Notes
Article history paragraph text
The research reported in this issue of the journal was funded by the HTA programme as project number 08/45/01. The contractual start date was in November 2009. The draft report began editorial review in February 2012 and was accepted for publication in September 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Simpson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Scientific summary
Background
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. AF may be asymptomatic, but may cause palpitations, chest pain, shortness of breath or fainting. If left untreated, AF is a significant risk factor for stroke and other morbidities.
Transthoracic echocardiography (TTE) allows imaging of the heart and blood flow. Echocardiography enables the diagnosis of cardiac abnormalities earlier than would be possible if symptoms were left to develop. Currently, only selected patients with AF are recommended for TTE: those who have clinically suspected heart disease or for whom further information is needed for treatment planning.
Objectives
The assessment investigated the clinical effectiveness and cost-effectiveness of performing routine TTE in all newly diagnosed patients with AF, in comparison with the current practice of selective testing.
Methods
Literature reviews were conducted on the diagnostic accuracy of TTE for clinically important pathologies in AF and their prevalence in patients with AF. A search of MEDLINE, and, for the prevalence review, of 11 other databases was conducted from March to August 2010, and reference lists of relevant articles were checked. For the diagnostic review, the intervention was conventional TTE, and the outcomes sensitivity or specificity. Results were tabulated and discussed in a narrative synthesis.
A mathematical model was constructed to assess the cost-effectiveness of TTE in patients with newly diagnosed AF. It was assumed that TTE would be of benefit only when patient management was changed. It was assumed that if a left atrial abnormality was detected then the patient was at a higher risk of stroke and should receive treatment. The estimated sensitivity and specificity of TTE in identifying left atrial abnormality was incorporated in the model.
A total of 14 separate paired comparisons, comparing a baseline strategy of not using TTE with a comparator strategy that did, were produced. These considered higher- and lower-risk groups, two different age groups, three different types of oral anticoagulant, and both males and females separately.
A simplified approach was also undertaken that evaluated the additional quality-adjusted life-years (QALYs) required in order for TTE to be perceived as cost-effective at a threshold of £20,000 per QALY.
Results
The literature reviews identified 44 diagnostic accuracy studies, five prognostic studies and 16 prevalence studies. Diagnostic accuracy showed high specificities for all selected pathologies, with the majority having specificity of 0.8 or higher, meaning a low proportion of false-positives. Specificity was lower for aortic dissection and pulmonary disease than for other pathologies. For most pathologies there was also quite high sensitivity, with the majority having sensitivity of ≥ 0.6, with the exceptions of atrial thrombi, atrial septal defect and pulmonary embolism (PE), for which sensitivity was lower. Prognostic studies indicated that TTE-diagnosed left ventricular (LV) dysfunction or increased left atrial diameter (LAD) was associated with significantly increased risks of thromboembolism or mortality. LV dysfunction also had a significantly increased risk of stroke, and valvular abnormality a significantly increased risk of mortality. Not all studies found a significant association between TTE-diagnosed mitral regurgitation (MR) and prognosis; however, there were reported a significantly increased risk of thromboembolism with mild MR, in contrast with a significantly protective effect of severe MR against stroke. Mitral annular calcification and mitral valve prolapse were not found to be associated with thromboembolism and stroke, respectively. There was a high prevalence (around 25–30%) of ischaemic heart disease, valvular heart disease and heart failure in patients with AF in the included prevalence studies.
The results of the mathematical model indicated that it may be cost-effective to use TTE to make the decision about whether to prescribe warfarin to patients with a CHADS2 (cardiac failure, hypertension, age, diabetes, stroke doubled) score of 1, or whether to prescribe rivaroxaban to patients aged ≥ 65 years with a CHADS2 score of 0.
In the simplified approach, a threshold of 0.0033 was required for a TTE to be cost-effective. This is a very small value, and if a clinician believes there will be some patient gain in addition to providing treatment to reduce stroke risk then TTE is likely to be cost-effective.
Discussion
Diagnostic accuracy of TTE and prevalence of pathologies in patients with AF indicate that routine TTE following AF diagnosis would identify pathologies in many patients, particularly with regard to valvular heart disease, ischaemic heart disease and heart failure. TTE seems to be a sufficient diagnostic tool for screening most pathologies included in this review. For completeness of screening, extra testing for PE by lung scan and for atrial thrombi and atrial septal hypertrophy by transoesophageal echocardiography would reduce risk of false-negatives from TTE. However, it is unclear whether identifying these pathologies, in addition to the many diagnosed by TTE, would lead to improvement above that of TTE screening.
It is clear that TTE has the potential to be cost-effective, and this has been indicated in the analyses that assume that the CHADS2 tool is used. The simplified approach indicates that very few QALYs are required for TTE to be perceived as cost-effective. The modelling undertaken focuses purely on the risks of stroke and of bleed events; if patients will benefit from TTE in other respects it is likely that this diagnostic test would be cost-effective.
Conclusions
Transthoracic echocardiography is a non-invasive procedure with the potential to accurately identify treatable pathologies in patients with AF.
Where the CHADS2 tool is used, the addition of TTE in identifying patients with left atrial abnormality appears to be cost-effective for informing some oral anticoagulation decisions. A simple analysis indicates that the QALYs required for TTE to be cost-effective is small, and that if benefits beyond those associated with a reduction in stroke (at the expense of greater number of bleed) are believed probable then TTE is likely to be cost-effective in all scenarios.
Our findings suggest that further research is needed to follow-up newly diagnosed patients with AF who have undergone TTE, to study treatments given as a result of TTE diagnoses and subsequent cardiovascular events, which could identify additional benefits of routine testing, beyond stroke prevention. Studies assessing the proportion of people with a CHADS2 scores of 0 or 1 that have left atrial abnormality would provide better estimates of the cost-effectiveness of TTE, and allow more accurate estimates of the sensitivity and specificity of TTE for identifying left atrial abnormality in AF to be obtained.
Study registration
This study is registered as PROSPERO CRD42011001354.
Funding
Funding for this study was provided by the Health Technology Assessment programme of the National Institute for Health Research.
Chapter 1 Background
Atrial fibrillation
Cardiac arrhythmias affect the heart, causing an irregular heartbeat. Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. 1 It is a form of tachyarrhythmia, meaning an abnormally rapid heartbeat accompanied by an irregular rhythm, and is characterised by uncoordinated atrial activation with consequent deterioration of atrial mechanical function. 2
Atrial fibrillation:
-
does not always cause symptoms but may cause palpitations, chest pain or discomfort, shortness of breath, dizziness, or fainting. 1 In extreme cases there may be loss of consciousness1
-
is sometimes associated with other arrhythmias, most commonly atrial flutter or atrial tachycardias, but may occur by itself3
-
is more common in older people, and at the age of 80–89 years, almost 9% of people have AF. 1 With the ageing population and increasing prevalence of chronic heart disease, AF has increased in frequency over the past few years. 2
Types of atrial fibrillation
The Working Group of Arrhythmia of the European Society of Cardiology (WGA-ESC) and the North American Society of Pacing and Electrophysiology (NASPE) created an international consensus on the classification of AF, applying to episodes of AF lasting more than 30 seconds. 3
The initial event of AF is the first detected episode. 3 AF may or may not recur after the initial event. 3 AF is considered recurrent on experiencing two or more episodes. 1,3
Paroxysmal AF is a recurrent form of AF that spontaneously terminates within 7 days, usually within 48 hours. 3
Persistent AF is a recurrent form of AF that does not self-terminate, or lasts longer than 7 days (without cardioversion). 3 This may be the first presentation of AF, or may follow paroxysmal AF. 3 A patient may have some episodes of paroxysmal AF and some episodes of persistent AF, in which case he or she may be classified according to the most frequent presentation. 2
Permanent AF is established AF that has not terminated, has terminated but recurred within 24 hours, or for which cardioversion has not been attempted (accepted AF). 3 This may be the first presentation of AF, or may follow self-terminating AF episodes. 3
Non-valvular (or non-rheumatic) AF refers to cases of AF with the absence of rheumatic valve disease, prosthetic valve or repaired mitral valve. 2
Aetiology, pathology and prognosis of atrial fibrillation
Atrial fibrillation may occur in the absence of any concomitant disease, in which case it is termed idiopathic AF. 3 Lone AF is a term used to describe AF in patients without concomitant heart disease3 and with normal echocardiogram. 2 This term is usually applied to younger patients with AF, that is < 60 years old. 2 AF may be triggered by atrial flutter or by other atrial tachycardias. 3
Atrial fibrillation can be caused by other medical conditions, such as cardiovascular disease, diabetes mellitus (DM), obesity or hypertension. 1 Cardiovascular conditions associated with AF include coronary artery disease, valvular heart disease, heart failure and hypertension. 2 AF may occur following surgery. 1 Alcohol and caffeine may predispose patients to AF. 2 Family history is a risk factor for AF. 4
Atrial fibrillation occurring in the context of acute myocardial infarction (AMI), cardiac surgery, pericarditis, myocarditis, hyperthyroidism, pulmonary embolism (PE), pneumonia, or other acute pulmonary disease is termed secondary AF. 2
For some patients with secondary AF, after curing the underlying cause the AF is unlikely to recur. 2,3 Examples of these causes include AMI, acute pericarditis, acute myocarditis or acute pulmonary embolus. 3 However, AF may occur independently of other diseases, for example in patients with hypothyroidism, even when the concomitant disorder is being treated. 2
Atrial fibrillation is associated with atrial fibrosis and loss of atrial muscle mass. 2 A coexistence of normal and fibrosed atrial fibres may explain non-homogeneity of conduction within the condition. 2
On electrocardiography (ECG), AF is described by the absence of consistent P waves. 1,3 Replacing consistent P waves on the ECG of a patient in AF, are rapid oscillations or fibrillatory waves that vary in size, shape and timing. 1,3 These are generally associated with an irregular ventricular response when atrioventricular (AV) conduction is intact. 1,3
In AF, the ventricular response depends on AV nodal properties, the level of vagal and sympathetic tone, and drugs that affect AV nodal conduction, such as beta-blockers, non-dihydropyridine calcium channel blockers (calcium antagonists) and digitalis glycosides. 1,3
Paroxysmal AF can progress to chronic AF (CAF). A study of the Canadian Registry of Atrial Fibrillation (CARAF) found the probability of progression to CAF by 1 year was 8.6% and thereafter there was a slow but steady progression to 24.7% by 5 years. 5 By 5 years, the probability of documented recurrence of any AF (chronic or paroxysmal) was 63.2%. 5 Increasing age, significant aortic stenosis or mitral regurgitation (MR), enlargement of the left atrial (LA) and diagnosis of cardiomyopathy were independently associated with progression to CAF. 5 A more rapid heart rate during AF was associated with decreased risk of progression. 5 If left untreated, AF may sometimes result in a degree of haemodynamic instability that can represent a critical condition that requires immediate intervention to alleviate symptoms of breathlessness, chest pain and loss of consciousness. 1
An irregular heartbeat makes the heart less efficient at circulating blood around the body. This can increase the risk of blood clots developing within the circulatory system. If left untreated, AF is a significant risk factor for thromboembolic events including stroke. 1
Atrial fibrillation can be a risk factor for stroke. The rate of ischaemic stroke has been estimated to be two to seven times higher among patients with non-valvular AF than in those without. 2 The risk is greater for those with rheumatic AF. 2
Guidelines produced by the National Collaborating Centre for Chronic Conditions (NCC-CC) for National Institute for Health and Care Excellence (NICE) on AF define risk of stroke in patients with AF as follows:
High risk Previous ischaemic stroke or transient ischaemic attack (TIA) or thromboembolic event, age ≥ 75 years with hypertension, diabetes or vascular disease (coronary or peripheral artery disease), clinical evidence of valve disease or heart failure, or impaired left ventricular (LV) function.
Moderate risk Age ≥ 65 years with no high risk factors, or age < 75 years with hypertension, diabetes or vascular disease.
Low risk Age < 65 years with no moderate- or high-risk factors. 1
Several sets of clinical criteria have been proposed for stratifying risk of stroke in patients with AF, including Atrial Fibrillation Investigators (AFI) criteria, Stroke Prevention in Atrial Fibrillation (SPAF) study criteria, and the CHADS2 (cardiac failure, hypertension, age, diabetes, stroke doubled) score, which is a clinical prediction rule for estimating the risk of stroke in patients with non-rheumatic AF. 2
European Society of Cardiology (ESC) 2010 guidelines6 recommend a risk factor-based approach for assessing stroke risk in patients with non-valvular AF based on CHA2 DS2-VASc [congestive heart failure (CHF), hypertension, age ≥ 75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 years, and sex category (female)]. Valvular AF is considered a major risk factor for stroke. 6
As well as thromboembolic complications, AF has been associated with an increased risk of dementia, heart failure and death. 4
An increased mortality rate in AF, compared with that of patients in normal sinus rhythm, has been linked to the severity of underlying heart disease. 2 Among patients with heart failure, AF has been associated with increased mortality rate in some, although not all, studies. 2,7
Echocardiography may be useful in predicting the prognosis of AF. A retrospective study of PE in CAF identified transthoracic echocardiography (TTE) variables that were associated with acute PE or chronic thromboembolic pulmonary hypertension in AF as increased right ventricular (RV) dimension, higher tricuspid pressure gradient and shorter pulmonary artery acceleration time. 8 CAF was also associated with PE in participants with significantly decreased LV dimension and better LV performance. 8 A study of CARAF found baseline echocardiographic variables were associated with progression to CAF, independently of age, cardiomyopathy and heart rate. 5
Transthoracic tissue Doppler imaging has associated the ratio of early transmitral flow velocity to early diastolic mitral annular velocity with the prognosis of non-valvular AF, in terms of overall survival, cardiac death and CHF. 9 Cerebral infarction in patients with non-valvular paroxysmal AF has been linked with TTE markers of a lower peak late diastolic flow velocity and a higher early to late ratio for transmitral flow. 10
Transoesophageal echocardiography has also been used to predict prognosis in patients with AF. 11,12
Providencia et al. 13 investigated TTE and transoesophageal echocardiography (TOE) in combination with the CHADS2 and CHA2 DS2-VASc scores as means of improving risk stratification for thromboembolic events in a cohort of patients with AF. They found that TTE diagnosed LV systolic function, and LA area measurement may provide a valuable addition to CHADS2 and CHA2 DS2-VASc scores. 13
Measurement of atrial fibrillation
Guidelines on the management of AF, published by NICE in 2006, state that diagnosis should be made by ECG. 1 Suspected paroxysmal AF not detected by standard ECG recording can be diagnosed by a 24-hour ambulatory ECG monitor where asymptomatic episodes are suspected or where episodes are < 24 hours apart, or by an event recorder ECG in which symptomatic episodes are > 24 hours apart. 1
On the ECG, AF is described by the absence of consistent P waves. 1,3
Regular relative risk (RR) intervals on the ECG may occur in some cases of AF, for example, in the presence of heart block associated with conduction disease or drug therapy. 1,3
Patients with permanent ventricular pacing may require temporary pacemaker inhibition in order to visualise AF activity and diagnose AF. 1,3 A rapid, irregular, sustained, wide QRS complex (combination of Q, R and S waves) tachycardia could suggest AF with conduction via an accessory pathway. 1,3
Atrial fibrillation is distinguished from atrial flutter on the ECG, as atrial flutter shows a pattern of atrial activity called ‘flutter waves’ visible on the ECG. 3 AF is distinguished from atrial tachycardia on the ECG, as in atrial tachycardia the P waves are well identified and separated by an isoelectric baseline. 3
Physical examination suggestive of AF includes irregular pulse, irregular jugular venous pulsations, and variation in the intensity of first heart sound or absence of fourth sound heard previously during sinus rhythm. 2
Incidence and/or prevalence of atrial fibrillation
Atrial fibrillation is a common cardiac arrhythmia associated with a substantial degree of morbidity and mortality. 14 On average, AF is present in 1–2% of the population. 15,16 Epidemiological data have often come from studies with small populations in developed countries with a minimal representation of ethnic minorities. 17,18 Additional concerns about previous studies have included limited age range of patients and unreliable ascertainment (such as self-reporting and/or examination of hospital records) for AF diagnosis. 15 However, two large studies with lengthy follow-up periods, the Framingham Heart Study in the USA19 and the Renfrew/Paisley study16 in the west of Scotland, have been notable sources of incidence data. The Framingham Heart Study estimated 2-yearly incidence rates of 0.9 per 1000 person-years and 1.9 per 1000 person-years in women and men aged 50–59 years, respectively. 19 Incidence rates, over a 4-year period, reported from the Renfrew/Paisley study16 were 0.44 per 1000 person-years and 1.31 per 1000 person-years for women and men aged 55–64 years. Data from the Framingham study19 showed that men were 1.5 times more likely to develop AF than women.
Atrial fibrillation affects approximately 6 million people in Europe and 2.3 million of the US population. 20 It is estimated that there are about 650,000 cases of AF in England and Wales, with the greatest number of affected patients aged between 75 and 84 years. 21
The incidence of AF is closely related to age. 22 Increasing age is more often than not associated with structural and physiological cardiac abnormalities that predispose to the development of AF. In addition, advanced age implies longer exposure to known risk factors. Available epidemiological evidence has demonstrated that AF is more common in those aged ≥ 50 years: reported rates in 50- to 59-year-olds and those aged between 80 and 89 years were 0.5% and 8.8%, respectively. 23 AF rates double with each successive decade of age, especially after the age of 50 years. 24 The prevalence of AF in patients who are 80–90 years of age is close to 9%,20 and this trend is often reported. 15,20,25 Factors influencing this include the increasingly ageing population and the greater proportion of patients living with cardiovascular and non-cardiac predisposing risk factors, such as hypertension, obesity and diabetes. 20 An underlying pathology may be absent in 15–30% of patients. 26
Impact of atrial fibrillation
Significance for patients in terms of ill health (burden of disease)
Atrial fibrillation is a common and significant cause of cardiovascular-related morbidity and mortality. The condition is a major predictor of atrial thrombosis, peripheral embolism and stroke, especially in elderly patients. 16,27,28 Evidence for the Framingham Heart Study noted a four- to fivefold rise in the risk of stroke in patients with AF. 14 The observed increase in risk has been attributed to the presence of LV hypertrophy, which is often associated with long-standing AF. 27 The risk of stroke also increases with age. 14,20
Coexisting cardiovascular disease and AF significantly reduce quality of life in those patients with symptoms such as palpitations, light-headedness and fatigue. Furthermore, patients with AF with underlying coronary heart disease and chronic lung disease are more likely to suffer from myocardial infarction (MI) and acute respiratory failure. 29 Evidence from a longitudinal cohort study has also suggested an increased risk of dementia in patients with AF. 30
Atrial fibrillation is also associated with an increase in mortality rate. In the Framingham study,19 AF was associated with 1.5- to 1.9-fold increase in the risk of mortality, following adjustments for the underlying cardiovascular diseases in affected patients. 31 This conferred risk of death is similar in both men and women and does not vary significantly by age. 31 Data from the Renfrew/Paisley cohort with a 20-year follow-up period demonstrated an increase of 1.8- to 2.8-fold and 1.5- to 2.2-fold in cardiovascular-related death and all-cause death, respectively. 32
Significance for the NHS
In many developed countries, AF is increasingly becoming a significant public health challenge. It is a major cause of increased hospitalisation in the UK. 18,33 The number of hospital admissions for patients with AF has at least doubled in recent times. 34
A study of the health and social care-related expenditure on patients with AF in 1995 showed that an estimated £244M, which accounted for 0.62% of the total NHS budget was spent on patients with AF. 35 Of this, half of the cost covered the hospitalisation of patients, whereas 20% of the total expenditure was for the cost of drug prescriptions. An extra £46.4M was used to provide long-term nursing home care following admission. Based on projections of AF expenditure in 1995, it was estimated that direct costs of the condition would be approximately £459M in the year 2000. 35
Current service provision
The setting for management may vary depending on the nature and the severity of the condition1,36 [National Service Framework (NSF)/NICE]; however, urgent referral of patients with persisting and complicated arrhythmia is required for prompt and appropriate treatment.
Relevant national guidelines and management of atrial fibrillation
Key guidance documents for the care of patients with AF have been developed by the ESC,6 American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA),2,37 NICE1 and the NSF committee. 36 Recommendations within these guidelines emphasise the importance of early and accurate diagnosis and appropriate management strategies for patients.
Immediate and early 12-lead ECG tracing is advised, even if symptoms have subsided. 1,36 ECG is also recommended in patients with documented AF. 2 The number and duration of ECG monitoring studies are usually based on clinical judgement. 2 Intense and prolonged monitoring is recommended in patients with:1,2
-
severe symptoms
-
documented or suspected underlying disease of cardiac or non-cardiac origin
-
complications due to ongoing or previous ‘silent’ AF
-
treatment with antiarrhythmic agents
-
treatment related to rate control.
The aim of treatment is to reduce symptoms and avert complications in patients with AF. To achieve this, the goal of treatment may include a number of desirable effects such as the control of ventricular rate, treatment of underlying conditions and the prevention of thromboembolic events. There are a range of options for the management of AF. Available therapies consist of a variety of pharmacological agents including antiarrhythmic and antithrombotic agents as well as the use of alternative non-pharmacological interventions, such as cardioversion. Treatment decisions depend on the type of AF. 1
For the control of ventricular rate, treatment may consist of a rate control or rhythm control strategy. Rhythm control is the recommended initial therapy for patients with paroxysmal AF, whereas rate control treatment is the choice of initial treatment for patients with permanent AF. 1 For patients with persistent AF, treatment with rhythm control or rate control strategies may be the initial approach. 1
Rate control strategies aim to control ventricular rate. 2 Generally, heart rate is considered to be controlled if it is between 60 and 80 beats per minute at rest and between 90 and 115 beats during moderate exercise. Treatment may be tailored to achieve a resting heart rate ≤ 80 beats per minute (strict rate control) or < 110 beats per minute (lenient rate control). 38 A study comparing the two strategies in 614 patients with permanent AF who were followed up for no less than 2 years reported similar clinical outcomes (based on a primary composite outcome of systemic embolism, bleeding, stroke, life-threatening arrhythmic event, hospitalisation and death from cardiovascular causes) for both interventions. 38 Rate control involves drug therapy with a beta-blocker [e.g. metoprolol (Lopresor®, Novartis)], a calcium channel blocker [e.g. verapamil (Calan®, Pfizer)] or a cardiac glycoside [e.g. digoxin (Lanoxin®, GlaxoSmithKline)]. Usually, a combination of different classes of drugs may be required to achieve adequate rate control. Rate control is recommended as initial treatment in elderly patients with minor symptoms. 39–41
Rhythm control treatments are used to achieve a sinus rhythm. Rhythm control is recommended to be tried first for patients who are symptomatic, present for the first time with lone AF, have CHF or AF secondary to a treated or corrected precipitant, or younger patients. 1 For patients with persistent AF, rhythm control may include cardioversion, followed by antiarrhythmic drug therapy if needed to maintain sinus rhythm. It is important that such patients undergo further investigations to identify coexisting underlying structural cardiac abnormalities. 1 Antiarrhythmic drug therapy usually includes a standard beta-blocker, unless this is ineffective or contraindicated. In this case, alternatives such as flecainide (Tambocor®, Meda), propafenone (Rythmol®, GlaxoSmithKline; Arthmol®, Abbott) sotalol (Sotacor®, Bristol-Myers Squibb) or amiodarone (Cordarone X®, Sanofi-Aventis) can be used. 1 In patients with paroxysmal AF, this antiarrhythmic drug therapy may be used. Alternatively, a patient may be considered for a ‘pill-in-the-pocket’ strategy if there is no history of infrequent symptomatic episodes of paroxysmal AF, LV dysfunction, valvular or ischaemic heart disease, a systolic blood pressure of > 100 mmHg and a resting heart rate of > 70 beats per minute. 1 A rhythm control strategy may also be used for postoperative AF after cardiothoracic surgery. 1
Cardioversion is a method for converting an abnormal heart rate to normal (sinus rhythm). 1,2,36 This may be achieved by pharmacological or electrical interventions. Pharmacological cardioversion involves the use of oral or intravenous agents to achieve a normal and regular heart rate. Examples of treatments include flecainide or intravenous amiodarone. The latter is recommended in patients with structural heart disease. 1 Electrical cardioversion, also referred to as direct-current (DC) cardioversion, involves the delivery of a ‘safe’ electrical shock to the heart. The electrical current may be delivered across the wall of the chest (external cardioversion) or through a tiny wire introduced into the heart through a peripheral vein (internal cardioversion). 36 Anticoagulation is essential in all patients undergoing elective cardioversion for AF of more than 48 hours' duration or an unknown duration. This is essential because of the associated risk of embolism related to the procedure. 2 In some cases, anticoagulation may need to be continued after cardioversion. 1
Ablation strategies are indicated for patients with AF who remain symptomatic following antiarrhythmic medication or those for whom pharmacological treatment is contraindicated because of intolerance or existing comorbidity. 42,43 The aim of treatment is to destroy heart muscles that generate abnormal electrical impulses leading to arrhythmic activity. A number of approaches may be used; these include ablation of the AV node, left atrium, right atrium or the focal pulmonary vein. Various energy sources, including ultrasound, microwave, radiofrequency and cryotherapy, are used in ablation techniques. Ablation generally involves the introduction of a fine flexible catheter into the heart via a peripheral vein (usually the femoral vein). However, in some cases, ablation can be used during open cardiac surgery.
Furthermore, AF may be prevented or controlled by the insertion of a pacemaker, an implantable device in contact with the heart by means of flexible wires. Artificial impulses generated by the pacemaker regulate and maintain the heart rate. Although a number of pacing algorithms and techniques exist, the role of permanent pacing in patients with AF is still uncertain. 44
Antithrombotic therapy may additionally be given to patients with AF in accordance with risk of stroke. The 2006 recommendations from the NCC-CC state that anticoagulation therapy with warfarin is recommended for patients at high risk of stroke, or to be considered for patients at moderate risk of stroke, unless the patient has contraindications to warfarin. For patients with low risk of stroke, aspirin is recommended. 1 On the other hand, inconsistencies in the evidence regarding the antiplatelet benefits of aspirin require that it is used cautiously in patients with an increased risk of thromboembolism. 1,2
Risk of stroke is defined as follows:1
-
High risk Previous ischaemic stroke or TIA or thromboembolic event, age ≥ 75 years with hypertension, diabetes or vascular disease (coronary or peripheral artery disease), clinical evidence of valve disease or heart failure, or impaired LV function.
-
Moderate risk Age ≥ 65 years with no high risk factors, or age < 75 years with hypertension, diabetes or vascular disease.
-
Low risk Age < 65 years with no moderate or high risk factors.
The 2006 NICE guidelines1,2 recommend use of warfarin for patients at high risk, and some patients at moderate risk, of stroke. NICE has recently approved both dabigatran etexilate (Pradaxa®, Boehringer Ingelheim) and rivaroxaban (Xarelto®, Bayer Schering) as alternatives for the prevention of stroke in people with AF45,46 (last accessed January 201247).
European Society of Cardiology 2010 guidelines6 recommend a risk factor-based approach for patients with non-valvular atrial fibrillation based on CHA2 DS2-VASc. Valvular AF is considered high risk for stroke. 6
The CHA2 DS2-VASc model uses a point system in which two points are allocated where a patient has a history of stroke or TIA, or is aged ≥ 75 years. One point is allocated for each of the following: aged 65–74 years, a history of hypertension, diabetes, recent cardiac failure, vascular disease comprising MI, complex aortic plaque, or peripheral arterial disease, and female sex. ESC 2010 guidelines6 recommend oral anticoagulant (OAC) for patients with AF with a CHA2 DS2-VASc score of ≥ 2, OAC or aspirin for those with a score of 1, and either no antithrombotic therapy or aspirin for those with a score of 0. Where oral anticoagulation is prescribed, this is generally a vitamin K agonist adjusted for international normalised ratio (INR) range 2.0–3.0 (target 2.5). 6
Alternative new OACs, the oral direct thrombin inhibitors (e.g. dabigatran etexilate and AZD0837) and the oral factor Xa inhibitors [rivaroxaban (Xarelto®, Bayer), apixaban (Eliquis®, Pfizer/Bristol-Myers Squibb), edoxaban (Lixiana®, Daiichi Sankyo), betrixaban (Portola Pharmaceuticals), YM150 (Darexaban®, Astellas Pharma)] are described in the 2010 ESC guidelines6 as investigational agents that may be considered following regulatory approval, if the patient has a low risk of bleeding. 6
Dabigatran is useful as an alternative to warfarin for the prevention of stroke and systemic thromboembolism in patients with paroxysmal to permanent AF and risk factors for stroke or systemic embolisation who do not have a prosthetic heart valve or haemodynamically significant valve disease, severe renal failure (creatinine clearance of < 15 ml/minute) or advanced liver disease (impaired baseline clotting function). 48
Current service cost and anticipated costs associated with intervention
The Healthcare Resource Group (HRG) cost code RA60Z (‘Simple Echocardiogram’) was used as the cost of TTE. The mean cost of this technology was estimated as £66. A second, more expensive estimate of £425 was listed for HRG code EA45Z Complex Echocardiogram (includes congenital, transoesophageal and fetal echocardiography), which was deemed not appropriate for TTE.
It is important to note that the costs associated with the intervention (indirect costs of TTE) are likely to greatly exceed the current service cost (direct costs of TTE). This is because the associated costs include those of acting on the clinical information provided by the diagnostic test, which may include the costs of surgical intervention, as well as additional costs of long-term medication for some patients who would not otherwise have received such treatments. For example, TTE may indicate that some additional patients should receive OACs. For illustration, the annual costs of both rivaroxaban and dabigatran are both estimated to be in the region of £800, so a single year's additional treatment cost as a result of a clinical indication provided by TTE can be much greater than the cost of TTE itself.
Description of transthoracic echocardiography
Summary of intervention
Aim of transthoracic echocardiography
Transthoracic echocardiography is used to assist the diagnosis and management of a broad range of heart conditions. The commonest indications are for heart failure, murmur, palpitations/arrhythmias/blackouts and hypertension. 49
Transthoracic echocardiography
The standard adult transthoracic echocardiogram measures structure and function of the heart. It should reliably describe quantitative LV systolic and diastolic function and assess all valves, including minor abnormalities that may progress or need follow-up, basic prosthetic valve function, and common congenital abnormalities cardiomyopathies, as well as detecting the presence and significance of pericardial fluid. 50
The following cardiac and vascular structures are routinely evaluated as part of a complete adult echocardiographic report: left ventricle, mitral valve, left atrium, aortic valve, aorta, right ventricle, tricuspid valve, right atrium, pulmonary valve, pulmonary artery, pericardium, inferior vena cava and pulmonary veins. LV size is one of the most important components of LV function quantification. Changes in LV dimensions are frequently interpreted as indices of progression or regression of a disease state that affects the left heart. 51
A complete transthoracic study includes two-dimensional (2D) and, usually, M-mode (motion mode) echocardiography, as well as spectral and colour Doppler techniques. M-mode supplies additional information when indicated; it is obtained by selecting any of the individual sector lines from which a 2D image is constructed. It is useful for quantifying linear dimensions of the cardiac chambers and walls when the correct direction is verified under 2D imaging. Doppler modalities provide functional information on intracardiac flow haemodynamics, including measurement of systolic and diastolic blood flow velocities and volumes, assessment of the severity of valvular lesions, and location and severity of intracardiac shunts. Pulsed-wave Doppler is useful for locating and timing blood flow within the physiological range of velocities. Continuous wave Doppler can accurately measure the highest flow velocities and estimate the gradients across valves or interventricular defects. Colour flow mapping provides a composite picture of flow over a larger area and is most useful for screening valves for regurgitation and stenosis, and detecting the presence of intracardiac shunts. Colour flow M-mode is useful for timing blood flow information. 51
Transthoracic echocardiography provides comprehensive evaluation of cardiac and vascular structures and function, and can immediately affect the diagnostic and management work-up of the patient. It is accepted that 2D TTE can accurately assess cardiac chamber size, wall thickness, ventricular function, valvular anatomy, and the size of great vessels. Pulsed-wave, continuous wave and colour flow Doppler echocardiography provide measurements of blood flow velocities and assessment of intracardiac pressures and haemodynamics, and can detect and quantify stenosis, regurgitation and other abnormal flow states. 51
The diagnosis of heart conditions requires the integration of clinical, laboratory and echocardiographic data. The contribution of TTE in the diagnosis of heart conditions depends on the particular condition. It is particularly useful for the assessment and management of valve disease, providing good structural information about the valve and its supporting structures. Doppler provides good information about the severity of the lesion and whether the valve is repairable. The impact of valve lesion on the heart as a whole can also be assessed. 52 The usefulness of TTE in an intensive care setting has been reported by Stanko et al. 53 TTE resulted in a change of diagnosis in 29% of studies, and a change of management in 41% of studies.
Indications
According to the British Society of Echocardiography (BSE),50 TTE is indicated for the following conditions if certain circumstances (relating to seriousness) are fulfilled: heart murmurs, native valvular stenosis, native valvular regurgitation, prosthetic valve assessment, infective endocarditis, ischaemic heart disease, cardiomyopathy, pericardial disease, cardiac masses, pulmonary disease, neurological disease, arrhythmia/palpitations/syncope, echocardiography before cardioversion, hypertension, aortic and major arterial disease, and preoperative echocardiography for elective and semi-urgent surgery.
Various guidelines for the use of TTE for all indications have been reported in recent years. Details of the clinical indications for echocardiography are provided by the BSE. 50 The Bedfordshire and Hertfordshire Cardiac Network49 describes the effective use of TTE in adults for indications including heart murmur and palpitations, and the National Imaging Board52 provides guidance relating to a number of cardiac imaging modalities, including echocardiography.
Technical difficulties
Some patients give poor images and the information derived using TTE from these patients can therefore be limited. Furthermore, the accuracy of TTE depends on the experience of the person reporting the images. 52 The risks associated with TTE are extremely low.
Setting and equipment required
Transthoracic echocardiography is a non-invasive imaging technique performed with the use of an ultrasound machine. It provides real-time images, is portable and of low cost. 51 It is usually performed in cardiology clinics and is less used in primary or non-specialist secondary care, and may be undertaken by a cardiologist, BSE-accredited echocardiographer or general practitioner (GP) with special interests. 1
Current usage in the NHS
A prospective survey of the management of AF in the ESC member countries, conducted in 2005, showed that 78% (n = 757) of patients with first-detected AF had been given a transthoracic echocardiogram. 54
Criteria for use
Patients currently meeting the criteria for recommended TTE in the NICE guidelines1 for AF comprise:
-
younger patients for whom a baseline echocardiogram is important for long-term management
-
patients for whom cardioversion (electrical or pharmacological) is being considered
-
patients in whom there is a high risk or a suspicion of underlying structural/functional heart disease (such as heart failure or heart murmur) that influences their subsequent management
-
patients in need of clinical risk stratification for antithrombotic therapy, where clinical evidence is needed of LV dysfunction or valve disease.
Guidelines from NICE1 state that TTE is not recommended for patients with AF for whom the need to initiate anticoagulation therapy has already been decided on clinical criteria.
Factors associated with successful screening programmes
Many of the issues facing routine testing of a specific patient group are issues shared by screening programmes, especially the impact of false-positives (FPs) and false-negatives (FNs). The UK National Screening Committee have set criteria for effective screening programmes. 55 These are as follows.
-
The condition being screened for should be an important health condition, with adequate clinical and epidemiological understanding, and, where possible, primary prevention interventions should be in place. 55 In addition, the health condition should have a detectable risk factor, disease marker, latent period or early symptomatic stage. 55
-
The diagnostic tool for the health condition should be validated, safe and acceptable to those being screened, with an agreed cut-off level defined to diagnose the health condition. 55 Policies should be in place for further diagnoses and patient choices in the event of a positive diagnosis. 55
-
The health condition should have an effective treatment available, for which early treatment is more advantageous than treatment if the health condition is not diagnosed until a later stage. 55 Appropriate treatment should be widely available. 55
-
The screening programme should be evidence based, clinically and socially acceptable, cost-effective, adequately resourced and should be monitored. 55 Informed consent should be obtained from all participants. Any potential adverse effects from the diagnostic test or subsequent treatment should be outweighed by the benefits of the screening programme. 55
-
False-positives can lead to unnecessary anxiety for the participant. It may lead to further diagnostic tests, some of which may be unpleasant for the participant. If unchecked, subsequent change in treatment may result in adverse effects. From a health provider's perspective, these are associated with costs of unnecessary diagnostic tests and/or treatment provision.
-
False-negatives may lead to false reassurance, diagnostic delay and subsequent treatment delay. 56 These may adversely affect the participant, including psychologically. 56 From a health provider's perspective this may be damaging by reducing public confidence in the screening programme or may result in legal action. 56
Chapter 2 Definition of the decision problem
Decision problem
The purpose of the assessment was to address the question ‘What is the clinical effectiveness and cost-effectiveness of performing a routine echocardiogram in all newly diagnosed patients with AF in preventing complications arising from AF, in comparison with current practice of selective testing?’
The population was newly diagnosed patients with AF.
Potential subgroups identified prior to the review were those patients in whom AF was diagnosed when they presented with associated medical conditions (heart failure, stroke or thromboembolism), as opposed to patients in whom AF was the primary diagnosis, whether asymptomatic or based on symptoms not requiring hospital visit, or patients receiving diagnoses of paroxysmal, persistent or permanent AF. Lack of data made analyses of these subgroups impractical.
The technology investigated was TTE.
Conventional TTE was the intervention. Included modes were M-mode, 2D/cross-sectional and the Doppler modes (colour flow mapping, continuous wave, pulsed wave).
Complex or invasive modes of TTE were excluded, such as stress/exercise echocardiography, contrast echocardiography, three-dimensional echocardiography and intraoperative echocardiography. These would not form the routine TTE. Invasive modes, such as contrast TTE requiring application of dobutamine or adenosine, would have a different impact on patients and may have adverse effects, unlike routine TTE, as well as differences in time taken and cost.
We excluded diagnostic assessments that used a combination of tests including TTE.
The intervention was defined as TTE in all newly diagnosed patients with AF. This included patients for whom TTE is not currently recommended, such as patients with AF for whom the need to initiate anticoagulation therapy has already been decided on clinical criteria.
The comparator was current practice, that is only selected subgroups of patients with AF undergoing TTE. These comprise:
-
younger patients for whom a baseline echocardiogram is important for long-term management
-
patients for whom cardioversion (electrical or pharmacological) is being considered
-
patients in whom there is a high risk or a suspicion of underlying structural/functional heart disease (such as heart failure or heart murmur) that influences their subsequent management
-
patients in need of clinical risk stratification for antithrombotic therapy.
The decision problem was essentially reduced to the cost-effectiveness of TTE in those patients where there would initially be a decision not to provide anticoagulation treatment (that is those patients with a CHADS2 or CHA2 DS2-VASC score of 0). In such a group the use of TTE could detect underlying conditions that are associated with a high risk of stroke and for which the use of anticoagulant treatment would be recommended.
Outcomes sought related to selected pathologies in patients with AF identifiable by TTE. Clinical outcome measures were diagnostic accuracy of TTE in identifying pathologies as measured in terms of sensitivity [proportion of true-positives (TPs)] and specificity [proportion of true-negatives (TNs)] and prognosis of AF populations based on diagnosis of pathology by TTE, and prevalence of these pathologies in AF.
Overall aims and objectives of assessment
The objectives of the review were to:
-
investigate, by systematic review, the diagnostic accuracy of TTE for clinically important pathologies in AF
-
investigate, by systematic review, the prevalence of these pathologies
-
estimate the potential benefits and harms due to altered treatment based on results of TTE
-
estimate the incremental cost-effectiveness of routine TTE for newly diagnosed compared with current practice of TTE in selected patients with AF.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
The purpose of the assessment report was to assess the effectiveness of performing routine echocardiography in all patients with newly diagnosed AF in enabling appropriate treatment for patients based on diagnoses of pathologies from TTE. As no clinical studies screening patients with AF with TTE were identified, the review question was broken down. Critical to the effectiveness of routine screening are the diagnostic accuracy of TTE and the prevalence within the AF population for the pathologies tested. Two systematic reviews were conducted to investigate clinical effectiveness of routine TTE in patients with newly diagnosed AF. These were reviews of:
-
diagnostic accuracy of TTE for clinically relevant pathologies in patients with AF (see Methods for diagnostic accuracy review, below)
-
prevalence of clinically relevant pathologies within the AF population (see Methods for reviewing prevalence of clinically relevant pathologies in atrial fibrillation patients, below).
Clinically relevant pathologies
In order for routine TTE screening of newly diagnosed patients with AF to be successful, the screening would need to identify pathologies which would not usually be identified by the time of AF diagnosis, and which would result in a change in clinical management. These were not restricted to pathologies affecting decisions about anticoagulation. Factors affecting routine screening programmes are reported in Chapter 1 (see Factors associated with successful screening programmes).
Pathologies were selected according to the following inclusion/exclusion criteria.
Inclusion:
-
The pathology could occur in patients with AF.
-
The pathology is detectable by TTE.
-
A positive diagnosis would lead to a change in clinical management.
Exclusion:
-
The pathology would necessarily be diagnosed prior to AF diagnosis (e.g. congenital abnormalities that would have been diagnosed in infancy) or at the time of AF diagnosis (i.e. would be diagnosed by ECG).
-
The pathology would necessarily be clinically diagnosed without echocardiography.
-
The pathology presents with symptoms that represent indications for which a patient would receive TTE regardless of AF diagnosis, including indications for emergency TTE.
Based on the above inclusion and exclusion criteria, the following pathologies were selected, and for ease of reporting were grouped into the following categories.
-
Structural heart defects This category comprised atrial septal defect, ventricular septal defect and rupture of the chordae tendineae or papillary muscle.
-
Ischaemia or thrombosis This category comprised atrial and ventricular thrombosis, atherosclerotic heart disease and aneurysm of the heart.
-
Pulmonary disease This category comprised PE and hypertension, and cor pulmonale.
-
Endocarditis This category comprised infective and non-infective endocarditis.
-
Valvular heart disease This category comprised valvular regurgitation/incompetence/insufficiency or stenosis of one or more of the mitral, aortic, tricuspid or pulmonary valves.
-
Cardiomyopathy This category comprised hypertrophic obstructive or non-obstructive or dilated cardiomyopathies, and included LV non-compaction.
-
Heart failure This category comprised CHF, LV dysfunction or impairment, LA enlargement and RV dysfunction.
-
Diseases of arteries This category comprised aortic dissection.
-
Cardiac masses This category comprised cardiac tumours or masses.
Examples of excluded pathologies are given in Appendix 1.
For some, but not all, of the selected pathologies, TTE/TOE are considered the gold standard for diagnosis (see Appendix 2).
Methods for diagnostic accuracy review
Identification of studies
A comprehensive search was undertaken to systematically identify studies assessing the diagnostic accuracy of TTE for the clinically relevant pathologies as described above (see Clinically relevant pathologies).
The search strategy comprised the following main elements: searching of an electronic database; contact with experts in the field; and scrutiny of bibliographies of retrieved papers. Owing to the large number of references identified by the search, the search was restricted to MEDLINE. The MEDLINE search strategy is presented in Appendix 3.
Literature searches were conducted from March to August 2010. References were collected in a database and duplicates removed.
Inclusion and exclusion criteria
Inclusion
Population
Studies of patients with AF were selected. Where studies of patients with AF were not available for a selected pathology, diagnostic accuracy studies were sought from other adult populations with suspected cardiac conditions. Only populations with AF were considered for prognostic studies.
Intervention
Conventional TTE was the intervention. Included modes were M-mode, 2D/cross-sectional and the Doppler modes (colour flow mapping, continuous wave, pulsed wave).
Comparators
Included comparators were diagnostic techniques appropriate for the selected pathology: autopsy, surgery, cardiac catheterisation, TOE, computerised tomography (CT), magnetic resonance imaging (MRI).
Outcomes
Included outcomes were the diagnostic accuracy of TTE for each pathology in terms of sensitivity (proportion of TPs) or specificity (proportion of TNs). Studies were accepted if they reported sensitivity or specificity, or if they provided sufficient data to calculate sensitivity or specificity. Sensitivity is calculated as the number of TPs divided by the sum of TPs and FNs. Specificity is calculated as the number of TNs divided by the sum of TNs and FPs.
Prognostic accuracy was also included (i.e. TTE diagnosis of pathology predicting later cardiovascular events or mortality in AF populations).
Study types
Diagnostic accuracy studies using TTE to diagnose any of the selected pathologies (see Clinically relevant pathologies, above) were sought.
For each pathology, we initially sought studies of diagnostic accuracy with a population of patients with AF. Where sensitivity or specificity data were lacking from studies of AF populations for a particular pathology, studies of populations with other suspected cardiac conditions were sought. Study types were accepted into the review according to the hierarchy of evidence published by Merlin et al. 57 For this, level 1 evidence is considered to be systematic reviews of level 2 evidence, with level 2 being diagnostic test accuracy studies with an independent, blinded comparator of a valid reference standard, tested on consecutive patients. Level 3 includes comparative studies with either non-consecutive patients, a comparator that has not been validated or is not blinded, or a case–control design. Level 4 refers to studies of diagnostic yield that do not compare with a reference standard. For studies of AF patients, study types of any of the four levels were included.
Prognostic accuracy studies were sought. For these, studies with a population of AF patients were sought. Study types of any of the four levels of prognostic accuracy study types according to the hierarchy of evidence published by Merlin et al. 57 were included. For this, level 1 evidence is considered to be systematic reviews of level 2 evidence, with level 2 being prospective cohort studies, level 3 being all-or-none studies, prognostic data from one arm of a controlled trial, or a retrospective cohort study. Level 4 refers to case series, or cohort studies with populations at different stages of disease.
Exclusion
Population
Infants and children were excluded. AF is very rare in infants and children unless concomitant structural or congenital heart disease is present. 1 Any AF presentation in an infant or child would lead to further investigations.
Intervention
Diagnostic assessments that used a combination of tests including TTE were excluded, when data were not available for TTE alone. Invasive or complex modes of TTE were excluded. These comprised stress/exercise echocardiography, contrast echocardiography, three-dimensional echocardiography, intraoperative echocardiography, or handheld echocardiography devices. TOE was excluded.
Study types
Studies looking solely at defining severity of previously confirmed diagnosed conditions, treatment studies (such as the use of echocardiography to assess effects of surgery) and animal studies.
The following publication types were excluded: studies only published in languages other than English, reports published as meeting abstracts only where insufficient details were reported, editorials and opinion pieces.
Study selection was made by one reviewer based on the above inclusion/exclusion criteria, and discussed with a second reviewer where needed.
Data abstraction, critical appraisal strategy and synthesis
Data were extracted by one reviewer using a standardised data extraction form and checked by another reviewer. Discrepancies were resolved by discussion. Where needed, sensitivity was calculated as the number of TPs divided by the sum of the number of TPs and the number of FNs. Specificity was calculated as the number of TNs divided by the sum of the number of TNs and the number of FPs. Where possible, confidence intervals (CIs) were calculated based on the Gaussian formula from Newcombe:58
Quality assessment involved assessing the study type according to the hierarchy of Merlin et al. 57 This takes into account whether studies of test accuracy use consecutive patients, and whether assessors are blinded to other test results (see study types in Inclusion and exclusion criteria, above).
Further quality assessment was based on QUADAS (quality assessment of studies of diagnostic accuracy included in systematic reviews) criteria. 59
Data extraction forms are in Appendix 4. Quality assessment forms are in Appendix 5.
Data were tabulated and discussed in a narrative review.
Methods for reviewing prevalence of clinically relevant pathologies in atrial fibrillation patients
Identification of studies
A comprehensive search was undertaken to systematically identify clinical effectiveness literature concerning the prevalence of clinically important pathologies in patients with AF. To obtain the best estimates, the search was restricted to studies with the objective of assessing prevalence.
The search strategy comprised the following main elements: searching of electronic databases; contact with experts in the field; and scrutiny of bibliographies of retrieved papers.
The following databases were searched from inception: MEDLINE; MEDLINE in Process (for latest publications); EMBASE; The Cochrane Library, including the Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register (CCTR), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) databases; NIHR Clinical Research Network Portfolio database; National Research Register (NRR) Archive; Web of Science (WoS) Conference Proceedings; Current Controlled Trials (CCT); ClinicalTrials.gov. Searches were not restricted by date or publication type.
The MEDLINE search strategy is presented in Appendix 3.
Literature searches were conducted from March to August 2010. References were collected in a database, and duplicates removed.
Inclusion and exclusion criteria
Inclusion
Population
Adult patients diagnosed with AF. Diagnosis of AF may be confirmed by ECG, which may be standard ECG, 24-hour ambulatory ECG or event recorder ECG.
Study types
Epidemiological studies of prevalence of selected pathologies (see Clinically relevant pathologies, above) were sought.
Outcome
Prevalence of selected pathologies (see Clinically relevant pathologies, above).
Exclusion
The following publication types were excluded: animal studies, editorials, opinion pieces, studies only published in languages other than English, and reports published as meeting abstracts only if insufficient details were reported.
Study selection was made by one reviewer based on the above inclusion/exclusion criteria, and checked with a second reviewer where needed.
Data abstraction, critical appraisal and synthesis
Data were extracted by one reviewer using a standardised data extraction form and checked by another reviewer. Discrepancies were resolved by discussion.
Quality assessment, for studies with the intended outcome of prevalence of a pathology, was based on criteria identified in the STROBE statement (Strengthening the Reporting of Observational Studies in Epidemiology). 60
Data extraction forms are provided in Appendix 6. Quality assessment forms are in Appendix 7.
Data from studies designed to detect the prevalence of a particular pathology were tabulated. Owing to heterogeneity of populations, pathologies and comparators, data synthesis was precluded. These data were discussed in a narrative review.
Results
Diagnostic accuracy of transthoracic echocardiography for clinically relevant pathologies
Quantity and quality of research available
The literature search yielded 15,824 article citations when duplicates had been removed. Figure 1 shows study selection, in a modified version of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram. 61 Citations presenting purely economic analyses were not included in this chapter. References excluded at the full paper screening stage (n = 38), with reason for exclusion, are presented in Appendix 8.
FIGURE 1.
Study selection for diagnostic review.
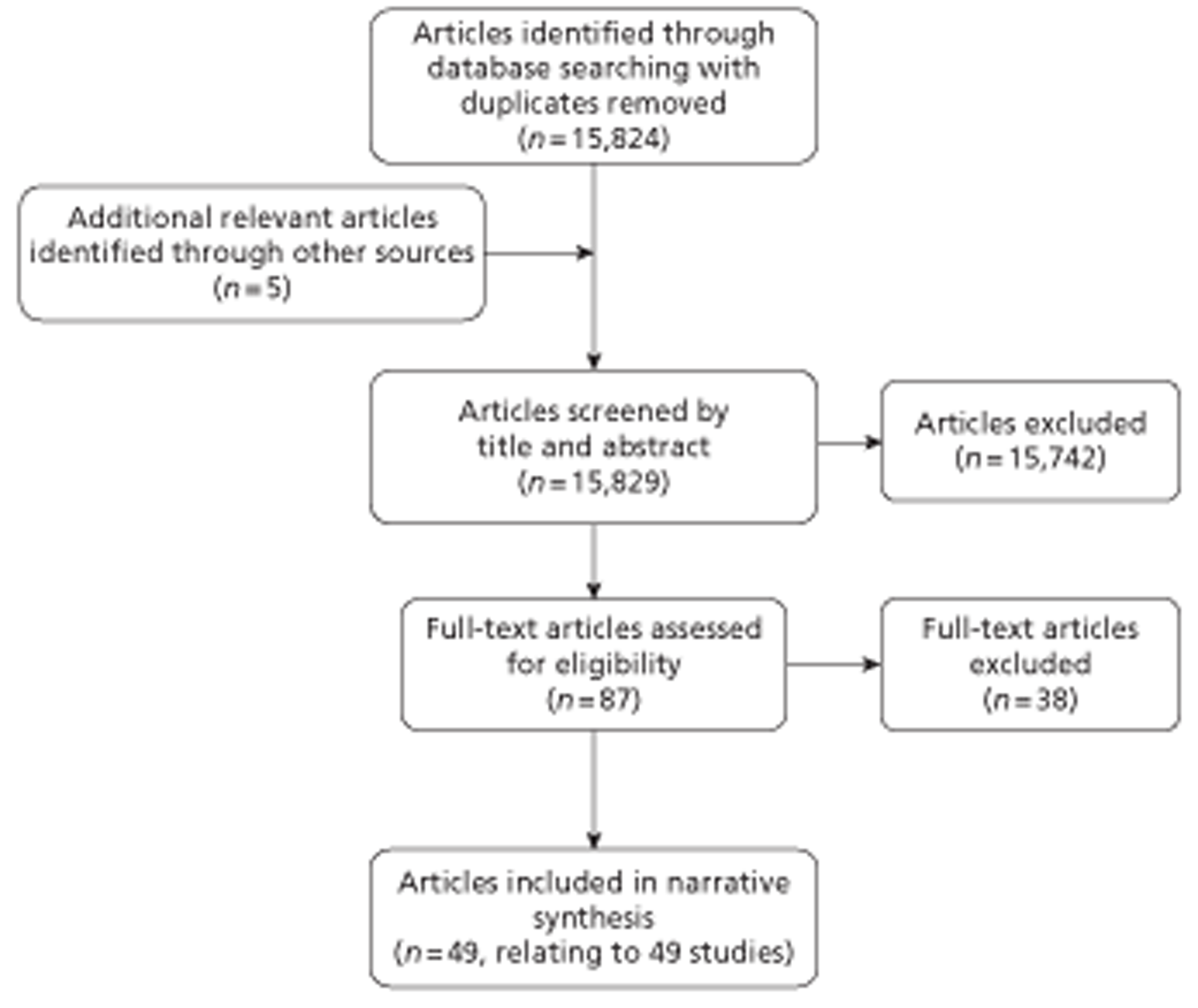
There were 44 diagnostic accuracy studies62–105 and five prognostic studies106–110 accepted into the review.
A summary of included diagnostic accuracy studies is presented in Table 1 and a summary of included prognostic studies is presented in Table 2.
| Study | Category of pathology | No. of participants enrolled in study | Population AF | Type of TTE | Percentage usable TTE images |
|---|---|---|---|---|---|
| Acar et al. 199162 | Ischaemia/thrombosis | 581 | 44.9% AF | 2D TTE | 100 |
| Arques et al. 200563 | Heart failure | 40 | 0% | TTE colour M-mode Doppler | 98 |
| Attenhofer Jost et al. 200064 | Structural defect and valvular heart disease | 100 | NR (all had heart murmur) | TTE 2D and continuous wave Doppler | 100 |
| Barron et al. 198865 | Valvular heart disease | 140 | NR | 2D and Doppler TTE | 100 |
| Bova et al. 200366 | Pulmonary disease | 162 | NR | TTE continuous wave Doppler | 97 |
| Casella et al. 200967 | Endocarditis | 75 | NR | Harmonic imaging TTE | 100 (81.5% good image quality) |
| Cassidy et al. 199268 | Valvular heart disease | 41 | NR (systolic murmur 100%) | TTE, M-mode, 2D and Doppler | 91 |
| Dittmann et al. 198769 | Valvular heart disease | 55 | 38% AF | M-mode, pulsed Doppler TTE | 100 |
| Enia et al. 198970 | Disease of arteries | 555 | NR | TTE | 100 |
| Erbel et al. 198471 | Heart failure | 110 | 0% | 2D echocardiography | 100 |
| Grossmann et al. 200272 | Valvular heart disease | 68 | 25% AF | Colour Doppler TTE | 100 |
| Groves et al. 200473 | Valvular heart disease | 61 | NR | TTE | 100 (selected for having usable data) |
| Guyer et al. 198474 | Valvular heart disease | 38 | 82% AF | 2D TTE | 100 (selected for having usable data) |
| Helmcke et al. 198775 | Valvular heart disease | 160 | 21% AF | Colour Doppler echocardiography | 92 |
| Jassal et al. 200776 | Endocarditis | 36 | NR | Harmonic imaging TTE | 100 (17% indeterminate diagnosis but included in analysis) |
| Kaymaz et al. 200177 | Ischaemia/thrombosis | 474 | 56.3% AF at time of study | TTE | 100 |
| Kishon et al. 199378 | Structural defect | 40 | NR (new systolic murmur in 68%) | 2D TTE, Doppler colour TTE | 100 (15% of VSD images suboptimal, but included in analysis) |
| Kitayama et al. 199779 | Ischaemia/thrombosis | 70 | 100% CAF | TTE M-mode, 2D and pulsed and colour Doppler | 100 (10% technically inadequate but included in analysis) |
| Lanzarini et al. 200580 | Pulmonary disease | 86 | 13% controlled AF | TTE standard M-mode, 2D and pulsed and continuous wave Doppler | 100 |
| Maestre et al. 200981 | Heart failure | 216 | NR | M-mode and 2D TTE | 100 |
| Mugge et al. 199582 | Ischaemia/thrombosis | 195 | 14.4% in AF | Colour Doppler TTE | 100 (patients selected from group with usable TTE) |
| Nienaber et al. 199383 | Disease of arteries | 110 | NR | Colour, Doppler TTE | 100 |
| Nienaber et al. 199484 | Disease of arteries | 35 | NR | M-mode, 2D, Doppler TTE | 100 |
| Okura et al. 200685 | Cardiomyopathy | 52 | NR | 2D and Doppler TTE | 85 |
| Pochis et al. 199286 | Structural defect | 116 | 53% atrial fibrillation or flutter, or paroxysmal atrial tachycardia | TTE | 92 |
| Reichek et al. 198187 | Heart failure | 34 | NR | M-mode echocardiography | 100 |
| Reichlin et al. 200488 | Valvular heart disease | 203 | NR (all had heart murmur) | Two-colour Doppler TTE (gold standard comparator) | 100 |
| Roudat et al. 198889 | Disease of arteries | 673 | NR | TTE 2D, M-mode | 98 |
| Saraste et al. 200590 | Ischaemia/thrombosis | 84 | 4% CAF | Doppler TTE, colour and 2D | 100 |
| Sharifi et al. 200391 | Ischaemia/thrombosis | 112 | 100% AF (24% CAF) | TTE | 100 (patients selected from group with usable TTE) |
| Sharma et al. 199292 | Structural defect | 53 | NR | TTE M-mode (pulsed and continuous wave Doppler and colour flow available only for some patients) | 85 |
| Sheiban et al. 198793 | Cardiac masses | 77 | NR | 2D TTE | 100 |
| Shively et al. 199194 | Endocarditis | 62 | NR | TTE 2D, M-mode and Doppler colour | 100 (at least 68% good quality) |
| Shrestha et al. 198395 | Ischaemia/thrombosis | 293 | 88% patients with thrombus had AF; NR whole population | 2D TTE | 100 |
| Shub et al. 198396 | Structural defect | 171 | NR | TTE 2D, pulsed Doppler | 95 |
| Shyu et al. 199297 | Structural defect | 60 | 77% AF | 2D and colour TTE | 100 |
| Smith et al. 198598 | Structural defect | 12 | NR (all post AMI) | Cross-sectional Doppler echocardiography | 100 |
| Sparrow et al. 200399 | Heart failure | 737 | NR | TTE | 87 |
| Stratton et al. 1982100 | Ischaemia/thrombosis | 88 | Some AF, per cent NR | 2D TTE | 89 |
| Veyrat et al. 1983101 | Valvular heart disease | 95 | 40% AF | Pulsed Doppler echocardiography | 100 |
| Vigna et al. 1993102 | Ischaemia/thrombosis | 59 | 59% in AF at time of study | TTE colour Doppler | 100 |
| Wong et al. 1983103 | Valvular heart disease | 113 | NR | 2D echocardiography | 100 |
| Zanolla et al. 1982104 | Valvular heart disease | 43 | NR | 2D echocardiography | 100 |
| Zotz et al. 1993105 | Structural defect | 17 (16 for colour Doppler) | NR (all post AMI) | Colour Doppler TTE | 100 |
| Study | Category of pathology | No. of participants | Population AF | Prospective or retrospective | Follow-up | Type of TTE |
|---|---|---|---|---|---|---|
| Atrial Fibrillation Investigators 1998106 | Heart failure and valvular heart disease | 1010 | Non-valvular AF | Prospective | Mean 1.6 years | TTE 2D, M-mode |
| Klem et al. 2003107 | Heart failure and valvular heart disease | 409 | Non-rheumatic AF | Prospective | Mean 9.6 years | TTE |
| Miyaska et al. 2000108 | Valvular heart disease | 173 | Non-rheumatic AF | Retrospective | NA | TTE 2D, M-mode |
| Nakagami et al. 1998109 | Heart failure and valvular heart disease | 290 | Non-rheumatic AF | Retrospective | Mean 7.4 years | TTE M-mode, 2D and colour Doppler |
| The Stroke Prevention in Atrial Fibrillation Investigators 1992110 | Heart failure and valvular heart disease | 568 | Non-rheumatic AF | Prospective | Mean 1.3 years | M-mode and 2D and Doppler |
Of the 44 included studies,62–105 there were 17 studies62,69,72,74,75,77,79,80,82,86,90,91,95,97,100–102 that included AF patients in the population. Of these, for two studies79,91 all participants had AF. Although two studies63,71 stated that there were no patients with AF in the population, in other studies it was not reported.
For all categories of pathologies sought, studies of diagnostic accuracy were identified. AF population studies were available for the categories of structural defect, ischaemia/thrombosis, pulmonary disease and valvular heart disease.
Methods of TTE represented were 2D, M-mode, pulsed and continuous wave Doppler, and colour Doppler. All studies had a high percentage of usable, good images from TTE.
All five prognostic accuracy studies included106–110 had a population of non-valvular/non-rheumatic AF. Of the categories of pathologies sought, only heart failure and valvular heart disease were represented. Three of the studies were prospective studies106,107,110 with follow-up ranging from mean 1.3 to 9.6 years. Two of the studies108,109 were retrospective. The TTE methods represented were 2D, M-mode and colour Doppler.
Quality of included studies
Quality assessment forms are in Appendix 5. According to the level of hierarchy proposed by Merlin et al. , 57 studies ranged from level 2 (higher quality) to level 3c (lower quality). Twelve studies were of level 2,64–67,76,80,81,83,88,91,94,102 a study of test accuracy with an independent, blinded comparison with a reference standard among consecutive patients. Six of the studies were level 3a,68,73,74,84,99,103 i.e. they differed from level 2 only in being among non-consecutive patients. Twenty-two of the studies were level 3b,62,69,71,72,77–79,82,85–87,89,90,92,93,95,96,98,100,101,104,105 comparisons with a reference standard that did not meet criteria for higher levels of evidence. There were four diagnostic case–control studies, level 3c. 63,70,75,97
Considering only diagnostic accuracy studies with AF populations, there were three level 2 studies,80,91,102 one level 3a study,74 11 level 3b studies62,69,72,77,79,82,86,90,95,100,101 and two level 3c studies. 75,97 As all AF population studies were included, and non-AF population studies selected according to hierarchy of evidence, this explains the higher proportion of level 2 and 3a studies with non-AF populations.
For the prognostic studies, one study was level 2,107 a prospective cohort study; two studies were level 3b;106,110 and two studies were level 3c,108,109 retrospective cohort studies.
Selected items from QUADAS were also addressed (see Appendix 5). We did not ask about representativeness of patients in the study for participants receiving the test in practice, as this review is concerned with screening patients with AF, and so an AF population, although relevant to this review, would not necessarily reflect quality of the diagnostic studies. All included diagnostic studies were of high quality in terms of all patients receiving TTE and a reference standard, and the reference standard being administered whatever the TTE results, and the reference standard being independent of TTE. More than half of the studies were blinded.
Some studies selected participants on the basis of having usable TTE images, and some excluded indeterminate images from the analysis of sensitivity or specificity, whereas six studies explicitly included either poorer images in analysis78,92,96–98,105 or provided separate analyses by the inclusion or exclusion of poor-image-quality TTE. 67
Diagnostic accuracy results
Eight studies64,78,86,92,96–98,105 reported diagnostic accuracy of TTE in structural defects (Table 3). TTE was presumed the gold standard for one study of ventricular septal defect. 64 Sensitivity ranged from 0.25 for atrial septal hypertrophy86 to 1 for ostium primum atrial septal defect96 or ventricular septal rupture. 98 Two studies86,97 reported specificity, which ranged from 0.9 for rupture of chordae tendineae97 to 0.909 for atrial septal hypertrophy. 86 Six78,92,96–98,105 of the eight studies used catheterisation, surgery or autopsy as the comparator diagnostic test, whereas one used clinical cardiac examination,64 and one used TOE86 (see Table 3).
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Attenhofer Jost et al. 200064 | Ventricular septal defect | 100 | NR (heart murmur 100%) | TTE | Clinical cardiac examination | TTE as gold standard (presumed sensitivity = 1) | TTE as gold standard (presumed specificity = 1) |
| Kishon et al. 199378 | Ventricular septal defect | 40 | NR (new systolic murmur in 68%) | TTE | Surgery or autopsy | 0.68 (95% CI 0.53 to 0.82) (if include suspected by TTE then 0.775) | NC |
| Rupture of papillary muscle | 22 | NR (new systolic murmur in 100%) | TTE | Surgery or autopsy | 0.46 (95% CI 0.25 to 0.66) (0.727 if include suspected by TTE) | NC | |
| Pochis et al. 199286 | Atrial septal hypertrophy | 107 | 53% atrial fibrillation or flutter, or paroxysmal atrial tachycardia | TTE | TOE | 0.25 (95% CI 0.17 to 0.33) | 0.91 (95% CI 0.85 to 0.96) |
| Sharma et al. 199292 | Atrial septal defect, sinus venosus defect | 45 | NR | TTE | Catheterisation | 0.62 (95% CI 0.48 to 0.76) | NC |
| Shub et al. 198396 | Atrial septal defect, ostium secundum | 105 | NR | TTE | Catheterisation or surgery | 0.89 (95% CI 0.82 to 0.95) | NC |
| Atrial septal defect, ostium primum | 32 | NR | TTE | Catheterisation or surgery | 1 | NC | |
| Atrial septal defect, sinus venosus | 16 | NR | TTE | Catheterisation or surgery | 0.434 (95% CI 0.19 to 0.68) | NC | |
| Shyu et al. 199297 | Rupture of chordae tendineae | 60 | 77% AF | TTE | Catheterisation or (valve repair) surgery | 0.65 (95% CI 0.53 to 0.77) | 0.9 (95% CI 0.82 to 0.98) |
| Smith et al. 198598 | Ventricular septal rupture | 12 | NR (all post AMI) | TTE | Catheterisation or autopsy | 1 | NC |
| Zotz et al. 1993105 | Ventricular septal rupture | 17 (16 for colour Doppler) | NR (all post AMI) | TTE | Surgery or autopsy | 0.71 (95% CI 0.49 to 0.92) (if TTE using only conventional view 0.235); by colour Doppler 0.938 | NC |
Nine studies62,77,79,82,90,91,95,100,102 reported diagnostic accuracy of TTE in ischaemic heart disease (Table 4). Sensitivity ranged from 0 for right atrial appendage (RAA)79 or left atrial appendage (LAA)102 thrombus to 0.955 for thrombosis of ventricle. 100 Specificity ranged from 0.857 for thrombosis of ventricle100 to 1 for LA79 or right atrial (RA)79 thrombus. Five of the studies62,77,90,95,100 used surgery or angiography, three used TOE82,91,102 and one used CT79 as comparators (see Table 4).
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Acar et al. 199162 | LA thrombus | 581 | 44.9% AF | TTE | Surgery | 0.28 (95% CI 0.24 to 0.32) (LA body 0.65, LAA 0.04) | 0.99 (95% CI 0.99 to 1.0) |
| Kaymaz et al. 200177 | LA thrombus | 474 | 56.3% AF at time of study | TTE | Surgery | 0.32 (95% CI 0.28 to 0.37) | 0.94 (95% CI 0.91 to 0.96) |
| Kitayama et al. 199779 | LA thrombus | 70 | 100% CAF | TTE | CT | 0.67 (95% CI 0.55 to 0.78) | 1 |
| RA thrombus | 70 | 100% CAF | TTE | CT | 0 | 1 | |
| Mugge et al. 199582 | Atrial septal aneurysm | 195 | 14.4% in AF | TTE | TOE | 0.47 (95% CI 0.41 to 0.53) | NC |
| Saraste et al. 200590 | Coronary artery stenosis (significant stenosis/occlusion in any coronary artery) | 84 | 4% CAF | TTE | Angiography | 0.82 (95% CI 0.74 to 0.90) | 0.92 (95% CI 0.86 to 0.98) |
| Sharifi et al. 200391 | Atrial thrombi | 112 | 100% AF (24% CAF) | TTE | TOE | 0.17 (95% CI 0.09 to 0.24) (if includes SEC as well as thrombus 0.714) | 1 (if includes SEC as well as thrombus 1) |
| Shrestha et al. 198395 | LA thrombus | 293 | NR whole population, 88% patients with thrombus | TTE | Surgery | 0.59 (95% CI 0.53 to 0.64) (LA body 0.75, LAA 0.00) | 0.99 (95% CI 0.97 to 1.0) |
| Stratton et al. 1982100 | Thrombosis of ventricle | 78 | Some AF, per cent NR | TTE | Surgery or indium-111 platelet imaging | 0.86 (95% CI 0.79 to 0.94) (0.955 if includes equivocal diagnoses) | 0.95 (95% CI 0.90 to 0.99) (0.857 if includes equivocal diagnoses) |
| Vigna et al. 1993102 | LA thrombus | 59 | 59% in AF at time of study | TTE | TOE | 0.33 (95% CI 0.21 to 0.45) (LA body 0.44, LAA 0.00) | 1 |
Two studies66,80 reported diagnostic accuracy of TTE in pulmonary disease (Table 5). Sensitivity ranged from 0.523 for PE66 to 1 for pulmonary hypertension. 80 Specificity ranged from 0.6 to 1 for pulmonary hypertension. 80 The study of PE66 used perfusion lung scan with radiography or pulmonary angiography as a comparator, whereas the study of pulmonary hypertension80 used catheterisation (see Table 5).
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Bova et al. 200366 | PE | 152 | NR | TTE | Perfusion lung scan with radiography, or pulmonary angiography | 0.52 (95% CI 0.44 to 0.60) | 0.87 (95% CI 0.82 to 0.93) |
| Lanzarini et al. 200580 | Pulmonary hypertension | 86 | 13% controlled AF | TTE | Catheterisation | 1 using PAPd/TR; 0.88 using PAPs | 0.6 using PAPd/TR; 1 using PAPs |
Three studies67,76,94 reported diagnostic accuracy of TTE in endocarditis (Table 6). Sensitivity ranged from 0.4494 to 0.871;67 specificity ranged from 0.61567 to 0.98. 94 Two of the studies used TOE as a comparator,67,76 whereas the other study94 used information obtained from clinical follow-up (see Table 6).
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Casella et al. 200967 | Native valve infective endocarditis | 75 | NR | TTE | TOE | 0.82 (95% CI 0.65 to 0.93). If indeterminate images excluded (n = 61) 0.871 (0.702 to 0.964) | 0.62 (95% CI 0.445 to 0.77). If indeterminate images excluded 0.857 (0.673 to 0.960) |
| Jassal et al. 200776 | Native valve infective endocarditis | 36 | NR | TTE | TOE | 0.84 (95% CI 0.72 to 0.96) | 0.88 (95% CI 0.77 to 0.98) |
| Shively et al. 199194 | Endocarditis | 66 episodes in 62 patients (four patients referred twice) | NR | TTE | Non-echocardiographic pathological data from the subsequent clinical course | 0.44 (95% CI 0.32 to 0.56) | 0.98 (95% CI 0.95 to 1.0) |
Twelve studies64,65,68,69,72–75,88,101,103,104 reported diagnostic accuracy of TTE in valvular heart disease (Table 7). TTE was presumed gold standard for four studies of MR,64,68 aortic stenosis,64,68 mitral valve prolapse (MVP),64 valvular heart disease,64,88 aortic regurgitation (AR)64,68 and tricuspid regurgitation. 73 Sensitivity ranged from 0.222 for mitral stenosis leaflet calcification103 to 1 for mitral stenosis104 or mitral regurgitation75 or severe AR. 69 Specificity ranged from 0.655 for mitral stenosis to 1 for AR or MR. Six of the studies used catheterisation/aortography or radiography/cinefluorography as comparators,69,74,75,101,103,104 four of the studies used clinical examination,64,65,68,88 one used TOE68 and one used CT73 (see Table 7).
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Attenhofer Jost et al. 200064 | Mitral regurgitation Aortic stenosis MVP Valvular heart disease, aortic and mitral valve AR |
100 | NR (all had heart murmur) | TTE | Clinical cardiac examination | TTE as gold standard (presumed sensitivity = 1) | TTE as gold standard (presumed specificity = 1) |
| Barron 1988 et al.65 | MVP | 140 | NR | TTE | Auscultation | 0.47 (95% CI 0.39 to 0.55) | 0.90 (95% CI 0.85 to 0.95) |
| Cassidy et al. 199268 | Mitral regurgitation AR Aortic stenosis |
37 | NR (all had systolic murmur) | TTE | Clinical cardiac examination | TTE as gold standard (presumed sensitivity = 1) | TTE as gold standard (presumed specificity = 1) |
| Dittmann et al. 198769 | AR | 55 | 38% AF | TTE | Aortography | Pulsed Doppler 0.93 (95% CI 0.86 to 0.99) (0.87 mild or moderate; 1 severe AR), M-mode 0.62 | Pulsed Doppler 1, M-mode 1 |
| Grossmann et al. 200272 | Mitral regurgitation | 68 | 25% AF | TTE | TOE | 0.79 (95% CI 0.69 to 0.89) | 1 |
| Groves et al. 200473 | Tricuspid regurgitation | 61 | NR | TTE | CT | TTE as gold standard (presumed sensitivity = 1) | TTE as gold standard (presumed specificity = 1) |
| Guyer et al. 198474 | Tricuspid stenosis | 38 | 82% AF | TTE | Catheterisation | 0.69 (95% CI 0.55 to 0.84) | 0.96 (95% CI 0.90 to 1.0) |
| Helmcke et al. 198775 | Mitral regurgitation | 147 | 21% AF | TTE | Catheterisation | 1 | 1 |
| Reichlin et al. 200488 | Valvular heart disease | 203 | NR (all had heart murmur) | TTE | Clinical cardiac examination (including auscultation) | TTE as gold standard (presumed sensitivity = 1) | TTE as gold standard (presumed specificity = 1) |
| Veyrat et al. 1983101 | AR | 95 | 40% AF | TTE | Aortography | 0.95 (95% CI 0.89 to 1.0) | 1 |
| Wong et al. 1983103 | Aortic stenosis, calcification Mitral stenosis, annulus calcification Mitral stenosis, leafet calcification |
113 | NR | TTE | Cinefluorography | 0.76 (95% CI 0.68 to 0.84) 0.77 (95% CI 0.69 to 0.84) 0.22 (95% CI 0.15 to 0.30) |
0.89 (95% CI 0.83 to 0.95) 0.94 (95% CI 0.89 to 0.98) 0.93 (95% CI 0.88 to 0.97) |
| Zanolla et al. 1982104 | Mitral stenosis | 43 | NR | TTE | Radiography | 1 | 0.66 (95% CI 0.51 to 0.80) |
One study85 reported the accuracy of TTE in differentiating between ischaemic and non-ischaemic cardiomyopathy (ICM and non-ICM) (Table 8). This study reported a sensitivity of 0.77 and specificity of 0.77 for differentiating between ICM and non-ICM with a comparator of angiography.
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Okura et al. 200685 | Cardiomyopathy, differentiating between ischaemic and non-ischaemic | 44 | NR | TTE | Angiography | 0.77 (95% CI 0.64 to 0.89) | 0.77 (95% CI 0.65 to 0.90) |
Five studies63,71,81,87,99 reported accuracy of TTE in the diagnosis of heart failure (Table 9). TTE was presumed the gold standard for two studies of CHF81 and LV dysfunction. 99 Sensitivity ranged from 0.737 for CHF63 to 0.93 for LV hypertrophy. 87 Specificity ranged from 0.75 for CHF63 to 1 for LV dysfunction. 71 Two of the studies used clinical diagnosis as comparators,81,99 two studies63,71 used radiography or catheterisation, and one used autopsy results87 (see Table 9).
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Arques et al. 200563 | CHF | 39 | 0% | TTE | Radiography and clinical signs | 0.74 (95% CI 0.60 to 0.88) | 0.75 (95% CI 0.61 to 0.89) |
| Erbel et al. 198471 | LV dysfunction, ejection fraction | 110 | 0% | TTE | Catheterisation to cineventriculograms | 0.81 (95% CI 0.73 to 0.88) | 1 |
| Maestre et al. 200981 | CHF | 216 | NR | TTE | Clinical criteria | TTE as gold standard (presumed sensitivity = 1) | TTE as gold standard (presumed specificity = 1) |
| Reichek et al. 198187 | LV hypertrophy | 34 | NR | TTE | Autopsy | 0.93 (95% CI 0.84 to 1) | 0.95 (95% CI 0.88 to 1) |
| Sparrow et al. 200399 | LV dysfunction | 621 | NR | TTE | Clinical diagnosis | TTE as gold standard (presumed sensitivity = 1) | TTE as gold standard (presumed specificity = 1) |
Four studies70,83,84,89 reported diagnostic accuracy of TTE in aortic dissection (Table 10). Sensitivity ranged from 0.59383 to 0.953. 89 Specificity ranged from 0.50889 to 0.977. 89 Three studies83,84,89 included surgery, autopsy or angiography as comparators; the other study used aortography70 (see Table 10).
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Enia et al. 198970 | Aortic dissection | 555 | NR | TTE | Aortography | 0.92 (95% CI 0.89 to 0.94) | 0.71 (95% CI 0.68 to 0.75) |
| Nienaber et al. 199484 | Aortic dissection | 35 | NR | TTE | Surgery, autopsy or angiography | 0.77 (95% CI 0.63 to 0.91) | 0.67 (95% CI 0.51 to 0.82) |
| Nienaber et al. 199383 | Aortic dissection, thoracic | 110 | NR | TTE | Surgery, autopsy or angiography | 0.59 (95% CI 0.51 to 0.69) | 0.83 (95% CI 0.76 to 0.90) |
| Roudat et al. 198889 | Aortic dissection | 660 | NR | TTE | Surgery, autopsy or angiography or CT | 0.95 (95% CI 0.94 to 0.97) using dilatation of segment of aorta (0.758 using abnormal linear image in lumen) | 0.51 (95% CI 0.47 to 0.55) using dilatation of segment of aorta (0.977 using abnormal linear image in lumen) |
One study93 reported the diagnostic accuracy of TTE for intracardiac masses (Table 11). This study reported sensitivity 0.882 and specificity 0.953, with a comparator of surgery. 93
| Study | Pathology | No. of patients analysed | Population AF | Intervention | Comparator diagnostic test | Sensitivity of TTE | Specificity of TTE |
|---|---|---|---|---|---|---|---|
| Sheiban et al. 198793 | Intracardiac masses | 77 | NR | TTE | Surgery | 0.88 (95% CI 0.81 to 0.95) | 0.95 (95% CI 0.91 to 1.0) |
Prognostic study results
Five studies106–110 reported prognosis based on TTE-diagnosed pathologies in AF populations.
The pathologies were left atrial diameter (LAD); mitral annular calcification; MVP global, moderate to severe or reduced LV systolic dysfunction; any or severe MR; and valvular abnormality (Table 12).
| Study | Pathology | No. of participants | Population AF | Follow-up | Results |
|---|---|---|---|---|---|
| Atrial Fibrillation Investigators 1998106 | Moderate to severe LV systolic dysfunction | 1010 (of whom 129 with moderate to severe LV dysfunction) | Non-valvular AF | Mean 1.6 years | Independent predictor of stroke (relative to none or mild LV dysfunction), unadjusted RR of 3.04 (p < 0.001), multivariate analysis RR of 2.5 (95% CI 1.5 to 4.4; p < 0.001) (multivariate analysis includes age, previous stroke/TIA, history of diabetes, history of heart failure, history of hypertension) |
| LAD | 1003 | Non-significant association with risk of stroke unadjusted RR of 1.02/mm (95% CI 0.99 to 1.06; p = 0.10), multivariate analysis p = 0.62 (multivariate analysis includes age, previous stroke/TIA, history of diabetes, history of heart failure, history of hypertension) | |||
| MVP | 991 (of whom 50 with MVP) | Non-significant association with risk of stroke unadjusted RR of 0.29 (p = 0.22) | |||
| Severe MR | 863 (of whom 86 with severe MR) | Non-significant association with risk of stroke (relative to none or mild MR) unadjusted RR of 1.7 (p = 0.59) | |||
| Klem et al. 2003107 | Reduced LV function | 409 (reduced LV function, of whom 31 of 73 diabetic, and 98 of 336 non-diabetic) | Non-rheumatic AF | Mean 9.6 years | HR for mortality, diabetic participants 1.52 (95% CI 0.85 to 2.70; p = 0.1598), non-diabetic participants HR of 2.28 (95% CI 1.58 to 3.29; p < 0.0001) |
| LAD | 409 (of whom 73 diabetic, 336 non-diabetic) | HR for mortality, diabetic participants 1.01 (95% CI 0.97 to 1.05; p = 0.6445), non-diabetic participants HR of 1.06 (95% CI 1.03 to 1.08; p < 0.0001) | |||
| Valvular abnormality | 409 (of whom valvular abnormality in 41 of 73 diabetic, and 136 of 336 non-diabetic) | HR for mortality, diabetic participants 2.05 (95% CI 1.10 to 3.82; p = 0.0229), non-diabetic participants HR of 1.88 (95% CI 1.30 to 2.70; p = 0.0007) | |||
| Miyaska et al. 2000108 | MR | 173 (of whom 104 no MR, 69 grade 1 MR) | Non-rheumatic AF | NA (patient records' study) | Grade 1 MR (compared with no MR) OR of 2.689 (95% CI 1.039 to 7.189; p = 0.0434) significantly associated with history of thromboembolic events |
| Nakagami et al. 1998109 | Severe MRLAD | 290 | Non-rheumatic AF | Mean 7.4 years | HR of stroke for increase in MR from mild to severe groups 0.45 (95% CI 0.20 to 0.97) (MR protective against stroke) by multivariate analysis (multivariate analysis includes MR, LAD, sex, age) |
| HR of stroke for every 10-mm increment in LA size 1.06 (95% CI 0.75 to 1.49) by multivariate analysis (multivariate analysis includes MR, LAD, sex, age) | |||||
| The Stroke Prevention in Atrial Fibrillation Investigators 1992110 | LAD | 539 | Non-rheumatic AF | Mean 1.3 years | LAD (corrected for body surface area) as a continuous variable by univariate analysis was significantly associated with thromboembolism (p = 0.01). By multivariate analysis p = 0.02 (multivariate analysis LAD and global LV diameter) |
| Mitral annular calcification | 568 (of whom 91 with mitral annular calcification) | Non-significantly associated with thromboembolism by age-adjusted analysis RR 0.6 (95% CI 0.2 to 1.5; p > 0.2) | |||
| Severe MR | 568 (of whom 37 with severe MR) | Non-significantly associated with thromboembolism by age-adjusted analysis RR 0.4 (95% CI 0.1 to 3.0; p > 0.2) | |||
| LV dysfunction (global) | 568 (of whom 132 with LV dysfunction) | By univariate analysis was significantly associated with thromboembolism (RR 2.9, 95% CI 1.6 to 5.3; p < 0.001); by multivariate analysis RR of 2.6 (95% CI 1.4 to 4.9; p = 0.003) (multivariate analysis LAD and global LV dysfunction) |
Prognosis was investigated by studies for the types of valvular heart disease, mitral annular calcification, MVP, MR and valvular abnormality. Mitral annular calcification was non-significantly associated with thromboembolism by age-adjusted analysis RR of 0.6 (95% CI 0.2 to 1.5; p > 0.2). 110 MVP had a non-significant association with risk of stroke unadjusted RR of 0.29 (p = 0.22). 106 For MR, grade 1 MR (compared with no MR) odds ratio (OR) of 2.689 (95% CI 1.039 to 7.189; p = 0.0434) was significantly associated with history of thromboembolic events. 108 Severe MR had a non-significant association with risk of stroke (relative to none or mild MR) unadjusted RR of 1.7 (p = 0.59),106 was found to be protective against stroke with hazard ratio (HR) of stroke for increase in MR from mild to severe groups 0.45 (95% CI 0.20 to 0.97) by multivariate analysis (multivariate analysis includes MR, LAD, sex and age),109 and was non-significantly associated with thromboembolism by age-adjusted analysis RR 0.4 (95% CI 0.1 to 3.0; p > 0.2). 110 For MR, the retrospective studies108,109 found significant associations with prognosis, whereas the prospective studies106,110 had non-significant results. Any detected valvular abnormalities had a reported HR for mortality: diabetic participants 2.05 (95% CI 1.10 to 3.82; p = 0.0229), non-diabetic participants HR 1.88 (95% CI 1.30 to 2.70; p = 0.0007). 107 For this study, diabetics and non-diabetic groups differed in that diabetic participants were older and had higher comorbidity, and more of them received oral anticoagulation; there was also a relatively small number of diabetics. 107
Left atrial diameter had a reported non-significant association with risk of stroke unadjusted RR of 1.02/mm (95% CI 0.99 to 1.06; p = 0.10106) and was reported to have HR of stroke for every 10-mm increment in LA size of 1.06 (95% CI 0.75 to 1.49) by multivariate analysis (multivariate analysis includes MR, LAD, sex, age). 109 LAD (corrected for body surface area) as a continuous variable by univariate analysis was significantly associated with thromboembolism (p = 0.01110), and had reported HR for mortality, diabetic participants 1.01 (95% CI 0.97 to 1.05; p = 0.6445), HR non-diabetic participants 1.06 (95% CI 1.03 to 1.08; p < 0.0001). 107 Moderate to severe LV dysfunction was associated with a significantly higher risk of stroke relative to normal LV function or mild dysfunction,106 and global LV dysfunction was significantly associated with risk of thromboembolism. 110 Reduced LV function had reported HR for mortality, diabetic participants 1.52 (95% CI 0.85 to 2.70; p = 0.1598), HR non-diabetic participants 2.28 (95% CI 1.58 to 3.29; p < 0.0001). 107
Results of prevalence review
Quantity and quality of research available
The literature search yielded 8316 article citations when duplicates had been removed. Figure 2 shows study selection, in a modified version of the PRISMA flow diagram. 61 References excluded at the full paper screening stage (n = 15), with reason for exclusion, are presented in Appendix 9.
FIGURE 2.
Study selection for prevalence review.
There were 16 prevalence studies28,111–125 accepted into the review. Some of the studies investigated the prevalence of more than one pathology.
A summary of included prevalence studies is presented in Table 13.
| Study | Category of pathology | Population sample size | Type of AF of population (where specified) |
|---|---|---|---|
| Agmon et al. 2001111 | Ischaemia/thrombosis | 42 | AF |
| Archer et al. 1995112 | Ischaemia/thrombosis | 55 | Non-rheumatic AF |
| Blackshear et al. 1999113 | Ischaemia/thrombosis | 770 | AF |
| Corrado et al. 2004114 | Ischaemia/thrombosis | 41 | AF or atrial flutter |
| Dang et al. 2004115 | Valvular heart disease | 737 | First hospitalisation associated with AF |
| de Divitiis et al. 199928 | Ischaemia/thrombosis | 90 | AF |
| Heppell et al. 1997116 | Ischaemia/thrombosis | 109 | AF |
| Kleeman et al. 2009117 | Ischaemia/thrombosis | 295 | Non-valvular AF |
| Levy et al. 1999118 | Ischaemia/thrombosis and valvular heart disease, and cardiomyopathy and heart failure | 756 | Chronic, paroxysmal or recent-onset AF |
| Lip et al. 1997119 | Structural defect and ischaemia/thrombosis, and valvular heart disease and cardiomyopathy | 111 | AF |
| Maltagliati et al. 2006120 | Ischaemia/thrombosis | 757 | AF or atrial flutter |
| Narumiya et al. 2003121 | Ischaemia/thrombosis | 50 | Lone AF (28%) or non-lone AF (72%) |
| Santiago et al. 1994122 | Ischaemia/thrombosis and valvular heart disease | 30 | AF |
| Scherr et al. 2009123 | Ischaemia/thrombosis | 732 catheter ablations for AF in 585 patients | AF (catheter ablations) |
| Shen et al. 2002124 | Ischaemia/thrombosis | 182 | AF and subtherapeutic INR |
| Tsai et al. 1997125 | Ischaemia/thrombosis | 219 | Chronic non-rheumatic AF |
Prevalence studies were found for the following categories of pathologies: structural defect, ischaemia/thrombosis, valvular heart disease, cardiomyopathy and heart failure.
The assessment of methodological quality of included studies was performed using the recommended guidelines in the checklist for the STROBE statement. 126 Features of the study considered were information regarding the study's rationale and objectives, study design (including methods of recruitment and assessment), reporting of results and measures used to address confounding factors. The criteria and characteristics of individual studies are shown in Appendix 7.
Of the 16 studies that provided data for the review of the prevalence of clinically significant pathologies in patients with AF, seven studies28,112,114,115,119,121,124 were retrospective in design, eight113,116–118,120,122,123,125 were prospective studies, and one111 was a case–control study.
Patients with AF were identified mainly by ECG, either at the time of recruitment or from hospital notes such as admissions notes or discharge records. Five studies did not report the methods used to verify the presence or history of AF in eligible patients112,114,120,124,125 although one study gave details in a prior publication98 and the others used candidates for cardioversion giving confidence in accuracy of diagnosis. Although two retrospective studies28,121 used TOE and TTE in diagnosing the presence of ischaemic heart disease, the methods used to diagnose the presence of coexisting clinically significant cardiac pathologies were not detailed in three studies. 115,118,119 The remaining studies relied on TOE and provided information on diagnostic criteria for pathologies of interest. Detailed descriptions of the assessors evaluating eligible patients regarding pathologies of interest were reported in four studies;113,114,116,123 for one of these studies,113 the relevant information was reported in a separate publication. 127 In one study,116 outcome data were incomplete; the reason was that TOE provided inadequate visualisation of the pathology of interest in a number of patients.
All methodological quality criteria of interest were met in six studies. 111,113,117,122,123,125 At least one of the criteria was not satisfied in three studies,28,114,116 the criteria were partially met in one study115 and information was unclear in two studies. 112,120
Prevalence results
One prevalence study119 was identified that sought to identify prevalence of atrial septal defect, as presented in Table 14. This study, by Lip et al. ,119 found a prevalence of 0.9% for atrial septal defect. This study looked at a cross-section of patient records in UK primary care.
| Study | Pathology | Population (n, type of AF) | Prevalence (%) |
|---|---|---|---|
| Lip et al. 1997119 | Atrial septal defect | 111 AF | 0.9 |
Fifteen studies28,111–114,116–125 investigated the prevalence of pathologies within the category ‘ischaemia/thrombosis’, as shown in Table 15. One study, that by Lip et al. 1997,119 found a 28.8% prevalence of ischaemic heart disease.
The six studies112,116,117,120,124,125 reporting prevalence of LA thrombus gave differing prevalences, ranging from 3%117 to 18%. 116 Both of these studies116,117 used TOE to diagnose thrombi. These six studies differed in terms of sample size and population, with Maltagliati et al. 120 including atrial flutter, Shen et al. 124 restricting the population to patients with subtherapeutic INR, and Kleeman et al. 117 using a population admitted for cardioversion.
| Study | Pathology | Population (n, type of AF) | Prevalence (%) |
|---|---|---|---|
| Agmon et al. 2001111 | Aortic atherosclerosis | 42 AF | 73.8 |
| Complex aortic atherosclerosis | 42 AF | 16.7 | |
| Archer et al. 1995112 | LA thrombus | 55 non-rheumatic AF | 9.1 |
| LV thrombus | 55 non-rheumatic AF | 3.6 | |
| Atrial septal aneurysm | 55 non-rheumatic AF | 7.3 | |
| Blackshear et al. 1999113 | Aortic atherosclerotic plaque | 770 AF | 56.6 |
| Complex aortic atherosclerotic plaque | 770 AF | 25.1 | |
| Corrado et al. 2004114 | LAA thrombus | 41 AF or atrial flutter | 9.8 |
| de Divitiis et al. 199928 | LAA thrombus | 90 AF | 12.2 |
| RAA thrombus | 90 AF | 6.7 | |
| Left and/or RAA thrombus | 90 AF | 13 | |
| Heppell et al. 1997116 | LA thrombus | 109 AF | 18 |
| Kleeman et al. 2009117 | LA thrombus | 295 non-valvular AF or atrial flutter | 3 |
| Levy et al. 1999118 | Coronary artery disease | 756 chronic, paroxysmal or recent-onset AF | 16.6 |
| Lip et al. 1997119 | Ischaemic heart disease | 111 AF | 28.8 |
| Maltagliati et al. 2006120 | LA thrombus | 757 AF or atrial flutter | 6.3 (if exclude LAA 0.3) |
| LAA thrombus | 757 AF or atrial flutter | 5.5 | |
| RAA thrombus | 757 AF or atrial flutter | 0.5 | |
| Narumiya et al. 2003121 | LAA thrombus | 50, of which 14 lone AF, 36 non-lone AF | 12 (16.7% non-lone AF; 0% lone AF) |
| Santiago et al. 1994122 | LAA thrombus | 30 AF | 40 |
| Scherr et al. 2009123 | LAA thrombus | 732 catheter ablations for AF in 585 patients | 1.6 |
| Shen et al. 2002124 | LA thrombus | 182 AF and subtherapeutic INR | 9.9 |
| Tsai et al. 1997125 | LA thrombus | 219 chronic non-rheumatic AF | 6.8 |
Six studies28,114,120–123 investigated prevalence of LAA thrombi; the lowest reported prevalence was for patients undergoing catheter ablations 1.6%123 and the highest was 40%,122 although this study had a small sample size (n = 30). Two studies28,120 looked at RAA thrombus, reporting prevalences of 0.5%120 and 6.7%. 28 Both of these studies used TOE to diagnose thrombi.
Four studies115,118,119,122 investigated the prevalence of valvular pathologies (Table 16). Two of these studies115,119 reported prevalence of valvular heart disease as 13.4%115 to 26.1%,119 and combining rheumatic and non-rheumatic valvular heart disease from Levy et al. 118 would give 18.8% prevalence. Mitral valve disease had a reported prevalence of 10.4%,115 and Santiago et al. 122 reported prevalence of 30% for MR.
| Study | Pathology | Population (n, type of AF) | Prevalence (%) |
|---|---|---|---|
| Dang et al. 2004115 | Mitral valve disease | 737 first hospitalisation associated with AF | 10.4 |
| All valve diseases | 737 first hospitalisation associated with AF | 13.4 | |
| Levy et al. 1999118 | Valvular heart disease rheumatic | 756 chronic, paroxysmal or recent-onset AF (167 paroxysmal, 389 chronic, 200 recent onset) | 15.2 (10% paroxysmal, 20% chronic, 12% recent onset) |
| Valvular heart disease non-rheumatic (including MVP) | 756 chronic, paroxysmal or recent-onset AF (167 paroxysmal, 389 chronic, 200 recent onset) | 3.3 (5% paroxysmal, 3% chronic, 3% recent onset) | |
| Lip et al. 1997119 | Valvular heart disease | 111 AF | 26.1 |
| Santiago et al. 1994122 | MR | 30 AF | 30 |
Three studies115,118,119 investigated the prevalence of pathologies within the category cardiomyopathy, as presented in Table 17. Dang et al. 115 and Lip et al. 119 reported prevalences of 4.5%115 to 5.4%. 119 Levy et al. 118 reported a prevalence of 4.5% for hypertrophic cardiomyopathy and of 9.2% for dilated cardiomyopathy.
| Study | Pathology | Population (n, type of AF) | Prevalence (%) |
|---|---|---|---|
| Dang et al. 2004115 | Cardiomyopathy | 737 first hospitalisation associated with AF | 4.5 |
| Levy et al. 1999118 | Hypertrophic cardiomyopathy | 756 chronic, paroxysmal or recent-onset AF (167 paroxysmal, 389 chronic, 200 recent onset) | 4.8 (3% paroxysmal, 4% chronic, 9% recent onset) |
| Dilated cardiomyopathy | 756 chronic, paroxysmal or recent-onset AF (167 paroxysmal, 389 chronic, 200 recent onset) | 9.2 (2% paroxysmal, 13% chronic, 9% recent onset) | |
| Cardiomyopathy (other) | 756 chronic, paroxysmal or recent-onset AF | 1.2 | |
| Lip et al. 1997119 | Cardiomyopathy | 111 AF | 5.4 |
Two studies115,118 investigated the prevalence of heart failure, as shown in Table 18. Dang et al. 115 reported a prevalence of 31.1% and Levy et al. 118 reported the prevalence of CHF to be 29.8%.
Discussion of clinical effectiveness
Diagnostic accuracy studies with AF populations were available for the pathologies atrial septal defect, atrial septal aneurysm, rupture of chordae tendineae, atrial thrombosis, ventricular thrombosis, coronary artery stenosis, pulmonary hypertension, aortic and MR, and tricuspid stenosis. Diagnostic accuracy studies without reported AF populations were available for other pathologies, including endocarditis, cardiomyopathy, heart failure, LV dysfunction, aortic dissection and cardiac masses. As the search was limited to MEDLINE, it is possible that the database search will have missed some studies, although additional bibliography and hand-searching identified only a small proportion of articles to be screened, and the database search identified diagnostic studies for almost all the pathologies selected as relevant. Thus diagnostic accuracy data were available for a range of relevant pathologies, although data were not available for all pathologies in an AF population. There was considerable heterogeneity between studies, especially in terms of population and pathology being identified, and in comparator diagnostic technique, with some heterogeneity in the type of TTE used and the study type.
Diagnostic accuracy showed high specificities for all selected pathologies, with the majority having specificity of 0.8 or higher, meaning a low proportion of FPs. For most pathologies there was also quite high sensitivity, with the majority having sensitivity of ≥ 0.6, with the exceptions of atrial thrombi, atrial septal defect and PE. Thus screening may result in considerable FNs for atrial thrombi, atrial septal hypertrophy/defect and PE. In general, sensitivity was lower for atrial thrombi, atrial septal defect and PE than for other pathologies, and specificity was lower for aortic dissection and pulmonary disease than for other pathologies. TTE seems to be a sufficient diagnostic tool for most pathologies included here, but there may need to be extra screening for PE by lung scan, and atrial thrombi and atrial septal hypertrophy by TOE.
All studies had a high percentage of usable, good images from TTE. Although for some studies participants were selected on the basis of having usable TTE images, even for other studies the lowest percentage of usable images was 85%.
In practice, accuracy will depend on the skill of the echocardiographer. Studies of diagnostic accuracy used experienced echocardiographers. It may be that when less-experienced staff are employed, there is lower accuracy. Even with skilled echocardiographers, there may be interobserver variations in the interpretation of images. 128,129–132
Diagnostic accuracy studies identified were published from 1981 to 2009; with many relatively old studies included, it may be that imaging techniques and equipment have improved since then. This means that the review may underestimate the accuracy of TTE.
Prognostic studies indicated that LV dysfunction as diagnosed by TTE was associated with a significantly increased risk of thromboembolism, stroke or mortality. Increased LAD as assessed by TTE was associated with a significantly increased risk of thromboembolism or mortality; however, it was not significantly associated with stroke when assessed in 10-mm increments. Valvular abnormality carried a significantly increased risk of mortality. MR was not significantly associated with stroke or thromboembolism in two studies; however, two other studies suggested a significantly increased risk of thromboembolism with mild MR, in contrast with a significantly protective effect of severe MR against stroke. There was no significant association found between mitral annular calcification and thromboembolism, or between MVP and stroke. These findings are consistent with the report of Providencia et al. ,13 published after the literature search was conducted, which found that TTE-diagnosed LV systolic function and LA area measurement may provide a valuable addition to CHADS2 and CHA2 DS2-VASc scores. 13
Prevalence studies were sought for AF populations, and were not found for all pathologies. Prevalence studies were found for atrial septal defect, atrial septal aneurysm, atrial thrombus, ventricular thrombus, ischaemic heart disease or thrombosis, valvular heart disease, cardiomyopathy and heart failure. The prevalence studies had relevance to the UK. Two of the prevalence studies had UK populations, although the most common setting for the prevalence studies was USA. The wide variations in prevalence rates for specific pathologies may be explained by the degree of heterogeneity in the studies considered in this review. The sources of dissimilarities stem from study designs, characteristics of patients studied and severity of illness (e.g. as assessed by CHADS2 scores). Although in most instances the diagnostic criteria for assessing pathologies of interest were outlined, there was a lack of detail regarding the description of the assessor or observer variability. Many of the studies used TOE to diagnose pathologies, and inadequate reporting of observer variation in transoesophageal echocardiographic examinations has been noted in available literature. 133
There was a high prevalence (around 25–30%) of ischaemic heart disease, valvular heart disease and heart failure in patients with AF in the included prevalence studies. Cardiomyopathy prevalence was around 5%, and atrial septal defect had a prevalence of < 1%.
Studies of AF have reported characteristics of patients with AF, including prevalences of cardiac pathologies. This review found a prevalence study only for atrial septal defect, whereas prevalence of structural heart disease, of any type, thus encompassing many difference pathologies, has been reported in 46%108 or 54%114 of patients with AF not experiencing thromboembolic event or LAA thrombus, respectively. Prevalence of ischaemic heart disease found in this review was broadly in line with other reports. Ischaemic heart disease has been reported in 25%113 or 12%,134 and coronary disease in 22%,28 56% of 188 patients with short-term (< 48 hours) AF,117 or 32% of first-detected AF,54 34% paroxysmal AF,54 29%54 persistent AF, 36%54 permanent AF. Atrial thrombus has been found in 3% AF135 or 12–20% in post-mortem studies of valvular AF,136 LA thrombus in 14% of new-onset AF,137 and LAA thrombus in 12% non-valvular AF or atrial flutter. 138 Results of thrombus prevalence studies within this review fell within these estimates. In the Scherr study123 all patients with atrial flutter had a larger LAD (> 4.5 cm) than those patients without LA thrombi. It was also noted that the prevalence of LAA thrombi increased with increasing CHADS2 score. Although the prevalence of LAA thrombi ranges between 0.3% and 1.4% for those with scores of 0 and 1, the prevalence of this pathology occurs in 5.3% of patients with a score of ≥ 2. Atrial septal aneurysm has been reported in 2%139 and LV aneurysm in 1% (SPAF Investigators 1992110) of patients with AF. This review did not find studies that set out to assess prevalence of pulmonary disease in patients with AF; however, pulmonary disease has been reported in 6% of patients with AF. 134 According to the Framingham heart study,19 approximately one-third of women with AF and one-fifth of men with AF have valvular heart disease. 19 Results of valvular heart disease prevalence studies within this review were broadly in line with other reported estimates. Valvular heart disease has been reported in 10% of asymptomatic patients with AF and 26% of symptomatic patients with AF,134 23% AF,140 21% of first-detected AF,54 19% paroxysmal AF, 24% persistent AF54 and 40% permanent AF,54 with a review estimate of up to 40% AF. 141 Cardiomyopathy has been reported in 8% of first-detected AF,54 7% paroxysmal AF,54 13% persistent AF54 and 16% permanent AF. 54 Dilated cardiomyopathy has been found in 11% AF28 and 17% patients with short-term (< 48 hours) AF. 117 Heart failure has been reported in 26% of first-detected AF,54 23% paroxysmal AF,54 35% persistent AF54 and 49% permanent AF. 54 According to the Framingham heart study,19 approximately one-quarter of men and women with AF have heart failure,19 with up to 42% patients with AF developing CHF during their lifetime. 142 CHF in AF study participants has been reported as 28%113 or 40%,122 similar to results of prevalence studies included in this review. This review did not find studies that set out to assess prevalence of aortic dissection in patients with AF; however, aortic dissection has been reported in 7% patients with AF. 122
Overall, diagnostic accuracy of TTE and prevalence of pathologies in patients with AF indicate that routine TTE following AF diagnosis would identify pathologies in many patients, particularly with regard to valvular heart disease, ischaemic heart disease and heart failure. TTE seems to be a sufficient diagnostic tool for screening most pathologies included in this review. For completeness of screening, extra testing for PE by lung scan, and for atrial thrombi and atrial septal hypertrophy by TOE, would reduce risk of FNs from TTE. However, it is unclear whether identifying these pathologies, in addition to the many diagnosed by TTE, would lead to improvement above that of TTE screening. In practice, some patients may have been diagnosed with a pathology prior to AF diagnosis. Patients may have more than one pathology in addition to AF. In practice, some diagnoses are likely to be checked with other diagnostic tools before treatment change, which will minimise the impact of FPs, although FPs may lead to some unnecessary diagnostic tests.
Chapter 4 Simulating clinical events and estimating cost-effectiveness ratios
Introduction
Key questions that are investigated
The model described here attempts to determine the cost-effectiveness of conducting TTE in all newly diagnosed patients by answering the following two linked questions:
-
Does the added information provided by performing TTE on everyone lead to better long-term clinical outcomes for patients with newly diagnosed AF? (Clinical effectiveness. )
-
Is any improvement in long-term clinical outcome [increased quality-adjusted life-years (QALYs)] worth the additional cost of performing TTE tests in all patients? (Cost-effectiveness. )
The relationship between information provided by a transthoracic echocardiography and clinical outcomes
With regard to the first question, the added information of performing a TTE in all patients can only lead to improvements in clinical outcomes if it leads to altered patient management. If by performing a TTE in a patient additional information about the structure and function of the heart is revealed, but this new information does not lead to any change in medical strategy, then the new information has not improved the clinical effectiveness of the patient's treatment.
Given this, it is important to identify situations where the identification of particular clinical features through TTE would lead to clear and consistent differences in clinical management. One example of this would be the identification of structural features within the heart that confer a greater risk of stroke than was previously estimated before TTE, where the updated risk level would recommend that pharmaceutical treatment should be provided, contrasting with a decision to not treat prior to the TTE. A further example would be a change in decision regarding surgical interventions; however, given the complexity of this area and paucity of data, our focus in the model has been on the way additional information provided by TTE is likely to change pharmaceutical management.
The decision to prescribe anticoagulants
Introduction
The specific focus of the model is the clinical decision whether or not to prescribe an OAC to a patient. Three types of OAC are considered: warfarin, dabigatran and rivaroxaban. The decision involves balancing competing clinical risks, as these drugs reduce the risk of stroke, but an adverse effect increases the risk of major bleeding events, which, in some cases, can lead to clinical outcomes as, or more, severe than the strokes that the treatment aims to prevent. In patients with an underlying low risk of stroke, the added risks of treatment in terms of bleeding events can outweigh the additional benefit caused by the reduced risk of stroke, and so it is neither clinically effective nor cost-effective to prescribe anticoagulants in this patient group. Within the context of this model, the added clinical benefit of performing TTE in a patient is a direct result of the increased appropriateness of the decision whether or not to prescribe anticoagulants, measured in terms of estimated QALYs.
Uncertainty about appropriate anticoagulants
Introduction
An important point to note is that the choice of OAC available to newly diagnosed patients with AF may affect the cost-effectiveness of the diagnostic technology. Three OACs are currently recommended for this patient group. These are warfarin, dabigatran and rivaroxaban. Each drug differs in terms of costs, clinical effectiveness in preventing strokes, and major bleeding event risks.
Diagnostic strategies
In the context of this clinical decision, TTE is best conceived as part of a diagnostic strategy. Two versions of the diagnostic strategy are compared: a ‘baseline’ strategy assuming that TTE is not undertaken, and a ‘baseline + TTE’ strategy that incorporates additional information provided by TTE. The diagnostic strategy will indicate that some patients should receive the drug, and others should not. This indication is appropriate in some cases (‘TPs’ and ‘TNs’) and not appropriate in others (‘FPs’ and ‘FNs’), as indicated in Table 19. This table focuses purely on the clinical issues; whether additional benefits are worth any additional costs will be detailed later in this report.
| Diagnostic strategy indicates that additional benefit: | In reality | |
|---|---|---|
| The additional benefit of treatment outweighs additional risk: patient should receive drug | The additional benefit of treatment does not outweigh additional risk: patient should not receive drug | |
| Outweighs additional risk | Correct decision to prescribe | Incorrect decision to prescribe |
| Patient prescribed drug | TP | FP |
| Does not outweigh additional risk | Incorrect decision not to prescribe | Correct decision not to prescribe |
| Do not prescribe drug | FN | TN |
The CHADS2 diagnostic tool for assessing the risk of stroke in patients with atrial fibrillation
The main diagnostic tool currently used to make the decision about whether to prescribe anticoagulants in newly diagnosed patients with AF is the CHADS2 instrument.
The CHADS2 instrument produces a risk score for each patient ranging from 0 to 6 points inclusive, according to the criteria shown in Table 20.
| Code | Condition | Points |
|---|---|---|
| C | CHF | 1 |
| H | Hypertension | 1 |
| A | Age ≥ 75 years | 1 |
| D | DM | 1 |
| S2 | Prior stroke or TIA | 2 |
CHADS2 decision rule and choice of oral anticoagulants
Although dabigatran and rivaroxaban are generally considered to be very similar OACs in terms of costs, clinical effectiveness, and risk of major bleeding events, the indications provided in the NICE guidance for each OAC differ slightly.
The guidance for rivaroxaban states:
-
Rivaroxaban is recommended as an option for the prevention of stroke and systemic embolism within its licensed indication, that is, people with non-valvular AF with one or more risk factors, such as:
-
CHF
-
hypertension
-
≥ 75 years of age
-
DM
-
prior stroke or transient ischaemic attack. 47
-
It is noted that this guidance is equivalent to stating that rivaroxaban is recommended for patients with AF with a CHADS2 score of ≥ 1.
The guidance for dabigatran states that:
-
Dabigatran etexilate is recommended as an option for the prevention of stroke and systemic embolism within its licensed indication (i.e. in people with non-valvular AF with one or more of the following risk factors):previous stroke, transient ischaemic attack or systemic embolism
-
LV ejection fraction of < 40%
-
symptomatic heart failure of New York Heart Association (NYHA) class 2 or above
-
age ≥ 75 years
-
age ≥ 65 years, with one of the following: DM, coronary artery disease or hypertension. 143
-
It is noted that an implication of the guidance is that dabigatran is indicated in patients who have a CHADS2 score of ≥ 1 if they are aged ≥ 65 years. In people aged < 65 years, the correspondence between CHADS2 score and OAC decision is not clear cut. For simplicity, and because the mathematical model developed does not model the progression of each individual disease state that is incorporated in the CHADS2 score, the mathematical model will be run in patients aged ≥ 65 years only when considering dabigatran as the OAC of choice.
Based on the above NICE recommendations, recent ESC guidance47 and broad recognition that warfarin carries a higher risk of major bleeding events for most patient groups than either dabigatran or rivaroxaban,6 different CHADS2 thresholds were used for each OAC, as shown in Table 21 below.
| CHADS2 score | Prescribe dabigatran | Prescribe warfarin | Prescribe rivaroxaban |
|---|---|---|---|
| 0 | No | No | No |
| 1 | Yes (≥ 65 years) | No | Yes |
| 2 or more | Yes | Yes | Yes |
Comparator strategy: CHADS2 plus transthoracic echocardiography
In our comparator strategy, ‘CHADS2 + TTE’, the decision to coagulate can also be made as a result of TTE identifying a structural feature of LA abnormality that predisposes an individual to a high risk of stroke. 144 For the purposes of this report we define LA abnormality as a patient having one or more of the following conditions:
-
LAA thrombi
-
dense spontaneous echo contrast
-
LAA low-flow velocities.
These conditions have been chosen as they are the ones used in a recent publication by Provedencia et al. 13 which provides key data for populating the model.
This means the comparator offers two alternative routes by which a decision to recommend OACs can be made:
-
a CHADS2 score at or above the threshold required to recommend OACs
-
a TTE indicating the presence of LA abnormality.
In effect, this means that some individuals who would not have received OAC with CHADS2 alone will receive OAC following CHADS2 + TTE due to detection of LA abnormality. It is noted that in no instance patients who would have received OAC using CHADS2 alone would have treatment withheld. As such, it has been assumed that TTE will only provide information that can alter patient management when the CHADS2 score does not indicate treatment with OAC. In the remaining patients we have not formally assessed the cost-effectiveness of TTE, noting that costs would be incurred for no assumed gain.
Scenarios modelled
The risks of each of the discrete events modelled in the discrete event simulation (DES) depend on factors such as the OAC chosen, the initial age of the patient when newly diagnosed with AF, gender, and whether or not the patient has another CHADS2 risk factor. Because of this, a number of different scenarios were considered incorporating different combinations of OAC, age, gender, and initial CHADS2 score. A total of 14 scenarios are presented, as described in Table 22.
| Scenario | OAC | Age (years) | Initial CHADS2 score | Gender |
|---|---|---|---|---|
| W_50_0_M | Warfarin | 50 | 0 | Male |
| W_50_0_F | Female | |||
| W_50_1_M | 50 | 1 | Male | |
| W_50_1_F | Female | |||
| W_65_0_M | 65 | 0 | Male | |
| W_65_0_F | Female | |||
| W_65_1_M | 65 | 1 | Male | |
| W_65_1_F | Female | |||
| R_50_0_M | Rivaroxaban | 50 | 0 | Male |
| R_50_0_F | Female | |||
| R_65_0_M | 65 | 0 | Male | |
| R_65_0_F | Female | |||
| D_65_0_M | Dabigatran | 65 | 0 | Male |
| D_65_0_F | Female |
CHA2 DS2-VASc score [Congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 years, and sex category (female)]
An alternative variation of the CHADS2 instrument exists, which uses additional information such as gender to make the decision. It was decided not to produce an additional 14 scenarios using CHA2 DS2-VASc rather than CHADS2 as the baseline strategy for a number of reasons. These include the already large number of scenarios considered; the fact the recent NICE guidance47 on rivaroxaban and dabigatran relate more clearly to CHADS2 than CHA2 DS2-VASc scores, and the fact that CHADS2 is the more established of the two instruments.
Detailing the mathematical model
Overall structure of model
An overview of the model is presented in Figure 3. The model involves two distinct stages:
-
A short-term stage in which the clinical characteristics of a patient are generated, and the decision whether or not to prescribe an OAC is made for both the baseline and the baseline + TTE strategy.
-
A long-term simulation of the clinical outcomes, and associated costs and utilities, which follow from the patient's clinical characteristics and the decision whether or not to prescribe an OAC.
FIGURE 3.
Mathematical model. GI, gastrointestinal; GOS, Glasgow Outcome Scale; ICH, intracranial haemorrhage; mRS, modified Rankin Scale.
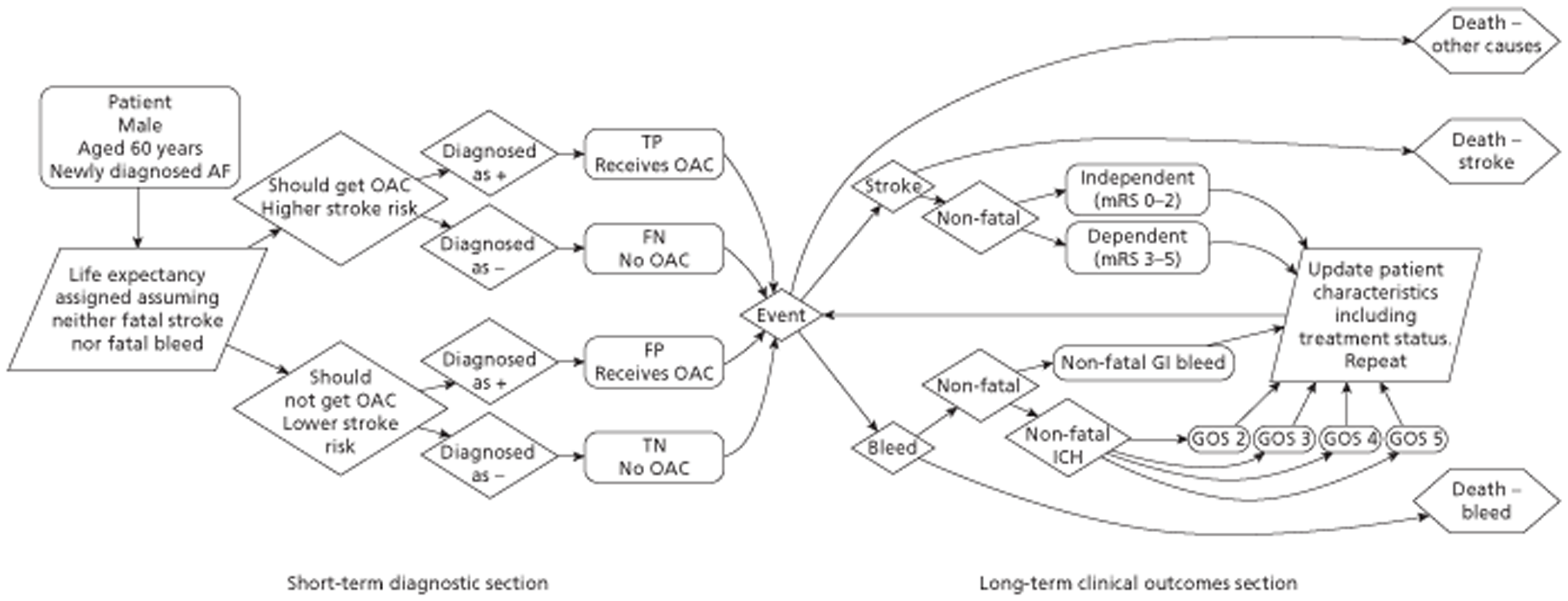
The cost-effectiveness of TTE in this context results from the differences in the long-term outcomes in a large cohort of individuals following the baseline + TTE diagnostic strategy compared with long-term outcomes in a similar cohort of individuals following the baseline diagnostic strategy.
Key structural assumptions made by the model are:
-
Patients who have a major clinical bleed while on OAC have treatment with OACs stopped.
-
Patients who have a stroke receive OAC, unless they have had a prior major clinical bleed.
In populating the model we elected to use data reported by Providencia et al. ,13 as this was a recent, internally consistent study, which used the CHADS2 tool and had also conducted TOE. Although the study was not large (n = 405) it was deemed to outweigh the limitations associated with using data from heterogeneous studies that would have required numerous assumptions.
Simulating patient characteristics
Introduction
The short-term diagnostic section of the model begins by simulating a series of male and female cohorts aged either 50 or 65 years old with newly diagnosed AF but with none of the following conditions: CHF, hypertension, DM, prior stroke or TIA, or vascular disease. This patient group has been selected as they would have a CHADS2 score of 0 and thus would not be currently recommended treatment with an OAC. Additionally, otherwise identical cohorts of individuals with a CHADS2 score of 1 are considered in the case of warfarin.
A summary of the sources of data used to populate the model is provided in Appendix 10.
For this patient group the age at death (assuming no AF- or AF treatment-related mortality) was simulated. The diagnosis, or not, of LA abnormality following TTE was additionally simulated.
Risk of all-cause mortality
The risks of all-cause mortality were estimated separately for males and females using gender-specific UK life table data. 145 These were converted into probabilities of males and females dying in each forthcoming year assuming initial ages of 50 and 65 years. These produced a range of distributions of death given. For simplicity, it was assumed that all remaining patients would die within their 101st year.
The assumed sensitivity and specificity of transthoracic echocardiography of diagnosing left atrial abnormality
Table 2 in the paper by Providencia et al. 13 provides sufficient information to calculate an estimate of sensitivity and specificity of TTE in diagnosing LA abnormality. These data were used to derive Tables 23 and 24. Patients were assessed using both TTE and TOE, and for TTE were assigned an echocardiographic risk score depending on how many structural features that are constituents of LA abnormality were identified through TTE. It was assumed that TOE was the gold standard and identified all patients with LA abnormality.
| Echocardiographic parameters: score | No. of patients | No. of patients with LA abnormality |
|---|---|---|
| 0 | 88 | 5 |
| ≥ 1 | 246 | 87 |
| Total | 334 | 92 |
| Feature | Patients with high-risk feature | Patients without high-risk feature |
|---|---|---|
| No high-risk feature identified by TTE | FN 5 | TN 88 − 5 = 83 |
| High-risk feature identified by TTE | TP 87 | FP 246 − 87 = 159 |
This allows calculation of sensitivity and specificity for this patient group:
-
sensitivity = TP/(TP + FN) = 87/(87 + 5) = 0.946 (to three decimal places)
-
specificity = TN/(TN + FP) = 83/(83 + 159) = 0.343 (to three decimal places).
The four cells in Table 25 also allow uncertainty to be estimated for the joint distribution of sensitivity and specificity. One thousand draws from a Dirichlet distribution using the cell counts as parameter values were used to jointly calculate sensitivity and specificity. The resulting joint distribution of sensitivity and specificity estimates is shown in Figure 4 as a contour plot. A contour plot represents variation in density by joining together points on the surface with equal heights, allowing three-dimensional information to be presented in a monochrome graph. This method of presenting the data was preferred to a scatterplot, as the relative density would have been relatively difficult to ascertain in a scatterplot containing 1000 points. In Figure 4 the peak density of the plot is, unsurprisingly, close to the point estimates of 0.95 for sensitivity and 0.34 for specificity.
| CHADS2 score | Annual risk (95% CI) |
|---|---|
| 0 | 0.6 (0.5 to 0.7) |
| 1 | 3.0 (2.9 to 3.2) |
| 2 | 4.2 (4.0 to 4.4) |
| 3 | 7.1 (6.7 to 7.5) |
| 4 | 11.1 (10.4 to 11.8) |
FIGURE 4.
Joint distribution of sensitivity and specificity based on 1000 draws from a Dirichlet distribution.
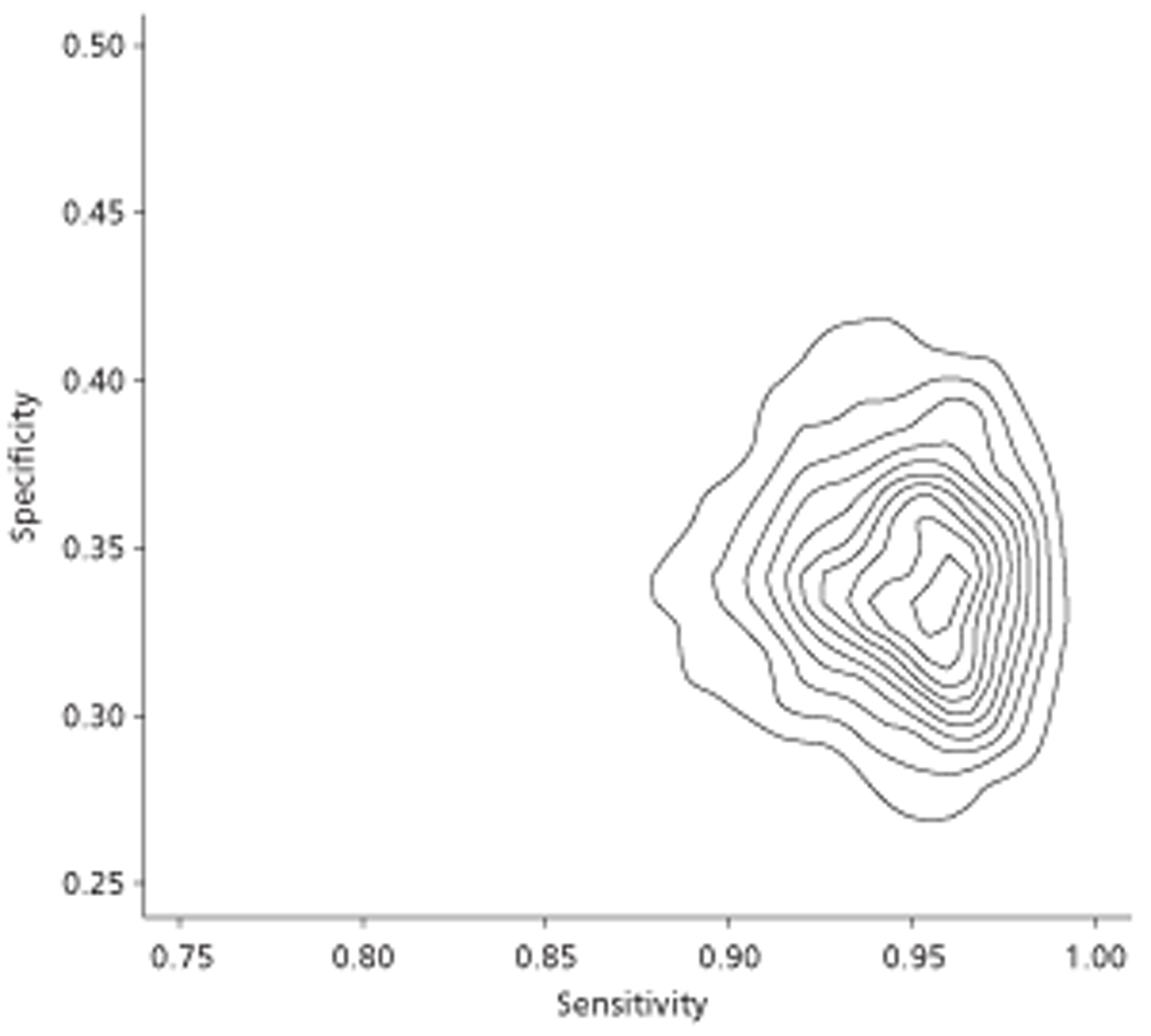
It was assumed that the derived distributions of sensitivity and specificity were applicable to all patients and were thus assumed applicable to patients who had a CHADS2 score of 0.
Data reported in table 2 of Provedencia et al. 13 indicate that of 24 patients with a CHADS2 score of 0, two had a LA abnormality. Given the small number of data available (the number in the LA abnormality group being < 5) an uninformative prior of 0.5 was added to each paired data set, culminating in an expected 2.5 out of 25 patients expected to have a LA abnormality among those with a CHADS2 score of 0. These values were used to populate beta distributions to allow for uncertainty in the true proportion of patients with LA abnormality within the CHADS2 = 0 score.
For patients with a CHADS2 score of 0 there were 17 patients out of 79 with a LA abnormality.
Estimating a patient's underlying risk of stroke
This section describes the risk of a stroke. The breakdown of types of stroke and the associated costs and utilities are detailed in later sections.
CHADS2-related stroke risk
We assumed that higher CHADS2 scores were associated with a higher risk of stroke. Our estimates were based on unadjusted stroke risk estimates presented in Friberg et al. 146 These estimates are presented in Table 25 (95% CIs were estimated from beta distributions).
Patients with LAA were assumed to have a risk of stroke independent of CHADS2 score. The risk was set as 8.0% (95% CI 7.26 to 8.31) per annum, as reported in Connelly et al. 147 For simplicity, the risk of stroke was assumed to apply throughout the lifetime of the patient.
Estimating uncertainty in CHADS2-related stroke risk using CHADS2 score
Introduction
In order to ensure no estimated risks were less than zero, we assumed that the above estimates followed a log-normal distribution, producing 10,000 simulated values for the stroke risk associated with each score, as shown in Figure 5.
FIGURE 5.
The estimated distribution of annual stroke risk by CHADS2 score.
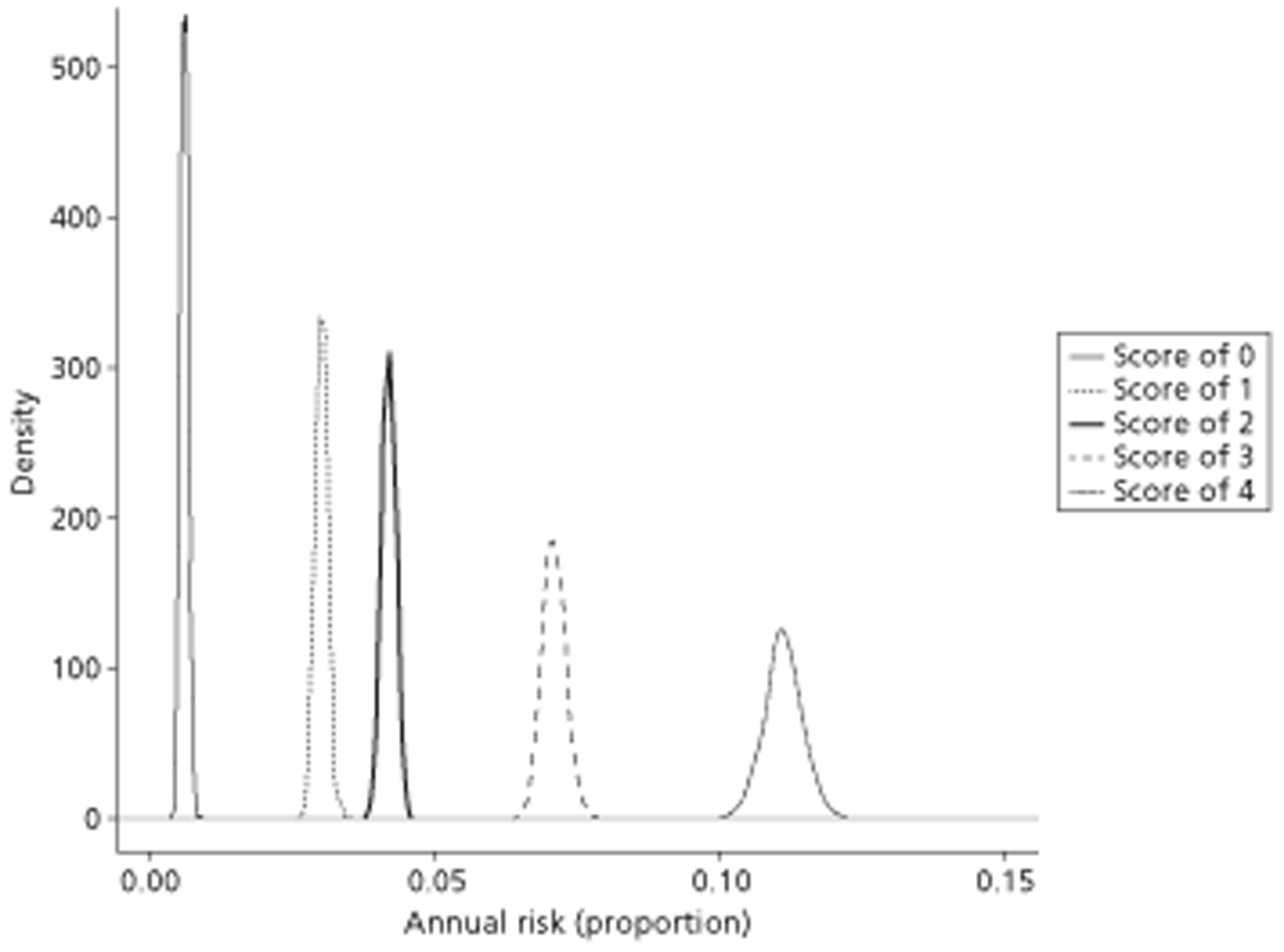
The estimated efficacy of each oral anticoagulant in preventing strokes
Effect of dabigatran on stroke risk
The effect of warfarin in preventing strokes was taken from a 2006 meta-analysis. 148 The effects of dabigatran and rivaroxaban compared with placebo (i.e. no treatment) were estimated using an indirect comparison approach. Estimates for the annual risk ratio of a stroke for patients given 150 mg of dabigatran twice daily compared with warfarin were taken from a paper based on the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study. 147 Data on the effect of rivaroxaban were taken from Patel et al. 149
Overall, 1000 simulated values were sampled from each distribution, with derived values for dabigatran compared with placebo estimated by multiplying the RRs of the sampled warfarin compared with placebo value and the dabigatran compared with warfarin value, to produce a distribution of estimates of the RR of dabigatran compared with placebo (Figure 6). An identical methodology was used to produce a distribution of the RR of rivaroxaban compared with placebo.
FIGURE 6.
The assumed distributions of each OAC vs. placebo.
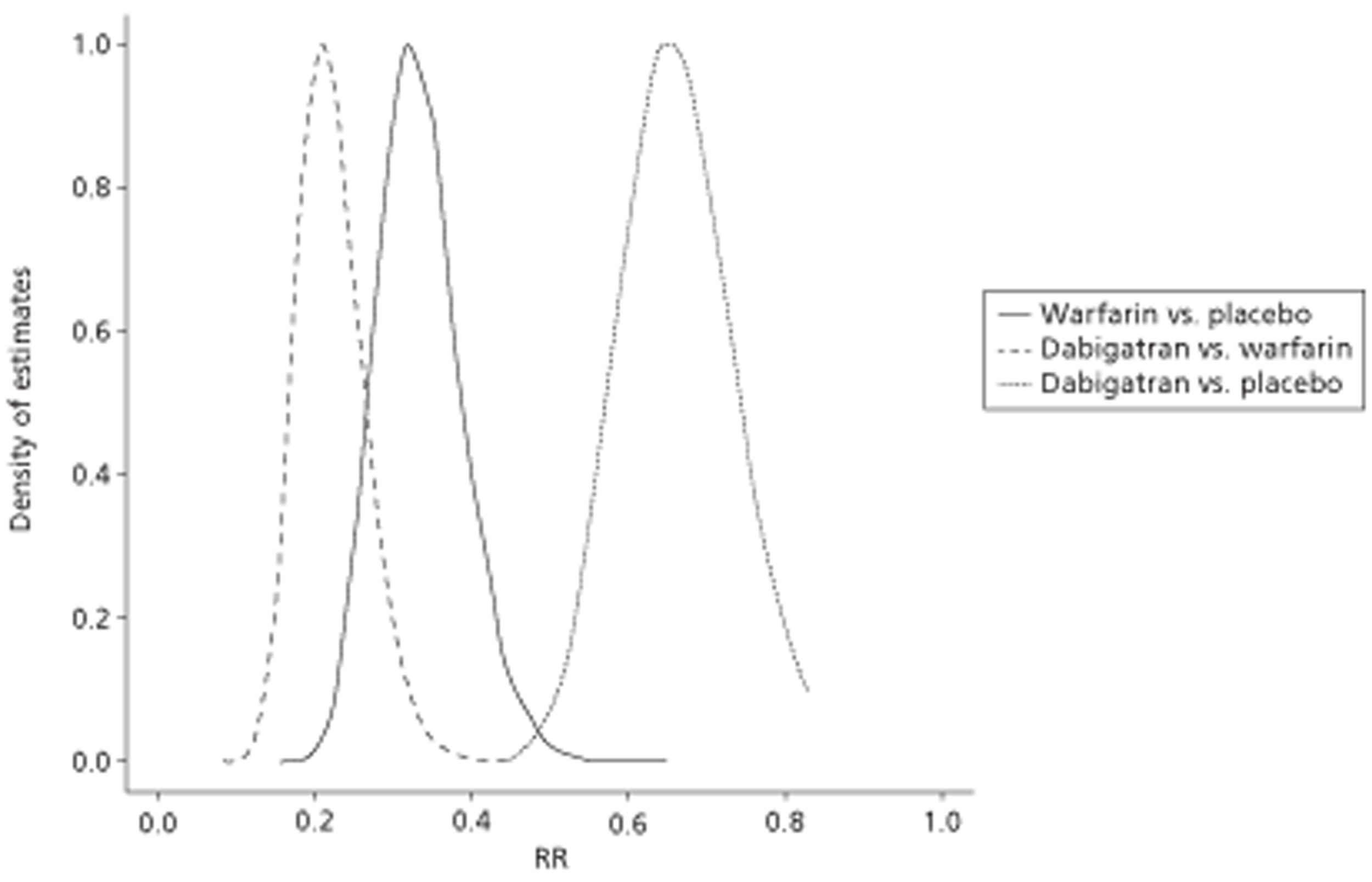
Table 26 shows summary statistics from these two papers, as well as for the simulated distribution produced by combining the two, whereas the density functions of the three distributions are shown below (Table 27).
| Comparison | RR (95% CI or CrI) | Assumed distribution | Source |
|---|---|---|---|
| RR: warfarin vs. placebo | 0.33 (0.24 to 0.45) | Log-normal | Lip and Edwards 2006148 |
| RR: dabigatran vs. warfarin | 0.66 (0.53 to 0.82) | Log-normal | Connolly et al. 2009147 |
| RR: dabigatran vs. placebo | 0.22 (0.15 to 0.32) | Log-normal | Derived from above |
| RR: rivaroxaban vs. warfarin | 0.88 (0.74 to 1.03) | Log-normal | Patel et al. 2011149 |
| RR: rivaroxaban vs. placebo | 0.30 (0.20 to 0.41) | Log-normal | Derived from above |
| Age group (years) | Central estimate (%) | Presumed sample size | 95% CrI |
|---|---|---|---|
| < 75 | 2.12 | a3618150 | 1.66% to 2.60% |
| ≥ 75 | 5.10 | b2419150 | 4.22% to 5.99% |
The estimated risk of bleed associated with each oral anticoagulant
This section describes the risk of a major bleeding event. The breakdown of types of bleed and the associated costs and utilities are detailed in later sections.
The estimated risk of bleeding associated with dabigatran
The risk of major bleeding events in patients receiving dabigatran was based on results published using data from the RE-LY trial. 150 This reported that major bleeding events occurred at a rate of 2.12% per year in patients given dabigatran and who were < 75 years of age, and at a rate of 5.10% in patients aged ≥ 75 years. A simulation approach, based on the binomial distribution and incorporating information about the different sample sizes of these two age groups within the trial, was used to represent uncertainty around these estimates for use within the probabilistic sensitivity analysis (PSA). Table 27 presents credible intervals (CrIs) for these two age groups, and Figure 7 shows these results graphically. CrIs were calculated using a presumed sample size: the paper reports 10,855 participants of < 75 years and 7258 participants aged ≥ 75 years; an equal probability of assignment between the three trial arms was assumed.
FIGURE 7.
Distribution of simulated estimates for the annual risk of bleed on dabigatran.
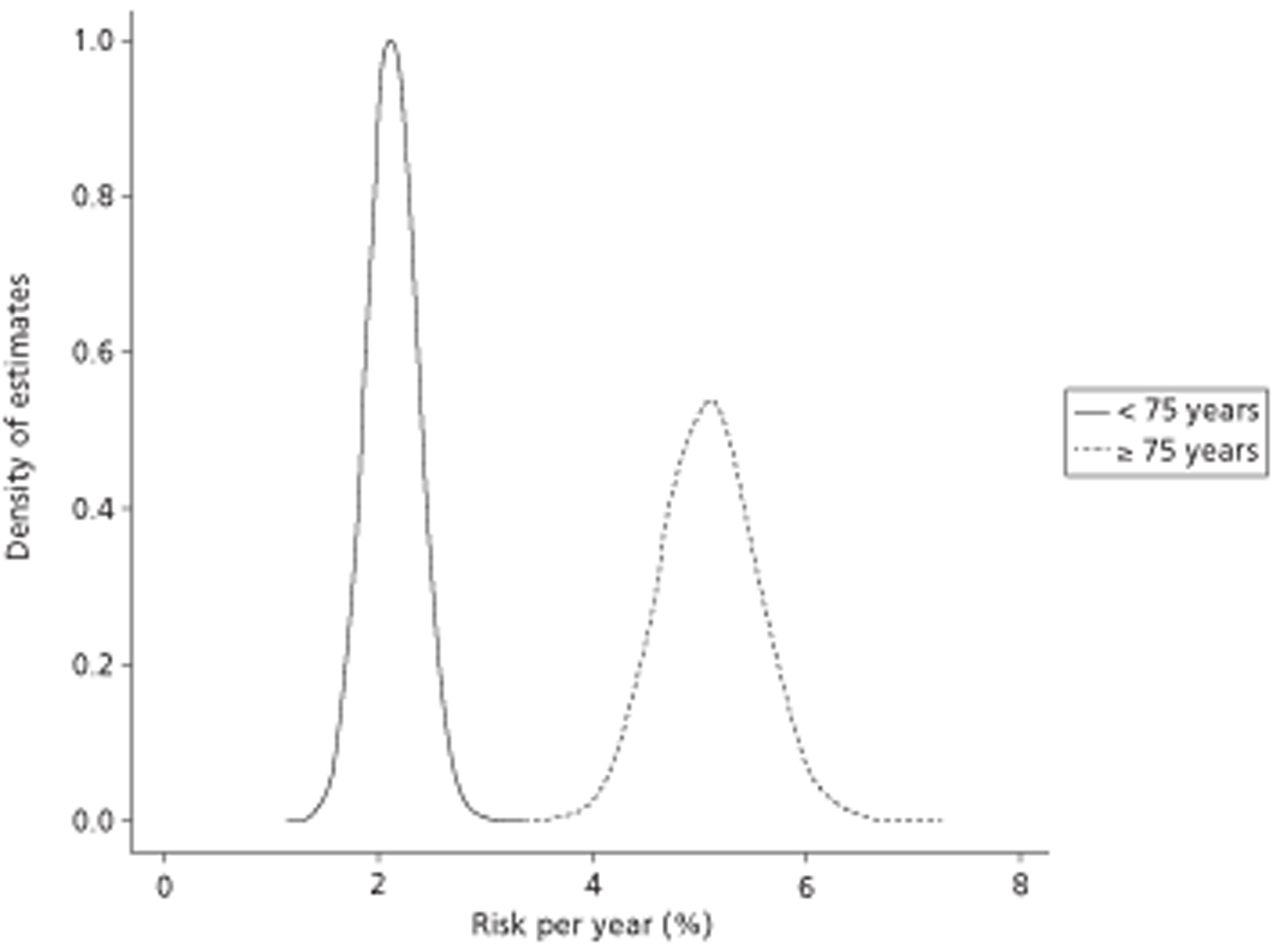
The estimated risk of bleeding associated with warfarin
Results from the RE-LY study150 were used to estimate the annual risk of bleed associated with warfarin, as shown in Table 28. The presumed sample sizes used for estimating the risk of bleeding in patients treated with dabigatran were also used in estimating the risk of bleeding associated with warfarin.
| Age group (years) | Central estimate (%) | 95% CrI |
|---|---|---|
| < 75 | 3.04 | 2.49% to 3.62% |
| ≥ 75 | 4.38 | 3.60% to 5.21% |
The estimated risk of bleeding associated with rivaroxaban
The annual risk of bleeding given rivaroxaban was estimated indirectly by combining estimates of the risk of bleed given warfarin compared with placebo148 with estimates of the risk of bleed given rivaroxaban compared with warfarin. 149 The central estimates and CrIs are shown in Table 29. The presumed sample sizes used for estimating the risk of bleeding in patients treated with dabigatran were also used in estimating the risk of bleeding associated with warfarin.
| Age group (years) | Central estimate (%) | 95% CrI |
|---|---|---|
| < 75 years | 3.15 | 2.46% to 3.96% |
| ≥ 75 years | 4.55 | 3.57% to 5.70% |
The assumed costs associated with each oral anticoagulant and with transthoracic echocardiography
Cost of dabigatran
This is assumed to cost £920.43 per year, assuming two 150-mg tablets daily at a cost of £2.52 per day. 142 This cost is fixed within all runs of the PSA.
Cost of rivaroxaban
The cost of rivaroxaban was assumed to be £767 per year, based on 20 mg per day. 151 This price was fixed in all runs.
Cost of warfarin
The annual cost of warfarin includes both drug costs and monitoring costs. The dosage received depends on the results of monitoring, although the costs of different dosages of drugs differ only marginally in comparison with the costs of monitoring. The annual monitoring costs were assumed to be £241 per annum, in line with assumptions made by the appraisal committee in the review of dabigatran. 142 The annual cost of the drug was taken from the British National Formulary (BNF) website, and suggested prices varied from 3.1 to 4.8 pence per tablet, depending on dose, equivalent to between £11.22 and £17.87 per year. 152
Including monitoring costs, this suggests a range for average total costs of between £252.22 and £258.87 per year. The average of this range (£255.54) was used as the central estimate. In the PSA the total costs were assumed to be drawn from a uniform distribution ranging from £252.22 to £258.87.
Cost of transthoracic echocardiography
TTE has been estimated to cost £66 using HRG code RA60Z Simple Echocardiogram. 153 A second, more expensive estimate of £425 was listed for HRG code EA45Z Complex Echocardiogram (including Congenital, Transoesophageal and Fetal Echocardiography), which was deemed not appropriate for TTE. Consideration was given to the use of alternative values for the cost of TTE, such as £100 to allow for variation in cost. However, on viewing the initial results it was seen that a small change in the cost of TTE would not materially alter the cost per QALY. This was due to the main component of incremental costs being the costs associated with prescribing OACs minus any savings in the reduced numbers of stroke plus any additional costs associated with bleeding episodes.
Simulating the patient experience
Introduction
The long-term part of the model uses an individual-level DES approach to simulate health trajectories experienced by a large series of patients who experience competing risks of major health events.
Within a DES, an individual begins in one of a range of discrete states. They remain in this state until the ‘next event’ occurs. The next event the individual experiences, and the time they remain in their current state, are both determined by the competing risks of possible events that may occur next given the individual's current state. In this DES, the individual begins aged 60 years. They are assumed to be newly diagnosed patients with AF, and not to have previously taken OACs, and thus not to have a risk of experiencing a major bleeding event.
Extended example
Within the DES, the patient who enters the model is assigned a life expectancy using data from national life tables. 145 This produces a time to event against which other competing risks are compared. For example, for a patient aged 60 years and assigned a life expectancy of 75 years, this baseline next event is assigned a value of 15 years (75–60 years).
This value of 15 years is compared against the risk of alternative next events. The two alternative next events in this model are:
-
risk of stroke
-
risk of major bleeds due to an OAC.
Only if a patient is receiving an OAC do they experience a risk of major bleeds due to that OAC, and so this event will not occur in patients who are not receiving OACs. As previously discussed, taking an OAC reduces the risk of stroke, so patients not treated with an OAC have no risk of bleed but a higher risk of stroke.
In the DES, higher risks are represented by, on average, shorter times to competing next events. For example, if an event has a 20% risk of occurring per year then it has an expected time to occurrence of 5 years (1/0.2). An event with a 50% risk of occurring per year, however, has an expected time to occurrence of just 2 years (1/0.5). As events do not all occur at the expected time to occurrence, and have a range of times to occurrence around this expected time, simulated values for each of these times of events are sampled from exponential distributions parameterised by the expected time to occurrence.
Within the DES, the ‘next event’ an individual experiences is the event out of a series of candidate events with the shortest simulated time to occurrence. As a hypothetical example, consider Table 30 for a 60-year-old patient on OACs.
| Candidate event name | Annual probability | Sampled time to occurrence (years) | Candidate event selected |
|---|---|---|---|
| Death, not bleed or stroke related | NA (using life table data145) | 15 | No |
| Stroke | 0.050 | 18 | No |
| Bleed | 0.100 | 12 | Yes |
Out of the three candidate next events, the event with shortest time to occurrence is that of a bleed. This means that the next event the individual experiences is a bleed, and that this event occurs 12 years from the patient's current age. In the model, the candidate's profile is updated to increase their age by 12 years and their most recent event to ‘bleed’.
Assuming the bleed is non-fatal (discussed later), this updating of the patient's profile has knock-on effects for the subsequent next events too. As the individual is now 12 years older, the time to occurrence of the baseline candidate ‘death due to other causes’ has to be reduced by 12 years, from 15 years to 3 years. Having experienced a major side effect from the OAC, it is assumed the individual will no longer be prescribed the drug, as later detailed, so the risk of major bleeds is set to zero. However, in no longer being prescribed the OAC, the risk of stroke is increased. Assuming, for example, the annual risk of stroke increases as a result of this from 5% per year to 12.5% per year, the table of candidate next events that the individual (now 72 years old) could experience is as follows (Table 31).
| Candidate event name | Annual probability | Sampled time to occurrence | Candidate event selected |
|---|---|---|---|
| Death, not bleed or stroke related | NA (using life table data145) | 3 years | Yes |
| Stroke | 0.125 | 6 years | No |
| Bleed | 0.000 | Infinite | No |
As ‘death from other causes’ is the next event candidate with the shortest time to occurrence, it is this next event for this individual, and occurs 3 years after the previous event, at the age of 75 years.
Over the course of the 15 years from the age of 60–75 years for which this simulated individual lives, it is assumed that resources are consumed and QALYs accrued. These patterns of resource use and utility depend on the events experienced and the order they are experienced in, which is partly determined both by the patient's underlying risk of stroke and the decision made about whether or not to prescribe OACs, on the basis either of the CHADS2-alone diagnostic strategy or the CHADS2 + TTE diagnostic strategy.
The differences between the costs and utilities following these two diagnostic strategies are considered to result from the addition of TTE to the diagnostic package. As this is just one of a range of ways that information from TTE could improve clinical management of the patient, the estimates provided are thus partial and conservative estimates of the cost-effectiveness of TTE for this patient group.
Dynamic features of the model
Dynamic features of the model include:
-
Updating the CHADS2 score when a patient reaches the age of 75 years. This is because being aged ≥ 75 years is a risk factor within CHADS2 , and increases the CHADS2 score by one point. This means the annual risk of stroke increases at the age of 75 years.
-
Updating the CHADS2 score by two points when a patient has a first stroke thus resulting in an increased risk of subsequent strokes.
-
If a patient suffers a major bleeding event after taking OACs, they stop being prescribed the OACs, leading to the risk of bleeds reducing to zero, but the risk of stroke increasing.
-
If a patient experiences a stroke and is not already taking an OAC, they are prescribed OACs, reducing the risk of stroke but increasing the risk of bleeds assuming that the patient had not previously had a bleed event.
-
The risk of a major bleeding event when taking dabigatran (150 mg twice daily) was also assumed to change at the age of 75 years.
-
The life expectancy given a Glasgow Outcome Scale state 2 (GOS2) was reduced to a maximum of 3.4 years.
The outcome following stroke
Not all strokes are the same in their consequences. The immediate outcome following a stroke is divided into three categories:
-
death from stroke
-
dependent state following stroke
-
independent state following stroke.
Determining the category of stroke
This section describes the methods used to estimate the probabilities of different mutually exclusive states following a stroke, and the costs and utilities associated with each of these states.
The outcome following a stroke is estimated using a two-stage process, using data from Rivero-Arias et al. 154 This paper154 reported that, of 1283 patients who had a stroke within the Oxford Vascular Study (OXVASC) cohort, 24.8% (319/1283) were dead within 24 months. Of those who survived, the degree of disability following the stroke was graded according to the modified Rankin Scale (mRS) 24 months after the event in 425 patients. For simplicity, this 24-month state is assumed to be the patient's permanent state until another event occurs, and the patients for whom mRS outcomes were reported were assumed to be representative of those for whom the data were not collected. The mRS has six discrete non-dead states, categorised 0–5, as shown in Table 32.
| mRS score | Category | Description |
|---|---|---|
| 0 | No symptoms | No symptoms at all |
| 1 | No significant disability | No significant disability despite symptoms; able to perform all usual duties and activities |
| 2 | Slight disability | Slight disability; unable to perform all normal activities but able to look after own affairs without assistance |
| 3 | Moderate disability | Moderate disability requiring some help but able to walk without assistance |
| 4 | Moderately severe disability | Moderately severe disability; unable to walk without assistance and unable to attend to own bodily needs without assistance |
| 5 | Severe disability | Severe disability; bedridden, incontinent, and requiring constant nursing care and attention |
By convention, mRS states 0–2 are categorised as ‘independent’ states, and states 3–5 as ‘dependent’ states. Of those with mRS states recorded at 24 months, 74.1% of those living after a stroke were in an independent state, and 25.9% were in a dependent state, as indicated in Figure 8.
FIGURE 8.
Distribution of stroke outcomes at 24 months (survivors at 24 months only).
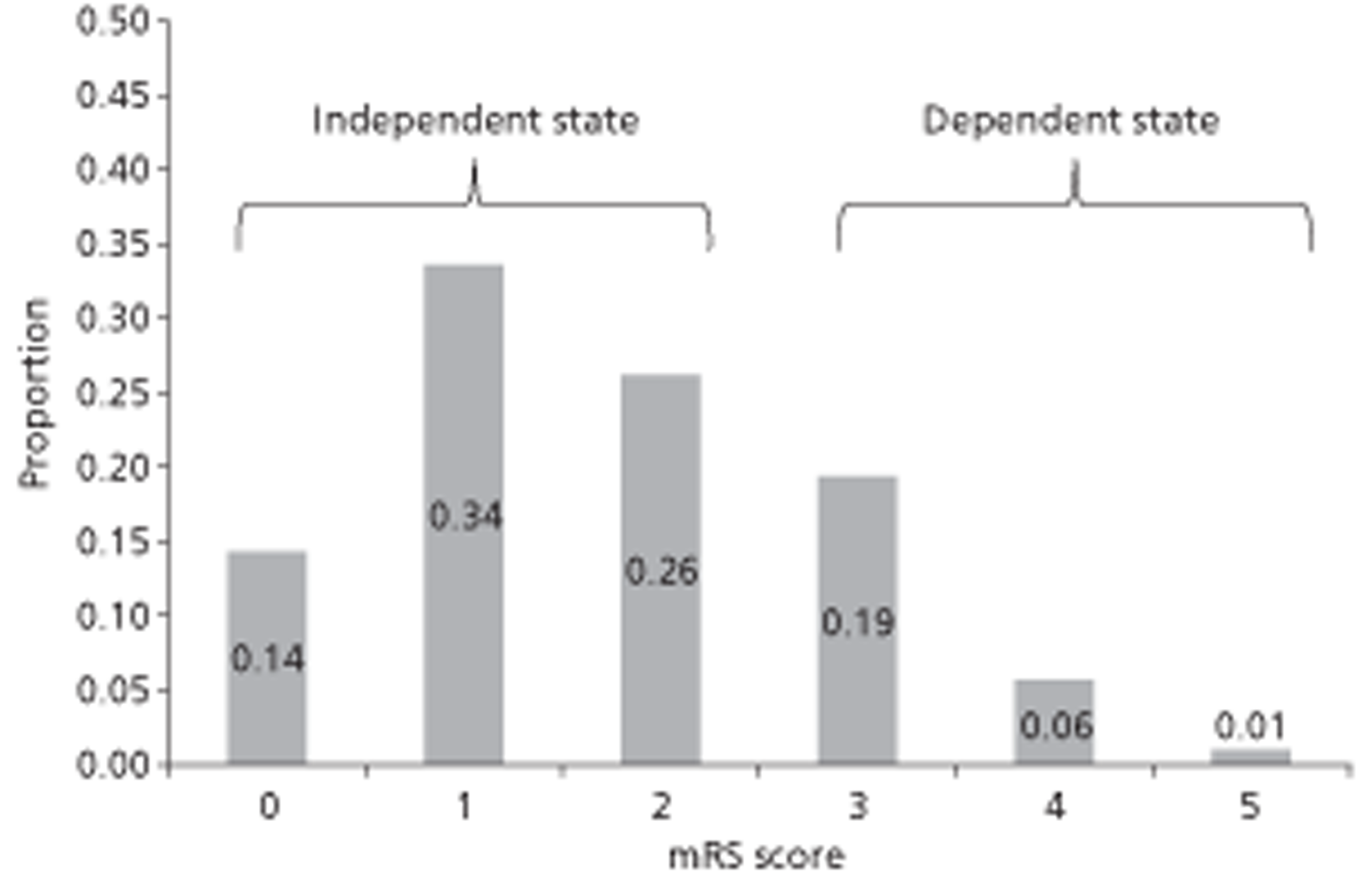
Uncertainty in the proportion of patients who survive a stroke was represented using a binomial distribution. As it is required for the accurate calculation of utility multipliers associated with dependent and independent states, the proportion of patients in each of the six mRS outcome states was used to parameterise a Dirichlet distribution in order to represent uncertainty in the distribution of non-dead outcome states following a stroke. These values were then converted back into estimated proportions of those alive in dependent and independent states following stroke. Results from the two-state (dead/alive) Binomial simulation and the six-state (mRS 0–6) Dirichlet simulation were used to estimate uncertainty in the proportions of patient outcomes following stroke in the three mutually exclusive categories: ‘dead’, ‘dependent stroke’, and ‘independent stroke’. The estimated proportions, together with 95% CrIs, are shown in Table 33 and graphically in Figure 9.
| State | Central estimate | 95% CrI |
|---|---|---|
| Dead | 0.25 | 0.23 to 0.27 |
| Independent | 0.56 | 0.52 to 0.59 |
| Dependent | 0.19 | 0.16 to 0.23 |
FIGURE 9.
The estimated distribution of patients 24 months after a stroke.
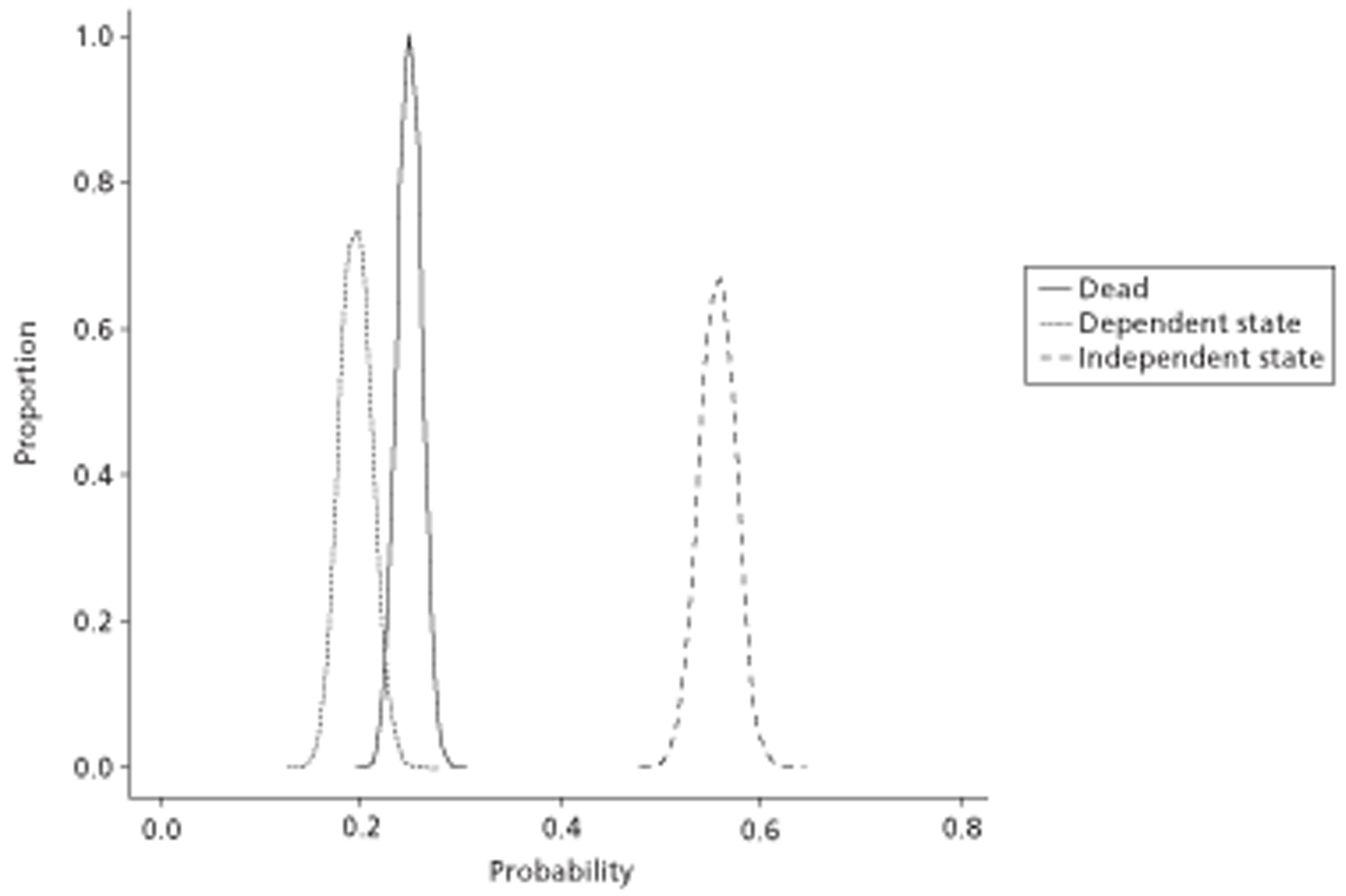
The effect of a stroke on a patient's utility
The utilities associated with independent and dependent states following strokes were estimated from the same data set used to determine the outcome following stroke. 154
Utility multipliers following a stroke were estimated from data that presented EuroQOL Five Dimension (EQ-5D) mapped utility estimates for the utility associated with each of the six mRS scores. As the mildest of these categories (mRS 0) is a full recovery, this is assumed to represent baseline patient utility. Multipliers for mRS 1–5 were thus calculated by dividing utility estimates of these worse states by the utility estimates of mRS 0. Uncertainty in both nominators and denominators were estimated using a simulation approach, with 10,000 random draws from EQ-5D estimates of each of the states mRS 1–5 divided by 10,000 random draws from the EQ-5D estimates for state mRS 0.
In order to derive estimates of the utility multiplier associated with both dependent and independent strokes, the proportions of each of the constituent mRS states within the dependent and independent stroke categories need to be estimated. Uncertainty in our knowledge of these proportions thus also needs to be represented. This is done as follows:
-
sample from a Dirichlet distribution with all six mRS states (as detailed in Introduction, above)
-
divide the six states into the independent stroke category (mRS 0–2) and dependent stroke category (mRS 3–5)
-
calculate the relative proportion of mRS states 0–2 within the independent stroke category, and relative proportion of mRS states 3–5 within the dependent stroke category
-
use weight utility multiplier estimates of mRS states 0, 1 and 2 in proportion to these states' relative prevalence within the independent stroke category, and weight utility multiplier estimates of mRS states 3, 4 and 5 in proportion to these states' relative prevalence within the dependent stroke category.
As our interest is in the mean utility multiplier for dependent and independent stroke multipliers, the mean values of 10,000 bootstraps of the distributions produced were then calculated in order to estimate both the means and uncertainty around the means. The mean utility multipliers produced are shown in Table 34.
| Category | Central utility estimate (95% CrI) |
|---|---|
| Independent state | 0.822 (0.819 to 0.824) |
| Dependent state | 0.482 (0.477 to 0.487) |
For simplicity, it was assumed that patients who had a fatal stroke accrued no further QALYs. This is a limitation as not all patients would have died instantly; however, data that could be used to accurately populate this parameter were not identified.
Comparison with previous utility multiplier estimates
Our estimated utility multipliers are very similar to those presented by Dorman et al. 155 for independent strokes, but somewhat higher than those reported in that paper for dependent strokes. This is largely due to the distribution of mRS states within the ‘independent stroke’ and ‘dependent stroke’ categories, which for both categories of stroke are weighted towards less severe mRS states (as shown in Figure 8). In the case of dependent strokes (mRS 3–5), for example, only around 4% were the worst category mRS 5, which has an estimated EQ-5D score of around 0, and around 75% were in the least severe category mRS 3, which has an estimated EQ-5D score of > 0.5. The discrepancy may reflect improvements in the prognosis following strokes in the decade that separates the studies used.
The assumed costs following stroke
Costs following categories of events were subdivided into one-off costs, such as the cost of admission to an emergency department, and ongoing costs, such as rehabilitation costs, which are assumed to continue indefinitely.
Cost of death due to stroke
The immediate costs associated with death due to a stroke were estimated using values reported in table 6 of a 2002 HTA report. 156 This reported that the mean length of intensive care hospital stay was between 33 and 34 days, and that the mean cost of stay was around £200 per day (95% CI £150 to £500). The mean length of stay was assumed to follow a uniform distribution ranging from 33 to 34 days, and the cost per night to follow a log-normal distribution owing to the asymmetry of the CIs and the fact that a negative cost is implausible.
A total of 10,000 draws from both distributions were combined in a random order to produce a distribution of estimated cost per death. Bootstrapping was used to simulate uncertainty in the mean of this distribution; 1000 draws from this bootstrapped distribution of means was used within the PSA. Costs were then inflation adjusted. 157 The estimated mean costs together with 95% CrIs are presented in Table 35.
| Value | Central estimate (£) (95% CrI) |
|---|---|
| Cost of death due to stroke | 9319 (9259 to 9378) |
Cost of dependent state due to stroke
The one-off cost of a patient entering a dependent state due to stroke was estimated using currency code AA22Z (‘Non-transient Stroke or Cerebrovascular Accident, Nervous system infections of Encephalopathy’) for long-stay non-elective inpatients from the NHS reference costs 2009–10. 153
The ongoing cost of a patient in a dependent state due to stroke was estimated using data reported in the National Stroke Strategy Impact Assessment,153 with costs inflation adjusted from 2005–6 to 2009–10 values. 157 A summary of these costs is presented in Table 36.
| Value | Central estimate (£) (95% CrI) |
|---|---|
| One-off cost of a patient in a dependent state | 2830 (2708 to 2952) |
| Ongoing annual cost of a patient in a dependent state | 6386 (5749 to 7023) |
Cost of independent state due to stroke
The one-off cost of a patient entering an independent state due to stroke was estimated using currency code AA22Z (‘Non-transient Stroke or Cerebrovascular Accident, Nervous system infections of Encephalopathy’) for short-stay non-elective inpatients, from the NHS reference costs 2009–10. 153
The ongoing cost of a patient in an independent state owing to stroke was estimated using data reported in the National Stroke Strategy Impact Assessment,157 with costs inflation adjusted from 2005–6 to 2009–10 values. A summary of these costs is presented in Table 37.
| Value | Central estimate (£) (95% CrI) |
|---|---|
| One-off cost of a patient in an independent state | 542 (513 to 571) |
| Ongoing cost of a patient in an independent state | 3195 (2871 to 3518) |
The outcome following major clinical bleed
Determining the category of major clinical bleed
Not all major clinical bleeds are the same in their consequences. The immediate outcome following a bleed is divided into three categories:
-
death from major bleed
-
non-fatal gastrointestinal (GI) haemorrhage
-
non-fatal intracranial haemorrhage (ICH).
There is a wide variation in the effects of an ICH. To represent this variation, outcomes following non-fatal ICH are further subdivided into four distinct states according to the GOS (described later):
-
GOS 2 vegetative state
-
GOS 3 severely disabled
-
GOS 4 moderately disabled
-
GOS 5 good recovery.
The probabilities of discrete states following a bleed were calculated using a two-stage approach as described below.
Initial stage
In the model, three possible outcomes are assumed to result from a major bleeding event:
-
death from bleed
-
non-fatal GI haemorrhage
-
non-fatal ICH.
The proportions of these three events are estimated from table 79 of a 2009 HTA monograph,158 which is derived from a 2003 meta-analysis. 159 Uncertainty about the relative distribution of these three outcomes was represented using a Dirichlet distribution. These results are shown in Table 38.
| Event category | Dirichlet distribution value | Central estimate (95% CrI) |
|---|---|---|
| Fatal bleed | 22.7 | 0.11 (0.08 to 0.16) |
| Non-fatal GI | 28.4 | 0.80 (0.74 to 0.84) |
| Non-fatal ICH | 198.9 | 0.09 (0.06 to 0.13) |
If a non-fatal ICH occurs, the effect of this bleed on patient outcome is simulated using data that maps outcomes on to the GOS, which categorises a patient's state after a traumatic brain injury. 160 Uncertainty about the relative distribution of these three outcomes was represented using a Dirichlet distribution. These results are shown in Table 39 and use data in Holmes et al. 161
A GOS state of 2 is associated with a severely reduced life expectancy. This was taken into account in the model by replicating an assumption in Holmes et al. ,161 reducing the life expectancy to 3.4 years where it was otherwise expected to be greater.
Utility multiplier following a major clinical bleed
Utility multiplier following a fatal bleed
It was assumed that the patient would die immediately following a bleeding-related mortality and that no further QALYs would be accrued.
Utility multiplier following a gastrointestinal haemorrhage
The effect of a GI haemorrhage on long-term quality of life is generally considered to be very small. A decision analysis by Goodacre et al. 162 assumed that the event resulted in no utility loss. A separate decision analysis model by Meenan et al. 163 used a utility multiplier of 0.997. Within the PSA, estimates were sampled from a uniform distribution with upper and lower bounds of 0.997 ± 0.003.
Utility multipliers following an intracranial haemorrhage
The utility following an ICH depends on the GOS state that follows from the haemorrhage. These range from a long-term vegetative state (GOS 2) to a good recovery. Both the GOS and mRS provide ordinal scales of disability and dependence following damage to the brain. By comparing the outcome descriptions for each of the non-fatal GOS states to those of the mRS states described in Table 39, we mapped each GOS state on to one or more mRS states. This allowed us to map utility values on to each GOS state using data from the same patient group that was used to inform the stroke utilities. 154 The methods used to derive the utility multipliers for each GOS state are very similar to those used to estimate utility multipliers following stroke, and also make the assumption that the distribution of the mRS states that the GOS states map on to is that reported at 24 months within the Rivero-Arias paper. 154 The assumed mapping between GOS scores and mRS scores, together with the estimated utility multipliers with 95% CrIs, are presented in Table 40.
| Event category | Dirichlet distribution value | Central estimate (95% CrI) |
|---|---|---|
| GOS 2 | 115.5 | 0.12 (0.10 to 0.14) |
| GOS 3 | 140 | 0.14 (0.12 to 0.16) |
| GOS 4 | 79.3 | 0.08 (0.06 to 0.10) |
| GOS 5 | 665.1 | 0.67 (0.64 to 0.70) |
| GOS state | Assumed equivalent to | Utility multiplier |
|---|---|---|
| GOS 2: vegetative state | mRS 6: dead | 0 |
| GOS 3: severely disabled | mRS 4: moderately severely disabled; and mRS 5: severely disabled | 0.226 (95% CI 0.221 to 0.231) |
| GOS 4: moderately disabled | mRS 2: slight disability and mRS 3: moderate disability |
0.642 (95% CI 0.638 to 0.645) |
| GOS 5: good recovery | mRS 0: no symptoms and mRS 1: no significant disability |
0.895 (95% CI 0.892 to 0.898 |
The assumed costs following a major clinical bleed
Costs following categories of events were subdivided into one-off costs (such as the cost of admission to an emergency department) and ongoing costs (such as nursing), which are assumed to continue indefinitely. Mean costs were calculated by adding together distributions from component costs associated with the event then using a bootstrapping approach to identify the mean and uncertainty around the mean of the distribution.
Costs of a fatal major clinical bleed
The costs of a death due to haemorrhage were assumed to be identical to the costs of death due to stroke. This was a mean of £7019 with a 95% CrI of £6975 to £7064.
Costs of a gastrointestinal haemorrhage
The one-off cost of a GI haemorrhage was derived from NHS Reference Costs 2009–2010,153 currency code FZ38E (‘Gastrointestinal Bleed with length of stay 2 days or more without major CC’) for non-elective inpatients. The central estimate plus 95% CIs are presented in Table 41.
| Value | Central estimate (£) (95% CI) |
|---|---|
| One-off cost of GI bleed | 1261 (1212 to 1310) |
Costs of an intracranial haemorrhage
The costs of an ICH were assumed to depend on the effects of the haemorrhage, as assessed using the GOS. As any intracranial bleed was assumed to be more costly than a GI haemorrhage, a cost equal to a GI bleed was added to the one-off costs of each of the GOS states. The specific one-off and ongoing costs associated with each GOS state are presented in Table 42.
| GOS state | One-off costs | Ongoing costs |
|---|---|---|
| GOS 2 | GI equivalent costs + GOS 2 intensive care costs + GOS 2 rehabilitation costs | Nursing home costs |
| GOS 3 | GI equivalent costs + intracranial procedures costs | GOS 3 annual care costs |
| GOS 4 | GI equivalent costs + intracranial procedures costs + GOS 4 rehabilitation costs | None |
| GOS 5 | GI equivalent costs | None |
Further details, including the reference sources and distributions of these component costs, are presented in Table 43.
| Cost component name | Distribution | Details | Source |
|---|---|---|---|
| GI equivalent costs | Normal (μ = 1261, σ = 25) | Code FZ38E | Department of Health153 |
| GOS 2 intensive care costs | Gamma (α = 165, β = 6) | Holmes161 | |
| GOS 2 rehabilitation costs | Gamma (α = 250, β = 120) | Holmes161 | |
| Nursing home costs | 52 × gamma (α = 159, β = 6) | Holmes161 | |
| Intracranial procedures costs | Normal (μ = 8829, σ = 633) | Code AA17Z | Department of Health153 |
| GOS 3 annual care cost | Gamma (α = 326, β = 104) | Holmes161 | |
| GOS 4 rehabilitation costs | Gamma (α = 385, β = 45) | Holmes161 |
The resulting combined cost estimates were bootstrapped. The bootstrapped estimates are shown in Table 44.
| State | Mean one-off costs (£) (95% CrI) | Mean ongoing cost (£) (95% CrI) |
|---|---|---|
| GOS 2 | 46,785 (40,895 to 53,250) | 50,047 (49,645 to 50,434) |
| GOS 3 | 10,096 (8849 to 11,363) | 33,949 (33,843 to 33,969) |
| GOS 4 | 27,419 (22,582 to 32,964) | None |
| GOS 5 | 1261 (1211 to 1309) | None |
Analyses undertaken
To facilitate the interpretation of results four cohorts of patients were simulated assuming that CHADS2 was the prevalent tool. These four cohorts were patients with a CHADS2 score of 0 (1 when considering warfarin) who:
-
have LA abnormality that was detected by TTE
-
have LA abnormality that was not detected by TTE
-
do not have LA abnormality that was not detected by TTE
-
do not have LA abnormality but where a TTE indicated that LA abnormality was present.
The first two cohorts represent higher risk patients, the remaining two cohorts represent low-risk patients. Cohort 1 represents a TP, cohort 2 represents a FN, cohort 3 represents a TN and cohort 4 represents a FN. For the baseline strategy where TTE is not used, all patients will be in cohorts 2 and 3, with the proportion in cohort 2 equal to the number of patients who actually have LA abnormality.
The results for each cohort were then weighted by the numbers of people in each cohort to form an overall estimation of costs and QALY for the entire population with the given CHADS2 score.
The main results section of the report presents, for each of the 14 pairs of comparisons made: summary statistics of the patient experience simulated in both the no-TTE and TTE strategies; a scatterplot of the output of the PSA;164 the cost-effectiveness acceptability frontier (CEAF) over willingness-to-pay thresholds ranging from £0/QALY to £50,000/QALY;165 and mean costs, QALYs and incremental cost-effectiveness ratios (ICERs) estimated from the mathematic models. The CEAF* was used as it incorporates information from both expected value of perfect information (EVPI) analyses and cost-effectiveness acceptability curves in a single measure. (*If there is no solid line in the figure, this indicates that the screening option is not optimal over all willingness-to-pay thresholds ranging from £0/QALY to £50,000/QALY.)
The EVPI was estimated. This provides the maximum level of investment that a funding body would be prepared to pay to eliminate all uncertainty in the decision problem. 166 In calculating EVPI an estimation of the number of patients who will be affected by the decision is required. We have performed a crude estimate that, assuming that the incidence of AF was 1 per 1000 person-years (approximately the pooled rate for women and men aged 55–64 years reported by the Renfrew/Paisley study),16 there are 6.7 million people aged between 55 and 64 years in England and Wales;145 6% of these people are in the CHADS2 0 category;13 and the information is relevant for 10 years. These broad estimates indicate that around 70,000 people would benefit from there being no uncertainty regarding whether TTE is cost-effective. Because a large number of subpopulations were considered, this was considered an upper estimate of the population EVPI to consider in each comparison. For illustration, the population EVPI is presented for each comparison assuming population sizes of 25,000, 50,000 and 75,000 people.
A more complex value of information analyses, EVPPI (expected value of partial perfect information), which indicates the maximum level of investment to reduce uncertainty in a subset of one or more parameters,167 could not be undertaken for computational reasons. This is because each PSA run of 1000 iterations, for each of the 14 groups considered, took approximately 1 hour to run, meaning that EVPPI would take approximately 14,000 hours to run, equivalent to over 1.5 years of uninterrupted computing time.
Instead, to provide further information, sensitivity analyses were undertaken on two key parameters: the proportion of patients with LA abnormality and the joint uncertainty in the sensitivity and specificity of TTE in detecting LA abnormality.
An alternative simplified methodology was also undertaken. This simply divided the assumed cost per QALY threshold £20,000 by the cost of performing a TTE (£66) to provide the threshold QALYs required for TTE to be cost-effective, were it assumed that there would be further benefits than in identifying LA abnormality.
Results
Main clinical effectiveness and cost-effectiveness results: comparison of transthoracic echocardiography and no-transthoracic echocardiography strategies
Fifty-year-old males, initial CHADS2 score of 0, informing the decision whether to treat patients with warfarin
Summary results for this treatment option and patient group are shown in Table 45 and Figure 10, below. They indicate that the majority of the PSA scatterplot is in the north-west quadrant, suggesting that the addition of TTE is ruled out by simple dominance in this patient group when using warfarin as the OAC. This is confirmed in the table of results, indicating that the mean cost of conducting full TTE screening is greater than not screening, whereas the mean QALY score is lower. Summary statistics from the patient experience simulation indicate that although the number of deaths from stroke is predicted to reduce as a result of using TTE in this way, the number of deaths due to major bleeding events is predicted to increase.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 28.840 | 11.7 | 1.3 | 87.1 | 0.120 | 0.242 | 0.010 | 0.075 |
| TTE with those diagnosed with LA abnormality treated | 28.928 | 10.8 | 1.8 | 87.4 | 0.111 | 0.223 | 0.014 | 0.112 |
FIGURE 10.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_50_0_M comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF (If there is no solid line in the figure, this indicates that the screening option using TTE is not optimal over all willingness-to-pay thresholds ranging from £0/QALY to £50,000/QALY.) (c) Mean costs, QALYs and ICERs.
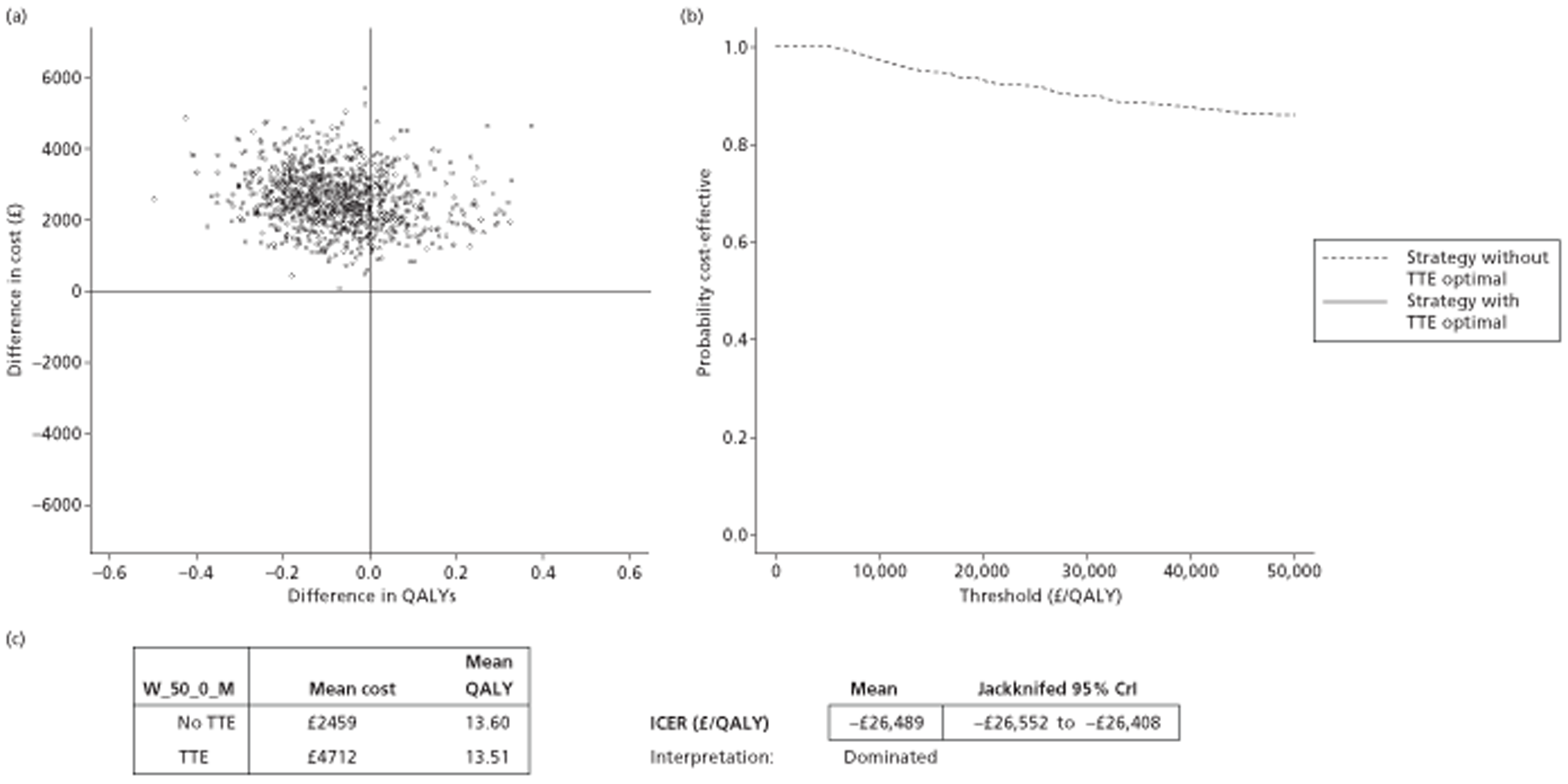
The CEAF indicates that not screening (dashed line) remains the optimal choice compared with screening (solid line) at willingness-to-pay thresholds varying from £0/QALY to £50,000/QALY. In this case, the addition of TTE is not predicted to be the optional choice at willingness-to-pay thresholds of either £20,000/QALY or £30,000/QALY
Fifty-year-old females, initial CHADS2 score of 0, treated with warfarin
Summary results for this treatment option and patient group are shown in Table 46 and Figure 11, below. They also indicate that the majority of the PSA scatterplot is in the north-west quadrant, suggesting that the addition of TTE is ruled out by simple dominance in this patient group when using warfarin as the OAC. This is confirmed in the table of results, indicating that the mean cost of conducting full TTE screening is greater than not screening, whereas the mean QALY score is lower. Summary statistics from the patient experience simulation indicate that although the number of deaths from stroke is predicted to reduce as a result of using TTE in this way, the number of deaths due to major bleeding events is predicted to increase.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent Strokes | Independent Strokes | ICH | NICH | ||
| No initial treatment | 31.633 | 13.5 | 1.6 | 84.9 | 0.139 | 0.278 | 0.012 | 0.091 |
| TTE with those diagnosed with LA abnormality treated | 31.734 | 12.6 | 2.1 | 85.2 | 0.130 | 0.259 | 0.017 | 0.130 |
FIGURE 11.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_50_0_F comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF (If there is no solid line in the figure, this indicates that the screening option using TTE is not optimal over all willingness-to-pay thresholds ranging from £0/QALY to £50,000 per QALY.) (c) Mean costs, QALYs and ICERs.
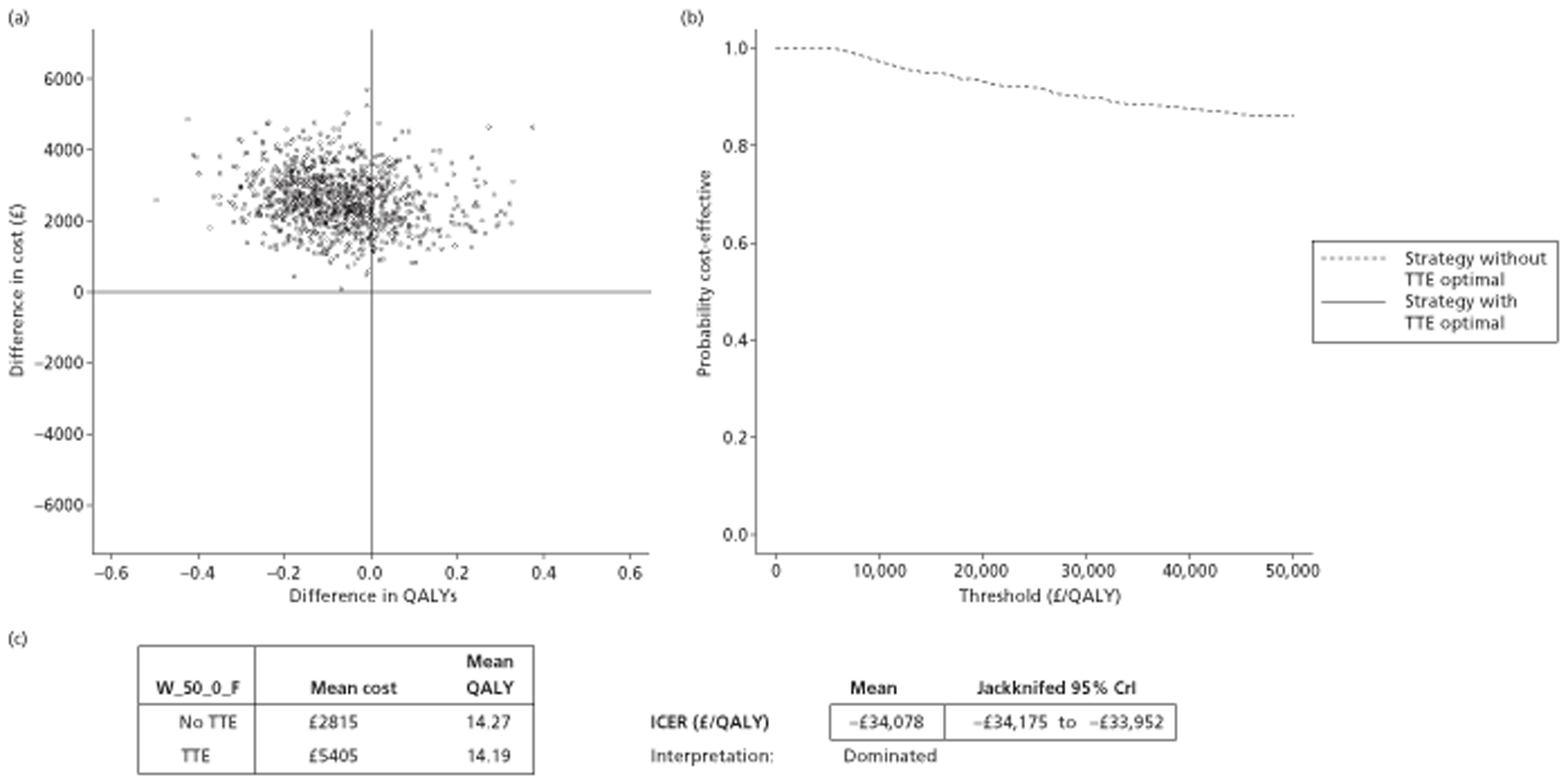
The CEAF indicates that not screening (dashed line) remains the optimal choice compared with screening (solid line) at willingness-to-pay thresholds varying from £0/QALY to £50,000/QALY. In this case, the addition of TTE is not predicted to be the optional choice at willingness-to-pay thresholds of either £20,000/QALY or £30,000/QALY.
Sixty-five-year-old males, initial CHADS2 score of 0, treated with warfarin
Summary results for this treatment option and patient group are shown in Table 47 and Figure 12. The PSA scatterplot suggests that although the use of TTE is clearly associated with increased costs, it is not associated with substantially increased predicted QALYs. This is confirmed by the table in Figure 12c, which shows the TTE strategy to cost almost £1000 more than the no-TTE strategy, but to result in almost no increase in QALYs. Summary statistics from the patient experience simulation indicate that although the number of deaths from stroke is predicted to reduce as a result of using TTE in this way, the number of deaths due to major bleeding events is predicted to increase.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 17.131 | 9.0 | 0.9 | 90.2 | 0.087 | 0.192 | 0.007 | 0.052 |
| TTE with those diagnosed with LA abnormality treated | 17.204 | 8.0 | 1.3 | 90.7 | 0.078 | 0.172 | 0.010 | 0.079 |
FIGURE 12.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_65_0_M comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF (If there is no solid line in the figure, this indicates that the screening option using TTE is not optimal over all willingness-to-pay thresholds ranging from £0/QALY to £50,000 per QALY.) (c) Mean costs, QALYs and ICERs.
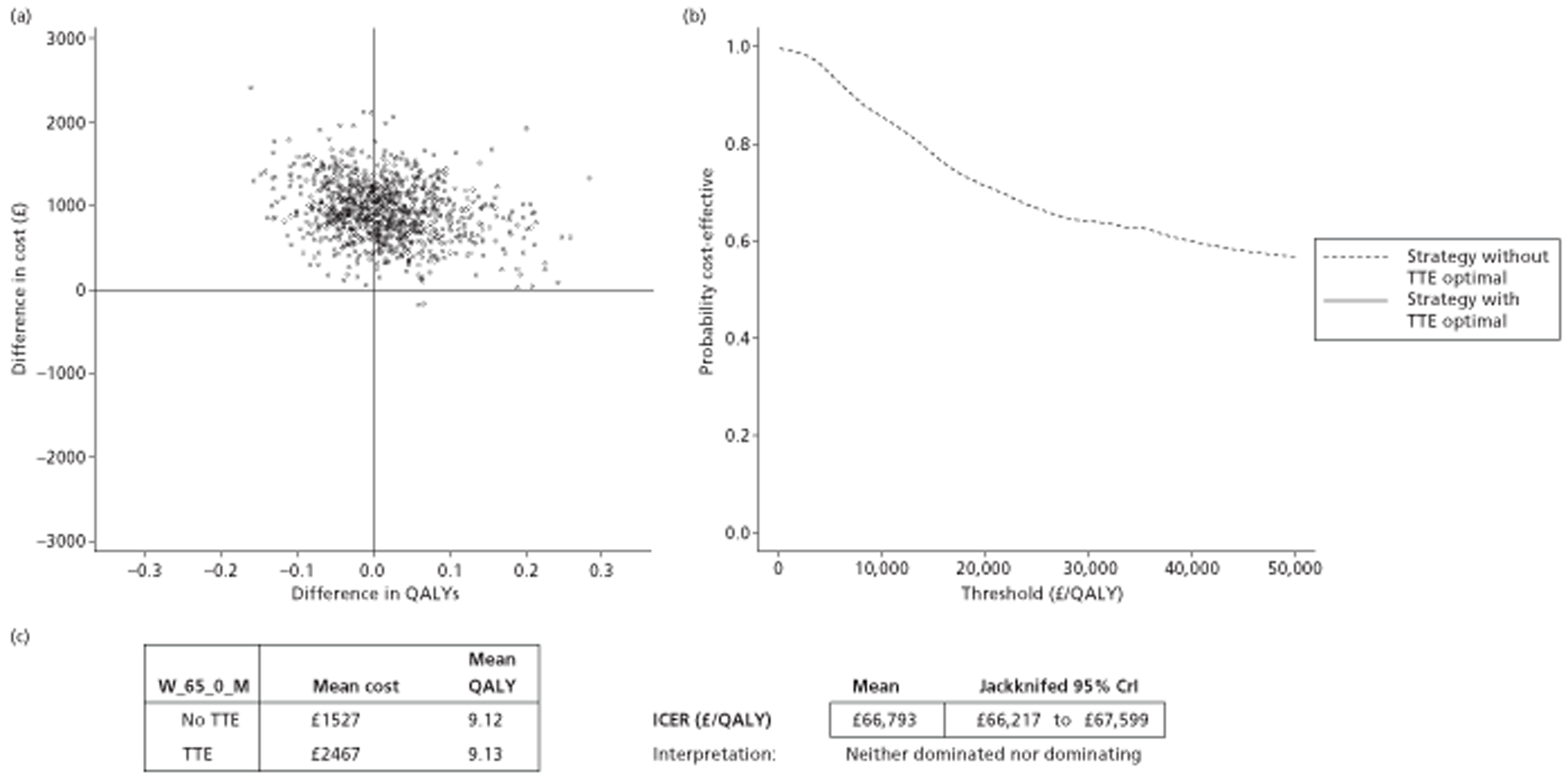
The CEAF indicates that not screening (dashed line) remains the optimal choice compared with screening (solid line) at willingness-to-pay thresholds varying from £0/QALY to £50,000/QALY. In this case, the addition of TTE is not predicted to be the optional choice at willingness-to-pay thresholds of either £20,000/QALY or £30,000/QALY.
Sixty-five-year-old females, initial CHADS2 score of 0, treated with warfarin
Summary results for this treatment option and patient group are shown in Table 48 and Figure 13. The PSA scatter indicates that slightly more of the estimates were in the north-east than the north-west quadrant. The table in part (c) of Figure 13 shows that the differences in mean costs between strategies are around £1000, but the differences in mean QALYs are less than one-tenth of a QALY. The mean ICER is around £40,000/QALY, and the CEAF indicates that the TTE strategy (solid line) is unlikely to be the optimal decision at standard NICE willingness-to-pay thresholds of £20,000/QALY and £30,000/QALY.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 19.447 | 10.6 | 1.1 | 88.3 | 0.105 | 0.225 | 0.009 | 0.065 |
| TTE with those diagnosed with LA abnormality treated | 19.531 | 9.6 | 1.6 | 88.8 | 0.096 | 0.205 | 0.012 | 0.095 |
FIGURE 13.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_65_0_F comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
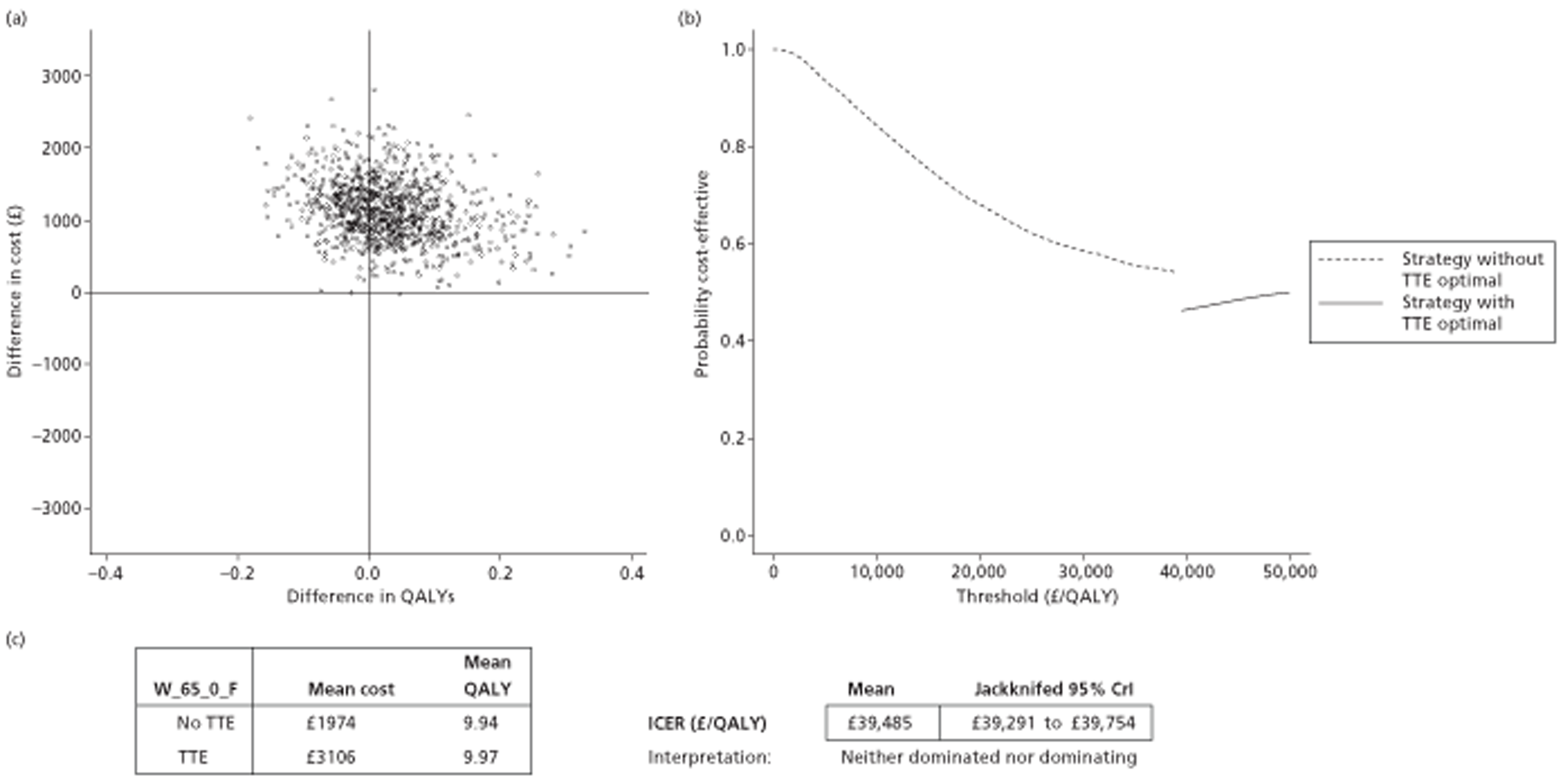
Fifty-year-old males, initial CHADS2 score of 1, treated with warfarin
In this patient group and treatment option (Table 49) the mathematical model shows that the majority of the estimates from the PSA (as shown in Figure 14a) are in the north-east quadrant, indicating that the TTE strategy is both more costly and more effective than the no-TTE strategy. There is a difference in mean QALYs between the two strategies of approximately half a QALY (the table shown in Figure 14c) and difference in mean costs of approximately £3000. The mean ICER is estimated to be slightly over £6000/QALY, and the CEAF indicates that, compared with the no-TTE strategy (dashed line), the TTE strategy (solid line) appears optimal at both the £20,000/QALY and £30,000/QALY willingness-to-pay thresholds; at both thresholds the probability that the TTE strategy is more cost-effective is estimated to be > 99%.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 25.921 | 22.6 | 2.7 | 74.7 | 0.235 | 0.463 | 0.019 | 0.156 |
| TTE with those diagnosed with LA abnormality treated | 26.250 | 20.8 | 3.5 | 75.7 | 0.218 | 0.424 | 0.025 | 0.208 |
FIGURE 14.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_50_1_M comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
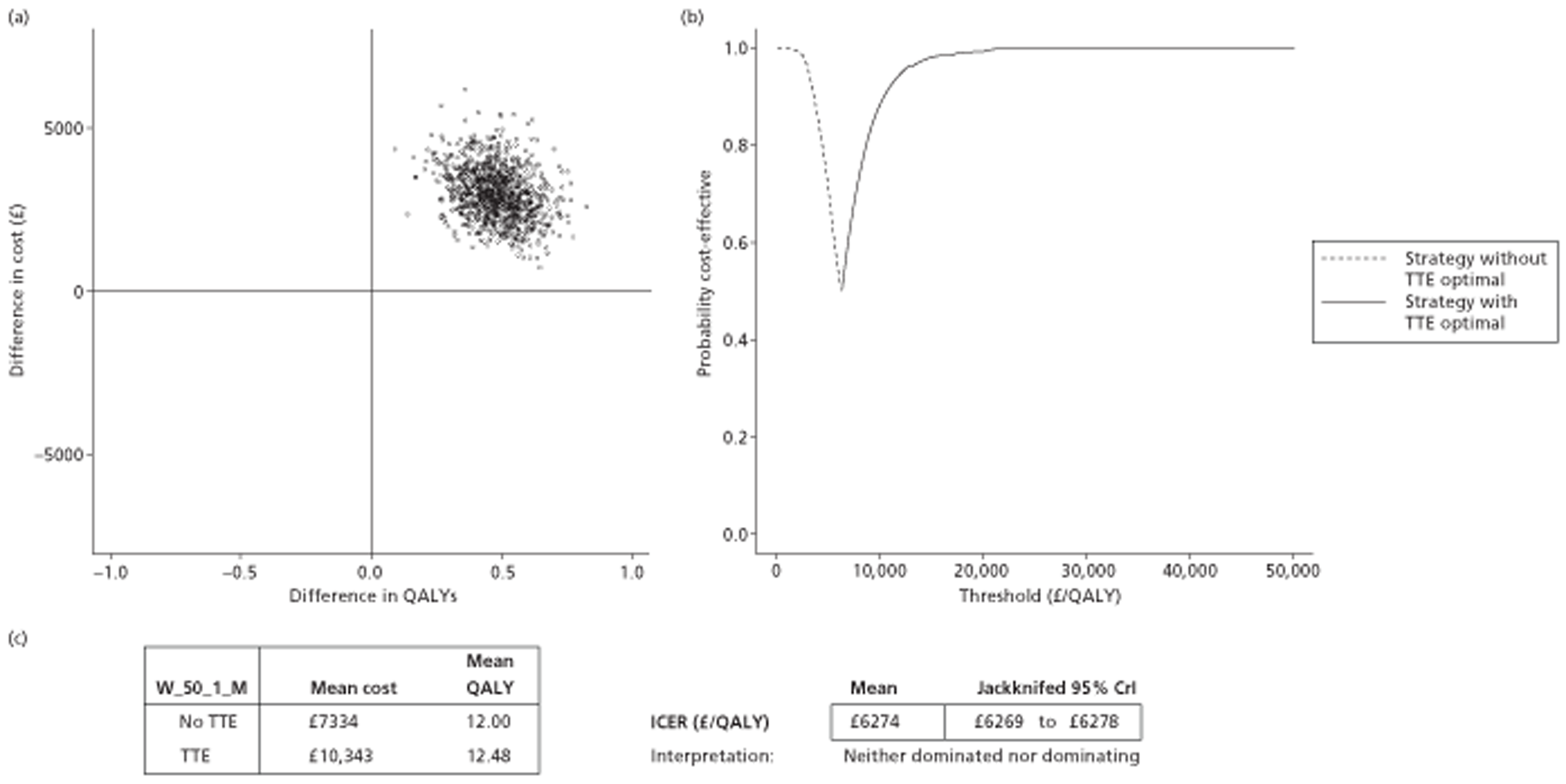
Fifty-year-old females, initial CHADS2 score of 1, treated with warfarin
In this patient group and treatment option (Table 50) the mathematical model shows that the majority of the estimates from the PSA (as shown in Figure 15a) are in the north-east quadrant, indicating that the TTE strategy is both more costly and more effective than the no-TTE strategy. There is a difference in mean QALYS between the two strategies of approximately half a QALY (the table in Figure 15c) and a difference in mean costs of approximately £3000 . The mean ICER is estimated to be slightly over £7000/QALY, and the CEAF indicates that, compared with the no-TTE strategy (dashed line), the TTE strategy (solid line) appears optimal at both the £20,000/QALY and £30,000/QALY willingness-to-pay thresholds; at both thresholds the probability that the TTE strategy is more cost-effective is estimated to be > 99%.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent Strokes | ICH | NICH | ||
| No initial treatment | 28.294 | 24.6 | 3.1 | 72.4 | 0.259 | 0.496 | 0.021 | 0.181 |
| TTE with those diagnosed with LA abnormality treated | 28.660 | 22.8 | 3.8 | 73.4 | 0.243 | 0.459 | 0.027 | 0.234 |
FIGURE 15.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_50_1_F comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
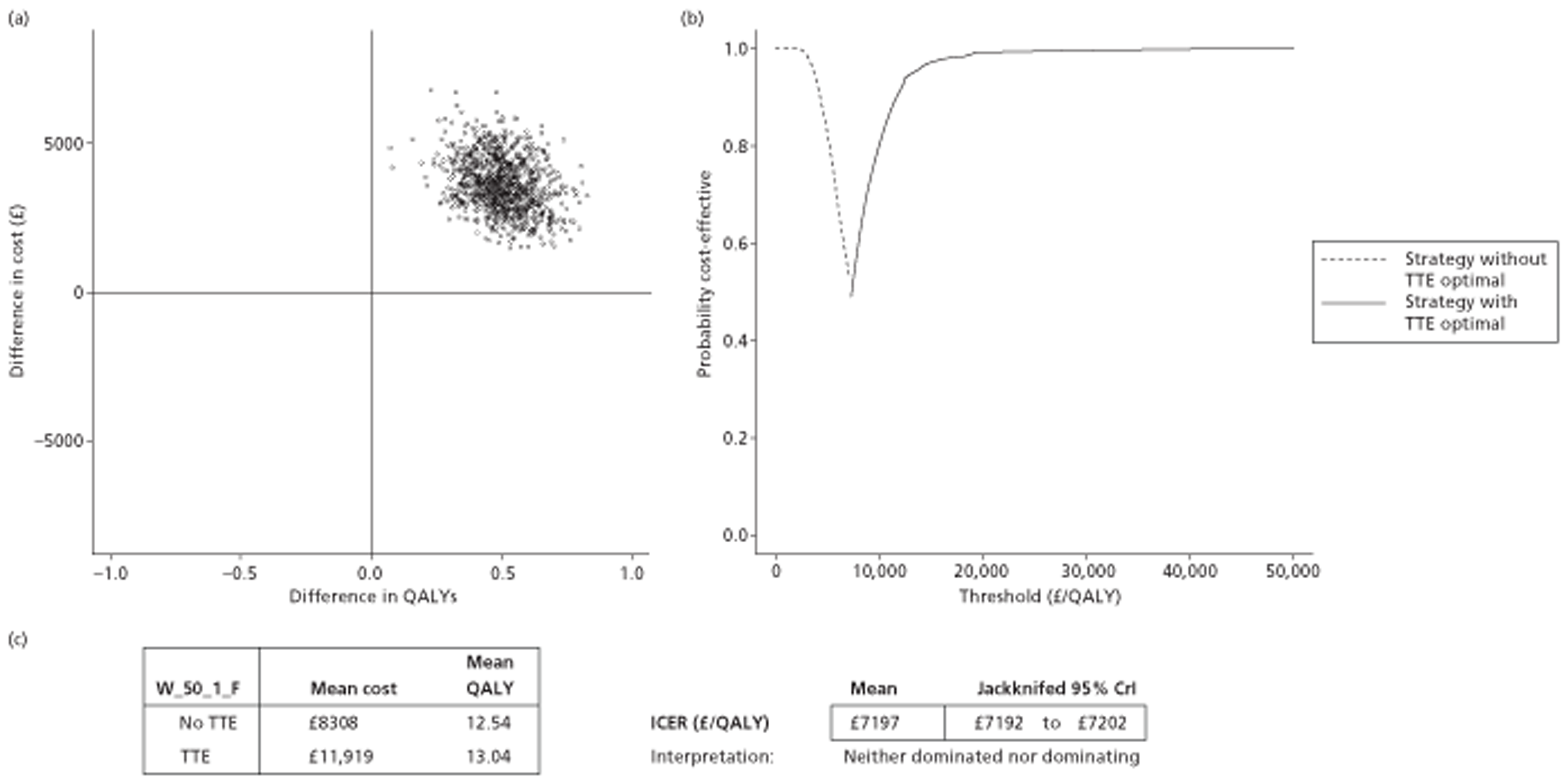
Sixty-five-year-old males, initial CHADS2 score of 1, treated with warfarin
In this patient group and treatment option (Table 51) the mathematical model shows that the majority of the estimates from the PSA (as shown in Figure 16a) are in the north-east quadrant, indicating that the TTE strategy is both more costly and more effective than the no-TTE strategy. There is a difference in mean QALYs between the two strategies (the table in Figure 16c) of approximately one-fifth of a QALY and a difference in mean costs of approximately £2500. The mean ICER is estimated to be around £11,000/QALY, and the CEAF indicates that, compared with the no-TTE strategy (dashed line), the TTE strategy (solid line) appears optimal at both the £20,000/QALY (93% probability most cost-effective) and £30,000/QALY (98.5% probability most cost-effective) willingness-to-pay thresholds.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 16.176 | 14.2 | 1.5 | 84.4 | 0.135 | 0.303 | 0.012 | 0.084 |
| TTE with those diagnosed with LA abnormality treated | 16.361 | 12.5 | 2.1 | 85.4 | 0.121 | 0.265 | 0.016 | 0.125 |
FIGURE 16.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_65_1_M comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
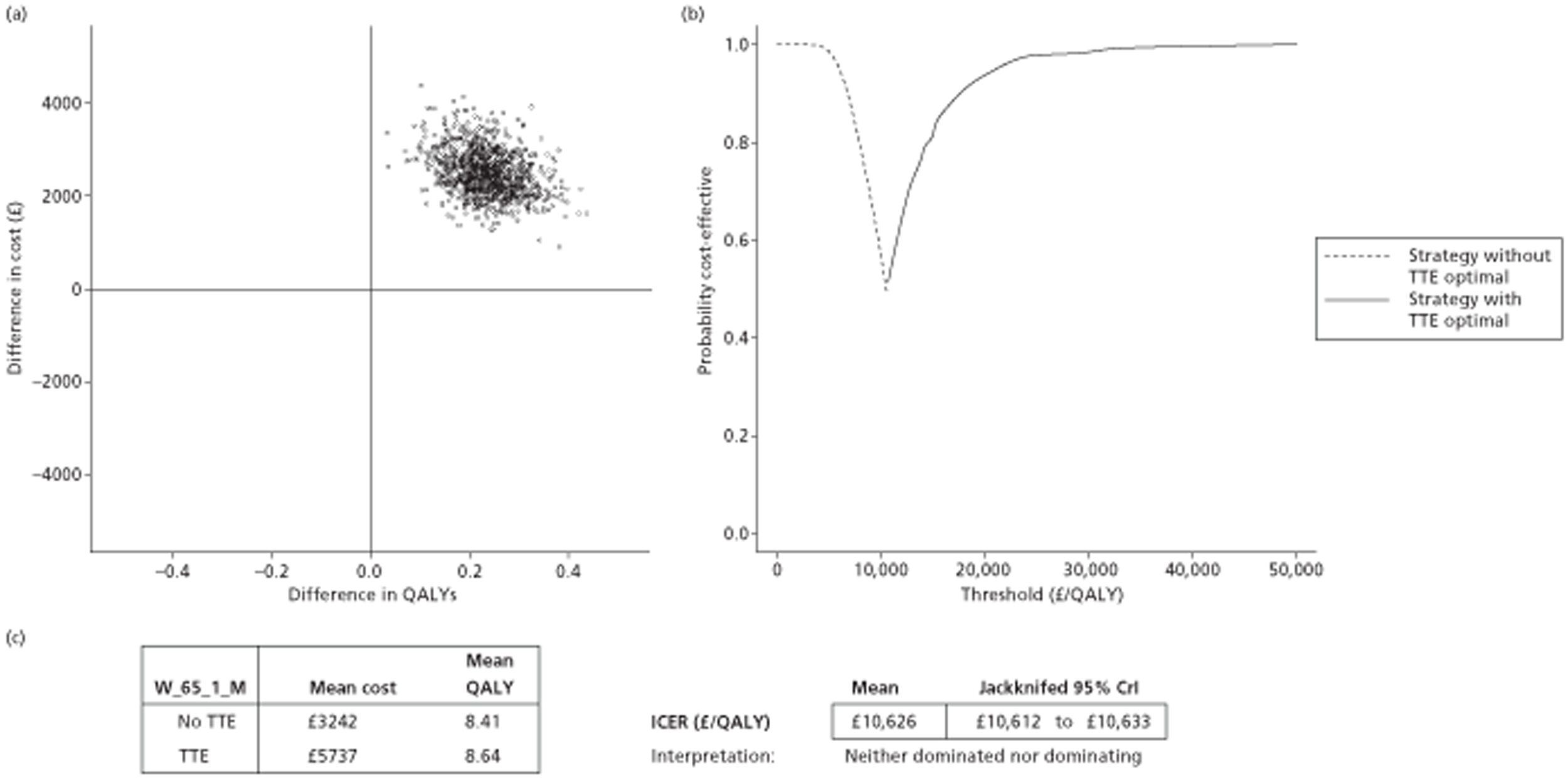
Sixty-five-year-old females, initial CHADS2 score of 1, treated with warfarin
In this patient group and treatment option (Table 52) the mathematical model the majority of the estimates from the PSA (as shown in Figure 17a) are in the north-east quadrant, indicating that the TTE strategy is both more costly and more effective than the no-TTE strategy. There is a difference in mean QALYs between the two strategies of approximately one-fifth of a QALY (the table in Figure 17c) and a difference in mean costs of approximately £4000. The mean ICER is estimated to be slightly < £15,000/QALY, and the CEAF indicates that, compared with the no-TTE strategy (dashed line), the TTE strategy (solid line) appears optimal at both the £20,000/QALY (73% probability most cost-effective) and £30,000/QALY (92% probability most cost-effective) willingness-to-pay thresholds.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 18.340 | 15.8 | 1.8 | 82.4 | 0.155 | 0.337 | 0.013 | 0.104 |
| TTE with those diagnosed with LA abnormality treated | 18.544 | 14.2 | 2.5 | 83.3 | 0.141 | 0.300 | 0.018 | 0.149 |
FIGURE 17.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the W_65_1_F comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
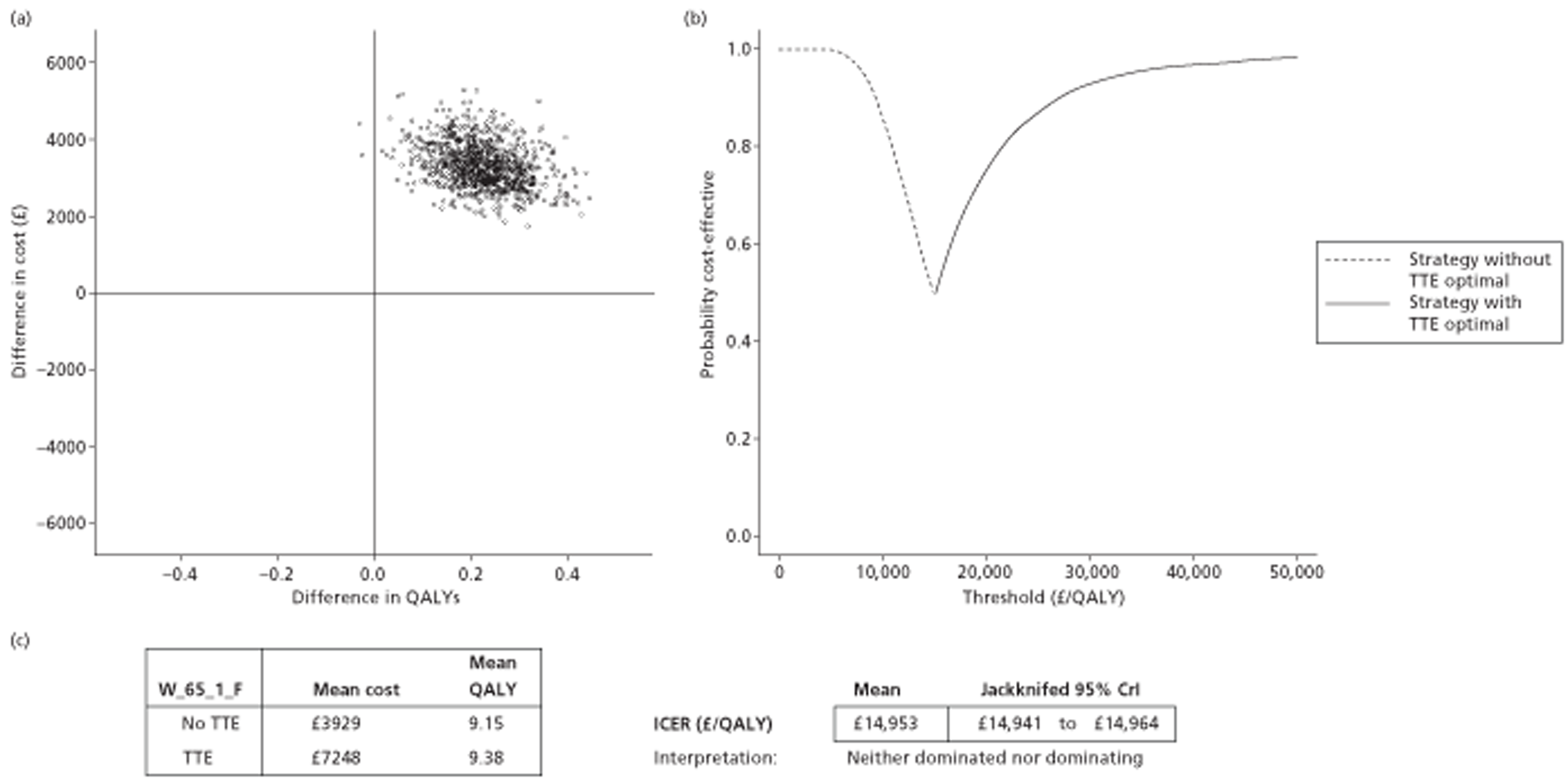
Fifty-year-old males, initial CHADS2 score of 0, treated with rivaroxaban
In this patient group and treatment option (Table 53) the mathematical model slightly more of the estimates from the PSA are in the north-west than north-east quadrant, implying that the TTE strategy is likely to be ruled out by simple dominance compared with the no-TTE strategy. This is confirmed by the mean values, which indicate that the TTE strategy is around £2000 more expensive and slightly less effective than the no-TTE strategy. The CEAF (Figure 18) indicates that the TTE strategy (solid line) does not appear to be the optimal strategy at all willingness-to-pay thresholds from £0/QALY to £50,000/QALY compared with the no-TTE strategy (dashed line).
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 28.861 | 11.5 | 1.3 | 87.2 | 0.117 | 0.239 | 0.010 | 0.075 |
| TTE with those diagnosed with LA abnormality treated | 28.963 | 10.5 | 1.8 | 87.6 | 0.108 | 0.219 | 0.014 | 0.113 |
FIGURE 18.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the R_50_0_M comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF (If there is no solid line in the figure, this indicates that the screening option using TTE is not optimal over all willingness-to-pay thresholds ranging from £0/QALY to £50,000 per QALY.) (c) Mean costs, QALYs and ICERs.
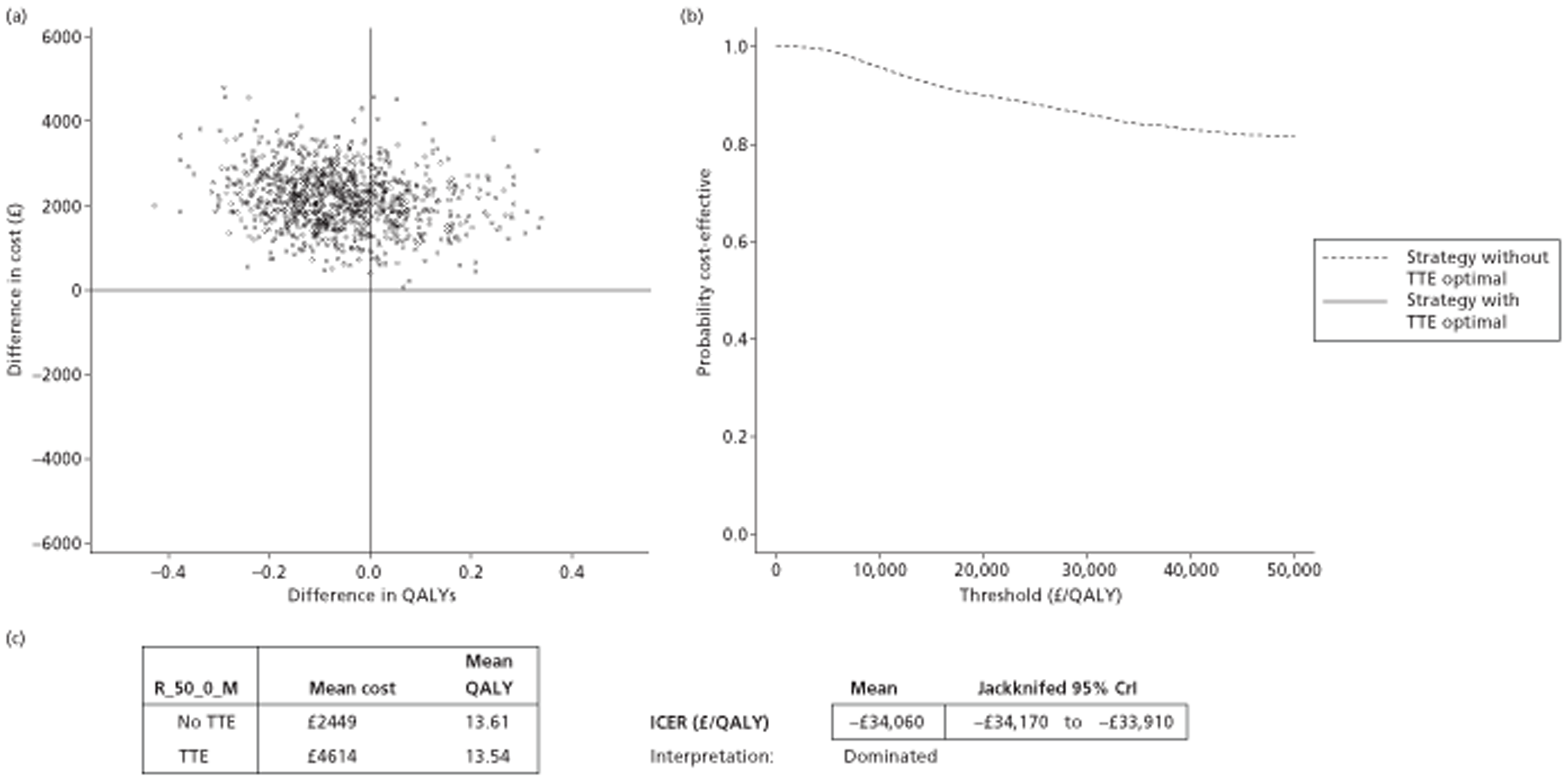
Fifty-year-old females, initial CHADS2 score of 0, treated with rivaroxaban
In this patient group and treatment option (Table 54) the mathematical model slightly more of the estimates from the PSA are in the north-west than north-east quadrant, implying that the TTE strategy is likely to be ruled out by simple dominance compared with the no-TTE strategy. This is confirmed by the mean values, which indicate that the TTE strategy is around £2500 more expensive and slightly less effective than the no-TTE strategy. The CEAF (Figure 19) indicates that the TTE strategy (solid line) does not appear to be the optimal strategy at all willingness-to-pay thresholds from £0/QALY to £50,000/QALY compared with the no-TTE strategy (dashed line).
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 31.657 | 13.3 | 1.6 | 85.1 | 0.136 | 0.275 | 0.012 | 0.091 |
| TTE with those diagnosed with LA abnormality treated | 31.772 | 12.4 | 2.1 | 85.5 | 0.127 | 0.255 | 0.017 | 0.130 |
FIGURE 19.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the R_50_0_F comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF (If there is no solid line in the figure, this indicates that the screening option using TTE is not optimal over all willingness-to-pay thresholds ranging from £0/QALY to £50,000 per QALY.) (c) Mean costs, QALYs and ICERs.
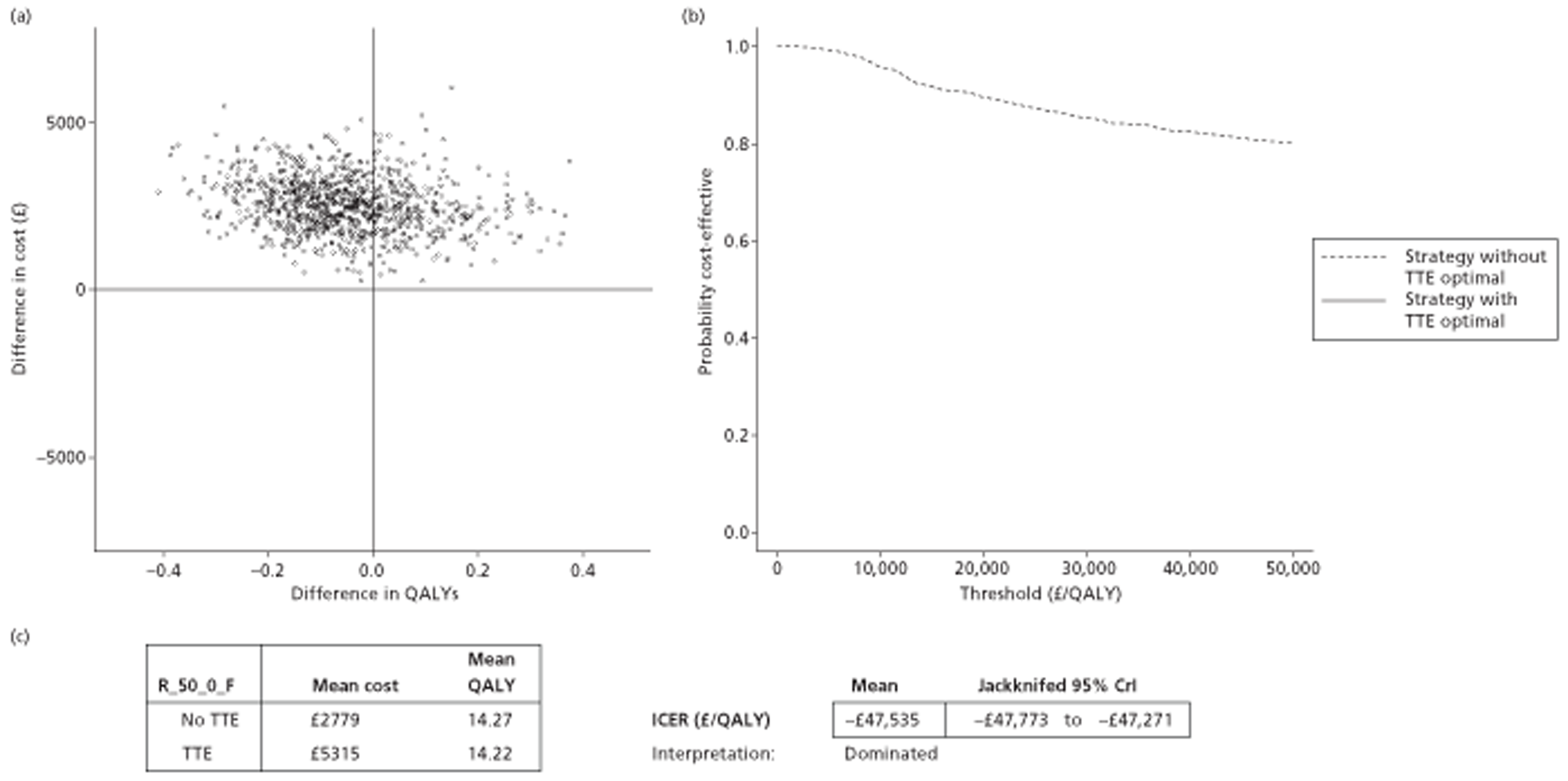
Sixty-five-year-old males, initial CHADS2 score of 0, treated with rivaroxaban
For this patient group and treatment combination (Table 55), the majority of the PSA scatterplot (Figure 20a) is either in the north-east or north-west quadrant. Slightly more of the scatterplot appears to be in the north-east quadrant, and the TTE strategy has both a greater mean cost and greater mean QALY estimate than the no-TTE strategy. The mean ICER is slightly over £30,000, and the CEAF (see Figure 20b) indicates that the TTE strategy is still not the optimal strategy compared with no TTE at the £30,000/QALY threshold; the TTE strategy is estimated to become optimal only at around £33,700/QALY.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 17.141 | 8.8 | 0.9 | 90.3 | 0.085 | 0.190 | 0.007 | 0.052 |
| TTE with those diagnosed with LA abnormality treated | 17.221 | 7.8 | 1.3 | 90.9 | 0.076 | 0.169 | 0.010 | 0.080 |
FIGURE 20.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the R_65_0_M comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
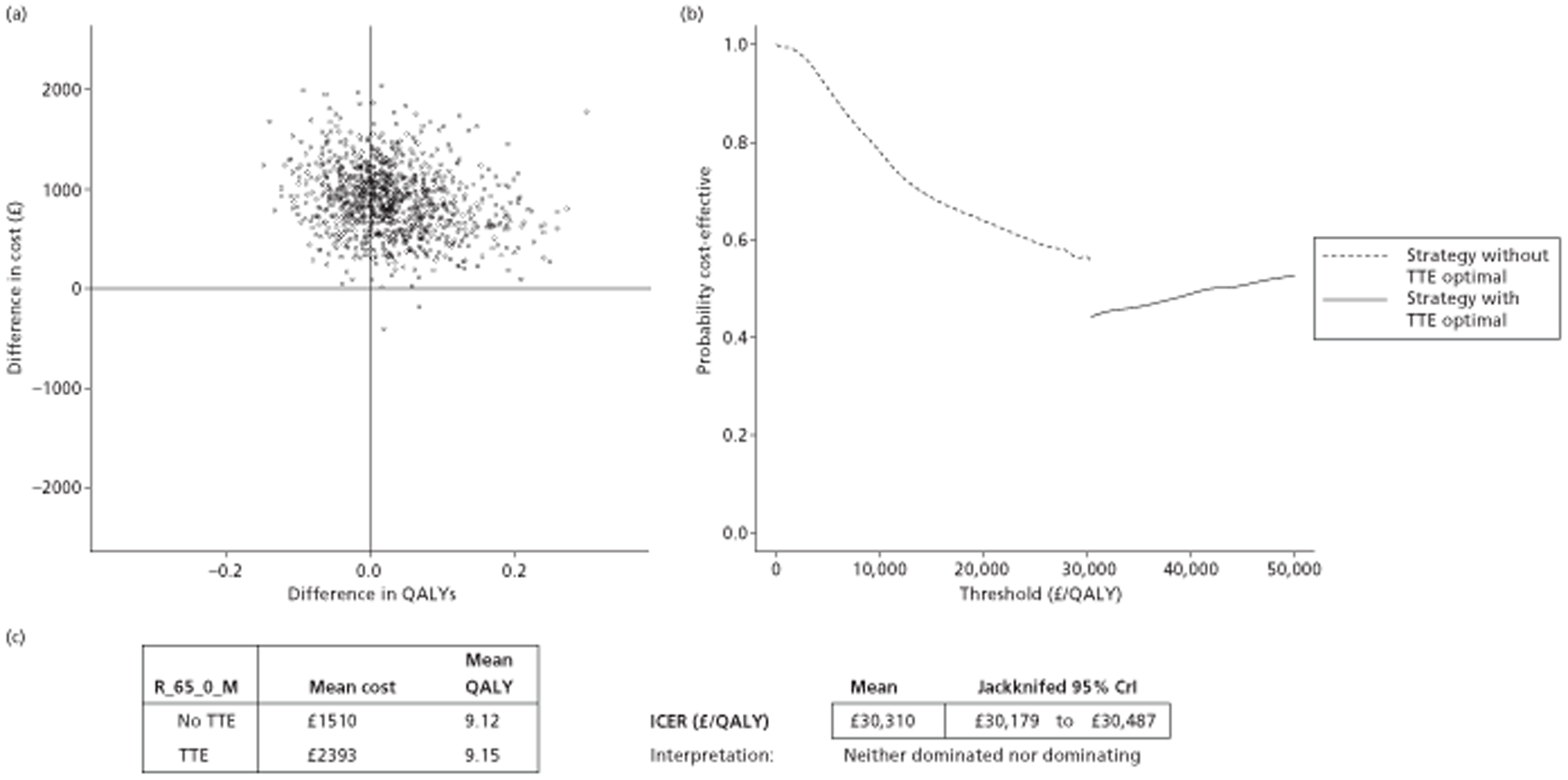
Sixty-five-year-old females, initial CHADS2 score of 0, treated with rivaroxaban
The mathematical model results (Table 56) suggest that the TTE strategy is more expensive and slightly more clinically effective, on the average, than the no-TTE strategy, with the PSA scatterplot appearing slightly more predominant in the north-east than the north-west quadrant. The mean ICER estimate is slightly > £20,000, and the CEAF indicates that the TTE strategy starts to become optimal at a willingness-to-pay threshold of approximately £21,000/QALY (Figure 21). If the willingness-to-pay threshold is £30,000 then the TTE strategy is estimated to have a 55% probability of being most cost-effective.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 19.460 | 10.5 | 1.1 | 88.4 | 0.103 | 0.223 | 0.009 | 0.066 |
| TTE with those diagnosed with LA abnormality treated | 19.554 | 9.4 | 1.6 | 89.0 | 0.093 | 0.201 | 0.012 | 0.096 |
FIGURE 21.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the R_65_0_F comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
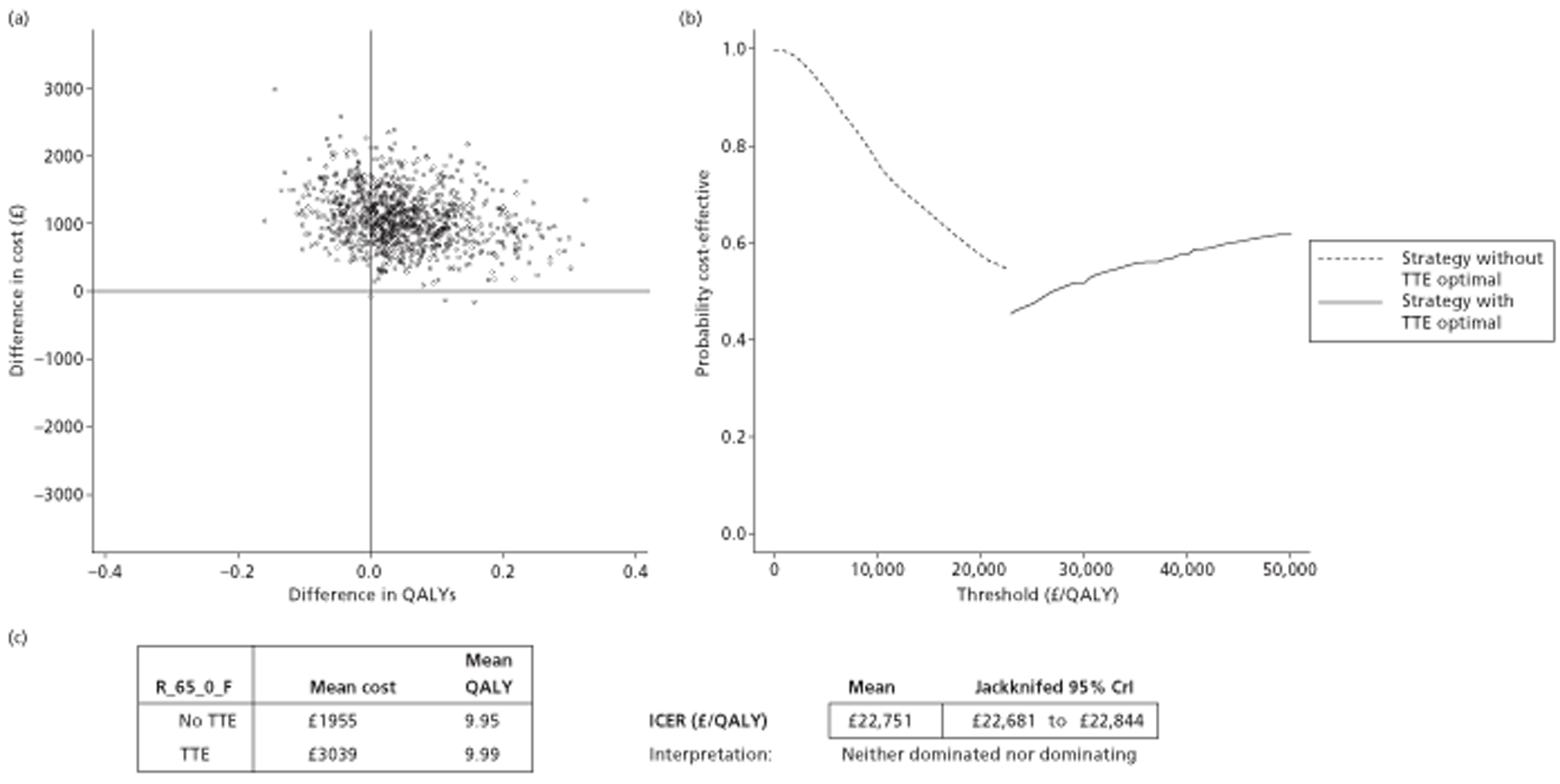
Sixty-five-year-old males, initial CHADS2 score of 0, treated with dabigatran
The mathematical model results (Table 57) suggest that the TTE strategy is more expensive and slightly more clinically effective, on the average, than the no-TTE strategy, with the PSA scatterplot appearing slightly more predominant in the north-east than the north-west quadrant. The mean ICER estimate is slightly < £15,000, and the CEAF indicates that the TTE strategy starts to become optimal at a willingness-to-pay threshold of approximately £13,800/QALY (Figure 22). If the willingness-to-pay threshold is £20,000, then the TTE strategy is estimated to have a 57% probability of being most cost-effective, and with a willingness-to-pay threshold of £30,000 the TTE strategy has a 65% probability of being most cost-effective.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 17.158 | 8.6 | 0.9 | 90.5 | 0.081 | 0.188 | 0.007 | 0.053 |
| TTE with those diagnosed with LA abnormality treated | 17.251 | 7.5 | 1.3 | 91.2 | 0.072 | 0.163 | 0.010 | 0.081 |
FIGURE 22.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the D_65_0_M comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
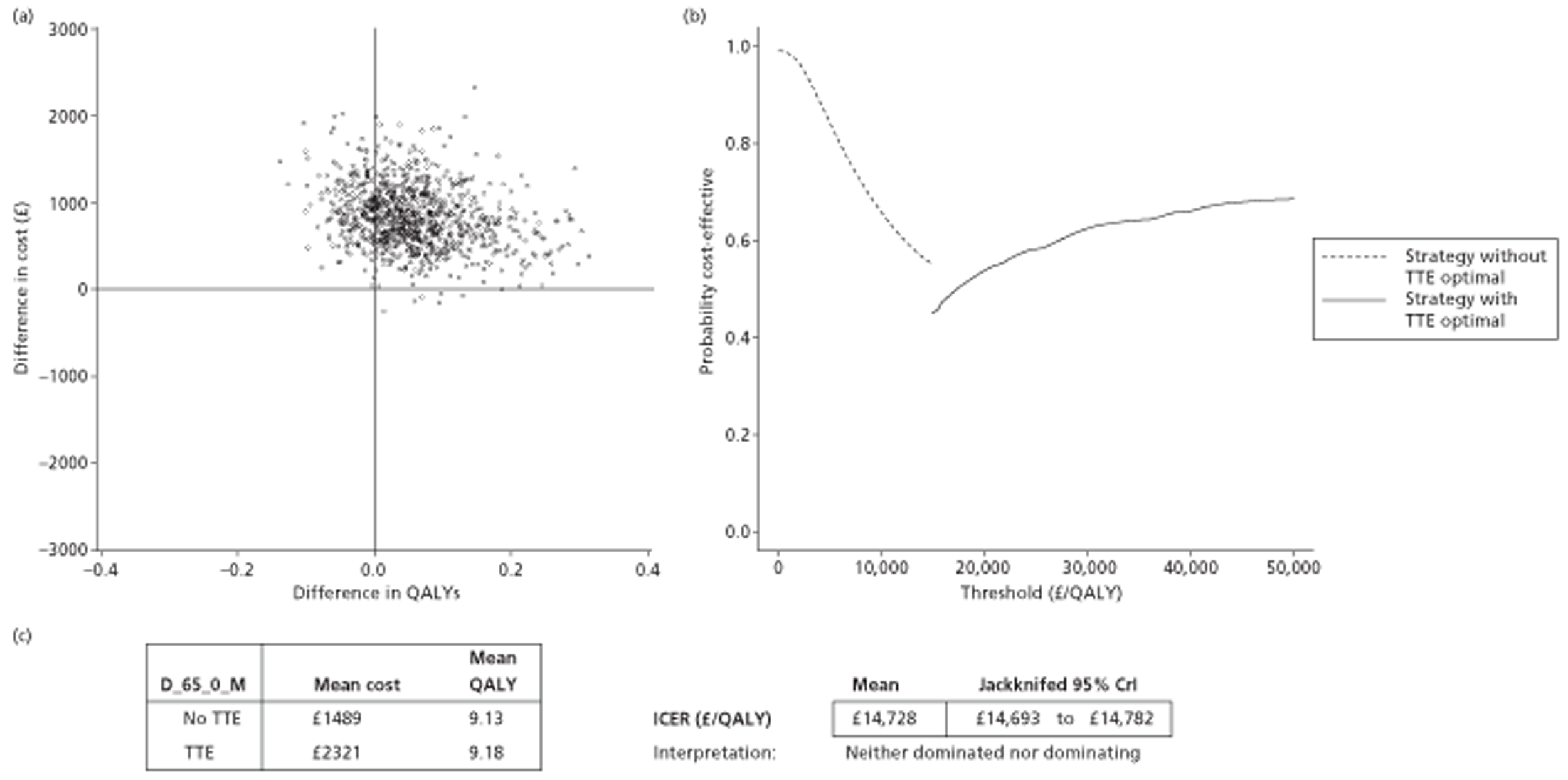
Sixty-five-year-old females, initial CHADS2 score of 0, treated with dabigatran
The mathematical model results (Table 58) suggest that the TTE strategy is more expensive and slightly more clinically effective, on the average, than the no-TTE strategy, with the PSA scatterplot appearing slightly more predominant in the north-east quadrant than in the north-west quadrant. The mean ICER estimate is slightly over £12,000, and the CEAF (Figure 23) indicates that the TTE strategy starts to become optimal at a willingness-to-pay threshold of approximately £11,800/QALY. If the willingness-to-pay threshold is £20,000 then the TTE strategy is estimated to have a 64% probability of being most cost-effective, and with a willingness-to-pay threshold of £30,000 the TTE strategy has a 72% probability of being most cost-effective.
| Strategy | Life-years | Cause of death (%) | Average no. of events | |||||
|---|---|---|---|---|---|---|---|---|
| Stroke | Bleed | Other | Dependent strokes | Independent strokes | ICH | NICH | ||
| No initial treatment | 19.485 | 10.2 | 1.1 | 88.7 | 0.099 | 0.220 | 0.009 | 0.066 |
| TTE with those diagnosed with LA abnormality treated | 19.598 | 9.0 | 1.6 | 89.4 | 0.089 | 0.195 | 0.012 | 0.097 |
FIGURE 23.
Probabilistic sensitivity analysis scatterplots, CEAFs and mean ICERs in the D_65_0_F comparison. (a) Scatterplot of difference in costs (£) against difference in QALYs. (b) CEAF. (c) Mean costs, QALYs and ICERs.
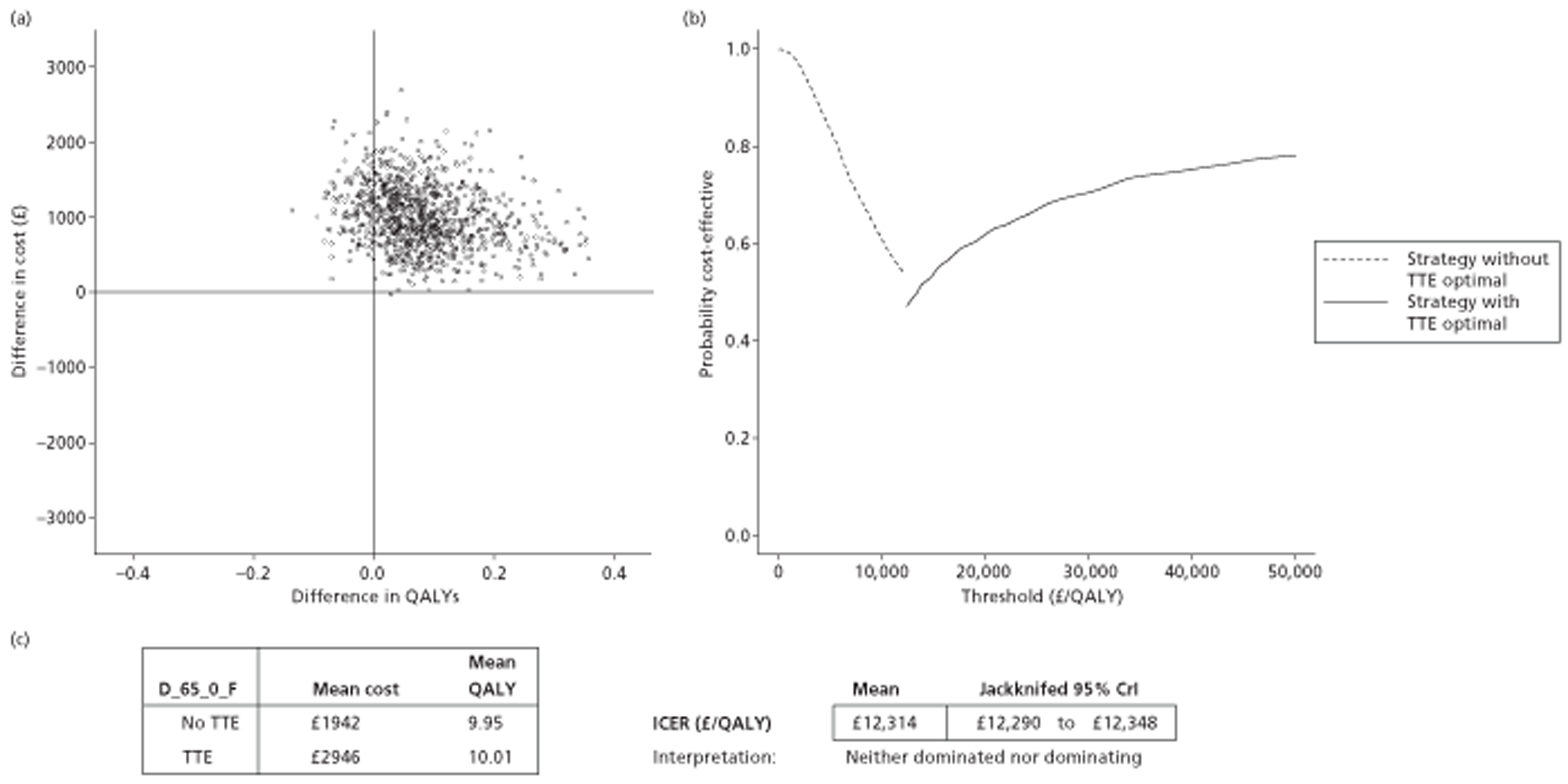
Expected value of perfect information results
The individual EVPI curves for maximum acceptable incremental cost-effectiveness ratios (MAICERs) varying from £0/QALY to £50,000/QALY are presented in Figure 24. In this table, the estimated individual EVPI at £20,000/QALY and £30,000/QALY, and corresponding population EVPIs assuming populations ranging from 25,000 to 75,000 people, are also presented for each of the 14 comparisons considered.
FIGURE 24.
Relationship between individual EVPI and MAICER for all 14 comparisons, together with individual and population EVPIs at MAICERs of £20,000/QALY and £30,000/QALY. (a) W_50_0_M; (b) W_50_0_F; (c) W_65_0_M; (d) W_65_0_F; (e) W_50_1_M; (f) W_50_1_F; (g) W_65_1_M; (h) W_65_1_F; (i) R_50_0_M; (j) R_50_0_F; (k) R_65_0_M; (l) R_65_0_F; (m) D_65_0_M; (n) D_65_0_F.

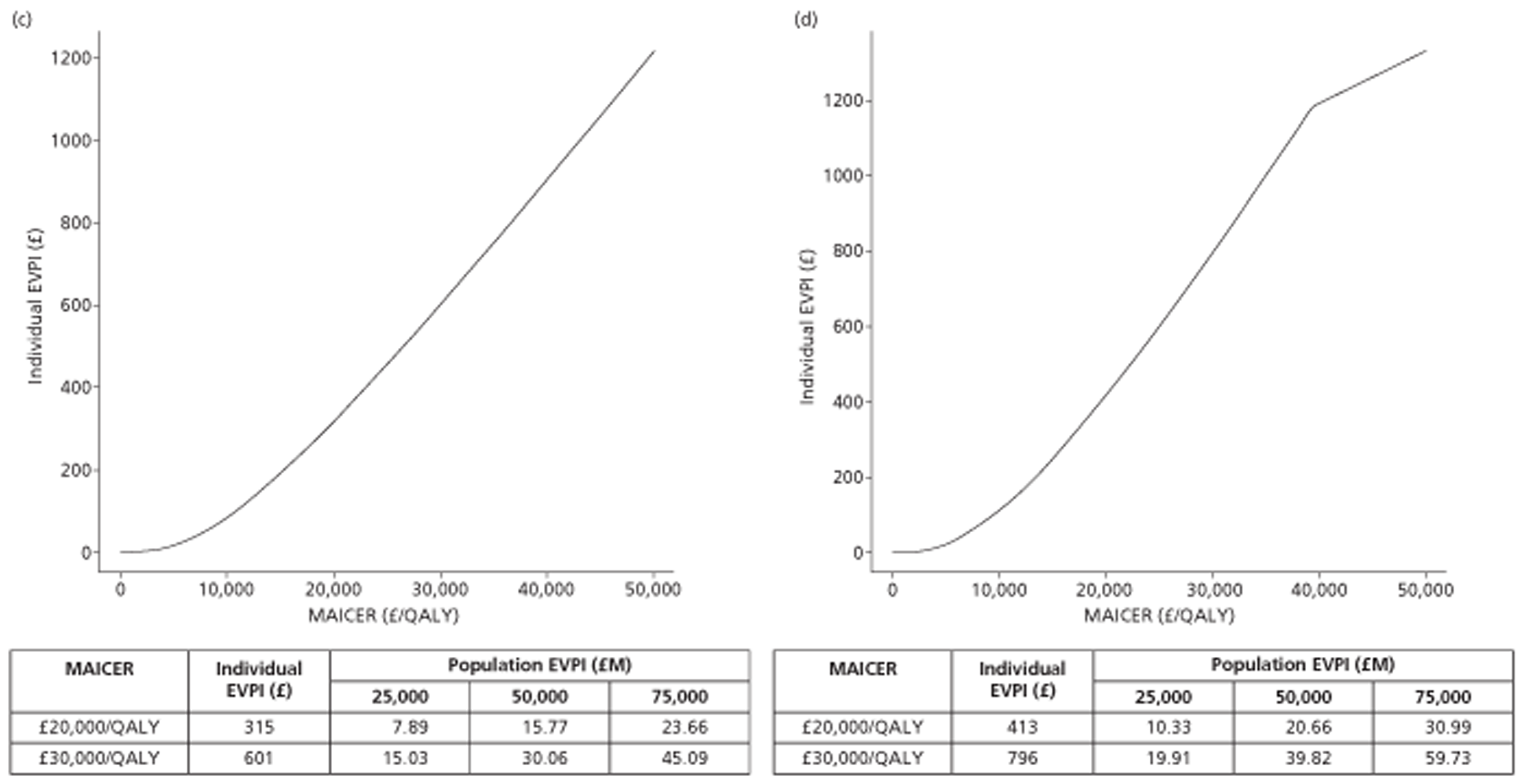
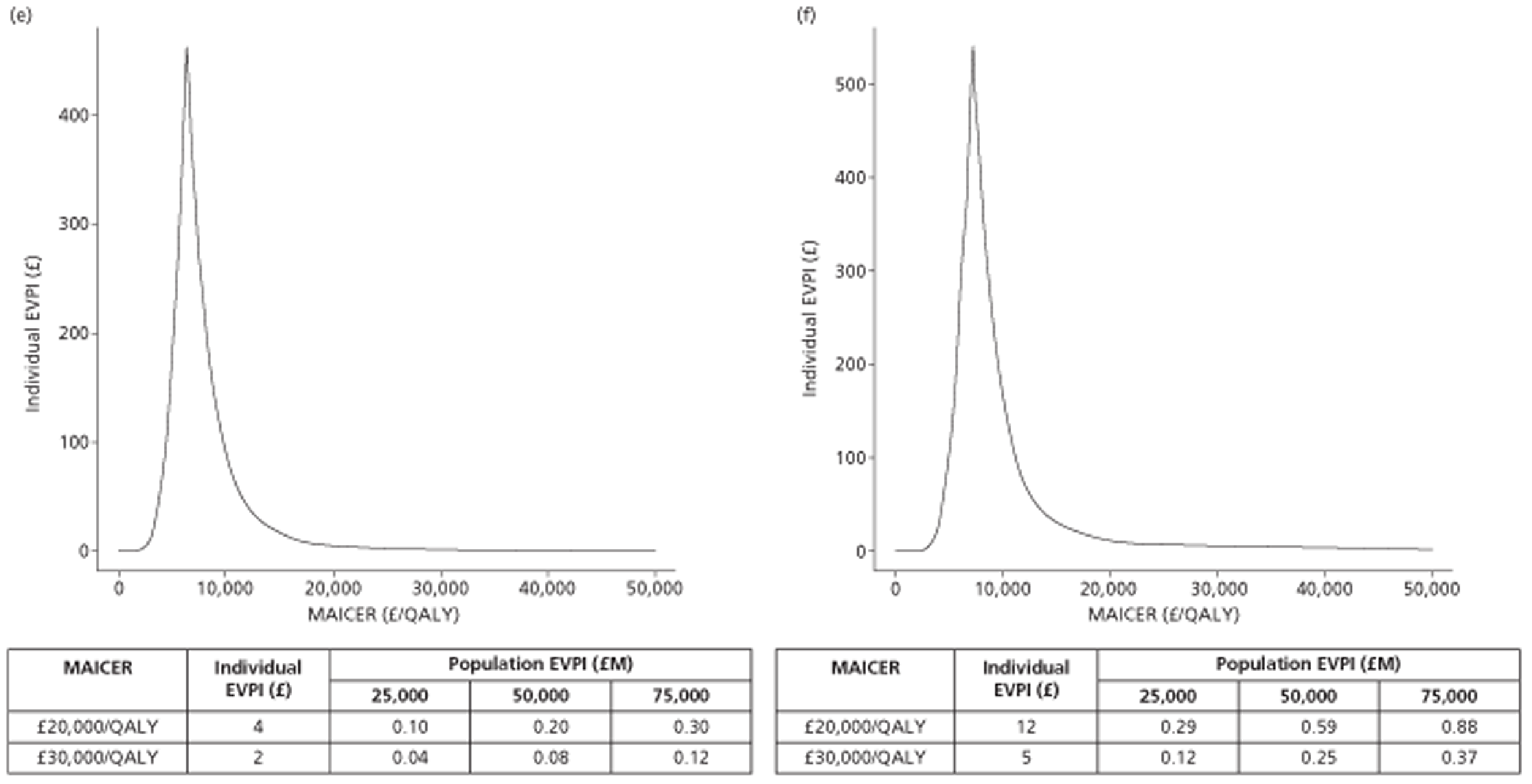
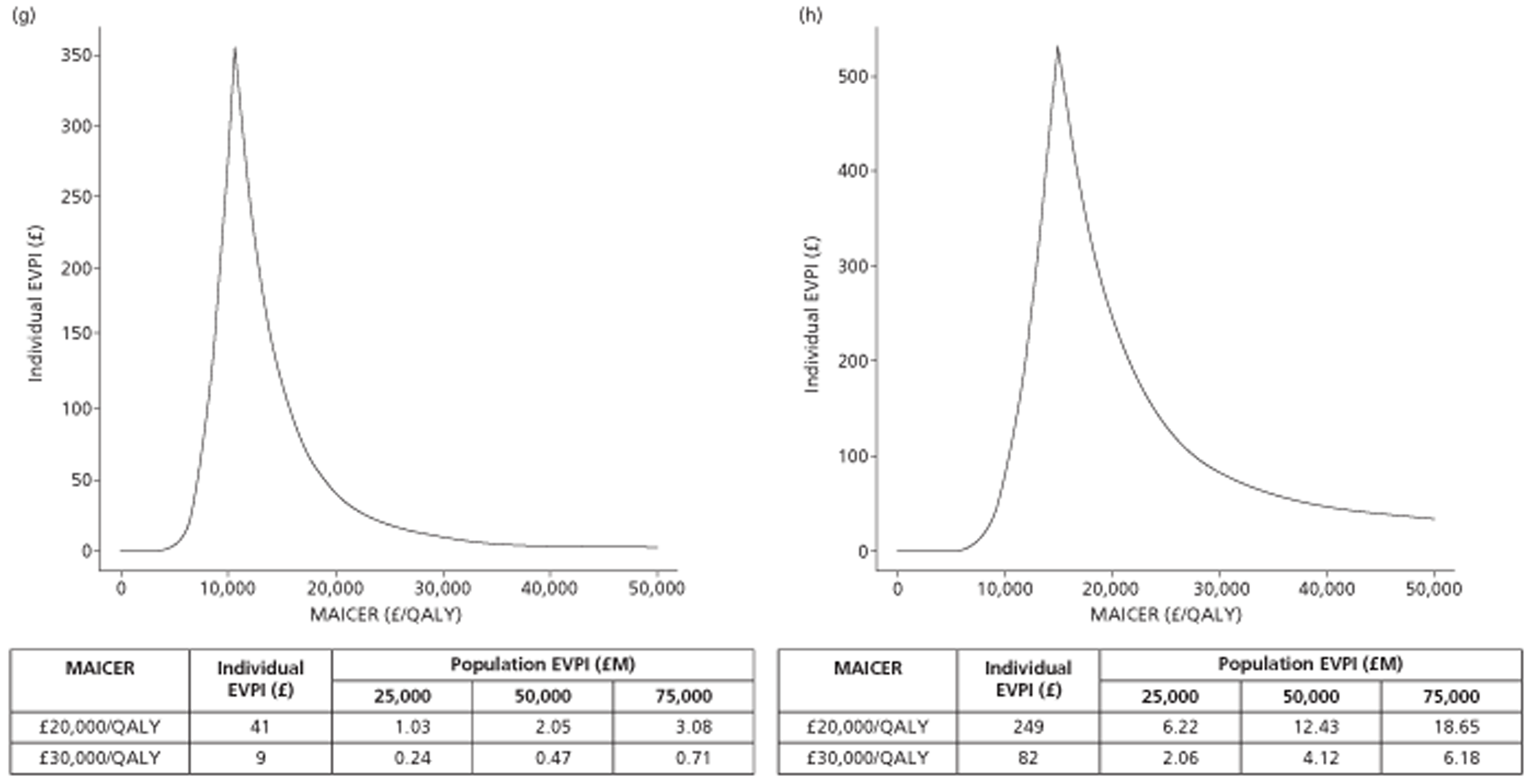
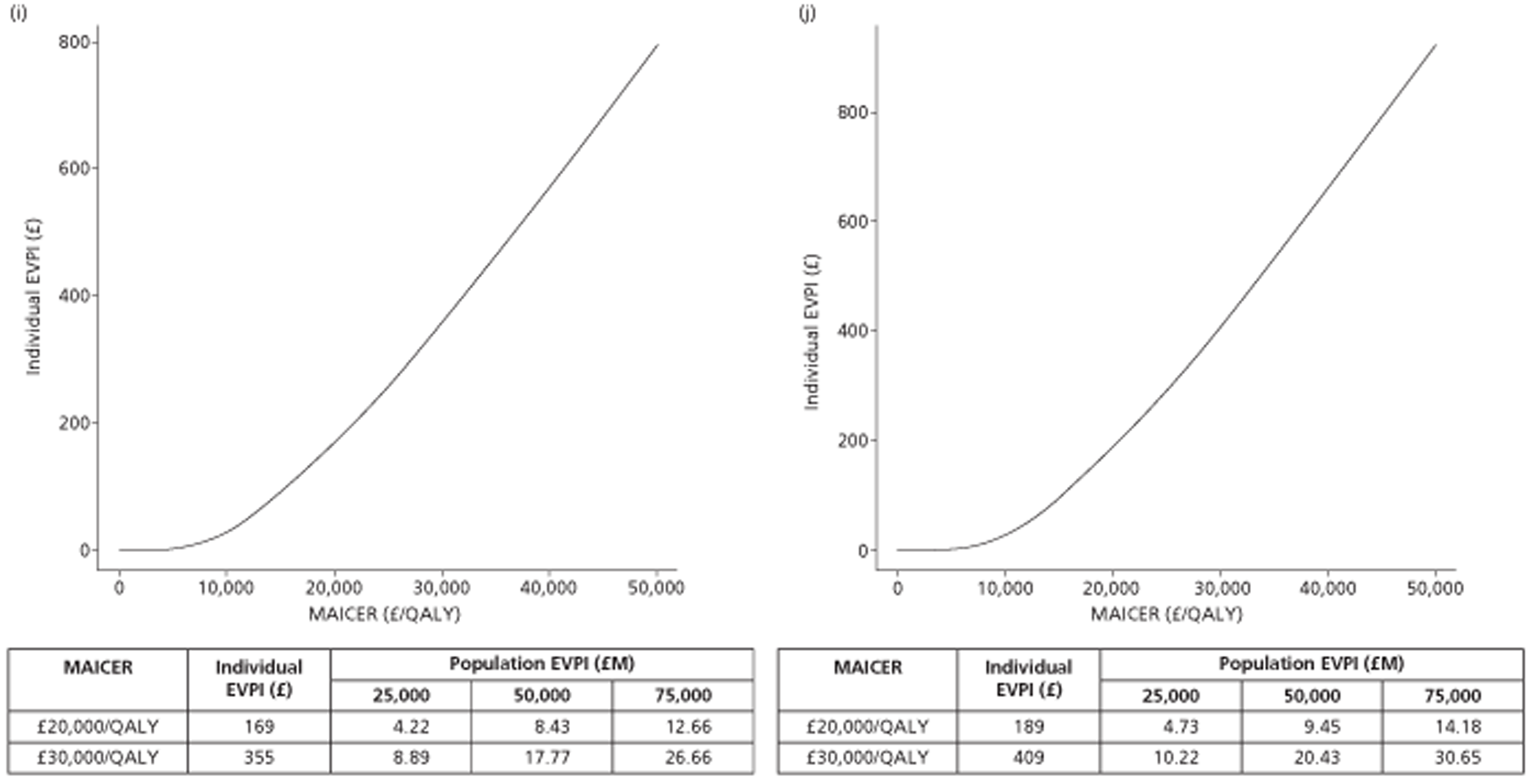
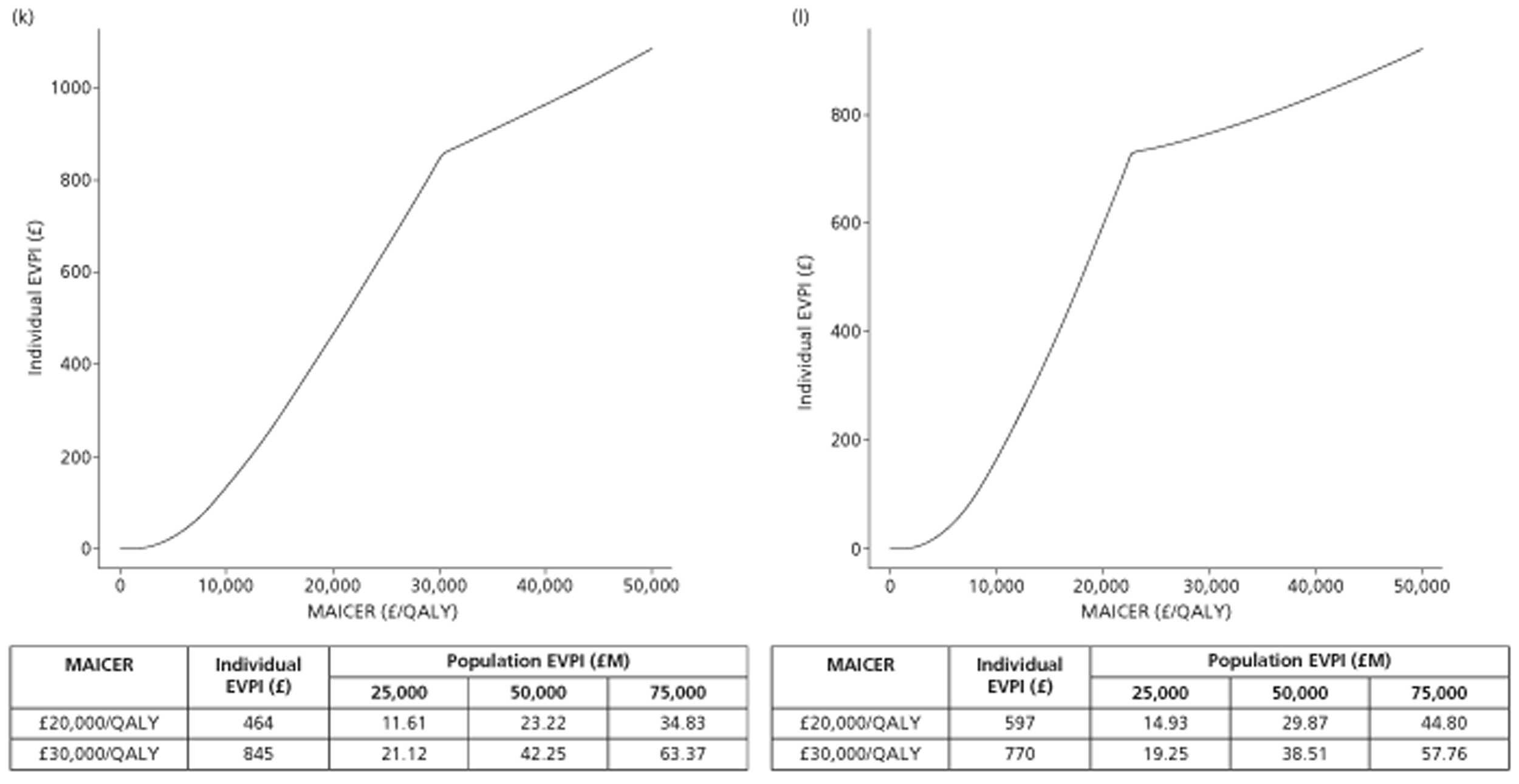
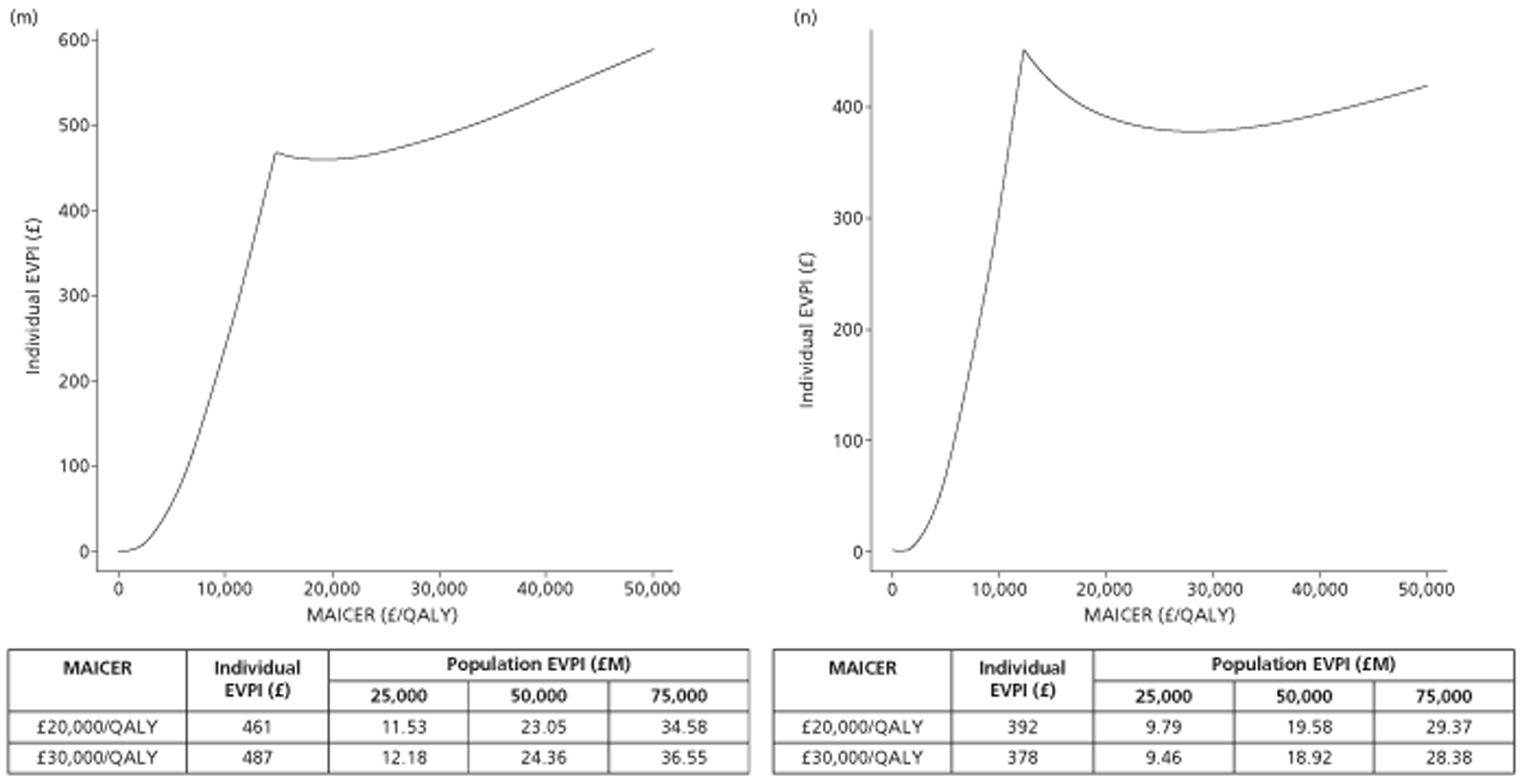
Sensitivity of incremental cost-effectiveness ratios to joint sensitivity and specificity of transthoracic echocardiography, and true prevalence of left atrial abnormality
In order to explore the influence of certain parameters on the cost-effectiveness estimates, the effects of changing the sensitivity and specificity estimates, holding all other values at their means, were calculated for each of the 14 comparisons. These are presented in Appendix 11.
Additionally, the influence of difference assumptions about the true proportion with LA abnormality was estimated in a similar way. These are presented in Figure 25 for people aged 50 years with a CHADS2 score of 0, Figure 26 for people aged 65 years with a CHADS2 score of 0, and in Figure 27 for people aged either 50 or 65 years with a CHADS2 score of 1.
FIGURE 25.
Effect of true proportion of population with LA abnormality on estimated ICER in 50-year-olds who have a CHADS2 score of 0.
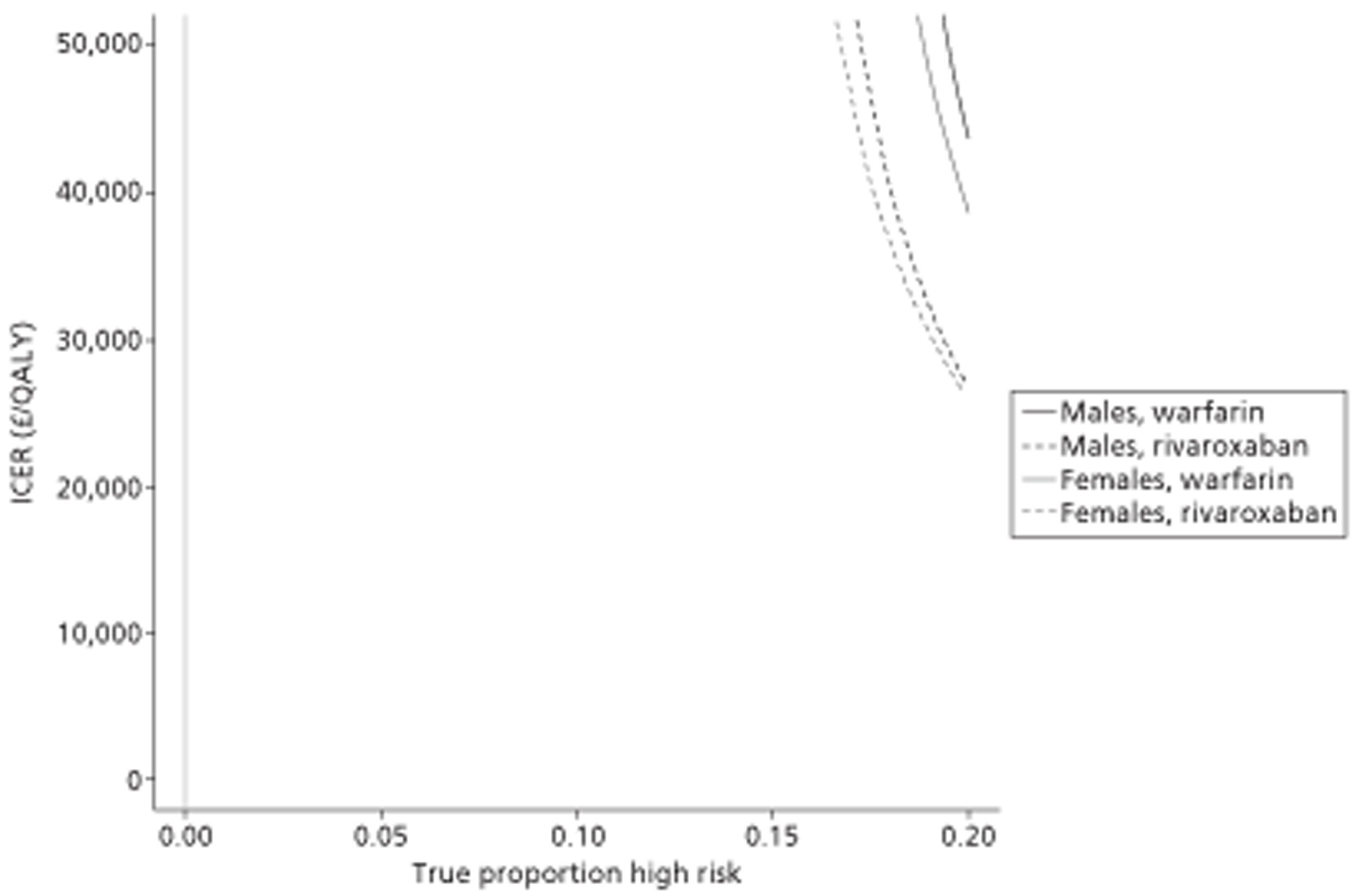
FIGURE 26.
Effect of true proportion of population with LA abnormality on estimated ICER in 65-year-olds who have a CHADS2 score of 0.
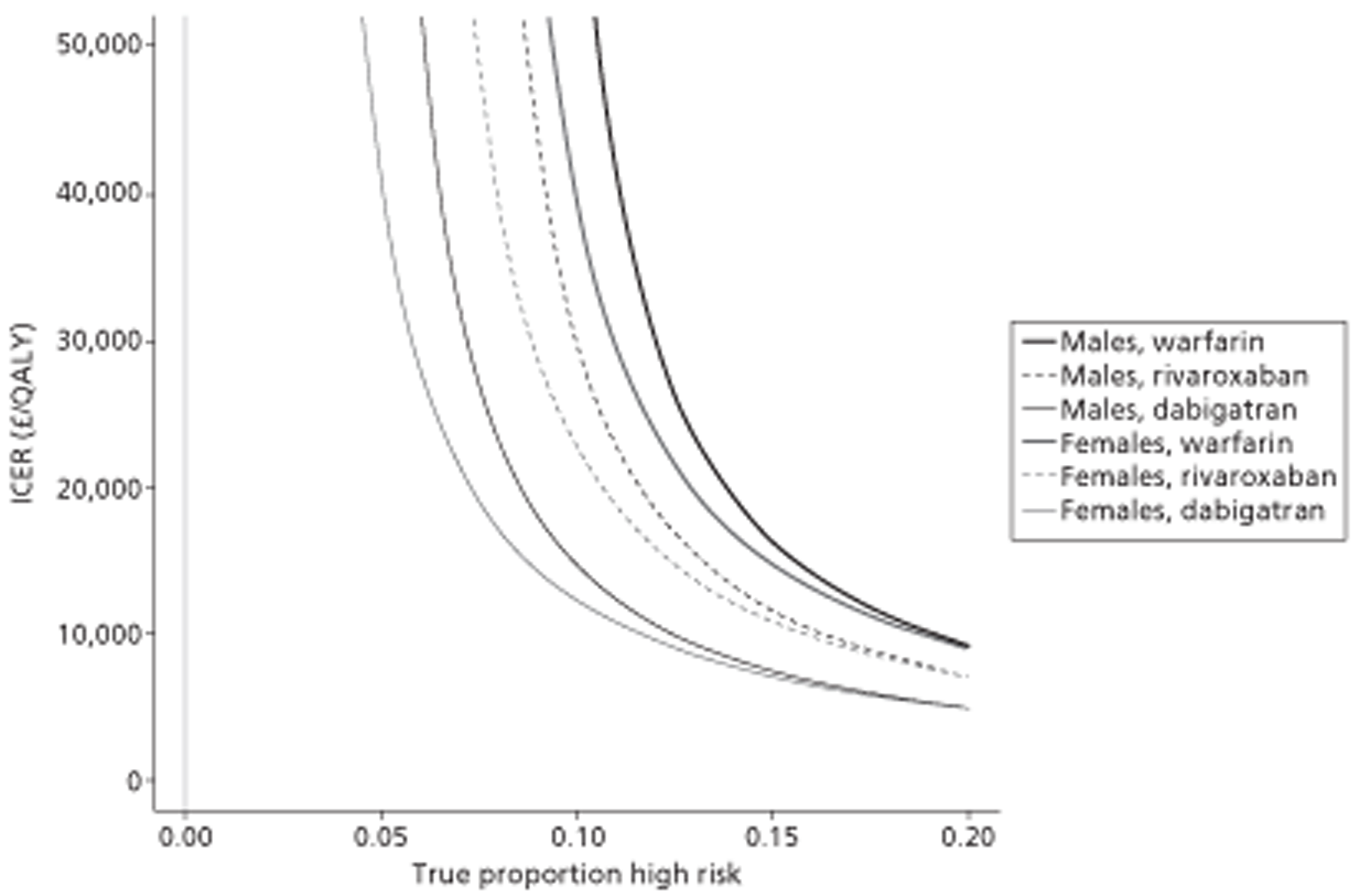
FIGURE 27.
Effect of true proportion of population with LA abnormality on estimated ICER in people aged either 50 or 65 years who have a CHADS2 score of 1.
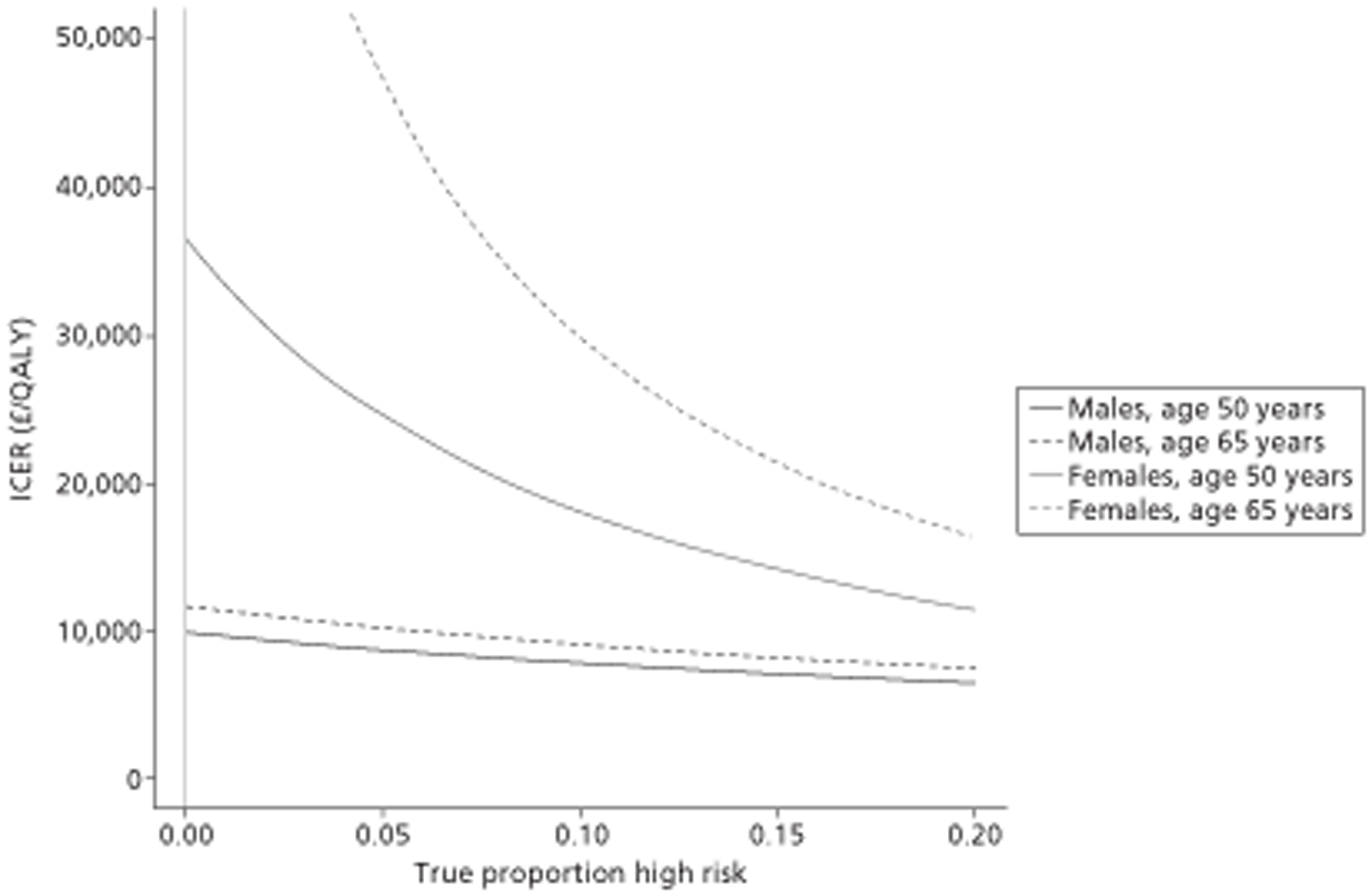
The figures indicate that, over the range of values considered here, the true prevalence of the LA abnormality could have a significant influence on the ICER in people aged 65 years with a CHADS2 score of 0, suggesting that identifying the true value of this parameter in these patient populations may be more important than in 50-year-olds or people with a CHADS2 score of 1. Among people with a CHADS2 score of 1, it may be more valuable to identify the true value of this parameter in females than in males.
Full incremental analyses
Performing a full incremental analysis was considered beyond the remit of this report, as warfarin, rivaroxaban and dabigatran have all been recommended by NICE through the single technology appraisal process. However, it is recognised that the choice of OAC affects the cost-effectiveness estimates of TTE. The above results can be categorised by patient population, each with differing OAC options considered, as shown in Table 59.
| Patient population | OACs considered |
|---|---|
| Males, age 50 years, CHADS2 score of 0 | Warfarin, rivaroxaban |
| Females, age 50 years, CHADS2 score of 0 | Warfarin, rivaroxaban |
| Male, age 65 years, CHADS2 score of 0 | Warfarin, rivaroxaban, dabigatran |
| Females, age 65 years, CHADS2 score of 0 | Warfarin, rivaroxaban, dabigatran |
| Males, age 50 years, CHADS2 score of 1 | Warfarin |
| Females, age 50 years, CHADS2 score of 1 | Warfarin |
| Male, age 65 years, CHADS2 score of 1 | Warfarin |
| Females, age 65 years, CHADS2 score of 1 | Warfarin |
A full incremental analysis would require that each with-TTE and without-TTE strategy be compared for each OAC for each patient population. For example, both male and female populations aged 65 years with a CHADS2 score of 0 would involve six comparisons. In addition, because rivaroxaban is effectively recommended by NICE at a CHADS2 score of 1, and dabigatran effectively recommended at a CHADS2 score of 1 in people aged ≥ 65 years, in these populations it may be appropriate to compare the decision to prescribe warfarin with and without TTE with people who are already receiving either rivaroxaban or dabigatran.
Results from the simplified method
The threshold QALYs required in order that a TTE would be deemed cost-effective was 0.0033 (£20,000/£66).
Interpretation of the results
The series of results presented above can be simplistically summarised as shown in Table 60.
| Age (years) | Gender | CHADS2 score of 1 | OAC | Ruled out by simple dominance | Likely to be cost-effective at £20,000/QALY |
|---|---|---|---|---|---|
| 50 | Male | No | Warfarin | Yes | No |
| Female | |||||
| 65 | Male | No | Warfarin | No | No |
| Female | No (possibly at £30,000/QALY) | ||||
| 50 | Male | Yes | Warfarin | No | Yes |
| Female | |||||
| 65 | Male | Yes | Warfarin | No | Yes |
| Female | |||||
| 50 | Male | No | Rivaroxaban | Yes | No |
| Female | |||||
| 65 | Male | No | Rivaroxaban | No | No (possibly at £30,000/QALY) |
| Female | Maybe | ||||
| 65 | Male | No | Dabigatran | No | Yes |
| Female |
These results suggest that:
-
In newly diagnosed patients with a CHADS2 score of 1, who are not already receiving warfarin, rivaroxaban, or dabigatran, it may be cost-effective to use TTE to help inform the decision whether to prescribe warfarin.
-
In newly diagnosed patients aged ≥ 65 years, it may be cost-effective to use TTE to help inform the decision about whether to prescribe dabigatran.
The threshold number of QALYs required for TTE to be cost-effective in the simplified analysis is a very small value and is below the sensitivity of standard preference-based utility measures, such as the EQ-5D. If there was clinical belief that there were benefits aside from identifying LA abnormality that were gained from the TTE then it is possible that TTE would be perceived as cost-effective.
Limitations in the modeling
Assumptions have been made within the modelling that have simplified the decision problem. Although it is unlikely that these assumptions would change the broad conclusions these are detailed for completeness. The assumptions are that:
-
Within the baseline strategies the decision was assumed to be made on the basis of CHADS2 scores alone. Alternative baseline strategies include the use of CHA2 DS2-VASc, and CHADS2 or CHA2 DS2-VASc scores in combination with bleed risk scores, such as Hypertension; Abnormal Liver/Renal Function; Stroke History; Bleeding Disposition; Labile INRs; Elderly; Drugs/Alcohol Usage (HAS-BLED). The baseline strategy could also be that a decision is made on the basis of the individual components within these scores.
-
The dose of dabigatran was set at 150 mg twice daily, rather than allowing some patients to receive a lower dose of 110 mg twice daily. The model could be adapted to reduce the dose when a patient reaches a specified age.
-
The stroke risk associated with patients with left atrial abnormalities is assumed to be constant at 8.0% (95% CI 7.26% to 8.31%) per year. Ideally differential rates by age or by the number (and type) of abnormalities would be used but these data were not identified.
-
The key data on which the economic evaluation was based came from a relatively small study, of fewer than 400 patients. Of these patients, fewer than 25 had a CHADS2 score of 0, and fewer than 80 patients had a CHADS2 score of 1. This has made the assessment of the benefits of TTE uncertain.
-
The risk of death unrelated to bleeding or stroke events was taken from life tables145 and was not adjusted for the probability of bleeding or stroke mortality. As such, the risk of mortality is likely to be slightly overestimated.
-
The sensitivity and specificity of TTE in identifying LA abnormality was estimated assuming that TOE had perfect sensitivity and specificity. If TOE was not a perfect gold standard then the accuracy of TTE would also change.
-
The full model assumed that the only benefit from TTE would be due to identification of LA abnormality. Any other conditions that may alter patient management have been ignored. To address this limitation a simplified approach was undertaken that calculated the additional QALY gain needed for TTE to be deemed cost-effective.
-
The mathematical model developed does not model the progression of each individual disease state that is incorporated in the CHADS2 score, and so the mathematical model was be run only in patients aged ≥ 65 years when considering dabigatran as the OAC of choice.
Comparison of our results with those in the published literature
A systematic literature review was conducted to identify, summarise and appraise existing economic studies for evaluating the cost-effectiveness of TTE in patients with AF.
Methodology
Search strategy
A comprehensive literature search was undertaken across five databases: MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), WoS, and Cochrane Database of Systematic Reviews (CDSR). Details of the full search strategy are provided in Appendix 12.
Inclusion/exclusion criteria
The inclusion criteria for this systematic review were as follows:
-
Population Patients with AF.
-
Intervention Transthoracic echocardiogram.
-
Comparators Conventional therapy.
-
Setting Interventions delivered within any geographical jurisdiction.
-
Outcomes Cost per QALY.
-
Study designs Studies reporting a full economic evaluation, with results expressed in terms of both costs and health outcomes.
Studies were excluded from this review if:
-
the patients suffered from cardiac problems other than AF
-
the intervention was not transthoracic echocardiogram
-
they reported only costs or outcomes
-
they were not of full economic evaluation, such as cost-minimisation analysis
-
they were not published papers, such as editorials, commentaries and letters
-
they were not published in the English language.
All of the potentially relevant citations were imported to Reference Manager Software, version 12 (Thomson ResearchSoft, San Francisco, CA, USA) and duplicates were removed. The titles and abstracts of the unique studies were then screened according to the predetermined inclusion criteria as outlined above. Any disagreements concerning possible inclusion of papers were resolved by discussion among the researchers of the team, or through retrieval and subsequent examination of the full study publication. Full papers of all the potentially relevant citations were retrieved for an in-depth assessment concerning study inclusion in the review.
Data extraction and evidence synthesis
Data concerning the characteristics of the population, interventions, comparators, outcomes, study location, time horizon, costs, outcomes and the perspective of the evaluations undertaken were extracted from the included study. These were then tabulated and discussed in a narrative manner.
Results of the systematic review
Number of studies identified and included
In total, 1038 studies were identified through the systematic searches. Following the removal of duplicate citations, 881 unique studies were retrieved. Of these, 43 potentially relevant citations were retrieved for a more detailed inspection. Further, 26 studies were excluded and 17 studies were retrieved for double screening. After double screening, 17 studies were excluded as they failed to satisfy one or more of the inclusion criteria.
Chapter 5 Assessment of factors relevant to the NHS and other parties
The assessment of newly diagnosed patients with AF using a TTE is unlikely to cause a significant impact on either the NHS or other parties. TTEs are relatively easily available, as well as both safe and non-invasive for patients, with staff who are trained in their use likely to be already available in hospitals.
The additional resources required are relatively small, at an estimated £66 per TTE performed. It is likely that additional bed-days are made available owing to the reduction in stroke following appropriate management, although there is likely to be an increase in bleed-related admissions.
Chapter 6 Discussion and conclusions
Statement of principal findings
Diagnostic accuracy showed high specificities for all selected pathologies, with the majority having specificity of ≥ 0.8, meaning a low proportion of FPs. Specificity was lower for aortic dissection and pulmonary disease than for other pathologies. For most pathologies there was also quite high sensitivity, with the majority having sensitivity of ≥ 0.6, with the exceptions of atrial thrombi, atrial septal defect and PE, for which sensitivity was lower. There was a high prevalence (around 25–30%) of ischaemic heart disease, valvular heart disease and heart failure in patients with AF in the included prevalence studies. TTE seems to be a sufficient diagnostic tool for most pathologies included here, but there may need to be extra screening for PE by lung scan, and atrial thrombi and atrial septal hypertrophy by TOE, to avoid FNs for these pathologies.
The results of the mathematical model indicated that in newly diagnosed patients with a CHADS2 score of 1, who are not already receiving warfarin, rivaroxaban, or dabigatran, it may be cost-effective to use TTE to help inform the decision whether to prescribe warfarin. In newly diagnosed patients aged ≥ 65 years it may be cost-effective to use TTE to help inform the decision about whether to prescribe dabigatran. A simplified approach indicated that only a small number of QALYs (0.0033) was required to deem a TTE to be cost-effective, and that incidental benefits may provide more than this number of QALYs.
Strengths and limitations of the assessment
A range of studies were identified that were of good quality and of relevance to UK populations.
It is possible that some studies were missed owing to limiting to studies published in the English language, and only one database being searched for diagnostic accuracy studies.
Data here are not a substitute for a trial of routine screening. In practice, the many different pathologies that could be identified may lead to many different treatment strategies. Patients may have more than one pathology in addition to AF, and may have been diagnosed with other conditions prior to AF diagnosis. It is also important that personnel performing these examinations receive adequate training to minimise bias and improve the quality of screening procedures. The outcome of screening in terms of treatment modification and subsequent prognostic impact will be complex. Receiving diagnoses may result in the patient making lifestyle changes as well as being provided with more appropriate medical treatment. In addition, there may be an emotional impact on patients in terms of undergoing testing, receiving additional diagnoses or being reassured where comorbidities are not diagnosed. Patients need to be provided with information about screening – including implications and limitations – before deciding whether to consent to testing. A trial of routine TTE screening in patients with newly diagnosed AF could address the impact on patients, which may go beyond simple changes in medical treatment, although any such trial would be costly owing to the large sample size and long length of follow-up needed to investigate outcomes including mortality; however, given the benefits and lack of adverse effects of TTE, it is unclear how useful additional evidence from a trial would be.
Our literature review identified no economic evaluations of TTE in patients with AF so it is believed that this is the first. One strength of the modelling is that it uses recent data assessing the sensitivity and specificity of TTE in identifying LA abnormality in patients categorised by CHADS2 score with confirmation provided by TOE. A limitation is that this study had a relatively small data set (n = 405) and was undertaken in Portugal, with the population not necessarily representative of a UK population.
A further strength is the simplified approach that was also undertaken. This showed that the QALYs required for TTE to be cost-effective were very small (< 0.005). Such values could be provided by many factors not incorporated into the mathematical model, and if clinicians believe that benefits other than those associated with reduced stroke rates (albeit at an increased risk of bleeding) are likely then it is probable that TTE is cost-effective.
Analyses have also been undertaken using different OACs with the conclusions remaining constant.
Uncertainties
There are a number of uncertainties within the economic evaluation of TTE. We elected to model the problem using data provided in Providencia et al. ,13 as this was a recent, internally consistent study using the CHADS2 tool, and it had also conducted TOE. However, the study was not large (n = 405). This meant that data on the specificity and sensitivity of TTE in identifying LA abnormality were sparse. Using TTE could only affect the decisions in the low risk categories: CHADS2 = 0, and CHADS2 = 1. The number of patients in these categories was small, each fewer than 80 patients, with three values of fewer than 25 patients
A key uncertainty is whether there are other benefits that are accrued from a TTE other than identifying LA abnormality. If these exist, and produce even small QALY gains (> 0.0033) then TTE would be cost-effective in all scenarios.
Other relevant factors
As TTE is relatively easily available, and is a safe and non-invasive diagnostic, no other relevant factors were identified.
Implications for service provision
Our conclusions have few implications for service provision. Should TTE be recommended for those patients with CHADS2 scores of 0 or 1 then this is unlikely to place a great burden on hospitals who are likely to have staff trained in the use of TTE machines. Capacity will depend on scheduling the use of existing TTE equipment and extra staff time needed.
Suggested research priorities
Following up patients with newly diagnosed AF who have undergone TTE to study treatments given as a result of TTE diagnoses and subsequent cardiovascular events could identify potential benefits of routine testing, beyond stroke prevention.
Our conclusions regarding the cost-effectiveness of TTE have been limited by the available data relating to the proportion of people with CHADS2 scores of 0 or 1 who have LA abnormality. These proportions have been shown to markedly affect the cost per QALY and there are few data available. In obtaining such data more accurate estimates of the sensitivity and specificity of TTE in identifying LA abnormality should be collected.
Any additional benefit of TTE beyond those associated with treatment for stroke prevention also needs to be researched. Even small gains would equate to TTE being perceived as cost-effective.
Acknowledgements
The authors would like to thank the clinical advisors who have contributed to the project, in alphabetical order:
Professor John Chambers, Consultant Cardiologist
Professor Gregory YH Lip, Professor of Cardiovascular Medicine
Dr Guy Lloyd, Consultant Cardiologist
Dr Rick Steeds, Consultant Cardiologist
The authors would also like to thank Andrea Shippam and Gill Rooney, Programme Administrators, ScHARR, for their help in preparing and formatting the report.
Contribution of authors
The literature search was conducted by P Evans.
The clinical reviews were conducted by EL Simpson, A Scope and E Poku.
The cost-effectiveness modelling was conducted by MD Stevenson and J Minton.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Atrial fibrillation: national clinical guideline for management in primary and secondary care. 2006.
- Fuster V, Ryden LE, Cannom, DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation 2011;123:e269-367. http://dx.doi.org/10.1161/CIR.0b013e318214876d.
- Levy S, Camm AJ, Saksena S, Aliot E, Breithardt G, Crijns H, et al. International consensus on nomenclature and classification of atrial fibrillation. Europace 2003;5:119-22. http://dx.doi.org/10.1053/eupc.2002.0300.
- Estes NAM, Sacco RL, Al-Khatib SM, Ellinor PT, Bezanson J, Alonso A, et al. American Heart Association Atrial Fibrillation Research Summit. Circulation 2011;124:363-72. http://dx.doi.org/10.1161/CIR.0b013e318224b037.
- Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J 2005;149:489-96. http://dx.doi.org/10.1016/j.ahj.2004.09.053.
- Task FM, Camm AJ, Kirchhof P, Lip GYH, . Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) . Guidelines for the management of atrial fibrillation. Eur Heart J 2010;31:2369-429.
- Olson JM, Samad BA, Alam M. Prognostic value of pulse-wave tissue Doppler parameters in patients with systolic heart failure. Am J Cardiol 2008;102:722-5. http://dx.doi.org/10.1016/j.amjcard.2008.04.054.
- Pizko P, Lewczuk J, Lenatowska L, Jagas J, Romaszkiewicz R, Blaszczyk D, et al. Pulmonary thromboembolism in 102 consecutive patients with chronic atrial fibrillation. Diagnostic value of echocardiography. Kardiol Pol 2007;65:246-51.
- Okura H, Takada Y, Kubo T, Iwata K, Mizoguchi S, Taguchi H, et al. Tissue Doppler-derived index of left ventricular filling pressure, E/E', predicts survival of patients with non-valvular atrial fibrillation. Heart 2006;92:1248-52. http://dx.doi.org/10.1136/hrt.2005.082594.
- Sakabe K, Fukuda N, Fukuda Y, Morishita S, Shinohara H, Tamura Y, et al. Transthoracic echocardiography during sinus rhythm identifies elderly patients with nonvalvular paroxysmal atrial fibrillation at high risk of cerebral infarction. Int J Cardiol 2009;136:346-8. http://dx.doi.org/10.1016/j.ijcard.2008.04.065.
- Thambidorai SK, Murray RD, Parakh K, Shah TK, Black IW, Jasper SE, et al. Utility of transesophageal echocardiography in identification of thrombogenic milieu in patients with atrial fibrillation (an ACUTE ancillary study). Am J Cardiol 2005;96:935-41. http://dx.doi.org/10.1016/j.amjcard.2005.05.051.
- Bernhardt P, Schmidt H, Hammerstingl C, Luderitz B, Omran H. Patients at high risk with atrial fibrillation: a prospective and serial follow-up during 12 months with transesophageal echocardiography and cerebral magnetic resonance imaging. J Am Soc Echocardiogr 2005;18:919-24. http://dx.doi.org/10.1016/j.echo.2005.01.028.
- Providencia R, Botelho A, Trigo J, Quintal N, Nascimento J, Mota P, et al. Possible refinement of clinical thromboembolism assessment in patients with atrial fibrillation using echocardiographic parameters. Europace 2012;14:36-45. http://dx.doi.org/10.1093/europace/eur272.
- Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin 2009;27:13-vii.
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5. http://dx.doi.org/10.1001/jama.285.18.2370.
- Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516-21. http://dx.doi.org/10.1136/heart.86.5.516.
- Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol 1999;84:131-8R. http://dx.doi.org/10.1016/S0002-9149(99)00713-4.
- Stewart S. Epidemiology and economic impact of atrial fibrillation. J Cardiovasc Nurs 2004;19:94-102.
- Kannel WBW. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N-9N. http://dx.doi.org/10.1016/S0002-9149(98)00583-9.
- Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin N Am 2008;92:17-40. http://dx.doi.org/10.1016/j.mcna.2007.09.002.
- Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart 2001;86:284-8. http://dx.doi.org/10.1136/heart.86.3.284.
- Murphy NF, Simpson CR, Jhund PS, Stewart S, Kirkpatrick M, Chalmers J, et al. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart 2007;93:606-12. http://dx.doi.org/10.1136/hrt.2006.107573.
- Lip GY, Beevers DG. ABC of atrial fibrillation. History, epidemiology, and importance of atrial fibrillation. BMJ 1995;311:1361-3. http://dx.doi.org/10.1136/bmj.311.7016.1361.
- Wolf PAB. Secular trends in the prevalence of atrial fibrillation: the Framingham study. Am Heart J 1996;131:790-5. http://dx.doi.org/10.1016/S0002-8703(96)90288-4.
- Lip GY, Bawden L, Hodson R, Rutland E, Snatchfold J, Beevers DG. Atrial fibrillation amongst the Indo-Asian general practice population. The West Birmingham Atrial Fibrillation Project. Int J Cardiol 1998;65:187-92.
- Rienstra M, Hagens VE, van Veldhuisen DJ, Bosker HA, Tijssen JG, Kamp O, et al. Clinical characteristics of persistent lone atrial fibrillation in the RACE study. Am J Cardiol 2004;94:1486-90. http://dx.doi.org/10.1016/j.amjcard.2004.08.024.
- Aronow WS, Ahn C, Kronzon I, Gutstein H. Association of left ventricular hypertrophy and chronic atrial fibrillation with the incidence of new thromboembolic stroke in 2,384 older persons. Am J Cardiol 1999;84:468-9.
- de Divitiis M, Omran H, Rabahieh R, Rang B, Illien S, Schimpf R, et al. Right atrial appendage thrombosis in atrial fibrillation: its frequency and its clinical predictors. Am J Cardiol 1999;84:1023-8. http://dx.doi.org/10.1016/S0002-9149(99)00492-0.
- Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol 2001;37:371-8. http://dx.doi.org/10.1016/S0735-1097(00)01107-4.
- Miyasaka Y, Barnes ME, Petersen RC, Cha SS, Bailey KR, Gersh BJ, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J 2007;28:1962-7. http://dx.doi.org/10.1093/eurheartj/ehm012.
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death. Circulation 1998;98:946-52. http://dx.doi.org/10.1161/01.CIR.98.10.946.
- Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359-64. http://dx.doi.org/10.1016/S0002-9343(02)01236-6.
- Mitra R, Leatham EW. Admissions with atrial fibrillation: disease patterns and outcomes in a District General Hospital. QJM 2005;98:153-4. http://dx.doi.org/10.1093/qjmed/hci021.
- Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospital activity, morbidity and case fatality related to atrial fibrillation in Scotland, 1986–1996. Eur Heart J 2001;22:693-701. http://dx.doi.org/10.1053/euhj.2000.2511.
- Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286-92. http://dx.doi.org/10.1136/hrt.2002.008748.
- National Service Framework . National Service Framework for Coronary Heart Disease. Chapter 8: Arrhythmias and Sudden Cardiac Death 2005. www.cardiomyopathy.org/assets/files/NSF%20Chapter%208.pdf (accessed November 2011).
- Fuster V, Ryden L, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation 2006;114:700-52. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.177031.
- Van Gelder I, Groenveld H, Crijns H, Tuininga Y, Tijssen J, Alings A, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010;362:1363-73. http://dx.doi.org/10.1056/NEJMoa1001337.
- AFFIRM Investigators . A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825-33. http://dx.doi.org/10.1056/NEJMoa021328.
- Van Gelder I, Hagens VE, Bosker H, Kingma H, Kamp O, Kingma T, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834-40. http://dx.doi.org/10.1056/NEJMoa021375.
- Roy D, Talajic M, Nattel S, Wyse D, Dorian P, Lee K, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667-77. http://dx.doi.org/10.1056/NEJMoa0708789.
- Cryoablation for atrial fibrillation in association with other cardiac surgery. London: NICE; 2006.
- Camm A, Kirchhof P, Lip G, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429. http://dx.doi.org/10.1093/eurheartj/ehq278.
- Knight BP, Gersh BJ, Carlson M, Friedman P, McNamara R, . American Heart Association (AHA) Science Advisory . Role of permanent pacing to prevent atrial fibrillation: science advisory from the American Heart Association Council on Clinical Cardiology (Subcommittee on Electrocardiography and Arrhythmias) and the Quality of Care and Outcomes Research Interdisciplinary Working Group, in collaboration with the Heart Rhythm Society. Circulation 2005;111:240-3. http://dx.doi.org/10.1161/01.CIR.0000151800.84945.47.
- Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation. London: NICE; 2012.
- Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation. London: NICE; 2012.
- Atrial fibrillation (stroke prevention) – rivaroxaban. London: NICE; 2012.
- Wann LS, Curtis AB, Ellenbogen KA, Estes NAM, Ezekowitz MD, Jackman WM, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;57:1330-7. http://dx.doi.org/10.1016/j.jacc.2011.01.010.
- Bayliss J. Bedfordshire and Hertfordshire Cardiac Network guidance. Effective Use of Transthoracic Echocardiography (TTE) in Adults – Summary 2006. www.bhhsnetwork.nhs.uk/UserFiles/File/StandardsGuidelines/Beds&Herts_Echo_guidelines_v2_Brief.pdf (accessed November 2011).
- British Society of Echocardiography . Clinical Indications for Echocardiography 2009. http://cdn1.cache.twofourdigital.net/u/bsecho/media/10465/clinical_indications_for_echocardiography.pdf (accessed November 2011).
- Evangelista A, Flachskampf FA, Lancellotti P, Badano LP, Aguilar R, Monaghan MJ, et al. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur J Echocardiogr 2008;9:438-48. http://dx.doi.org/10.1093/ejechocard/jen174.
- National Imaging Board . Cardiac Imaging: A Report from the National Imaging Board 2010. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_114380 (accessed November 2011).
- Stanko L, Jacobsohn E, Tam J, De Wet C, Avidan M. Transthoracic echocardiography: impact on diagnosis and management in tertiary care intensive care units. Anaesth Intensive Care 2005;33:492-6.
- Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2005;26:2422-34. http://dx.doi.org/10.1093/eurheartj/ehi505.
- UK National Screening Committee . Screening Criteria Set by the UK National Screening Committee 2011. www.scotland.gov.uk/Topics/Health/health/cancer/Cancer-Screening/criteria (accessed November 2011).
- Petticrew M, Sowden A, Lister-Sharp D, Wright K. False-negative results in screening programmes: systematic review of impact and implications. Health Technol Assess 2000;4.
- Merlin T, Weston A, Tooher R. Extending an evidence hierarchy to include topics other than treatment: revising the Australian ‘levels of evidence’. BMC Med Res Meth 2009;9. http://dx.doi.org/10.1186/1471-2288-9-34.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stats Med 1998;17:857-72. http://dx.doi.org/10.1002/(SICI)1097-0258(19980430)17:8⟨857::AID-SIM777⟩3.0.CO;2-E.
- Whiting P, Rutjes A, Reitsma J, Bossuyt P, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Meth 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Elm EV, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. http://dx.doi.org/10.1136/bmj.39335.541782.AD.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med 2009;151:65-94.
- Acar J, Cormier B, Grimberg D, Kawthekar G, Iung B, Scheuer B, et al. Diagnosis of left atrial thrombi in mitral stenosis – usefulness of ultrasound techniques compared with other methods. Eur Heart J 1991;12:70-6. http://dx.doi.org/10.1093/eurheartj/12.suppl_B.70.
- Arques S, Roux E, Sbragia P, Gelisse R, Ambrosi P, Pieri B, et al. Comparative accuracy of color M-mode and tissue Doppler echocardiography in the emergency diagnosis of congestive heart failure in chronic hypertensive patients with normal left ventricular ejection fraction. Am J Cardiol 2005;96:1456-9. http://dx.doi.org/10.1016/j.amjcard.2005.07.051.
- Attenhofer Jost CH, Turina J, Mayer K, Seifert B, Amann FW, Buechi M, et al. Echocardiography in the evaluation of systolic murmurs of unknown cause. Am J Med 2000;108:614-20. http://dx.doi.org/10.1016/S0002-9343(00)00361-2.
- Barron JT, Manrose DL, Liebson PR. Comparison of auscultation with two-dimensional and Doppler echocardiography in patients with suspected mitral valve prolapse. Clin Cardiol 1988;11:401-6. http://dx.doi.org/10.1002/clc.4960110608.
- Bova C, Greco F, Misuraca G, Serafini O, Crocco F, Greco A, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003;21:180-3. http://dx.doi.org/10.1016/S0735-6757(02)42257-7.
- Casella F, Rana B, Casazza G, Bhan A, Kapetanakis S, Omigie J, et al. The potential impact of contemporary transthoracic echocardiography on the management of patients with native valve endocarditis: a comparison with transesophageal echocardiography. Echocardiography 2009;26:900-6. http://dx.doi.org/10.1111/j.1540-8175.2009.00906.x.
- Cassidy TP, Hardwick DJ, Norris CA. Aortic stenosis in elderly people: an evaluation of clinical and echocardiographic diagnosis. Age Ageing 1992;21:440-4. http://dx.doi.org/10.1093/ageing/21.6.440.
- Dittmann H, Karsch KR, Seipel L. Diagnosis and quantification of aortic regurgitation by pulsed Doppler echocardiography in patients with mitral valve disease. Eur Heart J 1987;8:53-7. http://dx.doi.org/10.1093/eurheartj/8.suppl_C.53.
- Enia F, Ledda G, Lo MR, Matassa C, Raspanti G, Stabile A, et al. Utility of echocardiography in the diagnosis of aortic dissection involving the ascending aorta. Chest 1989;95:124-9. http://dx.doi.org/10.1378/chest.95.1.124.
- Erbel R, Schweizer P, Krebs W, Meyer J, Effert S, Erbel R, et al. Sensitivity and specificity of two-dimensional echocardiography in detection of impaired left ventricular function. Eur Heart J 1984;5:477-89.
- Grossmann G, Wohrle J, Kochs M, Giesler M, Hombach V, Hoher M, et al. Quantification of mitral regurgitation by the proximal flow convergence method – comparison of transthoracic and transesophageal echocardiography. Clin Cardiol 2002;25:517-24. http://dx.doi.org/10.1002/clc.4960251108.
- Groves AM, Win T, Charman SC, Wisbey C, Pepke-Zaba J, Coulden RA, et al. Semi-quantitative assessment of tricuspid regurgitation on contrast-enhanced multidetector CT. Clin Radiol 2004;59:715-19. http://dx.doi.org/10.1016/j.crad.2004.02.007.
- Guyer DE, Gillam LD, Foale RA, Clark MC, Dinsmore R, Palacios I, et al. Comparison of the echocardiographic and hemodynamic diagnosis of rheumatic tricuspid stenosis. J Am Coll Cardiol 1984;3:1135-44. http://dx.doi.org/10.1016/S0735-1097(84)80170-9.
- Helmcke F, Nanda NC, Hsiung MC, Soto B, Adey CK, Goyal RG, et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 1987;75:175-83. http://dx.doi.org/10.1161/01.CIR.75.1.175.
- Jassal DS, Aminbakhsh A, Fang T, Shaikh N, Embil JM, Mackenzie GS, et al. Diagnostic value of harmonic transthoracic echocardiography in native valve infective endocarditis: comparison with transesophageal echocardiography. Cardiovasc Ultrasound 2007;5. http://dx.doi.org/10.1186/1476-7120-5-20.
- Kaymaz C, Ozdemir N, Kirma C, Sismanoglu M, Daglar B, Ozkan M, et al. Location, size and morphological characteristics of left atrial thrombi as assessed by echocardiography in patients with rheumatic mitral valve disease. Eur J Echocardiogr 2001;2:270-6. http://dx.doi.org/10.1053/euje.2001.0093.
- Kishon Y, Iqbal A, Oh JK, Gersh BJ, Freeman WK, Seward JB, et al. Evolution of echocardiographic modalities in detection of postmyocardial infarction ventricular septal defect and papillary muscle rupture: study of 62 patients. Am Heart J 1993;126:667-75. http://dx.doi.org/10.1016/0002-8703(93)90417-8.
- Kitayama H, Kiuchi K, Endo T, Hayakawa H, Kitayama H, Kiuchi K, et al. Value of cardiac ultrafast computed tomography for detecting right atrial thrombi in chronic atrial fibrillation. Am J Cardiol 1997;79:1292-5. http://dx.doi.org/10.1016/S0002-9149(97)00107-0.
- Lanzarini L, Fontana A, Campana C, Klersy C, Lanzarini L, Fontana A, et al. Two simple echo-Doppler measurements can accurately identify pulmonary hypertension in the large majority of patients with chronic heart failure. J Heart Lung Transplant 2005;24:745-54. http://dx.doi.org/10.1016/j.healun.2004.03.026.
- Maestre A, Gil V, Gallego J, Aznar J, Mora A, Martin-Hidalgo A, et al. Diagnostic accuracy of clinical criteria for identifying systolic and diastolic heart failure: cross-sectional study. J Eval Clin Pract 2009;15:55-61. http://dx.doi.org/10.1111/j.1365-2753.2008.00954.x.
- Mugge A, Daniel WG, Angermann C, Spes C, Khandheria BK, Kronzon I, et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation 1995;91:2785-92. http://dx.doi.org/10.1161/01.CIR.91.11.2785.
- Nienaber CA, von Kodolitsch Y, Nicolas V, Siglow V, Piepho A, Brockhoff C, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med 1993;328:1-9. http://dx.doi.org/10.1056/NEJM199301073280101.
- Nienaber CA, von Kodolitsch Y, Brockhoff CJ, Koschyk DH, Spielmann RP. Comparison of conventional and transesophageal echocardiography with magnetic resonance imaging for anatomical mapping of thoracic aortic dissection. A dual noninvasive imaging study with anatomical and/or angiographic validation. Int J Card Imag 1994;10:1-14. http://dx.doi.org/10.1007/BF01151576.
- Okura H, Fuyuki H, Kubo T, Iwata K, Taguchi H, Toda I, et al. Noninvasive diagnosis of ischemic and nonischemic cardiomyopathy using coronary flow velocity measurements of the left anterior descending coronary artery by transthoracic Doppler echocardiography. J Am Soc Echocardiogr 2006;19:552-8. http://dx.doi.org/10.1016/j.echo.2005.12.013.
- Pochis WT, Saeian K, Sagar KB. Usefulness of transesophageal echocardiography in diagnosing lipomatous hypertrophy of the atrial septum with comparison to transthoracic echocardiography. Am J Cardiol 1992;70:396-8. http://dx.doi.org/10.1016/0002-9149(92)90629-D.
- Reichek N, Devereux RB. Left ventricular hypertrophy: relationship of anatomic, echocardiographic and electrocardiographic findings. Circulation 1981;63:1391-8. http://dx.doi.org/10.1161/01.CIR.63.6.1391.
- Reichlin S, Dieterle T, Camli C, Leimenstoll B, Schoenenberger RA, Martina B. Initial clinical evaluation of cardiac systolic murmurs in the ED by noncardiologists. Am J Emerg Med 2004;22:71-5. http://dx.doi.org/10.1016/S0735-6757(03)00093-7.
- Roudaut RP, Billes MA, Gosse P, Deville C, Baudet E, Fontan F, et al. Accuracy of M-mode and two-dimensional echocardiography in the diagnosis of aortic dissection: an experience with 128 cases. Clin Cardiol 1988;11:553-62. http://dx.doi.org/10.1002/clc.4960110809.
- Saraste M, Vesalainen RK, Ylitalo A, Saraste A, Koskenvuo JW, Toikka JO, et al. Transthoracic Doppler echocardiography as a noninvasive tool to assess coronary artery stenoses – a comparison with quantitative coronary angiography. J Am Soc Echocardiogr 2005;18:679-85. http://dx.doi.org/10.1016/j.echo.2004.09.016.
- Sharifi M, Parhizgar A, Gupta P, Mehdipour M, Emrani F, Baldwin D. Is transesophageal echocardiography necessary before D.C. cardioversion in patients with a normal transthoracic echocardiogram?. Echocardiography 2007;24:397-400. http://dx.doi.org/10.1111/j.1540-8175.2007.00400.x.
- Sharma S, Krishnan U. Diagnosis of the sinus venosus defect by echocardiography and angiocardiography. Indian Heart J 1992;44:395-8.
- Sheiban I, Casarotto D, Trevi G, Benussi P, Marini A, Accardi R, et al. Two-dimensional echocardiography in the diagnosis of intracardiac masses: a prospective study with anatomic validation. Cardiovasc Intervent Radiol 1987;10:157-61. http://dx.doi.org/10.1007/BF02577993.
- Shively BK, Gurule FT, Roldan CA, Leggett JH, Schiller NB, Shively BK, et al. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. J Am Coll Cardiol 1991;18:391-7. http://dx.doi.org/10.1016/0735-1097(91)90591-V.
- Shrestha NK, Moreno FL, Narciso FV, Torres L, Calleja HB. Two-dimensional echocardiographic diagnosis of left-atrial thrombus in rheumatic heart disease. A clinicopathologic study. Circulation 1983;67:341-7. http://dx.doi.org/10.1161/01.CIR.67.2.341.
- Shub C, Dimopoulos IN, Seward JB, Callahan JA, Tancredi RG, Schattenberg TT, et al. Sensitivity of two-dimensional echocardiography in the direct visualization of atrial septal defect utilizing the subcostal approach: experience with 154 patients. J Am Coll Cardiol 1983;2:127-35. http://dx.doi.org/10.1016/S0735-1097(83)80385-4.
- Shyu KG, Lei M, Hwang J, Lin SC, Kuan P, Lien WP. Morphologic characterization and quantitative assessment of mitral regurgitation with ruptured chordae tendineae by transesophageal echocardiography. Am J Cardiol 1992;70:1152-6. http://dx.doi.org/10.1016/0002-9149(92)90047-3.
- Smith G, Endresen K, Sivertssen E, Semb G. Ventricular septal rupture diagnosed by simultaneous cross-sectional echocardiography and Doppler ultrasound. Eur Heart J 1985;6:631-6.
- Sparrow N, Adlam D, Cowley A, Hampton JR. The diagnosis of heart failure in general practice: implications for the UK National Service Framework. Eur J Heart Fail 2003;5:349-54. http://dx.doi.org/10.1016/S1388-9842(03)00046-1.
- Stratton JR, Lighty GW, Pearlman AS, Ritchie JL. Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation 1982;66:156-66. http://dx.doi.org/10.1161/01.CIR.66.1.156.
- Veyrat C, Lessana A, Abitbol G, Ameur A, Benaim R, Kalmanson D, et al. New indexes for assessing aortic regurgitation with two-dimensional Doppler echocardiographic measurement of the regurgitant aortic valvular area. Circulation 1983;68:998-1005. http://dx.doi.org/10.1161/01.CIR.68.5.998.
- Vigna C, de Rito V, Criconia GM, Russo A, Testa M, Fanelli R, et al. Left atrial thrombus and spontaneous echo-contrast in nonanticoagulated mitral stenosis. A transesophageal echocardiographic study. Chest 1993;103:348-52. http://dx.doi.org/10.1378/chest.103.2.348.
- Wong M, Tei C, Shah PM. Sensitivity and specificity of two-dimensional echocardiography in the detection of valvular calcification. Chest 1983;84:423-7. http://dx.doi.org/10.1378/chest.84.4.423.
- Zanolla L, Marino P, Nicolosi GL, Peranzoni PF, Poppi A, Zanolla L, et al. Two-dimensional echocardiographic evaluation of mitral valve calcification. Sensitivity and specificity. Chest 1982;82:154-7. http://dx.doi.org/10.1378/chest.82.2.154.
- Zotz RJ, Dohmen G, Genth S, Erbel R, Dieterich HA, Meyer J. Transthoracic and transesophageal echocardiography to diagnose ventricular septal rupture: importance of right heart infarction. Coron Artery Dis 1993;4:911-17. http://dx.doi.org/10.1097/00019501-199310000-00011.
- Atrial Fibrillation Investigators . Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med 1998;158:1316-20. http://dx.doi.org/10.1001/archinte.158.12.1316.
- Klem I, Wehinger C, Schneider B, Hartl E, Finsterer J, Stollberger C, et al. Diabetic atrial fibrillation patients: mortality and risk for stroke or embolism during a 10-year follow-up. Diabetes Metabol Res Rev 2003;19:320-8. http://dx.doi.org/10.1002/dmrr.386.
- Miyasaka Y, Tsuji H, Tokunaga S, Nishiue T, Yamada K, Watanabe J, et al. Mild mitral regurgitation was associated with increased prevalence of thromboembolic events in patients with nonrheumatic atrial fibrillation. Int J Cardiol 2000;72:229-33.
- Nakagami H, Yamamoto K, Ikeda U, Mitsuhashi T, Goto T, Shimada K, et al. Mitral regurgitation reduces the risk of stroke in patients with nonrheumatic atrial fibrillation. Am Heart J 1998;136:528-32. http://dx.doi.org/10.1016/S0002-8703(98)70231-5.
- The Stroke Prevention in Atrial Fibrillation Investigators . Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann Intern Med 1992;116:6-12.
- Agmon Y, Khandheria BK, Meissner I, Schwartz GL, Petterson TM, O’Fallon WM, et al. Association of atrial fibrillation and aortic atherosclerosis: a population-based study. Mayo Clin Proc 2001;76:252-9. http://dx.doi.org/10.4065/76.3.252.
- Archer SL, James KE, Kvernen LR, Cohen IS, Ezekowitz MD, Gornick CC. Role of transesophageal echocardiography in the detection of left atrial thrombus in patients with chronic nonrheumatic atrial fibrillation. Am Heart J 1995;130:287-95. http://dx.doi.org/10.1016/0002-8703(95)90442-5.
- Blackshear JL, Pearce LA, Hart RG, Zabalgoitia M, Labovitz A, Asinger RW, et al. Aortic plaque in atrial fibrillation: prevalence, predictors, and thromboembolic implications. Stroke 1999;30:834-40. http://dx.doi.org/10.1161/01.STR.30.4.834.
- Corrado GB. Prevalence of atrial thrombi in patients with atrial fibrillation/flutter and subtherapeutic anticoagulation prior to cardioversion. Eur J Echocardiogr 2004;5:257-61.
- Dang D, Patel R. Atrial fibrillation in a multiethnic inpatient population of a large public hospital. J Natl Med Assoc 2004;96:1438-44.
- Heppell RM, Berkin KE, McLenachan JM, Davies JA. Haemostatic and haemodynamic abnormalities associated with left atrial thrombosis in non-rheumatic atrial fibrillation. Heart 1997;77:407-11.
- Kleemann T, Becker T, Strauss M, Schneider S, Seidl K. Prevalence of left atrial thrombus and dense spontaneous echo contrast in patients with short-term atrial fibrillation < 48 hours undergoing cardioversion: value of transesophageal echocardiography to guide cardioversion. J Am Soc Echocardiogr 2009;22:1403-8. http://dx.doi.org/10.1016/j.echo.2009.09.015.
- Levy S, Maarek M. Characterization of different subsets of atrial fibrillation in general practice in France: The ALFA study. Circulation 1999;99:3028-35. http://dx.doi.org/10.1161/01.CIR.99.23.3028.
- Lip GY, Golding DJ, Nazir M, Beevers DG, Child DL, Fletcher RI, et al. A survey of atrial fibrillation in general practice: the West Birmingham Atrial Fibrillation Project. Br J General Practice 1997;47:285-9.
- Maltagliati AG. Usefulness of transoesophageal echocardiography before cardioversion in patients with atrial fibrillation and different anticoagulant regimens. Heart 2006;92:933-8.
- Narumiya T, Sakamaki T, Sato Y, Kanmatsuse K. Relationship between left atrial appendage function and left atrial thrombus in patients with nonvalvular chronic atrial fibrillation and atrial flutter. Circ J 2003;67:68-72. http://dx.doi.org/10.1253/circj.67.68.
- Santiago D, Warshofsky M, Li MG, Di Tullio M, Coromilas J, Reiffel J, et al. Left atrial appendage function and thrombus formation in atrial fibrillation-flutter: a transesophageal echocardiographic study. J Am Coll Cardiol 1994;24:159-64. http://dx.doi.org/10.1016/0735-1097(94)90557-6.
- Scherr D, Dalal D, Chilukuri K, Dong J, Spragg D, Henrikson CA, et al. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009;20:379-84. http://dx.doi.org/10.1111/j.1540-8167.2008.01336.x.
- Shen X, Li H, Rovang K, Holmberg MJ, Mooss AN, Mohiuddin SM. Prevalence of intra-atrial thrombi in atrial fibrillation patients with subtherapeutic international normalized ratios while taking conventional anticoagulation. Am J Cardiol 2002;90:660-2.
- Tsai LM, Lin LJ, Teng JK, Chen JH. Prevalence and clinical significance of left atrial thrombus in nonrheumatic atrial fibrillation. Int J Cardiol 1997;58:163-9. http://dx.doi.org/10.1016/S0167-5273(96)02862-8.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. http://dx.doi.org/10.1016/j.jclinepi.2007.11.008.
- Stroke Prevention in Atrial Fibrillation Investigators . The Stroke Prevention in Atrial Fibrillation III randomized clinical trial: background, design and patient characteristics of the Stroke Prevention in Atrial Fibrillation III study. J Stroke Cerebrovasc Dis 1996;6:341-53.
- Higashiue S, Watanabe H, Yokoi Y, Takeuchi K, Yoshikawa J. Simple detection of severe coronary stenosis using transthoracic Doppler echocardiography at rest. Am J Cardiol 2001;87:1064-8. http://dx.doi.org/10.1016/S0002-9149(01)01462-X.
- Garg R, Khaja A, Madsen R, Alpert MA, Tejwani L, Aggarwal K, et al. Observer variation in the echocardiographic measurement of maximum atrial septal excursion: a comparison of M-mode with two-dimensional or transesophageal echocardiography. Echocardiography 2009;26:1122-6. http://dx.doi.org/10.1111/j.1540-8175.2009.00964.x.
- Hirata K, Pulerwitz T, Sciacca R, Otsuka R, Oe Y, Fujikura K, et al. Clinical utility of new real time three-dimensional transthoracic echocardiography in assessment of mitral valve prolapse. Echocardiography 2008;25:482-8. http://dx.doi.org/10.1111/j.1540-8175.2008.00630.x.
- Miniati M, Monti S, Pratali L, Di Ricco G, Marini C, Formichi B, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001;110:528-35. http://dx.doi.org/10.1016/S0002-9343(01)00693-3.
- Chamoun AJ, McCulloch M, Xie T, Shah S, Ahmad M. Real-time three-dimensional echocardiography versus two-dimensional echocardiography in the diagnosis of left ventricular apical thrombi: preliminary findings. J Clin Ultrasound 2003;31:412-18. http://dx.doi.org/10.1002/jcu.10199.
- Shiga T, Wajima Z, Inoue T, Ogawa R. Survey of observer variation in transesophageal echocardiography: comparison of anesthesiology and cardiology literature. J Cardiothorac Vasc Anesth 2003;17:430-42. http://dx.doi.org/10.1016/S1053-0770(03)00146-0.
- Frykman V, Frick M, Jensen-Urstad M, Ostergren J, Rosenqvist M. Asymptomatic versus symptomatic persistent atrial fibrillation: clinical and noninvasive characteristics. J Intern Med 2001;250:390-7. http://dx.doi.org/10.1046/j.1365-2796.2001.00893.x.
- Rozenberg V, Boccara F, Benhalima B, Lamisse N, Buyukoglu B, Cohen A, et al. Comparison of echocardiographic markers of embolism in atrial flutter and fibrillation: frequency of protruding atherosclerotic plaques in the thoracic aorta. Echocardiography 2000;17:555-62. http://dx.doi.org/10.1046/j.1540-8175.2000.00555.x.
- DiPasquale G, Urbinati S, Pinelli G. New echocardiographic markers of embolic risk in atrial fibrillation. Cerebrovasc Dis 1995;5:315-22. http://dx.doi.org/10.1159/000107874.
- Rubin DN, Katz SE, Riley MF, Douglas PS, Manning WJ. Evaluation of left atrial appendage anatomy and function in recent-onset atrial fibrillation by transesophageal echocardiography. Am J Cardiol 1996;78:774-8. http://dx.doi.org/10.1016/S0002-9149(96)00419-5.
- Black IW, Hopkins AP, Lee LC, Walsh WF. Evaluation of transesophageal echocardiography before cardioversion of atrial fibrillation and flutter in nonanticoagulated patients. Am Heart J 1993;126:375-81. http://dx.doi.org/10.1016/0002-8703(93)91054-I.
- Rostagno C, Galanti G, Bertini G, Olivo G, Gensini GF. Doppler echocardiography in suspected lone atrial fibrillation. Angiology 1998;49:637-40. http://dx.doi.org/10.1177/000331979804900808.
- Levy S. Epidemiology and classification of atrial fibrillation. J Cardiovasc Electrophysiol 1998;9:S78-82.
- Tops LF, Schalij MJ, Bax JJ. Imaging and atrial fibrillation: the role of multimodality imaging in patient evaluation and management of atrial fibrillation. Eur Heart J 2010;31:542-51. http://dx.doi.org/10.1093/eurheartj/ehq005.
- Tsang TS, Miyasaka Y, Barnes ME, Gersh BJ. Epidemiological profile of atrial fibrillation: a contemporary perspective. Prog Cardiovasc Dis 2005;48:1-8. http://dx.doi.org/10.1016/j.pcad.2005.06.001.
- National Institute for Health and Care Excellence (NICE) . Dabigatran Etexilate for the Prevention of Stroke and Systemic Embolism in Atrial Fibrillation. NICE Technology Appraisal Guidance 249 2012. www.nice.org.uk/nicemedia/live/13677/58470/58470.pdf (accessed 28 September 2012).
- The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography . Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med 1998;128:639-47.
- Office for National Statistics (ONS) . Life Tables n.d. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2008-2010/index.html (accessed February 2012).
- Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500-10. http://dx.doi.org/10.1093/eurheartj/ehr488.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. http://dx.doi.org/10.1056/NEJMoa0905561.
- Lip G, Edwards S. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Thromb Res 2006;118:321-33. http://dx.doi.org/10.1016/j.thromres.2005.08.007.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. http://dx.doi.org/10.1056/NEJMoa1009638.
- Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363-72. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.004747.
- A briefing paper on dabigatran and rivaroxaban: what we know so far. UK Medicines Information (UKMi); 2012.
- British national formulary. London: BMA and RPS; 2011.
- NHS reference costs 2009–2010. London: DoH; 2010.
- Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell P, Luengo-Fernandez R. Mapping the modified Rankin Scale (mRS) Measurement into the Generic EuroQol (EQ-5D) Health Outcome. Med Decis Making 2010;30:341-54. http://dx.doi.org/10.1177/0272989X09349961.
- Dorman P, Dennis M, Sandercock P. Are the modified ‘simple questions’ a valid and reliable measure of health related quality of life after stroke?. J Neurol Neurosurg Psychiatr 2000;69:487-93. http://dx.doi.org/10.1136/jnnp.69.4.487.
- Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS. Health Technol Assess 2002;6.
- Curtis L. Unit costs of health and social care 2010. Canterbury: PSSRU, University of Kent; 2011.
- Simpson E, Stevenson M, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess 2009;13.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism. Ann Intern Med 2003;139:893-900.
- Haselsberger K, Pucher R, Auer LM. Prognosis after acute subdural or epiduralhaemorrhage. Acta Neurochir (Wien) 1988;90:111-16. http://dx.doi.org/10.1007/BF01560563.
- Holmes M, Goodacre S, Stevenson M, Pandor A, Pickering A. The cost-effectiveness of diagnostic management strategies for adults with minor head injury. Injury 2012;43:1423-31. http://dx.doi.org/10.1016/j.injury.2011.07.017.
- Goodacre S, Stevenson M, Wailoo A, Sampson F, Sutton AJ, Thomas S. How should we diagnose suspected deep-vein thrombosis?. QJM 2006;99:377-88. http://dx.doi.org/10.1093/qjmed/hcl051.
- Meenan RT, Saha S, Chou R, Swarztrauber K, Pyle Krages K, O’Keeffe-Rosetti MC, et al. Cost-effectiveness of echocardiography to identify intracardiac thrombus among patients with first stroke or transient ischemic attack. Med Decis Making 2007;27:161-77. http://dx.doi.org/10.1177/0272989X06297388.
- Guide to the methods of technology appraisals. London: NICE; 2008.
- Fenwick E, Claxton K, Sculpher MJ. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. http://dx.doi.org/10.1002/(SICI)1099-1050(199611)5:6⟨513::AID-HEC237⟩3.0.CO;2-9.
- Felli JC, Hazen BG. Sensitivity analysis and the expected value of perfect information. Med Decis Making 1998;18:95-109. http://dx.doi.org/10.1177/0272989X9801800117.
- Spain MG, Smith MD, Graybum PA, Harlamert EA, Demaria AN. Quantitative assessment of mitral regurgitation by Doppler color flow imaging: angiographic and hemodynamic correlations. J Am Coll Cardiol 1989;13:585-90. http://dx.doi.org/10.1016/0735-1097(89)90597-4.
- Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography . Transesophageal echocardiography in atrial fibrillation: standards for acquisition and interpretation and assessment of interobserver variability. J Am Soc Echocardiogr 1996;9:556-66.
- Abbasi AS, Allen MW, DeCristofaro D, Ungar I. Detection and estimation of the degree of mitral regurgitation by range-gated pulsed doppler echocardiography. Circulation 1980;61:143-7. http://dx.doi.org/10.1161/01.CIR.61.1.143.
- Aghassi P, Aurigemma GP, Folland ED, Tighe DA. Catheterization-Doppler discrepancies in nonsimultaneous evaluations of aortic stenosis. Echocardiography 2005;22:367-73. http://dx.doi.org/10.1111/j.1540-8175.2005.04006.x.
- Agricola E, Meris A, Oppizzi M, Bombardini T, Pisani M, Fragasso G, et al. Rest and stress echocardiographic predictors of prognosis in patients with left ventricular dysfunction and functional mitral regurgitation. Int J Cardiol 2008;124:247-9. http://dx.doi.org/10.1016/j.ijcard.2006.11.234.
- Alkadhi H, Desbiolles L, Husmann L, Plass A, Leschka S, Scheffel H, et al. Aortic regurgitation: assessment with 64-section CT. Radiology 2007;245:111-21. http://dx.doi.org/10.1148/radiol.2451061523.
- Assey ME, Usher BW. Echocardiography in diagnosing and managing aortic valve endocarditis. South Med J 1981;74:558-65. http://dx.doi.org/10.1097/00007611-198105000-00014.
- Babic UU, Popovic Z, Vucinic M, Djurisic Z, Pejcic P, Grujicic S, et al. Selective coronary angiography versus two-dimensional echocardiography for diagnosis of left atrial thrombi. Angiology 1991;42:889-98. http://dx.doi.org/10.1177/000331979104201104.
- Badran HM, Mostafa A, Serage A, Fareed W, Abdelfatah E, Fathe A. Arterial mechanics in ischemic versus nonischemic cardiomyopathy: clinical and diagnostic impact. Echocardiography 2009;26:785-800. http://dx.doi.org/10.1111/j.1540-8175.2008.00888.x.
- Blanchard D, Diebold B, Peronneau P, Foult JM, Nee M, Guermonprez JL, et al. Non-invasive diagnosis of mitral regurgitation by Doppler echocardiography. Br Heart J 1981;45:589-93. http://dx.doi.org/10.1136/hrt.45.5.589.
- Blot-Souletie N, Hebrard A, Acar P, Carrie D, Puel J. Comparison of accuracy of aortic valve area assessment in aortic stenosis by real time three-dimensional echocardiography in biplane mode versus two-dimensional transthoracic and transesophageal echocardiography. Echocardiography 2007;24:1065-72. http://dx.doi.org/10.1111/j.1540-8175.2007.00526.x.
- Bogren HG, Demaria AN, Mason DT. Imaging procedures in the detection of cardiac tumors, with emphasis on echocardiography: a review. Cardiovasc Intervent Radiol 1980;3:107-25. http://dx.doi.org/10.1007/BF02552329.
- Buchner S, Debl K, Poschenrieder F, Feuerbach S, Riegger GA, Luchner A, et al. Cardiovascular magnetic resonance for direct assessment of anatomic regurgitant orifice in mitral regurgitation. Circ Cardiovasc Imaging 2008;1:148-55. http://dx.doi.org/10.1161/CIRCIMAGING.107.753103.
- Casiglia E, Schiavon L, Tikhonoff V, Bascelli A, Martini B, Mazza A, et al. Electrocardiographic criteria of left ventricular hypertrophy in general population. Eur J Epidemiol 2008;23:261-71. http://dx.doi.org/10.1007/s10654-008-9234-6.
- Charron P, Dubourg O, Desnos M, Isnard R, Hagege A, Millaire A, et al. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in a genotyped adult population. Circulation 1997;96:214-19. http://dx.doi.org/10.1161/01.CIR.96.1.214.
- Chen YZ, Sherrid MV, Dwyer EM. Value of two-dimensional echocardiography in evaluating coronary artery disease: a randomized blinded analysis. J Am Coll Cardiol 1985;5:911-17. http://dx.doi.org/10.1016/S0735-1097(85)80432-0.
- Egeblad H, Berning J, Saunamaki K, Jacobsen JR, Wennevold A. Assessment of rheumatic mitral valve disease. Value of echocardiography in patients clinically suspected of predominant stenosis. Br Heart J 1983;49:38-44. http://dx.doi.org/10.1136/hrt.49.1.38.
- Fleischmann KE, Goldman L, Robiolio PA, Lee RT, Johnson PA, Cook EF, et al. Echocardiographic correlates of survival in patients with chest pain. J Am Coll Cardiol 1994;23:1390-6. http://dx.doi.org/10.1016/0735-1097(94)90382-4.
- Gonzalez-Torrecilla E, Garcia-Fernandez MA, Perez-David E, Bermejo J, Moreno M, Delcan JL, et al. Predictors of left atrial spontaneous echo contrast and thrombi in patients with mitral stenosis and atrial fibrillation. Am J Cardiol 2000;86:529-34. http://dx.doi.org/10.1016/S0002-9149(00)01007-9.
- Hsiao SH, Chang SM, Lee CY, Yang SH, Lin SK, Chiou KR, et al. Usefulness of tissue Doppler parameters for identifying pulmonary embolism in patients with signs of pulmonary hypertension. Am J Cardiol 2006;98:685-90. http://dx.doi.org/10.1016/j.amjcard.2006.03.053.
- Irani WN, Grayburn PA, Afridi I, Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996;78:101-3. http://dx.doi.org/10.1016/S0002-9149(96)00236-6.
- Khanna D, Vengala S, Miller AP, Nanda NC, Lloyd SG, Ahmed S, et al. Quantification of mitral regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area. Echocardiography 2004;21:737-43. http://dx.doi.org/10.1111/j.0742-2822.2004.40027.x.
- Khumri TM, Idupulapati M, Rader VJ, Nayyar S, Stoner CN, Main ML, et al. Clinical and echocardiographic markers of mortality risk in patients with atrial fibrillation. Am J Cardiol 2007;99:1733-6. http://dx.doi.org/10.1016/j.amjcard.2007.01.055.
- Kruger S, Haage P, Hoffmann R, Breuer C, Bucker A, Hanrath P, et al. Diagnosis of pulmonary arterial hypertension and pulmonary embolism with magnetic resonance angiography. Chest 2001;120:1556-61. http://dx.doi.org/10.1378/chest.120.5.1556.
- Lembcke A, Thiele H, Lachnitt A, Enzweiler CN, Wagner M, Hein PA, et al. Precision of forty slice spiral computed tomography for quantifying aortic valve stenosis: comparison with echocardiography and validation against cardiac catheterization. Investig Radiol 2008;43:719-28. http://dx.doi.org/10.1097/RLI.0b013e318184d7c5.
- Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S, et al. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol 2002;84:69-75. http://dx.doi.org/10.1016/S0167-5273(02)00136-5.
- Miyatake K, Izumi S, Okamoto M, Kinoshita N, Asonuma H, Nakagawa H, et al. Semiquantitative grading of severity of mitral regurgitation by real-time two-dimensional Doppler flow imaging technique. J Am Coll Cardiol 1986;7:82-8. http://dx.doi.org/10.1016/S0735-1097(86)80263-7.
- Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009;119:2679-85. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.793471.
- Nitta M, Takamoto T, Taniguchi K, Hultgren HN. Diagnostic accuracy of continuous wave Doppler echocardiography in severe aortic stenosis in the elderly. Japanese Heart J 1988;29:169-78. http://dx.doi.org/10.1536/ihj.29.169.
- Pechacek LW. Comparison of two-dimensional echocardiography, radionuclide ventriculography and cineangiography in detecting surgically documented left ventricular thrombi. Texas Heart Inst J 1984;11:118-27.
- Peteiro J, Bendayan I, Marinas J, Campos R, Bouzas B, Castro-Beiras A, et al. Prognostic value of mitral regurgitation assessment during exercise echocardiography in patients with left ventricular dysfunction: a follow-up study of 1.7+/−1.5 years. Eur J Echocardiogr 2008;9:18-25.
- Roe MT, Abramson MA, Li J, Heinle SK, Kisslo J, Corey GR, et al. Clinical information determines the impact of transesophageal echocardiography on the diagnosis of infective endocarditis by the duke criteria. Am Heart J 2000;139:945-51. http://dx.doi.org/10.1067/mhj.2000.104762.
- Sallach JA, Puwanant S, Drinko JK, Jaffer S, Donal E, Thambidorai SK, et al. Comprehensive left atrial appendage optimization of thrombus using surface echocardiography: the CLOTS multicenter pilot trial. J Am Soc Echocardiogr 2009;22:1165-72. http://dx.doi.org/10.1016/j.echo.2009.05.028.
- Stafford WJ, Petch J, Radford DJ. Vegetations in infective endocarditis. Clinical relevance and diagnosis by cross sectional echocardiography. Br Heart J 1985;53:310-13. http://dx.doi.org/10.1136/hrt.53.3.310.
- Steckelberg JM, Murphy JG, Ballard D, Bailey K, Tajik AJ, Taliercio CP, et al. Emboli in infective endocarditis: the prognostic value of echocardiography. Ann Intern Med 1991;114:635-40.
- Stevens SM, Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul study. JACC Cardiovasc Imag 2009;2:11-20. http://dx.doi.org/10.1016/j.jcmg.2008.08.004.
- Takamoto T, Kim D, Urie PM, Guthaner DF, Gordon HJ, Keren A, et al. Comparative recognition of left ventricular thrombi by echocardiography and cineangiography. Br Heart J 1985;53:36-42. http://dx.doi.org/10.1136/hrt.53.1.36.
- Thuny F, Di Salvo G, Belliard O, Avierinos JF, Pergola V, Rosenberg V, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation 2005;112:69-75. http://dx.doi.org/10.1161/CIRCULATIONAHA.104.493155.
- Visser CA, Kan G, David GK, Lie KI, Durrer D. Two dimensional echocardiography in the diagnosis of left ventricular thrombus. A prospective study of 67 patients with anatomic validation. Chest 1983;83:228-32. http://dx.doi.org/10.1378/chest.83.2.228.
- Willens HJ, Chirinos JA, Schob A, Veerani A, Perez AJ, Chakko S, et al. The relation between mitral annular calcification and mortality in patients undergoing diagnostic coronary angiography. Echocardiography 2006;23:717-22. http://dx.doi.org/10.1111/j.1540-8175.2006.00300.x.
- Colkesen Y, Acil T, Demircan S, Sezgin AT, Ozin B, Muderrisoglu H, et al. Echocardiographic features of patients with paroxysmal atrial fibrillation. Int J Cardiovasc Imag 2008;24:159-63. http://dx.doi.org/10.1007/s10554-007-9247-3.
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey K, Seward J, et al. Coronary ischemic events after first atrial fibrillation: risk and survival. Am J Med 2007;120:357-63. http://dx.doi.org/10.1016/j.amjmed.2006.06.042.
- Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess 2009.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- National Institute for Health and Care Excellence (NICE) . Atrial Fibrillation – Dabigatran Etexilate (TA249) n.d. www.nice.org.uk/TA249 (accessed March 2012).
- Department of Health (DoH) . Impact Assessment: National Stroke Strategy 2007. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_081054.pdf (accessed April 2013).
Appendix 1 Inclusion and exclusion of pathologies
Appendix 2 Diagnosis of pathologies
Appendix 3 Search strategies
Appendix 4 Data abstraction tables: diagnostic review
Appendix 5 Quality assessment: diagnostic review
Appendix 6 Data abstraction tables: prevalence review
Appendix 7 Quality assessment: prevalence review
Appendix 8 Excluded studies: diagnostic review
Appendix 9 Excluded studies: prevalence review
Appendix 10 Parameters used in mathematical models
Appendix 11 Influence of assumed sensitivity and specificity of transthoracic echocardiography in identifying left atrial abnormality on incremental cost-effectiveness ratio estimates
Influence of assumed sensitivity and specificity of transthoracic echocardiography in identifying left atrial abnormality on incremental cost-effectiveness ratio estimates (PDF download)
Appendix 12 Search strategy for economic evaluations of transthoracic echocardiography in atrial fibrillation
Search strategy for economic evaluations of transthoracic echocardiography in atrial fibrillation (PDF download)
Appendix 13 Study protocol
Glossary
- Antiarrhythmic drugs
- Pharmacological treatment to correct irregular heartbeats and to slow rapid heartbeats
- Anticoagulant drugs
- Pharmacological treatment to reduce the risk of blood clotting.
- Arrhythmia
- Abnormality of the normal heart rhythm.
- Atrial fibrillation
- Arrhythmia characterised by rapid and irregular beating of the atria and absence of regular P waves on the electrocardiogram.
- Atrial flutter
- Arrhythmia characterised by ‘flutter waves’ on the electrocardiogram.
- Cardioversion
- Treatment to restore the heart to normal sinus rhythm using drugs or electric shock.
- Electrical cardioversion
- Treatment to restore the heart to normal sinus rhythm using electric shock.
- Electrocardiography
- Recording of the heart's electrical activity.
- Lone atrial fibrillation
- Atrial fibrillation with no identified cause.
- Paroxysmal atrial fibrillation
- Atrial fibrillation that spontaneously terminates within 7 days, usually within 48 hours.
- Permanent atrial fibrillation
- Established atrial fibrillation that has not terminated, has terminated but recurred, or for which cardioversion has not been attempted.
- Persistent atrial fibrillation
- Atrial fibrillation that does not self-terminate, or lasts > 7 days (without cardioversion).
- Pharmacological cardioversion
- Treatment to restore the heart to normal sinus rhythm using drugs.
- Rate control
- Management of arrhythmia that works to control heart rate.
- Rhythm control
- Management of arrhythmia that works to restore and maintain normal sinus rhythm.
- Sensitivity
- Proportion of true-positives, a measure of the accuracy of a diagnostic test.
- Sinus rhythm
- Normal heart rhythm.
- Specificity
- Proportion of true-negatives, a measure of the accuracy of a diagnostic test.
List of abbreviations
- 2D
- two-dimensional
- AF
- atrial fibrillation
- AMI
- acute myocardial infarction
- AR
- aortic regurgitation
- AV
- atrioventricular
- BSE
- British Society of Echocardiography
- CAF
- chronic atrial fibrillation
- CARAF
- Canadian Registry of Atrial Fibrillation
- CEAF
- cost-effectiveness acceptability frontier
- CHA2DS2-VASc
- congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 years, and sex category (female)
- CHADS2
- cardiac failure, hypertension, age, diabetes, stroke doubled
- CHF
- congestive heart failure
- CI
- confidence interval
- CrI
- credible interval
- CT
- computerised tomography
- DES
- discrete event simulation
- DM
- diabetes mellitus
- ECG
- electrocardiography
- ESC
- European Society of Cardiology
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- FN
- false-negative
- FP
- false-positive
- GI
- gastrointestinal
- GOS
- Glasgow Outcome Scale
- GP
- general practitioner
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracranial haemorrhage
- ICM
- ischaemic cardiomyopathy
- INR
- international normalised ratio
- LA
- left atrial
- LAA
- left atrial appendage
- LAD
- left atrial diameter
- LV
- left ventricular
- M-mode
- motion-mode echocardiography
- MAICER
- maximum acceptable incremental cost effectiveness ratio
- MI
- myocardial infarction
- MR
- mitral regurgitation
- MRI
- magnetic resonance imaging
- mRS
- modifed Rankin Scale
- MVP
- mitral valve prolapse
- NCC-CC
- National Collaborating Centre for Chronic Conditions
- NICE
- National Institute for Health and Care Excellence
- NSF
- National Service Framework
- OAC
- oral anticoagulant
- OR
- odds ratio
- PE
- pulmonary embolism
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QUADAS
- quality assessment of studies of diagnostic accuracy included in systematic reviews
- RA
- right atrial
- RAA
- right atrial appendage
- RCT
- randomised controlled trial
- RE-LY
- Randomized Evaluation of Long-Term Anticoagulation Therapy
- RR
- relative risk
- RV
- right ventricular
- SPAF
- Stroke Prevention in Atrial Fibrillation
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- TIA
- transient ischaemic attack
- TN
- true-negative
- TOE
- transoesophageal echocardiography
- TP
- true-positive
- TTE
- transthoracic echocardiography
- WoS
- Web of Science
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.