate for the with-PAS analysis for SRE-naive prostate cancer patients (commercial-in-confidence information has been removed) per QALY compared with BSC higher than the (commercial-in-confidence information has been removed) when applying the maximum decrement.
As for the breast cancer modelling, removing treatment discontinuations increases the net gain from denosumab over zoledronic acid, though this may be better viewed in effect as fewer patients receiving BSC. The net impact on the ICER is quite muted as net costs change roughly in proportion, but note that it tends to worsen the cost-effectiveness for the comparison with BSC but improve it for the comparison with zoledronic acid.
A reduction in the price of zoledronic acid (commercial-in-confidence information has been removed) is sufficient to result in the cost-effectiveness of denosumab compared with zoledronic acid for SRE-experienced prostate cancer patients including the PAS to worsen to levels that might not be considered cost-effective.
Applying the head-to-head SRE-naive- and -experienced-specific clinical effectiveness results for denosumab versus zoledronic acid, while retaining the remainder of the AG's NMA, gives the results in Table 111.
| Comparator | SREs | Net | QALYs | Net | Treatment costs | Net | All costs | Net | ICER |
|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||
| BSC | 2.185 | −0.529 | 1.065 | 0.035 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £3968 | £113,851 |
| Including PAS | CiC information has been removed | £2783 | £79,865 | ||||||
| Zoledronic acid | 1.772 | −0.116 | 1.090 | 0.009 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1076 | £117,021 |
| Including PAS | CiC information has been removed | −£109 | Dominant | ||||||
| Denosumab | 1.656 | 1.100 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE naive | |||||||||
| BSC | 2.049 | −0.526 | 1.088 | 0.039 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £3972 | £102,016 |
| Including PAS | CiC information has been removed | £2788 | £71,597 | ||||||
| Zoledronic acid | 1.650 | −0.126 | 1.116 | 0.011 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1064 | £96,209 |
| Including PAS | CiC information has been removed | −£121 | Dominant | ||||||
| Denosumab | 1.523 | 1.127 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE experienced | |||||||||
| BSC | 2.574 | −0.539 | 0.997 | 0.023 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £3955 | £170,340 |
| Including PAS | CiC information has been removed | £2770 | £119,327 | ||||||
| Zoledronic acid | 2.122 | −0.087 | 1.016 | 0.004 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £1111 | £285,209 |
| Including PAS | CiC information has been removed | −£74 | Dominant | ||||||
| Denosumab | 2.035 | 1.020 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
Cost-effectiveness results for prostate cancer are more sensitive to the application of the SRE-naive- and -experienced-specific HRs and RRs. Note that within the modelling the impact of this on the average cost-effectiveness across all patients does not broadly cancel out. This is because over the period of extrapolation SRE-naive patients experience SREs and so cross over to the SRE-experienced group. The baseline balance between SRE-naive and -experienced patients as drawn from the trial trends towards SRE-experienced patients as extrapolation within the model progresses. This also explains why applying the SRE-specific estimates worsens the cost-effectiveness estimate among those who were SRE naive at baseline.
But with the PAS, denosumab is still estimated to be cost saving across the patient groups and so dominates zoledronic acid (commercial-in-confidence information has been removed).
Other solid tumours including lung cancer base case
The modelling that applies the AG's NMA gives the results in Table 112.
| Comparator | SREs | Net | QALYs | Net | Treatment costs | Net | All costs | Net | ICER |
|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||
| BSC | 1.606 | −0.288 | 0.703 | 0.017 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2548 | £147,122 |
| Including PAS | CiC information has been removed | £1766 | £101,986 | ||||||
| Zoledronic acid | 1.410 | −0.092 | 0.714 | 0.006 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £836 | £139,739 |
| Including PAS | CiC information has been removed | £54 | £9004 | ||||||
| Denosumab | 1.318 | 0.720 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE naive | |||||||||
| BSC | 1.598 | −0.343 | 0.716 | 0.024 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2473 | £103,350 |
| Including PAS | CiC information has been removed | £1691 | £70,679 | ||||||
| Zoledronic acid | 1.358 | −0.103 | 0.732 | 0.008 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £823 | £106,812 |
| Including PAS | CiC information has been removed | £41 | £5337 | ||||||
| Denosumab | 1.255 | 0.740 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE experienced | |||||||||
| BSC | 1.614 | −0.235 | 0.691 | 0.011 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2620 | £238,840 |
| Including PAS | CiC information has been removed | £1839 | £167,587 | ||||||
| Zoledronic acid | 1.460 | −0.082 | 0.697 | 0.004 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £848 | £196,114 |
| Including PAS | CiC information has been removed | £66 | £15,282 | ||||||
| Denosumab | 1.378 | 0.702 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
For OSTs including lung cancer, possibly because of around 40% having lung cancer with the associated poor survival, the additional patient benefits from denosumab over zoledronic acid are muted: between 0.004 QALYs for SRE-experienced patients and 0.008 QALYs for SRE-naive patients. Without the PAS the additional cost of around £840 results in cost-effectiveness estimates of more than £100,000 per QALY.
(Commercial-in-confidence information has been removed.) This results in an additional average cost of around £50 and cost-effectiveness estimates of between £5400 per QALY and £15,300 per QALY. Probabilistic modelling is again in line with this, an average gain of 0.006 QALYs at an additional average cost of £56 resulting in a central estimate of £9391 per QALY across all patients. The likelihood of denosumab being cost-effective compared with the BPs across all patients is estimated as 75% for a willingness to pay of £20,000 per QALY and as 88% for a willingness to pay of £30,000 per QALY.
As would be anticipated given the preceding analysis, for those in whom BPs are contraindicated, even with the PAS denosumab is not estimated to be cost-effective compared with BSC. Across all patients the probabilistic modelling suggests similar central estimates of 0.017 QALYs and a net cost of £1771 to yield a cost-effectiveness estimate of £102,102 per QALY compared with BSC. The likelihood of denosumab being cost-effective compared with the BPs and BSC across all patients is estimated as 0% for a willingness to pay of £20,000 per QALY and as 0% for a willingness to pay of £30,000 per QALY (Figure 14).
FIGURE 14.
Other solid tumours + lung cancer CEAFs including the PAS. (a) CEAF excluding BSC: all patients; (b) CEAF including BSC: all patients; (c) CEAF excluding BSC: SRE-naive patients; (d) CEAF including BSC: SRE-naive patients; (e) CEAF excluding BSC: SRE-experienced patients; (f) CEAF including BSC: SRE-experienced patients.
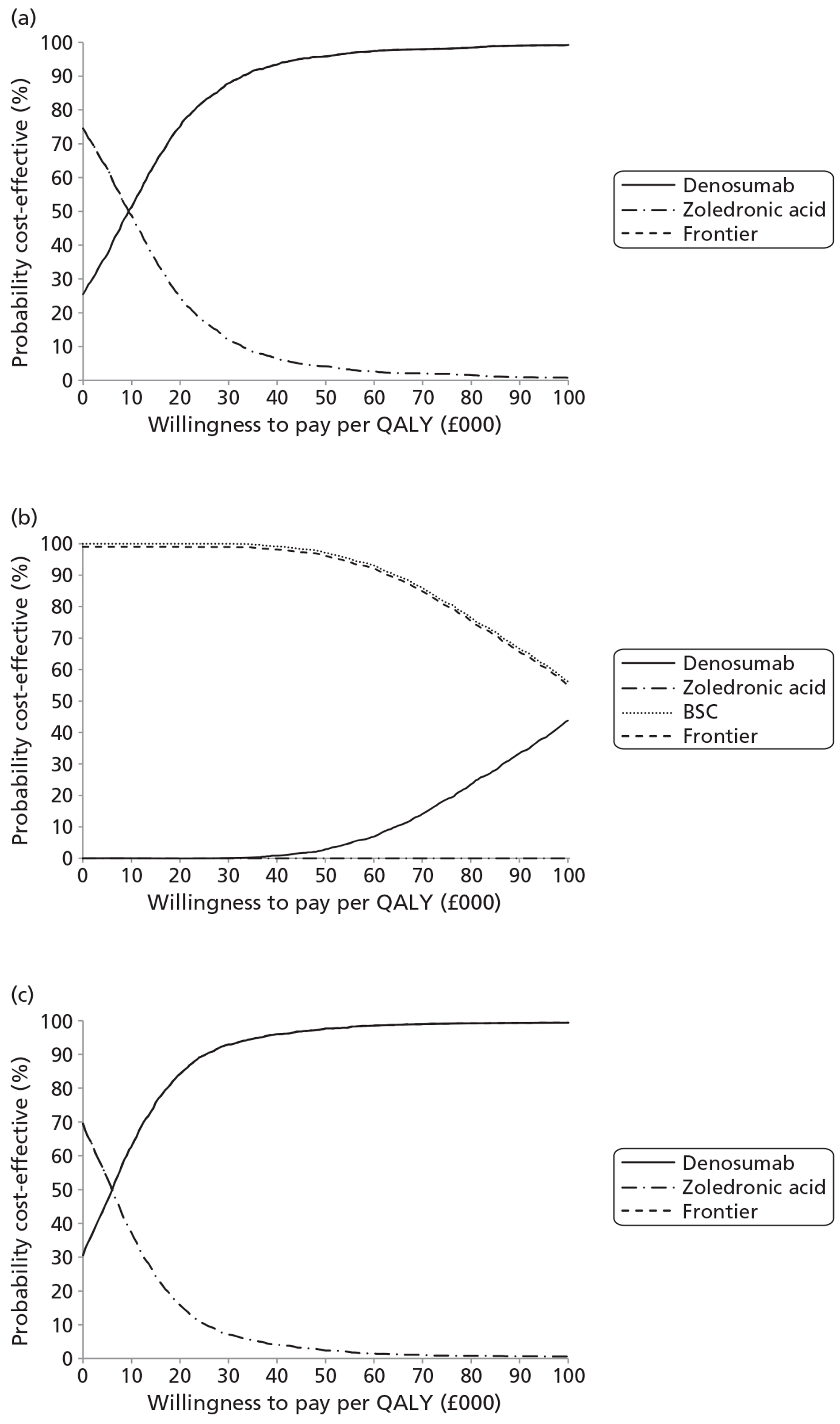
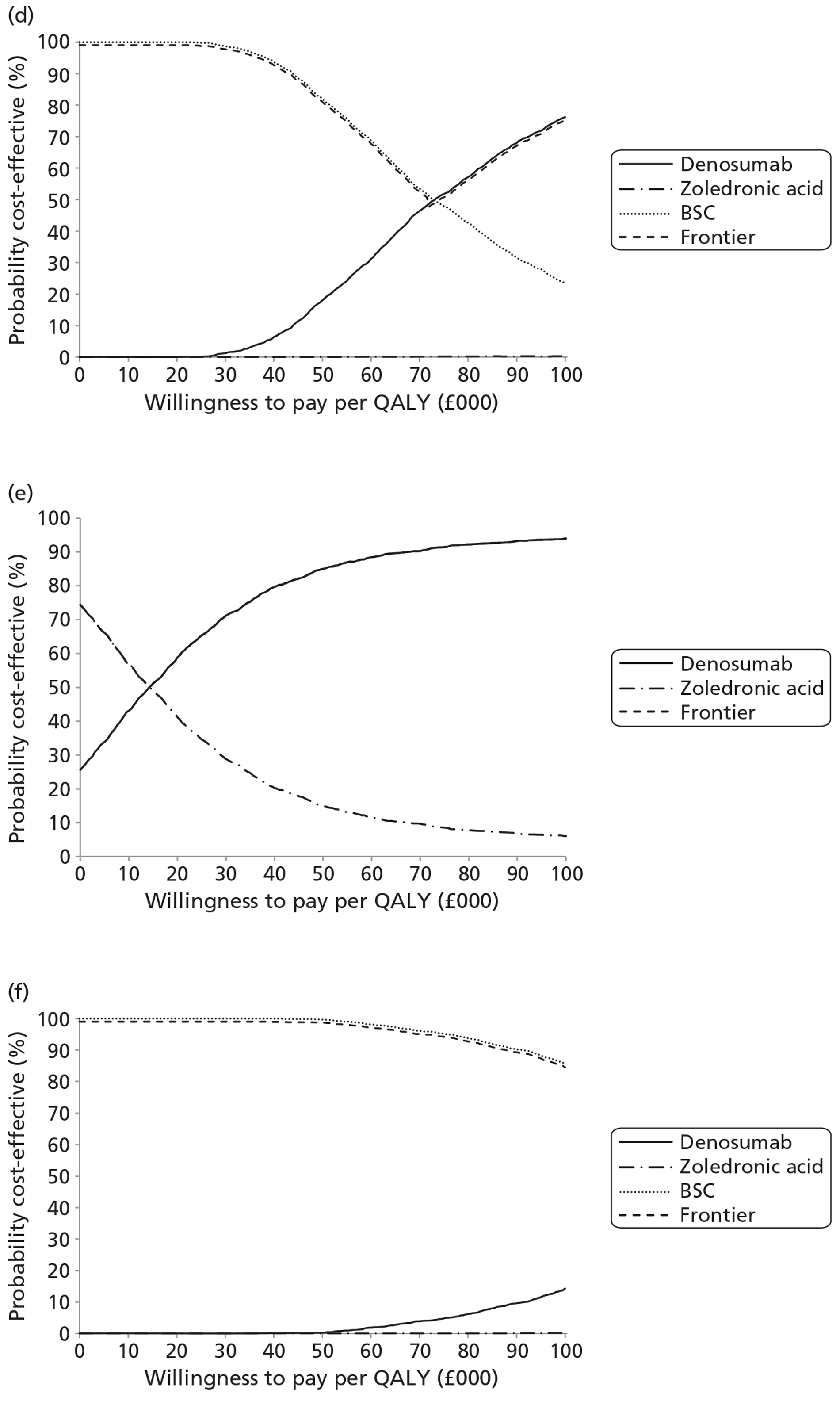
Other solid tumours including lung cancer sensitivity analyses
The univariate sensitivity analyses for the SRE-experienced patient modelling for the cost-effectiveness of denosumab are presented in Table 113.
| Sensitivity analyses | SRE-naive patients vs BSC | SRE-experienced patients vs zoledronic acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excluding PAS | Including PAS | SREs | QALYs | ICER excluding | ICER including | Excluding PAS | Including PAS | SREs | QALYs | ICER excluding | ICER including | |
| Base case | £2473 | £1691 | −0.343 | 0.024 | £103,350 | £70,679 | £848 | £66 | −0.082 | 0.004 | £196,114 | £15,282 |
| Amgen STARs | £2618 | £1836 | −0.343 | 0.024 | £109,409 | £76,737 | £869 | £87 | −0.082 | 0.004 | £200,948 | £20,115 |
| Amgen NMA | £2509 | £1727 | −0.320 | 0.022 | £112,789 | £77,644 | £849 | £68 | −0.081 | 0.004 | £198,534 | £15,801 |
| Amgen STARs+NMA | £2646 | £1864 | −0.320 | 0.022 | £118,943 | £83,798 | £870 | £88 | −0.081 | 0.004 | £203,338 | £20,606 |
| No naive util step | £2473 | £1691 | −0.343 | 0.018 | £135,660 | £92,775 | – | – | – | – | – | – |
| SCC ongoing mean | £2473 | £1691 | −0.343 | 0.027 | £90,853 | £62,132 | £848 | £66 | −0.082 | 0.005 | £164,375 | £12,808 |
| SCC ongoing max. | £2473 | £1691 | −0.343 | 0.030 | £82,514 | £56,429 | £848 | £66 | −0.082 | 0.006 | £144,789 | £11,282 |
| No gen. mortality | £2481 | £1696 | −0.344 | 0.024 | £103,033 | £70,452 | £851 | £67 | −0.082 | 0.004 | £195,987 | £15,403 |
| 5-year horizon | £2476 | £1695 | −0.338 | 0.024 | £105,289 | £72,086 | £845 | £65 | −0.082 | 0.004 | £196,090 | £15,025 |
| 2-year horizon | £2385 | £1639 | −0.311 | 0.021 | £113,714 | £78,167 | £788 | £42 | −0.076 | 0.004 | £195,766 | £10,537 |
| vd Hout utility | £2473 | £1691 | −0.343 | 0.020 | £124,310 | £85,013 | £848 | £66 | −0.082 | 0.004 | £237,589 | £18,514 |
| SAE P1+ | £2473 | £1691 | −0.343 | 0.020 | £122,918 | £84,061 | £848 | £66 | −0.082 | 0.008 | £107,304 | £8361 |
| No SAE | £2459 | £1671 | −0.345 | 0.025 | £98,978 | £67,269 | £846 | £58 | −0.083 | 0.004 | £204,488 | £14,044 |
| Gen. discs. EoT | £2941 | £2008 | −0.418 | 0.029 | £100,698 | £68,732 | £928 | −£5 | −0.091 | 0.005 | £190,376 | Dominant |
| No gen. discs. | £5895 | £4064 | −0.760 | 0.049 | £120,402 | £83,010 | £1630 | −£201 | −0.172 | 0.009 | £176,418 | Dominant |
| No discs. | £6040 | £4165 | −0.777 | 0.050 | £121,082 | £83,502 | £1696 | −£178 | −0.179 | 0.010 | £176,813 | Dominant |
| TTF form AG naive | – | – | – | – | – | – | – | – | – | – | – | – |
| TTF form AG all | £2475 | £1693 | −0.339 | 0.024 | £103,297 | £70,666 | – | – | – | – | – | – |
(Commercial-in-confidence information has been removed.) The slight increase in patient benefits is not sufficient to offset the increase in costs within the without-PAS scenario and the cost-effectiveness worsens as a consequence. But with the PAS the balance alters and the SRE and SAE effects come to the fore and the cost reductions result in cost-effectiveness estimates with the PAS seeing denosumab come to dominate zoledronic acid. This is mirrored to a more muted extent by the sensitivity analysis, which removes the impact of SAEs, causing the patient benefit to be reduced and cost-effectiveness estimates to worsen accordingly.
Assuming discontinuations occur at the end of the average trial duration of therapy, or removing discontinuations altogether, tends to worsen the cost-effectiveness for the comparison with BSC but improve it for the comparison with zoledronic acid. The latter is mainly due to the higher rate of discontinuations in the zoledronic arm than in the denosumab arm, causing more to move on to BSC. Given the poor cost-effectiveness of denosumab compared with BSC, this tends to also worsen the cost-effectiveness of the denosumab arm compared with the zoledronic acid arm. It can be argued that the apparent worsening of the cost-effectiveness of denosumab versus zoledronic acid, when compared with the breast cancer and prostate cancer estimates, is the result of the perverse impact of the differential discontinuation rates causing more patients in the zoledronic acid arm to discontinue and receive BSC.
A reduction in the price of zoledronic acid (commercial-in-confidence information has been removed) may be sufficient to result in the cost-effectiveness of denosumab compared with zoledronic acid for SRE-experienced patients with OSTs including lung cancer, including the PAS, worsening to levels that might not be considered cost-effective.
Applying the head-to-head SRE-naive- and -experienced-specific clinical effectiveness results for denosumab versus zoledronic acid, while retaining the remainder of the AG's NMA, gives the results in Table 114.
| Comparator | SREs | Net | QALYs | Net | Tx costs | Net | All costs | Net | ICER |
|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||
| BSC | 1.606 | −0.255 | 0.703 | 0.016 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2606 | £164,322 |
| Including PAS | CiC information has been removed | £1824 | £115,025 | ||||||
| Zoledronic acid | 1.410 | −0.059 | 0.714 | 0.005 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £893 | £197,725 |
| Including PAS | CiC information has been removed | £112 | £24,686 | ||||||
| Denosumab | 1.352 | 0.719 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE naive | |||||||||
| BSC | 1.598 | −0.341 | 0.716 | 0.024 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2477 | £102,060 |
| Including PAS | CiC information has been removed | £1695 | £69,845 | ||||||
| Zoledronic acid | 1.358 | −0.102 | 0.732 | 0.008 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £827 | £102,773 |
| Including PAS | CiC information has been removed | £45 | £5580 | ||||||
| Denosumab | 1.257 | 0.740 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE experienced | |||||||||
| BSC | 1.614 | −0.171 | 0.691 | 0.008 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2730 | £350,937 |
| Including PAS | CiC information has been removed | £1948 | £250,441 | ||||||
| Zoledronic acid | 1.460 | −0.018 | 0.697 | 0.001 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £957 | £846,749 |
| Including PAS | CiC information has been removed | £176 | £155,285 | ||||||
| Denosumab | 1.443 | 0.698 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
The SRE subgroup-specific clinical effectiveness estimates have the most dramatic impact on this group of cancers. As would be anticipated given the RR among the SRE-experienced subgroup their modelled benefits from denosumab over zoledronic acid are very slight and do not justify the additional cost.
Lung cancer base case
The results for lung cancer are broadly similar to the previous analysis (Table 115). For the comparison with zoledronic acid patient benefits are muted among SRE-experienced patients: 0.003 QALYs. This may be a factor in their short life expectancy, but with the PAS the additional costs of £43 result in a cost-effectiveness estimate of £12,742. This also applies to the SRE-naive subgroup where larger gains of 0.006 QALYs are achieved at minimal additional cost once the PAS is included. But the cost-effectiveness for these patients compared with BSC remains poor at an estimated £110,671 per QALY.
| Comparator | SREs | Net | QALYs | Net | Tx costs | Net | All costs | Net | ICER |
|---|---|---|---|---|---|---|---|---|---|
| All patients | |||||||||
| BSC | 0.952 | −0.218 | 0.441 | 0.012 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2262 | £191,412 |
| Including PAS | CiC information has been removed | £1583 | £133,926 | ||||||
| Zoledronic acid | 0.809 | −0.076 | 0.448 | 0.005 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £708 | £149,878 |
| Including PAS | CiC information has been removed | £28 | £5972 | ||||||
| Denosumab | 0.734 | 0.453 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE naive | |||||||||
| BSC | 0.886 | −0.228 | 0.455 | 0.014 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2257 | £158,333 |
| Including PAS | CiC information has been removed | £1578 | £110,671 | ||||||
| Zoledronic acid | 0.746 | −0.087 | 0.463 | 0.006 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £693 | £112,617 |
| Including PAS | CiC information has been removed | £13 | £2135 | ||||||
| Denosumab | 0.659 | 0.470 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
| SRE experienced | |||||||||
| BSC | 1.015 | −0.210 | 0.427 | 0.009 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £2268 | £239,211 |
| Including PAS | CiC information has been removed | £1588 | £167,529 | ||||||
| Zoledronic acid | 0.870 | −0.065 | 0.433 | 0.003 | CiC information has been removed | CiC information has been removed | CiC information has been removed | £722 | £215,614 |
| Including PAS | CiC information has been removed | £43 | £12,743 | ||||||
| Denosumab | 0.806 | 0.437 | CiC information has been removed | CiC information has been removed | |||||
| Including PAS | CiC information has been removed | CiC information has been removed | |||||||
As for the other analyses, the probabilistic modelling central estimates are broadly in line with those of the deterministic analysis. Across all patients the central estimate is of a 0.005 QALY gain compared with zoledronic acid and a 0.012 QALY gain compared with BSC. This is at an additional net cost central estimate of £32 and £1582, respectively, with the PAS.
The likelihood of denosumab being cost-effective compared with the BPs across all patients is estimated as 69% for a willingness to pay of £20,000 per QALY and as 77% for a willingness to pay of £30,000 per QALY. The likelihood of denosumab being cost-effective compared with the BPs and BSC across all patients is estimated as 0% for a willingness to pay of £20,000 per QALY and as 0% for a willingness to pay of £30,000 per QALY (Figure 15).
FIGURE 15.
Lung cancer CEAFs including the PAS. (a) CEAF excluding BSC: all patients; (b) CEAF including BSC: all patients; (c) CEAF excluding BSC: SRE-naive patients; (d) CEAF including BSC: SRE-naive patients; (e) CEAF excluding BSC: SRE-experienced patients; (f) CEAF including BSC: SRE-experienced patients.
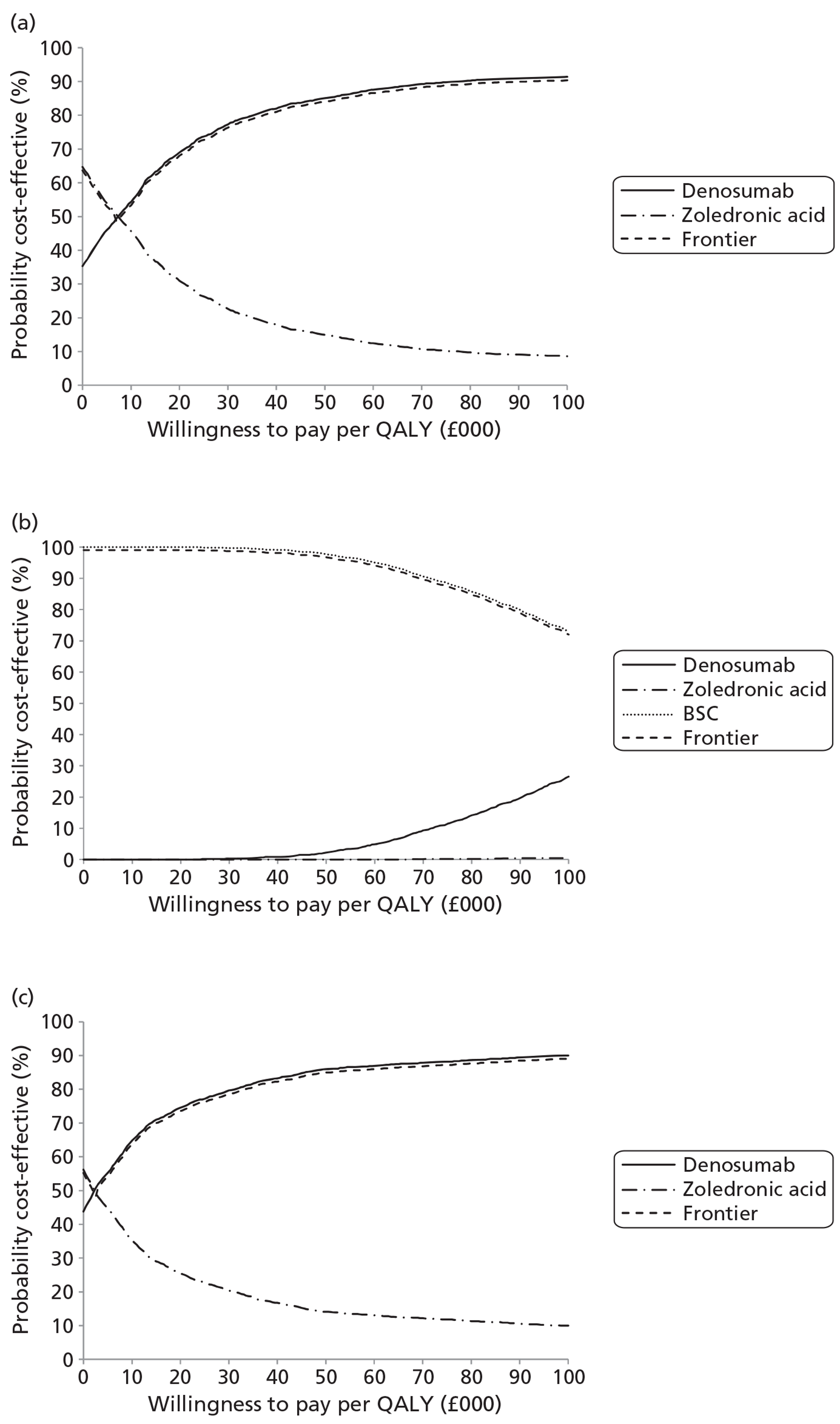
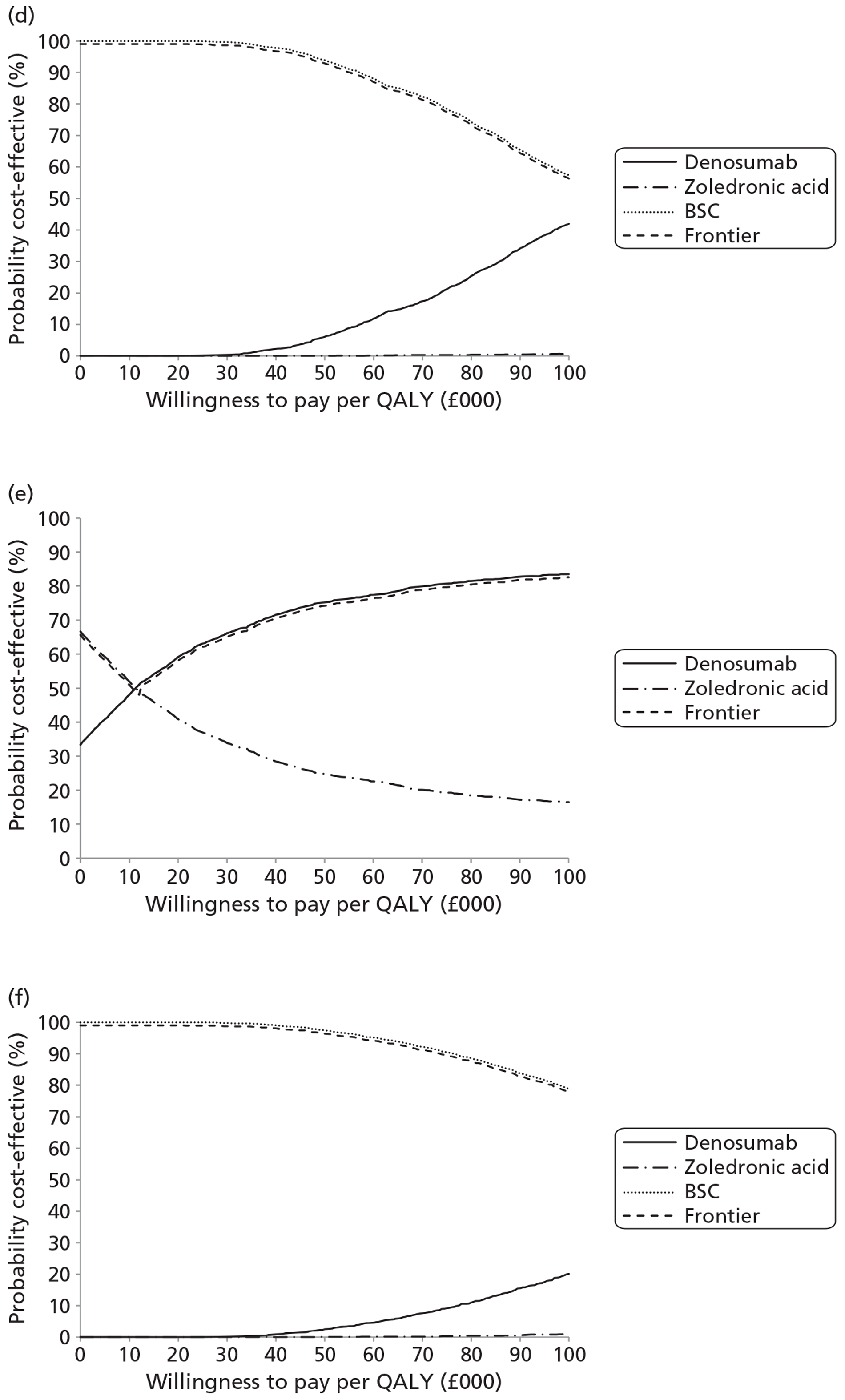
Lung cancer sensitivity analyses
The univariate sensitivity analyses for the SRE-experienced patient modelling for the cost-effectiveness of denosumab compared with zoledronic acid is presented in Table 116.
| Sensitivity analyses | SRE-naive patients vs BSC | SRE-experienced patients vs zoledronic acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excluding PAS | Including PAS | SREs | QALYs | ICER excluding | ICER including | Excluding PAS | Including PAS | SREs | QALYs | ICER excluding | ICER including | |
| Base case | £2257 | £1578 | −0.228 | 0.014 | £158,333 | £110,671 | £722 | £43 | −0.065 | 0.003 | £215,614 | £12,743 |
| Amgen STARs | £2359 | £1679 | −0.228 | 0.014 | £165,463 | £117,801 | £738 | £58 | −0.065 | 0.003 | £220,231 | £17,361 |
| Amgen NMA | £2257 | £1578 | −0.228 | 0.014 | £158,333 | £110,671 | £722 | £43 | −0.065 | 0.003 | £215,614 | £12,743 |
| Amgen STARs+NMA | £2359 | £1679 | −0.228 | 0.014 | £165,463 | £117,801 | £738 | £58 | −0.065 | 0.003 | £220,231 | £17,361 |
| No naive util step | £2257 | £1578 | −0.228 | 0.011 | £199,936 | £139,750 | – | – | – | – | – | – |
| SCC ongoing mean | £2257 | £1578 | −0.228 | 0.015 | £149,443 | £104,457 | £722 | £43 | −0.065 | 0.004 | £200,348 | £11,841 |
| SCC ongoing max. | £2257 | £1578 | −0.228 | 0.016 | £142,745 | £99,775 | £722 | £43 | −0.065 | 0.004 | £189,156 | £11,180 |
| No gen. mortality | £2263 | £1582 | −0.228 | 0.014 | £158,064 | £110,477 | £725 | £43 | −0.065 | 0.003 | £215,469 | £12,821 |
| 5-year horizon | £2257 | £1578 | −0.227 | 0.014 | £158,499 | £110,792 | £722 | £43 | −0.065 | 0.003 | £215,613 | £12,735 |
| 2-year horizon | £2227 | £1559 | −0.218 | 0.013 | £165,275 | £115,737 | £703 | £36 | −0.063 | 0.003 | £215,451 | £10,888 |
| vd Hout utility | £2257 | £1578 | −0.228 | 0.011 | £205,154 | £143,397 | £722 | £43 | −0.065 | 0.003 | £279,244 | £16,504 |
| SAE P1+ | £2257 | £1578 | −0.228 | 0.013 | £177,449 | £124,032 | £722 | £43 | −0.065 | 0.005 | £147,641 | £8726 |
| No SAE | £2243 | £1559 | −0.229 | 0.015 | £149,896 | £104,205 | £719 | £35 | −0.065 | 0.003 | £227,229 | £11,032 |
| Gen. discs. EoT | £2727 | £1910 | −0.266 | 0.017 | £164,689 | £115,359 | £798 | −£18 | −0.071 | 0.004 | £211,256 | Dominant |
| No gen. discs. | £3885 | £2737 | −0.343 | 0.020 | £191,622 | £135,008 | £1046 | −£102 | −0.096 | 0.005 | £204,827 | Dominant |
| No discs. | £3926 | £2766 | −0.346 | 0.020 | £192,291 | £135,497 | £1064 | −£96 | −0.097 | 0.005 | £204,801 | Dominant |
| TTF form AG naive | – | – | – | – | – | – | – | – | – | – | – | – |
| TTF form AG all | – | – | – | – | – | – | – | – | – | – | – | – |
The sensitivity analyses for lung cancer that remove the discontinuations have a similar impact as within the OSTs plus lung cancer modelling, given that in the absence of other data the lung cancer modelling assumes the adverse event rates and discontinuations of the OST plus lung cancer modelling. This may again argue that the apparent worsening of the cost-effectiveness of denosumab versus zoledronic acid, when compared with the breast cancer and prostate cancer estimates, is the result of the perverse impact of the differential discontinuation rates causing more patients in the zoledronic acid arm to discontinue and receive BSC.
The main sensitivities are in the treatment of utilities, with the removal of the step change going from naive to experienced reducing patient benefits by around one-quarter. Given the short life expectancy, the application of the van den Hout utility modifiers also has a reasonably large impact.
A reduction in the price of zoledronic acid of (commercial-in-confidence information has been removed) results in the cost-effectiveness of denosumab compared with zoledronic acid for SRE-experienced patients with OSTs including lung cancer including the PAS, which might not be considered cost-effective.
Sensitivity analyses
The sensitivity analyses are presented in greater detail within each of the cancer-specific modelling sections above.
In brief, the results of the AG for breast cancer are broadly in line with those of the manufacturer. There is some sensitivity in results to the rates of SAEs because of the higher rate of renal toxicity applied within the zoledronic acid arm. Discontinuations tend to increase net costs compared with zoledronic acid broadly in line with the net benefits and the cost-effectiveness estimates are reasonably stable. Applying the SRE-naive- and -experienced-specific HRs and RRs has only a muted impact.
For prostate cancer the AG base-case results are again broadly in line with those of the manufacturer. Results show some sensitivity to the utility decrements from SCC being extended to the end of life. Applying the SRE-naive- and -experienced-specific HRs and RRs has a more noticeable effect. Among the SRE-experienced patients this sees the net impact of denosumab compared with zoledronic acid fall from a reduction in SREs of 0.135 to a reduction of only 0.087, with a parallel impact on the anticipated patient benefits.
Within the modelling of OSTs including lung cancer, the benefit of denosumab over zoledronic acid is small and results become sensitive to the other parameters within the modelling, such as the treatment of SAEs. Results for denosumab compared with BSC are more stable as the analysis is driven more by the relative rates of SREs, particularly among SRE-naive patients.
Applying the SRE-naive- and -experienced-specific HRs and RRs has a relatively large impact on results for the SRE-experienced OSTs including lung cancer modelling. This may in itself be sufficient to render denosumab, even with the PAS, non-cost-effective compared with zoledronic acid for this group.
The OSTs plus NSCLC results are broadly mirrored in the modelling of lung cancer.
An aspect that may have an impact beyond that modelled is the treatment of SCC. Extending the average quality-of-life decrement measured in the 5 months subsequent to the compression through to death improves the estimated cost-effectiveness, particularly among SRE-naive prostate cancer patients. There remains uncertainty as to the rate of paralysis from SCC, the long-term quality-of-life impacts from SCC and the need for long-term care together with the associated costs.
Where the appropriate comparator is zoledronic acid, there is additional uncertainty concerning its likely price when it shortly comes off patent. (Commercial-in-confidence information has been removed.)
Probabilistic modelling suggests that within the usual range of cost-effectiveness thresholds there is relatively little uncertainty around the CEAF. The central estimates are also in line with those of the deterministic analyses.
Discussion
For ease of reference, the manufacturer's base-case results, the Evidence Review Group (ERG)'s base-case results and the ERG's structural sensitivity analyses that apply the SRE-naive- and -experienced-specific HRs and RRs are summarised for the comparison with zoledronic acid (Table 117) and the comparison with BSC (Table 118).
| Quantity | Breast cancer | Prostate cancer | OST + lung cancer | Lung cancer | ||||
|---|---|---|---|---|---|---|---|---|
| Excluding PAS | Including PAS | Excluding PAS | Including PAS | Excluding PAS | Including PAS | Excluding PAS | Including PAS | |
| Manufacturer: pooled RR and HR | ||||||||
| All | ||||||||
| Δ cost | £1484 | −£483 | ||||||
| Δ QALY | 0.007 | |||||||
| ICER | £203,387 | Dominant | ||||||
| Experienced | ||||||||
| Δ cost | £922 | −£281 | £757 | −£43 | ||||
| Δ QALY | 0.006 | 0.004 | ||||||
| ICER | £157,276 | Dominant | £205,580 | Dominant | ||||
| AG modelling: pooled RR and HR | ||||||||
| All | ||||||||
| Δ cost | £1707 | −£243 | £1059 | −£125 | £836 | £54 | £708 | £28 |
| Δ QALY | 0.007 | 0.007 | 0.009 | 0.009 | 0.006 | 0.006 | 0.005 | 0.005 |
| ICER | £245,264 | Dominant | £111,603 | Dominant | £139,739 | £9004 | £149,878 | £5972 |
| Naive | ||||||||
| Δ cost | £1747 | −£203 | £1061 | −£123 | £823 | £41 | £693 | £13 |
| Δ QALY | 0.008 | 0.008 | 0.011 | 0.011 | 0.008 | 0.008 | 0.006 | 0.006 |
| ICER | £209,345 | Dominant | £99,561 | Dominant | £106,812 | £5337 | £112,617 | £2135 |
| Experienced | ||||||||
| Δ cost | £1649 | −£301 | £1053 | −£131 | £848 | £66 | £722 | £43 |
| Δ QALY | 0.005 | 0.005 | 0.006 | 0.006 | 0.004 | 0.004 | 0.003 | 0.003 |
| ICER | £332,185 | Dominant | £170,854 | Dominant | £196,114 | £15,282 | £215,614 | £12,743 |
| AG modelling: SRE-naive- and -experienced-specific HRs and RRs | ||||||||
| All | ||||||||
| Δ cost | £1693 | −£258 | £1076 | −£109 | £893 | £112 | £708 | £28 |
| Δ QALY | 0.007 | 0.007 | 0.009 | 0.009 | 0.005 | 0.005 | 0.005 | 0.005 |
| ICER | £259,484 | Dominant | £117,021 | Dominant | £197,725 | £24,686 | £149,878 | £5972 |
| Naive | ||||||||
| Δ cost | £1763 | −£187 | £1064 | −£121 | £827 | £45 | £693 | £13 |
| Δ QALY | 0.007 | 0.007 | 0.011 | 0.011 | 0.008 | 0.008 | 0.006 | 0.006 |
| ICER | £247,591 | Dominant | £96,209 | Dominant | £102,773 | £5580 | £112,617 | £2135 |
| Experienced | ||||||||
| Δ cost | £1592 | -£359 | £1111 | −£74 | £957 | £176 | £722 | £43 |
| Δ QALY | 0.006 | 0.006 | 0.004 | 0.004 | 0.001 | 0.001 | 0.003 | 0.003 |
| ICER | £280,994 | Dominant | £285,209 | Dominant | £846,749 | £155,285 | £215,614 | £12,743 |
| Quantity | Breast cancer | Prostate cancer | OST + lung cancer | Lung cancer | ||||
|---|---|---|---|---|---|---|---|---|
| Excluding PAS | Including PAS | Excluding PAS | Including PAS | Excluding PAS | Including PAS | Excluding PAS | Including PAS | |
| Manufacturer: pooled RR and AR | ||||||||
| Naive | ||||||||
| Δ cost | £3993 | £2790 | £2530 | £1730 | ||||
| Δ QALY | 0.039 | 0.021 | ||||||
| ICER | £102,067 | £71,320 | £122,499 | £83,763 | ||||
| AG modelling: pooled RR and HR | ||||||||
| All | ||||||||
| Δ cost | £6242 | £4292 | £3951 | £2766 | £2548 | £1766 | £2262 | £1583 |
| Δ QALY | 0.027 | 0.027 | 0.035 | 0.035 | 0.017 | 0.017 | 0.012 | 0.012 |
| ICER | £229,547 | £157,829 | £112,415 | £78,713 | £147,122 | £101,986 | £191,412 | £133,926 |
| Naive | ||||||||
| Δ cost | £6308 | £4358 | £3969 | £2785 | £2473 | £1691 | £2257 | £1578 |
| Δ QALY | 0.035 | 0.035 | 0.039 | 0.039 | 0.024 | 0.024 | 0.014 | 0.014 |
| ICER | £181,092 | £125,109 | £103,003 | £72,269 | £103,350 | £70,679 | £158,333 | £110,671 |
| Experienced | ||||||||
| Δ cost | £6146 | £4196 | £3897 | £2713 | £2620 | £1839 | £2268 | £1588 |
| Δ QALY | 0.016 | 0.016 | 0.025 | 0.025 | 0.011 | 0.011 | 0.009 | 0.009 |
| ICER | £379,539 | £259,113 | £152,916 | £106,446 | £238,840 | £167,587 | £239,211 | £167,529 |
| AG modelling: SRE-naive- and -experienced-specific HRs and RRs | ||||||||
| All | ||||||||
| Δ cost | £6227 | £4277 | £3968 | £2783 | £2606 | £1824 | £2262 | £1583 |
| Δ QALY | 0.027 | 0.027 | 0.035 | 0.035 | 0.016 | 0.016 | 0.012 | 0.012 |
| ICER | £232,756 | £159,866 | £113,851 | £79,865 | £164,322 | £115,025 | £191,412 | £133,926 |
| Naive | ||||||||
| Δ cost | £6323 | £4373 | £3972 | £2788 | £2477 | £1695 | £2257 | £1578 |
| Δ QALY | 0.034 | 0.034 | 0.039 | 0.039 | 0.024 | 0.024 | 0.014 | 0.014 |
| ICER | £188,162 | £130,133 | £102,016 | £71,597 | £102,060 | £69,845 | £158,333 | £110,671 |
| Experienced | ||||||||
| Δ cost | £6089 | £4139 | £3955 | £2770 | £2730 | £1948 | £2268 | £1588 |
| Δ QALY | 0.017 | 0.017 | 0.023 | 0.023 | 0.008 | 0.008 | 0.009 | 0.009 |
| ICER | £360,413 | £244,979 | £170,340 | £119,327 | £350,937 | £250,441 | £239,211 | £167,529 |
The manufacturer's case is broadly that while the average patient benefits from the reduced number of SREs is not large, with the PAS denosumab will be cost saving compared with zoledronic acid. (Commercial-in-confidence information has been removed.) As a consequence, denosumab is estimated to dominate zoledronic acid among patients for whom zoledronic acid is indicated when the PAS is included.
But for patients for whom zoledronic acid is not indicated, the manufacturer accepts that even with the PAS the relatively small patient gains do not justify the additional cost of denosumab. The manufacturer's cost-effectiveness estimates for denosumab compared with BSC are typically in excess of £100,000 per QALY, and even with the PAS are closer to £100,000 per QALY than £50,000 per QALY.
Within-trial analyses by the AG suggest that for breast cancer patients denosumab results in a slightly lower average number of SREs than zoledronic acid, and that this will translate into a small average annual gain of perhaps 0.003–0.006 QALYs: roughly equivalent to 1–2 additional days in full health or 2–3 days at the SRE-naive average quality of life. Without the PAS, the additional cost of denosumab does not justify these relatively minor gains. With the PAS, denosumab is estimated to be broadly cost-neutral to slightly cost saving, and so cost-effective compared with zoledronic acid. (Commercial-in-confidence information has been removed.)
Within-trial analyses suggest that for prostate cancer patients, denosumab results in a slightly lower average number of SREs compared with zoledronic acid. This translates into a slightly larger additional average annual gain of perhaps 0.008–0.016 QALYs. The reason for this difference in prostate cancer is the greater proportion of SCCs within the overall number of SREs. (Academic-in-confidence information has been removed.) This aspect is not considered in either the manufacturer's model or the AG economic model.
Without the PAS the additional cost of denosumab still does not justify the relatively minor estimated gains. With the PAS, because of the average annual number of doses, denosumab is estimated to increase annual costs by around £100, which translates into cost-effectiveness estimates of between £6545 per QALY and £15,272 per QALY. But this AG within-trial analysis does not distinguish between SRE-naive and -experienced patients.
Given the slightly larger patient gains estimated for prostate cancer patients from denosumab, its cost-effectiveness compared with zoledronic acid is not as sensitive to the price of zoledronic acid as it is in breast cancer. (Commercial-in-confidence information has been removed.)
For the cost–utility modelling within breast cancer, the lifetime gains across all patients are estimated to be around 0.007 QALYs. This is again small, and does not justify the additional cost of £1707 per patient compared with zoledronic acid. With the PAS (commercial-in-confidence information has been removed), denosumab is estimated to dominate zoledronic acid. But for those in whom BPs are contraindicated the cost-effectiveness is poor: even with the PAS the cost-effectiveness is £157,829 per QALY. Applying the SRE-naive and -experienced subgroup-specific clinical effectiveness has little impact on the results, as these estimates are reasonably close to the pooled all-patient estimates.
For the cost–utility modelling within prostate cancer, across all patients the gain from denosumab over zoledronic acid is around 0.009 QALYs, while compared with BSC it is 0.035 QALYs, at net costs without the PAS of £1059 and £3951, respectively. Without the PAS, compared with zoledronic acid, this results in a cost-effectiveness of £111,603 per QALY. Cost-effectiveness is estimated to be slightly better among the SRE-naive patients, at £99,561 per QALY, but the quid pro quo is a worse cost-effectiveness among the SRE-experienced patients of £170,854 per QALY. This may arise in large part because of the estimated step change in HRQoL arising from a patient's first SRE.
With the PAS, denosumab is estimated to be cost saving compared with zoledronic acid and so dominate it. For those in whom BPs are contraindicated, denosumab is not estimated to be cost-effective compared with BSC.
Within the cost–utility modelling of OSTs including lung cancer, the gains from denosumab over zoledronic acid are estimated to be less than 0.01 QALYs. Without the PAS, denosumab is not cost-effective, but with it the small additional overall costs of around £50 result in cost-effectiveness estimates of between £5400 per QALY and £15,300 per QALY. The impact of applying the SRE subgroup-specific estimates within this group is quite large. While it improves the estimates of cost-effectiveness of denosumab compared with BSC for SRE-naive patients, even with the PAS it is not sufficient to render it cost-effective. (Academic-in-confidence information has been removed.) The cost-effectiveness estimate for denosumab worsens to £155,285 per QALY compared with zoledronic acid among these patients.
For lung cancer, possibly because of the short life expectancy, the patient gains from denosumab over zoledronic acid among SRE-experienced patients are estimated to be small: 0.003 QALYs. With the PAS, the additional cost of £43 results in a cost-effectiveness of £12,743 per QALY.
Some questions for possible consideration are:
-
To what extent do the available data on SRE-naive patients and SRE-experienced patients reflect the likely patient groups for whom zoledronic acid is used? Is the manufacturer's case review sufficient to conclude that most SRE-experienced patients within the cancers reviewed are typically receiving BPs, leading to zoledronic acid being the appropriate comparator?
-
Should the base case apply the SRE subgroup-specific clinical effectiveness estimates? This has little impact within breast cancer. But it has quite large adverse effects on the cost-effectiveness of denosumab for SRE-experienced patients in prostate cancer and OSTs including lung cancer.
-
To what extent should zoledronic acid coming off patent in 2013 be considered? The anticipated patient benefits from denosumab over zoledronic acid are small. Only a relatively small drop in the price of zoledronic acid would be sufficient to make denosumab not cost-effective when judged by conventional thresholds.
Chapter 10 Assessment of factors relevant to the National Health Service and other parties
Any change in the treatment pathway of bone metastases is likely to have an impact on the NHS and other parties. The impact of denosumab depends on whether the patient would otherwise have received an intravenous BP, oral BP or BSC.
Factors relevant to the National Health Service
For patients who would have received an intravenous BP, subcutaneous denosumab is advantageous. First, subcutaneous administration does not require inpatient administration. Denosumab could be given in an outpatient setting, in a general practitioner surgery or even potentially at home by a district nurse or other qualified health-care provider. Compared with intravenous injections, subcutaneous administration takes less time, is associated with few complications and is technically easier. This is not relevant to those patients who would have been prescribed an oral BP or who need to attend hospital for other reasons, such as intravenous chemotherapy. Any shift of care from acute hospitals into the community has implications for the NHS. Additional resources and training may be needed in the community. Denosumab is administered using the standard subcutaneous method. NHS staff need to be aware that in prostate cancer and OSTs BPs may be used for treatment of bone pain when conventional analgesics have failed. Denosumab is licensed for the prevention of SREs and not for the treatment of bone pain. It is conceivable that reduction in pain is a method of preventing the need for radiotherapy. However, evidence for the analgesic effects of denosumab is not consistent. Prescribers would also need to be aware of the potential adverse events, such as hypocalcaemia and ONJ.
Second, for patients who are prescribed oral BPs, adherence may increase if they are switched to denosumab. Oral BPs are inconvenient for patients to take because of adverse effects and the required technique. Subcutaneous injection avoids these unpleasant upper gastrointestinal adverse effects. However, it should be noted that, according to the Xgeva SPC, diarrhoeal adverse events are ‘very common’.
For those patients who would have otherwise been treated with BSC, administration of denosumab would require additional resources. Denosumab needs to be stored at 2–8 °C in a refrigerator. Most NHS premises have facilities to store medicinal products in a refrigerator. However, if any premises did not have these facilities or required more space, additional resources may be necessary.
Renal monitoring is required in patients receiving BPs. This has not only resource issues but also safety issues. Any medication that requires dose adjustment according to renal function increases the likelihood of human error. As denosumab is administered by fixed-dose single injection, the risk of human error is substantially reduced. Denosumab may reduce the need for laboratory services. However, patients with advanced cancer usually undergo frequent blood sampling, including measure of renal function.
Factors relevant to other parties
Delaying or preventing SREs may result in patients being mobile for longer. It should be noted that mobility has not been assessed in the pivotal trials. However, preventing pathological fractures, surgery to bone or SCC is likely to result in reduced immobility. In turn, this would reduce the burden on carers.
Patients who would have otherwise been prescribed an intravenous BP may have reduced need for hospital attendance. Administration may be possible in the community. This would reduce travelling time for both patients and carers. This is particularly important for patients who have problems with mobility or live in rural locations or areas with poor transport links. It may reduce the number of days off work for patients who are still employed or for carers who need to take time off to attend hospital appointments. For patients who are required to attend hospital, denosumab would shorten the time in hospital. Total time for administration of zoledronic acid may be 30–45 minutes depending on the time it takes to establish intravenous access, whereas a subcutaneous injection would take only a few minutes.
Subcutaneous administration may also be less unpleasant for many patients compared with intravenous or oral BP administration.
For patients who would have previously been treated with BSC alone, the addition of denosumab would usually mean additional health-care appointments. This may require the patient and carer travelling to an acute hospital or general practitioner surgery.
Chapter 11 Discussion
Clinical effectiveness
Statement of principal findings
Breast cancer
There was a statistically significant difference in favour of denosumab compared with zoledronic acid for the time to first on-study SRE for all patients (HR 0.82; 95% CI 0.71 to 0.95; not reached vs median 26.4 months) (Table 119). (Academic-in-confidence information has been removed) (Table 119) (academic-in-confidence information has been removed). For both time to first on-study SRE and risk of developing first and subsequent SREs, the distribution of type of SRE was similar across treatment groups, with pathological fracture (academic-in-confidence information has been removed) and radiation to bone (academic-in-confidence information has been removed) being the most common, while there were few occurrences of SCC (academic-in-confidence information has been removed) or surgery to bone. (Academic-in-confidence information has been removed.)
| Results | Breast cancer (study 136) | Prostate cancer (study 103) | NSCLC (study 244 subgroup) | OST excluding NSCLC (study 244 subgroup) | OST including NSCLC (study 244 post-hoc analysis) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Denosumab (n = 1026) | Zoledronic acid (n = 1020) | Denosumab (n = 950) | Zoledronic acid (n = 951) | Denosumab (n = 350) | Zoledronic acid (n = 352) | Denosumab (n = 449) | Zoledronic acid (n = 445) | Denosumab (n = 800) | Zoledronic acid (n = 797) | |
| Time to first on-study SRE | ||||||||||
| n (%) | 315 (30.7) | 372 (36.5) | 341 (35.9) | 386 (40.6) | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Median timea, months | NR | 26.4 | 20.7 | 17.1 | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 21.4 | 15.4 |
| HR (95% CI) | 0.82 (0.71 to 0.95) | 0.82 (0.71 to 0.95) | 0.84 (0.64 to 1.10) | 0.79 (0.62 to 0.99) | 0.81 (0.68 to 0.96) | |||||
| Risk of first and subsequent on-study SREs | ||||||||||
| No. of events | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | 328 | 374 |
| Mean no. of SREs per patient | 0.46 | 0.60 | 0.52 | 0.61 | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Rate ratio (95% CI) | 0.77 (0.66 to 0.89) | 0.82 (0.71 to 0.94) | AiC information has been removed | AiC information has been removed | 0.85 (0.72 to 1.00) | |||||
For the subgroup of patients with no or mild pain at baseline, denosumab delayed the time to development of moderate or severe worst pain (worst pain score of > 4 points) compared with zoledronic acid (HR 0.78; 95% CI 0.67 to 0.92; median 9.7 vs 5.8 months; p = 0.0024). The median time to worsening pain (≥ 2-point increase from baseline) was longer for denosumab (median 8.5 vs 7.4 months; p = 0.0822). In terms of quality of life, overall mean FACT scores remained similar between the groups (academic-in-confidence information has been removed).
In terms of adverse events, there were more occurrences of hypocalcaemia in the denosumab group than in the zoledronic acid group (5.5% vs 3.4%); rates of ONJ were also higher (2.0% vs 1.4%), but there were lower rates of events associated with renal impairment (4.9% vs 8.5%) or acute-phase reactions (10.4% vs 27.3%) (academic-in-confidence information has been removed). Overall survival was balanced between the denosumab and zoledronic acid groups (HR 0.95; 95% CI 0.81 to 1.11). (Academic-in-confidence information has been removed.)
In the AG's NMA, there was a statistically significant difference in favour of denosumab compared with zoledronic acid or placebo for time to first on-study SRE and for these comparisons plus denosumab versus disodium pamidronate for risk of first and subsequent SREs (Table 120). (Academic-in-confidence information has been removed.)
| Comparison | Time to first on-study SRE | Time to first and subsequent SRE | ||
|---|---|---|---|---|
| AG's NMA, HR (95% CI) | MS's NMA, HR (95% CI) | AG's NMA, RR (95% CI) | MS's NMA, RR (95% CI) | |
| Breast cancer | ||||
| Denosumab vs zoledronic acid | 0.82 (0.71 to 0.95) | AiC information has been removed | 0.77 (0.66 to 0.89) | AiC information has been removed |
| Denosumab vs disodium pamidronate | 0.79 (0.61 to 1.03) | AiC information has been removed | 0.62 (0.48 to 0.80) | AiC information has been removed |
| Denosumab vs placebo | 0.46 (0.29 to 0.72) | AiC information has been removed | 0.45 (0.28 to 0.72) | AiC information has been removed |
| Denosumab vs ibandronic acid | Not done | AiC information has been removed | Not done | AiC information has been removed |
| Prostate cancer | ||||
| Denosumab vs zoledronic acid | 0.82 (0.71 to 0.95) | AiC information has been removed | 0.82 (0.71 to 0.94) | AiC information has been removed |
| Denosumab vs placebo | 0.56 (0.40 to 0.77) | AiC information has been removed | 0.53 (0.39 to 0.72) | AiC information has been removed |
| NSCLC | ||||
| Denosumab vs zoledronic acid | 0.84 (0.64 to 1.10) | Not done | 0.87 (0.68 to 1.12) | Not done |
| Denosumab vs placebo | 0.68 (0.45 to 1.03) | Not done | 0.63 (0.42 to 0.97) | Not done |
| OST excluding NSCLC | ||||
| Denosumab vs zoledronic acid | 0.79 (0.62 to 0.99) | Not done | 0.83 (0.67 to 1.03) | Not done |
| Denosumab vs placebo | 0.30 (0.11 to 0.82) | Not done | 0.61 (0.39 to 0.97) | Not done |
| OST including NSCLC | ||||
| Denosumab vs zoledronic acid | 0.81 (0.68 to 0.96) | AiC information has been removed | 0.85 (0.72 to 1.00) | AiC information has been removed |
| Denosumab vs placebo | 0.49 (0.30 to 0.78) | AiC information has been removed | 0.62 (0.46 to 0.85) | AiC information has been removed |
Prostate cancer
There was a statistically significant difference in favour of denosumab compared with zoledronic acid for the time to first on-study SRE for all patients (HR 0.82; 95% CI 0.71 to 0.95; median 20.7 vs 17.1 months) (Table 119) and for those with no prior SRE (HR 0.80; 95% CI 0.67 to 0.95) but not for those with a prior SRE (HR 0.88; 95% CI 0.67 to 1.16). There was also a statistically significant difference in favour of denosumab for reducing the risk of developing first and subsequent SREs for all patients (RR 0.82; 95% CI 0.71 to 0.94) (Table 119) and for those with no prior SRE (RR 0.79; 95% CI 0.67 to 0.94), but not for those with a prior SRE (RR 0.88; 95% CI 0.68 to 1.13). For both time to first on-study SRE, and risk of first and subsequent SREs, the distribution of type of SRE was similar across treatment groups, with radiation to bone (academic-in-confidence information has been removed) and pathological fracture (academic-in-confidence information has been removed) being the most common, whereas there were fewer occurrences of SCC (academic-in-confidence information has been removed) or surgery to bone. (Academic-in-confidence information has been removed.)
The time to development of moderate or severe worst pain, in patients with no or mild pain at baseline, favoured denosumab compared with zoledronic acid (median 5.8 vs 4.9 months) without being statistically significant (HR 0.89; 95% CI 0.77 to 1.04). The median time to worsening pain was similar (academic-in-confidence information has been removed). In terms of quality of life, overall mean FACT scores remained similar between the groups (academic-in-confidence information has been removed).
In terms of adverse events, there were more occurrences of hypocalcaemia in the denosumab group compared with the zoledronic acid group (12.8% vs 5.8%), higher rates of ONJ (2.3% vs 1.3%) (academic-in-confidence information has been removed), whereas events associated with renal impairment (14.7% vs 16.2%) and acute-phase reactions (8.4% vs 17.8%) were lower. Overall survival was similar between the treatment groups (HR 1.03; 95% CI 0.91 to 1.17; median 19.4 months vs 19.8 months).
The AG's NMA reported a statistically significant difference in favour of denosumab compared with zoledronic acid or placebo for both time to first on-study SRE and risk of first and subsequent SREs (academic-in-confidence information has been removed) (Table 120).
Non-small cell lung cancer
For time to first on-study SRE for all patients, the difference favoured denosumab without being statistically significant [HR 0.84; 95% CI 0.64 to 1.10 (academic-in-confidence information has been removed)], (academic-in-confidence information has been removed) (Table 120). There was a statistically significant difference in favour of denosumab for overall survival (HR 0.79; 95% CI 0.65 to 0.95). The following outcomes were not reported for NSCLC: time to first on-study SRE or risk of first and subsequent SRE by history of SRE or type of SRE; pain scores or quality of life; hypercalcaemia; hypocalcaemia; ONJ; events associated with renal impairment; or acute-phase reactions.
The MS did not perform a NMA of NSCLC. In the AG's NMA, the direction of effect of the comparisons of denosumab compared with zoledronic acid or placebo favoured denosumab for both time to first on-study SRE and risk of first and subsequent SREs but only the comparison with placebo for risk of first and subsequent SRE was statistically significant (Table 120).
Other solid tumours (excluding non-small cell lung cancer)
There was a statistically significant difference in favour of denosumab for median time to first on-study SRE for all patients [HR 0.79; 95% CI 0.62 to 0.99; (academic-in-confidence information has been removed)] (Table 119). Overall survival was similar (HR 1.08; 95% CI 0.90 to 1.30). The following outcomes were not reported for OSTs excluding NSCLC: time to first on-study SRE or risk of first and subsequent SREs by history of SRE or type of SRE; pain scores or quality of life; hypercalcaemia, hypocalcaemia, ONJ, events associated with renal impairment or acute-phase reactions.
The MS did not perform a NMA of OSTs excluding NSCLC. In the AG's NMA there was a statistically significant difference in favour of denosumab compared with zoledronic acid or placebo for time to first on-study SRE and compared with placebo for risk of first and subsequent on-study SREs (Table 120).
Other solid tumours (including non-small cell lung cancer)
In the manufacturer's post-hoc analysis (excluding multiple myeloma) there was a statistically significant difference in favour of denosumab for time to first on-study SRE for all patients (HR 0.81; 95% CI 0.68 to 0.96; 21.4 vs 15.4 months) (Table 119). (Academic-in-confidence information has been removed.) For risk of developing first and subsequent SREs, for all patients, the difference was borderline significant in favour of denosumab (RR 0.85; 95% CI 0.72 to 1.00) (Table 119). (Academic-in-confidence information has been removed.) For both time to first on-study SRE and risk of first and subsequent SREs, the distribution of type of SRE was similar across treatment groups, with radiation to bone (academic-in-confidence information has been removed) and pathological fracture (academic-in-confidence information has been removed) being the most common while there were fewer occurrences of SCC (academic-in-confidence information has been removed) or surgery to bone (academic-in-confidence information has been removed).
Denosumab delayed the time to development of moderate or severe worst pain in patients with no or mild pain at baseline compared with zoledronic acid (median 3.7 months vs 2.8 months; p = 0.0369) and also the time to worsening pain (academic-in-confidence information has been removed; p = 0.04). In terms of quality of life, overall mean FACT scores remained similar between the groups. (Academic-in-confidence information has been removed.)
In terms of adverse events, there were more occurrences of hypocalcaemia in the denosumab group than in the zoledronic acid group (10.8% vs 5.8%), rates of (academic-in-confidence information has been removed). Rates of ONJ (1.3% vs 1.1%) were similar, while there were lower rates of events associated with renal impairment (8.3% vs 10.9%) or acute-phase reactions (6.9% vs 14.5%). Overall survival was similar [HR 0.92; 95% CI 0.81 to 1.05; median (academic-in-confidence information has been removed)].
The AG's NMA reported a statistically significant difference in favour of denosumab compared with zoledronic acid or placebo for time to first on-study SRE and compared with placebo for risk of first and subsequent SREs (academic-in-confidence information has been removed) (Table 120).
Strengths and limitations of the assessment
In terms of strengths, our review focused on RCTs, resulting in a high level of evidence. Where outcome data were not available from published reports, we attempted to source such data from the MS and CSRs. We undertook a NMA to provide an indirect estimate of the effectiveness of denosumab against appropriate comparators that were not considered in the direct evidence. A NMA of NSCLC and OSTs (excluding NSCLC) was undertaken which reduced the degree of methodological heterogeneity within the analysis. We did not assume a class effect for BPs and instead incorporated different types of BP, as appropriate for the type of primary cancer being considered in the NMA.
In terms of limitations, non-English-language studies were excluded from the review because of the tight timelines. Fewer outcomes were available for NSCLC and for OSTs excluding NSCLC than were reported for breast cancer, prostate cancer or OSTs including NSCLC. Definitions used by the studies of what constituted BSC varied both within and across each of the primary tumour types. The study by Saad and colleagues118 was used in the NMA for BSC. The control arm was randomised to receive placebo. Both groups received standard pain management, including analgesics, radiation or ‘other treatment’, at the discretion of the clinician. This standard treatment is consistent with the BSA described by the AG clinical expert (RJ).
The strength of a NMA is that all the available and relevant evidence (direct and indirect) can be considered in a single consistent analysis. However, a key limitation of the NMA in this assessment is the small number of trials included. Furthermore, network meta-analyses are not randomised comparisons but rather observational findings across studies and therefore the results are subject to considerable uncertainty and should be interpreted with caution.
Uncertainties
External validity of the denosumab randomised controlled trials
The three denosumab RCTs were large, international, multicentre trials. The participants all had advanced cancer (breast, prostate, lung or OSTs) with at least one bone metastasis, ECOG status ≤ 2 and a life expectancy of ≥ 6 months. Therefore, it is reasonable to expect that the results of the trials would be generalisable to patients meeting the above criteria. It is important to note that these results would not be generalisable to patients with a life expectancy of < 6 months. (Academic-in-confidence information has been removed.) It is unclear to what extent, if any, this might impact on the generalisability of the results to a UK setting. Patients with poor renal function (creatinine clearance < 30 ml/minute) were excluded from the trials on the basis that they could not be randomised to zoledronic acid because the drug would be contraindicated for them. Therefore, the effects of denosumab on patients with advanced cancer with bone metastases and poor renal function are unknown. However, it has been estimated that < 2% of patients with solid tumours have sufficiently poor renal function to avoid zoledronic acid. 207 The RCT for OSTs (excluding breast or prostate cancer) pooled data from patients with a range of different types of solid tumour.
In addition, the direct evidence from the trials comparing denosumab with zoledronic acid is generalisable only to those patients with advanced cancer and bone metastases for whom clinical guidance advocates the use of BPs. For breast cancer, this applies to all patients with advanced breast cancer and newly diagnosed bone metastases. 45 For prostate cancer, it applies to men with hormone-refractory prostate cancer with painful bone metastases for whom other treatments (including analgesics and palliative radiotherapy) have failed. 46 For lung cancer and OSTs there is no clear guidance on when BPs should be administered. 48 In the prostate cancer denosumab RCT (and the other two denosumab RCTs), in subgroup analysis, rather than presenting data on patients with painful bone metastases for whom other treatments have failed, the manufacturer presents data on patients with (1) no prior SRE and (2) prior SRE. The results would be more generalisable if effectiveness data were presented for patients who had painful bone metastases despite conventional analgesics.
Network meta-analysis
There are several uncertainties associated with the NMA. Although caution was exercised when selecting trials for inclusion in the NMA, some differences inevitably exist between included studies in terms of populations and trial methodologies, and this can lead to uncertainty in any meta-analysis with potential for further bias in a NMA. There were primary studies (other than those comparing denosumab) that did not report complete results, so some treatment effects used in the NMA (including levels of precision of the effects) were estimated and therefore subject to uncertainty although when missing data were treated as uncertain parameters the impact on the results was negligible. The small number of trials in each of the NMAs add to the uncertainty in the results, particularly as some of the individual trials were small themselves and there were no instances (for any comparison between two treatments within a NMA) where there was sufficient comparable direct evidence to include more than one trial. Further uncertainty may have resulted from the potential for different assumptions to be made when specifying NMA models (e.g. in relation to baseline prior distributions) and this could be illustrated by differences between the NMA results in this assessment and the manufacturer's analysis. Although a different approach to the manufacturer was taken, many of the results from the manufacturer's indirect comparisons can be accurately replicated, which may mitigate some of the uncertainty associated with the NMA.
Skeletal-related events as a composite end point
Skeletal-related events are composite end points used in research studies and generally defined as including pathological fracture, requirement for radiation therapy to bone, surgery to bone, or SCC. These end points include both complications of bone metastases (pathological fracture and SCC) and therapeutic or preventative measures (radiotherapy and surgery). In the three denosumab RCTs the distribution of type of SRE was similar across treatment groups, for both time to first on-study SRE and risk of first and subsequent SREs. The vast majority of SREs consisted of pathological fracture or radiation to bone, with far fewer occurrences of SCC or surgery to bone. The three RCTs reported a statistically significant difference in favour of denosumab for time to first on-study SRE. (Academic-in-confidence information has been removed.) Therefore, higher event rates and larger treatment effects that are associated with the less important components of a composite end point could result in a misleading impression of the treatment's effectiveness in relation to components that are clinically more important but occur less frequently. This could potentially create the impression that the treatment is equally effective for each component of the composite end point when in fact this may not be supported by the evidence.
Symptomatic versus non-symptomatic skeletal-related events
The impact on patients of pathological fractures varies from unnoticeable, asymptomatic fractures to vertebral fractures associated with SCC that result in paraplegia. Patients in the denosumab RCTs underwent radiography before treatment and at 12-weekly intervals during the study to detect the occurrence of pathological fractures or SCC. This skeletal survey frequency is unlikely to be the case in clinical practice. More frequent tests may have resulted in asymptomatic pathological fractures being detected that would have remained undetected in clinical practice. Also, in the RCTs once a SRE had been detected and classified as asymptomatic it could not later be reclassified as symptomatic – this could potentially lead to a rate of symptomatic SREs detected that was lower than that observed in clinical practice, on the basis that in clinical practice asymptomatic fractures would likely remain undetected until they had become symptomatic. Trinkaus and colleagues37 compared observational SRE frequency in clinical practice with SRE frequency in the intravenous BP trials and reported a higher rate of SREs in the trial setting compared with clinical practice.
The MS stated that clinical expert opinion indicated that in clinical practice SCCs were symptomatic. For pathological fractures, vertebral fractures were predominantly asymptomatic, whereas non-vertebral fractures were predominantly symptomatic, based on their skeletal locations. In the denosumab RCTs, for time to first on-study SRE, and risk of first and subsequent SREs respectively, the percentage of fractures that were vertebral were recorded in the breast cancer trial (academic-in-confidence information has been removed), in the prostate cancer trial (academic-in-confidence information has been removed) and in the OSTs trial (academic-in-confidence information has been removed).
Twenty-one-day window
More than one SRE may occur in relation to a single event. For example, an individual may suffer a pathological fracture, which is treated by radiotherapy or surgery (two SREs related to one event). Therefore, in order to provide an estimate of the number of SRE events rather than just the overall number of SREs, in the denosumab and BP trials a subsequent SRE was counted as a separate SRE only after a defined period (usually 21 days). When more than one SRE occurred within a 21-day period, the SRE that was taken to represent the event was the first SRE that occurred within the 21-day period.
Overall survival
In the three denosumab RCTs, overall survival was reported as similar. However, a post-hoc analysis of the NSCLC subgroup of the OSTs RCT by Henry and colleagues30 reported a statistically significant difference in favour of denosumab (HR 0.79; 95% CI 0.65 to 0.95). A recent paper by Scagliotti and colleagues208 reported this difference as a median 9.5 months for denosumab and 8.1 months for zoledronic acid (HR 0.78; 95% CI 0.65 to 0.94). Henry and colleagues30 postulated that the difference in survival observed in this post-hoc analysis might be a result of differences in prognostic variables at study entry in a highly heterogeneous population or of differences in specific antineoplastic treatments while on study. The AG is of the opinion that this result should be interpreted with caution until further evidence is available.
Appropriateness of analysing different tumour types together
The denosumab RCT of OSTs (post-hoc study 244) analysed a number of different primary tumour types together. The tumour types included NSCLC (44.0%). (Academic-in-confidence information has been removed.) Combining tumour types within a trial increases the risk of selection and performance bias. In addition, because of the small numbers of each tumour type, it is difficult to conclude if an intervention is more effective in one tumour type than another. However, it would not be practical to conduct sufficiently powered trials on each tumour type and combining tumour types would be required at some stage.
Bisphosphonates
It was our intention to compare denosumab with zoledronic acid, disodium pamidronate, ibandronic acid and sodium clodronate. However, head-to-head evidence was available only for denosumab compared with zoledronic acid. In breast cancer, disodium pamidronate was suitable for inclusion in the NMA and indirect comparison with denosumab was possible. Owing to lack of evidence, the assessment of the effectiveness of denosumab compared with ibandronic acid and sodium clodronate was not possible. In addition, it was not possible to compare the different routes of BP treatments because of the inadequacy of data for indirect comparison. However, based on advice from clinical experts, zoledronic acid is the most widely used BP and should be used as the primary BP comparator.
Other relevant factors
Place of denosumab in the care pathway
There are various points in the care pathway at which the use of denosumab could be considered. Current evidence assesses denosumab compared with zoledronic acid as a first-line treatment only for the prevention of SREs. Denosumab could also be considered in patients who have had a previous SRE. In the denosumab trials, individuals who had previously experienced a SRE at baseline were at higher risk than those who had not. Subgroups of patients with and without a history of SRE at baseline were reported. Denosumab significantly delayed the time to first SRE in those patients without a history of SRE and reduced the risk of first and subsequent SREs compared with zoledronic acid. However, for those patients with a history of SRE at baseline there was a significant difference in these outcomes only in those with breast cancer. It should be noted that the trials were not powered to detect differences in these subgroups.
Denosumab could also be considered in the care pathway as a second-line agent in those who continue to have SREs on current recommended treatment (BPs or BSC) or in patients who are contraindicated to BPs. All patients in the pivotal denosumab trials were naive to BPs for bone metastases. Therefore, no evidence was found for the use of denosumab in patients previously prescribed a BP. Patients with severe renal impairment were excluded from the pivotal trials. Therefore, the effectiveness of denosumab in patients with advanced cancer and severe renal impairment is unknown.
Potential for community-based treatment
Denosumab is administered by monthly subcutaneous injection, whereas zoledronic acid is administered in hospital by intravenous infusion over at least 15 minutes every 3–4 weeks. Therefore, patients receiving denosumab who were not otherwise required to attend hospital could potentially receive community-based treatment, which they (and their carers) might find more convenient in terms of, for example, having less distance to travel.
Physiology of bone metastases between tumour types
Bone metastases result in an imbalance of osteoclast and osteoblast activity. Traditionally it was thought that bone metastases could be osteolytic (also known as osteoclastic), osteoblastic or mixed. However, current opinion is that a spectrum exists, with no metastasis being purely osteolytic or osteoblastic. Prostate cancer generally results in predominantly osteoblastic lesions and breast cancer predominantly osteolytic lesions. Theoretically there may be a difference in the efficacy of denosumab depending on the predominant type of bone lesion. As denosumab inhibits osteoclasts, one might expect denosumab to be more effective in preventing complications associated with osteolytic lesions. However, osteoclasts also affect osteoblastic function. A subgroup of the study comparing zoledronic acid and disodium pamidronate in breast cancer found that patients with predominantly lytic lesions responded better to zoledronic acid. 109 The pivotal denosumab studies did not report a subgroup of patients by lesion type.
Bone markers
Despite the clinical benefits of denosumab and BPs, only a proportion of SREs are prevented, and some patients may not experience a skeletal event despite the presence of metastatic bone disease. It has been suggested that bone markers could be used to stratify risk to individuals with bone metastases. 27,28 There are several different types of bone markers, including BSAP, osteocalcin and PINP for monitoring bone formation and CTX and NTX for monitoring bone resorption. The ASCO guidelines34 currently do not recommend the use of bone markers in breast cancer outwith the trial setting.
Ongoing studies
Five ongoing studies of denosumab were reported by the manufacturer. Two studies are open-label extensions of the Stopeck trial31 and Fizazi trial. 29 One Phase III study is currently evaluating denosumab for prolonging bone metastasis-free survival in hormone-refractory prostate cancer. There are also two Phase II studies in progress, one investigating the use of denosumab for the treatment of hypercalcaemia and the other evaluating the effectiveness of denosumab in giant cell tumour of the bone.
Cost-effectiveness
Statement of principal findings
Within-trial analyses by the AG suggest that for breast cancer patients denosumab results in a slightly lower average number of SREs than zoledronic acid, and that this will translate into a small average annual gain of perhaps 0.003–0.006 QALYs: roughly equivalent to 1–2 additional days in full health or 2–3 days at the SRE-naive quality of life. Without the PAS, the additional cost of denosumab does not justify these relatively minor gains. With the PAS, denosumab is estimated to be broadly cost neutral to slightly cost saving, and so cost-effective compared with zoledronic acid.
Within-trial analyses suggest that, for prostate cancer patients, denosumab results in a slightly lower average number of SREs than zoledronic acid. This translates into a slightly larger additional average annual gain of perhaps 0.008–0.016 QALYs. The reason for this difference for prostate cancer is the greater proportion of SCCs within the overall number of SREs. However, there is a suggestion that there may be slightly fewer zoledronic acid administrations per annum than denosumab administrations. This triangulates with the higher proportion of zoledronic acid patients within the prostate cancer trial having doses withheld for creatine clearance. This aspect is not formally considered in either the manufacturer's or the AG's economic model.
Without the PAS, the additional cost of denosumab still does not justify the relatively minor estimated gains. With the PAS (commercial-in-confidence information has been removed), denosumab is estimated to increase annual costs by around £100, which translates into cost-effectiveness estimates of between £6545 per QALY and £15,272 per QALY. However, this AG within-trial analysis does not distinguish between SRE-naive and -experienced patients.
For the cost–utility modelling within breast cancer, the lifetime gains across all patients are estimated to be around 0.007 QALYs. This is again small, and does not justify the additional cost of £1707 per patient compared with zoledronic acid. With the PAS (commercial-in-confidence information has been removed), denosumab is estimated to dominate zoledronic acid. But for those in whom BPs are contraindicated the cost-effectiveness is poor: even with the PAS the cost-effectiveness is £157,829 per QALY. Applying the SRE-naive and -experienced subgroups, clinical effectiveness has little impact on the results, as these estimates are reasonably close to the pooled all-patient estimates.
For the cost–utility modelling within prostate cancer, across all patients the gain from denosumab over zoledronic acid is around 0.009 QALY, whereas compared with BSC it is 0.035 QALYs, at net costs without the PAS of £1059 and £3951, respectively. Without the PAS, compared with zoledronic acid this results in a cost-effectiveness of £111,603 per QALY. Cost-effectiveness is estimated to be slightly better among the SRE-naive patients, at £99,561 per QALY, but the quid pro quo is a worse cost-effectiveness among the SRE-experienced patients of £170,854 per QALY. This may arise in large part from the estimated step change in HRQoL arising from a patient's first SRE.
With the PAS, denosumab is estimated to be cost saving compared with zoledronic acid and so dominates it. For those in whom BPs are contraindicated, denosumab is not estimated to be cost-effective compared with BSC. The PAS (commercial-in-confidence information has been removed) results in denosumab being estimated to remain dominant over zoledronic acid.
Within the cost–utility modelling of OSTs including lung cancer, the gains from denosumab over zoledronic acid are estimated to be less than 0.01 QALYs. Without the PAS, denosumab is not cost-effective, but with it the small additional overall costs of around £50 result in cost-effectiveness estimates of between £5400 per QALY and £15,300 per QALY. The impact of applying the SRE-subgroup-specific estimates within this group is quite large. Although it improves the cost-effectiveness estimates of denosumab compared with BSC for SRE-naive patients, even with the PAS it is not sufficient to render it cost-effective because of the SRE-experienced RRs for SREs. (Academic-in-confidence information has been removed.)
For lung cancer, possibly because of the short life expectancy, the patient gains from denosumab over zoledronic acid among SRE-experienced patients are estimated to be small: 0.003 QALYs. With the PAS, the additional cost of £43 results in a cost-effectiveness of £12,743 per QALY.
If the price of zoledronic acid falls by only a reasonably small amount at patent expiry, the cost-effectiveness of denosumab will change dramatically in comparison owing to the very small estimate for patient gains.
Strengths and limitations of the assessment
The AG's analysis is in part framed by the manufacturer's analysis in terms of outlook and approach. The cost–utility modelling relies on it for the greater part of its input, because of a paucity of other data sources for elements such as quality-of-life values. But the broad conclusions of the assessment appear relatively insensitive to the approach adopted, as shown by the much simpler within-trial analyses.
Uncertainties
A concern within the modelling is BSC being assumed to have a zero incidence of the modelled SAEs. When the benefits from active treatments on SREs are muted, there is the possibility that SAEs come to the fore and require more detailed consideration. Sensitivity analyses that completely exclude SAEs from the analysis do improve the cost-effectiveness of denosumab compared with BSC, but this in itself is not sufficient to render denosumab cost-effective compared with BSC when this is the appropriate comparator.
There remains some structural uncertainty around the reasonableness of the utility estimates applied. In particular, the step change estimated between SRE-naive patients and SRE-experienced patients provides much of the gain anticipated from SRE-naive patients avoiding their first SRE. Whether or not this estimate is picking up the impact of other variables, such as progression, which are not considered in the utility estimates is currently an open question.
A key uncertainty is the rate of paralysis associated with SCC and the duration of quality-of-life impact from SCC. Extending the average quality-of-life decrement measured in the 5 months subsequent to the compression through to death improves the estimated cost-effectiveness, particularly among SRE-naive prostate cancer patients. Although not in itself sufficient to render denosumab cost-effective against BSC, extending the impacts of SCC does improve the cost-effectiveness. There are also some concerns that the ongoing costs of SCC may have been underestimated.
Probabilistic modelling suggests that within the usual range of acceptable cost-effectiveness thresholds there is relatively little uncertainty because the treatment with the highest probability of being cost-effective is also that with the highest probability of being optimal when compared with the alternatives, and this treatment does not change over the usual range of acceptable cost-effectiveness thresholds. The central estimates are also in line with those of the deterministic analyses.
Chapter 12 Conclusions
Implications for service provision
Denosumab is effective in delaying the time to first SRE and reducing the risk of developing first and subsequent SREs in patients with bone metastases from breast cancer and prostate cancer. For NSCLC, for time to first SRE the direction of effect favoured denosumab without being statistically significant. (Academic-in-confidence information has been removed.) For OSTs (excluding breast cancer, prostate cancer and NSCLC), denosumab was effective in delaying the time to first SRE. (Academic-in-confidence information has been removed.) The distribution of type of SRE was similar across treatment groups, with the vast majority consisting of pathological fracture or radiation to the bone. (Academic-in-confidence information has been removed), whereas there were few occurrences of SCC or surgery to bone.
Denosumab was also shown to be effective in delaying the time to development of moderate or severe pain (for the subgroup of patients with no or mild pain at baseline) in patients with breast cancer and those with OSTs (including NSCLC), but the difference was smaller for prostate cancer. The median time to worsening pain was generally similar for the treatment groups in the three studies. In terms of quality of life, across all three RCTs FACT scores remained similar between the groups. (Academic-in-confidence information has been removed.) Overall survival was reported to be similar in the studies apart from the post-hoc analysis of NSCLC, in which a statistically significant difference was reported in favour of denosumab.
In the AG's NMA, there was a statistically significant difference in favour of denosumab for both time to first SRE and risk of first and subsequent SRE for most comparisons. (Academic-in-confidence information has been removed.) However, the results of the network meta-analyses are subject to considerable uncertainty and should be interpreted with caution.
The effectiveness of denosumab compared with zoledronic acid and BSC in delaying time to first SRE and reducing the risk of first and subsequent SREs has been demonstrated. These results have mostly reached statistical significance and met the minimally clinically significant change described by clinical experts (delay of > 3 months or HR reduction of > 20%). However, the importance of the composite SRE outcome, and spectrum of corresponding possible health states, to an individual patient is not clear. Evidence for the effectiveness of denosumab compared with zoledronic acid in reducing pain and improving relative quality of life is less evident.
The manufacturer's model, the AG within-trials analyses and the AG cost–utility model all estimate denosumab to result in patient benefits from reduced SREs compared with denosumab, and larger benefits compared with BSC. But the estimates of the numbers of SREs avoided per patient are small when compared with zoledronic acid, typically less than 0.3 SREs over the patient's lifetime, and often a lot less than this. SCC is relatively rare. The QALY gains from the number of SREs avoided compared with zoledronic acid are small, typically less than 0.02 QALYs over the patient's lifetime, and again often quite a lot less than this.
(Commercial-in-confidence information has been removed.) Given the small QALY gains, denosumab is estimated to dominate or be cost-effective compared with zoledronic acid. But zoledronic acid comes off patent quite soon. (Commercial-in-confidence information has been removed.) A price reduction (commercial-in-confidence information has been removed) for zoledronic acid is required to result in the additional net costs from denosumab rendering it not cost-effective at current thresholds. For those patients for whom BPs are not currently recommended or are not used, possibly owing to contraindications, both the manufacturer and the AG conclude that denosumab is not cost-effective compared with BSC.
Suggested research priorities
Further research would be helpful in the following areas:
-
the effectiveness of denosumab compared with zoledronic acid in delaying time to first SRE and reducing the risk of first and subsequent SREs in patients with hormone-refractory prostate cancer and painful bone metastases for whom other treatments, including analgesics and palliative radiotherapy, have failed
-
whether or not there is an identifiable subgroup of patients at higher risk of SCC for whom denosumab might result in larger QALY gains
-
the safety and efficacy of denosumab in patients with severe renal impairment and advanced cancer (breast cancer, prostate cancer, NSCLC and OSTs)
-
the safety and efficacy of denosumab in patients with advanced cancer who have previously been exposed to a BP
-
the role of bone markers (including BSAP, PINP, CTX and NTX) to identify subgroups of patients with advanced cancer and bone metastases who may be likely to benefit from bone-targeting therapies
-
the effectiveness of denosumab compared with zoledronic acid for overall survival in patients with NSCLC and bone metastases.
Acknowledgements
We thank Lara Kemp for secretarial support. Graeme MacLennan provided oversight to the statistical analysis. Joyce Craig commented on the economic sections of the report. The Health Services Research Unit, Institute of Applied Health Sciences, University of Aberdeen is core-funded by the Chief Scientist Office of the Scottish Government Health Directorates.
Contributions of authors
Pam Royle developed the protocol, ran the search strategies and obtained papers.
Fiona Stewart obtained papers, managed the reference database and formatted references.
John Ford and Pam Royle screened the search results, assessed full-text studies for inclusion and along with Pawana Sharma undertook data extraction and quality assessment.
John Ford drafted the background chapter, Pawana Sharma drafted the methods chapter and Pawana Sharma, John Ford and Graham Mowatt drafted the clinical effectiveness results chapters.
Ewen Cummins undertook the economic modelling and drafted the chapters on cost-effectiveness and the critique of the MS.
Rhona Johnston helped to build the economic model.
Andrew Elders conducted the statistical analysis.
Rob Jones, Clive Mulatero and Radha Todd provided expert advice on clinical aspects of the review.
All authors assisted in preparing the manuscript and commenting on drafts.
Publication
Ford JA, Jones R, Elders A, Mulatero C, Royle P, Stewart F, et al. Denosumab for treatment of bone metastases secondary to solid tumours: systematic review and network meta-analysis. Eur J Cancer 2013;49:416–30.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Statistical bulletin: births and deaths in England and Wales. Newport: Office for National Statistics; 2009.
- Cancer Research UK. Latest UK cancer incidence (2008) and mortality (2008) summary. London: Cancer Research UK; 2011.
- Jensen AT, Jacobsen JB, Norgaard M, Yong M, Fryzek JP, Sorensen HT. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer 2011;11.
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:S1588-94.
- Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer 1998;77:336-40. http://dx.doi.org/10.1038/bjc.1998.52.
- TA101: docetaxel for the treatment of hormone-refractory metastatic prostate cancer. London: NICE; 2006.
- Bubendorf L, Schapfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum Pathol 2000;31:578-83. http://dx.doi.org/10.1053/hp.2000.6698.
- Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 2010;184:162-7. http://dx.doi.org/10.1016/j.juro.2010.03.034.
- Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <4.0 ng per milliliter. N Engl J Med 2004;350:2239-46. http://dx.doi.org/10.1056/NEJMoa031918.
- De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-200. http://dx.doi.org/10.1056/NEJMoa1014618.
- Cancer Research UK. Cancer survival statistics by cancer site. London: Cancer Research UK; 2009.
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165-76. http://dx.doi.org/10.1053/ctrv.2000.0210.
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:S6243-9. http://dx.doi.org/10.1158/1078-0432.CCR-06-0931.
- Kanthan R, Loewy J, Kanthan SC. Skeletal metastases in colorectal carcinomas: a Saskatchewan profile. Dis Colon Rectum 1999;42:1592-7. http://dx.doi.org/10.1007/BF02236213.
- Sundermeyer ML, Meropol NJ, Rogatko A, Wang H, Cohen SJ. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005;5:108-13. http://dx.doi.org/10.3816/CCC.2005.n.022.
- McCawley LJ, Matrisian LM. Tumor progression: defining the soil round the tumor seed. Curr Biol 2001;11:R25-7. http://dx.doi.org/10.1016/S0960-9822(00)00038-5.
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655-64. http://dx.doi.org/10.1056/NEJMra030831.
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. http://dx.doi.org/10.1038/nrc867.
- Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007;110:1860-7. http://dx.doi.org/10.1002/cncr.22991.
- Brown JE, Cook RJ, Lipton A, Costa L, Coleman RE. Prognostic factors for skeletal complications from metastatic bone disease in breast cancer. Breast Cancer Res Treat 2010;123:767-79. http://dx.doi.org/10.1007/s10549-010-0981-1.
- Koizumi M, Yoshimoto M, Kasumi F, Iwase T, Ogata E. Post-operative breast cancer patients diagnosed with skeletal metastasis without bone pain had fewer skeletal-related events and deaths than those with bone pain. BMC Cancer 2010;10. http://dx.doi.org/10.1186/1471-2407-10-423.
- Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 1996;335:1785-91. http://dx.doi.org/10.1056/NEJM199612123352401.
- Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 2011;14:177-83. http://dx.doi.org/10.1038/pcan.2011.7.
- Yong M, Jensen AO, Jacobsen JB, Norgaard M, Fryzek JP, Sorensen HT. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007). Breast Cancer Res Treat 2011;129:495-503. http://dx.doi.org/10.1007/s10549-011-1475-5.
- Costelloe CM, Rohren EM, Madewell JE, Hamaoka T, Theriault RL, Yu TK, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol 2009;10:606-14. http://dx.doi.org/10.1016/S1470-2045(09)70088-9.
- Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res 2008;14:6690-6. http://dx.doi.org/10.1158/1078-0432.CCR-07-5234.
- Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE. Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer 2003;89:2031-7. http://dx.doi.org/10.1038/sj.bjc.6601437.
- Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, et al. Bone turnover markers as predictors of skeketal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 2005;97:59-6. http://dx.doi.org/10.1093/jnci/dji002.
- Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-22. http://dx.doi.org/10.1016/S0140-6736(10)62344-6.
- Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011;29:1125-32. http://dx.doi.org/10.1200/JCO.2010.31.3304.
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132-9. http://dx.doi.org/10.1200/JCO.2010.29.7101.
- Coleman RE, Major P, Lipton A, Brown JE, Lee K-A, Smith M, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 2005;23:4925-35. http://dx.doi.org/10.1200/JCO.2005.06.091.
- Lipton A, Cook RJ, Major P, Smith MR, Coleman RE. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. Oncologist 2007;12:1035-43. http://dx.doi.org/10.1634/theoncologist.12-9-1035.
- Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 2011;29:1221-7. http://dx.doi.org/10.1200/JCO.2010.32.5209.
- Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine 2006;31:2849-56. http://dx.doi.org/10.1097/01.brs.0000245838.37817.40.
- Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. http://dx.doi.org/10.1016/j.ijrobp.2010.11.026.
- Trinkaus M, Simmons C, Myers J, Dranatisaris G, Clemons M. Skeletal-related events (SREs) in breast cancer patients with bone metastases treated in the nontrial setting. Support Care Cancer 2010;18:197-203. http://dx.doi.org/10.1007/s00520-009-0645-z.
- Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat 1982;10:1100-20. http://dx.doi.org/10.1214/aos/1176345976.
- Major PP, Cook RJ. Clinical endpoints for assessing bisphosphonate efficacy in the prevention of skeletal complications of bone metastases. Eur Urol Suppl 2004;3:34-9. http://dx.doi.org/10.1016/j.eursup.2004.08.010.
- Ghosh D, Lin DY. Nonparametric analysis of recurrent events and death. Biometrics 2000;56:554-62. http://dx.doi.org/10.1111/j.0006-341X.2000.00554.x.
- Cook RJ, Lawless JF. Marginal analysis of recurrent events and a terminating event. Stat Med 1997;16:911-24. http://dx.doi.org/10.1002/(SICI)1097-0258(19970430)16:8<911::AID-SIM544≥3.0.CO;2-I.
- Cook RJ, Major P. Methodology for treatment evaluation in patients with cancer metastatic to bone. J Natl Cancer Inst 2001;93:534-8. http://dx.doi.org/10.1093/jnci/93.7.534.
- Clemons M, Dranitsaris G, Cole D, Gainford MC. Too much, too little, too late to start again? Assessing the efficacy of bisphosphonates in patients with bone metastases from breast cancer. Oncologist 2006;11:227-33. http://dx.doi.org/10.1634/theoncologist.11-3-227.
- Pockett RD, Castellano D, McEwan P, Oglesby A, Barber BL, Chung K. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010;19:755-60. http://dx.doi.org/10.1111/j.1365-2354.2009.01135.x.
- CG81: Advanced breast cancer: diagnosis and treatment. London: NICE; 2009.
- CG58: Prostate cancer: diagnosis and treatment. London: NICE; 2008.
- CG75: Metastatic spinal cord compression: diagnosis and management of patients at risk of or with metastatic spinal cord compression. London: NICE; 2008.
- CG121: The diagnosis and treatment of lung cancer (update). London: NICE; 2011.
- CG121: The diagnosis and treatment of lung cancer (update). London: NICE; 2011.
- CG121: The diagnosis and treatment of lung cancer – Appendix 10. London: NICE; 2011.
- SIGN 106: Control of pain in adults with cancer. Edinburgh: SIGN; 2008.
- Scottish Intercollegiate Guidelines Network (SIGN) . SIGN 84: Management of Breast Cancer in Women. 2005. www.sign.ac.uk/pdf/sign84.pdf (accessed October 2011).
- De Marinis F, Eberhardt W, Harper PG, Sureda BM, Nackaerts K, Soerensen JB, et al. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. J Thorac Oncol 2009;4:1280-8. http://dx.doi.org/10.1097/JTO.0b013e3181b68e5a.
- Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008;83:1032-45. http://dx.doi.org/10.4065/83.9.1032.
- Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al. A systematic review of the role of bisphosphonates in metastatic disease. Health Technol Assess 2004;8.
- Lufkin EG, Argueta R, Whitaker MD, Cameron AL, Wong VH, Egan KS, et al. Pamidronate: an unrecognized problem in gastrointestinal tolerability. Osteoporosis Int 1994;4:320-2. http://dx.doi.org/10.1007/BF01622190.
- Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol 2002;42:1228-36. http://dx.doi.org/10.1177/009127002762491316.
- Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case–control analysis within a UK primary care cohort. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4444.
- Fallowfield L, Stebbing J, Braybrooke J, Langridge C, Jenkins V. The preferences and experiences of different bisphosphonate treatments in women with breast cancer. Psychooncology 2011;20:755-61. http://dx.doi.org/10.1002/pon.1781.
- Chern B, Joseph D, Joshua D, Pittman K, Richardson G, Schou M, et al. Bisphosphonate infusions: patient preference, safety and clinic use. Support Care Cancer 2004;12:463-6. http://dx.doi.org/10.1007/s00520-004-0628-z.
- McDermott RS, Kloth DD, Wang H, Hudes GR, Langer CJ. Impact of zoledronic acid on renal function in patients with cancer: clinical significance and development of a predictive model. J Support Oncol 2006;4:524-9.
- Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005;23:8580-7. http://dx.doi.org/10.1200/JCO.2005.02.8670.
- Non-Small Cell Lung Cancer Collaborative Group . Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010;5.
- Fallowfield L, Jenkins V, Coleman R. Which bisphosphonates do oncologists prescribe for women with metastatic breast cancer and why? Results of a UK survey. Breast 2008;17:459-63. http://dx.doi.org/10.1016/j.breast.2008.02.009.
- Mathias SD, Crosby RD, Qian Y, Jiang Q, Dansey R, Chung K. Estimating minimally important differences for the worst pain rating of the brief pain inventory-short form. J Support Oncol 2011;9:72-8. http://dx.doi.org/10.1016/j.snoc.2010.12.004.
- CG121 Appendix 10. London: NICE; 2011.
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. 2011.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. http://dx.doi.org/10.1002/sim.1875.
- Dias S, Welton NJ, Sutton AJ, Ades AE. Sheffield: NICE Decision Support Unit; 2011.
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-16.
- Body JJ, Diel IJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 2003;14:1399-405. http://dx.doi.org/10.1093/annonc/mdg367.
- Body JJ, Diel IJ, Bell R, Pecherstorfer M, Lichinitser MR, Lazarev AF, et al. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 2004;111:306-12. http://dx.doi.org/10.1016/j.pain.2004.07.011.
- Elomaa I, Blomqvist C, Porkka L, Holmström T, Taube T, Lamberg-Allardt C, et al. Clodronate for osteolytic metastases due to breast cancer. Biomed Pharmacother 1988;42:111-16.
- Heras P, Kritikos K, Hatzopoulos A, Georgopoulou AP. Efficacy of ibandronate for the treatment of skeletal events in patients with metastatic breast cancer. Eur J Cancer Care (Engl) 2009;18:653-6. http://dx.doi.org/10.1111/j.1365-2354.2008.00980.x.
- Kristensen B, Ejlertsen B, Groenvold M, Hein S, Loft H, Mouridsen HT. Oral clodronate in breast cancer patients with bone metastases: a randomized study. J Intern Med 1999;246:67-74. http://dx.doi.org/10.1046/j.1365-2796.1999.00507.x.
- Paterson AH, Powles TJ, Kanis JA, McCloskey E, Hanson J, Ashley S. Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer. J Clin Oncol 1993;11:59-65.
- Adami S, Mian M. Clodronate therapy of metastatic bone disease in patients with prostatic carcinoma. Recent Results Cancer Res 1989;116:67-72. http://dx.doi.org/10.1007/978-3-642-83668-8_6.
- Buchali K, Correns HJ, Schuerer M, Schnorr D, Lips H, Sydow K. Results of a double blind study of 89-strontium therapy of skeletal metastases of prostatic carcinoma. Eur J Nucl Med 1988;14:349-51. http://dx.doi.org/10.1007/BF00254382.
- Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson AC, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 2003;95:1300-11. http://dx.doi.org/10.1093/jnci/djg038.
- Elomaa I, Kylmala T, Tammela T, Viitanen J, Ottelin J, Ruutu M, et al. Effect of oral clodronate on bone pain. A controlled study in patients with metastic prostatic cancer. Int Urol Nephrol 1992;24:159-66. http://dx.doi.org/10.1007/BF02549644.
- Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol 2003;21:3335-42. http://dx.doi.org/10.1200/JCO.2003.03.042.
- Kylmala T, Tammela T, Risteli L, Risteli J, Taube T, Elomaa I. Evaluation of the effect of oral clodronate on skeletal metastases with type 1 collagen metabolites. A controlled trial of the Finnish Prostate Cancer Group. Eur J Cancer 1993;29A:821-5.
- Kylmala T, Taube T, Tammela TL, Risteli L, Risteli J, Elomaa I. Concomitant i.v. and oral clodronate in the relief of bone pain – a double-blind placebo-controlled study in patients with prostate cancer. Br J Cancer 1997;76:939-42. http://dx.doi.org/10.1038/bjc.1997.488.
- Nilsson S, Strang P, Ginman C, Zimmermann R, Edgren M, Nordstrom B, et al. Palliation of bone pain in prostate cancer using chemotherapy and strontium-89. A randomized phase II study. J Pain Symptom Manage 2005;29:352-7. http://dx.doi.org/10.1016/j.jpainsymman.2004.07.008.
- Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 1993;25:805-13. http://dx.doi.org/10.1016/0360-3016(93)90309-J.
- Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 1994;31:33-40. http://dx.doi.org/10.1016/0167-8140(94)90411-1.
- Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol 2003;21:4277-84. http://dx.doi.org/10.1200/JCO.2003.05.147.
- Smith JA. Palliation of painful bone metastases from prostate cancer using sodium etidronate: results of a randomized, prospective, double-blind, placebo-controlled study. J Urol 1989;141:85-7.
- Strang P, Nilsson S, Brandstedt S, Sehlin J, Borghede G, Varenhorst E, et al. The analgesic efficacy of clodronate compared with placebo in patients with painful bone metastases from prostatic cancer. Anticancer Res 1997;17:4717-21.
- Arican A, Icli F, Akbulut H, Cakir M, Sencan O, Samur M, et al. The effect of two different doses of oral clodronate on pain in patients with bone metastases. Med Oncol 1999;16:204-10. http://dx.doi.org/10.1007/BF02906133.
- Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 2001;91:1191-200. http://dx.doi.org/10.1002/1097-0142(20010401)91:7<1191::AID-CNCR1119>3.0.CO;2-0.
- Brown JE, McCloskey EV, Dewar JA, Body JJ, Cameron DA, Harnett AN, et al. The use of bone markers in a 6-week study to assess the efficacy of oral clodronate in patients with metastatic bone disease. Calcif Tissue Int 2007;81:341-51. http://dx.doi.org/10.1007/s00223-007-9061-x.
- Heras Rincon, Zubillaga RI, Castrillo Tambay M, Montalvo Moreno JJ. Osteonecrosis of the jaws and bisphosphonates. Report of fifteen cases. Therapeutic recommendations. Med Oral Patol Oral Cir Bucal 2007;12:E267-71.
- Jagdev SP, Purohit P, Heatley S, Herling C, Coleman RE. Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Ann Oncol 2001;12:1433-8. http://dx.doi.org/10.1023/A:1012506426440.
- Mystakidou K, Stathopoulou E, Parpa E, Kouloulias V, Kouskouni E, Vlahos L. Oral versus intravenous ibandronic acid: a comparison of treatment options for metastatic bone disease. J Cancer Res Clin Oncol 2008;134:1303-10. http://dx.doi.org/10.1007/s00432-008-0419-x.
- O’Rourke N, McCloskey E, Houghton F, Huss H, Kanis JA. Double-blind, placebo-controlled, dose–response trial of oral clodronate in patients with bone metastases. J Clin Oncol 1995;13:929-34.
- Piga A, Bracci R, Ferretti B, Sandri P, Nortilli R, Acito L, et al. A double blind randomized study of oral clodronate in the treatment of bone metastases from tumors poorly responsive to chemotherapy. J Exp Clin Cancer Res 1998;17:213-17.
- Robertson AG, Reed NS, Ralston SH. Effect of oral clodronate on metastatic bone pain: a double-blind, placebo-controlled study. J Clin Oncol 1995;13:2427-30.
- Zaghloul MS, Boutrus R, El-Hossieny H, Kader YA, El-Attar I, Nazmy M. A prospective, randomized, placebo-controlled trial of zoledronic acid in bony metastatic bladder cancer. Int J Clin Oncol 2010;15:382-9. http://dx.doi.org/10.1007/s10147-010-0074-5.
- Zhao YY, Xue C, Hou X, Liao H, Li S, Zhao HY, et al. Changes of bone resorption marker (NTX) in chemotherapy plus zoledronic acid versus chemotherapy alone for nasopharyngeal cancer patients with bone metastases. Eur J Cancer 2011;47:848-53. http://dx.doi.org/10.1016/j.ejca.2010.12.004.
- Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinom. Cancer 2003;98:962-9. http://dx.doi.org/10.1002/cncr.11571.
- Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 2005;23:3314-21. http://dx.doi.org/10.1200/JCO.2005.05.116.
- Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 2000;88:1082-90. http://dx.doi.org/10.1002/(SICI)1097-0142(20000301)88:5<1082::AID-CNCR20>3.0.CO;2-Z.
- Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 2003;98:1735-44. http://dx.doi.org/10.1002/cncr.11701.
- Fallowfield L, Patrick D, Body J, Lipton A, Tonkin KS, Qian Y, et al. Effects of denosumab versus zoledronic acid (ZA) on health-related quality of life (HRQL) in metastatic breast cancer: results from a randomized phase III trial. J Clin Oncol 2010;28.
- Fallowfield L, Patrick D, Body JJ, Lipton A, Tonkin KS, Qian Y, et al. The effect of treatment with denosumab or zoledronic acid on health-related quality of life in patients with metastatic breast cancer. Cancer Res 2010;70.
- Hortobagyi GN, Theriault RL, Lipton A, Porter L, Blayney D, Sinoff C, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 1998;16:2038-44.
- Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 2001;7:377-87.
- Rosen LS, Gordon DH, Dugan W, Major P, Eisenberg PD, Provencher L, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36-43. http://dx.doi.org/10.1002/cncr.11892.
- Stopeck A, Fallowfield L, Patrick D, Cleeland CS, de Boer RH, Steger GG, et al. Effects of denosumab versus zoledronic acid (ZA) on pain in patients (pts) with metastatic breast cancer: results from a phase III clinical trial. J Clin Oncol 2010;28.
- Stopeck A, Fallowfield L, Patrick D, Cleeland CS, de Boer RH, Steger GG, et al. Pain in patients (pts) with metastatic breast cancer: results from a phase III trial of denosumab versus zoledronic acid (ZA). 33rd Annual San Antiono Breast Cancer Symposium 8–12 December 2010. http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=100&abstractID=60225 (accessed September 2011).
- Stopeck A, Martin M, Ritchie D, Body JJ, Paterson A, Viniegra M, et al. Effect of denosumab versus zoledronic acid treatment in patients with breast cancer and bone metastases: Results from the extended blinded treatment phase. Cancer Res 2010;70.
- Stopeck A, Lipton AA, Campbell-Baird C, von Moos R, Fan M, Haddock B, et al. Acute-phase reactions following treatment with zoledronic acid or denosumab: Results from a randomized, controlled phase 3 study in patients with breast cancer and bone metastases. Cancer Res 2010;70.
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Reply to V. Fusco et al. J Clin Oncol 2011;29:e523-4. http://dx.doi.org/10.1200/JCO.2011.35.2203.
- Theriault RL, Lipton A, Hortobagyi GN, Leff R, Gluck S, Stewart JF, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol 1999;17:846-54.
- Martin M, Steger G, von Moos R, Stopeck A, de Boer R, Bourgeois H, et al. Benefit of denosumab therapy in patients with bone metastases from breast cancer: a number-needed-to-treat (NNT) analysis. Breast 2011;20. http://dx.doi.org/10.1016/S0960-9776(11)70283-1.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94:1458-68. http://dx.doi.org/10.1093/jnci/94.19.1458.
- Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urol 2010;76:1175-81. http://dx.doi.org/10.1016/j.urology.2010.05.026.
- Saad F, Chen YM, Gleason DM, Chin J. Continuing benefit of zoledronic acid in preventing skeletal complications in patients with bone metastases. Clin Genitourin Cancer 2007;5:390-6. http://dx.doi.org/10.3816/CGC.2007.n.022.
- Saad F. Clinical benefit of zoledronic acid for the prevention of skeletal complications in advanced prostate cancer. Clin Prostate Cancer 2005;4:31-7. http://dx.doi.org/10.3816/CGC.2005.n.009.
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004;96:879-82. http://dx.doi.org/10.1093/jnci/djh141.
- Brown JE, Cleeland CS, Fallowfield LJ, Patrick DL, Fizazi K, Smith MR, et al. Pain outcomes in patients with bone metastases from castrate-resistant prostate cancer: results from a phase 3 trial of denosumab vs. zoledronic acid. Eur Urol Suppl 2011;10. http://dx.doi.org/10.1016/S1569-9056(11)61070-1.
- Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur Urol 2004;46:731-9. http://dx.doi.org/10.1016/j.eururo.2004.08.016.
- Miller K, Fizazi K, Smith M, Moroto JP, Klotz L, Brown J, et al. Benefit of denosumab therapy in patients with bone metastases from castrate resistant prostate cancer: a number-needed-to-treat (NNT) analysis. J Urol 2011;185. http://dx.doi.org/10.1016/j.juro.2011.02.1550.
- Patrick D, Cleeland C, Fallowfield L, Smith MR, Trachtenberg J, Chilingirov P, et al. Effects of denosumab and zoledronic acid on pain interference with daily functioning in patients with castrate-resistant prostate cancer. J Urol 2011;185. http://dx.doi.org/10.1016/j.juro.2011.02.1679.
- Assessment report for zometa (zoledronic acid). London; 2010.
- Tadros S. Clinical Study Report: 20050103. A randomized, double-blind, multicenter study of denosumab compared with zoledronic acid (Zometa) in the treatment of bone metastases in men with hormone-refractory prostate. Thousand Oaks, CA: Amgen Inc.; 2010.
- Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F. Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Ann Oncol 2006;17:986-9. http://dx.doi.org/10.1093/annonc/mdl041.
- Shore ND, Smith MR, Jievaltas M, Fizazi K, Damiao R, Chin J, et al. Effect of denosumab versus zoledronic acid in patients with castrate-resistant prostate cancer and bone metastases: subgroup analyses by prior SRE and baseline pain. J Clin Oncol 2011;29.
- Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial – the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 2003;21:3150-7. http://dx.doi.org/10.1200/JCO.2003.04.105.
- O’Neill S. Clinical Study Report: 20050244. A randomized, double-Blind, multicenter study of denosumab compared with zoledronic acid (Zometa) in the treatment of bone metastases in subjects with advanced cancer excluding breast and prostate Cancer) or multiple myeloma. Thousand Oaks, CA: Amgen Inc.; 2010.
- Henry DH, von Moos R, Hungria V, Costa L, Woll PJ, Scagliotti G, et al. Delaying skeletal-related events in a randomized phase III study of denosumab versus zoledronic acid in patients with advanced cancer. J Clin Oncol 2010;15.
- Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer 2004;100:2613-21. http://dx.doi.org/10.1002/cncr.20308.
- von Moos R, Patrick D, Fallowfield L, Cleeland CS, Henry DH, Qian Y, et al. Effects of denosumab versus zoledronic acid (ZA) on pain in patients (pts) with advanced cancer (excluding breast and prostate) or multiple myeloma (MM): results from a randomized phase III clinical trial. J Clin Oncol 2010;28.
- Schulman CC. Efficacy of zoledronic acid in the treatment of bone metastases secondary to renal cell carcinoma. Eur Urol Suppl 2004;3:40-5. http://dx.doi.org/10.1016/j.eursup.2004.08.011.
- Aguiar Bujanda D, Bohn Sarmiento U, Cabrera Suárez M, Aguiar Morales J. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Ann Oncol 2007;18:556-60. http://dx.doi.org/10.1093/annonc/mdl408.
- Bonomi M, Nortilli R, Molino A, Sava T, Santo A, Caldara A, et al. Renal toxicity and osteonecrosis of the jaw in cancer patients treated with bisphosphonates: a long-term retrospective analysis. Med Oncol 2010;27:224-9. http://dx.doi.org/10.1007/s12032-009-9195-y.
- Chennuru S, Koduri J, Baumann MA. Risk factors for symptomatic hypocalcaemia complicating treatment with zoledronic acid. Int Med J 2008;38:635-7. http://dx.doi.org/10.1111/j.1445-5994.2007.01580.x.
- Coleman R, Woodward E, Brown J, Cameron D, Bell R, Dodwell D, et al. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01–04) for women with stage II/III breast cancer. Breast Cancer Res Treat 2011;127:429-38. http://dx.doi.org/10.1007/s10549-011-1429-y.
- Diel IJ, Body JJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, et al. Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer 2004;40:1704-12. http://dx.doi.org/10.1016/j.ejca.2004.03.025.
- Diel IJ, Weide R, Koppler H, Antras L, Smith M, Green J, et al. Risk of renal impairment after treatment with ibandronate versus zoledronic acid: a retrospective medical records review. Support Care Cancer 2009;17:719-25. http://dx.doi.org/10.1007/s00520-008-0553-7.
- Estilo CL, Van Poznak CH, Wiliams T, Bohle GC, Lwin PT, Zhou Q, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist 2008;13:911-20. http://dx.doi.org/10.1634/theoncologist.2008-0091.
- Francini F, Pascucci A, Francini E, Miano ST, Bargagli G, Ruggiero G, et al. Osteonecrosis of the jaw in patients with cancer who received zoledronic acid and bevacizumab. J Am Dental Assoc 2011;142:506-13.
- Garcia Saenz JA, Lopez TS, Garcia PB, Rodriguez LL, Villalobos L, Diaz RE. Osteonecrosis of the jaw as an adverse bisphosphonate event: three cases of bone metastatic prostate cancer patients treated with zoledronic acid. Med Oral Patol Oral Cir Bucal 2007;12:E351-6.
- Guarneri V, Donati S, Nicolini M, Giovannelli S, D’Amico R, Conte PF. Renal safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 years. Oncologist 2005;10:842-8. http://dx.doi.org/10.1634/theoncologist.10-10-842.
- Haidar A, Jonler M, Folkmar TB, Lund L. Bisphosphonate (zoledronic acid)-induced osteonecrosis of the jaw. Scand J Urol Nephrol 2009;43:442-4. http://dx.doi.org/10.3109/00365590903295193.
- Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Mineral Res 2008;23:826-36. http://dx.doi.org/10.1359/jbmr.080205.
- Houston S, Grieve RJ, Hickish T, Percival F, Hamilton E. Renal function changes and NHS resource use in breast cancer patients with metastatic bone disease treated with IV zoledronic acid or oral ibandronic acid: a four-centre non-interventional study. J Med Econ 2010;13:162-7. http://dx.doi.org/10.3111/13696991003640383.
- Ibrahim T, Barbanti F, Giorgio-Marrano G, Mercatali L, Ronconi S, Vicini C, et al. Osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: a retrospective study. Oncologist 2008;13:330-6. http://dx.doi.org/10.1634/theoncologist.2007-0159.
- Jackson GH. Renal safety of ibandronate. Oncologist 2005;10:14-8. http://dx.doi.org/10.1634/theoncologist.10-90001-14.
- La Verde N, Bareggi C, Garassino M, Borgonovo K, Sburlati P, Pedretti D, et al. Osteonecrosis of the jaw (ONJ) in cancer patients treated with bisphosphonates: how the knowledge of a phenomenon can change its evolution. Support Care Cancer 2008;16:1311-15. http://dx.doi.org/10.1007/s00520-008-0484-3.
- Oh WK, Proctor K, Nakabayashi M, Evan C, Tormey LK, Daskivich T, et al. The risk of renal impairment in hormone-refractory prostate cancer patients with bone metastases treated with zoledronic acid. Cancer 2007;109:1090-6. http://dx.doi.org/10.1002/cncr.22504.
- Pandey R, Dey S, Mukhopadhyay A. The better bisphosphonate in patients with bone metastasis: zoledronic acid or ibandronic acid? A study from Eastern India. J Clin Oncol 2009;27.
- Pecherstorfer M, Rivkin S, Body JJ, Diel I, Bergstrom B. Long-term safety of intravenous ibandronic acid for up to 4 years in metastatic breast cancer: an open-label trial. Clin Drug Investig 2006;26:315-22. http://dx.doi.org/10.2165/00044011-200626060-00002.
- Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol 2009;20:137-45. http://dx.doi.org/10.1093/annonc/mdn526.
- Shah SR, Jean GW, Keisner SV, Gressett Ussery SM, Dowell JE. Risk of renal failure in cancer patients with bone metastasis treated with renally adjusted zoledronic acid. Support Care Cancer 2012;20:87-93. http://dx.doi.org/10.1007/s00520-010-1067-7.
- Stumpe MR, Chandra RK, Yunus F, Samant S. Incidence and risk factors of bisphosphonate-associated osteonecrosis of the jaws. Head Neck 2009;31:202-6. http://dx.doi.org/10.1002/hed.20941.
- Tralongo P, Repetto L, Di MA, Mauceri G, Bollina R, Ferrau’ F, et al. Safety of long-term administration of bisphosphonates in elderly cancer patients. Oncology 2004;67:112-16. http://dx.doi.org/10.1159/000080996.
- Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol 2009;27:5356-62. ttp://dx.doi.org/10.1200/JCO.2009.21.9584.
- Walter C, Al-Nawas B, Grotz KA, Thomas C, Thuroff JW, Zinser V, et al. Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol 2008;54:1066-72. http://dx.doi.org/10.1016/j.eururo.2008.06.070.
- Zuradelli M, Masci G, Biancofiore G, Gullo G, Scorsetti M, Navarria P, et al. High incidence of hypocalcemia and serum creatinine increase in patients with bone metastases treated with zoledronic acid. Oncologist 2009;14:548-56. http://dx.doi.org/10.1634/theoncologist.2008-0227.
- Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol 2007;25:4431-7. http://dx.doi.org/10.1200/JCO.2007.11.8604.
- Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N-telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. J Urol 2009;182:509-15. http://dx.doi.org/10.1016/j.juro.2009.04.023.
- Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res 2010;25:440-6. http://dx.doi.org/10.1359/jbmr.090810.
- Carteni G, Bordonaro R, Giotta F, Lorusso V, Scalone S, Vinaccia V, et al. Efficacy and safety of zoledronic acid in patients with breast cancer metastatic to bone: a multicenter clinical trial. Oncologist 2006;11:841-8. http://dx.doi.org/10.1634/theoncologist.11-7-841.
- Tripathy D, Lichinitzer M, Lazarev A, MacLachlan SA, Apffelstaedt J, Budde M, et al. Oral ibandronate for the treatment of metastatic bone disease in breast cancer: efficacy and safety results from a randomized, double-blind, placebo-controlled trial. Ann Oncol 2004;15:743-50. http://dx.doi.org/10.1093/annonc/mdh173.
- Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit; 2010.
- Dranitsaris G, Hsu T. Cost utility analysis of prophylactic pamidronate for the prevention of skeletal related events in patients with advanced breast cancer. Support Care Cancer 1999;7:271-9. http://dx.doi.org/10.1007/s005200050260.
- Hillner BE, Weeks JC, Desch CE, Smith TJ. Pamidronate in prevention of bone complications in metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 2000;18:72-9.
- Reed SD, Radeva JI, Glendenning GA, Coleman RE, Schulman KA. Economic evaluation of zoledronic acid versus pamidronate for the prevention of skeletal-related events in metastatic breast cancer and multiple myeloma. Am J Clin Oncol 2005;28:8-16. http://dx.doi.org/10.1097/01.coc.0000138966.66780.3e.
- De Cock E, Hutton J, Canney P, Body JJ, Barrett-Lee P, Neary MP, et al. Cost-effectiveness of oral ibandronate versus IV zoledronic acid or IV pamidronate for bone metastases in patients receiving oral hormonal therapy for breast cancer in the United Kingdom. Clin Ther 2005;27:1295-310. http://dx.doi.org/10.1016/j.clinthera.2005.08.006.
- De Cock E, Hutton J, Canney P, Body JJ, Barrett-Lee P, Neary MP, et al. Cost-effectiveness of oral ibandronate compared with intravenous (i.v.) zoledronic acid or i.v. generic pamidronate in breast cancer patients with metastatic bone disease undergoing i.v. chemotherapy. Support Care Cancer 2005;13:975-86. http://dx.doi.org/10.1007/s00520-005-0828-1.
- Guest JF, Clegg JP, Davie AM, McCloskey E. Costs and consequences of using pamidronate compared with zoledronic acid in the management of breast cancer patients in the UK. Curr Med Res Opin 2005;21:805-15. http://dx.doi.org/10.1185/030079905X40472.
- Botteman M, Barghout V, Stephens J, Hay J, Brandman J, Aapro M. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol 2006;17:1072-82. http://dx.doi.org/10.1093/annonc/mdl093.
- Botteman MF, Stephens J, Foley I, Kaura S. Cost Effectiveness of Zoledronic Acid in the Prevention of Skeletal Related Events in Renal Cell Carcinoma Patients with Bone Metastases: An Exploratory Analysis for France. Eur Urol Suppl 2009;8.
- Botteman MF, Logman JFS, Kaura S. Cost-effectiveness assessment of zoledronic acid (zol) relative to placebo (Pbo) in the treatment of lung cancer patients with skeletal metastases in five European countries. Value Health 2009;12. http://dx.doi.org/10.1016/S1098-3015(10)74329-1.
- Botteman MF, Kaura S. Comparison of the cost-effectiveness of zoledronic acid therapy for renal cell carcinoma (Rcc) patients with bone metastases in French, German, and the UK populations. Value Health 2009;12:A270-1. http://dx.doi.org/10.1016/S1098-3015(10)74325-4.
- Botteman MF, Stephens J, Foley I, Kaura S. Cost effectiveness of zoledronic acid in the prevention of skeletal related events in renal cell carcinoma patients with bone metastases: an exploratory analysis for France. Eur Urol Suppl 2009;8. http://dx.doi.org/10.1016/S1569-9056(09)60151-2.
- Botteman MF, Stephens JM, Foley I, Kaura S. Zoledronic acid is cost effective for the prevention of skeletal-related events in patients with bone metastases from renal cell carcinoma: a French exploratory analysis. J Urol 2009;181. http://dx.doi.org/10.1016/S0022-5347(09)61414-2.
- Botteman MF, Kaura S. Cost-effectiveness of zoledronic acid in the prevention of skeletal-related events in patients with bone metastases from renal cell carcinoma: comparison between France, Germany, and the United Kingdom. J Clin Oncol 2009;27.
- Joshi AD, Carter JA, Botteman MF, Kaura S. Cost-effectiveness of zoledronic acid in the management of skeletal metastases in patients with lung cancer in France, Germany, Portugal, the Netherlands, and the United Kingdom. Clin Ther 2011;33:291-304. http://dx.doi.org/10.1016/j.clinthera.2011.04.002.
- DesHarnais Castel L, Bajwa K, Markle JP, Timbie JW, Zacker C, Schulman KA. A microcosting analysis of zoledronic acid and pamidronate therapy in patients with metastatic bone disease. Support Care Cancer 2001;9:545-51. http://dx.doi.org/10.1007/s005200100249.
- Carter JA, Snedecor SJ, Kaura S, Botteman M. Cost-effectiveness of zoledronic acid (ZOL) versus denosumab (Dmab) in prevention of skeletal-related events (SREs) in metastatic breast cancer (mBC). J Clin Oncol 2011;29.
- Reed SD, Radeva JI, Glendenning GA, Saad F, Schulman KA. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J Urol 2004;171:1537-42. http://dx.doi.org/10.1097/01.ju.0000116777.94426.60.
- Xie J, Namjoshi M, Wu EQ, Parikh K, Diener M, Yu AP, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. J Manag Care Pharm 2011;17:621-43.
- Tchekmedyian NS, Chen YM, Saad F. Disease progression increases the risk of skeletal-related events in patients with bone metastases from castration-resistant prostate cancer, lung cancer, or other solid tumors. Cancer Invest 2010;28:849-55. http://dx.doi.org/10.3109/07357907.2010.483508.
- Stephens J. Cost-effectiveness of zoledronic acid versus placebo in the management of skeletal metastases in lung cancer patients (LC pts): comparison across three European countries. J Clin Oncol 2009;27.
- Botteman MF, Kaura S. Assessment of the cost-effectiveness of zoledronic acid in the management of skeletal metastases in lung cancer patients in France, Germany, and the United Kingdom. J Thorac Oncol 2009;4:S542-3.
- Botteman M, Hay JW, Stephens JM, Barghout V, Quednau K. A Markov model to evaluate the cost effectiveness of five bisphosphonate therapies in the prevention of bone complications in breast cancer patients with bone metastases: a German outpatient perspective. Eur J Cancer Suppl 2005;3. http://dx.doi.org/10.1016/S1359-6349(05)80721-9.
- Botteman M, Carter J, Kaura S. A comparison of the cost-effectiveness of zoledronic acid for preventing skeletal-related events in patients with bone metastases from prostate cancer in 4 European countries. Value Health 2010;13. http://dx.doi.org/10.1016/S1098-3015(11)71966-0.
- El Ouagari K, Botteman M, Aapro M, Hay J, Stephens J, Brandman JP. Cost-effectiveness of zoledronic acid vs. other bisphosphonate agents for the prevention of bone complications in breast cancer: an application to Canada. Eur J Cancer Suppl 2005;3. http://dx.doi.org/10.1016/S1359-6349(05)80716-5.
- Meijboom M, Botteman MF, Kaura S. Cost effectiveness of zoledronic acid for the prevention of skeletal related events in prostate cancer patients with bone metastases in France and Germany. Eur Urol Suppl 2009;8. http://dx.doi.org/10.1016/S1569-9056(09)60055-5.
- Snedecor SJ, Carter JA, Kaura S, Botteman M. Cost-effectiveness of zoledronic acid (ZOL) versus denosumab (Dmab) in prevention of skeletal-related events (SREs) in castration-resistant prostate cancer metastatic to the bone (mCRPC)q. J Clin Oncol 2011;29.
- Yu AP, Namjoshi M, Xie J, Parikh K, Wu EQ, Guo A, et al. Economic evaluation of denosumab compared with zoledronic acid in patients with hormone-refractory prostate cancer with bone metastases. J Clin Oncol 2011;29.
- Clohisy DR, Le CT, Cheng EY, Dykes DC, Thompson RC. Evaluation of the feasibility of and results of measuring health-status changes in patients undergoing surgical treatment for skeletal metastases. J Orthop Res 2000;18:1-9. http://dx.doi.org/10.1002/jor.1100180102.
- Matza LS, van Brunt K, Chung K, Brazier J, Braun AH, Currie B, et al. Health state utilities for skeletal-related events associated with bone metastases. J Clin Oncol 2011;29.
- Miksad RA, Lai KC, Dodson TB, Woo SB, Treister NS, Akinyemi O, et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist 2011;16:121-32. http://dx.doi.org/10.1634/theoncologist.2010-0183.
- van den Hout WB, van der Linden YM, Steenland E, Wiggenraad RG, Kievit J, de Haes H, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst 2003;95:222-9. http://dx.doi.org/10.1093/jnci/95.3.222.
- Barrett-Lee P, Bloomfield D, Dougherty L, Harries M, Laing R, Patel H, et al. An audit to determine the time taken to administer intravenous bisphosphonate infusions in patients diagnosed with metastatic breast cancer to bone in a hospital setting. Curr Med Res Opin 2007;23:1575-82. http://dx.doi.org/10.1185/030079907X210543.
- Oglesby A, Sherif B, Odom D, Leahy M, Qian Y. Time and costs associated with preparing and administering zoledronic acid in patients with breast or prostate cancer and metastatic bone disease. Community Oncol 2009;6:494-502. http://dx.doi.org/10.1016/S1548-5315(11)70357-6.
- Malmberg I, Persson U, Ask A, Tennvall J, Abrahamsson PA. Painful bone metastases in hormone-refractory prostate cancer: economic costs of strontium-89 and/or external radiotherapy. Urology 1997;50:747-53. http://dx.doi.org/10.1016/S0090-4295(97)00326-9.
- Groot MT, Boeken Kruger CG, Pelger RC, Uyl-de Groot CA. Costs of prostate cancer, metastatic to the bone, in the Netherlands. Eur Urol 2003;43:226-32. http://dx.doi.org/10.1016/S0302-2838(03)00007-1.
- Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology 2004;67:390-6. http://dx.doi.org/10.1159/000082923.
- Delea T, McKiernan J, Brandman J, Edelsberg J, Sung J, Raut M, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol 2006;4:341-7.
- Lage MJ, Barber BL, Harrison DJ, Jun S. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manage Care 2008;14:317-22.
- Barlev A, Song X, Ivanov B, Setty V, Chung K. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm 2010;16:693-702.
- Janus N, Le Tourneau C, Launay-Vacher V, Gligorov J, Rixe O, Pourrat X, et al. Renal insufficiency in bone metastasis cancer patients: prevalence and implications on anticancer drugs management, subgroup analysis of the IRMA study. J Clin Oncol 2007;25.
- Scagliotti G, Hirsh V, Siena S, Henry D, Woll P, Manegold C, et al. Overall Survival Improvement in Patients With Lung Cancer Treated With Denosumab Versus Zoledronic Acid: Results from a Randomised Phase 3 Study n.d.
- Adami S, Salvagno G, Guarrera G. Dichloromethylene-diphosphonate in patients with prostatic carcinoma metastatic to the skeleton. J Urol 1985;134:1152-4.
- Zhao X, Xu X, Guo L, Ragaz J, Guo H, Wu J, et al. Biomarker alterations with metronomic use of low-dose zoledronic acid for breast cancer patients with bone metastases and potential clinical significance. Breast Cancer Res Treat 2010;124:733-43. http://dx.doi.org/10.1007/s10549-010-1183-6.
- Saad F. Should patients with prostate cancer without bone metastases receive sodium clodronate? Commentary. Nat Clin Pract Urol 2007;4:584-5. http://dx.doi.org/10.1038/ncpuro0911.
- Kotteas E, Alamara C, Kiagia M, Pantazopoulos K, Boufas A, Provata A, et al. Safety and efficacy of zoledronic acid rapid infusion in lung cancer patients with bone metastases: a single institution experience. Anticancer Res 2008;28:529-33.
Appendix 1 Search strategies
Appendix 2 Data extraction form
Appendix 3 The Cochrane collaboration's tool for assessing risk of bias
The Cochrane collaboration's tool for assessing risk of bias (PDF download)
Appendix 4 List of included studies
Appendix 5 List of excluded studies
Appendix 6 Characteristics of studies excluded from network meta-analysis
Characteristics of studies excluded from network meta-analysis (PDF download)
Appendix 7 Results from studies excluded from network meta-analysis
Results from studies excluded from network meta-analysis (PDF download)
Appendix 8 Characteristics of studies included in indirect comparison
Characteristics of studies included in indirect comparison (PDF download)
Appendix 9 Quality assessment results for the individual studies
Quality assessment results for the individual studies (PDF download)
Appendix 10 Breast cancer adverse events
Appendix 11 Prostate cancer adverse events
Appendix 12 Other solid tumours adverse events
Appendix 13 European Quality of Life-5 Dimensions health-related quality-of-life estimates presented by the manufacturer
European Quality of Life-5 Dimensions health-related quality-of-life estimates presented by the manufacturer (PDF download)
Appendix 14 Sensitivity analyses presented by the manufacturer
Sensitivity analyses presented by the manufacturer (PDF download)
Appendix 15 Univariate and probabilistic sensitivity analyses
Univariate and probabilistic sensitivity analyses (PDF download)
Appendix 16 Protocol
List of abbreviations
- AG
- assessment group
- ASCO
- American Society of Clinical Oncology
- BNF
- British National Formulary
- BP
- bisphosphonate
- BPI
- Brief Pain Inventory
- BPI-SF
- Brief Pain Inventory – Short Form
- BSAP
- bone-specific alkaline phosphatase
- BSC
- best supportive care
- CEAF
- cost-effectiveness acceptability frontier
- CG
- clinical guideline
- CI
- confidence interval
- CRPC
- castration-resistant prostate cancer
- CSR
- clinical study report
- CT
- computerised tomography
- CTCAE
- Common Terminology Criteria for Adverse Events
- CTX
- urinary or serum collagen type 1 cross-linked C-telopeptide
- ECOG
- Eastern Cooperative Oncology Group
- EQ-5D
- European Quality of Life-5 Dimensions
- ERG
- Evidence Review Group
- FACT
- Functional Assessment of Cancer Therapy
- FACT-B
- Functional Assessment of Cancer Therapy – Breast
- FACT-G
- Functional Assessment of Cancer Therapy – General
- FACT-P
- Functional Assessment of Cancer Therapy – Prostate
- HCM
- hypercalcaemia of malignancy
- HR
- hazard ratio
- HRG
- health-care resource group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- LDH
- lactate dehydrogenase
- MRI
- magnetic resonance imaging
- MS
- manufacturer's submission
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NSCLC
- non-small cell lung cancer
- NTX
- urinary collagen type 1 cross-linked N-telopeptide
- ONJ
- osteonecrosis of the jaw
- OST
- other solid tumour
- PAS
- Patient Access Scheme
- PINP
- N-terminal type 1 procollagen peptide
- PR
- progesterone receptor
- PSA
- prostate-specific antigen
- QALY
- quality-adjusted life-year
- RANKL
- receptor activator of nuclear factor kappa-B ligand
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SCC
- spinal cord compression
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMPR
- skeletal morbidity period rate
- SMR
- skeletal morbidity rate
- SRE
- skeletal-related event
- TNM
- tumour–node–metastasis
- TOI
- trial outcome index
- TTO
- time trade-off
- VAS
- visual analogue scale
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices in which case the abbreviation is defined in the figure legend or at the end of the table.