Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/112/03. The contractual start date was in November 2010. The draft report began editorial review in April 2012 and was accepted for publication in May 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors' report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen's Printer and Controller of HMSO 2013. This work was produced by Meads et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Aim of the report
The aims of this project were as follows:
-
To determine the accuracy of sentinel lymph node (SLN) biopsy with technetium-99 (99mTc) enhanced and/or blue dye lymphoscintigraphy for diagnosis of inguinofemoral lymphadenopathy (IFL) in vulval cancer through systematic review.
-
To assess, through systematic review, the diagnostic and therapeutic impact of SLN biopsy with 99mTc enhanced and/or blue dye lymphoscintigraphy in:
-
changing disease staging
-
changing planned treatment
-
reducing complications associated with IFL
-
improving morbidity and disease-free survival.
-
-
To determine the effectiveness of various interventions [e.g. surgery, radiotherapy (RT) and chemotherapy] in the management of vulval cancer.
-
To evaluate the cost-effectiveness of SLN biopsy with 99mTc enhanced and/or blue dye lymphoscintigraphy versus IFL using decision-analytic modelling.
The original protocol for the project is in Appendix 1.
Chapter 2 Background
Description of underlying health problem
Vulval cancer is a relatively rare gynaecological malignancy diagnosed mainly among elderly patients. In 90% of cases it develops as a squamous cell carcinoma (SCC) and the remaining 10% are melanomas, Paget's disease, Bartholin's gland tumours, adenocarcinomas and basal cell carcinomas. 1 Lesions mainly occur on the inner edges of the labia majora (around 50% of the cases), less often in the labia minora and very rarely on the clitoris or in the Bartholin glands. The symptoms of vulval cancer include a lump on the vulva, vulval bleeding, itching, pain or ulceration, and approximately 90% of women present with a visible tumour. 1 Diagnosis is by biopsy with histological examination of the sample. Histological examination can include immunohistochemical analysis which may enable more a precise interpretation of the degree of dysplasia compared with conventional haematoxylin and eosin (H&E) staining. 2
Vulval cancer can be locally invasive and can spread via the lymphatic system to the inguinal or femoral nodes. If the primary tumour is laterally located in the vulva, spread may be only to that side; if the tumour is central, spread may be to either side. Lymphatic spread is strongly related to lesion size: metastasis is present in 20–30% of tumours < 2 cm in diameter and in 44% of tumours > 2 cm. 3 Correlation of lymph node status with depth of invasion is also important. Tumours with a < 1-mm depth of invasion have a < 1% risk of nodal spread. 4,5 Lymph node status is regarded as an important predictor of survival. 6 Malignancy in the lymph nodes can result in invasion of the blood vessels of the groin, including rupture of the femoral blood vessels. Distal spread via the bloodstream is relatively rare. 2
Once vulval cancer is diagnosed, staging is important in order to plan treatment and estimate prognosis. Two staging systems are frequently used, one developed by the International Federation of Gynecology and Obstetrics (FIGO)7,8 and one by the tumour node metastasis (TNM) classification of malignant tumours7 (Tables 1 and 2). Both staging systems have gradually evolved over time. Table 3 gives a comparison of FIGO and TNM staging. 2
| Stage | FIGO staging 1969 | FIGO staging 1988 | FIGO staging 2000 | FIGO staging 2009 |
|---|---|---|---|---|
| Clinical staging | Surgical staging | Surgical staging | Surgical staging | |
| 0 | Carcinoma in situ | Carcinoma in situ | Carcinoma in situ | Carcinoma in situ |
| I | Tumour confined to vulva, 2 cm or less in largest diameter and no suspicious groin nodes | Tumour confined to vulva or perineum, < 2 cm in greatest dimension and nodes are negative | Ia: tumour confined to vulva or vulva and perineum, 2 cm or less in greatest dimension and with stromal invasion no greater than 1 mm. Nodes are negative Ib: tumour confined to vulva or vulva and perineum, 2 cm or less in greatest dimension and with stromal invasion greater than 1 mm. Nodes are negative |
IA: lesions ≤ 2 cm in size, with depth of invasion ≤ 1 mm IB: lesions > 2 cm in size, with depth of invasion > 1 mm |
| II | Tumour confined to vulva, more than 2 cm in diameter and no suspicious groin nodes | Tumour confined to vulva or perineum, > 2 cm in greatest dimension, nodes are negative | Tumour confined to vulva or vulva and perineum, more than 2 cm in greatest dimension. Nodes are negative | Tumour of any size with extension to adjacent perineal structures: one-third lower urethra, one-third lower vagina, anus. No lymph node metastases |
| III | Tumour of any size with (1) adjacent spread to the urethra and/or vagina, perineum, and anus, and/or (2) clinically suspicious lymph nodes in either groin | Tumour of any size with (1) adjacent spread to the lower urethra or anus, and/or (2) unilateral regional lymph nodes metastases | (1) Tumour invades any of the following: lower urethra, vagina, anus (2) Unilateral regional lymph nodes metastases |
Tumour of any size, with or without extension to adjacent perineal structures, with positive lymph nodes IIIA: one lymph node metastasis (≥ 5 mm) or 1–2 lymph node metastases (< 5 mm) IIIB: at least two lymph node metastases (≥ 5 mm) or at least three lymph node metastases (< 5 mm) IIIC: lymph node metastases with extracapsular spread |
| IVa | IV: tumour of any size, (1) infiltrating the bladder mucosa or the rectal mucosa, or both, including the upper part of the urethral mucosa, and/or (2) fixed to the bone, and/or (3) other distant metastases | Tumour invasion of any of the following: upper urethra, bladder mucosa, rectal mucosa, pelvic bone and/or bilateral regional node metastasis | Any size tumour with bilateral regional lymph node involvement or with invasion of any of the following: upper urethra, bladder or rectal mucosa, pelvic bone | Tumour invades any of the following: upper urethral and/or vaginal mucosa, bladder or rectal mucosa, or fixed to the pelvic bone. Or fixed or ulcerated lymph nodes |
| IVb | – | Any distant metastasis, including pelvic lymph nodes | Any distant metastasis, including pelvic lymph nodes | Any distant metastases including pelvic nodes |
| Stage | TNM 1969 | TNM 1988 | TNM 2000 |
|---|---|---|---|
| Clinical staging | Surgical staging | Surgical staging | |
| Tx | – | Primary tumour cannot be assessed | Primary tumour cannot be assessed |
| T0 | – | No evidence of primary tumour | No evidence of primary tumour |
| Tis | – | Carcinoma in situ | Carcinoma in situ |
| T1 | Tumour confined to the vulva, 2 cm in largest diameter | Tumour confined to the vulva and/or perineum, ≤ 2 cm in greatest dimension | T1a: tumour confined to the vulva and/or perineum, ≤ 2 cm in greatest dimension and with stromal invasion no greater than 1 mm T1b: tumour confined to the vulva and/or perineum, ≤ 2 cm in greatest dimension and with stromal invasion > 1 mm |
| T2 | Tumour confined to the vulva, 2 cm in largest diameter | Tumour confined to the vulva and/or perineum, > 2 cm in greatest dimension | Tumour confined to the vulva and/or perineum, > 2 cm in greatest dimension |
| T3 | Tumour of any size with adjacent spread to the urethra, and/or vagina, and/or perineum and/or anus | Tumour involves any of the following: the lower urethra, vagina, anus | Tumour invades any of the following: the lower urethra, vagina, anus |
| T4 | Tumour of any size infiltrating the bladder mucosa, and/or the rectal mucosa, or including the upper part of the urethral mucosa and/or fixed to the bone | Tumour involves any of the following: bladder mucosa, rectal mucosa, upper urethra, pelvic bone | Tumour invades any of the following: bladder mucosa, rectal mucosa, upper urethral mucosa or is fixed to bone |
| Nx | Regional (i.e. femoral and inguinal) lymph nodes cannot be assessed | Regional (i.e. femoral and inguinal) lymph nodes cannot be assessed | |
| N0 | No nodes palpable | No lymph node metastases | No lymph node metastases |
| N1 | Nodes palpable in either groin, not enlarged, mobile (not clinically suspicious for neoplasm) | Unilateral regional lymph node metastases | Unilateral regional lymph node metastases |
| N2 | Nodes palpable in either groin, enlarged, firm and mobile (clinically suspicious for neoplasm) | Bilateral regional lymph node metastases | Bilateral regional lymph node metastases |
| N3 | Fixed or ulcerated nodes | – | – |
| Mx | – | Distant metastases cannot be assessed | Distant metastases cannot be assessed |
| M0 | No clinical metastases | No distant metastases | No distant metastases |
| M1 | M1a: palpable deep pelvic lymph nodes M1b: other distant metastases |
Distant metastases, including pelvic lymph node metastases | Distant metastases, including pelvic lymph node metastases |
| FIGO stage | TNM status | ||
|---|---|---|---|
| Tumour | Node | Metastases | |
| 0 | Tis | N0 | M0 |
| IA | T1a | N0 | M0 |
| IB | T1b | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T3 | N0 | M0 |
| T3 | N1 | M0 | |
| T1 | N1 | M0 | |
| T2 | N1 | M0 | |
| IVA | Ta | N2 | M0 |
| T4 | Na | M0 | |
| IVB | Ta | Na | M1 |
The grade of malignancy refers to the extent of differentiation seen on microscopic examination and gives an indication of how fast the malignancy is likely to develop. In vulval cancer three grades are defined:
-
Grade 1: cells are low grade or well differentiated and histologically look very much like normal vulval cells.
-
Grade 2: cells are medium grade or moderately differentiated and look more abnormal than grade 1 cells, but not so much as grade 3 cells.
-
Grade 3: cells are high grade or poorly differentiated or can be undifferentiated, they are very unlike normal vulval cells.
Premalignant conditions
Vulval intraepithelial neoplasia (VIN) is a precancerous condition that can occur in the vulval area and may present as pigmented lesions. There are two main types of VIN, each with their own distinctive clinical and pathological features. The classifications in use are the World Health Organization (WHO)’s classification that refers to human papillomavirus (HPV)-associated VIN as warty/basaloid VIN and grades the disease as VIN1, VIN2 and VIN3. The International Society for the Study of Vulvovaginal Disease (ISSVD) has proposed a newer classification referring to VIN usual type and VIN differentiated type. The more commonly referred to classical or usual-type VIN is a disease of relatively young women and is associated with HPV. HPV deoxyribonucleic acid (DNA), mostly type 16, is present in up to 90% of classical VIN. Typically, classical VIN involves the vulva multifocally and is associated with multicentric involvement of the vagina and cervix. Classical VIN progresses to cancer in only 3–10% of treated patients. The other type of VIN is referred to as simplex or differentiated VIN and was described in the 1960s, but added to ISSVD classification in 2004/5. It constitutes 2–10% of VIN diagnoses, but is seen adjacent to up to 60% of vulval cancers. It characteristically occurs in postmenopausal women and is associated with lichen sclerosis. Histologically, it is a subtle lesion and is likely to be underdiagnosed because of its high degree of differentiation. 9–14
Aetiology
Several factors increase the risk of developing cancer of the vulva, but none confers high risk. Factors increasing risk of malignancy include HPV infection, smoking, human immunodeficiency virus (HIV) infection and other factors.
Smoking
Smoking is a risk factor for the development of many malignancies, including vulval cancer. 15,16 For women already infected with HPV, smoking further raises the risk of neoplasia. It has been reported that cigarette smoking increases the risk of vulval cancer development by 25 times for women smoking ≥ 20 cigarettes a day who also have serological evidence of HPV-16 infection. 16 Another study reported that active smoking was associated with an increased risk of developing vulval cancer [relative risk (RR) 2.03, 95% confidence interval (CI) 1.3 to 3.2]. 17
Human papillomavirus infection
Human papillomavirus infection is thought to cause up to half of vulval cancers, mostly in women under the age of 50 years. HPV is less likely to be a risk factor in older women. Vulval carcinoma development is associated with infection of high-risk HPV types. A case–control study found that over 30% of examined tumours contained HPV DNA of types 16, 18 or 33. Moreover, HPV DNA was found in 61% of tumours with adjacent intraepithelial neoplasia (VIN III) and in 13% of tumours without associated VIN III. 18 The prevailing evidence favours HPV as one causative factor in genital tract carcinoma. 19
Human immunodeficiency virus infections
Human immunodeficiency virus causes the acquired immune deficiency syndrome (AIDS). Owing to its destructive effect on the human immune system, women burdened with it are more prone to a HPV infection, which, in turn, may be easily linked with vulval cancer development. 20
Other factors
A higher risk of vulval cancer is associated with lower socioeconomic status and fewer years of education. 21 It has been found that a prior history of lichen sclerosus of the vulva and inflammation of the vulva or vagina are significantly associated with vulval cancer development. Some other evidence suggests that environmental exposures may also play an important role in vulval carcinogenesis. 22 In addition, some studies have shown a link between the risk of vulval cancer occurrence and psoriasis,23 or vulval cancer occurrence and transplant immunosuppression. 24
Epidemiology
Vulval cancer accounts for approximately 3–5% of all gynaecological malignancies and 1% of all cancers in women. 25 The worldwide prevalence of vulval cancer is around 3% and it is estimated that 27,000 women are diagnosed each year. 21 In the UK, the lifetime risk of developing vulval cancer is 1 in 316. 21 According to a recent report, the highest rate of occurrence is in North America, South America and Europe, with incidence rates of between 1 and 2 per 100,000 per year. 26
Vulval cancer is very rare in young women aged < 25 years. Incidence rates are around 1.1 per 100,000/year, among women aged 25–39 years, rising to 3.8 per 100,000/year in those aged 60–64 years and peaking at 24.5 per 100,000/year in women aged ≥ 85 years. The proportion of women diagnosed with vulval cancer under the age of 50 years has risen from 7% in the 1970s to 14% in 2006–8. 21
Each year in the UK, there are, on average, 373 deaths from vulval cancer. The European age-standardised death rate for vulval cancer in the UK was 0.64 per 100,000 female population over the 5-year period 2004–8. The mortality rate for vulval cancer is 0.1 per 100,000 women aged 25–44 years and 0.5 for women aged 45–64 years, rising to 6 per 100,000 women aged ≥ 65 years and 12 per 100,000 women aged ≥ 80 years. Mortality rates for vulval cancer in the UK have declined steadily since the early 1970s. The rate fell by almost half (48%) between 1971 and 2008, from 1.3 per 100,000 female population to 0.7 per 100,000. 27
Prognosis
Vulval cancer is highly curable when diagnosed at an early stage. 28 Survival is most dependent on primary lesion diameter and the pathological status of the inguinal nodes. 1 Linking staging to survival is not precise if it is unclear whether the nodes are involved (which would be stage III) or not (which would be stage II). In a study of 588 patients treated in 1977–84,29 survival of patients with FIGO stage I, II, III and IV disease was 98%, 87%, 75% and 29%, respectively. If nodes are involved, survival is linked to the number of nodes involved and unilaterality versus bilaterality. In patients with tumour diameter > 8 cm and three or more unilateral or two bilateral metastatic nodes, the relative survival at 5 years was 0%. 29 The following aspects have been accounted as the risk factors for node metastasis: clinical node status, age, degree of differentiation, tumour stage, tumour thickness and depth of stromal invasion. 29,30 The presence of capillary–lymphatic space invasion is associated with increased local recurrence in the vulva but not with increased risk of groin node metastases. 1
Another early study gave 5-year survival rates for RT of only 13.0%, simple vulvectomy of 30.8%, vulvectomy and RT of 32.5%, radical vulvectomy with IFL of 55.7% and radical vulvectomy with inguinal, femoral and pelvic lymphadenectomy of 63.3%. 31
Current treatment options
Since the late 1960s, the treatment of choice for vulval cancer has been surgical removal of the tumour and affected lymph nodes. 1 The actual treatment used for SCC depends on the stage of tumour found (Table 4). 3 Surgical management of non-SCC cancers vary by type of cancer. For carcinoma of the Bartholin's gland, treatment follows that for SCC tumours but the tumours are more likely to be deep and metastatic. For basal cell and verrucous carcinomas, wide local excision is usually used as metastases are rare. For malignant melanoma (MM), wide local excision is preferred, but there is a high rate of relapse. 1
| Stage | Definition | Treatment |
|---|---|---|
| 0 | Carcinoma in situ and less advanced precancerous changes, e.g. VIN | Laser surgery, wide local excision, or a skinning vulvectomy; alternatively fluorouracil ointment may be prescribed or imiquimod |
| I | Treatment depend on the size and depth of the cancer and VIN occurrence | – |
| IA – depth of invasion of 1 mm or less, and there are no other areas of cancer or VIN | Wide local excision. The cancer is being removed along with a 1-cm margin of the normal tissue surrounding | |
| IB – lesions > 2 cm in size with depth of invasion > 1 mm | Wide local excision/hemi/radical vulvectomy and inguinal lymph node dissection SLN biopsy may be performed instead of lymph node dissection when tumour size is < 4 cm and is unifocal |
|
| II | Cancer spread to structures near the vulva area | Partial radical vulvectomy |
| Optional removal of the lymph nodes in the groin on both sides of the body | ||
| If cancer cells are present near the margins the radiation therapy to the area of surgery is performed | ||
| Radiation therapy with surgery in order to remove remaining cancer tissues. Chemotherapy with fluorouracil and/or cisplatin | ||
| III | Cancer spread to nearby lymph nodes | Surgical removal of cancer and lymph nodes in the groin optionally followed by radiation therapy. Radiation and chemotherapy are applied for patients not able to undergo surgery |
| IVA | Cancer spread to organs and tissues in the pelvis: rectum, bladder, pelvic bone, upper part of the vagina and urethra. Tumours of type T1 and T2 with less severe nearby spread but with extensive spread to nearby lymph nodes | Removal of as much as possible of the affected tissue with possible pelvic exenteration. Usually operation includes vulvectomy, removal of the pelvic lymph nodes or, alternatively, lower colon, rectum, bladder, uterus, cervix and vagina Gold standard: combination of surgery, radiation and chemotherapy |
| IVB | Cancer spread to lymph nodes in the pelvis or to organs and tissues outside the pelvis | None of the approaches is assumed to cure the malignancy though they may be helpful in relieving some symptoms emerging from the disease |
| Recurrent | Cancer recurrence | Treatment depends on the recurrence time and location. Local recurrence: surgery or combination of three approaches. Unresectable recurrence: chemotherapy and/or radiation therapy |
Surgery to the vulva can be radical vulvectomy (complete removal of the vulva), hemivulvectomy or, more commonly, wide local excision. 1 If necessary, skin grafting using skin from the thigh can be used. The intention of surgery is complete removal of the lesion with a minimum margin of 15 mm disease-free tissue on all sides of the specimen. The side effects of vulvectomy are extensive pain, disfigurement and the risk of sphincter damage leading to urinary or faecal incontinence. There can be considerable psychological trauma and loss of psychosexual function. 32
Because of the risk of lymphatic spread to the groin nodes, lymphadenectomy of the inguinal and femoral nodes, either unilaterally or bilaterally, is usually undertaken, depending on the stage of the disease and the laterality of the tumour. IFL can be omitted safely if the tumour depth of invasion is < 1 mm. Unilateral IFL is performed for lateral tumours that are at least at a 1-cm distance from the midline of the vulva. Bilateral IFLs are performed for tumours encroaching within 1 cm of the midline. Complications affect over 50% of patients having IFL, including infection of groin wounds, subsequent wound breakdown, lymphoedema and cellulitis. 33–35 If the groin breaks down, patients need to stay in hospital for several days longer than otherwise and will need antibiotics and community care once discharged. As patients are often elderly, this additional morbidity can compromise overall recovery. Lymphoedema may be the most aggravating as it significantly limits overall mobility,36 is disfiguring, causes difficulties in daily living, can lead to lifestyle becoming severely limited and may also result in psychological distress. Over the last 20 years, use of the triple-incision technique in the groin, for which three cuts are made, leaving skin bridges between so that the skin can heal more quickly than one longer single (butterfly) incision, has reduced postoperative stay and improved recovery from IFL. 37
Patients with poor prognostic features may additionally receive RT covering the pelvis, groin and the perineum area. In cases of locally advanced disease, standard management includes surgery, chemotherapy and radiation therapy (sequentially or in combination). 1 If patients are unable to withstand surgery, RT alone or, occasionally, chemotherapy alone can be used.
Sentinel lymph node biopsy
Currently, clinical examination for the determination of metastatic involvement of groin lymph nodes is insufficiently accurate, particularly when the nodes may contain micrometastases but appear clinically normal. Out of all patients with clinically normal lymph nodes, between 16% and 24% will go on to develop metastases;30,38 therefore, interest has been shown in the use of imaging modalities and SLN biopsy in order to determine lymphatic spread and thereby more accurately stage vulval cancer and reduce the need for unnecessary surgery. If IFL is undertaken and nodes are negative for metastases, there is considerable morbidity from the IFL, which is associated with longer hospital stays. If IFL is not undertaken and there were micrometastases that were missed, survival rates are reduced. So IFL is a surgical procedure that serves to obtain lymph nodes for histopathology as a diagnostic test for metastases and IFL can also remove clinically suspicious enlarged nodes to improve treatment success rates.
A SLN refers to any lymph node that receives drainage directly from the primary tumour and is the first in the chain of lymph nodes in the groin and, therefore, has the highest probability of containing cancer cells from the tumour in the vulva. 39 SLNs can be identified by using a dye called isosulfan blue or a radioactive tracer called 99mTc in a procedure called lymphoscintigraphy. Blue dye and 99mTc can be used alone or in combination. 40 The blue dye/99mTc procedure only detects the SLN, but cannot determine whether or not the SLN has metastatic deposits. For this, histopathological examination is required. This is best done by routine histopathology using H&E staining, although, in some centres, frozen sections may also be used. Lymph nodes can be cut in a variety of slices, with thinner slices known as ultrastaging. Immunohistochemical techniques that will enhance the ability to detect metastatic deposits can also be used.
There is a risk with SLN biopsy that malignancy may be missed. It may be that the first draining lymph node was missed, or that the malignancy developed not in the SLN but in any of the groin nodes other than the SLNs biopsied and examined with histology. There has been one small survey of vulval cancer patients evaluating the acceptability of SLN biopsy compared with IFL at different levels of risk. 41 This 106 patients who were surveyed had fully recovered from vulval cancer (99 questionnaires could be evaluated) and had received IFL as part of their treatment. 41 It was found that 66% would recommend IFL if the risk of missing metastasis from SLN biopsy was 1 in 80, and 84% would recommend IFL if the risk of missing metastasis from SLN biopsy was one in eight. Age and the presence or degree of side effects experienced by the patients surveyed, which included severe lymphoedema in 39% and with severe pain in 28%, did not affect preferences for each procedure.
The extent of SLN biopsy being undertaken in the NHS for vulval cancer is currently unclear. The most recent guideline from the UK Royal College of Obstetricians and Gynaecologists states that ‘Dye studies and lymphoscintigraphy may be of value in the detection of SLNs although the outcome of this type of intervention is awaiting the outcome of controlled clinical evaluation’. 1
Chapter 3 Definition of the decision problem
If SLN biopsy could accurately identify those patients in whom cancer has spread to the groin nodes without extensive surgical removal of all of the groin nodes, this would be extremely valuable. If this technique was very accurate in detecting no metastases, no radical treatment would be necessary. In order to test the accuracy of SLN biopsy, the reference standard can be IFL for all node-positive and -negative patients. Alternatively, clinical follow-up could be used for node-negative patients if SLN was considered to be sufficiently accurate not to miss patients with metastases in the lymph nodes. The different possibilities are illustrated in Figure 1 for the situations in which a SLN was found at biopsy and Figure 2 in the case that a SLN was not found at biopsy. The histopathology of the SLN biopsy should ideally be compared with the same type of histopathology used for all of the lymph nodes examined after IFL because the histopathology is part of the SLN biopsy test as well as the reference standard. If frozen sections or immunohistochemistry were used for the SLN histopathology and routine histological techniques such as H&E staining were used only for the IFL nodes, then that would, in effect, mean a different reference standard was being used.
FIGURE 1.
Decision tree for SLN biopsy (part 1).
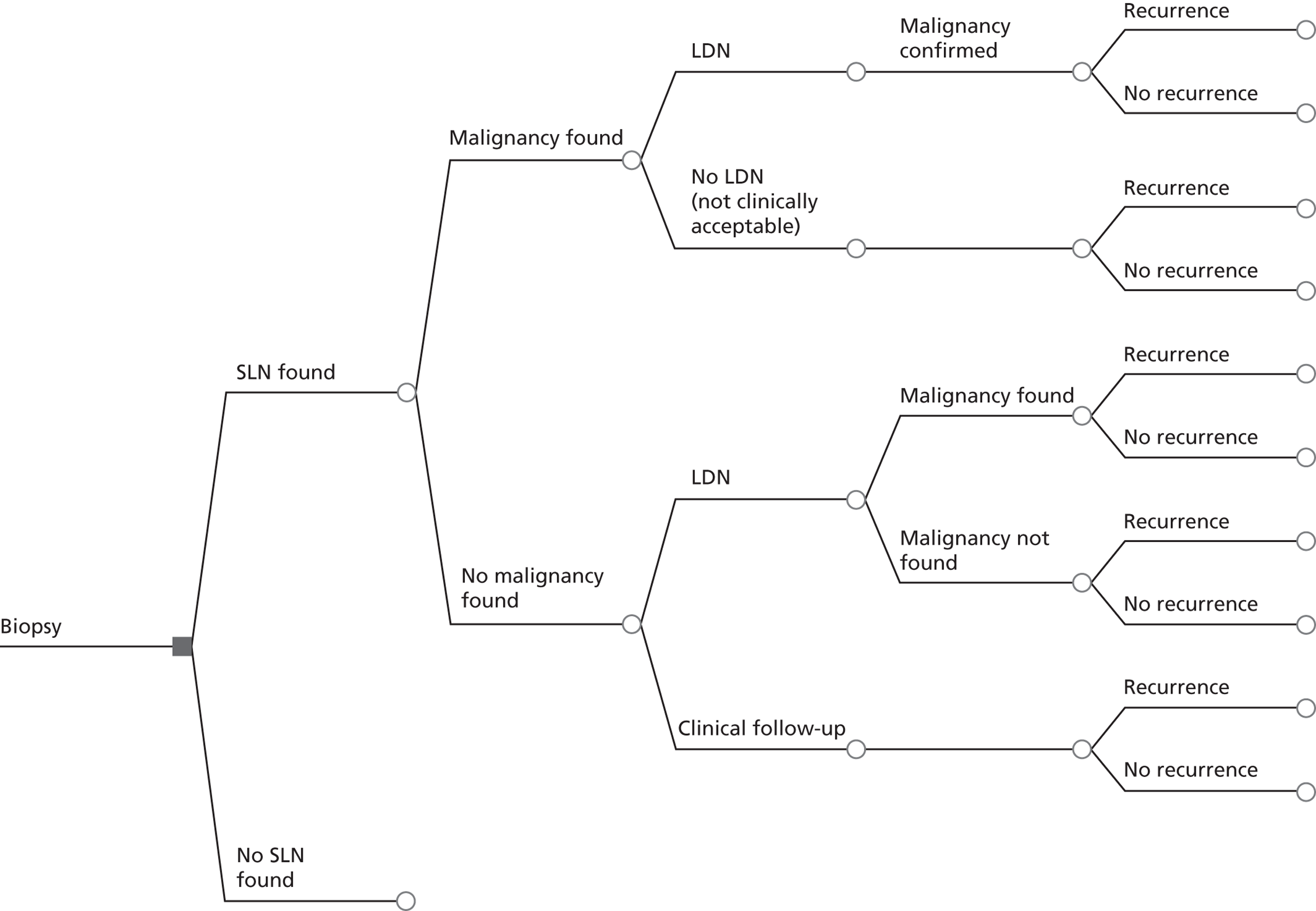
FIGURE 2.
Decision tree for SLN biopsy (part 2).
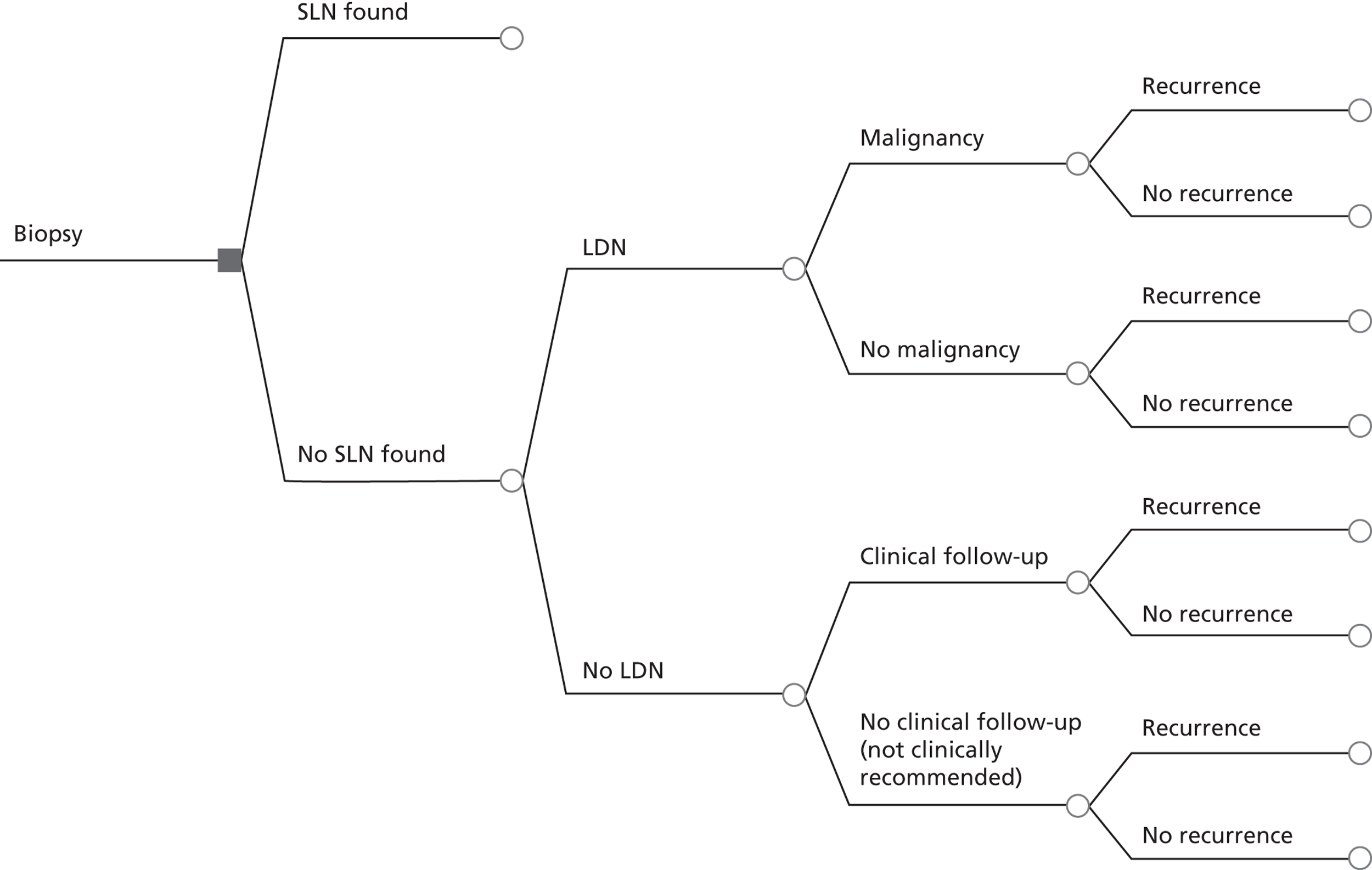
In the decision trees, recurrence refers to groin recurrence; however, any recurrence is important, and both groin and distant recurrence may be reduced following IFL, but not local recurrence in the vulva.
The aim of this health technology assessment (HTA) was to determine the test accuracy and cost-effectiveness of SLN biopsy in vulval cancer by systematic reviews and decision-analytic modelling.
Chapter 4 Systematic review methods
Protocol development and overview of review methods
A protocol was developed for undertaking systematic reviews of test accuracy, diagnostic and therapeutic impact and effectiveness of treatment for vulval cancer (see Appendix 1). Scoping searches for relevant systematic reviews were conducted in MEDLINE, EMBASE and The Cochrane Library [systematic reviews, HTA, Database of Abstracts of Reviews of Effects (DARE)] (see Appendix 2).
Systematic reviews were carried out using established methods in line with the recommendations of the NHS Centre for Reviews and Dissemination and the Cochrane Collaboration,42,43 including those of the Cochrane Methods Working Group on Screening and Diagnostic Tests. 44 Presentation of results is according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. 45
Inclusion of studies, data extraction and quality assessment were carried out in duplicate, with differences resolved by consensus and/or arbitration involving a third reviewer. The selection process was piloted by applying the inclusion criteria to a sample of papers first. A two-stage process was then followed. First titles and abstracts were screened, then, for all references categorised as ‘include’ or ‘uncertain’ by both reviewers, the full text was retrieved wherever possible and final inclusion decisions were made based on the full paper. Reference Manager version 12.0 (Thomson Reuters, New York, NY, USA) software was used to construct a database of citations for all systematic reviews.
Clinical, methodological and statistical data extraction was conducted into data extraction sheets by at least two reviewers and discrepancies were resolved through discussion. If consensus could not be reached, disagreements were resolved by arbitration by a third reviewer. For diagnostic studies, information regarding study design and methods, characteristics of participants, SLN biopsy and comparison tests, and outcomes of interest were extracted using data extraction forms (see Appendix 3). For the effectiveness review, separate data extraction forms were used for different study designs: comparative experimental study (part A), comparative observational study (part B) and non-comparative study (part C). The data extraction sheets used are shown in Appendix 4. The quality assessment questions for randomised controlled trials (RCTs) were included in the data extraction sheet, but a separate form was used for case series (see Appendix 4). Data extraction was managed with Microsoft Word 2003 (Microsoft Corporation, Redmond, WA, USA) and Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA). Quality was also assessed independently by two reviewers. Any disagreements were resolved through discussion or by arbitration by the third reviewer. For each review a comprehensive master database of articles was constructed using Reference Manager 12.0 software.
Methods for test accuracy, diagnostic and therapeutic impact review
Search strategy
A sensitive search was conducted to identify all relevant published and unpublished studies and studies in progress. All databases were searched from inception to January 2011. Search strategies were designed from a series of test searches and discussions of the results of those searches among the review team. Both medical subject heading (MeSH) terms and text words were used and included ‘vulva cancer’, ‘sentinel lymph node biopsy’ and ‘lymphoscintigraphy’. Search strategies can be found in Appendix 5. Literature was identified from several sources, including:
-
General health and biomedical databases: MEDLINE (Ovid), EMBASE (Ovid), Science Citation Index; and medical diagnostic studies database (MEDION).
-
Checking of reference lists of review articles and papers.
-
Specialist search gateways [Organizing Medical Networked Information (OMNI) and The National Cancer Institute], general search engine (Google) and metasearch engine (Copernic).
-
Searching a range of relevant databases including Clinical Trials.com and UK Clinical Research Network Portfolio to identify information about studies in progress, unpublished research or research reported in the grey literature.
-
Hand-searching of Gynecologic Oncology journal (1980 to January 2011).
-
Contact with authors of the included studies for information on any relevant published or unpublished studies.
Inclusion and exclusion criteria
Population
Included were:
-
women with early stages of vulval cancer: at least 75% of population with FIGO stage I and II or TNM categories T1–2, N0, M0.
Excluded were:
-
all patients with vulval melanomas
-
advanced stage vulval cancer (FIGO stage IV), inoperable tumours, tumours unsuitable for primary surgery
-
patients with clinical suspicion of metastases, i.e. with palpable inguinofemoral lymph nodes, enlarged lymph nodes (> 1.5 cm) on imaging or cytologically proven inguinofemoral lymph node metastases at the start of the study.
Index tests and comparator tests
Included were:
-
SLN biopsy with 99mTc, blue dye or combined technique (99mTc with blue dye), with histopathology by frozen section or other routine histopathological techniques. Where studies reported any of ultrastaging, serial sections, multiple slices, additional sections or step sections, these were all classified as ultrastaging.
Excluded were:
-
imaging modalities such as ultrasonography
-
novel techniques such as reverse transcriptase-polymerase chain reaction (RT-PCR).
Reference standard
Included were:
-
histopathology of inguinofemoral node dissection
-
follow-up for groin recurrence.
Excluded were:
-
imaging modalities such as ultrasonography.
Outcomes
Included were:
-
diagnostic accuracy
-
diagnostic impact: change in staging after SLN biopsy
-
therapeutic impact: change in treatment plan including avoidance of full IFL after SLN biopsy
-
complications
-
morbidity
-
mortality and disease-free survival
-
quality of life (QoL)
-
impact on surgeon's and team’s skills and experience (learning curve).
Excluded were:
-
non-clinical outcomes
-
outcomes reported per groin only.
Study design
Included were:
-
any prospective or retrospective test accuracy study designs
-
studies investigating the diagnostic and therapeutic impact with or without concurrent assessment of test accuracy
-
prospective cohort studies of outcomes of patients tested with 99mTc, blue dye or combined technique for SLN biopsy.
Excluded were:
-
case studies
-
studies with 10 or fewer patients.
Quality assessment
Test accuracy quality assessment followed the quality of diagnostic accuracy studies (QUADAS) guidelines46 and diagnostic and therapeutic impact followed those suggested by Meads and Davenport. 47 The items of methodological quality listed in the QUADAS guidelines are representative spectrum, selection criteria clearly described, acceptable reference standard, acceptable delay between tests, partial verification avoided, differential verification avoided, reference standard independent of the index test, index test described in sufficient detail, reference standard described in sufficient detail, index test results blinded, reference standard results blinded, relevant clinical information available, uninterpretable results reported and withdrawals explained. 46
These were tailored to assess the included studies because different aspects of quality are applicable to different topic areas. The actual quality items used for this report are listed below. For acceptable delay between tests, this included delay between the index test and reference standard (within 1 month). There will inevitably be a delay between index test and clinical follow-up (when available). Study quality was summarised in a table (see Table 10). No additional issues were thought to be useful in interpretetion of the results of these studies. The following items were included in study summaries and assessed using the three criteria listed under each item. 44
1. Representative spectrum
Yes: If patients were women in early-stage squamous cell vulval cancer.
No: If a few patients were in a higher stage of vulval cancer (T3–T4) or some patients had vulval melanoma or another type of cancer rather than squamous cell cancer.
Unclear: If there is insufficient information available to make a judgement about the spectrum of patients.
2. Selection criteria clearly described
Yes: If the selection criteria are described.
No: If the selection criteria are not described.
Unclear: If there is insufficient information available to clearly know the selection criteria.
3. Acceptable reference standard
Yes: Whether or not the reference standard used (histopathology, clinical follow-up) was adequately described to permit sufficient replication and was appropriate according to advice from our clinical experts (e.g. ultrastaging used, immunohistochemistry used).
No: The reference standards used do not include ultrastaging or immunohistochemistry.
Unclear: It is unclear exactly what reference standard was used.
4. Acceptable delay between sentinel lymph nodes biopsy and histopathology
Yes: If the time between tests was shorter than 1 month, at least for an acceptably high proportion of patients.
No: If the time between tests was longer than 1 month for an unacceptably high proportion of patients.
Unclear: If information on timing of tests was not provided.
5. Partial verification avoided
Yes: If all patients, or a random selection of patients, who received the index test went on to receive verification of their disease status using a reference standard, even if the reference standard was not the same for all patients.
No: If some of the patients who received the index test did not receive verification of their true disease state, and the selection of patients to receive the reference standard was not random.
Unclear: If this information is not reported by the study.
6. Differential verification avoided
Yes: If the same reference standard was used in all patients.
No: If the choice of reference standard varied between individuals.
Unclear: If it is unclear whether or not different reference standards were used.
7. Incorporation avoided
Yes: If the index test did not form part of the reference standard.
No: If the reference standard formally included the result of the index test.
Unclear: If it is unclear whether or not the results of the index test were used in the final diagnosis.
8. Whether or not there was sufficient information to replicate index test and reference standard
Yes: Sufficient information available.
No: Insufficient information available.
Unclear: If it is unclear whether or not there is enough information to permit replication.
9. Reference standard/index test results blinded
Yes: If test results (index or reference standard) were interpreted blind to the results of the other test or blinding is dictated by the test order.
No: If it is clear that one set of test results was interpreted with knowledge of the other.
Unclear: If it is unclear whether or not blinding took place.
10. Relevant clinical information
Yes: If the same clinical data available when test results were interpreted as would be available when the test is used in practice.
No: If clinical data usually available was withheld or if more information than is usually available was provided.
Unclear: If information about the clinical data available was not stated.
11. Uninterpretable results reported
Yes: If the number of uninterpretable test results is stated or if the number of results reported agrees with the number of patients recruited (indicating no uninterpretable test results).
No: If it states that uninterpretable test results occurred or were excluded and does not report how many.
Unclear: If it is not possible to work out whether or not uninterpretable results occurred.
12. Withdrawals explained
Yes: If it is clear what happened to all patients who entered the study, for example, if a flow diagram of study participants is reported explaining any withdrawals or exclusions, or the numbers recruited match those in the analysis.
No: If it appears that some of the patients who entered the study did not complete the study, i.e. did not receive both the index test and reference standard, and these patients were not accounted for.
Unclear: If it is unclear how many patients entered and, hence, whether or not there were any withdrawals.
Methods of statistical analysis
RevMan version 5.0 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) was used in the statistical analyses and Meta-Disc statistical package version 1.4 (Javier Zamora, Madrid, Spain) was used to conduct meta-analysis. Sensitivity, specificity, true-positives (TPs), false-positives (FPs), true-negatives (TNs) and false-negatives (FNs) were taken directly from the source papers. If that was not possible, values were calculated from data provided. Based on an investigation of heterogeneity, summary estimates of sensitivity, specificity and likelihood ratios (LRs) were derived as appropriate. Results are displayed graphically on summary receiver operator curve plots (see Figure 4). Summary SLN detection rates and their 95% CIs were calculated using Meta-Disc. Methods for meta-analysis used by Meta-Disc are as follows. Sensitivity and specificity are pooled by the formulae:
where a are TPs and d are TNs, D is total number with disease and ND is the total number without disease. These formulae correspond to weighted averages in which the weight of each study is its sample size. The CIs of sensitivity and specificity are calculated using the F distribution method to compute the exact confidence limits for the binomial proportion (x/n) and are given by the formulae below where LL is the lower limit and UL is the upper limit:
Bivariate meta-analysis can only be conducted when there are more than four studies. Only one group of studies were eligible [IFL for all, 99mTc with blue dye – ultrastaging with immunohistochemistry (see Table 16)]. However, the diagnostic test results for all of the studies have no FPs, so STATA (version 12.1; StataCorp, College Station, TX, USA) will only run a bivariate meta-analysis if a continuity correction is added (changing 0 to 1 in some of the studies). This was done for the last five studies (see Table 16).
Methods for effectiveness reviews
Search strategy
A sensitive search was conducted to identify all relevant published and unpublished trials and trials in progress. All databases were searched from inception to January 2011. Search strategies were designed from a series of test searches in a multistep process. Both MESH terms and text words were used and included a variety of synonyms for vulval cancer and the interventions (surgery, RT, chemotherapy). Search strategies can be found in Appendix 6. Studies were identified from several sources, including:
-
General health and biomedical databases: MEDLINE (Ovid), EMBASE (Ovid).
-
Specialist electronic databases: The Cochrane Library, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), DARE and HTA Database.
-
Checking of reference lists of review articles and papers.
-
Searching a range of relevant databases including ClinicalTrials.com and UK Clinical Research Network Portfolio to identify information about studies in progress, unpublished research or research reported in the grey literature.
-
Hand-searching (Gynecologic Oncology) from 1980 to January 2011.
-
Specialist search gateways (OMNI and the National Cancer Institute), general search engine (Google) and metasearch engine (Copernic) in January 2011.
Inclusion and exclusion criteria
Population
Included were:
-
women with early stages of vulval cancer (including squamous cell carcinoma, basal cell carcinoma, adenocarcinoma, vulval Paget's disease, Bartholin's gland cancer): at least 75% of population with FIGO stage I and II or TNM categories T1–2, N0–1, M0.
Excluded were:
-
all patients with vulval melanomas or VIN only
-
patients with advanced vulval cancer, inoperable tumours and those unsuitable for primary surgery
-
patients with clinical suspicion of metastases, i.e. with palpable inguinofemoral lymph nodes, enlarged lymph nodes (> 1.5 cm) on imaging or cytologically proven inguinofemoral lymph node metastases at the start of the study only
-
patients with multifocal tumours only
-
studies with 25% or more patients with clinical stages more advanced than FIGO stages I and II or TNM T1–2, N0–1, M0, unless the subgroup with these characteristics were clearly indicated and results given separately
-
studies with all patients treated before 1980.
Intervention
Included were:
-
surgery: vulvectomy (any form, with or without IFL)
-
RT (any type, to vulval area or groin).
Excluded were:
-
diagnostic treatment studies.
Comparator (when available)
Included were:
-
surgery (any form) with RT (adjuvant or neoadjuvant) or chemotherapy.
Excluded were:
-
same surgery as intervention. We did not include studies comparing different types of vulval excision for vulval cancer, as this was not relevant to the primary question to be addressed.
Outcomes
Included were:
-
deaths, overall survival, disease-free survival (presented as raw numbers, survival curves, etc.)
-
morbidity
-
recurrence
-
QoL
-
early and late complications.
Excluded were:
-
psychosexual outcomes.
Study design
Included were:
-
RCTs
-
non-RCTs
-
observational studies (cohort, case–control or case series).
Excluded were:
-
studies with five or fewer patients in the therapeutic group
-
studies in which the majority of patients were enrolled in 1970s or earlier.
Quality assessment
Quality assessment was performed appropriate to study designs. For RCTs, quality assessment was according to the Cochrane Handbook for Systematic Reviews of Interventions43 (Table 5). In all cases, a ‘yes’ answer indicated a low risk of bias and a ‘no’ indicated a high risk of bias. ‘Unclear’ was used if details were insufficient. Quality of studies was summarised in tables (see Tables 34 and 35). Case–control studies were evaluated using the Newcastle–Ottawa Scale48 (Table 6). A study was awarded with maximum one star [*] for each numbered item within the ‘selection’ and ‘exposure’ categories and a maximum of two stars [**] in the ‘comparability’ category. Each evaluated study could obtain a maximum of nine stars (four for the selection part, two for the comparability part and three for the exposure part). Qualitative description was also used. The detailed coding manual for this scale is in Appendix 7. Quality assessment of case series used criteria from a recent HTA report on methodological characteristics of case series. 49 A checklist composed of 13 items in five categories was used, and this is reproduced in Appendix 8.
| Section | Description | Question |
|---|---|---|
| Sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether or not it should produce comparable group | Adequate sequence generation? |
| Allocation concealment | Describe the method used to generate the allocation sequence in sufficient detail to determine whether or not intervention allocations could have been foreseen in advance of, or during, enrolment | Allocation concealment? |
| Blinding of participants, personnel and outcome assessors | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether or not the intended blinding was effective | Blinding? (Self-reported outcomes) |
| Blinding? (Objective outcomes) | ||
| Incomplete outcome data | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from analysis. State whether or not attrition and exclusions were reported, the numbers in each intervention group (compare with total randomised participants), reasons for attrition/exclusions when reported and any reinclusions in analyses performed by the review authors | Incomplete outcome data addressed? |
| Selective outcome reporting | State how the possibility of selective outcome reporting was examined by the review authors and what was found | Free of selective reporting? |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool | Free of other bias? |
| Section | Number | Question |
|---|---|---|
| Selection | 1. | Is the case definition adequate? |
| 2. | Representativeness of the cases | |
| 3. | Selection of controls | |
| 4. | Definition of controls | |
| Comparability | 1. | Comparability of cases and controls on the basis of the design or analysis |
| Exposure | 1. | Ascertainment of exposure |
| 2. | Same method of ascertainment for cases and controls | |
| 3. | Non-response rate |
Methods of statistical analysis
Separate analyses were performed on randomised and observational studies. RevMan version 5.0 was used in the statistical analyses. Information was analysed based on the group to which the participants were allocated, regardless of whether or not they received the allocated intervention. For dichotomous data, results are presented as summary RR with 95% CI (for comparative observational studies odds ratios were calculated when appropriate). For case–control studies and case series, a narrative summary of the findings is presented along with the numerical results.
Chapter 5 Diagnostic review
Study selection
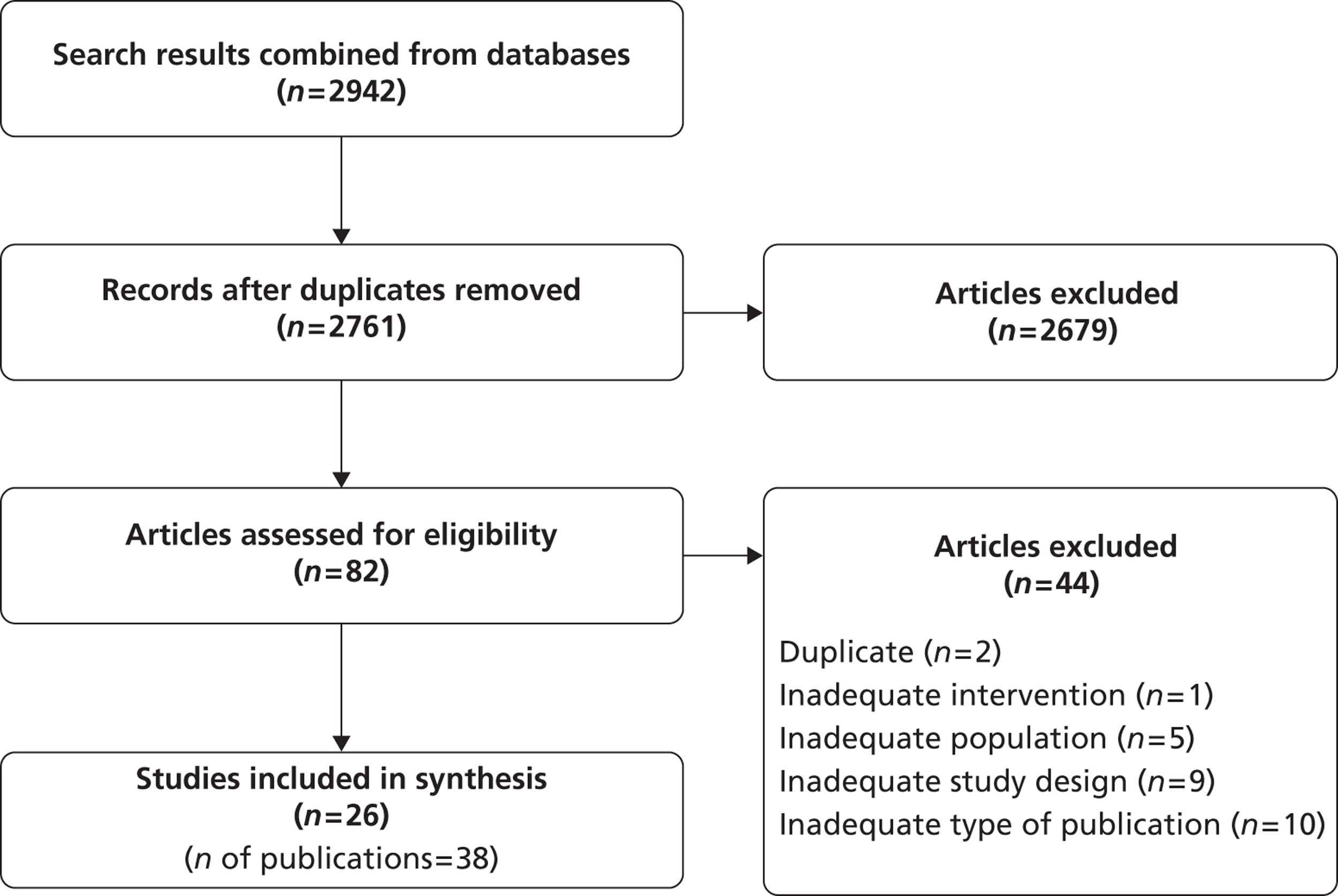
From the searches, 2942 citations were identified, of which 82 full papers were obtained. Included were 26 relevant studies (38 publications) (Figure 3). Excluded full-text articles are listed in Appendix 9 with reasons for exclusion, which were mostly because of small sample size or type of publication (reviews, abstracts). Some studies were excluded because they gave results only per groin rather than per patient.
FIGURE 3.
A PRISMA diagram for diagnostic review.
Characteristics of included studies
Index tests and histopathological techniques used for the index tests and reference standards used in each of the studies are given in Table 7. Although both 99mTc and blue dye were used in a number of studies, how the results were presented varied considerably. In some studies, only one was used. For example, De Cicco et al. ,55 Merisio et al. 64 and Vidal-Sicart et al. 74 used only 99mTc and Levenback et al. 61 used only blue dye. In six studies,39,52,62,66,67,69 a proportion of SLNs were diagnosed with blue dye or 99mTc separately and a proportion with both (see Table 7); the results for malignancy were given for the whole cohort irrespective of the test or tests actually used to find the malignancy. In such cases, only the sensitivity and specificity results can be given for the combination of tests used rather than only blue dye or 99mTc separately or only both used together in all patients. However, for the other 20 studies,50,51,53–61,63–65,68–74 detection rates per groin can be given for each test separately and both tests combined (see Table 7). It is noticeable that the histopathological techniques used for the full IFL specimens were either not given or were less detailed than those used for the SLNs. Only De Cicco et al. ,55 Johann et al. 59 and Radziszewski et al. 68 appeared to use the same techniques and very little detail is given in the first two. More details of index tests and reference standards used are given in Appendix 10.
| Study | 99mTc | Blue dye | Both together | Histopathological techniques: SLN | Histopathological techniques: remaining nodes | Type of surgery given (or RT) |
|---|---|---|---|---|---|---|
| Achimas-Cadariu et al., 200950 | – | – | ✗ | H&E, ultrastaging | NR | Radical vulvectomy (58%), modified (41%) |
| Basta et al., 200551 | ✗a | ✗a | ✗ | SLN immunochemical stain for micrometastases | NR | NR |
| Brunner et al., 200852 | ✗ (91%) | – | ✗ (9%) | Frozen sections, H&E and, if negative, immunohistochemistry for cytokeratins | Routine techniques | NR |
| Camara et al., 200953 | ✗ | ✗ | ✗ | Frozen section | NR | NR |
| Crosbie et al., 201054 | – | – | ✗ | H&E and, if negative, with additional sections and immunohistochemistry for cytokeratins AE1–3 | NR | Radical excision (47%), unclear (53%) |
| De Cicco et al., 200055 | ✗ | – | – | H&E | H&E | Wide radical excision, hemivulvectomy or radical vulvectomy (percentages not given) |
| de Hullu et al., 200056 | – | ✗ | ✗ | H&E and, if negative, with additional sections and immunohistochemistry for cytokeratins AE1–3 | H&E | Radical excision (100%) |
| Hampl et al., 200857 | ✗ | ✗ | ✗ | H&E and, if negative, with additional sections and immunohistochemistry for panycytokeratin antibody | NR | Hemivulvectomy (35%), vulvectomy (35%), local tumour resection (30%) |
| Hauspy et al., 200758 | ✗ | – | ✗ | Frozen section then serial sections H&E and immunohistochemistry for cytokeratins AE1–3 for some sections | H&E | Wide local excision (76%), radical vulvectomy (20%), RT (5%) |
| Johann et al., 200859 | – | – | ✗ | Step sectioning | Step sectioning | Radical vulvectomy (30%), hemivulvectomy (57%), wide excision (13%) |
| Klat et al., 200960 | – | – | ✗ | H&E, ultrastaging and immunohistochemistry for cytokeratins AE1–3 | NR | Radical surgery (100%) |
| Levenback et al., 200161 | – | ✗ | – | Frozen section if suspicious, step sectioning and some immunohistochemistry using several protocols | NR | NR |
| Lindell et al., 201039 | – | ✗ (22%) | ✗ (78%) | Step sections, H&E and if negative, immunohistochemistry for cytokeratin MNF116 | H&E | Vulvectomy (47%), hemivulvectomy (31%), wide local excision (22%) |
| Louis-Sylvestre et al., 200662 | ✗ (21%) | – | ✗ (79%) | Serial sections, H&E and immunohistochemistry for cytokeratins AE1 and AE3 | NR | NR |
| Martinez-Palones et al., 200663 | – | – | ✗ | 0.2 mm sections, H&E and if negative, immunohistochemistry for cytokeratin and membrane epithelial antigen | NR | NR |
| Merisio et al., 200564 | ✗ | – | H&E, ultrastaging, immunohistochemistry for cytokeratins in 50% of samples | Standard techniques | Radical vulvectomy or radical vulval excision (percentages NR) | |
| Moore et al., 200865 | – | – | ✗ | H&E and ultrastaging | NR | Radical vulvectomy or radical vulval excision (percentages NR) |
| Nyberg et al., 200766 | – | ✗ (20%) | ✗ (80%) | Histopathology | NR | NR |
| Pityński et al., 200367 | – | ✗ (14%) | ✗ (86%) | NR | NR | NR |
| Radziszewski et al., 201068 | – | – | ✗ | Multiple slices, H&E in 50% slices, H&E and immunohistochemistry in the other 50% of slices | H&E in 50% slices, H&E and immunohistochemistry in other 50% slices | NR |
| Rob et al., 200769 | – | ✗ (27%) | ✗ (73%) | Frozen section then serial sections, H&E, and immunohistochemistry on every third slide | H&E | NR |
| Terada et al., 200670 | – | – | ✗ | Multiple slices, H&E then if negative, immunohistochemistry with cytokeratin antigen | NR | NR |
| Vakselj et al., 200771 | – | – | ✗ | NR | NR | Tumour excised (100%) |
| Van den Eynden et al., 200372 | – | – | ✗ | H&E then if negative, ultrastaging and immunohistochemistry for cytokeratins AE1 and AE3 | NR | NR |
| Van der Zee et al., 200873 | – | – | ✗ | Frozen section or routine histopathology, ultrastaging | H&E | Radical excision (100%) |
| Vidal-Sicart et al., 200774 | – | – | ✗ | Multiple slices, H&E then if negative, H&E with immunohistochemistry | H&E | Radical vulvectomy or radical vulval excision (percentages NR) |
Details of included studies and baseline characteristics are presented in Tables 8 and 9. The studies were conducted in a variety of European countries and in the USA and Canada. The majority were small and from single centres. The largest was a recent multicentre study by Van der Zee et al. from the Netherlands [the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V)],73 which included 403 patients recruited between the years 2000 and 2006. Inclusion and exclusion criteria of the studies are given in Appendix 10.
| Study | Publications | Setting | Study design/patient selection | Recruitment dates |
|---|---|---|---|---|
| Achimas-Cadariu et al., 200950 | Achimas-Cadariu et al., 200950 | Dr Horst Schmidt Klinik, Wiesbaden, Germany | Retrospective/consecutive | June 2000 to May 2008 |
| Basta et al., 200551 | Basta et al., 200551 | Department of Gynaecology, Obstetrics and Oncology, Jagiellonian University Medical College, Krakow, Poland | Prospective | NR |
| Brunner et al., 200852 | Brunner et al., 2008;52 Sliutz et al., 2002;75 Hefler et al., 200876 | Department of Obstetrics and Gynaecology at the Medical University of Vienna, Vienna, Austria | Retrospective/consecutive | January 2001 to August 2007 |
| Camara et al., 200953 | Camara et al., 200953 | Friedrich-Schiller-University of Jena, Jena, Germany | Prospective | February 2003 to March 2007 |
| Crosbie et al., 201054 | Crosbie et al., 201054 | Gynaecology Clinic at The Christie NHS Foundation Trust in Manchester, Manchester, UK | Prospective | 2002–6 |
| De Cicco et al., 200055 | De Cicco et al., 200055 | San Gerardo Hospital, Monza, Italy | Prospective/consecutive | May 1996 to September 1998 |
| de Hullu et al., 200056 | de Hullu et al., 1998;77 de Hullu et al., 200056 | Groningen University Hospital, Groningen and Academic Hospital Vrije Universiteit, Amsterdam, the Netherlands | Prospective/consecutive | July 1996 to July 1999 |
| Hampl et al., 200857 | Hampl et al., 200857 | Department of Gynaecology and Obstetrics of Heinrich Heine Universit (Dusseldorf), University of Jena, Medizinischen Hochschule Hannover and Women's Hospital, Regional Hospital of Altötting, Germany | Prospective/consecutive | 2003–6 |
| Hauspy et al., 200758 | Hauspy et al., 200758 | Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada | Prospective | April 2004 to September 2006 |
| Johann et al., 200859 | Johann et al., 200859 | Department of Obstetrics and Gynaecology and of Nuclear Medicine, Inselspital, Bern University Hospital, Bern, Switzerland | Retrospective | January 1990 to March 2007 |
| Klat et al., 200960 | Klat et al., 200960 | University Hospital Ostrava, Ostrava, Czech Republic | Prospective | May 2004 to November 2007 |
| Levenback et al., 200161 | Levenback et al., 1994;78 Levenback et al., 1995;79 Levenback et al., 2001;61 Frumovitz et al., 200480 | Anderson Cancer Center and Southwestern Medical Centre, University of Texas, TX, USA | Prospective | 1993–9 |
| Lindell et al., 201039 | Lindell et al., 201039 | Department of Women's and Children's Health, of Pathology and Oncology and of Molecular Medicine and Surgery, Karolinska Institute, Stockholm, Sweden | Retrospective | 2000–7 |
| Louis-Sylvestre et al., 200662 | Louis-Sylvestre et al., 200662 | Centre Hospitalier Intercommunal Créteil, Créteil, France | Prospective | April 2002 to December 2005 |
| Martinez-Palones et al., 200663 | Martinez-Palones et al., 200663 | Hospital Materno-infantil Vall d'Hebron, Barcelona, Spain | Prospective/consecutive | January 1995 to July 2005 |
| Merisio et al., 200564 | Merisio et al., 200564 | Gynaecology Units of University of Parma and of Policlinico S Matteo of Pavia, Italy | Prospective | May 1999 to May 2003 |
| Moore et al., 200865 | Moore et al., 200865 | Women and Infants' Hospital, Brown University, Providence, RI, USA | Prospective/consecutive | 2002–7 |
| Nyberg et al., 200766 | Nyberg et al., 200766 | Tampere University Hospital, Tampere, Finland | Retrospective | 1 January 2001 to 30 June 2005 |
| Pityński et al., 200367 | Pityński et al., 200367 | Department of Gynaecology and Obstetrics and of Nuclear Medicine, Jagiellonian University Medical College, Krakow, Poland | Prospective | NR |
| Radziszewski et al., 201068 | Radziszewski et al., 201068 | Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology, Warsaw, Poland | Prospective/consecutive | January 2002 to December 2006 |
| Rob et al., 200769 | Rob et al., 2006;81 Rob et al., 200769 | Motol University Hospital, Second Medical Faculty, Charles University, Prague, Czech Republic | Prospective/consecutive | December 2001 to December 2005 |
| Terada et al., 200670 | Terada et al., 200670 | Department of Obstetrics and Gynaecology and Cancer Research Center, University of Hawaii School of Medicine, and Department of Pathology, Queens Medical Centre, Honolulu, HI, USA, | Retrospective | 1996–2003 |
| Vakselj et al., 200771 | Vakselj et al., 200771 | Institute of Oncology Ljubljana, Ljubljana, Slovenia | Prospective/consecutive | March 2006 to December 2006 |
| Van den Eynden et al., 200372 | Van den Eynden et al., 200372 | Department of Obstetrics-Gynaecology, University Hospital of Leuven, Leuven, Belgium | Retrospective | November 1999 to December 2002 |
| Van der Zee et al., 200873 | Van der Zee et al., 2008;73 Oonk et al., 2009;82 Oonk et al., 201083 | 15 centres registered at the University Medical Center Groningen, Groningen, Netherlands | Prospective/consecutive | March 2000 to May 2006 |
| Vidal-Sicart et al., 200774 | Vidal-Sicart et al., 2002;84 Vidal-Sicart et al., 2007;74 Vidal-Sicart et al., 2009;85 Puig-Tintore et al., 200386 | Section of Gynecologic Oncology, Instituto Clínic de Ginecología, Obstetrícia y Neonatología, Hospital Clinic, University of Barcelona, Barcelona, Spain | Prospective | May 1998 to June 2005 |
| Study | n | Percentage of patients in early stage available for analysis | Age, years (median or mean, range) | Tumour location (n) | Histological type of tumour (n) | TNM (n) | FIGO stage (I–IV) (n) | Grade (1–3) (n) |
|---|---|---|---|---|---|---|---|---|
| Achimas-Cadariu et al., 200950 | 46 | 95% | Median 66, range 34–94 | Midline: 17; lateral: 29 | SCC: 46 | T1–16, T2–28, T3–2 | I: 16; II: 19; III: 7; IV: 4 | 1: 8; 2: 29; 3: 9 |
| Basta et al., 200551 | 39 | 100% | NR | NR | NR | NR | All stage I and II | NR |
| Brunner et al., 200852 | 44 | 100% | Mean 70 | Midline: 34; lateral: 10 | SCC: 44 | T1–30, T2–14/N0–27, N1–17 | NR | 1: 14; 2: 27; 3: 3 |
| Camara et al., 200953 | 17 | 94.1% | Median 75, range 37–83 | NR | SCC: 16; melanoma: 1 | T1–7, T2–9, T3–1, N1–9, N2–8 | NR | NR |
| Crosbie et al., 201054 | 32 | 100% | Median 67, range 34–94 | Midline: 17; lateral: 15 | SCC: 32 | NR | I: 7; II: 5; III: 4; IV: 3 | NR |
| De Cicco et al., 200055 | 37 | 100% | NR | Midline: 19; lateral: 18 | SCC: 37 | T1–17, T2–20 | NR | NR |
| de Hullu et al., 200056 | 59 | 100% | Median 69, range 33–92 | NR | SCC: 59 | T1–25, T2–34 | NR | NR |
| Hampl et al., 200857 | 127 | 94.4% | Mean 61.4 | Midline: 33; lateral: 49 | SCC: 126; other: 1 | T1–56, T2–62, T3–7, N1–88, N2 + N3–39 | NR | 1: 15; 2: 86; 3: 23 |
| Hauspy et al., 200758 | 41 | 95% | Mean 65, range 34–92 | NR | SCC: 39; melanoma: 2 | T1–22, T2–19 | NR | 1: 18; 2: 17; 3: 4 |
| Johann et al., 200859 | 23 | 86% | Median 68.4, range 34.1–86.5 | NR | SCC: 23 | T1–9, T2–11, T3–3, N1–11, N2–11, N3–1 | NR | 1: 3; 2: 14; 3: 6 |
| Klat et al., 200960 | 23 | 100% | Median 67.5, range 38–92 | Midline: 18; lateral: 5 | SCC: 23 | T1–11, T2–12 | NR | NR |
| Levenback et al., 200161 | 52 | 87% | Median 58, range 18–92 | Midline: 25; lateral: 27 | SCC: 35; melanoma: 7; other: 10 | T1–22, T2–23, T3–7/N0–39, N1–9, N2–4 | NR | NR |
| Lindell et al., 201039 | 77 | 98% | Mean 71.2, range 40–92 | Midline: 22; lateral: 55 | SCC: 77 (other: 1a) | T1 + T2–76, T3–1 | NR | 1: 18; 2: 28; 3: 31 |
| Louis-Sylvestre et al., 200662 | 38 | 100% | Mean 66, range 34–90 | Midline: 26; lateral: 12 | NR | T1–29, T2–9, N1–32, N2–6 | NR | NR |
| Martinez-Palones et al., 200663 | 28 | 92.9% | Mean 71.3 ± 12, 7 (SD), range 30–84 | NR | SCC: 26; melanoma: 2 | T1–9, T2–19 | I: 9; II: 19 | 1: 19; 2: 6; 3: 1 |
| Merisio et al., 200564 | 20 | 100% | Mean 75, range 49–95 | Midline: 11; lateral: 9 | SCC: 20 | T1–9, T2–11/N0–20 | NR | NR |
| Moore et al., 200865 | 36 | 100% | Median 63, Mean 64, range 29–87 | NR | SCC: 35 | NR | I: 24; II: 8; III: 3; IV: 1 | NR |
| Nyberg et al., 200766 | 47 (results for 25 stage I and II only) | 100% | NR | NR | NR (full sample SCC: 46, other: 1) | NR | I: 11; II: 14 | 1: 15; 2: 8; 3: 2 |
| Pityński et al., 200367 | 37 | 100% | NR | NR | NR | NR | All stage I or II | NR |
| Radziszewski et al., 201068 | 62 | 100% | Median 68, range 37–94 | NR | SCC: 62 | T1–20, T2–42, N1–62 | NR | NR |
| Rob et al., 200769 | 43 | 100% | Median 70.9, range 26–95 | Midline: 21; lateral: 22 | SCC: 43 | T1–25, T2–18 | NR | NR |
| Terada et al., 200670 | 21 | 100% | Mean 72, range 42–86 | NR | SCC: 21 | T1–21 | NR | NR |
| Vakselj et al., 200771 | 35 | 92% | Median 65.8, range 36–88 | NR | SCC: 32; melanoma: 1; other: 2 | NR | I: 18; II: 6 | 1: 1; 2: 4; 3: 2b |
| Van den Eynden et al., 200372 | 32 | 100% | Mean 67, range 32–96 | NR | SCC: 31; other: 1 | T1 – 16, T2 – 16, N1 – 24, N2 + N3–8 | NR | NR |
| Van der Zee et al., 200873 | 403 | 100% | NR | Midline: 151; lateral: 252 | NR | T1 or 2–403, N0–276, N1–27 | NR | NR |
| Vidal-Sicart et al., 200774 | 50 | 86% | Mean 75, range 41–95 | NR | SCC: 50 (other: 8a) | NR | Ib: 23; II: 20; III: 8 | NR |
Data collection was prospective in 19 studies and retrospective in seven. Patients were recruited consecutively in 11 studies, prospectively in nine and retrospectively in two. Achimas-Cadariu et al. 50 described their study as retrospective, but data were collected prospectively from an in-house tumour registry. Recruitment dates varied between 1990 and 2008 and were not given in two studies51,67 (see Table 8). The percentage of patients with early-stage disease varied between 86% and 100%, being 100% in 16 studies. Median or mean ages varied between 58 and 75 years and individual ages varied between 18 and 95 years. Medians were given in most studies as vulval cancer is relatively rare in younger women. Where reported, tumour locations were relatively evenly spread between midline or lateral positions. The most commonly reported tumour type was SCC. Five studies included one or two melanomas53,58,61,63,71 and seven included other tumour types. 39,57,61,66,71,72,74 Either TNM, FIGO or grade staging, alone or in combination, was given in all studies. Most included patients with disease of varying severity and a few only included patients with early-stage disease, such as Terada et al. 70
Quality of included studies
Quality assessment is reported in Table 10. Of the 26 studies included, four53,66,67,71 provided no information about the histological staining method used. Brunner et al. ,52 Camara et al. ,53 Hauspy et al. 58 and Rob et al. 69 used frozen section as the reference standard, rather than more routine histopathological techniques. In 16 studies, on receipt of negative results by H&E procedures, immunohistochemical tests using specific protein antibodies such as AE1, AE3, S-100, human melanoma black monoclonal antibody (HMB)-45, monoclonal antibody, cytokine myocyte nuclear factor (CKMNF), cytokine (CK)-88 and epithelial membrane antigen were conducted. In others, ultrastaging was used if samples were negative by H&E staining and standard sectioning. The thickness of slices varied from one study to another so that some studies were more likely to find small metastatic deposits than others because of the thinner sections taken.
| Study | Quality factors | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Achimas-Cadariu et al., 200950 | N | Y | N | Y | Y | Y | Y | N | U | Y | Y | Y |
| Basta et al., 200551 | U | N | Y | Y | Y | Y | Y | N | U | N | Y | Y |
| Brunner et al., 200852 | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | N |
| Camara et al., 200953 | N | N | Y | Y | Y | Y | Y | N | U | Y | Y | Y |
| Crosbie et al., 201054 | Y | Y | Y | Y | Y | N | Y | N | U | U | Y | Y |
| De Cicco et al., 200055 | Y | Y | Y | Y | U | Y | Y | Y | N | N | Y | Y |
| de Hullu et al., 200056 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Hampl et al., 200857 | N | Y | Y | Y | U | Y | Y | N | U | Y | Y | N |
| Hauspy et al., 200758 | N | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Johann et al., 200859 | N | Y | U | Y | Y | Y | Y | N | U | Y | Y | N |
| Klat et al., 200960 | Y | Y | Y | Y | Y | Y | Y | N | U | Y | Y | Y |
| Levenback et al., 200161 | N | Y | Y | Y | Y | Y | Y | N | U | Y | Y | N |
| Lindell et al., 201039 | N | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | N |
| Louis-Sylvestre et al., 200662 | Y | Y | Y | U | Y | Y | Y | N | U | U | Y | N |
| Martinez-Palones et al., 200663 | N | Y | Y | U | Y | Y | Y | N | U | Y | Y | Y |
| Merisio et al., 200564 | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y |
| Moore et al., 200865 | Y | Y | N | N | Y | N | Y | N | U | Y | Y | Y |
| Nyberg et al., 200766 | Y | Y | U | Y | Y | Y | Y | N | U | U | Y | Y |
| Pityński et al., 200367 | U | U | U | U | U | Y | Y | N | U | N | Y | Y |
| Radziszewski et al., 201068 | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | N |
| Rob et al., 200769 | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | N |
| Terada et al., 200670 | Y | Y | N | U | Y | N | Y | N | U | Y | Y | Y |
| Vakselj et al., 200771 | N | N | U | Y | Y | N | Y | N | U | Y | Y | Y |
| Van den Eynden et al., 200372 | Y | U | N | Y | Y | N | Y | N | U | Y | Y | N |
| Van der Zee et al., 200873 | Y | Y | N | U | Y | N | Y | Y | U | Y | Y | N |
| Vidal-Sicart et al., 200774 | N | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | N |
There were four studies50,65,70,73 in which, if the SLN was found to be negative, no IFL was performed but patients were followed up clinically instead. In a study by Van den Eynden et al. ,72 10 out of 32 patients had a SLN biopsy plus full IFL. In the remaining 22 patients, an IFL was performed only if the SLN was positive or not found. In a study by Johann et al. 59 and another by Vidal-Sicart et al. ,74 some patients had SLN biopsy and full IFL regardless of node statistics and some only had IFL if the SLN was positive, but the results for the two groups were reported separately. Only the results for SLN biopsy and full IFL are reported here. In Crosbie et al. ,54 Klat et al. ,60 Martine-Palonez et al. ,63 Vakselj and Bebar71 and Vidal-Sicart et al. ,74 clinical follow-up was reported, and, for all except Vidal-Sicart et al. ,74 this was reported according to whether patients had been SLN positive or negative.
Because the main aim of the included studies was the analysis of diagnostic procedures, most did not report information about the number of patients who had undergone specific types of surgery or other treatment procedures (see Appendix 10, Table 53, for treatment descriptions). Usually, patients underwent radical vulvectomy, wide local excision or hemivulvectomy. RT was performed in only six studies (as adjuvant therapy in Hauspy et al. ,58 Levenback et al. ,61 Moore et al. ,65 Vakselj and Bebar71 and Van der Zee et al. 73 or as palliative treatment in Terada et al. 70). Additionally, a study by Levenback et al. 61 mentioned that, in one patient in whom SLN was grossly positive after SLN biopsy, the surgeon aborted IFL in favour of RT. Adverse events (AEs) were reported in five studies54,65,70,72,73 (see Appendix 10, Table 55).
With regard to blinding of index and reference test results, it would have been possible for the SLN and the full IFL nodes to be examined by different pathologists blind to each other's reports, but only de Hullu et al. 56 achieved this, and only De Cicco et al. 55 mentioned that they had not blinded pathologists. The remaining studies did not mention any blinding.
Test accuracy results
Results of test accuracy studies for which all patients had IFL as the reference standard are given in Table 11 and for which IFL was the reference standard in test positives and clinical follow-up in test negatives is given in Table 12. Most of the studies reported their results per groin rather than per patient; therefore, teasing out the results per patient was difficult in several of the papers (noted as numbers unclear in the comments column of Tables 11 and 12).
| Study | n | No. of patients with one or more SLNs found | No. of patients with SLN containing malignancies (frozen section) | No. of patients with SLN containing malignancies (histopathology) | No. with SLN found but negative and malignancy found at ILN | No. with no SLN found | No. with no SLN and malignancy from ILN (frozen section) | No. with no SLN and malignancy from ILN (histopathology) | No. with clinical follow-up | No. with malignancies at follow-up | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basta et al., 200551 | 39 | 38 | – | 12 | 0 | 1 | – | 0 | 0 | – | – |
| Brunner et al., 200852 | 44 | 44 | 17 | 0 | 3 | 0 | – | – | 0 | – | Numbers unclear |
| Camara et al., 200953 | 17 | 15 | 7 | - | 0 | 2 | – | 1 | 0 | – | Both |
| 17 | 13 | – | – | – | – | – | – | – | – | 99mTc | |
| 17 | 9 | – | – | – | – | – | – | – | – | Blue | |
| Crosbie et al., 201054 | 32 | 31 | – | 6 | 0 | 1 | – | 1 | 32 | SLN negative: 0 SLN positive: 5 |
– |
| De Cicco et al., 200055 | 37 | 37 | – | 8 | – | 0 | – | 0 | 0 | – | – |
| de Hullu et al., 200056 | 59 | 59 | – | 20 | 0 | 0 | – | - | 0 | – | – |
| Hampl et al., 200857 | 127 | 125 | 0 | 36 | 3 | 2 | – | 0 | 0 | – | Numbers unclear |
| Hauspy et al., 200758 | 41 | 39 | 15 | 0 | 0 | 2 | 0 | 1 | 0 | – | – |
| Johann et al., 200859 | 23 | 23 | ? | 10 | 1 | 0 | – | 0 | 0 | – | Numbers unclear |
| Klat et al., 200960 | 23 | 23 | – | 14 | 1 | 0 | – | - | 23 | SLN positive: 3 SLN negative: 1 |
– |
| Levenback et al., 200161 | 52 | 46 | – | 11 | 0 | 6 | 2 | 0 | – | Numbers unclear | |
| Lindell et al., 201039 | 77 | 75 | – | 21 | 2 | 2 | – | 0 | 0 | – | Numbers unclear |
| Louis-Sylvestre et al., 200662 | 38 | 36 | – | 11 | – | 2 | – | ? | 0 | – | Numbers unclear |
| Martinez-Palones et al., 200663 | 28 | 27 | – | 6 | 1 | 1 | – | – | 28 | SLN positive: 4 SLN negative: 3? No SLN: 1 |
|
| Merisio et al., 200564 | 20 | 20 | – | 3 | 1 | 0 | – | – | 0 | – | – |
| Nyberg et al., 200766 | 25 (47)b | 25 | 4 | 0 | 0 | – | 0 | 0 | – | Stage I and II only | |
| Pityński et al., 200367 | 37 | 37 | – | 11 | 0 | 0 | – | – | 0 | – | |
| Radziszewski et al., 201068 | 56 | 56 | – | 21 | ? | 0 | – | – | 0 | – | Six patients with metastases on FNA, numbers unclear |
| Rob et al., 200769 | 43 | 43 | 13 | 14 | 0 | 0 | – | – | 0 | – | Both |
| 16 | 11 | 0 | 4 | 1 | 5 | - | 1 | 0 | – | Blue only, numbers unclear | |
| Vakselj et al., 200771 | 35 | 35 | 0 | 10 | 0 | 0 | – | – | 35 | SLN positive: 6 SLN negative: 3 |
– |
| Vidal-Sicart et al., 200774 | 50 | 49 | 0 | 16 | 0 | 1 | – | Not found | (70)a | Total: 5 | – |
| Study | n | No. of patients with 1 or more SLNs found | No. of patients with SLN with malignancies (frozen section) | No. of patients with SLN with malignancies (histopathology) | No. with SLN found but negative and malignancy found at ILN | No. with no SLN found | No. with no SLN and malignancy from ILN (frozen section) | No. with no SLN and malignancy from ILN (histopathology) | No. with clinical follow-up | No. with malignancies at follow-up | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Achimas-Cadariu et al., 200950 | 46 | 43 | – | – | – | 3 | – | – | 46 | 8 | Mostly per groin analysis |
| Moore et al., 200865 | 36 | 35 | – | 4 | 0 | 1 | – | 0 | 31 (negative SLN) | SLN negative: 2 | – |
| Terada et al., 200670 | 21 | 21 | 0 | 3 | 0 | 0 | – | – | 21 | SLN positive: 2 SLN negative: 0 |
– |
| Van den Eynden et al., 200372 | 32 | 27 | – | 10 | 5 | 5 | – | ? | 17 | SLN negative: 4 | Numbers unclear |
| Van der Zee et al., 200873 | 403 | 403 | – | 127 (135 in Oonk et al. 201083) |
36 (micromets from 33 immunohistochemistry of SLN, Oonk et al. 201083) | 0 | – | – | 276 | SLN negative: 8 groin, 34 local SLN positive: 11 groin, 34 local, 7 distant |
Numbers unclear |
For calculation of sensitivity and specificity, studies have been categorised by the reference standards used, the index test used and the histopathological techniques used as follows:
-
IFL for all
-
99mTc with blue dye
-
99mTc only
-
blue dye only
-
immunohistochemistry (Table 20).
-
-
-
IFL for SLN positive and clinical follow-up for SLN negative
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Nyberg et al., 200766 | 25 | 25 | 4 | 0 | 21 | 0 | 100% (39.8% to 100%) | 100% (83.9% to 100%) |
| Pityński et al., 200367 | 37 | 37 | 11 | 0 | 26 | 0 | 100% (71.5% to 100%) | 100% (86.8% to 100% |
| Vakselj et al., 200771 | 35 | 35 | 10 | 0 | 25 | 0 | 100% (69.2% to 100%) | 100% (86.3% to 100%) |
| Pooled sensitivity = 100% (95% CI 86.3 to 100%); chi-squared test = 0.00 (degrees of freedom = 2); p = 1.000; I 2 = 0.0%. | ||||||||
| Pooled specificity = 100% (95% CI 95.0 to 100%); chi-squared test = 0.00 (degrees of freedom = 2); p = 1.000; I 2 = 0.0%. | ||||||||
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Basta et al., 200551 | 39 | 38 (82%) | 12 | 0 | 24 | 0 | 100% (73.5% to 100%) | 100% (85.8% to 100%) |
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Basta et al., 200551 | 17 | 15 | 7 | 0 | 8 | 0 | 100% (59.0% to 100%) | 100% (63.1% to 100%) |
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Crosbie et al., 201054 | 32 | 31 | 6 | 0 | 25 | 0 | 100% (54.1% to 100%) | 100% (86.3% to 100%) |
| de Hullu et al., 200056 | 59 | 59 | 20 | 0 | 39 | 0 | 100% (83.2% to 100%) | 100% (91.0% to 100%) |
| Hampl et al., 200857 | 127 | 125 | 36 | 0 | 86 | 3 | 92.3% (79.1% to 98.4%) | 100% (95.8% to 100%) |
| Hauspy et al., 200758 | 41 | 39 | 15 | 0 | 24 | 0 | 100% (78.2% to 100%) | 100% (85.8% to 100%) |
| Johann et al., 200859 | 23 | 23 | 10 | 0 | 12 | 1 | 90.9% (58.7% to 99.8%) | 100% (73.5% to 100%) |
| Klat et al., 200960 | 23 | 23 | 14 | 0 | 8 | 1 | 93.3% (68.1% to 99.8%) | 100% (63.1% to 100%) |
| Lindell et al., 201039 | 77 | 75 | 21 | 0 | 52 | 2 | 91.3% (72.0% to 98.9%) | 100% (93.2% to 100%) |
| Louis-Sylvestre et al., 200662 | 38 | 36 | 11 | 0 | 25 | 0 | 100% (71.5% to 100%) | 100% (86.3% to 100%) |
| Martinez-Palones et al., 200663 | 28 | 27 | 6 | 0 | 20 | 1 | 85.7% (42.1% to 99.6%) | 100% (83.2% to 100%) |
| Radziszewski et al., 201068 | 62 | 56 | 21 | 0 | 35? | 0? | 100% (83.9% to 100%) | 100% (90.0% to 100%) |
| Rob et al., 200769 | 43 | 43 | 14 | 0 | 29 | 0 | 100% (76.8% to 100%) | 100% (88.1% to 100%) |
| Pooled sensitivity = 95.6% (95% CI 91.5 to 98.1%); chi-squared test = 11.0 (degrees of freedom = 10); p = 0.35; I 2 = 9.9%. | ||||||||
| Pooled specificity = 100% (95% CI 99.0 to 100%); chi-squared test = 0.00 (degrees of freedom = 10); p = 1.000; I 2 = 0.0%. | ||||||||
| Negative predictive value = 97.8%; random-effects-positive LR = 51.368 (95% CI 22.440 to 117.586), negative LR = 0.088 (95% CI 0.053 to 0.146). | ||||||||
| Study | TP | FP | TN | FN |
|---|---|---|---|---|
| Crosbie et al., 201054 | 6 | 0 | 25 | 0 |
| de Hullu et al., 200056 | 20 | 0 | 39 | 0 |
| Hampl et al., 200857 | 36 | 0 | 86 | 3 |
| Hauspy et al., 200758 | 15 | 0 | 24 | 0 |
| Johann et al., 200859 | 10 | 0 | 12 | 1 |
| Klat et al., 200960 | 14 | 0 | 8 | 1 |
| Lindell et al., 201039 | 21 | 1 | 52 | 2 |
| Louis-Sylvestre et al., 200662 | 11 | 1 | 25 | 0 |
| Martinez-Palones et al., 200663 | 6 | 1 | 20 | 1 |
| Radziszewski et al., 201068 | 21 | 1 | 35 | 0 |
| Rob et al., 200769 | 14 | 1 | 29 | 0 |
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| De Cicco et al., 200055 | 37 | 37 | 8 | 0 | 29 | 0 | 100% (63.1% to 100%) | 100% (88.1% to 100%) |
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95%CI) | Specificity (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Brunner et al., 200852 | 44 | 44 | 17 | 0 | 24 | 3 | 85.0% (62.1% to 96.8%) | 100% (85.8% to 100%) |
| Merisio et al., 200564 | 20 | 20 | 3 | 0 | 16 | 1 | 75.0% (19.4% to 99.4%) | 100% (79.4% to 100%) |
| Vidal-Sicart et al., 200774 | 50 | 49 | 16 | 0 | 33 | 0 | 100% (79.4% to 100%) | 100% (89.4% to 100%) |
| Pooled sensitivity = 90.0% (95% CI 76.3 to 97.2%); chi-squared = 4.60 (degrees of freedom = 2); p = 0.10; I 2 = 56.5%. | ||||||||
| Pooled specificity = 100% (95% CI 95.1 to 100%); chi-squared = 0.00 (degrees of freedom = 1); p = 1.000; I 2 = 0.0%. | ||||||||
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Levenback et al., 200161 | 52 | 46 | 11 | 0 | 35 | 0 | 100% (71.5% to 100%) | 100% (90.0% to 100%) |
| Rob et al., 200769 | 16 | 11 | 4 | 0 | 6 | 1 | 80.0% (28.4% to 99.5%) | 100% (54.1% to 100%) |
| Pooled sensitivity = 93.8% (95% CI 69.8 to 99.8%); chi-squared test = 2.48 (degrees of freedom = 1); p = 0.115; I 2 = 59.6%. | ||||||||
| Pooled specificity = 100% (95% CI 91.4 to 100%); chi-squared test = 0.00 (degrees of freedom = 1); p = 1.000; I 2 = 0.0%. | ||||||||
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Moore et al., 200865 | 36 | 35 | 4 | 0 | 29 | 2 | 66.7% (22.3% to 95.7%) | 100% (88.1% to 100%) |
| Terada et al., 200670 | 21 | 21 | 3 | 0 | 18 | 0 | 100% (29.2% to 100%) | 100% (81.5% to 100%) |
| Van der Zee et al., 200873 | 403 | 403 | 127 | 0 | 234 | 42 | 75.1% (67.9% to 81.5%) | 100% (98.4% to 100%) |
| Pooled sensitivity = 75.3% (95% CI 68.3 to 81.4%); chi-squared test = 1.93 (degrees of freedom = 2); p = 0.381; I 2 = 0.0%. | ||||||||
| Pooled specificity = 100% (95% CI 98.7 to 100%); chi-squared test = 0.00 (degrees of freedom = 2); p = 1.000; I 2 = 0.0%. | ||||||||
| Study | n | No. of n patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Achimas-Cadariu et al., 200950 | 46 | 43 (94%) | NR | 0 | NR | 0 | Not calculable | Not calculable |
| Van den Eynden et al., 200372 | 32 | 27 | 10 | 0 | 12 | 5 | 66.7% (38.4% to 88.2%) | 100% (73.5% to 100%) |
| Study | n | No. of patients with SLNs found | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Achimas-Cadariu et al., 200950 | 46 | 43 (94%) | NR | 0 | NR | 0 | Not calculable | Not calculable |
| Moore et al., 200865 | 36 | 35 | 4 | 0 | 29 | 2 | 66.7% (22.3% to 95.7% | 100% (88.1% to 100%) |
| Terada et al., 200670 | 21 | 21 | 3 | 0 | 18 | 0 | 100% (29.%2 to 100%) | 100% (81.5% to 100%) |
| Van den Eynden et al., 200372 | 32 | 27 | 10 | 0 | 12 | 5 | 66.7% (38.4% to 88.2%) | 100% (73.5% to 100%) |
| Van der Zee et al., 200873 | 403 | 403 | 127 | 0 | 268 | 8 | 94.1% (88.7% to 81.5%) | 100% (98.6% to 100%) |
| Pooled sensitivity = 90.6% (95% CI 84.9 to 94.6%); chi-squared test 11.90 (degrees of freedom = 3); p = 0.008; I 2 = 74.8%. | ||||||||
| Pooled specificity = 100% (95% CI 98.9 to 100%); chi-squared test 0.00 (degrees of freedom = 3); p = 1.0000; I 2 = 0.0%. | ||||||||
The sensitivities and specificities in the results tables are based on the number of patients with one (or more) SLNs found, rather than the total number of patients. They could have had SLN biopsy in one or both groins; therefore, the results are giving the sensitivity and specificity of malignancy or none when a SLN (or more than one) has been found. It would not be possible for a SLN biopsy to be false-positive for malignancy, so all the point estimates of specificity are 100%. For this reason, only one receiver operating characteristic (ROC) plane is given, for the category of IFL for all, 99mTc with blue dye: ultrastaging and immunohistochemistry, in which there were 11 included studies (see Tables 16 and 17, Figure 4). All of the points are clustered along the top left-hand-side and the plot is not particularly informative. Unfortunately, there were insufficient studies of similar clinical characteristics to be able to conduct metaregression comparing 99mTc and blue dye results.
FIGURE 4.
Receiver operating characteristic plane for IFL for all, 99mTc with blue dye: ultrastaging and immunohistochemistry.
The results of sensitivities and specificities show that, although the point estimates are either 100% or close to 100%, the CIs are wide, reflecting the small samples available. However, the pooled sensitivity of the studies with clinical follow-up was less than the ones with IFL for all.
In a test for cancer, it is important not to miss malignancy. If a FN diagnosis is made, the patient is at risk of developing malignancy in the groin or disseminated malignancy. If a FP diagnosis is made, the patient would undergo unnecessary IFL with resulting morbidity. So FNs may be considered to be relatively more important than FPs. In SLN biopsy, there would be no false-positive diagnoses unless an error was made in the histological examination, which would be rare, so we have to evaluate only the FN diagnoses. The results suggest that, if we evaluate SLN biopsy with clinical follow-up for node-negative patients, many more FN diagnoses will be made because of the longer time of follow-up, enabling more development of observable metastases. However, one would assume that the accuracy of a test would be a function of the test itself, rather than the method of evaluation. It is known that differential verification usually leads to increased estimates of sensitivity and specificity. The opposite is seen in this example and may be because there is more time to develop a recurrence with a long clinical follow-up. In addition, total recurrence rates were used rather than groin recurrence rates only because some studies only gave total recurrence rates. It is reasonable to assume that IFL should not influence recurrence in the vulval area. Therefore, the recurrence rates for clinical follow-up studies were recalculated, using only groin and distant recurrence, where given (only available in the study by Van der Zee et al. 73). The results are shown in Table 23 and show a higher pooled sensitivity, comparable to the estimates for which all patients received IFL as the gold standard.
The probability of curing vulval cancer is greatest when it is diagnosed at an early stage. The studies included patients with disease at a variety of FIGO stages; some included only patients with early-stage disease51,63,67,71 and others patients with disease of all stages50,54,65 (when reported). A study by Nyberg et al. 66 had a mixture of stages but reported results for each stage separately so results for stages I and II are reported here. There was a trend for the studies with early FIGO stage patients to have higher sensitivities than studies with mixed-FIGO-stage patients, but this was not consistent across all studies.
The vast majority of vulval cancer (> 90%) is SCC. Vulval melanoma is the second most common vulval malignancy but, unlike SCC, melanoma has a high risk of metastasis and the overall prognosis is poor. However, studies that included malignancies other than SCCs did not appear to have noticeably different results to those with SCCs only, but this may be a result of small sample sizes.
Sentinel lymph node detection rate
The accuracy of a diagnostic test such as SLN biopsy depends on the ability of the surgeon to identify the SLN; therefore, SLN detection rates for each of the analysed techniques (blue dye, 99mTc, blue dye/99mTc) are presented (Table 24). The detection rate calculated per patient was available in all included studies. Some of the studies also gave detection rates per groin for 99mTc and blue dye separately, but these are not reported here. The detection rates do not obviously vary by whether or not patient groups included non-SCCs, such as melanomas, or whether both early and late stage were included rather than early only. However, it is clear that blue dye detects fewer SLNs than 99mTc and that both used together is the most successful strategy.
| Study | 99mTc | Blue dye | Both together | Study characteristics |
|---|---|---|---|---|
| Achimas-Cadariu et al., 200950 | – | – | 43/46 (94%) | Only SCC, early and late stage |
| Basta et al., 200551 | 38/39a (97%) | 32/39a (82%) | 38/39 (97%) | Not reported, early stage |
| Camara et al., 200953 | 13/17 (76%) | 9/17 (53%) | 15/17 (88%) | Mostly SCC, early and late stage |
| Crosbie et al., 201054 | – | – | 31/32 (97%) | Only SCC, early and late stage |
| De Cicco et al., 200055 | 37/37 (100%) | – | – | Only SCC, early and late stage |
| de Hullu et al., 200056 | – | 35/59 (60%) | 59/59 (100%) | Only SCC, early stage |
| Hampl et al., 200857 | 119/127 (94%) | 80/127 (63%) | 125/127 (98%) | Mostly SCC, early and late stage |
| Hauspy et al., 200758 | NR | – | 39/41 (95%) | Mostly SCC, early and late stage |
| Johann et al., 200859 | – | – | 23/23 (100%) | Only SCC, early stage |
| Klat et al., 200960 | – | – | 23/23 (100%) | Only SCC, early stage |
| Levenback et al., 200161 | – | 46/52 (88%) | – | Mostly SCC, early and late stage |
| Martinez-Palones et al., 200663 | – | – | 27/28 (96%) | Mostly SCC, early stage |
| Merisio et al., 200564 | 20/20 (100%) | – | – | Only SCC, early stage |
| Moore et al., 200865 | – | – | 35/36 (97%) | Only SCC, early and late stage |
| Radziszewski et al., 201068 | – | – | 56/62 (90%) | Only SCC, early stage |
| Terada et al., 200670 | – | – | 21/21 (100%) | Only SCC, early stage |
| Vakselj et al., 200771 | – | – | 35/35 (100%) | Mostly SCC, early stage |
| Van Den eynden et al., 200372 | – | – | 27/32 (84%) | Mostly SCC, early stage |
| Van der Zee et al., 200873 | – | – | 403/403 100% | Only SCC, early stage |
| Vidal-Sicart et al., 200774 | – | – | 49/50 (98%) | Mostly SCC, early and late stage |
| Combined rates | 94.6% | 68.7% | 97. 7% | – |
| 95% CI | 0.909 to 0.971 | 0.631 to 0.740 | 0.966 to 0.985 | – |
Subpopulation of stages I and II
Two studies66,74 gave detection rates for patients in the different FIGO stages separately (Table 25). The sample sizes are very small but suggest no obvious gradient of detection rate by FIGO stage.
| Stage | Nyberg et al. 200766 | Vidal-Sicart et al. 200774 | ||||
|---|---|---|---|---|---|---|
| Blue dye | 99mTc | Blue dye/99mTc | Blue dye | 99mTc | Blue dye/99mTc | |
| Stage I | 100% (11/11) | 100% (10/10) | 100% (11/11) | 74% (17/23) | 100% (23/23) | 100% (23/23) |
| Stage II | 93% (13/14) | 90% (9/10) | 100% (10/10) | 90% (18/20) | 100% (20/20) | 100% (20/20) |
| Stage III | 100% (21/21) | 90% (18/20) | 100% (20/20) | 71% (5/7) | 86% (6/7) | NR |
| Stage IV | 0% (0/1) | None | None | None | None | None |
Training and experience
There is a learning curve for surgeons performing SLN biopsy and IFL. Several of the included studies mention this (Table 26), but for most of the studies the learning curve was taken to mean that, after the first few cases, only SLN biopsy without full IFL was to be performed. Only Levenback et al. 61 calculated that the rate of SLN detection was worse in the first 2 years of the study but then continues with full IFL for all patients regardless of node status.
| Study | Learning curve mentioned |
|---|---|
| Achimas-Cadariu et al., 200950 | At least 20 inguinal SLN biopsies followed by full IFL regardless of node status; thereafter only SLN performed |
| Hampl et al., 200857 | At least 10 SLN biopsies followed by full IFL regardless of node status (before offering SLN biopsy) |
| Johann et al., 200859 | SLN biopsy followed by full IFL regardless of node status during a learning period of approximately 2 years, before SLN biopsy and full IFL if histology positive |
| Levenback et al., 200161 | Success of SLN identification varied by clinical experience of the procedure such that the failure rate was 16% (4/25) in the first 2 years and 7% (2/27) in subsequent years |
| Van der Zee et al., 200873 | At least 10 successful experiences of the SLN procedure with subsequent full IFL regardless of node status (this study design was SLN biopsy and full IFL if histology positive) |
| Vidal-Sicart et al., 200774 | In the first 50 patients, SLN procedure followed by a full IFL was performed, as a representing learning curve, thereafter only SLN biopsy performed |
Recurrence rates
Two groups of studies gave recurrences at follow-up. The first group are those that used full IFL regardless of node status at initial operation to establish diagnostic accuracy, but also followed up patients afterwards. The second are those that used clinical follow-up for SLN-negative patients to establish diagnostic accuracy.
In group 1 are Crosbie et al. ,54 Klat et al. ,60 Martinez-Palonez et al. ,63 Vakselj and Bebar71 and Vidal-Sicart et al. 74 Overall recurrence rates are given in Table 11. Vidal-Sicart et al. 74 did not give recurrences by SLN status. In Crosbie et al. ,54 6 out of 31 patients were SLN positive at biopsy and five SLN-positive patients developed recurrences. No SLN-negative patients developed recurrences. In Klat et al. ,60 14 out of 23 patients were SLN positive at biopsy (and one with a malignancy not in SLN) and three SLN-positive patients developed recurrences. One SLN-negative patient developed recurrence. In Martinez-Palonez et al. ,63 6 out of 27 patients were SLN positive at biopsy (and one with malignancy not in SLN) and four SLN-positive patients developed recurrences. Recurrences also developed in possibly three SLN-negative patients and in one patient for whom the SLN was not found (the numbers are unclear from the journal article). In Vakselj and Bebar,71 10 out of 35 patients were SLN-positive at biopsy and six SLN-positive patients developed recurrences. Three SLN-negative patients also developed recurrences.
In group 2 are Achimas-Cadariu et al. ,50 Moore et al. ,65 Terada et al. ,70 Van den Eynden et al. 72 and Van der Zee et al. 73 The overall recurrence rates are given in Tables 12 and 22. Achimas-Cadariu et al. 50 did not give recurrences by SLN status, so test accuracy could not be calculated. Van der Zee et al. 73 gave separate results for groin and local recurrences. In addition, Van der Zee et al. 73 gave recurrences for node-negative and node-positive patients in separate papers (node negative, Van der Zee et al. ;73 node positive, Oonk et al. 83). It was curious that there were 34 local recurrences in each category.
Given the recurrence results, it is reasonable to assume that the number of clinically apparent recurrences is likely to be smaller than the number of SLN-positive patients at biopsy. This may be because the subsequent IFL is removing malignancy that might otherwise develop into a recurrence. In addition, in a very small proportion of patients who do not undergo IFL, some recurrences will be distant rather than groin because the groin metastases may stay very small and not be noticeable.
In addition, it shows that some SLN-negative patients will develop recurrences so will be FNs. In Martinez-Palonez et al. 63 and Vakselj and Bebar,71 there was a higher rate of recurrences in SLN-negative patients than known FN SLN biopsies. In Crosbie et al. 54 and Klat et al. ,60 the rates were the same.
Survival rates
Nine studies gave information about survival. 50,54,60,63,65,70,71,73,74
In the study by Achimas-Cadariu et al. ,50 12 out of 46 patients died during follow-up; median survival was 61.2 months for the whole cohort and 16.2 months for the eight patients with relapse.
Crosbie et al. 54 reported that 2 out of 32 patients died from disease during a median follow-up period of 62 months (range 33–84 months).
Klat et al. 60 reported that 1 out of 23 patients died from disease, with a follow-up of 8–46 months.
Martinez-Palonez et al. 63 did not mention any deaths in a group of 28 patients followed up for a median of 22.5 months (range 0–64 months).
Moore et al. 65 reported that 1 out of 35 patients died from intercurrent disease, with a median follow-up of 29 months (range 8–51 months).
Terada et al. 70 gave overall survival and disease-free survival curves for all patients and node-negative patients. Median follow-up of 21 patients was 4.6 years (range 2–8 years) and largely reflects losses to follow-up, as none of the node-negative patients died of cancer. Two out of three node-positive patients died of cancer and three patients died of other illnesses.
Vakselj and Bebar71 gave the status of each of the node-positive and -negative patients and length of follow-up. Out of the 10 node-positive patients, six died of disease at follow-up times of 42, 10, unstated, 3, 18 and 10 months (total range of follow-up was 3–55 months). Of the 25 node-negative patients, one died of disease at 49 months and one died of another cause (no time given) (total range of follow-up 2–52 months).
Van der Zee et al. 73 gave a disease-specific survival curve for node-negative patients with a median follow-up of 35 months (range 2–87 months) for 202 of the 276 patients with at least 24 months of follow-up. Four patients were lost to follow-up, 10 patients died of vulval cancer and 16 died of intercurrent disease. Five out of 34 patients with local recurrents eventually died of distant metastases at 15, 18, 22, 41 and 41 months after primary treatment. The 3-year disease-specific survival rate for patients with unifocal vulval disease and negative SLNs was 97.0%. In the Oonk et al. paper,83 in the group of patients with positive SLNs, the 5-year disease-specific survival was 77.3%. Survival was varied depending on the pathology, and was 64.9% when the SLNs were identified by routine pathology and 92.1% when identified by ultrastaging. During the follow-up of median 31 months (range 0–109 months) 15 died of other causes and 28 died of vulval cancer.
In the study by Vidal-Sicart et al. ,74 1 out of 50 patients died of disease, with a mean follow-up of 20 months.
In summary, vulval cancer is largely a disease of older women, so a relatively large proportion die of other causes during follow-up. Survival rates are better in women with a node-negative status than in those with a node-positive status.
Quality of life
The Van der Zee et al. 73 study had a substudy investigating QoL that was published separately (Oonk et al. 82). The authors used the European Organisation for Research and Treatment of Cancer QoL Questionnaire-C30 (EORTC QLQ-C30), the Functional Assessment of Cancer Therapy – Vulvar (FACT-V) and a patient’s opinion questionnaire. The EORTC QLQ-C30 consists of five functional scales, global health status and nine symptom scales. The FACT-V consists of five functional scales and four symptom scales. In both of these questionnaires, a higher score indicates better functioning on the functional scales and worse functioning on the symptom scales. The patient opinion questionnaire asked what they would recommend to a friend or family member with vulval cancer: IFL or SLN biopsy (with the chance of missing metastases at a rate of 1 in 10, 1 in 100 or 1 in 1000). Patients included in the Van der Zee et al. 73 study between March 2000 and December 2005 were eligible. Questionnaires were sent to 37 patients with positive SLN and 37 age-matched patients with a negative SLN. Sixty-two of the 74 patients (84%) returned the completed questionnaires.
The results of the EORTC QLQ-C30 are in given Table 27, the results of the FACT-V in Table 28 and the results of the patient’s opinion questionnaire in Table 29. The most useful result was the global health status/QoL, which had a mean of 80 and a standard deviation (SD) of 18 for the SLN group and a mean of 80 and a SD of 23 for the IFL group. The authors state that ‘our present study does not support our original idea that a decrease in especially long-term morbidity also translates into an improved overall quality of life’. 82 It may be that there were too few participants to detect a small difference in QoL.
| Parameter | SLN patients (n = 35), mean (SD) | IFL patients (n = 27), mean (SD) | p-Value |
|---|---|---|---|
| Functional scalea | |||
| Physical functioning | 84 (21) | 80 (19) | 0.43 |
| Role functioning | 87 (22) | 85 (26) | 0.87 |
| Emotional functioning | 90 (14) | 89 (19) | 0.63 |
| Cognitive functioning | 94 (11) | 94 (14) | 0.90 |
| Concentration | 95 (12) | 96 (14) | 0.44 |
| Memory | 92 (16) | 91 (18) | 0.83 |
| Social functioning | 96 (13) | 90 (22) | 0.23 |
| Symptom scaleb | |||
| Fatigue | 23 (22) | 18 (24) | 0.23 |
| Nausea and vomiting | 3 (11) | 2 (10) | 0.61 |
| Nausea | 6 (19) | 4 (14) | 0.61 |
| Vomiting | 1 (6) | 1 (6) | 0.85 |
| Pain | 15 (21) | 14 (24) | 0.63 |
| Dyspnoea | 12 (24) | 12 (21) | 0.79 |
| Insomnia | 14 (25) | 23 (29) | 0.15 |
| Appetite loss | 10 (24) | 9 (22) | 0.65 |
| Constipation | 7 (16) | 5 (15) | 0.53 |
| Diarrhoea | 9 (20) | 2 (13) | 0.11 |
| Financial difficulties | 2 (11) | 12 (25) | 0.01c |
| Global health status/QoL | 80 (18) | 80 (23) | 0.62 |
| Parameter | SLN, mean (SD) | IFL, mean (SD) | p-Value |
|---|---|---|---|
| Functional scalea | |||
| Physical functioning | 85 (17) | 80 (18) | 0.27 |
| Discomfort groins/vulva/legs | 86 (20) | 69 (32) | 0.03 |
| Discomfort sitting | 87 (18) | 86 (28) | 0.46 |
| Discomfort bending | 83 (27) | 85 (20) | 0.83 |
| Sexual functioningb | 78 (19) | 81 (26) | 0.67 |
| Future perspective | 70 (27) | 64 (28) | 0.41 |
| Body image | 43 (35) | 59 (33) | 0.09 |
| Contentment | 90 (27) | 78 (31) | 0.04d |
| Sexual activeness | 6 (21) | 13 (21) | 0.06 |
| Symptom scalec | |||
| Vulval symptoms | 14 (14) | 10 (12) | 0.33 |
| Discharge/blood loss | 5 (12) | 1 (6) | 0.17 |
| Fetor | 7 (16) | 5 (12) | 0.77 |
| Itching | 24 (26) | 16 (19) | 0.29 |
| Pain/numbness | 22 (28) | 20 (27) | 0.76 |
| Oedema | 12 (22) | 35 (32) | 0.001d |
| Complaints | 12 (24) | 27 (29) | 0.01d |
| Stockings | 12 (30) | 43 (46) | 0.003d |
| Urination | 14 (18) | 18 (18) | 0.30 |
| Incontinence | 18 (30) | 26 (34) | 0.32 |
| Discomfort | 10 (21) | 10 (18) | 0.68 |
| Study | Maximum acceptable FN rate (%) | Patients who accept the FN rate (%) | SLN | IFL | p-Valuea |
|---|---|---|---|---|---|
| Oonk et al., 200982 | 10 | 69 | 84% | 48% | 0.005 |
| 1 | 82 | 97% | 62% | 0.001 | |
| 0.1 | 87 | 97% | 71% | 0.013 |
The EORTC QLQ-C30 showed very few differences between the two groups; only on the financial difficulties scale was the score statistically significantly worse in the IFL group. For the FACT-V questionnaire, results were significantly worse for the contentment functional scale, oedema, complaints and stockings symptom scales.
For the patient’s opinion questionnaire, the authors analysed the maximum FN rate of the SLN procedure that would be acceptable to patients. There were significant differences between the SLN biopsy patients and the IFL patients for all three FN rates, so that the SLN biopsy patients were more likely than the IFL patients to accept the FN rates.
Adverse events
Information about AEs was generally poorly reported. Eight studies provided data. 50,52,54,59,65,70,72,73 In Brunner et al. ,52 8.7% of patients' groins had postoperative inguinal morbidity (inguinal seromas, abscess, wound breakdown), but this information was not given by group.
Adverse events according to surgical procedures (SLN biopsy, SLN biopsy plus IFL) and time interval (short and long term) are as follows:
Short-term AEs:
-
For SLN biopsy only: transient lymph oedema (13%),59 wound breakdown (11.7%) and wound cellulitis (4.5%). 73
-
For SLN biopsy + IFL: transient lymph oedema (39%),59 postoperative groin lymphocele (5.5%) and cellulitis arising in the labia majora (2.8%),65 wound cellulitis (9.5%) and seroma (4.3%),70 wound breakdown (34%) and wound cellulitis (21.3%),73 cellulitis (5.9%) and lymphocele (11.8%). 72
Longer term AEs:
-
For SLN biopsy only: lymphoedema (1.9%) and recurrent erysipelas (0.4%). 73
-
For SLN biopsy + IFL: wound infection (31%), wound dehiscence (5%), lymphocyst (22%) and chronic lymphoedema (16%),54 lymphoedema (25.2%) and recurrent erysipelas (16.2%). 73
In Achimas-Cadariu et al. ,50 AEs were presented according to surgical procedures and by vulva and groin locations:
-
For SLN biopsy only: wound breakdown – 3% (vulva) and 3.6% (groin); haematoma – 3.6% (vulva) (no AEs from blue dye only).
-
For SLN biopsy + IFL: wound breakdown – 5% (vulva) and 6% (groin); haematoma – 1% (vulva) and 3.7% (groin); chronic lymphoedema – 3.7% (vulva) and 8% (groin).
-
For IFL only: wound breakdown – 2% (vulva) and 7% (groin); haematoma – 6.9% (vulva) and 6.9% (groin); chronic lymphoedema – 1.7% (vulva) and 4% (groin).
Chapter 6 Clinical effectiveness review
Study selection
The final search retrieved 14,038 potentially relevant citations, which were screened for relevance to the inclusion criteria. Relevant full-text articles for 313 citations were retrieved and 295 articles were subsequently excluded. The most common reasons for study exclusion were lack of the full-text version, wrong study design and wrong population. The list of excluded studies and reasons for their exclusion can be found in Appendix 11. Eighteen publications (corresponding to 17 studies) fulfilled the inclusion criteria (Figure 5). There was one RCT, three case–control studies and 13 case series included.
FIGURE 5.
A PRISMA diagram of the selection process for the clinical effectiveness systematic review.
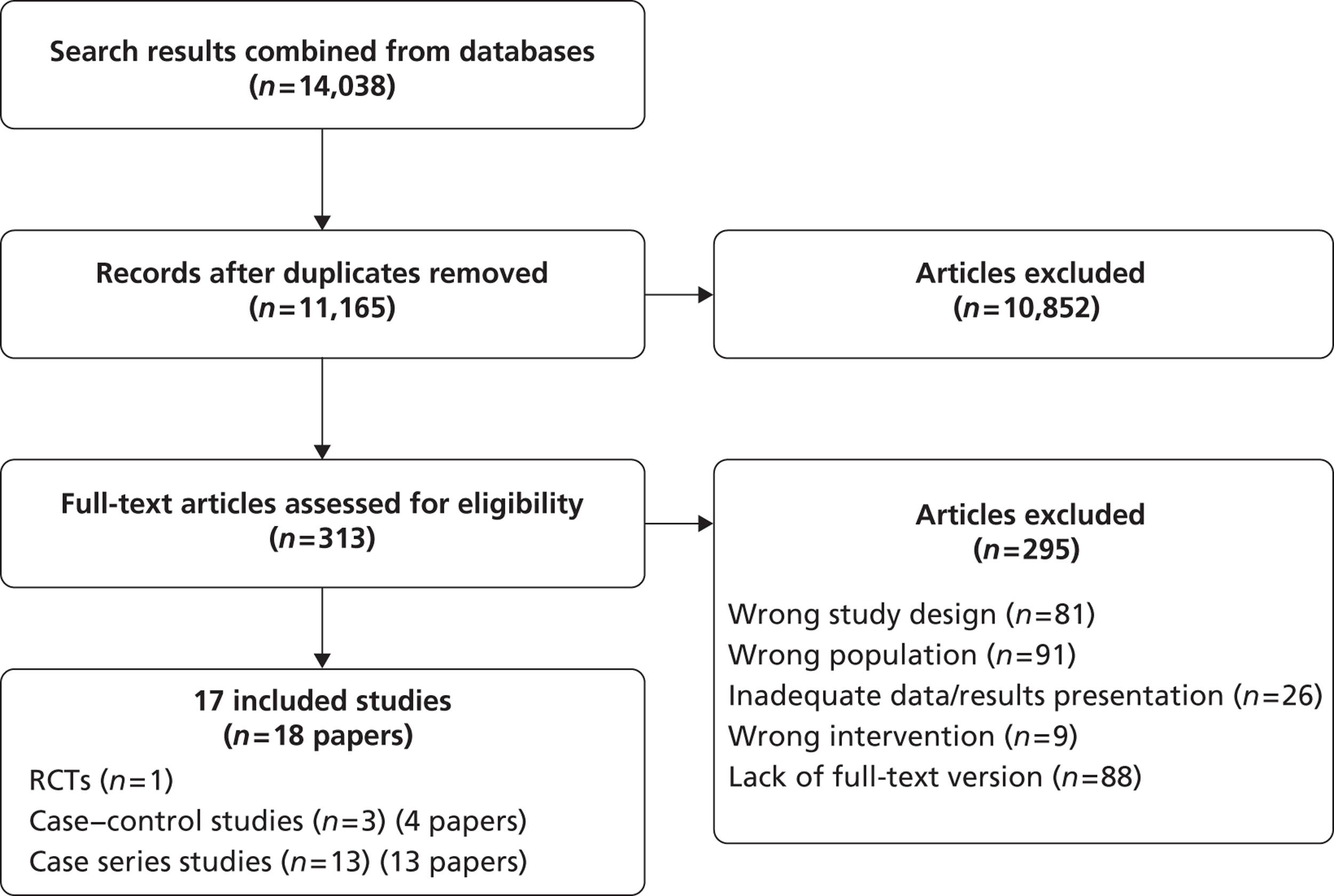
Randomised controlled trials
Characteristics of included study
Only one RCT (Stehman et al. 87) met the inclusion criteria for the clinical effectiveness systematic review. It included patients with primary vulval cancer at FIGO stage I, II and III and TNM classification T1–3, N0–1, M0 (percentages in each FIGO stage not given, but likely that I and II were > 75%). Patients were enrolled between 1986 and 1990. The intervention group received radical vulvectomy or modified radical hemivulvectomy with RT to both groins. The control group received radical vulvectomy or modified radical hemivulvectomy with bilateral groin IFL. Patients were followed up for at least 3 years, or until recurrence. Inclusion criteria and baseline characteristics are in Table 30. Characteristics were well balanced between the groups. Three patients in the RT arm did not receive the full doses of RT; however, all patients in the control arm received IFL. All patients were analysed for recurrence, survival, deaths, AEs and number of days spent in hospital after clinical intervention.
| Parameter | Intervention group | Control group | ||
|---|---|---|---|---|
| Patients | Inclusion criteria | Primary SCC of the vulva, previously untreated, T1–3 FIGO (according to guidelines from 1971), patients with T1 were eligible only if there was capillary–lymphatic space involvement or if there was > 5 mm of invasion. Lymph nodes, if palpable, must not have been suspicious and informed consent was gained for study in accordance with local Institutional review board guidelines and with the Treaty of Helsinki | ||
| Exclusion criteria | Patients with distant metastasis (M1), unsuitable for operation, have received any prior radiation or chemotherapy, with any prior malignancy other than non-melanoma skin cancer of a site other than the vulva | |||
| Number of patients | 25 | 27 | ||
| Age (years) | Median | 64 | ||
| Age ranges: n (%) | 31–40: 1 (4) 41–50: 1 (4) 51–60: 5 (20) 61–70: 7 (28) 71–80: 5 (20) 81–90: 6 (24) | 31–40: 3 (11.1) 41–50: 6 (22.2) 51–60: 4 (14.8) 61–70: 5 (18.5) 71–80: 6 (22.2) 81–90: 3 (11.1) | ||
| Disease | Location of primary tumour: n (%) | Labia: 14 (56) Clitoris: 6 (24) Perineum: 3 (12) Other: 2 (8) |
Labia: 16 (59.3) Clitoris: 5 (18.5) Perineum: 6 (22.2) |
|
| Size of tumour: n (%) | ≤ 2 cm: 2 (8) 2.1–4 cm: 18 (72.5) ≥ 4.1 cm: 5 (20) |
≤ 2 cm: 3 (11.1) 2.1–4 cm: 19 (70.4) ≥ 4.1 cm: 5 (18.5) |
||
| Depth of invasion | Not reported | |||
| Tumour histology type | Squamous cell carcinoma | |||
| Morphology of the nodes: n (%) | Not palpable: 20 (80) Palpable but normal: 5 (20) |
Not palpable: 20 (74.1) Palpable but normal: 7 (25.9) |
||
Quality of included study
In Stehman et al. 1992,87 the authors did not describe method of randomisation, apart from that it was conducted in blocks that were balanced within and between institutions (21 institutions). In addition, methods of allocation concealment or any blinding procedure that might have been used were not reported. However, it would not have been possible to blind the interventions and blinding the outcomes assessment may have made little difference. Results for all eligible patients are given.
Randomised controlled trial results
The median postoperative stay was 13 days [interquartile range (IQR) 8–20 days] and those in the RT arm had substantially shorter hospitalisations than those in the IFL arm (p = 0.0001). Ten patients had hospital stays of 7 days or fewer in the radiation arm compared with two in the IFL arm, whereas six patients had hospital stays of 13 or more days in the RT arm compared with 20 in the IFL arm. There were 10 episodes of grade 3 toxicity in the RT arm, including seven episodes of cutaneous toxicity. There were 22 episodes of grade 3 or 4 toxicity in the IFL arm including one death, five severe cardiovascular complications and 12 wound eruptions. There were also seven patients in this group with mild or moderate lymphoedema.
Survival curves are presented for progression-free survival, overall survival and relative survival (described as survival with intercurrent deaths censored) for up to 36 months. All three show that survival in the RT arm was worse than the IFL arm (log-rank tests: p = 0.033, p = 0.035 and p = 0.042, respectively). In the radiation arm there were eight deaths from vulval cancer and two from other causes. In the IFL arm there was one treatment-related death (pulmonary embolism), one death from vulval cancer and one death from other causes. The calculated relative risk for overall survival is 1.40 (95% CI 1.01 to 1.93) in favour of the IFL arm.
In the radiation arm, there was one vulval recurrence, five groin recurrences and two distant recurrences. In the IFL arm, there was one vulval recurrence and one distant recurrence. The calculated relative risk for recurrence of any sort is 0.27 (95% CI 0.07 to 0.99) in favour of the IFL arm.
Case–control studies and case series
Sixteen studies were included in this section (see Table 31). There was one matched case–control study (Helm et al. 37), two unmatched case–control studies (Manavi et al. 88 and Stehman et al. 89) and the remainder were retrospective case series. One study (Katz et al. 90) included more than 25% FIGO stage III and IV patients but the results for recurrence for stage I and II patients only by treatment received were clear, so this subgroup was reported here. The characteristics of the included studies are shown in Table 31. Almost all studies were conducted in the USA or Europe and the follow-up times varied between 1 and 30 years.
| Study | Study type | Population | Location | Length of follow-up [range (months)] |
|---|---|---|---|---|
| Anderson et al., 199591 | Retrospective, case series | Primary vulval cancer in stages I, II and III according to TNM classification | USA | Median: 54 months (2–212) |
| Andrews et al., 199492 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II, III and T1, T2 according to TNM classification | USA | Mean: 5 years (1–15) |
| Burke et al., 199593 | Retrospective, case series | Primary vulval cancer in stages T1, T2 according to TNM classification | USA | Median: 38 months |
| Busch et al., 200094 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II and T1N0–1, T2N0–1 according to TNM classification | Germany | Up to 30 years |
| de Hullu et al., 200295 | Retrospective case series | Primary vulval cancer in stages T1, T2 according to TNM classification | Netherlands | Median:110 months (3–220) |
| DeSimone et al., 200796 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II, III and T1, T2 according to TNM classification | USA | Mean: 59 months (10–195) |
| Farias-Eisner et al., 199497 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II and T1N0–1M0, T2N0–1M0 according to TNM classification | USA | Median: 12 months (6–77) |
| Hallak et al., 200798 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II | Sweden | Mean: 101 months (19–252) |
| Helm et al., 199237 | Matched case–control study | Primary vulval cancer in FIGO stages I, II, cases with triple incision IFL, controls with single incision | USA | Up to 8 years |
| Katz et al., 200390 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II, III and IV (stage I and II subgroup only) | USA | Up to 10 years |
| Kumar et al., 200999 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II | USA | Up to 220 months |
| Manavi et al., 199788 | Case–control study | Primary vulval cancer in stages T1, T2 according to TNM classification, cases with inguinofemoral RT, controls without | Austria | Up to 5 years |
| Scheistroen et al., 2002100 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II | Norway | Up to 80 months |
| Stehman et al., 199289 | Case–control study | Primary vulval cancer in FIGO stage I, cases had modified radical hemivulvectomy and ipsilateral inguinal lymphadenectomy, historical controls with vulvectomy and bilateral IFL | USA | 3 years or until death |
| Tantipalakorn et al., 2009101 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II | Australia | Median: 84 months |
| Vavra et al., 1990102 | Retrospective, case series | Primary vulval cancer in FIGO stages I, II and T1, T2–3 according to TNM classification | Austria | Up to 5 years |
The numbers of patients given different types of treatments are shown in Table 32. The table indicates that the different studies evaluated several interventions and many of the groups were small. Many of the case series compared radical vulvectomy with hemivulvectomy and wide excision and it was frequently difficult to determine how many patients had received unilateral or bilateral IFL. The largest study was that by Kumar et al. ,99 which reported results from the US Surveillance, Epidemiology and End Results (SEER) database between 1988 and 2005. With this paper, it was impossible to determine the number of women who had had IFL so the results have been placed in the surgery to primary site-only category.
| Study | Total number of patients | Surgery to primary site only | IFL – bilateral and surgery | IFL – unilateral and surgery | RT to groin and surgery to primary site | RT only | Surgery, IFL and RT | Comments |
|---|---|---|---|---|---|---|---|---|
| Anderson et al., 199591 | 47 | 13 | – | 23 | – | 11 | – | – |
| Andrews et al., 199492 | 84 | – | 49 | 28 | – | – | 7 | – |
| Burke et al., 199593 | 76 | – | 51 | 25 | – | – | – | – |
| Busch et al., 200094 | 92 | – | – | – | 92 | – | – | – |
| de Hullu et al., 200295 | 253 | – | 219 | 34 | – | – | – | – |
| DeSimone et al., 200796 | 122 | – | 60 | 62 | – | – | – | – |
| Farias-Eisner et al., 199497 | 74 | 3 | 58 | 13 | – | – | – | – |
| Hallak et al., 200798 | 294 | – | – | – | 267 | – | 27 | – |
| Helm et al., 199237 | 64 | – | 62 | – | – | – | 2 | Matched comparison |
| Katz et al., 200390 | 153 | – | Combined 104 | 153 | – | 29 | Stage I and II subgroup | |
| Kumar et al., 200999 | 6965 | 5253 | – | – | 1023 | 689 | – | Number of IFL not given |
| Manavi et al., 199788 | 135 | 70 | – | – | 65 | – | – | – |
| Scheistroen et al., 2002100 | 216 | 47 | 161 | 8 | – | – | – | – |
| Stehman et al., 199289 | 217 | – | 96 | 121 | – | – | – | – |
| Tantipalakorn et al., 2009101 | 121 | 19 | 48 | 54 | – | – | – | – |
| Vavra et al., 1990102 | 101 | 43 | – | – | 58 | – | – | – |
The case–control study by Helm et al. 37 matched 32 women who had a triple incision for IFL with 32 women of similar FIGO stage, lymph node status, greatest diameter of lesion and site of lesion with a single incision for IFL. The case–control study by Manavi et al. 199788 compared women who had had inguinofemoral RT with those who had no RT. IFL was not performed on any of the patients but all had had simple vulvectomy. The case–control study by Stehman et al. 89 compared women who had modified radical hemivulvectomy and ipsilateral superficial inguinal lymphadenectomy with a historical comparator group of women who had had radical vulvectomy and bilateral IFL. Cases and controls had comparable stage I disease, 5 mm or less invasion, no vascular space involvement and negative inguinal and femoral nodes.
The baseline characteristics of patients in the included studies are shown in Table 33. Their median or mean ages were mostly of 60 years, which reflects the fact that vulval cancer is mostly a disease of older women. However, some younger women were included down to age 20 years and Kumar et al. 99 compared characteristics of younger women (aged < 50 years) with older women (aged ≥ 50 years). Where location of primary tumour was specified in studies, the majority were on the labium majus and were medial rather than lateral. The vast majority of malignancies were SCCs but also included were a few adenocarcinomas, MMs and others in some of the studies. Some of the studies included just FIGO stage I, whereas others had a spread of stages from I to IV. Some of the studies gave TNM classification as well as FIGO stage but others just gave one or the other. Node status was given in eight studies only. 37,89,91,92,95,96,100,101
| Study | Patients | Diseases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Number of analysed patients | Age (years) | Location of primary tumour | Size of tumour (cm) [SD] | Depth of invasion (mm) [SD] | Histology | FIGO stage | TNM | Nodes status | ||||
| I | II | III | IV | ||||||||||
| Anderson et al., 199591 | 47 | 40 | Median: 71 (range 20–91) | NR | NR | NR | SCC | 15 (32%) | 13 (28%) | 12 (25%) | – | T1 | Positive: 7 (20%) |
| Andrews et al., 199492 | 84 | 84 | Median: 65 (range 28–89) | Labium majus: n = 46 (54.8%); labium minus: n = 26 (31%); both: n = 12 (14.2%) | T1: Median: 1.5 (range 0.5–2); T2: Median: 3.4 (range 2.2–9.0) | 1–2 mm: 17; 2,1–3 mm: 23; > 3 mm: 32; unknown: 12 | SCC | 32 (38%) | 31 (37%) | 21 (25%) | – | T1: n = 37(44%); T2: n = 47 (56%) | T1: 5 (13%) positive; T2: 16 (34%) positive |
| Burke et al., 199593 | 76 | 76 | Mean: 62 (range 22–87) | NR | Mean: 2.6 (range 0.5–6.5) | Mean: 4.4 | SCC alone | NR | NR | NR | NR | T1: n = 33 (43.3%); T2: n = 43 (56.6%) | NR |
| Busch et al., 200094 | 92 | 56 | Mean: 65 (range 23–81) | Labia major: n = 10 (10.9%); labia minor: n = 38 (41.3%); clitoris: n = 20 (21.7%); mons pubis and perineum: n = 24 (26.1%) | ≤ 2 cm: n = 42 (45.7%); > 2 cm: n = 50 (44.3%) | NR | NR | 42 (45.7%) | 50 (44.3%) | – | – | T1N0: n = 42 (45.7%), T2N0: n = 50 (44.3%) | NR |
| de Hullu et al., 200295 | 253 | 238 | Group I: Median: 71 (range 28–94) Group II: Median: 71 (range 34–94) | NR | NR | Group I: Median: 5 (range 1–4) Group II: Median: 5 (range 1–27) | SCC alone | NR | NR | NR | NR | Group I: T1 n = 53 (21%); T2 n = 115 (45.5%) Group II: T1 n = 25 (10%); T2 n = 60 (23.5%) | Invasion Group I: 29 (11.5%) Group II: 13 (5.1%) |
| DeSimone et al., 200796 | 122 | 122 | Mean: 67 (range 28–91) | Labium minus: n = 38 (31.1%); labium majus: n = 68 (55.7%); both: n = 16 (13.2%) | T1: Median: 1.5 (range 0.5–2.0) T2: Median: 3.6 (range 2.2–9.0) | T1: Median: 4 (range 1.2–6) T2: Median: 5.4 (range 1.1–15) | SCC alone | 54 (44%) | 42 (35%) | 26 (2%) | – | T1: n = 61 (50%) T2: n = 61 (50%) | Lymph vascular involvement −23 (19%); and perineural invasion −11 (9) |
| Farias-Eisner et al., 199497 | 74 | 74 | NR | NR | NR | NR | SCC alone | 39 (52.7%) | 35 (47.3%) | – | – | NR | NR |
| Hallak et al., 200798 | 294 | 225 | Mean: 71 (range 32–93) | NR | NR | NR | SCC: n = 269 (91.5%) MM: n = 10 (3.4%) Paget's disease: n = 8 (2.7%) Other: n = 7 (2.4%) | 110 (37.4%) | 115 (39.1%) | 43 (14.6%) | 26 (8.9%) | NR | NR |
| Helm et al., 199237 | 64 | 35 | Mean: 65 | Single incision group: vulva, n = 25 (39%); midline, n = 7 (10.9%) Triple incision group: vulva, n = 23 (35.9%); midline, n = 9 (14.2%) |
Single incision group: ≤ 1: n = 6 (9.4%); > 1–2: n = 11 (17.2%); > 2–4: n = 10 (15.6%); > 4: n = 5 (7.8%) Triple incision group: ≤ 1: n = 5 (7.8%); > 1–2: n = 13 (20.3%); > 2–4: n = 8 (12.5%); > 4: 6 (n = 9.4%) | NR | SCC alone | 32 (50%) | 20 (31.3%) | 8 (12.5%) | 4 (6.2%) | NR | Negative – 56 (87.4%) Positive (bilateral) – 4 (6.3%) (unilateral) – 4 (6.3%) |
| Katz et al., 200390 | 153 | 153 | NR | NR | NR | NR | NR | 153 (100%) | – | – | NR | NR | |
| Kumar et al., 200999 | 5620 | 3239 | Mean: 73 (range 50–102) | NR | NR | NR | SCC alone | 3239 (57.6%) | 1862 (33.1%) | 248 (0.4%) | – | NR | NR |
| Manavi et al., 199788 | 135 | 135 | Mean: 68.5 | Clitoris: group I, n = 6 (9.2%); group II, n = 1 (1.4%) | ≤ 2: n = 135 (100%) | Group I: ≤ 2: n = 17 (SD 26.15); > 2: n = 24 (SD 36.92) Group II: ≤ 2: n = 30 (SD 42.8); > 2: n = 29 (SD 41.42) | SCC: n = 130 (96.3%); adenocarcinoma: n = 1 (1%); other: n = 4 (3%) | NR | NR | NR | NR | T1, N0–1: n = 135 (100%) | NR |
| Scheistroen et al., 2002100 | 216 | 216 | Mean: 70 (range 27–96) | Medial: n = 132 (61%); lateral: n = 80 (37%); multifocal: n = 4 (2%) | ≤ 2: n = 91 (42%); > 2: n = 125 (58%) | NR | SCC | 95 (44%) | 121 (56%) | – | – | NR | Absent: 138 (64%); present: 31 (14%); not assessed: 47 (22%) |
| Stehman et al., 199289 | 217 | 217 | Median: 61 Group 1: (range 21–40) n = 21 (17.3%), (range 41–60) n = 39 (32.3%), (range 61–80) n = 53 (43.8%), (range ≤ 81) n = 8 (6.6%) Group 2: (range 21–40) n = 4 (4.1%), (range 41–60) n = 29 (29.6%), (range 61–80) n = 57 (58.2%), (range ≤ 81) n = 8 (8.2%) |
Group 1 labia minus: n = 31 (25.6%); labia majus: n = 58 (47.9%); labia majus and minus: n = 18 (14.9%); clitoris and/or labium: n = 2 (1.7%); perineum and/or labium: n = 12 (9.9%) Group 2 labia minus: n = 37 (37.8%); labia majus: n = 35 (35.7%); labia majus and minus: n = 12 (12.2%); clitoris and/or labium: n = 14 (14.3%); perineum and/or labium: n = 0 (0%) | Group 1: 0.1–1: n = 45 (37.2%); 1.1–2: n = 76 (62.8%) Group 2: 0.1–1: n = 36 (36.7%); 1.1–2: n = 62 (63.3%) | Group 1: 0–1 mm: n = 13 (10.8%); 1.1–2 mm: n = 43 (35.8%); 2.1–3 mm: n = 29 (24.2%); 3.1–4 mm: n = 25 (20.8%); 4.1–5 mm: n = 10 (8.3%); (1 missing) Group 2: 0–1 mm: n = 18 (18.4%); 1.1–2 mm: n = 22 (22.4%); 2.1–3 mm: n = 28 (28.6%); 3.1–4 mm: n = 13 (13.3%) | SCC and baso-SCC (number of patients with each not reported) | 217 (100%) | – | – | – | NR | All not palpable or palpable but normal |
| Tantipalakorn et al., 2009101 | 121 | 78 | Stage I: Mean: 62.9 (range 35–98) [15.4], Stage II: Mean: 67.9, (range 29–94) [16.2] |
NR | Stage I: 1.6 (range 0.5–2.0) [0.4] Stage II: 3.7 (range 2.5–6.5) [1.1] | < 1: n = 21 (17.4%); 1.1–2.0: n = 26 (21.5%); 2.1–3.0: n = 17 (14%); 3.1–4.0: n = 8 (6.6%); 4.1–5.0: n = 1 (1%); > 5: n = 5 (4%) | SCC alone | 78 (65%) | 43 (35%) | – | – | NR | Positive: 7 (6%); negative: 114 (94.2%) |
| Vavra et al., 1990102 | 101 | 81 | Mean: 64.9 (range 38–75) | NR | NR | < 5: n = 67 (66%); > 5–10: n = 34 (34%) | SCC alone | 54 (53%) | 27 (27%) | 18 (18%) | 2 (2%) | T1: n = 58 (57%); T2 + T3: n = 43 (44%), N0,N1: n = 82 (81%); N2,N3: n = 19 (19%) | NR |
Quality of included studies
Quality assessment of case series is shown in Table 34 and of case–control studies in Table 35. Because of these study designs, it is inevitable that there will be inherent biases. In addition, they were all retrospective studies conducted by chart review and there was no reported blinding of investigators. Most of the studies were from single institutions, so were small, and, in order to obtain a reasonable sample size, they covered a number of years of recruitment. During this time, treatment methods and success rates may well have changed. Some studies attempted to reflect this by comparing different types of treatment, in particular that by Stehman et al. 89 However, they often compared different surgical techniques rather than whether or not patients had unilateral or bilateral IFL, or RT compared with no RT. In general, the clarity of reporting of findings in both types of study designs was poor and it was difficult to extract precise data about how the patients were selected and treated. In a number of the studies, it was also difficult to establish which patients had which outcomes and results for the different treatment types were combined. The follow-up times were also confusingly reported in some studies so that the median follow-up was unclear and only the maximum follow-up could be obtained. Reporting of AEs was also patchy.
| Study | Quality factors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Anderson et al.,199591 | Y | Y | U | Y | Y | Y | Y | Y | N | Y | Y | Y | N |
| Andrews et al., 199492 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | N |
| Burke et al., 199593 | Y | Y | Y | U | Y | U | U | Y | N | Y | Y | Y | Y |
| Busch et al., 200094 | Y | Y | U | Y | Y | N | U | Y | N | Y | Y | Y | N |
| de Hullu et al., 200295 | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | N |
| DeSimone et al., 200796 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Farias-Eisner et al., 199497 | Y | U | U | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Hallak et al., 200798 | Y | Y | U | U | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Katz et al., 200390 | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y | Y | Y |
| Kumar et al., 200999 | Y | Y | U | N | N | N | Y | Y | N | Y | N | Y | N |
| Scheistroen et al., 2002100 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y |
| Tantipalakorn et al., 2009101 | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | N |
| Vavra et al., 1990102 | Y | Y | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y |
| Study | Quality factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5a | 5b | 6 | 7 | 8 | |
| Helm et al., 199237 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Manavi et al., 199788 | N | Y | Y | Y | Y | Y | Y | N | Y |
| Stehman et al., 199289 | Y | Y | N | N | N | N | Y | N | U |
Effectiveness results
Deaths, survival and recurrence
All of the included studies reported either deaths, survival or recurrence. Deaths and survival are given in Table 36 and recurrence in Table 37, together with the time frame that the results are reported within and any information on whether patients were node negative or positive at IFL (if conducted) and whether the deaths were following local, groin or distant recurrence (if reported). Many of the studies combined deaths or survival rates for different types of treatments. In addition, as studies were mostly small, it is difficult to establish any patterns of survival on different categories of treatment. The general trend was that there were more deaths in node-positive patients and in older patients. The two studies that compared RT with surgery on the primary site only (Manavi et al. 88 and Vavra et al. 102) found little difference in survival between the two treatments. In Stehman et al. ,89 the calculated odds ratio for overall deaths was 0.66 (95% CI 0.31 to 1.40) and was not statistically significant. The paper included a survival curve for cases and controls, which showed that survival of cases was lower than of controls, but the log-rank test was not significant. There is approximately a 50 : 50 chance of dying from other causes as vulval cancer, particularly in the case series with median ages over 60 years.
| Study | Total number patients | Surgery on primary site only | IFL – bilateral and surgery | IFL – unilateral and surgery | RT to groin and surgery to primary site | Radiation only | Surgery, IFL and RT | Comment |
|---|---|---|---|---|---|---|---|---|
| Anderson et al., 199591 | 47 (40) | NR | – | NR | – | 5-year overall survival 74% (range 43–100%) (n = 11) | – | There were 47 patients in the study and survival results given for 40 of these patients |
| Andrews et al., 199492 | 84 | – | Combined, two died from disease within 1 year (one local, one distant) (n = 77) | – | – | NR | – | |
| Burke et al., 199593 | 76 | – | Combined, four died from disease, nine from other causes (median follow-up 38 months) | – | – | – | – | |
| Busch et al., 200094 | 92 | – | – | – | 5-year overall survival 56%, 10-year overall survival 40%, 5-year cause-specific survival 61%, 10-year cause-specific survival 51% | – | – | |
| de Hullu et al., 200295 | 253 | – | Combined, 37 died from disease, 34 died from other causes, (follow-up at least 4 years) | – | – | – | – | |
| DeSimone et al., 200796 | 122 | – | Combined, five died from disease of which one negative nodes (groin), four positive nodes (distant) [mean follow-up 59 months (range 10–195 months)] | – | – | – | – | |
| Farias-Eisner et al., 199497 | 74 | Combined, 5-year overall survival 98% for stage I, 81% stage II. (Total? One death in stage I and five deaths in stage II, these five all had groin then distant recurrence) | – | – | – | Stage I node-positive 5-year overall survival 50%, node negative 100%, stage II survival by node status not given | ||
| Hallak et al., 200798 | 294 | Combined, 107 died from vulval cancer, 85 from other causes (mean follow-up 101 months (range 19–252 months). 5-year overall survival 53% | ||||||
| Helm et al., 199237 | 64 | – | Six died from disease (all with positive nodes), one from other causes | – | – | – | One died from disease (with positive nodes) (n=2) | Matched comparison (follow-up median 4.7 years in one group, 2.9 years in the other group) |
| Katz et al., 200390 | 153 | – | NR | NR | – | NR | Stage I and II subgroup | |
| Kumar et al., 200999 | 6965 | Combined, 2981 died. 5-year overall survival 87.5% in 1345 women aged under 50 years, 52.5% in 5620 older women (= 50 years) | – | Number of IFL not given | ||||
| Manavi et al., 199788 | 135 | 5-year survival 91.4% (65 out of 70 died) | – | – | 5-year survival 93.7% (61/65) | – | – | – |
| Scheistroen et al., 2002100 | 216 | Combined, 55 died from vulval cancer, 70 died from other causes. 5-year overall survival 76%, node negative 82%, node positive 57% | – | – | – | – | ||
| Stehman et al., 199289 | 217 | – | – | Seven died from vulval cancer (five from groin recurrence), seven died from other causes, one unclear (n = 121) | – | – | – | – |
| Tantipalakorn et al., 2009101 | 121 | Combined, seven died from vulval cancer (two from vulval, three from groin and two from distant), ? two died from other causes (median follow-up 84 months) | – | – | – | – | ||
| Vavra et al., 1990102 | 101 | 5-year survival 88.4% (n = 43) | – | – | 5-year survival 79.3% (n = 58) | – | – | 17 deaths in total |
| Study | Total number patients | Surgery on primary site only | IFL – bilateral and surgery | IFL – unilateral and surgery | RT to groin and surgery to primary site | Radiation only | Surgery, IFL and RT | Comment |
|---|---|---|---|---|---|---|---|---|
| Anderson et al., 199591 | 47 (40) | NR | – | NR | – | Three had recurrence out of the nine who had radiation therapy (33%) | – | There were 47 patients in the study and survival results given to 40 of these patients |
| Andrews et al., 199492 | 84 | – | Combined, seven local, up to 185 months (n = 77) | – | – | Groin 1, distant 1 within 1 year (n = 7) | ||
| Burke et al., 199593 | 76 | – | Combined, nine vulval, four groin (three were negative at IFL, one was not dissected) (median follow-up 38 months) | – | – | – | ||
| Busch et al., 200094 | 92 | – | – | – | NR | – | – | |
| de Hullu et al., 200295 | 253 | – | Combined, 38 within 2 years (of which 21 local, 11 groin, six distant) and 66 within 4 years | – | – | – | ||
| DeSimone et al., 200796 | 122 | – | Nine, of which eight local, one distant (mean follow-up 59 months (range 10–195) (n = 60) | Nine, of which five local, two groin, two distant (mean follow-up 59 months (range 10–195) (n = 62) | – | – | – | Recurrence in 12 out of 96 with negative nodes, 6 out of 26 positive nodes |
| Farias-Eisner et al., 199497 | 74 | Recurrence stage I NR, stage II 9 recurrences: location NR [median follow-up 12 months (range 6–77 months)] | – | – | – | |||
| Hallak et al., 200798 | 294 | Combined, 127 recurrences: 80 vulval, 55 inguinal, 20 distant [mean follow-up 101 months (range 19–252 months)] | ||||||
| Helm et al., 199237 | 64 | – | 13 recurrences (seven vulval, one distant and in five patients the site of recurrence was not reported) (n = 62) | – | – | – | NR (n = 2) | Matched comparison |
| Katz et al., 200390 | 153 | – | 17 had recurrence out of the 104 who had therapy (17%) | One had recurrence out of the 20 who had therapy (5%) | – | Five had recurrence out of the 29 who had therapy (20%) | Stage I and II subgroup | |
| Kumar et al., 200999 | 6965 | NR | – | – | NR | NR | – | Number of IFL not given |
| Manavi et al., 199788 | 135 | 13 recurrence: five local, seven groin, one distant (up to 5 years’ follow-up) (n = 70) | – | – | Eight recurrences: four local, three groin, one distant (up to 5 years’ follow-up) (n = 65) | – | – | |
| Scheistroen et al., 2002100 | 216 | Combined, 66 recurrences, 41 vulva, 16 groin, nine distant (up to 80 months' follow-up) | – | – | – | |||
| Stehman et al., 199289 | 217 | – | Six vulval and one distant recurrence (n = 96) | 10 vulval, nine groin, no distant recurrences (all node negative) (n = 121) | – | – | – | Follow-up 3 years or until death |
| Tantipalakorn et al., 2009101 | 121 | Combined, 28 recurrences, 26 local, two distant (median follow-up 84 months) | – | – | – | |||
| Vavra et al., 1990102 | 101 | 9.3% recurrence (n = 43) | – | – | 2.6% recurrence (n = 58) | – | – | Five vulval, nine inguinal, two distant) |
Recurrences were more likely in node-positive patients. The recurrence rates show that there is an approximate ratio of 4 : 2 : 1 rate of recurrence in vulva, groin and distant. As studies were small, some had no distant recurrences. Very few gave details about node status, but results from DeSimone et al. 96 suggested that recurrences were more likely in node-positive patients; however, in Stehman et al. ,89 all recurrences in cases were in node-negative patients. In this study, survival curves for recurrence-free interval for cases and controls was given in the paper and showed significantly worse recurrence-free intervals for the cases (p = 0.0028).
Hospital stay
In Burke et al. ,93 the mean hospital stay was 10 days. In DeSimone et al. ,96 the mean hospital stay was 9.6 days (range 4–14 days) in patients undergoing radical vulvectomy and 5.0 days (range 2–12 days) in patients undergoing radical hemivulvectomy. In Farias-Eisner et al. ,97 the median hospital stay was 19 days (range 12–33 days) for patients undergoing radical vulvectomy and 11 days (range 5–17 days) for patients undergoing modified radical vulvectomy. In Stehman et al. ,89 the median length of hospitalisation was 7 days (IQR 5–10 days, range 3–22 days) for cases given modified hemivulvectomy and ipsilateral inguinal lymphadenectomy and 18 days for controls (ranges not given) given radical vulvectomy and bilateral IFL.
Quality of life and adverse events
No studies reported QoL and not all studies reported AEs. Of the studies that did, their reported AEs are shown in Table 38. In Stehman et al. ,89 AEs were reported only for the cases. In other studies, only some AEs were reported (see Table 38). The rates varied considerably between studies, for example, the rate of lymphoedema varied between 41% in Farias-Eisner et al. 97 and 1% in Vavra et al. 102 Few studies gave the total number of women who suffered an AE and, in the ones that did, the rates varied between 39%100 and 19%. 89 Further details of AEs are reported in Appendix 12 (Tables 56 and 57).
| Characteristic | AEs | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Early surgical complication | Late surgical complication | ||||||||||||||||||
| Study | Population | Intervention | Wound break down/rupture (%) | Wound infection (%) | Groin breakdown (%) | Lymphocyst (%) | Cellulitis (%) | Bleeding (%) | None (%) | Rectovaginal fistula (%) | Major complication (%) | Inguinal pain (%) | Groin haematoma (%) | Lymphocyst (%) | Lymph oedema (%) | Lymphangitis (%) | Cellulitis (%) | Vaginal stenosis (%) | ||
| Burke et al., 199593 | I/II | Modified vulvectomy | – | 6 (8) | – | 8 (11) | 2 | 1 | 1 | – | – | – | – | – | 75 (80) | 5 (7) | 5 (4) | 2 (2) | – | |
| DeSimone et al., 200796 | I/II | Radical vulvectomy | – | 14 (23) | – | NR | 5 (7) | – | – | – | – | – | – | – | – | 16 (26) | – | – | – | |
| Radical hemivulvectomy | – | 5 (98) | – | NR | 2 (3) | – | – | – | – | – | – | – | – | 5 (8) | – | – | – | |||
| Farias-Eisner et al., 199497 | I/II | Radical local exision | – | 2 (11) | – | 5 (28) | – | – | – | – | – | 1 (1) | – | – | – | 7 (38) | – | – | – | |
| Radical vulvectomy | – | 14 (25) | – | 13 (23) | – | – | – | – | – | – | – | – | 24 (41) | – | – | – | ||||
| Hallak et al., 200798 | After IFL | 64 (22) | 5 (19) | 7 (26) | – | – | – | 2 (7) | 15 (56) | – | – | – | – | – | 1 (7) | – | – | 0 | ||
| No IFL | 11 (6) | 0 (5) | – | – | – | 4 (2) | 141 (77) | – | – | – | – | – | 0 | – | – | 2 (1) | ||||
| Helm et al., 199237 | Single incision group | Major – 2 (6) | 6 (19) | – | 11 (34) | – | 5(6) | – | – | – | – | – | – | – | – | – | – | – | ||
| Triple incision group | Major – 1 (3) | 2 (6) | – | 6 (19) | – | 7 (22) | – | – | – | – | – | – | – | – | – | – | – | |||
| Manavi et al., 199788 | I/II | RT (for groin) | 5 (8) | – | 1 (2) | – | – | – | – | – | 1 (2) | – | 1 (2) | – | – | – | – | – | 2 (3) | |
| No RT | 2 (3) | – | 0 | – | – | – | – | – | 0 | – | 0 | – | – | – | – | – | 2 (3) | |||
| Scheistroen et al., 2002100 | I/II | En block | 85 (39) | 45 (51) | 21 (24) | 16 (18) | – | – | – | – | – | – | 5 (6) | – | 3 (3) | – | – | – | – | – |
| Triple incision group | 18 (30) | 8 (13) | 8 (13) | – | – | – | – | – | – | 1 (2) | – | 1 (2) | – | – | – | – | – | |||
| Individual | 6 (30) | 1 (5) | 1 (5) | – | – | – | – | – | – | 0 | – | 1 (5) | – | – | – | – | – | |||
| Local excision | 16 (34) | 3 (6) | 3 (6) | – | – | – | – | – | – | 3 (6) | – | 2 (4) | – | – | – | – | – | |||
| Stehman et al., 199289 | Cases | All | 23 (19) | 9 (7) | 14 (12) | – | – | – | – | 67 (55) | – | – | – | – | – | Mild: 18 (15); moderate: 5 (4) | – | – | – | |
| Vavra et al., 1990102 | All | – | – | 2 (2) | – | – | – | – | – | – | – | – | – | – | – | 1 (1) | – | – | 4 (4) | |
Effectiveness in younger versus older women
Kumar et al. 99 investigated a variety of prognostic variables in 1345 younger women (< 50 years) compared with 5620 older women (≥ 50 years). The 5-year survival rate for any treatment in younger women was 87.5% compared with 52.5% in older women. If the malignancy was localised, the survival rates were 93% versus 66%, if regional 79% versus 38% and if distant 26% versus 11%. Younger women were more likely to have been treated with surgery (92.2% vs. 84.1%) and less likely to have had RT (16.2% vs. 26.9%) than older women. In surgically treated patients, the 5-year survival rates were 90% in younger women compared with 58% in older women.
Chapter 7 Economic evaluation
Objective
Patients with FIGO stage I or stage II vulval cancer may have an IFL as part of the treatment in order reduce the probability of groin and distant recurrence. As has been shown previously in this report, the morbidity of this procedure is high. Moreover, it is recognised that, although there is a possibility of groin lymph node metastases before IFL is conducted, for many patients the IFL is subsequently found to have been unnecessary since no metastases were found. Therefore, an accurate method of identifying which patients need IFL would help to reduce unnecessary morbidity in many patients.
The SLN biopsy may use one or both of blue dye and 99mTc and is intended to identify one or more SLNs. The SLN is then examined using histopathological techniques to identify patients with metastases that need an IFL. The potential advantage of SLN biopsy is that there is much less morbidity because of a smaller incision if patients have no metastases in the SLN, although there is a risk that patients may test false-negative for metastasis and then go onto have a groin recurrence with an associated higher risk of mortality. The important potential advantage with SLN biopsy is that only one lymph node is removed, thereby reducing the incision, the extent of surgical dissection required and the potential for subsequent lymphocyst and lymphoedema formation as a result of fewer lymph nodes being removed.
The objective of this economic evaluation is to compare the relative cost-effectiveness of undertaking a range of SLN biopsy options that examine for the presence of metastases compared with implementing a strategy of ILN for all without first identifying whether or not patients need such radical treatment.
Developing the model structure
A decision tree was developed in TreeAge Pro 2001 software (TreeAge Software Inc., Williamstown, MA, USA). It was felt that a decision tree approach would be most appropriate for this economic evaluation for two reasons:
-
The short time horizon.
-
There are no examples of multiple recurrences (the same event happening to the same patient many times) seen in the model structure.
Women enter the model having been identified with a previous biopsy with presumed FIGO stage T1 or T2 unifocal vulval cancer (but not T1a). Only patients with SCC are considered because the vast majority of vulval cancer is SCC. Patients with basal cell carcinoma and MM are excluded from this analysis because of the different characteristics of these types of tumours.
The patients are assumed to follow one of seven different pathways that describe alternative approaches to the SLN biopsy and the treatment of vulval cancer. The first pathway is the comparison arm, which is the implementation of an IFL without a SLN biopsy and is used to show how this more morbid treatment compares to different SLN biopsy options. In the case of pathways 2–7, a SLN biopsy is performed using either blue dye, 99mTc, or both, in order to identify the SLN. This is followed by histopathology, which is some combination of H&E staining and ultrastaging in order to test for the presence of metastasis, in which ultrastaging can be considered to be representative of more sensitive techniques such as immunohistochemistry. For patients with metastasis, an IFL is performed, with RT also given when necessary (for example, Van der Zee et al. ,73 in which RT was given when more than one intranodal metastasis and/or extranodal growth was detected). Following an IFL, patients are considered to be monitored every 3 months for 2 years for evidence of recurrence. If patients are only given a SLN biopsy and do not go on to IFL because no metastases were found, then patients are considered to be monitored every 2 months for 2 years. The seven patient treatment pathways are defined as follows:
-
IFL: this is performed on all patients, with no SLN biopsy being given.
-
Blue dye test and H&E: a blue dye test is administered intraoperatively to identify the SLN. This is followed by histopathology consisting of H&E staining of the SLN in order to identify the presence of metastasis.
-
Blue dye test and ultrastaging: a blue dye test administered intraoperatively to identify SLN, followed by histopathology consisting of H&E staining of the SLN. If no metastasis is detected then ultrastaging/staining is performed to confirm the absence or detect presence of metastases.
-
99mTc and H&E: a 99mTc test is administered, patient imaging preoperatively and then a radioactive probe used detect signal at surgery to identify the SLN. This is followed by histopathology consisting of H&E staining of the SLN to identify presence of metastasis.
-
99mTC and ultrastaging: a 99mTc test administered, patient imaging preoperatively and then a radioactive probe used detect signal at surgery to identify the SLN. This is followed by histopathology consisting of H&E staining of the SLN. If no metastasis is seen then ultrastaging/staining is administered to confirm absence or detect presence of metastases.
-
Blue dye and 99mTc and H&E: both blue dye and 99mTc test are administered to identify the SLN. Followed by H&E staining to identify the presence of metastasis.
-
Blue dye and 99mTc and ultrastaging: both blue dye and 99mTc test are administered to identify the SLN, followed by H&E staining. If no metastasis is seen, then ultrastaging/staining is administered to confirm absence or detect presence of metastases.
Morbidity can occur in the short and long term as a result of complications due to a SLN biopsy or an IFL. Local recurrence can occur at any time following either of these procedures, the probability of which is informed by data (see Table 42), whereas groin recurrence may occur depending on the outcome of biopsy result and the treatment response. RT may be implemented alongside an IFL, or in response to a recurrence among patients who have not previously received it. Chemotherapy is assumed to be administered to all patients who have a recurrence but have already received an IFL and RT. These points are illustrated in Figures 6–11:
FIGURE 6.
Summary of the decision pathway used in the economic model.
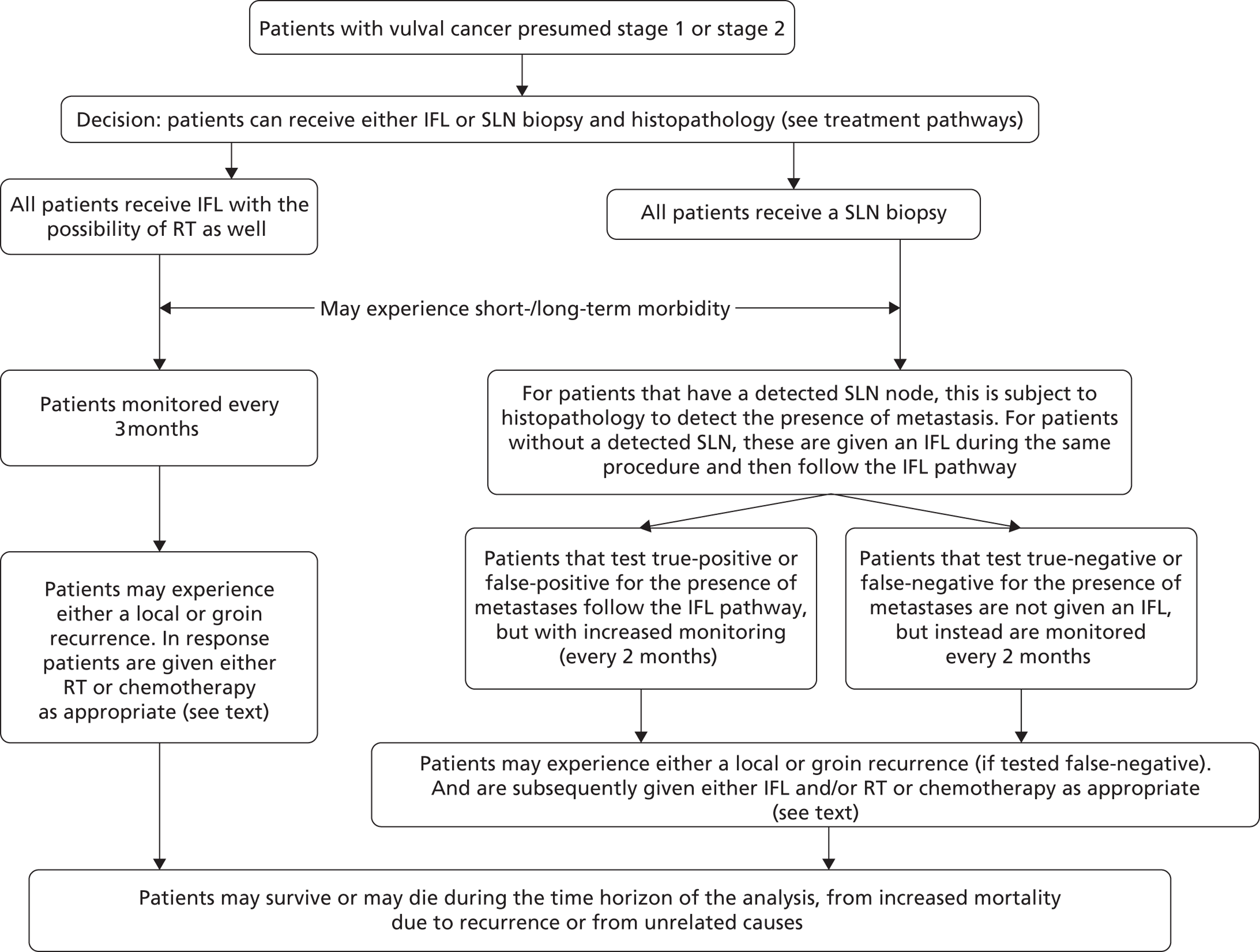
FIGURE 7.
Model Structure showing each of the seven treatment pathways and the treatment pathway for blue dye + H&E. This pathway is repeated for each of the pathways that include either blue dye and/or 99mTc.
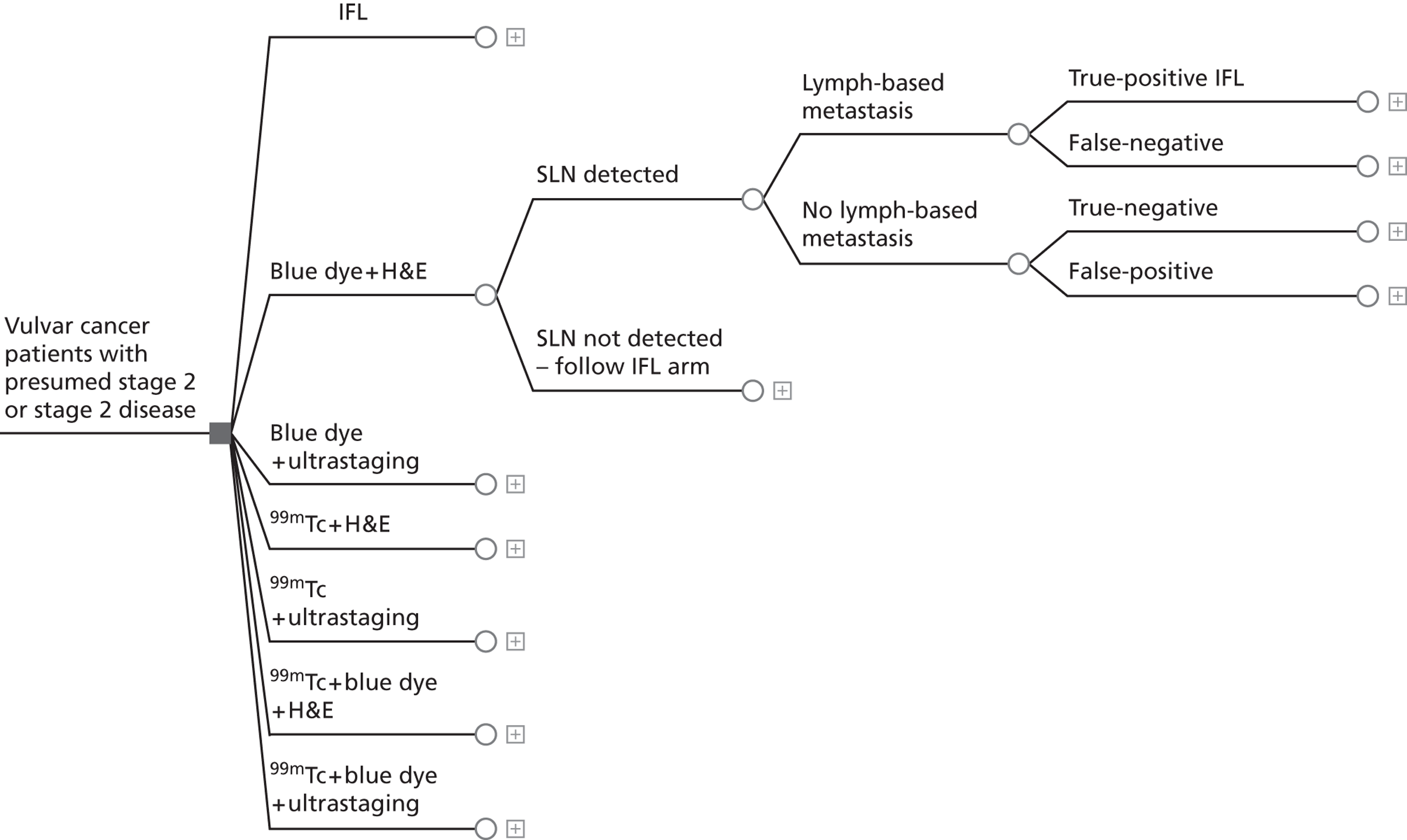
FIGURE 8.
Treatment pathway for IFL. Note: patients who have a local recurrence are given a primary excision. Patients who have a groin recurrence following IFL + RT are given chemotherapy. Patients who have a groin recurrence following an IFL only are given RT.
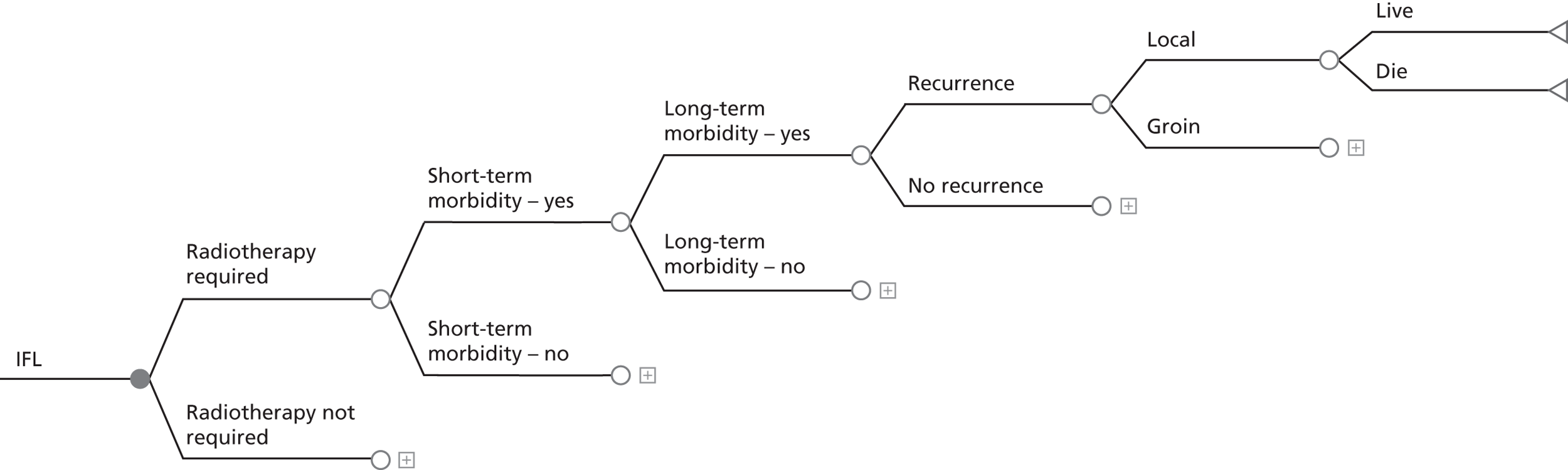
FIGURE 9.
Treatment pathway for strategies 2–7 following a TP result for metastasis. Note: patients who have an IFL with or without RT may still go on to have either a local or groin recurrence. In the case of a local recurrence, all patients are given a primary excision. Patients who have a groin recurrence having previously received an IFL + RT are given chemotherapy, whereas for those patients who have only previously received a IFL RT is given.
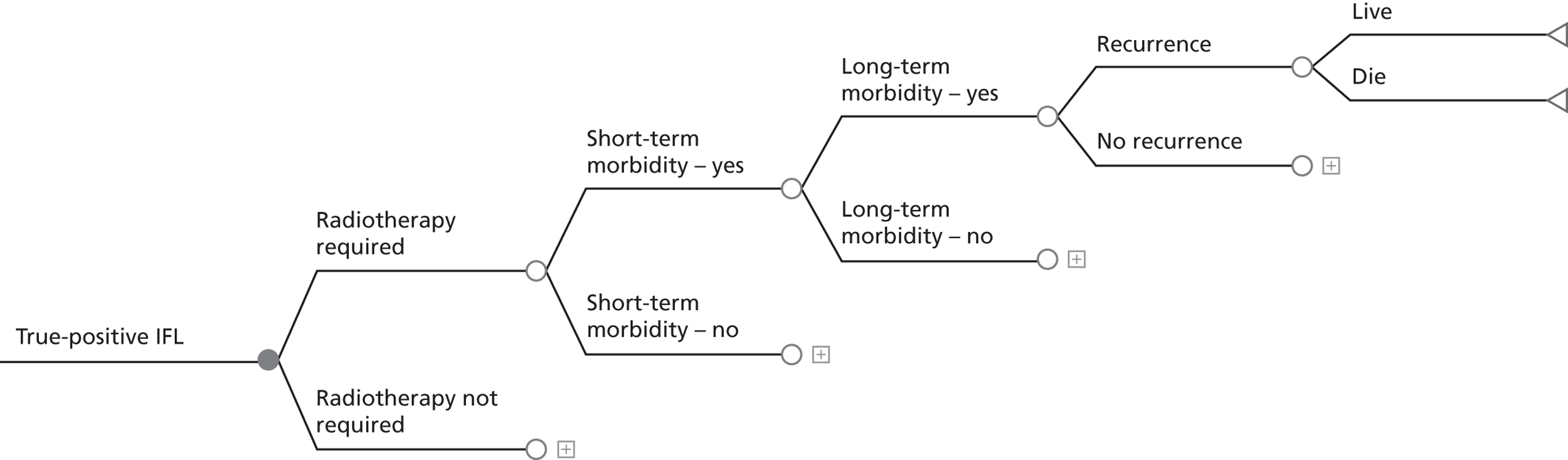
FIGURE 10.
Treatment pathway for strategies 2–6 following a FN result for metastasis. Note: patients who have a FN biopsy test result, subsequently go on to have a groin recurrence. In this case, all the patients receive both an IFL + RT.

FIGURE 11.
Treatment pathway for strategies 2–6 following a TN result for metastasis. Note: patients who have a TN biopsy test do not subsequently go on to have a groin recurrence. However, a local recurrence is still possible and under these circumstances the patients are given a primary excision.
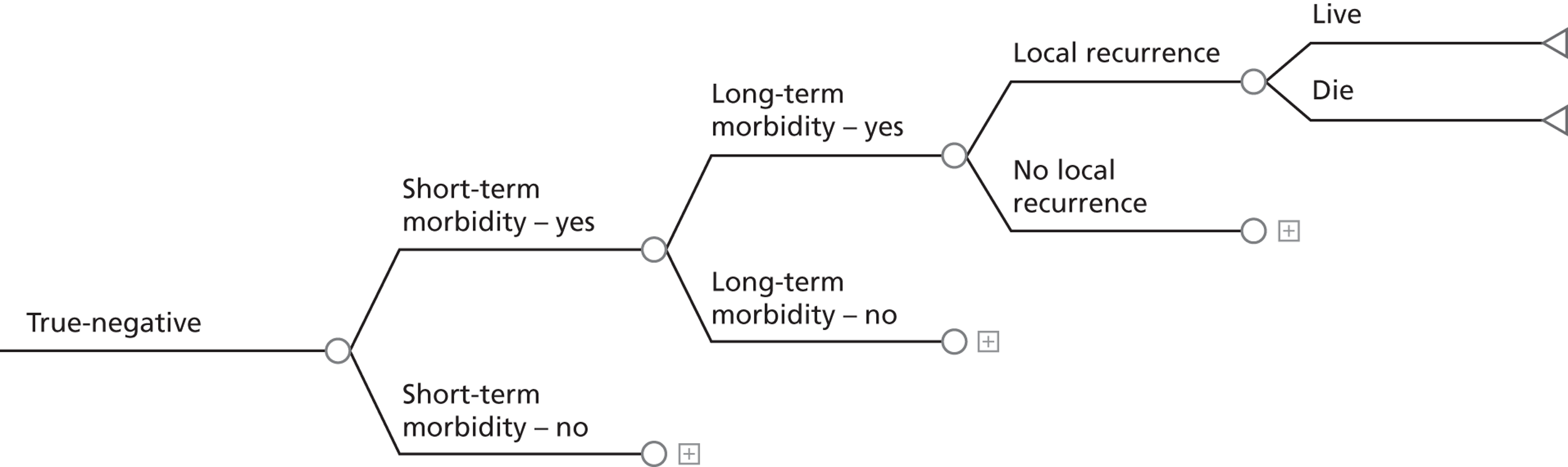
Model assumptions
A number of assumptions are required in order to develop a workable model structure and enable the analysis to be carried out. The assumptions made in this study are described below and grouped into those that refer to the general pathway, recurrence and the wider model:
General pathway: assumptions
-
Patients found to be FN during the SLN biopsy (blue dye and/or 99mTc), but are then subsequently found to have metastasis receive both an IFL and RT.
-
Patients are followed up every 2 months following a negative result for a SLN biopsy (and, therefore, are given no IFL) and every 3 months following an IFL.
-
There are no occasions in which RT would be administered to a patient who had not previously received an IFL (apart from following a recurrence).
-
Complications following a SLN biopsy (blue dye/99mTc) and then an IFL implemented during the same procedure will be the same as those experienced following just an IFL.
-
Complications following all types of SLN biopsy (e.g. blue dye/99mTc) will be the same.
Recurrence: assumptions
-
Recurrence will occur only in the groin or the vulva (local); distant recurrence will not be considered. This is because any distant recurrences are likely to occur following either a local or groin recurrence and rarely occur without either.
-
An additional primary excision will be required in the case of a local recurrence.
-
In the case of groin recurrence, the treatment is IFL + RT if it has not been administered already and chemotherapy if it has.
-
Mortality following recurrence within the 2-year time horizon is always due to vulval cancer, with these patients receiving palliative care as a result of their condition. Although it is acknowledged that the findings show that the death rate among vulval cancer patients due to vulval cancer or other causes is 50 : 50 following treatment, the risk of death following a recurrence is high enough for this assumption to be made.
Further modelling: assumptions
-
For the purpose of costing follow-up, all deaths from vulval cancer and all other causes occur at 12 months following screening.
-
All parameters in this model are independent of age, with the exception of the all-cause death rate. This assumption is made because of the paucity of age-specific data in this field.
-
Patients experience long-term complications independently of whether or not they experience short-term complications. This assumption is made owing to the paucity of data in the literature describing what proportion of patients experience both short- and long-term complications.
-
Short-term and long-term morbidity have no impact on the mortality of the patients. This assumption is made owing to the paucity of data; however, its impact is investigated through sensitivity analysis.
-
All patients in the patient cohort are aged 65 years. The impact of this assumption is investigated through sensitivity analysis by examining patients aged 55 and 75 years, respectively.
Data requirements
The data requirements for the economic evaluation are fulfilled by using the findings of the systematic review. However, when the results of many studies could not be generalisable to obtain an overall parameter value owing to differences in the study protocols, etc., the findings of larger and more recent studies have been preferred.
Characteristics of the patient cohort
The age of the patients in this analysis will have an impact on their all-cause death rate over the 2-year time horizon of this study. Age 65 years was chosen as it is in broad agreement with the mean/median age of the patients in the included studies in the systematic review, who were in the range from 58–75 years (see Table 9). The proportion of patients with metastasis is an important parameter that is subsequently used in the model in two ways: it influences what will be seen in the results from the histopathology following the SLN biopsy and will have an effect on the probability that a patient may have a later recurrence. Patient cohort characteristics are described in Table 39.
| Parameter | Value (range) | Reference | Notes |
|---|---|---|---|
| Patients with metastasis | 33.5% | Van der Zee et al., 200873 | – |
| Age of cohort (years) | 65 | – | Examined in sensitivity analysis |
Sentinel lymph node detection rate
Three approaches to the SLN biopsy are considered in this economic evaluation: blue dye and 99mTc alone, or both procedures implemented together. The aim of these procedures is to identify the SLN which can then be examined through histopathology to identify the presence/absence of metastasis. The following rates used in this study (Table 40) are informed by the findings of the systematic review (see Table 24).
| SLN biopsy | Detection rate |
|---|---|
| Blue dye | 202/294 (68.7%) |
| 99mTc | 227/240 (94.5%) |
| Blue dye + 99mTc | 1049/1074 (97.7%) |
Histopathology
Following the identification of the SLN(s) using a SLN biopsy, their histopathological assessment considered in this economic evaluation is the same as that described by Van der Zee et al. 73 H&E staining of the lymph node is used and then, if no metastasis is seen, ultrastaging with immunohistochemistry is then undertaken to confirm absence/presence of metastases. However, the impact of using routine histopathological examination alone is also considered. In this study, 80 out of 135 patients were found to have metastasis on routine examination (H&E) with the remaining positives (55 out of 135) being identified using ultrastaging (from Oonk et al. 83). In Van der Zee et al. ,73 6 out of 259 patients with unifocal vulval cancer and a negative SLN following H&E and ultrastaging were subsequently diagnosed with a groin recurrence, which can be used to help inform the probability of a FN test. Taking the prevalence of metastasis to be 33.5% (135 out of 403, see Table 39), the estimated probabilities for the different histopathology test results can be calculated as follows:
Calculation of histopathology test accuracy parameters
The following calculations describe the possible outcomes for patients who have a detected SLN that is then subject to histopathology.
As previously described, two approaches to histopathology are considered, these being H&E + ultrastaging and H&E alone. These are each considered in turn.
Among patients with an identified SLN that was subject to H&E + ultrastaging, Van der Zee et al. 73 describe that 6 out of 259 patients with unifocal vulval cancer and a negative SLN following H&E and ultrastaging were subsequently diagnosed with a groin recurrence (see Table 41); therefore, by definition:
Taking these values for FN and TN into account, this means that the assumed prevalence of metastasis in this study cannot fall below 2.3% (6/259).
The number of disease (metastasis) negative (DN) can be calculated from the sum of the patients who test TN and FP for metastasis (TN + FP):
However, given that the systematic review failed to find any evidence of patients testing FP for metastasis (see Tables 13–23), it is assumed here that FP = 0.
As described in Table 38, the proportion of patients with metastasis (p) is taken to be 0.335 (135/403). From this, the number of disease (metastasis) positive (DP) can be calculated:
Taking the previously calculated values for FN and DP, the number of TPs can now be calculated:
Now that we have values for FN, TN, FP and TP which are based values obtained from the literature that include the assumed prevalence of metastasis, it is straightforward to calculate what proportion of patients will test for each of these possibilities for H&E + ultrastaging, with the baseline parameters shown in Table 41.
| Test result | H&E | H&E if negative then ultrastaging |
|---|---|---|
| FN | 13.6% | 1.6% |
| TN | 66.5% | 66.5% |
| FP | 0.0% | 0.0% |
| TP | 19.9% | 31.9% |
As part of the study by Oonk et al. ,83 80 out of 135 patients who were found to have metastasis were found positive by H&E. This gives the sensitivity of H&E (SensH&E) to be 59% (80 out of 135).
Taking the number of DP described above for H&E + ultrastaging, the number of TPs and FNs detected by H&E can be given as follows:
Taking the number of TNs detected by H&E (TNH&E) to be the same as for H&E + ultrastaging and again assuming that FPH&E = 0.
The values for FNH&E, TNH&E, FPH&E and TPH&E allow the proportion of patients who test for each of these possibilities for H&E alone to be calculated, with the values used at baseline shown in Table 41.
In all cases the values taken from the literature, e.g. prevalence of metastasis, are varied as part of the probabilistic sensitivity analysis (PSA) in order to show their impact on the model results.
Cancer recurrence
A recurrence of cancer may occur along any of the patient pathways with varying probabilities. These are summarised in Table 42.
| Parameter | n/N (%) | Reference | Notes |
|---|---|---|---|
| Local recurrence | 34/276 (12.3) | Van der Zee et al., 200873 | The possibility of local recurrence is present in all arms of the model |
| Groin recurrence following IFL (no SLN biopsy) | 1/32 (3.1) | Crosbie et al., 201054 | Metastasis prevalence in this study found to be 6/31. Relative risk of groin recurrence given metastasis = 0.1546 |
| Groin recurrence following negative SLN biopsy result | 6/259 (2.3) | Van der Zee et al., 200873 | Patients with unifocal disease |
| Groin recurrence following positive SLN biopsy and IFL | 11/135 (8.1) | Oonk et al., 201083 | – |
| Groin recurrence following FN test | 100% | – | All patients will get a recurrence if the test is falsely negative |
Survival following treatment
Patient death is categorised as occurring as a result of a vulval cancer recurrence (local or groin) or from all other causes. All-cause death depends on the assumed age of the cohort with values calculated for the 2-year time horizon shown in Table 43.
| Death rate | Percentage | Reference | Note |
|---|---|---|---|
| Local recurrence | 5/34 (14.75%) | Van der Zee et al., 200873 | – |
| Groin recurrence | 9/11 (81.8%) | Oonk et al., 201083 | – |
| All cause | Age 55 years: 0.84% Age 65 years: 1.97% Age 75 years: 5.85% |
Office for National Statistics (2010)103 | Calculated from: Natural Death rates. Mid-year estimates published 30 June 2011 |
Radiotherapy
Radiotherapy may be given to patients following an IFL or following a recurrence. However, in all situations, RT is never administered to the same patient more than once. RT is assumed to always be implemented to a patient following a recurrence that has not previously received it, with the probability of RT at other points in the decision pathway being informed by data. This is summarised in Table 44.
| RT | Percentage | Reference | Notes |
|---|---|---|---|
| With an IFL Strategy 1 | 26/56 (46.4%) | Fonseca-Moutinho et al., 2000104 | – |
| After a TP SLN biopsy result and IFL | 49/117 (41.9%) | Van der Zee et al., 200873 | – |
| After a FP biopsy result and IFL | 0% | – | See Chapter 7, Model assumptions |
| Following a recurrence if not previously administered | 100% | – | See Chapter 7, Model assumptions |
Morbidity and complications
The reported complications following a procedure are used as a proxy for the additional morbidity experienced by patients. This in turn will have an impact on the costs to the health-care provider as a result of the extra resources needed to treat the patients. The definitions of short- and long-term morbidity in this study are the same as those proposed by Van der Zee et al. ,73 in which short-term morbidity is defined as the occurrence of wound breakdown or wound infection (requiring antibiotics) and long-term morbidity is defined as lymphoedema present over two consecutive visits more than 1 year after primary therapy or recurrent erysipelas (more than one episode of erysipelas requiring antibiotics) (see Chapter 5, Recurrence rates).
Complication-related morbidity can occur following an IFL or SLN biopsy in the short or long term. The percentage of patients with short- and long-term complications is calculated assuming that the probability of experiencing one type of short-term (or long-term) complication is independent of experiencing another at the same time and that the probability of experiencing long-term morbidity is independent of whether or not short-term morbidity was previously experienced (Table 45). This is informed by the findings of the study by Van der Zee et al. 2008. 73 These assumptions were made owing to the lack of information on the proportion of patients who have more than one complication at the same time or who experience both short-term and, then, long-term complications.
| Time frame | Procedure | Complication | % patients with complications (n/N) |
|---|---|---|---|
| Short term | IFL (with/without SLN biopsy) | Wound breakdown 34% Wound cellulitis 21.3% |
48.1 (22.6/47) |
| SLN biopsy | Wound breakdown 11.7% Wound cellulitis 4.5% |
15.7 (41.4/264) | |
| Long term | IFL (with/without SLN biopsy) and RT | Lymphoedema 25.5% Recurrent erysipelas 30.6% |
48.3 (23.7/49) |
| IFL (with/without SLN biopsy) no RT | Lymphoedema 25.5% Recurrent erysipelas 5.9% |
29.9 (20.9/70) | |
| SLN biopsy | Lymphoedema 1.9% | 2.3 (6.1/264) | |
| Recurrent erysipelas 0.4% | – |
Cost and resource data
Three sources of data were used to parameterise the cost component of the economic evaluation: NHS Reference Costs 2009/2010,105 information provided by the Histology Department at Birmingham City Hospital and data collected as part of United Kingdom Gynaecological Oncology Surgical Outcomes and Complications audit (UKGOSOC). The UKGOSOC is a prospective web-based audit looking at outcomes of surgery in gynaecological oncology, particularly focused on complications. All costs in this study are presented in values for the year 2010. In all cases, patients only require a maximum of one unit of each cost depending on their treatment pathway, with the itemised costs shown in Table 46.
| Item | Code | Cost (£) | Reference | Assumption |
|---|---|---|---|---|
| Radical excision | MB01B | 1971 | UKGOSOC data | 3.86 bed-days |
| IFL (+ radical excision) | MA06Z | 4129 | UKGOSOC data | 5.64 bed-days |
| RT | SC22Z + SC56Z | 1728 | NHS reference costs105 | ‘Day case and regular day/night’ 3 weeks of treatment, 5 days each week (assumption) |
| Chemotherapy | SB12Z + SB15Z | 1270 | NHS reference costs105 | ‘Inpatient’. Assume drugs from regime in band 6 procurement + delivery £779 + £207 + £284 |
| Monitoring of patients | 503 | 171 | NHS reference costs105 | Per consultation |
| SLN biopsy (+ radical excision) | ||||
| Blue dye | MA06Z | 3574 | UKGOSOC data | 3.86 bed-days |
| 99mTc | MA06Z + RA36Z | 3836 | UKGOSOC data | 3.86 bed-days |
| Blue dye + 99mTc | MA06Z + RA36Z | 4219 | UKGOSOC data | 3.86 bed-days 10% greater than 99mTc (assumption) |
| Morbidity and mortality | ||||
| Short term | MA06Z | 1635 | NHS reference costs105 | 5.24 bed-days |
| Long term | MA06Z TPCTCLFUMFF 502 gynaecology |
702 | NHS reference costs105 | Three outpatient visits + 1 bed-day (assumed) |
| Vulval cancer related death | SD01A | 436 | NHS reference costs105 | Specialist palliative care, inpatient |
| Histopathology | ||||
| H&E | 74.50 | Histology Department Birmingham City Hospital | – | |
| Ultrastaging | 86.75 | Histology Department Birmingham City Hospital | – | |
All patients receive a radical excision of the primary vulval cancer which is administered along with either the SLN biopsy or IFL depending on the treatment pathway. The prices for the SLN biopsies and IFL include the cost of the radical excision; however, as a radical excision is also administered in the case of a vulval recurrence, a separate price is also given.
Costs are given for blue dye and 99mTc when administered separately; however, no cost was available for the two procedures combined and, therefore, it is assumed that the cost for both blue dye and 99mTc is 10% greater than 99mTc alone (the more expensive of the two). The impact of this assumption on model results is examined through sensitivity analysis.
Outcomes
The main focus of this economic evaluation is how the different treatment scenarios impact on the mortality and morbidity of the patients. Therefore, the following outcomes have been examined in this analysis:
-
case of death avoided within 2 years
-
case of morbidity-free survival within 2 years
-
case of long-term morbidity-free survival within 2 years.
In each case, these are compared again the comparison scenario of implementing an IFL to all patients.
As has been highlighted in the systematic reviews, there are no studies that have measured a generic QoL such as Euroqol EQ-5D in vulval cancer. Furthermore, the only study to measure global health status (QoL)82 showed no difference for patients who received the different procedures that are considered in this analysis. Therefore, the option of using the quality-adjusted life-year (QALY) as an outcome measure was not available in this economic evaluation.
The outcome measures in this study are considered to be reasonable given that the main focus of this study is whether or not a negative result from a SLN biopsy and no further treatment, with the possibility of recurrence, is preferable to the highly morbid IFL which has a lower risk of recurrence after the procedure.
Analysis
The model used in the economic evaluation begins with a hypothetical cohort of women who are presumed to have FIGO stage I or stage II vulval cancer. The model estimates the mean costs associated with each of the treatment strategies and the base case assumes that all women entering the model are aged 65 years. The time horizon of the model is 2 years, which was chosen as it was felt that any groin recurrences that might appear as a result of a FN SLN biopsy would be detected within this time frame. Owing to this short time horizon, and with the majority of costs occurring in the first year, no discounting was applied. This economic evaluation takes the form of a cost-effectiveness analysis and is carried out from the UK NHS perspective. Therefore, only direct costs and resources associated with the intervention and outcomes are incorporated in the analysis. The results of the analysis are presented in terms of incremental cost-effective ratios (ICERs) for each of the three outcome measures considered.
Sensitivity analysis
The results described by the cost-effectiveness point estimates do not consider any uncertainty in relation to the model input parameters. PSA was therefore undertaken to assess the impact of the uncertainty in the model parameters on the results and conclusions obtained from the model. The costs in the model are all unit costs for specific procedures and are treated as fixed; however, the number of bed-days was varied. The probabilities in the tree, these being the proportions of patients who follow each branch, were also varied.
The standard distribution used in this analysis for the proportions is the beta distribution (Table 47). The beta distribution is described by two parameters, a and b. A beta distribution is able to precisely represent the uncertainty in a proportion when the only available information is alpha-positive cases and beta-negative cases. In all cases, in this study, exact numbers were available and so these were used to inform the parameters of each beta distribution directly. The bed-days were described by a gamma distribution. The method of moments approach was used to estimate the parameters of the gamma distribution, where:
| Parameter | Distribution | Alpha | Beta |
|---|---|---|---|
| Patients with metastasis | Beta | 135 | 268 |
| Blue dye detection rate | Beta | 202 | 92 |
| 99mTc detection rate | Beta | 227 | 13 |
| Blue dye + 99mTc detection rate | Beta | 1050 | 25 |
| Negative predictive value of H&E + ultrastaging | Beta | 253 | 6 |
| Sensitivity of H&E | Beta | 80 | 55 |
| Local recurrence | Beta | 34 | 242 |
| Groin recurrence following IFL (no SLN biopsy) | Beta | 1 | 31 |
| Groin recurrence following positive SLN biopsy and IFL | Beta | 11 | 124 |
| Death following a local recurrence | Beta | 5 | 29 |
| Death following a groin recurrence | Beta | 9 | 2 |
| RT following IFL in the comparison arm | Beta | 26 | 30 |
| RT with IFL following a TP histopathology result | Beta | 49 | 68 |
| Short-term morbidity following IFL | Beta | 22.6 | 24.4 |
| Short-term morbidity following SLN biopsy | Beta | 41.4 | 222.6 |
| Long-term morbidity following IFL + RT | Beta | 23.7 | 25.3 |
| Long-term morbidity following IFL without RT | Beta | 20.9 | 49.1 |
| Long-term morbidity following SLN Biopsy | Beta | 6.1 | 257.9 |
| Bed-days following a primary excision/SLN biopsy | Gamma | 1.925 | 2.007 |
| Bed-days following a IFL | Gamma | 3.504 | 1.6103 |
The following one-way sensitivity analyses were also carried out.
-
Age of the cohort: as a baseline. It is assumed that the age of the cohort is 65 years. The impact of the alternative ages of 55 and 75 years is examined as the all-cause death rate of these groups may impact on the conclusions drawn from the model.
-
Increased mortality due to patient morbidity. It has been assumed in this study that the morbidity experienced by the patients at baseline has no impact on their overall survival. In this sensitivity analysis, this assumption is relaxed, and instead it is assumed that, for all pathways in which the patients experience morbidity, their death rate is increased by 20%. To balance this out, the death rates for pathways in which there is no morbidity are reduced by 20%. The purpose of this analysis is solely to illustrate how this assumption may impact on the final conclusions drawn from the model.
-
Varying the cost of implementing 99mTc and blue dye together. It is assumed that the cost of implementing 99mTc and blue dye together is 10% more than the price of 99mTc alone and is £4219. The impact of this assumption on model results is examined by instead assuming that implementing 99mTc and blue dye together is equal to the cost of 99mTc (£3836) and by assuming that it costs 50% more than 99mTc (£5754).
-
Groin recurrence rate following a negative SLN biopsy result. Van der Zee et al. 73 found that 6 out of 259 (2.3%) patients with unifocal disease experienced a groin recurrence following a negative SLN biopsy result. Owing to the size of this study, this was used as the baseline figure in this economic evaluation. However, the results from the systematic review suggest that this result may be slightly low (see Chapter 5, Recurrence rates, in the test accuracy systematic review). A study by Moore et al. 65 found that 2 out of 31 (6.5%) patients experienced a groin recurrence following a negative SLN biopsy. This value was not used at baseline owing to the small sample size and the lack of clarity as to whether or not the disease was unifocal. The impact of this alternative value on the model results is examined.
Results
The base-case deterministic results for the seven different treatment strategies are calculated based on the following outcomes additional cost per case of patient survival at 2 years, cost per case of morbidity-free survival at 2 years and cost per case of survival free of long-term morbidity at 2 years. Incremental cost-effectiveness analysis then follows.
Deterministic results: base case
Table 48 shows the deterministic results obtained from the model. This shows the cost of each treatment strategy and its effectiveness in terms of each of the three outcome measures.
| Strategy | Cost (£) | Overall survival at 2 years | Morbidity-free survival at 2 years | Survival free of long-term morbidity at 2 years |
|---|---|---|---|---|
| IFL | 9367 | 0.9645 | 0.3512 | 0.6423 |
| Blue dye + ultrastaging | 9775 | 0.9427 | 0.5241 | 0.7534 |
| Blue dye + H&E | 9826 | 0.8782 | 0.5015 | 0.7105 |
| 99mTc + ultrastaging | 10,175 | 0.9345 | 0.6054 | 0.7985 |
| 99mTc + H&E | 10,245 | 0.8457 | 0.5744 | 0.7395 |
| 99mTc + blue dye + ultrastaging | 10,576 | 0.9335 | 0.6151 | 0.8039 |
| 99mTc + blue dye + H&E | 10,648 | 0.8418 | 0.5830 | 0.7430 |
Outcomes
Overall survival at 2 years following IFL was found to be the most effective strategy. This result is not surprising given that this procedure seeks to reduce the potential for future recurrences at the expensive of increased patient morbidity. For all types of morbidity-free survival, the 99mTc + blue dye + ultrastaging strategy was found to be the most effective. Again this is not a surprising result given that this uses the most robust procedures for identifying both the SLN (highest detection rate) and metastasis (highest sensitivity).
Costs
The IFL strategy was found to be the cheapest, costing £9367 per woman treated for presumed type I or type II vulval cancer. The most expensive was found to be the 99mTc + blue dye + H&E strategy, costing £10,648 per patient. Although this is not the most expensive treatment to administer, it is likely that these costs are due to extra costs associated with undetected recurrences that will occur with this type of treatment regimen.
Incremental analysis
A strategy is dominated by an alternative strategy if it is more expensive and less effective. It is not normally necessary to consider dominated strategies since they are supplanted by other strategies that are more cost-effective. The analysis below describes the treatment pathways that are dominated and, then, an incremental analysis is undertaken for the remaining strategies.
Results: overall survival at 2 years outcome
As can be seen in Figure 12 for the overall survival at 2 years outcome, the IFL strategy dominates all other strategies as it is both less expensive and averts the greatest mortality. Therefore, a further incremental analysis for overall survival for the baseline deterministic results is not undertaken.
FIGURE 12.
Cost-effectiveness plane showing the results of the deterministic analysis for the seven strategies with overall survival at 2 years as the outcome measure.
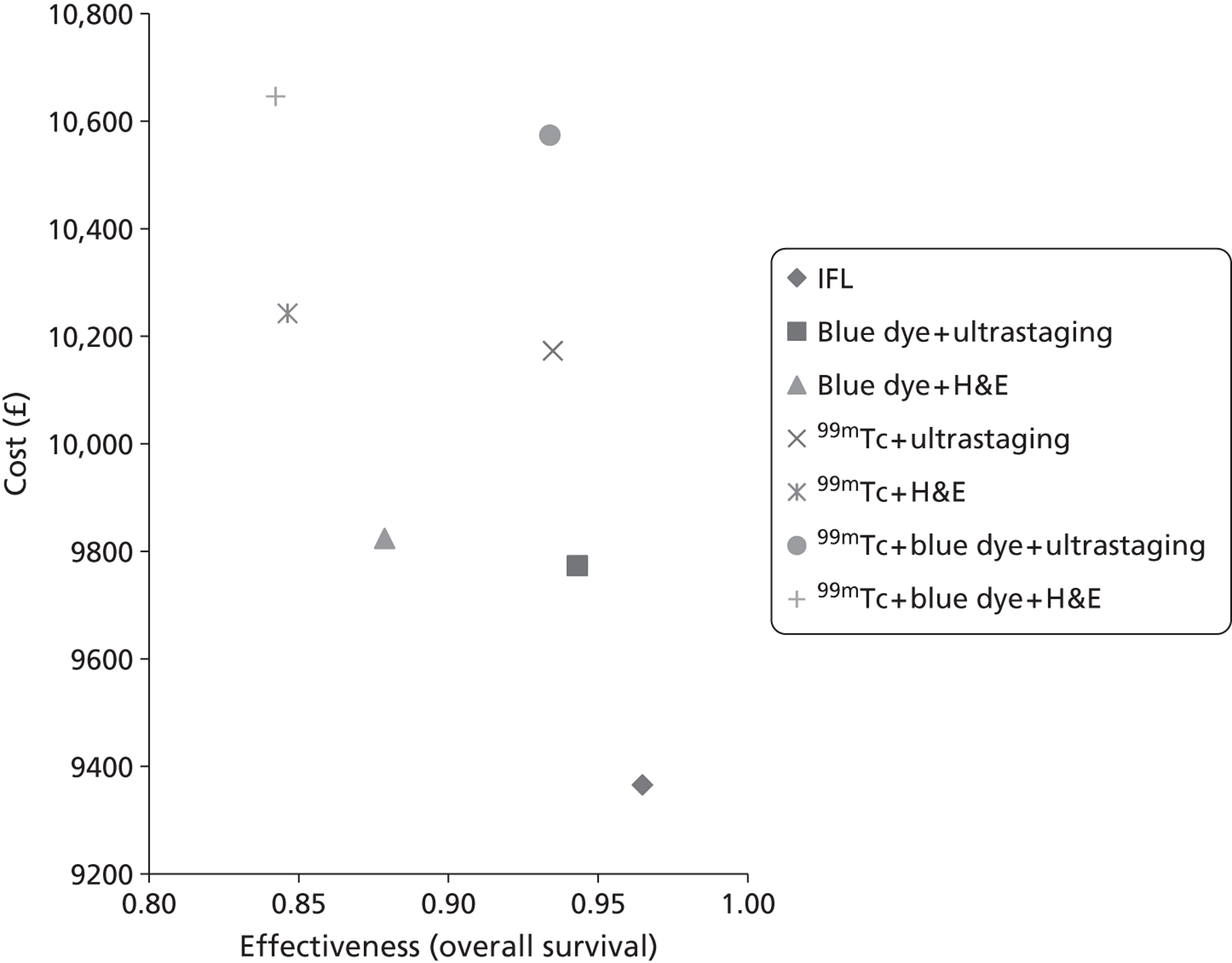
Results: overall morbidity-free survival at 2 years outcome
For the outcome of morbidity-free survival at 2 years, only the strategies of blue dye + ultrastaging, 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging remain undominated by any of the alternative treatment strategies. Table 49 presents the deterministic analysis for the morbidity-free survival at 2 years outcome restricted to the non-dominated competing strategies.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness | Incremental effectiveness | ICER (£) |
|---|---|---|---|---|---|
| IFL | 9367 | – | 0.3512 | – | – |
| Blue dye + ultrastaging | 9775 | 408 | 0.5241 | 0.1729 | 2400 |
| 99mTc + ultrastaging | 10,175 | 400 | 0.6054 | 0.0813 | 4900 |
| 99mTc + blue dye + ultrastaging | 10,576 | 400 | 0.6151 | 0.0097 | 41,200 |
In terms of morbidity-free survival at 2 years, the least expensive strategy is the base-case scenario of IFL. The most effective strategy is the 99mTc + blue dye + ultrastaging strategy, but this comes at a greater cost, generating an ICER of £41,200, i.e. the strategy requires an investment of £41,200 to generate one additional case of morbidity-free survival compared with the strategy of 99mTc + ultrastaging. The strategy of 99mTc + ultrastaging is both slightly less effective in terms of overall morbidity-free survival and slightly less costly than 99mTc + blue dye + ultrastaging. The ICER for 99mTc + ultrastaging is approximately £4900, i.e. a financial outlay of £4900 is necessary to generate one additional case of morbidity-free survival compared with the strategy of the blue dye + ultrastaging. The ICER for blue dye + ultrastaging is approximately £2400, i.e. a financial outlay of £2400 is necessary to generate one additional case of morbidity-free survival compared with the strategy of IFL.
Figure 13 shows the total costs and the effectiveness in terms of morbidity-free survival for the different treatment strategies considered in this analysis. The line on the graph joins the non-dominated strategies of blue dye + ultrastaging, 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging. Any strategy that appears above this line is not considered cost-effective in relation to the non-dominated alternatives.
FIGURE 13.
Cost-effectiveness plane showing the results of the deterministic analysis for the seven strategies with morbidity-free survival at 2 years as the outcome measure.
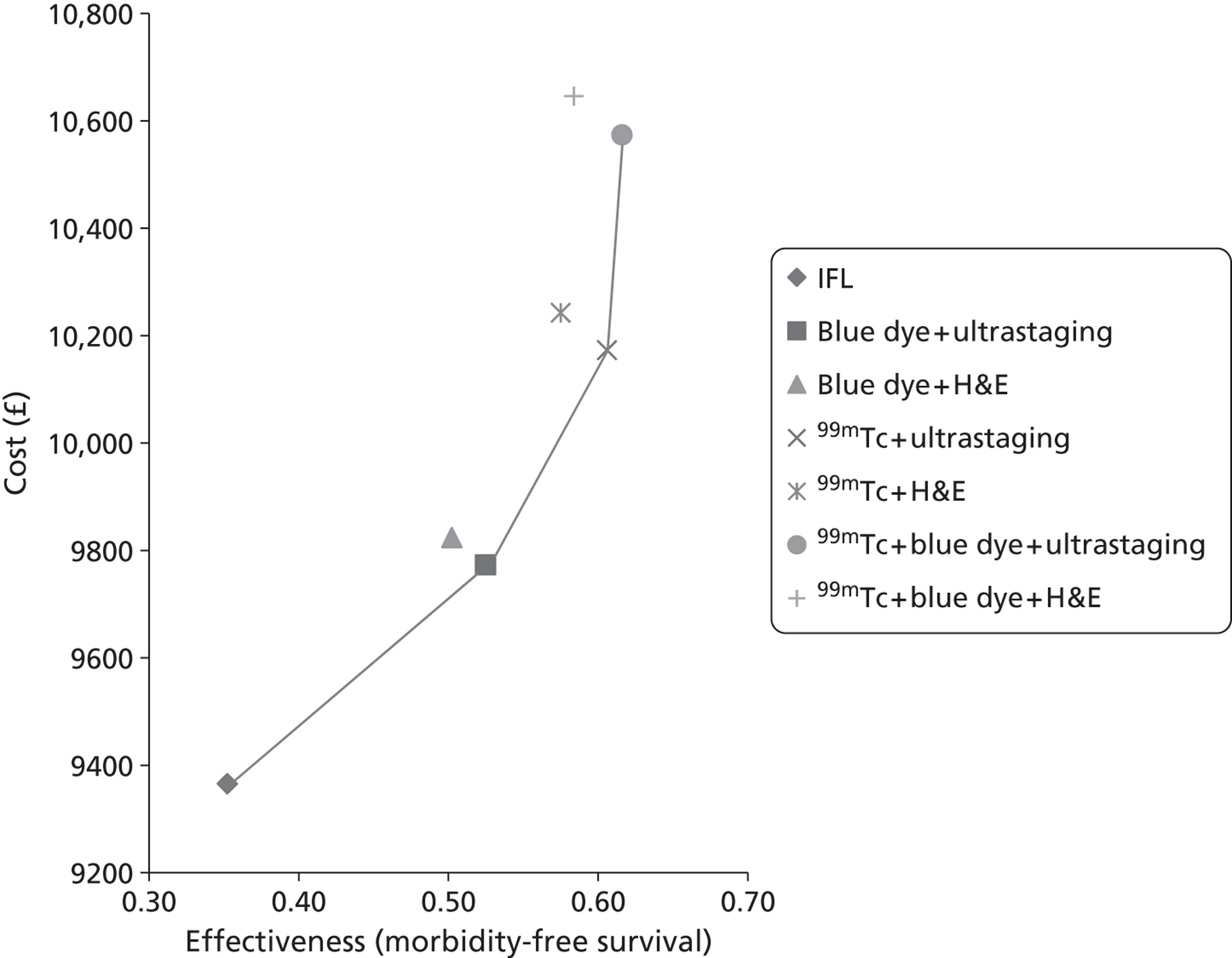
It is noted that the strategies blue dye + H&E, 99mTc + H&E and 99mTc + blue dye + H&E are sufficiently close to the boundary of dominance that the impact of parameter uncertainty on the model results between these dominated alternatives should be examined in addition to the non-dominated options.
Results: long-term morbidity-free survival at 2 years outcome
The dominance seen for morbidity-free survival at 2 years is repeated for long-term morbidity-free survival at 2 years in that the strategies of blue dye + ultrastaging, 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging remain undominated by any of the alternative treatment strategies (Figure 14).
FIGURE 14.
Cost-effectiveness plane showing the results of the deterministic analysis for the seven strategies with long-term morbidity-free survival at 2 years as the outcome measure.
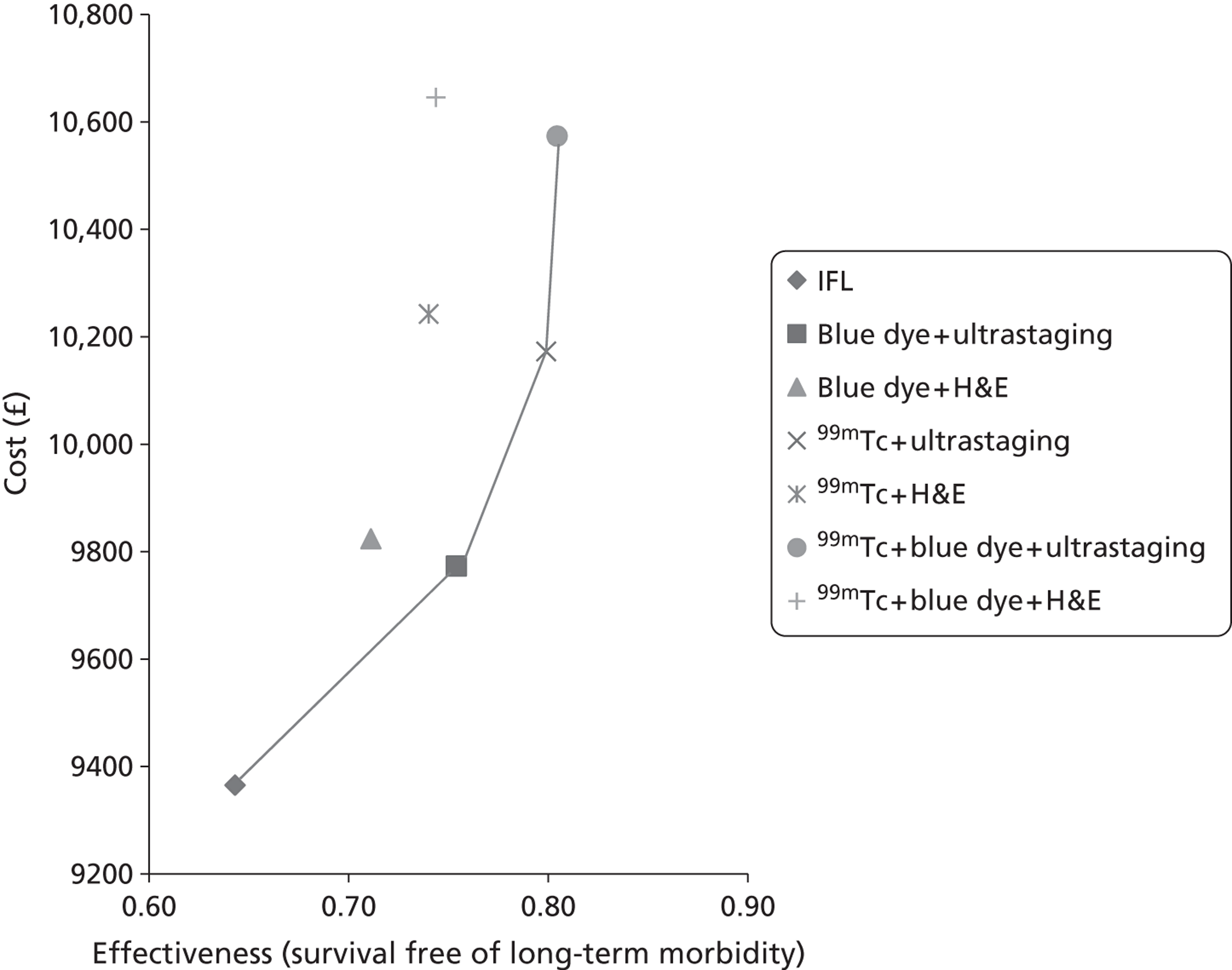
Considering the outcome measure to be long-term morbidity-free survival at 2 years does nothing to change the costs of the different strategies and so the cheapest strategy is still the base-case scenario of IFL (Table 50). The most effective strategy is the 99mTc + blue dye + ultrastaging strategy, but this comes at a greater cost, generating an ICER of £74,300, i.e. the strategy requires an investment of £74,300 to generate one additional case of long-term morbidity-free survival compared with the strategy of 99mTc + ultrastaging. The strategy of 99mTc + ultrastaging is both slightly less effective in terms of overall long-term morbidity-free survival and slightly less costly than 99mTc + blue dye + ultrastaging. The ICER for 99mTc + ultrastaging is approximately £8900, i.e. an additional financial outlay of £8900 is necessary to generate one case of long-term morbidity-free survival compared with the strategy of blue dye + ultrastaging. The strategy of blue dye + ultrastaging is again both slightly less effective in terms of overall long-term morbidity-free survival and slightly less costly than 99mTc + ultrastaging. The ICER for blue dye + ultrastaging is approximately £3700, i.e. an additional financial outlay of £3700 is necessary to generate one case of long-term morbidity-free survival compared with the strategy of IFL.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness | Incremental effectiveness | ICER (£) |
|---|---|---|---|---|---|
| IFL | 9367 | 0.6423 | |||
| Blue dye + ultrastaging | 9775 | 408 | 0.7534 | 0.1111 | 3700 |
| 99mTc + ultrastaging | 10,175 | 400 | 0.7985 | 0.0451 | 8900 |
| 99mTc + blue dye + ultrastaging | 10,576 | 400 | 0.8039 | 0.0054 | 74,300 |
Figure 14 shows the total costs and the effectiveness in terms of long-term morbidity-free survival at 2 years for the different treatment strategies considered in this analysis. The line on the graph joins the non-dominated strategies of blue dye + ultrastaging, 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging. Any strategy that appears above this line is not considered cost-effective in relation to the non-dominated alternatives.
Once again, it is noted that the strategies blue dye + H&E, 99mTc + H&E and 99mTc + blue dye + H&E are sufficiently close to the boundary of dominance that the impact of parameter uncertainty on the model results between these dominated alternatives should be examined in addition to the non-dominated options.
Probabilistic sensitivity analysis
The PSA is undertaken to assess the impact of the uncertainty in the model parameters on the results and conclusions obtained from the model. As with the deterministic analysis, the outcome measures of the overall survival at 2 years, morbidity-free survival at 2 years and survival free long-term morbidity at 2 years are considered.
Probabilistic sensitivity analysis: overall survival at 2 years outcome
Figure 15 illustrates the overall uncertainty in the model results with the outcome measure being overall survival at 2 years. It is clear from the degree of overlap of the results obtained from the different treatment strategies that there is uncertainty regarding which one may be considered most cost-effective when a range of values is sampled from the distributions that describe the data values. This output is therefore used to examine the overall uncertainty related to the optimal decision across a range of plausible willingness-to-pay (WTP) values, in which, for this outcome, the WTP is measured in pounds per additional case of survival achieved.
FIGURE 15.
Scatterplot showing the uncertainty in costs and effectiveness within the model for each of the seven strategies for 1000 runs with overall survival at 2 years as the outcome measure.

Figure 16 shows the cost-effectiveness acceptability frontier (CEAF) for the outcome of additional case of survival achieved and is generated as follows. First, for any value of the WTP, the optimal solution is obtained based on the mean results. Then the proportion of model replications for which that was the optimal solution was found and plotted. By definition, only the strategies that have been shown not to be dominated can appear on the CEAF. For the cost per case of survival achieved outcome, it can be seen that IFL is the optimal treatment strategy for all values of the WTP up to (and beyond) £100,000.
FIGURE 16.
Cost-effectiveness acceptability frontier showing the results of the sensitivity analysis examining the optimal investigative strategy across a range of WTP thresholds for the outcome of case of survival at 2 years achieved.
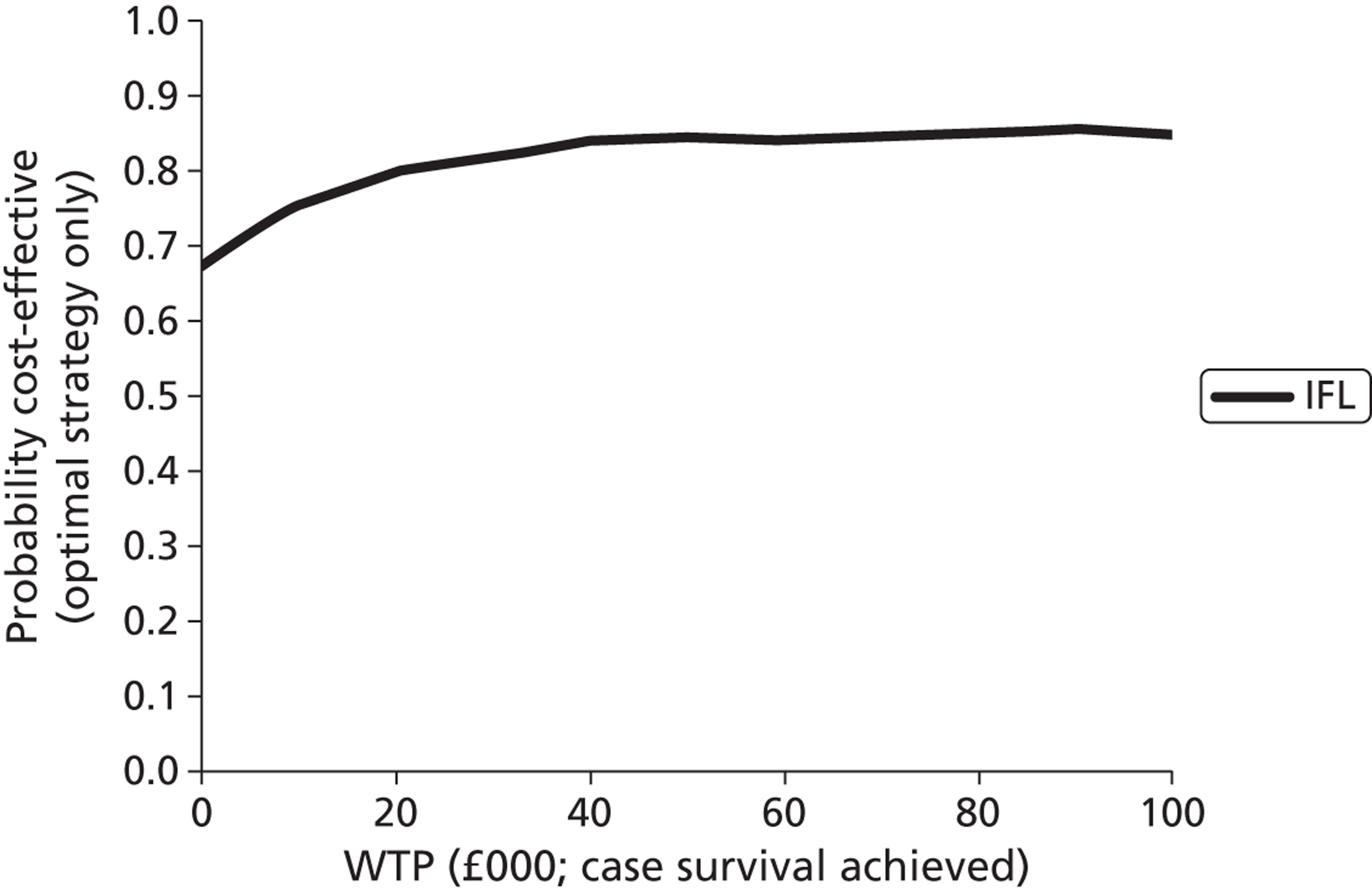
Probabilistic sensitivity analysis: morbidity-free survival outcome
Figure 17 illustrates the overall uncertainty in the model results for the outcome measure of morbidity-free survival at 2 years. Once again it is clear from the degree of overlap in the results obtained from the different treatment strategies that there is uncertainty regarding which one might be considered most cost-effective when a range of values is sampled from the distributions that describe the data values. This output is therefore used to examine the overall uncertainty related to the optimal decision across a range of plausible WTP values, in which, for this outcome, the WTP is measured in pounds per additional case of morbidity-free survival achieved.
FIGURE 17.
Scatterplot showing the uncertainty in costs and effectiveness within the model for each of the seven strategies for 1000 runs with morbidity-free survival at 2 years as the outcome measure.
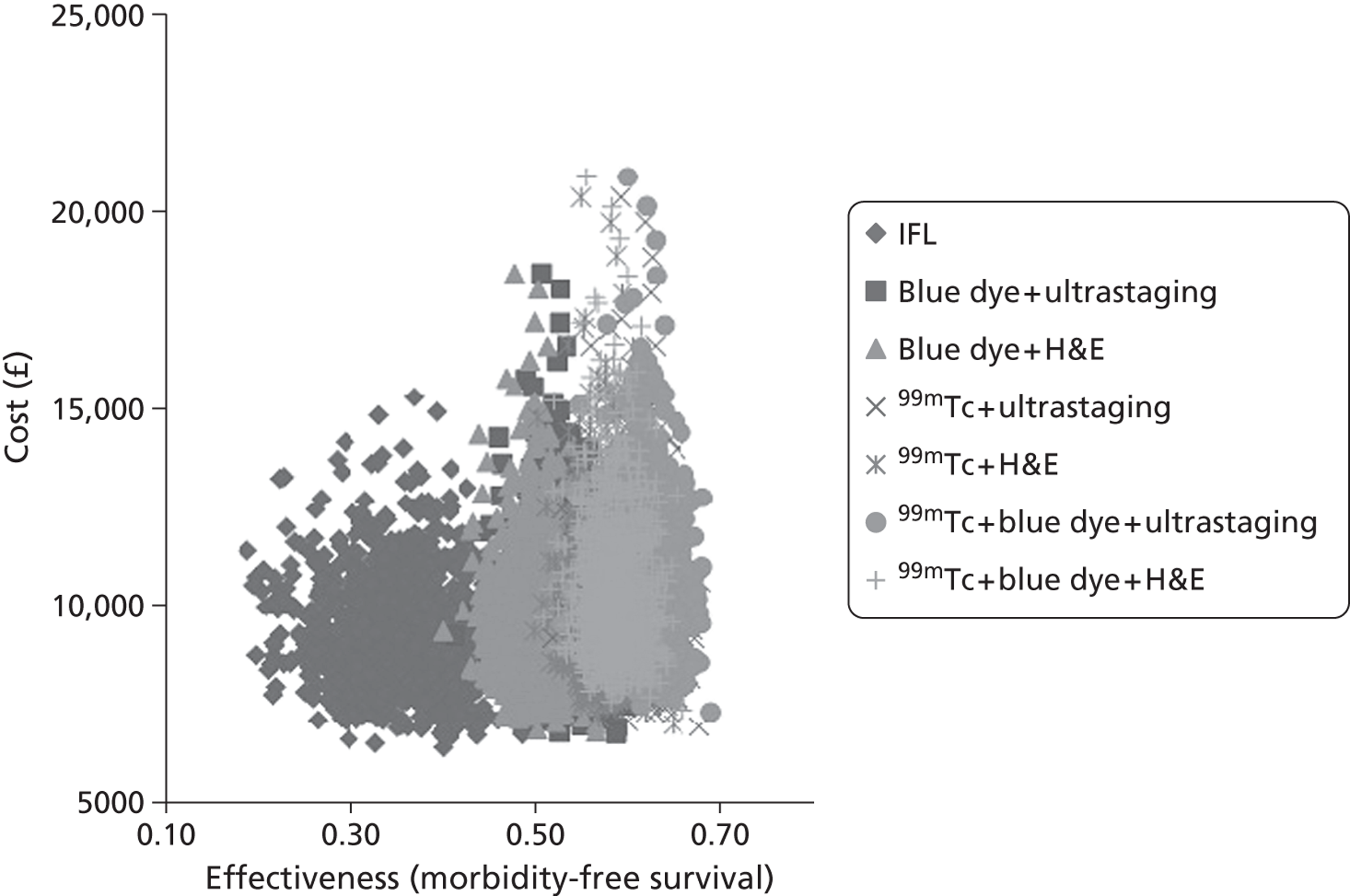
Using the results shown in Figure 17, which illustrate the overall uncertainty in the model results with the outcome measure being morbidity-free survival at 2 years, Figure 18 shows the CEAF for the outcome of additional case of morbidity averted. It can be seen that as the WTP crosses the ICER between two non-dominated strategies, the choice of optimal strategy changes, with a discontinuity in the curve being seen. Up to a WTP of £3500 the IFL strategy is the most cost-effective and, then, from £3500 to approximately £42,000 the 99mTc + ultrastaging strategy is the most cost-effective and, finally, for a WTP greater than approximately £42,000 the 99mTc + blue dye + ultrastaging strategy becomes the most cost-effective.
FIGURE 18.
Cost-effectiveness acceptability frontier showing the results of the sensitivity analysis examining the optimal treatment strategy across a range of WTP thresholds for the outcome of additional case of morbidity averted at 2 years.
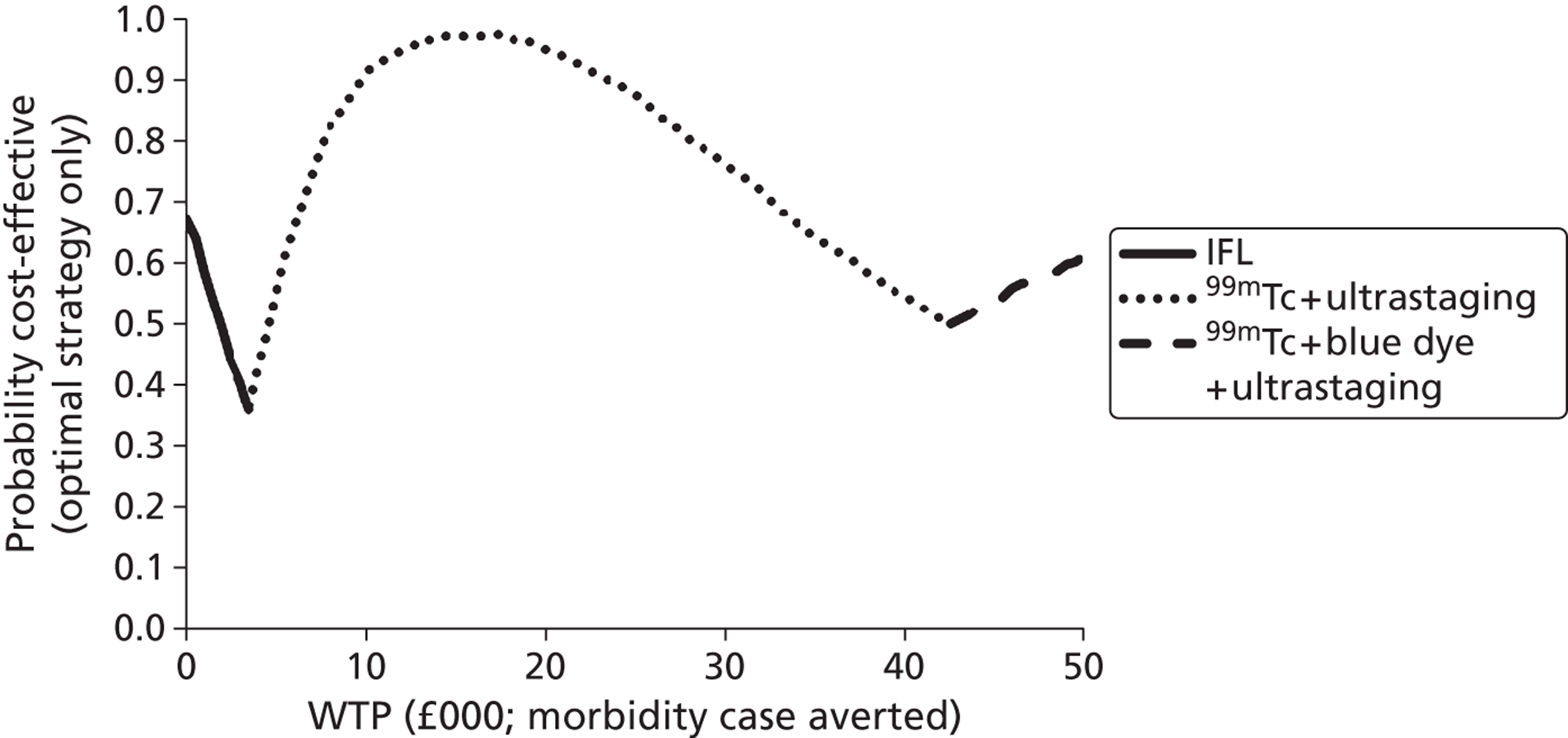
Probabilistic sensitivity analysis: long-term morbidity-free survival outcome
Using the results shown in Figure 19, which illustrates the overall uncertainty in the model results with the outcome measure being long-term morbidity-free survival at 2 years, Figure 20 shows the CEAF for the outcome of an additional case of long-term morbidity averted. It can be seen that for a WTP of less than approximately £5000 the IFL strategy is the most cost-effective. For a WTP in the range from £5000 to £77,500 the 99mTc + ultrastaging strategy is the most cost-effective and, then, for WTP values greater than £77,500 the 99mTc + blue dye + ultrastaging strategy is the most cost-effective.
FIGURE 19.
Scatterplot showing the uncertainty in costs and effectiveness within the model for each of the seven strategies for 1000 runs with long-term morbidity-free survival at 2 years as the outcome measure.
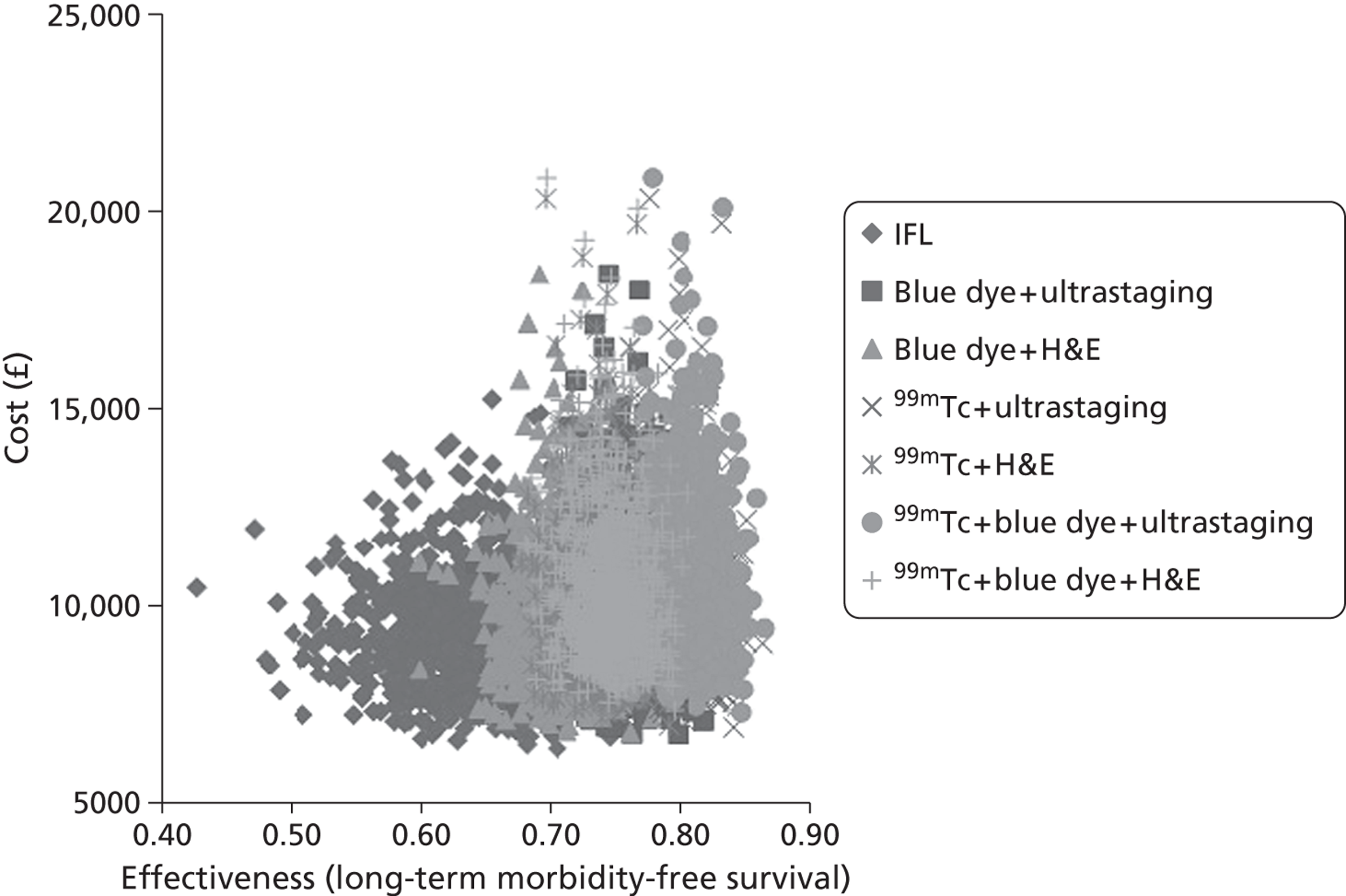
FIGURE 20.
Cost-effectiveness acceptability frontier showing the results of the sensitivity analysis examining the optimal investigative strategy across a range of WTP thresholds for the outcome of additional case of long-term morbidity averted at 2 years.
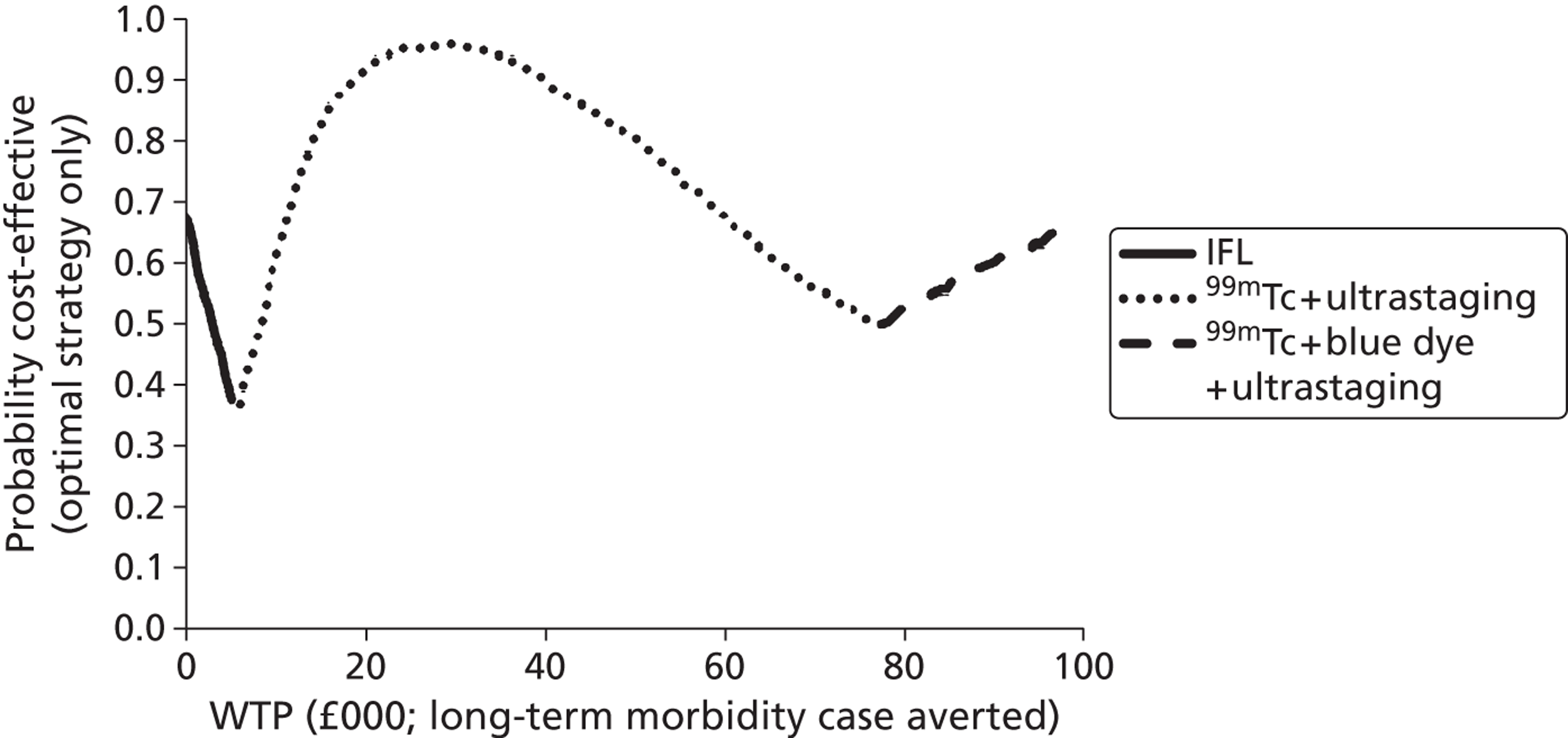
Incremental analysis
Bilateral comparisons were carried out for the two morbidity outcomes, as these were thought to be the most informative, with the outcome for overall survival being excluded from this incremental analysis.
1. Inguinofemoral lymphadenectomy versus blue dye + ultrastaging
Figure 21 shows the modelled uncertainty in the differences in the costs and effectiveness between IFL and blue dye + ultrastaging for morbidity-free survival at 2 years. It shows that blue dye + ultrastaging may or may not increase the cost but will also certainly increase the effectiveness in terms of morbidity-free survival. Figure 22 shows the proportion of model replications for which blue dye + ultrastaging is preferred to IFL. Blue dye+ ultrastaging is the preferred option at any WTP over £2000 although there is considerable uncertainty at any WTP around this figure. However, by the time the WTP exceeds £12,000, it is almost certain that blue dye + ultrastaging is preferred to IFL.
FIGURE 21.
Cost-effectiveness plane: IFL vs. blue dye + ultrastaging for the morbidity-free survival at 2 years outcome measure.
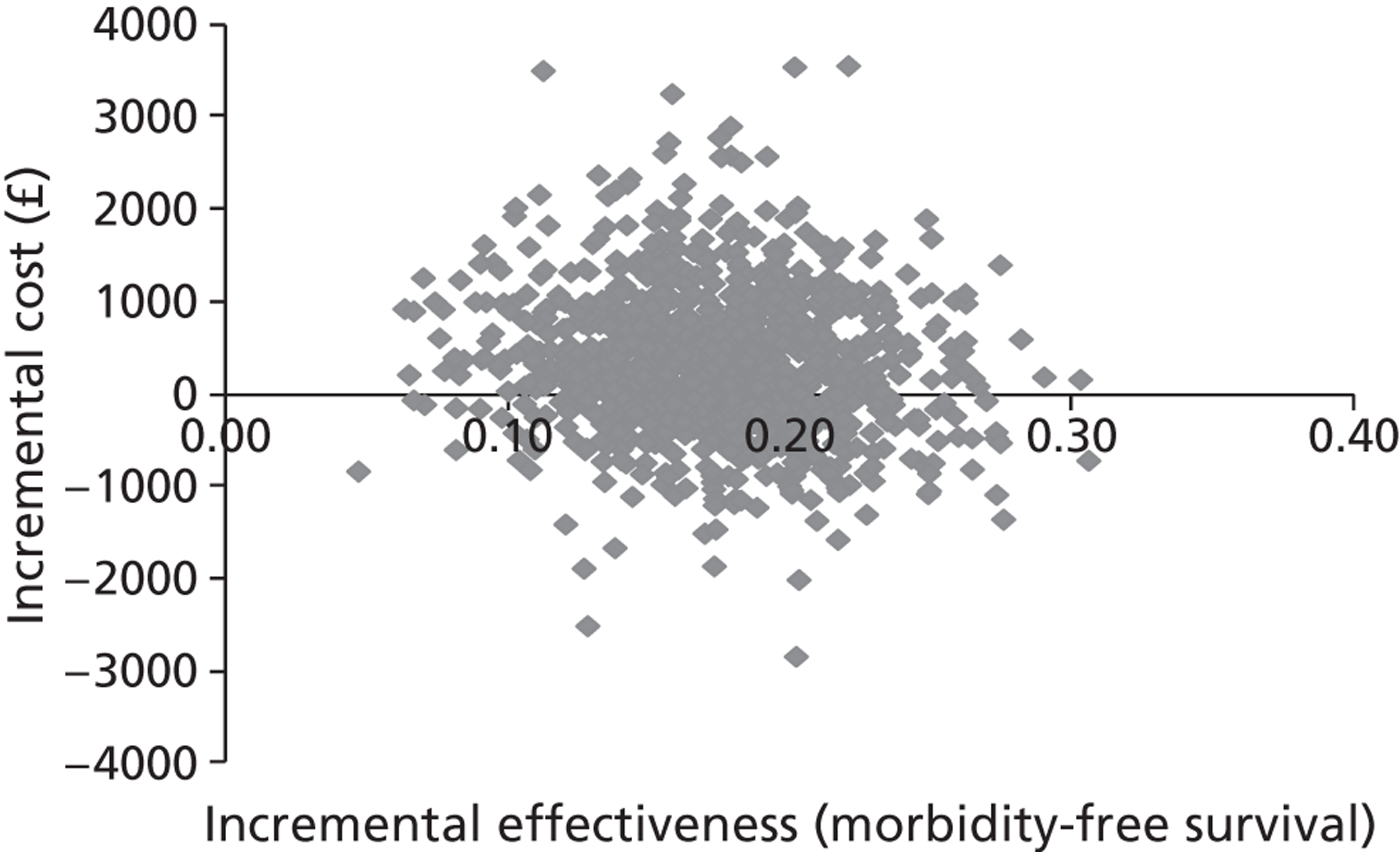
FIGURE 22.
Cost-effectiveness acceptability curve: IFL vs. blue dye + ultrastaging for the case of morbidity averted at 2 years outcome measure.
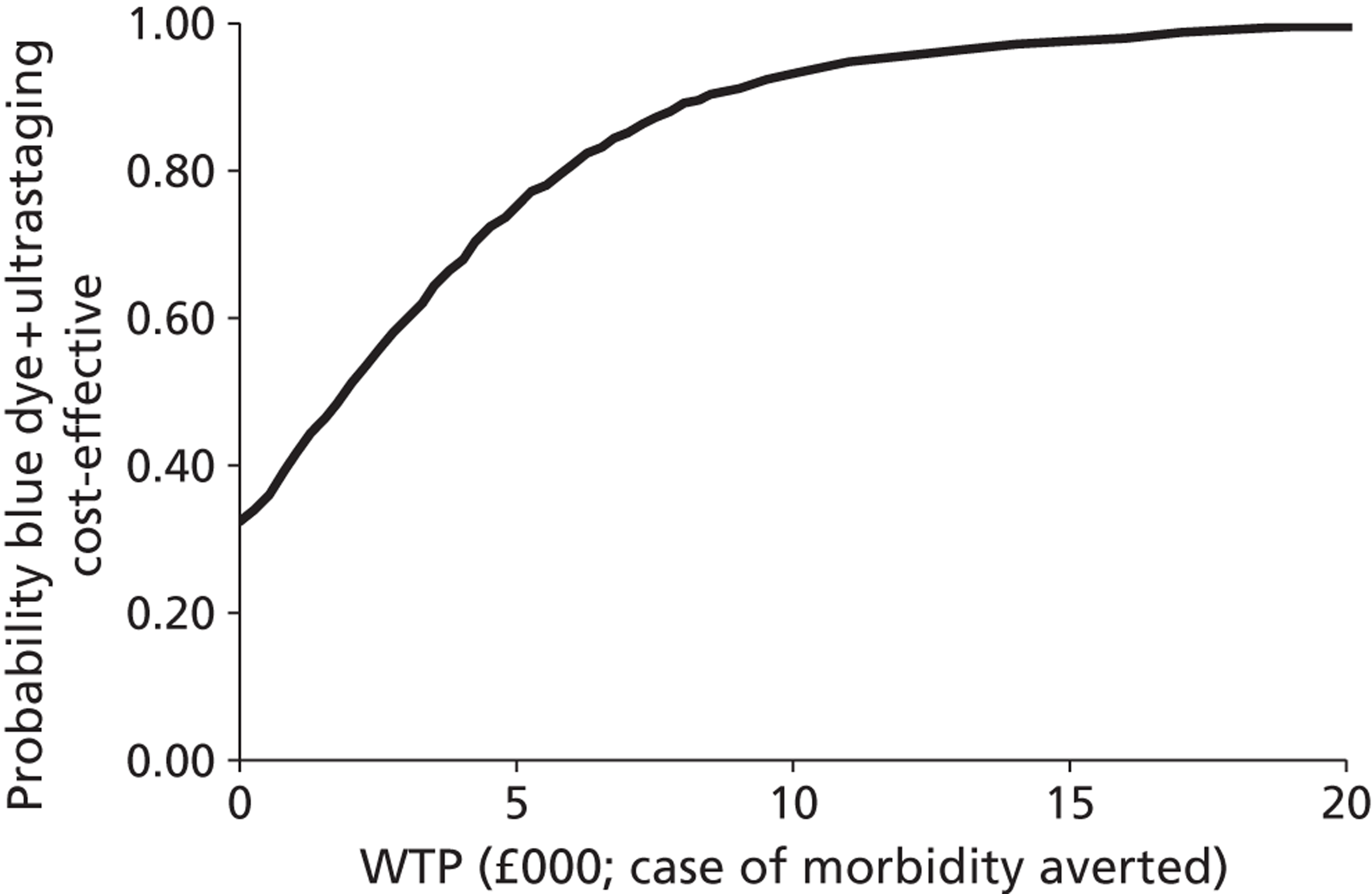
Figure 23 shows the modelled uncertainty in the differences in the costs and effectiveness between IFL and blue dye + ultrastaging for the long-term morbidity-free survival at 2 years outcome measure. It shows that blue dye + ultrastaging may or may not increase the cost but will also certainly increase the effectiveness in terms of long-term morbidity-free survival.
FIGURE 23.
Cost-effectiveness plane: IFL vs. blue dye + ultrastaging for the long-term morbidity-free survival at 2 years outcome measure.
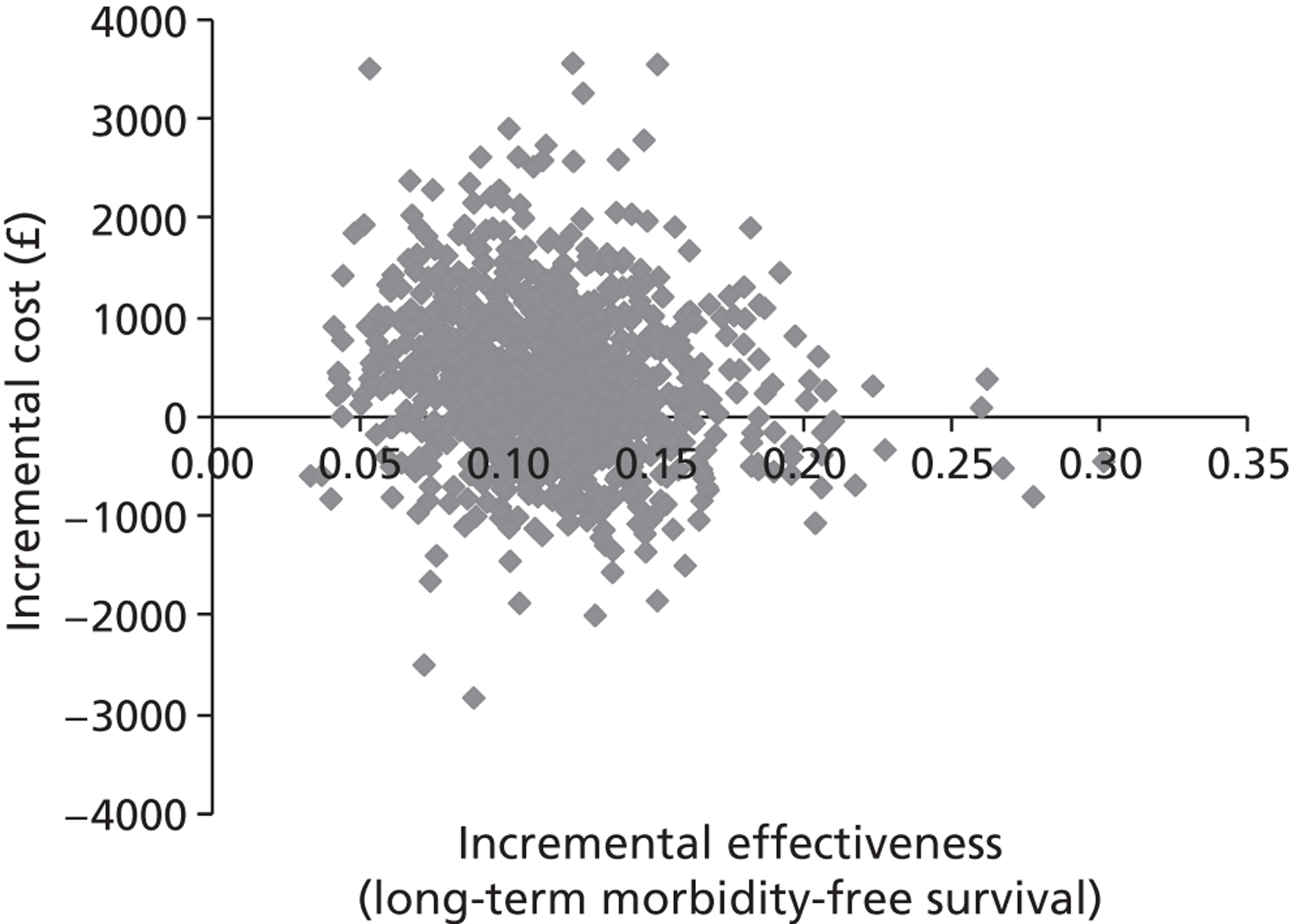
Figure 24 shows the proportion of model replications for which blue dye + ultrastaging is preferred to IFL for the case of long-term morbidity averted outcome measure. Blue dye + ultrastaging is the preferred option at any WTP over £3000, although there is considerable uncertainty around this figure. However, by the time the WTP exceeds £18,000, it is almost certain that 99mTc + ultrastaging is preferred to IFL.
FIGURE 24.
Cost-effectiveness acceptability curve: IFL vs. blue dye + ultrastaging for the case of long-term morbidity averted at 2 years outcome measure.
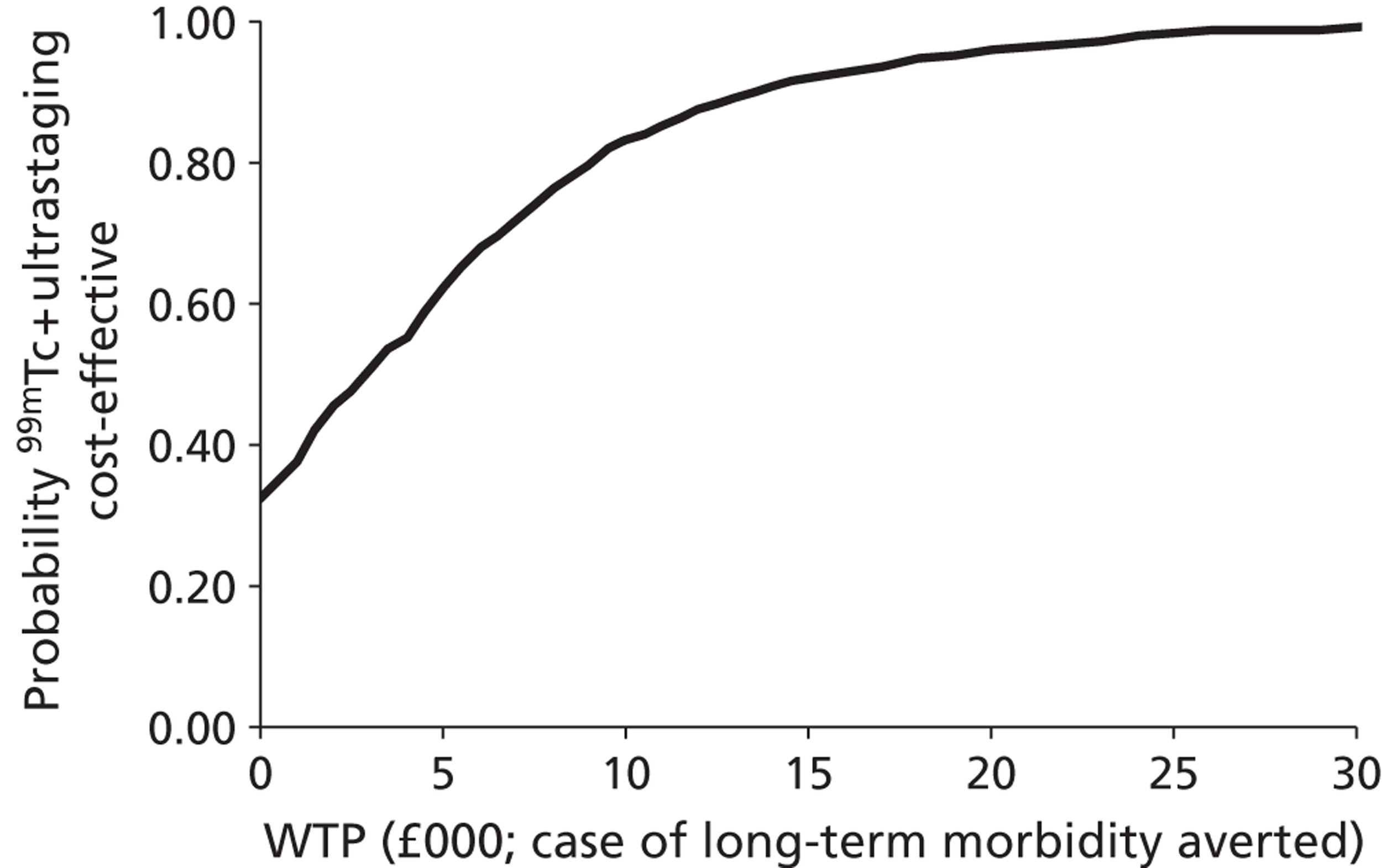
2. Blue dye + ultrastaging versus 99mTc + ultrastaging
Figure 25 shows the modelled uncertainty in the difference in costs between blue dye + ultrastaging and 99mTc + ultrastaging for the outcome measure of morbidity-free survival at 2 years. This shows that 99mTc + ultrastaging when compared with blue dye + ultrastaging will, on most occasions, increase the cost and will certainly increase the effectiveness in averting cases of morbidity. Figure 26 shows the proportion of model replications for which 99mTc + ultrastaging is preferred to blue dye + ultrastaging at any given WTP per case of morbidity averted. It is more likely than not that 99mTc + ultrastaging is cost-effective compared with blue dye + ultrastaging above a WTP threshold of around £5000. At a WTP greater than £12,000 it is almost certain that 99mTc + ultrastaging will be preferred to blue dye + ultrastaging.
FIGURE 25.
Cost-effectiveness plane: blue dye + ultrastaging vs. 99mTc + ultrastaging with morbidity-free survival at 2 years as the outcome measure.
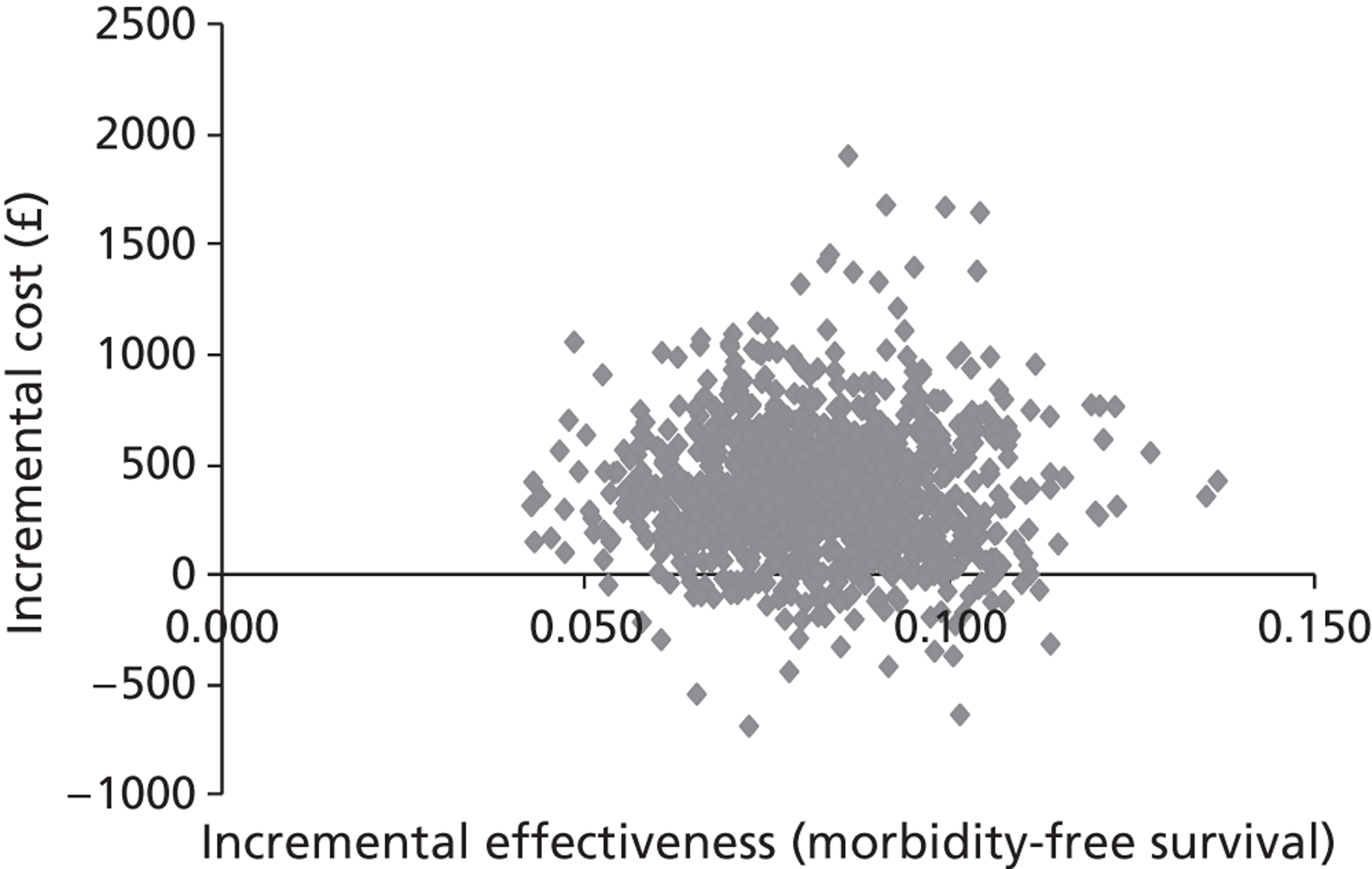
FIGURE 26.
Cost-effectiveness acceptability curve: blue dye + ultrastaging vs. 99mTc + ultrastaging with case of morbidity averted at 2 years as the outcome measure.
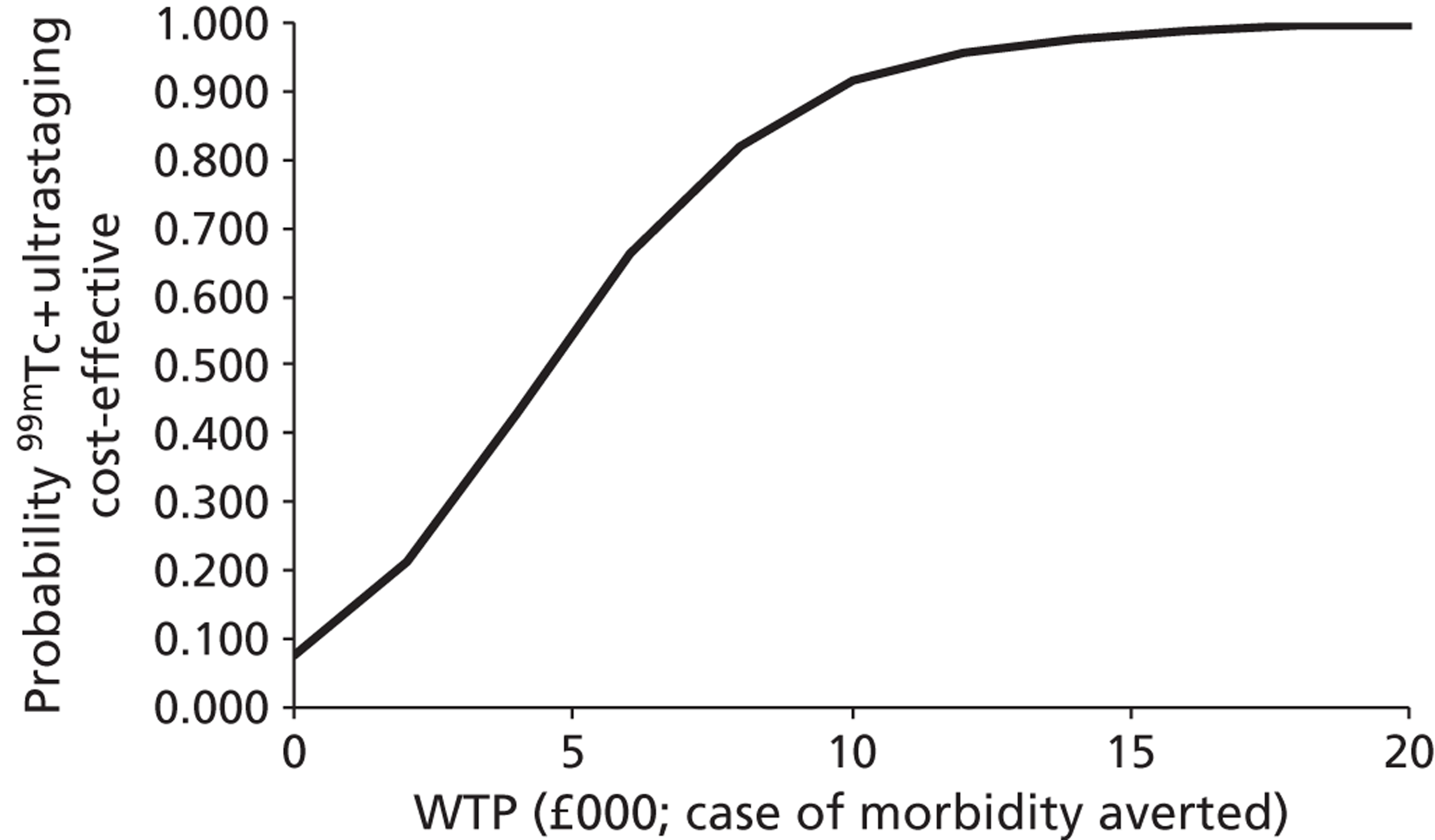
Figure 27 shows the modelled uncertainty in the difference in costs between blue dye + ultrastaging and 99mTc + ultrastaging for the outcome measure of long-term morbidity-free survival at 2 years. It shows that the 99mTc + ultrastaging when compared with the blue dye + ultrastaging is more likely than not to increase the cost and will certainly increase the effectiveness in averting cases of long-term morbidity. Figure 28 shows the proportion of model replications for which 99mTc + ultrastaging is preferred to blue dye + ultrastaging at any given WTP per case of long-term morbidity averted. It is more likely than not that 99mTc + ultrastaging is cost-effective compared with blue dye + ultrastaging above a WTP threshold of around £9000. At a WTP greater than £23,000 it is almost certain that 99mTc + ultrastaging will be preferred to blue dye + ultrastaging.
FIGURE 27.
Cost-effectiveness plane: blue dye + ultrastaging vs. 99mTc + ultrastaging with long-term morbidity-free survival at 2 years as the outcome measure.

FIGURE 28.
Cost-effectiveness acceptability curve: blue dye + ultrastaging vs. 99mTc + ultrastaging with case of long-term morbidity averted at 2 years as the outcome measure.

3. 99mTc + ultrastaging versus 99mTc + blue dye + ultrastaging
Figure 29 shows the modelled uncertainty in the differences in the costs and effectiveness between 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging for morbidity-free survival at 2 years. It shows that 99mTc + blue dye + ultrastaging will always increase the cost and will almost always increase the effectiveness in terms of morbidity-free survival. Figure 30 shows the proportion of model replications for which 99mTc + blue dye + ultrastaging is preferred to 99mTc + ultrastaging. 99mTc + blue dye+ ultrastaging is the preferred option at any WTP over £45,000, although there is considerable uncertainty at any WTP around this figure. Even when the WTP reaches £100,000, it still cannot be said that 99mTc + blue dye + ultrastaging is certainly preferred to 99mTc + ultrastaging.
FIGURE 29.
Cost-effectiveness plane: 99mTc + ultrastaging vs. 99mTc blue dye + ultrastaging with morbidity-free survival at 2 years as the outcome measure.
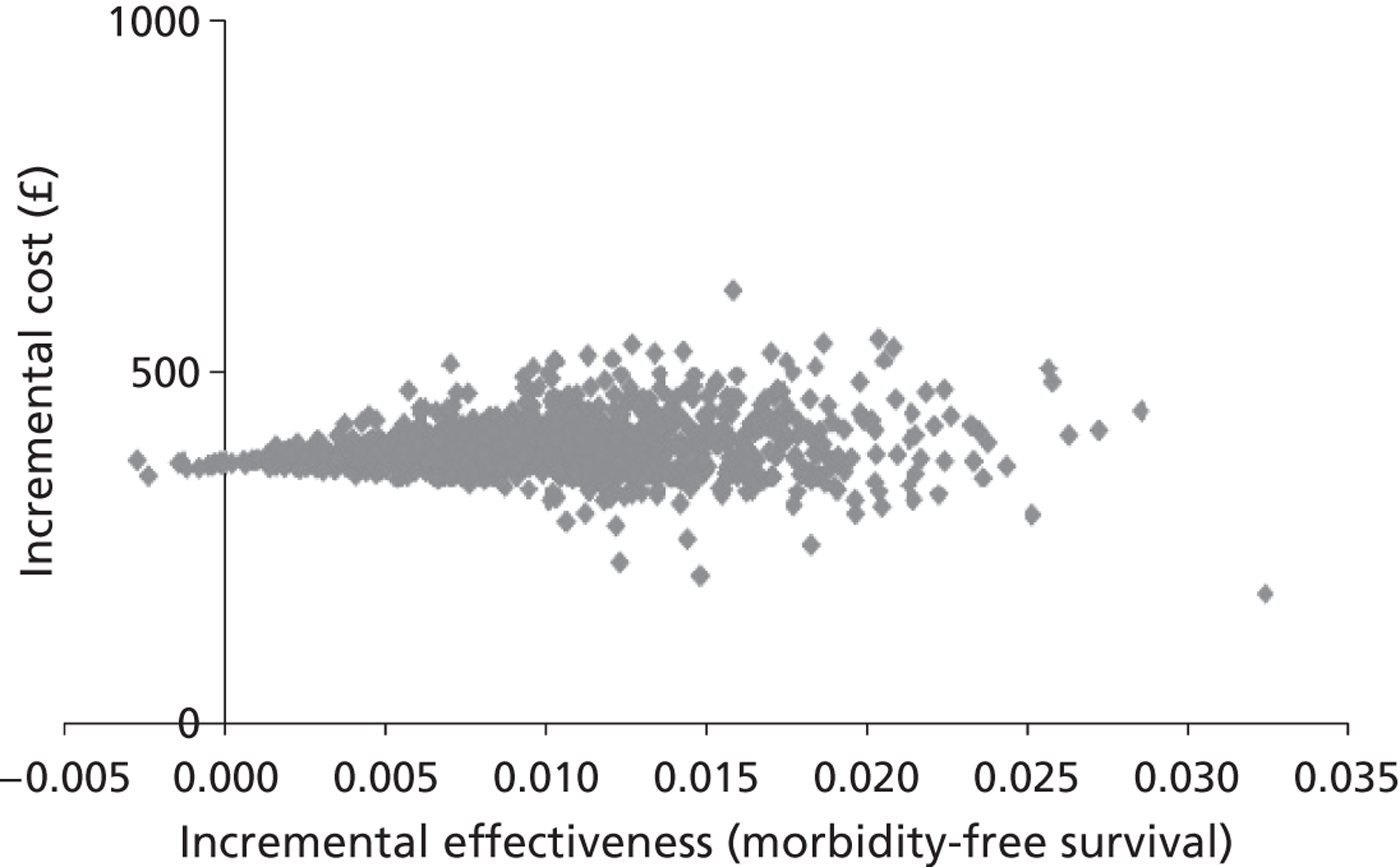
FIGURE 30.
Cost-effectiveness acceptability curve: 99mTc + ultrastaging vs. 99mTc + blue dye + ultrastaging with case of morbidity averted at 2 years as the outcome measure.
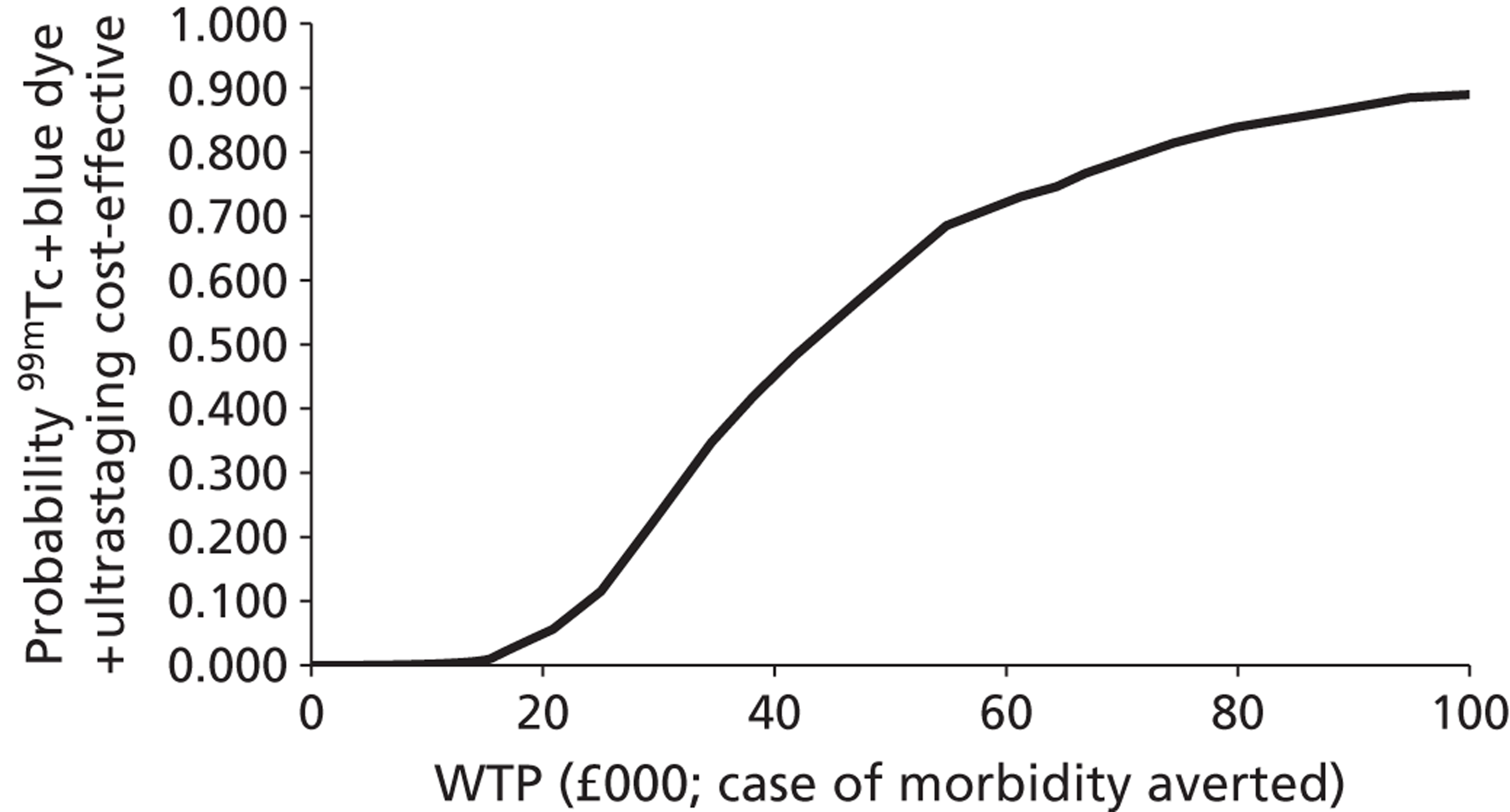
Figure 31 shows the modelled uncertainty in the differences in the costs and effectiveness between 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging for long-term morbidity-free survival. It shows that 99mTc + blue dye + ultrastaging will always increase the cost and will almost certainly increase the effectiveness in terms of long-term morbidity-free survival at 2 years. Figure 32 shows the proportion of model replications for which 99mTc + blue dye + ultrastaging is preferred to 99mTc + ultrastaging in terms of long-term morbidity-free survival. 99mTc + blue dye+ ultrastaging is the preferred option at any WTP over £75,000, although there is considerable uncertainty at any WTP around this figure. Even when the WTP reaches £100,000, it still cannot be said that 99mTc + blue dye + ultrastaging is certainly preferred to 99mTc + ultrastaging.
FIGURE 31.
Cost-effectiveness plane: 99mTc + ultrastaging vs. 99mTc + blue dye + ultrastaging with long-term morbidity-free survival at 2 years as the outcome measure.
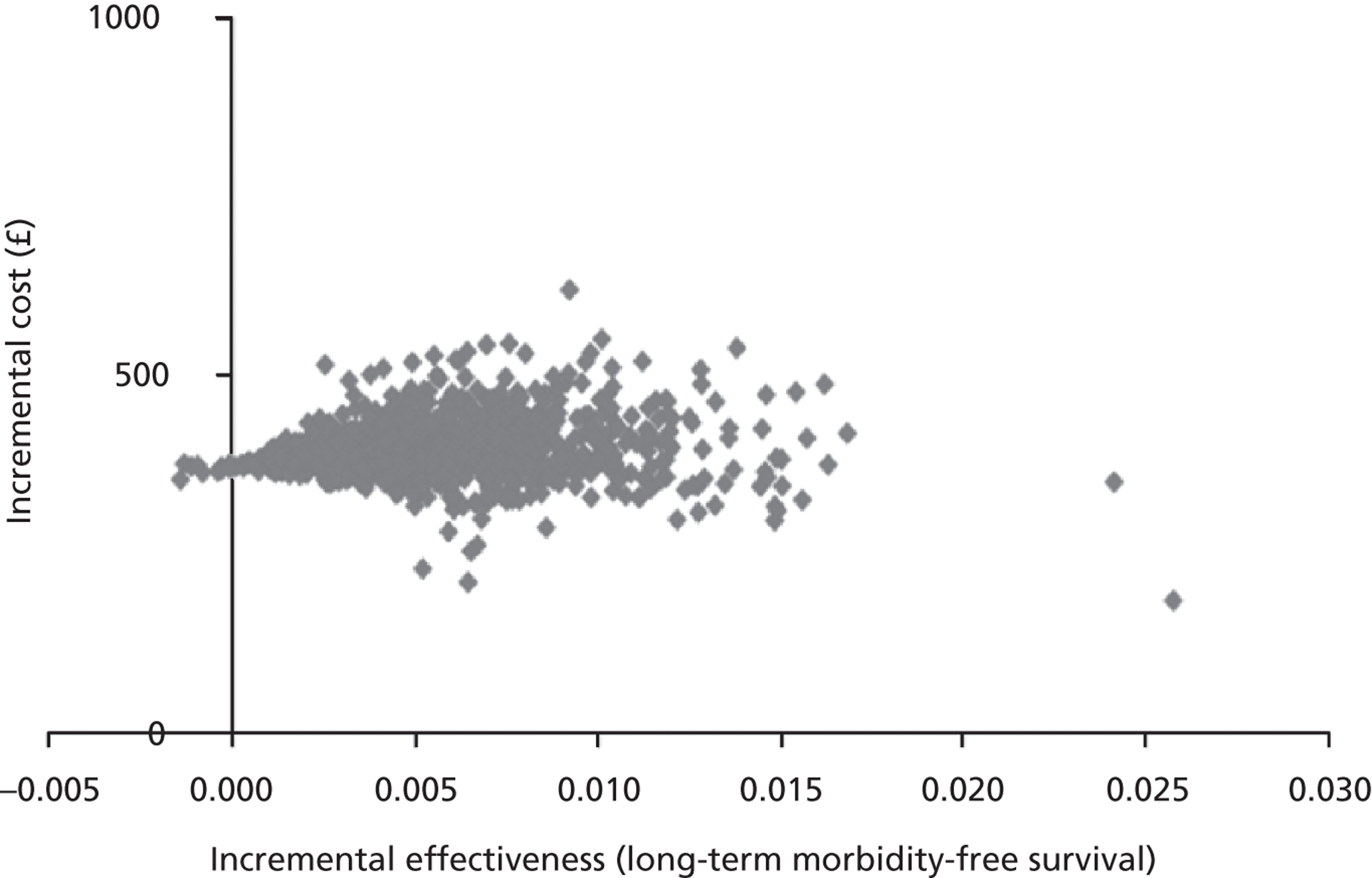
FIGURE 32.
Cost-effectiveness acceptability curve: 99mTc + ultrastaging vs. 99mTc+ blue dye + ultrastaging with case of long-term morbidity averted at 2 years as the outcome measure.
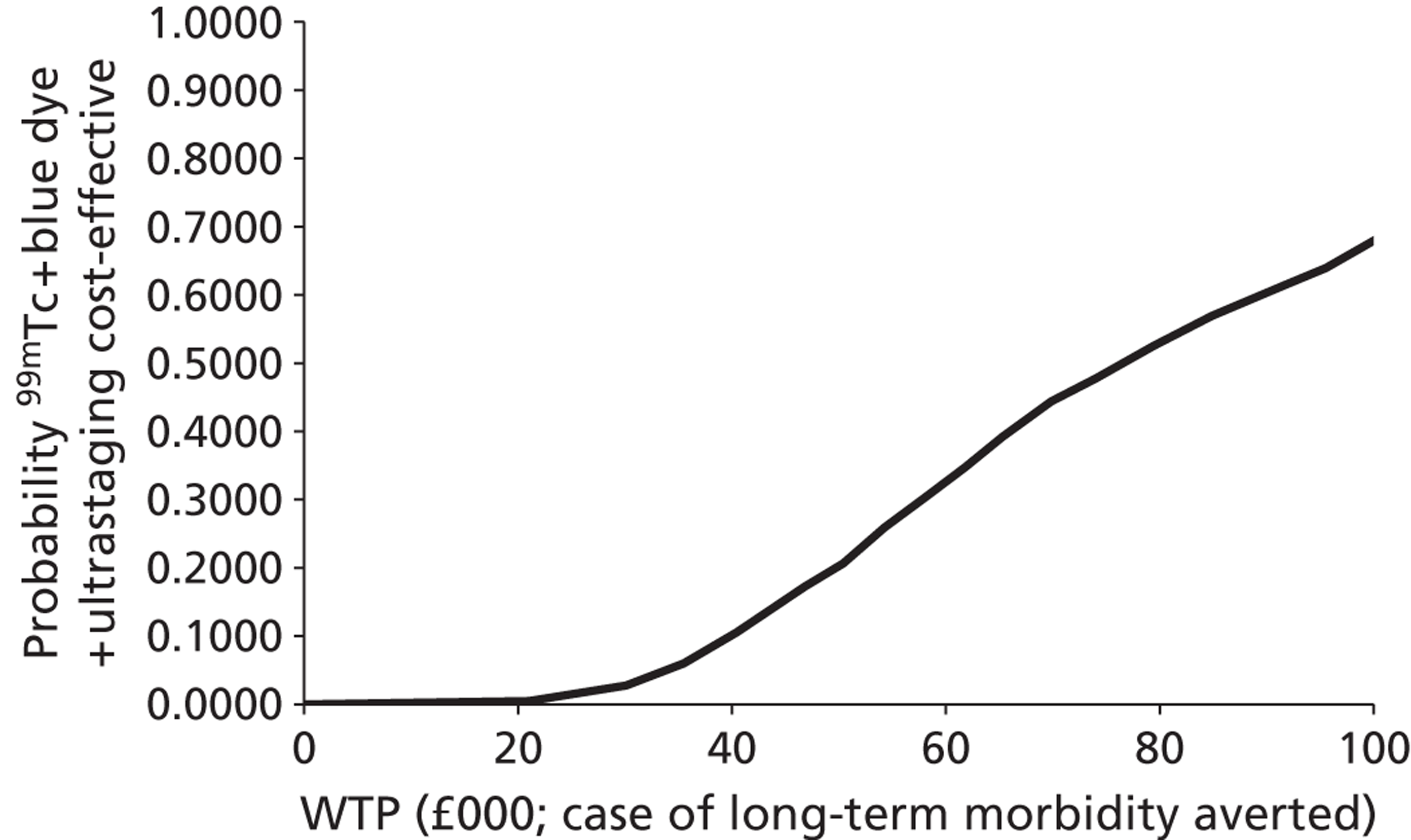
4. Blue dye + H&E versus blue dye + ultrastaging
Figures 33 and 34 shows the modelled uncertainty in the difference in costs between the strategies of blue dye + H&E and blue dye + ultrastaging for the outcome measures of morbidity and long-term morbidity-free survival at 2 years. This shows that the blue dye + ultrastaging strategy when compared with blue dye + H&E will almost always reduce the costs and will always increase the effectiveness at averting cases of morbidity and long-term morbidity. This is reflected in Figures 35 and 36, which show that across all values of the WTP for a case of morbidity and long-term morbidity averted blue dye + ultrastaging is preferred to blue dye + H&E.
FIGURE 33.
Cost-effectiveness plane: blue dye + H&E vs. blue dye + ultrastaging with morbidity-free survival at 2 years as the outcome measure.
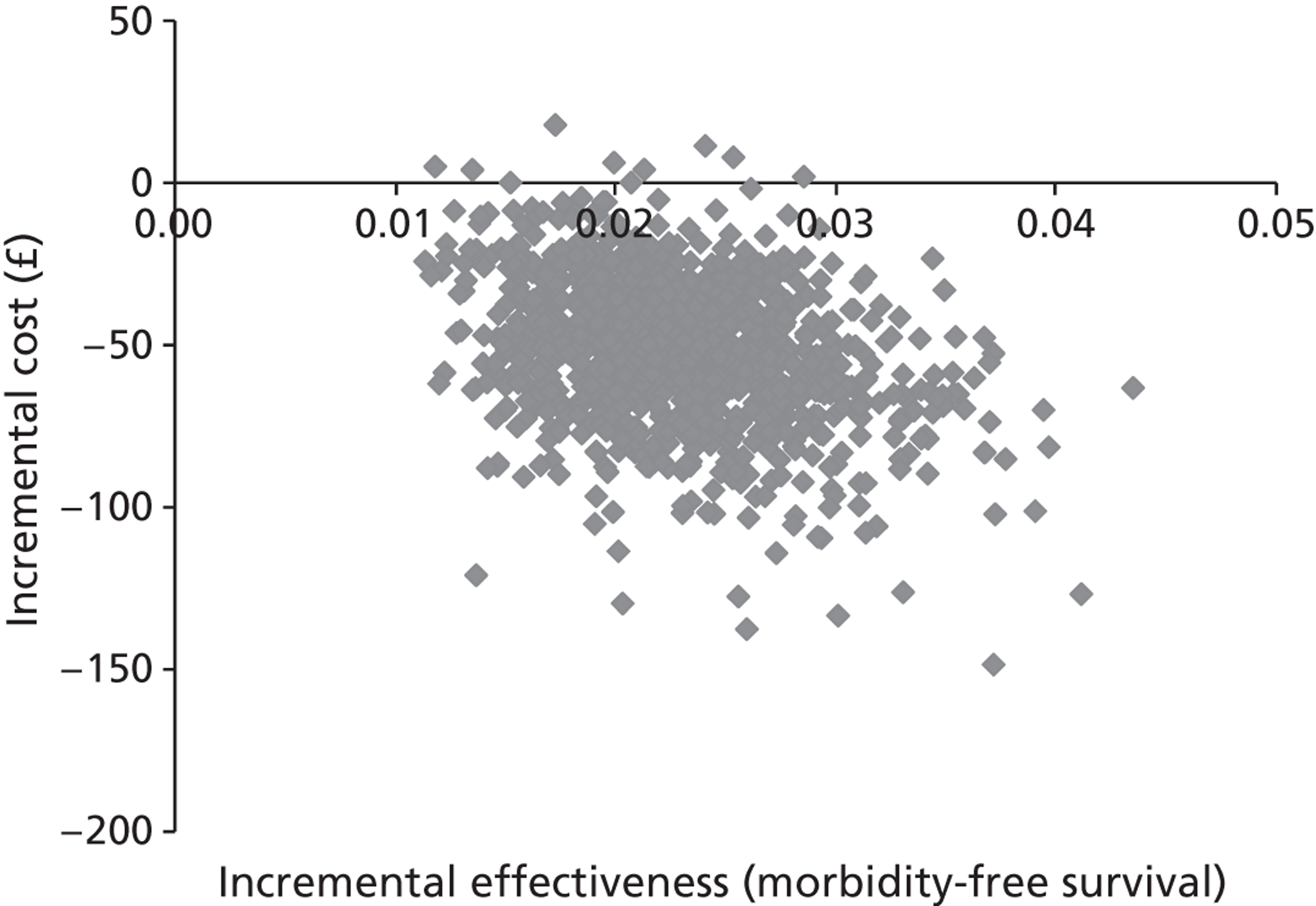
FIGURE 34.
Cost-effectiveness plane: blue dye + H&E vs. blue dye + ultrastaging with long-term morbidity case averted at 2 years as the outcome measure.
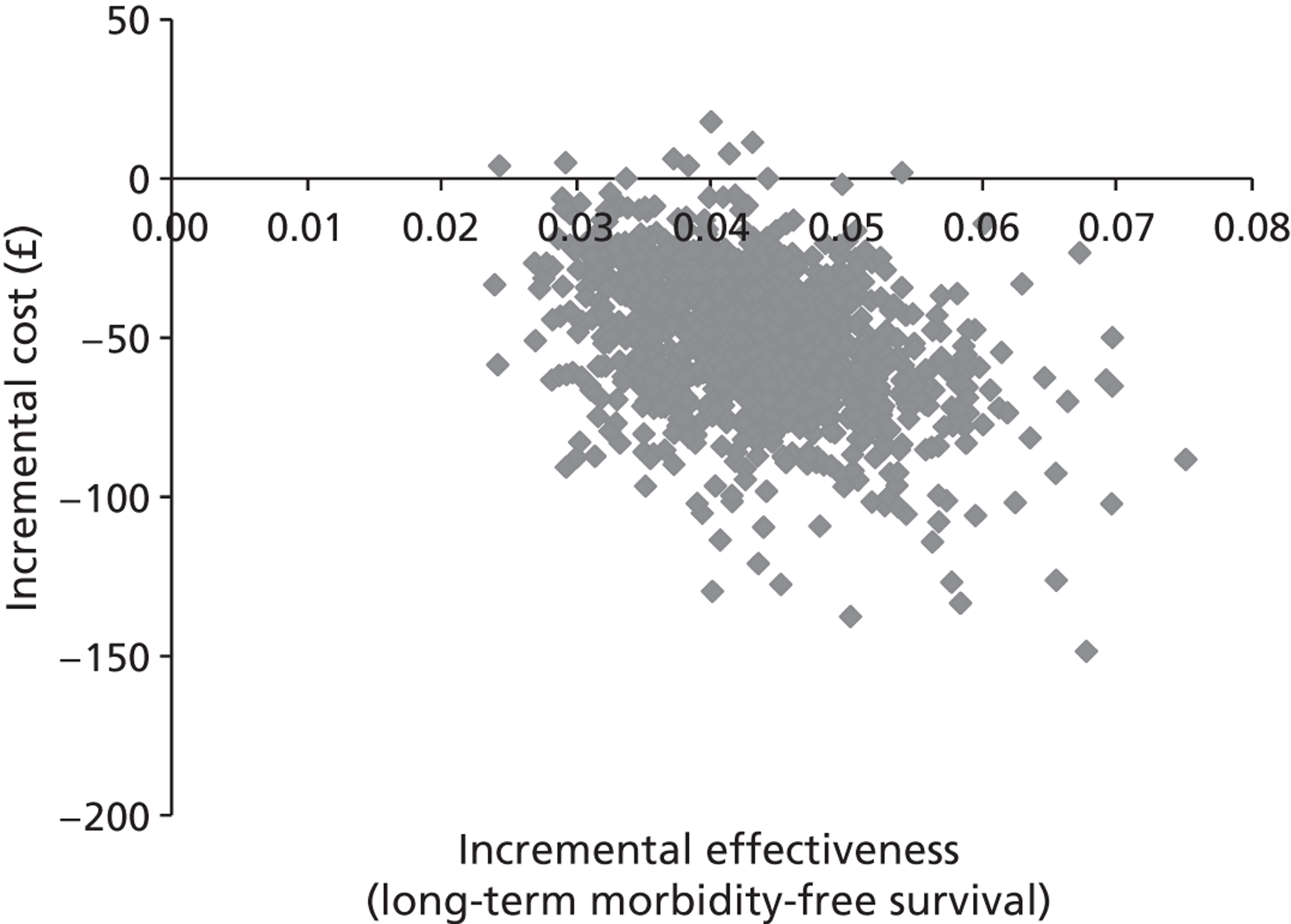
FIGURE 35.
Cost-effectiveness acceptability curve: blue dye + H&E vs. blue dye + ultrastaging with case of morbidity averted at 2 years as the outcome measure.
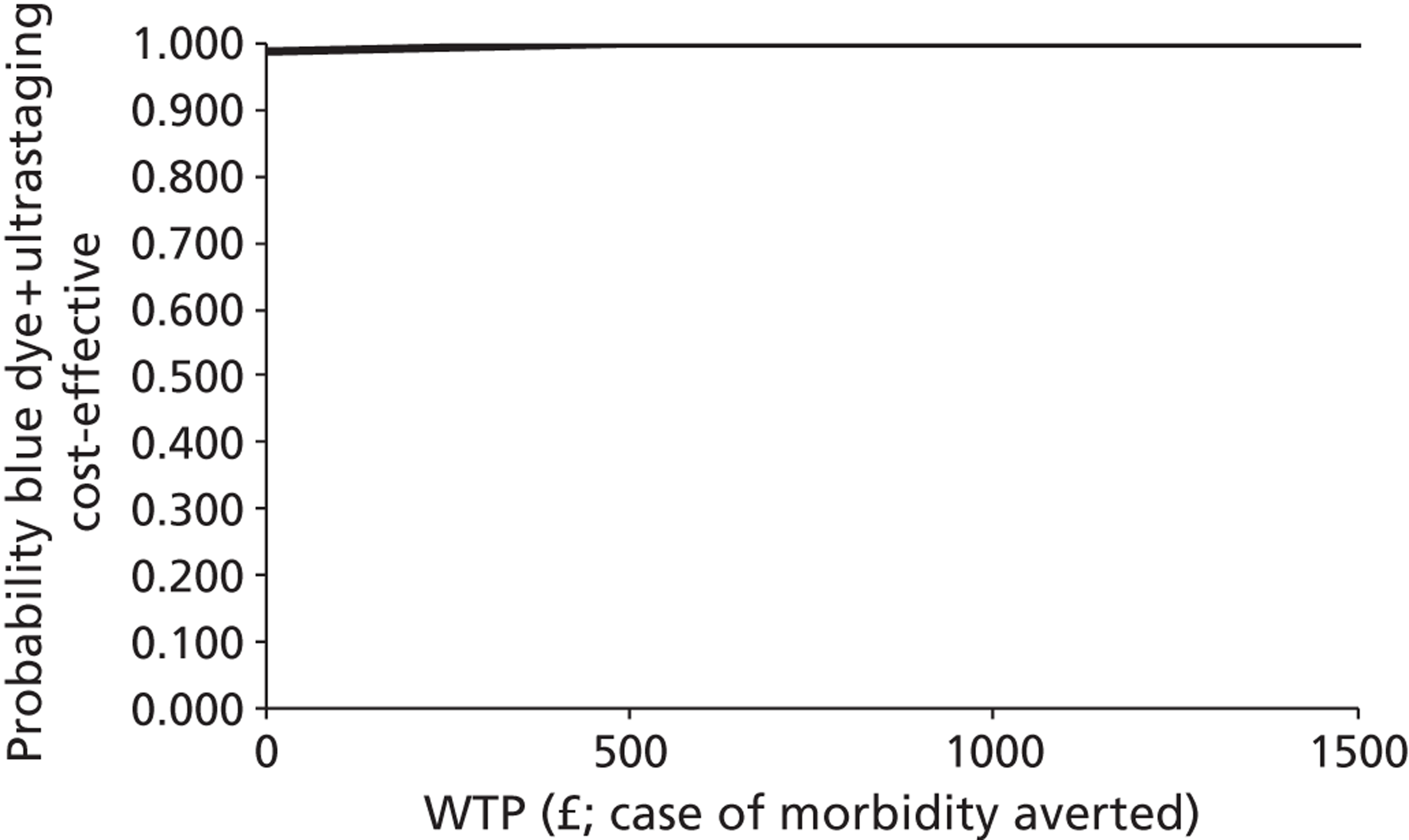
FIGURE 36.
Cost-effectiveness acceptability curve: blue dye + H&E vs. blue dye + ultrastaging with case of long-term morbidity averted at 2 years as the outcome measure.
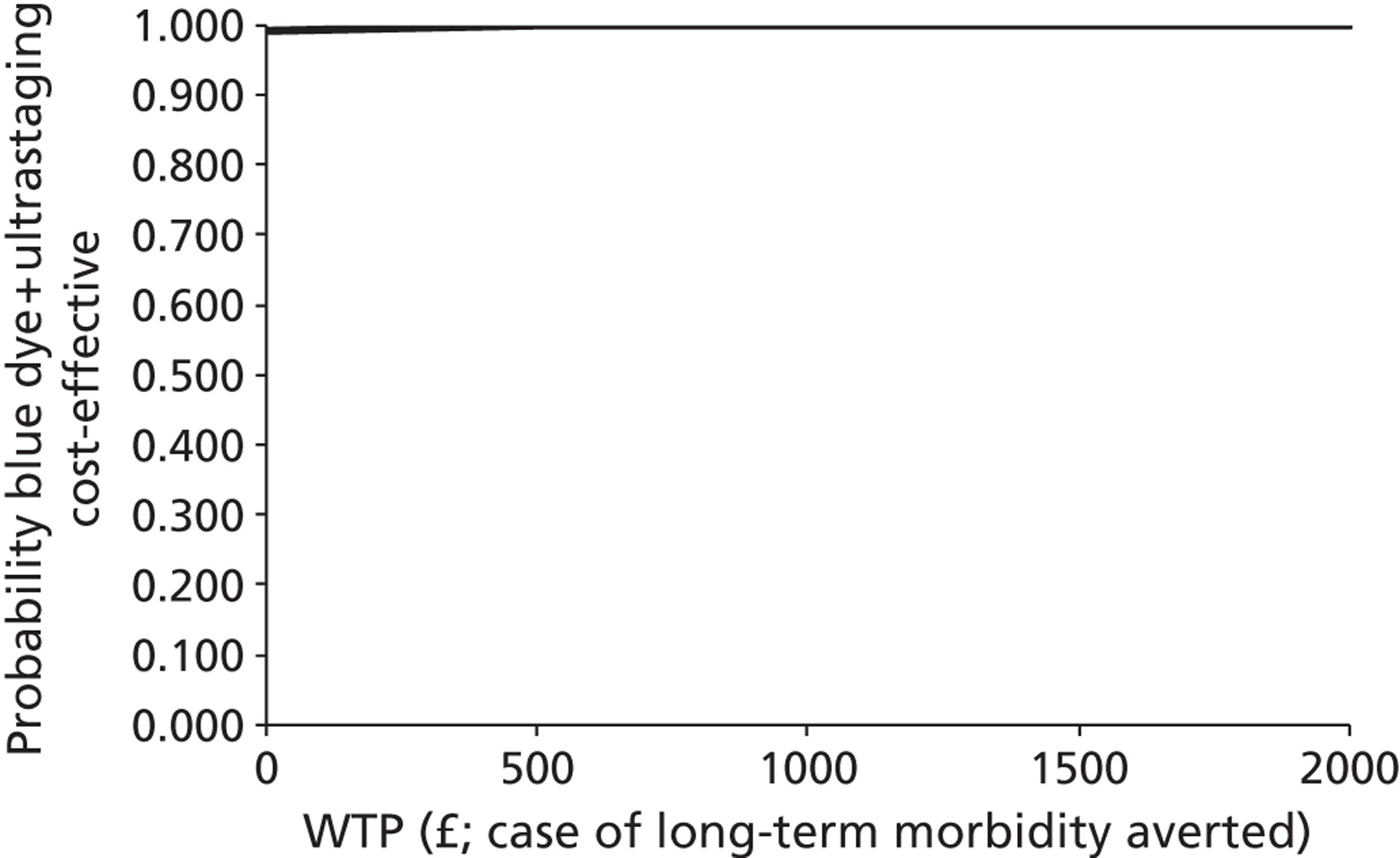
5. 99mTc + H&E versus 99mTc + ultrastaging
Figures 37 and 38 show the modelled uncertainty in the difference in costs between the strategies of 99mTc + H&E and 99mTc + ultrastaging for the outcome measures of morbidity and long-term morbidity-free survival at 2 years. This shows that the 99mTc + ultrastaging strategy when compared with 99mTc + H&E will almost always reduce the costs and will always increase the effectiveness at averting cases of morbidity and long-term morbidity. This is reflected in Figures 39 and 40, which show that across all values of the WTP for a case of morbidity and long-term morbidity averted, 99mTc + ultrastaging is preferred to 99mTc + H&E.
FIGURE 37.
Cost-effectiveness plane: 99mTc + H&E vs. 99mTc + ultrastaging with morbidity-free survival at 2 years as the outcome measure.
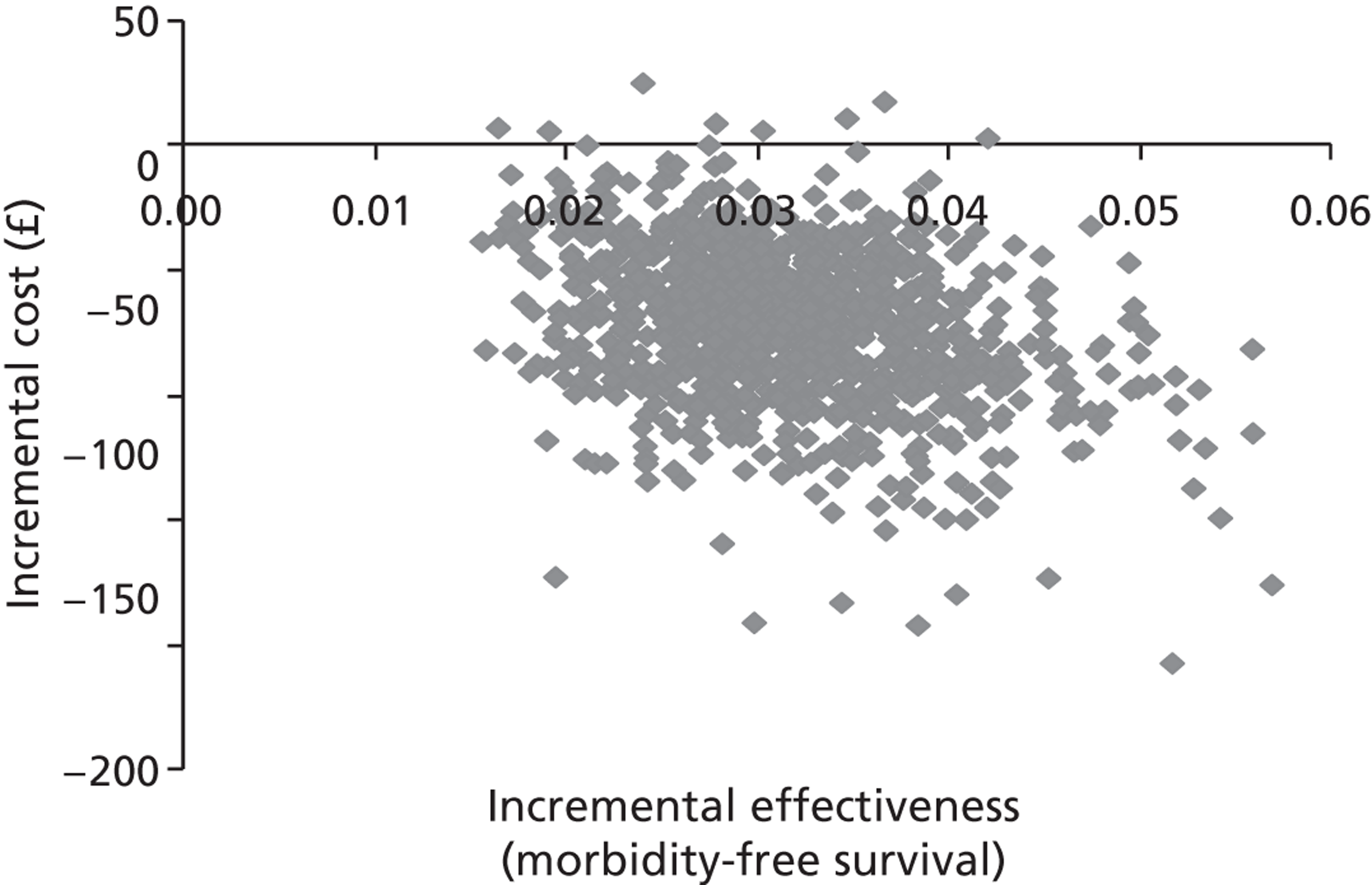
FIGURE 38.
Cost-effectiveness plane: 99mTc + H&E vs. 99mTc + ultrastaging with long-term morbidity-free survival at 2 years as the outcome measure.
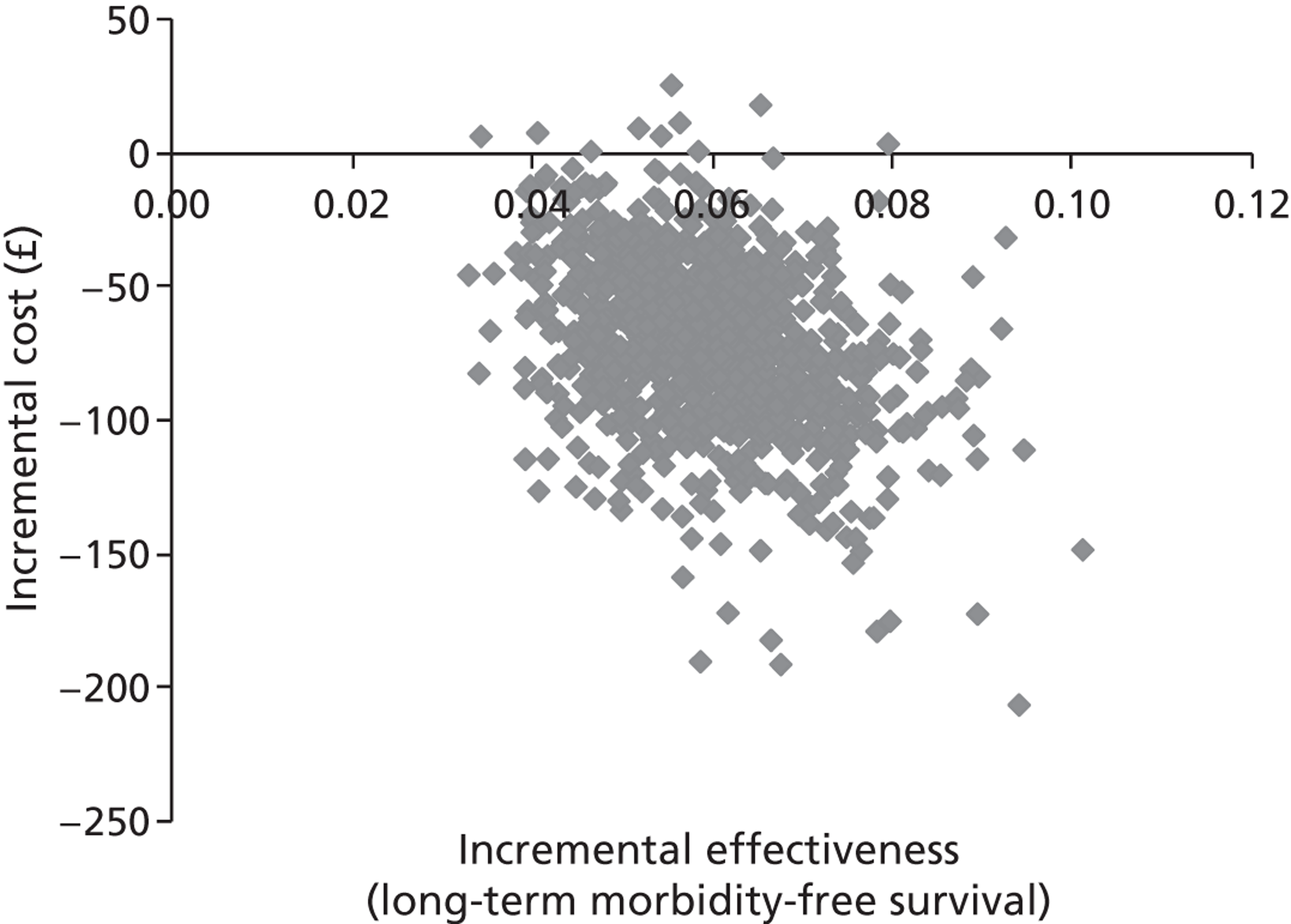
FIGURE 39.
Cost-effectiveness acceptability curve: 99mTc + H&E vs. 99mTc + ultrastaging with case of morbidity averted at 2 years as the outcome measure.

FIGURE 40.
Cost-effectiveness acceptability curve: 99mTc + H&E vs. 99mTc + ultrastaging with case of long-term morbidity averted at 2 years as the outcome measure.
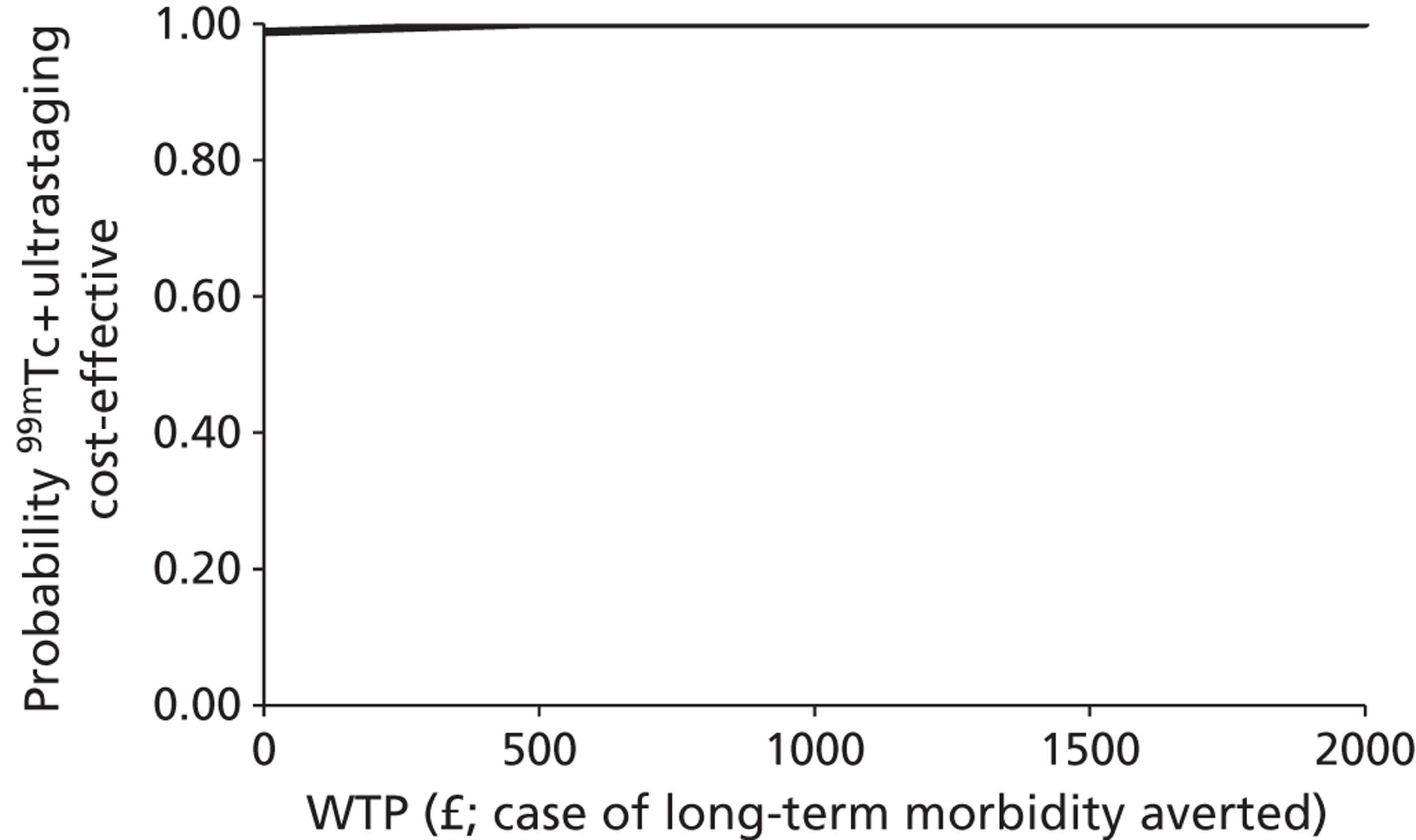
6. 99mTc + blue dye + H&E versus 99mTc + blue dye + ultrastaging
Figures 41 and 42 show the modelled uncertainty in the difference in costs between the strategies of 99mTc + blue dye + H&E and 99mTc + blue dye + ultrastaging for the outcome measures of morbidity and long-term morbidity-free survival at 2 years. This shows that the 99mTc + blue dye + ultrastaging strategy, when compared with 99mTc + blue dye + H&E, will almost always reduce the costs and will always increase the effectiveness at averting cases of morbidity and long-term morbidity. This is reflected in Figures 43 and 44, which show that across all values of the WTP for a case of morbidity and long-term morbidity averted, 99mTc + blue dye ultrastaging is preferred to 99mTc + blue dye + H&E.
FIGURE 41.
Cost-effectiveness plane: 99mTc + blue dye + H&E vs. 99mTc + blue dye + ultrastaging with morbidity-free survival at 2 years as the outcome measure.
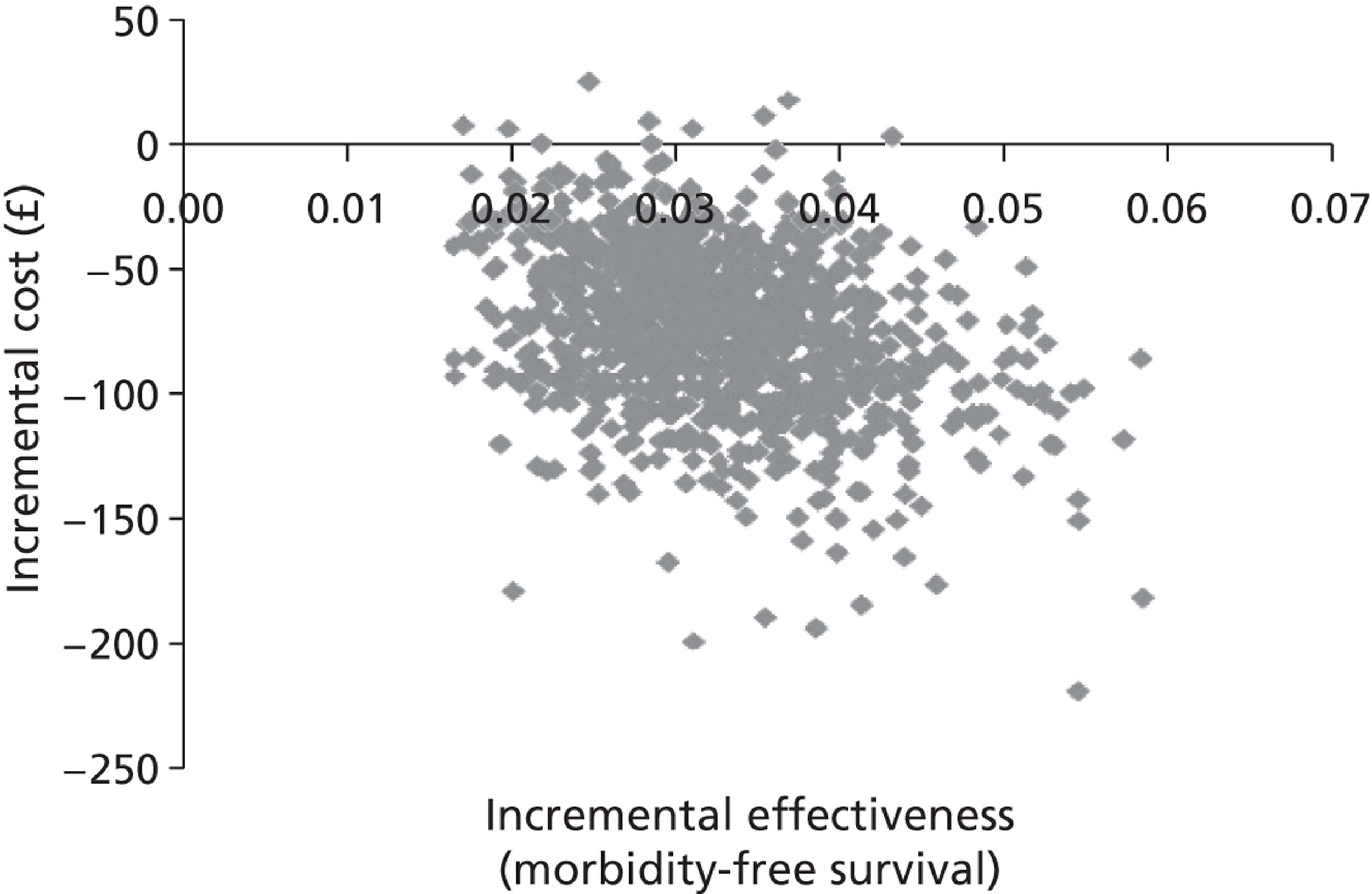
FIGURE 42.
Cost-effectiveness plane: 99mTc + blue dye + H&E vs. 99mTc + blue dye + ultrastaging] with long-term morbidity-free survival at 2 years as the outcome measure.
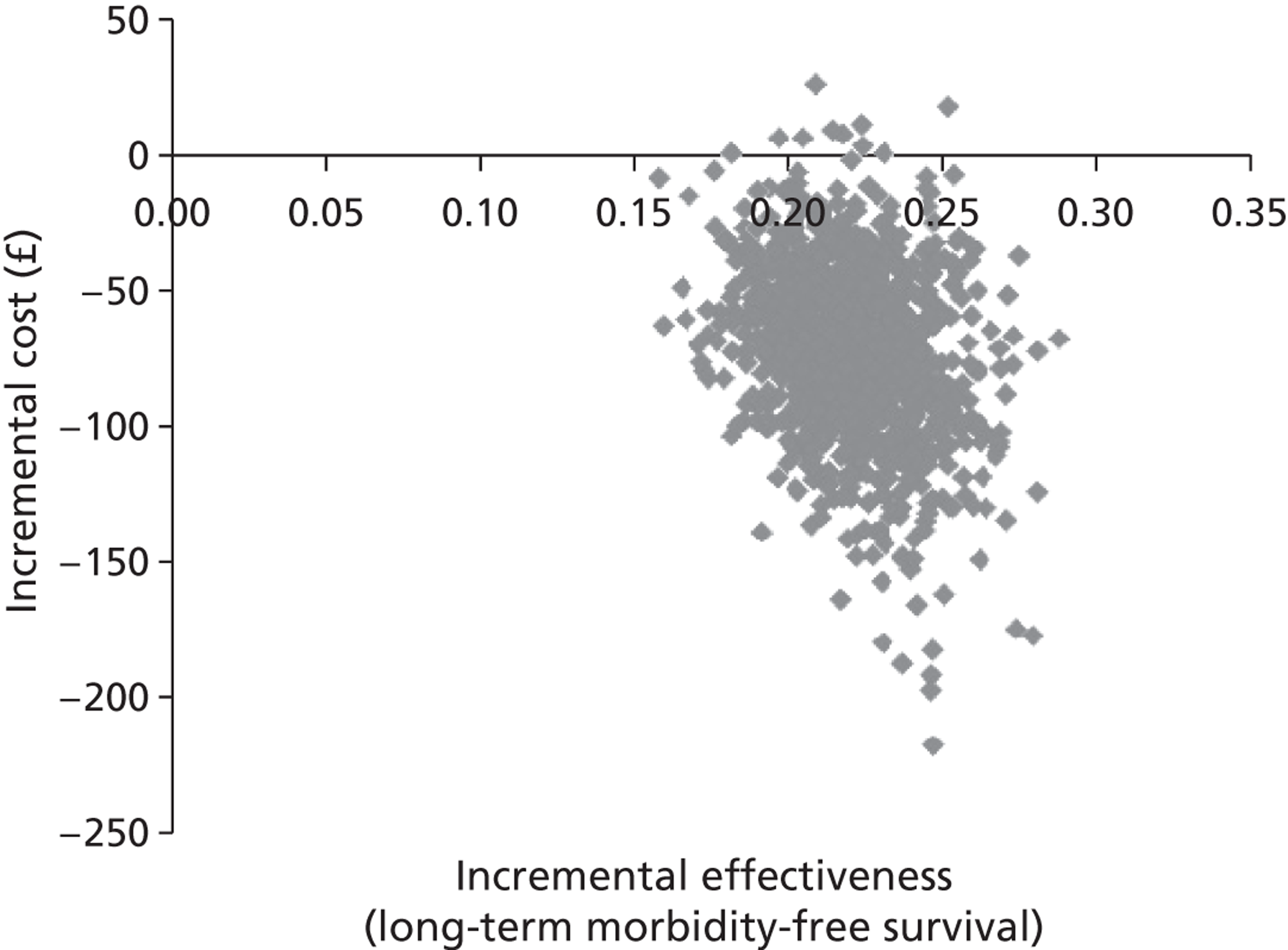
FIGURE 43.
Cost-effectiveness acceptability curve: 99mTc + blue dye + H&E vs. 99mTc + blue dye + ultrastaging with case of morbidity averted at 2 years as the outcome measure.
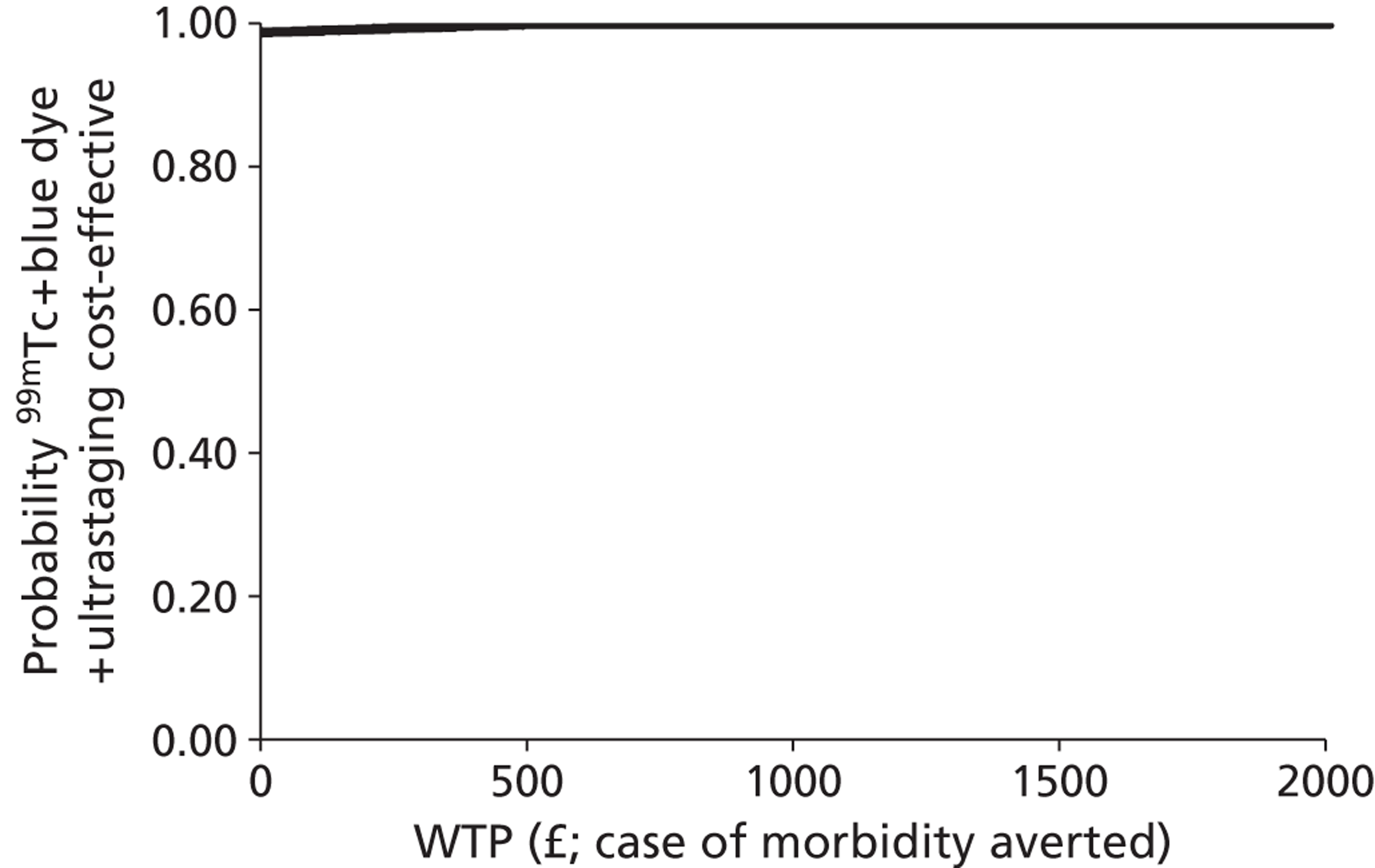
FIGURE 44.
Cost-effectiveness acceptability curve: 99mTc + blue dye + H&E vs. 99mTc + blue dye + ultrastaging with case of long-term morbidity averted at 2 years as the outcome measure.
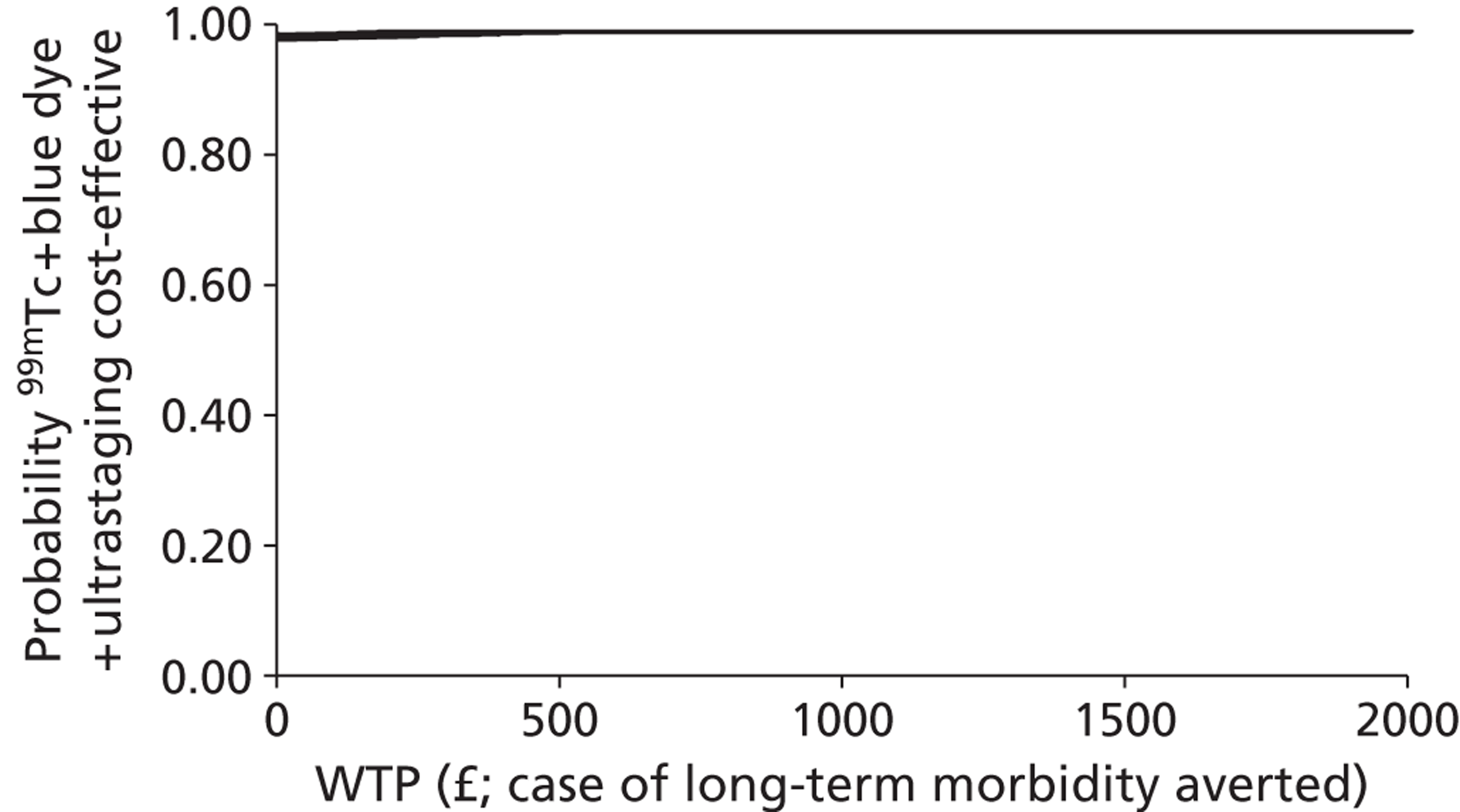
One-way sensitivity analysis
In order to examine the impact of a selection of model assumptions on the model results, the following sensitivity analysis was undertaken.
Assumed age of the cohort
The impact of the assumed age of the patient cohort on the costs and outcomes of each of the clinical pathways is shown in Table 51 for ages 55 and 75 years, respectively. It can be seen that for the deterministic results in all cases, the assumed age of the cohort has no impact on the conclusions from the model. IFL continues to be the option that is the least costly and sees that highest survival among the patients. The strategies of blue dye + ultrastaging, 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging show ICER values for morbidity and long-term morbidity that are very similar to those obtained at baseline.
| Scenario | ICER – overall survival at 2 years | ICER – morbidity-free survival at 2 years (£) | ICER – long-term morbidity-free survival at 2 years (£) |
|---|---|---|---|
| Baseline | |||
| IFL | Dominates | ||
| Blue dye + ultrastaging | 2400 | 3700 | |
| 99mTc + ultrastaging | 4900 | 8900 | |
| 99mTc + blue dye + ultrastaging | 41,200 | 74,300 | |
| Age = 55 years | |||
| IFL | Dominates | ||
| Blue dye + ultrastaging | 2300 | 3600 | |
| 99mTc + ultrastaging | 4900 | 8800 | |
| 99mTc + blue dye + ultrastaging | 40,900 | 73,800 | |
| Age = 75 years | |||
| IFL | Dominates | ||
| Blue dye + ultrastaging | 2400 | 4200 | |
| 99mTc + ultrastaging | 5100 | 9000 | |
| 99mTc + blue dye + ultrastaging | 42,600 | 76,000 | |
| Increased mortality due to patient morbidity | |||
| IFL | Dominates | ||
| Blue dye + ultrastaging | 2300 | 3700 | |
| 99mTc + ultrastaging | 4900 | 8700 | |
| 99mTc + blue dye + ultrastaging | 40,700 | 72,000 | |
| Cost of implementing 99mTc + blue dye together | |||
| Cost of 99mTc + blue dye = cost 99mTc (£3836) | |||
| IFL | Dominates | ||
| Blue dye + ultrastaging | 2400 | £3700 | |
| 99mTc + blue dye + ultrastaging | 4700 | 8400 | |
| Cost of 99mTc + blue dye = 50% more than the cost of 99mTc (£5754) | |||
| IFL | Dominates | ||
| Blue dye + ultrastaging | 2400 | 3700 | |
| 99mTc + ultrastaging | 4900 | 8900 | |
| 99mTc + blue dye + ultrastaging | 195,700 | 352,600 | |
| Groin recurrence rate following negative result for metastasis | |||
| IFL | Dominates | ||
| Blue dye + ultrastaging | 2600 | 4300 | |
| 99mTc + ultrastaging | 5200 | 9800 | |
| 99mTc + blue dye + ultrastaging | 42,400 | 81,300 | |
Increasing the death rate for morbidity
It has been assumed in this study at baseline that the morbidity experienced by the patients has no impact on their overall survival. It can be seen from the results obtained in Table 51 that adding this factor to the model parameterisation has very little impact on the conclusions drawn from the model. IFL continues to be the option that sees that highest survival among the patients, while the strategies of blue dye + ultrastaging, 99mTc + ultrastaging and 99mTc + blue dye + ultrastaging show ICER values for morbidity and long-term morbidity that are very similar to those obtained at baseline.
The cost of implementing 99mTc and blue dye together
It was assumed in this study at baseline that the cost of implementing 99mTc and blue dye together is 10% more than the cost of implementing 99mTc alone. In this sensitivity analysis the impact of the assumption on the model results is examined by varying this cost across a number of plausible values. In the first case, it is assumed that implementing 99mTc and blue dye together costs the same as implementing 99mTc on its own (£3836) and in the second case, it is assumed that 99mTc and blue dye together cost 50% more than 99mTc alone (£5754).
It can be seen from the results obtained in Table 51 in the case of overall survival that this change to the model parameterisation has very little impact on the conclusions drawn from the model. In terms of overall survival at 2 years, the IFL strategy continues to dominate all the other strategies. However, with respect to morbidity-free survival and long-term morbidity-free survival at 2 years, the cost of 99mTc and blue dye implemented together has the potential to have a significant impact on the conclusions drawn from the model. When the cost of 99mTc and blue dye implemented together is equal to the cost of 99mTc, this leads the strategy 99mTc + blue dye + ultrastaging to become far more acceptable in terms of its ICER value in comparison with the other strategies. However, when the cost of implementing 99mTc and blue dye together is increased to 50% greater, then the strategy of 99mTc + blue dye + ultrastaging becomes far less acceptable with an ICER value for morbidity and long-term morbidity survival of £195,700 and £352,600, respectively.
Groin recurrence rate following negative result for metastasis
It was assumed at baseline that the groin recurrence rate following a negative result from a SLN biopsy and histopathology is 6 out of 259 (2.3%). 73 To investigate the impact of this assumption on the model results, an alternative higher value of 2 out of 32 (6.5%), taken from a much smaller study,65 was used.
It can be seen from the results obtained in Table 51 that this change to the model parameterisation has very little impact on the conclusions drawn from the model. In terms of overall survival, the IFL strategy continues to dominate all the other strategies. In terms of morbidity-free survival and long-term morbidity-free survival at 2 years, the strategy of 99mTc + blue dye + ultrastaging is, again, the most effective option.
Chapter 8 Discussion
Principal findings
Test accuracy systematic review
Twenty-six studies gave information on test accuracy of 99mTc and/or blue dye identification of SLN biopsy with reference standard of either IFL for all or IFL for SLN-positive nodes (containing metastases) and clinical follow-up for SLN-negative nodes (see Table 7). Most of the studies included SCCs only (when reported), but 10 included up to eight patients with other forms of vulval cancer including melanoma and adenocarcinoma. 39,53,57,58,61,63,66,71,72,74 Most of the studies were small, with fewer than 50 patients, but one study involved 127 patients57 and one 403 patients. 73 The histopathological techniques used varied between studies and included frozen sections, ultrastaging, H&E staining and immunohistochemical techniques. Not all studies had the same histochemical techniques for the SLN as for the remaining lymph nodes. There were, in effect, three index tests (99mTc, blue dye, and 99mTc and blue dye) and five reference standard groups (H&E only or insufficient details to determine histopathological techniques used, frozen sections only, immunohistochemistry, ultrastaging and ultrastaging with immunohistochemistry). Therefore, calculating the sensitivity and specificity of finding metastases in a SLN biopsy compared with the reference standard was not straightforward and no meta-analysis of all 26 studies was appropriate. In addition, a SLN was not found in all patients, so information also needed to be collected on whether or not the patient had malignancy at IFL. Ultimately, each patient could be categorised as follows:
-
SLN found.
-
Has malignancy in SLN.
-
Has no malignancy in SLN.
-
Has malignancy in other lymph nodes found at IFL.
-
Has no malignancy in other lymph nodes found at IFL.
-
-
-
No SLN found.
-
Has malignancy in other lymph nodes found at IFL.
-
Has no malignancy in other lymph nodes found at IFL.
-
Has no IFL.
-
Has groin metastases at follow-up.
-
Has no groin metastases at follow-up.
-
-
Teasing out the results for all of these different categories of patients was extremely difficult, particularly when the studies were small and poorly reported. In some studies, it was obvious that these different possibilities were not clearly thought through when either designing or reporting the study; therefore, some of the categories were combined, for example patients with negative SLN and malignancy in other nodes, and patients with no SLN found and malignancy in other nodes.
If malignancy is found in a SLN, then the patient will have groin metastases, so no FPs would be possible and all point estimates of specificity were 100%. CIs were wide, usually because of small sample sizes. However, FN results maybe more important clinically because of the risk of groin metastases to the patient. Sensitivities approached 100% in most categories of index tests and reference standards. In the largest group of studies (11 studies), using 99mTc and blue dye, ultrastaging and using immunohistochemistry, the pooled sensitivity was 95.6% (95% CI 91.5% to 98.1%) with some heterogeneity and an estimate of the negative predictive value was 97.8% (see Tables 16 and 17). It is unclear whether or not the number of patients with metastases missed by biopsying the SLN is offset by the morbidity caused by IFL such as wound breakdown, cellulitis and lymphoedema. Where clinical follow-up is used, there will be more FNs at the time of the original operation, because of the longer time to develop a metastasis, so the sensitivity will be lower.
The SLN detection rate varied between studies: 76–100% for 99mTc only, 53–88% for blue dye only and 84–100% for both 99mTc and blue dye (see Table 24). The results from the 95% CIs of the combined rates suggest that, if SLN biopsy is going to be used, it is important that both tests are performed in every patient because not to do so risks missing the SLN in some patients. Protocols in more recent studies such as Levenback et al. 106 have tended to reflect this.
Recurrences can occur at the vulva, the groin and in distant organs. IFL is used not only to detect malignancies in groin nodes but also to remove them. Therefore, it is reasonable to assume that patients undergoing IFL are less likely to have groin recurrences than patients undergoing SLN biopsy only or no groin surgery (but this will have no impact on vulval recurrences). If metastases were found in the SLN, it would not be clinically acceptable to not perform an IFL (unless the patient refuses this procedure), so it is very difficult to demonstrate whether or not IFL does reduce groin recurrence rates because of lack of a comparator. Analysis of studies with clinical follow-up showed that groin recurrences do occur in patients who have had IFL and in patients with SLN-negative biopsies. In general, there was a higher rate of groin recurrences in SLN-positive patients than SLN-negative patients, but the numbers in the studies were small.
Survival rates were available in nine studies50,54,60,63,65,70,71,73,74 and showed that survival was worse in SLN-positive compared with SLN-negative patients. However, vulval cancer is largely a disease of older women so, of the deaths in the studies, approximately half were from vulval cancer and half were from other causes.
Quality of life was reported for a subset of patients82 in the largest included study. 73 QoL was lower in IFL patients than in SLN biopsy patients, at least according to some subscales of the FACT-V questionnaire, including discomfort in groins/vulva/legs, contentment, oedema, complaints and use of stockings. AEs were generally more frequent and more severe for the IFL patients than for the SLN biopsy patients.
Clinical effectiveness systematic review
All included studies had to report results for patients in whom more than 75% were FIGO stages I and II. Included in the systematic review was one RCT, three case–control studies and 13 case series (see Table 31 and Stehamn et al. 87). The RCT compared groin RT with bilateral IFL in patients who had undergone radical vulvectomy or modified radical vulvectomy and showed that IFL was associated with better survival and fewer recurrences than groin RT. The case–control studies and case series evaluated a variety of treatments, including surgery to the vulva (radical vulvectomy, modified radical vulvectomy, hemivulvectomy or wide local excision) alone or in combination with unilateral or bilateral IFL, RT or all three treatments. Two case series also evaluated RT only in some patients. 91,99 The smallest study enrolled 47 patients91 and the largest enrolled 6965 patients;99 patients from Kumar et al. 99 were from the SEER database. Follow-up of patients varied between 2 months92 and 30 years,94 but some studies did not report median follow-up times. The majority of patients had SCC, but three88,89,98 also had a small proportion with other tumour types such as melanoma and adenocarcinoma. The mean or median ages of patients were all over 60 years, but some younger patients were included in each of the case series as lower age ranges included 20 years,91 23 years,94 27 years,100 etc. In many of the studies, the rates of recurrence and survival in the different treatment groups were combined so that it was difficult to gain many insights into the effectiveness of different types of treatments.
The general trend of results was that there was more deaths in node-positive patients (but not for the cases in Stehman et al. 89) and in patients treated with RT. There were fewer deaths in younger patients. The approximate relative rates of recurrence in vulva, groin and distant were around 4 : 2 : 1, respectively. In addition, the numbers dying from vulval cancer and other causes were approximately equal. No studies reported QoL. It was very difficult to pick out any trends in AEs owing to the variability in methods and categories of reporting, as most compared different types of surgery to the vulva (radical vulvectomy, modified radical vulvectomy, hemivulvectomy) rather than comparing IFL and no IFL, for example.
Economic evaluation
No studies were identified that have previously considered the cost-effectiveness of the available technologies for the treatment of vulval cancer among presumed FIGO stage I and stage II patients and, therefore, appropriate comparisons with other existing studies were not possible.
The results of the base-case deterministic analyses based on the outcome of cost per death averted at 2 years showed that, for patients with presumed stage I and stage II vulval cancer, the treatment strategy of IFL is both less costly and more effective than any of the strategies that used SLN biopsy.
When considering the outcome measures of morbidity-free survival and long-term morbidity-free survival at 2 years, it was found that the strategy of 99mTc + ultrastaging, for which ultrastaging is administered in the case of a negative H&E test, was likely to be cost-effective. Note that ultrastaging here is used as a proxy for more involved histopathological techniques such as immunohistochemistry. Moreover, it was noted that the strategies that included blue dye only as the approach to the SLN biopsy and H&E only for the histopathology were never found to be cost-effective and were always dominated by other strategies (other strategies being less costly and more effective). This finding emphasises that using blue dye and H&E for the identification of the SLN and the identification of metastasis, respectively, are not sensitive enough to be used on their own.
The ICER based on the outcome of morbidity-free survival at 2 years for the strategy of blue dye + ultrastaging compared with IFL was £2400 per case of morbidity-free survival, the ICER for 99mTc + ultrastaging compared with blue dye + ultrastaging was £4900 per case of morbidity-free survival and the ICER for 99mTc + blue dye + ultrastaging compared with 99mTc + ultrastaging was £41,200 per case of morbidity-free survival. Similarly, the outcome measure of long-term morbidity-free survival at 2 years was £3700 per case of long-term morbidity-free survival, the ICER for 99mTc + ultrastaging compared with blue dye + ultrastaging was £8900 per case of long-term morbidity-free survival and the ICER for 99mTc + blue dye + ultrastaging compared with 99mTc + ultrastaging was £74,300 per case of long-term morbidity-free survival.
The PSA suggests that at a WTP threshold of less than £3500 for a case of morbidity averted the IFL strategy is the most cost-effective. Then, from £3500 to £42,000, the 99mTc + ultrastaging strategy is the preferred option, given the current model, and then for a WTP of greater than £42,000 the 99mTc + blue dye + ultrastaging strategy becomes most cost-effective. In the case of the long-term morbidity averted at 2 years outcome measure, the 99mTc + ultrastaging strategy is the preferred option for a WTP of greater than £5000 compared with the IFL strategy. If the WTP for a case of long-term morbidity averted exceeds £77,500, then the 99mTc + blue dye + ultrastaging strategy becomes most cost-effective compared with the 99mTc + ultrastaging strategy. These findings provide further evidence that in terms of morbidity and long-term morbidity averted at 2 years, 99mTc + ultrastaging is likely to be the most cost-effective approach to the treatment of early-stage vulval cancer patients.
In order to examine some of the assumptions made, further one-way deterministic sensitivity analysis was undertaken. It was found that changing the assumed age of the patients in the model made no difference to the conclusions drawn from the model. This was also the case when examining the potential impact of increased mortality due to morbidity experienced by the patients. In addition, varying the groin recurrence rate after a negative SLN test across plausible values did not impact on the conclusions drawn from the model. However, the cost of administering 99mTc and blue dye together has the potential to have a significant impact on the results obtained from this economic evaluation. During the parameterisation of this economic model, a cost for this specific procedure was not available and, so, at baseline it was assumed that this procedure would cost 10% more than the cost of 99mTc alone. When this cost was increased as part of sensitivity analysis, it was found that the 99mTc + blue dye + ultrastaging strategy was even less cost-effective with an ICER of £195,700 for the morbidity outcome compared with 99mTc + ultrastaging and £352,600 for the long-term morbidity outcome also compared with 99mTc + ultrastaging. The ICER values for the 99mTc + ultrastaging strategy remained unchanged. Although, conversely, when the cost of 99mTc and blue dye implemented together was set equal to 99mTc alone, then it was found that the strategy of 99mTc + blue dye + ultrastaging had an ICER of £4700 for morbidity averted and £8400 for long-term morbidity averted compared with blue dye + ultrastaging. However, it is probably not a reasonable assumption to assume that 99mTc and blue dye implemented together costs the same as when 99mTc is administered alone and, therefore, it can be argued that this provides further evidence of the robustness of the conclusion that 99mTc + ultrastaging is the most cost-effective strategy for the morbidity outcomes.
Strengths of the project
The strength of the test accuracy systematic review was the rigour of its conduct and the focus on comparing and contrasting the different versions of index test and reference standards. As much information as possible was extracted from each study including SLN detection rates, recurrence rates, survival, QoL and AEs in addition to test accuracy.
The strength of the clinical effectiveness systematic review included the rigorous efforts made to find as much relevant evidence as possible, demonstrated by the number of full papers examined and subsequently excluded (listed in Appendix 11). The focus was on FIGO stages I and II evidence to ensure compatibility with the test accuracy systematic review and also separation of the different treatment types.
The economic evaluation has had the advantage of being able to use the best available data in the model established in the systematic reviews of the evidence, particularly the sensitivity and specificity of the procedures used to identify a SLN and metastases. All assumptions used in the model were agreed by a panel of experts a priori, with key assumptions being examined through the use of sensitivity analysis. Owing to the scarcity of vulval cancer, many of the data points used in this modelling study were based on small samples. However, the resultant uncertainly in these parameter values was examined through the use of PSA and the conclusions were mainly robust.
Limitations of the project
In the test accuracy systematic review, some of the included studies had considerable methodological limitations, including lack an adequate description of inclusion criteria, population (especially stages of disease) and reference standard used. Other problems included the omission of results or failure to separate results for the different categories of patients. Areas for which information was often not reported included the blinding of test results and surgeons' experience. With histological examination, the thickness of sections taken was often not well reported so that some studies may have used much thicker sections than others. Thinner sections are more likely to find micrometastases. There was no information on the therapeutic impact of SLN biopsy.
The main limitation of the clinical effectiveness systematic review was the lack of good-quality information on the effectiveness of the different categories of treatment. Many of the case series were comparing different types of surgical techniques but tended not to compare surgery only with surgery plus IFL and, so, only combined results were available for many of the studies. This meant that the number of hospital days per treatment type was also unavailable. In the original protocol for this work, it was estimated that eliciting subjective probabilities from clinicians would be useful in determining diagnostic and therapeutic impact of SLN biopsy compared with IFL. During the project, and from our work in a previous project on positron emission tomography, computed tomography (PET-CT) for recurrent cervical cancer,107 it became obvious that the missing information was QoL from patients, and clinicians' estimates would probably not have been helpful.
Any systematic review must have a cut-off date for the searches, and it is unfortunate when a major study is published after this date. A large, good-quality diagnostic study of 452 patients comparing SLN biopsy with IFL has recently been published. 106 It used blue dye and 99mTc in most patients and ultrastaging with immunohistochemistry. The results from this paper are similar to those of the included studies. The sensitivity was 92.3% – very similar to that found by Klat et al. ,60 which had similar study characteristics.
The major limitation of the economic evaluation was the absence of overall QoL estimates that differentiate between the impact of the SLN biopsy and IFL on the morbidity of the patients. The only global QoL measure available was in a relatively small sample and showed no difference between SLN and IFL strategies. 82 Instead, three outcome measures were considered in this study – overall mortality, morbidity-free survival and long-term morbidity-free survival – all over a 2-year time horizon. The inevitable difficulty with this selection is that it is very difficult to know which should be the primary outcome measure. Intuitively, overall survival would seem to be best choice; however, in this setting, this outcome provided little insight into the clinical dilemma facing clinicians treating vulval cancer: is the extra morbidity from IFL worth it when compared with the slight increase in mortality and less morbidity from a SLN procedure? IFL, while the cheapest strategy considered in this analysis, is a highly morbid procedure that is most effective at reducing the probability of a groin recurrence in the future and hence patient mortality. The option of using SLN biopsy has been introduced into practice with the aim of reducing patient morbidity, but at the expense of the increased possibility of patients being diagnosed FN for metastasis. Therefore, it was inevitable that, in this study, IFL has been shown to be the most effective procedure in terms of overall survival. Perhaps the best outcome measure in this study is morbidity-free survival as this also incorporates all types of morbidity and the impact of overall survival into the outcome, although long-term morbidity-free survival has also been considered, since lymphoedema, which is a long-term complication, has been found to have some significant impacts on patients treated with an IFL compared with those receiving a SLN biopsy. 82
Cost data for the SLN biopsies were particularly difficult to identify as the NHS reference costs do not differentiate between the types of biopsy implemented. All the costs used in this study were checked by experts in the field to ensure that reasonable values had been adopted.
Uncertainties
The GROINSS-V study73 and a more recent diagnostic study of SLN biopsy in vulval cancer106 both discuss pathological characteristics of women in whom they consider SLN biopsy would be appropriate (in particular primary tumour size < 4 cm) and biopsy technique (SLN detected using 99mTc and blue dye, malignancy checked for with ultrastaging and immunohistochemistry, use of a high-volume centre of expertise) but it is uncertain whether or not patients would rather risk unremoved groin metastases by forgoing IFL if they are SLN negative at biopsy. The one published survey available shows that 100% of gynaecologists would accept a 1% FN rate from SLN biopsy, but only 33% of patients would accept a SLN biopsy in which the risk was 1 in 80. 41 It is clear that some metastases will be missed, but it is unclear whether or not the relative benefit of not having the morbidity of IFL is outweighed by the risk of missing metastases. This may depend on a number of factors, including the age of the patient, the stage of disease and the aggressiveness of the malignancy found in the vulval specimen.
The most effective treatment strategy for early vulval cancer is currently unclear, as is the relative effectiveness of vulval surgery only compared with vulval surgery with IFL. Much work has focused on the relative benefits of different types of surgical procedure, rather than the impact on mortality of IFL versus no IFL. From the RCT comparing RT with IFL, it is likely that IFL enhances survival but the sample sizes were small and the results were only just statistically significant so replication of this study would be useful to reduce some uncertainty.
The most important uncertainty in this project is the cost–utility of SLN biopsy compared with IFL, but this could not be calculated owing to lack of information on generic QoL.
Chapter 9 Overall conclusions
Recommendation for practice/implications for service provision
There is insufficient evidence to suggest that SLN biopsy should be used in routine clinical practice on health economic grounds. The strategy of IFL for all was found to be less costly and more effective when considering cost per death averted. Based on the findings of the current model and acknowledging the limitations that have been highlighted in terms of the inability to apply QALYs in this economic evaluation, the results of this analysis suggest that 99mTc + ultrastaging in the treatment of early-stage vulval cancer is likely to be cost-effective in terms of case of morbidity averted and long-term morbidity averted at 2 years. Note that ultrastaging has been used here as a proxy for more in-depth histopathological techniques such as immunohistochemistry. There is some uncertainty regarding the acceptability of the 99mTc + blue dye + ultrastaging strategy in terms of the outcome measures of case of morbidity and long-term morbidity averted at 2 years, as there is difficulty in attempting to apply these outcome measures to any acceptability threshold.
Recommendation for research
There needs to be further evaluation of patient preferences regarding the circumstance in which patients would rather risk unremoved groin metastases by forgoing IFL if they are SLN-negative at biopsy, incorporating a number of factors including the age of the patient, the stage of disease, persistency of side effects and the aggressiveness of the malignancy found in the vulval specimen.
There needs to be a robust prospective evaluation of the relative effectiveness of the different treatment strategies for vulval cancer, taking into account the uncertainty around the need for IFL in early-stage vulval cancer. As vulval cancer is uncommon, a multicentre RCT involving several countries will likely be needed to enrol sufficient patients in order to deal with the uncertainty.
There needs to be some information on the QoL in vulval cancer, using a generic QoL measure such as EQ-5D. This analysis has highlighted the importance of obtaining overall QoL values that describe the impact of the SLN biopsy and IFL and their related complications on patients over time. A previous study has attempted to identify these values but did not find a difference in the QoL estimates between 62 patients who received either a SLN biopsy or an IFL. 82 Intuitively, there would need to be a difference in QoL between these two groups because, if this were not the case, IFL – with its increased effectiveness at reducing the risk of a further groin recurrence and, therefore, patient mortality, but with its much higher risk of morbidity – would always be preferred. Therefore, future work should be undertaken to examine the QoL in these treatment groups perhaps by using an alternative type of questionnaire and through a larger study that includes more patients so would have better power to determine a small difference.
Good-quality, larger studies are required to more accurately estimate the test accuracy of SLN biopsy with histopathology. These studies need to distinguish carefully between finding the SLN and finding malignancy within the node.
Acknowledgements
Anne Fry-Smith for devising original searches, Shakila Thangaratinam for writing original bid and protocol, Marian Ellis for providing patient input to the protocol, Tara Selman for the original PhD work leading up to the development of this research and Kottekkattu Balan for providing clinical input to the protocol.
Contributions of authors
Catherine Meads supervised and co-ordinated the project, rewrote and edited the entire manuscript.
Andrew Sutton conducted the economic evaluation, wrote the economic evaluation chapter and the parts of the discussion chapter related to the economic evaluation.
Sylwia Małysiak conducted the original systematic review of test accuracy.
Monika Kowalska conducted the original systematic review of effectiveness.
Anna Zapalska conducted the original systematic review of test accuracy.
Ewelina Rogozińska wrote the original background section.
Peter Baldwin provided clinical advice for the project.
Adam Rosenthal provided clinical advice for the project.
Raji Ganesan provided pathology advice for the project and wrote the section on VIN.
Ewa Borowiack managed the original systematic reviews.
Pelham Barton provided economic evaluation supervision and edited the economic chapter.
Tracy Roberts managed the economic evaluation and edited the economic chapter.
Sudha Sundar provided clinical input for the project and the final report.
Khalid Khan provided the design of the HTA and overall management of project.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Management of vulval cancer. London, UK: Royal College of Obstetricians and Gynaecologists; 2006.
- Tyring SK. Vulvar squamous cell carcinoma: guidelines for early diagnosis and treatment. Am J Obstet Gynecol 2003;189:S17-23. http://dx.doi.org/10.1067/S0002-9378(03)00792-0.
- de Hullu JA, van der Avoort IA, Oonk MH, van der Zee AG. Management of vulvar cancers. Eur J Surg Oncol 2006;32:825-31. http://dx.doi.org/10.1016/j.ejso.2006.03.035.
- Gottleib WH. The assessment and surgical management of early-stage vulvar cancer. Best Pract Res Clin Obstet Gynaecol 2003;17:557-69. http://dx.doi.org/10.1016/S1521-6934(03)00066-X.
- Magrina JF, Gonzalez-Bosquet J, Weaver AL, Gaffey TA, Leslie KO, Webb MJ, et al. Squamous cell carcinoma of the vulva stage IA: long-term results. Gynecol Oncol 2000;76:24-7. http://dx.doi.org/10.1006/gyno.1999.5638.
- Reynolds RK, Loar PV, Doherty G. Current Diagnosis and Treatment: Surgery. New York, NY: McGraw-Hill; 2010.
- van Doorn HC, Ansink A, Verhaar-Langereis MM, Stalpers LL. Neoadjuvant chemoradiation for advanced primary vulvar cancer. Cochrane Database Syst Rev 2009;4:1-20.
- Oonk MH, de Hullu JA, van der Zee AG. current controversies in the management of patients with early-stage vulvar cancer. Curr Opin Oncol 2010;22:481-6. http://dx.doi.org/10.1097/CCO.0b013e32833c06da.
- Hart WR. Vulvar intraepithelial neoplasia: Historical aspects and current status. Int J Gynecol Pathol 2001;20:16-30. http://dx.doi.org/10.1097/00004347-200101000-00003.
- Sideri M, Wilkinson EJ. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med 2005;25:807-10.
- Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol 2000;24:429-41. http://dx.doi.org/10.1097/00000478-200003000-00013.
- de Bie RP, van de Niewenhof HP, Bekkers RL, Melchers WJ, Siebers AG, Bulten J, et al. Patients with usual vulvar intraepithelial neoplasia-related vulvar cancer have an increased risk of cervical abnormalities. Br J Cancer 2009;101:27-31. http://dx.doi.org/10.1038/sj.bjc.6605124.
- Eva LJ, Ganesan R, Chan KK, Honest LDM. Differentiated-type vulval intraepithelial neoplasia has a high-risk association with vulval squamous cell carcinoma. Int J Gynecol Cancer 2009;19:741-4. http://dx.doi.org/10.1111/IGC.0b013e3181a12fa2.
- Kokka F, Singh N, Faruqi A, Gibbon K, Rosenthal AN. Is differentiated vulval intraepithelial neoplasia the precursor lesion of human papillomavirus-negative vulval squamous cell carcinoma?. Int J Gynecol Cancer 2011;21:1297-305. http://dx.doi.org/10.1097/IGC.0b013e31822dbe26.
- Daling JR, Sherman K, Hislop TG, Maden C, Mandelson MT, Beckmann AM. Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol 1992;135:180-9.
- Madeleine MM, Daling JR, Schwarts SM. Cofactors with human papillomavirus in a population-based study of vulval cancer. J Natl Cancer Inst 1997;89:1516-23. http://dx.doi.org/10.1093/jnci/89.20.1516.
- Brinton LA, Nasca PC, Mallin K, Baptiste MS, Wilbanks GD, Richart RM. Case–control study of cancer of the vulva. Obstet Gynecol 1990;75:859-66.
- Hording U, Krigsholme B, Andreasson B, Visfeldt J, Daugaard S, Bock JE. Human papillomavirus in vulval squamous-cell carcinoma and in normal vulval tissues: a search for a possible impact of HPV on vulval cancer prognosis. Int J Cancer 1993;55. http://dx.doi.org/10.1002/ijc.2910550310.
- MacNab JC, Walkinshaw SA, Cordiner JW, Clements JB. Human papillomavirus in clinically and histologically normal tissue of patients with genital cancer. N Engl J Med 1986;315:1052-8. http://dx.doi.org/10.1056/NEJM198610233151703.
- Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000;92:1500-10. http://dx.doi.org/10.1093/jnci/92.18.1500.
- Cancer Research UK . Vulval Cancer Incidence Statistics n.d. http://info.cancerresearchuk.org/cancerstats/types/vulva/incidence/uk-vulva-cancer-incidence-statistics (accessed 1 April 2012).
- Mabuchi K, Bross DS, Kessler II. Epidemiology of cancer of the vulva. A case-control study. Cancer 1985;55:1843-8. http://dx.doi.org/10.1002/1097-0142(19850415)55:8<1843::AID-CNCR2820550833>3.0.CO;2-M.
- Boffetta P, Grindley G, Lindelof B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol 2001;117:1531-7.
- Penn I. Cancers of the anogenital region in renal transplant recipients. Analysis of 65 cases. Cancer 1986;58:611-16. http://dx.doi.org/10.1002/1097-0142(19860801)58:3<611::AID-CNCR2820580303>3.0.CO;2-M.
- Hacker NF, Berek JS, Hacker NF. Practical Gynaecologic Oncology. Philadelphia, PA: Williams & Wilkins; 2005.
- Handbook of Gynaecologic Oncology for Specialists and Trainees. Kuala Lumpur: Globalcrest Sdn Bhd; 2011.
- Cancer Research UK . Vulval Cancer Mortality Statistics n.d. http://info.cancerresearchuk.org/cancerstats/types/vulva/mortality/ (accessed 1st April 2012).
- National Cancer Institute . Vulvar Cancer Treatment (PDQ®) n.d. www.cancer.gov/cancertopics/pdq/treatment/vulvar/HealthProfessional/page5 (accessed 5 April 2011).
- Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study). Am J Obstet Gynecol 1991;164:997-1003. http://dx.doi.org/10.1016/0002-9378(91)90573-A.
- Homesley H, Bundy B, Sedlis A. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva. Gynecol Oncol 1993;49:279-83. http://dx.doi.org/10.1006/gyno.1993.1127.
- Lucas WE. Die stadienangepasste Behandlung des Vulvakarzinoms. Gynakologe 1981;14.
- Weijmar Schultz WC, van der Wiel HB, Bouma J, Janssens J, Littlewood J. Psychosexual functioning after the treatment of cancer of the vulva. Cancer 1990;66:402-7.
- Gould N, Kamelle S, Tillmanns T, Scribner D, Gold M, Walker J. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol 2001;82:329-32. http://dx.doi.org/10.1006/gyno.2001.6266.
- Gaarenstroom KN, Kenter GG, Trimbos JB, Agous I, Amant F, Peters AA, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer 2003;13:522-7. http://dx.doi.org/10.1046/j.1525-1438.2003.13304.x.
- Beesley V, Janda M, Eakin E, Obermair A, Battistutta D. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer 2007;109:2607-14. http://dx.doi.org/10.1002/cncr.22684.
- Pereira de Godoy BN, Braile DM, de Fatima Godoy M, Longo J. Quality of life and peripheral lymphoedema. Lymphology 2002;35:72-5.
- Helm CW, Hatch K, Austin JM, Partridge EE, Soong SJ, Elder JE, et al. A matched comparison of single and triple incision techniques for the surgical treatment of carcinoma of the vulva. Gynecol Oncol 1992;46:150-6. http://dx.doi.org/10.1016/0090-8258(92)90247-G.
- Sedlis A, Homesley H, Bundy B. Positive groin lymph nodes in superficial squamous vulvar cancer. Am J Obstet Gynecol 1987;156:1159-64. http://dx.doi.org/10.1016/0002-9378(87)90132-3.
- Lindell G, Jonsson C, Ehrsson RJ, Jacobsson H, Danielsson KG, Kallstrom BN, et al. Evaluation of preoperative lymphoscintigraphy and sentinel node procedure in vulvar cancer. Eur J Obstet Gynecol Reprod Biol 2010;152:91-5. http://dx.doi.org/10.1016/j.ejogrb.2010.05.011.
- Terada KY, Shimizu DM, Wong JH. Sentinel node dissection and ultrastaging in squamous cell Cancer of the vulva. Gynecol Oncol 2000;76:40-4.
- de Hullu JA, Ansink AC, Tymstra T, van der Zee AG. What doctors and patients think about false-negative sentinel lymph nodes in vulvar cancer. J Psychosom Obstet Gynecol 2001;22:199-203. http://dx.doi.org/10.3109/01674820109049974.
- Systematic reviews, CRD's Guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Higgins JT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley-Blackwell; 2008.
- Diagnostic Test Accuracy Working Group . Handbook for DTA Reviews 2012. http://srdta cochrane org/handbook-dta-reviews (accessed January 2012).
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. http://dx.doi.org/10.1016/j.jclinepi.2009.06.005.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Meads CA, Davenport CF. Quality assessment of diagnostic before-after studies: development of methodology in the context of a systematic review. BMC Med Res Methodol 2009;9. http://dx.doi.org/10.1186/1471-2288-9-3.
- Wells G, Shea B, O’Coonell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analysis n.d. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 5 April 2012).
- Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L. Do findings of case series studies vary significantly according to methodological characteristics?. Health Technol Assess 2005;9.
- Achimas-Cadariu P, Harter P, Fisseler-Eckhoff A, Beutel B, Traut A, Du BA. Assessment of the sentinel lymph node in patients with invasive squamous carcinoma of the vulva. Acta Obstet Gynecol Scand 2009;88:1209-14. http://dx.doi.org/10.3109/00016340903317982.
- Basta A, Pitynski K, Basta P, Hubalewska-Hola A, Oplawski M, Przesziakowski D. Sentinel node in gynaecological oncology. Rep Pract Oncol Radiother 2005;10:1-4.
- Brunner AH, Polterauer S, Tempfer C, Joura E, Reinthaller A, Horvat R, et al. The accuracy of intraoperative frozen section of the inguinal sentinel lymph node in vulvar cancer. Anticancer Res 2008;28:4091-4.
- Camara O, Gonnert H, Herrmann J, Egbe A, Diebolder H, Gajda M, et al. Sentinel lymph node biopsy in vulvar cancer: a pilot study. Eur J Gynaecol Oncol 2009;30:622-4.
- Crosbie EJ, Winter-Roach B, Sengupta P, Sikand KA, Carrington B, Murby B, et al. The accuracy of the sentinel node procedure after excision biopsy in squamous cell carcinoma of the vulva. Surg Oncol 2010;19:e150-4. http://dx.doi.org/10.1016/j.suronc.2010.08.003.
- De Cicco C, Sideri M, Bartolomei M, Grana C, Cremonesi M, Fiorenza M, et al. Sentinel node biopsy in early vulvar cancer. Br J Cancer 2000;82:295-9.
- de Hullu JA, Hollema H, Piers DA, Verheijen RH, van Diest PJ, Mourits MJ, et al. Sentinel lymph node procedure is highly accurate in squamous cell carcinoma of the vulva. J Clin Oncol 2000;18:2811-16.
- Hampl M, Hantschmann P, Michels W, Hillemanns P. Validation of the accuracy of the sentinel lymph node procedure in patients with vulvar cancer: results of a multicenter study in Germany. Gynecol Oncol 2008;111:282-8. http://dx.doi.org/10.1016/j.ygyno.2008.08.007.
- Hauspy J, Beiner M, Harley I, Ehrlich L, Rasty G, Covens A. Sentinel lymph node in vulvar cancer. Cancer 2007;110:1015-23. http://dx.doi.org/10.1002/cncr.22874.
- Johann S, Klaeser B, Krause T, Mueller MD. Comparison of outcome and recurrence-free survival after sentinel lymph node biopsy and lymphadenectomy in vulvar cancer. Gynecol Oncol 2008;110:324-8. http://dx.doi.org/10.1016/j.ygyno.2008.04.004.
- Klat J, Sevcik L, Simetka O, Graf P, Waloschek T, Kraft O, et al. Characteristics of sentinel lymph nodes' metastatic involvement in early stage of vulvar cancer. Aust N Z J Obstet Gynaecol 2009;49:672-6.
- Levenback C, Coleman RL, Burke TW, Bodurka-Bevers D, Wolf JK, Gershenson DM. Intraoperative lymphatic mapping and sentinel node identification with blue dye in patients with vulvar cancer. Gynecol Oncol 2001;83:276-81. http://dx.doi.org/10.1006/gyno.2001.6374.
- Louis-Sylvestre C, Evangelista E, Leonard F, Itti E, Meignan M, Paniel BJ. Interpretation of sentinel node identification in vulvar cancer. Gynecol Obstet Fertil 2006;34:706-10.
- Martinez-Palonez JM, Perez-Benavente MA, Gil-Moreno A, Diaz-Feijoo B, Roca I, Garcia-Jimenez A, et al. Comparison of recurrence after vulvectomy and lymphadenectomy with and without sentinel node biopsy in early stage vulvar cancer. Gynecol Oncol 2006;103:865-70. http://dx.doi.org/10.1016/j.ygyno.2006.05.024.
- Merisio C, Berretta R, Gualdi M, Pultrone DC, Anfuso S, Agnese G, et al. Radioguided sentinel lymph node detection in vulvar cancer. Int J Gynecol Cancer 2005;15:493-7. http://dx.doi.org/10.1111/j.1525-1438.2005.15314.x.
- Moore RG, Robison K, Brown AK, DiSilvestro P, Steinhoff M, Noto R, et al. Isolated sentinel lymph node dissection with conservative management in patients with squamous cell carcinoma of the vulva: a prospective trial. Gynecol Oncol 2008;109:65-70. http://dx.doi.org/10.1016/j.ygyno.2007.12.027.
- Nyberg RH, Iivonen M, Parkkinen J, Kuoppala T, Maenpaa JU. Sentinel node and vulvar cancer: a series of 47 patients. Acta Obstet Gynecol Scand 2007;86:615-19. http://dx.doi.org/10.1080/00016340701286793.
- Pitynski K, Basta A, Oplawski M, Przeszlakowski D, Hubalewska-Hola A, Krysztopowicz W. Lymph node mapping and sentinel node detection in carcinoma of the cervix, endometrium and vulva. Ginekol Pol 2003;74:830-5.
- Radziszewski J, Kowalewska M, Jedrzejczak T, Kozlowicz-Gudzinska I, Nasierowska-Guttmejer A, Bidzinski M, et al. The accuracy of the sentinel lymph node concept in early stage squamous cell vulvar carcinoma. Gynecol Oncol 2010;116:473-7. http://dx.doi.org/10.1016/j.ygyno.2009.10.072.
- Rob L, Robova H, Pluta M, Strnad P, Kacirek J, Skapa P, et al. Further data on sentinel lymph node mapping in vulvar cancer by blue dye and radiocolloid Tc99. Int J Gynecol Cancer 2007;17:147-53. http://dx.doi.org/10.1111/j.1525-1438.2007.00806.x.
- Terada KY, Shimizu DM, Jiang CS, Wong JH. Outcomes for patients with T1 squamous cell cancer of the vulva undergoing sentinel node biopsy. Gynecol Oncol 2006;102:200-3. http://dx.doi.org/10.1016/j.ygyno.2005.11.042.
- Vakselj A, Bebar S. The role of sentinel lymph node detection in vulvar carcinoma and the experiences at the Institute of Oncology Ljubljana. Radiol Oncol 2007;41:167-73. http://dx.doi.org/10.2478/v10019-007-0027-4.
- Van den Eynden J, Lannoo L, Amant F, Stroobants S, Vergote I. Sentinel node biopsy in the treatment of vulvar cancer. Tijdschr Voor Geneeskunde 2003;59:1169-78.
- Van der Zee AG, Oonk MH, de Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 2008;26:884-9. http://dx.doi.org/10.1200/JCO.2007.14.0566.
- Vidal-Sicart S, Puig-Tintore LM, Lejarcegui JA, Paredes P, Ortega ML, Munoz A, et al. Validation and application of the sentinel lymph node concept in malignant vulvar tumours. Eur J Nucl Med Mol Imaging 2007;34:384-91. http://dx.doi.org/10.1007/s00259-006-0237-9.
- Sliutz G, Reinthaller A, Lantzsch T, Mende T, Sinzinger H, Kainz C, et al. Lymphatic mapping of sentinel nodes in early vulvar cancer. Gynecol Oncol 2002;84:449-52. http://dx.doi.org/10.1006/gyno.2001.6572.
- Hefler LA, Grimm C, Six L, Seebacher V, Polterauer S, Joura E, et al. Inguinal sentinel lymph node dissection vs. complete inguinal lymph node dissection in patients with vulvar cancer. Anticancer Res 2008;28:515-17.
- de Hullu JA, Doting E, Piers DA, Hollema H, Aalders JG, Koops HS, et al. Sentinel lymph node identification with technetium-99m-labeled nanocolloid in squamous cell cancer of the vulva. J Nucl Med 1998;39:1381-5.
- Levenback C, Burke TW, Gershenson DM, Morris M, Malpica A, Ross MI. Intraoperative lymphatic mapping for vulvar cancer. Obstet Gynecol 1994;84:163-7.
- Levenback C, Burke TW, Morris M, Malpica A, Lucas KR, Gershenson DM. Potential applications of intraoperative lymphatic mapping in vulvar cancer. Gynecol Oncol 1995;59:216-20.
- Frumovitz M, Ramirez PT, Tortolero-Luna G, Malpica A, Eifel P, Burke TW, et al. Characteristics of recurrence in patients who underwent lymphatic mapping for vulvar cancer. Gynecol Oncol 2004;92:205-10. http://dx.doi.org/10.1016/j.ygyno.2003.09.022.
- Rob L, Robova H, Pluta M, Strnad P, Kacirek J, Chmel R, et al. Sentinel lymph nodes identification in vulvar cancer–methods and technique. Ceska Gynekol 2006;71:298-301.
- Oonk MH, van Os MA, de Bock GH, de Hullu JA, Ansink AC, van der Zee AG. A comparison of quality of life between vulvar cancer patients after sentinel lymph node procedure only and inguinofemoral lymphadenectomy. Gynecol Oncol 2009;113:301-5. http://dx.doi.org/10.1016/j.ygyno.2008.12.006.
- Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol 2010;11:646-52. http://dx.doi.org/10.1016/S1470-2045(10)70104-2.
- Vidal-Sicart S, Puig-Tintore LM, Ordi J, Lejarcegui JA. Localisation of sentinel lymph nodes in cancer of the vulva. Ginecol Clin Quir 2002;3:67-71.
- Vidal-Sicart S, Domenech B, Lujan B, Pahisa J, Torne A, Martinez-Roman S, et al. Sentinel node in gynaecological cancers. Our experience. Rev Esp Med Nucl 2009;28:221-8. http://dx.doi.org/10.1016/S1578-200X(09)70022-1.
- Puig-Tintore LM, Ordi J, Vidal-Sicart S, Lejarcegui JA, Torne A, Pahisa J, et al. Further data on the usefulness of sentinel lymph node identification and ultrastaging in vulvar squamous cell carcinoma. Gynecol Oncol 2003;88:29-34. http://dx.doi.org/10.1006/gyno.2002.6857.
- Stehman FB, Bundy BN, Thomas G, Varia M, Okagaki T, Roberts J, et al. Groin dissection versus groin radiation in carcinoma of the vulva: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys 1992;24:389-96. http://dx.doi.org/10.1016/0360-3016(92)90699-I.
- Manavi M, Berger A, Kucera E, Vavra N, Kucera H. Does T1, N0-1 vulvar cancer treated by vulvectomy but not lymphadenectomy need inguinofemoral radiation?. Int J Radiat Oncol Biol Phys 1997;38:749-53. http://dx.doi.org/10.1016/S0360-3016(97)00060-6.
- Stehman FB, Bundy BN, Dvoretsky PM, Creasman WT. Early stage I carcinoma of the vulva treated with ipsilateral superficial inguinal lymphadenectomy and modified radical hemivulvectomy: a prospective study of the Gynecologic Oncology Group. Obstet Gynecol 1992;79:490-7.
- Katz A, Eifel PJ, Jhingran A, Levenback CF. The role of radiation therapy in preventing regional recurrences of invasive squamous cell carcinoma of the vulva. Int J Radiat Oncol Biol Phys 2003;57:409-18. http://dx.doi.org/10.1016/S0360-3016(03)00591-1.
- Anderson JM, Cassady JR, Shimm DS, Stea B. Vulvar carcinoma. Int J Radiat Oncol Biol Phys 1995;32:1351-7. http://dx.doi.org/10.1016/0360-3016(95)00090-L.
- Andrews SJ, Williams BT, DePriest PD, Gallion HH, Hunter JE, Buckley SL, et al. Therapeutic implications of lymph nodal spread in lateral T1 and T2 squamous cell carcinoma of the vulva. Gynecol Oncol 1994;55:41-6. http://dx.doi.org/10.1006/gyno.1994.1244.
- Burke TW, Levenback C, Coleman RL, Morris M, Silva EG, Gershenson DM. Surgical therapy of T1 and T2 vulvar carcinoma: further experience with radical wide excision and selective inguinal lymphadenectomy. Gynecol Oncol 1995;57:215-20. http://dx.doi.org/10.1006/gyno.1995.1128.
- Busch M, Wagener B, Schaffer M, Duhmke E. Long-term impact of postoperative radiotherapy in carcinoma of the vulva FIGO I/II. Int J Radiat Oncol Biol Phys 2000;48:213-18. http://dx.doi.org/10.1016/S0360-3016(00)00586-1.
- de Hullu JA, Hollema H, Lolkema S, Boezen M, Boonstra H, Burger MP, et al. Vulvar carcinoma. The price of less radical surgery. Cancer 2002;95:2331-8. http://dx.doi.org/10.1002/cncr.10969.
- DeSimone CP, Van Ness JS, Cooper AL, Modesitt SC, DePriest PD, Ueland FR, et al. The treatment of lateral T1 and T2 squamous cell carcinomas of the vulva confined to the labium majus or minus. Gynecol Oncol 2007;104:390-5. http://dx.doi.org/10.1016/j.ygyno.2006.08.035.
- Farias-Eisner R, Cirisano FD, Grouse D, Leuchter RS, Karlan BY, Lagasse LD, et al. Conservative and individualized surgery for early squamous carcinoma of the vulva: the treatment of choice for stage I and II (T1-2N0-1M0) disease. Gynecol Oncol 1994;53:55-8.
- Hallak S, Ladi L, Sorbe B. Prophylactic inguinal-femoral irradiation as an alternative to primary lymphadenectomy in treatment of vulvar carcinoma. Int J Oncol 2007;31:1077-85.
- Kumar S, Shah JP, Bryant CS, Imudia AN, Morris RT, Malone JM. A comparison of younger vs. older women with vulvar cancer in the United States. Am J Obstet Gynecol 2009;200:e52-5. http://dx.doi.org/10.1016/j.ajog.2008.09.869.
- Scheistroen M, Nesland JM, Trope C. Have patients with early squamous carcinoma of the vulva been overtreated in the past? The Norwegian experience 1977–1991. Eur J Gynaecol Oncol 2002;23:93-103.
- Tantipalakorn C, Robertson G, Marsden DE, Gebski V, Hacker NF. Outcome and patterns of recurrence for International Federation of Gynecology and Obstetrics (FIGO) stages I and II squamous cell vulvar cancer. Obstet Gynecol 2009;113:895-901.
- Vavra N, Kucera H, Egarter C, Weghaupt K. Effect of inguinal lymph node irradiation on the treatment result of vulvar cancer with different risk factors. Geburtshilfe Frauenheilkd 1990;50:470-6.
- Office for National Statistics . Natural Death Rates. 2010 Mid-Year Estimates n.d. www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2010/index.html (accessed 1 November 2011).
- Fonseca-Moutinho JA, Coelho MC, Silva DP. Vulvar squamous cell carcinoma. Prognostic factors for local recurrence after primary en bloc radical vulvectomy and bilateral groin dissection. J Reprod Med 2000;45:672-8.
- NHS Reference Costs 2009/2010. London: Department of Health; 2010.
- Levenback CF, Ali S, Coleman RL, Gold M, Fowler JM, Judson PL, et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a Gynecologic Oncology Group study. J Clin Oncol 2012;30:3786-91. http://dx.doi.org/10.1200/JCO.2011.41.2528.
- Meads C, Auguste P, Davenport C, Malysiak S, Sundhar S, Kowalska M, et al. Positron emission tomography/computerised tomography imaging in detecting and managing recurrent cervical cancer: Systematic review of evidence, elicitation of subjective probabilities and economic modelling. Health Technol Assess 2013;17.
Appendix 1 Protocol
Sentinel lymph node (SLN) status in vulval cancer: Systematic quantitative reviews and decision-analytic model based economic evaluation
1 Clinical background
Vulval cancer accounts for approximately 3–5% of all gynaecological malignancies and 1% of all cancers in women, with an incidence rate of 1–2/100,000. 1 In the UK, it affects approximately 1,063 women every year with a 1 in 316 lifetime risk of developing vulval cancer. 2 Mortality data from 2007 shows 384 deaths in the UK. 2 Squamous cell carcinomas (SCC) account for more than 90% of vulval cancers;3 the other 10% include melanomas, sarcomas, basal cell carcinomas and adenocarcinomas. 4 Although its peak incidence is in the 7th decade, there has been a significant increase in rates of vulval cancer in younger women. The proportion of women diagnosed with this cancer under the age of 50 rose from 6% in 1975 to 15% in 2006. 2 This trend has been observed in many countries, and has been linked to the rising incidence of vulval intraepithelial neoplasia (VIN) in young women caused by infection with HPV. 5,6
Vulval cancer is curable when diagnosed at an early stage. The standard treatment for squamous cell carcinoma of the vulva is radical surgery, which in all but stage Ia or superficially invasive disease includes inguinofemoral lymphadenectomy. 7 The inguinal lymph node status has been identified as the single most important factor in predicting mortality attributable to vulval cancer. 8 Overall, about a third of patients with operable disease have nodal spread. 7 Those patients with primary lesions not more than 2 cm, who are inguinal node negative have a 98% 5-year survival rate, while those with any size lesion and three or more unilateral nodes or two or more bilateral nodes associated have a 29% 5-year survival rate. 9 Morbidity from lymphadenectomy is high with significant negative impact on the Quality of Life (QoL). Nodal assessment with biopsy is currently not routinely performed in practice.
The likelihood of metastasis is related to the size and the depth of the primary tumour. In stage Ia, this likelihood is almost zero, and rises once invasion extends beyond 1 mm depth. At the time of presentation, up to 25% of patients with vulval cancer are stage I, and of these, 30% are stage Ia. In absolute terms, this means that in any one year there will be a requirement for 700–750 groin lymph node dissections in the UK. 10 However, only around 30% of these operated cases will have evidence of nodal involvement;7 the rest being node negative. This project will assess if nodal biopsy can be accurately and efficiently performed to direct the need for further lymphadenectomy.
1.1 Current clinical practice
Traditionally, the management of vulval cancer involves radical surgery which includes the excision of the primary lesion and unilateral or bilateral superficial and deep inguinofemoral lymphadenectomy. 7 The efficacy of this treatment is good, with reported groin recurrence rates varying between 1% and 10%. 11 However, as only 25% – 35% of patients with early-stage disease will have lymph node metastases,7,12 and the remaining 65% – 75% possibly do not benefit from elective inguinofemoral lymphadenectomy while risking significant morbidity. 13 In the short term, wound healing in the groin is compromised by infection and breakdown in 20% to 40% of patients. 13 In the long term, lymphoedema of the legs with increased risk for erysipelas occurs in 30% to 70% of patients. 13 These complications can be incapacitating with major impact on sexual and psychological function. 14 Patients are also subjected to groin radiotherapy if cancer metastasis is detected on histopathological examination of lymph nodes. Patients treated with both complete inguinofemoral lymph node dissection and external beam radiotherapy to groin nodes suffer the morbidity of both treatments with a higher risk of lymphoedema and cellulitis.
Despite significant surgical morbidity and a low frequency of lymph node metastases, an elective inguinofemoral lymphadenectomy is regarded as standard of care. This is because unrecognised disease in the inguinofemoral lymph nodes is nearly always fatal. 13 An accurate test is needed that could identify those patients in whom the risk of metastases is low. Such a test could help exclude the need for lymphadenectomy and would be extremely valuable. A minimally invasive technique to detect metastasis to the groin lymph nodes has a huge potential to reduce unnecessary morbidity.
1.2 Tests for nodal involvement
Assessment of the nodal status by clinical palpation of the groins is inadequate; of patients with clinically normal lymph nodes, 16–24% has metastases, while 24–41% of those with clinically involved nodes are negative at histological examination. 15,16 There are several minimally and non-invasive tests available for the status of groin nodes in vulval cancer, but none are routinely used in clinical practice. These include ultrasonography with or without fine-needle aspiration, computerised tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) and SLN identification (using blue dye or technetium-99m-labelled nanocolloid—99mTc). 7 This call for proposals focuses on the value of sentinel node biopsy using 99mTc or blue dye.
1.3 Sentinel lymph node (SLN) identification by lymphoscintigraphy
SLN refers to any lymph node that receives drainage directly from the primary tumour17 (Fig 1). The SLN will be the lymph node with the highest probability of containing metastasis. Removal of SLN should therefore allow assessment of the status of lymphatic basin without the need to remove all the lymph nodes, providing an opportunity to avoid the morbidity associated with formal lymphadenectomy. If SLN is negative the rest of the groin should be at least risk of having subclinical metastasis. 18 The removal of fewer nodes (typically 1-3/groin) also permits more focussed pathological assessment of the SLN and direct pathological resources compared with 10-20 nodes removed by inguinofemoral lymphadenectomy. 18 SLNs are identified by isosulfan blue dye or 99mTc enhanced lymphoscintigraphy alone or in combination. 18

Pre operatively 99mTc nanocolloid is injected around the primary tumour and dynamic images are obtained. The first site of focal accumulation is considered to be the sentinel node. During surgery a gamma probe (typically a caesium iodide scintillator) is used to confirm the location of the SLN. Once the SLN have been removed the groin is rescanned to ensure removal of all SLNs. A background count after SLN removal of < 10% of the initial value is typically used to confirm that all relevant nodes have been sampled. The signal arising from the SLN will depend on the dose of isotope used, the time lapse since injection, the number of and rate of flow within lymphatic channels and the distance between the probe and the target SLN. Where the signal from the SLN is weak, removal of the primary injection site on the vulval may be performed first, to facilitate localisation of the SLN in the absence of background signal.
99mTc enhanced lymphoscintigraphy has the advantage that in comparison to blue dye it facilitates localisation of the SLN even prior to the skin incision, potentially enabling a smaller groin incision (2 cm) to be used. The use of isotope also permits detection of sentinel nodes outside the usual basin, with the identification of aberrant drainage in those cases of clitoral involvement. Use of radiocolloid alone in vulvar carcinoma can avoid complications from blue dye (allergic reactions, permanent staining, and false oximeter readings. 19 This technique has already become well established in breast cancer where it was found to be better than blue dye in the identification of the sentinel node20 and available data in vulval cancer would support the increased sensitivity afforded by the use of isotope.
Blue dye is injected intraoperatively around the site of primary tumour. This test is done in isolation or in combination with 99mTc enhanced lymphoscintigraphy. The use of blue dye to identify the sentinel node has the advantage that the entire test is performed under general anaesthetic at the time of the operation; hence additional preoperative assessment is not required. This technique does require a sizable skin incision to allow the surgeon dissection down to Camper's fascia and identification of the blue afferent channel to the blue/sentinel node.
1.4 Histopathological examination method of SLN
The accuracy of SLN biopsy in staging vulval cancer depends on the histopathological technique used for the examination of the sentinel nodes. Post-operative pathological assessment of the SLN may be combined with intra operative frozen section analysis. 18 Frozen section offers the advantage of performing synchronous complete lymphadenectomy and SLN biopsy if the SLNs are positive. The sensitivity of frozen section usually by single haematoxylin and eosin (H/E) staining is around 80%. 21 Apart from the concerns of accuracy with high false negative rates, there is a risk of loss of diagnostic tissue with intra operative methods. Standard pathologic examination of lymph nodes, i.e., H&E staining of a bivalved node, will sample only a fraction of the resected tissue. This sampling methodology could potentially fail to detect lymph node metastasis in the SLN producing a false negative result. Detailed analysis with serial sectioning and immunohistochemical staining can identify micrometastases that are not otherwise apparent. Enhanced pathologic analysis, termed “ultrastaging,” generally involves serial sections through the node and application of specific immunohistochemical staining for epithelial antigens. Post-operative ultrastaging is labour intensive and could not be performed on the large numbers of nodes removed at formal lymphadenectomy. It has been shown to improve the sensitivity in detecting micro and macro metastatic deposits compared with routinely stained sections. 22 Whilst the biological relevance of metastases detected by ultrastaging remains controversial, groin recurrence has been identified in patients with micrometastasis only in the SLN. 18 Reverse transcriptase-polymerase chain reaction (RT-PCR) has the potential to accurately detect micrometastasis. 20 Their use is limited for SLN biopsy in other tumours with very little data in vulval cancer. The therapeutic impact of stage migration due to detection of micrometastasis by the use of techniques with ever increasing sensitivity compared with standard histopathology needs further evaluation.
1.5 Role of SLN biopsy in clinical management of vulval cancer
Early vulval cancer may be treated with radical excision of the primary tumour in combination with SLN biopsy. Most protocols utilise ultrastaging for assessment of the SLN. Where the SLNs are negative no further treatment is employed and the patient is observed. Where such a protocol is followed for patients with small (< 4 cm) tumours and no obvious preoperative metastases, the groin recurrence rate is low (3% including multifocal disease; 2.3% in unifocal vulvar disease), there is excellent disease-specific survival rate of 97% at 3 years and minimal treatment-related morbidity. 13 If SLNs are positive for micro or macrometastasis, the patients typically undergo inguinofemoral lymphadenectomy. For those with a metastasis > 5 mm, more than one intranodal metastasis and/or extranodal spread, postoperative external-beam radiotherapy (50 Gy) to the groin/pelvis is recommended, possibly combined with chemotherapy. There are no randomised controlled trials (RCT) in vulval cancer that compare the effect of SLN biopsy on treatment and outcome due to the rarity of the condition. In fact, the EORTC (European Organisation for Research and Treatment of Cancer) withheld an attempt to assess SLN biopsy by an RCT as the power calculation estimated a prohibitively large sample size. 21 An ongoing study (GROINSS-V II GROningen INternational Study on Sentinel nodes in Vulvar cancer) is investigating the safety of omitting further surgery in such SLN positive patients but this is not yet within standard practice.
The performance of SLN biopsy is known to be associated with a learning curve. 23 Due to the consistent pattern of lymphatic drainage of the vulva the learning curve is considered to be much steeper for vulval cancer than other tumours. Introduction of SLN biopsy into routine practice will require quality control at each step. This multidisciplinary procedure, includes injection of radioactive tracer by either the surgeon or a nuclear medicine physician familiar with vulval anatomy, careful interpretation of lymphoscintigram, a surgeon with successful experience (sentinel node procedure followed by full lymphadenectomy) in at least 10 patients, and a pathology department experienced in ultrastaging (laterally sectioning of the nodes at 3-mm intervals and then each block cut at 400-μm intervals) of the sentinel nodes. 13
There is a need to systematically review the comparative accuracy of 99mTc and blue dye for SLN biopsy in staging for vulval cancer. Moreover it is important to review how testing of sentinel nodes will impact on staging, therapeutic options and outcomes. Fig 2 conceptualises the role of SLN biopsy in the management of vulval cancer to be evaluated in this project.
Fig 2.
SLN biopsy and treatment strategies in women with vulval cancer.
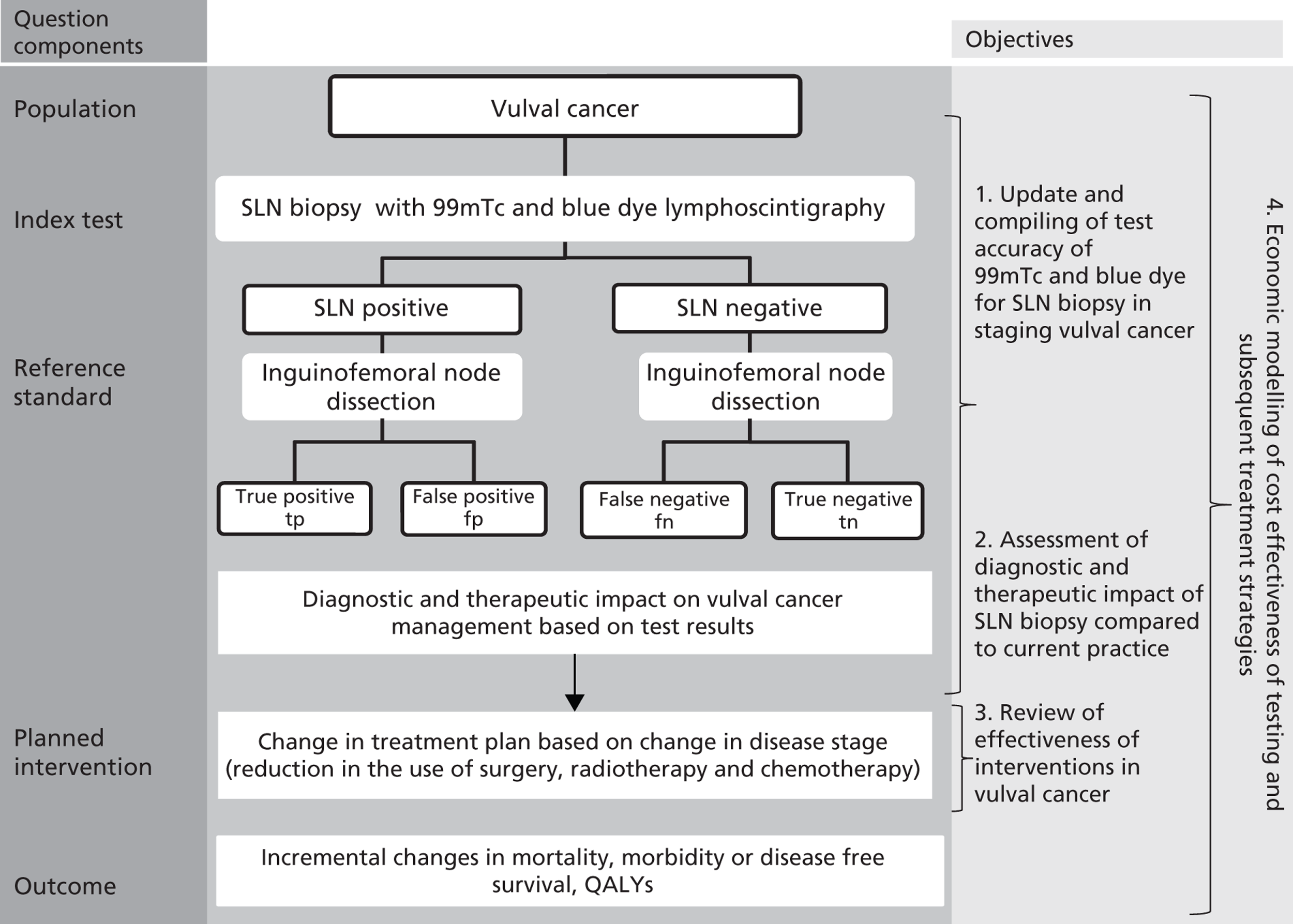
2 Work leading to the proposal
An MRC Fellowship awarded to TS supervised by KSK and TR conducted systematic reviews of accuracy of tests for lymph node metastasis in gynaecologic oncology24 (PhD awarded at University of Birmingham in 2009). We published a systematic review of accuracy of tests that evaluate node status in vulval cancer in 2005. We have also initiated a decision-analytic model based economic evaluation to determine the relative costs and effectiveness of a range of alternative preoperative tests and subsequent management strategies for inguinofemoral lymph nodes. 24 This HTA call for proposals gives us the opportunity to update the accuracy review, to use more robust statistical methods for meta analysis,25 to complete the economic evaluation initiated, and to undertake a probabilistic sensitivity analysis on the model.
2.1 Systematic review of accuracy of tests for sentinel node status
We identified relevant literature from 1974–2005 to conduct the review using a prospective protocol and widely recommended methods. 26,27 The initial search generated 1154 citations from which 82 articles were potentially relevant. After assessment of the full manuscripts, a total of 24 articles that reported 29 tests were selected. 10 Studies included in the review were those that compared the index test to the histological evaluation of inguinofemoral lymphadenectomy specimen. Eleven studies evaluated the accuracy of 99mTc and 8 studied blue dye for SLN biopsy in staging. SLN biopsy using 99mTc had a pooled sensitivity and negative Likelihood ratio (LR-) of 97% (91–100 95% CI) and 0.12 (0.053–0.28 95% CI), respectively, and was the most accurate of the tests reviewed. 10 Blue dye alone for identification of SLN had a pooled sensitivity of 95% (82–99 95% CI) and LR- of 0.16 (0.07–0.32 95% CI). 10 This review needs updating with reassessment of the study quality, use of bivariate meta analysis and metaregression to compare 99mTc vs blue dye.
| Study ( year) | TP | FP | TN | FN | Sensitivity (95% CI) | Specificity (95% CI) | LR + (95% CI) | LR– (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Isosulfan blue dye | ||||||||
| Levenback 1994 | 2 | 0 | 5 | 0 | 100% (63–100) | 100% (48–100) | 10 (0.67–149) | 0.18 (0.01–2.31) |
| Levenback 1995 | 3 | 0 | 15 | 0 | 100% (29–100) | 100% (78–100) | 28 (1.78–439.41) | 0.13 (0.01–1.73) |
| Ansink et al 1999 | 9 | 0 | 41 | 2 | 81% (48–98) | 100% (91–100) | 66.5 (4.17–1061) | 0.21 (0.07–0.64) |
| Echt 1999 | 2 | 0 | 7 | 0 | 100% (16–100) | 100% (59–100) | 13.3 (0.87–204.7) | 0.18 (0.01–2.25) |
| Molups 2001 | 2 | 0 | 6 | 0 | 100% (16–100) | 100% (54–100) | 11.6 (0.77–176.84) | 0.18 (0.01–2.27) |
| Levenback 2001 | 10 | 0 | 45 | 0 | 100% (69–100) | 100% (92–100) | 88 (5.6–1387) | 0.05 (0.003–0.69) |
| Puig-Tintore 2003 | 5 | 0 | 24 | 0 | 100% (48–100) | 100% (86–100) | 45.8 (2.92–720.2) | 0.08 (0.006–1.21) |
| Moore 2003 | 3 | 0 | 16 | 0 | 100% (29–100) | 100% (79–100) | 29.75 (1.89–456.0) | 0.13 (0.01–1.72) |
| Summary | 36 | 0 | 159 | 2 | 95% (82–99) | 100% (98–100) | 27.4 (10.4–72.2) | 0.16 (0.07–0.32) |
| 99mTc identification | ||||||||
| DeCesart 1997 | 10 | 0 | 0 | 0 | 96% (68–100) | 50% (6–95) | – | – |
| de Hullu 1998 | 2 | 0 | 8 | 0 | 100% (16–100) | 100% (63–100) | 15 (.96–232) | 0.18 (0.014–2.23) |
| De Cicco 2000 | 8 | 0 | 29 | 0 | 100% (16–100)) | 100% (88–100) | 50 (3.06–817.98) | 0.17 (0.013–2.13) |
| De Hullu 2000 | 27 | 0 | 68 | 0 | 100% (87–100) | 100% (95–100) | 135.54 (8.6–2146) | 0.018 (0.001–0.28) |
| Sideri 2000 | 13 | 0 | 31 | 0 | 100% (75–100) | 100% (89–100) | 61.7 (3.94–867.23) | 0.04 (0.002–0.52) |
| Molpus 2001 | 2 | 0 | 6 | 0 | 100% (16–100) | 100% (54–100) | 11.7 (0.78–176.84) | 0.18 (0.01–2.27) |
| Tavares 2002 | 3 | 0 | 12 | 0 | 100% (29–100) | 100% (73–1000) | 22.8 (1.46–353.43) | 0.13 (0.01–1.74) |
| Boran 2002 | 4 | 0 | 11 | 2 | 67% (22–96) | 100% (71–100) | 15.4 (0.97–245.88) | 0.37 (0.14–1.01) |
| Sliutz 2002 | 9 | 0 | 17 | 0 | 100% (66–100) | 100% (80–100) | 34.2 (2.22–527.99) | 0.05 (0.003–0.76) |
| Moore 2003 | 9 | 0 | 22 | 0 | 100% (66–100) | 100% (85–100) | 43.7 (2.81–680.31) | 0.05 (0.003–0.76) |
| Puig-Tintore 2003 | 5 | 0 | 25 | 0 | 100% (48–100) | 100% (86–100) | 47.7 (3.03–749.83) | 0.08 (0.006–1.21) |
| Summary | 91 | 0 | 229 | 2 | 97% (91–100) | 100% (98–100) | 33.4 (14.–79.8) | 0.12 (0.053–0.28) |
2.2 Prospective observational study of SLN biopsy in early vulval cancer (GROINSS-V GROningen INternational Study on Sentinel nodes in Vulvar cancer)
PB was the principal UK investigator of the GROINSS-V study and is now the chief investigator for the UK in the follow-on GROINSS-V II study. GROINSS-V was a large, prospective, multicentre observational study on SLN detection using radioactive tracer and blue dye in patients with early vulval cancer. 13 The study demonstrated that for appropriately selected patients, sentinel node dissection appears to be safe, reliable and associated with reduced morbidity as compared with formal inguinofemoral lymphadenectomy. The accuracy of SLN biopsy has been verified by two different reference standards. When SLN was found to be negative, inguinofemoral lymphadenectomy was omitted, and the patient was observed with follow-up for 2 years at intervals of every 2 months for groin recurrences. There is a risk of bias with this differential verification. Nevertheless GROINSS-V is the largest single well conducted study (n = 403) to date compared with other studies evaluating SLN biopsy. Furthermore it offers prospective data to evaluate the actual therapeutic impact of the alternate strategy of performing SLN biopsy without routine inguinofemoral lymphadenectomy.
2.3 Review of outcomes following inguinofemoral lymphadenectomy
We systematically reviewed the literature (1974–2005) for relevant clinical outcomes, Quality Adjusted Life Years (QALYs) and early and late complication rates following inguinal femoral lymphadenectomy. 24 The 5 year survival rate was estimated from published studies for node negative and node positive patients, the range of survival depending on the size of primary vulval lesion and the number of positive lymph nodes. For those with negative lymph nodes as point estimate for five year survival was 84% (70–98%) and for node positive patients 42.5% (25–60%). QALYs are the preferred outcome taking into account both the quantity and quality of life. 24 We failed to identify any studies that had used QALY data. As patients suffering for breast cancer also suffer similar lymphoedema a review of that literature was undertaken, but this also failed to provide relevant data. The data on complication rates came from studies specifically reporting on complications of lymphadenectomy excluding the radical vulvectomy procedure and from those that used a triple incision approach to the management of vulval cancer. 28 Two studies reported immediate post operative complication rates ranging from 44% to 66%. 29 A literature review of long-term complications found variation in rates from 12–51% with an average point estimate for the model of 34%. 30–32 The duration of inpatient stay was taken from local hospital statistics (Birmingham Women's Hospital NHS foundation Trust). We will obtain updated data from current inpatient statistics from the Pan Birmingham gynaecological cancer centre and the Addenbrookes NHS trust for this project. This review needs updating with recent searches and where published data are not available, we will contact the individual specialist centres to provide more information. This will enable us to evaluate the diagnostic and therapeutic impact of performing SLN biopsy over inguinofemoral lymphadenectomy.
2.4 Model based cost-effectiveness analysis of testing for sentinel nodes
We initiated an economic evaluation using a decision analytic model to compare strategies that used the results from various pre operative tests to determine the need for performing inguinofemoral lymphadenopathy in women over 70 years of age with vulval cancer. 24 This work is not prepared for publication as there are several areas for improvement identified below. Fig 3 shows a subset of the model to illustrate the approach developed.
Fig 3.
Branch of the decision tree model used in cost-effectiveness analysis of treatment strategies for inguinal femoral lymph nodes in women with vulval cancer.
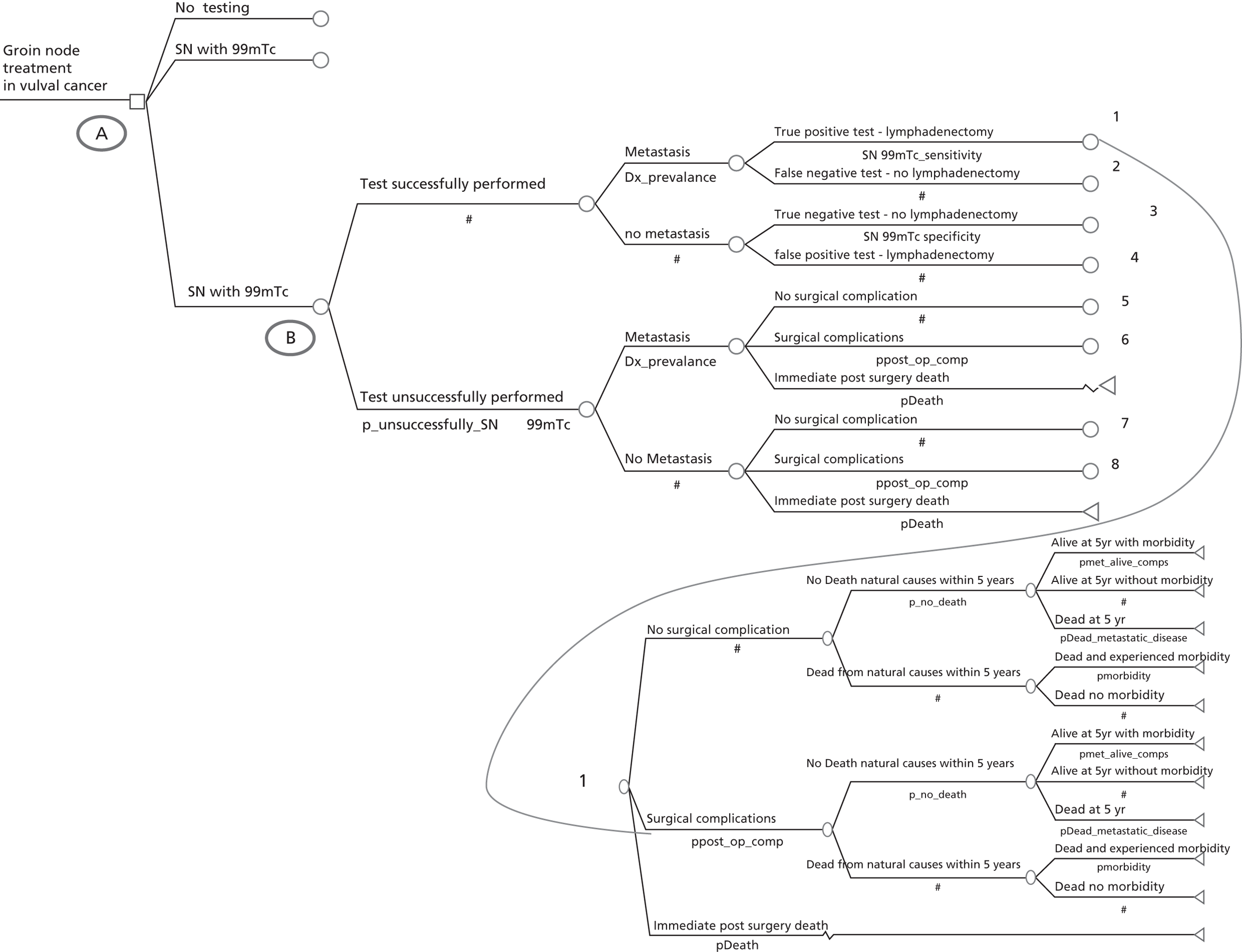
The outcomes were cost per morbidity-free 5 year survival and cost per death avoided at 5 year. Absence of relevant data prevented the estimation of cost per QALY. The strategy of inguinal femoral lymphadenectomy for all patients with vulval cancer without pre operative lymph node testing was the most expensive and not the most effective in avoiding death. It was the least effective in providing morbidity-free survival. The most effective strategy for both was that of pre operative SLN biopsy. This had an annual Incremental cost-effectiveness ratio (ICER) of £33,079 for the outcome of death avoided and an ICER of £100,888 for morbidity-free survival. Both ICERs were above the willingness to pay threshold set by NICE of £30,000. Through this exploratory work we have identified the following areas of additional work for a robust evaluation through this project. We will construct the model for women with vulval cancer of all age groups. We will evaluate the development of late complications in those women suffering early complications and incorporate data on inpatient stay. We will perform probabilistic sensitivity analysis. With this exhaustive evaluation we hope to have reliable answers to guide practice.
3 Research Objectives
The commissioning brief is for an evidence synthesis of the added value of 99mTc enhanced lymphoscintigraphy for SLN biopsy in staging for women with vulval cancer in comparison to the current practice of inguinofemoral lymphadenectomy. Our project will follow the key steps involved in health technology assessment of tests33 and will meet the commissioned brief by fulfilling the following objectives:
| Objectives | Plan of Research |
|---|---|
| To determine the accuracy of SLN biopsy with 99mTc enhanced and blue dye lymphoscintigraphy compared with the histopathology of inguinofemoral lymphadenectomy specimen in vulval cancer through systematic review, bivariate meta analysis and metaregression analysis. | Section 5.1 |
| To assess through systematic review the diagnostic and therapeutic impact of lymphoscintigraphy for SLN biopsy in changing disease staging changing planned treatment reducing complications associated with lymphadenectomy improving morbidity and disease free survival To determine the effectiveness of various interventions (surgery, radiotherapy, chemotherapy) in the management of vulval cancer |
Section 5.1 Section 5.2 |
| To evaluate the cost-effectiveness of lymphoscintigraphy directed treatment vs current treatment strategy in terms of both human and financial costs using decision-analytic modelling. | Section 5.4 |
The relationship of our objectives to the clinical process is shown in Fig 2.
4 Relevance to commissioning brief
The title of the HTA commissioning brief (09/112) refers to ‘The value of adding 99mTc enhanced lymphoscintigraphy for SLN biopsy to current methods of staging of vulval cancer’. It goes on to include the following in the scope of the work to be carried out: effect of staging on treatment planned, reduction in the need for lymphadenectomy, decision analysis and cost effectiveness of added value of 99mTc enhanced lymphoscintigraphy compared with blue dye and current practice of inguinofemoral lymphadenopathy. From this, we take it that the scope of the work is to be broad.
We have published systematic review of the diagnostic accuracy of 99mTc enhanced and blue dye lymphoscintigraphy directed SLN biopsy in the staging of vulval cancer in 2006. 10 We shall update the search to incorporate the findings of the primary studies published in the last 5 years. The brief has specified inguinofemoral lymph node dissection as the gold standard. It also asks researchers to ‘ensure data is included from the latest clinical trials and identify to what extent this will lead to a change in staging of the cancer and the subsequent treatment and quality of life of patients’. The largest and recent trial in this area, GROINSS-V, uses different reference standards - inguinofemoral lymph node dissection (if SLN positive) and follow up for groin recurrence (if SLN negative). 13 We have broadened our search and selection criteria to include studies that confirm SLN status by either inguinofemoral lymph node dissection or follow up and accounted for this variation in study design in our analysis and modelling.
In order to determine the value of SLN biopsy with 99mTc, information on diagnostic accuracy alone will not be sufficient. We will evaluate the potential of SLN biopsy in correctly identifying the sentinel node (localisation or mapping failure rates), the impact of surgeon and team's experience and skills on the accuracy, the impact of variation in disease characteristics, index test protocols and histopathological examination methods on the accuracy. In addition information on diagnostic impact, therapeutic and patient outcomes will be needed. Thus, it is crucial to review effectiveness of various interventions in patients with vulval cancer in addition to accuracy of 99mTc enhanced and blue dye lymphoscintigraphy directed SLN biopsy compared with inguinofemoral lymphadenopathy in staging of vulval cancer to inform decision analytic modelling. Through this project we will update the structure of our existing decision analytic model, will update the probability and cost input data, and will perform probabilistic sensitivity analysis.
We believe that it is feasible to undertake this work within the time scale with the resources we have requested. Our team has the necessary experience and expertise for fulfilling all the requirements in the HTA brief. We have a very strong, internationally renowned, group knowledgeable in systematic reviews of diagnostic and effectiveness data and in economic modelling. Through our MRC training fellowship project, we have the expertise to undertake and update systematic reviews on SLN status in vulval cancer. 24
5 Plan of research
The plan of research will be to update systematic reviews of the accuracy of 99mTc enhanced and blue dye lymphoscintigraphy for sentinel lymph node biopsy in vulval cancer and to undertake systematic reviews of the effectiveness of treatments for vulval cancer. Simultaneously a previously developed decision analytic model will be refined and additional rapid systematic reviews will be undertaken as necessary to populate the emerging model.
We will address the following structured question:
Population: Women with early-stage vulval cancer
Index Tests: 99mTc enhanced and blue dye lymphoscintigraphy for SLN biopsy
Reference standard: Histopathology of inguinofemoral node dissection
Follow up for groin recurrence
Interventions: Current practice of surgery with routine inguinofemoral lymphadenectomy compared with interventions based on SLN status with or without groin node dissection; radiation, or chemotherapy
Outcomes:
-
Test accuracy: Accuracy of 99mTc enhanced lymphoscintigraphy compared with blue dye in identifying potentially curable disease
-
Diagnostic impact: change in staging after 99mTc enhanced lymphoscintigraphy compared with blue dye or current practice
-
Therapeutic impact: change in treatment plan including avoidance of full inguinofemoral lymphadenectomy after 99mTc enhanced lymphoscintigraphy compared with blue dye and current practice by response to treatment that permits continuation or alteration of treatment or decision on clinical follow up only
-
Patient outcomes: mortality, morbidity-free survival, Quality of Life
-
Economic outcome: Use of resources, cost per death avoided, cost per complication free survival, cost per quality adjusted life years (costs per QALY)
Study design:
-
Test accuracy studies
-
Prospective cohort studies of outcomes of patients tested
-
Studies investigating diagnostic and therapeutic impact with or without concurrent assessment of test accuracy.
-
Randomised controlled trials and non randomised controlled studies assessing effectiveness of interventions.
-
Economic evaluations
Exclusions:
-
Advanced stage vulval cancer, inoperable tumours, tumours with diameter > 4 cm or those unsuitable for primary surgery
-
Clinical suspicion of metastases with palpable inguinofemoral lymph nodes, enlarged lymph nodes (> 1.5 cm) on imaging or cytologically proven inguinofemoral lymph node metastases.
-
Patients with multifocal tumours
Systematic reviews of test accuracy, diagnostic and therapeutic impact, and effectiveness will be updated/carried out using established methodology in line with the recommendations of the NHS Centre for Reviews and Dissemination and the Cochrane Collaboration including those of Cochrane Methods Working Group on Screening and Diagnostic tests. 27 Inclusion, data extraction and quality assessment will be carried out in duplicate with differences resolved by consensus and/or arbitration involving a third reviewer.
5.1 Reviews of test accuracy and impact of testing
Evidence on the accuracy of SLN biopsy with 99mTc and blue dye lymphoscintigraphy will be reviewed. Alongside this we will review the impact of SLN biopsy on staging and treatment in vulval cancer. Studies will be identified from a database of published and unpublished literature which will be assembled. We have published a systematic review of literature on diagnostic accuracy of SLN biopsy and have identified 24 relevant studies. 21 We will rerun our search strategy and update the accuracy review, seeking studies on diagnostic and therapeutic impact in addition.
5.1.1 Study identification and selection
Evidence on the accuracy of sentinel node biopsy using 99mTc and blue dye and their diagnostic and therapeutic impact in early vulval cancer will be identified from sensitive searches of published and unpublished sources. Language restrictions will not be applied to electronic searches. The following databases will be searched: MEDLINE, EMBASE, Science Citation Index, MEDION and Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment Database (HTA). Information on studies in progress, unpublished research or research reported in the grey literature will be sought by searching a range of relevant databases including Clinical Trials.com and UK Clinical Research Network Portfolio. A draft MEDLINE strategy is included in appendix A. Electronic searches will be supplemented by hand searching, contacting manufacturers and consultation with experts in the area. In addition authors of included studies will be contacted for information on relevant published or unpublished studies. The preliminary search undertaken to update our published accuracy review has yielded additional 142 citations with 85 on test accuracy. Citations identified by the search will be selected for inclusion in the review in a two-stage process using predefined and explicit criteria regarding populations, index tests, reference standard, outcomes and study design. These criteria have been piloted in our previous review.
5.1.2 Study quality assessment and data extraction
Methodological quality of the selected primary studies of test accuracy will be assessed based on elements of study design, conduct and analysis included in a validated assessment tool, QUADAS, which will be adapted to the topic area. 35 Existing studies of diagnostic and therapeutic impact are likely to be concurrent test accuracy studies evaluating SLN biopsy with either groin dissection or clinical follow up13 as reference standards. There are no validated assessment tools for studies that evaluate the impact of testing. We shall adapt the QUADAS criteria to evaluate the studies on impact adjusting for test, reference standard, treatment and outcome characteristics. Data extraction will be performed using pre-designed, piloted data extraction forms, drawing on existing pro-formas used by the project team in previous, completed reviews in the topic area. 10 Missing information will be obtained from investigators if is crucial to subsequent stages of analysis and modelling. To avoid introducing bias, unpublished information will be treated in the same fashion as published information. In addition to using double data extraction to ensure the reproducibility of the overview, sensitivity analyses around important or questionable judgements regarding quality assessment and data extraction will be performed.
5.1.3 Data synthesis
Sensitivity, specificity and LRs for individual studies comparing 99mTc and blue dye with inguinofemoral lymphadenopathy will be derived.
It is anticipated that the following will be important sources of variation in test accuracy estimates:
-
Population characteristics: Stage of vulval cancer, size of lesion, location of lesion, method of diagnosis of vulval cancer (clinical diagnosis, excision or punch biopsy)
-
Index test characteristics: Type of sentinel node biopsy, reporting of test execution and interpretation, number, training and expertise of the persons reading and executing the test, healthcare setting (secondary or tertiary)
-
Reference test: readers of histopathology of nodes from inguinofemoral lymphadenectomy blind to the index results, clinical follow up for groin recurrence in test negative patients
-
Study quality: study design (prospective or case-control) and study quality (high: meeting all assessment criteria; medium: meeting at least one assessment criteria; low: meeting no quality criteria). High-quality studies will be used as the reference category to determine whether or not medium- and low-quality studies have biased estimates of test accuracy.
Based on an investigation of heterogeneity summary estimates of sensitivity, specificity and summary ROC curves will be derived using bivariate method for meta-analysis. 25,36–38 LRs are considered more clinically meaningful as measures of test accuracy and they allow estimation of probabilities for economic modelling. Post test probabilities can be used to tailor the absolute effectiveness estimates according to test results. Presence of a threshold effect will be examined by plotting sensitivity against 1- specificity in a ROC analysis and by calculating Spearman correlation coefficients. 39
Heterogeneity of results between studies will be investigated qualitatively by examining the distribution of sensitivities and specificities in (ROC) space and variability of estimates of diagnostic odds ratios (DOR) across studies using the forest plot. 39 In addition, heterogeneity will be investigated quantitatively using meta-regression and subgroup analyses. Quantitative investigation will be undertaken based on variables defined a priori and including population characteristics, index and reference test characteristics and study quality. 40 Metaregression will allow us to test the hypothesis as to whether or not 99mTc is more accurate than blue dye. We will perform sensitivity analysis to assess the effect of study design and quality including those with differential reference standard on the overall accuracy, diagnostic and therapeutic impact. This data will be utilised in the development of the decision analytic model.
The risk of publication and related biases is expected to be high in reviews of test accuracy. Publication bias will be investigated using funnel plots of DOR against corresponding variances. 41,42 Qualitative investigation will be based on the premise that large gaps in the funnel indicate possible ‘missing’ publications. These omissions are usually due to small studies showing limited accuracy and are unlikely to be missing at random. Statistical investigation of publication bias will be undertaken in STATA based on templates of commands and instructions already developed by the project team.
5.2 Review of effectiveness of interventions
For evidence on the effectiveness of treatments for vulvar cancer we will begin by searching for existing systematic reviews. Any existing reviews will be examined for relevance and currency in order to inform further searching for primary studies. Existing reviews will be assessed for their quality and currency follow existing guidelines QUOROM and PRISMA. 43 Through this process we will identify gaps where reviews do not exist and where they need updating.
5.2.1 Study identification and selection
Where necessary effectiveness reviews of RCTs of treatments for vulval cancer will be undertaken following existing guidelines27 ensuring the output complies with the QUOROM statement. 43 Searches for further primary studies will be performed. The following databases will be searched: MEDLINE, EMBASE, Science Citation Index and the The Cochrane Library (all databases). On-going studies will be sought by searching Clinical Trials.com and the UK Clinical Research Network portfolio. Draft searches for MEDLINE are included in Appendix A. Studies will be selected for inclusion in the review in a two-stage process using predefined and explicit criteria regarding populations, interventions and outcomes using procedures similar to the ones outlined in the previous section 5.1.1.
5.2.2 Study quality assessment and data extraction
The quality of included reviews will be assessed against a validated tool and a reporting checklist, QUOROM. 43 Methodological quality of randomised and non-randomised trials will be assessed based on accepted criteria. Information on the adequacy of randomisation, sequence generation, concealment, blinding, description of withdrawals, and follow-up rates would be sought as these are elements most likely to have a direct relationship to bias in a RCT. 44 Procedures for obtaining missing information and resolving disagreements will be similar to the ones outlined in section 5.1.2.
5.2.3 Data synthesis
RevMan and STATA softwares will be used to conduct analyses. Heterogeneity of results between studies and investigation for publication bias will be statistically and graphically assessed using established methods. The decision to proceed to meta-analysis will depend on the degree of heterogeneity in the data set. It is anticipated that the following will be important sources of variation in the estimates of effectiveness:
-
Population characteristics: Stage of vulval cancer, age of patient, number of lymph node metastases, morphology of the nodes (size, extracapsular involvement)
-
Treatment characteristics: Type of intervention (surgery, radiotherapy or chemo radiotherapy), duration of therapy, healthcare setting (secondary or tertiary), timing of intervention
-
Outcome measures: Mortality, morbidity, Quality of life
Conclusions regarding the typical estimate of an effect size of the intervention will be interpreted cautiously if there is significant heterogeneity. Where uncertainty exists, the output from data syntheses will be employed following triangulation against subjective probability estimates, judiciously in decision analytic modelling.
5.3 Eliciting subjective probabilities
In anticipation of small numbers of effectiveness studies subjective probabilities will be elicited, using a group interview, from between 10 and 15 clinical experts in the fields of gynaecological cancer and oncology with no conflict of interest in the area, identified by clinicians in the project team and project advisors. The aim of the elicitation process will be to gather subjective views about the size and probability of diagnostic and therapeutic impact of SLN biopsy using 99mTc to current practice in the staging of vulval cancer and reduction in the need for lymphadenectomy.
A face-face group interview (behavioural aggregation) will be used in preference to individual interviews as this facilitates a common understanding of the problem and task from experts and will allow us to benefit from group discussion and interaction leading to a consensus of opinion. The expert group will be facilitated by both a clinical and non-clinical expert drawn from the project team with sufficient statistical expertise to provide probabilistic training to experts, validate their results and provide feedback. The interview will take place over two half days and will, briefly, comprise:
-
Training of experts (probability, probability distributions, judgement heuristics and biases)
-
Practicing elicitations
-
Eliciting probabilities
-
Presentation of results back to experts
-
Repeat elicitation of probabilities to check face validity and if necessary ensure a joint probability distribution.
Findings from the elicitation process will be triangulated with findings from the systematic reviews and probability distributions will assist with populating the decision analytic model. As well as expertise within the project team45 we have access to experts in the field, based at the University of Birmingham. 46 Furthermore the use of this method in the HTA funded project PET-CT imaging in restaging recurrent cervical cancer (HTA No 09/29/02) will help us to develop and refine the questionnaire for this project.
5.4 Model based economic evaluation
The objective of the economic evaluation will be to compare the relative cost effectiveness of undertaking SLN biopsy compared with current practice of intervention involving inguinofemoral lymphadenectomy without testing.
5.4.1 Perspective and data collection
If SLN biopsy is shown to be an accurate and effective alternative to the standard practice in staging vulval cancer then it is likely that important cost implications will be seen for the health care sector. For example, SLN biopsy may detect additional evidence of the extent of metastasis compared with standard investigations which could increase the number and extent of subsequent tests and treatment required by the individual. But the additional costs associated with more accurate staging of the cancer may lead to a reduction in costs associated with unnecessary or ineffective subsequent treatments and also prolong the life of the woman. Thus, if available data allow, the economic evaluation will be based on an outcome of cost per QALY and/or Cost per morbidity-free 5 year survival (this latter is an outcome we have used in our previous analysis24 due to the paucity of quality of life data)/or cost per ‘death due to recurrent cancer’ avoided. The analysis will adopt the perspective of the NHS.
Therefore data collection required for the model based economic evaluation will at least include:
-
The equipment, other resource use and costs associated with SLN biopsy
-
knock on costs associated with additional further tests and treatments that are required as a result of the staging
-
equipment, resource use and costs associated with current practice
-
Accuracy of the SLN biopsy and current practice package compared with the accuracy of current practice tests alone
-
Effectiveness of alternative intervention pathways that are followed as a result of the diagnosis
-
Outcomes such as quality of life associated with vulval cancer at various disease stages
Cost data will be collected from two principal sources. First, once the clinical evidence has been synthesised into the main strategies of diagnosis and treatment, relevant studies will be examined for their data on costs and resource use. These data will be subject to relevant quality criteria. Additional cost data will be available from other sources such as the National Schedule for Reference Costs. If necessary primary cost and resource data will be collected from Pan Birmingham Cancer network and Addenbrookes Hospital to complete any gaps in the information required for the modelling process.
Additional searches will be undertaken to help populate the decision model. The Information Officer will work in close liaison with the health economist to identify the model questions. Information to answer these questions will be provided by focused searching of appropriate databases, including reference cost databases, statistical sources and other sources of relevant information. The evidence found in the clinical accuracy and effectiveness reviews will provide the majority of the parameters required to carry out the economic evaluations of alternative test and treat packages. Additional data on early complications predisposing to late complications, and psychosexual problems will be systematically obtained by searching the relevant literature. Where there is paucity of evidence, we will elicit subjective probabilities as detailed above. The costs for lymphoedema and district nurse input will be gathered by liaising with the lymphoedema service and the centres.
5.4.2 Model and analysis
The economic evaluation will involve the development of an existing decision analytic simulation model as a framework for conducting cost-effectiveness analyses. 24 The economic evaluations will inform current treatment policy in this clinical area. A modelling framework is ideally suited to demonstrate and explore the importance of the inherent uncertainty. We will develop the model including women with vulval cancer of all age groups. This will be a development of the existing model which focuses only on women 70 years and older.
An incremental approach will be adopted with a focus on additional costs and gain in benefits associated with a move away from current practice to alternative test and treatment strategies. Using discounting, adjustments will be made to reflect the differential timing of costs and outcomes in terms of the extension to the length of life extend associated with the test and treat strategies. The base-case analysis will follow Treasury recommendations for public sector projects.
5.4.3 Presentation of results and sensitivity analysis
The results of these economic analyses will be presented using cost-effectiveness acceptability curves to reflect sampling variation and uncertainties in the appropriate threshold cost-effectiveness value. We shall also use both simple and probabilistic sensitivity analyses to explore the robustness of these results to plausible variations in key assumptions and variations in the analytical methods used, and to consider the broader issue of the generalisability of the results.
In addition to probabilistic sensitivity analysis on our base-case model, we shall include a range of alternative analyses to explore the robustness of these results to plausible variations in key assumptions and variations in analysis, and to consider generalisability of the results.
6 Expertise in the team
The applicants have a wide and appropriate range of expertise in systematic reviews, gynaecological oncology, clinical pathology, clinical epidemiology, health measurement, economic evaluation, medical statistics, information science and health technology assessment.
The team (KSK, ST, SS, TR, AF) has recently been awarded a HTA grant to undertake systematic review and economic modelling of clinical effectiveness of PET-CT imaging in restaging recurrent cervical cancer (HTA No 09/29/02). KSK and TR have successfully completed many HTA projects on systematic reviews of test and treatments including systematic reviews of tests for pre-eclampsia, intrapartum rapid tests for Group B streptococcus infection and preterm labour. 47–49 In addition KSK has experience of the process of eliciting subjective probabilities. His former student and current colleague TS was awarded MRC research training fellowship to undertake systematic reviews of accuracy of tests and treatment in gynaecologic cancer including vulval cancer and for undertaking decision analytic modelling and economic evaluation. 24 KSK has also led a grant on the methodology of evaluation of tests without gold standards by the NHS Research Methodology Programme. ST has undertaken many systematic reviews on tests and treatment in with pre-eclampsia, preterm labour and epilepsy. TS has conducted systematic review of accuracy of sentinel node biopsy with tests including 99mTc and blue dye in vulval cancer. She has been awarded PhD by the University of Birmingham for her work ‘Non invasive and minimally invasive diagnosis and treatment of lymphadenopathy in gynaecologic cancer. Systematic review of evidence’24
SS and PB are both gynaecologic oncologists involved in managing women with vulval cancer. They are members of the gynaecological cancer clinical studies group of the NCRI (National Cancer Research Institute) – the national group responsible for selecting national trials for inclusion in the NCRI portfolio and supporting and directing clinical research in gynaecological cancer. PB is a former trial group member of the large GROINSS-V prospective study investigating the role of SLN biopsy in early vulval cancer. His is also the Chief Investigator in the UK in the ongoing prospective multicentre international study GROINSS-V II which is evaluating the safety of omitting surgery in selected SLN positive patients. PB represents the study at the vulval subgroup of the NCRI. RG is a Consultant Histopathologist involved with Pan Birmingham Cancer network and Associate Director of Birmingham Cytology Training Centre (CTC). KB is a Consultant in Nuclear Medicine and has expertise in 99m Tc and blue dye lymphoscintigraphy for SLN biopsy. AF (information specialist) has extensive experience as an information specialist in providing support to a diagnostic and effectiveness technology assessments as a member of the West Midlands Health Technology Assessment Collaboration and the Aggressive Research Intelligence Facility (ARIF) based at the University of Birmingham. She is currently working on an HTA assessing the value of PET-CT for recurrent breast cancer and her expertise in devising the search strategy and database management will be of benefit to this proposal. Hilary Jeffries is a retired Lead cancer nurse and McMillan community nurse specialist with extensive experience of interaction with patients with vulval cancer. ME is a member of the Consumer liaison group, NCRI.
7 Contribution to Collective Research Effort
This systematic review on the value of SLN biopsy with 99mTc enhanced lymphoscintigraphy compared with blue dye and the current management of groin node dissection in vulval cancer and the cost effectiveness analysis of SLN biopsy using the above methods in comparison to current management fits comfortably with previously published HTA evaluations of sentinel node biopsy in other cancers. This research application complements existing National cancer research network portfolio research in gynaecological cancer. The ongoing GROINSS-V II study evaluates if inguinofemoral lymph node dissection can be omitted in the presence of a positive SLN and the treatment of groins with radiotherapy or chemoradiotherapy instead. This project will augment the current published evidence acquired through the MRC training fellowship on SLN biopsy in vulval cancer through update of the review and further comprehensive development of the decision analytic model. 24
Due to the multiple methods employed by the proposed evidence synthesis the project team expect that the outputs of the work would be of interest to a broad research and clinical community including experts in the areas of evidence synthesis and in particular synthesis of test accuracy, gynaecological cancer, and decision making. Outputs would be submitted for presentation at national and international conferences such as Health Technology Assessment international, Medical Decision Making, European Society of Gynaecological Oncology (EGSO) and Society of Gynaecological Oncology (SGO). Similarly the outputs of this work would be of interest to a variety of peer reviewed journals and the project team would aim for a minimum of 3 peer reviewed publications in addition to publication as an HTA monograph. The project team have involved members of the NCRI consumer liaison group and VACO (Vulva Awareness Campaign Organisation), an international support group dedicated to women with vulval cancer. Users will be represented in study conduct and planning of dissemination strategies. The team will benefit from the HJ in an advisory role who has worked closely with women with vulval cancer. She has recently published a qualitative study on the experiences of women with vulval cancer as part of her PhD. 50 Experience from previous research conducted by the team has already indicated that publication and dissemination needs careful consideration from the outset. 47–49 Publication strategy will also need to anticipate early the need for versions of the report, which can be, used by women themselves. For this we will seek input from relevant consumers.
8 Details about any related (planned or active) grants held by members of the research team
KSK (as supervisor) and TS were awarded MRC research training fellowship to undertake systematic reviews of accuracy of tests in gynaecologic cancer including vulval cancer. The resulting PhD has recently been awarded. 24 Information from the accuracy of tests in vulval cancer will be updated and the analysis will be refined. We have also developed a decision analytic modelling structure for tests in vulval cancer that will be improved upon. ST, KK, TR and SS have been successful in obtaining HTA grant for conducting systematic review and developing an economic model to evaluate the clinical effectiveness of PET CT in recurrent cervical cancer. (HTA No. 09/29/02) SS has a PhD student funded by the department of Health investigating the epigenetic changes induced by HPV in cervical cancer. PB holds a CRUK award to support the ongoing GROINSS-V II study. The expertise of SS and PB in gynaecologic oncology will be of use in providing subjective probabilistic estimates for test accuracy and effectiveness. AF (information specialist) is currently working on an HTA assessing the value of PET-CT for recurrent breast cancer and her expertise in devising the search strategy and database management will be of benefit to this proposal.
9 Summary for the non expert
Vulval cancer accounts for approximately 3–5% of all gynaecological cancers. In the UK, the lifetime risk of developing vulval cancer is 1 in 316. Although the peak incidence of this cancer is in the 7th decade, the proportion of women diagnosed with vulval cancer under the age of 50 has risen from 6% in 1975 to 15% in 2006. Vulval cancer is curable when diagnosed in an early stage. The current treatment for early-stage vulval cancer is extensive removal of the vulval tumour and excision of the groin nodes to check for spread of cancer. Cancer in the groin nodes has been identified as the single most important factor in predicting survival. Removal of the groin nodes is associated with complications in the short term (infection, wound breakdown) and long term (lymphoedema, cellulitis, sexual dysfunction) with significant negative impact on the Quality of Life. Only about a third of patients with operable disease have nodal spread and the rest are unlikely to benefit from routine removal of groin nodes. Despite the risk of significant complications and low probability of cancer spread to the nodes, groin nodes are routinely removed as missed cancer in the groin nodes is nearly always fatal. A test that could accurately identify those patients in whom cancer has spread to the groin nodes without extensive removal of all groin would be extremely valuable. There are several methods to check for involvement of the groin nodes, but none are routinely used in clinical practice. This HTA proposal focuses on the value of testing the groin node (sentinel node) with biopsy by locating them with radioactive substance (99m Technetium) or blue dye.
The sentinel node (SLN) refers to any node that receives lymphatic drainage directly from the vulval tumour and therefore has the highest probability of containing cancer cells. If the sentinel node is free from cancer, the rest of the groin should be at least risk of having spread of cancer. Identification and removal of the SLN(s) avoids the significant complications associated with complete groin dissection. In those patients negative for cancer in the sentinel nodes where extensive groin dissection was omitted, studies suggest that the risk of future disease in the groin is low, the survival rate is excellent (97% at 3 years) and that there are few complications associated with this smaller operation. If the SLN is negative no further treatment is therefore required and the patient will be followed up in the clinic.
There is a need to systematically review the accuracy of SLN biopsy with 99mTc and blue dye in identifying the spread of cancer to the groins. Moreover it is important to review how testing of SLN will have an impact on the extent of cancer spread, treatment decisions, clinical and cost outcomes.
For the proposed project our objectives are as follows:
-
In women who have been diagnosed to have vulval cancer, to systematically review the literature
-
To assess if SLN biopsy with 99mTc or blue dye can accurately diagnose spread of cancer to the groins compared with current practice of routine extensive removal of all groin nodes
-
To evaluate if the use of SLN biopsy results in change in (re)staging i.e. extent of disease compared with current practice
-
To assess the impact of performing SLN biopsy on the typical standard treatment
-
To summarise the effectiveness of available treatments in women with vulval cancer
-
To estimate the impact of SLN biopsy results on patient outcomes and the costs associated with its routine use in this patient group.
We plan to fulfil the above objectives by systematically identifying the available evidence on the diagnostic accuracy of SLN biopsy with 99mTc and blue dye in vulval cancer compared with the accuracy of existing practice of groin dissection used in this patients group and the effectiveness of treatments for vulval cancer. The evidence found will be used in an economic evaluation comparing existing testing and treatment strategies with SLN biopsy guided treatment strategies. This evaluation will inform current treatment policy in this clinical area and highlight future research need.
10 Project Timetable and Milestones
Fig 3 shows the project timetable and milestones for the accuracy and effectiveness reviews and economic modelling. We have carefully evaluated the ongoing work and the level of staffing within our departments and feel that we would be able to commence the work in Sep 2010 for a period of 18 months, if funded.
| Months | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Protocol development | ||||||||||
| Protocol peer review | ||||||||||
| Diagnostic reviews (Section 5.1) | ||||||||||
| Effectiveness reviews (Section 5.2) | ||||||||||
| Subjective probabilities (Section 5.3) | ||||||||||
| Economic reviews (Section 5.3) | ||||||||||
| Economic modelling (Section 5.4) | ||||||||||
| Report production |
11 Justification for the support required
Staff:
Supervisor, also providing support for researchers, for example: double data extraction, assisting with inclusion decisions and being the lead for producing the final report – 1 day per week for the duration of the project.
Researcher to perform systematic review of accuracy and effectiveness studies and to identify additional epidemiological and background information for input into the modelling exercise – 1 wte for 18 months.
Health economist to perform systematic review of cost-effectiveness literature and modelling – 1wte for 12 months.
Information support for searching and document retrieval –20 days.
Equipment and consumables:
two standard specification computers, printing cartridges, paper and photocopying,
telephone and fax calls, postage,
estimated 200 interlibrary loans.
Support:
-
Meeting room, refreshments and travel for the project team and consultants based on 4 face to face meetings over 12 months.
-
Meeting room, refreshments and travel for consumer group representatives
-
Administrative support, for steering group and preparation of final report –10 days over 12 months.
We are in an excellent position to gauge the level of resources required to deliver this type of project (systematic review and cost-effectiveness analysis) with several years experience in their delivery. We are able to draw on additional in-house expertise if necessary. Travel costs have included the cost of travel of experts and non experts for obtaining probabilistic estimates.
Appendix A Search strategy for electronic database identification of diagnostic studies for preoperative tests of lymph node status and therapeutic studies of interventions in vulval cancer
Test accuracy search – proposed MEDLINE strategy
Ovid MEDLINE(R) 1950 to November Week 3 2009
-
technetium.tw. (12014)
-
(radionuclide adj imag$).tw. (1508)
-
technetium/ (18834)
-
radionuclide imaging/ (23714)
-
sentinel lymph node biopsy/ (5545)
-
99m tc$.tw. (3979)
-
99mtc$.tw. (16283)
-
(sentinel adj2 lymph adj2 node$).tw. (4422)
-
or/1-8 (62808)
-
vulvar neoplasms/ (6493)
-
((vulva or vulval or vulvar) adj5 (cancer$ or carcinoma$ or adenocarcinoma$ or carcinogen$ or sarcoma$ or malignan$ or tumo?r$ or neoplas$)).tw. (4197)
-
or/10-11 (7219)
-
9 and 12 (192)
Effectiveness search (systematic reviews) – proposed MEDLINE strategy
Ovid MEDLINE(R) 1950 to November Week 3 2009>
-
vulvar neoplasms/ (6493)
-
(vulva or vulval or vulvar) adj5 (cancer$ or carcinoma$ or adenocarcinoma$ or carcinogen$ or sarcoma$ or malignan$ or tumo?r$ or neoplas$)).tw. (4197)
-
or/1-2 (7219)
-
limit 3 to "reviews (specificity)" (32)
Effectiveness search (RCTs) – proposed MEDLINE strategy
Ovid MEDLINE(R) 1950 to November Week 3 2009>
-
vulvar neoplasms/ (6493)
-
((vulva or vulval or vulvar) adj5 (cancer$ or carcinoma$ or adenocarcinoma$ or carcinogen$ or sarcoma$ or malignan$ or tumo?r$ or neoplas$)).tw. (4197)
-
or/1-2 (7219)
-
limit 3 to "therapy (optimized)" (91)
Reference List
- Hacker NF, Berek JS, Hacker NF. Practical Gynecologic Oncology 2005. Philadelphia, PA: Williams and Wilkins; 2005.
- n.d. URL: Http://cancerresearchuk.org/cancerstats/types/vulva/incidence/index.htm (accessed 2009).
- Darling JR, Sherman JH, Schottenfeld D, Fraumeni J. Cancer epidemiology and prevention. 1996.
- Woolas RP, Shepherd JH, O’Brien PMS. The yearbook of obstetrics and gynecology. 1999.
- Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstet Gynecol 1997;90:448-52.
- Joura EA, Losch A, Haider-Angeler MG, . Trends in vulvar neoplasia. Increased incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J Reprod Med 2000;45:613-5.
- de Hullu JA, van der Avoort IA, Oonk MH, van der Zee AG. Management of vulvar cancers. Eur J Surg Oncol 2006;32:825-31.
- RCOG Working Party report. Management of Vulval Cancer 1999.
- Homesley HD, Bundy BN, Sedlis A, . Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group Study). Am J OG 1991;164:997-1003.
- Selman TJ, Luesley DM, Acheson N, Khan KS, Mann CH. A systematic review of the accuracy of diagnostic tests for inguinal lymph node status in vulvar cancer. Gynecol Oncol 2005;99:206-14.
- Stehman FB, Ali S, DiSaia PJ. Node count and groin recurrence in early vulvar cancer: A Gynecologic Oncology group study. Gynecol Oncol 2009;113:52-6.
- de hullu JA, Doting E, Piers DA, . Sentinel lymph node identification with technetium 99m labelled nanocolloid in squamous cell cancer of the vulva. J Nucl Med 1998;39:1381-5.
- van der Zee AG, Oonk MH, de Hullu JA, . Sentinel node dissection is safe in the treatment of early stage vulvar cancer. J Clin Onc 2008;26:884-9.
- Barton DP. The prevention and management of treatment related morbidity in vulvar cancer. Best Pract Res Clin Obstet Gynaecol 2003;17:683-701.
- Homesley H, Bundy B, Sedlis A. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva. Gynecol Oncol 1993;49:279-83.
- Sedlis A, Homesley H, Bundy B. Positive groin lymph nodes in superficial squamous vulvar cancer. Am J OG 1987;156:1159-64.
- Thompson JF, Uren RF. What is a ‘sentinel’ lymph node?. Eur J Surg Oncol 2000;26:103-4.
- Terada KY, Shimizu DM, Wong JH. Sentinel node dissection and ultrastaging in squamous cell cancer of the vulva. Gynecol Oncol 2000;76:40-4.
- Blake Cady . Sentinel lymph node procedure in squamous cell carcinoma of the vulva. J Clin Onc 2000;18:2795-7.
- Medical Services Advisory Committee assessment report. Sentinel Lymph Node Biopsy in Breast Cancer 2005.
- de Hullu JA. Van der Zee AG. Groin surgery and the sentinel lymph node. Best Prac Res Clin Obstet Gynaecol 2003;17:571-89.
- de Hullu JA, Hollema H, Piers DA, . Sentinel lymph node procedure is highly accurate in squamous cell carcinoma of the vulva. J Clin Onc 2000;18:2811-6.
- Morton DL, Thompson JF, Essner R, . Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early stage melanoma: A multicenter trial. – Multicenter selective lymphadenectomy trial group. Ann Surg 1999;230:453-63.
- Selman TJ. Non-Invasive and Minimally Invasive Diagnosis and Therapy of Lymphadenopathy in Gynaecological Cancers. Systematic Reviews of the Evidence 2009.
- Rietsma JB, Glas AS, Rutjes AW, . Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90.
- Khan KS, ter Riet G, Glanville J, Soweden AJ, Kleijnen J. Undertaking systematic reviews of research on effectiveness. CRD's guidance for carrying out or commissioning reviews. York: NHS Centre for Reviews and dissemination, University of York; 2001.
- Fotiou SK, Tserkezoglou AJ, Fragakis G, Terzakis E, Stavrakakis E, Apostolikas N. “Butterfly” operation vs triple incision technique in vulvar cancer: a comparison of morbidity and clinical outcome. Eur J Gynaecol Oncol 1996;17:67-73.
- Lin JY, DuBeshter B, Angel C, Dvoretsky PM. Morbidity and recurrence with modifications of radical vulvectomy and groin dissection. Gynecol Oncol 1992;47:80-6.
- Beesley V, Janda M, Eakin E, Obermair A, Battistutta D. Lymphedema after gynecological cancer treatment: prevalence, correlates and supportive care needs. Cancer 2007;109:2607-14.
- Gould N, Kamelle S, Tillmanns T, Scribner D, Gold M, Walker J, et al. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol 2001;82:329-32.
- Ryan M, Stainton MC, Slaytor EK. Aetiology and prevalence of lower limb lymphoedema following treatment for gynecological cancer. Aust NZ J Obstet Gynaecol 2003;43:148-51.
- Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Dec Making 1991;11:88-94.
- Systematic reviews. Centre for Reviews and dissemination; 2009.
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JAC. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2006;1:1-21.
- van Houwelingen HC, Zwinderman AH, Stijnen T. A bivariate approach to meta-analysis. Stat Med 1993;12:2273-84.
- van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;28:589-624.
- Irwig L, Tostesen A, Gatsonis C. Guidelines for meta-analysis evaluating diagnostic tests. Ann Intern Med 1994;120:667-76.
- Lijmer JG, Bossuyt PM, Heistercamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 2002;21:1525-37.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93.
- Song FJ, Khan KS, Dinnes J, Sutton AJ. Asymetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol 2002;31:88-95.
- Moher D, Cook DJ, Eastwood S, . Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Br J Surg 2000;87:1448-54.
- Jadad AR, Moore RA, . Assessing the quality of reports of randomised clinical trials: is blinding necessary?. Control Clin Trials 1996;17:1-12.
- Lathe PM, Braunholtz DA, Hills RK, Khan KS, Lilford R. Measurement of beliefs about effectiveness of laparoscopic uterosacral nerve ablation. BJOG 2005;112:243-6.
- Lilford R. Formal measurements of clinical uncertainty: prelude to a trial in perinatal medicine. The Fetal Compromise Group. BMJ 1994;308:111-2.
- Honest H, Forbes CA, Duree KH, . Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2010;13:1-627.
- Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al. Methods of prediction and prevention of pre-eclampsia-systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2008;12.
- Daniels J, Gray J, Pattison H, Roberts T, . Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technol Assess 2009;13:1-178.
- Jeffries H. Searching the lived experience of women with cancer of the vulva. Cancer Nurse 2009;32:E30-6.
Appendix 2 Scoping searches for systematic reviews and Health Technology Assessments
Systematic review
MEDLINE (14 January 2011)
| 1. | exp Vulval Neoplasms | 6177 |
| 2. | ((vulva or vulval or vulval) adj5 (cancer$ or carcinoma$ or adenocarcinoma$ or carcinogen$ or sarcoma$ or malignan$ or tumo?r$ or neoplas$)).mp. [mp = title, original title, abstract, name of substance word, subject heading word, unique identifier] | 6992 |
| 3. | #1 or #2 | 6992 |
| 4. | ("review" or "review academic" or "review tutorial").pt. | 1,547,865 |
| 5. | cinahl.tw,sh. | 4605 |
| 6. | ((hand adj2 search$) or (manual$ adj2 search$)).tw,sh. | 4358 |
| 7. | (electronic database$ or bibliographic database$ or computeri?ed database$ or online database$).tw,sh. | 7263 |
| 8. | (pooling or pooled or mantel haenszel).tw,sh. | 34,931 |
| 9. | (retraction of publication or retracted publication).pt. | 3069 |
| 10. | (peto or dersimonian or der simonian or fixed effect).tw,sh. | 1811 |
| 11. | (medline or medlars or embase or pubmed).tw,sh. | 43,864 |
| 12. | #5 or #6 or #7 or #8 or #9 or #10 or #11 | 84,639 |
| 13. | #4 and #12 | 38,102 |
| 14. | meta-analysis.sh. | 25,963 |
| 15. | (meta-analys$ or meta analys$ or metaanalys$).tw,sh. | 45,017 |
| 16. | (systematic$ adj5 review$).tw,sh. | 26,728 |
| 17. | (systematic$ adj5 overview$).tw,sh. | 531 |
| 18. | (quantitativ$ adj5 overview$).tw,sh. | 129 |
| 19. | (quantitativ$ adj5 synthesis$).tw,sh. | 949 |
| 20. | (methodologic$ adj5 review$).tw,sh. | 2168 |
| 21. | (methodologic$ adj5 overview$).tw,sh. | 147 |
| 22. | (integrative research review$ or research integration).tw. | 67 |
| 23. | (quantitativ$ adj5 review$).tw,sh. | 2941 |
| 24. | #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 | 67,802 |
| 25. | #13 or #24 | 89,293 |
| 26. | #3 and #25 | 43 |
EMBASE (14 January 2011)
| 1. | 'vulva tumour'/exp | 6733 |
| 2. | ((vulva OR vulval OR vulval) NEAR/5 (cancer* OR carcinoma* OR adenocarcinoma* OR carcinogen* OR sarcoma* OR malignan* OR tumo?r* OR neoplas*)):lnk,ab,ti | 4922 |
| 3. | #1 OR #2 | 7756 |
| 4. | 'review'/exp | 1,696,863 |
| 5. | medline:lnk,ab,ti OR medlars:lnk,ab,ti OR embase:lnk,ab,ti OR pubmed:lnk,ab,ti | 53,845 |
| 6. | cinahl:lnk,ab,ti | 5675 |
| 7. | electronic:ab,ti AND adj:ab,ti AND database*:ab,ti OR (bibliographic NEAR/2 database*):ab,ti | 1169 |
| 8. | (pooled NEAR/2 analys*):ab,ti OR pooling: ab,ti | 11,058 |
| 9. | peto:ab,ti OR dersimonian:ab,ti OR (fixed NEAR/2 effect):ab,ti OR mantel:ab,ti AND haenszel:ab,ti | 2391 |
| 10. | #5 OR #6 OR #7 OR #8 OR #9 | 66,370 |
| 11. | #4 AND #10 | 34,213 |
| 12. | 'meta-analysis'/exp | 51,889 |
| 13. | meta AND analys*:lnk,ab,ti | 48,252 |
| 14. | (systematic* NEAR/5 review*):lnk,ab,ti | 34,901 |
| 15. | (systematic* NEAR/5 overview*):lnk,ab,ti | 667 |
| 16. | (quantitativ* NEAR/5 review*):lnk,ab,ti | 1906 |
| 17. | (quantitativ* NEAR/5 overview*):lnk,ab,ti | 162 |
| 18. | (methodologic* NEAR/5 review*):lnk,ab,ti | 2743 |
| 19. | (methodologic* NEAR/5 overview*):lnk,ab,ti | 188 |
| 20. | (integrative NEAR/5 (research OR review*)):ab,ti OR (research NEAR/5 integration):ab,ti | 2019 |
| 21. | (quantitativ* NEAR/5 synthesi*):lnk,ab,ti | 1437 |
| 22. | #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 | 102,029 |
| 23. | #11 OR #22 | 121,795 |
| 24. | #3 AND #23 | 63 |
| 25. | #3 AND #23 AND [embase]/lim | 54 |
Cochrane (14 January 2011)
| 1. | MeSH descriptor Vulval Neoplasms explode all trees | 53 |
| 2. | (vulva OR vulval OR vulval) NEAR/5 (cancer* OR carcinoma* OR adenocarcinoma *OR carcinogen* OR sarcoma* OR malignan* OR tumo?r* OR neoplas*) |
116 |
| 3. | (#1 OR #2) | 116 |
Appendix 3 Data extraction form for diagnostic reviews
Appendix 4 Data extraction form for effectiveness reviews
Appendix 5 Diagnostic search strategies
Diagnostic part
Searches Ovid MEDLINE (January 2011)
| 1. | exp Vulvar Neoplasms/ | 6177 |
| 2. | ((vulva or vulval or vulvar) adj5 (cancer$ or carcinoma$ or adenocarcinoma$ or carcinogen$ or sarcoma$ or malignan$ or tumo?r$ or neoplas$)).mp. [mp = title, original title, abstract, name of substance word, subject heading word, unique identifier] | 6992 |
| 3. | 1 or 2 | 6992 |
| 4. | exp Sentinel Lymph Node Biopsy/ | 5846 |
| 5. | (sentinel adj2 lymph$ adj2 node$).tw. | 4719 |
| 6. | (lymphatic adj2 mapping).tw. | 907 |
| 7. | (SLN adj2 biops$).tw. | 797 |
| 8. | lymphoscintigraphy.mp. | 1824 |
| 9. | 4 or 5 or 6 or 7 or 8 | 7903 |
| 10. | 3 and 9 | 202 |
Searches EMBASE (January 2011)
| 1. | 'vulva tumour'/exp | 6733 |
| 2. | ((vulva OR vulval OR vulvar) NEAR/5 (cancer* OR carcinoma* OR adenocarcinoma* OR carcinogen* OR sarcoma* OR malignan* OR tumo?r* OR neoplas*)):lnk,ab,ti | 4922 |
| 3. | #1 OR #2 | 7756 |
| 4. | 'sentinel lymph node biopsy'/exp | 5894 |
| 5. | sentinel:lnk,ab,ti AND lymph*:lnk,ab,ti AND node*:lnk,ab,ti | 7884 |
| 6. | (lymphatic NEAR/2 mapping):lnk,ab,ti | 1071 |
| 7. | (sln NEAR/2 biops*):lnk,ab,ti | 995 |
| 8. | lymphoscintigraphy:lnk,ab,ti | 2283 |
| 9. | #4 OR #5 OR #6 OR #7 OR #8 | 10,783 |
| 10. | #3 AND #9 AND [embase]/lim | 255 |
Appendix 6 Effectiveness search strategies
Primary studies effectiveness
EMBASE (14 January 2011)
| 1. | 'vulva tumour'/exp | 6733 |
| 2. | ((vulva OR vulval OR vulvar) NEAR/5 (cancer* OR carcinoma* OR adenocarcinoma* OR carcinogen* OR sarcoma* OR malignan* OR tumo?r* OR neoplas*)):lnk,ab,ti | 4922 |
| 3. | #1 OR #2 | 7756 |
| 4. | #1 OR #2 AND [embase]/lim | 5584 |
| 5. | #1 OR #2 AND [embase]/lim AND ([editorial]/lim OR [letter]/lim) | 252 |
| 6. | #1 OR #2 AND [embase]/lim AND [animals]/lim | 94 |
| 7. | #1 OR #2 NOT #5 NOT #6 AND [embase]/lim | 5238 |
| 8. | #1 OR #2 NOT #5 NOT #6 AND [embase]/lim AND ([erratum]/lim OR [note]/lim) | 69 |
| 9. | #1 OR #2 NOT #5 NOT #6 AND [embase]/lim NOT #8 | 5169 |
Ovid MEDLINE (17 January 2011)
| 1. | exp Vulvar Neoplasms/ | 6177 |
| 2. | ((vulva or vulval or vulvar) adj5 (cancer$ or carcinoma$ or adenocarcinoma$ or carcinogen$ or sarcoma$ or malignan$ or tumo?r$ or neoplas$)).mp. | 6992 |
| 3. | #1 or #2 | 6992 |
| 4. | limit #3 to (editorial or letter) | 253 |
| 5. | #3 not #4 | 6739 |
| 6. | limit #5 to animals | 239 |
| 7. | #5 not #6 | 6500 |
Appendix 7 Coding manual for case–control studies
A study was awarded with maximum one star (*) for each numbered item within the Section and Exposure categories and a maximum of two stars (**) in the Comparability category.
| Section | Question | |
|---|---|---|
| Selection | 1. Is the case definition adequate? | |
| Yes, with independent validation* | Requires some independent validation (e.g. > 1 person/record/time/process to extract information, or reference to primary record source such as X-rays or medical/hospital records)* | |
| Yes, e.g. record linkage or based on self reports | Record linkage (e.g. International Classification of Diseases codes in database) or self-report with no reference to primary record | |
| No description | No description | |
| 2. Representativeness of the cases | ||
| Consecutive or obviously representative series of cases* | All eligible cases with outcome of interest over a defined period of time, all cases in a defined catchment area, all cases in a defined hospital or clinic, group of hospitals, health maintenance organisation, or an appropriate sample of those cases (e.g. random sample)* | |
| Potential for selection biases or not stated | Not satisfying requirements in part (a), or not stated | |
| 3. Selection of controls | ||
| This item assesses whether or not the control series used in the study is derived from the same population as the cases and essentially would have been cases had the outcome been present | ||
| Community controls* | Community controls (i.e. same community as cases and would be cases if had outcome) | |
| Hospital controls | Hospital controls, within same community as cases (i.e. not another city) but derived from a hospitalised population | |
| No description | No description | |
| 4. Definition of controls | ||
| No history of disease (endpoint)* | If cases are first occurrence of outcome, then it must explicitly state that controls have no history of this outcome. If cases have new (not necessarily first) occurrence of outcome, then controls with previous occurrences of outcome of interest should not be excluded* | |
| No description of source | No mention of history of outcome | |
| Comparability | 1. Comparability of cases and controls on the basis of the design or analysis | |
| A. Study controls for . . . (select the most important factor)* | A maximum of 2 stars can be allotted in this category Either cases and controls must be matched in the design and/or confounders must be adjusted for in the analysis. Statements of no differences between groups or that differences were not statistically significant are not sufficient for establishing comparability Note: If the odds ratio for the exposure of interest is adjusted for the confounders listed, then the groups will be considered to be comparable on each variable used in the adjustment. There may be multiple ratings for this item for different categories of exposure (e.g. ever vs. never, current vs. previous or never) |
|
| B. Study controls for any additional factor.* (This criteria could be modified to indicate specific control for a second important factor)* | ||
| Exposure | 1. Ascertainment of exposure | |
| Secure record (e.g. surgical records)* | ||
| Structured interview where blind to case/control status* | ||
| Interview not blinded to case/control status | ||
| Written self report or medical record only | ||
| No description | ||
| 2. Same method of ascertainment for cases and controls | ||
| Yes* | ||
| No | ||
| 3. Non-response rate | ||
| Same rate for both groups* | ||
| Non respondents described | ||
| Rate different and no designation | ||
Appendix 8 Quality assessment questions for case series
| Section | Question | |
|---|---|---|
| Introduction | 1. Are the objective or the hypothesis of the study stated? | |
| YES | If the hypothesis was stated or describe | |
| NO | If the hypothesis wasn't stated or describe | |
| UNCLEAR | If the hypothesis wasn't stated or describe but study protocol was adequate for study population | |
| Population | 2. Was the study population clinically clearly describe? (age, FIGO stage, TNM classification) | |
| YES | If there were obligatory information of FIGO stage or TNM classification and at least one more clinically point was describe | |
| NO | If there wasn't any data about clinically significant information | |
| UNCLEAR | If authors gave information only about one point in patients clinically description | |
| 2. Was the study population pathologically clearly describe? (histology, tumour size, location, deep of invasion, node's status) | ||
| YES | If there were information about histology and one other points | |
| NO | If there wasn't any data about pathologically state | |
| UNCLEAR | If only one of points mentioned above was reported | |
| Intervention | 3. Was intervention of the vulval tumour clearly describe? | |
| YES | If there was information about type of treatment with manual protocol | |
| NO | If there was information only about type of intervention, e.g. surgery, RT, chemotherapy | |
| UNCLEAR | If there was information only about type of intervention but without description of treatment type, e.g. radical vulvectomy, hemivulvectomy | |
| 4. Was intervention for groin clearly describe? | ||
| YES | If there were information about type of treatment with description of technique, type of lymphadenectomy. | |
| NO | If in he study was only information about groins intervention, e.g. groin dissection | |
| UNCLEAR | If there were information about groins treatment with information about type of procedures but without description, e.g. IFL | |
| Follow-up | 5. Was follow-up time reported? | |
| YES | If there were information at least about mean time of observation for all study groups (intervention) patients | |
| NO | If there wasn't any information about follow-up time | |
| UNCLEAR | If information were reported only for part of patients, studies groups or there were problems with evaluation of adequate data, e.g. (…) patients were observed until the end of this study (…) | |
| 6. Were all included patients accounted at the end of follow-up? | ||
| YES | If all patients and data was estimate at the end | |
| NO | If not all patients accounted at the end of the study, and there were no information from authors about lost patients | |
| UNCLEAR | If not all patients accounted at the end of the study but information about lost were given | |
| Outcome | 7. Were clinically important outcomes considered? (e.g. survival, deaths) | |
| YES | If there were all necessary data | |
| NO | If there were not any information | |
| UNCLEAR | If information were given in inadequate way e.g. only p-value without count of treatment arm | |
| 8. Were definitions of the outcomes presented in the study? | ||
| YES | If the definitions were described | |
| NO | If there were not any descriptions of definitions | |
| UNCLEAR | If author only mentioned or give generally description, e.g. (…) we used this definition according Moore 2001 (…) | |
| 9. Were all outcomes reported in study consequently given? | ||
| YES | If all were given | |
| NO | If all were not given | |
| UNCLEAR | If only part were estimate | |
| 10. In case it was necessary, was it possible to analyse data for patients that meet our criteria separately? | ||
| YES | If patients were clinically described at the beginning of the study, and study data was presented for each group separately | |
| NO | If patients were not clinically described the beginning of the study, and study data wasn't presented for each group separately | |
| UNCLEAR | If patients were not clinically described at the beginning of the study, but study data was presented for each group separately | |
| 11. Were all clinically important date presented in way possible to estimate? | ||
| YES | If all were presented | |
| NO | If none of them were not possible to estimate | |
| UNCLEAR | If only part of them were possible to estimate | |
| 12. Were safety data presented? | ||
| YES | If such kind of data was presented with information of AEs an number of patients | |
| NO | If there weren't any information | |
| UNCLEAR | If such kind of data was reported but without information about count of patients or events | |
Appendix 9 Excluded studies with reasons, diagnostic systematic review
| Ansink AC, Sie-Go DM, van der Velden J, Sijmons EA, de Barros LA, Monaghan JM, et al. Identification of sentinel lymph nodes in vulval carcinoma patients with the aid of a patent blue V injection: a multicenter study. Cancer 1999;86:652–6 | Lack of data on population – no data about stage |
| Atienza Merino G. Applicability of the identification and biopsy technique of the sentinel-lymph-node in vulval cancer. Prog Obstet Ginecol 2010;53:403–11 | Wrong type of publication – review |
| Barton DP, Berman C, Cavanagh D, Roberts WS, Hoffman MS, Fiorica JV, et al. Lymphoscintigraphy in vulval cancer: a pilot study. Gynecol Oncol 1992;46:341–4 | Wrong population – stage I–II < 70% |
| Boran N, Kayikcioglu F, Kir M. Sentinel lymph node procedure in early vulval cancer. Gynecol Oncol 2003;90:492–3 | Lack of data on population – no data about stage |
| Bowles J, Terada KY, Coel MN, Wong JH. Preoperative lymphoscintigraphy in the evaluation of squamous cell cancer of the vulva. Clin Nucl Med 1999;24:235–8 | Wrong study design – small number of patients, no data about accuracy |
| Carcopino X, Houvenaeghel G, Buttarelli M, Charaffe-Jauffret E, Gonzague L, Rossi I. Feasibility and morbidity of sentinel lymph node detection in patients with vulval carcinoma. [French]. Bull Cancer 2005;92:489–97 | Wrong population – stage I–II < 70% |
| Cepni I, Kahraman N, Isiloglu H, Arvas M, Demirkiran F, Uzum F, et al. Preoperative assessment of lymph nodes metastases in gynecologic malignancies by pelvic lymphoscintigraphy. Eur J Lymphol Relat Probl 1992;3:111–18 | Small sample size |
| Crane LM, Pleijhuis RG, Themelis G, Harlaar NJ, Sarantopoulos A, Arts HG, et al. Detection of the sentinel lymph node in vulval cancer, using near-infrared fluorescence intraoperative imaging: a technical feasibility study. Mol Imaging Biol 2010;12:S1164 | Small sample size |
| Crane LM, Themelis G, Buddingh T, Harlaar NJ, Pleijhuis RG, Sarantopoulos A, et al. Multispectral real-time fluorescence imaging for intraoperative detection of the sentinel lymph node in gynecologic oncology. J Visualised Experiments 2010;44:2225 | Wrong study design – description of the technique |
| Crane LMA, Themelis G, Arts HJG, Buddingh KT, Brouwers AH, Ntziachristos V, et al. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulval cancer: First clinical results. Gynecol Oncol 2011;120:291–5 | Wrong study design – small number of patients |
| De Cesare SL, Fiorica JV, Roberts WS, Reintgen D, Arango H, Hoffman MS, et al. A pilot study utilising intraoperative lymphoscintigraphy for identification of the sentinel lymph nodes in vulval cancer. Gynecol Oncol 1997;66:425–8 | Small sample size |
| de Hullu JA, Piers DA, Hollema H, Aalders JG, Van der Zee AG. Sentinel lymph node detection in locally recurrent carcinoma of the vulva. BJOG 2001;108:766–8 | Wrong study design – case study |
| Echt ML, Finan MA, Hoffman MS, Kline RC, Roberts WS, Fiorica JV. Detection of sentinel lymph nodes with lymphazurin in cervical, uterine, and vulval malignancies. South Med J 1999;92:204–8 | Wrong population – stage I–II < 70% |
| Farrell C, Lee ST, Grant MP, Rowe C. Rapid localisation of sentinel lymph nodes in vulval lymphoscintigraphy. Intern Med J 2010;40:26–7 | Wrong type of publication – abstract |
| Fuste P, Ortega M, Vidal S, Mancebo G, Alameda F, Carreras R. Feasibility of the sentinel lymph node technique in cervical and vulval cancers. [Spanish]. Medicina Clinica 2007;128:569–71 | Small sample size |
| Garcia JD, Altolaguirre GR, Domingo JM, Ormaetxea GM, Goikoetxea NA, Gonzalvo EA, et al. First results in the sentinel lymph node procedure in vulval squamous cell carcinoma. Prog Obstet Ginecol 2009;52:675–80 | Small sample size |
| Hakam A, Nasir A, Raghuwanshi R, Smith PV, Crawley S, Kaiser HE, et al. Value of multilevel sectioning for improved detection of micrometastases in sentinel lymph nodes in invasive squamous cell carcinoma of the vulva. Anticancer Res 2004;24:1281–6 | Wrong population – stage I–II < 70% |
| Knopp S, Holm R, Trope C, Nesland JM. Occult lymph node metastases in early stage vulval carcinoma patients. Gynecol Oncol 2005;99:383–7 | Wrong intervention – non-sentinel lymph node examination |
| Kudo G, Toyama H, Hasegawa K, Kuroda M, Hattori H, Ishiguro M, et al. Sentinel lymph node navigation surgery in Paget’s disease of the vulva. Clin Nucl Med 2002;27:909–10 | Wrong study design – case study |
| Kurzl R, Friese K. Diseases of the vulva. Gynakologe 2009;42:245–6 | Wrong type of publication – review |
| Levenback CF, Tian C, Coleman RL, Gold MA, Fowler JM, Judson PL. Sentinel node (SN) biopsy in patients with vulval cancer: A Gynecologic Oncology Group (GOG) study. J Clin Oncol 2009;27:5505 | Wrong type of publication – abstract |
| Louis-Sylvestre C, Evangelista E, Leonard F, Ittu E, Meignan M, Paniel BJ. Sentinel node localisation should be interpreted with caution in midline vulval cancer. Gynecol Oncol 2005;97:151–4 | Duplicate of Louis-Sylvestre et al. 200665 |
| Makowski L, Tjahjadi M, Grubner S, Friedrich Gratz K, Hillemanns P, Hertel H. SPECT-CT improved the detection of inguinal sentinel lymph nodes in vulval cancer. Arch Gynecol Obstet 2010;282:S199 | Wrong type of publication – abstract |
| Maza S, Taupitz M, Taymoorian K, Winzer KJ, Ruckert J, Paschen C, et al. Multimodal fusion imaging ensemble for targeted sentinel lymph node management: Initial results of an innovative promising approach for anatomically difficult lymphatic drainage in different tumour entities. Eur J Nucl Med Mol Imaging 2007;34:378–83 | Wrong study design – case study |
| Molpus KL, Kelley MC, Johnson JE, Martin WH, Jones HW, III. Sentinel lymph node detection and microstaging in vulval carcinoma. J Reprod Med 2001;46:863–9 | Lack of data on population – no data about stage |
| Moore RG, Granai CO, Gajewski W, Gordinier M, Steinhoff MM. Pathologic evaluation of inguinal sentinel lymph nodes in vulval cancer patients: a comparison of immunohistochemical staining versus ultrastaging with haematoxylin and eosin staining. Gynecol Oncol 2003;91:378–82 | Lack of data on population – no data about stage |
| Moore RG, DePasquale SE, Steinhoff MM, Gajewski W, Steller M, Noto R, et al. Sentinel node identification and the ability to detect metastatic tumour to inguinal lymph nodes in squamous cell cancer of the vulva. Gynecol Oncol 2003;89:475–9 | Wrong population – stage I–II < 70% |
| Oonk MH, de Hullu JA, Van der Zee AG. Current controversies in the management of patients with early-stage vulval cancer. Curr Opin Oncol 2010;22:481–6 | Wrong type of publication – review |
| Oonk MHM, Hollema H, de Hullu JA, Van der Zee AGJ. Prediction of lymph node metastases in vulval cancer: A review. Int J Gynecol Cancer 2006;16:963–71 | Wrong type of publication – review |
| Oonk MHM, van de Nieuwenhof HP, de Hullu JA, Van der Zee AGJ. The role of sentinel node biopsy in gynecological cancer: a review. Curr Opin Oncol 2009;21:425–32 | Wrong type of publication – review |
| Radziszewski J, Bidzinski M, Panek G, Sobiczewski P, Derlatka P, Nasierowska-Guttmejer A, et al. Sentinel lymph node in vulval cancer – a pilot study to identify and assess the diagnostic value. Nowotwory 2003;53:270–4 | Lack of data on population |
| Regauer S. Histopathological work-up and interpretation of sentinel lymph nodes removed for vulval squamous cell carcinoma. Histopathology 2009;55:174–81 | Wrong study design |
| Robison K, Steinhoff MM, Granai CO, Brard L, Gajewski W, Moore RG. Inguinal sentinel node dissection versus standard inguinal node dissection in patients with vulval cancer: A comparison of the size of metastasis detected in inguinal lymph nodes. Gynecol Oncol 2006;101:24–7 | Wrong type of publication – no data about accuracy |
| Rodier JF, Janser JC, Routiot T, David E, Ott G, Schneegans O, et al. Sentinel node biopsy in vulval malignancies: a preliminary feasibility study. Oncol Rep 1999 Nov;6:1249–52 | Small sample size |
| Schmidt E, Zambo K, Hartmann T, Dehghani B, Bodis J. Preliminary experiences with sentinel nod detection in cases of vulval malignancy. Magy Noorv Lapja 2003;66:177–80 | Duplicate – published in English in Zambo 2002 (later also excluded) |
| Sideri M, De Cicco C, Maggioni A, Colombo N, Bocciolone L, Trifirò G, et al. Detection of sentinel nodes by lymphoscintigraphy and gamma probe guided surgery in vulval neoplasia. Tumori 2000;86:359–63 | Paper not received |
| Tavares MG, Sapienza MT, Galeb NA, Jr., Belfort FA, Costa RR, Osorio CA, et al. The use of 99mTc-phytate for sentinel node mapping in melanoma, breast cancer and vulval cancer: a study of 100 cases. Eur J Nucl Med 2001;28:1597–604 | Lack of data on population – no data about stage |
| Terada KY, Coel MN, Ko P, Wong JH. Combined use of intraoperative lymphatic mapping and lymphoscintigraphy in the management of squamous cell cancer of the vulva. Gynecol Oncol 1998;70:65–9 | Small sample size |
| Terada KY, Shimizu DM, Wong JH. Sentinel node dissection and ultrastaging in squamous cell cancer of the vulva. Gynecol Oncol 2000;76:40–4 | Small sample size |
| Tjin Asjoe FM, van BE, Ewing P, Burger CW, Ansink AC. Sentinel node procedure in vulval squamous cell carcinoma: a histomorphologic review of 32 cases. The significance of anucleate structures on immunohistochemistry. Int J Gynecol Cancer 2008;18:1032–6 | Wrong study design |
| Van Der Zee A, Oonk M, Van Hemel B, de Hullu J, Ansink A, Van Der Velde J, et al. Sentinel lymph node metastasis in patients with vulval cancer mandates adjuvant groin treatment, independent of size. Gynecol Oncol 2009;112:S14–15 | Wrong type of publication – abstract |
| Wydra D, Matuszewski R, Romanowicz G, Bandurski T. Evaluation of surgical gamma probes for sentinel node localisation in cervical and vulval cancer. Nucl Med Rev 2005;8:105–10 | Wrong study design – description of the technique |
| Wydra D, Sawicki S, Emerich J, Romanowicz G. Evaluation of sentinel node detection in vulval cancer. Nucl Med Rev 2005;8:128–30 | Small sample size |
| Zambo K, Schmidt E, Hartmann T, Kornya L, Dehghani B, Tinneberg HR, et al. Preliminary experiences with sentinel lymph node detection in cases of vulval malignancy. Eur J Nucl Med Mol Imaging 2002;29:1198–200 | Small sample size |
Appendix 10 Additional data from test accuracy systematic review
| Study (author, year) | Patients in correct clinical stage (%) | Inclusion criteria | Exclusion criteria |
|---|---|---|---|
| Achimas-Cadariu et al., 200950 | 95% | SCC; no bulky groin lymph nodes | Other than SCC or non-invasive cancer |
| Basta et al., 200551 | 100% | FIGO I–II | NR |
| Brunner et al., 200852 | 100% | SCC; T1–T2; without clinically suspicious inguinal nodes | Vulval cancer with an invasion depth < 1 mm, vulval melanoma, adenocarcinoma, basal cell cancer, verrucous carcinoma, prior chemotherapy, pelvic or inguinal RT or prior vulval surgery |
| Camara et al., 200953 | 94.1% | Clinical stage I–II | NR |
| Crosbie et al., 201054 | 100% | Clinical stage I–II; SCC, > 4 cm, stromal invasion < 1 mm; without clinically suggestive of metastasis | NR |
| De Cicco et al., 200055 | 100% | Stage T1–T2; SCC, without clinically suggestive of metastasis | Patients with clinically positive groin nodes, pregnant or lactating patients were excluded from the study |
| de Hullu et al., 200056 | 100% | Stage T1–T2; SCC, without clinically suggestive of metastasis | NR |
| Hampl et al., 200857 | 94.4% | SCC; T1–T3 | Unresectable tumours, suspicious nodes in the groin (detected by ultrasonography) or cytologically or histologically proven lymphatic metastases |
| Hauspy et al., 200758 | 95% | Stage T1–T2; SCC, without clinically suggestive of metastasis | NR |
| Johann et al., 200859 | 86% | SCC; T1–T2 | NR |
| Klat et al., 200960 | 100% | Stage T1B–T2; SCC | T3 or T4 tumours and with palpable and enlarged lymph nodes |
| Levenback et al., 200161 | 87% | Primary surgical treatment for vulval cancer, regardless of clinical stage or histological features | NR |
| Lindell et al., 201039 | 98% | With the intention to learn the procedure; T1–T3 without palpable lymph nodes in the groins, no upper limit for tumour size; (one patient with multifocal tumour) | NR |
| Louis-Sylvestre et al., 200662 | 100% | Stage T1–T2; stromal invasion < 1 mm | Prior vulval surgery |
| Martinez-Palones et al., 200663 | 92.9% | Stage T1–T2; SCC or melanoma | In situ carcinoma, depth invasion < 1 mm, prior chemotherapy or RT or vulval surgery, and vaginal, rectal or urinary bladder involvement (stages III–IV) |
| Merisio et al., 200564 | 100% | T1-T2; histologically confirmed invasive SCC, clinically negative groins, no prior chemotherapy or RT | Clinically positive groin nodes, stage T3–T4, pregnant or lactating |
| Moore et al., 200865 | 100% | Clinical stage I–II, SCC; > 4 cm, stromal invasion < 1 mm; without clinically suggestive of metastasis | NR |
| Nyberg et al., 200766 | 100% | Vulval cancer | Previous radiation therapy, no surgery at all, incomplete surgery, the primary operation performed at some other hospital or before 2001, or unknown origin of the malignant disease |
| Pityński et al., 200367 | 100% | Stage I–II | NR |
| Radziszewski et al., 201068 | 100% | Clinical stage I–II; SCC, > 4 cm, stromal invasion < 1 mm, T1–T2, N0, M0 | Prior vulval surgery, positive nodes |
| Rob et al., 200769 | 100% | Clinical stage I–II; SCC, > 4 cm, stromal invasion < 1 mm; without clinically suggestive of metastasis | NR |
| Terada et al., 200670 | 100% | SCC; T1; at least 1 mm of invasion | Locally advanced tumours (T2–4), gross adenopathy in the groin |
| Vakselj et al., 200771 | 92% | Vulval cancer | NR |
| Van den Eynden et al., 200372 | 100% | Vulval cancer | NR |
| Van der Zee et al., 200873 | 100% | SCC; T1–T2; < 4 cm; depth of invasion > 1 mm and clinically non-suspicious inguinofemoral lymph nodes; registered at the University Medical Centre Groningen (Groningen, the Netherlands); amendment – multifocal | NR |
| Vidal-Sicart et al., 200774 | 86% | Need to perform an IFL in patients initially proposed for a curative surgical procedure | NR |
| Study (author, year) | Treatment after SLN biopsy | IFL | ||
|---|---|---|---|---|
| Surgery | RT | Unilateral | Bilateral | |
| Achimas-Cadariu et al., 200950 | Modified hemivulvectomy or wide local excision n = 19, radical vulvectomy: 27 | NR | Lateral T1 | NR |
| Basta et al., 200551 | Radical vulvectomy: 39a | NR | NR | NR |
| Brunner et al., 200852 | NR | NR | 34 | 10 |
| Camara et al., 200953 | Hemivulvectomy: 5, radical vulvectomy: 12 | NR | NR | NR |
| Crosbie et al., 201054 | NR | NR | NR | NR |
| De Cicco et al., 200055 | Wide excision, hemivulvectomy, radical vulvectomy | NR | 18 | 19 |
| de Hullu et al., 200056 | Radical excision: 59 | NR | 11 | 48 |
| Hampl et al., 200857 | Hemivulvectomy (35%) or vulvectomy (35%), followed by local tumour resection in 30% of n = 127 | NR | 21 | 103 |
| Hauspy et al., 200758 | Wide excision: 31; radical vulvectomy: 8 | 2 | NR | NR |
| Johann et al., 200859 | Wide excision: 3; hemivulvectomy: 13; radical vulvectomy: 7 | NR | 1 | 22 |
| Klat et al., 200960 | Radical excision, radical vulvectomy | NR | NR | NR |
| Levenback et al., 200161 | NR | 1b | 28 | 24 |
| Lindell et al., 201039 | Wide excision: 17;c hemivulvectomy: 24 | NR | 24d | 53 |
| Louis-Sylvestre et al., 200662 | Radical vulvectomy or other | NR | NR | NR |
| Martinez-Palones et al., 200663 | Radical vulvectomy: 28 | NR | 16 | 12 |
| Merisio et al., 200564 | Radical vulvectomy: 20 | NR | 8 | 12 |
| Moore et al., 200865 | Radical wide excision or radical vulvectomy | RT (patients with SLN + ) | 16 | 19 |
| Nyberg et al., 200766 | NR | NR | NR | NR |
| Pityński et al., 200367 | Wide excision | NR | NR | NR |
| Radziszewski et al., 201068 | NR | NR | 5 | 51 |
| Rob et al., 200769 | Radical excision, radical vulvectomy, other | NR | NR | NR |
| Terada et al., 200670 | Radical excision | Palliative | 17 | 4 |
| Vakselj et al., 200771 | NR | 9 | 19 | 16 |
| Van den Eynden et al., 200372 | Wide excision, hemivulvectomy, radical vulvectomy | NR | 13 | 14 |
| Van der Zee et al., 200873 | Wide local excision: 358;c radical vulvectomy: 41; other: 4 | Four patients, > 1 intranodal metastasis and/or extra nodal growth was detected, postoperative external beam RT (50 Gy) to the groin/pelvis | 183 | NR |
| Vidal-Sicart et al., 200774 | Wide excision: 2; radical vulvectomy: 7; other: 17 | NR | 30 | 19 |
| Study ID | Reference standard | Index test (blue dye) | Index test (99mTc) | Lymphoscintigraphy | |||||
|---|---|---|---|---|---|---|---|---|---|
| IFL/follow-up | Follow-up | H&E/immunostained | Type | Dose | Type | Dose | Imaging | ||
| Achimas-Cadariu et al., 200950 | IFL for SLN + and follow-up for SLN- | Annually by screening the in-house records or contacting the office gynaecologists or family doctors | H&E for all and ultrastaging for SLN negative | Isosulfan blue dye | NR | 99mTc colloid albumin | NR | Handheld probe (Gammafinder, G100, World of Medicine, Berlin, Germany) | Performed |
| Basta et al., 200551 | IFL for all | NA | H&E and immunostained for all | Patent Blau (Guerbet GmbH) | 2–4 ml | Nanocolloid (Nycomed Amersham Sorin S.r.l., Saluggia, Italy) marked with 99mTc | 2.5 mCi | Handheld gamma probe (Navigator GPS, USSC; Tyco Healthcare, Mansfield, MA, USA) | Performed |
| Brunner et al., 200852 | IFL and follow-up for all | 3-month intervals, including inspection, vaginorectal and groin palpation, and in some cases, serum tumour marker evaluation. Mean 35.8 months, SD 122.1 months | H&E for all and immunostained for H&E negative | Isosulfan blue | 1 ml | 99mTc microcolloidal-containing albumin (Albures, Pharmaceutical Nycomed Amersham, Braunschweig, Germany) | 15 MBq tracer in 0.4 ml saline | Single- or dual-head gamma camera; handheld collimated gamma probe (ScintiProbe MR-100, Pol.Hi.Tech, Carsoli, Italy) | Preoperative lymphoscintigraphy was performed in all |
| Camara et al., 200953 | IFL for all | NA | NR | Blue dye | NR | 99mTc nanocolloid | NR | Handheld gamma probe | Not performed |
| Crosbie et al., 201054 | IFL and follow-up for all | Total follow-up: 5 years, assessment every 3 months for the first 2 years and every 6 months thereafter. Includes symptom enquiry and clinical examination of the vulva and groins | H&E and immunostained for negative on H&E | Patent blue dye | 3 ml | 99mTc-labelled human serum albumin nanocolloid (Solco Nuclear, Birfelden, Switzerland) | 40 MBq/0.2 ml | Gamma camera | Preoperative lymphoscintigraphy was performed in all |
| De Cicco et al., 200055 | IFL for all | NA | H&E | NA | NA | 99mTc-colloids Nanocoll (Nycomed Amersham Sorin S.R.L., Saluggia, Italy) | 0.1 ml of 5 MBq; total dose 10–20 MBq | Gamma-detecting probe (MR 100, Po.li.tech. L’Aquila, Italy) | Preoperative lymphoscintigraphy was performed in all |
| de Hullu et al., 200056 | IFL for all | NA | H&E for all and immunostained for negative on H&E | Blue V dye (Laboratoire Guerbet, Aulney-Sous-Bois, France) | 2 ml | 99mTc-labelled nanocolloid (Solco Nuclear, Birfelden, Switzerland) | 0.2–0.6 ml of 60 MBq | Handheld probe (Neoprobe; Neoprobe Corporation, Dublin, Ireland) | Preoperative lymphoscintigraphy was performed in all |
| Hampl et al., 200857 | IFL for all | NA | H&E for all and immunostained (unclear if for all) | Patentblue V 2.5% in aqua dest with 0.9 NaCl and 0.05% disodium phosphate (Laboratoire Guerbet, Aulney-Sous-Bois, France) | 0.5–1.0 ml | 99mTc-labelled nanocolloid with a particle size of b80 nm (Nycomed Amersham Sorin S.r.l., Saluggia, Italy) | 0.2 ml 60–120 MBq | Every 30 minutes, handheld gamma counter | Performed |
| Hauspy et al., 200758 | IFL for all | NA | H&E and immunostained for all | Lymphazurin blue dye | 4 ml | Technetium–sulfur colloid | 0.1–0.2 mCi | Handheld gamma probe (Navigator GPS; Tyco Healthcare, Mansfield, MA, USA) | Not routinely performed in all patients |
| Johann et al., 200859 | IFL for all | NA | Step sectioning, frozen section | Blue dye | NR | 99mTc-labelled nanocolloids, particle size ≤ 80 nm (Nanocoll™, GE Healthcare, Amersham Health, Braunschweig, Germany) | Four aliquots of 15 MBq | Dual-head gamma camera up to 2 hours post injection (SLN, standard gamma-probe) | Performed |
| Klat et al., 200960 | IFL for all | NA | H&E and immunostained for all | Bleu patenté V (2.5%, Guebert, Paris, France) | 4 ml | Radionuclide-labelled nanocolloid (Senti-Scint, FJC National Research Institute for Radiobiology and Radiohygiene, Budapest, Hungary) | 50 MBq | Handheld gamma counter (NEO 2000; Neoprobe Corporation, Dublin, Ohio OH, USA; Europrobe, Euromedical, Le Chesnay, France) | Preoperative lymphoscintigraphy was performed in all |
| Levenback et al., 200161 | IFL for all | NA | H&E for all and immunostained for negative on H&E | Isosulfan blue dye | 1–4 ml | NA | NA | NA | Not performed |
| Lindell et al., 201039 | IFL for all | NA | H&E for all and immunostained for negative on H&E | Blue dye (metyltionincloride, 10 mg/ml, ATL, Stockholm, Sweden) | 0.25 ml | Human serum albumin colloid labelled with 99mTc (Nanocoll, Nycomed Amersham Sorin Srl, Saluggia,Italy) | 40 MBq | One head of a Triad XLT gamma camera equipped with a parallel-hole collimator (Trionix Inc., Twinsburg, OH, USA); handheld gamma-probe (Europrobe1, Eurorad, France) | Performed |
| Louis-Sylvestre et al., 200662 | IFL for all | NA | H&E for all and immunostained for negative on H&E | Blue dye | 2 ml | 99mTc | 30 MBq | Manual probe de détection (Europrobe, Euromédical Instruments, The Chesnay France) | Preoperative lymphoscintigraphy was performed in all |
| Martinez-Palones et al., 200663 | IFL for all | NA | H&E all and immunostained for negative on H&E | Isosulfan blue dye (Lymphazurin 1%, United States Surgical Co., Norwalk, CT, USA) | 2–4 ml | 99mTc-labelled nanocolloid (Albu-res®, Pharmaceutical Nycomed Amersham, Bruanschweig, Germany) | NR | Gamma camera | Dynamic lymphoscientigraphy was performed in all |
| Merisio et al., 200564 | IFL for all | NA | H&E for all and immunostained for negative on H&E | NA | NA | Radioactively labelled 99mTc pertechnetate, nanocolloid particles (< 80 nµ in diameter) | Average total dose 11 MBq (range 10–20 MBq) diluted in a 20 : 1-l volume | Adac Thirus and Picker Prisma 2000 (Aurora, OH, USA) XP gamma cameras; ScintiProbe MR-100 surgical probe (IFL) | Preoperative lymphoscintigraphy was performed in all |
| Moore et al., 200865 | IFL for SLN positive and follow-up for SLN negative | At 3-month intervals for the first 2 years and then every 6 months thereafter | H&E for all and immunostained for negative on H&E | Methylene blue | NR | 99mTc–sulphur colloid | 2 mCi | Handheld gamma camera | Preoperative lymphoscintigraphy or examination with a handheld gamma counter regardless of the tumour location |
| Nyberg et al., 200766 | IFL for all | NA | NR | Patent blue dye | NR | 99mTc colloid | NR | Handheld gamma probe intraoperatively (Navigator GPS, Tyco HealthCare, Norwalk, CT, USA). | Not performed at all |
| Pityński et al., 200367 | IFL for all | NA | NR | Patent Blue | 2 ml | 99mTc nanocolloid | 1 ml; 50 MBq | Dual-head gamma camera (Siemens, Munich, Germany); gamma radiation detector (Navigator GPS; Tyco Healthcare, Mansfield, MA, USA) | Not routinely performed |
| Radziszewski et al., 201068 | IFL for all | NA | H&E and Immunostained for all | 2.5% of patent blue V | NR | 99mTc colloid albumin | 1.2 mCi | Double-headed high-resolution VariCam gamma probe (Elscint, Haifa, Israel) with a low-energy collimator | Preoperative lymphoscintigraphy was performed in all |
| Rob et al., 200769 | IFL for all | NA | H&E and immunostained for all | Blue dye (Patentblau V BYC, Gueden, Germany, or bleu patenté V 2.5%, Guerbet, France) | 2 ml | NA | NA | NA | NA |
| Terada et al., 200670 | IFL for SLN + and follow-up for all | Median follow-up 4.6 years (range 2–8 years) | H&E for all and immunostained for negative on H&E | Isosulfan blue dye | 0.5 ml | 99mTc-labelled sulphur colloid | 0.5 mCi | Handheld gamma probe (Neoprobe Corporation, Dublin, Ohio, OH, USA) | Performed |
| Vakselj et al., 200771 | IFL for SLN + and follow-up for SLN negative | Total follow-up: 4 years | NR | Methylene blue dye | NR | 99mTc-labelled nanocolloid | NR | Gamma camera | Static and dynamic lymphoscintigraphies were performed in all |
| Van den Eynden et al., 200372 | IFL for all (phase 1) IFL for SLN + and follow-up for SLN negative (phase 2) | Phase 2: short follow-up, maximum 17 months | H&E for all and immunostained for negative on H&E | Patent blue violet | 0.5–2.0 mL | 99mTc nanocolloid | 0.2 ml; 40 MBq | Handheld gamma probe | Performed |
| Van der Zee et al., 200873 | IFL for SLN + and follow-up for SLN negative | For 2 years at intervals of every 2 months. Stopping rules: occurrence of groin recurrences | H&E for all and immunostained for negative on H&E | Patent blue-V (Laboratoire Guerbet, Aulney-Sous-Bois, France) | 2.0 ml | 99mTc-labelled nanocolloid (Solco Nuclear, Birsfelden, Switzerland) | 0.5 mL of 100 MBq | Single-head gamma camera; handheld gamma-ray detection probe (Neoprobe, Dublin, Ohio, OH, USA) | Performed |
| Vidal-Sicart et al., 200774 | IFL and follow-up for all | Over a mean period of 24 months | H&E for all and immunostained for negative on H&E | Isosulfan blue (Lymphazurin, BenVenue Labs, Bedford, OH, USA), methylene blue (Laboratory Dr Carreras, Barcelona, Spain) | 1 ml | 99mTc nanocolloid (Lymphoscint Amersham, Saluggia, Italy) | 0.75–1 mCi | Handheld gamma probe (Navigator, USSC, Norwalk, CT, USA) | Performed |
| Outcome | Study | SLN biopsy + IFL | SLN biopsy only | SLN biopsy + IFL or follow-up |
|---|---|---|---|---|
| Short term | Moore et al., 200865n = 36 | NA | NA | Wound breakdown, n = 0 (0%) Wound cellulitis, n = 0 (0%) Cellulitis, n = 0 (0%) Postoperative groin lymphocele, n = 2 (5.5%) Cellulitis arising in the labia majora, n = 1 (2.8%) |
| Terada et al., 200670n = 21 | Wound cellulitis, n = 2 (9.5%) Seroma, n = 1 (4.8%) |
NA | NA | |
| Van den Eynden et al., 200372n = 17 | NA | NA | Cellulitis, n = 1 (5.9%) Lymphocele, n = 2 (11.8%) |
|
| Van der Zee et al., 200873n = 264 | Wound breakdown, n = 16 (34%)a Wound cellulitis, n = 10 (21.3%)a |
Wound breakdown, n = 31 (11.7%) Wound cellulitis, n = 12 (4.5) |
NA | |
| Long term | Crosbie et al., 201054n = 32 | NA | NA | Wound infection, n = 10 (31%) Wound dehiscence, n = 8 (25%) Lymphocyst, n = 7 (22%) Chronic lymphoedema, n = 5 (16%) |
| Van der Zee et al., 200873n = 264 | Lymphoedema, n = 30 (25.2%)b Recurrent erysipelas, n = 19 (16.2%)c |
Lymphoedema, n = 5 (1.9%) Recurrent erysipelas, n = 1 (0.4%) |
NA |
Appendix 11 Excluded studies with reasons, effectiveness systematic review
| Akashi K, Kudo R, Sato T, Tanaka S. Administration of bleomycin in cancer of the female sex organs and the follow-up results. Sanfujinka No Jissai – Pract Gynecol Obstet 2011;11:1970 | Paper not received |
| Akl A, Akl M, Boike G, Hebert III J, Graham J. Preliminary results of chemoradiation as a primary treatment for vulval carcinoma. Int J Radiat Oncol Biol Phys 2000;48:415–20 | Wrong study design: small study population, n = 8 |
| Andersen BL, Hacker NF. Psychosexual adjustment after vulval surgery. Obstet Gynecol 1983;62:457–62 | Wrong study design and data presentation: lack of data, only subjective description |
| Andreasson B, Bock JE, Visfeldt J. Prognostic role of histology in squamous cell carcinoma in the vulval region. Gynecol Oncol 1982;14:373–81 | Wrong study population: 54% patients met the included criteria, but most data were presented for all study group |
| Andreasson B, Bock JE, Weberg E. Invasive cancer in the vulval region. Acta Obstet Gynecol Scand 1982;61:113–19 | Wrong study population: 13% of patients had others malignancies |
| Andreasson B, Moth I, Jensen SB, Bock JE. Sexual function and somatopsychic reactions in vulvectomy-operated women and their partners. Acta Obstet Gynecolog Scand 1986;65:7–10 | Wrong study design: no information about FIGO stage |
| Andreasson B, Nyboe J. Value of prognostic parameters in squamous cell carcinoma of the vulva. Gynecol Oncol 1985;22:341–51 | Inadequate data presentation: no information about effectiveness of treatment |
| Ansink AC, Van Tinteren H, Aartsen EJ, Heintz APM. Outcome, complications and follow-up in surgically treated squamous cell carcinoma of the vulva 1956–1982. Eur J Obstet Gynecol Reprod Biol 1991;42:137–43 | Paper waiting to be received |
| Arvas M, Kose F, Gezer A, Demirkiran F, Tulunay G, Kosebay D. Radical versus conservative surgery for vular carcinoma. Int J Gynecol Obstet 2005;88:127–33 | More than 25% FIGO stage III and IV |
| Atlante G, Lombardi A, Mariani L, Vincenzoni C. Carcinoma of the vulva (1981–1985): Analysis of a radio-surgical approach. Eur J Gynaecol Oncol 1989;10:341–8 | Paper waiting to be received |
| Ayhan A, Tuncer ZS, Akarin R, Yucel I, Develioglu O, Mercan R et al. Complications of radical vulvectomy and inguinal lymphadenectomy for the treatment of carcinoma of the vulva. J Surg Oncol 1992;51:243–5 | Paper waiting to be received |
| Bafna UD, Devi K, Naik A, Hazra S, Sushma N, Babu N. Carcinoma of the vulva: a retrospective review of 37 cases at a regional cancer centre in South India. J Obstet Gynaecol 2004;24:403–7 | Wrong study population: too small a study group (n = 5), all patients presented as case reports |
| Bakalianou K, Salakos N, Iavazzo C, Paltoglou G, Papadias K, Gregoriou O, et al. Paget’s disease of the vulva. A ten-year experience. Eur J Gynaecol Oncol 2008;29:368–70 | Paper waiting to be received |
| Balat O, Edwards CL, Verschraegen C, Delclos L. The long term results of radiotherapy with or without surgery in management of advanced vulvar cancer: report of 76 patients. Eur J Gynaecol Oncol 2000;21:426–9 | More than 25% FIGO stage III and IV |
| Balat O, Edwards C, Delclos L. Complications following combined surgery (radical vulvectomy versus wide local excision) and radiotherapy for the treatment of carcinoma of the vulva: Report of 73 patients. Eur J Gynaecol Oncol 2000;21:501–3 | Paper waiting to be received |
| Baltzer J. Precancerous lesions and early stages of vulval cancer. Arch Gynecol Obstet 1989;245:498–503 | Paper waiting to be received |
| Barton DP. The prevention and management of treatment related morbidity in vulval cancer. Best Pract Res Clin Obstet Gynaecol 2003;17:683–701 | Wrong study design: review |
| Beissert M, Steinbach C, Neef G. Treatment of cancer of the vulva. Zentralbl Gynakol 1974;96:1268–73 | Wrong study design: lack of information about patients’ clinical stage |
| Bnedet JL, Turko JL, Fairey RN, Boyes DA. Squamous carcinoma of the vulva: results of treatment 1938 to 1976. Am J Obstet Gynecol 1979;134:201–7 | Sample recruited before 1980 |
| Bergen S, DiSaia PJ, Liao SY, Berman ML. Conservative management of extramammary Paget’s disease of the vulva. Gynecol Oncol 1989;33:151–6 | Inadequate data presentation: data format not allowing to obtain meaningful information |
| Berget A, Larsen JF, Pedersen PH. CO2 laser in Gynaecology. II. Practical experience. Ugeskr Laeg 1982;144:3350–3 | Paper waiting to be received |
| Beriwal S, Heron DE, Kim H, King G, Shogan J, Bahri S, et al. Intensity-modulated radiotherapy for the treatment of vulval carcinoma: A comparative dosimetric study with early clinical outcome. Int J Radiat Oncol Biol Phys 2006;64:1395–400 | Wrong study design: small study population, n < 10 |
| Berman ML, Soper JT, Creasman WT, Olt GT, SiSaia PJ. Conservative surgical management of superficially invasive stage I vulval carcinoma. Gynecol Oncol 1989;35:352–7 | Wrong study population: 16% of patients had concomitant cancer |
| Bienkiewicz A, Gottwald L, Akoel K M, Lech W, Welfel J, Suzin J. Clinical analysis of 105 cases of vulval cancer. Ginekol Pol 2002;73:913–18 | Inadequate data presentation: lack of information about each FIGO stage group treatment and adequate results presentation/mixed population; the problem and data format was inadequate |
| Black D, Tornos C, Soslow RA, Awtrey CS, Barakat R R, Chi DS. The outcomes of patients with positive margins after excision for intraepithelial Paget’s disease of the vulva. Gynecol Oncol 2007;104:547–50 | Wrong study design: lack of information about FIGO stage/women with vulval Paget's disease (80% primary), no FIGO staging; no comparison with other intervention (all patients underwent surgical excision of primary VI Paget's disease) |
| Blecharz P, Urbanski K, Karolewski K, Klimek M, Pudelek J, Bieda T, et al. Prognostic factors in patients with vulvar cancer in the material of the Crocow Division of Centre of Oncology. Ginecol Oncol 2007;5:22–8 | More than 25% FIGO stage III and IV |
| Bognel C, Prade M, Charpentier P, Michel G. Paget’s disease of the vulva. Seven clinicopathological cases. Gynecologie 1980;31:527–33 | Wrong study design: case report based on seven cases |
| Boidi Trotti A, Tardy A, Burke P, Tomassone W. Results of radiating treatment with fast electrons in 24 cases of cancer of the vulva. Minerva Ginecol 1980;32:1011–12 | Wrong study design: case report based on three cases |
| Bokhman J V, Maximov S J, Ebert AD. Efficiency of radical treatment in vulval carcinoma. Analysis of 148 cases. Zentralbl Gynakol 1997;119:166–72 | Paper waiting to be received |
| Bosquet JG, Kinney WK, Russell AH, Gaffey TA, Magrina JF, Podratz KC. Risk of occult inguinofemoral lymph node metastasis from squamous carcinoma of the vulva. Int J Radiat Oncol Biol Phys 2003;57:419–24 | Wrong study design: study is a retrospective follow-up |
| Bosquet JG, Magrina JF, Gaffey TA, Hernandez JL, Webb MJ, Cliby WA, et al. Long-term survival and disease recurrence in patients with primary squamous cell carcinoma of the vulva. Gynecol Oncol 2005;97:828–33 | Wrong study design: authors do not provide information about FIGO stage of disease, there are no opportunities to extract interesting data |
| Boutselis JG. Radical vulvectomy for invasive squamous cell carcinoma of the vulva. Obstet Gynecol 1972;39:827–36 | Inadequate data presentation: results presented are cumulative for all patients and all interventions |
| Boyce J, Fruchter RG, Kasambilides E, Nicastri AD, Sedlis A, Remy JC. Prognostic factors in carcinoma of the vulva. Gynecol Oncol 1985;20:364–77 | More than 25% FIGO stage III and IV |
| Bozzetti F, Lupi G, Di Re F. Evaluation of lymph node involvement in squamous cell carcinoma of the vulva and therapeutic implications. Tumori 1974;60:269–77 | Paper waiting to be received |
| Brinton LA, Nasca PC, Mallin K, Baptiste MS, Wilbanks GD, Richart RM. Case–control study of cancer of the vulva. Obstet Gynecol 1990;75:859–66 | Wrong study design: epidemiological investigation of rare reproductive tumours |
| Bryson SCP, Dembo AJ, Colgan TJ, Thomas GM, Deboer G, Lickrish GM. Invasive squamous-cell carcinoma of the vulva – defining low and high-risk groups for recurrence. Int J Gynecol Cancer 1991;1:25–31 | Paper waiting to be received |
| Burger MP, Hollema H, Emanuels AG, Krans M, Pras E, Bouma J. The importance of the groin node status for the survival of T1 and T2 vulval carcinoma patients. Gynecol Oncol 1995;57:327–34 | Wrong study design: the main aim was correlation between groin node status and risk factors |
| Burke TW, Morris M, Roh MS, Levenback C, Gershenson DM. Perineal reconstruction using single gracilis myocutaneous flaps. Gynecol Oncol 1995;57:221–5 | Wrong study population: patients after reconstruction |
| Busch M. Long-term results of radiotherapy alone for carcinoma of the vulva. Adv Ther 1999;16: 89–100 | Paper waiting to be received |
| Butler JSB, Milliken DA, Dina R, Eccles SA, Maghami SG, Jameson C, et al. Isolated groin recurrence in vulval squamous cell cancer (VSCC). The importance of node count. Eur J Gynaecol Oncol 2010;31:510–13 | Wrong study design: analyses of risk factors |
| Calista D. Topical 1% cidofovir for the treatment of vulval intraepidermal neoplasia (VIN1) developed on lichen sclerosus. Int J Dermatol 2009;48:535–6 | Wrong study design: case report, VIN1 treated with topical 1% cidofovir |
| Callies R, Kock U, Zeller GX. Results of treatment of vulval malignoma – retrospective analysis of 119 cases. Zentralbl Gynakol 1984;106:440–55 | Inadequate data presentation: population description |
| Campagnutta E, Scarabelli C, Juzzolino C. The treatment of carcinoma of the vulva using cis-platinum locoregional endoarterial chemotherapy. Minerva Ginecol 1984;36:407–9 | Wrong study design: case report |
| Carcopino X, Houvenaeghel G, Buttarelli M, Charaffe-Jauffret E, Gonzague L, Rossi I. Feasibility and morbidity of sentinel lymph node detection in patients with vulval carcinoma. Bull Cancer 2005;92:489–97 | Wrong intervention: diagnostic study |
| Cavanagh D, Fiorica JV, Hoffman MS, Roberts WS, Bryson SCP, LaPolla JP, et al. Invasive carcinoma of the vulva. Changing trends in surgical management. Am J Obstet Gynecol 1990;163:1007–15 | Wrong study population: only 67% patients met the inclusion criteria |
| Cavanagh D, Shepherd JH. The place of pelvic exenteration in the primary management of advanced carcinoma of the vulva. Gynecol Oncol 1982;13:318–22 | Wrong study design: data presented in cumulative way for all patients (also with advanced disease) |
| Chan JK, Sugiyama V, Pham H, Gu M, Rutgers J, Osann K, et al. Margin distance and other clinic-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol 2007;104:636–41 | More than 25% FIGO stage III and IV |
| Chardot C, Dartois D, Keil L. Long term results in vulva carcinoma and discussion about the extent of surgical exeresis. 92 Observations. Ann Med Nancy Est 1978;17:1323–30 | Paper waiting to be received |
| Cheng X, Zang RY, Wu XH, Li ZT, Cai SM, Zhang ZY. Recurrence patterns and prognostic factors in Chinese patients with squamous cell carcinoma of the vulva treated with primary surgery. Int J Gynecol Cancer 2009;19:158–62 | Wrong study population: only 35% patients met inclusion criteria |
| Choo YC. Invasive squamous carcinoma of the vulva in young patients. Gynecol Oncol 1982;13:158–64 | Sample recruited before 1980 |
| Chu J, Tamimi HK, Ek M, Figge DC. Stage I vulval cancer: criteria for microinvasion. Obstet Gynecol 1982;59:716–19 | Wrong study population: patients with other tumour |
| Crane LMA, Themelis G, Arts HJG, Buddingh KT, Brouwers AH, Ntziachristos V, et al. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulval cancer: first clinical results. Gynecol Oncol 2010;120:291–5 | Wrong intervention: diagnostic study |
| Crane LM, Pleijhuis RG, Themelis G, Harlaar NJ, Sarantopoulos A, Arts HG, et al. Detection of the sentinel lymph node in vulval cancer, using near-infrared fluorescence intraoperative imaging: a technical feasibility study. Mol Imaging Biol 2010;12:S1164 | Wrong study design: diagnostic study |
| Creasman WT. New 123 gynaecologic cancer staging. Obstetr Gynecol 1990;75:287–8. | Wrong study design: report from the FIGO Committee Meeting |
| Curtin JP, Rubin SC, Jones WB, Hoskins WJ, Lewis J. Paget’s disease of the vulva. Gynecol Oncol 1990;39:374–7 | Inadequate data presentation: all results presented per patient |
| Danby CS, Margesson LJ. Approach to the diagnosis and treatment of vulval pain. Dermatol Ther 2010;23:485–504 | Paper waiting to be received |
| de Hullu JA, Van Der Zee AG. Surgical treatment of early-stage vulva carcinoma and the complications of the operation. Ned Tijdschr Geneeskd 2005;149:336–42 | Paper waiting to be received |
| Dean RE, Taylor ES, Weisbrod DM, Martin JW. The treatment of premalignant and malignant lesions of the vulva. Am J Obstet Gynecol 1974;119:59–68 | Wrong study population: only 67% of patients met inclusion criteria |
| Dinh VT, Ton TP: Surgical treatment of cancer of the vulva. A study of 21 cases. J Gynecol Obstet Biol Reprod 1976;5:113–22 | Paper waiting to be received |
| DiSaia PJ, Creasman WT, Rich WM. An alternate approach to early cancer of the vulva. Am J Obstet Gynecol 1979;133:825–32 | Sample recruited before 1980 |
| Donaldson ES, Powell DE, Hanson MB, Van N, Jr. Prognostic parameters in invasive vulval cancer. Gynecol Oncol 1981;11:184–90 | Paper waiting to be received |
| Durdevic S, Hadzic B, Petrovic D. Radical vulvectomy with inguino-femoral lymphadenectomy in the surgical treatment of vulval carcinoma. Medicinski Pregled 2000;53:607–12 | Paper waiting to be received |
| Dvoretsky PM, Bonfiglio TA, Helmkamp BF. The pathology of superficially invasive, thin vulval squamous cell carcinoma. Int J Gynecol Pathol 1984;3:331–42 | Wrong study design: analyses of metastases’ pathology |
| Ebert A, Baege A, Maximov S and Bokhman JV. 148 cases of vulval cancer – results of radical surgical treatment. Eur J Cancer 1997;33:942 | Paper waiting to be received |
| Egwuatu VE, Ejeckam GC. An analysis of tumours of the female genital tract in Enugu, Nigeria (1973–1979): a hospital based tumour registry review. Bull Cancer 1980;67:535–9 | Inadequate data: no information about FIGO or TNM stage, patients without other gynaecological tumours |
| Eifel PJ. Radiotherapy versus radical surgery for 123 gynaecologic neoplasms: carcinomas of the cervix and vulva. Front Radiat Ther Oncol 1993;27:130–42 | Paper waiting to be received |
| Eke AC, Alabi-Isama LI, Akabuike JC. Management options for vulval carcinoma in a low resource setting. World J Surg Oncol 2010;8:94 | Wrong study population: all stages included in the investigation |
| Elit L, Hancock G, Carey M, Dal Bello D, Allen HH. Comparing the morbidity of single versus separate incision surgical approaches to vulval cancer. J Gynecol Tech 1999;5:147–50 | Paper waiting to be received |
| Eva LJ, Ganesan R, Chan KK, Honest H, Malik S, Luesley DM. Vulval squamous cell carcinoma occurring on a background of differentiated vulval intraepithelial neoplasia is more likely to recur: A review of 154 cases. J Reprod Med Obstet Gynecol 2008;53:397–401 | Inadequate data presentation |
| Eva LJ, Ganesan R, Chan KK, Honest H, Luesley DM. Differentiated-type vulval intraepithelial neoplasia has a high–risk association with vulval squamous cell carcinoma. Int J Gynecol Cancer 2009;19:741–4 | Wrong study design: inadequate study question |
| Fairey RN, MacKay PA, Benedet JL. Radiation treatment of carcinoma of the vulva, 1950–1980. Am J Obstet Gynecol 1985;151:591–7 | Paper waiting to be received |
| Fanning J, Lambert HCL, Hale TM, Morris PC, Schuerch C. Paget’s disease of the vulva: Prevalence of associated vulval adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol 1999;180:24–7 | Wrong study population: only 58% of patients met inclusion criteria |
| Fariaseisner R, Cirisano FD, Grouse D, Leuchter RS, Karlan BY, Lagasse LD, et al. Conservative and individualized surgery for early squamous carcinoma of the vulva – the treatment of choice for stage-I and II (T1–2N0–1M0) disease. Gynecol Oncol 1994;53:55–8 | Paper waiting to be received |
| Faul CM, Mirmow D, Huang Q, Gerszten K, Day R, Jones MW. Adjuvant radiation for vulvar carcinoma: improved local control. Int J Radiat Oncol Biol Phys 1997;38:381–9 | More than 25% FIGO stage III and IV |
| Feakins RM, Lowe DG. Basal cell carcinoma of the vulva: A clinicopathologic study of 45 cases. Int J Gynecol Pathol 1997;16:319–24 | Paper waiting to be received |
| Fioretti P, Gadducci A, Prato G, Tavella N, Fanucchi A, Facchini V. The influence of some prognostic factors on the clinical outcome of patients with squamous cell carcinoma of the vulva. Eur J Gynaecol Oncol 1992;8:97–104 | More than 25% FIGO stage III and IV |
| Fiorica JV, Roberts WS, LaPolla JP, Hoffman MS, Barton DPJ, Cavanagh D. Femoral vessel coverage with dura mater after inguinofemoral lymphadenectomy. Gynecol Oncol 1991:42:217–21 | Wrong study design: information only about primary risk factors of disease |
| Fonseca-Moutinho JA, Coelho MC, Dilva DP. Vulvar squamous cell carcinoma. Prognostic factors for local recurrence after primary en bloc radical vulvectomy and bilateral groin dissection. J Reprod Med 2000;45:672–8 | More than 25% FIGO stage III and IV |
| Frankendal B, Larsson LG, Westling P. Carcinoma of the vulva. Results of an individualized treatment schedule. Acta Radiol Ser Ther Phys Biol 1973;12:165–74 | Wrong study population: five patients had other tumours |
| Frankman O, Kabulski Z. Malignancy grading and prognosis from a biopsy only in cases of electrocoagulated squamous cell carcinoma of the vulva, stages I and II. Int J Gynecol Obstet 1983;21:119–24 | Paper waiting to be received |
| Friedrich J, Wilkinson EJ, Fu YS. Carcinoma in situ of the vulva: a continuing challenge. Am J Obstet Gynecol 1980;136:830–43 | Paper waiting to be received |
| Frischbier HJ, Thomsen K, Schmermund HJ, Oberheuser F, Hohne G, Lohbeck HU. Radiotherapy of carcinoma of the vulva – treatment results of electron therapy in 446 patients 1956 to 1978. Geburtsh Frauenheilk 1985;45:1–5 | Sample recruited before 1980 |
| Gaarenstroom KN, Kenter GG, Trimbos JB, Agous I, Amant F, Peters AA, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Oncol 2003;13:22–7 | Wrong study population: only 69.7% of patients met inclusion criteria |
| Gadducci A, Prato B, Fanucchi A, Bonuccelli A, Cristofani R, Facchini V. Disease-free survival in patients with squamous cell carcinoma of the vulva treated with radical vulvectomy and bilateral inguinal-femoral lymphadenectomy: Analysis of prognostic variables. Cancer J 1993;6:269–73 | Wrong study population: only 73% of patients met inclusion criteria |
| Garcia I, Tejerizo L, Garcia S, Hernandez H, Velasco M, Lanchares P. Prognosis factors in cancer of the vulva. Eur J Gynaecol Oncol 1993;14:386–91 | Paper waiting to be received |
| Geisler JP, Manahan KJ, Buller RE. Neoadjuvant chemotherapy in vulval cancer: Avoiding primary exenteration. Gynecol Oncol 2006;100:53–7 | Inadequate data presentation: no information about patients’ FIGO stage, all patients presented as case report |
| Gerbaulet A, Sendra F, Gallez D, Pejovi-Lenfant MH, Haie-Meder C, Michel G, et al. The role of radiotherapy in vulval carcinoma. Experience in the Institute Gustave-Roussy and review of the literature. Oncologia 1991;14:567–73 | Paper waiting to be received |
| Gerszten K, Selvaraj RN, Kelley J, Faul C. Preoperative chemoradiation for locally advanced carcinoma of the vulva. Gynecol Oncol 2005;99:640–4 | Wrong study population: only 33% of patients met inclusion criteria |
| Ghebre R, Petzel SV, Glubka B, Lindgren B. Quality of life, body image and sexual health for women with vulval cancer. Gynecol Oncol 2009;112:S165 | Paper waiting to be received |
| Goetze B, Ebert A. Treatment results in cancer of the vulva – analysis of the 113 cases. Eur J Cancer 1995;31:S249 | Wrong study design: poster |
| Gomez ED, Trincado JM. Effects of chemotherapy on cancer of the vulva. Prog Obstet Ginecol 1987;30:729–34 | Paper waiting to be received |
| Gonzalez Bosquet J, Magrina J F, Magtibay PM, Gaffey TA, Cha SS, Jones MB, et al. Patterns of inguinal groin metastases in squamous cell carcinoma of the vulva. Gynecol Oncol 2007;105:742–6 | Wrong study design: analyses of risk factors |
| Gordinier ME, Malpica A, Burke TW, Bodurka DC, Wolf JK, Jhingran A, et al. Groin recurrence in patients with vulval cancer with negative nodes on superficial inguinal lymphadenectomy. Gynecol Oncol 2003;90: 625–8 | Wrong study design: small population, n < 10 |
| Gould N, Kamelle S, Tillmanns T, Scribner D, Gold M, Walker J, et al. Predictors of complications after inguinal lymphadenectomy. Gynecol Oncol 2001;82:329–32 | Wrong study population: included also patients with advance stage of disease |
| Green MS, Naumann R W, Elliot M, Hall JB, Higgins RV, Grigsby JH. Sexual dysfunction following vulvectomy. Gynecol Oncol 2000;77:73–7 | Wrong study population: 51% patients of patients had vulval cancer and dysplasia |
| Grimshaw RN, Murdoch JB, Monaghan JM. Radical vulvectomy and bilateral inguinal-femoral lymphadenectomy through separate incisions – experience with 100 cases. Int J Gynecol Cancer 1993;3:18–23 | Paper waiting to be received |
| Gutierrez AR, Rodriguez OA, Rodriguez CS, Chicote MJV, Carreras P S, Fresnadillo J L R, et al. Vulval carcinoma: study of 35 patients treated in our service. Neoplasia 1997;14:211–14 | Paper waiting to be received |
| Hacker NF, Berek JS, Lagasse LD. Individualization of treatment for stage I squamous cell vulval carcinoma. Obstet Gynecol 1984;63:155–62 | Wrong study population: patients with other types of cancer |
| Hacker NF, Leuchter RS, Berek JS, Castaldo TW, Lagasse LD. Radical vulvectomy and bilateral inguinal lymphadenectomy through separate groin incisions. Obstet Gynecol 1981;58:574–9 | Wrong study population: patients with other types of cancer |
| Hacker NF, van der Velden J. Conservative management of early vulval cancer. Cancer 1993;71:1673–7 | Wrong study design: review |
| Hacker NF. Current treatment of small vulval cancers 3495. Oncology 1990;4:21–5 | Paper waiting to be received |
| Hampl M, Hantschmann P, Michels W, Hillemanns P. Validation of the accuracy of the sentinel lymph node procedure in patients with vulval cancer: results of a multicenter study in Germany. Gynecol Oncol 2008;111:282–8 | Wrong study population: only 44.8% of patients met inclusion criteria |
| Han SC, Kim DH, Higgins SA, Carcangiu ML, Kacinski BM. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int J Radiat Oncol Biol Phys 2000;47:1235–44 | Wrong study population: patients with local advanced vulval tumour |
| Hanprasertpong J, Chichareon S, Wootipoom V, Buhachat R, Tocharoenvanich S, Geater A. Clinico-pathological profile of vulval cancer in Southern Thailand: analysis of 66 cases. J Med Assoc Thailand 2005;88:575–81 | Paper waiting to be received |
| Harberthur F, Almendral AC, Ritter B. Therapy of vulval carcinoma. Eur J Gynaecol Oncol 1993;14:218–27 | Paper waiting to be received |
| Hatta N, Yamada M, Hirano T, Fujimoto A, Morita R. Extramammary Paget’s disease: treatment, prognostic factors and outcome in 76 patients. Br J Dermatol 2008;158:313–18 | Wrong study population: 28% of patients were men |
| Hauspy J, Beiner M, Harley I, Ehrlich L, Rasty G, Covens A. Sentinel lymph node in vulval cancer. Cancer 2007;110:1015–23 | Wrong intervention: only diagnostic procedures |
| Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol 1990;38:309–14 | Wrong study design: prognostic factors associated with the increased risk of cancer recurrence |
| Hefler LA, Grimm C, Six L, Seebacher V, Polterauer S, Joura E, et al. Inguinal sentinel lymph node dissection vs. Complete inguinal lymph node dissection in patients with vulval cancer. Anticancer Res 2008;28:515–17 | Paper waiting to be received |
| Heidenreich W, Majewski A. Treatment and results in carcinoma of the vulva – a survey of 234 patients treated from 1951 to 1976. Geburtsh Frauenheilk 1986;46:136–9 | Inadequate data presentation: results presented in cumulative way for patients with primary and advanced disease |
| Helgason NM, Hass AC, Latourette HB. Radiation therapy in carcinoma of the vulva. A review of 53 patients. Cancer 1972;30:997–1000 | Paper waiting to be received |
| Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol 1972;87:138–44 | Wrong study design: wrong population, 125 intervention and study subjects |
| Hoffman JS, Kumar NB, Morley GW. Microinvasive squamous carcinoma of the vulva: search for a definition. ObstetGynecol 1983;61:615–18 | Paper waiting to be received |
| Hoffman MS, Roberts WS, Finan MA, Fiorica JV, Bryson SCP, Ruffolo EH, et al. A comparative study of radical vulvectomy and modified radical vulvectomy for the treatment of invasive squamous cell carcinoma of the vulva. Gynecol Oncol 1992;45:192–7 | Wrong study population: only 64% of patients met inclusion criteria |
| Homesley HD, Bundy BN, Sedlis A, Adcock L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol 1986;68:733–40 | Paper waiting to be received |
| Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Assessment of current International Federation of gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (A Gynecologic Oncology Group study). Am J Obstet Gynecol 1991;164:997–1004 | More than 25% FIGO stage III and IV |
| Hopkins MP, Reid GC, Vettrano I, Morley GW. Squamous cell carcinoma of the vulva: prognostic factors influencing survival. Gynecol Oncol 1991;43:113–17 | More than 25% FIGO stage III and IV |
| Hopkins MP, Reid GC, Morley GW. Radical vulvectomy, the decision for the incision. Cancer 1993;72:799–803 | Same study as above |
| Hruby G, MacLeod C, Firth I. Radiation treatment in recurrent squamous cell cancer of the vulva. Int J Radiat Oncol Biol Phys 2000;46:1193–7 | Wrong study population: patients with recurrent vulval cancer |
| Hunter DJS. Carcinoma of the vulva: a review of 361 patients. Gynecol Oncol 1975;3:117–23 | Wrong study design: lack of information about FIGO or TNM stage |
| Husseinzadeh N, Wesseler T, Schneider D, Schellhas H, Nahhas W. Prognostic factors and the significance of cytologic grading in invasive squamous cell carcinoma of the vulva: a clinicopathologic study. Gynecol Oncol 1990;36:192–9 | Wrong study design: risk factors evaluation |
| Iglesias AG, Lopez LCT, Sanchez MHG, Hernandez JH, Martin MJV, Perez JLL. Prognosis factors in cancer of the vulva. Eur J Gynaecol Oncol 1993;14:386–91 | Paper waiting to be received |
| Iversen T, Aalders JG, Christensen A, Kolstad P. Squamous cell carcinoma of the vulva: a review of 424 patients, 1956–1974. Gynecol Oncol 1980;9:271–9 | Wrong study population: only 5% of patients met inclusion criteria |
| Iversen T, Abelier V, Aalders J. Individualised treatment of stage I carcinoma of the vulva. Obstetr Gynecol 1981;57:85–9 | Sample recruited before 1980 |
| Iversen T, Abeler V, Kolstad P. Squamous cell carcinoma in situ of the vulva. A clinical and histopathological study. Gynecol Oncol 1981;11:224–9 | Wrong study population: 80% of patients had carcinoma in situ |
| Jacek JS and Janusz E. Characteristic features of recurrences of squamous cell carcinoma of the vulva. Ginekol Pol 2010;81:12–19 | Wrong study design: prognostic factors evaluation, not diagnostic accuracy |
| Janda M, Obermair A, Cella D, Crandon AJ, Trimmel M. Vulval cancer patients’ quality of life: A qualitative assessment. Int J Gynecol Cancer 2004;14:875–81 | Inadequate data presentation: all results were given for all patients enrolled in the study |
| Janda M, Obermair A, Cella D, Perrin LC, Nicklin JL, Ward BG, etal. The functional assessment of cancer-vulval: reliability and validity. Gynecol Oncol 2005;97:568–75 | Wrong study population: 77.6% patients with SCC and 85% with adequate vulval tumour |
| Jeppesen JT, Sell A, Skjoldborg H. Treatment of cancer of the vulva. Acta Obstet Gynecol Scand 1972;51:101–7 | Wrong study population: < 50% of patients met inclusion criteria |
| Johann S, Klaeser B, Krause T, Mueller MD. Comparison of outcome and recurrence-free survival after sentinel lymph node biopsy and lymphadenectomy in vulval cancer. Gynecol Oncol 2008;110:324–8 | Wrong intervention: diagnostic treatment |
| Jolicoeur M, Nguyen TV, David S, Devieux A, Goffin F, Gauthier P, et al. Conservative treatment for vulval cancer: chemoradiation and high dose rate brachytherapy. European Society for Therapeutic Radiology and Oncology Joint Brachytherapy Meeting. Barcelona, Spain; May 2004 | Wrong study design: poster |
| Judson PL, Jonson AL, Paley PJ, Bliss RL, Murray KP, Downs LS Jr, et al. A prospective, randomised study analyzing sartorius transposition following inguinal–femoral lymphadenectomy. Gynecol Oncol 2004;95:226–30 | Wrong study population: only 52.4% of patients met inclusion criteria |
| Kacerovska D, Nemcova J, Petrik R, Michal M, Kazakov DV. Lymphoepithelioma-like carcinoma of the Bartholin gland. Am J Dermatopathol 2008;30:586–9 | Wrong study design: case report |
| Kaltenbach FJ, Keil G. Autoradiographic and histologic observations on vulva carcinoma under local bleomycin treatment. Strahlentherapie 1979;75:185–90 | Paper waiting to be received |
| Kaya S, Grillo M, Gent HJ. Results of the various treatment methods in vulval cancer. Arch Gynecol Obstet 1991;250:127–9 | Wrong study population: included patients after secondary treatment |
| Keys H. Gynecologic Oncology Group randomised trials of combined technique therapy for vulval cancer. Cancer 1993;71:1691–6 | Wrong study population: 55% of patients met inclusion criteria |
| Khobjai A, Srisomboon J, Charoenkwan K, Phongnarisorn C, Suprasert P, Siriaree S, et al. Radical surgery for T1 and T2 squamous cell carcinoma of the vulva through separate incisions. J Med Assoc Thai 2005;88(Suppl. 2):75–81 | Paper waiting to be received |
| Kirby TO, Rocconi RP, Numnum TM, Kendrick JE, Wright J, Fowler W, et al. Outcomes of Stage I/II vulval cancer patients after negative superficial inguinal lymphadenectomy. Gynecol Oncol 2005;98:309–12 | Wrong study design: letter to authors |
| Knopp S, Holm R, Trope C, Nesland JM. Occult lymph node metastases in early stage vulval carcinoma patients. Gynecol Oncol 2005;99:383–7 | Wrong study design: lack of information about proportion between patient in stage I, II and III |
| Kodama S, Kaneko T, Saito M, Yoshiya N, Honma S, Tanaka KA. A clinicopathologic study of 30 patients with Paget’s disease of the vulva. Gynecol Oncol 1995;56:63–70 | Wrong study design: case report |
| Kohler U, Schone M, Pawlowitsch T. Results of an individualized surgical treatment of carcinoma of the vulva from 1973 to 1993. Zentralbl Gynakol 1997;119(Suppl. 1):8–16 | Inadequate data presentation: cumulative for all patients (in all stages) |
| Konefka T, Olszewski J, Makarewicz H, Emerich J. Analysis of intra- and postoperative complications and postoperative course in patients surgically treated for vulval cancer. Przeglad Lekarski 1999;56:100–3 | Inadequate data presentation and population: only 60% of patients met inclusion criteria |
| Kouvaris JR, Kouloulias VE, Kondi-Pahpiti A, Kokakis JD, Vlahos LJ. Impact of inguinal dissection on prognosis of early-stage squamous cell carcinoma of the vulva – a retrospective analysis 1768. Onkologie 2003;26:564–7 | Paper waiting to be received |
| Kouvaris JR, Kouloulias VE, Plataniotis GA, Balafouta EJ, Vlahos LJ. Dermatitis during radiation for vulval carcinoma: Prevention and treatment with granulocyte–macrophage colony-stimulating factor impregnated gauze. Wound Repair Regen 2001;9:187–93 | Wrong study population: 22.9% of patients met inclusion criteria |
| Kouvaris J, Kouloulias V, Loghis C, Sykiotis C, Balafouta M, Vlahos L. Prognostic factors for survival in invasive squamous cell vulval carcinoma: A univariate analysis. Gynecol Obstet Invest 2001;51:262–5 | Inadequate data presentation: information only about statistical analyses |
| Kraemer B, Guengoer E, Solomayer EF, Wallwiener D, Hornung R. Stage I carcinoma of the Bartholin’s gland managed with the detection of inguinal and pelvic sentinel lymph node. Gynecol Oncol 2009;114:373–4 | Wrong study design: case report |
| Kreienberg R, Beck T, Bartzke G, Henne M and Friedberg F. Results of surgical treatment of carcinoma of the vulva. Geburtsh Frauenheilk 1990;50:375–82 | Wrong study design and data presentation |
| Kubicki J, Samborska B, Lembrych S. Clinical analysis of 58 cases of vulvectomy. Ginekol Pol 1987;58:816–19 | Wrong study population: only 17.9% of patients had vulval cancer |
| Kucera H, Weghaupt K. Radical vulvectomy using warm knife and irradiation of the inguinal lymph nodes for invasive squamous cell carcinoma of the vulva. Geburtsh Frauenheilk 1986;46:595–600 | Wrong study population: only 58.2% of patients met the inclusion criteria |
| Kucera H. Treatment of carcinoma of the vulva at the 1st University Clinic of Gynecology in Vienna (386 cases). Strahlentherapie 1980;156:598–600 | Wrong study design: literature review |
| Kunos C, Simpkins F, Gibbons H, Tian C, Homesley H. Radiation therapy compared with pelvic node resection for node-positive vulval cancer: a randomised controlled trial. Obstet Gynecol 2009;114:537–46 | Wrong study population: only patients with groin nodes tumour |
| Kuppers V, Bender HG. Principles of surgical treatment of vulval cancer and precancerous lesions. Gynakologe 1993;26:293–7 | Wrong study design: case report |
| Kurzl R, Messerer D. Prognostic factors in squamous cell carcinoma of the vulva: a multivariate analysis. Gynecol Oncol 1989;32:143–50 | Wrong study design and unclear information: only information about node status was given without any others clinical descriptions |
| Lahousen M, Pickel H. About treatment of vulval carcinoma. Zentralbl Gynakol 1988;110:1001–5 | Wrong study population: 66% of patients met inclusion criteria |
| Lahousen M. Invasive vulvar cancer: treatment and results. Arch Gynecol Obstet 1989;245:517–23 | Same study as above |
| Landrum LM, Lanneau GS, Skaggs VJ, Gould N, Walker JL, McMeekin DS, et al. Gynecologic Oncology Group risk groups for vulval carcinoma: Improvement in survival in the modern era. Gynecol Oncol 2007;106:521–5 | Wrong study population and data presentation |
| Lanneau GS, Argenta PA, Lanneau MS, Riffenburgh RH, Gold MA, McMeekin DS, et al. Vulval cancer in young women: demographic features and outcome evaluation editorial comment. Obstet Gynecolo Surv 2009;64:661–2 | Wrong study design: analyses of risk factors |
| Lanneau GS, Argenta PA, Lanneau MS, Riffenburgh RH, Gold MA, McMeekin DS, et al. Vulval cancer in young women: demographic features and outcome evaluation. Am J Obstet Gynecol 2009;200:645.e1–5 | More than 25% patients FIGO stage III and IV |
| Lasser A, Cornog JL, Morris JM. Adenoid squamous cell carcinoma of the vulva. Cancer 1974;33:224–7 | Wrong study design: no information about FIGO stage, some of the patients had other tumour |
| Le T, Elsugi R, Hopkins L, Faught W, Fung-Kee-Fung M. The definition of optimal inguinal femoral nodal dissection in the management of vulva squamous cell carcinoma. Ann Surg Oncol 2007;14:2128–32 | Paper waiting to be received |
| Leminen A, Forss M, Paavonen J. Wound complications in patients with carcinoma of the vulva: Comparison between radical and modified vulvectomies. Eur J Obstet Gynecol Reprod Biol 2000;93:193–7 | Inadequate data presentation: all results were presented for all cumulative patients |
| Leuchter RS, Hacker NF, Voet RL, Berek JS, Townsend DE, Lagasse LD. Primary carcinoma of the Bartholin gland: a report of 14 cases and review of the literature. Obstet Gynecol 1982;60:361–8 | Wrong study design: case report |
| Levato F, Bianchi A, Lenzi B, Pansini F, Randazzo F, Ferretti S, Grandi E, Mollica G. Surgical treatment of invasive carcinoma of the vulva. A reappraisal. Eur J Gynaecol Oncol 1992;13:99–104 | Paper waiting to be received |
| Levenback CF, Tian C, Coleman RL, Gold MA, Fowler JM, Judson PL. Sentinel node (SN) biopsy in patients with vulval cancer: a Gynecologic Oncology Group (GOG) study. J Clin Oncol 2009;27:5505 | Paper waiting to be received |
| Levin AO, Carpenter KM, Fowler JM, Brothers BM, Andersen BL, Maxwell GL. Sexual morbidity associated with poorer psychological adjustment among 127 gynaecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70 | Wrong study population: only 11.6% of patients were after vulval carcinoma treatment |
| Lifshitz S, Savage JE, Yates SJ, Buchsbaum HJ. Primary epidermoid carcinoma of the vulva. Surg Gynecol Obstet 1982;155:59–61 | Wrong study population: lack of any clinical data |
| Lin JY, DuBeshter B, Angel C, Dvoretsky PM. Morbidity and recurrence with modifications of radical vulvectomy and groin dissection. Gynecol Oncol 1992;47:80–6 | More than 25% patients FIGO stage III and IV |
| Lobraico RV, Waldow SM, Harris DM, Shuber S. Photodynamic therapy for cancer of the lower female genital tract. Colposcopy Gynecol Laser Surg 1986;2:185–99 | Paper waiting to be received |
| Luo B. Treatment and analysis of 54 cases of vulval carcinoma. Chung-Hua Fu Chan Ko Tsa Chih [Chinese J Obstet Gynecol] 1990;25:156–8 | Paper waiting to be received |
| Magrina JF, Gonzalez-Bosquet J, Weaver AL, Gaffey TA, Leslie KO, Webb MJ, et al. Squamous cell carcinoma of the vulva stage IA: long-term results. Gynecol Oncol 2000;76t:24–7 | Wrong study population: 20% patients after primary treatment |
| Magrina JF, Gonzalez-Bosquet J, Weaver AL, Gaffey TA, Webb MJ, Podratz KC, et al. Primary squamous cell cancer of the vulva: radical versus modified radical vulvar surgery. Gynecol Oncol 1998;71:116–21 | More than 25% patients FIGO stage III and IV |
| Magrina JF, Webb MJ, Gaffey TA, Symmonds RE. Stage I squamous cell cancer of the vulva. Am J Obstet Gynecol 1979;134:453–9 | Wrong study population: patients with other malignancies |
| Mak RH, Halasz LM, Tanaka CK, Ancukiewicz M, Schultz DJ, Russell AH, et al. Outcomes after radiation therapy with concurrent weekly platinum-based chemotherapy or every-3–4-week 5-fluorouracil-containing regimens for squamous cell carcinoma of the vulva. Gynecol Oncol 2011;120:101–7 | Wrong study population: 73% of patients with advanced disease |
| Makinem J, Salmi T, Gronroos M. Individually modified treatment of invasive squamous cell vulvar cancer: 10 year experience. Ann Chirurg Gynaecol 1987;76 (Suppl. 202):68–71 | More than 25% patients FIGO stage III and IV |
| Malfetano JH, Piver MS, Tsukada Y, Reese P. Univariate and multivariate analyses of 5-year survival, recurrence, and inguinal node metastases in stage I and II vulval carcinoma. J Surg Oncol 1985;30:124–31 | Paper waiting to be received |
| Malmstrom H, Janson H, Simonsen E, Stenson S, Stendahl U. Prognostic factors in invasive squamous cell carcinoma of the vulva treated with surgery and irradiation. Acta Oncol 1990;29:915–19 | Wrong study design: no data which could be extracted |
| Manci N, Marchetti C, Esposito F, De Falco C, Bellati F, Giorgini M, et al. Inguinofemoral lymphadenectomy: Randomised trial comparing inguinal skin access above or below the inguinal ligament. Ann Surg Oncol 2009;16:721–8 | Paper waiting to be received |
| Manci N, Marchetti C, Esposito F, et al. Inguinofemoral lymphadenectomy: randomised trial comparing inguinal skin access above or below the inguinal ligament. Ann Surg Oncol. 2009;16:721–8 | Wrong study population: included patients with advanced disease |
| Maricic Z, Kolaric K, Krusic J. Our experience in the treatment of carcinoma of the vulva. Strahlentherapie 1976;151:495–503 | Wrong study design and population: only 42% of patients met the inclusion criteria |
| Martinez-Palones JM, Perez-Benavente MA, Gil-Moreno A, Diaz-Feijoo B, Roca I, Garcia-Jimenez A, et al. Comparison of recurrence after vulvectomy and lymphadenectomy with and without sentinel node biopsy in early stage vulval cancer. Gynecol Oncol 2006;103: 865–70 | Wrong intervention: only diagnostic treatment |
| Marzetti L, Framarino d, Tagliaferri T, Khosravi L, Paolillo MF. Carcinoma of the vulva: our experience. Gior Ital Oncol 1989;9:35–8 | Paper waiting to be received |
| Matkowski R, Dryl J, Kornafel J. Analysis of treatment results of vulval cancer. Ginekol Pols 2004;75:720–8 | Inadequate data presentation: results were presented in a cumulative way for all FIGO stages |
| Meriggi G. Primary carcinoma of the vulva. Clinical study. Quad Clin Ostet Ginecol 1972;27:253–60 | Paper waiting to be received |
| Micheletti L, Borgno G, Barbero M, Preti M, Cavanna L, Nicolaci P, et al. Deep femoral lymphadenectomy with preservation of the fascia lata: Preliminary report on 42 invasive vulval carcinomas. J Reprod Med Obstet Gynecol 1990;35:1130–3 | Paper waiting to be received |
| Miecznikowski A, Starzewski J. Surgical treatment of vulval cancer. Eur J Gynaecol Oncol 1993;14:392–7 | Paper waiting to be received |
| Miyazawa K, Nori D, Hilaris BS, Lewis J. Role of radiation therapy in the treatment of advanced vulval carcinoma. J Reprod Med Obstet Gynecol 1983;28:539–41 | Paper waiting to be received |
| Mohr A, Rieken S, Hof H, Bischof M, Combs SE, Debus J, et al. Nodal state determines outcome in vulval cancer. Int J Radiat Oncol Biol Phys 2010;78:S418 | Paper waiting to be received |
| Molinie V, Paniel BJ, Lessana-Leibowitch M, Moyal-Barracco M, Pelisse M, Escande JP. Paget’s disease of the vulva. Ann Dermatol Venereol 1993;120:522–7 | Wrong study design and data presentation |
| Montana GS, Thomas GM, Moore DH, Saxer A, Mangan CE, Lentz SS, et al. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynaecologic oncology group study. Int J Radiat Oncol Biol Phys 2000;48:1007–13 | Wrong study population: patients with advanced disease |
| Montemaggi P, Luzi S, Morganti AG, Smaniotto D. Combined ERT and IRT in carcinoma of the vulva. Rays Int J Radiol Sci 1991;16:48–52 | Paper waiting to be received |
| Moore DH. Chemotherapy and radiation therapy in the treatment of squamous cell carcinoma of the vulva: are two therapies better than one? Gynecol Oncol 2009;113:379–83 | Wrong study design: review |
| Moore RG, Robison K, Brown AK, DiSilvestro P, Steinhoff M, Noto R, et al. Isolated sentinel lymph node dissection with conservative management in patients with squamous cell carcinoma of the vulva: a prospective trial. Gynecol Oncol 2008;109:65–70 | Wrong study design |
| Morris WIC. Experiences with vulval cancer. J R Coll Surg Edinburgh 1973;18:327–40 | Paper waiting to be received |
| Moth I, Andreasson B, Jensen SB, Bock JE. Sexual function and somatopsychic reactions after vulvectomy. A preliminary report. Danish Medical Bulletin 1983;30(Suppl. 2):27–30 | Wrong study design: no information about FIGO or TNM stage |
| Mulayim N, Silver DF, Schwartz PE, Higgins S. Chemoradiation with 5-fluorouracil and mitomycin C in the treatment of vulval squamous cell carcinoma. Gynecol Oncol 2004;93:659–66 | Wrong study population: patients with advanced disease |
| Muller RP, Fischedick AR, Schnepper E. Clinical symptoms and high-voltage therapy (electron therapy) of the vulval carcinoma. Strahlentherapie 1982;158:594–7 | Wrong study population: only 66% of patients met inclusion criteria |
| Nahhas WA and Brown M. Gynecologic surgery in the aged. J Reprod Med Obstet Gynecol 1990;35:550–4 | Paper waiting to be received |
| Narendra H, Ray S, Rao L, Geetha V. Malignant extrarenal rhabdoid tumour of the vulva in an adult. J Cancer Res Ther 2010;6:82–5 | Wrong study design: case report |
| Newcomb PA, Weiss NS, Daling JR. Incidence of vulval carcinoma in relation to menstrual, reproductive, and medical factors. J Natl Cancer Inst 1984;73:391–6 | Paper waiting to be received |
| Nicoletto MO, Parenti A, Bianco PD, Lombardi G, Pedrini L, Pizzi S et al. Vulvar cancer: prognostic factors. Anitcancer Res 2010;30:2311–8 | More than 25% patients FIGO stage III and IV |
| Nyberg RH, Iivonen M, Parkkinen J, Kuoppala T, Maenpaa JU. Sentinel node and vulval cancer: A series of 47 patients. Acta Obstet Gynecol Scand 2007;86:615–19 | Wrong intervention: diagnostic treatment |
| Ofodile FA, Oluwasanmi JO. Post-circumcision epidermoid inclusion cysts of the clitoris. Plast Reconstructive Surg 1979;63:485–6 | Paper waiting to be received |
| Onnis A, Marchetti M, Valente S: Surgical management of invasive vulval carcinoma. A new operative technique ‘non mutilant radical vulvectomy’. Eur J Gynaecol Oncol 1980;1:45–51 | Paper waiting to be received |
| Oonk MHM, van de Nieuwenhof HP, de Hullu JA, Van der Zee AGJ. The role of sentinel node biopsy in gynecological cancer: A review. Curr Opin Oncol 2009;21:425–32 | Wrong study design: diagnostic review |
| Oonk MHM, van Os MA, de Bock GH, de Hullu JA, Ansink AC, Van der Zee AGJ. A comparison of quality of life between vulval cancer patients after sentinel lymph node procedure only and inguinofemoral lymphadenectomy. Gynecol Oncol 2009;113:301–5 | Wrong study design: diagnostic study |
| Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulval cancer: Results from GROINSS-V, a multicentre observational study. Lancet Oncol 2010;11:646–52 | Wrong study design: diagnostic study |
| Origoni M, Dindelli M, Ferrari D, Frigerio L, Rossi M, Ferrari A. Surgical staging of invasive squamous cell carcinoma of the vulva. Analysis of treatment and survival. Int Surg 1996;81:67–70 | Paper waiting to be received |
| Paley PJ, Johnson PR, Adcock LL, Cosin JA, Chen MD, Fowler JM et al. The effect of sartorius transposition on wound morbidity following inguinal–femoral lymphadenectomy. Gynecol Oncol 1997;64:237–41 | Wrong study design: no information about patients’ clinical stage |
| Palli C, Lluch J, Valero S. Sexuality, communication and emotions: a situational study in women affected by gynecologic cancer. Psicooncologia 2010;7:153–73 | Paper waiting to be received |
| Papalas J, Selim M, Lewin M. Granular cell tumour of the VUVLA: A clinicopathologic study of 16 lesions. J Cutaneous Pathol 2009;36:112 | Wrong study population: patients with granular and Schwann cell tumours |
| Parker RT, Duncan I, Rampone J, Creasman W. Operative management of early invasive epidermoid carcinoma of the vulva. Am J Obstet Gynecol 1975;123:349–55 | Wrong study population and study design: 22% of patients had recurrent disease |
| Parmley TH, Woodruff JD, Julian CG. Invasive vulval Paget’s disease. Obstet Gynecol 1975;46:341–6 | Wrong study design and population: only seven patients had invasive vulval Paget's disease, other cases had intraepithelial changes |
| Parthasarathy A, Cheung MK, Osann K, Husain A, Teng NN, Berek JS, et al. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulval carcinoma. Gynecol Oncol 2006;103:1095–9 | Wrong study population: all patients had FIGO stage III disease |
| Patsner B, Mann J. Radical vulvectomy and ‘sneak’ superficial inguinal lymphadenectomy with a single elliptic incision. Am J Obstet Gynecol 1988;158:464–9 | More than 25% patients FIGO stage III and IV |
| Pellegrino A, Fruscio R, Maneo A, Corso S, Battistello M, Chiappa V, et al. Harmonic scalpel versus conventional electrosurgery in the treatment of vulval cancer. Int J Gynecol Obstet 2008;103:185–8 | Inadequate data presentation: all results were presented for all cumulative patients |
| Pengsaa P, Pothinam S, Udomthavornsuk B. Vulval carcinoma at Srinagarind Hospital, Khon Kaen, Thailand. Eur J Gynaecol Oncol 1993;14:56–62 | Paper waiting to be received |
| Perez CA, Grigsby PW, Chao C, Galakatos A, Garipagaoglu M, Mutch D, et al. Irradiation in carcinoma of the vulva: factors affecting outcome. Int J Radiat Oncol Biol Phys 1998;42:335–44 | More than 25% patients FIGO stage III and IV |
| Pescetto G. Criteria for the type of treatment of the vulval carcinoma. Eur J Gynaecol Oncol 1980;1:32–6 | Paper waiting to be received |
| Petereit DG, Mehta MP, Buchler DA and Kinsella TJ. A retrospective review of nodal treatment for vulval cancer. Am J Clin Oncol Cancer Clin Trials 1993;16:38–42 | Paper waiting to be received |
| Petereit DG, Mehta MP, Buchler DA, Kinsella TJ. Inguinofemoral radiation of N0,N1 vulval cancer may be equivalent to lymphadenectomy if proper radiation technique is used. Int J Radiat Oncol Biol Phys 1993;27:963–7 | Paper waiting to be received |
| Petru E. Changes of the FIGO staging of gynecologic malignancies 2009. Geburtsh Frauenheilk 2010;70:269–72 | Wrong study design: literature review |
| Petzel S, Gebre R, Austin S. Women’s health-related quality of life and sexual health following diagnosis and surgical treatment for vulval cancer and vulva dysplasia. Psycho-Oncology 2007;16:S1–110 | Paper waiting to be received |
| Pierson RL, Figge PK, Buchsbaum HJ. Surgery for gynecologic malignancy in the aged. Obstet Gynecol 1975;46:523–7 | Wrong study population: only 66% of patients met inclusion criteria |
| Pirtoli L, Rottoli ML. Results of radiation therapy for vulval carcinoma. Acta Radiol Ser Oncol Radiat Ther Phys Biol 1982;21:45–8 | Wrong study population and study design: poor information about patients’ clinical stage, only few of them were described |
| Piura B, Glezerman M. Cancer of the vulva in South Israel. A clinico-pathologic study of 28 cases. Cervix Lower Female Genital Tract 1989;7:21–8 | Paper waiting to be received |
| Pliskow S. Vulval Paget’s disease. Clinicopathological review of 14 cases. J Florida Med Assoc 1990;77:667–71 | Paper waiting to be received |
| Podratz KC, Symmonds RE, Taylor WF, Williams TJ. Carcinoma of the vulva: analysis of treatment and survival. Obstet Gynecol 1983;61:63–74 | Inadequate data presentation: results presented cumulative way for II, III and IV FIGO stage |
| Puig-Tintore LM, Ordi J, Vidal-Sicart S, Lejarcegui J A, Torne A, Pahisa J, et al. Further data on the usefulness of sentinel lymph node identification and ultrastaging in vulval squamous cell carcinoma. Gynecol Oncol 2003;88:29–34 | Wrong study design: diagnostic study |
| Rakar S. Surgical treatment of vulval cancer. Eur J Gynaecol Oncol 1992;13:319–21 | Paper waiting to be received |
| Raspagliesi F, Hanozet F, Ditto A, Solima E, Zanaboni F, Vecchione F, et al. Clinical and pathological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Oncol 2006;102:333–7 | Wrong study design: no information about patients’ FIGO stage disease |
| Ratner ES, Foran KA, Schwartz PE, Minkin MJ. Sexuality and intimacy after gynecological cancer. Maturitas 2010;66:23–6 | Paper waiting to be received |
| Redman M, Alheit HD, Winkler C, Herrmann T. Results in radiotherapy of vulvar carcinoma. Radiobiol Radiother 1986;27:189–95 | More than 25% patients FIGO stage III and IV |
| Reid GC, DeLancey JOL, Hopkins MP, Roberts JA, Morley GW. Urinary incontinence following radical vulvectomy. Obstet Gynecol 1990;75:852–8 | Inadequate data presentation: all results were given for all study group, unclear group description |
| Rhodes C A, Cummins C, Shafi MI. The management of squamous cell vulval cancer: a population based retrospective study of 411 cases. Br J Obstet Gynaecol 1998;105:200–5 | Wrong study population: 8% of patients had multifocal tumours |
| Roberts WS, Kavanagh JJ, Greenberg H, Bryson SC, LaPolla JP, Townsend PA, et al. Concomitant radiation therapy and chemotherapy in the treatment of advanced squamous carcinoma of the lower female genital tract. Gynecol Oncol 1989;34:183–6 | Wrong study population: most patients had advanced disease |
| Robison K, Steinhoff MM, Granai CO, Brard L, Gajewski W, Moore RG. Inguinal sentinel node dissection versus standard inguinal node dissection in patients with vulval cancer: A comparison of the size of metastasis detected in inguinal lymph nodes. Gynecol Oncol 2006;101:24–7 | Wrong study design: diagnostic study |
| Rodolakis A, Diakomanolis E, Voulgaris Z, Akrivos T, Vlachos G, Michalas S. Squamous vulvar cancer: a clinically based individualisation of treatment. Gynecol Oncol 2000;78:346–51 | More than 25% patients FIGO stage III and IV |
| Rogers L, Howard B, Van Wijk L, Wei W, Dehaeck K, Soeters R, et al. Chemoradiation in advanced vulval carcinoma. Int J Gynecol Obstet 2009;107:S323 | Inadequate data presentation: no possibilities to extract any data |
| Rosen C, Malmstrom H. Invasive cancer of the vulva. Gynecol Oncol 1997;65:213–17 | Wrong study population: only 59% of patients met inclusion criteria |
| Rouzier R, Haddad B, Dubernard G, Dubois P, Paniel BJ. Inguinofemoral dissection for carcinoma of the vulva: Effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg 2003;196:442–50 | Wrong study design: no information for effectiveness |
| Rowley KC, Gallion HH, Donaldson ES, Van Nagell JR, Higgins RV, Powell DE, et al. Prognostic factors in early vulval cancer. Gynecol Oncol 1988;31:43–9 | Wrong study design: no information about patients’ FIGO stage of disease |
| Scheistroen M, Trope C. Combined bleomycin and irradiation in preoperative treatment of advanced squamous cell carcinoma of the vulva. Acta Oncol 1993;32:657–61 | Wrong study population: only women with advanced disease |
| Schmeisser G, Krafft W, Schirmer A. Efficacy of supplementary treatment with bleomycin in carcinoma of the vulva. Zentralbl Gynakol 1979;101:350–3 | Wrong study design: population was described in an unclear way |
| Schmidt W, Schmid H, Villena-Heinsen C, Kuhn W, Jochum-Merger N, Von Fournier D. Treatment results in malignant tumours of the vulva 1970–1990. Geburtsh Frauenheilk 1992;52:749–57 | Wrong study population: < 70% of patients met inclusion criteria |
| Schnurch HG. Vulval cancer. diagnosis and treatment. Urologe A 2004;43:849–59 | Wrong study design: review |
| Sedlis A, Homesley H, Bundy BN, Marshall R, Yordan E, Hacker N, et al. Positive groin lymph nodes in superficial squamous cell vulval cancer. Am J Obstet Gynecol 1987;156:1159–64 | Wrong study population: 57.4% of patients met the inclusion criteria |
| Sengupta BS. Vulvar carcinoma in premenopausal Jamaican women. Int J Gynaecol Obstet 1980;17:526–30 | More than 25% patients FIGO stage III and IV |
| Senn B, Mueller MD, Cignacco EL, Eicher M. Period prevalence and risk factors for postoperative short-term wound complications in vulval cancer: a cross-sectional study 55. Int J Gynecol Cancer 2010;20:646–54 | Wrong study population: only 36.1% of patients met the inclusion criteria |
| Sesti F, Santeusanio G, Anemona L, Schiaroli S, Farne C, Piccione E. Verrucous carcinoma of the vulva. J Obstet Gynaecol 1991;11:79–80 | Wrong study design: case report |
| Shepherd JH, Crowther ME. Complications of gynaecological cancer surgery: a review. J R Soc Med 1986;79:289–93 | Paper waiting to be received |
| Sherman KJ, Daling JR, Chu J, Weiss NS, Ashley RL, Corey L. Genital warts, other sexually transmitted diseases, and vulval cancer. Epidemiology 1991;2:257–62 | Paper waiting to be received |
| Shimm DS, Fuller AF, Orlow EL. Prognostic variables in the treatment of squamous cell carcinoma of the vulva. Gynecol Oncol 1986;24:343–58 | Wrong study population: 66% patients met inclusion criteria, 86% had primary disease |
| Simonsen E, Johnsson JE and Trope C. Radical vulvectomy with warm-knife and open-wound techniques in vulval malignancies. Gynecol Oncol 1984;17:22–31 | Wrong study population: only 60% of patients met inclusion criteria |
| Simonsen E, Johnsson JE, Trope C. Stage I squamous cell carcinoma of the vulva. Acta Radiol Oncol 1984;23:443–8 | Wrong study population: also included patients with other malignancies |
| Simonsen E, Nordberg UB, Johnsson JE. Radiation therapy and surgery in the treatment of regional lymph nodes in squamous cell carcinoma of the vulva. Acta Radiol Oncol 1984;23:433–42 | Wrong study population: only 65% of patients met inclusion criteria |
| Slevin NJ, Pointon RCS. Radical radiotherapy for carcinoma of the vulva. Br J Radiol 1989;62:145–7 | Wrong study population: e.g. patients after primary treatment |
| Smith AM and Rauld HF. Radical vulvectomy and lymphadenectomy in the management of vulval cancer: The experience of a district general hospital. J Obstet Gynaecol 1985;6:57–61 | Paper waiting to be received |
| SmyczekGargya B, Volz B, Geppert M and Dietl J. A multivariate analysis of clinical and morphological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Obstet Invest 1997;43:261–7 | Wrong study population: only 53% of patients met the inclusion criteria |
| Song YN, Yang JX, Shen K, Huang HF, Pan LY. Clinical features of recurrence of vulval squamous cell carcinoma: Analysis of 18 cases. Nat Med J China 2008;88:1347–9 | Wrong study design and data presentation |
| Stegner HE. Ultrastructure of preneoplastic lesions of the vulva. J Reprod Med 1986;31:815–20 | Paper waiting to be received |
| Stehman FB, Bundy B N, Ball H, Clarke-Pearson DL, Lagasse LD. Sites of failure and times to failure in carcinoma of the vulva treated conservatively: A Gynecologic Oncology Group study. Am J Obstet Gynecol 1996;174:1128–33 | Inadequate data presentation: mixed for all stages |
| Stellman RE, Goodwin JM, Robinson J, Dansak D, Hilgers RD. Psychological effects of vulvectomy. Psychosomatics 1984;25:779–83 | Wrong study design: no information about FIGO stage |
| Stroup AM, Harlan LC, Trimble EL. Demographic, clinical, and treatment trends among women diagnosed with vulval cancer in the United States. Gynecol Oncol 2008;108:577–83 | Inadequate data presentation: unclear information about treatment and clinical patients’ stage |
| Sugawa T, Hashimoto M, Suzuki M. Clinical aspects and treatment of vulval cancer in Japan. Acta Obstet Gynaecol Jpn 1980;32:177–86 | Paper waiting to be received |
| Suzuki M, Watanabe M, Sato A. Effect of bleomycin on 131 gynaecologic 1311 carcinoma. Gann Monogr Cancer Res 1976;19:221–30 | Paper waiting to be received |
| Tamburini M, Filiberti A, Ventafridda V, De Palo G. Quality of life and psychological state after radical vulvectomy. J Psychosom Obstet Gynecol 1986;5:263–9 | Paper waiting to be received |
| Tatra G, Caucig H. Therapeutic results in primary carcinoma of the vulva. Wien Klin Wochenschr 1973;85:429–32 | Inadequate data presentation: no clear information about treatments and survival results, mixed data for patients with FIGO I, II and III |
| Tebes S, Cardosi R, Hoffman M. Paget’s disease of the vulva. Am J Obstet Gynecol 2002;187:281–4 | Wrong study design: lack of clinical information about patients disease stage |
| ten Bokkel Huinik WW. Chemotherapy and complications in gynaecologic cancer. Curr Options Gynecol 1991;3:930–2 | Wrong study design: review |
| Terada KY, Shimizu DM, Jiang CS, Wong JH. Outcomes for patients with T1 squamous cell cancer of the vulva undergoing sentinel node biopsy. Gynecol Oncol 2006;102:200–3 | Paper waiting to be received |
| Thomas G, Dembo A, DePetrillo A, Pringle J, Ackerman I, Bryson P, et al. Concurrent radiation and chemotherapy in vulval carcinoma. Gynecol Oncol 1989;34:263–7 | Wrong study population: 60% patients with recurrent disease |
| Tombolini V, Raffetto N, Santarelli M, Valerianai M, Necozione S, Masedu F, et al. Carcinoma of the vulva: clinical results of exclusive and adjuvant radiotherapy. Anticancer Res 2005;25:3089–94 | More than 25% patients FIGO stage III and IV |
| Trelford JD, Deer DA and Ordorica E. Ten-year prospective study in a management change of vulval carcinoma. Am J Obstet Gynecol 1984;150:288–96 | Wrong study design: diagnostic review |
| Van de Wiel HBM, Weijmar Schultz WCM, Hallensleben A, Thurkow FG, Bouma J, Verhoeven AC. Sexual functioning of women treated for cancer of the vulva. Sex Marit Ther 1990;5:73–82 | Paper waiting to be received |
| van der Velden J, Ansink AC. Re: ‘Outcomes of stage I/II vulval cancer patients after negative superficial inguinal lymphadenectomy’. Gynecol Oncol 2006;100:219–20 | Wrong study design: letter to authors |
| Van der Zee AGJ, Oonk MH, de Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. Sentinel node dissection is safe in the treatment of early-stage vulval cancer. J Clin Oncol 2008;26:884–9 | Wrong study design: diagnostic study |
| Vavra N, Kucera H, Weghaupt K. Evaluation of different risk factors in the follow-up of stage I vulval carcinoma and their influence on the results of therapy. Wien Klin Wochenschr 1990;102:289–94 | Inadequate data presentation: all results presented in cumulative way for all stages |
| Vidal-Sicart S, Puig-Tintore LM, Lejarcegui JA, Paredes P, Ortega ML, Munoz A, et al. Validation and application of the sentinel lymph node concept in malignant vulval tumours. Eur J Nucl Med Mol Imaging 2007;34:384–91 | Wrong intervention/study design: diagnostic only |
| Volk M, Schmidt Matthiesen H. Treatment of vulval cancer. Arch Gynakol 1977;223:145–62 | Wrong study design: unclear information about treatment and patients’ clinical stage |
| Wagner W, Prott FJ, Weissmann J, Niewohner-Desbordes U, Ostkamp K, Alfrink M. Vulval carcinoma: a retrospective analysis of 80 patients. Arch Gynecol Obstet 1999;262:99–104 | Inadequate data presentation: data format did not allow reader to obtain meaningful information |
| Weghaupt K. Electro-resection and -coagulation in the therapy of vulva carcinoma. Fortschr Med 1978;96:1629–34 | Wrong study design: review |
| Weghaupt K. Therapy of vulval carcinoma and its results. Arch Gynakol 1974;216:151–66 | Wrong study design: review |
| Weijmar S, van d, Bouma J, Janssens J, Littlewood J. Psychosexual functioning after the treatment of cancer of the vulva. A longitudinal study. Cancer 1990;66:402–7 | Paper waiting to be received |
| Weijmar Schultz WCM, Wijma K, Van de Wiel HBM. Sexual rehabilitation of radical vulvectomy patients. A pilot study. J Psychosom Obstet Gynecol 1986;5:119–26 | Wrong study design: small study group (n = 10), no information about FIGO sage |
| Wharton JT, Gallager S, Rutledge FN. Microinvasive carcinoma of the vulva. Am J Obstet Gynecol 1974;118:159–62 | Wrong study design/unclear information: lack of information about patients’ FIGO stage of disease |
| Weijmar Shultz WC, van der Wiel HB, Bouma J, Janssens J, Littlewood J. Psychosexual functioning after the treatment of cancer of the vulva. Cancer 1990;66:402–7 | Sample too small |
| Wolber L, Mahner S, Eulenburg C, Choschzick M, Hager M, Kock L, et al. Relevance of the number of metastatic lymph-nodes for disease recurrence in vulval cancer. Arch Gynecol Obstet 2010;282:S194 | Paper waiting to be received |
| Wydra D, Emerich J, Ciach K, Sawicki S, Marciniak A. The role of pelvic exenteration for treatment of pelvic malignancy – a nine-year experience. Eur J Gynaecol Oncol 2005;26:418–22 | Wrong study design: wrong study question |
| Wydra D, Matuszewski R, Romanowicz G, Bandurski T. Evaluation of surgical gamma probes for sentinel node localisation in cervical and vulval cancer. Nucl Med Rev 2005;8:105–10 | Paper waiting to be received |
| Yamashiro T, Hasumi K, Masubuchi K. Clinical study of 84 primary vulval carcinomas. Jpn J Cancer Chemother 1989;16:1677–82 | Paper waiting to be received |
| Yamashiro T, Teshima H, Yokosuka K, Shimizu Y, Hirai Y, Chen JT, et al. Clinical study of the vulval Paget’s disease: Report of 8 cases. J Jpn Soc Cancer Ther 1989;24:2563–8 | Paper waiting to be received |
| Yoder BJ, Rufforny I, Massoll NA, Wilkinson EJ. Stage IA vulval squamous cell carcinoma: An analysis of tumour invasive characteristics and risk. Am J Surg Pathol 2008;32:765–72 | Wrong study design: no information about treatment and effectiveness |
| Yoonessi M, Goodell T, Satchidanand S. Microinvasive squamous carcinoma of the vulva. J Surg Oncol 1983;24:315–21 | Wrong study design: unclear information about patients’ stage, intervention |
| Zerner J. Basal cell carcinoma of the vulva. A report of six cases and review of the literature. J Maine Med Assoc 1974;65:127–9 | Paper waiting to be received |
| Zhang SH, Sood AK, Sorosky JI, Anderson B, Buller RE. Preservation of the saphenous vein during inguinal lymphadenectomy decreases morbidity in patients with carcinoma of the vulva. Cancer 2000;89:1520–5 | Wrong study design: inadequate study question |
Appendix 12 Additional data from effectiveness systematic review
| Study | Intervention | Time of AE | AEs: n (%) | ||||
|---|---|---|---|---|---|---|---|
| Helm et al., 199237 | Single incision | Early surgical complication/RT Reaction |
Wound breakdown/rupture: 6 (19)a Groin breakdown: 11 (34)b Cellulitis: 5 (16) Seroma: 6 (19) Major complication: 2 (6)c |
||||
| Triple incision | Early surgical complication/RT Reaction |
Wound breakdown/rupture: 2 (6)a Groin breakdown: 6 (19)b Cellulitis: 7 (22) Seroma: 10 (31) Major complication: 1 (3)c |
|||||
| Katz et al., 200390 | Without IFL | Late surgical complication/RT Reaction |
Major complication: 2 (1)d | Lymphocyst or lymphoedema: 6 (5)d Skeletal complications: 2 (2)e |
|||
| With IFL and RT | Lymphocyst or lymphoedema: 4 (7)e Skeletal complications: 3 (5)f |
||||||
| RT | Skeletal complications: 8 (16)f | ||||||
| Hallak et al., 200798 | All interventions | Total number of patients who experienced an AE | 64 (21.8) | ||||
| After IFL | Early surgical complications | Wound breakdown/rupture: 5 (19); wound infection: 7 (26); bleeding: 2 (7); thrombosis: 0 (0); none: 15 (56) | |||||
| Late surgical complications | Lymphoedema: 1 (7); none: 20 (91); erysipelas: 0 (0); vaginal stenosis: 0 (0); other: 1 (5) | ||||||
| Without IFL | Early surgical complications | Wound breakdown/rupture: 11 (6); wound infection: 10 (5); bleeding: 4 (2), thrombosis: 2 (1); none: 141 (77) | |||||
| Late surgical complications | Lymphoedema: 0 (0); none: 161 (96); erysipelas: 1 (1); vaginal stenosis: 2 (1); other: 3 (2) | ||||||
| Vavra et al., 1990102 | All interventions | Total | 14 (13.8) | ||||
| Early surgical complications | Wound breakdown/rupture: 2 (2)g; postoperative urinary incontinence: 4 (4)g; major complication: 2 (2)g | ||||||
| Late surgicalcomplications | Lymphoedema: 1 (1)g; vaginal stenosis: 4 (4)g | ||||||
| Study | Timing of AEs | AEs: n (%) |
|---|---|---|
| Hallak et al., 200798 | Early complications | Acute erythema: 118 (51) Wet dermatitis: 72 (31) Cutaneous infection: 6 (3) Other (diarrhoea, urinary tract infection): 8 (4) |
| Late complications | Cutaneous pigmentation: 87 (38) Fibrosis (inguinal region): 63 (27) Telangiectasia: 17 (7) Lymphoedema: 16 (7)a Thrombosis: 2 (1) Other: 9 (4) |
List of abbreviations
- 99mTc
- technetium-99m
- AE
- adverse event
- AIDS
- acquired immune deficiency syndrome
- CDSR
- Cochrane Database of Systematic Reviews
- CEAF
- cost-effectiveness acceptability frontier
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CK
- cytokine
- CKMNF
- cytokine myocyte nuclear factor
- DARE
- Database of Abstracts of Reviews of Effects
- DN
- disease negative
- DNA
- deoxyribonucleic acid
- DP
- disease positive
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30
- FACT-V
- Functional Assessment of Cancer Therapy – Vulvar
- FIGO
- International Federation of Gynecology and Obstetrics
- FN
- false-negative
- FP
- false-positive
- GROINSS-V
- GROningen INternational Study on Sentinel nodes in Vulvar cancer
- H&E
- haematoxylin and eosin stain
- HIV
- human immunodeficiency virus
- HMB
- human melanoma black monoclonal antibody
- HPV
- human papillomavirus
- HTA
- health technology assessment
- ICER
- incremental cost-effectiveness ratio
- IFL
- inguinofemoral lymphadenectomy
- IQR
- interquartile range
- LR
- likelihood ratio
- MEDION
- medical diagnostic studies database
- MeSH
- medical subject heading
- MM
- malignant melanoma
- OMNI
- Organizing Medical Networked Information
- PET-CT
- positron emission tomography, computed tomography
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QUADAS
- quality of diagnostic accuracy studies
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- RR
- relative risk
- RT
- radiotherapy
- RT-PCR
- reverse transcription-polymerase chain reaction
- SCC
- squamous cell carcinoma
- SD
- standard deviation
- SEER
- US Surveillance, Epidemiology and End Results
- SLN
- sentinel lymph node
- TN
- true-negative
- TNM
- tumour node metastasis
- TP
- true-positive
- UKGOSOC
- United Kingdom Gynaecological Oncology Surgical Outcomes
- VIN
- vulval intraepithelial neoplasia
- WHO
- World Health Organization
- WTP
- willingness to pay